User login
Sharon Worcester is an award-winning medical journalist for MDedge News. She has been with the company since 1996, first as the Southeast Bureau Chief (1996-2009) when the company was known as International Medical News Group, then as a freelance writer (2010-2015) before returning as a reporter in 2015. She previously worked as a daily newspaper reporter covering health and local government. Sharon currently reports primarily on oncology and hematology. She has a BA from Eckerd College and an MA in Mass Communication/Print Journalism from the University of Florida. Connect with her via LinkedIn and follow her on twitter @SW_MedReporter.
VIDEO: Cannabidiol reduces convulsive seizures in Dravet syndrome
BOSTON – Adjunctive treatment with cannabidiol significantly reduced convulsive seizure frequency in Dravet syndrome patients in a randomized, double-blind, placebo-controlled trial.
Over a 14-week treatment period, including 2 weeks of titration and 12 weeks of maintenance, convulsive seizure frequency in 61 treated children and adolescents decreased from a median of 12.4 to 5.9 per month (median reduction of 39%), compared with a decrease from a median of 14.9 to 14.1 per month (median reduction of 13%) in 59 patients who received placebo, J. Helen Cross, MD, reported at the annual meeting of the American Academy of Neurology.
The proportion of patients with at least a 50% reduction in convulsive seizures was 42.6% with cannabidiol vs. 27.1% with placebo (odds ratio, 2.0), but this difference did not reach statistical significance, said Dr. Cross of the University College London Great Ormond Street Institute of Child Health and the Great Ormond Street Hospital for Children NHS Trust, London.
In a video interview, Dr. Cross discussed the findings and the importance of improving seizure control in patients with Dravet syndrome, a rare infantile-onset developmental and epileptic encephalopathy with very poor prognosis for long-term seizure control and neurodevelopmental outcomes.
Participants in the study (GWPCARE1) had a mean age of 10 years, but nearly a third were younger than 6 years. All had Dravet syndrome and drug-resistant seizures; the median number of antiepilepsy drugs previously tried was four, and the median number being used was three. Those randomized to the treatment group received cannabidiol oral solution up to 20 mg/kg per day.
Adverse events were common, occurring in 93.4% and 74.6% of treatment group and placebo group patients, respectively. But adverse events reported in the treatment group were mild or moderate in 84% of patients, and treatment was generally well tolerated.
“These are very complex patients with a high seizure burden... and therefore, to have another medication that looks as if it can be of benefit is really very exciting for this population,” Dr. Cross said, noting that cannabidiol was also shown in other studies presented at the AAN meeting (GWPCARE3 and GWPCARE4) to reduce seizure frequency in patients with Lennox-Gastaut syndrome.
GW Research sponsored the study. Dr. Cross is a member of the advisory boards for Eisai, GW Pharmaceuticals, Shire, and Zogenix.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
BOSTON – Adjunctive treatment with cannabidiol significantly reduced convulsive seizure frequency in Dravet syndrome patients in a randomized, double-blind, placebo-controlled trial.
Over a 14-week treatment period, including 2 weeks of titration and 12 weeks of maintenance, convulsive seizure frequency in 61 treated children and adolescents decreased from a median of 12.4 to 5.9 per month (median reduction of 39%), compared with a decrease from a median of 14.9 to 14.1 per month (median reduction of 13%) in 59 patients who received placebo, J. Helen Cross, MD, reported at the annual meeting of the American Academy of Neurology.
The proportion of patients with at least a 50% reduction in convulsive seizures was 42.6% with cannabidiol vs. 27.1% with placebo (odds ratio, 2.0), but this difference did not reach statistical significance, said Dr. Cross of the University College London Great Ormond Street Institute of Child Health and the Great Ormond Street Hospital for Children NHS Trust, London.
In a video interview, Dr. Cross discussed the findings and the importance of improving seizure control in patients with Dravet syndrome, a rare infantile-onset developmental and epileptic encephalopathy with very poor prognosis for long-term seizure control and neurodevelopmental outcomes.
Participants in the study (GWPCARE1) had a mean age of 10 years, but nearly a third were younger than 6 years. All had Dravet syndrome and drug-resistant seizures; the median number of antiepilepsy drugs previously tried was four, and the median number being used was three. Those randomized to the treatment group received cannabidiol oral solution up to 20 mg/kg per day.
Adverse events were common, occurring in 93.4% and 74.6% of treatment group and placebo group patients, respectively. But adverse events reported in the treatment group were mild or moderate in 84% of patients, and treatment was generally well tolerated.
“These are very complex patients with a high seizure burden... and therefore, to have another medication that looks as if it can be of benefit is really very exciting for this population,” Dr. Cross said, noting that cannabidiol was also shown in other studies presented at the AAN meeting (GWPCARE3 and GWPCARE4) to reduce seizure frequency in patients with Lennox-Gastaut syndrome.
GW Research sponsored the study. Dr. Cross is a member of the advisory boards for Eisai, GW Pharmaceuticals, Shire, and Zogenix.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
BOSTON – Adjunctive treatment with cannabidiol significantly reduced convulsive seizure frequency in Dravet syndrome patients in a randomized, double-blind, placebo-controlled trial.
Over a 14-week treatment period, including 2 weeks of titration and 12 weeks of maintenance, convulsive seizure frequency in 61 treated children and adolescents decreased from a median of 12.4 to 5.9 per month (median reduction of 39%), compared with a decrease from a median of 14.9 to 14.1 per month (median reduction of 13%) in 59 patients who received placebo, J. Helen Cross, MD, reported at the annual meeting of the American Academy of Neurology.
The proportion of patients with at least a 50% reduction in convulsive seizures was 42.6% with cannabidiol vs. 27.1% with placebo (odds ratio, 2.0), but this difference did not reach statistical significance, said Dr. Cross of the University College London Great Ormond Street Institute of Child Health and the Great Ormond Street Hospital for Children NHS Trust, London.
In a video interview, Dr. Cross discussed the findings and the importance of improving seizure control in patients with Dravet syndrome, a rare infantile-onset developmental and epileptic encephalopathy with very poor prognosis for long-term seizure control and neurodevelopmental outcomes.
Participants in the study (GWPCARE1) had a mean age of 10 years, but nearly a third were younger than 6 years. All had Dravet syndrome and drug-resistant seizures; the median number of antiepilepsy drugs previously tried was four, and the median number being used was three. Those randomized to the treatment group received cannabidiol oral solution up to 20 mg/kg per day.
Adverse events were common, occurring in 93.4% and 74.6% of treatment group and placebo group patients, respectively. But adverse events reported in the treatment group were mild or moderate in 84% of patients, and treatment was generally well tolerated.
“These are very complex patients with a high seizure burden... and therefore, to have another medication that looks as if it can be of benefit is really very exciting for this population,” Dr. Cross said, noting that cannabidiol was also shown in other studies presented at the AAN meeting (GWPCARE3 and GWPCARE4) to reduce seizure frequency in patients with Lennox-Gastaut syndrome.
GW Research sponsored the study. Dr. Cross is a member of the advisory boards for Eisai, GW Pharmaceuticals, Shire, and Zogenix.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
At ANN 2017
Key clinical point:
Major finding: Children and adolescents treated with cannabidiol had a decline in convulsive seizure frequency, from a median of 12.4 to 5.9 per month (median reduction of 39%), compared with a decrease from a median of 14.9 to 14.1 per month with placebo (median reduction of 13%).
Data source: A randomized, double-blind, placebo-controlled trial of adjunctive treatment with cannabidiol in 120 children and adolescents with Dravet syndrome.
Disclosures: GW Research sponsored the study. Dr. Cross is a member of the advisory boards for Eisai, GW Pharmaceuticals, Shire, and Zogenix.
In mantle cell lymphoma, triple therapy proves too toxic
Combined idelalisib, lenalidomide, and rituximab proved excessively toxic for the treatment of relapsed and refractory mantle cell and follicular lymphoma in two phase I trials conducted by the Alliance for Clinical Trials in Oncology.
The unexpected outcome, which led to early study termination, underscores the need for caution as new treatment combinations are proposed, Sonali M. Smith, MD, of the University of Chicago and her colleagues said in The Lancet Haematology.
In four of the first eight patients enrolled in the mantle cell lymphoma (A051201) and follicular lymphoma (A051202) phase I trials between July 9, 2013, and Sept. 30, 2014, unexpected dose-limiting toxicities occurred, including grade 4 sepsis syndrome, grade 4 hypotension with grade 3 rash and fevers, grade 4 aspartate aminotransferase (AST) or alanine aminotransferase (ALT) elevation with fevers, and grade 3 pulmonary infection with grade 3 maculopapular rash.
The adverse events occurred between 9 and 20 days after treatment initiation and coincided with rituximab infusions, the researchers said. No treatment-related deaths occurred (Lancet Haematol. 2017 Apr;4:e176-82).
Overall, 8 of 11 patients were removed from treatment because of an adverse event, and 3 of those required intensive care unit level of care.
Although rituximab was removed in both trials, two of the remaining three patients in the studies, including three with mantle cell lymphoma and eight with follicular lymphoma, experienced grade 3 rashes, and one had grade 3 AST elevations. In those with mantle cell lymphoma, the most common grade 3-4 adverse events were ALT elevations and rash. In those with follicular lymphoma, the most common grade 3-4 adverse events were neutropenia and rash.
“Given the inability to deliver treatment due to toxicity, both studies were permanently closed,” the researchers wrote, noting that the primary endpoint of safety and tolerability was not met.
The trials had the overall goal of developing targeted regimens to replace cytotoxic therapy.
“Both ... trials were designed to capitalize on the clinical synergy of lenalidomide and rituximab observed in previous trials by adding the highly specific PI3K delta inhibitor, idelalisib, for patients with relapsed mantle cell lymphoma and follicular lymphoma,” they said.
Previously available data implied that lenalidomide plus rituximab would be a safe backbone for therapy, and there was clinical rationale for adding idelalisib to that combination, they explained.
“Overall, our brief experience underscores the limited knowledge regarding drug interactions and off-target effects and serves as a cautionary note in developing biological agents in combination and against ad-hoc combinations outside of carefully monitored clinical trials,” they said.
The researchers noted that the nature of the toxicities observed in these trials supports an immune-activated state characterized by excessive inflammation.
“A more detailed assessment of effect on cytokines, T-cell subsets, natural killer cells, and clinical features predictive of toxicity and response should be included in any further testing of these classes of agents, and they should never be combined outside of a carefully designed and diligently monitored clinical trial setting,” they concluded.
The study was funded by the National Cancer Institute. Dr. Smith received research funding and consulting fees from Gilead and Celgene.
The findings by Dr. Smith and her colleagues add to several other reported studies that involved unexpected toxicities with various combinations of targeted agents in lymphoid malignancies.
Combinations of B-cell receptor signaling inhibitors can lead to immune dysregulation, which can be acute and severe when combined with immunomodulatory agents.
While the study of rational targeted combinations continues to hold immense potential in both untreated and relapsed/refractory disease, the combination must be thoroughly studied in the context of carefully and conservatively designed clinical trials.
Given the unpredictable nature of adverse events, the use of novel combinations outside of a clinical trial should be strongly discouraged.
Patrick M. Reagan, MD , and Paul M. Barr, MD , are with the James P. Wilmot Cancer Institute, University of Rochester, New York. Dr. Reagan reported having no disclosures. Dr. Barr has consulted for Gilead, Pharmacyclics, AbbVie, and Celgene. They made their remarks in an editorial that accompanied the article.
The findings by Dr. Smith and her colleagues add to several other reported studies that involved unexpected toxicities with various combinations of targeted agents in lymphoid malignancies.
Combinations of B-cell receptor signaling inhibitors can lead to immune dysregulation, which can be acute and severe when combined with immunomodulatory agents.
While the study of rational targeted combinations continues to hold immense potential in both untreated and relapsed/refractory disease, the combination must be thoroughly studied in the context of carefully and conservatively designed clinical trials.
Given the unpredictable nature of adverse events, the use of novel combinations outside of a clinical trial should be strongly discouraged.
Patrick M. Reagan, MD , and Paul M. Barr, MD , are with the James P. Wilmot Cancer Institute, University of Rochester, New York. Dr. Reagan reported having no disclosures. Dr. Barr has consulted for Gilead, Pharmacyclics, AbbVie, and Celgene. They made their remarks in an editorial that accompanied the article.
The findings by Dr. Smith and her colleagues add to several other reported studies that involved unexpected toxicities with various combinations of targeted agents in lymphoid malignancies.
Combinations of B-cell receptor signaling inhibitors can lead to immune dysregulation, which can be acute and severe when combined with immunomodulatory agents.
While the study of rational targeted combinations continues to hold immense potential in both untreated and relapsed/refractory disease, the combination must be thoroughly studied in the context of carefully and conservatively designed clinical trials.
Given the unpredictable nature of adverse events, the use of novel combinations outside of a clinical trial should be strongly discouraged.
Patrick M. Reagan, MD , and Paul M. Barr, MD , are with the James P. Wilmot Cancer Institute, University of Rochester, New York. Dr. Reagan reported having no disclosures. Dr. Barr has consulted for Gilead, Pharmacyclics, AbbVie, and Celgene. They made their remarks in an editorial that accompanied the article.
Combined idelalisib, lenalidomide, and rituximab proved excessively toxic for the treatment of relapsed and refractory mantle cell and follicular lymphoma in two phase I trials conducted by the Alliance for Clinical Trials in Oncology.
The unexpected outcome, which led to early study termination, underscores the need for caution as new treatment combinations are proposed, Sonali M. Smith, MD, of the University of Chicago and her colleagues said in The Lancet Haematology.
In four of the first eight patients enrolled in the mantle cell lymphoma (A051201) and follicular lymphoma (A051202) phase I trials between July 9, 2013, and Sept. 30, 2014, unexpected dose-limiting toxicities occurred, including grade 4 sepsis syndrome, grade 4 hypotension with grade 3 rash and fevers, grade 4 aspartate aminotransferase (AST) or alanine aminotransferase (ALT) elevation with fevers, and grade 3 pulmonary infection with grade 3 maculopapular rash.
The adverse events occurred between 9 and 20 days after treatment initiation and coincided with rituximab infusions, the researchers said. No treatment-related deaths occurred (Lancet Haematol. 2017 Apr;4:e176-82).
Overall, 8 of 11 patients were removed from treatment because of an adverse event, and 3 of those required intensive care unit level of care.
Although rituximab was removed in both trials, two of the remaining three patients in the studies, including three with mantle cell lymphoma and eight with follicular lymphoma, experienced grade 3 rashes, and one had grade 3 AST elevations. In those with mantle cell lymphoma, the most common grade 3-4 adverse events were ALT elevations and rash. In those with follicular lymphoma, the most common grade 3-4 adverse events were neutropenia and rash.
“Given the inability to deliver treatment due to toxicity, both studies were permanently closed,” the researchers wrote, noting that the primary endpoint of safety and tolerability was not met.
The trials had the overall goal of developing targeted regimens to replace cytotoxic therapy.
“Both ... trials were designed to capitalize on the clinical synergy of lenalidomide and rituximab observed in previous trials by adding the highly specific PI3K delta inhibitor, idelalisib, for patients with relapsed mantle cell lymphoma and follicular lymphoma,” they said.
Previously available data implied that lenalidomide plus rituximab would be a safe backbone for therapy, and there was clinical rationale for adding idelalisib to that combination, they explained.
“Overall, our brief experience underscores the limited knowledge regarding drug interactions and off-target effects and serves as a cautionary note in developing biological agents in combination and against ad-hoc combinations outside of carefully monitored clinical trials,” they said.
The researchers noted that the nature of the toxicities observed in these trials supports an immune-activated state characterized by excessive inflammation.
“A more detailed assessment of effect on cytokines, T-cell subsets, natural killer cells, and clinical features predictive of toxicity and response should be included in any further testing of these classes of agents, and they should never be combined outside of a carefully designed and diligently monitored clinical trial setting,” they concluded.
The study was funded by the National Cancer Institute. Dr. Smith received research funding and consulting fees from Gilead and Celgene.
Combined idelalisib, lenalidomide, and rituximab proved excessively toxic for the treatment of relapsed and refractory mantle cell and follicular lymphoma in two phase I trials conducted by the Alliance for Clinical Trials in Oncology.
The unexpected outcome, which led to early study termination, underscores the need for caution as new treatment combinations are proposed, Sonali M. Smith, MD, of the University of Chicago and her colleagues said in The Lancet Haematology.
In four of the first eight patients enrolled in the mantle cell lymphoma (A051201) and follicular lymphoma (A051202) phase I trials between July 9, 2013, and Sept. 30, 2014, unexpected dose-limiting toxicities occurred, including grade 4 sepsis syndrome, grade 4 hypotension with grade 3 rash and fevers, grade 4 aspartate aminotransferase (AST) or alanine aminotransferase (ALT) elevation with fevers, and grade 3 pulmonary infection with grade 3 maculopapular rash.
The adverse events occurred between 9 and 20 days after treatment initiation and coincided with rituximab infusions, the researchers said. No treatment-related deaths occurred (Lancet Haematol. 2017 Apr;4:e176-82).
Overall, 8 of 11 patients were removed from treatment because of an adverse event, and 3 of those required intensive care unit level of care.
Although rituximab was removed in both trials, two of the remaining three patients in the studies, including three with mantle cell lymphoma and eight with follicular lymphoma, experienced grade 3 rashes, and one had grade 3 AST elevations. In those with mantle cell lymphoma, the most common grade 3-4 adverse events were ALT elevations and rash. In those with follicular lymphoma, the most common grade 3-4 adverse events were neutropenia and rash.
“Given the inability to deliver treatment due to toxicity, both studies were permanently closed,” the researchers wrote, noting that the primary endpoint of safety and tolerability was not met.
The trials had the overall goal of developing targeted regimens to replace cytotoxic therapy.
“Both ... trials were designed to capitalize on the clinical synergy of lenalidomide and rituximab observed in previous trials by adding the highly specific PI3K delta inhibitor, idelalisib, for patients with relapsed mantle cell lymphoma and follicular lymphoma,” they said.
Previously available data implied that lenalidomide plus rituximab would be a safe backbone for therapy, and there was clinical rationale for adding idelalisib to that combination, they explained.
“Overall, our brief experience underscores the limited knowledge regarding drug interactions and off-target effects and serves as a cautionary note in developing biological agents in combination and against ad-hoc combinations outside of carefully monitored clinical trials,” they said.
The researchers noted that the nature of the toxicities observed in these trials supports an immune-activated state characterized by excessive inflammation.
“A more detailed assessment of effect on cytokines, T-cell subsets, natural killer cells, and clinical features predictive of toxicity and response should be included in any further testing of these classes of agents, and they should never be combined outside of a carefully designed and diligently monitored clinical trial setting,” they concluded.
The study was funded by the National Cancer Institute. Dr. Smith received research funding and consulting fees from Gilead and Celgene.
Key clinical point:
Major finding: Of 11 patients, 8 were removed from treatment because of an adverse event, and 3 of those required intensive care unit–level care.
Data source: Two phase I trials involving 11 patients.
Disclosures: The study was funded by the National Cancer Institute. Dr. Smith received research funding and consulting fees from Gilead and Celgene.
New guideline: Address GTCS frequency to reduce SUDEP risk
BOSTON – Generalized tonic-clonic seizures (GTCS) are a major risk factor for sudden unexpected death in epilepsy (SUDEP), which underscores the importance of advising people with epilepsy about controlling such seizures, according to a new practice guideline from the American Academy of Neurology and the American Epilepsy Society.
Though SUDEP is rare, with an incidence rate of 0.22/1,000 patient-years in children with epilepsy and 1.2/1,000 patient-years in adults with epilepsy, the guideline committee found that people with three or more GTCS per year are 15 times more likely to die suddenly than are those without this seizure type. The risk increases with increasing GTCS frequency. This translates to an absolute risk of up to 18 deaths per 1,000 patient-years for people with epilepsy who have frequent GTCS.
Specifically, the guideline recommends that , she said, adding that the guideline shows that “being free of seizures, particularly tonic-clonic seizures, is strongly associated with a decreased risk.”
Guideline coauthor, Elizabeth Donner, MD, added that, for this reason, the guideline recommends “that health professionals work with people who continue to have, specifically, these kind of seizures to try and reduce them with medications or with epilepsy surgery, actively weighing the risks and benefits of any new approach to seizure management.”
The recommendations are based on moderate (Level B) evidence.
The team also looked at numerous other risk factors for SUDEP and found that the strength of the evidence was too weak to support additional recommendations, said Dr. Donner, director of the comprehensive epilepsy program at The Hospital for Sick Children in Toronto and chair of the American Epilepsy Society SUDEP Task Force.
“More research is now needed to identify other preventable risk factors for SUDEP so that we can focus future studies on finding ways to reduce how often SUDEP occurs,” she added.
While the message regarding the importance of reducing seizure frequency is not new, it is important that this message be reiterated in the context of SUDEP, Dr. Donner said.
“It’s very important for it to be clear that the risk of frequent generalized tonic-clonic seizures – and we’re not talking about really frequent here; we’re talking about significant increased risk of death with only three per year – is not related only to maintaining a driver’s license, maintaining work, or other outcomes like that. It’s actually related to risk of death,” she said, noting that she hopes this is a motivator for pursuing treatments beyond medication when medication isn’t successful for treating seizures.
The guideline, which is endorsed by the International Child Neurology Association, is available online and in print (Neurology. 2017;88:1674–80).
Dr. Harden receives royalties from Wiley and Up-to-Date. Dr. Donner has received research support from the Canadian Institutes of Health Research, Dravet Canada, and SUDEP Aware. Other guideline authors reported numerous disclosures, including many industry sources.
BOSTON – Generalized tonic-clonic seizures (GTCS) are a major risk factor for sudden unexpected death in epilepsy (SUDEP), which underscores the importance of advising people with epilepsy about controlling such seizures, according to a new practice guideline from the American Academy of Neurology and the American Epilepsy Society.
Though SUDEP is rare, with an incidence rate of 0.22/1,000 patient-years in children with epilepsy and 1.2/1,000 patient-years in adults with epilepsy, the guideline committee found that people with three or more GTCS per year are 15 times more likely to die suddenly than are those without this seizure type. The risk increases with increasing GTCS frequency. This translates to an absolute risk of up to 18 deaths per 1,000 patient-years for people with epilepsy who have frequent GTCS.
Specifically, the guideline recommends that , she said, adding that the guideline shows that “being free of seizures, particularly tonic-clonic seizures, is strongly associated with a decreased risk.”
Guideline coauthor, Elizabeth Donner, MD, added that, for this reason, the guideline recommends “that health professionals work with people who continue to have, specifically, these kind of seizures to try and reduce them with medications or with epilepsy surgery, actively weighing the risks and benefits of any new approach to seizure management.”
The recommendations are based on moderate (Level B) evidence.
The team also looked at numerous other risk factors for SUDEP and found that the strength of the evidence was too weak to support additional recommendations, said Dr. Donner, director of the comprehensive epilepsy program at The Hospital for Sick Children in Toronto and chair of the American Epilepsy Society SUDEP Task Force.
“More research is now needed to identify other preventable risk factors for SUDEP so that we can focus future studies on finding ways to reduce how often SUDEP occurs,” she added.
While the message regarding the importance of reducing seizure frequency is not new, it is important that this message be reiterated in the context of SUDEP, Dr. Donner said.
“It’s very important for it to be clear that the risk of frequent generalized tonic-clonic seizures – and we’re not talking about really frequent here; we’re talking about significant increased risk of death with only three per year – is not related only to maintaining a driver’s license, maintaining work, or other outcomes like that. It’s actually related to risk of death,” she said, noting that she hopes this is a motivator for pursuing treatments beyond medication when medication isn’t successful for treating seizures.
The guideline, which is endorsed by the International Child Neurology Association, is available online and in print (Neurology. 2017;88:1674–80).
Dr. Harden receives royalties from Wiley and Up-to-Date. Dr. Donner has received research support from the Canadian Institutes of Health Research, Dravet Canada, and SUDEP Aware. Other guideline authors reported numerous disclosures, including many industry sources.
BOSTON – Generalized tonic-clonic seizures (GTCS) are a major risk factor for sudden unexpected death in epilepsy (SUDEP), which underscores the importance of advising people with epilepsy about controlling such seizures, according to a new practice guideline from the American Academy of Neurology and the American Epilepsy Society.
Though SUDEP is rare, with an incidence rate of 0.22/1,000 patient-years in children with epilepsy and 1.2/1,000 patient-years in adults with epilepsy, the guideline committee found that people with three or more GTCS per year are 15 times more likely to die suddenly than are those without this seizure type. The risk increases with increasing GTCS frequency. This translates to an absolute risk of up to 18 deaths per 1,000 patient-years for people with epilepsy who have frequent GTCS.
Specifically, the guideline recommends that , she said, adding that the guideline shows that “being free of seizures, particularly tonic-clonic seizures, is strongly associated with a decreased risk.”
Guideline coauthor, Elizabeth Donner, MD, added that, for this reason, the guideline recommends “that health professionals work with people who continue to have, specifically, these kind of seizures to try and reduce them with medications or with epilepsy surgery, actively weighing the risks and benefits of any new approach to seizure management.”
The recommendations are based on moderate (Level B) evidence.
The team also looked at numerous other risk factors for SUDEP and found that the strength of the evidence was too weak to support additional recommendations, said Dr. Donner, director of the comprehensive epilepsy program at The Hospital for Sick Children in Toronto and chair of the American Epilepsy Society SUDEP Task Force.
“More research is now needed to identify other preventable risk factors for SUDEP so that we can focus future studies on finding ways to reduce how often SUDEP occurs,” she added.
While the message regarding the importance of reducing seizure frequency is not new, it is important that this message be reiterated in the context of SUDEP, Dr. Donner said.
“It’s very important for it to be clear that the risk of frequent generalized tonic-clonic seizures – and we’re not talking about really frequent here; we’re talking about significant increased risk of death with only three per year – is not related only to maintaining a driver’s license, maintaining work, or other outcomes like that. It’s actually related to risk of death,” she said, noting that she hopes this is a motivator for pursuing treatments beyond medication when medication isn’t successful for treating seizures.
The guideline, which is endorsed by the International Child Neurology Association, is available online and in print (Neurology. 2017;88:1674–80).
Dr. Harden receives royalties from Wiley and Up-to-Date. Dr. Donner has received research support from the Canadian Institutes of Health Research, Dravet Canada, and SUDEP Aware. Other guideline authors reported numerous disclosures, including many industry sources.
AT AAN 2017
Prenotification, unequivocal stroke promote ultra-fast door-to-needle time
BOSTON – Ultra-fast door-to-needle times of 10 minutes or less for intravenous acute ischemic stroke thrombolysis can be safely achieved in carefully selected cases, according to a review of cases at an Austrian teaching hospital.
Raffi Topakian, MD, and his colleagues at the Academic Teaching Hospital Wels-Grieskirchen in Wels, Austria, followed a multidisciplinary intervention to reinforce key components of the well-known Helsinki model of acute stroke care to improve the intravenous thrombolysis rate and the median door-to-needle time (DNT) at the teaching hospital and analyzed data from 361 patients who underwent intravenous thrombolysis (IVT) for stroke there between July 2014 and September 2016. The IVT rate increased from 19% to about 27% after intervention, and the DNT during the study period was 60 minutes or less in 316 patients (87.5%), 30 minutes or less in 181 patients (50.1%), and 10 minutes or less in 63 patients (17.5%).
“Over the study period, we reduced the DNT time from 49 minutes to 25 minutes. This was significant, and the door-to-needle times were astonishingly similar for the in-hours service and the out-of-hour service,” he said at the annual meeting of the American Academy of Neurology.
Further, the rate of prenotifications from emergency medical services rose from about 30% to 63% during the study period.
Patients with ultra-fast DNT vs. those with slower DNT were older, had more chronic heart failure, had more severe stroke (National Institutes of Health Stroke Scale score of 10 vs. 5), had more anterior circulation stroke and cardioembolic stroke, and had clear onset of stroke. Independent predictors of ultra-fast DNT included prenotification by EMS, anterior circulation syndrome, chronic heart failure, and having a stroke neurologist on duty, Dr. Topakian said.
“Ultra short DNTs can be achieved safely. The key is that we are prenotified by the EMS, that we can get all the relevant history details during transport, that there is a dedicated multidisciplinary stroke team and EMS staff, and that we have a seemingly unequivocal clinical scenario,” he said. “Out-of-hours DNT matched in-hours DNT, but the caveat is we’re talking about highly selected candidates; safety must not be sacrificed for the sake of speed, in all of our patients.”
Dr. Topakian has received personal compensation for activities with Novartis and Shire-Baxalta as an advisory board member; from Novartis, Pfizer, AbbVie, and Bayer for conference support; and from Pfizer as a speaker.
BOSTON – Ultra-fast door-to-needle times of 10 minutes or less for intravenous acute ischemic stroke thrombolysis can be safely achieved in carefully selected cases, according to a review of cases at an Austrian teaching hospital.
Raffi Topakian, MD, and his colleagues at the Academic Teaching Hospital Wels-Grieskirchen in Wels, Austria, followed a multidisciplinary intervention to reinforce key components of the well-known Helsinki model of acute stroke care to improve the intravenous thrombolysis rate and the median door-to-needle time (DNT) at the teaching hospital and analyzed data from 361 patients who underwent intravenous thrombolysis (IVT) for stroke there between July 2014 and September 2016. The IVT rate increased from 19% to about 27% after intervention, and the DNT during the study period was 60 minutes or less in 316 patients (87.5%), 30 minutes or less in 181 patients (50.1%), and 10 minutes or less in 63 patients (17.5%).
“Over the study period, we reduced the DNT time from 49 minutes to 25 minutes. This was significant, and the door-to-needle times were astonishingly similar for the in-hours service and the out-of-hour service,” he said at the annual meeting of the American Academy of Neurology.
Further, the rate of prenotifications from emergency medical services rose from about 30% to 63% during the study period.
Patients with ultra-fast DNT vs. those with slower DNT were older, had more chronic heart failure, had more severe stroke (National Institutes of Health Stroke Scale score of 10 vs. 5), had more anterior circulation stroke and cardioembolic stroke, and had clear onset of stroke. Independent predictors of ultra-fast DNT included prenotification by EMS, anterior circulation syndrome, chronic heart failure, and having a stroke neurologist on duty, Dr. Topakian said.
“Ultra short DNTs can be achieved safely. The key is that we are prenotified by the EMS, that we can get all the relevant history details during transport, that there is a dedicated multidisciplinary stroke team and EMS staff, and that we have a seemingly unequivocal clinical scenario,” he said. “Out-of-hours DNT matched in-hours DNT, but the caveat is we’re talking about highly selected candidates; safety must not be sacrificed for the sake of speed, in all of our patients.”
Dr. Topakian has received personal compensation for activities with Novartis and Shire-Baxalta as an advisory board member; from Novartis, Pfizer, AbbVie, and Bayer for conference support; and from Pfizer as a speaker.
BOSTON – Ultra-fast door-to-needle times of 10 minutes or less for intravenous acute ischemic stroke thrombolysis can be safely achieved in carefully selected cases, according to a review of cases at an Austrian teaching hospital.
Raffi Topakian, MD, and his colleagues at the Academic Teaching Hospital Wels-Grieskirchen in Wels, Austria, followed a multidisciplinary intervention to reinforce key components of the well-known Helsinki model of acute stroke care to improve the intravenous thrombolysis rate and the median door-to-needle time (DNT) at the teaching hospital and analyzed data from 361 patients who underwent intravenous thrombolysis (IVT) for stroke there between July 2014 and September 2016. The IVT rate increased from 19% to about 27% after intervention, and the DNT during the study period was 60 minutes or less in 316 patients (87.5%), 30 minutes or less in 181 patients (50.1%), and 10 minutes or less in 63 patients (17.5%).
“Over the study period, we reduced the DNT time from 49 minutes to 25 minutes. This was significant, and the door-to-needle times were astonishingly similar for the in-hours service and the out-of-hour service,” he said at the annual meeting of the American Academy of Neurology.
Further, the rate of prenotifications from emergency medical services rose from about 30% to 63% during the study period.
Patients with ultra-fast DNT vs. those with slower DNT were older, had more chronic heart failure, had more severe stroke (National Institutes of Health Stroke Scale score of 10 vs. 5), had more anterior circulation stroke and cardioembolic stroke, and had clear onset of stroke. Independent predictors of ultra-fast DNT included prenotification by EMS, anterior circulation syndrome, chronic heart failure, and having a stroke neurologist on duty, Dr. Topakian said.
“Ultra short DNTs can be achieved safely. The key is that we are prenotified by the EMS, that we can get all the relevant history details during transport, that there is a dedicated multidisciplinary stroke team and EMS staff, and that we have a seemingly unequivocal clinical scenario,” he said. “Out-of-hours DNT matched in-hours DNT, but the caveat is we’re talking about highly selected candidates; safety must not be sacrificed for the sake of speed, in all of our patients.”
Dr. Topakian has received personal compensation for activities with Novartis and Shire-Baxalta as an advisory board member; from Novartis, Pfizer, AbbVie, and Bayer for conference support; and from Pfizer as a speaker.
Key clinical point:
Major finding: Door-to-needle time of 10 minutes or less was achieved in 63 patients (17.5%).
Data source: A retrospective review of prospectively collected data from 361 patients.
Disclosures: Dr. Topakian has received personal compensation for activities with Novartis and Shire-Baxalta as an advisory board member; from Novartis, Pfizer, AbbVie, and Bayer for conference support; and from Pfizer as a speaker.
Optimum antithymocyte globulin exposure after HTC affects survival
Optimum antithymocyte globulin exposure after allogeneic hemopoietic cell transplantation (HTC) is associated with a higher probability of survival as a result of reductions in transplant-related and relapse-related deaths, based on findings from a retrospective multicenter cohort study.
The findings come from a pharmacokinetic-pharmacodynamic analysis of data from 146 patients with acute lymphoid leukemia, acute myeloid leukemia, or myelodysplastic syndrome. All were receiving their first T cell–repleted allogeneic peripheral blood stem cell HCT with antithymocyte globulin (ATG) as part of their nonmyeloablative conditioning regimen. Based on hazard ratios for overall survival, nonrelapse mortality, and relapse-related mortality, the optimum range of ATG exposure was 60-95 arbitrary units per day/mL, Rick Admiraal, MD, of the University Medical Centre Utrecht, Netherlands, and his colleagues reported in the Lancet Haematology (Lancet Haematol. 2017 Apr;4[4]:e183-91).
The estimated 5-year survival was significantly greater with optimum ATG exposure than with below-optimum exposure (69% vs. 32%; hazard ratio, 2.41) or with above-optimum exposure (69% vs. 48%; hazard ratio, 2.11).
Optimum ATG exposure also was associated with a greater likelihood of event-free survival: The hazard ratio was 2.54 for those with below-optimum exposure and 1.83 for those with above-optimum exposure, the researchers said. Further, above-optimum exposure was associated with higher relapse-related mortality (hazard ratio, 2.66). Below-optimum exposure was associated with higher non-relapse mortality (hazard ratio, 4.36) as well as with a higher risk for grade 3-4 acute graft-versus-host disease (hazard ratio, 3.09), but not for chronic graft-versus-host disease (hazard ratio, 2.38).
Optimum target attainment was better with modeled dosing based on absolute lymphocyte counts than with weight-based dosing, the authors said.
The findings underscore the importance of optimum ATG dosing, as “survival after HCT is highly affected by ATG exposure after HCT,” and they suggest that survival chances may be improved with individualized dosing based on lymphocyte counts–a finding that requires assessment in a prospective study, they concluded.
This study was funded by the Dutch Organization for Scientific Research and the Queen Wilhelmina Fund for Cancer Research. Dr. Admiraal reported having no relevant disclosures.
The study by Dr. Admiraal and his associates introduces important concepts regarding ATG dosing, but the suggestion that dosing should be individualized based on lymphocyte count and should target the area under the time-versus-thymoglobulin-concentration curve of 60-95 arbitrary units per day/mL after HCT should not yet become the standard of care.
“As the authors correctly point out, first a prospective study is needed,” Dr. Storek wrote.
However, even if such a study confirms this approach – which the authors previously showed to be of benefit in children – it would apply only to the setting in which it was developed. The problem is that there are numerous HCT settings, each requiring a different dose. Further, there is no universally accepted assay for measuring thymoglobulin concentrations, he noted.
“Following the seminal observations in the paper … much work remains to be done,” he wrote.
Jan Storek, MD, is with the University of Calgary (Alta.) and Alberta Health Services, Calgary. He made his comments in an editorial (Lancet Haematol. 2017 Apr;4[4]:e154-5) published with the study and reported having no disclosures.
The study by Dr. Admiraal and his associates introduces important concepts regarding ATG dosing, but the suggestion that dosing should be individualized based on lymphocyte count and should target the area under the time-versus-thymoglobulin-concentration curve of 60-95 arbitrary units per day/mL after HCT should not yet become the standard of care.
“As the authors correctly point out, first a prospective study is needed,” Dr. Storek wrote.
However, even if such a study confirms this approach – which the authors previously showed to be of benefit in children – it would apply only to the setting in which it was developed. The problem is that there are numerous HCT settings, each requiring a different dose. Further, there is no universally accepted assay for measuring thymoglobulin concentrations, he noted.
“Following the seminal observations in the paper … much work remains to be done,” he wrote.
Jan Storek, MD, is with the University of Calgary (Alta.) and Alberta Health Services, Calgary. He made his comments in an editorial (Lancet Haematol. 2017 Apr;4[4]:e154-5) published with the study and reported having no disclosures.
The study by Dr. Admiraal and his associates introduces important concepts regarding ATG dosing, but the suggestion that dosing should be individualized based on lymphocyte count and should target the area under the time-versus-thymoglobulin-concentration curve of 60-95 arbitrary units per day/mL after HCT should not yet become the standard of care.
“As the authors correctly point out, first a prospective study is needed,” Dr. Storek wrote.
However, even if such a study confirms this approach – which the authors previously showed to be of benefit in children – it would apply only to the setting in which it was developed. The problem is that there are numerous HCT settings, each requiring a different dose. Further, there is no universally accepted assay for measuring thymoglobulin concentrations, he noted.
“Following the seminal observations in the paper … much work remains to be done,” he wrote.
Jan Storek, MD, is with the University of Calgary (Alta.) and Alberta Health Services, Calgary. He made his comments in an editorial (Lancet Haematol. 2017 Apr;4[4]:e154-5) published with the study and reported having no disclosures.
Optimum antithymocyte globulin exposure after allogeneic hemopoietic cell transplantation (HTC) is associated with a higher probability of survival as a result of reductions in transplant-related and relapse-related deaths, based on findings from a retrospective multicenter cohort study.
The findings come from a pharmacokinetic-pharmacodynamic analysis of data from 146 patients with acute lymphoid leukemia, acute myeloid leukemia, or myelodysplastic syndrome. All were receiving their first T cell–repleted allogeneic peripheral blood stem cell HCT with antithymocyte globulin (ATG) as part of their nonmyeloablative conditioning regimen. Based on hazard ratios for overall survival, nonrelapse mortality, and relapse-related mortality, the optimum range of ATG exposure was 60-95 arbitrary units per day/mL, Rick Admiraal, MD, of the University Medical Centre Utrecht, Netherlands, and his colleagues reported in the Lancet Haematology (Lancet Haematol. 2017 Apr;4[4]:e183-91).
The estimated 5-year survival was significantly greater with optimum ATG exposure than with below-optimum exposure (69% vs. 32%; hazard ratio, 2.41) or with above-optimum exposure (69% vs. 48%; hazard ratio, 2.11).
Optimum ATG exposure also was associated with a greater likelihood of event-free survival: The hazard ratio was 2.54 for those with below-optimum exposure and 1.83 for those with above-optimum exposure, the researchers said. Further, above-optimum exposure was associated with higher relapse-related mortality (hazard ratio, 2.66). Below-optimum exposure was associated with higher non-relapse mortality (hazard ratio, 4.36) as well as with a higher risk for grade 3-4 acute graft-versus-host disease (hazard ratio, 3.09), but not for chronic graft-versus-host disease (hazard ratio, 2.38).
Optimum target attainment was better with modeled dosing based on absolute lymphocyte counts than with weight-based dosing, the authors said.
The findings underscore the importance of optimum ATG dosing, as “survival after HCT is highly affected by ATG exposure after HCT,” and they suggest that survival chances may be improved with individualized dosing based on lymphocyte counts–a finding that requires assessment in a prospective study, they concluded.
This study was funded by the Dutch Organization for Scientific Research and the Queen Wilhelmina Fund for Cancer Research. Dr. Admiraal reported having no relevant disclosures.
Optimum antithymocyte globulin exposure after allogeneic hemopoietic cell transplantation (HTC) is associated with a higher probability of survival as a result of reductions in transplant-related and relapse-related deaths, based on findings from a retrospective multicenter cohort study.
The findings come from a pharmacokinetic-pharmacodynamic analysis of data from 146 patients with acute lymphoid leukemia, acute myeloid leukemia, or myelodysplastic syndrome. All were receiving their first T cell–repleted allogeneic peripheral blood stem cell HCT with antithymocyte globulin (ATG) as part of their nonmyeloablative conditioning regimen. Based on hazard ratios for overall survival, nonrelapse mortality, and relapse-related mortality, the optimum range of ATG exposure was 60-95 arbitrary units per day/mL, Rick Admiraal, MD, of the University Medical Centre Utrecht, Netherlands, and his colleagues reported in the Lancet Haematology (Lancet Haematol. 2017 Apr;4[4]:e183-91).
The estimated 5-year survival was significantly greater with optimum ATG exposure than with below-optimum exposure (69% vs. 32%; hazard ratio, 2.41) or with above-optimum exposure (69% vs. 48%; hazard ratio, 2.11).
Optimum ATG exposure also was associated with a greater likelihood of event-free survival: The hazard ratio was 2.54 for those with below-optimum exposure and 1.83 for those with above-optimum exposure, the researchers said. Further, above-optimum exposure was associated with higher relapse-related mortality (hazard ratio, 2.66). Below-optimum exposure was associated with higher non-relapse mortality (hazard ratio, 4.36) as well as with a higher risk for grade 3-4 acute graft-versus-host disease (hazard ratio, 3.09), but not for chronic graft-versus-host disease (hazard ratio, 2.38).
Optimum target attainment was better with modeled dosing based on absolute lymphocyte counts than with weight-based dosing, the authors said.
The findings underscore the importance of optimum ATG dosing, as “survival after HCT is highly affected by ATG exposure after HCT,” and they suggest that survival chances may be improved with individualized dosing based on lymphocyte counts–a finding that requires assessment in a prospective study, they concluded.
This study was funded by the Dutch Organization for Scientific Research and the Queen Wilhelmina Fund for Cancer Research. Dr. Admiraal reported having no relevant disclosures.
FROM THE LANCET HAEMATOLOGY
Key clinical point:
Major finding: The estimated 5-year survival with optimum ATG exposure was 69% vs. 32% and 48% with below- and above-optimum exposure (hazard ratios, 2.41 and 2.11, respectively).
Data source: A retrospective cohort study of 146 patients receiving their first T-cell repleted allogeneic peripheral blood stem cell HCT.
Disclosures: This study was funded by the Dutch Organization for Scientific Research and the Queen Wilhelmina Fund for Cancer Research. Dr. Admiraal reported having no disclosures.
Extended maraviroc helps prevent graft-versus-host disease
ORLANDO – The use of the CCR5 antagonist maraviroc for 90 days is safe and effective for graft-versus-host disease (GVHD) prophylaxis in patients undergoing allogeneic stem cell transplantation, according to findings from a phase II study.
An earlier study showed that CCR5 blockade using maraviroc for 33 days was associated with a low incidence of acute GVHD, as well as with absence of early liver and gut GVHD – although delayed severe cases of visceral GVHD still occurred.
The current study was performed because the prior findings raised concerns that brief blockade was insufficient for preventing GVHD over a longer period of time. The new findings show that an extended course may indeed provide additional benefits, Ran Reshef, MD, reported at the combined annual meetings of the Center for International Blood & Marrow Transplant Research and the American Society for Blood and Marrow Transplantation.
In 37 high-risk patients who received allogeneic stem cell transplantation from unrelated donors using fludarabine/busulfan (Flu/Bu2) conditioning followed by peripheral blood stem cells, maraviroc was given at a dose of 300 mg twice daily, in addition to standard tacrolimus and methotrexate.
The 180-day rates of grade 2-4 and grade 3-4 acute GVHD (the primary endpoint of the study) in these patients were 27% and 5%, respectively. These rates were very similar to the 24% and 6% rates seen in the first study at 6 months after 30 days of maraviroc treatment, said Dr. Reshef of Columbia University Medical Center, New York.
The earlier results were “driven not so much by a reduction in the rates of skin GVHD, but by low rates of visceral GVHD of the gut and the liver – with a striking absence of gut and liver GVHD in the first 100 days,” he said.
Dr. Reshef also noted that the current study had a less favorable donor mix, as no matched related donors were included because of the earlier study’s very low rates of GVHD – with or without maraviroc – in those with related donors, who composed a third of donors.
Long-term follow-up of results from the earlier study, with comparison of a large contemporary control cohort, showed that “there is in fact an impact ... on grade 2-4 and grade 3-4 [GVHD], although the number of events is small, and the study was not powered enough to reach statistical significance,” Dr. Reshef said. The rates of chronic GVHD did not differ between the study subjects and contemporary controls, he noted.
At 100 days in the current study, there were no cases of liver GVHD, two cases of mild upper-GI GVHD, and one case of severe gut GVHD. At 1 year, the disease relapse rate was “fairly reasonable” at 30%, nonrelapse mortality was 12% with only one case of death from GVHD, and the incidence of chronic GVHD was 8%, which was significantly lower than in the prior study, he said.
The low rate of chronic GVHD led to a GVHD/relapse-free survival (GRFS) rate of 49%.
“To put this in context, the [Center for International Blood & Marrow Transplant Research] data for reduced-intensity transplants ... have shown 25% for acute myeloid leukemia and 12% for myelodysplastic syndrome,” he said. “So, we feel that these are by far improved numbers, compared with this benchmark.”
To determine which patients develop GVHD despite chemotaxis blockade and why, Dr. Reshef and his colleagues developed a pharmacodynamic assay to assess the activity of maraviroc in fresh blood samples. They found that those with insufficient CCR5 blockade on day 0 were those with higher incidence of severe acute GVHD, nonrelapse mortality, GRFS, and overall survival.
The investigators performed pharmacokinetic analysis using combined data from both trials to improve understanding of why some patients have insufficient CCR5 blockade. This showed significant variability in day 0 trough of maraviroc among patients (median of 65 ng/mL, range 12-316 ng/mL); levels above the median were associated with a significantly lower incidence of acute grade 2-4 GVHD and a trend toward improved GRFS.
These studies of maraviroc, which was originally developed for the treatment of HIV infection, were done to test the belief that blocking lymphocyte migration might prevent GVHD without interfering with graft-versus-tumor activity. Based on the earlier findings, Dr. Reshef and his colleagues hypothesized that treatment up to day 90 would decrease the rate to less than 30%, from a historical rate of 52%.
Patients in the study were high risk by virtue of age (median, 64 years), HLA matching (matched unrelated, 84%; mismatched unrelated, 16%), and comorbidities (comorbidity index greater than 2 in 49%). Underlying diseases were acute leukemia (78%), myelodysplastic syndrome (16%), and myeloproliferative neoplasm and cutaneous T-cell lymphomas (3% each).
At a median follow-up of 21 months, the 3-month course of maraviroc was well tolerated. Eight patients did not complete treatment because of disease relapse (five patients), skin reaction (one patient), early infection-related death (one patient), or poor tolerance of oral drugs (one patient). Neutrophil, platelet, and T-cell engraftment were similar to historical controls, and rates of infections were also similar, Dr Reshef noted.
“To conclude, an extended course of maraviroc up to day 90 is feasible and safe in the majority of patients,” he said. “This study confirms the effect of CCR5 blockade on visceral GVHD. I’m still awaiting a randomized study to confirm that further.
“A long course of maraviroc does not necessarily affect the rates of acute GVHD, but may help reduce chronic GVHD and improve GRFS,” Dr. Reshef said. “We should look further into the pharmacodynamic and pharmacokinetic variables.”
Dr. Reshef reported receiving research funding from Pfizer.
ORLANDO – The use of the CCR5 antagonist maraviroc for 90 days is safe and effective for graft-versus-host disease (GVHD) prophylaxis in patients undergoing allogeneic stem cell transplantation, according to findings from a phase II study.
An earlier study showed that CCR5 blockade using maraviroc for 33 days was associated with a low incidence of acute GVHD, as well as with absence of early liver and gut GVHD – although delayed severe cases of visceral GVHD still occurred.
The current study was performed because the prior findings raised concerns that brief blockade was insufficient for preventing GVHD over a longer period of time. The new findings show that an extended course may indeed provide additional benefits, Ran Reshef, MD, reported at the combined annual meetings of the Center for International Blood & Marrow Transplant Research and the American Society for Blood and Marrow Transplantation.
In 37 high-risk patients who received allogeneic stem cell transplantation from unrelated donors using fludarabine/busulfan (Flu/Bu2) conditioning followed by peripheral blood stem cells, maraviroc was given at a dose of 300 mg twice daily, in addition to standard tacrolimus and methotrexate.
The 180-day rates of grade 2-4 and grade 3-4 acute GVHD (the primary endpoint of the study) in these patients were 27% and 5%, respectively. These rates were very similar to the 24% and 6% rates seen in the first study at 6 months after 30 days of maraviroc treatment, said Dr. Reshef of Columbia University Medical Center, New York.
The earlier results were “driven not so much by a reduction in the rates of skin GVHD, but by low rates of visceral GVHD of the gut and the liver – with a striking absence of gut and liver GVHD in the first 100 days,” he said.
Dr. Reshef also noted that the current study had a less favorable donor mix, as no matched related donors were included because of the earlier study’s very low rates of GVHD – with or without maraviroc – in those with related donors, who composed a third of donors.
Long-term follow-up of results from the earlier study, with comparison of a large contemporary control cohort, showed that “there is in fact an impact ... on grade 2-4 and grade 3-4 [GVHD], although the number of events is small, and the study was not powered enough to reach statistical significance,” Dr. Reshef said. The rates of chronic GVHD did not differ between the study subjects and contemporary controls, he noted.
At 100 days in the current study, there were no cases of liver GVHD, two cases of mild upper-GI GVHD, and one case of severe gut GVHD. At 1 year, the disease relapse rate was “fairly reasonable” at 30%, nonrelapse mortality was 12% with only one case of death from GVHD, and the incidence of chronic GVHD was 8%, which was significantly lower than in the prior study, he said.
The low rate of chronic GVHD led to a GVHD/relapse-free survival (GRFS) rate of 49%.
“To put this in context, the [Center for International Blood & Marrow Transplant Research] data for reduced-intensity transplants ... have shown 25% for acute myeloid leukemia and 12% for myelodysplastic syndrome,” he said. “So, we feel that these are by far improved numbers, compared with this benchmark.”
To determine which patients develop GVHD despite chemotaxis blockade and why, Dr. Reshef and his colleagues developed a pharmacodynamic assay to assess the activity of maraviroc in fresh blood samples. They found that those with insufficient CCR5 blockade on day 0 were those with higher incidence of severe acute GVHD, nonrelapse mortality, GRFS, and overall survival.
The investigators performed pharmacokinetic analysis using combined data from both trials to improve understanding of why some patients have insufficient CCR5 blockade. This showed significant variability in day 0 trough of maraviroc among patients (median of 65 ng/mL, range 12-316 ng/mL); levels above the median were associated with a significantly lower incidence of acute grade 2-4 GVHD and a trend toward improved GRFS.
These studies of maraviroc, which was originally developed for the treatment of HIV infection, were done to test the belief that blocking lymphocyte migration might prevent GVHD without interfering with graft-versus-tumor activity. Based on the earlier findings, Dr. Reshef and his colleagues hypothesized that treatment up to day 90 would decrease the rate to less than 30%, from a historical rate of 52%.
Patients in the study were high risk by virtue of age (median, 64 years), HLA matching (matched unrelated, 84%; mismatched unrelated, 16%), and comorbidities (comorbidity index greater than 2 in 49%). Underlying diseases were acute leukemia (78%), myelodysplastic syndrome (16%), and myeloproliferative neoplasm and cutaneous T-cell lymphomas (3% each).
At a median follow-up of 21 months, the 3-month course of maraviroc was well tolerated. Eight patients did not complete treatment because of disease relapse (five patients), skin reaction (one patient), early infection-related death (one patient), or poor tolerance of oral drugs (one patient). Neutrophil, platelet, and T-cell engraftment were similar to historical controls, and rates of infections were also similar, Dr Reshef noted.
“To conclude, an extended course of maraviroc up to day 90 is feasible and safe in the majority of patients,” he said. “This study confirms the effect of CCR5 blockade on visceral GVHD. I’m still awaiting a randomized study to confirm that further.
“A long course of maraviroc does not necessarily affect the rates of acute GVHD, but may help reduce chronic GVHD and improve GRFS,” Dr. Reshef said. “We should look further into the pharmacodynamic and pharmacokinetic variables.”
Dr. Reshef reported receiving research funding from Pfizer.
ORLANDO – The use of the CCR5 antagonist maraviroc for 90 days is safe and effective for graft-versus-host disease (GVHD) prophylaxis in patients undergoing allogeneic stem cell transplantation, according to findings from a phase II study.
An earlier study showed that CCR5 blockade using maraviroc for 33 days was associated with a low incidence of acute GVHD, as well as with absence of early liver and gut GVHD – although delayed severe cases of visceral GVHD still occurred.
The current study was performed because the prior findings raised concerns that brief blockade was insufficient for preventing GVHD over a longer period of time. The new findings show that an extended course may indeed provide additional benefits, Ran Reshef, MD, reported at the combined annual meetings of the Center for International Blood & Marrow Transplant Research and the American Society for Blood and Marrow Transplantation.
In 37 high-risk patients who received allogeneic stem cell transplantation from unrelated donors using fludarabine/busulfan (Flu/Bu2) conditioning followed by peripheral blood stem cells, maraviroc was given at a dose of 300 mg twice daily, in addition to standard tacrolimus and methotrexate.
The 180-day rates of grade 2-4 and grade 3-4 acute GVHD (the primary endpoint of the study) in these patients were 27% and 5%, respectively. These rates were very similar to the 24% and 6% rates seen in the first study at 6 months after 30 days of maraviroc treatment, said Dr. Reshef of Columbia University Medical Center, New York.
The earlier results were “driven not so much by a reduction in the rates of skin GVHD, but by low rates of visceral GVHD of the gut and the liver – with a striking absence of gut and liver GVHD in the first 100 days,” he said.
Dr. Reshef also noted that the current study had a less favorable donor mix, as no matched related donors were included because of the earlier study’s very low rates of GVHD – with or without maraviroc – in those with related donors, who composed a third of donors.
Long-term follow-up of results from the earlier study, with comparison of a large contemporary control cohort, showed that “there is in fact an impact ... on grade 2-4 and grade 3-4 [GVHD], although the number of events is small, and the study was not powered enough to reach statistical significance,” Dr. Reshef said. The rates of chronic GVHD did not differ between the study subjects and contemporary controls, he noted.
At 100 days in the current study, there were no cases of liver GVHD, two cases of mild upper-GI GVHD, and one case of severe gut GVHD. At 1 year, the disease relapse rate was “fairly reasonable” at 30%, nonrelapse mortality was 12% with only one case of death from GVHD, and the incidence of chronic GVHD was 8%, which was significantly lower than in the prior study, he said.
The low rate of chronic GVHD led to a GVHD/relapse-free survival (GRFS) rate of 49%.
“To put this in context, the [Center for International Blood & Marrow Transplant Research] data for reduced-intensity transplants ... have shown 25% for acute myeloid leukemia and 12% for myelodysplastic syndrome,” he said. “So, we feel that these are by far improved numbers, compared with this benchmark.”
To determine which patients develop GVHD despite chemotaxis blockade and why, Dr. Reshef and his colleagues developed a pharmacodynamic assay to assess the activity of maraviroc in fresh blood samples. They found that those with insufficient CCR5 blockade on day 0 were those with higher incidence of severe acute GVHD, nonrelapse mortality, GRFS, and overall survival.
The investigators performed pharmacokinetic analysis using combined data from both trials to improve understanding of why some patients have insufficient CCR5 blockade. This showed significant variability in day 0 trough of maraviroc among patients (median of 65 ng/mL, range 12-316 ng/mL); levels above the median were associated with a significantly lower incidence of acute grade 2-4 GVHD and a trend toward improved GRFS.
These studies of maraviroc, which was originally developed for the treatment of HIV infection, were done to test the belief that blocking lymphocyte migration might prevent GVHD without interfering with graft-versus-tumor activity. Based on the earlier findings, Dr. Reshef and his colleagues hypothesized that treatment up to day 90 would decrease the rate to less than 30%, from a historical rate of 52%.
Patients in the study were high risk by virtue of age (median, 64 years), HLA matching (matched unrelated, 84%; mismatched unrelated, 16%), and comorbidities (comorbidity index greater than 2 in 49%). Underlying diseases were acute leukemia (78%), myelodysplastic syndrome (16%), and myeloproliferative neoplasm and cutaneous T-cell lymphomas (3% each).
At a median follow-up of 21 months, the 3-month course of maraviroc was well tolerated. Eight patients did not complete treatment because of disease relapse (five patients), skin reaction (one patient), early infection-related death (one patient), or poor tolerance of oral drugs (one patient). Neutrophil, platelet, and T-cell engraftment were similar to historical controls, and rates of infections were also similar, Dr Reshef noted.
“To conclude, an extended course of maraviroc up to day 90 is feasible and safe in the majority of patients,” he said. “This study confirms the effect of CCR5 blockade on visceral GVHD. I’m still awaiting a randomized study to confirm that further.
“A long course of maraviroc does not necessarily affect the rates of acute GVHD, but may help reduce chronic GVHD and improve GRFS,” Dr. Reshef said. “We should look further into the pharmacodynamic and pharmacokinetic variables.”
Dr. Reshef reported receiving research funding from Pfizer.
AT THE 2017 BMT TANDEM MEETINGS
Key clinical point:
Major finding: The 180-day rates of grade 2-4 and grade 3-4 acute GVHD were 27% and 5%, respectively.
Data source: A phase II study of 37 patients.
Disclosures: Dr. Reshef reported receiving research funding from Pfizer.
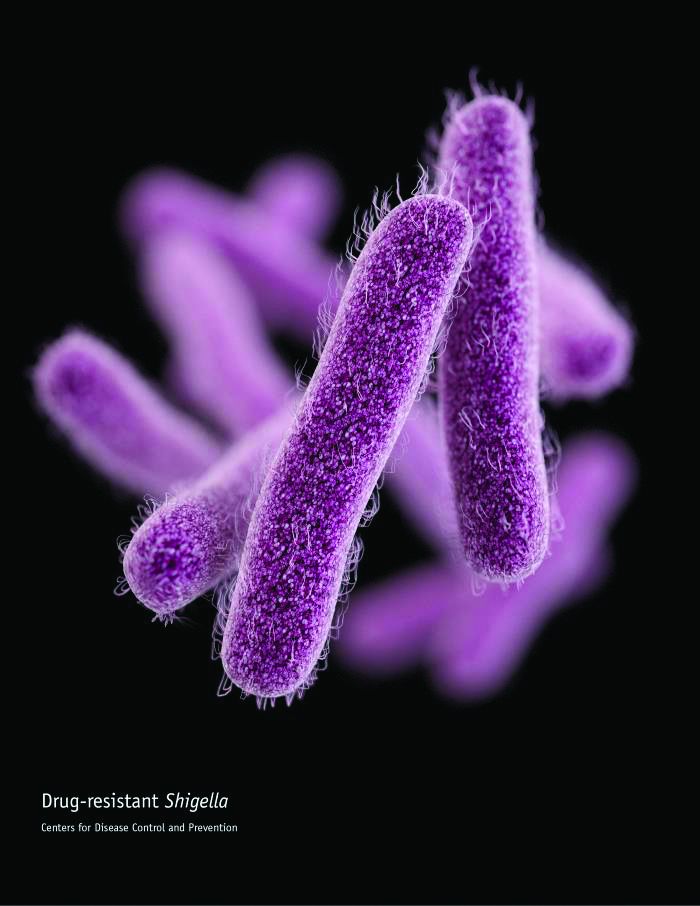
CDC: Some Shigella strains show reduced ciprofloxacin susceptibility
The Centers for Disease Control and Prevention has identified an increase in Shigella isolates with reduced susceptibility to ciprofloxacin, and has released an official health advisory outlining new recommendations for clinical diagnosis, management, and reporting, as well as for laboratories and public health officials.
The Shigella isolates of concern in the United States have minimum inhibitory concentration (MIC) values of 0.12-1 mcg/mL for ciprofloxacin, which is within the range considered susceptible. These strains, however, “often have a quinolone resistance gene that may lead to clinically significant reduced susceptibility to fluoroquinolone antibiotics,” such as ciprofloxacin, according to the CDC advisory.
It is possible that strains with MIC in the 0.12-1 mcg/mL range may have worse clinical outcome or increased risk of transmission, so the CDC made the following recommendations to clinicians:
• Order a stool culture to obtain isolates for antimicrobial susceptibility testing in suspected cases.
• Order antimicrobial susceptibility testing when ordering a stool culture for Shigella.
• Avoid routine prescribing of antibiotic therapy for Shigella infection, instead reserving antibiotics for patients with a clinical indication or when advised by public health officials in an outbreak setting.
• Tailor antibiotic choice (when antibiotics are indicated) to susceptibility results as soon as possible – with special attention given to the MIC for fluoroquinolone antibiotics.
• Obtain follow-up stool cultures in shigellosis patients who have continued or worsening symptoms despite antibiotic therapy.
• Consult local or state health departments for guidance regarding when patients may return to child care, school, or work.
• Counsel patients with active diarrhea on how they can prevent spreading the infection to others, regardless of whether antibiotic treatment is prescribed.
Additionally, the CDC noted that shigellosis is a nationally notifiable condition; all cases should be reported to the local health department. If a patient with shigellosis and a ciprofloxacin MIC of 0.12-1 mcg/mL is identified, this information should be included in the report to facilitate further testing of the isolate.
The CDC reported that it is working with state and local public health departments and clinical partners to determine if outcomes are indeed worse for patients treated with ciprofloxacin for Shigella strains harboring a quinolone resistance gene, and it will continue to monitor trends in susceptibility of Shigella isolates and to perform genetic testing on select strains to confirm the presence and type of resistance genes.
The Centers for Disease Control and Prevention has identified an increase in Shigella isolates with reduced susceptibility to ciprofloxacin, and has released an official health advisory outlining new recommendations for clinical diagnosis, management, and reporting, as well as for laboratories and public health officials.
The Shigella isolates of concern in the United States have minimum inhibitory concentration (MIC) values of 0.12-1 mcg/mL for ciprofloxacin, which is within the range considered susceptible. These strains, however, “often have a quinolone resistance gene that may lead to clinically significant reduced susceptibility to fluoroquinolone antibiotics,” such as ciprofloxacin, according to the CDC advisory.
It is possible that strains with MIC in the 0.12-1 mcg/mL range may have worse clinical outcome or increased risk of transmission, so the CDC made the following recommendations to clinicians:
• Order a stool culture to obtain isolates for antimicrobial susceptibility testing in suspected cases.
• Order antimicrobial susceptibility testing when ordering a stool culture for Shigella.
• Avoid routine prescribing of antibiotic therapy for Shigella infection, instead reserving antibiotics for patients with a clinical indication or when advised by public health officials in an outbreak setting.
• Tailor antibiotic choice (when antibiotics are indicated) to susceptibility results as soon as possible – with special attention given to the MIC for fluoroquinolone antibiotics.
• Obtain follow-up stool cultures in shigellosis patients who have continued or worsening symptoms despite antibiotic therapy.
• Consult local or state health departments for guidance regarding when patients may return to child care, school, or work.
• Counsel patients with active diarrhea on how they can prevent spreading the infection to others, regardless of whether antibiotic treatment is prescribed.
Additionally, the CDC noted that shigellosis is a nationally notifiable condition; all cases should be reported to the local health department. If a patient with shigellosis and a ciprofloxacin MIC of 0.12-1 mcg/mL is identified, this information should be included in the report to facilitate further testing of the isolate.
The CDC reported that it is working with state and local public health departments and clinical partners to determine if outcomes are indeed worse for patients treated with ciprofloxacin for Shigella strains harboring a quinolone resistance gene, and it will continue to monitor trends in susceptibility of Shigella isolates and to perform genetic testing on select strains to confirm the presence and type of resistance genes.
The Centers for Disease Control and Prevention has identified an increase in Shigella isolates with reduced susceptibility to ciprofloxacin, and has released an official health advisory outlining new recommendations for clinical diagnosis, management, and reporting, as well as for laboratories and public health officials.
The Shigella isolates of concern in the United States have minimum inhibitory concentration (MIC) values of 0.12-1 mcg/mL for ciprofloxacin, which is within the range considered susceptible. These strains, however, “often have a quinolone resistance gene that may lead to clinically significant reduced susceptibility to fluoroquinolone antibiotics,” such as ciprofloxacin, according to the CDC advisory.
It is possible that strains with MIC in the 0.12-1 mcg/mL range may have worse clinical outcome or increased risk of transmission, so the CDC made the following recommendations to clinicians:
• Order a stool culture to obtain isolates for antimicrobial susceptibility testing in suspected cases.
• Order antimicrobial susceptibility testing when ordering a stool culture for Shigella.
• Avoid routine prescribing of antibiotic therapy for Shigella infection, instead reserving antibiotics for patients with a clinical indication or when advised by public health officials in an outbreak setting.
• Tailor antibiotic choice (when antibiotics are indicated) to susceptibility results as soon as possible – with special attention given to the MIC for fluoroquinolone antibiotics.
• Obtain follow-up stool cultures in shigellosis patients who have continued or worsening symptoms despite antibiotic therapy.
• Consult local or state health departments for guidance regarding when patients may return to child care, school, or work.
• Counsel patients with active diarrhea on how they can prevent spreading the infection to others, regardless of whether antibiotic treatment is prescribed.
Additionally, the CDC noted that shigellosis is a nationally notifiable condition; all cases should be reported to the local health department. If a patient with shigellosis and a ciprofloxacin MIC of 0.12-1 mcg/mL is identified, this information should be included in the report to facilitate further testing of the isolate.
The CDC reported that it is working with state and local public health departments and clinical partners to determine if outcomes are indeed worse for patients treated with ciprofloxacin for Shigella strains harboring a quinolone resistance gene, and it will continue to monitor trends in susceptibility of Shigella isolates and to perform genetic testing on select strains to confirm the presence and type of resistance genes.
Pegylated interferon alfa-2a induces durable responses in MPNs
Pegylated interferon alfa-2a can induce durable hematologic and molecular responses in patients with advanced essential thrombocythemia and polycythemia vera, according to a post hoc analysis of data from a prospective, open-label, phase II trial.
Of 83 patients treated with pegylated interferon alfa-2a, 66 (80%) experienced hematological response, and of 55 of the 83 who were positive for the JAK2 Val617 mutation and who were evaluable for a molecular response, 35 (64%) experienced molecular response. The median response durations were 66 months and 53 months, respectively, wrote Lucia Masarova, MD, and her colleagues at MD Anderson Cancer Center, Houston.
Of the 66 hematological responders, 26 (39%) maintained some response during a median follow-up of 83 months. Among the 40 who lost their response, 19 had dose reductions or had the drug withheld because of intolerance or toxicity, 1 developed concurrent diffuse large B-cell lymphoma, and 20 progressed despite treatment with the highest tolerable dose of pegylated interferon alfa-2a. Of note, 7 (28%) of 25 patients who were treated for at least 46 months (median of 77 months) sustained their hematologic response for a median of 6 months after discontinuation of therapy, the investigators said (Lancet Haematol. 2017 Apr;4:e165-75).
Of the 35 molecular responders, 25 (71%) maintained some response during follow-up. Of the nine evaluable patients who did not maintain response (the 10th patient was taken off the study because of concurrent non-Hodgkin lymphoma) four lost response at a median of 2 years after the drug was withheld, and five lost response while on therapy. Three maintained their complete molecular remission – for 18, 55, and 79 months – after discontinuation of therapy.
“Only one patient who achieved a complete molecular remission has relapsed after stopping therapy for 16 months (complete molecular remission duration, 66 months). The other 9 of 10 patients had durable remissions (median duration 69 months),” the investigators wrote, noting that among the 20 patients with a partial molecular remission, 5 (25%) sustained best partial remission, 7 (28%) are in minor molecular remission, 8 (32%) lost their response, and 3 of 5 (60%) with minor molecular remission sustained that remission.
The study comprised adults over age 18 years who were diagnosed with essential thrombocythemia (40 patients) or polycythemia vera (43) and were enrolled during May 2005 to October 2009. Of the 83 patients, 52 (63%) had received some form of therapy prior to enrollment, including 14 who were treated with standard interferon alfa-2a and 1 who was treated with pegylated interferon alfa-2a. The initial starting dose of pegylated interferon alfa-2a used in the study was 450 mcg delivered subcutaneously once each week, but the dose was decreased in a stepwise manner to a final starting dose of 90 mcg per week due to toxicity; starting doses include 450 mcg in 3 patients, 360 mcg in 3 patients, 270 mcg in 19 patients, 180 mcg in 26 patients, and 90 mcg in 32 patients). Treatment continued as long as clinical benefit continued, and hematological responses were assessed every 3-6 months.
Treatment-related toxicities decreased over time, but five patients had treatment-limiting grade 3 or 4 toxicities after 60 months on therapy; overall 18 patients (22%) discontinued treatment due to toxicity.
The therapeutic approach to essential thrombocythemia and polycythemia vera has mainly focused on control of blood counts and reduction of the risk of thrombosis. Those at high risk for thrombosis generally undergo cytoreductive therapy with hydroxyurea. Recombinant interferon alfa is an alternative to hydroxyurea “given its biological, anti-proliferative, immunomodulating, and anticlonal effects,” the investigators explained.
“However, the widespread use of this biological drug has been limited by high rates of discontinuation due to side effects. Pegylated forms of interferon have a better pharmacological profile than short-acting interferons: they require less frequent injection, lower immunogenicity, and possibly fewer toxic effects,” they said.
Although pegylated interferon-alfa 2a has shown promise in several trials, most had short follow-up. The nearly 7 years of follow-up in the current trial is almost twice as long as in those prior studies.
During the current study, including follow-up, eight major vascular thromboembolic events occurred. One was associated with heart catheterization, one with elective chest surgery, and one with an angiogram. The remaining five occurred with no discernible cause after a median of 38 months of therapy for an incidence of 1.22 unprovoked vascular thromboembolic events per 100 person-years, and three of those were in patients with complete hematologic response. Two of the five patients were under age 60 years and had no history of thrombosis. Another patient had a serious unprovoked cerebrovascular hemorrhage after 3 years on therapy and while in complete hematological response.
In addition, 7 of the 83 patients in the study had disease progression on therapy; 6 progressed to myelofibrosis, and 1 developed acute myeloid leukemia. The median time to transformation in these patients was 40 months.
At the time of publication, 32 patient remained in the study and 24 were receiving treatment. Nineteen were in hematologic response at last follow-up, and most (75%) were on a dose of 90 mcg or less per week.
In addition to showing that some patients achieve durable responses on pegylated interferon alfa-2a, this study provided five important observations, the investigators said: 1) Patients might continue to derive clinical benefit from pegylated interferon alfa-2a even after losing response. 2) Only complete molecular remissions are durable, and some cases can be sustained after therapy discontinuation. 3) Clinical activity of pegylated interferon alfa-2a is not correlated with JAK2 mutation status. 4) Toxic effects unrelated to dose may develop and can be treatment-limiting, even after a long exposure to the drug. 5) Disease-related vascular complications or progression to myelofibrosis can still occur in patients on therapy.
“Our findings suggest that pegylated interferon alfa-2a is a viable treatment option, especially for young patients who want to avoid prolonged cytotoxic therapy. Lower doses minimize side effects while retaining efficacy,” they wrote, suggesting – based on these and other results – a starting dose of 45 mcg weekly to limit adverse events and maximize response.
They also noted that treated patients with a history of autoimmune disease and those with mood disorder should be monitored closely for side effects.
Future studies on pegylated interferon afla-2a alone or in combination with novel immunomodulatory drugs are needed to identify patients who would benefit most from treatment, and additional objective response criteria, such as measurement of spleen size, bone marrow histology, and quality of life should be used to better assess clinical benefit, they said.
The National Cancer Institute funded the study. The authors reported having no disclosures.
The finding by Masarova et al. that interferon alfa induces durable responses that persist even after stopping treatment in a relatively large proportion of patients contradicts the main issues raised against use of interferon alfa in this setting, Jean-Jacques Kiladjian, MD, said in an editorial.
For example, despite concerns about side effects and an inability of patients to tolerate interferon alfa, the findings confirm that it can control myeloproliferative neoplasms at reduced doses, with toxicity similar to that reported with hydroxyurea and, importantly, with no new safety issues noted, he said.
Further, the findings underscore the value of achieving a molecular response, which is a subject of debate.
“In particular, patients who achieved complete molecular response derived the longest clinical benefit and none of them had disease progression,” he wrote (Lancet Haematol. 2017 Apr;4:e150-1).
While the investigators did not note a clear decrease in the expected incidence of transformation to myelofibrosis or acute leukemia among study participants, they did “underline the limitations of this study for accurate estimation of these events,” and two ongoing studies comparing interferon alfa and hydroxyurea in much larger cohorts (the Proud-PV and MPD-RC 112 studies) should provide stronger evidence regarding the leukemogenic potential of the therapies, he said.
Dr. Kiladjian is with Assistance Publique–Hopitaux de Paris and Centre d’Investigations Cliniques, Hopital Saint-Louis, Université Paris Diderot, France. He reported receiving institutional research grants from Novartis and AOP Orphan, and serving as an advisory board member for Novartis, AOP Orphan, and Shire.
The finding by Masarova et al. that interferon alfa induces durable responses that persist even after stopping treatment in a relatively large proportion of patients contradicts the main issues raised against use of interferon alfa in this setting, Jean-Jacques Kiladjian, MD, said in an editorial.
For example, despite concerns about side effects and an inability of patients to tolerate interferon alfa, the findings confirm that it can control myeloproliferative neoplasms at reduced doses, with toxicity similar to that reported with hydroxyurea and, importantly, with no new safety issues noted, he said.
Further, the findings underscore the value of achieving a molecular response, which is a subject of debate.
“In particular, patients who achieved complete molecular response derived the longest clinical benefit and none of them had disease progression,” he wrote (Lancet Haematol. 2017 Apr;4:e150-1).
While the investigators did not note a clear decrease in the expected incidence of transformation to myelofibrosis or acute leukemia among study participants, they did “underline the limitations of this study for accurate estimation of these events,” and two ongoing studies comparing interferon alfa and hydroxyurea in much larger cohorts (the Proud-PV and MPD-RC 112 studies) should provide stronger evidence regarding the leukemogenic potential of the therapies, he said.
Dr. Kiladjian is with Assistance Publique–Hopitaux de Paris and Centre d’Investigations Cliniques, Hopital Saint-Louis, Université Paris Diderot, France. He reported receiving institutional research grants from Novartis and AOP Orphan, and serving as an advisory board member for Novartis, AOP Orphan, and Shire.
The finding by Masarova et al. that interferon alfa induces durable responses that persist even after stopping treatment in a relatively large proportion of patients contradicts the main issues raised against use of interferon alfa in this setting, Jean-Jacques Kiladjian, MD, said in an editorial.
For example, despite concerns about side effects and an inability of patients to tolerate interferon alfa, the findings confirm that it can control myeloproliferative neoplasms at reduced doses, with toxicity similar to that reported with hydroxyurea and, importantly, with no new safety issues noted, he said.
Further, the findings underscore the value of achieving a molecular response, which is a subject of debate.
“In particular, patients who achieved complete molecular response derived the longest clinical benefit and none of them had disease progression,” he wrote (Lancet Haematol. 2017 Apr;4:e150-1).
While the investigators did not note a clear decrease in the expected incidence of transformation to myelofibrosis or acute leukemia among study participants, they did “underline the limitations of this study for accurate estimation of these events,” and two ongoing studies comparing interferon alfa and hydroxyurea in much larger cohorts (the Proud-PV and MPD-RC 112 studies) should provide stronger evidence regarding the leukemogenic potential of the therapies, he said.
Dr. Kiladjian is with Assistance Publique–Hopitaux de Paris and Centre d’Investigations Cliniques, Hopital Saint-Louis, Université Paris Diderot, France. He reported receiving institutional research grants from Novartis and AOP Orphan, and serving as an advisory board member for Novartis, AOP Orphan, and Shire.
Pegylated interferon alfa-2a can induce durable hematologic and molecular responses in patients with advanced essential thrombocythemia and polycythemia vera, according to a post hoc analysis of data from a prospective, open-label, phase II trial.
Of 83 patients treated with pegylated interferon alfa-2a, 66 (80%) experienced hematological response, and of 55 of the 83 who were positive for the JAK2 Val617 mutation and who were evaluable for a molecular response, 35 (64%) experienced molecular response. The median response durations were 66 months and 53 months, respectively, wrote Lucia Masarova, MD, and her colleagues at MD Anderson Cancer Center, Houston.
Of the 66 hematological responders, 26 (39%) maintained some response during a median follow-up of 83 months. Among the 40 who lost their response, 19 had dose reductions or had the drug withheld because of intolerance or toxicity, 1 developed concurrent diffuse large B-cell lymphoma, and 20 progressed despite treatment with the highest tolerable dose of pegylated interferon alfa-2a. Of note, 7 (28%) of 25 patients who were treated for at least 46 months (median of 77 months) sustained their hematologic response for a median of 6 months after discontinuation of therapy, the investigators said (Lancet Haematol. 2017 Apr;4:e165-75).
Of the 35 molecular responders, 25 (71%) maintained some response during follow-up. Of the nine evaluable patients who did not maintain response (the 10th patient was taken off the study because of concurrent non-Hodgkin lymphoma) four lost response at a median of 2 years after the drug was withheld, and five lost response while on therapy. Three maintained their complete molecular remission – for 18, 55, and 79 months – after discontinuation of therapy.
“Only one patient who achieved a complete molecular remission has relapsed after stopping therapy for 16 months (complete molecular remission duration, 66 months). The other 9 of 10 patients had durable remissions (median duration 69 months),” the investigators wrote, noting that among the 20 patients with a partial molecular remission, 5 (25%) sustained best partial remission, 7 (28%) are in minor molecular remission, 8 (32%) lost their response, and 3 of 5 (60%) with minor molecular remission sustained that remission.
The study comprised adults over age 18 years who were diagnosed with essential thrombocythemia (40 patients) or polycythemia vera (43) and were enrolled during May 2005 to October 2009. Of the 83 patients, 52 (63%) had received some form of therapy prior to enrollment, including 14 who were treated with standard interferon alfa-2a and 1 who was treated with pegylated interferon alfa-2a. The initial starting dose of pegylated interferon alfa-2a used in the study was 450 mcg delivered subcutaneously once each week, but the dose was decreased in a stepwise manner to a final starting dose of 90 mcg per week due to toxicity; starting doses include 450 mcg in 3 patients, 360 mcg in 3 patients, 270 mcg in 19 patients, 180 mcg in 26 patients, and 90 mcg in 32 patients). Treatment continued as long as clinical benefit continued, and hematological responses were assessed every 3-6 months.
Treatment-related toxicities decreased over time, but five patients had treatment-limiting grade 3 or 4 toxicities after 60 months on therapy; overall 18 patients (22%) discontinued treatment due to toxicity.
The therapeutic approach to essential thrombocythemia and polycythemia vera has mainly focused on control of blood counts and reduction of the risk of thrombosis. Those at high risk for thrombosis generally undergo cytoreductive therapy with hydroxyurea. Recombinant interferon alfa is an alternative to hydroxyurea “given its biological, anti-proliferative, immunomodulating, and anticlonal effects,” the investigators explained.
“However, the widespread use of this biological drug has been limited by high rates of discontinuation due to side effects. Pegylated forms of interferon have a better pharmacological profile than short-acting interferons: they require less frequent injection, lower immunogenicity, and possibly fewer toxic effects,” they said.
Although pegylated interferon-alfa 2a has shown promise in several trials, most had short follow-up. The nearly 7 years of follow-up in the current trial is almost twice as long as in those prior studies.
During the current study, including follow-up, eight major vascular thromboembolic events occurred. One was associated with heart catheterization, one with elective chest surgery, and one with an angiogram. The remaining five occurred with no discernible cause after a median of 38 months of therapy for an incidence of 1.22 unprovoked vascular thromboembolic events per 100 person-years, and three of those were in patients with complete hematologic response. Two of the five patients were under age 60 years and had no history of thrombosis. Another patient had a serious unprovoked cerebrovascular hemorrhage after 3 years on therapy and while in complete hematological response.
In addition, 7 of the 83 patients in the study had disease progression on therapy; 6 progressed to myelofibrosis, and 1 developed acute myeloid leukemia. The median time to transformation in these patients was 40 months.
At the time of publication, 32 patient remained in the study and 24 were receiving treatment. Nineteen were in hematologic response at last follow-up, and most (75%) were on a dose of 90 mcg or less per week.
In addition to showing that some patients achieve durable responses on pegylated interferon alfa-2a, this study provided five important observations, the investigators said: 1) Patients might continue to derive clinical benefit from pegylated interferon alfa-2a even after losing response. 2) Only complete molecular remissions are durable, and some cases can be sustained after therapy discontinuation. 3) Clinical activity of pegylated interferon alfa-2a is not correlated with JAK2 mutation status. 4) Toxic effects unrelated to dose may develop and can be treatment-limiting, even after a long exposure to the drug. 5) Disease-related vascular complications or progression to myelofibrosis can still occur in patients on therapy.
“Our findings suggest that pegylated interferon alfa-2a is a viable treatment option, especially for young patients who want to avoid prolonged cytotoxic therapy. Lower doses minimize side effects while retaining efficacy,” they wrote, suggesting – based on these and other results – a starting dose of 45 mcg weekly to limit adverse events and maximize response.
They also noted that treated patients with a history of autoimmune disease and those with mood disorder should be monitored closely for side effects.
Future studies on pegylated interferon afla-2a alone or in combination with novel immunomodulatory drugs are needed to identify patients who would benefit most from treatment, and additional objective response criteria, such as measurement of spleen size, bone marrow histology, and quality of life should be used to better assess clinical benefit, they said.
The National Cancer Institute funded the study. The authors reported having no disclosures.
Pegylated interferon alfa-2a can induce durable hematologic and molecular responses in patients with advanced essential thrombocythemia and polycythemia vera, according to a post hoc analysis of data from a prospective, open-label, phase II trial.
Of 83 patients treated with pegylated interferon alfa-2a, 66 (80%) experienced hematological response, and of 55 of the 83 who were positive for the JAK2 Val617 mutation and who were evaluable for a molecular response, 35 (64%) experienced molecular response. The median response durations were 66 months and 53 months, respectively, wrote Lucia Masarova, MD, and her colleagues at MD Anderson Cancer Center, Houston.
Of the 66 hematological responders, 26 (39%) maintained some response during a median follow-up of 83 months. Among the 40 who lost their response, 19 had dose reductions or had the drug withheld because of intolerance or toxicity, 1 developed concurrent diffuse large B-cell lymphoma, and 20 progressed despite treatment with the highest tolerable dose of pegylated interferon alfa-2a. Of note, 7 (28%) of 25 patients who were treated for at least 46 months (median of 77 months) sustained their hematologic response for a median of 6 months after discontinuation of therapy, the investigators said (Lancet Haematol. 2017 Apr;4:e165-75).
Of the 35 molecular responders, 25 (71%) maintained some response during follow-up. Of the nine evaluable patients who did not maintain response (the 10th patient was taken off the study because of concurrent non-Hodgkin lymphoma) four lost response at a median of 2 years after the drug was withheld, and five lost response while on therapy. Three maintained their complete molecular remission – for 18, 55, and 79 months – after discontinuation of therapy.
“Only one patient who achieved a complete molecular remission has relapsed after stopping therapy for 16 months (complete molecular remission duration, 66 months). The other 9 of 10 patients had durable remissions (median duration 69 months),” the investigators wrote, noting that among the 20 patients with a partial molecular remission, 5 (25%) sustained best partial remission, 7 (28%) are in minor molecular remission, 8 (32%) lost their response, and 3 of 5 (60%) with minor molecular remission sustained that remission.
The study comprised adults over age 18 years who were diagnosed with essential thrombocythemia (40 patients) or polycythemia vera (43) and were enrolled during May 2005 to October 2009. Of the 83 patients, 52 (63%) had received some form of therapy prior to enrollment, including 14 who were treated with standard interferon alfa-2a and 1 who was treated with pegylated interferon alfa-2a. The initial starting dose of pegylated interferon alfa-2a used in the study was 450 mcg delivered subcutaneously once each week, but the dose was decreased in a stepwise manner to a final starting dose of 90 mcg per week due to toxicity; starting doses include 450 mcg in 3 patients, 360 mcg in 3 patients, 270 mcg in 19 patients, 180 mcg in 26 patients, and 90 mcg in 32 patients). Treatment continued as long as clinical benefit continued, and hematological responses were assessed every 3-6 months.
Treatment-related toxicities decreased over time, but five patients had treatment-limiting grade 3 or 4 toxicities after 60 months on therapy; overall 18 patients (22%) discontinued treatment due to toxicity.
The therapeutic approach to essential thrombocythemia and polycythemia vera has mainly focused on control of blood counts and reduction of the risk of thrombosis. Those at high risk for thrombosis generally undergo cytoreductive therapy with hydroxyurea. Recombinant interferon alfa is an alternative to hydroxyurea “given its biological, anti-proliferative, immunomodulating, and anticlonal effects,” the investigators explained.
“However, the widespread use of this biological drug has been limited by high rates of discontinuation due to side effects. Pegylated forms of interferon have a better pharmacological profile than short-acting interferons: they require less frequent injection, lower immunogenicity, and possibly fewer toxic effects,” they said.
Although pegylated interferon-alfa 2a has shown promise in several trials, most had short follow-up. The nearly 7 years of follow-up in the current trial is almost twice as long as in those prior studies.
During the current study, including follow-up, eight major vascular thromboembolic events occurred. One was associated with heart catheterization, one with elective chest surgery, and one with an angiogram. The remaining five occurred with no discernible cause after a median of 38 months of therapy for an incidence of 1.22 unprovoked vascular thromboembolic events per 100 person-years, and three of those were in patients with complete hematologic response. Two of the five patients were under age 60 years and had no history of thrombosis. Another patient had a serious unprovoked cerebrovascular hemorrhage after 3 years on therapy and while in complete hematological response.
In addition, 7 of the 83 patients in the study had disease progression on therapy; 6 progressed to myelofibrosis, and 1 developed acute myeloid leukemia. The median time to transformation in these patients was 40 months.
At the time of publication, 32 patient remained in the study and 24 were receiving treatment. Nineteen were in hematologic response at last follow-up, and most (75%) were on a dose of 90 mcg or less per week.
In addition to showing that some patients achieve durable responses on pegylated interferon alfa-2a, this study provided five important observations, the investigators said: 1) Patients might continue to derive clinical benefit from pegylated interferon alfa-2a even after losing response. 2) Only complete molecular remissions are durable, and some cases can be sustained after therapy discontinuation. 3) Clinical activity of pegylated interferon alfa-2a is not correlated with JAK2 mutation status. 4) Toxic effects unrelated to dose may develop and can be treatment-limiting, even after a long exposure to the drug. 5) Disease-related vascular complications or progression to myelofibrosis can still occur in patients on therapy.
“Our findings suggest that pegylated interferon alfa-2a is a viable treatment option, especially for young patients who want to avoid prolonged cytotoxic therapy. Lower doses minimize side effects while retaining efficacy,” they wrote, suggesting – based on these and other results – a starting dose of 45 mcg weekly to limit adverse events and maximize response.
They also noted that treated patients with a history of autoimmune disease and those with mood disorder should be monitored closely for side effects.
Future studies on pegylated interferon afla-2a alone or in combination with novel immunomodulatory drugs are needed to identify patients who would benefit most from treatment, and additional objective response criteria, such as measurement of spleen size, bone marrow histology, and quality of life should be used to better assess clinical benefit, they said.
The National Cancer Institute funded the study. The authors reported having no disclosures.
FROM THE LANCET HAEMATOLOGY
Key clinical point:
Major finding: Eighty percent of patients experienced hematological response and 64% experienced molecular response. The median response durations were 66 months and 53 months, respectively.
Data source: A post hoc analysis of data from an open-label, phase II study of 83 patients.
Disclosures: The National Cancer Institute funded the study. The authors reported having no disclosures.
Biomarker algorithm sharpens GVHD treatment outcomes prediction
ORLANDO – A biomarker algorithm was better than was clinical response at predicting outcomes after 1 week of systemic steroid treatment for graft-versus-host disease, according to findings from a multicenter study.
The findings have implications for early decision making regarding treatment course, Hannah Major-Monfried reported at the combined annual meetings of the Center for International Blood & Marrow Transplant Research and the American Society for Blood and Marrow Transplantation.
“The same GVHD algorithm that stratifies patients at day 7 after transplant, at [GVHD] diagnosis, also stratifies them after 1 week of steroid treatment into two groups with distinct risks for treatment failure, 6-month nonrelapse mortality, and overall survival,” she said.
The biomarker algorithm, which includes measures of ST2 and REG3-alpha, was previously shown to predict day-28 treatment response and 6-month non-relapse mortality (NRM) when applied at day 7 post transplant before the onset of GVHD and at the time of diagnosis, said Ms. Major-Monfried, a third-year medical student at Icahn School of Medicine at Mount Sinai, New York.
For the current analysis, levels of the biomarkers were measured after 1 week of treatment in 378 patients with acute GVHD from 11 centers in the Mount Sinai Acute GVHD International Consortium.
In a test cohort that included 236 of the patients, the measurements were used to generate a new predicted probability, or treatment score, for 6-month NRM, which had a value between 0 and 1.
Of the 236 patients, 93 (39%) were considered to have high posttreatment probability of NRM, and the remaining patients (61%) had low posttreatment probability of NRM, based on their treatment scores.
“High-risk patients were significantly less likely to respond to treatment than low-risk patients,” she said, noting that very similar results were found in a validation cohort of the remaining 142 patients, which had a similar proportion of high- and low-risk patients as did the test cohort.
The overall 6-month NRM for patients treated for GVHD was 27% in the test cohort. When the biomarker algorithm was used to separate the cohort into high- and low-risk groups, the NRM rate was found to be approximately 4 times higher among the high-risk patients than among the low-risk patients.
Overall survival was also significantly worse among high- vs. low-risk patients in both the test and validation cohorts.
“We can conclude that the increased NRM seen in the high-risk groups can explain these large differences in overall survival,” Ms. Major-Monfried said.
Because treatment decisions are often made after 1 week based on early clinical response, she and her colleagues also explored whether treatment response after 1 week could similarly predict NRM.
In the test cohort, early response – which includes complete or partial response in GVHD symptoms after 1 week of steroids – was observed in 48% of patients, while 52% were early nonresponders. NRM occurred in 17% of the early responders, compared with 36% of the nonresponders in the test cohort, and similar results were found in the validation cohort.
“These differences are independent of biomarkers,” she noted. “These are solely based on observed clinical response.”
When the biomarker algorithm was applied, prediction of NRM was more precise.
“We started with the early responders,” she explained. “When we used the biomarker algorithm to stratify these patients into high- and low-risk groups, we found that 28% of early responders were actually high risk, and that they experienced 38% NRM – significantly higher than the 8% observed in low-risk patients. Similar results were found again in the validation cohort.”
When the biomarker algorithm was used to stratify patients who were nonresponders at 7 days into high- and low-risk groups, 50% were found to be low risk, and those patients experienced 17% NRM, significantly lower than the 57% seen in the high-risk patients. Similar results were again seen in the validation cohort.
“Early responders with high posttreatment probability have high NRM, and perhaps should not be tapered despite the improvement of their clinical symptoms, while early nonresponders with low posttreatment probability have lower NRM and may not need treatment escalation,” Ms. Major-Monfried said.
“In data not shown, many of these patients are actually what we could call ‘slow responders’ who ultimately fare well,” she noted.
Ms. Major-Monfried reported having no disclosures.
ORLANDO – A biomarker algorithm was better than was clinical response at predicting outcomes after 1 week of systemic steroid treatment for graft-versus-host disease, according to findings from a multicenter study.
The findings have implications for early decision making regarding treatment course, Hannah Major-Monfried reported at the combined annual meetings of the Center for International Blood & Marrow Transplant Research and the American Society for Blood and Marrow Transplantation.
“The same GVHD algorithm that stratifies patients at day 7 after transplant, at [GVHD] diagnosis, also stratifies them after 1 week of steroid treatment into two groups with distinct risks for treatment failure, 6-month nonrelapse mortality, and overall survival,” she said.
The biomarker algorithm, which includes measures of ST2 and REG3-alpha, was previously shown to predict day-28 treatment response and 6-month non-relapse mortality (NRM) when applied at day 7 post transplant before the onset of GVHD and at the time of diagnosis, said Ms. Major-Monfried, a third-year medical student at Icahn School of Medicine at Mount Sinai, New York.
For the current analysis, levels of the biomarkers were measured after 1 week of treatment in 378 patients with acute GVHD from 11 centers in the Mount Sinai Acute GVHD International Consortium.
In a test cohort that included 236 of the patients, the measurements were used to generate a new predicted probability, or treatment score, for 6-month NRM, which had a value between 0 and 1.
Of the 236 patients, 93 (39%) were considered to have high posttreatment probability of NRM, and the remaining patients (61%) had low posttreatment probability of NRM, based on their treatment scores.
“High-risk patients were significantly less likely to respond to treatment than low-risk patients,” she said, noting that very similar results were found in a validation cohort of the remaining 142 patients, which had a similar proportion of high- and low-risk patients as did the test cohort.
The overall 6-month NRM for patients treated for GVHD was 27% in the test cohort. When the biomarker algorithm was used to separate the cohort into high- and low-risk groups, the NRM rate was found to be approximately 4 times higher among the high-risk patients than among the low-risk patients.
Overall survival was also significantly worse among high- vs. low-risk patients in both the test and validation cohorts.
“We can conclude that the increased NRM seen in the high-risk groups can explain these large differences in overall survival,” Ms. Major-Monfried said.
Because treatment decisions are often made after 1 week based on early clinical response, she and her colleagues also explored whether treatment response after 1 week could similarly predict NRM.
In the test cohort, early response – which includes complete or partial response in GVHD symptoms after 1 week of steroids – was observed in 48% of patients, while 52% were early nonresponders. NRM occurred in 17% of the early responders, compared with 36% of the nonresponders in the test cohort, and similar results were found in the validation cohort.
“These differences are independent of biomarkers,” she noted. “These are solely based on observed clinical response.”
When the biomarker algorithm was applied, prediction of NRM was more precise.
“We started with the early responders,” she explained. “When we used the biomarker algorithm to stratify these patients into high- and low-risk groups, we found that 28% of early responders were actually high risk, and that they experienced 38% NRM – significantly higher than the 8% observed in low-risk patients. Similar results were found again in the validation cohort.”
When the biomarker algorithm was used to stratify patients who were nonresponders at 7 days into high- and low-risk groups, 50% were found to be low risk, and those patients experienced 17% NRM, significantly lower than the 57% seen in the high-risk patients. Similar results were again seen in the validation cohort.
“Early responders with high posttreatment probability have high NRM, and perhaps should not be tapered despite the improvement of their clinical symptoms, while early nonresponders with low posttreatment probability have lower NRM and may not need treatment escalation,” Ms. Major-Monfried said.
“In data not shown, many of these patients are actually what we could call ‘slow responders’ who ultimately fare well,” she noted.
Ms. Major-Monfried reported having no disclosures.
ORLANDO – A biomarker algorithm was better than was clinical response at predicting outcomes after 1 week of systemic steroid treatment for graft-versus-host disease, according to findings from a multicenter study.
The findings have implications for early decision making regarding treatment course, Hannah Major-Monfried reported at the combined annual meetings of the Center for International Blood & Marrow Transplant Research and the American Society for Blood and Marrow Transplantation.
“The same GVHD algorithm that stratifies patients at day 7 after transplant, at [GVHD] diagnosis, also stratifies them after 1 week of steroid treatment into two groups with distinct risks for treatment failure, 6-month nonrelapse mortality, and overall survival,” she said.
The biomarker algorithm, which includes measures of ST2 and REG3-alpha, was previously shown to predict day-28 treatment response and 6-month non-relapse mortality (NRM) when applied at day 7 post transplant before the onset of GVHD and at the time of diagnosis, said Ms. Major-Monfried, a third-year medical student at Icahn School of Medicine at Mount Sinai, New York.
For the current analysis, levels of the biomarkers were measured after 1 week of treatment in 378 patients with acute GVHD from 11 centers in the Mount Sinai Acute GVHD International Consortium.
In a test cohort that included 236 of the patients, the measurements were used to generate a new predicted probability, or treatment score, for 6-month NRM, which had a value between 0 and 1.
Of the 236 patients, 93 (39%) were considered to have high posttreatment probability of NRM, and the remaining patients (61%) had low posttreatment probability of NRM, based on their treatment scores.
“High-risk patients were significantly less likely to respond to treatment than low-risk patients,” she said, noting that very similar results were found in a validation cohort of the remaining 142 patients, which had a similar proportion of high- and low-risk patients as did the test cohort.
The overall 6-month NRM for patients treated for GVHD was 27% in the test cohort. When the biomarker algorithm was used to separate the cohort into high- and low-risk groups, the NRM rate was found to be approximately 4 times higher among the high-risk patients than among the low-risk patients.
Overall survival was also significantly worse among high- vs. low-risk patients in both the test and validation cohorts.
“We can conclude that the increased NRM seen in the high-risk groups can explain these large differences in overall survival,” Ms. Major-Monfried said.
Because treatment decisions are often made after 1 week based on early clinical response, she and her colleagues also explored whether treatment response after 1 week could similarly predict NRM.
In the test cohort, early response – which includes complete or partial response in GVHD symptoms after 1 week of steroids – was observed in 48% of patients, while 52% were early nonresponders. NRM occurred in 17% of the early responders, compared with 36% of the nonresponders in the test cohort, and similar results were found in the validation cohort.
“These differences are independent of biomarkers,” she noted. “These are solely based on observed clinical response.”
When the biomarker algorithm was applied, prediction of NRM was more precise.
“We started with the early responders,” she explained. “When we used the biomarker algorithm to stratify these patients into high- and low-risk groups, we found that 28% of early responders were actually high risk, and that they experienced 38% NRM – significantly higher than the 8% observed in low-risk patients. Similar results were found again in the validation cohort.”
When the biomarker algorithm was used to stratify patients who were nonresponders at 7 days into high- and low-risk groups, 50% were found to be low risk, and those patients experienced 17% NRM, significantly lower than the 57% seen in the high-risk patients. Similar results were again seen in the validation cohort.
“Early responders with high posttreatment probability have high NRM, and perhaps should not be tapered despite the improvement of their clinical symptoms, while early nonresponders with low posttreatment probability have lower NRM and may not need treatment escalation,” Ms. Major-Monfried said.
“In data not shown, many of these patients are actually what we could call ‘slow responders’ who ultimately fare well,” she noted.
Ms. Major-Monfried reported having no disclosures.
Key clinical point:
Major finding: High- vs. low-risk patients, based on the biomarker algorithm, had a fourfold higher rate of nonrelapse mortality.
Data source: A multicenter study of 378 patients.
Disclosures: Ms. Major-Monfried reported having no disclosures.
Preparing for pancreatic cancer ‘tsunami’ ahead
ORLANDO – Overall cancer death rates are dropping dramatically, but pancreatic cancer mortality remains high.
“By 2020 we expect [pancreatic cancer] to be the second most common cause of cancer-related death, exceeded only by lung cancer, and if lung cancer deaths continue to fall – which we expect that they will – it will be the most common cause of cancer-related death,” Margaret A. Tempero, MD, said at the annual conference of the National Comprehensive Cancer Network.
Further, as the population ages there will be an increasing number of patients presenting with pancreatic cancer, said Dr. Tempero of the University of California, San Francisco.
Currently, 80% of pancreatic cancer patients are diagnosed with disease that is not amenable to resection, and 80% of those who have resection and adjuvant therapy experience relapse. The overall survival rate is only about 9%.
“This is really, really an aggressive malignancy,” Dr. Tempero said, noting that the survival among those with metastases who do not receive treatment is only about 3 months.
There are no early symptoms that direct attention to the pancreas. Additionally, some patients experience very early invasion and metastases; in fact, up to two-thirds of patients with lesions only 1 cm in size will already have lymph node metastasis, she said.
The disease tends to be chemoresistant, although this may be changing with treatment advances, but it is characterized by “a lot of desmoplastic stroma, so there’s a lot of microenvironment that’s perturbed around the malignancy,” making it difficult for drugs to permeate the stroma.
“That’s something we’re actively working on – ways that we can actually change the stroma so that we can get more drug into the cancer,” she said.
Another challenge is the disease’s “habit of elaborating a lot of cytokines that disable the person with the disease,” which can lead to frailty that makes it difficult to deliver potentially effective therapies that are well tolerated in patients with other types of cancer, she noted.
An important question is whether pancreatic cancer is diagnosed too late or metastasizes too early, and there is evidence to support both possibilities.
“Either way ... we need to work on better treatment,” she said.
Resectable/borderline resectable disease
One noteworthy recent advance for patients with resectable/borderline resectable disease is the addition of capecitabine to gemcitabine for adjuvant therapy. In the ESPAC 4 study published in March in the Lancet, the combination of gemcitabine and capecitabine showed a clear benefit over gemcitabine alone.
“So that has now entered [the NCCN] guidelines as an important option for adjuvant therapy,” said Dr. Tempero, chair of the NCCN pancreatic cancer guidelines panel.
Ongoing trials are also looking at the FOLFIRINOX (irinotecan plus 5-fluorouracil plus leucovorin plus oxaliplatin) and gemcitabine/nanoparticle albumin-bound paclitaxel (nab-P) regimens in the adjuvant setting. These have previously shown some efficacy in the metastatic setting.
“So we really wanted to get these therapies into the adjuvant setting as soon as we could,” she said.
In the ACCORD trial, FOLFIRINOX is being compared with gemcitabine in the postoperative adjuvant setting, and the APACT trial comparing gemcitabine/nab-P to gemcitabine monotherapy completed accrual last year.
“I’m pretty sure that we’ll have enough events on the APACT study by the end of this year, and hopefully we can present that data in the spring. I’m hoping it will be a positive trial for us,” she said.
Neoadjuvant therapy – a successful strategy used in many other malignancies – is also being looked at for pancreatic cancer.
A pilot study (A021101) completed last year suggested that chemotherapy (FOLFIRINOX for 2 months) and chemoradiation (capecitabine and radiation at 50.4 Gy) followed by surgical resection and adjuvant chemotherapy (gemcitabine for 2 months) provided some benefit in patients with borderline resectable pancreatic cancer. This trial led to another ongoing study (S1505) in which patients with borderline resectable disease will be treated with 4 months of FOLFIRINOX prior to resection, followed by chemoradiation and surgery or FOLFIRINOX and surgery, and patients with resectable disease will undergo resection followed by FOLFIRINOX and surgery or gemcitabine/nab-P and surgery.
“The goal is not to compare the two regimens; the goal is to identify the benchmarks that we get with these regimens. In other words, if you’re going to give FOLFIRINOX, what pathologic complete response rate can you expect? What will your R1 resection rate be? With gemcitabine and nab-paclitaxel, the same thing, because if you want to continue to build on these regimens in the neoadjuvant setting you need to know what you’re likely to get so you can make clinical trial assumptions when you add new drugs.
“Once we have these benchmarking data we can really sail in the neoadjuvant setting. It’s a great window-of-opportunity setting for new drugs, because you’re getting serial tissue,” Dr. Tempero said, explaining that in addition to resection of tissue, there is opportunity to get biopsies ahead of time and look at the effects of the drug.
Locally advanced disease
For locally advanced disease, studies have failed to show a benefit of adding radiation after chemotherapy, but radiation oncologists who argue that, “when your therapy gets better, my therapy gets better,” have a valid point, Dr. Tempero said.
For that reason, the Radiation Therapy Oncology group launched a trial to look at gemcitabine/nab-P with and without radiation, but had difficulty with enrollment due to resistance among some physicians who are opposed to radiation in this setting .
“So I don’t know that we will ever answer this question in locally advanced disease. What I can say ... is, in my mind, in locally advanced disease, the most important component is the chemotherapy,” she said.
Metastatic disease
When it comes to trials involving pancreatic cancer patients with metastatic disease, it is important to understand – and to convey to policymakers – that the goal is not only to provide better care in these patients who are at the end of their life, but also to identify strategies that can be used in the adjuvant and neoadjuvant settings, as this is part of the “overall mission of helping patients to feel better and live longer and be cured,” Dr. Tempero said.
One regimen currently used in the metastatic setting is FOLFIRINOX, which was shown in the Prodige 4-ACCORD 11 trial to be superior to gemcitabine monotherapy for survival (hazard ratio, 0.57).
“This is the first time we ever saw a hazard ratio below 0.6 in this disease,” she said, adding that for some patients this means “they can get tremendous benefit, they can come off chemotherapy and have a chemotherapy holiday,” she said.
That said, it’s a tough regimen, she added, explaining that it has dominating toxicities of myelosuppression, diarrhea, and neuropathy that can be irreversible.
Frail patients may not be able to tolerate the regimen, but modifications to the regimen may help. For example, the 5-fluorourasil bolus is often omitted, and doses are sometimes reduced. Chemotherapy holidays can also be of benefit.
Another regimen for the metastatic setting involves the use of nab-P plus gemcitabine, which was shown in a phase III trial to improve survival (HR for death, 0.72).
The results aren’t quite as dramatic as those seen with FOLFIRINOX, but the regimen is slightly easier to manage, Dr. Tempero said, adding: “It’s still not a walk in the park.”
Myelosuppression, arthralgias, and neuropathy still occur, she explained.
Gemcitabine/capecitabine, which has been shown to improve progression-free survival, can also be used, and may be preferable in elderly patients who aren’t fit enough for the other regimens, she said.
“When I select treatment, I really sit down with the patient, and I look at their comorbidities and let them review the toxicities. They decide,” she said, explaining that she provides recommendations based on their concerns and input. “We do have a conversation and we talk about the goals of treatment, we talk about the toxicities.”
Future efforts for metastatic disease should build upon both FOLFIRINOX and gemcitabine/nab-P, she said.
However, because of the difficulty with administering FOLFIRINOX, only two of the 54 open phase I-III trials ongoing in the United States for metastatic disease incorporate the regimen.
Other treatment options include gemcitabine/cisplatin, GTX (gemcitabine/docetaxel/capecitabine), and gemcitabine/erlotinib. The former remains in the NCCN guidelines, primarily for those with hereditary forms of pancreatic cancer, and in particular for those in the DNA repair pathway (BRCA patients, for example).
“We actually have trials now focusing on just BRCA-related pancreatic cancer,” she said, noting that these patients are “exquisitely sensitive to cisplatin and don’t need a harsh regimen like FOLFIRINOX to get the same benefit.
GTX is a very active regimen, although it has never been compared with gemcitabine monotherapy in a randomized trial. However, because of its clear activity it remains in the NCCN guidelines as an option.
Gemcitabine/erlotinib also remains in the guidelines because of a tiny trial that showed a small benefit, but it is not a preferred combination, she said.
Future therapies
Efforts going forward are focusing on finding drugs that inactivate activated RAS, which is “a really big driver” in many cancers, as well as on addressing the microenvironment (such as the desmoplastic stroma that may help encourage invasion of metastases and/or impede drug delivery to the cancer), Dr. Tempero said.
“So there is a lot of interest right now in various forms of immunotherapy or in stromal remodeling so we can see what impact that has on the progression of this disease,” she said, noting that new agents in registration trials include ibrutinib, laparib, PEGPH20, and insulinlike growth factor 1 (IGF-1) inhibitors, and those in planning stages include chemokine (C-C motif) receptor 2 (CCR2) inhibitors and palbociclib.
“And I think we have opportunities in the maintenance setting and in the neoadjuvant setting to do window-of-opportunity trials where we can test new concepts, where we can get pharmacodynamic and biologic data to understand what these new agents are doing,” she said.
Collaborative effort to identify patients at risk
Population-level screening strategies for pancreatic cancer aren’t feasible because of the relatively low incidence of the disease, but efforts are underway to identify and screen high-risk groups.
“We actually have some tests that you could screen with, but they’re not perfect, and with a disease that occurs at the rate that [pancreatic cancer] does, you can’t screen the whole population, because you will find false positives, and you will cause unnecessary procedures more than you’ll find the cancer, Dr. Tempero said during a meeting with press at the NCCN annual conference.
Instead, it is important to identify and screen only those at high risk, she added.
Such a group might include patients with new-onset diabetes who experience weight loss.
New-onset diabetes can be caused by pancreatic cancer, and weight loss with diabetes is unexpected and should raise a red flag, Dr. Temepero explained, adding that an education gap among community physicians means these patients are sometimes cheered for the weight loss instead – and then they end up in the pancreatic disease clinic with metastatic disease 2 years later.
In an effort to better define and characterize this and other high-risk groups, the National Institute for Diabetes and Digestive and Kidney Diseases and the National Cancer Institute are working together to fund a network of institutions that will develop high-risk cohorts and begin deploying screening strategies. The effort is partly in response to the Recalcitrant Cancer Act passed in 2012 to “force more attention on funding pancreatic cancer research through the branches of the NIH,” she explained.
In this particular high-risk group, the institutions will look at the character of diabetes and the clinical correlates with pancreatic cancer.
“Once we hone in on these, we can use those – what we hope are – early-detection biomarkers, and if those are positive, then we would ask the patient to have a CT scan to look for pancreatic cancer,” she said.
Dr. Tempero reported serving as a scientific advisor and/or receiving grant/research support, consulting fees, and/or honoraria from Celgene Corporation, Champion Oncology, Cornerstone Pharmaceuticals, Eli Lilly, EMD Serono, Gilead Sciences, Halozyme Therapeutics, MCS Biotech Resources, NeoHealth, Novocure, Opsona Therapeutics, Pfizer, Portola Pharmaceuticals, and Threshold Pharmaceuticals.
ORLANDO – Overall cancer death rates are dropping dramatically, but pancreatic cancer mortality remains high.
“By 2020 we expect [pancreatic cancer] to be the second most common cause of cancer-related death, exceeded only by lung cancer, and if lung cancer deaths continue to fall – which we expect that they will – it will be the most common cause of cancer-related death,” Margaret A. Tempero, MD, said at the annual conference of the National Comprehensive Cancer Network.
Further, as the population ages there will be an increasing number of patients presenting with pancreatic cancer, said Dr. Tempero of the University of California, San Francisco.
Currently, 80% of pancreatic cancer patients are diagnosed with disease that is not amenable to resection, and 80% of those who have resection and adjuvant therapy experience relapse. The overall survival rate is only about 9%.
“This is really, really an aggressive malignancy,” Dr. Tempero said, noting that the survival among those with metastases who do not receive treatment is only about 3 months.
There are no early symptoms that direct attention to the pancreas. Additionally, some patients experience very early invasion and metastases; in fact, up to two-thirds of patients with lesions only 1 cm in size will already have lymph node metastasis, she said.
The disease tends to be chemoresistant, although this may be changing with treatment advances, but it is characterized by “a lot of desmoplastic stroma, so there’s a lot of microenvironment that’s perturbed around the malignancy,” making it difficult for drugs to permeate the stroma.
“That’s something we’re actively working on – ways that we can actually change the stroma so that we can get more drug into the cancer,” she said.
Another challenge is the disease’s “habit of elaborating a lot of cytokines that disable the person with the disease,” which can lead to frailty that makes it difficult to deliver potentially effective therapies that are well tolerated in patients with other types of cancer, she noted.
An important question is whether pancreatic cancer is diagnosed too late or metastasizes too early, and there is evidence to support both possibilities.
“Either way ... we need to work on better treatment,” she said.
Resectable/borderline resectable disease
One noteworthy recent advance for patients with resectable/borderline resectable disease is the addition of capecitabine to gemcitabine for adjuvant therapy. In the ESPAC 4 study published in March in the Lancet, the combination of gemcitabine and capecitabine showed a clear benefit over gemcitabine alone.
“So that has now entered [the NCCN] guidelines as an important option for adjuvant therapy,” said Dr. Tempero, chair of the NCCN pancreatic cancer guidelines panel.
Ongoing trials are also looking at the FOLFIRINOX (irinotecan plus 5-fluorouracil plus leucovorin plus oxaliplatin) and gemcitabine/nanoparticle albumin-bound paclitaxel (nab-P) regimens in the adjuvant setting. These have previously shown some efficacy in the metastatic setting.
“So we really wanted to get these therapies into the adjuvant setting as soon as we could,” she said.
In the ACCORD trial, FOLFIRINOX is being compared with gemcitabine in the postoperative adjuvant setting, and the APACT trial comparing gemcitabine/nab-P to gemcitabine monotherapy completed accrual last year.
“I’m pretty sure that we’ll have enough events on the APACT study by the end of this year, and hopefully we can present that data in the spring. I’m hoping it will be a positive trial for us,” she said.
Neoadjuvant therapy – a successful strategy used in many other malignancies – is also being looked at for pancreatic cancer.
A pilot study (A021101) completed last year suggested that chemotherapy (FOLFIRINOX for 2 months) and chemoradiation (capecitabine and radiation at 50.4 Gy) followed by surgical resection and adjuvant chemotherapy (gemcitabine for 2 months) provided some benefit in patients with borderline resectable pancreatic cancer. This trial led to another ongoing study (S1505) in which patients with borderline resectable disease will be treated with 4 months of FOLFIRINOX prior to resection, followed by chemoradiation and surgery or FOLFIRINOX and surgery, and patients with resectable disease will undergo resection followed by FOLFIRINOX and surgery or gemcitabine/nab-P and surgery.
“The goal is not to compare the two regimens; the goal is to identify the benchmarks that we get with these regimens. In other words, if you’re going to give FOLFIRINOX, what pathologic complete response rate can you expect? What will your R1 resection rate be? With gemcitabine and nab-paclitaxel, the same thing, because if you want to continue to build on these regimens in the neoadjuvant setting you need to know what you’re likely to get so you can make clinical trial assumptions when you add new drugs.
“Once we have these benchmarking data we can really sail in the neoadjuvant setting. It’s a great window-of-opportunity setting for new drugs, because you’re getting serial tissue,” Dr. Tempero said, explaining that in addition to resection of tissue, there is opportunity to get biopsies ahead of time and look at the effects of the drug.
Locally advanced disease
For locally advanced disease, studies have failed to show a benefit of adding radiation after chemotherapy, but radiation oncologists who argue that, “when your therapy gets better, my therapy gets better,” have a valid point, Dr. Tempero said.
For that reason, the Radiation Therapy Oncology group launched a trial to look at gemcitabine/nab-P with and without radiation, but had difficulty with enrollment due to resistance among some physicians who are opposed to radiation in this setting .
“So I don’t know that we will ever answer this question in locally advanced disease. What I can say ... is, in my mind, in locally advanced disease, the most important component is the chemotherapy,” she said.
Metastatic disease
When it comes to trials involving pancreatic cancer patients with metastatic disease, it is important to understand – and to convey to policymakers – that the goal is not only to provide better care in these patients who are at the end of their life, but also to identify strategies that can be used in the adjuvant and neoadjuvant settings, as this is part of the “overall mission of helping patients to feel better and live longer and be cured,” Dr. Tempero said.
One regimen currently used in the metastatic setting is FOLFIRINOX, which was shown in the Prodige 4-ACCORD 11 trial to be superior to gemcitabine monotherapy for survival (hazard ratio, 0.57).
“This is the first time we ever saw a hazard ratio below 0.6 in this disease,” she said, adding that for some patients this means “they can get tremendous benefit, they can come off chemotherapy and have a chemotherapy holiday,” she said.
That said, it’s a tough regimen, she added, explaining that it has dominating toxicities of myelosuppression, diarrhea, and neuropathy that can be irreversible.
Frail patients may not be able to tolerate the regimen, but modifications to the regimen may help. For example, the 5-fluorourasil bolus is often omitted, and doses are sometimes reduced. Chemotherapy holidays can also be of benefit.
Another regimen for the metastatic setting involves the use of nab-P plus gemcitabine, which was shown in a phase III trial to improve survival (HR for death, 0.72).
The results aren’t quite as dramatic as those seen with FOLFIRINOX, but the regimen is slightly easier to manage, Dr. Tempero said, adding: “It’s still not a walk in the park.”
Myelosuppression, arthralgias, and neuropathy still occur, she explained.
Gemcitabine/capecitabine, which has been shown to improve progression-free survival, can also be used, and may be preferable in elderly patients who aren’t fit enough for the other regimens, she said.
“When I select treatment, I really sit down with the patient, and I look at their comorbidities and let them review the toxicities. They decide,” she said, explaining that she provides recommendations based on their concerns and input. “We do have a conversation and we talk about the goals of treatment, we talk about the toxicities.”
Future efforts for metastatic disease should build upon both FOLFIRINOX and gemcitabine/nab-P, she said.
However, because of the difficulty with administering FOLFIRINOX, only two of the 54 open phase I-III trials ongoing in the United States for metastatic disease incorporate the regimen.
Other treatment options include gemcitabine/cisplatin, GTX (gemcitabine/docetaxel/capecitabine), and gemcitabine/erlotinib. The former remains in the NCCN guidelines, primarily for those with hereditary forms of pancreatic cancer, and in particular for those in the DNA repair pathway (BRCA patients, for example).
“We actually have trials now focusing on just BRCA-related pancreatic cancer,” she said, noting that these patients are “exquisitely sensitive to cisplatin and don’t need a harsh regimen like FOLFIRINOX to get the same benefit.
GTX is a very active regimen, although it has never been compared with gemcitabine monotherapy in a randomized trial. However, because of its clear activity it remains in the NCCN guidelines as an option.
Gemcitabine/erlotinib also remains in the guidelines because of a tiny trial that showed a small benefit, but it is not a preferred combination, she said.
Future therapies
Efforts going forward are focusing on finding drugs that inactivate activated RAS, which is “a really big driver” in many cancers, as well as on addressing the microenvironment (such as the desmoplastic stroma that may help encourage invasion of metastases and/or impede drug delivery to the cancer), Dr. Tempero said.
“So there is a lot of interest right now in various forms of immunotherapy or in stromal remodeling so we can see what impact that has on the progression of this disease,” she said, noting that new agents in registration trials include ibrutinib, laparib, PEGPH20, and insulinlike growth factor 1 (IGF-1) inhibitors, and those in planning stages include chemokine (C-C motif) receptor 2 (CCR2) inhibitors and palbociclib.
“And I think we have opportunities in the maintenance setting and in the neoadjuvant setting to do window-of-opportunity trials where we can test new concepts, where we can get pharmacodynamic and biologic data to understand what these new agents are doing,” she said.
Collaborative effort to identify patients at risk
Population-level screening strategies for pancreatic cancer aren’t feasible because of the relatively low incidence of the disease, but efforts are underway to identify and screen high-risk groups.
“We actually have some tests that you could screen with, but they’re not perfect, and with a disease that occurs at the rate that [pancreatic cancer] does, you can’t screen the whole population, because you will find false positives, and you will cause unnecessary procedures more than you’ll find the cancer, Dr. Tempero said during a meeting with press at the NCCN annual conference.
Instead, it is important to identify and screen only those at high risk, she added.
Such a group might include patients with new-onset diabetes who experience weight loss.
New-onset diabetes can be caused by pancreatic cancer, and weight loss with diabetes is unexpected and should raise a red flag, Dr. Temepero explained, adding that an education gap among community physicians means these patients are sometimes cheered for the weight loss instead – and then they end up in the pancreatic disease clinic with metastatic disease 2 years later.
In an effort to better define and characterize this and other high-risk groups, the National Institute for Diabetes and Digestive and Kidney Diseases and the National Cancer Institute are working together to fund a network of institutions that will develop high-risk cohorts and begin deploying screening strategies. The effort is partly in response to the Recalcitrant Cancer Act passed in 2012 to “force more attention on funding pancreatic cancer research through the branches of the NIH,” she explained.
In this particular high-risk group, the institutions will look at the character of diabetes and the clinical correlates with pancreatic cancer.
“Once we hone in on these, we can use those – what we hope are – early-detection biomarkers, and if those are positive, then we would ask the patient to have a CT scan to look for pancreatic cancer,” she said.
Dr. Tempero reported serving as a scientific advisor and/or receiving grant/research support, consulting fees, and/or honoraria from Celgene Corporation, Champion Oncology, Cornerstone Pharmaceuticals, Eli Lilly, EMD Serono, Gilead Sciences, Halozyme Therapeutics, MCS Biotech Resources, NeoHealth, Novocure, Opsona Therapeutics, Pfizer, Portola Pharmaceuticals, and Threshold Pharmaceuticals.
ORLANDO – Overall cancer death rates are dropping dramatically, but pancreatic cancer mortality remains high.
“By 2020 we expect [pancreatic cancer] to be the second most common cause of cancer-related death, exceeded only by lung cancer, and if lung cancer deaths continue to fall – which we expect that they will – it will be the most common cause of cancer-related death,” Margaret A. Tempero, MD, said at the annual conference of the National Comprehensive Cancer Network.
Further, as the population ages there will be an increasing number of patients presenting with pancreatic cancer, said Dr. Tempero of the University of California, San Francisco.
Currently, 80% of pancreatic cancer patients are diagnosed with disease that is not amenable to resection, and 80% of those who have resection and adjuvant therapy experience relapse. The overall survival rate is only about 9%.
“This is really, really an aggressive malignancy,” Dr. Tempero said, noting that the survival among those with metastases who do not receive treatment is only about 3 months.
There are no early symptoms that direct attention to the pancreas. Additionally, some patients experience very early invasion and metastases; in fact, up to two-thirds of patients with lesions only 1 cm in size will already have lymph node metastasis, she said.
The disease tends to be chemoresistant, although this may be changing with treatment advances, but it is characterized by “a lot of desmoplastic stroma, so there’s a lot of microenvironment that’s perturbed around the malignancy,” making it difficult for drugs to permeate the stroma.
“That’s something we’re actively working on – ways that we can actually change the stroma so that we can get more drug into the cancer,” she said.
Another challenge is the disease’s “habit of elaborating a lot of cytokines that disable the person with the disease,” which can lead to frailty that makes it difficult to deliver potentially effective therapies that are well tolerated in patients with other types of cancer, she noted.
An important question is whether pancreatic cancer is diagnosed too late or metastasizes too early, and there is evidence to support both possibilities.
“Either way ... we need to work on better treatment,” she said.
Resectable/borderline resectable disease
One noteworthy recent advance for patients with resectable/borderline resectable disease is the addition of capecitabine to gemcitabine for adjuvant therapy. In the ESPAC 4 study published in March in the Lancet, the combination of gemcitabine and capecitabine showed a clear benefit over gemcitabine alone.
“So that has now entered [the NCCN] guidelines as an important option for adjuvant therapy,” said Dr. Tempero, chair of the NCCN pancreatic cancer guidelines panel.
Ongoing trials are also looking at the FOLFIRINOX (irinotecan plus 5-fluorouracil plus leucovorin plus oxaliplatin) and gemcitabine/nanoparticle albumin-bound paclitaxel (nab-P) regimens in the adjuvant setting. These have previously shown some efficacy in the metastatic setting.
“So we really wanted to get these therapies into the adjuvant setting as soon as we could,” she said.
In the ACCORD trial, FOLFIRINOX is being compared with gemcitabine in the postoperative adjuvant setting, and the APACT trial comparing gemcitabine/nab-P to gemcitabine monotherapy completed accrual last year.
“I’m pretty sure that we’ll have enough events on the APACT study by the end of this year, and hopefully we can present that data in the spring. I’m hoping it will be a positive trial for us,” she said.
Neoadjuvant therapy – a successful strategy used in many other malignancies – is also being looked at for pancreatic cancer.
A pilot study (A021101) completed last year suggested that chemotherapy (FOLFIRINOX for 2 months) and chemoradiation (capecitabine and radiation at 50.4 Gy) followed by surgical resection and adjuvant chemotherapy (gemcitabine for 2 months) provided some benefit in patients with borderline resectable pancreatic cancer. This trial led to another ongoing study (S1505) in which patients with borderline resectable disease will be treated with 4 months of FOLFIRINOX prior to resection, followed by chemoradiation and surgery or FOLFIRINOX and surgery, and patients with resectable disease will undergo resection followed by FOLFIRINOX and surgery or gemcitabine/nab-P and surgery.
“The goal is not to compare the two regimens; the goal is to identify the benchmarks that we get with these regimens. In other words, if you’re going to give FOLFIRINOX, what pathologic complete response rate can you expect? What will your R1 resection rate be? With gemcitabine and nab-paclitaxel, the same thing, because if you want to continue to build on these regimens in the neoadjuvant setting you need to know what you’re likely to get so you can make clinical trial assumptions when you add new drugs.
“Once we have these benchmarking data we can really sail in the neoadjuvant setting. It’s a great window-of-opportunity setting for new drugs, because you’re getting serial tissue,” Dr. Tempero said, explaining that in addition to resection of tissue, there is opportunity to get biopsies ahead of time and look at the effects of the drug.
Locally advanced disease
For locally advanced disease, studies have failed to show a benefit of adding radiation after chemotherapy, but radiation oncologists who argue that, “when your therapy gets better, my therapy gets better,” have a valid point, Dr. Tempero said.
For that reason, the Radiation Therapy Oncology group launched a trial to look at gemcitabine/nab-P with and without radiation, but had difficulty with enrollment due to resistance among some physicians who are opposed to radiation in this setting .
“So I don’t know that we will ever answer this question in locally advanced disease. What I can say ... is, in my mind, in locally advanced disease, the most important component is the chemotherapy,” she said.
Metastatic disease
When it comes to trials involving pancreatic cancer patients with metastatic disease, it is important to understand – and to convey to policymakers – that the goal is not only to provide better care in these patients who are at the end of their life, but also to identify strategies that can be used in the adjuvant and neoadjuvant settings, as this is part of the “overall mission of helping patients to feel better and live longer and be cured,” Dr. Tempero said.
One regimen currently used in the metastatic setting is FOLFIRINOX, which was shown in the Prodige 4-ACCORD 11 trial to be superior to gemcitabine monotherapy for survival (hazard ratio, 0.57).
“This is the first time we ever saw a hazard ratio below 0.6 in this disease,” she said, adding that for some patients this means “they can get tremendous benefit, they can come off chemotherapy and have a chemotherapy holiday,” she said.
That said, it’s a tough regimen, she added, explaining that it has dominating toxicities of myelosuppression, diarrhea, and neuropathy that can be irreversible.
Frail patients may not be able to tolerate the regimen, but modifications to the regimen may help. For example, the 5-fluorourasil bolus is often omitted, and doses are sometimes reduced. Chemotherapy holidays can also be of benefit.
Another regimen for the metastatic setting involves the use of nab-P plus gemcitabine, which was shown in a phase III trial to improve survival (HR for death, 0.72).
The results aren’t quite as dramatic as those seen with FOLFIRINOX, but the regimen is slightly easier to manage, Dr. Tempero said, adding: “It’s still not a walk in the park.”
Myelosuppression, arthralgias, and neuropathy still occur, she explained.
Gemcitabine/capecitabine, which has been shown to improve progression-free survival, can also be used, and may be preferable in elderly patients who aren’t fit enough for the other regimens, she said.
“When I select treatment, I really sit down with the patient, and I look at their comorbidities and let them review the toxicities. They decide,” she said, explaining that she provides recommendations based on their concerns and input. “We do have a conversation and we talk about the goals of treatment, we talk about the toxicities.”
Future efforts for metastatic disease should build upon both FOLFIRINOX and gemcitabine/nab-P, she said.
However, because of the difficulty with administering FOLFIRINOX, only two of the 54 open phase I-III trials ongoing in the United States for metastatic disease incorporate the regimen.
Other treatment options include gemcitabine/cisplatin, GTX (gemcitabine/docetaxel/capecitabine), and gemcitabine/erlotinib. The former remains in the NCCN guidelines, primarily for those with hereditary forms of pancreatic cancer, and in particular for those in the DNA repair pathway (BRCA patients, for example).
“We actually have trials now focusing on just BRCA-related pancreatic cancer,” she said, noting that these patients are “exquisitely sensitive to cisplatin and don’t need a harsh regimen like FOLFIRINOX to get the same benefit.
GTX is a very active regimen, although it has never been compared with gemcitabine monotherapy in a randomized trial. However, because of its clear activity it remains in the NCCN guidelines as an option.
Gemcitabine/erlotinib also remains in the guidelines because of a tiny trial that showed a small benefit, but it is not a preferred combination, she said.
Future therapies
Efforts going forward are focusing on finding drugs that inactivate activated RAS, which is “a really big driver” in many cancers, as well as on addressing the microenvironment (such as the desmoplastic stroma that may help encourage invasion of metastases and/or impede drug delivery to the cancer), Dr. Tempero said.
“So there is a lot of interest right now in various forms of immunotherapy or in stromal remodeling so we can see what impact that has on the progression of this disease,” she said, noting that new agents in registration trials include ibrutinib, laparib, PEGPH20, and insulinlike growth factor 1 (IGF-1) inhibitors, and those in planning stages include chemokine (C-C motif) receptor 2 (CCR2) inhibitors and palbociclib.
“And I think we have opportunities in the maintenance setting and in the neoadjuvant setting to do window-of-opportunity trials where we can test new concepts, where we can get pharmacodynamic and biologic data to understand what these new agents are doing,” she said.
Collaborative effort to identify patients at risk
Population-level screening strategies for pancreatic cancer aren’t feasible because of the relatively low incidence of the disease, but efforts are underway to identify and screen high-risk groups.
“We actually have some tests that you could screen with, but they’re not perfect, and with a disease that occurs at the rate that [pancreatic cancer] does, you can’t screen the whole population, because you will find false positives, and you will cause unnecessary procedures more than you’ll find the cancer, Dr. Tempero said during a meeting with press at the NCCN annual conference.
Instead, it is important to identify and screen only those at high risk, she added.
Such a group might include patients with new-onset diabetes who experience weight loss.
New-onset diabetes can be caused by pancreatic cancer, and weight loss with diabetes is unexpected and should raise a red flag, Dr. Temepero explained, adding that an education gap among community physicians means these patients are sometimes cheered for the weight loss instead – and then they end up in the pancreatic disease clinic with metastatic disease 2 years later.
In an effort to better define and characterize this and other high-risk groups, the National Institute for Diabetes and Digestive and Kidney Diseases and the National Cancer Institute are working together to fund a network of institutions that will develop high-risk cohorts and begin deploying screening strategies. The effort is partly in response to the Recalcitrant Cancer Act passed in 2012 to “force more attention on funding pancreatic cancer research through the branches of the NIH,” she explained.
In this particular high-risk group, the institutions will look at the character of diabetes and the clinical correlates with pancreatic cancer.
“Once we hone in on these, we can use those – what we hope are – early-detection biomarkers, and if those are positive, then we would ask the patient to have a CT scan to look for pancreatic cancer,” she said.
Dr. Tempero reported serving as a scientific advisor and/or receiving grant/research support, consulting fees, and/or honoraria from Celgene Corporation, Champion Oncology, Cornerstone Pharmaceuticals, Eli Lilly, EMD Serono, Gilead Sciences, Halozyme Therapeutics, MCS Biotech Resources, NeoHealth, Novocure, Opsona Therapeutics, Pfizer, Portola Pharmaceuticals, and Threshold Pharmaceuticals.
AT THE NCCN ANNUAL CONFERENCE
Key clinical point:
Disclosures: Dr. Tempero reported serving as a scientific advisor and/or receiving grant/research support, consulting fees, and/or honoraria from Celgene Corporation, Champion Oncology, Cornerstone Pharmaceuticals, Eli Lilly, EMD Serono, Gilead Sciences, Halozyme Therapeutics, MCS Biotech Resources, NeoHealth, Novocure, Opsona Therapeutics, Pfizer, Portola Pharmaceuticals, and Threshold Pharmaceuticals.