User login
Sharon Worcester is an award-winning medical journalist for MDedge News. She has been with the company since 1996, first as the Southeast Bureau Chief (1996-2009) when the company was known as International Medical News Group, then as a freelance writer (2010-2015) before returning as a reporter in 2015. She previously worked as a daily newspaper reporter covering health and local government. Sharon currently reports primarily on oncology and hematology. She has a BA from Eckerd College and an MA in Mass Communication/Print Journalism from the University of Florida. Connect with her via LinkedIn and follow her on twitter @SW_MedReporter.
CTCs help predict breast cancer outcomes in neoadjuvant setting
SAN ANTONIO – Circulating tumor cells are a useful prognostic biomarker in early breast cancer patients treated with neoadjuvant chemotherapy, according to findings from an international meta-analysis of individual patient data.
The cells (CTCs), which can be measured using a Food and Drug Administration–approved assay, are known to seed distant metastases and to be prognostic before and during therapy for patients with metastatic breast cancer, and prognostic before adjuvant therapy for patients with nonmetastatic breast cancer.
However, findings in the neoadjuvant setting have been variable, Francois-Clement Bidard, MD, of Institut Curie, Paris, reported at the San Antonio Breast Cancer Symposium.
In the study (the international meta-analysis of circulating tumor cell detection in early breast cancer patients treated by neoadjuvant chemotherapy, or IMENEO), CTCs were useful, independent of pathologic complete response, for predicting overall survival and distant disease-free survival in the neoadjuvant setting. Further, IMENEO showed for the first time that CTCs also predict locoregional relapse-free survival,
Based on the analysis of data from 2,156 patients from 21 studies and 16 centers in 10 countries, the CTC positivity rates using thresholds of one or more, two or more, and five or more, respectively, were 25%, 13%, and 6% in 1,574 patients tested at baseline, 17%, 6%, and 3% in 290 tested after neoadjuvant chemotherapy, 15%, 5%, and 1% in 1,200 tested before surgery, and 11%, 4%, and 1% in 285 tested after surgery, Dr. Bidard said.
Prior to neoadjuvant chemotherapy, at least one CTC was found in 19%, 22%, 24%, 29% and 41% of cT1, T2, T3, T4a-c, and T4d breast cancers, respectively, and this was marginally associated with hormone receptor negativity, he said, noting that later CTC detection rates were not associated with any patient baseline characteristics.
Nearly one in four patients (24%) achieved pathologic complete response, but this was not associated at any time point with CTC count.
For the primary study endpoint of overall survival, a significant association was found with the presence of at least two CTCs at baseline (hazard ratio, 2.6 for two CTCs; 3.84 for three to four CTCs; and 6.25 for five or more CTCs). Similar associations were found for distant disease-free survival (hazard ratios, 2.4, 3.4, and 5.0, respectively) and for locoregional relapse-free interval with two CTCs and five or more CTCs (hazard ratios, 2.4 and 4.2, respectively).
Similar results were found using later time points, such as after the start of neoadjuvant chemotherapy or before surgery, he said.
On multivariate analysis, baseline CTC detection using any of the thresholds remained an independent predictor of overall and distant disease-free survival and locoregional relapse-free interval when considered together with pathologic complete response, cT, cN, and tumor subtype, suggesting that CTC measurement adds value to comprehensive prognostic models.
That is, they complement rather than duplicate usual prognostic factors and pathologic complete response rates to better predict outcomes in patients with early breast cancer in the neoadjuvant setting, Dr. Bidard said.
This study was supported by a research grant from Janssen Diagnostics. Dr. Bidard reported having no disclosures.
SAN ANTONIO – Circulating tumor cells are a useful prognostic biomarker in early breast cancer patients treated with neoadjuvant chemotherapy, according to findings from an international meta-analysis of individual patient data.
The cells (CTCs), which can be measured using a Food and Drug Administration–approved assay, are known to seed distant metastases and to be prognostic before and during therapy for patients with metastatic breast cancer, and prognostic before adjuvant therapy for patients with nonmetastatic breast cancer.
However, findings in the neoadjuvant setting have been variable, Francois-Clement Bidard, MD, of Institut Curie, Paris, reported at the San Antonio Breast Cancer Symposium.
In the study (the international meta-analysis of circulating tumor cell detection in early breast cancer patients treated by neoadjuvant chemotherapy, or IMENEO), CTCs were useful, independent of pathologic complete response, for predicting overall survival and distant disease-free survival in the neoadjuvant setting. Further, IMENEO showed for the first time that CTCs also predict locoregional relapse-free survival,
Based on the analysis of data from 2,156 patients from 21 studies and 16 centers in 10 countries, the CTC positivity rates using thresholds of one or more, two or more, and five or more, respectively, were 25%, 13%, and 6% in 1,574 patients tested at baseline, 17%, 6%, and 3% in 290 tested after neoadjuvant chemotherapy, 15%, 5%, and 1% in 1,200 tested before surgery, and 11%, 4%, and 1% in 285 tested after surgery, Dr. Bidard said.
Prior to neoadjuvant chemotherapy, at least one CTC was found in 19%, 22%, 24%, 29% and 41% of cT1, T2, T3, T4a-c, and T4d breast cancers, respectively, and this was marginally associated with hormone receptor negativity, he said, noting that later CTC detection rates were not associated with any patient baseline characteristics.
Nearly one in four patients (24%) achieved pathologic complete response, but this was not associated at any time point with CTC count.
For the primary study endpoint of overall survival, a significant association was found with the presence of at least two CTCs at baseline (hazard ratio, 2.6 for two CTCs; 3.84 for three to four CTCs; and 6.25 for five or more CTCs). Similar associations were found for distant disease-free survival (hazard ratios, 2.4, 3.4, and 5.0, respectively) and for locoregional relapse-free interval with two CTCs and five or more CTCs (hazard ratios, 2.4 and 4.2, respectively).
Similar results were found using later time points, such as after the start of neoadjuvant chemotherapy or before surgery, he said.
On multivariate analysis, baseline CTC detection using any of the thresholds remained an independent predictor of overall and distant disease-free survival and locoregional relapse-free interval when considered together with pathologic complete response, cT, cN, and tumor subtype, suggesting that CTC measurement adds value to comprehensive prognostic models.
That is, they complement rather than duplicate usual prognostic factors and pathologic complete response rates to better predict outcomes in patients with early breast cancer in the neoadjuvant setting, Dr. Bidard said.
This study was supported by a research grant from Janssen Diagnostics. Dr. Bidard reported having no disclosures.
SAN ANTONIO – Circulating tumor cells are a useful prognostic biomarker in early breast cancer patients treated with neoadjuvant chemotherapy, according to findings from an international meta-analysis of individual patient data.
The cells (CTCs), which can be measured using a Food and Drug Administration–approved assay, are known to seed distant metastases and to be prognostic before and during therapy for patients with metastatic breast cancer, and prognostic before adjuvant therapy for patients with nonmetastatic breast cancer.
However, findings in the neoadjuvant setting have been variable, Francois-Clement Bidard, MD, of Institut Curie, Paris, reported at the San Antonio Breast Cancer Symposium.
In the study (the international meta-analysis of circulating tumor cell detection in early breast cancer patients treated by neoadjuvant chemotherapy, or IMENEO), CTCs were useful, independent of pathologic complete response, for predicting overall survival and distant disease-free survival in the neoadjuvant setting. Further, IMENEO showed for the first time that CTCs also predict locoregional relapse-free survival,
Based on the analysis of data from 2,156 patients from 21 studies and 16 centers in 10 countries, the CTC positivity rates using thresholds of one or more, two or more, and five or more, respectively, were 25%, 13%, and 6% in 1,574 patients tested at baseline, 17%, 6%, and 3% in 290 tested after neoadjuvant chemotherapy, 15%, 5%, and 1% in 1,200 tested before surgery, and 11%, 4%, and 1% in 285 tested after surgery, Dr. Bidard said.
Prior to neoadjuvant chemotherapy, at least one CTC was found in 19%, 22%, 24%, 29% and 41% of cT1, T2, T3, T4a-c, and T4d breast cancers, respectively, and this was marginally associated with hormone receptor negativity, he said, noting that later CTC detection rates were not associated with any patient baseline characteristics.
Nearly one in four patients (24%) achieved pathologic complete response, but this was not associated at any time point with CTC count.
For the primary study endpoint of overall survival, a significant association was found with the presence of at least two CTCs at baseline (hazard ratio, 2.6 for two CTCs; 3.84 for three to four CTCs; and 6.25 for five or more CTCs). Similar associations were found for distant disease-free survival (hazard ratios, 2.4, 3.4, and 5.0, respectively) and for locoregional relapse-free interval with two CTCs and five or more CTCs (hazard ratios, 2.4 and 4.2, respectively).
Similar results were found using later time points, such as after the start of neoadjuvant chemotherapy or before surgery, he said.
On multivariate analysis, baseline CTC detection using any of the thresholds remained an independent predictor of overall and distant disease-free survival and locoregional relapse-free interval when considered together with pathologic complete response, cT, cN, and tumor subtype, suggesting that CTC measurement adds value to comprehensive prognostic models.
That is, they complement rather than duplicate usual prognostic factors and pathologic complete response rates to better predict outcomes in patients with early breast cancer in the neoadjuvant setting, Dr. Bidard said.
This study was supported by a research grant from Janssen Diagnostics. Dr. Bidard reported having no disclosures.
AT SABCS 2016
Key clinical point:
Major finding: Overall survival was associated with the presence of at least two CTCs at baseline (hazard ratio, 2.6 for two CTCs; 3.84 for three to four CTCs; and 6.25 for five or more CTCs).
Data source: A meta-analysis of data for 2,156 patients.
Disclosures: This study was supported by a research grant from Janssen Diagnostics. Dr. Bidard reported having no disclosures.
Neoadjuvant AI plus Ki67 info could deescalate adjuvant breast cancer treatment
Triage to chemotherapy using a Ki67-based Preoperative Endocrine Prognostic Index (PEPI), which also integrates residual disease burden, appears useful for deescalating adjuvant treatment after neoadjuvant aromatase inhibitor (AI) therapy for breast cancer, investigators report in the Journal of Clinical Oncology.
Only 4 of 109 patients (3.7%) in the American College of Surgeons Oncology Group Z1031B trial with a PEPI score of 0 relapsed after a median of 5.5 years (and were therefore unlikely to benefit from adjuvant chemotherapy), compared with 49 (14.4%) of 341 patients with a PEPI score greater than 0 (recurrence hazard ratio, 0.27), reported Matthew J. Ellis, MD, of Baylor College of Medicine, Houston, and his associates.
“The low pCR rate for ACOSOG Z1031B patients who switched to neoadjuvant chemotherapy contradicts the hypothesis that AI-resistant proliferation in ER-rich tumors is associated with enhanced chemotherapy response,” they said (J Clin Oncol. 2016 Jan 3. doi: 10.1200/JCO.2016.69.4406).
Study subjects were postmenopausal women with stage II or III ER+ breast cancer who were randomly assigned to receive neoadjuvant AI therapy with anastrozole, exemestane, or letrozole, then triaged to neoadjuvant chemotherapy based on Ki67 level. The efficacy of chemotherapy was lower than expected in this population, suggesting that optimal therapy for intrinsically AI-resistant disease – which will “likely depend on new insights into the molecular basis for primary endocrine therapy resistance”– should be further investigated, they wrote, noting that the triage approaches used in this study are being evaluated in the ongoing phase III ALTERNATE trial.
Dr. Ellis disclosed financial and other relationships with Bioclassifier, Novartis, Pfizer, Celgene, AstraZeneca, NanoString Technologies, and Prosigna.
Triage to chemotherapy using a Ki67-based Preoperative Endocrine Prognostic Index (PEPI), which also integrates residual disease burden, appears useful for deescalating adjuvant treatment after neoadjuvant aromatase inhibitor (AI) therapy for breast cancer, investigators report in the Journal of Clinical Oncology.
Only 4 of 109 patients (3.7%) in the American College of Surgeons Oncology Group Z1031B trial with a PEPI score of 0 relapsed after a median of 5.5 years (and were therefore unlikely to benefit from adjuvant chemotherapy), compared with 49 (14.4%) of 341 patients with a PEPI score greater than 0 (recurrence hazard ratio, 0.27), reported Matthew J. Ellis, MD, of Baylor College of Medicine, Houston, and his associates.
“The low pCR rate for ACOSOG Z1031B patients who switched to neoadjuvant chemotherapy contradicts the hypothesis that AI-resistant proliferation in ER-rich tumors is associated with enhanced chemotherapy response,” they said (J Clin Oncol. 2016 Jan 3. doi: 10.1200/JCO.2016.69.4406).
Study subjects were postmenopausal women with stage II or III ER+ breast cancer who were randomly assigned to receive neoadjuvant AI therapy with anastrozole, exemestane, or letrozole, then triaged to neoadjuvant chemotherapy based on Ki67 level. The efficacy of chemotherapy was lower than expected in this population, suggesting that optimal therapy for intrinsically AI-resistant disease – which will “likely depend on new insights into the molecular basis for primary endocrine therapy resistance”– should be further investigated, they wrote, noting that the triage approaches used in this study are being evaluated in the ongoing phase III ALTERNATE trial.
Dr. Ellis disclosed financial and other relationships with Bioclassifier, Novartis, Pfizer, Celgene, AstraZeneca, NanoString Technologies, and Prosigna.
Triage to chemotherapy using a Ki67-based Preoperative Endocrine Prognostic Index (PEPI), which also integrates residual disease burden, appears useful for deescalating adjuvant treatment after neoadjuvant aromatase inhibitor (AI) therapy for breast cancer, investigators report in the Journal of Clinical Oncology.
Only 4 of 109 patients (3.7%) in the American College of Surgeons Oncology Group Z1031B trial with a PEPI score of 0 relapsed after a median of 5.5 years (and were therefore unlikely to benefit from adjuvant chemotherapy), compared with 49 (14.4%) of 341 patients with a PEPI score greater than 0 (recurrence hazard ratio, 0.27), reported Matthew J. Ellis, MD, of Baylor College of Medicine, Houston, and his associates.
“The low pCR rate for ACOSOG Z1031B patients who switched to neoadjuvant chemotherapy contradicts the hypothesis that AI-resistant proliferation in ER-rich tumors is associated with enhanced chemotherapy response,” they said (J Clin Oncol. 2016 Jan 3. doi: 10.1200/JCO.2016.69.4406).
Study subjects were postmenopausal women with stage II or III ER+ breast cancer who were randomly assigned to receive neoadjuvant AI therapy with anastrozole, exemestane, or letrozole, then triaged to neoadjuvant chemotherapy based on Ki67 level. The efficacy of chemotherapy was lower than expected in this population, suggesting that optimal therapy for intrinsically AI-resistant disease – which will “likely depend on new insights into the molecular basis for primary endocrine therapy resistance”– should be further investigated, they wrote, noting that the triage approaches used in this study are being evaluated in the ongoing phase III ALTERNATE trial.
Dr. Ellis disclosed financial and other relationships with Bioclassifier, Novartis, Pfizer, Celgene, AstraZeneca, NanoString Technologies, and Prosigna.
FROM THE JOURNAL OF CLINICAL ONCOLOGY
Key clinical point:
Major finding: Four of 109 patients (3.7%) with a PEPI score of 0 relapsed after a median of 5.5 years (and were therefore unlikely to benefit from adjuvant chemotherapy), compared with 49 (14.4%) of 341 patients with a PEPI score greater than 0 (recurrence HR, 0.27).
Data source: The randomized phase II ACOSOG Z1031B trial.
Disclosures: Dr. Ellis disclosed financial and other relationships with Bioclassifier, Novartis, Pfizer, Celgene, AstraZeneca, NanoString Technologies, and Prosigna.
Gene, risk signatures could predict PARP inhibitor response in breast cancer
SAN ANTONIO – PARPi 7, BRCAness, and MammaPrint high1/(ultra)high2 signatures could help predict response to combination therapy with the poly ADP ribose polymerase (PARP) inhibitors veliparib and carboplatin among high-risk breast cancer patients and thereby improve patient care, according to findings from the I-SPY 2 clinical trial.
“I-SPY 2 is a phase II adaptively randomized neoadjuvant clinical trial with a shared control arm where patients receive standard neoadjuvant chemotherapy, and up to 4 simultaneous investigational arms. The primary endpoint of the trial is pathologic complete response, or pCR. The goal is to match therapies with the most responsive breast cancer subtypes,” Denise M. Wolf, PhD, of the University of California, San Francisco, explained at the San Antonio Breast Cancer Symposium.
The current analysis of I-Spy 2 data focuses on veliparib/carboplatin (VC), and the subtypes Dr. Wolf mentioned are defined by hormone receptor (HR) and HER2 expression, and by MammaPrint high1 or (ultra)high2 risk status, which, “roughly speaking, is a further stratification of the poor prognosis group into high- and extra-high-risk groups,” she said.
“This arm was open to HER2-negative patients, and graduated successfully in the triple-negative subset,” she noted, explaining that “agents or combinations graduate for efficacy if they reach 85% predicted probability of success in a subsequent phase III trial in the most responsive patient subset.”
The biomarker component of the trial aimed to evaluate biomarkers associated with the mechanisms of action of each investigational agent along with the predefined subsets.
Although the findings require verification in a larger trial, Dr. Wolf and her colleagues found that three biomarkers – the PARPi 7 gene signature, the 77-gene BRCAness signature (which both relate to DNA damage repair deficiency), and the MammaPrint high1 and (ultra)high2 risk categories – were each moderately correlated with treatment response in 72 patients receiving veliparib/carboplatin (VC), but not in 44 controls, and that the treatment-biomarker interactions retained statistical significance after adjusting for hormone receptor status.
“And so we asked the question, ‘Are these signatures identifying the same patients – and if not, might there be a way to combine them to identify a subset of patients who are especially likely to respond to VC?’” she said.
Further analysis showed that even though each of the biomarkers was a predictor of response, the biomarkers did not appear to identify exactly the same patients, therefore combining them might be of benefit.
“We did this using a simple voting scheme to combine pairs of biomarkers,” she said, adding that if the two paired biomarkers predicted resistance, the biomarker also predicted resistance. If only 1 predicted resistance, the combination predicted resistance, and only if both biomarkers predicted sensitivity did the combination predict sensitivity.
In the graduated triple-negative subset, for example, the 40% of patients who were PARPi 7–high and MammaPrint high2 (the two biomarkers most predictive of response) a dramatic separation was seen in the pCR probability curves, with an estimated pCR of 79% with VC treatment vs. 23% in the control arm.
“In contrast, triple-negative patients who were negative for one or more of the sensitivity markers had nearly overlapping probability response curves, from which we conclude that nearly all of the specific sensitivity to VC seen in the triple-negative patients is found in that subset who are positive for both sensitivity markers,” she said.
Additionally, although only 9% of HR-positive/HER2-negative patients were PARPi 7-high and MammaPrint high2, those patients also appeared to be more responsive to VC vs. the control arm (49% vs. 15%).
The results also demonstrate the value of an exploratory voting method for combining multiple biomarkers for the same treatment, Dr. Wolf noted.
However, the findings are limited by the small sample size and need to be evaluated in larger trials, and the biomarkers also need to be evaluated in carboplatin-only arms in order to “tease out whether the biomarkers are really specific to a PARP inhibitor with carboplatin or whether they might also apply to carboplatin alone.
“Our ongoing and future work is to develop predictive biomarkers for other I-Spy 2 agents,” she said.
Dr. Wolf reported having no disclosures.
SAN ANTONIO – PARPi 7, BRCAness, and MammaPrint high1/(ultra)high2 signatures could help predict response to combination therapy with the poly ADP ribose polymerase (PARP) inhibitors veliparib and carboplatin among high-risk breast cancer patients and thereby improve patient care, according to findings from the I-SPY 2 clinical trial.
“I-SPY 2 is a phase II adaptively randomized neoadjuvant clinical trial with a shared control arm where patients receive standard neoadjuvant chemotherapy, and up to 4 simultaneous investigational arms. The primary endpoint of the trial is pathologic complete response, or pCR. The goal is to match therapies with the most responsive breast cancer subtypes,” Denise M. Wolf, PhD, of the University of California, San Francisco, explained at the San Antonio Breast Cancer Symposium.
The current analysis of I-Spy 2 data focuses on veliparib/carboplatin (VC), and the subtypes Dr. Wolf mentioned are defined by hormone receptor (HR) and HER2 expression, and by MammaPrint high1 or (ultra)high2 risk status, which, “roughly speaking, is a further stratification of the poor prognosis group into high- and extra-high-risk groups,” she said.
“This arm was open to HER2-negative patients, and graduated successfully in the triple-negative subset,” she noted, explaining that “agents or combinations graduate for efficacy if they reach 85% predicted probability of success in a subsequent phase III trial in the most responsive patient subset.”
The biomarker component of the trial aimed to evaluate biomarkers associated with the mechanisms of action of each investigational agent along with the predefined subsets.
Although the findings require verification in a larger trial, Dr. Wolf and her colleagues found that three biomarkers – the PARPi 7 gene signature, the 77-gene BRCAness signature (which both relate to DNA damage repair deficiency), and the MammaPrint high1 and (ultra)high2 risk categories – were each moderately correlated with treatment response in 72 patients receiving veliparib/carboplatin (VC), but not in 44 controls, and that the treatment-biomarker interactions retained statistical significance after adjusting for hormone receptor status.
“And so we asked the question, ‘Are these signatures identifying the same patients – and if not, might there be a way to combine them to identify a subset of patients who are especially likely to respond to VC?’” she said.
Further analysis showed that even though each of the biomarkers was a predictor of response, the biomarkers did not appear to identify exactly the same patients, therefore combining them might be of benefit.
“We did this using a simple voting scheme to combine pairs of biomarkers,” she said, adding that if the two paired biomarkers predicted resistance, the biomarker also predicted resistance. If only 1 predicted resistance, the combination predicted resistance, and only if both biomarkers predicted sensitivity did the combination predict sensitivity.
In the graduated triple-negative subset, for example, the 40% of patients who were PARPi 7–high and MammaPrint high2 (the two biomarkers most predictive of response) a dramatic separation was seen in the pCR probability curves, with an estimated pCR of 79% with VC treatment vs. 23% in the control arm.
“In contrast, triple-negative patients who were negative for one or more of the sensitivity markers had nearly overlapping probability response curves, from which we conclude that nearly all of the specific sensitivity to VC seen in the triple-negative patients is found in that subset who are positive for both sensitivity markers,” she said.
Additionally, although only 9% of HR-positive/HER2-negative patients were PARPi 7-high and MammaPrint high2, those patients also appeared to be more responsive to VC vs. the control arm (49% vs. 15%).
The results also demonstrate the value of an exploratory voting method for combining multiple biomarkers for the same treatment, Dr. Wolf noted.
However, the findings are limited by the small sample size and need to be evaluated in larger trials, and the biomarkers also need to be evaluated in carboplatin-only arms in order to “tease out whether the biomarkers are really specific to a PARP inhibitor with carboplatin or whether they might also apply to carboplatin alone.
“Our ongoing and future work is to develop predictive biomarkers for other I-Spy 2 agents,” she said.
Dr. Wolf reported having no disclosures.
SAN ANTONIO – PARPi 7, BRCAness, and MammaPrint high1/(ultra)high2 signatures could help predict response to combination therapy with the poly ADP ribose polymerase (PARP) inhibitors veliparib and carboplatin among high-risk breast cancer patients and thereby improve patient care, according to findings from the I-SPY 2 clinical trial.
“I-SPY 2 is a phase II adaptively randomized neoadjuvant clinical trial with a shared control arm where patients receive standard neoadjuvant chemotherapy, and up to 4 simultaneous investigational arms. The primary endpoint of the trial is pathologic complete response, or pCR. The goal is to match therapies with the most responsive breast cancer subtypes,” Denise M. Wolf, PhD, of the University of California, San Francisco, explained at the San Antonio Breast Cancer Symposium.
The current analysis of I-Spy 2 data focuses on veliparib/carboplatin (VC), and the subtypes Dr. Wolf mentioned are defined by hormone receptor (HR) and HER2 expression, and by MammaPrint high1 or (ultra)high2 risk status, which, “roughly speaking, is a further stratification of the poor prognosis group into high- and extra-high-risk groups,” she said.
“This arm was open to HER2-negative patients, and graduated successfully in the triple-negative subset,” she noted, explaining that “agents or combinations graduate for efficacy if they reach 85% predicted probability of success in a subsequent phase III trial in the most responsive patient subset.”
The biomarker component of the trial aimed to evaluate biomarkers associated with the mechanisms of action of each investigational agent along with the predefined subsets.
Although the findings require verification in a larger trial, Dr. Wolf and her colleagues found that three biomarkers – the PARPi 7 gene signature, the 77-gene BRCAness signature (which both relate to DNA damage repair deficiency), and the MammaPrint high1 and (ultra)high2 risk categories – were each moderately correlated with treatment response in 72 patients receiving veliparib/carboplatin (VC), but not in 44 controls, and that the treatment-biomarker interactions retained statistical significance after adjusting for hormone receptor status.
“And so we asked the question, ‘Are these signatures identifying the same patients – and if not, might there be a way to combine them to identify a subset of patients who are especially likely to respond to VC?’” she said.
Further analysis showed that even though each of the biomarkers was a predictor of response, the biomarkers did not appear to identify exactly the same patients, therefore combining them might be of benefit.
“We did this using a simple voting scheme to combine pairs of biomarkers,” she said, adding that if the two paired biomarkers predicted resistance, the biomarker also predicted resistance. If only 1 predicted resistance, the combination predicted resistance, and only if both biomarkers predicted sensitivity did the combination predict sensitivity.
In the graduated triple-negative subset, for example, the 40% of patients who were PARPi 7–high and MammaPrint high2 (the two biomarkers most predictive of response) a dramatic separation was seen in the pCR probability curves, with an estimated pCR of 79% with VC treatment vs. 23% in the control arm.
“In contrast, triple-negative patients who were negative for one or more of the sensitivity markers had nearly overlapping probability response curves, from which we conclude that nearly all of the specific sensitivity to VC seen in the triple-negative patients is found in that subset who are positive for both sensitivity markers,” she said.
Additionally, although only 9% of HR-positive/HER2-negative patients were PARPi 7-high and MammaPrint high2, those patients also appeared to be more responsive to VC vs. the control arm (49% vs. 15%).
The results also demonstrate the value of an exploratory voting method for combining multiple biomarkers for the same treatment, Dr. Wolf noted.
However, the findings are limited by the small sample size and need to be evaluated in larger trials, and the biomarkers also need to be evaluated in carboplatin-only arms in order to “tease out whether the biomarkers are really specific to a PARP inhibitor with carboplatin or whether they might also apply to carboplatin alone.
“Our ongoing and future work is to develop predictive biomarkers for other I-Spy 2 agents,” she said.
Dr. Wolf reported having no disclosures.
AT SABCS 2016
Key clinical point:
Major finding: Estimated pCR in PARPi 7–high and MammaPrint 2 triple-negative patients was 79% vs. 23% for the VC arm vs. control arm.
Data source: The phase II adaptively randomized I-Spy 2 clinical trial of 116 subjects.
Disclosures: Dr. Wolf reported having no disclosures.
SLND after neoadjuvant chemo is feasible, but more study needed
SAN ANTONIO – Sentinel lymph node detection after neoadjuvant chemotherapy (NAC) is a safe and feasible strategy for preventing unnecessary systematic lymphadenectomy in patients with operable breast cancer and no clinical signs of cancer in the axillary lymph nodes prior to NAC, according to findings from the French prospective multicenter GANEA 2 trial.
However, further study is needed to assess the clinical impact of the 12% false negative rate associated with sentinel lymph node detection (SLND) in the current study, according to Jean-Marc Classe, MD, who reported the findings at the San Antonio Breast Cancer Symposium.
SLND was feasible in that it was achieved in 570 of 590 women (97%) with large operable breast tumors and negative findings on axillary sonography with fine needle cytology who were enrolled in the study, said Dr. Classe of Institut Cancerologie de l’Ouest Rene Gauducheau, Nantes, France.
Cancer cells were detected by SLND in 139 subjects after NAC and surgery, and all of those patients underwent axillary lymph node dissection. Another 418 had no sentinel node involvement after NAC and surgery, and had adequate follow-up; among those, overall 3-year survival was 97.8% and 3-year disease-free survival was 94.8%,
“In this group of patients ... we found only one axillary relapse,” he said.
These rates are comparable to historical survival rates among those without axillary involvement who undergo axillary lymph node dissection rather than sentinel lymph node detection, and the findings suggest that women with no clinical signs of axillary involvement could be spared systematic lymphadenectomy, he said.
“The standard surgical treatment after neoadjuvant chemotherapy is breast cancer surgery and lymphadenectomy level 1 and 2, but since the [National Surgical Adjuvant Breast and Bowel Project] B-27 trial, we all know that after neoadjuvant chemotherapy there are not any involved nodes in 50%-58% of patients,” he said, adding that about half of all lymphadenectomies in these patients are therefore unnecessary.
That percentage increases to more than 70% in “the very specific situation of patients treated for HER2+ breast cancer with cytologically proved axillary metastases after neoadjuvant chemotherapy,” he said.
“So we know that there is a place for sentinel lymph node biopsy after neoadjuvant chemotherapy in order to avoid unnecessary lymphadenectomy,” he said.
However, the high false negative rate associated with SLND in this and in prior studies, including the first GANEA trial, remains a concern. In fact, the most recent guidelines stated that the proof was too weak to strongly recommend sentinel lymph node biopsy after NAC, he noted.
The GANEA 2 trial was performed in response to a call in those guidelines for additional studies to assess the long-term risks of this strategy.
Study subjects included patients with FIGO stage T1-T3 infiltrating breast cancer who were enrolled from 15 French institutions between July 2010 and February 2014. Those with inflammatory cancer, local relapse, contraindications for NAC, or interrupted NAC due to progressive disease were excluded.
Follow-up included a medical visit with clinical assessment every 6 months and annual mammography.
The findings suggest that in patients with no proof of node involvement before treatment, SLND “seems to be safe within the limits of the short-term follow-up of this study,” Dr. Classe said, noting that given the concerns about the false negative rate and the uncertainty about the clinical impact of that, this approach “is not proved to be a safe procedure outside of trials.”
The strategy will be further evaluated, with a focus on eliminating false negative results, in the GANEA 3 trial, he said.
Dr. Classe reported having no disclosures.
SAN ANTONIO – Sentinel lymph node detection after neoadjuvant chemotherapy (NAC) is a safe and feasible strategy for preventing unnecessary systematic lymphadenectomy in patients with operable breast cancer and no clinical signs of cancer in the axillary lymph nodes prior to NAC, according to findings from the French prospective multicenter GANEA 2 trial.
However, further study is needed to assess the clinical impact of the 12% false negative rate associated with sentinel lymph node detection (SLND) in the current study, according to Jean-Marc Classe, MD, who reported the findings at the San Antonio Breast Cancer Symposium.
SLND was feasible in that it was achieved in 570 of 590 women (97%) with large operable breast tumors and negative findings on axillary sonography with fine needle cytology who were enrolled in the study, said Dr. Classe of Institut Cancerologie de l’Ouest Rene Gauducheau, Nantes, France.
Cancer cells were detected by SLND in 139 subjects after NAC and surgery, and all of those patients underwent axillary lymph node dissection. Another 418 had no sentinel node involvement after NAC and surgery, and had adequate follow-up; among those, overall 3-year survival was 97.8% and 3-year disease-free survival was 94.8%,
“In this group of patients ... we found only one axillary relapse,” he said.
These rates are comparable to historical survival rates among those without axillary involvement who undergo axillary lymph node dissection rather than sentinel lymph node detection, and the findings suggest that women with no clinical signs of axillary involvement could be spared systematic lymphadenectomy, he said.
“The standard surgical treatment after neoadjuvant chemotherapy is breast cancer surgery and lymphadenectomy level 1 and 2, but since the [National Surgical Adjuvant Breast and Bowel Project] B-27 trial, we all know that after neoadjuvant chemotherapy there are not any involved nodes in 50%-58% of patients,” he said, adding that about half of all lymphadenectomies in these patients are therefore unnecessary.
That percentage increases to more than 70% in “the very specific situation of patients treated for HER2+ breast cancer with cytologically proved axillary metastases after neoadjuvant chemotherapy,” he said.
“So we know that there is a place for sentinel lymph node biopsy after neoadjuvant chemotherapy in order to avoid unnecessary lymphadenectomy,” he said.
However, the high false negative rate associated with SLND in this and in prior studies, including the first GANEA trial, remains a concern. In fact, the most recent guidelines stated that the proof was too weak to strongly recommend sentinel lymph node biopsy after NAC, he noted.
The GANEA 2 trial was performed in response to a call in those guidelines for additional studies to assess the long-term risks of this strategy.
Study subjects included patients with FIGO stage T1-T3 infiltrating breast cancer who were enrolled from 15 French institutions between July 2010 and February 2014. Those with inflammatory cancer, local relapse, contraindications for NAC, or interrupted NAC due to progressive disease were excluded.
Follow-up included a medical visit with clinical assessment every 6 months and annual mammography.
The findings suggest that in patients with no proof of node involvement before treatment, SLND “seems to be safe within the limits of the short-term follow-up of this study,” Dr. Classe said, noting that given the concerns about the false negative rate and the uncertainty about the clinical impact of that, this approach “is not proved to be a safe procedure outside of trials.”
The strategy will be further evaluated, with a focus on eliminating false negative results, in the GANEA 3 trial, he said.
Dr. Classe reported having no disclosures.
SAN ANTONIO – Sentinel lymph node detection after neoadjuvant chemotherapy (NAC) is a safe and feasible strategy for preventing unnecessary systematic lymphadenectomy in patients with operable breast cancer and no clinical signs of cancer in the axillary lymph nodes prior to NAC, according to findings from the French prospective multicenter GANEA 2 trial.
However, further study is needed to assess the clinical impact of the 12% false negative rate associated with sentinel lymph node detection (SLND) in the current study, according to Jean-Marc Classe, MD, who reported the findings at the San Antonio Breast Cancer Symposium.
SLND was feasible in that it was achieved in 570 of 590 women (97%) with large operable breast tumors and negative findings on axillary sonography with fine needle cytology who were enrolled in the study, said Dr. Classe of Institut Cancerologie de l’Ouest Rene Gauducheau, Nantes, France.
Cancer cells were detected by SLND in 139 subjects after NAC and surgery, and all of those patients underwent axillary lymph node dissection. Another 418 had no sentinel node involvement after NAC and surgery, and had adequate follow-up; among those, overall 3-year survival was 97.8% and 3-year disease-free survival was 94.8%,
“In this group of patients ... we found only one axillary relapse,” he said.
These rates are comparable to historical survival rates among those without axillary involvement who undergo axillary lymph node dissection rather than sentinel lymph node detection, and the findings suggest that women with no clinical signs of axillary involvement could be spared systematic lymphadenectomy, he said.
“The standard surgical treatment after neoadjuvant chemotherapy is breast cancer surgery and lymphadenectomy level 1 and 2, but since the [National Surgical Adjuvant Breast and Bowel Project] B-27 trial, we all know that after neoadjuvant chemotherapy there are not any involved nodes in 50%-58% of patients,” he said, adding that about half of all lymphadenectomies in these patients are therefore unnecessary.
That percentage increases to more than 70% in “the very specific situation of patients treated for HER2+ breast cancer with cytologically proved axillary metastases after neoadjuvant chemotherapy,” he said.
“So we know that there is a place for sentinel lymph node biopsy after neoadjuvant chemotherapy in order to avoid unnecessary lymphadenectomy,” he said.
However, the high false negative rate associated with SLND in this and in prior studies, including the first GANEA trial, remains a concern. In fact, the most recent guidelines stated that the proof was too weak to strongly recommend sentinel lymph node biopsy after NAC, he noted.
The GANEA 2 trial was performed in response to a call in those guidelines for additional studies to assess the long-term risks of this strategy.
Study subjects included patients with FIGO stage T1-T3 infiltrating breast cancer who were enrolled from 15 French institutions between July 2010 and February 2014. Those with inflammatory cancer, local relapse, contraindications for NAC, or interrupted NAC due to progressive disease were excluded.
Follow-up included a medical visit with clinical assessment every 6 months and annual mammography.
The findings suggest that in patients with no proof of node involvement before treatment, SLND “seems to be safe within the limits of the short-term follow-up of this study,” Dr. Classe said, noting that given the concerns about the false negative rate and the uncertainty about the clinical impact of that, this approach “is not proved to be a safe procedure outside of trials.”
The strategy will be further evaluated, with a focus on eliminating false negative results, in the GANEA 3 trial, he said.
Dr. Classe reported having no disclosures.
AT SABCS 2016
Key clinical point:
Major finding: Overall 3-year survival was 97.8% and 3-year disease-free survival was 94.8% in 418 breast cancer patients who had no sentinel node involvement after NAC and surgery.
Data source: The prospective multicenter GANEA 2 trial of 590 patients.
Disclosures: Dr. Classe reported having no disclosures.
Cooling device reduces breast cancer–related alopecia during chemotherapy
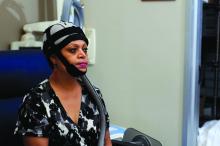
SAN ANTONIO – About half of the women with stage 1 or 2 breast cancer who were treated using a scalp cooling device during chemotherapy retained their hair in the prospective, randomized Scalp Cooling Alopecia Prevention (SCALP) trial.
Of 229 women who were enrolled from seven U.S. sites between December 2013 and September 2016 and who planned to undergo at least four cycles of anthracycline- or taxane-based chemotherapy, 182 met eligibility criteria; 119 were randomized to undergo scalp cooling using the Orbis Paxman Hair Loss Prevention System (OPHLPS; Paxman Coolers) and 63 were assigned to a control group. Of those, 95 and 47, respectively, were evaluable and had completed four cycles of chemotherapy at the time of a preplanned interim analysis.
The study was stopped after the interim analysis because of the positive superiority in the treatment group, Dr. Nangia said.
Study subjects were women with early-stage breast cancer and planned neoadjuvant or adjuvant chemotherapy. Most (65%) received taxane-based chemotherapy and 35% received anthracycline-based chemotherapy; the latter has been shown to be associated with higher rates of hair loss.
The OPHLPS system involves the use of a “cold cap” that is cooled using a refrigeration system and fitted to a patient’s head during chemotherapy treatment. For the current study, subjects underwent scalp cooling for 30 minutes before chemotherapy, during chemotherapy, and for 90 minutes after.
Most subjects (39%-52%) reported that they were reasonably comfortable during use of the device during treatment cycles 1-4. Only 2.4% reported being very uncomfortable, and that was only during cycle 2.
An analysis based on type of therapy showed that 65.1% of those receiving a taxane experienced hair preservation, compared with 21.9% of those receiving an anthracycline.
Scalp cooling, which reduces blood flow and chemoexposure to the hair follicles and thereby reduces hair loss, is widely used in Europe and, despite a great deal of interest in the technology in the United States, the Food and Drug Administration has been slow to get on board because of concerns over the potential for scalp metastasis. However, long-term outcomes data from Europe demonstrate that scalp metastasis is “exceedingly rare” and has occurred only in those with metastasis throughout the body, Dr. Nangia said.
Those data opened the door to the current study – the first prospective randomized trial of scalp-cooling.
In response to questions about the effects of scalp cooling across additional chemotherapy cycles (for example, those who undergo eight cycles), Dr. Nangia said that all patients completed four cycles, and data for those with eight cycles planned will be included in a final analysis.
“In other countries, they do have success rates with eight cycles, which is standard for some women,” she said. SCALP trial subjects will also be followed for 5 years to monitor overall survival, recurrence of cancer, and potential metastasis to the scalp, she said.
Based on the findings of the study, the maker of the device is seeking FDA clearance. If approved, the OPHLPS would compete with the DigniCap (Dignitana), which has already received approval.
“Scalp cooling devices are highly effective and should become available to women with breast cancer receiving chemotherapy ... Further study should be done exploring the technology for other types of tumors and with other chemotherapy regimens. More study looking at the impact of chemotherapy-induced alopecia on psyche and body image should be performed as well,” Dr. Nangia concluded, noting that tailored quality-of-life tools are needed to evaluate the impact of alopecia on quality of life.
Dr. Nangia reported receiving research funding from Paxman, the sponsor of the study, to her institution.
SAN ANTONIO – About half of the women with stage 1 or 2 breast cancer who were treated using a scalp cooling device during chemotherapy retained their hair in the prospective, randomized Scalp Cooling Alopecia Prevention (SCALP) trial.
Of 229 women who were enrolled from seven U.S. sites between December 2013 and September 2016 and who planned to undergo at least four cycles of anthracycline- or taxane-based chemotherapy, 182 met eligibility criteria; 119 were randomized to undergo scalp cooling using the Orbis Paxman Hair Loss Prevention System (OPHLPS; Paxman Coolers) and 63 were assigned to a control group. Of those, 95 and 47, respectively, were evaluable and had completed four cycles of chemotherapy at the time of a preplanned interim analysis.
The study was stopped after the interim analysis because of the positive superiority in the treatment group, Dr. Nangia said.
Study subjects were women with early-stage breast cancer and planned neoadjuvant or adjuvant chemotherapy. Most (65%) received taxane-based chemotherapy and 35% received anthracycline-based chemotherapy; the latter has been shown to be associated with higher rates of hair loss.
The OPHLPS system involves the use of a “cold cap” that is cooled using a refrigeration system and fitted to a patient’s head during chemotherapy treatment. For the current study, subjects underwent scalp cooling for 30 minutes before chemotherapy, during chemotherapy, and for 90 minutes after.
Most subjects (39%-52%) reported that they were reasonably comfortable during use of the device during treatment cycles 1-4. Only 2.4% reported being very uncomfortable, and that was only during cycle 2.
An analysis based on type of therapy showed that 65.1% of those receiving a taxane experienced hair preservation, compared with 21.9% of those receiving an anthracycline.
Scalp cooling, which reduces blood flow and chemoexposure to the hair follicles and thereby reduces hair loss, is widely used in Europe and, despite a great deal of interest in the technology in the United States, the Food and Drug Administration has been slow to get on board because of concerns over the potential for scalp metastasis. However, long-term outcomes data from Europe demonstrate that scalp metastasis is “exceedingly rare” and has occurred only in those with metastasis throughout the body, Dr. Nangia said.
Those data opened the door to the current study – the first prospective randomized trial of scalp-cooling.
In response to questions about the effects of scalp cooling across additional chemotherapy cycles (for example, those who undergo eight cycles), Dr. Nangia said that all patients completed four cycles, and data for those with eight cycles planned will be included in a final analysis.
“In other countries, they do have success rates with eight cycles, which is standard for some women,” she said. SCALP trial subjects will also be followed for 5 years to monitor overall survival, recurrence of cancer, and potential metastasis to the scalp, she said.
Based on the findings of the study, the maker of the device is seeking FDA clearance. If approved, the OPHLPS would compete with the DigniCap (Dignitana), which has already received approval.
“Scalp cooling devices are highly effective and should become available to women with breast cancer receiving chemotherapy ... Further study should be done exploring the technology for other types of tumors and with other chemotherapy regimens. More study looking at the impact of chemotherapy-induced alopecia on psyche and body image should be performed as well,” Dr. Nangia concluded, noting that tailored quality-of-life tools are needed to evaluate the impact of alopecia on quality of life.
Dr. Nangia reported receiving research funding from Paxman, the sponsor of the study, to her institution.
SAN ANTONIO – About half of the women with stage 1 or 2 breast cancer who were treated using a scalp cooling device during chemotherapy retained their hair in the prospective, randomized Scalp Cooling Alopecia Prevention (SCALP) trial.
Of 229 women who were enrolled from seven U.S. sites between December 2013 and September 2016 and who planned to undergo at least four cycles of anthracycline- or taxane-based chemotherapy, 182 met eligibility criteria; 119 were randomized to undergo scalp cooling using the Orbis Paxman Hair Loss Prevention System (OPHLPS; Paxman Coolers) and 63 were assigned to a control group. Of those, 95 and 47, respectively, were evaluable and had completed four cycles of chemotherapy at the time of a preplanned interim analysis.
The study was stopped after the interim analysis because of the positive superiority in the treatment group, Dr. Nangia said.
Study subjects were women with early-stage breast cancer and planned neoadjuvant or adjuvant chemotherapy. Most (65%) received taxane-based chemotherapy and 35% received anthracycline-based chemotherapy; the latter has been shown to be associated with higher rates of hair loss.
The OPHLPS system involves the use of a “cold cap” that is cooled using a refrigeration system and fitted to a patient’s head during chemotherapy treatment. For the current study, subjects underwent scalp cooling for 30 minutes before chemotherapy, during chemotherapy, and for 90 minutes after.
Most subjects (39%-52%) reported that they were reasonably comfortable during use of the device during treatment cycles 1-4. Only 2.4% reported being very uncomfortable, and that was only during cycle 2.
An analysis based on type of therapy showed that 65.1% of those receiving a taxane experienced hair preservation, compared with 21.9% of those receiving an anthracycline.
Scalp cooling, which reduces blood flow and chemoexposure to the hair follicles and thereby reduces hair loss, is widely used in Europe and, despite a great deal of interest in the technology in the United States, the Food and Drug Administration has been slow to get on board because of concerns over the potential for scalp metastasis. However, long-term outcomes data from Europe demonstrate that scalp metastasis is “exceedingly rare” and has occurred only in those with metastasis throughout the body, Dr. Nangia said.
Those data opened the door to the current study – the first prospective randomized trial of scalp-cooling.
In response to questions about the effects of scalp cooling across additional chemotherapy cycles (for example, those who undergo eight cycles), Dr. Nangia said that all patients completed four cycles, and data for those with eight cycles planned will be included in a final analysis.
“In other countries, they do have success rates with eight cycles, which is standard for some women,” she said. SCALP trial subjects will also be followed for 5 years to monitor overall survival, recurrence of cancer, and potential metastasis to the scalp, she said.
Based on the findings of the study, the maker of the device is seeking FDA clearance. If approved, the OPHLPS would compete with the DigniCap (Dignitana), which has already received approval.
“Scalp cooling devices are highly effective and should become available to women with breast cancer receiving chemotherapy ... Further study should be done exploring the technology for other types of tumors and with other chemotherapy regimens. More study looking at the impact of chemotherapy-induced alopecia on psyche and body image should be performed as well,” Dr. Nangia concluded, noting that tailored quality-of-life tools are needed to evaluate the impact of alopecia on quality of life.
Dr. Nangia reported receiving research funding from Paxman, the sponsor of the study, to her institution.
AT SABCS 2016
Key clinical point:
Major finding: 50.5% of treated patients experienced no more than grade 1 alopecia (less than 50% hair loss), compared with 0% of control subjects.
Data source: The prospective, randomized SCALP trial involving 182 women.
Disclosures: Dr. Nangia reported receiving research funding from Paxman, sponsor of the study, to her institution.
VIDEO: Abemaciclib reduces Ki67 expression in early HR+/HER2– breast cancer
SAN ANTONIO – Abemaciclib, both alone and in combination with anastrozole, significantly reduced Ki67 expression vs. anastrozole monotherapy after 2 weeks of treatment in the NeoMONARCH phase II neoadjuvant clinical trial of postmenopausal patients with hormone receptor–positive, HER2-negative early-stage breast cancer.
The findings, given that a change in Ki67 at 2 weeks in neoadjuvant studies appears to predict improved disease-free survival in adjuvant studies, support continued evaluation of the cyclin-dependent kinase-4 (CDK4) inhibitor for the treatment of patients with early-stage breast cancer, Sara Hurvitz, MD, reported at the San Antonio Breast Cancer Symposium.
“In hormone receptor–positive breast cancers, estrogen stimulates D-type cyclins, resulting in increased activity of CDK4 and CDK6, and then phosphorylate RB – the tumor suppressor protein retinoblastoma – which releases the E2F transcription factor,” explained Dr. Hurvitz of the University of California, Los Angeles.
This in turn ultimately leads to cell cycle progression from G1 to S.
“This increased rate of proliferation can be observed in tumor tissue samples by measuring the expression of Ki67. Blocking CDK4 and CDK6 should lead to a decrease in E2F expression, as well as a drop in the cell cycling and a drop in Ki67,” she said, adding that cell cycle arrest may induce senescence, which may also induce a phenotype that’s characterized by an immune cell infiltrate.
Indeed, in study subjects randomized to receive abemaciclib, treatment was shown to induce profound cell cycle arrest defined by decreased Ki67 and E2F targeted proliferation messenger RNAs, and reduction of expression of genes associated with senescence.
“Abemaciclib alone or in combination with anastrozole significantly reduced the Ki67 expression compared to anastrozole alone after 2 weeks of therapy, based on the geometric mean change and complete cell cycle arrest, and the study did meet its primary endpoint,” she said.
In a video interview, Dr. Hurvitz discussed the study methodology, results, and safety findings, as well as an intriguing observation regarding the effects of treatment on tumor differentiation and immune infiltrates over time.
This study was sponsored by Eli Lilly. Dr. Hurvitz has received renumeration for research and/or travel from Amgen, Bayer, BioMarin, Boehringer Ingelheim, Dignitana, Eli Lilly, Genentech, GSK, Medivation, Merrimack, Novartis, OBI Pharma, Pfizer, Puma Biotechnology, and Roche.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
SAN ANTONIO – Abemaciclib, both alone and in combination with anastrozole, significantly reduced Ki67 expression vs. anastrozole monotherapy after 2 weeks of treatment in the NeoMONARCH phase II neoadjuvant clinical trial of postmenopausal patients with hormone receptor–positive, HER2-negative early-stage breast cancer.
The findings, given that a change in Ki67 at 2 weeks in neoadjuvant studies appears to predict improved disease-free survival in adjuvant studies, support continued evaluation of the cyclin-dependent kinase-4 (CDK4) inhibitor for the treatment of patients with early-stage breast cancer, Sara Hurvitz, MD, reported at the San Antonio Breast Cancer Symposium.
“In hormone receptor–positive breast cancers, estrogen stimulates D-type cyclins, resulting in increased activity of CDK4 and CDK6, and then phosphorylate RB – the tumor suppressor protein retinoblastoma – which releases the E2F transcription factor,” explained Dr. Hurvitz of the University of California, Los Angeles.
This in turn ultimately leads to cell cycle progression from G1 to S.
“This increased rate of proliferation can be observed in tumor tissue samples by measuring the expression of Ki67. Blocking CDK4 and CDK6 should lead to a decrease in E2F expression, as well as a drop in the cell cycling and a drop in Ki67,” she said, adding that cell cycle arrest may induce senescence, which may also induce a phenotype that’s characterized by an immune cell infiltrate.
Indeed, in study subjects randomized to receive abemaciclib, treatment was shown to induce profound cell cycle arrest defined by decreased Ki67 and E2F targeted proliferation messenger RNAs, and reduction of expression of genes associated with senescence.
“Abemaciclib alone or in combination with anastrozole significantly reduced the Ki67 expression compared to anastrozole alone after 2 weeks of therapy, based on the geometric mean change and complete cell cycle arrest, and the study did meet its primary endpoint,” she said.
In a video interview, Dr. Hurvitz discussed the study methodology, results, and safety findings, as well as an intriguing observation regarding the effects of treatment on tumor differentiation and immune infiltrates over time.
This study was sponsored by Eli Lilly. Dr. Hurvitz has received renumeration for research and/or travel from Amgen, Bayer, BioMarin, Boehringer Ingelheim, Dignitana, Eli Lilly, Genentech, GSK, Medivation, Merrimack, Novartis, OBI Pharma, Pfizer, Puma Biotechnology, and Roche.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
SAN ANTONIO – Abemaciclib, both alone and in combination with anastrozole, significantly reduced Ki67 expression vs. anastrozole monotherapy after 2 weeks of treatment in the NeoMONARCH phase II neoadjuvant clinical trial of postmenopausal patients with hormone receptor–positive, HER2-negative early-stage breast cancer.
The findings, given that a change in Ki67 at 2 weeks in neoadjuvant studies appears to predict improved disease-free survival in adjuvant studies, support continued evaluation of the cyclin-dependent kinase-4 (CDK4) inhibitor for the treatment of patients with early-stage breast cancer, Sara Hurvitz, MD, reported at the San Antonio Breast Cancer Symposium.
“In hormone receptor–positive breast cancers, estrogen stimulates D-type cyclins, resulting in increased activity of CDK4 and CDK6, and then phosphorylate RB – the tumor suppressor protein retinoblastoma – which releases the E2F transcription factor,” explained Dr. Hurvitz of the University of California, Los Angeles.
This in turn ultimately leads to cell cycle progression from G1 to S.
“This increased rate of proliferation can be observed in tumor tissue samples by measuring the expression of Ki67. Blocking CDK4 and CDK6 should lead to a decrease in E2F expression, as well as a drop in the cell cycling and a drop in Ki67,” she said, adding that cell cycle arrest may induce senescence, which may also induce a phenotype that’s characterized by an immune cell infiltrate.
Indeed, in study subjects randomized to receive abemaciclib, treatment was shown to induce profound cell cycle arrest defined by decreased Ki67 and E2F targeted proliferation messenger RNAs, and reduction of expression of genes associated with senescence.
“Abemaciclib alone or in combination with anastrozole significantly reduced the Ki67 expression compared to anastrozole alone after 2 weeks of therapy, based on the geometric mean change and complete cell cycle arrest, and the study did meet its primary endpoint,” she said.
In a video interview, Dr. Hurvitz discussed the study methodology, results, and safety findings, as well as an intriguing observation regarding the effects of treatment on tumor differentiation and immune infiltrates over time.
This study was sponsored by Eli Lilly. Dr. Hurvitz has received renumeration for research and/or travel from Amgen, Bayer, BioMarin, Boehringer Ingelheim, Dignitana, Eli Lilly, Genentech, GSK, Medivation, Merrimack, Novartis, OBI Pharma, Pfizer, Puma Biotechnology, and Roche.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
AT SABCS 2016
VIDEO: No effect of BRCA status on overall outcomes in early-onset breast cancer
SAN ANTONIO – No difference in outcomes was seen among young BRCA gene mutation carriers and noncarriers with early-stage invasive breast cancer in a large cohort in the United Kingdom, but a subgroup analysis of those with triple-negative breast cancer showed a clear survival advantage among BRCA mutation carriers.
The findings of the POSH (Prospective Study of Outcomes in Sporadic Versus Hereditary Breast Cancer) trial have important implications for treatment decision making – particularly with respect to surgery – for younger women with breast cancer, according to Diana M. Eccles, MD, of the University of Southampton (England) and University Hospital Southampton Foundation Trust.
The overall finding of no difference in outcomes – including for the primary endpoint of overall survival and secondary endpoints of overall and distant disease-free survival – was true in both univariate and multivariable analysis of data for 2,759 women (14% with BRCA mutations), aged 40 years and younger, who were enrolled over an 8-year period, beginning in 2000, from 126 oncology centers across the United Kingdom, and who were followed for a median of 8.2 years, Dr. Eccles reported at the San Antonio Breast Cancer Symposium.
“We looked at BRCA1 and BRCA2 separately, and we still could see absolutely no difference in survival between BRCA1 and BRCA2, and we were well powered to show a difference,” she said, noting that 99% of these patients were diagnosed based on symptomatic presentation and did not know they were BRCA mutation carriers.
The findings were different among 511 patients with triple-negative breast cancer, however,
“Here we did see a clear difference in survival in favor of BRCA gene carriers – 11% at 10 years. And what was interesting was this clear time-varying hazard of relapse, with the maximum benefit of surviving in the BRCA gene carriers observed in the first few years with a hazard for relapse at 5 years equivalent between the two groups,” she said.
The primary treatment approach in these patients was no different than that in the group as a whole, Dr. Eccles noted, explaining that those with and without triple-negative disease had similar usage of breast conserving vs. unilateral surgery and similar usage of anthracycline-based chemotherapy regimens, with taxane added in a small proportion, which was “very typical for treatment regimens at the time.”
A proportion of women with BRCA gene mutations that were found in the clinical setting had opted to have bilateral mastectomy (15% vs. 3% of noncarriers) “closely following or during their primary treatment diagnosis,” and a separate analysis showed an intriguing survival benefit among those who did not have bilateral mastectomy; survival was 2% better among those women at 5 and 10 years, again with a time-varying hazard, Dr. Eccles said, noting, however, that the study was not powered to show a difference in this measure.
“It’s intriguing that bilateral mastectomy after diagnosis does not seem to improve survival in these young gene carriers. These are very small numbers so we have to be careful, but this is good news for patients. Many patients believe that being a BRCA gene carrier, or even just having a family history is going to give them an adverse prognosis, and that’s clearly not true – and it’s also important that patients who are facing the difficult, difficult decisions around chemotherapy and breast cancer treatment don’t feel compelled to make a decision about bilateral mastectomy within that time shortly after their diagnosis and can reasonably reserve judgment about the extent of surgery until a later date,” she said.
She discussed her findings further in a video interview.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
SAN ANTONIO – No difference in outcomes was seen among young BRCA gene mutation carriers and noncarriers with early-stage invasive breast cancer in a large cohort in the United Kingdom, but a subgroup analysis of those with triple-negative breast cancer showed a clear survival advantage among BRCA mutation carriers.
The findings of the POSH (Prospective Study of Outcomes in Sporadic Versus Hereditary Breast Cancer) trial have important implications for treatment decision making – particularly with respect to surgery – for younger women with breast cancer, according to Diana M. Eccles, MD, of the University of Southampton (England) and University Hospital Southampton Foundation Trust.
The overall finding of no difference in outcomes – including for the primary endpoint of overall survival and secondary endpoints of overall and distant disease-free survival – was true in both univariate and multivariable analysis of data for 2,759 women (14% with BRCA mutations), aged 40 years and younger, who were enrolled over an 8-year period, beginning in 2000, from 126 oncology centers across the United Kingdom, and who were followed for a median of 8.2 years, Dr. Eccles reported at the San Antonio Breast Cancer Symposium.
“We looked at BRCA1 and BRCA2 separately, and we still could see absolutely no difference in survival between BRCA1 and BRCA2, and we were well powered to show a difference,” she said, noting that 99% of these patients were diagnosed based on symptomatic presentation and did not know they were BRCA mutation carriers.
The findings were different among 511 patients with triple-negative breast cancer, however,
“Here we did see a clear difference in survival in favor of BRCA gene carriers – 11% at 10 years. And what was interesting was this clear time-varying hazard of relapse, with the maximum benefit of surviving in the BRCA gene carriers observed in the first few years with a hazard for relapse at 5 years equivalent between the two groups,” she said.
The primary treatment approach in these patients was no different than that in the group as a whole, Dr. Eccles noted, explaining that those with and without triple-negative disease had similar usage of breast conserving vs. unilateral surgery and similar usage of anthracycline-based chemotherapy regimens, with taxane added in a small proportion, which was “very typical for treatment regimens at the time.”
A proportion of women with BRCA gene mutations that were found in the clinical setting had opted to have bilateral mastectomy (15% vs. 3% of noncarriers) “closely following or during their primary treatment diagnosis,” and a separate analysis showed an intriguing survival benefit among those who did not have bilateral mastectomy; survival was 2% better among those women at 5 and 10 years, again with a time-varying hazard, Dr. Eccles said, noting, however, that the study was not powered to show a difference in this measure.
“It’s intriguing that bilateral mastectomy after diagnosis does not seem to improve survival in these young gene carriers. These are very small numbers so we have to be careful, but this is good news for patients. Many patients believe that being a BRCA gene carrier, or even just having a family history is going to give them an adverse prognosis, and that’s clearly not true – and it’s also important that patients who are facing the difficult, difficult decisions around chemotherapy and breast cancer treatment don’t feel compelled to make a decision about bilateral mastectomy within that time shortly after their diagnosis and can reasonably reserve judgment about the extent of surgery until a later date,” she said.
She discussed her findings further in a video interview.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
SAN ANTONIO – No difference in outcomes was seen among young BRCA gene mutation carriers and noncarriers with early-stage invasive breast cancer in a large cohort in the United Kingdom, but a subgroup analysis of those with triple-negative breast cancer showed a clear survival advantage among BRCA mutation carriers.
The findings of the POSH (Prospective Study of Outcomes in Sporadic Versus Hereditary Breast Cancer) trial have important implications for treatment decision making – particularly with respect to surgery – for younger women with breast cancer, according to Diana M. Eccles, MD, of the University of Southampton (England) and University Hospital Southampton Foundation Trust.
The overall finding of no difference in outcomes – including for the primary endpoint of overall survival and secondary endpoints of overall and distant disease-free survival – was true in both univariate and multivariable analysis of data for 2,759 women (14% with BRCA mutations), aged 40 years and younger, who were enrolled over an 8-year period, beginning in 2000, from 126 oncology centers across the United Kingdom, and who were followed for a median of 8.2 years, Dr. Eccles reported at the San Antonio Breast Cancer Symposium.
“We looked at BRCA1 and BRCA2 separately, and we still could see absolutely no difference in survival between BRCA1 and BRCA2, and we were well powered to show a difference,” she said, noting that 99% of these patients were diagnosed based on symptomatic presentation and did not know they were BRCA mutation carriers.
The findings were different among 511 patients with triple-negative breast cancer, however,
“Here we did see a clear difference in survival in favor of BRCA gene carriers – 11% at 10 years. And what was interesting was this clear time-varying hazard of relapse, with the maximum benefit of surviving in the BRCA gene carriers observed in the first few years with a hazard for relapse at 5 years equivalent between the two groups,” she said.
The primary treatment approach in these patients was no different than that in the group as a whole, Dr. Eccles noted, explaining that those with and without triple-negative disease had similar usage of breast conserving vs. unilateral surgery and similar usage of anthracycline-based chemotherapy regimens, with taxane added in a small proportion, which was “very typical for treatment regimens at the time.”
A proportion of women with BRCA gene mutations that were found in the clinical setting had opted to have bilateral mastectomy (15% vs. 3% of noncarriers) “closely following or during their primary treatment diagnosis,” and a separate analysis showed an intriguing survival benefit among those who did not have bilateral mastectomy; survival was 2% better among those women at 5 and 10 years, again with a time-varying hazard, Dr. Eccles said, noting, however, that the study was not powered to show a difference in this measure.
“It’s intriguing that bilateral mastectomy after diagnosis does not seem to improve survival in these young gene carriers. These are very small numbers so we have to be careful, but this is good news for patients. Many patients believe that being a BRCA gene carrier, or even just having a family history is going to give them an adverse prognosis, and that’s clearly not true – and it’s also important that patients who are facing the difficult, difficult decisions around chemotherapy and breast cancer treatment don’t feel compelled to make a decision about bilateral mastectomy within that time shortly after their diagnosis and can reasonably reserve judgment about the extent of surgery until a later date,” she said.
She discussed her findings further in a video interview.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
AT SABCS 2016
Key clinical point:
Major finding: A significant 11% improvement in overall survival was seen among BRCA mutation carriers vs. noncarriers with triple-negative breast cancer.
Data source: The prospective POSH trial included 2,759 women with early-stage invasive breast cancer.
Disclosures: Dr. Eccles has been a consultant for AstraZeneca.
Next-generation sequencing highlights evolution of ER+ breast cancer
SAN ANTONIO – The “genomic landscape” of resistant estrogen receptor–positive (ER+) metastatic breast cancer differs significantly from that of ER+ primary breast cancer, according to findings from a study involving whole-exome sequencing and transcriptome sequencing of such cancers.
Multiple genes were recurrently altered in ER+ metastatic breast cancers at a rate significantly higher than in ER+ primary breast cancers (with 3- to 33-fold enrichment in metastatic vs. primary samples), Ofir Cohen, PhD, explained at the San Antonio Breast Cancer Symposium.
This finding, which suggests that recurrent alterations in genes in ER+ metastatic breast cancers are often acquired after treatment and thus may play a role in resistance to ER-directed therapies and/or metastasis, highlights the value of genomic profiling of metastatic biopsies and has implications for drug development, said Dr. Cohen of the Broad Institute of MIT (Massachusetts Institute of Technology) and Harvard in Cambridge.
Although the genomic and molecular landscape of ER+ primary breast cancer is better understood, that of ER+ metastatic breast cancer is underexplored, he noted.
Thus, he and his colleagues performed whole-exome sequencing on 149 metastatic tumor biopsies from patients with ER+ metastatic tumors and resistance to at least one ER-directed therapy and compared the results with those from 44 matched primary samples from the same patient. They also performed transcriptome sequencing (RNA-seq) of 128 metastatic biopsies.
A key observation from the study is that ESR1 genes were mutated in 24% of the cohort, and the mutations were acquired in 14 of the 15 samples with matched primary samples.
“This emphasized the important role that these mutations may play, specifically in the metastatic and drug-resistant settings,” Dr. Cohen said.
Similarly, ERBB2 mutations occurred in 7% of samples, and seemed to be acquired in five of six metastatic samples with matched primary samples, he said.
“For both of these genes, other than being acquired and suggestive of importance, they also have clear clinical implications, as finding those mutations may guide treatment choices for those patients,” he said.
Another example involves RB1 mutations, which were found in 5% of samples, and which appeared to be acquired in three of five of those with matched primaries. More frequent alterations were also found in PIK3CA, PTEN, and AKTI genes, among others, in metastatic vs. primary cancers.
The observation that these tumors evolve and have different mutations in the primary and metastatic settings may be important, because this suggests that knowledge of the alterations and mutations in the primary setting is insufficient to guide treatment in the metastatic setting.
“So the take-home message here is that tumors do evolve, and that the metastatic setting is different than the primary setting,” Dr. Cohen said.
Sequencing both the exome and the transcriptome may “give us a handle to assess that,” he said.
“Our goal is really to understand evolved resistance. That is, to try and understand the mechanisms that drive the evolution of resistance in ER+ metastatic breast cancer, and, once we understand that, [to determine] how we can translate that knowledge in the clinic,” he said, noting that resistance also may develop through other nongenomic mechanisms, such as epigenetic and regulatory mechanisms.
“As long as those mechanisms leave a footprint on the transcriptome … sequencing the transcriptome together with the exome may yield relevant insights into resistance states that do not derive specifically from exome mutations,” he added.
The findings are important because, despite tremendous advances in the treatment of ER+ metastatic breast cancer, therapeutic resistance remains a common problem, and improved understanding of the underlying resistance mechanisms is critical for enabling durable control of this disease, Dr. Cohen said, explaining that resistant tumors remain the most common cause of breast cancer death and that there is an urgent need to develop new therapeutic strategies for patients who no longer respond to existing therapies.
In a written statement, senior investigator Nikhil Wagle, MD, of Dana-Farber Cancer Institute and Harvard Medical School, Boston, said that the ultimate goal of the project is “to integrate the functional and clinical findings into a unified ‘Resistance Atlas’ for ER+ metastatic breast cancer, which should help inform treatment decisions for individual patients as well as propel the development of new combination treatment strategies for ER-positive metastatic breast cancer.”
This study was funded by the National Cancer Institute, the National Human Genome Research Institute, the Department of Defense Breast Cancer Research Program, Susan G. Komen, the V Foundation, the Breast Cancer Alliance, the AACR-Landon Foundation, the Friends of Dana-Farber Cancer Institute, and the Breast Cancer Research Foundation. Dr. Cohen reported having no conflicts of interest. Dr. Wagle is an equity holder in Foundation Medicine, a consultant to Novartis, and a recipient of sponsored research support from Novartis, Genentech, and Merck.
SAN ANTONIO – The “genomic landscape” of resistant estrogen receptor–positive (ER+) metastatic breast cancer differs significantly from that of ER+ primary breast cancer, according to findings from a study involving whole-exome sequencing and transcriptome sequencing of such cancers.
Multiple genes were recurrently altered in ER+ metastatic breast cancers at a rate significantly higher than in ER+ primary breast cancers (with 3- to 33-fold enrichment in metastatic vs. primary samples), Ofir Cohen, PhD, explained at the San Antonio Breast Cancer Symposium.
This finding, which suggests that recurrent alterations in genes in ER+ metastatic breast cancers are often acquired after treatment and thus may play a role in resistance to ER-directed therapies and/or metastasis, highlights the value of genomic profiling of metastatic biopsies and has implications for drug development, said Dr. Cohen of the Broad Institute of MIT (Massachusetts Institute of Technology) and Harvard in Cambridge.
Although the genomic and molecular landscape of ER+ primary breast cancer is better understood, that of ER+ metastatic breast cancer is underexplored, he noted.
Thus, he and his colleagues performed whole-exome sequencing on 149 metastatic tumor biopsies from patients with ER+ metastatic tumors and resistance to at least one ER-directed therapy and compared the results with those from 44 matched primary samples from the same patient. They also performed transcriptome sequencing (RNA-seq) of 128 metastatic biopsies.
A key observation from the study is that ESR1 genes were mutated in 24% of the cohort, and the mutations were acquired in 14 of the 15 samples with matched primary samples.
“This emphasized the important role that these mutations may play, specifically in the metastatic and drug-resistant settings,” Dr. Cohen said.
Similarly, ERBB2 mutations occurred in 7% of samples, and seemed to be acquired in five of six metastatic samples with matched primary samples, he said.
“For both of these genes, other than being acquired and suggestive of importance, they also have clear clinical implications, as finding those mutations may guide treatment choices for those patients,” he said.
Another example involves RB1 mutations, which were found in 5% of samples, and which appeared to be acquired in three of five of those with matched primaries. More frequent alterations were also found in PIK3CA, PTEN, and AKTI genes, among others, in metastatic vs. primary cancers.
The observation that these tumors evolve and have different mutations in the primary and metastatic settings may be important, because this suggests that knowledge of the alterations and mutations in the primary setting is insufficient to guide treatment in the metastatic setting.
“So the take-home message here is that tumors do evolve, and that the metastatic setting is different than the primary setting,” Dr. Cohen said.
Sequencing both the exome and the transcriptome may “give us a handle to assess that,” he said.
“Our goal is really to understand evolved resistance. That is, to try and understand the mechanisms that drive the evolution of resistance in ER+ metastatic breast cancer, and, once we understand that, [to determine] how we can translate that knowledge in the clinic,” he said, noting that resistance also may develop through other nongenomic mechanisms, such as epigenetic and regulatory mechanisms.
“As long as those mechanisms leave a footprint on the transcriptome … sequencing the transcriptome together with the exome may yield relevant insights into resistance states that do not derive specifically from exome mutations,” he added.
The findings are important because, despite tremendous advances in the treatment of ER+ metastatic breast cancer, therapeutic resistance remains a common problem, and improved understanding of the underlying resistance mechanisms is critical for enabling durable control of this disease, Dr. Cohen said, explaining that resistant tumors remain the most common cause of breast cancer death and that there is an urgent need to develop new therapeutic strategies for patients who no longer respond to existing therapies.
In a written statement, senior investigator Nikhil Wagle, MD, of Dana-Farber Cancer Institute and Harvard Medical School, Boston, said that the ultimate goal of the project is “to integrate the functional and clinical findings into a unified ‘Resistance Atlas’ for ER+ metastatic breast cancer, which should help inform treatment decisions for individual patients as well as propel the development of new combination treatment strategies for ER-positive metastatic breast cancer.”
This study was funded by the National Cancer Institute, the National Human Genome Research Institute, the Department of Defense Breast Cancer Research Program, Susan G. Komen, the V Foundation, the Breast Cancer Alliance, the AACR-Landon Foundation, the Friends of Dana-Farber Cancer Institute, and the Breast Cancer Research Foundation. Dr. Cohen reported having no conflicts of interest. Dr. Wagle is an equity holder in Foundation Medicine, a consultant to Novartis, and a recipient of sponsored research support from Novartis, Genentech, and Merck.
SAN ANTONIO – The “genomic landscape” of resistant estrogen receptor–positive (ER+) metastatic breast cancer differs significantly from that of ER+ primary breast cancer, according to findings from a study involving whole-exome sequencing and transcriptome sequencing of such cancers.
Multiple genes were recurrently altered in ER+ metastatic breast cancers at a rate significantly higher than in ER+ primary breast cancers (with 3- to 33-fold enrichment in metastatic vs. primary samples), Ofir Cohen, PhD, explained at the San Antonio Breast Cancer Symposium.
This finding, which suggests that recurrent alterations in genes in ER+ metastatic breast cancers are often acquired after treatment and thus may play a role in resistance to ER-directed therapies and/or metastasis, highlights the value of genomic profiling of metastatic biopsies and has implications for drug development, said Dr. Cohen of the Broad Institute of MIT (Massachusetts Institute of Technology) and Harvard in Cambridge.
Although the genomic and molecular landscape of ER+ primary breast cancer is better understood, that of ER+ metastatic breast cancer is underexplored, he noted.
Thus, he and his colleagues performed whole-exome sequencing on 149 metastatic tumor biopsies from patients with ER+ metastatic tumors and resistance to at least one ER-directed therapy and compared the results with those from 44 matched primary samples from the same patient. They also performed transcriptome sequencing (RNA-seq) of 128 metastatic biopsies.
A key observation from the study is that ESR1 genes were mutated in 24% of the cohort, and the mutations were acquired in 14 of the 15 samples with matched primary samples.
“This emphasized the important role that these mutations may play, specifically in the metastatic and drug-resistant settings,” Dr. Cohen said.
Similarly, ERBB2 mutations occurred in 7% of samples, and seemed to be acquired in five of six metastatic samples with matched primary samples, he said.
“For both of these genes, other than being acquired and suggestive of importance, they also have clear clinical implications, as finding those mutations may guide treatment choices for those patients,” he said.
Another example involves RB1 mutations, which were found in 5% of samples, and which appeared to be acquired in three of five of those with matched primaries. More frequent alterations were also found in PIK3CA, PTEN, and AKTI genes, among others, in metastatic vs. primary cancers.
The observation that these tumors evolve and have different mutations in the primary and metastatic settings may be important, because this suggests that knowledge of the alterations and mutations in the primary setting is insufficient to guide treatment in the metastatic setting.
“So the take-home message here is that tumors do evolve, and that the metastatic setting is different than the primary setting,” Dr. Cohen said.
Sequencing both the exome and the transcriptome may “give us a handle to assess that,” he said.
“Our goal is really to understand evolved resistance. That is, to try and understand the mechanisms that drive the evolution of resistance in ER+ metastatic breast cancer, and, once we understand that, [to determine] how we can translate that knowledge in the clinic,” he said, noting that resistance also may develop through other nongenomic mechanisms, such as epigenetic and regulatory mechanisms.
“As long as those mechanisms leave a footprint on the transcriptome … sequencing the transcriptome together with the exome may yield relevant insights into resistance states that do not derive specifically from exome mutations,” he added.
The findings are important because, despite tremendous advances in the treatment of ER+ metastatic breast cancer, therapeutic resistance remains a common problem, and improved understanding of the underlying resistance mechanisms is critical for enabling durable control of this disease, Dr. Cohen said, explaining that resistant tumors remain the most common cause of breast cancer death and that there is an urgent need to develop new therapeutic strategies for patients who no longer respond to existing therapies.
In a written statement, senior investigator Nikhil Wagle, MD, of Dana-Farber Cancer Institute and Harvard Medical School, Boston, said that the ultimate goal of the project is “to integrate the functional and clinical findings into a unified ‘Resistance Atlas’ for ER+ metastatic breast cancer, which should help inform treatment decisions for individual patients as well as propel the development of new combination treatment strategies for ER-positive metastatic breast cancer.”
This study was funded by the National Cancer Institute, the National Human Genome Research Institute, the Department of Defense Breast Cancer Research Program, Susan G. Komen, the V Foundation, the Breast Cancer Alliance, the AACR-Landon Foundation, the Friends of Dana-Farber Cancer Institute, and the Breast Cancer Research Foundation. Dr. Cohen reported having no conflicts of interest. Dr. Wagle is an equity holder in Foundation Medicine, a consultant to Novartis, and a recipient of sponsored research support from Novartis, Genentech, and Merck.
AT SABCS 2016
Key clinical point:
Major finding: ESR1 genes were mutated in 24% of the cohort, and the mutations were acquired in 14 of the 15 samples with matched primary samples.
Data source: Whole-exome sequencing of 149 metastatic breast cancer biopsies and 44 matched primary tumor biopsies, and transcriptome sequencing of 128 metastatic biopsies.
Disclosures: This study was funded by the National Cancer Institute, the National Human Genome Research Institute, the Department of Defense Breast Cancer Research Program, Susan G. Komen, the V Foundation, the Breast Cancer Alliance, the AACR-Landon Foundation, the Friends of Dana-Farber Cancer Institute, and the Breast Cancer Research Foundation. Dr. Cohen reported having no conflicts of interest. Dr. Wagle is an equity holder in Foundation Medicine, a consultant to Novartis, and a recipient of sponsored research support from Novartis, Genentech, and Merck.
VIDEO: Veliparib misses PFS endpoint, advances to phase III trial
SAN ANTONIO – The investigational selective PARP-1 and PARP-2 inhibitor veliparib failed to significantly improve progression-free or overall survival, compared with placebo, when added to carboplatin and paclitaxel in patients with BRCA1 or BRCA2 mutations and locally recurrent or metastatic breast cancer in the randomized phase II BROCADE study.
The potent, orally bioavailable drug did, however, show a trend toward improvement on these measures, as well as a significant improvement in overall response-rate findings that warrant further investigation in a phase III trial, Hyo Han, MD, said at the San Antonio Breast Cancer Symposium.
Patients were randomized to receive placebo (98 patients) or 120 mg veliparib twice daily on days 1-7 (95 patients) in addition to carboplatin and 175 mg/m2 of paclitaxel every 3 weeks.
Median progression-free survival – the primary endpoint of the study – was 12.3 months in the placebo group and 14.1 months in the veliparib group (hazard ratio, 0.789), and overall survival was 25.9 and 28.3 months, respectively (HR, 0.750), said Dr. Han of Moffitt Cancer Center, Tampa, Fla.
The overall response rate was 61.3% vs. 77.8% in the groups, respectively.
Although the study did not meet its primary endpoint, the findings are encouraging as it was powered to detect only dramatic differences between the groups, Dr. Han said.
In a video interview, she discussed veliparib, its safety, the BROCADE findings – including data from a third study arm looking at veliparib in combination with temozolomide, and plans for evaluating veliparib in the ongoing phase III BROCADE3 trial.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
SAN ANTONIO – The investigational selective PARP-1 and PARP-2 inhibitor veliparib failed to significantly improve progression-free or overall survival, compared with placebo, when added to carboplatin and paclitaxel in patients with BRCA1 or BRCA2 mutations and locally recurrent or metastatic breast cancer in the randomized phase II BROCADE study.
The potent, orally bioavailable drug did, however, show a trend toward improvement on these measures, as well as a significant improvement in overall response-rate findings that warrant further investigation in a phase III trial, Hyo Han, MD, said at the San Antonio Breast Cancer Symposium.
Patients were randomized to receive placebo (98 patients) or 120 mg veliparib twice daily on days 1-7 (95 patients) in addition to carboplatin and 175 mg/m2 of paclitaxel every 3 weeks.
Median progression-free survival – the primary endpoint of the study – was 12.3 months in the placebo group and 14.1 months in the veliparib group (hazard ratio, 0.789), and overall survival was 25.9 and 28.3 months, respectively (HR, 0.750), said Dr. Han of Moffitt Cancer Center, Tampa, Fla.
The overall response rate was 61.3% vs. 77.8% in the groups, respectively.
Although the study did not meet its primary endpoint, the findings are encouraging as it was powered to detect only dramatic differences between the groups, Dr. Han said.
In a video interview, she discussed veliparib, its safety, the BROCADE findings – including data from a third study arm looking at veliparib in combination with temozolomide, and plans for evaluating veliparib in the ongoing phase III BROCADE3 trial.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
SAN ANTONIO – The investigational selective PARP-1 and PARP-2 inhibitor veliparib failed to significantly improve progression-free or overall survival, compared with placebo, when added to carboplatin and paclitaxel in patients with BRCA1 or BRCA2 mutations and locally recurrent or metastatic breast cancer in the randomized phase II BROCADE study.
The potent, orally bioavailable drug did, however, show a trend toward improvement on these measures, as well as a significant improvement in overall response-rate findings that warrant further investigation in a phase III trial, Hyo Han, MD, said at the San Antonio Breast Cancer Symposium.
Patients were randomized to receive placebo (98 patients) or 120 mg veliparib twice daily on days 1-7 (95 patients) in addition to carboplatin and 175 mg/m2 of paclitaxel every 3 weeks.
Median progression-free survival – the primary endpoint of the study – was 12.3 months in the placebo group and 14.1 months in the veliparib group (hazard ratio, 0.789), and overall survival was 25.9 and 28.3 months, respectively (HR, 0.750), said Dr. Han of Moffitt Cancer Center, Tampa, Fla.
The overall response rate was 61.3% vs. 77.8% in the groups, respectively.
Although the study did not meet its primary endpoint, the findings are encouraging as it was powered to detect only dramatic differences between the groups, Dr. Han said.
In a video interview, she discussed veliparib, its safety, the BROCADE findings – including data from a third study arm looking at veliparib in combination with temozolomide, and plans for evaluating veliparib in the ongoing phase III BROCADE3 trial.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
AT SABCS 2016
Low-dose IL-2 shows promise for refractory lupus
WASHINGTON – A novel biologic treatment strategy involving subcutaneous low-dose interleukin-2 therapy for refractory systemic lupus erythematosus (SLE) showed promise in a single-center, combined phase I/IIa trial.
In 12 patients with active and refractory SLE – that is, patients with SLE disease activity index (SLEDAI) score of at least 6 who were on at least two different immunosuppressive therapies – low-dose IL-2 treatment led to an effective and cycle-dependent increase in the percentage of CD25hi cells among regulatory T cells (Treg). The increase was statistically significant (P less than .001), Jens Humrich, MD, and his colleagues reported in a late-breaking poster at the annual meeting of the American College of Rheumatology.
However, a reduction in levels of anti-dsDNA antibodies was not observed, said Dr. Humrich of University Hospital Schleswig-Holstein in Lubeck, Germany.
Treatment was safe; treatment-related adverse events were generally mild and transient, Dr. Humrich noted.
Study subjects received four treatment cycles each, with daily subcutaneous injections of recombinant human IL-2 (aldesleukin) at single doses of 0.75, 1.5, or 3.0 million IU on 5 consecutive days. Cycles were separated by a washout period of 9-16 days. Subjects were then followed for 9 weeks.
IL-2 is crucial for the growth and survival of Treg (and thus for the control of autoimmunity). Prior studies demonstrated the significance of acquired IL-2 deficiency and related Treg defects in the pathogenesis of SLE – and that compensation for IL-2 deficiency with low-dose IL-2 can correct these defects, he explained.
In the current study, the primary aim was to show at least a twofold increase in the percentage of CD25hi cells among CD3+CD4+Foxp3+CD127lo Treg cells after the fourth treatment cycle vs. baseline, and secondary aims included clinical responses assessed by SLEDAI and changes in serologic and other immunologic parameters, he said.
The findings suggest that low-dose IL-2 therapy can safely and selectively expand the Treg population and decrease disease activity in patients with active and refractory SLE.
“This study provides the basis for larger and placebo-controlled clinical studies aiming to prove the efficacy of this novel biologic treatment strategy,” the investigators concluded.
Dr. Humrich reported having no disclosures.
WASHINGTON – A novel biologic treatment strategy involving subcutaneous low-dose interleukin-2 therapy for refractory systemic lupus erythematosus (SLE) showed promise in a single-center, combined phase I/IIa trial.
In 12 patients with active and refractory SLE – that is, patients with SLE disease activity index (SLEDAI) score of at least 6 who were on at least two different immunosuppressive therapies – low-dose IL-2 treatment led to an effective and cycle-dependent increase in the percentage of CD25hi cells among regulatory T cells (Treg). The increase was statistically significant (P less than .001), Jens Humrich, MD, and his colleagues reported in a late-breaking poster at the annual meeting of the American College of Rheumatology.
However, a reduction in levels of anti-dsDNA antibodies was not observed, said Dr. Humrich of University Hospital Schleswig-Holstein in Lubeck, Germany.
Treatment was safe; treatment-related adverse events were generally mild and transient, Dr. Humrich noted.
Study subjects received four treatment cycles each, with daily subcutaneous injections of recombinant human IL-2 (aldesleukin) at single doses of 0.75, 1.5, or 3.0 million IU on 5 consecutive days. Cycles were separated by a washout period of 9-16 days. Subjects were then followed for 9 weeks.
IL-2 is crucial for the growth and survival of Treg (and thus for the control of autoimmunity). Prior studies demonstrated the significance of acquired IL-2 deficiency and related Treg defects in the pathogenesis of SLE – and that compensation for IL-2 deficiency with low-dose IL-2 can correct these defects, he explained.
In the current study, the primary aim was to show at least a twofold increase in the percentage of CD25hi cells among CD3+CD4+Foxp3+CD127lo Treg cells after the fourth treatment cycle vs. baseline, and secondary aims included clinical responses assessed by SLEDAI and changes in serologic and other immunologic parameters, he said.
The findings suggest that low-dose IL-2 therapy can safely and selectively expand the Treg population and decrease disease activity in patients with active and refractory SLE.
“This study provides the basis for larger and placebo-controlled clinical studies aiming to prove the efficacy of this novel biologic treatment strategy,” the investigators concluded.
Dr. Humrich reported having no disclosures.
WASHINGTON – A novel biologic treatment strategy involving subcutaneous low-dose interleukin-2 therapy for refractory systemic lupus erythematosus (SLE) showed promise in a single-center, combined phase I/IIa trial.
In 12 patients with active and refractory SLE – that is, patients with SLE disease activity index (SLEDAI) score of at least 6 who were on at least two different immunosuppressive therapies – low-dose IL-2 treatment led to an effective and cycle-dependent increase in the percentage of CD25hi cells among regulatory T cells (Treg). The increase was statistically significant (P less than .001), Jens Humrich, MD, and his colleagues reported in a late-breaking poster at the annual meeting of the American College of Rheumatology.
However, a reduction in levels of anti-dsDNA antibodies was not observed, said Dr. Humrich of University Hospital Schleswig-Holstein in Lubeck, Germany.
Treatment was safe; treatment-related adverse events were generally mild and transient, Dr. Humrich noted.
Study subjects received four treatment cycles each, with daily subcutaneous injections of recombinant human IL-2 (aldesleukin) at single doses of 0.75, 1.5, or 3.0 million IU on 5 consecutive days. Cycles were separated by a washout period of 9-16 days. Subjects were then followed for 9 weeks.
IL-2 is crucial for the growth and survival of Treg (and thus for the control of autoimmunity). Prior studies demonstrated the significance of acquired IL-2 deficiency and related Treg defects in the pathogenesis of SLE – and that compensation for IL-2 deficiency with low-dose IL-2 can correct these defects, he explained.
In the current study, the primary aim was to show at least a twofold increase in the percentage of CD25hi cells among CD3+CD4+Foxp3+CD127lo Treg cells after the fourth treatment cycle vs. baseline, and secondary aims included clinical responses assessed by SLEDAI and changes in serologic and other immunologic parameters, he said.
The findings suggest that low-dose IL-2 therapy can safely and selectively expand the Treg population and decrease disease activity in patients with active and refractory SLE.
“This study provides the basis for larger and placebo-controlled clinical studies aiming to prove the efficacy of this novel biologic treatment strategy,” the investigators concluded.
Dr. Humrich reported having no disclosures.
Key clinical point:
Major finding: A reduction in SLEDAI was seen in 10 patients (83.3%), and a clinical response occurred in 8 (66.7%).
Data source: A combined phase I/IIa trial involving 12 patients.
Disclosures: Dr. Humrich reported having no disclosures.