User login
Multiple Fungating Plaques on the Face, Arms, and Legs
THE DIAGNOSIS: Mpox
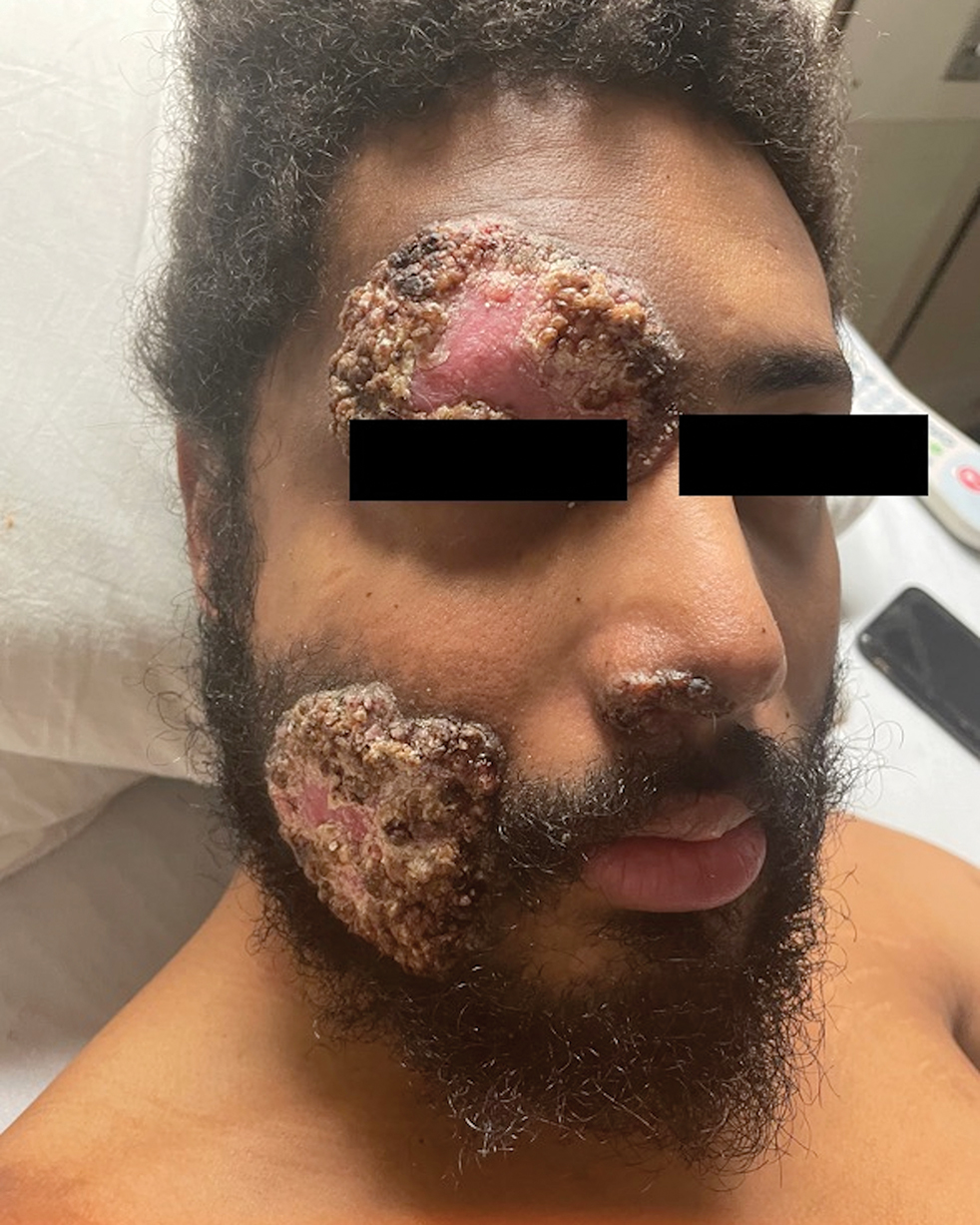
Histologic examination demonstrated dense aggregates of necrotic cellular debris composed of karyorrhectic nuclear fragments intermixed with neutrophils, lymphocytes, and histiocytes. Eosinophilic intracytoplasmic inclusions also were observed (Figure 1). The bacterial, fungal, and mycobacterial histologic special stains and cultures were negative. Three weeks after the initial visit with dermatology, the patient was admitted to the hospital for worsening symptoms of fever, chills, and painful erythema surrounding the skin lesions. Serology and viral workup revealed a positive mpox polymerase chain reaction test, suggesting a diagnosis of mpox. Following the Centers for Disease Control and Prevention protocol, the patient was started on oral tecovirimat 200 mg twice daily for 3 weeks and intravenous infusions of cidofovir 345 mg once weekly for 2 weeks. After treatment was initiated, the skin lesions showed rapid improvement (Figure 2), and he was discharged from the hospital after finishing the second dose of cidofovir. Four months after the initial dermatology consultation, the lesions had resolved completely with residual scarring. At that time, the patient had full movement of the right eye.
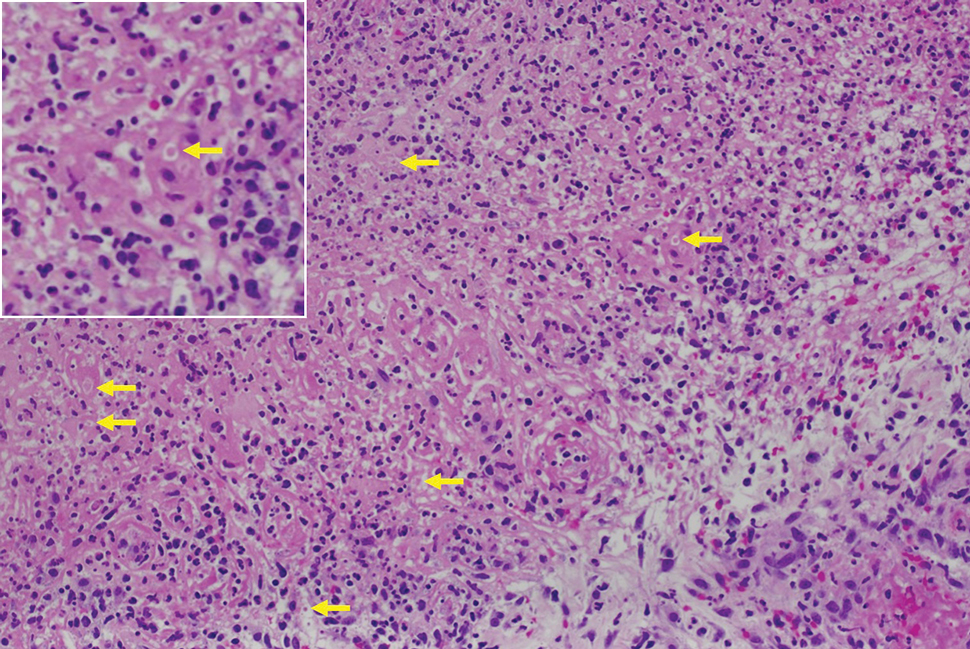
shows higher digital magnification of eosinophilic inclusions observed throughout the biopsy specimen (original magnification ×400).
Mpox virus is a member of the Poxviridae family of zoonotic viruses, which are transmitted from animals to humans. The mpox virus is brick-shaped (rectangular) and has a genome of linear double-stranded DNA encoding 180 proteins.1 Primates and rodents are the typical host reservoirs for viral circulation of mpox.2 Animal-to-human transmission occurs through direct contact with mucous membranes, bodily fluids, or tissues of an infected animal. Human-to-human transmission occurs through direct contact with infected mucous membranes, bodily fluids, respiratory droplets, and contaminated fomites.2
Symptoms typically occur within 1 week of exposure to the mpox virus. Prodromal symptoms of fever, sore throat, body aches, and headaches last for 3 days.1 Many patients experience a facial rash that spreads to the arms and legs over a period of 2 to 4 weeks. The rash initially manifests as small papules that progress to painful pustules and vesicles measuring 0.5 to 1.0 cm in diameter.3 The mpox virus is transmitted through these skin lesions until they crust over and re-epithelialize.1 The case fatality rate for mpox infection remains low (0.18%).4
Mpox outbreaks mainly were limited to central and western Africa prior to 2022. From May 17, 2022, through October 6, 2022, 26,384 cases of mpox were reported in the United States.5 During this outbreak, immunocompromised patients diagnosed with HIV and men who have sex with men were disproportionately affected.5
Due to the similarities between the smallpox virus and other orthopoxviruses, certain smallpox vaccines have been indicated for pre-exposure prophylaxis.6 The efficacy of prophylactic vaccination is believed to stem from the production of neutralizing antibodies that are cross-protective against other orthopoxviruses, including mpox.7 The 2 vaccines approved in the United States for mpox prophylaxis are JYNNEOS and ACAM2000, which are both live attenuated vaccines. Pre-exposure prophylaxis is indicated for patients at risk for severe disease, including men who have sex with men, individuals diagnosed with HIV or other immunosuppressive disorders, and individuals with recent diagnoses of one or more sexually transmitted diseases.8
Most mpox cases resolve within 2 to 4 weeks and only require supportive care (eg, nonsteroidal anti-inflammatory drugs, topical steroids, topical anesthetics) to treat pain.8 For patients at risk for severe disease, antiviral medications are warranted. Tecovirimat, brincidofovir, and cidofovir are antiviral medications used to treat smallpox that are thought to be effective against mpox.8,9 Tecovirimat and cidofovir have been shown to be effective against mpox in animal trials, but randomized or nonrandomized trials have not been performed in humans.9-11 Tecovirimat currently is available for the treatment of severe mpox in patients who meet the Centers for Disease Control and Prevention’s Investigational New Drug protocol; for these patients, a 200-mg course is administered orally or intravenously every 12 hours for 2 weeks.8
- Lu J, Xing H, Wang C, et al. Mpox (formerly monkeypox): pathogenesis, prevention, and treatment. Signal Transduct Target Ther. 2023;8:458. doi:10.1038/s41392-023-01675-
- Lim CK, Roberts J, Moso M, et al. Mpox diagnostics: review of current and emerging technologies. J Med Virol. 2023;95:e28429. doi:10.1002/jmv.28429
- Brown K, Leggat PA. Human monkeypox: current state of knowledge and implications for the future. Trop Med Infect Dis. 2016;1:8. doi:10.3390/tropicalmed1010008
- World Health Organization. Mpox (monkeypox) World Health Organization. Published April 18, 2023. Accessed May 28, 2025. https://www.who.int/news-room/fact-sheets/detail/monkeypox
- Kava CM, Rohraff DM, Wallace B, et al. Epidemiologic features of the monkeypox outbreak and the public health response—United States, May 17–October 6, 2022. 2022:1449-1456. https://www.cdc.gov/mmwr/volumes/71/wr/mm7145a4.htm?s_cid=mm7145a4_w
- Rizk JG, Lippi G, Henry BM, et al. Prevention and treatment of monkeypox. Drugs. 2022;82:957-963. doi:10.1007/s40265-022-01742-y
- Edghill-Smith Y, Golding H, Manischewitz J, et al. Smallpox vaccine-induced antibodies are necessary and sufficient for protection against monkeypox virus. Nat Med. 2005;11:740-747. doi:10.1038 /nm1261
- Centers for Disease Control and Prevention. Mpox treatment information for healthcare professionals. Updated June 18, 2024. Accessed May 28, 2025. https://www.cdc.gov/mpox/hcp/clinical-care/?CDC_AAref_Val=https://www.cdc.gov/poxvirus/mpox/clinicians/treatment.html
- Mitja O, Ogoina D, Titanji BK, et al. Monkeypox. Lancet. 2023;401:60-74. doi:10.1016/S0140-6736(22)02075-X
- Huggins J, Goff A, Hensley L, et al. Nonhuman primates are protected from smallpox virus or monkeypox virus challenges by the antiviral drug ST-246. Antimicrob Agents Chemother. 2009;53:2620-2625. doi:10.1128/aac.00021-09
- Grosenbach DW, Honeychurch K, Rose EA, et al. Oral tecovirimat for the treatment of smallpox. N Engl J Med. 2018;379:44-53. doi:10.1056 /nejmoa1705688
THE DIAGNOSIS: Mpox
Histologic examination demonstrated dense aggregates of necrotic cellular debris composed of karyorrhectic nuclear fragments intermixed with neutrophils, lymphocytes, and histiocytes. Eosinophilic intracytoplasmic inclusions also were observed (Figure 1). The bacterial, fungal, and mycobacterial histologic special stains and cultures were negative. Three weeks after the initial visit with dermatology, the patient was admitted to the hospital for worsening symptoms of fever, chills, and painful erythema surrounding the skin lesions. Serology and viral workup revealed a positive mpox polymerase chain reaction test, suggesting a diagnosis of mpox. Following the Centers for Disease Control and Prevention protocol, the patient was started on oral tecovirimat 200 mg twice daily for 3 weeks and intravenous infusions of cidofovir 345 mg once weekly for 2 weeks. After treatment was initiated, the skin lesions showed rapid improvement (Figure 2), and he was discharged from the hospital after finishing the second dose of cidofovir. Four months after the initial dermatology consultation, the lesions had resolved completely with residual scarring. At that time, the patient had full movement of the right eye.

shows higher digital magnification of eosinophilic inclusions observed throughout the biopsy specimen (original magnification ×400).

Mpox virus is a member of the Poxviridae family of zoonotic viruses, which are transmitted from animals to humans. The mpox virus is brick-shaped (rectangular) and has a genome of linear double-stranded DNA encoding 180 proteins.1 Primates and rodents are the typical host reservoirs for viral circulation of mpox.2 Animal-to-human transmission occurs through direct contact with mucous membranes, bodily fluids, or tissues of an infected animal. Human-to-human transmission occurs through direct contact with infected mucous membranes, bodily fluids, respiratory droplets, and contaminated fomites.2
Symptoms typically occur within 1 week of exposure to the mpox virus. Prodromal symptoms of fever, sore throat, body aches, and headaches last for 3 days.1 Many patients experience a facial rash that spreads to the arms and legs over a period of 2 to 4 weeks. The rash initially manifests as small papules that progress to painful pustules and vesicles measuring 0.5 to 1.0 cm in diameter.3 The mpox virus is transmitted through these skin lesions until they crust over and re-epithelialize.1 The case fatality rate for mpox infection remains low (0.18%).4
Mpox outbreaks mainly were limited to central and western Africa prior to 2022. From May 17, 2022, through October 6, 2022, 26,384 cases of mpox were reported in the United States.5 During this outbreak, immunocompromised patients diagnosed with HIV and men who have sex with men were disproportionately affected.5
Due to the similarities between the smallpox virus and other orthopoxviruses, certain smallpox vaccines have been indicated for pre-exposure prophylaxis.6 The efficacy of prophylactic vaccination is believed to stem from the production of neutralizing antibodies that are cross-protective against other orthopoxviruses, including mpox.7 The 2 vaccines approved in the United States for mpox prophylaxis are JYNNEOS and ACAM2000, which are both live attenuated vaccines. Pre-exposure prophylaxis is indicated for patients at risk for severe disease, including men who have sex with men, individuals diagnosed with HIV or other immunosuppressive disorders, and individuals with recent diagnoses of one or more sexually transmitted diseases.8
Most mpox cases resolve within 2 to 4 weeks and only require supportive care (eg, nonsteroidal anti-inflammatory drugs, topical steroids, topical anesthetics) to treat pain.8 For patients at risk for severe disease, antiviral medications are warranted. Tecovirimat, brincidofovir, and cidofovir are antiviral medications used to treat smallpox that are thought to be effective against mpox.8,9 Tecovirimat and cidofovir have been shown to be effective against mpox in animal trials, but randomized or nonrandomized trials have not been performed in humans.9-11 Tecovirimat currently is available for the treatment of severe mpox in patients who meet the Centers for Disease Control and Prevention’s Investigational New Drug protocol; for these patients, a 200-mg course is administered orally or intravenously every 12 hours for 2 weeks.8
THE DIAGNOSIS: Mpox
Histologic examination demonstrated dense aggregates of necrotic cellular debris composed of karyorrhectic nuclear fragments intermixed with neutrophils, lymphocytes, and histiocytes. Eosinophilic intracytoplasmic inclusions also were observed (Figure 1). The bacterial, fungal, and mycobacterial histologic special stains and cultures were negative. Three weeks after the initial visit with dermatology, the patient was admitted to the hospital for worsening symptoms of fever, chills, and painful erythema surrounding the skin lesions. Serology and viral workup revealed a positive mpox polymerase chain reaction test, suggesting a diagnosis of mpox. Following the Centers for Disease Control and Prevention protocol, the patient was started on oral tecovirimat 200 mg twice daily for 3 weeks and intravenous infusions of cidofovir 345 mg once weekly for 2 weeks. After treatment was initiated, the skin lesions showed rapid improvement (Figure 2), and he was discharged from the hospital after finishing the second dose of cidofovir. Four months after the initial dermatology consultation, the lesions had resolved completely with residual scarring. At that time, the patient had full movement of the right eye.

shows higher digital magnification of eosinophilic inclusions observed throughout the biopsy specimen (original magnification ×400).

Mpox virus is a member of the Poxviridae family of zoonotic viruses, which are transmitted from animals to humans. The mpox virus is brick-shaped (rectangular) and has a genome of linear double-stranded DNA encoding 180 proteins.1 Primates and rodents are the typical host reservoirs for viral circulation of mpox.2 Animal-to-human transmission occurs through direct contact with mucous membranes, bodily fluids, or tissues of an infected animal. Human-to-human transmission occurs through direct contact with infected mucous membranes, bodily fluids, respiratory droplets, and contaminated fomites.2
Symptoms typically occur within 1 week of exposure to the mpox virus. Prodromal symptoms of fever, sore throat, body aches, and headaches last for 3 days.1 Many patients experience a facial rash that spreads to the arms and legs over a period of 2 to 4 weeks. The rash initially manifests as small papules that progress to painful pustules and vesicles measuring 0.5 to 1.0 cm in diameter.3 The mpox virus is transmitted through these skin lesions until they crust over and re-epithelialize.1 The case fatality rate for mpox infection remains low (0.18%).4
Mpox outbreaks mainly were limited to central and western Africa prior to 2022. From May 17, 2022, through October 6, 2022, 26,384 cases of mpox were reported in the United States.5 During this outbreak, immunocompromised patients diagnosed with HIV and men who have sex with men were disproportionately affected.5
Due to the similarities between the smallpox virus and other orthopoxviruses, certain smallpox vaccines have been indicated for pre-exposure prophylaxis.6 The efficacy of prophylactic vaccination is believed to stem from the production of neutralizing antibodies that are cross-protective against other orthopoxviruses, including mpox.7 The 2 vaccines approved in the United States for mpox prophylaxis are JYNNEOS and ACAM2000, which are both live attenuated vaccines. Pre-exposure prophylaxis is indicated for patients at risk for severe disease, including men who have sex with men, individuals diagnosed with HIV or other immunosuppressive disorders, and individuals with recent diagnoses of one or more sexually transmitted diseases.8
Most mpox cases resolve within 2 to 4 weeks and only require supportive care (eg, nonsteroidal anti-inflammatory drugs, topical steroids, topical anesthetics) to treat pain.8 For patients at risk for severe disease, antiviral medications are warranted. Tecovirimat, brincidofovir, and cidofovir are antiviral medications used to treat smallpox that are thought to be effective against mpox.8,9 Tecovirimat and cidofovir have been shown to be effective against mpox in animal trials, but randomized or nonrandomized trials have not been performed in humans.9-11 Tecovirimat currently is available for the treatment of severe mpox in patients who meet the Centers for Disease Control and Prevention’s Investigational New Drug protocol; for these patients, a 200-mg course is administered orally or intravenously every 12 hours for 2 weeks.8
- Lu J, Xing H, Wang C, et al. Mpox (formerly monkeypox): pathogenesis, prevention, and treatment. Signal Transduct Target Ther. 2023;8:458. doi:10.1038/s41392-023-01675-
- Lim CK, Roberts J, Moso M, et al. Mpox diagnostics: review of current and emerging technologies. J Med Virol. 2023;95:e28429. doi:10.1002/jmv.28429
- Brown K, Leggat PA. Human monkeypox: current state of knowledge and implications for the future. Trop Med Infect Dis. 2016;1:8. doi:10.3390/tropicalmed1010008
- World Health Organization. Mpox (monkeypox) World Health Organization. Published April 18, 2023. Accessed May 28, 2025. https://www.who.int/news-room/fact-sheets/detail/monkeypox
- Kava CM, Rohraff DM, Wallace B, et al. Epidemiologic features of the monkeypox outbreak and the public health response—United States, May 17–October 6, 2022. 2022:1449-1456. https://www.cdc.gov/mmwr/volumes/71/wr/mm7145a4.htm?s_cid=mm7145a4_w
- Rizk JG, Lippi G, Henry BM, et al. Prevention and treatment of monkeypox. Drugs. 2022;82:957-963. doi:10.1007/s40265-022-01742-y
- Edghill-Smith Y, Golding H, Manischewitz J, et al. Smallpox vaccine-induced antibodies are necessary and sufficient for protection against monkeypox virus. Nat Med. 2005;11:740-747. doi:10.1038 /nm1261
- Centers for Disease Control and Prevention. Mpox treatment information for healthcare professionals. Updated June 18, 2024. Accessed May 28, 2025. https://www.cdc.gov/mpox/hcp/clinical-care/?CDC_AAref_Val=https://www.cdc.gov/poxvirus/mpox/clinicians/treatment.html
- Mitja O, Ogoina D, Titanji BK, et al. Monkeypox. Lancet. 2023;401:60-74. doi:10.1016/S0140-6736(22)02075-X
- Huggins J, Goff A, Hensley L, et al. Nonhuman primates are protected from smallpox virus or monkeypox virus challenges by the antiviral drug ST-246. Antimicrob Agents Chemother. 2009;53:2620-2625. doi:10.1128/aac.00021-09
- Grosenbach DW, Honeychurch K, Rose EA, et al. Oral tecovirimat for the treatment of smallpox. N Engl J Med. 2018;379:44-53. doi:10.1056 /nejmoa1705688
- Lu J, Xing H, Wang C, et al. Mpox (formerly monkeypox): pathogenesis, prevention, and treatment. Signal Transduct Target Ther. 2023;8:458. doi:10.1038/s41392-023-01675-
- Lim CK, Roberts J, Moso M, et al. Mpox diagnostics: review of current and emerging technologies. J Med Virol. 2023;95:e28429. doi:10.1002/jmv.28429
- Brown K, Leggat PA. Human monkeypox: current state of knowledge and implications for the future. Trop Med Infect Dis. 2016;1:8. doi:10.3390/tropicalmed1010008
- World Health Organization. Mpox (monkeypox) World Health Organization. Published April 18, 2023. Accessed May 28, 2025. https://www.who.int/news-room/fact-sheets/detail/monkeypox
- Kava CM, Rohraff DM, Wallace B, et al. Epidemiologic features of the monkeypox outbreak and the public health response—United States, May 17–October 6, 2022. 2022:1449-1456. https://www.cdc.gov/mmwr/volumes/71/wr/mm7145a4.htm?s_cid=mm7145a4_w
- Rizk JG, Lippi G, Henry BM, et al. Prevention and treatment of monkeypox. Drugs. 2022;82:957-963. doi:10.1007/s40265-022-01742-y
- Edghill-Smith Y, Golding H, Manischewitz J, et al. Smallpox vaccine-induced antibodies are necessary and sufficient for protection against monkeypox virus. Nat Med. 2005;11:740-747. doi:10.1038 /nm1261
- Centers for Disease Control and Prevention. Mpox treatment information for healthcare professionals. Updated June 18, 2024. Accessed May 28, 2025. https://www.cdc.gov/mpox/hcp/clinical-care/?CDC_AAref_Val=https://www.cdc.gov/poxvirus/mpox/clinicians/treatment.html
- Mitja O, Ogoina D, Titanji BK, et al. Monkeypox. Lancet. 2023;401:60-74. doi:10.1016/S0140-6736(22)02075-X
- Huggins J, Goff A, Hensley L, et al. Nonhuman primates are protected from smallpox virus or monkeypox virus challenges by the antiviral drug ST-246. Antimicrob Agents Chemother. 2009;53:2620-2625. doi:10.1128/aac.00021-09
- Grosenbach DW, Honeychurch K, Rose EA, et al. Oral tecovirimat for the treatment of smallpox. N Engl J Med. 2018;379:44-53. doi:10.1056 /nejmoa1705688
Multiple Fungating Plaques on the Face, Arms, and Legs
Multiple Fungating Plaques on the Face, Arms, and Legs
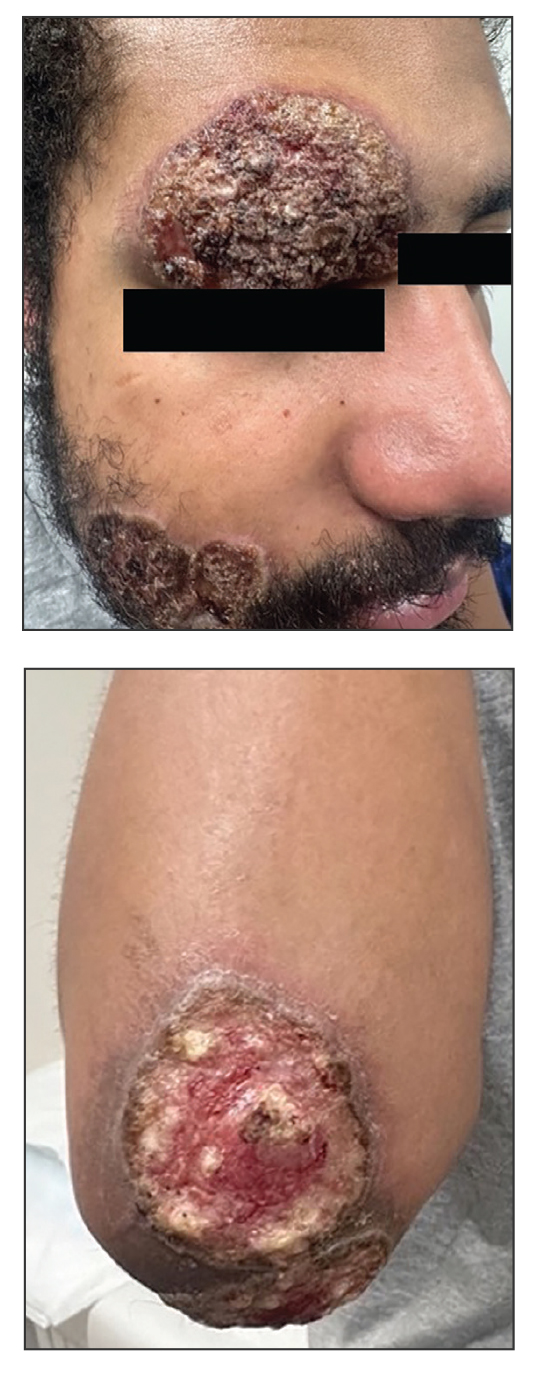
A 27-year-old man presented to his primary care physician after he was struck in the head by a tree branch while working outside. The next day, ulcerating lesions emerged on the right supraorbital ridge, along with subjective fevers, chills, fatigue, and shortness of breath. The patient reported a history of unprotected sexual intercourse with a male partner who was HIV positive. His medical history included syphilis status posttreatment with a course of 5 penicillin injections, hepatitis C, and HIV diagnosed one month prior to presentation (CD4 count, 169 cells/mm3 [reference range, 500-1500 cells/mm3]). A punch biopsy performed by the primary care physician revealed suppurative granulomatous inflammation, and the patient was prescribed antibiotics with mild improvement. He then was referred to dermatology for further evaluation of the ulcerating lesions.
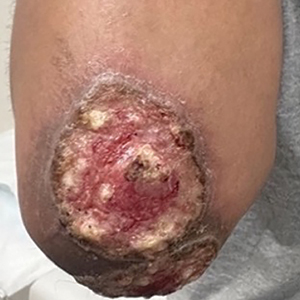
Three months after the initial trauma, the patient presented to the dermatology clinic for evaluation of multiple large fungating plaques affecting multiple sites on the face (top), arms (bottom), and legs. Physical examination revealed large circinate verrucous plaques involving the right supraorbital ridge and eyelid. The patient was unable to fully open the right eye. Similar plaques also were observed on the right malar cheek, arms, and feet. Four 5-mm punch biopsies from lesions on the right elbow and left ankle were obtained with fungal and bacterial cultures.