User login
22nd European Stroke Conference
Poststroke prevention strategies cut dementia risk in half
LONDON – Cognitive dysfunction following a stroke could be reduced significantly if treatment with antihypertensive, antithrombotic, and lipid-lowering drugs were optimized, according to a study looking at the long-term impact of secondary prevention strategies.
The relative risk for cognitive dysfunction was 0.8 with the optimal use of anticoagulants, and 0.9, 0.9, and 0.9 with the optimal use of dual antihypertensive therapy, dual antiplatelet therapy, and lipid-lowering treatment, respectively.
"The combination of antihypertensives, antithrombotics, and lipid-lowering drugs reduced the risk of cognitive impairment by about half," Abdel Douiri, Ph.D., said at the annual European Stroke Conference. The benefit was seen in most stroke subtypes, although not in those with hemorrhagic stroke or in patients with stroke due to atrial fibrillation.
Dr. Douri of King’s College London and coinvestigators looked at whether preventing vascular events could be associated with a protective effect on patients’ overall cognitive function after a stroke. They used the population-based South London Stroke Register to identify 4,413 patients who had experienced a first stroke between 1995 and 2011. Patients were assessed for cognitive function using the Abbreviated Mental Test or Mini-Mental State Examination at about 3 months after their stroke and annually thereafter.
The mean age of patients was 70 years, 49% of the cohort were female, and 70.5% were white. Blacks (21.2%) and other ethnicities made up the remainder of the patient population.
Cognitive impairment rates after stroke vary by study, but have been shown to be relatively consistent over time, affecting up to a quarter of patients overall, Dr. Douri noted (Stroke 2013;44:138-45). Approximately 10% of stroke patients have cognitive impairment prior to their first stroke, 10% develop dementia after a single stroke, and 30% develop dementia after recurrent strokes (Lancet Neurol. 2009;8:1006-18).
Current treatment strategies for preventing cognitive impairment after stroke tend to focus on reducing the risk of recurrent stroke or other vascular events, although most studies to date have had short follow-up or too few patients, with only a small number of these having vascular causes for their cognitive problems.
"The use of recommended therapies after stroke appears to be associated with a protective effect," said Dr. Douiri.
Suboptimal lipid-lowering post stroke
Although optimizing poststroke medications might improve cognitive outcomes, it is not always achieved in routine care. The results of a separate prospective population-based study showed that lipid-lowering targets are not always being achieved.
"The suboptimal lipid control we observed, both preceding and following a stroke or TIA [transient ischemic attack], even where there was established vascular disease or risk factors, highlights the need for improved lipid management in patients who are at risk of stroke or TIA," said Dr. Danielle Ní Chróinín of Mater University Hospital and University College Dublin.
Dr. Chróinín and colleagues assessed patients’ lipid profiles and clinicians’ adherence to evidence-based guidelines for lipid-lowering medications after a stroke or transient ischemic attack using data from the North Dublin Population Stroke Study.
Over the course of the 1-year study, the medical records of 428 patients who had had an ischemic stroke and 188 who had had a TIA were analyzed. The mean age of patients was 71 years.
Lipid measurement at presentation and prescription of statin therapy at discharge were found to be less likely in female patients, those who were older, those with poorer modified Rankin scores before the event, and those with higher National Institutes of Health Stroke Scale scores. There was no difference in the likelihood of measurement or statin treatment based on the type of event or if the patient required hospitalization.
At presentation, only 33.7% of high-risk patients were being treated with lipid-lowering medications. Although 75.5% of patients were discharged on statin therapy, approximately one in four patients who should have been prescribed this medication were not taking a statin at discharge.
Of patients who were on lipid-lowering therapy, less than half (44%-46%) were achieving recommended target levels set by U.S. or European guidelines.
Statin treatment at discharge was more likely in patients who had concomitant diabetes or atherothrombotic stroke.
The study presented by Dr. Chróinín was supported by the Health Research Board, the Irish Heart Foundation, and a Mater University postgraduate research and education grant. Dr. Chróinín and Dr. Douiri said they had no relevant financial disclosures.
LONDON – Cognitive dysfunction following a stroke could be reduced significantly if treatment with antihypertensive, antithrombotic, and lipid-lowering drugs were optimized, according to a study looking at the long-term impact of secondary prevention strategies.
The relative risk for cognitive dysfunction was 0.8 with the optimal use of anticoagulants, and 0.9, 0.9, and 0.9 with the optimal use of dual antihypertensive therapy, dual antiplatelet therapy, and lipid-lowering treatment, respectively.
"The combination of antihypertensives, antithrombotics, and lipid-lowering drugs reduced the risk of cognitive impairment by about half," Abdel Douiri, Ph.D., said at the annual European Stroke Conference. The benefit was seen in most stroke subtypes, although not in those with hemorrhagic stroke or in patients with stroke due to atrial fibrillation.
Dr. Douri of King’s College London and coinvestigators looked at whether preventing vascular events could be associated with a protective effect on patients’ overall cognitive function after a stroke. They used the population-based South London Stroke Register to identify 4,413 patients who had experienced a first stroke between 1995 and 2011. Patients were assessed for cognitive function using the Abbreviated Mental Test or Mini-Mental State Examination at about 3 months after their stroke and annually thereafter.
The mean age of patients was 70 years, 49% of the cohort were female, and 70.5% were white. Blacks (21.2%) and other ethnicities made up the remainder of the patient population.
Cognitive impairment rates after stroke vary by study, but have been shown to be relatively consistent over time, affecting up to a quarter of patients overall, Dr. Douri noted (Stroke 2013;44:138-45). Approximately 10% of stroke patients have cognitive impairment prior to their first stroke, 10% develop dementia after a single stroke, and 30% develop dementia after recurrent strokes (Lancet Neurol. 2009;8:1006-18).
Current treatment strategies for preventing cognitive impairment after stroke tend to focus on reducing the risk of recurrent stroke or other vascular events, although most studies to date have had short follow-up or too few patients, with only a small number of these having vascular causes for their cognitive problems.
"The use of recommended therapies after stroke appears to be associated with a protective effect," said Dr. Douiri.
Suboptimal lipid-lowering post stroke
Although optimizing poststroke medications might improve cognitive outcomes, it is not always achieved in routine care. The results of a separate prospective population-based study showed that lipid-lowering targets are not always being achieved.
"The suboptimal lipid control we observed, both preceding and following a stroke or TIA [transient ischemic attack], even where there was established vascular disease or risk factors, highlights the need for improved lipid management in patients who are at risk of stroke or TIA," said Dr. Danielle Ní Chróinín of Mater University Hospital and University College Dublin.
Dr. Chróinín and colleagues assessed patients’ lipid profiles and clinicians’ adherence to evidence-based guidelines for lipid-lowering medications after a stroke or transient ischemic attack using data from the North Dublin Population Stroke Study.
Over the course of the 1-year study, the medical records of 428 patients who had had an ischemic stroke and 188 who had had a TIA were analyzed. The mean age of patients was 71 years.
Lipid measurement at presentation and prescription of statin therapy at discharge were found to be less likely in female patients, those who were older, those with poorer modified Rankin scores before the event, and those with higher National Institutes of Health Stroke Scale scores. There was no difference in the likelihood of measurement or statin treatment based on the type of event or if the patient required hospitalization.
At presentation, only 33.7% of high-risk patients were being treated with lipid-lowering medications. Although 75.5% of patients were discharged on statin therapy, approximately one in four patients who should have been prescribed this medication were not taking a statin at discharge.
Of patients who were on lipid-lowering therapy, less than half (44%-46%) were achieving recommended target levels set by U.S. or European guidelines.
Statin treatment at discharge was more likely in patients who had concomitant diabetes or atherothrombotic stroke.
The study presented by Dr. Chróinín was supported by the Health Research Board, the Irish Heart Foundation, and a Mater University postgraduate research and education grant. Dr. Chróinín and Dr. Douiri said they had no relevant financial disclosures.
LONDON – Cognitive dysfunction following a stroke could be reduced significantly if treatment with antihypertensive, antithrombotic, and lipid-lowering drugs were optimized, according to a study looking at the long-term impact of secondary prevention strategies.
The relative risk for cognitive dysfunction was 0.8 with the optimal use of anticoagulants, and 0.9, 0.9, and 0.9 with the optimal use of dual antihypertensive therapy, dual antiplatelet therapy, and lipid-lowering treatment, respectively.
"The combination of antihypertensives, antithrombotics, and lipid-lowering drugs reduced the risk of cognitive impairment by about half," Abdel Douiri, Ph.D., said at the annual European Stroke Conference. The benefit was seen in most stroke subtypes, although not in those with hemorrhagic stroke or in patients with stroke due to atrial fibrillation.
Dr. Douri of King’s College London and coinvestigators looked at whether preventing vascular events could be associated with a protective effect on patients’ overall cognitive function after a stroke. They used the population-based South London Stroke Register to identify 4,413 patients who had experienced a first stroke between 1995 and 2011. Patients were assessed for cognitive function using the Abbreviated Mental Test or Mini-Mental State Examination at about 3 months after their stroke and annually thereafter.
The mean age of patients was 70 years, 49% of the cohort were female, and 70.5% were white. Blacks (21.2%) and other ethnicities made up the remainder of the patient population.
Cognitive impairment rates after stroke vary by study, but have been shown to be relatively consistent over time, affecting up to a quarter of patients overall, Dr. Douri noted (Stroke 2013;44:138-45). Approximately 10% of stroke patients have cognitive impairment prior to their first stroke, 10% develop dementia after a single stroke, and 30% develop dementia after recurrent strokes (Lancet Neurol. 2009;8:1006-18).
Current treatment strategies for preventing cognitive impairment after stroke tend to focus on reducing the risk of recurrent stroke or other vascular events, although most studies to date have had short follow-up or too few patients, with only a small number of these having vascular causes for their cognitive problems.
"The use of recommended therapies after stroke appears to be associated with a protective effect," said Dr. Douiri.
Suboptimal lipid-lowering post stroke
Although optimizing poststroke medications might improve cognitive outcomes, it is not always achieved in routine care. The results of a separate prospective population-based study showed that lipid-lowering targets are not always being achieved.
"The suboptimal lipid control we observed, both preceding and following a stroke or TIA [transient ischemic attack], even where there was established vascular disease or risk factors, highlights the need for improved lipid management in patients who are at risk of stroke or TIA," said Dr. Danielle Ní Chróinín of Mater University Hospital and University College Dublin.
Dr. Chróinín and colleagues assessed patients’ lipid profiles and clinicians’ adherence to evidence-based guidelines for lipid-lowering medications after a stroke or transient ischemic attack using data from the North Dublin Population Stroke Study.
Over the course of the 1-year study, the medical records of 428 patients who had had an ischemic stroke and 188 who had had a TIA were analyzed. The mean age of patients was 71 years.
Lipid measurement at presentation and prescription of statin therapy at discharge were found to be less likely in female patients, those who were older, those with poorer modified Rankin scores before the event, and those with higher National Institutes of Health Stroke Scale scores. There was no difference in the likelihood of measurement or statin treatment based on the type of event or if the patient required hospitalization.
At presentation, only 33.7% of high-risk patients were being treated with lipid-lowering medications. Although 75.5% of patients were discharged on statin therapy, approximately one in four patients who should have been prescribed this medication were not taking a statin at discharge.
Of patients who were on lipid-lowering therapy, less than half (44%-46%) were achieving recommended target levels set by U.S. or European guidelines.
Statin treatment at discharge was more likely in patients who had concomitant diabetes or atherothrombotic stroke.
The study presented by Dr. Chróinín was supported by the Health Research Board, the Irish Heart Foundation, and a Mater University postgraduate research and education grant. Dr. Chróinín and Dr. Douiri said they had no relevant financial disclosures.
AT THE EUROPEAN STROKE CONFERENCE
Major finding: The relative risk for cognitive dysfunction was 0.8-0.9 with the use of recommended antihypertensive, antithrombotic, and lipid-lowering drugs.
Data source: A prospective, population-based South London Stroke Register of 4,413 patients who had a first-ever stroke between 1995 and 2011 and completed a global measure of cognitive function.
Disclosures: The study presented by Dr. Chróinín was supported by the Health Research Board, the Irish Heart Foundation, and a Mater University postgraduate research and education grant. Dr. Chróinín and Dr. Douiri said they had no relevant financial disclosures.
TPA improved stroke patients' functional outcomes, quality of life
LONDON – Intravenous thrombolysis administered within 6 hours of a stroke resulted in a significantly greater percentage of patients with better functional outcomes and quality of life after 18 months than did standard stroke care in an analysis of secondary endpoints in the Third International Stroke Trial.
At 18 months’ follow-up, a significant overall difference in quality of life on the EuroQoL-5 Dimensions (EQ-5D) instrument was seen in favor of tissue plasminogen activator (TPA) rather than placebo treatment (P = .03).
Furthermore, four out of the five domains measured by the EQ-5D were significantly improved, including mobility (P = .02), self-care (P = .001), usual activities (P = .008), and pain or discomfort (P = .03). The results of the Third International Stroke Trial (IST-3) at 18 months’ follow-up were recently published (Lancet Neurol. 2013;12:768-76).
"If you survive a stroke, you have to live with it for rather a very long time," said IST-3 chief investigator Dr. Peter Sandercock, who presented these secondary endpoint findings at the annual European Stroke Conference.
"I think this is something that many trials today have forgotten," said Dr. Sandercock, who is professor of medical neurology in the Centre for Clinical Brain Sciences at the University of Edinburgh (Scotland) and director of Edinburgh Neuroscience there. He added that treatments that produce relatively modest improvements in pathological outcomes in the short term might turn out to be cost effective in the long term.
Indeed, few trials in stroke to date have considered the longer-term quality-of-life effects of stroke treatment. The INTERACT2 trial (N. Engl. J. Med. 2013;368:2355-65) recently reported positive findings for blood pressure reduction in intracerebral hemorrhage, Dr. Sandercock noted, but IST-3 is the largest, and first, to report on the long-term benefits of thrombolysis in a stroke population. IST-3 is a randomized, controlled, open-treatment trial of 3,035 patients enrolled at 156 hospitals in 12 countries who were treated with intravenous thrombolysis or placebo within 6 hours of a stroke.
The primary endpoint results of IST-3 were published last year (Lancet 2012;379:2352-63) and showed a similar percentage of patients treated with TPA (37%) or placebo (35%) were "alive and independent" at 6 months, as signified by a score of 0-2 on the Oxford Handicap Scale (OHS). The OHS is similar to the modified Rankin Scale, with lower scores indicating no or minimal functional problems.
Now with longer follow-up out to 18 months, however, a higher percentage of TPA-treated patients had an OHS score of 0-2 (35% vs. 31% for the control group; odds ratio, 1.28; P = .024).
This result equates to an absolute difference of 36 more patients per 1,000 being alive and independent at 18 months if they received thrombolysis, Dr. Sandercock said. At 6 months, the absolute difference was not significant, with 14 more patients per 1,000 being independent if they received thrombolysis.
An ordinal shift analysis of OHS scores at 18 months showed that there was a similar percentage of deaths, at 37% for both treatment arms, but that all other scores were shifted in favor of TPA treatment (OR, 1.3; P = .002).
Of the 3,035 patients who participated in IST-3, 2,348 from 10 recruiting countries were eligible for inclusion in the 18-month follow-on study. Of these, 1,169 were treated with intravenous TPA, and the remainder were given a placebo injection.
Follow-up was by postal questionnaires, 44% of which were completed by a patient and 56% by a caregiver. If there was no response, a telephone-based interview was conducted. The investigators assessed QoL by the EQ-5D, EQ index, and EQ visual analog scale, as well as by an assessment of where the patient was living.
There was a marginal difference in patients’ living situations in favor of TPA treatment, with the agent being administered to 85.1% of patients living at home rather than in an institution versus 83.9% of patients given placebo (P = .05).
Boehringer Ingelheim donated the thrombolytic agent alteplase (Actilyse) to the first 300 patients but had no other role in the study. Dr. Sandercock disclosed that he has served as a speaker for Boehringer Ingelheim.
LONDON – Intravenous thrombolysis administered within 6 hours of a stroke resulted in a significantly greater percentage of patients with better functional outcomes and quality of life after 18 months than did standard stroke care in an analysis of secondary endpoints in the Third International Stroke Trial.
At 18 months’ follow-up, a significant overall difference in quality of life on the EuroQoL-5 Dimensions (EQ-5D) instrument was seen in favor of tissue plasminogen activator (TPA) rather than placebo treatment (P = .03).
Furthermore, four out of the five domains measured by the EQ-5D were significantly improved, including mobility (P = .02), self-care (P = .001), usual activities (P = .008), and pain or discomfort (P = .03). The results of the Third International Stroke Trial (IST-3) at 18 months’ follow-up were recently published (Lancet Neurol. 2013;12:768-76).
"If you survive a stroke, you have to live with it for rather a very long time," said IST-3 chief investigator Dr. Peter Sandercock, who presented these secondary endpoint findings at the annual European Stroke Conference.
"I think this is something that many trials today have forgotten," said Dr. Sandercock, who is professor of medical neurology in the Centre for Clinical Brain Sciences at the University of Edinburgh (Scotland) and director of Edinburgh Neuroscience there. He added that treatments that produce relatively modest improvements in pathological outcomes in the short term might turn out to be cost effective in the long term.
Indeed, few trials in stroke to date have considered the longer-term quality-of-life effects of stroke treatment. The INTERACT2 trial (N. Engl. J. Med. 2013;368:2355-65) recently reported positive findings for blood pressure reduction in intracerebral hemorrhage, Dr. Sandercock noted, but IST-3 is the largest, and first, to report on the long-term benefits of thrombolysis in a stroke population. IST-3 is a randomized, controlled, open-treatment trial of 3,035 patients enrolled at 156 hospitals in 12 countries who were treated with intravenous thrombolysis or placebo within 6 hours of a stroke.
The primary endpoint results of IST-3 were published last year (Lancet 2012;379:2352-63) and showed a similar percentage of patients treated with TPA (37%) or placebo (35%) were "alive and independent" at 6 months, as signified by a score of 0-2 on the Oxford Handicap Scale (OHS). The OHS is similar to the modified Rankin Scale, with lower scores indicating no or minimal functional problems.
Now with longer follow-up out to 18 months, however, a higher percentage of TPA-treated patients had an OHS score of 0-2 (35% vs. 31% for the control group; odds ratio, 1.28; P = .024).
This result equates to an absolute difference of 36 more patients per 1,000 being alive and independent at 18 months if they received thrombolysis, Dr. Sandercock said. At 6 months, the absolute difference was not significant, with 14 more patients per 1,000 being independent if they received thrombolysis.
An ordinal shift analysis of OHS scores at 18 months showed that there was a similar percentage of deaths, at 37% for both treatment arms, but that all other scores were shifted in favor of TPA treatment (OR, 1.3; P = .002).
Of the 3,035 patients who participated in IST-3, 2,348 from 10 recruiting countries were eligible for inclusion in the 18-month follow-on study. Of these, 1,169 were treated with intravenous TPA, and the remainder were given a placebo injection.
Follow-up was by postal questionnaires, 44% of which were completed by a patient and 56% by a caregiver. If there was no response, a telephone-based interview was conducted. The investigators assessed QoL by the EQ-5D, EQ index, and EQ visual analog scale, as well as by an assessment of where the patient was living.
There was a marginal difference in patients’ living situations in favor of TPA treatment, with the agent being administered to 85.1% of patients living at home rather than in an institution versus 83.9% of patients given placebo (P = .05).
Boehringer Ingelheim donated the thrombolytic agent alteplase (Actilyse) to the first 300 patients but had no other role in the study. Dr. Sandercock disclosed that he has served as a speaker for Boehringer Ingelheim.
LONDON – Intravenous thrombolysis administered within 6 hours of a stroke resulted in a significantly greater percentage of patients with better functional outcomes and quality of life after 18 months than did standard stroke care in an analysis of secondary endpoints in the Third International Stroke Trial.
At 18 months’ follow-up, a significant overall difference in quality of life on the EuroQoL-5 Dimensions (EQ-5D) instrument was seen in favor of tissue plasminogen activator (TPA) rather than placebo treatment (P = .03).
Furthermore, four out of the five domains measured by the EQ-5D were significantly improved, including mobility (P = .02), self-care (P = .001), usual activities (P = .008), and pain or discomfort (P = .03). The results of the Third International Stroke Trial (IST-3) at 18 months’ follow-up were recently published (Lancet Neurol. 2013;12:768-76).
"If you survive a stroke, you have to live with it for rather a very long time," said IST-3 chief investigator Dr. Peter Sandercock, who presented these secondary endpoint findings at the annual European Stroke Conference.
"I think this is something that many trials today have forgotten," said Dr. Sandercock, who is professor of medical neurology in the Centre for Clinical Brain Sciences at the University of Edinburgh (Scotland) and director of Edinburgh Neuroscience there. He added that treatments that produce relatively modest improvements in pathological outcomes in the short term might turn out to be cost effective in the long term.
Indeed, few trials in stroke to date have considered the longer-term quality-of-life effects of stroke treatment. The INTERACT2 trial (N. Engl. J. Med. 2013;368:2355-65) recently reported positive findings for blood pressure reduction in intracerebral hemorrhage, Dr. Sandercock noted, but IST-3 is the largest, and first, to report on the long-term benefits of thrombolysis in a stroke population. IST-3 is a randomized, controlled, open-treatment trial of 3,035 patients enrolled at 156 hospitals in 12 countries who were treated with intravenous thrombolysis or placebo within 6 hours of a stroke.
The primary endpoint results of IST-3 were published last year (Lancet 2012;379:2352-63) and showed a similar percentage of patients treated with TPA (37%) or placebo (35%) were "alive and independent" at 6 months, as signified by a score of 0-2 on the Oxford Handicap Scale (OHS). The OHS is similar to the modified Rankin Scale, with lower scores indicating no or minimal functional problems.
Now with longer follow-up out to 18 months, however, a higher percentage of TPA-treated patients had an OHS score of 0-2 (35% vs. 31% for the control group; odds ratio, 1.28; P = .024).
This result equates to an absolute difference of 36 more patients per 1,000 being alive and independent at 18 months if they received thrombolysis, Dr. Sandercock said. At 6 months, the absolute difference was not significant, with 14 more patients per 1,000 being independent if they received thrombolysis.
An ordinal shift analysis of OHS scores at 18 months showed that there was a similar percentage of deaths, at 37% for both treatment arms, but that all other scores were shifted in favor of TPA treatment (OR, 1.3; P = .002).
Of the 3,035 patients who participated in IST-3, 2,348 from 10 recruiting countries were eligible for inclusion in the 18-month follow-on study. Of these, 1,169 were treated with intravenous TPA, and the remainder were given a placebo injection.
Follow-up was by postal questionnaires, 44% of which were completed by a patient and 56% by a caregiver. If there was no response, a telephone-based interview was conducted. The investigators assessed QoL by the EQ-5D, EQ index, and EQ visual analog scale, as well as by an assessment of where the patient was living.
There was a marginal difference in patients’ living situations in favor of TPA treatment, with the agent being administered to 85.1% of patients living at home rather than in an institution versus 83.9% of patients given placebo (P = .05).
Boehringer Ingelheim donated the thrombolytic agent alteplase (Actilyse) to the first 300 patients but had no other role in the study. Dr. Sandercock disclosed that he has served as a speaker for Boehringer Ingelheim.
AT THE EUROPEAN STROKE CONFERENCE
Major finding: At 18 months’ follow-up, 35% of TPA-treated patients were alive and independent with an Oxford Handicap Scale score of 0-2, compared with 31% of the control group (odds ratio, 1.28; P = .024).
Data source: IST-3 is a randomized, controlled, open-treatment trial of 3,035 patients enrolled at 156 hospitals in 12 countries who were treated with intravenous thrombolysis or placebo within 6 hours of a stroke.
Disclosures: Boehringer Ingelheim donated the thrombolytic agent alteplase (Actilyse) to the first 300 patients but had no other role in the study. Dr. Sandercock disclosed that he has served as a speaker for Boehringer Ingelheim.
Aphasic stroke patients' mood lifted by behavioral therapy
LONDON – People with aphasia and low mood after a stroke can benefit from behavioral therapy, the results of a multicenter, randomized controlled trial suggested.
In the Communication and Low Mood (CALM) study, patients who were in the behavioral therapy group reported better mood, as measured by the 21-item hospital version of the Stroke Aphasic Depression Questionnaire (SADQ) at both 3 and 6 months’ follow-up. Mean SADQ scores in the behavioral therapy versus the usual care arm were 16.9 and 19.2 at 3 months (P less than .05) and 17.4 and 21.9 at 6 months (P = .002), with a lower score indicating a better level of mood.
Depression is estimated to affect up to a third of patients after a stroke and can have detrimental effects on patients’ rehabilitation. It can cause psychological distress for patients and caregivers and is linked to higher mortality.
"People with aphasia may be particularly susceptible to depression, but they are often excluded from research," Dr. Shirley Thomas said at the annual European Stroke Conference.
Dr. Thomas of the Institute of Work, Health, and Organisations at the University of Nottingham (England) noted that few studies have looked at the use of psychological interventions for depression after a stroke. The studies that have been conducted have typically excluded people with aphasia because the interventions are often talk based and require good communication skills.
"Behavioral therapy is quite a practical and concrete approach that doesn’t require intact communication skills," Dr. Thomas said. She explained that it "is based on a behavior model of depression, the idea being that people develop depression because they are not getting positive reward and reinforcement from their environment." This "fits" with having aphasia following a stroke, she commented.
The aim of the CALM study, therefore, was to compare usual care alone with usual care plus the addition of a behavioral intervention to address low mood in patients with aphasia.
A total of 511 patients who had aphasia after a stroke were screened for signs of depression, with 105 identified as having "low mood" and consenting to participate in the trial. The mean age of the enrolled patients was 67 years and 63% were men. The trial began a median of 9 months after a stroke.
The behavioral therapy involved one-on-one sessions between a patient and a psychologist in the patient’s home, with up to 20 sessions occurring over a period of 3 months. Each session lasted for 1 hour and included patient education, which involved asking patients how they spent their time, identifying mood-lifting activities, scheduling these activities into each week, helping patients break down large tasks into graded steps, and giving people tasks to complete before the next session. Therapy was tailored to patients’ needs and guided by a manual specifically designed for the trial, which outlined all the various methods that could be used.
Two main instruments were used to assess patients’ mood in the trial: the SADQ and the ‘sad’ item of the Visual Analog Mood Scales (VAMS). Other measures used included the Visual Analog Self-Esteem Scale (VASES) and the Nottingham Leisure Questionnaire (NLQ).
When the VAMS was used, behavioral therapy was associated with significantly better mood at 3 months (P = .033) but not at 6 months. VASES scores were higher at 3 months in the behavioral therapy group than in those who got usual care, indicating better self-esteem, although results did not remain significant at 6 months. The results from the NLQ showed no significant differences between the groups.
A similar percentage of patients in the behavioral therapy and usual care groups took antidepressants (29% and 26%), so the differences that were seen in favor of behavioral therapy are not influenced by the use of these drugs, Dr. Thomas said.
"Overall, we found that the behavioral approaches [used] were appropriate and could be used in patients with aphasia," Dr. Thomas said. "This is important because this is a group of patients that are usually excluded from psychological interventions for mood problems," she added.
"Behavioral therapy appears to be promising, but going forward we need to look in more detail at the duration and content of treatment," Dr. Thomas said. It was unclear when the trial started how many sessions might be needed – no patient actually needed 20 sessions and most received about 10. An intervention period longer than 3 months might be better to integrate behavioral therapy into clinical practice and to ensure the effects are sustained, Dr. Thomas noted, but this needs to be confirmed by additional, larger trials with more statistical power.
The CALM study findings have been published (Clin. Rehabil. 2013;27:398-408). The Stroke Association in London funded the study. Dr. Thomas said she had no relevant financial disclosures.
LONDON – People with aphasia and low mood after a stroke can benefit from behavioral therapy, the results of a multicenter, randomized controlled trial suggested.
In the Communication and Low Mood (CALM) study, patients who were in the behavioral therapy group reported better mood, as measured by the 21-item hospital version of the Stroke Aphasic Depression Questionnaire (SADQ) at both 3 and 6 months’ follow-up. Mean SADQ scores in the behavioral therapy versus the usual care arm were 16.9 and 19.2 at 3 months (P less than .05) and 17.4 and 21.9 at 6 months (P = .002), with a lower score indicating a better level of mood.
Depression is estimated to affect up to a third of patients after a stroke and can have detrimental effects on patients’ rehabilitation. It can cause psychological distress for patients and caregivers and is linked to higher mortality.
"People with aphasia may be particularly susceptible to depression, but they are often excluded from research," Dr. Shirley Thomas said at the annual European Stroke Conference.
Dr. Thomas of the Institute of Work, Health, and Organisations at the University of Nottingham (England) noted that few studies have looked at the use of psychological interventions for depression after a stroke. The studies that have been conducted have typically excluded people with aphasia because the interventions are often talk based and require good communication skills.
"Behavioral therapy is quite a practical and concrete approach that doesn’t require intact communication skills," Dr. Thomas said. She explained that it "is based on a behavior model of depression, the idea being that people develop depression because they are not getting positive reward and reinforcement from their environment." This "fits" with having aphasia following a stroke, she commented.
The aim of the CALM study, therefore, was to compare usual care alone with usual care plus the addition of a behavioral intervention to address low mood in patients with aphasia.
A total of 511 patients who had aphasia after a stroke were screened for signs of depression, with 105 identified as having "low mood" and consenting to participate in the trial. The mean age of the enrolled patients was 67 years and 63% were men. The trial began a median of 9 months after a stroke.
The behavioral therapy involved one-on-one sessions between a patient and a psychologist in the patient’s home, with up to 20 sessions occurring over a period of 3 months. Each session lasted for 1 hour and included patient education, which involved asking patients how they spent their time, identifying mood-lifting activities, scheduling these activities into each week, helping patients break down large tasks into graded steps, and giving people tasks to complete before the next session. Therapy was tailored to patients’ needs and guided by a manual specifically designed for the trial, which outlined all the various methods that could be used.
Two main instruments were used to assess patients’ mood in the trial: the SADQ and the ‘sad’ item of the Visual Analog Mood Scales (VAMS). Other measures used included the Visual Analog Self-Esteem Scale (VASES) and the Nottingham Leisure Questionnaire (NLQ).
When the VAMS was used, behavioral therapy was associated with significantly better mood at 3 months (P = .033) but not at 6 months. VASES scores were higher at 3 months in the behavioral therapy group than in those who got usual care, indicating better self-esteem, although results did not remain significant at 6 months. The results from the NLQ showed no significant differences between the groups.
A similar percentage of patients in the behavioral therapy and usual care groups took antidepressants (29% and 26%), so the differences that were seen in favor of behavioral therapy are not influenced by the use of these drugs, Dr. Thomas said.
"Overall, we found that the behavioral approaches [used] were appropriate and could be used in patients with aphasia," Dr. Thomas said. "This is important because this is a group of patients that are usually excluded from psychological interventions for mood problems," she added.
"Behavioral therapy appears to be promising, but going forward we need to look in more detail at the duration and content of treatment," Dr. Thomas said. It was unclear when the trial started how many sessions might be needed – no patient actually needed 20 sessions and most received about 10. An intervention period longer than 3 months might be better to integrate behavioral therapy into clinical practice and to ensure the effects are sustained, Dr. Thomas noted, but this needs to be confirmed by additional, larger trials with more statistical power.
The CALM study findings have been published (Clin. Rehabil. 2013;27:398-408). The Stroke Association in London funded the study. Dr. Thomas said she had no relevant financial disclosures.
LONDON – People with aphasia and low mood after a stroke can benefit from behavioral therapy, the results of a multicenter, randomized controlled trial suggested.
In the Communication and Low Mood (CALM) study, patients who were in the behavioral therapy group reported better mood, as measured by the 21-item hospital version of the Stroke Aphasic Depression Questionnaire (SADQ) at both 3 and 6 months’ follow-up. Mean SADQ scores in the behavioral therapy versus the usual care arm were 16.9 and 19.2 at 3 months (P less than .05) and 17.4 and 21.9 at 6 months (P = .002), with a lower score indicating a better level of mood.
Depression is estimated to affect up to a third of patients after a stroke and can have detrimental effects on patients’ rehabilitation. It can cause psychological distress for patients and caregivers and is linked to higher mortality.
"People with aphasia may be particularly susceptible to depression, but they are often excluded from research," Dr. Shirley Thomas said at the annual European Stroke Conference.
Dr. Thomas of the Institute of Work, Health, and Organisations at the University of Nottingham (England) noted that few studies have looked at the use of psychological interventions for depression after a stroke. The studies that have been conducted have typically excluded people with aphasia because the interventions are often talk based and require good communication skills.
"Behavioral therapy is quite a practical and concrete approach that doesn’t require intact communication skills," Dr. Thomas said. She explained that it "is based on a behavior model of depression, the idea being that people develop depression because they are not getting positive reward and reinforcement from their environment." This "fits" with having aphasia following a stroke, she commented.
The aim of the CALM study, therefore, was to compare usual care alone with usual care plus the addition of a behavioral intervention to address low mood in patients with aphasia.
A total of 511 patients who had aphasia after a stroke were screened for signs of depression, with 105 identified as having "low mood" and consenting to participate in the trial. The mean age of the enrolled patients was 67 years and 63% were men. The trial began a median of 9 months after a stroke.
The behavioral therapy involved one-on-one sessions between a patient and a psychologist in the patient’s home, with up to 20 sessions occurring over a period of 3 months. Each session lasted for 1 hour and included patient education, which involved asking patients how they spent their time, identifying mood-lifting activities, scheduling these activities into each week, helping patients break down large tasks into graded steps, and giving people tasks to complete before the next session. Therapy was tailored to patients’ needs and guided by a manual specifically designed for the trial, which outlined all the various methods that could be used.
Two main instruments were used to assess patients’ mood in the trial: the SADQ and the ‘sad’ item of the Visual Analog Mood Scales (VAMS). Other measures used included the Visual Analog Self-Esteem Scale (VASES) and the Nottingham Leisure Questionnaire (NLQ).
When the VAMS was used, behavioral therapy was associated with significantly better mood at 3 months (P = .033) but not at 6 months. VASES scores were higher at 3 months in the behavioral therapy group than in those who got usual care, indicating better self-esteem, although results did not remain significant at 6 months. The results from the NLQ showed no significant differences between the groups.
A similar percentage of patients in the behavioral therapy and usual care groups took antidepressants (29% and 26%), so the differences that were seen in favor of behavioral therapy are not influenced by the use of these drugs, Dr. Thomas said.
"Overall, we found that the behavioral approaches [used] were appropriate and could be used in patients with aphasia," Dr. Thomas said. "This is important because this is a group of patients that are usually excluded from psychological interventions for mood problems," she added.
"Behavioral therapy appears to be promising, but going forward we need to look in more detail at the duration and content of treatment," Dr. Thomas said. It was unclear when the trial started how many sessions might be needed – no patient actually needed 20 sessions and most received about 10. An intervention period longer than 3 months might be better to integrate behavioral therapy into clinical practice and to ensure the effects are sustained, Dr. Thomas noted, but this needs to be confirmed by additional, larger trials with more statistical power.
The CALM study findings have been published (Clin. Rehabil. 2013;27:398-408). The Stroke Association in London funded the study. Dr. Thomas said she had no relevant financial disclosures.
AT THE EUROPEAN STROKE CONFERENCE
Major finding: Mean Stroke Aphasic Depression Questionnaire scores were significantly lower at 6 months among patients in the behavioral therapy arm than among those in the usual care arm (17.4 vs. 21.9; P = .002).
Data source: A multicenter, randomized, controlled study that compared behavioral therapy with usual care in 105 patients with aphasia and low mood.
Disclosures: The Stroke Association in London funded the study. Dr. Thomas said she had no relevant financial disclosures.
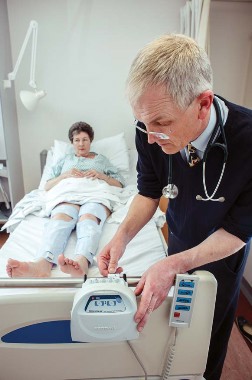
Device cuts DVT risk, saves stroke patients' lives
LONDON – Intermittent pneumatic compression reduced the absolute risk of proximal deep vein thrombosis in patients who had suffered a stroke and were immobile by 3.6% in a large, randomized trial.
The incidence of proximal DVT at 30 days in the CLOTS 3 study was 8.5% with intermittent pneumatic compression (IPC) and 12.1% with routine poststroke care alone (P = .001). The adjusted odds ratio was 0.65.
There was also a 14% reduction in the risk of death seen at 6 months favoring IPC use over routine care alone (P = .042). This was a surprising finding, said principal investigator Dr. Martin Dennis of the University of Edinburgh’s clinical neurosciences division and Western General Hospital in Edinburgh, Scotland.
Importantly, IPC appears to be effective across a variety of prespecified subgroups, including both ischemic and hemorrhagic stroke.
Findings will change practice
The findings, which were published online in the Lancet (2013 May 31 [doi:10.1016/ S0140-6736(13)61050-8]) to coincide with their presentation at the annual European Stroke Conference, are practice changing and suggest that national stroke guidelines need to be updated.
"This study is a major breakthrough, showing how a simple and safe treatment can save lives," Dr. Tony Rudd, a consultant stroke physician at Guy’s and St. Thomas’ NHS Foundation Trust, London, said in a statement issued by the University of Edinburgh.
"The challenge now will be to ensure that all patients who might benefit are offered treatment," added Dr. Rudd, who chairs the Royal College of Physicians’ Intercollegiate Stroke Working Party. "It is one of the most important research studies to emerge in the field of stroke in recent years," he noted.
Dr. Christine Roffe, consultant in stroke medicine and professor of medicine at Keele University in Stoke-on-Trent, England, also praised the study’s results. "That something as simple as a compressive sleeve saves lives after stroke is fascinating," she said in an interview at the conference. Dr. Roffe was not involved in the study.
The CLOTS 3 study
CLOTS 3 follows on from two other trials performed by the CLOTS (Clots in Legs or Stockings after Stroke) Trials Collaboration, in which compression stockings were examined as a possible means of preventing thrombotic complications in patients who had suffered a stroke. Results of CLOTS 1 (Lancet 2009;373:1958-65) and CLOTS 2 were negative, however, and no benefit of compression stockings was seen in stroke patients.
Between December 2008 and September 2012, a total of 2,876 patients were enrolled in CLOTS 3. For inclusion, patients had to be immobile and randomized within 0-3 days of having had a stroke. Immobility was defined as being unable to walk to the bathroom without the help of another person.
Patients were randomized to receive either routine poststroke care alone or with additional IPC delivered by Covidien’s Kendall SCD Express Sequential Compression System. The latter involved wearing thigh-high, inflatable sleeves continuously for up to 30 days, during which time the device automatically provided IPC depending on the position of the patient. The mean and median durations of wear were 12.5 days and 9.0 days, respectively.
DVT was assessed using duplex ultrasound at 7-10 days and again at 25-30 days if possible. Both patient groups wore compression sleeves to ensure that the ultrasound technicians remained blinded to the treatment group. Follow-up was at 6 months via postal questionnaires sent to patients’ primary care physicians asking about vital status and the occurrence of venous thromboembolism since hospital discharge. Patients were also sent a postal questionnaire and telephoned if they did not respond.
DVT risk reduced
The effect on proximal DVT at 30 days was the primary outcome measure, but IPC also reduced the incidence of symptomatic DVT (4.6% vs. 6.3%; P = .0045) and any DVT (16.2% vs. 21.1%; P = .001) versus routine care. There was no significant difference in the incidence of pulmonary embolism between study arms (2.0% vs. 2.4%, respectively, P = .453).
In terms of safety, there was no difference between the treatment groups in the number of falls with injury or fractures as a result of constantly wearing the compression sleeves. There was a significant difference in skin ulcers (3.1% with IPC vs. 1.4% without, P = .002), but close inspection of the data suggested that only 10 (0.7%) cases were due to IPC.
"During the study, [the manufacturers of the IPC device] brought out a new comfort sleeve," Dr. Dennis noted in an interview.
"Normally these sleeves were being used for short periods in surgical patients, but we were using them for longer periods, so they brought out a softer sleeve," he observed. Anecdotally, he conceded that some people found the sleeves uncomfortable, too hot, or the system "noisy" to use.
The bottom line is that "intermittent pneumatic compression in people who are immobile with stroke reduces the risk of deep vein thrombosis," Dr. Dennis said.
He emphasized that "IPC is feasible in stroke patients, and it is relatively safe. It is an effective means of reducing venous thromboembolism after stroke, with a number needed to treat of about 28 for proximal DVT."
Intriguingly, it may also improve overall survival, "although we weren’t expecting to see that effect," Dr. Dennis said. The number needed to treat to prevent 1 death in 30 days was 43.
Dr. Dennis, Dr. Rudd, and Dr. Roffe had no relevant disclosures. The University of Edinburgh and NHS Lothian sponsored the study with funding from the Chief Scientist Office of the Scottish Government, the National Institute of Heath Research Health Technology Assessment Programme, and the Scottish Stroke Research Network. Covidien provided the equipment used in the study free of charge.
Venous thromboembolism is a common cause of hospital-related morbidity. Anticoagulants (e.g., heparin, low-molecular-weight heparin [LMWH]) reduce VTE with acceptable safety. Stroke patients have a high risk of VTE, but also a heightened bleeding risk in the setting of anticoagulants. Mechanical devices (elastic stockings, sequential compression devices) are attractive alternatives, but efficacy is unproven in many settings. Unlike previous trials that demonstrated a lack of efficacy of elastic stockings, the CLOTS 3 study provides convincing evidence that SCDs reduce VTE and decrease mortality in patients with a stroke. Although encouraging, VTE event rates remained high, 8.5%, in contrast to VTE rates of 4.8% in a prior study of enoxaparin in stroke patients.
Together, these findings support recommendations by the American College of Chest Physicians: In patients with acute ischemic stroke and restricted mobility, use prophylactic-dose subcutaneous heparin or LMWH or intermittent SCDs as opposed to no prophylaxis.
Dr. Robert Pendleton is chief medical quality officer for University of Utah Health Care, Salt Lake City, and a member of the Hospitalist News advisory board.
Venous thromboembolism is a common cause of hospital-related morbidity. Anticoagulants (e.g., heparin, low-molecular-weight heparin [LMWH]) reduce VTE with acceptable safety. Stroke patients have a high risk of VTE, but also a heightened bleeding risk in the setting of anticoagulants. Mechanical devices (elastic stockings, sequential compression devices) are attractive alternatives, but efficacy is unproven in many settings. Unlike previous trials that demonstrated a lack of efficacy of elastic stockings, the CLOTS 3 study provides convincing evidence that SCDs reduce VTE and decrease mortality in patients with a stroke. Although encouraging, VTE event rates remained high, 8.5%, in contrast to VTE rates of 4.8% in a prior study of enoxaparin in stroke patients.
Together, these findings support recommendations by the American College of Chest Physicians: In patients with acute ischemic stroke and restricted mobility, use prophylactic-dose subcutaneous heparin or LMWH or intermittent SCDs as opposed to no prophylaxis.
Dr. Robert Pendleton is chief medical quality officer for University of Utah Health Care, Salt Lake City, and a member of the Hospitalist News advisory board.
Venous thromboembolism is a common cause of hospital-related morbidity. Anticoagulants (e.g., heparin, low-molecular-weight heparin [LMWH]) reduce VTE with acceptable safety. Stroke patients have a high risk of VTE, but also a heightened bleeding risk in the setting of anticoagulants. Mechanical devices (elastic stockings, sequential compression devices) are attractive alternatives, but efficacy is unproven in many settings. Unlike previous trials that demonstrated a lack of efficacy of elastic stockings, the CLOTS 3 study provides convincing evidence that SCDs reduce VTE and decrease mortality in patients with a stroke. Although encouraging, VTE event rates remained high, 8.5%, in contrast to VTE rates of 4.8% in a prior study of enoxaparin in stroke patients.
Together, these findings support recommendations by the American College of Chest Physicians: In patients with acute ischemic stroke and restricted mobility, use prophylactic-dose subcutaneous heparin or LMWH or intermittent SCDs as opposed to no prophylaxis.
Dr. Robert Pendleton is chief medical quality officer for University of Utah Health Care, Salt Lake City, and a member of the Hospitalist News advisory board.
LONDON – Intermittent pneumatic compression reduced the absolute risk of proximal deep vein thrombosis in patients who had suffered a stroke and were immobile by 3.6% in a large, randomized trial.
The incidence of proximal DVT at 30 days in the CLOTS 3 study was 8.5% with intermittent pneumatic compression (IPC) and 12.1% with routine poststroke care alone (P = .001). The adjusted odds ratio was 0.65.
There was also a 14% reduction in the risk of death seen at 6 months favoring IPC use over routine care alone (P = .042). This was a surprising finding, said principal investigator Dr. Martin Dennis of the University of Edinburgh’s clinical neurosciences division and Western General Hospital in Edinburgh, Scotland.
Importantly, IPC appears to be effective across a variety of prespecified subgroups, including both ischemic and hemorrhagic stroke.
Findings will change practice
The findings, which were published online in the Lancet (2013 May 31 [doi:10.1016/ S0140-6736(13)61050-8]) to coincide with their presentation at the annual European Stroke Conference, are practice changing and suggest that national stroke guidelines need to be updated.
"This study is a major breakthrough, showing how a simple and safe treatment can save lives," Dr. Tony Rudd, a consultant stroke physician at Guy’s and St. Thomas’ NHS Foundation Trust, London, said in a statement issued by the University of Edinburgh.
"The challenge now will be to ensure that all patients who might benefit are offered treatment," added Dr. Rudd, who chairs the Royal College of Physicians’ Intercollegiate Stroke Working Party. "It is one of the most important research studies to emerge in the field of stroke in recent years," he noted.
Dr. Christine Roffe, consultant in stroke medicine and professor of medicine at Keele University in Stoke-on-Trent, England, also praised the study’s results. "That something as simple as a compressive sleeve saves lives after stroke is fascinating," she said in an interview at the conference. Dr. Roffe was not involved in the study.
The CLOTS 3 study
CLOTS 3 follows on from two other trials performed by the CLOTS (Clots in Legs or Stockings after Stroke) Trials Collaboration, in which compression stockings were examined as a possible means of preventing thrombotic complications in patients who had suffered a stroke. Results of CLOTS 1 (Lancet 2009;373:1958-65) and CLOTS 2 were negative, however, and no benefit of compression stockings was seen in stroke patients.
Between December 2008 and September 2012, a total of 2,876 patients were enrolled in CLOTS 3. For inclusion, patients had to be immobile and randomized within 0-3 days of having had a stroke. Immobility was defined as being unable to walk to the bathroom without the help of another person.
Patients were randomized to receive either routine poststroke care alone or with additional IPC delivered by Covidien’s Kendall SCD Express Sequential Compression System. The latter involved wearing thigh-high, inflatable sleeves continuously for up to 30 days, during which time the device automatically provided IPC depending on the position of the patient. The mean and median durations of wear were 12.5 days and 9.0 days, respectively.
DVT was assessed using duplex ultrasound at 7-10 days and again at 25-30 days if possible. Both patient groups wore compression sleeves to ensure that the ultrasound technicians remained blinded to the treatment group. Follow-up was at 6 months via postal questionnaires sent to patients’ primary care physicians asking about vital status and the occurrence of venous thromboembolism since hospital discharge. Patients were also sent a postal questionnaire and telephoned if they did not respond.
DVT risk reduced
The effect on proximal DVT at 30 days was the primary outcome measure, but IPC also reduced the incidence of symptomatic DVT (4.6% vs. 6.3%; P = .0045) and any DVT (16.2% vs. 21.1%; P = .001) versus routine care. There was no significant difference in the incidence of pulmonary embolism between study arms (2.0% vs. 2.4%, respectively, P = .453).
In terms of safety, there was no difference between the treatment groups in the number of falls with injury or fractures as a result of constantly wearing the compression sleeves. There was a significant difference in skin ulcers (3.1% with IPC vs. 1.4% without, P = .002), but close inspection of the data suggested that only 10 (0.7%) cases were due to IPC.
"During the study, [the manufacturers of the IPC device] brought out a new comfort sleeve," Dr. Dennis noted in an interview.
"Normally these sleeves were being used for short periods in surgical patients, but we were using them for longer periods, so they brought out a softer sleeve," he observed. Anecdotally, he conceded that some people found the sleeves uncomfortable, too hot, or the system "noisy" to use.
The bottom line is that "intermittent pneumatic compression in people who are immobile with stroke reduces the risk of deep vein thrombosis," Dr. Dennis said.
He emphasized that "IPC is feasible in stroke patients, and it is relatively safe. It is an effective means of reducing venous thromboembolism after stroke, with a number needed to treat of about 28 for proximal DVT."
Intriguingly, it may also improve overall survival, "although we weren’t expecting to see that effect," Dr. Dennis said. The number needed to treat to prevent 1 death in 30 days was 43.
Dr. Dennis, Dr. Rudd, and Dr. Roffe had no relevant disclosures. The University of Edinburgh and NHS Lothian sponsored the study with funding from the Chief Scientist Office of the Scottish Government, the National Institute of Heath Research Health Technology Assessment Programme, and the Scottish Stroke Research Network. Covidien provided the equipment used in the study free of charge.
LONDON – Intermittent pneumatic compression reduced the absolute risk of proximal deep vein thrombosis in patients who had suffered a stroke and were immobile by 3.6% in a large, randomized trial.
The incidence of proximal DVT at 30 days in the CLOTS 3 study was 8.5% with intermittent pneumatic compression (IPC) and 12.1% with routine poststroke care alone (P = .001). The adjusted odds ratio was 0.65.
There was also a 14% reduction in the risk of death seen at 6 months favoring IPC use over routine care alone (P = .042). This was a surprising finding, said principal investigator Dr. Martin Dennis of the University of Edinburgh’s clinical neurosciences division and Western General Hospital in Edinburgh, Scotland.
Importantly, IPC appears to be effective across a variety of prespecified subgroups, including both ischemic and hemorrhagic stroke.
Findings will change practice
The findings, which were published online in the Lancet (2013 May 31 [doi:10.1016/ S0140-6736(13)61050-8]) to coincide with their presentation at the annual European Stroke Conference, are practice changing and suggest that national stroke guidelines need to be updated.
"This study is a major breakthrough, showing how a simple and safe treatment can save lives," Dr. Tony Rudd, a consultant stroke physician at Guy’s and St. Thomas’ NHS Foundation Trust, London, said in a statement issued by the University of Edinburgh.
"The challenge now will be to ensure that all patients who might benefit are offered treatment," added Dr. Rudd, who chairs the Royal College of Physicians’ Intercollegiate Stroke Working Party. "It is one of the most important research studies to emerge in the field of stroke in recent years," he noted.
Dr. Christine Roffe, consultant in stroke medicine and professor of medicine at Keele University in Stoke-on-Trent, England, also praised the study’s results. "That something as simple as a compressive sleeve saves lives after stroke is fascinating," she said in an interview at the conference. Dr. Roffe was not involved in the study.
The CLOTS 3 study
CLOTS 3 follows on from two other trials performed by the CLOTS (Clots in Legs or Stockings after Stroke) Trials Collaboration, in which compression stockings were examined as a possible means of preventing thrombotic complications in patients who had suffered a stroke. Results of CLOTS 1 (Lancet 2009;373:1958-65) and CLOTS 2 were negative, however, and no benefit of compression stockings was seen in stroke patients.
Between December 2008 and September 2012, a total of 2,876 patients were enrolled in CLOTS 3. For inclusion, patients had to be immobile and randomized within 0-3 days of having had a stroke. Immobility was defined as being unable to walk to the bathroom without the help of another person.
Patients were randomized to receive either routine poststroke care alone or with additional IPC delivered by Covidien’s Kendall SCD Express Sequential Compression System. The latter involved wearing thigh-high, inflatable sleeves continuously for up to 30 days, during which time the device automatically provided IPC depending on the position of the patient. The mean and median durations of wear were 12.5 days and 9.0 days, respectively.
DVT was assessed using duplex ultrasound at 7-10 days and again at 25-30 days if possible. Both patient groups wore compression sleeves to ensure that the ultrasound technicians remained blinded to the treatment group. Follow-up was at 6 months via postal questionnaires sent to patients’ primary care physicians asking about vital status and the occurrence of venous thromboembolism since hospital discharge. Patients were also sent a postal questionnaire and telephoned if they did not respond.
DVT risk reduced
The effect on proximal DVT at 30 days was the primary outcome measure, but IPC also reduced the incidence of symptomatic DVT (4.6% vs. 6.3%; P = .0045) and any DVT (16.2% vs. 21.1%; P = .001) versus routine care. There was no significant difference in the incidence of pulmonary embolism between study arms (2.0% vs. 2.4%, respectively, P = .453).
In terms of safety, there was no difference between the treatment groups in the number of falls with injury or fractures as a result of constantly wearing the compression sleeves. There was a significant difference in skin ulcers (3.1% with IPC vs. 1.4% without, P = .002), but close inspection of the data suggested that only 10 (0.7%) cases were due to IPC.
"During the study, [the manufacturers of the IPC device] brought out a new comfort sleeve," Dr. Dennis noted in an interview.
"Normally these sleeves were being used for short periods in surgical patients, but we were using them for longer periods, so they brought out a softer sleeve," he observed. Anecdotally, he conceded that some people found the sleeves uncomfortable, too hot, or the system "noisy" to use.
The bottom line is that "intermittent pneumatic compression in people who are immobile with stroke reduces the risk of deep vein thrombosis," Dr. Dennis said.
He emphasized that "IPC is feasible in stroke patients, and it is relatively safe. It is an effective means of reducing venous thromboembolism after stroke, with a number needed to treat of about 28 for proximal DVT."
Intriguingly, it may also improve overall survival, "although we weren’t expecting to see that effect," Dr. Dennis said. The number needed to treat to prevent 1 death in 30 days was 43.
Dr. Dennis, Dr. Rudd, and Dr. Roffe had no relevant disclosures. The University of Edinburgh and NHS Lothian sponsored the study with funding from the Chief Scientist Office of the Scottish Government, the National Institute of Heath Research Health Technology Assessment Programme, and the Scottish Stroke Research Network. Covidien provided the equipment used in the study free of charge.
AT THE EUROPEAN STROKE CONFERENCE
Major finding: Fewer proximal DVTs occurred at 30 days with intermittent pneumatic compression than with usual care (8.5% vs. 12.1%, P = .001).
Data source: CLOTS 3, a multicenter trial of 2,876 immobile patients with stroke treated with or without intermittent pneumatic compression in addition to usual care.
Disclosures: Dr. Dennis, Dr. Rudd, and Dr. Roffe had no relevant disclosures. The University of Edinburgh and NHS Lothian sponsored the study with funding from the Chief Scientist Office of the Scottish Government, the National Institute of Heath Research Health Technology Assessment Programme, and the Scottish Stroke Research Network. Covidien provided the equipment used in the study free of charge.
Early, intensive BP drop benefits stroke patients
LONDON – Receipt of early, intensive blood pressure–lowering treatment improved functional outcomes in patients with acute intracerebral hemorrhage in a large, randomized trial.
There was a significant, favorable shift in the distribution of modified Rankin Scale (mRS) scores with a more intensive approach to blood pressuring lowering than guideline-recommended treatment, resulting in lower levels of disability (odds ratio, 0.87; P = .04).
However, the results of the eagerly anticipated INTERACT2 trial showed only a modest and nonsignificant reduction in the primary endpoint of death and disability (OR, 0.87; P less than .06).
The trial’s findings are still clinically relevant, the study’s investigators believe, and the approach now warrants implementation in routine clinical practice in most patients with acute intracerebral hemorrhage (ICH), particularly as there were no undue safety concerns when compared with the guideline-recommended approach.
"Blood pressure lowering in acute ICH is safe, so go early, go intensive, and go sustained in most of our patients because it improves the chances of a better recovery in survivors," said Dr. Craig Anderson, of the George Institute for Global Health in Sydney, Australia. "Ultimately, that’s what patients and families want us to do."
Dr. Anderson, who is the study’s chief investigator and is also professor of stroke medicine and clinical neuroscience at the University of Sydney, presented the study’s findings on May 29 at the annual European Stroke Conference; the results were also published simultaneously in the New England Journal of Medicine (doi: 10.1056/NEJMoa1214609).
Design of INTERACT2
INTERACT2 (Second Intensive Blood Pressure Reduction in Acute Cerebral Haemorrhage Trial) was an international, multicenter, prospective, open-label, blinded-endpoint, randomized trial of 2,839 patients. Patients were recruited within 6 hours of onset of spontaneous ICH and had elevated systolic blood pressure (150-220 mm Hg). The mean age was 63.5 years, and most patients were men (63%), Chinese (68%), and hypertensive (72%).
Patients were centrally allocated to randomly receive either intensive (n = 1,399) or guideline-recommended (n = 1,430) blood pressure–lowering treatment. Intensive treatment aimed to lower the systolic blood pressure rapidly, within 1 hour, to a target of less than 140 mm Hg. By contrast, guideline-recommended treatment aimed to reduce systolic blood pressure below a target of 180 mm Hg with no time limit.
The antihypertensive agents used were not stipulated in the study protocol, and hence a variety of agents were used by the 141 hospitals that participated in the trial. Intravenous agents were used in 90% of patients in the intensive-treatment arm versus 43% in the conventionally treated arm. Intensively managed patients were more likely to receive an IV bolus dose and an infusion (30% vs. 18%) or multiple agents (27% vs. 8%). The most commonly used agent was urapidil, an alpha-blocker available in some parts of Europe, but not in the United States.
Key findings
The primary measure of a poor outcome, defined as an mRS score of 3-6 at 90 days, was observed in 719 (52%) of intensively treated patients versus 785 (55.6%) of guideline-treated patients. An mRS score of 6 indicates death, and 3-5 denotes major disability.
An ordinal analysis of the mRS showed lower scores with the intensive than with the guideline-recommended blood pressure–lowering approach, with more patients able to remain independent despite having some level of disability (mRS score 0-2 in 48% of intensively treated vs. 44% of guideline-treated patients).
Health-related quality of life was assessed using the European Quality of Life-5 Dimensions questionnaire, and intensively treated patients reported fewer problems in mobility (64% vs. 67%; P = .13), self-care (47% vs. 52%; P = .02), usual activities (61% vs. 66%; P = .006), pain or discomfort (40% vs. 45%; P = .01), and anxiety or depression (34% vs. 38%, P = .05) than did those in the standard-treatment arm.
"I’ve never seen this in a clinical trial before," Dr. Anderson observed. "It’s very hard to find a signal in clinical trials, and I guess I was surprised by the degree of significance in benefit on usual activities, which is a very high measure of functional recovery when the patient is home."
Nonfatal serious adverse events occurred in a similar proportion of intensively treated and guideline-treated patients (23.3% and 23.6%, respectively). "The important thing is that we tried to look for any hazard, and we found no excess harms any way we looked at it," Dr. Anderson reported.
Implications for current practice
"If the results of this study with respect to the primary outcome were not as robust as some may have hoped, practitioners should be reassured by the safety data," Dr. Jennifer Frontera of the Cleveland Clinic Foundation commented in an editorial accompanying the NEJM paper.
There were no significant differences between the two treatment approaches in terms of neurologic deterioration, expansion of the ICH, ischemic stroke, cardiovascular events, or severe symptomatic hypotension, she noted.
The ongoing ATACH II (Antihypertensive Treatment of Acute Cerebral Hemorrhage) trial should hopefully shed more light on the benefit of early blood pressure lowering after ICH onset. ATACH II has similar blood pressure–lowering targets and primary and secondary endpoints as INTERACT2. A key difference, however, is that nicardipine is being used as the sole blood pressure–lowering agent. Results should be available in 2016.
Dr. Philip Bath, professor of stroke medicine and chair of the division of stroke at the University of Nottingham, England, who chaired the session in which the INTERACT2 findings were revealed, said that it is still too early to change the guidelines, particularly in view of the fact that several other trials are also ongoing. These include the ENOS (Efficacy of Nitric Oxide on Stroke) trial, of which he is the principal investigator.
"I think randomized data that are near are probably more important than guidelines, so I would always favor more data. It’s not as if we are going to have to wait a very long time; they will be there in the next year or two," Dr. Bath argued.
Study investigator Dr. Christian Stapf, professor of neurology at Université Diderot–Sorbonne Paris Cité, France, commented that, as a clinical scientist, he agreed that more data would be preferable before changing practice.
As a clinician, however, he said, "At this stage I don’t see the risk of any harm. I have no reason not to do it, and if anything it will only help."
Dr. Anderson further commented at a press briefing: "We have presented, for the first time, a strategy that can improve recovery from ICH, the most devastating type of stroke." He emphasized that it was rapid and intensive blood pressure lowering, rather than the use of any specific treatment, that was important in the trial.
This is very good news for the stroke community, Dr. Anderson argued. "We would expect guidelines to change and clinical practice to change as a result of this strategy."
The National Health and Medical Research Council of Australia funded INTERACT2, which was an investigator-led trial. The experts cited in this report had no relevant disclosures.
LONDON – Receipt of early, intensive blood pressure–lowering treatment improved functional outcomes in patients with acute intracerebral hemorrhage in a large, randomized trial.
There was a significant, favorable shift in the distribution of modified Rankin Scale (mRS) scores with a more intensive approach to blood pressuring lowering than guideline-recommended treatment, resulting in lower levels of disability (odds ratio, 0.87; P = .04).
However, the results of the eagerly anticipated INTERACT2 trial showed only a modest and nonsignificant reduction in the primary endpoint of death and disability (OR, 0.87; P less than .06).
The trial’s findings are still clinically relevant, the study’s investigators believe, and the approach now warrants implementation in routine clinical practice in most patients with acute intracerebral hemorrhage (ICH), particularly as there were no undue safety concerns when compared with the guideline-recommended approach.
"Blood pressure lowering in acute ICH is safe, so go early, go intensive, and go sustained in most of our patients because it improves the chances of a better recovery in survivors," said Dr. Craig Anderson, of the George Institute for Global Health in Sydney, Australia. "Ultimately, that’s what patients and families want us to do."
Dr. Anderson, who is the study’s chief investigator and is also professor of stroke medicine and clinical neuroscience at the University of Sydney, presented the study’s findings on May 29 at the annual European Stroke Conference; the results were also published simultaneously in the New England Journal of Medicine (doi: 10.1056/NEJMoa1214609).
Design of INTERACT2
INTERACT2 (Second Intensive Blood Pressure Reduction in Acute Cerebral Haemorrhage Trial) was an international, multicenter, prospective, open-label, blinded-endpoint, randomized trial of 2,839 patients. Patients were recruited within 6 hours of onset of spontaneous ICH and had elevated systolic blood pressure (150-220 mm Hg). The mean age was 63.5 years, and most patients were men (63%), Chinese (68%), and hypertensive (72%).
Patients were centrally allocated to randomly receive either intensive (n = 1,399) or guideline-recommended (n = 1,430) blood pressure–lowering treatment. Intensive treatment aimed to lower the systolic blood pressure rapidly, within 1 hour, to a target of less than 140 mm Hg. By contrast, guideline-recommended treatment aimed to reduce systolic blood pressure below a target of 180 mm Hg with no time limit.
The antihypertensive agents used were not stipulated in the study protocol, and hence a variety of agents were used by the 141 hospitals that participated in the trial. Intravenous agents were used in 90% of patients in the intensive-treatment arm versus 43% in the conventionally treated arm. Intensively managed patients were more likely to receive an IV bolus dose and an infusion (30% vs. 18%) or multiple agents (27% vs. 8%). The most commonly used agent was urapidil, an alpha-blocker available in some parts of Europe, but not in the United States.
Key findings
The primary measure of a poor outcome, defined as an mRS score of 3-6 at 90 days, was observed in 719 (52%) of intensively treated patients versus 785 (55.6%) of guideline-treated patients. An mRS score of 6 indicates death, and 3-5 denotes major disability.
An ordinal analysis of the mRS showed lower scores with the intensive than with the guideline-recommended blood pressure–lowering approach, with more patients able to remain independent despite having some level of disability (mRS score 0-2 in 48% of intensively treated vs. 44% of guideline-treated patients).
Health-related quality of life was assessed using the European Quality of Life-5 Dimensions questionnaire, and intensively treated patients reported fewer problems in mobility (64% vs. 67%; P = .13), self-care (47% vs. 52%; P = .02), usual activities (61% vs. 66%; P = .006), pain or discomfort (40% vs. 45%; P = .01), and anxiety or depression (34% vs. 38%, P = .05) than did those in the standard-treatment arm.
"I’ve never seen this in a clinical trial before," Dr. Anderson observed. "It’s very hard to find a signal in clinical trials, and I guess I was surprised by the degree of significance in benefit on usual activities, which is a very high measure of functional recovery when the patient is home."
Nonfatal serious adverse events occurred in a similar proportion of intensively treated and guideline-treated patients (23.3% and 23.6%, respectively). "The important thing is that we tried to look for any hazard, and we found no excess harms any way we looked at it," Dr. Anderson reported.
Implications for current practice
"If the results of this study with respect to the primary outcome were not as robust as some may have hoped, practitioners should be reassured by the safety data," Dr. Jennifer Frontera of the Cleveland Clinic Foundation commented in an editorial accompanying the NEJM paper.
There were no significant differences between the two treatment approaches in terms of neurologic deterioration, expansion of the ICH, ischemic stroke, cardiovascular events, or severe symptomatic hypotension, she noted.
The ongoing ATACH II (Antihypertensive Treatment of Acute Cerebral Hemorrhage) trial should hopefully shed more light on the benefit of early blood pressure lowering after ICH onset. ATACH II has similar blood pressure–lowering targets and primary and secondary endpoints as INTERACT2. A key difference, however, is that nicardipine is being used as the sole blood pressure–lowering agent. Results should be available in 2016.
Dr. Philip Bath, professor of stroke medicine and chair of the division of stroke at the University of Nottingham, England, who chaired the session in which the INTERACT2 findings were revealed, said that it is still too early to change the guidelines, particularly in view of the fact that several other trials are also ongoing. These include the ENOS (Efficacy of Nitric Oxide on Stroke) trial, of which he is the principal investigator.
"I think randomized data that are near are probably more important than guidelines, so I would always favor more data. It’s not as if we are going to have to wait a very long time; they will be there in the next year or two," Dr. Bath argued.
Study investigator Dr. Christian Stapf, professor of neurology at Université Diderot–Sorbonne Paris Cité, France, commented that, as a clinical scientist, he agreed that more data would be preferable before changing practice.
As a clinician, however, he said, "At this stage I don’t see the risk of any harm. I have no reason not to do it, and if anything it will only help."
Dr. Anderson further commented at a press briefing: "We have presented, for the first time, a strategy that can improve recovery from ICH, the most devastating type of stroke." He emphasized that it was rapid and intensive blood pressure lowering, rather than the use of any specific treatment, that was important in the trial.
This is very good news for the stroke community, Dr. Anderson argued. "We would expect guidelines to change and clinical practice to change as a result of this strategy."
The National Health and Medical Research Council of Australia funded INTERACT2, which was an investigator-led trial. The experts cited in this report had no relevant disclosures.
LONDON – Receipt of early, intensive blood pressure–lowering treatment improved functional outcomes in patients with acute intracerebral hemorrhage in a large, randomized trial.
There was a significant, favorable shift in the distribution of modified Rankin Scale (mRS) scores with a more intensive approach to blood pressuring lowering than guideline-recommended treatment, resulting in lower levels of disability (odds ratio, 0.87; P = .04).
However, the results of the eagerly anticipated INTERACT2 trial showed only a modest and nonsignificant reduction in the primary endpoint of death and disability (OR, 0.87; P less than .06).
The trial’s findings are still clinically relevant, the study’s investigators believe, and the approach now warrants implementation in routine clinical practice in most patients with acute intracerebral hemorrhage (ICH), particularly as there were no undue safety concerns when compared with the guideline-recommended approach.
"Blood pressure lowering in acute ICH is safe, so go early, go intensive, and go sustained in most of our patients because it improves the chances of a better recovery in survivors," said Dr. Craig Anderson, of the George Institute for Global Health in Sydney, Australia. "Ultimately, that’s what patients and families want us to do."
Dr. Anderson, who is the study’s chief investigator and is also professor of stroke medicine and clinical neuroscience at the University of Sydney, presented the study’s findings on May 29 at the annual European Stroke Conference; the results were also published simultaneously in the New England Journal of Medicine (doi: 10.1056/NEJMoa1214609).
Design of INTERACT2
INTERACT2 (Second Intensive Blood Pressure Reduction in Acute Cerebral Haemorrhage Trial) was an international, multicenter, prospective, open-label, blinded-endpoint, randomized trial of 2,839 patients. Patients were recruited within 6 hours of onset of spontaneous ICH and had elevated systolic blood pressure (150-220 mm Hg). The mean age was 63.5 years, and most patients were men (63%), Chinese (68%), and hypertensive (72%).
Patients were centrally allocated to randomly receive either intensive (n = 1,399) or guideline-recommended (n = 1,430) blood pressure–lowering treatment. Intensive treatment aimed to lower the systolic blood pressure rapidly, within 1 hour, to a target of less than 140 mm Hg. By contrast, guideline-recommended treatment aimed to reduce systolic blood pressure below a target of 180 mm Hg with no time limit.
The antihypertensive agents used were not stipulated in the study protocol, and hence a variety of agents were used by the 141 hospitals that participated in the trial. Intravenous agents were used in 90% of patients in the intensive-treatment arm versus 43% in the conventionally treated arm. Intensively managed patients were more likely to receive an IV bolus dose and an infusion (30% vs. 18%) or multiple agents (27% vs. 8%). The most commonly used agent was urapidil, an alpha-blocker available in some parts of Europe, but not in the United States.
Key findings
The primary measure of a poor outcome, defined as an mRS score of 3-6 at 90 days, was observed in 719 (52%) of intensively treated patients versus 785 (55.6%) of guideline-treated patients. An mRS score of 6 indicates death, and 3-5 denotes major disability.
An ordinal analysis of the mRS showed lower scores with the intensive than with the guideline-recommended blood pressure–lowering approach, with more patients able to remain independent despite having some level of disability (mRS score 0-2 in 48% of intensively treated vs. 44% of guideline-treated patients).
Health-related quality of life was assessed using the European Quality of Life-5 Dimensions questionnaire, and intensively treated patients reported fewer problems in mobility (64% vs. 67%; P = .13), self-care (47% vs. 52%; P = .02), usual activities (61% vs. 66%; P = .006), pain or discomfort (40% vs. 45%; P = .01), and anxiety or depression (34% vs. 38%, P = .05) than did those in the standard-treatment arm.
"I’ve never seen this in a clinical trial before," Dr. Anderson observed. "It’s very hard to find a signal in clinical trials, and I guess I was surprised by the degree of significance in benefit on usual activities, which is a very high measure of functional recovery when the patient is home."
Nonfatal serious adverse events occurred in a similar proportion of intensively treated and guideline-treated patients (23.3% and 23.6%, respectively). "The important thing is that we tried to look for any hazard, and we found no excess harms any way we looked at it," Dr. Anderson reported.
Implications for current practice
"If the results of this study with respect to the primary outcome were not as robust as some may have hoped, practitioners should be reassured by the safety data," Dr. Jennifer Frontera of the Cleveland Clinic Foundation commented in an editorial accompanying the NEJM paper.
There were no significant differences between the two treatment approaches in terms of neurologic deterioration, expansion of the ICH, ischemic stroke, cardiovascular events, or severe symptomatic hypotension, she noted.
The ongoing ATACH II (Antihypertensive Treatment of Acute Cerebral Hemorrhage) trial should hopefully shed more light on the benefit of early blood pressure lowering after ICH onset. ATACH II has similar blood pressure–lowering targets and primary and secondary endpoints as INTERACT2. A key difference, however, is that nicardipine is being used as the sole blood pressure–lowering agent. Results should be available in 2016.
Dr. Philip Bath, professor of stroke medicine and chair of the division of stroke at the University of Nottingham, England, who chaired the session in which the INTERACT2 findings were revealed, said that it is still too early to change the guidelines, particularly in view of the fact that several other trials are also ongoing. These include the ENOS (Efficacy of Nitric Oxide on Stroke) trial, of which he is the principal investigator.
"I think randomized data that are near are probably more important than guidelines, so I would always favor more data. It’s not as if we are going to have to wait a very long time; they will be there in the next year or two," Dr. Bath argued.
Study investigator Dr. Christian Stapf, professor of neurology at Université Diderot–Sorbonne Paris Cité, France, commented that, as a clinical scientist, he agreed that more data would be preferable before changing practice.
As a clinician, however, he said, "At this stage I don’t see the risk of any harm. I have no reason not to do it, and if anything it will only help."
Dr. Anderson further commented at a press briefing: "We have presented, for the first time, a strategy that can improve recovery from ICH, the most devastating type of stroke." He emphasized that it was rapid and intensive blood pressure lowering, rather than the use of any specific treatment, that was important in the trial.
This is very good news for the stroke community, Dr. Anderson argued. "We would expect guidelines to change and clinical practice to change as a result of this strategy."
The National Health and Medical Research Council of Australia funded INTERACT2, which was an investigator-led trial. The experts cited in this report had no relevant disclosures.
AT THE EUROPEAN STROKE CONFERENCE
Major finding: The odds ratio for intensive blood pressure lowering preventing death or disability, compared with guideline-recommended treatment, was 0.87 (P = .06).
Data source: INTERACT2, an international, multicenter, prospective, open-label, blinded-endpoint, randomized trial of 2,839 patients with elevated systolic blood pressure after spontaneous intracerebral hemorrhage, who were treated with an intensive or guideline-recommended blood pressure–lowering approach.
Disclosures: The National Health and Medical Research Council of Australia funded INTERACT2, which was an investigator-led trial. The experts cited in this report had no relevant disclosures.