User login
Sharon Worcester is an award-winning medical journalist for MDedge News. She has been with the company since 1996, first as the Southeast Bureau Chief (1996-2009) when the company was known as International Medical News Group, then as a freelance writer (2010-2015) before returning as a reporter in 2015. She previously worked as a daily newspaper reporter covering health and local government. Sharon currently reports primarily on oncology and hematology. She has a BA from Eckerd College and an MA in Mass Communication/Print Journalism from the University of Florida. Connect with her via LinkedIn and follow her on twitter @SW_MedReporter.
Survey highlights interest in diet’s effects on RA
Nearly one-quarter of patients with long-standing rheumatoid arthritis who participated in a recent survey reported that their diets affect their RA symptoms.
Of 217 participants with a median disease duration of 17 years, 52 (24%) reported that certain foods either improve or worsen symptoms. Foods most commonly associated with improved symptoms were blueberries (11.1%), fish (10.9%), and spinach; foods most commonly associated with exacerbated symptoms were desserts (12.7%) and soda with sugar (12.4%, ), Sara K. Tedeschi, MD, of Brigham and Women’s Hospital, Boston, and her colleagues reported online in Arthritis Care & Research.
Participants came from a single-center RA registry (the Brigham RA Sequential Study, or BRASS) at a large academic center and were surveyed between May 2015 and December 2015. They were asked about the effects of 20 different foods that have been popularized as “inflammatory” or “anti-inflammatory” and about the effects of four lifestyle/environment factors. Most (83%) were women, and 58% were using a biologic disease-modifying antirheumatic drug.
The findings indicate that there is substantial patient interest in the effects of diet on RA symptoms and highlight the need for prospective studies on the topic, the investigators concluded. While strong conclusions cannot be drawn based on this survey, further study regarding a potential link between sugar consumption and inflammation is warranted.
Dr. Tedeschi’s work on this project was supported by the National Institutes of Health. The Brigham RA Sequential Study received funding from UCB, Crescendo Biosciences, Bristol-Myers Squibb, Amgen, and DxTerity.
Nearly one-quarter of patients with long-standing rheumatoid arthritis who participated in a recent survey reported that their diets affect their RA symptoms.
Of 217 participants with a median disease duration of 17 years, 52 (24%) reported that certain foods either improve or worsen symptoms. Foods most commonly associated with improved symptoms were blueberries (11.1%), fish (10.9%), and spinach; foods most commonly associated with exacerbated symptoms were desserts (12.7%) and soda with sugar (12.4%, ), Sara K. Tedeschi, MD, of Brigham and Women’s Hospital, Boston, and her colleagues reported online in Arthritis Care & Research.
Participants came from a single-center RA registry (the Brigham RA Sequential Study, or BRASS) at a large academic center and were surveyed between May 2015 and December 2015. They were asked about the effects of 20 different foods that have been popularized as “inflammatory” or “anti-inflammatory” and about the effects of four lifestyle/environment factors. Most (83%) were women, and 58% were using a biologic disease-modifying antirheumatic drug.
The findings indicate that there is substantial patient interest in the effects of diet on RA symptoms and highlight the need for prospective studies on the topic, the investigators concluded. While strong conclusions cannot be drawn based on this survey, further study regarding a potential link between sugar consumption and inflammation is warranted.
Dr. Tedeschi’s work on this project was supported by the National Institutes of Health. The Brigham RA Sequential Study received funding from UCB, Crescendo Biosciences, Bristol-Myers Squibb, Amgen, and DxTerity.
Nearly one-quarter of patients with long-standing rheumatoid arthritis who participated in a recent survey reported that their diets affect their RA symptoms.
Of 217 participants with a median disease duration of 17 years, 52 (24%) reported that certain foods either improve or worsen symptoms. Foods most commonly associated with improved symptoms were blueberries (11.1%), fish (10.9%), and spinach; foods most commonly associated with exacerbated symptoms were desserts (12.7%) and soda with sugar (12.4%, ), Sara K. Tedeschi, MD, of Brigham and Women’s Hospital, Boston, and her colleagues reported online in Arthritis Care & Research.
Participants came from a single-center RA registry (the Brigham RA Sequential Study, or BRASS) at a large academic center and were surveyed between May 2015 and December 2015. They were asked about the effects of 20 different foods that have been popularized as “inflammatory” or “anti-inflammatory” and about the effects of four lifestyle/environment factors. Most (83%) were women, and 58% were using a biologic disease-modifying antirheumatic drug.
The findings indicate that there is substantial patient interest in the effects of diet on RA symptoms and highlight the need for prospective studies on the topic, the investigators concluded. While strong conclusions cannot be drawn based on this survey, further study regarding a potential link between sugar consumption and inflammation is warranted.
Dr. Tedeschi’s work on this project was supported by the National Institutes of Health. The Brigham RA Sequential Study received funding from UCB, Crescendo Biosciences, Bristol-Myers Squibb, Amgen, and DxTerity.
FROM ARTHRITIS CARE & RESEARCH
Key clinical point:
Major finding: 24% of respondents reported that diet affects RA symptoms.
Data source: A survey of 217 participants in the Brigham RA Sequential Study.
Disclosures: Dr. Tedeschi’s work on this project was supported by the National Institutes of Health. The Brigham RA Sequential Study received funding from UCB, Crescendo Biosciences, Bristol-Myers Squibb, Amgen, and DxTerity.
Multiple myeloma: Lenalidomide approved as maintenance therapy after auto-HSCT
The Food and Drug Administration has approved the use of lenalidomide (Revlimid) for maintenance therapy following autologous hematopoietic stem cell transplant in patients with multiple myeloma.
The expanded indication, announced Feb. 22, makes the immunomodulatory agent the first and only approved treatment for post autologous hematopoietic stem cell transplant (auto-HSCT) maintenance. It was initially approved in 2006 for use in combination with dexamethasone in patients with multiple myeloma who have received at least one prior therapy, and that indication was expanded in 2015 to include those with newly diagnosed multiple myeloma.
According to Celgene, the maker of Revlimid, the latest approval was based on data showing that lenalidomide maintenance therapy delays disease progression following auto-HSCT. Updated phase III randomized controlled trial data from two studies including more than 1,000 patients demonstrated median progression-free survival (PFS) advantages with lenalidomide maintenance vs. no maintenance. In one study – the U.S.-based CALGB 1001014 – median PFS was 5.7 vs. 1.9 years for a difference of 3.8 years (hazard ratio, 0.38). In the second study – the European IFM 2005-02 – median PFS was 3.9 vs. 2 years, for a difference of 1.9 years (HR, 0.53).
In both studies lenalidomide was given as a 10-mg daily oral dose (increased to 15 mg daily after 3 months if tolerated) until disease progression or unacceptable toxicity after auto-HSCT.
Lenalidomide, a derivative of thalidomide, can cause fetal harm and is contraindicated in women who are pregnant. It is available only through a restricted distribution program.
The most frequently reported adverse reactions in the two studies were neutropenia, thrombocytopenia, leukopenia, anemia, upper respiratory tract infection, bronchitis, nasopharyngitis, cough, gastroenteritis, diarrhea, rash, fatigue, muscle spasm, and pyrexia. The most frequently reported grade 3 or 4 reactions occurring in more than 20% of patients in the lenalidomide arms included neutropenia, thrombocytopenia, and leukopenia.
“Autologous stem cell transplant after induction therapy is part of the continuum of care for transplant-eligible multiple myeloma patients. However, most patients will still see their disease recur or progress after this treatment,” Philip McCarthy, MD, of the Roswell Park Cancer Institute in Buffalo, N.Y., said in a Celgene press statement. “Lenalidomide maintenance therapy ... can be considered a standard of care for these patients.”
The Food and Drug Administration has approved the use of lenalidomide (Revlimid) for maintenance therapy following autologous hematopoietic stem cell transplant in patients with multiple myeloma.
The expanded indication, announced Feb. 22, makes the immunomodulatory agent the first and only approved treatment for post autologous hematopoietic stem cell transplant (auto-HSCT) maintenance. It was initially approved in 2006 for use in combination with dexamethasone in patients with multiple myeloma who have received at least one prior therapy, and that indication was expanded in 2015 to include those with newly diagnosed multiple myeloma.
According to Celgene, the maker of Revlimid, the latest approval was based on data showing that lenalidomide maintenance therapy delays disease progression following auto-HSCT. Updated phase III randomized controlled trial data from two studies including more than 1,000 patients demonstrated median progression-free survival (PFS) advantages with lenalidomide maintenance vs. no maintenance. In one study – the U.S.-based CALGB 1001014 – median PFS was 5.7 vs. 1.9 years for a difference of 3.8 years (hazard ratio, 0.38). In the second study – the European IFM 2005-02 – median PFS was 3.9 vs. 2 years, for a difference of 1.9 years (HR, 0.53).
In both studies lenalidomide was given as a 10-mg daily oral dose (increased to 15 mg daily after 3 months if tolerated) until disease progression or unacceptable toxicity after auto-HSCT.
Lenalidomide, a derivative of thalidomide, can cause fetal harm and is contraindicated in women who are pregnant. It is available only through a restricted distribution program.
The most frequently reported adverse reactions in the two studies were neutropenia, thrombocytopenia, leukopenia, anemia, upper respiratory tract infection, bronchitis, nasopharyngitis, cough, gastroenteritis, diarrhea, rash, fatigue, muscle spasm, and pyrexia. The most frequently reported grade 3 or 4 reactions occurring in more than 20% of patients in the lenalidomide arms included neutropenia, thrombocytopenia, and leukopenia.
“Autologous stem cell transplant after induction therapy is part of the continuum of care for transplant-eligible multiple myeloma patients. However, most patients will still see their disease recur or progress after this treatment,” Philip McCarthy, MD, of the Roswell Park Cancer Institute in Buffalo, N.Y., said in a Celgene press statement. “Lenalidomide maintenance therapy ... can be considered a standard of care for these patients.”
The Food and Drug Administration has approved the use of lenalidomide (Revlimid) for maintenance therapy following autologous hematopoietic stem cell transplant in patients with multiple myeloma.
The expanded indication, announced Feb. 22, makes the immunomodulatory agent the first and only approved treatment for post autologous hematopoietic stem cell transplant (auto-HSCT) maintenance. It was initially approved in 2006 for use in combination with dexamethasone in patients with multiple myeloma who have received at least one prior therapy, and that indication was expanded in 2015 to include those with newly diagnosed multiple myeloma.
According to Celgene, the maker of Revlimid, the latest approval was based on data showing that lenalidomide maintenance therapy delays disease progression following auto-HSCT. Updated phase III randomized controlled trial data from two studies including more than 1,000 patients demonstrated median progression-free survival (PFS) advantages with lenalidomide maintenance vs. no maintenance. In one study – the U.S.-based CALGB 1001014 – median PFS was 5.7 vs. 1.9 years for a difference of 3.8 years (hazard ratio, 0.38). In the second study – the European IFM 2005-02 – median PFS was 3.9 vs. 2 years, for a difference of 1.9 years (HR, 0.53).
In both studies lenalidomide was given as a 10-mg daily oral dose (increased to 15 mg daily after 3 months if tolerated) until disease progression or unacceptable toxicity after auto-HSCT.
Lenalidomide, a derivative of thalidomide, can cause fetal harm and is contraindicated in women who are pregnant. It is available only through a restricted distribution program.
The most frequently reported adverse reactions in the two studies were neutropenia, thrombocytopenia, leukopenia, anemia, upper respiratory tract infection, bronchitis, nasopharyngitis, cough, gastroenteritis, diarrhea, rash, fatigue, muscle spasm, and pyrexia. The most frequently reported grade 3 or 4 reactions occurring in more than 20% of patients in the lenalidomide arms included neutropenia, thrombocytopenia, and leukopenia.
“Autologous stem cell transplant after induction therapy is part of the continuum of care for transplant-eligible multiple myeloma patients. However, most patients will still see their disease recur or progress after this treatment,” Philip McCarthy, MD, of the Roswell Park Cancer Institute in Buffalo, N.Y., said in a Celgene press statement. “Lenalidomide maintenance therapy ... can be considered a standard of care for these patients.”
Urgent colonoscopy for LGIB: Consider case by case
Colonoscopy performed within 24 hours of lower gastrointestinal bleeding appears safe and well tolerated but does not appear to improve a number of important clinical outcomes when compared with elective colonoscopy, according to the findings of a systematic review and meta-analysis.
Such “urgent colonoscopy” may, however, reduce hospital length of stay and cost, Abdul M. Kouanda, MD, of the University of California, San Francisco, and his colleagues reported online in Gastrointestinal Endoscopy.
In a pooled analysis of data from 12 studies with a total of 10,172 patients who underwent urgent colonoscopy, and 14,224 patients who underwent elective colonoscopy, the former was associated with increased use of endoscopic therapeutic interventions, compared with elective colonoscopy (relative risk, 1.70), but not with improved bleeding source localization (RR, 1.08), adverse event rates (RR, 1.05), rebleeding rates (RR, 1.14), transfusion requirements (RR, 1.02), or mortality (RR, 1.17), the investigators found (Gastrointest Endosc. 2017 Feb 4. doi: 10.1016/j.gie.2017.01.035).
The findings are based on nine studies from the United States, two from Japan, and one from Spain. Nine were retrospective cohort studies, two were randomized controlled trials, and one was a prospective cohort study.
With respect to the 70% greater use of therapeutic interventions with urgent colonoscopy, a subanalysis showed that the difference between urgent and elective colonoscopy was evident only in the randomized trials; no difference was seen in the prospective trials. With further stratification of urgent colonoscopy into procedures performed within 12 hours, the observation of increased therapeutic interventions was no longer statistically significant (RR, 3.46), they said.
As for bleeding source localization, the outcomes remained similar when retrospective studies were analyzed separately, and with colonoscopy performed within 12 hours. Blood transfusions decreased with urgent colonoscopy when only retrospective studies were analyzed (RR, 0.84).
The investigators noted that there was a trend toward decreased length of hospital stay among those undergoing urgent colonoscopy (mean of 4.8 days vs. 6.4 days with elective colonoscopy). Only two studies looked at cost: One showed a decrease in hospital costs with urgent vs. elective colonoscopy, while one showed no difference.
“In our pooled analysis, the mean hospital costs in the urgent colonoscopy group were $24,866, compared with $27,691 in the elective group; however, the difference between the two was not statistically significant,” they wrote.
The annual incidence of lower gastrointestinal bleeding (LGIB) in the United States is 20.5-35.7 out of 100,000 patients, and the incidence increases with age; there is a 200-fold increase in incidence from the 3rd to 9th decade of life, the investigators said, adding that the incidence is rising as the population ages.
“Such a trend has important implications for both the quality of care for treating LGIB and the associated costs to the overall U.S. health care system,” they wrote, noting that while colonoscopy is appropriate for evaluating LGIB in most cases, no clear consensus exists with respect to timing of colonoscopy.
Even a recent American Society for Gastrointestinal Endoscopy guideline recommending that initial colonoscopy for severe and hemodynamically stable hematochezia be performed within 8-24 hours of admission is based only on moderate-quality level evidence that is “fraught with a number of limitations,” they wrote.
The current study was designed to “further clarify the utility of urgent versus elective colonoscopy in evaluating patients hospitalized with a lower GI bleed,” they added.
The lack of clinical benefit seen in this study “may be secondary to the benign, often self-resolving natural history in the majority of LGIB cases. However, there may be a subset of patients who could benefit from early intervention (such as severe blood loss, hemodynamically unstable patients), and thus the decision to pursue urgent colonoscopy should be made on a case-by-case basis,” they said.
Further, although several critical patient outcomes did not appear to be impacted by urgent vs. elective colonoscopy in this study, the trends toward a decrease in length of stay suggest that earlier performance of colonoscopy may lead to earlier and better identification of low-risk and high-risk stigmata, allowing those with low-risk lesions to be discharged much earlier.
“Additionally, earlier discharge of patients could also reduce their risk of health care–associated infections and adverse events,” the investigators noted.
The findings with respect to length of stay and cost “align perfectly with the new focus in health care on providing high quality and safe care to patients while at the same time containing medical costs,” they wrote, adding that clinicians should carefully consider all factors when deciding to pursue urgent colonoscopy.”
The study is limited by heterogeneity and publications bias, and by factors inherent in meta-analyses, but it also has several strengths, including a large number of studies and patients. Also, it is the first of its kind to examine “all of the available literature to elucidate the time frame for performing colonoscopy in patients with hematochezia,” the investigators said, concluding that further research is needed to identify subsets of patients who will benefit from early intervention, to evaluate the cost effectiveness of urgent colonoscopy, and to look at – in larger randomized controlled trials – the overall benefit of urgent colonoscopy.
The authors reported having no disclosures.
Colonoscopy performed within 24 hours of lower gastrointestinal bleeding appears safe and well tolerated but does not appear to improve a number of important clinical outcomes when compared with elective colonoscopy, according to the findings of a systematic review and meta-analysis.
Such “urgent colonoscopy” may, however, reduce hospital length of stay and cost, Abdul M. Kouanda, MD, of the University of California, San Francisco, and his colleagues reported online in Gastrointestinal Endoscopy.
In a pooled analysis of data from 12 studies with a total of 10,172 patients who underwent urgent colonoscopy, and 14,224 patients who underwent elective colonoscopy, the former was associated with increased use of endoscopic therapeutic interventions, compared with elective colonoscopy (relative risk, 1.70), but not with improved bleeding source localization (RR, 1.08), adverse event rates (RR, 1.05), rebleeding rates (RR, 1.14), transfusion requirements (RR, 1.02), or mortality (RR, 1.17), the investigators found (Gastrointest Endosc. 2017 Feb 4. doi: 10.1016/j.gie.2017.01.035).
The findings are based on nine studies from the United States, two from Japan, and one from Spain. Nine were retrospective cohort studies, two were randomized controlled trials, and one was a prospective cohort study.
With respect to the 70% greater use of therapeutic interventions with urgent colonoscopy, a subanalysis showed that the difference between urgent and elective colonoscopy was evident only in the randomized trials; no difference was seen in the prospective trials. With further stratification of urgent colonoscopy into procedures performed within 12 hours, the observation of increased therapeutic interventions was no longer statistically significant (RR, 3.46), they said.
As for bleeding source localization, the outcomes remained similar when retrospective studies were analyzed separately, and with colonoscopy performed within 12 hours. Blood transfusions decreased with urgent colonoscopy when only retrospective studies were analyzed (RR, 0.84).
The investigators noted that there was a trend toward decreased length of hospital stay among those undergoing urgent colonoscopy (mean of 4.8 days vs. 6.4 days with elective colonoscopy). Only two studies looked at cost: One showed a decrease in hospital costs with urgent vs. elective colonoscopy, while one showed no difference.
“In our pooled analysis, the mean hospital costs in the urgent colonoscopy group were $24,866, compared with $27,691 in the elective group; however, the difference between the two was not statistically significant,” they wrote.
The annual incidence of lower gastrointestinal bleeding (LGIB) in the United States is 20.5-35.7 out of 100,000 patients, and the incidence increases with age; there is a 200-fold increase in incidence from the 3rd to 9th decade of life, the investigators said, adding that the incidence is rising as the population ages.
“Such a trend has important implications for both the quality of care for treating LGIB and the associated costs to the overall U.S. health care system,” they wrote, noting that while colonoscopy is appropriate for evaluating LGIB in most cases, no clear consensus exists with respect to timing of colonoscopy.
Even a recent American Society for Gastrointestinal Endoscopy guideline recommending that initial colonoscopy for severe and hemodynamically stable hematochezia be performed within 8-24 hours of admission is based only on moderate-quality level evidence that is “fraught with a number of limitations,” they wrote.
The current study was designed to “further clarify the utility of urgent versus elective colonoscopy in evaluating patients hospitalized with a lower GI bleed,” they added.
The lack of clinical benefit seen in this study “may be secondary to the benign, often self-resolving natural history in the majority of LGIB cases. However, there may be a subset of patients who could benefit from early intervention (such as severe blood loss, hemodynamically unstable patients), and thus the decision to pursue urgent colonoscopy should be made on a case-by-case basis,” they said.
Further, although several critical patient outcomes did not appear to be impacted by urgent vs. elective colonoscopy in this study, the trends toward a decrease in length of stay suggest that earlier performance of colonoscopy may lead to earlier and better identification of low-risk and high-risk stigmata, allowing those with low-risk lesions to be discharged much earlier.
“Additionally, earlier discharge of patients could also reduce their risk of health care–associated infections and adverse events,” the investigators noted.
The findings with respect to length of stay and cost “align perfectly with the new focus in health care on providing high quality and safe care to patients while at the same time containing medical costs,” they wrote, adding that clinicians should carefully consider all factors when deciding to pursue urgent colonoscopy.”
The study is limited by heterogeneity and publications bias, and by factors inherent in meta-analyses, but it also has several strengths, including a large number of studies and patients. Also, it is the first of its kind to examine “all of the available literature to elucidate the time frame for performing colonoscopy in patients with hematochezia,” the investigators said, concluding that further research is needed to identify subsets of patients who will benefit from early intervention, to evaluate the cost effectiveness of urgent colonoscopy, and to look at – in larger randomized controlled trials – the overall benefit of urgent colonoscopy.
The authors reported having no disclosures.
Colonoscopy performed within 24 hours of lower gastrointestinal bleeding appears safe and well tolerated but does not appear to improve a number of important clinical outcomes when compared with elective colonoscopy, according to the findings of a systematic review and meta-analysis.
Such “urgent colonoscopy” may, however, reduce hospital length of stay and cost, Abdul M. Kouanda, MD, of the University of California, San Francisco, and his colleagues reported online in Gastrointestinal Endoscopy.
In a pooled analysis of data from 12 studies with a total of 10,172 patients who underwent urgent colonoscopy, and 14,224 patients who underwent elective colonoscopy, the former was associated with increased use of endoscopic therapeutic interventions, compared with elective colonoscopy (relative risk, 1.70), but not with improved bleeding source localization (RR, 1.08), adverse event rates (RR, 1.05), rebleeding rates (RR, 1.14), transfusion requirements (RR, 1.02), or mortality (RR, 1.17), the investigators found (Gastrointest Endosc. 2017 Feb 4. doi: 10.1016/j.gie.2017.01.035).
The findings are based on nine studies from the United States, two from Japan, and one from Spain. Nine were retrospective cohort studies, two were randomized controlled trials, and one was a prospective cohort study.
With respect to the 70% greater use of therapeutic interventions with urgent colonoscopy, a subanalysis showed that the difference between urgent and elective colonoscopy was evident only in the randomized trials; no difference was seen in the prospective trials. With further stratification of urgent colonoscopy into procedures performed within 12 hours, the observation of increased therapeutic interventions was no longer statistically significant (RR, 3.46), they said.
As for bleeding source localization, the outcomes remained similar when retrospective studies were analyzed separately, and with colonoscopy performed within 12 hours. Blood transfusions decreased with urgent colonoscopy when only retrospective studies were analyzed (RR, 0.84).
The investigators noted that there was a trend toward decreased length of hospital stay among those undergoing urgent colonoscopy (mean of 4.8 days vs. 6.4 days with elective colonoscopy). Only two studies looked at cost: One showed a decrease in hospital costs with urgent vs. elective colonoscopy, while one showed no difference.
“In our pooled analysis, the mean hospital costs in the urgent colonoscopy group were $24,866, compared with $27,691 in the elective group; however, the difference between the two was not statistically significant,” they wrote.
The annual incidence of lower gastrointestinal bleeding (LGIB) in the United States is 20.5-35.7 out of 100,000 patients, and the incidence increases with age; there is a 200-fold increase in incidence from the 3rd to 9th decade of life, the investigators said, adding that the incidence is rising as the population ages.
“Such a trend has important implications for both the quality of care for treating LGIB and the associated costs to the overall U.S. health care system,” they wrote, noting that while colonoscopy is appropriate for evaluating LGIB in most cases, no clear consensus exists with respect to timing of colonoscopy.
Even a recent American Society for Gastrointestinal Endoscopy guideline recommending that initial colonoscopy for severe and hemodynamically stable hematochezia be performed within 8-24 hours of admission is based only on moderate-quality level evidence that is “fraught with a number of limitations,” they wrote.
The current study was designed to “further clarify the utility of urgent versus elective colonoscopy in evaluating patients hospitalized with a lower GI bleed,” they added.
The lack of clinical benefit seen in this study “may be secondary to the benign, often self-resolving natural history in the majority of LGIB cases. However, there may be a subset of patients who could benefit from early intervention (such as severe blood loss, hemodynamically unstable patients), and thus the decision to pursue urgent colonoscopy should be made on a case-by-case basis,” they said.
Further, although several critical patient outcomes did not appear to be impacted by urgent vs. elective colonoscopy in this study, the trends toward a decrease in length of stay suggest that earlier performance of colonoscopy may lead to earlier and better identification of low-risk and high-risk stigmata, allowing those with low-risk lesions to be discharged much earlier.
“Additionally, earlier discharge of patients could also reduce their risk of health care–associated infections and adverse events,” the investigators noted.
The findings with respect to length of stay and cost “align perfectly with the new focus in health care on providing high quality and safe care to patients while at the same time containing medical costs,” they wrote, adding that clinicians should carefully consider all factors when deciding to pursue urgent colonoscopy.”
The study is limited by heterogeneity and publications bias, and by factors inherent in meta-analyses, but it also has several strengths, including a large number of studies and patients. Also, it is the first of its kind to examine “all of the available literature to elucidate the time frame for performing colonoscopy in patients with hematochezia,” the investigators said, concluding that further research is needed to identify subsets of patients who will benefit from early intervention, to evaluate the cost effectiveness of urgent colonoscopy, and to look at – in larger randomized controlled trials – the overall benefit of urgent colonoscopy.
The authors reported having no disclosures.
FROM GASTROINTESTINAL ENDOSCOPY
Key clinical point:
Major finding: Urgent vs. elective colonoscopy was not associated with improved bleeding source localization (RR, 1.08), adverse event rates (RR, 1.05), rebleeding rates (RR, 1.14), transfusion requirements (RR, 1.02) or mortality (RR, 1.17).
Data source: A systematic review and meta-analysis of 12 studies including more than 24,000 patients.
Disclosures: The authors reported having no disclosures.
PADI2: A potential therapeutic target in multiple myeloma?
Researchers have identified a novel mechanism by which increased expression of peptidyl arginine deiminase 2 (PADI2) by bone marrow mesenchymal stem cells in patients with monoclonal gammopathy of undetermined significance (MGUS) and multiple myeloma leads directly to pro-malignancy signaling.
Specifically, increased PADI2 expression by bone marrow mesenchymal stem cells in patients with MGUS (a benign condition that precedes multiple myeloma) or with multiple myeloma directly induces upregulation of interleukin-6 expression through its enzymatic deimination of histone H3 arginine 26, which leads to acquired resistance to bortezomib – a “highly clinically relevant anti-myeloma drug”– by malignant plasma cells, Gavin McNee, PhD, of the University of Birmingham, England, and colleagues reported (Leukemia. 2017;31[2]:373-81).
The findings, based on transcriptomic analysis of mRNA extracted from bone marrow mesenchymal stem cells cultured from MGUS and multiple myeloma patients and controls, could have implications for therapeutic targeting of PADI2 in MGUS and multiple myeloma, the investigators said.
The findings, in the context of those from prior studies, “highlight the significant similarities between the microenvironment of the bone marrow in MGUS and multiple myeloma, suggesting that transformation of the bone marrow microenvironment is an early event in disease etiology, raising the potential of interfering with this process in order to delay or prevent progression of patients with MGUS to multiple myeloma,” they wrote, adding that the data “further highlight a need to identify those biological determinants in the [bone marrow] that actually contribute to the progression of this disease.”
The investigators concluded that PADI2 may therefore represent a good therapeutic target in MGUS and multiple myeloma patients, which “may act by removing a significant proportion of the supportive signaling required by malignant plasma cells for survival and proliferation.”
The authors were supported by grants from Bloodwise, Cancer Research UK, and the Medical Research Council, They reported having no other disclosures.
Researchers have identified a novel mechanism by which increased expression of peptidyl arginine deiminase 2 (PADI2) by bone marrow mesenchymal stem cells in patients with monoclonal gammopathy of undetermined significance (MGUS) and multiple myeloma leads directly to pro-malignancy signaling.
Specifically, increased PADI2 expression by bone marrow mesenchymal stem cells in patients with MGUS (a benign condition that precedes multiple myeloma) or with multiple myeloma directly induces upregulation of interleukin-6 expression through its enzymatic deimination of histone H3 arginine 26, which leads to acquired resistance to bortezomib – a “highly clinically relevant anti-myeloma drug”– by malignant plasma cells, Gavin McNee, PhD, of the University of Birmingham, England, and colleagues reported (Leukemia. 2017;31[2]:373-81).
The findings, based on transcriptomic analysis of mRNA extracted from bone marrow mesenchymal stem cells cultured from MGUS and multiple myeloma patients and controls, could have implications for therapeutic targeting of PADI2 in MGUS and multiple myeloma, the investigators said.
The findings, in the context of those from prior studies, “highlight the significant similarities between the microenvironment of the bone marrow in MGUS and multiple myeloma, suggesting that transformation of the bone marrow microenvironment is an early event in disease etiology, raising the potential of interfering with this process in order to delay or prevent progression of patients with MGUS to multiple myeloma,” they wrote, adding that the data “further highlight a need to identify those biological determinants in the [bone marrow] that actually contribute to the progression of this disease.”
The investigators concluded that PADI2 may therefore represent a good therapeutic target in MGUS and multiple myeloma patients, which “may act by removing a significant proportion of the supportive signaling required by malignant plasma cells for survival and proliferation.”
The authors were supported by grants from Bloodwise, Cancer Research UK, and the Medical Research Council, They reported having no other disclosures.
Researchers have identified a novel mechanism by which increased expression of peptidyl arginine deiminase 2 (PADI2) by bone marrow mesenchymal stem cells in patients with monoclonal gammopathy of undetermined significance (MGUS) and multiple myeloma leads directly to pro-malignancy signaling.
Specifically, increased PADI2 expression by bone marrow mesenchymal stem cells in patients with MGUS (a benign condition that precedes multiple myeloma) or with multiple myeloma directly induces upregulation of interleukin-6 expression through its enzymatic deimination of histone H3 arginine 26, which leads to acquired resistance to bortezomib – a “highly clinically relevant anti-myeloma drug”– by malignant plasma cells, Gavin McNee, PhD, of the University of Birmingham, England, and colleagues reported (Leukemia. 2017;31[2]:373-81).
The findings, based on transcriptomic analysis of mRNA extracted from bone marrow mesenchymal stem cells cultured from MGUS and multiple myeloma patients and controls, could have implications for therapeutic targeting of PADI2 in MGUS and multiple myeloma, the investigators said.
The findings, in the context of those from prior studies, “highlight the significant similarities between the microenvironment of the bone marrow in MGUS and multiple myeloma, suggesting that transformation of the bone marrow microenvironment is an early event in disease etiology, raising the potential of interfering with this process in order to delay or prevent progression of patients with MGUS to multiple myeloma,” they wrote, adding that the data “further highlight a need to identify those biological determinants in the [bone marrow] that actually contribute to the progression of this disease.”
The investigators concluded that PADI2 may therefore represent a good therapeutic target in MGUS and multiple myeloma patients, which “may act by removing a significant proportion of the supportive signaling required by malignant plasma cells for survival and proliferation.”
The authors were supported by grants from Bloodwise, Cancer Research UK, and the Medical Research Council, They reported having no other disclosures.
FROM LEUKEMIA
Key clinical point:
Major finding: Increased PADI2 expression by bone marrow mesenchymal stem cells in patients with MGUS or multiple myeloma directly induces upregulation of interleukin-6 expression through its enzymatic deimination of histone H3 arginine 26, which leads to acquired resistance to bortezomib by malignant plasma cells.
Data source: Transcriptomic analysis of mRNA extracted from bone marrow mesenchymal stem cells cultured from MGUS and multiple myeloma patients and controls.
Disclosures: The authors were supported by grants from Bloodwise, Cancer Research UK, and the Medical Research Council, They reported having no other disclosures.
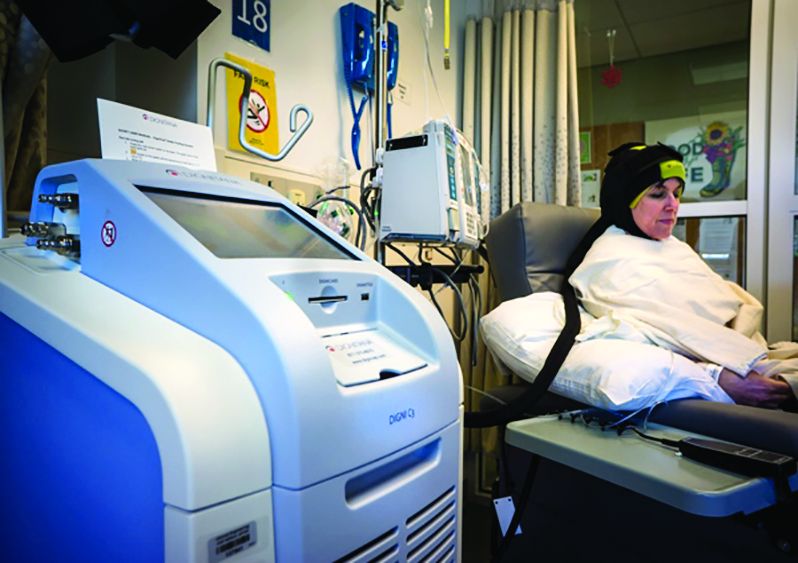
Scalp cooling reduces hair loss in 50% or more of women in separate studies
Scalp cooling resulted in significant reductions in hair loss in about half of all women who were treated before, during, and after chemotherapy for breast cancer in both the Scalp Cooling Alopecia Prevention (SCALP) randomized clinical trial and in a multicenter prospective cohort study.
However, the effects of the reduced alopecia on quality of life measures were mixed, according to the findings of the studies, which were published online in JAMA.
The findings of the multicenter SCALP trial are from a planned interim analysis of data from 95 women with breast cancer who were undergoing chemotherapy and who were randomized to undergo scalp cooling using the Orbis Paxman Hair Loss Prevention System (OPHLPS), and 47 controls. Successful hair preservation occurred in 50.5% of those in the scalp cooling group, compared with 0% of those in the control group – results which led to early study termination, reported Julie Nangia, MD, of Baylor College of Medicine, Houston, and her colleagues (JAMA 2017 Feb 14. doi: 10.1001/jama.2016.20939).
No serious adverse device-related events occurred in the cooling group, but there also were no significant differences between the groups with respect to changes in quality of life scales from baseline to chemotherapy cycle 4, the investigators found.
Study subjects were women with a mean age of 52.6 years who were receiving anthracycline-based chemotherapy (36%) or taxane-based chemotherapy (64%). Successful hair preservation was defined as grade 0 or 1 based on the Common Terminology Criteria for Adverse Events version 4.1 scale, representing no hair loss or less than 50% hair loss not requiring a wig, respectively. Five women had grade 0 hair loss, and 43 had grade 1 hair loss.
Quality of life was measured using the European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire-Core 30 (EORTC-QLQ-30), the Hospital Anxiety and Depression Scale (HADS), and a summary scale of the Body Image Scale. Changes in emotional and social functioning as measured using the EORTC-QLQ-30 did not differ between the groups, including among those with and without hair preservation, after four treatment cycles, and HADS anxiety and depression summary scores were normal both at baseline and after four cycles in both groups, regardless of hair preservation.
Patients will be followed for 5 years to assess for safety and overall survival, the investigators noted.
During a presentation of these findings at the San Antonio Breast Cancer Symposium in December, Dr. Nangia noted that the maker of the OPHLPS is seeking Food and Drug Administration clearance based on the findings, and if approved, the system would compete with the DigniCap (Dignitana AB), which has already received Food and Drug Administration clearance.*
The DigniCap was the device evaluated (at the time under an FDA investigational device exemption) in the prospective cohort study also published in JAMA.
In that study, 106 women receiving adjuvant or neoadjuvant chemotherapy for stage 1 or II breast cancer were treated with scalp cooling between August 2013 and October 2014, and, along with 16 control subjects, were followed for a median of 29.5 months. Self-estimated hair loss at 4 weeks after the last chemotherapy dose was 50% or less, based on the Dean scale (score of 0-2) in 67 of 101 evaluable patients in the scalp cooling group, vs. 0 of 16 in the control group, reported Dr. Hope S. Rugo, MD, of the Helen Diller Family Comprehensive Cancer Center, San Francisco, and her colleagues.
Five patients had no hair loss, and 62 had less than 50% hair loss, the investigators said (JAMA. 2017 Feb 14. doi: 10.1001/jama.2016.21038).
Three of five quality of life measures, as assessed using the EORTC-QLQ, were significantly better at 1 month after the end of chemotherapy vs. at baseline in the cooling group, compared with the control group. For example, 27.3% vs. 56.3% of treatment and control subjects, respectively, reported feeling less physically attractive. The results were similar among those with 50% or less hair loss vs. controls, they noted.
Adverse events associated with cooling included mild headache in four patients. Three patients discontinued treatment due to feeling cold.
The mean age of the women was 53 years. None of those in the treatment group received anthracyclines, thus further research is needed to assess scalp cooling outcomes after treatment with anthracycline regimens, the investigators said, noting that additional research is also needed to assess long-term measures of alopecia and adverse effects. Patients will be followed for a total of 5 years.
Taken together, the findings of these two studies suggest that increased use of scalp cooling is warranted, as it has the potential to both reduce a troublesome side effect of chemotherapy and to remove a common concern – and sometimes a deterrent – among women considering chemotherapy, according to Dawn L. Hershman, MD, of the Herbert Irving Comprehensive Cancer Center at Columbia University.
In an editorial, Dr. Hershman noted that adjuvant chemotherapy reduces the 10-year relative risk of death from breast cancer by about 35%, but that “a substantial number of women who are advised to undergo chemotherapy choose not to receive treatment because of concerns about adverse effects.”
About 50% of patients consider hair loss the most traumatic aspect of chemotherapy, and about 8% said they would decline chemotherapy because of concerns about hair loss, she said.
“Therefore an intervention that could reduce the adverse effects of chemotherapy may lead to improvement in the initiation and completion of therapy, in quality of life, and in survival outcomes,” she wrote (JAMA. 2017 Feb 14;317[6]:587-8).
At face value, the “reassuringly similar” findings from these two studies appear to represent a major step forward for improving the quality of life for individuals with cancer, she added, explaining that while the quality of life data suggest a limited effect, they should be interpreted with caution as the overall effects of the patients’ diagnoses, surgery, and treatment may have influenced the patient-reported outcomes, diminishing the likelihood of detecting differences in quality of life associated with lower rates of alopecia.
Further, the unblinded nature of the intervention may also bias patient-reported outcomes results, she said, adding that “better measures may be needed to capture the effect of treatments on outcomes that are meaningful to patients so that important adverse effects are fully captured in comparative clinical trials.”
Although questions about cost and coverage of scalp cooling remain, Dr. Hershman concluded that until chemotherapy is no longer necessary and some of the distressing adverse effects of cancer treatment can be avoided, interventions such as scalp cooling that can reduce or eliminate toxic effects will help ease the distress and may thereby improve outcomes for patients with breast cancer.
In a separate editorial, Howard (Jack) West, MD, of the Swedish Cancer Institute in Seattle, further notes that a “lingering concern” with respect to scalp cooling is “the speculated potential for increased scalp metastases ... owing to poor local circulation of chemotherapy.”
However, this has not been observed in studies to date, he noted (JAMA. 2017 Feb 14. doi: 10.1001/jamaoncol.2017.0051).
“While some may argue that we need long-term data on timing and patterns of recurrence as well as overall survival to ensure that there is no increased risk of scalp metastases or otherwise compromised outcomes for scalp cooling, there is no evidence thus far to suggest this,” he said, concluding that “it is arguable that growing attention on interventions to reduce chemotherapy-induced alopecia are reaching an inflection point that justifies far more widespread adoption.”
The SCALP trial was supported by Paxman Coolers Ltd. Dr. Nangia reported having no disclosures. The study by Dr. Rugo et al. was funded in part by Dignitana AB, the Laszlo Tauber Family Foundation, the Anne Moore Breast Cancer Research Fund, and the Friedman Family Foundation. Dr. Rugo, Dr. Hershman, and Dr. West reported having no disclosures.
*Correction, 4/5/17: An earlier version of this article misstated the device's FDA status.
Scalp cooling resulted in significant reductions in hair loss in about half of all women who were treated before, during, and after chemotherapy for breast cancer in both the Scalp Cooling Alopecia Prevention (SCALP) randomized clinical trial and in a multicenter prospective cohort study.
However, the effects of the reduced alopecia on quality of life measures were mixed, according to the findings of the studies, which were published online in JAMA.
The findings of the multicenter SCALP trial are from a planned interim analysis of data from 95 women with breast cancer who were undergoing chemotherapy and who were randomized to undergo scalp cooling using the Orbis Paxman Hair Loss Prevention System (OPHLPS), and 47 controls. Successful hair preservation occurred in 50.5% of those in the scalp cooling group, compared with 0% of those in the control group – results which led to early study termination, reported Julie Nangia, MD, of Baylor College of Medicine, Houston, and her colleagues (JAMA 2017 Feb 14. doi: 10.1001/jama.2016.20939).
No serious adverse device-related events occurred in the cooling group, but there also were no significant differences between the groups with respect to changes in quality of life scales from baseline to chemotherapy cycle 4, the investigators found.
Study subjects were women with a mean age of 52.6 years who were receiving anthracycline-based chemotherapy (36%) or taxane-based chemotherapy (64%). Successful hair preservation was defined as grade 0 or 1 based on the Common Terminology Criteria for Adverse Events version 4.1 scale, representing no hair loss or less than 50% hair loss not requiring a wig, respectively. Five women had grade 0 hair loss, and 43 had grade 1 hair loss.
Quality of life was measured using the European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire-Core 30 (EORTC-QLQ-30), the Hospital Anxiety and Depression Scale (HADS), and a summary scale of the Body Image Scale. Changes in emotional and social functioning as measured using the EORTC-QLQ-30 did not differ between the groups, including among those with and without hair preservation, after four treatment cycles, and HADS anxiety and depression summary scores were normal both at baseline and after four cycles in both groups, regardless of hair preservation.
Patients will be followed for 5 years to assess for safety and overall survival, the investigators noted.
During a presentation of these findings at the San Antonio Breast Cancer Symposium in December, Dr. Nangia noted that the maker of the OPHLPS is seeking Food and Drug Administration clearance based on the findings, and if approved, the system would compete with the DigniCap (Dignitana AB), which has already received Food and Drug Administration clearance.*
The DigniCap was the device evaluated (at the time under an FDA investigational device exemption) in the prospective cohort study also published in JAMA.
In that study, 106 women receiving adjuvant or neoadjuvant chemotherapy for stage 1 or II breast cancer were treated with scalp cooling between August 2013 and October 2014, and, along with 16 control subjects, were followed for a median of 29.5 months. Self-estimated hair loss at 4 weeks after the last chemotherapy dose was 50% or less, based on the Dean scale (score of 0-2) in 67 of 101 evaluable patients in the scalp cooling group, vs. 0 of 16 in the control group, reported Dr. Hope S. Rugo, MD, of the Helen Diller Family Comprehensive Cancer Center, San Francisco, and her colleagues.
Five patients had no hair loss, and 62 had less than 50% hair loss, the investigators said (JAMA. 2017 Feb 14. doi: 10.1001/jama.2016.21038).
Three of five quality of life measures, as assessed using the EORTC-QLQ, were significantly better at 1 month after the end of chemotherapy vs. at baseline in the cooling group, compared with the control group. For example, 27.3% vs. 56.3% of treatment and control subjects, respectively, reported feeling less physically attractive. The results were similar among those with 50% or less hair loss vs. controls, they noted.
Adverse events associated with cooling included mild headache in four patients. Three patients discontinued treatment due to feeling cold.
The mean age of the women was 53 years. None of those in the treatment group received anthracyclines, thus further research is needed to assess scalp cooling outcomes after treatment with anthracycline regimens, the investigators said, noting that additional research is also needed to assess long-term measures of alopecia and adverse effects. Patients will be followed for a total of 5 years.
Taken together, the findings of these two studies suggest that increased use of scalp cooling is warranted, as it has the potential to both reduce a troublesome side effect of chemotherapy and to remove a common concern – and sometimes a deterrent – among women considering chemotherapy, according to Dawn L. Hershman, MD, of the Herbert Irving Comprehensive Cancer Center at Columbia University.
In an editorial, Dr. Hershman noted that adjuvant chemotherapy reduces the 10-year relative risk of death from breast cancer by about 35%, but that “a substantial number of women who are advised to undergo chemotherapy choose not to receive treatment because of concerns about adverse effects.”
About 50% of patients consider hair loss the most traumatic aspect of chemotherapy, and about 8% said they would decline chemotherapy because of concerns about hair loss, she said.
“Therefore an intervention that could reduce the adverse effects of chemotherapy may lead to improvement in the initiation and completion of therapy, in quality of life, and in survival outcomes,” she wrote (JAMA. 2017 Feb 14;317[6]:587-8).
At face value, the “reassuringly similar” findings from these two studies appear to represent a major step forward for improving the quality of life for individuals with cancer, she added, explaining that while the quality of life data suggest a limited effect, they should be interpreted with caution as the overall effects of the patients’ diagnoses, surgery, and treatment may have influenced the patient-reported outcomes, diminishing the likelihood of detecting differences in quality of life associated with lower rates of alopecia.
Further, the unblinded nature of the intervention may also bias patient-reported outcomes results, she said, adding that “better measures may be needed to capture the effect of treatments on outcomes that are meaningful to patients so that important adverse effects are fully captured in comparative clinical trials.”
Although questions about cost and coverage of scalp cooling remain, Dr. Hershman concluded that until chemotherapy is no longer necessary and some of the distressing adverse effects of cancer treatment can be avoided, interventions such as scalp cooling that can reduce or eliminate toxic effects will help ease the distress and may thereby improve outcomes for patients with breast cancer.
In a separate editorial, Howard (Jack) West, MD, of the Swedish Cancer Institute in Seattle, further notes that a “lingering concern” with respect to scalp cooling is “the speculated potential for increased scalp metastases ... owing to poor local circulation of chemotherapy.”
However, this has not been observed in studies to date, he noted (JAMA. 2017 Feb 14. doi: 10.1001/jamaoncol.2017.0051).
“While some may argue that we need long-term data on timing and patterns of recurrence as well as overall survival to ensure that there is no increased risk of scalp metastases or otherwise compromised outcomes for scalp cooling, there is no evidence thus far to suggest this,” he said, concluding that “it is arguable that growing attention on interventions to reduce chemotherapy-induced alopecia are reaching an inflection point that justifies far more widespread adoption.”
The SCALP trial was supported by Paxman Coolers Ltd. Dr. Nangia reported having no disclosures. The study by Dr. Rugo et al. was funded in part by Dignitana AB, the Laszlo Tauber Family Foundation, the Anne Moore Breast Cancer Research Fund, and the Friedman Family Foundation. Dr. Rugo, Dr. Hershman, and Dr. West reported having no disclosures.
*Correction, 4/5/17: An earlier version of this article misstated the device's FDA status.
Scalp cooling resulted in significant reductions in hair loss in about half of all women who were treated before, during, and after chemotherapy for breast cancer in both the Scalp Cooling Alopecia Prevention (SCALP) randomized clinical trial and in a multicenter prospective cohort study.
However, the effects of the reduced alopecia on quality of life measures were mixed, according to the findings of the studies, which were published online in JAMA.
The findings of the multicenter SCALP trial are from a planned interim analysis of data from 95 women with breast cancer who were undergoing chemotherapy and who were randomized to undergo scalp cooling using the Orbis Paxman Hair Loss Prevention System (OPHLPS), and 47 controls. Successful hair preservation occurred in 50.5% of those in the scalp cooling group, compared with 0% of those in the control group – results which led to early study termination, reported Julie Nangia, MD, of Baylor College of Medicine, Houston, and her colleagues (JAMA 2017 Feb 14. doi: 10.1001/jama.2016.20939).
No serious adverse device-related events occurred in the cooling group, but there also were no significant differences between the groups with respect to changes in quality of life scales from baseline to chemotherapy cycle 4, the investigators found.
Study subjects were women with a mean age of 52.6 years who were receiving anthracycline-based chemotherapy (36%) or taxane-based chemotherapy (64%). Successful hair preservation was defined as grade 0 or 1 based on the Common Terminology Criteria for Adverse Events version 4.1 scale, representing no hair loss or less than 50% hair loss not requiring a wig, respectively. Five women had grade 0 hair loss, and 43 had grade 1 hair loss.
Quality of life was measured using the European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire-Core 30 (EORTC-QLQ-30), the Hospital Anxiety and Depression Scale (HADS), and a summary scale of the Body Image Scale. Changes in emotional and social functioning as measured using the EORTC-QLQ-30 did not differ between the groups, including among those with and without hair preservation, after four treatment cycles, and HADS anxiety and depression summary scores were normal both at baseline and after four cycles in both groups, regardless of hair preservation.
Patients will be followed for 5 years to assess for safety and overall survival, the investigators noted.
During a presentation of these findings at the San Antonio Breast Cancer Symposium in December, Dr. Nangia noted that the maker of the OPHLPS is seeking Food and Drug Administration clearance based on the findings, and if approved, the system would compete with the DigniCap (Dignitana AB), which has already received Food and Drug Administration clearance.*
The DigniCap was the device evaluated (at the time under an FDA investigational device exemption) in the prospective cohort study also published in JAMA.
In that study, 106 women receiving adjuvant or neoadjuvant chemotherapy for stage 1 or II breast cancer were treated with scalp cooling between August 2013 and October 2014, and, along with 16 control subjects, were followed for a median of 29.5 months. Self-estimated hair loss at 4 weeks after the last chemotherapy dose was 50% or less, based on the Dean scale (score of 0-2) in 67 of 101 evaluable patients in the scalp cooling group, vs. 0 of 16 in the control group, reported Dr. Hope S. Rugo, MD, of the Helen Diller Family Comprehensive Cancer Center, San Francisco, and her colleagues.
Five patients had no hair loss, and 62 had less than 50% hair loss, the investigators said (JAMA. 2017 Feb 14. doi: 10.1001/jama.2016.21038).
Three of five quality of life measures, as assessed using the EORTC-QLQ, were significantly better at 1 month after the end of chemotherapy vs. at baseline in the cooling group, compared with the control group. For example, 27.3% vs. 56.3% of treatment and control subjects, respectively, reported feeling less physically attractive. The results were similar among those with 50% or less hair loss vs. controls, they noted.
Adverse events associated with cooling included mild headache in four patients. Three patients discontinued treatment due to feeling cold.
The mean age of the women was 53 years. None of those in the treatment group received anthracyclines, thus further research is needed to assess scalp cooling outcomes after treatment with anthracycline regimens, the investigators said, noting that additional research is also needed to assess long-term measures of alopecia and adverse effects. Patients will be followed for a total of 5 years.
Taken together, the findings of these two studies suggest that increased use of scalp cooling is warranted, as it has the potential to both reduce a troublesome side effect of chemotherapy and to remove a common concern – and sometimes a deterrent – among women considering chemotherapy, according to Dawn L. Hershman, MD, of the Herbert Irving Comprehensive Cancer Center at Columbia University.
In an editorial, Dr. Hershman noted that adjuvant chemotherapy reduces the 10-year relative risk of death from breast cancer by about 35%, but that “a substantial number of women who are advised to undergo chemotherapy choose not to receive treatment because of concerns about adverse effects.”
About 50% of patients consider hair loss the most traumatic aspect of chemotherapy, and about 8% said they would decline chemotherapy because of concerns about hair loss, she said.
“Therefore an intervention that could reduce the adverse effects of chemotherapy may lead to improvement in the initiation and completion of therapy, in quality of life, and in survival outcomes,” she wrote (JAMA. 2017 Feb 14;317[6]:587-8).
At face value, the “reassuringly similar” findings from these two studies appear to represent a major step forward for improving the quality of life for individuals with cancer, she added, explaining that while the quality of life data suggest a limited effect, they should be interpreted with caution as the overall effects of the patients’ diagnoses, surgery, and treatment may have influenced the patient-reported outcomes, diminishing the likelihood of detecting differences in quality of life associated with lower rates of alopecia.
Further, the unblinded nature of the intervention may also bias patient-reported outcomes results, she said, adding that “better measures may be needed to capture the effect of treatments on outcomes that are meaningful to patients so that important adverse effects are fully captured in comparative clinical trials.”
Although questions about cost and coverage of scalp cooling remain, Dr. Hershman concluded that until chemotherapy is no longer necessary and some of the distressing adverse effects of cancer treatment can be avoided, interventions such as scalp cooling that can reduce or eliminate toxic effects will help ease the distress and may thereby improve outcomes for patients with breast cancer.
In a separate editorial, Howard (Jack) West, MD, of the Swedish Cancer Institute in Seattle, further notes that a “lingering concern” with respect to scalp cooling is “the speculated potential for increased scalp metastases ... owing to poor local circulation of chemotherapy.”
However, this has not been observed in studies to date, he noted (JAMA. 2017 Feb 14. doi: 10.1001/jamaoncol.2017.0051).
“While some may argue that we need long-term data on timing and patterns of recurrence as well as overall survival to ensure that there is no increased risk of scalp metastases or otherwise compromised outcomes for scalp cooling, there is no evidence thus far to suggest this,” he said, concluding that “it is arguable that growing attention on interventions to reduce chemotherapy-induced alopecia are reaching an inflection point that justifies far more widespread adoption.”
The SCALP trial was supported by Paxman Coolers Ltd. Dr. Nangia reported having no disclosures. The study by Dr. Rugo et al. was funded in part by Dignitana AB, the Laszlo Tauber Family Foundation, the Anne Moore Breast Cancer Research Fund, and the Friedman Family Foundation. Dr. Rugo, Dr. Hershman, and Dr. West reported having no disclosures.
*Correction, 4/5/17: An earlier version of this article misstated the device's FDA status.
FROM JAMA
Key clinical point:
Major finding: About half of the treated women in both studies experienced reduced or no hair loss during chemotherapy.
Data source: A randomized clinical trial involving 142 women, and a prospective cohort study involving 122 women.
Disclosures: The SCALP trial was supported by Paxman Coolers Ltd. Dr. Nangia reported having no disclosures. The study by Dr. Rugo et al. was funded in part by Dignitana AB, the Laszlo Tauber Family Foundation, the Anne Moore Breast Cancer Research Fund, and the Friedman Family Foundation. Dr. Rugo, Dr. Hershman, and Dr. West reported having no disclosures.
Reversion mutations detected in cfDNA could guide HGSC treatment
BRCA1/2 reversion mutations that restore protein function and lead to clinically significant rates of acquired resistance can be detected in an unbiased analysis of cell-free DNA, an analysis of plasma and tumor samples from 30 patients with high-grade serous ovarian cancer (HGSC) and BRCA1 or BRCA2 germline mutation shows.
The findings suggest that detection of these mutations could be useful for predicting chemotherapy response in recurrent HGSC, reported Elizabeth L. Christie, PhD, of Peter MacCallum Cancer Centre, Melbourne, and her colleagues.
The findings of this study suggest that detection of BRCA1/2 reversion mutations in cfDNA by targeted amplicon sequencing is feasible and predictive of poor response to platin-based therapy or PARP inhibition, which is important given the current lack of predictive markers of response to guide drug selection in patients with recurrent HGSC, the investigators said.
Further evaluation is needed, but the findings suggest that analysis of cell-free DNA has the potential to be used to direct treatment in recurrent HGSC, they concluded.
Dr. Christie reported having no conflicts of interest.
BRCA1/2 reversion mutations that restore protein function and lead to clinically significant rates of acquired resistance can be detected in an unbiased analysis of cell-free DNA, an analysis of plasma and tumor samples from 30 patients with high-grade serous ovarian cancer (HGSC) and BRCA1 or BRCA2 germline mutation shows.
The findings suggest that detection of these mutations could be useful for predicting chemotherapy response in recurrent HGSC, reported Elizabeth L. Christie, PhD, of Peter MacCallum Cancer Centre, Melbourne, and her colleagues.
The findings of this study suggest that detection of BRCA1/2 reversion mutations in cfDNA by targeted amplicon sequencing is feasible and predictive of poor response to platin-based therapy or PARP inhibition, which is important given the current lack of predictive markers of response to guide drug selection in patients with recurrent HGSC, the investigators said.
Further evaluation is needed, but the findings suggest that analysis of cell-free DNA has the potential to be used to direct treatment in recurrent HGSC, they concluded.
Dr. Christie reported having no conflicts of interest.
BRCA1/2 reversion mutations that restore protein function and lead to clinically significant rates of acquired resistance can be detected in an unbiased analysis of cell-free DNA, an analysis of plasma and tumor samples from 30 patients with high-grade serous ovarian cancer (HGSC) and BRCA1 or BRCA2 germline mutation shows.
The findings suggest that detection of these mutations could be useful for predicting chemotherapy response in recurrent HGSC, reported Elizabeth L. Christie, PhD, of Peter MacCallum Cancer Centre, Melbourne, and her colleagues.
The findings of this study suggest that detection of BRCA1/2 reversion mutations in cfDNA by targeted amplicon sequencing is feasible and predictive of poor response to platin-based therapy or PARP inhibition, which is important given the current lack of predictive markers of response to guide drug selection in patients with recurrent HGSC, the investigators said.
Further evaluation is needed, but the findings suggest that analysis of cell-free DNA has the potential to be used to direct treatment in recurrent HGSC, they concluded.
Dr. Christie reported having no conflicts of interest.
FROM THE JOURNAL OF CLINICAL ONCOLOGY
Key clinical point:
Major finding: Five of 30 patients – all in the recurrent disease group – had reversion mutations. Reversion mutations were detected in the cell-free DNA only from those with tumor-detected reversion.
Data source: Plasma and tumor samples from 30 women in the Australian Ovarian Cancer Study.
Disclosures: Dr. Christie reported having no conflicts of interest.
Specific polymorphisms excluded in hemophilic arthropathy
Carriers of a hemochromatosis (HFE) gene mutation or a long (GT)n-repeat length within the HMOX1 promoter regions did not appear to have an increase in hemophilic arthropathy.
In 201 blood samples from patients with severe hemophilia A or B and 37 from patients with moderate disease, neither the presence of an HFE mutation nor a long (GT)n-repeat length was associated with an increase in joint damage. The assessment was based on Pettersson score after adjustment for disease severity, presence of inhibitors, annual joint bleeding rate (AJBR), age at Pettersson score and at clinic entrance, and birth cohort (standardized beta = 0.033 and -0.022, respectively), Lize F. van Vulpen, MD, of University Medical Center Utrecht, the Netherlands, reported at the annual meeting of the European Association of Haemophilia and Allied Disorders.
Study subjects had a median age of 43 years, median AJBRs of 2.5 and 0.5 for severe and moderate disease, respectively, and median Pettersson scores of 22 and 4 in the groups, respectively. An HFE mutation was detected in 91 patients, and their levels of ferritin, iron, and transferrin saturation were significantly increased, but other baseline characteristic were similar in those with and without HFE mutation, and regardless of (GT)n-repeat length.
The marked heterogeneity in joint damage seen among hemophilia patients was hypothesized in this study to be associated with differences in iron handling, but the findings failed to support that hypothesis, Dr. van Vulpen concluded.
Dr. van Vulpen reported having no disclosures.
Carriers of a hemochromatosis (HFE) gene mutation or a long (GT)n-repeat length within the HMOX1 promoter regions did not appear to have an increase in hemophilic arthropathy.
In 201 blood samples from patients with severe hemophilia A or B and 37 from patients with moderate disease, neither the presence of an HFE mutation nor a long (GT)n-repeat length was associated with an increase in joint damage. The assessment was based on Pettersson score after adjustment for disease severity, presence of inhibitors, annual joint bleeding rate (AJBR), age at Pettersson score and at clinic entrance, and birth cohort (standardized beta = 0.033 and -0.022, respectively), Lize F. van Vulpen, MD, of University Medical Center Utrecht, the Netherlands, reported at the annual meeting of the European Association of Haemophilia and Allied Disorders.
Study subjects had a median age of 43 years, median AJBRs of 2.5 and 0.5 for severe and moderate disease, respectively, and median Pettersson scores of 22 and 4 in the groups, respectively. An HFE mutation was detected in 91 patients, and their levels of ferritin, iron, and transferrin saturation were significantly increased, but other baseline characteristic were similar in those with and without HFE mutation, and regardless of (GT)n-repeat length.
The marked heterogeneity in joint damage seen among hemophilia patients was hypothesized in this study to be associated with differences in iron handling, but the findings failed to support that hypothesis, Dr. van Vulpen concluded.
Dr. van Vulpen reported having no disclosures.
Carriers of a hemochromatosis (HFE) gene mutation or a long (GT)n-repeat length within the HMOX1 promoter regions did not appear to have an increase in hemophilic arthropathy.
In 201 blood samples from patients with severe hemophilia A or B and 37 from patients with moderate disease, neither the presence of an HFE mutation nor a long (GT)n-repeat length was associated with an increase in joint damage. The assessment was based on Pettersson score after adjustment for disease severity, presence of inhibitors, annual joint bleeding rate (AJBR), age at Pettersson score and at clinic entrance, and birth cohort (standardized beta = 0.033 and -0.022, respectively), Lize F. van Vulpen, MD, of University Medical Center Utrecht, the Netherlands, reported at the annual meeting of the European Association of Haemophilia and Allied Disorders.
Study subjects had a median age of 43 years, median AJBRs of 2.5 and 0.5 for severe and moderate disease, respectively, and median Pettersson scores of 22 and 4 in the groups, respectively. An HFE mutation was detected in 91 patients, and their levels of ferritin, iron, and transferrin saturation were significantly increased, but other baseline characteristic were similar in those with and without HFE mutation, and regardless of (GT)n-repeat length.
The marked heterogeneity in joint damage seen among hemophilia patients was hypothesized in this study to be associated with differences in iron handling, but the findings failed to support that hypothesis, Dr. van Vulpen concluded.
Dr. van Vulpen reported having no disclosures.
FROM EAHAD 2017
Key clinical point:
Major finding: Neither the presence of an HFE mutation, nor a long (GT)n-repeat length was associated with an increase in joint damage as assessed by Pettersson score (standardized beta = 0.033 and –0.022, respectively).
Data source: An evaluation of blood samples from a cohort of 238 patients.
Disclosures: Dr. van Vulpen reported having no disclosures.
Low inhibitor incidence seen with new generation rhFVIII
Human-cl rhFVIII, a new generation recombinant factor VIII of human origin, appears to be associated with a low inhibitor incidence in patients with severe hemophilia A, according to interim findings from the ongoing NuProtect study.
In 66 evaluable previously untreated patients with at least 20 days of exposure to human-cl rhFVIII, the cumulative incidence of high-titer and low-titer inhibitor development was 12.8% and 8.4%, respectively, Ellis J. Neufeld, MD, reported at the annual meeting of the European Association for Haemophilia and Allied Disorders.
The median age at first treatment was 13 months (range of 3-135 months). Of 59 patients with available F8 gene analysis, 44 had high-risk mutations and 47 had null mutations; 1 had no mutation identified. After a median of 11.5 treatment exposure days, 8 of the 66 patients developed high-titer inhibitors and 5 developed low-titer inhibitors; 4 of those were transient, said Dr. Neufeld of Harvard Medical School, Boston.
Only two of the patients developed an inhibitor, including one high- and one low-titer inhibitor, after 20 exposure days. Among the 13 inhibitor patients, 12 had an F8 mutation identified; all were null, and 11 were high risk.
In a prior study of 201 patients with previously treated severe hemophilia A, no inhibitors were reported with human-cl rhFVIII – which is produced in human cells without chemical modification or protein fusion. The NuProtect study is looking at immunogenicity, efficacy, and safety in previously untreated patients. Final data are expected in 2018, Dr. Neufeld noted.
Dr. Neufeld disclosed financial ties to Octapharma, the sponsor of NuProtect, and numerous other pharmaceutical companies.
Human-cl rhFVIII, a new generation recombinant factor VIII of human origin, appears to be associated with a low inhibitor incidence in patients with severe hemophilia A, according to interim findings from the ongoing NuProtect study.
In 66 evaluable previously untreated patients with at least 20 days of exposure to human-cl rhFVIII, the cumulative incidence of high-titer and low-titer inhibitor development was 12.8% and 8.4%, respectively, Ellis J. Neufeld, MD, reported at the annual meeting of the European Association for Haemophilia and Allied Disorders.
The median age at first treatment was 13 months (range of 3-135 months). Of 59 patients with available F8 gene analysis, 44 had high-risk mutations and 47 had null mutations; 1 had no mutation identified. After a median of 11.5 treatment exposure days, 8 of the 66 patients developed high-titer inhibitors and 5 developed low-titer inhibitors; 4 of those were transient, said Dr. Neufeld of Harvard Medical School, Boston.
Only two of the patients developed an inhibitor, including one high- and one low-titer inhibitor, after 20 exposure days. Among the 13 inhibitor patients, 12 had an F8 mutation identified; all were null, and 11 were high risk.
In a prior study of 201 patients with previously treated severe hemophilia A, no inhibitors were reported with human-cl rhFVIII – which is produced in human cells without chemical modification or protein fusion. The NuProtect study is looking at immunogenicity, efficacy, and safety in previously untreated patients. Final data are expected in 2018, Dr. Neufeld noted.
Dr. Neufeld disclosed financial ties to Octapharma, the sponsor of NuProtect, and numerous other pharmaceutical companies.
Human-cl rhFVIII, a new generation recombinant factor VIII of human origin, appears to be associated with a low inhibitor incidence in patients with severe hemophilia A, according to interim findings from the ongoing NuProtect study.
In 66 evaluable previously untreated patients with at least 20 days of exposure to human-cl rhFVIII, the cumulative incidence of high-titer and low-titer inhibitor development was 12.8% and 8.4%, respectively, Ellis J. Neufeld, MD, reported at the annual meeting of the European Association for Haemophilia and Allied Disorders.
The median age at first treatment was 13 months (range of 3-135 months). Of 59 patients with available F8 gene analysis, 44 had high-risk mutations and 47 had null mutations; 1 had no mutation identified. After a median of 11.5 treatment exposure days, 8 of the 66 patients developed high-titer inhibitors and 5 developed low-titer inhibitors; 4 of those were transient, said Dr. Neufeld of Harvard Medical School, Boston.
Only two of the patients developed an inhibitor, including one high- and one low-titer inhibitor, after 20 exposure days. Among the 13 inhibitor patients, 12 had an F8 mutation identified; all were null, and 11 were high risk.
In a prior study of 201 patients with previously treated severe hemophilia A, no inhibitors were reported with human-cl rhFVIII – which is produced in human cells without chemical modification or protein fusion. The NuProtect study is looking at immunogenicity, efficacy, and safety in previously untreated patients. Final data are expected in 2018, Dr. Neufeld noted.
Dr. Neufeld disclosed financial ties to Octapharma, the sponsor of NuProtect, and numerous other pharmaceutical companies.
Key clinical point:
Major finding: The cumulative incidence of high-titer and low-titer inhibitor development was 12.8% and 8.4%, respectively.
Data source: The ongoing NuProtect study of 66 patients.
Disclosures: Dr. Neufeld disclosed financial ties to Octapharma, the sponsor of NuProtect, and numerous other pharmaceutical companies.
Study highlights importance of genotyping in von Willebrand disease
Patients with genetically confirmed von Willebrand disease (VWD) type 2M have a relatively mild clinical phenotype, according to findings from a retrospective cross-sectional study.
In fact, three of 31 patients included in the study had a near normal laboratory phenotype. Additionally, patients with the p.Val1360Ala mutation had significantly higher values of all von Willebrand factor (VWF)-related laboratory parameters, compared with those with the p.Arg1374Cys or p.Phe1293Leu mutation, Dominique Maas reported at the European Association for Haemophilia and Allied Disorders annual meeting.
The findings underscore the importance of genotyping for making a correct diagnosis of VWD type 2M, said Ms. Maas of Radboud University Medical Center, Nijmegen, The Netherlands.
The study subjects, who had a least one VWD type 2M mutation, had a median age of 34 years and a median bleeding score of 6. Most suffered from mucocutaneous bleeding, but with low frequency compared with other VWD subtypes. The incidence of muscle hematomas, postpartum hemorrhage, and postsurgery bleeding were relatively high, however. Age and bleeding score were strongly positively correlated.
Subjects had a median VWF antigen level of 24 IU dL-1, median VWF ristocetin cofactor activity of 6 IU dL-1, and median VSF:RCo/VWF:Ag ratio of 0.29. The VSF collagen binding activity and VWF:Ag ratio was normal, she said.
Genotyping is a powerful diagnostic tool for making an appropriate diagnosis and classification of VWD. The current findings are important because understanding the correlation between genotype and phenotype improves the understanding of VWD pathogenesis and has important implications for treatment, follow-up, and genetic counseling, but has not been throughly investigated in fully genotyped patients with VWD type 2M, she said.
Ms. Maas reported having no disclosures.
Patients with genetically confirmed von Willebrand disease (VWD) type 2M have a relatively mild clinical phenotype, according to findings from a retrospective cross-sectional study.
In fact, three of 31 patients included in the study had a near normal laboratory phenotype. Additionally, patients with the p.Val1360Ala mutation had significantly higher values of all von Willebrand factor (VWF)-related laboratory parameters, compared with those with the p.Arg1374Cys or p.Phe1293Leu mutation, Dominique Maas reported at the European Association for Haemophilia and Allied Disorders annual meeting.
The findings underscore the importance of genotyping for making a correct diagnosis of VWD type 2M, said Ms. Maas of Radboud University Medical Center, Nijmegen, The Netherlands.
The study subjects, who had a least one VWD type 2M mutation, had a median age of 34 years and a median bleeding score of 6. Most suffered from mucocutaneous bleeding, but with low frequency compared with other VWD subtypes. The incidence of muscle hematomas, postpartum hemorrhage, and postsurgery bleeding were relatively high, however. Age and bleeding score were strongly positively correlated.
Subjects had a median VWF antigen level of 24 IU dL-1, median VWF ristocetin cofactor activity of 6 IU dL-1, and median VSF:RCo/VWF:Ag ratio of 0.29. The VSF collagen binding activity and VWF:Ag ratio was normal, she said.
Genotyping is a powerful diagnostic tool for making an appropriate diagnosis and classification of VWD. The current findings are important because understanding the correlation between genotype and phenotype improves the understanding of VWD pathogenesis and has important implications for treatment, follow-up, and genetic counseling, but has not been throughly investigated in fully genotyped patients with VWD type 2M, she said.
Ms. Maas reported having no disclosures.
Patients with genetically confirmed von Willebrand disease (VWD) type 2M have a relatively mild clinical phenotype, according to findings from a retrospective cross-sectional study.
In fact, three of 31 patients included in the study had a near normal laboratory phenotype. Additionally, patients with the p.Val1360Ala mutation had significantly higher values of all von Willebrand factor (VWF)-related laboratory parameters, compared with those with the p.Arg1374Cys or p.Phe1293Leu mutation, Dominique Maas reported at the European Association for Haemophilia and Allied Disorders annual meeting.
The findings underscore the importance of genotyping for making a correct diagnosis of VWD type 2M, said Ms. Maas of Radboud University Medical Center, Nijmegen, The Netherlands.
The study subjects, who had a least one VWD type 2M mutation, had a median age of 34 years and a median bleeding score of 6. Most suffered from mucocutaneous bleeding, but with low frequency compared with other VWD subtypes. The incidence of muscle hematomas, postpartum hemorrhage, and postsurgery bleeding were relatively high, however. Age and bleeding score were strongly positively correlated.
Subjects had a median VWF antigen level of 24 IU dL-1, median VWF ristocetin cofactor activity of 6 IU dL-1, and median VSF:RCo/VWF:Ag ratio of 0.29. The VSF collagen binding activity and VWF:Ag ratio was normal, she said.
Genotyping is a powerful diagnostic tool for making an appropriate diagnosis and classification of VWD. The current findings are important because understanding the correlation between genotype and phenotype improves the understanding of VWD pathogenesis and has important implications for treatment, follow-up, and genetic counseling, but has not been throughly investigated in fully genotyped patients with VWD type 2M, she said.
Ms. Maas reported having no disclosures.
FROM EAHAD 2017
Key clinical point:
Major finding: Three of 31 patients included in the study had a near normal laboratory phenotype.
Data source: A retrospective cross-sectional study of 31 patients.
Disclosures: Ms. Maas reported having no disclosures.
Solulin variants activate TAFI in vitro
A new generation of solulin variants is showing promise for the treatment of severe hemophilia A.
The in vitro production and characterization of these solulin variants – F376A-, M388A-, and F376A/M388A-solulin – showed that while they lost their abilities to activate protein C (an inhibitor of thrombin generation), they were still capable of activating thrombin activatable fibrinolysis inhibitor (TAFI), Yohann Repesse, PhD reported at the annual meeting of the European Association for Haemophilia and Allied Disorders.
Additionally, the double mutant F376A/M388A-solulin, which was tested ex vivo using blood samples from hemophilic A patients, was found on thromboelastography to increase clot firmness and stability. As opposed to wild type solulin, the effects were maintained even at high concentrations of F376A/M388A-solulin, said Dr. Repesse of the National Institute of Health and Medical Research, Caen, France.
An important aggravating factor when it comes to treating hemophilia involves premature fibrinolysis, which means that antifibrinolytics are of interest, he explained. Thrombomodulin is a key player in the coagulation cascade, because it activates protein C, and also in the fibrinolytic cascade, as it activates TAFI. Solulin, a soluble form of thrombomodulin, has both capabilities.
The findings with respect to the new generation of solulin variants tested in this study open new opportunities for the development of specific medications for hemophilia patients, he concluded.
Dr. Repesse reported having no disclosures.
A new generation of solulin variants is showing promise for the treatment of severe hemophilia A.
The in vitro production and characterization of these solulin variants – F376A-, M388A-, and F376A/M388A-solulin – showed that while they lost their abilities to activate protein C (an inhibitor of thrombin generation), they were still capable of activating thrombin activatable fibrinolysis inhibitor (TAFI), Yohann Repesse, PhD reported at the annual meeting of the European Association for Haemophilia and Allied Disorders.
Additionally, the double mutant F376A/M388A-solulin, which was tested ex vivo using blood samples from hemophilic A patients, was found on thromboelastography to increase clot firmness and stability. As opposed to wild type solulin, the effects were maintained even at high concentrations of F376A/M388A-solulin, said Dr. Repesse of the National Institute of Health and Medical Research, Caen, France.
An important aggravating factor when it comes to treating hemophilia involves premature fibrinolysis, which means that antifibrinolytics are of interest, he explained. Thrombomodulin is a key player in the coagulation cascade, because it activates protein C, and also in the fibrinolytic cascade, as it activates TAFI. Solulin, a soluble form of thrombomodulin, has both capabilities.
The findings with respect to the new generation of solulin variants tested in this study open new opportunities for the development of specific medications for hemophilia patients, he concluded.
Dr. Repesse reported having no disclosures.
A new generation of solulin variants is showing promise for the treatment of severe hemophilia A.
The in vitro production and characterization of these solulin variants – F376A-, M388A-, and F376A/M388A-solulin – showed that while they lost their abilities to activate protein C (an inhibitor of thrombin generation), they were still capable of activating thrombin activatable fibrinolysis inhibitor (TAFI), Yohann Repesse, PhD reported at the annual meeting of the European Association for Haemophilia and Allied Disorders.
Additionally, the double mutant F376A/M388A-solulin, which was tested ex vivo using blood samples from hemophilic A patients, was found on thromboelastography to increase clot firmness and stability. As opposed to wild type solulin, the effects were maintained even at high concentrations of F376A/M388A-solulin, said Dr. Repesse of the National Institute of Health and Medical Research, Caen, France.
An important aggravating factor when it comes to treating hemophilia involves premature fibrinolysis, which means that antifibrinolytics are of interest, he explained. Thrombomodulin is a key player in the coagulation cascade, because it activates protein C, and also in the fibrinolytic cascade, as it activates TAFI. Solulin, a soluble form of thrombomodulin, has both capabilities.
The findings with respect to the new generation of solulin variants tested in this study open new opportunities for the development of specific medications for hemophilia patients, he concluded.
Dr. Repesse reported having no disclosures.
FROM EAHAD 2017
Key clinical point:
Major finding: F376A-, M388A-, and F376A/M388A-solulin lost their abilities to activate protein C but were still capable of activating TAFI.
Data source: An in vitro and ex vivo evaluation of solulin variants.
Disclosures: Dr. Repesse reported having no disclosures.