User login
Diabetes Drugs Promising for Alcohol Use Disorder
TOPLINE:
Use of the glucagon-like peptide 1 (GLP-1) receptor agonists semaglutide and liraglutide is linked to a lower risk for alcohol use disorder (AUD)–related hospitalizations, compared with traditional AUD medications, a new study suggested.
METHODOLOGY:
- Researchers conducted a nationwide cohort study from 2006 to 2023 in Sweden that included more than 220,000 individuals with AUD (mean age, 40 years; 64% men).
- Data were obtained from registers of inpatient and specialized outpatient care, sickness absence, and disability pension, with a median follow-up period of 8.8 years.
- The primary exposure measured was the use of individual GLP-1 receptor agonists — commonly used to treat type 2 diabetes and obesity — compared with nonuse.
- The secondary exposure examined was the use of medications indicated for AUD.
- The primary outcome was AUD-related hospitalization; secondary outcomes included hospitalization due to substance use disorder (SUD), somatic hospitalization, and suicide attempts.
TAKEAWAY:
- About 59% of participants experienced AUD-related hospitalization.
- Semaglutide users (n = 4321) had the lowest risk for hospitalization related to AUD (adjusted hazard ratio [aHR], 0.64; 95% CI, 0.50-0.83) and to any SUD (aHR, 0.68; 95% CI, 0.54-0.85).
- Liraglutide users (n = 2509) had the second lowest risk for both AUD-related (aHR, 0.72; 95% CI, 0.57-0.92) and SUD-related (aHR, 0.78; 95% CI, 0.64-0.97) hospitalizations.
- The use of both semaglutide (aHR, 0.78; 95% CI, 0.68-0.90) and liraglutide (aHR, 0.79; 95% CI, 0.69-0.91) was linked to a reduced risk for hospitalization because of somatic reasons but was not associated with the risk of suicide attempts.
- Traditional AUD medications showed modest effectiveness with a slightly decreased but nonsignificant risk for AUD-related hospitalization (aHR, 0.98).
IN PRACTICE:
“AUDs and SUDs are undertreated pharmacologically, despite the availability of effective treatments. However, novel treatments are also needed because existing treatments may not be suitable for all patients. Semaglutide and liraglutide may be effective in the treatment of AUD, and clinical trials are urgently needed to confirm these findings,” the investigators wrote.
SOURCE:
This study was led by Markku Lähteenvuo, MD, PhD, University of Eastern Finland, Niuvanniemi Hospital, Kuopio. It was published online on November 13 in JAMA Psychiatry.
LIMITATIONS:
The observational nature of this study limited causal inferences.
DISCLOSURES:
The data used in this study were obtained from the REWHARD consortium, supported by the Swedish Research Council. Four of the six authors reported receiving grants or personal fees from various sources outside the submitted work, which are fully listed in the original article.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
Use of the glucagon-like peptide 1 (GLP-1) receptor agonists semaglutide and liraglutide is linked to a lower risk for alcohol use disorder (AUD)–related hospitalizations, compared with traditional AUD medications, a new study suggested.
METHODOLOGY:
- Researchers conducted a nationwide cohort study from 2006 to 2023 in Sweden that included more than 220,000 individuals with AUD (mean age, 40 years; 64% men).
- Data were obtained from registers of inpatient and specialized outpatient care, sickness absence, and disability pension, with a median follow-up period of 8.8 years.
- The primary exposure measured was the use of individual GLP-1 receptor agonists — commonly used to treat type 2 diabetes and obesity — compared with nonuse.
- The secondary exposure examined was the use of medications indicated for AUD.
- The primary outcome was AUD-related hospitalization; secondary outcomes included hospitalization due to substance use disorder (SUD), somatic hospitalization, and suicide attempts.
TAKEAWAY:
- About 59% of participants experienced AUD-related hospitalization.
- Semaglutide users (n = 4321) had the lowest risk for hospitalization related to AUD (adjusted hazard ratio [aHR], 0.64; 95% CI, 0.50-0.83) and to any SUD (aHR, 0.68; 95% CI, 0.54-0.85).
- Liraglutide users (n = 2509) had the second lowest risk for both AUD-related (aHR, 0.72; 95% CI, 0.57-0.92) and SUD-related (aHR, 0.78; 95% CI, 0.64-0.97) hospitalizations.
- The use of both semaglutide (aHR, 0.78; 95% CI, 0.68-0.90) and liraglutide (aHR, 0.79; 95% CI, 0.69-0.91) was linked to a reduced risk for hospitalization because of somatic reasons but was not associated with the risk of suicide attempts.
- Traditional AUD medications showed modest effectiveness with a slightly decreased but nonsignificant risk for AUD-related hospitalization (aHR, 0.98).
IN PRACTICE:
“AUDs and SUDs are undertreated pharmacologically, despite the availability of effective treatments. However, novel treatments are also needed because existing treatments may not be suitable for all patients. Semaglutide and liraglutide may be effective in the treatment of AUD, and clinical trials are urgently needed to confirm these findings,” the investigators wrote.
SOURCE:
This study was led by Markku Lähteenvuo, MD, PhD, University of Eastern Finland, Niuvanniemi Hospital, Kuopio. It was published online on November 13 in JAMA Psychiatry.
LIMITATIONS:
The observational nature of this study limited causal inferences.
DISCLOSURES:
The data used in this study were obtained from the REWHARD consortium, supported by the Swedish Research Council. Four of the six authors reported receiving grants or personal fees from various sources outside the submitted work, which are fully listed in the original article.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
Use of the glucagon-like peptide 1 (GLP-1) receptor agonists semaglutide and liraglutide is linked to a lower risk for alcohol use disorder (AUD)–related hospitalizations, compared with traditional AUD medications, a new study suggested.
METHODOLOGY:
- Researchers conducted a nationwide cohort study from 2006 to 2023 in Sweden that included more than 220,000 individuals with AUD (mean age, 40 years; 64% men).
- Data were obtained from registers of inpatient and specialized outpatient care, sickness absence, and disability pension, with a median follow-up period of 8.8 years.
- The primary exposure measured was the use of individual GLP-1 receptor agonists — commonly used to treat type 2 diabetes and obesity — compared with nonuse.
- The secondary exposure examined was the use of medications indicated for AUD.
- The primary outcome was AUD-related hospitalization; secondary outcomes included hospitalization due to substance use disorder (SUD), somatic hospitalization, and suicide attempts.
TAKEAWAY:
- About 59% of participants experienced AUD-related hospitalization.
- Semaglutide users (n = 4321) had the lowest risk for hospitalization related to AUD (adjusted hazard ratio [aHR], 0.64; 95% CI, 0.50-0.83) and to any SUD (aHR, 0.68; 95% CI, 0.54-0.85).
- Liraglutide users (n = 2509) had the second lowest risk for both AUD-related (aHR, 0.72; 95% CI, 0.57-0.92) and SUD-related (aHR, 0.78; 95% CI, 0.64-0.97) hospitalizations.
- The use of both semaglutide (aHR, 0.78; 95% CI, 0.68-0.90) and liraglutide (aHR, 0.79; 95% CI, 0.69-0.91) was linked to a reduced risk for hospitalization because of somatic reasons but was not associated with the risk of suicide attempts.
- Traditional AUD medications showed modest effectiveness with a slightly decreased but nonsignificant risk for AUD-related hospitalization (aHR, 0.98).
IN PRACTICE:
“AUDs and SUDs are undertreated pharmacologically, despite the availability of effective treatments. However, novel treatments are also needed because existing treatments may not be suitable for all patients. Semaglutide and liraglutide may be effective in the treatment of AUD, and clinical trials are urgently needed to confirm these findings,” the investigators wrote.
SOURCE:
This study was led by Markku Lähteenvuo, MD, PhD, University of Eastern Finland, Niuvanniemi Hospital, Kuopio. It was published online on November 13 in JAMA Psychiatry.
LIMITATIONS:
The observational nature of this study limited causal inferences.
DISCLOSURES:
The data used in this study were obtained from the REWHARD consortium, supported by the Swedish Research Council. Four of the six authors reported receiving grants or personal fees from various sources outside the submitted work, which are fully listed in the original article.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
Hoarding Disorder: A Looming National Crisis?
A report published in July 2024 by the US Senate Special Committee on Aging is calling for a national coordinated response to what the authors claim may be an emerging hoarding disorder (HD) crisis.
While millions of US adults are estimated to have HD, it is the disorder’s prevalence and severity among older adults that sounded the alarm for the Committee Chair Sen. Bob Casey (D-PA).
the report stated. Older adults made up about 16% of the US population in 2019. By 2060, that proportion is projected to soar to 25%.
The country’s aging population alone “could fuel a rise in hoarding in the coming decades,” the report authors noted.
These findings underscore the pressing need for a deeper understanding of HD, particularly as reports of its impact continue to rise. The Senate report also raises critical questions about the nature of HD: What is known about the condition? What evidence-based treatments are currently available, and are there national strategies that will prevent it from becoming a systemic crisis?
Why the Urgency?
An increase in anecdotal reports of HD in his home state prompted Casey, chair of the Senate Committee on Aging, to launch the investigation into the incidence and consequences of HD. Soon after the committee began its work, it became evident that the problem was not unique to communities in Pennsylvania. It was a nationwide issue.
“Communities throughout the United States are already grappling with HD,” the report noted.
HD is characterized by persistent difficulty discarding possessions, regardless of their monetary value. For individuals with HD, such items frequently hold meaningful reminders of past events and provide a sense of security. Difficulties with emotional regulation, executive functioning, and impulse control all contribute to the excessive buildup of clutter. Problems with attention, organization, and problem-solving are also common.
As individuals with HD age, physical limitations or disabilities may hinder their ability to discard clutter. As the accumulation increases, it can pose serious risks not only to their safety but also to public health.
Dozens of statements submitted to the Senate committee by those with HD, clinicians and social workers, first responders, social service organizations, state and federal agencies, and professional societies paint a concerning picture about the impact of hoarding on emergency and community services.
Data from the National Fire Incident Reporting System show the number of hoarding-related residential structural fires increased 26% between 2014 and 2022. Some 5242 residential fires connected to cluttered environments during that time resulted in 1367 fire service injuries, 1119 civilian injuries, and over $396 million in damages.
“For older adults, those consequences include health and safety risks, social isolation, eviction, and homelessness,” the report authors noted. “For communities, those consequences include public health concerns, increased risk of fire, and dangers to emergency responders.”
What Causes HD?
HD was once classified as a symptom of obsessive-compulsive personality disorder, with extreme causes meeting the diagnostic criteria for obsessive-compulsive disorder. That changed in 2010 when a working group recommended that HD be added to the Diagnostic and Statistical Manual of Mental Disorders (DSM), Fifth Edition, as a stand-alone disorder. That recommendation was approved in 2012.
However, a decade later, much about HD’s etiology remains unknown.
Often beginning in early adolescence, HD is a chronic and progressive condition, with genetics and trauma playing a role in its onset and course, Sanjaya Saxena, MD, director of Clinical and Research Affairs at the International OCD Foundation, said in an interview.
Between 50% and 85% of people with HD symptoms have family members with similar behavior. HD is often comorbid with other psychiatric and medical disorders, which can complicate treatment.
Results of a 2022 study showed that, compared with healthy control individuals, people with HD had widespread abnormalities in the prefrontal white matter tract which connects cortical regions involved in executive functioning, including working memory, attention, reward processing, and decision-making.
Some research also suggests that dysregulation of serotonin transmission may contribute to compulsive behaviors and the difficulty in letting go of possessions.
“We do know that there are factors that contribute to worsening of hoarding symptoms, but that’s not the same thing as what really causes it. So unfortunately, it’s still very understudied, and we don’t have great knowledge of what causes it,” Saxena said.
What Treatments Are Available?
There are currently no Food and Drug Administration–approved medications to treat HD, although some research has shown antidepressants paroxetine and venlafaxine may have some benefit. Methylphenidate and atomoxetine are also under study for HD.
Nonpharmacological therapies have shown more promising results. Among the first was a specialized cognitive-behavioral therapy (CBT) program developed by Randy Frost, PhD, professor emeritus of psychology at Smith College in Northampton, Massachusetts, and Gail Steketee, PhD, dean emerita and professor emerita of social work at Boston University in Massachusetts.
First published in 2007 and the subject of many clinical trials and studies since, the 26-session program has served as a model for psychosocial treatments for HD. The evidence-based therapy addresses various symptoms, including impulse control. One module encourages participants to develop a set of questions to consider before acquiring new items, gradually helping them build resistance to the urge to accumulate more possessions, said Frost, whose early work on HD was cited by those who supported adding the condition to the DSM in 2012.
“There are several features that I think are important including exercises in resisting acquiring and processing information when making decisions about discarding,” Frost said in an interview.
A number of studies have demonstrated the efficacy of CBT for HD, including a 2015 meta-analysis coauthored by Frost. The research showed symptom severity decreased significantly following CBT, with the largest gains in difficulty discarding and moderate improvements in clutter and acquiring.
Responses were better among women and younger patients, and although symptoms improved, posttreatment scores remained closer to the clinical range, researchers noted. It’s possible that more intervention beyond what is usually included in clinical trials — such as more sessions or adding home decluttering visits — could improve treatment response, they added.
A workshop based on the specialized CBT program has expanded the reach of the treatment. The group therapy project, Buried in Treasures (BiT), was developed by Frost, Steketee, and David Tolin, PhD, founder and director of the Anxiety Disorders Center at the Institute of Living, Hartford, and an adjunct professor of psychiatry at Yale School of Medicine, New Haven, Connecticut. The workshop is designed as a facilitated treatment that can be delivered by clinicians or trained nonclinician facilitators.
A study published in May found that more than half the participants with HD responded to the treatment, and of those, 39% reported significant reductions in HD symptoms. BiT sessions were led by trained facilitators, and the study included in-home decluttering sessions, also led by trained volunteers. Researchers said adding the home intervention could increase engagement with the group therapy.
Another study of a modified version of BiT found a 32% decrease in HD symptoms after 15 weeks of treatment delivered via video teleconference.
“The BiT workshop has been expanding around the world and has the advantage of being relatively inexpensive,” Frost said. Another advantage is that it can be run by nonclinicians, which expands treatment options in areas where mental health professionals trained to treat HD are in short supply.
However, the workshop “is not perfect, and clients usually still have symptoms at the end of the workshop,” Frost noted.
“The point is that the BiT workshop is the first step in changing a lifestyle related to possessions,” he continued. “We do certainly need to train more people in how to treat hoarding, and we need to facilitate research to make our treatments more effective.”
What’s New in the Field?
One novel program currently under study combines CBT with a cognitive rehabilitation protocol. Called Cognitive Rehabilitation and Exposure/Sorting Therapy (CREST), the program has been shown to help older adults with HD who don’t respond to traditional CBT for HD.
The program, led by Catherine Ayers, PhD, professor of clinical psychiatry at University of California, San Diego, involves memory training and problem-solving combined with exposure therapy to help participants learn how to tolerate distress associated with discarding their possessions.
Early findings pointed to symptom improvement in older adults following 24 sessions with CREST. The program fared better than geriatric case management in a 2018 study — the first randomized controlled trial of a treatment for HD in older adults — and offered additional benefits compared with exposure therapy in a study published in February 2024.
Virtual reality is also helping people with HD. A program developed at Stanford University in California, allows people with HD to work with a therapist as they practice decluttering in a three-dimensional virtual environment created using photographs and videos of actual hoarded objects and cluttered rooms in patients’ homes.
In a small pilot study, nine people older than 55 years with HD attended 16 weeks of online facilitated therapy where they learned to better understand their attachment to those items. They practiced decluttering by selecting virtual items for recycling, donation, or trash. A virtual garbage truck even hauled away the items they had placed in the trash.
Participants were then asked to discard the actual items at home. Most participants reported a decrease in hoarding symptoms, which was confirmed following a home assessment by a clinician.
“When you pick up an object from a loved one, it still maybe has the scent of the loved one. It has these tactile cues, colors. But in the virtual world, you can take a little bit of a step back,” lead researchers Carolyn Rodriguez, MD, PhD, director of Stanford’s Hoarding Disorders Research Program, said in an interview.
“It’s a little ramp to help people practice these skills. And then what we find is that it actually translated really well. They were able to go home and actually do the real uncluttering,” Rodriguez added.
What Else Can Be Done?
While researchers like Rodriguez continue studies of new and existing treatments, the Senate report draws attention to other responses that could aid people with HD. Because of its significant impact on emergency responders, adult protective services, aging services, and housing providers, the report recommends a nationwide response to older adults with HD.
Currently, federal agencies in charge of mental and community health are not doing enough to address HD, the report’s authors noted.
The report demonstrates “the scope and severity of these challenges and offers a path forward for how we can help people, communities, and local governments contend with this condition,” Casey said.
Specifically, the document cites a lack of HD services and tracking by the Substance Abuse and Mental Health Services Administration, the Administration for Community Living, and the Centers for Disease Control and Prevention.
The committee recommended these agencies collaborate to improve HD data collection, which will be critical to managing a potential spike in cases as the population ages. The committee also suggested awareness and training campaigns to better educate clinicians, social service providers, court officials, and first responders about HD.
Further, the report’s authors called for the Department of Housing and Urban Development to provide guidance and technical assistance on HD for landlords and housing assistance programs and urged Congress to collaborate with the Centers for Medicare & Medicaid Services to expand coverage for hoarding treatments.
Finally, the committee encouraged policymakers to engage directly with individuals affected by HD and their families to better understand the impact of the disorder and inform policy development.
“I think the Senate report focuses on education, not just for therapists, but other stakeholders too,” Frost said. “There are lots of other professionals who have a stake in this process, housing specialists, elder service folks, health and human services. Awareness of this problem is something that’s important for them as well.”
Rodriguez characterized the report’s recommendations as “potentially lifesaving” for individuals with HD. She added that it represents the first step in an ongoing effort to address an impending public health crisis related to HD in older adults and its broader impact on communities.
A spokesperson with Casey’s office said it’s unclear whether any federal agencies have acted on the report recommendations since it was released in June. It’s also unknown whether the Senate Committee on Aging will pursue any additional work on HD when new committee leaders are appointed in 2025.
“Although some federal agencies have taken steps to address HD, those steps are frequently limited. Other relevant agencies have not addressed HD at all in recent years,” report authors wrote. “The federal government can, and should, do more to bolster the response to HD.”
Frost agreed.
“I think federal agencies can have a positive effect by promoting, supporting, and tracking local efforts in dealing with this problem,” he said.
With reporting from Eve Bender.
A version of this article appeared on Medscape.com.
A report published in July 2024 by the US Senate Special Committee on Aging is calling for a national coordinated response to what the authors claim may be an emerging hoarding disorder (HD) crisis.
While millions of US adults are estimated to have HD, it is the disorder’s prevalence and severity among older adults that sounded the alarm for the Committee Chair Sen. Bob Casey (D-PA).
the report stated. Older adults made up about 16% of the US population in 2019. By 2060, that proportion is projected to soar to 25%.
The country’s aging population alone “could fuel a rise in hoarding in the coming decades,” the report authors noted.
These findings underscore the pressing need for a deeper understanding of HD, particularly as reports of its impact continue to rise. The Senate report also raises critical questions about the nature of HD: What is known about the condition? What evidence-based treatments are currently available, and are there national strategies that will prevent it from becoming a systemic crisis?
Why the Urgency?
An increase in anecdotal reports of HD in his home state prompted Casey, chair of the Senate Committee on Aging, to launch the investigation into the incidence and consequences of HD. Soon after the committee began its work, it became evident that the problem was not unique to communities in Pennsylvania. It was a nationwide issue.
“Communities throughout the United States are already grappling with HD,” the report noted.
HD is characterized by persistent difficulty discarding possessions, regardless of their monetary value. For individuals with HD, such items frequently hold meaningful reminders of past events and provide a sense of security. Difficulties with emotional regulation, executive functioning, and impulse control all contribute to the excessive buildup of clutter. Problems with attention, organization, and problem-solving are also common.
As individuals with HD age, physical limitations or disabilities may hinder their ability to discard clutter. As the accumulation increases, it can pose serious risks not only to their safety but also to public health.
Dozens of statements submitted to the Senate committee by those with HD, clinicians and social workers, first responders, social service organizations, state and federal agencies, and professional societies paint a concerning picture about the impact of hoarding on emergency and community services.
Data from the National Fire Incident Reporting System show the number of hoarding-related residential structural fires increased 26% between 2014 and 2022. Some 5242 residential fires connected to cluttered environments during that time resulted in 1367 fire service injuries, 1119 civilian injuries, and over $396 million in damages.
“For older adults, those consequences include health and safety risks, social isolation, eviction, and homelessness,” the report authors noted. “For communities, those consequences include public health concerns, increased risk of fire, and dangers to emergency responders.”
What Causes HD?
HD was once classified as a symptom of obsessive-compulsive personality disorder, with extreme causes meeting the diagnostic criteria for obsessive-compulsive disorder. That changed in 2010 when a working group recommended that HD be added to the Diagnostic and Statistical Manual of Mental Disorders (DSM), Fifth Edition, as a stand-alone disorder. That recommendation was approved in 2012.
However, a decade later, much about HD’s etiology remains unknown.
Often beginning in early adolescence, HD is a chronic and progressive condition, with genetics and trauma playing a role in its onset and course, Sanjaya Saxena, MD, director of Clinical and Research Affairs at the International OCD Foundation, said in an interview.
Between 50% and 85% of people with HD symptoms have family members with similar behavior. HD is often comorbid with other psychiatric and medical disorders, which can complicate treatment.
Results of a 2022 study showed that, compared with healthy control individuals, people with HD had widespread abnormalities in the prefrontal white matter tract which connects cortical regions involved in executive functioning, including working memory, attention, reward processing, and decision-making.
Some research also suggests that dysregulation of serotonin transmission may contribute to compulsive behaviors and the difficulty in letting go of possessions.
“We do know that there are factors that contribute to worsening of hoarding symptoms, but that’s not the same thing as what really causes it. So unfortunately, it’s still very understudied, and we don’t have great knowledge of what causes it,” Saxena said.
What Treatments Are Available?
There are currently no Food and Drug Administration–approved medications to treat HD, although some research has shown antidepressants paroxetine and venlafaxine may have some benefit. Methylphenidate and atomoxetine are also under study for HD.
Nonpharmacological therapies have shown more promising results. Among the first was a specialized cognitive-behavioral therapy (CBT) program developed by Randy Frost, PhD, professor emeritus of psychology at Smith College in Northampton, Massachusetts, and Gail Steketee, PhD, dean emerita and professor emerita of social work at Boston University in Massachusetts.
First published in 2007 and the subject of many clinical trials and studies since, the 26-session program has served as a model for psychosocial treatments for HD. The evidence-based therapy addresses various symptoms, including impulse control. One module encourages participants to develop a set of questions to consider before acquiring new items, gradually helping them build resistance to the urge to accumulate more possessions, said Frost, whose early work on HD was cited by those who supported adding the condition to the DSM in 2012.
“There are several features that I think are important including exercises in resisting acquiring and processing information when making decisions about discarding,” Frost said in an interview.
A number of studies have demonstrated the efficacy of CBT for HD, including a 2015 meta-analysis coauthored by Frost. The research showed symptom severity decreased significantly following CBT, with the largest gains in difficulty discarding and moderate improvements in clutter and acquiring.
Responses were better among women and younger patients, and although symptoms improved, posttreatment scores remained closer to the clinical range, researchers noted. It’s possible that more intervention beyond what is usually included in clinical trials — such as more sessions or adding home decluttering visits — could improve treatment response, they added.
A workshop based on the specialized CBT program has expanded the reach of the treatment. The group therapy project, Buried in Treasures (BiT), was developed by Frost, Steketee, and David Tolin, PhD, founder and director of the Anxiety Disorders Center at the Institute of Living, Hartford, and an adjunct professor of psychiatry at Yale School of Medicine, New Haven, Connecticut. The workshop is designed as a facilitated treatment that can be delivered by clinicians or trained nonclinician facilitators.
A study published in May found that more than half the participants with HD responded to the treatment, and of those, 39% reported significant reductions in HD symptoms. BiT sessions were led by trained facilitators, and the study included in-home decluttering sessions, also led by trained volunteers. Researchers said adding the home intervention could increase engagement with the group therapy.
Another study of a modified version of BiT found a 32% decrease in HD symptoms after 15 weeks of treatment delivered via video teleconference.
“The BiT workshop has been expanding around the world and has the advantage of being relatively inexpensive,” Frost said. Another advantage is that it can be run by nonclinicians, which expands treatment options in areas where mental health professionals trained to treat HD are in short supply.
However, the workshop “is not perfect, and clients usually still have symptoms at the end of the workshop,” Frost noted.
“The point is that the BiT workshop is the first step in changing a lifestyle related to possessions,” he continued. “We do certainly need to train more people in how to treat hoarding, and we need to facilitate research to make our treatments more effective.”
What’s New in the Field?
One novel program currently under study combines CBT with a cognitive rehabilitation protocol. Called Cognitive Rehabilitation and Exposure/Sorting Therapy (CREST), the program has been shown to help older adults with HD who don’t respond to traditional CBT for HD.
The program, led by Catherine Ayers, PhD, professor of clinical psychiatry at University of California, San Diego, involves memory training and problem-solving combined with exposure therapy to help participants learn how to tolerate distress associated with discarding their possessions.
Early findings pointed to symptom improvement in older adults following 24 sessions with CREST. The program fared better than geriatric case management in a 2018 study — the first randomized controlled trial of a treatment for HD in older adults — and offered additional benefits compared with exposure therapy in a study published in February 2024.
Virtual reality is also helping people with HD. A program developed at Stanford University in California, allows people with HD to work with a therapist as they practice decluttering in a three-dimensional virtual environment created using photographs and videos of actual hoarded objects and cluttered rooms in patients’ homes.
In a small pilot study, nine people older than 55 years with HD attended 16 weeks of online facilitated therapy where they learned to better understand their attachment to those items. They practiced decluttering by selecting virtual items for recycling, donation, or trash. A virtual garbage truck even hauled away the items they had placed in the trash.
Participants were then asked to discard the actual items at home. Most participants reported a decrease in hoarding symptoms, which was confirmed following a home assessment by a clinician.
“When you pick up an object from a loved one, it still maybe has the scent of the loved one. It has these tactile cues, colors. But in the virtual world, you can take a little bit of a step back,” lead researchers Carolyn Rodriguez, MD, PhD, director of Stanford’s Hoarding Disorders Research Program, said in an interview.
“It’s a little ramp to help people practice these skills. And then what we find is that it actually translated really well. They were able to go home and actually do the real uncluttering,” Rodriguez added.
What Else Can Be Done?
While researchers like Rodriguez continue studies of new and existing treatments, the Senate report draws attention to other responses that could aid people with HD. Because of its significant impact on emergency responders, adult protective services, aging services, and housing providers, the report recommends a nationwide response to older adults with HD.
Currently, federal agencies in charge of mental and community health are not doing enough to address HD, the report’s authors noted.
The report demonstrates “the scope and severity of these challenges and offers a path forward for how we can help people, communities, and local governments contend with this condition,” Casey said.
Specifically, the document cites a lack of HD services and tracking by the Substance Abuse and Mental Health Services Administration, the Administration for Community Living, and the Centers for Disease Control and Prevention.
The committee recommended these agencies collaborate to improve HD data collection, which will be critical to managing a potential spike in cases as the population ages. The committee also suggested awareness and training campaigns to better educate clinicians, social service providers, court officials, and first responders about HD.
Further, the report’s authors called for the Department of Housing and Urban Development to provide guidance and technical assistance on HD for landlords and housing assistance programs and urged Congress to collaborate with the Centers for Medicare & Medicaid Services to expand coverage for hoarding treatments.
Finally, the committee encouraged policymakers to engage directly with individuals affected by HD and their families to better understand the impact of the disorder and inform policy development.
“I think the Senate report focuses on education, not just for therapists, but other stakeholders too,” Frost said. “There are lots of other professionals who have a stake in this process, housing specialists, elder service folks, health and human services. Awareness of this problem is something that’s important for them as well.”
Rodriguez characterized the report’s recommendations as “potentially lifesaving” for individuals with HD. She added that it represents the first step in an ongoing effort to address an impending public health crisis related to HD in older adults and its broader impact on communities.
A spokesperson with Casey’s office said it’s unclear whether any federal agencies have acted on the report recommendations since it was released in June. It’s also unknown whether the Senate Committee on Aging will pursue any additional work on HD when new committee leaders are appointed in 2025.
“Although some federal agencies have taken steps to address HD, those steps are frequently limited. Other relevant agencies have not addressed HD at all in recent years,” report authors wrote. “The federal government can, and should, do more to bolster the response to HD.”
Frost agreed.
“I think federal agencies can have a positive effect by promoting, supporting, and tracking local efforts in dealing with this problem,” he said.
With reporting from Eve Bender.
A version of this article appeared on Medscape.com.
A report published in July 2024 by the US Senate Special Committee on Aging is calling for a national coordinated response to what the authors claim may be an emerging hoarding disorder (HD) crisis.
While millions of US adults are estimated to have HD, it is the disorder’s prevalence and severity among older adults that sounded the alarm for the Committee Chair Sen. Bob Casey (D-PA).
the report stated. Older adults made up about 16% of the US population in 2019. By 2060, that proportion is projected to soar to 25%.
The country’s aging population alone “could fuel a rise in hoarding in the coming decades,” the report authors noted.
These findings underscore the pressing need for a deeper understanding of HD, particularly as reports of its impact continue to rise. The Senate report also raises critical questions about the nature of HD: What is known about the condition? What evidence-based treatments are currently available, and are there national strategies that will prevent it from becoming a systemic crisis?
Why the Urgency?
An increase in anecdotal reports of HD in his home state prompted Casey, chair of the Senate Committee on Aging, to launch the investigation into the incidence and consequences of HD. Soon after the committee began its work, it became evident that the problem was not unique to communities in Pennsylvania. It was a nationwide issue.
“Communities throughout the United States are already grappling with HD,” the report noted.
HD is characterized by persistent difficulty discarding possessions, regardless of their monetary value. For individuals with HD, such items frequently hold meaningful reminders of past events and provide a sense of security. Difficulties with emotional regulation, executive functioning, and impulse control all contribute to the excessive buildup of clutter. Problems with attention, organization, and problem-solving are also common.
As individuals with HD age, physical limitations or disabilities may hinder their ability to discard clutter. As the accumulation increases, it can pose serious risks not only to their safety but also to public health.
Dozens of statements submitted to the Senate committee by those with HD, clinicians and social workers, first responders, social service organizations, state and federal agencies, and professional societies paint a concerning picture about the impact of hoarding on emergency and community services.
Data from the National Fire Incident Reporting System show the number of hoarding-related residential structural fires increased 26% between 2014 and 2022. Some 5242 residential fires connected to cluttered environments during that time resulted in 1367 fire service injuries, 1119 civilian injuries, and over $396 million in damages.
“For older adults, those consequences include health and safety risks, social isolation, eviction, and homelessness,” the report authors noted. “For communities, those consequences include public health concerns, increased risk of fire, and dangers to emergency responders.”
What Causes HD?
HD was once classified as a symptom of obsessive-compulsive personality disorder, with extreme causes meeting the diagnostic criteria for obsessive-compulsive disorder. That changed in 2010 when a working group recommended that HD be added to the Diagnostic and Statistical Manual of Mental Disorders (DSM), Fifth Edition, as a stand-alone disorder. That recommendation was approved in 2012.
However, a decade later, much about HD’s etiology remains unknown.
Often beginning in early adolescence, HD is a chronic and progressive condition, with genetics and trauma playing a role in its onset and course, Sanjaya Saxena, MD, director of Clinical and Research Affairs at the International OCD Foundation, said in an interview.
Between 50% and 85% of people with HD symptoms have family members with similar behavior. HD is often comorbid with other psychiatric and medical disorders, which can complicate treatment.
Results of a 2022 study showed that, compared with healthy control individuals, people with HD had widespread abnormalities in the prefrontal white matter tract which connects cortical regions involved in executive functioning, including working memory, attention, reward processing, and decision-making.
Some research also suggests that dysregulation of serotonin transmission may contribute to compulsive behaviors and the difficulty in letting go of possessions.
“We do know that there are factors that contribute to worsening of hoarding symptoms, but that’s not the same thing as what really causes it. So unfortunately, it’s still very understudied, and we don’t have great knowledge of what causes it,” Saxena said.
What Treatments Are Available?
There are currently no Food and Drug Administration–approved medications to treat HD, although some research has shown antidepressants paroxetine and venlafaxine may have some benefit. Methylphenidate and atomoxetine are also under study for HD.
Nonpharmacological therapies have shown more promising results. Among the first was a specialized cognitive-behavioral therapy (CBT) program developed by Randy Frost, PhD, professor emeritus of psychology at Smith College in Northampton, Massachusetts, and Gail Steketee, PhD, dean emerita and professor emerita of social work at Boston University in Massachusetts.
First published in 2007 and the subject of many clinical trials and studies since, the 26-session program has served as a model for psychosocial treatments for HD. The evidence-based therapy addresses various symptoms, including impulse control. One module encourages participants to develop a set of questions to consider before acquiring new items, gradually helping them build resistance to the urge to accumulate more possessions, said Frost, whose early work on HD was cited by those who supported adding the condition to the DSM in 2012.
“There are several features that I think are important including exercises in resisting acquiring and processing information when making decisions about discarding,” Frost said in an interview.
A number of studies have demonstrated the efficacy of CBT for HD, including a 2015 meta-analysis coauthored by Frost. The research showed symptom severity decreased significantly following CBT, with the largest gains in difficulty discarding and moderate improvements in clutter and acquiring.
Responses were better among women and younger patients, and although symptoms improved, posttreatment scores remained closer to the clinical range, researchers noted. It’s possible that more intervention beyond what is usually included in clinical trials — such as more sessions or adding home decluttering visits — could improve treatment response, they added.
A workshop based on the specialized CBT program has expanded the reach of the treatment. The group therapy project, Buried in Treasures (BiT), was developed by Frost, Steketee, and David Tolin, PhD, founder and director of the Anxiety Disorders Center at the Institute of Living, Hartford, and an adjunct professor of psychiatry at Yale School of Medicine, New Haven, Connecticut. The workshop is designed as a facilitated treatment that can be delivered by clinicians or trained nonclinician facilitators.
A study published in May found that more than half the participants with HD responded to the treatment, and of those, 39% reported significant reductions in HD symptoms. BiT sessions were led by trained facilitators, and the study included in-home decluttering sessions, also led by trained volunteers. Researchers said adding the home intervention could increase engagement with the group therapy.
Another study of a modified version of BiT found a 32% decrease in HD symptoms after 15 weeks of treatment delivered via video teleconference.
“The BiT workshop has been expanding around the world and has the advantage of being relatively inexpensive,” Frost said. Another advantage is that it can be run by nonclinicians, which expands treatment options in areas where mental health professionals trained to treat HD are in short supply.
However, the workshop “is not perfect, and clients usually still have symptoms at the end of the workshop,” Frost noted.
“The point is that the BiT workshop is the first step in changing a lifestyle related to possessions,” he continued. “We do certainly need to train more people in how to treat hoarding, and we need to facilitate research to make our treatments more effective.”
What’s New in the Field?
One novel program currently under study combines CBT with a cognitive rehabilitation protocol. Called Cognitive Rehabilitation and Exposure/Sorting Therapy (CREST), the program has been shown to help older adults with HD who don’t respond to traditional CBT for HD.
The program, led by Catherine Ayers, PhD, professor of clinical psychiatry at University of California, San Diego, involves memory training and problem-solving combined with exposure therapy to help participants learn how to tolerate distress associated with discarding their possessions.
Early findings pointed to symptom improvement in older adults following 24 sessions with CREST. The program fared better than geriatric case management in a 2018 study — the first randomized controlled trial of a treatment for HD in older adults — and offered additional benefits compared with exposure therapy in a study published in February 2024.
Virtual reality is also helping people with HD. A program developed at Stanford University in California, allows people with HD to work with a therapist as they practice decluttering in a three-dimensional virtual environment created using photographs and videos of actual hoarded objects and cluttered rooms in patients’ homes.
In a small pilot study, nine people older than 55 years with HD attended 16 weeks of online facilitated therapy where they learned to better understand their attachment to those items. They practiced decluttering by selecting virtual items for recycling, donation, or trash. A virtual garbage truck even hauled away the items they had placed in the trash.
Participants were then asked to discard the actual items at home. Most participants reported a decrease in hoarding symptoms, which was confirmed following a home assessment by a clinician.
“When you pick up an object from a loved one, it still maybe has the scent of the loved one. It has these tactile cues, colors. But in the virtual world, you can take a little bit of a step back,” lead researchers Carolyn Rodriguez, MD, PhD, director of Stanford’s Hoarding Disorders Research Program, said in an interview.
“It’s a little ramp to help people practice these skills. And then what we find is that it actually translated really well. They were able to go home and actually do the real uncluttering,” Rodriguez added.
What Else Can Be Done?
While researchers like Rodriguez continue studies of new and existing treatments, the Senate report draws attention to other responses that could aid people with HD. Because of its significant impact on emergency responders, adult protective services, aging services, and housing providers, the report recommends a nationwide response to older adults with HD.
Currently, federal agencies in charge of mental and community health are not doing enough to address HD, the report’s authors noted.
The report demonstrates “the scope and severity of these challenges and offers a path forward for how we can help people, communities, and local governments contend with this condition,” Casey said.
Specifically, the document cites a lack of HD services and tracking by the Substance Abuse and Mental Health Services Administration, the Administration for Community Living, and the Centers for Disease Control and Prevention.
The committee recommended these agencies collaborate to improve HD data collection, which will be critical to managing a potential spike in cases as the population ages. The committee also suggested awareness and training campaigns to better educate clinicians, social service providers, court officials, and first responders about HD.
Further, the report’s authors called for the Department of Housing and Urban Development to provide guidance and technical assistance on HD for landlords and housing assistance programs and urged Congress to collaborate with the Centers for Medicare & Medicaid Services to expand coverage for hoarding treatments.
Finally, the committee encouraged policymakers to engage directly with individuals affected by HD and their families to better understand the impact of the disorder and inform policy development.
“I think the Senate report focuses on education, not just for therapists, but other stakeholders too,” Frost said. “There are lots of other professionals who have a stake in this process, housing specialists, elder service folks, health and human services. Awareness of this problem is something that’s important for them as well.”
Rodriguez characterized the report’s recommendations as “potentially lifesaving” for individuals with HD. She added that it represents the first step in an ongoing effort to address an impending public health crisis related to HD in older adults and its broader impact on communities.
A spokesperson with Casey’s office said it’s unclear whether any federal agencies have acted on the report recommendations since it was released in June. It’s also unknown whether the Senate Committee on Aging will pursue any additional work on HD when new committee leaders are appointed in 2025.
“Although some federal agencies have taken steps to address HD, those steps are frequently limited. Other relevant agencies have not addressed HD at all in recent years,” report authors wrote. “The federal government can, and should, do more to bolster the response to HD.”
Frost agreed.
“I think federal agencies can have a positive effect by promoting, supporting, and tracking local efforts in dealing with this problem,” he said.
With reporting from Eve Bender.
A version of this article appeared on Medscape.com.
US Alcohol-Related Deaths Double Over 2 Decades, With Notable Age and Gender Disparities
TOPLINE:
US alcohol-related mortality rates increased from 10.7 to 21.6 per 100,000 between 1999 and 2020, with the largest rise of 3.8-fold observed in adults aged 25-34 years. Women experienced a 2.5-fold increase, while the Midwest region showed a similar rise in mortality rates.
METHODOLOGY:
- Analysis utilized the US Centers for Disease Control and Prevention Wide-Ranging Online Data for Epidemiologic Research to examine alcohol-related mortality trends from 1999 to 2020.
- Researchers analyzed data from a total US population of 180,408,769 people aged 25 to 85+ years in 1999 and 226,635,013 people in 2020.
- International Classification of Diseases, Tenth Revision, codes were used to identify deaths with alcohol attribution, including mental and behavioral disorders, alcoholic organ damage, and alcohol-related poisoning.
TAKEAWAY:
- Overall mortality rates increased from 10.7 (95% CI, 10.6-10.8) per 100,000 in 1999 to 21.6 (95% CI, 21.4-21.8) per 100,000 in 2020, representing a significant twofold increase.
- Adults aged 55-64 years demonstrated both the steepest increase and highest absolute rates in both 1999 and 2020.
- American Indian and Alaska Native individuals experienced the steepest increase and highest absolute rates among all racial groups.
- The West region maintained the highest absolute rates in both 1999 and 2020, despite the Midwest showing the largest increase.
IN PRACTICE:
“Individuals who consume large amounts of alcohol tend to have the highest risks of total mortality as well as deaths from cardiovascular disease. Cardiovascular disease deaths are predominantly due to myocardial infarction and stroke. To mitigate these risks, health providers may wish to implement screening for alcohol use in primary care and other healthcare settings. By providing brief interventions and referrals to treatment, healthcare providers would be able to achieve the early identification of individuals at risk of alcohol-related harm and offer them the support and resources they need to reduce their alcohol consumption,” wrote the authors of the study.
SOURCE:
The study was led by Alexandra Matarazzo, BS, Charles E. Schmidt College of Medicine, Florida Atlantic University, Boca Raton. It was published online in The American Journal of Medicine.
LIMITATIONS:
According to the authors, the cross-sectional nature of the data limits the study to descriptive analysis only, making it suitable for hypothesis generation but not hypothesis testing. While the validity and generalizability within the United States are secure because of the use of complete population data, potential bias and uncontrolled confounding may exist because of different population mixes between the two time points.
DISCLOSURES:
The authors reported no relevant conflicts of interest. One coauthor disclosed serving as an independent scientist in an advisory role to investigators and sponsors as Chair of Data Monitoring Committees for Amgen and UBC, to the Food and Drug Administration, and to Up to Date. Additional disclosures are noted in the original article.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
US alcohol-related mortality rates increased from 10.7 to 21.6 per 100,000 between 1999 and 2020, with the largest rise of 3.8-fold observed in adults aged 25-34 years. Women experienced a 2.5-fold increase, while the Midwest region showed a similar rise in mortality rates.
METHODOLOGY:
- Analysis utilized the US Centers for Disease Control and Prevention Wide-Ranging Online Data for Epidemiologic Research to examine alcohol-related mortality trends from 1999 to 2020.
- Researchers analyzed data from a total US population of 180,408,769 people aged 25 to 85+ years in 1999 and 226,635,013 people in 2020.
- International Classification of Diseases, Tenth Revision, codes were used to identify deaths with alcohol attribution, including mental and behavioral disorders, alcoholic organ damage, and alcohol-related poisoning.
TAKEAWAY:
- Overall mortality rates increased from 10.7 (95% CI, 10.6-10.8) per 100,000 in 1999 to 21.6 (95% CI, 21.4-21.8) per 100,000 in 2020, representing a significant twofold increase.
- Adults aged 55-64 years demonstrated both the steepest increase and highest absolute rates in both 1999 and 2020.
- American Indian and Alaska Native individuals experienced the steepest increase and highest absolute rates among all racial groups.
- The West region maintained the highest absolute rates in both 1999 and 2020, despite the Midwest showing the largest increase.
IN PRACTICE:
“Individuals who consume large amounts of alcohol tend to have the highest risks of total mortality as well as deaths from cardiovascular disease. Cardiovascular disease deaths are predominantly due to myocardial infarction and stroke. To mitigate these risks, health providers may wish to implement screening for alcohol use in primary care and other healthcare settings. By providing brief interventions and referrals to treatment, healthcare providers would be able to achieve the early identification of individuals at risk of alcohol-related harm and offer them the support and resources they need to reduce their alcohol consumption,” wrote the authors of the study.
SOURCE:
The study was led by Alexandra Matarazzo, BS, Charles E. Schmidt College of Medicine, Florida Atlantic University, Boca Raton. It was published online in The American Journal of Medicine.
LIMITATIONS:
According to the authors, the cross-sectional nature of the data limits the study to descriptive analysis only, making it suitable for hypothesis generation but not hypothesis testing. While the validity and generalizability within the United States are secure because of the use of complete population data, potential bias and uncontrolled confounding may exist because of different population mixes between the two time points.
DISCLOSURES:
The authors reported no relevant conflicts of interest. One coauthor disclosed serving as an independent scientist in an advisory role to investigators and sponsors as Chair of Data Monitoring Committees for Amgen and UBC, to the Food and Drug Administration, and to Up to Date. Additional disclosures are noted in the original article.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
US alcohol-related mortality rates increased from 10.7 to 21.6 per 100,000 between 1999 and 2020, with the largest rise of 3.8-fold observed in adults aged 25-34 years. Women experienced a 2.5-fold increase, while the Midwest region showed a similar rise in mortality rates.
METHODOLOGY:
- Analysis utilized the US Centers for Disease Control and Prevention Wide-Ranging Online Data for Epidemiologic Research to examine alcohol-related mortality trends from 1999 to 2020.
- Researchers analyzed data from a total US population of 180,408,769 people aged 25 to 85+ years in 1999 and 226,635,013 people in 2020.
- International Classification of Diseases, Tenth Revision, codes were used to identify deaths with alcohol attribution, including mental and behavioral disorders, alcoholic organ damage, and alcohol-related poisoning.
TAKEAWAY:
- Overall mortality rates increased from 10.7 (95% CI, 10.6-10.8) per 100,000 in 1999 to 21.6 (95% CI, 21.4-21.8) per 100,000 in 2020, representing a significant twofold increase.
- Adults aged 55-64 years demonstrated both the steepest increase and highest absolute rates in both 1999 and 2020.
- American Indian and Alaska Native individuals experienced the steepest increase and highest absolute rates among all racial groups.
- The West region maintained the highest absolute rates in both 1999 and 2020, despite the Midwest showing the largest increase.
IN PRACTICE:
“Individuals who consume large amounts of alcohol tend to have the highest risks of total mortality as well as deaths from cardiovascular disease. Cardiovascular disease deaths are predominantly due to myocardial infarction and stroke. To mitigate these risks, health providers may wish to implement screening for alcohol use in primary care and other healthcare settings. By providing brief interventions and referrals to treatment, healthcare providers would be able to achieve the early identification of individuals at risk of alcohol-related harm and offer them the support and resources they need to reduce their alcohol consumption,” wrote the authors of the study.
SOURCE:
The study was led by Alexandra Matarazzo, BS, Charles E. Schmidt College of Medicine, Florida Atlantic University, Boca Raton. It was published online in The American Journal of Medicine.
LIMITATIONS:
According to the authors, the cross-sectional nature of the data limits the study to descriptive analysis only, making it suitable for hypothesis generation but not hypothesis testing. While the validity and generalizability within the United States are secure because of the use of complete population data, potential bias and uncontrolled confounding may exist because of different population mixes between the two time points.
DISCLOSURES:
The authors reported no relevant conflicts of interest. One coauthor disclosed serving as an independent scientist in an advisory role to investigators and sponsors as Chair of Data Monitoring Committees for Amgen and UBC, to the Food and Drug Administration, and to Up to Date. Additional disclosures are noted in the original article.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
We Haven’t Kicked Our Pandemic Drinking Habit
This transcript has been edited for clarity.
You’re stuck in your house. Work is closed or you’re working remotely. Your kids’ school is closed or is offering an hour or two a day of Zoom-based instruction. You have a bit of cabin fever which, you suppose, is better than the actual fever that comes with COVID infections, which are running rampant during the height of the pandemic. But still — it’s stressful. What do you do?
We all coped in our own way. We baked sourdough bread. We built that tree house we’d been meaning to build. We started podcasts. And ... we drank. Quite a bit, actually.
During the first year of the pandemic, alcohol sales increased 3%, the largest year-on-year increase in more than 50 years. There was also an increase in drunkenness across the board, though it was most pronounced in those who were already at risk from alcohol use disorder.
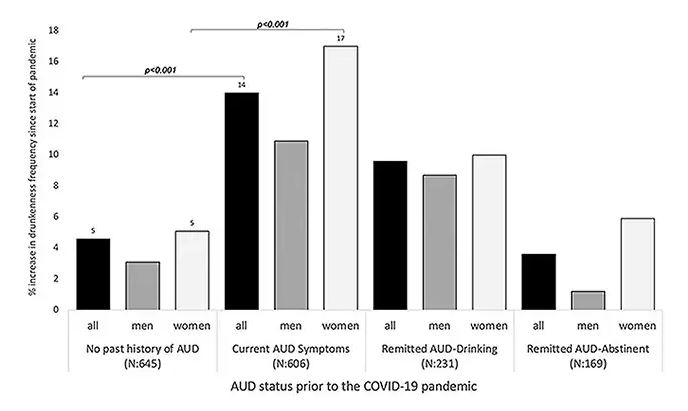
Alcohol-associated deaths increased by around 10% from 2019 to 2020. Obviously, this is a small percentage of COVID-associated deaths, but it is nothing to sneeze at.
But look, we were anxious. And say what you will about alcohol as a risk factor for liver disease, heart disease, and cancer — not to mention traffic accidents — it is an anxiolytic, at least in the short term.
But as the pandemic waned, as society reopened, as we got back to work and reintegrated into our social circles and escaped the confines of our houses and apartments, our drinking habits went back to normal, right?
Americans’ love affair with alcohol has been a torrid one, as this graph showing gallons of ethanol consumed per capita over time shows you.
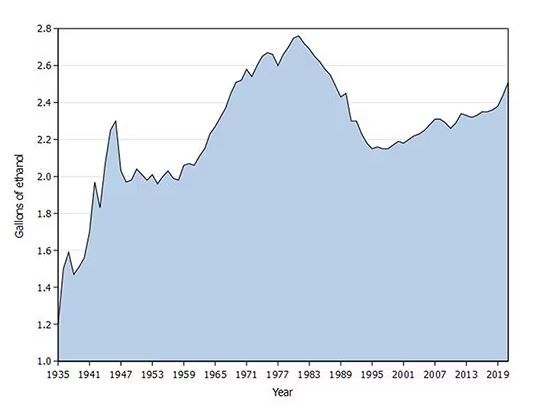
What you see is a steady increase in alcohol consumption from the end of prohibition in 1933 to its peak in the heady days of the early 1980s, followed by a steady decline until the mid-1990s. Since then, there has been another increase with, as you will note, a notable uptick during the early part of the COVID pandemic.
What came across my desk this week was updated data, appearing in a research letter in Annals of Internal Medicine, that compared alcohol consumption in 2020 — the first year of the COVID pandemic — with that in 2022 (the latest available data). And it looks like not much has changed.
This was a population-based survey study leveraging the National Health Interview Survey, including around 80,000 respondents from 2018, 2020, and 2022.
They created two main categories of drinking: drinking any alcohol at all and heavy drinking.
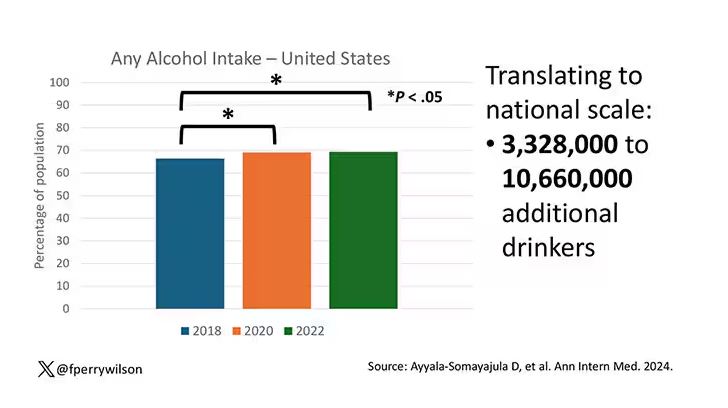
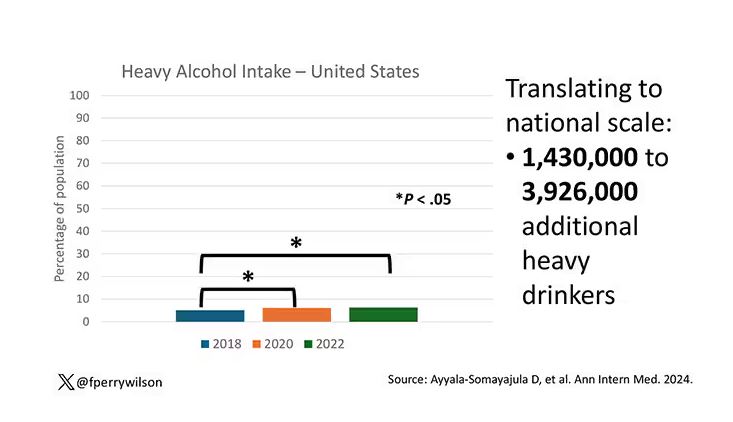
In 2018, 66% of Americans reported drinking any alcohol. That had risen to 69% by 2020, and it stayed at that level even after the lockdown had ended, as you can see here. This may seem like a small increase, but this was a highly significant result. Translating into absolute numbers, it suggests that we have added between 3,328,000 and 10,660,000 net additional drinkers to the population over this time period.
This trend was seen across basically every demographic group, with some notably larger increases among Black and Hispanic individuals, and marginally higher rates among people under age 30.
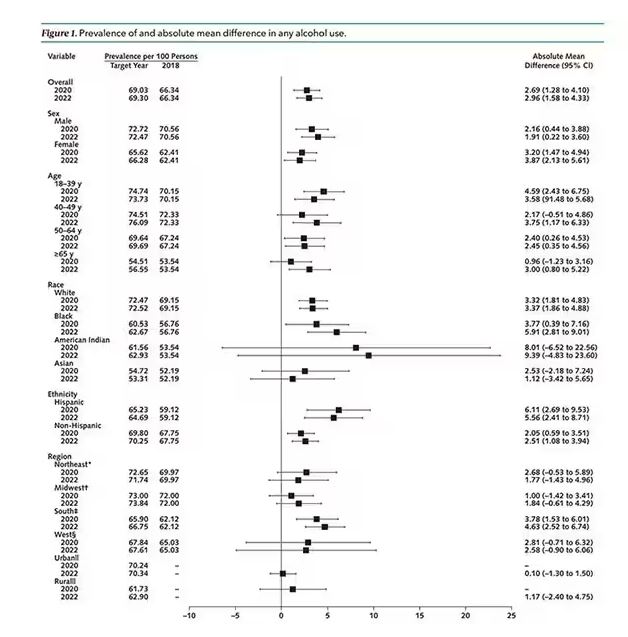
But far be it from me to deny someone a tot of brandy on a cold winter’s night. More interesting is the rate of heavy alcohol use reported in the study. For context, the definitions of heavy alcohol use appear here. For men, it’s any one day with five or more drinks or 15 or more drinks per week. For women it’s four or more drinks on a given day or eight drinks or more per week.
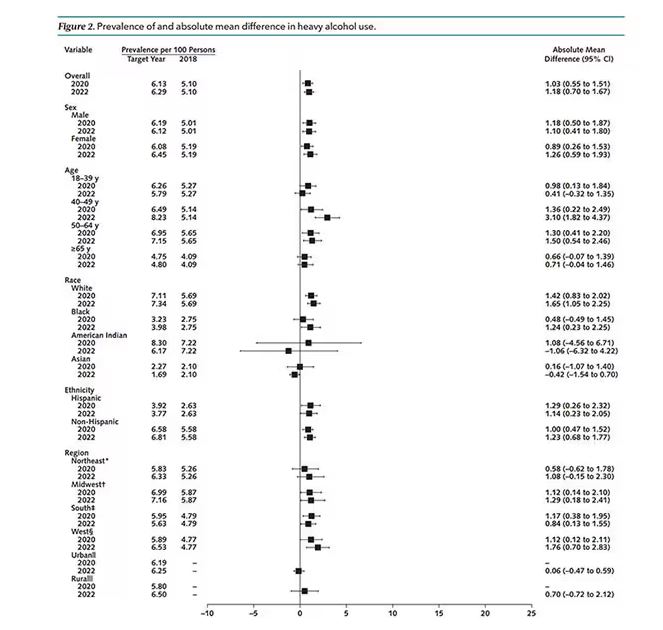
The overall rate of heavy drinking was about 5.1% in 2018 before the start of the pandemic. That rose to more than 6% in 2020 and it rose a bit more into 2022. The net change here, on a population level, is from 1,430,000 to 3,926,000 new heavy drinkers. That’s a number that rises to the level of an actual public health issue.
Again, this trend was fairly broad across demographic groups. Although in this case, the changes were a bit larger among White people and those in the 40- to 49-year age group. This is my cohort, I guess. Cheers.
The information we have from this study is purely descriptive. It tells us that people are drinking more since the pandemic. It doesn’t tell us why, or the impact that this excess drinking will have on subsequent health outcomes, although other studies would suggest that it will contribute to certain chronic conditions, both physical and mental.
Maybe more important is that it reminds us that habits are sticky. Once we become accustomed to something — that glass of wine or two with dinner, and before bed — it has a tendency to stay with us. There’s an upside to that phenomenon as well, of course; it means that we can train good habits too. And those, once they become ingrained, can be just as hard to break. We just need to be mindful of the habits we pick. New Year 2025 is just around the corner. Start brainstorming those resolutions now.
Wilson is associate professor of medicine and public health and director of the Clinical and Translational Research Accelerator at Yale University, New Haven, Conn. He has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
You’re stuck in your house. Work is closed or you’re working remotely. Your kids’ school is closed or is offering an hour or two a day of Zoom-based instruction. You have a bit of cabin fever which, you suppose, is better than the actual fever that comes with COVID infections, which are running rampant during the height of the pandemic. But still — it’s stressful. What do you do?
We all coped in our own way. We baked sourdough bread. We built that tree house we’d been meaning to build. We started podcasts. And ... we drank. Quite a bit, actually.
During the first year of the pandemic, alcohol sales increased 3%, the largest year-on-year increase in more than 50 years. There was also an increase in drunkenness across the board, though it was most pronounced in those who were already at risk from alcohol use disorder.

Alcohol-associated deaths increased by around 10% from 2019 to 2020. Obviously, this is a small percentage of COVID-associated deaths, but it is nothing to sneeze at.

But look, we were anxious. And say what you will about alcohol as a risk factor for liver disease, heart disease, and cancer — not to mention traffic accidents — it is an anxiolytic, at least in the short term.
But as the pandemic waned, as society reopened, as we got back to work and reintegrated into our social circles and escaped the confines of our houses and apartments, our drinking habits went back to normal, right?
Americans’ love affair with alcohol has been a torrid one, as this graph showing gallons of ethanol consumed per capita over time shows you.

What you see is a steady increase in alcohol consumption from the end of prohibition in 1933 to its peak in the heady days of the early 1980s, followed by a steady decline until the mid-1990s. Since then, there has been another increase with, as you will note, a notable uptick during the early part of the COVID pandemic.
What came across my desk this week was updated data, appearing in a research letter in Annals of Internal Medicine, that compared alcohol consumption in 2020 — the first year of the COVID pandemic — with that in 2022 (the latest available data). And it looks like not much has changed.
This was a population-based survey study leveraging the National Health Interview Survey, including around 80,000 respondents from 2018, 2020, and 2022.
They created two main categories of drinking: drinking any alcohol at all and heavy drinking.
In 2018, 66% of Americans reported drinking any alcohol. That had risen to 69% by 2020, and it stayed at that level even after the lockdown had ended, as you can see here. This may seem like a small increase, but this was a highly significant result. Translating into absolute numbers, it suggests that we have added between 3,328,000 and 10,660,000 net additional drinkers to the population over this time period.

This trend was seen across basically every demographic group, with some notably larger increases among Black and Hispanic individuals, and marginally higher rates among people under age 30.

But far be it from me to deny someone a tot of brandy on a cold winter’s night. More interesting is the rate of heavy alcohol use reported in the study. For context, the definitions of heavy alcohol use appear here. For men, it’s any one day with five or more drinks or 15 or more drinks per week. For women it’s four or more drinks on a given day or eight drinks or more per week.
The overall rate of heavy drinking was about 5.1% in 2018 before the start of the pandemic. That rose to more than 6% in 2020 and it rose a bit more into 2022. The net change here, on a population level, is from 1,430,000 to 3,926,000 new heavy drinkers. That’s a number that rises to the level of an actual public health issue.

Again, this trend was fairly broad across demographic groups. Although in this case, the changes were a bit larger among White people and those in the 40- to 49-year age group. This is my cohort, I guess. Cheers.

The information we have from this study is purely descriptive. It tells us that people are drinking more since the pandemic. It doesn’t tell us why, or the impact that this excess drinking will have on subsequent health outcomes, although other studies would suggest that it will contribute to certain chronic conditions, both physical and mental.
Maybe more important is that it reminds us that habits are sticky. Once we become accustomed to something — that glass of wine or two with dinner, and before bed — it has a tendency to stay with us. There’s an upside to that phenomenon as well, of course; it means that we can train good habits too. And those, once they become ingrained, can be just as hard to break. We just need to be mindful of the habits we pick. New Year 2025 is just around the corner. Start brainstorming those resolutions now.
Wilson is associate professor of medicine and public health and director of the Clinical and Translational Research Accelerator at Yale University, New Haven, Conn. He has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
You’re stuck in your house. Work is closed or you’re working remotely. Your kids’ school is closed or is offering an hour or two a day of Zoom-based instruction. You have a bit of cabin fever which, you suppose, is better than the actual fever that comes with COVID infections, which are running rampant during the height of the pandemic. But still — it’s stressful. What do you do?
We all coped in our own way. We baked sourdough bread. We built that tree house we’d been meaning to build. We started podcasts. And ... we drank. Quite a bit, actually.
During the first year of the pandemic, alcohol sales increased 3%, the largest year-on-year increase in more than 50 years. There was also an increase in drunkenness across the board, though it was most pronounced in those who were already at risk from alcohol use disorder.

Alcohol-associated deaths increased by around 10% from 2019 to 2020. Obviously, this is a small percentage of COVID-associated deaths, but it is nothing to sneeze at.

But look, we were anxious. And say what you will about alcohol as a risk factor for liver disease, heart disease, and cancer — not to mention traffic accidents — it is an anxiolytic, at least in the short term.
But as the pandemic waned, as society reopened, as we got back to work and reintegrated into our social circles and escaped the confines of our houses and apartments, our drinking habits went back to normal, right?
Americans’ love affair with alcohol has been a torrid one, as this graph showing gallons of ethanol consumed per capita over time shows you.

What you see is a steady increase in alcohol consumption from the end of prohibition in 1933 to its peak in the heady days of the early 1980s, followed by a steady decline until the mid-1990s. Since then, there has been another increase with, as you will note, a notable uptick during the early part of the COVID pandemic.
What came across my desk this week was updated data, appearing in a research letter in Annals of Internal Medicine, that compared alcohol consumption in 2020 — the first year of the COVID pandemic — with that in 2022 (the latest available data). And it looks like not much has changed.
This was a population-based survey study leveraging the National Health Interview Survey, including around 80,000 respondents from 2018, 2020, and 2022.
They created two main categories of drinking: drinking any alcohol at all and heavy drinking.
In 2018, 66% of Americans reported drinking any alcohol. That had risen to 69% by 2020, and it stayed at that level even after the lockdown had ended, as you can see here. This may seem like a small increase, but this was a highly significant result. Translating into absolute numbers, it suggests that we have added between 3,328,000 and 10,660,000 net additional drinkers to the population over this time period.

This trend was seen across basically every demographic group, with some notably larger increases among Black and Hispanic individuals, and marginally higher rates among people under age 30.

But far be it from me to deny someone a tot of brandy on a cold winter’s night. More interesting is the rate of heavy alcohol use reported in the study. For context, the definitions of heavy alcohol use appear here. For men, it’s any one day with five or more drinks or 15 or more drinks per week. For women it’s four or more drinks on a given day or eight drinks or more per week.
The overall rate of heavy drinking was about 5.1% in 2018 before the start of the pandemic. That rose to more than 6% in 2020 and it rose a bit more into 2022. The net change here, on a population level, is from 1,430,000 to 3,926,000 new heavy drinkers. That’s a number that rises to the level of an actual public health issue.

Again, this trend was fairly broad across demographic groups. Although in this case, the changes were a bit larger among White people and those in the 40- to 49-year age group. This is my cohort, I guess. Cheers.

The information we have from this study is purely descriptive. It tells us that people are drinking more since the pandemic. It doesn’t tell us why, or the impact that this excess drinking will have on subsequent health outcomes, although other studies would suggest that it will contribute to certain chronic conditions, both physical and mental.
Maybe more important is that it reminds us that habits are sticky. Once we become accustomed to something — that glass of wine or two with dinner, and before bed — it has a tendency to stay with us. There’s an upside to that phenomenon as well, of course; it means that we can train good habits too. And those, once they become ingrained, can be just as hard to break. We just need to be mindful of the habits we pick. New Year 2025 is just around the corner. Start brainstorming those resolutions now.
Wilson is associate professor of medicine and public health and director of the Clinical and Translational Research Accelerator at Yale University, New Haven, Conn. He has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
Case Series Highlight Necrotic Wounds Associated with Xylazine-Tainted Fentanyl
TOPLINE:
including 9% that involved exposed deep structures such as bone or tendon.
METHODOLOGY:
- The alpha-2 agonist xylazine, a veterinary sedative, is increasingly detected in fentanyl used illicitly in the United States and may be causing necrotizing wounds in drug users.
- To characterize specific clinical features of xylazine-associated wounds, researchers conducted a case series at three academic medical hospitals in Philadelphia from April 2022 to February 2023.
- They included 29 patients with confirmed xylazine exposure and a chief complaint that was wound-related, seen as inpatients or in the emergency department.
TAKEAWAY:
- The 29 patients (mean age, 39.4 years; 52% men) had a total of 59 wounds, 90% were located on the arms and legs, and 69% were on the posterior upper or anterior lower extremities. Five wounds (9%) involved exposed deep structures such as the bone or tendon.
- Of the 57 wounds with available photographs, 60% had wound beds with predominantly devitalized tissue (eschar or slough), 11% were blisters, 9% had granulation tissue, and 21% had mixed tissue or other types of wound beds. Devitalized tissue was more commonly observed in medium or large wounds (odds ratio [OR], 5.2; P = .02) than in small wounds.
- As reported by patients, 48% were acute wounds, 20% were subacute, and 29% were chronic (present for 3 months or longer). Subacute and chronic wounds were often medium or large compared with acute wounds (OR, 48.5; P < .001) and contained devitalized tissue (OR, 9.5; P < .001).
- Of the 39 wounds with patient-reported etiology, 34 (87%) occurred at drug injection sites.
IN PRACTICE:
To the best of their knowledge, this is “the largest study of wounds among patients with confirmed exposure to xylazine and the first to systematically describe wound characteristics,” the authors wrote. The results, they concluded, “may help identify xylazine exposure and can guide research on the etiology and management of these wounds.”
SOURCE:
This study was conducted by Lydia Lutz, MD, Johns Hopkins University School of Medicine, Baltimore, Maryland, and coinvestigators and was published online in JAMA Dermatology.
LIMITATIONS:
This single-city, retrospective study limited generalizability, and the selection of the largest wounds may bias results. Additionally, chronicity data relied on patient recall, potentially introducing recall bias.
DISCLOSURES:
Two authors received support from the National Institute on Drug Abuse for the study. The authors declared no competing interests.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
including 9% that involved exposed deep structures such as bone or tendon.
METHODOLOGY:
- The alpha-2 agonist xylazine, a veterinary sedative, is increasingly detected in fentanyl used illicitly in the United States and may be causing necrotizing wounds in drug users.
- To characterize specific clinical features of xylazine-associated wounds, researchers conducted a case series at three academic medical hospitals in Philadelphia from April 2022 to February 2023.
- They included 29 patients with confirmed xylazine exposure and a chief complaint that was wound-related, seen as inpatients or in the emergency department.
TAKEAWAY:
- The 29 patients (mean age, 39.4 years; 52% men) had a total of 59 wounds, 90% were located on the arms and legs, and 69% were on the posterior upper or anterior lower extremities. Five wounds (9%) involved exposed deep structures such as the bone or tendon.
- Of the 57 wounds with available photographs, 60% had wound beds with predominantly devitalized tissue (eschar or slough), 11% were blisters, 9% had granulation tissue, and 21% had mixed tissue or other types of wound beds. Devitalized tissue was more commonly observed in medium or large wounds (odds ratio [OR], 5.2; P = .02) than in small wounds.
- As reported by patients, 48% were acute wounds, 20% were subacute, and 29% were chronic (present for 3 months or longer). Subacute and chronic wounds were often medium or large compared with acute wounds (OR, 48.5; P < .001) and contained devitalized tissue (OR, 9.5; P < .001).
- Of the 39 wounds with patient-reported etiology, 34 (87%) occurred at drug injection sites.
IN PRACTICE:
To the best of their knowledge, this is “the largest study of wounds among patients with confirmed exposure to xylazine and the first to systematically describe wound characteristics,” the authors wrote. The results, they concluded, “may help identify xylazine exposure and can guide research on the etiology and management of these wounds.”
SOURCE:
This study was conducted by Lydia Lutz, MD, Johns Hopkins University School of Medicine, Baltimore, Maryland, and coinvestigators and was published online in JAMA Dermatology.
LIMITATIONS:
This single-city, retrospective study limited generalizability, and the selection of the largest wounds may bias results. Additionally, chronicity data relied on patient recall, potentially introducing recall bias.
DISCLOSURES:
Two authors received support from the National Institute on Drug Abuse for the study. The authors declared no competing interests.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
including 9% that involved exposed deep structures such as bone or tendon.
METHODOLOGY:
- The alpha-2 agonist xylazine, a veterinary sedative, is increasingly detected in fentanyl used illicitly in the United States and may be causing necrotizing wounds in drug users.
- To characterize specific clinical features of xylazine-associated wounds, researchers conducted a case series at three academic medical hospitals in Philadelphia from April 2022 to February 2023.
- They included 29 patients with confirmed xylazine exposure and a chief complaint that was wound-related, seen as inpatients or in the emergency department.
TAKEAWAY:
- The 29 patients (mean age, 39.4 years; 52% men) had a total of 59 wounds, 90% were located on the arms and legs, and 69% were on the posterior upper or anterior lower extremities. Five wounds (9%) involved exposed deep structures such as the bone or tendon.
- Of the 57 wounds with available photographs, 60% had wound beds with predominantly devitalized tissue (eschar or slough), 11% were blisters, 9% had granulation tissue, and 21% had mixed tissue or other types of wound beds. Devitalized tissue was more commonly observed in medium or large wounds (odds ratio [OR], 5.2; P = .02) than in small wounds.
- As reported by patients, 48% were acute wounds, 20% were subacute, and 29% were chronic (present for 3 months or longer). Subacute and chronic wounds were often medium or large compared with acute wounds (OR, 48.5; P < .001) and contained devitalized tissue (OR, 9.5; P < .001).
- Of the 39 wounds with patient-reported etiology, 34 (87%) occurred at drug injection sites.
IN PRACTICE:
To the best of their knowledge, this is “the largest study of wounds among patients with confirmed exposure to xylazine and the first to systematically describe wound characteristics,” the authors wrote. The results, they concluded, “may help identify xylazine exposure and can guide research on the etiology and management of these wounds.”
SOURCE:
This study was conducted by Lydia Lutz, MD, Johns Hopkins University School of Medicine, Baltimore, Maryland, and coinvestigators and was published online in JAMA Dermatology.
LIMITATIONS:
This single-city, retrospective study limited generalizability, and the selection of the largest wounds may bias results. Additionally, chronicity data relied on patient recall, potentially introducing recall bias.
DISCLOSURES:
Two authors received support from the National Institute on Drug Abuse for the study. The authors declared no competing interests.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
Alcohol-Associated Liver Disease and Alcohol Use Disorder on the Rise in Older Adults
SAN DIEGO — according to the results of a new study.
Even as mortality rates decline globally, AUD deaths rose in the United States, increasing 1.63% per year between 2010 and 2019. Deaths from cirrhosis increased by 0.56% each year, and deaths from primary liver cancer associated with alcohol increased by 3.09% per year.
Several factors, such as an aging US population and increasing alcohol consumption, play a major role in the uptick in mortality, said lead author Pojsakorn Danpanichkul, MD, an internal medicine resident at Texas Tech University Health Sciences Center, Lubbock, who presented the findings at The Liver Meeting 2024: American Association for the Study of Liver Diseases (AASLD).
“Healthcare providers should increase screening for alcohol use among older adults and consider the added risks of alcohol consumption. Public health strategies should target alcohol prevention and treatment programs tailored to older adults,” he said.
“Older adults are more vulnerable to the harmful effects of alcohol due to natural declines in liver function and metabolism, leading to a higher risk of liver disease and complications,” he explained. However, “little research has focused on this issue.”
Trends in US Not Seen Globally
Danpanichkul and colleagues analyzed data from the Global Burden of Disease Study for 2010-2019, calculating the annual percent change for the burden of AUD, ALD, and liver cancer from alcohol in patients age 70 and older. The research team then compared data in the United States to global estimates for these same diseases.
In 2019, there were 556,340 cases of AUD, 112,560 cases of ALD, and 3720 cases of liver cancer from alcohol in older adults in the United States. In addition, there were 1750 deaths attributed to AUD, 4860 deaths from ALD, and 3010 deaths caused by primary liver cancer from alcohol.
The age-standardized prevalence rates (ASPRs) per 100,000 people were 1547 cases of AUD, 313 cases of ALD, and 10 cases of primary liver cancer caused by alcohol.
The age-standardized death rates (ASDRs) per 100,000 people were 4.88 for AUD, 13.52 for ALD, and 8.38 for primary liver cancer.
During the time period studied, upward trends occurred in the United States, with annual ASPRs increasing by 2.52% for AUD, 1.78% for ALD, and 3.31% for primary liver cancer due to alcohol. Globally, the trends were lower, with annual increases of 0.2% for AUD, 0.38% for ALD, and 0.67% for primary liver cancer from alcohol.
During the same time, ASDRs also increased in all three categories in the United States, while global trends showed a 0.91% decline in AUD deaths and 0.6% decline in ALD deaths. Liver cancer deaths, however, increased by 0.3% worldwide.
Targeted strategies are essential to reduce this growing health burden, especially in an aging population, Danpanichkul said. “These interventions should focus on early detection, intervention, and management for individuals at risk or already affected by ALD and AUD.”
Future studies should investigate alcohol consumption and mortality trends in other age groups, including by sex, location (such as state or territory), and race and ethnicity, he said. Data for more recent years would be compelling as well.
Increased Alcohol Use During and After Pandemic
Numerous studies have indicated that alcohol use increased in 2020 during the COVID-19 pandemic and has remained elevated since then.
In a study published in the Annals of Internal Medicine, for instance, alcohol use per 100 people increased 2.69% in 2020 and 2.96% in 2022, as compared with 2018. Increases occurred across all subgroups, including age, sex, race, ethnicity, and US region.
“During the COVID-19 pandemic, many people stayed at home, watched the television, and increased their alcohol intake” — in the United States and also in Japan — said Hisanori Muto, MD, senior assistant professor of gastroenterology at Fujita Health University in Nagoya, Japan, who wasn’t involved with this study.
“Although the global numbers may appear lower, we’re also seeing an increase in AUD and ALD in Japan, similar to the United States,” he said. “It’s very important to watch these trends and address these diseases.”
Danpanichkul and Muto reported no relevant disclosures.
A version of this article appeared on Medscape.com.
SAN DIEGO — according to the results of a new study.
Even as mortality rates decline globally, AUD deaths rose in the United States, increasing 1.63% per year between 2010 and 2019. Deaths from cirrhosis increased by 0.56% each year, and deaths from primary liver cancer associated with alcohol increased by 3.09% per year.
Several factors, such as an aging US population and increasing alcohol consumption, play a major role in the uptick in mortality, said lead author Pojsakorn Danpanichkul, MD, an internal medicine resident at Texas Tech University Health Sciences Center, Lubbock, who presented the findings at The Liver Meeting 2024: American Association for the Study of Liver Diseases (AASLD).
“Healthcare providers should increase screening for alcohol use among older adults and consider the added risks of alcohol consumption. Public health strategies should target alcohol prevention and treatment programs tailored to older adults,” he said.
“Older adults are more vulnerable to the harmful effects of alcohol due to natural declines in liver function and metabolism, leading to a higher risk of liver disease and complications,” he explained. However, “little research has focused on this issue.”
Trends in US Not Seen Globally
Danpanichkul and colleagues analyzed data from the Global Burden of Disease Study for 2010-2019, calculating the annual percent change for the burden of AUD, ALD, and liver cancer from alcohol in patients age 70 and older. The research team then compared data in the United States to global estimates for these same diseases.
In 2019, there were 556,340 cases of AUD, 112,560 cases of ALD, and 3720 cases of liver cancer from alcohol in older adults in the United States. In addition, there were 1750 deaths attributed to AUD, 4860 deaths from ALD, and 3010 deaths caused by primary liver cancer from alcohol.
The age-standardized prevalence rates (ASPRs) per 100,000 people were 1547 cases of AUD, 313 cases of ALD, and 10 cases of primary liver cancer caused by alcohol.
The age-standardized death rates (ASDRs) per 100,000 people were 4.88 for AUD, 13.52 for ALD, and 8.38 for primary liver cancer.
During the time period studied, upward trends occurred in the United States, with annual ASPRs increasing by 2.52% for AUD, 1.78% for ALD, and 3.31% for primary liver cancer due to alcohol. Globally, the trends were lower, with annual increases of 0.2% for AUD, 0.38% for ALD, and 0.67% for primary liver cancer from alcohol.
During the same time, ASDRs also increased in all three categories in the United States, while global trends showed a 0.91% decline in AUD deaths and 0.6% decline in ALD deaths. Liver cancer deaths, however, increased by 0.3% worldwide.
Targeted strategies are essential to reduce this growing health burden, especially in an aging population, Danpanichkul said. “These interventions should focus on early detection, intervention, and management for individuals at risk or already affected by ALD and AUD.”
Future studies should investigate alcohol consumption and mortality trends in other age groups, including by sex, location (such as state or territory), and race and ethnicity, he said. Data for more recent years would be compelling as well.
Increased Alcohol Use During and After Pandemic
Numerous studies have indicated that alcohol use increased in 2020 during the COVID-19 pandemic and has remained elevated since then.
In a study published in the Annals of Internal Medicine, for instance, alcohol use per 100 people increased 2.69% in 2020 and 2.96% in 2022, as compared with 2018. Increases occurred across all subgroups, including age, sex, race, ethnicity, and US region.
“During the COVID-19 pandemic, many people stayed at home, watched the television, and increased their alcohol intake” — in the United States and also in Japan — said Hisanori Muto, MD, senior assistant professor of gastroenterology at Fujita Health University in Nagoya, Japan, who wasn’t involved with this study.
“Although the global numbers may appear lower, we’re also seeing an increase in AUD and ALD in Japan, similar to the United States,” he said. “It’s very important to watch these trends and address these diseases.”
Danpanichkul and Muto reported no relevant disclosures.
A version of this article appeared on Medscape.com.
SAN DIEGO — according to the results of a new study.
Even as mortality rates decline globally, AUD deaths rose in the United States, increasing 1.63% per year between 2010 and 2019. Deaths from cirrhosis increased by 0.56% each year, and deaths from primary liver cancer associated with alcohol increased by 3.09% per year.
Several factors, such as an aging US population and increasing alcohol consumption, play a major role in the uptick in mortality, said lead author Pojsakorn Danpanichkul, MD, an internal medicine resident at Texas Tech University Health Sciences Center, Lubbock, who presented the findings at The Liver Meeting 2024: American Association for the Study of Liver Diseases (AASLD).
“Healthcare providers should increase screening for alcohol use among older adults and consider the added risks of alcohol consumption. Public health strategies should target alcohol prevention and treatment programs tailored to older adults,” he said.
“Older adults are more vulnerable to the harmful effects of alcohol due to natural declines in liver function and metabolism, leading to a higher risk of liver disease and complications,” he explained. However, “little research has focused on this issue.”
Trends in US Not Seen Globally
Danpanichkul and colleagues analyzed data from the Global Burden of Disease Study for 2010-2019, calculating the annual percent change for the burden of AUD, ALD, and liver cancer from alcohol in patients age 70 and older. The research team then compared data in the United States to global estimates for these same diseases.
In 2019, there were 556,340 cases of AUD, 112,560 cases of ALD, and 3720 cases of liver cancer from alcohol in older adults in the United States. In addition, there were 1750 deaths attributed to AUD, 4860 deaths from ALD, and 3010 deaths caused by primary liver cancer from alcohol.
The age-standardized prevalence rates (ASPRs) per 100,000 people were 1547 cases of AUD, 313 cases of ALD, and 10 cases of primary liver cancer caused by alcohol.
The age-standardized death rates (ASDRs) per 100,000 people were 4.88 for AUD, 13.52 for ALD, and 8.38 for primary liver cancer.
During the time period studied, upward trends occurred in the United States, with annual ASPRs increasing by 2.52% for AUD, 1.78% for ALD, and 3.31% for primary liver cancer due to alcohol. Globally, the trends were lower, with annual increases of 0.2% for AUD, 0.38% for ALD, and 0.67% for primary liver cancer from alcohol.
During the same time, ASDRs also increased in all three categories in the United States, while global trends showed a 0.91% decline in AUD deaths and 0.6% decline in ALD deaths. Liver cancer deaths, however, increased by 0.3% worldwide.
Targeted strategies are essential to reduce this growing health burden, especially in an aging population, Danpanichkul said. “These interventions should focus on early detection, intervention, and management for individuals at risk or already affected by ALD and AUD.”
Future studies should investigate alcohol consumption and mortality trends in other age groups, including by sex, location (such as state or territory), and race and ethnicity, he said. Data for more recent years would be compelling as well.
Increased Alcohol Use During and After Pandemic
Numerous studies have indicated that alcohol use increased in 2020 during the COVID-19 pandemic and has remained elevated since then.
In a study published in the Annals of Internal Medicine, for instance, alcohol use per 100 people increased 2.69% in 2020 and 2.96% in 2022, as compared with 2018. Increases occurred across all subgroups, including age, sex, race, ethnicity, and US region.
“During the COVID-19 pandemic, many people stayed at home, watched the television, and increased their alcohol intake” — in the United States and also in Japan — said Hisanori Muto, MD, senior assistant professor of gastroenterology at Fujita Health University in Nagoya, Japan, who wasn’t involved with this study.
“Although the global numbers may appear lower, we’re also seeing an increase in AUD and ALD in Japan, similar to the United States,” he said. “It’s very important to watch these trends and address these diseases.”
Danpanichkul and Muto reported no relevant disclosures.
A version of this article appeared on Medscape.com.
FROM AASLD 2024
Alcohol Use Disorder Therapy Remains Underutilized in Alcohol-Associated Liver Disease
PHILADELPHIA — according to a study presented at the American College of Gastroenterology (ACG) 2024 Annual Scientific Meeting.
In an analysis of commercially insured Americans, AUD medications were prescribed to only 1 in 50 patients with ALD and about 1 in 10 patients with acute alcohol-associated hepatitis (AAH).
“Providers caring for these patients should consider early initiation of this therapy in select cases,” said lead author Alex R. Jones, MD, chief resident of internal medicine at the University of Texas Southwestern Medical Center in Dallas.
“Based on additional analyses looking at the prescriber subspecialty, we didn’t identify any gastroenterologists or hepatologists who prescribed pharmacotherapy,” he said. “This could be a great opportunity for hepatologists to engage in the pharmacologic treatment of AUD.”
Jones and colleagues analyzed 2006-2021 data from IQVIA PharMetrics Plus for Academics, a nationally representative database of commercially insured patients in the United States. They looked for AUD pharmacologic treatment at any time after AUD diagnosis, including prescriptions for gabapentin, naltrexone, topiramate, acamprosate, baclofen, and disulfiram.
Among 28,625 patients with AUD (defined as at least two outpatient codes or at least one inpatient code), 1201 had ALD with cirrhosis and 439 had AAH.
Pharmacologic therapy was prescribed in 3924 (14.5%) patients without ALD, 28 (2.3%) with ALD, and 42 (9.8%) with AAH.
In addition, one-time prescriptions were observed in 1113 (28.4%) patients without ALD, three patients (10.7%) with ALD, and eight patients (18.6%) with AAH.
Overall, 64.5% of the general population consisted of men. About 46% had a psychiatric diagnosis other than substance use disorder (SUD), and 35.7% had a non-AUD SUD.
Patients who received AUD pharmacotherapy tended to be older, at a median age of 45 years, than those aged 42 years without a prescription.
The median time to prescription was 302 days, with no significant differences based on the presence of liver disease.
By medication, gabapentin was prescribed most often (9.4%), followed by oral naltrexone (2.6%) and topiramate (2%). Oral naltrexone was prescribed at a lower rate in patients with ALD and at a higher rate in patients with AAH than in patients without ALD. Baclofen was also prescribed at lower rates in patients with ALD and AAH.
In a multivariable logistic regression analysis, several characteristics were more significantly associated with pharmacologic therapy, such as age ≥ 50 years (adjusted odds ratio [aOR], 1.33), female sex (aOR, 1.31), a non-liver Charlson Comorbidity Index ≥ 3 (aOR, 2.21), and psychiatric comorbidities (aOR, 2.76).
On the other hand, the presence of hepatic decompensation — defined as ascites, hepatic encephalopathy, or bleeding varices — was associated with lower odds of receiving pharmacotherapy (aOR, 0.08). ALD cirrhosis (non-AAH) also had lower odds (aOR, 0.24).
The study was limited by only incorporating patients with commercial insurance, lacking demographic details related to race or ethnicity, and potentially misclassifying patients despite validated definitions of ALD and AUD, Jones said.
As the study couldn’t determine the indications for prescriptions, such as gabapentin use for migraines or diabetes-associated neuropathy, for instance, future studies could look at these precise details, he added.
“It’s important to know we’re underutilizing therapies that we have a lot of information about, such as gabapentin, which is an old medication that we should feel fairly comfortable using,” said Patricia Jones, MD, a hepatologist and associate professor of clinical medicine at the University of Miami Miller School of Medicine, in Florida. Patricia Jones comoderated the plenary session on small intestine, functional, and liver research.
“I also expect that, if a future study reviewed this data and excluded people with valid indications, such as migraines or diabetic neuropathy, we’d see even lower rates of prescription,” she said.
From a clinical perspective, patient communication and clinical decision-making are key, Patricia Jones added, particularly when clinical gastroenterologists and hepatologists may not offer this type of therapy or patients refuse this type of therapy.
“We need to think about our practice patterns and how we can offer therapy,” she said. “In general, we know these medications are very safe. Even though they’re not widely used in people with cirrhosis, there’s not enough evidence to suggest we shouldn’t use them.”
Alex Jones and Patricia Jones reported no relevant disclosures.
A version of this article first appeared on Medscape.com.
PHILADELPHIA — according to a study presented at the American College of Gastroenterology (ACG) 2024 Annual Scientific Meeting.
In an analysis of commercially insured Americans, AUD medications were prescribed to only 1 in 50 patients with ALD and about 1 in 10 patients with acute alcohol-associated hepatitis (AAH).
“Providers caring for these patients should consider early initiation of this therapy in select cases,” said lead author Alex R. Jones, MD, chief resident of internal medicine at the University of Texas Southwestern Medical Center in Dallas.
“Based on additional analyses looking at the prescriber subspecialty, we didn’t identify any gastroenterologists or hepatologists who prescribed pharmacotherapy,” he said. “This could be a great opportunity for hepatologists to engage in the pharmacologic treatment of AUD.”
Jones and colleagues analyzed 2006-2021 data from IQVIA PharMetrics Plus for Academics, a nationally representative database of commercially insured patients in the United States. They looked for AUD pharmacologic treatment at any time after AUD diagnosis, including prescriptions for gabapentin, naltrexone, topiramate, acamprosate, baclofen, and disulfiram.
Among 28,625 patients with AUD (defined as at least two outpatient codes or at least one inpatient code), 1201 had ALD with cirrhosis and 439 had AAH.
Pharmacologic therapy was prescribed in 3924 (14.5%) patients without ALD, 28 (2.3%) with ALD, and 42 (9.8%) with AAH.
In addition, one-time prescriptions were observed in 1113 (28.4%) patients without ALD, three patients (10.7%) with ALD, and eight patients (18.6%) with AAH.
Overall, 64.5% of the general population consisted of men. About 46% had a psychiatric diagnosis other than substance use disorder (SUD), and 35.7% had a non-AUD SUD.
Patients who received AUD pharmacotherapy tended to be older, at a median age of 45 years, than those aged 42 years without a prescription.
The median time to prescription was 302 days, with no significant differences based on the presence of liver disease.
By medication, gabapentin was prescribed most often (9.4%), followed by oral naltrexone (2.6%) and topiramate (2%). Oral naltrexone was prescribed at a lower rate in patients with ALD and at a higher rate in patients with AAH than in patients without ALD. Baclofen was also prescribed at lower rates in patients with ALD and AAH.
In a multivariable logistic regression analysis, several characteristics were more significantly associated with pharmacologic therapy, such as age ≥ 50 years (adjusted odds ratio [aOR], 1.33), female sex (aOR, 1.31), a non-liver Charlson Comorbidity Index ≥ 3 (aOR, 2.21), and psychiatric comorbidities (aOR, 2.76).
On the other hand, the presence of hepatic decompensation — defined as ascites, hepatic encephalopathy, or bleeding varices — was associated with lower odds of receiving pharmacotherapy (aOR, 0.08). ALD cirrhosis (non-AAH) also had lower odds (aOR, 0.24).
The study was limited by only incorporating patients with commercial insurance, lacking demographic details related to race or ethnicity, and potentially misclassifying patients despite validated definitions of ALD and AUD, Jones said.
As the study couldn’t determine the indications for prescriptions, such as gabapentin use for migraines or diabetes-associated neuropathy, for instance, future studies could look at these precise details, he added.
“It’s important to know we’re underutilizing therapies that we have a lot of information about, such as gabapentin, which is an old medication that we should feel fairly comfortable using,” said Patricia Jones, MD, a hepatologist and associate professor of clinical medicine at the University of Miami Miller School of Medicine, in Florida. Patricia Jones comoderated the plenary session on small intestine, functional, and liver research.
“I also expect that, if a future study reviewed this data and excluded people with valid indications, such as migraines or diabetic neuropathy, we’d see even lower rates of prescription,” she said.
From a clinical perspective, patient communication and clinical decision-making are key, Patricia Jones added, particularly when clinical gastroenterologists and hepatologists may not offer this type of therapy or patients refuse this type of therapy.
“We need to think about our practice patterns and how we can offer therapy,” she said. “In general, we know these medications are very safe. Even though they’re not widely used in people with cirrhosis, there’s not enough evidence to suggest we shouldn’t use them.”
Alex Jones and Patricia Jones reported no relevant disclosures.
A version of this article first appeared on Medscape.com.
PHILADELPHIA — according to a study presented at the American College of Gastroenterology (ACG) 2024 Annual Scientific Meeting.
In an analysis of commercially insured Americans, AUD medications were prescribed to only 1 in 50 patients with ALD and about 1 in 10 patients with acute alcohol-associated hepatitis (AAH).
“Providers caring for these patients should consider early initiation of this therapy in select cases,” said lead author Alex R. Jones, MD, chief resident of internal medicine at the University of Texas Southwestern Medical Center in Dallas.
“Based on additional analyses looking at the prescriber subspecialty, we didn’t identify any gastroenterologists or hepatologists who prescribed pharmacotherapy,” he said. “This could be a great opportunity for hepatologists to engage in the pharmacologic treatment of AUD.”
Jones and colleagues analyzed 2006-2021 data from IQVIA PharMetrics Plus for Academics, a nationally representative database of commercially insured patients in the United States. They looked for AUD pharmacologic treatment at any time after AUD diagnosis, including prescriptions for gabapentin, naltrexone, topiramate, acamprosate, baclofen, and disulfiram.
Among 28,625 patients with AUD (defined as at least two outpatient codes or at least one inpatient code), 1201 had ALD with cirrhosis and 439 had AAH.
Pharmacologic therapy was prescribed in 3924 (14.5%) patients without ALD, 28 (2.3%) with ALD, and 42 (9.8%) with AAH.
In addition, one-time prescriptions were observed in 1113 (28.4%) patients without ALD, three patients (10.7%) with ALD, and eight patients (18.6%) with AAH.
Overall, 64.5% of the general population consisted of men. About 46% had a psychiatric diagnosis other than substance use disorder (SUD), and 35.7% had a non-AUD SUD.
Patients who received AUD pharmacotherapy tended to be older, at a median age of 45 years, than those aged 42 years without a prescription.
The median time to prescription was 302 days, with no significant differences based on the presence of liver disease.
By medication, gabapentin was prescribed most often (9.4%), followed by oral naltrexone (2.6%) and topiramate (2%). Oral naltrexone was prescribed at a lower rate in patients with ALD and at a higher rate in patients with AAH than in patients without ALD. Baclofen was also prescribed at lower rates in patients with ALD and AAH.
In a multivariable logistic regression analysis, several characteristics were more significantly associated with pharmacologic therapy, such as age ≥ 50 years (adjusted odds ratio [aOR], 1.33), female sex (aOR, 1.31), a non-liver Charlson Comorbidity Index ≥ 3 (aOR, 2.21), and psychiatric comorbidities (aOR, 2.76).
On the other hand, the presence of hepatic decompensation — defined as ascites, hepatic encephalopathy, or bleeding varices — was associated with lower odds of receiving pharmacotherapy (aOR, 0.08). ALD cirrhosis (non-AAH) also had lower odds (aOR, 0.24).
The study was limited by only incorporating patients with commercial insurance, lacking demographic details related to race or ethnicity, and potentially misclassifying patients despite validated definitions of ALD and AUD, Jones said.
As the study couldn’t determine the indications for prescriptions, such as gabapentin use for migraines or diabetes-associated neuropathy, for instance, future studies could look at these precise details, he added.
“It’s important to know we’re underutilizing therapies that we have a lot of information about, such as gabapentin, which is an old medication that we should feel fairly comfortable using,” said Patricia Jones, MD, a hepatologist and associate professor of clinical medicine at the University of Miami Miller School of Medicine, in Florida. Patricia Jones comoderated the plenary session on small intestine, functional, and liver research.
“I also expect that, if a future study reviewed this data and excluded people with valid indications, such as migraines or diabetic neuropathy, we’d see even lower rates of prescription,” she said.
From a clinical perspective, patient communication and clinical decision-making are key, Patricia Jones added, particularly when clinical gastroenterologists and hepatologists may not offer this type of therapy or patients refuse this type of therapy.
“We need to think about our practice patterns and how we can offer therapy,” she said. “In general, we know these medications are very safe. Even though they’re not widely used in people with cirrhosis, there’s not enough evidence to suggest we shouldn’t use them.”
Alex Jones and Patricia Jones reported no relevant disclosures.
A version of this article first appeared on Medscape.com.
FROM ACG 2024
Canadian Guideline on Managing Opioid Use Disorder Updated
Canada’s National Guideline for the Clinical Management of Opioid Use Disorder (OUD) has been updated to reflect the latest literature. The new document recommends buprenorphine and methadone as first-line treatments for OUD.
Opioid use and OUD remain the leading causes of drug-related death worldwide. In Canada, the number of apparent opioid-related deaths increased from 2831 in 2016 to 8049 in 2023. Despite the expansion of treatment options, including the lifting of restrictions on methadone prescribing in 2018, there has been a substantial surge in opioid-related harms, the authors wrote.
“OUD and opioid-related harms have devastating outcomes for our communities across Canada,” author Ginette Poulin, MD, a family physician at the University of Manitoba in Winnipeg, Manitoba, Canada, said in a statement. “With the growing dangers associated with the illicit market, we need to ensure we are sharing the most relevant therapeutic tools and up-to-date knowledge to help providers and communities address this complex issue.”
The 2024 update, which was drafted by the Canadian Research Initiative in Substance Matters (CRISM), was published in CMAJ.
Expanding Access
The COVID-19 pandemic marked an increase in opioid-related harms, senior author Julie Bruneau, MD, Canada research chair in addiction medicine and professor of family and emergency medicine at the Université de Montréal, in Quebec, Canada, told this news organization. Access to essential services and support for people with OUD became restricted, and the drug supply became toxic and volatile.
“In March 2018, CRISM published the first Canadian national clinical practice guideline to assist clinicians in making informed decisions regarding the clinical management of OUD, and recommendations were made in light of existing evidence on prioritizing available treatments,” said Bruneau.
“This guideline is intended for use by healthcare providers, including physicians, nurse practitioners, pharmacists, clinical psychologists, social workers, medical educators, and clinical care case managers with or without specialized experience in addiction treatment. We hope it will help expand access to evidence-based interventions for people with OUD beyond tertiary care,” she said.
Bruneau added that integrating first-line opioid agonist treatment into primary care could reduce stigma, increase early screening and patient retention, and help reduce Canada’s opioid crisis.
The CRISM guideline development team carried out a comprehensive systematic review of the literature published from January 1, 2017, to September 14, 2023. The team, which included patients with OUD, drafted and graded their recommendations using the Grading of Recommendations, Assessment, Development and Evaluation approach.
“First, OUD management should be based on a patient-centered approach, which includes respect for the patient’s rights, preferences, and dignity,” said Bruneau.
Highlights of the guideline include the following recommendations:
- Buprenorphine, with or without naloxone, and methadone can be used as standard first-line treatment options.
- Opioid agonist treatment with slow-release oral morphine should be made available and offered as a second-line option.
- Patients with OUD should not be offered withdrawal management as stand-alone treatment because it is associated with increased rates of relapse, morbidity, and mortality.
- Psychosocial treatment, interventions, and supports can be offered as adjunct treatments but should not be a mandatory component of standard treatment for OUD and should not prevent access to opioid agonist therapy.
- Harm reduction strategies should be offered as part of the continuum of care for patients with OUD.
- Pregnant people can be offered buprenorphine or methadone as treatment options.
Treating More Patients
“Too many people die from untreated opioid addiction in Canada,” coauthor Peter Selby, MD, director of medical education at the Centre for Addiction and Mental Health, said in a statement. “We have medicines that help people stop using, but too few patients are treated due to stigma and lack of prescribers knowing what to do. These national guidelines help them use proven medications to not only prevent death but also help people recover.”
“That both buprenorphine and methadone are now to be considered first-line therapy for the management of OUD is an important change to the guideline,” said Abhimanyu Sud, MD, PhD, research chair in primary care and population health systems at Humber River Health and assistant professor of family and community medicine at the University of Toronto. He did not participate in drafting the guidelines.
“There is a lot of good evidence that these agents are effective for the management of OUD. We had this idea that methadone was harder or somehow more unsafe than buprenorphine, and that buprenorphine was therefore a safer therapy that should be used more widely. Now we have very high-potency opioids that are circulating, and methadone, as a strong opioid agonist, has an important role to play. Clinical experience has borne that out, and this is reflected in the guidelines,” said Sud.
“When we treat patients who are using fentanyl, for example, or fentanyl analogs, or they’re not sure what they are using because the drug supply has been so contaminated, you sometimes need another agent. Also, a lot of patients do not respond very well to buprenorphine, so for many people, a full agonist like methadone is needed,” he added.
Giving higher priority to slow-release morphine is a good move, and the drug’s use is likely to be safe when administered by a skilled clinician, said Akash Goel, MD, staff physician in the Department of Anesthesiology and Pain Medicine at St. Michael’s Hospital and assistant professor of anesthesiology and pain medicine at the University of Toronto. Goel was not involved in drafting the guideline.
The updated document will empower patients to make informed decisions about their care, he said. “Buprenorphine, for example, may not be the right selection for all patients. The updated guideline recognizes this. So, for patients who are at risk of failing OUD therapy and going back to using, buprenorphine may not be the best option. The new guideline gives patients the opportunity to have a conversation with their healthcare providers and then decide what’s the best way forward for them.”
The guideline was supported by Health Canada and the Canadian Institutes of Health Research (CIHR) via CRISM. Poulin reported receiving honoraria for presentations from the Master Clinician Alliance and Indivior outside this work. Bruneau reported receiving a CIHR research grant and a grant from Health Canada’s Substance Use and Addictions Program. Outside this work, Bruneau received a National Institutes of Health research grant and consulting fees for Gilead Sciences and AbbVie.
A version of this article first appeared on Medscape.com.
Canada’s National Guideline for the Clinical Management of Opioid Use Disorder (OUD) has been updated to reflect the latest literature. The new document recommends buprenorphine and methadone as first-line treatments for OUD.
Opioid use and OUD remain the leading causes of drug-related death worldwide. In Canada, the number of apparent opioid-related deaths increased from 2831 in 2016 to 8049 in 2023. Despite the expansion of treatment options, including the lifting of restrictions on methadone prescribing in 2018, there has been a substantial surge in opioid-related harms, the authors wrote.
“OUD and opioid-related harms have devastating outcomes for our communities across Canada,” author Ginette Poulin, MD, a family physician at the University of Manitoba in Winnipeg, Manitoba, Canada, said in a statement. “With the growing dangers associated with the illicit market, we need to ensure we are sharing the most relevant therapeutic tools and up-to-date knowledge to help providers and communities address this complex issue.”
The 2024 update, which was drafted by the Canadian Research Initiative in Substance Matters (CRISM), was published in CMAJ.
Expanding Access
The COVID-19 pandemic marked an increase in opioid-related harms, senior author Julie Bruneau, MD, Canada research chair in addiction medicine and professor of family and emergency medicine at the Université de Montréal, in Quebec, Canada, told this news organization. Access to essential services and support for people with OUD became restricted, and the drug supply became toxic and volatile.
“In March 2018, CRISM published the first Canadian national clinical practice guideline to assist clinicians in making informed decisions regarding the clinical management of OUD, and recommendations were made in light of existing evidence on prioritizing available treatments,” said Bruneau.
“This guideline is intended for use by healthcare providers, including physicians, nurse practitioners, pharmacists, clinical psychologists, social workers, medical educators, and clinical care case managers with or without specialized experience in addiction treatment. We hope it will help expand access to evidence-based interventions for people with OUD beyond tertiary care,” she said.
Bruneau added that integrating first-line opioid agonist treatment into primary care could reduce stigma, increase early screening and patient retention, and help reduce Canada’s opioid crisis.
The CRISM guideline development team carried out a comprehensive systematic review of the literature published from January 1, 2017, to September 14, 2023. The team, which included patients with OUD, drafted and graded their recommendations using the Grading of Recommendations, Assessment, Development and Evaluation approach.
“First, OUD management should be based on a patient-centered approach, which includes respect for the patient’s rights, preferences, and dignity,” said Bruneau.
Highlights of the guideline include the following recommendations:
- Buprenorphine, with or without naloxone, and methadone can be used as standard first-line treatment options.
- Opioid agonist treatment with slow-release oral morphine should be made available and offered as a second-line option.
- Patients with OUD should not be offered withdrawal management as stand-alone treatment because it is associated with increased rates of relapse, morbidity, and mortality.
- Psychosocial treatment, interventions, and supports can be offered as adjunct treatments but should not be a mandatory component of standard treatment for OUD and should not prevent access to opioid agonist therapy.
- Harm reduction strategies should be offered as part of the continuum of care for patients with OUD.
- Pregnant people can be offered buprenorphine or methadone as treatment options.
Treating More Patients
“Too many people die from untreated opioid addiction in Canada,” coauthor Peter Selby, MD, director of medical education at the Centre for Addiction and Mental Health, said in a statement. “We have medicines that help people stop using, but too few patients are treated due to stigma and lack of prescribers knowing what to do. These national guidelines help them use proven medications to not only prevent death but also help people recover.”
“That both buprenorphine and methadone are now to be considered first-line therapy for the management of OUD is an important change to the guideline,” said Abhimanyu Sud, MD, PhD, research chair in primary care and population health systems at Humber River Health and assistant professor of family and community medicine at the University of Toronto. He did not participate in drafting the guidelines.
“There is a lot of good evidence that these agents are effective for the management of OUD. We had this idea that methadone was harder or somehow more unsafe than buprenorphine, and that buprenorphine was therefore a safer therapy that should be used more widely. Now we have very high-potency opioids that are circulating, and methadone, as a strong opioid agonist, has an important role to play. Clinical experience has borne that out, and this is reflected in the guidelines,” said Sud.
“When we treat patients who are using fentanyl, for example, or fentanyl analogs, or they’re not sure what they are using because the drug supply has been so contaminated, you sometimes need another agent. Also, a lot of patients do not respond very well to buprenorphine, so for many people, a full agonist like methadone is needed,” he added.
Giving higher priority to slow-release morphine is a good move, and the drug’s use is likely to be safe when administered by a skilled clinician, said Akash Goel, MD, staff physician in the Department of Anesthesiology and Pain Medicine at St. Michael’s Hospital and assistant professor of anesthesiology and pain medicine at the University of Toronto. Goel was not involved in drafting the guideline.
The updated document will empower patients to make informed decisions about their care, he said. “Buprenorphine, for example, may not be the right selection for all patients. The updated guideline recognizes this. So, for patients who are at risk of failing OUD therapy and going back to using, buprenorphine may not be the best option. The new guideline gives patients the opportunity to have a conversation with their healthcare providers and then decide what’s the best way forward for them.”
The guideline was supported by Health Canada and the Canadian Institutes of Health Research (CIHR) via CRISM. Poulin reported receiving honoraria for presentations from the Master Clinician Alliance and Indivior outside this work. Bruneau reported receiving a CIHR research grant and a grant from Health Canada’s Substance Use and Addictions Program. Outside this work, Bruneau received a National Institutes of Health research grant and consulting fees for Gilead Sciences and AbbVie.
A version of this article first appeared on Medscape.com.
Canada’s National Guideline for the Clinical Management of Opioid Use Disorder (OUD) has been updated to reflect the latest literature. The new document recommends buprenorphine and methadone as first-line treatments for OUD.
Opioid use and OUD remain the leading causes of drug-related death worldwide. In Canada, the number of apparent opioid-related deaths increased from 2831 in 2016 to 8049 in 2023. Despite the expansion of treatment options, including the lifting of restrictions on methadone prescribing in 2018, there has been a substantial surge in opioid-related harms, the authors wrote.
“OUD and opioid-related harms have devastating outcomes for our communities across Canada,” author Ginette Poulin, MD, a family physician at the University of Manitoba in Winnipeg, Manitoba, Canada, said in a statement. “With the growing dangers associated with the illicit market, we need to ensure we are sharing the most relevant therapeutic tools and up-to-date knowledge to help providers and communities address this complex issue.”
The 2024 update, which was drafted by the Canadian Research Initiative in Substance Matters (CRISM), was published in CMAJ.
Expanding Access
The COVID-19 pandemic marked an increase in opioid-related harms, senior author Julie Bruneau, MD, Canada research chair in addiction medicine and professor of family and emergency medicine at the Université de Montréal, in Quebec, Canada, told this news organization. Access to essential services and support for people with OUD became restricted, and the drug supply became toxic and volatile.
“In March 2018, CRISM published the first Canadian national clinical practice guideline to assist clinicians in making informed decisions regarding the clinical management of OUD, and recommendations were made in light of existing evidence on prioritizing available treatments,” said Bruneau.
“This guideline is intended for use by healthcare providers, including physicians, nurse practitioners, pharmacists, clinical psychologists, social workers, medical educators, and clinical care case managers with or without specialized experience in addiction treatment. We hope it will help expand access to evidence-based interventions for people with OUD beyond tertiary care,” she said.
Bruneau added that integrating first-line opioid agonist treatment into primary care could reduce stigma, increase early screening and patient retention, and help reduce Canada’s opioid crisis.
The CRISM guideline development team carried out a comprehensive systematic review of the literature published from January 1, 2017, to September 14, 2023. The team, which included patients with OUD, drafted and graded their recommendations using the Grading of Recommendations, Assessment, Development and Evaluation approach.
“First, OUD management should be based on a patient-centered approach, which includes respect for the patient’s rights, preferences, and dignity,” said Bruneau.
Highlights of the guideline include the following recommendations:
- Buprenorphine, with or without naloxone, and methadone can be used as standard first-line treatment options.
- Opioid agonist treatment with slow-release oral morphine should be made available and offered as a second-line option.
- Patients with OUD should not be offered withdrawal management as stand-alone treatment because it is associated with increased rates of relapse, morbidity, and mortality.
- Psychosocial treatment, interventions, and supports can be offered as adjunct treatments but should not be a mandatory component of standard treatment for OUD and should not prevent access to opioid agonist therapy.
- Harm reduction strategies should be offered as part of the continuum of care for patients with OUD.
- Pregnant people can be offered buprenorphine or methadone as treatment options.
Treating More Patients
“Too many people die from untreated opioid addiction in Canada,” coauthor Peter Selby, MD, director of medical education at the Centre for Addiction and Mental Health, said in a statement. “We have medicines that help people stop using, but too few patients are treated due to stigma and lack of prescribers knowing what to do. These national guidelines help them use proven medications to not only prevent death but also help people recover.”
“That both buprenorphine and methadone are now to be considered first-line therapy for the management of OUD is an important change to the guideline,” said Abhimanyu Sud, MD, PhD, research chair in primary care and population health systems at Humber River Health and assistant professor of family and community medicine at the University of Toronto. He did not participate in drafting the guidelines.
“There is a lot of good evidence that these agents are effective for the management of OUD. We had this idea that methadone was harder or somehow more unsafe than buprenorphine, and that buprenorphine was therefore a safer therapy that should be used more widely. Now we have very high-potency opioids that are circulating, and methadone, as a strong opioid agonist, has an important role to play. Clinical experience has borne that out, and this is reflected in the guidelines,” said Sud.
“When we treat patients who are using fentanyl, for example, or fentanyl analogs, or they’re not sure what they are using because the drug supply has been so contaminated, you sometimes need another agent. Also, a lot of patients do not respond very well to buprenorphine, so for many people, a full agonist like methadone is needed,” he added.
Giving higher priority to slow-release morphine is a good move, and the drug’s use is likely to be safe when administered by a skilled clinician, said Akash Goel, MD, staff physician in the Department of Anesthesiology and Pain Medicine at St. Michael’s Hospital and assistant professor of anesthesiology and pain medicine at the University of Toronto. Goel was not involved in drafting the guideline.
The updated document will empower patients to make informed decisions about their care, he said. “Buprenorphine, for example, may not be the right selection for all patients. The updated guideline recognizes this. So, for patients who are at risk of failing OUD therapy and going back to using, buprenorphine may not be the best option. The new guideline gives patients the opportunity to have a conversation with their healthcare providers and then decide what’s the best way forward for them.”
The guideline was supported by Health Canada and the Canadian Institutes of Health Research (CIHR) via CRISM. Poulin reported receiving honoraria for presentations from the Master Clinician Alliance and Indivior outside this work. Bruneau reported receiving a CIHR research grant and a grant from Health Canada’s Substance Use and Addictions Program. Outside this work, Bruneau received a National Institutes of Health research grant and consulting fees for Gilead Sciences and AbbVie.
A version of this article first appeared on Medscape.com.
Do Patients on Anti-Obesity Drugs Decrease Alcohol Use?
SAN ANTONIO —
The findings, from surveys of more than 14,000 participants in WeightWatchers’ telehealth weight management program, were presented on November 6 at the Obesity Society’s Obesity Week 2024 meeting by the company’s Chief Nutrition Officer, Michelle I. Cardel, PhD, RD, based in Gainesville, Florida.
Similar reductions in alcohol consumption were seen in people taking different classes of AOMs, suggesting “an additional mechanism by which AOMs reduce energy intake, and also signal a potential role for these medications to reduce alcohol use,” Cardel said, adding “Clinicians treating individuals for obesity may consider anti-obesity medications particularly among those who report higher alcohol intake.”
Asked to comment, session moderator and obesity researcher Joseph A. Skelton, MD, professor of pediatrics at Wake Forest University School of Medicine, Winston-Salem, North Carolina, said, “I think there are some overlapping pathways there, possibly a reward system or something like that in the brain. I don’t think we know exactly what the end result will be as a potential use of the medications. But there’s a signal that needs to be investigated more.”
Cardel noted that there was one previous large cohort study finding that semaglutide was associated with a lower risk for alcohol use disorder, and another study that analyzed social media threads of people saying they’d quit drinking after starting a GLP-1 drug. But this new study is the first to examine the relationship with different classes of AOMs and to quantify the amount of alcohol consumed.
About Half Reported Reduced Alcohol Consumption, Regardless the AOM Class
The study included 14,053 WeightWatchers’ telehealth program participants who initiated an AOM between January 2022 and August 2023 and refilled the same AOM between October and November 2023. Those who had previously used AOMs before coming to the program or who had undergone bariatric surgery were excluded.
Participants had a mean age of 43 years, were 86% women, were 60% White, and had a mean body mass index of 36. They were surveyed about their weekly alcohol use prior to AOM initiation and again at the time of AOM refill.
At baseline, they were divided into categories of 0 (no alcohol use; n = 6562), category 1 (one to three drinks for women and one to six for men; n = 5948), category 2 (4-6 for women and 7-14 for men; n = 1216), and category 3 (≥ 7 for women and ≥ 15 for men; n = 327).
At the second survey, 24% reported decreased drinking after starting an AOM, 71% reported no change, and 4% reported increased drinking (P < .0001). But when just the 7491 individuals who reported any alcohol use at baseline were included, 45% reported decreased drinking after starting an AOM, 52% reported no change, and only 2% reported increased drinking.
The decrease in drinking with AOM use rose with greater alcohol use at baseline, from 37% for category 1, 76% for category 2, and 91% for category 3. The proportions reporting increased drinking were just 3%, 1%, and 0%, respectively. The adjusted odds ratios (ORs) for decreasing drinking were 5.97 for category 2 (P < .0001) and 19.18 for category 3 (P < .0001) vs category 1.
The proportions reporting reduced drinking were similar across AOM classes: 51% for metformin, 46% for bupropion/naltrexone, 46% for first-generation GLP-1s (Saxenda, Trulicity, and Victoza), and 45% for the second-generation GLP-1 drugs (Mounjaro, Ozempic, Rybelsus, Wegovy, and Zepbound). All were statistically significant at P < .0001.
The highest proportion reporting increased drinking was 4% for bupropion/naltrexone. Compared with women, men were significantly more likely to report decreased drinking with AOM use (adjusted OR, 0.74; P < .001), but there were no differences by race/ethnicity or age.
Compared with those who had overweight, those in obesity classes I, II, and III were all more likely to decrease drinking with AOM use, with adjusted ORs of 1.26 (P = .0045), 1.49 (P < .001), and 1.63 (P < .001), respectively.
Mechanisms Appear Both Biological and Behavioral
During the discussion, Cardel said that qualitative assessments with participants suggest that there are at least two mechanisms behind this phenomenon: One biological and the other intentional.
“What we hear from them is twofold, one, particularly amongst those folks on GLP-1 medications, we’re hearing that physiologically, they feel different with the medications, that their cravings for alcohol are decreased, and that when they do choose to drink that there’s often a very much a negative reinforcement ... I’ve had a patient tell me, ‘I used to be able to have two or three margaritas, and maybe I didn’t feel like the best I’d ever felt in the morning, but I was okay. And now if I have two or three drinks, I will be throwing up for 5 hours, and it’s the worst hangover I’ve ever had in my life.’ And so it very much creates that negative reinforcement loop.”
But at the same time, “folks who are coming to us and seeking these medications are very much on a on a health-based journey. That’s what they tell us. The majority of our patients are there to improve their health. We rarely hear about the vanity or aesthetic part of it. So perhaps it’s that, in terms of trying to improve their health, they’re also trying to reduce their alcohol consumption, either just for their overall health or also as a means of trying to decrease their overall calorie consumption.”
In future research, Cardel said, “we want to examine whether the anti-obesity medications are more successful at reducing alcohol use compared to non-pharmacological weight management interventions, as we know that people often reduce their alcohol consumption on a weight management journey as a means of prioritizing their calories for food and decreasing the calories from alcohol.”
Cardel and all the study coauthors were employees and shareholders at WeightWatchers at the time the research was conducted. Skelton is editor in chief of the journal Childhood Obesity.
A version of this article appeared on Medscape.com.
SAN ANTONIO —
The findings, from surveys of more than 14,000 participants in WeightWatchers’ telehealth weight management program, were presented on November 6 at the Obesity Society’s Obesity Week 2024 meeting by the company’s Chief Nutrition Officer, Michelle I. Cardel, PhD, RD, based in Gainesville, Florida.
Similar reductions in alcohol consumption were seen in people taking different classes of AOMs, suggesting “an additional mechanism by which AOMs reduce energy intake, and also signal a potential role for these medications to reduce alcohol use,” Cardel said, adding “Clinicians treating individuals for obesity may consider anti-obesity medications particularly among those who report higher alcohol intake.”
Asked to comment, session moderator and obesity researcher Joseph A. Skelton, MD, professor of pediatrics at Wake Forest University School of Medicine, Winston-Salem, North Carolina, said, “I think there are some overlapping pathways there, possibly a reward system or something like that in the brain. I don’t think we know exactly what the end result will be as a potential use of the medications. But there’s a signal that needs to be investigated more.”
Cardel noted that there was one previous large cohort study finding that semaglutide was associated with a lower risk for alcohol use disorder, and another study that analyzed social media threads of people saying they’d quit drinking after starting a GLP-1 drug. But this new study is the first to examine the relationship with different classes of AOMs and to quantify the amount of alcohol consumed.
About Half Reported Reduced Alcohol Consumption, Regardless the AOM Class
The study included 14,053 WeightWatchers’ telehealth program participants who initiated an AOM between January 2022 and August 2023 and refilled the same AOM between October and November 2023. Those who had previously used AOMs before coming to the program or who had undergone bariatric surgery were excluded.
Participants had a mean age of 43 years, were 86% women, were 60% White, and had a mean body mass index of 36. They were surveyed about their weekly alcohol use prior to AOM initiation and again at the time of AOM refill.
At baseline, they were divided into categories of 0 (no alcohol use; n = 6562), category 1 (one to three drinks for women and one to six for men; n = 5948), category 2 (4-6 for women and 7-14 for men; n = 1216), and category 3 (≥ 7 for women and ≥ 15 for men; n = 327).
At the second survey, 24% reported decreased drinking after starting an AOM, 71% reported no change, and 4% reported increased drinking (P < .0001). But when just the 7491 individuals who reported any alcohol use at baseline were included, 45% reported decreased drinking after starting an AOM, 52% reported no change, and only 2% reported increased drinking.
The decrease in drinking with AOM use rose with greater alcohol use at baseline, from 37% for category 1, 76% for category 2, and 91% for category 3. The proportions reporting increased drinking were just 3%, 1%, and 0%, respectively. The adjusted odds ratios (ORs) for decreasing drinking were 5.97 for category 2 (P < .0001) and 19.18 for category 3 (P < .0001) vs category 1.
The proportions reporting reduced drinking were similar across AOM classes: 51% for metformin, 46% for bupropion/naltrexone, 46% for first-generation GLP-1s (Saxenda, Trulicity, and Victoza), and 45% for the second-generation GLP-1 drugs (Mounjaro, Ozempic, Rybelsus, Wegovy, and Zepbound). All were statistically significant at P < .0001.
The highest proportion reporting increased drinking was 4% for bupropion/naltrexone. Compared with women, men were significantly more likely to report decreased drinking with AOM use (adjusted OR, 0.74; P < .001), but there were no differences by race/ethnicity or age.
Compared with those who had overweight, those in obesity classes I, II, and III were all more likely to decrease drinking with AOM use, with adjusted ORs of 1.26 (P = .0045), 1.49 (P < .001), and 1.63 (P < .001), respectively.
Mechanisms Appear Both Biological and Behavioral
During the discussion, Cardel said that qualitative assessments with participants suggest that there are at least two mechanisms behind this phenomenon: One biological and the other intentional.
“What we hear from them is twofold, one, particularly amongst those folks on GLP-1 medications, we’re hearing that physiologically, they feel different with the medications, that their cravings for alcohol are decreased, and that when they do choose to drink that there’s often a very much a negative reinforcement ... I’ve had a patient tell me, ‘I used to be able to have two or three margaritas, and maybe I didn’t feel like the best I’d ever felt in the morning, but I was okay. And now if I have two or three drinks, I will be throwing up for 5 hours, and it’s the worst hangover I’ve ever had in my life.’ And so it very much creates that negative reinforcement loop.”
But at the same time, “folks who are coming to us and seeking these medications are very much on a on a health-based journey. That’s what they tell us. The majority of our patients are there to improve their health. We rarely hear about the vanity or aesthetic part of it. So perhaps it’s that, in terms of trying to improve their health, they’re also trying to reduce their alcohol consumption, either just for their overall health or also as a means of trying to decrease their overall calorie consumption.”
In future research, Cardel said, “we want to examine whether the anti-obesity medications are more successful at reducing alcohol use compared to non-pharmacological weight management interventions, as we know that people often reduce their alcohol consumption on a weight management journey as a means of prioritizing their calories for food and decreasing the calories from alcohol.”
Cardel and all the study coauthors were employees and shareholders at WeightWatchers at the time the research was conducted. Skelton is editor in chief of the journal Childhood Obesity.
A version of this article appeared on Medscape.com.
SAN ANTONIO —
The findings, from surveys of more than 14,000 participants in WeightWatchers’ telehealth weight management program, were presented on November 6 at the Obesity Society’s Obesity Week 2024 meeting by the company’s Chief Nutrition Officer, Michelle I. Cardel, PhD, RD, based in Gainesville, Florida.
Similar reductions in alcohol consumption were seen in people taking different classes of AOMs, suggesting “an additional mechanism by which AOMs reduce energy intake, and also signal a potential role for these medications to reduce alcohol use,” Cardel said, adding “Clinicians treating individuals for obesity may consider anti-obesity medications particularly among those who report higher alcohol intake.”
Asked to comment, session moderator and obesity researcher Joseph A. Skelton, MD, professor of pediatrics at Wake Forest University School of Medicine, Winston-Salem, North Carolina, said, “I think there are some overlapping pathways there, possibly a reward system or something like that in the brain. I don’t think we know exactly what the end result will be as a potential use of the medications. But there’s a signal that needs to be investigated more.”
Cardel noted that there was one previous large cohort study finding that semaglutide was associated with a lower risk for alcohol use disorder, and another study that analyzed social media threads of people saying they’d quit drinking after starting a GLP-1 drug. But this new study is the first to examine the relationship with different classes of AOMs and to quantify the amount of alcohol consumed.
About Half Reported Reduced Alcohol Consumption, Regardless the AOM Class
The study included 14,053 WeightWatchers’ telehealth program participants who initiated an AOM between January 2022 and August 2023 and refilled the same AOM between October and November 2023. Those who had previously used AOMs before coming to the program or who had undergone bariatric surgery were excluded.
Participants had a mean age of 43 years, were 86% women, were 60% White, and had a mean body mass index of 36. They were surveyed about their weekly alcohol use prior to AOM initiation and again at the time of AOM refill.
At baseline, they were divided into categories of 0 (no alcohol use; n = 6562), category 1 (one to three drinks for women and one to six for men; n = 5948), category 2 (4-6 for women and 7-14 for men; n = 1216), and category 3 (≥ 7 for women and ≥ 15 for men; n = 327).
At the second survey, 24% reported decreased drinking after starting an AOM, 71% reported no change, and 4% reported increased drinking (P < .0001). But when just the 7491 individuals who reported any alcohol use at baseline were included, 45% reported decreased drinking after starting an AOM, 52% reported no change, and only 2% reported increased drinking.
The decrease in drinking with AOM use rose with greater alcohol use at baseline, from 37% for category 1, 76% for category 2, and 91% for category 3. The proportions reporting increased drinking were just 3%, 1%, and 0%, respectively. The adjusted odds ratios (ORs) for decreasing drinking were 5.97 for category 2 (P < .0001) and 19.18 for category 3 (P < .0001) vs category 1.
The proportions reporting reduced drinking were similar across AOM classes: 51% for metformin, 46% for bupropion/naltrexone, 46% for first-generation GLP-1s (Saxenda, Trulicity, and Victoza), and 45% for the second-generation GLP-1 drugs (Mounjaro, Ozempic, Rybelsus, Wegovy, and Zepbound). All were statistically significant at P < .0001.
The highest proportion reporting increased drinking was 4% for bupropion/naltrexone. Compared with women, men were significantly more likely to report decreased drinking with AOM use (adjusted OR, 0.74; P < .001), but there were no differences by race/ethnicity or age.
Compared with those who had overweight, those in obesity classes I, II, and III were all more likely to decrease drinking with AOM use, with adjusted ORs of 1.26 (P = .0045), 1.49 (P < .001), and 1.63 (P < .001), respectively.
Mechanisms Appear Both Biological and Behavioral
During the discussion, Cardel said that qualitative assessments with participants suggest that there are at least two mechanisms behind this phenomenon: One biological and the other intentional.
“What we hear from them is twofold, one, particularly amongst those folks on GLP-1 medications, we’re hearing that physiologically, they feel different with the medications, that their cravings for alcohol are decreased, and that when they do choose to drink that there’s often a very much a negative reinforcement ... I’ve had a patient tell me, ‘I used to be able to have two or three margaritas, and maybe I didn’t feel like the best I’d ever felt in the morning, but I was okay. And now if I have two or three drinks, I will be throwing up for 5 hours, and it’s the worst hangover I’ve ever had in my life.’ And so it very much creates that negative reinforcement loop.”
But at the same time, “folks who are coming to us and seeking these medications are very much on a on a health-based journey. That’s what they tell us. The majority of our patients are there to improve their health. We rarely hear about the vanity or aesthetic part of it. So perhaps it’s that, in terms of trying to improve their health, they’re also trying to reduce their alcohol consumption, either just for their overall health or also as a means of trying to decrease their overall calorie consumption.”
In future research, Cardel said, “we want to examine whether the anti-obesity medications are more successful at reducing alcohol use compared to non-pharmacological weight management interventions, as we know that people often reduce their alcohol consumption on a weight management journey as a means of prioritizing their calories for food and decreasing the calories from alcohol.”
Cardel and all the study coauthors were employees and shareholders at WeightWatchers at the time the research was conducted. Skelton is editor in chief of the journal Childhood Obesity.
A version of this article appeared on Medscape.com.
FROM OBESITY WEEK 2024
Can Weight Loss Drugs Also Treat Addiction?
A new study provides more evidence that glucagon-like peptide 1 receptor agonists (GLP-1 RAs) used to treat diabetes and obesity could be repurposed for opioid use disorder (OUD) and alcohol use disorder (AUD).
Researchers found that patients with OUD or AUD who were taking semaglutide (Ozempic, Novo Nordisk) or similar medications for diabetes or weight-related conditions had a 40% lower rate of opioid overdose and a 50% lower rate of alcohol intoxication than their peers with OUD or AUD who were not taking these medications.
Their real-world study of more than 1 million adults with a history of OUD or AUD provide “foundational” estimates of the association between glucose-dependent insulinotropic polypeptide (GIP)/GLP-1 RA prescriptions and opioid overdose/alcohol intoxication “and introduce the idea that GLP-1 RA and other related drugs should be investigated as a novel pharmacotherapy treatment option for individuals with OUD or AUD,” wrote the investigators, led by Fares Qeadan, PhD, Parkinson School of Health Sciences and Public Health, Loyola University Chicago, Maywood, Illinois.
The study was published online in the journal Addiction.
Protective Effect?
As previously reported by Medscape Medical News, earlier studies have pointed to a link between weight loss drugs and reduced overdose risk in people with OUD and decreased alcohol intake in people with AUD.
Until now, most studies on GLP-1 RAs and GIP agonists like tirzepatide (Mounjaro) to treat substance use disorders consisted of animal studies and small-scale clinical trials, investigators noted.
This new retrospective cohort study analyzed de-identified electronic health record data from the Oracle Health Real-World Data.
Participants, all aged 18 years or older, included 503,747 patients with a history of OUD, of whom 8103 had a GLP-1 RA or GIP prescription, and 817,309 patients with a history of AUD, of whom 5621 had a GLP-1 RA or GIP prescription.
Patients with OUD who were prescribed GLP-1 RAs had a 40% lower rate of opioid overdose than those without such prescriptions (adjusted incidence rate ratio [aIRR], 0.60; 95% CI, 0.43-0.83), the study team found.
In addition, patients with AUD and a GLP-1 RA prescription exhibited a 50% lower rate of alcohol intoxication (aIRR, 0.50; 95% CI, 0.40-0.63).
The protective effect of GLP-1 RA on opioid overdose and alcohol intoxication was maintained across patients with comorbid conditions, such as type 2 diabetes and obesity.
“Future research should focus on prospective clinical trials to validate these findings, explore the underlying mechanisms, and determine the long-term efficacy and safety of GIP/GLP-1 RA medications in diverse populations,” Qeadan and colleagues concluded.
“Additionally, the study highlights the importance of interdisciplinary research in understanding the neurobiological links between metabolic disorders and problematic substance use, potentially leading to more effective treatment strategies within healthcare systems,” they added.
Questions Remain
In a statement from the UK nonprofit Science Media Centre, Matt Field, DPhil, professor of psychology, The University of Sheffield, in England, noted that the findings “add to those from other studies, particularly animal research, which suggest that this and similar drugs might one day be prescribed to help people with addiction.”
However, “a note of caution is that the outcomes are very extreme instances of substance intoxication,” added Field, who wasn’t involved in the study.
“Those outcomes are very different from the outcomes used when researchers test new treatments for addiction, in which case we might look at whether the treatment helps people to stop taking the substance altogether (complete abstinence), or if it helps people to reduce the amount of substance they consume, or how often they consume it. Those things could not be measured in this study,” he continued.
“This leaves open the possibility that while Ozempic may — for reasons currently unknown — prevent people from taking so much alcohol or heroin that they overdose and end up in hospital, it may not actually help them to reduce their substance use, or to abstain altogether,” Field said.
The study had no specific funding. The study authors and Field declared no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
A new study provides more evidence that glucagon-like peptide 1 receptor agonists (GLP-1 RAs) used to treat diabetes and obesity could be repurposed for opioid use disorder (OUD) and alcohol use disorder (AUD).
Researchers found that patients with OUD or AUD who were taking semaglutide (Ozempic, Novo Nordisk) or similar medications for diabetes or weight-related conditions had a 40% lower rate of opioid overdose and a 50% lower rate of alcohol intoxication than their peers with OUD or AUD who were not taking these medications.
Their real-world study of more than 1 million adults with a history of OUD or AUD provide “foundational” estimates of the association between glucose-dependent insulinotropic polypeptide (GIP)/GLP-1 RA prescriptions and opioid overdose/alcohol intoxication “and introduce the idea that GLP-1 RA and other related drugs should be investigated as a novel pharmacotherapy treatment option for individuals with OUD or AUD,” wrote the investigators, led by Fares Qeadan, PhD, Parkinson School of Health Sciences and Public Health, Loyola University Chicago, Maywood, Illinois.
The study was published online in the journal Addiction.
Protective Effect?
As previously reported by Medscape Medical News, earlier studies have pointed to a link between weight loss drugs and reduced overdose risk in people with OUD and decreased alcohol intake in people with AUD.
Until now, most studies on GLP-1 RAs and GIP agonists like tirzepatide (Mounjaro) to treat substance use disorders consisted of animal studies and small-scale clinical trials, investigators noted.
This new retrospective cohort study analyzed de-identified electronic health record data from the Oracle Health Real-World Data.
Participants, all aged 18 years or older, included 503,747 patients with a history of OUD, of whom 8103 had a GLP-1 RA or GIP prescription, and 817,309 patients with a history of AUD, of whom 5621 had a GLP-1 RA or GIP prescription.
Patients with OUD who were prescribed GLP-1 RAs had a 40% lower rate of opioid overdose than those without such prescriptions (adjusted incidence rate ratio [aIRR], 0.60; 95% CI, 0.43-0.83), the study team found.
In addition, patients with AUD and a GLP-1 RA prescription exhibited a 50% lower rate of alcohol intoxication (aIRR, 0.50; 95% CI, 0.40-0.63).
The protective effect of GLP-1 RA on opioid overdose and alcohol intoxication was maintained across patients with comorbid conditions, such as type 2 diabetes and obesity.
“Future research should focus on prospective clinical trials to validate these findings, explore the underlying mechanisms, and determine the long-term efficacy and safety of GIP/GLP-1 RA medications in diverse populations,” Qeadan and colleagues concluded.
“Additionally, the study highlights the importance of interdisciplinary research in understanding the neurobiological links between metabolic disorders and problematic substance use, potentially leading to more effective treatment strategies within healthcare systems,” they added.
Questions Remain
In a statement from the UK nonprofit Science Media Centre, Matt Field, DPhil, professor of psychology, The University of Sheffield, in England, noted that the findings “add to those from other studies, particularly animal research, which suggest that this and similar drugs might one day be prescribed to help people with addiction.”
However, “a note of caution is that the outcomes are very extreme instances of substance intoxication,” added Field, who wasn’t involved in the study.
“Those outcomes are very different from the outcomes used when researchers test new treatments for addiction, in which case we might look at whether the treatment helps people to stop taking the substance altogether (complete abstinence), or if it helps people to reduce the amount of substance they consume, or how often they consume it. Those things could not be measured in this study,” he continued.
“This leaves open the possibility that while Ozempic may — for reasons currently unknown — prevent people from taking so much alcohol or heroin that they overdose and end up in hospital, it may not actually help them to reduce their substance use, or to abstain altogether,” Field said.
The study had no specific funding. The study authors and Field declared no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
A new study provides more evidence that glucagon-like peptide 1 receptor agonists (GLP-1 RAs) used to treat diabetes and obesity could be repurposed for opioid use disorder (OUD) and alcohol use disorder (AUD).
Researchers found that patients with OUD or AUD who were taking semaglutide (Ozempic, Novo Nordisk) or similar medications for diabetes or weight-related conditions had a 40% lower rate of opioid overdose and a 50% lower rate of alcohol intoxication than their peers with OUD or AUD who were not taking these medications.
Their real-world study of more than 1 million adults with a history of OUD or AUD provide “foundational” estimates of the association between glucose-dependent insulinotropic polypeptide (GIP)/GLP-1 RA prescriptions and opioid overdose/alcohol intoxication “and introduce the idea that GLP-1 RA and other related drugs should be investigated as a novel pharmacotherapy treatment option for individuals with OUD or AUD,” wrote the investigators, led by Fares Qeadan, PhD, Parkinson School of Health Sciences and Public Health, Loyola University Chicago, Maywood, Illinois.
The study was published online in the journal Addiction.
Protective Effect?
As previously reported by Medscape Medical News, earlier studies have pointed to a link between weight loss drugs and reduced overdose risk in people with OUD and decreased alcohol intake in people with AUD.
Until now, most studies on GLP-1 RAs and GIP agonists like tirzepatide (Mounjaro) to treat substance use disorders consisted of animal studies and small-scale clinical trials, investigators noted.
This new retrospective cohort study analyzed de-identified electronic health record data from the Oracle Health Real-World Data.
Participants, all aged 18 years or older, included 503,747 patients with a history of OUD, of whom 8103 had a GLP-1 RA or GIP prescription, and 817,309 patients with a history of AUD, of whom 5621 had a GLP-1 RA or GIP prescription.
Patients with OUD who were prescribed GLP-1 RAs had a 40% lower rate of opioid overdose than those without such prescriptions (adjusted incidence rate ratio [aIRR], 0.60; 95% CI, 0.43-0.83), the study team found.
In addition, patients with AUD and a GLP-1 RA prescription exhibited a 50% lower rate of alcohol intoxication (aIRR, 0.50; 95% CI, 0.40-0.63).
The protective effect of GLP-1 RA on opioid overdose and alcohol intoxication was maintained across patients with comorbid conditions, such as type 2 diabetes and obesity.
“Future research should focus on prospective clinical trials to validate these findings, explore the underlying mechanisms, and determine the long-term efficacy and safety of GIP/GLP-1 RA medications in diverse populations,” Qeadan and colleagues concluded.
“Additionally, the study highlights the importance of interdisciplinary research in understanding the neurobiological links between metabolic disorders and problematic substance use, potentially leading to more effective treatment strategies within healthcare systems,” they added.
Questions Remain
In a statement from the UK nonprofit Science Media Centre, Matt Field, DPhil, professor of psychology, The University of Sheffield, in England, noted that the findings “add to those from other studies, particularly animal research, which suggest that this and similar drugs might one day be prescribed to help people with addiction.”
However, “a note of caution is that the outcomes are very extreme instances of substance intoxication,” added Field, who wasn’t involved in the study.
“Those outcomes are very different from the outcomes used when researchers test new treatments for addiction, in which case we might look at whether the treatment helps people to stop taking the substance altogether (complete abstinence), or if it helps people to reduce the amount of substance they consume, or how often they consume it. Those things could not be measured in this study,” he continued.
“This leaves open the possibility that while Ozempic may — for reasons currently unknown — prevent people from taking so much alcohol or heroin that they overdose and end up in hospital, it may not actually help them to reduce their substance use, or to abstain altogether,” Field said.
The study had no specific funding. The study authors and Field declared no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
FROM ADDICTION
