User login
Pending further study, caution recommended in treating vitiligo patients with lasers, IPL
SAN DIEGO – The .
Those are the preliminary conclusions from a systematic review and survey of experts that Albert Wolkerstorfer, MD, presented during a clinical abstract session at the annual conference of the American Society for Laser Medicine and Surgery.
According to Dr. Wolkerstorfer, a dermatologist at Amsterdam University Medical Center, clinicians are reluctant to perform laser/intense pulsed light (IPL) treatments in patients with vitiligo because of the absence of clear guidelines, so he and his colleagues set out to investigate the risks of laser/IPL-induced vitiligo in patients with vitiligo and to seek out international consensus on recommendations from experts. “There is hardly any literature about it and certainly no guidelines,” he pointed out.
Dr. Wolkerstorfer and his colleagues designed three consecutive studies: A systematic review of laser/IPL-induced vitiligo; an international survey among 14 vitiligo experts from 10 countries about the occurrence of laser‐induced vitiligo, and a Delphi technique aimed at establishing a broad consensus about recommendations for safe use of lasers in vitiligo patients. At the time of the meeting, the Delphi process was still being carried out, so he did not discuss that study.
For the systematic review, the researchers found 11,073 unique hits on PubMed, Embase, and CINAHL using the terms “vitiligo,” “depigmentation,” “hypopigmentation,” and “leukoderma.” Only six case reports of laser/IPL-induced vitiligo were included in the final analysis. Of these, three had de novo vitiligo and three had vitiligo/halo nevi. These cases included two that occurred following treatment of port wine stains with the 585-nm laser; one that occurred following treatment of dyspigmentation with IPL; one that occurred following treatment of hypertrichosis with the 1,064-nm laser, one that occurred following treatment of hypertrichosis with the 755-nm laser, and one case that occurred following treatment of melasma with the ablative laser.
For the international survey of 14 experts from 10 countries, respondents said they had 10,670 new face-to-face vitiligo consultations in the past year. They reported that 30 of the vitiligo cases (0.3%) were likely caused by laser/IPL. Of these 30 cases, 18 (60%) had de novo vitiligo.
Of these cases, vitiligo occurred most frequently after laser hair reduction (47%), followed by use of the fractional laser (17%), and the ablative laser (13%). The interval between laser/IPL treatment and onset of vitiligo was 0-4 weeks in 27% of cases and 4-12 weeks in 57% of cases. Direct complications such as blistering, crusting, and erosions occurred in 57% of cases.
“Our conclusion is that laser and IPL-induced vitiligo is a rare phenomenon, and it often affects patients without prior vitiligo, which was really a surprise to us,” Dr. Wolkerstorfer said. “Complications seem to increase the risk,” he added.
“Despite the apparently low risk, we recommend caution [in patients with vitiligo], especially with aggressive laser procedures,” he said. “We recommend using conservative settings, not to treat active vitiligo patients ... and to perform test spots prior to treating large areas.” But he characterized this recommendation as “totally preliminary” pending results of the Delphi technique aimed at building consensus about laser/IPL treatments in vitiligo.
In an interview at the meeting, one of the session moderators, Oge Onwudiwe, MD, a dermatologist who practices in Alexandria, Va., said that as clinicians await results of the study’s Delphi consensus, current use of lasers and IPL in patients with vitiligo “is based on your clinical judgment and whether the vitiligo is active or inactive. If the patient has vitiligo and you’re doing laser hair removal in the armpit, they may get active lesions in that area, but they can cover it. So, they may take that as a ‘win’ with the risk. But if it can erupt in other areas, that’s a risk they must be willing to take.”
Dr. Wolkerstorfer disclosed that he has received grant or research funding from Lumenis, Novartis, and Avita Medical. He is an advisory board member for Incyte. Dr. Onwudiwe reported having no disclosures.
SAN DIEGO – The .
Those are the preliminary conclusions from a systematic review and survey of experts that Albert Wolkerstorfer, MD, presented during a clinical abstract session at the annual conference of the American Society for Laser Medicine and Surgery.
According to Dr. Wolkerstorfer, a dermatologist at Amsterdam University Medical Center, clinicians are reluctant to perform laser/intense pulsed light (IPL) treatments in patients with vitiligo because of the absence of clear guidelines, so he and his colleagues set out to investigate the risks of laser/IPL-induced vitiligo in patients with vitiligo and to seek out international consensus on recommendations from experts. “There is hardly any literature about it and certainly no guidelines,” he pointed out.
Dr. Wolkerstorfer and his colleagues designed three consecutive studies: A systematic review of laser/IPL-induced vitiligo; an international survey among 14 vitiligo experts from 10 countries about the occurrence of laser‐induced vitiligo, and a Delphi technique aimed at establishing a broad consensus about recommendations for safe use of lasers in vitiligo patients. At the time of the meeting, the Delphi process was still being carried out, so he did not discuss that study.
For the systematic review, the researchers found 11,073 unique hits on PubMed, Embase, and CINAHL using the terms “vitiligo,” “depigmentation,” “hypopigmentation,” and “leukoderma.” Only six case reports of laser/IPL-induced vitiligo were included in the final analysis. Of these, three had de novo vitiligo and three had vitiligo/halo nevi. These cases included two that occurred following treatment of port wine stains with the 585-nm laser; one that occurred following treatment of dyspigmentation with IPL; one that occurred following treatment of hypertrichosis with the 1,064-nm laser, one that occurred following treatment of hypertrichosis with the 755-nm laser, and one case that occurred following treatment of melasma with the ablative laser.
For the international survey of 14 experts from 10 countries, respondents said they had 10,670 new face-to-face vitiligo consultations in the past year. They reported that 30 of the vitiligo cases (0.3%) were likely caused by laser/IPL. Of these 30 cases, 18 (60%) had de novo vitiligo.
Of these cases, vitiligo occurred most frequently after laser hair reduction (47%), followed by use of the fractional laser (17%), and the ablative laser (13%). The interval between laser/IPL treatment and onset of vitiligo was 0-4 weeks in 27% of cases and 4-12 weeks in 57% of cases. Direct complications such as blistering, crusting, and erosions occurred in 57% of cases.
“Our conclusion is that laser and IPL-induced vitiligo is a rare phenomenon, and it often affects patients without prior vitiligo, which was really a surprise to us,” Dr. Wolkerstorfer said. “Complications seem to increase the risk,” he added.
“Despite the apparently low risk, we recommend caution [in patients with vitiligo], especially with aggressive laser procedures,” he said. “We recommend using conservative settings, not to treat active vitiligo patients ... and to perform test spots prior to treating large areas.” But he characterized this recommendation as “totally preliminary” pending results of the Delphi technique aimed at building consensus about laser/IPL treatments in vitiligo.
In an interview at the meeting, one of the session moderators, Oge Onwudiwe, MD, a dermatologist who practices in Alexandria, Va., said that as clinicians await results of the study’s Delphi consensus, current use of lasers and IPL in patients with vitiligo “is based on your clinical judgment and whether the vitiligo is active or inactive. If the patient has vitiligo and you’re doing laser hair removal in the armpit, they may get active lesions in that area, but they can cover it. So, they may take that as a ‘win’ with the risk. But if it can erupt in other areas, that’s a risk they must be willing to take.”
Dr. Wolkerstorfer disclosed that he has received grant or research funding from Lumenis, Novartis, and Avita Medical. He is an advisory board member for Incyte. Dr. Onwudiwe reported having no disclosures.
SAN DIEGO – The .
Those are the preliminary conclusions from a systematic review and survey of experts that Albert Wolkerstorfer, MD, presented during a clinical abstract session at the annual conference of the American Society for Laser Medicine and Surgery.
According to Dr. Wolkerstorfer, a dermatologist at Amsterdam University Medical Center, clinicians are reluctant to perform laser/intense pulsed light (IPL) treatments in patients with vitiligo because of the absence of clear guidelines, so he and his colleagues set out to investigate the risks of laser/IPL-induced vitiligo in patients with vitiligo and to seek out international consensus on recommendations from experts. “There is hardly any literature about it and certainly no guidelines,” he pointed out.
Dr. Wolkerstorfer and his colleagues designed three consecutive studies: A systematic review of laser/IPL-induced vitiligo; an international survey among 14 vitiligo experts from 10 countries about the occurrence of laser‐induced vitiligo, and a Delphi technique aimed at establishing a broad consensus about recommendations for safe use of lasers in vitiligo patients. At the time of the meeting, the Delphi process was still being carried out, so he did not discuss that study.
For the systematic review, the researchers found 11,073 unique hits on PubMed, Embase, and CINAHL using the terms “vitiligo,” “depigmentation,” “hypopigmentation,” and “leukoderma.” Only six case reports of laser/IPL-induced vitiligo were included in the final analysis. Of these, three had de novo vitiligo and three had vitiligo/halo nevi. These cases included two that occurred following treatment of port wine stains with the 585-nm laser; one that occurred following treatment of dyspigmentation with IPL; one that occurred following treatment of hypertrichosis with the 1,064-nm laser, one that occurred following treatment of hypertrichosis with the 755-nm laser, and one case that occurred following treatment of melasma with the ablative laser.
For the international survey of 14 experts from 10 countries, respondents said they had 10,670 new face-to-face vitiligo consultations in the past year. They reported that 30 of the vitiligo cases (0.3%) were likely caused by laser/IPL. Of these 30 cases, 18 (60%) had de novo vitiligo.
Of these cases, vitiligo occurred most frequently after laser hair reduction (47%), followed by use of the fractional laser (17%), and the ablative laser (13%). The interval between laser/IPL treatment and onset of vitiligo was 0-4 weeks in 27% of cases and 4-12 weeks in 57% of cases. Direct complications such as blistering, crusting, and erosions occurred in 57% of cases.
“Our conclusion is that laser and IPL-induced vitiligo is a rare phenomenon, and it often affects patients without prior vitiligo, which was really a surprise to us,” Dr. Wolkerstorfer said. “Complications seem to increase the risk,” he added.
“Despite the apparently low risk, we recommend caution [in patients with vitiligo], especially with aggressive laser procedures,” he said. “We recommend using conservative settings, not to treat active vitiligo patients ... and to perform test spots prior to treating large areas.” But he characterized this recommendation as “totally preliminary” pending results of the Delphi technique aimed at building consensus about laser/IPL treatments in vitiligo.
In an interview at the meeting, one of the session moderators, Oge Onwudiwe, MD, a dermatologist who practices in Alexandria, Va., said that as clinicians await results of the study’s Delphi consensus, current use of lasers and IPL in patients with vitiligo “is based on your clinical judgment and whether the vitiligo is active or inactive. If the patient has vitiligo and you’re doing laser hair removal in the armpit, they may get active lesions in that area, but they can cover it. So, they may take that as a ‘win’ with the risk. But if it can erupt in other areas, that’s a risk they must be willing to take.”
Dr. Wolkerstorfer disclosed that he has received grant or research funding from Lumenis, Novartis, and Avita Medical. He is an advisory board member for Incyte. Dr. Onwudiwe reported having no disclosures.
AT ASLMS 2022
What’s ahead for laser-assisted drug delivery?
SAN DIEGO – Twelve years ago, Merete Haedersdal, MD, PhD, and colleagues published data from a swine study, which showed for the first time that the ablative fractional laser can be used to boost the uptake of drugs into the skin.
That discovery paved the way for what are now well-established clinical applications of laser-assisted drug delivery for treating actinic keratoses and scars. According to Dr. Haedersdal, professor of dermatology at the University of Copenhagen, evolving clinical indications for laser-assisted drug delivery include rejuvenation, local anesthesia, melasma, onychomycosis, hyperhidrosis, alopecia, and vitiligo, while emerging indications include treatment of skin cancer with PD-1 inhibitors and combination chemotherapy regimens, and vaccinations.
During a presentation at the annual conference of the American Society for Laser Medicine and Surgery, she said that researchers have much to learn about laser-assisted drug delivery, including biodistribution of the drug being delivered. Pointing out that so far, “what we have been dealing with is primarily looking at the skin as a black box,” she asked, “what happens when we drill the holes and drugs are applied on top of the skin and swim through the tiny channels?”
By using high-performance liquid chromatography (HPLC) and HPLC mass spectrometry to measure drug concentration in the skin, she and her colleagues have observed enhanced uptake of drugs – 4-fold to 40-fold greater – primarily in ex vivo pig skin. “We do know from ex vivo models that it’s much easier to boost the uptake in the skin” when compared with in vivo human use, where much lower drug concentrations are detected, said Dr. Haedersdal, who, along with Emily Wenande, MD, PhD, and R. Rox Anderson, MD, at the Wellman Center for Photomedicine, at Massachusetts General Hospital, Boston, authored a clinical review, published in 2020, on the basics of laser-assisted drug delivery.
“What we are working on now is visualizing what’s taking place when we apply the holes and the drugs in the skin. This is the key to tailoring laser-assisted uptake to specific dermatologic diseases being treated,” she said. To date, she and her colleagues have examined the interaction with tissue using different devices, including ex vivo confocal microscopy, to view the thermal response to ablative fractional laser and radiofrequency. “We want to take that to the next level and look at the drug biodistribution.”
Efforts are underway to compare the pattern of drug distribution with different modes of delivery, such as comparing ablative fractional laser to intradermal needle injection. “We are also working on pneumatic jet injection, which creates a focal drug distribution,” said Dr. Haedersdal, who is a visiting scientist at the Wellman Center. “In the future, we may take advantage of device-tailored biodistribution, depending on which clinical indication we are treating.”
Another important aspect to consider is drug retention in the skin. In a study presented as an abstract at the meeting, led by Dr. Wenande, she, Dr. Haedersdal, and colleagues used a pig model to evaluate the effect of three vasoregulative interventions on ablative fractional laser-assisted 5-fluororacil concentrations in in vivo skin. The three interventions were brimonidine 0.33% solution, epinephrine 10 mcg/mL gel, and a 595-nm pulsed dye laser (PDL) in designated treatment areas.
“What we learned from that was in the short term – 1-4 hours – the ablative fractional laser enhanced the uptake of 5-FU, but it was very transient,” with a twofold increased concentration of 5-FU, Dr. Haedersdal said. Over 48-72 hours, after PDL, there was “sustained enhancement of drug in the skin by three to four times,” she noted.
The synergy of systemic drugs with ablative fractional laser therapy is also being evaluated. In a mouse study led by Dr. Haedersdal’s colleague, senior researcher Uffe H. Olesen, PhD, the treatment of advanced squamous cell carcinoma tumors with a combination of ablative fractional laser and systemic treatment with PD-1 inhibitors resulted in the clearance of more tumors than with either treatment as monotherapy. “What we want to explore is the laser-induced tumor immune response in keratinocyte cancers,” she added.
“When you shine the laser on the skin, there is a robust increase of neutrophilic granulocytes.” Combining this topical immune-boosting response with systemic delivery of PD-1 inhibitors in a mouse model with basal cell carcinoma, she said, “we learned that, when we compare systemic PD-1 inhibitors alone to the laser alone and then with combination therapy, there was an increased tumor clearance of basal cell carcinomas and also enhanced survival of the mice” with the combination, she said. There were also “enhanced neutrophilic counts and both CD4- and CD8-positive cells were increased,” she added.
Dr. Haedersdal disclosed that she has received grants or research funding from Lutronic, Venus Concept, Leo Pharma, and Mirai Medical.
SAN DIEGO – Twelve years ago, Merete Haedersdal, MD, PhD, and colleagues published data from a swine study, which showed for the first time that the ablative fractional laser can be used to boost the uptake of drugs into the skin.
That discovery paved the way for what are now well-established clinical applications of laser-assisted drug delivery for treating actinic keratoses and scars. According to Dr. Haedersdal, professor of dermatology at the University of Copenhagen, evolving clinical indications for laser-assisted drug delivery include rejuvenation, local anesthesia, melasma, onychomycosis, hyperhidrosis, alopecia, and vitiligo, while emerging indications include treatment of skin cancer with PD-1 inhibitors and combination chemotherapy regimens, and vaccinations.
During a presentation at the annual conference of the American Society for Laser Medicine and Surgery, she said that researchers have much to learn about laser-assisted drug delivery, including biodistribution of the drug being delivered. Pointing out that so far, “what we have been dealing with is primarily looking at the skin as a black box,” she asked, “what happens when we drill the holes and drugs are applied on top of the skin and swim through the tiny channels?”
By using high-performance liquid chromatography (HPLC) and HPLC mass spectrometry to measure drug concentration in the skin, she and her colleagues have observed enhanced uptake of drugs – 4-fold to 40-fold greater – primarily in ex vivo pig skin. “We do know from ex vivo models that it’s much easier to boost the uptake in the skin” when compared with in vivo human use, where much lower drug concentrations are detected, said Dr. Haedersdal, who, along with Emily Wenande, MD, PhD, and R. Rox Anderson, MD, at the Wellman Center for Photomedicine, at Massachusetts General Hospital, Boston, authored a clinical review, published in 2020, on the basics of laser-assisted drug delivery.
“What we are working on now is visualizing what’s taking place when we apply the holes and the drugs in the skin. This is the key to tailoring laser-assisted uptake to specific dermatologic diseases being treated,” she said. To date, she and her colleagues have examined the interaction with tissue using different devices, including ex vivo confocal microscopy, to view the thermal response to ablative fractional laser and radiofrequency. “We want to take that to the next level and look at the drug biodistribution.”
Efforts are underway to compare the pattern of drug distribution with different modes of delivery, such as comparing ablative fractional laser to intradermal needle injection. “We are also working on pneumatic jet injection, which creates a focal drug distribution,” said Dr. Haedersdal, who is a visiting scientist at the Wellman Center. “In the future, we may take advantage of device-tailored biodistribution, depending on which clinical indication we are treating.”
Another important aspect to consider is drug retention in the skin. In a study presented as an abstract at the meeting, led by Dr. Wenande, she, Dr. Haedersdal, and colleagues used a pig model to evaluate the effect of three vasoregulative interventions on ablative fractional laser-assisted 5-fluororacil concentrations in in vivo skin. The three interventions were brimonidine 0.33% solution, epinephrine 10 mcg/mL gel, and a 595-nm pulsed dye laser (PDL) in designated treatment areas.
“What we learned from that was in the short term – 1-4 hours – the ablative fractional laser enhanced the uptake of 5-FU, but it was very transient,” with a twofold increased concentration of 5-FU, Dr. Haedersdal said. Over 48-72 hours, after PDL, there was “sustained enhancement of drug in the skin by three to four times,” she noted.
The synergy of systemic drugs with ablative fractional laser therapy is also being evaluated. In a mouse study led by Dr. Haedersdal’s colleague, senior researcher Uffe H. Olesen, PhD, the treatment of advanced squamous cell carcinoma tumors with a combination of ablative fractional laser and systemic treatment with PD-1 inhibitors resulted in the clearance of more tumors than with either treatment as monotherapy. “What we want to explore is the laser-induced tumor immune response in keratinocyte cancers,” she added.
“When you shine the laser on the skin, there is a robust increase of neutrophilic granulocytes.” Combining this topical immune-boosting response with systemic delivery of PD-1 inhibitors in a mouse model with basal cell carcinoma, she said, “we learned that, when we compare systemic PD-1 inhibitors alone to the laser alone and then with combination therapy, there was an increased tumor clearance of basal cell carcinomas and also enhanced survival of the mice” with the combination, she said. There were also “enhanced neutrophilic counts and both CD4- and CD8-positive cells were increased,” she added.
Dr. Haedersdal disclosed that she has received grants or research funding from Lutronic, Venus Concept, Leo Pharma, and Mirai Medical.
SAN DIEGO – Twelve years ago, Merete Haedersdal, MD, PhD, and colleagues published data from a swine study, which showed for the first time that the ablative fractional laser can be used to boost the uptake of drugs into the skin.
That discovery paved the way for what are now well-established clinical applications of laser-assisted drug delivery for treating actinic keratoses and scars. According to Dr. Haedersdal, professor of dermatology at the University of Copenhagen, evolving clinical indications for laser-assisted drug delivery include rejuvenation, local anesthesia, melasma, onychomycosis, hyperhidrosis, alopecia, and vitiligo, while emerging indications include treatment of skin cancer with PD-1 inhibitors and combination chemotherapy regimens, and vaccinations.
During a presentation at the annual conference of the American Society for Laser Medicine and Surgery, she said that researchers have much to learn about laser-assisted drug delivery, including biodistribution of the drug being delivered. Pointing out that so far, “what we have been dealing with is primarily looking at the skin as a black box,” she asked, “what happens when we drill the holes and drugs are applied on top of the skin and swim through the tiny channels?”
By using high-performance liquid chromatography (HPLC) and HPLC mass spectrometry to measure drug concentration in the skin, she and her colleagues have observed enhanced uptake of drugs – 4-fold to 40-fold greater – primarily in ex vivo pig skin. “We do know from ex vivo models that it’s much easier to boost the uptake in the skin” when compared with in vivo human use, where much lower drug concentrations are detected, said Dr. Haedersdal, who, along with Emily Wenande, MD, PhD, and R. Rox Anderson, MD, at the Wellman Center for Photomedicine, at Massachusetts General Hospital, Boston, authored a clinical review, published in 2020, on the basics of laser-assisted drug delivery.
“What we are working on now is visualizing what’s taking place when we apply the holes and the drugs in the skin. This is the key to tailoring laser-assisted uptake to specific dermatologic diseases being treated,” she said. To date, she and her colleagues have examined the interaction with tissue using different devices, including ex vivo confocal microscopy, to view the thermal response to ablative fractional laser and radiofrequency. “We want to take that to the next level and look at the drug biodistribution.”
Efforts are underway to compare the pattern of drug distribution with different modes of delivery, such as comparing ablative fractional laser to intradermal needle injection. “We are also working on pneumatic jet injection, which creates a focal drug distribution,” said Dr. Haedersdal, who is a visiting scientist at the Wellman Center. “In the future, we may take advantage of device-tailored biodistribution, depending on which clinical indication we are treating.”
Another important aspect to consider is drug retention in the skin. In a study presented as an abstract at the meeting, led by Dr. Wenande, she, Dr. Haedersdal, and colleagues used a pig model to evaluate the effect of three vasoregulative interventions on ablative fractional laser-assisted 5-fluororacil concentrations in in vivo skin. The three interventions were brimonidine 0.33% solution, epinephrine 10 mcg/mL gel, and a 595-nm pulsed dye laser (PDL) in designated treatment areas.
“What we learned from that was in the short term – 1-4 hours – the ablative fractional laser enhanced the uptake of 5-FU, but it was very transient,” with a twofold increased concentration of 5-FU, Dr. Haedersdal said. Over 48-72 hours, after PDL, there was “sustained enhancement of drug in the skin by three to four times,” she noted.
The synergy of systemic drugs with ablative fractional laser therapy is also being evaluated. In a mouse study led by Dr. Haedersdal’s colleague, senior researcher Uffe H. Olesen, PhD, the treatment of advanced squamous cell carcinoma tumors with a combination of ablative fractional laser and systemic treatment with PD-1 inhibitors resulted in the clearance of more tumors than with either treatment as monotherapy. “What we want to explore is the laser-induced tumor immune response in keratinocyte cancers,” she added.
“When you shine the laser on the skin, there is a robust increase of neutrophilic granulocytes.” Combining this topical immune-boosting response with systemic delivery of PD-1 inhibitors in a mouse model with basal cell carcinoma, she said, “we learned that, when we compare systemic PD-1 inhibitors alone to the laser alone and then with combination therapy, there was an increased tumor clearance of basal cell carcinomas and also enhanced survival of the mice” with the combination, she said. There were also “enhanced neutrophilic counts and both CD4- and CD8-positive cells were increased,” she added.
Dr. Haedersdal disclosed that she has received grants or research funding from Lutronic, Venus Concept, Leo Pharma, and Mirai Medical.
AT ASLMS 2022
Can lasers be used to measure nerve sensitivity in the skin?
SAN DIEGO – In a 2006 report of complications from laser dermatologic surgery, one of the authors, Dieter Manstein, MD, PhD, who had subjected his forearm to treatment with a fractional laser skin resurfacing prototype device, was included as 1 of the 19 featured cases.
Dr. Manstein, of the Cutaneous Biology Research Center in the department of dermatology at Massachusetts General Hospital, Boston, was exposed to three test spots in the evaluation of the effects of different microscopic thermal zone densities for the prototype device, emitting at 1,450 nm and an energy per MTZ of 3 mJ.
Two years later, hypopigmentation persisted at the test site treated with the highest MTZ density, while two other sites treated with the lower MTZ densities did not show any dyspigmentation. But he noticed something else during the experiment: He felt minimal to no pain as each test site was being treated.
“It took 7 minutes without any cooling or anesthesia,” Dr. Manstein recalled at the annual meeting of the American Society for Laser Medicine and Surgery. “It was not completely painless, but each time the laser was applied, sometimes I felt a little prick, sometimes I felt nothing.” Essentially, he added, “we created cell injury with a focused laser beam without anesthesia,” but this could also indicate that if skin is treated with a fractional laser very slowly, anesthesia is not needed. “Current devices are meant to treat very quickly, but if we [treat] slowly, maybe you could remove lesions painlessly without anesthesia.”
The observation from that experiment also led Dr. Manstein and colleagues to wonder: Could a focused laser beam pattern be used to assess cutaneous innervation? If so, they postulated, perhaps it could be used to not only assess nerve sensitivity of candidates for dermatologic surgery, but as a tool to help diagnose small fiber neuropathies such as diabetic neuropathy, and neuropathies in patients with HIV and sarcoidosis.
The current gold standard for making these diagnoses involves a skin biopsy, immunohistochemical analysis, and nerve fiber quantification, which is not widely available. It also requires strict histologic processing and nerve counting rules. Confocal microscopy of nerve fibers in the cornea is another approach, but is very difficult to perform, “so it would be nice if there was a simple way” to determine nerve fiber density in the skin using a focused laser beam, Dr. Manstein said.
With help from Payal Patel, MD, a dermatology research fellow at MGH, records each subject’s perception of a stimulus, and maps the areas of stimulus response. Current diameters being studied range from 0.076-1.15 mm and depths less than 0.71 mm. “We can focus the laser beam, preset the beam diameter, and very slowly, in a controlled manner, make a rectangular pattern, and after each time, inquire if the subject felt the pulse or not,” Dr. Manstein explained.
“This laser could become a new method for diagnosing nerve fiber neuropathies. If this works well, I think we can miniaturize the device,” he added.
Dr. Manstein disclosed that he is a consultant for Blossom Innovations, R2 Dermatology, and AVAVA. He is also a member of the advisory board for Blossom Innovations.
SAN DIEGO – In a 2006 report of complications from laser dermatologic surgery, one of the authors, Dieter Manstein, MD, PhD, who had subjected his forearm to treatment with a fractional laser skin resurfacing prototype device, was included as 1 of the 19 featured cases.
Dr. Manstein, of the Cutaneous Biology Research Center in the department of dermatology at Massachusetts General Hospital, Boston, was exposed to three test spots in the evaluation of the effects of different microscopic thermal zone densities for the prototype device, emitting at 1,450 nm and an energy per MTZ of 3 mJ.
Two years later, hypopigmentation persisted at the test site treated with the highest MTZ density, while two other sites treated with the lower MTZ densities did not show any dyspigmentation. But he noticed something else during the experiment: He felt minimal to no pain as each test site was being treated.
“It took 7 minutes without any cooling or anesthesia,” Dr. Manstein recalled at the annual meeting of the American Society for Laser Medicine and Surgery. “It was not completely painless, but each time the laser was applied, sometimes I felt a little prick, sometimes I felt nothing.” Essentially, he added, “we created cell injury with a focused laser beam without anesthesia,” but this could also indicate that if skin is treated with a fractional laser very slowly, anesthesia is not needed. “Current devices are meant to treat very quickly, but if we [treat] slowly, maybe you could remove lesions painlessly without anesthesia.”
The observation from that experiment also led Dr. Manstein and colleagues to wonder: Could a focused laser beam pattern be used to assess cutaneous innervation? If so, they postulated, perhaps it could be used to not only assess nerve sensitivity of candidates for dermatologic surgery, but as a tool to help diagnose small fiber neuropathies such as diabetic neuropathy, and neuropathies in patients with HIV and sarcoidosis.
The current gold standard for making these diagnoses involves a skin biopsy, immunohistochemical analysis, and nerve fiber quantification, which is not widely available. It also requires strict histologic processing and nerve counting rules. Confocal microscopy of nerve fibers in the cornea is another approach, but is very difficult to perform, “so it would be nice if there was a simple way” to determine nerve fiber density in the skin using a focused laser beam, Dr. Manstein said.
With help from Payal Patel, MD, a dermatology research fellow at MGH, records each subject’s perception of a stimulus, and maps the areas of stimulus response. Current diameters being studied range from 0.076-1.15 mm and depths less than 0.71 mm. “We can focus the laser beam, preset the beam diameter, and very slowly, in a controlled manner, make a rectangular pattern, and after each time, inquire if the subject felt the pulse or not,” Dr. Manstein explained.
“This laser could become a new method for diagnosing nerve fiber neuropathies. If this works well, I think we can miniaturize the device,” he added.
Dr. Manstein disclosed that he is a consultant for Blossom Innovations, R2 Dermatology, and AVAVA. He is also a member of the advisory board for Blossom Innovations.
SAN DIEGO – In a 2006 report of complications from laser dermatologic surgery, one of the authors, Dieter Manstein, MD, PhD, who had subjected his forearm to treatment with a fractional laser skin resurfacing prototype device, was included as 1 of the 19 featured cases.
Dr. Manstein, of the Cutaneous Biology Research Center in the department of dermatology at Massachusetts General Hospital, Boston, was exposed to three test spots in the evaluation of the effects of different microscopic thermal zone densities for the prototype device, emitting at 1,450 nm and an energy per MTZ of 3 mJ.
Two years later, hypopigmentation persisted at the test site treated with the highest MTZ density, while two other sites treated with the lower MTZ densities did not show any dyspigmentation. But he noticed something else during the experiment: He felt minimal to no pain as each test site was being treated.
“It took 7 minutes without any cooling or anesthesia,” Dr. Manstein recalled at the annual meeting of the American Society for Laser Medicine and Surgery. “It was not completely painless, but each time the laser was applied, sometimes I felt a little prick, sometimes I felt nothing.” Essentially, he added, “we created cell injury with a focused laser beam without anesthesia,” but this could also indicate that if skin is treated with a fractional laser very slowly, anesthesia is not needed. “Current devices are meant to treat very quickly, but if we [treat] slowly, maybe you could remove lesions painlessly without anesthesia.”
The observation from that experiment also led Dr. Manstein and colleagues to wonder: Could a focused laser beam pattern be used to assess cutaneous innervation? If so, they postulated, perhaps it could be used to not only assess nerve sensitivity of candidates for dermatologic surgery, but as a tool to help diagnose small fiber neuropathies such as diabetic neuropathy, and neuropathies in patients with HIV and sarcoidosis.
The current gold standard for making these diagnoses involves a skin biopsy, immunohistochemical analysis, and nerve fiber quantification, which is not widely available. It also requires strict histologic processing and nerve counting rules. Confocal microscopy of nerve fibers in the cornea is another approach, but is very difficult to perform, “so it would be nice if there was a simple way” to determine nerve fiber density in the skin using a focused laser beam, Dr. Manstein said.
With help from Payal Patel, MD, a dermatology research fellow at MGH, records each subject’s perception of a stimulus, and maps the areas of stimulus response. Current diameters being studied range from 0.076-1.15 mm and depths less than 0.71 mm. “We can focus the laser beam, preset the beam diameter, and very slowly, in a controlled manner, make a rectangular pattern, and after each time, inquire if the subject felt the pulse or not,” Dr. Manstein explained.
“This laser could become a new method for diagnosing nerve fiber neuropathies. If this works well, I think we can miniaturize the device,” he added.
Dr. Manstein disclosed that he is a consultant for Blossom Innovations, R2 Dermatology, and AVAVA. He is also a member of the advisory board for Blossom Innovations.
AT ASLMS 2022
Fractional lasers appear to treat more than a fraction of skin, expert says
SAN DIEGO – Using the according to Molly Wanner, MD, MBA.
As a case in point, Dr. Wanner discussed the results of a trial of 48 people over aged 60 years with actinic damage, who received ablative fractional laser treatment on one arm and no treatment on the other arm, which served as the control. At 24 months, only two nonmelanoma skin cancers (NMSCs) developed on the treated arms, compared with 26 on the treated arms.
“What I find interesting is that the treated arm did not develop basal cell carcinoma, only squamous cell carcinoma,” she said at the annual meeting of the American Society for Laser Medicine and Surgery. “It appears that this is working through more than just treatment of the AK precursor lesions, for which fractional lasers are cleared for use. It appears to impact both types of NMSCs.”
The ablative fractional laser and other wounding therapies can modulate a response to UV light – a process that naturally diminishes with age, according to Dr. Wanner, a dermatologist at Massachusetts General Hospital’s Dermatology Laser and Cosmetic Center in Boston. “This ability to repair DNA is actually modulated by insulin-like growth factor 1,” she said. “IGF-1 is produced by papillary dermal fibroblasts and communicates with keratinocytes. If keratinocytes are exposed to UV light and there is no IGF-1 around, you get a mutated cell, and that keeps spreading, and you could potentially get a skin cancer.”
On the other hand, she continued, if IGF-1 is injected around the keratinocytes, they are able to respond. “Keratinocytes, which are the most superficial layer of the skin, are really active,” noted Dr. Wanner, who is also an assistant professor of dermatology at Harvard Medical School, Boston. “They’re dividing and replicating, whereas fibroblasts are more non-proliferative and more long-lived. They stick around for a long time. I think of them as the adults in the room, giving these new keratinocytes direction.”
In a review of wounding therapies for the prevention of photocarcinogenesis, she and her coauthors noted that IGF-1 increases nucleotide excision repair of damaged DNA, promotes checkpoint signaling and suppression of DNA synthesis, favors specialized polymerases that are better able to repair DNA damage, and enhances p53-dependent transcriptional responses to DNA damage.
“Older fibroblasts produce less IGF-1 and lead to a situation where keratinocytes can grow unchecked,” she said. “We can use fractional laser to help with this. Fractional laser increases fibroblast production and decreases senescent fibroblasts.”
In a 2017 review on the impact of age and IGF-1 on DNA damage responses in UV-irradiated skin, the authors noted the high levels of IGF-1 in the skin of younger individuals and lower levels in the skin of their older counterparts.
“But once older skin has been treated with either dermabrasion or fractional laser, the levels of IGF-1 are restored to that of a young adult,” Dr. Wanner said. “The restoration of IGF-1 then restores that level of appropriate response to UV light. So, what’s interesting is that fractional lasers treat more than a fraction [of skin]. Fractional lasers were developed to have an easier way to improve wound healing by leaving the skin intact around these columns [of treated skin]. It turns out that treatment of these columns of skin does not just impact the cells in that area. There is a true global effect that’s allowing us to almost normalize skin.”
Dr. Wanner now thinks of fractional lasers as stimulating a laser-cell biology interaction, not just a laser-tissue interaction. “It’s incredible that we can use these photons to not only impact the tissue itself but how the cells actually respond,” she said. “What’s going to be interesting for us in the next few years is to look at how lasers impact our cellular biology. How can we harness it to help our patients?”
She and her colleagues are conducting a trial of different wounding modalities to assess their impact on IGF-1. “Does depth matter? Does density matter? Does the wavelength matter?” she asked. “The bottom line is, it turns out that when the skin looks healthier, it is healthier. Cosmetic treatments can impact medical outcomes.”
Dr. Wanner disclosed that she is a consultant and advisor to Nu Skin. She has also received research funding and equipment from Solta.
SAN DIEGO – Using the according to Molly Wanner, MD, MBA.
As a case in point, Dr. Wanner discussed the results of a trial of 48 people over aged 60 years with actinic damage, who received ablative fractional laser treatment on one arm and no treatment on the other arm, which served as the control. At 24 months, only two nonmelanoma skin cancers (NMSCs) developed on the treated arms, compared with 26 on the treated arms.
“What I find interesting is that the treated arm did not develop basal cell carcinoma, only squamous cell carcinoma,” she said at the annual meeting of the American Society for Laser Medicine and Surgery. “It appears that this is working through more than just treatment of the AK precursor lesions, for which fractional lasers are cleared for use. It appears to impact both types of NMSCs.”
The ablative fractional laser and other wounding therapies can modulate a response to UV light – a process that naturally diminishes with age, according to Dr. Wanner, a dermatologist at Massachusetts General Hospital’s Dermatology Laser and Cosmetic Center in Boston. “This ability to repair DNA is actually modulated by insulin-like growth factor 1,” she said. “IGF-1 is produced by papillary dermal fibroblasts and communicates with keratinocytes. If keratinocytes are exposed to UV light and there is no IGF-1 around, you get a mutated cell, and that keeps spreading, and you could potentially get a skin cancer.”
On the other hand, she continued, if IGF-1 is injected around the keratinocytes, they are able to respond. “Keratinocytes, which are the most superficial layer of the skin, are really active,” noted Dr. Wanner, who is also an assistant professor of dermatology at Harvard Medical School, Boston. “They’re dividing and replicating, whereas fibroblasts are more non-proliferative and more long-lived. They stick around for a long time. I think of them as the adults in the room, giving these new keratinocytes direction.”
In a review of wounding therapies for the prevention of photocarcinogenesis, she and her coauthors noted that IGF-1 increases nucleotide excision repair of damaged DNA, promotes checkpoint signaling and suppression of DNA synthesis, favors specialized polymerases that are better able to repair DNA damage, and enhances p53-dependent transcriptional responses to DNA damage.
“Older fibroblasts produce less IGF-1 and lead to a situation where keratinocytes can grow unchecked,” she said. “We can use fractional laser to help with this. Fractional laser increases fibroblast production and decreases senescent fibroblasts.”
In a 2017 review on the impact of age and IGF-1 on DNA damage responses in UV-irradiated skin, the authors noted the high levels of IGF-1 in the skin of younger individuals and lower levels in the skin of their older counterparts.
“But once older skin has been treated with either dermabrasion or fractional laser, the levels of IGF-1 are restored to that of a young adult,” Dr. Wanner said. “The restoration of IGF-1 then restores that level of appropriate response to UV light. So, what’s interesting is that fractional lasers treat more than a fraction [of skin]. Fractional lasers were developed to have an easier way to improve wound healing by leaving the skin intact around these columns [of treated skin]. It turns out that treatment of these columns of skin does not just impact the cells in that area. There is a true global effect that’s allowing us to almost normalize skin.”
Dr. Wanner now thinks of fractional lasers as stimulating a laser-cell biology interaction, not just a laser-tissue interaction. “It’s incredible that we can use these photons to not only impact the tissue itself but how the cells actually respond,” she said. “What’s going to be interesting for us in the next few years is to look at how lasers impact our cellular biology. How can we harness it to help our patients?”
She and her colleagues are conducting a trial of different wounding modalities to assess their impact on IGF-1. “Does depth matter? Does density matter? Does the wavelength matter?” she asked. “The bottom line is, it turns out that when the skin looks healthier, it is healthier. Cosmetic treatments can impact medical outcomes.”
Dr. Wanner disclosed that she is a consultant and advisor to Nu Skin. She has also received research funding and equipment from Solta.
SAN DIEGO – Using the according to Molly Wanner, MD, MBA.
As a case in point, Dr. Wanner discussed the results of a trial of 48 people over aged 60 years with actinic damage, who received ablative fractional laser treatment on one arm and no treatment on the other arm, which served as the control. At 24 months, only two nonmelanoma skin cancers (NMSCs) developed on the treated arms, compared with 26 on the treated arms.
“What I find interesting is that the treated arm did not develop basal cell carcinoma, only squamous cell carcinoma,” she said at the annual meeting of the American Society for Laser Medicine and Surgery. “It appears that this is working through more than just treatment of the AK precursor lesions, for which fractional lasers are cleared for use. It appears to impact both types of NMSCs.”
The ablative fractional laser and other wounding therapies can modulate a response to UV light – a process that naturally diminishes with age, according to Dr. Wanner, a dermatologist at Massachusetts General Hospital’s Dermatology Laser and Cosmetic Center in Boston. “This ability to repair DNA is actually modulated by insulin-like growth factor 1,” she said. “IGF-1 is produced by papillary dermal fibroblasts and communicates with keratinocytes. If keratinocytes are exposed to UV light and there is no IGF-1 around, you get a mutated cell, and that keeps spreading, and you could potentially get a skin cancer.”
On the other hand, she continued, if IGF-1 is injected around the keratinocytes, they are able to respond. “Keratinocytes, which are the most superficial layer of the skin, are really active,” noted Dr. Wanner, who is also an assistant professor of dermatology at Harvard Medical School, Boston. “They’re dividing and replicating, whereas fibroblasts are more non-proliferative and more long-lived. They stick around for a long time. I think of them as the adults in the room, giving these new keratinocytes direction.”
In a review of wounding therapies for the prevention of photocarcinogenesis, she and her coauthors noted that IGF-1 increases nucleotide excision repair of damaged DNA, promotes checkpoint signaling and suppression of DNA synthesis, favors specialized polymerases that are better able to repair DNA damage, and enhances p53-dependent transcriptional responses to DNA damage.
“Older fibroblasts produce less IGF-1 and lead to a situation where keratinocytes can grow unchecked,” she said. “We can use fractional laser to help with this. Fractional laser increases fibroblast production and decreases senescent fibroblasts.”
In a 2017 review on the impact of age and IGF-1 on DNA damage responses in UV-irradiated skin, the authors noted the high levels of IGF-1 in the skin of younger individuals and lower levels in the skin of their older counterparts.
“But once older skin has been treated with either dermabrasion or fractional laser, the levels of IGF-1 are restored to that of a young adult,” Dr. Wanner said. “The restoration of IGF-1 then restores that level of appropriate response to UV light. So, what’s interesting is that fractional lasers treat more than a fraction [of skin]. Fractional lasers were developed to have an easier way to improve wound healing by leaving the skin intact around these columns [of treated skin]. It turns out that treatment of these columns of skin does not just impact the cells in that area. There is a true global effect that’s allowing us to almost normalize skin.”
Dr. Wanner now thinks of fractional lasers as stimulating a laser-cell biology interaction, not just a laser-tissue interaction. “It’s incredible that we can use these photons to not only impact the tissue itself but how the cells actually respond,” she said. “What’s going to be interesting for us in the next few years is to look at how lasers impact our cellular biology. How can we harness it to help our patients?”
She and her colleagues are conducting a trial of different wounding modalities to assess their impact on IGF-1. “Does depth matter? Does density matter? Does the wavelength matter?” she asked. “The bottom line is, it turns out that when the skin looks healthier, it is healthier. Cosmetic treatments can impact medical outcomes.”
Dr. Wanner disclosed that she is a consultant and advisor to Nu Skin. She has also received research funding and equipment from Solta.
AT ASLMS 2022
‘Cool’ way of eradicating fat a promising therapy for many medical conditions
SAN DIEGO – During her third year in the combined Harvard/Massachusetts General Hospital dermatology residency program in 2011, Lilit Garibyan, MD, PhD, attended a lecture presented by R. Rox Anderson, MD, director of the Wellman Center for Photomedicine at MGH. He described the concept of selective cryolipolysis – the method of removing fat by topical cooling that eventually led to the development of the CoolSculpting device.
“He was saying that this is such a great noninvasive technology for fat removal and that patients love it,” Dr. Garibyan recalled at the annual meeting of the American Society for Laser Medicine and Surgery. “But one of the most common side effects after cryolipolysis that is long-lasting, but completely reversible, is hypoesthesia. I was intrigued by this because even as a dermatology resident, I had seen how pain and itch symptoms are present in many dermatologic diseases, and we don’t have great treatments for them. I thought to myself, not the fat.
Following Dr. Anderson’s lecture, Dr. Garibyan asked him if anyone knew the mechanism of action or if anyone was working to find out. He did not, but Dr. Anderson invited her to join his lab to investigate. “I didn’t have a background in lasers or energy devices, but I thought this was such a great opportunity” and addressed an unmet need, she said at the meeting.
Dr. Garibyan then led a clinical trial to characterize the effect of a single cryolipolysis treatment in 11 healthy people and to quantitatively analyze what sensory functions change with treatment over a period of 56 days. Skin biopsies revealed that cryolipolysis mainly decreased myelinated dermal nerve fiber density, which persisted throughout the study.
“The conclusion was that yes, controlled topical cooling does lead to significant and long-lasting but reversible reduction of sensory function, including pain,” said Dr. Garibyan, who is now an assistant professor of dermatology at Harvard Medical School, Boston, and director of the Magic Wand Initiative at the Wellman Center.
Ice slurry injections
Enter ice slurry, a chilly mix of ice, saline, and glycol that can be directly injected into adipose tissue. In a swine study published online in January 2020, Dr. Garibyan and colleagues at the Wellman Center injected ice slurry into the flanks of swine and followed them for up to 8 weeks, using ultrasound imaging to quantify and show the location of fat loss. The researchers observed about 40%-50% loss of fat in the treated area, compared with a 60% increase of fat in controls. “On histology, this was very selective,” she said. “Only adipose tissue was affected. There was no damage to the underlying muscle or to the dermis or epidermis.”
In 2021, researchers tested the injection of ice slurry in 12 humans for the first time, injected into tissue, and followed them for 12 weeks. As observed by thermal imaging, ultrasound, and tissue histology, they concluded that ice slurry injection was feasible and safe as a way of inducing cryolipolysis, and was well tolerated by patients.
“This can become a promising treatment for a precise, effective, and customizable way of removing unwanted fat for aesthetic application,” Dr. Garibyan said. However, she added, it is not approved by the Food and Drug Administration and more studies are needed, “but it’s promising and encouraging to see this move forward in patients.”
Potential nonaesthetic uses
The potential applications of injectable ice slurry extend well beyond cosmetic dermatology, she continued, noting that it is being explored as a treatment for many medical conditions including obstructive sleep apnea (OSA). At the University of Pennsylvania, Philadelphia, researchers used MRI to image the tongue fat in a case-control study of 31 obese patients without OSA and 90 obese patients with OSA. They found that patients with OSA had increased deposition of fat at the base of their tongue, which can lead to airway obstruction in this subset of patients with OSA, pointed out Dr. Garibyan, who was not involved with the study. “This also gave us a hint. If we can remove that tongue fat, we could potentially help reduce severity or even cure OSA in this population of patients. This points to tongue fat as a therapeutic target.”
With help from researchers at Uniformed Services University of the Health Sciences, Bethesda, Md., she and her Wellman Center colleagues recently completed a swine study that showed the safety and feasibility of injecting the base of the tongue with ice slurry, targeting adipose tissue. The work has been submitted for publication in a journal, but at the meeting, she said that, 8 weeks after injecting the ice slurry, there were no changes to any tongue tissue other than fat.
“On histology, we only see selective damage to the adipose tissue,” she said. “It is very promising that it’s safe in animal models and we’re hoping to conduct a human trial later this year to test the ability of this injectable ice slurry to remove fat at the base of the tongue with the hope that this will treat OSA.”
Another potential application of this technology is in the cardiology field. Dr. Garibyan is part of a multidisciplinary team at MGH that includes cardiac surgeons, cardiologists, and imaging experts who plan to investigate whether injecting ice slurry into fat around the heart can modify heart disease in humans. “Visceral fat around the heart – pericardial fat and epicardial fat – is involved in cardiovascular disease, arrhythmias, and many other unwanted effects on the heart,” she said. “Imagine if you could inject this around the heart, ablate the fat, and halt cardiovascular disease?”
She led a study that examined the effect of injecting ice slurry into swine with significant amounts of adipose tissue around their hearts, based on baseline CT scans. She and her coinvestigators observed a significant loss of that fat tissue on follow-up CT scans 8 weeks later. “On average, there was about a 30% reduction of this pericardial adipose tissue after a single injection,” and the procedure “was safe and well tolerated by the animals,” she added.
Ice slurry could also play a role in managing pain by targeting peripheral nerves. Peripheral nerves are composed of 75%-80% lipids, such as the myelin sheaths around the nerves, she noted. “That’s lipid-rich tissue. We think that by targeting that we’re able to block pain.”
She led a study that showed that a single injection of ice slurry around the sciatic nerve in rats served as a sustained anesthetic by blocking mechanical pain sensation for up to 56 days. They imaged the peripheral nerves in the rats and showed that the mechanism involved was loss of the lipid-rich myelin tissue around the nerves, which blocks the signaling of the nerve, she said.
Dr. Garibyan disclosed that she is a member of the advisory board for Brixton Biosciences, Vyome Therapeutics, and Aegle Therapeutics. She is also a consultant for Aegle Therapeutics and Blossom Innovations and holds equity in Brixton Biosciences and EyeCool Therapeutics.
SAN DIEGO – During her third year in the combined Harvard/Massachusetts General Hospital dermatology residency program in 2011, Lilit Garibyan, MD, PhD, attended a lecture presented by R. Rox Anderson, MD, director of the Wellman Center for Photomedicine at MGH. He described the concept of selective cryolipolysis – the method of removing fat by topical cooling that eventually led to the development of the CoolSculpting device.
“He was saying that this is such a great noninvasive technology for fat removal and that patients love it,” Dr. Garibyan recalled at the annual meeting of the American Society for Laser Medicine and Surgery. “But one of the most common side effects after cryolipolysis that is long-lasting, but completely reversible, is hypoesthesia. I was intrigued by this because even as a dermatology resident, I had seen how pain and itch symptoms are present in many dermatologic diseases, and we don’t have great treatments for them. I thought to myself, not the fat.
Following Dr. Anderson’s lecture, Dr. Garibyan asked him if anyone knew the mechanism of action or if anyone was working to find out. He did not, but Dr. Anderson invited her to join his lab to investigate. “I didn’t have a background in lasers or energy devices, but I thought this was such a great opportunity” and addressed an unmet need, she said at the meeting.
Dr. Garibyan then led a clinical trial to characterize the effect of a single cryolipolysis treatment in 11 healthy people and to quantitatively analyze what sensory functions change with treatment over a period of 56 days. Skin biopsies revealed that cryolipolysis mainly decreased myelinated dermal nerve fiber density, which persisted throughout the study.
“The conclusion was that yes, controlled topical cooling does lead to significant and long-lasting but reversible reduction of sensory function, including pain,” said Dr. Garibyan, who is now an assistant professor of dermatology at Harvard Medical School, Boston, and director of the Magic Wand Initiative at the Wellman Center.
Ice slurry injections
Enter ice slurry, a chilly mix of ice, saline, and glycol that can be directly injected into adipose tissue. In a swine study published online in January 2020, Dr. Garibyan and colleagues at the Wellman Center injected ice slurry into the flanks of swine and followed them for up to 8 weeks, using ultrasound imaging to quantify and show the location of fat loss. The researchers observed about 40%-50% loss of fat in the treated area, compared with a 60% increase of fat in controls. “On histology, this was very selective,” she said. “Only adipose tissue was affected. There was no damage to the underlying muscle or to the dermis or epidermis.”
In 2021, researchers tested the injection of ice slurry in 12 humans for the first time, injected into tissue, and followed them for 12 weeks. As observed by thermal imaging, ultrasound, and tissue histology, they concluded that ice slurry injection was feasible and safe as a way of inducing cryolipolysis, and was well tolerated by patients.
“This can become a promising treatment for a precise, effective, and customizable way of removing unwanted fat for aesthetic application,” Dr. Garibyan said. However, she added, it is not approved by the Food and Drug Administration and more studies are needed, “but it’s promising and encouraging to see this move forward in patients.”
Potential nonaesthetic uses
The potential applications of injectable ice slurry extend well beyond cosmetic dermatology, she continued, noting that it is being explored as a treatment for many medical conditions including obstructive sleep apnea (OSA). At the University of Pennsylvania, Philadelphia, researchers used MRI to image the tongue fat in a case-control study of 31 obese patients without OSA and 90 obese patients with OSA. They found that patients with OSA had increased deposition of fat at the base of their tongue, which can lead to airway obstruction in this subset of patients with OSA, pointed out Dr. Garibyan, who was not involved with the study. “This also gave us a hint. If we can remove that tongue fat, we could potentially help reduce severity or even cure OSA in this population of patients. This points to tongue fat as a therapeutic target.”
With help from researchers at Uniformed Services University of the Health Sciences, Bethesda, Md., she and her Wellman Center colleagues recently completed a swine study that showed the safety and feasibility of injecting the base of the tongue with ice slurry, targeting adipose tissue. The work has been submitted for publication in a journal, but at the meeting, she said that, 8 weeks after injecting the ice slurry, there were no changes to any tongue tissue other than fat.
“On histology, we only see selective damage to the adipose tissue,” she said. “It is very promising that it’s safe in animal models and we’re hoping to conduct a human trial later this year to test the ability of this injectable ice slurry to remove fat at the base of the tongue with the hope that this will treat OSA.”
Another potential application of this technology is in the cardiology field. Dr. Garibyan is part of a multidisciplinary team at MGH that includes cardiac surgeons, cardiologists, and imaging experts who plan to investigate whether injecting ice slurry into fat around the heart can modify heart disease in humans. “Visceral fat around the heart – pericardial fat and epicardial fat – is involved in cardiovascular disease, arrhythmias, and many other unwanted effects on the heart,” she said. “Imagine if you could inject this around the heart, ablate the fat, and halt cardiovascular disease?”
She led a study that examined the effect of injecting ice slurry into swine with significant amounts of adipose tissue around their hearts, based on baseline CT scans. She and her coinvestigators observed a significant loss of that fat tissue on follow-up CT scans 8 weeks later. “On average, there was about a 30% reduction of this pericardial adipose tissue after a single injection,” and the procedure “was safe and well tolerated by the animals,” she added.
Ice slurry could also play a role in managing pain by targeting peripheral nerves. Peripheral nerves are composed of 75%-80% lipids, such as the myelin sheaths around the nerves, she noted. “That’s lipid-rich tissue. We think that by targeting that we’re able to block pain.”
She led a study that showed that a single injection of ice slurry around the sciatic nerve in rats served as a sustained anesthetic by blocking mechanical pain sensation for up to 56 days. They imaged the peripheral nerves in the rats and showed that the mechanism involved was loss of the lipid-rich myelin tissue around the nerves, which blocks the signaling of the nerve, she said.
Dr. Garibyan disclosed that she is a member of the advisory board for Brixton Biosciences, Vyome Therapeutics, and Aegle Therapeutics. She is also a consultant for Aegle Therapeutics and Blossom Innovations and holds equity in Brixton Biosciences and EyeCool Therapeutics.
SAN DIEGO – During her third year in the combined Harvard/Massachusetts General Hospital dermatology residency program in 2011, Lilit Garibyan, MD, PhD, attended a lecture presented by R. Rox Anderson, MD, director of the Wellman Center for Photomedicine at MGH. He described the concept of selective cryolipolysis – the method of removing fat by topical cooling that eventually led to the development of the CoolSculpting device.
“He was saying that this is such a great noninvasive technology for fat removal and that patients love it,” Dr. Garibyan recalled at the annual meeting of the American Society for Laser Medicine and Surgery. “But one of the most common side effects after cryolipolysis that is long-lasting, but completely reversible, is hypoesthesia. I was intrigued by this because even as a dermatology resident, I had seen how pain and itch symptoms are present in many dermatologic diseases, and we don’t have great treatments for them. I thought to myself, not the fat.
Following Dr. Anderson’s lecture, Dr. Garibyan asked him if anyone knew the mechanism of action or if anyone was working to find out. He did not, but Dr. Anderson invited her to join his lab to investigate. “I didn’t have a background in lasers or energy devices, but I thought this was such a great opportunity” and addressed an unmet need, she said at the meeting.
Dr. Garibyan then led a clinical trial to characterize the effect of a single cryolipolysis treatment in 11 healthy people and to quantitatively analyze what sensory functions change with treatment over a period of 56 days. Skin biopsies revealed that cryolipolysis mainly decreased myelinated dermal nerve fiber density, which persisted throughout the study.
“The conclusion was that yes, controlled topical cooling does lead to significant and long-lasting but reversible reduction of sensory function, including pain,” said Dr. Garibyan, who is now an assistant professor of dermatology at Harvard Medical School, Boston, and director of the Magic Wand Initiative at the Wellman Center.
Ice slurry injections
Enter ice slurry, a chilly mix of ice, saline, and glycol that can be directly injected into adipose tissue. In a swine study published online in January 2020, Dr. Garibyan and colleagues at the Wellman Center injected ice slurry into the flanks of swine and followed them for up to 8 weeks, using ultrasound imaging to quantify and show the location of fat loss. The researchers observed about 40%-50% loss of fat in the treated area, compared with a 60% increase of fat in controls. “On histology, this was very selective,” she said. “Only adipose tissue was affected. There was no damage to the underlying muscle or to the dermis or epidermis.”
In 2021, researchers tested the injection of ice slurry in 12 humans for the first time, injected into tissue, and followed them for 12 weeks. As observed by thermal imaging, ultrasound, and tissue histology, they concluded that ice slurry injection was feasible and safe as a way of inducing cryolipolysis, and was well tolerated by patients.
“This can become a promising treatment for a precise, effective, and customizable way of removing unwanted fat for aesthetic application,” Dr. Garibyan said. However, she added, it is not approved by the Food and Drug Administration and more studies are needed, “but it’s promising and encouraging to see this move forward in patients.”
Potential nonaesthetic uses
The potential applications of injectable ice slurry extend well beyond cosmetic dermatology, she continued, noting that it is being explored as a treatment for many medical conditions including obstructive sleep apnea (OSA). At the University of Pennsylvania, Philadelphia, researchers used MRI to image the tongue fat in a case-control study of 31 obese patients without OSA and 90 obese patients with OSA. They found that patients with OSA had increased deposition of fat at the base of their tongue, which can lead to airway obstruction in this subset of patients with OSA, pointed out Dr. Garibyan, who was not involved with the study. “This also gave us a hint. If we can remove that tongue fat, we could potentially help reduce severity or even cure OSA in this population of patients. This points to tongue fat as a therapeutic target.”
With help from researchers at Uniformed Services University of the Health Sciences, Bethesda, Md., she and her Wellman Center colleagues recently completed a swine study that showed the safety and feasibility of injecting the base of the tongue with ice slurry, targeting adipose tissue. The work has been submitted for publication in a journal, but at the meeting, she said that, 8 weeks after injecting the ice slurry, there were no changes to any tongue tissue other than fat.
“On histology, we only see selective damage to the adipose tissue,” she said. “It is very promising that it’s safe in animal models and we’re hoping to conduct a human trial later this year to test the ability of this injectable ice slurry to remove fat at the base of the tongue with the hope that this will treat OSA.”
Another potential application of this technology is in the cardiology field. Dr. Garibyan is part of a multidisciplinary team at MGH that includes cardiac surgeons, cardiologists, and imaging experts who plan to investigate whether injecting ice slurry into fat around the heart can modify heart disease in humans. “Visceral fat around the heart – pericardial fat and epicardial fat – is involved in cardiovascular disease, arrhythmias, and many other unwanted effects on the heart,” she said. “Imagine if you could inject this around the heart, ablate the fat, and halt cardiovascular disease?”
She led a study that examined the effect of injecting ice slurry into swine with significant amounts of adipose tissue around their hearts, based on baseline CT scans. She and her coinvestigators observed a significant loss of that fat tissue on follow-up CT scans 8 weeks later. “On average, there was about a 30% reduction of this pericardial adipose tissue after a single injection,” and the procedure “was safe and well tolerated by the animals,” she added.
Ice slurry could also play a role in managing pain by targeting peripheral nerves. Peripheral nerves are composed of 75%-80% lipids, such as the myelin sheaths around the nerves, she noted. “That’s lipid-rich tissue. We think that by targeting that we’re able to block pain.”
She led a study that showed that a single injection of ice slurry around the sciatic nerve in rats served as a sustained anesthetic by blocking mechanical pain sensation for up to 56 days. They imaged the peripheral nerves in the rats and showed that the mechanism involved was loss of the lipid-rich myelin tissue around the nerves, which blocks the signaling of the nerve, she said.
Dr. Garibyan disclosed that she is a member of the advisory board for Brixton Biosciences, Vyome Therapeutics, and Aegle Therapeutics. She is also a consultant for Aegle Therapeutics and Blossom Innovations and holds equity in Brixton Biosciences and EyeCool Therapeutics.
AT ASLMS 2022
Forceps for Milia Extraction
To the Editor:
Several techniques can be used to destroy milia including electrocautery, electrodesiccation, and laser therapy. Manual extraction of milia uses a scalpel blade, needle, or stylet followed by the application of pressure to the lesion with a curette, comedone extractor, paper clip, cotton-tipped applicator, tongue blade, or hypodermic needle.1-4 Many of these techniques fail to stabilize milia, particularly in sensitive areas such as around the eyes or mouth, which can make extraction challenging, inefficient, and painful for the patient. We report a novel technique that quickly and effectively removes milia with equipment commonly used in the practice of clinical dermatology.
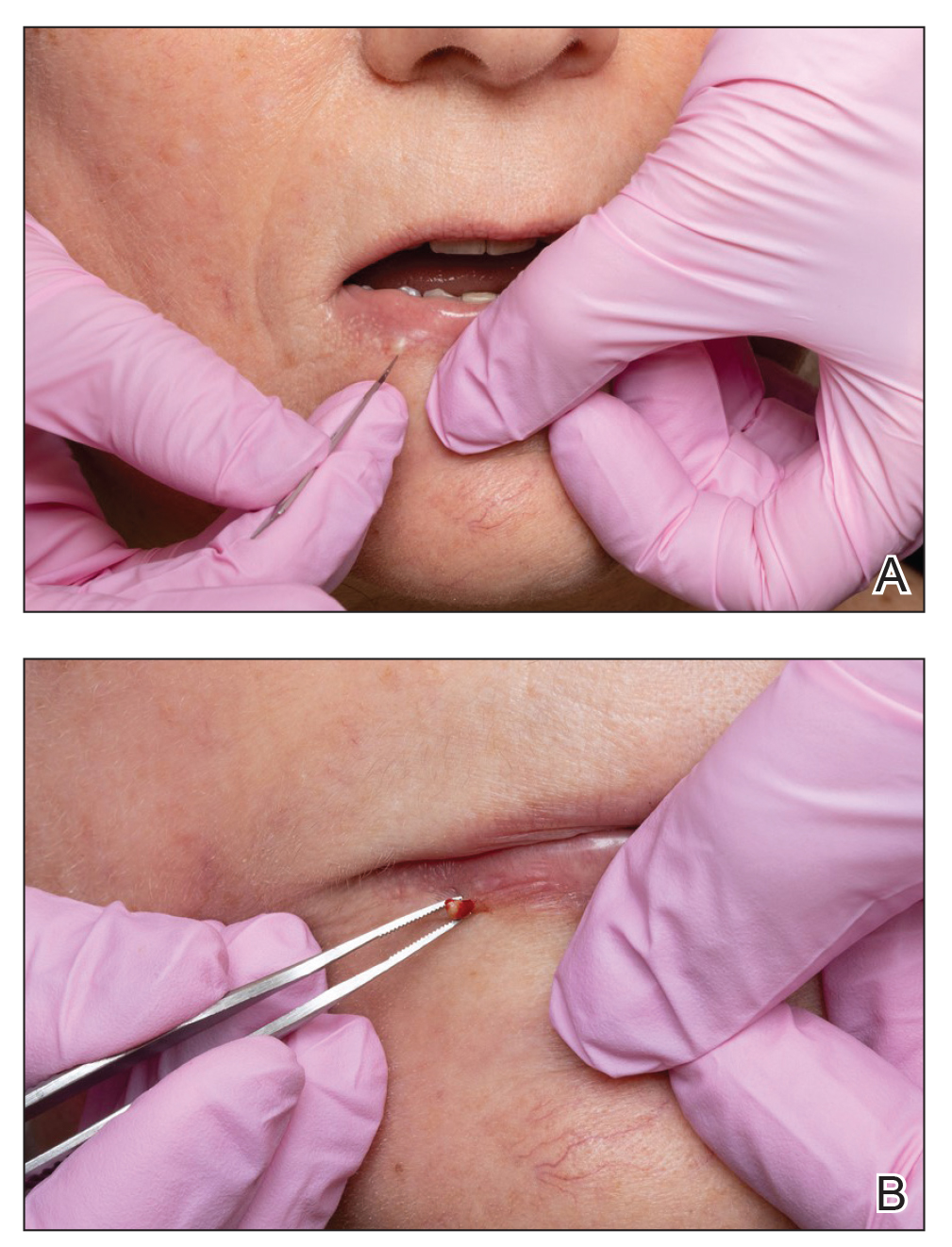
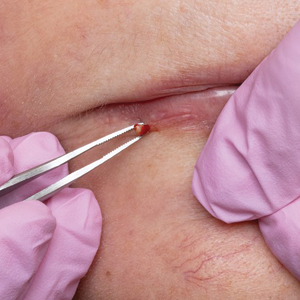
A 74-year-old woman presented with an asymptomatic papule on the right lower vermilion border of several years' duration. Physical examination of the lesion revealed a 3-mm, firm, white, dome-shaped papule. Clinical features were most consistent with a benign acquired milium. The patient desired removal for cosmesis. The area was cleaned with an alcohol swab, the surface of the milium was nicked with a No. 11 blade (Figure, A), and then tips of nontoothed Adson forceps were used to gently secure and pinch the base of the papule (Figure, B). The intact cyst was quickly and effortlessly expressed through the epidermal nick. The patient tolerated the procedure well, experiencing minimal pain and bleeding.
Histologically, milia represent infundibular keratin-filled cysts lined with stratified squamous epithelial tissue that contains a granular cell layer. These lesions are classified as primary or secondary; the former represent spontaneous occurrence, and the latter are associated with medications, trauma, or genodermatoses.2 Multiple milia are associated with conditions such as Bazex-Dupré-Christol syndrome, Rombo syndrome, Brooke-Spiegler syndrome, oro-facial-digital syndrome type I, atrichia with papular lesions, pachyonychia congenita type 2, basal cell nevus syndrome, basaloid follicular hamartoma syndrome, and hereditary vitamin D–dependent rickets type 2.5-9 The most common subtype seen in clinical practice includes benign primary milia, which tends to favor the cheeks and eyelids.2
Although these lesions are benign, many patients seek extraction for cosmesis. Milia extraction is a common procedure performed in dermatology clinical practice. Proposed extraction techniques using destructive methods include electrocautery, electrodesiccation, and laser therapy, and manual methods include nicking the surface of the lesion with a scalpel blade, needle, or stylet and then applying tangential pressure with a curette, comedone extractor, paper clip, cotton-tipped applicator, tongue blade, or hypodermic needle.1-4 Topical retinoids have been proposed as treatment of multiple milia.10 Many of these techniques do not use equipment common to clinical practice, or they fail to stabilize milia in sensitive areas, which makes extraction challenging. We describe a case with a new manual technique that successfully extracts milia in an efficient and safe manner.
- Parlette HL III. Management of cutaneous cysts. In: Wheeland RG, ed. Cutaneous Surgery. WB Saunders; 1994:651-652.
- Berk DR, Bayliss SJ. Milia: a review and classification. J Am Acad Dermatol. 2008;59:1050-1063.
- George DE, Wasko CA, Hsu S. Surgical pearl: evacuation of milia with a paper clip. J Am Acad Dermatol. 2006;54:326.
- Thami GP, Kaur S, Kanwar AJ. Surgical pearl: enucleation of milia with a disposable hypodermic needle. J Am Acad Dermatol. 2002;47:602-603.
- Goeteyn M, Geerts ML, Kint A, et al. The Bazex-Dupré-Christol syndrome. Arch Dermatol. 1994;130:337-342.
- Michaëlsson G, Olsson E, Westermark P. The Rombo syndrome: a familial disorder with vermiculate atrophoderma, milia, hypotrichosis, trichoepitheliomas, basal cell carcinomas and peripheral vasodilation with cyanosis. Acta Derm Venereol. 1981;61:497-503.
- Gurrieri F, Franco B, Toriello H, et al. Oral-facial-digital syndromes: review and diagnostic guidelines. Am J Med Genet A. 2007;143A:3314-3323.
- Zlotogorski A, Panteleyev AA, Aita VM, et al. Clinical and molecular diagnostic criteria of congenital atrichia with papular lesions. J Invest Dermatol. 2001;117:1662-1665.
- Paller AS, Moore JA, Scher R. Pachyonychia congenita tarda. alate-onset form of pachyonychia congenita. Arch Dermatol. 1991;127:701-703.
- Connelly T. Eruptive milia and rapid response to topical tretinoin. Arch Dermatol. 2008;144:816-817.
To the Editor:
Several techniques can be used to destroy milia including electrocautery, electrodesiccation, and laser therapy. Manual extraction of milia uses a scalpel blade, needle, or stylet followed by the application of pressure to the lesion with a curette, comedone extractor, paper clip, cotton-tipped applicator, tongue blade, or hypodermic needle.1-4 Many of these techniques fail to stabilize milia, particularly in sensitive areas such as around the eyes or mouth, which can make extraction challenging, inefficient, and painful for the patient. We report a novel technique that quickly and effectively removes milia with equipment commonly used in the practice of clinical dermatology.
A 74-year-old woman presented with an asymptomatic papule on the right lower vermilion border of several years' duration. Physical examination of the lesion revealed a 3-mm, firm, white, dome-shaped papule. Clinical features were most consistent with a benign acquired milium. The patient desired removal for cosmesis. The area was cleaned with an alcohol swab, the surface of the milium was nicked with a No. 11 blade (Figure, A), and then tips of nontoothed Adson forceps were used to gently secure and pinch the base of the papule (Figure, B). The intact cyst was quickly and effortlessly expressed through the epidermal nick. The patient tolerated the procedure well, experiencing minimal pain and bleeding.
Histologically, milia represent infundibular keratin-filled cysts lined with stratified squamous epithelial tissue that contains a granular cell layer. These lesions are classified as primary or secondary; the former represent spontaneous occurrence, and the latter are associated with medications, trauma, or genodermatoses.2 Multiple milia are associated with conditions such as Bazex-Dupré-Christol syndrome, Rombo syndrome, Brooke-Spiegler syndrome, oro-facial-digital syndrome type I, atrichia with papular lesions, pachyonychia congenita type 2, basal cell nevus syndrome, basaloid follicular hamartoma syndrome, and hereditary vitamin D–dependent rickets type 2.5-9 The most common subtype seen in clinical practice includes benign primary milia, which tends to favor the cheeks and eyelids.2
Although these lesions are benign, many patients seek extraction for cosmesis. Milia extraction is a common procedure performed in dermatology clinical practice. Proposed extraction techniques using destructive methods include electrocautery, electrodesiccation, and laser therapy, and manual methods include nicking the surface of the lesion with a scalpel blade, needle, or stylet and then applying tangential pressure with a curette, comedone extractor, paper clip, cotton-tipped applicator, tongue blade, or hypodermic needle.1-4 Topical retinoids have been proposed as treatment of multiple milia.10 Many of these techniques do not use equipment common to clinical practice, or they fail to stabilize milia in sensitive areas, which makes extraction challenging. We describe a case with a new manual technique that successfully extracts milia in an efficient and safe manner.
To the Editor:
Several techniques can be used to destroy milia including electrocautery, electrodesiccation, and laser therapy. Manual extraction of milia uses a scalpel blade, needle, or stylet followed by the application of pressure to the lesion with a curette, comedone extractor, paper clip, cotton-tipped applicator, tongue blade, or hypodermic needle.1-4 Many of these techniques fail to stabilize milia, particularly in sensitive areas such as around the eyes or mouth, which can make extraction challenging, inefficient, and painful for the patient. We report a novel technique that quickly and effectively removes milia with equipment commonly used in the practice of clinical dermatology.
A 74-year-old woman presented with an asymptomatic papule on the right lower vermilion border of several years' duration. Physical examination of the lesion revealed a 3-mm, firm, white, dome-shaped papule. Clinical features were most consistent with a benign acquired milium. The patient desired removal for cosmesis. The area was cleaned with an alcohol swab, the surface of the milium was nicked with a No. 11 blade (Figure, A), and then tips of nontoothed Adson forceps were used to gently secure and pinch the base of the papule (Figure, B). The intact cyst was quickly and effortlessly expressed through the epidermal nick. The patient tolerated the procedure well, experiencing minimal pain and bleeding.
Histologically, milia represent infundibular keratin-filled cysts lined with stratified squamous epithelial tissue that contains a granular cell layer. These lesions are classified as primary or secondary; the former represent spontaneous occurrence, and the latter are associated with medications, trauma, or genodermatoses.2 Multiple milia are associated with conditions such as Bazex-Dupré-Christol syndrome, Rombo syndrome, Brooke-Spiegler syndrome, oro-facial-digital syndrome type I, atrichia with papular lesions, pachyonychia congenita type 2, basal cell nevus syndrome, basaloid follicular hamartoma syndrome, and hereditary vitamin D–dependent rickets type 2.5-9 The most common subtype seen in clinical practice includes benign primary milia, which tends to favor the cheeks and eyelids.2
Although these lesions are benign, many patients seek extraction for cosmesis. Milia extraction is a common procedure performed in dermatology clinical practice. Proposed extraction techniques using destructive methods include electrocautery, electrodesiccation, and laser therapy, and manual methods include nicking the surface of the lesion with a scalpel blade, needle, or stylet and then applying tangential pressure with a curette, comedone extractor, paper clip, cotton-tipped applicator, tongue blade, or hypodermic needle.1-4 Topical retinoids have been proposed as treatment of multiple milia.10 Many of these techniques do not use equipment common to clinical practice, or they fail to stabilize milia in sensitive areas, which makes extraction challenging. We describe a case with a new manual technique that successfully extracts milia in an efficient and safe manner.
- Parlette HL III. Management of cutaneous cysts. In: Wheeland RG, ed. Cutaneous Surgery. WB Saunders; 1994:651-652.
- Berk DR, Bayliss SJ. Milia: a review and classification. J Am Acad Dermatol. 2008;59:1050-1063.
- George DE, Wasko CA, Hsu S. Surgical pearl: evacuation of milia with a paper clip. J Am Acad Dermatol. 2006;54:326.
- Thami GP, Kaur S, Kanwar AJ. Surgical pearl: enucleation of milia with a disposable hypodermic needle. J Am Acad Dermatol. 2002;47:602-603.
- Goeteyn M, Geerts ML, Kint A, et al. The Bazex-Dupré-Christol syndrome. Arch Dermatol. 1994;130:337-342.
- Michaëlsson G, Olsson E, Westermark P. The Rombo syndrome: a familial disorder with vermiculate atrophoderma, milia, hypotrichosis, trichoepitheliomas, basal cell carcinomas and peripheral vasodilation with cyanosis. Acta Derm Venereol. 1981;61:497-503.
- Gurrieri F, Franco B, Toriello H, et al. Oral-facial-digital syndromes: review and diagnostic guidelines. Am J Med Genet A. 2007;143A:3314-3323.
- Zlotogorski A, Panteleyev AA, Aita VM, et al. Clinical and molecular diagnostic criteria of congenital atrichia with papular lesions. J Invest Dermatol. 2001;117:1662-1665.
- Paller AS, Moore JA, Scher R. Pachyonychia congenita tarda. alate-onset form of pachyonychia congenita. Arch Dermatol. 1991;127:701-703.
- Connelly T. Eruptive milia and rapid response to topical tretinoin. Arch Dermatol. 2008;144:816-817.
- Parlette HL III. Management of cutaneous cysts. In: Wheeland RG, ed. Cutaneous Surgery. WB Saunders; 1994:651-652.
- Berk DR, Bayliss SJ. Milia: a review and classification. J Am Acad Dermatol. 2008;59:1050-1063.
- George DE, Wasko CA, Hsu S. Surgical pearl: evacuation of milia with a paper clip. J Am Acad Dermatol. 2006;54:326.
- Thami GP, Kaur S, Kanwar AJ. Surgical pearl: enucleation of milia with a disposable hypodermic needle. J Am Acad Dermatol. 2002;47:602-603.
- Goeteyn M, Geerts ML, Kint A, et al. The Bazex-Dupré-Christol syndrome. Arch Dermatol. 1994;130:337-342.
- Michaëlsson G, Olsson E, Westermark P. The Rombo syndrome: a familial disorder with vermiculate atrophoderma, milia, hypotrichosis, trichoepitheliomas, basal cell carcinomas and peripheral vasodilation with cyanosis. Acta Derm Venereol. 1981;61:497-503.
- Gurrieri F, Franco B, Toriello H, et al. Oral-facial-digital syndromes: review and diagnostic guidelines. Am J Med Genet A. 2007;143A:3314-3323.
- Zlotogorski A, Panteleyev AA, Aita VM, et al. Clinical and molecular diagnostic criteria of congenital atrichia with papular lesions. J Invest Dermatol. 2001;117:1662-1665.
- Paller AS, Moore JA, Scher R. Pachyonychia congenita tarda. alate-onset form of pachyonychia congenita. Arch Dermatol. 1991;127:701-703.
- Connelly T. Eruptive milia and rapid response to topical tretinoin. Arch Dermatol. 2008;144:816-817.
Practice Points
- Milia are common benign lesions that are cosmetically undesirable to some patients.
- Although some methods of milia removal can be painful, removal with forceps is quick and effective.
Expect Ellacor’s applications to be wide-ranging, expert predicts
SAN DIEGO – When the Food and Drug Administration gave the nod to a first-in-class tissue removal device in July of 2021, clearance was limited to the treatment of moderate to severe wrinkles in the mid to lower face.
Jill S. Waibel, MD, a dermatologist with the Miami Dermatology and Laser Institute, predicted at the annual conference of the American Society for Laser Medicine and Surgery. “I’m using it in my practice more for laxity and jowls,” she said. Eventually, “I think it’s going to be preventative for 30- to 50-year-olds but that hasn’t been studied. I think it’s going to have a pre- and postrhytidectomy role, so I think the plastic surgeons are going to love this in their practice.”
Developed by Cytrellis, and based on research conducted by William G. Austen Jr., MD, chief of plastic and reconstructive surgery at Massachusetts General Hospital (MGH), Boston, and R. Rox Anderson, MD, director of the Wellman Center for Photomedicine at MGH, the company’s scientific founders, the device uses hollow needles contained in a handpiece to create thousands of microexcisions to physically remove small cores of skin – a process known as microcoring. Dr. Austin and Dr. Anderson were the senior authors, respectively, of seminal trials of the device in swine, published in 2013 and 2015.
This can result in immediate physical hole closures (mechanical closure), which may lead to skin tightening.
“We’re removing dermis and epidermis,” said Dr. Waibel, who noted that the technology has been studied mostly for skin laxity and rhytids. “There are no other devices that are doing this.”
The immediate closure of tiny holes in the skin results in a quantitative and directional reduction in the treated area of skin, she said, which leads to wrinkle improvement, tightening, and smoothing of lax skin. The device contains three 22-guage needles that are less than 500 micrometers in diameter. “Based on optical coherence tomography work we did, these channels of treated skin stay open for about 1.5 minutes,” she added, noting that the tunable depth of the device ranges from 0 to 4 mm. “I tend to treat only with the 4-mm depth,” said Dr. Waibel, who is subsection chief of dermatology at Baptist Hospital of Miami.
The device features a disposable tip that can remove up to 24,000 cores of skin, and the amount of skin removed in a treated area can be 1%, 3%, 5%, 7%, and 8%. “The more cores you do, the more wrinkle improvement we saw in pivotal trials,” she said. “A minimal core count of 12,000 per treatment is recommended for the mid and lower face. Interestingly, higher core counts do not result in more patient downtime.” In her office, she said that the treatment takes about 20 minutes. Recommended postoperative care involves application of petrolatum or Aquaphor over the treated area for 24 hours, or until the holes have closed. “There is very low postoperative downtime,” she noted.
According to pivotal clinical data from Cytrellis on 51 patients treated with Ellacor, patients experienced a mean 1.3-grade improvement on the Lemperle Rating Scale, 86% said that they were satisfied with the procedure, and investigators rated their Global Aesthetic Improvement as 90%.
To date, Dr. Waibel and her colleagues in Miami have treated 102 patients with Ellacor, mostly for wrinkles and skin laxity. In these patients, the minimal downtime experienced was 3.8 days, 75% of patients did not miss any work, and 46% did not miss any social activities. The worst part for patients is the preprocedure numbing, she said. “We do lidocaine injections. Some people do nerve blocks. Once you do the lidocaine injection, the average pain is about 0.36 on a scale of 1-10 during treatment and 0 for all subsequent time points.”
At the meeting, she presented a set of before and after photos that showed improvement of moderate facial wrinkles in a female patient 90 days after one treatment with Ellacor, which removed about 5% of skin in the area of the jowls. “It’s pretty incredible,” Dr. Waibel remarked.
“I don’t have anything in my practice that can help with that kind of laxity other than sending them to a plastic surgeon, and I have about 80 devices.”
At the 2019 meeting of the American Society for Dermatologic Surgery, Dr. Waibel and Roy G. Geronemus, MD, director of the Laser & Skin Surgery Center of New York, presented a small pilot study on the successful use of Ellacor for acne scars and striae. Dr. Waibel said that she and her colleagues in Miami have been using the device to treat skin laxity in several anatomical areas, including the neck, nose, inner thigh, above the knee, elbow, and the axillary region. They have used the device to treat tattoos, rhytidectomy scars, abdominal striae, acne and surgical scars, and idiopathic guttate hypomelanosis, she added.
“We do a lot of combinations with other devices on the same day, and I think this list will increase over the next few years,” she said. “Probably my favorite use in the past 5 months has been doing microcoring and, separated by a month, doing resurfacing.”
Clinical trials of Ellacor were conducted in patients with types I-IV skin, but she has treated several patients with types V-VI skin “with absolutely no safety issues,” which includes treatment of epidermal nevi.
Which variables are the most important for patient selection and procedural success remain unclear, she continued, including patient age, elastic recoil, body mass index, history of a prior procedure (such as radiofrequency or ultrasound), the amount of laxity and rhytids, and overall health, which have not been studied, Dr. Waibel said.
“We have patients that don’t have the same response as others. For the modest improvement seen in some patients, is that their elastic recoil or are we choosing the wrong patients? Do they need more treatments? We are also still learning about the ideal treatment for scars and other indications.”
The device is expected to launch in fourth quarter of 2022.
Dr. Waibel disclosed that she is an advisory board member for Cytrellis. She has conducted clinical trials for and is a consultant to many pharmaceutical and device companies.
SAN DIEGO – When the Food and Drug Administration gave the nod to a first-in-class tissue removal device in July of 2021, clearance was limited to the treatment of moderate to severe wrinkles in the mid to lower face.
Jill S. Waibel, MD, a dermatologist with the Miami Dermatology and Laser Institute, predicted at the annual conference of the American Society for Laser Medicine and Surgery. “I’m using it in my practice more for laxity and jowls,” she said. Eventually, “I think it’s going to be preventative for 30- to 50-year-olds but that hasn’t been studied. I think it’s going to have a pre- and postrhytidectomy role, so I think the plastic surgeons are going to love this in their practice.”
Developed by Cytrellis, and based on research conducted by William G. Austen Jr., MD, chief of plastic and reconstructive surgery at Massachusetts General Hospital (MGH), Boston, and R. Rox Anderson, MD, director of the Wellman Center for Photomedicine at MGH, the company’s scientific founders, the device uses hollow needles contained in a handpiece to create thousands of microexcisions to physically remove small cores of skin – a process known as microcoring. Dr. Austin and Dr. Anderson were the senior authors, respectively, of seminal trials of the device in swine, published in 2013 and 2015.
This can result in immediate physical hole closures (mechanical closure), which may lead to skin tightening.
“We’re removing dermis and epidermis,” said Dr. Waibel, who noted that the technology has been studied mostly for skin laxity and rhytids. “There are no other devices that are doing this.”
The immediate closure of tiny holes in the skin results in a quantitative and directional reduction in the treated area of skin, she said, which leads to wrinkle improvement, tightening, and smoothing of lax skin. The device contains three 22-guage needles that are less than 500 micrometers in diameter. “Based on optical coherence tomography work we did, these channels of treated skin stay open for about 1.5 minutes,” she added, noting that the tunable depth of the device ranges from 0 to 4 mm. “I tend to treat only with the 4-mm depth,” said Dr. Waibel, who is subsection chief of dermatology at Baptist Hospital of Miami.
The device features a disposable tip that can remove up to 24,000 cores of skin, and the amount of skin removed in a treated area can be 1%, 3%, 5%, 7%, and 8%. “The more cores you do, the more wrinkle improvement we saw in pivotal trials,” she said. “A minimal core count of 12,000 per treatment is recommended for the mid and lower face. Interestingly, higher core counts do not result in more patient downtime.” In her office, she said that the treatment takes about 20 minutes. Recommended postoperative care involves application of petrolatum or Aquaphor over the treated area for 24 hours, or until the holes have closed. “There is very low postoperative downtime,” she noted.
According to pivotal clinical data from Cytrellis on 51 patients treated with Ellacor, patients experienced a mean 1.3-grade improvement on the Lemperle Rating Scale, 86% said that they were satisfied with the procedure, and investigators rated their Global Aesthetic Improvement as 90%.
To date, Dr. Waibel and her colleagues in Miami have treated 102 patients with Ellacor, mostly for wrinkles and skin laxity. In these patients, the minimal downtime experienced was 3.8 days, 75% of patients did not miss any work, and 46% did not miss any social activities. The worst part for patients is the preprocedure numbing, she said. “We do lidocaine injections. Some people do nerve blocks. Once you do the lidocaine injection, the average pain is about 0.36 on a scale of 1-10 during treatment and 0 for all subsequent time points.”
At the meeting, she presented a set of before and after photos that showed improvement of moderate facial wrinkles in a female patient 90 days after one treatment with Ellacor, which removed about 5% of skin in the area of the jowls. “It’s pretty incredible,” Dr. Waibel remarked.
“I don’t have anything in my practice that can help with that kind of laxity other than sending them to a plastic surgeon, and I have about 80 devices.”
At the 2019 meeting of the American Society for Dermatologic Surgery, Dr. Waibel and Roy G. Geronemus, MD, director of the Laser & Skin Surgery Center of New York, presented a small pilot study on the successful use of Ellacor for acne scars and striae. Dr. Waibel said that she and her colleagues in Miami have been using the device to treat skin laxity in several anatomical areas, including the neck, nose, inner thigh, above the knee, elbow, and the axillary region. They have used the device to treat tattoos, rhytidectomy scars, abdominal striae, acne and surgical scars, and idiopathic guttate hypomelanosis, she added.
“We do a lot of combinations with other devices on the same day, and I think this list will increase over the next few years,” she said. “Probably my favorite use in the past 5 months has been doing microcoring and, separated by a month, doing resurfacing.”
Clinical trials of Ellacor were conducted in patients with types I-IV skin, but she has treated several patients with types V-VI skin “with absolutely no safety issues,” which includes treatment of epidermal nevi.
Which variables are the most important for patient selection and procedural success remain unclear, she continued, including patient age, elastic recoil, body mass index, history of a prior procedure (such as radiofrequency or ultrasound), the amount of laxity and rhytids, and overall health, which have not been studied, Dr. Waibel said.
“We have patients that don’t have the same response as others. For the modest improvement seen in some patients, is that their elastic recoil or are we choosing the wrong patients? Do they need more treatments? We are also still learning about the ideal treatment for scars and other indications.”
The device is expected to launch in fourth quarter of 2022.
Dr. Waibel disclosed that she is an advisory board member for Cytrellis. She has conducted clinical trials for and is a consultant to many pharmaceutical and device companies.
SAN DIEGO – When the Food and Drug Administration gave the nod to a first-in-class tissue removal device in July of 2021, clearance was limited to the treatment of moderate to severe wrinkles in the mid to lower face.
Jill S. Waibel, MD, a dermatologist with the Miami Dermatology and Laser Institute, predicted at the annual conference of the American Society for Laser Medicine and Surgery. “I’m using it in my practice more for laxity and jowls,” she said. Eventually, “I think it’s going to be preventative for 30- to 50-year-olds but that hasn’t been studied. I think it’s going to have a pre- and postrhytidectomy role, so I think the plastic surgeons are going to love this in their practice.”
Developed by Cytrellis, and based on research conducted by William G. Austen Jr., MD, chief of plastic and reconstructive surgery at Massachusetts General Hospital (MGH), Boston, and R. Rox Anderson, MD, director of the Wellman Center for Photomedicine at MGH, the company’s scientific founders, the device uses hollow needles contained in a handpiece to create thousands of microexcisions to physically remove small cores of skin – a process known as microcoring. Dr. Austin and Dr. Anderson were the senior authors, respectively, of seminal trials of the device in swine, published in 2013 and 2015.
This can result in immediate physical hole closures (mechanical closure), which may lead to skin tightening.
“We’re removing dermis and epidermis,” said Dr. Waibel, who noted that the technology has been studied mostly for skin laxity and rhytids. “There are no other devices that are doing this.”
The immediate closure of tiny holes in the skin results in a quantitative and directional reduction in the treated area of skin, she said, which leads to wrinkle improvement, tightening, and smoothing of lax skin. The device contains three 22-guage needles that are less than 500 micrometers in diameter. “Based on optical coherence tomography work we did, these channels of treated skin stay open for about 1.5 minutes,” she added, noting that the tunable depth of the device ranges from 0 to 4 mm. “I tend to treat only with the 4-mm depth,” said Dr. Waibel, who is subsection chief of dermatology at Baptist Hospital of Miami.
The device features a disposable tip that can remove up to 24,000 cores of skin, and the amount of skin removed in a treated area can be 1%, 3%, 5%, 7%, and 8%. “The more cores you do, the more wrinkle improvement we saw in pivotal trials,” she said. “A minimal core count of 12,000 per treatment is recommended for the mid and lower face. Interestingly, higher core counts do not result in more patient downtime.” In her office, she said that the treatment takes about 20 minutes. Recommended postoperative care involves application of petrolatum or Aquaphor over the treated area for 24 hours, or until the holes have closed. “There is very low postoperative downtime,” she noted.
According to pivotal clinical data from Cytrellis on 51 patients treated with Ellacor, patients experienced a mean 1.3-grade improvement on the Lemperle Rating Scale, 86% said that they were satisfied with the procedure, and investigators rated their Global Aesthetic Improvement as 90%.
To date, Dr. Waibel and her colleagues in Miami have treated 102 patients with Ellacor, mostly for wrinkles and skin laxity. In these patients, the minimal downtime experienced was 3.8 days, 75% of patients did not miss any work, and 46% did not miss any social activities. The worst part for patients is the preprocedure numbing, she said. “We do lidocaine injections. Some people do nerve blocks. Once you do the lidocaine injection, the average pain is about 0.36 on a scale of 1-10 during treatment and 0 for all subsequent time points.”
At the meeting, she presented a set of before and after photos that showed improvement of moderate facial wrinkles in a female patient 90 days after one treatment with Ellacor, which removed about 5% of skin in the area of the jowls. “It’s pretty incredible,” Dr. Waibel remarked.
“I don’t have anything in my practice that can help with that kind of laxity other than sending them to a plastic surgeon, and I have about 80 devices.”
At the 2019 meeting of the American Society for Dermatologic Surgery, Dr. Waibel and Roy G. Geronemus, MD, director of the Laser & Skin Surgery Center of New York, presented a small pilot study on the successful use of Ellacor for acne scars and striae. Dr. Waibel said that she and her colleagues in Miami have been using the device to treat skin laxity in several anatomical areas, including the neck, nose, inner thigh, above the knee, elbow, and the axillary region. They have used the device to treat tattoos, rhytidectomy scars, abdominal striae, acne and surgical scars, and idiopathic guttate hypomelanosis, she added.
“We do a lot of combinations with other devices on the same day, and I think this list will increase over the next few years,” she said. “Probably my favorite use in the past 5 months has been doing microcoring and, separated by a month, doing resurfacing.”
Clinical trials of Ellacor were conducted in patients with types I-IV skin, but she has treated several patients with types V-VI skin “with absolutely no safety issues,” which includes treatment of epidermal nevi.
Which variables are the most important for patient selection and procedural success remain unclear, she continued, including patient age, elastic recoil, body mass index, history of a prior procedure (such as radiofrequency or ultrasound), the amount of laxity and rhytids, and overall health, which have not been studied, Dr. Waibel said.
“We have patients that don’t have the same response as others. For the modest improvement seen in some patients, is that their elastic recoil or are we choosing the wrong patients? Do they need more treatments? We are also still learning about the ideal treatment for scars and other indications.”
The device is expected to launch in fourth quarter of 2022.
Dr. Waibel disclosed that she is an advisory board member for Cytrellis. She has conducted clinical trials for and is a consultant to many pharmaceutical and device companies.
AT ASLMS 2022
20th anniversary and history of cosmetic botulinum toxin type A
The timeline of botulinum toxin discovery began with deadly outbreaks related to contaminated food across Europe in the late 1700s, the largest of which occurred in 1793 in Wildebrad, in southern Germany. In 1811, “prussic acid” was named as the culprit in what was referred to as sausage poisoning. Between 1817 and 1822, German physician Justinus Kerner noted that the active substance interrupted signals from motor nerves to muscles, but spared sensory and cognitive abilities, accurately describing botulism. He hypothesized that this substance could possibly be used as treatment for medical conditions when ingested orally. It wasn’t until 1895 that microbiologist Emile Pierre Van Ermengem, a professor of bacteriology in Belgium, identified the bacterium responsible as Bacillus botulinus, later renamed C. botulinum.
In 1905, it was discovered that C. botulinum produced a substance that affected neurotransmitter function, and between 1895 and 1915, seven toxin serotypes were recognized. In 1928, Herman Sommer, PhD, at the Hooper Foundation, at the University of California, San Francisco, isolated the most potent serotype: botulinum toxin type A (BoNT-A).
In 1946, Carl Lamanna and James Duff developed concentration and crystallization techniques that were subsequently used by Edward Schantz, PhD, at Fort Detrick, Md., for a possible biologic weapon. In 1972, Dr. Schantz took his research to the University of Wisconsin, where he produced a large batch of BoNT-A that remained in clinical use until December 1997.
In the late 1960s and early 1970s, an ophthalmologist in San Francisco, Alan Scott, MD, began animal studies with BoNT-A supplied by Dr. Schantz, as a possible treatment for strabismus, publishing his first report of BoNT-A in primates in 1973. In 1978, the Food and Drug Administration granted approval to begin testing small amounts of the toxin in human volunteers. In 1980, a landmark paper was published demonstrating that BoNT-A corrects gaze misalignment in humans. By 1989, it was approved as Oculinum by the FDA for the treatment of strabismus and blepharospasm.
Keen clinical observation and a serendipitous discovery led to botulinum toxin’s first uses for cosmetic purposes. In the mid-1980s, Jean Carruthers, MD, an ophthalmologist in Vancouver, noted an unexpected concomitant improvement of glabellar rhytids in a patient treated with BoNT for blepharospasm. The result of the treatment was a more serene, untroubled expression. Dr. Carruthers discussed the observation with her dermatologist spouse, Alastair Carruthers, MD, who was attempting to use soft tissue–augmenting agents available at the time to soften forehead wrinkles. Intrigued by the possibilities, the Carruthers subsequently injected a small amount of BoNT-A between the eyebrows of their assistant, Cathy Bickerton Swann, also now known as “patient zero” and awaited the results.
Seventeen additional patients followed, aged 34-51, who collectively, would become part of the first report on the efficacy of BoNT-A for cosmetic use – for the treatment of glabellar frown lines – published in 1992.
Between 1992 and 1997, the popularity of off-label use of BoNT-A grew so rapidly that Allergan’s supply temporarily ran out. By 2002, safety and efficacy profiles of use in medical conditions such as strabismus, blepharospasm, hemifacial spasm, cervical dystonia, cerebral palsy, poststroke spasticity, hyperhidrosis, headache, and back pain had been well-established, facilitating the comfort and use for cosmetic purposes.
By 2002, open-label studies of more than 800 patients confirmed the efficacy and safety of BoNT for the treatment of dynamic facial rhytids. And in April 2002, the FDA granted approval of BoNT for the nonsurgical reduction of glabellar rhytids. The rest, some would say, is history. On this 20th-year anniversary of the approval of botulinum toxin for cosmetic use, special recognition is given here for the physicians and scientists who were astute enough to make this discovery, as botulinum toxin use remains one of the most popular and effective nonsurgical cosmetic procedures today.
Dr. Wesley and Dr. Lily Talakoub are cocontributors to this column. Dr. Wesley practices dermatology in Beverly Hills, Calif. Dr. Talakoub is in private practice in McLean, Va. This month’s column is by Dr. Wesley. Write to them at dermnews@mdedge.com. Dr. Wesley disclosed that she has been a clinical investigator and consultant for Botox manufacturer Allergan in the past, and manufacturers of other brands of botulinum toxins available for cosmetic use; Dysport (Galderma), Xeomin (Merz), and Jeuveau (Evolus). Dr. Talakoub had no disclosures.
Reference
“Botulinum Toxin: Procedures in Cosmetic Dermatology Series 3rd Edition” (Philadelphia: Saunders Elsevier, 2013)
The timeline of botulinum toxin discovery began with deadly outbreaks related to contaminated food across Europe in the late 1700s, the largest of which occurred in 1793 in Wildebrad, in southern Germany. In 1811, “prussic acid” was named as the culprit in what was referred to as sausage poisoning. Between 1817 and 1822, German physician Justinus Kerner noted that the active substance interrupted signals from motor nerves to muscles, but spared sensory and cognitive abilities, accurately describing botulism. He hypothesized that this substance could possibly be used as treatment for medical conditions when ingested orally. It wasn’t until 1895 that microbiologist Emile Pierre Van Ermengem, a professor of bacteriology in Belgium, identified the bacterium responsible as Bacillus botulinus, later renamed C. botulinum.
In 1905, it was discovered that C. botulinum produced a substance that affected neurotransmitter function, and between 1895 and 1915, seven toxin serotypes were recognized. In 1928, Herman Sommer, PhD, at the Hooper Foundation, at the University of California, San Francisco, isolated the most potent serotype: botulinum toxin type A (BoNT-A).
In 1946, Carl Lamanna and James Duff developed concentration and crystallization techniques that were subsequently used by Edward Schantz, PhD, at Fort Detrick, Md., for a possible biologic weapon. In 1972, Dr. Schantz took his research to the University of Wisconsin, where he produced a large batch of BoNT-A that remained in clinical use until December 1997.
In the late 1960s and early 1970s, an ophthalmologist in San Francisco, Alan Scott, MD, began animal studies with BoNT-A supplied by Dr. Schantz, as a possible treatment for strabismus, publishing his first report of BoNT-A in primates in 1973. In 1978, the Food and Drug Administration granted approval to begin testing small amounts of the toxin in human volunteers. In 1980, a landmark paper was published demonstrating that BoNT-A corrects gaze misalignment in humans. By 1989, it was approved as Oculinum by the FDA for the treatment of strabismus and blepharospasm.
Keen clinical observation and a serendipitous discovery led to botulinum toxin’s first uses for cosmetic purposes. In the mid-1980s, Jean Carruthers, MD, an ophthalmologist in Vancouver, noted an unexpected concomitant improvement of glabellar rhytids in a patient treated with BoNT for blepharospasm. The result of the treatment was a more serene, untroubled expression. Dr. Carruthers discussed the observation with her dermatologist spouse, Alastair Carruthers, MD, who was attempting to use soft tissue–augmenting agents available at the time to soften forehead wrinkles. Intrigued by the possibilities, the Carruthers subsequently injected a small amount of BoNT-A between the eyebrows of their assistant, Cathy Bickerton Swann, also now known as “patient zero” and awaited the results.
Seventeen additional patients followed, aged 34-51, who collectively, would become part of the first report on the efficacy of BoNT-A for cosmetic use – for the treatment of glabellar frown lines – published in 1992.
Between 1992 and 1997, the popularity of off-label use of BoNT-A grew so rapidly that Allergan’s supply temporarily ran out. By 2002, safety and efficacy profiles of use in medical conditions such as strabismus, blepharospasm, hemifacial spasm, cervical dystonia, cerebral palsy, poststroke spasticity, hyperhidrosis, headache, and back pain had been well-established, facilitating the comfort and use for cosmetic purposes.
By 2002, open-label studies of more than 800 patients confirmed the efficacy and safety of BoNT for the treatment of dynamic facial rhytids. And in April 2002, the FDA granted approval of BoNT for the nonsurgical reduction of glabellar rhytids. The rest, some would say, is history. On this 20th-year anniversary of the approval of botulinum toxin for cosmetic use, special recognition is given here for the physicians and scientists who were astute enough to make this discovery, as botulinum toxin use remains one of the most popular and effective nonsurgical cosmetic procedures today.
Dr. Wesley and Dr. Lily Talakoub are cocontributors to this column. Dr. Wesley practices dermatology in Beverly Hills, Calif. Dr. Talakoub is in private practice in McLean, Va. This month’s column is by Dr. Wesley. Write to them at dermnews@mdedge.com. Dr. Wesley disclosed that she has been a clinical investigator and consultant for Botox manufacturer Allergan in the past, and manufacturers of other brands of botulinum toxins available for cosmetic use; Dysport (Galderma), Xeomin (Merz), and Jeuveau (Evolus). Dr. Talakoub had no disclosures.
Reference
“Botulinum Toxin: Procedures in Cosmetic Dermatology Series 3rd Edition” (Philadelphia: Saunders Elsevier, 2013)
The timeline of botulinum toxin discovery began with deadly outbreaks related to contaminated food across Europe in the late 1700s, the largest of which occurred in 1793 in Wildebrad, in southern Germany. In 1811, “prussic acid” was named as the culprit in what was referred to as sausage poisoning. Between 1817 and 1822, German physician Justinus Kerner noted that the active substance interrupted signals from motor nerves to muscles, but spared sensory and cognitive abilities, accurately describing botulism. He hypothesized that this substance could possibly be used as treatment for medical conditions when ingested orally. It wasn’t until 1895 that microbiologist Emile Pierre Van Ermengem, a professor of bacteriology in Belgium, identified the bacterium responsible as Bacillus botulinus, later renamed C. botulinum.
In 1905, it was discovered that C. botulinum produced a substance that affected neurotransmitter function, and between 1895 and 1915, seven toxin serotypes were recognized. In 1928, Herman Sommer, PhD, at the Hooper Foundation, at the University of California, San Francisco, isolated the most potent serotype: botulinum toxin type A (BoNT-A).
In 1946, Carl Lamanna and James Duff developed concentration and crystallization techniques that were subsequently used by Edward Schantz, PhD, at Fort Detrick, Md., for a possible biologic weapon. In 1972, Dr. Schantz took his research to the University of Wisconsin, where he produced a large batch of BoNT-A that remained in clinical use until December 1997.
In the late 1960s and early 1970s, an ophthalmologist in San Francisco, Alan Scott, MD, began animal studies with BoNT-A supplied by Dr. Schantz, as a possible treatment for strabismus, publishing his first report of BoNT-A in primates in 1973. In 1978, the Food and Drug Administration granted approval to begin testing small amounts of the toxin in human volunteers. In 1980, a landmark paper was published demonstrating that BoNT-A corrects gaze misalignment in humans. By 1989, it was approved as Oculinum by the FDA for the treatment of strabismus and blepharospasm.
Keen clinical observation and a serendipitous discovery led to botulinum toxin’s first uses for cosmetic purposes. In the mid-1980s, Jean Carruthers, MD, an ophthalmologist in Vancouver, noted an unexpected concomitant improvement of glabellar rhytids in a patient treated with BoNT for blepharospasm. The result of the treatment was a more serene, untroubled expression. Dr. Carruthers discussed the observation with her dermatologist spouse, Alastair Carruthers, MD, who was attempting to use soft tissue–augmenting agents available at the time to soften forehead wrinkles. Intrigued by the possibilities, the Carruthers subsequently injected a small amount of BoNT-A between the eyebrows of their assistant, Cathy Bickerton Swann, also now known as “patient zero” and awaited the results.
Seventeen additional patients followed, aged 34-51, who collectively, would become part of the first report on the efficacy of BoNT-A for cosmetic use – for the treatment of glabellar frown lines – published in 1992.
Between 1992 and 1997, the popularity of off-label use of BoNT-A grew so rapidly that Allergan’s supply temporarily ran out. By 2002, safety and efficacy profiles of use in medical conditions such as strabismus, blepharospasm, hemifacial spasm, cervical dystonia, cerebral palsy, poststroke spasticity, hyperhidrosis, headache, and back pain had been well-established, facilitating the comfort and use for cosmetic purposes.
By 2002, open-label studies of more than 800 patients confirmed the efficacy and safety of BoNT for the treatment of dynamic facial rhytids. And in April 2002, the FDA granted approval of BoNT for the nonsurgical reduction of glabellar rhytids. The rest, some would say, is history. On this 20th-year anniversary of the approval of botulinum toxin for cosmetic use, special recognition is given here for the physicians and scientists who were astute enough to make this discovery, as botulinum toxin use remains one of the most popular and effective nonsurgical cosmetic procedures today.
Dr. Wesley and Dr. Lily Talakoub are cocontributors to this column. Dr. Wesley practices dermatology in Beverly Hills, Calif. Dr. Talakoub is in private practice in McLean, Va. This month’s column is by Dr. Wesley. Write to them at dermnews@mdedge.com. Dr. Wesley disclosed that she has been a clinical investigator and consultant for Botox manufacturer Allergan in the past, and manufacturers of other brands of botulinum toxins available for cosmetic use; Dysport (Galderma), Xeomin (Merz), and Jeuveau (Evolus). Dr. Talakoub had no disclosures.
Reference
“Botulinum Toxin: Procedures in Cosmetic Dermatology Series 3rd Edition” (Philadelphia: Saunders Elsevier, 2013)
Is benzophenone safe in skin care? Part 1: Risks to humans
Benzophenones are a family of compounds that include dixoxybenzone, sulisobenzone, and benzophenone-3, or oxybenzone. These . Benzophenones (BPs) act as penetration enhancers, as they modify the structure of the skin and facilitate the absorption of other chemical ingredients into the body. The best known uses of these compounds are as perfume fixatives and sunscreen agents.
Sunscreens and benzophenones
BP-2, -3 and -4 are used as sunscreens but have many downsides. They are well known photoallergens, are toxic to aquatic animals (especially BP-3), and are found in urine. BP-2 has weak estrogenic effects, and some studies suggest that it decreases fertility in men. BP-4 can increase absorption of pesticides. BP-3 is banned in Hawaii because of the risk to coral and is the most worrisome.
In particular, BP-3 is known to protect skin and hair from UV radiation-induced harm.1 Unfortunately, BPs are also associated with photocontact allergies, hypersensitivity, hives, contact urticaria, anaphylaxis, hormone disruption, and DNA damage.2,3 BP-3 has also been implicated as an environmental contaminant. This column will focus on recent studies pertaining to effects on humans, primarily, and on the role of BPs in sunscreen agents.
Effects of BPs in animals
A recent study on the cytotoxicity of BP-3 against thymocytes in rats revealed that cell mortality increased significantly after 3 hours of exposure to 300 μM BP-3, but the membrane potential of thymocytes was unchanged by BP-3 exposure. In a concentration-dependent fashion, intracellular Zn2+ levels increased significantly after administration of at least 30 μM BP-3. The investigators concluded that the cytotoxicity engendered by BP-3 could be the result of oxidative stress linked to elevated intracellular Zn2+ levels.1
Effects of BPs in humans and systemic absorption
In multiple studies, exposure to BP-3, as well as to octinoxate, has been linked to endocrine and hormonal disruptions in humans and animals.4,5 Motivated by several notable observations (global increase in the use of sunscreens with UV filters; rapid rise in malignant melanoma, against which sunscreens should protect; increase in reported experimental findings of UV filters acting as endocrine disruptors), Krause et al. in 2012 reviewed animal and human data on the UV filters BP-3, 3-benzylidene camphor (3-BC), 3-(4-methyl-benzylidene) camphor (4-MBC), 2-ethylhexyl 4-methoxy cinnamate (OMC), homosalate (HMS), 2-ethylhexyl 4-dimethylaminobenzoate, and 4-aminobenzoic acid (PABA). Importantly, BP-3 was present in 96% of human urine samples in the United States, and various filters were found in 85% of the human breast milk samples in Switzerland.6
A 2019 analysis by Wang and Ganley reported that systemic absorption of the active sunscreen ingredient BP-3 can be substantial, justifying the assessment and understanding of systemic exposure to characterize the risks of long-term usage.7
Between January and February 2019, Matta et al. conducted a randomized clinical trial with 48 healthy participants to evaluate the systemic absorption and pharmacokinetics of six active ingredients in four sunscreen formulations, including avobenzone and BP-3. The researchers found that all ingredients were systemically absorbed, with plasma concentrations exceeding the Food and Drug Administration threshold for considering the waiving of further safety studies. They concluded that these results did not warrant discontinuing the use of the tested sunscreen ingredients.8 Yeager and Lim add that, while BP-3 has been incorporated into sunscreen formulations for sale in the United States since 1978, there have been no reports of adverse systemic reactions in human beings.3
However, topical reactions have elicited a different assessment. That is, in 2014, the American Contact Dermatitis Society labeled BPs the Contact Allergen of the Year, as they were identified as the most common source of photoallergic and contact allergic reactions of all UV filters.3,9
Risks of BPs in sunscreens and other skincare products
In 2015, Amar et al. investigated the photogenotoxicity and apoptotic effects in human keratinocytes (HaCaT cells) of BP-1, which is used as a UV blocker in sunscreens. They found that BP-1, when exposed to UV radiation, photosensitized cells and yielded intracellular reactive oxygen species. Significant reductions in cell viability were also seen with exposure to sunlight, UVA, and UVB. The researchers also confirmed genotoxic activity, with BP-1 augmenting lipid peroxidation and upregulating apoptotic proteins. They concluded that sunscreen users should be advised to avoid products that contain BP-1.10
In 2019, Amar et al. evaluated the effects of BPs on the differential expression of proteins in HaCaT cells exposed to UVA. Their findings indicated the expression of novel proteins that helped to initiate or promote apoptosis. They concluded that, because of the predilection to render such effects in human skin keratinocytes, consumers should avoid the use of sunscreens that contain BPs as UV blocking ingredients.11
Still widely used as an effective filter against UVA2 and UVB, BP-3 was believed to be present in two thirds of nonmineral sunscreens in the United States in 2018.3,12
Notably, BP-1 and BP-3 were found in small proportions (3.7% and 4.9%, respectively) among a total of 283 products culled from various stores in Lecce, Italy, in a survey of the potentially dangerous chemicals found in rinse-off, leave-on, and makeup products in 2019.13 The authors added that the International Agency for Research on Cancer, in 2010, classified BP as potentially carcinogenic to humans (2B group).13,14
Promising use of nanocapsules
The widespread concern about the phototoxicity of BP has prompted some interesting research into workarounds. Specifically, in 2019, Barbosa et al. reported on the creation of a new sunscreen formulation using polymeric nanocapsules loading BP-3. The nanocapsules are made of poly(ε-caprolactone) carrot oil and Pluronic F68 (nonionic surfactant used in suspension cultures), and the BP-3–loaded capsules were found to be noncytotoxic in L929 fibroblast cell lines with a sun protection factor of 8.64. The researchers concluded that this promising nanocapsule may be an effective and safe way to use lipophilic sunscreen ingredients such as BP-3.15
Conclusion
The body of evidence is weighted against the use of BPs. Luckily, we have safe sunscreen choices that allow us to protect our skin without using these compounds.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann has written two textbooks and a New York Times Best Sellers book for consumers. Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Galderma, Revance, Evolus, and Burt’s Bees. She is the CEO of Skin Type Solutions Inc., a company that independently tests skin care products and makes recommendations to physicians on which skin care technologies are best. Write to her at dermnews@mdedge.com.
References
1. Utsunomiya H et al. Chem Biol Interact. 2019 Jan 25;298:52-6.
2. Schneider SL and Lim HW. J Am Acad Dermatol. 2019 Jan;80(1):266-71.
3. Yeager DG and Lim HW. Dermatol Clin. 2019 Apr;37(2):149-57.
4. Ramos S et al. Sci Total Environ. 2015 Sep 1;526:278-311.
5. Siller A et al. Plast Surg Nur. 2019 Oct/Dec;39(4):157-60.
6. Krause M et al. Int J Androl. 2012 Jun;35(3):424-36.
7. Wang J and Ganley CJ. Clin Pharmacol Ther. 2019 Jan;105(1):161-7.
8. Matta MK et al. JAMA. 2020 Jan 21;323(3):256-67.
9. Warshaw EM et al. Dermatitis. 2013 Jul-Aug;24(4):176-82.
10. Amar SK et al. Toxicol Lett. 2015 Dec 15;239(3):182-93.
11. Amar SK et al. Toxicol Ind Health. 2019 Jul;35(7):457-65.
12. EWG. The trouble with ingredients in sunscreens. Accessed on 4 April 2020.
13. Panico A et al. J Prev Med Hyg. 2019 Mar 29;60(1):E50-7.
14. International Agency for Research on Cancer (IARC). Benzophenone. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. WHO, IARC Press, Lyon, France. 2010;101:285-304.
15. Barbosa TC et al. Toxics. 2019 Sep 22;7(4):51.
Benzophenones are a family of compounds that include dixoxybenzone, sulisobenzone, and benzophenone-3, or oxybenzone. These . Benzophenones (BPs) act as penetration enhancers, as they modify the structure of the skin and facilitate the absorption of other chemical ingredients into the body. The best known uses of these compounds are as perfume fixatives and sunscreen agents.
Sunscreens and benzophenones
BP-2, -3 and -4 are used as sunscreens but have many downsides. They are well known photoallergens, are toxic to aquatic animals (especially BP-3), and are found in urine. BP-2 has weak estrogenic effects, and some studies suggest that it decreases fertility in men. BP-4 can increase absorption of pesticides. BP-3 is banned in Hawaii because of the risk to coral and is the most worrisome.
In particular, BP-3 is known to protect skin and hair from UV radiation-induced harm.1 Unfortunately, BPs are also associated with photocontact allergies, hypersensitivity, hives, contact urticaria, anaphylaxis, hormone disruption, and DNA damage.2,3 BP-3 has also been implicated as an environmental contaminant. This column will focus on recent studies pertaining to effects on humans, primarily, and on the role of BPs in sunscreen agents.
Effects of BPs in animals
A recent study on the cytotoxicity of BP-3 against thymocytes in rats revealed that cell mortality increased significantly after 3 hours of exposure to 300 μM BP-3, but the membrane potential of thymocytes was unchanged by BP-3 exposure. In a concentration-dependent fashion, intracellular Zn2+ levels increased significantly after administration of at least 30 μM BP-3. The investigators concluded that the cytotoxicity engendered by BP-3 could be the result of oxidative stress linked to elevated intracellular Zn2+ levels.1
Effects of BPs in humans and systemic absorption
In multiple studies, exposure to BP-3, as well as to octinoxate, has been linked to endocrine and hormonal disruptions in humans and animals.4,5 Motivated by several notable observations (global increase in the use of sunscreens with UV filters; rapid rise in malignant melanoma, against which sunscreens should protect; increase in reported experimental findings of UV filters acting as endocrine disruptors), Krause et al. in 2012 reviewed animal and human data on the UV filters BP-3, 3-benzylidene camphor (3-BC), 3-(4-methyl-benzylidene) camphor (4-MBC), 2-ethylhexyl 4-methoxy cinnamate (OMC), homosalate (HMS), 2-ethylhexyl 4-dimethylaminobenzoate, and 4-aminobenzoic acid (PABA). Importantly, BP-3 was present in 96% of human urine samples in the United States, and various filters were found in 85% of the human breast milk samples in Switzerland.6
A 2019 analysis by Wang and Ganley reported that systemic absorption of the active sunscreen ingredient BP-3 can be substantial, justifying the assessment and understanding of systemic exposure to characterize the risks of long-term usage.7
Between January and February 2019, Matta et al. conducted a randomized clinical trial with 48 healthy participants to evaluate the systemic absorption and pharmacokinetics of six active ingredients in four sunscreen formulations, including avobenzone and BP-3. The researchers found that all ingredients were systemically absorbed, with plasma concentrations exceeding the Food and Drug Administration threshold for considering the waiving of further safety studies. They concluded that these results did not warrant discontinuing the use of the tested sunscreen ingredients.8 Yeager and Lim add that, while BP-3 has been incorporated into sunscreen formulations for sale in the United States since 1978, there have been no reports of adverse systemic reactions in human beings.3
However, topical reactions have elicited a different assessment. That is, in 2014, the American Contact Dermatitis Society labeled BPs the Contact Allergen of the Year, as they were identified as the most common source of photoallergic and contact allergic reactions of all UV filters.3,9
Risks of BPs in sunscreens and other skincare products
In 2015, Amar et al. investigated the photogenotoxicity and apoptotic effects in human keratinocytes (HaCaT cells) of BP-1, which is used as a UV blocker in sunscreens. They found that BP-1, when exposed to UV radiation, photosensitized cells and yielded intracellular reactive oxygen species. Significant reductions in cell viability were also seen with exposure to sunlight, UVA, and UVB. The researchers also confirmed genotoxic activity, with BP-1 augmenting lipid peroxidation and upregulating apoptotic proteins. They concluded that sunscreen users should be advised to avoid products that contain BP-1.10
In 2019, Amar et al. evaluated the effects of BPs on the differential expression of proteins in HaCaT cells exposed to UVA. Their findings indicated the expression of novel proteins that helped to initiate or promote apoptosis. They concluded that, because of the predilection to render such effects in human skin keratinocytes, consumers should avoid the use of sunscreens that contain BPs as UV blocking ingredients.11
Still widely used as an effective filter against UVA2 and UVB, BP-3 was believed to be present in two thirds of nonmineral sunscreens in the United States in 2018.3,12
Notably, BP-1 and BP-3 were found in small proportions (3.7% and 4.9%, respectively) among a total of 283 products culled from various stores in Lecce, Italy, in a survey of the potentially dangerous chemicals found in rinse-off, leave-on, and makeup products in 2019.13 The authors added that the International Agency for Research on Cancer, in 2010, classified BP as potentially carcinogenic to humans (2B group).13,14
Promising use of nanocapsules
The widespread concern about the phototoxicity of BP has prompted some interesting research into workarounds. Specifically, in 2019, Barbosa et al. reported on the creation of a new sunscreen formulation using polymeric nanocapsules loading BP-3. The nanocapsules are made of poly(ε-caprolactone) carrot oil and Pluronic F68 (nonionic surfactant used in suspension cultures), and the BP-3–loaded capsules were found to be noncytotoxic in L929 fibroblast cell lines with a sun protection factor of 8.64. The researchers concluded that this promising nanocapsule may be an effective and safe way to use lipophilic sunscreen ingredients such as BP-3.15
Conclusion
The body of evidence is weighted against the use of BPs. Luckily, we have safe sunscreen choices that allow us to protect our skin without using these compounds.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann has written two textbooks and a New York Times Best Sellers book for consumers. Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Galderma, Revance, Evolus, and Burt’s Bees. She is the CEO of Skin Type Solutions Inc., a company that independently tests skin care products and makes recommendations to physicians on which skin care technologies are best. Write to her at dermnews@mdedge.com.
References
1. Utsunomiya H et al. Chem Biol Interact. 2019 Jan 25;298:52-6.
2. Schneider SL and Lim HW. J Am Acad Dermatol. 2019 Jan;80(1):266-71.
3. Yeager DG and Lim HW. Dermatol Clin. 2019 Apr;37(2):149-57.
4. Ramos S et al. Sci Total Environ. 2015 Sep 1;526:278-311.
5. Siller A et al. Plast Surg Nur. 2019 Oct/Dec;39(4):157-60.
6. Krause M et al. Int J Androl. 2012 Jun;35(3):424-36.
7. Wang J and Ganley CJ. Clin Pharmacol Ther. 2019 Jan;105(1):161-7.
8. Matta MK et al. JAMA. 2020 Jan 21;323(3):256-67.
9. Warshaw EM et al. Dermatitis. 2013 Jul-Aug;24(4):176-82.
10. Amar SK et al. Toxicol Lett. 2015 Dec 15;239(3):182-93.
11. Amar SK et al. Toxicol Ind Health. 2019 Jul;35(7):457-65.
12. EWG. The trouble with ingredients in sunscreens. Accessed on 4 April 2020.
13. Panico A et al. J Prev Med Hyg. 2019 Mar 29;60(1):E50-7.
14. International Agency for Research on Cancer (IARC). Benzophenone. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. WHO, IARC Press, Lyon, France. 2010;101:285-304.
15. Barbosa TC et al. Toxics. 2019 Sep 22;7(4):51.
Benzophenones are a family of compounds that include dixoxybenzone, sulisobenzone, and benzophenone-3, or oxybenzone. These . Benzophenones (BPs) act as penetration enhancers, as they modify the structure of the skin and facilitate the absorption of other chemical ingredients into the body. The best known uses of these compounds are as perfume fixatives and sunscreen agents.
Sunscreens and benzophenones
BP-2, -3 and -4 are used as sunscreens but have many downsides. They are well known photoallergens, are toxic to aquatic animals (especially BP-3), and are found in urine. BP-2 has weak estrogenic effects, and some studies suggest that it decreases fertility in men. BP-4 can increase absorption of pesticides. BP-3 is banned in Hawaii because of the risk to coral and is the most worrisome.
In particular, BP-3 is known to protect skin and hair from UV radiation-induced harm.1 Unfortunately, BPs are also associated with photocontact allergies, hypersensitivity, hives, contact urticaria, anaphylaxis, hormone disruption, and DNA damage.2,3 BP-3 has also been implicated as an environmental contaminant. This column will focus on recent studies pertaining to effects on humans, primarily, and on the role of BPs in sunscreen agents.
Effects of BPs in animals
A recent study on the cytotoxicity of BP-3 against thymocytes in rats revealed that cell mortality increased significantly after 3 hours of exposure to 300 μM BP-3, but the membrane potential of thymocytes was unchanged by BP-3 exposure. In a concentration-dependent fashion, intracellular Zn2+ levels increased significantly after administration of at least 30 μM BP-3. The investigators concluded that the cytotoxicity engendered by BP-3 could be the result of oxidative stress linked to elevated intracellular Zn2+ levels.1
Effects of BPs in humans and systemic absorption
In multiple studies, exposure to BP-3, as well as to octinoxate, has been linked to endocrine and hormonal disruptions in humans and animals.4,5 Motivated by several notable observations (global increase in the use of sunscreens with UV filters; rapid rise in malignant melanoma, against which sunscreens should protect; increase in reported experimental findings of UV filters acting as endocrine disruptors), Krause et al. in 2012 reviewed animal and human data on the UV filters BP-3, 3-benzylidene camphor (3-BC), 3-(4-methyl-benzylidene) camphor (4-MBC), 2-ethylhexyl 4-methoxy cinnamate (OMC), homosalate (HMS), 2-ethylhexyl 4-dimethylaminobenzoate, and 4-aminobenzoic acid (PABA). Importantly, BP-3 was present in 96% of human urine samples in the United States, and various filters were found in 85% of the human breast milk samples in Switzerland.6
A 2019 analysis by Wang and Ganley reported that systemic absorption of the active sunscreen ingredient BP-3 can be substantial, justifying the assessment and understanding of systemic exposure to characterize the risks of long-term usage.7
Between January and February 2019, Matta et al. conducted a randomized clinical trial with 48 healthy participants to evaluate the systemic absorption and pharmacokinetics of six active ingredients in four sunscreen formulations, including avobenzone and BP-3. The researchers found that all ingredients were systemically absorbed, with plasma concentrations exceeding the Food and Drug Administration threshold for considering the waiving of further safety studies. They concluded that these results did not warrant discontinuing the use of the tested sunscreen ingredients.8 Yeager and Lim add that, while BP-3 has been incorporated into sunscreen formulations for sale in the United States since 1978, there have been no reports of adverse systemic reactions in human beings.3
However, topical reactions have elicited a different assessment. That is, in 2014, the American Contact Dermatitis Society labeled BPs the Contact Allergen of the Year, as they were identified as the most common source of photoallergic and contact allergic reactions of all UV filters.3,9
Risks of BPs in sunscreens and other skincare products
In 2015, Amar et al. investigated the photogenotoxicity and apoptotic effects in human keratinocytes (HaCaT cells) of BP-1, which is used as a UV blocker in sunscreens. They found that BP-1, when exposed to UV radiation, photosensitized cells and yielded intracellular reactive oxygen species. Significant reductions in cell viability were also seen with exposure to sunlight, UVA, and UVB. The researchers also confirmed genotoxic activity, with BP-1 augmenting lipid peroxidation and upregulating apoptotic proteins. They concluded that sunscreen users should be advised to avoid products that contain BP-1.10
In 2019, Amar et al. evaluated the effects of BPs on the differential expression of proteins in HaCaT cells exposed to UVA. Their findings indicated the expression of novel proteins that helped to initiate or promote apoptosis. They concluded that, because of the predilection to render such effects in human skin keratinocytes, consumers should avoid the use of sunscreens that contain BPs as UV blocking ingredients.11
Still widely used as an effective filter against UVA2 and UVB, BP-3 was believed to be present in two thirds of nonmineral sunscreens in the United States in 2018.3,12
Notably, BP-1 and BP-3 were found in small proportions (3.7% and 4.9%, respectively) among a total of 283 products culled from various stores in Lecce, Italy, in a survey of the potentially dangerous chemicals found in rinse-off, leave-on, and makeup products in 2019.13 The authors added that the International Agency for Research on Cancer, in 2010, classified BP as potentially carcinogenic to humans (2B group).13,14
Promising use of nanocapsules
The widespread concern about the phototoxicity of BP has prompted some interesting research into workarounds. Specifically, in 2019, Barbosa et al. reported on the creation of a new sunscreen formulation using polymeric nanocapsules loading BP-3. The nanocapsules are made of poly(ε-caprolactone) carrot oil and Pluronic F68 (nonionic surfactant used in suspension cultures), and the BP-3–loaded capsules were found to be noncytotoxic in L929 fibroblast cell lines with a sun protection factor of 8.64. The researchers concluded that this promising nanocapsule may be an effective and safe way to use lipophilic sunscreen ingredients such as BP-3.15
Conclusion
The body of evidence is weighted against the use of BPs. Luckily, we have safe sunscreen choices that allow us to protect our skin without using these compounds.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann has written two textbooks and a New York Times Best Sellers book for consumers. Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Galderma, Revance, Evolus, and Burt’s Bees. She is the CEO of Skin Type Solutions Inc., a company that independently tests skin care products and makes recommendations to physicians on which skin care technologies are best. Write to her at dermnews@mdedge.com.
References
1. Utsunomiya H et al. Chem Biol Interact. 2019 Jan 25;298:52-6.
2. Schneider SL and Lim HW. J Am Acad Dermatol. 2019 Jan;80(1):266-71.
3. Yeager DG and Lim HW. Dermatol Clin. 2019 Apr;37(2):149-57.
4. Ramos S et al. Sci Total Environ. 2015 Sep 1;526:278-311.
5. Siller A et al. Plast Surg Nur. 2019 Oct/Dec;39(4):157-60.
6. Krause M et al. Int J Androl. 2012 Jun;35(3):424-36.
7. Wang J and Ganley CJ. Clin Pharmacol Ther. 2019 Jan;105(1):161-7.
8. Matta MK et al. JAMA. 2020 Jan 21;323(3):256-67.
9. Warshaw EM et al. Dermatitis. 2013 Jul-Aug;24(4):176-82.
10. Amar SK et al. Toxicol Lett. 2015 Dec 15;239(3):182-93.
11. Amar SK et al. Toxicol Ind Health. 2019 Jul;35(7):457-65.
12. EWG. The trouble with ingredients in sunscreens. Accessed on 4 April 2020.
13. Panico A et al. J Prev Med Hyg. 2019 Mar 29;60(1):E50-7.
14. International Agency for Research on Cancer (IARC). Benzophenone. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. WHO, IARC Press, Lyon, France. 2010;101:285-304.
15. Barbosa TC et al. Toxics. 2019 Sep 22;7(4):51.
Restoring dignity to sex trafficking survivors, one tattoo removal at a time
SAN DIEGO – , according to the results of an online survey evaluating the need for and impact of tattoo removal in this population.
Sex trafficking involves the use of force, fraud, or coercion to compel another person to engage in commercial sex acts, and traffickers often brand their victims with tattoos that convey ownership, including tattoos of names, symbols, and barcodes. According to data from Polaris, a nonprofit organization that works to combat and prevent sex and labor trafficking in the United States, 16,658 sex trafficking victims were identified in the country in 2020, but tens of thousands go unreported.
“Given the inherently covert nature of this crime, it is difficult to determine exact statistics,” Emily L. Guo, MD, a cosmetic dermatologic surgery fellow at the Dermatology and Laser Surgery Center in Houston, said during a clinical abstract session at the annual meeting of the American Society for Laser Medicine and Surgery. “We have been working with sex trafficking survivors local to our practice in Houston providing pro bono tattoo removal, and we’ve observed how impactful that is in their recovery. We wanted to see if there was a national need for support of these survivors, allowing them to reclaim their lives.”
In collaboration with Elizabeth Kream, MD, a dermatology resident at the University of Illinois at Chicago, and Paul M. Friedman, MD, director of the Dermatology and Laser Surgery Center and the current ASLMS president, Dr. Guo conducted an online needs and impact survey regarding laser removal of branding tattoos. With assistance from the National Trafficking Sheltered Alliance, the researchers distributed the survey to U.S. organizations that support sex trafficking survivors. Representatives from 40 organizations responded to the survey. Most were based in the South (45%), followed by the West (20%) and Midwest (20%), and the Northeast (15%).
“On average, these programs support 81 survivors per year, which translates into 3,240 victims per year,” Dr. Guo said. Survey respondents estimated that 47% of sex trafficking survivors had branding tattoos. Of those, 67% were in a stable situation that would make it possible to undergo tattoo removal.
On a scale of 1 to 10 with 10 being the highest, “pro bono removal of branding tattoos received a survivor impact recovery score of 9.2 by these respondents,” Dr. Guo said. “Breaking down these numbers, there are at least 1,200 survivors per year who would benefit from tattoo removal during recovery. Qualitative responses to our survey echoed the same messages: There is a great need and a large impact for pro bono tattoo removal.”
For example, one survey respondent wrote, “Thank you for being willing to remove tattoos, allowing them to feel as though they are no longer owned by their trafficker.” Another wrote, “Erasing or revising the mark of her trafficker is a critical part of every survivor’s recovery journey.”
Sometimes branding tattoos are placed in highly visible locations. One sex trafficking survivor presented to Dr. Guo with a large dark blue tattoo above an eyebrow. “She shared with me that because the tattoo was so highly visible, nobody would offer her a job,” Dr. Guo said. Another survivor had her trafficker’s initial tattooed on her left ring finger. Yet another had a large tattoo on her forearm branded with her trafficker’s name as well as the word cash, “indicating that she is source of money for him,” she said, noting that on average, one sex trafficking victim generates about $100,000 per year for their trafficker.
Although there has been work published on recognition of branding tattoos in the medical community, including the difficulty in differentiating branding tattoos from voluntary tattoos, Dr. Friedman said that there have not been any studies evaluating the need and impact of laser branding tattoo removal in the recovery of sex trafficking survivors. Findings from the current survey “illuminate that the removal of branding tattoos is highly impactful on recovery and may be preferred over tattoo cover-ups,” Dr. Friedman told this news organization.
“Furthermore, survivors frequently move during their recovery process, so a national partnership is essential to allowing survivors to continue the removal process wherever they may be.”
The findings support a proposed ASLMS campaign that intends to connect sex trafficking survivors with board-certified physicians for pro bono removal of branding tattoos. “This will not only aid in survivors’ recovery, but this work will also be beneficial to allow for an avenue to create a repository of sex trafficking tattoo images to improve branding tattoo identification competency among health care providers,” Dr. Friedman said.
He acknowledged certain limitations of the survey, including the fact that “thorough and exact data collection regarding human trafficking is challenging given the inherently covert and underground nature of this crime.” In addition, the study involved surveying organizations supporting sex trafficking survivors rather than the survivors themselves. However, he noted, “we felt for this initial study we wanted to be sensitive to the survivors.”
In an interview at the meeting, one of the session moderators, Oge Onwudiwe, MD, a dermatologist who practices at AllPhases Dermatology in Alexandria, Va., said that pro bono laser removal of branding tattoos “is something that a lot of us can work on and do, and have an impact on. There’s no reason why we shouldn’t help. I can only imagine the psychological impact of having a daily reminder of that [in the form of a branding tattoo]. That’s like PTSD every day almost. You have a trigger there.”
Another session moderator, Eliot Battle, MD, CEO of Cultura Dermatology and Laser Center in Washington, is a board member of Innocents at Risk, a nonprofit that works to fight child exploitation and human trafficking. With pro bono laser removal of a branded tattoo, “this is not just a cosmetic correction you’re making,” Dr. Battle said. “It’s so much deeper than that. It changes people’s lives.”
The researchers and Dr. Onwudiwe reported having no financial disclosures. Dr. Battle disclosed that he conducts research for Cynosure, and has received discounts from Cynosure, Cutera, Solta Medical, Lumenis, Be Inc., and Sciton.
SAN DIEGO – , according to the results of an online survey evaluating the need for and impact of tattoo removal in this population.
Sex trafficking involves the use of force, fraud, or coercion to compel another person to engage in commercial sex acts, and traffickers often brand their victims with tattoos that convey ownership, including tattoos of names, symbols, and barcodes. According to data from Polaris, a nonprofit organization that works to combat and prevent sex and labor trafficking in the United States, 16,658 sex trafficking victims were identified in the country in 2020, but tens of thousands go unreported.
“Given the inherently covert nature of this crime, it is difficult to determine exact statistics,” Emily L. Guo, MD, a cosmetic dermatologic surgery fellow at the Dermatology and Laser Surgery Center in Houston, said during a clinical abstract session at the annual meeting of the American Society for Laser Medicine and Surgery. “We have been working with sex trafficking survivors local to our practice in Houston providing pro bono tattoo removal, and we’ve observed how impactful that is in their recovery. We wanted to see if there was a national need for support of these survivors, allowing them to reclaim their lives.”
In collaboration with Elizabeth Kream, MD, a dermatology resident at the University of Illinois at Chicago, and Paul M. Friedman, MD, director of the Dermatology and Laser Surgery Center and the current ASLMS president, Dr. Guo conducted an online needs and impact survey regarding laser removal of branding tattoos. With assistance from the National Trafficking Sheltered Alliance, the researchers distributed the survey to U.S. organizations that support sex trafficking survivors. Representatives from 40 organizations responded to the survey. Most were based in the South (45%), followed by the West (20%) and Midwest (20%), and the Northeast (15%).
“On average, these programs support 81 survivors per year, which translates into 3,240 victims per year,” Dr. Guo said. Survey respondents estimated that 47% of sex trafficking survivors had branding tattoos. Of those, 67% were in a stable situation that would make it possible to undergo tattoo removal.
On a scale of 1 to 10 with 10 being the highest, “pro bono removal of branding tattoos received a survivor impact recovery score of 9.2 by these respondents,” Dr. Guo said. “Breaking down these numbers, there are at least 1,200 survivors per year who would benefit from tattoo removal during recovery. Qualitative responses to our survey echoed the same messages: There is a great need and a large impact for pro bono tattoo removal.”
For example, one survey respondent wrote, “Thank you for being willing to remove tattoos, allowing them to feel as though they are no longer owned by their trafficker.” Another wrote, “Erasing or revising the mark of her trafficker is a critical part of every survivor’s recovery journey.”
Sometimes branding tattoos are placed in highly visible locations. One sex trafficking survivor presented to Dr. Guo with a large dark blue tattoo above an eyebrow. “She shared with me that because the tattoo was so highly visible, nobody would offer her a job,” Dr. Guo said. Another survivor had her trafficker’s initial tattooed on her left ring finger. Yet another had a large tattoo on her forearm branded with her trafficker’s name as well as the word cash, “indicating that she is source of money for him,” she said, noting that on average, one sex trafficking victim generates about $100,000 per year for their trafficker.
Although there has been work published on recognition of branding tattoos in the medical community, including the difficulty in differentiating branding tattoos from voluntary tattoos, Dr. Friedman said that there have not been any studies evaluating the need and impact of laser branding tattoo removal in the recovery of sex trafficking survivors. Findings from the current survey “illuminate that the removal of branding tattoos is highly impactful on recovery and may be preferred over tattoo cover-ups,” Dr. Friedman told this news organization.
“Furthermore, survivors frequently move during their recovery process, so a national partnership is essential to allowing survivors to continue the removal process wherever they may be.”
The findings support a proposed ASLMS campaign that intends to connect sex trafficking survivors with board-certified physicians for pro bono removal of branding tattoos. “This will not only aid in survivors’ recovery, but this work will also be beneficial to allow for an avenue to create a repository of sex trafficking tattoo images to improve branding tattoo identification competency among health care providers,” Dr. Friedman said.
He acknowledged certain limitations of the survey, including the fact that “thorough and exact data collection regarding human trafficking is challenging given the inherently covert and underground nature of this crime.” In addition, the study involved surveying organizations supporting sex trafficking survivors rather than the survivors themselves. However, he noted, “we felt for this initial study we wanted to be sensitive to the survivors.”
In an interview at the meeting, one of the session moderators, Oge Onwudiwe, MD, a dermatologist who practices at AllPhases Dermatology in Alexandria, Va., said that pro bono laser removal of branding tattoos “is something that a lot of us can work on and do, and have an impact on. There’s no reason why we shouldn’t help. I can only imagine the psychological impact of having a daily reminder of that [in the form of a branding tattoo]. That’s like PTSD every day almost. You have a trigger there.”
Another session moderator, Eliot Battle, MD, CEO of Cultura Dermatology and Laser Center in Washington, is a board member of Innocents at Risk, a nonprofit that works to fight child exploitation and human trafficking. With pro bono laser removal of a branded tattoo, “this is not just a cosmetic correction you’re making,” Dr. Battle said. “It’s so much deeper than that. It changes people’s lives.”
The researchers and Dr. Onwudiwe reported having no financial disclosures. Dr. Battle disclosed that he conducts research for Cynosure, and has received discounts from Cynosure, Cutera, Solta Medical, Lumenis, Be Inc., and Sciton.
SAN DIEGO – , according to the results of an online survey evaluating the need for and impact of tattoo removal in this population.
Sex trafficking involves the use of force, fraud, or coercion to compel another person to engage in commercial sex acts, and traffickers often brand their victims with tattoos that convey ownership, including tattoos of names, symbols, and barcodes. According to data from Polaris, a nonprofit organization that works to combat and prevent sex and labor trafficking in the United States, 16,658 sex trafficking victims were identified in the country in 2020, but tens of thousands go unreported.
“Given the inherently covert nature of this crime, it is difficult to determine exact statistics,” Emily L. Guo, MD, a cosmetic dermatologic surgery fellow at the Dermatology and Laser Surgery Center in Houston, said during a clinical abstract session at the annual meeting of the American Society for Laser Medicine and Surgery. “We have been working with sex trafficking survivors local to our practice in Houston providing pro bono tattoo removal, and we’ve observed how impactful that is in their recovery. We wanted to see if there was a national need for support of these survivors, allowing them to reclaim their lives.”
In collaboration with Elizabeth Kream, MD, a dermatology resident at the University of Illinois at Chicago, and Paul M. Friedman, MD, director of the Dermatology and Laser Surgery Center and the current ASLMS president, Dr. Guo conducted an online needs and impact survey regarding laser removal of branding tattoos. With assistance from the National Trafficking Sheltered Alliance, the researchers distributed the survey to U.S. organizations that support sex trafficking survivors. Representatives from 40 organizations responded to the survey. Most were based in the South (45%), followed by the West (20%) and Midwest (20%), and the Northeast (15%).
“On average, these programs support 81 survivors per year, which translates into 3,240 victims per year,” Dr. Guo said. Survey respondents estimated that 47% of sex trafficking survivors had branding tattoos. Of those, 67% were in a stable situation that would make it possible to undergo tattoo removal.
On a scale of 1 to 10 with 10 being the highest, “pro bono removal of branding tattoos received a survivor impact recovery score of 9.2 by these respondents,” Dr. Guo said. “Breaking down these numbers, there are at least 1,200 survivors per year who would benefit from tattoo removal during recovery. Qualitative responses to our survey echoed the same messages: There is a great need and a large impact for pro bono tattoo removal.”
For example, one survey respondent wrote, “Thank you for being willing to remove tattoos, allowing them to feel as though they are no longer owned by their trafficker.” Another wrote, “Erasing or revising the mark of her trafficker is a critical part of every survivor’s recovery journey.”
Sometimes branding tattoos are placed in highly visible locations. One sex trafficking survivor presented to Dr. Guo with a large dark blue tattoo above an eyebrow. “She shared with me that because the tattoo was so highly visible, nobody would offer her a job,” Dr. Guo said. Another survivor had her trafficker’s initial tattooed on her left ring finger. Yet another had a large tattoo on her forearm branded with her trafficker’s name as well as the word cash, “indicating that she is source of money for him,” she said, noting that on average, one sex trafficking victim generates about $100,000 per year for their trafficker.
Although there has been work published on recognition of branding tattoos in the medical community, including the difficulty in differentiating branding tattoos from voluntary tattoos, Dr. Friedman said that there have not been any studies evaluating the need and impact of laser branding tattoo removal in the recovery of sex trafficking survivors. Findings from the current survey “illuminate that the removal of branding tattoos is highly impactful on recovery and may be preferred over tattoo cover-ups,” Dr. Friedman told this news organization.
“Furthermore, survivors frequently move during their recovery process, so a national partnership is essential to allowing survivors to continue the removal process wherever they may be.”
The findings support a proposed ASLMS campaign that intends to connect sex trafficking survivors with board-certified physicians for pro bono removal of branding tattoos. “This will not only aid in survivors’ recovery, but this work will also be beneficial to allow for an avenue to create a repository of sex trafficking tattoo images to improve branding tattoo identification competency among health care providers,” Dr. Friedman said.
He acknowledged certain limitations of the survey, including the fact that “thorough and exact data collection regarding human trafficking is challenging given the inherently covert and underground nature of this crime.” In addition, the study involved surveying organizations supporting sex trafficking survivors rather than the survivors themselves. However, he noted, “we felt for this initial study we wanted to be sensitive to the survivors.”
In an interview at the meeting, one of the session moderators, Oge Onwudiwe, MD, a dermatologist who practices at AllPhases Dermatology in Alexandria, Va., said that pro bono laser removal of branding tattoos “is something that a lot of us can work on and do, and have an impact on. There’s no reason why we shouldn’t help. I can only imagine the psychological impact of having a daily reminder of that [in the form of a branding tattoo]. That’s like PTSD every day almost. You have a trigger there.”
Another session moderator, Eliot Battle, MD, CEO of Cultura Dermatology and Laser Center in Washington, is a board member of Innocents at Risk, a nonprofit that works to fight child exploitation and human trafficking. With pro bono laser removal of a branded tattoo, “this is not just a cosmetic correction you’re making,” Dr. Battle said. “It’s so much deeper than that. It changes people’s lives.”
The researchers and Dr. Onwudiwe reported having no financial disclosures. Dr. Battle disclosed that he conducts research for Cynosure, and has received discounts from Cynosure, Cutera, Solta Medical, Lumenis, Be Inc., and Sciton.
AT ASLMS 2022