User login
The Integration of Extended Reality in Arthroplasty: Reviewing Technological Progress and Clinical Benefits
The Integration of Extended Reality in Arthroplasty: Reviewing Technological Progress and Clinical Benefits
The introduction of extended reality (XR) to the operating room (OR) has proved promising for enhancing surgical precision and improving patient outcomes. In the field of orthopedic surgery, precise alignment of implants is integral to maintaining functional range of motion and preventing impingement of adjacent neurovascular structures. XR systems have shown promise in arthroplasty including by improving precision and streamlining surgery by allowing surgeons to create 3D preoperative plans that are accessible intraoperatively. This article explores the current applications of XR in arthroplasty, highlights recent advancements and benefits, and describes limitations in comparison to traditional techniques.
Methods
A literature search identified studies involving the use of XR in arthroplasty and current US Food and Drug Administration (FDA)-approved XR systems. Multiple electronic databases were used, including PubMed, Google Scholar, and IEEE Xplore. Search terms included: extended reality, augmented reality, virtual reality, arthroplasty, joint replacement, total knee arthroplasty, total shoulder arthroplasty, and total hip arthroplasty. The study design, intervention details, outcomes, and comparisons with traditional surgical techniques were thematically analyzed, with identification of common ideas associated with XR use in arthroplasty. This narrative report highlights the integration of XR in arthroplasty.
Extended Reality Fundamentals
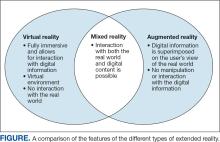
XR encompasses augmented reality (AR), virtual reality (VR), and mixed reality (MR). AR involves superimposing digitally rendered information and images onto the surgeon’s view of the real world, typically through the use of a headset and smart glasses.1 AR allows the surgeon to move and interact freely within the OR, removing the need for additional screens or devices to display patient information or imaging. VR is a fully immersive simulation using a headset that obstructs the view of the real world but allows the user to move freely within this virtual setting, often with audio or other sensory stimuli. MR combines AR and VR to create a digital model that allows for real-world interaction, with the advantage of adapting information and models in real time.2 Whereas in AR the surgeon can view the data projected from the headset, MR provides the ability to interact with and manipulate the digital content (Figure). Both AR and MR have been adapted for use in the OR, while VR has been adapted for use in surgical planning and training.
Extended Reality Use in Orthopedics
The HipNav system was introduced in 1995 to create preoperative plans that assist surgeons in accurately implanting the acetabular cup during total hip arthroplasty (THA).3 Although not commercially successful, this system spurred surgeons to experiment with XR to improve the accuracy and alignment of orthopedic implants. Systems capable of displaying the desired intraoperative implant placement have flourished, with applications in fracture reduction, arthroplasty, solid tumor resection, and hardware placement.4-7 Accurate alignment has been linked to improvements in patient outcomes.8-10 XR has great potential within the field of arthroplasty, with multiple new systems approved by the FDA and currently available in the US (Table).
Hip Arthroplasty
Orientation of the acetabular cup is a technically challenging part of THA. Accuracy in the anteversion and inclination angles of the acetabular cup is required to maintain implant stability, preserve functional range of motion (ROM), and prevent precocious wear.11,12 Despite preoperative planning, surgeons often overestimate the inclination angle and underestimate anteversion.13 Improper implantation of the acetabular cup can lead to joint instability caused by aseptic loosening, increasing the risk of dislocation and the need for revision surgery.14,15 Dislocations typically present to the emergency department, but primary care practitioners may encounter patients with pain or diminished sensation due to impingement or instability.16
The introduction of XR into the OR has provided the opportunity for real-time navigation and adjustment of the acetabular cup to maximize anteversion and inclination angles. Currently, 2 FDA-approved systems are available for THA: the Zimmer and Surgical Planning Associates HipInsight system, and the Insight Augmented Reality Visualization and Information System (ARVIS). The HipInsight system consists of a hologram projection using the Microsoft HoloLens2 device and optimizes preoperative planning, producing accuracy of anteversion and inclination angles within 3°.17 ARVIS employs existing surgical helmets and 2 mounted tracking cameras to provide navigation intraoperatively. ARVIS has also been approved for use in total knee arthroplasty (TKA) and unicompartmental knee arthroplasty.18
HipInsight has shown utility in increasing the accuracy of acetabular cup placement along with the use of biplanar radiographic scans.19 However, there are no studies validating the efficacy of ARVIS and HipInsight and assessing long-term disease-oriented or patient-oriented outcomes.
Knee Arthroplasty
In the setting of TKA, XR is most effective in ensuring accurate resection of the tibial and femoral components. Achieving the planned femoral coronal, axial, and sagittal angles allows the prosthesis to be on the femoral axis of rotation, improving functional outcomes. XR systems for TKA have been shown to increase the accuracy of distal femoral resection with a limited increase in surgery duration.20,21 For TKA in particular, patients are often less satisfied with the result than surgeons expect.22 Accurate alignment can improve patient satisfaction and reduce return-to-clinic rates for postoperative pain management, a factor that primary care practitioners should consider when recommending a patient for TKA.23
Along with ARVIS, 3 additional XR systems are FDA-approved for use in TKA. The Pixee Medical Knee+ system uses smart glasses and trackers to aid in the positioning of instruments for improved accuracy while allowing real-time navigation.24 The Medacta NextAR Knee’s single-use tracking system allows for intraoperative navigation with the use of AR glasses.25 The Polaris STELLAR Knee uses MR and avoids the need for preoperative imaging by capturing real-time anatomic data.26
The Pixee Medical Knee+ system was commercially available in Europe for several years prior to FDA approval, so more research exists on its efficacy. One study found that the Pixee Medical Knee+ system initially demonstrated an inferior clinical outcome, attributed to the learning curve associated with using the system.27 However, more recent studies have shown its utility in improving alignment, regardless of implant specifications.28,29 The Medacta NextAR Knee system has been shown to improve accuracy of tibial rotation and soft tissue balance and even increase OR efficiency.30,31 The Polaris STELLAR Knee system received FDA approval in 2023; no published research exists on its accuracy and outcomes.26
Shoulder Arthroplasty
Minimally invasive techniques are favored in total shoulder arthroplasty (TSA) due to the vitality of maintaining the surrounding soft tissue to maximize preservation of motility and strength.32 However, this complicates the procedure by decreasing the ability to effectively access and visualize key structures of the shoulder. Accordingly, issues with implant positioning and alignment are more common with TSA than other joint arthroplasties, making XR particularly promising.33 Some studies report that up to 67% of patients experience glenohumeral instability, which can clinically present as weakness, decreased range of motion, and persistent shoulder pain.34,35 The use of preoperative computed tomography to improve understanding of glenoid anatomy and glenohumeral subluxation is becoming increasingly common, and it can be combined with XR to improve accuracy.36,37
Two FDA-approved systems are available. The Stryker Blueprint MR system is used for intraoperative guidance and integration for patient imaging used for preoperative planning. The Medacta NextAR Shoulder system is a parallel of the company’s TKA system. The Stryker Blueprint MR system combines the Microsoft HoloLens 2 headset to display preoperative plans with a secondary display for coordination with the rest of the surgical team.38 Similar to the Medacta NextAR Knee, the Medacta NextAR Shoulder system uses the same single-use tracking system and AR glasses for intraoperative guidance.39
Data on the long-term outcomes of using these systems are still limited, but the Stryker Blueprint MR system has not been shown to accurately predict postoperative ROM.40 Cadaveric studies have demonstrated that the Medacta NextAR Shoulder system can provide accurate inclination, retroversion, entry point, depth, and rotation values based on the preoperative planned values.41,42 However, this accuracy has yet to be confirmed in vivo, and the impact of using XR in TSA on long-term outcomes is still unknown.
Challenges and Limitations
Though XR has proven to be promising in arthroplasty, several limitations regarding widespread implementation exist. In particular, there is a steep learning curve associated with the use of XR systems, which can cause increased operative time and even initial inferior outcomes, as demonstrated with the Pixee Medical Knee+ system. The need for extensive practice and training prior to use could delay widespread adoption and may cause discrepancies in surgical outcomes. Unfamiliarity with the system and technological difficulties that may require troubleshooting can also increase operative time, particularly for surgeons new to using the XR system. Though intraoperative navigation is expected to improve accuracy of implant alignment, its added complexity may also result in longer surgeries.
In addition to the steep learning curve and increased operative time, there is a high upfront cost associated with XR systems. Exact costs of XR systems are not typically disclosed, but available estimates suggest an average sales price of about $1000 per case. Given the proprietary nature of these technologies, publicly available cost data are limited, making it challenging to fully assess the financial burden on health care institutions. Though some systems, such as ARVIS, can be integrated with existing surgical helmets, many require the purchase of AR glasses and secondary displays. This can cause further variation in the total expense for each system. In low-resource settings, this represents a significant challenge to widespread implementation. To justify this cost, additional research on long-term patient outcomes is needed to ensure the benefits of XR systems outweigh the cost.
Although early studies on XR systems in arthroplasty have shown improvements in precision and short-term outcomes, long-term data regarding effectiveness remains. Even systems such as ARVIS and HipInsight have limited long-term follow-up, making it difficult to assess whether the improved accuracy with these XR systems translates into improved patient outcomes compared with traditional arthroplasty.
CONCLUSIONS
XR technologies have shown significant potential in enhancing precision and patient outcomes. Through the integration of XR in the OR, surgeons can visualize preoperative plans and even make intraoperative changes, with the benefit of improving implant alignment.
There are some disadvantages to its use, however, including high cost and increased operative time. Despite this, the integration of XR into surgical practice can deliver more precise implant alignment and address other challenges faced with conventional techniques. As these technologies evolve and studies on long-term outcomes validate their utility, XR has the potential to transform the field of arthroplasty.
Azuma RT. A survey of augmented reality. Presence-Teleop Virt. 1997;6:355-385. doi:10.1162/pres.1997.6.4.355
Speicher M, Hall BD, Nebeling M. What is Mixed Reality? In: Proceedings of the 2019 CHI Conference on Human Factors in Computing Systems. Association for Computing Machinery; 2019:1-15. doi:10.1145/3290605.3300767
Digioia AM, Jaramaz B, Nikou C, et al. Surgical navigation for total hip replacement with the use of hipnav. Oper Tech Orthop. 2000;10:3-8. doi:10.1016/S1048-6666(00)80036-1
Ogawa H, Hasegawa S, Tsukada S, et al. A pilot study of augmented reality technology applied to the acetabular cup placement during total hip arthroplasty. J Arthroplasty. 2018;33:1833-1837. doi:10.1016/j.arth.2018.01.067
Shen F, Chen B, Guo Q, et al. Augmented reality patient-specific reconstruction plate design for pelvic and acetabular fracture surgery. Int J CARS. 2013;8:169-179. doi:10.1007/s11548-012-0775-5
Cho HS, Park YK, Gupta S, et al. Augmented reality in bone tumour resection: an experimental study. Bone Joint Res. 2017;6:137-143. doi:10.1302/2046-3758.63.bjr-2016-0289.r1
Wu X, Liu R, Yu J, et al. Mixed reality technology launches in orthopedic surgery for comprehensive preoperative management of complicated cervical fractures. Surg Innov. 2018;25:421-422. doi:10.1177/1553350618761758
Dossett HG, Arthur JR, Makovicka JL, et al. A randomized controlled trial of kinematically and mechanically aligned total knee arthroplasties: long-term follow-up. J Arthroplasty. 2023;38:S209-S214. doi:10.1016/j.arth.2023.03.065
Kazarian GS, Haddad FS, Donaldson MJ, et al. Implant malalignment may be a risk factor for poor patient-reported outcomes measures (PROMs) following total knee arthroplasty (TKA). J Arthroplasty. 2022;37:S129-S133. doi:10.1016/j.arth.2022.02.087
Peng Y, Arauz P, An S, et al. Does component alignment affect patient reported outcomes following bicruciate retaining total knee arthroplasty? An in vivo three-dimensional analysis. J Knee Surg. 2020;33:798-803. doi:10.1055/s-0039-1688500
D’Lima DD, Urquhart AG, Buehler KO, et al. The effect of the orientation of the acetabular and femoral components on the range of motion of the hip at different head-neck ratios. J Bone Joint Surg Am. 2000;82:315-321. doi:10.2106/00004623-200003000-00003
Yamaguchi M, Akisue T, Bauer TW, et al. The spatial location of impingement in total hip arthroplasty. J Arthroplasty. 2000;15:305-313. doi:10.1016/s0883-5403(00)90601-6
Grammatopoulos G, Alvand A, Monk AP, et al. Surgeons’ accuracy in achieving their desired acetabular component orientation. J Bone Joint Surg. 2016;98:e72. doi:10.2106/JBJS.15.01080
Kennedy JG, Rogers WB, Soffe KE, et al. Effect of acetabular component orientation on recurrent dislocation, pelvic osteolysis, polyethylene wear, and component migration. J Arthroplasty. 1998;13:530-534. doi:10.1016/S0883-5403(98)90052-3
Del Schutte H, Lipman AJ, Bannar SM, et al. Effects of acetabular abduction on cup wear rates in total hip arthroplasty. J Arthroplasty. 1998;13:621-626. doi:10.1016/S0883-5403(98)80003-X
Aresti N, Kassam J, Bartlett D, et al. Primary care management of postoperative shoulder, hip, and knee arthroplasty. BMJ. 2017;359:j4431. doi:10.1136/bmj.j4431
HipInsightTM System. Zimmer Biomet. Accessed September 3, 2025. https://www.zimmerbiomet.com/en/products-and-solutions/zb-edge/mixed-reality-portfolio/hipinsight-system.html
ARVIS. Insight Medical Systems. Accessed September 3, 2025. https://www.insightmedsys.com/arvis
Sun DC, Murphy WS, Amundson AJ, et al. Validation of a novel method of measuring cup orientation using biplanar simultaneous radiographic images. J Arthroplasty. 2023;38:S252-S256. doi:10.1016/j.arth.2023.04.011
Tsukada S, Ogawa H, Nishino M, et al. Augmented reality-assisted femoral bone resection in total knee arthroplasty. JBJS Open Access. 2021;6:e21.00001. doi:10.2106/JBJS.OA.21.00001
Castellarin G, Bori E, Barbieux E, et al. Is total knee arthroplasty surgical performance enhanced using augmented reality? A single-center study on 76 consecutive patients. J Arthroplasty. 2024;39:332-335. doi:10.1016/j.arth.2023.08.013
Choi YJ, Ra HJ. Patient satisfaction after total knee arthroplasty. Knee Surg Relat Res. 2016;28:1. doi:10.5792/ksrr.2016.28.1.1
Hazratwala K, Gouk C, Wilkinson MPR, et al. Navigated functional alignment total knee arthroplasty achieves reliable, reproducible and accurate results with high patient satisfaction. Knee Surg Sports Traumatol Arthrosc. 2023;31:3861-3870. doi:10.1007/s00167-023-07327-w
Knee+. Pixee Medical. Accessed September 3, 2025. https://www.pixee-medical.com/en/products/knee-nexsight/
KNEE | NEXTAR. Nextar. Accessed September 3, 2025. https://nextar.medacta.com/knee
POLARIS AR receives clearance from the U.S. Food and Drug Administration for STELLAR Knee. News release. PRNewswire. November 3, 2023. Accessed September 3, 2025. https://www.prnewswire.com/news-releases/polarisar-receives-clearance-from-the-us-food-and-drug-administration-for-stellar-knee-301976747.html
van Overschelde P, Vansintjan P, Byn P, Lapierre C, van Lysebettens W. Does augmented reality improve clinical outcome in TKA? A prospective observational report. In: The 20th Annual Meeting of the International Society for Computer Assisted Orthopaedic Surgery. 2022:170-174.
Sakellariou E, Alevrogiannis P, Alevrogianni F, et al. Single-center experience with Knee+TM augmented reality navigation system in primary total knee arthroplasty. World J Orthop. 2024;15:247-256. doi:10.5312/wjo.v15.i3.247
León-Muñoz VJ, Moya-Angeler J, López-López M, et al. Integration of square fiducial markers in patient-specific instrumentation and their applicability in knee surgery. J Pers Med. 2023;13:727. doi:10.3390/jpm13050727
Fucentese SF, Koch PP. A novel augmented reality-based surgical guidance system for total knee arthroplasty. Arch Orthop Trauma Surg. 2021;141:2227-2233. doi:10.1007/s00402-021-04204-4
Sabatini L, Ascani D, Vezza D, et al. Novel surgical technique for total knee arthroplasty integrating kinematic alignment and real-time elongation of the ligaments using the NextAR system. J Pers Med. 2024;14:794. doi:10.3390/jpm14080794
Daher M, Ghanimeh J, Otayek J, et al. Augmented reality and shoulder replacement: a state-of-the-art review article. JSES Rev Rep Tech. 2023;3:274-278. doi:10.1016/j.xrrt.2023.01.008
Atmani H, Merienne F, Fofi D, et al. Computer aided surgery system for shoulder prosthesis placement. Comput Aided Surg. 2007;12:60-70. doi:10.3109/10929080701210832
Eichinger JK, Galvin JW. Management of complications after total shoulder arthroplasty. Curr Rev Musculoskelet Med. 2015;8:83-91. doi:10.1007/s12178-014-9251-x
Bonnevialle N, Melis B, Neyton L, et al. Aseptic glenoid loosening or failure in total shoulder arthroplasty: revision with glenoid reimplantation. J Shoulder Elbow Surg. 2013;22:745-751. doi:10.1016/j.jse.2012.08.009
Erickson BJ, Chalmers PN, Denard P, et al. Does commercially available shoulder arthroplasty preoperative planning software agree with surgeon measurements of version, inclination, and subluxation? J Shoulder Elbow Surg. 2021;30:413-420. doi:10.1016/j.jse.2020.05.027
Werner BS, Hudek R, Burkhart KJ, et al. The influence of three-dimensional planning on decision-making in total shoulder arthroplasty. J Shoulder Elbow Surg. 2017;26:1477-1483. doi:10.1016/j.jse.2017.01.006
Blueprint. Stryker. Updated August 2025. Accessed September 3, 2025. https://www.stryker.com/us/en/trauma-and-extremities/products/blueprint.html
NextAR Shoulder. Medacta. Accessed September 3, 2025. https://nextar.medacta.com/shoulder
Baumgarten KM. Accuracy of Blueprint software in predicting range of motion 1 year after reverse total shoulder arthroplasty. J Shoulder Elbow Surg. 2023;32:1088-1094. doi:10.1016/j.jse.2022.12.009
Rojas JT, Jost B, Zipeto C, et al. Glenoid component placement in reverse shoulder arthroplasty assisted with augmented reality through a head-mounted display leads to low deviation between planned and postoperative parameters. J Shoulder Elbow Surg. 2023;32:e587-e596. doi:10.1016/j.jse.2023.05.002
Dey Hazra RO, Paksoy A, Imiolczyk JP, et al. Augmented reality–assisted intraoperative navigation increases precision of glenoid inclination in reverse shoulder arthroplasty. J Shoulder Elbow Surg. 2025;34(2):577-583. doi:10.1016/j.jse.2024.05.039
The introduction of extended reality (XR) to the operating room (OR) has proved promising for enhancing surgical precision and improving patient outcomes. In the field of orthopedic surgery, precise alignment of implants is integral to maintaining functional range of motion and preventing impingement of adjacent neurovascular structures. XR systems have shown promise in arthroplasty including by improving precision and streamlining surgery by allowing surgeons to create 3D preoperative plans that are accessible intraoperatively. This article explores the current applications of XR in arthroplasty, highlights recent advancements and benefits, and describes limitations in comparison to traditional techniques.
Methods
A literature search identified studies involving the use of XR in arthroplasty and current US Food and Drug Administration (FDA)-approved XR systems. Multiple electronic databases were used, including PubMed, Google Scholar, and IEEE Xplore. Search terms included: extended reality, augmented reality, virtual reality, arthroplasty, joint replacement, total knee arthroplasty, total shoulder arthroplasty, and total hip arthroplasty. The study design, intervention details, outcomes, and comparisons with traditional surgical techniques were thematically analyzed, with identification of common ideas associated with XR use in arthroplasty. This narrative report highlights the integration of XR in arthroplasty.
Extended Reality Fundamentals
XR encompasses augmented reality (AR), virtual reality (VR), and mixed reality (MR). AR involves superimposing digitally rendered information and images onto the surgeon’s view of the real world, typically through the use of a headset and smart glasses.1 AR allows the surgeon to move and interact freely within the OR, removing the need for additional screens or devices to display patient information or imaging. VR is a fully immersive simulation using a headset that obstructs the view of the real world but allows the user to move freely within this virtual setting, often with audio or other sensory stimuli. MR combines AR and VR to create a digital model that allows for real-world interaction, with the advantage of adapting information and models in real time.2 Whereas in AR the surgeon can view the data projected from the headset, MR provides the ability to interact with and manipulate the digital content (Figure). Both AR and MR have been adapted for use in the OR, while VR has been adapted for use in surgical planning and training.
Extended Reality Use in Orthopedics
The HipNav system was introduced in 1995 to create preoperative plans that assist surgeons in accurately implanting the acetabular cup during total hip arthroplasty (THA).3 Although not commercially successful, this system spurred surgeons to experiment with XR to improve the accuracy and alignment of orthopedic implants. Systems capable of displaying the desired intraoperative implant placement have flourished, with applications in fracture reduction, arthroplasty, solid tumor resection, and hardware placement.4-7 Accurate alignment has been linked to improvements in patient outcomes.8-10 XR has great potential within the field of arthroplasty, with multiple new systems approved by the FDA and currently available in the US (Table).
Hip Arthroplasty
Orientation of the acetabular cup is a technically challenging part of THA. Accuracy in the anteversion and inclination angles of the acetabular cup is required to maintain implant stability, preserve functional range of motion (ROM), and prevent precocious wear.11,12 Despite preoperative planning, surgeons often overestimate the inclination angle and underestimate anteversion.13 Improper implantation of the acetabular cup can lead to joint instability caused by aseptic loosening, increasing the risk of dislocation and the need for revision surgery.14,15 Dislocations typically present to the emergency department, but primary care practitioners may encounter patients with pain or diminished sensation due to impingement or instability.16
The introduction of XR into the OR has provided the opportunity for real-time navigation and adjustment of the acetabular cup to maximize anteversion and inclination angles. Currently, 2 FDA-approved systems are available for THA: the Zimmer and Surgical Planning Associates HipInsight system, and the Insight Augmented Reality Visualization and Information System (ARVIS). The HipInsight system consists of a hologram projection using the Microsoft HoloLens2 device and optimizes preoperative planning, producing accuracy of anteversion and inclination angles within 3°.17 ARVIS employs existing surgical helmets and 2 mounted tracking cameras to provide navigation intraoperatively. ARVIS has also been approved for use in total knee arthroplasty (TKA) and unicompartmental knee arthroplasty.18
HipInsight has shown utility in increasing the accuracy of acetabular cup placement along with the use of biplanar radiographic scans.19 However, there are no studies validating the efficacy of ARVIS and HipInsight and assessing long-term disease-oriented or patient-oriented outcomes.
Knee Arthroplasty
In the setting of TKA, XR is most effective in ensuring accurate resection of the tibial and femoral components. Achieving the planned femoral coronal, axial, and sagittal angles allows the prosthesis to be on the femoral axis of rotation, improving functional outcomes. XR systems for TKA have been shown to increase the accuracy of distal femoral resection with a limited increase in surgery duration.20,21 For TKA in particular, patients are often less satisfied with the result than surgeons expect.22 Accurate alignment can improve patient satisfaction and reduce return-to-clinic rates for postoperative pain management, a factor that primary care practitioners should consider when recommending a patient for TKA.23
Along with ARVIS, 3 additional XR systems are FDA-approved for use in TKA. The Pixee Medical Knee+ system uses smart glasses and trackers to aid in the positioning of instruments for improved accuracy while allowing real-time navigation.24 The Medacta NextAR Knee’s single-use tracking system allows for intraoperative navigation with the use of AR glasses.25 The Polaris STELLAR Knee uses MR and avoids the need for preoperative imaging by capturing real-time anatomic data.26
The Pixee Medical Knee+ system was commercially available in Europe for several years prior to FDA approval, so more research exists on its efficacy. One study found that the Pixee Medical Knee+ system initially demonstrated an inferior clinical outcome, attributed to the learning curve associated with using the system.27 However, more recent studies have shown its utility in improving alignment, regardless of implant specifications.28,29 The Medacta NextAR Knee system has been shown to improve accuracy of tibial rotation and soft tissue balance and even increase OR efficiency.30,31 The Polaris STELLAR Knee system received FDA approval in 2023; no published research exists on its accuracy and outcomes.26
Shoulder Arthroplasty
Minimally invasive techniques are favored in total shoulder arthroplasty (TSA) due to the vitality of maintaining the surrounding soft tissue to maximize preservation of motility and strength.32 However, this complicates the procedure by decreasing the ability to effectively access and visualize key structures of the shoulder. Accordingly, issues with implant positioning and alignment are more common with TSA than other joint arthroplasties, making XR particularly promising.33 Some studies report that up to 67% of patients experience glenohumeral instability, which can clinically present as weakness, decreased range of motion, and persistent shoulder pain.34,35 The use of preoperative computed tomography to improve understanding of glenoid anatomy and glenohumeral subluxation is becoming increasingly common, and it can be combined with XR to improve accuracy.36,37
Two FDA-approved systems are available. The Stryker Blueprint MR system is used for intraoperative guidance and integration for patient imaging used for preoperative planning. The Medacta NextAR Shoulder system is a parallel of the company’s TKA system. The Stryker Blueprint MR system combines the Microsoft HoloLens 2 headset to display preoperative plans with a secondary display for coordination with the rest of the surgical team.38 Similar to the Medacta NextAR Knee, the Medacta NextAR Shoulder system uses the same single-use tracking system and AR glasses for intraoperative guidance.39
Data on the long-term outcomes of using these systems are still limited, but the Stryker Blueprint MR system has not been shown to accurately predict postoperative ROM.40 Cadaveric studies have demonstrated that the Medacta NextAR Shoulder system can provide accurate inclination, retroversion, entry point, depth, and rotation values based on the preoperative planned values.41,42 However, this accuracy has yet to be confirmed in vivo, and the impact of using XR in TSA on long-term outcomes is still unknown.
Challenges and Limitations
Though XR has proven to be promising in arthroplasty, several limitations regarding widespread implementation exist. In particular, there is a steep learning curve associated with the use of XR systems, which can cause increased operative time and even initial inferior outcomes, as demonstrated with the Pixee Medical Knee+ system. The need for extensive practice and training prior to use could delay widespread adoption and may cause discrepancies in surgical outcomes. Unfamiliarity with the system and technological difficulties that may require troubleshooting can also increase operative time, particularly for surgeons new to using the XR system. Though intraoperative navigation is expected to improve accuracy of implant alignment, its added complexity may also result in longer surgeries.
In addition to the steep learning curve and increased operative time, there is a high upfront cost associated with XR systems. Exact costs of XR systems are not typically disclosed, but available estimates suggest an average sales price of about $1000 per case. Given the proprietary nature of these technologies, publicly available cost data are limited, making it challenging to fully assess the financial burden on health care institutions. Though some systems, such as ARVIS, can be integrated with existing surgical helmets, many require the purchase of AR glasses and secondary displays. This can cause further variation in the total expense for each system. In low-resource settings, this represents a significant challenge to widespread implementation. To justify this cost, additional research on long-term patient outcomes is needed to ensure the benefits of XR systems outweigh the cost.
Although early studies on XR systems in arthroplasty have shown improvements in precision and short-term outcomes, long-term data regarding effectiveness remains. Even systems such as ARVIS and HipInsight have limited long-term follow-up, making it difficult to assess whether the improved accuracy with these XR systems translates into improved patient outcomes compared with traditional arthroplasty.
CONCLUSIONS
XR technologies have shown significant potential in enhancing precision and patient outcomes. Through the integration of XR in the OR, surgeons can visualize preoperative plans and even make intraoperative changes, with the benefit of improving implant alignment.
There are some disadvantages to its use, however, including high cost and increased operative time. Despite this, the integration of XR into surgical practice can deliver more precise implant alignment and address other challenges faced with conventional techniques. As these technologies evolve and studies on long-term outcomes validate their utility, XR has the potential to transform the field of arthroplasty.
The introduction of extended reality (XR) to the operating room (OR) has proved promising for enhancing surgical precision and improving patient outcomes. In the field of orthopedic surgery, precise alignment of implants is integral to maintaining functional range of motion and preventing impingement of adjacent neurovascular structures. XR systems have shown promise in arthroplasty including by improving precision and streamlining surgery by allowing surgeons to create 3D preoperative plans that are accessible intraoperatively. This article explores the current applications of XR in arthroplasty, highlights recent advancements and benefits, and describes limitations in comparison to traditional techniques.
Methods
A literature search identified studies involving the use of XR in arthroplasty and current US Food and Drug Administration (FDA)-approved XR systems. Multiple electronic databases were used, including PubMed, Google Scholar, and IEEE Xplore. Search terms included: extended reality, augmented reality, virtual reality, arthroplasty, joint replacement, total knee arthroplasty, total shoulder arthroplasty, and total hip arthroplasty. The study design, intervention details, outcomes, and comparisons with traditional surgical techniques were thematically analyzed, with identification of common ideas associated with XR use in arthroplasty. This narrative report highlights the integration of XR in arthroplasty.
Extended Reality Fundamentals
XR encompasses augmented reality (AR), virtual reality (VR), and mixed reality (MR). AR involves superimposing digitally rendered information and images onto the surgeon’s view of the real world, typically through the use of a headset and smart glasses.1 AR allows the surgeon to move and interact freely within the OR, removing the need for additional screens or devices to display patient information or imaging. VR is a fully immersive simulation using a headset that obstructs the view of the real world but allows the user to move freely within this virtual setting, often with audio or other sensory stimuli. MR combines AR and VR to create a digital model that allows for real-world interaction, with the advantage of adapting information and models in real time.2 Whereas in AR the surgeon can view the data projected from the headset, MR provides the ability to interact with and manipulate the digital content (Figure). Both AR and MR have been adapted for use in the OR, while VR has been adapted for use in surgical planning and training.
Extended Reality Use in Orthopedics
The HipNav system was introduced in 1995 to create preoperative plans that assist surgeons in accurately implanting the acetabular cup during total hip arthroplasty (THA).3 Although not commercially successful, this system spurred surgeons to experiment with XR to improve the accuracy and alignment of orthopedic implants. Systems capable of displaying the desired intraoperative implant placement have flourished, with applications in fracture reduction, arthroplasty, solid tumor resection, and hardware placement.4-7 Accurate alignment has been linked to improvements in patient outcomes.8-10 XR has great potential within the field of arthroplasty, with multiple new systems approved by the FDA and currently available in the US (Table).
Hip Arthroplasty
Orientation of the acetabular cup is a technically challenging part of THA. Accuracy in the anteversion and inclination angles of the acetabular cup is required to maintain implant stability, preserve functional range of motion (ROM), and prevent precocious wear.11,12 Despite preoperative planning, surgeons often overestimate the inclination angle and underestimate anteversion.13 Improper implantation of the acetabular cup can lead to joint instability caused by aseptic loosening, increasing the risk of dislocation and the need for revision surgery.14,15 Dislocations typically present to the emergency department, but primary care practitioners may encounter patients with pain or diminished sensation due to impingement or instability.16
The introduction of XR into the OR has provided the opportunity for real-time navigation and adjustment of the acetabular cup to maximize anteversion and inclination angles. Currently, 2 FDA-approved systems are available for THA: the Zimmer and Surgical Planning Associates HipInsight system, and the Insight Augmented Reality Visualization and Information System (ARVIS). The HipInsight system consists of a hologram projection using the Microsoft HoloLens2 device and optimizes preoperative planning, producing accuracy of anteversion and inclination angles within 3°.17 ARVIS employs existing surgical helmets and 2 mounted tracking cameras to provide navigation intraoperatively. ARVIS has also been approved for use in total knee arthroplasty (TKA) and unicompartmental knee arthroplasty.18
HipInsight has shown utility in increasing the accuracy of acetabular cup placement along with the use of biplanar radiographic scans.19 However, there are no studies validating the efficacy of ARVIS and HipInsight and assessing long-term disease-oriented or patient-oriented outcomes.
Knee Arthroplasty
In the setting of TKA, XR is most effective in ensuring accurate resection of the tibial and femoral components. Achieving the planned femoral coronal, axial, and sagittal angles allows the prosthesis to be on the femoral axis of rotation, improving functional outcomes. XR systems for TKA have been shown to increase the accuracy of distal femoral resection with a limited increase in surgery duration.20,21 For TKA in particular, patients are often less satisfied with the result than surgeons expect.22 Accurate alignment can improve patient satisfaction and reduce return-to-clinic rates for postoperative pain management, a factor that primary care practitioners should consider when recommending a patient for TKA.23
Along with ARVIS, 3 additional XR systems are FDA-approved for use in TKA. The Pixee Medical Knee+ system uses smart glasses and trackers to aid in the positioning of instruments for improved accuracy while allowing real-time navigation.24 The Medacta NextAR Knee’s single-use tracking system allows for intraoperative navigation with the use of AR glasses.25 The Polaris STELLAR Knee uses MR and avoids the need for preoperative imaging by capturing real-time anatomic data.26
The Pixee Medical Knee+ system was commercially available in Europe for several years prior to FDA approval, so more research exists on its efficacy. One study found that the Pixee Medical Knee+ system initially demonstrated an inferior clinical outcome, attributed to the learning curve associated with using the system.27 However, more recent studies have shown its utility in improving alignment, regardless of implant specifications.28,29 The Medacta NextAR Knee system has been shown to improve accuracy of tibial rotation and soft tissue balance and even increase OR efficiency.30,31 The Polaris STELLAR Knee system received FDA approval in 2023; no published research exists on its accuracy and outcomes.26
Shoulder Arthroplasty
Minimally invasive techniques are favored in total shoulder arthroplasty (TSA) due to the vitality of maintaining the surrounding soft tissue to maximize preservation of motility and strength.32 However, this complicates the procedure by decreasing the ability to effectively access and visualize key structures of the shoulder. Accordingly, issues with implant positioning and alignment are more common with TSA than other joint arthroplasties, making XR particularly promising.33 Some studies report that up to 67% of patients experience glenohumeral instability, which can clinically present as weakness, decreased range of motion, and persistent shoulder pain.34,35 The use of preoperative computed tomography to improve understanding of glenoid anatomy and glenohumeral subluxation is becoming increasingly common, and it can be combined with XR to improve accuracy.36,37
Two FDA-approved systems are available. The Stryker Blueprint MR system is used for intraoperative guidance and integration for patient imaging used for preoperative planning. The Medacta NextAR Shoulder system is a parallel of the company’s TKA system. The Stryker Blueprint MR system combines the Microsoft HoloLens 2 headset to display preoperative plans with a secondary display for coordination with the rest of the surgical team.38 Similar to the Medacta NextAR Knee, the Medacta NextAR Shoulder system uses the same single-use tracking system and AR glasses for intraoperative guidance.39
Data on the long-term outcomes of using these systems are still limited, but the Stryker Blueprint MR system has not been shown to accurately predict postoperative ROM.40 Cadaveric studies have demonstrated that the Medacta NextAR Shoulder system can provide accurate inclination, retroversion, entry point, depth, and rotation values based on the preoperative planned values.41,42 However, this accuracy has yet to be confirmed in vivo, and the impact of using XR in TSA on long-term outcomes is still unknown.
Challenges and Limitations
Though XR has proven to be promising in arthroplasty, several limitations regarding widespread implementation exist. In particular, there is a steep learning curve associated with the use of XR systems, which can cause increased operative time and even initial inferior outcomes, as demonstrated with the Pixee Medical Knee+ system. The need for extensive practice and training prior to use could delay widespread adoption and may cause discrepancies in surgical outcomes. Unfamiliarity with the system and technological difficulties that may require troubleshooting can also increase operative time, particularly for surgeons new to using the XR system. Though intraoperative navigation is expected to improve accuracy of implant alignment, its added complexity may also result in longer surgeries.
In addition to the steep learning curve and increased operative time, there is a high upfront cost associated with XR systems. Exact costs of XR systems are not typically disclosed, but available estimates suggest an average sales price of about $1000 per case. Given the proprietary nature of these technologies, publicly available cost data are limited, making it challenging to fully assess the financial burden on health care institutions. Though some systems, such as ARVIS, can be integrated with existing surgical helmets, many require the purchase of AR glasses and secondary displays. This can cause further variation in the total expense for each system. In low-resource settings, this represents a significant challenge to widespread implementation. To justify this cost, additional research on long-term patient outcomes is needed to ensure the benefits of XR systems outweigh the cost.
Although early studies on XR systems in arthroplasty have shown improvements in precision and short-term outcomes, long-term data regarding effectiveness remains. Even systems such as ARVIS and HipInsight have limited long-term follow-up, making it difficult to assess whether the improved accuracy with these XR systems translates into improved patient outcomes compared with traditional arthroplasty.
CONCLUSIONS
XR technologies have shown significant potential in enhancing precision and patient outcomes. Through the integration of XR in the OR, surgeons can visualize preoperative plans and even make intraoperative changes, with the benefit of improving implant alignment.
There are some disadvantages to its use, however, including high cost and increased operative time. Despite this, the integration of XR into surgical practice can deliver more precise implant alignment and address other challenges faced with conventional techniques. As these technologies evolve and studies on long-term outcomes validate their utility, XR has the potential to transform the field of arthroplasty.
Azuma RT. A survey of augmented reality. Presence-Teleop Virt. 1997;6:355-385. doi:10.1162/pres.1997.6.4.355
Speicher M, Hall BD, Nebeling M. What is Mixed Reality? In: Proceedings of the 2019 CHI Conference on Human Factors in Computing Systems. Association for Computing Machinery; 2019:1-15. doi:10.1145/3290605.3300767
Digioia AM, Jaramaz B, Nikou C, et al. Surgical navigation for total hip replacement with the use of hipnav. Oper Tech Orthop. 2000;10:3-8. doi:10.1016/S1048-6666(00)80036-1
Ogawa H, Hasegawa S, Tsukada S, et al. A pilot study of augmented reality technology applied to the acetabular cup placement during total hip arthroplasty. J Arthroplasty. 2018;33:1833-1837. doi:10.1016/j.arth.2018.01.067
Shen F, Chen B, Guo Q, et al. Augmented reality patient-specific reconstruction plate design for pelvic and acetabular fracture surgery. Int J CARS. 2013;8:169-179. doi:10.1007/s11548-012-0775-5
Cho HS, Park YK, Gupta S, et al. Augmented reality in bone tumour resection: an experimental study. Bone Joint Res. 2017;6:137-143. doi:10.1302/2046-3758.63.bjr-2016-0289.r1
Wu X, Liu R, Yu J, et al. Mixed reality technology launches in orthopedic surgery for comprehensive preoperative management of complicated cervical fractures. Surg Innov. 2018;25:421-422. doi:10.1177/1553350618761758
Dossett HG, Arthur JR, Makovicka JL, et al. A randomized controlled trial of kinematically and mechanically aligned total knee arthroplasties: long-term follow-up. J Arthroplasty. 2023;38:S209-S214. doi:10.1016/j.arth.2023.03.065
Kazarian GS, Haddad FS, Donaldson MJ, et al. Implant malalignment may be a risk factor for poor patient-reported outcomes measures (PROMs) following total knee arthroplasty (TKA). J Arthroplasty. 2022;37:S129-S133. doi:10.1016/j.arth.2022.02.087
Peng Y, Arauz P, An S, et al. Does component alignment affect patient reported outcomes following bicruciate retaining total knee arthroplasty? An in vivo three-dimensional analysis. J Knee Surg. 2020;33:798-803. doi:10.1055/s-0039-1688500
D’Lima DD, Urquhart AG, Buehler KO, et al. The effect of the orientation of the acetabular and femoral components on the range of motion of the hip at different head-neck ratios. J Bone Joint Surg Am. 2000;82:315-321. doi:10.2106/00004623-200003000-00003
Yamaguchi M, Akisue T, Bauer TW, et al. The spatial location of impingement in total hip arthroplasty. J Arthroplasty. 2000;15:305-313. doi:10.1016/s0883-5403(00)90601-6
Grammatopoulos G, Alvand A, Monk AP, et al. Surgeons’ accuracy in achieving their desired acetabular component orientation. J Bone Joint Surg. 2016;98:e72. doi:10.2106/JBJS.15.01080
Kennedy JG, Rogers WB, Soffe KE, et al. Effect of acetabular component orientation on recurrent dislocation, pelvic osteolysis, polyethylene wear, and component migration. J Arthroplasty. 1998;13:530-534. doi:10.1016/S0883-5403(98)90052-3
Del Schutte H, Lipman AJ, Bannar SM, et al. Effects of acetabular abduction on cup wear rates in total hip arthroplasty. J Arthroplasty. 1998;13:621-626. doi:10.1016/S0883-5403(98)80003-X
Aresti N, Kassam J, Bartlett D, et al. Primary care management of postoperative shoulder, hip, and knee arthroplasty. BMJ. 2017;359:j4431. doi:10.1136/bmj.j4431
HipInsightTM System. Zimmer Biomet. Accessed September 3, 2025. https://www.zimmerbiomet.com/en/products-and-solutions/zb-edge/mixed-reality-portfolio/hipinsight-system.html
ARVIS. Insight Medical Systems. Accessed September 3, 2025. https://www.insightmedsys.com/arvis
Sun DC, Murphy WS, Amundson AJ, et al. Validation of a novel method of measuring cup orientation using biplanar simultaneous radiographic images. J Arthroplasty. 2023;38:S252-S256. doi:10.1016/j.arth.2023.04.011
Tsukada S, Ogawa H, Nishino M, et al. Augmented reality-assisted femoral bone resection in total knee arthroplasty. JBJS Open Access. 2021;6:e21.00001. doi:10.2106/JBJS.OA.21.00001
Castellarin G, Bori E, Barbieux E, et al. Is total knee arthroplasty surgical performance enhanced using augmented reality? A single-center study on 76 consecutive patients. J Arthroplasty. 2024;39:332-335. doi:10.1016/j.arth.2023.08.013
Choi YJ, Ra HJ. Patient satisfaction after total knee arthroplasty. Knee Surg Relat Res. 2016;28:1. doi:10.5792/ksrr.2016.28.1.1
Hazratwala K, Gouk C, Wilkinson MPR, et al. Navigated functional alignment total knee arthroplasty achieves reliable, reproducible and accurate results with high patient satisfaction. Knee Surg Sports Traumatol Arthrosc. 2023;31:3861-3870. doi:10.1007/s00167-023-07327-w
Knee+. Pixee Medical. Accessed September 3, 2025. https://www.pixee-medical.com/en/products/knee-nexsight/
KNEE | NEXTAR. Nextar. Accessed September 3, 2025. https://nextar.medacta.com/knee
POLARIS AR receives clearance from the U.S. Food and Drug Administration for STELLAR Knee. News release. PRNewswire. November 3, 2023. Accessed September 3, 2025. https://www.prnewswire.com/news-releases/polarisar-receives-clearance-from-the-us-food-and-drug-administration-for-stellar-knee-301976747.html
van Overschelde P, Vansintjan P, Byn P, Lapierre C, van Lysebettens W. Does augmented reality improve clinical outcome in TKA? A prospective observational report. In: The 20th Annual Meeting of the International Society for Computer Assisted Orthopaedic Surgery. 2022:170-174.
Sakellariou E, Alevrogiannis P, Alevrogianni F, et al. Single-center experience with Knee+TM augmented reality navigation system in primary total knee arthroplasty. World J Orthop. 2024;15:247-256. doi:10.5312/wjo.v15.i3.247
León-Muñoz VJ, Moya-Angeler J, López-López M, et al. Integration of square fiducial markers in patient-specific instrumentation and their applicability in knee surgery. J Pers Med. 2023;13:727. doi:10.3390/jpm13050727
Fucentese SF, Koch PP. A novel augmented reality-based surgical guidance system for total knee arthroplasty. Arch Orthop Trauma Surg. 2021;141:2227-2233. doi:10.1007/s00402-021-04204-4
Sabatini L, Ascani D, Vezza D, et al. Novel surgical technique for total knee arthroplasty integrating kinematic alignment and real-time elongation of the ligaments using the NextAR system. J Pers Med. 2024;14:794. doi:10.3390/jpm14080794
Daher M, Ghanimeh J, Otayek J, et al. Augmented reality and shoulder replacement: a state-of-the-art review article. JSES Rev Rep Tech. 2023;3:274-278. doi:10.1016/j.xrrt.2023.01.008
Atmani H, Merienne F, Fofi D, et al. Computer aided surgery system for shoulder prosthesis placement. Comput Aided Surg. 2007;12:60-70. doi:10.3109/10929080701210832
Eichinger JK, Galvin JW. Management of complications after total shoulder arthroplasty. Curr Rev Musculoskelet Med. 2015;8:83-91. doi:10.1007/s12178-014-9251-x
Bonnevialle N, Melis B, Neyton L, et al. Aseptic glenoid loosening or failure in total shoulder arthroplasty: revision with glenoid reimplantation. J Shoulder Elbow Surg. 2013;22:745-751. doi:10.1016/j.jse.2012.08.009
Erickson BJ, Chalmers PN, Denard P, et al. Does commercially available shoulder arthroplasty preoperative planning software agree with surgeon measurements of version, inclination, and subluxation? J Shoulder Elbow Surg. 2021;30:413-420. doi:10.1016/j.jse.2020.05.027
Werner BS, Hudek R, Burkhart KJ, et al. The influence of three-dimensional planning on decision-making in total shoulder arthroplasty. J Shoulder Elbow Surg. 2017;26:1477-1483. doi:10.1016/j.jse.2017.01.006
Blueprint. Stryker. Updated August 2025. Accessed September 3, 2025. https://www.stryker.com/us/en/trauma-and-extremities/products/blueprint.html
NextAR Shoulder. Medacta. Accessed September 3, 2025. https://nextar.medacta.com/shoulder
Baumgarten KM. Accuracy of Blueprint software in predicting range of motion 1 year after reverse total shoulder arthroplasty. J Shoulder Elbow Surg. 2023;32:1088-1094. doi:10.1016/j.jse.2022.12.009
Rojas JT, Jost B, Zipeto C, et al. Glenoid component placement in reverse shoulder arthroplasty assisted with augmented reality through a head-mounted display leads to low deviation between planned and postoperative parameters. J Shoulder Elbow Surg. 2023;32:e587-e596. doi:10.1016/j.jse.2023.05.002
Dey Hazra RO, Paksoy A, Imiolczyk JP, et al. Augmented reality–assisted intraoperative navigation increases precision of glenoid inclination in reverse shoulder arthroplasty. J Shoulder Elbow Surg. 2025;34(2):577-583. doi:10.1016/j.jse.2024.05.039
Azuma RT. A survey of augmented reality. Presence-Teleop Virt. 1997;6:355-385. doi:10.1162/pres.1997.6.4.355
Speicher M, Hall BD, Nebeling M. What is Mixed Reality? In: Proceedings of the 2019 CHI Conference on Human Factors in Computing Systems. Association for Computing Machinery; 2019:1-15. doi:10.1145/3290605.3300767
Digioia AM, Jaramaz B, Nikou C, et al. Surgical navigation for total hip replacement with the use of hipnav. Oper Tech Orthop. 2000;10:3-8. doi:10.1016/S1048-6666(00)80036-1
Ogawa H, Hasegawa S, Tsukada S, et al. A pilot study of augmented reality technology applied to the acetabular cup placement during total hip arthroplasty. J Arthroplasty. 2018;33:1833-1837. doi:10.1016/j.arth.2018.01.067
Shen F, Chen B, Guo Q, et al. Augmented reality patient-specific reconstruction plate design for pelvic and acetabular fracture surgery. Int J CARS. 2013;8:169-179. doi:10.1007/s11548-012-0775-5
Cho HS, Park YK, Gupta S, et al. Augmented reality in bone tumour resection: an experimental study. Bone Joint Res. 2017;6:137-143. doi:10.1302/2046-3758.63.bjr-2016-0289.r1
Wu X, Liu R, Yu J, et al. Mixed reality technology launches in orthopedic surgery for comprehensive preoperative management of complicated cervical fractures. Surg Innov. 2018;25:421-422. doi:10.1177/1553350618761758
Dossett HG, Arthur JR, Makovicka JL, et al. A randomized controlled trial of kinematically and mechanically aligned total knee arthroplasties: long-term follow-up. J Arthroplasty. 2023;38:S209-S214. doi:10.1016/j.arth.2023.03.065
Kazarian GS, Haddad FS, Donaldson MJ, et al. Implant malalignment may be a risk factor for poor patient-reported outcomes measures (PROMs) following total knee arthroplasty (TKA). J Arthroplasty. 2022;37:S129-S133. doi:10.1016/j.arth.2022.02.087
Peng Y, Arauz P, An S, et al. Does component alignment affect patient reported outcomes following bicruciate retaining total knee arthroplasty? An in vivo three-dimensional analysis. J Knee Surg. 2020;33:798-803. doi:10.1055/s-0039-1688500
D’Lima DD, Urquhart AG, Buehler KO, et al. The effect of the orientation of the acetabular and femoral components on the range of motion of the hip at different head-neck ratios. J Bone Joint Surg Am. 2000;82:315-321. doi:10.2106/00004623-200003000-00003
Yamaguchi M, Akisue T, Bauer TW, et al. The spatial location of impingement in total hip arthroplasty. J Arthroplasty. 2000;15:305-313. doi:10.1016/s0883-5403(00)90601-6
Grammatopoulos G, Alvand A, Monk AP, et al. Surgeons’ accuracy in achieving their desired acetabular component orientation. J Bone Joint Surg. 2016;98:e72. doi:10.2106/JBJS.15.01080
Kennedy JG, Rogers WB, Soffe KE, et al. Effect of acetabular component orientation on recurrent dislocation, pelvic osteolysis, polyethylene wear, and component migration. J Arthroplasty. 1998;13:530-534. doi:10.1016/S0883-5403(98)90052-3
Del Schutte H, Lipman AJ, Bannar SM, et al. Effects of acetabular abduction on cup wear rates in total hip arthroplasty. J Arthroplasty. 1998;13:621-626. doi:10.1016/S0883-5403(98)80003-X
Aresti N, Kassam J, Bartlett D, et al. Primary care management of postoperative shoulder, hip, and knee arthroplasty. BMJ. 2017;359:j4431. doi:10.1136/bmj.j4431
HipInsightTM System. Zimmer Biomet. Accessed September 3, 2025. https://www.zimmerbiomet.com/en/products-and-solutions/zb-edge/mixed-reality-portfolio/hipinsight-system.html
ARVIS. Insight Medical Systems. Accessed September 3, 2025. https://www.insightmedsys.com/arvis
Sun DC, Murphy WS, Amundson AJ, et al. Validation of a novel method of measuring cup orientation using biplanar simultaneous radiographic images. J Arthroplasty. 2023;38:S252-S256. doi:10.1016/j.arth.2023.04.011
Tsukada S, Ogawa H, Nishino M, et al. Augmented reality-assisted femoral bone resection in total knee arthroplasty. JBJS Open Access. 2021;6:e21.00001. doi:10.2106/JBJS.OA.21.00001
Castellarin G, Bori E, Barbieux E, et al. Is total knee arthroplasty surgical performance enhanced using augmented reality? A single-center study on 76 consecutive patients. J Arthroplasty. 2024;39:332-335. doi:10.1016/j.arth.2023.08.013
Choi YJ, Ra HJ. Patient satisfaction after total knee arthroplasty. Knee Surg Relat Res. 2016;28:1. doi:10.5792/ksrr.2016.28.1.1
Hazratwala K, Gouk C, Wilkinson MPR, et al. Navigated functional alignment total knee arthroplasty achieves reliable, reproducible and accurate results with high patient satisfaction. Knee Surg Sports Traumatol Arthrosc. 2023;31:3861-3870. doi:10.1007/s00167-023-07327-w
Knee+. Pixee Medical. Accessed September 3, 2025. https://www.pixee-medical.com/en/products/knee-nexsight/
KNEE | NEXTAR. Nextar. Accessed September 3, 2025. https://nextar.medacta.com/knee
POLARIS AR receives clearance from the U.S. Food and Drug Administration for STELLAR Knee. News release. PRNewswire. November 3, 2023. Accessed September 3, 2025. https://www.prnewswire.com/news-releases/polarisar-receives-clearance-from-the-us-food-and-drug-administration-for-stellar-knee-301976747.html
van Overschelde P, Vansintjan P, Byn P, Lapierre C, van Lysebettens W. Does augmented reality improve clinical outcome in TKA? A prospective observational report. In: The 20th Annual Meeting of the International Society for Computer Assisted Orthopaedic Surgery. 2022:170-174.
Sakellariou E, Alevrogiannis P, Alevrogianni F, et al. Single-center experience with Knee+TM augmented reality navigation system in primary total knee arthroplasty. World J Orthop. 2024;15:247-256. doi:10.5312/wjo.v15.i3.247
León-Muñoz VJ, Moya-Angeler J, López-López M, et al. Integration of square fiducial markers in patient-specific instrumentation and their applicability in knee surgery. J Pers Med. 2023;13:727. doi:10.3390/jpm13050727
Fucentese SF, Koch PP. A novel augmented reality-based surgical guidance system for total knee arthroplasty. Arch Orthop Trauma Surg. 2021;141:2227-2233. doi:10.1007/s00402-021-04204-4
Sabatini L, Ascani D, Vezza D, et al. Novel surgical technique for total knee arthroplasty integrating kinematic alignment and real-time elongation of the ligaments using the NextAR system. J Pers Med. 2024;14:794. doi:10.3390/jpm14080794
Daher M, Ghanimeh J, Otayek J, et al. Augmented reality and shoulder replacement: a state-of-the-art review article. JSES Rev Rep Tech. 2023;3:274-278. doi:10.1016/j.xrrt.2023.01.008
Atmani H, Merienne F, Fofi D, et al. Computer aided surgery system for shoulder prosthesis placement. Comput Aided Surg. 2007;12:60-70. doi:10.3109/10929080701210832
Eichinger JK, Galvin JW. Management of complications after total shoulder arthroplasty. Curr Rev Musculoskelet Med. 2015;8:83-91. doi:10.1007/s12178-014-9251-x
Bonnevialle N, Melis B, Neyton L, et al. Aseptic glenoid loosening or failure in total shoulder arthroplasty: revision with glenoid reimplantation. J Shoulder Elbow Surg. 2013;22:745-751. doi:10.1016/j.jse.2012.08.009
Erickson BJ, Chalmers PN, Denard P, et al. Does commercially available shoulder arthroplasty preoperative planning software agree with surgeon measurements of version, inclination, and subluxation? J Shoulder Elbow Surg. 2021;30:413-420. doi:10.1016/j.jse.2020.05.027
Werner BS, Hudek R, Burkhart KJ, et al. The influence of three-dimensional planning on decision-making in total shoulder arthroplasty. J Shoulder Elbow Surg. 2017;26:1477-1483. doi:10.1016/j.jse.2017.01.006
Blueprint. Stryker. Updated August 2025. Accessed September 3, 2025. https://www.stryker.com/us/en/trauma-and-extremities/products/blueprint.html
NextAR Shoulder. Medacta. Accessed September 3, 2025. https://nextar.medacta.com/shoulder
Baumgarten KM. Accuracy of Blueprint software in predicting range of motion 1 year after reverse total shoulder arthroplasty. J Shoulder Elbow Surg. 2023;32:1088-1094. doi:10.1016/j.jse.2022.12.009
Rojas JT, Jost B, Zipeto C, et al. Glenoid component placement in reverse shoulder arthroplasty assisted with augmented reality through a head-mounted display leads to low deviation between planned and postoperative parameters. J Shoulder Elbow Surg. 2023;32:e587-e596. doi:10.1016/j.jse.2023.05.002
Dey Hazra RO, Paksoy A, Imiolczyk JP, et al. Augmented reality–assisted intraoperative navigation increases precision of glenoid inclination in reverse shoulder arthroplasty. J Shoulder Elbow Surg. 2025;34(2):577-583. doi:10.1016/j.jse.2024.05.039
The Integration of Extended Reality in Arthroplasty: Reviewing Technological Progress and Clinical Benefits
The Integration of Extended Reality in Arthroplasty: Reviewing Technological Progress and Clinical Benefits
Diagnostic Testing for Patients With Suspected Ocular Manifestations of Lyme Disease
Diagnostic Testing for Patients With Suspected Ocular Manifestations of Lyme Disease
Since Lyme disease (LD) was first identified in 1975, there has been uncertainty regarding the proper diagnostic testing for suspected cases.1 Challenges involved with ordering Lyme serology testing include navigating tests with an array of false negatives and false positives.2 Confounding these challenges is the wide variety of ocular manifestations of LD, ranging from nonspecific conjunctivitis, cranial palsies, and anterior and posterior segment inflammation.2,3 This article provides diagnostic testing guidelines for eye care clinicians who encounter patients with suspected LD.
BACKGROUND
LD is a bacterial infection caused by the spirochete Borrelia burgdorferi sensu lato complex transmitted by the Ixodes tick genus. There are 4 species of Ixodes ticks that can infect humans, and only 2 have been identified as principal vectors in North America: Ixodes scapularis and Ixodes pacificus. The incidence of LD is on the rise due to increasing global temperatures and expanding geographic borders for the organism. Cases in endemic areas range from 10 per 100,000 people to 50 per 100,000 people.4
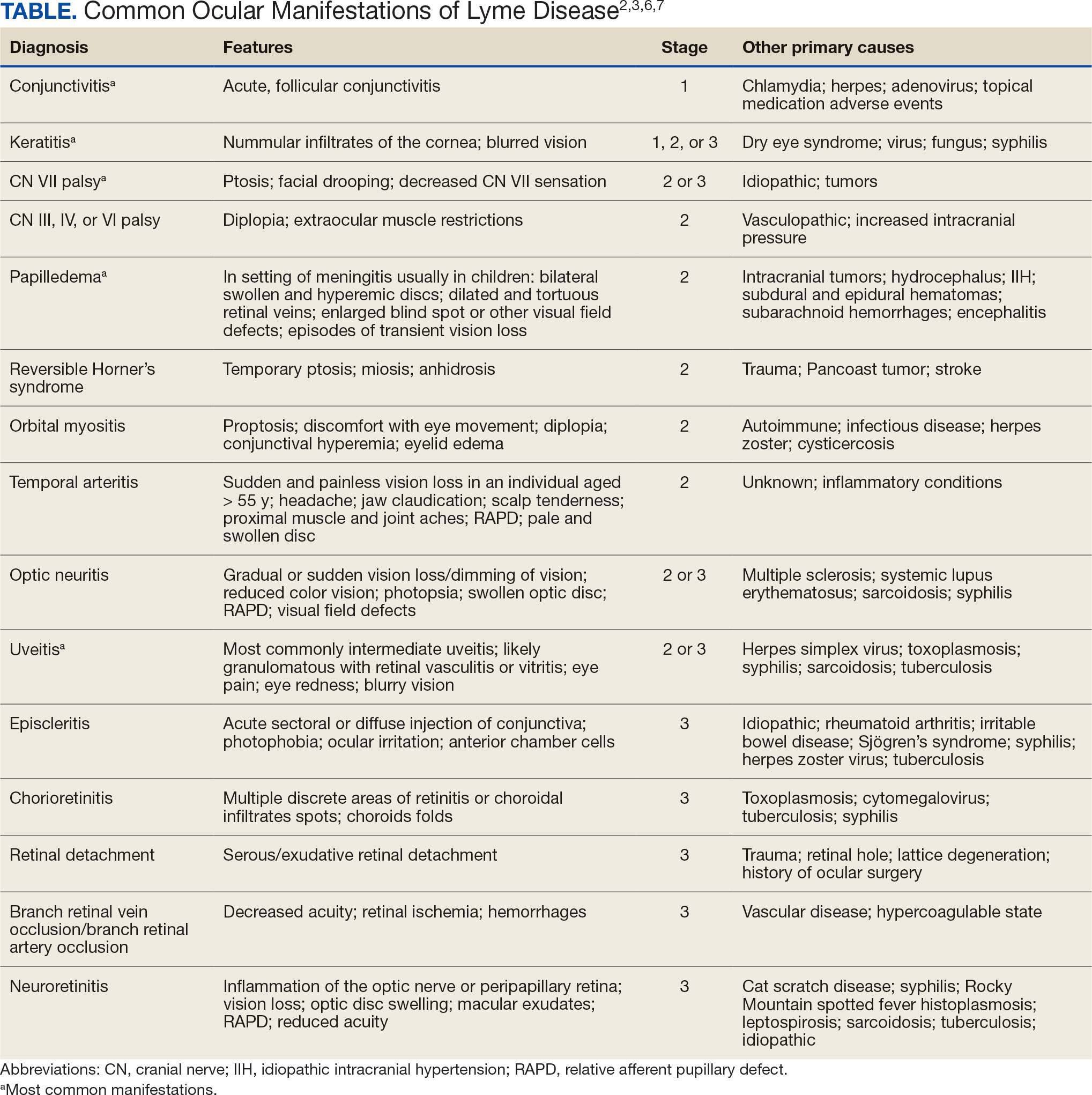
LD occurs in 3 stages: early localized (stage 1), early disseminated (stage 2), and late disseminated (stage 3). In stage 1, patients typically present with erythema migrans (EM) rash (bull’s-eye cutaneous rash) and other nonspecific flu-like symptoms of fever, fatigue, and arthralgia. Stage 2 occurs several weeks to months after the initial infection and the infection has invaded other systemic organs, causing conditions like carditis, meningitis, and arthritis. A small subset of patients may progress to stage 3, which is characterized by chronic arthritis and chronic neurological LD.2,4,5 Ocular manifestations have been well-documented in all stages of LD but are more prevalent in early disseminated disease (Table).2,3,6,7
Indications
Recognizing common ocular manifestations associated with LD will allow eye care practitioners to make a timely diagnosis and initiate treatment. The most common ocular findings from LD include conjunctivitis, keratitis, cranial nerve VII palsy, optic neuritis, granulomatous iridocyclitis, and pars planitis.2,6 While retrospective studies suggest that up to 10% of patients with early localized LD have a nonspecific follicular conjunctivitis, those patients are unlikely to present for ocular evaluation. If a patient does present with an acute conjunctivitis, many clinicians do not consider LD in their differential diagnosis.8 In endemic areas, it is important to query patients for additional symptoms that may indicate LD.
Obtaining a complete patient history is vital in aiding a clinician’s decision to order Lyme serology for suspected LD. Epidemiology, history of geography/travel, pet exposure, sexual history (necessary to rule out other conditions [ie, syphilis] to direct appropriate diagnostic testing), and a complete review of systems should be obtained.2,4 LD may mimic other inflammatory autoimmune conditions or infectious diseases such as syphilis.2,5 This can lead to obtaining unnecessary Lyme serologies or failing to diagnose LD.5,7
Diagnostic testing is not indicated when a patient presents with an asymptomatic tick bite (ie, has no fever, malaise, or EM rash) or if a patient does not live in or has not recently traveled to an endemic area because it would be highly unlikely the patient has LD.9,10 If the patient reports known contact with a tick and has a rash suspicious for EM, the diagnosis may be made without confirmatory testing because EM is pathognomonic for LD.7,11 Serologic testing is not recommended in these cases, particularly if there is a single EM lesion, since the lesion often presents prior to development of an immune response leading to seronegative results.8
Lyme serology is necessary if a patient presents with ocular manifestations known to be associated with LD and resides in, or has recently traveled to, an area where LD is endemic (ie, New England, Minnesota, or Wisconsin).7,12 These criteria are of particular importance: about 50% of patients do not recall a tick bite and 20% to 40% do not present with an EM.2,9
Diagnostic Testing
In 2019 the Centers for Disease Control and Prevention (CDC) updated their testing guidelines to the modified 2-tier testing (MTTT) method. The MTTT first recommends a Lyme enzyme immunoassay (EIA), with a second EIA recommended only if the first is positive.12-14 The MTTT method has better sensitivity in early localized LD compared to standard 2-tier testing.9,11,12 The CDC advises against the use of any laboratory serology tests not approved by the US Food and Drug Administration.13 The CDC also advises that LD serology testing should not be performed as a “test for cure,” because even after successful treatment, an individual may still test positive.1,9 Follow-up testing in patients treated early in the disease course (ie, in the setting of EM) may never have an antibody response. In these cases, a negative test should not exclude an LD diagnosis. 9 For patients with suspected neuroborreliosis, a lumbar puncture may not be needed if a patient already has a positive peripheral serology via the MTTT method.12 The Figure depicts a flow chart for the process of ordering and interpreting testing.
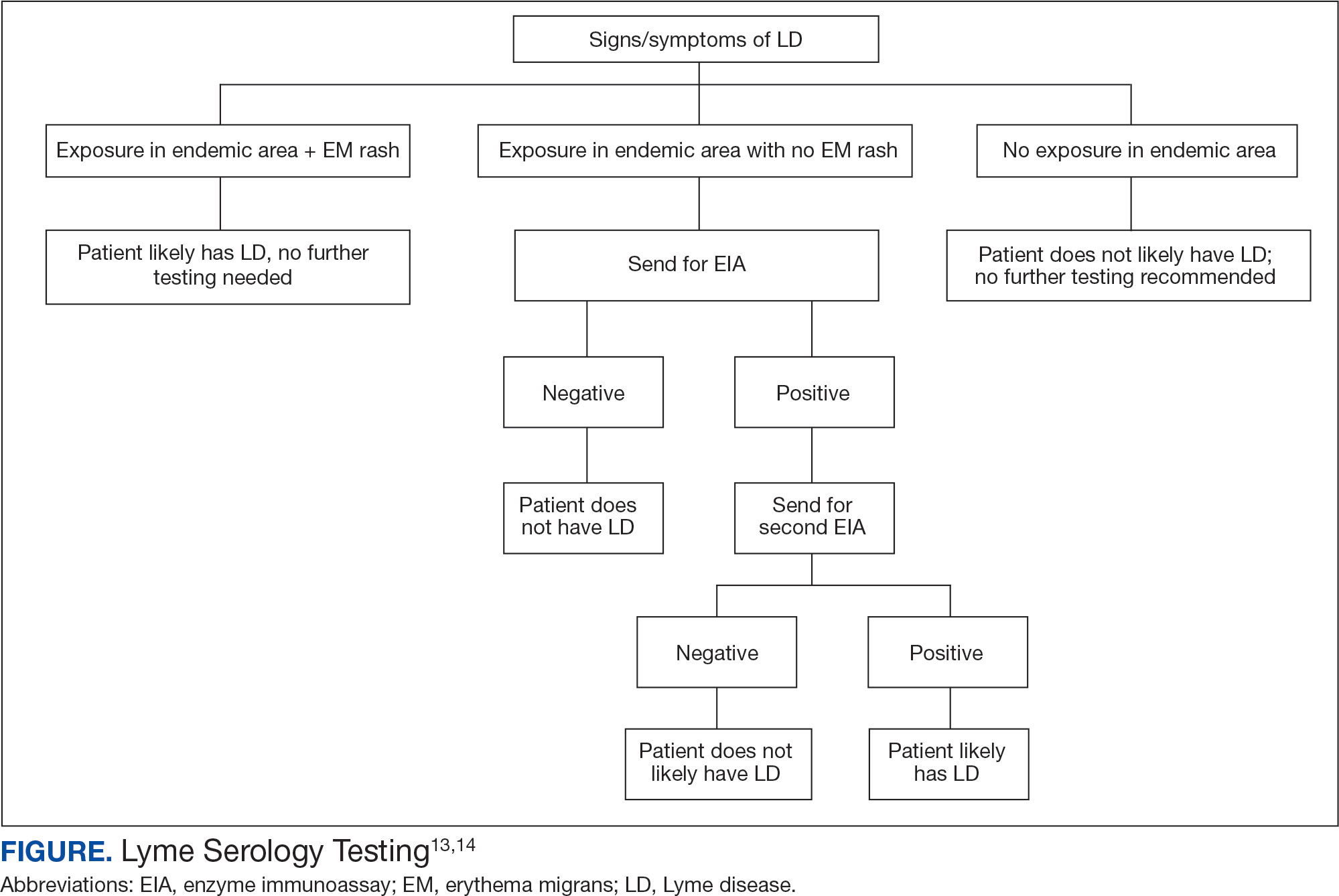
Most LD testing, if correlated with clinical disease, is positive after 4 to 6 weeks.9 If an eye disease is noted and the patient has positive Lyme serology, the patient should still be screened for Lyme neuroborreliosis of the central nervous system (CNS). Examination of the fundus for papilledema, review of symptoms of aseptic meningitis, and a careful neurologic examination should be performed.15
If CNS disease is suspected, the patient may need additional CNS testing to support treatment decisions. The 2020 Infectious Diseases Society of America Lyme guidelines recommend to: (1) obtain simultaneous samples of cerebrospinal fluid (CSF) and serum for determination of the CSF:serum antibody index; (2) do not obtain CSF serology without measurement of the CSF:serum antibody index; and (3) do not obtain routine polymerase chain reaction or culture of CSF or serum.15 Once an LD diagnosis is confirmed, the CDC recommends a course of 100 mg of oral doxycycline twice daily for 14 to 21 days or an antimicrobial equivalent (eg, amoxicillin) if doxycycline is contraindicated. However, the antimicrobial dosage may vary depending on the stage of LD.11 Patients with confirmed neuroborreliosis should be admitted for 14 days of intravenous ceftriaxone or intravenous penicillin.2
CONCLUSIONS
To ensure timely diagnosis and treatment, eye care clinicians should be familiar with the appropriate diagnostic testing for patients suspected to have ocular manifestations of LD. For patients with suspected LD and a high pretest probability, clinicians should obtain a first-order Lyme EIA.12-14 If testing confirms LD, refer the patient to an infectious disease specialist for antimicrobial treatment and additional management.11
- Kullberg BJ, Vrijmoeth HD, van de Schoor F, Hovius JW. Lyme borreliosis: diagnosis and management. BMJ. 2020;369:m1041. doi:10.1136/bmj.m1041
- Zaidman GW. The ocular manifestations of Lyme disease. Int Ophthalmol Clin. 1993;33(1):9-22. doi:10.1097/00004397-199303310-00004
- Lesser RL. Ocular manifestations of Lyme disease. Am J Med. 1995; 98(4A):60S-62S. doi:10.1016/s0002-9343(99)80045-x
- Mead P. Epidemiology of Lyme disease. Infect Dis Clin North Am. 2022;36(3):495-521. doi:10.1016/j.idc.2022.03.004
- Klig JE. Ophthalmologic complications of systemic disease. Emerg Med Clin North Am. 2008;26(1):217-viii. doi:10.1016/j.emc.2007.10.003
- Raja H, Starr MR, Bakri SJ. Ocular manifestations of tickborne diseases. Surv Ophthalmol. 2016;61(6):726-744. doi:10.1016/j.survophthal.2016.03.011
- Mora P, Carta A. Ocular manifestations of Lyme borreliosis in Europe. Int J Med Sci. 2009;6(3):124-125. doi:10.7150/ijms.6.124
- Mikkilä HO, Seppälä IJ, Viljanen MK, Peltomaa MP, Karma A. The expanding clinical spectrum of ocular lyme borreliosis. Ophthalmology. 2000;107(3):581-587. doi:10.1016/s0161-6420(99)00128-1
- Schriefer ME. Lyme disease diagnosis: serology. Clin Lab Med. 2015;35(4):797-814. doi:10.1016/j.cll.2015.08.001
- Beck AR, Marx GE, Hinckley AF. Diagnosis, treatment, and prevention practices for Lyme disease by clinicians, United States, 2013-2015. Public Health Rep. 2021;136(5):609- 617. doi:10.1177/0033354920973235
- Wormser GP, McKenna D, Nowakowski J. Management approaches for suspected and established Lyme disease used at the Lyme disease diagnostic center. Wien Klin Wochenschr. 2018;130(15-16):463-467. doi:10.1007/s00508-015-0936-y
- Kobayashi T, Auwaerter PG. Diagnostic testing for Lyme disease. Infect Dis Clin North Am. 2022;36(3):605-620. doi:10.1016/j.idc.2022.04.001
- Mead P, Petersen J, Hinckley A. Updated CDC recommendation for serologic diagnosis of Lyme disease. MMWR Morb Mortal Wkly Rep. 2019;68(32):703. doi:10.15585/mmwr.mm6832a4
- Association of Public Health Laboratories. Suggested Reporting Language, Interpretation and Guidance Regarding Lyme Disease Serologic Test Results. April 2024. Accessed December 3, 2024. https://www.aphl.org/aboutAPHL/publications/Documents/ID-2024-Lyme-Disease-Serologic-Testing-Reporting.pdf
- Lantos PM, Rumbaugh P, Bockenstedt L, et al. Clinical practice guidelines by the Infectious Diseases Society of America (IDSA), American Academy of Neurology (AAN), and American College of Rheumatology (ACR): 2020 guidelines for the prevention, diagnosis and treatment of Lyme Disease. Clin Infect Dis. 2021;72(1):e1-e48. doi:10.1093/cid/ciaa1215
Since Lyme disease (LD) was first identified in 1975, there has been uncertainty regarding the proper diagnostic testing for suspected cases.1 Challenges involved with ordering Lyme serology testing include navigating tests with an array of false negatives and false positives.2 Confounding these challenges is the wide variety of ocular manifestations of LD, ranging from nonspecific conjunctivitis, cranial palsies, and anterior and posterior segment inflammation.2,3 This article provides diagnostic testing guidelines for eye care clinicians who encounter patients with suspected LD.
BACKGROUND
LD is a bacterial infection caused by the spirochete Borrelia burgdorferi sensu lato complex transmitted by the Ixodes tick genus. There are 4 species of Ixodes ticks that can infect humans, and only 2 have been identified as principal vectors in North America: Ixodes scapularis and Ixodes pacificus. The incidence of LD is on the rise due to increasing global temperatures and expanding geographic borders for the organism. Cases in endemic areas range from 10 per 100,000 people to 50 per 100,000 people.4
LD occurs in 3 stages: early localized (stage 1), early disseminated (stage 2), and late disseminated (stage 3). In stage 1, patients typically present with erythema migrans (EM) rash (bull’s-eye cutaneous rash) and other nonspecific flu-like symptoms of fever, fatigue, and arthralgia. Stage 2 occurs several weeks to months after the initial infection and the infection has invaded other systemic organs, causing conditions like carditis, meningitis, and arthritis. A small subset of patients may progress to stage 3, which is characterized by chronic arthritis and chronic neurological LD.2,4,5 Ocular manifestations have been well-documented in all stages of LD but are more prevalent in early disseminated disease (Table).2,3,6,7

Indications
Recognizing common ocular manifestations associated with LD will allow eye care practitioners to make a timely diagnosis and initiate treatment. The most common ocular findings from LD include conjunctivitis, keratitis, cranial nerve VII palsy, optic neuritis, granulomatous iridocyclitis, and pars planitis.2,6 While retrospective studies suggest that up to 10% of patients with early localized LD have a nonspecific follicular conjunctivitis, those patients are unlikely to present for ocular evaluation. If a patient does present with an acute conjunctivitis, many clinicians do not consider LD in their differential diagnosis.8 In endemic areas, it is important to query patients for additional symptoms that may indicate LD.
Obtaining a complete patient history is vital in aiding a clinician’s decision to order Lyme serology for suspected LD. Epidemiology, history of geography/travel, pet exposure, sexual history (necessary to rule out other conditions [ie, syphilis] to direct appropriate diagnostic testing), and a complete review of systems should be obtained.2,4 LD may mimic other inflammatory autoimmune conditions or infectious diseases such as syphilis.2,5 This can lead to obtaining unnecessary Lyme serologies or failing to diagnose LD.5,7
Diagnostic testing is not indicated when a patient presents with an asymptomatic tick bite (ie, has no fever, malaise, or EM rash) or if a patient does not live in or has not recently traveled to an endemic area because it would be highly unlikely the patient has LD.9,10 If the patient reports known contact with a tick and has a rash suspicious for EM, the diagnosis may be made without confirmatory testing because EM is pathognomonic for LD.7,11 Serologic testing is not recommended in these cases, particularly if there is a single EM lesion, since the lesion often presents prior to development of an immune response leading to seronegative results.8
Lyme serology is necessary if a patient presents with ocular manifestations known to be associated with LD and resides in, or has recently traveled to, an area where LD is endemic (ie, New England, Minnesota, or Wisconsin).7,12 These criteria are of particular importance: about 50% of patients do not recall a tick bite and 20% to 40% do not present with an EM.2,9
Diagnostic Testing
In 2019 the Centers for Disease Control and Prevention (CDC) updated their testing guidelines to the modified 2-tier testing (MTTT) method. The MTTT first recommends a Lyme enzyme immunoassay (EIA), with a second EIA recommended only if the first is positive.12-14 The MTTT method has better sensitivity in early localized LD compared to standard 2-tier testing.9,11,12 The CDC advises against the use of any laboratory serology tests not approved by the US Food and Drug Administration.13 The CDC also advises that LD serology testing should not be performed as a “test for cure,” because even after successful treatment, an individual may still test positive.1,9 Follow-up testing in patients treated early in the disease course (ie, in the setting of EM) may never have an antibody response. In these cases, a negative test should not exclude an LD diagnosis. 9 For patients with suspected neuroborreliosis, a lumbar puncture may not be needed if a patient already has a positive peripheral serology via the MTTT method.12 The Figure depicts a flow chart for the process of ordering and interpreting testing.

Most LD testing, if correlated with clinical disease, is positive after 4 to 6 weeks.9 If an eye disease is noted and the patient has positive Lyme serology, the patient should still be screened for Lyme neuroborreliosis of the central nervous system (CNS). Examination of the fundus for papilledema, review of symptoms of aseptic meningitis, and a careful neurologic examination should be performed.15
If CNS disease is suspected, the patient may need additional CNS testing to support treatment decisions. The 2020 Infectious Diseases Society of America Lyme guidelines recommend to: (1) obtain simultaneous samples of cerebrospinal fluid (CSF) and serum for determination of the CSF:serum antibody index; (2) do not obtain CSF serology without measurement of the CSF:serum antibody index; and (3) do not obtain routine polymerase chain reaction or culture of CSF or serum.15 Once an LD diagnosis is confirmed, the CDC recommends a course of 100 mg of oral doxycycline twice daily for 14 to 21 days or an antimicrobial equivalent (eg, amoxicillin) if doxycycline is contraindicated. However, the antimicrobial dosage may vary depending on the stage of LD.11 Patients with confirmed neuroborreliosis should be admitted for 14 days of intravenous ceftriaxone or intravenous penicillin.2
CONCLUSIONS
To ensure timely diagnosis and treatment, eye care clinicians should be familiar with the appropriate diagnostic testing for patients suspected to have ocular manifestations of LD. For patients with suspected LD and a high pretest probability, clinicians should obtain a first-order Lyme EIA.12-14 If testing confirms LD, refer the patient to an infectious disease specialist for antimicrobial treatment and additional management.11
Since Lyme disease (LD) was first identified in 1975, there has been uncertainty regarding the proper diagnostic testing for suspected cases.1 Challenges involved with ordering Lyme serology testing include navigating tests with an array of false negatives and false positives.2 Confounding these challenges is the wide variety of ocular manifestations of LD, ranging from nonspecific conjunctivitis, cranial palsies, and anterior and posterior segment inflammation.2,3 This article provides diagnostic testing guidelines for eye care clinicians who encounter patients with suspected LD.
BACKGROUND
LD is a bacterial infection caused by the spirochete Borrelia burgdorferi sensu lato complex transmitted by the Ixodes tick genus. There are 4 species of Ixodes ticks that can infect humans, and only 2 have been identified as principal vectors in North America: Ixodes scapularis and Ixodes pacificus. The incidence of LD is on the rise due to increasing global temperatures and expanding geographic borders for the organism. Cases in endemic areas range from 10 per 100,000 people to 50 per 100,000 people.4
LD occurs in 3 stages: early localized (stage 1), early disseminated (stage 2), and late disseminated (stage 3). In stage 1, patients typically present with erythema migrans (EM) rash (bull’s-eye cutaneous rash) and other nonspecific flu-like symptoms of fever, fatigue, and arthralgia. Stage 2 occurs several weeks to months after the initial infection and the infection has invaded other systemic organs, causing conditions like carditis, meningitis, and arthritis. A small subset of patients may progress to stage 3, which is characterized by chronic arthritis and chronic neurological LD.2,4,5 Ocular manifestations have been well-documented in all stages of LD but are more prevalent in early disseminated disease (Table).2,3,6,7

Indications
Recognizing common ocular manifestations associated with LD will allow eye care practitioners to make a timely diagnosis and initiate treatment. The most common ocular findings from LD include conjunctivitis, keratitis, cranial nerve VII palsy, optic neuritis, granulomatous iridocyclitis, and pars planitis.2,6 While retrospective studies suggest that up to 10% of patients with early localized LD have a nonspecific follicular conjunctivitis, those patients are unlikely to present for ocular evaluation. If a patient does present with an acute conjunctivitis, many clinicians do not consider LD in their differential diagnosis.8 In endemic areas, it is important to query patients for additional symptoms that may indicate LD.
Obtaining a complete patient history is vital in aiding a clinician’s decision to order Lyme serology for suspected LD. Epidemiology, history of geography/travel, pet exposure, sexual history (necessary to rule out other conditions [ie, syphilis] to direct appropriate diagnostic testing), and a complete review of systems should be obtained.2,4 LD may mimic other inflammatory autoimmune conditions or infectious diseases such as syphilis.2,5 This can lead to obtaining unnecessary Lyme serologies or failing to diagnose LD.5,7
Diagnostic testing is not indicated when a patient presents with an asymptomatic tick bite (ie, has no fever, malaise, or EM rash) or if a patient does not live in or has not recently traveled to an endemic area because it would be highly unlikely the patient has LD.9,10 If the patient reports known contact with a tick and has a rash suspicious for EM, the diagnosis may be made without confirmatory testing because EM is pathognomonic for LD.7,11 Serologic testing is not recommended in these cases, particularly if there is a single EM lesion, since the lesion often presents prior to development of an immune response leading to seronegative results.8
Lyme serology is necessary if a patient presents with ocular manifestations known to be associated with LD and resides in, or has recently traveled to, an area where LD is endemic (ie, New England, Minnesota, or Wisconsin).7,12 These criteria are of particular importance: about 50% of patients do not recall a tick bite and 20% to 40% do not present with an EM.2,9
Diagnostic Testing
In 2019 the Centers for Disease Control and Prevention (CDC) updated their testing guidelines to the modified 2-tier testing (MTTT) method. The MTTT first recommends a Lyme enzyme immunoassay (EIA), with a second EIA recommended only if the first is positive.12-14 The MTTT method has better sensitivity in early localized LD compared to standard 2-tier testing.9,11,12 The CDC advises against the use of any laboratory serology tests not approved by the US Food and Drug Administration.13 The CDC also advises that LD serology testing should not be performed as a “test for cure,” because even after successful treatment, an individual may still test positive.1,9 Follow-up testing in patients treated early in the disease course (ie, in the setting of EM) may never have an antibody response. In these cases, a negative test should not exclude an LD diagnosis. 9 For patients with suspected neuroborreliosis, a lumbar puncture may not be needed if a patient already has a positive peripheral serology via the MTTT method.12 The Figure depicts a flow chart for the process of ordering and interpreting testing.

Most LD testing, if correlated with clinical disease, is positive after 4 to 6 weeks.9 If an eye disease is noted and the patient has positive Lyme serology, the patient should still be screened for Lyme neuroborreliosis of the central nervous system (CNS). Examination of the fundus for papilledema, review of symptoms of aseptic meningitis, and a careful neurologic examination should be performed.15
If CNS disease is suspected, the patient may need additional CNS testing to support treatment decisions. The 2020 Infectious Diseases Society of America Lyme guidelines recommend to: (1) obtain simultaneous samples of cerebrospinal fluid (CSF) and serum for determination of the CSF:serum antibody index; (2) do not obtain CSF serology without measurement of the CSF:serum antibody index; and (3) do not obtain routine polymerase chain reaction or culture of CSF or serum.15 Once an LD diagnosis is confirmed, the CDC recommends a course of 100 mg of oral doxycycline twice daily for 14 to 21 days or an antimicrobial equivalent (eg, amoxicillin) if doxycycline is contraindicated. However, the antimicrobial dosage may vary depending on the stage of LD.11 Patients with confirmed neuroborreliosis should be admitted for 14 days of intravenous ceftriaxone or intravenous penicillin.2
CONCLUSIONS
To ensure timely diagnosis and treatment, eye care clinicians should be familiar with the appropriate diagnostic testing for patients suspected to have ocular manifestations of LD. For patients with suspected LD and a high pretest probability, clinicians should obtain a first-order Lyme EIA.12-14 If testing confirms LD, refer the patient to an infectious disease specialist for antimicrobial treatment and additional management.11
- Kullberg BJ, Vrijmoeth HD, van de Schoor F, Hovius JW. Lyme borreliosis: diagnosis and management. BMJ. 2020;369:m1041. doi:10.1136/bmj.m1041
- Zaidman GW. The ocular manifestations of Lyme disease. Int Ophthalmol Clin. 1993;33(1):9-22. doi:10.1097/00004397-199303310-00004
- Lesser RL. Ocular manifestations of Lyme disease. Am J Med. 1995; 98(4A):60S-62S. doi:10.1016/s0002-9343(99)80045-x
- Mead P. Epidemiology of Lyme disease. Infect Dis Clin North Am. 2022;36(3):495-521. doi:10.1016/j.idc.2022.03.004
- Klig JE. Ophthalmologic complications of systemic disease. Emerg Med Clin North Am. 2008;26(1):217-viii. doi:10.1016/j.emc.2007.10.003
- Raja H, Starr MR, Bakri SJ. Ocular manifestations of tickborne diseases. Surv Ophthalmol. 2016;61(6):726-744. doi:10.1016/j.survophthal.2016.03.011
- Mora P, Carta A. Ocular manifestations of Lyme borreliosis in Europe. Int J Med Sci. 2009;6(3):124-125. doi:10.7150/ijms.6.124
- Mikkilä HO, Seppälä IJ, Viljanen MK, Peltomaa MP, Karma A. The expanding clinical spectrum of ocular lyme borreliosis. Ophthalmology. 2000;107(3):581-587. doi:10.1016/s0161-6420(99)00128-1
- Schriefer ME. Lyme disease diagnosis: serology. Clin Lab Med. 2015;35(4):797-814. doi:10.1016/j.cll.2015.08.001
- Beck AR, Marx GE, Hinckley AF. Diagnosis, treatment, and prevention practices for Lyme disease by clinicians, United States, 2013-2015. Public Health Rep. 2021;136(5):609- 617. doi:10.1177/0033354920973235
- Wormser GP, McKenna D, Nowakowski J. Management approaches for suspected and established Lyme disease used at the Lyme disease diagnostic center. Wien Klin Wochenschr. 2018;130(15-16):463-467. doi:10.1007/s00508-015-0936-y
- Kobayashi T, Auwaerter PG. Diagnostic testing for Lyme disease. Infect Dis Clin North Am. 2022;36(3):605-620. doi:10.1016/j.idc.2022.04.001
- Mead P, Petersen J, Hinckley A. Updated CDC recommendation for serologic diagnosis of Lyme disease. MMWR Morb Mortal Wkly Rep. 2019;68(32):703. doi:10.15585/mmwr.mm6832a4
- Association of Public Health Laboratories. Suggested Reporting Language, Interpretation and Guidance Regarding Lyme Disease Serologic Test Results. April 2024. Accessed December 3, 2024. https://www.aphl.org/aboutAPHL/publications/Documents/ID-2024-Lyme-Disease-Serologic-Testing-Reporting.pdf
- Lantos PM, Rumbaugh P, Bockenstedt L, et al. Clinical practice guidelines by the Infectious Diseases Society of America (IDSA), American Academy of Neurology (AAN), and American College of Rheumatology (ACR): 2020 guidelines for the prevention, diagnosis and treatment of Lyme Disease. Clin Infect Dis. 2021;72(1):e1-e48. doi:10.1093/cid/ciaa1215
- Kullberg BJ, Vrijmoeth HD, van de Schoor F, Hovius JW. Lyme borreliosis: diagnosis and management. BMJ. 2020;369:m1041. doi:10.1136/bmj.m1041
- Zaidman GW. The ocular manifestations of Lyme disease. Int Ophthalmol Clin. 1993;33(1):9-22. doi:10.1097/00004397-199303310-00004
- Lesser RL. Ocular manifestations of Lyme disease. Am J Med. 1995; 98(4A):60S-62S. doi:10.1016/s0002-9343(99)80045-x
- Mead P. Epidemiology of Lyme disease. Infect Dis Clin North Am. 2022;36(3):495-521. doi:10.1016/j.idc.2022.03.004
- Klig JE. Ophthalmologic complications of systemic disease. Emerg Med Clin North Am. 2008;26(1):217-viii. doi:10.1016/j.emc.2007.10.003
- Raja H, Starr MR, Bakri SJ. Ocular manifestations of tickborne diseases. Surv Ophthalmol. 2016;61(6):726-744. doi:10.1016/j.survophthal.2016.03.011
- Mora P, Carta A. Ocular manifestations of Lyme borreliosis in Europe. Int J Med Sci. 2009;6(3):124-125. doi:10.7150/ijms.6.124
- Mikkilä HO, Seppälä IJ, Viljanen MK, Peltomaa MP, Karma A. The expanding clinical spectrum of ocular lyme borreliosis. Ophthalmology. 2000;107(3):581-587. doi:10.1016/s0161-6420(99)00128-1
- Schriefer ME. Lyme disease diagnosis: serology. Clin Lab Med. 2015;35(4):797-814. doi:10.1016/j.cll.2015.08.001
- Beck AR, Marx GE, Hinckley AF. Diagnosis, treatment, and prevention practices for Lyme disease by clinicians, United States, 2013-2015. Public Health Rep. 2021;136(5):609- 617. doi:10.1177/0033354920973235
- Wormser GP, McKenna D, Nowakowski J. Management approaches for suspected and established Lyme disease used at the Lyme disease diagnostic center. Wien Klin Wochenschr. 2018;130(15-16):463-467. doi:10.1007/s00508-015-0936-y
- Kobayashi T, Auwaerter PG. Diagnostic testing for Lyme disease. Infect Dis Clin North Am. 2022;36(3):605-620. doi:10.1016/j.idc.2022.04.001
- Mead P, Petersen J, Hinckley A. Updated CDC recommendation for serologic diagnosis of Lyme disease. MMWR Morb Mortal Wkly Rep. 2019;68(32):703. doi:10.15585/mmwr.mm6832a4
- Association of Public Health Laboratories. Suggested Reporting Language, Interpretation and Guidance Regarding Lyme Disease Serologic Test Results. April 2024. Accessed December 3, 2024. https://www.aphl.org/aboutAPHL/publications/Documents/ID-2024-Lyme-Disease-Serologic-Testing-Reporting.pdf
- Lantos PM, Rumbaugh P, Bockenstedt L, et al. Clinical practice guidelines by the Infectious Diseases Society of America (IDSA), American Academy of Neurology (AAN), and American College of Rheumatology (ACR): 2020 guidelines for the prevention, diagnosis and treatment of Lyme Disease. Clin Infect Dis. 2021;72(1):e1-e48. doi:10.1093/cid/ciaa1215
Diagnostic Testing for Patients With Suspected Ocular Manifestations of Lyme Disease
Diagnostic Testing for Patients With Suspected Ocular Manifestations of Lyme Disease
Sim and Learn: Simulation and its Value in Neurology Education
Sim and Learn: Simulation and its Value in Neurology Education
Clinical simulation is a technique, not a technology, used to replace or amplify real experiences with guided experiences that evoke or replicate substantial aspects of the real world in a fully interactive fashion.1 Simulation is widely used in medical education and spans a spectrum of sophistication, from simple reproduction of isolated body parts to high-fidelity human patient simulators that replicate whole body appearance and variable physiological parameters.2,3
Simulation-based medical education can be a valuable tool for safe health care delivery.4Simulation-based education is typically provided via 5 modalities: mannequins, computer-based mannequins, standardized patients, computer-based simulators, and software-based simulations. Simulation technology increases procedural skill by allowing for deliberate practice in a safe environment.5 Mastery learning is a stringent form of competency-based education that requires trainees to acquire clinical skill measured against a fixed achievement standard.6 In mastery learning, educational practice time varies but results are uniform. This approach improves patient outcomes and is more effective than clinical training alone.7-9
Advanced simulation models are helpful tools for neurologic education and training, especially for emergency department encounters.10 In recent years, advanced simulation models have been applied in various fields of medicine, especially emergency medicine and anesthesia.11-14
Acute neurology
In acute neurologic conditions (eg, stroke, intracerebral hemorrhage, status epilepticus, and neuromuscular respiratory failure) clinical outcomes are highly time dependent; consequently, a reduction in treatment delays can improve patient care. The application of simulation methodology allows trainees to address acute and potentially life-threatening emergencies in a safe, controlled, and reproducible environment. In addition to improving trainees’ knowledge base, simulation also helps to enhance team skills, communication, multidisciplinary collaboration, and leadership. Research has shown that deliberate practice leads to a decrease in clinical errors and improved procedural performance in the operating room.8,15 These results can be extrapolated to acute neurology settings to improve adherence to set protocols, thus streamlining management in acute settings.
Scenarios can be built to teach skills such as eliciting an appropriate history, establishing inclusion or exclusion criteria for the use of certain medications, evaluating neuroimaging and laboratory studies (while avoiding related common pitfalls), and managing treatment complications. Simulation also provides an opportunity for interprofessional education by training nurses and collaborative staff. It can be used to enhance nontechnical skills (eg, communication, situation awareness, decision making, and leadership) that further contribute to patient safety.
Simulation can be performed with the help of mannequins such as the SimMan 3G(Laerdal), which can display neurologic symptoms and physiological findings, or live actors who portray a patient by mimicking focal neurologic deficits.16,17 A briefing familiarizes the trainees with the equipment and explains the simulation process. The documentation and equipment are the same as that which is used in emergency departments or intensive care units.
Once the simulation is completed, a trainee’s performance is checked against a critical action checklist before a debriefing process during which the scenario is reviewed and learning goals are assessed. Immediate feedback is given to trainees to identify weaknesses and the simulation is repeated if multiple critical action items are missed. (Figure).17
RESIDENCY TRAINING
Simulation training in stroke is mandatory in some residency programs for neurology postgraduate year (PGY) 2 residents.18 These simulations are a part of a boot camp for incoming neurology residents after completing an internal medicine internship. The simulation program is not standardized across various training programs. The European Stroke Organization Simulation Committee has published an opinion paper with a consensus of experts about the implementation of simulation techniques in the stroke field.19,20 Residents participating in these mandatory programs are required to complete certification in the National Institutes of Health Stroke Scale (NIHSS) and the modified Rankin Scale, including a pretest that assesses their knowledge of acute stroke protocols prior to live simulation.17 A stepwise algorithm that incorporates faculty specialized in the field is used to evaluate and debrief the simulation.
Stroke vignettes are typically selected by the vascular neurology attending physician to cover thrombolytic therapy (indications and contraindications), mechanical thrombectomy, early arterial blood pressure management, anticoagulant reversal protocols, and management of thrombolytic complications (eg, neurologic worsening). Nursing staff is educated on the acute stroke protocol. Computed tomography (CT) and CT angiography scans are retrieved from teaching files. These are provided as live responses along with pertinent laboratory work, vital signs, and electrocardiogram tracings. Trainee performance is based on adherence to a critical action checklist, which includes (but is not limited to) identification of relative and absolute contraindications of thrombolytic treatments, estimation of NIHSS within 5 minutes of arrival, and consideration of candidacy for endovascular intervention.17
EVIDENCE FOR SIMULATION TRAINING
Simulations for acute ischemic stroke also improve cohesive teamwork to improve the door-to-needle and door-to-puncture time. A retrospective analysis involving first-year neurology residents at a comprehensive stroke center that compared patient cohort data before and after implementation of simulation training found that there was an improvement in door-to-needle time after implementation of stroke simulation training program by nearly 10 minutes.17 This was likely due to improvement in the comfort of the flow of management across multidisciplinary teams.
Discussing goals of care, communicating poor prognosis or complex decisions with distraught family members or patients requires practice. Simulation programs with video playback help focus on trainee’s body language, avoiding medical jargon and handling ethical dilemmas while adjusting the communication style to the patient’s personality.20 Enhanced communication skills improve patient satisfaction, trust, and adherence to treatments, all of which lead to better outcomes.21
Simulation has been effectively used as a training tool for recognizing and managing acute neuromuscular respiratory failure. These scenarios emphasize the importance of obtaining a focused clinical history, performing key neurological assessments (such as neck flexion strength and breath counting), evaluating pulmonary function tests, and identifying when to initiate ventilatory support.22 In a study designed as a simulation-based learning curriculum for status epilepticus, there was an improvement in the performance of PGY-2 residents after completing the curriculum from a median of 44.2% at pretest to 94.2% at posttest.23 In this curriculum, an emphasis was placed on the following: recognizing the delay in identification and treatment of status epilepticus; evaluating contraindications of certain antiseizure medication (ASM) based on history or laboratory work; giving first-line ASM within 5 minutes of seizure onset; airway and blood pressure assessment; suctioning the patient; use of second-line ASMs after first-line has failed; ordering a head CT and re-evaluating the case with postload ASM level; ordering a stat electroencephalography (EEG); and communicating the decision regarding patient disposition/level of care.24
There is a growing need for well designed simulation education programs targeted at the management of disorders requiring acute neurologic care, including not only stroke and status epilepticus, but also traumatic brain injury, subarachnoid hemorrhage, neuromuscular respiratory failure, flare of multiple sclerosis, acutely elevated intracranial pressure, malignant cerebral infarction, deterioration of Parkinson disease, and brain death evaluation with family counseling.25 This novel approach to teaching provides an opportunity to learn in addition to remediation with repetition of scenario and might be used for maintenance of recertification programs.
PROCEDURAL SKILLs
Perhaps one of the most studied uses for simulation in neurology is in procedural skills. This extends beyond neurology trainees and can include pulmonary critical care fellows, pediatric residents, and internal medicine residents receiving training in neurology-based procedures such as lumbar punctures (LPs). Other examples of neurology procedures and protocols in which simulation has been studied include fundoscopy, brain death evaluation, EEG interpretation in context of status epilepticus, and simulated stroke code responses. Additional procedures that lack research but may benefit from simulation-based training include the use of Doppler ultrasound and botulinum toxin injections practiced on mannequins.
Proficiency in LP procedural skills has been extensively studied by multiple institutions, with trainee levels ranging from medical students to fellows. One study in France enrolled 115 medical students without prior LP experience and randomized them to either a simulation or a control group.26 Those in the simulation group received instruction using a mannequin, and those in the control group received clinical training through hospital rotations. Both groups received an email containing literature-based information on the procedure as well as a self-assessment questionnaire before participating in either educational program.
The study showed that those students who received simulation training had a success rate of 67% on their first LP on a live patient compared with a success rate of 14% in those with traditional training. Students receiving simulation training required less assistance during the procedure from a supervisor and had higher satisfaction rates and confidence in their procedural skills.26
Another study of 128 medical students at the University of Pittsburgh found that a hybrid LP simulation significantly improved students’ confidence and perceived skill in performing LPs, obtaining informed consent, and electronic order entry. For example, confidence with LP increased from 5.95% presimulation to 90% postsimulation, with 58.24% of students reporting an improvement from minimal or no confidence to average or better (P < .001). Similarly, the proportion of students who felt able to perform LP with minimal or no assistance rose from 0% to 38.57% (P < .001). Confidence and perceived skill in obtaining informed consent and electronic order entry also saw significant gains. Although real-world skill assessments were limited by low survey response rates, preceptor evaluations and follow-up surveys suggested that students who participated in the simulation were more likely to perform these tasks independently or with minimal supervision during clinical rotations.27
Research on simulation training involving nonneurology residents is also encouraging. One study compared the LP skills of traditionally trained neurology residents (PGY-2 to PGY-4) to internal medicine residents (PGY-1) who underwent simulation on a mannequin.28 The internal medicine residents first underwent a pretest on LP performance, watched an educational video, underwent an LP demonstration, and practiced on a mannequin with feedback. The neurology residents completed the checklist-style pretest and performed an LP on a mannequin. Internal medicine residents were found to increase their pretest scores from a mean of 46.3% to 95.7% following training, whereas neurology residents scored a mean of 65.4%. More than half of neurology residents were unable to identify the correct anatomic location or standard cerebrospinal fluid (CSF) tests to be ordered on a routine LP.28
A pediatric resident study in Canada found that following simulation-based training, LP procedural skill improved in 15 of 16 residents tested, and PGY-1 residents showed a reduction in anxiety related to performing the procedure.29
Virtual Reality
An additional tool for simulation is the use of virtual reality (VR) in combination with mannequins. A French study used videos of LPs on actual patients, from equipment set up to final CSF collection and termination of the procedure.30 These videos included a 360-degree view of the procedure. The short video was administered through a VR device (the Oculus Go headset by Microsoft) or by a YouTube video (if VR was not desired).
Participants in the study watched the video then performed an LP on a mannequin. Those who used the VR option had minimal adverse effects (eg, low rates of cybersickness, blurred vision, nausea) and high satisfaction regarding their training environment.30Another VR-based program is the vascular intervention system trainer, which allows clinicians to use endovascular devices and simulate procedures such as thrombectomies. VR simulation is used for trainees and to retrain experienced physicians in performance of high-risk procedures.31
Fundoscopic and Ultrasound Simulations
The AR403 eye stimulator device for fundoscopic examinations is a mannequin-based simulation.32 In a single-center, prospective, single-blind study of neurology and pediatric neurology residents, trainees were split into control and intervention groups, with the intervention group receiving simulator training. Both groups received video lectures on fundoscopy techniques. Pre- and postintervention measurements included knowledge, skill, and total scores on the skills assessment. Of the 48 trainees who participated, the intervention group demonstrated significantly higher increases in skills (P = .01) and total (P = .02) scores, although knowledge scores did not improve. The intervention group also reported higher comfort levels, higher confidence, and higher success rates.
Areas that would benefit from simulation training and development include ultrasound training, such as transcranial Doppler evaluation. In a national survey of residents in anesthesia and critical care, trainees reported that simulation was not frequently used in ultrasound training and that bedside teaching was more common. Interestingly, there was a discrepancy between the opinions of residents and program directors. The program directors felt simulation was in fact used (18.2% of program directors reported this vs 5.3% of trainees).33
A new program, the NewroSim (Gaumard), is a computer-based model of cerebral perfusion that may be a useful tool in this setting. It can simulate blood flow velocities, including pathologic ones, both with a mannequin or without.34
Another potential area for development is the use of mannequins to teach botulinum toxin injections for migraine, dystonia and spasticity in a training environment This is typically led by pharmaceutical representatives who are not necessarily clinicians. Residents and fellows may benefit instead from clinician-led education during their training programs.
Simulation in Patient Communication
Simulation provides a realistic environment for teaching rapid decision-making, leadership, and appropriate management of acutely ill neurologic patients; this includes the communication skills needed in response to neurologic injury.35 Simulation can be particularly useful in situations involving brain death determination, where the communication techniques differ significantly from those used in shared decision-making. Simulation provides a low-stakes setting for clinicians to practice the process of brain death determination and communication, leading to improved confidence and knowledge.36
In the context of acute neurologic emergencies, simulation exercises have been used to investigate the consistency of prognostication across a spectrum of neurology physicians. These exercises revealed that acute neuroprognostication is highly variable and often inaccurate among neurology clinicians, suggesting a potential area for improvement through further simulation training.37
FUTURE DIRECTIONS
Simulation education in neurology can be directed towards learners at all levels, including medical students, residents, fellows, nurses, and medical technologists. In addition, simulation has great value to different disciplines, including emergency medicine, intensive care, and psychiatry. In our view simulation is not being used to full potential in neurology.
Simulation can be used to expose clinicians to rare pathology, play an integral role in competency-based evaluations, and serve as the foundation for simulation-based neurology curriculums, teleneurology simulation training programs, and team training for neurologic emergencies.38Another under-recognized aspect of neurology education is teaching interpersonal communication and professionalism. A survey conducted at a neurology department (20 residents and 73 faculty respondents) asked about residents’ comfort level in performing a number of interpersonal communication and professionalism tasks.38 While none of the residents said they were “very uncomfortable” with these tasks, only 1 resident reported being “very comfortable.” In addition, fewer than 50% noted that they had been directly observed by a faculty member while performing these tasks. The results prompted the facility to develop a simulation curriculum that including observation and feedback from 8 objective structured clinical examinations at a simulation center. A standardized professional simulated the role of a patient, caregiver, medical student, or a faculty member. Residents indicated in postsimulation surveys that it was very useful, and a majority voted for the activity to be repeated for future classes.38
Simulation models may also provide a more objective method to evaluate neurology residents. Accreditation Council for Graduate Medical Education has provided Milestones that are used for assessment of neurology residents. Most of the programs rely on end-of-rotation faculty evaluations. These are subjective evaluations, rely on chance evaluations and may not reflect the exact caliber of a trainee in different clinical situations. Simulation models can serve as alternatives to provide an objective and accurate assessment of resident’s competency in different neurologic scenarios.
In a study of PGY-4 neurology residents from 3 tertiary care academic medical centers were evaluated using simulation-based assessment. Their skills in identifying and managing status epilepticus were assessed via a simulation-based model and compared with clinical experience. No graduating neurology residents were able to meet or exceed the minimum passing score during the testing. It was suggested that end-of-rotation evaluations are inadequate for assigning level of Milestones.24 To move forward with use of simulation-based assessments, these models need to be trialed more extensively and validated.
Morris et al developed simulations for assessment in neurocritical care.39 Ten evaluative simulation cases were developed. Researchers reported on 64 trainee participants in 274 evaluative simulation scenarios. The participants were very satisfied with the cases, found them to be very realistic and appropriately difficult. Interrater reliability was acceptable for both checklist action items and global rating scales. The researchers concluded that they were able to demonstrate validity evidence via the 10 simulation cases for assessment in neurologic emergencies.39 It is the authors’ belief that the future of residents’ competency assessment should include more widespread use of similar simulation models.
Finally, VR and augmented reality (AR) have shown promise in various fields, including neurology. In neurology, these technologies are being explored for applications in rehabilitation, therapy, and medical training. Ongoing research aims to leverage these technologies for improved patient outcomes and medical education. Virtual simulations can recreate neurologic scenarios, allowing learners to interact with 3-dimensional (3D) models of the brain or experience virtual patient cases. AR can enhance traditional learning materials by overlaying digital information onto real-world objects, aiding in the understanding of complex neuroanatomy and medical concepts. These technologies contribute to more engaging and effective neurology education.39In a study of 84 graduate medical students divided into 3 groups, the first group attended a traditional lecture on neuroanatomy, the second group was shown VR-based 3D images, and the third group had a VR-based, interactive and stereoscopic session.40 Groups 2 and 3 showed the highest mean scores in evaluations and differed significantly from Group 1 (P < .05). Groups 2 and 3 did not differ significantly from each other. The researchers concluded that VR-based resources for teaching neuroanatomy fostered significantly higher learning when compared to the traditional methods.40
- Corvetto M, Bravo MP, Montaña R, et al. Simulación en educación médica: una sinopsis. Rev Med Chil. 2013;141:70-79. doi:10.4067/S0034-98872013000100010
- Lane JL, Slavin S, Ziv A. Simulation in medical education: a review. Simul Gaming. 2001;32:297-314. doi:10.1177/104687810103200302
- Bradley P. The history of simulation in medical education and possible future directions. Med Educ. 2006;40:254-262. doi:10.1111/j.1365-2929.2006.02394.x
- Jones F, Passos-Neto C, Melro Braghiroli O. Simulation in medical education: brief history and methodology. Princ Pract Clin Res J. 2015;1:46-54. doi:10.21801/ppcrj.2015.12.8
- Issenberg SB. Simulation technology for health care professional skills training and assessment. JAMA. 1999;28:861-866. doi:10.1001/jama.282.9.861
- McGaghie WC, Miller GE, Sajid AW, et al. Competency-based curriculum development on medical education: an introduction. Public Health Pap. 1978;68:11-91.
- Barsuk JH, Cohen ER, Feinglass J, et al. Use of simulation-based education to reduce catheter-related bloodstream infections. Arch Intern Med. 2009;169:1420-1423. doi:10.1001/archinternmed.2009.215
- Wayne DB, Didwania A, Feinglass J, et al. Simulation-based education improves quality of care during cardiac arrest team responses at an academic teaching hospital: a case-control study. Chest. 2008;133:56-61. doi:10.1378/chest.07-0131
- McGaghie WC, Issenberg SB, Cohen ER, et al. Does simulation-based medical education with deliberate practice yield better results than traditional clinical education? A meta-analytic comparative review of the evidence. Acad Med. 2011;86:706-711. doi:10.1097/ACM.0b013e318217e119
- Micieli G, Cavallini A, Santalucia P, et al. Simulation in neurology. Neurol Sci. 2015;36:1967-1971. doi:10.1007/s10072-015-2228-8
- Bond WF, Lammers RL, Spillane LL, et al. The use of simulation in emergency medicine: a research agenda. Acad Emerg Med. 2007;14:353-363. doi:10.1197/j.aem.2006.11.02112.
- McLaughlin SA, Doezema D, Sklar DP. Human simulation in emergency medicine training: a model curriculum. Acad Emerg Med. 2002;9:1310-1318. doi:10.1111/j.1553-2712.2002.tb01593.x
- Howard SK, Gaba DM, Fish KJ, et al. Anesthesia crisis resource management training: teaching anesthesiologists to handle critical incidents. Aviat Space Environ Med. 1992;63:763-770.
- Gaba DM. Anaesthesiology as a model for patient safety in health care. BMJ. 2000;320:785-788. doi:10.1136/bmj.320.7237.785
- Sedlack RE, Kolars JC. Computer simulator training enhances the competency of gastroenterology fellows at colonoscopy: results of a pilot study. Am J Gastroenterol. 2004;99:33-37. doi:10.1111/j.1572-0241.2004.04007.x
- Tchopev ZN, Nelson AE, Hunninghake JC, et al. Curriculum innovations: high-fidelity simulation of acute neurology enhances rising resident confidence: results from a multicohort study. Neurol Educ. 2022;1:e200022. doi:10.1212/ne9.0000000000200022
- Mehta T, Strauss S, Beland D, et al. Stroke simulation improves acute stroke management: a systems-based practice experience. J Grad Med Educ. 2018;10:57-62. doi:10.4300/JGME-D-17-00167.1
- Pergakis MB, Chang WTW, Tabatabai A, et al. Simulation-based assessment of graduate neurology trainees’ performance managing acute ischemic stroke. Neurology. 2021;97:e2414-e2422. doi:10.1212/WNL.0000000000012972
- Casolla B. Simulation for neurology training: acute setting and beyond. Rev Neurol (Paris). 2021;177:1207-1213. doi:10.1016/j.neurol.2021.03.008
- Casolla B, de Leciñana MA, Neves R, et al. Simulation training programs for acute stroke care: Objectives and standards of methodology. Eur Stroke J. 2020;5:328-335. doi:10.1177/2396987320971105
- Zolnierek KB, Dimatteo MR. Physician communication and patient adherence to treatment: a meta-analysis. Med Care. 2009;47:826-834.doi:10.1097/MLR.0b013e31819a5acc
- Patel RA, Mohl L, Paetow G, Maiser S. Acute neuromuscular respiratory weakness due to acute inflammatory demyelinating polyneuropathy (AIDP): a simulation scenario for neurology providers. MedEdPORTAL. 2019;15:10811. doi:10.15766/mep_2374-8265.10811
- Mikhaeil-Demo Y, Barsuk JH, Culler GW, et al. Use of a simulation-based mastery learning curriculum for neurology residents to improve the identification and management of status epilepticus. Epilepsy Behav. 2020;111:107247. doi:10.1016/j.yebeh.2020.107247
- Mikhaeil-Demo Y, Holmboe E, Gerard EE, et al. Simulation-based assessments and graduating neurology residents’ milestones: status epilepticus milestones. J Grad Med Educ. 2021;13:223-230. doi:10.4300/JGME-D-20-00832.1
- Hocker S, Wijdicks EFM, Feske SK, et al. Use of simulation in acute neurology training: point and counterpoint. Ann Neurol. 2015;78:337-342. doi:10.1002/ana.24473
- Gaubert S, Blet A, Dib F, et al. Positive effects of lumbar puncture simulation training for medical students in clinical practice. BMC Med Educ. 2021;21:1-6. doi:10.1186/S12909-020-02452-327.
- Yanta C, Knepper L, Van Deusen R, et al. The use of hybrid lumbar puncture simulation to teach entrustable professional activities during a medical student neurology clerkship. MedEdPublish (2016). 2021;9:266. doi:10.15694/mep.2020.000266.2
- Barsuk JH, Cohen ER, Caprio T, et al. Simulation-based education with mastery learning improves residents’ lumbar puncture skills. Neurology. 2012;79:132-137. doi:10.1212/WNL.0B013E31825DD39D
- McMillan HJ, Writer H, Moreau KA, et al. Lumbar puncture simulation in pediatric residency training: improving procedural competence and decreasing anxiety. BMC Med Educ. 2016;16:198. doi:10.1186/S12909-016-0722-1
- Vrillon A, Gonzales-Marabal L, Ceccaldi PF, et al. Using virtual reality in lumbar puncture training improves students learning experience. BMC Med Educ. 2022;22:244. doi:10.1186/S12909-022-03317-7
- Liebig T, Holtmannspötter M, Crossley R, et al. Metric-based virtual reality simulation: a paradigm shift in training for mechanical thrombectomy in acute stroke. Stroke. 2018;49:e239-e242.doi:10.1161/STROKEAHA.118.021089
- Gupta DK, Khandker N, Stacy K, et al. Utility of combining a simulation-based method with a lecture-based method for fundoscopy training in neurology residency. JAMA Neurol. 2017;74:1223-1227. doi:10.1001/JAMANEUROL.2017.2073
- Mongodi S, Bonomi F, Vaschetto R, et al. Point-of-care ultrasound training for residents in anaesthesia and critical care: results of a national survey comparing residents and training program directors’ perspectives. BMC Med Educ. 2022;22:647. doi:10.1186/S12909-022-03708-W
- Morris NA, Czeisler BM, Sarwal A. Simulation in neurocritical care: past, present, and future. Neurocrit Care. 2019;30:522-533. doi:10.1007/S12028-018-0629-2
- Wijdicks EFM, Hocker SE. A future for simulation in acute neurology. Semin Neurol. 2018;38:465-470. doi:10.1055/s-0038-1666986
- Kramer NM, O’Mahony S, Deamant C. Brain death determination and communication: an innovative approach using simulation and standardized patients. J Pain Symptom Manage. 2022;63:e765-e772. doi:10.1016/j.jpainsymman.2022.01.020
- Sloane KL, Miller JJ, Piquet A, et al. Prognostication in acute neurological emergencies. J Stroke Cerebrovasc Dis. 2022;31:106277. doi:10.1016/J.JSTROKECEREBROVASDIS.2021.106277
- Kurzweil AM, Lewis A, Pleninger P, et al. Education research: teaching and assessing communication and professionalism in neurology residency with simulation. Neurology. 2020;94:229-232. doi:10.1212/WNL.0000000000008895
- Morris NA, Chang WT, Tabatabai A, et al. Development of neurological emergency simulations for assessment: content evidence and response process. Neurocrit Care. 2021;35:389-396. doi:10.1007/S12028-020-01176-Y
- De Faria JWV, Teixeira MJ, De Moura Sousa Júnior L, et al. Virtual and stereoscopic anatomy: when virtual reality meets medical education. J Neurosurg. 2016;125:1105-1111. doi:10.3171/2015.8.JNS141563
Clinical simulation is a technique, not a technology, used to replace or amplify real experiences with guided experiences that evoke or replicate substantial aspects of the real world in a fully interactive fashion.1 Simulation is widely used in medical education and spans a spectrum of sophistication, from simple reproduction of isolated body parts to high-fidelity human patient simulators that replicate whole body appearance and variable physiological parameters.2,3
Simulation-based medical education can be a valuable tool for safe health care delivery.4Simulation-based education is typically provided via 5 modalities: mannequins, computer-based mannequins, standardized patients, computer-based simulators, and software-based simulations. Simulation technology increases procedural skill by allowing for deliberate practice in a safe environment.5 Mastery learning is a stringent form of competency-based education that requires trainees to acquire clinical skill measured against a fixed achievement standard.6 In mastery learning, educational practice time varies but results are uniform. This approach improves patient outcomes and is more effective than clinical training alone.7-9
Advanced simulation models are helpful tools for neurologic education and training, especially for emergency department encounters.10 In recent years, advanced simulation models have been applied in various fields of medicine, especially emergency medicine and anesthesia.11-14
Acute neurology
In acute neurologic conditions (eg, stroke, intracerebral hemorrhage, status epilepticus, and neuromuscular respiratory failure) clinical outcomes are highly time dependent; consequently, a reduction in treatment delays can improve patient care. The application of simulation methodology allows trainees to address acute and potentially life-threatening emergencies in a safe, controlled, and reproducible environment. In addition to improving trainees’ knowledge base, simulation also helps to enhance team skills, communication, multidisciplinary collaboration, and leadership. Research has shown that deliberate practice leads to a decrease in clinical errors and improved procedural performance in the operating room.8,15 These results can be extrapolated to acute neurology settings to improve adherence to set protocols, thus streamlining management in acute settings.
Scenarios can be built to teach skills such as eliciting an appropriate history, establishing inclusion or exclusion criteria for the use of certain medications, evaluating neuroimaging and laboratory studies (while avoiding related common pitfalls), and managing treatment complications. Simulation also provides an opportunity for interprofessional education by training nurses and collaborative staff. It can be used to enhance nontechnical skills (eg, communication, situation awareness, decision making, and leadership) that further contribute to patient safety.
Simulation can be performed with the help of mannequins such as the SimMan 3G(Laerdal), which can display neurologic symptoms and physiological findings, or live actors who portray a patient by mimicking focal neurologic deficits.16,17 A briefing familiarizes the trainees with the equipment and explains the simulation process. The documentation and equipment are the same as that which is used in emergency departments or intensive care units.
Once the simulation is completed, a trainee’s performance is checked against a critical action checklist before a debriefing process during which the scenario is reviewed and learning goals are assessed. Immediate feedback is given to trainees to identify weaknesses and the simulation is repeated if multiple critical action items are missed. (Figure).17

RESIDENCY TRAINING
Simulation training in stroke is mandatory in some residency programs for neurology postgraduate year (PGY) 2 residents.18 These simulations are a part of a boot camp for incoming neurology residents after completing an internal medicine internship. The simulation program is not standardized across various training programs. The European Stroke Organization Simulation Committee has published an opinion paper with a consensus of experts about the implementation of simulation techniques in the stroke field.19,20 Residents participating in these mandatory programs are required to complete certification in the National Institutes of Health Stroke Scale (NIHSS) and the modified Rankin Scale, including a pretest that assesses their knowledge of acute stroke protocols prior to live simulation.17 A stepwise algorithm that incorporates faculty specialized in the field is used to evaluate and debrief the simulation.
Stroke vignettes are typically selected by the vascular neurology attending physician to cover thrombolytic therapy (indications and contraindications), mechanical thrombectomy, early arterial blood pressure management, anticoagulant reversal protocols, and management of thrombolytic complications (eg, neurologic worsening). Nursing staff is educated on the acute stroke protocol. Computed tomography (CT) and CT angiography scans are retrieved from teaching files. These are provided as live responses along with pertinent laboratory work, vital signs, and electrocardiogram tracings. Trainee performance is based on adherence to a critical action checklist, which includes (but is not limited to) identification of relative and absolute contraindications of thrombolytic treatments, estimation of NIHSS within 5 minutes of arrival, and consideration of candidacy for endovascular intervention.17
EVIDENCE FOR SIMULATION TRAINING
Simulations for acute ischemic stroke also improve cohesive teamwork to improve the door-to-needle and door-to-puncture time. A retrospective analysis involving first-year neurology residents at a comprehensive stroke center that compared patient cohort data before and after implementation of simulation training found that there was an improvement in door-to-needle time after implementation of stroke simulation training program by nearly 10 minutes.17 This was likely due to improvement in the comfort of the flow of management across multidisciplinary teams.
Discussing goals of care, communicating poor prognosis or complex decisions with distraught family members or patients requires practice. Simulation programs with video playback help focus on trainee’s body language, avoiding medical jargon and handling ethical dilemmas while adjusting the communication style to the patient’s personality.20 Enhanced communication skills improve patient satisfaction, trust, and adherence to treatments, all of which lead to better outcomes.21
Simulation has been effectively used as a training tool for recognizing and managing acute neuromuscular respiratory failure. These scenarios emphasize the importance of obtaining a focused clinical history, performing key neurological assessments (such as neck flexion strength and breath counting), evaluating pulmonary function tests, and identifying when to initiate ventilatory support.22 In a study designed as a simulation-based learning curriculum for status epilepticus, there was an improvement in the performance of PGY-2 residents after completing the curriculum from a median of 44.2% at pretest to 94.2% at posttest.23 In this curriculum, an emphasis was placed on the following: recognizing the delay in identification and treatment of status epilepticus; evaluating contraindications of certain antiseizure medication (ASM) based on history or laboratory work; giving first-line ASM within 5 minutes of seizure onset; airway and blood pressure assessment; suctioning the patient; use of second-line ASMs after first-line has failed; ordering a head CT and re-evaluating the case with postload ASM level; ordering a stat electroencephalography (EEG); and communicating the decision regarding patient disposition/level of care.24
There is a growing need for well designed simulation education programs targeted at the management of disorders requiring acute neurologic care, including not only stroke and status epilepticus, but also traumatic brain injury, subarachnoid hemorrhage, neuromuscular respiratory failure, flare of multiple sclerosis, acutely elevated intracranial pressure, malignant cerebral infarction, deterioration of Parkinson disease, and brain death evaluation with family counseling.25 This novel approach to teaching provides an opportunity to learn in addition to remediation with repetition of scenario and might be used for maintenance of recertification programs.
PROCEDURAL SKILLs
Perhaps one of the most studied uses for simulation in neurology is in procedural skills. This extends beyond neurology trainees and can include pulmonary critical care fellows, pediatric residents, and internal medicine residents receiving training in neurology-based procedures such as lumbar punctures (LPs). Other examples of neurology procedures and protocols in which simulation has been studied include fundoscopy, brain death evaluation, EEG interpretation in context of status epilepticus, and simulated stroke code responses. Additional procedures that lack research but may benefit from simulation-based training include the use of Doppler ultrasound and botulinum toxin injections practiced on mannequins.
Proficiency in LP procedural skills has been extensively studied by multiple institutions, with trainee levels ranging from medical students to fellows. One study in France enrolled 115 medical students without prior LP experience and randomized them to either a simulation or a control group.26 Those in the simulation group received instruction using a mannequin, and those in the control group received clinical training through hospital rotations. Both groups received an email containing literature-based information on the procedure as well as a self-assessment questionnaire before participating in either educational program.
The study showed that those students who received simulation training had a success rate of 67% on their first LP on a live patient compared with a success rate of 14% in those with traditional training. Students receiving simulation training required less assistance during the procedure from a supervisor and had higher satisfaction rates and confidence in their procedural skills.26
Another study of 128 medical students at the University of Pittsburgh found that a hybrid LP simulation significantly improved students’ confidence and perceived skill in performing LPs, obtaining informed consent, and electronic order entry. For example, confidence with LP increased from 5.95% presimulation to 90% postsimulation, with 58.24% of students reporting an improvement from minimal or no confidence to average or better (P < .001). Similarly, the proportion of students who felt able to perform LP with minimal or no assistance rose from 0% to 38.57% (P < .001). Confidence and perceived skill in obtaining informed consent and electronic order entry also saw significant gains. Although real-world skill assessments were limited by low survey response rates, preceptor evaluations and follow-up surveys suggested that students who participated in the simulation were more likely to perform these tasks independently or with minimal supervision during clinical rotations.27
Research on simulation training involving nonneurology residents is also encouraging. One study compared the LP skills of traditionally trained neurology residents (PGY-2 to PGY-4) to internal medicine residents (PGY-1) who underwent simulation on a mannequin.28 The internal medicine residents first underwent a pretest on LP performance, watched an educational video, underwent an LP demonstration, and practiced on a mannequin with feedback. The neurology residents completed the checklist-style pretest and performed an LP on a mannequin. Internal medicine residents were found to increase their pretest scores from a mean of 46.3% to 95.7% following training, whereas neurology residents scored a mean of 65.4%. More than half of neurology residents were unable to identify the correct anatomic location or standard cerebrospinal fluid (CSF) tests to be ordered on a routine LP.28
A pediatric resident study in Canada found that following simulation-based training, LP procedural skill improved in 15 of 16 residents tested, and PGY-1 residents showed a reduction in anxiety related to performing the procedure.29
Virtual Reality
An additional tool for simulation is the use of virtual reality (VR) in combination with mannequins. A French study used videos of LPs on actual patients, from equipment set up to final CSF collection and termination of the procedure.30 These videos included a 360-degree view of the procedure. The short video was administered through a VR device (the Oculus Go headset by Microsoft) or by a YouTube video (if VR was not desired).
Participants in the study watched the video then performed an LP on a mannequin. Those who used the VR option had minimal adverse effects (eg, low rates of cybersickness, blurred vision, nausea) and high satisfaction regarding their training environment.30Another VR-based program is the vascular intervention system trainer, which allows clinicians to use endovascular devices and simulate procedures such as thrombectomies. VR simulation is used for trainees and to retrain experienced physicians in performance of high-risk procedures.31
Fundoscopic and Ultrasound Simulations
The AR403 eye stimulator device for fundoscopic examinations is a mannequin-based simulation.32 In a single-center, prospective, single-blind study of neurology and pediatric neurology residents, trainees were split into control and intervention groups, with the intervention group receiving simulator training. Both groups received video lectures on fundoscopy techniques. Pre- and postintervention measurements included knowledge, skill, and total scores on the skills assessment. Of the 48 trainees who participated, the intervention group demonstrated significantly higher increases in skills (P = .01) and total (P = .02) scores, although knowledge scores did not improve. The intervention group also reported higher comfort levels, higher confidence, and higher success rates.
Areas that would benefit from simulation training and development include ultrasound training, such as transcranial Doppler evaluation. In a national survey of residents in anesthesia and critical care, trainees reported that simulation was not frequently used in ultrasound training and that bedside teaching was more common. Interestingly, there was a discrepancy between the opinions of residents and program directors. The program directors felt simulation was in fact used (18.2% of program directors reported this vs 5.3% of trainees).33
A new program, the NewroSim (Gaumard), is a computer-based model of cerebral perfusion that may be a useful tool in this setting. It can simulate blood flow velocities, including pathologic ones, both with a mannequin or without.34
Another potential area for development is the use of mannequins to teach botulinum toxin injections for migraine, dystonia and spasticity in a training environment This is typically led by pharmaceutical representatives who are not necessarily clinicians. Residents and fellows may benefit instead from clinician-led education during their training programs.
Simulation in Patient Communication
Simulation provides a realistic environment for teaching rapid decision-making, leadership, and appropriate management of acutely ill neurologic patients; this includes the communication skills needed in response to neurologic injury.35 Simulation can be particularly useful in situations involving brain death determination, where the communication techniques differ significantly from those used in shared decision-making. Simulation provides a low-stakes setting for clinicians to practice the process of brain death determination and communication, leading to improved confidence and knowledge.36
In the context of acute neurologic emergencies, simulation exercises have been used to investigate the consistency of prognostication across a spectrum of neurology physicians. These exercises revealed that acute neuroprognostication is highly variable and often inaccurate among neurology clinicians, suggesting a potential area for improvement through further simulation training.37
FUTURE DIRECTIONS
Simulation education in neurology can be directed towards learners at all levels, including medical students, residents, fellows, nurses, and medical technologists. In addition, simulation has great value to different disciplines, including emergency medicine, intensive care, and psychiatry. In our view simulation is not being used to full potential in neurology.
Simulation can be used to expose clinicians to rare pathology, play an integral role in competency-based evaluations, and serve as the foundation for simulation-based neurology curriculums, teleneurology simulation training programs, and team training for neurologic emergencies.38Another under-recognized aspect of neurology education is teaching interpersonal communication and professionalism. A survey conducted at a neurology department (20 residents and 73 faculty respondents) asked about residents’ comfort level in performing a number of interpersonal communication and professionalism tasks.38 While none of the residents said they were “very uncomfortable” with these tasks, only 1 resident reported being “very comfortable.” In addition, fewer than 50% noted that they had been directly observed by a faculty member while performing these tasks. The results prompted the facility to develop a simulation curriculum that including observation and feedback from 8 objective structured clinical examinations at a simulation center. A standardized professional simulated the role of a patient, caregiver, medical student, or a faculty member. Residents indicated in postsimulation surveys that it was very useful, and a majority voted for the activity to be repeated for future classes.38
Simulation models may also provide a more objective method to evaluate neurology residents. Accreditation Council for Graduate Medical Education has provided Milestones that are used for assessment of neurology residents. Most of the programs rely on end-of-rotation faculty evaluations. These are subjective evaluations, rely on chance evaluations and may not reflect the exact caliber of a trainee in different clinical situations. Simulation models can serve as alternatives to provide an objective and accurate assessment of resident’s competency in different neurologic scenarios.
In a study of PGY-4 neurology residents from 3 tertiary care academic medical centers were evaluated using simulation-based assessment. Their skills in identifying and managing status epilepticus were assessed via a simulation-based model and compared with clinical experience. No graduating neurology residents were able to meet or exceed the minimum passing score during the testing. It was suggested that end-of-rotation evaluations are inadequate for assigning level of Milestones.24 To move forward with use of simulation-based assessments, these models need to be trialed more extensively and validated.
Morris et al developed simulations for assessment in neurocritical care.39 Ten evaluative simulation cases were developed. Researchers reported on 64 trainee participants in 274 evaluative simulation scenarios. The participants were very satisfied with the cases, found them to be very realistic and appropriately difficult. Interrater reliability was acceptable for both checklist action items and global rating scales. The researchers concluded that they were able to demonstrate validity evidence via the 10 simulation cases for assessment in neurologic emergencies.39 It is the authors’ belief that the future of residents’ competency assessment should include more widespread use of similar simulation models.
Finally, VR and augmented reality (AR) have shown promise in various fields, including neurology. In neurology, these technologies are being explored for applications in rehabilitation, therapy, and medical training. Ongoing research aims to leverage these technologies for improved patient outcomes and medical education. Virtual simulations can recreate neurologic scenarios, allowing learners to interact with 3-dimensional (3D) models of the brain or experience virtual patient cases. AR can enhance traditional learning materials by overlaying digital information onto real-world objects, aiding in the understanding of complex neuroanatomy and medical concepts. These technologies contribute to more engaging and effective neurology education.39In a study of 84 graduate medical students divided into 3 groups, the first group attended a traditional lecture on neuroanatomy, the second group was shown VR-based 3D images, and the third group had a VR-based, interactive and stereoscopic session.40 Groups 2 and 3 showed the highest mean scores in evaluations and differed significantly from Group 1 (P < .05). Groups 2 and 3 did not differ significantly from each other. The researchers concluded that VR-based resources for teaching neuroanatomy fostered significantly higher learning when compared to the traditional methods.40
Clinical simulation is a technique, not a technology, used to replace or amplify real experiences with guided experiences that evoke or replicate substantial aspects of the real world in a fully interactive fashion.1 Simulation is widely used in medical education and spans a spectrum of sophistication, from simple reproduction of isolated body parts to high-fidelity human patient simulators that replicate whole body appearance and variable physiological parameters.2,3
Simulation-based medical education can be a valuable tool for safe health care delivery.4Simulation-based education is typically provided via 5 modalities: mannequins, computer-based mannequins, standardized patients, computer-based simulators, and software-based simulations. Simulation technology increases procedural skill by allowing for deliberate practice in a safe environment.5 Mastery learning is a stringent form of competency-based education that requires trainees to acquire clinical skill measured against a fixed achievement standard.6 In mastery learning, educational practice time varies but results are uniform. This approach improves patient outcomes and is more effective than clinical training alone.7-9
Advanced simulation models are helpful tools for neurologic education and training, especially for emergency department encounters.10 In recent years, advanced simulation models have been applied in various fields of medicine, especially emergency medicine and anesthesia.11-14
Acute neurology
In acute neurologic conditions (eg, stroke, intracerebral hemorrhage, status epilepticus, and neuromuscular respiratory failure) clinical outcomes are highly time dependent; consequently, a reduction in treatment delays can improve patient care. The application of simulation methodology allows trainees to address acute and potentially life-threatening emergencies in a safe, controlled, and reproducible environment. In addition to improving trainees’ knowledge base, simulation also helps to enhance team skills, communication, multidisciplinary collaboration, and leadership. Research has shown that deliberate practice leads to a decrease in clinical errors and improved procedural performance in the operating room.8,15 These results can be extrapolated to acute neurology settings to improve adherence to set protocols, thus streamlining management in acute settings.
Scenarios can be built to teach skills such as eliciting an appropriate history, establishing inclusion or exclusion criteria for the use of certain medications, evaluating neuroimaging and laboratory studies (while avoiding related common pitfalls), and managing treatment complications. Simulation also provides an opportunity for interprofessional education by training nurses and collaborative staff. It can be used to enhance nontechnical skills (eg, communication, situation awareness, decision making, and leadership) that further contribute to patient safety.
Simulation can be performed with the help of mannequins such as the SimMan 3G(Laerdal), which can display neurologic symptoms and physiological findings, or live actors who portray a patient by mimicking focal neurologic deficits.16,17 A briefing familiarizes the trainees with the equipment and explains the simulation process. The documentation and equipment are the same as that which is used in emergency departments or intensive care units.
Once the simulation is completed, a trainee’s performance is checked against a critical action checklist before a debriefing process during which the scenario is reviewed and learning goals are assessed. Immediate feedback is given to trainees to identify weaknesses and the simulation is repeated if multiple critical action items are missed. (Figure).17

RESIDENCY TRAINING
Simulation training in stroke is mandatory in some residency programs for neurology postgraduate year (PGY) 2 residents.18 These simulations are a part of a boot camp for incoming neurology residents after completing an internal medicine internship. The simulation program is not standardized across various training programs. The European Stroke Organization Simulation Committee has published an opinion paper with a consensus of experts about the implementation of simulation techniques in the stroke field.19,20 Residents participating in these mandatory programs are required to complete certification in the National Institutes of Health Stroke Scale (NIHSS) and the modified Rankin Scale, including a pretest that assesses their knowledge of acute stroke protocols prior to live simulation.17 A stepwise algorithm that incorporates faculty specialized in the field is used to evaluate and debrief the simulation.
Stroke vignettes are typically selected by the vascular neurology attending physician to cover thrombolytic therapy (indications and contraindications), mechanical thrombectomy, early arterial blood pressure management, anticoagulant reversal protocols, and management of thrombolytic complications (eg, neurologic worsening). Nursing staff is educated on the acute stroke protocol. Computed tomography (CT) and CT angiography scans are retrieved from teaching files. These are provided as live responses along with pertinent laboratory work, vital signs, and electrocardiogram tracings. Trainee performance is based on adherence to a critical action checklist, which includes (but is not limited to) identification of relative and absolute contraindications of thrombolytic treatments, estimation of NIHSS within 5 minutes of arrival, and consideration of candidacy for endovascular intervention.17
EVIDENCE FOR SIMULATION TRAINING
Simulations for acute ischemic stroke also improve cohesive teamwork to improve the door-to-needle and door-to-puncture time. A retrospective analysis involving first-year neurology residents at a comprehensive stroke center that compared patient cohort data before and after implementation of simulation training found that there was an improvement in door-to-needle time after implementation of stroke simulation training program by nearly 10 minutes.17 This was likely due to improvement in the comfort of the flow of management across multidisciplinary teams.
Discussing goals of care, communicating poor prognosis or complex decisions with distraught family members or patients requires practice. Simulation programs with video playback help focus on trainee’s body language, avoiding medical jargon and handling ethical dilemmas while adjusting the communication style to the patient’s personality.20 Enhanced communication skills improve patient satisfaction, trust, and adherence to treatments, all of which lead to better outcomes.21
Simulation has been effectively used as a training tool for recognizing and managing acute neuromuscular respiratory failure. These scenarios emphasize the importance of obtaining a focused clinical history, performing key neurological assessments (such as neck flexion strength and breath counting), evaluating pulmonary function tests, and identifying when to initiate ventilatory support.22 In a study designed as a simulation-based learning curriculum for status epilepticus, there was an improvement in the performance of PGY-2 residents after completing the curriculum from a median of 44.2% at pretest to 94.2% at posttest.23 In this curriculum, an emphasis was placed on the following: recognizing the delay in identification and treatment of status epilepticus; evaluating contraindications of certain antiseizure medication (ASM) based on history or laboratory work; giving first-line ASM within 5 minutes of seizure onset; airway and blood pressure assessment; suctioning the patient; use of second-line ASMs after first-line has failed; ordering a head CT and re-evaluating the case with postload ASM level; ordering a stat electroencephalography (EEG); and communicating the decision regarding patient disposition/level of care.24
There is a growing need for well designed simulation education programs targeted at the management of disorders requiring acute neurologic care, including not only stroke and status epilepticus, but also traumatic brain injury, subarachnoid hemorrhage, neuromuscular respiratory failure, flare of multiple sclerosis, acutely elevated intracranial pressure, malignant cerebral infarction, deterioration of Parkinson disease, and brain death evaluation with family counseling.25 This novel approach to teaching provides an opportunity to learn in addition to remediation with repetition of scenario and might be used for maintenance of recertification programs.
PROCEDURAL SKILLs
Perhaps one of the most studied uses for simulation in neurology is in procedural skills. This extends beyond neurology trainees and can include pulmonary critical care fellows, pediatric residents, and internal medicine residents receiving training in neurology-based procedures such as lumbar punctures (LPs). Other examples of neurology procedures and protocols in which simulation has been studied include fundoscopy, brain death evaluation, EEG interpretation in context of status epilepticus, and simulated stroke code responses. Additional procedures that lack research but may benefit from simulation-based training include the use of Doppler ultrasound and botulinum toxin injections practiced on mannequins.
Proficiency in LP procedural skills has been extensively studied by multiple institutions, with trainee levels ranging from medical students to fellows. One study in France enrolled 115 medical students without prior LP experience and randomized them to either a simulation or a control group.26 Those in the simulation group received instruction using a mannequin, and those in the control group received clinical training through hospital rotations. Both groups received an email containing literature-based information on the procedure as well as a self-assessment questionnaire before participating in either educational program.
The study showed that those students who received simulation training had a success rate of 67% on their first LP on a live patient compared with a success rate of 14% in those with traditional training. Students receiving simulation training required less assistance during the procedure from a supervisor and had higher satisfaction rates and confidence in their procedural skills.26
Another study of 128 medical students at the University of Pittsburgh found that a hybrid LP simulation significantly improved students’ confidence and perceived skill in performing LPs, obtaining informed consent, and electronic order entry. For example, confidence with LP increased from 5.95% presimulation to 90% postsimulation, with 58.24% of students reporting an improvement from minimal or no confidence to average or better (P < .001). Similarly, the proportion of students who felt able to perform LP with minimal or no assistance rose from 0% to 38.57% (P < .001). Confidence and perceived skill in obtaining informed consent and electronic order entry also saw significant gains. Although real-world skill assessments were limited by low survey response rates, preceptor evaluations and follow-up surveys suggested that students who participated in the simulation were more likely to perform these tasks independently or with minimal supervision during clinical rotations.27
Research on simulation training involving nonneurology residents is also encouraging. One study compared the LP skills of traditionally trained neurology residents (PGY-2 to PGY-4) to internal medicine residents (PGY-1) who underwent simulation on a mannequin.28 The internal medicine residents first underwent a pretest on LP performance, watched an educational video, underwent an LP demonstration, and practiced on a mannequin with feedback. The neurology residents completed the checklist-style pretest and performed an LP on a mannequin. Internal medicine residents were found to increase their pretest scores from a mean of 46.3% to 95.7% following training, whereas neurology residents scored a mean of 65.4%. More than half of neurology residents were unable to identify the correct anatomic location or standard cerebrospinal fluid (CSF) tests to be ordered on a routine LP.28
A pediatric resident study in Canada found that following simulation-based training, LP procedural skill improved in 15 of 16 residents tested, and PGY-1 residents showed a reduction in anxiety related to performing the procedure.29
Virtual Reality
An additional tool for simulation is the use of virtual reality (VR) in combination with mannequins. A French study used videos of LPs on actual patients, from equipment set up to final CSF collection and termination of the procedure.30 These videos included a 360-degree view of the procedure. The short video was administered through a VR device (the Oculus Go headset by Microsoft) or by a YouTube video (if VR was not desired).
Participants in the study watched the video then performed an LP on a mannequin. Those who used the VR option had minimal adverse effects (eg, low rates of cybersickness, blurred vision, nausea) and high satisfaction regarding their training environment.30Another VR-based program is the vascular intervention system trainer, which allows clinicians to use endovascular devices and simulate procedures such as thrombectomies. VR simulation is used for trainees and to retrain experienced physicians in performance of high-risk procedures.31
Fundoscopic and Ultrasound Simulations
The AR403 eye stimulator device for fundoscopic examinations is a mannequin-based simulation.32 In a single-center, prospective, single-blind study of neurology and pediatric neurology residents, trainees were split into control and intervention groups, with the intervention group receiving simulator training. Both groups received video lectures on fundoscopy techniques. Pre- and postintervention measurements included knowledge, skill, and total scores on the skills assessment. Of the 48 trainees who participated, the intervention group demonstrated significantly higher increases in skills (P = .01) and total (P = .02) scores, although knowledge scores did not improve. The intervention group also reported higher comfort levels, higher confidence, and higher success rates.
Areas that would benefit from simulation training and development include ultrasound training, such as transcranial Doppler evaluation. In a national survey of residents in anesthesia and critical care, trainees reported that simulation was not frequently used in ultrasound training and that bedside teaching was more common. Interestingly, there was a discrepancy between the opinions of residents and program directors. The program directors felt simulation was in fact used (18.2% of program directors reported this vs 5.3% of trainees).33
A new program, the NewroSim (Gaumard), is a computer-based model of cerebral perfusion that may be a useful tool in this setting. It can simulate blood flow velocities, including pathologic ones, both with a mannequin or without.34
Another potential area for development is the use of mannequins to teach botulinum toxin injections for migraine, dystonia and spasticity in a training environment This is typically led by pharmaceutical representatives who are not necessarily clinicians. Residents and fellows may benefit instead from clinician-led education during their training programs.
Simulation in Patient Communication
Simulation provides a realistic environment for teaching rapid decision-making, leadership, and appropriate management of acutely ill neurologic patients; this includes the communication skills needed in response to neurologic injury.35 Simulation can be particularly useful in situations involving brain death determination, where the communication techniques differ significantly from those used in shared decision-making. Simulation provides a low-stakes setting for clinicians to practice the process of brain death determination and communication, leading to improved confidence and knowledge.36
In the context of acute neurologic emergencies, simulation exercises have been used to investigate the consistency of prognostication across a spectrum of neurology physicians. These exercises revealed that acute neuroprognostication is highly variable and often inaccurate among neurology clinicians, suggesting a potential area for improvement through further simulation training.37
FUTURE DIRECTIONS
Simulation education in neurology can be directed towards learners at all levels, including medical students, residents, fellows, nurses, and medical technologists. In addition, simulation has great value to different disciplines, including emergency medicine, intensive care, and psychiatry. In our view simulation is not being used to full potential in neurology.
Simulation can be used to expose clinicians to rare pathology, play an integral role in competency-based evaluations, and serve as the foundation for simulation-based neurology curriculums, teleneurology simulation training programs, and team training for neurologic emergencies.38Another under-recognized aspect of neurology education is teaching interpersonal communication and professionalism. A survey conducted at a neurology department (20 residents and 73 faculty respondents) asked about residents’ comfort level in performing a number of interpersonal communication and professionalism tasks.38 While none of the residents said they were “very uncomfortable” with these tasks, only 1 resident reported being “very comfortable.” In addition, fewer than 50% noted that they had been directly observed by a faculty member while performing these tasks. The results prompted the facility to develop a simulation curriculum that including observation and feedback from 8 objective structured clinical examinations at a simulation center. A standardized professional simulated the role of a patient, caregiver, medical student, or a faculty member. Residents indicated in postsimulation surveys that it was very useful, and a majority voted for the activity to be repeated for future classes.38
Simulation models may also provide a more objective method to evaluate neurology residents. Accreditation Council for Graduate Medical Education has provided Milestones that are used for assessment of neurology residents. Most of the programs rely on end-of-rotation faculty evaluations. These are subjective evaluations, rely on chance evaluations and may not reflect the exact caliber of a trainee in different clinical situations. Simulation models can serve as alternatives to provide an objective and accurate assessment of resident’s competency in different neurologic scenarios.
In a study of PGY-4 neurology residents from 3 tertiary care academic medical centers were evaluated using simulation-based assessment. Their skills in identifying and managing status epilepticus were assessed via a simulation-based model and compared with clinical experience. No graduating neurology residents were able to meet or exceed the minimum passing score during the testing. It was suggested that end-of-rotation evaluations are inadequate for assigning level of Milestones.24 To move forward with use of simulation-based assessments, these models need to be trialed more extensively and validated.
Morris et al developed simulations for assessment in neurocritical care.39 Ten evaluative simulation cases were developed. Researchers reported on 64 trainee participants in 274 evaluative simulation scenarios. The participants were very satisfied with the cases, found them to be very realistic and appropriately difficult. Interrater reliability was acceptable for both checklist action items and global rating scales. The researchers concluded that they were able to demonstrate validity evidence via the 10 simulation cases for assessment in neurologic emergencies.39 It is the authors’ belief that the future of residents’ competency assessment should include more widespread use of similar simulation models.
Finally, VR and augmented reality (AR) have shown promise in various fields, including neurology. In neurology, these technologies are being explored for applications in rehabilitation, therapy, and medical training. Ongoing research aims to leverage these technologies for improved patient outcomes and medical education. Virtual simulations can recreate neurologic scenarios, allowing learners to interact with 3-dimensional (3D) models of the brain or experience virtual patient cases. AR can enhance traditional learning materials by overlaying digital information onto real-world objects, aiding in the understanding of complex neuroanatomy and medical concepts. These technologies contribute to more engaging and effective neurology education.39In a study of 84 graduate medical students divided into 3 groups, the first group attended a traditional lecture on neuroanatomy, the second group was shown VR-based 3D images, and the third group had a VR-based, interactive and stereoscopic session.40 Groups 2 and 3 showed the highest mean scores in evaluations and differed significantly from Group 1 (P < .05). Groups 2 and 3 did not differ significantly from each other. The researchers concluded that VR-based resources for teaching neuroanatomy fostered significantly higher learning when compared to the traditional methods.40
- Corvetto M, Bravo MP, Montaña R, et al. Simulación en educación médica: una sinopsis. Rev Med Chil. 2013;141:70-79. doi:10.4067/S0034-98872013000100010
- Lane JL, Slavin S, Ziv A. Simulation in medical education: a review. Simul Gaming. 2001;32:297-314. doi:10.1177/104687810103200302
- Bradley P. The history of simulation in medical education and possible future directions. Med Educ. 2006;40:254-262. doi:10.1111/j.1365-2929.2006.02394.x
- Jones F, Passos-Neto C, Melro Braghiroli O. Simulation in medical education: brief history and methodology. Princ Pract Clin Res J. 2015;1:46-54. doi:10.21801/ppcrj.2015.12.8
- Issenberg SB. Simulation technology for health care professional skills training and assessment. JAMA. 1999;28:861-866. doi:10.1001/jama.282.9.861
- McGaghie WC, Miller GE, Sajid AW, et al. Competency-based curriculum development on medical education: an introduction. Public Health Pap. 1978;68:11-91.
- Barsuk JH, Cohen ER, Feinglass J, et al. Use of simulation-based education to reduce catheter-related bloodstream infections. Arch Intern Med. 2009;169:1420-1423. doi:10.1001/archinternmed.2009.215
- Wayne DB, Didwania A, Feinglass J, et al. Simulation-based education improves quality of care during cardiac arrest team responses at an academic teaching hospital: a case-control study. Chest. 2008;133:56-61. doi:10.1378/chest.07-0131
- McGaghie WC, Issenberg SB, Cohen ER, et al. Does simulation-based medical education with deliberate practice yield better results than traditional clinical education? A meta-analytic comparative review of the evidence. Acad Med. 2011;86:706-711. doi:10.1097/ACM.0b013e318217e119
- Micieli G, Cavallini A, Santalucia P, et al. Simulation in neurology. Neurol Sci. 2015;36:1967-1971. doi:10.1007/s10072-015-2228-8
- Bond WF, Lammers RL, Spillane LL, et al. The use of simulation in emergency medicine: a research agenda. Acad Emerg Med. 2007;14:353-363. doi:10.1197/j.aem.2006.11.02112.
- McLaughlin SA, Doezema D, Sklar DP. Human simulation in emergency medicine training: a model curriculum. Acad Emerg Med. 2002;9:1310-1318. doi:10.1111/j.1553-2712.2002.tb01593.x
- Howard SK, Gaba DM, Fish KJ, et al. Anesthesia crisis resource management training: teaching anesthesiologists to handle critical incidents. Aviat Space Environ Med. 1992;63:763-770.
- Gaba DM. Anaesthesiology as a model for patient safety in health care. BMJ. 2000;320:785-788. doi:10.1136/bmj.320.7237.785
- Sedlack RE, Kolars JC. Computer simulator training enhances the competency of gastroenterology fellows at colonoscopy: results of a pilot study. Am J Gastroenterol. 2004;99:33-37. doi:10.1111/j.1572-0241.2004.04007.x
- Tchopev ZN, Nelson AE, Hunninghake JC, et al. Curriculum innovations: high-fidelity simulation of acute neurology enhances rising resident confidence: results from a multicohort study. Neurol Educ. 2022;1:e200022. doi:10.1212/ne9.0000000000200022
- Mehta T, Strauss S, Beland D, et al. Stroke simulation improves acute stroke management: a systems-based practice experience. J Grad Med Educ. 2018;10:57-62. doi:10.4300/JGME-D-17-00167.1
- Pergakis MB, Chang WTW, Tabatabai A, et al. Simulation-based assessment of graduate neurology trainees’ performance managing acute ischemic stroke. Neurology. 2021;97:e2414-e2422. doi:10.1212/WNL.0000000000012972
- Casolla B. Simulation for neurology training: acute setting and beyond. Rev Neurol (Paris). 2021;177:1207-1213. doi:10.1016/j.neurol.2021.03.008
- Casolla B, de Leciñana MA, Neves R, et al. Simulation training programs for acute stroke care: Objectives and standards of methodology. Eur Stroke J. 2020;5:328-335. doi:10.1177/2396987320971105
- Zolnierek KB, Dimatteo MR. Physician communication and patient adherence to treatment: a meta-analysis. Med Care. 2009;47:826-834.doi:10.1097/MLR.0b013e31819a5acc
- Patel RA, Mohl L, Paetow G, Maiser S. Acute neuromuscular respiratory weakness due to acute inflammatory demyelinating polyneuropathy (AIDP): a simulation scenario for neurology providers. MedEdPORTAL. 2019;15:10811. doi:10.15766/mep_2374-8265.10811
- Mikhaeil-Demo Y, Barsuk JH, Culler GW, et al. Use of a simulation-based mastery learning curriculum for neurology residents to improve the identification and management of status epilepticus. Epilepsy Behav. 2020;111:107247. doi:10.1016/j.yebeh.2020.107247
- Mikhaeil-Demo Y, Holmboe E, Gerard EE, et al. Simulation-based assessments and graduating neurology residents’ milestones: status epilepticus milestones. J Grad Med Educ. 2021;13:223-230. doi:10.4300/JGME-D-20-00832.1
- Hocker S, Wijdicks EFM, Feske SK, et al. Use of simulation in acute neurology training: point and counterpoint. Ann Neurol. 2015;78:337-342. doi:10.1002/ana.24473
- Gaubert S, Blet A, Dib F, et al. Positive effects of lumbar puncture simulation training for medical students in clinical practice. BMC Med Educ. 2021;21:1-6. doi:10.1186/S12909-020-02452-327.
- Yanta C, Knepper L, Van Deusen R, et al. The use of hybrid lumbar puncture simulation to teach entrustable professional activities during a medical student neurology clerkship. MedEdPublish (2016). 2021;9:266. doi:10.15694/mep.2020.000266.2
- Barsuk JH, Cohen ER, Caprio T, et al. Simulation-based education with mastery learning improves residents’ lumbar puncture skills. Neurology. 2012;79:132-137. doi:10.1212/WNL.0B013E31825DD39D
- McMillan HJ, Writer H, Moreau KA, et al. Lumbar puncture simulation in pediatric residency training: improving procedural competence and decreasing anxiety. BMC Med Educ. 2016;16:198. doi:10.1186/S12909-016-0722-1
- Vrillon A, Gonzales-Marabal L, Ceccaldi PF, et al. Using virtual reality in lumbar puncture training improves students learning experience. BMC Med Educ. 2022;22:244. doi:10.1186/S12909-022-03317-7
- Liebig T, Holtmannspötter M, Crossley R, et al. Metric-based virtual reality simulation: a paradigm shift in training for mechanical thrombectomy in acute stroke. Stroke. 2018;49:e239-e242.doi:10.1161/STROKEAHA.118.021089
- Gupta DK, Khandker N, Stacy K, et al. Utility of combining a simulation-based method with a lecture-based method for fundoscopy training in neurology residency. JAMA Neurol. 2017;74:1223-1227. doi:10.1001/JAMANEUROL.2017.2073
- Mongodi S, Bonomi F, Vaschetto R, et al. Point-of-care ultrasound training for residents in anaesthesia and critical care: results of a national survey comparing residents and training program directors’ perspectives. BMC Med Educ. 2022;22:647. doi:10.1186/S12909-022-03708-W
- Morris NA, Czeisler BM, Sarwal A. Simulation in neurocritical care: past, present, and future. Neurocrit Care. 2019;30:522-533. doi:10.1007/S12028-018-0629-2
- Wijdicks EFM, Hocker SE. A future for simulation in acute neurology. Semin Neurol. 2018;38:465-470. doi:10.1055/s-0038-1666986
- Kramer NM, O’Mahony S, Deamant C. Brain death determination and communication: an innovative approach using simulation and standardized patients. J Pain Symptom Manage. 2022;63:e765-e772. doi:10.1016/j.jpainsymman.2022.01.020
- Sloane KL, Miller JJ, Piquet A, et al. Prognostication in acute neurological emergencies. J Stroke Cerebrovasc Dis. 2022;31:106277. doi:10.1016/J.JSTROKECEREBROVASDIS.2021.106277
- Kurzweil AM, Lewis A, Pleninger P, et al. Education research: teaching and assessing communication and professionalism in neurology residency with simulation. Neurology. 2020;94:229-232. doi:10.1212/WNL.0000000000008895
- Morris NA, Chang WT, Tabatabai A, et al. Development of neurological emergency simulations for assessment: content evidence and response process. Neurocrit Care. 2021;35:389-396. doi:10.1007/S12028-020-01176-Y
- De Faria JWV, Teixeira MJ, De Moura Sousa Júnior L, et al. Virtual and stereoscopic anatomy: when virtual reality meets medical education. J Neurosurg. 2016;125:1105-1111. doi:10.3171/2015.8.JNS141563
- Corvetto M, Bravo MP, Montaña R, et al. Simulación en educación médica: una sinopsis. Rev Med Chil. 2013;141:70-79. doi:10.4067/S0034-98872013000100010
- Lane JL, Slavin S, Ziv A. Simulation in medical education: a review. Simul Gaming. 2001;32:297-314. doi:10.1177/104687810103200302
- Bradley P. The history of simulation in medical education and possible future directions. Med Educ. 2006;40:254-262. doi:10.1111/j.1365-2929.2006.02394.x
- Jones F, Passos-Neto C, Melro Braghiroli O. Simulation in medical education: brief history and methodology. Princ Pract Clin Res J. 2015;1:46-54. doi:10.21801/ppcrj.2015.12.8
- Issenberg SB. Simulation technology for health care professional skills training and assessment. JAMA. 1999;28:861-866. doi:10.1001/jama.282.9.861
- McGaghie WC, Miller GE, Sajid AW, et al. Competency-based curriculum development on medical education: an introduction. Public Health Pap. 1978;68:11-91.
- Barsuk JH, Cohen ER, Feinglass J, et al. Use of simulation-based education to reduce catheter-related bloodstream infections. Arch Intern Med. 2009;169:1420-1423. doi:10.1001/archinternmed.2009.215
- Wayne DB, Didwania A, Feinglass J, et al. Simulation-based education improves quality of care during cardiac arrest team responses at an academic teaching hospital: a case-control study. Chest. 2008;133:56-61. doi:10.1378/chest.07-0131
- McGaghie WC, Issenberg SB, Cohen ER, et al. Does simulation-based medical education with deliberate practice yield better results than traditional clinical education? A meta-analytic comparative review of the evidence. Acad Med. 2011;86:706-711. doi:10.1097/ACM.0b013e318217e119
- Micieli G, Cavallini A, Santalucia P, et al. Simulation in neurology. Neurol Sci. 2015;36:1967-1971. doi:10.1007/s10072-015-2228-8
- Bond WF, Lammers RL, Spillane LL, et al. The use of simulation in emergency medicine: a research agenda. Acad Emerg Med. 2007;14:353-363. doi:10.1197/j.aem.2006.11.02112.
- McLaughlin SA, Doezema D, Sklar DP. Human simulation in emergency medicine training: a model curriculum. Acad Emerg Med. 2002;9:1310-1318. doi:10.1111/j.1553-2712.2002.tb01593.x
- Howard SK, Gaba DM, Fish KJ, et al. Anesthesia crisis resource management training: teaching anesthesiologists to handle critical incidents. Aviat Space Environ Med. 1992;63:763-770.
- Gaba DM. Anaesthesiology as a model for patient safety in health care. BMJ. 2000;320:785-788. doi:10.1136/bmj.320.7237.785
- Sedlack RE, Kolars JC. Computer simulator training enhances the competency of gastroenterology fellows at colonoscopy: results of a pilot study. Am J Gastroenterol. 2004;99:33-37. doi:10.1111/j.1572-0241.2004.04007.x
- Tchopev ZN, Nelson AE, Hunninghake JC, et al. Curriculum innovations: high-fidelity simulation of acute neurology enhances rising resident confidence: results from a multicohort study. Neurol Educ. 2022;1:e200022. doi:10.1212/ne9.0000000000200022
- Mehta T, Strauss S, Beland D, et al. Stroke simulation improves acute stroke management: a systems-based practice experience. J Grad Med Educ. 2018;10:57-62. doi:10.4300/JGME-D-17-00167.1
- Pergakis MB, Chang WTW, Tabatabai A, et al. Simulation-based assessment of graduate neurology trainees’ performance managing acute ischemic stroke. Neurology. 2021;97:e2414-e2422. doi:10.1212/WNL.0000000000012972
- Casolla B. Simulation for neurology training: acute setting and beyond. Rev Neurol (Paris). 2021;177:1207-1213. doi:10.1016/j.neurol.2021.03.008
- Casolla B, de Leciñana MA, Neves R, et al. Simulation training programs for acute stroke care: Objectives and standards of methodology. Eur Stroke J. 2020;5:328-335. doi:10.1177/2396987320971105
- Zolnierek KB, Dimatteo MR. Physician communication and patient adherence to treatment: a meta-analysis. Med Care. 2009;47:826-834.doi:10.1097/MLR.0b013e31819a5acc
- Patel RA, Mohl L, Paetow G, Maiser S. Acute neuromuscular respiratory weakness due to acute inflammatory demyelinating polyneuropathy (AIDP): a simulation scenario for neurology providers. MedEdPORTAL. 2019;15:10811. doi:10.15766/mep_2374-8265.10811
- Mikhaeil-Demo Y, Barsuk JH, Culler GW, et al. Use of a simulation-based mastery learning curriculum for neurology residents to improve the identification and management of status epilepticus. Epilepsy Behav. 2020;111:107247. doi:10.1016/j.yebeh.2020.107247
- Mikhaeil-Demo Y, Holmboe E, Gerard EE, et al. Simulation-based assessments and graduating neurology residents’ milestones: status epilepticus milestones. J Grad Med Educ. 2021;13:223-230. doi:10.4300/JGME-D-20-00832.1
- Hocker S, Wijdicks EFM, Feske SK, et al. Use of simulation in acute neurology training: point and counterpoint. Ann Neurol. 2015;78:337-342. doi:10.1002/ana.24473
- Gaubert S, Blet A, Dib F, et al. Positive effects of lumbar puncture simulation training for medical students in clinical practice. BMC Med Educ. 2021;21:1-6. doi:10.1186/S12909-020-02452-327.
- Yanta C, Knepper L, Van Deusen R, et al. The use of hybrid lumbar puncture simulation to teach entrustable professional activities during a medical student neurology clerkship. MedEdPublish (2016). 2021;9:266. doi:10.15694/mep.2020.000266.2
- Barsuk JH, Cohen ER, Caprio T, et al. Simulation-based education with mastery learning improves residents’ lumbar puncture skills. Neurology. 2012;79:132-137. doi:10.1212/WNL.0B013E31825DD39D
- McMillan HJ, Writer H, Moreau KA, et al. Lumbar puncture simulation in pediatric residency training: improving procedural competence and decreasing anxiety. BMC Med Educ. 2016;16:198. doi:10.1186/S12909-016-0722-1
- Vrillon A, Gonzales-Marabal L, Ceccaldi PF, et al. Using virtual reality in lumbar puncture training improves students learning experience. BMC Med Educ. 2022;22:244. doi:10.1186/S12909-022-03317-7
- Liebig T, Holtmannspötter M, Crossley R, et al. Metric-based virtual reality simulation: a paradigm shift in training for mechanical thrombectomy in acute stroke. Stroke. 2018;49:e239-e242.doi:10.1161/STROKEAHA.118.021089
- Gupta DK, Khandker N, Stacy K, et al. Utility of combining a simulation-based method with a lecture-based method for fundoscopy training in neurology residency. JAMA Neurol. 2017;74:1223-1227. doi:10.1001/JAMANEUROL.2017.2073
- Mongodi S, Bonomi F, Vaschetto R, et al. Point-of-care ultrasound training for residents in anaesthesia and critical care: results of a national survey comparing residents and training program directors’ perspectives. BMC Med Educ. 2022;22:647. doi:10.1186/S12909-022-03708-W
- Morris NA, Czeisler BM, Sarwal A. Simulation in neurocritical care: past, present, and future. Neurocrit Care. 2019;30:522-533. doi:10.1007/S12028-018-0629-2
- Wijdicks EFM, Hocker SE. A future for simulation in acute neurology. Semin Neurol. 2018;38:465-470. doi:10.1055/s-0038-1666986
- Kramer NM, O’Mahony S, Deamant C. Brain death determination and communication: an innovative approach using simulation and standardized patients. J Pain Symptom Manage. 2022;63:e765-e772. doi:10.1016/j.jpainsymman.2022.01.020
- Sloane KL, Miller JJ, Piquet A, et al. Prognostication in acute neurological emergencies. J Stroke Cerebrovasc Dis. 2022;31:106277. doi:10.1016/J.JSTROKECEREBROVASDIS.2021.106277
- Kurzweil AM, Lewis A, Pleninger P, et al. Education research: teaching and assessing communication and professionalism in neurology residency with simulation. Neurology. 2020;94:229-232. doi:10.1212/WNL.0000000000008895
- Morris NA, Chang WT, Tabatabai A, et al. Development of neurological emergency simulations for assessment: content evidence and response process. Neurocrit Care. 2021;35:389-396. doi:10.1007/S12028-020-01176-Y
- De Faria JWV, Teixeira MJ, De Moura Sousa Júnior L, et al. Virtual and stereoscopic anatomy: when virtual reality meets medical education. J Neurosurg. 2016;125:1105-1111. doi:10.3171/2015.8.JNS141563
Sim and Learn: Simulation and its Value in Neurology Education
Sim and Learn: Simulation and its Value in Neurology Education
Updates in Multiple Sclerosis Imaging
Updates in Multiple Sclerosis Imaging
Multiple sclerosis (MS) is a complex, chronic immune-mediated disease of the central nervous system characterized by focal inflammation, demyelination, and neurodegeneration. Magnetic resonance imaging (MRI), first incorporated into the McDonald Criteria for the diagnosis of MS in 2001, is an integral tool in the diagnosis, prognosis, and therapeutic monitoring of people with MS (PwMS).1
MRI research in MS is rapidly expanding and offers insights into the pathophysiology of MS with important implications for the routine clinical care of PwMS. At the Consortium of Multiple Sclerosis Centers 2024 Annual Meeting, the US Department of Veterans Affairs (VA) MS Centers of Excellence hosted an educational symposium highlighting MRI biomarkers in MS, including T2-lesions, chronic black holes (cBHs), brain atrophy, paramagnetic rim lesions (PRLs), and the central vein sign (CVS). The symposium also provided a brief overview of quantitative MRI techniques used to characterize MS lesion severity and research applications of these techniques. This clinical review summarizes the main points of that symposium with the goal of introducing key concepts to federal health care practitioners caring for PwMS.
MRI Biomarkers in MS
T2-lesions, Chronic Black Holes, and Brain Atrophy
Focal immune-mediated inflammation and demyelination in MS may be detected by MRI as hyperintense foci on T2-weighted (T2-w) imaging (eg, T2-w turbo spin echo or T2-w fluid attenuated inversion recovery sequences). These T2-lesions, critical for diagnosing MS, are typically ovoid and occur in the periventricular, juxtacortical, infratentorial spinal cord white matter (Figure 1A). T2-lesion number and volume show some association with disability and optic nerve.
Wattjes et al highlight 2 cases to demonstrate this point: a man aged 52 years with MS for 23 years and a woman aged 50 years with MS for 11 years. Despite having MS for a much shorter duration, the woman had worse disability due to a higher lesion number and volume.2 T2-lesion volume also impacts disability progression in PwMS. Gauthier et al compared the probability of progression in 3 women, all of whom were aged 39 years and had MS for 6 years. The profile with highest probability of disability progression had the highest quartile of T2-lesion volume.3 T2-lesion volume over 2 years correlates with worse scores on disability metrics such as the MS functional composite, paced auditory serial addition task, and brain volume.4 A 2024 systematic review and meta-analysis demonstrated that T2-lesion volume is significantly correlated with clinical disability in PwMS.5
Select T2-lesions are also hypointense on T1-w spin echo images and are known as cBHs (Figure 1B). Histologically, T2-lesions with cBHs have more severe architectural disruption than those without cBHs.6 cBH number and volume are significantly correlated with disability, regardless of the degree of hypointensity on T1-w imaging.5,7 A 10-year longitudinal study demonstrated that cBHs were associated with disease progression after 5 years while T2-lesion volume was not, indicating that cBHs may be a more accurate predictor of disability.8
Brain atrophy, another imaging biomarker of MS, affects both the cerebral white and gray matter. White matter fraction (the volume of white matter relative to the intracranial compartment volume) and gray matter fraction (the volume of gray matter relative to the intracranial compartment) are significantly lower among PwMS compared with healthy controls. In addition, gray matter fraction is lower among patients with primary and secondary progressive MS compared with those with relapsing-remitting MS, clinically isolated syndrome (CIS), and radiologically isolated syndrome (RIS). Gray matter fraction is also correlated with several motor and cognitive disability indices.9
Paramagnetic Rim Lesions
Neurologic worsening in PwMS occurs by 2 distinct mechanisms: relapse-associated worsening, a stepwise worsening of symptoms due to incomplete recovery following a relapse; and progression independent of relapse activity (PIRA), which is an irreversible neurologic deterioration in the absence of clinical or radiological relapses.10 PIRA is associated with neurodegeneration and predominates in both primary and secondary progressive MS. However, recent data demonstrated that PIRA may contribute to as much as 50% of disability worsening in relapsing MS and occurs early in the RMS disease course.10,11 Current high-efficacy disease modifying therapy, such as ocrelizumab, are extraordinarily successful at preventing focal inflammation and relapses but are less effective for preventing the slow march of disability progression characterizing PIRA.12,13 The prevention of PIRA is therefore an unmet treatment need.
Chronic active lesions (CALs) are an important driver of PIRA. When an acute gadolinium-enhancing lesion develops in PwMS, there are 3 possible fates of this lesion. The lesion may become chronically inactive, remyelinate, or transition to CALs.14 The histopathologic signature of CALs is compartmentalized, low-grade inflammation behind an intact blood-brain barrier with evidence of both active and chronic components.15 CALs may be found not only in cerebral white matter but also in the cerebral cortex and spinal cord.16,17 Combined MRI and histopathological studies have shown that iron-laden microglia/macrophages can be detected by susceptibility-based MRI as a rim of paramagnetic signal surrounding select T2-lesions.19 These PRLs represent an in vivo imaging biomarker of CAL (Figure 1C). According to the North American Imaging in MS Cooperative (NAIMS) consensus criteria, a PRL must surround at least two-thirds of the outer edge of a T2-lesion, be visible in ≥ 2 consecutive MRI slices, and cannot be contrast enhancing.20
PRLs can be visualized on multiple susceptibility-based imaging methods, including multiecho derived R2*/T2*, phase maps, susceptibility-weighted imaging, and quantitative susceptibility mapping.21-23 Retrospective analyses have shown no significant differences in sensitivity across these imaging modalities.24 Although first visualized with 7T MRI, PRLs may also be detected by 1.5T and 3T MRI with comparable sensitivities.25-27 However, there remains a significant knowledge gap regarding the accuracy of each imaging modality. Systematic, prospectively designed studies are needed to ascertain the comparative value of each method.
The presence of PRL is a poor prognostic indicator. PwMS without PRLs have higher levels of disability, are more likely to progress, and demonstrate greater gray matter atrophy and cognitive dysfunction when compared with PwMS with PRLs.27-29 Lesions with PRL tend to slowly expand, exhibit greater demyelination, and have diminished white matter integrity.21,22,30
PRLs may also be used as a diagnostic tool. PRLs are highly specific for MS/CIS with a 99.7% specificity and 98.4% positive predictive value, although the sensitivity is limited to 24%.31 Taken together, these data indicate that the presence of a PRL substantially increases the likelihood of an MS/CIS diagnosis, whereas the absence of a PRL does not exclude these diagnoses.
Several unanswered questions remain: Why do select acute MS lesions transition to CALs? How may investigators utilize PRLs as outcome measures in future clinical trials? How should PRLs be incorporated into the routine care of PwMS? As the role of this imaging biomarker is clarified both in the research and clinical settings, clinicians caring for PwMS can expect to increasingly encounter the topic of PRLs in the near future.
Central Vein Sign
A CVS is defined by the presence of a central vessel within a demyelinating plaque (Figure 1D). As early as the 1820s, MS plaques on gross pathology were noted to follow the course of a vessel. Early histological studies reported that up to 91% of MS plaques had a central vessel present.32 Lesion formation is dependent on the movement of lymphocytes and other inflammatory cells from the systemic circulation across the blood brain barrier into the perivascular space, a privileged site where immune cells interact with antigen presenting cells to launch an inflammatory cascade and eventual demyelinating lesion.33
CVS can be visualized on 1.5T, 3T and 7T MRI. However, 7T MRI is superior to 3T in the detection of CVS, with 85% of MS lesions having CVS visible compared with 45% on 3T.34 With advances in 7T MRI, fluid attenuated inversion recovery and T2* susceptibility, weighted sequences can be overlaid, allowing simultaneous visualization of the vessel and the demyelinating lesion. With higher density of parenchymal veins in the periventricular regions, the CVS is most seen in lesions of this territory but can also be present in juxtacortical, thalamic and infratentorial lesions with decreasing prevalence as these approach the cortex.35
MS lesions are more likely to have CVS than T2 hyperintense white matter lesions of other causes, with a large study reporting 78% of MS lesions were CVS positive. Further, CVS positive lesions can be found across all MS phenotypes including relapsing remitting, primary progressive, and secondary progressive.35 The CVS is also specific to MS lesions and is an effective tool for differentiating MS lesions from other common causes of T2 hyperintense lesions including chronic ischemic white matter disease,36 migraines,37 neuromyelitis optica spectrum disorders,38,39 Susac syndrome,40 and systemic autoimmune diseases (Behcet disease, systemic lupus erythematosus, and antiphospholipid syndrome).41
With CVS emerging as a promising radiographic biomarker for MS, NAIMS issued a consensus statement on necessary properties of a CVS. These criteria included appearance of a thin hypointense line or small dot, visualized in ≥ 2 perpendicular planes, with diameter < 2 mm, and running partially or entirely through the center of the lesion. They also clarified that lesions < 3 mm, confluent lesions, lesions with multiple vessels present or poorly visualized lesions were excluded.42
A shared CVS definition was a necessary step toward routine use of CVS as a radiographic biomarker and its incorporation in the 2024 revised McDonald criteria.43 Remaining limitations including 7T MRI is primarily available in research settings and the lack of consensus on a diagnostic threshold. There have been many proposed methods, including a 40% cut off,44 60% cut off,45 and Select 3* or Select 6* methods.46 The goal of each method is to optimize sensitivity and specificity while not compromising efficiency of MRI review for both neurologists and radiologists.
The CVS has significant potential as a radiographic biomarker for MS and may allow the early stages of MS to be differentiated from other common causes of white matter lesions on MRI. However, it remains unclear whether CVS holds prognostic value for patients, if CVS is suggestive of differing underlying pathology, or if the presence of a CVS is dynamic over time. Progress in these areas is anticipated as CVS is incorporated into routine clinical practice.
Quantitative MRI Techniques
In the research setting, several imaging modalities can be used to quantify the degree of microstructural injury in PwMS. The goal of these methods is to identify and quantify myelin and axonal damage, the major drivers of neurodegeneration. Among these methods, diffusion-based imaging is a measure of the amount of diffusion or fluid mobility across the tissues of the brain.47 Diffusion-weighted imaging (DWI) yields several parametric maps including axial diffusivity (AD), radial diffusivity (RD), and mean diffusivity (Figure 2 A, B, and C). These parametric maps provide information on different directions of water molecules’ movements. Myelin surrounds the axons preventing water molecules diffusion perpendicular to axons (RD) while axonal content prevents water diffusion horizontal to the axons (AD).Thus, AD is considered more specific to axonal injury, whereas RD is specific to myelin content.48 A higher value of any of these metrics is associated with a higher degree of tissue injury.
Although sensitive to axonal and myelin injury, AD and RD computed from single b-shell DWI experience several limitations including being affected by nonpathologic factors such as fiber orientation, distribution, and crossing, and by various nonmyelin specific pathologies including fluid accumulation during inflammation, myelin sheath thickness, and axonal intactness.48 Several multi b-shell methods have been developed to overcome diffusion imaging limitations. For example, work at the Nashville VA MS Center of Excellence has focused on the use of the multicompartment diffusion MRI with spherical mean technique (SMT). This method removes the orientation dependency of the diffusion MRI signal, increasing the signal-to-noise ratio and reducing biases from fiber undulation, crossing, and dispersion.49 SMT generates the apparent axonal volume fraction (Vax), which is a direct measure of axonal integrity with lower values indicating lower axonal content and higher tissue destruction (Figure 2D). Vax was previously validated in MS as a measure of axonal integrity.49
In terms of myelin, several other specific measures have been developed. Magnetization transfer ratio (MTR) is another measure of tissue integrity that has been validated as a measure of tissue injury in MS (Figure 2E).50,51 Zheng et al found that the percentage of lesions with low MTR was significantly higher among patients whose disease disability progressed compared with patients who did not.52Selective inversion recovery with quantitative magnetization transfer (SIR-qMT) was developed to account for the limitations of MTR, including its sensitivity to edema and axonal density.52 Germane to myelin measurements, SIR-qMT generates the macromolecular to free size ratio (PSR). PSR represents the ratio of protons bound to macromolecules (myelin) to free protons (Figure 2F). PSR is considered a marker of myelin integrity, with lower values correlating with disability severity and indicating higher tissue damage and lower myelin content. Previous studies from the Nashville VA MS Center of Excellence validated the use of SIR-qMT among patients with MS, CIS, RIS, and healthy controls.53
Quantitative MRI has several research applications in the field of MS. We demonstrated that PRL harbor a higher degree of myelin injury indicated by PSR compared with rimless lesions.54 These MRI techniques are also helpful to investigate tissues surrounding the lesions, called normal appearing white matter (NAWM). Using quantitative MRI techniques such as MTR,52 PSR,53 and Vax,49 investigators have demonstrated that NAWM is injured in PwMS, and proximal NAWM may have higher degree of tissue damage compared with distant NAWM.55
Anticipated Innovations and Challenges
In the field of quantitative MRI, several new techniques are being adopted. Researchers are developing techniques such as myelin water fraction which evaluates the interaction between water and protons to measure myelin content. This is considered an advancement as it takes into account edema resulting from MS injury.56 Another example is multicompartment diffusion imaging, such as standard model imaging,57 and neurite orientation dispersion and density imaging,58 which considers water as an additional compartment compared with the SMT derived Vax. For PRL identification, more advanced methodologic techniques are developing such quantitative susceptibility mapping (QSM), which can detect iron deposits that surround the lesions with relatively high sensitivity and specificity of identifying PRL.59
Despite these innovations, several challenges remain before possible incorporation into the clinical setting. These limitations include longer scan time, familiarity of clinicians in using these maps, higher financial cost, and the necessity of advanced imaging processing skills. Artificial intelligence is a promising tool that may overcome these challenges through creating automated processing pipelines and developing synthetic maps without the need for additional acquisition.60
Conclusions
MRI is the most important tool for diagnosing and treating PwMS. Imaging biomarkers such as T2-lesions, cBHs, brain atrophy, PRLs, and CVS provide insight into the disease’s pathogenesis and are invaluable for the accurate diagnosis and prognostication of MS. Quantitative MRI techniques, while not available in the clinical setting, are important tools for translational research that may help direct the development of future therapeutics. In the near future, clinicians caring for PwMS should expect to encounter these imaging biomarkers more frequently in the clinical setting, especially with the inclusion of PRLs and CVS in the next iteration of the McDonald diagnostic criteria.
McDonald WI, Compston A, Edan G, et al. Recommended diagnostic criteria for multiple sclerosis: guidelines from the International Panel on the diagnosis of multiple sclerosis. Ann Neurol. 2001;50:121-127. doi:10.1002/ana.1032
Wattjes MP, Steenwijk MD, Stangel M. MRI in the diagnosis and monitoring of multiple sclerosis: an update. Clin Neuroradiol. 2015;25:157-165. doi:10.1007/s00062-015-0430-y
Gauthier SA, Mandel M, Guttmann CR, et al. Predicting short-term disability in multiple sclerosis. Neurology. 2007;68:2059-2065.doi:10.1212/01.wnl.0000264890.97479.b1
Rudick RA, Lee JC, Simon J, Fisher E. Significance of T2 lesions in multiple sclerosis: a 13-year longitudinal study. Ann Neurol. 2006;60:236-242. doi:10.1002/ana.20883
Nabizadeh F, Zafari R, Mohamadi M, et al. MRI features and disability in multiple sclerosis: a systematic review and meta-analysis. J Neuroradiol. 2024;51:24-37. doi:10.1016/j.neurad.2023.11.007
Bagnato F, Jeffries N, Richert ND, et al. Evolution of T1 black holes in patients with multiple sclerosis imaged monthly for 4 years. Brain. 2003;126:1782-1789. doi:10.1093/brain/awg182
Jacobsen C, Hagemeier J, Myhr KM, et al. Brain atrophy and disability progression in multiple sclerosis patients: a 10-year follow-up study. J Neurol Neurosurg Psychiatry. 2014;85:1109-1115. doi:10.1136/jnnp-2013-306906
Rovaris M, Gass A, Bammer R, et al. Diffusion MRI in multiple sclerosis. Neurology. 2005;65:1526-1532. doi:10.1212/01.wnl.0000184471.83948.e0
Fisniku LK, Chard DT, Jackson JS, et al. Gray matter atrophy is related to long-term disability in multiple sclerosis. Ann Neurol. 2008;64:247-254. doi:10.1002/ana.21423
Lublin FD, Häring DA, Ganjgahi H, et al. How patients with multiple sclerosis acquire disability. Brain. 2022;145:3147-3161. doi:10.1093/brain/awac016
Kappos L, Wolinsky JS, Giovannoni G, et al. Contribution of relapse-independent progression vs relapse-associated worsening to overall confirmed disability accumulation in typical relapsing multiple sclerosis in a pooled analysis of 2 randomized clinical trials. JAMA Neurol. 2020;77:1132-1140. doi:10.1001/jamaneurol.2020.1568
Hauser SL, Bar-Or A, Comi G, et al. Ocrelizumab versus interferon beta-1a in relapsing multiple sclerosis. N Engl J Med. 2017;376:221-234. doi:10.1056/NEJMoa1601277
Montalban X, Hauser SL, Kappos L, et al. Ocrelizumab versus placebo in primary progressive multiple sclerosis. N Engl J Med. 2017;376:209-220. doi:10.1056/NEJMoa1606468
Prineas JW, Kwon EE, Cho ES, et al. Immunopathology of secondary-progressive multiple sclerosis. Ann Neurol. 2001;50:646-657. doi:10.1002/ana.1255
Kuhlmann T, Ludwin S, Prat A, Antel J, Brück W, Lassmann H. An updated histological classification system for multiple sclerosis lesions. Acta Neuropathol. 2017;133:13-24. doi:10.1007/s00401-016-1653-y
Pitt D, Boster A, Pei W, et al. Imaging cortical lesions in multiple sclerosis with ultra-high-field magnetic resonance imaging. Arch Neurol. 2010;67:812-818. doi:10.1001/archneurol.2010.148
Gilmore CP, Geurts JJ, Evangelou N, et al. Spinal cord grey matter lesions in multiple sclerosis detected by post-mortem high field MR imaging. Mult Scler. 2009;15:180-188. doi:10.1177/1352458508096876
Lassmann H, Brück W, Lucchinetti CF. The immunopathology of multiple sclerosis: an overview. Brain Pathol. 2007;17:210-218. doi:10.1111/j.1750-3639.2007.00064.x
Bagnato F, Hametner S, Yao B, et al. Tracking iron in multiple sclerosis: a combined imaging and histopathological study at 7 Tesla. Brain. 2011;134:3602-3615. doi:10.1093/brain/awr278
Bagnato F, Sati P, Hemond CC, et al. Imaging chronic active lesions in multiple sclerosis: a consensus statement. Brain. 2024;147:2913-2933. doi:10.1093/brain/awae013
Dal-Bianco A, Grabner G, Kronnerwetter C, et al. Slow expansion of multiple sclerosis iron rim lesions: pathology and 7 T magnetic resonance imaging. Acta Neuropathol. 2017;133:25-42. doi:10.1007/s00401-016-1636-z
Absinta M, Sati P, Schindler M, et al. Persistent 7-tesla phase rim predicts poor outcome in new multiple sclerosis patient lesions. J Clin Invest. 2016;126:2597-2609. doi:10.1172/JCI86198
Gillen KM, Mubarak M, Park C, et al. QSM is an imaging biomarker for chronic glial activation in multiple sclerosis lesions. Ann Clin Transl Neurol. 2021;8:877-886. doi:10.1002/acn3.51338
Ng Kee Kwong KC, Mollison D, Meijboom R, et al. The prevalence of paramagnetic rim lesions in multiple sclerosis: a systematic review and meta-analysis. PLoS One. 2021;16:e0256845. doi:10.1371/journal.pone.0256845
Absinta M, Sati P, Fechner A, et al. Identification of chronic active multiple sclerosis lesions on 3T MRI. AJNR Am J Neuroradiol. 2018;39:1233-1238. doi:10.3174/ajnr.A5660
Hemond CC, Reich DS, Dundamadappa SK. Paramagnetic rim lesions in multiple sclerosis: comparison of visualization at 1.5-T and 3-T MRI. AJR Am J Roentgenol. 2022;219:120-131. doi:10.2214/AJR.21.26777
Altokhis AI, Hibbert AM, Allen CM, et al. Longitudinal clinical study of patients with iron rim lesions in multiple sclerosis. Mult Scler. 2022;28:2202-2211. doi:10.1177/13524585221114750
Choi S, Lake S, Harrison DM. Evaluation of the blood-brain barrier, demyelination, and neurodegeneration in paramagnetic rim lesions in multiple sclerosis on 7 tesla MRI. J Magn Reson Imaging. 2024;59:941-951. doi:10.1002/jmri.28847
Kazimuddin HF, Wang J, Hernandez B, et al. Paramagnetic rim lesions and their relationship with neurodegeneration and clinical disability at the time of multiple sclerosis diagnosis. Poster presented at: 2024 Americas Committee for Treatment and Research in Multiple Sclerosis (ACTRIMS) Forum; February 26-March 2; West Palm Beach, FL.
Rohm Z, Koch C, Kazimuddin H, et al. Longitudinal characterization of paramagnetic rim lesions in early multiple sclerosis. Poster presented at: 2024 Americas Committee for Treatment and Research in Multiple Sclerosis (ACTRIMS) Forum; February 26-March 2; West Palm Beach, FL.
Meaton I, Altokhis A, Allen CM, et al. Paramagnetic rims are a promising diagnostic imaging biomarker in multiple sclerosis. Mult Scler. 2022;28:2212-2220. doi:10.1177/13524585221118677
Fog T. On the vessel-plaque relationships in the brain in multiple sclerosis. Acta Neurol Scand Suppl. 1964;40:9-15.
Ineichen BV, Okar SV, Proulx ST, et al. Perivascular spaces and their role in neuroinflammation. Neuron. 2022;110:3566-3581. doi:10.1016/j.neuron.2022.10.024
Tallantyre EC, Morgan PS, Dixon JE, et al. A comparison of 3T and 7T in the detection of small parenchymal veins within MS lesions. Invest Radiol. 2009;44:491-494. doi:10.1097/RLI.0b013e3181b4c144
Kilsdonk ID, Lopez-Soriano A, Kuijer JP, et al. Morphological features of MS lesions on FLAIR* at 7 T and their relation to patient characteristics. J Neurol. 2014;261:1356-1364. doi:10.1007/s00415-014-7351-6
Tallantyre EC, Dixon JE, Donaldson I, et al. Ultra-high-field imaging distinguishes MS lesions from asymptomatic white matter lesions. Neurology. 2011;76:534-539. doi:10.1212/WNL.0b013e31820b7630
Solomon AJ, Schindler MK, Howard DB, et al. “Central vessel sign” on 3T FLAIR* MRI for the differentiation of multiple sclerosis from migraine. Ann Clin Transl Neurol. 2015;3:82-87. doi:10.1002/acn3.273
Sinnecker T, Dörr J, Pfueller CF, et al. Distinct lesion morphology at 7-T MRI differentiates neuromyelitis optica from multiple sclerosis. Neurology. 2012;79:708-714. doi:10.1212/WNL.0b013e3182648bc8
Kister I, Herbert J, Zhou Y, Ge Y. Ultrahigh-field MR (7 T) imaging of brain lesions in neuromyelitis optica. Mult Scler Int. 2013;2013:398259. doi:10.1155/2013/398259
Wuerfel J, Sinnecker T, Ringelstein EB, et al. Lesion morphology at 7 Tesla MRI differentiates Susac syndrome from multiple sclerosis. Mult Scler. 2012;18:1592-1599. doi:10.1177/1352458512441270
Massacesi L. Perivenular distribution of white matter lesions evaluated by MRI can differentiate MS lesions from inflammatory small vessel diseases. Eur J Neurol. 2016;23:86. doi:10.1212/WNL.86.16_supplement.P6.121
Sati P, Oh J, Constable RT, et al. The central vein sign and its clinical evaluation for the diagnosis of multiple sclerosis: a consensus statement from the North American Imaging in Multiple Sclerosis Cooperative. Nat Rev Neurol. 2016;12:714-722. doi:10.1038/nrneurol.2016.166
Montalban X, Lebrun-Frénay C, Oh J, et al. Diagnosis of multiple sclerosis: 2024 revisions of the McDonald criteria. Lancet Neurol. 2025;24:850-865. doi:10.1016/S1474-4422(25)00270-4
Mistry N, Dixon J, Tallantyre E, et al. Central veins in brain lesions visualized with high-field magnetic resonance imaging: a pathologically specific diagnostic biomarker for inflammatory demyelination in the brain. JAMA Neurol. 2013;70:623-628. doi:10.1001/jamaneurol.2013.1405
Campion T, Smith RJP, Altmann DR, et al. FLAIR* to visualize veins in white matter lesions: a new tool for the diagnosis of multiple sclerosis? Eur Radiol. 2017;27:4257-4263. doi:10.1007/s00330-017-4822-z
Solomon AJ, Watts R, Ontaneda D, et al. Diagnostic performance of central vein sign for multiple sclerosis with a simplified three-lesion algorithm. Mult Scler. 2018;24:750-757. doi:10.1177/1352458517726383
Cercignani M, Bozzali M, Iannucci G, Comi G, Filippi M. Intra-voxel and inter-voxel coherence in patients with multiple sclerosis assessed using diffusion tensor MRI. J Neurol. 2002;249:875-883. doi:10.1007/s00415-002-0752-y
Song SK, Yoshino J, Le TQ, et al. Demyelination increases radial diffusivity in corpus callosum of mouse brain. Neuroimage. 2005;26:132-140. doi:10.1016/j.neuroimage.2005.01.028
Bagnato F, Franco G, Li H, et al. Probing axons using multi-compartmental diffusion in multiple sclerosis. Ann Clin Transl Neurol. 2019;6:1595-1605. doi:10.1002/acn3.50836
Filippi M, Cercignani M, Inglese M, et al. Diffusion tensor magnetic resonance imaging in multiple sclerosis. Neurology. 2001;56:304-311. doi:10.1212/wnl.56.3.304
Bagnato F. Clinical application of magnetization transfer imaging. In: Advanced Neuro MR Techniques and Applications. Elsevier; 2022:403-417. doi:10.1016/B978-0-12-822479-3.00041-5
Zheng Y, Lee JC, Rudick R, Fisher E. Long-term magnetization transfer ratio evolution in multiple sclerosis white matter lesions. J Neuroimaging. 2018;28:191-198. doi:10.1111/jon.12480
Bagnato F, Hametner S, Franco G, et al. Selective inversion recovery quantitative magnetization transfer brain MRI at 7T: clinical and postmortem validation in multiple sclerosis. J Neuroimaging. 2018;28:380-388. doi:10.1111/jon.12511
Clarke MA, Cheek R, Hernandez B, et al. Paramagnetic rim lesions and the central vein sign: characterizing multiple sclerosis imaging markers. J Neuroimaging. 2024;34:86-94. doi:10.1111/jon.13173
Clarke MA, Lakhani DA, Wen S, et al. Perilesional neurodegenerative injury in multiple sclerosis: relation to focal lesions and impact on disability. Mult Scler Relat Disord. 2021;49:102738. doi:10.1016/j.msard.2021.102738
Laule C, Moore GRW. Myelin water imaging to detect demyelination and remyelination and its validation in pathology. Brain Pathol. 2018;28:750-764. doi:10.1111/bpa.12645
Coelho S, Baete SH, Lemberskiy G, et al. Reproducibility of the standard model of diffusion in white matter on clinical MRI systems. Neuroimage. 2022;257:119290. doi:10.1016/j.neuroimage.2022.119290
Novikov DS, Veraart J, Jelescu IO, et al. Rotationally-invariant mapping of scalar and orientational metrics of neuronal microstructure with diffusion MRI. Neuroimage. 2018;174:518-538. doi:10.1016/j.neuroimage.2018.03.006
Langkammer C, Liu T, Khalil M, et al. Quantitative susceptibility mapping in multiple sclerosis. Radiology. 2013;267:551-559. doi:10.1148/radiol.12120707
Collorone S, Coll L, Lorenzi M, et al. Artificial intelligence applied to MRI data to tackle key challenges in multiple sclerosis. Mult Scler. 2024;30:767-784. doi:10.1177/13524585241249422
Multiple sclerosis (MS) is a complex, chronic immune-mediated disease of the central nervous system characterized by focal inflammation, demyelination, and neurodegeneration. Magnetic resonance imaging (MRI), first incorporated into the McDonald Criteria for the diagnosis of MS in 2001, is an integral tool in the diagnosis, prognosis, and therapeutic monitoring of people with MS (PwMS).1
MRI research in MS is rapidly expanding and offers insights into the pathophysiology of MS with important implications for the routine clinical care of PwMS. At the Consortium of Multiple Sclerosis Centers 2024 Annual Meeting, the US Department of Veterans Affairs (VA) MS Centers of Excellence hosted an educational symposium highlighting MRI biomarkers in MS, including T2-lesions, chronic black holes (cBHs), brain atrophy, paramagnetic rim lesions (PRLs), and the central vein sign (CVS). The symposium also provided a brief overview of quantitative MRI techniques used to characterize MS lesion severity and research applications of these techniques. This clinical review summarizes the main points of that symposium with the goal of introducing key concepts to federal health care practitioners caring for PwMS.
MRI Biomarkers in MS
T2-lesions, Chronic Black Holes, and Brain Atrophy
Focal immune-mediated inflammation and demyelination in MS may be detected by MRI as hyperintense foci on T2-weighted (T2-w) imaging (eg, T2-w turbo spin echo or T2-w fluid attenuated inversion recovery sequences). These T2-lesions, critical for diagnosing MS, are typically ovoid and occur in the periventricular, juxtacortical, infratentorial spinal cord white matter (Figure 1A). T2-lesion number and volume show some association with disability and optic nerve.
Wattjes et al highlight 2 cases to demonstrate this point: a man aged 52 years with MS for 23 years and a woman aged 50 years with MS for 11 years. Despite having MS for a much shorter duration, the woman had worse disability due to a higher lesion number and volume.2 T2-lesion volume also impacts disability progression in PwMS. Gauthier et al compared the probability of progression in 3 women, all of whom were aged 39 years and had MS for 6 years. The profile with highest probability of disability progression had the highest quartile of T2-lesion volume.3 T2-lesion volume over 2 years correlates with worse scores on disability metrics such as the MS functional composite, paced auditory serial addition task, and brain volume.4 A 2024 systematic review and meta-analysis demonstrated that T2-lesion volume is significantly correlated with clinical disability in PwMS.5
Select T2-lesions are also hypointense on T1-w spin echo images and are known as cBHs (Figure 1B). Histologically, T2-lesions with cBHs have more severe architectural disruption than those without cBHs.6 cBH number and volume are significantly correlated with disability, regardless of the degree of hypointensity on T1-w imaging.5,7 A 10-year longitudinal study demonstrated that cBHs were associated with disease progression after 5 years while T2-lesion volume was not, indicating that cBHs may be a more accurate predictor of disability.8
Brain atrophy, another imaging biomarker of MS, affects both the cerebral white and gray matter. White matter fraction (the volume of white matter relative to the intracranial compartment volume) and gray matter fraction (the volume of gray matter relative to the intracranial compartment) are significantly lower among PwMS compared with healthy controls. In addition, gray matter fraction is lower among patients with primary and secondary progressive MS compared with those with relapsing-remitting MS, clinically isolated syndrome (CIS), and radiologically isolated syndrome (RIS). Gray matter fraction is also correlated with several motor and cognitive disability indices.9
Paramagnetic Rim Lesions
Neurologic worsening in PwMS occurs by 2 distinct mechanisms: relapse-associated worsening, a stepwise worsening of symptoms due to incomplete recovery following a relapse; and progression independent of relapse activity (PIRA), which is an irreversible neurologic deterioration in the absence of clinical or radiological relapses.10 PIRA is associated with neurodegeneration and predominates in both primary and secondary progressive MS. However, recent data demonstrated that PIRA may contribute to as much as 50% of disability worsening in relapsing MS and occurs early in the RMS disease course.10,11 Current high-efficacy disease modifying therapy, such as ocrelizumab, are extraordinarily successful at preventing focal inflammation and relapses but are less effective for preventing the slow march of disability progression characterizing PIRA.12,13 The prevention of PIRA is therefore an unmet treatment need.
Chronic active lesions (CALs) are an important driver of PIRA. When an acute gadolinium-enhancing lesion develops in PwMS, there are 3 possible fates of this lesion. The lesion may become chronically inactive, remyelinate, or transition to CALs.14 The histopathologic signature of CALs is compartmentalized, low-grade inflammation behind an intact blood-brain barrier with evidence of both active and chronic components.15 CALs may be found not only in cerebral white matter but also in the cerebral cortex and spinal cord.16,17 Combined MRI and histopathological studies have shown that iron-laden microglia/macrophages can be detected by susceptibility-based MRI as a rim of paramagnetic signal surrounding select T2-lesions.19 These PRLs represent an in vivo imaging biomarker of CAL (Figure 1C). According to the North American Imaging in MS Cooperative (NAIMS) consensus criteria, a PRL must surround at least two-thirds of the outer edge of a T2-lesion, be visible in ≥ 2 consecutive MRI slices, and cannot be contrast enhancing.20
PRLs can be visualized on multiple susceptibility-based imaging methods, including multiecho derived R2*/T2*, phase maps, susceptibility-weighted imaging, and quantitative susceptibility mapping.21-23 Retrospective analyses have shown no significant differences in sensitivity across these imaging modalities.24 Although first visualized with 7T MRI, PRLs may also be detected by 1.5T and 3T MRI with comparable sensitivities.25-27 However, there remains a significant knowledge gap regarding the accuracy of each imaging modality. Systematic, prospectively designed studies are needed to ascertain the comparative value of each method.
The presence of PRL is a poor prognostic indicator. PwMS without PRLs have higher levels of disability, are more likely to progress, and demonstrate greater gray matter atrophy and cognitive dysfunction when compared with PwMS with PRLs.27-29 Lesions with PRL tend to slowly expand, exhibit greater demyelination, and have diminished white matter integrity.21,22,30
PRLs may also be used as a diagnostic tool. PRLs are highly specific for MS/CIS with a 99.7% specificity and 98.4% positive predictive value, although the sensitivity is limited to 24%.31 Taken together, these data indicate that the presence of a PRL substantially increases the likelihood of an MS/CIS diagnosis, whereas the absence of a PRL does not exclude these diagnoses.
Several unanswered questions remain: Why do select acute MS lesions transition to CALs? How may investigators utilize PRLs as outcome measures in future clinical trials? How should PRLs be incorporated into the routine care of PwMS? As the role of this imaging biomarker is clarified both in the research and clinical settings, clinicians caring for PwMS can expect to increasingly encounter the topic of PRLs in the near future.
Central Vein Sign
A CVS is defined by the presence of a central vessel within a demyelinating plaque (Figure 1D). As early as the 1820s, MS plaques on gross pathology were noted to follow the course of a vessel. Early histological studies reported that up to 91% of MS plaques had a central vessel present.32 Lesion formation is dependent on the movement of lymphocytes and other inflammatory cells from the systemic circulation across the blood brain barrier into the perivascular space, a privileged site where immune cells interact with antigen presenting cells to launch an inflammatory cascade and eventual demyelinating lesion.33
CVS can be visualized on 1.5T, 3T and 7T MRI. However, 7T MRI is superior to 3T in the detection of CVS, with 85% of MS lesions having CVS visible compared with 45% on 3T.34 With advances in 7T MRI, fluid attenuated inversion recovery and T2* susceptibility, weighted sequences can be overlaid, allowing simultaneous visualization of the vessel and the demyelinating lesion. With higher density of parenchymal veins in the periventricular regions, the CVS is most seen in lesions of this territory but can also be present in juxtacortical, thalamic and infratentorial lesions with decreasing prevalence as these approach the cortex.35
MS lesions are more likely to have CVS than T2 hyperintense white matter lesions of other causes, with a large study reporting 78% of MS lesions were CVS positive. Further, CVS positive lesions can be found across all MS phenotypes including relapsing remitting, primary progressive, and secondary progressive.35 The CVS is also specific to MS lesions and is an effective tool for differentiating MS lesions from other common causes of T2 hyperintense lesions including chronic ischemic white matter disease,36 migraines,37 neuromyelitis optica spectrum disorders,38,39 Susac syndrome,40 and systemic autoimmune diseases (Behcet disease, systemic lupus erythematosus, and antiphospholipid syndrome).41
With CVS emerging as a promising radiographic biomarker for MS, NAIMS issued a consensus statement on necessary properties of a CVS. These criteria included appearance of a thin hypointense line or small dot, visualized in ≥ 2 perpendicular planes, with diameter < 2 mm, and running partially or entirely through the center of the lesion. They also clarified that lesions < 3 mm, confluent lesions, lesions with multiple vessels present or poorly visualized lesions were excluded.42
A shared CVS definition was a necessary step toward routine use of CVS as a radiographic biomarker and its incorporation in the 2024 revised McDonald criteria.43 Remaining limitations including 7T MRI is primarily available in research settings and the lack of consensus on a diagnostic threshold. There have been many proposed methods, including a 40% cut off,44 60% cut off,45 and Select 3* or Select 6* methods.46 The goal of each method is to optimize sensitivity and specificity while not compromising efficiency of MRI review for both neurologists and radiologists.
The CVS has significant potential as a radiographic biomarker for MS and may allow the early stages of MS to be differentiated from other common causes of white matter lesions on MRI. However, it remains unclear whether CVS holds prognostic value for patients, if CVS is suggestive of differing underlying pathology, or if the presence of a CVS is dynamic over time. Progress in these areas is anticipated as CVS is incorporated into routine clinical practice.
Quantitative MRI Techniques
In the research setting, several imaging modalities can be used to quantify the degree of microstructural injury in PwMS. The goal of these methods is to identify and quantify myelin and axonal damage, the major drivers of neurodegeneration. Among these methods, diffusion-based imaging is a measure of the amount of diffusion or fluid mobility across the tissues of the brain.47 Diffusion-weighted imaging (DWI) yields several parametric maps including axial diffusivity (AD), radial diffusivity (RD), and mean diffusivity (Figure 2 A, B, and C). These parametric maps provide information on different directions of water molecules’ movements. Myelin surrounds the axons preventing water molecules diffusion perpendicular to axons (RD) while axonal content prevents water diffusion horizontal to the axons (AD).Thus, AD is considered more specific to axonal injury, whereas RD is specific to myelin content.48 A higher value of any of these metrics is associated with a higher degree of tissue injury.
Although sensitive to axonal and myelin injury, AD and RD computed from single b-shell DWI experience several limitations including being affected by nonpathologic factors such as fiber orientation, distribution, and crossing, and by various nonmyelin specific pathologies including fluid accumulation during inflammation, myelin sheath thickness, and axonal intactness.48 Several multi b-shell methods have been developed to overcome diffusion imaging limitations. For example, work at the Nashville VA MS Center of Excellence has focused on the use of the multicompartment diffusion MRI with spherical mean technique (SMT). This method removes the orientation dependency of the diffusion MRI signal, increasing the signal-to-noise ratio and reducing biases from fiber undulation, crossing, and dispersion.49 SMT generates the apparent axonal volume fraction (Vax), which is a direct measure of axonal integrity with lower values indicating lower axonal content and higher tissue destruction (Figure 2D). Vax was previously validated in MS as a measure of axonal integrity.49
In terms of myelin, several other specific measures have been developed. Magnetization transfer ratio (MTR) is another measure of tissue integrity that has been validated as a measure of tissue injury in MS (Figure 2E).50,51 Zheng et al found that the percentage of lesions with low MTR was significantly higher among patients whose disease disability progressed compared with patients who did not.52Selective inversion recovery with quantitative magnetization transfer (SIR-qMT) was developed to account for the limitations of MTR, including its sensitivity to edema and axonal density.52 Germane to myelin measurements, SIR-qMT generates the macromolecular to free size ratio (PSR). PSR represents the ratio of protons bound to macromolecules (myelin) to free protons (Figure 2F). PSR is considered a marker of myelin integrity, with lower values correlating with disability severity and indicating higher tissue damage and lower myelin content. Previous studies from the Nashville VA MS Center of Excellence validated the use of SIR-qMT among patients with MS, CIS, RIS, and healthy controls.53
Quantitative MRI has several research applications in the field of MS. We demonstrated that PRL harbor a higher degree of myelin injury indicated by PSR compared with rimless lesions.54 These MRI techniques are also helpful to investigate tissues surrounding the lesions, called normal appearing white matter (NAWM). Using quantitative MRI techniques such as MTR,52 PSR,53 and Vax,49 investigators have demonstrated that NAWM is injured in PwMS, and proximal NAWM may have higher degree of tissue damage compared with distant NAWM.55
Anticipated Innovations and Challenges
In the field of quantitative MRI, several new techniques are being adopted. Researchers are developing techniques such as myelin water fraction which evaluates the interaction between water and protons to measure myelin content. This is considered an advancement as it takes into account edema resulting from MS injury.56 Another example is multicompartment diffusion imaging, such as standard model imaging,57 and neurite orientation dispersion and density imaging,58 which considers water as an additional compartment compared with the SMT derived Vax. For PRL identification, more advanced methodologic techniques are developing such quantitative susceptibility mapping (QSM), which can detect iron deposits that surround the lesions with relatively high sensitivity and specificity of identifying PRL.59
Despite these innovations, several challenges remain before possible incorporation into the clinical setting. These limitations include longer scan time, familiarity of clinicians in using these maps, higher financial cost, and the necessity of advanced imaging processing skills. Artificial intelligence is a promising tool that may overcome these challenges through creating automated processing pipelines and developing synthetic maps without the need for additional acquisition.60
Conclusions
MRI is the most important tool for diagnosing and treating PwMS. Imaging biomarkers such as T2-lesions, cBHs, brain atrophy, PRLs, and CVS provide insight into the disease’s pathogenesis and are invaluable for the accurate diagnosis and prognostication of MS. Quantitative MRI techniques, while not available in the clinical setting, are important tools for translational research that may help direct the development of future therapeutics. In the near future, clinicians caring for PwMS should expect to encounter these imaging biomarkers more frequently in the clinical setting, especially with the inclusion of PRLs and CVS in the next iteration of the McDonald diagnostic criteria.
Multiple sclerosis (MS) is a complex, chronic immune-mediated disease of the central nervous system characterized by focal inflammation, demyelination, and neurodegeneration. Magnetic resonance imaging (MRI), first incorporated into the McDonald Criteria for the diagnosis of MS in 2001, is an integral tool in the diagnosis, prognosis, and therapeutic monitoring of people with MS (PwMS).1
MRI research in MS is rapidly expanding and offers insights into the pathophysiology of MS with important implications for the routine clinical care of PwMS. At the Consortium of Multiple Sclerosis Centers 2024 Annual Meeting, the US Department of Veterans Affairs (VA) MS Centers of Excellence hosted an educational symposium highlighting MRI biomarkers in MS, including T2-lesions, chronic black holes (cBHs), brain atrophy, paramagnetic rim lesions (PRLs), and the central vein sign (CVS). The symposium also provided a brief overview of quantitative MRI techniques used to characterize MS lesion severity and research applications of these techniques. This clinical review summarizes the main points of that symposium with the goal of introducing key concepts to federal health care practitioners caring for PwMS.
MRI Biomarkers in MS
T2-lesions, Chronic Black Holes, and Brain Atrophy
Focal immune-mediated inflammation and demyelination in MS may be detected by MRI as hyperintense foci on T2-weighted (T2-w) imaging (eg, T2-w turbo spin echo or T2-w fluid attenuated inversion recovery sequences). These T2-lesions, critical for diagnosing MS, are typically ovoid and occur in the periventricular, juxtacortical, infratentorial spinal cord white matter (Figure 1A). T2-lesion number and volume show some association with disability and optic nerve.
Wattjes et al highlight 2 cases to demonstrate this point: a man aged 52 years with MS for 23 years and a woman aged 50 years with MS for 11 years. Despite having MS for a much shorter duration, the woman had worse disability due to a higher lesion number and volume.2 T2-lesion volume also impacts disability progression in PwMS. Gauthier et al compared the probability of progression in 3 women, all of whom were aged 39 years and had MS for 6 years. The profile with highest probability of disability progression had the highest quartile of T2-lesion volume.3 T2-lesion volume over 2 years correlates with worse scores on disability metrics such as the MS functional composite, paced auditory serial addition task, and brain volume.4 A 2024 systematic review and meta-analysis demonstrated that T2-lesion volume is significantly correlated with clinical disability in PwMS.5
Select T2-lesions are also hypointense on T1-w spin echo images and are known as cBHs (Figure 1B). Histologically, T2-lesions with cBHs have more severe architectural disruption than those without cBHs.6 cBH number and volume are significantly correlated with disability, regardless of the degree of hypointensity on T1-w imaging.5,7 A 10-year longitudinal study demonstrated that cBHs were associated with disease progression after 5 years while T2-lesion volume was not, indicating that cBHs may be a more accurate predictor of disability.8
Brain atrophy, another imaging biomarker of MS, affects both the cerebral white and gray matter. White matter fraction (the volume of white matter relative to the intracranial compartment volume) and gray matter fraction (the volume of gray matter relative to the intracranial compartment) are significantly lower among PwMS compared with healthy controls. In addition, gray matter fraction is lower among patients with primary and secondary progressive MS compared with those with relapsing-remitting MS, clinically isolated syndrome (CIS), and radiologically isolated syndrome (RIS). Gray matter fraction is also correlated with several motor and cognitive disability indices.9
Paramagnetic Rim Lesions
Neurologic worsening in PwMS occurs by 2 distinct mechanisms: relapse-associated worsening, a stepwise worsening of symptoms due to incomplete recovery following a relapse; and progression independent of relapse activity (PIRA), which is an irreversible neurologic deterioration in the absence of clinical or radiological relapses.10 PIRA is associated with neurodegeneration and predominates in both primary and secondary progressive MS. However, recent data demonstrated that PIRA may contribute to as much as 50% of disability worsening in relapsing MS and occurs early in the RMS disease course.10,11 Current high-efficacy disease modifying therapy, such as ocrelizumab, are extraordinarily successful at preventing focal inflammation and relapses but are less effective for preventing the slow march of disability progression characterizing PIRA.12,13 The prevention of PIRA is therefore an unmet treatment need.
Chronic active lesions (CALs) are an important driver of PIRA. When an acute gadolinium-enhancing lesion develops in PwMS, there are 3 possible fates of this lesion. The lesion may become chronically inactive, remyelinate, or transition to CALs.14 The histopathologic signature of CALs is compartmentalized, low-grade inflammation behind an intact blood-brain barrier with evidence of both active and chronic components.15 CALs may be found not only in cerebral white matter but also in the cerebral cortex and spinal cord.16,17 Combined MRI and histopathological studies have shown that iron-laden microglia/macrophages can be detected by susceptibility-based MRI as a rim of paramagnetic signal surrounding select T2-lesions.19 These PRLs represent an in vivo imaging biomarker of CAL (Figure 1C). According to the North American Imaging in MS Cooperative (NAIMS) consensus criteria, a PRL must surround at least two-thirds of the outer edge of a T2-lesion, be visible in ≥ 2 consecutive MRI slices, and cannot be contrast enhancing.20
PRLs can be visualized on multiple susceptibility-based imaging methods, including multiecho derived R2*/T2*, phase maps, susceptibility-weighted imaging, and quantitative susceptibility mapping.21-23 Retrospective analyses have shown no significant differences in sensitivity across these imaging modalities.24 Although first visualized with 7T MRI, PRLs may also be detected by 1.5T and 3T MRI with comparable sensitivities.25-27 However, there remains a significant knowledge gap regarding the accuracy of each imaging modality. Systematic, prospectively designed studies are needed to ascertain the comparative value of each method.
The presence of PRL is a poor prognostic indicator. PwMS without PRLs have higher levels of disability, are more likely to progress, and demonstrate greater gray matter atrophy and cognitive dysfunction when compared with PwMS with PRLs.27-29 Lesions with PRL tend to slowly expand, exhibit greater demyelination, and have diminished white matter integrity.21,22,30
PRLs may also be used as a diagnostic tool. PRLs are highly specific for MS/CIS with a 99.7% specificity and 98.4% positive predictive value, although the sensitivity is limited to 24%.31 Taken together, these data indicate that the presence of a PRL substantially increases the likelihood of an MS/CIS diagnosis, whereas the absence of a PRL does not exclude these diagnoses.
Several unanswered questions remain: Why do select acute MS lesions transition to CALs? How may investigators utilize PRLs as outcome measures in future clinical trials? How should PRLs be incorporated into the routine care of PwMS? As the role of this imaging biomarker is clarified both in the research and clinical settings, clinicians caring for PwMS can expect to increasingly encounter the topic of PRLs in the near future.
Central Vein Sign
A CVS is defined by the presence of a central vessel within a demyelinating plaque (Figure 1D). As early as the 1820s, MS plaques on gross pathology were noted to follow the course of a vessel. Early histological studies reported that up to 91% of MS plaques had a central vessel present.32 Lesion formation is dependent on the movement of lymphocytes and other inflammatory cells from the systemic circulation across the blood brain barrier into the perivascular space, a privileged site where immune cells interact with antigen presenting cells to launch an inflammatory cascade and eventual demyelinating lesion.33
CVS can be visualized on 1.5T, 3T and 7T MRI. However, 7T MRI is superior to 3T in the detection of CVS, with 85% of MS lesions having CVS visible compared with 45% on 3T.34 With advances in 7T MRI, fluid attenuated inversion recovery and T2* susceptibility, weighted sequences can be overlaid, allowing simultaneous visualization of the vessel and the demyelinating lesion. With higher density of parenchymal veins in the periventricular regions, the CVS is most seen in lesions of this territory but can also be present in juxtacortical, thalamic and infratentorial lesions with decreasing prevalence as these approach the cortex.35
MS lesions are more likely to have CVS than T2 hyperintense white matter lesions of other causes, with a large study reporting 78% of MS lesions were CVS positive. Further, CVS positive lesions can be found across all MS phenotypes including relapsing remitting, primary progressive, and secondary progressive.35 The CVS is also specific to MS lesions and is an effective tool for differentiating MS lesions from other common causes of T2 hyperintense lesions including chronic ischemic white matter disease,36 migraines,37 neuromyelitis optica spectrum disorders,38,39 Susac syndrome,40 and systemic autoimmune diseases (Behcet disease, systemic lupus erythematosus, and antiphospholipid syndrome).41
With CVS emerging as a promising radiographic biomarker for MS, NAIMS issued a consensus statement on necessary properties of a CVS. These criteria included appearance of a thin hypointense line or small dot, visualized in ≥ 2 perpendicular planes, with diameter < 2 mm, and running partially or entirely through the center of the lesion. They also clarified that lesions < 3 mm, confluent lesions, lesions with multiple vessels present or poorly visualized lesions were excluded.42
A shared CVS definition was a necessary step toward routine use of CVS as a radiographic biomarker and its incorporation in the 2024 revised McDonald criteria.43 Remaining limitations including 7T MRI is primarily available in research settings and the lack of consensus on a diagnostic threshold. There have been many proposed methods, including a 40% cut off,44 60% cut off,45 and Select 3* or Select 6* methods.46 The goal of each method is to optimize sensitivity and specificity while not compromising efficiency of MRI review for both neurologists and radiologists.
The CVS has significant potential as a radiographic biomarker for MS and may allow the early stages of MS to be differentiated from other common causes of white matter lesions on MRI. However, it remains unclear whether CVS holds prognostic value for patients, if CVS is suggestive of differing underlying pathology, or if the presence of a CVS is dynamic over time. Progress in these areas is anticipated as CVS is incorporated into routine clinical practice.
Quantitative MRI Techniques
In the research setting, several imaging modalities can be used to quantify the degree of microstructural injury in PwMS. The goal of these methods is to identify and quantify myelin and axonal damage, the major drivers of neurodegeneration. Among these methods, diffusion-based imaging is a measure of the amount of diffusion or fluid mobility across the tissues of the brain.47 Diffusion-weighted imaging (DWI) yields several parametric maps including axial diffusivity (AD), radial diffusivity (RD), and mean diffusivity (Figure 2 A, B, and C). These parametric maps provide information on different directions of water molecules’ movements. Myelin surrounds the axons preventing water molecules diffusion perpendicular to axons (RD) while axonal content prevents water diffusion horizontal to the axons (AD).Thus, AD is considered more specific to axonal injury, whereas RD is specific to myelin content.48 A higher value of any of these metrics is associated with a higher degree of tissue injury.
Although sensitive to axonal and myelin injury, AD and RD computed from single b-shell DWI experience several limitations including being affected by nonpathologic factors such as fiber orientation, distribution, and crossing, and by various nonmyelin specific pathologies including fluid accumulation during inflammation, myelin sheath thickness, and axonal intactness.48 Several multi b-shell methods have been developed to overcome diffusion imaging limitations. For example, work at the Nashville VA MS Center of Excellence has focused on the use of the multicompartment diffusion MRI with spherical mean technique (SMT). This method removes the orientation dependency of the diffusion MRI signal, increasing the signal-to-noise ratio and reducing biases from fiber undulation, crossing, and dispersion.49 SMT generates the apparent axonal volume fraction (Vax), which is a direct measure of axonal integrity with lower values indicating lower axonal content and higher tissue destruction (Figure 2D). Vax was previously validated in MS as a measure of axonal integrity.49
In terms of myelin, several other specific measures have been developed. Magnetization transfer ratio (MTR) is another measure of tissue integrity that has been validated as a measure of tissue injury in MS (Figure 2E).50,51 Zheng et al found that the percentage of lesions with low MTR was significantly higher among patients whose disease disability progressed compared with patients who did not.52Selective inversion recovery with quantitative magnetization transfer (SIR-qMT) was developed to account for the limitations of MTR, including its sensitivity to edema and axonal density.52 Germane to myelin measurements, SIR-qMT generates the macromolecular to free size ratio (PSR). PSR represents the ratio of protons bound to macromolecules (myelin) to free protons (Figure 2F). PSR is considered a marker of myelin integrity, with lower values correlating with disability severity and indicating higher tissue damage and lower myelin content. Previous studies from the Nashville VA MS Center of Excellence validated the use of SIR-qMT among patients with MS, CIS, RIS, and healthy controls.53
Quantitative MRI has several research applications in the field of MS. We demonstrated that PRL harbor a higher degree of myelin injury indicated by PSR compared with rimless lesions.54 These MRI techniques are also helpful to investigate tissues surrounding the lesions, called normal appearing white matter (NAWM). Using quantitative MRI techniques such as MTR,52 PSR,53 and Vax,49 investigators have demonstrated that NAWM is injured in PwMS, and proximal NAWM may have higher degree of tissue damage compared with distant NAWM.55
Anticipated Innovations and Challenges
In the field of quantitative MRI, several new techniques are being adopted. Researchers are developing techniques such as myelin water fraction which evaluates the interaction between water and protons to measure myelin content. This is considered an advancement as it takes into account edema resulting from MS injury.56 Another example is multicompartment diffusion imaging, such as standard model imaging,57 and neurite orientation dispersion and density imaging,58 which considers water as an additional compartment compared with the SMT derived Vax. For PRL identification, more advanced methodologic techniques are developing such quantitative susceptibility mapping (QSM), which can detect iron deposits that surround the lesions with relatively high sensitivity and specificity of identifying PRL.59
Despite these innovations, several challenges remain before possible incorporation into the clinical setting. These limitations include longer scan time, familiarity of clinicians in using these maps, higher financial cost, and the necessity of advanced imaging processing skills. Artificial intelligence is a promising tool that may overcome these challenges through creating automated processing pipelines and developing synthetic maps without the need for additional acquisition.60
Conclusions
MRI is the most important tool for diagnosing and treating PwMS. Imaging biomarkers such as T2-lesions, cBHs, brain atrophy, PRLs, and CVS provide insight into the disease’s pathogenesis and are invaluable for the accurate diagnosis and prognostication of MS. Quantitative MRI techniques, while not available in the clinical setting, are important tools for translational research that may help direct the development of future therapeutics. In the near future, clinicians caring for PwMS should expect to encounter these imaging biomarkers more frequently in the clinical setting, especially with the inclusion of PRLs and CVS in the next iteration of the McDonald diagnostic criteria.
McDonald WI, Compston A, Edan G, et al. Recommended diagnostic criteria for multiple sclerosis: guidelines from the International Panel on the diagnosis of multiple sclerosis. Ann Neurol. 2001;50:121-127. doi:10.1002/ana.1032
Wattjes MP, Steenwijk MD, Stangel M. MRI in the diagnosis and monitoring of multiple sclerosis: an update. Clin Neuroradiol. 2015;25:157-165. doi:10.1007/s00062-015-0430-y
Gauthier SA, Mandel M, Guttmann CR, et al. Predicting short-term disability in multiple sclerosis. Neurology. 2007;68:2059-2065.doi:10.1212/01.wnl.0000264890.97479.b1
Rudick RA, Lee JC, Simon J, Fisher E. Significance of T2 lesions in multiple sclerosis: a 13-year longitudinal study. Ann Neurol. 2006;60:236-242. doi:10.1002/ana.20883
Nabizadeh F, Zafari R, Mohamadi M, et al. MRI features and disability in multiple sclerosis: a systematic review and meta-analysis. J Neuroradiol. 2024;51:24-37. doi:10.1016/j.neurad.2023.11.007
Bagnato F, Jeffries N, Richert ND, et al. Evolution of T1 black holes in patients with multiple sclerosis imaged monthly for 4 years. Brain. 2003;126:1782-1789. doi:10.1093/brain/awg182
Jacobsen C, Hagemeier J, Myhr KM, et al. Brain atrophy and disability progression in multiple sclerosis patients: a 10-year follow-up study. J Neurol Neurosurg Psychiatry. 2014;85:1109-1115. doi:10.1136/jnnp-2013-306906
Rovaris M, Gass A, Bammer R, et al. Diffusion MRI in multiple sclerosis. Neurology. 2005;65:1526-1532. doi:10.1212/01.wnl.0000184471.83948.e0
Fisniku LK, Chard DT, Jackson JS, et al. Gray matter atrophy is related to long-term disability in multiple sclerosis. Ann Neurol. 2008;64:247-254. doi:10.1002/ana.21423
Lublin FD, Häring DA, Ganjgahi H, et al. How patients with multiple sclerosis acquire disability. Brain. 2022;145:3147-3161. doi:10.1093/brain/awac016
Kappos L, Wolinsky JS, Giovannoni G, et al. Contribution of relapse-independent progression vs relapse-associated worsening to overall confirmed disability accumulation in typical relapsing multiple sclerosis in a pooled analysis of 2 randomized clinical trials. JAMA Neurol. 2020;77:1132-1140. doi:10.1001/jamaneurol.2020.1568
Hauser SL, Bar-Or A, Comi G, et al. Ocrelizumab versus interferon beta-1a in relapsing multiple sclerosis. N Engl J Med. 2017;376:221-234. doi:10.1056/NEJMoa1601277
Montalban X, Hauser SL, Kappos L, et al. Ocrelizumab versus placebo in primary progressive multiple sclerosis. N Engl J Med. 2017;376:209-220. doi:10.1056/NEJMoa1606468
Prineas JW, Kwon EE, Cho ES, et al. Immunopathology of secondary-progressive multiple sclerosis. Ann Neurol. 2001;50:646-657. doi:10.1002/ana.1255
Kuhlmann T, Ludwin S, Prat A, Antel J, Brück W, Lassmann H. An updated histological classification system for multiple sclerosis lesions. Acta Neuropathol. 2017;133:13-24. doi:10.1007/s00401-016-1653-y
Pitt D, Boster A, Pei W, et al. Imaging cortical lesions in multiple sclerosis with ultra-high-field magnetic resonance imaging. Arch Neurol. 2010;67:812-818. doi:10.1001/archneurol.2010.148
Gilmore CP, Geurts JJ, Evangelou N, et al. Spinal cord grey matter lesions in multiple sclerosis detected by post-mortem high field MR imaging. Mult Scler. 2009;15:180-188. doi:10.1177/1352458508096876
Lassmann H, Brück W, Lucchinetti CF. The immunopathology of multiple sclerosis: an overview. Brain Pathol. 2007;17:210-218. doi:10.1111/j.1750-3639.2007.00064.x
Bagnato F, Hametner S, Yao B, et al. Tracking iron in multiple sclerosis: a combined imaging and histopathological study at 7 Tesla. Brain. 2011;134:3602-3615. doi:10.1093/brain/awr278
Bagnato F, Sati P, Hemond CC, et al. Imaging chronic active lesions in multiple sclerosis: a consensus statement. Brain. 2024;147:2913-2933. doi:10.1093/brain/awae013
Dal-Bianco A, Grabner G, Kronnerwetter C, et al. Slow expansion of multiple sclerosis iron rim lesions: pathology and 7 T magnetic resonance imaging. Acta Neuropathol. 2017;133:25-42. doi:10.1007/s00401-016-1636-z
Absinta M, Sati P, Schindler M, et al. Persistent 7-tesla phase rim predicts poor outcome in new multiple sclerosis patient lesions. J Clin Invest. 2016;126:2597-2609. doi:10.1172/JCI86198
Gillen KM, Mubarak M, Park C, et al. QSM is an imaging biomarker for chronic glial activation in multiple sclerosis lesions. Ann Clin Transl Neurol. 2021;8:877-886. doi:10.1002/acn3.51338
Ng Kee Kwong KC, Mollison D, Meijboom R, et al. The prevalence of paramagnetic rim lesions in multiple sclerosis: a systematic review and meta-analysis. PLoS One. 2021;16:e0256845. doi:10.1371/journal.pone.0256845
Absinta M, Sati P, Fechner A, et al. Identification of chronic active multiple sclerosis lesions on 3T MRI. AJNR Am J Neuroradiol. 2018;39:1233-1238. doi:10.3174/ajnr.A5660
Hemond CC, Reich DS, Dundamadappa SK. Paramagnetic rim lesions in multiple sclerosis: comparison of visualization at 1.5-T and 3-T MRI. AJR Am J Roentgenol. 2022;219:120-131. doi:10.2214/AJR.21.26777
Altokhis AI, Hibbert AM, Allen CM, et al. Longitudinal clinical study of patients with iron rim lesions in multiple sclerosis. Mult Scler. 2022;28:2202-2211. doi:10.1177/13524585221114750
Choi S, Lake S, Harrison DM. Evaluation of the blood-brain barrier, demyelination, and neurodegeneration in paramagnetic rim lesions in multiple sclerosis on 7 tesla MRI. J Magn Reson Imaging. 2024;59:941-951. doi:10.1002/jmri.28847
Kazimuddin HF, Wang J, Hernandez B, et al. Paramagnetic rim lesions and their relationship with neurodegeneration and clinical disability at the time of multiple sclerosis diagnosis. Poster presented at: 2024 Americas Committee for Treatment and Research in Multiple Sclerosis (ACTRIMS) Forum; February 26-March 2; West Palm Beach, FL.
Rohm Z, Koch C, Kazimuddin H, et al. Longitudinal characterization of paramagnetic rim lesions in early multiple sclerosis. Poster presented at: 2024 Americas Committee for Treatment and Research in Multiple Sclerosis (ACTRIMS) Forum; February 26-March 2; West Palm Beach, FL.
Meaton I, Altokhis A, Allen CM, et al. Paramagnetic rims are a promising diagnostic imaging biomarker in multiple sclerosis. Mult Scler. 2022;28:2212-2220. doi:10.1177/13524585221118677
Fog T. On the vessel-plaque relationships in the brain in multiple sclerosis. Acta Neurol Scand Suppl. 1964;40:9-15.
Ineichen BV, Okar SV, Proulx ST, et al. Perivascular spaces and their role in neuroinflammation. Neuron. 2022;110:3566-3581. doi:10.1016/j.neuron.2022.10.024
Tallantyre EC, Morgan PS, Dixon JE, et al. A comparison of 3T and 7T in the detection of small parenchymal veins within MS lesions. Invest Radiol. 2009;44:491-494. doi:10.1097/RLI.0b013e3181b4c144
Kilsdonk ID, Lopez-Soriano A, Kuijer JP, et al. Morphological features of MS lesions on FLAIR* at 7 T and their relation to patient characteristics. J Neurol. 2014;261:1356-1364. doi:10.1007/s00415-014-7351-6
Tallantyre EC, Dixon JE, Donaldson I, et al. Ultra-high-field imaging distinguishes MS lesions from asymptomatic white matter lesions. Neurology. 2011;76:534-539. doi:10.1212/WNL.0b013e31820b7630
Solomon AJ, Schindler MK, Howard DB, et al. “Central vessel sign” on 3T FLAIR* MRI for the differentiation of multiple sclerosis from migraine. Ann Clin Transl Neurol. 2015;3:82-87. doi:10.1002/acn3.273
Sinnecker T, Dörr J, Pfueller CF, et al. Distinct lesion morphology at 7-T MRI differentiates neuromyelitis optica from multiple sclerosis. Neurology. 2012;79:708-714. doi:10.1212/WNL.0b013e3182648bc8
Kister I, Herbert J, Zhou Y, Ge Y. Ultrahigh-field MR (7 T) imaging of brain lesions in neuromyelitis optica. Mult Scler Int. 2013;2013:398259. doi:10.1155/2013/398259
Wuerfel J, Sinnecker T, Ringelstein EB, et al. Lesion morphology at 7 Tesla MRI differentiates Susac syndrome from multiple sclerosis. Mult Scler. 2012;18:1592-1599. doi:10.1177/1352458512441270
Massacesi L. Perivenular distribution of white matter lesions evaluated by MRI can differentiate MS lesions from inflammatory small vessel diseases. Eur J Neurol. 2016;23:86. doi:10.1212/WNL.86.16_supplement.P6.121
Sati P, Oh J, Constable RT, et al. The central vein sign and its clinical evaluation for the diagnosis of multiple sclerosis: a consensus statement from the North American Imaging in Multiple Sclerosis Cooperative. Nat Rev Neurol. 2016;12:714-722. doi:10.1038/nrneurol.2016.166
Montalban X, Lebrun-Frénay C, Oh J, et al. Diagnosis of multiple sclerosis: 2024 revisions of the McDonald criteria. Lancet Neurol. 2025;24:850-865. doi:10.1016/S1474-4422(25)00270-4
Mistry N, Dixon J, Tallantyre E, et al. Central veins in brain lesions visualized with high-field magnetic resonance imaging: a pathologically specific diagnostic biomarker for inflammatory demyelination in the brain. JAMA Neurol. 2013;70:623-628. doi:10.1001/jamaneurol.2013.1405
Campion T, Smith RJP, Altmann DR, et al. FLAIR* to visualize veins in white matter lesions: a new tool for the diagnosis of multiple sclerosis? Eur Radiol. 2017;27:4257-4263. doi:10.1007/s00330-017-4822-z
Solomon AJ, Watts R, Ontaneda D, et al. Diagnostic performance of central vein sign for multiple sclerosis with a simplified three-lesion algorithm. Mult Scler. 2018;24:750-757. doi:10.1177/1352458517726383
Cercignani M, Bozzali M, Iannucci G, Comi G, Filippi M. Intra-voxel and inter-voxel coherence in patients with multiple sclerosis assessed using diffusion tensor MRI. J Neurol. 2002;249:875-883. doi:10.1007/s00415-002-0752-y
Song SK, Yoshino J, Le TQ, et al. Demyelination increases radial diffusivity in corpus callosum of mouse brain. Neuroimage. 2005;26:132-140. doi:10.1016/j.neuroimage.2005.01.028
Bagnato F, Franco G, Li H, et al. Probing axons using multi-compartmental diffusion in multiple sclerosis. Ann Clin Transl Neurol. 2019;6:1595-1605. doi:10.1002/acn3.50836
Filippi M, Cercignani M, Inglese M, et al. Diffusion tensor magnetic resonance imaging in multiple sclerosis. Neurology. 2001;56:304-311. doi:10.1212/wnl.56.3.304
Bagnato F. Clinical application of magnetization transfer imaging. In: Advanced Neuro MR Techniques and Applications. Elsevier; 2022:403-417. doi:10.1016/B978-0-12-822479-3.00041-5
Zheng Y, Lee JC, Rudick R, Fisher E. Long-term magnetization transfer ratio evolution in multiple sclerosis white matter lesions. J Neuroimaging. 2018;28:191-198. doi:10.1111/jon.12480
Bagnato F, Hametner S, Franco G, et al. Selective inversion recovery quantitative magnetization transfer brain MRI at 7T: clinical and postmortem validation in multiple sclerosis. J Neuroimaging. 2018;28:380-388. doi:10.1111/jon.12511
Clarke MA, Cheek R, Hernandez B, et al. Paramagnetic rim lesions and the central vein sign: characterizing multiple sclerosis imaging markers. J Neuroimaging. 2024;34:86-94. doi:10.1111/jon.13173
Clarke MA, Lakhani DA, Wen S, et al. Perilesional neurodegenerative injury in multiple sclerosis: relation to focal lesions and impact on disability. Mult Scler Relat Disord. 2021;49:102738. doi:10.1016/j.msard.2021.102738
Laule C, Moore GRW. Myelin water imaging to detect demyelination and remyelination and its validation in pathology. Brain Pathol. 2018;28:750-764. doi:10.1111/bpa.12645
Coelho S, Baete SH, Lemberskiy G, et al. Reproducibility of the standard model of diffusion in white matter on clinical MRI systems. Neuroimage. 2022;257:119290. doi:10.1016/j.neuroimage.2022.119290
Novikov DS, Veraart J, Jelescu IO, et al. Rotationally-invariant mapping of scalar and orientational metrics of neuronal microstructure with diffusion MRI. Neuroimage. 2018;174:518-538. doi:10.1016/j.neuroimage.2018.03.006
Langkammer C, Liu T, Khalil M, et al. Quantitative susceptibility mapping in multiple sclerosis. Radiology. 2013;267:551-559. doi:10.1148/radiol.12120707
Collorone S, Coll L, Lorenzi M, et al. Artificial intelligence applied to MRI data to tackle key challenges in multiple sclerosis. Mult Scler. 2024;30:767-784. doi:10.1177/13524585241249422
McDonald WI, Compston A, Edan G, et al. Recommended diagnostic criteria for multiple sclerosis: guidelines from the International Panel on the diagnosis of multiple sclerosis. Ann Neurol. 2001;50:121-127. doi:10.1002/ana.1032
Wattjes MP, Steenwijk MD, Stangel M. MRI in the diagnosis and monitoring of multiple sclerosis: an update. Clin Neuroradiol. 2015;25:157-165. doi:10.1007/s00062-015-0430-y
Gauthier SA, Mandel M, Guttmann CR, et al. Predicting short-term disability in multiple sclerosis. Neurology. 2007;68:2059-2065.doi:10.1212/01.wnl.0000264890.97479.b1
Rudick RA, Lee JC, Simon J, Fisher E. Significance of T2 lesions in multiple sclerosis: a 13-year longitudinal study. Ann Neurol. 2006;60:236-242. doi:10.1002/ana.20883
Nabizadeh F, Zafari R, Mohamadi M, et al. MRI features and disability in multiple sclerosis: a systematic review and meta-analysis. J Neuroradiol. 2024;51:24-37. doi:10.1016/j.neurad.2023.11.007
Bagnato F, Jeffries N, Richert ND, et al. Evolution of T1 black holes in patients with multiple sclerosis imaged monthly for 4 years. Brain. 2003;126:1782-1789. doi:10.1093/brain/awg182
Jacobsen C, Hagemeier J, Myhr KM, et al. Brain atrophy and disability progression in multiple sclerosis patients: a 10-year follow-up study. J Neurol Neurosurg Psychiatry. 2014;85:1109-1115. doi:10.1136/jnnp-2013-306906
Rovaris M, Gass A, Bammer R, et al. Diffusion MRI in multiple sclerosis. Neurology. 2005;65:1526-1532. doi:10.1212/01.wnl.0000184471.83948.e0
Fisniku LK, Chard DT, Jackson JS, et al. Gray matter atrophy is related to long-term disability in multiple sclerosis. Ann Neurol. 2008;64:247-254. doi:10.1002/ana.21423
Lublin FD, Häring DA, Ganjgahi H, et al. How patients with multiple sclerosis acquire disability. Brain. 2022;145:3147-3161. doi:10.1093/brain/awac016
Kappos L, Wolinsky JS, Giovannoni G, et al. Contribution of relapse-independent progression vs relapse-associated worsening to overall confirmed disability accumulation in typical relapsing multiple sclerosis in a pooled analysis of 2 randomized clinical trials. JAMA Neurol. 2020;77:1132-1140. doi:10.1001/jamaneurol.2020.1568
Hauser SL, Bar-Or A, Comi G, et al. Ocrelizumab versus interferon beta-1a in relapsing multiple sclerosis. N Engl J Med. 2017;376:221-234. doi:10.1056/NEJMoa1601277
Montalban X, Hauser SL, Kappos L, et al. Ocrelizumab versus placebo in primary progressive multiple sclerosis. N Engl J Med. 2017;376:209-220. doi:10.1056/NEJMoa1606468
Prineas JW, Kwon EE, Cho ES, et al. Immunopathology of secondary-progressive multiple sclerosis. Ann Neurol. 2001;50:646-657. doi:10.1002/ana.1255
Kuhlmann T, Ludwin S, Prat A, Antel J, Brück W, Lassmann H. An updated histological classification system for multiple sclerosis lesions. Acta Neuropathol. 2017;133:13-24. doi:10.1007/s00401-016-1653-y
Pitt D, Boster A, Pei W, et al. Imaging cortical lesions in multiple sclerosis with ultra-high-field magnetic resonance imaging. Arch Neurol. 2010;67:812-818. doi:10.1001/archneurol.2010.148
Gilmore CP, Geurts JJ, Evangelou N, et al. Spinal cord grey matter lesions in multiple sclerosis detected by post-mortem high field MR imaging. Mult Scler. 2009;15:180-188. doi:10.1177/1352458508096876
Lassmann H, Brück W, Lucchinetti CF. The immunopathology of multiple sclerosis: an overview. Brain Pathol. 2007;17:210-218. doi:10.1111/j.1750-3639.2007.00064.x
Bagnato F, Hametner S, Yao B, et al. Tracking iron in multiple sclerosis: a combined imaging and histopathological study at 7 Tesla. Brain. 2011;134:3602-3615. doi:10.1093/brain/awr278
Bagnato F, Sati P, Hemond CC, et al. Imaging chronic active lesions in multiple sclerosis: a consensus statement. Brain. 2024;147:2913-2933. doi:10.1093/brain/awae013
Dal-Bianco A, Grabner G, Kronnerwetter C, et al. Slow expansion of multiple sclerosis iron rim lesions: pathology and 7 T magnetic resonance imaging. Acta Neuropathol. 2017;133:25-42. doi:10.1007/s00401-016-1636-z
Absinta M, Sati P, Schindler M, et al. Persistent 7-tesla phase rim predicts poor outcome in new multiple sclerosis patient lesions. J Clin Invest. 2016;126:2597-2609. doi:10.1172/JCI86198
Gillen KM, Mubarak M, Park C, et al. QSM is an imaging biomarker for chronic glial activation in multiple sclerosis lesions. Ann Clin Transl Neurol. 2021;8:877-886. doi:10.1002/acn3.51338
Ng Kee Kwong KC, Mollison D, Meijboom R, et al. The prevalence of paramagnetic rim lesions in multiple sclerosis: a systematic review and meta-analysis. PLoS One. 2021;16:e0256845. doi:10.1371/journal.pone.0256845
Absinta M, Sati P, Fechner A, et al. Identification of chronic active multiple sclerosis lesions on 3T MRI. AJNR Am J Neuroradiol. 2018;39:1233-1238. doi:10.3174/ajnr.A5660
Hemond CC, Reich DS, Dundamadappa SK. Paramagnetic rim lesions in multiple sclerosis: comparison of visualization at 1.5-T and 3-T MRI. AJR Am J Roentgenol. 2022;219:120-131. doi:10.2214/AJR.21.26777
Altokhis AI, Hibbert AM, Allen CM, et al. Longitudinal clinical study of patients with iron rim lesions in multiple sclerosis. Mult Scler. 2022;28:2202-2211. doi:10.1177/13524585221114750
Choi S, Lake S, Harrison DM. Evaluation of the blood-brain barrier, demyelination, and neurodegeneration in paramagnetic rim lesions in multiple sclerosis on 7 tesla MRI. J Magn Reson Imaging. 2024;59:941-951. doi:10.1002/jmri.28847
Kazimuddin HF, Wang J, Hernandez B, et al. Paramagnetic rim lesions and their relationship with neurodegeneration and clinical disability at the time of multiple sclerosis diagnosis. Poster presented at: 2024 Americas Committee for Treatment and Research in Multiple Sclerosis (ACTRIMS) Forum; February 26-March 2; West Palm Beach, FL.
Rohm Z, Koch C, Kazimuddin H, et al. Longitudinal characterization of paramagnetic rim lesions in early multiple sclerosis. Poster presented at: 2024 Americas Committee for Treatment and Research in Multiple Sclerosis (ACTRIMS) Forum; February 26-March 2; West Palm Beach, FL.
Meaton I, Altokhis A, Allen CM, et al. Paramagnetic rims are a promising diagnostic imaging biomarker in multiple sclerosis. Mult Scler. 2022;28:2212-2220. doi:10.1177/13524585221118677
Fog T. On the vessel-plaque relationships in the brain in multiple sclerosis. Acta Neurol Scand Suppl. 1964;40:9-15.
Ineichen BV, Okar SV, Proulx ST, et al. Perivascular spaces and their role in neuroinflammation. Neuron. 2022;110:3566-3581. doi:10.1016/j.neuron.2022.10.024
Tallantyre EC, Morgan PS, Dixon JE, et al. A comparison of 3T and 7T in the detection of small parenchymal veins within MS lesions. Invest Radiol. 2009;44:491-494. doi:10.1097/RLI.0b013e3181b4c144
Kilsdonk ID, Lopez-Soriano A, Kuijer JP, et al. Morphological features of MS lesions on FLAIR* at 7 T and their relation to patient characteristics. J Neurol. 2014;261:1356-1364. doi:10.1007/s00415-014-7351-6
Tallantyre EC, Dixon JE, Donaldson I, et al. Ultra-high-field imaging distinguishes MS lesions from asymptomatic white matter lesions. Neurology. 2011;76:534-539. doi:10.1212/WNL.0b013e31820b7630
Solomon AJ, Schindler MK, Howard DB, et al. “Central vessel sign” on 3T FLAIR* MRI for the differentiation of multiple sclerosis from migraine. Ann Clin Transl Neurol. 2015;3:82-87. doi:10.1002/acn3.273
Sinnecker T, Dörr J, Pfueller CF, et al. Distinct lesion morphology at 7-T MRI differentiates neuromyelitis optica from multiple sclerosis. Neurology. 2012;79:708-714. doi:10.1212/WNL.0b013e3182648bc8
Kister I, Herbert J, Zhou Y, Ge Y. Ultrahigh-field MR (7 T) imaging of brain lesions in neuromyelitis optica. Mult Scler Int. 2013;2013:398259. doi:10.1155/2013/398259
Wuerfel J, Sinnecker T, Ringelstein EB, et al. Lesion morphology at 7 Tesla MRI differentiates Susac syndrome from multiple sclerosis. Mult Scler. 2012;18:1592-1599. doi:10.1177/1352458512441270
Massacesi L. Perivenular distribution of white matter lesions evaluated by MRI can differentiate MS lesions from inflammatory small vessel diseases. Eur J Neurol. 2016;23:86. doi:10.1212/WNL.86.16_supplement.P6.121
Sati P, Oh J, Constable RT, et al. The central vein sign and its clinical evaluation for the diagnosis of multiple sclerosis: a consensus statement from the North American Imaging in Multiple Sclerosis Cooperative. Nat Rev Neurol. 2016;12:714-722. doi:10.1038/nrneurol.2016.166
Montalban X, Lebrun-Frénay C, Oh J, et al. Diagnosis of multiple sclerosis: 2024 revisions of the McDonald criteria. Lancet Neurol. 2025;24:850-865. doi:10.1016/S1474-4422(25)00270-4
Mistry N, Dixon J, Tallantyre E, et al. Central veins in brain lesions visualized with high-field magnetic resonance imaging: a pathologically specific diagnostic biomarker for inflammatory demyelination in the brain. JAMA Neurol. 2013;70:623-628. doi:10.1001/jamaneurol.2013.1405
Campion T, Smith RJP, Altmann DR, et al. FLAIR* to visualize veins in white matter lesions: a new tool for the diagnosis of multiple sclerosis? Eur Radiol. 2017;27:4257-4263. doi:10.1007/s00330-017-4822-z
Solomon AJ, Watts R, Ontaneda D, et al. Diagnostic performance of central vein sign for multiple sclerosis with a simplified three-lesion algorithm. Mult Scler. 2018;24:750-757. doi:10.1177/1352458517726383
Cercignani M, Bozzali M, Iannucci G, Comi G, Filippi M. Intra-voxel and inter-voxel coherence in patients with multiple sclerosis assessed using diffusion tensor MRI. J Neurol. 2002;249:875-883. doi:10.1007/s00415-002-0752-y
Song SK, Yoshino J, Le TQ, et al. Demyelination increases radial diffusivity in corpus callosum of mouse brain. Neuroimage. 2005;26:132-140. doi:10.1016/j.neuroimage.2005.01.028
Bagnato F, Franco G, Li H, et al. Probing axons using multi-compartmental diffusion in multiple sclerosis. Ann Clin Transl Neurol. 2019;6:1595-1605. doi:10.1002/acn3.50836
Filippi M, Cercignani M, Inglese M, et al. Diffusion tensor magnetic resonance imaging in multiple sclerosis. Neurology. 2001;56:304-311. doi:10.1212/wnl.56.3.304
Bagnato F. Clinical application of magnetization transfer imaging. In: Advanced Neuro MR Techniques and Applications. Elsevier; 2022:403-417. doi:10.1016/B978-0-12-822479-3.00041-5
Zheng Y, Lee JC, Rudick R, Fisher E. Long-term magnetization transfer ratio evolution in multiple sclerosis white matter lesions. J Neuroimaging. 2018;28:191-198. doi:10.1111/jon.12480
Bagnato F, Hametner S, Franco G, et al. Selective inversion recovery quantitative magnetization transfer brain MRI at 7T: clinical and postmortem validation in multiple sclerosis. J Neuroimaging. 2018;28:380-388. doi:10.1111/jon.12511
Clarke MA, Cheek R, Hernandez B, et al. Paramagnetic rim lesions and the central vein sign: characterizing multiple sclerosis imaging markers. J Neuroimaging. 2024;34:86-94. doi:10.1111/jon.13173
Clarke MA, Lakhani DA, Wen S, et al. Perilesional neurodegenerative injury in multiple sclerosis: relation to focal lesions and impact on disability. Mult Scler Relat Disord. 2021;49:102738. doi:10.1016/j.msard.2021.102738
Laule C, Moore GRW. Myelin water imaging to detect demyelination and remyelination and its validation in pathology. Brain Pathol. 2018;28:750-764. doi:10.1111/bpa.12645
Coelho S, Baete SH, Lemberskiy G, et al. Reproducibility of the standard model of diffusion in white matter on clinical MRI systems. Neuroimage. 2022;257:119290. doi:10.1016/j.neuroimage.2022.119290
Novikov DS, Veraart J, Jelescu IO, et al. Rotationally-invariant mapping of scalar and orientational metrics of neuronal microstructure with diffusion MRI. Neuroimage. 2018;174:518-538. doi:10.1016/j.neuroimage.2018.03.006
Langkammer C, Liu T, Khalil M, et al. Quantitative susceptibility mapping in multiple sclerosis. Radiology. 2013;267:551-559. doi:10.1148/radiol.12120707
Collorone S, Coll L, Lorenzi M, et al. Artificial intelligence applied to MRI data to tackle key challenges in multiple sclerosis. Mult Scler. 2024;30:767-784. doi:10.1177/13524585241249422
Updates in Multiple Sclerosis Imaging
Updates in Multiple Sclerosis Imaging
Steatocystomas: Update on Clinical Manifestations, Diagnosis, and Management
Steatocystomas: Update on Clinical Manifestations, Diagnosis, and Management
Steatocystomas are small sebum-filled cysts that typically manifest in the dermis and originate from sebaceous follicles. Although commonly asymptomatic, these lesions can manifest with pruritus or become infected, predisposing patients to further complications.1 Steatocystomas can manifest as single (steatocystoma simplex [SS]) or numerous (steatocystoma multiplex [SM]) lesions; the lesions also can spontaneously rupture with characteristics that resemble hidradenitis suppurativa (HS)(steatocystoma multiplex suppurativa [SMS]).1,2
Steatocystomas are relatively rare, and there is limited consensus in the published literature on the etiology and management of this condition. In this article, we present a comprehensive review of steatocystomas in the current literature. We highlight important features to consider when making the diagnosis and also offer recommendations for best-practice treatment.
Historical Background
Although not explicitly identified by name, the first documentation of steatocystomas is a case report published in 1873. In this account, the author described a patient who presented with approximately 250 flesh-colored dermal cysts across the body that varied in size.3 In 1899, the term steatocystoma multiple—derived from Greek roots meaning “fatty bag”—was first used.4
In 1982, almost a century later, Brownstein5 reported some of the earliest cases of SS. This solitary subtype is identical to SM on a microscopic level; however, unlike SM, this variant occurs as a single lesion that typically forms in adulthood and in the absence of family history. Other benign adnexal tumors (eg, pilomatricomas, pilar cysts, and sebaceous hyperplasias) also can manifest as either solitary or multiple lesions.
In 1976, McDonald and Reed6 reported the first known cases of patients with both SM and HS. At the time, the co-occurrence of these conditions was viewed as coincidental, but there were postulations of a shared inflammatory process and hereditary link6; it was not until 1982 that the term steatocystoma multiplex suppurativum was coined to describe this variant.7 Although rare, there have been multiple documented instances of SMS since. It has been suggested that the convergence of these conditions may indicate a shared follicular proliferation defect.8 Ongoing investigation is warranted to explain the underlying pathogenesis of this unique variant.
Epidemiology
The available epidemiologic data primarily relate to SM, the most common steatocystoma variant. Nevertheless, SM is a relatively rare condition, and the exact incidence and prevalence remain unknown.8,9 Steatocystomas typically manifest in the first and second decades of life and have been observed in patients of both sexes, with studies demonstrating no notable sex bias.4,9
Etiology and Pathophysiology
Steatocystomas can occur sporadically or may be inherited as an autosomal-dominant condition.4 Typically, SS tends to manifest as an isolated occurrence without any inherent genetic predisposition.5 Alternatively, SM may develop sporadically or be associated with a mutation in the keratin 17 gene (KRT17).4 Steatocystoma multiplex also has been associated with at least 4 different missense mutations, including N92H, R94H, and R94C, located on the long (q) arm of chromosome 17.4,10-12
The keratin 17 gene is responsible for encoding the keratin 17 protein, a type I intermediate filament predominantly synthesized in the basal cells of epithelial tissue. This fibrous structural protein can regulate many processes, including inflammation and cell proliferation, and is found in regions such as the sebaceous glands, hair follicles, and eccrine sweat glands. Overexpression of KRT17 has been suggested in other cutaneous conditions, most notably psoriasis.12 Despite KRT17’s many roles, it remains unclear why SM typically manifests with a myriad of sebum-containing cysts as the primary symptom.12 Continued investigation into the genetic underpinnings of SM and the keratin 17 protein is necessary to further elucidate a more comprehensive understanding of this condition.
Hormonal influences have been suggested as a potential trigger for steatocystoma growth.4,13 This condition is associated with dysfunction of the sebaceous glands, and, correspondingly, the incidence of disease is highest in pubertal patients, in whom androgen levels and sebum production are elevated.4,13,14 Two cases of transgender men taking testosterone therapy presenting with steatocystomas provide additional clinical support for this association.15
Additionally, the use of immunomodulatory agents, such as ustekinumab (anti–interleukin 12/interleukin 23), has been shown to trigger SM. It is predicted that the reduced expression of certain interferons and interleukins may lead to downstream consequences in the keratin 17 pathway and lead to SM lesion formation in genetically susceptible individuals.16 Targeting these potential causes in the future may prove efficacious in the secondary prevention of familial SM manifestation or exacerbations.
Mutations in the KRT17 gene also have been implicated in pachyonychia congenita type 2 (PC-2).4 Marked by extensive systemic hyperkeratosis, PC-2 has been observed to coincide with SM in certain patients.4,5 Interestingly, the location of the KRT17 mutations are identical in both PC-2 and SM.4 Although most individuals with hereditary SM do not exhibit the characteristic features of PC-2, mild nail and dental abnormalities have been observed in some SM cases.4,10 This relationship suggests that SM may be a less severe variant of PC-2 or part of a complex polygenetic spectrum of disease.10 Further research is imperative to determine the exact nature and extent of the relationship between these conditions.
Clinical Manifestations
Steatocystomas are flesh-colored subcutaneous cysts that range in size from less than 3 mm to larger than 3 cm in diameter (Figure). They form within a single pilosebaceous unit and typically display firm attachment due to their origination in the dermis.2,7,17 Steatocystomas generally contain lipid material, and less frequently, keratin and hair shafts, distinguishing them as the only “true” sebaceous cysts.18 Their color can range from flesh-toned to yellow, with reports of occasional dark-blue shades and calcifications.19,20 Steatocystomas can persist indefinitely, and they usually are asymptomatic.
Diagnosis of steatocystoma is confirmed by biopsy.4 Steatocystomas are characterized by a dermal cyst lined by stratified squamous cell epithelium (eFigures 1 and 2).21 Classically they feature flattened sebaceous lobules, multinucleated giant cells, and abortive hair follicles. The lining of these cysts is marked by lymphocytic infiltrate and a dense, wrinkled, eosinophilic keratin cuticle that replaces the granular layer.22 The cyst maintains an epidermal connection through a follicular infundibulum characterized by clumps of keratinocytes, sebocytes, corneocytes, and/or hair follicles.7 Aspirated contents reveal crystalline structures and anucleate squamous cells upon microscopic analysis. That being said, variable histologic findings of steatocystomas have been described.23
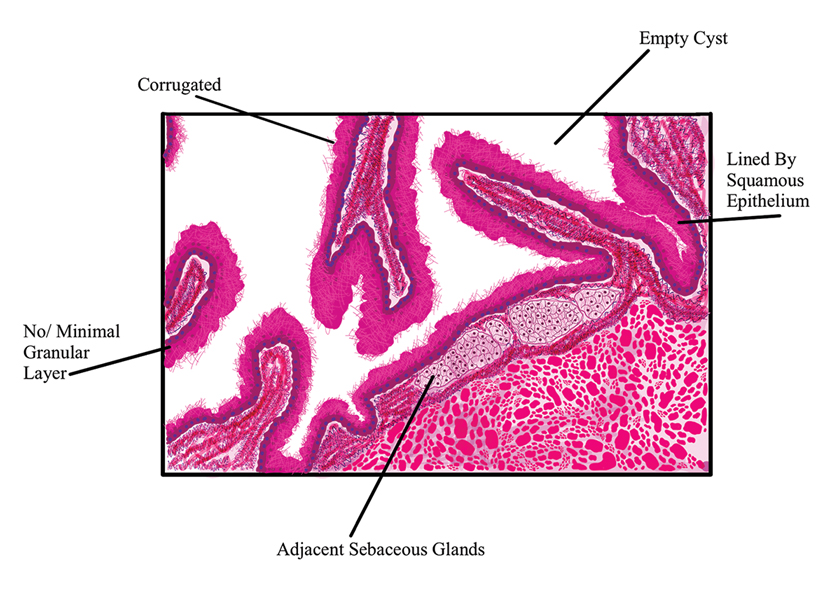
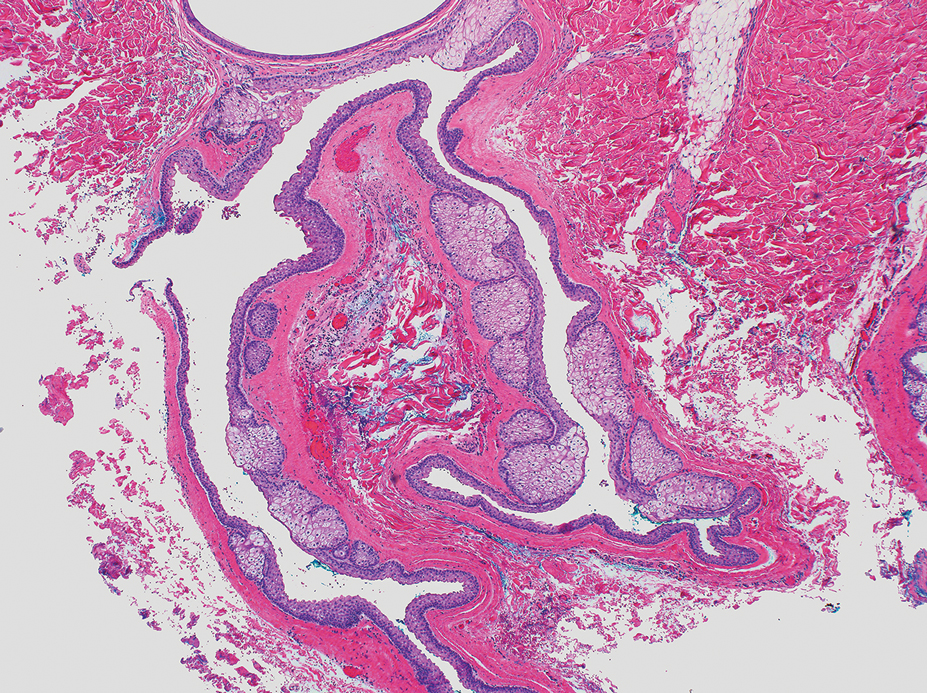
Steatocystoma simplex, as the name implies, classifies a single isolated steatocystoma. This subtype exhibits similar histopathologic and clinical features to the other subtypes of steatocystomas. Notably, SS is not associated with a genetic mutation and is not an inherited condition within families.5 Steatocystoma multiplex manifests with many steatocystomas, often distributed widely across the body.3,4 The chest, axillae, and groin are the most common locations; however, these cysts can manifest on the face, back, abdomen, and extremities.4,18-22 Rare occurrences of SM limited to the face, scalp, and distal extremities have been documented.18,21,24,25 Due to the possibility of an autosomal-dominant inheritance, it is advisable to take a comprehensive family history in patients for whom SM is in the differential.17
Steatocystoma multiplex—especially familial variants—has been shown to develop in conjunction with other dermatologic conditions, including eruptive vellus hair (EVH) cysts, persistent infantile milia, and epidermoid/dermoid cysts.26 While some investigators regard these as separate entities due to their varied genetic etiology, it has been suggested that these conditions may be related and that the diagnosis is determined by the location of cyst origin along the sebaceous ducts.26,27 Other dermatologic conditions and lesions that frequently manifest comorbidly with SM include hidrocystomas, syringomas, pilonidal cysts, lichen planus, nodulocystic acne, trichotillomania, trichoblastomas, trichoepithelioma, HS, keratoacanthomas, acrokeratosis verruciformis of Hopf, and embryonal hair formation. Steatocystoma multiplex, manifesting comorbidly with dental and orofacial malformations (eg, partial noneruption of secondary teeth, natal and defective teeth, and bilateral preauricular sinuses) has been classified as SM natal teeth syndrome.6
Steatocystoma multiplex suppurativa is a rare and serious variant of SM characterized by inflammation, cyst rupture, sinus tract formation, and scarring.24 Patients with SMS typically have multiple intact SM cysts, which can aid in differentiation from HS.2,24 Steatocystoma multiplex suppurativa is associated with more complications than SS and SM, including cyst perforation, development of purulent and/or foul-smelling discharge, infection, scarring, pain, and overall discomfort.2
Given its rarity and the potential manifestations that overlap with other conditions, steatocystomas easily can be misdiagnosed. In some clinical instances, EVHs may share similar characteristics with SM; however, certain distinguishing features exist, including a central tuft of protruding hairs and different expressed contents, such as the vellus hair shafts, from the cyst’s lumen.28 Furthermore, histologic examination of EVHs reveals epidermoid keratinization of the lining as well as a lack of sebaceous glands within the wall.28,29 Other similar conditions include epidermoid cysts, pilar cysts, lipomas, epidermal inclusion cysts, dermoid cysts, sebaceous hyperplasia, folliculitis, xanthomas, neurofibromatosis, and syringomas.30 Occasionally, SMS can be mistaken for HS or acne conglobata, and SM lesions with a facial distribution can mimic acne vulgaris.1,31 These conditions should be excluded before a diagnosis of SS, SM, or SMS is made.
Importantly, SM is visually indistinguishable from subcutaneous metastasis on physical examination, and there are reports of oncologic conditions (eg, pulmonary adenocarcinoma metastasized to the skin) being mistaken for SS or SM.32 Therefore, a thorough clinical examination, histopathologic analysis, and potential use of other imaging modalities such as ultrasonography (US) are needed to ensure an accurate diagnosis.
Ultrasonography has demonstrated utility in diagnosing steatocystomas.33-35 Steatocystomas have incidentally been found on routine mammograms and can demonstrate well-defined circular nodules with radiolucent characteristics and a thin radiodense outline.33,36 Homogeneous hypoechoic nodules within the dermis without posterior acoustic features generally are observed (eFigure 3).33,37 In patients declining biopsy, US may be useful in further characterization of an unknown lesion. Color Doppler US can be used to distinguish SMS from HS. Specifically, SM typically exhibits an absence of Doppler signaling due to a lack of vascularity, providing a helpful diagnostic clue for the SMS variant.33
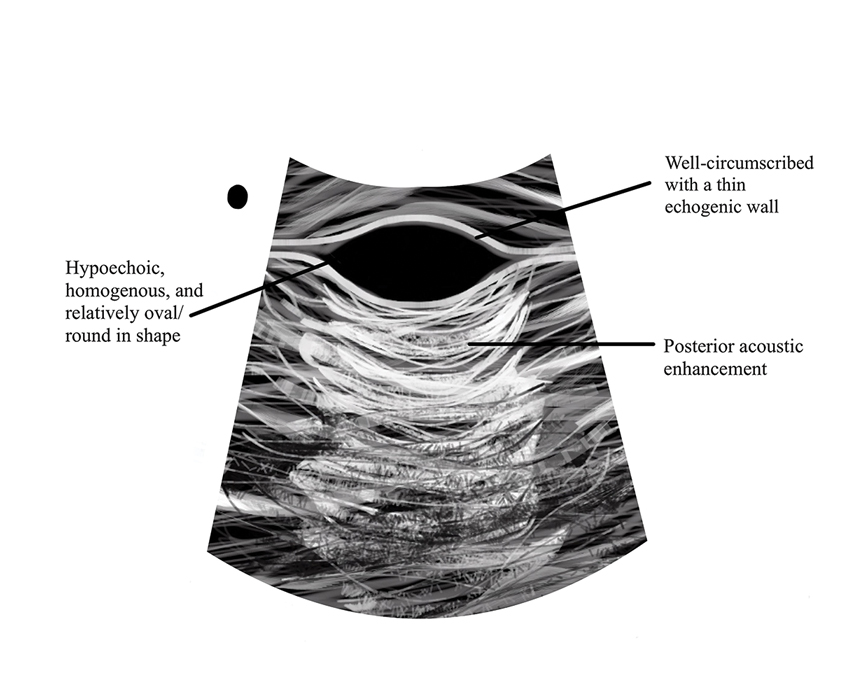
Management and Treatment Options
There is no established standard treatment for steatocystomas; therefore, the approach to management is contingent on clinical presentation and patient preferences. Various medical, surgical, and laser management options are available, each with its own advantages and limitations. Treatment of SM is difficult due to the large number of lesions.38 In many cases, continued observation is a viable treatment option, as most SS and SM lesions are asymptomatic; however, cosmetic concerns can be debilitating for patients with SM and may warrant intervention.39 More extensive medical and surgical management often are necessary in SMS due to associated morbidity. Discussing options and goals as well as setting realistic expectations with the patient are essential in determining the optimal approach.
Medical Management—In medical literature, oral isotretinoin (13-cis-retinoic acid) has been the mainstay of therapy for steatocystoma, as its effect on the size and activity of sebaceous glands is hypothesized to decrease disease activity.38,40 Interventional studies and case reports have exhibited varying degrees of effectiveness.1,38-41 Some reports depict a reduction in the formation of new lesions and a decrease in the size of pre-existing lesions, some show mild delayed therapeutic efficacy, and others suggest exacerbation of the condition.1,38-41 This outcome variability is attributed to isotretinoin’s preferential efficacy in treating inflammatory lesions.40,42
Tetracycline derivatives and intralesional steroid injections also have been employed with some efficacy in patients with focal inflammatory SM and SMS.43 There is limited evidence on the long-term outcomes of these interventions, and intralesional injections often are not recommended in conditions such as SM, in which there are many lesions present.
Surgical Management—Minimally invasive surgical procedures including drainage and resections have been used with varying efficacy in SS and SM. Typically, a 2- to 3-mm incision or sharp-tipped cautery is employed to puncture the cyst. Alternatively, radiofrequency probes with a 2.4-MHz frequency setting have been used to minimize incision size.44 The contents then are expressed with manual pressure or forceps, and the cyst sac is extracted using forceps and/or a vein hook (eFigure 4).44,45 The specific surgical techniques and their respective advantages and limitations are summarized in the eTable. Reported advantages and limitations of surgical techniques are derived from information provided by the authors of steatocystoma case reports, which are based on observations of a very limited sample size.
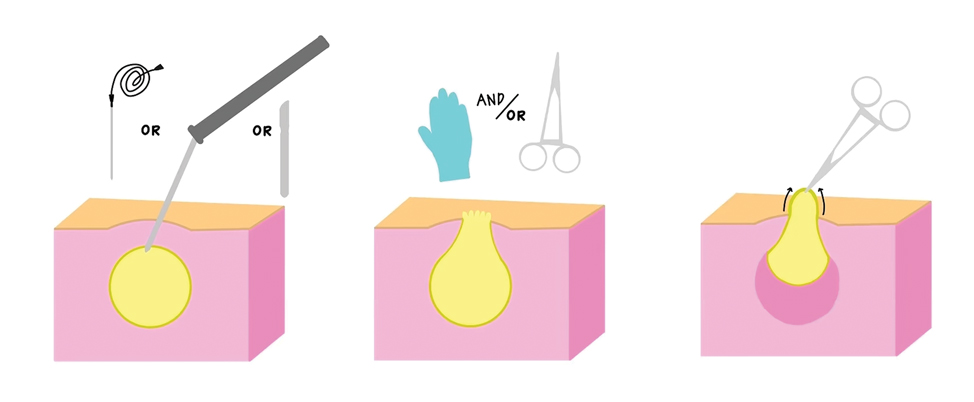
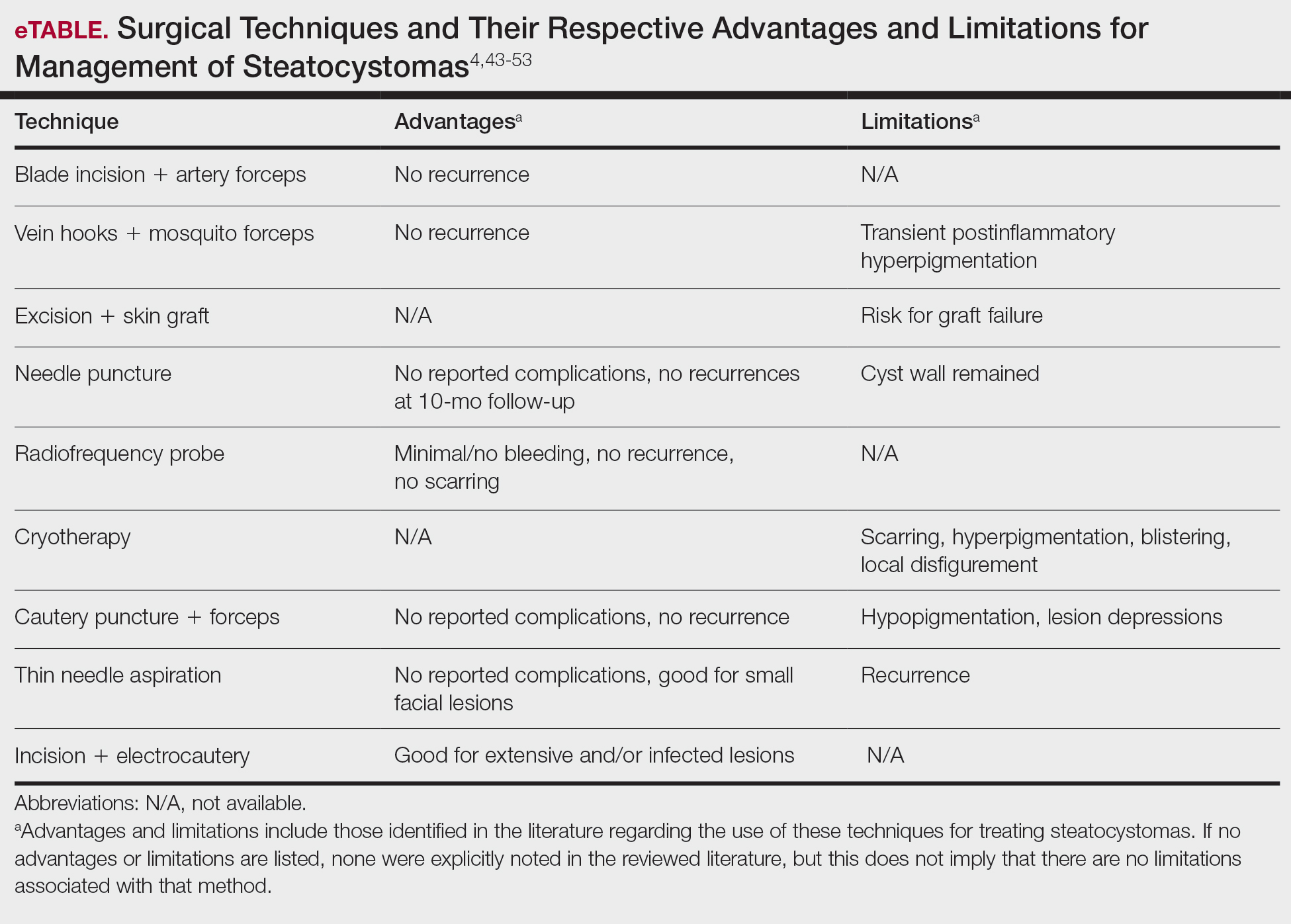
Laser Treatment—Various laser modalities have been used in the management of steatocystomas, including carbon dioxide lasers, erbium-doped yttrium aluminum garnet lasers, 1450-nm diode plus 1550-nm fractionated erbium-doped fiber lasers, and 1927-nm diode lasers.54,55-57 These lasers are used to perforate the cyst before extirpation and have displayed advantages in minimizing scar length.58 The super-pulse mode of carbon dioxide lasers demonstrates efficacy with minimal scarring and recurrence, and this mode is preferred to minimize thermal damage.54,59 Furthermore, this modality can be especially useful in patients whose condition is refractory to other noninvasive options.59 Similarly, the erbium-doped yttrium aluminum garnet laser was well tolerated with no complications noted.55 The 1927-nm diode laser also displayed good outcomes as well as no recurrence.57 With laser use, it is important to note that multiple treatments are needed to see optimal outcomes.54 Moreover, laser settings must be carefully considered, especially in patients with Fitzpatrick skin type III or higher, and topical anti-inflammatory agents should be considered posttreatment to minimize complications.54,59,60
Recommendations
For management of SS, we recommend conservative therapy of watchful observation, as scarring or postinflammatory pigment change may be brought on by medical or surgical therapy; however, if SS is cosmetically bothersome, laser or surgical excision can be done (eFigure 4).4,43-53 It is important to counsel the patient on risks/benefits. For SM, watchful observation also is indicated; however, systemic therapies aimed at prevention may be the most efficacious by limiting disease progression, and oral tetracycline or isotretinoin may be tried.4 Tetracyclines have the risk for photosensitivity and are teratogenic, while isotretinoin is extremely teratogenic, requires laboratory monitoring and regular pregnancy tests in women, and often causes substantial mucosal dryness. If lesions are bothersome or refractory to these therapies, intralesional steroids or surgical/laser procedures can be tried throughout multiple visits.43-53 For SMS, systemic therapies frequently are recommended. The risks of systemic tetracycline and isotretinoin therapies must be discussed. Patients with treatment-refractory SMS may require surgical excision or deroofing of sinus tracts.43-53 This management is similar to that of HS and must be tailored to the patient.
Conclusion
Overall, steatocystomas are a relatively rare pathology, with a limited consensus on their etiology and management. This review summarizes the current knowledge on the condition to support clinicians in diagnosis and management, ranging from watchful waiting to surgical removal. By individualizing treatment plans, clinicians ultimately can optimize outcomes in patients with steatocystomas.
- Santana CN, Pereira DD, Lisboa AP, et al. Steatocystoma multiplex suppurativa: case report of a rare condition. An Bras Dermatol. 2016;91(5 suppl 1):51-53.
- Atzori L, Zanniello R, Pilloni L, et al. Steatocystoma multiplex suppurativa associated with hidradenitis suppurativa successfully treated with adalimumab. J Eur Acad Dermatol Venereol. 2019;33(Suppl 6):42-44.
- Jamieson WA. Case of numerous cutaneous cysts scattered over the body. Edinb Med J. 1873;19:223-225.
- Kamra HT, Gadgil PA, Ovhal AG, et al. Steatocystoma multiplex-a rare genetic disorder: a case report and review of the literature. J Clin Diagn Res. 2013;7:166-168.
- Brownstein MH. Steatocystoma simplex. A solitary steatocystoma. Arch Dermatol. 1982;118:409-411.
- McDonald RM, Reed WB. Natal teeth and steatocystoma multiplex complicated by hidradenitis suppurativa. A new syndrome. Arch Dermatol. 1976;112:1132-1134.
- Plewig G, Wolff HH, Braun-Falco O. Steatocystoma multiplex: anatomic reevaluation, electron microscopy, and autoradiography. Arch Dermatol. 1982;272:363-380.
- Fletcher J, Posso-De Los Rios C, Jambrosic J, A, et al. Coexistence of hidradenitis suppurativa and steatocystoma multiplex: is it a new variant of hidradenitis suppurativa? J Cutan Med Surg. 2021;25:586-590.
- Cho S, Chang SE, Choi JH, et al. Clinical and histologic features of 64 cases of steatocystoma multiplex. J Dermatol. 2002;29:152-156.
- Covello SP, Smith FJ, Sillevis Smitt JH, et al. Keratin 17 mutations cause either steatocystoma multiplex or pachyonychia congenita type 2. Br J Dermatol. 1998;139:475-480.
- Liu Q, Wu W, Lu J, et al. Steatocystoma multiplex is associated with the R94C mutation in the KRTl7 gene. Mol Med Rep. 2015;12:5072-5076.
- Yang L, Zhang S, Wang G. Keratin 17 in disease pathogenesis: from cancer to dermatoses. J Pathol. 2019;247:158-165.
- Shamloul G, Khachemoune A. An updated review of the sebaceous gland and its role in health and diseases Part 1: embryology, evolution, structure, and function of sebaceous glands. Dermatol Ther. 2021;34:e14695.
- Del Rosso JQ, Kircik LH, Stein Gold L, et al. Androgens, androgen receptors, and the skin: from the laboratory to the clinic with emphasis on clinical and therapeutic implications. J Drugs Dermatol. 2020;19:30-35.
- Porras Fimbres DC, Wolfe SA, Kelley CE. Proliferation of steatocystomas in 2 transgender men. JAAD Case Rep. 2022;26:70-72.
- Marasca C, Megna M, Donnarumma M, et al. A case of steatocystoma multiplex in a psoriatic patient during treatment with anti-IL-12/23. Skin Appendage Disord. 2020;6:309-311.
- Gordon Spratt EA, Kaplan J, Patel RR, et al. Steatocystoma. Dermatol Online J. 2013;19:20721.
- Sharma A, Agrawal S, Dhurat R, et al. An unusual case of facial steatocystoma multiplex: a clinicopathologic and dermoscopic report. Dermatopathology (Basel). 2018;5:58-63.
- Rahman MH, Islam MS, Ansari NP. Atypical steatocystoma multiplex with calcification. ISRN Dermatol. 2011;2011:381901.
- Beyer AV, Vossmann D. Steatocystoma multiplex. Article in German. Hautarzt. 1996;47:469-471.
- Yanagi T, Matsumura T. Steatocystoma multiplex presenting as acral subcutaneous nodules. Acta Derm Venereol. 2006;86:374-375.
- Marzano AV, Tavecchio S, Balice Y, et al. Acral subcutaneous steatocystoma multiplex: a distinct subtype of the disease? Australas J Dermatol. 2012;53:198-201.
- Ferrandiz C, Peyri J. Steatocystoma multiplex. Article in Spanish. Med Cutan Ibero Lat Am. 1984;12:173-176.
- Alotaibi L, Alsaif M, Alhumidi A, et al. Steatocystoma multiplex suppurativa: a case with unusual giant cysts over the scalp and neck. Case Rep Dermatol. 2019;11:71-76.
- Kim SJ, Park HJ, Oh ST, et al. A case of steatocystoma multiplex limited to scalp. Ann Dermatol. 2009;21:106-109.
- Patrizi A, Neri I, Guerrini V, et al. Persistent milia, steatocystoma multiplex and eruptive vellus hair cysts: variable expression of multiple pilosebaceous cysts within an affected family. Dermatology. 1998;196:392-396.
- Tomková H, Fujimoto W, Arata J. Expression of keratins (K10 and K17) in steatocystoma multiplex, eruptive vellus hair cysts, and epidermoid and trichilemmal cysts. Am J Dermatopathol. 1997;19:250-253.
- Patokar AS, Holani AR, Khandait GH, et al. Eruptive vellus hair cysts: an underdiagnosed entity. Int J Trichology. 2022;14:31-33.
- Ohtake N, Kubota Y, Takayama O, et al. Relationship between steatocystoma multiplex and eruptive vellus hair cysts. J Am Acad Dermatol. 1992;26(5 Pt 2):876-878.
- Yoon H, Kang Y, Park H, et al. Sonographic appearance of steatocystoma: an analysis of 14 pathologically confirmed lesions. Taehan Yongsang Uihakhoe Chi. 2021;82:382-392.
- Varshney M, Aziz M, Maheshwari V, et al. Steatocystoma multiplex. BMJ Case Rep. 2011;2011:bcr0420114165.
- Tsai MH, Hsiao YP, Lin WL, et al. Steatocystoma multiplex as initial impression of non-small cell lung cancer with complete response to gefitinib. Chin J Cancer Res. 2014;26:E5-E9.
- Zussino M, Nazzaro G, Moltrasio C, et al. Coexistence of steatocystoma multiplex and hidradenitis suppurativa: assessment of this unique association by means of ultrasonography and color Doppler. Skin Res Technol. 2019;25:877-880.
- Whittle C, Silva-Hirschberg C, Loyola K, et al. Ultrasonographic spectrum of cutaneous cysts with stratified squamous epithelium in pediatric dermatology: pictorial essay. J Ultrasound Med. 2023;42:923-930.
- Arceu M, Martinez G, Alfaro D, et al. Ultrasound morphologic features of steatocystoma multiplex with clinical correlation. J Ultrasound Med. 2020;39:2255-2260.
- Reick-Mitrisin V, Reddy A, Shah BA. A breast imaging case of steatocystoma multiplex: a rare condition involving multiple anatomic regions. Cureus. 2022;14:E27756.
- Yoon H, Kang Y, Park H, et al. Sonographic appearance of steatocystoma: an analysis of 14 pathologically confirmed lesions. Taehan Yongsang Uihakhoe Chi. 2021;82:382-392.
- Apaydin R, Bilen N, Bayramgurler D, et al. Steatocystoma multiplex suppurativum: oral isotretinoin treatment combined with cryotherapy. Australas J Dermatol. 2000;41:98-100.
- Sharma A, Agrawal S, Dhurat R, et al. An unusual case of facial steatocystoma multiplex: a clinicopathologic and dermoscopic report. Dermatopathology (Basel). 2018;5:58-63.
- Moritz DL, Silverman RA. Steatocystoma multiplex treated with isotretinoin: a delayed response. Cutis. 1988;42:437-439.
- Schwartz JL, Goldsmith LA. Steatocystoma multiplex suppurativum: treatment with isotretinoin. Cutis. 1984;34:149-153.
- Kim SJ, Park HJ, Oh ST, et al. A case of steatocystoma multiplex limited to the scalp. Ann Dermatol. 2009;21:106-109.
- Fekete GL, Fekete JE. Steatocystoma multiplex generalisata partially suppurativa--case report. Acta Dermatovenerol Croat. 2010;18:114-119.
- Choudhary S, Koley S, Salodkar A. A modified surgical technique for steatocystoma multiplex. J Cutan Aesthet Surg. 2010;3:25-28.
- Kaya TI, Ikizoglu G, Kokturk A, et al. A simple surgical technique for the treatment of steatocystoma multiplex. Int J Dermatol. 2001;40:785-788.
- Oertel YC, Scott DM. Cytologic-pathologic correlations: fine needle aspiration of three cases of steatocystoma multiplex. Ann Diagn Pathol. 1998;2:318-320.
- Egbert BM, Price NM, Segal RJ. Steatocystoma multiplex. Report of a florid case and a review. Arch Dermatol. 1979;115:334-335.
- Adams BB, Mutasim DF, Nordlund JJ. Steatocystoma multiplex: a quick removal technique. Cutis. 1999;64:127-130.
- Lee SJ, Choe YS, Park BC, et al. The vein hook successfully used for eradication of steatocystoma multiplex. Dermatol Surg. 2007;33:82-84.
- Bettes PSL, Lopes SL, Prestes MA, et al. Treatment of a facial variant of the multiple steatocystoma with skin graft: case report. Rev Bras Cir Plást. 1998;13:31-36
- Düzova AN, Sentürk GB. Suggestion for the treatment of steatocystoma multiplex located exclusively on the face. Int J Dermatol. 2004;43:60-62. doi:10.1111/j.1365-4632.2004.02068.x
- Choudhary S, Koley S, Salodkar A. A modified surgical technique for steatocystoma multiplex. J Cutan Aesthet Surg. 2010;3:25-28.
- Kaya TI, Ikizoglu G, Kokturk A, et al. A simple surgical technique for the treatment of steatocystoma multiplex. Int J Dermatol. 2001;40:785-788.
- Bakkour W, Madan V. Carbon dioxide laser perforation and extirpation of steatocystoma multiplex. Dermatol Surg. 2014;40:658-662.
- Mumcuog?lu CT, Gurel MS, Kiremitci U, et al. Er: yag laser therapy for steatocystoma multiplex. Indian J Dermatol. 2010;55:300-301.
- Moody MN, Landau JM, Goldberg LH, et al. 1,450-nm diode laser in combination with the 1550-nm fractionated erbium-doped fiber laser for the treatment of steatocystoma multiplex: a case report. Dermatol Surg. 2012;38(7 Pt 1):1104-1106.
- Cheon DU, Ko JY. 1927-nm fiber-optic diode laser: a novel therapeutic option for facial steatocystoma multiplex. J Cosmet Dermatol. 2019;18:1326-1329.
- Kim KT, Sun H, Chung EH. Comparison of complete surgical excision and minimally invasive excision using CO2 laser for removal of epidermal cysts on the face. Arch Craniofac Surg. 2019;20:84-88.
- Kassira S, Korta DZ, de Feraudy S, et al. Fractionated ablative carbon dioxide laser treatment of steatocystoma multiplex. J Cosmet Laser Ther. 2016;18:364-366.
- Dixit N, Sardana K, Paliwal P. The rationale of ideal pulse duration and pulse interval in the treatment of steatocystoma multiplex using the carbon dioxide laser in a super-pulse mode as opposedto the ultra-pulse mode. Indian J Dermatol Venereol Leprol. 2020;86:454-456.
Steatocystomas are small sebum-filled cysts that typically manifest in the dermis and originate from sebaceous follicles. Although commonly asymptomatic, these lesions can manifest with pruritus or become infected, predisposing patients to further complications.1 Steatocystomas can manifest as single (steatocystoma simplex [SS]) or numerous (steatocystoma multiplex [SM]) lesions; the lesions also can spontaneously rupture with characteristics that resemble hidradenitis suppurativa (HS)(steatocystoma multiplex suppurativa [SMS]).1,2
Steatocystomas are relatively rare, and there is limited consensus in the published literature on the etiology and management of this condition. In this article, we present a comprehensive review of steatocystomas in the current literature. We highlight important features to consider when making the diagnosis and also offer recommendations for best-practice treatment.
Historical Background
Although not explicitly identified by name, the first documentation of steatocystomas is a case report published in 1873. In this account, the author described a patient who presented with approximately 250 flesh-colored dermal cysts across the body that varied in size.3 In 1899, the term steatocystoma multiple—derived from Greek roots meaning “fatty bag”—was first used.4
In 1982, almost a century later, Brownstein5 reported some of the earliest cases of SS. This solitary subtype is identical to SM on a microscopic level; however, unlike SM, this variant occurs as a single lesion that typically forms in adulthood and in the absence of family history. Other benign adnexal tumors (eg, pilomatricomas, pilar cysts, and sebaceous hyperplasias) also can manifest as either solitary or multiple lesions.
In 1976, McDonald and Reed6 reported the first known cases of patients with both SM and HS. At the time, the co-occurrence of these conditions was viewed as coincidental, but there were postulations of a shared inflammatory process and hereditary link6; it was not until 1982 that the term steatocystoma multiplex suppurativum was coined to describe this variant.7 Although rare, there have been multiple documented instances of SMS since. It has been suggested that the convergence of these conditions may indicate a shared follicular proliferation defect.8 Ongoing investigation is warranted to explain the underlying pathogenesis of this unique variant.
Epidemiology
The available epidemiologic data primarily relate to SM, the most common steatocystoma variant. Nevertheless, SM is a relatively rare condition, and the exact incidence and prevalence remain unknown.8,9 Steatocystomas typically manifest in the first and second decades of life and have been observed in patients of both sexes, with studies demonstrating no notable sex bias.4,9
Etiology and Pathophysiology
Steatocystomas can occur sporadically or may be inherited as an autosomal-dominant condition.4 Typically, SS tends to manifest as an isolated occurrence without any inherent genetic predisposition.5 Alternatively, SM may develop sporadically or be associated with a mutation in the keratin 17 gene (KRT17).4 Steatocystoma multiplex also has been associated with at least 4 different missense mutations, including N92H, R94H, and R94C, located on the long (q) arm of chromosome 17.4,10-12
The keratin 17 gene is responsible for encoding the keratin 17 protein, a type I intermediate filament predominantly synthesized in the basal cells of epithelial tissue. This fibrous structural protein can regulate many processes, including inflammation and cell proliferation, and is found in regions such as the sebaceous glands, hair follicles, and eccrine sweat glands. Overexpression of KRT17 has been suggested in other cutaneous conditions, most notably psoriasis.12 Despite KRT17’s many roles, it remains unclear why SM typically manifests with a myriad of sebum-containing cysts as the primary symptom.12 Continued investigation into the genetic underpinnings of SM and the keratin 17 protein is necessary to further elucidate a more comprehensive understanding of this condition.
Hormonal influences have been suggested as a potential trigger for steatocystoma growth.4,13 This condition is associated with dysfunction of the sebaceous glands, and, correspondingly, the incidence of disease is highest in pubertal patients, in whom androgen levels and sebum production are elevated.4,13,14 Two cases of transgender men taking testosterone therapy presenting with steatocystomas provide additional clinical support for this association.15
Additionally, the use of immunomodulatory agents, such as ustekinumab (anti–interleukin 12/interleukin 23), has been shown to trigger SM. It is predicted that the reduced expression of certain interferons and interleukins may lead to downstream consequences in the keratin 17 pathway and lead to SM lesion formation in genetically susceptible individuals.16 Targeting these potential causes in the future may prove efficacious in the secondary prevention of familial SM manifestation or exacerbations.
Mutations in the KRT17 gene also have been implicated in pachyonychia congenita type 2 (PC-2).4 Marked by extensive systemic hyperkeratosis, PC-2 has been observed to coincide with SM in certain patients.4,5 Interestingly, the location of the KRT17 mutations are identical in both PC-2 and SM.4 Although most individuals with hereditary SM do not exhibit the characteristic features of PC-2, mild nail and dental abnormalities have been observed in some SM cases.4,10 This relationship suggests that SM may be a less severe variant of PC-2 or part of a complex polygenetic spectrum of disease.10 Further research is imperative to determine the exact nature and extent of the relationship between these conditions.
Clinical Manifestations
Steatocystomas are flesh-colored subcutaneous cysts that range in size from less than 3 mm to larger than 3 cm in diameter (Figure). They form within a single pilosebaceous unit and typically display firm attachment due to their origination in the dermis.2,7,17 Steatocystomas generally contain lipid material, and less frequently, keratin and hair shafts, distinguishing them as the only “true” sebaceous cysts.18 Their color can range from flesh-toned to yellow, with reports of occasional dark-blue shades and calcifications.19,20 Steatocystomas can persist indefinitely, and they usually are asymptomatic.

Diagnosis of steatocystoma is confirmed by biopsy.4 Steatocystomas are characterized by a dermal cyst lined by stratified squamous cell epithelium (eFigures 1 and 2).21 Classically they feature flattened sebaceous lobules, multinucleated giant cells, and abortive hair follicles. The lining of these cysts is marked by lymphocytic infiltrate and a dense, wrinkled, eosinophilic keratin cuticle that replaces the granular layer.22 The cyst maintains an epidermal connection through a follicular infundibulum characterized by clumps of keratinocytes, sebocytes, corneocytes, and/or hair follicles.7 Aspirated contents reveal crystalline structures and anucleate squamous cells upon microscopic analysis. That being said, variable histologic findings of steatocystomas have been described.23


Steatocystoma simplex, as the name implies, classifies a single isolated steatocystoma. This subtype exhibits similar histopathologic and clinical features to the other subtypes of steatocystomas. Notably, SS is not associated with a genetic mutation and is not an inherited condition within families.5 Steatocystoma multiplex manifests with many steatocystomas, often distributed widely across the body.3,4 The chest, axillae, and groin are the most common locations; however, these cysts can manifest on the face, back, abdomen, and extremities.4,18-22 Rare occurrences of SM limited to the face, scalp, and distal extremities have been documented.18,21,24,25 Due to the possibility of an autosomal-dominant inheritance, it is advisable to take a comprehensive family history in patients for whom SM is in the differential.17
Steatocystoma multiplex—especially familial variants—has been shown to develop in conjunction with other dermatologic conditions, including eruptive vellus hair (EVH) cysts, persistent infantile milia, and epidermoid/dermoid cysts.26 While some investigators regard these as separate entities due to their varied genetic etiology, it has been suggested that these conditions may be related and that the diagnosis is determined by the location of cyst origin along the sebaceous ducts.26,27 Other dermatologic conditions and lesions that frequently manifest comorbidly with SM include hidrocystomas, syringomas, pilonidal cysts, lichen planus, nodulocystic acne, trichotillomania, trichoblastomas, trichoepithelioma, HS, keratoacanthomas, acrokeratosis verruciformis of Hopf, and embryonal hair formation. Steatocystoma multiplex, manifesting comorbidly with dental and orofacial malformations (eg, partial noneruption of secondary teeth, natal and defective teeth, and bilateral preauricular sinuses) has been classified as SM natal teeth syndrome.6
Steatocystoma multiplex suppurativa is a rare and serious variant of SM characterized by inflammation, cyst rupture, sinus tract formation, and scarring.24 Patients with SMS typically have multiple intact SM cysts, which can aid in differentiation from HS.2,24 Steatocystoma multiplex suppurativa is associated with more complications than SS and SM, including cyst perforation, development of purulent and/or foul-smelling discharge, infection, scarring, pain, and overall discomfort.2
Given its rarity and the potential manifestations that overlap with other conditions, steatocystomas easily can be misdiagnosed. In some clinical instances, EVHs may share similar characteristics with SM; however, certain distinguishing features exist, including a central tuft of protruding hairs and different expressed contents, such as the vellus hair shafts, from the cyst’s lumen.28 Furthermore, histologic examination of EVHs reveals epidermoid keratinization of the lining as well as a lack of sebaceous glands within the wall.28,29 Other similar conditions include epidermoid cysts, pilar cysts, lipomas, epidermal inclusion cysts, dermoid cysts, sebaceous hyperplasia, folliculitis, xanthomas, neurofibromatosis, and syringomas.30 Occasionally, SMS can be mistaken for HS or acne conglobata, and SM lesions with a facial distribution can mimic acne vulgaris.1,31 These conditions should be excluded before a diagnosis of SS, SM, or SMS is made.
Importantly, SM is visually indistinguishable from subcutaneous metastasis on physical examination, and there are reports of oncologic conditions (eg, pulmonary adenocarcinoma metastasized to the skin) being mistaken for SS or SM.32 Therefore, a thorough clinical examination, histopathologic analysis, and potential use of other imaging modalities such as ultrasonography (US) are needed to ensure an accurate diagnosis.
Ultrasonography has demonstrated utility in diagnosing steatocystomas.33-35 Steatocystomas have incidentally been found on routine mammograms and can demonstrate well-defined circular nodules with radiolucent characteristics and a thin radiodense outline.33,36 Homogeneous hypoechoic nodules within the dermis without posterior acoustic features generally are observed (eFigure 3).33,37 In patients declining biopsy, US may be useful in further characterization of an unknown lesion. Color Doppler US can be used to distinguish SMS from HS. Specifically, SM typically exhibits an absence of Doppler signaling due to a lack of vascularity, providing a helpful diagnostic clue for the SMS variant.33

Management and Treatment Options
There is no established standard treatment for steatocystomas; therefore, the approach to management is contingent on clinical presentation and patient preferences. Various medical, surgical, and laser management options are available, each with its own advantages and limitations. Treatment of SM is difficult due to the large number of lesions.38 In many cases, continued observation is a viable treatment option, as most SS and SM lesions are asymptomatic; however, cosmetic concerns can be debilitating for patients with SM and may warrant intervention.39 More extensive medical and surgical management often are necessary in SMS due to associated morbidity. Discussing options and goals as well as setting realistic expectations with the patient are essential in determining the optimal approach.
Medical Management—In medical literature, oral isotretinoin (13-cis-retinoic acid) has been the mainstay of therapy for steatocystoma, as its effect on the size and activity of sebaceous glands is hypothesized to decrease disease activity.38,40 Interventional studies and case reports have exhibited varying degrees of effectiveness.1,38-41 Some reports depict a reduction in the formation of new lesions and a decrease in the size of pre-existing lesions, some show mild delayed therapeutic efficacy, and others suggest exacerbation of the condition.1,38-41 This outcome variability is attributed to isotretinoin’s preferential efficacy in treating inflammatory lesions.40,42
Tetracycline derivatives and intralesional steroid injections also have been employed with some efficacy in patients with focal inflammatory SM and SMS.43 There is limited evidence on the long-term outcomes of these interventions, and intralesional injections often are not recommended in conditions such as SM, in which there are many lesions present.
Surgical Management—Minimally invasive surgical procedures including drainage and resections have been used with varying efficacy in SS and SM. Typically, a 2- to 3-mm incision or sharp-tipped cautery is employed to puncture the cyst. Alternatively, radiofrequency probes with a 2.4-MHz frequency setting have been used to minimize incision size.44 The contents then are expressed with manual pressure or forceps, and the cyst sac is extracted using forceps and/or a vein hook (eFigure 4).44,45 The specific surgical techniques and their respective advantages and limitations are summarized in the eTable. Reported advantages and limitations of surgical techniques are derived from information provided by the authors of steatocystoma case reports, which are based on observations of a very limited sample size.


Laser Treatment—Various laser modalities have been used in the management of steatocystomas, including carbon dioxide lasers, erbium-doped yttrium aluminum garnet lasers, 1450-nm diode plus 1550-nm fractionated erbium-doped fiber lasers, and 1927-nm diode lasers.54,55-57 These lasers are used to perforate the cyst before extirpation and have displayed advantages in minimizing scar length.58 The super-pulse mode of carbon dioxide lasers demonstrates efficacy with minimal scarring and recurrence, and this mode is preferred to minimize thermal damage.54,59 Furthermore, this modality can be especially useful in patients whose condition is refractory to other noninvasive options.59 Similarly, the erbium-doped yttrium aluminum garnet laser was well tolerated with no complications noted.55 The 1927-nm diode laser also displayed good outcomes as well as no recurrence.57 With laser use, it is important to note that multiple treatments are needed to see optimal outcomes.54 Moreover, laser settings must be carefully considered, especially in patients with Fitzpatrick skin type III or higher, and topical anti-inflammatory agents should be considered posttreatment to minimize complications.54,59,60
Recommendations
For management of SS, we recommend conservative therapy of watchful observation, as scarring or postinflammatory pigment change may be brought on by medical or surgical therapy; however, if SS is cosmetically bothersome, laser or surgical excision can be done (eFigure 4).4,43-53 It is important to counsel the patient on risks/benefits. For SM, watchful observation also is indicated; however, systemic therapies aimed at prevention may be the most efficacious by limiting disease progression, and oral tetracycline or isotretinoin may be tried.4 Tetracyclines have the risk for photosensitivity and are teratogenic, while isotretinoin is extremely teratogenic, requires laboratory monitoring and regular pregnancy tests in women, and often causes substantial mucosal dryness. If lesions are bothersome or refractory to these therapies, intralesional steroids or surgical/laser procedures can be tried throughout multiple visits.43-53 For SMS, systemic therapies frequently are recommended. The risks of systemic tetracycline and isotretinoin therapies must be discussed. Patients with treatment-refractory SMS may require surgical excision or deroofing of sinus tracts.43-53 This management is similar to that of HS and must be tailored to the patient.
Conclusion
Overall, steatocystomas are a relatively rare pathology, with a limited consensus on their etiology and management. This review summarizes the current knowledge on the condition to support clinicians in diagnosis and management, ranging from watchful waiting to surgical removal. By individualizing treatment plans, clinicians ultimately can optimize outcomes in patients with steatocystomas.
Steatocystomas are small sebum-filled cysts that typically manifest in the dermis and originate from sebaceous follicles. Although commonly asymptomatic, these lesions can manifest with pruritus or become infected, predisposing patients to further complications.1 Steatocystomas can manifest as single (steatocystoma simplex [SS]) or numerous (steatocystoma multiplex [SM]) lesions; the lesions also can spontaneously rupture with characteristics that resemble hidradenitis suppurativa (HS)(steatocystoma multiplex suppurativa [SMS]).1,2
Steatocystomas are relatively rare, and there is limited consensus in the published literature on the etiology and management of this condition. In this article, we present a comprehensive review of steatocystomas in the current literature. We highlight important features to consider when making the diagnosis and also offer recommendations for best-practice treatment.
Historical Background
Although not explicitly identified by name, the first documentation of steatocystomas is a case report published in 1873. In this account, the author described a patient who presented with approximately 250 flesh-colored dermal cysts across the body that varied in size.3 In 1899, the term steatocystoma multiple—derived from Greek roots meaning “fatty bag”—was first used.4
In 1982, almost a century later, Brownstein5 reported some of the earliest cases of SS. This solitary subtype is identical to SM on a microscopic level; however, unlike SM, this variant occurs as a single lesion that typically forms in adulthood and in the absence of family history. Other benign adnexal tumors (eg, pilomatricomas, pilar cysts, and sebaceous hyperplasias) also can manifest as either solitary or multiple lesions.
In 1976, McDonald and Reed6 reported the first known cases of patients with both SM and HS. At the time, the co-occurrence of these conditions was viewed as coincidental, but there were postulations of a shared inflammatory process and hereditary link6; it was not until 1982 that the term steatocystoma multiplex suppurativum was coined to describe this variant.7 Although rare, there have been multiple documented instances of SMS since. It has been suggested that the convergence of these conditions may indicate a shared follicular proliferation defect.8 Ongoing investigation is warranted to explain the underlying pathogenesis of this unique variant.
Epidemiology
The available epidemiologic data primarily relate to SM, the most common steatocystoma variant. Nevertheless, SM is a relatively rare condition, and the exact incidence and prevalence remain unknown.8,9 Steatocystomas typically manifest in the first and second decades of life and have been observed in patients of both sexes, with studies demonstrating no notable sex bias.4,9
Etiology and Pathophysiology
Steatocystomas can occur sporadically or may be inherited as an autosomal-dominant condition.4 Typically, SS tends to manifest as an isolated occurrence without any inherent genetic predisposition.5 Alternatively, SM may develop sporadically or be associated with a mutation in the keratin 17 gene (KRT17).4 Steatocystoma multiplex also has been associated with at least 4 different missense mutations, including N92H, R94H, and R94C, located on the long (q) arm of chromosome 17.4,10-12
The keratin 17 gene is responsible for encoding the keratin 17 protein, a type I intermediate filament predominantly synthesized in the basal cells of epithelial tissue. This fibrous structural protein can regulate many processes, including inflammation and cell proliferation, and is found in regions such as the sebaceous glands, hair follicles, and eccrine sweat glands. Overexpression of KRT17 has been suggested in other cutaneous conditions, most notably psoriasis.12 Despite KRT17’s many roles, it remains unclear why SM typically manifests with a myriad of sebum-containing cysts as the primary symptom.12 Continued investigation into the genetic underpinnings of SM and the keratin 17 protein is necessary to further elucidate a more comprehensive understanding of this condition.
Hormonal influences have been suggested as a potential trigger for steatocystoma growth.4,13 This condition is associated with dysfunction of the sebaceous glands, and, correspondingly, the incidence of disease is highest in pubertal patients, in whom androgen levels and sebum production are elevated.4,13,14 Two cases of transgender men taking testosterone therapy presenting with steatocystomas provide additional clinical support for this association.15
Additionally, the use of immunomodulatory agents, such as ustekinumab (anti–interleukin 12/interleukin 23), has been shown to trigger SM. It is predicted that the reduced expression of certain interferons and interleukins may lead to downstream consequences in the keratin 17 pathway and lead to SM lesion formation in genetically susceptible individuals.16 Targeting these potential causes in the future may prove efficacious in the secondary prevention of familial SM manifestation or exacerbations.
Mutations in the KRT17 gene also have been implicated in pachyonychia congenita type 2 (PC-2).4 Marked by extensive systemic hyperkeratosis, PC-2 has been observed to coincide with SM in certain patients.4,5 Interestingly, the location of the KRT17 mutations are identical in both PC-2 and SM.4 Although most individuals with hereditary SM do not exhibit the characteristic features of PC-2, mild nail and dental abnormalities have been observed in some SM cases.4,10 This relationship suggests that SM may be a less severe variant of PC-2 or part of a complex polygenetic spectrum of disease.10 Further research is imperative to determine the exact nature and extent of the relationship between these conditions.
Clinical Manifestations
Steatocystomas are flesh-colored subcutaneous cysts that range in size from less than 3 mm to larger than 3 cm in diameter (Figure). They form within a single pilosebaceous unit and typically display firm attachment due to their origination in the dermis.2,7,17 Steatocystomas generally contain lipid material, and less frequently, keratin and hair shafts, distinguishing them as the only “true” sebaceous cysts.18 Their color can range from flesh-toned to yellow, with reports of occasional dark-blue shades and calcifications.19,20 Steatocystomas can persist indefinitely, and they usually are asymptomatic.

Diagnosis of steatocystoma is confirmed by biopsy.4 Steatocystomas are characterized by a dermal cyst lined by stratified squamous cell epithelium (eFigures 1 and 2).21 Classically they feature flattened sebaceous lobules, multinucleated giant cells, and abortive hair follicles. The lining of these cysts is marked by lymphocytic infiltrate and a dense, wrinkled, eosinophilic keratin cuticle that replaces the granular layer.22 The cyst maintains an epidermal connection through a follicular infundibulum characterized by clumps of keratinocytes, sebocytes, corneocytes, and/or hair follicles.7 Aspirated contents reveal crystalline structures and anucleate squamous cells upon microscopic analysis. That being said, variable histologic findings of steatocystomas have been described.23


Steatocystoma simplex, as the name implies, classifies a single isolated steatocystoma. This subtype exhibits similar histopathologic and clinical features to the other subtypes of steatocystomas. Notably, SS is not associated with a genetic mutation and is not an inherited condition within families.5 Steatocystoma multiplex manifests with many steatocystomas, often distributed widely across the body.3,4 The chest, axillae, and groin are the most common locations; however, these cysts can manifest on the face, back, abdomen, and extremities.4,18-22 Rare occurrences of SM limited to the face, scalp, and distal extremities have been documented.18,21,24,25 Due to the possibility of an autosomal-dominant inheritance, it is advisable to take a comprehensive family history in patients for whom SM is in the differential.17
Steatocystoma multiplex—especially familial variants—has been shown to develop in conjunction with other dermatologic conditions, including eruptive vellus hair (EVH) cysts, persistent infantile milia, and epidermoid/dermoid cysts.26 While some investigators regard these as separate entities due to their varied genetic etiology, it has been suggested that these conditions may be related and that the diagnosis is determined by the location of cyst origin along the sebaceous ducts.26,27 Other dermatologic conditions and lesions that frequently manifest comorbidly with SM include hidrocystomas, syringomas, pilonidal cysts, lichen planus, nodulocystic acne, trichotillomania, trichoblastomas, trichoepithelioma, HS, keratoacanthomas, acrokeratosis verruciformis of Hopf, and embryonal hair formation. Steatocystoma multiplex, manifesting comorbidly with dental and orofacial malformations (eg, partial noneruption of secondary teeth, natal and defective teeth, and bilateral preauricular sinuses) has been classified as SM natal teeth syndrome.6
Steatocystoma multiplex suppurativa is a rare and serious variant of SM characterized by inflammation, cyst rupture, sinus tract formation, and scarring.24 Patients with SMS typically have multiple intact SM cysts, which can aid in differentiation from HS.2,24 Steatocystoma multiplex suppurativa is associated with more complications than SS and SM, including cyst perforation, development of purulent and/or foul-smelling discharge, infection, scarring, pain, and overall discomfort.2
Given its rarity and the potential manifestations that overlap with other conditions, steatocystomas easily can be misdiagnosed. In some clinical instances, EVHs may share similar characteristics with SM; however, certain distinguishing features exist, including a central tuft of protruding hairs and different expressed contents, such as the vellus hair shafts, from the cyst’s lumen.28 Furthermore, histologic examination of EVHs reveals epidermoid keratinization of the lining as well as a lack of sebaceous glands within the wall.28,29 Other similar conditions include epidermoid cysts, pilar cysts, lipomas, epidermal inclusion cysts, dermoid cysts, sebaceous hyperplasia, folliculitis, xanthomas, neurofibromatosis, and syringomas.30 Occasionally, SMS can be mistaken for HS or acne conglobata, and SM lesions with a facial distribution can mimic acne vulgaris.1,31 These conditions should be excluded before a diagnosis of SS, SM, or SMS is made.
Importantly, SM is visually indistinguishable from subcutaneous metastasis on physical examination, and there are reports of oncologic conditions (eg, pulmonary adenocarcinoma metastasized to the skin) being mistaken for SS or SM.32 Therefore, a thorough clinical examination, histopathologic analysis, and potential use of other imaging modalities such as ultrasonography (US) are needed to ensure an accurate diagnosis.
Ultrasonography has demonstrated utility in diagnosing steatocystomas.33-35 Steatocystomas have incidentally been found on routine mammograms and can demonstrate well-defined circular nodules with radiolucent characteristics and a thin radiodense outline.33,36 Homogeneous hypoechoic nodules within the dermis without posterior acoustic features generally are observed (eFigure 3).33,37 In patients declining biopsy, US may be useful in further characterization of an unknown lesion. Color Doppler US can be used to distinguish SMS from HS. Specifically, SM typically exhibits an absence of Doppler signaling due to a lack of vascularity, providing a helpful diagnostic clue for the SMS variant.33

Management and Treatment Options
There is no established standard treatment for steatocystomas; therefore, the approach to management is contingent on clinical presentation and patient preferences. Various medical, surgical, and laser management options are available, each with its own advantages and limitations. Treatment of SM is difficult due to the large number of lesions.38 In many cases, continued observation is a viable treatment option, as most SS and SM lesions are asymptomatic; however, cosmetic concerns can be debilitating for patients with SM and may warrant intervention.39 More extensive medical and surgical management often are necessary in SMS due to associated morbidity. Discussing options and goals as well as setting realistic expectations with the patient are essential in determining the optimal approach.
Medical Management—In medical literature, oral isotretinoin (13-cis-retinoic acid) has been the mainstay of therapy for steatocystoma, as its effect on the size and activity of sebaceous glands is hypothesized to decrease disease activity.38,40 Interventional studies and case reports have exhibited varying degrees of effectiveness.1,38-41 Some reports depict a reduction in the formation of new lesions and a decrease in the size of pre-existing lesions, some show mild delayed therapeutic efficacy, and others suggest exacerbation of the condition.1,38-41 This outcome variability is attributed to isotretinoin’s preferential efficacy in treating inflammatory lesions.40,42
Tetracycline derivatives and intralesional steroid injections also have been employed with some efficacy in patients with focal inflammatory SM and SMS.43 There is limited evidence on the long-term outcomes of these interventions, and intralesional injections often are not recommended in conditions such as SM, in which there are many lesions present.
Surgical Management—Minimally invasive surgical procedures including drainage and resections have been used with varying efficacy in SS and SM. Typically, a 2- to 3-mm incision or sharp-tipped cautery is employed to puncture the cyst. Alternatively, radiofrequency probes with a 2.4-MHz frequency setting have been used to minimize incision size.44 The contents then are expressed with manual pressure or forceps, and the cyst sac is extracted using forceps and/or a vein hook (eFigure 4).44,45 The specific surgical techniques and their respective advantages and limitations are summarized in the eTable. Reported advantages and limitations of surgical techniques are derived from information provided by the authors of steatocystoma case reports, which are based on observations of a very limited sample size.


Laser Treatment—Various laser modalities have been used in the management of steatocystomas, including carbon dioxide lasers, erbium-doped yttrium aluminum garnet lasers, 1450-nm diode plus 1550-nm fractionated erbium-doped fiber lasers, and 1927-nm diode lasers.54,55-57 These lasers are used to perforate the cyst before extirpation and have displayed advantages in minimizing scar length.58 The super-pulse mode of carbon dioxide lasers demonstrates efficacy with minimal scarring and recurrence, and this mode is preferred to minimize thermal damage.54,59 Furthermore, this modality can be especially useful in patients whose condition is refractory to other noninvasive options.59 Similarly, the erbium-doped yttrium aluminum garnet laser was well tolerated with no complications noted.55 The 1927-nm diode laser also displayed good outcomes as well as no recurrence.57 With laser use, it is important to note that multiple treatments are needed to see optimal outcomes.54 Moreover, laser settings must be carefully considered, especially in patients with Fitzpatrick skin type III or higher, and topical anti-inflammatory agents should be considered posttreatment to minimize complications.54,59,60
Recommendations
For management of SS, we recommend conservative therapy of watchful observation, as scarring or postinflammatory pigment change may be brought on by medical or surgical therapy; however, if SS is cosmetically bothersome, laser or surgical excision can be done (eFigure 4).4,43-53 It is important to counsel the patient on risks/benefits. For SM, watchful observation also is indicated; however, systemic therapies aimed at prevention may be the most efficacious by limiting disease progression, and oral tetracycline or isotretinoin may be tried.4 Tetracyclines have the risk for photosensitivity and are teratogenic, while isotretinoin is extremely teratogenic, requires laboratory monitoring and regular pregnancy tests in women, and often causes substantial mucosal dryness. If lesions are bothersome or refractory to these therapies, intralesional steroids or surgical/laser procedures can be tried throughout multiple visits.43-53 For SMS, systemic therapies frequently are recommended. The risks of systemic tetracycline and isotretinoin therapies must be discussed. Patients with treatment-refractory SMS may require surgical excision or deroofing of sinus tracts.43-53 This management is similar to that of HS and must be tailored to the patient.
Conclusion
Overall, steatocystomas are a relatively rare pathology, with a limited consensus on their etiology and management. This review summarizes the current knowledge on the condition to support clinicians in diagnosis and management, ranging from watchful waiting to surgical removal. By individualizing treatment plans, clinicians ultimately can optimize outcomes in patients with steatocystomas.
- Santana CN, Pereira DD, Lisboa AP, et al. Steatocystoma multiplex suppurativa: case report of a rare condition. An Bras Dermatol. 2016;91(5 suppl 1):51-53.
- Atzori L, Zanniello R, Pilloni L, et al. Steatocystoma multiplex suppurativa associated with hidradenitis suppurativa successfully treated with adalimumab. J Eur Acad Dermatol Venereol. 2019;33(Suppl 6):42-44.
- Jamieson WA. Case of numerous cutaneous cysts scattered over the body. Edinb Med J. 1873;19:223-225.
- Kamra HT, Gadgil PA, Ovhal AG, et al. Steatocystoma multiplex-a rare genetic disorder: a case report and review of the literature. J Clin Diagn Res. 2013;7:166-168.
- Brownstein MH. Steatocystoma simplex. A solitary steatocystoma. Arch Dermatol. 1982;118:409-411.
- McDonald RM, Reed WB. Natal teeth and steatocystoma multiplex complicated by hidradenitis suppurativa. A new syndrome. Arch Dermatol. 1976;112:1132-1134.
- Plewig G, Wolff HH, Braun-Falco O. Steatocystoma multiplex: anatomic reevaluation, electron microscopy, and autoradiography. Arch Dermatol. 1982;272:363-380.
- Fletcher J, Posso-De Los Rios C, Jambrosic J, A, et al. Coexistence of hidradenitis suppurativa and steatocystoma multiplex: is it a new variant of hidradenitis suppurativa? J Cutan Med Surg. 2021;25:586-590.
- Cho S, Chang SE, Choi JH, et al. Clinical and histologic features of 64 cases of steatocystoma multiplex. J Dermatol. 2002;29:152-156.
- Covello SP, Smith FJ, Sillevis Smitt JH, et al. Keratin 17 mutations cause either steatocystoma multiplex or pachyonychia congenita type 2. Br J Dermatol. 1998;139:475-480.
- Liu Q, Wu W, Lu J, et al. Steatocystoma multiplex is associated with the R94C mutation in the KRTl7 gene. Mol Med Rep. 2015;12:5072-5076.
- Yang L, Zhang S, Wang G. Keratin 17 in disease pathogenesis: from cancer to dermatoses. J Pathol. 2019;247:158-165.
- Shamloul G, Khachemoune A. An updated review of the sebaceous gland and its role in health and diseases Part 1: embryology, evolution, structure, and function of sebaceous glands. Dermatol Ther. 2021;34:e14695.
- Del Rosso JQ, Kircik LH, Stein Gold L, et al. Androgens, androgen receptors, and the skin: from the laboratory to the clinic with emphasis on clinical and therapeutic implications. J Drugs Dermatol. 2020;19:30-35.
- Porras Fimbres DC, Wolfe SA, Kelley CE. Proliferation of steatocystomas in 2 transgender men. JAAD Case Rep. 2022;26:70-72.
- Marasca C, Megna M, Donnarumma M, et al. A case of steatocystoma multiplex in a psoriatic patient during treatment with anti-IL-12/23. Skin Appendage Disord. 2020;6:309-311.
- Gordon Spratt EA, Kaplan J, Patel RR, et al. Steatocystoma. Dermatol Online J. 2013;19:20721.
- Sharma A, Agrawal S, Dhurat R, et al. An unusual case of facial steatocystoma multiplex: a clinicopathologic and dermoscopic report. Dermatopathology (Basel). 2018;5:58-63.
- Rahman MH, Islam MS, Ansari NP. Atypical steatocystoma multiplex with calcification. ISRN Dermatol. 2011;2011:381901.
- Beyer AV, Vossmann D. Steatocystoma multiplex. Article in German. Hautarzt. 1996;47:469-471.
- Yanagi T, Matsumura T. Steatocystoma multiplex presenting as acral subcutaneous nodules. Acta Derm Venereol. 2006;86:374-375.
- Marzano AV, Tavecchio S, Balice Y, et al. Acral subcutaneous steatocystoma multiplex: a distinct subtype of the disease? Australas J Dermatol. 2012;53:198-201.
- Ferrandiz C, Peyri J. Steatocystoma multiplex. Article in Spanish. Med Cutan Ibero Lat Am. 1984;12:173-176.
- Alotaibi L, Alsaif M, Alhumidi A, et al. Steatocystoma multiplex suppurativa: a case with unusual giant cysts over the scalp and neck. Case Rep Dermatol. 2019;11:71-76.
- Kim SJ, Park HJ, Oh ST, et al. A case of steatocystoma multiplex limited to scalp. Ann Dermatol. 2009;21:106-109.
- Patrizi A, Neri I, Guerrini V, et al. Persistent milia, steatocystoma multiplex and eruptive vellus hair cysts: variable expression of multiple pilosebaceous cysts within an affected family. Dermatology. 1998;196:392-396.
- Tomková H, Fujimoto W, Arata J. Expression of keratins (K10 and K17) in steatocystoma multiplex, eruptive vellus hair cysts, and epidermoid and trichilemmal cysts. Am J Dermatopathol. 1997;19:250-253.
- Patokar AS, Holani AR, Khandait GH, et al. Eruptive vellus hair cysts: an underdiagnosed entity. Int J Trichology. 2022;14:31-33.
- Ohtake N, Kubota Y, Takayama O, et al. Relationship between steatocystoma multiplex and eruptive vellus hair cysts. J Am Acad Dermatol. 1992;26(5 Pt 2):876-878.
- Yoon H, Kang Y, Park H, et al. Sonographic appearance of steatocystoma: an analysis of 14 pathologically confirmed lesions. Taehan Yongsang Uihakhoe Chi. 2021;82:382-392.
- Varshney M, Aziz M, Maheshwari V, et al. Steatocystoma multiplex. BMJ Case Rep. 2011;2011:bcr0420114165.
- Tsai MH, Hsiao YP, Lin WL, et al. Steatocystoma multiplex as initial impression of non-small cell lung cancer with complete response to gefitinib. Chin J Cancer Res. 2014;26:E5-E9.
- Zussino M, Nazzaro G, Moltrasio C, et al. Coexistence of steatocystoma multiplex and hidradenitis suppurativa: assessment of this unique association by means of ultrasonography and color Doppler. Skin Res Technol. 2019;25:877-880.
- Whittle C, Silva-Hirschberg C, Loyola K, et al. Ultrasonographic spectrum of cutaneous cysts with stratified squamous epithelium in pediatric dermatology: pictorial essay. J Ultrasound Med. 2023;42:923-930.
- Arceu M, Martinez G, Alfaro D, et al. Ultrasound morphologic features of steatocystoma multiplex with clinical correlation. J Ultrasound Med. 2020;39:2255-2260.
- Reick-Mitrisin V, Reddy A, Shah BA. A breast imaging case of steatocystoma multiplex: a rare condition involving multiple anatomic regions. Cureus. 2022;14:E27756.
- Yoon H, Kang Y, Park H, et al. Sonographic appearance of steatocystoma: an analysis of 14 pathologically confirmed lesions. Taehan Yongsang Uihakhoe Chi. 2021;82:382-392.
- Apaydin R, Bilen N, Bayramgurler D, et al. Steatocystoma multiplex suppurativum: oral isotretinoin treatment combined with cryotherapy. Australas J Dermatol. 2000;41:98-100.
- Sharma A, Agrawal S, Dhurat R, et al. An unusual case of facial steatocystoma multiplex: a clinicopathologic and dermoscopic report. Dermatopathology (Basel). 2018;5:58-63.
- Moritz DL, Silverman RA. Steatocystoma multiplex treated with isotretinoin: a delayed response. Cutis. 1988;42:437-439.
- Schwartz JL, Goldsmith LA. Steatocystoma multiplex suppurativum: treatment with isotretinoin. Cutis. 1984;34:149-153.
- Kim SJ, Park HJ, Oh ST, et al. A case of steatocystoma multiplex limited to the scalp. Ann Dermatol. 2009;21:106-109.
- Fekete GL, Fekete JE. Steatocystoma multiplex generalisata partially suppurativa--case report. Acta Dermatovenerol Croat. 2010;18:114-119.
- Choudhary S, Koley S, Salodkar A. A modified surgical technique for steatocystoma multiplex. J Cutan Aesthet Surg. 2010;3:25-28.
- Kaya TI, Ikizoglu G, Kokturk A, et al. A simple surgical technique for the treatment of steatocystoma multiplex. Int J Dermatol. 2001;40:785-788.
- Oertel YC, Scott DM. Cytologic-pathologic correlations: fine needle aspiration of three cases of steatocystoma multiplex. Ann Diagn Pathol. 1998;2:318-320.
- Egbert BM, Price NM, Segal RJ. Steatocystoma multiplex. Report of a florid case and a review. Arch Dermatol. 1979;115:334-335.
- Adams BB, Mutasim DF, Nordlund JJ. Steatocystoma multiplex: a quick removal technique. Cutis. 1999;64:127-130.
- Lee SJ, Choe YS, Park BC, et al. The vein hook successfully used for eradication of steatocystoma multiplex. Dermatol Surg. 2007;33:82-84.
- Bettes PSL, Lopes SL, Prestes MA, et al. Treatment of a facial variant of the multiple steatocystoma with skin graft: case report. Rev Bras Cir Plást. 1998;13:31-36
- Düzova AN, Sentürk GB. Suggestion for the treatment of steatocystoma multiplex located exclusively on the face. Int J Dermatol. 2004;43:60-62. doi:10.1111/j.1365-4632.2004.02068.x
- Choudhary S, Koley S, Salodkar A. A modified surgical technique for steatocystoma multiplex. J Cutan Aesthet Surg. 2010;3:25-28.
- Kaya TI, Ikizoglu G, Kokturk A, et al. A simple surgical technique for the treatment of steatocystoma multiplex. Int J Dermatol. 2001;40:785-788.
- Bakkour W, Madan V. Carbon dioxide laser perforation and extirpation of steatocystoma multiplex. Dermatol Surg. 2014;40:658-662.
- Mumcuog?lu CT, Gurel MS, Kiremitci U, et al. Er: yag laser therapy for steatocystoma multiplex. Indian J Dermatol. 2010;55:300-301.
- Moody MN, Landau JM, Goldberg LH, et al. 1,450-nm diode laser in combination with the 1550-nm fractionated erbium-doped fiber laser for the treatment of steatocystoma multiplex: a case report. Dermatol Surg. 2012;38(7 Pt 1):1104-1106.
- Cheon DU, Ko JY. 1927-nm fiber-optic diode laser: a novel therapeutic option for facial steatocystoma multiplex. J Cosmet Dermatol. 2019;18:1326-1329.
- Kim KT, Sun H, Chung EH. Comparison of complete surgical excision and minimally invasive excision using CO2 laser for removal of epidermal cysts on the face. Arch Craniofac Surg. 2019;20:84-88.
- Kassira S, Korta DZ, de Feraudy S, et al. Fractionated ablative carbon dioxide laser treatment of steatocystoma multiplex. J Cosmet Laser Ther. 2016;18:364-366.
- Dixit N, Sardana K, Paliwal P. The rationale of ideal pulse duration and pulse interval in the treatment of steatocystoma multiplex using the carbon dioxide laser in a super-pulse mode as opposedto the ultra-pulse mode. Indian J Dermatol Venereol Leprol. 2020;86:454-456.
- Santana CN, Pereira DD, Lisboa AP, et al. Steatocystoma multiplex suppurativa: case report of a rare condition. An Bras Dermatol. 2016;91(5 suppl 1):51-53.
- Atzori L, Zanniello R, Pilloni L, et al. Steatocystoma multiplex suppurativa associated with hidradenitis suppurativa successfully treated with adalimumab. J Eur Acad Dermatol Venereol. 2019;33(Suppl 6):42-44.
- Jamieson WA. Case of numerous cutaneous cysts scattered over the body. Edinb Med J. 1873;19:223-225.
- Kamra HT, Gadgil PA, Ovhal AG, et al. Steatocystoma multiplex-a rare genetic disorder: a case report and review of the literature. J Clin Diagn Res. 2013;7:166-168.
- Brownstein MH. Steatocystoma simplex. A solitary steatocystoma. Arch Dermatol. 1982;118:409-411.
- McDonald RM, Reed WB. Natal teeth and steatocystoma multiplex complicated by hidradenitis suppurativa. A new syndrome. Arch Dermatol. 1976;112:1132-1134.
- Plewig G, Wolff HH, Braun-Falco O. Steatocystoma multiplex: anatomic reevaluation, electron microscopy, and autoradiography. Arch Dermatol. 1982;272:363-380.
- Fletcher J, Posso-De Los Rios C, Jambrosic J, A, et al. Coexistence of hidradenitis suppurativa and steatocystoma multiplex: is it a new variant of hidradenitis suppurativa? J Cutan Med Surg. 2021;25:586-590.
- Cho S, Chang SE, Choi JH, et al. Clinical and histologic features of 64 cases of steatocystoma multiplex. J Dermatol. 2002;29:152-156.
- Covello SP, Smith FJ, Sillevis Smitt JH, et al. Keratin 17 mutations cause either steatocystoma multiplex or pachyonychia congenita type 2. Br J Dermatol. 1998;139:475-480.
- Liu Q, Wu W, Lu J, et al. Steatocystoma multiplex is associated with the R94C mutation in the KRTl7 gene. Mol Med Rep. 2015;12:5072-5076.
- Yang L, Zhang S, Wang G. Keratin 17 in disease pathogenesis: from cancer to dermatoses. J Pathol. 2019;247:158-165.
- Shamloul G, Khachemoune A. An updated review of the sebaceous gland and its role in health and diseases Part 1: embryology, evolution, structure, and function of sebaceous glands. Dermatol Ther. 2021;34:e14695.
- Del Rosso JQ, Kircik LH, Stein Gold L, et al. Androgens, androgen receptors, and the skin: from the laboratory to the clinic with emphasis on clinical and therapeutic implications. J Drugs Dermatol. 2020;19:30-35.
- Porras Fimbres DC, Wolfe SA, Kelley CE. Proliferation of steatocystomas in 2 transgender men. JAAD Case Rep. 2022;26:70-72.
- Marasca C, Megna M, Donnarumma M, et al. A case of steatocystoma multiplex in a psoriatic patient during treatment with anti-IL-12/23. Skin Appendage Disord. 2020;6:309-311.
- Gordon Spratt EA, Kaplan J, Patel RR, et al. Steatocystoma. Dermatol Online J. 2013;19:20721.
- Sharma A, Agrawal S, Dhurat R, et al. An unusual case of facial steatocystoma multiplex: a clinicopathologic and dermoscopic report. Dermatopathology (Basel). 2018;5:58-63.
- Rahman MH, Islam MS, Ansari NP. Atypical steatocystoma multiplex with calcification. ISRN Dermatol. 2011;2011:381901.
- Beyer AV, Vossmann D. Steatocystoma multiplex. Article in German. Hautarzt. 1996;47:469-471.
- Yanagi T, Matsumura T. Steatocystoma multiplex presenting as acral subcutaneous nodules. Acta Derm Venereol. 2006;86:374-375.
- Marzano AV, Tavecchio S, Balice Y, et al. Acral subcutaneous steatocystoma multiplex: a distinct subtype of the disease? Australas J Dermatol. 2012;53:198-201.
- Ferrandiz C, Peyri J. Steatocystoma multiplex. Article in Spanish. Med Cutan Ibero Lat Am. 1984;12:173-176.
- Alotaibi L, Alsaif M, Alhumidi A, et al. Steatocystoma multiplex suppurativa: a case with unusual giant cysts over the scalp and neck. Case Rep Dermatol. 2019;11:71-76.
- Kim SJ, Park HJ, Oh ST, et al. A case of steatocystoma multiplex limited to scalp. Ann Dermatol. 2009;21:106-109.
- Patrizi A, Neri I, Guerrini V, et al. Persistent milia, steatocystoma multiplex and eruptive vellus hair cysts: variable expression of multiple pilosebaceous cysts within an affected family. Dermatology. 1998;196:392-396.
- Tomková H, Fujimoto W, Arata J. Expression of keratins (K10 and K17) in steatocystoma multiplex, eruptive vellus hair cysts, and epidermoid and trichilemmal cysts. Am J Dermatopathol. 1997;19:250-253.
- Patokar AS, Holani AR, Khandait GH, et al. Eruptive vellus hair cysts: an underdiagnosed entity. Int J Trichology. 2022;14:31-33.
- Ohtake N, Kubota Y, Takayama O, et al. Relationship between steatocystoma multiplex and eruptive vellus hair cysts. J Am Acad Dermatol. 1992;26(5 Pt 2):876-878.
- Yoon H, Kang Y, Park H, et al. Sonographic appearance of steatocystoma: an analysis of 14 pathologically confirmed lesions. Taehan Yongsang Uihakhoe Chi. 2021;82:382-392.
- Varshney M, Aziz M, Maheshwari V, et al. Steatocystoma multiplex. BMJ Case Rep. 2011;2011:bcr0420114165.
- Tsai MH, Hsiao YP, Lin WL, et al. Steatocystoma multiplex as initial impression of non-small cell lung cancer with complete response to gefitinib. Chin J Cancer Res. 2014;26:E5-E9.
- Zussino M, Nazzaro G, Moltrasio C, et al. Coexistence of steatocystoma multiplex and hidradenitis suppurativa: assessment of this unique association by means of ultrasonography and color Doppler. Skin Res Technol. 2019;25:877-880.
- Whittle C, Silva-Hirschberg C, Loyola K, et al. Ultrasonographic spectrum of cutaneous cysts with stratified squamous epithelium in pediatric dermatology: pictorial essay. J Ultrasound Med. 2023;42:923-930.
- Arceu M, Martinez G, Alfaro D, et al. Ultrasound morphologic features of steatocystoma multiplex with clinical correlation. J Ultrasound Med. 2020;39:2255-2260.
- Reick-Mitrisin V, Reddy A, Shah BA. A breast imaging case of steatocystoma multiplex: a rare condition involving multiple anatomic regions. Cureus. 2022;14:E27756.
- Yoon H, Kang Y, Park H, et al. Sonographic appearance of steatocystoma: an analysis of 14 pathologically confirmed lesions. Taehan Yongsang Uihakhoe Chi. 2021;82:382-392.
- Apaydin R, Bilen N, Bayramgurler D, et al. Steatocystoma multiplex suppurativum: oral isotretinoin treatment combined with cryotherapy. Australas J Dermatol. 2000;41:98-100.
- Sharma A, Agrawal S, Dhurat R, et al. An unusual case of facial steatocystoma multiplex: a clinicopathologic and dermoscopic report. Dermatopathology (Basel). 2018;5:58-63.
- Moritz DL, Silverman RA. Steatocystoma multiplex treated with isotretinoin: a delayed response. Cutis. 1988;42:437-439.
- Schwartz JL, Goldsmith LA. Steatocystoma multiplex suppurativum: treatment with isotretinoin. Cutis. 1984;34:149-153.
- Kim SJ, Park HJ, Oh ST, et al. A case of steatocystoma multiplex limited to the scalp. Ann Dermatol. 2009;21:106-109.
- Fekete GL, Fekete JE. Steatocystoma multiplex generalisata partially suppurativa--case report. Acta Dermatovenerol Croat. 2010;18:114-119.
- Choudhary S, Koley S, Salodkar A. A modified surgical technique for steatocystoma multiplex. J Cutan Aesthet Surg. 2010;3:25-28.
- Kaya TI, Ikizoglu G, Kokturk A, et al. A simple surgical technique for the treatment of steatocystoma multiplex. Int J Dermatol. 2001;40:785-788.
- Oertel YC, Scott DM. Cytologic-pathologic correlations: fine needle aspiration of three cases of steatocystoma multiplex. Ann Diagn Pathol. 1998;2:318-320.
- Egbert BM, Price NM, Segal RJ. Steatocystoma multiplex. Report of a florid case and a review. Arch Dermatol. 1979;115:334-335.
- Adams BB, Mutasim DF, Nordlund JJ. Steatocystoma multiplex: a quick removal technique. Cutis. 1999;64:127-130.
- Lee SJ, Choe YS, Park BC, et al. The vein hook successfully used for eradication of steatocystoma multiplex. Dermatol Surg. 2007;33:82-84.
- Bettes PSL, Lopes SL, Prestes MA, et al. Treatment of a facial variant of the multiple steatocystoma with skin graft: case report. Rev Bras Cir Plást. 1998;13:31-36
- Düzova AN, Sentürk GB. Suggestion for the treatment of steatocystoma multiplex located exclusively on the face. Int J Dermatol. 2004;43:60-62. doi:10.1111/j.1365-4632.2004.02068.x
- Choudhary S, Koley S, Salodkar A. A modified surgical technique for steatocystoma multiplex. J Cutan Aesthet Surg. 2010;3:25-28.
- Kaya TI, Ikizoglu G, Kokturk A, et al. A simple surgical technique for the treatment of steatocystoma multiplex. Int J Dermatol. 2001;40:785-788.
- Bakkour W, Madan V. Carbon dioxide laser perforation and extirpation of steatocystoma multiplex. Dermatol Surg. 2014;40:658-662.
- Mumcuog?lu CT, Gurel MS, Kiremitci U, et al. Er: yag laser therapy for steatocystoma multiplex. Indian J Dermatol. 2010;55:300-301.
- Moody MN, Landau JM, Goldberg LH, et al. 1,450-nm diode laser in combination with the 1550-nm fractionated erbium-doped fiber laser for the treatment of steatocystoma multiplex: a case report. Dermatol Surg. 2012;38(7 Pt 1):1104-1106.
- Cheon DU, Ko JY. 1927-nm fiber-optic diode laser: a novel therapeutic option for facial steatocystoma multiplex. J Cosmet Dermatol. 2019;18:1326-1329.
- Kim KT, Sun H, Chung EH. Comparison of complete surgical excision and minimally invasive excision using CO2 laser for removal of epidermal cysts on the face. Arch Craniofac Surg. 2019;20:84-88.
- Kassira S, Korta DZ, de Feraudy S, et al. Fractionated ablative carbon dioxide laser treatment of steatocystoma multiplex. J Cosmet Laser Ther. 2016;18:364-366.
- Dixit N, Sardana K, Paliwal P. The rationale of ideal pulse duration and pulse interval in the treatment of steatocystoma multiplex using the carbon dioxide laser in a super-pulse mode as opposedto the ultra-pulse mode. Indian J Dermatol Venereol Leprol. 2020;86:454-456.
Steatocystomas: Update on Clinical Manifestations, Diagnosis, and Management
Steatocystomas: Update on Clinical Manifestations, Diagnosis, and Management
Practice Points
- Steatocystomas, which manifest as single or multiple flesh-colored subcutaneous cysts ranging from less than 3 mm to more than 3 cm, typically are asymptomatic and can persist indefinitely.
- Treatment options for steatocystomas include oral isotretinoin, tetracycline derivatives, and intralesional steroid injections. Minimally invasive procedures such as drainage and resection also are available, employing techniques such as blade incision, radiofrequency probes, and laser treatments to minimize scarring and recurrence.
- Conservative therapies such as watchful waiting are recommended for the simplex and multiplex variants, while more aggressive management such as surgical removal is recommended for the multiplex suppurativa variant due to its elevated risk for complications.
Type VII Collagen Disorders Simplified
Type VII Collagen Disorders Simplified
There are 3 uncommon types of mechanobullous skin diseases caused by relative reduction or complete loss of functional type VII collagen, which is the main component of anchoring fibrils in the lamina densa of the basement membrane zone (BMZ) of the skin and mucous membrane epithelium.1 The function of the anchoring fibrils is to maintain adherence of the basement membrane of the epithelium to the connective tissue of the papillary dermis and submucosa.1 The mechanism of action of the loss of type VII collagen function is via autoimmunity in epidermolysis bullosa acquisita (EBA)2 and
Epidermolysis Bullosa
Epidermolysis bullosa consists of a heterogeneous family of 4 major genetic mechanobullous diseases that affect the skin and mucous membranes with more than 30 subtypes.1 Dystrophic EB is caused by mutations in the COL7A1 gene, which encodes for the α-1 chain of collagen type VII. Classically, EB is divided into 4 main variants based on the location of the cleavage plane or split occurring in the epithelium, which in turn helps to predict the severity of the illness.
Epidermolysis bullosa may be inherited in an autosomal-dominant or autosomal-recessive fashion, or it may occur as a spontaneous mutation. All sexes and races are affected equally. Patients present at birth or in early childhood with fragile skin and mucous membranes that may develop blisters, erosions, and ulcerations after minor trauma.7 These lesions are marked by slow healing and scar formation and often are associated with itching and pain.
Dystrophic Epidermolysis Bullosa
Dystrophic EB accounts for approximately 25%6 of all EB cases in the United States and may be inherited as either a dominant or recessive trait. Hundreds of different pathogenic mutations have been discovered in the COL7A1 gene in the subtypes of DEB.4,8 Dominant DEB tends to cause milder disease because the patients retain one normal COL7A1 allele and produce some type VII collagen (Figure 1), whereas patients with recessive DEB lack type VII collagen completely.9 The cleavage plane is between the lamina densa and the superficial dermis or submucosa. Severity is variable and ranges from localization to the hands and feet to severe generalized blistering and painful ulcerations depending on which of the many possible gene mutations have been inherited. Sequelae include mitten deformities, malalignment and tooth decay, and the development of early aggressive squamous cell carcinomas, which may be fatal. The most severe cases of recessive DEB also may have internal organ involvement.
Epidermolysis Bullosa Simplex
Epidermolysis bullosa simplex is the most common variant, comprising approximately 70%of EB cases in the United States.6 Epidermolysis bullosa simplex usually is inherited as autosomal-dominant mutations in the keratin 5 or keratin 14 genes,10 not COL7A1. Skin blistering results from cleavage within the basal cell layer where the keratin genes are primarily expressed. Blisters tend to occur in acral areas such as hands and feet and may heal without scarring in the localized form of epidermolysis bullosa simplex (Figure 2).
Junctional Epidermolysis Bullosa and Kindler Syndrome
Junctional epidermolysis bullosa (JEB) and Kindler syndrome11 are the rarest of the autosomal-recessive EB variants.6 The plane of cleavage in JEB is through the lamina lucida of the BMZ. Junctional epidermolysis bullosa is caused by mutations of the genes that encode for the 3 chains of laminin 332 protein and type XVII collagen,5,12 not to be confused with type VII collagen. As with DEB, there is a wide range of severity in JEB, from localized effects on the eyes, oral cavity, and tooth enamel to widespread blistering and skin cancers. In JEB cases involving newborns, nonhealing wounds on the face, buttocks, fingers, and toes may be seen, with devastating complications in the oral cavity, esophagus, and larynx. Life expectancy is limited to 2 years or less.6 There have only been approximately 40013 cases of Kindler syndrome reported worldwide6 and there is clinical overlap with DEB. Patients also may demonstrate poikiloderma and photosensitivity. Kindler syndrome is caused by mutations in the FERMT1 gene which encodes for kindlin-1. This protein mediates anchorage between the actin cytoskeleton and the extracellular matrix.5,11 Loss of function produces variable cleavage planes around the dermoepidermal junction.
Clinical management of all EB variants, especially the severe recessive types, traditionally has been limited to the prevention of trauma to the skin and mucous membranes and supportive care, including dressing changes to erosions and ulcerations, antibiotic ointments as needed, and amelioration of pain and pruritus. Bone marrow and pluripotential stem cell transplants have been attempted.12 Complications of EB, such as deformities of the hands and feet caused by excessive scarring, esophageal strictures, poor dentition, and squamous cell carcinomas, must be addressed by a multidisciplinary team of specialists, including plastic surgery, gastroenterology, dentistry/oral surgery, ophthalmology, and dermatology/Mohs surgery.
Until recently, there were no medications approved by the US Food and Drug Administration (FDA) specifically indicated for EB. In 2023, topical gene therapy was approved by the FDA for both recessive and dominant forms of DEB. Normal COL7A1 sequences are delivered by an attenuated herpes simplex virus 1 vector (beremagene geperpavec) in a gel applied directly to the wounds of patients with DEB. In a clinical trial, matching wounds on 31 patients (62 wounds total) were treated with the active agent or placebo gel. After 6 months, complete wound closure was observed in 67% (21/31) of those treated with the active agent and 22% (7/31) of those treated with placebo (P=.002).14 In a single case report, a patient with recessive DEB and cicatrizing conjunctivitis (Figure 3) was given ophthalmic beremagene geperpavec after surgery and had improved visual acuity.15 A topical gel consisting of birch triterpenes to promote healing of partial-thickness wounds also was approved for patients with DEB and JEB by the FDA and the European Commission. In a study of 223 patients, 41% of those using active gel and 29% of those using placebo gel achieved the primary end point of percentage of target wounds that had first complete closure at 45 days.16
The most recent FDA approval for DEB involves transferring the functional COL7A1 gene to the patient’s skin cells, then expanding the gene-corrected cells into sheets of keratinocytes that can be surgically applied to the chronic wound sites. In a phase 3 trial of prademagene zamikeracel (pz-cel), 11 patients with 86 matched wounds were randomized to receive pz-cel (50%) or standard wound care (50%). After 24 weeks, 35 wounds treated with pz-cel were at least 50% healed compared to 7 control wounds.17 The results for healing and reduction of pain were statistically significant (P<.0001 and P<.0002, respectively).17 Recombinant collagen VII as replacement therapy also is under study to be given by intravenous infusion to increase tissue collagen VII where it is lacking. This treatment has shown early biologic and therapeutic effects.9,18 Larger long-term follow-up studies are necessary to confirm persistence of the gene-corrected skin cells, the functionality of the replacement collagen VII, and the potential risk for the development of autoantibodies to type VII collagen.
Epidermolysis Bullosa Acquisita
Epidermolysis bullosa acquisita is a rare autoimmune subepithelial bullous disease that primarily affects middle-aged adults but also has been reported in children.19 Epidermolysis bullosa acquisita is caused by circulating pathogenic IgG autoantibodies that target and bind to type VII collagen in the anchoring fibrils,20-22 thereby disrupting the attachment of the epithelium to its underlying connective tissue.
The 2 major clinical manifestations of EBA include a mechanobullous disease resembling inherited forms of DEB (Figure 4) and an inflammatory bullous pemphigoid (BP)–like disease,23 as well as a combination of both types of skin lesions (Figure 5). The skin and mucous membranes of the oral cavity, esophagus, eyes, and urogenital areas are affected in both types; scarring may cause functional disabilities. In the mechanobullous type of EBA, it is common for blisters and erosions to develop in trauma-prone areas such as the hands, feet, elbows, and knees. The blisters tend to heal with scarring and milia formation as might be seen in porphyria cutanea tarda or cicatricial pemphigoid, which are in the differential diagnosis. Dystrophy of the fingernails or complete nail loss may be observed, resembling DEB. In the BP-like presentation, tense blisters arise upon inflamed or urticarial skin and mucous membranes, which may then become generalized.
Histopathology in both forms of EBA demonstrates subepithelial separation as clefts or blisters. The mechanobullous type shows a sparse inflammatory infiltrate compared to large collections of neutrophils and eosinophils in the blister cavity and in the superficial dermis in the BP-like cases. The final diagnosis rests on the results of immunopathology testing.24 Direct immunofluorescence of perilesional skin and mucosa shows a linear-granular band of IgG and C3 and other conjugates along the BMZ. Deposits of IgA alone in EBA occur in only about 2.4% of cases and are observed more often when there is mucous membrane involvement.2 Indirect immunofluorescence of sera against salt-split skin substrates detects immunoreactants in the floor of the blister rather than in the roof, as would be seen in BP. Highly specific and sensitive enzyme-linked immunosorbent assay (ELISA) kits now are commercially available and can detect autoantibodies against the N-terminal domain of type VII collagen in more than 90% of cases of EBA.25
Inflammatory bowel disease (IBD), particularly Crohn disease (CD), precedes the onset of EBA in approximately 25% of cases.26,27 Ulcerative colitis is much less common. Type VII collagen is normally present in the basement membrane of intestinal epithelium. In a survey of patients with IBD, 68% of those with CD and 13% of those with ulcerative colitis had circulating anti–type VII collagen antibodies detected by ELISA without having symptoms of EBA.28 A case report of a patient with both well-proven EBA and CD highlighted the clinical difficulty of controlling EBA: treatment with prednisolone and sulfasalazine improved the CD but had little effect on the skin blisters.29 A variety of malignancies have been reported in association with EBA, including cancers of the uterine cervix,30 thyroid, and pancreas,31 lymphoma, and chronic lymphatic leukemia. Some of these cases have met the criteria for classification as paraneoplastic, whereas others may have been coincidental.
Treatment for chronic EBA generally has been limited.2,24 Putative antineutrophil drugs such as dapsone and colchicine combined with systemic corticosteroids may be useful in milder or juvenile cases, which tend to have a better prognosis than adult cases.19 In more severe EBA, systemic corticosteroids and/or immunosuppressive drugs such as azathioprine,23 cyclophosphamide,23 mycophenolate mofetil,31 methotrexate,23 cyclosporine,33 and infliximab23 have been used. More recently, rituximab infusion monotherapy33 and rituximab combined with intravenous immunoglobulin or
Bullous Systemic Lupus Erythematosus
Bullous systemic lupus erythematosus is a rare and specific autoimmune skin complication that mostly is seen in patients with an established diagnosis of systemic lupus erythematosus (SLE) who are experiencing a disease flare. Although more common in women, it has been reported in all sexes and races as well as in children. Vesicles and bullae may arise on sun-exposed (Figure 6) and sun-protected areas of skin.
Histopathology shows subepidermal separation with collections of neutrophils and nuclear fragments in the blister cavity. The differential diagnosis of BSLE includes EBA, BP, dermatitis herpetiformis, and linear IgA bullous dermatosis. Direct immunofluorescence testing shows linear-granular deposits of IgG and/or IgM and IgA along the BMZ.34 When utilizing the indirect immunofluorescence split-skin assay, the autoantibody to type VII collagen would be detected in the floor of the blister if the serum titer was sufficiently high.3 Proposed criteria for the diagnosis of BSLE have been published: 1) diagnosis of SLE now based on the 2019 European League Against Rheumatism/American College of Rheumatology classification35; 2) vesicles and bullae arising upon but not limited to sun-exposed skin; 3) histopathology featuring neutrophil-rich subepithelial bullae; 4) positive indirect immunofluorescence for circulating BMZ antibodies using separated human skin as substrate; 5) and direct immunofluorescence showing IgG and/or IgM and often IgA at the BMZ.36 Using ELISA to detect circulating antibodies against type VII collagen24 should now be added to the criteria. The new criteria for SLE34 do not include BSLE, perhaps because it occurs in less than 1% of patients with SLE.37
Further investigation by Gammon et al3 confirmed that the autoantibodies in BSLE are identical to those found in EBA (ie, directed against type VII collagen in the lamina densa). Bullous systemic lupus erythematosus is not considered to be the coexistence of EBA with SLE but rather a specific entity wherein type VII collagen autoantibodies are expressed in the autoimmune spectrum of SLE. It is especially important to make the diagnosis of BSLE because it is predictive of more serious systemic complications of SLE (eg, hematologic and renal disease is found in up to 90% of cases).38
The natural course of BSLE is variable. Treatments include systemic corticosteroids, dapsone, and immunosuppressive drugs such as azathioprine, methotrexate, mycophenolate mofetil, and cyclophosphamide, especially in cases with nephritis.37 There may be spontaneous resolution of the rash as the inflammatory activity of SLE subsides. Rituximab has been used effectively in several refractory cases of BSLE that failed to respond to all other conventional treatments.39
Conclusion
Anchoring fibrils are composed primarily of type VII collagen. Their role is to maintain the attachment of epithelium to the upper dermis and submucosa. The reduction or complete loss of type VII collagen caused by mutations of the COL7A1 gene results in dominant DEB or recessive DEB, respectively. Two distinct non-heritable immunobullous diseases, EBA and BSLE, are caused by autoantibodies that target type VII collagen. A comparison of the 4 type VII collagen disorders can be found in the eTable.
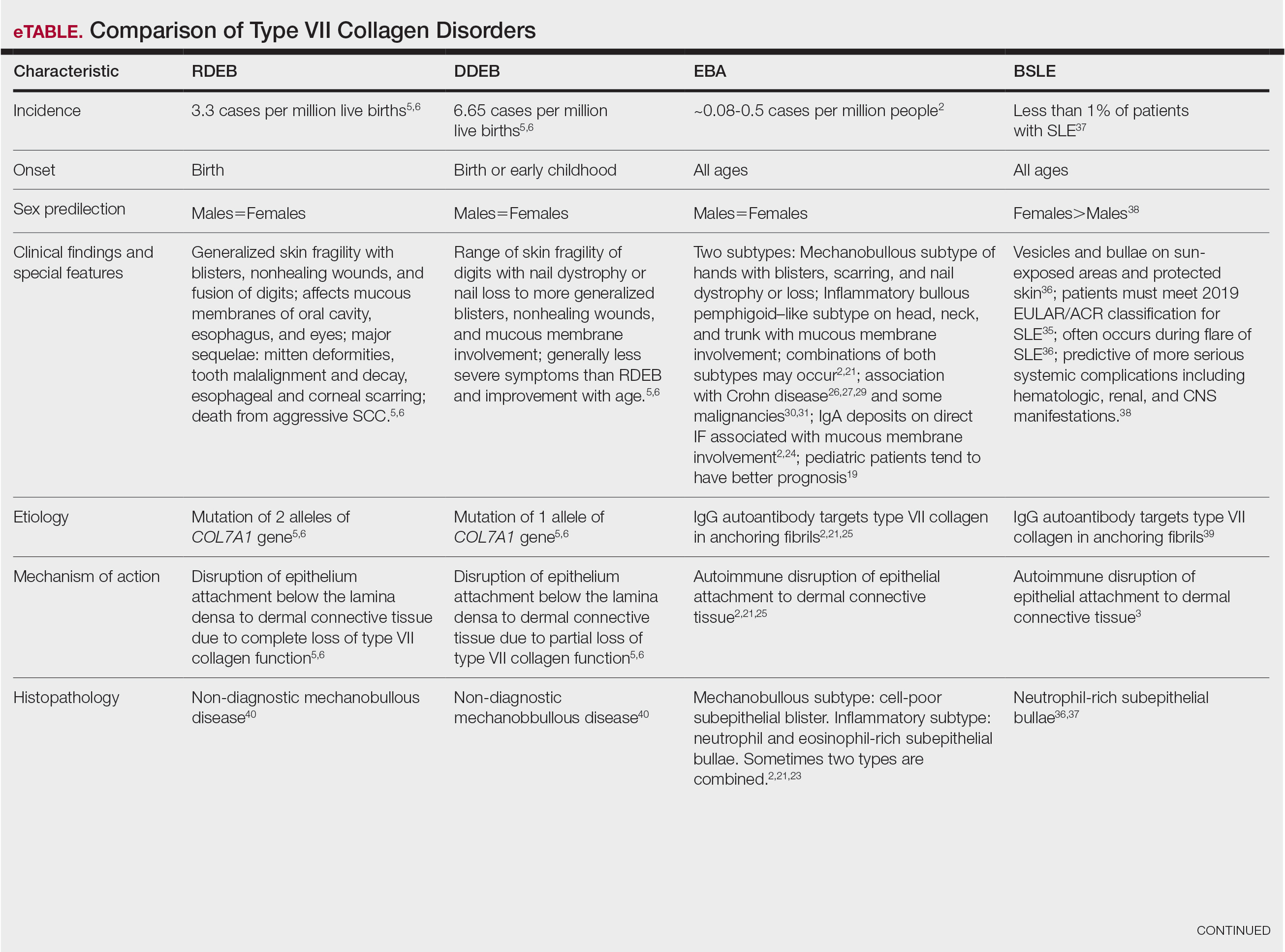
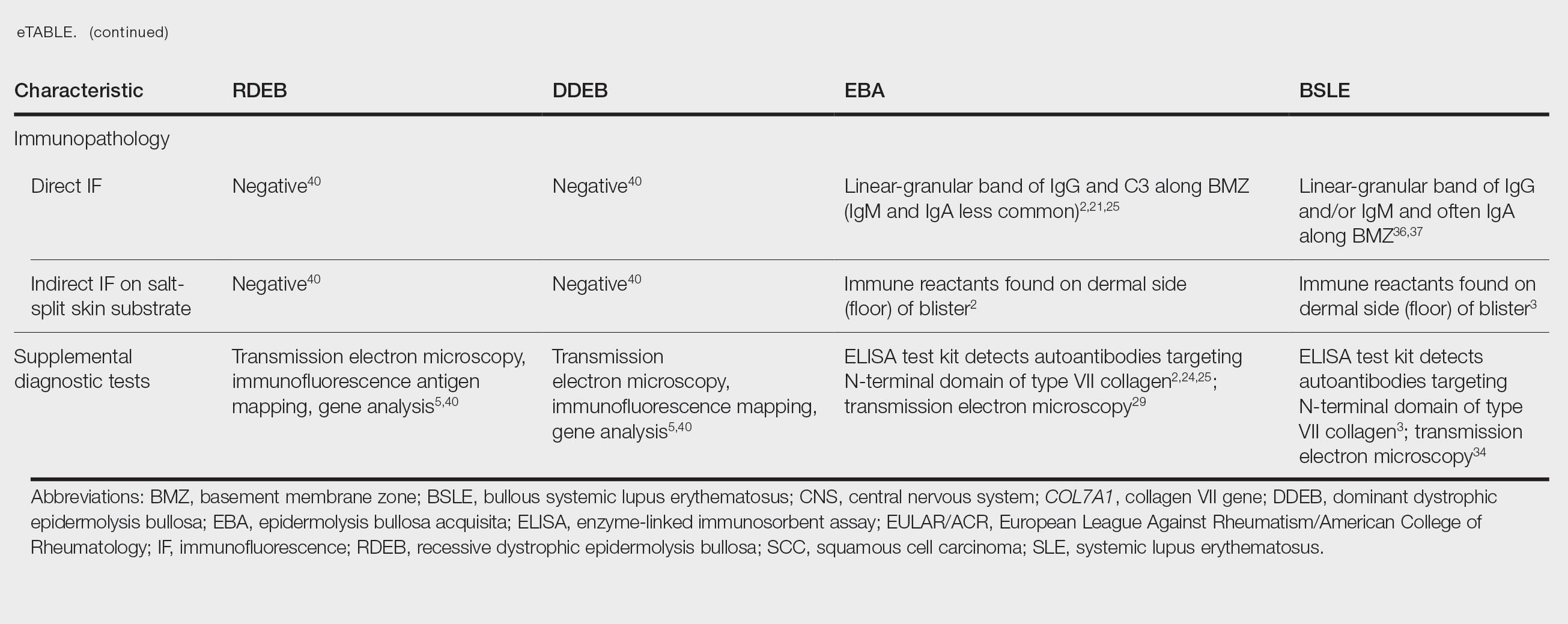
- Bardhan A, Bruckner-Tuderman L, Chapple ILC, et al. Epidermolysis bullosa. Nat Rev Dis Primers. 2020;6:78. doi:10.1038/s41572-020-0210-0
- Miyamoto D, Gordilho JO, Santi CG, et al. Epidermolysis bullosa acquisita. An Bras Dermatol. 2022;97:409-423. doi:10.1016/j.abd.2021.09.010.
- Gammon WR, Woodley DT, Dole KC, et al. Evidence that anti-basement membrane zone antibodies in bullous eruption of systemic lupus erythematosus recognize epidermolysis bullosa acquisita autoantigen. J Invest Dermatol. 1985;84:472-476. doi:10.1111/1523-1747.ep12272402.
- Yadav RS, Jaswal A, Shrestha S, et al. Dystrophic epidermolysis bullosa. J Nepal Med Assoc. 2018;56:879-882. doi:10.31729/jnma.3791
- Mariath LM, Santin JT, Schuler-Faccini L, et al. Inherited epidermolysis bullosa: update on the clinical and genetic aspects. An Bras Dermatol. 2020;95:551-569. doi:10.1016/j.abd.2020.05.001
- Understanding epidermolysis bullosa (EB). DEBRA website. Accessed August 17, 2025. https://www.debra.org/about-eb/understanding-epidermolysis-bullosa-eb
- Hon KL, Chu S, Leung AKC. Epidermolysis bullosa: pediatric perspectives. Curr Pediatr Rev. 2022;18:182-190. doi:10.2174/1573396317666210525161252
- Dang N, Klingberg S, Marr P, et al. Review of collagen VII sequence variants found in Australasian patients with dystrophic epidermolysis bullosa reveals nine COL7A1 variants. J Dermatol Sci. 2007;46:169-178. doi:10.1016/j.jdermsci.2007.02.006
- Payne AS. Topical gene therapy for epidermolysis bullosa. N Engl J Med. 2022;387:2281-2284. doi:10.1056/NEJMe2213203
- Khani P, Ghazi F, Zekri A, et al. Keratins and epidermolysis bullosa simplex. J Cell Physiol. 2018;234:289-297. doi:10.1002/jcp.26898
- Lai-Cheong JE, Tanaka A, Hawche G, et al. Kindler syndrome: a focal adhesion genodermatosis. Br J Dermatol. 2009;160:233-242. doi:10.1111/j.1365-2133.2008.08976.x
- Hou P-C, Wang H-T, Abhee S, et al. Investigational treatments for epidermolysis bullosa. Am J Clin Dermatol. 2021;22:801-817. doi:10.1007/s40257-021-00626-3
- Youseffian L, Vahidnezhad H, Uitto J. Kindler Syndrome. GeneReviews [Internet]. Updated January 6, 2022. Accessed August 21, 2025.
- Guide SV, Gonzalez ME, Bagci S, et al. Trial of beremagene geperpavec (B-VEC) for dystrophic epidermolysis bullosa. N Engl J Med. 2022;387:2211-2219. doi:10.1056/NEJMoa2206663
- Vetencourt AT, Sayed-Ahmed I, Gomez J, et al. Ocular gene therapy in a patient with dystrophic epidermolysis bullosa. N Engl J Med. 2024;390:530-535. doi:10.1056/NEJMoa2301244
- Kern JS, Sprecher E, Fernandez MF, et al. Efficacy and safety of Oleogel-S10 (birch triterpenes for epidermolysis bullosa: results from the phase III randomized double-blind phase of the EASE study. Br J Dermatol. 2023;188:12-21. doi:10.1093/bjd/ljac001
- Tang JY, Marinkovich MP, Wiss K, et al. Prademagene zamikeracel for recessive dystrophic epidermolysis bullosa wounds (VIITAL): a two-centre, randomized, open-label, intrapatient-controlled phase 3 trial. Lancet. 2025;406:163-173. doi:10.1016/S0140-6736(25)00778-0
- Gretzmeier C, Pin D, Kern JS, et al. Systemic collagen VII replacement therapy for advanced recessive dystrophic epidermolysis bullosa. J Invest Dermatol. 2022;142:1094-1102. doi:10.1016/j.jid.2021.09.008
- Hignett E, Sami N. Pediatric epidermolysis bullosa acquisita. A review. Pediatr Dermatol. 2021;38:1047-1050. doi:10.1111/pde.14722
- Chen M, Kim GH, Prakash L, et al. Autoimmunity to anchoring fibril collagen. Autoimmunity. 2012;45:91-101. doi:10.1007/s12016-007-0027-6.
- Kridin K, Kneiber D, Kowalski EH, et al. Epidermolysis bullosa acquisita: a comprehensive review. Autoimmun Rev. 2019;18:786-795. doi:10.1016/j.autrev.2019.06.007
- Hofmann SC, Weidinger A. Epidermolysis bullosa acquisita. Hautarzt. 2019;70:265-270. doi:10.1007/s00105-019-4387-7
- Ishi N, Hamada T, Dainichi T, et al. Epidermolysis bullosa acquisita: what’s new? J Dermatol. 2010;37:220-230. doi:10.1111/j.1346-8138.2009.00799.x
- Iwata H, Vorobyev A, Koga H, et al. Meta-analysis of the clinical and immunopathological characteristics and treatment outcomes in epidermolysis bullosa acquisita patients. Orphanet J Rare Dis. 2018;13:153. doi:10.1186/s13023-018-0896-1
- Komorowski L, Muller R, Vorobyev A, et al. Sensitive and specific assays for routine serological diagnosis of epidermolysis bullosa acquisita. J Am Acad Dermatol. 2013;68:e89-95. doi:10.1016/j.jaad.2011.12.032
- Antonelli E, Bassotti G, Tramontana M, et al. Dermatological manifestations in inflammatory bowel diseases. J Clin Med. 2021;10:364-390. doi:10.3390/jcm10020364
- Bezzio C, Della Corte C, Vernero M, et al. Inflammatory bowel disease and immune-mediated inflammatory diseases: looking at less frequent associations. Therap Adv Gastroenterol. 2022;15:17562848221115312. doi:10.1177/17562848221115312
- Chen M, O’Toole EA, Sanghavi J, et al. The epidermolysis acquisita antigen (type VII collagen) is present in human colon and patients with Crohn’s disease have antibodies to type VII collagen. J Invest Dermatol. 2002;118:1059-1064. doi:10.1046/j.1523-1747.2002.01772.x
- Labeille B, Gineston JL, Denoeux JP, et al. Epidermolysis bullosa acquisita and Crohn’s disease. A case report with immunological and electron microscopic studies. Arch Intern Med. 1988;148:1457-1459.
- Etienne A, Ruffieux P, Didierjean L, et al. Epidermolysis bullosa acquisita and metastatic cancer of the uterine cervix. Ann Dermatol Venereol. 1998;125:321-323.
- Busch J-O, Sticherling M. Epidermolysis bullosa acquisita and neuroendocrine pancreatic cancer-Coincidence or patho-genetic relationship? J Dtsch Dermatol Ges. 2007;5:916-918. doi:10.111/j.1610-0387.2007.06338.x
- Bevans SL, Sami N. The use of rituximab in treatment of epidermolysis bullosa acquisita: three new cases and a review of the literature. Dermatol Ther. 2018;31:e12726. doi:10.1111/j.1610-0387.2007.06338.x
- Yang A, Kim M, Craig P, et al. A case report of the use of rituximab and the epidermolysis bullosa disease activity scoring index (EBDASI) in a patient with epidermolysis bullosa acquisita with extensive esophageal involvement. Arch Dermatovenerol Croat. 2018;26:325-328.
- Burrows NP, Bhogal BS, Black MM, et al. Bullous eruption of systemic lupus erythematosus: a clinicopathological study of four cases. Br J Dermatol. 1993;128:332-338. doi:10.1111/j.1365-2133.1993.tb00180.x
- Aringer M, Leuchten N, Johnson SR. New criteria for lupus. Curr Rheum Rep. 2020;22:18. doi:10.1007/s11926-020-00896-6
- Camisa C. Vesiculobullous systemic lupus erythematosus. A report of four cases. J Am Acad Dermatol. 1988;18:93-100. doi:10.1016/s0190-9622(88)70014-6
- Duan L, Chen L, Zhong S, et al. Treatment of bullous systemic lupus erythematosus. J Immunol Res. 2015;2015:167064. doi:10.1155/2015/167064
- Sprow G, Afarideh M, Dan J, et al. Bullous systemic lupus erythematosus in females. Int J Womens Dermatol. 2022;8:e034. doi:10.1097/JW9.0000000000000034
- Contestable JJ, Edhegard KD, Meyerle JH. Bullous systemic lupus erythematosus: a review and update to diagnosis and treatment. Am J Clin Dermatol. 2014;15:517-524. doi:10.1007/s40257-014-0098-0
- Fine JD, Mellerio JE. Epidermolysis bullosa. In: Bolognia JL, Jorizzo JL, Schaffer JV (eds), Dermatology (ed 3), Elsevier Saunders; 2012: 501-513.
There are 3 uncommon types of mechanobullous skin diseases caused by relative reduction or complete loss of functional type VII collagen, which is the main component of anchoring fibrils in the lamina densa of the basement membrane zone (BMZ) of the skin and mucous membrane epithelium.1 The function of the anchoring fibrils is to maintain adherence of the basement membrane of the epithelium to the connective tissue of the papillary dermis and submucosa.1 The mechanism of action of the loss of type VII collagen function is via autoimmunity in epidermolysis bullosa acquisita (EBA)2 and
Epidermolysis Bullosa
Epidermolysis bullosa consists of a heterogeneous family of 4 major genetic mechanobullous diseases that affect the skin and mucous membranes with more than 30 subtypes.1 Dystrophic EB is caused by mutations in the COL7A1 gene, which encodes for the α-1 chain of collagen type VII. Classically, EB is divided into 4 main variants based on the location of the cleavage plane or split occurring in the epithelium, which in turn helps to predict the severity of the illness.
Epidermolysis bullosa may be inherited in an autosomal-dominant or autosomal-recessive fashion, or it may occur as a spontaneous mutation. All sexes and races are affected equally. Patients present at birth or in early childhood with fragile skin and mucous membranes that may develop blisters, erosions, and ulcerations after minor trauma.7 These lesions are marked by slow healing and scar formation and often are associated with itching and pain.
Dystrophic Epidermolysis Bullosa
Dystrophic EB accounts for approximately 25%6 of all EB cases in the United States and may be inherited as either a dominant or recessive trait. Hundreds of different pathogenic mutations have been discovered in the COL7A1 gene in the subtypes of DEB.4,8 Dominant DEB tends to cause milder disease because the patients retain one normal COL7A1 allele and produce some type VII collagen (Figure 1), whereas patients with recessive DEB lack type VII collagen completely.9 The cleavage plane is between the lamina densa and the superficial dermis or submucosa. Severity is variable and ranges from localization to the hands and feet to severe generalized blistering and painful ulcerations depending on which of the many possible gene mutations have been inherited. Sequelae include mitten deformities, malalignment and tooth decay, and the development of early aggressive squamous cell carcinomas, which may be fatal. The most severe cases of recessive DEB also may have internal organ involvement.

Epidermolysis Bullosa Simplex
Epidermolysis bullosa simplex is the most common variant, comprising approximately 70%of EB cases in the United States.6 Epidermolysis bullosa simplex usually is inherited as autosomal-dominant mutations in the keratin 5 or keratin 14 genes,10 not COL7A1. Skin blistering results from cleavage within the basal cell layer where the keratin genes are primarily expressed. Blisters tend to occur in acral areas such as hands and feet and may heal without scarring in the localized form of epidermolysis bullosa simplex (Figure 2).

Junctional Epidermolysis Bullosa and Kindler Syndrome
Junctional epidermolysis bullosa (JEB) and Kindler syndrome11 are the rarest of the autosomal-recessive EB variants.6 The plane of cleavage in JEB is through the lamina lucida of the BMZ. Junctional epidermolysis bullosa is caused by mutations of the genes that encode for the 3 chains of laminin 332 protein and type XVII collagen,5,12 not to be confused with type VII collagen. As with DEB, there is a wide range of severity in JEB, from localized effects on the eyes, oral cavity, and tooth enamel to widespread blistering and skin cancers. In JEB cases involving newborns, nonhealing wounds on the face, buttocks, fingers, and toes may be seen, with devastating complications in the oral cavity, esophagus, and larynx. Life expectancy is limited to 2 years or less.6 There have only been approximately 40013 cases of Kindler syndrome reported worldwide6 and there is clinical overlap with DEB. Patients also may demonstrate poikiloderma and photosensitivity. Kindler syndrome is caused by mutations in the FERMT1 gene which encodes for kindlin-1. This protein mediates anchorage between the actin cytoskeleton and the extracellular matrix.5,11 Loss of function produces variable cleavage planes around the dermoepidermal junction.
Clinical management of all EB variants, especially the severe recessive types, traditionally has been limited to the prevention of trauma to the skin and mucous membranes and supportive care, including dressing changes to erosions and ulcerations, antibiotic ointments as needed, and amelioration of pain and pruritus. Bone marrow and pluripotential stem cell transplants have been attempted.12 Complications of EB, such as deformities of the hands and feet caused by excessive scarring, esophageal strictures, poor dentition, and squamous cell carcinomas, must be addressed by a multidisciplinary team of specialists, including plastic surgery, gastroenterology, dentistry/oral surgery, ophthalmology, and dermatology/Mohs surgery.
Until recently, there were no medications approved by the US Food and Drug Administration (FDA) specifically indicated for EB. In 2023, topical gene therapy was approved by the FDA for both recessive and dominant forms of DEB. Normal COL7A1 sequences are delivered by an attenuated herpes simplex virus 1 vector (beremagene geperpavec) in a gel applied directly to the wounds of patients with DEB. In a clinical trial, matching wounds on 31 patients (62 wounds total) were treated with the active agent or placebo gel. After 6 months, complete wound closure was observed in 67% (21/31) of those treated with the active agent and 22% (7/31) of those treated with placebo (P=.002).14 In a single case report, a patient with recessive DEB and cicatrizing conjunctivitis (Figure 3) was given ophthalmic beremagene geperpavec after surgery and had improved visual acuity.15 A topical gel consisting of birch triterpenes to promote healing of partial-thickness wounds also was approved for patients with DEB and JEB by the FDA and the European Commission. In a study of 223 patients, 41% of those using active gel and 29% of those using placebo gel achieved the primary end point of percentage of target wounds that had first complete closure at 45 days.16

The most recent FDA approval for DEB involves transferring the functional COL7A1 gene to the patient’s skin cells, then expanding the gene-corrected cells into sheets of keratinocytes that can be surgically applied to the chronic wound sites. In a phase 3 trial of prademagene zamikeracel (pz-cel), 11 patients with 86 matched wounds were randomized to receive pz-cel (50%) or standard wound care (50%). After 24 weeks, 35 wounds treated with pz-cel were at least 50% healed compared to 7 control wounds.17 The results for healing and reduction of pain were statistically significant (P<.0001 and P<.0002, respectively).17 Recombinant collagen VII as replacement therapy also is under study to be given by intravenous infusion to increase tissue collagen VII where it is lacking. This treatment has shown early biologic and therapeutic effects.9,18 Larger long-term follow-up studies are necessary to confirm persistence of the gene-corrected skin cells, the functionality of the replacement collagen VII, and the potential risk for the development of autoantibodies to type VII collagen.
Epidermolysis Bullosa Acquisita
Epidermolysis bullosa acquisita is a rare autoimmune subepithelial bullous disease that primarily affects middle-aged adults but also has been reported in children.19 Epidermolysis bullosa acquisita is caused by circulating pathogenic IgG autoantibodies that target and bind to type VII collagen in the anchoring fibrils,20-22 thereby disrupting the attachment of the epithelium to its underlying connective tissue.
The 2 major clinical manifestations of EBA include a mechanobullous disease resembling inherited forms of DEB (Figure 4) and an inflammatory bullous pemphigoid (BP)–like disease,23 as well as a combination of both types of skin lesions (Figure 5). The skin and mucous membranes of the oral cavity, esophagus, eyes, and urogenital areas are affected in both types; scarring may cause functional disabilities. In the mechanobullous type of EBA, it is common for blisters and erosions to develop in trauma-prone areas such as the hands, feet, elbows, and knees. The blisters tend to heal with scarring and milia formation as might be seen in porphyria cutanea tarda or cicatricial pemphigoid, which are in the differential diagnosis. Dystrophy of the fingernails or complete nail loss may be observed, resembling DEB. In the BP-like presentation, tense blisters arise upon inflamed or urticarial skin and mucous membranes, which may then become generalized.


Histopathology in both forms of EBA demonstrates subepithelial separation as clefts or blisters. The mechanobullous type shows a sparse inflammatory infiltrate compared to large collections of neutrophils and eosinophils in the blister cavity and in the superficial dermis in the BP-like cases. The final diagnosis rests on the results of immunopathology testing.24 Direct immunofluorescence of perilesional skin and mucosa shows a linear-granular band of IgG and C3 and other conjugates along the BMZ. Deposits of IgA alone in EBA occur in only about 2.4% of cases and are observed more often when there is mucous membrane involvement.2 Indirect immunofluorescence of sera against salt-split skin substrates detects immunoreactants in the floor of the blister rather than in the roof, as would be seen in BP. Highly specific and sensitive enzyme-linked immunosorbent assay (ELISA) kits now are commercially available and can detect autoantibodies against the N-terminal domain of type VII collagen in more than 90% of cases of EBA.25
Inflammatory bowel disease (IBD), particularly Crohn disease (CD), precedes the onset of EBA in approximately 25% of cases.26,27 Ulcerative colitis is much less common. Type VII collagen is normally present in the basement membrane of intestinal epithelium. In a survey of patients with IBD, 68% of those with CD and 13% of those with ulcerative colitis had circulating anti–type VII collagen antibodies detected by ELISA without having symptoms of EBA.28 A case report of a patient with both well-proven EBA and CD highlighted the clinical difficulty of controlling EBA: treatment with prednisolone and sulfasalazine improved the CD but had little effect on the skin blisters.29 A variety of malignancies have been reported in association with EBA, including cancers of the uterine cervix,30 thyroid, and pancreas,31 lymphoma, and chronic lymphatic leukemia. Some of these cases have met the criteria for classification as paraneoplastic, whereas others may have been coincidental.
Treatment for chronic EBA generally has been limited.2,24 Putative antineutrophil drugs such as dapsone and colchicine combined with systemic corticosteroids may be useful in milder or juvenile cases, which tend to have a better prognosis than adult cases.19 In more severe EBA, systemic corticosteroids and/or immunosuppressive drugs such as azathioprine,23 cyclophosphamide,23 mycophenolate mofetil,31 methotrexate,23 cyclosporine,33 and infliximab23 have been used. More recently, rituximab infusion monotherapy33 and rituximab combined with intravenous immunoglobulin or
Bullous Systemic Lupus Erythematosus
Bullous systemic lupus erythematosus is a rare and specific autoimmune skin complication that mostly is seen in patients with an established diagnosis of systemic lupus erythematosus (SLE) who are experiencing a disease flare. Although more common in women, it has been reported in all sexes and races as well as in children. Vesicles and bullae may arise on sun-exposed (Figure 6) and sun-protected areas of skin.

Histopathology shows subepidermal separation with collections of neutrophils and nuclear fragments in the blister cavity. The differential diagnosis of BSLE includes EBA, BP, dermatitis herpetiformis, and linear IgA bullous dermatosis. Direct immunofluorescence testing shows linear-granular deposits of IgG and/or IgM and IgA along the BMZ.34 When utilizing the indirect immunofluorescence split-skin assay, the autoantibody to type VII collagen would be detected in the floor of the blister if the serum titer was sufficiently high.3 Proposed criteria for the diagnosis of BSLE have been published: 1) diagnosis of SLE now based on the 2019 European League Against Rheumatism/American College of Rheumatology classification35; 2) vesicles and bullae arising upon but not limited to sun-exposed skin; 3) histopathology featuring neutrophil-rich subepithelial bullae; 4) positive indirect immunofluorescence for circulating BMZ antibodies using separated human skin as substrate; 5) and direct immunofluorescence showing IgG and/or IgM and often IgA at the BMZ.36 Using ELISA to detect circulating antibodies against type VII collagen24 should now be added to the criteria. The new criteria for SLE34 do not include BSLE, perhaps because it occurs in less than 1% of patients with SLE.37
Further investigation by Gammon et al3 confirmed that the autoantibodies in BSLE are identical to those found in EBA (ie, directed against type VII collagen in the lamina densa). Bullous systemic lupus erythematosus is not considered to be the coexistence of EBA with SLE but rather a specific entity wherein type VII collagen autoantibodies are expressed in the autoimmune spectrum of SLE. It is especially important to make the diagnosis of BSLE because it is predictive of more serious systemic complications of SLE (eg, hematologic and renal disease is found in up to 90% of cases).38
The natural course of BSLE is variable. Treatments include systemic corticosteroids, dapsone, and immunosuppressive drugs such as azathioprine, methotrexate, mycophenolate mofetil, and cyclophosphamide, especially in cases with nephritis.37 There may be spontaneous resolution of the rash as the inflammatory activity of SLE subsides. Rituximab has been used effectively in several refractory cases of BSLE that failed to respond to all other conventional treatments.39
Conclusion
Anchoring fibrils are composed primarily of type VII collagen. Their role is to maintain the attachment of epithelium to the upper dermis and submucosa. The reduction or complete loss of type VII collagen caused by mutations of the COL7A1 gene results in dominant DEB or recessive DEB, respectively. Two distinct non-heritable immunobullous diseases, EBA and BSLE, are caused by autoantibodies that target type VII collagen. A comparison of the 4 type VII collagen disorders can be found in the eTable.


There are 3 uncommon types of mechanobullous skin diseases caused by relative reduction or complete loss of functional type VII collagen, which is the main component of anchoring fibrils in the lamina densa of the basement membrane zone (BMZ) of the skin and mucous membrane epithelium.1 The function of the anchoring fibrils is to maintain adherence of the basement membrane of the epithelium to the connective tissue of the papillary dermis and submucosa.1 The mechanism of action of the loss of type VII collagen function is via autoimmunity in epidermolysis bullosa acquisita (EBA)2 and
Epidermolysis Bullosa
Epidermolysis bullosa consists of a heterogeneous family of 4 major genetic mechanobullous diseases that affect the skin and mucous membranes with more than 30 subtypes.1 Dystrophic EB is caused by mutations in the COL7A1 gene, which encodes for the α-1 chain of collagen type VII. Classically, EB is divided into 4 main variants based on the location of the cleavage plane or split occurring in the epithelium, which in turn helps to predict the severity of the illness.
Epidermolysis bullosa may be inherited in an autosomal-dominant or autosomal-recessive fashion, or it may occur as a spontaneous mutation. All sexes and races are affected equally. Patients present at birth or in early childhood with fragile skin and mucous membranes that may develop blisters, erosions, and ulcerations after minor trauma.7 These lesions are marked by slow healing and scar formation and often are associated with itching and pain.
Dystrophic Epidermolysis Bullosa
Dystrophic EB accounts for approximately 25%6 of all EB cases in the United States and may be inherited as either a dominant or recessive trait. Hundreds of different pathogenic mutations have been discovered in the COL7A1 gene in the subtypes of DEB.4,8 Dominant DEB tends to cause milder disease because the patients retain one normal COL7A1 allele and produce some type VII collagen (Figure 1), whereas patients with recessive DEB lack type VII collagen completely.9 The cleavage plane is between the lamina densa and the superficial dermis or submucosa. Severity is variable and ranges from localization to the hands and feet to severe generalized blistering and painful ulcerations depending on which of the many possible gene mutations have been inherited. Sequelae include mitten deformities, malalignment and tooth decay, and the development of early aggressive squamous cell carcinomas, which may be fatal. The most severe cases of recessive DEB also may have internal organ involvement.

Epidermolysis Bullosa Simplex
Epidermolysis bullosa simplex is the most common variant, comprising approximately 70%of EB cases in the United States.6 Epidermolysis bullosa simplex usually is inherited as autosomal-dominant mutations in the keratin 5 or keratin 14 genes,10 not COL7A1. Skin blistering results from cleavage within the basal cell layer where the keratin genes are primarily expressed. Blisters tend to occur in acral areas such as hands and feet and may heal without scarring in the localized form of epidermolysis bullosa simplex (Figure 2).

Junctional Epidermolysis Bullosa and Kindler Syndrome
Junctional epidermolysis bullosa (JEB) and Kindler syndrome11 are the rarest of the autosomal-recessive EB variants.6 The plane of cleavage in JEB is through the lamina lucida of the BMZ. Junctional epidermolysis bullosa is caused by mutations of the genes that encode for the 3 chains of laminin 332 protein and type XVII collagen,5,12 not to be confused with type VII collagen. As with DEB, there is a wide range of severity in JEB, from localized effects on the eyes, oral cavity, and tooth enamel to widespread blistering and skin cancers. In JEB cases involving newborns, nonhealing wounds on the face, buttocks, fingers, and toes may be seen, with devastating complications in the oral cavity, esophagus, and larynx. Life expectancy is limited to 2 years or less.6 There have only been approximately 40013 cases of Kindler syndrome reported worldwide6 and there is clinical overlap with DEB. Patients also may demonstrate poikiloderma and photosensitivity. Kindler syndrome is caused by mutations in the FERMT1 gene which encodes for kindlin-1. This protein mediates anchorage between the actin cytoskeleton and the extracellular matrix.5,11 Loss of function produces variable cleavage planes around the dermoepidermal junction.
Clinical management of all EB variants, especially the severe recessive types, traditionally has been limited to the prevention of trauma to the skin and mucous membranes and supportive care, including dressing changes to erosions and ulcerations, antibiotic ointments as needed, and amelioration of pain and pruritus. Bone marrow and pluripotential stem cell transplants have been attempted.12 Complications of EB, such as deformities of the hands and feet caused by excessive scarring, esophageal strictures, poor dentition, and squamous cell carcinomas, must be addressed by a multidisciplinary team of specialists, including plastic surgery, gastroenterology, dentistry/oral surgery, ophthalmology, and dermatology/Mohs surgery.
Until recently, there were no medications approved by the US Food and Drug Administration (FDA) specifically indicated for EB. In 2023, topical gene therapy was approved by the FDA for both recessive and dominant forms of DEB. Normal COL7A1 sequences are delivered by an attenuated herpes simplex virus 1 vector (beremagene geperpavec) in a gel applied directly to the wounds of patients with DEB. In a clinical trial, matching wounds on 31 patients (62 wounds total) were treated with the active agent or placebo gel. After 6 months, complete wound closure was observed in 67% (21/31) of those treated with the active agent and 22% (7/31) of those treated with placebo (P=.002).14 In a single case report, a patient with recessive DEB and cicatrizing conjunctivitis (Figure 3) was given ophthalmic beremagene geperpavec after surgery and had improved visual acuity.15 A topical gel consisting of birch triterpenes to promote healing of partial-thickness wounds also was approved for patients with DEB and JEB by the FDA and the European Commission. In a study of 223 patients, 41% of those using active gel and 29% of those using placebo gel achieved the primary end point of percentage of target wounds that had first complete closure at 45 days.16

The most recent FDA approval for DEB involves transferring the functional COL7A1 gene to the patient’s skin cells, then expanding the gene-corrected cells into sheets of keratinocytes that can be surgically applied to the chronic wound sites. In a phase 3 trial of prademagene zamikeracel (pz-cel), 11 patients with 86 matched wounds were randomized to receive pz-cel (50%) or standard wound care (50%). After 24 weeks, 35 wounds treated with pz-cel were at least 50% healed compared to 7 control wounds.17 The results for healing and reduction of pain were statistically significant (P<.0001 and P<.0002, respectively).17 Recombinant collagen VII as replacement therapy also is under study to be given by intravenous infusion to increase tissue collagen VII where it is lacking. This treatment has shown early biologic and therapeutic effects.9,18 Larger long-term follow-up studies are necessary to confirm persistence of the gene-corrected skin cells, the functionality of the replacement collagen VII, and the potential risk for the development of autoantibodies to type VII collagen.
Epidermolysis Bullosa Acquisita
Epidermolysis bullosa acquisita is a rare autoimmune subepithelial bullous disease that primarily affects middle-aged adults but also has been reported in children.19 Epidermolysis bullosa acquisita is caused by circulating pathogenic IgG autoantibodies that target and bind to type VII collagen in the anchoring fibrils,20-22 thereby disrupting the attachment of the epithelium to its underlying connective tissue.
The 2 major clinical manifestations of EBA include a mechanobullous disease resembling inherited forms of DEB (Figure 4) and an inflammatory bullous pemphigoid (BP)–like disease,23 as well as a combination of both types of skin lesions (Figure 5). The skin and mucous membranes of the oral cavity, esophagus, eyes, and urogenital areas are affected in both types; scarring may cause functional disabilities. In the mechanobullous type of EBA, it is common for blisters and erosions to develop in trauma-prone areas such as the hands, feet, elbows, and knees. The blisters tend to heal with scarring and milia formation as might be seen in porphyria cutanea tarda or cicatricial pemphigoid, which are in the differential diagnosis. Dystrophy of the fingernails or complete nail loss may be observed, resembling DEB. In the BP-like presentation, tense blisters arise upon inflamed or urticarial skin and mucous membranes, which may then become generalized.


Histopathology in both forms of EBA demonstrates subepithelial separation as clefts or blisters. The mechanobullous type shows a sparse inflammatory infiltrate compared to large collections of neutrophils and eosinophils in the blister cavity and in the superficial dermis in the BP-like cases. The final diagnosis rests on the results of immunopathology testing.24 Direct immunofluorescence of perilesional skin and mucosa shows a linear-granular band of IgG and C3 and other conjugates along the BMZ. Deposits of IgA alone in EBA occur in only about 2.4% of cases and are observed more often when there is mucous membrane involvement.2 Indirect immunofluorescence of sera against salt-split skin substrates detects immunoreactants in the floor of the blister rather than in the roof, as would be seen in BP. Highly specific and sensitive enzyme-linked immunosorbent assay (ELISA) kits now are commercially available and can detect autoantibodies against the N-terminal domain of type VII collagen in more than 90% of cases of EBA.25
Inflammatory bowel disease (IBD), particularly Crohn disease (CD), precedes the onset of EBA in approximately 25% of cases.26,27 Ulcerative colitis is much less common. Type VII collagen is normally present in the basement membrane of intestinal epithelium. In a survey of patients with IBD, 68% of those with CD and 13% of those with ulcerative colitis had circulating anti–type VII collagen antibodies detected by ELISA without having symptoms of EBA.28 A case report of a patient with both well-proven EBA and CD highlighted the clinical difficulty of controlling EBA: treatment with prednisolone and sulfasalazine improved the CD but had little effect on the skin blisters.29 A variety of malignancies have been reported in association with EBA, including cancers of the uterine cervix,30 thyroid, and pancreas,31 lymphoma, and chronic lymphatic leukemia. Some of these cases have met the criteria for classification as paraneoplastic, whereas others may have been coincidental.
Treatment for chronic EBA generally has been limited.2,24 Putative antineutrophil drugs such as dapsone and colchicine combined with systemic corticosteroids may be useful in milder or juvenile cases, which tend to have a better prognosis than adult cases.19 In more severe EBA, systemic corticosteroids and/or immunosuppressive drugs such as azathioprine,23 cyclophosphamide,23 mycophenolate mofetil,31 methotrexate,23 cyclosporine,33 and infliximab23 have been used. More recently, rituximab infusion monotherapy33 and rituximab combined with intravenous immunoglobulin or
Bullous Systemic Lupus Erythematosus
Bullous systemic lupus erythematosus is a rare and specific autoimmune skin complication that mostly is seen in patients with an established diagnosis of systemic lupus erythematosus (SLE) who are experiencing a disease flare. Although more common in women, it has been reported in all sexes and races as well as in children. Vesicles and bullae may arise on sun-exposed (Figure 6) and sun-protected areas of skin.

Histopathology shows subepidermal separation with collections of neutrophils and nuclear fragments in the blister cavity. The differential diagnosis of BSLE includes EBA, BP, dermatitis herpetiformis, and linear IgA bullous dermatosis. Direct immunofluorescence testing shows linear-granular deposits of IgG and/or IgM and IgA along the BMZ.34 When utilizing the indirect immunofluorescence split-skin assay, the autoantibody to type VII collagen would be detected in the floor of the blister if the serum titer was sufficiently high.3 Proposed criteria for the diagnosis of BSLE have been published: 1) diagnosis of SLE now based on the 2019 European League Against Rheumatism/American College of Rheumatology classification35; 2) vesicles and bullae arising upon but not limited to sun-exposed skin; 3) histopathology featuring neutrophil-rich subepithelial bullae; 4) positive indirect immunofluorescence for circulating BMZ antibodies using separated human skin as substrate; 5) and direct immunofluorescence showing IgG and/or IgM and often IgA at the BMZ.36 Using ELISA to detect circulating antibodies against type VII collagen24 should now be added to the criteria. The new criteria for SLE34 do not include BSLE, perhaps because it occurs in less than 1% of patients with SLE.37
Further investigation by Gammon et al3 confirmed that the autoantibodies in BSLE are identical to those found in EBA (ie, directed against type VII collagen in the lamina densa). Bullous systemic lupus erythematosus is not considered to be the coexistence of EBA with SLE but rather a specific entity wherein type VII collagen autoantibodies are expressed in the autoimmune spectrum of SLE. It is especially important to make the diagnosis of BSLE because it is predictive of more serious systemic complications of SLE (eg, hematologic and renal disease is found in up to 90% of cases).38
The natural course of BSLE is variable. Treatments include systemic corticosteroids, dapsone, and immunosuppressive drugs such as azathioprine, methotrexate, mycophenolate mofetil, and cyclophosphamide, especially in cases with nephritis.37 There may be spontaneous resolution of the rash as the inflammatory activity of SLE subsides. Rituximab has been used effectively in several refractory cases of BSLE that failed to respond to all other conventional treatments.39
Conclusion
Anchoring fibrils are composed primarily of type VII collagen. Their role is to maintain the attachment of epithelium to the upper dermis and submucosa. The reduction or complete loss of type VII collagen caused by mutations of the COL7A1 gene results in dominant DEB or recessive DEB, respectively. Two distinct non-heritable immunobullous diseases, EBA and BSLE, are caused by autoantibodies that target type VII collagen. A comparison of the 4 type VII collagen disorders can be found in the eTable.


- Bardhan A, Bruckner-Tuderman L, Chapple ILC, et al. Epidermolysis bullosa. Nat Rev Dis Primers. 2020;6:78. doi:10.1038/s41572-020-0210-0
- Miyamoto D, Gordilho JO, Santi CG, et al. Epidermolysis bullosa acquisita. An Bras Dermatol. 2022;97:409-423. doi:10.1016/j.abd.2021.09.010.
- Gammon WR, Woodley DT, Dole KC, et al. Evidence that anti-basement membrane zone antibodies in bullous eruption of systemic lupus erythematosus recognize epidermolysis bullosa acquisita autoantigen. J Invest Dermatol. 1985;84:472-476. doi:10.1111/1523-1747.ep12272402.
- Yadav RS, Jaswal A, Shrestha S, et al. Dystrophic epidermolysis bullosa. J Nepal Med Assoc. 2018;56:879-882. doi:10.31729/jnma.3791
- Mariath LM, Santin JT, Schuler-Faccini L, et al. Inherited epidermolysis bullosa: update on the clinical and genetic aspects. An Bras Dermatol. 2020;95:551-569. doi:10.1016/j.abd.2020.05.001
- Understanding epidermolysis bullosa (EB). DEBRA website. Accessed August 17, 2025. https://www.debra.org/about-eb/understanding-epidermolysis-bullosa-eb
- Hon KL, Chu S, Leung AKC. Epidermolysis bullosa: pediatric perspectives. Curr Pediatr Rev. 2022;18:182-190. doi:10.2174/1573396317666210525161252
- Dang N, Klingberg S, Marr P, et al. Review of collagen VII sequence variants found in Australasian patients with dystrophic epidermolysis bullosa reveals nine COL7A1 variants. J Dermatol Sci. 2007;46:169-178. doi:10.1016/j.jdermsci.2007.02.006
- Payne AS. Topical gene therapy for epidermolysis bullosa. N Engl J Med. 2022;387:2281-2284. doi:10.1056/NEJMe2213203
- Khani P, Ghazi F, Zekri A, et al. Keratins and epidermolysis bullosa simplex. J Cell Physiol. 2018;234:289-297. doi:10.1002/jcp.26898
- Lai-Cheong JE, Tanaka A, Hawche G, et al. Kindler syndrome: a focal adhesion genodermatosis. Br J Dermatol. 2009;160:233-242. doi:10.1111/j.1365-2133.2008.08976.x
- Hou P-C, Wang H-T, Abhee S, et al. Investigational treatments for epidermolysis bullosa. Am J Clin Dermatol. 2021;22:801-817. doi:10.1007/s40257-021-00626-3
- Youseffian L, Vahidnezhad H, Uitto J. Kindler Syndrome. GeneReviews [Internet]. Updated January 6, 2022. Accessed August 21, 2025.
- Guide SV, Gonzalez ME, Bagci S, et al. Trial of beremagene geperpavec (B-VEC) for dystrophic epidermolysis bullosa. N Engl J Med. 2022;387:2211-2219. doi:10.1056/NEJMoa2206663
- Vetencourt AT, Sayed-Ahmed I, Gomez J, et al. Ocular gene therapy in a patient with dystrophic epidermolysis bullosa. N Engl J Med. 2024;390:530-535. doi:10.1056/NEJMoa2301244
- Kern JS, Sprecher E, Fernandez MF, et al. Efficacy and safety of Oleogel-S10 (birch triterpenes for epidermolysis bullosa: results from the phase III randomized double-blind phase of the EASE study. Br J Dermatol. 2023;188:12-21. doi:10.1093/bjd/ljac001
- Tang JY, Marinkovich MP, Wiss K, et al. Prademagene zamikeracel for recessive dystrophic epidermolysis bullosa wounds (VIITAL): a two-centre, randomized, open-label, intrapatient-controlled phase 3 trial. Lancet. 2025;406:163-173. doi:10.1016/S0140-6736(25)00778-0
- Gretzmeier C, Pin D, Kern JS, et al. Systemic collagen VII replacement therapy for advanced recessive dystrophic epidermolysis bullosa. J Invest Dermatol. 2022;142:1094-1102. doi:10.1016/j.jid.2021.09.008
- Hignett E, Sami N. Pediatric epidermolysis bullosa acquisita. A review. Pediatr Dermatol. 2021;38:1047-1050. doi:10.1111/pde.14722
- Chen M, Kim GH, Prakash L, et al. Autoimmunity to anchoring fibril collagen. Autoimmunity. 2012;45:91-101. doi:10.1007/s12016-007-0027-6.
- Kridin K, Kneiber D, Kowalski EH, et al. Epidermolysis bullosa acquisita: a comprehensive review. Autoimmun Rev. 2019;18:786-795. doi:10.1016/j.autrev.2019.06.007
- Hofmann SC, Weidinger A. Epidermolysis bullosa acquisita. Hautarzt. 2019;70:265-270. doi:10.1007/s00105-019-4387-7
- Ishi N, Hamada T, Dainichi T, et al. Epidermolysis bullosa acquisita: what’s new? J Dermatol. 2010;37:220-230. doi:10.1111/j.1346-8138.2009.00799.x
- Iwata H, Vorobyev A, Koga H, et al. Meta-analysis of the clinical and immunopathological characteristics and treatment outcomes in epidermolysis bullosa acquisita patients. Orphanet J Rare Dis. 2018;13:153. doi:10.1186/s13023-018-0896-1
- Komorowski L, Muller R, Vorobyev A, et al. Sensitive and specific assays for routine serological diagnosis of epidermolysis bullosa acquisita. J Am Acad Dermatol. 2013;68:e89-95. doi:10.1016/j.jaad.2011.12.032
- Antonelli E, Bassotti G, Tramontana M, et al. Dermatological manifestations in inflammatory bowel diseases. J Clin Med. 2021;10:364-390. doi:10.3390/jcm10020364
- Bezzio C, Della Corte C, Vernero M, et al. Inflammatory bowel disease and immune-mediated inflammatory diseases: looking at less frequent associations. Therap Adv Gastroenterol. 2022;15:17562848221115312. doi:10.1177/17562848221115312
- Chen M, O’Toole EA, Sanghavi J, et al. The epidermolysis acquisita antigen (type VII collagen) is present in human colon and patients with Crohn’s disease have antibodies to type VII collagen. J Invest Dermatol. 2002;118:1059-1064. doi:10.1046/j.1523-1747.2002.01772.x
- Labeille B, Gineston JL, Denoeux JP, et al. Epidermolysis bullosa acquisita and Crohn’s disease. A case report with immunological and electron microscopic studies. Arch Intern Med. 1988;148:1457-1459.
- Etienne A, Ruffieux P, Didierjean L, et al. Epidermolysis bullosa acquisita and metastatic cancer of the uterine cervix. Ann Dermatol Venereol. 1998;125:321-323.
- Busch J-O, Sticherling M. Epidermolysis bullosa acquisita and neuroendocrine pancreatic cancer-Coincidence or patho-genetic relationship? J Dtsch Dermatol Ges. 2007;5:916-918. doi:10.111/j.1610-0387.2007.06338.x
- Bevans SL, Sami N. The use of rituximab in treatment of epidermolysis bullosa acquisita: three new cases and a review of the literature. Dermatol Ther. 2018;31:e12726. doi:10.1111/j.1610-0387.2007.06338.x
- Yang A, Kim M, Craig P, et al. A case report of the use of rituximab and the epidermolysis bullosa disease activity scoring index (EBDASI) in a patient with epidermolysis bullosa acquisita with extensive esophageal involvement. Arch Dermatovenerol Croat. 2018;26:325-328.
- Burrows NP, Bhogal BS, Black MM, et al. Bullous eruption of systemic lupus erythematosus: a clinicopathological study of four cases. Br J Dermatol. 1993;128:332-338. doi:10.1111/j.1365-2133.1993.tb00180.x
- Aringer M, Leuchten N, Johnson SR. New criteria for lupus. Curr Rheum Rep. 2020;22:18. doi:10.1007/s11926-020-00896-6
- Camisa C. Vesiculobullous systemic lupus erythematosus. A report of four cases. J Am Acad Dermatol. 1988;18:93-100. doi:10.1016/s0190-9622(88)70014-6
- Duan L, Chen L, Zhong S, et al. Treatment of bullous systemic lupus erythematosus. J Immunol Res. 2015;2015:167064. doi:10.1155/2015/167064
- Sprow G, Afarideh M, Dan J, et al. Bullous systemic lupus erythematosus in females. Int J Womens Dermatol. 2022;8:e034. doi:10.1097/JW9.0000000000000034
- Contestable JJ, Edhegard KD, Meyerle JH. Bullous systemic lupus erythematosus: a review and update to diagnosis and treatment. Am J Clin Dermatol. 2014;15:517-524. doi:10.1007/s40257-014-0098-0
- Fine JD, Mellerio JE. Epidermolysis bullosa. In: Bolognia JL, Jorizzo JL, Schaffer JV (eds), Dermatology (ed 3), Elsevier Saunders; 2012: 501-513.
- Bardhan A, Bruckner-Tuderman L, Chapple ILC, et al. Epidermolysis bullosa. Nat Rev Dis Primers. 2020;6:78. doi:10.1038/s41572-020-0210-0
- Miyamoto D, Gordilho JO, Santi CG, et al. Epidermolysis bullosa acquisita. An Bras Dermatol. 2022;97:409-423. doi:10.1016/j.abd.2021.09.010.
- Gammon WR, Woodley DT, Dole KC, et al. Evidence that anti-basement membrane zone antibodies in bullous eruption of systemic lupus erythematosus recognize epidermolysis bullosa acquisita autoantigen. J Invest Dermatol. 1985;84:472-476. doi:10.1111/1523-1747.ep12272402.
- Yadav RS, Jaswal A, Shrestha S, et al. Dystrophic epidermolysis bullosa. J Nepal Med Assoc. 2018;56:879-882. doi:10.31729/jnma.3791
- Mariath LM, Santin JT, Schuler-Faccini L, et al. Inherited epidermolysis bullosa: update on the clinical and genetic aspects. An Bras Dermatol. 2020;95:551-569. doi:10.1016/j.abd.2020.05.001
- Understanding epidermolysis bullosa (EB). DEBRA website. Accessed August 17, 2025. https://www.debra.org/about-eb/understanding-epidermolysis-bullosa-eb
- Hon KL, Chu S, Leung AKC. Epidermolysis bullosa: pediatric perspectives. Curr Pediatr Rev. 2022;18:182-190. doi:10.2174/1573396317666210525161252
- Dang N, Klingberg S, Marr P, et al. Review of collagen VII sequence variants found in Australasian patients with dystrophic epidermolysis bullosa reveals nine COL7A1 variants. J Dermatol Sci. 2007;46:169-178. doi:10.1016/j.jdermsci.2007.02.006
- Payne AS. Topical gene therapy for epidermolysis bullosa. N Engl J Med. 2022;387:2281-2284. doi:10.1056/NEJMe2213203
- Khani P, Ghazi F, Zekri A, et al. Keratins and epidermolysis bullosa simplex. J Cell Physiol. 2018;234:289-297. doi:10.1002/jcp.26898
- Lai-Cheong JE, Tanaka A, Hawche G, et al. Kindler syndrome: a focal adhesion genodermatosis. Br J Dermatol. 2009;160:233-242. doi:10.1111/j.1365-2133.2008.08976.x
- Hou P-C, Wang H-T, Abhee S, et al. Investigational treatments for epidermolysis bullosa. Am J Clin Dermatol. 2021;22:801-817. doi:10.1007/s40257-021-00626-3
- Youseffian L, Vahidnezhad H, Uitto J. Kindler Syndrome. GeneReviews [Internet]. Updated January 6, 2022. Accessed August 21, 2025.
- Guide SV, Gonzalez ME, Bagci S, et al. Trial of beremagene geperpavec (B-VEC) for dystrophic epidermolysis bullosa. N Engl J Med. 2022;387:2211-2219. doi:10.1056/NEJMoa2206663
- Vetencourt AT, Sayed-Ahmed I, Gomez J, et al. Ocular gene therapy in a patient with dystrophic epidermolysis bullosa. N Engl J Med. 2024;390:530-535. doi:10.1056/NEJMoa2301244
- Kern JS, Sprecher E, Fernandez MF, et al. Efficacy and safety of Oleogel-S10 (birch triterpenes for epidermolysis bullosa: results from the phase III randomized double-blind phase of the EASE study. Br J Dermatol. 2023;188:12-21. doi:10.1093/bjd/ljac001
- Tang JY, Marinkovich MP, Wiss K, et al. Prademagene zamikeracel for recessive dystrophic epidermolysis bullosa wounds (VIITAL): a two-centre, randomized, open-label, intrapatient-controlled phase 3 trial. Lancet. 2025;406:163-173. doi:10.1016/S0140-6736(25)00778-0
- Gretzmeier C, Pin D, Kern JS, et al. Systemic collagen VII replacement therapy for advanced recessive dystrophic epidermolysis bullosa. J Invest Dermatol. 2022;142:1094-1102. doi:10.1016/j.jid.2021.09.008
- Hignett E, Sami N. Pediatric epidermolysis bullosa acquisita. A review. Pediatr Dermatol. 2021;38:1047-1050. doi:10.1111/pde.14722
- Chen M, Kim GH, Prakash L, et al. Autoimmunity to anchoring fibril collagen. Autoimmunity. 2012;45:91-101. doi:10.1007/s12016-007-0027-6.
- Kridin K, Kneiber D, Kowalski EH, et al. Epidermolysis bullosa acquisita: a comprehensive review. Autoimmun Rev. 2019;18:786-795. doi:10.1016/j.autrev.2019.06.007
- Hofmann SC, Weidinger A. Epidermolysis bullosa acquisita. Hautarzt. 2019;70:265-270. doi:10.1007/s00105-019-4387-7
- Ishi N, Hamada T, Dainichi T, et al. Epidermolysis bullosa acquisita: what’s new? J Dermatol. 2010;37:220-230. doi:10.1111/j.1346-8138.2009.00799.x
- Iwata H, Vorobyev A, Koga H, et al. Meta-analysis of the clinical and immunopathological characteristics and treatment outcomes in epidermolysis bullosa acquisita patients. Orphanet J Rare Dis. 2018;13:153. doi:10.1186/s13023-018-0896-1
- Komorowski L, Muller R, Vorobyev A, et al. Sensitive and specific assays for routine serological diagnosis of epidermolysis bullosa acquisita. J Am Acad Dermatol. 2013;68:e89-95. doi:10.1016/j.jaad.2011.12.032
- Antonelli E, Bassotti G, Tramontana M, et al. Dermatological manifestations in inflammatory bowel diseases. J Clin Med. 2021;10:364-390. doi:10.3390/jcm10020364
- Bezzio C, Della Corte C, Vernero M, et al. Inflammatory bowel disease and immune-mediated inflammatory diseases: looking at less frequent associations. Therap Adv Gastroenterol. 2022;15:17562848221115312. doi:10.1177/17562848221115312
- Chen M, O’Toole EA, Sanghavi J, et al. The epidermolysis acquisita antigen (type VII collagen) is present in human colon and patients with Crohn’s disease have antibodies to type VII collagen. J Invest Dermatol. 2002;118:1059-1064. doi:10.1046/j.1523-1747.2002.01772.x
- Labeille B, Gineston JL, Denoeux JP, et al. Epidermolysis bullosa acquisita and Crohn’s disease. A case report with immunological and electron microscopic studies. Arch Intern Med. 1988;148:1457-1459.
- Etienne A, Ruffieux P, Didierjean L, et al. Epidermolysis bullosa acquisita and metastatic cancer of the uterine cervix. Ann Dermatol Venereol. 1998;125:321-323.
- Busch J-O, Sticherling M. Epidermolysis bullosa acquisita and neuroendocrine pancreatic cancer-Coincidence or patho-genetic relationship? J Dtsch Dermatol Ges. 2007;5:916-918. doi:10.111/j.1610-0387.2007.06338.x
- Bevans SL, Sami N. The use of rituximab in treatment of epidermolysis bullosa acquisita: three new cases and a review of the literature. Dermatol Ther. 2018;31:e12726. doi:10.1111/j.1610-0387.2007.06338.x
- Yang A, Kim M, Craig P, et al. A case report of the use of rituximab and the epidermolysis bullosa disease activity scoring index (EBDASI) in a patient with epidermolysis bullosa acquisita with extensive esophageal involvement. Arch Dermatovenerol Croat. 2018;26:325-328.
- Burrows NP, Bhogal BS, Black MM, et al. Bullous eruption of systemic lupus erythematosus: a clinicopathological study of four cases. Br J Dermatol. 1993;128:332-338. doi:10.1111/j.1365-2133.1993.tb00180.x
- Aringer M, Leuchten N, Johnson SR. New criteria for lupus. Curr Rheum Rep. 2020;22:18. doi:10.1007/s11926-020-00896-6
- Camisa C. Vesiculobullous systemic lupus erythematosus. A report of four cases. J Am Acad Dermatol. 1988;18:93-100. doi:10.1016/s0190-9622(88)70014-6
- Duan L, Chen L, Zhong S, et al. Treatment of bullous systemic lupus erythematosus. J Immunol Res. 2015;2015:167064. doi:10.1155/2015/167064
- Sprow G, Afarideh M, Dan J, et al. Bullous systemic lupus erythematosus in females. Int J Womens Dermatol. 2022;8:e034. doi:10.1097/JW9.0000000000000034
- Contestable JJ, Edhegard KD, Meyerle JH. Bullous systemic lupus erythematosus: a review and update to diagnosis and treatment. Am J Clin Dermatol. 2014;15:517-524. doi:10.1007/s40257-014-0098-0
- Fine JD, Mellerio JE. Epidermolysis bullosa. In: Bolognia JL, Jorizzo JL, Schaffer JV (eds), Dermatology (ed 3), Elsevier Saunders; 2012: 501-513.
Type VII Collagen Disorders Simplified
Type VII Collagen Disorders Simplified
PRACTICE POINTS
- The full complement of type VII collagen is required for the normal assembly of anchoring fibrils, whose function is to adhere the basement membrane to the underlying connective tissue of skin and mucous membranes.
- In the heritable epidermolysis bullosa (EB) family of diseases, only dominant and recessive dystrophic epidermolysis bullosa are caused by partial or total loss of type VII collagen function.
- New treatments that have been approved for EB include topical gene therapy with COL7A1, topical birch triterpene gel, and skin cells from patients that are genetically corrected with a functional COL7A1 gene.
- Epidermolysis bullosa acquisita and bullous systemic lupus erythematosus are rare distinct autoimmune subepithelial bullous diseases caused by IgG antibodies that target type VII collagen in the anchoring fibrils.
Sniffing Out Skin Disease: Odors in Dermatologic Conditions
Sniffing Out Skin Disease: Odors in Dermatologic Conditions
Humans possess the ability to recognize and distinguish a large range of odors that can be utilized in a wide range of applications. For example, sommeliers can classify more than 88 smells specific to the roughly 800 volatile organic compounds (VOCs) in wine. Thorough physical examination is essential in dermatology, and although sight and touch play the most important diagnostic roles, the sense of smell often is overlooked. Dermatologists are rigorously trained on the many visual aspects of skin disease and have a plethora of terms to describe these features while there is minimal characterization of odors. Research on odors and the role of olfaction in dermatologic practice is limited.1,2 We conducted a literature review of PubMed and Google Scholar for peer-reviewed articles discussing the role of odors in dermatologic diseases. Keywords included odor + dermatology, smell + dermatology, cutaneous odor, odor + diagnosis, and disease odor. Relevant studies were identified by screening their abstracts, followed by a full-text review. A total of 38 articles written in English that presented information on the odor associated with dermatologic diseases were included. Articles that were unrelated to the topic or written in a language other than English were excluded.
Common Skin Odors
The human body emits odorants—small VOCs—in various forms (skin/sweat, breath, urine, reproductive fluids). Human odor originates from the oxidation and bacterial metabolism of sweat and sebum on the skin.3 While many odors are physiologic and not cause for concern, others can signal underlying dermatologic pathologies.4 Odor-producing conditions can be categorized broadly into infectious diseases, disorders of keratinization and acantholysis, metabolic disorders, and organ dysfunction (Table). Infectious causes include bacterial infections and chronic wounds, which commonly emit characteristic offensive odors. For example, coryneform infections produce methanethiol, causing a cheesy odor of putrid fruit, and pseudomonal pyoderma infections emit a grape juice–like or mousy odor.
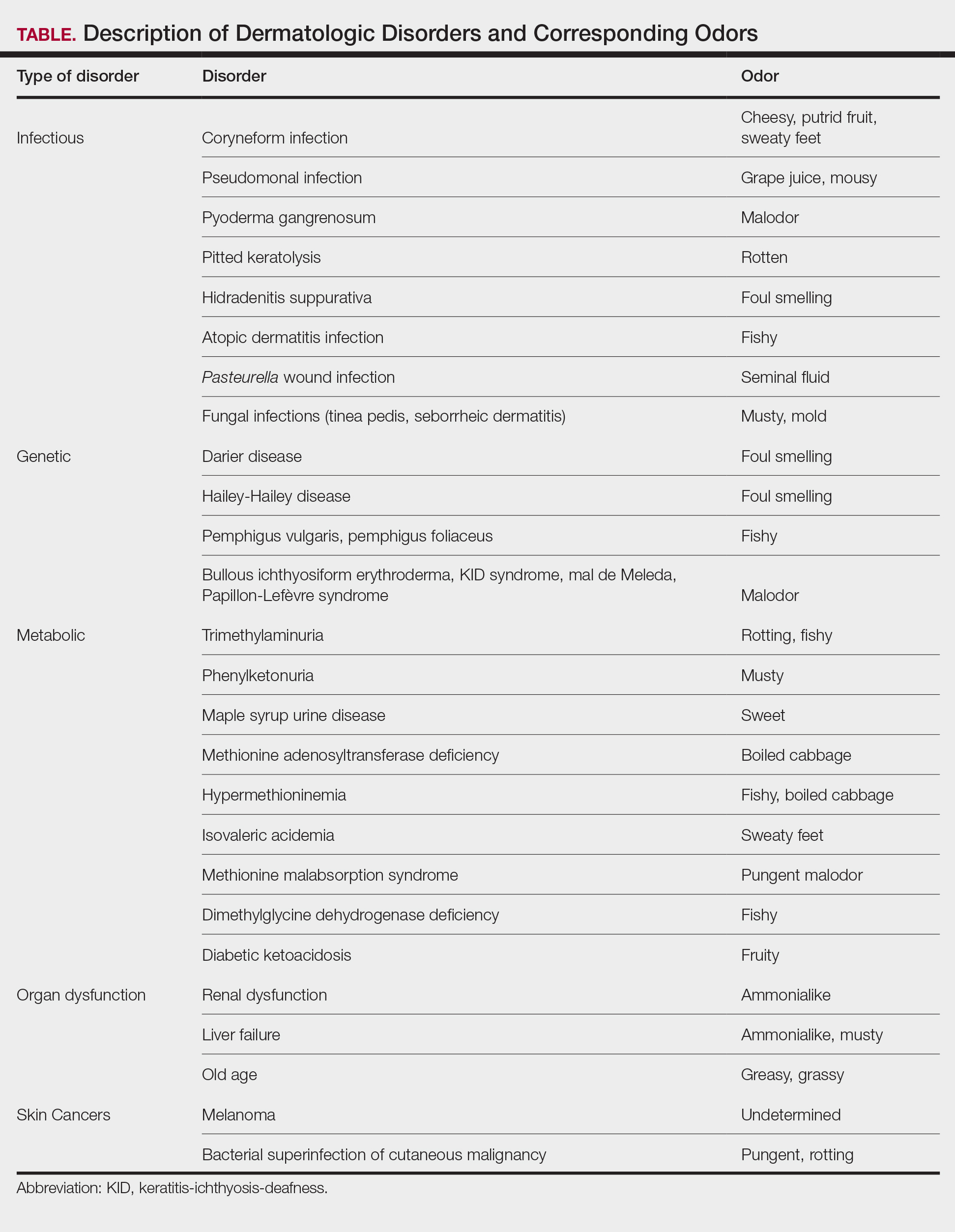
Bacterial and Fungal Infections
Bacterial and fungal infections often have distinct smells. Coryneform infections emit an odor of sweaty feet, pseudomonal infections emit a grape juice–like or mousy odor, and trichomycosis infections (caused by Corynebacterium tenuis) present with malodor.5 Pseudomonas can infect pyoderma gangrenosum lesions, producing a characteristic malodor.5 These smells can be clues for infectious etiology and guide further workup.
Pitted keratolysis, a malodorous pitted rash characterized by infection of the stratum corneum by Kytococcus sedentarius, Dermatophilus congolensis, or Corynebacterium species, is associated with a rotten smell. Its pungent odor, clinical location, and characteristic appearance often are enough to make a diagnosis. The amount of bacteria maintained in the stratum corneum is correlated with the extent of the lesion. Controlling excessive moisture in footwear, aluminum chloride, and topical microbial agents work together to eliminate the skin eruption.6
Hidradenitis suppurativa, a chronic inflammatory disease of apocrine gland–containing skin, can manifest with abscesses, draining sinuses, and nodules that produce a foul-smelling, purulent discharge. The disease can be debilitating, largely impacting patients’ quality of life, making early diagnosis and treatment critical.7,8 Therapy is dependent on disease severity and includes topical antibiotics, systemic therapies, and biologics.8
Patients with atopic dermatitis often experience bacterial superinfection with Staphylococcus aureus. A case report described a patient who developed a fishy odor in this setting that resolved with antibiotic treatment, implicating S aureus in the etiology of the smell.9
A seminal fluid odor has been reported in cases of Pasteurella wound infection. In such cases, Pasteurella multocida subspecies septica was identified in the wounds caused by a dog scratch and a cat bite. The seminal fluid–like odor was apparent hours after the inciting incident and resolved after treatment with antibiotics.10
Fungal infections frequently emit musty or moldy odors. Tinea pedis (athlete’s foot) is the most prevalent cutaneous fungal infection. The presence of tinea pedis is associated with an intense foul-smelling odor, itching, fissuring, scaling, or maceration of the interdigital regions. The rash and odor resolve with use of topical antifungal agents.11,12 Seborrheic dermatitis, a prevalent and chronic dermatosis, is characterized by yellow greasy scaling on an erythematous base. In severe cases, a greasy crust with an offensive odor can cover the entire scalp.13 The specific cause of this odor is unclear, but it is thought that sebum production and the immunological response to specific Malassezia yeast species may play a role.14
Genetic and Metabolic Disorders
An array of disorders of keratinization and acantholysis can manifest with distinctive smells that dermatologists frequently encounter. For example, Darier disease, characterized by keratotic papules progressing to crusted plaques, has a signature foul-smelling odor associated with cutaneous bacterial colonization.15 Similarly, Hailey-Hailey disease, an autosomal-dominant disorder with crusted erosions in skinfold areas, produces a distinct foul smell.16 Disorders such as pemphigus vulgaris and pemphigus foliaceus emit a peculiar fishy odor that can be helpful in making a diagnosis.17 Additionally, bullous ichthyosiform erythroderma, keratitis-ichthyosis-deafness syndrome, mal de Meleda, and Papillon-Lefèvre syndrome are all associated with malodor.5
Certain metabolic disorders can manifest and present initially with identifiable odors. Trimethylaminuria is a psychologically disabling disease known for its rotting fishy smell due to high amounts of trimethylamine appearing in affected individuals’ sweat, urine, and breath. Previously considered to be very rare, Messenger et al18 reported the disorder is likely underdiagnosed in those with idiopathic malodor production. Detection and treatment can greatly improve patient quality of life.
Phenylketonuria is an autosomal-recessive inborn error of phenylalanine metabolism that produces a musty body and urine odor as well as other neurologic and dermatologic symptoms.19,20 Patients can present with eczematous rashes, fair skin, and blue eyes. Phenylacetic acid produces the characteristic odor in the bodily fluids, and the disease is treated with a phenylalanine-free diet.21
Maple syrup urine disease is a disorder of the oxidative decarboxylation of valine, leucine, and isoleucine (branched-chain amino acids) characterized by urine that smells sweet, resembling maple syrup, in afflicted individuals. The odor also can be present in other bodily secretions, such as sweat. Patients present early in infancy with poor feeding and vomiting as well as neurologic symptoms, eventually leading to intellectual disability. These individuals must avoid the branched-chain amino acids in their diets.21
Other metabolic storage disorders linked with specific odors are methionine adenosyltransferase deficiency (boiled cabbage), hypermethioninemia (fishy, boiled cabbage), isovaleric acidemia (sweaty feet), methionine malabsorption syndrome (pungent malodor), and dimethylglycine dehydrogenase deficiency (fishy).5,21,22
In diabetic ketoacidosis, a life-threatening complication of diabetes, the excess of ketone bodies produced causes patients to have a distinct fruity breath and urine odor, as well as fatigue, polyuria, polydipsia, nausea, and vomiting.22 Although patients with type 1 diabetes typically comprise the cohort of patients presenting with diabetic ketoacidosis, patients with type 2 diabetes can exhibit cutaneous manifestations such as infection, xerosis, and inflammatory skin diseases.23,24
Organ Dysfunction
A peculiar body odor can be a sign of organ dysfunction. Renal dysfunction may present with both an odor and dermatologic manifestations. Patients with end-stage renal disease can have an ammonialike uremic breath odor as the result of excessive nitrogenous waste products and increased concentrations of urea in their saliva.4,22 These patients also can exhibit pruritus, xerosis, pigmentation changes, nail changes, other dermatoses, and rarely uremic frost with white urate crystals present on the skin.25,26
Liver failure has been associated with an ammonialike musty breath odor termed fetor hepaticus. Shimamoto et al27 reported notably higher levels of breath ammonia levels in patients with hepatic encephalopathy, indicating that excess ammonia is responsible for the odor. Fetor hepaticus has unique characteristics that can permit a diagnosis of liver disease, though it has been reported in cases in which a liver injury could not be identified.28
Aging patients typically have a distinctive smell. Haze et al29 analyzed the body odor of patients aged 26 to 75 years and discovered the compound 2-nonenal—an unsaturated aldehyde with a smell described as greasy and grassy—was found only in patients older than 40 years. The researchers’ analysis of skin-surface lipids also revealed that the presence of ω7 unsaturated fatty acids and lipid peroxides increased with age. They concluded that 2-nonenal is generated from the oxidative degradation of ω7 unsaturated fatty acids by lipid peroxides, suggesting that 2-nonenal may be a cause of the odor of old age.29
Cutaneous Malignancies
Research shows that the profiles of the body’s continuously released VOCs change in the presence of malignancy. Some studies suggest that melanoma may have a unique odor. Willis et al30 reported that after a 13-month training period, a dog was able to correctly identify melanoma and distinguish it from basal cell carcinoma, benign nevi, and healthy skin based on olfaction alone. Additional cases have been reported in which dogs have been able to identify melanoma based on smell, suggesting that canine olfactory detection of melanoma could possibly aid in the diagnosis of skin cancer, which warrants further investigation.31,32 There is limited evidence on the specific odors of other cutaneous malignancies, such as basal cell carcinoma and squamous cell carcinoma.
Bacterial superinfection of cutaneous malignancy can secrete pungent odors. An offensive rotting odor has been associated with necrotic malignant ulcers of the vagina. This malodor likely is a result of the formation of putrescine, cadaverine, short-chain fatty acids (isovaleric and butyric acids) and sulfur-containing compounds by bacteria.33 Recognition of similar smells may aid in management of these infections.
Diagnostic Techniques
Evaluating human skin odor is challenging, as the components of VOCs are complicated and typically found at trace levels. Studies indicate that gas chromatography–mass spectrometry is the most effective way to analyze human odor. This method separates, quantifies, and analyzes VOCs from samples containing odors.34 Gas chromatography–mass spectrometry, however, has limitations, as the time for analysis is lengthy, the equipment is large, and the process is expensive.3 Research supports the usefulness and validity of quantitative gas chromatography–olfactometry to detect odorants and evaluate odor activity of VOCs in various samples.35 With this technique, human assessors act in place of more conventional detectors, such as mass spectrometers. This method has been used to evaluate odorants in human urine with the goal of increasing understanding of metabolization and excretion processes.36 However, gas chromatography–olfactometry typically is used in the analysis of food and drink, and future research should be aimed at applying this method to medicine.
Zheng et al3 proposed a wearable electronic nose as a tool to identify human odor to emulate the odor recognition of a canine’s nose. They developed a sensor array based on the composites of carbon nanotubes and polymers able to examine and identify odors in the air. Study participants wore the electronic nose on the arm with the sensory array facing the armpits while they walked on a treadmill. Although many issues regarding odor measurement were not addressed in this study, the research suggests further studies are warranted to improve analysis of odor.3
Clinical Cases
Patient 1—Arseculeratne et al37 described a 41-year-old man who presented with a fishy odor that others had noticed since the age of 13 years but that the patient could not smell himself. Based on his presentation, he was worked up for trimethylaminuria and found to have elevated levels of urinary trimethylamine (TMA) with a raised TMA/TMA-oxidase ratio. These findings were consistent with a diagnosis of primary trimethylaminuria, and the patient was referred to a dietician for counseling on foods that contain low amounts of choline and lecithin. Initially his urinary TMA level fell but then rose again, indicating possible relaxation of his diet. He then took a 10-day course of metronidazole, which helped reduce some of the malodor. The authors reported that the most impactful therapy for the patient was being able to discuss the disorder with his friends and family members.37 This case highlighted the importance of confirming the diagnosis and early initiation of dietary and pharmacologic interventions in patients with trimethylaminuria. In patients reporting a persistent fishy body odor, trimethylaminuria should be on the differential.
Patient 2—In 1999, Schissel et al6 described a 20-year-old active-duty soldier who presented to the dermatology department with smelly trench foot and tinea pedis. The soldier reported having this malodorous pitted rash for more than 10 years. He also reported occasional interdigital burning and itching and noted no improvement despite using various topical antifungals. Physical examination revealed an “overpowering pungent odor” when the patient removed his shoes. He had many tender, white, and wet plaques with scalloped borders coalescing into shallow pits on the plantar surface of the feet and great toes. Potassium hydroxide preparation of the great toe plaques and interdigital web spaces were positive for fungal elements, and bacterial cultures isolated moderate coagulase-negative staphylococcal and Corynebacterium species. Additionally, fungal cultures identified Acremonium species. The patient was started on clotrimazole cream twice daily, clindamycin solution twice daily, and topical ammonium chloride nightly. Two weeks later, the patient reported resolution of symptoms, including the malodor.6 In pitted keratolysis, warm and wet environments within boots or shoes allow for the growth of bacteria and fungi. The extent of the lesions is related to the amount of bacteria within the stratum corneum. The diagnosis often is made based on odor, location, and appearance of the rash alone. The most common organisms implicated as causal agents in the condition are Kytococcus sedentarius, Dermatophilus congolensis, and species of Corynebacterium and Actinomyces. It is thought that these organisms release proteolytic enzymes that degrade the horny layer, releasing a mixture of thiols, thioesters, and sulfides, which cause the pungent odor. Familiarity with the characteristic odor aids in prompt diagnosis and treatment, which will ultimately heal the skin eruption.
Patient 3—Srivastava et al32 described a 43-year-old woman who presented with a nevus on the back since childhood. She noticed that it had changed and grown over the past few years and reported that her dog would often sniff the lesion and try to scratch and bite the lesion. This reaction from her dog led the patient to seek out evaluation from a dermatologist. The patient had no personal history of skin cancer, bad sunburns, tanning bed use, or use of immunosuppressants. She reported that her father had a history of basal cell carcinoma. Physical examination revealed a 1.2×1.5-cm brown patch with an ulcerated nodule located on the lower aspect of the lesion. The patient underwent a wide local excision and sentinel lymph node biopsy with pathology showing a 4-mm-thick melanoma with positive lymph nodes. She then underwent a right axillary lymphadenectomy and was diagnosed with stage IIIB malignant melanoma. Following the surgery, the patient’s dog would sniff the back and calmly rest his head in her lap. She has not had a recurrence and credits her dog for saving her life.32 Canine olfaction may play a role in detecting skin cancers, as evidenced by this case. Patients and dermatologists should pay attention to the behavior of dogs toward skin lesions. Harnessing this sense into a method to noninvasively screen for melanoma in humans should be further investigated.
Patient 4—Matthews et al38 described a 32-year-old woman who presented to an emergency eye clinic with a white “lump” on the left upper eyelid of 6 months’ duration. Physical examination revealed 3 nodular and cystic lesions oozing a thick yellow-white discharge. Cultures were taken, and the patient was started on chloramphenicol ointment once daily to the skin. At follow-up, the lesions had not changed, and the cultures were negative. The patient reported an intermittent malodorous discharge and noted multiple similar lesions on her body. Excisional biopsy demonstrated histologic findings including dyskeratosis, papillomatosis, and suprabasal acantholysis associated with focal underlying chronic inflammatory infiltrate. She was referred to a dermatologist and was diagnosed with Darier disease. She was started on clobetasone butyrate when necessary and adapalene nocte. Understanding the smell associated with Darier disease in conjunction with the cutaneous findings may aid in earlier diagnosis, improving outcomes for affected patients.38
Conclusion
The sense of smell may be an overlooked diagnostic tool that dermatologists innately possess. Odors detected when examining patients should be considered, as these odors may help guide a diagnosis. Early diagnosis and treatment are important in many dermatologic diseases, so it is imperative to consider all diagnostic clues. Although physician olfaction may aid in diagnosis, its utility remains challenging, as there is a lack of consensus and terminology regarding odor in disease. A limitation of training to identify disease-specific odors is the requirement of engaging in often unpleasant odors. Methods to objectively measure odor are expensive and still in the early stages of development. Further research and exploration of olfactory-based diagnostic techniques is warranted to potentially improve dermatologic diagnosis.
- Stitt WZ, Goldsmith A. Scratch and sniff: the dynamic duo. Arch Dermatol. 1995;131:997-999.
- Delahunty CM, Eyres G, Dufour JP. Gas chromatography-olfactometry. J Sep Sci. 2006;29:2107-2125.
- Zheng Y, Li H, Shen W, et al. Wearable electronic nose for human skin odor identification: a preliminary study. Sens Actuators A Phys. 2019;285:395-405.
- Mogilnicka I, Bogucki P, Ufnal M. Microbiota and malodor—etiology and management. Int J Mol Sci. 2020;21:2886. doi:10.3390/ijms21082886
- Ravindra K, Gandhi S, Sivuni A. Olfactory diagnosis in skin. Clin Derm Rev. 2018;2:38-40.
- Schissel DJ, Aydelotte J, Keller R. Road rash with a rotten odor. Mil Med. 1999;164:65-67.
- Buyukasik O, Osmanoglu CG, Polat Y, et al. A life-threatening multilocalized hidradenitis suppurativa case. MedGenMed. 2005;7:19.
- Napolitano M, Megna M, Timoshchuk EA, et al. Hidradenitis suppurativa: from pathogenesis to diagnosis and treatment. Clin Cosmet Investig Dermatol. 2017;10:105-115.
- Hon KLE, Leung AKC, Kong AYF, et al. Atopic dermatitis complicated by methicillin-resistant Staphylococcus aureus infection. J Natl Med Assoc. 2008;100:797-800.
- Arashima Y, Kumasaka K, Tutchiya T, et al. Two cases of pasteurellosis accompanied by exudate with semen-like odor from the wound. Article in Japanese. Kansenshogaku Zasshi. 1999;73:623-625.
- Goldstein AO, Smith KM, Ives TJ, et al. Mycotic infections. Effective management of conditions involving the skin, hair, and nails. Geriatrics. 2000;55:40-42, 45-47, 51-52.
- Kircik LH. Observational evaluation of sertaconazole nitrate cream 2% in the treatment of pruritus related to tinea pedis. Cutis. 2009;84:279-283.
- James WD, Elston DM, Treat JR, et al. Andrews’ Diseases of the Skin: Clinical Dermatology. Elsevier Health Sciences; 2019.
- Sameen K. A clinical study on the efficacy of homoeopathic medicines in the treatment of seborrhiec eczema. Int J Hom Sci. 2022;6:209-212.
- Burge S. Management of Darier’s disease. Clin Exp Dermatol. 1999;24:53-56.
- Nanda KB, Saldanha CS, Jacintha M, et al. Hailey-Hailey disease responding to thalidomide. Indian J Dermatol. 2014;59:190-192.
- Kanwar AJ, Ghosh S, Dhar S, et al. Odor in pemphigus. Dermatology. 1992;185:215.
- Messenger J, Clark S, Massick S, et al. A review of trimethylaminuria: (fish odor syndrome). J Clin Aesthet Dermatol. 2013;6:45-48.
- Stone WL, Basit H, Los E. Phenylketonuria. StatPearls [Internet]. Updated August 8, 2023. Accessed August 12, 2025. https://www.ncbi.nlm.nih.gov/books/NBK535378/
- Williams RA, Mamotte CDS, Burnett JR. Phenylketonuria: an inborn error of phenylalanine metabolism. Clin Biochem Rev. 2008;29:31-41.
- Cone TE Jr. Diagnosis and treatment: some diseases, syndromes, and conditions associated with an unusual odor. Pediatrics. 1968;41:993-995.
- Shirasu M, Touhara K. The scent of disease: volatile organic compounds of the human body related to disease and disorder. J Biochem. 2011;150:257-266.
- Ghimire P, Dhamoon AS. Ketoacidosis. StatPearls [Internet]. Updated August 8, 2023. Accessed August 12, 2025. https://www.ncbi.nlm.nih.gov/books/NBK534848/
- Duff M, Demidova O, Blackburn S, et al. Cutaneous manifestations of diabetes mellitus. Clin Diabetes. 2015;33:40-48.
- Raina S, Chauhan V, Sharma R, et al. Uremic frost. Indian Dermatol Online J. 2014;5(suppl 1):S58.
- Blaha T, Nigwekar S, Combs S, et al. Dermatologic manifestations in end stage renal disease. Hemodial Int. 2019;23:3-18.
- Shimamoto C, Hirata I, Katsu K. Breath and blood ammonia in liver cirrhosis. Hepatogastroenterology. 2000;47:443-445.
- Butt HR, Mason HL. Fetor hepaticus: its clinical significance and attempts at chemical isolation. Gastroenterology. 1954;26:829-845.
- Haze S, Gozu Y, Nakamura S, et al. 2-nonenal newly found in human body odor tends to increase with aging. J Invest Dermatol. 2001;116:520-524.
- Willis CM, Britton LE, Swindells MA, et al. Invasive melanoma in vivo can be distinguished from basal cell carcinoma, benign naevi and healthy skin by canine olfaction: a proof-of-principle study of differential volatile organic compound emission. Br J Dermatol. 2016;175:1020-1029.
- Campbell LF, Farmery L, George SMC, et al. Canine olfactory detection of malignant melanoma. BMJ Case Rep. 2013;2013:bcr2013008566. doi:10.1136/bcr-2013-008566
- Srivastava R, John JJ, Reilly C, et al. Sniffing out malignant melanoma: a case of canine olfactory detection. Cutis. 2019;104:E4-E6.
- Fleck CA. Fighting odor in wounds. Adv Skin Wound Care. 2006;19:242-244.
- Gallagher M, Wysocki CJ, Leyden JJ, et al. Analyses of volatile organic compounds from human skin. Br J Dermatol. 2008;159:780-791.
- Campo E, Ferreira V, Escudero A, et al. Quantitative gas chromatography–olfactometry and chemical quantitative study of the aroma of four Madeira wines. Anal Chim Acta. 2006;563:180-187.
- Wagenstaller M, Buettner A. Characterization of odorants in human urine using a combined chemo-analytical and human-sensory approach: a potential diagnostic strategy. Metabolomics. 2012;9:9-20.
- Arseculeratne G, Wong AKC, Goudie DR, et al. Trimethylaminuria (fish-odor syndrome): a case report. Arch Dermatol. 2007;143:81-84.
- Mathews D, Perera LP, Irion LD, et al. Darier disease: beware the cyst that smells. Ophthal Plast Reconstr Surg. 2010;26:206-207.
Humans possess the ability to recognize and distinguish a large range of odors that can be utilized in a wide range of applications. For example, sommeliers can classify more than 88 smells specific to the roughly 800 volatile organic compounds (VOCs) in wine. Thorough physical examination is essential in dermatology, and although sight and touch play the most important diagnostic roles, the sense of smell often is overlooked. Dermatologists are rigorously trained on the many visual aspects of skin disease and have a plethora of terms to describe these features while there is minimal characterization of odors. Research on odors and the role of olfaction in dermatologic practice is limited.1,2 We conducted a literature review of PubMed and Google Scholar for peer-reviewed articles discussing the role of odors in dermatologic diseases. Keywords included odor + dermatology, smell + dermatology, cutaneous odor, odor + diagnosis, and disease odor. Relevant studies were identified by screening their abstracts, followed by a full-text review. A total of 38 articles written in English that presented information on the odor associated with dermatologic diseases were included. Articles that were unrelated to the topic or written in a language other than English were excluded.
Common Skin Odors
The human body emits odorants—small VOCs—in various forms (skin/sweat, breath, urine, reproductive fluids). Human odor originates from the oxidation and bacterial metabolism of sweat and sebum on the skin.3 While many odors are physiologic and not cause for concern, others can signal underlying dermatologic pathologies.4 Odor-producing conditions can be categorized broadly into infectious diseases, disorders of keratinization and acantholysis, metabolic disorders, and organ dysfunction (Table). Infectious causes include bacterial infections and chronic wounds, which commonly emit characteristic offensive odors. For example, coryneform infections produce methanethiol, causing a cheesy odor of putrid fruit, and pseudomonal pyoderma infections emit a grape juice–like or mousy odor.

Bacterial and Fungal Infections
Bacterial and fungal infections often have distinct smells. Coryneform infections emit an odor of sweaty feet, pseudomonal infections emit a grape juice–like or mousy odor, and trichomycosis infections (caused by Corynebacterium tenuis) present with malodor.5 Pseudomonas can infect pyoderma gangrenosum lesions, producing a characteristic malodor.5 These smells can be clues for infectious etiology and guide further workup.
Pitted keratolysis, a malodorous pitted rash characterized by infection of the stratum corneum by Kytococcus sedentarius, Dermatophilus congolensis, or Corynebacterium species, is associated with a rotten smell. Its pungent odor, clinical location, and characteristic appearance often are enough to make a diagnosis. The amount of bacteria maintained in the stratum corneum is correlated with the extent of the lesion. Controlling excessive moisture in footwear, aluminum chloride, and topical microbial agents work together to eliminate the skin eruption.6
Hidradenitis suppurativa, a chronic inflammatory disease of apocrine gland–containing skin, can manifest with abscesses, draining sinuses, and nodules that produce a foul-smelling, purulent discharge. The disease can be debilitating, largely impacting patients’ quality of life, making early diagnosis and treatment critical.7,8 Therapy is dependent on disease severity and includes topical antibiotics, systemic therapies, and biologics.8
Patients with atopic dermatitis often experience bacterial superinfection with Staphylococcus aureus. A case report described a patient who developed a fishy odor in this setting that resolved with antibiotic treatment, implicating S aureus in the etiology of the smell.9
A seminal fluid odor has been reported in cases of Pasteurella wound infection. In such cases, Pasteurella multocida subspecies septica was identified in the wounds caused by a dog scratch and a cat bite. The seminal fluid–like odor was apparent hours after the inciting incident and resolved after treatment with antibiotics.10
Fungal infections frequently emit musty or moldy odors. Tinea pedis (athlete’s foot) is the most prevalent cutaneous fungal infection. The presence of tinea pedis is associated with an intense foul-smelling odor, itching, fissuring, scaling, or maceration of the interdigital regions. The rash and odor resolve with use of topical antifungal agents.11,12 Seborrheic dermatitis, a prevalent and chronic dermatosis, is characterized by yellow greasy scaling on an erythematous base. In severe cases, a greasy crust with an offensive odor can cover the entire scalp.13 The specific cause of this odor is unclear, but it is thought that sebum production and the immunological response to specific Malassezia yeast species may play a role.14
Genetic and Metabolic Disorders
An array of disorders of keratinization and acantholysis can manifest with distinctive smells that dermatologists frequently encounter. For example, Darier disease, characterized by keratotic papules progressing to crusted plaques, has a signature foul-smelling odor associated with cutaneous bacterial colonization.15 Similarly, Hailey-Hailey disease, an autosomal-dominant disorder with crusted erosions in skinfold areas, produces a distinct foul smell.16 Disorders such as pemphigus vulgaris and pemphigus foliaceus emit a peculiar fishy odor that can be helpful in making a diagnosis.17 Additionally, bullous ichthyosiform erythroderma, keratitis-ichthyosis-deafness syndrome, mal de Meleda, and Papillon-Lefèvre syndrome are all associated with malodor.5
Certain metabolic disorders can manifest and present initially with identifiable odors. Trimethylaminuria is a psychologically disabling disease known for its rotting fishy smell due to high amounts of trimethylamine appearing in affected individuals’ sweat, urine, and breath. Previously considered to be very rare, Messenger et al18 reported the disorder is likely underdiagnosed in those with idiopathic malodor production. Detection and treatment can greatly improve patient quality of life.
Phenylketonuria is an autosomal-recessive inborn error of phenylalanine metabolism that produces a musty body and urine odor as well as other neurologic and dermatologic symptoms.19,20 Patients can present with eczematous rashes, fair skin, and blue eyes. Phenylacetic acid produces the characteristic odor in the bodily fluids, and the disease is treated with a phenylalanine-free diet.21
Maple syrup urine disease is a disorder of the oxidative decarboxylation of valine, leucine, and isoleucine (branched-chain amino acids) characterized by urine that smells sweet, resembling maple syrup, in afflicted individuals. The odor also can be present in other bodily secretions, such as sweat. Patients present early in infancy with poor feeding and vomiting as well as neurologic symptoms, eventually leading to intellectual disability. These individuals must avoid the branched-chain amino acids in their diets.21
Other metabolic storage disorders linked with specific odors are methionine adenosyltransferase deficiency (boiled cabbage), hypermethioninemia (fishy, boiled cabbage), isovaleric acidemia (sweaty feet), methionine malabsorption syndrome (pungent malodor), and dimethylglycine dehydrogenase deficiency (fishy).5,21,22
In diabetic ketoacidosis, a life-threatening complication of diabetes, the excess of ketone bodies produced causes patients to have a distinct fruity breath and urine odor, as well as fatigue, polyuria, polydipsia, nausea, and vomiting.22 Although patients with type 1 diabetes typically comprise the cohort of patients presenting with diabetic ketoacidosis, patients with type 2 diabetes can exhibit cutaneous manifestations such as infection, xerosis, and inflammatory skin diseases.23,24
Organ Dysfunction
A peculiar body odor can be a sign of organ dysfunction. Renal dysfunction may present with both an odor and dermatologic manifestations. Patients with end-stage renal disease can have an ammonialike uremic breath odor as the result of excessive nitrogenous waste products and increased concentrations of urea in their saliva.4,22 These patients also can exhibit pruritus, xerosis, pigmentation changes, nail changes, other dermatoses, and rarely uremic frost with white urate crystals present on the skin.25,26
Liver failure has been associated with an ammonialike musty breath odor termed fetor hepaticus. Shimamoto et al27 reported notably higher levels of breath ammonia levels in patients with hepatic encephalopathy, indicating that excess ammonia is responsible for the odor. Fetor hepaticus has unique characteristics that can permit a diagnosis of liver disease, though it has been reported in cases in which a liver injury could not be identified.28
Aging patients typically have a distinctive smell. Haze et al29 analyzed the body odor of patients aged 26 to 75 years and discovered the compound 2-nonenal—an unsaturated aldehyde with a smell described as greasy and grassy—was found only in patients older than 40 years. The researchers’ analysis of skin-surface lipids also revealed that the presence of ω7 unsaturated fatty acids and lipid peroxides increased with age. They concluded that 2-nonenal is generated from the oxidative degradation of ω7 unsaturated fatty acids by lipid peroxides, suggesting that 2-nonenal may be a cause of the odor of old age.29
Cutaneous Malignancies
Research shows that the profiles of the body’s continuously released VOCs change in the presence of malignancy. Some studies suggest that melanoma may have a unique odor. Willis et al30 reported that after a 13-month training period, a dog was able to correctly identify melanoma and distinguish it from basal cell carcinoma, benign nevi, and healthy skin based on olfaction alone. Additional cases have been reported in which dogs have been able to identify melanoma based on smell, suggesting that canine olfactory detection of melanoma could possibly aid in the diagnosis of skin cancer, which warrants further investigation.31,32 There is limited evidence on the specific odors of other cutaneous malignancies, such as basal cell carcinoma and squamous cell carcinoma.
Bacterial superinfection of cutaneous malignancy can secrete pungent odors. An offensive rotting odor has been associated with necrotic malignant ulcers of the vagina. This malodor likely is a result of the formation of putrescine, cadaverine, short-chain fatty acids (isovaleric and butyric acids) and sulfur-containing compounds by bacteria.33 Recognition of similar smells may aid in management of these infections.
Diagnostic Techniques
Evaluating human skin odor is challenging, as the components of VOCs are complicated and typically found at trace levels. Studies indicate that gas chromatography–mass spectrometry is the most effective way to analyze human odor. This method separates, quantifies, and analyzes VOCs from samples containing odors.34 Gas chromatography–mass spectrometry, however, has limitations, as the time for analysis is lengthy, the equipment is large, and the process is expensive.3 Research supports the usefulness and validity of quantitative gas chromatography–olfactometry to detect odorants and evaluate odor activity of VOCs in various samples.35 With this technique, human assessors act in place of more conventional detectors, such as mass spectrometers. This method has been used to evaluate odorants in human urine with the goal of increasing understanding of metabolization and excretion processes.36 However, gas chromatography–olfactometry typically is used in the analysis of food and drink, and future research should be aimed at applying this method to medicine.
Zheng et al3 proposed a wearable electronic nose as a tool to identify human odor to emulate the odor recognition of a canine’s nose. They developed a sensor array based on the composites of carbon nanotubes and polymers able to examine and identify odors in the air. Study participants wore the electronic nose on the arm with the sensory array facing the armpits while they walked on a treadmill. Although many issues regarding odor measurement were not addressed in this study, the research suggests further studies are warranted to improve analysis of odor.3
Clinical Cases
Patient 1—Arseculeratne et al37 described a 41-year-old man who presented with a fishy odor that others had noticed since the age of 13 years but that the patient could not smell himself. Based on his presentation, he was worked up for trimethylaminuria and found to have elevated levels of urinary trimethylamine (TMA) with a raised TMA/TMA-oxidase ratio. These findings were consistent with a diagnosis of primary trimethylaminuria, and the patient was referred to a dietician for counseling on foods that contain low amounts of choline and lecithin. Initially his urinary TMA level fell but then rose again, indicating possible relaxation of his diet. He then took a 10-day course of metronidazole, which helped reduce some of the malodor. The authors reported that the most impactful therapy for the patient was being able to discuss the disorder with his friends and family members.37 This case highlighted the importance of confirming the diagnosis and early initiation of dietary and pharmacologic interventions in patients with trimethylaminuria. In patients reporting a persistent fishy body odor, trimethylaminuria should be on the differential.
Patient 2—In 1999, Schissel et al6 described a 20-year-old active-duty soldier who presented to the dermatology department with smelly trench foot and tinea pedis. The soldier reported having this malodorous pitted rash for more than 10 years. He also reported occasional interdigital burning and itching and noted no improvement despite using various topical antifungals. Physical examination revealed an “overpowering pungent odor” when the patient removed his shoes. He had many tender, white, and wet plaques with scalloped borders coalescing into shallow pits on the plantar surface of the feet and great toes. Potassium hydroxide preparation of the great toe plaques and interdigital web spaces were positive for fungal elements, and bacterial cultures isolated moderate coagulase-negative staphylococcal and Corynebacterium species. Additionally, fungal cultures identified Acremonium species. The patient was started on clotrimazole cream twice daily, clindamycin solution twice daily, and topical ammonium chloride nightly. Two weeks later, the patient reported resolution of symptoms, including the malodor.6 In pitted keratolysis, warm and wet environments within boots or shoes allow for the growth of bacteria and fungi. The extent of the lesions is related to the amount of bacteria within the stratum corneum. The diagnosis often is made based on odor, location, and appearance of the rash alone. The most common organisms implicated as causal agents in the condition are Kytococcus sedentarius, Dermatophilus congolensis, and species of Corynebacterium and Actinomyces. It is thought that these organisms release proteolytic enzymes that degrade the horny layer, releasing a mixture of thiols, thioesters, and sulfides, which cause the pungent odor. Familiarity with the characteristic odor aids in prompt diagnosis and treatment, which will ultimately heal the skin eruption.
Patient 3—Srivastava et al32 described a 43-year-old woman who presented with a nevus on the back since childhood. She noticed that it had changed and grown over the past few years and reported that her dog would often sniff the lesion and try to scratch and bite the lesion. This reaction from her dog led the patient to seek out evaluation from a dermatologist. The patient had no personal history of skin cancer, bad sunburns, tanning bed use, or use of immunosuppressants. She reported that her father had a history of basal cell carcinoma. Physical examination revealed a 1.2×1.5-cm brown patch with an ulcerated nodule located on the lower aspect of the lesion. The patient underwent a wide local excision and sentinel lymph node biopsy with pathology showing a 4-mm-thick melanoma with positive lymph nodes. She then underwent a right axillary lymphadenectomy and was diagnosed with stage IIIB malignant melanoma. Following the surgery, the patient’s dog would sniff the back and calmly rest his head in her lap. She has not had a recurrence and credits her dog for saving her life.32 Canine olfaction may play a role in detecting skin cancers, as evidenced by this case. Patients and dermatologists should pay attention to the behavior of dogs toward skin lesions. Harnessing this sense into a method to noninvasively screen for melanoma in humans should be further investigated.
Patient 4—Matthews et al38 described a 32-year-old woman who presented to an emergency eye clinic with a white “lump” on the left upper eyelid of 6 months’ duration. Physical examination revealed 3 nodular and cystic lesions oozing a thick yellow-white discharge. Cultures were taken, and the patient was started on chloramphenicol ointment once daily to the skin. At follow-up, the lesions had not changed, and the cultures were negative. The patient reported an intermittent malodorous discharge and noted multiple similar lesions on her body. Excisional biopsy demonstrated histologic findings including dyskeratosis, papillomatosis, and suprabasal acantholysis associated with focal underlying chronic inflammatory infiltrate. She was referred to a dermatologist and was diagnosed with Darier disease. She was started on clobetasone butyrate when necessary and adapalene nocte. Understanding the smell associated with Darier disease in conjunction with the cutaneous findings may aid in earlier diagnosis, improving outcomes for affected patients.38
Conclusion
The sense of smell may be an overlooked diagnostic tool that dermatologists innately possess. Odors detected when examining patients should be considered, as these odors may help guide a diagnosis. Early diagnosis and treatment are important in many dermatologic diseases, so it is imperative to consider all diagnostic clues. Although physician olfaction may aid in diagnosis, its utility remains challenging, as there is a lack of consensus and terminology regarding odor in disease. A limitation of training to identify disease-specific odors is the requirement of engaging in often unpleasant odors. Methods to objectively measure odor are expensive and still in the early stages of development. Further research and exploration of olfactory-based diagnostic techniques is warranted to potentially improve dermatologic diagnosis.
Humans possess the ability to recognize and distinguish a large range of odors that can be utilized in a wide range of applications. For example, sommeliers can classify more than 88 smells specific to the roughly 800 volatile organic compounds (VOCs) in wine. Thorough physical examination is essential in dermatology, and although sight and touch play the most important diagnostic roles, the sense of smell often is overlooked. Dermatologists are rigorously trained on the many visual aspects of skin disease and have a plethora of terms to describe these features while there is minimal characterization of odors. Research on odors and the role of olfaction in dermatologic practice is limited.1,2 We conducted a literature review of PubMed and Google Scholar for peer-reviewed articles discussing the role of odors in dermatologic diseases. Keywords included odor + dermatology, smell + dermatology, cutaneous odor, odor + diagnosis, and disease odor. Relevant studies were identified by screening their abstracts, followed by a full-text review. A total of 38 articles written in English that presented information on the odor associated with dermatologic diseases were included. Articles that were unrelated to the topic or written in a language other than English were excluded.
Common Skin Odors
The human body emits odorants—small VOCs—in various forms (skin/sweat, breath, urine, reproductive fluids). Human odor originates from the oxidation and bacterial metabolism of sweat and sebum on the skin.3 While many odors are physiologic and not cause for concern, others can signal underlying dermatologic pathologies.4 Odor-producing conditions can be categorized broadly into infectious diseases, disorders of keratinization and acantholysis, metabolic disorders, and organ dysfunction (Table). Infectious causes include bacterial infections and chronic wounds, which commonly emit characteristic offensive odors. For example, coryneform infections produce methanethiol, causing a cheesy odor of putrid fruit, and pseudomonal pyoderma infections emit a grape juice–like or mousy odor.

Bacterial and Fungal Infections
Bacterial and fungal infections often have distinct smells. Coryneform infections emit an odor of sweaty feet, pseudomonal infections emit a grape juice–like or mousy odor, and trichomycosis infections (caused by Corynebacterium tenuis) present with malodor.5 Pseudomonas can infect pyoderma gangrenosum lesions, producing a characteristic malodor.5 These smells can be clues for infectious etiology and guide further workup.
Pitted keratolysis, a malodorous pitted rash characterized by infection of the stratum corneum by Kytococcus sedentarius, Dermatophilus congolensis, or Corynebacterium species, is associated with a rotten smell. Its pungent odor, clinical location, and characteristic appearance often are enough to make a diagnosis. The amount of bacteria maintained in the stratum corneum is correlated with the extent of the lesion. Controlling excessive moisture in footwear, aluminum chloride, and topical microbial agents work together to eliminate the skin eruption.6
Hidradenitis suppurativa, a chronic inflammatory disease of apocrine gland–containing skin, can manifest with abscesses, draining sinuses, and nodules that produce a foul-smelling, purulent discharge. The disease can be debilitating, largely impacting patients’ quality of life, making early diagnosis and treatment critical.7,8 Therapy is dependent on disease severity and includes topical antibiotics, systemic therapies, and biologics.8
Patients with atopic dermatitis often experience bacterial superinfection with Staphylococcus aureus. A case report described a patient who developed a fishy odor in this setting that resolved with antibiotic treatment, implicating S aureus in the etiology of the smell.9
A seminal fluid odor has been reported in cases of Pasteurella wound infection. In such cases, Pasteurella multocida subspecies septica was identified in the wounds caused by a dog scratch and a cat bite. The seminal fluid–like odor was apparent hours after the inciting incident and resolved after treatment with antibiotics.10
Fungal infections frequently emit musty or moldy odors. Tinea pedis (athlete’s foot) is the most prevalent cutaneous fungal infection. The presence of tinea pedis is associated with an intense foul-smelling odor, itching, fissuring, scaling, or maceration of the interdigital regions. The rash and odor resolve with use of topical antifungal agents.11,12 Seborrheic dermatitis, a prevalent and chronic dermatosis, is characterized by yellow greasy scaling on an erythematous base. In severe cases, a greasy crust with an offensive odor can cover the entire scalp.13 The specific cause of this odor is unclear, but it is thought that sebum production and the immunological response to specific Malassezia yeast species may play a role.14
Genetic and Metabolic Disorders
An array of disorders of keratinization and acantholysis can manifest with distinctive smells that dermatologists frequently encounter. For example, Darier disease, characterized by keratotic papules progressing to crusted plaques, has a signature foul-smelling odor associated with cutaneous bacterial colonization.15 Similarly, Hailey-Hailey disease, an autosomal-dominant disorder with crusted erosions in skinfold areas, produces a distinct foul smell.16 Disorders such as pemphigus vulgaris and pemphigus foliaceus emit a peculiar fishy odor that can be helpful in making a diagnosis.17 Additionally, bullous ichthyosiform erythroderma, keratitis-ichthyosis-deafness syndrome, mal de Meleda, and Papillon-Lefèvre syndrome are all associated with malodor.5
Certain metabolic disorders can manifest and present initially with identifiable odors. Trimethylaminuria is a psychologically disabling disease known for its rotting fishy smell due to high amounts of trimethylamine appearing in affected individuals’ sweat, urine, and breath. Previously considered to be very rare, Messenger et al18 reported the disorder is likely underdiagnosed in those with idiopathic malodor production. Detection and treatment can greatly improve patient quality of life.
Phenylketonuria is an autosomal-recessive inborn error of phenylalanine metabolism that produces a musty body and urine odor as well as other neurologic and dermatologic symptoms.19,20 Patients can present with eczematous rashes, fair skin, and blue eyes. Phenylacetic acid produces the characteristic odor in the bodily fluids, and the disease is treated with a phenylalanine-free diet.21
Maple syrup urine disease is a disorder of the oxidative decarboxylation of valine, leucine, and isoleucine (branched-chain amino acids) characterized by urine that smells sweet, resembling maple syrup, in afflicted individuals. The odor also can be present in other bodily secretions, such as sweat. Patients present early in infancy with poor feeding and vomiting as well as neurologic symptoms, eventually leading to intellectual disability. These individuals must avoid the branched-chain amino acids in their diets.21
Other metabolic storage disorders linked with specific odors are methionine adenosyltransferase deficiency (boiled cabbage), hypermethioninemia (fishy, boiled cabbage), isovaleric acidemia (sweaty feet), methionine malabsorption syndrome (pungent malodor), and dimethylglycine dehydrogenase deficiency (fishy).5,21,22
In diabetic ketoacidosis, a life-threatening complication of diabetes, the excess of ketone bodies produced causes patients to have a distinct fruity breath and urine odor, as well as fatigue, polyuria, polydipsia, nausea, and vomiting.22 Although patients with type 1 diabetes typically comprise the cohort of patients presenting with diabetic ketoacidosis, patients with type 2 diabetes can exhibit cutaneous manifestations such as infection, xerosis, and inflammatory skin diseases.23,24
Organ Dysfunction
A peculiar body odor can be a sign of organ dysfunction. Renal dysfunction may present with both an odor and dermatologic manifestations. Patients with end-stage renal disease can have an ammonialike uremic breath odor as the result of excessive nitrogenous waste products and increased concentrations of urea in their saliva.4,22 These patients also can exhibit pruritus, xerosis, pigmentation changes, nail changes, other dermatoses, and rarely uremic frost with white urate crystals present on the skin.25,26
Liver failure has been associated with an ammonialike musty breath odor termed fetor hepaticus. Shimamoto et al27 reported notably higher levels of breath ammonia levels in patients with hepatic encephalopathy, indicating that excess ammonia is responsible for the odor. Fetor hepaticus has unique characteristics that can permit a diagnosis of liver disease, though it has been reported in cases in which a liver injury could not be identified.28
Aging patients typically have a distinctive smell. Haze et al29 analyzed the body odor of patients aged 26 to 75 years and discovered the compound 2-nonenal—an unsaturated aldehyde with a smell described as greasy and grassy—was found only in patients older than 40 years. The researchers’ analysis of skin-surface lipids also revealed that the presence of ω7 unsaturated fatty acids and lipid peroxides increased with age. They concluded that 2-nonenal is generated from the oxidative degradation of ω7 unsaturated fatty acids by lipid peroxides, suggesting that 2-nonenal may be a cause of the odor of old age.29
Cutaneous Malignancies
Research shows that the profiles of the body’s continuously released VOCs change in the presence of malignancy. Some studies suggest that melanoma may have a unique odor. Willis et al30 reported that after a 13-month training period, a dog was able to correctly identify melanoma and distinguish it from basal cell carcinoma, benign nevi, and healthy skin based on olfaction alone. Additional cases have been reported in which dogs have been able to identify melanoma based on smell, suggesting that canine olfactory detection of melanoma could possibly aid in the diagnosis of skin cancer, which warrants further investigation.31,32 There is limited evidence on the specific odors of other cutaneous malignancies, such as basal cell carcinoma and squamous cell carcinoma.
Bacterial superinfection of cutaneous malignancy can secrete pungent odors. An offensive rotting odor has been associated with necrotic malignant ulcers of the vagina. This malodor likely is a result of the formation of putrescine, cadaverine, short-chain fatty acids (isovaleric and butyric acids) and sulfur-containing compounds by bacteria.33 Recognition of similar smells may aid in management of these infections.
Diagnostic Techniques
Evaluating human skin odor is challenging, as the components of VOCs are complicated and typically found at trace levels. Studies indicate that gas chromatography–mass spectrometry is the most effective way to analyze human odor. This method separates, quantifies, and analyzes VOCs from samples containing odors.34 Gas chromatography–mass spectrometry, however, has limitations, as the time for analysis is lengthy, the equipment is large, and the process is expensive.3 Research supports the usefulness and validity of quantitative gas chromatography–olfactometry to detect odorants and evaluate odor activity of VOCs in various samples.35 With this technique, human assessors act in place of more conventional detectors, such as mass spectrometers. This method has been used to evaluate odorants in human urine with the goal of increasing understanding of metabolization and excretion processes.36 However, gas chromatography–olfactometry typically is used in the analysis of food and drink, and future research should be aimed at applying this method to medicine.
Zheng et al3 proposed a wearable electronic nose as a tool to identify human odor to emulate the odor recognition of a canine’s nose. They developed a sensor array based on the composites of carbon nanotubes and polymers able to examine and identify odors in the air. Study participants wore the electronic nose on the arm with the sensory array facing the armpits while they walked on a treadmill. Although many issues regarding odor measurement were not addressed in this study, the research suggests further studies are warranted to improve analysis of odor.3
Clinical Cases
Patient 1—Arseculeratne et al37 described a 41-year-old man who presented with a fishy odor that others had noticed since the age of 13 years but that the patient could not smell himself. Based on his presentation, he was worked up for trimethylaminuria and found to have elevated levels of urinary trimethylamine (TMA) with a raised TMA/TMA-oxidase ratio. These findings were consistent with a diagnosis of primary trimethylaminuria, and the patient was referred to a dietician for counseling on foods that contain low amounts of choline and lecithin. Initially his urinary TMA level fell but then rose again, indicating possible relaxation of his diet. He then took a 10-day course of metronidazole, which helped reduce some of the malodor. The authors reported that the most impactful therapy for the patient was being able to discuss the disorder with his friends and family members.37 This case highlighted the importance of confirming the diagnosis and early initiation of dietary and pharmacologic interventions in patients with trimethylaminuria. In patients reporting a persistent fishy body odor, trimethylaminuria should be on the differential.
Patient 2—In 1999, Schissel et al6 described a 20-year-old active-duty soldier who presented to the dermatology department with smelly trench foot and tinea pedis. The soldier reported having this malodorous pitted rash for more than 10 years. He also reported occasional interdigital burning and itching and noted no improvement despite using various topical antifungals. Physical examination revealed an “overpowering pungent odor” when the patient removed his shoes. He had many tender, white, and wet plaques with scalloped borders coalescing into shallow pits on the plantar surface of the feet and great toes. Potassium hydroxide preparation of the great toe plaques and interdigital web spaces were positive for fungal elements, and bacterial cultures isolated moderate coagulase-negative staphylococcal and Corynebacterium species. Additionally, fungal cultures identified Acremonium species. The patient was started on clotrimazole cream twice daily, clindamycin solution twice daily, and topical ammonium chloride nightly. Two weeks later, the patient reported resolution of symptoms, including the malodor.6 In pitted keratolysis, warm and wet environments within boots or shoes allow for the growth of bacteria and fungi. The extent of the lesions is related to the amount of bacteria within the stratum corneum. The diagnosis often is made based on odor, location, and appearance of the rash alone. The most common organisms implicated as causal agents in the condition are Kytococcus sedentarius, Dermatophilus congolensis, and species of Corynebacterium and Actinomyces. It is thought that these organisms release proteolytic enzymes that degrade the horny layer, releasing a mixture of thiols, thioesters, and sulfides, which cause the pungent odor. Familiarity with the characteristic odor aids in prompt diagnosis and treatment, which will ultimately heal the skin eruption.
Patient 3—Srivastava et al32 described a 43-year-old woman who presented with a nevus on the back since childhood. She noticed that it had changed and grown over the past few years and reported that her dog would often sniff the lesion and try to scratch and bite the lesion. This reaction from her dog led the patient to seek out evaluation from a dermatologist. The patient had no personal history of skin cancer, bad sunburns, tanning bed use, or use of immunosuppressants. She reported that her father had a history of basal cell carcinoma. Physical examination revealed a 1.2×1.5-cm brown patch with an ulcerated nodule located on the lower aspect of the lesion. The patient underwent a wide local excision and sentinel lymph node biopsy with pathology showing a 4-mm-thick melanoma with positive lymph nodes. She then underwent a right axillary lymphadenectomy and was diagnosed with stage IIIB malignant melanoma. Following the surgery, the patient’s dog would sniff the back and calmly rest his head in her lap. She has not had a recurrence and credits her dog for saving her life.32 Canine olfaction may play a role in detecting skin cancers, as evidenced by this case. Patients and dermatologists should pay attention to the behavior of dogs toward skin lesions. Harnessing this sense into a method to noninvasively screen for melanoma in humans should be further investigated.
Patient 4—Matthews et al38 described a 32-year-old woman who presented to an emergency eye clinic with a white “lump” on the left upper eyelid of 6 months’ duration. Physical examination revealed 3 nodular and cystic lesions oozing a thick yellow-white discharge. Cultures were taken, and the patient was started on chloramphenicol ointment once daily to the skin. At follow-up, the lesions had not changed, and the cultures were negative. The patient reported an intermittent malodorous discharge and noted multiple similar lesions on her body. Excisional biopsy demonstrated histologic findings including dyskeratosis, papillomatosis, and suprabasal acantholysis associated with focal underlying chronic inflammatory infiltrate. She was referred to a dermatologist and was diagnosed with Darier disease. She was started on clobetasone butyrate when necessary and adapalene nocte. Understanding the smell associated with Darier disease in conjunction with the cutaneous findings may aid in earlier diagnosis, improving outcomes for affected patients.38
Conclusion
The sense of smell may be an overlooked diagnostic tool that dermatologists innately possess. Odors detected when examining patients should be considered, as these odors may help guide a diagnosis. Early diagnosis and treatment are important in many dermatologic diseases, so it is imperative to consider all diagnostic clues. Although physician olfaction may aid in diagnosis, its utility remains challenging, as there is a lack of consensus and terminology regarding odor in disease. A limitation of training to identify disease-specific odors is the requirement of engaging in often unpleasant odors. Methods to objectively measure odor are expensive and still in the early stages of development. Further research and exploration of olfactory-based diagnostic techniques is warranted to potentially improve dermatologic diagnosis.
- Stitt WZ, Goldsmith A. Scratch and sniff: the dynamic duo. Arch Dermatol. 1995;131:997-999.
- Delahunty CM, Eyres G, Dufour JP. Gas chromatography-olfactometry. J Sep Sci. 2006;29:2107-2125.
- Zheng Y, Li H, Shen W, et al. Wearable electronic nose for human skin odor identification: a preliminary study. Sens Actuators A Phys. 2019;285:395-405.
- Mogilnicka I, Bogucki P, Ufnal M. Microbiota and malodor—etiology and management. Int J Mol Sci. 2020;21:2886. doi:10.3390/ijms21082886
- Ravindra K, Gandhi S, Sivuni A. Olfactory diagnosis in skin. Clin Derm Rev. 2018;2:38-40.
- Schissel DJ, Aydelotte J, Keller R. Road rash with a rotten odor. Mil Med. 1999;164:65-67.
- Buyukasik O, Osmanoglu CG, Polat Y, et al. A life-threatening multilocalized hidradenitis suppurativa case. MedGenMed. 2005;7:19.
- Napolitano M, Megna M, Timoshchuk EA, et al. Hidradenitis suppurativa: from pathogenesis to diagnosis and treatment. Clin Cosmet Investig Dermatol. 2017;10:105-115.
- Hon KLE, Leung AKC, Kong AYF, et al. Atopic dermatitis complicated by methicillin-resistant Staphylococcus aureus infection. J Natl Med Assoc. 2008;100:797-800.
- Arashima Y, Kumasaka K, Tutchiya T, et al. Two cases of pasteurellosis accompanied by exudate with semen-like odor from the wound. Article in Japanese. Kansenshogaku Zasshi. 1999;73:623-625.
- Goldstein AO, Smith KM, Ives TJ, et al. Mycotic infections. Effective management of conditions involving the skin, hair, and nails. Geriatrics. 2000;55:40-42, 45-47, 51-52.
- Kircik LH. Observational evaluation of sertaconazole nitrate cream 2% in the treatment of pruritus related to tinea pedis. Cutis. 2009;84:279-283.
- James WD, Elston DM, Treat JR, et al. Andrews’ Diseases of the Skin: Clinical Dermatology. Elsevier Health Sciences; 2019.
- Sameen K. A clinical study on the efficacy of homoeopathic medicines in the treatment of seborrhiec eczema. Int J Hom Sci. 2022;6:209-212.
- Burge S. Management of Darier’s disease. Clin Exp Dermatol. 1999;24:53-56.
- Nanda KB, Saldanha CS, Jacintha M, et al. Hailey-Hailey disease responding to thalidomide. Indian J Dermatol. 2014;59:190-192.
- Kanwar AJ, Ghosh S, Dhar S, et al. Odor in pemphigus. Dermatology. 1992;185:215.
- Messenger J, Clark S, Massick S, et al. A review of trimethylaminuria: (fish odor syndrome). J Clin Aesthet Dermatol. 2013;6:45-48.
- Stone WL, Basit H, Los E. Phenylketonuria. StatPearls [Internet]. Updated August 8, 2023. Accessed August 12, 2025. https://www.ncbi.nlm.nih.gov/books/NBK535378/
- Williams RA, Mamotte CDS, Burnett JR. Phenylketonuria: an inborn error of phenylalanine metabolism. Clin Biochem Rev. 2008;29:31-41.
- Cone TE Jr. Diagnosis and treatment: some diseases, syndromes, and conditions associated with an unusual odor. Pediatrics. 1968;41:993-995.
- Shirasu M, Touhara K. The scent of disease: volatile organic compounds of the human body related to disease and disorder. J Biochem. 2011;150:257-266.
- Ghimire P, Dhamoon AS. Ketoacidosis. StatPearls [Internet]. Updated August 8, 2023. Accessed August 12, 2025. https://www.ncbi.nlm.nih.gov/books/NBK534848/
- Duff M, Demidova O, Blackburn S, et al. Cutaneous manifestations of diabetes mellitus. Clin Diabetes. 2015;33:40-48.
- Raina S, Chauhan V, Sharma R, et al. Uremic frost. Indian Dermatol Online J. 2014;5(suppl 1):S58.
- Blaha T, Nigwekar S, Combs S, et al. Dermatologic manifestations in end stage renal disease. Hemodial Int. 2019;23:3-18.
- Shimamoto C, Hirata I, Katsu K. Breath and blood ammonia in liver cirrhosis. Hepatogastroenterology. 2000;47:443-445.
- Butt HR, Mason HL. Fetor hepaticus: its clinical significance and attempts at chemical isolation. Gastroenterology. 1954;26:829-845.
- Haze S, Gozu Y, Nakamura S, et al. 2-nonenal newly found in human body odor tends to increase with aging. J Invest Dermatol. 2001;116:520-524.
- Willis CM, Britton LE, Swindells MA, et al. Invasive melanoma in vivo can be distinguished from basal cell carcinoma, benign naevi and healthy skin by canine olfaction: a proof-of-principle study of differential volatile organic compound emission. Br J Dermatol. 2016;175:1020-1029.
- Campbell LF, Farmery L, George SMC, et al. Canine olfactory detection of malignant melanoma. BMJ Case Rep. 2013;2013:bcr2013008566. doi:10.1136/bcr-2013-008566
- Srivastava R, John JJ, Reilly C, et al. Sniffing out malignant melanoma: a case of canine olfactory detection. Cutis. 2019;104:E4-E6.
- Fleck CA. Fighting odor in wounds. Adv Skin Wound Care. 2006;19:242-244.
- Gallagher M, Wysocki CJ, Leyden JJ, et al. Analyses of volatile organic compounds from human skin. Br J Dermatol. 2008;159:780-791.
- Campo E, Ferreira V, Escudero A, et al. Quantitative gas chromatography–olfactometry and chemical quantitative study of the aroma of four Madeira wines. Anal Chim Acta. 2006;563:180-187.
- Wagenstaller M, Buettner A. Characterization of odorants in human urine using a combined chemo-analytical and human-sensory approach: a potential diagnostic strategy. Metabolomics. 2012;9:9-20.
- Arseculeratne G, Wong AKC, Goudie DR, et al. Trimethylaminuria (fish-odor syndrome): a case report. Arch Dermatol. 2007;143:81-84.
- Mathews D, Perera LP, Irion LD, et al. Darier disease: beware the cyst that smells. Ophthal Plast Reconstr Surg. 2010;26:206-207.
- Stitt WZ, Goldsmith A. Scratch and sniff: the dynamic duo. Arch Dermatol. 1995;131:997-999.
- Delahunty CM, Eyres G, Dufour JP. Gas chromatography-olfactometry. J Sep Sci. 2006;29:2107-2125.
- Zheng Y, Li H, Shen W, et al. Wearable electronic nose for human skin odor identification: a preliminary study. Sens Actuators A Phys. 2019;285:395-405.
- Mogilnicka I, Bogucki P, Ufnal M. Microbiota and malodor—etiology and management. Int J Mol Sci. 2020;21:2886. doi:10.3390/ijms21082886
- Ravindra K, Gandhi S, Sivuni A. Olfactory diagnosis in skin. Clin Derm Rev. 2018;2:38-40.
- Schissel DJ, Aydelotte J, Keller R. Road rash with a rotten odor. Mil Med. 1999;164:65-67.
- Buyukasik O, Osmanoglu CG, Polat Y, et al. A life-threatening multilocalized hidradenitis suppurativa case. MedGenMed. 2005;7:19.
- Napolitano M, Megna M, Timoshchuk EA, et al. Hidradenitis suppurativa: from pathogenesis to diagnosis and treatment. Clin Cosmet Investig Dermatol. 2017;10:105-115.
- Hon KLE, Leung AKC, Kong AYF, et al. Atopic dermatitis complicated by methicillin-resistant Staphylococcus aureus infection. J Natl Med Assoc. 2008;100:797-800.
- Arashima Y, Kumasaka K, Tutchiya T, et al. Two cases of pasteurellosis accompanied by exudate with semen-like odor from the wound. Article in Japanese. Kansenshogaku Zasshi. 1999;73:623-625.
- Goldstein AO, Smith KM, Ives TJ, et al. Mycotic infections. Effective management of conditions involving the skin, hair, and nails. Geriatrics. 2000;55:40-42, 45-47, 51-52.
- Kircik LH. Observational evaluation of sertaconazole nitrate cream 2% in the treatment of pruritus related to tinea pedis. Cutis. 2009;84:279-283.
- James WD, Elston DM, Treat JR, et al. Andrews’ Diseases of the Skin: Clinical Dermatology. Elsevier Health Sciences; 2019.
- Sameen K. A clinical study on the efficacy of homoeopathic medicines in the treatment of seborrhiec eczema. Int J Hom Sci. 2022;6:209-212.
- Burge S. Management of Darier’s disease. Clin Exp Dermatol. 1999;24:53-56.
- Nanda KB, Saldanha CS, Jacintha M, et al. Hailey-Hailey disease responding to thalidomide. Indian J Dermatol. 2014;59:190-192.
- Kanwar AJ, Ghosh S, Dhar S, et al. Odor in pemphigus. Dermatology. 1992;185:215.
- Messenger J, Clark S, Massick S, et al. A review of trimethylaminuria: (fish odor syndrome). J Clin Aesthet Dermatol. 2013;6:45-48.
- Stone WL, Basit H, Los E. Phenylketonuria. StatPearls [Internet]. Updated August 8, 2023. Accessed August 12, 2025. https://www.ncbi.nlm.nih.gov/books/NBK535378/
- Williams RA, Mamotte CDS, Burnett JR. Phenylketonuria: an inborn error of phenylalanine metabolism. Clin Biochem Rev. 2008;29:31-41.
- Cone TE Jr. Diagnosis and treatment: some diseases, syndromes, and conditions associated with an unusual odor. Pediatrics. 1968;41:993-995.
- Shirasu M, Touhara K. The scent of disease: volatile organic compounds of the human body related to disease and disorder. J Biochem. 2011;150:257-266.
- Ghimire P, Dhamoon AS. Ketoacidosis. StatPearls [Internet]. Updated August 8, 2023. Accessed August 12, 2025. https://www.ncbi.nlm.nih.gov/books/NBK534848/
- Duff M, Demidova O, Blackburn S, et al. Cutaneous manifestations of diabetes mellitus. Clin Diabetes. 2015;33:40-48.
- Raina S, Chauhan V, Sharma R, et al. Uremic frost. Indian Dermatol Online J. 2014;5(suppl 1):S58.
- Blaha T, Nigwekar S, Combs S, et al. Dermatologic manifestations in end stage renal disease. Hemodial Int. 2019;23:3-18.
- Shimamoto C, Hirata I, Katsu K. Breath and blood ammonia in liver cirrhosis. Hepatogastroenterology. 2000;47:443-445.
- Butt HR, Mason HL. Fetor hepaticus: its clinical significance and attempts at chemical isolation. Gastroenterology. 1954;26:829-845.
- Haze S, Gozu Y, Nakamura S, et al. 2-nonenal newly found in human body odor tends to increase with aging. J Invest Dermatol. 2001;116:520-524.
- Willis CM, Britton LE, Swindells MA, et al. Invasive melanoma in vivo can be distinguished from basal cell carcinoma, benign naevi and healthy skin by canine olfaction: a proof-of-principle study of differential volatile organic compound emission. Br J Dermatol. 2016;175:1020-1029.
- Campbell LF, Farmery L, George SMC, et al. Canine olfactory detection of malignant melanoma. BMJ Case Rep. 2013;2013:bcr2013008566. doi:10.1136/bcr-2013-008566
- Srivastava R, John JJ, Reilly C, et al. Sniffing out malignant melanoma: a case of canine olfactory detection. Cutis. 2019;104:E4-E6.
- Fleck CA. Fighting odor in wounds. Adv Skin Wound Care. 2006;19:242-244.
- Gallagher M, Wysocki CJ, Leyden JJ, et al. Analyses of volatile organic compounds from human skin. Br J Dermatol. 2008;159:780-791.
- Campo E, Ferreira V, Escudero A, et al. Quantitative gas chromatography–olfactometry and chemical quantitative study of the aroma of four Madeira wines. Anal Chim Acta. 2006;563:180-187.
- Wagenstaller M, Buettner A. Characterization of odorants in human urine using a combined chemo-analytical and human-sensory approach: a potential diagnostic strategy. Metabolomics. 2012;9:9-20.
- Arseculeratne G, Wong AKC, Goudie DR, et al. Trimethylaminuria (fish-odor syndrome): a case report. Arch Dermatol. 2007;143:81-84.
- Mathews D, Perera LP, Irion LD, et al. Darier disease: beware the cyst that smells. Ophthal Plast Reconstr Surg. 2010;26:206-207.
Sniffing Out Skin Disease: Odors in Dermatologic Conditions
Sniffing Out Skin Disease: Odors in Dermatologic Conditions
PRACTICE POINTS
- Olfaction may be underutilized in making dermatologic diagnoses. Clinicians should include smell in their physical examination, as characteristic odors are associated with infectious disorders, disorders of keratinization and acantholysis, and metabolic disorders.
- Recognizing distinctive smells can help narrow the differential diagnosis and prompt targeted testing in dermatology.
- Canines and electronic noses have demonstrated the potential to detect certain malignancies, including melanoma, based on unique volatile organic compound profiles.
Fluoroscopy-Induced Chronic Radiation Dermatitis: A Comprehensive Review and Reappraisal
Fluoroscopy-Induced Chronic Radiation Dermatitis: A Comprehensive Review and Reappraisal
Fluoroscopy is an imaging technique that allows for real-time visualization of internal structures in the body using continuous radiography beams. More than 1 million fluoroscopy-guided procedures are performed annually in the United States.1 Utilization of these procedures continues to increase, and so does the probability of related complications, as prolonged exposure to ionizing radiation can cause skin injuries.2 Fortunately, the incidence of radiation-induced skin injuries compared with the total number of fluoroscopic procedures performed remains small,2 although one study suggested the incidence may be as high as 8.9% in at-risk populations.3
Radiation dermatitis is well recognized in dermatology as a complication of oncologic management; however, radiation dermatitis as a complication of fluoroscopic procedures is underrecognized.4 Fluoroscopy-induced radiation dermatitis can be categorized as acute, subacute, or chronic.5 Common fluoroscopic procedures that have been associated with fluoroscopy-induced radiation dermatitis include interventional cardiac procedures, neurovascular procedures, transjugular intrahepatic portosystemic shunt procedures, and endovascular abdominal aortic aneurysm repairs.6,7
Patients with fluoroscopy-induced radiation dermatitis, particularly fluoroscopy-induced chronic radiation dermatitis (FICRD), can present to dermatology up to several years after the initial fluoroscopy procedure with no awareness of the association between the procedure and their skin findings. This presents a diagnostic challenge, and FICRD often is overlooked.5,8-10
We conducted a literature search of PubMed articles indexed for MEDLINE using the search terms fluoroscopy and dermatitis. In this reappraisal, we will provide a comprehensive overview of fluoroscopy-induced radiation dermatitis with an emphasis on FICRD, covering its clinical manifestations, pathophysiology, risk factors, differential diagnosis, histology, and management. The aim of this review is to highlight the salient features and mimickers of FICRD and inform readers how to approach suspected cases, leading to accurate diagnosis and effective management.
Pathophysiology
Fluoroscopy-induced radiation dermatitis is the result of dose-dependent radiation-induced tissue damage. As the peak skin dosage (PSD) of radiation increases over the course of a procedure or multiple procedures, the severity of skin injury predictably increases. During fluoroscopic procedures, the standard irradiation dosage ranges from 0.02 Gy/min to 0.05 Gy/min.11 Transient skin changes may start to be seen around 2 Gy of cumulative exposure. Fluoroscopic procedures typically range in duration from 60 to 120 minutes; however, complex cases may exceed that. Additionally, multiple procedures performed within shorter intervals can result in greater PSD accumulation. Shorter intervals between procedures do not allow enough time for damage repair from the previous procedure and can result in further severe damage when the skin is re-exposed to radiation.2 The American College of Radiology recommends medical follow-up after 10 Gy of cumulative exposure, while cumulative exposure above 15 Gy within a 6- to 12-month period is defined as a sentinel event, according to The Joint Commission.12-14
Depending on the patient’s total radiation dosage during one or more procedures, the result of the tissue damage manifests differently at varying times: early skin changes are categorized as fluoroscopy-induced acute radiation dermatitis, and late skin changes are categorized as FICRD (Table 1).
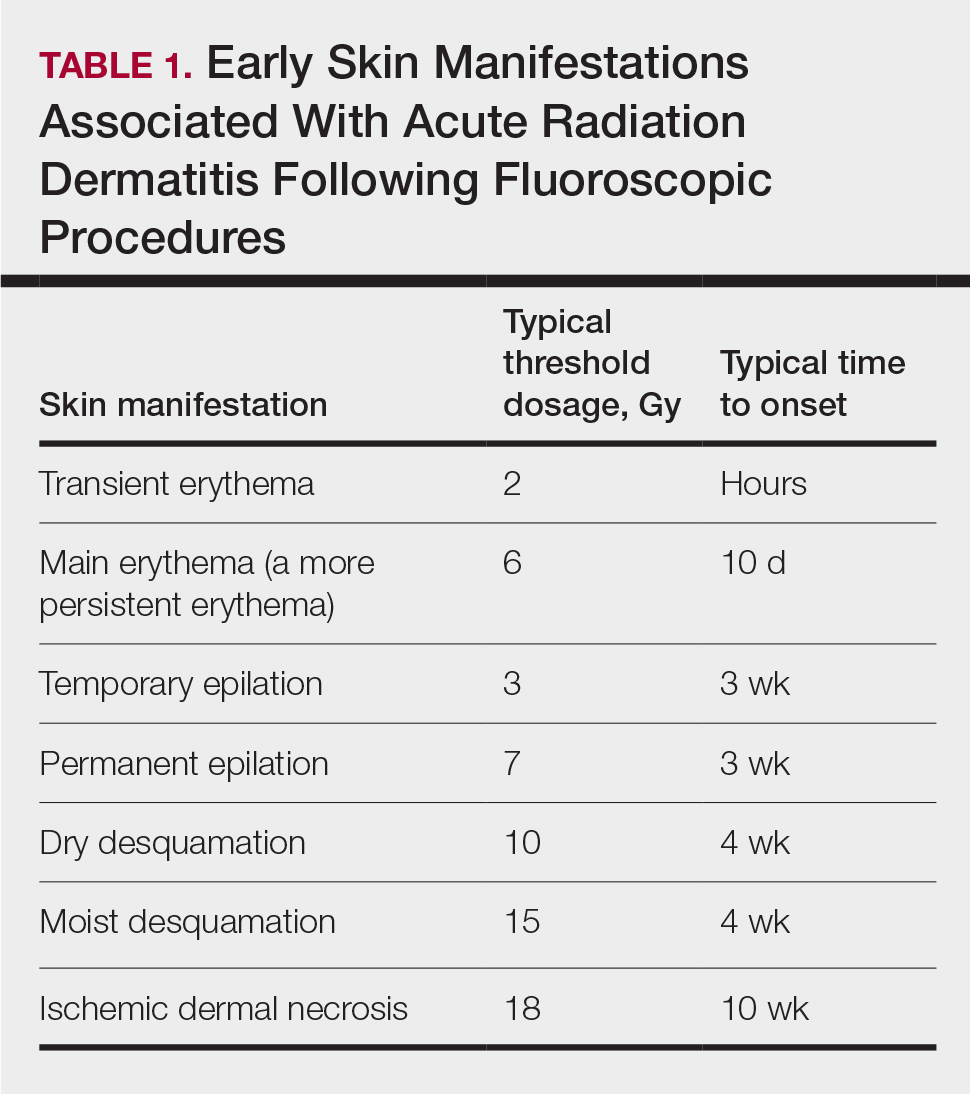
Clinical Manifestations
Acute radiation dermatitis from fluoroscopic procedures manifests within hours to days up to 90 days following radiation exposure and can be characterized by erythema with blistering, desquamation, epilation, pigmentation changes, and even necrosis if the accumulated dosage exceeds 15 Gy.15 Chronic radiation dermatitis (which as related to fluoroscopic procedures is termed FICRD) has a longer onset of weeks to years and is clinically characterized by telangiectasias, permanent erythema, dermal atrophy, or ulcerations. Clinically, subacute radiation dermatitis shares features of both acute and chronic radiation dermatitis; therefore, it is differentiated based on its histologic features.5,16
Although fluoroscopy-induced acute radiation dermatitis (Table 1) may precede FICRD, acute manifestations of fluoroscopy-related dermatitis can be subtle and often manifest in areas not easily visualized. Because referrals to dermatologists for full-skin examinations after fluoroscopy procedures are not standard, patients may not be aware of the association between these procedures and the development of skin lesions. Nonetheless, some patients may report a history of skin changes such as redness days or weeks after a fluoroscopic procedure with accompanying pain and pruritus limited to the fluoroscopy-exposed region, which tend to self-resolve.17 The risk for FICRD is thought to increase if a history of fluoroscopy-induced acute radiation dermatitis is present.18
The location of the skin findings correlates to the area exposed to prolonged radiation during the procedure(s). The most common areas include the scapular and subscapular regions, the right lateral trunk inferior to the axilla, the mid back, and the right anterolateral chest.16,19,20 These regions are associated with more complex (eg, cardiac) procedures that have been reported to lead to prolonged radiation exposure. The skin findings in FICRD are described as geometric, corresponding to the squarish or rectangular radiography beam that is directed at the patient. Additionally, radiography beams spread outward as they travel in space; therefore, skin injuries are common at the region more distal to the path of origination of the beam.21-23 Subsequently, a geometric, dyspigmented, indurated or atrophic plaque with telangiectasias and erosions or ulcerations with progressive worsening is a common manifestation of FICRD.5,16,23 Patients also commonly present with pruritus or severe pain associated with the lesion.24,25
Dermatologic Manifestations of FICRD
Skin responses seen weeks to years after a fluoroscopic procedure and typically after cumulative radiation exposure of 10 Gy or greater are categorized as FICRD (Table 2). These changes also can be clinically graded based on the Radiation Therapy Oncology Group classification of radiation dermatitis (Tables 3 and 4).26 Chronic changes in the skin largely result from remodeling of the vasculature and the subcutaneous tissue over time. Unlike acute changes, chronic changes typically persist and continue to worsen.27

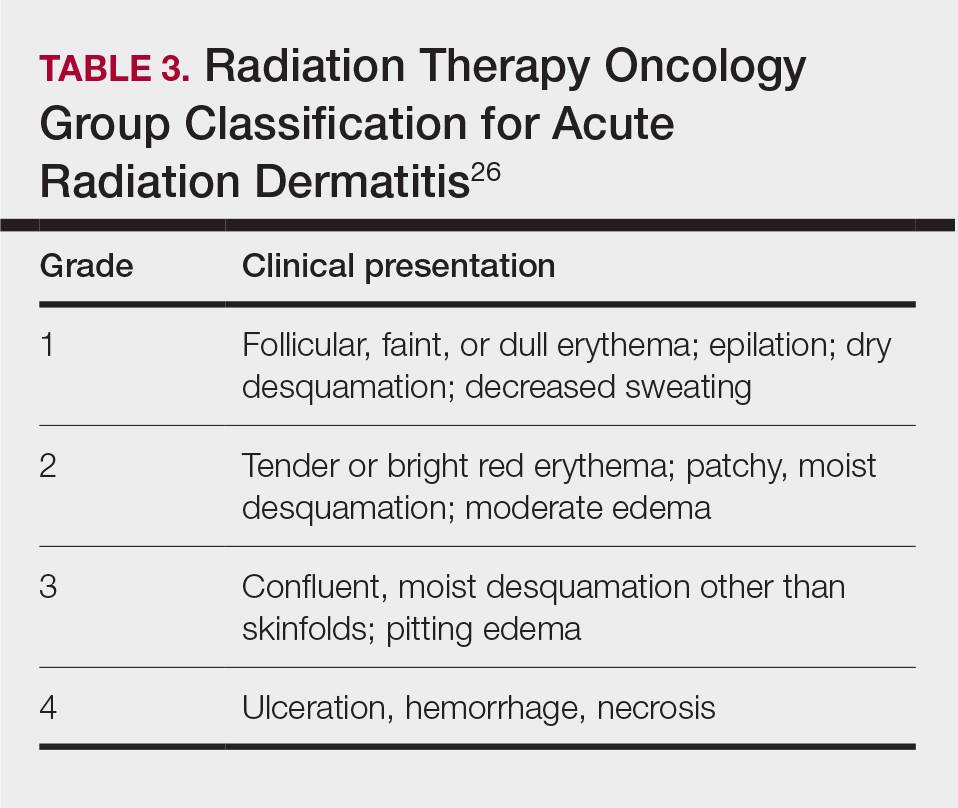
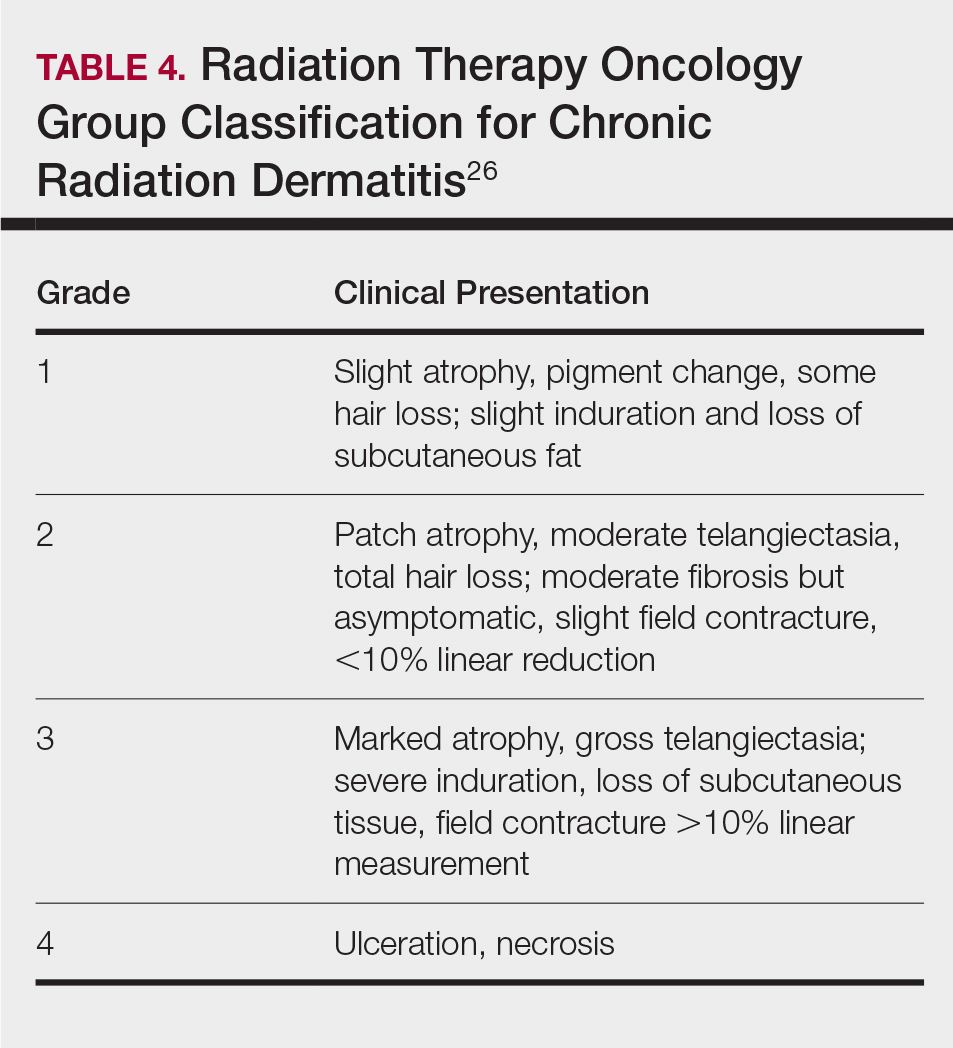
Telangiectasias—Anywhere from months to 1 year after exposure to 10 Gy of radiation, proliferation of atypical superficial vessels in the dermis can be seen, typically manifesting as telangiectasias on physical examination. Telangiectasias can increase with time and can even exhibit a dose-dependent relationship to the radiation exposure.28
Atrophy—Atrophic-appearing skin after radiation exposure is the result of direct injury to both the epidermis and fibroblasts in the dermis. The destruction of keratinocytes leads to a thin epidermis, and destruction of dermal fibroblasts causes insufficient collagen production.29 Clinically, this process manifests as an atrophic plaque that can be seen 12 weeks to 1 year after the procedure.
Fibrosis—Approximately 1 year after the exposure, the initial damage can lead to disruption of molecular pathways, causing fibrosis. Transforming growth factor (TGF) β1 is the main factor involved.29 Damage to the endothelial cells results in increased TGF-β1 levels, which causes increased stimulation of remaining atypical fibroblasts and thus increased irregular collagen deposition.30 Further adding to this knowledge, Wei et al31 recently proposed that damage to the epidermal keratinocytes leads to disruption of yes-associated protein 1, which is a protective factor released from keratinocytes that regulates the dermal fibroblasts. However, extensive damage to the keratinocytes can lead to lower yes-associated protein 1 levels and its downstream activity, leading to increased levels of TGF-β1 and fibroblast activity.31 Clinically, this fibrotic stage is seen as indurated plaques in patients.
Necrosis—There are 2 forms of necrosis that can be seen. Ischemic dermal necrosis typically occurs in the acute phase after 10 weeks and approximately 18 Gy of cumulative exposure. It results from substantial skin damage, including microvascular damage and reduction in dermal capillaries, leading to ischemia of the tissue.2 Late dermal necrosis is the process seen in the chronic stage of FICRD and radiation dermatitis not related to fluoroscopy. It results from the inability of the fibrotic dermis to vascularly support the epidermis above it.2 It can be seen anywhere from 1 to 4 years after the procedure. This stage clinically manifests as worsening ulcerations with major pain and increased risk for secondary infections.16
Dyspigmentation—Dyspigmentation at the site of the radiation exposure can be seen acutely and chronically. Dosage above 15 to 18 Gy can lead to destruction of melanocytes, which can cause hypopigmentation in exposed areas. However, melanocytes are relatively resistant to radiation; therefore, dosages below the threshold of destruction of 15 to 18 Gy can cause melanocytic hyperactivity leading to hyperpigmentation.32 Hence, pigmentary changes can vary greatly. Classically, a central area of hypopigmentation with surrounding hyperpigmentation is seen.
Histology
Histologic appearance of radiation dermatitis varies depending on its stage. Acute radiation dermatitis primarily demonstrates superficial dermal edema, damage to the basal cell layer, small vessel dilation with thrombi, and hemorrhage along with a sparse inflammatory cell infiltrate.33 Histology typically is the only way to characterize subacute radiation dermatitis.5 Lichenoid tissue reaction is its characteristic feature. Mononuclear cells are found adjected to necrotic keratinocytes along with prominent vacuolization of the basal cell layer.33
The key histologic features of chronic radiation dermatitis include epidermal atrophy, hyperkeratosis, telangiectasias, loss of adnexal structures, and dermal fibrosis along with sparse atypical stellate fibroblasts.34 However, clinical context of fluoroscopic exposure is required for the dermatopathologist to differentiate chronic radiation dermatitis from its histologic differential of morphea and lichen sclerosus. In a cross-sectional study, only 1 of 6 cases (16.7%) was correctly diagnosed as chronic radiation dermatitis in the absence of correlating clinical history.35
Risk Factors for FICRD
Since the diagnosis of FICRD can be a clinical challenge, understanding the risk factors can be helpful. The general likelihood of developing FICRD is related to the duration, frequency, interval, intensity, and area of radiation exposure. Procedures exceeding the normal duration of 60 to 120 minutes have been well documented as a substantial risk factor for radiation dermatitis and FICRD.36-38 The risk tends to be higher in longer procedures because they result in more radiation exposure and higher accumulated PSD. Obesity (ie, body mass index >26) is the major risk factor that has been associated with longer procedure times, as higher radiation dosages are necessary to penetrate the body of a larger patient and a larger skin surface area is exposed.37-39
Other risk factors associated with FICRD relate to how prone a patient is to radiation-induced DNA damage. Older patients are at higher risk due to lower intrinsic ability of the tissue to repair itself.11 Patients with a history of connective tissue diseases—particularly lupus, scleroderma, and mixed connective tissue disease—are at an increased risk.40 Furthermore, patients with genetic disorders that impair DNA repair are more susceptible to radiation-induced DNA damage; therefore, patients with ataxia-telangiectasia, xeroderma pigmentosum, Fanconi anemia, and hereditary nevoid basal cell carcinoma are at higher risk for FICRD.39 Similarly, medications that can affect DNA repair also have been shown to be risk factors. These medications include chemotherapeutic agents such as actinomycin D, cyclophosphamide, doxorubicin, methotrexate, and 5-fluorouracil.2,39 Diabetes, hyperthyroidism, and tobacco use also have been shown to increase a patient’s risk for FICRD.39 It also is reasonable to believe that patients with defects in fibroblasts or with elastin or collagen disorders (eg, Ehlers-Danlos syndrome) would be at higher risk, but there are no known studies highlighting the association in the literature.
Differential Diagnosis of FICRD
Acute allergic or irritant contact dermatitis manifests with a localized area of erythematous skin accompanied by pruritus.41 Patients with FICRD can present with a localized area of erythema and hyperpigmentation with minimal atrophy. The lesion may accompany substantial pruritus, which can favor the more common diagnosis of contact dermatitis.35,42,43
Fixed-drug eruption manifests as a well-defined, hyperpigmented plaque in a fixed location that occurs upon ingestion of a drug.44 Fluoroscopy-induced chronic radiation dermatitis lesions are well demarcated and geometrically shaped and therefore can mimic lesions seen in fixed-drug eruptions.45 Additionally, the patient population undergoing fluoroscopic procedures tends to have major comorbidities requiring multiple medications.4
Decubitus ulcers are a result of vascular compromise to an area of skin due to constant pressure and are most commonly seen in the sacral region of patients with obesity.46 Ulcerated FICRD lesions can manifest on the lower midback. These lesions can be seen after endovascular repair of abdominal aortic aneurysm or prostatic artery embolization.20,21 The location of these lesions can mimic decubitus ulcers if fluoroscopic history is unknown. As mentioned, obesity also increases the risk for FICRD.
Morphea can manifest as a localized area of induration and hyperpigmentation of the skin.47 When FICRD has progressed to dermal fibrosis, patients can present with indurated plaques without ulcerations, which can be hard to differentiate from morphea.16,48 However, the presence of ulcerations or hyperkeratosis can differentiate morphea from FICRD.16
Ultimately, it is the location of FICRD lesions that remains the biggest diagnostic clue. Any suspicious lesion present on the scapular or subscapular areas, anterolateral chest, and/or mid back should prompt an investigation into recent or remote history of fluoroscopic procedures.
Management of FICRD
Diagnosis of FICRD should be made clinically based on the history and physical examination whenever possible, since a biopsy is not recommended.35 Wound healing in FICRD is delayed, and biopsies can lead to ulcerations or secondary infections.17 Therefore, it is important to remain suspicious for FICRD. Management of FICRD should correspond to the clinical findings outlined by a recent Delphi consensus survey.49 Regardless, the core of FICRD management framework should always include good hygiene, maintenance of skin hydration to improve epithelialization, and sufficient photoprotection.49,50
Among the first signs of FICRD are telangiectasias. Although asymptomatic, their appearance can be distressing for patients. Pulsed dye laser therapy is a first-line option that has been studied and has shown clinical efficacy for treatment of telangiectasias and vascular changes in patients with FICRD.49,51
If patients develop fibrotic changes, treatment options are limited. Fibrosis is hard to reverse, and the management approach is limited to symptomatic relief. Mechanical and deep-friction massages have been shown to be effective at reducing skin induration in patients.52 Fractional ablative lasers also may be utilized for skin contractures, especially if range of motion is affected.53,54 Although it comes with its own challenges, autologous fat grafting has shown promise in reducing postradiation fibrosis and inducing angiogenesis in tissue.55 Oral pentoxifylline also has shown mild efficacy, as it may be able to suppress TGF-β1 levels.53 However, prevention of fibrotic changes may be the most important. Wei et al31 suggested that low-dose oral prednisolone at 5 mg twice daily for 3 weeks might be an option to prevent the progression of skin changes and even reverse fibrosis to an extent; however, further evidence regarding its efficacy still is necessary. Additionally, no evidence was identified to support the use of topical corticosteroids for fibrotic changes seen in FICRD.56
Patients with FICRD or even acute radiation dermatitis after fluoroscopy tend to develop superficial ulcerations from minor traumas. Good wound hygiene, antiseptic care, and absorbent dressings, such as hydrogel and hydrocolloid, may be sufficient for treating these wounds, as seen in the Figure.42,48 However, once patients develop refractory ulcerations or necrosis, treatment options are then limited to surgical removal with a flap or graft.5,33,42,45
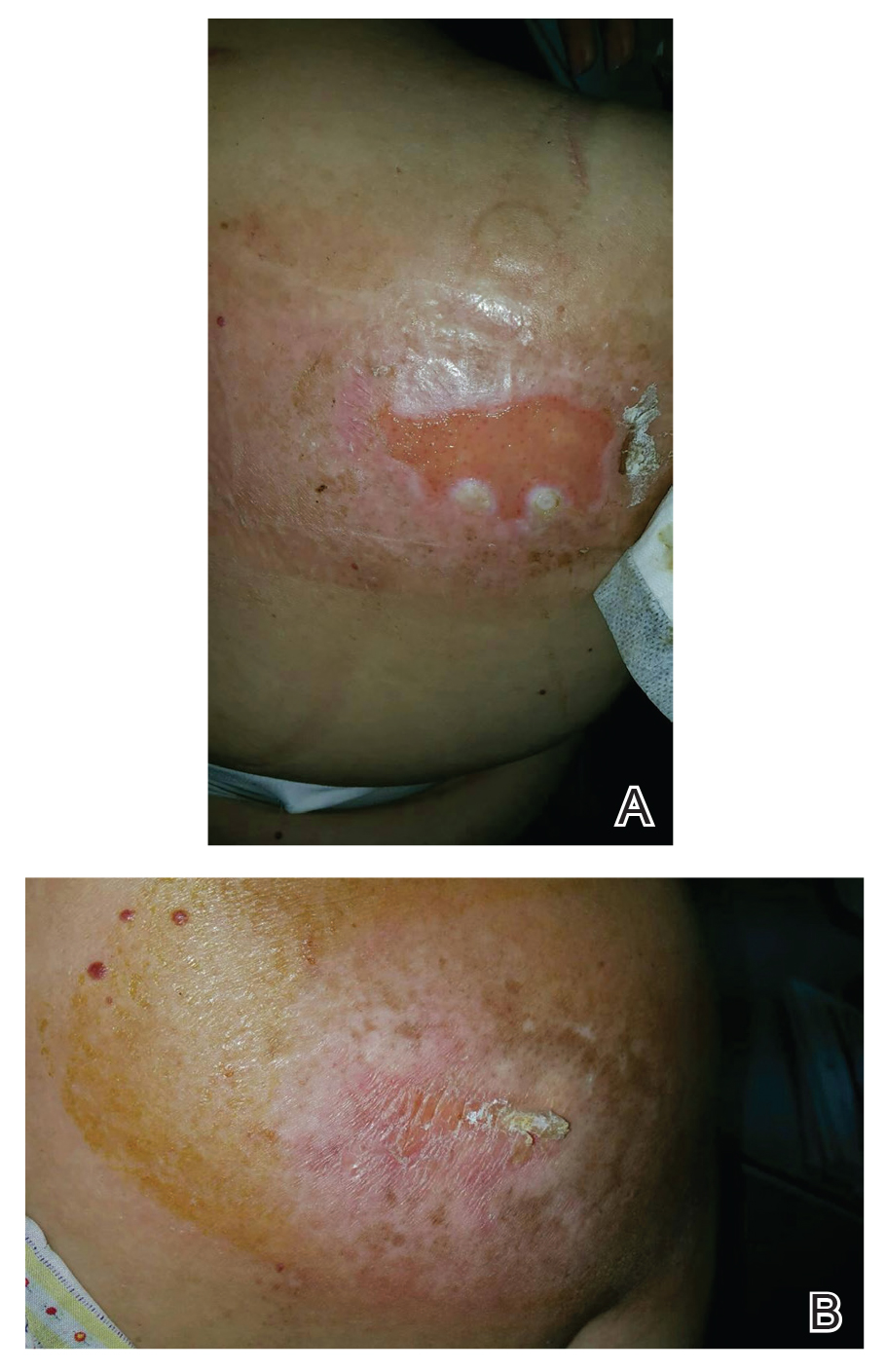
Risk for basal cell carcinomas and squamous cell carcinomas is higher in patients with radiation exposure; however, the exact risk from fluoroscopic procedures is unknown. One study demonstrated an increased risk of 6.9% in development of skin cancer after a median radiation exposure of 15.5 Gy and a mean latency period of 38.3 years,57 and in another retrospective study, the risk was higher in Fitzpatrick skin types I and II.58 Unlike the development of radiodermatitis itself, which shows a dose-dependent response, development of skin cancers follows a stochastic pattern (not dose dependent).59 Therefore, it is important to identify these high-risk patients and establish follow-up.
Conclusion
Fluoroscopy-induced chronic radiation dermatitis can be a diagnostic challenge, as skin changes may not be readily associated with the procedure by patients. Therefore, any lesion with a geometric shape and accompanying chronic radiation dermatitis features located on the scapular or subscapular areas, anterolateral chest, and midback should prompt an investigation into history of fluoroscopic procedures. Treatment of chronic skin changes in FICRD depends on the clinical manifestations. Good hygiene, skin hydration, and sufficient photoprotection are crucial. Finally, long-term monitoring with skin examinations is important to assess for the development of skin cancers in the treated area.
- Benjamin EJ, Muntner P, Alonso A, et al. Heart Disease and Stroke Statistics-2019 Update: a report from the American Heart Association. Circulation. 2019;139:E56-E528. doi:10.1161/CIR.0000000000000659. Published correction appears in Circulation. 2020;141:E33.
- Koenig TR, Wolff D, Mettler FA, et al. Skin injuries from fluoroscopically guided procedures: part 1, characteristics of radiation injury. AJR Am J Roentgenol. 2001;177:3-11. doi:10.2214/ajr.177.1.1770003
- Guesnier-Dopagne M, Boyer L, Pereira B, et al. Incidence of chronic radiodermatitis after fluoroscopically guided interventions: a retrospective study. J Vasc Interv Radiol. 2019;30:692-698.e13. doi:10.1016/j.jvir.2019.01.010
- Cunha N, Cardoso P, Cabete J. Subacute radiation dermatitis following an interventional cardiology procedure. Cutan Ocul Toxicol. 2017;36:297-299. doi:10.1080/15569527.2016.1254649
- Frazier TH, Richardson JB, Fabré VC, et al. Fluoroscopy-induced chronic radiation skin injury: a disease perhaps often overlooked. Arch Dermatol. 2007;143:637-640. doi:10.1001/archderm.143.5.637
- Koenig TR, Mettler FA, Wagner LK. Skin injuries from fluoroscopically guided procedures: part 2, review of 73 cases and recommendations for minimizing dose delivered to patient. AJR Am J Roentgenol. 2001;177:13-20. doi:10.2214/ajr.177.1.1770013
- Shope TB. Radiation-induced skin injuries from fluoroscopy. Radiographics. 1996;16:1195-1199. doi:10.1148/radiographics.16.5.8888398
- Tchanque-Fossuo CN, Isseroff RR, Silverstein MA. Fluoroscopy induced chronic radiation dermatitis should be included in the differential diagnosis of notalgia paresthetica. Dermatol Online J. 2016;22:13030/qt0kh726m9.
- Berlin L. Radiation-induced skin injuries and fluoroscopy. AJR Am J Roentgenol. 2001;177:21-25. doi:10.2214/ajr.177.1.1770021
- Tchanque-Fossuo CN, Kamangar F, Ho B, et al. Fluoroscopy-induced radionecrosis. Dermatol Online J. 2016;22:13030/qt68w910t2.
- Wagner LK, Eifel PJ, Geise RA. Potential biological effects following high X-ray dose interventional procedures. J Vasc Interv Radiol. 1994;5:71-84. doi:10.1016/s1051-0443(94)71456-1
- Balter S, Hopewell JW, Miller DL, et al. Fluoroscopically guided interventional procedures: a review of radiation effects on patients’ skin and hair. Radiology. 2010;254:326-341. doi:10.1148/radiol.2542082312
- Vance AZ, Weinberg BD, Arbique GM, et al. Fluoroscopic sentinel events in neuroendovascular procedures: how to screen, prevent, and address occurrence. AJNR Am J Neuroradiol. 2013;34:1513-1515. doi:10.3174/ajnr.A3185
- Aerts A, Decraene T, van den Oord JJ, et al. Chronic radiodermatitis following percutaneous coronary interventions: a report of two cases. J Eur Acad Dermatol Venereol. 2003;17:340-343. doi:10.1046/j.1468-3083.2003.00687.x
- Rosenthal A, Israilevich R, Moy R. Management of acute radiation dermatitis: a review of the literature and proposal for treatment algorithm. J Am Acad Dermatol. 2019;81:558-567. doi:10.1016/j.jaad.2019.02.047
- Boncher J, Bergfeld WF. Fluoroscopy-induced chronic radiation dermatitis: a report of two additional cases and a brief review of the literature. J Cutan Pathol. 2012;39:63-67. doi:10.1111/j.1600-0560.2011.01754.x
- Spiker A, Zinn Z, Carter WH, et al. Fluoroscopy-induced chronic radiation dermatitis. Am J Cardiol. 2012;110:1861-1863. doi:10.1016/j.amjcard.2012.08.023
- Batrani M, Kubba A, Sundharam J. Fluoroscopy-induced chronic radiation dermatitis masquerading as morphea: a diagnostic pitfall. Indian J Pathol Microbiol. 2018;61:393-396. doi:10.4103/IJPM.IJPM_566_17
- Jeskowiak A, Hubmer M, Prenner G, et al. Radiation induced cutaneous ulcer on the back in a patient with congenital anomaly of the upper cava system. Interact Cardiovasc Thorac Surg. 2011;12:290-292.
- Laborda A, De Assis AM, Ioakeim I, et al. Radiodermitis after prostatic artery embolization: case report and review of the literature. Cardiovasc Intervent Radiol. 2015;38:755-759. doi:10.1007/s00270-015-1083-6
- Lyons AB, Harvey VM, Gusev J. Fluoroscopy-induced chronic radiation dermatitis (FICRD) after endovascular abdominal aortic aneurysm endoleak repair. JAAD Case Rep. 2015;1:403-405. doi:10.1016/j.jdcr.2015.09.022
- Mossman KL. Analysis of risk in computerized tomography and other diagnostic radiology procedures. Comput Radiol. 1982;6:251-256. doi:10.1016/0730-4862(82)90109-3
- Henry MF, Maender JL, Shen Y, et al. Fluoroscopy-induced chronic radiation dermatitis: a report of three cases. Dermatol Online J. 2009;15:3.
- Balter S, Miller DL. Patient skin reactions from interventional fluoroscopy procedures. AJR Am J Roentgenol. 2014;202:W335-W342. doi:10.2214/AJR.13.12029
- Nishimoto S, Fukuda K, Kawai K, et al. Supplementation of bone marrow aspirate-derived platelet-rich plasma for treating radiation-induced ulcer after cardiac fluoroscopic procedures: a preliminary report. Indian J Plast Surg. 2012;45:109-114. doi:10.4103/0970-0358.96599
- Cox JD, Stetz J, Pajak TF. Toxicity criteria of the Radiation Therapy Oncology Group (RTOG) and the European Organization for Research and Treatment of Cancer (EORTC). Int J Radiat Oncol Biol Phys. 1995;31:1341-1346. doi:10.1016/0360-3016(95)00060-C
- Wong RK, Bensadoun RJ, Boers-Doets CB, et al. Clinical practice guidelines for the prevention and treatment of acute and late radiation reactions from the MASCC Skin Toxicity Study Group. Support Care Cancer. 2013;21:2933-2948. doi:10.1007/s00520-013-1896-2
- Turesson I, Notter G. The predictive value of skin telangiectasia for late radiation effects in different normal tissues. Int J Radiat Oncol Biol Phys. 1986;12:603-609. doi:10.1016/0360-3016(86)90069-6
- Hegedus F, Mathew LM, Schwartz RA. Radiation dermatitis: an overview. Int J Dermatol. 2017;56:909-914. doi:10.1111/ijd.13371
- Denham JW, Hauer-Jensen M. The radiotherapeutic injury—a complex ‘wound.’ Radiother Oncol. 2002;63:129-145. doi:10.1016/s0167-8140(02)00060-9
- Wei KC, Lai SF, Huang WL, et al. An innovative targeted therapy for fluoroscopy-induced chronic radiation dermatitis. J Mol Med (Berl). 2022;100:135-146. doi:10.1007/s00109-021-02146-3
- Sitton E. Early and late radiation-induced skin alterations. part I: mechanisms of skin changes. Oncol Nurs Forum. 1992;19:801-807.
- Pruitt LG, Rogers W, Byarlay JA, et al. Subacute radiation dermatitis after fluoroscopy. J Cutan Pathol. 2016;43:1091-1095. doi:10.1111/cup.12815
- Anderson EB, Draft KS, Lee RA, et al. Update in dermatopathology. Am J Clin Pathol. 2006;125(Suppl):S50-S70. doi:10.1309/GMUFNP6LFMPNR86R
- Wei KC, Yang KC, Mar GY, et al. STROBE—radiation ulcer: an overlooked complication of fluoroscopic intervention: a cross-sectional study. Medicine (Baltimore). 2015;94:e2178. doi:10.1097/MD.0000000000002178
- Otterburn D, Losken A. Iatrogenic fluoroscopy injury to the skin. Ann Plast Surg. 2010;65:462-465. doi:10.1097/SAP.0b013e3181d6e2d3
- Cha MJ, Jo SJ, Cho Y, et al. Patient characteristics and the incidence of radiation-induced dermatitis following radiofrequency catheter ablation. Korean Circ J. 2016;46:646-653. doi:10.4070/kcj.2016.46.5.646
- Dehen L, Vilmer C, Humilière C, et al. Chronic radiodermatitis following cardiac catheterisation: a report of two cases and a brief review of the literature. Heart. 1999;81:308-312. doi:10.1136/hrt.81.3.308
- Brown KR, Rzucidlo E. Acute and chronic radiation injury. J Vasc Surg. 2011;53(Suppl 1):15S-21S. doi:10.1016/j.jvs.2010.06.175. Published correction appears in J Vasc Surg. 2012;55:627.
- Hymes SR, Strom EA, Fife C. Radiation dermatitis: clinical presentation, pathophysiology, and treatment 2006. J Am Acad Dermatol. 2006;54:28-46. doi:10.1016/j.jaad.2005.08.054
- Scheinman PL, Vocanson M, Thyssen JP, et al. Contact dermatitis. Nat Rev Dis Primers. 2021;7:38. doi:10.1038/s41572-021-00271-4
- Cheng TT, Yang HJ. Chronic radiation dermatitis induced by cardiac catheterization: a case report and literature review. Acta Dermatovenerol Alp Pannonica Adriat. 2022;31:147-149.
- Minni JP, Nowak M, Usmani A, et al. A unique case of subacute radiodermatitis. Cutis. 2013;91:230-232.
- Flowers H, Brodell R, Brents M, et al. Fixed drug eruptions: presentation, diagnosis, and management. South Med J. 2014;107:724-727. doi:10.14423/SMJ.0000000000000195
- Hashimoto I, Sedo H, Inatsugi K, et al. Severe radiation-induced injury after cardiac catheter ablation: a case requiring free anterolateral thigh flap and vastus lateralis muscle flap reconstruction on the upper arm. J Plast Reconstr Aesthet Surg. 2008;61:704-708. doi:10.1016/j.bjps.2007.01.003
- Mervis JS, Phillips TJ. Pressure ulcers: pathophysiology, epidemiology, risk factors, and presentation. J Am Acad Dermatol. 2019;81:881-890. doi:10.1016/j.jaad.2018.12.069
- Careta MF, Romiti R. Localized scleroderma: clinical spectrum and therapeutic update. An Bras Dermatol. 2015;90:62-73. doi:10.1590/abd1806-4841.20152890
- Herz-Ruelas ME, Gómez-Flores M, Moxica-Del Angel J, et al. Ulcerated radiodermatitis induced after fluoroscopically guided stent implantation angioplasty. Case Rep Dermatol Med. 2014;2014:768624. doi:10.1155/2014/768624
- Wilson BN, Shah R, Menzer C, et al. Consensus on the clinical management of chronic radiation dermatitis and radiation fibrosis: a Delphi survey. Br J Dermatol. 2022;187:1054-1056. doi:10.1111/bjd.21852
- Khanna NR, Kumar DP, Laskar SG, et al. Radiation dermatitis: an overview. Indian J Burns. 2013;21:24-31. doi:10.4103/0971-653x.121877
- Spalek M. Chronic radiation-induced dermatitis: challenges and solutions. Clin Cosmet Investig Dermatol. 2016;9:473-482. doi:10.2147/CCID.S94320
- Bourgeois JF, Gourgou S, Kramar A, et al. A randomized, prospective study using the LPG technique in treating radiation-induced skin fibrosis: clinical and profilometric analysis. Skin Res Technol. 2008;14:71-76. doi:10.1111/j.1600-0846.2007.00263.x
- Borrelli MR, Shen AH, Lee GK, et al. Radiation-induced skinfibrosis: pathogenesis, current treatment options, and emerging therapeutics. Ann Plast Surg. 2019;83(4S Suppl 1):S59-S64. doi:10.1097/SAP.0000000000002098
- Wilson B, Shah R, Menzer C, et al. Laser therapy as a treatment for chronic radiation fibrosis. Lasers Surg Med. 2023;55:82-88. doi:10.1002/lsm.23617
- Rigotti G, Marchi A, Galiè M, et al. Clinical treatment of radiotherapy tissue damage by lipoaspirate transplant: a healing process mediated by adipose-derived adult stem cells. Plast Reconstr Surg. 2007;119:1409-1422. doi:10.1097/01.prs.0000256047.47909.71
- Leventhal J, Young MR. Radiation dermatitis: recognition, prevention, and management. Oncology (Williston Park). 2017;31:885-899.
- van Vloten WA, Hermans J, van Daal WA. Radiation-induced skin cancer and radiodermatitis of the head and neck. Cancer. 1987;59:411-414. doi:10.1002/1097-0142(19870201)59:3<411::aid-cncr2820590310>3.0.co;2-z
- Davis MM, Hanke CW, Zollinger TW, et al. Skin cancer in patients with chronic radiation dermatitis. J Am Acad Dermatol. 1989;20:608-616. doi:10.1016/s0190-9622(89)70072-4
- Miller DL, Balter S, Schueler BA, et al. Clinical radiation management for fluoroscopically guided interventional procedures. Radiology. 2010;257:321-332. doi:10.1148/radiol.10091269
Fluoroscopy is an imaging technique that allows for real-time visualization of internal structures in the body using continuous radiography beams. More than 1 million fluoroscopy-guided procedures are performed annually in the United States.1 Utilization of these procedures continues to increase, and so does the probability of related complications, as prolonged exposure to ionizing radiation can cause skin injuries.2 Fortunately, the incidence of radiation-induced skin injuries compared with the total number of fluoroscopic procedures performed remains small,2 although one study suggested the incidence may be as high as 8.9% in at-risk populations.3
Radiation dermatitis is well recognized in dermatology as a complication of oncologic management; however, radiation dermatitis as a complication of fluoroscopic procedures is underrecognized.4 Fluoroscopy-induced radiation dermatitis can be categorized as acute, subacute, or chronic.5 Common fluoroscopic procedures that have been associated with fluoroscopy-induced radiation dermatitis include interventional cardiac procedures, neurovascular procedures, transjugular intrahepatic portosystemic shunt procedures, and endovascular abdominal aortic aneurysm repairs.6,7
Patients with fluoroscopy-induced radiation dermatitis, particularly fluoroscopy-induced chronic radiation dermatitis (FICRD), can present to dermatology up to several years after the initial fluoroscopy procedure with no awareness of the association between the procedure and their skin findings. This presents a diagnostic challenge, and FICRD often is overlooked.5,8-10
We conducted a literature search of PubMed articles indexed for MEDLINE using the search terms fluoroscopy and dermatitis. In this reappraisal, we will provide a comprehensive overview of fluoroscopy-induced radiation dermatitis with an emphasis on FICRD, covering its clinical manifestations, pathophysiology, risk factors, differential diagnosis, histology, and management. The aim of this review is to highlight the salient features and mimickers of FICRD and inform readers how to approach suspected cases, leading to accurate diagnosis and effective management.
Pathophysiology
Fluoroscopy-induced radiation dermatitis is the result of dose-dependent radiation-induced tissue damage. As the peak skin dosage (PSD) of radiation increases over the course of a procedure or multiple procedures, the severity of skin injury predictably increases. During fluoroscopic procedures, the standard irradiation dosage ranges from 0.02 Gy/min to 0.05 Gy/min.11 Transient skin changes may start to be seen around 2 Gy of cumulative exposure. Fluoroscopic procedures typically range in duration from 60 to 120 minutes; however, complex cases may exceed that. Additionally, multiple procedures performed within shorter intervals can result in greater PSD accumulation. Shorter intervals between procedures do not allow enough time for damage repair from the previous procedure and can result in further severe damage when the skin is re-exposed to radiation.2 The American College of Radiology recommends medical follow-up after 10 Gy of cumulative exposure, while cumulative exposure above 15 Gy within a 6- to 12-month period is defined as a sentinel event, according to The Joint Commission.12-14
Depending on the patient’s total radiation dosage during one or more procedures, the result of the tissue damage manifests differently at varying times: early skin changes are categorized as fluoroscopy-induced acute radiation dermatitis, and late skin changes are categorized as FICRD (Table 1).

Clinical Manifestations
Acute radiation dermatitis from fluoroscopic procedures manifests within hours to days up to 90 days following radiation exposure and can be characterized by erythema with blistering, desquamation, epilation, pigmentation changes, and even necrosis if the accumulated dosage exceeds 15 Gy.15 Chronic radiation dermatitis (which as related to fluoroscopic procedures is termed FICRD) has a longer onset of weeks to years and is clinically characterized by telangiectasias, permanent erythema, dermal atrophy, or ulcerations. Clinically, subacute radiation dermatitis shares features of both acute and chronic radiation dermatitis; therefore, it is differentiated based on its histologic features.5,16
Although fluoroscopy-induced acute radiation dermatitis (Table 1) may precede FICRD, acute manifestations of fluoroscopy-related dermatitis can be subtle and often manifest in areas not easily visualized. Because referrals to dermatologists for full-skin examinations after fluoroscopy procedures are not standard, patients may not be aware of the association between these procedures and the development of skin lesions. Nonetheless, some patients may report a history of skin changes such as redness days or weeks after a fluoroscopic procedure with accompanying pain and pruritus limited to the fluoroscopy-exposed region, which tend to self-resolve.17 The risk for FICRD is thought to increase if a history of fluoroscopy-induced acute radiation dermatitis is present.18
The location of the skin findings correlates to the area exposed to prolonged radiation during the procedure(s). The most common areas include the scapular and subscapular regions, the right lateral trunk inferior to the axilla, the mid back, and the right anterolateral chest.16,19,20 These regions are associated with more complex (eg, cardiac) procedures that have been reported to lead to prolonged radiation exposure. The skin findings in FICRD are described as geometric, corresponding to the squarish or rectangular radiography beam that is directed at the patient. Additionally, radiography beams spread outward as they travel in space; therefore, skin injuries are common at the region more distal to the path of origination of the beam.21-23 Subsequently, a geometric, dyspigmented, indurated or atrophic plaque with telangiectasias and erosions or ulcerations with progressive worsening is a common manifestation of FICRD.5,16,23 Patients also commonly present with pruritus or severe pain associated with the lesion.24,25
Dermatologic Manifestations of FICRD
Skin responses seen weeks to years after a fluoroscopic procedure and typically after cumulative radiation exposure of 10 Gy or greater are categorized as FICRD (Table 2). These changes also can be clinically graded based on the Radiation Therapy Oncology Group classification of radiation dermatitis (Tables 3 and 4).26 Chronic changes in the skin largely result from remodeling of the vasculature and the subcutaneous tissue over time. Unlike acute changes, chronic changes typically persist and continue to worsen.27



Telangiectasias—Anywhere from months to 1 year after exposure to 10 Gy of radiation, proliferation of atypical superficial vessels in the dermis can be seen, typically manifesting as telangiectasias on physical examination. Telangiectasias can increase with time and can even exhibit a dose-dependent relationship to the radiation exposure.28
Atrophy—Atrophic-appearing skin after radiation exposure is the result of direct injury to both the epidermis and fibroblasts in the dermis. The destruction of keratinocytes leads to a thin epidermis, and destruction of dermal fibroblasts causes insufficient collagen production.29 Clinically, this process manifests as an atrophic plaque that can be seen 12 weeks to 1 year after the procedure.
Fibrosis—Approximately 1 year after the exposure, the initial damage can lead to disruption of molecular pathways, causing fibrosis. Transforming growth factor (TGF) β1 is the main factor involved.29 Damage to the endothelial cells results in increased TGF-β1 levels, which causes increased stimulation of remaining atypical fibroblasts and thus increased irregular collagen deposition.30 Further adding to this knowledge, Wei et al31 recently proposed that damage to the epidermal keratinocytes leads to disruption of yes-associated protein 1, which is a protective factor released from keratinocytes that regulates the dermal fibroblasts. However, extensive damage to the keratinocytes can lead to lower yes-associated protein 1 levels and its downstream activity, leading to increased levels of TGF-β1 and fibroblast activity.31 Clinically, this fibrotic stage is seen as indurated plaques in patients.
Necrosis—There are 2 forms of necrosis that can be seen. Ischemic dermal necrosis typically occurs in the acute phase after 10 weeks and approximately 18 Gy of cumulative exposure. It results from substantial skin damage, including microvascular damage and reduction in dermal capillaries, leading to ischemia of the tissue.2 Late dermal necrosis is the process seen in the chronic stage of FICRD and radiation dermatitis not related to fluoroscopy. It results from the inability of the fibrotic dermis to vascularly support the epidermis above it.2 It can be seen anywhere from 1 to 4 years after the procedure. This stage clinically manifests as worsening ulcerations with major pain and increased risk for secondary infections.16
Dyspigmentation—Dyspigmentation at the site of the radiation exposure can be seen acutely and chronically. Dosage above 15 to 18 Gy can lead to destruction of melanocytes, which can cause hypopigmentation in exposed areas. However, melanocytes are relatively resistant to radiation; therefore, dosages below the threshold of destruction of 15 to 18 Gy can cause melanocytic hyperactivity leading to hyperpigmentation.32 Hence, pigmentary changes can vary greatly. Classically, a central area of hypopigmentation with surrounding hyperpigmentation is seen.
Histology
Histologic appearance of radiation dermatitis varies depending on its stage. Acute radiation dermatitis primarily demonstrates superficial dermal edema, damage to the basal cell layer, small vessel dilation with thrombi, and hemorrhage along with a sparse inflammatory cell infiltrate.33 Histology typically is the only way to characterize subacute radiation dermatitis.5 Lichenoid tissue reaction is its characteristic feature. Mononuclear cells are found adjected to necrotic keratinocytes along with prominent vacuolization of the basal cell layer.33
The key histologic features of chronic radiation dermatitis include epidermal atrophy, hyperkeratosis, telangiectasias, loss of adnexal structures, and dermal fibrosis along with sparse atypical stellate fibroblasts.34 However, clinical context of fluoroscopic exposure is required for the dermatopathologist to differentiate chronic radiation dermatitis from its histologic differential of morphea and lichen sclerosus. In a cross-sectional study, only 1 of 6 cases (16.7%) was correctly diagnosed as chronic radiation dermatitis in the absence of correlating clinical history.35
Risk Factors for FICRD
Since the diagnosis of FICRD can be a clinical challenge, understanding the risk factors can be helpful. The general likelihood of developing FICRD is related to the duration, frequency, interval, intensity, and area of radiation exposure. Procedures exceeding the normal duration of 60 to 120 minutes have been well documented as a substantial risk factor for radiation dermatitis and FICRD.36-38 The risk tends to be higher in longer procedures because they result in more radiation exposure and higher accumulated PSD. Obesity (ie, body mass index >26) is the major risk factor that has been associated with longer procedure times, as higher radiation dosages are necessary to penetrate the body of a larger patient and a larger skin surface area is exposed.37-39
Other risk factors associated with FICRD relate to how prone a patient is to radiation-induced DNA damage. Older patients are at higher risk due to lower intrinsic ability of the tissue to repair itself.11 Patients with a history of connective tissue diseases—particularly lupus, scleroderma, and mixed connective tissue disease—are at an increased risk.40 Furthermore, patients with genetic disorders that impair DNA repair are more susceptible to radiation-induced DNA damage; therefore, patients with ataxia-telangiectasia, xeroderma pigmentosum, Fanconi anemia, and hereditary nevoid basal cell carcinoma are at higher risk for FICRD.39 Similarly, medications that can affect DNA repair also have been shown to be risk factors. These medications include chemotherapeutic agents such as actinomycin D, cyclophosphamide, doxorubicin, methotrexate, and 5-fluorouracil.2,39 Diabetes, hyperthyroidism, and tobacco use also have been shown to increase a patient’s risk for FICRD.39 It also is reasonable to believe that patients with defects in fibroblasts or with elastin or collagen disorders (eg, Ehlers-Danlos syndrome) would be at higher risk, but there are no known studies highlighting the association in the literature.
Differential Diagnosis of FICRD
Acute allergic or irritant contact dermatitis manifests with a localized area of erythematous skin accompanied by pruritus.41 Patients with FICRD can present with a localized area of erythema and hyperpigmentation with minimal atrophy. The lesion may accompany substantial pruritus, which can favor the more common diagnosis of contact dermatitis.35,42,43
Fixed-drug eruption manifests as a well-defined, hyperpigmented plaque in a fixed location that occurs upon ingestion of a drug.44 Fluoroscopy-induced chronic radiation dermatitis lesions are well demarcated and geometrically shaped and therefore can mimic lesions seen in fixed-drug eruptions.45 Additionally, the patient population undergoing fluoroscopic procedures tends to have major comorbidities requiring multiple medications.4
Decubitus ulcers are a result of vascular compromise to an area of skin due to constant pressure and are most commonly seen in the sacral region of patients with obesity.46 Ulcerated FICRD lesions can manifest on the lower midback. These lesions can be seen after endovascular repair of abdominal aortic aneurysm or prostatic artery embolization.20,21 The location of these lesions can mimic decubitus ulcers if fluoroscopic history is unknown. As mentioned, obesity also increases the risk for FICRD.
Morphea can manifest as a localized area of induration and hyperpigmentation of the skin.47 When FICRD has progressed to dermal fibrosis, patients can present with indurated plaques without ulcerations, which can be hard to differentiate from morphea.16,48 However, the presence of ulcerations or hyperkeratosis can differentiate morphea from FICRD.16
Ultimately, it is the location of FICRD lesions that remains the biggest diagnostic clue. Any suspicious lesion present on the scapular or subscapular areas, anterolateral chest, and/or mid back should prompt an investigation into recent or remote history of fluoroscopic procedures.
Management of FICRD
Diagnosis of FICRD should be made clinically based on the history and physical examination whenever possible, since a biopsy is not recommended.35 Wound healing in FICRD is delayed, and biopsies can lead to ulcerations or secondary infections.17 Therefore, it is important to remain suspicious for FICRD. Management of FICRD should correspond to the clinical findings outlined by a recent Delphi consensus survey.49 Regardless, the core of FICRD management framework should always include good hygiene, maintenance of skin hydration to improve epithelialization, and sufficient photoprotection.49,50
Among the first signs of FICRD are telangiectasias. Although asymptomatic, their appearance can be distressing for patients. Pulsed dye laser therapy is a first-line option that has been studied and has shown clinical efficacy for treatment of telangiectasias and vascular changes in patients with FICRD.49,51
If patients develop fibrotic changes, treatment options are limited. Fibrosis is hard to reverse, and the management approach is limited to symptomatic relief. Mechanical and deep-friction massages have been shown to be effective at reducing skin induration in patients.52 Fractional ablative lasers also may be utilized for skin contractures, especially if range of motion is affected.53,54 Although it comes with its own challenges, autologous fat grafting has shown promise in reducing postradiation fibrosis and inducing angiogenesis in tissue.55 Oral pentoxifylline also has shown mild efficacy, as it may be able to suppress TGF-β1 levels.53 However, prevention of fibrotic changes may be the most important. Wei et al31 suggested that low-dose oral prednisolone at 5 mg twice daily for 3 weeks might be an option to prevent the progression of skin changes and even reverse fibrosis to an extent; however, further evidence regarding its efficacy still is necessary. Additionally, no evidence was identified to support the use of topical corticosteroids for fibrotic changes seen in FICRD.56
Patients with FICRD or even acute radiation dermatitis after fluoroscopy tend to develop superficial ulcerations from minor traumas. Good wound hygiene, antiseptic care, and absorbent dressings, such as hydrogel and hydrocolloid, may be sufficient for treating these wounds, as seen in the Figure.42,48 However, once patients develop refractory ulcerations or necrosis, treatment options are then limited to surgical removal with a flap or graft.5,33,42,45

Risk for basal cell carcinomas and squamous cell carcinomas is higher in patients with radiation exposure; however, the exact risk from fluoroscopic procedures is unknown. One study demonstrated an increased risk of 6.9% in development of skin cancer after a median radiation exposure of 15.5 Gy and a mean latency period of 38.3 years,57 and in another retrospective study, the risk was higher in Fitzpatrick skin types I and II.58 Unlike the development of radiodermatitis itself, which shows a dose-dependent response, development of skin cancers follows a stochastic pattern (not dose dependent).59 Therefore, it is important to identify these high-risk patients and establish follow-up.
Conclusion
Fluoroscopy-induced chronic radiation dermatitis can be a diagnostic challenge, as skin changes may not be readily associated with the procedure by patients. Therefore, any lesion with a geometric shape and accompanying chronic radiation dermatitis features located on the scapular or subscapular areas, anterolateral chest, and midback should prompt an investigation into history of fluoroscopic procedures. Treatment of chronic skin changes in FICRD depends on the clinical manifestations. Good hygiene, skin hydration, and sufficient photoprotection are crucial. Finally, long-term monitoring with skin examinations is important to assess for the development of skin cancers in the treated area.
Fluoroscopy is an imaging technique that allows for real-time visualization of internal structures in the body using continuous radiography beams. More than 1 million fluoroscopy-guided procedures are performed annually in the United States.1 Utilization of these procedures continues to increase, and so does the probability of related complications, as prolonged exposure to ionizing radiation can cause skin injuries.2 Fortunately, the incidence of radiation-induced skin injuries compared with the total number of fluoroscopic procedures performed remains small,2 although one study suggested the incidence may be as high as 8.9% in at-risk populations.3
Radiation dermatitis is well recognized in dermatology as a complication of oncologic management; however, radiation dermatitis as a complication of fluoroscopic procedures is underrecognized.4 Fluoroscopy-induced radiation dermatitis can be categorized as acute, subacute, or chronic.5 Common fluoroscopic procedures that have been associated with fluoroscopy-induced radiation dermatitis include interventional cardiac procedures, neurovascular procedures, transjugular intrahepatic portosystemic shunt procedures, and endovascular abdominal aortic aneurysm repairs.6,7
Patients with fluoroscopy-induced radiation dermatitis, particularly fluoroscopy-induced chronic radiation dermatitis (FICRD), can present to dermatology up to several years after the initial fluoroscopy procedure with no awareness of the association between the procedure and their skin findings. This presents a diagnostic challenge, and FICRD often is overlooked.5,8-10
We conducted a literature search of PubMed articles indexed for MEDLINE using the search terms fluoroscopy and dermatitis. In this reappraisal, we will provide a comprehensive overview of fluoroscopy-induced radiation dermatitis with an emphasis on FICRD, covering its clinical manifestations, pathophysiology, risk factors, differential diagnosis, histology, and management. The aim of this review is to highlight the salient features and mimickers of FICRD and inform readers how to approach suspected cases, leading to accurate diagnosis and effective management.
Pathophysiology
Fluoroscopy-induced radiation dermatitis is the result of dose-dependent radiation-induced tissue damage. As the peak skin dosage (PSD) of radiation increases over the course of a procedure or multiple procedures, the severity of skin injury predictably increases. During fluoroscopic procedures, the standard irradiation dosage ranges from 0.02 Gy/min to 0.05 Gy/min.11 Transient skin changes may start to be seen around 2 Gy of cumulative exposure. Fluoroscopic procedures typically range in duration from 60 to 120 minutes; however, complex cases may exceed that. Additionally, multiple procedures performed within shorter intervals can result in greater PSD accumulation. Shorter intervals between procedures do not allow enough time for damage repair from the previous procedure and can result in further severe damage when the skin is re-exposed to radiation.2 The American College of Radiology recommends medical follow-up after 10 Gy of cumulative exposure, while cumulative exposure above 15 Gy within a 6- to 12-month period is defined as a sentinel event, according to The Joint Commission.12-14
Depending on the patient’s total radiation dosage during one or more procedures, the result of the tissue damage manifests differently at varying times: early skin changes are categorized as fluoroscopy-induced acute radiation dermatitis, and late skin changes are categorized as FICRD (Table 1).

Clinical Manifestations
Acute radiation dermatitis from fluoroscopic procedures manifests within hours to days up to 90 days following radiation exposure and can be characterized by erythema with blistering, desquamation, epilation, pigmentation changes, and even necrosis if the accumulated dosage exceeds 15 Gy.15 Chronic radiation dermatitis (which as related to fluoroscopic procedures is termed FICRD) has a longer onset of weeks to years and is clinically characterized by telangiectasias, permanent erythema, dermal atrophy, or ulcerations. Clinically, subacute radiation dermatitis shares features of both acute and chronic radiation dermatitis; therefore, it is differentiated based on its histologic features.5,16
Although fluoroscopy-induced acute radiation dermatitis (Table 1) may precede FICRD, acute manifestations of fluoroscopy-related dermatitis can be subtle and often manifest in areas not easily visualized. Because referrals to dermatologists for full-skin examinations after fluoroscopy procedures are not standard, patients may not be aware of the association between these procedures and the development of skin lesions. Nonetheless, some patients may report a history of skin changes such as redness days or weeks after a fluoroscopic procedure with accompanying pain and pruritus limited to the fluoroscopy-exposed region, which tend to self-resolve.17 The risk for FICRD is thought to increase if a history of fluoroscopy-induced acute radiation dermatitis is present.18
The location of the skin findings correlates to the area exposed to prolonged radiation during the procedure(s). The most common areas include the scapular and subscapular regions, the right lateral trunk inferior to the axilla, the mid back, and the right anterolateral chest.16,19,20 These regions are associated with more complex (eg, cardiac) procedures that have been reported to lead to prolonged radiation exposure. The skin findings in FICRD are described as geometric, corresponding to the squarish or rectangular radiography beam that is directed at the patient. Additionally, radiography beams spread outward as they travel in space; therefore, skin injuries are common at the region more distal to the path of origination of the beam.21-23 Subsequently, a geometric, dyspigmented, indurated or atrophic plaque with telangiectasias and erosions or ulcerations with progressive worsening is a common manifestation of FICRD.5,16,23 Patients also commonly present with pruritus or severe pain associated with the lesion.24,25
Dermatologic Manifestations of FICRD
Skin responses seen weeks to years after a fluoroscopic procedure and typically after cumulative radiation exposure of 10 Gy or greater are categorized as FICRD (Table 2). These changes also can be clinically graded based on the Radiation Therapy Oncology Group classification of radiation dermatitis (Tables 3 and 4).26 Chronic changes in the skin largely result from remodeling of the vasculature and the subcutaneous tissue over time. Unlike acute changes, chronic changes typically persist and continue to worsen.27



Telangiectasias—Anywhere from months to 1 year after exposure to 10 Gy of radiation, proliferation of atypical superficial vessels in the dermis can be seen, typically manifesting as telangiectasias on physical examination. Telangiectasias can increase with time and can even exhibit a dose-dependent relationship to the radiation exposure.28
Atrophy—Atrophic-appearing skin after radiation exposure is the result of direct injury to both the epidermis and fibroblasts in the dermis. The destruction of keratinocytes leads to a thin epidermis, and destruction of dermal fibroblasts causes insufficient collagen production.29 Clinically, this process manifests as an atrophic plaque that can be seen 12 weeks to 1 year after the procedure.
Fibrosis—Approximately 1 year after the exposure, the initial damage can lead to disruption of molecular pathways, causing fibrosis. Transforming growth factor (TGF) β1 is the main factor involved.29 Damage to the endothelial cells results in increased TGF-β1 levels, which causes increased stimulation of remaining atypical fibroblasts and thus increased irregular collagen deposition.30 Further adding to this knowledge, Wei et al31 recently proposed that damage to the epidermal keratinocytes leads to disruption of yes-associated protein 1, which is a protective factor released from keratinocytes that regulates the dermal fibroblasts. However, extensive damage to the keratinocytes can lead to lower yes-associated protein 1 levels and its downstream activity, leading to increased levels of TGF-β1 and fibroblast activity.31 Clinically, this fibrotic stage is seen as indurated plaques in patients.
Necrosis—There are 2 forms of necrosis that can be seen. Ischemic dermal necrosis typically occurs in the acute phase after 10 weeks and approximately 18 Gy of cumulative exposure. It results from substantial skin damage, including microvascular damage and reduction in dermal capillaries, leading to ischemia of the tissue.2 Late dermal necrosis is the process seen in the chronic stage of FICRD and radiation dermatitis not related to fluoroscopy. It results from the inability of the fibrotic dermis to vascularly support the epidermis above it.2 It can be seen anywhere from 1 to 4 years after the procedure. This stage clinically manifests as worsening ulcerations with major pain and increased risk for secondary infections.16
Dyspigmentation—Dyspigmentation at the site of the radiation exposure can be seen acutely and chronically. Dosage above 15 to 18 Gy can lead to destruction of melanocytes, which can cause hypopigmentation in exposed areas. However, melanocytes are relatively resistant to radiation; therefore, dosages below the threshold of destruction of 15 to 18 Gy can cause melanocytic hyperactivity leading to hyperpigmentation.32 Hence, pigmentary changes can vary greatly. Classically, a central area of hypopigmentation with surrounding hyperpigmentation is seen.
Histology
Histologic appearance of radiation dermatitis varies depending on its stage. Acute radiation dermatitis primarily demonstrates superficial dermal edema, damage to the basal cell layer, small vessel dilation with thrombi, and hemorrhage along with a sparse inflammatory cell infiltrate.33 Histology typically is the only way to characterize subacute radiation dermatitis.5 Lichenoid tissue reaction is its characteristic feature. Mononuclear cells are found adjected to necrotic keratinocytes along with prominent vacuolization of the basal cell layer.33
The key histologic features of chronic radiation dermatitis include epidermal atrophy, hyperkeratosis, telangiectasias, loss of adnexal structures, and dermal fibrosis along with sparse atypical stellate fibroblasts.34 However, clinical context of fluoroscopic exposure is required for the dermatopathologist to differentiate chronic radiation dermatitis from its histologic differential of morphea and lichen sclerosus. In a cross-sectional study, only 1 of 6 cases (16.7%) was correctly diagnosed as chronic radiation dermatitis in the absence of correlating clinical history.35
Risk Factors for FICRD
Since the diagnosis of FICRD can be a clinical challenge, understanding the risk factors can be helpful. The general likelihood of developing FICRD is related to the duration, frequency, interval, intensity, and area of radiation exposure. Procedures exceeding the normal duration of 60 to 120 minutes have been well documented as a substantial risk factor for radiation dermatitis and FICRD.36-38 The risk tends to be higher in longer procedures because they result in more radiation exposure and higher accumulated PSD. Obesity (ie, body mass index >26) is the major risk factor that has been associated with longer procedure times, as higher radiation dosages are necessary to penetrate the body of a larger patient and a larger skin surface area is exposed.37-39
Other risk factors associated with FICRD relate to how prone a patient is to radiation-induced DNA damage. Older patients are at higher risk due to lower intrinsic ability of the tissue to repair itself.11 Patients with a history of connective tissue diseases—particularly lupus, scleroderma, and mixed connective tissue disease—are at an increased risk.40 Furthermore, patients with genetic disorders that impair DNA repair are more susceptible to radiation-induced DNA damage; therefore, patients with ataxia-telangiectasia, xeroderma pigmentosum, Fanconi anemia, and hereditary nevoid basal cell carcinoma are at higher risk for FICRD.39 Similarly, medications that can affect DNA repair also have been shown to be risk factors. These medications include chemotherapeutic agents such as actinomycin D, cyclophosphamide, doxorubicin, methotrexate, and 5-fluorouracil.2,39 Diabetes, hyperthyroidism, and tobacco use also have been shown to increase a patient’s risk for FICRD.39 It also is reasonable to believe that patients with defects in fibroblasts or with elastin or collagen disorders (eg, Ehlers-Danlos syndrome) would be at higher risk, but there are no known studies highlighting the association in the literature.
Differential Diagnosis of FICRD
Acute allergic or irritant contact dermatitis manifests with a localized area of erythematous skin accompanied by pruritus.41 Patients with FICRD can present with a localized area of erythema and hyperpigmentation with minimal atrophy. The lesion may accompany substantial pruritus, which can favor the more common diagnosis of contact dermatitis.35,42,43
Fixed-drug eruption manifests as a well-defined, hyperpigmented plaque in a fixed location that occurs upon ingestion of a drug.44 Fluoroscopy-induced chronic radiation dermatitis lesions are well demarcated and geometrically shaped and therefore can mimic lesions seen in fixed-drug eruptions.45 Additionally, the patient population undergoing fluoroscopic procedures tends to have major comorbidities requiring multiple medications.4
Decubitus ulcers are a result of vascular compromise to an area of skin due to constant pressure and are most commonly seen in the sacral region of patients with obesity.46 Ulcerated FICRD lesions can manifest on the lower midback. These lesions can be seen after endovascular repair of abdominal aortic aneurysm or prostatic artery embolization.20,21 The location of these lesions can mimic decubitus ulcers if fluoroscopic history is unknown. As mentioned, obesity also increases the risk for FICRD.
Morphea can manifest as a localized area of induration and hyperpigmentation of the skin.47 When FICRD has progressed to dermal fibrosis, patients can present with indurated plaques without ulcerations, which can be hard to differentiate from morphea.16,48 However, the presence of ulcerations or hyperkeratosis can differentiate morphea from FICRD.16
Ultimately, it is the location of FICRD lesions that remains the biggest diagnostic clue. Any suspicious lesion present on the scapular or subscapular areas, anterolateral chest, and/or mid back should prompt an investigation into recent or remote history of fluoroscopic procedures.
Management of FICRD
Diagnosis of FICRD should be made clinically based on the history and physical examination whenever possible, since a biopsy is not recommended.35 Wound healing in FICRD is delayed, and biopsies can lead to ulcerations or secondary infections.17 Therefore, it is important to remain suspicious for FICRD. Management of FICRD should correspond to the clinical findings outlined by a recent Delphi consensus survey.49 Regardless, the core of FICRD management framework should always include good hygiene, maintenance of skin hydration to improve epithelialization, and sufficient photoprotection.49,50
Among the first signs of FICRD are telangiectasias. Although asymptomatic, their appearance can be distressing for patients. Pulsed dye laser therapy is a first-line option that has been studied and has shown clinical efficacy for treatment of telangiectasias and vascular changes in patients with FICRD.49,51
If patients develop fibrotic changes, treatment options are limited. Fibrosis is hard to reverse, and the management approach is limited to symptomatic relief. Mechanical and deep-friction massages have been shown to be effective at reducing skin induration in patients.52 Fractional ablative lasers also may be utilized for skin contractures, especially if range of motion is affected.53,54 Although it comes with its own challenges, autologous fat grafting has shown promise in reducing postradiation fibrosis and inducing angiogenesis in tissue.55 Oral pentoxifylline also has shown mild efficacy, as it may be able to suppress TGF-β1 levels.53 However, prevention of fibrotic changes may be the most important. Wei et al31 suggested that low-dose oral prednisolone at 5 mg twice daily for 3 weeks might be an option to prevent the progression of skin changes and even reverse fibrosis to an extent; however, further evidence regarding its efficacy still is necessary. Additionally, no evidence was identified to support the use of topical corticosteroids for fibrotic changes seen in FICRD.56
Patients with FICRD or even acute radiation dermatitis after fluoroscopy tend to develop superficial ulcerations from minor traumas. Good wound hygiene, antiseptic care, and absorbent dressings, such as hydrogel and hydrocolloid, may be sufficient for treating these wounds, as seen in the Figure.42,48 However, once patients develop refractory ulcerations or necrosis, treatment options are then limited to surgical removal with a flap or graft.5,33,42,45

Risk for basal cell carcinomas and squamous cell carcinomas is higher in patients with radiation exposure; however, the exact risk from fluoroscopic procedures is unknown. One study demonstrated an increased risk of 6.9% in development of skin cancer after a median radiation exposure of 15.5 Gy and a mean latency period of 38.3 years,57 and in another retrospective study, the risk was higher in Fitzpatrick skin types I and II.58 Unlike the development of radiodermatitis itself, which shows a dose-dependent response, development of skin cancers follows a stochastic pattern (not dose dependent).59 Therefore, it is important to identify these high-risk patients and establish follow-up.
Conclusion
Fluoroscopy-induced chronic radiation dermatitis can be a diagnostic challenge, as skin changes may not be readily associated with the procedure by patients. Therefore, any lesion with a geometric shape and accompanying chronic radiation dermatitis features located on the scapular or subscapular areas, anterolateral chest, and midback should prompt an investigation into history of fluoroscopic procedures. Treatment of chronic skin changes in FICRD depends on the clinical manifestations. Good hygiene, skin hydration, and sufficient photoprotection are crucial. Finally, long-term monitoring with skin examinations is important to assess for the development of skin cancers in the treated area.
- Benjamin EJ, Muntner P, Alonso A, et al. Heart Disease and Stroke Statistics-2019 Update: a report from the American Heart Association. Circulation. 2019;139:E56-E528. doi:10.1161/CIR.0000000000000659. Published correction appears in Circulation. 2020;141:E33.
- Koenig TR, Wolff D, Mettler FA, et al. Skin injuries from fluoroscopically guided procedures: part 1, characteristics of radiation injury. AJR Am J Roentgenol. 2001;177:3-11. doi:10.2214/ajr.177.1.1770003
- Guesnier-Dopagne M, Boyer L, Pereira B, et al. Incidence of chronic radiodermatitis after fluoroscopically guided interventions: a retrospective study. J Vasc Interv Radiol. 2019;30:692-698.e13. doi:10.1016/j.jvir.2019.01.010
- Cunha N, Cardoso P, Cabete J. Subacute radiation dermatitis following an interventional cardiology procedure. Cutan Ocul Toxicol. 2017;36:297-299. doi:10.1080/15569527.2016.1254649
- Frazier TH, Richardson JB, Fabré VC, et al. Fluoroscopy-induced chronic radiation skin injury: a disease perhaps often overlooked. Arch Dermatol. 2007;143:637-640. doi:10.1001/archderm.143.5.637
- Koenig TR, Mettler FA, Wagner LK. Skin injuries from fluoroscopically guided procedures: part 2, review of 73 cases and recommendations for minimizing dose delivered to patient. AJR Am J Roentgenol. 2001;177:13-20. doi:10.2214/ajr.177.1.1770013
- Shope TB. Radiation-induced skin injuries from fluoroscopy. Radiographics. 1996;16:1195-1199. doi:10.1148/radiographics.16.5.8888398
- Tchanque-Fossuo CN, Isseroff RR, Silverstein MA. Fluoroscopy induced chronic radiation dermatitis should be included in the differential diagnosis of notalgia paresthetica. Dermatol Online J. 2016;22:13030/qt0kh726m9.
- Berlin L. Radiation-induced skin injuries and fluoroscopy. AJR Am J Roentgenol. 2001;177:21-25. doi:10.2214/ajr.177.1.1770021
- Tchanque-Fossuo CN, Kamangar F, Ho B, et al. Fluoroscopy-induced radionecrosis. Dermatol Online J. 2016;22:13030/qt68w910t2.
- Wagner LK, Eifel PJ, Geise RA. Potential biological effects following high X-ray dose interventional procedures. J Vasc Interv Radiol. 1994;5:71-84. doi:10.1016/s1051-0443(94)71456-1
- Balter S, Hopewell JW, Miller DL, et al. Fluoroscopically guided interventional procedures: a review of radiation effects on patients’ skin and hair. Radiology. 2010;254:326-341. doi:10.1148/radiol.2542082312
- Vance AZ, Weinberg BD, Arbique GM, et al. Fluoroscopic sentinel events in neuroendovascular procedures: how to screen, prevent, and address occurrence. AJNR Am J Neuroradiol. 2013;34:1513-1515. doi:10.3174/ajnr.A3185
- Aerts A, Decraene T, van den Oord JJ, et al. Chronic radiodermatitis following percutaneous coronary interventions: a report of two cases. J Eur Acad Dermatol Venereol. 2003;17:340-343. doi:10.1046/j.1468-3083.2003.00687.x
- Rosenthal A, Israilevich R, Moy R. Management of acute radiation dermatitis: a review of the literature and proposal for treatment algorithm. J Am Acad Dermatol. 2019;81:558-567. doi:10.1016/j.jaad.2019.02.047
- Boncher J, Bergfeld WF. Fluoroscopy-induced chronic radiation dermatitis: a report of two additional cases and a brief review of the literature. J Cutan Pathol. 2012;39:63-67. doi:10.1111/j.1600-0560.2011.01754.x
- Spiker A, Zinn Z, Carter WH, et al. Fluoroscopy-induced chronic radiation dermatitis. Am J Cardiol. 2012;110:1861-1863. doi:10.1016/j.amjcard.2012.08.023
- Batrani M, Kubba A, Sundharam J. Fluoroscopy-induced chronic radiation dermatitis masquerading as morphea: a diagnostic pitfall. Indian J Pathol Microbiol. 2018;61:393-396. doi:10.4103/IJPM.IJPM_566_17
- Jeskowiak A, Hubmer M, Prenner G, et al. Radiation induced cutaneous ulcer on the back in a patient with congenital anomaly of the upper cava system. Interact Cardiovasc Thorac Surg. 2011;12:290-292.
- Laborda A, De Assis AM, Ioakeim I, et al. Radiodermitis after prostatic artery embolization: case report and review of the literature. Cardiovasc Intervent Radiol. 2015;38:755-759. doi:10.1007/s00270-015-1083-6
- Lyons AB, Harvey VM, Gusev J. Fluoroscopy-induced chronic radiation dermatitis (FICRD) after endovascular abdominal aortic aneurysm endoleak repair. JAAD Case Rep. 2015;1:403-405. doi:10.1016/j.jdcr.2015.09.022
- Mossman KL. Analysis of risk in computerized tomography and other diagnostic radiology procedures. Comput Radiol. 1982;6:251-256. doi:10.1016/0730-4862(82)90109-3
- Henry MF, Maender JL, Shen Y, et al. Fluoroscopy-induced chronic radiation dermatitis: a report of three cases. Dermatol Online J. 2009;15:3.
- Balter S, Miller DL. Patient skin reactions from interventional fluoroscopy procedures. AJR Am J Roentgenol. 2014;202:W335-W342. doi:10.2214/AJR.13.12029
- Nishimoto S, Fukuda K, Kawai K, et al. Supplementation of bone marrow aspirate-derived platelet-rich plasma for treating radiation-induced ulcer after cardiac fluoroscopic procedures: a preliminary report. Indian J Plast Surg. 2012;45:109-114. doi:10.4103/0970-0358.96599
- Cox JD, Stetz J, Pajak TF. Toxicity criteria of the Radiation Therapy Oncology Group (RTOG) and the European Organization for Research and Treatment of Cancer (EORTC). Int J Radiat Oncol Biol Phys. 1995;31:1341-1346. doi:10.1016/0360-3016(95)00060-C
- Wong RK, Bensadoun RJ, Boers-Doets CB, et al. Clinical practice guidelines for the prevention and treatment of acute and late radiation reactions from the MASCC Skin Toxicity Study Group. Support Care Cancer. 2013;21:2933-2948. doi:10.1007/s00520-013-1896-2
- Turesson I, Notter G. The predictive value of skin telangiectasia for late radiation effects in different normal tissues. Int J Radiat Oncol Biol Phys. 1986;12:603-609. doi:10.1016/0360-3016(86)90069-6
- Hegedus F, Mathew LM, Schwartz RA. Radiation dermatitis: an overview. Int J Dermatol. 2017;56:909-914. doi:10.1111/ijd.13371
- Denham JW, Hauer-Jensen M. The radiotherapeutic injury—a complex ‘wound.’ Radiother Oncol. 2002;63:129-145. doi:10.1016/s0167-8140(02)00060-9
- Wei KC, Lai SF, Huang WL, et al. An innovative targeted therapy for fluoroscopy-induced chronic radiation dermatitis. J Mol Med (Berl). 2022;100:135-146. doi:10.1007/s00109-021-02146-3
- Sitton E. Early and late radiation-induced skin alterations. part I: mechanisms of skin changes. Oncol Nurs Forum. 1992;19:801-807.
- Pruitt LG, Rogers W, Byarlay JA, et al. Subacute radiation dermatitis after fluoroscopy. J Cutan Pathol. 2016;43:1091-1095. doi:10.1111/cup.12815
- Anderson EB, Draft KS, Lee RA, et al. Update in dermatopathology. Am J Clin Pathol. 2006;125(Suppl):S50-S70. doi:10.1309/GMUFNP6LFMPNR86R
- Wei KC, Yang KC, Mar GY, et al. STROBE—radiation ulcer: an overlooked complication of fluoroscopic intervention: a cross-sectional study. Medicine (Baltimore). 2015;94:e2178. doi:10.1097/MD.0000000000002178
- Otterburn D, Losken A. Iatrogenic fluoroscopy injury to the skin. Ann Plast Surg. 2010;65:462-465. doi:10.1097/SAP.0b013e3181d6e2d3
- Cha MJ, Jo SJ, Cho Y, et al. Patient characteristics and the incidence of radiation-induced dermatitis following radiofrequency catheter ablation. Korean Circ J. 2016;46:646-653. doi:10.4070/kcj.2016.46.5.646
- Dehen L, Vilmer C, Humilière C, et al. Chronic radiodermatitis following cardiac catheterisation: a report of two cases and a brief review of the literature. Heart. 1999;81:308-312. doi:10.1136/hrt.81.3.308
- Brown KR, Rzucidlo E. Acute and chronic radiation injury. J Vasc Surg. 2011;53(Suppl 1):15S-21S. doi:10.1016/j.jvs.2010.06.175. Published correction appears in J Vasc Surg. 2012;55:627.
- Hymes SR, Strom EA, Fife C. Radiation dermatitis: clinical presentation, pathophysiology, and treatment 2006. J Am Acad Dermatol. 2006;54:28-46. doi:10.1016/j.jaad.2005.08.054
- Scheinman PL, Vocanson M, Thyssen JP, et al. Contact dermatitis. Nat Rev Dis Primers. 2021;7:38. doi:10.1038/s41572-021-00271-4
- Cheng TT, Yang HJ. Chronic radiation dermatitis induced by cardiac catheterization: a case report and literature review. Acta Dermatovenerol Alp Pannonica Adriat. 2022;31:147-149.
- Minni JP, Nowak M, Usmani A, et al. A unique case of subacute radiodermatitis. Cutis. 2013;91:230-232.
- Flowers H, Brodell R, Brents M, et al. Fixed drug eruptions: presentation, diagnosis, and management. South Med J. 2014;107:724-727. doi:10.14423/SMJ.0000000000000195
- Hashimoto I, Sedo H, Inatsugi K, et al. Severe radiation-induced injury after cardiac catheter ablation: a case requiring free anterolateral thigh flap and vastus lateralis muscle flap reconstruction on the upper arm. J Plast Reconstr Aesthet Surg. 2008;61:704-708. doi:10.1016/j.bjps.2007.01.003
- Mervis JS, Phillips TJ. Pressure ulcers: pathophysiology, epidemiology, risk factors, and presentation. J Am Acad Dermatol. 2019;81:881-890. doi:10.1016/j.jaad.2018.12.069
- Careta MF, Romiti R. Localized scleroderma: clinical spectrum and therapeutic update. An Bras Dermatol. 2015;90:62-73. doi:10.1590/abd1806-4841.20152890
- Herz-Ruelas ME, Gómez-Flores M, Moxica-Del Angel J, et al. Ulcerated radiodermatitis induced after fluoroscopically guided stent implantation angioplasty. Case Rep Dermatol Med. 2014;2014:768624. doi:10.1155/2014/768624
- Wilson BN, Shah R, Menzer C, et al. Consensus on the clinical management of chronic radiation dermatitis and radiation fibrosis: a Delphi survey. Br J Dermatol. 2022;187:1054-1056. doi:10.1111/bjd.21852
- Khanna NR, Kumar DP, Laskar SG, et al. Radiation dermatitis: an overview. Indian J Burns. 2013;21:24-31. doi:10.4103/0971-653x.121877
- Spalek M. Chronic radiation-induced dermatitis: challenges and solutions. Clin Cosmet Investig Dermatol. 2016;9:473-482. doi:10.2147/CCID.S94320
- Bourgeois JF, Gourgou S, Kramar A, et al. A randomized, prospective study using the LPG technique in treating radiation-induced skin fibrosis: clinical and profilometric analysis. Skin Res Technol. 2008;14:71-76. doi:10.1111/j.1600-0846.2007.00263.x
- Borrelli MR, Shen AH, Lee GK, et al. Radiation-induced skinfibrosis: pathogenesis, current treatment options, and emerging therapeutics. Ann Plast Surg. 2019;83(4S Suppl 1):S59-S64. doi:10.1097/SAP.0000000000002098
- Wilson B, Shah R, Menzer C, et al. Laser therapy as a treatment for chronic radiation fibrosis. Lasers Surg Med. 2023;55:82-88. doi:10.1002/lsm.23617
- Rigotti G, Marchi A, Galiè M, et al. Clinical treatment of radiotherapy tissue damage by lipoaspirate transplant: a healing process mediated by adipose-derived adult stem cells. Plast Reconstr Surg. 2007;119:1409-1422. doi:10.1097/01.prs.0000256047.47909.71
- Leventhal J, Young MR. Radiation dermatitis: recognition, prevention, and management. Oncology (Williston Park). 2017;31:885-899.
- van Vloten WA, Hermans J, van Daal WA. Radiation-induced skin cancer and radiodermatitis of the head and neck. Cancer. 1987;59:411-414. doi:10.1002/1097-0142(19870201)59:3<411::aid-cncr2820590310>3.0.co;2-z
- Davis MM, Hanke CW, Zollinger TW, et al. Skin cancer in patients with chronic radiation dermatitis. J Am Acad Dermatol. 1989;20:608-616. doi:10.1016/s0190-9622(89)70072-4
- Miller DL, Balter S, Schueler BA, et al. Clinical radiation management for fluoroscopically guided interventional procedures. Radiology. 2010;257:321-332. doi:10.1148/radiol.10091269
- Benjamin EJ, Muntner P, Alonso A, et al. Heart Disease and Stroke Statistics-2019 Update: a report from the American Heart Association. Circulation. 2019;139:E56-E528. doi:10.1161/CIR.0000000000000659. Published correction appears in Circulation. 2020;141:E33.
- Koenig TR, Wolff D, Mettler FA, et al. Skin injuries from fluoroscopically guided procedures: part 1, characteristics of radiation injury. AJR Am J Roentgenol. 2001;177:3-11. doi:10.2214/ajr.177.1.1770003
- Guesnier-Dopagne M, Boyer L, Pereira B, et al. Incidence of chronic radiodermatitis after fluoroscopically guided interventions: a retrospective study. J Vasc Interv Radiol. 2019;30:692-698.e13. doi:10.1016/j.jvir.2019.01.010
- Cunha N, Cardoso P, Cabete J. Subacute radiation dermatitis following an interventional cardiology procedure. Cutan Ocul Toxicol. 2017;36:297-299. doi:10.1080/15569527.2016.1254649
- Frazier TH, Richardson JB, Fabré VC, et al. Fluoroscopy-induced chronic radiation skin injury: a disease perhaps often overlooked. Arch Dermatol. 2007;143:637-640. doi:10.1001/archderm.143.5.637
- Koenig TR, Mettler FA, Wagner LK. Skin injuries from fluoroscopically guided procedures: part 2, review of 73 cases and recommendations for minimizing dose delivered to patient. AJR Am J Roentgenol. 2001;177:13-20. doi:10.2214/ajr.177.1.1770013
- Shope TB. Radiation-induced skin injuries from fluoroscopy. Radiographics. 1996;16:1195-1199. doi:10.1148/radiographics.16.5.8888398
- Tchanque-Fossuo CN, Isseroff RR, Silverstein MA. Fluoroscopy induced chronic radiation dermatitis should be included in the differential diagnosis of notalgia paresthetica. Dermatol Online J. 2016;22:13030/qt0kh726m9.
- Berlin L. Radiation-induced skin injuries and fluoroscopy. AJR Am J Roentgenol. 2001;177:21-25. doi:10.2214/ajr.177.1.1770021
- Tchanque-Fossuo CN, Kamangar F, Ho B, et al. Fluoroscopy-induced radionecrosis. Dermatol Online J. 2016;22:13030/qt68w910t2.
- Wagner LK, Eifel PJ, Geise RA. Potential biological effects following high X-ray dose interventional procedures. J Vasc Interv Radiol. 1994;5:71-84. doi:10.1016/s1051-0443(94)71456-1
- Balter S, Hopewell JW, Miller DL, et al. Fluoroscopically guided interventional procedures: a review of radiation effects on patients’ skin and hair. Radiology. 2010;254:326-341. doi:10.1148/radiol.2542082312
- Vance AZ, Weinberg BD, Arbique GM, et al. Fluoroscopic sentinel events in neuroendovascular procedures: how to screen, prevent, and address occurrence. AJNR Am J Neuroradiol. 2013;34:1513-1515. doi:10.3174/ajnr.A3185
- Aerts A, Decraene T, van den Oord JJ, et al. Chronic radiodermatitis following percutaneous coronary interventions: a report of two cases. J Eur Acad Dermatol Venereol. 2003;17:340-343. doi:10.1046/j.1468-3083.2003.00687.x
- Rosenthal A, Israilevich R, Moy R. Management of acute radiation dermatitis: a review of the literature and proposal for treatment algorithm. J Am Acad Dermatol. 2019;81:558-567. doi:10.1016/j.jaad.2019.02.047
- Boncher J, Bergfeld WF. Fluoroscopy-induced chronic radiation dermatitis: a report of two additional cases and a brief review of the literature. J Cutan Pathol. 2012;39:63-67. doi:10.1111/j.1600-0560.2011.01754.x
- Spiker A, Zinn Z, Carter WH, et al. Fluoroscopy-induced chronic radiation dermatitis. Am J Cardiol. 2012;110:1861-1863. doi:10.1016/j.amjcard.2012.08.023
- Batrani M, Kubba A, Sundharam J. Fluoroscopy-induced chronic radiation dermatitis masquerading as morphea: a diagnostic pitfall. Indian J Pathol Microbiol. 2018;61:393-396. doi:10.4103/IJPM.IJPM_566_17
- Jeskowiak A, Hubmer M, Prenner G, et al. Radiation induced cutaneous ulcer on the back in a patient with congenital anomaly of the upper cava system. Interact Cardiovasc Thorac Surg. 2011;12:290-292.
- Laborda A, De Assis AM, Ioakeim I, et al. Radiodermitis after prostatic artery embolization: case report and review of the literature. Cardiovasc Intervent Radiol. 2015;38:755-759. doi:10.1007/s00270-015-1083-6
- Lyons AB, Harvey VM, Gusev J. Fluoroscopy-induced chronic radiation dermatitis (FICRD) after endovascular abdominal aortic aneurysm endoleak repair. JAAD Case Rep. 2015;1:403-405. doi:10.1016/j.jdcr.2015.09.022
- Mossman KL. Analysis of risk in computerized tomography and other diagnostic radiology procedures. Comput Radiol. 1982;6:251-256. doi:10.1016/0730-4862(82)90109-3
- Henry MF, Maender JL, Shen Y, et al. Fluoroscopy-induced chronic radiation dermatitis: a report of three cases. Dermatol Online J. 2009;15:3.
- Balter S, Miller DL. Patient skin reactions from interventional fluoroscopy procedures. AJR Am J Roentgenol. 2014;202:W335-W342. doi:10.2214/AJR.13.12029
- Nishimoto S, Fukuda K, Kawai K, et al. Supplementation of bone marrow aspirate-derived platelet-rich plasma for treating radiation-induced ulcer after cardiac fluoroscopic procedures: a preliminary report. Indian J Plast Surg. 2012;45:109-114. doi:10.4103/0970-0358.96599
- Cox JD, Stetz J, Pajak TF. Toxicity criteria of the Radiation Therapy Oncology Group (RTOG) and the European Organization for Research and Treatment of Cancer (EORTC). Int J Radiat Oncol Biol Phys. 1995;31:1341-1346. doi:10.1016/0360-3016(95)00060-C
- Wong RK, Bensadoun RJ, Boers-Doets CB, et al. Clinical practice guidelines for the prevention and treatment of acute and late radiation reactions from the MASCC Skin Toxicity Study Group. Support Care Cancer. 2013;21:2933-2948. doi:10.1007/s00520-013-1896-2
- Turesson I, Notter G. The predictive value of skin telangiectasia for late radiation effects in different normal tissues. Int J Radiat Oncol Biol Phys. 1986;12:603-609. doi:10.1016/0360-3016(86)90069-6
- Hegedus F, Mathew LM, Schwartz RA. Radiation dermatitis: an overview. Int J Dermatol. 2017;56:909-914. doi:10.1111/ijd.13371
- Denham JW, Hauer-Jensen M. The radiotherapeutic injury—a complex ‘wound.’ Radiother Oncol. 2002;63:129-145. doi:10.1016/s0167-8140(02)00060-9
- Wei KC, Lai SF, Huang WL, et al. An innovative targeted therapy for fluoroscopy-induced chronic radiation dermatitis. J Mol Med (Berl). 2022;100:135-146. doi:10.1007/s00109-021-02146-3
- Sitton E. Early and late radiation-induced skin alterations. part I: mechanisms of skin changes. Oncol Nurs Forum. 1992;19:801-807.
- Pruitt LG, Rogers W, Byarlay JA, et al. Subacute radiation dermatitis after fluoroscopy. J Cutan Pathol. 2016;43:1091-1095. doi:10.1111/cup.12815
- Anderson EB, Draft KS, Lee RA, et al. Update in dermatopathology. Am J Clin Pathol. 2006;125(Suppl):S50-S70. doi:10.1309/GMUFNP6LFMPNR86R
- Wei KC, Yang KC, Mar GY, et al. STROBE—radiation ulcer: an overlooked complication of fluoroscopic intervention: a cross-sectional study. Medicine (Baltimore). 2015;94:e2178. doi:10.1097/MD.0000000000002178
- Otterburn D, Losken A. Iatrogenic fluoroscopy injury to the skin. Ann Plast Surg. 2010;65:462-465. doi:10.1097/SAP.0b013e3181d6e2d3
- Cha MJ, Jo SJ, Cho Y, et al. Patient characteristics and the incidence of radiation-induced dermatitis following radiofrequency catheter ablation. Korean Circ J. 2016;46:646-653. doi:10.4070/kcj.2016.46.5.646
- Dehen L, Vilmer C, Humilière C, et al. Chronic radiodermatitis following cardiac catheterisation: a report of two cases and a brief review of the literature. Heart. 1999;81:308-312. doi:10.1136/hrt.81.3.308
- Brown KR, Rzucidlo E. Acute and chronic radiation injury. J Vasc Surg. 2011;53(Suppl 1):15S-21S. doi:10.1016/j.jvs.2010.06.175. Published correction appears in J Vasc Surg. 2012;55:627.
- Hymes SR, Strom EA, Fife C. Radiation dermatitis: clinical presentation, pathophysiology, and treatment 2006. J Am Acad Dermatol. 2006;54:28-46. doi:10.1016/j.jaad.2005.08.054
- Scheinman PL, Vocanson M, Thyssen JP, et al. Contact dermatitis. Nat Rev Dis Primers. 2021;7:38. doi:10.1038/s41572-021-00271-4
- Cheng TT, Yang HJ. Chronic radiation dermatitis induced by cardiac catheterization: a case report and literature review. Acta Dermatovenerol Alp Pannonica Adriat. 2022;31:147-149.
- Minni JP, Nowak M, Usmani A, et al. A unique case of subacute radiodermatitis. Cutis. 2013;91:230-232.
- Flowers H, Brodell R, Brents M, et al. Fixed drug eruptions: presentation, diagnosis, and management. South Med J. 2014;107:724-727. doi:10.14423/SMJ.0000000000000195
- Hashimoto I, Sedo H, Inatsugi K, et al. Severe radiation-induced injury after cardiac catheter ablation: a case requiring free anterolateral thigh flap and vastus lateralis muscle flap reconstruction on the upper arm. J Plast Reconstr Aesthet Surg. 2008;61:704-708. doi:10.1016/j.bjps.2007.01.003
- Mervis JS, Phillips TJ. Pressure ulcers: pathophysiology, epidemiology, risk factors, and presentation. J Am Acad Dermatol. 2019;81:881-890. doi:10.1016/j.jaad.2018.12.069
- Careta MF, Romiti R. Localized scleroderma: clinical spectrum and therapeutic update. An Bras Dermatol. 2015;90:62-73. doi:10.1590/abd1806-4841.20152890
- Herz-Ruelas ME, Gómez-Flores M, Moxica-Del Angel J, et al. Ulcerated radiodermatitis induced after fluoroscopically guided stent implantation angioplasty. Case Rep Dermatol Med. 2014;2014:768624. doi:10.1155/2014/768624
- Wilson BN, Shah R, Menzer C, et al. Consensus on the clinical management of chronic radiation dermatitis and radiation fibrosis: a Delphi survey. Br J Dermatol. 2022;187:1054-1056. doi:10.1111/bjd.21852
- Khanna NR, Kumar DP, Laskar SG, et al. Radiation dermatitis: an overview. Indian J Burns. 2013;21:24-31. doi:10.4103/0971-653x.121877
- Spalek M. Chronic radiation-induced dermatitis: challenges and solutions. Clin Cosmet Investig Dermatol. 2016;9:473-482. doi:10.2147/CCID.S94320
- Bourgeois JF, Gourgou S, Kramar A, et al. A randomized, prospective study using the LPG technique in treating radiation-induced skin fibrosis: clinical and profilometric analysis. Skin Res Technol. 2008;14:71-76. doi:10.1111/j.1600-0846.2007.00263.x
- Borrelli MR, Shen AH, Lee GK, et al. Radiation-induced skinfibrosis: pathogenesis, current treatment options, and emerging therapeutics. Ann Plast Surg. 2019;83(4S Suppl 1):S59-S64. doi:10.1097/SAP.0000000000002098
- Wilson B, Shah R, Menzer C, et al. Laser therapy as a treatment for chronic radiation fibrosis. Lasers Surg Med. 2023;55:82-88. doi:10.1002/lsm.23617
- Rigotti G, Marchi A, Galiè M, et al. Clinical treatment of radiotherapy tissue damage by lipoaspirate transplant: a healing process mediated by adipose-derived adult stem cells. Plast Reconstr Surg. 2007;119:1409-1422. doi:10.1097/01.prs.0000256047.47909.71
- Leventhal J, Young MR. Radiation dermatitis: recognition, prevention, and management. Oncology (Williston Park). 2017;31:885-899.
- van Vloten WA, Hermans J, van Daal WA. Radiation-induced skin cancer and radiodermatitis of the head and neck. Cancer. 1987;59:411-414. doi:10.1002/1097-0142(19870201)59:3<411::aid-cncr2820590310>3.0.co;2-z
- Davis MM, Hanke CW, Zollinger TW, et al. Skin cancer in patients with chronic radiation dermatitis. J Am Acad Dermatol. 1989;20:608-616. doi:10.1016/s0190-9622(89)70072-4
- Miller DL, Balter S, Schueler BA, et al. Clinical radiation management for fluoroscopically guided interventional procedures. Radiology. 2010;257:321-332. doi:10.1148/radiol.10091269
Fluoroscopy-Induced Chronic Radiation Dermatitis: A Comprehensive Review and Reappraisal
Fluoroscopy-Induced Chronic Radiation Dermatitis: A Comprehensive Review and Reappraisal
PRACTICE POINTS
- Fluoroscopy-induced chronic radiation dermatitis poses diagnostic challenges, as patients often are unable to associate a history of fluoroscopic procedures with the development of skin lesions.
- Scapular and subscapular lesions as well as those on the anterolateral chest and mid back should prompt clinicians to inquire about the patient’s history of fluoroscopic procedures.
- Because lesions can remain refractory to treatment, longterm monitoring is necessary if they are not excised.
Consider Cultural Practices and Barriers to Care When Treating Alopecia Areata
Consider Cultural Practices and Barriers to Care When Treating Alopecia Areata
The Comparison
A. Alopecia areata in a young girl with a lighter skin tone. The fine white vellus hairs are signs of regrowth.
B. Alopecia areata in a 49-year-old man with tightly coiled hair and darker skin tone. Coiled white hairs are noted in the alopecia patches.
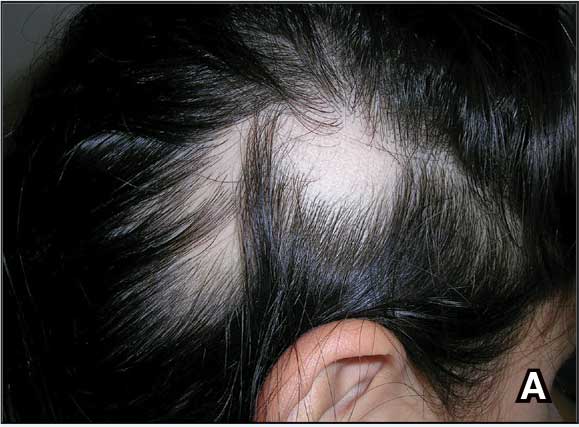
young girl with a lighter skin
tone. The fine white vellus
hairs are signs of regrowth. Photographs courtesy of
Richard P. Usatine, MD.

49-year-old man with tightly
coiled hair and darker skin
tone. Coiled white hairs
are noted in the alopecia
patches. Photographs courtesy of
Richard P. Usatine, MD.
Alopecia areata (AA) is a common autoimmune condition characterized by hair loss resulting from a T cell–mediated attack on the hair follicles. It manifests as nonscarring patches of hair loss on the scalp, eyebrows, eyelashes, and beard area as well as more extensive complete loss of scalp and body hair. While AA may affect individuals of any age, most patients develop their first patch(es) of hair loss during childhood.1 The treatment landscape for AA has evolved considerably in recent years, but barriers to access to newer treatments persist.
Epidemiology
AA is most prevalent among pediatric and adult individuals of African, Asian, or Hispanic/Latino descent.2-4 In some studies, Black individuals had higher odds and Asian individuals had lower odds of developing AA, while other studies have reported the highest standardized prevalence among Asian individuals.5 In the United States, AA affects about 1.47% of adults and as many as 0.11% of children.6-8 In Black patients, AA often manifests early with a female predominance.5
AA frequently is associated with autoimmune comorbidities, the most common being thyroid disease.3,5 In Black patients, AA is associated with more atopic comorbidities, including asthma, atopic dermatitis, and allergic rhinitis.5
Key Clinical Features
AA clinically manifests similarly across different skin tones; however, in patients with more tightly coiled or curly hair, the extent of scalp hair loss may be underestimated without a full examination. Culturally sensitive approaches to hair and scalp evaluation are essential, especially for Black women, whose hair care practices and scalp conditions may be overlooked or misunderstood during visits to evaluate hair loss. A thoughtful history and gentle examination of the hair and scalp that considers hair texture, cultural practices such as head coverings (eg, headwraps, turbans, hijabs), use of hair adornments (eg, clips, beads, bows), traditional braiding, and use of natural oils or herbal treatments, as well as styling methods including tight hairstyles, use of heat styling tools (eg, flat irons, curling irons), chemical application (eg, straighteners, hair color), and washing or styling frequency can improve diagnostic accuracy and help build trust in the patient-provider relationship.
Classic signs of AA visualized with dermoscopy include yellow and/or black dots on the scalp and exclamation point hairs. The appearance of fine white vellus hairs within the alopecic patches also may indicate early regrowth. On scalp trichoscopy, black dots are more prominent, and yellow dots are less prominent, in individuals with darker skin tones vs lighter skin tones.9
Worth Noting
In addition to a full examination of the scalp, documenting the extent of hair loss using validated severity scales, including the severity of alopecia tool (SALT), AA severity index (AASI), clinician-reported outcome assessment, and patient-reported outcome measures, can standardize disease severity assessment, facilitate timely insurance or medication approvals, and support objective tracking of treatment response, which may ultimately enhance access to care.10
Prompt treatment of AA is essential. Not surprisingly, patients given a diagnosis of AA may experience considerable emotional and psychological distress—regardless of the extent of the loss.11 Treatment options include mid- to high-potency topical or intralesional corticosteroids and newer and more targeted systemic options, including 3 Janus kinase (JAK) inhibitors—baricitinib, ritlecitinib, and deuruxolitinib—for more extensive disease.12 Treatment with intralesional corticosteroids may cause transient hypopigmentation, which may be more noticeable in patients with darker skin tones. Delays in treatment with JAK inhibitors can lead to a less-than-optimal response. Of the 3 JAK inhibitors that are approved by the US Food and Drug Administration for AA, only ritlecitinib is approved for children 12 years and older, leaving a therapeutic gap for younger patients that often leads to uncomfortable scalp injections, delayed or no treatment, off-label use of JAK inhibitors as well as the pairing of off-label dupilumab with oral minoxidil.12
Based on adult data, patients with severe disease and a shorter duration of hair loss (ie, < 4 years) tend to respond better to JAK inhibitors than those experiencing hair loss for longer periods. Also, those with more severe AA tend to have poorer outcomes than those with less severe disease.13 If treatment proves less than optimal, wigs and hair pieces may need to be considered. It is worth noting that some insurance companies will cover the cost of wigs for patients when prescribed as cranial prostheses.
Health Disparity Highlight
Health disparities in AA can be influenced by socioeconomic status and access to care. Patients from lower-income backgrounds often face barriers to accessing dermatologic care and treatments such as JAK inhibitors, which may remain inaccessible due to high costs and insurance limitations.14 These barriers can intersect with other factors such as age, sex, and race, potentially exacerbating disparities. Women with skin of color in underserved communities may experience delayed diagnosis, limited treatment options, and greater psychosocial distress from hair loss.14 Addressing these inequities requires advocacy, education for both patients and clinicians, and improved access to treatment to ensure comprehensive care for all patients.
- Kara T, Topkarcı Z. Interactions between posttraumatic stress disorder and alopecia areata in child with trauma exposure: two case reports. Int J Trichology. 2018;10:131-134. doi:10.4103/ijt.ijt_2_18
- Sy N, Mastacouris N, Strunk A, et al. Overall and racial and ethnic subgroup prevalences of alopecia areata, alopecia totalis, and alopecia universalis. JAMA Dermatol. 2023;159:419-423.
- Lee H, Jung SJ, Patel AB, et al. Racial characteristics of alopecia areata in the United States. J Am Acad Dermatol. 2020;83:1064-1070.
- Feaster B, McMichael AJ. Epidemiology of alopecia areata in Black patients: a retrospective chart review. J Am Acad Dermatol. 2022;87:1121-1123.
- Lee HH, Gwillim E, Patel KR, et al. Epidemiology of alopecia areata, ophiasis, totalis, and universalis: a systematic review and meta-analysis. J Am Acad Dermatol. 2020;82:675-682.
- Mostaghimi A, Gao W, Ray M, et al. Trends in prevalence and incidence of alopecia areata, alopecia totalis, and alopecia universalis among adults and children in a US employer-sponsored insured population. JAMA Dermatol. 2023;159:411-418.
- Adhanom R, Ansbro B, Castelo-Soccio L. Epidemiology of pediatric alopecia areata. Pediatr Dermatol. 2025;42 suppl 1(suppl 1):12-23.
- Karampinis E, Toli O, Georgopoulou KE, et al. Exploring pediatric dermatology in skin of color: focus on dermoscopy. Life (Basel). 2024;14:1604.
- King BA, Senna MM, Ohyama M, et al. Defining severity in alopecia areata: current perspectives and a multidimensional framework. Dermatol Ther (Heidelb). 2022;12:825-834.
- Toussi A, Barton VR, Le ST, et al. Psychosocial and psychiatric comorbidities and health-related quality of life in alopecia areata: a systematic review. J Am Acad Dermatol. 2021;85:162-175.
- Kalil L, Welch D, Heath CR, et al. Systemic therapies for pediatric alopecia areata. Pediatr Dermatol. 2025;42 suppl 1:36-42.
- King BA, Craiglow BG. Janus kinase inhibitors for alopecia areata. J Am Acad Dermatol. 2023;89:S29-S32.
- Klein EJ, Taiwò D, Kakpovbia E, et al. Disparities in Janus kinase inhibitor access for alopecia areata: a retrospective analysis. Int J Womens Dermatol. 2024;10:E155.
- McKenzie PL, Maltenfort M, Bruckner AL, et al. Evaluation of the prevalence and incidence of pediatric alopecia areata using electronic health record data. JAMA Dermatol. 2022;158:547-551. doi:10.1001/jamadermatol.2022.0351
The Comparison
A. Alopecia areata in a young girl with a lighter skin tone. The fine white vellus hairs are signs of regrowth.
B. Alopecia areata in a 49-year-old man with tightly coiled hair and darker skin tone. Coiled white hairs are noted in the alopecia patches.

young girl with a lighter skin
tone. The fine white vellus
hairs are signs of regrowth. Photographs courtesy of
Richard P. Usatine, MD.

49-year-old man with tightly
coiled hair and darker skin
tone. Coiled white hairs
are noted in the alopecia
patches. Photographs courtesy of
Richard P. Usatine, MD.
Alopecia areata (AA) is a common autoimmune condition characterized by hair loss resulting from a T cell–mediated attack on the hair follicles. It manifests as nonscarring patches of hair loss on the scalp, eyebrows, eyelashes, and beard area as well as more extensive complete loss of scalp and body hair. While AA may affect individuals of any age, most patients develop their first patch(es) of hair loss during childhood.1 The treatment landscape for AA has evolved considerably in recent years, but barriers to access to newer treatments persist.
Epidemiology
AA is most prevalent among pediatric and adult individuals of African, Asian, or Hispanic/Latino descent.2-4 In some studies, Black individuals had higher odds and Asian individuals had lower odds of developing AA, while other studies have reported the highest standardized prevalence among Asian individuals.5 In the United States, AA affects about 1.47% of adults and as many as 0.11% of children.6-8 In Black patients, AA often manifests early with a female predominance.5
AA frequently is associated with autoimmune comorbidities, the most common being thyroid disease.3,5 In Black patients, AA is associated with more atopic comorbidities, including asthma, atopic dermatitis, and allergic rhinitis.5
Key Clinical Features
AA clinically manifests similarly across different skin tones; however, in patients with more tightly coiled or curly hair, the extent of scalp hair loss may be underestimated without a full examination. Culturally sensitive approaches to hair and scalp evaluation are essential, especially for Black women, whose hair care practices and scalp conditions may be overlooked or misunderstood during visits to evaluate hair loss. A thoughtful history and gentle examination of the hair and scalp that considers hair texture, cultural practices such as head coverings (eg, headwraps, turbans, hijabs), use of hair adornments (eg, clips, beads, bows), traditional braiding, and use of natural oils or herbal treatments, as well as styling methods including tight hairstyles, use of heat styling tools (eg, flat irons, curling irons), chemical application (eg, straighteners, hair color), and washing or styling frequency can improve diagnostic accuracy and help build trust in the patient-provider relationship.
Classic signs of AA visualized with dermoscopy include yellow and/or black dots on the scalp and exclamation point hairs. The appearance of fine white vellus hairs within the alopecic patches also may indicate early regrowth. On scalp trichoscopy, black dots are more prominent, and yellow dots are less prominent, in individuals with darker skin tones vs lighter skin tones.9
Worth Noting
In addition to a full examination of the scalp, documenting the extent of hair loss using validated severity scales, including the severity of alopecia tool (SALT), AA severity index (AASI), clinician-reported outcome assessment, and patient-reported outcome measures, can standardize disease severity assessment, facilitate timely insurance or medication approvals, and support objective tracking of treatment response, which may ultimately enhance access to care.10
Prompt treatment of AA is essential. Not surprisingly, patients given a diagnosis of AA may experience considerable emotional and psychological distress—regardless of the extent of the loss.11 Treatment options include mid- to high-potency topical or intralesional corticosteroids and newer and more targeted systemic options, including 3 Janus kinase (JAK) inhibitors—baricitinib, ritlecitinib, and deuruxolitinib—for more extensive disease.12 Treatment with intralesional corticosteroids may cause transient hypopigmentation, which may be more noticeable in patients with darker skin tones. Delays in treatment with JAK inhibitors can lead to a less-than-optimal response. Of the 3 JAK inhibitors that are approved by the US Food and Drug Administration for AA, only ritlecitinib is approved for children 12 years and older, leaving a therapeutic gap for younger patients that often leads to uncomfortable scalp injections, delayed or no treatment, off-label use of JAK inhibitors as well as the pairing of off-label dupilumab with oral minoxidil.12
Based on adult data, patients with severe disease and a shorter duration of hair loss (ie, < 4 years) tend to respond better to JAK inhibitors than those experiencing hair loss for longer periods. Also, those with more severe AA tend to have poorer outcomes than those with less severe disease.13 If treatment proves less than optimal, wigs and hair pieces may need to be considered. It is worth noting that some insurance companies will cover the cost of wigs for patients when prescribed as cranial prostheses.
Health Disparity Highlight
Health disparities in AA can be influenced by socioeconomic status and access to care. Patients from lower-income backgrounds often face barriers to accessing dermatologic care and treatments such as JAK inhibitors, which may remain inaccessible due to high costs and insurance limitations.14 These barriers can intersect with other factors such as age, sex, and race, potentially exacerbating disparities. Women with skin of color in underserved communities may experience delayed diagnosis, limited treatment options, and greater psychosocial distress from hair loss.14 Addressing these inequities requires advocacy, education for both patients and clinicians, and improved access to treatment to ensure comprehensive care for all patients.
The Comparison
A. Alopecia areata in a young girl with a lighter skin tone. The fine white vellus hairs are signs of regrowth.
B. Alopecia areata in a 49-year-old man with tightly coiled hair and darker skin tone. Coiled white hairs are noted in the alopecia patches.

young girl with a lighter skin
tone. The fine white vellus
hairs are signs of regrowth. Photographs courtesy of
Richard P. Usatine, MD.

49-year-old man with tightly
coiled hair and darker skin
tone. Coiled white hairs
are noted in the alopecia
patches. Photographs courtesy of
Richard P. Usatine, MD.
Alopecia areata (AA) is a common autoimmune condition characterized by hair loss resulting from a T cell–mediated attack on the hair follicles. It manifests as nonscarring patches of hair loss on the scalp, eyebrows, eyelashes, and beard area as well as more extensive complete loss of scalp and body hair. While AA may affect individuals of any age, most patients develop their first patch(es) of hair loss during childhood.1 The treatment landscape for AA has evolved considerably in recent years, but barriers to access to newer treatments persist.
Epidemiology
AA is most prevalent among pediatric and adult individuals of African, Asian, or Hispanic/Latino descent.2-4 In some studies, Black individuals had higher odds and Asian individuals had lower odds of developing AA, while other studies have reported the highest standardized prevalence among Asian individuals.5 In the United States, AA affects about 1.47% of adults and as many as 0.11% of children.6-8 In Black patients, AA often manifests early with a female predominance.5
AA frequently is associated with autoimmune comorbidities, the most common being thyroid disease.3,5 In Black patients, AA is associated with more atopic comorbidities, including asthma, atopic dermatitis, and allergic rhinitis.5
Key Clinical Features
AA clinically manifests similarly across different skin tones; however, in patients with more tightly coiled or curly hair, the extent of scalp hair loss may be underestimated without a full examination. Culturally sensitive approaches to hair and scalp evaluation are essential, especially for Black women, whose hair care practices and scalp conditions may be overlooked or misunderstood during visits to evaluate hair loss. A thoughtful history and gentle examination of the hair and scalp that considers hair texture, cultural practices such as head coverings (eg, headwraps, turbans, hijabs), use of hair adornments (eg, clips, beads, bows), traditional braiding, and use of natural oils or herbal treatments, as well as styling methods including tight hairstyles, use of heat styling tools (eg, flat irons, curling irons), chemical application (eg, straighteners, hair color), and washing or styling frequency can improve diagnostic accuracy and help build trust in the patient-provider relationship.
Classic signs of AA visualized with dermoscopy include yellow and/or black dots on the scalp and exclamation point hairs. The appearance of fine white vellus hairs within the alopecic patches also may indicate early regrowth. On scalp trichoscopy, black dots are more prominent, and yellow dots are less prominent, in individuals with darker skin tones vs lighter skin tones.9
Worth Noting
In addition to a full examination of the scalp, documenting the extent of hair loss using validated severity scales, including the severity of alopecia tool (SALT), AA severity index (AASI), clinician-reported outcome assessment, and patient-reported outcome measures, can standardize disease severity assessment, facilitate timely insurance or medication approvals, and support objective tracking of treatment response, which may ultimately enhance access to care.10
Prompt treatment of AA is essential. Not surprisingly, patients given a diagnosis of AA may experience considerable emotional and psychological distress—regardless of the extent of the loss.11 Treatment options include mid- to high-potency topical or intralesional corticosteroids and newer and more targeted systemic options, including 3 Janus kinase (JAK) inhibitors—baricitinib, ritlecitinib, and deuruxolitinib—for more extensive disease.12 Treatment with intralesional corticosteroids may cause transient hypopigmentation, which may be more noticeable in patients with darker skin tones. Delays in treatment with JAK inhibitors can lead to a less-than-optimal response. Of the 3 JAK inhibitors that are approved by the US Food and Drug Administration for AA, only ritlecitinib is approved for children 12 years and older, leaving a therapeutic gap for younger patients that often leads to uncomfortable scalp injections, delayed or no treatment, off-label use of JAK inhibitors as well as the pairing of off-label dupilumab with oral minoxidil.12
Based on adult data, patients with severe disease and a shorter duration of hair loss (ie, < 4 years) tend to respond better to JAK inhibitors than those experiencing hair loss for longer periods. Also, those with more severe AA tend to have poorer outcomes than those with less severe disease.13 If treatment proves less than optimal, wigs and hair pieces may need to be considered. It is worth noting that some insurance companies will cover the cost of wigs for patients when prescribed as cranial prostheses.
Health Disparity Highlight
Health disparities in AA can be influenced by socioeconomic status and access to care. Patients from lower-income backgrounds often face barriers to accessing dermatologic care and treatments such as JAK inhibitors, which may remain inaccessible due to high costs and insurance limitations.14 These barriers can intersect with other factors such as age, sex, and race, potentially exacerbating disparities. Women with skin of color in underserved communities may experience delayed diagnosis, limited treatment options, and greater psychosocial distress from hair loss.14 Addressing these inequities requires advocacy, education for both patients and clinicians, and improved access to treatment to ensure comprehensive care for all patients.
- Kara T, Topkarcı Z. Interactions between posttraumatic stress disorder and alopecia areata in child with trauma exposure: two case reports. Int J Trichology. 2018;10:131-134. doi:10.4103/ijt.ijt_2_18
- Sy N, Mastacouris N, Strunk A, et al. Overall and racial and ethnic subgroup prevalences of alopecia areata, alopecia totalis, and alopecia universalis. JAMA Dermatol. 2023;159:419-423.
- Lee H, Jung SJ, Patel AB, et al. Racial characteristics of alopecia areata in the United States. J Am Acad Dermatol. 2020;83:1064-1070.
- Feaster B, McMichael AJ. Epidemiology of alopecia areata in Black patients: a retrospective chart review. J Am Acad Dermatol. 2022;87:1121-1123.
- Lee HH, Gwillim E, Patel KR, et al. Epidemiology of alopecia areata, ophiasis, totalis, and universalis: a systematic review and meta-analysis. J Am Acad Dermatol. 2020;82:675-682.
- Mostaghimi A, Gao W, Ray M, et al. Trends in prevalence and incidence of alopecia areata, alopecia totalis, and alopecia universalis among adults and children in a US employer-sponsored insured population. JAMA Dermatol. 2023;159:411-418.
- Adhanom R, Ansbro B, Castelo-Soccio L. Epidemiology of pediatric alopecia areata. Pediatr Dermatol. 2025;42 suppl 1(suppl 1):12-23.
- Karampinis E, Toli O, Georgopoulou KE, et al. Exploring pediatric dermatology in skin of color: focus on dermoscopy. Life (Basel). 2024;14:1604.
- King BA, Senna MM, Ohyama M, et al. Defining severity in alopecia areata: current perspectives and a multidimensional framework. Dermatol Ther (Heidelb). 2022;12:825-834.
- Toussi A, Barton VR, Le ST, et al. Psychosocial and psychiatric comorbidities and health-related quality of life in alopecia areata: a systematic review. J Am Acad Dermatol. 2021;85:162-175.
- Kalil L, Welch D, Heath CR, et al. Systemic therapies for pediatric alopecia areata. Pediatr Dermatol. 2025;42 suppl 1:36-42.
- King BA, Craiglow BG. Janus kinase inhibitors for alopecia areata. J Am Acad Dermatol. 2023;89:S29-S32.
- Klein EJ, Taiwò D, Kakpovbia E, et al. Disparities in Janus kinase inhibitor access for alopecia areata: a retrospective analysis. Int J Womens Dermatol. 2024;10:E155.
- McKenzie PL, Maltenfort M, Bruckner AL, et al. Evaluation of the prevalence and incidence of pediatric alopecia areata using electronic health record data. JAMA Dermatol. 2022;158:547-551. doi:10.1001/jamadermatol.2022.0351
- Kara T, Topkarcı Z. Interactions between posttraumatic stress disorder and alopecia areata in child with trauma exposure: two case reports. Int J Trichology. 2018;10:131-134. doi:10.4103/ijt.ijt_2_18
- Sy N, Mastacouris N, Strunk A, et al. Overall and racial and ethnic subgroup prevalences of alopecia areata, alopecia totalis, and alopecia universalis. JAMA Dermatol. 2023;159:419-423.
- Lee H, Jung SJ, Patel AB, et al. Racial characteristics of alopecia areata in the United States. J Am Acad Dermatol. 2020;83:1064-1070.
- Feaster B, McMichael AJ. Epidemiology of alopecia areata in Black patients: a retrospective chart review. J Am Acad Dermatol. 2022;87:1121-1123.
- Lee HH, Gwillim E, Patel KR, et al. Epidemiology of alopecia areata, ophiasis, totalis, and universalis: a systematic review and meta-analysis. J Am Acad Dermatol. 2020;82:675-682.
- Mostaghimi A, Gao W, Ray M, et al. Trends in prevalence and incidence of alopecia areata, alopecia totalis, and alopecia universalis among adults and children in a US employer-sponsored insured population. JAMA Dermatol. 2023;159:411-418.
- Adhanom R, Ansbro B, Castelo-Soccio L. Epidemiology of pediatric alopecia areata. Pediatr Dermatol. 2025;42 suppl 1(suppl 1):12-23.
- Karampinis E, Toli O, Georgopoulou KE, et al. Exploring pediatric dermatology in skin of color: focus on dermoscopy. Life (Basel). 2024;14:1604.
- King BA, Senna MM, Ohyama M, et al. Defining severity in alopecia areata: current perspectives and a multidimensional framework. Dermatol Ther (Heidelb). 2022;12:825-834.
- Toussi A, Barton VR, Le ST, et al. Psychosocial and psychiatric comorbidities and health-related quality of life in alopecia areata: a systematic review. J Am Acad Dermatol. 2021;85:162-175.
- Kalil L, Welch D, Heath CR, et al. Systemic therapies for pediatric alopecia areata. Pediatr Dermatol. 2025;42 suppl 1:36-42.
- King BA, Craiglow BG. Janus kinase inhibitors for alopecia areata. J Am Acad Dermatol. 2023;89:S29-S32.
- Klein EJ, Taiwò D, Kakpovbia E, et al. Disparities in Janus kinase inhibitor access for alopecia areata: a retrospective analysis. Int J Womens Dermatol. 2024;10:E155.
- McKenzie PL, Maltenfort M, Bruckner AL, et al. Evaluation of the prevalence and incidence of pediatric alopecia areata using electronic health record data. JAMA Dermatol. 2022;158:547-551. doi:10.1001/jamadermatol.2022.0351
Consider Cultural Practices and Barriers to Care When Treating Alopecia Areata
Consider Cultural Practices and Barriers to Care When Treating Alopecia Areata
Enhancing Patient Satisfaction and Quality of Life With Mohs Micrographic Surgery: A Systematic Review of Patient Education, Communication, and Anxiety Management
Enhancing Patient Satisfaction and Quality of Life With Mohs Micrographic Surgery: A Systematic Review of Patient Education, Communication, and Anxiety Management
Mohs micrographic surgery (MMS)—developed by Dr. Frederic Mohs in the 1930s—is the gold standard for treating various cutaneous malignancies. It provides maximal conservation of uninvolved tissues while producing higher cure rates compared to wide local excision.1,2
We sought to assess the various characteristics that impact patient satisfaction to help Mohs surgeons incorporate relatively simple yet clinically significant practices into their patient encounters. We conducted a systematic literature search of peer-reviewed PubMed articles indexed for MEDLINE from database inception through November 2023 using the terms Mohs micrographic surgery and patient satisfaction. Among the inclusion criteria were studies involving participants having undergone MMS, with objective assessments on patient-reported satisfaction or preferences related to patient education, communication, anxiety-alleviating measures, or QOL in MMS. Studies were excluded if they failed to meet these criteria, were outdated and no longer clinically relevant, or measured unalterable factors with no significant impact on how Mohs surgeons could change clinical practice. Of the 157 nonreplicated studies identified, 34 met inclusion criteria.
Perioperative Patient Communication and Education Techniques
Perioperative Patient Communication—Many studies have evaluated the impact of perioperative patient-provider communication and education on patient satisfaction in those undergoing MMS. Studies focusing on preoperative and postoperative telephone calls, patient consultation formats, and patient-perceived impact of such communication modalities have been well documented (Table 1).3-8 The importance of the patient follow-up after MMS was further supported by a retrospective study concluding that 88.7% (86/97) of patients regarded follow-up visits as important, and 80% (77/97) desired additional follow-up 3 months after MMS.9 Additional studies have highlighted the importance of thorough and open perioperative patient-provider communication during MMS (Table 2).10-12
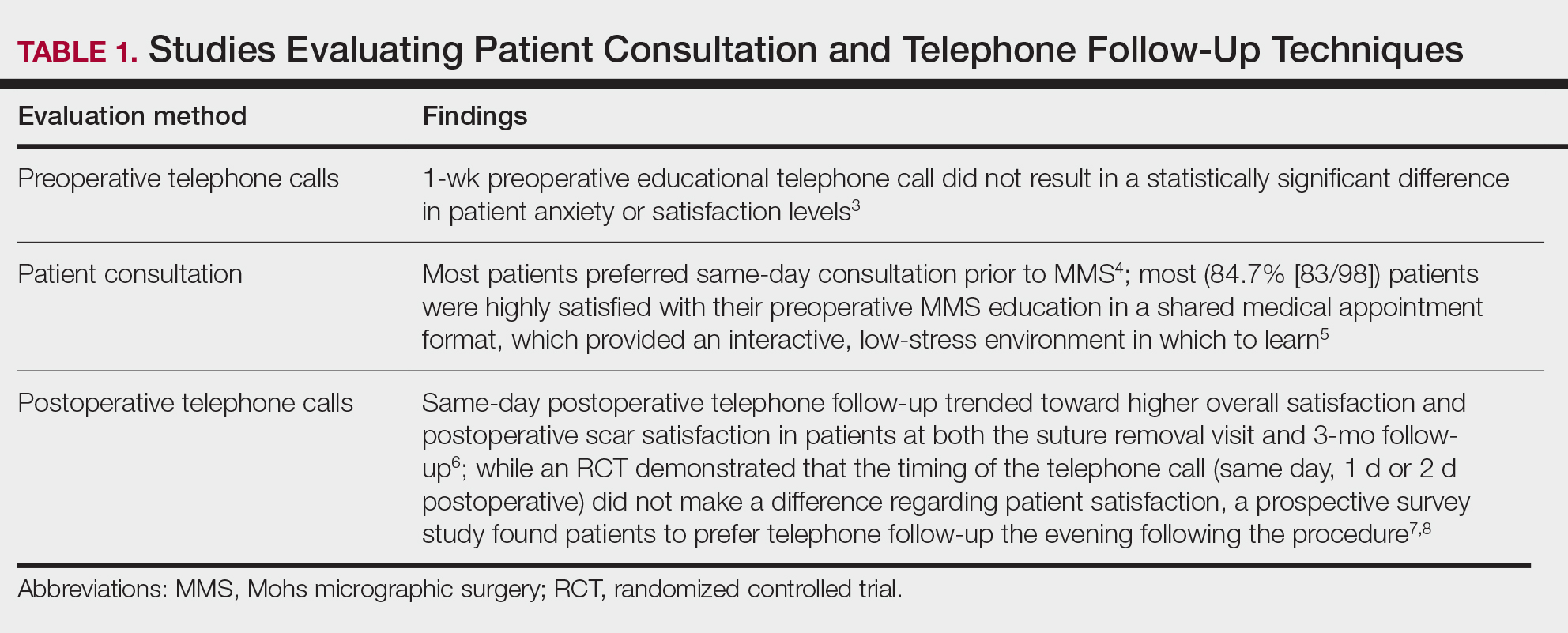
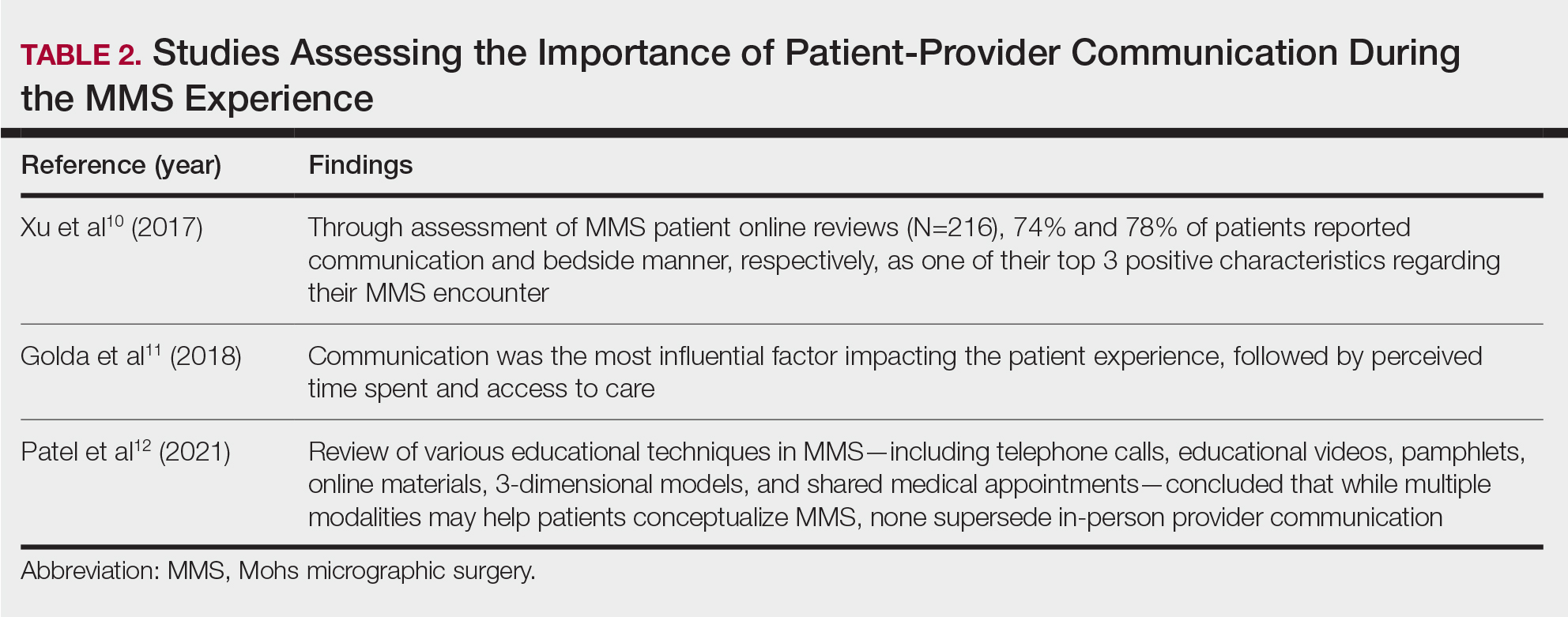
Patient-Education Techniques—Many studies have assessed the use of visual models to aid in patient education on MMS, specifically the preprocedural consent process (Table 3).13-16 Additionally, 2 randomized controlled trials assessing the use of at-home and same-day in-office preoperative educational videos concluded that these interventions increased patient knowledge and confidence regarding procedural risks and benefits, with no statistically significant differences in patient anxiety or satisfaction.17,18
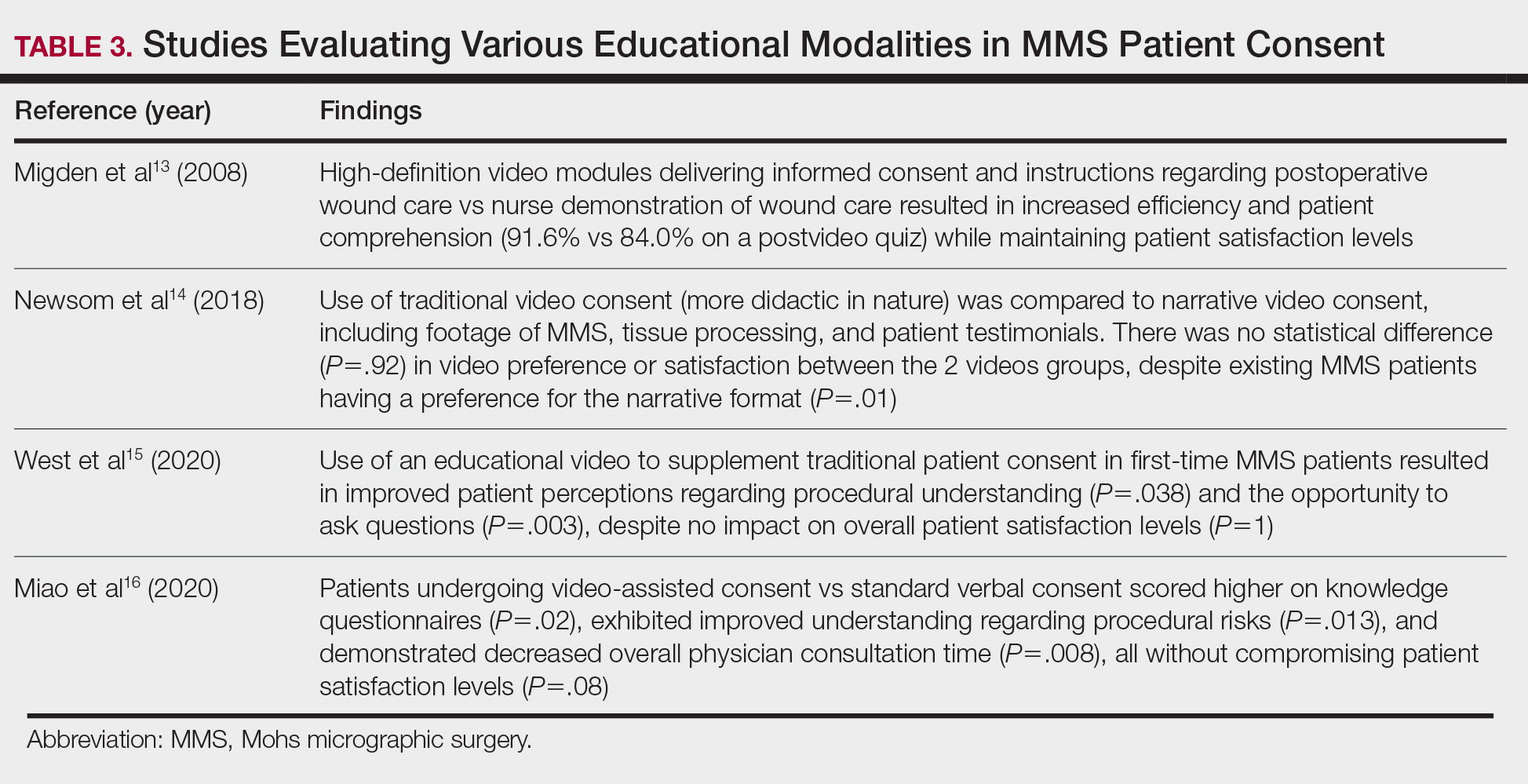
Despite the availability of these educational videos, many patients often turn to online resources for self-education, which is problematic if reader literacy is incongruent with online readability. One study assessing readability of online MMS resources concluded that the most accessed articles exceeded the recommended reading level for adequate patient comprehension.19 A survey studying a wide range of variables related to patient satisfaction (eg, demographics, socioeconomics, health status) in 339 MMS patients found that those who considered themselves more involved in the decision-making process were more satisfied in the short-term, and married patients had even higher long-term satisfaction. Interestingly, this study also concluded that undergoing 3 or more MMS stages was associated with higher short- and long-term satisfaction, likely secondary to perceived effects of increased overall care, medical attention, and time spent with the provider.20
Synthesis of this information with emphasis on the higher evidence-based studies—including systematic reviews, meta-analyses, and randomized controlled trials—yields the following beneficial interventions regarding patient education and communication13-20:
- Preoperative and same-day postoperative telephone follow-up (TFU) do not show statistically significant impacts on patient satisfaction; however, TFU allows for identification of postoperative concerns and inadequate pain management, which may have downstream effects on long-term perception of the overall patient experience.
- The use of video-assisted consent yields improved patient satisfaction and knowledge, while video content—traditional or didactic—has no impact on satisfaction in new MMS patients.
- The use of at-home or same-day in-office preoperative educational videos can improve procedural knowledge and risk-benefit understanding of MMS while having no impact on satisfaction.
- Bedside manner and effective in-person communication by the provider often takes precedence in the patient experience; however, implementation of additional educational modalities should be considered.
Patient Anxiety and QOL
Reducing Patient Anxiety—The use of perioperative distractors to reduce patient anxiety may play an integral role when patients undergo MMS, as there often are prolonged waiting periods between stages when patients may feel increasingly vulnerable or anxious. Table 4 reviews studies on perioperative distractors that showed a statistically significant reduction in MMS patient anxiety.21-24
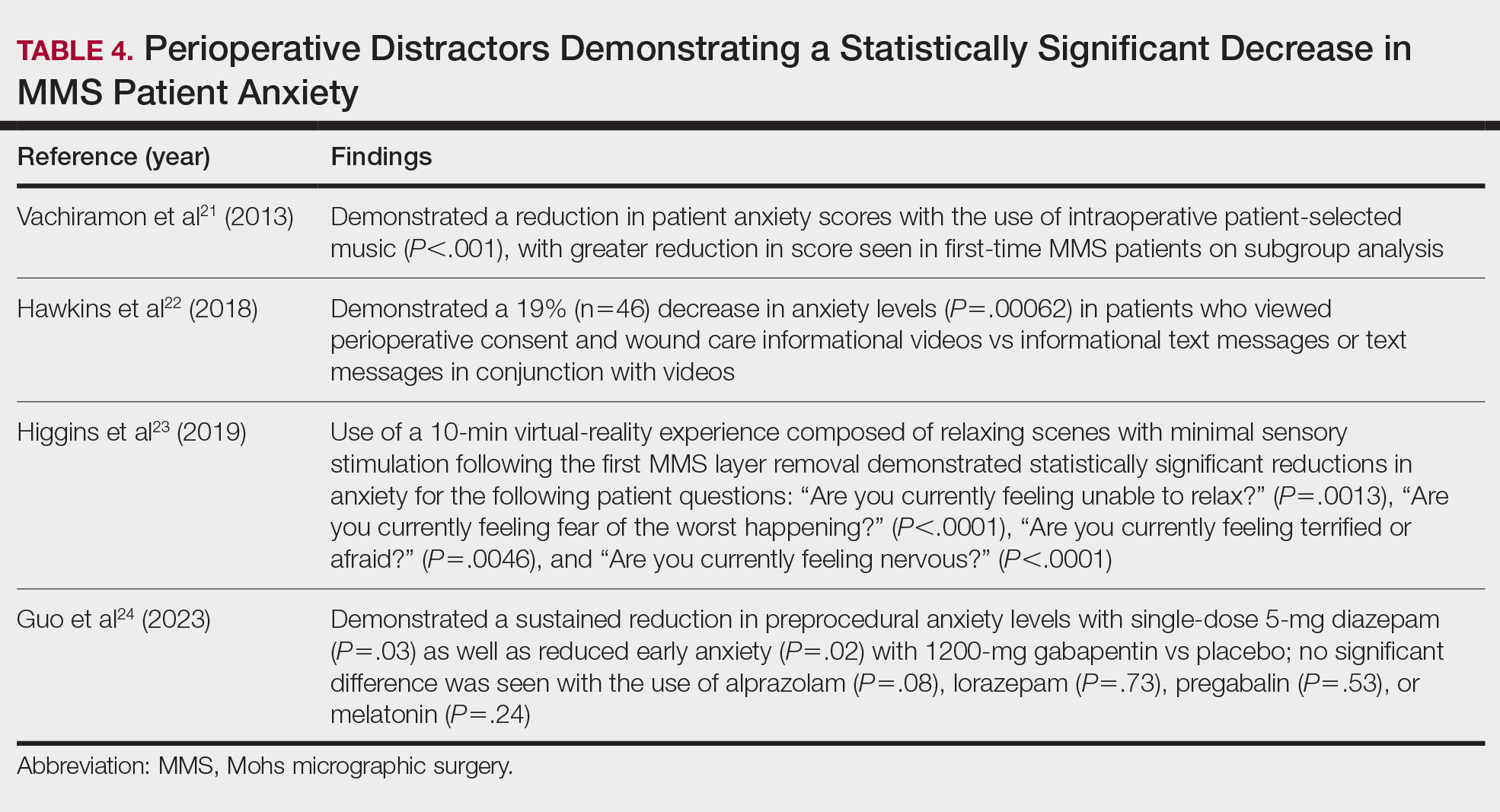
Although not statistically significant, additional studies evaluating the use of intraoperative anxiety-reduction methods in MMS have demonstrated a downtrend in patient anxiety with the following interventions: engaging in small talk with clinic staff, bringing a guest, eating, watching television, communicating surgical expectations with the provider, handholding, use of a stress ball, and use of 3-dimensional educational MMS models.25-27 Similarly, a survey of 73 patients undergoing MMS found that patients tended to enjoy complimentary beverages preprocedurally in the waiting room, reading, speaking with their guest, watching television, or using their telephone during wait times.28 Table 5 lists additional perioperative factors encompassing specific patient and surgical characteristics that help reduce patient anxiety.29-32
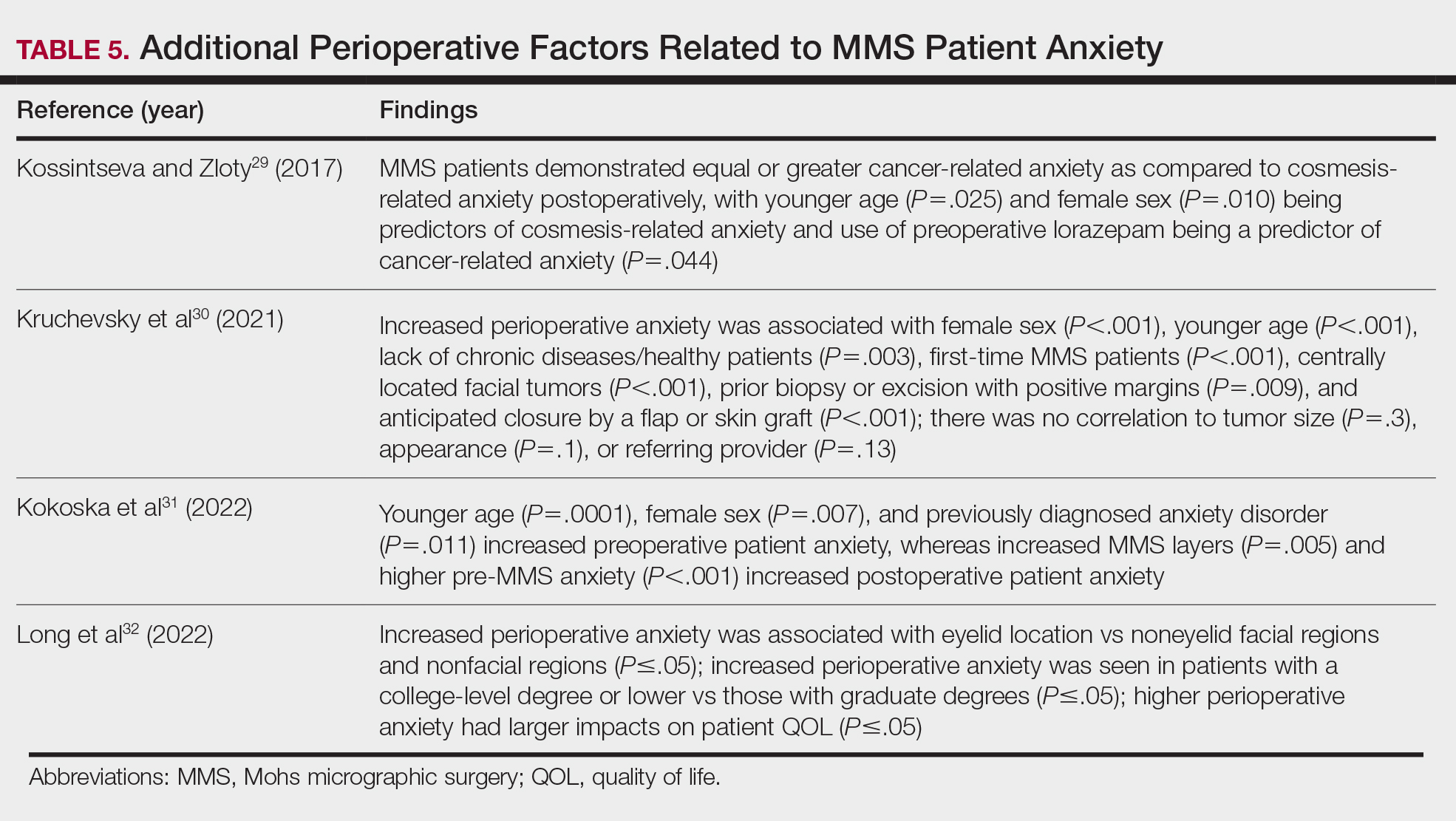
Patient QOL—Many methods aimed at decreasing MMS-related patient anxiety often show no direct impact on patient satisfaction, likely due to the multifactorial nature of the patient-perceived experience. A prospective observational study of MMS patients noted a statistically significant improvement in patient QOL scores 3 months postsurgery (P=.0007), demonstrating that MMS generally results in positive patient outcomes despite preprocedural anxiety.33 An additional prospective study in MMS patients with nonmelanoma skin cancer concluded that sex, age, and closure type—factors often shown to affect anxiety levels—did not significantly impact patient satisfaction.34 Similarly, high satisfaction levels can be expected among MMS patients undergoing treatment of melanoma in situ, with more than 90% of patients rating their treatment experience a 4 (agree) or 5 (strongly agree) out of 5 in short- and long-term satisfaction assessments (38/41 and 40/42, respectively).35 This assessment, conducted 3 months postoperatively, asked patients to score the statement, “I am completely satisfied with the treatment of my skin problem,” on a scale ranging from 1 (strongly disagree) to 5 (strongly agree).
Lastly, patient perception of their surgeon’s skill may contribute to levels of patient satisfaction. Although suture spacing has not been shown to affect surgical outcomes, it has been demonstrated to impact the patient’s perception of surgical skill and is further supported by a study concluding that closures with 2-mm spacing were ranked significantly lower by patients compared with closures with either 4- or 6-mm spacing (P=.005 and P=.012, respectively).36
Synthesis of this information with emphasis on the higher evidence-based studies—including systematic reviews, meta-analyses, and randomized controlled trials—yields the following beneficial interventions regarding anxiety-reducing measures and patient-perceived QOL21-36:
- Factors shown to decrease patient anxiety include patient personalized music, virtual-reality experience, perioperative informational videos, and 3-dimensional–printed MMS models.
- Many methods aimed at decreasing MMS-related patient anxiety show no direct impact on patient satisfaction, likely due to the multifactorial nature of the patient-perceived experience.
- Higher anxiety can be associated with worse QOL scores in MMS patients, and additional factors that may have a negative impact on anxiety include female sex, younger age, and tumor location on the face.
Conclusion
Many factors affect patient satisfaction in MMS. Increased awareness and acknowledgement of these factors can foster improved clinical practice and patient experience, which can have downstream effects on patient compliance and overall psychosocial and medical well-being. With the movement toward value-based health care, patient satisfaction ratings are likely to play an increasingly important role in physician reimbursement. Adapting one’s practice to include high-quality, time-efficient, patient-centered care goes hand in hand with increasing MMS patient satisfaction. Careful evaluation and scrutiny of one’s current practices while remaining cognizant of patient population, resource availability, and clinical limitations often reveal opportunities for small adjustments that can have a great impact on patient satisfaction. This thorough assessment and review of the published literature aims to assist MMS surgeons in understanding the role that certain factors—(1) perioperative patient communication and education techniques and (2) patient anxiety, QOL, and additional considerations—have on overall satisfaction with MMS. Specific consideration should be placed on the fact that patient satisfaction is multifactorial, and many different interventions can have a positive impact on the overall patient experience.
- Trost LB, Bailin PL. History of Mohs surgery. Dermatol Clin. 2011; 29:135-139, vii. doi:10.1016/j.det.2011.01.010
- Leslie DF, Greenway HT. Mohs micrographic surgery for skin cancer. Australas J Dermatol. 1991;32:159-164. doi:10.1111/j.1440 -0960.1991.tb01783.x
- Sobanko JF, Da Silva D, Chiesa Fuxench ZC, et al. Preoperative telephone consultation does not decrease patient anxiety before Mohs micrographic surgery. J Am Acad Dermatol. 2017;76:519-526. doi:10.1016/j.jaad.2016.09.027
- Sharon VR, Armstrong AW, Jim On SC, et al. Separate- versus same-day preoperative consultation in dermatologic surgery: a patient-centered investigation in an academic practice. Dermatol Surg. 2013;39:240-247. doi:10.1111/dsu.12083
- Knackstedt TJ, Samie FH. Shared medical appointments for the preoperative consultation visit of Mohs micrographic surgery. J Am Acad Dermatol. 2015;72:340-344. doi:10.1016/j.jaad.2014.10.022
- Vance S, Fontecilla N, Samie FH, et al. Effect of postoperative telephone calls on patient satisfaction and scar satisfaction after Mohs micrographic surgery. Dermatol Surg. 2019;45:1459-1464. doi:10.1097/DSS.0000000000001913
- Hafiji J, Salmon P, Hussain W. Patient satisfaction with post-operative telephone calls after Mohs micrographic surgery: a New Zealand and U.K. experience. Br J Dermatol. 2012;167:570-574. doi:10.1111 /j.1365-2133.2012.11011.x
- Bednarek R, Jonak C, Golda N. Optimal timing of postoperative patient telephone calls after Mohs micrographic surgery: a randomized controlled trial. J Am Acad Dermatol. 2021;85:220-221. doi:10.1016 /j.jaad.2020.07.106
- Sharon VR, Armstrong AW, Jim-On S, et al. Postoperative preferences in cutaneous surgery: a patient-centered investigation from an academic dermatologic surgery practice. Dermatol Surg. 2013;39:773-778. doi:10.1111/dsu.12136
- Xu S, Atanelov Z, Bhatia AC. Online patient-reported reviews of Mohs micrographic surgery: qualitative analysis of positive and negative experiences. Cutis. 2017;99:E25-E29.
- Golda N, Beeson S, Kohli N, et al. Recommendations for improving the patient experience in specialty encounters. J Am Acad Dermatol. 2018;78:653-659. doi:10.1016/j.jaad.2017.05.040
- Patel P, Malik K, Khachemoune A. Patient education in Mohs surgery: a review and critical evaluation of techniques. Arch Dermatol Res. 2021;313:217-224. doi:10.1007/s00403-020-02119-5
- Migden M, Chavez-Frazier A, Nguyen T. The use of high definition video modules for delivery of informed consent and wound care education in the Mohs surgery unit. Semin Cutan Med Surg. 2008;27:89-93. doi:10.1016/j.sder.2008.02.001
- Newsom E, Lee E, Rossi A, et al. Modernizing the Mohs surgery consultation: instituting a video module for improved patient education and satisfaction. Dermatol Surg. 2018;44:778-784. doi:10.1097/DSS.0000000000001473
- West L, Srivastava D, Goldberg LH, et al. Multimedia technology used to supplement patient consent for Mohs micrographic surgery. Dermatol Surg. 2020;46:586-590. doi:10.1097/DSS.0000000000002134
- Miao Y, Venning VL, Mallitt KA, et al. A randomized controlled trial comparing video-assisted informed consent with standard consent for Mohs micrographic surgery. JAAD Int. 2020;1:13-20. doi:10.1016 /j.jdin.2020.03.005
- Mann J, Li L, Kulakov E, et al. Home viewing of educational video improves patient understanding of Mohs micrographic surgery. Clin Exp Dermatol. 2022;47:93-97. doi:10.1111/ced.14845
- Delcambre M, Haynes D, Hajar T, et al. Using a multimedia tool for informed consent in Mohs surgery: a randomized trial measuring effects on patient anxiety, knowledge, and satisfaction. Dermatol Surg. 2020;46:591-598. doi:10.1097/DSS.0000000000002213
- Vargas CR, DePry J, Lee BT, et al. The readability of online patient information about Mohs micrographic surgery. Dermatol Surg. 2016;42:1135-1141. doi:10.1097/DSS.0000000000000866
- Asgari MM, Warton EM, Neugebauer R, et al. Predictors of patient satisfaction with Mohs surgery: analysis of preoperative, intraoperative, and postoperative factors in a prospective cohort. Arch Dermatol. 2011;147:1387-1394.
- Vachiramon V, Sobanko JF, Rattanaumpawan P, et al. Music reduces patient anxiety during Mohs surgery: an open-label randomized controlled trial. Dermatol Surg. 2013;39:298-305. doi:10.1111/dsu.12047
- Hawkins SD, Koch SB, Williford PM, et al. Web app- and text message-based patient education in Mohs micrographic surgery-a randomized controlled trial. Dermatol Surg. 2018;44:924-932. doi:10.1097/DSS.0000000000001489
- Higgins S, Feinstein S, Hawkins M, et al. Virtual reality to improve the experience of the Mohs patient-a prospective interventional study. Dermatol Surg. 2019;45:1009-1018. doi:10.1097 /DSS.0000000000001854
- Guo D, Zloty DM, Kossintseva I. Efficacy and safety of anxiolytics in Mohs micrographic surgery: a randomized, double-blinded, placebo-controlled trial. Dermatol Surg. 2023;49:989-994. doi:10.1097 /DSS.0000000000003905
- Locke MC, Wilkerson EC, Mistur RL, et al. 2015 Arte Poster Competition first place winner: assessing the correlation between patient anxiety and satisfaction for Mohs surgery. J Drugs Dermatol. 2015;14:1070-1072.
- Yanes AF, Weil A, Furlan KC, et al. Effect of stress ball use or hand-holding on anxiety during skin cancer excision: a randomized clinical trial. JAMA Dermatol. 2018;154:1045-1049. doi:10.1001 /jamadermatol.2018.1783
- Biro M, Kim I, Huynh A, et al. The use of 3-dimensionally printed models to optimize patient education and alleviate perioperative anxiety in Mohs micrographic surgery: a randomized controlled trial. J Am Acad Dermatol. 2019;81:1339-1345. doi:10.1016/j.jaad.2019.05.085
- Ali FR, Al-Niaimi F, Craythorne EE, et al. Patient satisfaction and the waiting room in Mohs surgery: appropriate prewarning may abrogate boredom. J Eur Acad Dermatol Venereol. 2017;31:e337-e338.
- Kossintseva I, Zloty D. Determinants and timeline of perioperative anxiety in Mohs surgery. Dermatol Surg. 2017;43:1029-1035.
- Kruchevsky D, Hirth J, Capucha T, et al. Triggers of preoperative anxiety in patients undergoing Mohs micrographic surgery. Dermatol Surg. 2021;47:1110-1112.
- Kokoska RE, Szeto MD, Steadman L, et al. Analysis of factors contributing to perioperative Mohs micrographic surgery anxiety: patient survey study at an academic center. Dermatol Surg. 2022;48:1279-1282.
- Long J, Rajabi-Estarabadi A, Levin A, et al. Perioperative anxiety associated with Mohs micrographic surgery: a survey-based study. Dermatol Surg. 2022;48:711-715.
- Zhang J, Miller CJ, O’Malley V, et al. Patient quality of life fluctuates before and after Mohs micrographic surgery: a longitudinal assessment of the patient experience. J Am Acad Dermatol. 2018;78:1060-1067.
- Lee EB, Ford A, Clarey D, et al. Patient outcomes and satisfaction after Mohs micrographic surgery in patients with nonmelanoma skin cancer. Dermatol Sur. 2021;47:1190-1194.
- Condie D, West L, Hynan LS, et al. Patient satisfaction with Mohs surgery for melanoma in situ. Dermatol Surg. 2021;47:288-290.
- Arshanapalli A, Tra n JM, Aylward JL, et al. The effect of suture spacing on patient perception of surgical skill. J Am Acad Dermatol. 2021;84:735-736.
Mohs micrographic surgery (MMS)—developed by Dr. Frederic Mohs in the 1930s—is the gold standard for treating various cutaneous malignancies. It provides maximal conservation of uninvolved tissues while producing higher cure rates compared to wide local excision.1,2
We sought to assess the various characteristics that impact patient satisfaction to help Mohs surgeons incorporate relatively simple yet clinically significant practices into their patient encounters. We conducted a systematic literature search of peer-reviewed PubMed articles indexed for MEDLINE from database inception through November 2023 using the terms Mohs micrographic surgery and patient satisfaction. Among the inclusion criteria were studies involving participants having undergone MMS, with objective assessments on patient-reported satisfaction or preferences related to patient education, communication, anxiety-alleviating measures, or QOL in MMS. Studies were excluded if they failed to meet these criteria, were outdated and no longer clinically relevant, or measured unalterable factors with no significant impact on how Mohs surgeons could change clinical practice. Of the 157 nonreplicated studies identified, 34 met inclusion criteria.
Perioperative Patient Communication and Education Techniques
Perioperative Patient Communication—Many studies have evaluated the impact of perioperative patient-provider communication and education on patient satisfaction in those undergoing MMS. Studies focusing on preoperative and postoperative telephone calls, patient consultation formats, and patient-perceived impact of such communication modalities have been well documented (Table 1).3-8 The importance of the patient follow-up after MMS was further supported by a retrospective study concluding that 88.7% (86/97) of patients regarded follow-up visits as important, and 80% (77/97) desired additional follow-up 3 months after MMS.9 Additional studies have highlighted the importance of thorough and open perioperative patient-provider communication during MMS (Table 2).10-12


Patient-Education Techniques—Many studies have assessed the use of visual models to aid in patient education on MMS, specifically the preprocedural consent process (Table 3).13-16 Additionally, 2 randomized controlled trials assessing the use of at-home and same-day in-office preoperative educational videos concluded that these interventions increased patient knowledge and confidence regarding procedural risks and benefits, with no statistically significant differences in patient anxiety or satisfaction.17,18

Despite the availability of these educational videos, many patients often turn to online resources for self-education, which is problematic if reader literacy is incongruent with online readability. One study assessing readability of online MMS resources concluded that the most accessed articles exceeded the recommended reading level for adequate patient comprehension.19 A survey studying a wide range of variables related to patient satisfaction (eg, demographics, socioeconomics, health status) in 339 MMS patients found that those who considered themselves more involved in the decision-making process were more satisfied in the short-term, and married patients had even higher long-term satisfaction. Interestingly, this study also concluded that undergoing 3 or more MMS stages was associated with higher short- and long-term satisfaction, likely secondary to perceived effects of increased overall care, medical attention, and time spent with the provider.20
Synthesis of this information with emphasis on the higher evidence-based studies—including systematic reviews, meta-analyses, and randomized controlled trials—yields the following beneficial interventions regarding patient education and communication13-20:
- Preoperative and same-day postoperative telephone follow-up (TFU) do not show statistically significant impacts on patient satisfaction; however, TFU allows for identification of postoperative concerns and inadequate pain management, which may have downstream effects on long-term perception of the overall patient experience.
- The use of video-assisted consent yields improved patient satisfaction and knowledge, while video content—traditional or didactic—has no impact on satisfaction in new MMS patients.
- The use of at-home or same-day in-office preoperative educational videos can improve procedural knowledge and risk-benefit understanding of MMS while having no impact on satisfaction.
- Bedside manner and effective in-person communication by the provider often takes precedence in the patient experience; however, implementation of additional educational modalities should be considered.
Patient Anxiety and QOL
Reducing Patient Anxiety—The use of perioperative distractors to reduce patient anxiety may play an integral role when patients undergo MMS, as there often are prolonged waiting periods between stages when patients may feel increasingly vulnerable or anxious. Table 4 reviews studies on perioperative distractors that showed a statistically significant reduction in MMS patient anxiety.21-24

Although not statistically significant, additional studies evaluating the use of intraoperative anxiety-reduction methods in MMS have demonstrated a downtrend in patient anxiety with the following interventions: engaging in small talk with clinic staff, bringing a guest, eating, watching television, communicating surgical expectations with the provider, handholding, use of a stress ball, and use of 3-dimensional educational MMS models.25-27 Similarly, a survey of 73 patients undergoing MMS found that patients tended to enjoy complimentary beverages preprocedurally in the waiting room, reading, speaking with their guest, watching television, or using their telephone during wait times.28 Table 5 lists additional perioperative factors encompassing specific patient and surgical characteristics that help reduce patient anxiety.29-32

Patient QOL—Many methods aimed at decreasing MMS-related patient anxiety often show no direct impact on patient satisfaction, likely due to the multifactorial nature of the patient-perceived experience. A prospective observational study of MMS patients noted a statistically significant improvement in patient QOL scores 3 months postsurgery (P=.0007), demonstrating that MMS generally results in positive patient outcomes despite preprocedural anxiety.33 An additional prospective study in MMS patients with nonmelanoma skin cancer concluded that sex, age, and closure type—factors often shown to affect anxiety levels—did not significantly impact patient satisfaction.34 Similarly, high satisfaction levels can be expected among MMS patients undergoing treatment of melanoma in situ, with more than 90% of patients rating their treatment experience a 4 (agree) or 5 (strongly agree) out of 5 in short- and long-term satisfaction assessments (38/41 and 40/42, respectively).35 This assessment, conducted 3 months postoperatively, asked patients to score the statement, “I am completely satisfied with the treatment of my skin problem,” on a scale ranging from 1 (strongly disagree) to 5 (strongly agree).
Lastly, patient perception of their surgeon’s skill may contribute to levels of patient satisfaction. Although suture spacing has not been shown to affect surgical outcomes, it has been demonstrated to impact the patient’s perception of surgical skill and is further supported by a study concluding that closures with 2-mm spacing were ranked significantly lower by patients compared with closures with either 4- or 6-mm spacing (P=.005 and P=.012, respectively).36
Synthesis of this information with emphasis on the higher evidence-based studies—including systematic reviews, meta-analyses, and randomized controlled trials—yields the following beneficial interventions regarding anxiety-reducing measures and patient-perceived QOL21-36:
- Factors shown to decrease patient anxiety include patient personalized music, virtual-reality experience, perioperative informational videos, and 3-dimensional–printed MMS models.
- Many methods aimed at decreasing MMS-related patient anxiety show no direct impact on patient satisfaction, likely due to the multifactorial nature of the patient-perceived experience.
- Higher anxiety can be associated with worse QOL scores in MMS patients, and additional factors that may have a negative impact on anxiety include female sex, younger age, and tumor location on the face.
Conclusion
Many factors affect patient satisfaction in MMS. Increased awareness and acknowledgement of these factors can foster improved clinical practice and patient experience, which can have downstream effects on patient compliance and overall psychosocial and medical well-being. With the movement toward value-based health care, patient satisfaction ratings are likely to play an increasingly important role in physician reimbursement. Adapting one’s practice to include high-quality, time-efficient, patient-centered care goes hand in hand with increasing MMS patient satisfaction. Careful evaluation and scrutiny of one’s current practices while remaining cognizant of patient population, resource availability, and clinical limitations often reveal opportunities for small adjustments that can have a great impact on patient satisfaction. This thorough assessment and review of the published literature aims to assist MMS surgeons in understanding the role that certain factors—(1) perioperative patient communication and education techniques and (2) patient anxiety, QOL, and additional considerations—have on overall satisfaction with MMS. Specific consideration should be placed on the fact that patient satisfaction is multifactorial, and many different interventions can have a positive impact on the overall patient experience.
Mohs micrographic surgery (MMS)—developed by Dr. Frederic Mohs in the 1930s—is the gold standard for treating various cutaneous malignancies. It provides maximal conservation of uninvolved tissues while producing higher cure rates compared to wide local excision.1,2
We sought to assess the various characteristics that impact patient satisfaction to help Mohs surgeons incorporate relatively simple yet clinically significant practices into their patient encounters. We conducted a systematic literature search of peer-reviewed PubMed articles indexed for MEDLINE from database inception through November 2023 using the terms Mohs micrographic surgery and patient satisfaction. Among the inclusion criteria were studies involving participants having undergone MMS, with objective assessments on patient-reported satisfaction or preferences related to patient education, communication, anxiety-alleviating measures, or QOL in MMS. Studies were excluded if they failed to meet these criteria, were outdated and no longer clinically relevant, or measured unalterable factors with no significant impact on how Mohs surgeons could change clinical practice. Of the 157 nonreplicated studies identified, 34 met inclusion criteria.
Perioperative Patient Communication and Education Techniques
Perioperative Patient Communication—Many studies have evaluated the impact of perioperative patient-provider communication and education on patient satisfaction in those undergoing MMS. Studies focusing on preoperative and postoperative telephone calls, patient consultation formats, and patient-perceived impact of such communication modalities have been well documented (Table 1).3-8 The importance of the patient follow-up after MMS was further supported by a retrospective study concluding that 88.7% (86/97) of patients regarded follow-up visits as important, and 80% (77/97) desired additional follow-up 3 months after MMS.9 Additional studies have highlighted the importance of thorough and open perioperative patient-provider communication during MMS (Table 2).10-12


Patient-Education Techniques—Many studies have assessed the use of visual models to aid in patient education on MMS, specifically the preprocedural consent process (Table 3).13-16 Additionally, 2 randomized controlled trials assessing the use of at-home and same-day in-office preoperative educational videos concluded that these interventions increased patient knowledge and confidence regarding procedural risks and benefits, with no statistically significant differences in patient anxiety or satisfaction.17,18

Despite the availability of these educational videos, many patients often turn to online resources for self-education, which is problematic if reader literacy is incongruent with online readability. One study assessing readability of online MMS resources concluded that the most accessed articles exceeded the recommended reading level for adequate patient comprehension.19 A survey studying a wide range of variables related to patient satisfaction (eg, demographics, socioeconomics, health status) in 339 MMS patients found that those who considered themselves more involved in the decision-making process were more satisfied in the short-term, and married patients had even higher long-term satisfaction. Interestingly, this study also concluded that undergoing 3 or more MMS stages was associated with higher short- and long-term satisfaction, likely secondary to perceived effects of increased overall care, medical attention, and time spent with the provider.20
Synthesis of this information with emphasis on the higher evidence-based studies—including systematic reviews, meta-analyses, and randomized controlled trials—yields the following beneficial interventions regarding patient education and communication13-20:
- Preoperative and same-day postoperative telephone follow-up (TFU) do not show statistically significant impacts on patient satisfaction; however, TFU allows for identification of postoperative concerns and inadequate pain management, which may have downstream effects on long-term perception of the overall patient experience.
- The use of video-assisted consent yields improved patient satisfaction and knowledge, while video content—traditional or didactic—has no impact on satisfaction in new MMS patients.
- The use of at-home or same-day in-office preoperative educational videos can improve procedural knowledge and risk-benefit understanding of MMS while having no impact on satisfaction.
- Bedside manner and effective in-person communication by the provider often takes precedence in the patient experience; however, implementation of additional educational modalities should be considered.
Patient Anxiety and QOL
Reducing Patient Anxiety—The use of perioperative distractors to reduce patient anxiety may play an integral role when patients undergo MMS, as there often are prolonged waiting periods between stages when patients may feel increasingly vulnerable or anxious. Table 4 reviews studies on perioperative distractors that showed a statistically significant reduction in MMS patient anxiety.21-24

Although not statistically significant, additional studies evaluating the use of intraoperative anxiety-reduction methods in MMS have demonstrated a downtrend in patient anxiety with the following interventions: engaging in small talk with clinic staff, bringing a guest, eating, watching television, communicating surgical expectations with the provider, handholding, use of a stress ball, and use of 3-dimensional educational MMS models.25-27 Similarly, a survey of 73 patients undergoing MMS found that patients tended to enjoy complimentary beverages preprocedurally in the waiting room, reading, speaking with their guest, watching television, or using their telephone during wait times.28 Table 5 lists additional perioperative factors encompassing specific patient and surgical characteristics that help reduce patient anxiety.29-32

Patient QOL—Many methods aimed at decreasing MMS-related patient anxiety often show no direct impact on patient satisfaction, likely due to the multifactorial nature of the patient-perceived experience. A prospective observational study of MMS patients noted a statistically significant improvement in patient QOL scores 3 months postsurgery (P=.0007), demonstrating that MMS generally results in positive patient outcomes despite preprocedural anxiety.33 An additional prospective study in MMS patients with nonmelanoma skin cancer concluded that sex, age, and closure type—factors often shown to affect anxiety levels—did not significantly impact patient satisfaction.34 Similarly, high satisfaction levels can be expected among MMS patients undergoing treatment of melanoma in situ, with more than 90% of patients rating their treatment experience a 4 (agree) or 5 (strongly agree) out of 5 in short- and long-term satisfaction assessments (38/41 and 40/42, respectively).35 This assessment, conducted 3 months postoperatively, asked patients to score the statement, “I am completely satisfied with the treatment of my skin problem,” on a scale ranging from 1 (strongly disagree) to 5 (strongly agree).
Lastly, patient perception of their surgeon’s skill may contribute to levels of patient satisfaction. Although suture spacing has not been shown to affect surgical outcomes, it has been demonstrated to impact the patient’s perception of surgical skill and is further supported by a study concluding that closures with 2-mm spacing were ranked significantly lower by patients compared with closures with either 4- or 6-mm spacing (P=.005 and P=.012, respectively).36
Synthesis of this information with emphasis on the higher evidence-based studies—including systematic reviews, meta-analyses, and randomized controlled trials—yields the following beneficial interventions regarding anxiety-reducing measures and patient-perceived QOL21-36:
- Factors shown to decrease patient anxiety include patient personalized music, virtual-reality experience, perioperative informational videos, and 3-dimensional–printed MMS models.
- Many methods aimed at decreasing MMS-related patient anxiety show no direct impact on patient satisfaction, likely due to the multifactorial nature of the patient-perceived experience.
- Higher anxiety can be associated with worse QOL scores in MMS patients, and additional factors that may have a negative impact on anxiety include female sex, younger age, and tumor location on the face.
Conclusion
Many factors affect patient satisfaction in MMS. Increased awareness and acknowledgement of these factors can foster improved clinical practice and patient experience, which can have downstream effects on patient compliance and overall psychosocial and medical well-being. With the movement toward value-based health care, patient satisfaction ratings are likely to play an increasingly important role in physician reimbursement. Adapting one’s practice to include high-quality, time-efficient, patient-centered care goes hand in hand with increasing MMS patient satisfaction. Careful evaluation and scrutiny of one’s current practices while remaining cognizant of patient population, resource availability, and clinical limitations often reveal opportunities for small adjustments that can have a great impact on patient satisfaction. This thorough assessment and review of the published literature aims to assist MMS surgeons in understanding the role that certain factors—(1) perioperative patient communication and education techniques and (2) patient anxiety, QOL, and additional considerations—have on overall satisfaction with MMS. Specific consideration should be placed on the fact that patient satisfaction is multifactorial, and many different interventions can have a positive impact on the overall patient experience.
- Trost LB, Bailin PL. History of Mohs surgery. Dermatol Clin. 2011; 29:135-139, vii. doi:10.1016/j.det.2011.01.010
- Leslie DF, Greenway HT. Mohs micrographic surgery for skin cancer. Australas J Dermatol. 1991;32:159-164. doi:10.1111/j.1440 -0960.1991.tb01783.x
- Sobanko JF, Da Silva D, Chiesa Fuxench ZC, et al. Preoperative telephone consultation does not decrease patient anxiety before Mohs micrographic surgery. J Am Acad Dermatol. 2017;76:519-526. doi:10.1016/j.jaad.2016.09.027
- Sharon VR, Armstrong AW, Jim On SC, et al. Separate- versus same-day preoperative consultation in dermatologic surgery: a patient-centered investigation in an academic practice. Dermatol Surg. 2013;39:240-247. doi:10.1111/dsu.12083
- Knackstedt TJ, Samie FH. Shared medical appointments for the preoperative consultation visit of Mohs micrographic surgery. J Am Acad Dermatol. 2015;72:340-344. doi:10.1016/j.jaad.2014.10.022
- Vance S, Fontecilla N, Samie FH, et al. Effect of postoperative telephone calls on patient satisfaction and scar satisfaction after Mohs micrographic surgery. Dermatol Surg. 2019;45:1459-1464. doi:10.1097/DSS.0000000000001913
- Hafiji J, Salmon P, Hussain W. Patient satisfaction with post-operative telephone calls after Mohs micrographic surgery: a New Zealand and U.K. experience. Br J Dermatol. 2012;167:570-574. doi:10.1111 /j.1365-2133.2012.11011.x
- Bednarek R, Jonak C, Golda N. Optimal timing of postoperative patient telephone calls after Mohs micrographic surgery: a randomized controlled trial. J Am Acad Dermatol. 2021;85:220-221. doi:10.1016 /j.jaad.2020.07.106
- Sharon VR, Armstrong AW, Jim-On S, et al. Postoperative preferences in cutaneous surgery: a patient-centered investigation from an academic dermatologic surgery practice. Dermatol Surg. 2013;39:773-778. doi:10.1111/dsu.12136
- Xu S, Atanelov Z, Bhatia AC. Online patient-reported reviews of Mohs micrographic surgery: qualitative analysis of positive and negative experiences. Cutis. 2017;99:E25-E29.
- Golda N, Beeson S, Kohli N, et al. Recommendations for improving the patient experience in specialty encounters. J Am Acad Dermatol. 2018;78:653-659. doi:10.1016/j.jaad.2017.05.040
- Patel P, Malik K, Khachemoune A. Patient education in Mohs surgery: a review and critical evaluation of techniques. Arch Dermatol Res. 2021;313:217-224. doi:10.1007/s00403-020-02119-5
- Migden M, Chavez-Frazier A, Nguyen T. The use of high definition video modules for delivery of informed consent and wound care education in the Mohs surgery unit. Semin Cutan Med Surg. 2008;27:89-93. doi:10.1016/j.sder.2008.02.001
- Newsom E, Lee E, Rossi A, et al. Modernizing the Mohs surgery consultation: instituting a video module for improved patient education and satisfaction. Dermatol Surg. 2018;44:778-784. doi:10.1097/DSS.0000000000001473
- West L, Srivastava D, Goldberg LH, et al. Multimedia technology used to supplement patient consent for Mohs micrographic surgery. Dermatol Surg. 2020;46:586-590. doi:10.1097/DSS.0000000000002134
- Miao Y, Venning VL, Mallitt KA, et al. A randomized controlled trial comparing video-assisted informed consent with standard consent for Mohs micrographic surgery. JAAD Int. 2020;1:13-20. doi:10.1016 /j.jdin.2020.03.005
- Mann J, Li L, Kulakov E, et al. Home viewing of educational video improves patient understanding of Mohs micrographic surgery. Clin Exp Dermatol. 2022;47:93-97. doi:10.1111/ced.14845
- Delcambre M, Haynes D, Hajar T, et al. Using a multimedia tool for informed consent in Mohs surgery: a randomized trial measuring effects on patient anxiety, knowledge, and satisfaction. Dermatol Surg. 2020;46:591-598. doi:10.1097/DSS.0000000000002213
- Vargas CR, DePry J, Lee BT, et al. The readability of online patient information about Mohs micrographic surgery. Dermatol Surg. 2016;42:1135-1141. doi:10.1097/DSS.0000000000000866
- Asgari MM, Warton EM, Neugebauer R, et al. Predictors of patient satisfaction with Mohs surgery: analysis of preoperative, intraoperative, and postoperative factors in a prospective cohort. Arch Dermatol. 2011;147:1387-1394.
- Vachiramon V, Sobanko JF, Rattanaumpawan P, et al. Music reduces patient anxiety during Mohs surgery: an open-label randomized controlled trial. Dermatol Surg. 2013;39:298-305. doi:10.1111/dsu.12047
- Hawkins SD, Koch SB, Williford PM, et al. Web app- and text message-based patient education in Mohs micrographic surgery-a randomized controlled trial. Dermatol Surg. 2018;44:924-932. doi:10.1097/DSS.0000000000001489
- Higgins S, Feinstein S, Hawkins M, et al. Virtual reality to improve the experience of the Mohs patient-a prospective interventional study. Dermatol Surg. 2019;45:1009-1018. doi:10.1097 /DSS.0000000000001854
- Guo D, Zloty DM, Kossintseva I. Efficacy and safety of anxiolytics in Mohs micrographic surgery: a randomized, double-blinded, placebo-controlled trial. Dermatol Surg. 2023;49:989-994. doi:10.1097 /DSS.0000000000003905
- Locke MC, Wilkerson EC, Mistur RL, et al. 2015 Arte Poster Competition first place winner: assessing the correlation between patient anxiety and satisfaction for Mohs surgery. J Drugs Dermatol. 2015;14:1070-1072.
- Yanes AF, Weil A, Furlan KC, et al. Effect of stress ball use or hand-holding on anxiety during skin cancer excision: a randomized clinical trial. JAMA Dermatol. 2018;154:1045-1049. doi:10.1001 /jamadermatol.2018.1783
- Biro M, Kim I, Huynh A, et al. The use of 3-dimensionally printed models to optimize patient education and alleviate perioperative anxiety in Mohs micrographic surgery: a randomized controlled trial. J Am Acad Dermatol. 2019;81:1339-1345. doi:10.1016/j.jaad.2019.05.085
- Ali FR, Al-Niaimi F, Craythorne EE, et al. Patient satisfaction and the waiting room in Mohs surgery: appropriate prewarning may abrogate boredom. J Eur Acad Dermatol Venereol. 2017;31:e337-e338.
- Kossintseva I, Zloty D. Determinants and timeline of perioperative anxiety in Mohs surgery. Dermatol Surg. 2017;43:1029-1035.
- Kruchevsky D, Hirth J, Capucha T, et al. Triggers of preoperative anxiety in patients undergoing Mohs micrographic surgery. Dermatol Surg. 2021;47:1110-1112.
- Kokoska RE, Szeto MD, Steadman L, et al. Analysis of factors contributing to perioperative Mohs micrographic surgery anxiety: patient survey study at an academic center. Dermatol Surg. 2022;48:1279-1282.
- Long J, Rajabi-Estarabadi A, Levin A, et al. Perioperative anxiety associated with Mohs micrographic surgery: a survey-based study. Dermatol Surg. 2022;48:711-715.
- Zhang J, Miller CJ, O’Malley V, et al. Patient quality of life fluctuates before and after Mohs micrographic surgery: a longitudinal assessment of the patient experience. J Am Acad Dermatol. 2018;78:1060-1067.
- Lee EB, Ford A, Clarey D, et al. Patient outcomes and satisfaction after Mohs micrographic surgery in patients with nonmelanoma skin cancer. Dermatol Sur. 2021;47:1190-1194.
- Condie D, West L, Hynan LS, et al. Patient satisfaction with Mohs surgery for melanoma in situ. Dermatol Surg. 2021;47:288-290.
- Arshanapalli A, Tra n JM, Aylward JL, et al. The effect of suture spacing on patient perception of surgical skill. J Am Acad Dermatol. 2021;84:735-736.
- Trost LB, Bailin PL. History of Mohs surgery. Dermatol Clin. 2011; 29:135-139, vii. doi:10.1016/j.det.2011.01.010
- Leslie DF, Greenway HT. Mohs micrographic surgery for skin cancer. Australas J Dermatol. 1991;32:159-164. doi:10.1111/j.1440 -0960.1991.tb01783.x
- Sobanko JF, Da Silva D, Chiesa Fuxench ZC, et al. Preoperative telephone consultation does not decrease patient anxiety before Mohs micrographic surgery. J Am Acad Dermatol. 2017;76:519-526. doi:10.1016/j.jaad.2016.09.027
- Sharon VR, Armstrong AW, Jim On SC, et al. Separate- versus same-day preoperative consultation in dermatologic surgery: a patient-centered investigation in an academic practice. Dermatol Surg. 2013;39:240-247. doi:10.1111/dsu.12083
- Knackstedt TJ, Samie FH. Shared medical appointments for the preoperative consultation visit of Mohs micrographic surgery. J Am Acad Dermatol. 2015;72:340-344. doi:10.1016/j.jaad.2014.10.022
- Vance S, Fontecilla N, Samie FH, et al. Effect of postoperative telephone calls on patient satisfaction and scar satisfaction after Mohs micrographic surgery. Dermatol Surg. 2019;45:1459-1464. doi:10.1097/DSS.0000000000001913
- Hafiji J, Salmon P, Hussain W. Patient satisfaction with post-operative telephone calls after Mohs micrographic surgery: a New Zealand and U.K. experience. Br J Dermatol. 2012;167:570-574. doi:10.1111 /j.1365-2133.2012.11011.x
- Bednarek R, Jonak C, Golda N. Optimal timing of postoperative patient telephone calls after Mohs micrographic surgery: a randomized controlled trial. J Am Acad Dermatol. 2021;85:220-221. doi:10.1016 /j.jaad.2020.07.106
- Sharon VR, Armstrong AW, Jim-On S, et al. Postoperative preferences in cutaneous surgery: a patient-centered investigation from an academic dermatologic surgery practice. Dermatol Surg. 2013;39:773-778. doi:10.1111/dsu.12136
- Xu S, Atanelov Z, Bhatia AC. Online patient-reported reviews of Mohs micrographic surgery: qualitative analysis of positive and negative experiences. Cutis. 2017;99:E25-E29.
- Golda N, Beeson S, Kohli N, et al. Recommendations for improving the patient experience in specialty encounters. J Am Acad Dermatol. 2018;78:653-659. doi:10.1016/j.jaad.2017.05.040
- Patel P, Malik K, Khachemoune A. Patient education in Mohs surgery: a review and critical evaluation of techniques. Arch Dermatol Res. 2021;313:217-224. doi:10.1007/s00403-020-02119-5
- Migden M, Chavez-Frazier A, Nguyen T. The use of high definition video modules for delivery of informed consent and wound care education in the Mohs surgery unit. Semin Cutan Med Surg. 2008;27:89-93. doi:10.1016/j.sder.2008.02.001
- Newsom E, Lee E, Rossi A, et al. Modernizing the Mohs surgery consultation: instituting a video module for improved patient education and satisfaction. Dermatol Surg. 2018;44:778-784. doi:10.1097/DSS.0000000000001473
- West L, Srivastava D, Goldberg LH, et al. Multimedia technology used to supplement patient consent for Mohs micrographic surgery. Dermatol Surg. 2020;46:586-590. doi:10.1097/DSS.0000000000002134
- Miao Y, Venning VL, Mallitt KA, et al. A randomized controlled trial comparing video-assisted informed consent with standard consent for Mohs micrographic surgery. JAAD Int. 2020;1:13-20. doi:10.1016 /j.jdin.2020.03.005
- Mann J, Li L, Kulakov E, et al. Home viewing of educational video improves patient understanding of Mohs micrographic surgery. Clin Exp Dermatol. 2022;47:93-97. doi:10.1111/ced.14845
- Delcambre M, Haynes D, Hajar T, et al. Using a multimedia tool for informed consent in Mohs surgery: a randomized trial measuring effects on patient anxiety, knowledge, and satisfaction. Dermatol Surg. 2020;46:591-598. doi:10.1097/DSS.0000000000002213
- Vargas CR, DePry J, Lee BT, et al. The readability of online patient information about Mohs micrographic surgery. Dermatol Surg. 2016;42:1135-1141. doi:10.1097/DSS.0000000000000866
- Asgari MM, Warton EM, Neugebauer R, et al. Predictors of patient satisfaction with Mohs surgery: analysis of preoperative, intraoperative, and postoperative factors in a prospective cohort. Arch Dermatol. 2011;147:1387-1394.
- Vachiramon V, Sobanko JF, Rattanaumpawan P, et al. Music reduces patient anxiety during Mohs surgery: an open-label randomized controlled trial. Dermatol Surg. 2013;39:298-305. doi:10.1111/dsu.12047
- Hawkins SD, Koch SB, Williford PM, et al. Web app- and text message-based patient education in Mohs micrographic surgery-a randomized controlled trial. Dermatol Surg. 2018;44:924-932. doi:10.1097/DSS.0000000000001489
- Higgins S, Feinstein S, Hawkins M, et al. Virtual reality to improve the experience of the Mohs patient-a prospective interventional study. Dermatol Surg. 2019;45:1009-1018. doi:10.1097 /DSS.0000000000001854
- Guo D, Zloty DM, Kossintseva I. Efficacy and safety of anxiolytics in Mohs micrographic surgery: a randomized, double-blinded, placebo-controlled trial. Dermatol Surg. 2023;49:989-994. doi:10.1097 /DSS.0000000000003905
- Locke MC, Wilkerson EC, Mistur RL, et al. 2015 Arte Poster Competition first place winner: assessing the correlation between patient anxiety and satisfaction for Mohs surgery. J Drugs Dermatol. 2015;14:1070-1072.
- Yanes AF, Weil A, Furlan KC, et al. Effect of stress ball use or hand-holding on anxiety during skin cancer excision: a randomized clinical trial. JAMA Dermatol. 2018;154:1045-1049. doi:10.1001 /jamadermatol.2018.1783
- Biro M, Kim I, Huynh A, et al. The use of 3-dimensionally printed models to optimize patient education and alleviate perioperative anxiety in Mohs micrographic surgery: a randomized controlled trial. J Am Acad Dermatol. 2019;81:1339-1345. doi:10.1016/j.jaad.2019.05.085
- Ali FR, Al-Niaimi F, Craythorne EE, et al. Patient satisfaction and the waiting room in Mohs surgery: appropriate prewarning may abrogate boredom. J Eur Acad Dermatol Venereol. 2017;31:e337-e338.
- Kossintseva I, Zloty D. Determinants and timeline of perioperative anxiety in Mohs surgery. Dermatol Surg. 2017;43:1029-1035.
- Kruchevsky D, Hirth J, Capucha T, et al. Triggers of preoperative anxiety in patients undergoing Mohs micrographic surgery. Dermatol Surg. 2021;47:1110-1112.
- Kokoska RE, Szeto MD, Steadman L, et al. Analysis of factors contributing to perioperative Mohs micrographic surgery anxiety: patient survey study at an academic center. Dermatol Surg. 2022;48:1279-1282.
- Long J, Rajabi-Estarabadi A, Levin A, et al. Perioperative anxiety associated with Mohs micrographic surgery: a survey-based study. Dermatol Surg. 2022;48:711-715.
- Zhang J, Miller CJ, O’Malley V, et al. Patient quality of life fluctuates before and after Mohs micrographic surgery: a longitudinal assessment of the patient experience. J Am Acad Dermatol. 2018;78:1060-1067.
- Lee EB, Ford A, Clarey D, et al. Patient outcomes and satisfaction after Mohs micrographic surgery in patients with nonmelanoma skin cancer. Dermatol Sur. 2021;47:1190-1194.
- Condie D, West L, Hynan LS, et al. Patient satisfaction with Mohs surgery for melanoma in situ. Dermatol Surg. 2021;47:288-290.
- Arshanapalli A, Tra n JM, Aylward JL, et al. The effect of suture spacing on patient perception of surgical skill. J Am Acad Dermatol. 2021;84:735-736.
Enhancing Patient Satisfaction and Quality of Life With Mohs Micrographic Surgery: A Systematic Review of Patient Education, Communication, and Anxiety Management
Enhancing Patient Satisfaction and Quality of Life With Mohs Micrographic Surgery: A Systematic Review of Patient Education, Communication, and Anxiety Management
PRACTICE POINTS
- When patients are treated with Mohs micrographic surgery (MMS), thorough in-person dialogue augmented by pre- and same-day telephone follow-ups can help them feel heard and better supported, even though follow-up calls alone may not drive satisfaction scores.
- Increased awareness and implementation of the various factors influencing patient satisfaction and quality of life in MMS can enhance clinical practice and improve patient experiences, with potential impacts on compliance, psychosocial well-being, medical outcomes, and physician reimbursement.
- Patient satisfaction and procedural understanding can be improved with video and visual-based education. Anxiety-reducing methods help lower perioperative stress.