User login
Are Breast Cancer Survivors Vulnerable to Alzheimer’s Disease?
Despite concerns about cognitive decline after cancer treatment, most breast cancer survivors show no increased risk of developing Alzheimer’s disease, and some may have a slightly lower risk than their cancer-free peers, according to a large retrospective study from Korea.
However, any apparent protective effect faded with time, the investigators reported online in JAMA Network Open.
Overall, this is “reassuring news for cancer survivors,” Tim Ahles, PhD, a psychologist with Memorial Sloan Kettering Cancer Center, New York City, who wasn’t involved in the study, told this news organization.
“I get this question from patients a lot,” Ahles said. And based on these findings, “it doesn’t look like a history of breast cancer and breast cancer treatment increases your risk for Alzheimer’s disease.”
Breast cancer survivors often report cancer-related cognitive impairment, such as difficulties with concentration and memory, both during and after cancer treatment. But evidence surrounding patients’ risk for Alzheimer’s disease is mixed. One large study based in Sweden, for instance, reported a 35% increased risk for Alzheimer’s disease among patients diagnosed with breast cancer after the age of 65 years, but not among younger patients. A population-based study from Taiwan, however, found no increase in the risk for dementia overall compared with cancer-free individuals but did note a lower dementia risk in patients who had received tamoxifen.
To help clarify the evidence, investigators assessed Alzheimer’s disease risk in a large cohort of patients and explored the association by treatment type, age, and important risk factors.
Using the Korean National Health Insurance Service database, the researchers matched 70,701 patients who underwent breast cancer surgery between 2010 and 2016 with 180,360 cancer-free control individuals.
The mean age of breast cancer survivors was 53.1 years. Overall, 72% received radiotherapy. Cyclophosphamide (57%) and anthracycline (50%) were the most commonly used chemotherapies, and tamoxifen (47%) and aromatase inhibitors (30%) were the most commonly used endocrine therapies.
The primary outcome of this study was the incidence of newly diagnosed Alzheimer’s disease, which was defined on the basis of at least one prescription for medications to manage dementia associated with Alzheimer’s disease (donepezil, rivastigmine, galantamine, or memantine).
During a median follow-up of about 7 years, 1229 newly diagnosed Alzheimer’s disease cases were detected in breast cancer survivors and 3430 cases in control individuals — incidence rates of 2.45 and 2.63 per 1000 person-years, respectively.
This corresponded to an 8% lower risk for Alzheimer’s disease in breast cancer survivors compared with cancer-free control individuals at 6 months (subdistribution hazard ratio [SHR], 0.92; 95% CI, 0.86-0.98). The association was especially notable in survivors older than 65 years (SHR, 0.92; 95% CI, 0.85-0.99).
Looking at individual treatment modalities, only radiation therapy was associated with significantly lower risk for Alzheimer’s disease among breast cancer survivors (adjusted HR [aHR], 0.77).
Several risk factors were associated with a significantly higher risk for Alzheimer’s disease: current smoker vs never or ex-smokers (aHR, 2.04), diabetes (aHR, 1.58), and chronic kidney disease (aHR, 3.11). Notably, alcohol use, physical activity level, and hypertension were not associated with Alzheimer’s disease risk.
However, any potential protective effect may be short-lived. The reduced risk for Alzheimer’s disease was no longer significant at 1 year (SHR, 0.94; 95% CI, 0.87-1.01), 3 years (SHR, 0.97; 95% CI, 0.90-1.05), or 5 years (SHR, 0.98; 95% CI, 0.89-1.08).
Even so, breast cancer survivors can still feel reassured by the findings.
“Concerns about chemobrain and the long-term adverse effects of breast cancer treatment on cognition are common, but our findings suggest that this treatment does not directly lead to Alzheimer’s disease,” wrote the authors, led by Su-Min Jeong, MD, with Seoul National University College of Medicine, Seoul, South Korea.
Ahles agreed. The general takeaway from this study is that there is “no strong evidence that the cancer treatment is going to increase your risk for developing Alzheimer’s,” Ahles said. When patients ask about the risk for Alzheimer’s disease, “I can say, ‘Here’s yet another new study that supports the idea that there’s no increased risk.’”
He cautioned, however, that the study doesn’t address whether people with a genetic predisposition to Alzheimer’s might develop it sooner due to cancer treatment.
“Does the cancer treatment increase your probability or nudge you along? The study doesn’t answer that question,” Ahles said.
The study reported having no commercial funding. Jeong and Ahles reported having no relevant disclosures.
A version of this article first appeared on Medscape.com.
Despite concerns about cognitive decline after cancer treatment, most breast cancer survivors show no increased risk of developing Alzheimer’s disease, and some may have a slightly lower risk than their cancer-free peers, according to a large retrospective study from Korea.
However, any apparent protective effect faded with time, the investigators reported online in JAMA Network Open.
Overall, this is “reassuring news for cancer survivors,” Tim Ahles, PhD, a psychologist with Memorial Sloan Kettering Cancer Center, New York City, who wasn’t involved in the study, told this news organization.
“I get this question from patients a lot,” Ahles said. And based on these findings, “it doesn’t look like a history of breast cancer and breast cancer treatment increases your risk for Alzheimer’s disease.”
Breast cancer survivors often report cancer-related cognitive impairment, such as difficulties with concentration and memory, both during and after cancer treatment. But evidence surrounding patients’ risk for Alzheimer’s disease is mixed. One large study based in Sweden, for instance, reported a 35% increased risk for Alzheimer’s disease among patients diagnosed with breast cancer after the age of 65 years, but not among younger patients. A population-based study from Taiwan, however, found no increase in the risk for dementia overall compared with cancer-free individuals but did note a lower dementia risk in patients who had received tamoxifen.
To help clarify the evidence, investigators assessed Alzheimer’s disease risk in a large cohort of patients and explored the association by treatment type, age, and important risk factors.
Using the Korean National Health Insurance Service database, the researchers matched 70,701 patients who underwent breast cancer surgery between 2010 and 2016 with 180,360 cancer-free control individuals.
The mean age of breast cancer survivors was 53.1 years. Overall, 72% received radiotherapy. Cyclophosphamide (57%) and anthracycline (50%) were the most commonly used chemotherapies, and tamoxifen (47%) and aromatase inhibitors (30%) were the most commonly used endocrine therapies.
The primary outcome of this study was the incidence of newly diagnosed Alzheimer’s disease, which was defined on the basis of at least one prescription for medications to manage dementia associated with Alzheimer’s disease (donepezil, rivastigmine, galantamine, or memantine).
During a median follow-up of about 7 years, 1229 newly diagnosed Alzheimer’s disease cases were detected in breast cancer survivors and 3430 cases in control individuals — incidence rates of 2.45 and 2.63 per 1000 person-years, respectively.
This corresponded to an 8% lower risk for Alzheimer’s disease in breast cancer survivors compared with cancer-free control individuals at 6 months (subdistribution hazard ratio [SHR], 0.92; 95% CI, 0.86-0.98). The association was especially notable in survivors older than 65 years (SHR, 0.92; 95% CI, 0.85-0.99).
Looking at individual treatment modalities, only radiation therapy was associated with significantly lower risk for Alzheimer’s disease among breast cancer survivors (adjusted HR [aHR], 0.77).
Several risk factors were associated with a significantly higher risk for Alzheimer’s disease: current smoker vs never or ex-smokers (aHR, 2.04), diabetes (aHR, 1.58), and chronic kidney disease (aHR, 3.11). Notably, alcohol use, physical activity level, and hypertension were not associated with Alzheimer’s disease risk.
However, any potential protective effect may be short-lived. The reduced risk for Alzheimer’s disease was no longer significant at 1 year (SHR, 0.94; 95% CI, 0.87-1.01), 3 years (SHR, 0.97; 95% CI, 0.90-1.05), or 5 years (SHR, 0.98; 95% CI, 0.89-1.08).
Even so, breast cancer survivors can still feel reassured by the findings.
“Concerns about chemobrain and the long-term adverse effects of breast cancer treatment on cognition are common, but our findings suggest that this treatment does not directly lead to Alzheimer’s disease,” wrote the authors, led by Su-Min Jeong, MD, with Seoul National University College of Medicine, Seoul, South Korea.
Ahles agreed. The general takeaway from this study is that there is “no strong evidence that the cancer treatment is going to increase your risk for developing Alzheimer’s,” Ahles said. When patients ask about the risk for Alzheimer’s disease, “I can say, ‘Here’s yet another new study that supports the idea that there’s no increased risk.’”
He cautioned, however, that the study doesn’t address whether people with a genetic predisposition to Alzheimer’s might develop it sooner due to cancer treatment.
“Does the cancer treatment increase your probability or nudge you along? The study doesn’t answer that question,” Ahles said.
The study reported having no commercial funding. Jeong and Ahles reported having no relevant disclosures.
A version of this article first appeared on Medscape.com.
Despite concerns about cognitive decline after cancer treatment, most breast cancer survivors show no increased risk of developing Alzheimer’s disease, and some may have a slightly lower risk than their cancer-free peers, according to a large retrospective study from Korea.
However, any apparent protective effect faded with time, the investigators reported online in JAMA Network Open.
Overall, this is “reassuring news for cancer survivors,” Tim Ahles, PhD, a psychologist with Memorial Sloan Kettering Cancer Center, New York City, who wasn’t involved in the study, told this news organization.
“I get this question from patients a lot,” Ahles said. And based on these findings, “it doesn’t look like a history of breast cancer and breast cancer treatment increases your risk for Alzheimer’s disease.”
Breast cancer survivors often report cancer-related cognitive impairment, such as difficulties with concentration and memory, both during and after cancer treatment. But evidence surrounding patients’ risk for Alzheimer’s disease is mixed. One large study based in Sweden, for instance, reported a 35% increased risk for Alzheimer’s disease among patients diagnosed with breast cancer after the age of 65 years, but not among younger patients. A population-based study from Taiwan, however, found no increase in the risk for dementia overall compared with cancer-free individuals but did note a lower dementia risk in patients who had received tamoxifen.
To help clarify the evidence, investigators assessed Alzheimer’s disease risk in a large cohort of patients and explored the association by treatment type, age, and important risk factors.
Using the Korean National Health Insurance Service database, the researchers matched 70,701 patients who underwent breast cancer surgery between 2010 and 2016 with 180,360 cancer-free control individuals.
The mean age of breast cancer survivors was 53.1 years. Overall, 72% received radiotherapy. Cyclophosphamide (57%) and anthracycline (50%) were the most commonly used chemotherapies, and tamoxifen (47%) and aromatase inhibitors (30%) were the most commonly used endocrine therapies.
The primary outcome of this study was the incidence of newly diagnosed Alzheimer’s disease, which was defined on the basis of at least one prescription for medications to manage dementia associated with Alzheimer’s disease (donepezil, rivastigmine, galantamine, or memantine).
During a median follow-up of about 7 years, 1229 newly diagnosed Alzheimer’s disease cases were detected in breast cancer survivors and 3430 cases in control individuals — incidence rates of 2.45 and 2.63 per 1000 person-years, respectively.
This corresponded to an 8% lower risk for Alzheimer’s disease in breast cancer survivors compared with cancer-free control individuals at 6 months (subdistribution hazard ratio [SHR], 0.92; 95% CI, 0.86-0.98). The association was especially notable in survivors older than 65 years (SHR, 0.92; 95% CI, 0.85-0.99).
Looking at individual treatment modalities, only radiation therapy was associated with significantly lower risk for Alzheimer’s disease among breast cancer survivors (adjusted HR [aHR], 0.77).
Several risk factors were associated with a significantly higher risk for Alzheimer’s disease: current smoker vs never or ex-smokers (aHR, 2.04), diabetes (aHR, 1.58), and chronic kidney disease (aHR, 3.11). Notably, alcohol use, physical activity level, and hypertension were not associated with Alzheimer’s disease risk.
However, any potential protective effect may be short-lived. The reduced risk for Alzheimer’s disease was no longer significant at 1 year (SHR, 0.94; 95% CI, 0.87-1.01), 3 years (SHR, 0.97; 95% CI, 0.90-1.05), or 5 years (SHR, 0.98; 95% CI, 0.89-1.08).
Even so, breast cancer survivors can still feel reassured by the findings.
“Concerns about chemobrain and the long-term adverse effects of breast cancer treatment on cognition are common, but our findings suggest that this treatment does not directly lead to Alzheimer’s disease,” wrote the authors, led by Su-Min Jeong, MD, with Seoul National University College of Medicine, Seoul, South Korea.
Ahles agreed. The general takeaway from this study is that there is “no strong evidence that the cancer treatment is going to increase your risk for developing Alzheimer’s,” Ahles said. When patients ask about the risk for Alzheimer’s disease, “I can say, ‘Here’s yet another new study that supports the idea that there’s no increased risk.’”
He cautioned, however, that the study doesn’t address whether people with a genetic predisposition to Alzheimer’s might develop it sooner due to cancer treatment.
“Does the cancer treatment increase your probability or nudge you along? The study doesn’t answer that question,” Ahles said.
The study reported having no commercial funding. Jeong and Ahles reported having no relevant disclosures.
A version of this article first appeared on Medscape.com.
Strength Training Can Improve Lymphedema in Breast Cancer
TOPLINE:
A recent study found that 3 months of resistance training did not worsen lymphedema in breast cancer survivors and instead significantly improved fluid balance and increased upper extremity muscle mass. The edema index also improved, suggesting potential therapeutic benefits of intense resistance training for managing lymphedema.
METHODOLOGY:
- Lymphedema is a common adverse effect of breast cancer treatment that can limit mobility. Although strength training can have multiple benefits for breast cancer survivors, such as increased bone density and metabolism, data on whether more intense resistance training exacerbates lymphedema in this population are limited. Worries that more intense training will lead to or worsen lymphedema have typically led to cautious recommendations.
- Researchers conducted a cohort study involving 115 women with breast cancer (median age, 54 years; 96% White; 4% Black) between September 2022 and March 2024. Most (83%) underwent sentinel lymph node biopsy (SLNB), while 12% had axillary lymph node dissection (ALND). At baseline, 13% had clinical lymphedema, including 37% in the ALND group and 8% in the SLNB group.
- Participants attended resistance training sessions three times a week, with intensity escalation over 3 months. Exercises involved hand weights, resistance bands, and body weight (eg, pushups) to promote strength, mobility, and muscle hypertrophy.
- Bioimpedance analysis measured intracellular water, extracellular water, and total body water before and after exercise. Lymphedema was defined as more than a 3% increase in arm circumference discrepancy relative to preoperative ipsilateral arm measurements, along with an elevated edema index (extracellular water to total body water ratio).
TAKEAWAY:
- No participants experienced subjective or clinical worsening of lymphedema after completing the resistance training regimen.
- Lean mass in the affected arm increased from a median of 5.45 lb to 5.64 lb (P < .001), while lean mass in the unaffected arm rose from 5.51 lb to 5.53 lb (P < .001) after the resistance training.
- Overall, participants’ fluid balance improved. The edema index in both arms showed a significant reduction at training completion (mean, 0.383) vs baseline (mean, 0.385), indicating reduced lymphedema. Subgroup analysis of women who underwent SLNB showed similar improvements in the edema index.
IN PRACTICE:
“These findings highlight the safety of strength and resistance training in a large group of patients with breast cancer during and after treatment,” the authors wrote. Beyond that, the authors noted, the results point to a potential role for resistance training in reducing lymphedema.
SOURCE:
This study, led by Parisa Shamsesfandabadi, MD, Allegheny Health Network, Pittsburgh, was published online in JAMA Network Open.
LIMITATIONS:
A major limitation was the absence of a control group, which prevented a direct comparison between the effects of exercise and the natural progression of lymphedema. The 3-month intervention provided limited insight into the long-term sustainability of benefits. Patient-reported outcomes were not included. Additionally, potential confounding variables such as diet, medication use, and baseline physical activity levels were not controlled for in the analysis.
DISCLOSURES:
The authors did not disclose any funding information. Several authors reported having ties with various sources. Additional disclosures are noted in the original article.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
A recent study found that 3 months of resistance training did not worsen lymphedema in breast cancer survivors and instead significantly improved fluid balance and increased upper extremity muscle mass. The edema index also improved, suggesting potential therapeutic benefits of intense resistance training for managing lymphedema.
METHODOLOGY:
- Lymphedema is a common adverse effect of breast cancer treatment that can limit mobility. Although strength training can have multiple benefits for breast cancer survivors, such as increased bone density and metabolism, data on whether more intense resistance training exacerbates lymphedema in this population are limited. Worries that more intense training will lead to or worsen lymphedema have typically led to cautious recommendations.
- Researchers conducted a cohort study involving 115 women with breast cancer (median age, 54 years; 96% White; 4% Black) between September 2022 and March 2024. Most (83%) underwent sentinel lymph node biopsy (SLNB), while 12% had axillary lymph node dissection (ALND). At baseline, 13% had clinical lymphedema, including 37% in the ALND group and 8% in the SLNB group.
- Participants attended resistance training sessions three times a week, with intensity escalation over 3 months. Exercises involved hand weights, resistance bands, and body weight (eg, pushups) to promote strength, mobility, and muscle hypertrophy.
- Bioimpedance analysis measured intracellular water, extracellular water, and total body water before and after exercise. Lymphedema was defined as more than a 3% increase in arm circumference discrepancy relative to preoperative ipsilateral arm measurements, along with an elevated edema index (extracellular water to total body water ratio).
TAKEAWAY:
- No participants experienced subjective or clinical worsening of lymphedema after completing the resistance training regimen.
- Lean mass in the affected arm increased from a median of 5.45 lb to 5.64 lb (P < .001), while lean mass in the unaffected arm rose from 5.51 lb to 5.53 lb (P < .001) after the resistance training.
- Overall, participants’ fluid balance improved. The edema index in both arms showed a significant reduction at training completion (mean, 0.383) vs baseline (mean, 0.385), indicating reduced lymphedema. Subgroup analysis of women who underwent SLNB showed similar improvements in the edema index.
IN PRACTICE:
“These findings highlight the safety of strength and resistance training in a large group of patients with breast cancer during and after treatment,” the authors wrote. Beyond that, the authors noted, the results point to a potential role for resistance training in reducing lymphedema.
SOURCE:
This study, led by Parisa Shamsesfandabadi, MD, Allegheny Health Network, Pittsburgh, was published online in JAMA Network Open.
LIMITATIONS:
A major limitation was the absence of a control group, which prevented a direct comparison between the effects of exercise and the natural progression of lymphedema. The 3-month intervention provided limited insight into the long-term sustainability of benefits. Patient-reported outcomes were not included. Additionally, potential confounding variables such as diet, medication use, and baseline physical activity levels were not controlled for in the analysis.
DISCLOSURES:
The authors did not disclose any funding information. Several authors reported having ties with various sources. Additional disclosures are noted in the original article.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
A recent study found that 3 months of resistance training did not worsen lymphedema in breast cancer survivors and instead significantly improved fluid balance and increased upper extremity muscle mass. The edema index also improved, suggesting potential therapeutic benefits of intense resistance training for managing lymphedema.
METHODOLOGY:
- Lymphedema is a common adverse effect of breast cancer treatment that can limit mobility. Although strength training can have multiple benefits for breast cancer survivors, such as increased bone density and metabolism, data on whether more intense resistance training exacerbates lymphedema in this population are limited. Worries that more intense training will lead to or worsen lymphedema have typically led to cautious recommendations.
- Researchers conducted a cohort study involving 115 women with breast cancer (median age, 54 years; 96% White; 4% Black) between September 2022 and March 2024. Most (83%) underwent sentinel lymph node biopsy (SLNB), while 12% had axillary lymph node dissection (ALND). At baseline, 13% had clinical lymphedema, including 37% in the ALND group and 8% in the SLNB group.
- Participants attended resistance training sessions three times a week, with intensity escalation over 3 months. Exercises involved hand weights, resistance bands, and body weight (eg, pushups) to promote strength, mobility, and muscle hypertrophy.
- Bioimpedance analysis measured intracellular water, extracellular water, and total body water before and after exercise. Lymphedema was defined as more than a 3% increase in arm circumference discrepancy relative to preoperative ipsilateral arm measurements, along with an elevated edema index (extracellular water to total body water ratio).
TAKEAWAY:
- No participants experienced subjective or clinical worsening of lymphedema after completing the resistance training regimen.
- Lean mass in the affected arm increased from a median of 5.45 lb to 5.64 lb (P < .001), while lean mass in the unaffected arm rose from 5.51 lb to 5.53 lb (P < .001) after the resistance training.
- Overall, participants’ fluid balance improved. The edema index in both arms showed a significant reduction at training completion (mean, 0.383) vs baseline (mean, 0.385), indicating reduced lymphedema. Subgroup analysis of women who underwent SLNB showed similar improvements in the edema index.
IN PRACTICE:
“These findings highlight the safety of strength and resistance training in a large group of patients with breast cancer during and after treatment,” the authors wrote. Beyond that, the authors noted, the results point to a potential role for resistance training in reducing lymphedema.
SOURCE:
This study, led by Parisa Shamsesfandabadi, MD, Allegheny Health Network, Pittsburgh, was published online in JAMA Network Open.
LIMITATIONS:
A major limitation was the absence of a control group, which prevented a direct comparison between the effects of exercise and the natural progression of lymphedema. The 3-month intervention provided limited insight into the long-term sustainability of benefits. Patient-reported outcomes were not included. Additionally, potential confounding variables such as diet, medication use, and baseline physical activity levels were not controlled for in the analysis.
DISCLOSURES:
The authors did not disclose any funding information. Several authors reported having ties with various sources. Additional disclosures are noted in the original article.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
Rethinking the Scalpel: Advancing Non-Surgical Strategies for Early Breast Cancer
Breast cancer is the most common cancer in women worldwide and a leading cause of cancer-related deaths. The most common form of breast cancer is invasive ductal carcinoma, which accounts for 75%-80% of breast cancers. The second most common form is invasive lobular carcinoma, which accounts for 10%-15% of cases.
Surgical treatment of breast cancer involves removal and pathological staging of the cancerous tissue. Breast-conserving surgery and mastectomy are two surgical treatment options for patients with breast cancer. Breast-conserving surgery, which involves resection of the tumor and the surrounding margin of healthy tissue to achieve clean margins, is usually combined with radiotherapy. Mastectomy is considered in patients with relative and absolute contraindications to breast-conserving therapeutic options (eg, patients with a genetic predisposition to breast cancer, tumors > 5 cm, extensive margins, prior radiation to breast or chest wall, first-trimester pregnancy, extensive ductal carcinoma in situ, inflammatory breast cancer). Although surgical treatment of breast cancer is widely used, there have been calls to minimize unnecessary invasive surgical interventions in patients with early-stage breast cancer.
Reassessing the Role of Surgery in the Early Stages
Some surgical procedures, including axillary lymph node dissection (ALND) and contralateral prophylactic mastectomy (CPM), once considered standard treatment for early-stage breast cancer, are now being recognized as unnecessary in most cases of early-stage breast cancer without sentinel node metastases. Although ALND, which involves removal of all lymphatic tissue in the axilla, has been used for decades in the surgical management of early-stage breast cancer, this intervention typically results in lymphedema and significant morbidity.
Contralateral prophylactic mastectomy is a surgical option chosen by some women with early-stage unilateral breast cancer. However, this procedure is considered controversial in this patient population since evidence shows no survival advantage with CPM. A large-scale survey by Jagsi et al of female patients with in situ or early-stage breast cancer concluded that CPM was more common in patients who were White, had a higher level of education, and had private health insurance. In the study, 598 of the 1569 patients without an identified mutation or high genetic risk reported that a surgeon recommended against CPM. Of this group, only 1.9% underwent CPM. In contrast, of the 746 patients who reported that they did not receive any recommendation from a surgeon, 19% underwent CPM.
Re-excision and mastectomy are considered in patients with early-stage breast cancer when clear margins are not achieved with breast-conserving surgery. To prevent unnecessary reoperations and mastectomies, the 2013 invasive cancer margin consensus guideline by the American Society for Radiation Oncology (ASTRO) and the Society of Surgical Oncology, defined adequate margins in breast-conserving surgery in invasive breast cancer as “no ink on tumor.” The guideline is endorsed by the American Society of Breast Surgeons, ASTRO, and the St Gallen Consensus Conference.
A Shift in Practice: Moving Away From Routine Node Dissection
Based on findings from multiple clinical trials, experts recommend sentinel lymph node biopsy (SLNB) over ANLD and omit axillary surgery in certain patients. Findings from ACOSOG Z1071, SENTINA, and SN FNAC prospective multi-institutional trials support the use of SLNB as the initial diagnostic procedure. Sentinel lobe biopsy involves removal and evaluation of the first lymph node which receives lymphatic drainage from the breast cancer site. Negative biopsy findings on SLNB can avoid ALND as it is less likely that metastasis has occurred.
Although SLNB is preferred in younger patients with early-stage breast cancer, it is not routinely recommended for women aged ≥ 70 years of age with clinically node-negative, early-stage, HR-positive and HER2-negative breast cancer. This recommendation is based on study findings showing no difference in survival of women aged > 70 years with HR-positive clinical stage I breast cancer who did and did not undergo axillary evaluation.
The Z0011 trial by the American College of Surgeons Oncology Group found SLNB alone was not inferior to ALND regarding overall and disease-free survival in patients with clinically node-negative cancer undergoing breast conservation surgery and radiation therapy.
SLNB: A Less Invasive Alternative to ALND
Compared to SLNB, ALND is associated with more morbidity, physical symptoms, and poorer quality of life. A systemic review by Bakri et al evaluating the impact of ALND vs SLNB found higher rates of lymphedema, pain, reduced strength, and range of motion in patients who underwent ALND. In addition, an analysis of the National Cancer Database by Cocco et al found that patients with limited CN+ T1-2 breast cancer had favorable survival outcomes after undergoing SLNB and regional node irradiation vs ALND.
Rethinking First Steps: Non-Surgical Strategies
While surgical intervention with or without radiation therapy remains a primary treatment in early-stage breast cancer, there is an increased emphasis on de-escalation to minimize surgery and consider nonsurgical options in this patient population. A neoadjuvant systemic therapeutic approach by Kuerer et al for HER2-positive breast cancer and triple-negative breast cancer yielded a pathological complete response in 62% of patients. This multicenter, single-arm, phase 2 trial evaluated patients with HER2-positive breast cancer and a residual breast lesion < 2 cm or unicentric cT1-2N0-1M0 triple-negative breast cancer. Patients in the study underwent radiotherapy alone after excluding invasive in-situ disease.
The Clinician’s Role in Shaping Conservative Surgical Approaches
De-escalating surgery in breast cancer should involve acknowledging the patient’s fears and misperceptions regarding the risks of cancer recurrence that can lead them to opt for more invasive surgical treatments. Patients may not or fully regard the long-term effects of electing an invasive procedure in the absence of clinical indications. For example, patients undergoing more invasive interventions may experience worse body image and quality of life.
Clinicians may also not adequately estimate other harms associated with unnecessary surgical interventions. Providing clinicians with data that focuses on the psychological outcomes and satisfaction of patients post surgery may help them to better interpret and consider patient values and wishes and minimize future unnecessary surgeries.
Breast cancer remains one of the best-studied cancers with multiple high-quality randomized controlled trials supporting de-escalation of surgery. De-escalation of breast cancer surgery has been successful in multiple ways, including the implementation of ALND in early-stage breast cancer. However, other options such as CPM remain common. Proper patient and physician education involving data from clinical trials and reports of patient satisfaction may further decrease unnecessary surgical interventions.
Nameera Temkar has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Breast cancer is the most common cancer in women worldwide and a leading cause of cancer-related deaths. The most common form of breast cancer is invasive ductal carcinoma, which accounts for 75%-80% of breast cancers. The second most common form is invasive lobular carcinoma, which accounts for 10%-15% of cases.
Surgical treatment of breast cancer involves removal and pathological staging of the cancerous tissue. Breast-conserving surgery and mastectomy are two surgical treatment options for patients with breast cancer. Breast-conserving surgery, which involves resection of the tumor and the surrounding margin of healthy tissue to achieve clean margins, is usually combined with radiotherapy. Mastectomy is considered in patients with relative and absolute contraindications to breast-conserving therapeutic options (eg, patients with a genetic predisposition to breast cancer, tumors > 5 cm, extensive margins, prior radiation to breast or chest wall, first-trimester pregnancy, extensive ductal carcinoma in situ, inflammatory breast cancer). Although surgical treatment of breast cancer is widely used, there have been calls to minimize unnecessary invasive surgical interventions in patients with early-stage breast cancer.
Reassessing the Role of Surgery in the Early Stages
Some surgical procedures, including axillary lymph node dissection (ALND) and contralateral prophylactic mastectomy (CPM), once considered standard treatment for early-stage breast cancer, are now being recognized as unnecessary in most cases of early-stage breast cancer without sentinel node metastases. Although ALND, which involves removal of all lymphatic tissue in the axilla, has been used for decades in the surgical management of early-stage breast cancer, this intervention typically results in lymphedema and significant morbidity.
Contralateral prophylactic mastectomy is a surgical option chosen by some women with early-stage unilateral breast cancer. However, this procedure is considered controversial in this patient population since evidence shows no survival advantage with CPM. A large-scale survey by Jagsi et al of female patients with in situ or early-stage breast cancer concluded that CPM was more common in patients who were White, had a higher level of education, and had private health insurance. In the study, 598 of the 1569 patients without an identified mutation or high genetic risk reported that a surgeon recommended against CPM. Of this group, only 1.9% underwent CPM. In contrast, of the 746 patients who reported that they did not receive any recommendation from a surgeon, 19% underwent CPM.
Re-excision and mastectomy are considered in patients with early-stage breast cancer when clear margins are not achieved with breast-conserving surgery. To prevent unnecessary reoperations and mastectomies, the 2013 invasive cancer margin consensus guideline by the American Society for Radiation Oncology (ASTRO) and the Society of Surgical Oncology, defined adequate margins in breast-conserving surgery in invasive breast cancer as “no ink on tumor.” The guideline is endorsed by the American Society of Breast Surgeons, ASTRO, and the St Gallen Consensus Conference.
A Shift in Practice: Moving Away From Routine Node Dissection
Based on findings from multiple clinical trials, experts recommend sentinel lymph node biopsy (SLNB) over ANLD and omit axillary surgery in certain patients. Findings from ACOSOG Z1071, SENTINA, and SN FNAC prospective multi-institutional trials support the use of SLNB as the initial diagnostic procedure. Sentinel lobe biopsy involves removal and evaluation of the first lymph node which receives lymphatic drainage from the breast cancer site. Negative biopsy findings on SLNB can avoid ALND as it is less likely that metastasis has occurred.
Although SLNB is preferred in younger patients with early-stage breast cancer, it is not routinely recommended for women aged ≥ 70 years of age with clinically node-negative, early-stage, HR-positive and HER2-negative breast cancer. This recommendation is based on study findings showing no difference in survival of women aged > 70 years with HR-positive clinical stage I breast cancer who did and did not undergo axillary evaluation.
The Z0011 trial by the American College of Surgeons Oncology Group found SLNB alone was not inferior to ALND regarding overall and disease-free survival in patients with clinically node-negative cancer undergoing breast conservation surgery and radiation therapy.
SLNB: A Less Invasive Alternative to ALND
Compared to SLNB, ALND is associated with more morbidity, physical symptoms, and poorer quality of life. A systemic review by Bakri et al evaluating the impact of ALND vs SLNB found higher rates of lymphedema, pain, reduced strength, and range of motion in patients who underwent ALND. In addition, an analysis of the National Cancer Database by Cocco et al found that patients with limited CN+ T1-2 breast cancer had favorable survival outcomes after undergoing SLNB and regional node irradiation vs ALND.
Rethinking First Steps: Non-Surgical Strategies
While surgical intervention with or without radiation therapy remains a primary treatment in early-stage breast cancer, there is an increased emphasis on de-escalation to minimize surgery and consider nonsurgical options in this patient population. A neoadjuvant systemic therapeutic approach by Kuerer et al for HER2-positive breast cancer and triple-negative breast cancer yielded a pathological complete response in 62% of patients. This multicenter, single-arm, phase 2 trial evaluated patients with HER2-positive breast cancer and a residual breast lesion < 2 cm or unicentric cT1-2N0-1M0 triple-negative breast cancer. Patients in the study underwent radiotherapy alone after excluding invasive in-situ disease.
The Clinician’s Role in Shaping Conservative Surgical Approaches
De-escalating surgery in breast cancer should involve acknowledging the patient’s fears and misperceptions regarding the risks of cancer recurrence that can lead them to opt for more invasive surgical treatments. Patients may not or fully regard the long-term effects of electing an invasive procedure in the absence of clinical indications. For example, patients undergoing more invasive interventions may experience worse body image and quality of life.
Clinicians may also not adequately estimate other harms associated with unnecessary surgical interventions. Providing clinicians with data that focuses on the psychological outcomes and satisfaction of patients post surgery may help them to better interpret and consider patient values and wishes and minimize future unnecessary surgeries.
Breast cancer remains one of the best-studied cancers with multiple high-quality randomized controlled trials supporting de-escalation of surgery. De-escalation of breast cancer surgery has been successful in multiple ways, including the implementation of ALND in early-stage breast cancer. However, other options such as CPM remain common. Proper patient and physician education involving data from clinical trials and reports of patient satisfaction may further decrease unnecessary surgical interventions.
Nameera Temkar has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Breast cancer is the most common cancer in women worldwide and a leading cause of cancer-related deaths. The most common form of breast cancer is invasive ductal carcinoma, which accounts for 75%-80% of breast cancers. The second most common form is invasive lobular carcinoma, which accounts for 10%-15% of cases.
Surgical treatment of breast cancer involves removal and pathological staging of the cancerous tissue. Breast-conserving surgery and mastectomy are two surgical treatment options for patients with breast cancer. Breast-conserving surgery, which involves resection of the tumor and the surrounding margin of healthy tissue to achieve clean margins, is usually combined with radiotherapy. Mastectomy is considered in patients with relative and absolute contraindications to breast-conserving therapeutic options (eg, patients with a genetic predisposition to breast cancer, tumors > 5 cm, extensive margins, prior radiation to breast or chest wall, first-trimester pregnancy, extensive ductal carcinoma in situ, inflammatory breast cancer). Although surgical treatment of breast cancer is widely used, there have been calls to minimize unnecessary invasive surgical interventions in patients with early-stage breast cancer.
Reassessing the Role of Surgery in the Early Stages
Some surgical procedures, including axillary lymph node dissection (ALND) and contralateral prophylactic mastectomy (CPM), once considered standard treatment for early-stage breast cancer, are now being recognized as unnecessary in most cases of early-stage breast cancer without sentinel node metastases. Although ALND, which involves removal of all lymphatic tissue in the axilla, has been used for decades in the surgical management of early-stage breast cancer, this intervention typically results in lymphedema and significant morbidity.
Contralateral prophylactic mastectomy is a surgical option chosen by some women with early-stage unilateral breast cancer. However, this procedure is considered controversial in this patient population since evidence shows no survival advantage with CPM. A large-scale survey by Jagsi et al of female patients with in situ or early-stage breast cancer concluded that CPM was more common in patients who were White, had a higher level of education, and had private health insurance. In the study, 598 of the 1569 patients without an identified mutation or high genetic risk reported that a surgeon recommended against CPM. Of this group, only 1.9% underwent CPM. In contrast, of the 746 patients who reported that they did not receive any recommendation from a surgeon, 19% underwent CPM.
Re-excision and mastectomy are considered in patients with early-stage breast cancer when clear margins are not achieved with breast-conserving surgery. To prevent unnecessary reoperations and mastectomies, the 2013 invasive cancer margin consensus guideline by the American Society for Radiation Oncology (ASTRO) and the Society of Surgical Oncology, defined adequate margins in breast-conserving surgery in invasive breast cancer as “no ink on tumor.” The guideline is endorsed by the American Society of Breast Surgeons, ASTRO, and the St Gallen Consensus Conference.
A Shift in Practice: Moving Away From Routine Node Dissection
Based on findings from multiple clinical trials, experts recommend sentinel lymph node biopsy (SLNB) over ANLD and omit axillary surgery in certain patients. Findings from ACOSOG Z1071, SENTINA, and SN FNAC prospective multi-institutional trials support the use of SLNB as the initial diagnostic procedure. Sentinel lobe biopsy involves removal and evaluation of the first lymph node which receives lymphatic drainage from the breast cancer site. Negative biopsy findings on SLNB can avoid ALND as it is less likely that metastasis has occurred.
Although SLNB is preferred in younger patients with early-stage breast cancer, it is not routinely recommended for women aged ≥ 70 years of age with clinically node-negative, early-stage, HR-positive and HER2-negative breast cancer. This recommendation is based on study findings showing no difference in survival of women aged > 70 years with HR-positive clinical stage I breast cancer who did and did not undergo axillary evaluation.
The Z0011 trial by the American College of Surgeons Oncology Group found SLNB alone was not inferior to ALND regarding overall and disease-free survival in patients with clinically node-negative cancer undergoing breast conservation surgery and radiation therapy.
SLNB: A Less Invasive Alternative to ALND
Compared to SLNB, ALND is associated with more morbidity, physical symptoms, and poorer quality of life. A systemic review by Bakri et al evaluating the impact of ALND vs SLNB found higher rates of lymphedema, pain, reduced strength, and range of motion in patients who underwent ALND. In addition, an analysis of the National Cancer Database by Cocco et al found that patients with limited CN+ T1-2 breast cancer had favorable survival outcomes after undergoing SLNB and regional node irradiation vs ALND.
Rethinking First Steps: Non-Surgical Strategies
While surgical intervention with or without radiation therapy remains a primary treatment in early-stage breast cancer, there is an increased emphasis on de-escalation to minimize surgery and consider nonsurgical options in this patient population. A neoadjuvant systemic therapeutic approach by Kuerer et al for HER2-positive breast cancer and triple-negative breast cancer yielded a pathological complete response in 62% of patients. This multicenter, single-arm, phase 2 trial evaluated patients with HER2-positive breast cancer and a residual breast lesion < 2 cm or unicentric cT1-2N0-1M0 triple-negative breast cancer. Patients in the study underwent radiotherapy alone after excluding invasive in-situ disease.
The Clinician’s Role in Shaping Conservative Surgical Approaches
De-escalating surgery in breast cancer should involve acknowledging the patient’s fears and misperceptions regarding the risks of cancer recurrence that can lead them to opt for more invasive surgical treatments. Patients may not or fully regard the long-term effects of electing an invasive procedure in the absence of clinical indications. For example, patients undergoing more invasive interventions may experience worse body image and quality of life.
Clinicians may also not adequately estimate other harms associated with unnecessary surgical interventions. Providing clinicians with data that focuses on the psychological outcomes and satisfaction of patients post surgery may help them to better interpret and consider patient values and wishes and minimize future unnecessary surgeries.
Breast cancer remains one of the best-studied cancers with multiple high-quality randomized controlled trials supporting de-escalation of surgery. De-escalation of breast cancer surgery has been successful in multiple ways, including the implementation of ALND in early-stage breast cancer. However, other options such as CPM remain common. Proper patient and physician education involving data from clinical trials and reports of patient satisfaction may further decrease unnecessary surgical interventions.
Nameera Temkar has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Veterans and Nonveterans Show Similar Mammogram Rates
TOPLINE: A national survey of 8996 females reveals comparable mammography screening rates between those who identify as veterans (57.9%) and nonveterans (55.2%).
METHODOLOGY:
Researchers analyzed data from the 2019 National Health Interview Survey, a cross-sectional national survey tracking health information.
Female respondents aged 40 to 74 years without history of breast cancer were included in the analysis.
Analysis evaluated the association between screening and veteran status through logistic regression, adjusting for potential confounders.
Survey procedures accounted for complex sampling design to obtain valid estimates for the civilian, noninstitutionalized US population.
TAKEAWAY:
Analysis included 8996 female survey respondents, including 169 veterans (1.9%) and 320 (3.2%) reported having military health coverage.
Mammography screening rates within the last year were comparable between veterans (57.9%) and nonveterans (55.2%).
Veteran status showed no significant association with differences in mammography screening percentages (P = .96).
Among insured participants, military health insurance demonstrated no significant association with mammography screening percentages (P = .13).
The authors suggest that radiology practices should design proactive outreach strategies to address the needs of the growing number of female veterans who may face increased breast cancer risk due to military environmental exposures.
IN PRACTICE: “Although the results from our study demonstrate comparable mammography screening percentages, veterans may face additional risk factors for breast cancer due to occupational,” the authors argue.
SOURCE: This summary is based on a preprint published online in the Journal of the American College of Radiology: Milton A, Miles R, Gettle LM, Van Geertruyden P, Narayan AK. Utilization of Mammography Screening in Female Veterans: Cross-Sectional Survey Results from the National Health Interview Survey. J Am Coll Radiol. Published online April 24, 2025. doi:10.1016/j.jacr.2025.04.017
LIMITATIONS: The study relied on self-reported adherence data, which could overestimate screening percentages. Data collection occurred prior to updated United States Preventive Services Task Force guidelines recommending routine mammography screening for women starting at age 40 years every 2 years. The relatively small number of female veteran respondents limited the precision of population estimates. Additionally, the data were collected before the COVID-19 pandemic, which has been associated with reduced mammographic screening, particularly in medically underserved populations.
DISCLOSURES: Anand Narayan disclosed receiving financial support from Susan G. Komen Breast Cancer Foundation and National Academy of Medicine. The study did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. The remaining authors reported no potential conflicts of interest. Additional disclosures are noted in the original article.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
TOPLINE: A national survey of 8996 females reveals comparable mammography screening rates between those who identify as veterans (57.9%) and nonveterans (55.2%).
METHODOLOGY:
Researchers analyzed data from the 2019 National Health Interview Survey, a cross-sectional national survey tracking health information.
Female respondents aged 40 to 74 years without history of breast cancer were included in the analysis.
Analysis evaluated the association between screening and veteran status through logistic regression, adjusting for potential confounders.
Survey procedures accounted for complex sampling design to obtain valid estimates for the civilian, noninstitutionalized US population.
TAKEAWAY:
Analysis included 8996 female survey respondents, including 169 veterans (1.9%) and 320 (3.2%) reported having military health coverage.
Mammography screening rates within the last year were comparable between veterans (57.9%) and nonveterans (55.2%).
Veteran status showed no significant association with differences in mammography screening percentages (P = .96).
Among insured participants, military health insurance demonstrated no significant association with mammography screening percentages (P = .13).
The authors suggest that radiology practices should design proactive outreach strategies to address the needs of the growing number of female veterans who may face increased breast cancer risk due to military environmental exposures.
IN PRACTICE: “Although the results from our study demonstrate comparable mammography screening percentages, veterans may face additional risk factors for breast cancer due to occupational,” the authors argue.
SOURCE: This summary is based on a preprint published online in the Journal of the American College of Radiology: Milton A, Miles R, Gettle LM, Van Geertruyden P, Narayan AK. Utilization of Mammography Screening in Female Veterans: Cross-Sectional Survey Results from the National Health Interview Survey. J Am Coll Radiol. Published online April 24, 2025. doi:10.1016/j.jacr.2025.04.017
LIMITATIONS: The study relied on self-reported adherence data, which could overestimate screening percentages. Data collection occurred prior to updated United States Preventive Services Task Force guidelines recommending routine mammography screening for women starting at age 40 years every 2 years. The relatively small number of female veteran respondents limited the precision of population estimates. Additionally, the data were collected before the COVID-19 pandemic, which has been associated with reduced mammographic screening, particularly in medically underserved populations.
DISCLOSURES: Anand Narayan disclosed receiving financial support from Susan G. Komen Breast Cancer Foundation and National Academy of Medicine. The study did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. The remaining authors reported no potential conflicts of interest. Additional disclosures are noted in the original article.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
TOPLINE: A national survey of 8996 females reveals comparable mammography screening rates between those who identify as veterans (57.9%) and nonveterans (55.2%).
METHODOLOGY:
Researchers analyzed data from the 2019 National Health Interview Survey, a cross-sectional national survey tracking health information.
Female respondents aged 40 to 74 years without history of breast cancer were included in the analysis.
Analysis evaluated the association between screening and veteran status through logistic regression, adjusting for potential confounders.
Survey procedures accounted for complex sampling design to obtain valid estimates for the civilian, noninstitutionalized US population.
TAKEAWAY:
Analysis included 8996 female survey respondents, including 169 veterans (1.9%) and 320 (3.2%) reported having military health coverage.
Mammography screening rates within the last year were comparable between veterans (57.9%) and nonveterans (55.2%).
Veteran status showed no significant association with differences in mammography screening percentages (P = .96).
Among insured participants, military health insurance demonstrated no significant association with mammography screening percentages (P = .13).
The authors suggest that radiology practices should design proactive outreach strategies to address the needs of the growing number of female veterans who may face increased breast cancer risk due to military environmental exposures.
IN PRACTICE: “Although the results from our study demonstrate comparable mammography screening percentages, veterans may face additional risk factors for breast cancer due to occupational,” the authors argue.
SOURCE: This summary is based on a preprint published online in the Journal of the American College of Radiology: Milton A, Miles R, Gettle LM, Van Geertruyden P, Narayan AK. Utilization of Mammography Screening in Female Veterans: Cross-Sectional Survey Results from the National Health Interview Survey. J Am Coll Radiol. Published online April 24, 2025. doi:10.1016/j.jacr.2025.04.017
LIMITATIONS: The study relied on self-reported adherence data, which could overestimate screening percentages. Data collection occurred prior to updated United States Preventive Services Task Force guidelines recommending routine mammography screening for women starting at age 40 years every 2 years. The relatively small number of female veteran respondents limited the precision of population estimates. Additionally, the data were collected before the COVID-19 pandemic, which has been associated with reduced mammographic screening, particularly in medically underserved populations.
DISCLOSURES: Anand Narayan disclosed receiving financial support from Susan G. Komen Breast Cancer Foundation and National Academy of Medicine. The study did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. The remaining authors reported no potential conflicts of interest. Additional disclosures are noted in the original article.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
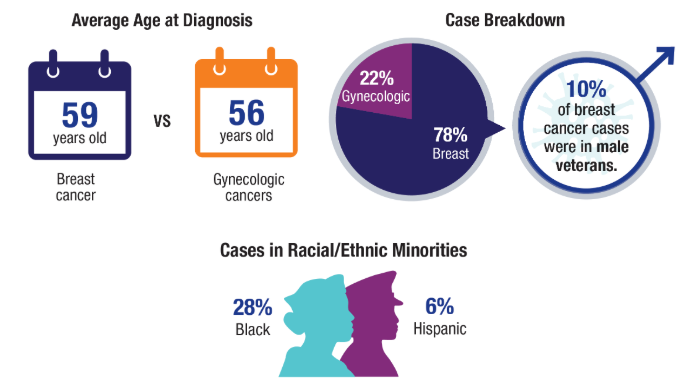
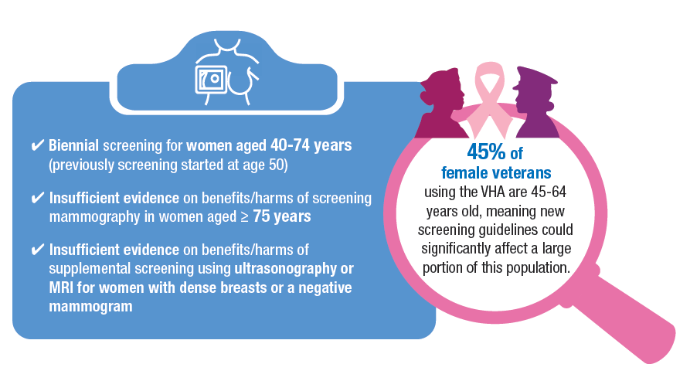
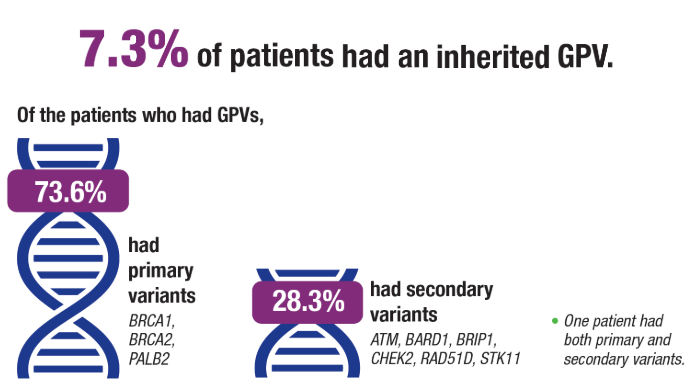
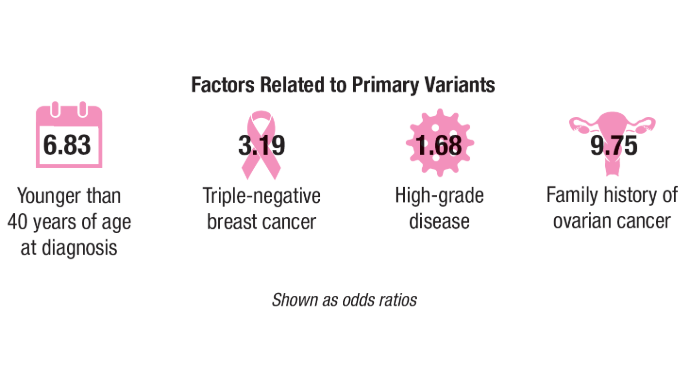
Breast and Uterine Cancer: Screening Guidelines, Genetic Testing, and Mortality Trends
Breast and Uterine Cancer: Screening Guidelines, Genetic Testing, and Mortality Trends
Click to view more from Cancer Data Trends 2025.
- Shepherd-Banigan M, Zullig LL, Berkowitz TSZ, et al. Improving Cancer Care
for Women Seeking Services in the Veterans Health Administration Through the
Breast and Gynecological Oncology System of Excellence. Mil Med. 2024:usae447.
doi:10.1093/milmed/usae447 - US Preventive Services Task Force, Nicholson WK, Silverstein M, et al. Screening
for Breast Cancer: US Preventive Services Task Force Recommendation Statement.
JAMA. 2024;331(22):1918-1930. doi:10.1001/jama.2024.5534 - VA announces steps to increase life-saving screening, access to benefits for
Veterans with cancer. VA News. March 8, 2024. Accessed January 14, 2025. https://
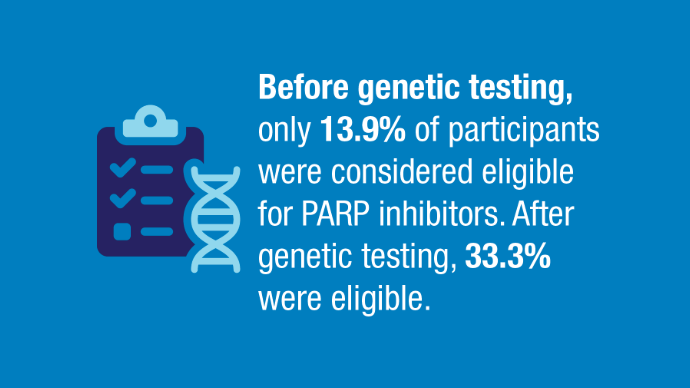
news.va.gov/press-room/va-expands-health-care-benefits-veterans-cancer/ - Rezoug Z, Totten SP, Szlachtycz D, et al. Universal Genetic Testing for Newly
Diagnosed Invasive Breast Cancer. JAMA Netw Open. 2024;7(9):e2431427.
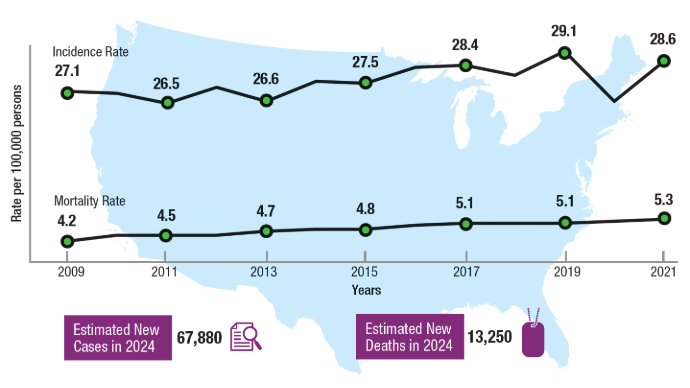
doi:10.1001/jamanetworkopen.2024.31427 - National Institutes of Health. National Cancer Institute. Surveillance, Epidemiology,
and End Results Program. Cancer Stat Facts: Uterine Cancer. Accessed January 14,
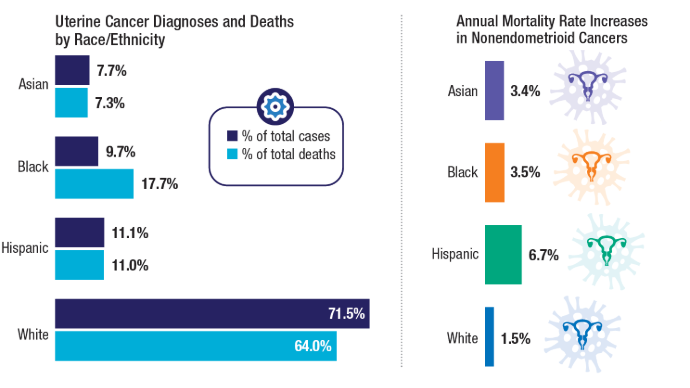
2025. https://seer.cancer.gov/statfacts/html/corp.html - Clarke MA, Devesa SS, Hammer A, Wentzensen N. Racial and Ethnic Differences in
Hysterectomy-Corrected Uterine Corpus Cancer Mortality by Stage and Histologic
Subtype. JAMA Oncol. 2022;8(6):895-903. doi:10.1001/jamaoncol.2022.0009 - Moss HA, Rasmussen, KM, Patil, V, et al. Demographic Characteristics of Veterans
Diagnosed With Breast and Gynecologic Cancers: A Comparative Analysis With the
General Population. Abstract presented at: Annual Meeting of the Association of
VA Hematology/Oncology (AVAHO); September 29–October 1, 2023; Chicago, IL.
Abstract 47. - Breland JY, Frayne SM, Saechao F, Gujral K, Vashi AA, Shaw JG, Gray KM, Illarmo SS,
Urech T, Grant N, Berg E, Offer C, Veldanda S, Schoemaker L, Dalton AL, Esmaeili
A, Phibbs CS, Hayes PM, Haskell S. Sourcebook: Women Veterans in the Veterans
Health Administration. Volume 5: Longitudinal Trends in Sociodemographics and
Utilization, Including Type, Modality, and Source of Care. Women’s Health Evaluation
Initiative, Office of Women’s Health, Veterans Health Administration, Department of
Veterans Affairs, Washington DC. June 2024. - NCCN: National Comprehensive Cancer Network. Breast Cancer Screening and
Diagnosis. V2.2024 April 9, 2024. Accessed January 14, 2025. https://www.nccn.
org/professionals/physician_gls/pdf/breast-screening.pdf - ACS: American Cancer Society. Breast Cancer Early Detection and Diagnosis.
Revised December 19, 2023. Accessed January 14, 2025. https://www.cancer.org/
cancer/types/breast-cancer/screening-tests-and-early-detection/american-cancersociety-
recommendations-for-the-early-detection-of-breast-cancer.html - Somasegar S, Bashi A, Lang SM, et al. Trends in Uterine Cancer Mortality
in the United States: A 50-Year Population-Based Analysis. Obstet Gynecol.
2023;142(4):978-986. doi:10.1097/AOG.0000000000005321
Click to view more from Cancer Data Trends 2025.
Click to view more from Cancer Data Trends 2025.
- Shepherd-Banigan M, Zullig LL, Berkowitz TSZ, et al. Improving Cancer Care
for Women Seeking Services in the Veterans Health Administration Through the
Breast and Gynecological Oncology System of Excellence. Mil Med. 2024:usae447.
doi:10.1093/milmed/usae447 - US Preventive Services Task Force, Nicholson WK, Silverstein M, et al. Screening
for Breast Cancer: US Preventive Services Task Force Recommendation Statement.
JAMA. 2024;331(22):1918-1930. doi:10.1001/jama.2024.5534 - VA announces steps to increase life-saving screening, access to benefits for
Veterans with cancer. VA News. March 8, 2024. Accessed January 14, 2025. https://
news.va.gov/press-room/va-expands-health-care-benefits-veterans-cancer/ - Rezoug Z, Totten SP, Szlachtycz D, et al. Universal Genetic Testing for Newly
Diagnosed Invasive Breast Cancer. JAMA Netw Open. 2024;7(9):e2431427.
doi:10.1001/jamanetworkopen.2024.31427 - National Institutes of Health. National Cancer Institute. Surveillance, Epidemiology,
and End Results Program. Cancer Stat Facts: Uterine Cancer. Accessed January 14,
2025. https://seer.cancer.gov/statfacts/html/corp.html - Clarke MA, Devesa SS, Hammer A, Wentzensen N. Racial and Ethnic Differences in
Hysterectomy-Corrected Uterine Corpus Cancer Mortality by Stage and Histologic
Subtype. JAMA Oncol. 2022;8(6):895-903. doi:10.1001/jamaoncol.2022.0009 - Moss HA, Rasmussen, KM, Patil, V, et al. Demographic Characteristics of Veterans
Diagnosed With Breast and Gynecologic Cancers: A Comparative Analysis With the
General Population. Abstract presented at: Annual Meeting of the Association of
VA Hematology/Oncology (AVAHO); September 29–October 1, 2023; Chicago, IL.
Abstract 47. - Breland JY, Frayne SM, Saechao F, Gujral K, Vashi AA, Shaw JG, Gray KM, Illarmo SS,
Urech T, Grant N, Berg E, Offer C, Veldanda S, Schoemaker L, Dalton AL, Esmaeili
A, Phibbs CS, Hayes PM, Haskell S. Sourcebook: Women Veterans in the Veterans
Health Administration. Volume 5: Longitudinal Trends in Sociodemographics and
Utilization, Including Type, Modality, and Source of Care. Women’s Health Evaluation
Initiative, Office of Women’s Health, Veterans Health Administration, Department of
Veterans Affairs, Washington DC. June 2024. - NCCN: National Comprehensive Cancer Network. Breast Cancer Screening and
Diagnosis. V2.2024 April 9, 2024. Accessed January 14, 2025. https://www.nccn.
org/professionals/physician_gls/pdf/breast-screening.pdf - ACS: American Cancer Society. Breast Cancer Early Detection and Diagnosis.
Revised December 19, 2023. Accessed January 14, 2025. https://www.cancer.org/
cancer/types/breast-cancer/screening-tests-and-early-detection/american-cancersociety-
recommendations-for-the-early-detection-of-breast-cancer.html - Somasegar S, Bashi A, Lang SM, et al. Trends in Uterine Cancer Mortality
in the United States: A 50-Year Population-Based Analysis. Obstet Gynecol.
2023;142(4):978-986. doi:10.1097/AOG.0000000000005321
- Shepherd-Banigan M, Zullig LL, Berkowitz TSZ, et al. Improving Cancer Care
for Women Seeking Services in the Veterans Health Administration Through the
Breast and Gynecological Oncology System of Excellence. Mil Med. 2024:usae447.
doi:10.1093/milmed/usae447 - US Preventive Services Task Force, Nicholson WK, Silverstein M, et al. Screening
for Breast Cancer: US Preventive Services Task Force Recommendation Statement.
JAMA. 2024;331(22):1918-1930. doi:10.1001/jama.2024.5534 - VA announces steps to increase life-saving screening, access to benefits for
Veterans with cancer. VA News. March 8, 2024. Accessed January 14, 2025. https://
news.va.gov/press-room/va-expands-health-care-benefits-veterans-cancer/ - Rezoug Z, Totten SP, Szlachtycz D, et al. Universal Genetic Testing for Newly
Diagnosed Invasive Breast Cancer. JAMA Netw Open. 2024;7(9):e2431427.
doi:10.1001/jamanetworkopen.2024.31427 - National Institutes of Health. National Cancer Institute. Surveillance, Epidemiology,
and End Results Program. Cancer Stat Facts: Uterine Cancer. Accessed January 14,
2025. https://seer.cancer.gov/statfacts/html/corp.html - Clarke MA, Devesa SS, Hammer A, Wentzensen N. Racial and Ethnic Differences in
Hysterectomy-Corrected Uterine Corpus Cancer Mortality by Stage and Histologic
Subtype. JAMA Oncol. 2022;8(6):895-903. doi:10.1001/jamaoncol.2022.0009 - Moss HA, Rasmussen, KM, Patil, V, et al. Demographic Characteristics of Veterans
Diagnosed With Breast and Gynecologic Cancers: A Comparative Analysis With the
General Population. Abstract presented at: Annual Meeting of the Association of
VA Hematology/Oncology (AVAHO); September 29–October 1, 2023; Chicago, IL.
Abstract 47. - Breland JY, Frayne SM, Saechao F, Gujral K, Vashi AA, Shaw JG, Gray KM, Illarmo SS,
Urech T, Grant N, Berg E, Offer C, Veldanda S, Schoemaker L, Dalton AL, Esmaeili
A, Phibbs CS, Hayes PM, Haskell S. Sourcebook: Women Veterans in the Veterans
Health Administration. Volume 5: Longitudinal Trends in Sociodemographics and
Utilization, Including Type, Modality, and Source of Care. Women’s Health Evaluation
Initiative, Office of Women’s Health, Veterans Health Administration, Department of
Veterans Affairs, Washington DC. June 2024. - NCCN: National Comprehensive Cancer Network. Breast Cancer Screening and
Diagnosis. V2.2024 April 9, 2024. Accessed January 14, 2025. https://www.nccn.
org/professionals/physician_gls/pdf/breast-screening.pdf - ACS: American Cancer Society. Breast Cancer Early Detection and Diagnosis.
Revised December 19, 2023. Accessed January 14, 2025. https://www.cancer.org/
cancer/types/breast-cancer/screening-tests-and-early-detection/american-cancersociety-
recommendations-for-the-early-detection-of-breast-cancer.html - Somasegar S, Bashi A, Lang SM, et al. Trends in Uterine Cancer Mortality
in the United States: A 50-Year Population-Based Analysis. Obstet Gynecol.
2023;142(4):978-986. doi:10.1097/AOG.0000000000005321
Breast and Uterine Cancer: Screening Guidelines, Genetic Testing, and Mortality Trends
Breast and Uterine Cancer: Screening Guidelines, Genetic Testing, and Mortality Trends
PATINA Trial Shifts Paradigm in HER2+/ER+ Breast Cancer Treatment, Prolonging Survival With Targeted Combination Therapy
This is a transcript of a video essay, which can be found on Medscape.
I’m here with you today to talk about what I think was one of the most important trials reported at the December San Antonio Breast Cancer Symposium meeting, the PATINA trial.
This is a trial that was not on our radar as we were looking forward to the meeting. In fact, it wasn’t on the agenda because the results didn’t become available until about a week and a half before the meeting kicked off. Kudos to the authors for getting these data out there, and to the organizers for recognizing the importance and finding a way to add this to the program.
The PATINA trial enrolled patients whose tumors were both HER2 positive and ER positive. That is about half of our patients with HER2-positive disease.
Almost all of our trials looking at HER2-targeted therapies did not allow patients to continue antiestrogen therapy. Patients could have had antiestrogen therapy before they came to those HER2-focused trials. Some did, some may not have. It was not a requirement, but they could not continue it.
The same is true for patients with ER-positive disease. If your disease was ER positive and HER2 positive, you were excluded from all of our recent trials focusing on ER-positive disease. That includes those looking at the benefit of cyclin-dependent kinase inhibitors.
It also includes those looking at PI3 kinase inhibitors, AKT inhibitors, and selective estrogen receptor downregulators in their oral formulations. We›ve had to pick: Do we want to focus on HER2 or do we want to focus on ER? The PATINA trial results are not only important for practice, but they also show us the problem in that dichotomy.
PATINA enrolled patients who were receiving their first chemotherapy and HER2-targeted therapy for metastatic disease. Once they had received at least four cycles of combined therapy, they could receive additional chemotherapy, but they could also move into a maintenance phase if their disease was responding or stable, continuing HER2-targeted therapy alone without chemotherapy.
At that point, hormone therapy was reintroduced. This is a common practice for many of us. Those patients were then randomized to either palbociclib or not. This was a large effort, with 518 patients in this randomized trial. The expectations of progression-free survival were based on the results of the CLEOPATRA trial.
The trial assumed about a 15-month progression-free survival in those randomized to the control arm. What was actually observed was a 29-month progression-free survival. Two things might have contributed to this difference.
First, the CLEOPATRA trial did not allow patients to receive concurrent hormone therapy, and that may have had a major impact on its own. Also, CLEOPATRA reported the PFS for all of the patients enrolled. To get into PATINA, you had to be responding or stable to your initial combined modality therapy. Those patients with really resistant disease who progressed early were excluded, and that may have had an impact as well.
With the addition of palbociclib, that 29-month progression-free survival became 44 months. Stop and think about this. There was almost a 4-year period of time where patients were on trastuzumab and pertuzumab, an aromatase inhibitor, and a cyclin-dependent kinase inhibitor. No chemotherapy, much less day-to-day toxicities — not no toxicity, but less of the day-to-day toxicities that patients are really troubled by.
We don’t yet have mature overall survival data. Those will be coming. You can imagine with progression-free survival nearing 4 years, overall survival data will be some months or years hence until there are enough events for us to look at that evaluation.
Realizing that there are going to be issues with insurance approval and regulatory approvals, I would like to take these results into account for my patients in that situation.
It also challenges those of us who are developing clinical trials and drugs to realize that studying targets in isolation is needed early in the development of new agents. To get the maximum benefit for our patients, you need to put those building blocks back together and stop this forced dichotomy.
That doesn’t serve our patients well and it’s not where we will need to be in the future.
Kathy D. Miller, Professor of Medicine, Indiana University School of Medicine; Co-Director, Breast Cancer Program, Indiana University Simon Cancer Center, Indianapolis, Indiana, has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
This is a transcript of a video essay, which can be found on Medscape.
I’m here with you today to talk about what I think was one of the most important trials reported at the December San Antonio Breast Cancer Symposium meeting, the PATINA trial.
This is a trial that was not on our radar as we were looking forward to the meeting. In fact, it wasn’t on the agenda because the results didn’t become available until about a week and a half before the meeting kicked off. Kudos to the authors for getting these data out there, and to the organizers for recognizing the importance and finding a way to add this to the program.
The PATINA trial enrolled patients whose tumors were both HER2 positive and ER positive. That is about half of our patients with HER2-positive disease.
Almost all of our trials looking at HER2-targeted therapies did not allow patients to continue antiestrogen therapy. Patients could have had antiestrogen therapy before they came to those HER2-focused trials. Some did, some may not have. It was not a requirement, but they could not continue it.
The same is true for patients with ER-positive disease. If your disease was ER positive and HER2 positive, you were excluded from all of our recent trials focusing on ER-positive disease. That includes those looking at the benefit of cyclin-dependent kinase inhibitors.
It also includes those looking at PI3 kinase inhibitors, AKT inhibitors, and selective estrogen receptor downregulators in their oral formulations. We›ve had to pick: Do we want to focus on HER2 or do we want to focus on ER? The PATINA trial results are not only important for practice, but they also show us the problem in that dichotomy.
PATINA enrolled patients who were receiving their first chemotherapy and HER2-targeted therapy for metastatic disease. Once they had received at least four cycles of combined therapy, they could receive additional chemotherapy, but they could also move into a maintenance phase if their disease was responding or stable, continuing HER2-targeted therapy alone without chemotherapy.
At that point, hormone therapy was reintroduced. This is a common practice for many of us. Those patients were then randomized to either palbociclib or not. This was a large effort, with 518 patients in this randomized trial. The expectations of progression-free survival were based on the results of the CLEOPATRA trial.
The trial assumed about a 15-month progression-free survival in those randomized to the control arm. What was actually observed was a 29-month progression-free survival. Two things might have contributed to this difference.
First, the CLEOPATRA trial did not allow patients to receive concurrent hormone therapy, and that may have had a major impact on its own. Also, CLEOPATRA reported the PFS for all of the patients enrolled. To get into PATINA, you had to be responding or stable to your initial combined modality therapy. Those patients with really resistant disease who progressed early were excluded, and that may have had an impact as well.
With the addition of palbociclib, that 29-month progression-free survival became 44 months. Stop and think about this. There was almost a 4-year period of time where patients were on trastuzumab and pertuzumab, an aromatase inhibitor, and a cyclin-dependent kinase inhibitor. No chemotherapy, much less day-to-day toxicities — not no toxicity, but less of the day-to-day toxicities that patients are really troubled by.
We don’t yet have mature overall survival data. Those will be coming. You can imagine with progression-free survival nearing 4 years, overall survival data will be some months or years hence until there are enough events for us to look at that evaluation.
Realizing that there are going to be issues with insurance approval and regulatory approvals, I would like to take these results into account for my patients in that situation.
It also challenges those of us who are developing clinical trials and drugs to realize that studying targets in isolation is needed early in the development of new agents. To get the maximum benefit for our patients, you need to put those building blocks back together and stop this forced dichotomy.
That doesn’t serve our patients well and it’s not where we will need to be in the future.
Kathy D. Miller, Professor of Medicine, Indiana University School of Medicine; Co-Director, Breast Cancer Program, Indiana University Simon Cancer Center, Indianapolis, Indiana, has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
This is a transcript of a video essay, which can be found on Medscape.
I’m here with you today to talk about what I think was one of the most important trials reported at the December San Antonio Breast Cancer Symposium meeting, the PATINA trial.
This is a trial that was not on our radar as we were looking forward to the meeting. In fact, it wasn’t on the agenda because the results didn’t become available until about a week and a half before the meeting kicked off. Kudos to the authors for getting these data out there, and to the organizers for recognizing the importance and finding a way to add this to the program.
The PATINA trial enrolled patients whose tumors were both HER2 positive and ER positive. That is about half of our patients with HER2-positive disease.
Almost all of our trials looking at HER2-targeted therapies did not allow patients to continue antiestrogen therapy. Patients could have had antiestrogen therapy before they came to those HER2-focused trials. Some did, some may not have. It was not a requirement, but they could not continue it.
The same is true for patients with ER-positive disease. If your disease was ER positive and HER2 positive, you were excluded from all of our recent trials focusing on ER-positive disease. That includes those looking at the benefit of cyclin-dependent kinase inhibitors.
It also includes those looking at PI3 kinase inhibitors, AKT inhibitors, and selective estrogen receptor downregulators in their oral formulations. We›ve had to pick: Do we want to focus on HER2 or do we want to focus on ER? The PATINA trial results are not only important for practice, but they also show us the problem in that dichotomy.
PATINA enrolled patients who were receiving their first chemotherapy and HER2-targeted therapy for metastatic disease. Once they had received at least four cycles of combined therapy, they could receive additional chemotherapy, but they could also move into a maintenance phase if their disease was responding or stable, continuing HER2-targeted therapy alone without chemotherapy.
At that point, hormone therapy was reintroduced. This is a common practice for many of us. Those patients were then randomized to either palbociclib or not. This was a large effort, with 518 patients in this randomized trial. The expectations of progression-free survival were based on the results of the CLEOPATRA trial.
The trial assumed about a 15-month progression-free survival in those randomized to the control arm. What was actually observed was a 29-month progression-free survival. Two things might have contributed to this difference.
First, the CLEOPATRA trial did not allow patients to receive concurrent hormone therapy, and that may have had a major impact on its own. Also, CLEOPATRA reported the PFS for all of the patients enrolled. To get into PATINA, you had to be responding or stable to your initial combined modality therapy. Those patients with really resistant disease who progressed early were excluded, and that may have had an impact as well.
With the addition of palbociclib, that 29-month progression-free survival became 44 months. Stop and think about this. There was almost a 4-year period of time where patients were on trastuzumab and pertuzumab, an aromatase inhibitor, and a cyclin-dependent kinase inhibitor. No chemotherapy, much less day-to-day toxicities — not no toxicity, but less of the day-to-day toxicities that patients are really troubled by.
We don’t yet have mature overall survival data. Those will be coming. You can imagine with progression-free survival nearing 4 years, overall survival data will be some months or years hence until there are enough events for us to look at that evaluation.
Realizing that there are going to be issues with insurance approval and regulatory approvals, I would like to take these results into account for my patients in that situation.
It also challenges those of us who are developing clinical trials and drugs to realize that studying targets in isolation is needed early in the development of new agents. To get the maximum benefit for our patients, you need to put those building blocks back together and stop this forced dichotomy.
That doesn’t serve our patients well and it’s not where we will need to be in the future.
Kathy D. Miller, Professor of Medicine, Indiana University School of Medicine; Co-Director, Breast Cancer Program, Indiana University Simon Cancer Center, Indianapolis, Indiana, has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Red Wine May Not Be a Health Tonic, But Is It a Cancer Risk?
Earlier this month, US surgeon general Vivek Murthy, MD, issued an advisory, calling for alcoholic beverages to carry a warning label about cancer risk. The advisory flagged alcohol as the third leading preventable cause of cancer in the United States, after tobacco and obesity, and highlighted people’s limited awareness about the relationship between alcohol and cancer risk.
But, when it comes to cancer risk, are all types of alcohol created equal?
For many years, red wine seemed to be an outlier, with studies indicating that, in moderation, it might even be good for you. Red wine has anti-inflammatory and antioxidant properties — most notably, it contains the antioxidant resveratrol. Starting in the 1990s, research began to hint that the compound might protect against heart disease, aging, and cancer, though much of this work was done in animals or test tubes.
The idea that red wine carries health benefits, however, has been called into question more recently. A recent meta-analysis, for instance, suggests that many previous studies touting the health benefits of more moderate drinking were likely biased, potentially leading to “misleading positive health associations.” And one recent study found that alcohol consumption, largely red wine and beer, at all levels was linked to an increased risk for cardiovascular disease.
Although wine’s health halo is dwindling, there might be an exception: Cancer risk.
Overall, research shows that even light to moderate drinking increases the risk for at least seven types of cancer, but when focusing on red wine, in particular, that risk calculus can look different.
“It’s very complicated and nuanced,” said Timothy Rebbeck, PhD, professor of cancer prevention, Harvard T.H. Chan School of Public Health, Boston. “And ‘complicated and nuanced’ doesn’t work very well in public health messages.”
The Knowns About Alcohol and Cancer Risk
Some things about the relationship between alcohol and cancer risk are crystal clear. “There’s no question that alcohol is a group 1 carcinogen,” Rebbeck said. “Alcohol can cause cancer.”
Groups including the International Agency for Research on Cancer (IARC) and American Cancer Society agree that alcohol use is an established cause of seven types of cancer: Those of the oral cavity, larynx, pharynx, esophagus (squamous cell carcinoma), liver (hepatocellular carcinoma), breast, and colon/rectum. Heavy drinking — at least 8 standard drinks a week for women and 15 for men — and binge drinking — 4 or more drinks in 2 hours for women and 5 or more for men — only amplify that risk. (A “standard” drink has 14 g of alcohol, which translates to a 5-oz glass of wine.)
“We’re most concerned about high-risk drinking — more than 2 drinks a day — and/or binge drinking,” said Noelle LoConte, MD, of the Division of Hematology, Medical Oncology and Palliative Care, University of Wisconsin School of Medicine and Public Health, Madison, who authored a 2018 statement on alcohol and cancer risk from the American Society of Clinical Oncology (ASCO).
Compared with not drinking, heavy drinking is linked with a roughly fivefold increase in the risk for oral cavity, pharyngeal, and esophageal cancers, and a 61% increase in the risk for breast cancer, according to LoConte and colleagues.
Things get murkier when it comes to moderate drinking — defined as up to 1 standard drink per day for women and 2 per day for men. There is evidence, LoConte said, that moderate drinking is associated with increased cancer risks, though the magnitude is generally much less than heavier drinking.
Cancer type also matters. One analysis found that the risk for breast cancer increased with even light to moderate alcohol consumption. Compared with no drinking, light to moderate drinking has also been linked to increased risks for oral cavity, pharynx, larynx, and esophageal cancers.
As for whether the type of alcoholic beverage matters, LoConte said, there’s no clear physiological reason that wine would be less risky than beer or liquor. Research indicates that ethanol is the problematic ingredient: Once ingested, it’s metabolized into acetaldehyde, a DNA-damaging substance that’s considered a probable human carcinogen. Ethanol can also alter circulating levels of estrogens and androgens, LoConte said, which is thought to drive its association with breast cancer risk.
“It likely doesn’t matter how you choose to get your ethanol,” she said. “It’s a question of volume.”
Hints That Wine Is an Outlier
Still, some studies suggest that how people ingest ethanol could make a difference.
A study published in August in JAMA Network Open is a case in point. The study found that, among older adults, light to heavy drinkers had an increased risk of dying from cancer, compared with occasional drinkers (though the increased risk among light to moderate drinkers occurred only among people who also had chronic health conditions, such as diabetes or high blood pressure, or were of lower socioeconomic status).
Wine drinkers fared differently. Most notably, drinkers who “preferred” wine — consuming over 80% of total ethanol from wine — or those who drank only with meals showed a small reduction in their risk for cancer mortality and all-cause mortality (hazard ratio [HR], 0.94 for both). The small protective association was somewhat stronger among people who reported both patterns (HR, 0.88), especially if they were of lower socioeconomic status (HR, 0.79).
The findings are in line with other research suggesting that wine drinkers may be outliers when it comes to cancer risk. A 2023 meta-analysis of 26 observational studies, for instance, found no association between wine consumption and any cancer type, with the caveat that there was «substantial» heterogeneity among the studies.
This heterogeneity caveat speaks to the inherent limitations of observational research, said Tim Stockwell, PhD, of the Canadian Institute for Substance Use Research, University of Victoria in British Columbia, Canada.
“Individual studies of alcohol and cancer risk do find differences by type of drink, or patterns of drinking,” Stockwell said. “But it’s so hard to unpack the confounding that goes along with the type of person who’s a wine drinker or a beer drinker or a spirit drinker. The beverage of choice seems to come with a lot of baggage.”
Compared with people who favor beer or liquor, he noted, wine aficionados are typically higher-income, exercise more often, smoke less, and have different diets, for example. The “best” studies, Rebbeck said, try to adjust for those differences, but it’s challenging.
The authors of the 2023 meta-analysis noted that “many components in wine could have anticarcinogenic effects” that theoretically could counter the ill effects of ethanol. Besides resveratrol, which is mainly found in red wine, the list includes anthocyanins, quercetin, and tannins. However, the authors also acknowledged that they couldn’t account for whether other lifestyle habits might explain why wine drinkers, overall, showed no increased cancer risks and sometimes lower risks.
Still, groups such as the IARC and ASCO hold that there is no known “safe” level, or type, of alcohol when it comes to cancer.
In the latest Canadian guidelines on alcohol use, the scientific panel calculated that people who have 6 drinks a week throughout adulthood (whatever the source of the alcohol) could shave 11 weeks from their life expectancy, on average, said Stockwell, who was on the guideline panel. Compare that with heavy drinking, where 4 drinks a day could rob the average person of 2 or 3 years. “If you’re drinking a lot, you could get huge benefits from cutting down,” Stockwell explained. “If you’re a moderate drinker, the benefits would obviously be less.”
Stockwell said that choices around drinking and breast cancer risk, specifically, can be “tough.” Unlike many of the other alcohol-associated cancers, he noted, breast cancer is common — so even small relative risk increases may be concerning. Based on a 2020 meta-analysis of 22 cohort studies, the risk for breast cancer rises by about 10%, on average, for every 10 g of alcohol a woman drinks per day. This study also found no evidence that wine is any different from other types of alcohol.
In real life, the calculus around wine consumption and cancer risk will probably vary widely from person to person, Rebbeck said. One woman with a family history of breast cancer might decide that having wine with dinner isn’t worth it. Another with the same family history might see that glass of wine as a stress reliever and opt to focus on other ways to reduce her breast cancer risk — by exercising and maintaining a healthy weight, for example.
“The bottom line is, in human studies, the data on light to moderate drinking and cancer are limited and messy, and you can’t draw firm conclusions from them,” Rebbeck said. “It probably raises risk in some people, but we don’t know who those people are. And the risk increases are relatively small.”
A Conversation Few Are Having
Even with many studies highlighting the connection between alcohol consumption and cancer risk, most people remain unaware about this risk.
A 2023 study by the National Cancer Institute found that only a minority of US adults knew that drinking alcohol is linked to increased cancer risk, and they were much less likely to say that was true of wine: Only 20% did, vs 31% who said that liquor can boost cancer risk. Meanwhile, 10% believed that wine helps prevent cancer. Other studies show that even among cancer survivors and patients undergoing active cancer treatment, many drink — often heavily.
“What we know right now is, physicians almost never talk about this,” LoConte said.
That could be due to time constraints, according to Rebbeck, or clinicians’ perceptions that the subject is too complicated and/or their own confusion about the data. There could also be some “cognitive dissonance” at play, LoConte noted, because many doctors drink alcohol.
It’s critical, she said, that conversations about drinking habits become “normalized,” and that should include informing patients that alcohol use is associated with certain cancers. Again, LoConte said, it’s high-risk drinking that’s most concerning and where reducing intake could have the biggest impact on cancer risk and other health outcomes.
“From a cancer prevention standpoint, it’s probably best not to drink,” she said. “But people don’t make choices based solely on cancer risk. We don’t want to come out with recommendations saying no one should drink. I don’t think the data support that, and people would buck against that advice.”
Rebbeck made a similar point. Even if there’s uncertainty about the risks for a daily glass of wine, he said, people can use that information to make decisions. “Everybody’s preferences and choices are going to be different,” Rebbeck said. “And that’s all we can really do.”
A version of this article appeared on Medscape.com.
Earlier this month, US surgeon general Vivek Murthy, MD, issued an advisory, calling for alcoholic beverages to carry a warning label about cancer risk. The advisory flagged alcohol as the third leading preventable cause of cancer in the United States, after tobacco and obesity, and highlighted people’s limited awareness about the relationship between alcohol and cancer risk.
But, when it comes to cancer risk, are all types of alcohol created equal?
For many years, red wine seemed to be an outlier, with studies indicating that, in moderation, it might even be good for you. Red wine has anti-inflammatory and antioxidant properties — most notably, it contains the antioxidant resveratrol. Starting in the 1990s, research began to hint that the compound might protect against heart disease, aging, and cancer, though much of this work was done in animals or test tubes.
The idea that red wine carries health benefits, however, has been called into question more recently. A recent meta-analysis, for instance, suggests that many previous studies touting the health benefits of more moderate drinking were likely biased, potentially leading to “misleading positive health associations.” And one recent study found that alcohol consumption, largely red wine and beer, at all levels was linked to an increased risk for cardiovascular disease.
Although wine’s health halo is dwindling, there might be an exception: Cancer risk.
Overall, research shows that even light to moderate drinking increases the risk for at least seven types of cancer, but when focusing on red wine, in particular, that risk calculus can look different.
“It’s very complicated and nuanced,” said Timothy Rebbeck, PhD, professor of cancer prevention, Harvard T.H. Chan School of Public Health, Boston. “And ‘complicated and nuanced’ doesn’t work very well in public health messages.”
The Knowns About Alcohol and Cancer Risk
Some things about the relationship between alcohol and cancer risk are crystal clear. “There’s no question that alcohol is a group 1 carcinogen,” Rebbeck said. “Alcohol can cause cancer.”
Groups including the International Agency for Research on Cancer (IARC) and American Cancer Society agree that alcohol use is an established cause of seven types of cancer: Those of the oral cavity, larynx, pharynx, esophagus (squamous cell carcinoma), liver (hepatocellular carcinoma), breast, and colon/rectum. Heavy drinking — at least 8 standard drinks a week for women and 15 for men — and binge drinking — 4 or more drinks in 2 hours for women and 5 or more for men — only amplify that risk. (A “standard” drink has 14 g of alcohol, which translates to a 5-oz glass of wine.)
“We’re most concerned about high-risk drinking — more than 2 drinks a day — and/or binge drinking,” said Noelle LoConte, MD, of the Division of Hematology, Medical Oncology and Palliative Care, University of Wisconsin School of Medicine and Public Health, Madison, who authored a 2018 statement on alcohol and cancer risk from the American Society of Clinical Oncology (ASCO).
Compared with not drinking, heavy drinking is linked with a roughly fivefold increase in the risk for oral cavity, pharyngeal, and esophageal cancers, and a 61% increase in the risk for breast cancer, according to LoConte and colleagues.
Things get murkier when it comes to moderate drinking — defined as up to 1 standard drink per day for women and 2 per day for men. There is evidence, LoConte said, that moderate drinking is associated with increased cancer risks, though the magnitude is generally much less than heavier drinking.
Cancer type also matters. One analysis found that the risk for breast cancer increased with even light to moderate alcohol consumption. Compared with no drinking, light to moderate drinking has also been linked to increased risks for oral cavity, pharynx, larynx, and esophageal cancers.
As for whether the type of alcoholic beverage matters, LoConte said, there’s no clear physiological reason that wine would be less risky than beer or liquor. Research indicates that ethanol is the problematic ingredient: Once ingested, it’s metabolized into acetaldehyde, a DNA-damaging substance that’s considered a probable human carcinogen. Ethanol can also alter circulating levels of estrogens and androgens, LoConte said, which is thought to drive its association with breast cancer risk.
“It likely doesn’t matter how you choose to get your ethanol,” she said. “It’s a question of volume.”
Hints That Wine Is an Outlier
Still, some studies suggest that how people ingest ethanol could make a difference.
A study published in August in JAMA Network Open is a case in point. The study found that, among older adults, light to heavy drinkers had an increased risk of dying from cancer, compared with occasional drinkers (though the increased risk among light to moderate drinkers occurred only among people who also had chronic health conditions, such as diabetes or high blood pressure, or were of lower socioeconomic status).
Wine drinkers fared differently. Most notably, drinkers who “preferred” wine — consuming over 80% of total ethanol from wine — or those who drank only with meals showed a small reduction in their risk for cancer mortality and all-cause mortality (hazard ratio [HR], 0.94 for both). The small protective association was somewhat stronger among people who reported both patterns (HR, 0.88), especially if they were of lower socioeconomic status (HR, 0.79).
The findings are in line with other research suggesting that wine drinkers may be outliers when it comes to cancer risk. A 2023 meta-analysis of 26 observational studies, for instance, found no association between wine consumption and any cancer type, with the caveat that there was «substantial» heterogeneity among the studies.
This heterogeneity caveat speaks to the inherent limitations of observational research, said Tim Stockwell, PhD, of the Canadian Institute for Substance Use Research, University of Victoria in British Columbia, Canada.
“Individual studies of alcohol and cancer risk do find differences by type of drink, or patterns of drinking,” Stockwell said. “But it’s so hard to unpack the confounding that goes along with the type of person who’s a wine drinker or a beer drinker or a spirit drinker. The beverage of choice seems to come with a lot of baggage.”
Compared with people who favor beer or liquor, he noted, wine aficionados are typically higher-income, exercise more often, smoke less, and have different diets, for example. The “best” studies, Rebbeck said, try to adjust for those differences, but it’s challenging.
The authors of the 2023 meta-analysis noted that “many components in wine could have anticarcinogenic effects” that theoretically could counter the ill effects of ethanol. Besides resveratrol, which is mainly found in red wine, the list includes anthocyanins, quercetin, and tannins. However, the authors also acknowledged that they couldn’t account for whether other lifestyle habits might explain why wine drinkers, overall, showed no increased cancer risks and sometimes lower risks.
Still, groups such as the IARC and ASCO hold that there is no known “safe” level, or type, of alcohol when it comes to cancer.
In the latest Canadian guidelines on alcohol use, the scientific panel calculated that people who have 6 drinks a week throughout adulthood (whatever the source of the alcohol) could shave 11 weeks from their life expectancy, on average, said Stockwell, who was on the guideline panel. Compare that with heavy drinking, where 4 drinks a day could rob the average person of 2 or 3 years. “If you’re drinking a lot, you could get huge benefits from cutting down,” Stockwell explained. “If you’re a moderate drinker, the benefits would obviously be less.”
Stockwell said that choices around drinking and breast cancer risk, specifically, can be “tough.” Unlike many of the other alcohol-associated cancers, he noted, breast cancer is common — so even small relative risk increases may be concerning. Based on a 2020 meta-analysis of 22 cohort studies, the risk for breast cancer rises by about 10%, on average, for every 10 g of alcohol a woman drinks per day. This study also found no evidence that wine is any different from other types of alcohol.
In real life, the calculus around wine consumption and cancer risk will probably vary widely from person to person, Rebbeck said. One woman with a family history of breast cancer might decide that having wine with dinner isn’t worth it. Another with the same family history might see that glass of wine as a stress reliever and opt to focus on other ways to reduce her breast cancer risk — by exercising and maintaining a healthy weight, for example.
“The bottom line is, in human studies, the data on light to moderate drinking and cancer are limited and messy, and you can’t draw firm conclusions from them,” Rebbeck said. “It probably raises risk in some people, but we don’t know who those people are. And the risk increases are relatively small.”
A Conversation Few Are Having
Even with many studies highlighting the connection between alcohol consumption and cancer risk, most people remain unaware about this risk.
A 2023 study by the National Cancer Institute found that only a minority of US adults knew that drinking alcohol is linked to increased cancer risk, and they were much less likely to say that was true of wine: Only 20% did, vs 31% who said that liquor can boost cancer risk. Meanwhile, 10% believed that wine helps prevent cancer. Other studies show that even among cancer survivors and patients undergoing active cancer treatment, many drink — often heavily.
“What we know right now is, physicians almost never talk about this,” LoConte said.
That could be due to time constraints, according to Rebbeck, or clinicians’ perceptions that the subject is too complicated and/or their own confusion about the data. There could also be some “cognitive dissonance” at play, LoConte noted, because many doctors drink alcohol.
It’s critical, she said, that conversations about drinking habits become “normalized,” and that should include informing patients that alcohol use is associated with certain cancers. Again, LoConte said, it’s high-risk drinking that’s most concerning and where reducing intake could have the biggest impact on cancer risk and other health outcomes.
“From a cancer prevention standpoint, it’s probably best not to drink,” she said. “But people don’t make choices based solely on cancer risk. We don’t want to come out with recommendations saying no one should drink. I don’t think the data support that, and people would buck against that advice.”
Rebbeck made a similar point. Even if there’s uncertainty about the risks for a daily glass of wine, he said, people can use that information to make decisions. “Everybody’s preferences and choices are going to be different,” Rebbeck said. “And that’s all we can really do.”
A version of this article appeared on Medscape.com.
Earlier this month, US surgeon general Vivek Murthy, MD, issued an advisory, calling for alcoholic beverages to carry a warning label about cancer risk. The advisory flagged alcohol as the third leading preventable cause of cancer in the United States, after tobacco and obesity, and highlighted people’s limited awareness about the relationship between alcohol and cancer risk.
But, when it comes to cancer risk, are all types of alcohol created equal?
For many years, red wine seemed to be an outlier, with studies indicating that, in moderation, it might even be good for you. Red wine has anti-inflammatory and antioxidant properties — most notably, it contains the antioxidant resveratrol. Starting in the 1990s, research began to hint that the compound might protect against heart disease, aging, and cancer, though much of this work was done in animals or test tubes.
The idea that red wine carries health benefits, however, has been called into question more recently. A recent meta-analysis, for instance, suggests that many previous studies touting the health benefits of more moderate drinking were likely biased, potentially leading to “misleading positive health associations.” And one recent study found that alcohol consumption, largely red wine and beer, at all levels was linked to an increased risk for cardiovascular disease.
Although wine’s health halo is dwindling, there might be an exception: Cancer risk.
Overall, research shows that even light to moderate drinking increases the risk for at least seven types of cancer, but when focusing on red wine, in particular, that risk calculus can look different.
“It’s very complicated and nuanced,” said Timothy Rebbeck, PhD, professor of cancer prevention, Harvard T.H. Chan School of Public Health, Boston. “And ‘complicated and nuanced’ doesn’t work very well in public health messages.”
The Knowns About Alcohol and Cancer Risk
Some things about the relationship between alcohol and cancer risk are crystal clear. “There’s no question that alcohol is a group 1 carcinogen,” Rebbeck said. “Alcohol can cause cancer.”
Groups including the International Agency for Research on Cancer (IARC) and American Cancer Society agree that alcohol use is an established cause of seven types of cancer: Those of the oral cavity, larynx, pharynx, esophagus (squamous cell carcinoma), liver (hepatocellular carcinoma), breast, and colon/rectum. Heavy drinking — at least 8 standard drinks a week for women and 15 for men — and binge drinking — 4 or more drinks in 2 hours for women and 5 or more for men — only amplify that risk. (A “standard” drink has 14 g of alcohol, which translates to a 5-oz glass of wine.)
“We’re most concerned about high-risk drinking — more than 2 drinks a day — and/or binge drinking,” said Noelle LoConte, MD, of the Division of Hematology, Medical Oncology and Palliative Care, University of Wisconsin School of Medicine and Public Health, Madison, who authored a 2018 statement on alcohol and cancer risk from the American Society of Clinical Oncology (ASCO).
Compared with not drinking, heavy drinking is linked with a roughly fivefold increase in the risk for oral cavity, pharyngeal, and esophageal cancers, and a 61% increase in the risk for breast cancer, according to LoConte and colleagues.
Things get murkier when it comes to moderate drinking — defined as up to 1 standard drink per day for women and 2 per day for men. There is evidence, LoConte said, that moderate drinking is associated with increased cancer risks, though the magnitude is generally much less than heavier drinking.
Cancer type also matters. One analysis found that the risk for breast cancer increased with even light to moderate alcohol consumption. Compared with no drinking, light to moderate drinking has also been linked to increased risks for oral cavity, pharynx, larynx, and esophageal cancers.
As for whether the type of alcoholic beverage matters, LoConte said, there’s no clear physiological reason that wine would be less risky than beer or liquor. Research indicates that ethanol is the problematic ingredient: Once ingested, it’s metabolized into acetaldehyde, a DNA-damaging substance that’s considered a probable human carcinogen. Ethanol can also alter circulating levels of estrogens and androgens, LoConte said, which is thought to drive its association with breast cancer risk.
“It likely doesn’t matter how you choose to get your ethanol,” she said. “It’s a question of volume.”
Hints That Wine Is an Outlier
Still, some studies suggest that how people ingest ethanol could make a difference.
A study published in August in JAMA Network Open is a case in point. The study found that, among older adults, light to heavy drinkers had an increased risk of dying from cancer, compared with occasional drinkers (though the increased risk among light to moderate drinkers occurred only among people who also had chronic health conditions, such as diabetes or high blood pressure, or were of lower socioeconomic status).
Wine drinkers fared differently. Most notably, drinkers who “preferred” wine — consuming over 80% of total ethanol from wine — or those who drank only with meals showed a small reduction in their risk for cancer mortality and all-cause mortality (hazard ratio [HR], 0.94 for both). The small protective association was somewhat stronger among people who reported both patterns (HR, 0.88), especially if they were of lower socioeconomic status (HR, 0.79).
The findings are in line with other research suggesting that wine drinkers may be outliers when it comes to cancer risk. A 2023 meta-analysis of 26 observational studies, for instance, found no association between wine consumption and any cancer type, with the caveat that there was «substantial» heterogeneity among the studies.
This heterogeneity caveat speaks to the inherent limitations of observational research, said Tim Stockwell, PhD, of the Canadian Institute for Substance Use Research, University of Victoria in British Columbia, Canada.
“Individual studies of alcohol and cancer risk do find differences by type of drink, or patterns of drinking,” Stockwell said. “But it’s so hard to unpack the confounding that goes along with the type of person who’s a wine drinker or a beer drinker or a spirit drinker. The beverage of choice seems to come with a lot of baggage.”
Compared with people who favor beer or liquor, he noted, wine aficionados are typically higher-income, exercise more often, smoke less, and have different diets, for example. The “best” studies, Rebbeck said, try to adjust for those differences, but it’s challenging.
The authors of the 2023 meta-analysis noted that “many components in wine could have anticarcinogenic effects” that theoretically could counter the ill effects of ethanol. Besides resveratrol, which is mainly found in red wine, the list includes anthocyanins, quercetin, and tannins. However, the authors also acknowledged that they couldn’t account for whether other lifestyle habits might explain why wine drinkers, overall, showed no increased cancer risks and sometimes lower risks.
Still, groups such as the IARC and ASCO hold that there is no known “safe” level, or type, of alcohol when it comes to cancer.
In the latest Canadian guidelines on alcohol use, the scientific panel calculated that people who have 6 drinks a week throughout adulthood (whatever the source of the alcohol) could shave 11 weeks from their life expectancy, on average, said Stockwell, who was on the guideline panel. Compare that with heavy drinking, where 4 drinks a day could rob the average person of 2 or 3 years. “If you’re drinking a lot, you could get huge benefits from cutting down,” Stockwell explained. “If you’re a moderate drinker, the benefits would obviously be less.”
Stockwell said that choices around drinking and breast cancer risk, specifically, can be “tough.” Unlike many of the other alcohol-associated cancers, he noted, breast cancer is common — so even small relative risk increases may be concerning. Based on a 2020 meta-analysis of 22 cohort studies, the risk for breast cancer rises by about 10%, on average, for every 10 g of alcohol a woman drinks per day. This study also found no evidence that wine is any different from other types of alcohol.
In real life, the calculus around wine consumption and cancer risk will probably vary widely from person to person, Rebbeck said. One woman with a family history of breast cancer might decide that having wine with dinner isn’t worth it. Another with the same family history might see that glass of wine as a stress reliever and opt to focus on other ways to reduce her breast cancer risk — by exercising and maintaining a healthy weight, for example.
“The bottom line is, in human studies, the data on light to moderate drinking and cancer are limited and messy, and you can’t draw firm conclusions from them,” Rebbeck said. “It probably raises risk in some people, but we don’t know who those people are. And the risk increases are relatively small.”
A Conversation Few Are Having
Even with many studies highlighting the connection between alcohol consumption and cancer risk, most people remain unaware about this risk.
A 2023 study by the National Cancer Institute found that only a minority of US adults knew that drinking alcohol is linked to increased cancer risk, and they were much less likely to say that was true of wine: Only 20% did, vs 31% who said that liquor can boost cancer risk. Meanwhile, 10% believed that wine helps prevent cancer. Other studies show that even among cancer survivors and patients undergoing active cancer treatment, many drink — often heavily.
“What we know right now is, physicians almost never talk about this,” LoConte said.
That could be due to time constraints, according to Rebbeck, or clinicians’ perceptions that the subject is too complicated and/or their own confusion about the data. There could also be some “cognitive dissonance” at play, LoConte noted, because many doctors drink alcohol.
It’s critical, she said, that conversations about drinking habits become “normalized,” and that should include informing patients that alcohol use is associated with certain cancers. Again, LoConte said, it’s high-risk drinking that’s most concerning and where reducing intake could have the biggest impact on cancer risk and other health outcomes.
“From a cancer prevention standpoint, it’s probably best not to drink,” she said. “But people don’t make choices based solely on cancer risk. We don’t want to come out with recommendations saying no one should drink. I don’t think the data support that, and people would buck against that advice.”
Rebbeck made a similar point. Even if there’s uncertainty about the risks for a daily glass of wine, he said, people can use that information to make decisions. “Everybody’s preferences and choices are going to be different,” Rebbeck said. “And that’s all we can really do.”
A version of this article appeared on Medscape.com.
CDK 4/6 Blocker Prolongs Survival in HER2+ Metastatic Breast Cancer
according to the results of the phase 3 PATINA study.
This regimen “may represent a new standard of care” for these patients, said principal investigator and presenter Otto Metzger, MD, a medical breast oncologist at the Dana-Farber Cancer Institute in Boston, Massachusetts, who presented the findings at the San Antonio Breast Cancer Symposium (SABCS) 2024.
The open-label PATINA trial, which was conducted in Europe, Australia, New Zealand, and the United States, included a total of 518 patients. Patients received first-line treatment of six to eight cycles of induction chemotherapy plus anti-HER2 therapy. Researchers then randomized patients to either palbociclib plus anti-HER2 and endocrine therapy (n = 261) or to anti-HER2 and endocrine therapy alone (n = 257).
Patients did not progress on induction therapy, which likely would have signaled early resistance to anti-HER2 treatment. For anti-HER2 therapy, 97.3% received a combination of trastuzumab and pertuzumab. For endocrine therapy, 90.9% received an aromatase inhibitor.
Metzger and colleagues found that median progression-free survival was 1.3 years longer in patients receiving palbociclib — 3.7 years in the palbociclib arm vs 2.4 years in the control group (hazard ratio [HR], 0.74; P = .0074).
Although overall survival outcomes are immature, 5-year survival rates were slightly better in the palbociclib arm — 74.3% with palbociclib vs 69.8% without it — but the difference was not statistically significant.
Grade 3 neutropenia was the most frequent adverse event in the palbociclib arm (63.2% vs 2%). Grades 2 and 3 fatigue, stomatitis, and diarrhea were also more common with palbociclib. Grade 4 adverse events occurred in 12.3% of those receiving palbociclib and 8.9% of those who did not. There were no treatment-related deaths.
“We’re very impressed with the results,” said Metzger.
On the basis of previous studies, it’s believed that CDK 4/6 inhibition counteracts the development of resistance to anti-HER2 and endocrine therapies, which likely explains the benefit found in the trial.
But even without CDK 4/6 inhibition, the progression-free survival of 2.4 years in the control arm “far exceed[ed] our expectations,” Metzger reported. This may have occurred because the control arm received endocrine therapy, something previous trials of anti-HER2 therapy have avoided because of tolerability and other concerns.
These findings, however, support “the common use of endocrine therapy,” Metzger said.
‘Incredible’ Results
The progression-free survival as well as overall survival results in the trial are “incredible,” said study discussant Sara Hurvitz, MD, a medical breast oncologist at the Fred Hutch Cancer Center in Seattle, Washington. This is “historic and very important data.”
Hurvitz even suggested the results might mean that patients who fit the PATINA criteria can avoid the toxicity of upfront trastuzumab deruxtecan and use the PATINA regimen instead, potentially preserving their quality of life for longer.
Another study discussant, Virginia Kaklamani, MD, a medical breast oncologist at the University of Texas MD Anderson Cancer Center, San Antonio, had a similar thought.
In PATINA, “we’re talking about patients being on a treatment that’s well tolerated, where patients continue to work and continue with their lives despite being on treatment for metastatic breast cancer for 4 years, which is remarkable,” Kaklamani said.
Many of us have dabbled with giving CDK 4/6 inhibitors in triple-positive breast cancer, but “now we have more definitive data,” she said. The approach can help “maintain the quality of life of our patients for a longer period of time” and delay the use of chemotherapy in the second line, she added.
Metzger said Pfizer, the maker of palbociclib, plans to file for a HER2-positive indication with the Food and Drug Administration based on the trial results.
For now, the CDK 4/6 blocker is only indicated in combination with endocrine therapy for HR-positive, HER2-negative metastatic disease.
In response to a question about using the PATINA regimen in patients who don’t get chemotherapy induction, Metzger noted that, “while the study didn’t test this directly, I would argue that this data is quite compelling” for using palbociclib plus anti-HER2 and endocrine therapy, even without chemotherapy induction.
The work was funded by palbociclib maker Pfizer. Metzger had no disclosures. Hurvitz has numerous industry ties, including being a researcher and advisor to Pfizer. Kaklamani also has numerous industry ties, including reporting personal/consulting fees from Pfizer Canada.
A version of this article first appeared on Medscape.com.
according to the results of the phase 3 PATINA study.
This regimen “may represent a new standard of care” for these patients, said principal investigator and presenter Otto Metzger, MD, a medical breast oncologist at the Dana-Farber Cancer Institute in Boston, Massachusetts, who presented the findings at the San Antonio Breast Cancer Symposium (SABCS) 2024.
The open-label PATINA trial, which was conducted in Europe, Australia, New Zealand, and the United States, included a total of 518 patients. Patients received first-line treatment of six to eight cycles of induction chemotherapy plus anti-HER2 therapy. Researchers then randomized patients to either palbociclib plus anti-HER2 and endocrine therapy (n = 261) or to anti-HER2 and endocrine therapy alone (n = 257).
Patients did not progress on induction therapy, which likely would have signaled early resistance to anti-HER2 treatment. For anti-HER2 therapy, 97.3% received a combination of trastuzumab and pertuzumab. For endocrine therapy, 90.9% received an aromatase inhibitor.
Metzger and colleagues found that median progression-free survival was 1.3 years longer in patients receiving palbociclib — 3.7 years in the palbociclib arm vs 2.4 years in the control group (hazard ratio [HR], 0.74; P = .0074).
Although overall survival outcomes are immature, 5-year survival rates were slightly better in the palbociclib arm — 74.3% with palbociclib vs 69.8% without it — but the difference was not statistically significant.
Grade 3 neutropenia was the most frequent adverse event in the palbociclib arm (63.2% vs 2%). Grades 2 and 3 fatigue, stomatitis, and diarrhea were also more common with palbociclib. Grade 4 adverse events occurred in 12.3% of those receiving palbociclib and 8.9% of those who did not. There were no treatment-related deaths.
“We’re very impressed with the results,” said Metzger.
On the basis of previous studies, it’s believed that CDK 4/6 inhibition counteracts the development of resistance to anti-HER2 and endocrine therapies, which likely explains the benefit found in the trial.
But even without CDK 4/6 inhibition, the progression-free survival of 2.4 years in the control arm “far exceed[ed] our expectations,” Metzger reported. This may have occurred because the control arm received endocrine therapy, something previous trials of anti-HER2 therapy have avoided because of tolerability and other concerns.
These findings, however, support “the common use of endocrine therapy,” Metzger said.
‘Incredible’ Results
The progression-free survival as well as overall survival results in the trial are “incredible,” said study discussant Sara Hurvitz, MD, a medical breast oncologist at the Fred Hutch Cancer Center in Seattle, Washington. This is “historic and very important data.”
Hurvitz even suggested the results might mean that patients who fit the PATINA criteria can avoid the toxicity of upfront trastuzumab deruxtecan and use the PATINA regimen instead, potentially preserving their quality of life for longer.
Another study discussant, Virginia Kaklamani, MD, a medical breast oncologist at the University of Texas MD Anderson Cancer Center, San Antonio, had a similar thought.
In PATINA, “we’re talking about patients being on a treatment that’s well tolerated, where patients continue to work and continue with their lives despite being on treatment for metastatic breast cancer for 4 years, which is remarkable,” Kaklamani said.
Many of us have dabbled with giving CDK 4/6 inhibitors in triple-positive breast cancer, but “now we have more definitive data,” she said. The approach can help “maintain the quality of life of our patients for a longer period of time” and delay the use of chemotherapy in the second line, she added.
Metzger said Pfizer, the maker of palbociclib, plans to file for a HER2-positive indication with the Food and Drug Administration based on the trial results.
For now, the CDK 4/6 blocker is only indicated in combination with endocrine therapy for HR-positive, HER2-negative metastatic disease.
In response to a question about using the PATINA regimen in patients who don’t get chemotherapy induction, Metzger noted that, “while the study didn’t test this directly, I would argue that this data is quite compelling” for using palbociclib plus anti-HER2 and endocrine therapy, even without chemotherapy induction.
The work was funded by palbociclib maker Pfizer. Metzger had no disclosures. Hurvitz has numerous industry ties, including being a researcher and advisor to Pfizer. Kaklamani also has numerous industry ties, including reporting personal/consulting fees from Pfizer Canada.
A version of this article first appeared on Medscape.com.
according to the results of the phase 3 PATINA study.
This regimen “may represent a new standard of care” for these patients, said principal investigator and presenter Otto Metzger, MD, a medical breast oncologist at the Dana-Farber Cancer Institute in Boston, Massachusetts, who presented the findings at the San Antonio Breast Cancer Symposium (SABCS) 2024.
The open-label PATINA trial, which was conducted in Europe, Australia, New Zealand, and the United States, included a total of 518 patients. Patients received first-line treatment of six to eight cycles of induction chemotherapy plus anti-HER2 therapy. Researchers then randomized patients to either palbociclib plus anti-HER2 and endocrine therapy (n = 261) or to anti-HER2 and endocrine therapy alone (n = 257).
Patients did not progress on induction therapy, which likely would have signaled early resistance to anti-HER2 treatment. For anti-HER2 therapy, 97.3% received a combination of trastuzumab and pertuzumab. For endocrine therapy, 90.9% received an aromatase inhibitor.
Metzger and colleagues found that median progression-free survival was 1.3 years longer in patients receiving palbociclib — 3.7 years in the palbociclib arm vs 2.4 years in the control group (hazard ratio [HR], 0.74; P = .0074).
Although overall survival outcomes are immature, 5-year survival rates were slightly better in the palbociclib arm — 74.3% with palbociclib vs 69.8% without it — but the difference was not statistically significant.
Grade 3 neutropenia was the most frequent adverse event in the palbociclib arm (63.2% vs 2%). Grades 2 and 3 fatigue, stomatitis, and diarrhea were also more common with palbociclib. Grade 4 adverse events occurred in 12.3% of those receiving palbociclib and 8.9% of those who did not. There were no treatment-related deaths.
“We’re very impressed with the results,” said Metzger.
On the basis of previous studies, it’s believed that CDK 4/6 inhibition counteracts the development of resistance to anti-HER2 and endocrine therapies, which likely explains the benefit found in the trial.
But even without CDK 4/6 inhibition, the progression-free survival of 2.4 years in the control arm “far exceed[ed] our expectations,” Metzger reported. This may have occurred because the control arm received endocrine therapy, something previous trials of anti-HER2 therapy have avoided because of tolerability and other concerns.
These findings, however, support “the common use of endocrine therapy,” Metzger said.
‘Incredible’ Results
The progression-free survival as well as overall survival results in the trial are “incredible,” said study discussant Sara Hurvitz, MD, a medical breast oncologist at the Fred Hutch Cancer Center in Seattle, Washington. This is “historic and very important data.”
Hurvitz even suggested the results might mean that patients who fit the PATINA criteria can avoid the toxicity of upfront trastuzumab deruxtecan and use the PATINA regimen instead, potentially preserving their quality of life for longer.
Another study discussant, Virginia Kaklamani, MD, a medical breast oncologist at the University of Texas MD Anderson Cancer Center, San Antonio, had a similar thought.
In PATINA, “we’re talking about patients being on a treatment that’s well tolerated, where patients continue to work and continue with their lives despite being on treatment for metastatic breast cancer for 4 years, which is remarkable,” Kaklamani said.
Many of us have dabbled with giving CDK 4/6 inhibitors in triple-positive breast cancer, but “now we have more definitive data,” she said. The approach can help “maintain the quality of life of our patients for a longer period of time” and delay the use of chemotherapy in the second line, she added.
Metzger said Pfizer, the maker of palbociclib, plans to file for a HER2-positive indication with the Food and Drug Administration based on the trial results.
For now, the CDK 4/6 blocker is only indicated in combination with endocrine therapy for HR-positive, HER2-negative metastatic disease.
In response to a question about using the PATINA regimen in patients who don’t get chemotherapy induction, Metzger noted that, “while the study didn’t test this directly, I would argue that this data is quite compelling” for using palbociclib plus anti-HER2 and endocrine therapy, even without chemotherapy induction.
The work was funded by palbociclib maker Pfizer. Metzger had no disclosures. Hurvitz has numerous industry ties, including being a researcher and advisor to Pfizer. Kaklamani also has numerous industry ties, including reporting personal/consulting fees from Pfizer Canada.
A version of this article first appeared on Medscape.com.
FROM SABCS 2024
MRI-Invisible Prostate Lesions: Are They Dangerous?
MRI-invisible prostate lesions. It sounds like the stuff of science fiction and fantasy, a creation from the minds of H.G. Wells, who wrote The Invisible Man, or J.K. Rowling, who authored the Harry Potter series.
But MRI-invisible prostate lesions are real. And what these lesions may, or may not, indicate is the subject of intense debate.
MRI plays an increasingly important role in detecting and diagnosing prostate cancer, staging prostate cancer as well as monitoring disease progression. However, on occasion, a puzzling phenomenon arises. Certain prostate lesions that appear when pathologists examine biopsied tissue samples under a microscope are not visible on MRI. The prostate tissue will, instead, appear normal to a radiologist’s eye.
Some experts believe these MRI-invisible lesions are nothing to worry about.
If the clinician can’t see the cancer on MRI, then it simply isn’t a threat, according to Mark Emberton, MD, a pioneer in prostate MRIs and director of interventional oncology at University College London, England.
Laurence Klotz, MD, of the University of Toronto, Ontario, Canada, agreed, noting that “invisible cancers are clinically insignificant and don’t require systematic biopsies.”
Emberton and Klotz compared MRI-invisible lesions to grade group 1 prostate cancer (Gleason score ≤ 6) — the least aggressive category that indicates the cancer that is not likely to spread or kill. For patients on active surveillance, those with MRI-invisible cancers do drastically better than those with visible cancers, Klotz explained.
But other experts in the field are skeptical that MRI-invisible lesions are truly innocuous.
Although statistically an MRI-visible prostate lesion indicates a more aggressive tumor, that is not always the case for every individual, said Brian Helfand, MD, PhD, chief of urology at NorthShore University Health System, Evanston, Illinois.
MRIs can lead to false negatives in about 10%-20% of patients who have clinically significant prostate cancer, though estimates vary.
In one analysis, 16% of men with no suspicious lesions on MRI had clinically significant prostate cancer identified after undergoing a systematic biopsy. Another analysis found that about 35% of MRI-invisible prostate cancers identified via biopsy were clinically significant.
Other studies, however, have indicated that negative MRI results accurately indicate patients at low risk of developing clinically significant cancers. A recent JAMA Oncology analysis, for instance, found that only seven of 233 men (3%) with negative MRI results at baseline who completed 3 years of monitoring were diagnosed with clinically significant prostate cancer.
When a patient has an MRI-invisible prostate tumor, there are a couple of reasons the MRI may not be picking it up, said urologic oncologist Alexander Putnam Cole, MD, assistant professor of surgery, Harvard Medical School, Boston, Massachusetts. “One is that the cancer is aggressive but just very small,” said Cole.
“Another possibility is that the cancer looks very similar to background prostate tissue, which is something that you might expect if you think about more of a low-grade cancer,” he explained.
The experience level of the radiologist interpreting the MRI can also play into the accuracy of the reading.
But Cole agreed that “in general, MRI visibility is associated with molecular and histologic features of progression and aggressiveness and non-visible cancers are less likely to have aggressive features.”
The genomic profiles of MRI-visible and -invisible cancers bear this out.
According to Todd Morgan, MD, chief of urologic oncology at Michigan Medicine, University of Michigan, Ann Arbor, the gene expression in visible disease tends to be linked to more aggressive prostate tumors whereas gene expression in invisible disease does not.
In one analysis, for instance, researchers found that four genes — PHYHD1, CENPF, ALDH2, and GDF15 — associated with worse progression-free survival and metastasis-free survival in prostate cancer also predicted MRI visibility.
“Genes that are associated with visibility are essentially the same genes that are associated with aggressive cancers,” Klotz said.
Next Steps After Negative MRI Result
What do MRI-invisible lesions mean for patient care? If, for instance, a patient has elevated PSA levels but a normal MRI, is a targeted or systematic biopsy warranted?
The overarching message, according to Klotz, is that “you don’t need to find them.” Klotz noted, however, that patients with a negative MRI result should still be followed with periodic repeat imaging.
Several trials support this approach of using MRI to decide who needs a biopsy and delaying a biopsy in men with normal MRIs.
The recent JAMA Oncology analysis found that, among men with negative MRI results, 86% avoided a biopsy over 3 years, with clinically significant prostate cancer detected in only 4% of men across the study period — four in the initial diagnostic phase and seven in the 3-year monitoring phase. However, during the initial diagnostic phase, more than half the men with positive MRI findings had clinically significant prostate cancer detected.
Another recent study found that patients with negative MRI results were much less likely to upgrade to higher Gleason scores over time. Among 522 patients who underwent a systematic and targeted biopsy within 18 months of their grade group 1 designation, 9.2% with negative MRI findings had tumors reclassified as grade group 2 or higher vs 27% with positive MRI findings, and 2.3% with negative MRI findings had tumors reclassified as grade group 3 or higher vs 7.8% with positive MRI findings.
These data suggest that men with grade group 1 cancer and negative MRI result “may be able to avoid confirmatory biopsies until a routine surveillance biopsy in 2-3 years,” according to study author Christian Pavlovich, MD, professor of urologic oncology at the Johns Hopkins University School of Medicine, Baltimore.
Cole used MRI findings to triage who gets a biopsy. When a biopsy is warranted, “I usually recommend adding in some systematic sampling of the other side to assess for nonvisible cancers,” he noted.
Sampling prostate tissue outside the target area “adds maybe 1-2 minutes to the procedure and doesn’t drastically increase the morbidity or risks,” Cole said. It also can help “confirm there is cancer in the MRI target and also confirm there is no cancer in the nonvisible areas.”
According to Klotz, if imaging demonstrates progression, patients should receive a biopsy — in most cases, a targeted biopsy only. And, Klotz noted, skipping routine prostate biopsies in men with negative MRI results can save thousands of men from these procedures, which carry risks for infections and sepsis.
Looking beyond Gleason scores for risk prediction, MRI “visibility is a very powerful risk stratifier,” he said.
A version of this article appeared on Medscape.com.
MRI-invisible prostate lesions. It sounds like the stuff of science fiction and fantasy, a creation from the minds of H.G. Wells, who wrote The Invisible Man, or J.K. Rowling, who authored the Harry Potter series.
But MRI-invisible prostate lesions are real. And what these lesions may, or may not, indicate is the subject of intense debate.
MRI plays an increasingly important role in detecting and diagnosing prostate cancer, staging prostate cancer as well as monitoring disease progression. However, on occasion, a puzzling phenomenon arises. Certain prostate lesions that appear when pathologists examine biopsied tissue samples under a microscope are not visible on MRI. The prostate tissue will, instead, appear normal to a radiologist’s eye.
Some experts believe these MRI-invisible lesions are nothing to worry about.
If the clinician can’t see the cancer on MRI, then it simply isn’t a threat, according to Mark Emberton, MD, a pioneer in prostate MRIs and director of interventional oncology at University College London, England.
Laurence Klotz, MD, of the University of Toronto, Ontario, Canada, agreed, noting that “invisible cancers are clinically insignificant and don’t require systematic biopsies.”
Emberton and Klotz compared MRI-invisible lesions to grade group 1 prostate cancer (Gleason score ≤ 6) — the least aggressive category that indicates the cancer that is not likely to spread or kill. For patients on active surveillance, those with MRI-invisible cancers do drastically better than those with visible cancers, Klotz explained.
But other experts in the field are skeptical that MRI-invisible lesions are truly innocuous.
Although statistically an MRI-visible prostate lesion indicates a more aggressive tumor, that is not always the case for every individual, said Brian Helfand, MD, PhD, chief of urology at NorthShore University Health System, Evanston, Illinois.
MRIs can lead to false negatives in about 10%-20% of patients who have clinically significant prostate cancer, though estimates vary.
In one analysis, 16% of men with no suspicious lesions on MRI had clinically significant prostate cancer identified after undergoing a systematic biopsy. Another analysis found that about 35% of MRI-invisible prostate cancers identified via biopsy were clinically significant.
Other studies, however, have indicated that negative MRI results accurately indicate patients at low risk of developing clinically significant cancers. A recent JAMA Oncology analysis, for instance, found that only seven of 233 men (3%) with negative MRI results at baseline who completed 3 years of monitoring were diagnosed with clinically significant prostate cancer.
When a patient has an MRI-invisible prostate tumor, there are a couple of reasons the MRI may not be picking it up, said urologic oncologist Alexander Putnam Cole, MD, assistant professor of surgery, Harvard Medical School, Boston, Massachusetts. “One is that the cancer is aggressive but just very small,” said Cole.
“Another possibility is that the cancer looks very similar to background prostate tissue, which is something that you might expect if you think about more of a low-grade cancer,” he explained.
The experience level of the radiologist interpreting the MRI can also play into the accuracy of the reading.
But Cole agreed that “in general, MRI visibility is associated with molecular and histologic features of progression and aggressiveness and non-visible cancers are less likely to have aggressive features.”
The genomic profiles of MRI-visible and -invisible cancers bear this out.
According to Todd Morgan, MD, chief of urologic oncology at Michigan Medicine, University of Michigan, Ann Arbor, the gene expression in visible disease tends to be linked to more aggressive prostate tumors whereas gene expression in invisible disease does not.
In one analysis, for instance, researchers found that four genes — PHYHD1, CENPF, ALDH2, and GDF15 — associated with worse progression-free survival and metastasis-free survival in prostate cancer also predicted MRI visibility.
“Genes that are associated with visibility are essentially the same genes that are associated with aggressive cancers,” Klotz said.
Next Steps After Negative MRI Result
What do MRI-invisible lesions mean for patient care? If, for instance, a patient has elevated PSA levels but a normal MRI, is a targeted or systematic biopsy warranted?
The overarching message, according to Klotz, is that “you don’t need to find them.” Klotz noted, however, that patients with a negative MRI result should still be followed with periodic repeat imaging.
Several trials support this approach of using MRI to decide who needs a biopsy and delaying a biopsy in men with normal MRIs.
The recent JAMA Oncology analysis found that, among men with negative MRI results, 86% avoided a biopsy over 3 years, with clinically significant prostate cancer detected in only 4% of men across the study period — four in the initial diagnostic phase and seven in the 3-year monitoring phase. However, during the initial diagnostic phase, more than half the men with positive MRI findings had clinically significant prostate cancer detected.
Another recent study found that patients with negative MRI results were much less likely to upgrade to higher Gleason scores over time. Among 522 patients who underwent a systematic and targeted biopsy within 18 months of their grade group 1 designation, 9.2% with negative MRI findings had tumors reclassified as grade group 2 or higher vs 27% with positive MRI findings, and 2.3% with negative MRI findings had tumors reclassified as grade group 3 or higher vs 7.8% with positive MRI findings.
These data suggest that men with grade group 1 cancer and negative MRI result “may be able to avoid confirmatory biopsies until a routine surveillance biopsy in 2-3 years,” according to study author Christian Pavlovich, MD, professor of urologic oncology at the Johns Hopkins University School of Medicine, Baltimore.
Cole used MRI findings to triage who gets a biopsy. When a biopsy is warranted, “I usually recommend adding in some systematic sampling of the other side to assess for nonvisible cancers,” he noted.
Sampling prostate tissue outside the target area “adds maybe 1-2 minutes to the procedure and doesn’t drastically increase the morbidity or risks,” Cole said. It also can help “confirm there is cancer in the MRI target and also confirm there is no cancer in the nonvisible areas.”
According to Klotz, if imaging demonstrates progression, patients should receive a biopsy — in most cases, a targeted biopsy only. And, Klotz noted, skipping routine prostate biopsies in men with negative MRI results can save thousands of men from these procedures, which carry risks for infections and sepsis.
Looking beyond Gleason scores for risk prediction, MRI “visibility is a very powerful risk stratifier,” he said.
A version of this article appeared on Medscape.com.
MRI-invisible prostate lesions. It sounds like the stuff of science fiction and fantasy, a creation from the minds of H.G. Wells, who wrote The Invisible Man, or J.K. Rowling, who authored the Harry Potter series.
But MRI-invisible prostate lesions are real. And what these lesions may, or may not, indicate is the subject of intense debate.
MRI plays an increasingly important role in detecting and diagnosing prostate cancer, staging prostate cancer as well as monitoring disease progression. However, on occasion, a puzzling phenomenon arises. Certain prostate lesions that appear when pathologists examine biopsied tissue samples under a microscope are not visible on MRI. The prostate tissue will, instead, appear normal to a radiologist’s eye.
Some experts believe these MRI-invisible lesions are nothing to worry about.
If the clinician can’t see the cancer on MRI, then it simply isn’t a threat, according to Mark Emberton, MD, a pioneer in prostate MRIs and director of interventional oncology at University College London, England.
Laurence Klotz, MD, of the University of Toronto, Ontario, Canada, agreed, noting that “invisible cancers are clinically insignificant and don’t require systematic biopsies.”
Emberton and Klotz compared MRI-invisible lesions to grade group 1 prostate cancer (Gleason score ≤ 6) — the least aggressive category that indicates the cancer that is not likely to spread or kill. For patients on active surveillance, those with MRI-invisible cancers do drastically better than those with visible cancers, Klotz explained.
But other experts in the field are skeptical that MRI-invisible lesions are truly innocuous.
Although statistically an MRI-visible prostate lesion indicates a more aggressive tumor, that is not always the case for every individual, said Brian Helfand, MD, PhD, chief of urology at NorthShore University Health System, Evanston, Illinois.
MRIs can lead to false negatives in about 10%-20% of patients who have clinically significant prostate cancer, though estimates vary.
In one analysis, 16% of men with no suspicious lesions on MRI had clinically significant prostate cancer identified after undergoing a systematic biopsy. Another analysis found that about 35% of MRI-invisible prostate cancers identified via biopsy were clinically significant.
Other studies, however, have indicated that negative MRI results accurately indicate patients at low risk of developing clinically significant cancers. A recent JAMA Oncology analysis, for instance, found that only seven of 233 men (3%) with negative MRI results at baseline who completed 3 years of monitoring were diagnosed with clinically significant prostate cancer.
When a patient has an MRI-invisible prostate tumor, there are a couple of reasons the MRI may not be picking it up, said urologic oncologist Alexander Putnam Cole, MD, assistant professor of surgery, Harvard Medical School, Boston, Massachusetts. “One is that the cancer is aggressive but just very small,” said Cole.
“Another possibility is that the cancer looks very similar to background prostate tissue, which is something that you might expect if you think about more of a low-grade cancer,” he explained.
The experience level of the radiologist interpreting the MRI can also play into the accuracy of the reading.
But Cole agreed that “in general, MRI visibility is associated with molecular and histologic features of progression and aggressiveness and non-visible cancers are less likely to have aggressive features.”
The genomic profiles of MRI-visible and -invisible cancers bear this out.
According to Todd Morgan, MD, chief of urologic oncology at Michigan Medicine, University of Michigan, Ann Arbor, the gene expression in visible disease tends to be linked to more aggressive prostate tumors whereas gene expression in invisible disease does not.
In one analysis, for instance, researchers found that four genes — PHYHD1, CENPF, ALDH2, and GDF15 — associated with worse progression-free survival and metastasis-free survival in prostate cancer also predicted MRI visibility.
“Genes that are associated with visibility are essentially the same genes that are associated with aggressive cancers,” Klotz said.
Next Steps After Negative MRI Result
What do MRI-invisible lesions mean for patient care? If, for instance, a patient has elevated PSA levels but a normal MRI, is a targeted or systematic biopsy warranted?
The overarching message, according to Klotz, is that “you don’t need to find them.” Klotz noted, however, that patients with a negative MRI result should still be followed with periodic repeat imaging.
Several trials support this approach of using MRI to decide who needs a biopsy and delaying a biopsy in men with normal MRIs.
The recent JAMA Oncology analysis found that, among men with negative MRI results, 86% avoided a biopsy over 3 years, with clinically significant prostate cancer detected in only 4% of men across the study period — four in the initial diagnostic phase and seven in the 3-year monitoring phase. However, during the initial diagnostic phase, more than half the men with positive MRI findings had clinically significant prostate cancer detected.
Another recent study found that patients with negative MRI results were much less likely to upgrade to higher Gleason scores over time. Among 522 patients who underwent a systematic and targeted biopsy within 18 months of their grade group 1 designation, 9.2% with negative MRI findings had tumors reclassified as grade group 2 or higher vs 27% with positive MRI findings, and 2.3% with negative MRI findings had tumors reclassified as grade group 3 or higher vs 7.8% with positive MRI findings.
These data suggest that men with grade group 1 cancer and negative MRI result “may be able to avoid confirmatory biopsies until a routine surveillance biopsy in 2-3 years,” according to study author Christian Pavlovich, MD, professor of urologic oncology at the Johns Hopkins University School of Medicine, Baltimore.
Cole used MRI findings to triage who gets a biopsy. When a biopsy is warranted, “I usually recommend adding in some systematic sampling of the other side to assess for nonvisible cancers,” he noted.
Sampling prostate tissue outside the target area “adds maybe 1-2 minutes to the procedure and doesn’t drastically increase the morbidity or risks,” Cole said. It also can help “confirm there is cancer in the MRI target and also confirm there is no cancer in the nonvisible areas.”
According to Klotz, if imaging demonstrates progression, patients should receive a biopsy — in most cases, a targeted biopsy only. And, Klotz noted, skipping routine prostate biopsies in men with negative MRI results can save thousands of men from these procedures, which carry risks for infections and sepsis.
Looking beyond Gleason scores for risk prediction, MRI “visibility is a very powerful risk stratifier,” he said.
A version of this article appeared on Medscape.com.
A Cancer Patient’s Bittersweet Reminder
Recently, a 40-year-old woman took to Facebook to announce that she had died.
Rachel Davies, of Wales, wrote: “If you’re reading this, then it means I’m no longer here. What a life I’ve had, and surprisingly, since cancer entered my life. When I look through my photos, I’ve done and seen so much since cancer, and probably some of my best memories are from this period. In so many ways, I have to thank it for learning how to live fully. What I wish is that everyone can experience the same but without needing cancer. Get out there, experience life fully, and wear that dress!!! I’m so sad to leave my family and friends, I wish I never had to go. I’m so grateful to have had Charlie young so that I’ve watched him grow into the man he is today. I’m unbelievably proud of him. I am thankful I had the opportunity to have Kacey and Jacob in my life. Lastly, I was blessed to meet the love of my life, my husband, and my best friend. I have no regrets, I have had a wonderful life. So to all of you, don’t be sad I’ve gone. Live your life and live it well. Love, Rachel x.”
I didn’t know Ms. Davies, but am likely among many who wish I had. In a terrible situation she kept trying.
She had HER2 metastatic breast cancer, which can respond to the drug Enhertu (trastuzumab). Unfortunately, she never had the chance, because it wasn’t available to her in Wales. In the United Kingdom it’s available only in Scotland.
I’m not saying it was a cure. Statistically, it likely would have bought her another 6 months of family time. But that’s still another half year.
I’m not blaming the Welsh NHS, though they made the decision not to cover it because of cost. The jobs of such committees is a thankless one, trying to decide where the limited money goes — vaccines for many children that are proven to lessen morbidity and mortality over the course of a lifetime, or to add 6 months to the lives of comparatively fewer women with HER2 metastatic breast cancer.
I’m not blaming the company that makes Enhertu, though it was the cost that kept her from getting it. Bringing a drug to market, with all the labs and clinical research behind it, ain’t cheap. If the company can’t keep the lights on they’re not going to able to develop future pharmaceuticals to help others, though I do wonder if a better price could have been negotiated. (I’m not trying to justify the salaries of insurance CEOs — don’t even get me started on those.)
Money is always limited, and human suffering is infinite. Every health care organization, public or private, has to face that simple fact. There is no right place to draw the line, so we use the greatest good for the greatest many as our best guess.
In her last post, though, Ms. Davies didn’t dwell on any of this. She reflected on her joys and blessings, and encouraged others to live life fully. Things we should all focus on.
Thank you, Ms. Davies, for the reminder.
Allan M. Block, MD, has a solo neurology practice in Scottsdale, Arizona.
Recently, a 40-year-old woman took to Facebook to announce that she had died.
Rachel Davies, of Wales, wrote: “If you’re reading this, then it means I’m no longer here. What a life I’ve had, and surprisingly, since cancer entered my life. When I look through my photos, I’ve done and seen so much since cancer, and probably some of my best memories are from this period. In so many ways, I have to thank it for learning how to live fully. What I wish is that everyone can experience the same but without needing cancer. Get out there, experience life fully, and wear that dress!!! I’m so sad to leave my family and friends, I wish I never had to go. I’m so grateful to have had Charlie young so that I’ve watched him grow into the man he is today. I’m unbelievably proud of him. I am thankful I had the opportunity to have Kacey and Jacob in my life. Lastly, I was blessed to meet the love of my life, my husband, and my best friend. I have no regrets, I have had a wonderful life. So to all of you, don’t be sad I’ve gone. Live your life and live it well. Love, Rachel x.”
I didn’t know Ms. Davies, but am likely among many who wish I had. In a terrible situation she kept trying.
She had HER2 metastatic breast cancer, which can respond to the drug Enhertu (trastuzumab). Unfortunately, she never had the chance, because it wasn’t available to her in Wales. In the United Kingdom it’s available only in Scotland.
I’m not saying it was a cure. Statistically, it likely would have bought her another 6 months of family time. But that’s still another half year.
I’m not blaming the Welsh NHS, though they made the decision not to cover it because of cost. The jobs of such committees is a thankless one, trying to decide where the limited money goes — vaccines for many children that are proven to lessen morbidity and mortality over the course of a lifetime, or to add 6 months to the lives of comparatively fewer women with HER2 metastatic breast cancer.
I’m not blaming the company that makes Enhertu, though it was the cost that kept her from getting it. Bringing a drug to market, with all the labs and clinical research behind it, ain’t cheap. If the company can’t keep the lights on they’re not going to able to develop future pharmaceuticals to help others, though I do wonder if a better price could have been negotiated. (I’m not trying to justify the salaries of insurance CEOs — don’t even get me started on those.)
Money is always limited, and human suffering is infinite. Every health care organization, public or private, has to face that simple fact. There is no right place to draw the line, so we use the greatest good for the greatest many as our best guess.
In her last post, though, Ms. Davies didn’t dwell on any of this. She reflected on her joys and blessings, and encouraged others to live life fully. Things we should all focus on.
Thank you, Ms. Davies, for the reminder.
Allan M. Block, MD, has a solo neurology practice in Scottsdale, Arizona.
Recently, a 40-year-old woman took to Facebook to announce that she had died.
Rachel Davies, of Wales, wrote: “If you’re reading this, then it means I’m no longer here. What a life I’ve had, and surprisingly, since cancer entered my life. When I look through my photos, I’ve done and seen so much since cancer, and probably some of my best memories are from this period. In so many ways, I have to thank it for learning how to live fully. What I wish is that everyone can experience the same but without needing cancer. Get out there, experience life fully, and wear that dress!!! I’m so sad to leave my family and friends, I wish I never had to go. I’m so grateful to have had Charlie young so that I’ve watched him grow into the man he is today. I’m unbelievably proud of him. I am thankful I had the opportunity to have Kacey and Jacob in my life. Lastly, I was blessed to meet the love of my life, my husband, and my best friend. I have no regrets, I have had a wonderful life. So to all of you, don’t be sad I’ve gone. Live your life and live it well. Love, Rachel x.”
I didn’t know Ms. Davies, but am likely among many who wish I had. In a terrible situation she kept trying.
She had HER2 metastatic breast cancer, which can respond to the drug Enhertu (trastuzumab). Unfortunately, she never had the chance, because it wasn’t available to her in Wales. In the United Kingdom it’s available only in Scotland.
I’m not saying it was a cure. Statistically, it likely would have bought her another 6 months of family time. But that’s still another half year.
I’m not blaming the Welsh NHS, though they made the decision not to cover it because of cost. The jobs of such committees is a thankless one, trying to decide where the limited money goes — vaccines for many children that are proven to lessen morbidity and mortality over the course of a lifetime, or to add 6 months to the lives of comparatively fewer women with HER2 metastatic breast cancer.
I’m not blaming the company that makes Enhertu, though it was the cost that kept her from getting it. Bringing a drug to market, with all the labs and clinical research behind it, ain’t cheap. If the company can’t keep the lights on they’re not going to able to develop future pharmaceuticals to help others, though I do wonder if a better price could have been negotiated. (I’m not trying to justify the salaries of insurance CEOs — don’t even get me started on those.)
Money is always limited, and human suffering is infinite. Every health care organization, public or private, has to face that simple fact. There is no right place to draw the line, so we use the greatest good for the greatest many as our best guess.
In her last post, though, Ms. Davies didn’t dwell on any of this. She reflected on her joys and blessings, and encouraged others to live life fully. Things we should all focus on.
Thank you, Ms. Davies, for the reminder.
Allan M. Block, MD, has a solo neurology practice in Scottsdale, Arizona.