User login
MEDS Score for Sepsis Might Best Predict ED Mortality
Clinical question: Which illness severity score best predicts outcomes in emergency department (ED) patients presenting with infection?
Background: Several scoring models have been developed to predict illness severity and mortality in patients with infection. Some scores were developed specifically for patients with sepsis and others for patients in a general critical care setting. These different scoring models have not been specifically compared and validated in the ED setting in patients with infection of various severities.
Study design: Prospective, observational study.
Setting: Adult ED in a metropolitan tertiary, university-affiliated hospital.
Synopsis: Investigators prospectively identified 8,871 adult inpatients with infection from a single-center ED. Data to calculate five prediction models were collected. The models were:
- Mortality in Emergency Department Sepsis (MEDS) score;
- Acute Physiology and Chronic Health Evaluation II (APACHE II);
- Simplified Acute Physiology Score II (SAPS II);
- Sequential Organ Failure Assessment (SOFA); and
- Severe Sepsis Score (SSS).
Severity score performance was assessed for the overall cohort and for subgroups, including infection without systemic inflammatory response syndrome, sepsis, severe sepsis, and septic shock. The MEDS score best predicted mortality in the cohort, with an area under the receiver operating characteristics curve of 0.92. However, older scoring models such as the APACHE II and SAPS II still discriminated well, especially in patients who were admitted to the ICU. All scores tended to overestimate mortality.
Bottom line: The MEDS score may best predict illness severity in septic patients presenting to the ED, but other scoring models may be better-suited for specific patient populations.
Citation: Williams JM, Greenslade JH, Chu K, Brown AF, Lipman J. Severity scores in emergency department patients with presumed infection: a prospective validation study. Crit Care Med. 2016;44(3):539-547.
Clinical question: Which illness severity score best predicts outcomes in emergency department (ED) patients presenting with infection?
Background: Several scoring models have been developed to predict illness severity and mortality in patients with infection. Some scores were developed specifically for patients with sepsis and others for patients in a general critical care setting. These different scoring models have not been specifically compared and validated in the ED setting in patients with infection of various severities.
Study design: Prospective, observational study.
Setting: Adult ED in a metropolitan tertiary, university-affiliated hospital.
Synopsis: Investigators prospectively identified 8,871 adult inpatients with infection from a single-center ED. Data to calculate five prediction models were collected. The models were:
- Mortality in Emergency Department Sepsis (MEDS) score;
- Acute Physiology and Chronic Health Evaluation II (APACHE II);
- Simplified Acute Physiology Score II (SAPS II);
- Sequential Organ Failure Assessment (SOFA); and
- Severe Sepsis Score (SSS).
Severity score performance was assessed for the overall cohort and for subgroups, including infection without systemic inflammatory response syndrome, sepsis, severe sepsis, and septic shock. The MEDS score best predicted mortality in the cohort, with an area under the receiver operating characteristics curve of 0.92. However, older scoring models such as the APACHE II and SAPS II still discriminated well, especially in patients who were admitted to the ICU. All scores tended to overestimate mortality.
Bottom line: The MEDS score may best predict illness severity in septic patients presenting to the ED, but other scoring models may be better-suited for specific patient populations.
Citation: Williams JM, Greenslade JH, Chu K, Brown AF, Lipman J. Severity scores in emergency department patients with presumed infection: a prospective validation study. Crit Care Med. 2016;44(3):539-547.
Clinical question: Which illness severity score best predicts outcomes in emergency department (ED) patients presenting with infection?
Background: Several scoring models have been developed to predict illness severity and mortality in patients with infection. Some scores were developed specifically for patients with sepsis and others for patients in a general critical care setting. These different scoring models have not been specifically compared and validated in the ED setting in patients with infection of various severities.
Study design: Prospective, observational study.
Setting: Adult ED in a metropolitan tertiary, university-affiliated hospital.
Synopsis: Investigators prospectively identified 8,871 adult inpatients with infection from a single-center ED. Data to calculate five prediction models were collected. The models were:
- Mortality in Emergency Department Sepsis (MEDS) score;
- Acute Physiology and Chronic Health Evaluation II (APACHE II);
- Simplified Acute Physiology Score II (SAPS II);
- Sequential Organ Failure Assessment (SOFA); and
- Severe Sepsis Score (SSS).
Severity score performance was assessed for the overall cohort and for subgroups, including infection without systemic inflammatory response syndrome, sepsis, severe sepsis, and septic shock. The MEDS score best predicted mortality in the cohort, with an area under the receiver operating characteristics curve of 0.92. However, older scoring models such as the APACHE II and SAPS II still discriminated well, especially in patients who were admitted to the ICU. All scores tended to overestimate mortality.
Bottom line: The MEDS score may best predict illness severity in septic patients presenting to the ED, but other scoring models may be better-suited for specific patient populations.
Citation: Williams JM, Greenslade JH, Chu K, Brown AF, Lipman J. Severity scores in emergency department patients with presumed infection: a prospective validation study. Crit Care Med. 2016;44(3):539-547.
'Everything We Say and Do': Soliciting Goals from Our Patients and Their Families
Editor’s note: “Everything We Say and Do” is an informational series developed by SHM’s Patient Experience Committee to provide readers with thoughtful and actionable communication tactics that have great potential to positively impact patients’ experience of care. Each article will focus on how the contributor applies one ormore of the “key communication” tactics in practice to maintain provider accountability for “Everything we say and do that affects our patients’ thoughts, feelings and well-being.”
Read more from the “Everything We Say and Do” series.
What I Say and Do
Why I Do It
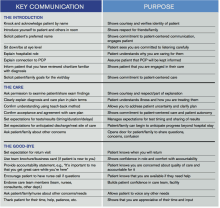
The literature shows that, in most cases, doctors interrupt within 18 to 23 seconds and that when we interrupt, patients often never get back to what they were saying, the course of their story changes, and our diagnostic accuracy decreases. I also do it because I learn things that I wouldn’t otherwise know, and my patients feel heard and treated with respect. Listening has a healing effect, and in medicine, it can be equally if not more therapeutic than the medicines and clinical care we provide. I find that it helps me to be a more effective doctor, one who is helping my patient in the way that is most meaningful and helpful to them. It is very easy to navigate an encounter from the physician point of view and to make assumptions about what people want and need from me, but in reality, what is most important to me is not always what is most important to them.
Allowing patients to tell me what is important shows them respect and also sets me up for success as I am more likely to know and meet their needs. Doing this up front saves time by preventing the “doorknob” questions on the way out. The human connection that follows keeps me connected to my purpose as a doctor. People often worry that listening will take too much time, but we know from the literature that most patients will talk for no more than 90 seconds. It’s really a very short amount of time for a gold mine of information.
How I Do It
Before I jump into my agenda, I make sure to know what is on the patient’s and family’s mind. What is most important to them to address? Once we have agreed on what we will be discussing or doing with our time in a way that includes both what I and the patient/family find important, I start by asking the patient to tell me everything about the first item at hand. I do not interrupt by asking questions, making comments, or “fixing.” I approach them with authentic curiosity, encouraging more without directing what they say.
I start with, “I’m here to talk to you about _____, but first, can you tell me what you’d like to make sure we talk about today?” Or, “Tell me a list of things that you’d like to make sure we talk about today.”
I follow that with, “What else?” until there is nothing else. Once we have negotiated what we will discuss, I say, “Tell me all about _______.” I do not interrupt or think about my response while I am listening. My only response is to use nonverbal continuers (“Uh huh,” “mmmm”), reflections (“That sounds really hard”), verbal continuers (“Tell me more”), empathic statements (“I can see why you would feel that way”), and body language that shows I am with them (sitting at eye level, facing them, looking at them rather than at my phone, pager or a computer screen.)
All of this happens before I jump into any of my own focused or clarifying questions.
Dr. Sliwka is medical director of patient and provider experience, medical director of the Goldman Medical Service, and associate clinical professor of medicine in the Division of Hospital Medicine at the UCSF Medical Center in San Francisco.
Editor’s note: “Everything We Say and Do” is an informational series developed by SHM’s Patient Experience Committee to provide readers with thoughtful and actionable communication tactics that have great potential to positively impact patients’ experience of care. Each article will focus on how the contributor applies one ormore of the “key communication” tactics in practice to maintain provider accountability for “Everything we say and do that affects our patients’ thoughts, feelings and well-being.”
Read more from the “Everything We Say and Do” series.
What I Say and Do
Why I Do It
The literature shows that, in most cases, doctors interrupt within 18 to 23 seconds and that when we interrupt, patients often never get back to what they were saying, the course of their story changes, and our diagnostic accuracy decreases. I also do it because I learn things that I wouldn’t otherwise know, and my patients feel heard and treated with respect. Listening has a healing effect, and in medicine, it can be equally if not more therapeutic than the medicines and clinical care we provide. I find that it helps me to be a more effective doctor, one who is helping my patient in the way that is most meaningful and helpful to them. It is very easy to navigate an encounter from the physician point of view and to make assumptions about what people want and need from me, but in reality, what is most important to me is not always what is most important to them.
Allowing patients to tell me what is important shows them respect and also sets me up for success as I am more likely to know and meet their needs. Doing this up front saves time by preventing the “doorknob” questions on the way out. The human connection that follows keeps me connected to my purpose as a doctor. People often worry that listening will take too much time, but we know from the literature that most patients will talk for no more than 90 seconds. It’s really a very short amount of time for a gold mine of information.
How I Do It
Before I jump into my agenda, I make sure to know what is on the patient’s and family’s mind. What is most important to them to address? Once we have agreed on what we will be discussing or doing with our time in a way that includes both what I and the patient/family find important, I start by asking the patient to tell me everything about the first item at hand. I do not interrupt by asking questions, making comments, or “fixing.” I approach them with authentic curiosity, encouraging more without directing what they say.
I start with, “I’m here to talk to you about _____, but first, can you tell me what you’d like to make sure we talk about today?” Or, “Tell me a list of things that you’d like to make sure we talk about today.”
I follow that with, “What else?” until there is nothing else. Once we have negotiated what we will discuss, I say, “Tell me all about _______.” I do not interrupt or think about my response while I am listening. My only response is to use nonverbal continuers (“Uh huh,” “mmmm”), reflections (“That sounds really hard”), verbal continuers (“Tell me more”), empathic statements (“I can see why you would feel that way”), and body language that shows I am with them (sitting at eye level, facing them, looking at them rather than at my phone, pager or a computer screen.)
All of this happens before I jump into any of my own focused or clarifying questions.
Dr. Sliwka is medical director of patient and provider experience, medical director of the Goldman Medical Service, and associate clinical professor of medicine in the Division of Hospital Medicine at the UCSF Medical Center in San Francisco.
Editor’s note: “Everything We Say and Do” is an informational series developed by SHM’s Patient Experience Committee to provide readers with thoughtful and actionable communication tactics that have great potential to positively impact patients’ experience of care. Each article will focus on how the contributor applies one ormore of the “key communication” tactics in practice to maintain provider accountability for “Everything we say and do that affects our patients’ thoughts, feelings and well-being.”
Read more from the “Everything We Say and Do” series.
What I Say and Do
Why I Do It
The literature shows that, in most cases, doctors interrupt within 18 to 23 seconds and that when we interrupt, patients often never get back to what they were saying, the course of their story changes, and our diagnostic accuracy decreases. I also do it because I learn things that I wouldn’t otherwise know, and my patients feel heard and treated with respect. Listening has a healing effect, and in medicine, it can be equally if not more therapeutic than the medicines and clinical care we provide. I find that it helps me to be a more effective doctor, one who is helping my patient in the way that is most meaningful and helpful to them. It is very easy to navigate an encounter from the physician point of view and to make assumptions about what people want and need from me, but in reality, what is most important to me is not always what is most important to them.
Allowing patients to tell me what is important shows them respect and also sets me up for success as I am more likely to know and meet their needs. Doing this up front saves time by preventing the “doorknob” questions on the way out. The human connection that follows keeps me connected to my purpose as a doctor. People often worry that listening will take too much time, but we know from the literature that most patients will talk for no more than 90 seconds. It’s really a very short amount of time for a gold mine of information.
How I Do It
Before I jump into my agenda, I make sure to know what is on the patient’s and family’s mind. What is most important to them to address? Once we have agreed on what we will be discussing or doing with our time in a way that includes both what I and the patient/family find important, I start by asking the patient to tell me everything about the first item at hand. I do not interrupt by asking questions, making comments, or “fixing.” I approach them with authentic curiosity, encouraging more without directing what they say.
I start with, “I’m here to talk to you about _____, but first, can you tell me what you’d like to make sure we talk about today?” Or, “Tell me a list of things that you’d like to make sure we talk about today.”
I follow that with, “What else?” until there is nothing else. Once we have negotiated what we will discuss, I say, “Tell me all about _______.” I do not interrupt or think about my response while I am listening. My only response is to use nonverbal continuers (“Uh huh,” “mmmm”), reflections (“That sounds really hard”), verbal continuers (“Tell me more”), empathic statements (“I can see why you would feel that way”), and body language that shows I am with them (sitting at eye level, facing them, looking at them rather than at my phone, pager or a computer screen.)
All of this happens before I jump into any of my own focused or clarifying questions.
Dr. Sliwka is medical director of patient and provider experience, medical director of the Goldman Medical Service, and associate clinical professor of medicine in the Division of Hospital Medicine at the UCSF Medical Center in San Francisco.
VTEP Guidelines Can Be Systematically Implemented in Pediatric Inpatients
Clinical question: Can venous thromboembolism prophylaxis (VTEP) guidelines be systematically implemented in a pediatric inpatient population?
Background: VTEP for hospitalized adult medical patients has been characterized in the literature as being safe and efficacious, although mortality benefits are unclear.1 Systematic risk stratification based on electronic medical records (EMRs) with resultant implementation of pharmacologic and mechanical thromboprophylaxis has been shown to improve appropriate VTEP ordering in the adult inpatient population.2
Although the incidence of VTE is known to be increasing in the pediatric population, systematic VTEP implementation in hospitalized children is not well-described. Prior studies have shown the safety of systematic VTEP implementation through a protocol identifying high-risk pediatric inpatients with resultant initiation of appropriate VTEP. 3,4
Risk stratification in prior studies has taken into consideration risk factors such as altered mobility, presence of a central venous catheter (CVC), spinal cord injury (SCI), major lower-extremity orthopedic surgery, major trauma, active malignancy, acute infection, obesity, estrogen use, inflammatory bowel disease (IBD), prior VTE, and family history of VTE.4
Study design: Prospective cohort study using QI methodology.
Setting: A 455-bed, tertiary, freestanding children’s hospital.
Synopsis: After reviewing current literature for VTEP in adults and children and existing institutional pathways for VTEP in adults, traumatic brain injury, and SCI, a multidisciplinary committee formulated VTEP guidelines for 12- to 17-year-old patients. Pharmacologic prophylaxis was considered appropriate in the absence of contraindications and only if CVC and altered mobility were present as risk factors. Using a previously published logistic regression model evaluating VTE risk factors, patients were further categorized as high, moderate, or low risk.
Initial risk-factor categorization was via EMR-based order set, where risk factors were displayed, but subsequently was performed by an integrated tool based on an initial screening form completed by providers upon admission. Logic rules applied by the EMR led to specific VTEP recommendations, which were then selectable by the provider.
Over the first 17 months of EMR tool use, 148 patients on average were admitted each month. VTEP screening rates via the EMR tool increased from 48% in the first month to 81% in the final month. Despite EMR tool usage, VTEP orders did not always correlate with recommendations. Although not a stated objective of the study, none of the screened patients developed a VTE (compared to three cases of VTE in patients between 12 and 17 years of age the year prior).
Bottom line: VTEP guidelines can be systematically implemented via an EMR-based tool in a pediatric inpatient population.
Citation: Mahajerin A, Webber E, Morris J, Taylor K, Saysana M. Development and implementation results of a venous thromboembolism prophylaxis guideline in a tertiary care pediatric hospital. Hosp Pediatr. 2015;5(12):630-636.
References
- Spyropoulos AC, Mahan C. Venous thromboembolism prophylaxis in the medical patient: controversies and perspectives. Am J Med. 2009;122(12):1077-1084.
- Kahn SR, Morrison DR, Cohen JM, et al. Interventions for implementation of thromboprophylaxis in hospitalized medical and surgical patients at risk for venous thromboembolism. Cochrane Database Syst Rev. 2013;7:CD008201.
- Takemoto CM, Sohi S, Desai K, et al. Hospital-associated venous thromboembolism in children: incidence and clinical characteristics. J Pediatr. 2014;164(2):332-338.
- Raffini L, Trimarchi T, Beliveau J, Davis D. Thromboprophylaxis in a pediatric hospital: a patient-safety and quality-improvement initiative. Pediatrics. 2011;127(5):e1326-1332.

Clinical question: Can venous thromboembolism prophylaxis (VTEP) guidelines be systematically implemented in a pediatric inpatient population?
Background: VTEP for hospitalized adult medical patients has been characterized in the literature as being safe and efficacious, although mortality benefits are unclear.1 Systematic risk stratification based on electronic medical records (EMRs) with resultant implementation of pharmacologic and mechanical thromboprophylaxis has been shown to improve appropriate VTEP ordering in the adult inpatient population.2
Although the incidence of VTE is known to be increasing in the pediatric population, systematic VTEP implementation in hospitalized children is not well-described. Prior studies have shown the safety of systematic VTEP implementation through a protocol identifying high-risk pediatric inpatients with resultant initiation of appropriate VTEP. 3,4
Risk stratification in prior studies has taken into consideration risk factors such as altered mobility, presence of a central venous catheter (CVC), spinal cord injury (SCI), major lower-extremity orthopedic surgery, major trauma, active malignancy, acute infection, obesity, estrogen use, inflammatory bowel disease (IBD), prior VTE, and family history of VTE.4
Study design: Prospective cohort study using QI methodology.
Setting: A 455-bed, tertiary, freestanding children’s hospital.
Synopsis: After reviewing current literature for VTEP in adults and children and existing institutional pathways for VTEP in adults, traumatic brain injury, and SCI, a multidisciplinary committee formulated VTEP guidelines for 12- to 17-year-old patients. Pharmacologic prophylaxis was considered appropriate in the absence of contraindications and only if CVC and altered mobility were present as risk factors. Using a previously published logistic regression model evaluating VTE risk factors, patients were further categorized as high, moderate, or low risk.
Initial risk-factor categorization was via EMR-based order set, where risk factors were displayed, but subsequently was performed by an integrated tool based on an initial screening form completed by providers upon admission. Logic rules applied by the EMR led to specific VTEP recommendations, which were then selectable by the provider.
Over the first 17 months of EMR tool use, 148 patients on average were admitted each month. VTEP screening rates via the EMR tool increased from 48% in the first month to 81% in the final month. Despite EMR tool usage, VTEP orders did not always correlate with recommendations. Although not a stated objective of the study, none of the screened patients developed a VTE (compared to three cases of VTE in patients between 12 and 17 years of age the year prior).
Bottom line: VTEP guidelines can be systematically implemented via an EMR-based tool in a pediatric inpatient population.
Citation: Mahajerin A, Webber E, Morris J, Taylor K, Saysana M. Development and implementation results of a venous thromboembolism prophylaxis guideline in a tertiary care pediatric hospital. Hosp Pediatr. 2015;5(12):630-636.
References
- Spyropoulos AC, Mahan C. Venous thromboembolism prophylaxis in the medical patient: controversies and perspectives. Am J Med. 2009;122(12):1077-1084.
- Kahn SR, Morrison DR, Cohen JM, et al. Interventions for implementation of thromboprophylaxis in hospitalized medical and surgical patients at risk for venous thromboembolism. Cochrane Database Syst Rev. 2013;7:CD008201.
- Takemoto CM, Sohi S, Desai K, et al. Hospital-associated venous thromboembolism in children: incidence and clinical characteristics. J Pediatr. 2014;164(2):332-338.
- Raffini L, Trimarchi T, Beliveau J, Davis D. Thromboprophylaxis in a pediatric hospital: a patient-safety and quality-improvement initiative. Pediatrics. 2011;127(5):e1326-1332.

Clinical question: Can venous thromboembolism prophylaxis (VTEP) guidelines be systematically implemented in a pediatric inpatient population?
Background: VTEP for hospitalized adult medical patients has been characterized in the literature as being safe and efficacious, although mortality benefits are unclear.1 Systematic risk stratification based on electronic medical records (EMRs) with resultant implementation of pharmacologic and mechanical thromboprophylaxis has been shown to improve appropriate VTEP ordering in the adult inpatient population.2
Although the incidence of VTE is known to be increasing in the pediatric population, systematic VTEP implementation in hospitalized children is not well-described. Prior studies have shown the safety of systematic VTEP implementation through a protocol identifying high-risk pediatric inpatients with resultant initiation of appropriate VTEP. 3,4
Risk stratification in prior studies has taken into consideration risk factors such as altered mobility, presence of a central venous catheter (CVC), spinal cord injury (SCI), major lower-extremity orthopedic surgery, major trauma, active malignancy, acute infection, obesity, estrogen use, inflammatory bowel disease (IBD), prior VTE, and family history of VTE.4
Study design: Prospective cohort study using QI methodology.
Setting: A 455-bed, tertiary, freestanding children’s hospital.
Synopsis: After reviewing current literature for VTEP in adults and children and existing institutional pathways for VTEP in adults, traumatic brain injury, and SCI, a multidisciplinary committee formulated VTEP guidelines for 12- to 17-year-old patients. Pharmacologic prophylaxis was considered appropriate in the absence of contraindications and only if CVC and altered mobility were present as risk factors. Using a previously published logistic regression model evaluating VTE risk factors, patients were further categorized as high, moderate, or low risk.
Initial risk-factor categorization was via EMR-based order set, where risk factors were displayed, but subsequently was performed by an integrated tool based on an initial screening form completed by providers upon admission. Logic rules applied by the EMR led to specific VTEP recommendations, which were then selectable by the provider.
Over the first 17 months of EMR tool use, 148 patients on average were admitted each month. VTEP screening rates via the EMR tool increased from 48% in the first month to 81% in the final month. Despite EMR tool usage, VTEP orders did not always correlate with recommendations. Although not a stated objective of the study, none of the screened patients developed a VTE (compared to three cases of VTE in patients between 12 and 17 years of age the year prior).
Bottom line: VTEP guidelines can be systematically implemented via an EMR-based tool in a pediatric inpatient population.
Citation: Mahajerin A, Webber E, Morris J, Taylor K, Saysana M. Development and implementation results of a venous thromboembolism prophylaxis guideline in a tertiary care pediatric hospital. Hosp Pediatr. 2015;5(12):630-636.
References
- Spyropoulos AC, Mahan C. Venous thromboembolism prophylaxis in the medical patient: controversies and perspectives. Am J Med. 2009;122(12):1077-1084.
- Kahn SR, Morrison DR, Cohen JM, et al. Interventions for implementation of thromboprophylaxis in hospitalized medical and surgical patients at risk for venous thromboembolism. Cochrane Database Syst Rev. 2013;7:CD008201.
- Takemoto CM, Sohi S, Desai K, et al. Hospital-associated venous thromboembolism in children: incidence and clinical characteristics. J Pediatr. 2014;164(2):332-338.
- Raffini L, Trimarchi T, Beliveau J, Davis D. Thromboprophylaxis in a pediatric hospital: a patient-safety and quality-improvement initiative. Pediatrics. 2011;127(5):e1326-1332.

Continuous Chest Compressions Do Not Improve Outcome Compared to Chest Compressions Interrupted for Ventilation
Clinical question: In cardiopulmonary resuscitation, do continuous chest compressions improve survival or neurologic outcome compared to chest compressions interrupted for ventilation?
Background: Animal models have demonstrated that interruptions in chest compressions are associated with decreased survival and worse neurologic outcome in cardiac arrests. Observational studies in humans have suggested that for out-of-hospital cardiac arrests, continuous compressions result in improved survival.
Study Design: Unblinded, randomized, cluster design with crossover.
Setting: One hundred fourteen emergency medical service (EMS) agencies across eight clinical sites in North America.
Synopsis: Patients with out-of-hospital cardiac arrest received either continuous chest compressions with asynchronous positive-pressure ventilations or interrupted compressions at a rate of 30 compressions to two ventilations. EMS agencies were divided into clusters and randomly assigned to deliver either resuscitation strategy. Twice per year, each cluster switched treatment strategies.
During the active enrollment phase, 12,653 patients were enrolled in the intervention arm and 11,058 were enrolled in the control arm. The primary outcome of survival to hospital discharge was comparable between the two groups, with 9.0% survival rate in the intervention group as compared to 9.7% in the control group (P=0.07). The secondary outcome of survivorship with favorable neurologic status was similar at 7.0% in the intervention group and 7.7% in the control group.
There was only a small difference in the proportion of minutes devoted to compressions between the two groups, so the similarity in outcomes may be reflective of high-quality chest compressions. Additional limitations include a lack of standardization of post-resuscitation care and a lack of measurement of oxygen or ventilation delivered.
Bottom line: For out-of-hospital cardiac arrests, continuous chest compressions with positive-pressure ventilation did not increase survival or improve neurologic outcome compared to interrupted chest compressions.
Citation: Nichol G, Lerou B, Wang H, et al. Trial of continuous or interrupted chest compressions during CPR. N Engl J Med. 2015;373(23):2203-2214.
Clinical question: In cardiopulmonary resuscitation, do continuous chest compressions improve survival or neurologic outcome compared to chest compressions interrupted for ventilation?
Background: Animal models have demonstrated that interruptions in chest compressions are associated with decreased survival and worse neurologic outcome in cardiac arrests. Observational studies in humans have suggested that for out-of-hospital cardiac arrests, continuous compressions result in improved survival.
Study Design: Unblinded, randomized, cluster design with crossover.
Setting: One hundred fourteen emergency medical service (EMS) agencies across eight clinical sites in North America.
Synopsis: Patients with out-of-hospital cardiac arrest received either continuous chest compressions with asynchronous positive-pressure ventilations or interrupted compressions at a rate of 30 compressions to two ventilations. EMS agencies were divided into clusters and randomly assigned to deliver either resuscitation strategy. Twice per year, each cluster switched treatment strategies.
During the active enrollment phase, 12,653 patients were enrolled in the intervention arm and 11,058 were enrolled in the control arm. The primary outcome of survival to hospital discharge was comparable between the two groups, with 9.0% survival rate in the intervention group as compared to 9.7% in the control group (P=0.07). The secondary outcome of survivorship with favorable neurologic status was similar at 7.0% in the intervention group and 7.7% in the control group.
There was only a small difference in the proportion of minutes devoted to compressions between the two groups, so the similarity in outcomes may be reflective of high-quality chest compressions. Additional limitations include a lack of standardization of post-resuscitation care and a lack of measurement of oxygen or ventilation delivered.
Bottom line: For out-of-hospital cardiac arrests, continuous chest compressions with positive-pressure ventilation did not increase survival or improve neurologic outcome compared to interrupted chest compressions.
Citation: Nichol G, Lerou B, Wang H, et al. Trial of continuous or interrupted chest compressions during CPR. N Engl J Med. 2015;373(23):2203-2214.
Clinical question: In cardiopulmonary resuscitation, do continuous chest compressions improve survival or neurologic outcome compared to chest compressions interrupted for ventilation?
Background: Animal models have demonstrated that interruptions in chest compressions are associated with decreased survival and worse neurologic outcome in cardiac arrests. Observational studies in humans have suggested that for out-of-hospital cardiac arrests, continuous compressions result in improved survival.
Study Design: Unblinded, randomized, cluster design with crossover.
Setting: One hundred fourteen emergency medical service (EMS) agencies across eight clinical sites in North America.
Synopsis: Patients with out-of-hospital cardiac arrest received either continuous chest compressions with asynchronous positive-pressure ventilations or interrupted compressions at a rate of 30 compressions to two ventilations. EMS agencies were divided into clusters and randomly assigned to deliver either resuscitation strategy. Twice per year, each cluster switched treatment strategies.
During the active enrollment phase, 12,653 patients were enrolled in the intervention arm and 11,058 were enrolled in the control arm. The primary outcome of survival to hospital discharge was comparable between the two groups, with 9.0% survival rate in the intervention group as compared to 9.7% in the control group (P=0.07). The secondary outcome of survivorship with favorable neurologic status was similar at 7.0% in the intervention group and 7.7% in the control group.
There was only a small difference in the proportion of minutes devoted to compressions between the two groups, so the similarity in outcomes may be reflective of high-quality chest compressions. Additional limitations include a lack of standardization of post-resuscitation care and a lack of measurement of oxygen or ventilation delivered.
Bottom line: For out-of-hospital cardiac arrests, continuous chest compressions with positive-pressure ventilation did not increase survival or improve neurologic outcome compared to interrupted chest compressions.
Citation: Nichol G, Lerou B, Wang H, et al. Trial of continuous or interrupted chest compressions during CPR. N Engl J Med. 2015;373(23):2203-2214.
ATRIA Better at Predicting Stroke Risk in Patients with Atrial Fibrillation Than CHADS2, CHA2DS2-VAS
Clinical question: Does the Anticoagulation and Risk Factors in Atrial Fibrillation (ATRIA) risk score more accurately identify patients with atrial fibrillation (Afib) who are at low risk for ischemic stroke than the CHADS2 or CHA2DS2-VASc score?
Background: More accurate and reliable stroke risk prediction tools are needed to optimize anticoagulation decision making in patients with Afib. Recently, a new clinically based risk score, the ATRIA, has been developed and validated. This risk score assigns points based on four age categories (as well as an interaction of age and prior stroke); female gender; renal function; and history of diabetes, congestive heart failure, and hypertension. This study compared the predictive ability of the ATRIA risk score with the CHADS2 and CHA2DS2-VASc risk scores and their implications for anticoagulant treatment in Afib patients.
Study Design: Retrospective cohort study.
Setting: Afib patients not using warfarin from the United Kingdom’s Clinical Practice Research Datalink (CPRD) database, January 1998 to January 2012.
Synopsis: A total of 60,594 patients with Afib were followed until occurrence of ischemic stroke, prescription of warfarin, death, or the study’s end. The annualized stroke rate was 2.99%. Patients with moderate and high-risk CHA2DS2-VASc scores had lower event rates than those with corresponding ATRIA and CHADS2 scores. C-statistics for full point scores were 0.70 (95% CI, 0.69–0.71) for ATRIA and 0.68 (95% CI, 0.67–0.69) for both CHADS2 and CHA2DS2-VASc scores. The net reclassification index of ATRIA compared with CHADS2 and CHA2DS2-VASc risk scores were 0.137 and 0.233, respectively, reflecting that the ATRIA risk score better categorizes patients developing an event.
ATRIA risk score more accurately identified low-risk patients than the CHA2DS2-VASc score assigned to higher-risk categories. The results persisted even after restricting analysis to more recent follow-up, excluding unspecified strokes and excluding renal dysfunction as a predictor. Most improvements with ATRIA were the result of “down classification,” suggesting that using the CHA2DS2-VASc risk score could lead to overtreatment of patients at very low risk of stroke.
Bottom line: The ATRIA risk score better identifies Afib patients who are at low risk for stroke compared to CHADS2 and CHA2DS2-VASc scores.
Citation: van den Ham HA, Klungel OH, Singer DE, Leufkens HG, van Staa TP. Comparative performance of ATRIA, CHADS2, and CHA2DS2-VASc risk scores predicting stroke in patients with atrial fibrillation: results from a national primary care database. J Am Coll Cardiol. 2015;66(17):1851-1959.
Clinical question: Does the Anticoagulation and Risk Factors in Atrial Fibrillation (ATRIA) risk score more accurately identify patients with atrial fibrillation (Afib) who are at low risk for ischemic stroke than the CHADS2 or CHA2DS2-VASc score?
Background: More accurate and reliable stroke risk prediction tools are needed to optimize anticoagulation decision making in patients with Afib. Recently, a new clinically based risk score, the ATRIA, has been developed and validated. This risk score assigns points based on four age categories (as well as an interaction of age and prior stroke); female gender; renal function; and history of diabetes, congestive heart failure, and hypertension. This study compared the predictive ability of the ATRIA risk score with the CHADS2 and CHA2DS2-VASc risk scores and their implications for anticoagulant treatment in Afib patients.
Study Design: Retrospective cohort study.
Setting: Afib patients not using warfarin from the United Kingdom’s Clinical Practice Research Datalink (CPRD) database, January 1998 to January 2012.
Synopsis: A total of 60,594 patients with Afib were followed until occurrence of ischemic stroke, prescription of warfarin, death, or the study’s end. The annualized stroke rate was 2.99%. Patients with moderate and high-risk CHA2DS2-VASc scores had lower event rates than those with corresponding ATRIA and CHADS2 scores. C-statistics for full point scores were 0.70 (95% CI, 0.69–0.71) for ATRIA and 0.68 (95% CI, 0.67–0.69) for both CHADS2 and CHA2DS2-VASc scores. The net reclassification index of ATRIA compared with CHADS2 and CHA2DS2-VASc risk scores were 0.137 and 0.233, respectively, reflecting that the ATRIA risk score better categorizes patients developing an event.
ATRIA risk score more accurately identified low-risk patients than the CHA2DS2-VASc score assigned to higher-risk categories. The results persisted even after restricting analysis to more recent follow-up, excluding unspecified strokes and excluding renal dysfunction as a predictor. Most improvements with ATRIA were the result of “down classification,” suggesting that using the CHA2DS2-VASc risk score could lead to overtreatment of patients at very low risk of stroke.
Bottom line: The ATRIA risk score better identifies Afib patients who are at low risk for stroke compared to CHADS2 and CHA2DS2-VASc scores.
Citation: van den Ham HA, Klungel OH, Singer DE, Leufkens HG, van Staa TP. Comparative performance of ATRIA, CHADS2, and CHA2DS2-VASc risk scores predicting stroke in patients with atrial fibrillation: results from a national primary care database. J Am Coll Cardiol. 2015;66(17):1851-1959.
Clinical question: Does the Anticoagulation and Risk Factors in Atrial Fibrillation (ATRIA) risk score more accurately identify patients with atrial fibrillation (Afib) who are at low risk for ischemic stroke than the CHADS2 or CHA2DS2-VASc score?
Background: More accurate and reliable stroke risk prediction tools are needed to optimize anticoagulation decision making in patients with Afib. Recently, a new clinically based risk score, the ATRIA, has been developed and validated. This risk score assigns points based on four age categories (as well as an interaction of age and prior stroke); female gender; renal function; and history of diabetes, congestive heart failure, and hypertension. This study compared the predictive ability of the ATRIA risk score with the CHADS2 and CHA2DS2-VASc risk scores and their implications for anticoagulant treatment in Afib patients.
Study Design: Retrospective cohort study.
Setting: Afib patients not using warfarin from the United Kingdom’s Clinical Practice Research Datalink (CPRD) database, January 1998 to January 2012.
Synopsis: A total of 60,594 patients with Afib were followed until occurrence of ischemic stroke, prescription of warfarin, death, or the study’s end. The annualized stroke rate was 2.99%. Patients with moderate and high-risk CHA2DS2-VASc scores had lower event rates than those with corresponding ATRIA and CHADS2 scores. C-statistics for full point scores were 0.70 (95% CI, 0.69–0.71) for ATRIA and 0.68 (95% CI, 0.67–0.69) for both CHADS2 and CHA2DS2-VASc scores. The net reclassification index of ATRIA compared with CHADS2 and CHA2DS2-VASc risk scores were 0.137 and 0.233, respectively, reflecting that the ATRIA risk score better categorizes patients developing an event.
ATRIA risk score more accurately identified low-risk patients than the CHA2DS2-VASc score assigned to higher-risk categories. The results persisted even after restricting analysis to more recent follow-up, excluding unspecified strokes and excluding renal dysfunction as a predictor. Most improvements with ATRIA were the result of “down classification,” suggesting that using the CHA2DS2-VASc risk score could lead to overtreatment of patients at very low risk of stroke.
Bottom line: The ATRIA risk score better identifies Afib patients who are at low risk for stroke compared to CHADS2 and CHA2DS2-VASc scores.
Citation: van den Ham HA, Klungel OH, Singer DE, Leufkens HG, van Staa TP. Comparative performance of ATRIA, CHADS2, and CHA2DS2-VASc risk scores predicting stroke in patients with atrial fibrillation: results from a national primary care database. J Am Coll Cardiol. 2015;66(17):1851-1959.
New Clinical Guides Available on Anemia, Congestive Heart Failure
SHM’s implementation tool kits provide hospitalists the information and tools they need to lead quality improvement projects on specific clinical topics, including two recent releases focused on anemia and congestive heart failure.
One third of the world’s population suffers from anemia, and SHM’s Center for Hospital Innovation and Improvement recently released a tool kit to improve outcomes by providing a framework for hospital-based anemia management quality improvement projects. It reviews each step of the process from forming a multidisciplinary team, obtaining institutional support, assessing baseline performance, and defining key metrics to implementing changes and monitoring their effects, with a focus on blood transfusion best practices.
Such projects can be expected to improve patient outcomes, improve the utilization of scarce resources such as allogeneic blood, and decrease transfusion-related adverse events, enabling hospitals to provide a better quality of care at a lower cost. In today’s competitive healthcare environment, these are quality gains and cost savings that hospitals cannot afford to miss.
Another recent release from the center, the congestive heart failure implementation guide, reviews methods to optimize heart failure care during and after hospital admission episodes. Congestive heart failure is responsible for 11 million physician visits annually and more hospitalizations than all forms of cancer combined.
Other recently added tool kits cover COPD, glycemic control, opioid monitoring, delirium, and pain management.
For more information on these tool kits and how the Center for Hospital Innovation and Improvement can share its proven best practices with your hospital, visit www.hospitalmedicine.org/CenterTJ16.
SHM’s implementation tool kits provide hospitalists the information and tools they need to lead quality improvement projects on specific clinical topics, including two recent releases focused on anemia and congestive heart failure.
One third of the world’s population suffers from anemia, and SHM’s Center for Hospital Innovation and Improvement recently released a tool kit to improve outcomes by providing a framework for hospital-based anemia management quality improvement projects. It reviews each step of the process from forming a multidisciplinary team, obtaining institutional support, assessing baseline performance, and defining key metrics to implementing changes and monitoring their effects, with a focus on blood transfusion best practices.
Such projects can be expected to improve patient outcomes, improve the utilization of scarce resources such as allogeneic blood, and decrease transfusion-related adverse events, enabling hospitals to provide a better quality of care at a lower cost. In today’s competitive healthcare environment, these are quality gains and cost savings that hospitals cannot afford to miss.
Another recent release from the center, the congestive heart failure implementation guide, reviews methods to optimize heart failure care during and after hospital admission episodes. Congestive heart failure is responsible for 11 million physician visits annually and more hospitalizations than all forms of cancer combined.
Other recently added tool kits cover COPD, glycemic control, opioid monitoring, delirium, and pain management.
For more information on these tool kits and how the Center for Hospital Innovation and Improvement can share its proven best practices with your hospital, visit www.hospitalmedicine.org/CenterTJ16.
SHM’s implementation tool kits provide hospitalists the information and tools they need to lead quality improvement projects on specific clinical topics, including two recent releases focused on anemia and congestive heart failure.
One third of the world’s population suffers from anemia, and SHM’s Center for Hospital Innovation and Improvement recently released a tool kit to improve outcomes by providing a framework for hospital-based anemia management quality improvement projects. It reviews each step of the process from forming a multidisciplinary team, obtaining institutional support, assessing baseline performance, and defining key metrics to implementing changes and monitoring their effects, with a focus on blood transfusion best practices.
Such projects can be expected to improve patient outcomes, improve the utilization of scarce resources such as allogeneic blood, and decrease transfusion-related adverse events, enabling hospitals to provide a better quality of care at a lower cost. In today’s competitive healthcare environment, these are quality gains and cost savings that hospitals cannot afford to miss.
Another recent release from the center, the congestive heart failure implementation guide, reviews methods to optimize heart failure care during and after hospital admission episodes. Congestive heart failure is responsible for 11 million physician visits annually and more hospitalizations than all forms of cancer combined.
Other recently added tool kits cover COPD, glycemic control, opioid monitoring, delirium, and pain management.
For more information on these tool kits and how the Center for Hospital Innovation and Improvement can share its proven best practices with your hospital, visit www.hospitalmedicine.org/CenterTJ16.
Should a Patient Who Requests Alcohol Detoxification Be Admitted or Treated as Outpatient?
Case
A 42-year-old man with a history of posttraumatic stress disorder (PTSD), hypertension, and alcohol use disorder (AUD) presents to the ED requesting alcohol detoxification. He has had six admissions in the last six months for alcohol detoxification. Two years ago, the patient had a documented alcohol withdrawal seizure. His last drink was eight hours ago, and he currently drinks a liter of vodka a day. On exam, his pulse rate is 126 bpm, and his blood pressure is 162/91 mm Hg. He appears anxious and has bilateral hand tremors. His serum ethanol level is 388.6 mg/dL.
Overview
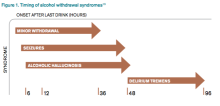
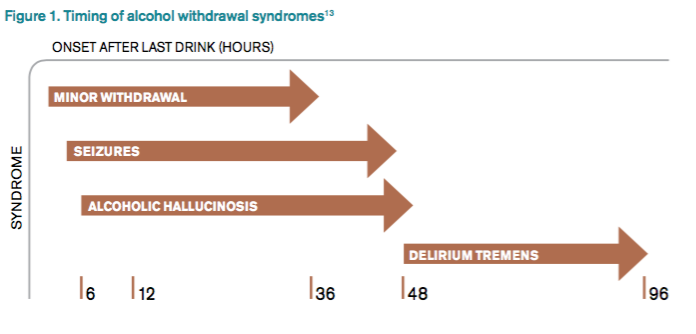
DSM-5 integrated alcohol abuse and alcohol dependence that were previously classified in DSM-IV into AUDs with mild, moderate, and severe subclassifications. AUDs are the most serious substance abuse problem in the U.S. In the general population, the lifetime prevalence of alcohol abuse is 17.8% and of alcohol dependence is 12.5%.1–3 One study estimates that 24% of adult patients brought to the ED by ambulance suffer from alcoholism, and approximately 10% to 32% of hospitalized medical patients have an AUD.4–8 Patients who stop drinking will develop alcohol withdrawal as early as six hours after their last drink (see Figure 1). The majority of patients at risk of alcohol withdrawal syndrome (AWS) will develop only minor uncomplicated symptoms, but up to 20% will develop symptoms associated with complicated AWS, including withdrawal seizures and delirium tremens (DT).9 It is not entirely clear why some individuals suffer from more severe withdrawal symptoms than others, but genetic predisposition may play a role.10
DT is a syndrome characterized by agitation, disorientation, hallucinations, and autonomic instability (tachycardia, hypertension, hyperthermia, and diaphoresis) in the setting of acute reduction or abstinence from alcohol and is associated with a mortality rate as high as 20%.11 Complicated AWS is associated with increased in-hospital morbidity and mortality, longer lengths of stay, inflated costs of care, increased burden and frustration of nursing and medical staff, and worse cognitive functioning.9 In 80% of cases, the symptoms of uncomplicated alcohol withdrawal do not require aggressive medical intervention and usually disappear within two to seven days of the last drink.12 Physicians making triage decisions for patients who present to the ED in need of detoxification face a difficult dilemma concerning inpatient versus outpatient treatment.
Review of the Data
The literature on both inpatient and outpatient management and treatment of AWS is well-described. Currently, there are no guidelines or consensus on whether to admit patients with alcohol abuse syndromes to the hospital when the request for detoxification is made. Admission should be considered for all patients experiencing alcohol withdrawal who present to the ED.13 Patients with mild AWS may be discharged if they do not require admission for an additional medical condition, but patients experiencing moderate to severe withdrawal require admission for monitoring and treatment. Many physicians use a simple assessment of past history of DT and pulse rate, which may be easily evaluated in clinical settings, to readily identify patients who are at high risk of developing DT during an alcohol dependence period.14
Since 1978, the Clinical Institute Withdrawal Assessment for Alcohol (CIWA) has been consistently used for both monitoring patients with alcohol withdrawal and for making an initial assessment. CIWA-Ar was developed as a revised scale and is frequently used to monitor the severity of ongoing alcohol withdrawal and the response to treatment for the clinical care of patients in alcohol withdrawal (see Figure 2). CIWA-Ar was not developed to identify patients at risk for AWS but is frequently used to determine if patients require admission to the hospital for detoxification.15 Patients with CIWA-Ar scores > 15 require inpatient detoxification. Patients with scores between 8 and 15 should be admitted if they have a history of prior seizures or DT but could otherwise be considered for outpatient detoxification. Patients with scores < 8, which are considered mild alcohol withdrawal, can likely be safely treated as outpatients unless they have a history of DT or alcohol withdrawal seizures.16 Because symptoms of severe alcohol withdrawal are often not present for more than six hours after the patient’s last drink, or often longer, CIWA-Ar is limited and does not identify patients who are otherwise at high risk for complicated withdrawal. A protocol was developed incorporating the patient’s history of alcohol withdrawal seizure, DT, and the CIWA to evaluate the outcome of outpatient versus inpatient detoxification.16
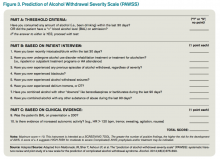
The most promising tool to screen patients for AWS was developed recently by researchers at Stanford University in Stanford, Calif., using an extensive systematic literature search to identify evidence-based clinical factors associated with the development of AWS.15 The Prediction of Alcohol Withdrawal Severity Scale (PAWSS) was subsequently constructed from 10 items correlating with complicated AWS (see Figure 3). When using a PAWSS score cutoff of ≥ 4, the predictive value of identifying a patient who is at risk for complicated withdrawal is significantly increased to 93.1%. This tool has only been used in medically ill patients but could be extrapolated for use in patients who present to an acute-care setting requesting inpatient detoxification.
Patients presenting to the ED with alcohol withdrawal seizures have been shown to have an associated 35% risk of progression to DT when found to have a low platelet count, low blood pyridoxine, and a high blood level of homocysteine. In another retrospective cohort study in Hepatology, three clinical features were identified to be associated with an increased risk for DT: alcohol dependence, a prior history of DT, and a higher pulse rate at admission (> 100 bpm).14
Instructions for the assessment of the patient who requests detoxification are as follows:
- A patient whose last drink of alcohol was more than five days ago and who shows no signs of withdrawal is unlikely to develop significant withdrawal symptoms and does not require inpatient detoxification.
- Other medical and psychiatric conditions should be evaluated for admission including alcohol use disorder complications.
- Calculate CIWA-Ar score:
Scores < 8 may not need detoxification; consider calculating PAWSS score.
Scores of 8 to 15 without symptoms of DT or seizures can be treated as an outpatient detoxification if no contraindication.
Scores of ≥ 15 should be admitted to the hospital.
- Calculate PAWSS score:
Scores ≥ 4 suggest high risk for moderate to severe complicated AWS, and admission should be considered.
Scores < 4 suggest lower risk for complicated AWS, and outpatient treatment should be considered if patients do not have a medical or surgical diagnosis requiring admission.
Back to the Case
At the time of his presentation, the patient was beginning to show signs of early withdrawal symptoms, including tremor and tachycardia, despite having an elevated blood alcohol level. This patient had a PAWSS score of 6, placing him at increased risk of complicated AWS, and a CIWA-Ar score of 13. He was subsequently admitted to the hospital, and symptom-triggered therapy for treatment of his alcohol withdrawal was used. The patient’s CIWA-Ar score peaked at 21 some 24 hours after his last drink. The patient otherwise had an uncomplicated four-day hospital course due to persistent nausea.
Bottom Line
Hospitalists unsure of which patients should be admitted for alcohol detoxification can use the PAWSS tool and an initial CIWA-Ar score to help determine a patient’s risk for developing complicated AWS. TH
Dr. Velasquez and Dr. Kornsawad are assistant professors and hospitalists at the University of Texas Health Science Center at San Antonio. Dr. Velasquez also serves as assistant professor and hospitalist at the South Texas Veterans Health Care System serving the San Antonio area.
References
- Grant BF, Stinson FS, Dawson DA, et al. Prevalence and co-occurrence of substance use disorder and independent mood and anxiety disorders: results from the National Epidemiologic Survey on Alcohol and Related Conditions. Arch Gen Psychiatry. 2004;61(8):807-816.
- Lieber CS. Medical disorders of alcoholism. N Engl J Med. 1995;333(16):1058-1065.
- Hasin SD, Stinson SF, Ogburn E, Grant BF. Prevalence, correlates, disability, and comorbidity of DSM-IV alcohol abuse and dependence in the United States: results from the National Epidemiologic Survey on Alcohol and Related Conditions. Arch Gen Psychiatry. 2007;64(7):830-842.
- Whiteman PJ, Hoffman RS, Goldfrank LR. Alcoholism in the emergency department: an epidemiologic study. Acad Emerg Med. 2000;7(1):14-20.
- Nielson SD, Storgarrd H, Moesgarrd F, Gluud C. Prevalence of alcohol problems among adult somatic in-patients of a Copenhagen hospital. Alcohol Alcohol. 1994;29(5):583-590.
- Smothers BA, Yahr HT, Ruhl CE. Detection of alcohol use disorders in general hospital admissions in the United States. Arch Intern Med. 2004;164(7):749-756.
- Dolman JM, Hawkes ND. Combining the audit questionnaire and biochemical markers to assess alcohol use and risk of alcohol withdrawal in medical inpatients. Alcohol Alcohol. 2005;40(6):515-519.
- Doering-Silveira J, Fidalgo TM, Nascimento CL, et al. Assessing alcohol dependence in hospitalized patients. Int J Environ Res Public Health. 2014;11(6):5783-5791.
- Maldonado JR, Sher Y, Das S, et al. Prospective validation study of the prediction of alcohol withdrawal severity scale (PAWSS) in medically ill inpatients: a new scale for the prediction of complicated alcohol withdrawal syndrome. Alcohol Alcohol. 2015;50(5):509-518.
- Saitz R, O’Malley SS. Pharmacotherapies for alcohol abuse. Withdrawal and treatment. Med Clin North Am. 1997;81(4):881-907.
- Turner RC, Lichstein PR, Pedan Jr JG, Busher JT, Waivers LE. Alcohol withdrawal syndromes: a review of pathophysiology, clinical presentation, and treatment. J Gen Intern Med. 1989;4(5):432-444.
- Schuckit MA. Alcohol-use disorders. Lancet. 2009;373(9662):492-501.
- Stehman CR, Mycyk MB. A rational approach to the treatment of alcohol withdrawal in the ED. Am J Emerg Med. 2013;31(4):734-742.
- Lee JH, Jang MK, Lee JY, et al. Clinical predictors for delirium tremens in alcohol dependence. J Gastroenterol Hepatol. 2005;20(12):1833-1837.
- Maldonado JR, Sher Y, Ashouri JF, et al. The “prediction of alcohol withdrawal severity scale” (PAWSS): systematic literature review and pilot study of a new scale for the prediction of complicated alcohol withdrawal syndrome. Alcohol. 2014;48(4):375-390.
- Stephens JR, Liles AE, Dancel R, Gilchrist M, Kirsch J, DeWalt DA. Who needs inpatient detox? Development and implementation of a hospitalist protocol for the evaluation of patients for alcohol detoxification. J Gen Intern Med. 2014;29(4):587-593.
Case
A 42-year-old man with a history of posttraumatic stress disorder (PTSD), hypertension, and alcohol use disorder (AUD) presents to the ED requesting alcohol detoxification. He has had six admissions in the last six months for alcohol detoxification. Two years ago, the patient had a documented alcohol withdrawal seizure. His last drink was eight hours ago, and he currently drinks a liter of vodka a day. On exam, his pulse rate is 126 bpm, and his blood pressure is 162/91 mm Hg. He appears anxious and has bilateral hand tremors. His serum ethanol level is 388.6 mg/dL.
Overview
DSM-5 integrated alcohol abuse and alcohol dependence that were previously classified in DSM-IV into AUDs with mild, moderate, and severe subclassifications. AUDs are the most serious substance abuse problem in the U.S. In the general population, the lifetime prevalence of alcohol abuse is 17.8% and of alcohol dependence is 12.5%.1–3 One study estimates that 24% of adult patients brought to the ED by ambulance suffer from alcoholism, and approximately 10% to 32% of hospitalized medical patients have an AUD.4–8 Patients who stop drinking will develop alcohol withdrawal as early as six hours after their last drink (see Figure 1). The majority of patients at risk of alcohol withdrawal syndrome (AWS) will develop only minor uncomplicated symptoms, but up to 20% will develop symptoms associated with complicated AWS, including withdrawal seizures and delirium tremens (DT).9 It is not entirely clear why some individuals suffer from more severe withdrawal symptoms than others, but genetic predisposition may play a role.10
DT is a syndrome characterized by agitation, disorientation, hallucinations, and autonomic instability (tachycardia, hypertension, hyperthermia, and diaphoresis) in the setting of acute reduction or abstinence from alcohol and is associated with a mortality rate as high as 20%.11 Complicated AWS is associated with increased in-hospital morbidity and mortality, longer lengths of stay, inflated costs of care, increased burden and frustration of nursing and medical staff, and worse cognitive functioning.9 In 80% of cases, the symptoms of uncomplicated alcohol withdrawal do not require aggressive medical intervention and usually disappear within two to seven days of the last drink.12 Physicians making triage decisions for patients who present to the ED in need of detoxification face a difficult dilemma concerning inpatient versus outpatient treatment.
Review of the Data
The literature on both inpatient and outpatient management and treatment of AWS is well-described. Currently, there are no guidelines or consensus on whether to admit patients with alcohol abuse syndromes to the hospital when the request for detoxification is made. Admission should be considered for all patients experiencing alcohol withdrawal who present to the ED.13 Patients with mild AWS may be discharged if they do not require admission for an additional medical condition, but patients experiencing moderate to severe withdrawal require admission for monitoring and treatment. Many physicians use a simple assessment of past history of DT and pulse rate, which may be easily evaluated in clinical settings, to readily identify patients who are at high risk of developing DT during an alcohol dependence period.14
Since 1978, the Clinical Institute Withdrawal Assessment for Alcohol (CIWA) has been consistently used for both monitoring patients with alcohol withdrawal and for making an initial assessment. CIWA-Ar was developed as a revised scale and is frequently used to monitor the severity of ongoing alcohol withdrawal and the response to treatment for the clinical care of patients in alcohol withdrawal (see Figure 2). CIWA-Ar was not developed to identify patients at risk for AWS but is frequently used to determine if patients require admission to the hospital for detoxification.15 Patients with CIWA-Ar scores > 15 require inpatient detoxification. Patients with scores between 8 and 15 should be admitted if they have a history of prior seizures or DT but could otherwise be considered for outpatient detoxification. Patients with scores < 8, which are considered mild alcohol withdrawal, can likely be safely treated as outpatients unless they have a history of DT or alcohol withdrawal seizures.16 Because symptoms of severe alcohol withdrawal are often not present for more than six hours after the patient’s last drink, or often longer, CIWA-Ar is limited and does not identify patients who are otherwise at high risk for complicated withdrawal. A protocol was developed incorporating the patient’s history of alcohol withdrawal seizure, DT, and the CIWA to evaluate the outcome of outpatient versus inpatient detoxification.16
The most promising tool to screen patients for AWS was developed recently by researchers at Stanford University in Stanford, Calif., using an extensive systematic literature search to identify evidence-based clinical factors associated with the development of AWS.15 The Prediction of Alcohol Withdrawal Severity Scale (PAWSS) was subsequently constructed from 10 items correlating with complicated AWS (see Figure 3). When using a PAWSS score cutoff of ≥ 4, the predictive value of identifying a patient who is at risk for complicated withdrawal is significantly increased to 93.1%. This tool has only been used in medically ill patients but could be extrapolated for use in patients who present to an acute-care setting requesting inpatient detoxification.
Patients presenting to the ED with alcohol withdrawal seizures have been shown to have an associated 35% risk of progression to DT when found to have a low platelet count, low blood pyridoxine, and a high blood level of homocysteine. In another retrospective cohort study in Hepatology, three clinical features were identified to be associated with an increased risk for DT: alcohol dependence, a prior history of DT, and a higher pulse rate at admission (> 100 bpm).14
Instructions for the assessment of the patient who requests detoxification are as follows:
- A patient whose last drink of alcohol was more than five days ago and who shows no signs of withdrawal is unlikely to develop significant withdrawal symptoms and does not require inpatient detoxification.
- Other medical and psychiatric conditions should be evaluated for admission including alcohol use disorder complications.
- Calculate CIWA-Ar score:
Scores < 8 may not need detoxification; consider calculating PAWSS score.
Scores of 8 to 15 without symptoms of DT or seizures can be treated as an outpatient detoxification if no contraindication.
Scores of ≥ 15 should be admitted to the hospital.
- Calculate PAWSS score:
Scores ≥ 4 suggest high risk for moderate to severe complicated AWS, and admission should be considered.
Scores < 4 suggest lower risk for complicated AWS, and outpatient treatment should be considered if patients do not have a medical or surgical diagnosis requiring admission.
Back to the Case
At the time of his presentation, the patient was beginning to show signs of early withdrawal symptoms, including tremor and tachycardia, despite having an elevated blood alcohol level. This patient had a PAWSS score of 6, placing him at increased risk of complicated AWS, and a CIWA-Ar score of 13. He was subsequently admitted to the hospital, and symptom-triggered therapy for treatment of his alcohol withdrawal was used. The patient’s CIWA-Ar score peaked at 21 some 24 hours after his last drink. The patient otherwise had an uncomplicated four-day hospital course due to persistent nausea.
Bottom Line
Hospitalists unsure of which patients should be admitted for alcohol detoxification can use the PAWSS tool and an initial CIWA-Ar score to help determine a patient’s risk for developing complicated AWS. TH
Dr. Velasquez and Dr. Kornsawad are assistant professors and hospitalists at the University of Texas Health Science Center at San Antonio. Dr. Velasquez also serves as assistant professor and hospitalist at the South Texas Veterans Health Care System serving the San Antonio area.
References
- Grant BF, Stinson FS, Dawson DA, et al. Prevalence and co-occurrence of substance use disorder and independent mood and anxiety disorders: results from the National Epidemiologic Survey on Alcohol and Related Conditions. Arch Gen Psychiatry. 2004;61(8):807-816.
- Lieber CS. Medical disorders of alcoholism. N Engl J Med. 1995;333(16):1058-1065.
- Hasin SD, Stinson SF, Ogburn E, Grant BF. Prevalence, correlates, disability, and comorbidity of DSM-IV alcohol abuse and dependence in the United States: results from the National Epidemiologic Survey on Alcohol and Related Conditions. Arch Gen Psychiatry. 2007;64(7):830-842.
- Whiteman PJ, Hoffman RS, Goldfrank LR. Alcoholism in the emergency department: an epidemiologic study. Acad Emerg Med. 2000;7(1):14-20.
- Nielson SD, Storgarrd H, Moesgarrd F, Gluud C. Prevalence of alcohol problems among adult somatic in-patients of a Copenhagen hospital. Alcohol Alcohol. 1994;29(5):583-590.
- Smothers BA, Yahr HT, Ruhl CE. Detection of alcohol use disorders in general hospital admissions in the United States. Arch Intern Med. 2004;164(7):749-756.
- Dolman JM, Hawkes ND. Combining the audit questionnaire and biochemical markers to assess alcohol use and risk of alcohol withdrawal in medical inpatients. Alcohol Alcohol. 2005;40(6):515-519.
- Doering-Silveira J, Fidalgo TM, Nascimento CL, et al. Assessing alcohol dependence in hospitalized patients. Int J Environ Res Public Health. 2014;11(6):5783-5791.
- Maldonado JR, Sher Y, Das S, et al. Prospective validation study of the prediction of alcohol withdrawal severity scale (PAWSS) in medically ill inpatients: a new scale for the prediction of complicated alcohol withdrawal syndrome. Alcohol Alcohol. 2015;50(5):509-518.
- Saitz R, O’Malley SS. Pharmacotherapies for alcohol abuse. Withdrawal and treatment. Med Clin North Am. 1997;81(4):881-907.
- Turner RC, Lichstein PR, Pedan Jr JG, Busher JT, Waivers LE. Alcohol withdrawal syndromes: a review of pathophysiology, clinical presentation, and treatment. J Gen Intern Med. 1989;4(5):432-444.
- Schuckit MA. Alcohol-use disorders. Lancet. 2009;373(9662):492-501.
- Stehman CR, Mycyk MB. A rational approach to the treatment of alcohol withdrawal in the ED. Am J Emerg Med. 2013;31(4):734-742.
- Lee JH, Jang MK, Lee JY, et al. Clinical predictors for delirium tremens in alcohol dependence. J Gastroenterol Hepatol. 2005;20(12):1833-1837.
- Maldonado JR, Sher Y, Ashouri JF, et al. The “prediction of alcohol withdrawal severity scale” (PAWSS): systematic literature review and pilot study of a new scale for the prediction of complicated alcohol withdrawal syndrome. Alcohol. 2014;48(4):375-390.
- Stephens JR, Liles AE, Dancel R, Gilchrist M, Kirsch J, DeWalt DA. Who needs inpatient detox? Development and implementation of a hospitalist protocol for the evaluation of patients for alcohol detoxification. J Gen Intern Med. 2014;29(4):587-593.
Case
A 42-year-old man with a history of posttraumatic stress disorder (PTSD), hypertension, and alcohol use disorder (AUD) presents to the ED requesting alcohol detoxification. He has had six admissions in the last six months for alcohol detoxification. Two years ago, the patient had a documented alcohol withdrawal seizure. His last drink was eight hours ago, and he currently drinks a liter of vodka a day. On exam, his pulse rate is 126 bpm, and his blood pressure is 162/91 mm Hg. He appears anxious and has bilateral hand tremors. His serum ethanol level is 388.6 mg/dL.
Overview
DSM-5 integrated alcohol abuse and alcohol dependence that were previously classified in DSM-IV into AUDs with mild, moderate, and severe subclassifications. AUDs are the most serious substance abuse problem in the U.S. In the general population, the lifetime prevalence of alcohol abuse is 17.8% and of alcohol dependence is 12.5%.1–3 One study estimates that 24% of adult patients brought to the ED by ambulance suffer from alcoholism, and approximately 10% to 32% of hospitalized medical patients have an AUD.4–8 Patients who stop drinking will develop alcohol withdrawal as early as six hours after their last drink (see Figure 1). The majority of patients at risk of alcohol withdrawal syndrome (AWS) will develop only minor uncomplicated symptoms, but up to 20% will develop symptoms associated with complicated AWS, including withdrawal seizures and delirium tremens (DT).9 It is not entirely clear why some individuals suffer from more severe withdrawal symptoms than others, but genetic predisposition may play a role.10
DT is a syndrome characterized by agitation, disorientation, hallucinations, and autonomic instability (tachycardia, hypertension, hyperthermia, and diaphoresis) in the setting of acute reduction or abstinence from alcohol and is associated with a mortality rate as high as 20%.11 Complicated AWS is associated with increased in-hospital morbidity and mortality, longer lengths of stay, inflated costs of care, increased burden and frustration of nursing and medical staff, and worse cognitive functioning.9 In 80% of cases, the symptoms of uncomplicated alcohol withdrawal do not require aggressive medical intervention and usually disappear within two to seven days of the last drink.12 Physicians making triage decisions for patients who present to the ED in need of detoxification face a difficult dilemma concerning inpatient versus outpatient treatment.
Review of the Data
The literature on both inpatient and outpatient management and treatment of AWS is well-described. Currently, there are no guidelines or consensus on whether to admit patients with alcohol abuse syndromes to the hospital when the request for detoxification is made. Admission should be considered for all patients experiencing alcohol withdrawal who present to the ED.13 Patients with mild AWS may be discharged if they do not require admission for an additional medical condition, but patients experiencing moderate to severe withdrawal require admission for monitoring and treatment. Many physicians use a simple assessment of past history of DT and pulse rate, which may be easily evaluated in clinical settings, to readily identify patients who are at high risk of developing DT during an alcohol dependence period.14
Since 1978, the Clinical Institute Withdrawal Assessment for Alcohol (CIWA) has been consistently used for both monitoring patients with alcohol withdrawal and for making an initial assessment. CIWA-Ar was developed as a revised scale and is frequently used to monitor the severity of ongoing alcohol withdrawal and the response to treatment for the clinical care of patients in alcohol withdrawal (see Figure 2). CIWA-Ar was not developed to identify patients at risk for AWS but is frequently used to determine if patients require admission to the hospital for detoxification.15 Patients with CIWA-Ar scores > 15 require inpatient detoxification. Patients with scores between 8 and 15 should be admitted if they have a history of prior seizures or DT but could otherwise be considered for outpatient detoxification. Patients with scores < 8, which are considered mild alcohol withdrawal, can likely be safely treated as outpatients unless they have a history of DT or alcohol withdrawal seizures.16 Because symptoms of severe alcohol withdrawal are often not present for more than six hours after the patient’s last drink, or often longer, CIWA-Ar is limited and does not identify patients who are otherwise at high risk for complicated withdrawal. A protocol was developed incorporating the patient’s history of alcohol withdrawal seizure, DT, and the CIWA to evaluate the outcome of outpatient versus inpatient detoxification.16
The most promising tool to screen patients for AWS was developed recently by researchers at Stanford University in Stanford, Calif., using an extensive systematic literature search to identify evidence-based clinical factors associated with the development of AWS.15 The Prediction of Alcohol Withdrawal Severity Scale (PAWSS) was subsequently constructed from 10 items correlating with complicated AWS (see Figure 3). When using a PAWSS score cutoff of ≥ 4, the predictive value of identifying a patient who is at risk for complicated withdrawal is significantly increased to 93.1%. This tool has only been used in medically ill patients but could be extrapolated for use in patients who present to an acute-care setting requesting inpatient detoxification.
Patients presenting to the ED with alcohol withdrawal seizures have been shown to have an associated 35% risk of progression to DT when found to have a low platelet count, low blood pyridoxine, and a high blood level of homocysteine. In another retrospective cohort study in Hepatology, three clinical features were identified to be associated with an increased risk for DT: alcohol dependence, a prior history of DT, and a higher pulse rate at admission (> 100 bpm).14
Instructions for the assessment of the patient who requests detoxification are as follows:
- A patient whose last drink of alcohol was more than five days ago and who shows no signs of withdrawal is unlikely to develop significant withdrawal symptoms and does not require inpatient detoxification.
- Other medical and psychiatric conditions should be evaluated for admission including alcohol use disorder complications.
- Calculate CIWA-Ar score:
Scores < 8 may not need detoxification; consider calculating PAWSS score.
Scores of 8 to 15 without symptoms of DT or seizures can be treated as an outpatient detoxification if no contraindication.
Scores of ≥ 15 should be admitted to the hospital.
- Calculate PAWSS score:
Scores ≥ 4 suggest high risk for moderate to severe complicated AWS, and admission should be considered.
Scores < 4 suggest lower risk for complicated AWS, and outpatient treatment should be considered if patients do not have a medical or surgical diagnosis requiring admission.
Back to the Case
At the time of his presentation, the patient was beginning to show signs of early withdrawal symptoms, including tremor and tachycardia, despite having an elevated blood alcohol level. This patient had a PAWSS score of 6, placing him at increased risk of complicated AWS, and a CIWA-Ar score of 13. He was subsequently admitted to the hospital, and symptom-triggered therapy for treatment of his alcohol withdrawal was used. The patient’s CIWA-Ar score peaked at 21 some 24 hours after his last drink. The patient otherwise had an uncomplicated four-day hospital course due to persistent nausea.
Bottom Line
Hospitalists unsure of which patients should be admitted for alcohol detoxification can use the PAWSS tool and an initial CIWA-Ar score to help determine a patient’s risk for developing complicated AWS. TH
Dr. Velasquez and Dr. Kornsawad are assistant professors and hospitalists at the University of Texas Health Science Center at San Antonio. Dr. Velasquez also serves as assistant professor and hospitalist at the South Texas Veterans Health Care System serving the San Antonio area.
References
- Grant BF, Stinson FS, Dawson DA, et al. Prevalence and co-occurrence of substance use disorder and independent mood and anxiety disorders: results from the National Epidemiologic Survey on Alcohol and Related Conditions. Arch Gen Psychiatry. 2004;61(8):807-816.
- Lieber CS. Medical disorders of alcoholism. N Engl J Med. 1995;333(16):1058-1065.
- Hasin SD, Stinson SF, Ogburn E, Grant BF. Prevalence, correlates, disability, and comorbidity of DSM-IV alcohol abuse and dependence in the United States: results from the National Epidemiologic Survey on Alcohol and Related Conditions. Arch Gen Psychiatry. 2007;64(7):830-842.
- Whiteman PJ, Hoffman RS, Goldfrank LR. Alcoholism in the emergency department: an epidemiologic study. Acad Emerg Med. 2000;7(1):14-20.
- Nielson SD, Storgarrd H, Moesgarrd F, Gluud C. Prevalence of alcohol problems among adult somatic in-patients of a Copenhagen hospital. Alcohol Alcohol. 1994;29(5):583-590.
- Smothers BA, Yahr HT, Ruhl CE. Detection of alcohol use disorders in general hospital admissions in the United States. Arch Intern Med. 2004;164(7):749-756.
- Dolman JM, Hawkes ND. Combining the audit questionnaire and biochemical markers to assess alcohol use and risk of alcohol withdrawal in medical inpatients. Alcohol Alcohol. 2005;40(6):515-519.
- Doering-Silveira J, Fidalgo TM, Nascimento CL, et al. Assessing alcohol dependence in hospitalized patients. Int J Environ Res Public Health. 2014;11(6):5783-5791.
- Maldonado JR, Sher Y, Das S, et al. Prospective validation study of the prediction of alcohol withdrawal severity scale (PAWSS) in medically ill inpatients: a new scale for the prediction of complicated alcohol withdrawal syndrome. Alcohol Alcohol. 2015;50(5):509-518.
- Saitz R, O’Malley SS. Pharmacotherapies for alcohol abuse. Withdrawal and treatment. Med Clin North Am. 1997;81(4):881-907.
- Turner RC, Lichstein PR, Pedan Jr JG, Busher JT, Waivers LE. Alcohol withdrawal syndromes: a review of pathophysiology, clinical presentation, and treatment. J Gen Intern Med. 1989;4(5):432-444.
- Schuckit MA. Alcohol-use disorders. Lancet. 2009;373(9662):492-501.
- Stehman CR, Mycyk MB. A rational approach to the treatment of alcohol withdrawal in the ED. Am J Emerg Med. 2013;31(4):734-742.
- Lee JH, Jang MK, Lee JY, et al. Clinical predictors for delirium tremens in alcohol dependence. J Gastroenterol Hepatol. 2005;20(12):1833-1837.
- Maldonado JR, Sher Y, Ashouri JF, et al. The “prediction of alcohol withdrawal severity scale” (PAWSS): systematic literature review and pilot study of a new scale for the prediction of complicated alcohol withdrawal syndrome. Alcohol. 2014;48(4):375-390.
- Stephens JR, Liles AE, Dancel R, Gilchrist M, Kirsch J, DeWalt DA. Who needs inpatient detox? Development and implementation of a hospitalist protocol for the evaluation of patients for alcohol detoxification. J Gen Intern Med. 2014;29(4):587-593.
HM16 Session Analysis: Medical, Behavioral Management of Eating Disorders
Presenter: Kyung E. Rhee, MD, MSc, MA
Summary: Eating disorders (ED) are common and have significant morbidity and mortality. EDs are the third most common psychiatric disorder of adolescents with a prevalence of 0.5-2% for anorexia and 0.9-3% for bulimia; 90% of patients are female. Mortality rate can be as high as 10% for anorexia and 1% for bulimia. Diagnosis is formally guided by DSM 5 criteria, but the mnemonic SCOFF can be useful:
- Do you feel or make yourself SICK when eating?
- Do you feel you’ve lost CONTROL of your eating?
- Have you lost one STONE (14 lbs. developed by the British) of weight?
- Do you feel FAT?
- Does FOOD dominate your life?
A detailed history is needed as patients with ED may engage in secretive behaviors to hide their illness. After diagnosis, treatment may be outpatient or inpatient. Medical issues hospitalists are likely to see with inpatients include re-feeding syndrome, various metabolic disturbances, secondary amenorrhea, sleep disturbances, and for patients with bulimia, evidence of dental or esophageal trauma from purging. Differential diagnoses include: IBD, thyroid disease, celiac, diabetes, and Addison’s disease.
Hospitalists’ role in treatment is as part of a multidisciplinary group to manage the medical complications. Inpatient management includes individual and group therapy, monitored group meals, daily blind weights, bathroom visits, and focused lab studies. There is no “cure” and only ~50% of patients are free of ongoing symptoms after treatment.
Key Takeaways
- Eating disorders are common in adolescent females and have significant morbidity and mortality.
- Hospitalists’ role is diagnosis via careful history and management of medical complications with an eating disorder team. TH
Dr. Pressel is a pediatric hospitalist and inpatient medical director at Nemours/Alfred I. duPont Hospital for Children in Wilmington, Del., and a member of Team Hospitalist.
Presenter: Kyung E. Rhee, MD, MSc, MA
Summary: Eating disorders (ED) are common and have significant morbidity and mortality. EDs are the third most common psychiatric disorder of adolescents with a prevalence of 0.5-2% for anorexia and 0.9-3% for bulimia; 90% of patients are female. Mortality rate can be as high as 10% for anorexia and 1% for bulimia. Diagnosis is formally guided by DSM 5 criteria, but the mnemonic SCOFF can be useful:
- Do you feel or make yourself SICK when eating?
- Do you feel you’ve lost CONTROL of your eating?
- Have you lost one STONE (14 lbs. developed by the British) of weight?
- Do you feel FAT?
- Does FOOD dominate your life?
A detailed history is needed as patients with ED may engage in secretive behaviors to hide their illness. After diagnosis, treatment may be outpatient or inpatient. Medical issues hospitalists are likely to see with inpatients include re-feeding syndrome, various metabolic disturbances, secondary amenorrhea, sleep disturbances, and for patients with bulimia, evidence of dental or esophageal trauma from purging. Differential diagnoses include: IBD, thyroid disease, celiac, diabetes, and Addison’s disease.
Hospitalists’ role in treatment is as part of a multidisciplinary group to manage the medical complications. Inpatient management includes individual and group therapy, monitored group meals, daily blind weights, bathroom visits, and focused lab studies. There is no “cure” and only ~50% of patients are free of ongoing symptoms after treatment.
Key Takeaways
- Eating disorders are common in adolescent females and have significant morbidity and mortality.
- Hospitalists’ role is diagnosis via careful history and management of medical complications with an eating disorder team. TH
Dr. Pressel is a pediatric hospitalist and inpatient medical director at Nemours/Alfred I. duPont Hospital for Children in Wilmington, Del., and a member of Team Hospitalist.
Presenter: Kyung E. Rhee, MD, MSc, MA
Summary: Eating disorders (ED) are common and have significant morbidity and mortality. EDs are the third most common psychiatric disorder of adolescents with a prevalence of 0.5-2% for anorexia and 0.9-3% for bulimia; 90% of patients are female. Mortality rate can be as high as 10% for anorexia and 1% for bulimia. Diagnosis is formally guided by DSM 5 criteria, but the mnemonic SCOFF can be useful:
- Do you feel or make yourself SICK when eating?
- Do you feel you’ve lost CONTROL of your eating?
- Have you lost one STONE (14 lbs. developed by the British) of weight?
- Do you feel FAT?
- Does FOOD dominate your life?
A detailed history is needed as patients with ED may engage in secretive behaviors to hide their illness. After diagnosis, treatment may be outpatient or inpatient. Medical issues hospitalists are likely to see with inpatients include re-feeding syndrome, various metabolic disturbances, secondary amenorrhea, sleep disturbances, and for patients with bulimia, evidence of dental or esophageal trauma from purging. Differential diagnoses include: IBD, thyroid disease, celiac, diabetes, and Addison’s disease.
Hospitalists’ role in treatment is as part of a multidisciplinary group to manage the medical complications. Inpatient management includes individual and group therapy, monitored group meals, daily blind weights, bathroom visits, and focused lab studies. There is no “cure” and only ~50% of patients are free of ongoing symptoms after treatment.
Key Takeaways
- Eating disorders are common in adolescent females and have significant morbidity and mortality.
- Hospitalists’ role is diagnosis via careful history and management of medical complications with an eating disorder team. TH
Dr. Pressel is a pediatric hospitalist and inpatient medical director at Nemours/Alfred I. duPont Hospital for Children in Wilmington, Del., and a member of Team Hospitalist.
HM16 Session Analysis: Stay Calm, Safe During Inpatient Behavioral Emergencies
Presenters: David Pressel, MD, PhD, FAAP, FHM, Emily Fingado, MD, FAAP, and Jessica Tomaszewski, MD, FAAP
Summary: Patients may engage in violent behaviors that pose a danger to themselves or others. Behavioral emergencies may be rare, can be dangerous, and staff may feel ill-trained to respond appropriately. Patients with ingestions, or underlying psychiatric or developmental difficulties, are at highest risk for developing a behavioral emergency.
The first strategy in handling a potentially violent patient is de-escalation, i.e., trying to identify and rectify the behavioral trigger. If de-escalation is not successful, personal safety is paramount. Get away from the patient and get help. If a patient needs to be physically restrained, minimally there should be one staff member per limb. Various physical devices, including soft restraints, four-point leathers, hand mittens, and spit hoods may be used to control a violent patient. A violent restraint is characterized by the indication, not the device. Medications may be used to treat the underlying mental health issue and should not be used as PRN chemical restraints.
After a violent patient is safely restrained, further steps need to be taken, including notification of the attending or legal guardian if a minor; documentation of the event, including a debrief of what occurred; a room sweep to ensure securing any dangerous items (metal eating utensils); and modification of the care plan to strategize on removal of the restraints as soon as is safe.
Hospitals should view behavioral emergencies similarly to a Code Blue. Have a specialized team that responds and undergoes regular training.
Key Takeaways
- Behavioral emergencies occur when a patient becomes violent.
- De-escalation is the best response.
- If not successful, maintain personal safety, control and medicate the patient as appropriate, and document clearly. TH
Dr. Pressel is a pediatric hospitalist and inpatient medical director at Nemours/Alfred I. duPont Hospital for Children in Wilmington, Del., and a member of Team Hospitalist.
Presenters: David Pressel, MD, PhD, FAAP, FHM, Emily Fingado, MD, FAAP, and Jessica Tomaszewski, MD, FAAP
Summary: Patients may engage in violent behaviors that pose a danger to themselves or others. Behavioral emergencies may be rare, can be dangerous, and staff may feel ill-trained to respond appropriately. Patients with ingestions, or underlying psychiatric or developmental difficulties, are at highest risk for developing a behavioral emergency.
The first strategy in handling a potentially violent patient is de-escalation, i.e., trying to identify and rectify the behavioral trigger. If de-escalation is not successful, personal safety is paramount. Get away from the patient and get help. If a patient needs to be physically restrained, minimally there should be one staff member per limb. Various physical devices, including soft restraints, four-point leathers, hand mittens, and spit hoods may be used to control a violent patient. A violent restraint is characterized by the indication, not the device. Medications may be used to treat the underlying mental health issue and should not be used as PRN chemical restraints.
After a violent patient is safely restrained, further steps need to be taken, including notification of the attending or legal guardian if a minor; documentation of the event, including a debrief of what occurred; a room sweep to ensure securing any dangerous items (metal eating utensils); and modification of the care plan to strategize on removal of the restraints as soon as is safe.
Hospitals should view behavioral emergencies similarly to a Code Blue. Have a specialized team that responds and undergoes regular training.
Key Takeaways
- Behavioral emergencies occur when a patient becomes violent.
- De-escalation is the best response.
- If not successful, maintain personal safety, control and medicate the patient as appropriate, and document clearly. TH
Dr. Pressel is a pediatric hospitalist and inpatient medical director at Nemours/Alfred I. duPont Hospital for Children in Wilmington, Del., and a member of Team Hospitalist.
Presenters: David Pressel, MD, PhD, FAAP, FHM, Emily Fingado, MD, FAAP, and Jessica Tomaszewski, MD, FAAP
Summary: Patients may engage in violent behaviors that pose a danger to themselves or others. Behavioral emergencies may be rare, can be dangerous, and staff may feel ill-trained to respond appropriately. Patients with ingestions, or underlying psychiatric or developmental difficulties, are at highest risk for developing a behavioral emergency.
The first strategy in handling a potentially violent patient is de-escalation, i.e., trying to identify and rectify the behavioral trigger. If de-escalation is not successful, personal safety is paramount. Get away from the patient and get help. If a patient needs to be physically restrained, minimally there should be one staff member per limb. Various physical devices, including soft restraints, four-point leathers, hand mittens, and spit hoods may be used to control a violent patient. A violent restraint is characterized by the indication, not the device. Medications may be used to treat the underlying mental health issue and should not be used as PRN chemical restraints.
After a violent patient is safely restrained, further steps need to be taken, including notification of the attending or legal guardian if a minor; documentation of the event, including a debrief of what occurred; a room sweep to ensure securing any dangerous items (metal eating utensils); and modification of the care plan to strategize on removal of the restraints as soon as is safe.
Hospitals should view behavioral emergencies similarly to a Code Blue. Have a specialized team that responds and undergoes regular training.
Key Takeaways
- Behavioral emergencies occur when a patient becomes violent.
- De-escalation is the best response.
- If not successful, maintain personal safety, control and medicate the patient as appropriate, and document clearly. TH
Dr. Pressel is a pediatric hospitalist and inpatient medical director at Nemours/Alfred I. duPont Hospital for Children in Wilmington, Del., and a member of Team Hospitalist.
HM16 Session Analysis: Update in Pulmonary Medicine
Presenter: Daniel D. Dressler, MD, MSc, SFHM
Summary: This presentation focused on pulmonary updates specific to hospitalist practice, from end of 2014 to early 2016.
New research on community-acquired pneumonia suggest that only 38% of cases a presumptive pathogen will be isolated. Virus account for 23%, bacteria 11% (including S. pneumonia, S. Aureus and Enterobacteriaceae), both (virus and bacteria) 3%, and fungus or mycobacterium 1%. It is important to notice no recent data on etiology was available since mid-1990.
There is also a new pragmatic trial suggesting that B-lactam monotherapy is not inferior to either B-lactam in combination with macrolides or fluoroquinolones. The study reported an 11%, 90-day mortality with B-lactam monotherapy compared with 11% when combined with macrolides and 8.8% when using quinolones monotherapy.
Update evidence supports the use of corticosteroids for hospitalized patients with community-acquired pneumonia, at a dose of 20-60 mg day for 5-7 days. The study showed decreased mortality in patients with clinical criteria for severe pneumonia with NNT 7; it also showed decrease need for mechanical ventilation and development of ARDS.
An additional, interesting finding was a decrease in length of stay (LOS) in the steroid group. In patients with acute hypoxemic respiratory failure, high flow nasal cannula reduced mortality and likely reduces intubation in severely hypoxemic patients when compared to NPPV.
In patients with first unprovoked VTE, extending anticoagulation to two years or adding aspirin after initial anticoagulation might reduce recurrent VTE without significant increasing in risk for major bleeding.
Key Takeaways:
- B-lactam monotherapy for hospitalized non-ICU CAP might be reasonable choice.
- Moderate short course of steroids in CAP, reduce ARDS, intubation, LOS in all hospitalized patients (and mortality on severe CAP)
- A trial of high flow NC is indicated in acute hypoxemic respiratory failure
- Aspirin prophylaxis following anticoagulation (most benefit first year), or extended anticoagulation for 2 years reduce recurrent VTE without much additional bleeding risk.
Dr. Villagra is a hospitalist in Batesville, Ark., and a member of Team Hospitalist.
Presenter: Daniel D. Dressler, MD, MSc, SFHM
Summary: This presentation focused on pulmonary updates specific to hospitalist practice, from end of 2014 to early 2016.
New research on community-acquired pneumonia suggest that only 38% of cases a presumptive pathogen will be isolated. Virus account for 23%, bacteria 11% (including S. pneumonia, S. Aureus and Enterobacteriaceae), both (virus and bacteria) 3%, and fungus or mycobacterium 1%. It is important to notice no recent data on etiology was available since mid-1990.
There is also a new pragmatic trial suggesting that B-lactam monotherapy is not inferior to either B-lactam in combination with macrolides or fluoroquinolones. The study reported an 11%, 90-day mortality with B-lactam monotherapy compared with 11% when combined with macrolides and 8.8% when using quinolones monotherapy.
Update evidence supports the use of corticosteroids for hospitalized patients with community-acquired pneumonia, at a dose of 20-60 mg day for 5-7 days. The study showed decreased mortality in patients with clinical criteria for severe pneumonia with NNT 7; it also showed decrease need for mechanical ventilation and development of ARDS.
An additional, interesting finding was a decrease in length of stay (LOS) in the steroid group. In patients with acute hypoxemic respiratory failure, high flow nasal cannula reduced mortality and likely reduces intubation in severely hypoxemic patients when compared to NPPV.
In patients with first unprovoked VTE, extending anticoagulation to two years or adding aspirin after initial anticoagulation might reduce recurrent VTE without significant increasing in risk for major bleeding.
Key Takeaways:
- B-lactam monotherapy for hospitalized non-ICU CAP might be reasonable choice.
- Moderate short course of steroids in CAP, reduce ARDS, intubation, LOS in all hospitalized patients (and mortality on severe CAP)
- A trial of high flow NC is indicated in acute hypoxemic respiratory failure
- Aspirin prophylaxis following anticoagulation (most benefit first year), or extended anticoagulation for 2 years reduce recurrent VTE without much additional bleeding risk.
Dr. Villagra is a hospitalist in Batesville, Ark., and a member of Team Hospitalist.
Presenter: Daniel D. Dressler, MD, MSc, SFHM
Summary: This presentation focused on pulmonary updates specific to hospitalist practice, from end of 2014 to early 2016.
New research on community-acquired pneumonia suggest that only 38% of cases a presumptive pathogen will be isolated. Virus account for 23%, bacteria 11% (including S. pneumonia, S. Aureus and Enterobacteriaceae), both (virus and bacteria) 3%, and fungus or mycobacterium 1%. It is important to notice no recent data on etiology was available since mid-1990.
There is also a new pragmatic trial suggesting that B-lactam monotherapy is not inferior to either B-lactam in combination with macrolides or fluoroquinolones. The study reported an 11%, 90-day mortality with B-lactam monotherapy compared with 11% when combined with macrolides and 8.8% when using quinolones monotherapy.
Update evidence supports the use of corticosteroids for hospitalized patients with community-acquired pneumonia, at a dose of 20-60 mg day for 5-7 days. The study showed decreased mortality in patients with clinical criteria for severe pneumonia with NNT 7; it also showed decrease need for mechanical ventilation and development of ARDS.
An additional, interesting finding was a decrease in length of stay (LOS) in the steroid group. In patients with acute hypoxemic respiratory failure, high flow nasal cannula reduced mortality and likely reduces intubation in severely hypoxemic patients when compared to NPPV.
In patients with first unprovoked VTE, extending anticoagulation to two years or adding aspirin after initial anticoagulation might reduce recurrent VTE without significant increasing in risk for major bleeding.
Key Takeaways:
- B-lactam monotherapy for hospitalized non-ICU CAP might be reasonable choice.
- Moderate short course of steroids in CAP, reduce ARDS, intubation, LOS in all hospitalized patients (and mortality on severe CAP)
- A trial of high flow NC is indicated in acute hypoxemic respiratory failure
- Aspirin prophylaxis following anticoagulation (most benefit first year), or extended anticoagulation for 2 years reduce recurrent VTE without much additional bleeding risk.
Dr. Villagra is a hospitalist in Batesville, Ark., and a member of Team Hospitalist.