User login
Causal Relationship Exists Between Atopic Dermatitis and Brain Cancer
Key clinical point: A causal relationship was observed between genetically related atopic dermatitis (AD) and brain cancer, delineating AD as a potential risk factor for brain cancer.
Major finding: The presence of AD led to an increased risk for brain cancer (odds ratio 1.0005; P = .0096); however, no significant causal association was observed on conducting reverse Mendelian randomization analysis.
Study details: This cohort study analyzed the data on AD-associated single nucleotide polymorphisms of patients with AD (n = 15,208) and control individuals without AD (n = 367,046) from the FinnGen database (10th release) and the summary data of patients with brain cancer (n = 606) and control individuals without cancer (n = 372,016) from the IEU Open GWAS database.
Disclosures: This study did not disclose any funding source. The authors declared no conflicts of interest.
Source: Xin Y, Yuan T, Wang J. The causal relationship between atopic dermatitis and brain cancer: A bidirectional Mendelian randomization study. Skin Res Technol. 2024;30(4):e13715. doi: 10.1111/srt.13715 Source
Key clinical point: A causal relationship was observed between genetically related atopic dermatitis (AD) and brain cancer, delineating AD as a potential risk factor for brain cancer.
Major finding: The presence of AD led to an increased risk for brain cancer (odds ratio 1.0005; P = .0096); however, no significant causal association was observed on conducting reverse Mendelian randomization analysis.
Study details: This cohort study analyzed the data on AD-associated single nucleotide polymorphisms of patients with AD (n = 15,208) and control individuals without AD (n = 367,046) from the FinnGen database (10th release) and the summary data of patients with brain cancer (n = 606) and control individuals without cancer (n = 372,016) from the IEU Open GWAS database.
Disclosures: This study did not disclose any funding source. The authors declared no conflicts of interest.
Source: Xin Y, Yuan T, Wang J. The causal relationship between atopic dermatitis and brain cancer: A bidirectional Mendelian randomization study. Skin Res Technol. 2024;30(4):e13715. doi: 10.1111/srt.13715 Source
Key clinical point: A causal relationship was observed between genetically related atopic dermatitis (AD) and brain cancer, delineating AD as a potential risk factor for brain cancer.
Major finding: The presence of AD led to an increased risk for brain cancer (odds ratio 1.0005; P = .0096); however, no significant causal association was observed on conducting reverse Mendelian randomization analysis.
Study details: This cohort study analyzed the data on AD-associated single nucleotide polymorphisms of patients with AD (n = 15,208) and control individuals without AD (n = 367,046) from the FinnGen database (10th release) and the summary data of patients with brain cancer (n = 606) and control individuals without cancer (n = 372,016) from the IEU Open GWAS database.
Disclosures: This study did not disclose any funding source. The authors declared no conflicts of interest.
Source: Xin Y, Yuan T, Wang J. The causal relationship between atopic dermatitis and brain cancer: A bidirectional Mendelian randomization study. Skin Res Technol. 2024;30(4):e13715. doi: 10.1111/srt.13715 Source
Preventive Effect of Maternal Probiotic Supplementation in Atopic Dermatitis
Key clinical point: Maternal probiotic supplementation was effective in preventing atopic dermatitis (AD) in children regardless of their filaggrin (FLG) gene mutation status.
Major finding: Heterozygous FLG mutations were observed in 7% of children. The risk for AD after maternal probiotic supplementation was similar between children who expressed a FLG mutation (risk ratio [RR] 0.6; 95% CI 0.1-4.1) and those having a wild-type FLG (RR 0.6; 95% CI 0.4-0.9).
Study details: This exploratory study included the data of 228 children from the Probiotic in the Prevention of Allergy among Children in Trondheim (ProPACT) study who did or did not have FLG mutations and whose mothers received probiotic or placebo milk from 36 weeks of gestation until 3 months post delivery while breastfeeding.
Disclosures: This study was funded by the Liaison Committee between the Central Norway Regional Health Authority and the Norwegian University of Science and Technology, and the Norwegian Research Council. The authors declared no conflicts of interest.
Source: Zakiudin DP, Thyssen JP, Zachariae C, Videm V, Øien T, Simpson MR. Filaggrin mutation status and prevention of atopic dermatitis with maternal probiotic supplementation. Acta Derm Venereol. 2024;104:adv24360 (Apr 24). doi: 10.2340/actadv.v104.24360 Source
Key clinical point: Maternal probiotic supplementation was effective in preventing atopic dermatitis (AD) in children regardless of their filaggrin (FLG) gene mutation status.
Major finding: Heterozygous FLG mutations were observed in 7% of children. The risk for AD after maternal probiotic supplementation was similar between children who expressed a FLG mutation (risk ratio [RR] 0.6; 95% CI 0.1-4.1) and those having a wild-type FLG (RR 0.6; 95% CI 0.4-0.9).
Study details: This exploratory study included the data of 228 children from the Probiotic in the Prevention of Allergy among Children in Trondheim (ProPACT) study who did or did not have FLG mutations and whose mothers received probiotic or placebo milk from 36 weeks of gestation until 3 months post delivery while breastfeeding.
Disclosures: This study was funded by the Liaison Committee between the Central Norway Regional Health Authority and the Norwegian University of Science and Technology, and the Norwegian Research Council. The authors declared no conflicts of interest.
Source: Zakiudin DP, Thyssen JP, Zachariae C, Videm V, Øien T, Simpson MR. Filaggrin mutation status and prevention of atopic dermatitis with maternal probiotic supplementation. Acta Derm Venereol. 2024;104:adv24360 (Apr 24). doi: 10.2340/actadv.v104.24360 Source
Key clinical point: Maternal probiotic supplementation was effective in preventing atopic dermatitis (AD) in children regardless of their filaggrin (FLG) gene mutation status.
Major finding: Heterozygous FLG mutations were observed in 7% of children. The risk for AD after maternal probiotic supplementation was similar between children who expressed a FLG mutation (risk ratio [RR] 0.6; 95% CI 0.1-4.1) and those having a wild-type FLG (RR 0.6; 95% CI 0.4-0.9).
Study details: This exploratory study included the data of 228 children from the Probiotic in the Prevention of Allergy among Children in Trondheim (ProPACT) study who did or did not have FLG mutations and whose mothers received probiotic or placebo milk from 36 weeks of gestation until 3 months post delivery while breastfeeding.
Disclosures: This study was funded by the Liaison Committee between the Central Norway Regional Health Authority and the Norwegian University of Science and Technology, and the Norwegian Research Council. The authors declared no conflicts of interest.
Source: Zakiudin DP, Thyssen JP, Zachariae C, Videm V, Øien T, Simpson MR. Filaggrin mutation status and prevention of atopic dermatitis with maternal probiotic supplementation. Acta Derm Venereol. 2024;104:adv24360 (Apr 24). doi: 10.2340/actadv.v104.24360 Source
Pharmacological Interventions in Atopic Dermatitis Reduce Anxiety and Depression
Key clinical point: Pharmacological interventions aimed at reducing disease severity in patients with moderate to severe atopic dermatitis (AD) are also effective for improving anxiety and depression.
Major finding: Pharmacologic interventions for AD led to significant improvements in anxiety levels (standardized mean difference [SMD] −0.29; 95% CI −0.49 to −0.09) and depression severity (SMD −0.27; 95% CI −0.45 to −0.08) and an overall significant improvement in Hospital Anxiety and Depression scale scores (SMD −0.50; 95% CI −0.064 to −0.35).
Study details: This meta-analysis of seven phase 2b or 3 randomized controlled trials included 4723 patients with AD who were treated with either abrocitinib, baricitinib, dupilumab, tralokinumab, or placebo.
Disclosures: This study did not disclose any funding source. The authors declared no conflicts of interest.
Source: Hartono SP, Chatrath S, Aktas ON, et al. Interventions for anxiety and depression in patients with atopic dermatitis: A systematic review and meta-analysis. Sci Rep. 2024;14:8844 (Apr 17). Source
Key clinical point: Pharmacological interventions aimed at reducing disease severity in patients with moderate to severe atopic dermatitis (AD) are also effective for improving anxiety and depression.
Major finding: Pharmacologic interventions for AD led to significant improvements in anxiety levels (standardized mean difference [SMD] −0.29; 95% CI −0.49 to −0.09) and depression severity (SMD −0.27; 95% CI −0.45 to −0.08) and an overall significant improvement in Hospital Anxiety and Depression scale scores (SMD −0.50; 95% CI −0.064 to −0.35).
Study details: This meta-analysis of seven phase 2b or 3 randomized controlled trials included 4723 patients with AD who were treated with either abrocitinib, baricitinib, dupilumab, tralokinumab, or placebo.
Disclosures: This study did not disclose any funding source. The authors declared no conflicts of interest.
Source: Hartono SP, Chatrath S, Aktas ON, et al. Interventions for anxiety and depression in patients with atopic dermatitis: A systematic review and meta-analysis. Sci Rep. 2024;14:8844 (Apr 17). Source
Key clinical point: Pharmacological interventions aimed at reducing disease severity in patients with moderate to severe atopic dermatitis (AD) are also effective for improving anxiety and depression.
Major finding: Pharmacologic interventions for AD led to significant improvements in anxiety levels (standardized mean difference [SMD] −0.29; 95% CI −0.49 to −0.09) and depression severity (SMD −0.27; 95% CI −0.45 to −0.08) and an overall significant improvement in Hospital Anxiety and Depression scale scores (SMD −0.50; 95% CI −0.064 to −0.35).
Study details: This meta-analysis of seven phase 2b or 3 randomized controlled trials included 4723 patients with AD who were treated with either abrocitinib, baricitinib, dupilumab, tralokinumab, or placebo.
Disclosures: This study did not disclose any funding source. The authors declared no conflicts of interest.
Source: Hartono SP, Chatrath S, Aktas ON, et al. Interventions for anxiety and depression in patients with atopic dermatitis: A systematic review and meta-analysis. Sci Rep. 2024;14:8844 (Apr 17). Source
Comparable Efficacy of Tralokinumab and Dupilumab in Moderate to Severe Atopic Dermatitis
Key clinical point: When combined with topical corticosteroids (TCS), tralokinumab and dupilumab demonstrate similar efficacy in the treatment of patients with moderate to severe atopic dermatitis (AD) at 32 weeks of therapy.
Major finding: At week 32, tralokinumab and dupilumab treatment, both in combination with TCS, led to a similar proportion of patients achieving an Investigator's Global Assessment score of 0 or 1 (49.9% vs 39.3%; P = .95) or 75% improvement in the Eczema Area Severity Index scores (71.5% vs 71.9%; P = .95).
Study details: This unanchored matching-adjusted indirect comparison study analyzed the individual patient data of adults with moderate to severe AD (sample size 123.4) treated with tralokinumab plus TCS in ECZTRA 3, which were matched with the aggregate data of 106 patients treated with dupilumab plus TCS in the LIBERTY AD CHRONOS trial.
Disclosures: This study was funded by LEO Pharma. Four authors declared being employees of LEO Pharma. The other authors declared receiving consultancy or speaker honoraria from or having other ties with various sources, including LEO Pharma.
Source: Torres T, Sohrt Petersen A, Ivens U, et al. Matching-adjusted indirect comparison of the efficacy at week 32 of tralokinumab and dupilumab in the treatment of moderate-to-severe atopic dermatitis. Dermatol Ther (Heidelb). 2024;14:983-992 (Apr 13). doi: 10.1007/s13555-024-01143-x Source
Key clinical point: When combined with topical corticosteroids (TCS), tralokinumab and dupilumab demonstrate similar efficacy in the treatment of patients with moderate to severe atopic dermatitis (AD) at 32 weeks of therapy.
Major finding: At week 32, tralokinumab and dupilumab treatment, both in combination with TCS, led to a similar proportion of patients achieving an Investigator's Global Assessment score of 0 or 1 (49.9% vs 39.3%; P = .95) or 75% improvement in the Eczema Area Severity Index scores (71.5% vs 71.9%; P = .95).
Study details: This unanchored matching-adjusted indirect comparison study analyzed the individual patient data of adults with moderate to severe AD (sample size 123.4) treated with tralokinumab plus TCS in ECZTRA 3, which were matched with the aggregate data of 106 patients treated with dupilumab plus TCS in the LIBERTY AD CHRONOS trial.
Disclosures: This study was funded by LEO Pharma. Four authors declared being employees of LEO Pharma. The other authors declared receiving consultancy or speaker honoraria from or having other ties with various sources, including LEO Pharma.
Source: Torres T, Sohrt Petersen A, Ivens U, et al. Matching-adjusted indirect comparison of the efficacy at week 32 of tralokinumab and dupilumab in the treatment of moderate-to-severe atopic dermatitis. Dermatol Ther (Heidelb). 2024;14:983-992 (Apr 13). doi: 10.1007/s13555-024-01143-x Source
Key clinical point: When combined with topical corticosteroids (TCS), tralokinumab and dupilumab demonstrate similar efficacy in the treatment of patients with moderate to severe atopic dermatitis (AD) at 32 weeks of therapy.
Major finding: At week 32, tralokinumab and dupilumab treatment, both in combination with TCS, led to a similar proportion of patients achieving an Investigator's Global Assessment score of 0 or 1 (49.9% vs 39.3%; P = .95) or 75% improvement in the Eczema Area Severity Index scores (71.5% vs 71.9%; P = .95).
Study details: This unanchored matching-adjusted indirect comparison study analyzed the individual patient data of adults with moderate to severe AD (sample size 123.4) treated with tralokinumab plus TCS in ECZTRA 3, which were matched with the aggregate data of 106 patients treated with dupilumab plus TCS in the LIBERTY AD CHRONOS trial.
Disclosures: This study was funded by LEO Pharma. Four authors declared being employees of LEO Pharma. The other authors declared receiving consultancy or speaker honoraria from or having other ties with various sources, including LEO Pharma.
Source: Torres T, Sohrt Petersen A, Ivens U, et al. Matching-adjusted indirect comparison of the efficacy at week 32 of tralokinumab and dupilumab in the treatment of moderate-to-severe atopic dermatitis. Dermatol Ther (Heidelb). 2024;14:983-992 (Apr 13). doi: 10.1007/s13555-024-01143-x Source
Topical Ruxolitinib Provides Long-Term Disease Control in Adolescents With Atopic Dermatitis
Key clinical point: Topical 1.5% ruxolitinib was effective and well-tolerated and offered long-term disease control with as-needed use in adolescents with atopic dermatitis (AD).
Major finding: At week 8, a substantially higher number of patients receiving 1.5% ruxolitinib vs vehicle achieved an Investigator's Global Assessment (IGA) score of 0 or 1 with ≥2 grade improvement from baseline (50.6% vs 14.0%) and ≥75% improvement in the Eczema Area and Severity Index score (60.9% vs 34.9%), with sustained or increased proportion of patients achieving an IGA score of 0 or 1 during the long-term safety (LTS) period. No serious adverse events were reported.
Study details: This study used pooled data from two phase 3 trials (TRuE-AD1 and TRuE-AD2) and included 137 adolescents (age, 12-17 years) with AD who were randomly assigned to receive 0.75% or 1.5% ruxolitinib cream or vehicle twice daily for 8 weeks, followed by an LTS period lasting up to 52 weeks.
Disclosures: This study was funded by Incyte Corporation. Four authors declared being employees or shareholders of Incyte Corporation. Several authors declared ties with various sources, including Incyte Corporation.
Source: Eichenfield LF, Simpson EL, Papp K, et al. Efficacy, safety, and long-term disease control of ruxolitinib cream among adolescents with atopic dermatitis: Pooled results from two randomized phase 3 studies. Am J Clin Dermatol. 2024 (May 2). doi: 10.1007/s40257-024-00855-2 Source
Key clinical point: Topical 1.5% ruxolitinib was effective and well-tolerated and offered long-term disease control with as-needed use in adolescents with atopic dermatitis (AD).
Major finding: At week 8, a substantially higher number of patients receiving 1.5% ruxolitinib vs vehicle achieved an Investigator's Global Assessment (IGA) score of 0 or 1 with ≥2 grade improvement from baseline (50.6% vs 14.0%) and ≥75% improvement in the Eczema Area and Severity Index score (60.9% vs 34.9%), with sustained or increased proportion of patients achieving an IGA score of 0 or 1 during the long-term safety (LTS) period. No serious adverse events were reported.
Study details: This study used pooled data from two phase 3 trials (TRuE-AD1 and TRuE-AD2) and included 137 adolescents (age, 12-17 years) with AD who were randomly assigned to receive 0.75% or 1.5% ruxolitinib cream or vehicle twice daily for 8 weeks, followed by an LTS period lasting up to 52 weeks.
Disclosures: This study was funded by Incyte Corporation. Four authors declared being employees or shareholders of Incyte Corporation. Several authors declared ties with various sources, including Incyte Corporation.
Source: Eichenfield LF, Simpson EL, Papp K, et al. Efficacy, safety, and long-term disease control of ruxolitinib cream among adolescents with atopic dermatitis: Pooled results from two randomized phase 3 studies. Am J Clin Dermatol. 2024 (May 2). doi: 10.1007/s40257-024-00855-2 Source
Key clinical point: Topical 1.5% ruxolitinib was effective and well-tolerated and offered long-term disease control with as-needed use in adolescents with atopic dermatitis (AD).
Major finding: At week 8, a substantially higher number of patients receiving 1.5% ruxolitinib vs vehicle achieved an Investigator's Global Assessment (IGA) score of 0 or 1 with ≥2 grade improvement from baseline (50.6% vs 14.0%) and ≥75% improvement in the Eczema Area and Severity Index score (60.9% vs 34.9%), with sustained or increased proportion of patients achieving an IGA score of 0 or 1 during the long-term safety (LTS) period. No serious adverse events were reported.
Study details: This study used pooled data from two phase 3 trials (TRuE-AD1 and TRuE-AD2) and included 137 adolescents (age, 12-17 years) with AD who were randomly assigned to receive 0.75% or 1.5% ruxolitinib cream or vehicle twice daily for 8 weeks, followed by an LTS period lasting up to 52 weeks.
Disclosures: This study was funded by Incyte Corporation. Four authors declared being employees or shareholders of Incyte Corporation. Several authors declared ties with various sources, including Incyte Corporation.
Source: Eichenfield LF, Simpson EL, Papp K, et al. Efficacy, safety, and long-term disease control of ruxolitinib cream among adolescents with atopic dermatitis: Pooled results from two randomized phase 3 studies. Am J Clin Dermatol. 2024 (May 2). doi: 10.1007/s40257-024-00855-2 Source
Obesity Associated With Disease Severity in Moderate to Severe Atopic Dermatitis
Key clinical point: Obesity is significantly associated with patient- and physician-assessed measures of atopic dermatitis (AD) disease severity.
Major finding: Increased body mass index (BMI) values were associated with higher disease severity as assessed by objective Scoring AD (adjusted β 1.24; P = .013) and patient-oriented eczema measure (adjusted β 1.09; P = .038) scores.
Study details: This study based on data from the prospective observational TREATgermany registry included 1416 patients with moderate to severe AD who were either underweight (BMI < 18.5 kg/m2; n = 33), normal weight or overweight (nonobese; BMI ≥ 18.5 and < 30 kg/m2; n = 1149), or obese (BMI ≥ 30 kg/m2; n = 234).
Disclosures: The TREATgermany registry is supported by AbbVie Deutschland GmbH & Co. KG, Galderma SA, LEO Pharma GmbH, Lilly Deutschland GmbH, Pfizer Inc., and Sanofi. Eight authors declared serving as consultants or lecturers for or receiving research grants, personal fees, or lecture or consulting honoraria from various sources, including some of the supporters of TREATgermany.
Source: Traidl S, Hollstein MM, Kroeger N, et al, and The TREATgermany Study Group. Obesity is linked to disease severity in moderate to severe atopic dermatitis—Data from the prospective observational TREATgermany registry. J Eur Acad Dermatol Venereol. 2024 (Apr 25). doi: 10.1111/jdv.20042 Source
Key clinical point: Obesity is significantly associated with patient- and physician-assessed measures of atopic dermatitis (AD) disease severity.
Major finding: Increased body mass index (BMI) values were associated with higher disease severity as assessed by objective Scoring AD (adjusted β 1.24; P = .013) and patient-oriented eczema measure (adjusted β 1.09; P = .038) scores.
Study details: This study based on data from the prospective observational TREATgermany registry included 1416 patients with moderate to severe AD who were either underweight (BMI < 18.5 kg/m2; n = 33), normal weight or overweight (nonobese; BMI ≥ 18.5 and < 30 kg/m2; n = 1149), or obese (BMI ≥ 30 kg/m2; n = 234).
Disclosures: The TREATgermany registry is supported by AbbVie Deutschland GmbH & Co. KG, Galderma SA, LEO Pharma GmbH, Lilly Deutschland GmbH, Pfizer Inc., and Sanofi. Eight authors declared serving as consultants or lecturers for or receiving research grants, personal fees, or lecture or consulting honoraria from various sources, including some of the supporters of TREATgermany.
Source: Traidl S, Hollstein MM, Kroeger N, et al, and The TREATgermany Study Group. Obesity is linked to disease severity in moderate to severe atopic dermatitis—Data from the prospective observational TREATgermany registry. J Eur Acad Dermatol Venereol. 2024 (Apr 25). doi: 10.1111/jdv.20042 Source
Key clinical point: Obesity is significantly associated with patient- and physician-assessed measures of atopic dermatitis (AD) disease severity.
Major finding: Increased body mass index (BMI) values were associated with higher disease severity as assessed by objective Scoring AD (adjusted β 1.24; P = .013) and patient-oriented eczema measure (adjusted β 1.09; P = .038) scores.
Study details: This study based on data from the prospective observational TREATgermany registry included 1416 patients with moderate to severe AD who were either underweight (BMI < 18.5 kg/m2; n = 33), normal weight or overweight (nonobese; BMI ≥ 18.5 and < 30 kg/m2; n = 1149), or obese (BMI ≥ 30 kg/m2; n = 234).
Disclosures: The TREATgermany registry is supported by AbbVie Deutschland GmbH & Co. KG, Galderma SA, LEO Pharma GmbH, Lilly Deutschland GmbH, Pfizer Inc., and Sanofi. Eight authors declared serving as consultants or lecturers for or receiving research grants, personal fees, or lecture or consulting honoraria from various sources, including some of the supporters of TREATgermany.
Source: Traidl S, Hollstein MM, Kroeger N, et al, and The TREATgermany Study Group. Obesity is linked to disease severity in moderate to severe atopic dermatitis—Data from the prospective observational TREATgermany registry. J Eur Acad Dermatol Venereol. 2024 (Apr 25). doi: 10.1111/jdv.20042 Source
Antibiotics in Early Infancy Disrupt Gut Microbiome and Increase Risk for Atopic Dermatitis
Key clinical point: Antibiotic use early in life, especially within one year of age, disrupts the gut microbiome and increases the risk for atopic dermatitis (AD) at 5 years of age.
Major finding: Children who received antibiotics during the first year of life vs later were significantly more likely to develop AD at 5 years of age (adjusted odds ratio [aOR] 1.81; P < .001), with an increased number of antibiotic courses leading to a dose-response-like increased risk for AD (1 course: aOR 1.67; P = .0044; ≥ 2 courses: aOR 2.16; P = .0030).
Study details: This study analyzed the clinical data for AD diagnosis at age 5 years of 2484 children from the prospective, general population CHILD birth cohort, which enrolled pregnant women and infants with no congenital abnormalities born at ≥ 34 weeks of gestation.
Disclosures: The CHILD Study is funded by the Canadian Institutes of Health Research, the Allergy, Genes, and Environment Network of Centres of Excellence, Debbie and Don Morrison, and others. The authors declared no conflicts of interest.
Source: Hoskinson C, Medeleanu MV, Reyna ME, et al. Antibiotics within first year are linked to infant gut microbiome disruption and elevated atopic dermatitis risk. J Allergy Clin Immunol. 2024 (Apr 24). doi: 10.1016/j.jaci.2024.03.025 Source
Key clinical point: Antibiotic use early in life, especially within one year of age, disrupts the gut microbiome and increases the risk for atopic dermatitis (AD) at 5 years of age.
Major finding: Children who received antibiotics during the first year of life vs later were significantly more likely to develop AD at 5 years of age (adjusted odds ratio [aOR] 1.81; P < .001), with an increased number of antibiotic courses leading to a dose-response-like increased risk for AD (1 course: aOR 1.67; P = .0044; ≥ 2 courses: aOR 2.16; P = .0030).
Study details: This study analyzed the clinical data for AD diagnosis at age 5 years of 2484 children from the prospective, general population CHILD birth cohort, which enrolled pregnant women and infants with no congenital abnormalities born at ≥ 34 weeks of gestation.
Disclosures: The CHILD Study is funded by the Canadian Institutes of Health Research, the Allergy, Genes, and Environment Network of Centres of Excellence, Debbie and Don Morrison, and others. The authors declared no conflicts of interest.
Source: Hoskinson C, Medeleanu MV, Reyna ME, et al. Antibiotics within first year are linked to infant gut microbiome disruption and elevated atopic dermatitis risk. J Allergy Clin Immunol. 2024 (Apr 24). doi: 10.1016/j.jaci.2024.03.025 Source
Key clinical point: Antibiotic use early in life, especially within one year of age, disrupts the gut microbiome and increases the risk for atopic dermatitis (AD) at 5 years of age.
Major finding: Children who received antibiotics during the first year of life vs later were significantly more likely to develop AD at 5 years of age (adjusted odds ratio [aOR] 1.81; P < .001), with an increased number of antibiotic courses leading to a dose-response-like increased risk for AD (1 course: aOR 1.67; P = .0044; ≥ 2 courses: aOR 2.16; P = .0030).
Study details: This study analyzed the clinical data for AD diagnosis at age 5 years of 2484 children from the prospective, general population CHILD birth cohort, which enrolled pregnant women and infants with no congenital abnormalities born at ≥ 34 weeks of gestation.
Disclosures: The CHILD Study is funded by the Canadian Institutes of Health Research, the Allergy, Genes, and Environment Network of Centres of Excellence, Debbie and Don Morrison, and others. The authors declared no conflicts of interest.
Source: Hoskinson C, Medeleanu MV, Reyna ME, et al. Antibiotics within first year are linked to infant gut microbiome disruption and elevated atopic dermatitis risk. J Allergy Clin Immunol. 2024 (Apr 24). doi: 10.1016/j.jaci.2024.03.025 Source
High-Potency Cannabis Tied to Impaired Brain Development, Psychosis, Cannabis-Use Disorder
It’s becoming clear that (CUD).
That was the message delivered by Yasmin Hurd, PhD, director of the Addiction Institute at Mount Sinai in New York, during a press briefing at the American Psychiatric Association (APA) 2024 annual meeting.
“We’re actually in historic times in that we now have highly concentrated, highly potent cannabis products that are administered in various routes,” Dr. Hurd told reporters.
Tetrahydrocannabinol (THC) concentrations in cannabis products have increased over the years, from around 2%-4% to 15%-24% now, Dr. Hurd noted.
Dr. Hurd and colleagues wrote in a commentary on the developmental trajectory of CUD published simultaneously in the American Journal of Psychiatry.
Dramatic Increase in Teen Cannabis Use
A recent study from Oregon Health & Science University showed that adolescent cannabis abuse in the United States has increased dramatically, by about 245%, since 2000.
“Drug abuse is often driven by what is in front of you,” Nora Volkow, MD, director of the National Institute on Drug Abuse, noted in an interview.
“Right now, cannabis is widely available. So, guess what? Cannabis becomes the drug that people take. Nicotine is much harder to get. It is regulated to a much greater extent than cannabis, so fewer teenagers are consuming nicotine than are consuming cannabis,” Dr. Volkow said.
Cannabis exposure during neurodevelopment has the potential to alter the endocannabinoid system, which in turn, can affect the development of neural pathways that mediate reward; emotional regulation; and multiple cognitive domains including executive functioning and decision-making, learning, abstraction, and attention — all processes central to substance use disorder and other psychiatric disorders, Dr. Hurd said at the briefing.
Dr. Volkow said that cannabis use in adolescence and young adulthood is “very concerning because that’s also the age of risk for psychosis, particularly schizophrenia, with one study showing that use of cannabis in high doses can trigger psychotic episodes, particularly among young males.”
Dr. Hurd noted that not all young people who use cannabis develop CUD, “but a significant number do,” and large-scale studies have consistently reported two main factors associated with CUD risk.
The first is age, both for the onset and frequency of use at younger age. Those who start using cannabis before age 16 years are at the highest risk for CUD. The risk for CUD also increases significantly among youth who use cannabis at least weekly, with the highest prevalence among youth who use cannabis daily. One large study linked increased frequency of use with up to a 17-fold increased risk for CUD.
The second factor consistently associated with the risk for CUD is biologic sex, with CUD rates typically higher in male individuals.
Treatment Challenges
For young people who develop CUD, access to and uptake of treatment can be challenging.
“Given that the increased potency of cannabis and cannabinoid products is expected to increase CUD risk, it is disturbing that less than 10% of youth who meet the criteria for a substance use disorder, including CUD, receive treatment,” Dr. Hurd and colleagues point out in their commentary.
Another challenge is that treatment strategies for CUD are currently limited and consist mainly of motivational enhancement and cognitive-behavioral therapies.
“Clearly new treatment strategies are needed to address the mounting challenge of CUD risk in teens and young adults,” Dr. Hurd and colleagues wrote.
Summing up, Dr. Hurd told reporters, “We now know that most psychiatric disorders have a developmental origin, and the adolescent time period is a critical window for cannabis use disorder risk.”
Yet, on a positive note, the “plasticity of the developing brain that makes it vulnerable to cannabis use disorder and psychiatric comorbidities also provides an opportunity for prevention and early intervention to change that trajectory,” Dr. Hurd said.
The changing legal landscape of cannabis — the US Drug Enforcement Agency is moving forward with plans to move marijuana from a Schedule I to a Schedule III controlled substance under the Controlled Substance Act — makes addressing these risks all the timelier.
“As states vie to leverage tax dollars from the growing cannabis industry, a significant portion of such funds must be used for early intervention/prevention strategies to reduce the impact of cannabis on the developing brain,” Dr. Hurd and colleagues wrote.
This research was supported in part by the National Institute on Drug Abuse and the National Institutes of Health. Dr. Hurd and Dr. Volkow have no relevant disclosures.
A version of this article appeared on Medscape.com.
It’s becoming clear that (CUD).
That was the message delivered by Yasmin Hurd, PhD, director of the Addiction Institute at Mount Sinai in New York, during a press briefing at the American Psychiatric Association (APA) 2024 annual meeting.
“We’re actually in historic times in that we now have highly concentrated, highly potent cannabis products that are administered in various routes,” Dr. Hurd told reporters.
Tetrahydrocannabinol (THC) concentrations in cannabis products have increased over the years, from around 2%-4% to 15%-24% now, Dr. Hurd noted.
Dr. Hurd and colleagues wrote in a commentary on the developmental trajectory of CUD published simultaneously in the American Journal of Psychiatry.
Dramatic Increase in Teen Cannabis Use
A recent study from Oregon Health & Science University showed that adolescent cannabis abuse in the United States has increased dramatically, by about 245%, since 2000.
“Drug abuse is often driven by what is in front of you,” Nora Volkow, MD, director of the National Institute on Drug Abuse, noted in an interview.
“Right now, cannabis is widely available. So, guess what? Cannabis becomes the drug that people take. Nicotine is much harder to get. It is regulated to a much greater extent than cannabis, so fewer teenagers are consuming nicotine than are consuming cannabis,” Dr. Volkow said.
Cannabis exposure during neurodevelopment has the potential to alter the endocannabinoid system, which in turn, can affect the development of neural pathways that mediate reward; emotional regulation; and multiple cognitive domains including executive functioning and decision-making, learning, abstraction, and attention — all processes central to substance use disorder and other psychiatric disorders, Dr. Hurd said at the briefing.
Dr. Volkow said that cannabis use in adolescence and young adulthood is “very concerning because that’s also the age of risk for psychosis, particularly schizophrenia, with one study showing that use of cannabis in high doses can trigger psychotic episodes, particularly among young males.”
Dr. Hurd noted that not all young people who use cannabis develop CUD, “but a significant number do,” and large-scale studies have consistently reported two main factors associated with CUD risk.
The first is age, both for the onset and frequency of use at younger age. Those who start using cannabis before age 16 years are at the highest risk for CUD. The risk for CUD also increases significantly among youth who use cannabis at least weekly, with the highest prevalence among youth who use cannabis daily. One large study linked increased frequency of use with up to a 17-fold increased risk for CUD.
The second factor consistently associated with the risk for CUD is biologic sex, with CUD rates typically higher in male individuals.
Treatment Challenges
For young people who develop CUD, access to and uptake of treatment can be challenging.
“Given that the increased potency of cannabis and cannabinoid products is expected to increase CUD risk, it is disturbing that less than 10% of youth who meet the criteria for a substance use disorder, including CUD, receive treatment,” Dr. Hurd and colleagues point out in their commentary.
Another challenge is that treatment strategies for CUD are currently limited and consist mainly of motivational enhancement and cognitive-behavioral therapies.
“Clearly new treatment strategies are needed to address the mounting challenge of CUD risk in teens and young adults,” Dr. Hurd and colleagues wrote.
Summing up, Dr. Hurd told reporters, “We now know that most psychiatric disorders have a developmental origin, and the adolescent time period is a critical window for cannabis use disorder risk.”
Yet, on a positive note, the “plasticity of the developing brain that makes it vulnerable to cannabis use disorder and psychiatric comorbidities also provides an opportunity for prevention and early intervention to change that trajectory,” Dr. Hurd said.
The changing legal landscape of cannabis — the US Drug Enforcement Agency is moving forward with plans to move marijuana from a Schedule I to a Schedule III controlled substance under the Controlled Substance Act — makes addressing these risks all the timelier.
“As states vie to leverage tax dollars from the growing cannabis industry, a significant portion of such funds must be used for early intervention/prevention strategies to reduce the impact of cannabis on the developing brain,” Dr. Hurd and colleagues wrote.
This research was supported in part by the National Institute on Drug Abuse and the National Institutes of Health. Dr. Hurd and Dr. Volkow have no relevant disclosures.
A version of this article appeared on Medscape.com.
It’s becoming clear that (CUD).
That was the message delivered by Yasmin Hurd, PhD, director of the Addiction Institute at Mount Sinai in New York, during a press briefing at the American Psychiatric Association (APA) 2024 annual meeting.
“We’re actually in historic times in that we now have highly concentrated, highly potent cannabis products that are administered in various routes,” Dr. Hurd told reporters.
Tetrahydrocannabinol (THC) concentrations in cannabis products have increased over the years, from around 2%-4% to 15%-24% now, Dr. Hurd noted.
Dr. Hurd and colleagues wrote in a commentary on the developmental trajectory of CUD published simultaneously in the American Journal of Psychiatry.
Dramatic Increase in Teen Cannabis Use
A recent study from Oregon Health & Science University showed that adolescent cannabis abuse in the United States has increased dramatically, by about 245%, since 2000.
“Drug abuse is often driven by what is in front of you,” Nora Volkow, MD, director of the National Institute on Drug Abuse, noted in an interview.
“Right now, cannabis is widely available. So, guess what? Cannabis becomes the drug that people take. Nicotine is much harder to get. It is regulated to a much greater extent than cannabis, so fewer teenagers are consuming nicotine than are consuming cannabis,” Dr. Volkow said.
Cannabis exposure during neurodevelopment has the potential to alter the endocannabinoid system, which in turn, can affect the development of neural pathways that mediate reward; emotional regulation; and multiple cognitive domains including executive functioning and decision-making, learning, abstraction, and attention — all processes central to substance use disorder and other psychiatric disorders, Dr. Hurd said at the briefing.
Dr. Volkow said that cannabis use in adolescence and young adulthood is “very concerning because that’s also the age of risk for psychosis, particularly schizophrenia, with one study showing that use of cannabis in high doses can trigger psychotic episodes, particularly among young males.”
Dr. Hurd noted that not all young people who use cannabis develop CUD, “but a significant number do,” and large-scale studies have consistently reported two main factors associated with CUD risk.
The first is age, both for the onset and frequency of use at younger age. Those who start using cannabis before age 16 years are at the highest risk for CUD. The risk for CUD also increases significantly among youth who use cannabis at least weekly, with the highest prevalence among youth who use cannabis daily. One large study linked increased frequency of use with up to a 17-fold increased risk for CUD.
The second factor consistently associated with the risk for CUD is biologic sex, with CUD rates typically higher in male individuals.
Treatment Challenges
For young people who develop CUD, access to and uptake of treatment can be challenging.
“Given that the increased potency of cannabis and cannabinoid products is expected to increase CUD risk, it is disturbing that less than 10% of youth who meet the criteria for a substance use disorder, including CUD, receive treatment,” Dr. Hurd and colleagues point out in their commentary.
Another challenge is that treatment strategies for CUD are currently limited and consist mainly of motivational enhancement and cognitive-behavioral therapies.
“Clearly new treatment strategies are needed to address the mounting challenge of CUD risk in teens and young adults,” Dr. Hurd and colleagues wrote.
Summing up, Dr. Hurd told reporters, “We now know that most psychiatric disorders have a developmental origin, and the adolescent time period is a critical window for cannabis use disorder risk.”
Yet, on a positive note, the “plasticity of the developing brain that makes it vulnerable to cannabis use disorder and psychiatric comorbidities also provides an opportunity for prevention and early intervention to change that trajectory,” Dr. Hurd said.
The changing legal landscape of cannabis — the US Drug Enforcement Agency is moving forward with plans to move marijuana from a Schedule I to a Schedule III controlled substance under the Controlled Substance Act — makes addressing these risks all the timelier.
“As states vie to leverage tax dollars from the growing cannabis industry, a significant portion of such funds must be used for early intervention/prevention strategies to reduce the impact of cannabis on the developing brain,” Dr. Hurd and colleagues wrote.
This research was supported in part by the National Institute on Drug Abuse and the National Institutes of Health. Dr. Hurd and Dr. Volkow have no relevant disclosures.
A version of this article appeared on Medscape.com.
FROM APA 2024
Follow-Up for Pediatric Depression Doubles With New Quality Initiative
TORONTO — An ambitious effort at a busy pediatrics clinic to improve follow-up in children and adolescents with a positive depression screen improved this quality metric, and it produced a fundamental change in approach.
“It was a big culture shift,” reported Landon B. Krantz, MD, a clinical fellow in the Division of General and Community Pediatrics at Cincinnati Children’s Hospital in Ohio. From a baseline position of screening, risk identification, and then referral, “we are now taking ownership of the process.”
Based on the substantial risk posed by significant levels of depression, guidelines recommend follow-up for any patient 12 years or older who has a positive screen, according to Dr. Krantz. At his center, they found only 19% had a documented follow-up within 30 days, even though timely intervention is important.
“Nearly half of suicide events in adolescents occur within 30 days after a positive PHQ-9 [9-question Patient Health Questionnaire] is completed,” said Dr. Krantz when presenting his data at the Pediatric Academic Societies annual meeting.
The issue has gained more urgency because of the substantial increase over the past several years in children presenting with depression and suicidal thoughts, according to Dr. Krantz. He said many are characterizing the upsurge as a mental health crisis in the pediatric age group.
Improving Follow-Up
. The goal at the outset was to increase the proportion to 35%.
“We know that a lot of children would receive follow-up at centers outside of our system,” said Dr. Krantz, explaining why the goal was relatively modest. Based on the likelihood that many follow-up visits would not be captured, he expected the final data would represent an underestimate.
Depression at baseline was defined as a score of 10 or higher on the PHQ-9 or any positive answer to item 9 on this screening tool, which asks specifically about thoughts of self-harm.
To be counted, follow-up had to be a documented encounter, whether by phone call, in-person visit, or telehealth visit.
“We needed patients to be checked. We did not count a prescription refill as a true follow-up,” Dr. Krantz specified.
There were numerous strategies implemented to improve follow-up, not least of which was an educational program to reinforce the importance and value of follow-up that was disseminated to clinicians in all of the participating clinics. Medical assistants were instructed to schedule a follow-up appointment for all patients who tested positive before they left the office. A target of 3 weeks was a strategy of overcorrection when so many patients were missing the initial 30-day window by just a few days.
The approach also involved an enhanced collaboration with psychologists to which patients were referred. Asking for expedited appointments when appropriate ensured that those at highest risk were prioritized, although Dr. Krantz said that this step was planned carefully to avoid overwhelming the mental health team.
“We monitored this and made sure it was not increasing the burden for psychologists from a capacity standpoint,” he said.
Other steps, like a depression action plan, which Dr. Krantz compared to an asthma action plan, were also implemented to reduce the risk of losing symptomatic patients before the chance for an effective treatment.
When compared with the 19% 30-day follow-up rate in the preintervention sample of 589 children, the 43.8% 30-day follow-up rate achieved in the 764 patients identified after implementation beat the original goal.
The improvement in follow-up was relatively consistent across all six clinics, which Dr. Krantz believes reflected a broad and shared change in a sense of responsibility for confirming that symptoms of depression were being addressed. Patients were still referred for psychological help, but referral was no longer considered enough.
“Children with mental health issues are still our patients in primary care,” said Dr. Krantz, who considers this an important change in orientation.
While the goal was to schedule patients for a follow-up at the time of a positive depression screen, Dr. Krantz described one important accommodation.
“The screen for depression was being performed in most cases during well visits, so patients and their families were not expecting to be discussing this issue,” he said. The diagnosis might be a particular surprise to parents who were not aware of any symptoms. In this case, Dr. Krantz said patients and families were given time to process the information and were contacted after a week to discuss further workup.
It is also notable that about one third of patients met the criteria for depression by answering positively to the PHQ-9 item on self-harm when they did not meet the 10 or more threshold depression score overall. In other words, these patients would have been missed without this criterion.
In the participating Cincinnati pediatric clinics, about 12%-13% of adolescents met the criteria for depression, which Dr. Krantz said is consistent with reports in the literature. He said the range is about 6%-24%.
Although outcomes were not tracked, there is evidence that early intervention for depression yields better outcomes than delayed intervention, according to Dr. Krantz. Based on approximately 600 positive screens for depression per year at his pediatric clinics, he estimated that his data predict at least 25% more patients will receive timely follow-up.
Seeking Solutions to a Growing Problem
There are several studies documenting the growing problem of adolescent depression and suicide and, for this reason, the topic is attracting a lot of attention, according to Corinna Rea, MD, MPH, a pediatrician working in the primary care center at Boston Children’s Hospital in Massachusetts.
Dr. Rea was not involved with the study, but when asked to comment, she said: “The results of this study were encouraging because we know that getting patients to care quickly is probably important.” She also agreed that referring patients with depression for care might not be enough, noting that a lot of patients do not follow up on recommendations to pursue a consultation or treatment.
“I am now involved in a project with the American Academy of Pediatrics to address this issue,” Dr. Rae said. She thinks that more work in this area is needed and agreed with Dr. Krantz that pediatricians should verify that children with depression are getting help even when other specialists are providing the treatment.
Dr. Krantz and Dr. Rae report no potential conflicts of interest.
TORONTO — An ambitious effort at a busy pediatrics clinic to improve follow-up in children and adolescents with a positive depression screen improved this quality metric, and it produced a fundamental change in approach.
“It was a big culture shift,” reported Landon B. Krantz, MD, a clinical fellow in the Division of General and Community Pediatrics at Cincinnati Children’s Hospital in Ohio. From a baseline position of screening, risk identification, and then referral, “we are now taking ownership of the process.”
Based on the substantial risk posed by significant levels of depression, guidelines recommend follow-up for any patient 12 years or older who has a positive screen, according to Dr. Krantz. At his center, they found only 19% had a documented follow-up within 30 days, even though timely intervention is important.
“Nearly half of suicide events in adolescents occur within 30 days after a positive PHQ-9 [9-question Patient Health Questionnaire] is completed,” said Dr. Krantz when presenting his data at the Pediatric Academic Societies annual meeting.
The issue has gained more urgency because of the substantial increase over the past several years in children presenting with depression and suicidal thoughts, according to Dr. Krantz. He said many are characterizing the upsurge as a mental health crisis in the pediatric age group.
Improving Follow-Up
. The goal at the outset was to increase the proportion to 35%.
“We know that a lot of children would receive follow-up at centers outside of our system,” said Dr. Krantz, explaining why the goal was relatively modest. Based on the likelihood that many follow-up visits would not be captured, he expected the final data would represent an underestimate.
Depression at baseline was defined as a score of 10 or higher on the PHQ-9 or any positive answer to item 9 on this screening tool, which asks specifically about thoughts of self-harm.
To be counted, follow-up had to be a documented encounter, whether by phone call, in-person visit, or telehealth visit.
“We needed patients to be checked. We did not count a prescription refill as a true follow-up,” Dr. Krantz specified.
There were numerous strategies implemented to improve follow-up, not least of which was an educational program to reinforce the importance and value of follow-up that was disseminated to clinicians in all of the participating clinics. Medical assistants were instructed to schedule a follow-up appointment for all patients who tested positive before they left the office. A target of 3 weeks was a strategy of overcorrection when so many patients were missing the initial 30-day window by just a few days.
The approach also involved an enhanced collaboration with psychologists to which patients were referred. Asking for expedited appointments when appropriate ensured that those at highest risk were prioritized, although Dr. Krantz said that this step was planned carefully to avoid overwhelming the mental health team.
“We monitored this and made sure it was not increasing the burden for psychologists from a capacity standpoint,” he said.
Other steps, like a depression action plan, which Dr. Krantz compared to an asthma action plan, were also implemented to reduce the risk of losing symptomatic patients before the chance for an effective treatment.
When compared with the 19% 30-day follow-up rate in the preintervention sample of 589 children, the 43.8% 30-day follow-up rate achieved in the 764 patients identified after implementation beat the original goal.
The improvement in follow-up was relatively consistent across all six clinics, which Dr. Krantz believes reflected a broad and shared change in a sense of responsibility for confirming that symptoms of depression were being addressed. Patients were still referred for psychological help, but referral was no longer considered enough.
“Children with mental health issues are still our patients in primary care,” said Dr. Krantz, who considers this an important change in orientation.
While the goal was to schedule patients for a follow-up at the time of a positive depression screen, Dr. Krantz described one important accommodation.
“The screen for depression was being performed in most cases during well visits, so patients and their families were not expecting to be discussing this issue,” he said. The diagnosis might be a particular surprise to parents who were not aware of any symptoms. In this case, Dr. Krantz said patients and families were given time to process the information and were contacted after a week to discuss further workup.
It is also notable that about one third of patients met the criteria for depression by answering positively to the PHQ-9 item on self-harm when they did not meet the 10 or more threshold depression score overall. In other words, these patients would have been missed without this criterion.
In the participating Cincinnati pediatric clinics, about 12%-13% of adolescents met the criteria for depression, which Dr. Krantz said is consistent with reports in the literature. He said the range is about 6%-24%.
Although outcomes were not tracked, there is evidence that early intervention for depression yields better outcomes than delayed intervention, according to Dr. Krantz. Based on approximately 600 positive screens for depression per year at his pediatric clinics, he estimated that his data predict at least 25% more patients will receive timely follow-up.
Seeking Solutions to a Growing Problem
There are several studies documenting the growing problem of adolescent depression and suicide and, for this reason, the topic is attracting a lot of attention, according to Corinna Rea, MD, MPH, a pediatrician working in the primary care center at Boston Children’s Hospital in Massachusetts.
Dr. Rea was not involved with the study, but when asked to comment, she said: “The results of this study were encouraging because we know that getting patients to care quickly is probably important.” She also agreed that referring patients with depression for care might not be enough, noting that a lot of patients do not follow up on recommendations to pursue a consultation or treatment.
“I am now involved in a project with the American Academy of Pediatrics to address this issue,” Dr. Rae said. She thinks that more work in this area is needed and agreed with Dr. Krantz that pediatricians should verify that children with depression are getting help even when other specialists are providing the treatment.
Dr. Krantz and Dr. Rae report no potential conflicts of interest.
TORONTO — An ambitious effort at a busy pediatrics clinic to improve follow-up in children and adolescents with a positive depression screen improved this quality metric, and it produced a fundamental change in approach.
“It was a big culture shift,” reported Landon B. Krantz, MD, a clinical fellow in the Division of General and Community Pediatrics at Cincinnati Children’s Hospital in Ohio. From a baseline position of screening, risk identification, and then referral, “we are now taking ownership of the process.”
Based on the substantial risk posed by significant levels of depression, guidelines recommend follow-up for any patient 12 years or older who has a positive screen, according to Dr. Krantz. At his center, they found only 19% had a documented follow-up within 30 days, even though timely intervention is important.
“Nearly half of suicide events in adolescents occur within 30 days after a positive PHQ-9 [9-question Patient Health Questionnaire] is completed,” said Dr. Krantz when presenting his data at the Pediatric Academic Societies annual meeting.
The issue has gained more urgency because of the substantial increase over the past several years in children presenting with depression and suicidal thoughts, according to Dr. Krantz. He said many are characterizing the upsurge as a mental health crisis in the pediatric age group.
Improving Follow-Up
. The goal at the outset was to increase the proportion to 35%.
“We know that a lot of children would receive follow-up at centers outside of our system,” said Dr. Krantz, explaining why the goal was relatively modest. Based on the likelihood that many follow-up visits would not be captured, he expected the final data would represent an underestimate.
Depression at baseline was defined as a score of 10 or higher on the PHQ-9 or any positive answer to item 9 on this screening tool, which asks specifically about thoughts of self-harm.
To be counted, follow-up had to be a documented encounter, whether by phone call, in-person visit, or telehealth visit.
“We needed patients to be checked. We did not count a prescription refill as a true follow-up,” Dr. Krantz specified.
There were numerous strategies implemented to improve follow-up, not least of which was an educational program to reinforce the importance and value of follow-up that was disseminated to clinicians in all of the participating clinics. Medical assistants were instructed to schedule a follow-up appointment for all patients who tested positive before they left the office. A target of 3 weeks was a strategy of overcorrection when so many patients were missing the initial 30-day window by just a few days.
The approach also involved an enhanced collaboration with psychologists to which patients were referred. Asking for expedited appointments when appropriate ensured that those at highest risk were prioritized, although Dr. Krantz said that this step was planned carefully to avoid overwhelming the mental health team.
“We monitored this and made sure it was not increasing the burden for psychologists from a capacity standpoint,” he said.
Other steps, like a depression action plan, which Dr. Krantz compared to an asthma action plan, were also implemented to reduce the risk of losing symptomatic patients before the chance for an effective treatment.
When compared with the 19% 30-day follow-up rate in the preintervention sample of 589 children, the 43.8% 30-day follow-up rate achieved in the 764 patients identified after implementation beat the original goal.
The improvement in follow-up was relatively consistent across all six clinics, which Dr. Krantz believes reflected a broad and shared change in a sense of responsibility for confirming that symptoms of depression were being addressed. Patients were still referred for psychological help, but referral was no longer considered enough.
“Children with mental health issues are still our patients in primary care,” said Dr. Krantz, who considers this an important change in orientation.
While the goal was to schedule patients for a follow-up at the time of a positive depression screen, Dr. Krantz described one important accommodation.
“The screen for depression was being performed in most cases during well visits, so patients and their families were not expecting to be discussing this issue,” he said. The diagnosis might be a particular surprise to parents who were not aware of any symptoms. In this case, Dr. Krantz said patients and families were given time to process the information and were contacted after a week to discuss further workup.
It is also notable that about one third of patients met the criteria for depression by answering positively to the PHQ-9 item on self-harm when they did not meet the 10 or more threshold depression score overall. In other words, these patients would have been missed without this criterion.
In the participating Cincinnati pediatric clinics, about 12%-13% of adolescents met the criteria for depression, which Dr. Krantz said is consistent with reports in the literature. He said the range is about 6%-24%.
Although outcomes were not tracked, there is evidence that early intervention for depression yields better outcomes than delayed intervention, according to Dr. Krantz. Based on approximately 600 positive screens for depression per year at his pediatric clinics, he estimated that his data predict at least 25% more patients will receive timely follow-up.
Seeking Solutions to a Growing Problem
There are several studies documenting the growing problem of adolescent depression and suicide and, for this reason, the topic is attracting a lot of attention, according to Corinna Rea, MD, MPH, a pediatrician working in the primary care center at Boston Children’s Hospital in Massachusetts.
Dr. Rea was not involved with the study, but when asked to comment, she said: “The results of this study were encouraging because we know that getting patients to care quickly is probably important.” She also agreed that referring patients with depression for care might not be enough, noting that a lot of patients do not follow up on recommendations to pursue a consultation or treatment.
“I am now involved in a project with the American Academy of Pediatrics to address this issue,” Dr. Rae said. She thinks that more work in this area is needed and agreed with Dr. Krantz that pediatricians should verify that children with depression are getting help even when other specialists are providing the treatment.
Dr. Krantz and Dr. Rae report no potential conflicts of interest.
FROM PAS 2024
Reactive Granulomatous Dermatitis: Variability of the Predominant Inflammatory Cell Type
To the Editor:
The term palisaded neutrophilic and granulomatous dermatitis (PNGD) has been proposed to encompass various conditions, including Winkelmann granuloma and superficial ulcerating rheumatoid necrobiosis. More recently, PNGD has been classified along with interstitial granulomatous dermatitis and interstitial granulomatous drug reaction under a unifying rubric of reactive granulomatous dermatitis (RGD).1-4 The diagnosis of RGD can be challenging because of a range of clinical and histopathologic features as well as variable nomenclature.1-3,5
Palisaded neutrophilic and granulomatous dermatitis classically manifests with papules and small plaques on the extensor extremities, with histopathology showing characteristic necrobiosis with both neutrophils and histiocytes.1,2,6 We report 6 cases of RGD, including an index case in which a predominance of neutrophils in the infiltrate impeded the diagnosis.
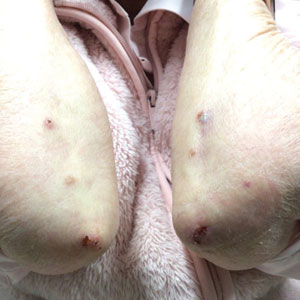
An 85-year-old woman (the index patient) presented with a several-week history of asymmetric crusted papules on the right upper extremity—3 lesions on the elbow and forearm and 1 lesion on a finger. She was an avid gardener with severe rheumatoid arthritis treated with Janus kinase (JAK) inhibitor therapy. An initial biopsy of the elbow revealed a dense infiltrate of neutrophils and sparse eosinophils within the dermis. Special stains for bacterial, fungal, and acid-fast organisms were negative.
Because infection with sporotrichoid spread remained high in the differential diagnosis, the JAK inhibitor was discontinued and an antifungal agent was initiated. Given the persistence of the lesions, a subsequent biopsy of the right finger revealed scarce neutrophils and predominant histiocytes with rare foci of degenerated collagen. Sporotrichosis remained the leading diagnosis for these unilateral lesions. The patient subsequently developed additional crusted papules on the left arm (Figure 1). A biopsy of a left elbow lesion revealed palisades of histiocytes around degenerated collagen and collections of neutrophils compatible with RGD (Figures 2 and 3). Incidentally, the patient also presented with bilateral lower extremity palpable purpura, with a biopsy showing leukocytoclastic vasculitis. Antifungal therapy was discontinued and JAK inhibitor therapy resumed, with partial resolution of both the arm and right finger lesions and complete resolution of the lower extremity palpable purpura over several months.
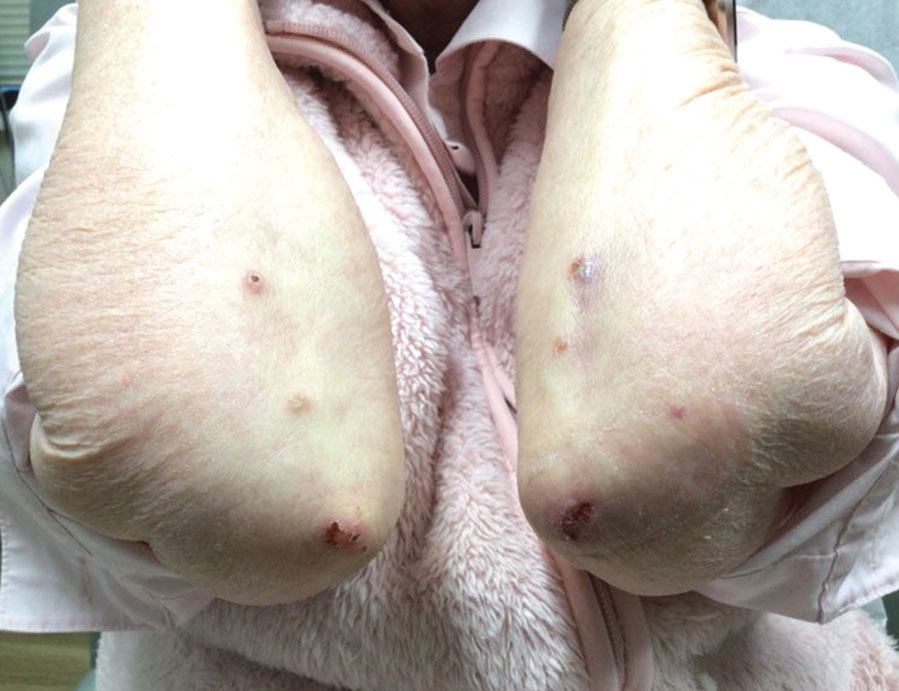
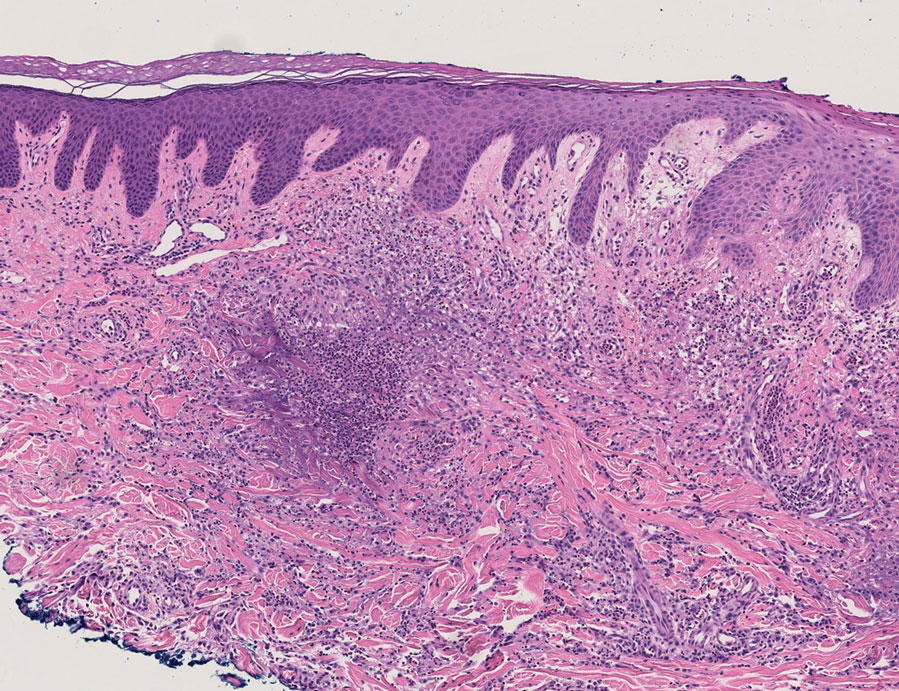
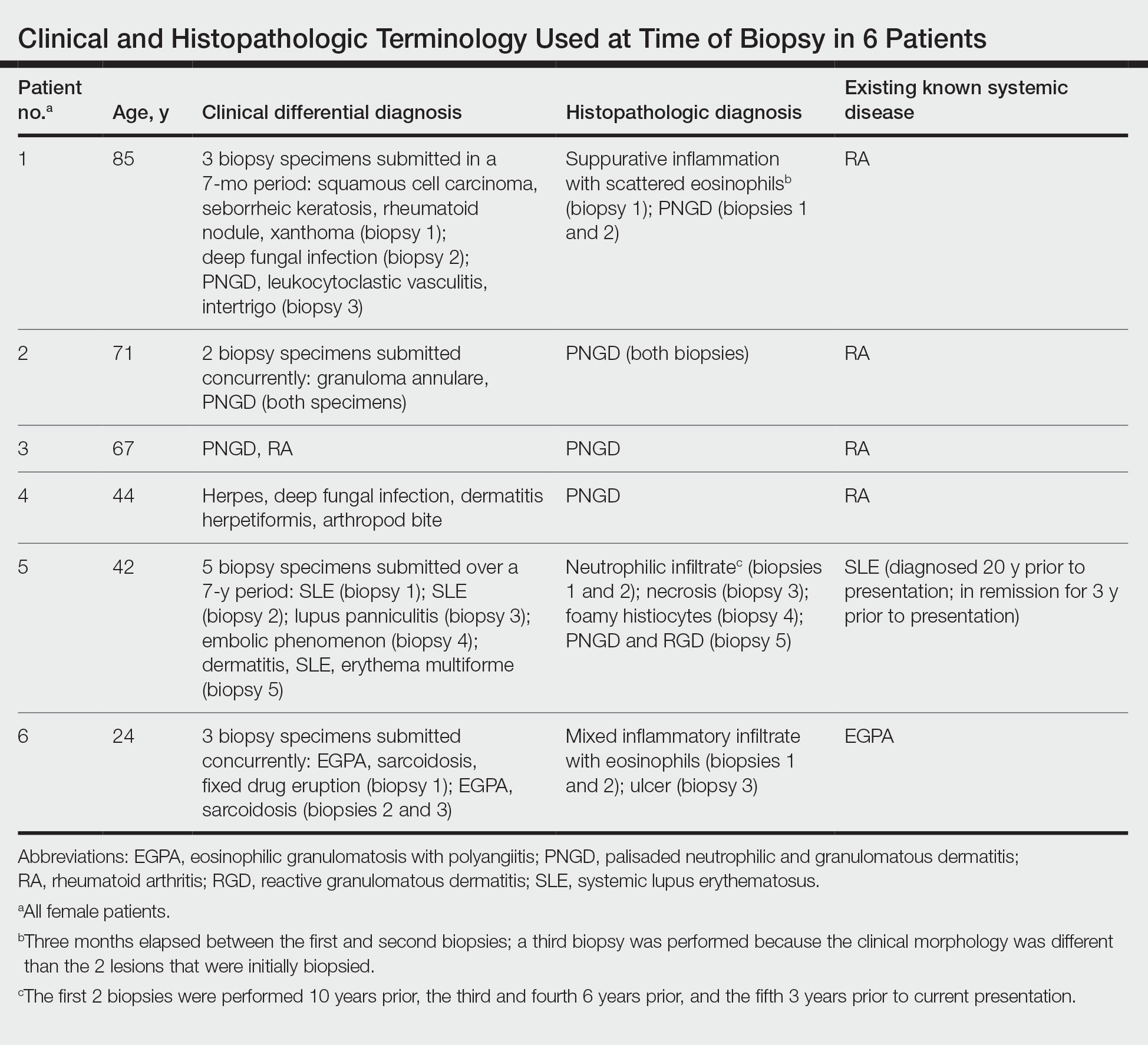
The dense neutrophilic infiltrate and asymmetric presentation seen in our index patient’s initial biopsy hindered categorization of the cutaneous findings as RGD in association with her rheumatoid arthritis rather than as an infectious process. To ascertain whether diagnosis also was difficult in other cases of RGD, we conducted a search of the Yale Dermatopathology database for the diagnosis palisaded neutrophilic and granulomatous dermatitis, a term consistently used at our institution over the past decade. This study was approved by the institutional review board of Yale University (New Haven, Connecticut), and informed consent was waived. The search covered a 10-year period; 13 patients were found. Eight patients were eliminated because further clinical information or follow-up could not be obtained, leaving 5 additional cases (Table). The 8 eliminated cases were consultations submitted to the laboratory by outside pathologists from other institutions.
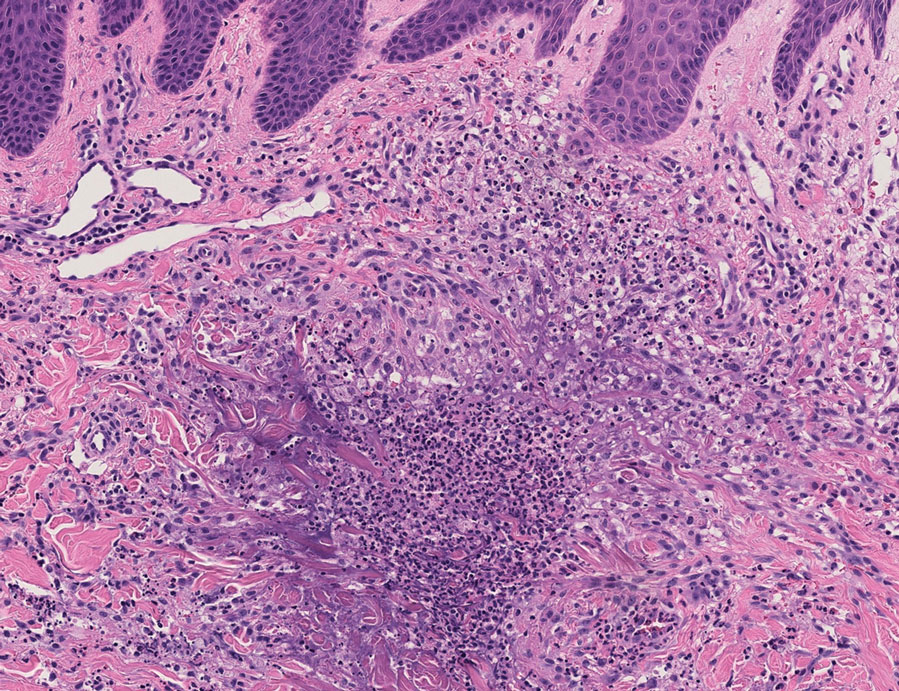
In one case (patient 5), the diagnosis of RGD was delayed for 7 years from first documentation of an RGD-compatible neutrophil-predominant infiltrate (Table). In 3 other cases, PNGD was in the clinical differential diagnosis. In patient 6 with known eosinophilic granulomatosis with polyangiitis, biopsy findings included a mixed inflammatory infiltrate with eosinophils, and the clinical and histopathologic findings were deemed compatible with RGD by group consensus at Grand Rounds.
In practice, a consistent unifying nomenclature has not been achieved for RGD and the diseases it encompasses—PNGD, interstitial granulomatous dermatitis, and interstitial granulomatous drug reaction. In this small series, a diagnosis of PNGD was given in the dermatopathology report only when biopsy specimens were characterized by histiocytes, neutrophils, and necrobiosis. Histopathology reports for neutrophil-predominant, histiocyte-predominant, and eosinophil-predominant cases did not mention PNGD or RGD, though potential association with systemic disease generally was noted.
Given the variability in the predominant inflammatory cell type in these patients, adding a qualifier to the histopathologic diagnosis—“RGD, eosinophil rich,” “RGD, histiocyte rich,” or “RGD, neutrophil rich”1—would underscore the range of inflammatory cells in this entity. Employing this terminology rather than stating a solely descriptive diagnosis such as neutrophilic infiltrate, which may bias clinicians toward an infectious process, would aid in the association of a given rash with systemic disease and may prevent unnecessary tissue sampling. Indeed, 3 patients in this small series underwent more than 2 biopsies; multiple procedures might have been avoided had there been better communication about the spectrum of inflammatory cells compatible with RGD.
The inflammatory infiltrate in biopsy specimens of RGD can be solely neutrophil or histiocyte predominant or even have prominent eosinophils depending on the stage of disease. Awareness of variability in the predominant inflammatory cell in RGD may facilitate an accurate diagnosis as well as an association with any underlying autoimmune process, thereby allowing better management and treatment.1
- Rosenbach M, English JC. Reactive granulomatous dermatitis: a review of palisaded neutrophilic and granulomatous dermatitis, interstitial granulomatous dermatitis, interstitial granulomatous drug reaction, and a proposed reclassification. Dermatol Clin. 2015;33:373-387. doi:10.1016/j.det.2015.03.005
- Wanat KA, Caplan A, Messenger E, et al. Reactive granulomatous dermatitis: a useful and encompassing term. JAAD Intl. 2022;7:126-128. doi:10.1016/j.jdin.2022.03.004
- Chu P, Connolly MK, LeBoit PE. The histopathologic spectrum of palisaded neutrophilic and granulomatous dermatitis in patients with collagen vascular disease. Arch Dermatol. 1994;130:1278-1283. doi:10.1001/archderm.1994.01690100062010
- Dykman CJ, Galens GJ, Good AE. Linear subcutaneous bands in rheumatoid arthritis: an unusual form of rheumatoid granuloma. Ann Intern Med. 1965;63:134-140. doi:10.7326/0003-4819-63-1-134
- Rodríguez-Garijo N, Bielsa I, Mascaró JM Jr, et al. Reactive granulomatous dermatitis as a histological pattern including manifestations of interstitial granulomatous dermatitis and palisaded neutrophilic and granulomtous dermatitis: a study of 52 patients. J Eur Acad Dermatol Venereol. 2021;35:988-994. doi:10.1111/jdv.17010
- Kalen JE, Shokeen D, Ramos-Caro F, et al. Palisaded neutrophilic granulomatous dermatitis: spectrum of histologic findings in a single patient. JAAD Case Rep. 2017;3:425. doi:10.1016/j.jdcr.2017.06.010
To the Editor:
The term palisaded neutrophilic and granulomatous dermatitis (PNGD) has been proposed to encompass various conditions, including Winkelmann granuloma and superficial ulcerating rheumatoid necrobiosis. More recently, PNGD has been classified along with interstitial granulomatous dermatitis and interstitial granulomatous drug reaction under a unifying rubric of reactive granulomatous dermatitis (RGD).1-4 The diagnosis of RGD can be challenging because of a range of clinical and histopathologic features as well as variable nomenclature.1-3,5
Palisaded neutrophilic and granulomatous dermatitis classically manifests with papules and small plaques on the extensor extremities, with histopathology showing characteristic necrobiosis with both neutrophils and histiocytes.1,2,6 We report 6 cases of RGD, including an index case in which a predominance of neutrophils in the infiltrate impeded the diagnosis.
An 85-year-old woman (the index patient) presented with a several-week history of asymmetric crusted papules on the right upper extremity—3 lesions on the elbow and forearm and 1 lesion on a finger. She was an avid gardener with severe rheumatoid arthritis treated with Janus kinase (JAK) inhibitor therapy. An initial biopsy of the elbow revealed a dense infiltrate of neutrophils and sparse eosinophils within the dermis. Special stains for bacterial, fungal, and acid-fast organisms were negative.
Because infection with sporotrichoid spread remained high in the differential diagnosis, the JAK inhibitor was discontinued and an antifungal agent was initiated. Given the persistence of the lesions, a subsequent biopsy of the right finger revealed scarce neutrophils and predominant histiocytes with rare foci of degenerated collagen. Sporotrichosis remained the leading diagnosis for these unilateral lesions. The patient subsequently developed additional crusted papules on the left arm (Figure 1). A biopsy of a left elbow lesion revealed palisades of histiocytes around degenerated collagen and collections of neutrophils compatible with RGD (Figures 2 and 3). Incidentally, the patient also presented with bilateral lower extremity palpable purpura, with a biopsy showing leukocytoclastic vasculitis. Antifungal therapy was discontinued and JAK inhibitor therapy resumed, with partial resolution of both the arm and right finger lesions and complete resolution of the lower extremity palpable purpura over several months.


The dense neutrophilic infiltrate and asymmetric presentation seen in our index patient’s initial biopsy hindered categorization of the cutaneous findings as RGD in association with her rheumatoid arthritis rather than as an infectious process. To ascertain whether diagnosis also was difficult in other cases of RGD, we conducted a search of the Yale Dermatopathology database for the diagnosis palisaded neutrophilic and granulomatous dermatitis, a term consistently used at our institution over the past decade. This study was approved by the institutional review board of Yale University (New Haven, Connecticut), and informed consent was waived. The search covered a 10-year period; 13 patients were found. Eight patients were eliminated because further clinical information or follow-up could not be obtained, leaving 5 additional cases (Table). The 8 eliminated cases were consultations submitted to the laboratory by outside pathologists from other institutions.

In one case (patient 5), the diagnosis of RGD was delayed for 7 years from first documentation of an RGD-compatible neutrophil-predominant infiltrate (Table). In 3 other cases, PNGD was in the clinical differential diagnosis. In patient 6 with known eosinophilic granulomatosis with polyangiitis, biopsy findings included a mixed inflammatory infiltrate with eosinophils, and the clinical and histopathologic findings were deemed compatible with RGD by group consensus at Grand Rounds.
In practice, a consistent unifying nomenclature has not been achieved for RGD and the diseases it encompasses—PNGD, interstitial granulomatous dermatitis, and interstitial granulomatous drug reaction. In this small series, a diagnosis of PNGD was given in the dermatopathology report only when biopsy specimens were characterized by histiocytes, neutrophils, and necrobiosis. Histopathology reports for neutrophil-predominant, histiocyte-predominant, and eosinophil-predominant cases did not mention PNGD or RGD, though potential association with systemic disease generally was noted.
Given the variability in the predominant inflammatory cell type in these patients, adding a qualifier to the histopathologic diagnosis—“RGD, eosinophil rich,” “RGD, histiocyte rich,” or “RGD, neutrophil rich”1—would underscore the range of inflammatory cells in this entity. Employing this terminology rather than stating a solely descriptive diagnosis such as neutrophilic infiltrate, which may bias clinicians toward an infectious process, would aid in the association of a given rash with systemic disease and may prevent unnecessary tissue sampling. Indeed, 3 patients in this small series underwent more than 2 biopsies; multiple procedures might have been avoided had there been better communication about the spectrum of inflammatory cells compatible with RGD.

The inflammatory infiltrate in biopsy specimens of RGD can be solely neutrophil or histiocyte predominant or even have prominent eosinophils depending on the stage of disease. Awareness of variability in the predominant inflammatory cell in RGD may facilitate an accurate diagnosis as well as an association with any underlying autoimmune process, thereby allowing better management and treatment.1
To the Editor:
The term palisaded neutrophilic and granulomatous dermatitis (PNGD) has been proposed to encompass various conditions, including Winkelmann granuloma and superficial ulcerating rheumatoid necrobiosis. More recently, PNGD has been classified along with interstitial granulomatous dermatitis and interstitial granulomatous drug reaction under a unifying rubric of reactive granulomatous dermatitis (RGD).1-4 The diagnosis of RGD can be challenging because of a range of clinical and histopathologic features as well as variable nomenclature.1-3,5
Palisaded neutrophilic and granulomatous dermatitis classically manifests with papules and small plaques on the extensor extremities, with histopathology showing characteristic necrobiosis with both neutrophils and histiocytes.1,2,6 We report 6 cases of RGD, including an index case in which a predominance of neutrophils in the infiltrate impeded the diagnosis.
An 85-year-old woman (the index patient) presented with a several-week history of asymmetric crusted papules on the right upper extremity—3 lesions on the elbow and forearm and 1 lesion on a finger. She was an avid gardener with severe rheumatoid arthritis treated with Janus kinase (JAK) inhibitor therapy. An initial biopsy of the elbow revealed a dense infiltrate of neutrophils and sparse eosinophils within the dermis. Special stains for bacterial, fungal, and acid-fast organisms were negative.
Because infection with sporotrichoid spread remained high in the differential diagnosis, the JAK inhibitor was discontinued and an antifungal agent was initiated. Given the persistence of the lesions, a subsequent biopsy of the right finger revealed scarce neutrophils and predominant histiocytes with rare foci of degenerated collagen. Sporotrichosis remained the leading diagnosis for these unilateral lesions. The patient subsequently developed additional crusted papules on the left arm (Figure 1). A biopsy of a left elbow lesion revealed palisades of histiocytes around degenerated collagen and collections of neutrophils compatible with RGD (Figures 2 and 3). Incidentally, the patient also presented with bilateral lower extremity palpable purpura, with a biopsy showing leukocytoclastic vasculitis. Antifungal therapy was discontinued and JAK inhibitor therapy resumed, with partial resolution of both the arm and right finger lesions and complete resolution of the lower extremity palpable purpura over several months.


The dense neutrophilic infiltrate and asymmetric presentation seen in our index patient’s initial biopsy hindered categorization of the cutaneous findings as RGD in association with her rheumatoid arthritis rather than as an infectious process. To ascertain whether diagnosis also was difficult in other cases of RGD, we conducted a search of the Yale Dermatopathology database for the diagnosis palisaded neutrophilic and granulomatous dermatitis, a term consistently used at our institution over the past decade. This study was approved by the institutional review board of Yale University (New Haven, Connecticut), and informed consent was waived. The search covered a 10-year period; 13 patients were found. Eight patients were eliminated because further clinical information or follow-up could not be obtained, leaving 5 additional cases (Table). The 8 eliminated cases were consultations submitted to the laboratory by outside pathologists from other institutions.

In one case (patient 5), the diagnosis of RGD was delayed for 7 years from first documentation of an RGD-compatible neutrophil-predominant infiltrate (Table). In 3 other cases, PNGD was in the clinical differential diagnosis. In patient 6 with known eosinophilic granulomatosis with polyangiitis, biopsy findings included a mixed inflammatory infiltrate with eosinophils, and the clinical and histopathologic findings were deemed compatible with RGD by group consensus at Grand Rounds.
In practice, a consistent unifying nomenclature has not been achieved for RGD and the diseases it encompasses—PNGD, interstitial granulomatous dermatitis, and interstitial granulomatous drug reaction. In this small series, a diagnosis of PNGD was given in the dermatopathology report only when biopsy specimens were characterized by histiocytes, neutrophils, and necrobiosis. Histopathology reports for neutrophil-predominant, histiocyte-predominant, and eosinophil-predominant cases did not mention PNGD or RGD, though potential association with systemic disease generally was noted.
Given the variability in the predominant inflammatory cell type in these patients, adding a qualifier to the histopathologic diagnosis—“RGD, eosinophil rich,” “RGD, histiocyte rich,” or “RGD, neutrophil rich”1—would underscore the range of inflammatory cells in this entity. Employing this terminology rather than stating a solely descriptive diagnosis such as neutrophilic infiltrate, which may bias clinicians toward an infectious process, would aid in the association of a given rash with systemic disease and may prevent unnecessary tissue sampling. Indeed, 3 patients in this small series underwent more than 2 biopsies; multiple procedures might have been avoided had there been better communication about the spectrum of inflammatory cells compatible with RGD.

The inflammatory infiltrate in biopsy specimens of RGD can be solely neutrophil or histiocyte predominant or even have prominent eosinophils depending on the stage of disease. Awareness of variability in the predominant inflammatory cell in RGD may facilitate an accurate diagnosis as well as an association with any underlying autoimmune process, thereby allowing better management and treatment.1
- Rosenbach M, English JC. Reactive granulomatous dermatitis: a review of palisaded neutrophilic and granulomatous dermatitis, interstitial granulomatous dermatitis, interstitial granulomatous drug reaction, and a proposed reclassification. Dermatol Clin. 2015;33:373-387. doi:10.1016/j.det.2015.03.005
- Wanat KA, Caplan A, Messenger E, et al. Reactive granulomatous dermatitis: a useful and encompassing term. JAAD Intl. 2022;7:126-128. doi:10.1016/j.jdin.2022.03.004
- Chu P, Connolly MK, LeBoit PE. The histopathologic spectrum of palisaded neutrophilic and granulomatous dermatitis in patients with collagen vascular disease. Arch Dermatol. 1994;130:1278-1283. doi:10.1001/archderm.1994.01690100062010
- Dykman CJ, Galens GJ, Good AE. Linear subcutaneous bands in rheumatoid arthritis: an unusual form of rheumatoid granuloma. Ann Intern Med. 1965;63:134-140. doi:10.7326/0003-4819-63-1-134
- Rodríguez-Garijo N, Bielsa I, Mascaró JM Jr, et al. Reactive granulomatous dermatitis as a histological pattern including manifestations of interstitial granulomatous dermatitis and palisaded neutrophilic and granulomtous dermatitis: a study of 52 patients. J Eur Acad Dermatol Venereol. 2021;35:988-994. doi:10.1111/jdv.17010
- Kalen JE, Shokeen D, Ramos-Caro F, et al. Palisaded neutrophilic granulomatous dermatitis: spectrum of histologic findings in a single patient. JAAD Case Rep. 2017;3:425. doi:10.1016/j.jdcr.2017.06.010
- Rosenbach M, English JC. Reactive granulomatous dermatitis: a review of palisaded neutrophilic and granulomatous dermatitis, interstitial granulomatous dermatitis, interstitial granulomatous drug reaction, and a proposed reclassification. Dermatol Clin. 2015;33:373-387. doi:10.1016/j.det.2015.03.005
- Wanat KA, Caplan A, Messenger E, et al. Reactive granulomatous dermatitis: a useful and encompassing term. JAAD Intl. 2022;7:126-128. doi:10.1016/j.jdin.2022.03.004
- Chu P, Connolly MK, LeBoit PE. The histopathologic spectrum of palisaded neutrophilic and granulomatous dermatitis in patients with collagen vascular disease. Arch Dermatol. 1994;130:1278-1283. doi:10.1001/archderm.1994.01690100062010
- Dykman CJ, Galens GJ, Good AE. Linear subcutaneous bands in rheumatoid arthritis: an unusual form of rheumatoid granuloma. Ann Intern Med. 1965;63:134-140. doi:10.7326/0003-4819-63-1-134
- Rodríguez-Garijo N, Bielsa I, Mascaró JM Jr, et al. Reactive granulomatous dermatitis as a histological pattern including manifestations of interstitial granulomatous dermatitis and palisaded neutrophilic and granulomtous dermatitis: a study of 52 patients. J Eur Acad Dermatol Venereol. 2021;35:988-994. doi:10.1111/jdv.17010
- Kalen JE, Shokeen D, Ramos-Caro F, et al. Palisaded neutrophilic granulomatous dermatitis: spectrum of histologic findings in a single patient. JAAD Case Rep. 2017;3:425. doi:10.1016/j.jdcr.2017.06.010
Practice Points
- The term reactive granulomatous dermatitis (RGD) provides a unifying rubric for palisaded neutrophilic and granulomatous dermatitis, interstitial granulomatous dermatitis, and interstitial granulomatous drug reaction.
- Reactive granulomatous dermatitis can have a variable infiltrate that includes neutrophils, histiocytes, and/or eosinophils.
- Awareness of the variability in inflammatory cell type is important for the diagnosis of RGD.