User login
Sharon Worcester is an award-winning medical journalist for MDedge News. She has been with the company since 1996, first as the Southeast Bureau Chief (1996-2009) when the company was known as International Medical News Group, then as a freelance writer (2010-2015) before returning as a reporter in 2015. She previously worked as a daily newspaper reporter covering health and local government. Sharon currently reports primarily on oncology and hematology. She has a BA from Eckerd College and an MA in Mass Communication/Print Journalism from the University of Florida. Connect with her via LinkedIn and follow her on twitter @SW_MedReporter.
ECG finding predicts AFib in ibrutinib-treated CLL
NEW YORK – A left atrial abnormality on a pretreatment electrocardiogram (ECG) is a moderately specific and sensitive finding that independently predicts risk for developing atrial fibrillation in chronic lymphocytic leukemia patients starting on ibrutinib, findings from a retrospective cohort study indicate.
ECGs are inexpensive and available in most physician’s offices. Routinely checking for a left atrial abnormality before starting ibrutinib would identify a patient subgroup that would benefit from increased monitoring and allow for proactive intervention strategies to reduce complications should atrial fibrillation develop, Anthony Mato, MD, said at the annual meeting of the International Workshop on Chronic Lymphocytic Leukemia.
Prior studies, including the RESONATE and RESONATE 2 trials, have clearly demonstrated a link between ibrutinib exposure and the development of AFib. Long-term follow-up data suggest an estimated incidence of 9% to 11%.
Dr. Mato and his colleagues used a case-control design within a two-center retrospective cohort study to test the hypothesis that pre-ibrutinib left atrial abnormality, as determined by the ECG, can identify patients at increased risk for AFib during ibrutinib-based therapy.
Of 153 consecutive CLL patients who were treated with ibrutinib 420 mg/day, 11% developed new AFib at a median of 7 months after starting treatment. Discontinuation of ibrutinib because of AFib was low, with less than 2% of the entire cohort discontinuing treatment.
Based on findings in 20 case patients and 24 controls with an available pretreatment ECG, the presence of a left atrial abnormality before ibrutinib therapy was associated with a nine times increased risk of subsequently developing AFib.
“We looked at baseline hypertension, coronary disease, diabetes, age, and sex, and, although hypertension, coronary disease, and age appeared to have some effect, they weren’t as significant as left atrial abnormality” for predicting risk of AFib, Dr. Mato noted.
On multivariate analysis, controlling for hypertension, coronary disease, and age, a left atrial abnormality continued to be a significant predictor of AFib (odds ratio, 6.6).
“We then wanted to make this more practical for clinicians who may potentially perform an ECG to estimate risk,” he said, noting that ECG test characteristics associated with left atrial abnormality were defined: Sensitivity was estimated to be 79%, specificity was 71%, positive and negative likelihood ratios were 2.7 and 0.3, respectively. Positive predictive value was 68%, and negative predictive value was 81%.
The area under the ROC curve for this single predictor was 75%, he said.
The median age of the cohort at CLL diagnosis was 61 years, and the median age at ibrutinib start was 70. Patients had undergone a median of 2 prior lines of therapy, and 87% were treated in the relapsed/refractory setting.
The median follow-up was 17 months, and the median time from CLL diagnosis to the start of ibrutinib was 73 months.
Cardiovascular characteristics prior to treatment included smoking or former smoking in 49%, hypertension in 42%, hyperlipidemia in 39%, diabetes in 17%, coronary artery disease in 12%, and valvular heart disease in 5%.
Controls were matched to cases on baseline characteristics, and only those with no pretreatment history of AFib, a pretreatment ECG, and therapeutic ibrutinib dosing (420 mg/day for at least 4 months) were included.
To minimize bias, all ECGs were reviewed by a cardio-oncologist blinded to clinical outcomes.
The findings need prospective validation, as they are limited by the retrospective study design, lack of balance with respect to cardiovascular characteristics among cases and controls, a small number of atrial fibrillation cases, and variable timing of pre-ibrutinib ECG, he said.
Patients should be educated about the signs and symptoms of AFib. “The development of AFib during ibrutinib treatment should not prevent its continuation. These patients should be managed medically,” he added.
Dr. Mato reported having no disclosures.
NEW YORK – A left atrial abnormality on a pretreatment electrocardiogram (ECG) is a moderately specific and sensitive finding that independently predicts risk for developing atrial fibrillation in chronic lymphocytic leukemia patients starting on ibrutinib, findings from a retrospective cohort study indicate.
ECGs are inexpensive and available in most physician’s offices. Routinely checking for a left atrial abnormality before starting ibrutinib would identify a patient subgroup that would benefit from increased monitoring and allow for proactive intervention strategies to reduce complications should atrial fibrillation develop, Anthony Mato, MD, said at the annual meeting of the International Workshop on Chronic Lymphocytic Leukemia.
Prior studies, including the RESONATE and RESONATE 2 trials, have clearly demonstrated a link between ibrutinib exposure and the development of AFib. Long-term follow-up data suggest an estimated incidence of 9% to 11%.
Dr. Mato and his colleagues used a case-control design within a two-center retrospective cohort study to test the hypothesis that pre-ibrutinib left atrial abnormality, as determined by the ECG, can identify patients at increased risk for AFib during ibrutinib-based therapy.
Of 153 consecutive CLL patients who were treated with ibrutinib 420 mg/day, 11% developed new AFib at a median of 7 months after starting treatment. Discontinuation of ibrutinib because of AFib was low, with less than 2% of the entire cohort discontinuing treatment.
Based on findings in 20 case patients and 24 controls with an available pretreatment ECG, the presence of a left atrial abnormality before ibrutinib therapy was associated with a nine times increased risk of subsequently developing AFib.
“We looked at baseline hypertension, coronary disease, diabetes, age, and sex, and, although hypertension, coronary disease, and age appeared to have some effect, they weren’t as significant as left atrial abnormality” for predicting risk of AFib, Dr. Mato noted.
On multivariate analysis, controlling for hypertension, coronary disease, and age, a left atrial abnormality continued to be a significant predictor of AFib (odds ratio, 6.6).
“We then wanted to make this more practical for clinicians who may potentially perform an ECG to estimate risk,” he said, noting that ECG test characteristics associated with left atrial abnormality were defined: Sensitivity was estimated to be 79%, specificity was 71%, positive and negative likelihood ratios were 2.7 and 0.3, respectively. Positive predictive value was 68%, and negative predictive value was 81%.
The area under the ROC curve for this single predictor was 75%, he said.
The median age of the cohort at CLL diagnosis was 61 years, and the median age at ibrutinib start was 70. Patients had undergone a median of 2 prior lines of therapy, and 87% were treated in the relapsed/refractory setting.
The median follow-up was 17 months, and the median time from CLL diagnosis to the start of ibrutinib was 73 months.
Cardiovascular characteristics prior to treatment included smoking or former smoking in 49%, hypertension in 42%, hyperlipidemia in 39%, diabetes in 17%, coronary artery disease in 12%, and valvular heart disease in 5%.
Controls were matched to cases on baseline characteristics, and only those with no pretreatment history of AFib, a pretreatment ECG, and therapeutic ibrutinib dosing (420 mg/day for at least 4 months) were included.
To minimize bias, all ECGs were reviewed by a cardio-oncologist blinded to clinical outcomes.
The findings need prospective validation, as they are limited by the retrospective study design, lack of balance with respect to cardiovascular characteristics among cases and controls, a small number of atrial fibrillation cases, and variable timing of pre-ibrutinib ECG, he said.
Patients should be educated about the signs and symptoms of AFib. “The development of AFib during ibrutinib treatment should not prevent its continuation. These patients should be managed medically,” he added.
Dr. Mato reported having no disclosures.
NEW YORK – A left atrial abnormality on a pretreatment electrocardiogram (ECG) is a moderately specific and sensitive finding that independently predicts risk for developing atrial fibrillation in chronic lymphocytic leukemia patients starting on ibrutinib, findings from a retrospective cohort study indicate.
ECGs are inexpensive and available in most physician’s offices. Routinely checking for a left atrial abnormality before starting ibrutinib would identify a patient subgroup that would benefit from increased monitoring and allow for proactive intervention strategies to reduce complications should atrial fibrillation develop, Anthony Mato, MD, said at the annual meeting of the International Workshop on Chronic Lymphocytic Leukemia.
Prior studies, including the RESONATE and RESONATE 2 trials, have clearly demonstrated a link between ibrutinib exposure and the development of AFib. Long-term follow-up data suggest an estimated incidence of 9% to 11%.
Dr. Mato and his colleagues used a case-control design within a two-center retrospective cohort study to test the hypothesis that pre-ibrutinib left atrial abnormality, as determined by the ECG, can identify patients at increased risk for AFib during ibrutinib-based therapy.
Of 153 consecutive CLL patients who were treated with ibrutinib 420 mg/day, 11% developed new AFib at a median of 7 months after starting treatment. Discontinuation of ibrutinib because of AFib was low, with less than 2% of the entire cohort discontinuing treatment.
Based on findings in 20 case patients and 24 controls with an available pretreatment ECG, the presence of a left atrial abnormality before ibrutinib therapy was associated with a nine times increased risk of subsequently developing AFib.
“We looked at baseline hypertension, coronary disease, diabetes, age, and sex, and, although hypertension, coronary disease, and age appeared to have some effect, they weren’t as significant as left atrial abnormality” for predicting risk of AFib, Dr. Mato noted.
On multivariate analysis, controlling for hypertension, coronary disease, and age, a left atrial abnormality continued to be a significant predictor of AFib (odds ratio, 6.6).
“We then wanted to make this more practical for clinicians who may potentially perform an ECG to estimate risk,” he said, noting that ECG test characteristics associated with left atrial abnormality were defined: Sensitivity was estimated to be 79%, specificity was 71%, positive and negative likelihood ratios were 2.7 and 0.3, respectively. Positive predictive value was 68%, and negative predictive value was 81%.
The area under the ROC curve for this single predictor was 75%, he said.
The median age of the cohort at CLL diagnosis was 61 years, and the median age at ibrutinib start was 70. Patients had undergone a median of 2 prior lines of therapy, and 87% were treated in the relapsed/refractory setting.
The median follow-up was 17 months, and the median time from CLL diagnosis to the start of ibrutinib was 73 months.
Cardiovascular characteristics prior to treatment included smoking or former smoking in 49%, hypertension in 42%, hyperlipidemia in 39%, diabetes in 17%, coronary artery disease in 12%, and valvular heart disease in 5%.
Controls were matched to cases on baseline characteristics, and only those with no pretreatment history of AFib, a pretreatment ECG, and therapeutic ibrutinib dosing (420 mg/day for at least 4 months) were included.
To minimize bias, all ECGs were reviewed by a cardio-oncologist blinded to clinical outcomes.
The findings need prospective validation, as they are limited by the retrospective study design, lack of balance with respect to cardiovascular characteristics among cases and controls, a small number of atrial fibrillation cases, and variable timing of pre-ibrutinib ECG, he said.
Patients should be educated about the signs and symptoms of AFib. “The development of AFib during ibrutinib treatment should not prevent its continuation. These patients should be managed medically,” he added.
Dr. Mato reported having no disclosures.
AT THE IWCLL MEETING
Key clinical point:
Major finding: Left atrial abnormality as measured using ECG was a significant predictor of AFIB (adjusted odds ratio, 6.6).
Data source: A case-control study of 44 patients within a retrospective cohort.
Disclosures: Dr. Mato reported having no disclosures.
In good-candidate CLL, don’t wait too long for alloHCT
NEW YORK – Allogeneic hematopoietic stem cell transplantation (alloHCT) using HLA-compatible donors results in excellent long-term progression-free survival in younger high-risk chronic lymphocytic leukemia (CLL) patients, an analysis of data from a European Society for Blood and Marrow Transplantation registry cohort suggests.
AlloHCT may, in some patients, be preferable to sequential targeted therapy, according to Michel van Gelder, MD.
This is especially true for those progressing with Richter’s syndrome, who comprise about one-third of patients, he noted.
“On the other hand, allogeneic stem cell transplantation can induce prolonged progression-free survival,” said Dr. van Gelder of Maastricht (the Netherlands) University Medical Center.
Further, most alloHCT patients become minimal residual disease negative, which predicts prolonged progression-free survival (PFS).
“The down-side, of course, is nonrelapse mortality,” he said, noting that NRM depends on factors such as age, performance status, and HLA match.
In a recent risk factor analysis currently pending publication, he and his colleagues found, in a large group of patients, that age, performance status, remission at time of transplant, donor relationship, HLA and sex match each had an impact on 5-year PFS after alloHCT.
The more risk factors a patient had, the worse the outcome, he said.
Based on current knowledge, the place for alloHCT in CLL treatment is in patients with high-risk cytogenetics. Patients can be treated first with a kinase inhibitor or venetoclax followed by transplant, or they can wait for progression and then do the transplant, he said.
Those without high risk cytogenetics but with short PFS after treatment with a kinase inhibitor or venetoclax may also be candidates for alloHCT, he added.
“Preferably they should be young [and] have a good matched donor and low comorbidity,” he said.
In the current study, the focus was on younger CLL patients. “We tried to identify factors that predict for a low 2-year NRM and a high 8-year PFS. We studied the impact of high risk cytogenetics, and, for this study, we chose del(17p) and del(11q), and we tried to officialize the PFS, the relapse incidence, and the nonrelapse mortality of so-called ‘good transplant risk CLL patients’ with these high cytogenetic risk factors,” he explained.
In 197 patients under age 50 years (median 46 years) with a median follow-up of 90 months in an updated EBMT registry cohort, the most important relevant prognostic factor for 2-year NRM was the donor HLA match (adjusted hazard ratio, 2.5 for a matched unrelated donor, 4.0 for a partially matched unrelated donor, both vs. a matched sibling), and predictors for poor 8-year PFS were no remission at the time of alloHCT (hazard ratio, 1.7), and partially HLA matched unrelated donor (HR, 2.8).
High-risk cytogenetics did not significantly impact 8-year PFS, Dr. van Gelder said, noting that this confirms findings from prior studies.
Most of the patients included in the analysis were fludarabine refractory, 70% had del(17p), 35% had del(11q), and the median number of prior treatments was 3. Additionally, 12% had previous autologous transplant, 62% had remission at time of transplant, and most had good performance status, he said.
Conditioning regimens varied by site, 42% of patients had an HLA-matched sibling donor, and 50% had a matched unrelated donor.
Based on the regression model, a reference patient with high risk cytogenetics (del[17p] and/or del[11q]) and good transplant characteristics (age 46 years, no prior autologous stem cell transplantation, remission at the time of alloHCT and HLA- and sex-matched sibling donor) was created. A reference patient with poor transplant characteristics (not in remission at the time of transplant, with an unrelated, non-sex-matched donor) was also created. The predicted 2-year NRM for the good transplant risk patient was 12.1%, and 8-year PFS was more than 50%, Dr. van Gelder said.
For the poor risk patient, 2-year NRM was 37%, and PFS was below 50%, he said.
“So, in conclusion ... good transplant risk young patients with a low nonrelapse mortality and high 8-year progression-free survival can be identified,” he said.
The problem in clinical practice is determining whether – and when – to do a transplant in a young patient, he continued.
“There are a lot of possibilities. Nobody knows, of course, what is the best regimen, but a problem in these patients is that, if they have progression with Richter’s transformation, then you are lost,” he said. “So, if you would like to prevent this, and you have a patient with a low nonrelapse mortality risk, maybe it is better to do the transplant before.”
As for whether alloHCT can be done after kinase inhibitor therapy, the data are limited, but data presented at EBMT 2017 suggest the approach is feasible and effective. In 43 younger patients who underwent alloHCT after ibrutinib treatment, including 37% with TP53 mutation, the 1-year NRM and PFS rates were 9% and 63%, which is “in the same range as in the era before kinase inhibitors,” Dr. van Gelder said regarding the abstract presented by Peter Dreger, MD.
In 32 patients who underwent alloHCT after idelalisib treatment, including 44% with del(17p)/del(11q) and 85% in remission at the time of alloHCT, early follow-up showed that 6-month NRM and PFS was 7% and 83%, respectively, according to another abstract presented by Johannes Schetelig, MD.
“It’s all about balancing the risks. On the one hand you can use sequential therapies. On the other, if you have patients with high-risk cytogenetics [and] CLL in remission and you have a well-matched donor, maybe you should consider the transplant earlier, Dr. van Gelder said. “If you have a good transplant patient in remission, I would propose [that you] don’t wait too long.”
Dr. van Gelder reported having no relevant disclosures.
NEW YORK – Allogeneic hematopoietic stem cell transplantation (alloHCT) using HLA-compatible donors results in excellent long-term progression-free survival in younger high-risk chronic lymphocytic leukemia (CLL) patients, an analysis of data from a European Society for Blood and Marrow Transplantation registry cohort suggests.
AlloHCT may, in some patients, be preferable to sequential targeted therapy, according to Michel van Gelder, MD.
This is especially true for those progressing with Richter’s syndrome, who comprise about one-third of patients, he noted.
“On the other hand, allogeneic stem cell transplantation can induce prolonged progression-free survival,” said Dr. van Gelder of Maastricht (the Netherlands) University Medical Center.
Further, most alloHCT patients become minimal residual disease negative, which predicts prolonged progression-free survival (PFS).
“The down-side, of course, is nonrelapse mortality,” he said, noting that NRM depends on factors such as age, performance status, and HLA match.
In a recent risk factor analysis currently pending publication, he and his colleagues found, in a large group of patients, that age, performance status, remission at time of transplant, donor relationship, HLA and sex match each had an impact on 5-year PFS after alloHCT.
The more risk factors a patient had, the worse the outcome, he said.
Based on current knowledge, the place for alloHCT in CLL treatment is in patients with high-risk cytogenetics. Patients can be treated first with a kinase inhibitor or venetoclax followed by transplant, or they can wait for progression and then do the transplant, he said.
Those without high risk cytogenetics but with short PFS after treatment with a kinase inhibitor or venetoclax may also be candidates for alloHCT, he added.
“Preferably they should be young [and] have a good matched donor and low comorbidity,” he said.
In the current study, the focus was on younger CLL patients. “We tried to identify factors that predict for a low 2-year NRM and a high 8-year PFS. We studied the impact of high risk cytogenetics, and, for this study, we chose del(17p) and del(11q), and we tried to officialize the PFS, the relapse incidence, and the nonrelapse mortality of so-called ‘good transplant risk CLL patients’ with these high cytogenetic risk factors,” he explained.
In 197 patients under age 50 years (median 46 years) with a median follow-up of 90 months in an updated EBMT registry cohort, the most important relevant prognostic factor for 2-year NRM was the donor HLA match (adjusted hazard ratio, 2.5 for a matched unrelated donor, 4.0 for a partially matched unrelated donor, both vs. a matched sibling), and predictors for poor 8-year PFS were no remission at the time of alloHCT (hazard ratio, 1.7), and partially HLA matched unrelated donor (HR, 2.8).
High-risk cytogenetics did not significantly impact 8-year PFS, Dr. van Gelder said, noting that this confirms findings from prior studies.
Most of the patients included in the analysis were fludarabine refractory, 70% had del(17p), 35% had del(11q), and the median number of prior treatments was 3. Additionally, 12% had previous autologous transplant, 62% had remission at time of transplant, and most had good performance status, he said.
Conditioning regimens varied by site, 42% of patients had an HLA-matched sibling donor, and 50% had a matched unrelated donor.
Based on the regression model, a reference patient with high risk cytogenetics (del[17p] and/or del[11q]) and good transplant characteristics (age 46 years, no prior autologous stem cell transplantation, remission at the time of alloHCT and HLA- and sex-matched sibling donor) was created. A reference patient with poor transplant characteristics (not in remission at the time of transplant, with an unrelated, non-sex-matched donor) was also created. The predicted 2-year NRM for the good transplant risk patient was 12.1%, and 8-year PFS was more than 50%, Dr. van Gelder said.
For the poor risk patient, 2-year NRM was 37%, and PFS was below 50%, he said.
“So, in conclusion ... good transplant risk young patients with a low nonrelapse mortality and high 8-year progression-free survival can be identified,” he said.
The problem in clinical practice is determining whether – and when – to do a transplant in a young patient, he continued.
“There are a lot of possibilities. Nobody knows, of course, what is the best regimen, but a problem in these patients is that, if they have progression with Richter’s transformation, then you are lost,” he said. “So, if you would like to prevent this, and you have a patient with a low nonrelapse mortality risk, maybe it is better to do the transplant before.”
As for whether alloHCT can be done after kinase inhibitor therapy, the data are limited, but data presented at EBMT 2017 suggest the approach is feasible and effective. In 43 younger patients who underwent alloHCT after ibrutinib treatment, including 37% with TP53 mutation, the 1-year NRM and PFS rates were 9% and 63%, which is “in the same range as in the era before kinase inhibitors,” Dr. van Gelder said regarding the abstract presented by Peter Dreger, MD.
In 32 patients who underwent alloHCT after idelalisib treatment, including 44% with del(17p)/del(11q) and 85% in remission at the time of alloHCT, early follow-up showed that 6-month NRM and PFS was 7% and 83%, respectively, according to another abstract presented by Johannes Schetelig, MD.
“It’s all about balancing the risks. On the one hand you can use sequential therapies. On the other, if you have patients with high-risk cytogenetics [and] CLL in remission and you have a well-matched donor, maybe you should consider the transplant earlier, Dr. van Gelder said. “If you have a good transplant patient in remission, I would propose [that you] don’t wait too long.”
Dr. van Gelder reported having no relevant disclosures.
NEW YORK – Allogeneic hematopoietic stem cell transplantation (alloHCT) using HLA-compatible donors results in excellent long-term progression-free survival in younger high-risk chronic lymphocytic leukemia (CLL) patients, an analysis of data from a European Society for Blood and Marrow Transplantation registry cohort suggests.
AlloHCT may, in some patients, be preferable to sequential targeted therapy, according to Michel van Gelder, MD.
This is especially true for those progressing with Richter’s syndrome, who comprise about one-third of patients, he noted.
“On the other hand, allogeneic stem cell transplantation can induce prolonged progression-free survival,” said Dr. van Gelder of Maastricht (the Netherlands) University Medical Center.
Further, most alloHCT patients become minimal residual disease negative, which predicts prolonged progression-free survival (PFS).
“The down-side, of course, is nonrelapse mortality,” he said, noting that NRM depends on factors such as age, performance status, and HLA match.
In a recent risk factor analysis currently pending publication, he and his colleagues found, in a large group of patients, that age, performance status, remission at time of transplant, donor relationship, HLA and sex match each had an impact on 5-year PFS after alloHCT.
The more risk factors a patient had, the worse the outcome, he said.
Based on current knowledge, the place for alloHCT in CLL treatment is in patients with high-risk cytogenetics. Patients can be treated first with a kinase inhibitor or venetoclax followed by transplant, or they can wait for progression and then do the transplant, he said.
Those without high risk cytogenetics but with short PFS after treatment with a kinase inhibitor or venetoclax may also be candidates for alloHCT, he added.
“Preferably they should be young [and] have a good matched donor and low comorbidity,” he said.
In the current study, the focus was on younger CLL patients. “We tried to identify factors that predict for a low 2-year NRM and a high 8-year PFS. We studied the impact of high risk cytogenetics, and, for this study, we chose del(17p) and del(11q), and we tried to officialize the PFS, the relapse incidence, and the nonrelapse mortality of so-called ‘good transplant risk CLL patients’ with these high cytogenetic risk factors,” he explained.
In 197 patients under age 50 years (median 46 years) with a median follow-up of 90 months in an updated EBMT registry cohort, the most important relevant prognostic factor for 2-year NRM was the donor HLA match (adjusted hazard ratio, 2.5 for a matched unrelated donor, 4.0 for a partially matched unrelated donor, both vs. a matched sibling), and predictors for poor 8-year PFS were no remission at the time of alloHCT (hazard ratio, 1.7), and partially HLA matched unrelated donor (HR, 2.8).
High-risk cytogenetics did not significantly impact 8-year PFS, Dr. van Gelder said, noting that this confirms findings from prior studies.
Most of the patients included in the analysis were fludarabine refractory, 70% had del(17p), 35% had del(11q), and the median number of prior treatments was 3. Additionally, 12% had previous autologous transplant, 62% had remission at time of transplant, and most had good performance status, he said.
Conditioning regimens varied by site, 42% of patients had an HLA-matched sibling donor, and 50% had a matched unrelated donor.
Based on the regression model, a reference patient with high risk cytogenetics (del[17p] and/or del[11q]) and good transplant characteristics (age 46 years, no prior autologous stem cell transplantation, remission at the time of alloHCT and HLA- and sex-matched sibling donor) was created. A reference patient with poor transplant characteristics (not in remission at the time of transplant, with an unrelated, non-sex-matched donor) was also created. The predicted 2-year NRM for the good transplant risk patient was 12.1%, and 8-year PFS was more than 50%, Dr. van Gelder said.
For the poor risk patient, 2-year NRM was 37%, and PFS was below 50%, he said.
“So, in conclusion ... good transplant risk young patients with a low nonrelapse mortality and high 8-year progression-free survival can be identified,” he said.
The problem in clinical practice is determining whether – and when – to do a transplant in a young patient, he continued.
“There are a lot of possibilities. Nobody knows, of course, what is the best regimen, but a problem in these patients is that, if they have progression with Richter’s transformation, then you are lost,” he said. “So, if you would like to prevent this, and you have a patient with a low nonrelapse mortality risk, maybe it is better to do the transplant before.”
As for whether alloHCT can be done after kinase inhibitor therapy, the data are limited, but data presented at EBMT 2017 suggest the approach is feasible and effective. In 43 younger patients who underwent alloHCT after ibrutinib treatment, including 37% with TP53 mutation, the 1-year NRM and PFS rates were 9% and 63%, which is “in the same range as in the era before kinase inhibitors,” Dr. van Gelder said regarding the abstract presented by Peter Dreger, MD.
In 32 patients who underwent alloHCT after idelalisib treatment, including 44% with del(17p)/del(11q) and 85% in remission at the time of alloHCT, early follow-up showed that 6-month NRM and PFS was 7% and 83%, respectively, according to another abstract presented by Johannes Schetelig, MD.
“It’s all about balancing the risks. On the one hand you can use sequential therapies. On the other, if you have patients with high-risk cytogenetics [and] CLL in remission and you have a well-matched donor, maybe you should consider the transplant earlier, Dr. van Gelder said. “If you have a good transplant patient in remission, I would propose [that you] don’t wait too long.”
Dr. van Gelder reported having no relevant disclosures.
AT THE IWCLL MEETING
Key clinical point:
Major finding: The predicted 2-year nonrelapse mortality was 12.1% for a patient who is a good transplant risk and predicted 8-year PFS was more than 50%.
Data source: An analysis of updated registry data for 197 patients.
Disclosures: Dr. van Gelder reported having no relevant disclosures
Benefit of rtPA in acute ischemic stroke doesn’t diminish with weight over 100 kg
BOSTON – Body weight over 100 kg in acute ischemic stroke patients does not reduce the clinical benefit derived from a 90-mg fixed dose of intravenous recombinant tissue plasminogen activator, according to a pooled analysis of data from three randomized clinical trials.
Prior small studies have suggested that the magnitude of benefit with intravenous recombinant tissue plasminogen activator (IV rtPA) is reduced in patients with body weight over 100 kg who receive less than 0.9 mg/kg of IV rtPA under current guidelines. However, in the current study, the rate of favorable outcomes at 90 days – defined as modified Rankin scale score of 0-2 – did not differ significantly between 872 patients with weight at or below 100 kg and 105 with body weight over 100 kg (and up to 190 kg) after adjustment for patient demographics, stroke severity, and 90-day modified Rankin scale score (adjusted odds ratio, 0.99), Shahram Majidi, MD, said at the annual meeting of the American Academy of Neurology.
The results were similar when patients with NIHSS score less than 8 were excluded, and when those with weight over 150 kg were compared with those with weight at 100 kg or less, Dr. Majidi said.
There were only eight patients who weighed more than 150 kg, but those patients did very well at 90 days, and had favorable outcomes that were comparable to those in the lower weight group, he noted.
“Body weight more than 100 kg, and receiving less than 0.9 mg/kg of IV rtPA, did not reduce the benefit of IV rtPA in acute ischemic stroke patients, and our results support the current recommendations from the American Stroke Association,” Dr. Majidi concluded.
Dr. Majidi reported having no disclosures.
BOSTON – Body weight over 100 kg in acute ischemic stroke patients does not reduce the clinical benefit derived from a 90-mg fixed dose of intravenous recombinant tissue plasminogen activator, according to a pooled analysis of data from three randomized clinical trials.
Prior small studies have suggested that the magnitude of benefit with intravenous recombinant tissue plasminogen activator (IV rtPA) is reduced in patients with body weight over 100 kg who receive less than 0.9 mg/kg of IV rtPA under current guidelines. However, in the current study, the rate of favorable outcomes at 90 days – defined as modified Rankin scale score of 0-2 – did not differ significantly between 872 patients with weight at or below 100 kg and 105 with body weight over 100 kg (and up to 190 kg) after adjustment for patient demographics, stroke severity, and 90-day modified Rankin scale score (adjusted odds ratio, 0.99), Shahram Majidi, MD, said at the annual meeting of the American Academy of Neurology.
The results were similar when patients with NIHSS score less than 8 were excluded, and when those with weight over 150 kg were compared with those with weight at 100 kg or less, Dr. Majidi said.
There were only eight patients who weighed more than 150 kg, but those patients did very well at 90 days, and had favorable outcomes that were comparable to those in the lower weight group, he noted.
“Body weight more than 100 kg, and receiving less than 0.9 mg/kg of IV rtPA, did not reduce the benefit of IV rtPA in acute ischemic stroke patients, and our results support the current recommendations from the American Stroke Association,” Dr. Majidi concluded.
Dr. Majidi reported having no disclosures.
BOSTON – Body weight over 100 kg in acute ischemic stroke patients does not reduce the clinical benefit derived from a 90-mg fixed dose of intravenous recombinant tissue plasminogen activator, according to a pooled analysis of data from three randomized clinical trials.
Prior small studies have suggested that the magnitude of benefit with intravenous recombinant tissue plasminogen activator (IV rtPA) is reduced in patients with body weight over 100 kg who receive less than 0.9 mg/kg of IV rtPA under current guidelines. However, in the current study, the rate of favorable outcomes at 90 days – defined as modified Rankin scale score of 0-2 – did not differ significantly between 872 patients with weight at or below 100 kg and 105 with body weight over 100 kg (and up to 190 kg) after adjustment for patient demographics, stroke severity, and 90-day modified Rankin scale score (adjusted odds ratio, 0.99), Shahram Majidi, MD, said at the annual meeting of the American Academy of Neurology.
The results were similar when patients with NIHSS score less than 8 were excluded, and when those with weight over 150 kg were compared with those with weight at 100 kg or less, Dr. Majidi said.
There were only eight patients who weighed more than 150 kg, but those patients did very well at 90 days, and had favorable outcomes that were comparable to those in the lower weight group, he noted.
“Body weight more than 100 kg, and receiving less than 0.9 mg/kg of IV rtPA, did not reduce the benefit of IV rtPA in acute ischemic stroke patients, and our results support the current recommendations from the American Stroke Association,” Dr. Majidi concluded.
Dr. Majidi reported having no disclosures.
Key clinical point:
Major finding: Patients with weight at or below 100 kg and those with weight over 100 kg had a similar rate of favorable outcome at 90 days (adjusted OR, 0.99).
Data source: A pooled analysis of data from 977 patients in three randomized trials.
Disclosures: Dr. Majidi reported having no disclosures.
Ibrutinib response in CLL/SLL less affected by select risk factors
NEW YORK – Risk factors associated with poor outcomes in chronic lymphocytic leukemia/small lymphocytic leukemia patients treated with standard therapies appear to have less relevance with ibrutinib treatment, according to an integrated analysis of data from the randomized, phase III RESONATE, RESONATE 2, and HELIOS trials.
In the combined analysis, at a median follow-up of 21 months, progression-free survival (PFS), overall survival (OS), overall response rate (ORR), and complete response rate (CRR) were better in ibrutinib-treated patients than in comparator-treated patients – and, in the ibrutinib-treated patients, the outcomes did not differ based on the adverse genomic factors examined, Thomas J. Kipps, MD, PhD, said at the annual International Workshop on Chronic Lymphocytic Leukemia.
The trials compared well with each other, but differed in terms of number of prior therapies received by patients, he said. Furthermore, the analysis did not examine the effect of del(17p); patients with that deletion were included only in the RESONATE trial.
In RESONATE, ibrutinib was superior to ofatumumab in relapsed/refractory CLL/SLL. In RESONATE 2, ibrutinib was superior to chlorambucil in treatment-naive patients with CLL/SLL. In HELIOS, ibrutinib with bendamustine/rituximab was superior to placebo with bendamustine/rituximab in patients with relapsed/refractory CLL/SLL.
In the new multivariate analysis of the pooled data from these trials – adjusting for the four genomic risk factors and age, sex, ECOG performance status, cytopenia, lactate dehydrogenase (LDH), bulky disease, and number of prior therapies – only having had one or more vs. no prior therapies, and having two or more vs. one prior therapies was associated with shorter PFS and OS in ibrutinib-treated patients, with a trend toward significance.
In comparator-treated patients, however, unmutated IGHV, del(11q), complex karyotype, male sex, two or more prior therapies, and bulky disease all were associated with significantly shorter PFS. Complex karyotype, male sex, bulky disease, ECOG performance status greater than 1, and elevated LDH were associated with significantly shorter OS.
“We need to debate on what the significance of this is and how that can be incorporated into our idea about first-line therapies,” said Dr. Kipps, who was an investigator in both RESONATE trials and is a professor of medicine at the University of California, San Diego.
In univariate analysis of data from ibrutinib-treated patients, unmutated IGHV, del(11q), trisomy 12, and complex karyotype were generally not associated with shorter PFS, OS, or lower ORR or CRR.
Overall survival with and without unmutated IGHV was 78% and 84%, respectively; with and without trisomy 12 was 82% and 80%, respectively; and with and without complex karyotype was 77% and 78%, respectively.
ORR, for example, was comparable in the presence (90%) and absence (89%) of unmutated IGHV in ibrutinib-treated patients, as was CRR, at 29% and 26%, respectively.
In the presence and absence of trisomy 12, ORR was 85% and 91%, respectively; CRR was 33% and 22%.
In the presence and absence of complex karyotype, ORR was 88% and 89%, respectively, and CRR was 18% and 24%.
In the presence and absence of del(11q), ORR was 91% and 90%, respectively, and CRR was 22% and 27%.
The only difference that reached statistical significance was the complete response rate with trisomy 12, which favored the presence of trisomy 12.
Interestingly, the ibrutinib-treated patients with del(11q) had a trend toward longer PFS and OS, compared with those without del(11q), said Dr. Kipps.
At 36 months, PFS was 74% with the presence of del(11q) and 68% with the absence of del(11q) (hazard ratio, 0.73 vs. 1.88 in comparator-treated patients), and overall survival at 42 months was 80% in patients with del(11q) and 78% in those without del(11q) (HR, 0.73), Dr. Kipps said.
“The [finding in the] patients with the complex karyotype was a bit surprising, and I think this requires further analysis,” he said, explaining that complex karyotype actually was associated with a shorter PFS in patients treated on the comparator arm, and that this finding conflicts with earlier data.
The findings suggest ibrutinib-treated patients with trisomy 12, for reasons that are unclear, had a significantly higher complete response rate, but not greater PFS or OS vs. those without trisomy 12, Dr. Kipps said.
“It’s also interesting that ... unmutated antibody genes or del(11q) or complex karyotype were adverse prognostic factors in patients treated with comparator treatments, but not necessarily in patients treated with ibrutinib-based therapy,” he said.
Furthermore, although a prior phase II study involving heavily pretreated patients suggested that del(11q) may have adverse prognostic influence on PFS, that finding may not be borne out in patients with fewer lines of prior therapy.
The findings suggest that genomic risk factors associated with poor outcomes with initial chemoimmunotherapy may be less apparent in patients treated with ibrutinib.
“I think this is important, because it may then turn a prognostic factor into a predictive factor, meaning, a predictor of adverse outcomes for a given type of therapy as opposed to adverse prognostic value overall,” he concluded.
Ibrutinib (Imbruvica, Pharmacyclics) was approved by the Food and Drug Administration in January 2015 for the treatment of CLL after previous therapy. Dr. Kipps has received research funding from and/or served as a consultant or advisor for AbbVie, Genentech, Gilead, and Pharmacyclics, an AbbVie company.
sworcester@frontlinemedcom.com
NEW YORK – Risk factors associated with poor outcomes in chronic lymphocytic leukemia/small lymphocytic leukemia patients treated with standard therapies appear to have less relevance with ibrutinib treatment, according to an integrated analysis of data from the randomized, phase III RESONATE, RESONATE 2, and HELIOS trials.
In the combined analysis, at a median follow-up of 21 months, progression-free survival (PFS), overall survival (OS), overall response rate (ORR), and complete response rate (CRR) were better in ibrutinib-treated patients than in comparator-treated patients – and, in the ibrutinib-treated patients, the outcomes did not differ based on the adverse genomic factors examined, Thomas J. Kipps, MD, PhD, said at the annual International Workshop on Chronic Lymphocytic Leukemia.
The trials compared well with each other, but differed in terms of number of prior therapies received by patients, he said. Furthermore, the analysis did not examine the effect of del(17p); patients with that deletion were included only in the RESONATE trial.
In RESONATE, ibrutinib was superior to ofatumumab in relapsed/refractory CLL/SLL. In RESONATE 2, ibrutinib was superior to chlorambucil in treatment-naive patients with CLL/SLL. In HELIOS, ibrutinib with bendamustine/rituximab was superior to placebo with bendamustine/rituximab in patients with relapsed/refractory CLL/SLL.
In the new multivariate analysis of the pooled data from these trials – adjusting for the four genomic risk factors and age, sex, ECOG performance status, cytopenia, lactate dehydrogenase (LDH), bulky disease, and number of prior therapies – only having had one or more vs. no prior therapies, and having two or more vs. one prior therapies was associated with shorter PFS and OS in ibrutinib-treated patients, with a trend toward significance.
In comparator-treated patients, however, unmutated IGHV, del(11q), complex karyotype, male sex, two or more prior therapies, and bulky disease all were associated with significantly shorter PFS. Complex karyotype, male sex, bulky disease, ECOG performance status greater than 1, and elevated LDH were associated with significantly shorter OS.
“We need to debate on what the significance of this is and how that can be incorporated into our idea about first-line therapies,” said Dr. Kipps, who was an investigator in both RESONATE trials and is a professor of medicine at the University of California, San Diego.
In univariate analysis of data from ibrutinib-treated patients, unmutated IGHV, del(11q), trisomy 12, and complex karyotype were generally not associated with shorter PFS, OS, or lower ORR or CRR.
Overall survival with and without unmutated IGHV was 78% and 84%, respectively; with and without trisomy 12 was 82% and 80%, respectively; and with and without complex karyotype was 77% and 78%, respectively.
ORR, for example, was comparable in the presence (90%) and absence (89%) of unmutated IGHV in ibrutinib-treated patients, as was CRR, at 29% and 26%, respectively.
In the presence and absence of trisomy 12, ORR was 85% and 91%, respectively; CRR was 33% and 22%.
In the presence and absence of complex karyotype, ORR was 88% and 89%, respectively, and CRR was 18% and 24%.
In the presence and absence of del(11q), ORR was 91% and 90%, respectively, and CRR was 22% and 27%.
The only difference that reached statistical significance was the complete response rate with trisomy 12, which favored the presence of trisomy 12.
Interestingly, the ibrutinib-treated patients with del(11q) had a trend toward longer PFS and OS, compared with those without del(11q), said Dr. Kipps.
At 36 months, PFS was 74% with the presence of del(11q) and 68% with the absence of del(11q) (hazard ratio, 0.73 vs. 1.88 in comparator-treated patients), and overall survival at 42 months was 80% in patients with del(11q) and 78% in those without del(11q) (HR, 0.73), Dr. Kipps said.
“The [finding in the] patients with the complex karyotype was a bit surprising, and I think this requires further analysis,” he said, explaining that complex karyotype actually was associated with a shorter PFS in patients treated on the comparator arm, and that this finding conflicts with earlier data.
The findings suggest ibrutinib-treated patients with trisomy 12, for reasons that are unclear, had a significantly higher complete response rate, but not greater PFS or OS vs. those without trisomy 12, Dr. Kipps said.
“It’s also interesting that ... unmutated antibody genes or del(11q) or complex karyotype were adverse prognostic factors in patients treated with comparator treatments, but not necessarily in patients treated with ibrutinib-based therapy,” he said.
Furthermore, although a prior phase II study involving heavily pretreated patients suggested that del(11q) may have adverse prognostic influence on PFS, that finding may not be borne out in patients with fewer lines of prior therapy.
The findings suggest that genomic risk factors associated with poor outcomes with initial chemoimmunotherapy may be less apparent in patients treated with ibrutinib.
“I think this is important, because it may then turn a prognostic factor into a predictive factor, meaning, a predictor of adverse outcomes for a given type of therapy as opposed to adverse prognostic value overall,” he concluded.
Ibrutinib (Imbruvica, Pharmacyclics) was approved by the Food and Drug Administration in January 2015 for the treatment of CLL after previous therapy. Dr. Kipps has received research funding from and/or served as a consultant or advisor for AbbVie, Genentech, Gilead, and Pharmacyclics, an AbbVie company.
sworcester@frontlinemedcom.com
NEW YORK – Risk factors associated with poor outcomes in chronic lymphocytic leukemia/small lymphocytic leukemia patients treated with standard therapies appear to have less relevance with ibrutinib treatment, according to an integrated analysis of data from the randomized, phase III RESONATE, RESONATE 2, and HELIOS trials.
In the combined analysis, at a median follow-up of 21 months, progression-free survival (PFS), overall survival (OS), overall response rate (ORR), and complete response rate (CRR) were better in ibrutinib-treated patients than in comparator-treated patients – and, in the ibrutinib-treated patients, the outcomes did not differ based on the adverse genomic factors examined, Thomas J. Kipps, MD, PhD, said at the annual International Workshop on Chronic Lymphocytic Leukemia.
The trials compared well with each other, but differed in terms of number of prior therapies received by patients, he said. Furthermore, the analysis did not examine the effect of del(17p); patients with that deletion were included only in the RESONATE trial.
In RESONATE, ibrutinib was superior to ofatumumab in relapsed/refractory CLL/SLL. In RESONATE 2, ibrutinib was superior to chlorambucil in treatment-naive patients with CLL/SLL. In HELIOS, ibrutinib with bendamustine/rituximab was superior to placebo with bendamustine/rituximab in patients with relapsed/refractory CLL/SLL.
In the new multivariate analysis of the pooled data from these trials – adjusting for the four genomic risk factors and age, sex, ECOG performance status, cytopenia, lactate dehydrogenase (LDH), bulky disease, and number of prior therapies – only having had one or more vs. no prior therapies, and having two or more vs. one prior therapies was associated with shorter PFS and OS in ibrutinib-treated patients, with a trend toward significance.
In comparator-treated patients, however, unmutated IGHV, del(11q), complex karyotype, male sex, two or more prior therapies, and bulky disease all were associated with significantly shorter PFS. Complex karyotype, male sex, bulky disease, ECOG performance status greater than 1, and elevated LDH were associated with significantly shorter OS.
“We need to debate on what the significance of this is and how that can be incorporated into our idea about first-line therapies,” said Dr. Kipps, who was an investigator in both RESONATE trials and is a professor of medicine at the University of California, San Diego.
In univariate analysis of data from ibrutinib-treated patients, unmutated IGHV, del(11q), trisomy 12, and complex karyotype were generally not associated with shorter PFS, OS, or lower ORR or CRR.
Overall survival with and without unmutated IGHV was 78% and 84%, respectively; with and without trisomy 12 was 82% and 80%, respectively; and with and without complex karyotype was 77% and 78%, respectively.
ORR, for example, was comparable in the presence (90%) and absence (89%) of unmutated IGHV in ibrutinib-treated patients, as was CRR, at 29% and 26%, respectively.
In the presence and absence of trisomy 12, ORR was 85% and 91%, respectively; CRR was 33% and 22%.
In the presence and absence of complex karyotype, ORR was 88% and 89%, respectively, and CRR was 18% and 24%.
In the presence and absence of del(11q), ORR was 91% and 90%, respectively, and CRR was 22% and 27%.
The only difference that reached statistical significance was the complete response rate with trisomy 12, which favored the presence of trisomy 12.
Interestingly, the ibrutinib-treated patients with del(11q) had a trend toward longer PFS and OS, compared with those without del(11q), said Dr. Kipps.
At 36 months, PFS was 74% with the presence of del(11q) and 68% with the absence of del(11q) (hazard ratio, 0.73 vs. 1.88 in comparator-treated patients), and overall survival at 42 months was 80% in patients with del(11q) and 78% in those without del(11q) (HR, 0.73), Dr. Kipps said.
“The [finding in the] patients with the complex karyotype was a bit surprising, and I think this requires further analysis,” he said, explaining that complex karyotype actually was associated with a shorter PFS in patients treated on the comparator arm, and that this finding conflicts with earlier data.
The findings suggest ibrutinib-treated patients with trisomy 12, for reasons that are unclear, had a significantly higher complete response rate, but not greater PFS or OS vs. those without trisomy 12, Dr. Kipps said.
“It’s also interesting that ... unmutated antibody genes or del(11q) or complex karyotype were adverse prognostic factors in patients treated with comparator treatments, but not necessarily in patients treated with ibrutinib-based therapy,” he said.
Furthermore, although a prior phase II study involving heavily pretreated patients suggested that del(11q) may have adverse prognostic influence on PFS, that finding may not be borne out in patients with fewer lines of prior therapy.
The findings suggest that genomic risk factors associated with poor outcomes with initial chemoimmunotherapy may be less apparent in patients treated with ibrutinib.
“I think this is important, because it may then turn a prognostic factor into a predictive factor, meaning, a predictor of adverse outcomes for a given type of therapy as opposed to adverse prognostic value overall,” he concluded.
Ibrutinib (Imbruvica, Pharmacyclics) was approved by the Food and Drug Administration in January 2015 for the treatment of CLL after previous therapy. Dr. Kipps has received research funding from and/or served as a consultant or advisor for AbbVie, Genentech, Gilead, and Pharmacyclics, an AbbVie company.
sworcester@frontlinemedcom.com
AT THE IWCLL MEETING
Key clinical point:
Major finding: In ibrutinib-treated patients, overall survival with and without unmutated IGHV was 78% and 84%, respectively; with and without trisomy 12 was 82% and 80%, respectively; and with and without complex karyotype was 77% and 78%, respectively.
Data source: A pooled analysis of data from 1,210 patients from three randomized phase III trials.
Disclosures: Dr. Kipps has received research funding from and/or served as a consultant or advisor for AbbVie, Genentech, Gilead, and Pharmacyclics, an AbbVie company.
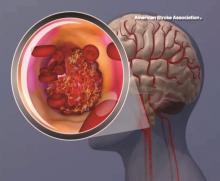
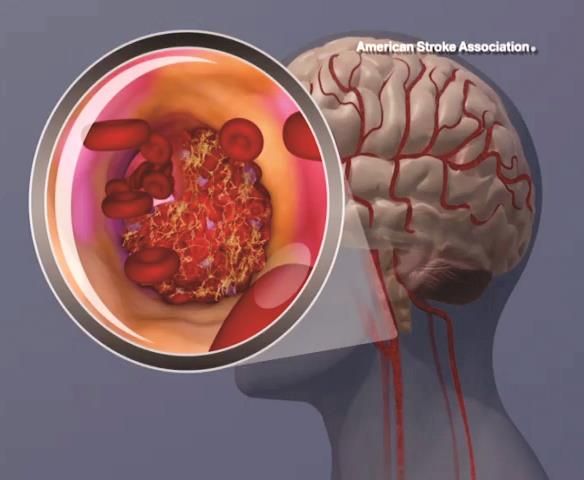
Intravenous tPA ups mortality in children with acute ischemic stroke
BOSTON – Intravenous thrombolysis with tissue plasminogen activator (tPA) is associated with adverse outcomes, including an increased risk of death, in children with acute ischemic stroke, based on a review of cases from the 2006-2010 Nationwide Inpatient Survey.
Of 20,587 patients aged 0-17 years who were included in the survey, 198 received an intervention, including tPA in 169 patients, intra-arterial thrombectomy (IAT) in 5 patients, and both tPA and IAT in 24 patients. The overall mortality was 7.8%, but in those who received tPA it was 13.8%, compared with 7.7% in those who did not, Kathryn Ess, MD, reported at the annual meeting of the American Academy of Neurology.
Other outcomes were also worse in those who received tPA. For example, untreated patients were more likely to be discharged home than were tPA-treated patients (67.8% vs. 47.5%), and intracerebral hemorrhage was more common in treated vs. untreated patients (10.1% vs. 3.8%). Costs for treated patients averaged $200,346 vs. $123,015 for untreated patients.
Children included in the review had a mean age of 6 years, 43.9% were girls, and 47.7% were white. Treated patients were older (10 years vs. 5.9 years), and comorbidities included Moyamoya disease in 12.4% of patients, cardiac valvular disease in 6.6%, and sickle cell disease in 6.5%. Those who received tPA had a higher prevalence of procoagulable conditions (15.2% vs. 2%). Of note, the higher prevalence of intracerebral hemorrhage in treated patients was not explained by Moyamoya or sickle cell disease, as patients with those comorbidities were less likely than those without those conditions to receive treatment, Dr. Ess said.
Though limited by the retrospective study design, small numbers of treated patients, a lack of data on stroke severity or functional outcomes, and the inclusion of data from years before newer thrombectomy devices became available, the findings highlight concerns about the safety and efficacy of tPA in children with ischemic stroke, she said, noting that few studies have looked at the utility of tPA with or without IAT in the pediatric population.
“Studies of the efficacy of ischemic stroke treatment in adults can’t necessarily be extrapolated to children,” she said, adding that this is especially true given the difference in etiologies of pediatric acute ischemic stroke.
Indeed, the findings underscore “the age-old adage that children are not just little adults,” said Andrew Southerland, MD, of the University of Virginia, Charlottesville, who was the discussant for the session.
“We need prospective clinical trials in children,” he said.
Dr. Ess and Dr. Southerland reported having no relevant financial disclosures.
BOSTON – Intravenous thrombolysis with tissue plasminogen activator (tPA) is associated with adverse outcomes, including an increased risk of death, in children with acute ischemic stroke, based on a review of cases from the 2006-2010 Nationwide Inpatient Survey.
Of 20,587 patients aged 0-17 years who were included in the survey, 198 received an intervention, including tPA in 169 patients, intra-arterial thrombectomy (IAT) in 5 patients, and both tPA and IAT in 24 patients. The overall mortality was 7.8%, but in those who received tPA it was 13.8%, compared with 7.7% in those who did not, Kathryn Ess, MD, reported at the annual meeting of the American Academy of Neurology.
Other outcomes were also worse in those who received tPA. For example, untreated patients were more likely to be discharged home than were tPA-treated patients (67.8% vs. 47.5%), and intracerebral hemorrhage was more common in treated vs. untreated patients (10.1% vs. 3.8%). Costs for treated patients averaged $200,346 vs. $123,015 for untreated patients.
Children included in the review had a mean age of 6 years, 43.9% were girls, and 47.7% were white. Treated patients were older (10 years vs. 5.9 years), and comorbidities included Moyamoya disease in 12.4% of patients, cardiac valvular disease in 6.6%, and sickle cell disease in 6.5%. Those who received tPA had a higher prevalence of procoagulable conditions (15.2% vs. 2%). Of note, the higher prevalence of intracerebral hemorrhage in treated patients was not explained by Moyamoya or sickle cell disease, as patients with those comorbidities were less likely than those without those conditions to receive treatment, Dr. Ess said.
Though limited by the retrospective study design, small numbers of treated patients, a lack of data on stroke severity or functional outcomes, and the inclusion of data from years before newer thrombectomy devices became available, the findings highlight concerns about the safety and efficacy of tPA in children with ischemic stroke, she said, noting that few studies have looked at the utility of tPA with or without IAT in the pediatric population.
“Studies of the efficacy of ischemic stroke treatment in adults can’t necessarily be extrapolated to children,” she said, adding that this is especially true given the difference in etiologies of pediatric acute ischemic stroke.
Indeed, the findings underscore “the age-old adage that children are not just little adults,” said Andrew Southerland, MD, of the University of Virginia, Charlottesville, who was the discussant for the session.
“We need prospective clinical trials in children,” he said.
Dr. Ess and Dr. Southerland reported having no relevant financial disclosures.
BOSTON – Intravenous thrombolysis with tissue plasminogen activator (tPA) is associated with adverse outcomes, including an increased risk of death, in children with acute ischemic stroke, based on a review of cases from the 2006-2010 Nationwide Inpatient Survey.
Of 20,587 patients aged 0-17 years who were included in the survey, 198 received an intervention, including tPA in 169 patients, intra-arterial thrombectomy (IAT) in 5 patients, and both tPA and IAT in 24 patients. The overall mortality was 7.8%, but in those who received tPA it was 13.8%, compared with 7.7% in those who did not, Kathryn Ess, MD, reported at the annual meeting of the American Academy of Neurology.
Other outcomes were also worse in those who received tPA. For example, untreated patients were more likely to be discharged home than were tPA-treated patients (67.8% vs. 47.5%), and intracerebral hemorrhage was more common in treated vs. untreated patients (10.1% vs. 3.8%). Costs for treated patients averaged $200,346 vs. $123,015 for untreated patients.
Children included in the review had a mean age of 6 years, 43.9% were girls, and 47.7% were white. Treated patients were older (10 years vs. 5.9 years), and comorbidities included Moyamoya disease in 12.4% of patients, cardiac valvular disease in 6.6%, and sickle cell disease in 6.5%. Those who received tPA had a higher prevalence of procoagulable conditions (15.2% vs. 2%). Of note, the higher prevalence of intracerebral hemorrhage in treated patients was not explained by Moyamoya or sickle cell disease, as patients with those comorbidities were less likely than those without those conditions to receive treatment, Dr. Ess said.
Though limited by the retrospective study design, small numbers of treated patients, a lack of data on stroke severity or functional outcomes, and the inclusion of data from years before newer thrombectomy devices became available, the findings highlight concerns about the safety and efficacy of tPA in children with ischemic stroke, she said, noting that few studies have looked at the utility of tPA with or without IAT in the pediatric population.
“Studies of the efficacy of ischemic stroke treatment in adults can’t necessarily be extrapolated to children,” she said, adding that this is especially true given the difference in etiologies of pediatric acute ischemic stroke.
Indeed, the findings underscore “the age-old adage that children are not just little adults,” said Andrew Southerland, MD, of the University of Virginia, Charlottesville, who was the discussant for the session.
“We need prospective clinical trials in children,” he said.
Dr. Ess and Dr. Southerland reported having no relevant financial disclosures.
AT AAN 2017
Key clinical point:
Major finding: Mortality for pediatric acute ischemic stroke was 7.8% overall, 7.7% in those who did not receive tPA, and 13.8% in those who did receive tPA.
Data source: A retrospective review of cases from the 2006-2010 Nationwide Inpatient Sample.
Disclosures: Dr. Ess and Dr. Southerland reported having no relevant financial disclosures.
Study supports link between pediatric MS and remote viral infections
BOSTON – Prior Epstein-Barr virus (EBV) infection and prior herpes simplex virus (HSV) infection each appear to be associated with development of pediatric-onset multiple sclerosis (MS), according to findings from a large national case-control study.
Samples from 360 children with MS or clinically isolated syndrome and 496 frequency-matched controls recruited from 16 pediatric MS centers across the United States were tested for EBV, cytomegalovirus (CMV), and HSV antibodies and for 25-(OH)-vitamin D levels. After adjusting for age, sex, and race/ethnicity, evidence of a remote infection with EBV was strongly associated with higher risk of pediatric-onset MS (odds ratio, 3.6), Bardia Nourbakhsh, MD, reported at the annual meeting of the American Academy of Neurology.
“We didn’t see an association between CMV and the risk of developing pediatric MS,” he said, noting that prior studies had shown a protective effect of prior CMV.
There was a trend toward an association between lower serum vitamin D levels and the risk of developing pediatric MS, but the findings are questionable because of vitamin D supplementation started after diagnosis in most patients, he noted.
Further, analysis showed that race also played a role in the relationships between prior infections and MS.
The association between HSV-1 and -2 infection was significant only among white patients, the association between prior EBV and MS was much stronger in whites than non-whites, and the association between EBV and MS was stronger in non-Hispanics than in Hispanics, he said.
The MS risk variant HLA DRB1*1501 also played a role in the associations. The association between prior HSV-1 and -2 infection and MS risk was apparent only in DRB1-negative individuals, and, conversely, the association between prior EBV and MS risk was much stronger in those who were DRB1-positive, he said.
Patients included in the study had a mean age of 15.2 years, 64% were girls, and the mean disease duration was 354 days. Controls had a mean age of 14.3 years.
“Remote viral infections have been known as one of the most commonly cited risk factors for adult and pediatric MS,” Dr. Nourbakhsh said, noting that a prior case-control study showed these associations and that other studies suggested associations with vitamin D deficiency.
The current study was conducted in an attempt to replicate those prior findings, he said.
The results of this large study support an association between prior EBV and HSV infections and MS risk and a possible association between vitamin D deficiency and MS risk but are limited by lack of testing before disease development and by vitamin D supplementation in almost all patients after diagnosis, he said.
“In the future, hopefully, we can look further at the interaction of genes and environment and the heterogeneity of the effect of risk factors in different subpopulations,” he concluded.
Dr. Nourbakhsh reported having no disclosures.
BOSTON – Prior Epstein-Barr virus (EBV) infection and prior herpes simplex virus (HSV) infection each appear to be associated with development of pediatric-onset multiple sclerosis (MS), according to findings from a large national case-control study.
Samples from 360 children with MS or clinically isolated syndrome and 496 frequency-matched controls recruited from 16 pediatric MS centers across the United States were tested for EBV, cytomegalovirus (CMV), and HSV antibodies and for 25-(OH)-vitamin D levels. After adjusting for age, sex, and race/ethnicity, evidence of a remote infection with EBV was strongly associated with higher risk of pediatric-onset MS (odds ratio, 3.6), Bardia Nourbakhsh, MD, reported at the annual meeting of the American Academy of Neurology.
“We didn’t see an association between CMV and the risk of developing pediatric MS,” he said, noting that prior studies had shown a protective effect of prior CMV.
There was a trend toward an association between lower serum vitamin D levels and the risk of developing pediatric MS, but the findings are questionable because of vitamin D supplementation started after diagnosis in most patients, he noted.
Further, analysis showed that race also played a role in the relationships between prior infections and MS.
The association between HSV-1 and -2 infection was significant only among white patients, the association between prior EBV and MS was much stronger in whites than non-whites, and the association between EBV and MS was stronger in non-Hispanics than in Hispanics, he said.
The MS risk variant HLA DRB1*1501 also played a role in the associations. The association between prior HSV-1 and -2 infection and MS risk was apparent only in DRB1-negative individuals, and, conversely, the association between prior EBV and MS risk was much stronger in those who were DRB1-positive, he said.
Patients included in the study had a mean age of 15.2 years, 64% were girls, and the mean disease duration was 354 days. Controls had a mean age of 14.3 years.
“Remote viral infections have been known as one of the most commonly cited risk factors for adult and pediatric MS,” Dr. Nourbakhsh said, noting that a prior case-control study showed these associations and that other studies suggested associations with vitamin D deficiency.
The current study was conducted in an attempt to replicate those prior findings, he said.
The results of this large study support an association between prior EBV and HSV infections and MS risk and a possible association between vitamin D deficiency and MS risk but are limited by lack of testing before disease development and by vitamin D supplementation in almost all patients after diagnosis, he said.
“In the future, hopefully, we can look further at the interaction of genes and environment and the heterogeneity of the effect of risk factors in different subpopulations,” he concluded.
Dr. Nourbakhsh reported having no disclosures.
BOSTON – Prior Epstein-Barr virus (EBV) infection and prior herpes simplex virus (HSV) infection each appear to be associated with development of pediatric-onset multiple sclerosis (MS), according to findings from a large national case-control study.
Samples from 360 children with MS or clinically isolated syndrome and 496 frequency-matched controls recruited from 16 pediatric MS centers across the United States were tested for EBV, cytomegalovirus (CMV), and HSV antibodies and for 25-(OH)-vitamin D levels. After adjusting for age, sex, and race/ethnicity, evidence of a remote infection with EBV was strongly associated with higher risk of pediatric-onset MS (odds ratio, 3.6), Bardia Nourbakhsh, MD, reported at the annual meeting of the American Academy of Neurology.
“We didn’t see an association between CMV and the risk of developing pediatric MS,” he said, noting that prior studies had shown a protective effect of prior CMV.
There was a trend toward an association between lower serum vitamin D levels and the risk of developing pediatric MS, but the findings are questionable because of vitamin D supplementation started after diagnosis in most patients, he noted.
Further, analysis showed that race also played a role in the relationships between prior infections and MS.
The association between HSV-1 and -2 infection was significant only among white patients, the association between prior EBV and MS was much stronger in whites than non-whites, and the association between EBV and MS was stronger in non-Hispanics than in Hispanics, he said.
The MS risk variant HLA DRB1*1501 also played a role in the associations. The association between prior HSV-1 and -2 infection and MS risk was apparent only in DRB1-negative individuals, and, conversely, the association between prior EBV and MS risk was much stronger in those who were DRB1-positive, he said.
Patients included in the study had a mean age of 15.2 years, 64% were girls, and the mean disease duration was 354 days. Controls had a mean age of 14.3 years.
“Remote viral infections have been known as one of the most commonly cited risk factors for adult and pediatric MS,” Dr. Nourbakhsh said, noting that a prior case-control study showed these associations and that other studies suggested associations with vitamin D deficiency.
The current study was conducted in an attempt to replicate those prior findings, he said.
The results of this large study support an association between prior EBV and HSV infections and MS risk and a possible association between vitamin D deficiency and MS risk but are limited by lack of testing before disease development and by vitamin D supplementation in almost all patients after diagnosis, he said.
“In the future, hopefully, we can look further at the interaction of genes and environment and the heterogeneity of the effect of risk factors in different subpopulations,” he concluded.
Dr. Nourbakhsh reported having no disclosures.
Key clinical point:
Major finding: Remote infections with EBV and HSV were associated with higher risk of pediatric-onset MS (odds ratios, 3.6 and 1.5, respectively).
Data source: A study of 360 pediatric MS patients and 496 controls.
Disclosures: Dr. Nourbakhsh reported having no disclosures.
Intensive BP lowering may reduce larger hematoma expansion in ICH
BOSTON – Intensive systolic blood pressure reduction did not significantly reduce hematoma expansion, compared with standard systolic blood pressure reduction in the Antihypertensive Treatment of Acute Cerebral Hemorrhage (ATACH) II trial, but, in a post hoc analysis, it did show a strong trend toward reducing hematoma expansion in those with a larger initial hematoma volume.
In 450 patients randomized to receive intensive treatment and 426 randomized to receive standard treatment in the large, international, phase III randomized trial, the proportion of patients with any hematoma expansion was 46.4% and 52.3% , respectively (relative risk, 0.89; 95% confidence interval, 0.73-1.07). The proportion with hematoma expansion with an increase of greater than 33% was 18.9% and 24.4%, respectively (RR, 0.77; 95% CI, 0.58-1.03), Joshua N. Goldstein, MD, reported at the annual meeting of the American Academy of Neurology.
The confidence intervals suggested only a trend toward improvement, as the difference between the groups did not reach statistical significance, he said.
To determine if intensive systolic blood pressure reduction might have more of an impact among higher-risk patients, the investigators conducted a post hoc, secondary analysis in those with relatively larger hematomas. Among patients with an initial hematoma volume of at least 10 mL (at least half of the study population had smaller hematomas), the proportion with any hematoma expansion was 53.8% in the intensive treatment group and 61.3% in the standard treatment group (RR, 0.88; 95% CI, 0.67-1.13), and the proportion of patients with hematoma expansion of greater than 33% was 18% and 27.6%, respectively (RR, 0.67; 95% CI, 0.45-1.00), said Dr. Goldstein of Massachusetts General Hospital, Boston.
In those with an expansion greater than 33% and a hematoma volume of at least 6 cc, the finding was similar.
“The trend looks a little bit more aggressive,” he said, but the 95% confidence interval in those with larger hematomas reached 1.
Study subjects had elevated blood pressure at arrival, a Glasgow coma scale score of at least 5, and a hematoma volume of less than 60 cc. They were enrolled and randomized within 4.5 hours of symptom onset. Those in the intensive treatment group were treated with a goal of achieving between 110 and 139 mm Hg within 24 hours, and those in the standard treatment group were treated with a goal of achieving between 140 mm Hg and 179 mm Hg within 24 hours. They underwent baseline and 24-hour computed tomography scans, which were analyzed centrally by blinded investigators who recorded any increase of 0.5 mL or more, an increase of more than 33% , an increase of more than 33% or more than 6 mL, and an intraventricular hemorrhage volume greater than 2 mL.
These measures were correlated with death and disability, and hematoma enlargement was shown to be significantly associated with those outcomes at 3 months after randomization (RR, 1.59; 95% CI, 1.25-2.02), Dr. Goldstein said.
Previous studies have suggested that intensive lowering of systolic BP in patients with intracerebral hemorrhage can reduce the rate of hematoma expansion. As such, the hypothesis of the current study was that the intensive treatment of elevated blood pressures – arriving systolic blood pressure of greater than 180 mm Hg – would reduce the likelihood of death and disability at 3 months, Dr. Goldstein said,
The question is whether the biomarker – hematoma expansion – is really linked to clinical outcome, he said, “because a lot of our attempts to treat hematoma expansion are based on the assumption that, if we reduce hematoma expansion, we’re going to improve clinical outcomes.”
“In this trial ... the expanders had more death and disability than nonexpanders, and this was statistically significant, so hematoma expansion does seem to be a statistically significant predictor of poor outcome,” he said.
Thus, the strong trend toward a reduced risk of hematoma expansion with intensive blood pressure lowering in patients with larger hematoma volumes at baseline in this analysis is noteworthy, he said.
“It appears that the treatment is affecting the biomarker, that the treatment is affecting hematoma expansion ... and hematoma expansion was a significant predictor of death and disability ... so why didn’t we get the result we wanted from the trial?” he asked.
It may be that a greater magnitude of reduction in the risk of hematoma expansion was necessary, he said.
“In other words, even if our treatment is having an effect, it’s just not having a big enough effect to change outcomes,” he said, adding that future trials probably need to involve a much bigger impact on the risk of expansion to translate to a change in clinical outcomes.”
This study was sponsored by the National Institute of Neurological Disorders and Stroke. Dr. Goldstein reported having no disclosures.
BOSTON – Intensive systolic blood pressure reduction did not significantly reduce hematoma expansion, compared with standard systolic blood pressure reduction in the Antihypertensive Treatment of Acute Cerebral Hemorrhage (ATACH) II trial, but, in a post hoc analysis, it did show a strong trend toward reducing hematoma expansion in those with a larger initial hematoma volume.
In 450 patients randomized to receive intensive treatment and 426 randomized to receive standard treatment in the large, international, phase III randomized trial, the proportion of patients with any hematoma expansion was 46.4% and 52.3% , respectively (relative risk, 0.89; 95% confidence interval, 0.73-1.07). The proportion with hematoma expansion with an increase of greater than 33% was 18.9% and 24.4%, respectively (RR, 0.77; 95% CI, 0.58-1.03), Joshua N. Goldstein, MD, reported at the annual meeting of the American Academy of Neurology.
The confidence intervals suggested only a trend toward improvement, as the difference between the groups did not reach statistical significance, he said.
To determine if intensive systolic blood pressure reduction might have more of an impact among higher-risk patients, the investigators conducted a post hoc, secondary analysis in those with relatively larger hematomas. Among patients with an initial hematoma volume of at least 10 mL (at least half of the study population had smaller hematomas), the proportion with any hematoma expansion was 53.8% in the intensive treatment group and 61.3% in the standard treatment group (RR, 0.88; 95% CI, 0.67-1.13), and the proportion of patients with hematoma expansion of greater than 33% was 18% and 27.6%, respectively (RR, 0.67; 95% CI, 0.45-1.00), said Dr. Goldstein of Massachusetts General Hospital, Boston.
In those with an expansion greater than 33% and a hematoma volume of at least 6 cc, the finding was similar.
“The trend looks a little bit more aggressive,” he said, but the 95% confidence interval in those with larger hematomas reached 1.
Study subjects had elevated blood pressure at arrival, a Glasgow coma scale score of at least 5, and a hematoma volume of less than 60 cc. They were enrolled and randomized within 4.5 hours of symptom onset. Those in the intensive treatment group were treated with a goal of achieving between 110 and 139 mm Hg within 24 hours, and those in the standard treatment group were treated with a goal of achieving between 140 mm Hg and 179 mm Hg within 24 hours. They underwent baseline and 24-hour computed tomography scans, which were analyzed centrally by blinded investigators who recorded any increase of 0.5 mL or more, an increase of more than 33% , an increase of more than 33% or more than 6 mL, and an intraventricular hemorrhage volume greater than 2 mL.
These measures were correlated with death and disability, and hematoma enlargement was shown to be significantly associated with those outcomes at 3 months after randomization (RR, 1.59; 95% CI, 1.25-2.02), Dr. Goldstein said.
Previous studies have suggested that intensive lowering of systolic BP in patients with intracerebral hemorrhage can reduce the rate of hematoma expansion. As such, the hypothesis of the current study was that the intensive treatment of elevated blood pressures – arriving systolic blood pressure of greater than 180 mm Hg – would reduce the likelihood of death and disability at 3 months, Dr. Goldstein said,
The question is whether the biomarker – hematoma expansion – is really linked to clinical outcome, he said, “because a lot of our attempts to treat hematoma expansion are based on the assumption that, if we reduce hematoma expansion, we’re going to improve clinical outcomes.”
“In this trial ... the expanders had more death and disability than nonexpanders, and this was statistically significant, so hematoma expansion does seem to be a statistically significant predictor of poor outcome,” he said.
Thus, the strong trend toward a reduced risk of hematoma expansion with intensive blood pressure lowering in patients with larger hematoma volumes at baseline in this analysis is noteworthy, he said.
“It appears that the treatment is affecting the biomarker, that the treatment is affecting hematoma expansion ... and hematoma expansion was a significant predictor of death and disability ... so why didn’t we get the result we wanted from the trial?” he asked.
It may be that a greater magnitude of reduction in the risk of hematoma expansion was necessary, he said.
“In other words, even if our treatment is having an effect, it’s just not having a big enough effect to change outcomes,” he said, adding that future trials probably need to involve a much bigger impact on the risk of expansion to translate to a change in clinical outcomes.”
This study was sponsored by the National Institute of Neurological Disorders and Stroke. Dr. Goldstein reported having no disclosures.
BOSTON – Intensive systolic blood pressure reduction did not significantly reduce hematoma expansion, compared with standard systolic blood pressure reduction in the Antihypertensive Treatment of Acute Cerebral Hemorrhage (ATACH) II trial, but, in a post hoc analysis, it did show a strong trend toward reducing hematoma expansion in those with a larger initial hematoma volume.
In 450 patients randomized to receive intensive treatment and 426 randomized to receive standard treatment in the large, international, phase III randomized trial, the proportion of patients with any hematoma expansion was 46.4% and 52.3% , respectively (relative risk, 0.89; 95% confidence interval, 0.73-1.07). The proportion with hematoma expansion with an increase of greater than 33% was 18.9% and 24.4%, respectively (RR, 0.77; 95% CI, 0.58-1.03), Joshua N. Goldstein, MD, reported at the annual meeting of the American Academy of Neurology.
The confidence intervals suggested only a trend toward improvement, as the difference between the groups did not reach statistical significance, he said.
To determine if intensive systolic blood pressure reduction might have more of an impact among higher-risk patients, the investigators conducted a post hoc, secondary analysis in those with relatively larger hematomas. Among patients with an initial hematoma volume of at least 10 mL (at least half of the study population had smaller hematomas), the proportion with any hematoma expansion was 53.8% in the intensive treatment group and 61.3% in the standard treatment group (RR, 0.88; 95% CI, 0.67-1.13), and the proportion of patients with hematoma expansion of greater than 33% was 18% and 27.6%, respectively (RR, 0.67; 95% CI, 0.45-1.00), said Dr. Goldstein of Massachusetts General Hospital, Boston.
In those with an expansion greater than 33% and a hematoma volume of at least 6 cc, the finding was similar.
“The trend looks a little bit more aggressive,” he said, but the 95% confidence interval in those with larger hematomas reached 1.
Study subjects had elevated blood pressure at arrival, a Glasgow coma scale score of at least 5, and a hematoma volume of less than 60 cc. They were enrolled and randomized within 4.5 hours of symptom onset. Those in the intensive treatment group were treated with a goal of achieving between 110 and 139 mm Hg within 24 hours, and those in the standard treatment group were treated with a goal of achieving between 140 mm Hg and 179 mm Hg within 24 hours. They underwent baseline and 24-hour computed tomography scans, which were analyzed centrally by blinded investigators who recorded any increase of 0.5 mL or more, an increase of more than 33% , an increase of more than 33% or more than 6 mL, and an intraventricular hemorrhage volume greater than 2 mL.
These measures were correlated with death and disability, and hematoma enlargement was shown to be significantly associated with those outcomes at 3 months after randomization (RR, 1.59; 95% CI, 1.25-2.02), Dr. Goldstein said.
Previous studies have suggested that intensive lowering of systolic BP in patients with intracerebral hemorrhage can reduce the rate of hematoma expansion. As such, the hypothesis of the current study was that the intensive treatment of elevated blood pressures – arriving systolic blood pressure of greater than 180 mm Hg – would reduce the likelihood of death and disability at 3 months, Dr. Goldstein said,
The question is whether the biomarker – hematoma expansion – is really linked to clinical outcome, he said, “because a lot of our attempts to treat hematoma expansion are based on the assumption that, if we reduce hematoma expansion, we’re going to improve clinical outcomes.”
“In this trial ... the expanders had more death and disability than nonexpanders, and this was statistically significant, so hematoma expansion does seem to be a statistically significant predictor of poor outcome,” he said.
Thus, the strong trend toward a reduced risk of hematoma expansion with intensive blood pressure lowering in patients with larger hematoma volumes at baseline in this analysis is noteworthy, he said.
“It appears that the treatment is affecting the biomarker, that the treatment is affecting hematoma expansion ... and hematoma expansion was a significant predictor of death and disability ... so why didn’t we get the result we wanted from the trial?” he asked.
It may be that a greater magnitude of reduction in the risk of hematoma expansion was necessary, he said.
“In other words, even if our treatment is having an effect, it’s just not having a big enough effect to change outcomes,” he said, adding that future trials probably need to involve a much bigger impact on the risk of expansion to translate to a change in clinical outcomes.”
This study was sponsored by the National Institute of Neurological Disorders and Stroke. Dr. Goldstein reported having no disclosures.
Key clinical point:
Major finding: Among patients with larger initial hematoma volume, expansion greater than 33% occurred in 18% vs. 28% in those with intense vs. standard blood pressure lowering, respectively (RR, 0.67).
Data source: The randomized phase III ATACH II trial involving 876 patients.
Disclosures: This study was sponsored by the National Institute of Neurological Disorders and Stroke. Dr. Goldstein reported having no disclosures.
PATH study: Subcutaneous immunoglobulin safe, effective for CIDP maintenance
BOSTON – Subcutaneously administered immunoglobulin was effective, well tolerated, and preferred over intravenous administration as maintenance treatment for chronic inflammatory demyelinating polyneuropathy in the phase III, randomized, placebo-controlled PATH study.
The 172-patient trial tested a high and low dose of subcutaneous immunoglobulin (SCIg) over the course of 25 weeks to determine their effect on the primary outcome of chronic inflammatory demyelinating polyneuropathy (CIDP) relapse or withdrawal from treatment for any reason. In this evaluation of using SCIg for maintenance of response, relapses or treatment withdrawal occurred in 63% with placebo, 39% with low dose SCIg (0.2 g/kg weekly), and 33% with high dose (0.4 g/kg weekly), Ivo N. van Schaik, MD, reported at the annual meeting of the American Academy of Neurology.
Patients in the trial had received at lease one dose of intravenous immunoglobulin (IVIg) within 8 weeks before screening. They then underwent a screening period first, followed by an IgG dependency period of up to 12 weeks to test for ongoing need for IgG. The patients who experienced CIDP relapse during this test period were administered a standardized IVIG regimen during a 10- to 13-week restabilization period, and those who improved and maintained their Inflammatory Neuropathy Cause and Treatment (INCAT) score continued to the randomized subcutaneous treatment period of the study.
CIDP relapse occurred in 56% of patients in the placebo group, compared with 33% in the low- and 19% in the high-dose SCIg groups, said Dr. van Schaik of the University of Amsterdam (the Netherlands).
“Both [SCIg] doses were effective in preventing relapse. The higher dose performed better than the lower dose, but the difference was not statistically significant,” he said.
Both doses were significantly more effective than placebo.
Study participants were adults with definite or probable CIDP enrolled from 69 neuromuscular centers worldwide between March 2012 and November 2015. Weekly self-administered subcutaneous infusions of SCIg (IgPro20Hizentra) were performed during 1 or 2 consecutive days in two separate sessions using special infusion pumps. Patients reported that learning the self-administration technique was easy, Dr. van Schaik said.
Adverse effects included mainly local reactions, which occurred in 19% of patients, but these were generally mild and rarely resulted in therapy discontinuation, and local reactions decreased considerably over time, he said, noting that systemic effects are reduced with SCIg vs. IVIg.
Subcutaneous administration of immunoglobulin is not new. In fact, it has been used successfully in patients with immunodeficiency syndromes for more than 2 decades and can increase patient autonomy and reduce costs by reducing hospital and infusion center visits, but this is the first study to assess efficacy, safety, and tolerability of this approach in an adequately powered, randomized, clinical trial, he said.
“Subcutaneous immunoglobulin can be used ... for maintenance treatment of patients with CIDP,” he concluded, adding that weekly doses of 0.2-0.4 g/kg are supported by these data, and that maintenance doses should be individualized based on patient factors and previous IVIg dose and frequency.
The PATH study was sponsored by CSL-Behring. Dr. van Schaik chairs a steering committee for CSL-Behring and received departmental honoraria for serving on scientific advisory boards for CSL-Behring, Baxalta, and UCB. He also received speakers fees from CSL-Behring and Kedrion.
BOSTON – Subcutaneously administered immunoglobulin was effective, well tolerated, and preferred over intravenous administration as maintenance treatment for chronic inflammatory demyelinating polyneuropathy in the phase III, randomized, placebo-controlled PATH study.
The 172-patient trial tested a high and low dose of subcutaneous immunoglobulin (SCIg) over the course of 25 weeks to determine their effect on the primary outcome of chronic inflammatory demyelinating polyneuropathy (CIDP) relapse or withdrawal from treatment for any reason. In this evaluation of using SCIg for maintenance of response, relapses or treatment withdrawal occurred in 63% with placebo, 39% with low dose SCIg (0.2 g/kg weekly), and 33% with high dose (0.4 g/kg weekly), Ivo N. van Schaik, MD, reported at the annual meeting of the American Academy of Neurology.
Patients in the trial had received at lease one dose of intravenous immunoglobulin (IVIg) within 8 weeks before screening. They then underwent a screening period first, followed by an IgG dependency period of up to 12 weeks to test for ongoing need for IgG. The patients who experienced CIDP relapse during this test period were administered a standardized IVIG regimen during a 10- to 13-week restabilization period, and those who improved and maintained their Inflammatory Neuropathy Cause and Treatment (INCAT) score continued to the randomized subcutaneous treatment period of the study.
CIDP relapse occurred in 56% of patients in the placebo group, compared with 33% in the low- and 19% in the high-dose SCIg groups, said Dr. van Schaik of the University of Amsterdam (the Netherlands).
“Both [SCIg] doses were effective in preventing relapse. The higher dose performed better than the lower dose, but the difference was not statistically significant,” he said.
Both doses were significantly more effective than placebo.
Study participants were adults with definite or probable CIDP enrolled from 69 neuromuscular centers worldwide between March 2012 and November 2015. Weekly self-administered subcutaneous infusions of SCIg (IgPro20Hizentra) were performed during 1 or 2 consecutive days in two separate sessions using special infusion pumps. Patients reported that learning the self-administration technique was easy, Dr. van Schaik said.
Adverse effects included mainly local reactions, which occurred in 19% of patients, but these were generally mild and rarely resulted in therapy discontinuation, and local reactions decreased considerably over time, he said, noting that systemic effects are reduced with SCIg vs. IVIg.
Subcutaneous administration of immunoglobulin is not new. In fact, it has been used successfully in patients with immunodeficiency syndromes for more than 2 decades and can increase patient autonomy and reduce costs by reducing hospital and infusion center visits, but this is the first study to assess efficacy, safety, and tolerability of this approach in an adequately powered, randomized, clinical trial, he said.
“Subcutaneous immunoglobulin can be used ... for maintenance treatment of patients with CIDP,” he concluded, adding that weekly doses of 0.2-0.4 g/kg are supported by these data, and that maintenance doses should be individualized based on patient factors and previous IVIg dose and frequency.
The PATH study was sponsored by CSL-Behring. Dr. van Schaik chairs a steering committee for CSL-Behring and received departmental honoraria for serving on scientific advisory boards for CSL-Behring, Baxalta, and UCB. He also received speakers fees from CSL-Behring and Kedrion.
BOSTON – Subcutaneously administered immunoglobulin was effective, well tolerated, and preferred over intravenous administration as maintenance treatment for chronic inflammatory demyelinating polyneuropathy in the phase III, randomized, placebo-controlled PATH study.
The 172-patient trial tested a high and low dose of subcutaneous immunoglobulin (SCIg) over the course of 25 weeks to determine their effect on the primary outcome of chronic inflammatory demyelinating polyneuropathy (CIDP) relapse or withdrawal from treatment for any reason. In this evaluation of using SCIg for maintenance of response, relapses or treatment withdrawal occurred in 63% with placebo, 39% with low dose SCIg (0.2 g/kg weekly), and 33% with high dose (0.4 g/kg weekly), Ivo N. van Schaik, MD, reported at the annual meeting of the American Academy of Neurology.
Patients in the trial had received at lease one dose of intravenous immunoglobulin (IVIg) within 8 weeks before screening. They then underwent a screening period first, followed by an IgG dependency period of up to 12 weeks to test for ongoing need for IgG. The patients who experienced CIDP relapse during this test period were administered a standardized IVIG regimen during a 10- to 13-week restabilization period, and those who improved and maintained their Inflammatory Neuropathy Cause and Treatment (INCAT) score continued to the randomized subcutaneous treatment period of the study.
CIDP relapse occurred in 56% of patients in the placebo group, compared with 33% in the low- and 19% in the high-dose SCIg groups, said Dr. van Schaik of the University of Amsterdam (the Netherlands).
“Both [SCIg] doses were effective in preventing relapse. The higher dose performed better than the lower dose, but the difference was not statistically significant,” he said.
Both doses were significantly more effective than placebo.
Study participants were adults with definite or probable CIDP enrolled from 69 neuromuscular centers worldwide between March 2012 and November 2015. Weekly self-administered subcutaneous infusions of SCIg (IgPro20Hizentra) were performed during 1 or 2 consecutive days in two separate sessions using special infusion pumps. Patients reported that learning the self-administration technique was easy, Dr. van Schaik said.
Adverse effects included mainly local reactions, which occurred in 19% of patients, but these were generally mild and rarely resulted in therapy discontinuation, and local reactions decreased considerably over time, he said, noting that systemic effects are reduced with SCIg vs. IVIg.
Subcutaneous administration of immunoglobulin is not new. In fact, it has been used successfully in patients with immunodeficiency syndromes for more than 2 decades and can increase patient autonomy and reduce costs by reducing hospital and infusion center visits, but this is the first study to assess efficacy, safety, and tolerability of this approach in an adequately powered, randomized, clinical trial, he said.
“Subcutaneous immunoglobulin can be used ... for maintenance treatment of patients with CIDP,” he concluded, adding that weekly doses of 0.2-0.4 g/kg are supported by these data, and that maintenance doses should be individualized based on patient factors and previous IVIg dose and frequency.
The PATH study was sponsored by CSL-Behring. Dr. van Schaik chairs a steering committee for CSL-Behring and received departmental honoraria for serving on scientific advisory boards for CSL-Behring, Baxalta, and UCB. He also received speakers fees from CSL-Behring and Kedrion.
Key clinical point:
Major finding: CIDP relapse occurred in 56% of patients in the placebo group, compared with 33% in the low- and 19% in the high-dose SCIg groups.
Data source: The randomized, placebo-controlled phase III PATH study of 172 CIDP patients.
Disclosures: The PATH study was sponsored by CSL-Behring. Dr. van Schaik chairs a steering committee for CSL-Behring and received departmental honoraria for serving on scientific advisory boards for CSL-Behring, Baxalta, and UCB. He also received speakers fees from CSL-Behring and Kedrion.
24/7 neurologist coverage improved community hospital stroke outcomes
BOSTON – Around-the-clock availability of a neurologist for acute stroke treatment significantly reduced door-to-needle times at a community-based primary stroke center serving as a regional tertiary referral center for western North Carolina.
The program involving the 24/7 availability of a neurologist in the hospital was implemented in October 2015 at Mission Hospital, a 763-bed hospital in Asheville, N.C., where nighttime emergency stroke coverage had historically been provided by a neurologist on-call from home. The implementation was partly in preparation for an application for Joint Commission–certified comprehensive stroke center status, which was approved in 2016.
A review of 2,022 Code Stroke activations in the emergency department from January 2012 through September 2016 that included only patients treated with intravenous tissue plasminogen activator revealed a significant decrease in door-to-neurologist time from an average of 7.1 minutes before the 24/7 in-hospital availability to 2.5 minutes after implementation. The analysis included 1,524 cases occurring prior to implementation and 498 cases occurring after.
The impact was most significant at night.
“Our nighttime reduction to the bedside went down from 13.6 minutes to 3.4 minutes, and our daytime reduction also improved from 5.2 minutes to 2.2 minutes,” said Dr. Schneider, a vascular neurologist at Mission Hospital.
Door-to-needle times were significantly reduced from 48.3 to 37.8 minutes overall, from 52 minutes to 40 minutes at night, and from 46.6 to 34.5 minutes during the day.
“We think that the trend toward significance at nighttime was mitigated by the fact there were just lesser numbers at night,” he said.
In-hospital mortality was reduced by 31% from 8.94% to 6.13%, he said.
Another variable that improved after the intervention was timing of prenotification by emergency medical services. The overall rate of prenotification did not improve (likely because of a ceiling effect; the rate of notification was already about 90%), but notifications improved from 12.5 minutes before arrival to 10.7 minutes.
There was no significant change in door-to-computed tomography times, which averaged about 15 minutes, Dr. Schneider said.
Though limited by a smaller number of postimplementation cases and lack of 3-month poststroke functional outcome data, the findings suggest that 24/7 in-hospital availability of a neurologist improves door to intravenous tissue plasminogen activator treatment times and in-hospital stroke mortality, Dr. Schneider concluded, noting that ongoing monitoring of outcomes will be necessary to assess for enduring impact.
Dr. Schneider reported having no disclosures.
BOSTON – Around-the-clock availability of a neurologist for acute stroke treatment significantly reduced door-to-needle times at a community-based primary stroke center serving as a regional tertiary referral center for western North Carolina.
The program involving the 24/7 availability of a neurologist in the hospital was implemented in October 2015 at Mission Hospital, a 763-bed hospital in Asheville, N.C., where nighttime emergency stroke coverage had historically been provided by a neurologist on-call from home. The implementation was partly in preparation for an application for Joint Commission–certified comprehensive stroke center status, which was approved in 2016.
A review of 2,022 Code Stroke activations in the emergency department from January 2012 through September 2016 that included only patients treated with intravenous tissue plasminogen activator revealed a significant decrease in door-to-neurologist time from an average of 7.1 minutes before the 24/7 in-hospital availability to 2.5 minutes after implementation. The analysis included 1,524 cases occurring prior to implementation and 498 cases occurring after.
The impact was most significant at night.
“Our nighttime reduction to the bedside went down from 13.6 minutes to 3.4 minutes, and our daytime reduction also improved from 5.2 minutes to 2.2 minutes,” said Dr. Schneider, a vascular neurologist at Mission Hospital.
Door-to-needle times were significantly reduced from 48.3 to 37.8 minutes overall, from 52 minutes to 40 minutes at night, and from 46.6 to 34.5 minutes during the day.
“We think that the trend toward significance at nighttime was mitigated by the fact there were just lesser numbers at night,” he said.
In-hospital mortality was reduced by 31% from 8.94% to 6.13%, he said.
Another variable that improved after the intervention was timing of prenotification by emergency medical services. The overall rate of prenotification did not improve (likely because of a ceiling effect; the rate of notification was already about 90%), but notifications improved from 12.5 minutes before arrival to 10.7 minutes.
There was no significant change in door-to-computed tomography times, which averaged about 15 minutes, Dr. Schneider said.
Though limited by a smaller number of postimplementation cases and lack of 3-month poststroke functional outcome data, the findings suggest that 24/7 in-hospital availability of a neurologist improves door to intravenous tissue plasminogen activator treatment times and in-hospital stroke mortality, Dr. Schneider concluded, noting that ongoing monitoring of outcomes will be necessary to assess for enduring impact.
Dr. Schneider reported having no disclosures.
BOSTON – Around-the-clock availability of a neurologist for acute stroke treatment significantly reduced door-to-needle times at a community-based primary stroke center serving as a regional tertiary referral center for western North Carolina.
The program involving the 24/7 availability of a neurologist in the hospital was implemented in October 2015 at Mission Hospital, a 763-bed hospital in Asheville, N.C., where nighttime emergency stroke coverage had historically been provided by a neurologist on-call from home. The implementation was partly in preparation for an application for Joint Commission–certified comprehensive stroke center status, which was approved in 2016.
A review of 2,022 Code Stroke activations in the emergency department from January 2012 through September 2016 that included only patients treated with intravenous tissue plasminogen activator revealed a significant decrease in door-to-neurologist time from an average of 7.1 minutes before the 24/7 in-hospital availability to 2.5 minutes after implementation. The analysis included 1,524 cases occurring prior to implementation and 498 cases occurring after.
The impact was most significant at night.
“Our nighttime reduction to the bedside went down from 13.6 minutes to 3.4 minutes, and our daytime reduction also improved from 5.2 minutes to 2.2 minutes,” said Dr. Schneider, a vascular neurologist at Mission Hospital.
Door-to-needle times were significantly reduced from 48.3 to 37.8 minutes overall, from 52 minutes to 40 minutes at night, and from 46.6 to 34.5 minutes during the day.
“We think that the trend toward significance at nighttime was mitigated by the fact there were just lesser numbers at night,” he said.
In-hospital mortality was reduced by 31% from 8.94% to 6.13%, he said.
Another variable that improved after the intervention was timing of prenotification by emergency medical services. The overall rate of prenotification did not improve (likely because of a ceiling effect; the rate of notification was already about 90%), but notifications improved from 12.5 minutes before arrival to 10.7 minutes.
There was no significant change in door-to-computed tomography times, which averaged about 15 minutes, Dr. Schneider said.
Though limited by a smaller number of postimplementation cases and lack of 3-month poststroke functional outcome data, the findings suggest that 24/7 in-hospital availability of a neurologist improves door to intravenous tissue plasminogen activator treatment times and in-hospital stroke mortality, Dr. Schneider concluded, noting that ongoing monitoring of outcomes will be necessary to assess for enduring impact.
Dr. Schneider reported having no disclosures.
AT AAN 2017
Key clinical point:
Major finding: Door-to-needle times were significantly reduced from 48.3 to 37.8 minutes overall, from 52 minutes to 40 minutes at night, and from 46.6 to 34.5 minutes during the day.
Data source: A review of 498 preintervention cases and 1,524 postintervention Code Stroke cases.
Disclosures: Dr. Schneider reported having no disclosures.
Cannabidiol cuts drop seizure frequency in Lennox-Gastaut syndrome
BOSTON – Cannabidiol add-on treatment significantly reduces the frequency of drop seizures in patients with Lennox-Gastaut syndrome, according to findings from the randomized, double-blind, placebo-controlled GWPCARE4 and GWPCARE3 trials.
In GWPCARE4 – the first controlled trial of cannabidiol in Lennox-Gastaut syndrome (LGS) – 171 patients were randomized to receive either placebo or cannabidiol oral solution at a dose of 20 mg/kg per day for 14 weeks, including 2 weeks of titration and 12 weeks of maintenance therapy. The drop seizure frequency decreased by a median of 44% in the cannabidiol group vs. 22% in the placebo group. The difference between the groups was statistically significant and was apparent within the first 4 weeks of the maintenance period, Jacqueline French, MD, director of translational research and clinical trials in epilepsy at New York University Langone Medical Center reported at the annual meeting of the American Academy of Neurology.
Study participants had a mean age of 15 years, and 34% were aged 18 years or older. The median drop seizure frequency was 74 per month at baseline.
“That is a very high seizure burden,” Dr. French said, noting that the median total drop and non-drop seizure frequency was in the mid to high 100s. “So you can see that these children were very afflicted by seizures.”
Interestingly, there was a “very substantial drop” in non-drop seizures, as well, she said.
“And that’s really hard to demonstrate, so that’s impressive to me, at least,” she added.
Patients had taken a median of six antiepileptic drugs (AEDs) in their past and were taking a median of three AEDs in addition to the cannabidiol throughout the study period.
Adverse events were common, occurring in 86% of cannabidiol patients and 69% of placebo patients. The most common were diarrhea, somnolence, pyrexia, decreased appetite, and vomiting, but most were mild or moderate. Treatment-related serious adverse events were reported in nine cannabidiol patients and one placebo patient. All patients who completed the trial entered an open-label extension study, Dr. French said.
GWPCARE4 was followed by GWPCARE3, which compared both 20 mg/kg per day and 10 mg/kg per day doses of cannabidiol with placebo.
In that study, 225 patients with a mean age of 16 years (30% were aged 18 years or older) were randomized, and the median drop in seizure frequency was 42% with 20 mg/kg of cannabidiol and 37% with 10 mg/kg of cannabidiol versus 17% with placebo, Anup D. Patel, MD, reported at the meeting.
Approximately 40% and 36% of patients in the 20 mg/kg and 10 mg/kg groups, respectively, achieved the secondary outcome of a 50% or greater drop in seizure frequency, compared with 15% of placebo patients.
“Overall, 5 patients in the 20 mg/kg per day arm, 3 in the 10 [mg/kg per day arm], and 1 in the placebo arm achieved drop seizure freedom during the maintenance period of our study,” said Dr. Patel, a pediatric neurologist at Nationwide Children’s Hospital, Columbus, Ohio.
The median monthly drop seizure frequency at baseline was 80-87 in the three groups, and patients had previously failed a median of six to seven AEDs. They took a median of three AEDs during the study.
Adverse events occurred in 94% of patients in the 20-mg/kg cannabidiol group, 84% of those in the 10-mg/kg group, and 72% of placebo patients, and most were mild or moderate. The most common were somnolence and decreased appetite.
Treatment-related serious adverse events were reported in 5, 2, and 0 patients in the groups, respectively. Some elevations in transaminases were seen (in 11 patients in the 20-mg/kg dose group, and 2 in the 10-mg/kg dose group), but no patients met criteria for drug-induced liver injury with concurrent elevated bilirubin, and all cases resolved, Dr. Patel said.
Similar findings were noted in GWPCARE4, and both Dr. Patel and Dr. French noted that most patients with elevated transaminases were also taking valproic acid.
No deaths occurred in either GWPCARE4 or GWPCARE3.
“We feel both doses... produce significantly greater reductions in drop seizures as compared to placebo,” Dr. Patel said.
In an interview, he noted that the two cannabidiol doses were not compared directly to determine if the differences in response rates and adverse events were statistically significant, but said that the rates appeared comparable, and that the adverse event rates were high in all of the groups, reflecting a very sick patient population.
Of the 212 patients who completed GWPCARE3, 99% entered an open-label extension study.
Eligible patients in both trials were children and adults aged 2-55 years with a clinical diagnosis of LGS, at least two drop seizures (including tonic, atonic, and tonic-clonic seizures) each week at baseline, and documented failures on at least one AED.
Patients and caregivers in both trials were more likely to report improvement in overall condition among treated vs. placebo patients, as measured using the Subject/Caregiver Global Impression of Change scale.
The findings provide class 1 evidence that cannabidiol as add-on therapy in LGS is efficacious for reducing seizure frequency, and is generally well tolerated, Dr. French and Dr. Patel said.
Dr. French noted that enthusiasm for the trials was high, with many study sites reporting waiting lists for patient enrollment.
Some of the authors in each study have consulted for, conducted studies funded by, or received honoraria from GW Pharmaceuticals, which funded the GWPCARE4 and GWPCARE3 trials and manufacturers the oral cannabidiol solution. Several authors in each study were employees of GW Pharmaceuticals.
sworcester@frontlinemedcom.com
BOSTON – Cannabidiol add-on treatment significantly reduces the frequency of drop seizures in patients with Lennox-Gastaut syndrome, according to findings from the randomized, double-blind, placebo-controlled GWPCARE4 and GWPCARE3 trials.
In GWPCARE4 – the first controlled trial of cannabidiol in Lennox-Gastaut syndrome (LGS) – 171 patients were randomized to receive either placebo or cannabidiol oral solution at a dose of 20 mg/kg per day for 14 weeks, including 2 weeks of titration and 12 weeks of maintenance therapy. The drop seizure frequency decreased by a median of 44% in the cannabidiol group vs. 22% in the placebo group. The difference between the groups was statistically significant and was apparent within the first 4 weeks of the maintenance period, Jacqueline French, MD, director of translational research and clinical trials in epilepsy at New York University Langone Medical Center reported at the annual meeting of the American Academy of Neurology.
Study participants had a mean age of 15 years, and 34% were aged 18 years or older. The median drop seizure frequency was 74 per month at baseline.
“That is a very high seizure burden,” Dr. French said, noting that the median total drop and non-drop seizure frequency was in the mid to high 100s. “So you can see that these children were very afflicted by seizures.”
Interestingly, there was a “very substantial drop” in non-drop seizures, as well, she said.
“And that’s really hard to demonstrate, so that’s impressive to me, at least,” she added.
Patients had taken a median of six antiepileptic drugs (AEDs) in their past and were taking a median of three AEDs in addition to the cannabidiol throughout the study period.
Adverse events were common, occurring in 86% of cannabidiol patients and 69% of placebo patients. The most common were diarrhea, somnolence, pyrexia, decreased appetite, and vomiting, but most were mild or moderate. Treatment-related serious adverse events were reported in nine cannabidiol patients and one placebo patient. All patients who completed the trial entered an open-label extension study, Dr. French said.
GWPCARE4 was followed by GWPCARE3, which compared both 20 mg/kg per day and 10 mg/kg per day doses of cannabidiol with placebo.
In that study, 225 patients with a mean age of 16 years (30% were aged 18 years or older) were randomized, and the median drop in seizure frequency was 42% with 20 mg/kg of cannabidiol and 37% with 10 mg/kg of cannabidiol versus 17% with placebo, Anup D. Patel, MD, reported at the meeting.
Approximately 40% and 36% of patients in the 20 mg/kg and 10 mg/kg groups, respectively, achieved the secondary outcome of a 50% or greater drop in seizure frequency, compared with 15% of placebo patients.
“Overall, 5 patients in the 20 mg/kg per day arm, 3 in the 10 [mg/kg per day arm], and 1 in the placebo arm achieved drop seizure freedom during the maintenance period of our study,” said Dr. Patel, a pediatric neurologist at Nationwide Children’s Hospital, Columbus, Ohio.
The median monthly drop seizure frequency at baseline was 80-87 in the three groups, and patients had previously failed a median of six to seven AEDs. They took a median of three AEDs during the study.
Adverse events occurred in 94% of patients in the 20-mg/kg cannabidiol group, 84% of those in the 10-mg/kg group, and 72% of placebo patients, and most were mild or moderate. The most common were somnolence and decreased appetite.
Treatment-related serious adverse events were reported in 5, 2, and 0 patients in the groups, respectively. Some elevations in transaminases were seen (in 11 patients in the 20-mg/kg dose group, and 2 in the 10-mg/kg dose group), but no patients met criteria for drug-induced liver injury with concurrent elevated bilirubin, and all cases resolved, Dr. Patel said.
Similar findings were noted in GWPCARE4, and both Dr. Patel and Dr. French noted that most patients with elevated transaminases were also taking valproic acid.
No deaths occurred in either GWPCARE4 or GWPCARE3.
“We feel both doses... produce significantly greater reductions in drop seizures as compared to placebo,” Dr. Patel said.
In an interview, he noted that the two cannabidiol doses were not compared directly to determine if the differences in response rates and adverse events were statistically significant, but said that the rates appeared comparable, and that the adverse event rates were high in all of the groups, reflecting a very sick patient population.
Of the 212 patients who completed GWPCARE3, 99% entered an open-label extension study.
Eligible patients in both trials were children and adults aged 2-55 years with a clinical diagnosis of LGS, at least two drop seizures (including tonic, atonic, and tonic-clonic seizures) each week at baseline, and documented failures on at least one AED.
Patients and caregivers in both trials were more likely to report improvement in overall condition among treated vs. placebo patients, as measured using the Subject/Caregiver Global Impression of Change scale.
The findings provide class 1 evidence that cannabidiol as add-on therapy in LGS is efficacious for reducing seizure frequency, and is generally well tolerated, Dr. French and Dr. Patel said.
Dr. French noted that enthusiasm for the trials was high, with many study sites reporting waiting lists for patient enrollment.
Some of the authors in each study have consulted for, conducted studies funded by, or received honoraria from GW Pharmaceuticals, which funded the GWPCARE4 and GWPCARE3 trials and manufacturers the oral cannabidiol solution. Several authors in each study were employees of GW Pharmaceuticals.
sworcester@frontlinemedcom.com
BOSTON – Cannabidiol add-on treatment significantly reduces the frequency of drop seizures in patients with Lennox-Gastaut syndrome, according to findings from the randomized, double-blind, placebo-controlled GWPCARE4 and GWPCARE3 trials.
In GWPCARE4 – the first controlled trial of cannabidiol in Lennox-Gastaut syndrome (LGS) – 171 patients were randomized to receive either placebo or cannabidiol oral solution at a dose of 20 mg/kg per day for 14 weeks, including 2 weeks of titration and 12 weeks of maintenance therapy. The drop seizure frequency decreased by a median of 44% in the cannabidiol group vs. 22% in the placebo group. The difference between the groups was statistically significant and was apparent within the first 4 weeks of the maintenance period, Jacqueline French, MD, director of translational research and clinical trials in epilepsy at New York University Langone Medical Center reported at the annual meeting of the American Academy of Neurology.
Study participants had a mean age of 15 years, and 34% were aged 18 years or older. The median drop seizure frequency was 74 per month at baseline.
“That is a very high seizure burden,” Dr. French said, noting that the median total drop and non-drop seizure frequency was in the mid to high 100s. “So you can see that these children were very afflicted by seizures.”
Interestingly, there was a “very substantial drop” in non-drop seizures, as well, she said.
“And that’s really hard to demonstrate, so that’s impressive to me, at least,” she added.
Patients had taken a median of six antiepileptic drugs (AEDs) in their past and were taking a median of three AEDs in addition to the cannabidiol throughout the study period.
Adverse events were common, occurring in 86% of cannabidiol patients and 69% of placebo patients. The most common were diarrhea, somnolence, pyrexia, decreased appetite, and vomiting, but most were mild or moderate. Treatment-related serious adverse events were reported in nine cannabidiol patients and one placebo patient. All patients who completed the trial entered an open-label extension study, Dr. French said.
GWPCARE4 was followed by GWPCARE3, which compared both 20 mg/kg per day and 10 mg/kg per day doses of cannabidiol with placebo.
In that study, 225 patients with a mean age of 16 years (30% were aged 18 years or older) were randomized, and the median drop in seizure frequency was 42% with 20 mg/kg of cannabidiol and 37% with 10 mg/kg of cannabidiol versus 17% with placebo, Anup D. Patel, MD, reported at the meeting.
Approximately 40% and 36% of patients in the 20 mg/kg and 10 mg/kg groups, respectively, achieved the secondary outcome of a 50% or greater drop in seizure frequency, compared with 15% of placebo patients.
“Overall, 5 patients in the 20 mg/kg per day arm, 3 in the 10 [mg/kg per day arm], and 1 in the placebo arm achieved drop seizure freedom during the maintenance period of our study,” said Dr. Patel, a pediatric neurologist at Nationwide Children’s Hospital, Columbus, Ohio.
The median monthly drop seizure frequency at baseline was 80-87 in the three groups, and patients had previously failed a median of six to seven AEDs. They took a median of three AEDs during the study.
Adverse events occurred in 94% of patients in the 20-mg/kg cannabidiol group, 84% of those in the 10-mg/kg group, and 72% of placebo patients, and most were mild or moderate. The most common were somnolence and decreased appetite.
Treatment-related serious adverse events were reported in 5, 2, and 0 patients in the groups, respectively. Some elevations in transaminases were seen (in 11 patients in the 20-mg/kg dose group, and 2 in the 10-mg/kg dose group), but no patients met criteria for drug-induced liver injury with concurrent elevated bilirubin, and all cases resolved, Dr. Patel said.
Similar findings were noted in GWPCARE4, and both Dr. Patel and Dr. French noted that most patients with elevated transaminases were also taking valproic acid.
No deaths occurred in either GWPCARE4 or GWPCARE3.
“We feel both doses... produce significantly greater reductions in drop seizures as compared to placebo,” Dr. Patel said.
In an interview, he noted that the two cannabidiol doses were not compared directly to determine if the differences in response rates and adverse events were statistically significant, but said that the rates appeared comparable, and that the adverse event rates were high in all of the groups, reflecting a very sick patient population.
Of the 212 patients who completed GWPCARE3, 99% entered an open-label extension study.
Eligible patients in both trials were children and adults aged 2-55 years with a clinical diagnosis of LGS, at least two drop seizures (including tonic, atonic, and tonic-clonic seizures) each week at baseline, and documented failures on at least one AED.
Patients and caregivers in both trials were more likely to report improvement in overall condition among treated vs. placebo patients, as measured using the Subject/Caregiver Global Impression of Change scale.
The findings provide class 1 evidence that cannabidiol as add-on therapy in LGS is efficacious for reducing seizure frequency, and is generally well tolerated, Dr. French and Dr. Patel said.
Dr. French noted that enthusiasm for the trials was high, with many study sites reporting waiting lists for patient enrollment.
Some of the authors in each study have consulted for, conducted studies funded by, or received honoraria from GW Pharmaceuticals, which funded the GWPCARE4 and GWPCARE3 trials and manufacturers the oral cannabidiol solution. Several authors in each study were employees of GW Pharmaceuticals.
sworcester@frontlinemedcom.com
Key clinical point:
Major finding: The drop seizure frequency decreased by a median of 42%-44% in the 20 mg/kg per day cannabidiol groups vs. 17%-22% in the placebo groups.
Data source: The randomized, placebo-controlled GWPCARE4 and GWPCARE3 trials, including 171 and 225 patients, respectively.
Disclosures: Some of the authors in each study have consulted for, conducted studies funded by, or received honoraria from GW Pharmaceuticals, which funded the GWPCARE4 and GWPCARE3 trials and manufactures the oral cannabidiol solution. Several authors in each study were employees of GW Pharmaceuticals.