User login
Does the current age cutoff for screening miss too many cases of cervical cancer in older women?
Cooley JJ, Maguire FB, Morris CR, et al. Cervical cancer stage at diagnosis and survival among women ≥65 years in California. Cancer Epidemiol Biomarkers Prev. 2023;32:91-97. doi:10.1158/1055-9965.EPI-22-0793.
EXPERT COMMENTARY
Cervical cancer screening guidelines recommend screening cessation at age 65 once specific exit criteria are met. (According to the American Cancer Society, individuals aged >65 years who have no history of cervical intraepithelial neoplasia [CIN] grade 2 or more severe disease within the past 25 years, and who have documented adequate negative prior screening in the prior 10 years, discontinue all cervical cancer screening.)1 We know, however, that about one-fifth of all cervical cancer cases are diagnosed among individuals aged 65 or older, and for Black women that proportion is even higher when data are appropriately adjusted to account for the increased rate of hysterectomy among Black versus White women.2-4
Early-stage cervical cancer is largely a curable disease with very high 5-year overall survival rates. Unfortunately, more than half of all cervical cancer is diagnosed at a more advanced stage, and survival rates are much lower for this population.5
Cervical cancer incidence rates plummeted in the United States after the introduction of the Pap test for cervical cancer screening. However, the percentage of women who are not up to date with cervical cancer screening may now be increasing, from 14% in 2005 to 23% in 2019 according to one study from the US Preventive Services Task Force.6 When looking at cervical cancer screening rates by age, researchers from the Centers for Disease Control and Prevention estimate that the proportion of patients who have not been recently screened goes up as patients get older, with approximately 845,000 American women aged 61 to 65 not adequately screened in 2015 alone.7
Details of the study
Cooley and colleagues sought to better characterize the cohort of women diagnosed with cervical cancer at a later age, specifically the stage at diagnosis and survival.8 They used data from the California Cancer Registry (CCR), a large state-mandated, population-based data repository that is affiliated with the Surveillance, Epidemiology, and End Results (SEER) program.
The researchers identified 12,442 womenin the CCR who were newly diagnosed with cervical cancer from 2009 to 2018, 17.4% of whom were age 65 or older. They looked at cancer stage at diagnosis as it relates to relative survival rate (“the ratio of the observed survival rate among those who have cancer divided by the expected survival rate for people of the same sex, race/ethnicity, and age who do not have cancer”), Charlson comorbidity score, socioeconomic status, health insurance status, urbanicity, and race/ethnicity.
Results. In this study, 71% of women aged 65 or older presented with advanced-stage disease (FIGO [International Federation of Gynecology and Obstetrics] stage II–IV) as compared with only 48% in those aged 21 to 64. Five-year relative survival rates also were lower in the older cohort—23% to 37%, compared with 42% to 52% in the younger patients. In a sensitivity analysis, late-stage disease was associated with older age, increasing medical comorbidities, and nonadenocarcinoma histology.
Interestingly, older women of Hispanic ethnicity were less likely to be diagnosed with late-stage disease when compared with non-Hispanic White women.
Study strengths and limitations
Although this study’s conclusions—that patients with advanced-stage cancer are more likely to do poorly than those with early-stage cancer—may seem obvious to some even without the proven data, it is still important to highlight what a clinician may intuit with data to support that intuition. It is particularly important to emphasize this risk in older women in light of the aging population in the United States, with adults older than age 65 expected to account for more than 20% of the nation’s population by 2030.9
The study by Cooley and colleagues adds value to the existing literature due to its large study population, which included more than 12,000 patients diagnosed with cervical cancer.8 And although its results may not be completely generalizable as the data were gathered from only a California-specific population, the sample was diverse with significant portions of Hispanic and Black patients. This study supports previous data that showed high rates of advanced cervical cancer in women older than age 65, with resultant worse 5-year relative survival in this population of older women specifically.4 ●
Cervical cancer is both common and deadly in older women. Although current cervical cancer screening guidelines recommend screening cessation after age 65, remember that this is based on strict exit criteria. Consider screening older women (especially with human papillomavirus [HPV] testing) for cervical cancer if they have risk factors (such as smoking, multiple sexual partners, inconsistent or infrequent screening, history of abnormal Pap or HPV tests), and keep cervical cancer on your differential diagnosis in women who present with postmenopausal bleeding, vaginal discharge, pelvic pain, recurrent urinary tract infections, or other concerning symptoms.
SARAH DILLEY, MD, MPH, AND WARNER HUH, MD
- Fontham ETH, Wolf AMD, Church TR, et al. Cervical cancer screening for individuals at average risk: 2020 guideline update from the American Cancer Society. CA Cancer J Clin. 2020;70:321-346. doi:10.3322/caac.21628.
- Dilley S, Huh W, Blechter B, et al. It’s time to re-evaluate cervical cancer screening after age 65. Gynecol Oncol. 2021;162:200-202. doi:10.1016/j.ygyno.2021.04.027.
- Rositch AF, Nowak RG, Gravitt PE. Increased age and racespecific incidence of cervical cancer after correction for hysterectomy prevalence in the United States from 2000 to 2009. Cancer. 2014;120:2032-2038. doi:10.1002/cncr.28548.
- Beavis AL, Gravitt PE, Rositch AF. Hysterectomy-corrected cervical cancer mortality rates reveal a larger racial disparity in the United States. Cancer. 2017;123:1044-1050. doi:10.1002 /cncr.30507.
- Cancer Stat Facts. National Cancer Institute Surveillance, Epidemiology, and End Results Program. https://seer.cancer .gov/statfacts/html/cervix.html
- Suk R, Hong YR, Rajan SS, et al. Assessment of US Preventive Services Task Force guideline-concordant cervical cancer screening rates and reasons for underscreening by age, race and ethnicity, sexual orientation, rurality, and insurance, 2005 to 2019. JAMA Netw Open. 2022;5:e2143582. doi:10.1001 /jamanetworkopen.2021.43582.
- White MC, Shoemaker ML, Benard VB. Cervical cancer screening and incidence by age: unmet needs near and after the stopping age for screening. Am J Prev Med. 2017;53:392395. doi:10.1016/j.amepre.2017.02.024.
- Cooley JJ, Maguire FB, Morris CR, et al. Cervical cancer stage at diagnosis and survival among women ≥65 years in California. Cancer Epidemiol Biomarkers Prev. 2023;32:91-97. doi:10.1158/1055-9965.EPI-22-0793.
- Ortman JM, Velkoff VA, Hogan H. An aging nation: the older population in the United States. May 2014. United States Census Bureau. Accessed April 12, 2023. https://www.census .gov/library/publications/2014/demo/p25-1140.html
Cooley JJ, Maguire FB, Morris CR, et al. Cervical cancer stage at diagnosis and survival among women ≥65 years in California. Cancer Epidemiol Biomarkers Prev. 2023;32:91-97. doi:10.1158/1055-9965.EPI-22-0793.
EXPERT COMMENTARY
Cervical cancer screening guidelines recommend screening cessation at age 65 once specific exit criteria are met. (According to the American Cancer Society, individuals aged >65 years who have no history of cervical intraepithelial neoplasia [CIN] grade 2 or more severe disease within the past 25 years, and who have documented adequate negative prior screening in the prior 10 years, discontinue all cervical cancer screening.)1 We know, however, that about one-fifth of all cervical cancer cases are diagnosed among individuals aged 65 or older, and for Black women that proportion is even higher when data are appropriately adjusted to account for the increased rate of hysterectomy among Black versus White women.2-4
Early-stage cervical cancer is largely a curable disease with very high 5-year overall survival rates. Unfortunately, more than half of all cervical cancer is diagnosed at a more advanced stage, and survival rates are much lower for this population.5
Cervical cancer incidence rates plummeted in the United States after the introduction of the Pap test for cervical cancer screening. However, the percentage of women who are not up to date with cervical cancer screening may now be increasing, from 14% in 2005 to 23% in 2019 according to one study from the US Preventive Services Task Force.6 When looking at cervical cancer screening rates by age, researchers from the Centers for Disease Control and Prevention estimate that the proportion of patients who have not been recently screened goes up as patients get older, with approximately 845,000 American women aged 61 to 65 not adequately screened in 2015 alone.7
Details of the study
Cooley and colleagues sought to better characterize the cohort of women diagnosed with cervical cancer at a later age, specifically the stage at diagnosis and survival.8 They used data from the California Cancer Registry (CCR), a large state-mandated, population-based data repository that is affiliated with the Surveillance, Epidemiology, and End Results (SEER) program.
The researchers identified 12,442 womenin the CCR who were newly diagnosed with cervical cancer from 2009 to 2018, 17.4% of whom were age 65 or older. They looked at cancer stage at diagnosis as it relates to relative survival rate (“the ratio of the observed survival rate among those who have cancer divided by the expected survival rate for people of the same sex, race/ethnicity, and age who do not have cancer”), Charlson comorbidity score, socioeconomic status, health insurance status, urbanicity, and race/ethnicity.
Results. In this study, 71% of women aged 65 or older presented with advanced-stage disease (FIGO [International Federation of Gynecology and Obstetrics] stage II–IV) as compared with only 48% in those aged 21 to 64. Five-year relative survival rates also were lower in the older cohort—23% to 37%, compared with 42% to 52% in the younger patients. In a sensitivity analysis, late-stage disease was associated with older age, increasing medical comorbidities, and nonadenocarcinoma histology.
Interestingly, older women of Hispanic ethnicity were less likely to be diagnosed with late-stage disease when compared with non-Hispanic White women.
Study strengths and limitations
Although this study’s conclusions—that patients with advanced-stage cancer are more likely to do poorly than those with early-stage cancer—may seem obvious to some even without the proven data, it is still important to highlight what a clinician may intuit with data to support that intuition. It is particularly important to emphasize this risk in older women in light of the aging population in the United States, with adults older than age 65 expected to account for more than 20% of the nation’s population by 2030.9
The study by Cooley and colleagues adds value to the existing literature due to its large study population, which included more than 12,000 patients diagnosed with cervical cancer.8 And although its results may not be completely generalizable as the data were gathered from only a California-specific population, the sample was diverse with significant portions of Hispanic and Black patients. This study supports previous data that showed high rates of advanced cervical cancer in women older than age 65, with resultant worse 5-year relative survival in this population of older women specifically.4 ●
Cervical cancer is both common and deadly in older women. Although current cervical cancer screening guidelines recommend screening cessation after age 65, remember that this is based on strict exit criteria. Consider screening older women (especially with human papillomavirus [HPV] testing) for cervical cancer if they have risk factors (such as smoking, multiple sexual partners, inconsistent or infrequent screening, history of abnormal Pap or HPV tests), and keep cervical cancer on your differential diagnosis in women who present with postmenopausal bleeding, vaginal discharge, pelvic pain, recurrent urinary tract infections, or other concerning symptoms.
SARAH DILLEY, MD, MPH, AND WARNER HUH, MD
Cooley JJ, Maguire FB, Morris CR, et al. Cervical cancer stage at diagnosis and survival among women ≥65 years in California. Cancer Epidemiol Biomarkers Prev. 2023;32:91-97. doi:10.1158/1055-9965.EPI-22-0793.
EXPERT COMMENTARY
Cervical cancer screening guidelines recommend screening cessation at age 65 once specific exit criteria are met. (According to the American Cancer Society, individuals aged >65 years who have no history of cervical intraepithelial neoplasia [CIN] grade 2 or more severe disease within the past 25 years, and who have documented adequate negative prior screening in the prior 10 years, discontinue all cervical cancer screening.)1 We know, however, that about one-fifth of all cervical cancer cases are diagnosed among individuals aged 65 or older, and for Black women that proportion is even higher when data are appropriately adjusted to account for the increased rate of hysterectomy among Black versus White women.2-4
Early-stage cervical cancer is largely a curable disease with very high 5-year overall survival rates. Unfortunately, more than half of all cervical cancer is diagnosed at a more advanced stage, and survival rates are much lower for this population.5
Cervical cancer incidence rates plummeted in the United States after the introduction of the Pap test for cervical cancer screening. However, the percentage of women who are not up to date with cervical cancer screening may now be increasing, from 14% in 2005 to 23% in 2019 according to one study from the US Preventive Services Task Force.6 When looking at cervical cancer screening rates by age, researchers from the Centers for Disease Control and Prevention estimate that the proportion of patients who have not been recently screened goes up as patients get older, with approximately 845,000 American women aged 61 to 65 not adequately screened in 2015 alone.7
Details of the study
Cooley and colleagues sought to better characterize the cohort of women diagnosed with cervical cancer at a later age, specifically the stage at diagnosis and survival.8 They used data from the California Cancer Registry (CCR), a large state-mandated, population-based data repository that is affiliated with the Surveillance, Epidemiology, and End Results (SEER) program.
The researchers identified 12,442 womenin the CCR who were newly diagnosed with cervical cancer from 2009 to 2018, 17.4% of whom were age 65 or older. They looked at cancer stage at diagnosis as it relates to relative survival rate (“the ratio of the observed survival rate among those who have cancer divided by the expected survival rate for people of the same sex, race/ethnicity, and age who do not have cancer”), Charlson comorbidity score, socioeconomic status, health insurance status, urbanicity, and race/ethnicity.
Results. In this study, 71% of women aged 65 or older presented with advanced-stage disease (FIGO [International Federation of Gynecology and Obstetrics] stage II–IV) as compared with only 48% in those aged 21 to 64. Five-year relative survival rates also were lower in the older cohort—23% to 37%, compared with 42% to 52% in the younger patients. In a sensitivity analysis, late-stage disease was associated with older age, increasing medical comorbidities, and nonadenocarcinoma histology.
Interestingly, older women of Hispanic ethnicity were less likely to be diagnosed with late-stage disease when compared with non-Hispanic White women.
Study strengths and limitations
Although this study’s conclusions—that patients with advanced-stage cancer are more likely to do poorly than those with early-stage cancer—may seem obvious to some even without the proven data, it is still important to highlight what a clinician may intuit with data to support that intuition. It is particularly important to emphasize this risk in older women in light of the aging population in the United States, with adults older than age 65 expected to account for more than 20% of the nation’s population by 2030.9
The study by Cooley and colleagues adds value to the existing literature due to its large study population, which included more than 12,000 patients diagnosed with cervical cancer.8 And although its results may not be completely generalizable as the data were gathered from only a California-specific population, the sample was diverse with significant portions of Hispanic and Black patients. This study supports previous data that showed high rates of advanced cervical cancer in women older than age 65, with resultant worse 5-year relative survival in this population of older women specifically.4 ●
Cervical cancer is both common and deadly in older women. Although current cervical cancer screening guidelines recommend screening cessation after age 65, remember that this is based on strict exit criteria. Consider screening older women (especially with human papillomavirus [HPV] testing) for cervical cancer if they have risk factors (such as smoking, multiple sexual partners, inconsistent or infrequent screening, history of abnormal Pap or HPV tests), and keep cervical cancer on your differential diagnosis in women who present with postmenopausal bleeding, vaginal discharge, pelvic pain, recurrent urinary tract infections, or other concerning symptoms.
SARAH DILLEY, MD, MPH, AND WARNER HUH, MD
- Fontham ETH, Wolf AMD, Church TR, et al. Cervical cancer screening for individuals at average risk: 2020 guideline update from the American Cancer Society. CA Cancer J Clin. 2020;70:321-346. doi:10.3322/caac.21628.
- Dilley S, Huh W, Blechter B, et al. It’s time to re-evaluate cervical cancer screening after age 65. Gynecol Oncol. 2021;162:200-202. doi:10.1016/j.ygyno.2021.04.027.
- Rositch AF, Nowak RG, Gravitt PE. Increased age and racespecific incidence of cervical cancer after correction for hysterectomy prevalence in the United States from 2000 to 2009. Cancer. 2014;120:2032-2038. doi:10.1002/cncr.28548.
- Beavis AL, Gravitt PE, Rositch AF. Hysterectomy-corrected cervical cancer mortality rates reveal a larger racial disparity in the United States. Cancer. 2017;123:1044-1050. doi:10.1002 /cncr.30507.
- Cancer Stat Facts. National Cancer Institute Surveillance, Epidemiology, and End Results Program. https://seer.cancer .gov/statfacts/html/cervix.html
- Suk R, Hong YR, Rajan SS, et al. Assessment of US Preventive Services Task Force guideline-concordant cervical cancer screening rates and reasons for underscreening by age, race and ethnicity, sexual orientation, rurality, and insurance, 2005 to 2019. JAMA Netw Open. 2022;5:e2143582. doi:10.1001 /jamanetworkopen.2021.43582.
- White MC, Shoemaker ML, Benard VB. Cervical cancer screening and incidence by age: unmet needs near and after the stopping age for screening. Am J Prev Med. 2017;53:392395. doi:10.1016/j.amepre.2017.02.024.
- Cooley JJ, Maguire FB, Morris CR, et al. Cervical cancer stage at diagnosis and survival among women ≥65 years in California. Cancer Epidemiol Biomarkers Prev. 2023;32:91-97. doi:10.1158/1055-9965.EPI-22-0793.
- Ortman JM, Velkoff VA, Hogan H. An aging nation: the older population in the United States. May 2014. United States Census Bureau. Accessed April 12, 2023. https://www.census .gov/library/publications/2014/demo/p25-1140.html
- Fontham ETH, Wolf AMD, Church TR, et al. Cervical cancer screening for individuals at average risk: 2020 guideline update from the American Cancer Society. CA Cancer J Clin. 2020;70:321-346. doi:10.3322/caac.21628.
- Dilley S, Huh W, Blechter B, et al. It’s time to re-evaluate cervical cancer screening after age 65. Gynecol Oncol. 2021;162:200-202. doi:10.1016/j.ygyno.2021.04.027.
- Rositch AF, Nowak RG, Gravitt PE. Increased age and racespecific incidence of cervical cancer after correction for hysterectomy prevalence in the United States from 2000 to 2009. Cancer. 2014;120:2032-2038. doi:10.1002/cncr.28548.
- Beavis AL, Gravitt PE, Rositch AF. Hysterectomy-corrected cervical cancer mortality rates reveal a larger racial disparity in the United States. Cancer. 2017;123:1044-1050. doi:10.1002 /cncr.30507.
- Cancer Stat Facts. National Cancer Institute Surveillance, Epidemiology, and End Results Program. https://seer.cancer .gov/statfacts/html/cervix.html
- Suk R, Hong YR, Rajan SS, et al. Assessment of US Preventive Services Task Force guideline-concordant cervical cancer screening rates and reasons for underscreening by age, race and ethnicity, sexual orientation, rurality, and insurance, 2005 to 2019. JAMA Netw Open. 2022;5:e2143582. doi:10.1001 /jamanetworkopen.2021.43582.
- White MC, Shoemaker ML, Benard VB. Cervical cancer screening and incidence by age: unmet needs near and after the stopping age for screening. Am J Prev Med. 2017;53:392395. doi:10.1016/j.amepre.2017.02.024.
- Cooley JJ, Maguire FB, Morris CR, et al. Cervical cancer stage at diagnosis and survival among women ≥65 years in California. Cancer Epidemiol Biomarkers Prev. 2023;32:91-97. doi:10.1158/1055-9965.EPI-22-0793.
- Ortman JM, Velkoff VA, Hogan H. An aging nation: the older population in the United States. May 2014. United States Census Bureau. Accessed April 12, 2023. https://www.census .gov/library/publications/2014/demo/p25-1140.html
Federal rules don’t require period product ingredients on packaging labels. States are stepping in.
Tens of millions of Americans use menstrual products, and while manufacturers contend they are safe, most disclose little about the chemicals they contain. Now, amid calls for more disclosure and research into the health effects of these products, some states require more transparency.
The manufacture and sale of period and related products is a big business, with revenue expected to top $4.5 billion in the United States this year. On average, a person uses up to 17,000 tampons or pads in their lifetime, and they might also use rubber or silicone cups, or absorbent period underwear.
The FDA regulates and classifies menstrual products as medical devices, meaning they are not subject to the same labeling laws as other consumer items. But companies can voluntarily disclose what’s in their products.
Now, some states are stepping into the breach. In 2021, New York became the first state to enact a menstrual product disclosure law requiring companies to list all intentionally added ingredients on packaging. California’s governor signed a similar law that took effect this year, but it gives manufacturers trade secret protections, so not all ingredients are necessarily disclosed. At least six other states have introduced legislation to address safety and disclosure of ingredients in these products.
Shruthi Mahalingaiah, an assistant professor of environmental, reproductive, and women’s health at Harvard University, Boston, evaluates endocrine disruptors in personal care products and studies menstrual health. She said the health risk depends on the dose, duration, and sensitivity of a person to the ingredients and their mixtures.
Harmful chemicals could come from manufacturing processes, through materials and shipping, from equipment cleaners, from contact with contaminants, or from companies adding them intentionally, said Alexandra Scranton, director of science and research for Women’s Voices for the Earth, a Montana-based nonprofit focused on eliminating toxic chemicals that affect women’s health.
Vaginal and vulvar tissues are capable of absorbing fluids at a higher rate than skin, which can lead to rapid chemical exposure. Ms. Scranton said scarcity of clinical studies and funding for vaginal health research limits understanding about the long-term effects of the ingredients and additives in period products.
“We think manufacturers should do better and be more careful with the ingredients they choose to use,” Ms. Scranton said. “The presence of toxic and hormone-disrupting chemicals in menstrual products is unsettling. We know that chemicals can cause disease, and exposures do add up over time.”
Ms. Scranton’s organization advocates for labels to include the chemical name of the ingredient, the component in which the ingredient is used, and the function of the ingredient.
K. Malaika Walton, operations director for the Center for Baby and Adult Hygiene Products, a trade industry group, said in an email, “BAHP supports accurate and transparent information for users of period products and many of our member companies list ingredients on their packages and websites.”
In a written statement, Procter & Gamble, a major manufacturer of menstrual products, said that ingredients it uses go through rigorous safety evaluations and are continuously tested, and that all fragrance components are added at levels the industry considers safe.
Even though manufacturing of scented tampons for the U.S. market has mostly stopped, companies still use fragrances in other menstrual products. Laws protecting trade secrets keep details about fragrances in pads and tampons confidential so competitors can’t copy the formulas. The Children’s Environmental Health Network lists phthalates, a group of chemicals commonly called plasticizers, that are suspected hormone disruptors, as an ingredient found in fragrances.
Manufacturers follow regulatory guidance issued in 2005 by registering with the Food and Drug Administration and submitting a detailed risk assessment of their products’ components and design, and a safety profile, before being cleared to sell in the United States.
Pads and menstrual cups are considered exempt from regulatory guidance and do not require premarket review, according to FDA spokesperson Carly Kempler. While tampons do require review, the FDA “does not clear or approve individual materials that are used in the fabrication of medical devices.”
“There’s an understanding that the FDA is regulating these products, and they are; it’s just not very adequate,” said Laura Strausfeld, an attorney and a cofounder of Period Law, an organization working to advance state and federal period-equity policies that would stop taxation of products and make them freely available in places like schools and prisons. “The consumer is supposed to trust that when these products are put on shelves they’ve been vetted by the government. But it’s basically a rubber stamp.”
In a 2022 report, a congressional committee directed the FDA to update its guidance for menstrual products to recommend that labels disclose intentionally added ingredients, such as fragrances, and test for contaminants. The FDA is reviewing the directives outlined by the House Appropriations Committee and will update the 2005 guidance as soon as possible, Ms. Kempler said. “We will share additional details when we are able to.”
At least one period product company makes disclosure of its ingredients a selling point. Alex Friedman, cofounder of Lola, said a lack of knowledge is a problem, and more action and awareness are needed to keep people safe.
“The hardest part to swallow is why this is even up for debate. We should all know what’s in these products,” Ms. Friedman said.
New York’s law requires companies to disclose all intentionally added ingredients no matter how much is used, with no trade secret protections for fragrances. Though it applies only to products sold in that state, similar detailed labeling is appearing elsewhere, advocates said.
“We’re also seeing similar or identical disclosure on packaging in other states outside of New York, which is a testament to the power of the law,” said Jamie McConnell, deputy director of Women’s Voices for the Earth.
Manufacturers have 18 months from the passage of the New York law to comply, and some products on shelves in New York still list few ingredients other than “absorbent material,” “surfactant,” “ink,” and “adhesive.”
“We’re like, ‘OK, what is that exactly?’ ” Ms. McConnell said.
Her organization is calling for a federal law at least as strong as New York’s. Previous federal legislation failed to advance, including the most recent, the Menstrual Products Right to Know Act, introduced in 2022.
BAHP, the trade group, supported the federal legislation and the California law. Ms. McConnell said she opposed both bills because they didn’t require companies to list all fragrance ingredients.
“I think what it boiled down to at the federal level was the support of corporate interests over public health,” she said.
KFF Health News is a national newsroom that produces in-depth journalism about health issues and is one of the core operating programs at KFF—an independent source of health policy research, polling, and journalism. Learn more about KFF.
Tens of millions of Americans use menstrual products, and while manufacturers contend they are safe, most disclose little about the chemicals they contain. Now, amid calls for more disclosure and research into the health effects of these products, some states require more transparency.
The manufacture and sale of period and related products is a big business, with revenue expected to top $4.5 billion in the United States this year. On average, a person uses up to 17,000 tampons or pads in their lifetime, and they might also use rubber or silicone cups, or absorbent period underwear.
The FDA regulates and classifies menstrual products as medical devices, meaning they are not subject to the same labeling laws as other consumer items. But companies can voluntarily disclose what’s in their products.
Now, some states are stepping into the breach. In 2021, New York became the first state to enact a menstrual product disclosure law requiring companies to list all intentionally added ingredients on packaging. California’s governor signed a similar law that took effect this year, but it gives manufacturers trade secret protections, so not all ingredients are necessarily disclosed. At least six other states have introduced legislation to address safety and disclosure of ingredients in these products.
Shruthi Mahalingaiah, an assistant professor of environmental, reproductive, and women’s health at Harvard University, Boston, evaluates endocrine disruptors in personal care products and studies menstrual health. She said the health risk depends on the dose, duration, and sensitivity of a person to the ingredients and their mixtures.
Harmful chemicals could come from manufacturing processes, through materials and shipping, from equipment cleaners, from contact with contaminants, or from companies adding them intentionally, said Alexandra Scranton, director of science and research for Women’s Voices for the Earth, a Montana-based nonprofit focused on eliminating toxic chemicals that affect women’s health.
Vaginal and vulvar tissues are capable of absorbing fluids at a higher rate than skin, which can lead to rapid chemical exposure. Ms. Scranton said scarcity of clinical studies and funding for vaginal health research limits understanding about the long-term effects of the ingredients and additives in period products.
“We think manufacturers should do better and be more careful with the ingredients they choose to use,” Ms. Scranton said. “The presence of toxic and hormone-disrupting chemicals in menstrual products is unsettling. We know that chemicals can cause disease, and exposures do add up over time.”
Ms. Scranton’s organization advocates for labels to include the chemical name of the ingredient, the component in which the ingredient is used, and the function of the ingredient.
K. Malaika Walton, operations director for the Center for Baby and Adult Hygiene Products, a trade industry group, said in an email, “BAHP supports accurate and transparent information for users of period products and many of our member companies list ingredients on their packages and websites.”
In a written statement, Procter & Gamble, a major manufacturer of menstrual products, said that ingredients it uses go through rigorous safety evaluations and are continuously tested, and that all fragrance components are added at levels the industry considers safe.
Even though manufacturing of scented tampons for the U.S. market has mostly stopped, companies still use fragrances in other menstrual products. Laws protecting trade secrets keep details about fragrances in pads and tampons confidential so competitors can’t copy the formulas. The Children’s Environmental Health Network lists phthalates, a group of chemicals commonly called plasticizers, that are suspected hormone disruptors, as an ingredient found in fragrances.
Manufacturers follow regulatory guidance issued in 2005 by registering with the Food and Drug Administration and submitting a detailed risk assessment of their products’ components and design, and a safety profile, before being cleared to sell in the United States.
Pads and menstrual cups are considered exempt from regulatory guidance and do not require premarket review, according to FDA spokesperson Carly Kempler. While tampons do require review, the FDA “does not clear or approve individual materials that are used in the fabrication of medical devices.”
“There’s an understanding that the FDA is regulating these products, and they are; it’s just not very adequate,” said Laura Strausfeld, an attorney and a cofounder of Period Law, an organization working to advance state and federal period-equity policies that would stop taxation of products and make them freely available in places like schools and prisons. “The consumer is supposed to trust that when these products are put on shelves they’ve been vetted by the government. But it’s basically a rubber stamp.”
In a 2022 report, a congressional committee directed the FDA to update its guidance for menstrual products to recommend that labels disclose intentionally added ingredients, such as fragrances, and test for contaminants. The FDA is reviewing the directives outlined by the House Appropriations Committee and will update the 2005 guidance as soon as possible, Ms. Kempler said. “We will share additional details when we are able to.”
At least one period product company makes disclosure of its ingredients a selling point. Alex Friedman, cofounder of Lola, said a lack of knowledge is a problem, and more action and awareness are needed to keep people safe.
“The hardest part to swallow is why this is even up for debate. We should all know what’s in these products,” Ms. Friedman said.
New York’s law requires companies to disclose all intentionally added ingredients no matter how much is used, with no trade secret protections for fragrances. Though it applies only to products sold in that state, similar detailed labeling is appearing elsewhere, advocates said.
“We’re also seeing similar or identical disclosure on packaging in other states outside of New York, which is a testament to the power of the law,” said Jamie McConnell, deputy director of Women’s Voices for the Earth.
Manufacturers have 18 months from the passage of the New York law to comply, and some products on shelves in New York still list few ingredients other than “absorbent material,” “surfactant,” “ink,” and “adhesive.”
“We’re like, ‘OK, what is that exactly?’ ” Ms. McConnell said.
Her organization is calling for a federal law at least as strong as New York’s. Previous federal legislation failed to advance, including the most recent, the Menstrual Products Right to Know Act, introduced in 2022.
BAHP, the trade group, supported the federal legislation and the California law. Ms. McConnell said she opposed both bills because they didn’t require companies to list all fragrance ingredients.
“I think what it boiled down to at the federal level was the support of corporate interests over public health,” she said.
KFF Health News is a national newsroom that produces in-depth journalism about health issues and is one of the core operating programs at KFF—an independent source of health policy research, polling, and journalism. Learn more about KFF.
Tens of millions of Americans use menstrual products, and while manufacturers contend they are safe, most disclose little about the chemicals they contain. Now, amid calls for more disclosure and research into the health effects of these products, some states require more transparency.
The manufacture and sale of period and related products is a big business, with revenue expected to top $4.5 billion in the United States this year. On average, a person uses up to 17,000 tampons or pads in their lifetime, and they might also use rubber or silicone cups, or absorbent period underwear.
The FDA regulates and classifies menstrual products as medical devices, meaning they are not subject to the same labeling laws as other consumer items. But companies can voluntarily disclose what’s in their products.
Now, some states are stepping into the breach. In 2021, New York became the first state to enact a menstrual product disclosure law requiring companies to list all intentionally added ingredients on packaging. California’s governor signed a similar law that took effect this year, but it gives manufacturers trade secret protections, so not all ingredients are necessarily disclosed. At least six other states have introduced legislation to address safety and disclosure of ingredients in these products.
Shruthi Mahalingaiah, an assistant professor of environmental, reproductive, and women’s health at Harvard University, Boston, evaluates endocrine disruptors in personal care products and studies menstrual health. She said the health risk depends on the dose, duration, and sensitivity of a person to the ingredients and their mixtures.
Harmful chemicals could come from manufacturing processes, through materials and shipping, from equipment cleaners, from contact with contaminants, or from companies adding them intentionally, said Alexandra Scranton, director of science and research for Women’s Voices for the Earth, a Montana-based nonprofit focused on eliminating toxic chemicals that affect women’s health.
Vaginal and vulvar tissues are capable of absorbing fluids at a higher rate than skin, which can lead to rapid chemical exposure. Ms. Scranton said scarcity of clinical studies and funding for vaginal health research limits understanding about the long-term effects of the ingredients and additives in period products.
“We think manufacturers should do better and be more careful with the ingredients they choose to use,” Ms. Scranton said. “The presence of toxic and hormone-disrupting chemicals in menstrual products is unsettling. We know that chemicals can cause disease, and exposures do add up over time.”
Ms. Scranton’s organization advocates for labels to include the chemical name of the ingredient, the component in which the ingredient is used, and the function of the ingredient.
K. Malaika Walton, operations director for the Center for Baby and Adult Hygiene Products, a trade industry group, said in an email, “BAHP supports accurate and transparent information for users of period products and many of our member companies list ingredients on their packages and websites.”
In a written statement, Procter & Gamble, a major manufacturer of menstrual products, said that ingredients it uses go through rigorous safety evaluations and are continuously tested, and that all fragrance components are added at levels the industry considers safe.
Even though manufacturing of scented tampons for the U.S. market has mostly stopped, companies still use fragrances in other menstrual products. Laws protecting trade secrets keep details about fragrances in pads and tampons confidential so competitors can’t copy the formulas. The Children’s Environmental Health Network lists phthalates, a group of chemicals commonly called plasticizers, that are suspected hormone disruptors, as an ingredient found in fragrances.
Manufacturers follow regulatory guidance issued in 2005 by registering with the Food and Drug Administration and submitting a detailed risk assessment of their products’ components and design, and a safety profile, before being cleared to sell in the United States.
Pads and menstrual cups are considered exempt from regulatory guidance and do not require premarket review, according to FDA spokesperson Carly Kempler. While tampons do require review, the FDA “does not clear or approve individual materials that are used in the fabrication of medical devices.”
“There’s an understanding that the FDA is regulating these products, and they are; it’s just not very adequate,” said Laura Strausfeld, an attorney and a cofounder of Period Law, an organization working to advance state and federal period-equity policies that would stop taxation of products and make them freely available in places like schools and prisons. “The consumer is supposed to trust that when these products are put on shelves they’ve been vetted by the government. But it’s basically a rubber stamp.”
In a 2022 report, a congressional committee directed the FDA to update its guidance for menstrual products to recommend that labels disclose intentionally added ingredients, such as fragrances, and test for contaminants. The FDA is reviewing the directives outlined by the House Appropriations Committee and will update the 2005 guidance as soon as possible, Ms. Kempler said. “We will share additional details when we are able to.”
At least one period product company makes disclosure of its ingredients a selling point. Alex Friedman, cofounder of Lola, said a lack of knowledge is a problem, and more action and awareness are needed to keep people safe.
“The hardest part to swallow is why this is even up for debate. We should all know what’s in these products,” Ms. Friedman said.
New York’s law requires companies to disclose all intentionally added ingredients no matter how much is used, with no trade secret protections for fragrances. Though it applies only to products sold in that state, similar detailed labeling is appearing elsewhere, advocates said.
“We’re also seeing similar or identical disclosure on packaging in other states outside of New York, which is a testament to the power of the law,” said Jamie McConnell, deputy director of Women’s Voices for the Earth.
Manufacturers have 18 months from the passage of the New York law to comply, and some products on shelves in New York still list few ingredients other than “absorbent material,” “surfactant,” “ink,” and “adhesive.”
“We’re like, ‘OK, what is that exactly?’ ” Ms. McConnell said.
Her organization is calling for a federal law at least as strong as New York’s. Previous federal legislation failed to advance, including the most recent, the Menstrual Products Right to Know Act, introduced in 2022.
BAHP, the trade group, supported the federal legislation and the California law. Ms. McConnell said she opposed both bills because they didn’t require companies to list all fragrance ingredients.
“I think what it boiled down to at the federal level was the support of corporate interests over public health,” she said.
KFF Health News is a national newsroom that produces in-depth journalism about health issues and is one of the core operating programs at KFF—an independent source of health policy research, polling, and journalism. Learn more about KFF.
Wireless neurostimulation safe for urge incontinence
CHICAGO – , according to new findings presented at the 2023 annual meeting of the American Urological Association.
As many as half of women in the United States aged 60 and older will experience urinary incontinence. Of those, roughly one in four experience urge urinary incontinence, marked by a sudden need to void that cannot be fully suppressed.
Researchers studied the benefits of the RENOVA iStim (BlueWind Medical) implantable tibial neuromodulation system for the treatment of overactive bladder in the OASIS trial.
Study investigator Roger R. Dmochowski, MD, MMHC, professor of urology and surgery and associate surgeon-in-chief at Vanderbilt University Medical Center, Nashville, Tenn., said the first-line treatment of urinary incontinence is lifestyle changes to retrain the bladder or physical therapy, including pelvic floor and Kegel exercises, per AUA guidelines. He said the success rate is about 30% and is not sustained. Second-line treatments include medications, which most (60%) patients stop taking by 6 months.
More than three-quarters of the 151 women who received the device responded to therapy at 1 year, and 84.6% of the patients showed improvement, according to Dr. Dmochowski.
The participants (mean age, 58.8) demonstrated a mean baseline of 4.8 urge incidents per day (standard deviation, 2.9) and 10 voids/day (SD, 3.3). No device or procedure-related serious adverse events were reported at 12 months. Half of the women no longer had symptoms on three consecutive days, Dr. Dmochowski said.
Because urge urinary incontinence is a chronic condition, “treatment with the BlueWind System will be ongoing, with frequency determined based on the patient’s response,” Dr. Dmochowski said. “The patient is then empowered to control when and where they perform therapy.”
“The device is activated by the external wearable. It’s like an on-off switch. It has a receiver within it that basically has the capacity to be turned on and off by the wearable, which is the control device. The device is in an off-position until the wearable is applied,” he said.
He said the device should be worn twice a day for about 20 minutes, with many patients using it less.
Only one implanted tibial neuromodulation device has been approved by the Food and Drug Administration – eCOIN (Valencia Technologies). The RENOVA iStim is an investigational device under review by the FDA, Dr. Dmochowski said.
In installing the device, Dr. Dmochowski said urologists use a subfascial technique to enable direct visualization of the tibial nerve and suture fixation that increases the possibility of a predictable placement. Patients use an external wearable, which activates the implant, without concern for battery longevity or replacement.
“This therapy is not associated with any adverse effects and may be beneficial for patients who do not respond to other treatments for OAB such as medications or Botox,” said Carol E. Bretschneider, MD, a urogynecologic and pelvic surgeon at Northwestern Medicine Central DuPage Hospital, outside Chicago. “Neurostimulators can be a great advanced therapy option for patients who do not respond to more conservative treatments or cannot take or tolerate a medication.”
The devices do not stimulate or strengthen muscles but act by modulating the reflexes that influence the bladder, sphincter, and pelvic floor, added Dr. Bretschneider, who was not involved in the study.
Other treatments for urge incontinence can include acupuncture, or percutaneous tibial nerve stimulation, to target the posterior tibial nerve in the ankle, which shares the same nerve root that controls the bladder, according to Aron Liaw, MD, a reconstructive urologist and assistant professor of urology at Wayne State University in Detroit. This treatment has been shown to be at least as effective as available medications, but with fewer side effects, he said.
But regular stimulation is necessary to achieve and preserve efficacy, he said.
Dr. Liaw, who was not involved in the neuromodulation study, said the benefits of a device like Renova iStim are that implantation is relatively easy and can be performed in office settings, and patients can then treat themselves at home. However, because the new study did not compare the device to other treatments or a placebo device, its relative benefits are unclear, he said,
Other treatments for urge urinary incontinence, such as bladder Botox and sacral neuromodulation, also are minimally invasive and have proven benefit, “so a device like this could well be less effective with little other advantage,” he said.
“Lifestyle changes can make a big difference, but making big lifestyle changes is not always easy,” added Dr. Liaw. “I have found neuromodulation [to be] very effective, especially in conjunction with lifestyle changes.”
BlueWind Medical funds the OASIS trial. Dr. Dmochowski reported he received no grants nor has any relevant financial relationships. Dr. Bretschneider and Dr. Liaw report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
CHICAGO – , according to new findings presented at the 2023 annual meeting of the American Urological Association.
As many as half of women in the United States aged 60 and older will experience urinary incontinence. Of those, roughly one in four experience urge urinary incontinence, marked by a sudden need to void that cannot be fully suppressed.
Researchers studied the benefits of the RENOVA iStim (BlueWind Medical) implantable tibial neuromodulation system for the treatment of overactive bladder in the OASIS trial.
Study investigator Roger R. Dmochowski, MD, MMHC, professor of urology and surgery and associate surgeon-in-chief at Vanderbilt University Medical Center, Nashville, Tenn., said the first-line treatment of urinary incontinence is lifestyle changes to retrain the bladder or physical therapy, including pelvic floor and Kegel exercises, per AUA guidelines. He said the success rate is about 30% and is not sustained. Second-line treatments include medications, which most (60%) patients stop taking by 6 months.
More than three-quarters of the 151 women who received the device responded to therapy at 1 year, and 84.6% of the patients showed improvement, according to Dr. Dmochowski.
The participants (mean age, 58.8) demonstrated a mean baseline of 4.8 urge incidents per day (standard deviation, 2.9) and 10 voids/day (SD, 3.3). No device or procedure-related serious adverse events were reported at 12 months. Half of the women no longer had symptoms on three consecutive days, Dr. Dmochowski said.
Because urge urinary incontinence is a chronic condition, “treatment with the BlueWind System will be ongoing, with frequency determined based on the patient’s response,” Dr. Dmochowski said. “The patient is then empowered to control when and where they perform therapy.”
“The device is activated by the external wearable. It’s like an on-off switch. It has a receiver within it that basically has the capacity to be turned on and off by the wearable, which is the control device. The device is in an off-position until the wearable is applied,” he said.
He said the device should be worn twice a day for about 20 minutes, with many patients using it less.
Only one implanted tibial neuromodulation device has been approved by the Food and Drug Administration – eCOIN (Valencia Technologies). The RENOVA iStim is an investigational device under review by the FDA, Dr. Dmochowski said.
In installing the device, Dr. Dmochowski said urologists use a subfascial technique to enable direct visualization of the tibial nerve and suture fixation that increases the possibility of a predictable placement. Patients use an external wearable, which activates the implant, without concern for battery longevity or replacement.
“This therapy is not associated with any adverse effects and may be beneficial for patients who do not respond to other treatments for OAB such as medications or Botox,” said Carol E. Bretschneider, MD, a urogynecologic and pelvic surgeon at Northwestern Medicine Central DuPage Hospital, outside Chicago. “Neurostimulators can be a great advanced therapy option for patients who do not respond to more conservative treatments or cannot take or tolerate a medication.”
The devices do not stimulate or strengthen muscles but act by modulating the reflexes that influence the bladder, sphincter, and pelvic floor, added Dr. Bretschneider, who was not involved in the study.
Other treatments for urge incontinence can include acupuncture, or percutaneous tibial nerve stimulation, to target the posterior tibial nerve in the ankle, which shares the same nerve root that controls the bladder, according to Aron Liaw, MD, a reconstructive urologist and assistant professor of urology at Wayne State University in Detroit. This treatment has been shown to be at least as effective as available medications, but with fewer side effects, he said.
But regular stimulation is necessary to achieve and preserve efficacy, he said.
Dr. Liaw, who was not involved in the neuromodulation study, said the benefits of a device like Renova iStim are that implantation is relatively easy and can be performed in office settings, and patients can then treat themselves at home. However, because the new study did not compare the device to other treatments or a placebo device, its relative benefits are unclear, he said,
Other treatments for urge urinary incontinence, such as bladder Botox and sacral neuromodulation, also are minimally invasive and have proven benefit, “so a device like this could well be less effective with little other advantage,” he said.
“Lifestyle changes can make a big difference, but making big lifestyle changes is not always easy,” added Dr. Liaw. “I have found neuromodulation [to be] very effective, especially in conjunction with lifestyle changes.”
BlueWind Medical funds the OASIS trial. Dr. Dmochowski reported he received no grants nor has any relevant financial relationships. Dr. Bretschneider and Dr. Liaw report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
CHICAGO – , according to new findings presented at the 2023 annual meeting of the American Urological Association.
As many as half of women in the United States aged 60 and older will experience urinary incontinence. Of those, roughly one in four experience urge urinary incontinence, marked by a sudden need to void that cannot be fully suppressed.
Researchers studied the benefits of the RENOVA iStim (BlueWind Medical) implantable tibial neuromodulation system for the treatment of overactive bladder in the OASIS trial.
Study investigator Roger R. Dmochowski, MD, MMHC, professor of urology and surgery and associate surgeon-in-chief at Vanderbilt University Medical Center, Nashville, Tenn., said the first-line treatment of urinary incontinence is lifestyle changes to retrain the bladder or physical therapy, including pelvic floor and Kegel exercises, per AUA guidelines. He said the success rate is about 30% and is not sustained. Second-line treatments include medications, which most (60%) patients stop taking by 6 months.
More than three-quarters of the 151 women who received the device responded to therapy at 1 year, and 84.6% of the patients showed improvement, according to Dr. Dmochowski.
The participants (mean age, 58.8) demonstrated a mean baseline of 4.8 urge incidents per day (standard deviation, 2.9) and 10 voids/day (SD, 3.3). No device or procedure-related serious adverse events were reported at 12 months. Half of the women no longer had symptoms on three consecutive days, Dr. Dmochowski said.
Because urge urinary incontinence is a chronic condition, “treatment with the BlueWind System will be ongoing, with frequency determined based on the patient’s response,” Dr. Dmochowski said. “The patient is then empowered to control when and where they perform therapy.”
“The device is activated by the external wearable. It’s like an on-off switch. It has a receiver within it that basically has the capacity to be turned on and off by the wearable, which is the control device. The device is in an off-position until the wearable is applied,” he said.
He said the device should be worn twice a day for about 20 minutes, with many patients using it less.
Only one implanted tibial neuromodulation device has been approved by the Food and Drug Administration – eCOIN (Valencia Technologies). The RENOVA iStim is an investigational device under review by the FDA, Dr. Dmochowski said.
In installing the device, Dr. Dmochowski said urologists use a subfascial technique to enable direct visualization of the tibial nerve and suture fixation that increases the possibility of a predictable placement. Patients use an external wearable, which activates the implant, without concern for battery longevity or replacement.
“This therapy is not associated with any adverse effects and may be beneficial for patients who do not respond to other treatments for OAB such as medications or Botox,” said Carol E. Bretschneider, MD, a urogynecologic and pelvic surgeon at Northwestern Medicine Central DuPage Hospital, outside Chicago. “Neurostimulators can be a great advanced therapy option for patients who do not respond to more conservative treatments or cannot take or tolerate a medication.”
The devices do not stimulate or strengthen muscles but act by modulating the reflexes that influence the bladder, sphincter, and pelvic floor, added Dr. Bretschneider, who was not involved in the study.
Other treatments for urge incontinence can include acupuncture, or percutaneous tibial nerve stimulation, to target the posterior tibial nerve in the ankle, which shares the same nerve root that controls the bladder, according to Aron Liaw, MD, a reconstructive urologist and assistant professor of urology at Wayne State University in Detroit. This treatment has been shown to be at least as effective as available medications, but with fewer side effects, he said.
But regular stimulation is necessary to achieve and preserve efficacy, he said.
Dr. Liaw, who was not involved in the neuromodulation study, said the benefits of a device like Renova iStim are that implantation is relatively easy and can be performed in office settings, and patients can then treat themselves at home. However, because the new study did not compare the device to other treatments or a placebo device, its relative benefits are unclear, he said,
Other treatments for urge urinary incontinence, such as bladder Botox and sacral neuromodulation, also are minimally invasive and have proven benefit, “so a device like this could well be less effective with little other advantage,” he said.
“Lifestyle changes can make a big difference, but making big lifestyle changes is not always easy,” added Dr. Liaw. “I have found neuromodulation [to be] very effective, especially in conjunction with lifestyle changes.”
BlueWind Medical funds the OASIS trial. Dr. Dmochowski reported he received no grants nor has any relevant financial relationships. Dr. Bretschneider and Dr. Liaw report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
AT AUA 2023
Neuropsychiatric side effects of hormonal contraceptives: More common than you think!
Since its introduction in 1950, the combined oral contraceptive pill has been used by countless women as a method for birth control (Liao P. Can Fam Physician. 2012 Dec; 58[12]:e757-e760).
Hormonal contraception (HC) provides women with both contraceptive and noncontraceptive benefits, most notably a method for avoiding unintended pregnancy. In addition to being an effective method of contraception, oral contraceptive pills (OCPs) are well established for treating conditions such as hirsutism, pain symptoms associated with endometriosis and adenomyosis, and pelvic inflammatory disease, among others (Schindler A. Int J Endocrinol Metab. 2013 Winter;11[1]:41-7).
Combined hormonal contraceptives are also first-line treatment for women with menstrual disorders, and in women with polycystic ovary syndrome, can offer an effective long-term method to regulate their menstrual cycle, decrease androgens, clear up oily skin and acne, and reduce facial hair while also providing them with effective contraception (de Melo et al. Open Access J Contracept. 2017;8:13-23).
Associations between ‘the pill’ and mood effects remain controversial
More than 100 million women worldwide use hormonal contraceptives today, yet despite this, the data are mixed regarding the prevalence and extent of neuropsychiatric symptoms and mood changes associated with use of “the pill.” Some studies show combined oral contraceptives are associated with a decrease in general well-being, but had no effect on depression, in women compared with placebo (Zethraeus N et al. Fertil Steril. 2017 May;107[5]:1238-45).
However, a large Danish study published in JAMA Psychiatry of more than 1 million women found a significant association between use of hormonal contraception and antidepressant use or first diagnosis of depression, with adolescents having a higher rate of first depression diagnosis and antidepressant use compared with women 20–30 years old (Skovlund C et al. JAMA Psychiatry. 2016 Nov 1;73[11]:1154-62).
Studies have also shown long-term exposure to levonorgestrel is significantly associated with anxiety and sleep problems in women without a history of these issues (Slattery J et al. Drug Saf. 2018 Oct;41[10]:951-8). A recent small nationwide cohort study in France suggests this may also be true of levonorgestrel delivered by intrauterine devices (IUD) and the association may be dose-dependent (Roland N et al. JAMA. 2023;329[3]:257-9).
Of note, a study published in the American Journal of Psychiatry found a nearly twofold risk of suicide attempt and over threefold risk of suicide among women taking hormonal contraception compared with women who had never used hormonal contraceptives (Skovlund et al. Am J Psychiatry. 2017 Nov 17:appiajp201717060616).
Knowledge gaps make drawing conclusions difficult
The latest information on use of antidepressant and antianxiety medications in women of reproductive age (18-44 years) is sparse and, in some cases, outdated. According to data from the National Health and Nutrition Examination Survey, 18.6% of adult women 18 years or older reported using antidepressant medications within the last 30 days in 2017-2018, an increase from 13.8% in 2009-2010. Among women aged 15-44 year with private employer–sponsored insurance surveyed during 2008-2013, the results showed 15.4% of women filled a prescription for an antidepressant. We must look back further to find data on antianxiety medication use among women aged 18-44 years where use of antianxiety drugs (anxiolytics, sedatives, and hypnotics) was 4.3% between 2005 and 2008.
A lack of literature in this area is likely due to significant underreporting, and an inability to select patients who are sensitive to or at risk of developing neuropsychiatric symptoms resulting from hormonal contraception use because the true pathophysiology is unknown. Existing studies tend to use varying methods to assess mood changes, and do not usually specify hormonal contraceptive use type in their analyses (Schaffir J et al. Eur J Contracept Reprod Health Care. 2016 Oct;21[5]:347-55).
Studies of this nature also require large sample sizes, but the percentage of women who develop neuropsychiatric symptoms from hormonal contraceptive use has historically been relatively small. In the late 1990s, Rosenberg and colleagues found 46% of 1,657 women discontinued oral contraceptives due to side effects within 6 months of starting a new prescription; of these women, 5% reported mood changes as their reason for discontinuing oral contraceptives (Rosenberg M et al. Am J Obstet Gynecol. 1998 Sep;179[3 Pt 1]:577-82).
One might expect that, as lower dosage combined hormonal contraceptives were developed in the 1980s, that the rate of reporting psychological side effects would continue to decrease as well. Yet greater awareness of the potential for mood changes while on “the pill” as outlined by the lay press and social media may be leading to increased reporting of neuropsychiatric effects in women. In a recent cross-sectional survey of 188 women in New York, 43.6% said they experienced mood changes while on hormonal contraceptives, and 61.2% of women with histories of psychiatric illness reported mood changes they attributed to hormonal contraceptives (Martell S et al. Contracept Reprod Med. 2023;8:9).
Martell and colleagues found 48.3% of women cited side effects as a reason for discontinuing hormonal contraception, and 43 participants mentioned psychological side effects unprompted, including 2 patients with suicidal thoughts. The authors said this suggests “psychological side effects, at least in part, may have impacted” HC users’ decisions to switch from OCPs to an alternative method of contraception.
It is also not clear what risk factors exist for women who develop neuropsychiatric symptoms from hormonal contraceptive use. First, it is important to note that both progestin-only contraceptives and combined hormonal contraceptives are classified by the Centers for Disease Control and Prevention’s US Medical Eligibility Criteria for Contraceptive Use, 2016 as having no restrictions for use, including among patients with depression. While women in a smaller subgroup have significant neuropsychiatric symptoms related to their hormonal contraceptives, the underlying mechanism is unknown, and is thought to be largely related to the progestogen component of combined hormonal contraceptives or progestogen-only contraceptives (Mu E. Aust Prescr. 2022 Jun; 45[3]:75-9). We know that some women are hormone sensitive, while others are less so, and some not at all. Progestogens could affect mood as a direct action of the progestogen, because progestogens can be neurosteroids, or the progestogen effect could be mediated secondarily through a change in that woman’s own production of or bioavailability of androgens or naturally occurring estrogens (Giatti S. J Mol Endocrinol. 2016 Aug;57[2]:R109-26).
Here, we also find that currently available evidence limits our ability to draw firm conclusions. A study by Berry-Bibee and colleagues found a “low concern for clinically significant interactions” between hormonal contraception and psychotropic drugs, but was limited by quality/quantity of evidence (Berry-Bibee E et al. Contraception. 2016 Dec;94[6]:650-67). Interestingly, a study by Robinson and colleagues from the mid-2000s posited based on low evidence that “psychological response to the practice of contraception” was a potential explanation for the side effect profile of hormonal contraception (Robinson S et al. Med Hypotheses. 2004;63[2]:268-73).
Further, it may be that women with premenstrual dysphoric disorder (PMDD) might be selected for oral contraceptives, and they are predisposed to other neuropsychiatric problems. Estimates have placed the prevalence of comorbid psychiatric disorders such as anxiety, major depression, bipolar disorder, and posttraumatic stress disorder as high as 70% for women with PMDD (Sepede G et al. Neuropsychiatr Dis Treat. 2020;16:415-26). This phenomenon is not new, having been characterized in the lay literature nearly 20 years ago, by endocrinologist Geoffrey P. Redmond, MD (Redmond GP. The Hormonally Vulnerable Woman. New York: HarperCollins; 2005).
While the cause is not exactly idiosyncratic, They tend to have an entire spectrum of responses to the progestogens in combined or progestin-only contraceptives, ranging from just a flattened affect – which could easily be explained by their flattened level of endogenous hormones – to frank depression. Their frank depression, in turn, can be demonstrated to include suicidal ideation and actual suicide.
Compounding this issue is a woman’s perception of her sexuality. Some women with low sexual desire or sexual problems who are younger may have more distress about their problems compared with women of older reproductive age. While the reason for that is not clear, it may be that in the sexual arena, it is more important for some younger women to be a sexual person than in perimenopausal women, or that women who are younger are more likely to be partnered than women of older reproductive age. While the European Society of Sexual Medicine concluded in a 2019 position statement that there is inconclusive evidence whether hormonal contraception may be contributing to changes in sexual desire and sexual dysfunction, it appears that “a minority of women” experience “better or worse sexual functioning” from taking combined oral contraceptives (Both S et al. J Sex Med. 2019 Nov;16[11]:1681-95), suggesting that the majority of women report no significant changes.
Practitioners should discuss mood effects during consultation
An ob.gyn., primary care physicians, or others with prescriptive authority (i.e. nurse practitioners and physician assistants) in clinical practice may encounter a patient who seems to have mood side effects owing to progestogen-containing contraceptives that they prescribe. However, many ob.gyns. are likely unaware of the prevalence, or that some of those same patients can have such significant mood effects that they would become or are suicidal.
I believe questioning patients about mood effects during consultation and particularly during follow-up following the initiation of any hormonal contraceptive is worth a passing comment for every patient, which should include mood effects in broader discussion for anyone currently using an antidepressant, patients with a history of antidepressant use, and patients who have considered suicide. As we do with other drugs, these questions can be posed in the form of a questionnaire followed up by the practitioner in counseling.
Practitioners who encounter a patient with mood changes as a result of hormonal contraceptive use can consider changing to a nonhormonal method of birth control, or recommending the patient use a barrier method during sexual activity, as none of these options have neuropsychiatric side effects.
Ultimately, practitioners of all types need to engage in shared decision-making to identify the key benefits and risks of hormonal contraceptive use for each patient, which may involve trial and error to determine the ideal treatment. It is critical that practitioners of all types strike a balance between alleviating patient concerns about potential mood changes, monitoring patients with an appreciable risk of mood changes, and continuing patients on hormonal contraception for whom the benefits outweigh the risks.
Dr. Simon is a clinical professor at George Washington University and the medical director and founder of IntimMedicine Specialists in Washington, which provides patient-focused care for women across the reproductive life cycle. He is a past president of the International Society for the Study of Women’s Sexual Health and the North American Menopause Society. Dr. Simon has been a consultant to, received grant and research support from, and served on the speakers bureau for various pharmaceutical companies that develop combination hormonal contraceptives. Email Dr. Simon at obnews@mdedge.com.
Since its introduction in 1950, the combined oral contraceptive pill has been used by countless women as a method for birth control (Liao P. Can Fam Physician. 2012 Dec; 58[12]:e757-e760).
Hormonal contraception (HC) provides women with both contraceptive and noncontraceptive benefits, most notably a method for avoiding unintended pregnancy. In addition to being an effective method of contraception, oral contraceptive pills (OCPs) are well established for treating conditions such as hirsutism, pain symptoms associated with endometriosis and adenomyosis, and pelvic inflammatory disease, among others (Schindler A. Int J Endocrinol Metab. 2013 Winter;11[1]:41-7).
Combined hormonal contraceptives are also first-line treatment for women with menstrual disorders, and in women with polycystic ovary syndrome, can offer an effective long-term method to regulate their menstrual cycle, decrease androgens, clear up oily skin and acne, and reduce facial hair while also providing them with effective contraception (de Melo et al. Open Access J Contracept. 2017;8:13-23).
Associations between ‘the pill’ and mood effects remain controversial
More than 100 million women worldwide use hormonal contraceptives today, yet despite this, the data are mixed regarding the prevalence and extent of neuropsychiatric symptoms and mood changes associated with use of “the pill.” Some studies show combined oral contraceptives are associated with a decrease in general well-being, but had no effect on depression, in women compared with placebo (Zethraeus N et al. Fertil Steril. 2017 May;107[5]:1238-45).
However, a large Danish study published in JAMA Psychiatry of more than 1 million women found a significant association between use of hormonal contraception and antidepressant use or first diagnosis of depression, with adolescents having a higher rate of first depression diagnosis and antidepressant use compared with women 20–30 years old (Skovlund C et al. JAMA Psychiatry. 2016 Nov 1;73[11]:1154-62).
Studies have also shown long-term exposure to levonorgestrel is significantly associated with anxiety and sleep problems in women without a history of these issues (Slattery J et al. Drug Saf. 2018 Oct;41[10]:951-8). A recent small nationwide cohort study in France suggests this may also be true of levonorgestrel delivered by intrauterine devices (IUD) and the association may be dose-dependent (Roland N et al. JAMA. 2023;329[3]:257-9).
Of note, a study published in the American Journal of Psychiatry found a nearly twofold risk of suicide attempt and over threefold risk of suicide among women taking hormonal contraception compared with women who had never used hormonal contraceptives (Skovlund et al. Am J Psychiatry. 2017 Nov 17:appiajp201717060616).
Knowledge gaps make drawing conclusions difficult
The latest information on use of antidepressant and antianxiety medications in women of reproductive age (18-44 years) is sparse and, in some cases, outdated. According to data from the National Health and Nutrition Examination Survey, 18.6% of adult women 18 years or older reported using antidepressant medications within the last 30 days in 2017-2018, an increase from 13.8% in 2009-2010. Among women aged 15-44 year with private employer–sponsored insurance surveyed during 2008-2013, the results showed 15.4% of women filled a prescription for an antidepressant. We must look back further to find data on antianxiety medication use among women aged 18-44 years where use of antianxiety drugs (anxiolytics, sedatives, and hypnotics) was 4.3% between 2005 and 2008.
A lack of literature in this area is likely due to significant underreporting, and an inability to select patients who are sensitive to or at risk of developing neuropsychiatric symptoms resulting from hormonal contraception use because the true pathophysiology is unknown. Existing studies tend to use varying methods to assess mood changes, and do not usually specify hormonal contraceptive use type in their analyses (Schaffir J et al. Eur J Contracept Reprod Health Care. 2016 Oct;21[5]:347-55).
Studies of this nature also require large sample sizes, but the percentage of women who develop neuropsychiatric symptoms from hormonal contraceptive use has historically been relatively small. In the late 1990s, Rosenberg and colleagues found 46% of 1,657 women discontinued oral contraceptives due to side effects within 6 months of starting a new prescription; of these women, 5% reported mood changes as their reason for discontinuing oral contraceptives (Rosenberg M et al. Am J Obstet Gynecol. 1998 Sep;179[3 Pt 1]:577-82).
One might expect that, as lower dosage combined hormonal contraceptives were developed in the 1980s, that the rate of reporting psychological side effects would continue to decrease as well. Yet greater awareness of the potential for mood changes while on “the pill” as outlined by the lay press and social media may be leading to increased reporting of neuropsychiatric effects in women. In a recent cross-sectional survey of 188 women in New York, 43.6% said they experienced mood changes while on hormonal contraceptives, and 61.2% of women with histories of psychiatric illness reported mood changes they attributed to hormonal contraceptives (Martell S et al. Contracept Reprod Med. 2023;8:9).
Martell and colleagues found 48.3% of women cited side effects as a reason for discontinuing hormonal contraception, and 43 participants mentioned psychological side effects unprompted, including 2 patients with suicidal thoughts. The authors said this suggests “psychological side effects, at least in part, may have impacted” HC users’ decisions to switch from OCPs to an alternative method of contraception.
It is also not clear what risk factors exist for women who develop neuropsychiatric symptoms from hormonal contraceptive use. First, it is important to note that both progestin-only contraceptives and combined hormonal contraceptives are classified by the Centers for Disease Control and Prevention’s US Medical Eligibility Criteria for Contraceptive Use, 2016 as having no restrictions for use, including among patients with depression. While women in a smaller subgroup have significant neuropsychiatric symptoms related to their hormonal contraceptives, the underlying mechanism is unknown, and is thought to be largely related to the progestogen component of combined hormonal contraceptives or progestogen-only contraceptives (Mu E. Aust Prescr. 2022 Jun; 45[3]:75-9). We know that some women are hormone sensitive, while others are less so, and some not at all. Progestogens could affect mood as a direct action of the progestogen, because progestogens can be neurosteroids, or the progestogen effect could be mediated secondarily through a change in that woman’s own production of or bioavailability of androgens or naturally occurring estrogens (Giatti S. J Mol Endocrinol. 2016 Aug;57[2]:R109-26).
Here, we also find that currently available evidence limits our ability to draw firm conclusions. A study by Berry-Bibee and colleagues found a “low concern for clinically significant interactions” between hormonal contraception and psychotropic drugs, but was limited by quality/quantity of evidence (Berry-Bibee E et al. Contraception. 2016 Dec;94[6]:650-67). Interestingly, a study by Robinson and colleagues from the mid-2000s posited based on low evidence that “psychological response to the practice of contraception” was a potential explanation for the side effect profile of hormonal contraception (Robinson S et al. Med Hypotheses. 2004;63[2]:268-73).
Further, it may be that women with premenstrual dysphoric disorder (PMDD) might be selected for oral contraceptives, and they are predisposed to other neuropsychiatric problems. Estimates have placed the prevalence of comorbid psychiatric disorders such as anxiety, major depression, bipolar disorder, and posttraumatic stress disorder as high as 70% for women with PMDD (Sepede G et al. Neuropsychiatr Dis Treat. 2020;16:415-26). This phenomenon is not new, having been characterized in the lay literature nearly 20 years ago, by endocrinologist Geoffrey P. Redmond, MD (Redmond GP. The Hormonally Vulnerable Woman. New York: HarperCollins; 2005).
While the cause is not exactly idiosyncratic, They tend to have an entire spectrum of responses to the progestogens in combined or progestin-only contraceptives, ranging from just a flattened affect – which could easily be explained by their flattened level of endogenous hormones – to frank depression. Their frank depression, in turn, can be demonstrated to include suicidal ideation and actual suicide.
Compounding this issue is a woman’s perception of her sexuality. Some women with low sexual desire or sexual problems who are younger may have more distress about their problems compared with women of older reproductive age. While the reason for that is not clear, it may be that in the sexual arena, it is more important for some younger women to be a sexual person than in perimenopausal women, or that women who are younger are more likely to be partnered than women of older reproductive age. While the European Society of Sexual Medicine concluded in a 2019 position statement that there is inconclusive evidence whether hormonal contraception may be contributing to changes in sexual desire and sexual dysfunction, it appears that “a minority of women” experience “better or worse sexual functioning” from taking combined oral contraceptives (Both S et al. J Sex Med. 2019 Nov;16[11]:1681-95), suggesting that the majority of women report no significant changes.
Practitioners should discuss mood effects during consultation
An ob.gyn., primary care physicians, or others with prescriptive authority (i.e. nurse practitioners and physician assistants) in clinical practice may encounter a patient who seems to have mood side effects owing to progestogen-containing contraceptives that they prescribe. However, many ob.gyns. are likely unaware of the prevalence, or that some of those same patients can have such significant mood effects that they would become or are suicidal.
I believe questioning patients about mood effects during consultation and particularly during follow-up following the initiation of any hormonal contraceptive is worth a passing comment for every patient, which should include mood effects in broader discussion for anyone currently using an antidepressant, patients with a history of antidepressant use, and patients who have considered suicide. As we do with other drugs, these questions can be posed in the form of a questionnaire followed up by the practitioner in counseling.
Practitioners who encounter a patient with mood changes as a result of hormonal contraceptive use can consider changing to a nonhormonal method of birth control, or recommending the patient use a barrier method during sexual activity, as none of these options have neuropsychiatric side effects.
Ultimately, practitioners of all types need to engage in shared decision-making to identify the key benefits and risks of hormonal contraceptive use for each patient, which may involve trial and error to determine the ideal treatment. It is critical that practitioners of all types strike a balance between alleviating patient concerns about potential mood changes, monitoring patients with an appreciable risk of mood changes, and continuing patients on hormonal contraception for whom the benefits outweigh the risks.
Dr. Simon is a clinical professor at George Washington University and the medical director and founder of IntimMedicine Specialists in Washington, which provides patient-focused care for women across the reproductive life cycle. He is a past president of the International Society for the Study of Women’s Sexual Health and the North American Menopause Society. Dr. Simon has been a consultant to, received grant and research support from, and served on the speakers bureau for various pharmaceutical companies that develop combination hormonal contraceptives. Email Dr. Simon at obnews@mdedge.com.
Since its introduction in 1950, the combined oral contraceptive pill has been used by countless women as a method for birth control (Liao P. Can Fam Physician. 2012 Dec; 58[12]:e757-e760).
Hormonal contraception (HC) provides women with both contraceptive and noncontraceptive benefits, most notably a method for avoiding unintended pregnancy. In addition to being an effective method of contraception, oral contraceptive pills (OCPs) are well established for treating conditions such as hirsutism, pain symptoms associated with endometriosis and adenomyosis, and pelvic inflammatory disease, among others (Schindler A. Int J Endocrinol Metab. 2013 Winter;11[1]:41-7).
Combined hormonal contraceptives are also first-line treatment for women with menstrual disorders, and in women with polycystic ovary syndrome, can offer an effective long-term method to regulate their menstrual cycle, decrease androgens, clear up oily skin and acne, and reduce facial hair while also providing them with effective contraception (de Melo et al. Open Access J Contracept. 2017;8:13-23).
Associations between ‘the pill’ and mood effects remain controversial
More than 100 million women worldwide use hormonal contraceptives today, yet despite this, the data are mixed regarding the prevalence and extent of neuropsychiatric symptoms and mood changes associated with use of “the pill.” Some studies show combined oral contraceptives are associated with a decrease in general well-being, but had no effect on depression, in women compared with placebo (Zethraeus N et al. Fertil Steril. 2017 May;107[5]:1238-45).
However, a large Danish study published in JAMA Psychiatry of more than 1 million women found a significant association between use of hormonal contraception and antidepressant use or first diagnosis of depression, with adolescents having a higher rate of first depression diagnosis and antidepressant use compared with women 20–30 years old (Skovlund C et al. JAMA Psychiatry. 2016 Nov 1;73[11]:1154-62).
Studies have also shown long-term exposure to levonorgestrel is significantly associated with anxiety and sleep problems in women without a history of these issues (Slattery J et al. Drug Saf. 2018 Oct;41[10]:951-8). A recent small nationwide cohort study in France suggests this may also be true of levonorgestrel delivered by intrauterine devices (IUD) and the association may be dose-dependent (Roland N et al. JAMA. 2023;329[3]:257-9).
Of note, a study published in the American Journal of Psychiatry found a nearly twofold risk of suicide attempt and over threefold risk of suicide among women taking hormonal contraception compared with women who had never used hormonal contraceptives (Skovlund et al. Am J Psychiatry. 2017 Nov 17:appiajp201717060616).
Knowledge gaps make drawing conclusions difficult
The latest information on use of antidepressant and antianxiety medications in women of reproductive age (18-44 years) is sparse and, in some cases, outdated. According to data from the National Health and Nutrition Examination Survey, 18.6% of adult women 18 years or older reported using antidepressant medications within the last 30 days in 2017-2018, an increase from 13.8% in 2009-2010. Among women aged 15-44 year with private employer–sponsored insurance surveyed during 2008-2013, the results showed 15.4% of women filled a prescription for an antidepressant. We must look back further to find data on antianxiety medication use among women aged 18-44 years where use of antianxiety drugs (anxiolytics, sedatives, and hypnotics) was 4.3% between 2005 and 2008.
A lack of literature in this area is likely due to significant underreporting, and an inability to select patients who are sensitive to or at risk of developing neuropsychiatric symptoms resulting from hormonal contraception use because the true pathophysiology is unknown. Existing studies tend to use varying methods to assess mood changes, and do not usually specify hormonal contraceptive use type in their analyses (Schaffir J et al. Eur J Contracept Reprod Health Care. 2016 Oct;21[5]:347-55).
Studies of this nature also require large sample sizes, but the percentage of women who develop neuropsychiatric symptoms from hormonal contraceptive use has historically been relatively small. In the late 1990s, Rosenberg and colleagues found 46% of 1,657 women discontinued oral contraceptives due to side effects within 6 months of starting a new prescription; of these women, 5% reported mood changes as their reason for discontinuing oral contraceptives (Rosenberg M et al. Am J Obstet Gynecol. 1998 Sep;179[3 Pt 1]:577-82).
One might expect that, as lower dosage combined hormonal contraceptives were developed in the 1980s, that the rate of reporting psychological side effects would continue to decrease as well. Yet greater awareness of the potential for mood changes while on “the pill” as outlined by the lay press and social media may be leading to increased reporting of neuropsychiatric effects in women. In a recent cross-sectional survey of 188 women in New York, 43.6% said they experienced mood changes while on hormonal contraceptives, and 61.2% of women with histories of psychiatric illness reported mood changes they attributed to hormonal contraceptives (Martell S et al. Contracept Reprod Med. 2023;8:9).
Martell and colleagues found 48.3% of women cited side effects as a reason for discontinuing hormonal contraception, and 43 participants mentioned psychological side effects unprompted, including 2 patients with suicidal thoughts. The authors said this suggests “psychological side effects, at least in part, may have impacted” HC users’ decisions to switch from OCPs to an alternative method of contraception.
It is also not clear what risk factors exist for women who develop neuropsychiatric symptoms from hormonal contraceptive use. First, it is important to note that both progestin-only contraceptives and combined hormonal contraceptives are classified by the Centers for Disease Control and Prevention’s US Medical Eligibility Criteria for Contraceptive Use, 2016 as having no restrictions for use, including among patients with depression. While women in a smaller subgroup have significant neuropsychiatric symptoms related to their hormonal contraceptives, the underlying mechanism is unknown, and is thought to be largely related to the progestogen component of combined hormonal contraceptives or progestogen-only contraceptives (Mu E. Aust Prescr. 2022 Jun; 45[3]:75-9). We know that some women are hormone sensitive, while others are less so, and some not at all. Progestogens could affect mood as a direct action of the progestogen, because progestogens can be neurosteroids, or the progestogen effect could be mediated secondarily through a change in that woman’s own production of or bioavailability of androgens or naturally occurring estrogens (Giatti S. J Mol Endocrinol. 2016 Aug;57[2]:R109-26).
Here, we also find that currently available evidence limits our ability to draw firm conclusions. A study by Berry-Bibee and colleagues found a “low concern for clinically significant interactions” between hormonal contraception and psychotropic drugs, but was limited by quality/quantity of evidence (Berry-Bibee E et al. Contraception. 2016 Dec;94[6]:650-67). Interestingly, a study by Robinson and colleagues from the mid-2000s posited based on low evidence that “psychological response to the practice of contraception” was a potential explanation for the side effect profile of hormonal contraception (Robinson S et al. Med Hypotheses. 2004;63[2]:268-73).
Further, it may be that women with premenstrual dysphoric disorder (PMDD) might be selected for oral contraceptives, and they are predisposed to other neuropsychiatric problems. Estimates have placed the prevalence of comorbid psychiatric disorders such as anxiety, major depression, bipolar disorder, and posttraumatic stress disorder as high as 70% for women with PMDD (Sepede G et al. Neuropsychiatr Dis Treat. 2020;16:415-26). This phenomenon is not new, having been characterized in the lay literature nearly 20 years ago, by endocrinologist Geoffrey P. Redmond, MD (Redmond GP. The Hormonally Vulnerable Woman. New York: HarperCollins; 2005).
While the cause is not exactly idiosyncratic, They tend to have an entire spectrum of responses to the progestogens in combined or progestin-only contraceptives, ranging from just a flattened affect – which could easily be explained by their flattened level of endogenous hormones – to frank depression. Their frank depression, in turn, can be demonstrated to include suicidal ideation and actual suicide.
Compounding this issue is a woman’s perception of her sexuality. Some women with low sexual desire or sexual problems who are younger may have more distress about their problems compared with women of older reproductive age. While the reason for that is not clear, it may be that in the sexual arena, it is more important for some younger women to be a sexual person than in perimenopausal women, or that women who are younger are more likely to be partnered than women of older reproductive age. While the European Society of Sexual Medicine concluded in a 2019 position statement that there is inconclusive evidence whether hormonal contraception may be contributing to changes in sexual desire and sexual dysfunction, it appears that “a minority of women” experience “better or worse sexual functioning” from taking combined oral contraceptives (Both S et al. J Sex Med. 2019 Nov;16[11]:1681-95), suggesting that the majority of women report no significant changes.
Practitioners should discuss mood effects during consultation
An ob.gyn., primary care physicians, or others with prescriptive authority (i.e. nurse practitioners and physician assistants) in clinical practice may encounter a patient who seems to have mood side effects owing to progestogen-containing contraceptives that they prescribe. However, many ob.gyns. are likely unaware of the prevalence, or that some of those same patients can have such significant mood effects that they would become or are suicidal.
I believe questioning patients about mood effects during consultation and particularly during follow-up following the initiation of any hormonal contraceptive is worth a passing comment for every patient, which should include mood effects in broader discussion for anyone currently using an antidepressant, patients with a history of antidepressant use, and patients who have considered suicide. As we do with other drugs, these questions can be posed in the form of a questionnaire followed up by the practitioner in counseling.
Practitioners who encounter a patient with mood changes as a result of hormonal contraceptive use can consider changing to a nonhormonal method of birth control, or recommending the patient use a barrier method during sexual activity, as none of these options have neuropsychiatric side effects.
Ultimately, practitioners of all types need to engage in shared decision-making to identify the key benefits and risks of hormonal contraceptive use for each patient, which may involve trial and error to determine the ideal treatment. It is critical that practitioners of all types strike a balance between alleviating patient concerns about potential mood changes, monitoring patients with an appreciable risk of mood changes, and continuing patients on hormonal contraception for whom the benefits outweigh the risks.
Dr. Simon is a clinical professor at George Washington University and the medical director and founder of IntimMedicine Specialists in Washington, which provides patient-focused care for women across the reproductive life cycle. He is a past president of the International Society for the Study of Women’s Sexual Health and the North American Menopause Society. Dr. Simon has been a consultant to, received grant and research support from, and served on the speakers bureau for various pharmaceutical companies that develop combination hormonal contraceptives. Email Dr. Simon at obnews@mdedge.com.
Premenopausal women benefit from ovarian conservation with benign hysterectomies
Although bilateral salpingo-oophorectomy (BSO) with hysterectomy has been shown to reduce the risk for ovarian cancer in women at increased risk, current guidelines are touting ovarian conservation, especially in premenopausal women, wrote Mathilde Gottschau, MD, of the Danish Cancer Society Research Center, Copenhagen, and colleagues. However, post-hysterectomy outcomes in women with and without BSO have not been well examined.
In a study published in the Annals of Internal Medicine, the researchers reviewed data from a nationwide registry of women in Denmark aged 20 years and older who underwent benign hysterectomies with BSO (22,974 women) and without BSO (120,011 women) between 1977 and 2017. The women were divided into subgroups based on age; those younger than 45 years were defined as premenopausal, those aged 45-54 years were defined as perimenopausal, those aged 55-64 were defined as early postmenopausal, and those aged 65 and older were defined as late menopausal.
The primary outcomes were hospitalization for cardiovascular disease, cancer incidence, and all-cause mortality over a median follow-up period of 22 years.
For women younger than 45 years, the 10-year cumulative risk for all cancer was lower with BSO than without, but the risk of overall cardiovascular disease was higher with BSO, with higher levels of ischemic heart disease and stroke, compared with women without BSO. The 10-year cumulative mortality was higher with BSO than without (2.16% vs. 1.94%).
For women aged 45-54 years, the 10-year cumulative cancer risk was higher in those with BSO than those without BSO (risk difference, 0.73 percentage points) associated mainly with nonbreast cancer, and both 10-year and 20-year mortality were higher in those with BSO than those without.
For women aged 55-65 years, the 10-year cumulative cancer risk was higher in those with BSO than those without BSO. Cumulative overall mortality was higher at 10 years for those with BSO, but lower at 20 years.
For women aged 65 years and older, both 10-year and 20-year cumulative overall cancer risk was higher with BSO than without (RD, 2.54 and 4.57 percentage points, respectively). Cumulative mortality was higher in the BSO group at 10 years, but lower at 20 years.
The study findings were limited by several factors including the use of age to determine menopausal status and the lack of genetic predisposition data, and the focus only on a relatively homogeneous population that may not be generalizable to other populations, the researchers noted.
However, the results were strengthened by the use of a nationwide registry and the long-term follow-up period, they said. The current study indicates that the health risks outweigh the potential benefits of BSO with benign hysterectomy for premenopausal women and supports the current guidelines for ovarian conservation in these women with low lifetime ovarian cancer risk, they said. For postmenopausal women, the data support a cautious approach to BSO given the lack of a clear survival benefit and cancer excess, they concluded.
Delayed diagnosis of ovarian cancers favors BSO
“The question of removing ovaries at the time of benign hysterectomy to prevent ovarian cancer in low-risk women has been widely debated,” which has contributed to the variation in incidence rates of unilateral and bilateral oophorectomy over time, wrote Elizabeth Casiano Evans, MD, of the University of Texas, San Antonio, and Deslyn T.G. Hobson, MD, of Wayne State University, Detroit, in an accompanying editorial.
Ovarian cancer often goes undiagnosed until an advanced stage, and BSO can significantly reduce risk in women with BRCA1 and BRCA2 mutations, they noted.
For women without increased risk, those who are premenopausal may wish to preserve ovarian function, but women also may benefit from improvements in a range of menopause-related symptoms including vasomotor and urogenital symptoms, sexual dysfunction, and psychiatric and cognitive symptoms, they said.
“In addition, salpingectomy alone has a role in significantly reducing ovarian cancer incidence without compromising ovarian function because the fallopian tube has been found to be at the origin of many ovarian cancer cases,” they noted. In the current study, “the crude ovarian cancer risk was lower with BSO” across all age groups, the editorialists said.
The choice of whether to include BSO at the time of benign hysterectomy is complicated, with many factors to consider, the editorialists wrote, and the current study supports the need for informed, shared decision-making between clinicians and patients.
The study was supported by the Danish Cancer Society’s Scientific Committee and the Mermaid Project. The researchers had no financial conflicts to disclose. The editorial authors had no financial conflicts to disclose.
Although bilateral salpingo-oophorectomy (BSO) with hysterectomy has been shown to reduce the risk for ovarian cancer in women at increased risk, current guidelines are touting ovarian conservation, especially in premenopausal women, wrote Mathilde Gottschau, MD, of the Danish Cancer Society Research Center, Copenhagen, and colleagues. However, post-hysterectomy outcomes in women with and without BSO have not been well examined.
In a study published in the Annals of Internal Medicine, the researchers reviewed data from a nationwide registry of women in Denmark aged 20 years and older who underwent benign hysterectomies with BSO (22,974 women) and without BSO (120,011 women) between 1977 and 2017. The women were divided into subgroups based on age; those younger than 45 years were defined as premenopausal, those aged 45-54 years were defined as perimenopausal, those aged 55-64 were defined as early postmenopausal, and those aged 65 and older were defined as late menopausal.
The primary outcomes were hospitalization for cardiovascular disease, cancer incidence, and all-cause mortality over a median follow-up period of 22 years.
For women younger than 45 years, the 10-year cumulative risk for all cancer was lower with BSO than without, but the risk of overall cardiovascular disease was higher with BSO, with higher levels of ischemic heart disease and stroke, compared with women without BSO. The 10-year cumulative mortality was higher with BSO than without (2.16% vs. 1.94%).
For women aged 45-54 years, the 10-year cumulative cancer risk was higher in those with BSO than those without BSO (risk difference, 0.73 percentage points) associated mainly with nonbreast cancer, and both 10-year and 20-year mortality were higher in those with BSO than those without.
For women aged 55-65 years, the 10-year cumulative cancer risk was higher in those with BSO than those without BSO. Cumulative overall mortality was higher at 10 years for those with BSO, but lower at 20 years.
For women aged 65 years and older, both 10-year and 20-year cumulative overall cancer risk was higher with BSO than without (RD, 2.54 and 4.57 percentage points, respectively). Cumulative mortality was higher in the BSO group at 10 years, but lower at 20 years.
The study findings were limited by several factors including the use of age to determine menopausal status and the lack of genetic predisposition data, and the focus only on a relatively homogeneous population that may not be generalizable to other populations, the researchers noted.
However, the results were strengthened by the use of a nationwide registry and the long-term follow-up period, they said. The current study indicates that the health risks outweigh the potential benefits of BSO with benign hysterectomy for premenopausal women and supports the current guidelines for ovarian conservation in these women with low lifetime ovarian cancer risk, they said. For postmenopausal women, the data support a cautious approach to BSO given the lack of a clear survival benefit and cancer excess, they concluded.
Delayed diagnosis of ovarian cancers favors BSO
“The question of removing ovaries at the time of benign hysterectomy to prevent ovarian cancer in low-risk women has been widely debated,” which has contributed to the variation in incidence rates of unilateral and bilateral oophorectomy over time, wrote Elizabeth Casiano Evans, MD, of the University of Texas, San Antonio, and Deslyn T.G. Hobson, MD, of Wayne State University, Detroit, in an accompanying editorial.
Ovarian cancer often goes undiagnosed until an advanced stage, and BSO can significantly reduce risk in women with BRCA1 and BRCA2 mutations, they noted.
For women without increased risk, those who are premenopausal may wish to preserve ovarian function, but women also may benefit from improvements in a range of menopause-related symptoms including vasomotor and urogenital symptoms, sexual dysfunction, and psychiatric and cognitive symptoms, they said.
“In addition, salpingectomy alone has a role in significantly reducing ovarian cancer incidence without compromising ovarian function because the fallopian tube has been found to be at the origin of many ovarian cancer cases,” they noted. In the current study, “the crude ovarian cancer risk was lower with BSO” across all age groups, the editorialists said.
The choice of whether to include BSO at the time of benign hysterectomy is complicated, with many factors to consider, the editorialists wrote, and the current study supports the need for informed, shared decision-making between clinicians and patients.
The study was supported by the Danish Cancer Society’s Scientific Committee and the Mermaid Project. The researchers had no financial conflicts to disclose. The editorial authors had no financial conflicts to disclose.
Although bilateral salpingo-oophorectomy (BSO) with hysterectomy has been shown to reduce the risk for ovarian cancer in women at increased risk, current guidelines are touting ovarian conservation, especially in premenopausal women, wrote Mathilde Gottschau, MD, of the Danish Cancer Society Research Center, Copenhagen, and colleagues. However, post-hysterectomy outcomes in women with and without BSO have not been well examined.
In a study published in the Annals of Internal Medicine, the researchers reviewed data from a nationwide registry of women in Denmark aged 20 years and older who underwent benign hysterectomies with BSO (22,974 women) and without BSO (120,011 women) between 1977 and 2017. The women were divided into subgroups based on age; those younger than 45 years were defined as premenopausal, those aged 45-54 years were defined as perimenopausal, those aged 55-64 were defined as early postmenopausal, and those aged 65 and older were defined as late menopausal.
The primary outcomes were hospitalization for cardiovascular disease, cancer incidence, and all-cause mortality over a median follow-up period of 22 years.
For women younger than 45 years, the 10-year cumulative risk for all cancer was lower with BSO than without, but the risk of overall cardiovascular disease was higher with BSO, with higher levels of ischemic heart disease and stroke, compared with women without BSO. The 10-year cumulative mortality was higher with BSO than without (2.16% vs. 1.94%).
For women aged 45-54 years, the 10-year cumulative cancer risk was higher in those with BSO than those without BSO (risk difference, 0.73 percentage points) associated mainly with nonbreast cancer, and both 10-year and 20-year mortality were higher in those with BSO than those without.
For women aged 55-65 years, the 10-year cumulative cancer risk was higher in those with BSO than those without BSO. Cumulative overall mortality was higher at 10 years for those with BSO, but lower at 20 years.
For women aged 65 years and older, both 10-year and 20-year cumulative overall cancer risk was higher with BSO than without (RD, 2.54 and 4.57 percentage points, respectively). Cumulative mortality was higher in the BSO group at 10 years, but lower at 20 years.
The study findings were limited by several factors including the use of age to determine menopausal status and the lack of genetic predisposition data, and the focus only on a relatively homogeneous population that may not be generalizable to other populations, the researchers noted.
However, the results were strengthened by the use of a nationwide registry and the long-term follow-up period, they said. The current study indicates that the health risks outweigh the potential benefits of BSO with benign hysterectomy for premenopausal women and supports the current guidelines for ovarian conservation in these women with low lifetime ovarian cancer risk, they said. For postmenopausal women, the data support a cautious approach to BSO given the lack of a clear survival benefit and cancer excess, they concluded.
Delayed diagnosis of ovarian cancers favors BSO
“The question of removing ovaries at the time of benign hysterectomy to prevent ovarian cancer in low-risk women has been widely debated,” which has contributed to the variation in incidence rates of unilateral and bilateral oophorectomy over time, wrote Elizabeth Casiano Evans, MD, of the University of Texas, San Antonio, and Deslyn T.G. Hobson, MD, of Wayne State University, Detroit, in an accompanying editorial.
Ovarian cancer often goes undiagnosed until an advanced stage, and BSO can significantly reduce risk in women with BRCA1 and BRCA2 mutations, they noted.
For women without increased risk, those who are premenopausal may wish to preserve ovarian function, but women also may benefit from improvements in a range of menopause-related symptoms including vasomotor and urogenital symptoms, sexual dysfunction, and psychiatric and cognitive symptoms, they said.
“In addition, salpingectomy alone has a role in significantly reducing ovarian cancer incidence without compromising ovarian function because the fallopian tube has been found to be at the origin of many ovarian cancer cases,” they noted. In the current study, “the crude ovarian cancer risk was lower with BSO” across all age groups, the editorialists said.
The choice of whether to include BSO at the time of benign hysterectomy is complicated, with many factors to consider, the editorialists wrote, and the current study supports the need for informed, shared decision-making between clinicians and patients.
The study was supported by the Danish Cancer Society’s Scientific Committee and the Mermaid Project. The researchers had no financial conflicts to disclose. The editorial authors had no financial conflicts to disclose.
FROM THE ANNALS OF INTERNAL MEDICINE
More states nix nonconsensual pelvic exams by med students
Performing intimate exams under anesthesia (EUA) is a standard part of medical training. Yet,
“Whenever I talk about this at conferences around the country, people always come up to me and say it’s still happening at their institutions,” Lori Bruce, MA, MBE, HEC-C, associate director of the Interdisciplinary Center for Bioethics at Yale University, New Haven, Conn., told this news organization.
Most think this is a women’s issue, which occurs only in unconscious patients, she said. But Ms. Bruce found otherwise in a survey last year in which she polled the general public about their intimate exam experiences.
“Unconsented exams happen much more than we imagined, and they happen as often to men [having] prostate exams without consent as to women. Black [respondents] were nearly four times more likely to have reported receiving an unconsented intimate pelvic or prostate exam,” she said, based on her research. And Ms. Bruce believes it can happen across the economic spectrum.
Concern about unconsented EUAs arose in the early 2000s. In a study at that time, 75% of medical students reported that their patients had not given consent to be examined during surgical procedures. An ethics committee of the American College of Obstetricians and Gynecologists published guidelines for EUAs and states began passing legislation with patient protections and medical training consent policies.
California is believed to be the first to adopt legislation outlawing unconsented pelvic exams for training purposes in 2003, followed by Virginia in 2007, along with a handful of other states.
In 2019, on the heels of the #MeToo movement and renewed calls to end unconsented exams, more patients and providers began to speak publicly about their experiences with the practice. Some posted on social media using the #MeTooPelvic hashtag. In 2022, an award-winning documentary was also released about consent, “At Your Cervix.”More states subsequently passed legislation, and some medical schools strengthened their EUA consent policies.
Today, nearly half the states in the country have enacted laws against unconsented intimate EUAs, with some carrying misdemeanor charges for both the individual conducting the exam and the supervising physician. Other states leave open the option to fine the physician and revoke or suspend medical licenses.
Much of the new legislation requires explicit consent for intimate exams involving the pelvis, prostate, and rectum, with exceptions for emergency procedures and, in some cases, the collection of court-ordered forensic evidence. In addition, several states, including Colorado, Indiana, and Ohio, have pending or recently introduced bills. Last month, sister bills in Missouri passed the House and Senate, gaining more traction than previous legislative attempts. A similar bill was introduced in the Kansas House several times, including this year, and is expected to be on the agenda again in the next session.
Intimate exams on patients without consent are “unethical and unacceptable,” said Alison Whelan, MD, chief academic officer of the Association of American Medical Colleges. Although medical students learn sensitive procedures through simulation labs and gynecological teaching associates – individuals specifically trained to help students develop physical exam skills – EUAs require strict adherence to widely accepted guidelines.
“Learners in the clinical setting should only perform such examinations for teaching purposes when the exam is explicitly consented to, related to the planned procedure, performed by a student who is recognized by the patient as a part of their care team, and done under direct supervision by an educator,” Dr. Whelan said.
Medical students bear moral burden
Arthur Caplan, PhD, director of medical ethics at New York University, has called unconsented intimate exams a “cousin issue” to abusive predatory behavior.
If the public is outraged that physicians “have misused their authority with athletes, then we should be equally outraged if that authority, even for a higher purpose [like] teaching and training, is still misused in terms of getting permission and consent,” he said in a video discussing Connecticut’s legislation to strengthen intimate exam requirements, which went into effect Jan. 1.
Advocates of stricter EUA consent policies say the variability in consent practices destroys patient trust by ignoring the basic principles of respect and autonomy. Because patients are usually unaware a violation has occurred, reporting typically depends on medical students raising questions with educators and attendings, which they may hesitate to do for fear of repercussions.
Current practices, such as patients signing consent documents in the outpatient setting where students aren’t always privy to the discussion, contribute to the lack of transparency, Karampreet Kaur, MD, a 2nd-year ob.gyn. resident at the Hospital of the University of Pennsylvania, Philadelphia, said.
A 2019 survey of medical students by Elle magazine found that nearly half did not meet patients before conducting an intimate EUA. Of the 92% who performed a pelvic EUA, 61% reported doing so without obtaining explicit patient consent.
Dr. Kaur recently coauthored a survey of students from six medical schools and found that 84% completed at least one pelvic EUA during their ob.gyn. clerkships. About half of the students surveyed observed patients giving informed consent most or every time. Of those, 67% reported they never or rarely witnessed an explicit explanation that a medical student may perform a pelvic EUA.
This burden weighs on the consciences of medical students. Respondents reported that they wanted to honor patient autonomy but felt they lacked the authority to object to pelvic EUAs when consent was unclear, which led to significant emotional distress.
“It’s not that physicians don’t care,” Dr. Kaur said. “I think most want to make sure patients feel safe and fully informed of the care they are receiving.”
To consent or not
Incorporating a separate EUA consent form, typically signed during a preoperative visit but occasionally on the day of surgery, offers one potential solution as it ensures “clear and consistent language is used and forces documentation of this conversation,” said Dr. Kaur. At her current institution, providers and medical students must review charted EUA documentation, then that information is “made clear to attendings, fellows, residents, students, and even the OR staff,” she said.
In Dr. Kaur’s survey, 11% of respondents supported a separate consent. Another study of 3rd- and 4th-year medical students published last year found that 45% agreed with having a separate signature line on the surgical consent form.
Legislation introduced recently in Colorado states that medical students must meet the patient, and patients must receive a written or electronic document titled, in at least 18-point bolded font, “consent for examination of breasts, pelvic region, rectum, and/or prostate.” The form must also include the names of medical students performing or observing an intimate exam for educational purposes.
Elizabeth Newman, MPP, public policy director at the Colorado Coalition Against Sexual Assault and supporter of the state’s intimate exam bill, said the legislation will allow medical students to learn the intricacies of these sensitive body systems and provide better patient care, particularly following the rollback of Roe v. Wade.
“Abortion is available and accessible in Colorado, and we are surrounded by states where it’s not,” said Ms. Newman. “Medical students in states where it’s outright banned are coming to Colorado to learn how to provide abortion care in their residencies and fellowships, so we want to maintain that access and not take those learning opportunities away with this law.”
Opponents of a separate form say it complicates the consent process. Dr. Kaur said she originally thought it would involve a lot of extra work, but it only takes 3-5 minutes. Few patients decline the exam after the conversation, and students benefit from the clear guidelines and transparency, she said.
“I had hoped that the many medical association guidelines [supporting] explicit consent would have influenced hospital policy, but it did not have that effect,” said Ms. Bruce, adding that recent legislative efforts have largely been driven by concerned bioethicists, lawmakers, and some medical students and physicians. “It all circles back to the patient having the right to refuse; it’s their body.”
A version of this article first appeared on Medscape.com.
Performing intimate exams under anesthesia (EUA) is a standard part of medical training. Yet,
“Whenever I talk about this at conferences around the country, people always come up to me and say it’s still happening at their institutions,” Lori Bruce, MA, MBE, HEC-C, associate director of the Interdisciplinary Center for Bioethics at Yale University, New Haven, Conn., told this news organization.
Most think this is a women’s issue, which occurs only in unconscious patients, she said. But Ms. Bruce found otherwise in a survey last year in which she polled the general public about their intimate exam experiences.
“Unconsented exams happen much more than we imagined, and they happen as often to men [having] prostate exams without consent as to women. Black [respondents] were nearly four times more likely to have reported receiving an unconsented intimate pelvic or prostate exam,” she said, based on her research. And Ms. Bruce believes it can happen across the economic spectrum.
Concern about unconsented EUAs arose in the early 2000s. In a study at that time, 75% of medical students reported that their patients had not given consent to be examined during surgical procedures. An ethics committee of the American College of Obstetricians and Gynecologists published guidelines for EUAs and states began passing legislation with patient protections and medical training consent policies.
California is believed to be the first to adopt legislation outlawing unconsented pelvic exams for training purposes in 2003, followed by Virginia in 2007, along with a handful of other states.
In 2019, on the heels of the #MeToo movement and renewed calls to end unconsented exams, more patients and providers began to speak publicly about their experiences with the practice. Some posted on social media using the #MeTooPelvic hashtag. In 2022, an award-winning documentary was also released about consent, “At Your Cervix.”More states subsequently passed legislation, and some medical schools strengthened their EUA consent policies.
Today, nearly half the states in the country have enacted laws against unconsented intimate EUAs, with some carrying misdemeanor charges for both the individual conducting the exam and the supervising physician. Other states leave open the option to fine the physician and revoke or suspend medical licenses.
Much of the new legislation requires explicit consent for intimate exams involving the pelvis, prostate, and rectum, with exceptions for emergency procedures and, in some cases, the collection of court-ordered forensic evidence. In addition, several states, including Colorado, Indiana, and Ohio, have pending or recently introduced bills. Last month, sister bills in Missouri passed the House and Senate, gaining more traction than previous legislative attempts. A similar bill was introduced in the Kansas House several times, including this year, and is expected to be on the agenda again in the next session.
Intimate exams on patients without consent are “unethical and unacceptable,” said Alison Whelan, MD, chief academic officer of the Association of American Medical Colleges. Although medical students learn sensitive procedures through simulation labs and gynecological teaching associates – individuals specifically trained to help students develop physical exam skills – EUAs require strict adherence to widely accepted guidelines.
“Learners in the clinical setting should only perform such examinations for teaching purposes when the exam is explicitly consented to, related to the planned procedure, performed by a student who is recognized by the patient as a part of their care team, and done under direct supervision by an educator,” Dr. Whelan said.
Medical students bear moral burden
Arthur Caplan, PhD, director of medical ethics at New York University, has called unconsented intimate exams a “cousin issue” to abusive predatory behavior.
If the public is outraged that physicians “have misused their authority with athletes, then we should be equally outraged if that authority, even for a higher purpose [like] teaching and training, is still misused in terms of getting permission and consent,” he said in a video discussing Connecticut’s legislation to strengthen intimate exam requirements, which went into effect Jan. 1.
Advocates of stricter EUA consent policies say the variability in consent practices destroys patient trust by ignoring the basic principles of respect and autonomy. Because patients are usually unaware a violation has occurred, reporting typically depends on medical students raising questions with educators and attendings, which they may hesitate to do for fear of repercussions.
Current practices, such as patients signing consent documents in the outpatient setting where students aren’t always privy to the discussion, contribute to the lack of transparency, Karampreet Kaur, MD, a 2nd-year ob.gyn. resident at the Hospital of the University of Pennsylvania, Philadelphia, said.
A 2019 survey of medical students by Elle magazine found that nearly half did not meet patients before conducting an intimate EUA. Of the 92% who performed a pelvic EUA, 61% reported doing so without obtaining explicit patient consent.
Dr. Kaur recently coauthored a survey of students from six medical schools and found that 84% completed at least one pelvic EUA during their ob.gyn. clerkships. About half of the students surveyed observed patients giving informed consent most or every time. Of those, 67% reported they never or rarely witnessed an explicit explanation that a medical student may perform a pelvic EUA.
This burden weighs on the consciences of medical students. Respondents reported that they wanted to honor patient autonomy but felt they lacked the authority to object to pelvic EUAs when consent was unclear, which led to significant emotional distress.
“It’s not that physicians don’t care,” Dr. Kaur said. “I think most want to make sure patients feel safe and fully informed of the care they are receiving.”
To consent or not
Incorporating a separate EUA consent form, typically signed during a preoperative visit but occasionally on the day of surgery, offers one potential solution as it ensures “clear and consistent language is used and forces documentation of this conversation,” said Dr. Kaur. At her current institution, providers and medical students must review charted EUA documentation, then that information is “made clear to attendings, fellows, residents, students, and even the OR staff,” she said.
In Dr. Kaur’s survey, 11% of respondents supported a separate consent. Another study of 3rd- and 4th-year medical students published last year found that 45% agreed with having a separate signature line on the surgical consent form.
Legislation introduced recently in Colorado states that medical students must meet the patient, and patients must receive a written or electronic document titled, in at least 18-point bolded font, “consent for examination of breasts, pelvic region, rectum, and/or prostate.” The form must also include the names of medical students performing or observing an intimate exam for educational purposes.
Elizabeth Newman, MPP, public policy director at the Colorado Coalition Against Sexual Assault and supporter of the state’s intimate exam bill, said the legislation will allow medical students to learn the intricacies of these sensitive body systems and provide better patient care, particularly following the rollback of Roe v. Wade.
“Abortion is available and accessible in Colorado, and we are surrounded by states where it’s not,” said Ms. Newman. “Medical students in states where it’s outright banned are coming to Colorado to learn how to provide abortion care in their residencies and fellowships, so we want to maintain that access and not take those learning opportunities away with this law.”
Opponents of a separate form say it complicates the consent process. Dr. Kaur said she originally thought it would involve a lot of extra work, but it only takes 3-5 minutes. Few patients decline the exam after the conversation, and students benefit from the clear guidelines and transparency, she said.
“I had hoped that the many medical association guidelines [supporting] explicit consent would have influenced hospital policy, but it did not have that effect,” said Ms. Bruce, adding that recent legislative efforts have largely been driven by concerned bioethicists, lawmakers, and some medical students and physicians. “It all circles back to the patient having the right to refuse; it’s their body.”
A version of this article first appeared on Medscape.com.
Performing intimate exams under anesthesia (EUA) is a standard part of medical training. Yet,
“Whenever I talk about this at conferences around the country, people always come up to me and say it’s still happening at their institutions,” Lori Bruce, MA, MBE, HEC-C, associate director of the Interdisciplinary Center for Bioethics at Yale University, New Haven, Conn., told this news organization.
Most think this is a women’s issue, which occurs only in unconscious patients, she said. But Ms. Bruce found otherwise in a survey last year in which she polled the general public about their intimate exam experiences.
“Unconsented exams happen much more than we imagined, and they happen as often to men [having] prostate exams without consent as to women. Black [respondents] were nearly four times more likely to have reported receiving an unconsented intimate pelvic or prostate exam,” she said, based on her research. And Ms. Bruce believes it can happen across the economic spectrum.
Concern about unconsented EUAs arose in the early 2000s. In a study at that time, 75% of medical students reported that their patients had not given consent to be examined during surgical procedures. An ethics committee of the American College of Obstetricians and Gynecologists published guidelines for EUAs and states began passing legislation with patient protections and medical training consent policies.
California is believed to be the first to adopt legislation outlawing unconsented pelvic exams for training purposes in 2003, followed by Virginia in 2007, along with a handful of other states.
In 2019, on the heels of the #MeToo movement and renewed calls to end unconsented exams, more patients and providers began to speak publicly about their experiences with the practice. Some posted on social media using the #MeTooPelvic hashtag. In 2022, an award-winning documentary was also released about consent, “At Your Cervix.”More states subsequently passed legislation, and some medical schools strengthened their EUA consent policies.
Today, nearly half the states in the country have enacted laws against unconsented intimate EUAs, with some carrying misdemeanor charges for both the individual conducting the exam and the supervising physician. Other states leave open the option to fine the physician and revoke or suspend medical licenses.
Much of the new legislation requires explicit consent for intimate exams involving the pelvis, prostate, and rectum, with exceptions for emergency procedures and, in some cases, the collection of court-ordered forensic evidence. In addition, several states, including Colorado, Indiana, and Ohio, have pending or recently introduced bills. Last month, sister bills in Missouri passed the House and Senate, gaining more traction than previous legislative attempts. A similar bill was introduced in the Kansas House several times, including this year, and is expected to be on the agenda again in the next session.
Intimate exams on patients without consent are “unethical and unacceptable,” said Alison Whelan, MD, chief academic officer of the Association of American Medical Colleges. Although medical students learn sensitive procedures through simulation labs and gynecological teaching associates – individuals specifically trained to help students develop physical exam skills – EUAs require strict adherence to widely accepted guidelines.
“Learners in the clinical setting should only perform such examinations for teaching purposes when the exam is explicitly consented to, related to the planned procedure, performed by a student who is recognized by the patient as a part of their care team, and done under direct supervision by an educator,” Dr. Whelan said.
Medical students bear moral burden
Arthur Caplan, PhD, director of medical ethics at New York University, has called unconsented intimate exams a “cousin issue” to abusive predatory behavior.
If the public is outraged that physicians “have misused their authority with athletes, then we should be equally outraged if that authority, even for a higher purpose [like] teaching and training, is still misused in terms of getting permission and consent,” he said in a video discussing Connecticut’s legislation to strengthen intimate exam requirements, which went into effect Jan. 1.
Advocates of stricter EUA consent policies say the variability in consent practices destroys patient trust by ignoring the basic principles of respect and autonomy. Because patients are usually unaware a violation has occurred, reporting typically depends on medical students raising questions with educators and attendings, which they may hesitate to do for fear of repercussions.
Current practices, such as patients signing consent documents in the outpatient setting where students aren’t always privy to the discussion, contribute to the lack of transparency, Karampreet Kaur, MD, a 2nd-year ob.gyn. resident at the Hospital of the University of Pennsylvania, Philadelphia, said.
A 2019 survey of medical students by Elle magazine found that nearly half did not meet patients before conducting an intimate EUA. Of the 92% who performed a pelvic EUA, 61% reported doing so without obtaining explicit patient consent.
Dr. Kaur recently coauthored a survey of students from six medical schools and found that 84% completed at least one pelvic EUA during their ob.gyn. clerkships. About half of the students surveyed observed patients giving informed consent most or every time. Of those, 67% reported they never or rarely witnessed an explicit explanation that a medical student may perform a pelvic EUA.
This burden weighs on the consciences of medical students. Respondents reported that they wanted to honor patient autonomy but felt they lacked the authority to object to pelvic EUAs when consent was unclear, which led to significant emotional distress.
“It’s not that physicians don’t care,” Dr. Kaur said. “I think most want to make sure patients feel safe and fully informed of the care they are receiving.”
To consent or not
Incorporating a separate EUA consent form, typically signed during a preoperative visit but occasionally on the day of surgery, offers one potential solution as it ensures “clear and consistent language is used and forces documentation of this conversation,” said Dr. Kaur. At her current institution, providers and medical students must review charted EUA documentation, then that information is “made clear to attendings, fellows, residents, students, and even the OR staff,” she said.
In Dr. Kaur’s survey, 11% of respondents supported a separate consent. Another study of 3rd- and 4th-year medical students published last year found that 45% agreed with having a separate signature line on the surgical consent form.
Legislation introduced recently in Colorado states that medical students must meet the patient, and patients must receive a written or electronic document titled, in at least 18-point bolded font, “consent for examination of breasts, pelvic region, rectum, and/or prostate.” The form must also include the names of medical students performing or observing an intimate exam for educational purposes.
Elizabeth Newman, MPP, public policy director at the Colorado Coalition Against Sexual Assault and supporter of the state’s intimate exam bill, said the legislation will allow medical students to learn the intricacies of these sensitive body systems and provide better patient care, particularly following the rollback of Roe v. Wade.
“Abortion is available and accessible in Colorado, and we are surrounded by states where it’s not,” said Ms. Newman. “Medical students in states where it’s outright banned are coming to Colorado to learn how to provide abortion care in their residencies and fellowships, so we want to maintain that access and not take those learning opportunities away with this law.”
Opponents of a separate form say it complicates the consent process. Dr. Kaur said she originally thought it would involve a lot of extra work, but it only takes 3-5 minutes. Few patients decline the exam after the conversation, and students benefit from the clear guidelines and transparency, she said.
“I had hoped that the many medical association guidelines [supporting] explicit consent would have influenced hospital policy, but it did not have that effect,” said Ms. Bruce, adding that recent legislative efforts have largely been driven by concerned bioethicists, lawmakers, and some medical students and physicians. “It all circles back to the patient having the right to refuse; it’s their body.”
A version of this article first appeared on Medscape.com.
Vaginal microbiome does not affect infant gut microbiome
The findings suggest that practices such as vaginal seeding are ineffective.
A longitudinal, prospective cohort study of more than 600 pregnant Canadian women and their newborns showed significant differences in an infant’s stool composition by delivery mode at 10 days post partum, but the differences could not be explained by the mother’s vaginal microbiome, and they effectively disappeared by 3 months.
The findings were surprising, Scott Dos Santos, a PhD candidate at the University of Saskatchewan in Saskatoon, told this news organization. “The bacteria living in the maternal vagina are the first microbes that vaginally delivered infants are exposed to. … so it sounds intuitive that different kinds of vaginal microbiomes could end up influencing the development of a baby’s gut microbiome in different ways. But the maternal vaginal microbiome didn’t seem to have any role in predicting what the infant stool microbiome looked like.”
Therefore, women should not be concerned about cesarean delivery having an adverse effect on their baby’s gut microbiome, said Mr. Dos Santos. Moreover, “vaginal seeding is not safe or advised. Professional bodies, including the Society of Obstetricians and Gynecologists of Canada and the American College of Obstetricians and Gynecologists, strongly advise against this practice.”
The study was published online in Frontiers in Cellular and Infection Microbiology.
Independent communities
The investigators analyzed vaginal and stool microbiome profiles from 442 mother-infant dyads. The mothers were healthy, low-risk women who delivered at term. They were recruited into the Maternal Microbiome LEGACY Project from three hospitals in British Columbia.
The mean age of the mothers at delivery was 34.6 years, which is typical of the study hospitals’ delivery populations. Participants identified themselves as White (54.7%), Asian (21.2%), South Asian (8.3%), and of other ethnicities.
A nurse, midwife, or clinician collected maternal vaginal swabs of the posterior fornix and lateral vaginal wall at first presentation to the labor and delivery area. Neonatal meconium, which was defined as the first stool specimen collected within 72 hours of birth, and two infant stool samples were collected at follow-up visits at 10 days and 3 months post partum.
A principal component analysis of infant stool microbiomes showed no significant clustering of microbiome profiles at 10 days or 3 months by maternal community state types (that is, microbial species).
Correspondence analyses also showed no coclustering of maternal and infant clusters at either time. In addition, there were no differences in the distribution of maternal vaginal microbiome clusters among infant stool microbiome clusters, regardless of delivery mode.
Vaginal microbiome clusters were distributed across infant stool clusters in proportion to their frequency in the overall maternal population, indicating that the two communities were independent of each other.
Intrapartum antibiotic administration was identified as a confounder of infant stool microbiome differences and was associated with lower abundances of Escherichia coli, Bacteroides vulgatus, Bifidobacterium longum, and Parabacteroides distasonis.
“Our findings demonstrate that maternal vaginal microbiome composition at delivery does not affect infant stool microbiome composition and development, suggesting that practices to amend infant stool microbiome composition focus on factors other than maternal vaginal microbes,” the authors conclude.
More evidence needed
Commenting on the study, Emily H. Adhikari, MD, assistant professor of obstetrics and gynecology at UT Southwestern Medical Center in Dallas, and medical director of perinatal infectious diseases for the Parkland Health and Hospital System, said, “These findings contribute significantly more data to an understudied area of research into factors that affect the infant gut microbiome from the earliest hours of life. Prior studies have been small and often conflicting, and the authors reference recent larger studies, which corroborate their findings.”
The data regarding whether delivery mode or antibiotic-associated differences in infant microbiomes persist remain controversial, said Dr. Adhikari. “More evidence is needed involving a more ethnically diverse sampling of patients.” In addition, prospectively evaluating vaginal seeding in a rigorously designed clinical trial setting is “imperative to understand any potential benefit and certainly to understand the potential harms of the practice. To date, this does not exist.”
The study was funded by a Canadian Institutes of Health Research grant. Mr. Dos Santos and Dr. Adhikari have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
The findings suggest that practices such as vaginal seeding are ineffective.
A longitudinal, prospective cohort study of more than 600 pregnant Canadian women and their newborns showed significant differences in an infant’s stool composition by delivery mode at 10 days post partum, but the differences could not be explained by the mother’s vaginal microbiome, and they effectively disappeared by 3 months.
The findings were surprising, Scott Dos Santos, a PhD candidate at the University of Saskatchewan in Saskatoon, told this news organization. “The bacteria living in the maternal vagina are the first microbes that vaginally delivered infants are exposed to. … so it sounds intuitive that different kinds of vaginal microbiomes could end up influencing the development of a baby’s gut microbiome in different ways. But the maternal vaginal microbiome didn’t seem to have any role in predicting what the infant stool microbiome looked like.”
Therefore, women should not be concerned about cesarean delivery having an adverse effect on their baby’s gut microbiome, said Mr. Dos Santos. Moreover, “vaginal seeding is not safe or advised. Professional bodies, including the Society of Obstetricians and Gynecologists of Canada and the American College of Obstetricians and Gynecologists, strongly advise against this practice.”
The study was published online in Frontiers in Cellular and Infection Microbiology.
Independent communities
The investigators analyzed vaginal and stool microbiome profiles from 442 mother-infant dyads. The mothers were healthy, low-risk women who delivered at term. They were recruited into the Maternal Microbiome LEGACY Project from three hospitals in British Columbia.
The mean age of the mothers at delivery was 34.6 years, which is typical of the study hospitals’ delivery populations. Participants identified themselves as White (54.7%), Asian (21.2%), South Asian (8.3%), and of other ethnicities.
A nurse, midwife, or clinician collected maternal vaginal swabs of the posterior fornix and lateral vaginal wall at first presentation to the labor and delivery area. Neonatal meconium, which was defined as the first stool specimen collected within 72 hours of birth, and two infant stool samples were collected at follow-up visits at 10 days and 3 months post partum.
A principal component analysis of infant stool microbiomes showed no significant clustering of microbiome profiles at 10 days or 3 months by maternal community state types (that is, microbial species).
Correspondence analyses also showed no coclustering of maternal and infant clusters at either time. In addition, there were no differences in the distribution of maternal vaginal microbiome clusters among infant stool microbiome clusters, regardless of delivery mode.
Vaginal microbiome clusters were distributed across infant stool clusters in proportion to their frequency in the overall maternal population, indicating that the two communities were independent of each other.
Intrapartum antibiotic administration was identified as a confounder of infant stool microbiome differences and was associated with lower abundances of Escherichia coli, Bacteroides vulgatus, Bifidobacterium longum, and Parabacteroides distasonis.
“Our findings demonstrate that maternal vaginal microbiome composition at delivery does not affect infant stool microbiome composition and development, suggesting that practices to amend infant stool microbiome composition focus on factors other than maternal vaginal microbes,” the authors conclude.
More evidence needed
Commenting on the study, Emily H. Adhikari, MD, assistant professor of obstetrics and gynecology at UT Southwestern Medical Center in Dallas, and medical director of perinatal infectious diseases for the Parkland Health and Hospital System, said, “These findings contribute significantly more data to an understudied area of research into factors that affect the infant gut microbiome from the earliest hours of life. Prior studies have been small and often conflicting, and the authors reference recent larger studies, which corroborate their findings.”
The data regarding whether delivery mode or antibiotic-associated differences in infant microbiomes persist remain controversial, said Dr. Adhikari. “More evidence is needed involving a more ethnically diverse sampling of patients.” In addition, prospectively evaluating vaginal seeding in a rigorously designed clinical trial setting is “imperative to understand any potential benefit and certainly to understand the potential harms of the practice. To date, this does not exist.”
The study was funded by a Canadian Institutes of Health Research grant. Mr. Dos Santos and Dr. Adhikari have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
The findings suggest that practices such as vaginal seeding are ineffective.
A longitudinal, prospective cohort study of more than 600 pregnant Canadian women and their newborns showed significant differences in an infant’s stool composition by delivery mode at 10 days post partum, but the differences could not be explained by the mother’s vaginal microbiome, and they effectively disappeared by 3 months.
The findings were surprising, Scott Dos Santos, a PhD candidate at the University of Saskatchewan in Saskatoon, told this news organization. “The bacteria living in the maternal vagina are the first microbes that vaginally delivered infants are exposed to. … so it sounds intuitive that different kinds of vaginal microbiomes could end up influencing the development of a baby’s gut microbiome in different ways. But the maternal vaginal microbiome didn’t seem to have any role in predicting what the infant stool microbiome looked like.”
Therefore, women should not be concerned about cesarean delivery having an adverse effect on their baby’s gut microbiome, said Mr. Dos Santos. Moreover, “vaginal seeding is not safe or advised. Professional bodies, including the Society of Obstetricians and Gynecologists of Canada and the American College of Obstetricians and Gynecologists, strongly advise against this practice.”
The study was published online in Frontiers in Cellular and Infection Microbiology.
Independent communities
The investigators analyzed vaginal and stool microbiome profiles from 442 mother-infant dyads. The mothers were healthy, low-risk women who delivered at term. They were recruited into the Maternal Microbiome LEGACY Project from three hospitals in British Columbia.
The mean age of the mothers at delivery was 34.6 years, which is typical of the study hospitals’ delivery populations. Participants identified themselves as White (54.7%), Asian (21.2%), South Asian (8.3%), and of other ethnicities.
A nurse, midwife, or clinician collected maternal vaginal swabs of the posterior fornix and lateral vaginal wall at first presentation to the labor and delivery area. Neonatal meconium, which was defined as the first stool specimen collected within 72 hours of birth, and two infant stool samples were collected at follow-up visits at 10 days and 3 months post partum.
A principal component analysis of infant stool microbiomes showed no significant clustering of microbiome profiles at 10 days or 3 months by maternal community state types (that is, microbial species).
Correspondence analyses also showed no coclustering of maternal and infant clusters at either time. In addition, there were no differences in the distribution of maternal vaginal microbiome clusters among infant stool microbiome clusters, regardless of delivery mode.
Vaginal microbiome clusters were distributed across infant stool clusters in proportion to their frequency in the overall maternal population, indicating that the two communities were independent of each other.
Intrapartum antibiotic administration was identified as a confounder of infant stool microbiome differences and was associated with lower abundances of Escherichia coli, Bacteroides vulgatus, Bifidobacterium longum, and Parabacteroides distasonis.
“Our findings demonstrate that maternal vaginal microbiome composition at delivery does not affect infant stool microbiome composition and development, suggesting that practices to amend infant stool microbiome composition focus on factors other than maternal vaginal microbes,” the authors conclude.
More evidence needed
Commenting on the study, Emily H. Adhikari, MD, assistant professor of obstetrics and gynecology at UT Southwestern Medical Center in Dallas, and medical director of perinatal infectious diseases for the Parkland Health and Hospital System, said, “These findings contribute significantly more data to an understudied area of research into factors that affect the infant gut microbiome from the earliest hours of life. Prior studies have been small and often conflicting, and the authors reference recent larger studies, which corroborate their findings.”
The data regarding whether delivery mode or antibiotic-associated differences in infant microbiomes persist remain controversial, said Dr. Adhikari. “More evidence is needed involving a more ethnically diverse sampling of patients.” In addition, prospectively evaluating vaginal seeding in a rigorously designed clinical trial setting is “imperative to understand any potential benefit and certainly to understand the potential harms of the practice. To date, this does not exist.”
The study was funded by a Canadian Institutes of Health Research grant. Mr. Dos Santos and Dr. Adhikari have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
FROM FRONTIERS IN CELLULAR AND INFECTION MICROBIOLOGY
IUD-released levonorgestrel eases heavy menstrual periods
Median blood loss decreased by more than 90% in the first three cycles. Overall, treatment was successful in 81.8% of 99 patients (95% confidence interval, 74.2%-89.4%), according to findings published in Obstetrics & Gynecology.
Already approved for contraception, the IUD (Liletta) had substantial benefits for quality of life in measures such as sleep, pain/cramping, and daily functioning, wrote a group led by Mitchell D. Creinin, MD, a professor in the department of obstetrics and gynecology at University of California, Davis.
“This study provides evidence of high efficacy, as expected, for the Liletta levonorgestrel 52 mg IUD for heavy menstrual bleeding treatment,” Dr. Creinin said in an interview.
Racially diverse cohort
Conducted at 29 U.S. sites prior to seeking FDA registration for this new use, the phase 3 open-label trial of the 52 mg progestin-releasing IUD enrolled 105 participants with a mean age of 35.4 years. Unlike previous trials, this one included obese or severely obese women (44.8%), with 42 participants having a body mass index (BMI) of more than 35 kg/m2, and also 28 nulliparous women (27.6%).
Those with abnormalities such as fibroids or coagulopathies were excluded. Although most of the cohort was White (n = 68), the study included Black (n = 25), Asian (n = 4), and Hispanic (n = 10) women, plus 7 from other minorities, suggesting the results would be widely applicable.
Mean baseline blood loss in the cohort ranged from 73 mL to 520 mL (median, 143 mL). Of 89 treated women with follow-up, participants had a median absolute blood-loss decreases of 93.3% (86.1%-97.8%) at cycle three and 97.6% (90.4%-100%) at cycle six. Median bleeding reductions at cycle six were similar between women with and without obesity at 97.6% and 97.5%, respectively, and between nulliparous and parous women at 97.0% and 98.1%, respectively (P = .43). The study, however, was not sufficiently powered to fully analyze these subgroups, the authors acknowledged.
Although results were overall comparable with those of a previous study on a different IUD, the expulsion rate was somewhat higher, at 9%, than the 6% reported in the earlier study.
“Although this strategy for reducing blood loss is not new, this study is notable because it looked at high-BMI women and nulliparous women,” said Kathryn J. Gray, MD, PhD, an attending physician in the department of obstetrics and gynecology at Brigham and Women’s Hospital in Boston, who was not involved in the research.“No prior trials have included patients with BMIs exceeding 35 kg/m2 or nulliparous patients, while this study enrolled a full array of patients, which allowed exploratory analyses of these subpopulations,” Dr. Creinin confirmed.
According to Dr. Gray, the IUD approach has advantages over systemic treatment with oral medication. “First, treatment is not user-dependent so the user doesn’t have to remember to take it. In addition, because the medication is locally targeted in the uterus, it is more effective and there is less fluctuation and variability in drug levels than when taken orally.”
As to treatment durability, Dr. Creinin said, “Long-term studies in a population being treated for heavy menstrual bleeding would be helpful to have an idea of how long this effect lasts. Still, there is no reason to expect that the effect will not last for many years.”
And with this treatment, he added, both patient and clinician can readily detect its effect. “If bleeding begins to increase, they will know!”
Would there be a lingering residual effect even after removal of the IUD? “That is an excellent question that remains to be answered,” Dr. Creinin said. “There are no data on when the heavy bleeding returns, but it would be expected to do so.”
This study was funded, designed, and supervised by Medicines360, which also provided the study treatment. Dr. Creinin disclosed financial relationships with various private-sector companies, including Medicines360, Organon, Fuji Pharma, GlaxoSmithKline, and Merck & Co. Multiple study coauthors disclosed similar financial ties to industry partners, including Medicines360. Dr. Gray had no potential conflicts of interest with regard to her comments.
Median blood loss decreased by more than 90% in the first three cycles. Overall, treatment was successful in 81.8% of 99 patients (95% confidence interval, 74.2%-89.4%), according to findings published in Obstetrics & Gynecology.
Already approved for contraception, the IUD (Liletta) had substantial benefits for quality of life in measures such as sleep, pain/cramping, and daily functioning, wrote a group led by Mitchell D. Creinin, MD, a professor in the department of obstetrics and gynecology at University of California, Davis.
“This study provides evidence of high efficacy, as expected, for the Liletta levonorgestrel 52 mg IUD for heavy menstrual bleeding treatment,” Dr. Creinin said in an interview.
Racially diverse cohort
Conducted at 29 U.S. sites prior to seeking FDA registration for this new use, the phase 3 open-label trial of the 52 mg progestin-releasing IUD enrolled 105 participants with a mean age of 35.4 years. Unlike previous trials, this one included obese or severely obese women (44.8%), with 42 participants having a body mass index (BMI) of more than 35 kg/m2, and also 28 nulliparous women (27.6%).
Those with abnormalities such as fibroids or coagulopathies were excluded. Although most of the cohort was White (n = 68), the study included Black (n = 25), Asian (n = 4), and Hispanic (n = 10) women, plus 7 from other minorities, suggesting the results would be widely applicable.
Mean baseline blood loss in the cohort ranged from 73 mL to 520 mL (median, 143 mL). Of 89 treated women with follow-up, participants had a median absolute blood-loss decreases of 93.3% (86.1%-97.8%) at cycle three and 97.6% (90.4%-100%) at cycle six. Median bleeding reductions at cycle six were similar between women with and without obesity at 97.6% and 97.5%, respectively, and between nulliparous and parous women at 97.0% and 98.1%, respectively (P = .43). The study, however, was not sufficiently powered to fully analyze these subgroups, the authors acknowledged.
Although results were overall comparable with those of a previous study on a different IUD, the expulsion rate was somewhat higher, at 9%, than the 6% reported in the earlier study.
“Although this strategy for reducing blood loss is not new, this study is notable because it looked at high-BMI women and nulliparous women,” said Kathryn J. Gray, MD, PhD, an attending physician in the department of obstetrics and gynecology at Brigham and Women’s Hospital in Boston, who was not involved in the research.“No prior trials have included patients with BMIs exceeding 35 kg/m2 or nulliparous patients, while this study enrolled a full array of patients, which allowed exploratory analyses of these subpopulations,” Dr. Creinin confirmed.
According to Dr. Gray, the IUD approach has advantages over systemic treatment with oral medication. “First, treatment is not user-dependent so the user doesn’t have to remember to take it. In addition, because the medication is locally targeted in the uterus, it is more effective and there is less fluctuation and variability in drug levels than when taken orally.”
As to treatment durability, Dr. Creinin said, “Long-term studies in a population being treated for heavy menstrual bleeding would be helpful to have an idea of how long this effect lasts. Still, there is no reason to expect that the effect will not last for many years.”
And with this treatment, he added, both patient and clinician can readily detect its effect. “If bleeding begins to increase, they will know!”
Would there be a lingering residual effect even after removal of the IUD? “That is an excellent question that remains to be answered,” Dr. Creinin said. “There are no data on when the heavy bleeding returns, but it would be expected to do so.”
This study was funded, designed, and supervised by Medicines360, which also provided the study treatment. Dr. Creinin disclosed financial relationships with various private-sector companies, including Medicines360, Organon, Fuji Pharma, GlaxoSmithKline, and Merck & Co. Multiple study coauthors disclosed similar financial ties to industry partners, including Medicines360. Dr. Gray had no potential conflicts of interest with regard to her comments.
Median blood loss decreased by more than 90% in the first three cycles. Overall, treatment was successful in 81.8% of 99 patients (95% confidence interval, 74.2%-89.4%), according to findings published in Obstetrics & Gynecology.
Already approved for contraception, the IUD (Liletta) had substantial benefits for quality of life in measures such as sleep, pain/cramping, and daily functioning, wrote a group led by Mitchell D. Creinin, MD, a professor in the department of obstetrics and gynecology at University of California, Davis.
“This study provides evidence of high efficacy, as expected, for the Liletta levonorgestrel 52 mg IUD for heavy menstrual bleeding treatment,” Dr. Creinin said in an interview.
Racially diverse cohort
Conducted at 29 U.S. sites prior to seeking FDA registration for this new use, the phase 3 open-label trial of the 52 mg progestin-releasing IUD enrolled 105 participants with a mean age of 35.4 years. Unlike previous trials, this one included obese or severely obese women (44.8%), with 42 participants having a body mass index (BMI) of more than 35 kg/m2, and also 28 nulliparous women (27.6%).
Those with abnormalities such as fibroids or coagulopathies were excluded. Although most of the cohort was White (n = 68), the study included Black (n = 25), Asian (n = 4), and Hispanic (n = 10) women, plus 7 from other minorities, suggesting the results would be widely applicable.
Mean baseline blood loss in the cohort ranged from 73 mL to 520 mL (median, 143 mL). Of 89 treated women with follow-up, participants had a median absolute blood-loss decreases of 93.3% (86.1%-97.8%) at cycle three and 97.6% (90.4%-100%) at cycle six. Median bleeding reductions at cycle six were similar between women with and without obesity at 97.6% and 97.5%, respectively, and between nulliparous and parous women at 97.0% and 98.1%, respectively (P = .43). The study, however, was not sufficiently powered to fully analyze these subgroups, the authors acknowledged.
Although results were overall comparable with those of a previous study on a different IUD, the expulsion rate was somewhat higher, at 9%, than the 6% reported in the earlier study.
“Although this strategy for reducing blood loss is not new, this study is notable because it looked at high-BMI women and nulliparous women,” said Kathryn J. Gray, MD, PhD, an attending physician in the department of obstetrics and gynecology at Brigham and Women’s Hospital in Boston, who was not involved in the research.“No prior trials have included patients with BMIs exceeding 35 kg/m2 or nulliparous patients, while this study enrolled a full array of patients, which allowed exploratory analyses of these subpopulations,” Dr. Creinin confirmed.
According to Dr. Gray, the IUD approach has advantages over systemic treatment with oral medication. “First, treatment is not user-dependent so the user doesn’t have to remember to take it. In addition, because the medication is locally targeted in the uterus, it is more effective and there is less fluctuation and variability in drug levels than when taken orally.”
As to treatment durability, Dr. Creinin said, “Long-term studies in a population being treated for heavy menstrual bleeding would be helpful to have an idea of how long this effect lasts. Still, there is no reason to expect that the effect will not last for many years.”
And with this treatment, he added, both patient and clinician can readily detect its effect. “If bleeding begins to increase, they will know!”
Would there be a lingering residual effect even after removal of the IUD? “That is an excellent question that remains to be answered,” Dr. Creinin said. “There are no data on when the heavy bleeding returns, but it would be expected to do so.”
This study was funded, designed, and supervised by Medicines360, which also provided the study treatment. Dr. Creinin disclosed financial relationships with various private-sector companies, including Medicines360, Organon, Fuji Pharma, GlaxoSmithKline, and Merck & Co. Multiple study coauthors disclosed similar financial ties to industry partners, including Medicines360. Dr. Gray had no potential conflicts of interest with regard to her comments.
FROM OBSTETRICS & GYNECOLOGY
Spotting STIs: Vaginal swabs work best
Vaginal swabs are more effective than urine analysis in detecting certain types of sexually transmitted infections, researchers have found.
In the study, which was published online in the Annals of Family Medicine, investigators found that the diagnostic sensitivity of commercially available vaginal swabs was significantly greater than that of urine tests in detecting certain infections, including those caused by Chlamydia trachomatis, Neisseria gonorrhoeae, and Trichomonas vaginalis.
Researchers studied chlamydia and gonorrhea, which are two of the most frequently reported STIs in the United States. Trichomoniasis is the most curable nonviral STI globally, with 156 million cases worldwide in 2016.
The Centers for Disease Control and Prevention has long recommended that vaginal swabs be used to produce optimal samples.
But despite the CDC’s recommendation, urine analysis for these STIs is more commonly used than vaginal swabs among U.S. health care providers.
“We’re using a poor sample type, and we can do better,” said Barbara Van Der Pol, PhD, a professor of medicine and public health at the University of Alabama at Birmingham and an author of the new study, a meta-analysis of 97 studies published between 1995 and 2021.
Vaginal swabs for chlamydia trachomatis had a diagnostic sensitivity of 94.1% (95% confidence interval, 93.2%-94.9%; P < .001), higher than urine testing (86.9%; 95% CI, 85.6%-88.0%; P < .001). The pooled sensitivity estimates for Neisseria gonorrhoeae were 96.5% (95% CI, 94.8%-97.7%; P < .001) for vaginal swabs and 90.7% (95% CI, 88.4%-92.5%; P < .001) for urine specimens.
The difference in pooled sensitivity estimates between vaginal swabs and urine analyses for Trichomonas vaginalis was 98% (95% CI, 97.0%-98.7%; P < .001) for vaginal swabs and 95.1% (95% CI, 93.6%-96.3%) for urine specimens.
STIs included in the study are not typically found in the urethra and appear in urine analyses only if cervical or vaginal cells have dripped into a urine sample. Dr. Van Der Pol and her colleagues estimated that the use of urine samples rather than vaginal swabs may result in more than 400,000 undiagnosed infections annually.
Undiagnosed and untreated STIs can lead to transmissions of the infection as well as infertility and can have negative effects on romantic relationships, according to Dr. Van Der Pol.
Sarah Wood, MD, an attending physician at Children’s Hospital of Philadelphia, said some health care providers may use urine analysis because patients may be more comfortable with this method. The approach also can be more convenient for medical offices: All they must do is hand a specimen container to the patient.
Conversations between clinicians and patients about vaginal swabbing may be considered “sensitive” and the swabbing more invasive, Dr. Wood, an author of an editorial accompanying the journal article, said. Clinicians may also lack awareness that the swab is a more sensitive method of detecting these STIs.
“We all want to do what’s right for our patient, but we often don’t know what’s right for the patient,” Dr. Wood said. “I don’t think people are really aware of a potential real difference in outcomes with one method over the other.”
Dr. Wood advised making STI screening using vaginal swabs more common by “offering universal opt-out screening, so not waiting until you find out if someone’s having sex but just sort of saying, ‘Hey, across our practice, we screen everybody for chlamydia. Is that something that you want to do today?’ That approach sort of takes out the piece of talking about sex, talking about sexual activity.”
Dr. Van Der Pol, who said she has worked in STI diagnostics for 40 years, said she was not surprised by the results and hopes the study changes how samples are collected and used.
“I really hope that it influences practice so that we really start using vaginal swabs, because it gives us better diagnostics for chlamydia and gonorrhea,” Dr. Van Der Pol said.
“Also, then starting to think about comprehensive women’s care in such a way that they actually order other tests on that same sample if a woman is presenting with complaints.”
A version of this article originally appeared on Medscape.com.
Vaginal swabs are more effective than urine analysis in detecting certain types of sexually transmitted infections, researchers have found.
In the study, which was published online in the Annals of Family Medicine, investigators found that the diagnostic sensitivity of commercially available vaginal swabs was significantly greater than that of urine tests in detecting certain infections, including those caused by Chlamydia trachomatis, Neisseria gonorrhoeae, and Trichomonas vaginalis.
Researchers studied chlamydia and gonorrhea, which are two of the most frequently reported STIs in the United States. Trichomoniasis is the most curable nonviral STI globally, with 156 million cases worldwide in 2016.
The Centers for Disease Control and Prevention has long recommended that vaginal swabs be used to produce optimal samples.
But despite the CDC’s recommendation, urine analysis for these STIs is more commonly used than vaginal swabs among U.S. health care providers.
“We’re using a poor sample type, and we can do better,” said Barbara Van Der Pol, PhD, a professor of medicine and public health at the University of Alabama at Birmingham and an author of the new study, a meta-analysis of 97 studies published between 1995 and 2021.
Vaginal swabs for chlamydia trachomatis had a diagnostic sensitivity of 94.1% (95% confidence interval, 93.2%-94.9%; P < .001), higher than urine testing (86.9%; 95% CI, 85.6%-88.0%; P < .001). The pooled sensitivity estimates for Neisseria gonorrhoeae were 96.5% (95% CI, 94.8%-97.7%; P < .001) for vaginal swabs and 90.7% (95% CI, 88.4%-92.5%; P < .001) for urine specimens.
The difference in pooled sensitivity estimates between vaginal swabs and urine analyses for Trichomonas vaginalis was 98% (95% CI, 97.0%-98.7%; P < .001) for vaginal swabs and 95.1% (95% CI, 93.6%-96.3%) for urine specimens.
STIs included in the study are not typically found in the urethra and appear in urine analyses only if cervical or vaginal cells have dripped into a urine sample. Dr. Van Der Pol and her colleagues estimated that the use of urine samples rather than vaginal swabs may result in more than 400,000 undiagnosed infections annually.
Undiagnosed and untreated STIs can lead to transmissions of the infection as well as infertility and can have negative effects on romantic relationships, according to Dr. Van Der Pol.
Sarah Wood, MD, an attending physician at Children’s Hospital of Philadelphia, said some health care providers may use urine analysis because patients may be more comfortable with this method. The approach also can be more convenient for medical offices: All they must do is hand a specimen container to the patient.
Conversations between clinicians and patients about vaginal swabbing may be considered “sensitive” and the swabbing more invasive, Dr. Wood, an author of an editorial accompanying the journal article, said. Clinicians may also lack awareness that the swab is a more sensitive method of detecting these STIs.
“We all want to do what’s right for our patient, but we often don’t know what’s right for the patient,” Dr. Wood said. “I don’t think people are really aware of a potential real difference in outcomes with one method over the other.”
Dr. Wood advised making STI screening using vaginal swabs more common by “offering universal opt-out screening, so not waiting until you find out if someone’s having sex but just sort of saying, ‘Hey, across our practice, we screen everybody for chlamydia. Is that something that you want to do today?’ That approach sort of takes out the piece of talking about sex, talking about sexual activity.”
Dr. Van Der Pol, who said she has worked in STI diagnostics for 40 years, said she was not surprised by the results and hopes the study changes how samples are collected and used.
“I really hope that it influences practice so that we really start using vaginal swabs, because it gives us better diagnostics for chlamydia and gonorrhea,” Dr. Van Der Pol said.
“Also, then starting to think about comprehensive women’s care in such a way that they actually order other tests on that same sample if a woman is presenting with complaints.”
A version of this article originally appeared on Medscape.com.
Vaginal swabs are more effective than urine analysis in detecting certain types of sexually transmitted infections, researchers have found.
In the study, which was published online in the Annals of Family Medicine, investigators found that the diagnostic sensitivity of commercially available vaginal swabs was significantly greater than that of urine tests in detecting certain infections, including those caused by Chlamydia trachomatis, Neisseria gonorrhoeae, and Trichomonas vaginalis.
Researchers studied chlamydia and gonorrhea, which are two of the most frequently reported STIs in the United States. Trichomoniasis is the most curable nonviral STI globally, with 156 million cases worldwide in 2016.
The Centers for Disease Control and Prevention has long recommended that vaginal swabs be used to produce optimal samples.
But despite the CDC’s recommendation, urine analysis for these STIs is more commonly used than vaginal swabs among U.S. health care providers.
“We’re using a poor sample type, and we can do better,” said Barbara Van Der Pol, PhD, a professor of medicine and public health at the University of Alabama at Birmingham and an author of the new study, a meta-analysis of 97 studies published between 1995 and 2021.
Vaginal swabs for chlamydia trachomatis had a diagnostic sensitivity of 94.1% (95% confidence interval, 93.2%-94.9%; P < .001), higher than urine testing (86.9%; 95% CI, 85.6%-88.0%; P < .001). The pooled sensitivity estimates for Neisseria gonorrhoeae were 96.5% (95% CI, 94.8%-97.7%; P < .001) for vaginal swabs and 90.7% (95% CI, 88.4%-92.5%; P < .001) for urine specimens.
The difference in pooled sensitivity estimates between vaginal swabs and urine analyses for Trichomonas vaginalis was 98% (95% CI, 97.0%-98.7%; P < .001) for vaginal swabs and 95.1% (95% CI, 93.6%-96.3%) for urine specimens.
STIs included in the study are not typically found in the urethra and appear in urine analyses only if cervical or vaginal cells have dripped into a urine sample. Dr. Van Der Pol and her colleagues estimated that the use of urine samples rather than vaginal swabs may result in more than 400,000 undiagnosed infections annually.
Undiagnosed and untreated STIs can lead to transmissions of the infection as well as infertility and can have negative effects on romantic relationships, according to Dr. Van Der Pol.
Sarah Wood, MD, an attending physician at Children’s Hospital of Philadelphia, said some health care providers may use urine analysis because patients may be more comfortable with this method. The approach also can be more convenient for medical offices: All they must do is hand a specimen container to the patient.
Conversations between clinicians and patients about vaginal swabbing may be considered “sensitive” and the swabbing more invasive, Dr. Wood, an author of an editorial accompanying the journal article, said. Clinicians may also lack awareness that the swab is a more sensitive method of detecting these STIs.
“We all want to do what’s right for our patient, but we often don’t know what’s right for the patient,” Dr. Wood said. “I don’t think people are really aware of a potential real difference in outcomes with one method over the other.”
Dr. Wood advised making STI screening using vaginal swabs more common by “offering universal opt-out screening, so not waiting until you find out if someone’s having sex but just sort of saying, ‘Hey, across our practice, we screen everybody for chlamydia. Is that something that you want to do today?’ That approach sort of takes out the piece of talking about sex, talking about sexual activity.”
Dr. Van Der Pol, who said she has worked in STI diagnostics for 40 years, said she was not surprised by the results and hopes the study changes how samples are collected and used.
“I really hope that it influences practice so that we really start using vaginal swabs, because it gives us better diagnostics for chlamydia and gonorrhea,” Dr. Van Der Pol said.
“Also, then starting to think about comprehensive women’s care in such a way that they actually order other tests on that same sample if a woman is presenting with complaints.”
A version of this article originally appeared on Medscape.com.
Product updates and reviews
REVIEW
FEMCERV®: Well-designed technology that can minimize patient discomfort
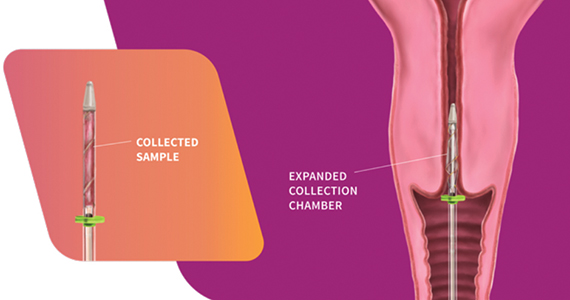
The FemCerv® Endocervical Sampler, developed by Femasys, Inc (Suwanee, Georgia) expands options for colposcopy biopsy.
Background. In the United States, approximately 3 million women per year undergo colposcopic evaluation to work-up abnormal screening cytology. While some controversy exists regarding the exact role of endocervical curettage (ECC) within each age group and clinical situation, it is nonetheless an important component of the colposcopy-biopsy examination in many cases. For over a century, the 3 mm metal endocervical curette has been the primary tool employed to obtain the tissue sample from the endocervical canal. Unfortunately, sharp curettage with a metal curette can have inadequate sampling rates as high as 14%,1 it runs the risk of ectocervical contamination, and it is painful, with almost half the participants in one study rating the procedural pain 3 ̶ 5 out of 8 on a VAS scale.2 So, maybe there is a better way.
Design/Functionality. According to Femasys, the FemCerv® Endocervical Sampler was designed to be the better way of performing an endocervical curettage. FemCerv is a single-use sterile device that comes in a standard 13 F size as well as an 11 F size for the narrower/stenotic os. In truth, at first glance it looks pretty complicated compared with a Kevorkian curette or an endocervical brush. The user end has a handle with a rotatable knob that transitions to a shaft with a flange at the end right before the sampling mechanism. To use the device, the sampling end is inserted into the endocervical canal up to the flange. The knob on the handle is then turned clockwise to open the sheath, thereby exposing sharp rigid plastic edges. The device is then rotated 360° clockwise and then 360° counterclockwise to “curette” the endocervical canal. Finally, the knob on the handle is turned counterclockwise to close the sheath and the device is removed. The specimen is then transferred to a standard vial for processing.
In my experience with its use, it actually exceeded my expectations. The device was easy to use, and the specimens were more than adequate. Truth be told, I came into the trial with a negative mindset having already convinced myself that this device was a waste of money given that doing an ECC with traditional methods is so straightforward. What I had not anticipated was the complete lack of patient discomfort when I used the FemCerv compared with a Kevorkian device.
Innovation. From an innovation standpoint, FemCerv is not super-disruptive technology, but it is well designed and pretty clever in that the opening and closing sheath prevents ectocervical cellular contamination, and the rotational sampling, rather than in-and-out sampling, does dramatically reduce the patient discomfort.
Summary. As I previously noted, before trying it, I did not anticipate liking FemCerv as much as I did. Does it add some non-reimbursable cost to a relatively low-reimbursing procedure? Absolutely. But it is not too expensive and, for me, making a painful procedure relatively painless is good value every time. I think all our patients would agree.
For more information, visit https://femasys.com/.
UPDATE
Hologic, Inc. announces that they received the following 2022 IMV ServiceTrakTM Awards for Mammography: Best Service, Best Customer Satisfaction, and Best System Performance. In addition, Hologic announces that their Affirm Contrast Biopsy software is commercially available in the United States. Tissue samples may be targeted and acquired using the Affirm Contrast Biopsy software from lesions identified using Hologic’s I-View Contrast Enhanced Mammography software. The latter software allows health care facilities an alternative to breast magnetic resonance imaging, which is used as supplemental imaging to mammography and/or ultrasonography.
For more information, visit https://www.hologic.com/
- Damkjaer M, Laursen JB, Petersen LK, et al. Endocervical sampling in women with suspected cervical neoplasia: a systematic review and meta-analysis of diagnostic test accuracy studies. Am J Obstet Gynecol. 2022;227:839-848.e4. doi: 10.1016/j.ajog.2022.07.036.
- Goksedef BP, Api M, Kaya O, et al. Diagnostic accuracy of two endocervical sampling method: randomized controlled trial. Arch Gynecol Obstet. 2013;287:117-22. doi: 10.1007/s00404-012-2542-9.
REVIEW
FEMCERV®: Well-designed technology that can minimize patient discomfort

The FemCerv® Endocervical Sampler, developed by Femasys, Inc (Suwanee, Georgia) expands options for colposcopy biopsy.
Background. In the United States, approximately 3 million women per year undergo colposcopic evaluation to work-up abnormal screening cytology. While some controversy exists regarding the exact role of endocervical curettage (ECC) within each age group and clinical situation, it is nonetheless an important component of the colposcopy-biopsy examination in many cases. For over a century, the 3 mm metal endocervical curette has been the primary tool employed to obtain the tissue sample from the endocervical canal. Unfortunately, sharp curettage with a metal curette can have inadequate sampling rates as high as 14%,1 it runs the risk of ectocervical contamination, and it is painful, with almost half the participants in one study rating the procedural pain 3 ̶ 5 out of 8 on a VAS scale.2 So, maybe there is a better way.
Design/Functionality. According to Femasys, the FemCerv® Endocervical Sampler was designed to be the better way of performing an endocervical curettage. FemCerv is a single-use sterile device that comes in a standard 13 F size as well as an 11 F size for the narrower/stenotic os. In truth, at first glance it looks pretty complicated compared with a Kevorkian curette or an endocervical brush. The user end has a handle with a rotatable knob that transitions to a shaft with a flange at the end right before the sampling mechanism. To use the device, the sampling end is inserted into the endocervical canal up to the flange. The knob on the handle is then turned clockwise to open the sheath, thereby exposing sharp rigid plastic edges. The device is then rotated 360° clockwise and then 360° counterclockwise to “curette” the endocervical canal. Finally, the knob on the handle is turned counterclockwise to close the sheath and the device is removed. The specimen is then transferred to a standard vial for processing.
In my experience with its use, it actually exceeded my expectations. The device was easy to use, and the specimens were more than adequate. Truth be told, I came into the trial with a negative mindset having already convinced myself that this device was a waste of money given that doing an ECC with traditional methods is so straightforward. What I had not anticipated was the complete lack of patient discomfort when I used the FemCerv compared with a Kevorkian device.
Innovation. From an innovation standpoint, FemCerv is not super-disruptive technology, but it is well designed and pretty clever in that the opening and closing sheath prevents ectocervical cellular contamination, and the rotational sampling, rather than in-and-out sampling, does dramatically reduce the patient discomfort.
Summary. As I previously noted, before trying it, I did not anticipate liking FemCerv as much as I did. Does it add some non-reimbursable cost to a relatively low-reimbursing procedure? Absolutely. But it is not too expensive and, for me, making a painful procedure relatively painless is good value every time. I think all our patients would agree.
For more information, visit https://femasys.com/.
UPDATE
Hologic, Inc. announces that they received the following 2022 IMV ServiceTrakTM Awards for Mammography: Best Service, Best Customer Satisfaction, and Best System Performance. In addition, Hologic announces that their Affirm Contrast Biopsy software is commercially available in the United States. Tissue samples may be targeted and acquired using the Affirm Contrast Biopsy software from lesions identified using Hologic’s I-View Contrast Enhanced Mammography software. The latter software allows health care facilities an alternative to breast magnetic resonance imaging, which is used as supplemental imaging to mammography and/or ultrasonography.
For more information, visit https://www.hologic.com/
REVIEW
FEMCERV®: Well-designed technology that can minimize patient discomfort

The FemCerv® Endocervical Sampler, developed by Femasys, Inc (Suwanee, Georgia) expands options for colposcopy biopsy.
Background. In the United States, approximately 3 million women per year undergo colposcopic evaluation to work-up abnormal screening cytology. While some controversy exists regarding the exact role of endocervical curettage (ECC) within each age group and clinical situation, it is nonetheless an important component of the colposcopy-biopsy examination in many cases. For over a century, the 3 mm metal endocervical curette has been the primary tool employed to obtain the tissue sample from the endocervical canal. Unfortunately, sharp curettage with a metal curette can have inadequate sampling rates as high as 14%,1 it runs the risk of ectocervical contamination, and it is painful, with almost half the participants in one study rating the procedural pain 3 ̶ 5 out of 8 on a VAS scale.2 So, maybe there is a better way.
Design/Functionality. According to Femasys, the FemCerv® Endocervical Sampler was designed to be the better way of performing an endocervical curettage. FemCerv is a single-use sterile device that comes in a standard 13 F size as well as an 11 F size for the narrower/stenotic os. In truth, at first glance it looks pretty complicated compared with a Kevorkian curette or an endocervical brush. The user end has a handle with a rotatable knob that transitions to a shaft with a flange at the end right before the sampling mechanism. To use the device, the sampling end is inserted into the endocervical canal up to the flange. The knob on the handle is then turned clockwise to open the sheath, thereby exposing sharp rigid plastic edges. The device is then rotated 360° clockwise and then 360° counterclockwise to “curette” the endocervical canal. Finally, the knob on the handle is turned counterclockwise to close the sheath and the device is removed. The specimen is then transferred to a standard vial for processing.
In my experience with its use, it actually exceeded my expectations. The device was easy to use, and the specimens were more than adequate. Truth be told, I came into the trial with a negative mindset having already convinced myself that this device was a waste of money given that doing an ECC with traditional methods is so straightforward. What I had not anticipated was the complete lack of patient discomfort when I used the FemCerv compared with a Kevorkian device.
Innovation. From an innovation standpoint, FemCerv is not super-disruptive technology, but it is well designed and pretty clever in that the opening and closing sheath prevents ectocervical cellular contamination, and the rotational sampling, rather than in-and-out sampling, does dramatically reduce the patient discomfort.
Summary. As I previously noted, before trying it, I did not anticipate liking FemCerv as much as I did. Does it add some non-reimbursable cost to a relatively low-reimbursing procedure? Absolutely. But it is not too expensive and, for me, making a painful procedure relatively painless is good value every time. I think all our patients would agree.
For more information, visit https://femasys.com/.
UPDATE
Hologic, Inc. announces that they received the following 2022 IMV ServiceTrakTM Awards for Mammography: Best Service, Best Customer Satisfaction, and Best System Performance. In addition, Hologic announces that their Affirm Contrast Biopsy software is commercially available in the United States. Tissue samples may be targeted and acquired using the Affirm Contrast Biopsy software from lesions identified using Hologic’s I-View Contrast Enhanced Mammography software. The latter software allows health care facilities an alternative to breast magnetic resonance imaging, which is used as supplemental imaging to mammography and/or ultrasonography.
For more information, visit https://www.hologic.com/
- Damkjaer M, Laursen JB, Petersen LK, et al. Endocervical sampling in women with suspected cervical neoplasia: a systematic review and meta-analysis of diagnostic test accuracy studies. Am J Obstet Gynecol. 2022;227:839-848.e4. doi: 10.1016/j.ajog.2022.07.036.
- Goksedef BP, Api M, Kaya O, et al. Diagnostic accuracy of two endocervical sampling method: randomized controlled trial. Arch Gynecol Obstet. 2013;287:117-22. doi: 10.1007/s00404-012-2542-9.
- Damkjaer M, Laursen JB, Petersen LK, et al. Endocervical sampling in women with suspected cervical neoplasia: a systematic review and meta-analysis of diagnostic test accuracy studies. Am J Obstet Gynecol. 2022;227:839-848.e4. doi: 10.1016/j.ajog.2022.07.036.
- Goksedef BP, Api M, Kaya O, et al. Diagnostic accuracy of two endocervical sampling method: randomized controlled trial. Arch Gynecol Obstet. 2013;287:117-22. doi: 10.1007/s00404-012-2542-9.








