User login
Sharon Worcester is an award-winning medical journalist for MDedge News. She has been with the company since 1996, first as the Southeast Bureau Chief (1996-2009) when the company was known as International Medical News Group, then as a freelance writer (2010-2015) before returning as a reporter in 2015. She previously worked as a daily newspaper reporter covering health and local government. Sharon currently reports primarily on oncology and hematology. She has a BA from Eckerd College and an MA in Mass Communication/Print Journalism from the University of Florida. Connect with her via LinkedIn and follow her on twitter @SW_MedReporter.
WPR may benefit patients with stage IVB cervical cancer
NATIONAL HARBOR, MD. – Whole pelvic radiation delivered in addition to chemotherapy in women who present with stage IVB cervical cancer confers significant 12-month overall and progression-free survival benefits, according to findings from a multi-institutional retrospective review.
Of 127 patients diagnosed during 2005-2015 at four academic high-volume centers, 31 received no treatment or elected hospice care and, of the remaining patients, 34 received whole pelvic radiation (WPR) in addition to chemotherapy, and 62 received chemotherapy alone, which is the standard of care. The median overall survival was 14.1 vs. 6.9 months with vs. without WPR, and the median progression-free survival was 10 vs. 5 months, respectively, Victoria B. Perkins, MD, said at the annual meeting of the Society of Gynecologic Oncology.
Of note, the rates of pelvic-related morbidity, including ureteral obstruction, vaginal or rectal bleeding, pelvic infection, pelvic pain, and fistula, did not differ significantly between the groups, said Dr. Perkins of the University of Oklahoma Health Sciences Center, Oklahoma City.
Study subjects were women with a median age of 54 years. A little more than a third (36%) were white, 35% were Hispanic, and 16% were black. Most (75%) had squamous cell carcinoma, and 95% were grade 2 or 3.
There were no significant differences in the demographics, location of [metastatic] disease, or distribution of chemotherapy type between the two groups, she said, noting that “interestingly, 26% of patients in the chemotherapy alone group received additional bevacizumab, compared to only 12% in the whole pelvic radiation with chemotherapy group.
“This could reflect the change in treatment strategy with the approval of bevacizumab during the treatment period,” Dr. Perkins noted.
About 5% of women have stage IVB cervical cancer at the time of diagnosis, but 5-year survival in these women is only about 15%.
“The mainstay of treatment is platinum and taxane with or without bevacizumab. In clinical practice, treatment of stage IVB disease varies,” Dr. Perkins said.
One strategy follows the guidance of phase III trials looking at chemotherapy with palliative radiation as needed.
“However, a significant number of patients experience morbidity and mortality directly related to their pelvic disease, such as vaginal bleeding, ureteral obstruction, fistulization, infections, and pain. Thus, an alternative approach is aimed at treating bulky pelvic disease with whole pelvic radiation followed by systemic chemotherapy with the goal to control symptoms and theoretically reduce recurrences in the pelvic field, which can be highly problematic in terms of symptomatic control,” she said, noting that this novel approach has not been formally studied.
The aim of the current review was to determine if WPR with chemotherapy would reduce pelvic morbidity and improve overall and progression-free survival.
“Survival in stage IVB disease remains extremely poor. Perhaps adding whole pelvic radiation to systemic chemotherapy has utility without increasing morbidity. However, the addition of whole pelvic radiation did not improve pelvic-related morbidity as previously hypothesized,” she said.
The study was limited by varied chemotherapy regimens in both groups and by changes in standard treatment practice during the span of study. It also was limited by the retrospective design, small sample size, and lack of quality of life data, but the findings support further study regarding subgroups of patients who could benefit the most from this treatment strategy, she concluded, noting, however, that such study is challenging because of the rarity of stage IVB disease.
Dr. Perkins reported having no disclosures.
NATIONAL HARBOR, MD. – Whole pelvic radiation delivered in addition to chemotherapy in women who present with stage IVB cervical cancer confers significant 12-month overall and progression-free survival benefits, according to findings from a multi-institutional retrospective review.
Of 127 patients diagnosed during 2005-2015 at four academic high-volume centers, 31 received no treatment or elected hospice care and, of the remaining patients, 34 received whole pelvic radiation (WPR) in addition to chemotherapy, and 62 received chemotherapy alone, which is the standard of care. The median overall survival was 14.1 vs. 6.9 months with vs. without WPR, and the median progression-free survival was 10 vs. 5 months, respectively, Victoria B. Perkins, MD, said at the annual meeting of the Society of Gynecologic Oncology.
Of note, the rates of pelvic-related morbidity, including ureteral obstruction, vaginal or rectal bleeding, pelvic infection, pelvic pain, and fistula, did not differ significantly between the groups, said Dr. Perkins of the University of Oklahoma Health Sciences Center, Oklahoma City.
Study subjects were women with a median age of 54 years. A little more than a third (36%) were white, 35% were Hispanic, and 16% were black. Most (75%) had squamous cell carcinoma, and 95% were grade 2 or 3.
There were no significant differences in the demographics, location of [metastatic] disease, or distribution of chemotherapy type between the two groups, she said, noting that “interestingly, 26% of patients in the chemotherapy alone group received additional bevacizumab, compared to only 12% in the whole pelvic radiation with chemotherapy group.
“This could reflect the change in treatment strategy with the approval of bevacizumab during the treatment period,” Dr. Perkins noted.
About 5% of women have stage IVB cervical cancer at the time of diagnosis, but 5-year survival in these women is only about 15%.
“The mainstay of treatment is platinum and taxane with or without bevacizumab. In clinical practice, treatment of stage IVB disease varies,” Dr. Perkins said.
One strategy follows the guidance of phase III trials looking at chemotherapy with palliative radiation as needed.
“However, a significant number of patients experience morbidity and mortality directly related to their pelvic disease, such as vaginal bleeding, ureteral obstruction, fistulization, infections, and pain. Thus, an alternative approach is aimed at treating bulky pelvic disease with whole pelvic radiation followed by systemic chemotherapy with the goal to control symptoms and theoretically reduce recurrences in the pelvic field, which can be highly problematic in terms of symptomatic control,” she said, noting that this novel approach has not been formally studied.
The aim of the current review was to determine if WPR with chemotherapy would reduce pelvic morbidity and improve overall and progression-free survival.
“Survival in stage IVB disease remains extremely poor. Perhaps adding whole pelvic radiation to systemic chemotherapy has utility without increasing morbidity. However, the addition of whole pelvic radiation did not improve pelvic-related morbidity as previously hypothesized,” she said.
The study was limited by varied chemotherapy regimens in both groups and by changes in standard treatment practice during the span of study. It also was limited by the retrospective design, small sample size, and lack of quality of life data, but the findings support further study regarding subgroups of patients who could benefit the most from this treatment strategy, she concluded, noting, however, that such study is challenging because of the rarity of stage IVB disease.
Dr. Perkins reported having no disclosures.
NATIONAL HARBOR, MD. – Whole pelvic radiation delivered in addition to chemotherapy in women who present with stage IVB cervical cancer confers significant 12-month overall and progression-free survival benefits, according to findings from a multi-institutional retrospective review.
Of 127 patients diagnosed during 2005-2015 at four academic high-volume centers, 31 received no treatment or elected hospice care and, of the remaining patients, 34 received whole pelvic radiation (WPR) in addition to chemotherapy, and 62 received chemotherapy alone, which is the standard of care. The median overall survival was 14.1 vs. 6.9 months with vs. without WPR, and the median progression-free survival was 10 vs. 5 months, respectively, Victoria B. Perkins, MD, said at the annual meeting of the Society of Gynecologic Oncology.
Of note, the rates of pelvic-related morbidity, including ureteral obstruction, vaginal or rectal bleeding, pelvic infection, pelvic pain, and fistula, did not differ significantly between the groups, said Dr. Perkins of the University of Oklahoma Health Sciences Center, Oklahoma City.
Study subjects were women with a median age of 54 years. A little more than a third (36%) were white, 35% were Hispanic, and 16% were black. Most (75%) had squamous cell carcinoma, and 95% were grade 2 or 3.
There were no significant differences in the demographics, location of [metastatic] disease, or distribution of chemotherapy type between the two groups, she said, noting that “interestingly, 26% of patients in the chemotherapy alone group received additional bevacizumab, compared to only 12% in the whole pelvic radiation with chemotherapy group.
“This could reflect the change in treatment strategy with the approval of bevacizumab during the treatment period,” Dr. Perkins noted.
About 5% of women have stage IVB cervical cancer at the time of diagnosis, but 5-year survival in these women is only about 15%.
“The mainstay of treatment is platinum and taxane with or without bevacizumab. In clinical practice, treatment of stage IVB disease varies,” Dr. Perkins said.
One strategy follows the guidance of phase III trials looking at chemotherapy with palliative radiation as needed.
“However, a significant number of patients experience morbidity and mortality directly related to their pelvic disease, such as vaginal bleeding, ureteral obstruction, fistulization, infections, and pain. Thus, an alternative approach is aimed at treating bulky pelvic disease with whole pelvic radiation followed by systemic chemotherapy with the goal to control symptoms and theoretically reduce recurrences in the pelvic field, which can be highly problematic in terms of symptomatic control,” she said, noting that this novel approach has not been formally studied.
The aim of the current review was to determine if WPR with chemotherapy would reduce pelvic morbidity and improve overall and progression-free survival.
“Survival in stage IVB disease remains extremely poor. Perhaps adding whole pelvic radiation to systemic chemotherapy has utility without increasing morbidity. However, the addition of whole pelvic radiation did not improve pelvic-related morbidity as previously hypothesized,” she said.
The study was limited by varied chemotherapy regimens in both groups and by changes in standard treatment practice during the span of study. It also was limited by the retrospective design, small sample size, and lack of quality of life data, but the findings support further study regarding subgroups of patients who could benefit the most from this treatment strategy, she concluded, noting, however, that such study is challenging because of the rarity of stage IVB disease.
Dr. Perkins reported having no disclosures.
AT THE ANNUAL MEETING ON WOMEN’S CANCERS
Key clinical point:
Major finding: Median overall and progression-free survival with vs. without WPR: 14.1 vs. 6.9 months and 10 vs. 5 months, respectively, but no difference in pelvic-related morbidity.
Data source: A retrospective review of 127 cases.
Disclosures: Dr. Perkins reported having no disclosures.
Replacement factors, bypassing agents safely manage fitusiran bleed events
Fitusiran appears to promote hemostasis and reduce the frequency of bleeding in patients with hemophilia. In a phase I trial of the investigational agent, breakthrough bleeds were treated effectively and safely with replacement factor or bypassing agent.
Bleed events were rare among patients achieving target antithrombin lowering of greater than 75% on fitusiran. Those that did occur were treated with factor concentrates, including recombinant Factor VIII or recombinant Factor IX, or with bypassing agents, including recombinant Factor VIIa or activated prothrombin complex–concentrates, Savita Rangarajan, MD, reported at the annual meeting of the European Association for Haemophilia and Allied Disorders.
The study included 41 patients with hemophilia A or B – 25 patients with inhibitors and 16 without inhibitors – who received either 50 mg or 80 mg of fitusiran. Early multiple ascending dose–cohorts received weekly subcutaneous dosing, and later cohorts received monthly dosing. All patients tolerated treatment well, with no serious adverse events related to the study drug. No thromboembolic events occurred, and the majority of adverse events were mild or moderate in severity, she noted.
Among patients with inhibitors, eight bleeds occurred in five patients with hemophilia A who were treated with Factor VIII, and three bleeds occurred in two patients with hemophilia B who were treated with Factor IX. Among those without inhibitors, six bleeds occurred in three patients treated with activated prothrombin complex–concentrates, and four occurred in three patients treated with recombinant Factor VIIa, said Dr. Rangarajan of Hampshire Hospitals NHS Foundation Trust, Basingstoke, England.
The ranges of factor replacement used per injection were 7-32 IU/kg of Factor VIII and 7-43 IU/kg of Factor IX.
The ranges of bypassing agents used per injection were 14-75 U/kg of activated prothrombin complex–concentrates (mean, 2.2 administrations per bleed) and 93-133 μg/kg of recombinant Factor VIIa (mean, 1.5 administrations per bleed), she said.
Doses of the factor concentrates and bypassing agents used were at or below those recommended by the World Federation of Hemophilia.
This phase I study of fitusiran, which targets and lowers antithrombin to improve thrombin generation and promote hemostasis in patients with hemophilia, is being conducted in four parts: Part A with healthy volunteers, parts B and C with patients with moderate to severe hemophilia A or B, and part D with patients with hemophilia A or B with inhibitors.
Findings from the current exploratory analysis of the data are encouraging as they demonstrate good treatment effect in the absence of identified safety concerns, Dr. Rangarajan said, noting that fitusiran should advance to pivotal studies in 2017 and that data on bleed management from a phase I and phase II open label extension will guide protocol on bleed management in phase III.
Dr. Rangarajan has received grant or research support from Alnylam Pharmaceuticals, BioMarin Pharmaceutical, Novo Nordisk, Pfizer, and Shire.
Fitusiran appears to promote hemostasis and reduce the frequency of bleeding in patients with hemophilia. In a phase I trial of the investigational agent, breakthrough bleeds were treated effectively and safely with replacement factor or bypassing agent.
Bleed events were rare among patients achieving target antithrombin lowering of greater than 75% on fitusiran. Those that did occur were treated with factor concentrates, including recombinant Factor VIII or recombinant Factor IX, or with bypassing agents, including recombinant Factor VIIa or activated prothrombin complex–concentrates, Savita Rangarajan, MD, reported at the annual meeting of the European Association for Haemophilia and Allied Disorders.
The study included 41 patients with hemophilia A or B – 25 patients with inhibitors and 16 without inhibitors – who received either 50 mg or 80 mg of fitusiran. Early multiple ascending dose–cohorts received weekly subcutaneous dosing, and later cohorts received monthly dosing. All patients tolerated treatment well, with no serious adverse events related to the study drug. No thromboembolic events occurred, and the majority of adverse events were mild or moderate in severity, she noted.
Among patients with inhibitors, eight bleeds occurred in five patients with hemophilia A who were treated with Factor VIII, and three bleeds occurred in two patients with hemophilia B who were treated with Factor IX. Among those without inhibitors, six bleeds occurred in three patients treated with activated prothrombin complex–concentrates, and four occurred in three patients treated with recombinant Factor VIIa, said Dr. Rangarajan of Hampshire Hospitals NHS Foundation Trust, Basingstoke, England.
The ranges of factor replacement used per injection were 7-32 IU/kg of Factor VIII and 7-43 IU/kg of Factor IX.
The ranges of bypassing agents used per injection were 14-75 U/kg of activated prothrombin complex–concentrates (mean, 2.2 administrations per bleed) and 93-133 μg/kg of recombinant Factor VIIa (mean, 1.5 administrations per bleed), she said.
Doses of the factor concentrates and bypassing agents used were at or below those recommended by the World Federation of Hemophilia.
This phase I study of fitusiran, which targets and lowers antithrombin to improve thrombin generation and promote hemostasis in patients with hemophilia, is being conducted in four parts: Part A with healthy volunteers, parts B and C with patients with moderate to severe hemophilia A or B, and part D with patients with hemophilia A or B with inhibitors.
Findings from the current exploratory analysis of the data are encouraging as they demonstrate good treatment effect in the absence of identified safety concerns, Dr. Rangarajan said, noting that fitusiran should advance to pivotal studies in 2017 and that data on bleed management from a phase I and phase II open label extension will guide protocol on bleed management in phase III.
Dr. Rangarajan has received grant or research support from Alnylam Pharmaceuticals, BioMarin Pharmaceutical, Novo Nordisk, Pfizer, and Shire.
Fitusiran appears to promote hemostasis and reduce the frequency of bleeding in patients with hemophilia. In a phase I trial of the investigational agent, breakthrough bleeds were treated effectively and safely with replacement factor or bypassing agent.
Bleed events were rare among patients achieving target antithrombin lowering of greater than 75% on fitusiran. Those that did occur were treated with factor concentrates, including recombinant Factor VIII or recombinant Factor IX, or with bypassing agents, including recombinant Factor VIIa or activated prothrombin complex–concentrates, Savita Rangarajan, MD, reported at the annual meeting of the European Association for Haemophilia and Allied Disorders.
The study included 41 patients with hemophilia A or B – 25 patients with inhibitors and 16 without inhibitors – who received either 50 mg or 80 mg of fitusiran. Early multiple ascending dose–cohorts received weekly subcutaneous dosing, and later cohorts received monthly dosing. All patients tolerated treatment well, with no serious adverse events related to the study drug. No thromboembolic events occurred, and the majority of adverse events were mild or moderate in severity, she noted.
Among patients with inhibitors, eight bleeds occurred in five patients with hemophilia A who were treated with Factor VIII, and three bleeds occurred in two patients with hemophilia B who were treated with Factor IX. Among those without inhibitors, six bleeds occurred in three patients treated with activated prothrombin complex–concentrates, and four occurred in three patients treated with recombinant Factor VIIa, said Dr. Rangarajan of Hampshire Hospitals NHS Foundation Trust, Basingstoke, England.
The ranges of factor replacement used per injection were 7-32 IU/kg of Factor VIII and 7-43 IU/kg of Factor IX.
The ranges of bypassing agents used per injection were 14-75 U/kg of activated prothrombin complex–concentrates (mean, 2.2 administrations per bleed) and 93-133 μg/kg of recombinant Factor VIIa (mean, 1.5 administrations per bleed), she said.
Doses of the factor concentrates and bypassing agents used were at or below those recommended by the World Federation of Hemophilia.
This phase I study of fitusiran, which targets and lowers antithrombin to improve thrombin generation and promote hemostasis in patients with hemophilia, is being conducted in four parts: Part A with healthy volunteers, parts B and C with patients with moderate to severe hemophilia A or B, and part D with patients with hemophilia A or B with inhibitors.
Findings from the current exploratory analysis of the data are encouraging as they demonstrate good treatment effect in the absence of identified safety concerns, Dr. Rangarajan said, noting that fitusiran should advance to pivotal studies in 2017 and that data on bleed management from a phase I and phase II open label extension will guide protocol on bleed management in phase III.
Dr. Rangarajan has received grant or research support from Alnylam Pharmaceuticals, BioMarin Pharmaceutical, Novo Nordisk, Pfizer, and Shire.
Key clinical point:
Major finding: 21 bleeds occurred in 13 patients, and all were treated effectively and safely.
Data source: An exploratory analysis of data from a four-part phase I trial.
Disclosures: Dr. Rangarajan has received grant or research support from Alnylam Pharmaceuticals, BioMarin Pharmaceutical, Novo Nordisk, Pfizer, and Shire.
VIDEO: Rucaparib benefits HGOC with BRCA mutations
National Harbor, MD. – The PARP inhibitor rucaparib is safe and effective in patients with primary platinum-sensitive high-grade ovarian carcinoma who have germline or somatic BRCA mutations, according to integrated summary data from parts 1 and 2 of the phase II ARIEL2 study.
Prior analyses of ARIEL2 data included 493 patients with germline/somatic BRCA mutations and BRCA wild-type. The current analysis included the 41 patients from ARIEL2 part 1 and the 93 patients from ARIEL2 part 2 who had germline or somatic BRCA mutations, and overall response rates in these patients ranged from 52% to 86% depending on the number of prior therapies, Gottfried E. Konecny, MD, reported at the annual meeting of the Society of Gynecologic Oncology.
The highest overall response rates were seen in platinum-sensitive vs. platinum-resistant and platinum-refractory patients, said Dr. Konecny of the University of California, Los Angeles.
Median progression-free survival was 12.7 months in the platinum-sensitive patients vs. 7.3 and 5.0 months in platinum-resistant and platinum-refractory patients, respectively, he said.
Treatment was generally safe and well tolerated. The most common treatment-emergent adverse events were nausea, fatigue, vomiting, and anemia; the most common grade 3/4 events included anemia, increased ALT/AST, and fatigue.
Previous findings from ARIEL2 and other studies of rucaparib led to conditional approval of the drug (pending further confirmation of the data), first for patients with germline or somatic BRCA mutations who fail at least three prior lines of chemotherapy, then for those who fail two or more prior therapies.
In this video, Dr. Konecny discusses his findings and the next steps with respect to the study of rucaparib for high-grade ovarian carcinoma.
ARIEL2 was supported by Clovis Oncology. Dr. Konecny is on the speakers’ bureau for AstraZeneca and Clovis Oncology and has received research funding or honorarium from Amgen, Merck, and Novartis.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
National Harbor, MD. – The PARP inhibitor rucaparib is safe and effective in patients with primary platinum-sensitive high-grade ovarian carcinoma who have germline or somatic BRCA mutations, according to integrated summary data from parts 1 and 2 of the phase II ARIEL2 study.
Prior analyses of ARIEL2 data included 493 patients with germline/somatic BRCA mutations and BRCA wild-type. The current analysis included the 41 patients from ARIEL2 part 1 and the 93 patients from ARIEL2 part 2 who had germline or somatic BRCA mutations, and overall response rates in these patients ranged from 52% to 86% depending on the number of prior therapies, Gottfried E. Konecny, MD, reported at the annual meeting of the Society of Gynecologic Oncology.
The highest overall response rates were seen in platinum-sensitive vs. platinum-resistant and platinum-refractory patients, said Dr. Konecny of the University of California, Los Angeles.
Median progression-free survival was 12.7 months in the platinum-sensitive patients vs. 7.3 and 5.0 months in platinum-resistant and platinum-refractory patients, respectively, he said.
Treatment was generally safe and well tolerated. The most common treatment-emergent adverse events were nausea, fatigue, vomiting, and anemia; the most common grade 3/4 events included anemia, increased ALT/AST, and fatigue.
Previous findings from ARIEL2 and other studies of rucaparib led to conditional approval of the drug (pending further confirmation of the data), first for patients with germline or somatic BRCA mutations who fail at least three prior lines of chemotherapy, then for those who fail two or more prior therapies.
In this video, Dr. Konecny discusses his findings and the next steps with respect to the study of rucaparib for high-grade ovarian carcinoma.
ARIEL2 was supported by Clovis Oncology. Dr. Konecny is on the speakers’ bureau for AstraZeneca and Clovis Oncology and has received research funding or honorarium from Amgen, Merck, and Novartis.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
National Harbor, MD. – The PARP inhibitor rucaparib is safe and effective in patients with primary platinum-sensitive high-grade ovarian carcinoma who have germline or somatic BRCA mutations, according to integrated summary data from parts 1 and 2 of the phase II ARIEL2 study.
Prior analyses of ARIEL2 data included 493 patients with germline/somatic BRCA mutations and BRCA wild-type. The current analysis included the 41 patients from ARIEL2 part 1 and the 93 patients from ARIEL2 part 2 who had germline or somatic BRCA mutations, and overall response rates in these patients ranged from 52% to 86% depending on the number of prior therapies, Gottfried E. Konecny, MD, reported at the annual meeting of the Society of Gynecologic Oncology.
The highest overall response rates were seen in platinum-sensitive vs. platinum-resistant and platinum-refractory patients, said Dr. Konecny of the University of California, Los Angeles.
Median progression-free survival was 12.7 months in the platinum-sensitive patients vs. 7.3 and 5.0 months in platinum-resistant and platinum-refractory patients, respectively, he said.
Treatment was generally safe and well tolerated. The most common treatment-emergent adverse events were nausea, fatigue, vomiting, and anemia; the most common grade 3/4 events included anemia, increased ALT/AST, and fatigue.
Previous findings from ARIEL2 and other studies of rucaparib led to conditional approval of the drug (pending further confirmation of the data), first for patients with germline or somatic BRCA mutations who fail at least three prior lines of chemotherapy, then for those who fail two or more prior therapies.
In this video, Dr. Konecny discusses his findings and the next steps with respect to the study of rucaparib for high-grade ovarian carcinoma.
ARIEL2 was supported by Clovis Oncology. Dr. Konecny is on the speakers’ bureau for AstraZeneca and Clovis Oncology and has received research funding or honorarium from Amgen, Merck, and Novartis.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
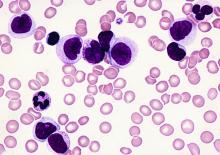
Post-transplant drug combo eyed for high-risk AML
ORLANDO – Azacitadine and valproic acid can be safely coadministered as maintenance therapy after allogeneic stem cell transplantation in patients with high-risk acute myelogenous leukemia (AML), according to interim findings from an investigator-initiated phase II study.
One-year relapse-free and overall survival rates were about 80%, and no significant toxicities were reported in 28 such patients who began the treatment at least 40 days after transplant and were treated for 4 months, Patrick A. Hagen, MD, reported at the combined annual meetings of the Center for International Blood & Marrow Transplant Research and the American Society for Blood and Marrow Transplantation.
“As we all know, relapse after allogeneic transplant for AML is a huge and ongoing problem. It’s the primary cause of death following transplant, and unfortunately it really hasn’t decreased over the past couple decades,” Dr. Hagen said, adding that relapse is of increasing concern for biologically high-risk patients and is a “pretty ripe area for improved methodologies or approaches.” The decision to pursue maintenance therapy after transplant is complicated: It’s a good opportunity to improve outcomes, but there are many challenges, including problems with drug interactions and myelosuppression, he said.
The study was undertaken based in part on previously reported findings of synergism of a demethylating agent and a histone dacetylase inhibitor in patients with AML, he said.
The maintenance therapy included up to four 28-day cycles of azacitadine at 40 mg/m2 daily on days 1-5, along with oral valproic acid daily throughout the cycle at a 15-mg/kg dose adjusted to achieve a 100-mcg/mL trough level of bound valproic acid as tolerated. Nineteen patients completed all four cycles of treatment.
“The regimen was pretty well tolerated. The vast majority of the toxicities were grade I/II – 70%. The only grade-4 toxicities were cytopenia related and did not lead to a delay in treatment,” he said.
No patients developed acute graft-versus-host disease after therapy, although 11 (39%) developed chronic GVHD, which was extensive in 8, Dr. Hagen said.
Study participants were adults with high-risk AML with no grade 3-4 acute GVHD. All had adequate organ function after allogeneic stem cell transplantation (allo-SCT) performed 40-60 days prior to the start of maintenance therapy. Those with active or uncontrolled infections, low-risk AML in first relapse, neutrophil counts below 1,500, and platelets below 50,000 were excluded, he said.
The patients’ median age was 44 years, they had a median of two prior chemotherapy regimens, and their median Sorror Comorbidity Index was 2.5. Cytogenetics were mostly intermediate and adverse; only one patient had favorable cytogenetics.
Graft type was mixed, with most patients having matched unrelated donors. Conditioning regimens also were mixed, although they “skewed toward myeloablative,” he said. GVHD prophylaxis was standard for the institution and included tacrolimus and methotrexate.
The promising 1-year overall survival and relapse rate without significant dose-limiting toxicities warrants further evaluation in a phase III trial, Dr. Hagen concluded.
He reported having no disclosures.
ORLANDO – Azacitadine and valproic acid can be safely coadministered as maintenance therapy after allogeneic stem cell transplantation in patients with high-risk acute myelogenous leukemia (AML), according to interim findings from an investigator-initiated phase II study.
One-year relapse-free and overall survival rates were about 80%, and no significant toxicities were reported in 28 such patients who began the treatment at least 40 days after transplant and were treated for 4 months, Patrick A. Hagen, MD, reported at the combined annual meetings of the Center for International Blood & Marrow Transplant Research and the American Society for Blood and Marrow Transplantation.
“As we all know, relapse after allogeneic transplant for AML is a huge and ongoing problem. It’s the primary cause of death following transplant, and unfortunately it really hasn’t decreased over the past couple decades,” Dr. Hagen said, adding that relapse is of increasing concern for biologically high-risk patients and is a “pretty ripe area for improved methodologies or approaches.” The decision to pursue maintenance therapy after transplant is complicated: It’s a good opportunity to improve outcomes, but there are many challenges, including problems with drug interactions and myelosuppression, he said.
The study was undertaken based in part on previously reported findings of synergism of a demethylating agent and a histone dacetylase inhibitor in patients with AML, he said.
The maintenance therapy included up to four 28-day cycles of azacitadine at 40 mg/m2 daily on days 1-5, along with oral valproic acid daily throughout the cycle at a 15-mg/kg dose adjusted to achieve a 100-mcg/mL trough level of bound valproic acid as tolerated. Nineteen patients completed all four cycles of treatment.
“The regimen was pretty well tolerated. The vast majority of the toxicities were grade I/II – 70%. The only grade-4 toxicities were cytopenia related and did not lead to a delay in treatment,” he said.
No patients developed acute graft-versus-host disease after therapy, although 11 (39%) developed chronic GVHD, which was extensive in 8, Dr. Hagen said.
Study participants were adults with high-risk AML with no grade 3-4 acute GVHD. All had adequate organ function after allogeneic stem cell transplantation (allo-SCT) performed 40-60 days prior to the start of maintenance therapy. Those with active or uncontrolled infections, low-risk AML in first relapse, neutrophil counts below 1,500, and platelets below 50,000 were excluded, he said.
The patients’ median age was 44 years, they had a median of two prior chemotherapy regimens, and their median Sorror Comorbidity Index was 2.5. Cytogenetics were mostly intermediate and adverse; only one patient had favorable cytogenetics.
Graft type was mixed, with most patients having matched unrelated donors. Conditioning regimens also were mixed, although they “skewed toward myeloablative,” he said. GVHD prophylaxis was standard for the institution and included tacrolimus and methotrexate.
The promising 1-year overall survival and relapse rate without significant dose-limiting toxicities warrants further evaluation in a phase III trial, Dr. Hagen concluded.
He reported having no disclosures.
ORLANDO – Azacitadine and valproic acid can be safely coadministered as maintenance therapy after allogeneic stem cell transplantation in patients with high-risk acute myelogenous leukemia (AML), according to interim findings from an investigator-initiated phase II study.
One-year relapse-free and overall survival rates were about 80%, and no significant toxicities were reported in 28 such patients who began the treatment at least 40 days after transplant and were treated for 4 months, Patrick A. Hagen, MD, reported at the combined annual meetings of the Center for International Blood & Marrow Transplant Research and the American Society for Blood and Marrow Transplantation.
“As we all know, relapse after allogeneic transplant for AML is a huge and ongoing problem. It’s the primary cause of death following transplant, and unfortunately it really hasn’t decreased over the past couple decades,” Dr. Hagen said, adding that relapse is of increasing concern for biologically high-risk patients and is a “pretty ripe area for improved methodologies or approaches.” The decision to pursue maintenance therapy after transplant is complicated: It’s a good opportunity to improve outcomes, but there are many challenges, including problems with drug interactions and myelosuppression, he said.
The study was undertaken based in part on previously reported findings of synergism of a demethylating agent and a histone dacetylase inhibitor in patients with AML, he said.
The maintenance therapy included up to four 28-day cycles of azacitadine at 40 mg/m2 daily on days 1-5, along with oral valproic acid daily throughout the cycle at a 15-mg/kg dose adjusted to achieve a 100-mcg/mL trough level of bound valproic acid as tolerated. Nineteen patients completed all four cycles of treatment.
“The regimen was pretty well tolerated. The vast majority of the toxicities were grade I/II – 70%. The only grade-4 toxicities were cytopenia related and did not lead to a delay in treatment,” he said.
No patients developed acute graft-versus-host disease after therapy, although 11 (39%) developed chronic GVHD, which was extensive in 8, Dr. Hagen said.
Study participants were adults with high-risk AML with no grade 3-4 acute GVHD. All had adequate organ function after allogeneic stem cell transplantation (allo-SCT) performed 40-60 days prior to the start of maintenance therapy. Those with active or uncontrolled infections, low-risk AML in first relapse, neutrophil counts below 1,500, and platelets below 50,000 were excluded, he said.
The patients’ median age was 44 years, they had a median of two prior chemotherapy regimens, and their median Sorror Comorbidity Index was 2.5. Cytogenetics were mostly intermediate and adverse; only one patient had favorable cytogenetics.
Graft type was mixed, with most patients having matched unrelated donors. Conditioning regimens also were mixed, although they “skewed toward myeloablative,” he said. GVHD prophylaxis was standard for the institution and included tacrolimus and methotrexate.
The promising 1-year overall survival and relapse rate without significant dose-limiting toxicities warrants further evaluation in a phase III trial, Dr. Hagen concluded.
He reported having no disclosures.
AT THE 2017 BMT TANDEM MEETINGS
Key clinical point:
Major finding: 1-year relapse-free survival and 1-year overall survival were about 80%.
Data source: An interim analysis of data from 28 patients in a phase II study.
Disclosures: Dr. Hagen reported having no disclosures.
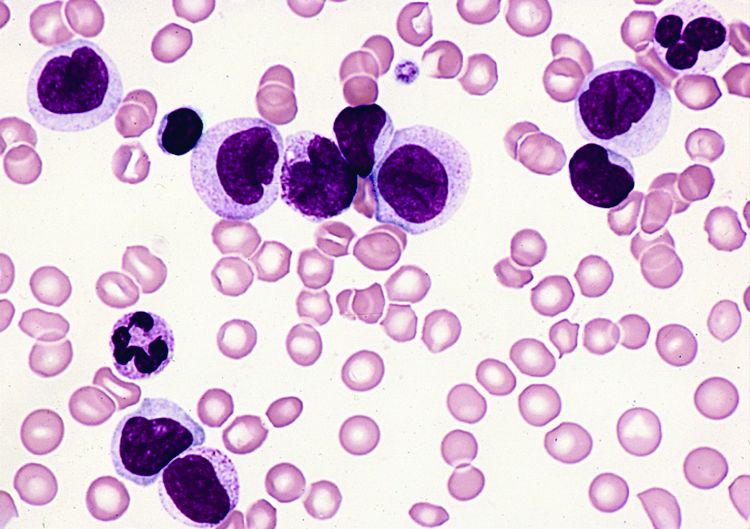
Mixed leukemias can benefit from allo-HST
ORLANDO – Allogeneic hematopoietic stem cell transplantation using a matched donor is a valid treatment option – and potential cure – for leukemias with markers of both myeloid and lymphoid lineages, or mixed phenotype acute leukemias, according to findings from the Acute Leukemia Working Party of the European Group for Blood and Marrow Transplantation (ALWP-EBMT) database.
Treatment outcomes at 3 years in 519 patients from the database who received an allogeneic transplant (allo-HCT) for mixed-phenotype acute leukemia (MPAL) between 2000 and 2014 and were transplanted in complete remission (CR1) included an overall survival of 56.3%, a leukemia-free survival of 46.5%, a relapse incidence of 31.4%, a nonrelapse mortality of 22.1%, and an incidence of chronic graft-versus-host disease (GVHD) of 37.5%, Reinhold Munker, MD, reported at the combined annual meetings of the Center for International Blood & Marrow Transplant Research and the American Society for Blood and Marrow Transplantation.
“The outcome in this large adult study is pretty favorable based upon 519 patients; 45%-65% can expect overall survival at 5 years,” he said.
The median age of the study subjects was 38.1 years (range, 18-75). Transplants were from a matched sibling donor in 54.5% of cases, and from a matched unrelated donor in 45.5% of cases. Myeloablative conditioning was used in 400 patients and included only chemotherapy in 140 patients and chemotherapy with total body irradiation in 260 patients. The remaining patients received nonmyeloablative conditioning, said Dr. Munker of Tulane University, New Orleans.
The source of stem cells was bone marrow in 26% of patients, and peripheral blood in 73%. Grade II-IV acute GVHD developed in 32.5% of patients. Median follow-up was 32 months, he noted.
In univariate analysis, age at transplant was strongly associated with leukemia-free survival, nonrelapse mortality, relapse incidence, and overall survival. The best outcomes were among those aged 18-35 years. The nonrelapse mortality rate and overall survival rate were lower for transplants done in 2005-2014 vs. 2000-2004 (20% vs 33.2% and 58.3 vs 44.7%, respectively). No differences in outcomes were found between related and unrelated donors, but chronic GVHD was more common with female donors and male recipients, with no in vivo T-cell depletion, and with use of peripheral blood stem cells – findings which are not unexpected, Dr. Munker noted.
Use of myeloablative conditioning with total-body irradiation correlated with a lower relapse incidence and with better leukemia-free survival vs. both myeloablative conditioning with only chemotherapy and reduced-intensity conditioning, he said.
In multivariate analysis, younger age and more recent year of transplant were associated with a better leukemia-free survival and overall survival, and use of myeloablative conditioning with total-body irradiation was associated with better leukemia-free survival and with a trend for higher overall survival.
MPALs are rare, accounting for only 2%-5% of all acute leukemias, Dr. Munker said, noting that prognosis is considered to be intermediate in children and unfavorable in adults.
The diagnostic criteria for MPAL were revised by the World Health Organization (WHO) in 2008 and accepted by most centers, but until recently data were lacking with respect to the recommended treatment strategy of induction regimens similar to those used in acute lymphoblastic leukemia, and consolidation by allogeneic transplant, he explained.
However, the Center for International Blood and Marrow Transplant Research last year published a series of 95 cases showing encouraging long-term survival with allo-HCT in MPAL patients with a median age of 20 years.
The current findings confirm and extend those prior findings, Dr. Munker said.
Dr. Munker reported having no disclosures.
ORLANDO – Allogeneic hematopoietic stem cell transplantation using a matched donor is a valid treatment option – and potential cure – for leukemias with markers of both myeloid and lymphoid lineages, or mixed phenotype acute leukemias, according to findings from the Acute Leukemia Working Party of the European Group for Blood and Marrow Transplantation (ALWP-EBMT) database.
Treatment outcomes at 3 years in 519 patients from the database who received an allogeneic transplant (allo-HCT) for mixed-phenotype acute leukemia (MPAL) between 2000 and 2014 and were transplanted in complete remission (CR1) included an overall survival of 56.3%, a leukemia-free survival of 46.5%, a relapse incidence of 31.4%, a nonrelapse mortality of 22.1%, and an incidence of chronic graft-versus-host disease (GVHD) of 37.5%, Reinhold Munker, MD, reported at the combined annual meetings of the Center for International Blood & Marrow Transplant Research and the American Society for Blood and Marrow Transplantation.
“The outcome in this large adult study is pretty favorable based upon 519 patients; 45%-65% can expect overall survival at 5 years,” he said.
The median age of the study subjects was 38.1 years (range, 18-75). Transplants were from a matched sibling donor in 54.5% of cases, and from a matched unrelated donor in 45.5% of cases. Myeloablative conditioning was used in 400 patients and included only chemotherapy in 140 patients and chemotherapy with total body irradiation in 260 patients. The remaining patients received nonmyeloablative conditioning, said Dr. Munker of Tulane University, New Orleans.
The source of stem cells was bone marrow in 26% of patients, and peripheral blood in 73%. Grade II-IV acute GVHD developed in 32.5% of patients. Median follow-up was 32 months, he noted.
In univariate analysis, age at transplant was strongly associated with leukemia-free survival, nonrelapse mortality, relapse incidence, and overall survival. The best outcomes were among those aged 18-35 years. The nonrelapse mortality rate and overall survival rate were lower for transplants done in 2005-2014 vs. 2000-2004 (20% vs 33.2% and 58.3 vs 44.7%, respectively). No differences in outcomes were found between related and unrelated donors, but chronic GVHD was more common with female donors and male recipients, with no in vivo T-cell depletion, and with use of peripheral blood stem cells – findings which are not unexpected, Dr. Munker noted.
Use of myeloablative conditioning with total-body irradiation correlated with a lower relapse incidence and with better leukemia-free survival vs. both myeloablative conditioning with only chemotherapy and reduced-intensity conditioning, he said.
In multivariate analysis, younger age and more recent year of transplant were associated with a better leukemia-free survival and overall survival, and use of myeloablative conditioning with total-body irradiation was associated with better leukemia-free survival and with a trend for higher overall survival.
MPALs are rare, accounting for only 2%-5% of all acute leukemias, Dr. Munker said, noting that prognosis is considered to be intermediate in children and unfavorable in adults.
The diagnostic criteria for MPAL were revised by the World Health Organization (WHO) in 2008 and accepted by most centers, but until recently data were lacking with respect to the recommended treatment strategy of induction regimens similar to those used in acute lymphoblastic leukemia, and consolidation by allogeneic transplant, he explained.
However, the Center for International Blood and Marrow Transplant Research last year published a series of 95 cases showing encouraging long-term survival with allo-HCT in MPAL patients with a median age of 20 years.
The current findings confirm and extend those prior findings, Dr. Munker said.
Dr. Munker reported having no disclosures.
ORLANDO – Allogeneic hematopoietic stem cell transplantation using a matched donor is a valid treatment option – and potential cure – for leukemias with markers of both myeloid and lymphoid lineages, or mixed phenotype acute leukemias, according to findings from the Acute Leukemia Working Party of the European Group for Blood and Marrow Transplantation (ALWP-EBMT) database.
Treatment outcomes at 3 years in 519 patients from the database who received an allogeneic transplant (allo-HCT) for mixed-phenotype acute leukemia (MPAL) between 2000 and 2014 and were transplanted in complete remission (CR1) included an overall survival of 56.3%, a leukemia-free survival of 46.5%, a relapse incidence of 31.4%, a nonrelapse mortality of 22.1%, and an incidence of chronic graft-versus-host disease (GVHD) of 37.5%, Reinhold Munker, MD, reported at the combined annual meetings of the Center for International Blood & Marrow Transplant Research and the American Society for Blood and Marrow Transplantation.
“The outcome in this large adult study is pretty favorable based upon 519 patients; 45%-65% can expect overall survival at 5 years,” he said.
The median age of the study subjects was 38.1 years (range, 18-75). Transplants were from a matched sibling donor in 54.5% of cases, and from a matched unrelated donor in 45.5% of cases. Myeloablative conditioning was used in 400 patients and included only chemotherapy in 140 patients and chemotherapy with total body irradiation in 260 patients. The remaining patients received nonmyeloablative conditioning, said Dr. Munker of Tulane University, New Orleans.
The source of stem cells was bone marrow in 26% of patients, and peripheral blood in 73%. Grade II-IV acute GVHD developed in 32.5% of patients. Median follow-up was 32 months, he noted.
In univariate analysis, age at transplant was strongly associated with leukemia-free survival, nonrelapse mortality, relapse incidence, and overall survival. The best outcomes were among those aged 18-35 years. The nonrelapse mortality rate and overall survival rate were lower for transplants done in 2005-2014 vs. 2000-2004 (20% vs 33.2% and 58.3 vs 44.7%, respectively). No differences in outcomes were found between related and unrelated donors, but chronic GVHD was more common with female donors and male recipients, with no in vivo T-cell depletion, and with use of peripheral blood stem cells – findings which are not unexpected, Dr. Munker noted.
Use of myeloablative conditioning with total-body irradiation correlated with a lower relapse incidence and with better leukemia-free survival vs. both myeloablative conditioning with only chemotherapy and reduced-intensity conditioning, he said.
In multivariate analysis, younger age and more recent year of transplant were associated with a better leukemia-free survival and overall survival, and use of myeloablative conditioning with total-body irradiation was associated with better leukemia-free survival and with a trend for higher overall survival.
MPALs are rare, accounting for only 2%-5% of all acute leukemias, Dr. Munker said, noting that prognosis is considered to be intermediate in children and unfavorable in adults.
The diagnostic criteria for MPAL were revised by the World Health Organization (WHO) in 2008 and accepted by most centers, but until recently data were lacking with respect to the recommended treatment strategy of induction regimens similar to those used in acute lymphoblastic leukemia, and consolidation by allogeneic transplant, he explained.
However, the Center for International Blood and Marrow Transplant Research last year published a series of 95 cases showing encouraging long-term survival with allo-HCT in MPAL patients with a median age of 20 years.
The current findings confirm and extend those prior findings, Dr. Munker said.
Dr. Munker reported having no disclosures.
Key clinical point:
Major finding: Treatment outcomes at 3 years included overall survival of 56.3%, leukemia-free survival of 46.5%, relapse incidence of 31.4%, nonrelapse mortality of 22.1%, and incidence of chronic graft-versus-host disease of 37.5%.
Data source: A review of 519 cases from the ALWP-EBMT database.
Disclosures: Dr. Munker reported having no disclosures.
Pre- and post-HCT MRD levels predict ALL survival
ORLANDO – Minimal residual disease (MRD) measured before and after allogeneic hematopoietic stem cell transplantation (HCT) is a powerful predictor of survival in children with acute lymphoblastic leukemia (ALL), according to a review of hundreds of cases from around the world.
The findings could have implications for using minimal residual disease measures to guide posttransplant interventions, Michael A. Pulsipher, MD, reported at the combined annual meetings of the Center for International Blood & Marrow Transplant Research and the American Society for Blood and Marrow Transplantation.
“MRD pretransplant was a very powerful predictor of outcomes. MRD posttransplant highlights individual patients at risk,” Dr. Pulsipher said. Results comparing reverse transcriptase–polymerase chain reaction with flow cytometry require validation by direct comparison in the same patient cohort, but “the new risk scores ... very nicely predict outcomes both pre- and post-transplant and can guide study planning and patient counseling.”
A total of 2,960 bone marrow MRD measurements were performed in the 747 patients included in the study. MRD was assessed prior to HCT and on or near days 30, 60, 100, 180, and 365 and beyond after HCT.
Patients were grouped for analysis according to MRD level: Group 1 had no detectable MRD, group 2 had low detectable MRD levels (less than 10E-4, or 0.01% by flow cytometry), and group 3 had high detectable MRD levels (10E-4 or higher). A second analysis compared findings in those tested by flow cytometry and those tested by real-time quantitative PCR (RQ-PCR), said Dr. Pulsipher of Children’s Hospital Los Angeles.
In 648 patients with pre-HCT MRD measurements available, the 4-year probability of event-free survival was 62%, 67%, and 37% for groups 1, 2, and 3, respectively. Group 3 – the high MRD level group – had 2.47 times the increased hazard ratio for relapse and 1.67 times the increased risk of treatment-related mortality, Dr. Pulsipher said, adding that pre-HCT MRD and remission status both significantly influenced survival, while age, sex, relapse site, cytogenetics, donor type, and stem cell source did not influence outcome.
Post-HCT MRD values were analyzed as time-dependent covariates.
“As time went by more and more, any detectable level of MRD led to a very poor prognosis, whereas patients arriving at day 365 with no detectable MRD had exceptional prognosis with survival approaching 90%,” he said.
Specifically, the 4-year probability of event-free survival for groups 1, 2, and 3, respectively, were 59%, 65%, and 43% at day 30; 64%, 47%, and 36% at day 60; 65%, 69%, and 44% at day 90; 79%, 40%, and 12% at day 180; and 87%, 36%, and 25% at day 365.
Of note, a very significant interaction was seen between acute graft-versus-host disease (GVHD) and MRD, Dr. Pulsipher said, explaining that patients who were MRD positive and had developed GVHD had a significant decrease in the cumulative incidence of relapse, compared with those with no GVHD.
“This translated into improved event-free survival with patients post transplant, who were MRD-positive [and] developing GVHD, still having a reasonable chance of survival, whereas patients post transplant who had MRD measured who did not develop GVHD had a very poor chance of survival,” he added.
Additionally, based on detailed multivariate analysis including a number of clinical factors, risk predictive scores were developed for event-free survival risk at 18 months or cumulative incidence of relapse at 18 months. Multiple scores were developed for each, but, as an example of factors that had an important effect on outcomes, patients with very early pretransplant relapse (those who went into remission but relapsed within 18 months) or with greater than 2nd relapse had a high risk for poor event-free survival. Mismatched donors and unrelated cord-blood stem cell transplant recipients also had high risk, he said, noting that, “of course, MRD had a significant effect” and was the most important factor prior to transplant.
These patients, who had a 4-point or greater risk score, were the poorest risk group, with survival that was less than 50%, as opposed to better risk groups that exceeded 90%, he said.
“A score of greater than 5 could identify 80% of patients who were going to relapse after transplant, and of course, event-free and overall survival in those patients were very poor,” he added.
As time went by, the early risk of GVHD diminished somewhat, as did the risk of mismatched donors.
“Most of the risk was associated with any MRD detection,” he said.
Flow cytometry and RQ-PCR levels of at least 10-4 were highly predictive of relapse at all pre- and post-HCT time points; however, RQ-PCR values between 10-4 and 10-3, in cases where adequate numbers were available for comparison, better predicted relapse as compared with flow cytometry results.
For example, before HCT, hazard ratios were 1.26 and 2.41 with flow cytometry vs. RQ-PCR. At day 30, the hazard ratios were 1.33 and 2.53, and at day 365, they were 3.54 and 31.84, Dr. Pulsipher reported.
The findings provide important information for improving outcomes in children with high-risk ALL undergoing HCT, he said.
“Older prognostic models for relapsed and refractory high-risk ALL have focused on timing and location of relapse, as well as disease phenotype. But it is clear that, in order to treat children with very high risk ALL with transplantation, MRD has become the most important thing to look at in the pretreatment setting. The challenges that we face in assessing MRD, however, have been hampered by the fact that we have differing MRD measurements,” he said, noting that RQ-PCR is often used in Europe, while flow cytometry is more often used in the United States. As such, direct comparisons are lacking, as are T-cell and posttransplant data.
The current study represents a “tremendous effort” by international collaborators to address these shortcoming, he said.
“This is a great opportunity, as our goal, of course, is to avoid futility in transplantation, but, more importantly, to find opportunities to identify groups for which we can improve our outcomes,” he added.
Patients included in the study were treated in Europe, North America, and Australia and were transplanted during Sept. 1999-May 2016. Most were in first or second remission, and most (586) had pre-B ALL. A notable 145 had T-cell ALL – “more than ever has been analyzed previously” – and 16 had B-lineage or biphenotypic ALL. About half were under age 10 years, 62% were boys, and stem cell sources were typical, although 20% received a cord blood transplant.
Dr. Pulsipher reported serving as an advisor and/or consultant for Chimerix, Novartis, Jazz Pharmaceutical, and receiving housing support from Medac Pharma for an educational meeting.
ORLANDO – Minimal residual disease (MRD) measured before and after allogeneic hematopoietic stem cell transplantation (HCT) is a powerful predictor of survival in children with acute lymphoblastic leukemia (ALL), according to a review of hundreds of cases from around the world.
The findings could have implications for using minimal residual disease measures to guide posttransplant interventions, Michael A. Pulsipher, MD, reported at the combined annual meetings of the Center for International Blood & Marrow Transplant Research and the American Society for Blood and Marrow Transplantation.
“MRD pretransplant was a very powerful predictor of outcomes. MRD posttransplant highlights individual patients at risk,” Dr. Pulsipher said. Results comparing reverse transcriptase–polymerase chain reaction with flow cytometry require validation by direct comparison in the same patient cohort, but “the new risk scores ... very nicely predict outcomes both pre- and post-transplant and can guide study planning and patient counseling.”
A total of 2,960 bone marrow MRD measurements were performed in the 747 patients included in the study. MRD was assessed prior to HCT and on or near days 30, 60, 100, 180, and 365 and beyond after HCT.
Patients were grouped for analysis according to MRD level: Group 1 had no detectable MRD, group 2 had low detectable MRD levels (less than 10E-4, or 0.01% by flow cytometry), and group 3 had high detectable MRD levels (10E-4 or higher). A second analysis compared findings in those tested by flow cytometry and those tested by real-time quantitative PCR (RQ-PCR), said Dr. Pulsipher of Children’s Hospital Los Angeles.
In 648 patients with pre-HCT MRD measurements available, the 4-year probability of event-free survival was 62%, 67%, and 37% for groups 1, 2, and 3, respectively. Group 3 – the high MRD level group – had 2.47 times the increased hazard ratio for relapse and 1.67 times the increased risk of treatment-related mortality, Dr. Pulsipher said, adding that pre-HCT MRD and remission status both significantly influenced survival, while age, sex, relapse site, cytogenetics, donor type, and stem cell source did not influence outcome.
Post-HCT MRD values were analyzed as time-dependent covariates.
“As time went by more and more, any detectable level of MRD led to a very poor prognosis, whereas patients arriving at day 365 with no detectable MRD had exceptional prognosis with survival approaching 90%,” he said.
Specifically, the 4-year probability of event-free survival for groups 1, 2, and 3, respectively, were 59%, 65%, and 43% at day 30; 64%, 47%, and 36% at day 60; 65%, 69%, and 44% at day 90; 79%, 40%, and 12% at day 180; and 87%, 36%, and 25% at day 365.
Of note, a very significant interaction was seen between acute graft-versus-host disease (GVHD) and MRD, Dr. Pulsipher said, explaining that patients who were MRD positive and had developed GVHD had a significant decrease in the cumulative incidence of relapse, compared with those with no GVHD.
“This translated into improved event-free survival with patients post transplant, who were MRD-positive [and] developing GVHD, still having a reasonable chance of survival, whereas patients post transplant who had MRD measured who did not develop GVHD had a very poor chance of survival,” he added.
Additionally, based on detailed multivariate analysis including a number of clinical factors, risk predictive scores were developed for event-free survival risk at 18 months or cumulative incidence of relapse at 18 months. Multiple scores were developed for each, but, as an example of factors that had an important effect on outcomes, patients with very early pretransplant relapse (those who went into remission but relapsed within 18 months) or with greater than 2nd relapse had a high risk for poor event-free survival. Mismatched donors and unrelated cord-blood stem cell transplant recipients also had high risk, he said, noting that, “of course, MRD had a significant effect” and was the most important factor prior to transplant.
These patients, who had a 4-point or greater risk score, were the poorest risk group, with survival that was less than 50%, as opposed to better risk groups that exceeded 90%, he said.
“A score of greater than 5 could identify 80% of patients who were going to relapse after transplant, and of course, event-free and overall survival in those patients were very poor,” he added.
As time went by, the early risk of GVHD diminished somewhat, as did the risk of mismatched donors.
“Most of the risk was associated with any MRD detection,” he said.
Flow cytometry and RQ-PCR levels of at least 10-4 were highly predictive of relapse at all pre- and post-HCT time points; however, RQ-PCR values between 10-4 and 10-3, in cases where adequate numbers were available for comparison, better predicted relapse as compared with flow cytometry results.
For example, before HCT, hazard ratios were 1.26 and 2.41 with flow cytometry vs. RQ-PCR. At day 30, the hazard ratios were 1.33 and 2.53, and at day 365, they were 3.54 and 31.84, Dr. Pulsipher reported.
The findings provide important information for improving outcomes in children with high-risk ALL undergoing HCT, he said.
“Older prognostic models for relapsed and refractory high-risk ALL have focused on timing and location of relapse, as well as disease phenotype. But it is clear that, in order to treat children with very high risk ALL with transplantation, MRD has become the most important thing to look at in the pretreatment setting. The challenges that we face in assessing MRD, however, have been hampered by the fact that we have differing MRD measurements,” he said, noting that RQ-PCR is often used in Europe, while flow cytometry is more often used in the United States. As such, direct comparisons are lacking, as are T-cell and posttransplant data.
The current study represents a “tremendous effort” by international collaborators to address these shortcoming, he said.
“This is a great opportunity, as our goal, of course, is to avoid futility in transplantation, but, more importantly, to find opportunities to identify groups for which we can improve our outcomes,” he added.
Patients included in the study were treated in Europe, North America, and Australia and were transplanted during Sept. 1999-May 2016. Most were in first or second remission, and most (586) had pre-B ALL. A notable 145 had T-cell ALL – “more than ever has been analyzed previously” – and 16 had B-lineage or biphenotypic ALL. About half were under age 10 years, 62% were boys, and stem cell sources were typical, although 20% received a cord blood transplant.
Dr. Pulsipher reported serving as an advisor and/or consultant for Chimerix, Novartis, Jazz Pharmaceutical, and receiving housing support from Medac Pharma for an educational meeting.
ORLANDO – Minimal residual disease (MRD) measured before and after allogeneic hematopoietic stem cell transplantation (HCT) is a powerful predictor of survival in children with acute lymphoblastic leukemia (ALL), according to a review of hundreds of cases from around the world.
The findings could have implications for using minimal residual disease measures to guide posttransplant interventions, Michael A. Pulsipher, MD, reported at the combined annual meetings of the Center for International Blood & Marrow Transplant Research and the American Society for Blood and Marrow Transplantation.
“MRD pretransplant was a very powerful predictor of outcomes. MRD posttransplant highlights individual patients at risk,” Dr. Pulsipher said. Results comparing reverse transcriptase–polymerase chain reaction with flow cytometry require validation by direct comparison in the same patient cohort, but “the new risk scores ... very nicely predict outcomes both pre- and post-transplant and can guide study planning and patient counseling.”
A total of 2,960 bone marrow MRD measurements were performed in the 747 patients included in the study. MRD was assessed prior to HCT and on or near days 30, 60, 100, 180, and 365 and beyond after HCT.
Patients were grouped for analysis according to MRD level: Group 1 had no detectable MRD, group 2 had low detectable MRD levels (less than 10E-4, or 0.01% by flow cytometry), and group 3 had high detectable MRD levels (10E-4 or higher). A second analysis compared findings in those tested by flow cytometry and those tested by real-time quantitative PCR (RQ-PCR), said Dr. Pulsipher of Children’s Hospital Los Angeles.
In 648 patients with pre-HCT MRD measurements available, the 4-year probability of event-free survival was 62%, 67%, and 37% for groups 1, 2, and 3, respectively. Group 3 – the high MRD level group – had 2.47 times the increased hazard ratio for relapse and 1.67 times the increased risk of treatment-related mortality, Dr. Pulsipher said, adding that pre-HCT MRD and remission status both significantly influenced survival, while age, sex, relapse site, cytogenetics, donor type, and stem cell source did not influence outcome.
Post-HCT MRD values were analyzed as time-dependent covariates.
“As time went by more and more, any detectable level of MRD led to a very poor prognosis, whereas patients arriving at day 365 with no detectable MRD had exceptional prognosis with survival approaching 90%,” he said.
Specifically, the 4-year probability of event-free survival for groups 1, 2, and 3, respectively, were 59%, 65%, and 43% at day 30; 64%, 47%, and 36% at day 60; 65%, 69%, and 44% at day 90; 79%, 40%, and 12% at day 180; and 87%, 36%, and 25% at day 365.
Of note, a very significant interaction was seen between acute graft-versus-host disease (GVHD) and MRD, Dr. Pulsipher said, explaining that patients who were MRD positive and had developed GVHD had a significant decrease in the cumulative incidence of relapse, compared with those with no GVHD.
“This translated into improved event-free survival with patients post transplant, who were MRD-positive [and] developing GVHD, still having a reasonable chance of survival, whereas patients post transplant who had MRD measured who did not develop GVHD had a very poor chance of survival,” he added.
Additionally, based on detailed multivariate analysis including a number of clinical factors, risk predictive scores were developed for event-free survival risk at 18 months or cumulative incidence of relapse at 18 months. Multiple scores were developed for each, but, as an example of factors that had an important effect on outcomes, patients with very early pretransplant relapse (those who went into remission but relapsed within 18 months) or with greater than 2nd relapse had a high risk for poor event-free survival. Mismatched donors and unrelated cord-blood stem cell transplant recipients also had high risk, he said, noting that, “of course, MRD had a significant effect” and was the most important factor prior to transplant.
These patients, who had a 4-point or greater risk score, were the poorest risk group, with survival that was less than 50%, as opposed to better risk groups that exceeded 90%, he said.
“A score of greater than 5 could identify 80% of patients who were going to relapse after transplant, and of course, event-free and overall survival in those patients were very poor,” he added.
As time went by, the early risk of GVHD diminished somewhat, as did the risk of mismatched donors.
“Most of the risk was associated with any MRD detection,” he said.
Flow cytometry and RQ-PCR levels of at least 10-4 were highly predictive of relapse at all pre- and post-HCT time points; however, RQ-PCR values between 10-4 and 10-3, in cases where adequate numbers were available for comparison, better predicted relapse as compared with flow cytometry results.
For example, before HCT, hazard ratios were 1.26 and 2.41 with flow cytometry vs. RQ-PCR. At day 30, the hazard ratios were 1.33 and 2.53, and at day 365, they were 3.54 and 31.84, Dr. Pulsipher reported.
The findings provide important information for improving outcomes in children with high-risk ALL undergoing HCT, he said.
“Older prognostic models for relapsed and refractory high-risk ALL have focused on timing and location of relapse, as well as disease phenotype. But it is clear that, in order to treat children with very high risk ALL with transplantation, MRD has become the most important thing to look at in the pretreatment setting. The challenges that we face in assessing MRD, however, have been hampered by the fact that we have differing MRD measurements,” he said, noting that RQ-PCR is often used in Europe, while flow cytometry is more often used in the United States. As such, direct comparisons are lacking, as are T-cell and posttransplant data.
The current study represents a “tremendous effort” by international collaborators to address these shortcoming, he said.
“This is a great opportunity, as our goal, of course, is to avoid futility in transplantation, but, more importantly, to find opportunities to identify groups for which we can improve our outcomes,” he added.
Patients included in the study were treated in Europe, North America, and Australia and were transplanted during Sept. 1999-May 2016. Most were in first or second remission, and most (586) had pre-B ALL. A notable 145 had T-cell ALL – “more than ever has been analyzed previously” – and 16 had B-lineage or biphenotypic ALL. About half were under age 10 years, 62% were boys, and stem cell sources were typical, although 20% received a cord blood transplant.
Dr. Pulsipher reported serving as an advisor and/or consultant for Chimerix, Novartis, Jazz Pharmaceutical, and receiving housing support from Medac Pharma for an educational meeting.
Key clinical point:
Major finding: Patients with high pretransplant MRD levels had a 2.47-fold increased hazard ratio for relapse and a 1.67-fold increased risk of treatment-related mortality.
Data source: A review of data from 747 pediatric high-risk ALL cases.
Disclosures: Dr. Pulsipher reported serving as an adviser and/or consultant for Chimerix, Novartis, and Jazz Pharmaceuticals and receiving housing support from Medac Pharma for an educational meeting.
Promising ibrutinib data prompt frontline cGVHD therapy study
ORLANDO – Ibrutinib was associated with clinically meaningful and durable responses in patients with chronic graft-versus-host disease that did not respond to frontline systemic therapy, based on the final results of a phase II study.
Preliminary findings from that study led in 2016 to a Food and Drug Administration Breakthrough Therapy Designation for ibrutinib for chronic graft-versus-host disease (cGVHD) after the failure of one or more lines of systemic therapy, and the responses seen in this pretreated, high-risk population support the study of ibrutinib for frontline treatment of cGVHD, said David Miklos, MD, of Stanford (Calif.) University.
Of 20 patients with multiple organ involvement, 25 (80%) had responses in at least two organs, and of 9 patients with three or more involved organs, 5 (56%) had responses in at least three organs, he reported at the combined annual meetings of the Center for International Blood & Marrow Transplant Research and the American Society for Blood and Marrow Transplantation..
“We think that the responses across different organ involvement as well as multiple organ responses speaks to the underlying impact of ibrutinib on the pathogenic pathway and not to masking symptoms or indirect effect,” he said.
The median steroid dose among responders decreased from 0.29 mg/kg daily at baseline to 0.19 mg/kg daily and 0.12 mg/kg daily at weeks 25 and 49, respectively. Overall, 62% of all patients reached steroid doses less than 0.15 mg/kg daily, and five responders discontinued steroid treatment.
“Patients also had clinically meaningful improvement as assessed by the Lee symptoms scale,” he said, noting that scores improved in 61% of responders and 11% of nonresponders.
Study participants had a median age of 56 years and a median of 7.6 months from allogeneic transplant to diagnosis of cGVHD. All had been treated with up to three prior cGVHD regimens (median, two) and had either a rash that exceeded 25% of their body surface area or a National Institutes of Health consensus mouth score greater than 4. They were treated with ibrutinib at a dose of 420 mg/day until cGVHD progression or unacceptable toxicity. The cGVHD response – the primary endpoint of the study – was measured using 2005 NIH response criteria.
Adverse events occurring in at least 20% of patients included fatigue, diarrhea, muscle spasms, nausea, and bruising. Grade 3 or higher adverse events occurring in at least 10% of patients included pneumonia, fatigue, and diarrhea.
Serious adverse events occurred in 52% of patients. Grade 3 or higher serious adverse events occurred in 40% of patients and included pneumonia, septic shock, and pyrexia. Two fatal events were reported and included one case of multilobular pneumonia and one case of bronchopulmonary aspergillosis.
Twelve patients (29%) remained on ibrutinib at 14 months; Of those who discontinued therapy, 5 discontinued because of progressive cGVHD, and 14 because of adverse events.
Patients who have cGVHD and don’t respond to frontline therapy have previously had no effective options. Ibrutinib showed promise in preclinical models; it reduced the severity of cGVHD through inhibition of Bruton’s tyrosine kinase and interleukin-2–inducible T-cell kinase, Dr. Miklos explained, noting that both B and T cells play a role in the pathophysiology of cGVHD.
The findings from this phase II trial demonstrate that ibrutinib does indeed lead to durable improvement in this patient population, and its safety profile is consistent with that previously reported for B-cell malignancies treated with ibrutinib and for cGVHD patients treated with concomitant steroids, he said.
“We think the efficacy of ibrutinib in this population supports further study in frontline treatment of cGVHD in a randomized, double-blinded study,” he concluded.
A phase III study – the INTEGRATE clinical trial – is now open. The international study will compare ibrutinib and prednisone with placebo and prednisone as a frontline therapy for moderate and severe cGVHD with a primary endpoint of response rate at 24 weeks.
The study was sponsored by Pharmacyclics in collaboration with Janssen Research & Development. Dr. Miklos reported various financial relationships with Pharmacyclics (the maker of ibrutinib [Imbruvica]), Velos, Kite Pharma, Sanofi Oncology, Adaptive Biotechnologies, and Genentech.
sworcester@frontlinemedcom.com
ORLANDO – Ibrutinib was associated with clinically meaningful and durable responses in patients with chronic graft-versus-host disease that did not respond to frontline systemic therapy, based on the final results of a phase II study.
Preliminary findings from that study led in 2016 to a Food and Drug Administration Breakthrough Therapy Designation for ibrutinib for chronic graft-versus-host disease (cGVHD) after the failure of one or more lines of systemic therapy, and the responses seen in this pretreated, high-risk population support the study of ibrutinib for frontline treatment of cGVHD, said David Miklos, MD, of Stanford (Calif.) University.
Of 20 patients with multiple organ involvement, 25 (80%) had responses in at least two organs, and of 9 patients with three or more involved organs, 5 (56%) had responses in at least three organs, he reported at the combined annual meetings of the Center for International Blood & Marrow Transplant Research and the American Society for Blood and Marrow Transplantation..
“We think that the responses across different organ involvement as well as multiple organ responses speaks to the underlying impact of ibrutinib on the pathogenic pathway and not to masking symptoms or indirect effect,” he said.
The median steroid dose among responders decreased from 0.29 mg/kg daily at baseline to 0.19 mg/kg daily and 0.12 mg/kg daily at weeks 25 and 49, respectively. Overall, 62% of all patients reached steroid doses less than 0.15 mg/kg daily, and five responders discontinued steroid treatment.
“Patients also had clinically meaningful improvement as assessed by the Lee symptoms scale,” he said, noting that scores improved in 61% of responders and 11% of nonresponders.
Study participants had a median age of 56 years and a median of 7.6 months from allogeneic transplant to diagnosis of cGVHD. All had been treated with up to three prior cGVHD regimens (median, two) and had either a rash that exceeded 25% of their body surface area or a National Institutes of Health consensus mouth score greater than 4. They were treated with ibrutinib at a dose of 420 mg/day until cGVHD progression or unacceptable toxicity. The cGVHD response – the primary endpoint of the study – was measured using 2005 NIH response criteria.
Adverse events occurring in at least 20% of patients included fatigue, diarrhea, muscle spasms, nausea, and bruising. Grade 3 or higher adverse events occurring in at least 10% of patients included pneumonia, fatigue, and diarrhea.
Serious adverse events occurred in 52% of patients. Grade 3 or higher serious adverse events occurred in 40% of patients and included pneumonia, septic shock, and pyrexia. Two fatal events were reported and included one case of multilobular pneumonia and one case of bronchopulmonary aspergillosis.
Twelve patients (29%) remained on ibrutinib at 14 months; Of those who discontinued therapy, 5 discontinued because of progressive cGVHD, and 14 because of adverse events.
Patients who have cGVHD and don’t respond to frontline therapy have previously had no effective options. Ibrutinib showed promise in preclinical models; it reduced the severity of cGVHD through inhibition of Bruton’s tyrosine kinase and interleukin-2–inducible T-cell kinase, Dr. Miklos explained, noting that both B and T cells play a role in the pathophysiology of cGVHD.
The findings from this phase II trial demonstrate that ibrutinib does indeed lead to durable improvement in this patient population, and its safety profile is consistent with that previously reported for B-cell malignancies treated with ibrutinib and for cGVHD patients treated with concomitant steroids, he said.
“We think the efficacy of ibrutinib in this population supports further study in frontline treatment of cGVHD in a randomized, double-blinded study,” he concluded.
A phase III study – the INTEGRATE clinical trial – is now open. The international study will compare ibrutinib and prednisone with placebo and prednisone as a frontline therapy for moderate and severe cGVHD with a primary endpoint of response rate at 24 weeks.
The study was sponsored by Pharmacyclics in collaboration with Janssen Research & Development. Dr. Miklos reported various financial relationships with Pharmacyclics (the maker of ibrutinib [Imbruvica]), Velos, Kite Pharma, Sanofi Oncology, Adaptive Biotechnologies, and Genentech.
sworcester@frontlinemedcom.com
ORLANDO – Ibrutinib was associated with clinically meaningful and durable responses in patients with chronic graft-versus-host disease that did not respond to frontline systemic therapy, based on the final results of a phase II study.
Preliminary findings from that study led in 2016 to a Food and Drug Administration Breakthrough Therapy Designation for ibrutinib for chronic graft-versus-host disease (cGVHD) after the failure of one or more lines of systemic therapy, and the responses seen in this pretreated, high-risk population support the study of ibrutinib for frontline treatment of cGVHD, said David Miklos, MD, of Stanford (Calif.) University.
Of 20 patients with multiple organ involvement, 25 (80%) had responses in at least two organs, and of 9 patients with three or more involved organs, 5 (56%) had responses in at least three organs, he reported at the combined annual meetings of the Center for International Blood & Marrow Transplant Research and the American Society for Blood and Marrow Transplantation..
“We think that the responses across different organ involvement as well as multiple organ responses speaks to the underlying impact of ibrutinib on the pathogenic pathway and not to masking symptoms or indirect effect,” he said.
The median steroid dose among responders decreased from 0.29 mg/kg daily at baseline to 0.19 mg/kg daily and 0.12 mg/kg daily at weeks 25 and 49, respectively. Overall, 62% of all patients reached steroid doses less than 0.15 mg/kg daily, and five responders discontinued steroid treatment.
“Patients also had clinically meaningful improvement as assessed by the Lee symptoms scale,” he said, noting that scores improved in 61% of responders and 11% of nonresponders.
Study participants had a median age of 56 years and a median of 7.6 months from allogeneic transplant to diagnosis of cGVHD. All had been treated with up to three prior cGVHD regimens (median, two) and had either a rash that exceeded 25% of their body surface area or a National Institutes of Health consensus mouth score greater than 4. They were treated with ibrutinib at a dose of 420 mg/day until cGVHD progression or unacceptable toxicity. The cGVHD response – the primary endpoint of the study – was measured using 2005 NIH response criteria.
Adverse events occurring in at least 20% of patients included fatigue, diarrhea, muscle spasms, nausea, and bruising. Grade 3 or higher adverse events occurring in at least 10% of patients included pneumonia, fatigue, and diarrhea.
Serious adverse events occurred in 52% of patients. Grade 3 or higher serious adverse events occurred in 40% of patients and included pneumonia, septic shock, and pyrexia. Two fatal events were reported and included one case of multilobular pneumonia and one case of bronchopulmonary aspergillosis.
Twelve patients (29%) remained on ibrutinib at 14 months; Of those who discontinued therapy, 5 discontinued because of progressive cGVHD, and 14 because of adverse events.
Patients who have cGVHD and don’t respond to frontline therapy have previously had no effective options. Ibrutinib showed promise in preclinical models; it reduced the severity of cGVHD through inhibition of Bruton’s tyrosine kinase and interleukin-2–inducible T-cell kinase, Dr. Miklos explained, noting that both B and T cells play a role in the pathophysiology of cGVHD.
The findings from this phase II trial demonstrate that ibrutinib does indeed lead to durable improvement in this patient population, and its safety profile is consistent with that previously reported for B-cell malignancies treated with ibrutinib and for cGVHD patients treated with concomitant steroids, he said.
“We think the efficacy of ibrutinib in this population supports further study in frontline treatment of cGVHD in a randomized, double-blinded study,” he concluded.
A phase III study – the INTEGRATE clinical trial – is now open. The international study will compare ibrutinib and prednisone with placebo and prednisone as a frontline therapy for moderate and severe cGVHD with a primary endpoint of response rate at 24 weeks.
The study was sponsored by Pharmacyclics in collaboration with Janssen Research & Development. Dr. Miklos reported various financial relationships with Pharmacyclics (the maker of ibrutinib [Imbruvica]), Velos, Kite Pharma, Sanofi Oncology, Adaptive Biotechnologies, and Genentech.
sworcester@frontlinemedcom.com
AT THE 2017 BMT TANDEM MEETINGS
Key clinical point:
Major finding: At a median follow-up of 14 months, the overall response rate among 42 patients treated with ibrutinib for cGVHD was 67%, with a third of responders achieving a complete response.
Data source: A phase II study of 42 patients.
Disclosures: The study was sponsored by Pharmacyclics in collaboration with Janssen Research & Development. Dr. Miklos reported various financial relationships with Pharmacyclics (the maker of ibrutinib [Imbruvica]), Velos, Kite Pharma, Sanofi Oncology, Adaptive Biotechnologies, and Genentech.
Follistatin, endoglin predict postallogeneic HCT NRM
ORLANDO – A composite score based on day 28 plasma levels of the angiogenic factors follistatin and endoglin predicts 1-year nonrelapse mortality in patients who have undergone myeloablative allogeneic hematopoietic cell transplantation, based on findings from the randomized Blood and Marrow Transplant Clinical Trials Network acute graft-versus-host prophylaxis study 0402 (BMT CTN 0402).
Elevations in these factors at day 28 may reflect susceptibility to regimen-related and other toxicities that adversely affect tissue repair and survival, Shernan Holtan, MD, said at the combined annual meetings of the Center for International Blood & Marrow Transplant Research and the American Society for Blood and Marrow Transplantation.
“Without important trophic angiogenic factors, the body may not be able to heal well after injury such as graft-versus-host disease [GVHD]. We previously reported that such angiogenic factors are indeed altered at the onset of acute graft-versus-host disease based upon samples from BMT CTN 0302 and 0802,” said Dr. Holtan of the University of Minnesota, Minneapolis. “Specifically, we found that repair factors of epidermal growth factor and VEGF-A are low at the onset of acute GVHD, and that damage-associated angiogenic factors are high at the onset of acute graft-versus-host disease.”
These damage-associated factors include follistatin, endoglin, placental growth factor, and angiopoietin-2, she added.
Based on the previous results, Dr. Holtan and her colleagues hypothesized that a pattern of tissue damage as illustrated by these markers at 28 days after treatment would be associated with 1-year nonrelapse mortality.
Of 221 patients from BMT CTN 0402 with pretreatment and day 28 plasma samples available for analysis, 25 had died at 1 year of causes unrelated to relapse. In a univariate analysis, nonrelapse mortality was associated with levels of follistatin, endoglin, and angiopoietin-2. When combined to assess for an overall pattern of damage, only follistatin and endoglin were significantly associated with nonrelapse mortality.
The relative risk of death unrelated to relapse was 4.5-fold higher in patients with the highest score (score of 3) on multivariate regression analysis of follistatin and endoglin levels. Grade II-IV acute GVHD was not significantly associated with 1-year nonrelapse mortality in multivariate analyses, but age over 50 years was.
“Notably, the composite score was a better predictor than any factor alone,” Dr. Holtan said.
The composite score was also predictive of the development of acute GVHD.
“We found that a moderate score of 2 was associated with a 2.3-fold increased risk of acute GVHD prior to day 100. Interestingly, the higher score [3] was not associated with acute GVHD. There was no association of the composite score with chronic GVHD,” she said.
The risk of nonrelapse mortality was less than 10% in patients with a composite score of 1.
Among patients in the study with a score of 3, more than half of the deaths were related to organ toxicity, including liver failure and respiratory failure, which were predominantly infection-related. Those with a low composite score had very few deaths associated with organ toxicity, she noted.
While there are many unanswered questions, these findings highlight possible opportunities to improve survival after allogeneic hematopoietic cell transplantation that warrant further study, she said.
“We need to learn how to constrain this angiogenic inflammatory response with the ultimate goal of hopefully identifying novel treatment strategies to mitigate nonrelapse mortality in our patients,” she concluded.
Dr. Holtan is an investigator for Alexion and is site principal investigator on the GI GVHD clinical trial.
ORLANDO – A composite score based on day 28 plasma levels of the angiogenic factors follistatin and endoglin predicts 1-year nonrelapse mortality in patients who have undergone myeloablative allogeneic hematopoietic cell transplantation, based on findings from the randomized Blood and Marrow Transplant Clinical Trials Network acute graft-versus-host prophylaxis study 0402 (BMT CTN 0402).
Elevations in these factors at day 28 may reflect susceptibility to regimen-related and other toxicities that adversely affect tissue repair and survival, Shernan Holtan, MD, said at the combined annual meetings of the Center for International Blood & Marrow Transplant Research and the American Society for Blood and Marrow Transplantation.
“Without important trophic angiogenic factors, the body may not be able to heal well after injury such as graft-versus-host disease [GVHD]. We previously reported that such angiogenic factors are indeed altered at the onset of acute graft-versus-host disease based upon samples from BMT CTN 0302 and 0802,” said Dr. Holtan of the University of Minnesota, Minneapolis. “Specifically, we found that repair factors of epidermal growth factor and VEGF-A are low at the onset of acute GVHD, and that damage-associated angiogenic factors are high at the onset of acute graft-versus-host disease.”
These damage-associated factors include follistatin, endoglin, placental growth factor, and angiopoietin-2, she added.
Based on the previous results, Dr. Holtan and her colleagues hypothesized that a pattern of tissue damage as illustrated by these markers at 28 days after treatment would be associated with 1-year nonrelapse mortality.
Of 221 patients from BMT CTN 0402 with pretreatment and day 28 plasma samples available for analysis, 25 had died at 1 year of causes unrelated to relapse. In a univariate analysis, nonrelapse mortality was associated with levels of follistatin, endoglin, and angiopoietin-2. When combined to assess for an overall pattern of damage, only follistatin and endoglin were significantly associated with nonrelapse mortality.
The relative risk of death unrelated to relapse was 4.5-fold higher in patients with the highest score (score of 3) on multivariate regression analysis of follistatin and endoglin levels. Grade II-IV acute GVHD was not significantly associated with 1-year nonrelapse mortality in multivariate analyses, but age over 50 years was.
“Notably, the composite score was a better predictor than any factor alone,” Dr. Holtan said.
The composite score was also predictive of the development of acute GVHD.
“We found that a moderate score of 2 was associated with a 2.3-fold increased risk of acute GVHD prior to day 100. Interestingly, the higher score [3] was not associated with acute GVHD. There was no association of the composite score with chronic GVHD,” she said.
The risk of nonrelapse mortality was less than 10% in patients with a composite score of 1.
Among patients in the study with a score of 3, more than half of the deaths were related to organ toxicity, including liver failure and respiratory failure, which were predominantly infection-related. Those with a low composite score had very few deaths associated with organ toxicity, she noted.
While there are many unanswered questions, these findings highlight possible opportunities to improve survival after allogeneic hematopoietic cell transplantation that warrant further study, she said.
“We need to learn how to constrain this angiogenic inflammatory response with the ultimate goal of hopefully identifying novel treatment strategies to mitigate nonrelapse mortality in our patients,” she concluded.
Dr. Holtan is an investigator for Alexion and is site principal investigator on the GI GVHD clinical trial.
ORLANDO – A composite score based on day 28 plasma levels of the angiogenic factors follistatin and endoglin predicts 1-year nonrelapse mortality in patients who have undergone myeloablative allogeneic hematopoietic cell transplantation, based on findings from the randomized Blood and Marrow Transplant Clinical Trials Network acute graft-versus-host prophylaxis study 0402 (BMT CTN 0402).
Elevations in these factors at day 28 may reflect susceptibility to regimen-related and other toxicities that adversely affect tissue repair and survival, Shernan Holtan, MD, said at the combined annual meetings of the Center for International Blood & Marrow Transplant Research and the American Society for Blood and Marrow Transplantation.
“Without important trophic angiogenic factors, the body may not be able to heal well after injury such as graft-versus-host disease [GVHD]. We previously reported that such angiogenic factors are indeed altered at the onset of acute graft-versus-host disease based upon samples from BMT CTN 0302 and 0802,” said Dr. Holtan of the University of Minnesota, Minneapolis. “Specifically, we found that repair factors of epidermal growth factor and VEGF-A are low at the onset of acute GVHD, and that damage-associated angiogenic factors are high at the onset of acute graft-versus-host disease.”
These damage-associated factors include follistatin, endoglin, placental growth factor, and angiopoietin-2, she added.
Based on the previous results, Dr. Holtan and her colleagues hypothesized that a pattern of tissue damage as illustrated by these markers at 28 days after treatment would be associated with 1-year nonrelapse mortality.
Of 221 patients from BMT CTN 0402 with pretreatment and day 28 plasma samples available for analysis, 25 had died at 1 year of causes unrelated to relapse. In a univariate analysis, nonrelapse mortality was associated with levels of follistatin, endoglin, and angiopoietin-2. When combined to assess for an overall pattern of damage, only follistatin and endoglin were significantly associated with nonrelapse mortality.
The relative risk of death unrelated to relapse was 4.5-fold higher in patients with the highest score (score of 3) on multivariate regression analysis of follistatin and endoglin levels. Grade II-IV acute GVHD was not significantly associated with 1-year nonrelapse mortality in multivariate analyses, but age over 50 years was.
“Notably, the composite score was a better predictor than any factor alone,” Dr. Holtan said.
The composite score was also predictive of the development of acute GVHD.
“We found that a moderate score of 2 was associated with a 2.3-fold increased risk of acute GVHD prior to day 100. Interestingly, the higher score [3] was not associated with acute GVHD. There was no association of the composite score with chronic GVHD,” she said.
The risk of nonrelapse mortality was less than 10% in patients with a composite score of 1.
Among patients in the study with a score of 3, more than half of the deaths were related to organ toxicity, including liver failure and respiratory failure, which were predominantly infection-related. Those with a low composite score had very few deaths associated with organ toxicity, she noted.
While there are many unanswered questions, these findings highlight possible opportunities to improve survival after allogeneic hematopoietic cell transplantation that warrant further study, she said.
“We need to learn how to constrain this angiogenic inflammatory response with the ultimate goal of hopefully identifying novel treatment strategies to mitigate nonrelapse mortality in our patients,” she concluded.
Dr. Holtan is an investigator for Alexion and is site principal investigator on the GI GVHD clinical trial.
AT THE 2017 BMT TANDEM MEETINGS
Key clinical point:
Major finding: Patients with a composite score of 3 had a 4.5-fold higher relative risk of nonrelapse mortality.
Data source: The randomized BMT CTN 0402 study of 221 patients.
Disclosures: Dr. Holtan is an investigator for Alexion and is site principal investigator on the GI GVHD clinical trial.
FIRST trial: Education unaffected by flexible duty hours
Flexible duty hour policies did not affect general surgery resident performance on examinations during the prospective cluster-randomized FIRST (Flexibility in Duty Hour Requirements for Surgical Trainees) trial.
However, more years under such policies may be required to observe an effect, Eddie Blay Jr., MD, of Northwestern University, Chicago, and his colleagues reported in the Journal of the American College of Surgeons.
Further, pass rates for the QE were 90.5% and 90.4%, and for the CE were 88.6% and 86.3%, respectively, they noted.
Residents from 117 programs participated in the FIRST trial. Those in the standard policy group had daily duty hour limits as outlined by current Accreditation Council for Graduate Medical Education requirements, with a limit of 16 hours daily for interns and 28 hours for senior residents, and with at least 8 hours between daily shifts and 14 hours off after a 24-hour call. Those in the flexible policy group were exempt from these limits by a formal waiver.
The findings suggest that flexible duty hour policies do not result in significantly different educational outcomes but are limited by concerns about the generalizability of the results, and by a focus on limited aspects of surgeon training and performance, the investigators said.
Measuring actual surgeon education and training quality is challenging and might not be possible with the ABSITE, QE, and CE, they added, noting that future FIRST trial analyses will examine the impact of multiple years of flexible duty hour policies.
The FIRST trial was funded by the American Board of Surgery, the American College of Surgeons, and the ACGME. Stipends for individual authors were supported by a National Institutes of Health grant. The authors reported having no other disclosures.
Flexible duty hour policies did not affect general surgery resident performance on examinations during the prospective cluster-randomized FIRST (Flexibility in Duty Hour Requirements for Surgical Trainees) trial.
However, more years under such policies may be required to observe an effect, Eddie Blay Jr., MD, of Northwestern University, Chicago, and his colleagues reported in the Journal of the American College of Surgeons.
Further, pass rates for the QE were 90.5% and 90.4%, and for the CE were 88.6% and 86.3%, respectively, they noted.
Residents from 117 programs participated in the FIRST trial. Those in the standard policy group had daily duty hour limits as outlined by current Accreditation Council for Graduate Medical Education requirements, with a limit of 16 hours daily for interns and 28 hours for senior residents, and with at least 8 hours between daily shifts and 14 hours off after a 24-hour call. Those in the flexible policy group were exempt from these limits by a formal waiver.
The findings suggest that flexible duty hour policies do not result in significantly different educational outcomes but are limited by concerns about the generalizability of the results, and by a focus on limited aspects of surgeon training and performance, the investigators said.
Measuring actual surgeon education and training quality is challenging and might not be possible with the ABSITE, QE, and CE, they added, noting that future FIRST trial analyses will examine the impact of multiple years of flexible duty hour policies.
The FIRST trial was funded by the American Board of Surgery, the American College of Surgeons, and the ACGME. Stipends for individual authors were supported by a National Institutes of Health grant. The authors reported having no other disclosures.
Flexible duty hour policies did not affect general surgery resident performance on examinations during the prospective cluster-randomized FIRST (Flexibility in Duty Hour Requirements for Surgical Trainees) trial.
However, more years under such policies may be required to observe an effect, Eddie Blay Jr., MD, of Northwestern University, Chicago, and his colleagues reported in the Journal of the American College of Surgeons.
Further, pass rates for the QE were 90.5% and 90.4%, and for the CE were 88.6% and 86.3%, respectively, they noted.
Residents from 117 programs participated in the FIRST trial. Those in the standard policy group had daily duty hour limits as outlined by current Accreditation Council for Graduate Medical Education requirements, with a limit of 16 hours daily for interns and 28 hours for senior residents, and with at least 8 hours between daily shifts and 14 hours off after a 24-hour call. Those in the flexible policy group were exempt from these limits by a formal waiver.
The findings suggest that flexible duty hour policies do not result in significantly different educational outcomes but are limited by concerns about the generalizability of the results, and by a focus on limited aspects of surgeon training and performance, the investigators said.
Measuring actual surgeon education and training quality is challenging and might not be possible with the ABSITE, QE, and CE, they added, noting that future FIRST trial analyses will examine the impact of multiple years of flexible duty hour policies.
The FIRST trial was funded by the American Board of Surgery, the American College of Surgeons, and the ACGME. Stipends for individual authors were supported by a National Institutes of Health grant. The authors reported having no other disclosures.
FROM JOURNAL OF THE AMERICAN COLLEGE OF SURGEONS
Key clinical point:
Major finding: Mean overall scores on the ABSITE in 2016 were 502.7 and 502.6 in the standard policy and flexible policy groups, respectively.
Data source: The prospective cluster-randomized FIRST trial of more than 4,300 residents.
Disclosures: The FIRST trial was funded by the American Board of Surgery, the American College of Surgeons, and the ACGME. Stipends for individual authors were supported by a National Institutes of Health grant. The authors reported having no other disclosures.
Phase III trial: VZV protects auto-HCT patients
ORLANDO – An inactivated varicella zoster virus vaccine currently in development for adult patients undergoing autologous hematopoietic stem cell transplantation is efficacious and well tolerated, according to findings from a randomized, placebo-controlled, phase III trial.
During the course of the 2 1/2-year pivotal multicenter trial, confirmed herpes zoster infections occurred in 42 of 560 patients who were randomized to receive inactivated varicella zoster virus vaccine (ZVIN) consistency lot (overall incidence of 32.8 cases/1,000 patient-years), compared with 113 of 564 patients who received placebo (overall incidence of 91.8/1,000 patient-years). The estimated vaccine efficacy was 63.8% after adjusting for age and duration of antiviral prophylaxis, Drew J. Winston, MD, reported at the combined annual meetings of the Center for International Blood & Marrow Transplant Research and the American Society for Blood and Marrow Transplantation.
The vaccine also was effective for reducing moderate and severe herpes zoster pain (estimated vaccine efficacy, 69.5%), for preventing postherpetic neuralgia (estimated vaccine efficacy, 83.7%), and for prevention of herpes zoster–related complications (estimated vaccine efficacy, 73.5%), he noted.
Study subjects were adults aged 18 years or older who were undergoing autologous hematopoietic stem cell transplantation (auto-HCT) for a malignancy or other indication. The most common underlying diseases were lymphoma and multiple myeloma. All patients had a history of varicella infection or were seropositive for varicella zoster virus (VZV) antibody, and had no history of VZV vaccine or herpes zoster infection within the prior year.
They were randomized to receive a four-dose regimen of either ZVIN consistency lot, ZVIN high-antigen lot, or placebo. A group of 106 patients who received the ZVIN high-antigen lot were included in the safety analysis only. The first ZVIN dose was administered about a month before transplantation, and doses two through four were administered about 30, 60, and 90 days after transplantation. About 90% in each group received antiviral agents after transplantation, and the duration of the use of antivirals also was similar in the groups. All patients were followed for the duration of the study, and those who developed herpes zoster were followed for 6 months after onset.
Herpes zoster cases were confirmed by polymerase chain reaction or by blinded endpoint committee adjudication.
Serious adverse events and vaccine-related serious adverse events occurred in a similar proportion of patients in the treatment and placebo groups (32.9% and 32.7%, and 0.8% and 0.9%, respectively). Vaccine-related events were primarily injection-site reactions. Systemic adverse events that occurred up to 28 days after vaccination were mainly gastrointestinal side effects, such as diarrhea, nausea, and vomiting. Pyrexia, oral mucositis, thrombocytopenia, and febrile neutropenia also were reported.
The most common serious adverse events were infectious complications, such as febrile neutropenia and relapse of underlying disease.
The findings are notable, as patients undergoing auto-HCT have an increased risk of developing herpes zoster infection and its complications, including postherpetic neuralgia, secondary bacterial infections, and disseminated VZV infection, as well as an increased risk of hospitalization and mortality, Dr. Winston explained.
Herpes zoster infections are associated primarily with cell-mediated immunity, and in older studies done prior to the routine use of antiviral prophylaxis, the reported incidence in auto-HCT patients was between 16% and 25%. Because of this high risk, current guidelines call for antiviral prophylaxis during auto-HCT, but even in this current era of acyclovir or valacyclovir prophylaxis, infections occur at relatively high rates after auto-HCT, he noted.
“Now another approach to prevention of herpes zoster infection is vaccination,” he said.
The live attenuated vaccine currently on the market is generally contraindicated in immunocompromised patients – at least in early period after transplantation, but ZVIN showed promise with respect to safety in earlier studies, which led to the current trial.
“This study demonstrated that the inactivated varicella vaccine is very effective for preventing herpes zoster after autologous stem cell transplantation,” Dr. Winston said, noting that efficacy was observed both in those younger than age 50 years and in those aged 50 and older, and also in those who received prophylaxis for less than 3 months and for 3-6 months.
“Finally!” said one audience member, who noted during a discussion of the findings that there has long been a need for a vaccine to prevent herpes zoster in auto-HCT patients.
Dr. Winston reported receiving research funding from Oxford, and serving as a consultant to Merck and Chimerix.
ORLANDO – An inactivated varicella zoster virus vaccine currently in development for adult patients undergoing autologous hematopoietic stem cell transplantation is efficacious and well tolerated, according to findings from a randomized, placebo-controlled, phase III trial.
During the course of the 2 1/2-year pivotal multicenter trial, confirmed herpes zoster infections occurred in 42 of 560 patients who were randomized to receive inactivated varicella zoster virus vaccine (ZVIN) consistency lot (overall incidence of 32.8 cases/1,000 patient-years), compared with 113 of 564 patients who received placebo (overall incidence of 91.8/1,000 patient-years). The estimated vaccine efficacy was 63.8% after adjusting for age and duration of antiviral prophylaxis, Drew J. Winston, MD, reported at the combined annual meetings of the Center for International Blood & Marrow Transplant Research and the American Society for Blood and Marrow Transplantation.
The vaccine also was effective for reducing moderate and severe herpes zoster pain (estimated vaccine efficacy, 69.5%), for preventing postherpetic neuralgia (estimated vaccine efficacy, 83.7%), and for prevention of herpes zoster–related complications (estimated vaccine efficacy, 73.5%), he noted.
Study subjects were adults aged 18 years or older who were undergoing autologous hematopoietic stem cell transplantation (auto-HCT) for a malignancy or other indication. The most common underlying diseases were lymphoma and multiple myeloma. All patients had a history of varicella infection or were seropositive for varicella zoster virus (VZV) antibody, and had no history of VZV vaccine or herpes zoster infection within the prior year.
They were randomized to receive a four-dose regimen of either ZVIN consistency lot, ZVIN high-antigen lot, or placebo. A group of 106 patients who received the ZVIN high-antigen lot were included in the safety analysis only. The first ZVIN dose was administered about a month before transplantation, and doses two through four were administered about 30, 60, and 90 days after transplantation. About 90% in each group received antiviral agents after transplantation, and the duration of the use of antivirals also was similar in the groups. All patients were followed for the duration of the study, and those who developed herpes zoster were followed for 6 months after onset.
Herpes zoster cases were confirmed by polymerase chain reaction or by blinded endpoint committee adjudication.
Serious adverse events and vaccine-related serious adverse events occurred in a similar proportion of patients in the treatment and placebo groups (32.9% and 32.7%, and 0.8% and 0.9%, respectively). Vaccine-related events were primarily injection-site reactions. Systemic adverse events that occurred up to 28 days after vaccination were mainly gastrointestinal side effects, such as diarrhea, nausea, and vomiting. Pyrexia, oral mucositis, thrombocytopenia, and febrile neutropenia also were reported.
The most common serious adverse events were infectious complications, such as febrile neutropenia and relapse of underlying disease.
The findings are notable, as patients undergoing auto-HCT have an increased risk of developing herpes zoster infection and its complications, including postherpetic neuralgia, secondary bacterial infections, and disseminated VZV infection, as well as an increased risk of hospitalization and mortality, Dr. Winston explained.
Herpes zoster infections are associated primarily with cell-mediated immunity, and in older studies done prior to the routine use of antiviral prophylaxis, the reported incidence in auto-HCT patients was between 16% and 25%. Because of this high risk, current guidelines call for antiviral prophylaxis during auto-HCT, but even in this current era of acyclovir or valacyclovir prophylaxis, infections occur at relatively high rates after auto-HCT, he noted.
“Now another approach to prevention of herpes zoster infection is vaccination,” he said.
The live attenuated vaccine currently on the market is generally contraindicated in immunocompromised patients – at least in early period after transplantation, but ZVIN showed promise with respect to safety in earlier studies, which led to the current trial.
“This study demonstrated that the inactivated varicella vaccine is very effective for preventing herpes zoster after autologous stem cell transplantation,” Dr. Winston said, noting that efficacy was observed both in those younger than age 50 years and in those aged 50 and older, and also in those who received prophylaxis for less than 3 months and for 3-6 months.
“Finally!” said one audience member, who noted during a discussion of the findings that there has long been a need for a vaccine to prevent herpes zoster in auto-HCT patients.
Dr. Winston reported receiving research funding from Oxford, and serving as a consultant to Merck and Chimerix.
ORLANDO – An inactivated varicella zoster virus vaccine currently in development for adult patients undergoing autologous hematopoietic stem cell transplantation is efficacious and well tolerated, according to findings from a randomized, placebo-controlled, phase III trial.
During the course of the 2 1/2-year pivotal multicenter trial, confirmed herpes zoster infections occurred in 42 of 560 patients who were randomized to receive inactivated varicella zoster virus vaccine (ZVIN) consistency lot (overall incidence of 32.8 cases/1,000 patient-years), compared with 113 of 564 patients who received placebo (overall incidence of 91.8/1,000 patient-years). The estimated vaccine efficacy was 63.8% after adjusting for age and duration of antiviral prophylaxis, Drew J. Winston, MD, reported at the combined annual meetings of the Center for International Blood & Marrow Transplant Research and the American Society for Blood and Marrow Transplantation.
The vaccine also was effective for reducing moderate and severe herpes zoster pain (estimated vaccine efficacy, 69.5%), for preventing postherpetic neuralgia (estimated vaccine efficacy, 83.7%), and for prevention of herpes zoster–related complications (estimated vaccine efficacy, 73.5%), he noted.
Study subjects were adults aged 18 years or older who were undergoing autologous hematopoietic stem cell transplantation (auto-HCT) for a malignancy or other indication. The most common underlying diseases were lymphoma and multiple myeloma. All patients had a history of varicella infection or were seropositive for varicella zoster virus (VZV) antibody, and had no history of VZV vaccine or herpes zoster infection within the prior year.
They were randomized to receive a four-dose regimen of either ZVIN consistency lot, ZVIN high-antigen lot, or placebo. A group of 106 patients who received the ZVIN high-antigen lot were included in the safety analysis only. The first ZVIN dose was administered about a month before transplantation, and doses two through four were administered about 30, 60, and 90 days after transplantation. About 90% in each group received antiviral agents after transplantation, and the duration of the use of antivirals also was similar in the groups. All patients were followed for the duration of the study, and those who developed herpes zoster were followed for 6 months after onset.
Herpes zoster cases were confirmed by polymerase chain reaction or by blinded endpoint committee adjudication.
Serious adverse events and vaccine-related serious adverse events occurred in a similar proportion of patients in the treatment and placebo groups (32.9% and 32.7%, and 0.8% and 0.9%, respectively). Vaccine-related events were primarily injection-site reactions. Systemic adverse events that occurred up to 28 days after vaccination were mainly gastrointestinal side effects, such as diarrhea, nausea, and vomiting. Pyrexia, oral mucositis, thrombocytopenia, and febrile neutropenia also were reported.
The most common serious adverse events were infectious complications, such as febrile neutropenia and relapse of underlying disease.
The findings are notable, as patients undergoing auto-HCT have an increased risk of developing herpes zoster infection and its complications, including postherpetic neuralgia, secondary bacterial infections, and disseminated VZV infection, as well as an increased risk of hospitalization and mortality, Dr. Winston explained.
Herpes zoster infections are associated primarily with cell-mediated immunity, and in older studies done prior to the routine use of antiviral prophylaxis, the reported incidence in auto-HCT patients was between 16% and 25%. Because of this high risk, current guidelines call for antiviral prophylaxis during auto-HCT, but even in this current era of acyclovir or valacyclovir prophylaxis, infections occur at relatively high rates after auto-HCT, he noted.
“Now another approach to prevention of herpes zoster infection is vaccination,” he said.
The live attenuated vaccine currently on the market is generally contraindicated in immunocompromised patients – at least in early period after transplantation, but ZVIN showed promise with respect to safety in earlier studies, which led to the current trial.
“This study demonstrated that the inactivated varicella vaccine is very effective for preventing herpes zoster after autologous stem cell transplantation,” Dr. Winston said, noting that efficacy was observed both in those younger than age 50 years and in those aged 50 and older, and also in those who received prophylaxis for less than 3 months and for 3-6 months.
“Finally!” said one audience member, who noted during a discussion of the findings that there has long been a need for a vaccine to prevent herpes zoster in auto-HCT patients.
Dr. Winston reported receiving research funding from Oxford, and serving as a consultant to Merck and Chimerix.
AT THE 2017 BMT TANDEM MEETINGS
Key clinical point:
Major finding: Overall incidence of herpes zoster was 32.8 cases/1,000 patient-years vs. 91.8/1,000 patient-years in patients in the vaccine and placebo groups, respectively.
Data source: A randomized, placebo-controlled phase III trial involving 1,230 patients.
Disclosures: Dr. Winston reported receiving research funding from Oxford, and serving as a consultant to Merck and Chimerix.