User login
Digital Pathology Seminar Focuses on Federal Practice
Recognizing the increasing importance of digital pathology and its potential impact to transform federal health care, government, military, and university digital pathology specialists convened in May 2023 to share expertise to advance the use of digital pathology in federal health care.
The seminar was hosted by the University of Pittsburgh and led by Ronald Poropatich, MD, Director of the Center for Military Medicine Research, Health Sciences, and Professor of Medicine at the University of Pittsburgh Medical Center, and Douglas Hartman, MD, Vice Chair of Pathology Informatics, Associate Director of the Center for AI Innovation in Medical Imaging, and Professor of Pathology at the University of Pittsburgh/University of Pittsburgh Medical Center (UPMC).
Invitees included senior federal government pathologists, laboratory scientists, IT leaders, and stakeholders from the VA, DoD, HHS (NIH, CDC, IHS, FDA) and other federal agencies. The speakers for the conference were CDR Roger Boodoo, MD, Chief of Innovation, Defense Health Agency; Ryan Collins, MD, Pathologist, Williamsport Pathology Association; Pat Flanders, Chief Information Officer, J6, Defense Health Agency; Matthew Hanna, MD, Director, Digital Pathology Informatics, Memorial Sloan Kettering Cancer Center; Stephanie Harmon, PhD, Staff Scientist, NIH NCI, Imaging/Data Scientist in Molecular Imaging; Douglas Hartman, MD, Vice Chair of Pathology Informatics, University of Pittsburgh; Stephen Hewitt, MD, PhD, Head, Experimental Pathology Laboratory, NIH NCI, Center for Cancer Research; Jason Hipp, MD, PhD, Chief Digital Innovation Officer, Mayo Collaborate Services, Mayo Clinic; Brian Lein, MD, Assistant Director, Healthcare Administration, Defense Health Agency; Col Mark Lyman, MD, Pathology Consultant to the US Air Force Surgeon General; COL Joel Moncur, MD, Director, Joint Pathology Center; Ronald Poropatich, MD, Director of the Center for Military Medicine Research, Health Sciences; Professor of Medicine, University of Pittsburgh; David Shulkin, MD, Ninth U.S. Secretary of Veterans Affairs; Eliot Siegel, MD, Chief of Radiology and Nuclear Medicine, Veterans Affairs Maryland Healthcare System; Professor and Vice Chair, University of Maryland School of Medicine; CDR Jenny Smith, DO, Pathologist, US Naval Medical Center Portsmouth; Shandong Wu, PhD, Associate Professor, Departments of Radiology, Biomedical Informatics, and Bioengineering, Director of Center for Artificial Intelligence Innovation in Medical Imaging, University of Pittsburgh; LCDR Victoria Mahar MD, Pathologist, US Army.
Throughout the 1.5-day meeting, topics such as the integration of systems, the value of single vendor solutions vs multiple vendors, and the interconnectedness of radiology and pathology in health care were discussed. The speakers addressed the challenges of adopting digital pathology, including workflow improvement, quality control, and the generalizability of algorithms. The importance of collaboration, leadership, data analytics, compliance with clinical practice guidelines, and research and development efforts were stressed. The increasingly important role of artificial intelligence (AI) in digital pathology, its applications, and its benefits were also highlighted. Continuing education credits were offered to participants.
Overall, the meeting provided valuable insights into the advancements, challenges, and potential of digital pathology, AI, and technology integration in the federal health care ecosystem. However, this cannot be achieved without leadership from and close collaboration between key industry, academic, and government stakeholders.
Uses of Digital Pathology
Digital pathology refers to the practice of digitizing glass slides containing tissue samples and using digital imaging technology to analyze and interpret them. It involves capturing high-resolution images of microscopic slides and storing them in a digital format. These digitized images can be accessed and analyzed using computer-based tools and software.
While traditional pathology involves examining tissue samples under a microscope to make diagnoses and provide insights into diseases and conditions, digital pathology uses digital scanners that capture all relevant tissue on the glass slide at high magnification. This process generates a high-fidelity digital representation of the tissue sample that can be navigated akin to how glass slides are reviewed on a brightfield microscope in current practice (eg, panning, zooming, etc). Microscopic review of patient specimens in pathology allows for identifying patterns and markers that may not be easily detectable with manual examination alone.
The digitized slides can be stored in a database or a slide management system, allowing pathologists and other healthcare professionals to access and review them remotely, thus creating the potential to improve collaboration among pathologists, facilitate second opinions, and enable easier access to archived slides for research purposes.
Potential Benefits
Digital pathology also opens the door to advanced image analysis techniques, such as computer-aided diagnosis, machine learning, and AI algorithms, with the potential for the following outcomes and benefits:
- Improved accuracy AI algorithms can analyze large volumes of digital pathology data with great precision, reducing the chances of human error and subjective interpretation. This can lead to more accurate and consistent diagnoses, especially in challenging cases where subtle patterns or features may be difficult to detect.
- Automated detection and classification AI algorithms can be trained to detect and classify specific features or abnormalities in digital pathology images. For example, AI models can identify cancerous cells, tissue patterns associated with different diseases, or specific biomarkers. This can assist pathologists in diagnosing diseases more accurately and efficiently.
- Quantitative analysis AI can analyze large quantities of digital pathology data and extract quantitative measurements. For instance, it can calculate the percentage of tumor cells in a sample, assess the density of immune cells, or measure the extent of tissue damage. These objective measurements can aid in prognosis prediction and treatment planning.
- Image segmentation AI algorithms can segment digital pathology images into different regions or structures, such as nuclei, cytoplasm, or blood vessels. This segmentation allows for precise analysis and extraction of features for further study. It can also facilitate the identification of specific cell types or tissue components.
- Image enhancement AI techniques can enhance the quality of digital pathology images by improving clarity and reducing noise or artifacts. This can help pathologists visualize and interpret slides more effectively, especially in challenging cases with low-quality or complex images.
- Decision support systems AI-powered decision support systems can assist pathologists by providing recommendations or second opinions based on the analysis of digital pathology data. These systems can offer insights, suggest potential diagnoses, or provide relevant research references, augmenting the pathologist’s expertise and improving diagnostic accuracy.
- Collaboration and second opinions Digital pathology, combined with AI, facilitates remote access to digitized slides, enabling pathologists to seek second opinions or collaborate with experts from around the world. This can enhance the quality of diagnoses by leveraging the collective expertise of pathologists and fostering knowledge sharing.
- Education and training AI algorithms can be utilized in virtual microscopy platforms to create interactive and educational experiences. Pathology residents and students can learn from annotated cases, receive real-time feedback, and develop their skills in a digital environment.
- Research and discovery AI can assist in identifying patterns, correlations, and novel biomarkers in digital pathology data. By analyzing large datasets, AI algorithms can help uncover new insights, contribute to research advancements, and aid in the development of personalized medicine approaches.
- Predictive modeling AI can analyze vast amounts of digital pathology data, patient records, and outcomes to develop predictive models. These models can estimate disease progression, treatment response, or patient survival rates based on various factors. They can contribute to personalized medicine by assisting in treatment decisions and prognosis assessment.
It is important to note that while AI has shown promising results, it is not intended to replace human pathologists but to augment their capabilities. Overall, the combination of AI technology with the expertise of pathologists can lead to improved diagnosis, better patient care, and more efficient workflows in digital pathology.
Recognizing the increasing importance of digital pathology and its potential impact to transform federal health care, government, military, and university digital pathology specialists convened in May 2023 to share expertise to advance the use of digital pathology in federal health care.
The seminar was hosted by the University of Pittsburgh and led by Ronald Poropatich, MD, Director of the Center for Military Medicine Research, Health Sciences, and Professor of Medicine at the University of Pittsburgh Medical Center, and Douglas Hartman, MD, Vice Chair of Pathology Informatics, Associate Director of the Center for AI Innovation in Medical Imaging, and Professor of Pathology at the University of Pittsburgh/University of Pittsburgh Medical Center (UPMC).
Invitees included senior federal government pathologists, laboratory scientists, IT leaders, and stakeholders from the VA, DoD, HHS (NIH, CDC, IHS, FDA) and other federal agencies. The speakers for the conference were CDR Roger Boodoo, MD, Chief of Innovation, Defense Health Agency; Ryan Collins, MD, Pathologist, Williamsport Pathology Association; Pat Flanders, Chief Information Officer, J6, Defense Health Agency; Matthew Hanna, MD, Director, Digital Pathology Informatics, Memorial Sloan Kettering Cancer Center; Stephanie Harmon, PhD, Staff Scientist, NIH NCI, Imaging/Data Scientist in Molecular Imaging; Douglas Hartman, MD, Vice Chair of Pathology Informatics, University of Pittsburgh; Stephen Hewitt, MD, PhD, Head, Experimental Pathology Laboratory, NIH NCI, Center for Cancer Research; Jason Hipp, MD, PhD, Chief Digital Innovation Officer, Mayo Collaborate Services, Mayo Clinic; Brian Lein, MD, Assistant Director, Healthcare Administration, Defense Health Agency; Col Mark Lyman, MD, Pathology Consultant to the US Air Force Surgeon General; COL Joel Moncur, MD, Director, Joint Pathology Center; Ronald Poropatich, MD, Director of the Center for Military Medicine Research, Health Sciences; Professor of Medicine, University of Pittsburgh; David Shulkin, MD, Ninth U.S. Secretary of Veterans Affairs; Eliot Siegel, MD, Chief of Radiology and Nuclear Medicine, Veterans Affairs Maryland Healthcare System; Professor and Vice Chair, University of Maryland School of Medicine; CDR Jenny Smith, DO, Pathologist, US Naval Medical Center Portsmouth; Shandong Wu, PhD, Associate Professor, Departments of Radiology, Biomedical Informatics, and Bioengineering, Director of Center for Artificial Intelligence Innovation in Medical Imaging, University of Pittsburgh; LCDR Victoria Mahar MD, Pathologist, US Army.
Throughout the 1.5-day meeting, topics such as the integration of systems, the value of single vendor solutions vs multiple vendors, and the interconnectedness of radiology and pathology in health care were discussed. The speakers addressed the challenges of adopting digital pathology, including workflow improvement, quality control, and the generalizability of algorithms. The importance of collaboration, leadership, data analytics, compliance with clinical practice guidelines, and research and development efforts were stressed. The increasingly important role of artificial intelligence (AI) in digital pathology, its applications, and its benefits were also highlighted. Continuing education credits were offered to participants.
Overall, the meeting provided valuable insights into the advancements, challenges, and potential of digital pathology, AI, and technology integration in the federal health care ecosystem. However, this cannot be achieved without leadership from and close collaboration between key industry, academic, and government stakeholders.
Uses of Digital Pathology
Digital pathology refers to the practice of digitizing glass slides containing tissue samples and using digital imaging technology to analyze and interpret them. It involves capturing high-resolution images of microscopic slides and storing them in a digital format. These digitized images can be accessed and analyzed using computer-based tools and software.
While traditional pathology involves examining tissue samples under a microscope to make diagnoses and provide insights into diseases and conditions, digital pathology uses digital scanners that capture all relevant tissue on the glass slide at high magnification. This process generates a high-fidelity digital representation of the tissue sample that can be navigated akin to how glass slides are reviewed on a brightfield microscope in current practice (eg, panning, zooming, etc). Microscopic review of patient specimens in pathology allows for identifying patterns and markers that may not be easily detectable with manual examination alone.
The digitized slides can be stored in a database or a slide management system, allowing pathologists and other healthcare professionals to access and review them remotely, thus creating the potential to improve collaboration among pathologists, facilitate second opinions, and enable easier access to archived slides for research purposes.
Potential Benefits
Digital pathology also opens the door to advanced image analysis techniques, such as computer-aided diagnosis, machine learning, and AI algorithms, with the potential for the following outcomes and benefits:
- Improved accuracy AI algorithms can analyze large volumes of digital pathology data with great precision, reducing the chances of human error and subjective interpretation. This can lead to more accurate and consistent diagnoses, especially in challenging cases where subtle patterns or features may be difficult to detect.
- Automated detection and classification AI algorithms can be trained to detect and classify specific features or abnormalities in digital pathology images. For example, AI models can identify cancerous cells, tissue patterns associated with different diseases, or specific biomarkers. This can assist pathologists in diagnosing diseases more accurately and efficiently.
- Quantitative analysis AI can analyze large quantities of digital pathology data and extract quantitative measurements. For instance, it can calculate the percentage of tumor cells in a sample, assess the density of immune cells, or measure the extent of tissue damage. These objective measurements can aid in prognosis prediction and treatment planning.
- Image segmentation AI algorithms can segment digital pathology images into different regions or structures, such as nuclei, cytoplasm, or blood vessels. This segmentation allows for precise analysis and extraction of features for further study. It can also facilitate the identification of specific cell types or tissue components.
- Image enhancement AI techniques can enhance the quality of digital pathology images by improving clarity and reducing noise or artifacts. This can help pathologists visualize and interpret slides more effectively, especially in challenging cases with low-quality or complex images.
- Decision support systems AI-powered decision support systems can assist pathologists by providing recommendations or second opinions based on the analysis of digital pathology data. These systems can offer insights, suggest potential diagnoses, or provide relevant research references, augmenting the pathologist’s expertise and improving diagnostic accuracy.
- Collaboration and second opinions Digital pathology, combined with AI, facilitates remote access to digitized slides, enabling pathologists to seek second opinions or collaborate with experts from around the world. This can enhance the quality of diagnoses by leveraging the collective expertise of pathologists and fostering knowledge sharing.
- Education and training AI algorithms can be utilized in virtual microscopy platforms to create interactive and educational experiences. Pathology residents and students can learn from annotated cases, receive real-time feedback, and develop their skills in a digital environment.
- Research and discovery AI can assist in identifying patterns, correlations, and novel biomarkers in digital pathology data. By analyzing large datasets, AI algorithms can help uncover new insights, contribute to research advancements, and aid in the development of personalized medicine approaches.
- Predictive modeling AI can analyze vast amounts of digital pathology data, patient records, and outcomes to develop predictive models. These models can estimate disease progression, treatment response, or patient survival rates based on various factors. They can contribute to personalized medicine by assisting in treatment decisions and prognosis assessment.
It is important to note that while AI has shown promising results, it is not intended to replace human pathologists but to augment their capabilities. Overall, the combination of AI technology with the expertise of pathologists can lead to improved diagnosis, better patient care, and more efficient workflows in digital pathology.
Recognizing the increasing importance of digital pathology and its potential impact to transform federal health care, government, military, and university digital pathology specialists convened in May 2023 to share expertise to advance the use of digital pathology in federal health care.
The seminar was hosted by the University of Pittsburgh and led by Ronald Poropatich, MD, Director of the Center for Military Medicine Research, Health Sciences, and Professor of Medicine at the University of Pittsburgh Medical Center, and Douglas Hartman, MD, Vice Chair of Pathology Informatics, Associate Director of the Center for AI Innovation in Medical Imaging, and Professor of Pathology at the University of Pittsburgh/University of Pittsburgh Medical Center (UPMC).
Invitees included senior federal government pathologists, laboratory scientists, IT leaders, and stakeholders from the VA, DoD, HHS (NIH, CDC, IHS, FDA) and other federal agencies. The speakers for the conference were CDR Roger Boodoo, MD, Chief of Innovation, Defense Health Agency; Ryan Collins, MD, Pathologist, Williamsport Pathology Association; Pat Flanders, Chief Information Officer, J6, Defense Health Agency; Matthew Hanna, MD, Director, Digital Pathology Informatics, Memorial Sloan Kettering Cancer Center; Stephanie Harmon, PhD, Staff Scientist, NIH NCI, Imaging/Data Scientist in Molecular Imaging; Douglas Hartman, MD, Vice Chair of Pathology Informatics, University of Pittsburgh; Stephen Hewitt, MD, PhD, Head, Experimental Pathology Laboratory, NIH NCI, Center for Cancer Research; Jason Hipp, MD, PhD, Chief Digital Innovation Officer, Mayo Collaborate Services, Mayo Clinic; Brian Lein, MD, Assistant Director, Healthcare Administration, Defense Health Agency; Col Mark Lyman, MD, Pathology Consultant to the US Air Force Surgeon General; COL Joel Moncur, MD, Director, Joint Pathology Center; Ronald Poropatich, MD, Director of the Center for Military Medicine Research, Health Sciences; Professor of Medicine, University of Pittsburgh; David Shulkin, MD, Ninth U.S. Secretary of Veterans Affairs; Eliot Siegel, MD, Chief of Radiology and Nuclear Medicine, Veterans Affairs Maryland Healthcare System; Professor and Vice Chair, University of Maryland School of Medicine; CDR Jenny Smith, DO, Pathologist, US Naval Medical Center Portsmouth; Shandong Wu, PhD, Associate Professor, Departments of Radiology, Biomedical Informatics, and Bioengineering, Director of Center for Artificial Intelligence Innovation in Medical Imaging, University of Pittsburgh; LCDR Victoria Mahar MD, Pathologist, US Army.
Throughout the 1.5-day meeting, topics such as the integration of systems, the value of single vendor solutions vs multiple vendors, and the interconnectedness of radiology and pathology in health care were discussed. The speakers addressed the challenges of adopting digital pathology, including workflow improvement, quality control, and the generalizability of algorithms. The importance of collaboration, leadership, data analytics, compliance with clinical practice guidelines, and research and development efforts were stressed. The increasingly important role of artificial intelligence (AI) in digital pathology, its applications, and its benefits were also highlighted. Continuing education credits were offered to participants.
Overall, the meeting provided valuable insights into the advancements, challenges, and potential of digital pathology, AI, and technology integration in the federal health care ecosystem. However, this cannot be achieved without leadership from and close collaboration between key industry, academic, and government stakeholders.
Uses of Digital Pathology
Digital pathology refers to the practice of digitizing glass slides containing tissue samples and using digital imaging technology to analyze and interpret them. It involves capturing high-resolution images of microscopic slides and storing them in a digital format. These digitized images can be accessed and analyzed using computer-based tools and software.
While traditional pathology involves examining tissue samples under a microscope to make diagnoses and provide insights into diseases and conditions, digital pathology uses digital scanners that capture all relevant tissue on the glass slide at high magnification. This process generates a high-fidelity digital representation of the tissue sample that can be navigated akin to how glass slides are reviewed on a brightfield microscope in current practice (eg, panning, zooming, etc). Microscopic review of patient specimens in pathology allows for identifying patterns and markers that may not be easily detectable with manual examination alone.
The digitized slides can be stored in a database or a slide management system, allowing pathologists and other healthcare professionals to access and review them remotely, thus creating the potential to improve collaboration among pathologists, facilitate second opinions, and enable easier access to archived slides for research purposes.
Potential Benefits
Digital pathology also opens the door to advanced image analysis techniques, such as computer-aided diagnosis, machine learning, and AI algorithms, with the potential for the following outcomes and benefits:
- Improved accuracy AI algorithms can analyze large volumes of digital pathology data with great precision, reducing the chances of human error and subjective interpretation. This can lead to more accurate and consistent diagnoses, especially in challenging cases where subtle patterns or features may be difficult to detect.
- Automated detection and classification AI algorithms can be trained to detect and classify specific features or abnormalities in digital pathology images. For example, AI models can identify cancerous cells, tissue patterns associated with different diseases, or specific biomarkers. This can assist pathologists in diagnosing diseases more accurately and efficiently.
- Quantitative analysis AI can analyze large quantities of digital pathology data and extract quantitative measurements. For instance, it can calculate the percentage of tumor cells in a sample, assess the density of immune cells, or measure the extent of tissue damage. These objective measurements can aid in prognosis prediction and treatment planning.
- Image segmentation AI algorithms can segment digital pathology images into different regions or structures, such as nuclei, cytoplasm, or blood vessels. This segmentation allows for precise analysis and extraction of features for further study. It can also facilitate the identification of specific cell types or tissue components.
- Image enhancement AI techniques can enhance the quality of digital pathology images by improving clarity and reducing noise or artifacts. This can help pathologists visualize and interpret slides more effectively, especially in challenging cases with low-quality or complex images.
- Decision support systems AI-powered decision support systems can assist pathologists by providing recommendations or second opinions based on the analysis of digital pathology data. These systems can offer insights, suggest potential diagnoses, or provide relevant research references, augmenting the pathologist’s expertise and improving diagnostic accuracy.
- Collaboration and second opinions Digital pathology, combined with AI, facilitates remote access to digitized slides, enabling pathologists to seek second opinions or collaborate with experts from around the world. This can enhance the quality of diagnoses by leveraging the collective expertise of pathologists and fostering knowledge sharing.
- Education and training AI algorithms can be utilized in virtual microscopy platforms to create interactive and educational experiences. Pathology residents and students can learn from annotated cases, receive real-time feedback, and develop their skills in a digital environment.
- Research and discovery AI can assist in identifying patterns, correlations, and novel biomarkers in digital pathology data. By analyzing large datasets, AI algorithms can help uncover new insights, contribute to research advancements, and aid in the development of personalized medicine approaches.
- Predictive modeling AI can analyze vast amounts of digital pathology data, patient records, and outcomes to develop predictive models. These models can estimate disease progression, treatment response, or patient survival rates based on various factors. They can contribute to personalized medicine by assisting in treatment decisions and prognosis assessment.
It is important to note that while AI has shown promising results, it is not intended to replace human pathologists but to augment their capabilities. Overall, the combination of AI technology with the expertise of pathologists can lead to improved diagnosis, better patient care, and more efficient workflows in digital pathology.
Overuse of Hematocrit Testing After Elective General Surgery at a Veterans Affairs Medical Center
It is common practice to routinely measure postoperative hematocrit levels at US Department of Veterans Affairs (VA) hospitals for a wide range of elective general surgeries. While hematocrit measurement is a low-cost test, the high frequency with which these tests are performed may drastically increase overall costs.
Numerous studies have suggested that physicians overuse laboratory testing.1-10 Kohli and colleagues recommended that the routine practice of obtaining postoperative hematocrit tests following elective gynecologic surgery be abandoned.1 A similar recommendation was made by Olus and colleagues after studying uneventful, unplanned cesarean sections and by Wu and colleagues after investigating routine laboratory tests post total hip arthroplasty.2,3
To our knowledge, a study assessing routine postoperative hematocrit testing in elective general surgery has not yet been conducted. Many laboratory tests ordered in the perioperative period are not indicated, including complete blood count (CBC), electrolytes, and coagulation studies.4 Based on the results of these studies, we expected that the routine measurement of postoperative hematocrit levels after elective general surgeries at VA medical centers would not be cost effective. A PubMed search for articles published from 1990 to 2023 using the search terms “hematocrit,” “hemoglobin,” “general,” “surgery,” “routine,” and “cost” or “cost-effectiveness,” suggests that the clinical usefulness of postoperative hematocrit testing has not been well studied in the general surgery setting. The purpose of this study was to determine the clinical utility and associated cost of measuring routine postoperative hematocrit levels in order to generate a guide as to when the practice is warranted following common elective general surgery.
Although gynecologic textbooks may describe recommendations of routine hematocrit checking after elective gynecologic operations, one has difficulty finding the same recommendations in general surgery textbooks.1 However, it is common practice for surgical residents and attending surgeons to routinely order hematocrit on postoperative day-1 to ensure that the operation did not result in unsuspected anemia that then would need treatment (either with fluids or a blood transfusion). Many other surgeons rely on clinical factors such as tachycardia, oliguria, or hypotension to trigger a hematocrit (and other laboratory) tests. Our hypothesis is that the latter group has chosen the most cost-effective and prudent practice. One problem with checking the hematocrit routinely, as with any other screening test, is what to do with an abnormal result, assuming an asymptomatic patient? If the postoperative hematocrit is lower than expected given the estimated blood loss (EBL), what is one to do?
Methods
This retrospective case-control study conducted at the New Mexico VA Health Care System (NMVAHCS) in Albuquerque compared data for patients who received transfusion within 72 hours of elective surgeries vs patients who did not. Patients who underwent elective general surgery from January 2011 through December 2014 were included. An elective general surgery was defined as surgery performed following an outpatient preoperative anesthesia evaluation ≥ 30 days prior to operation. Patients who underwent emergency operations, and those with baseline anemia (preoperative hematocrit < 30%), and those transfused > 72 hours after their operation were excluded. The NMVAHCSInstitutional Review Board approved this study (No. 15-H184).
A detailed record review was conducted to collect data on demographics and other preoperative risk factors, including age, sex, body mass index (BMI), race and ethnicity, cardiac and pulmonary comorbidities, tobacco use, alcohol intake, diabetes, American Society of Anesthesiologists Physical Status Classification, metabolic equivalent of task, hematologic conditions, and renal disease.
For each procedure, we recorded the type of elective general surgery performed, the diagnosis/indication, pre- and postoperative hemoglobin/hematocrit, intraoperative EBL, length of operation, surgical wound class, length of hospital stay (LOS), intensive care unit (ICU) status, number of hematocrit tests, cardiovascular risk of operation (defined by anesthesia assessment), presence or absence of malignancy, preoperative platelet count, albumin level, preoperative prothrombin time/activated partial thromboplastin time (aPTT), international normalized ratio (INR), hemoglobin A1c, and incidence of transfusion. Signs and symptoms of anemia were recorded as present if the postoperative vital signs suggested low intravascular volume (pulse > 120 beats/minute, systolic blood pressure < 90 mm Hg, or vasoactive medication requirement [per anesthesia postoperative note]) or if the patient reported or exhibited symptoms of dizziness or fatigue or evidence of clinically apparent bleeding (ie, hematoma formation). Laboratory charges for hematocrit tests and CBC at the NMAVAHCS were used to assess cost.11
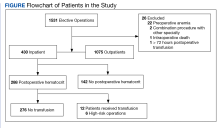
To stratify the transfusion risk, patients were distributed among 3 groups based on the following criteria: discharged home the same day as surgery; admitted but did not have postoperative hematocrit testing; and admitted and had postoperative hematocrit testing. We also stratified operations into low or high risk based on the risk for postoperative transfusion (Figure). Recognizing that the American College of Chest Physicians guidelines for perioperative management of antithrombotic therapy places bowel resection in a high-risk category, we designated a surgery as high risk when ≥ 2 patients in the transfusion group had that type of surgery over the 4 years of the study.12 Otherwise, the operations were deemed low risk.
Statistical Analysis
Numeric analysis used t tests and Binary and categorical variables used Fisher exact tests. P value ≤ .05 was considered statistically significant. SAS software was used for all statistical analyses.
Results
From 2011 through 2014, 1531 patients had elective general surgery at NMVAHCS. Twenty-two patients with preoperative anemia (hematocrit < 30%) and 1 patient who received a transfusion > 72 hours after the operation were excluded. Most elective operations (70%, n = 1075) were performed on an outpatient basis; none involved transfusion. Inguinal hernia repair was most common with 479 operations; 17 patients were treated inpatient of which 2 patients had routine postoperative hematocrit checks; (neither received transfusion). One patient with inguinal hernia surgery received transfusion without routine postoperative hematocrit monitoring.
Of 112 partial colon resections, 1 patient had a postoperative transfusion; and all but 3 received postoperative hematocrit monitoring. Nineteen patients undergoing partial colon resection had a clinical indication for postoperative hematocrit monitoring. None of the 5 patients with partial gastrectomy received a postoperative transfusion. Of 121 elective cholecystectomies, no patients had postoperative transfusion, whereas 34 had postoperative hematocrit monitoring; only 2 patients had a clinical reason for the hematocrit monitoring.
Of 430 elective inpatient operations, 12 received transfusions and 288 patients had ≥ 1 postoperative hematocrit test (67%). All hematocrit tests were requested by the attending surgeon, resident surgeon, or the surgical ICU team. Of the group that had postoperative hematocrit monitoring, there was an average of 4.4 postoperative hematocrit tests per patient (range, 1-44).
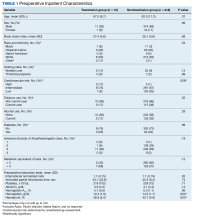
There were 12 transfusions for inpatients (2.8%), which is similar to the findings of a recent study of VA general surgery (2.3%).13 Five of the 12 patients received intraoperative transfusions while 7 were transfused within 72 hours postoperation. All but 1 patient receiving transfusion had EBL > 199 mL (range, 5-3000; mean, 950 mL; median, 500 mL) and/or signs or symptoms of anemia or other indications for measurement of the postoperative hematocrit. There were no statistically significant differences in patients’ age, sex, BMI, or race and ethnicity between groups receiving and not receiving transfusion (Table 1).
When comparing the transfusion vs the nontransfusion groups (after excluding those with clinical preoperative anemia) the risk factors for transfusion included: relatively low mean preoperative hematocrit (mean, 36.9% vs 42.7%, respectively; P = .003), low postoperative hematocrit (mean, 30.2% vs 37.1%, respectively; P < .001), high EBL (mean, 844 mL vs 109 mL, respectively; P = .005), large infusion of intraoperative fluids (mean, 4625 mL vs 2505 mL, respectively; P = .005), longer duration of operation (mean, 397 min vs 183 min, respectively; P < .001), and longer LOS (mean, 14.5 d vs 4.9 d, respectively; P < .001) (Table 2). Similarly, we found an increased risk for transfusion with high/intermediate cardiovascular risk (vs low), any wound not classified as clean, ICU stay, and postoperative symptoms of anemia.
We found no increased risk for transfusion with ethanol, tobacco, warfarin, or clopidogrel use; polycythemia; thrombocytopenia; preoperative INR; preoperative aPTT; preoperative albumin; Hemoglobin A1c; or diabetes mellitus; or for operations performed for malignancy. Ten patients in the ICU received transfusion (5.8%) compared with 2 patients (0.8%) not admitted to the ICU.
Operations were deemed high risk when ≥ 2 of patients having that operation received transfusions within 72 hours of their operation. There were 15 abdominoperineal resections; 3 of these received transfusions (20%). There were 7 total abdominal colectomies; 3 of these received transfusions (43%). We therefore had 22 high-risk operations, 6 of which were transfused (27%).
Discussion
Routine measurement of postoperative hematocrit levels after elective general surgery at NMVAHCS was not necessary. There were 12 transfusions for inpatients (2.8%), which is similar to the findings of a recent study of VA general surgery (2.3%).13 We found that routine postoperative hematocrit measurements to assess anemia had little or no effect on clinical decision-making or clinical outcomes.
According to our results, 88% of initial hematocrit tests after elective partial colectomies could have been eliminated; only 32 of 146 patients demonstrated a clinical reason for postoperative hematocrit testing. Similarly, 36 of 40 postcholecystectomy hematocrit tests (90%) could have been eliminated had the surgeons relied on clinical signs indicating possible postoperative anemia (none were transfused). Excluding patients with major intraoperative blood loss (> 300 mL), only 29 of 288 (10%) patients who had postoperative hematocrit tests had a clinical indication for a postoperative hematocrit test (ie, symptoms of anemia and/or active bleeding). One patient with inguinal hernia surgery who received transfusion was taking an anticoagulant and had a clinically indicated hematocrit test for a large hematoma that eventually required reoperation.
Our study found that routine hematocrit checks may actually increase the risk that a patient would receive an unnecessary transfusion. For instance, one elderly patient, after a right colectomy, had 6 hematocrit levels while on a heparin drip and received transfusion despite being asymptomatic. His lowest hematocrit level prior to transfusion was 23.7%. This patient had a total of 18 hematocrit tests. His EBL was 350 mL and his first postoperative HCT level was 33.1%. In another instance, a patient undergoing abdominoperineal resection had a transfusion on postoperative day 1, despite being hypertensive, with a hematocrit that ranged from 26% before transfusion to 31% after the transfusion. These 2 cases illustrate what has been shown in a recent study: A substantial number of patients with colorectal cancer receive unnecessary transfusions.14 On the other hand, one ileostomy closure patient had 33 hematocrit tests, yet his initial postoperative hematocrit was 37%, and he never received a transfusion. With low-risk surgeries, clinical judgment should dictate when a postoperative hematocrit level is needed. This strategy would have eliminated 206 unnecessary initial postoperative hematocrit tests (72%), could have decreased the number of unnecessary transfusions, and would have saved NMVAHCS about $1600 annually.
Abdominoperineal resections and total abdominal colectomies accounted for a high proportion of transfusions in our study. Inpatient elective operations can be risk stratified and have routine hematocrit tests ordered for patients at high risk. The probability of transfusion was greater in high-risk vs low-risk surgeries; 27% (6 of 22 patients) vs 2% (6 of 408 patients), respectively (P < .001). Since 14 of the 22 patients undergoing high-risk operation already had clinical reasons for a postoperative hematocrit test, we only need to add the remaining 8 patients with high-risk operations to the 74 who had a clinical reason for a hematocrit test and conclude that 82 of 430 patients (19%) had a clinical reason for a hematocrit test, either from signs or symptoms of blood loss or because they were in a high-risk group.
While our elective general surgery cases may not represent many general surgery programs in the US and VA health care systems, we can extrapolate cost savings using the same cost analyses outlined by Kohli and colleagues.1 Assuming 1.9 million elective inpatient general surgeries per year in the United States with an average cost of $21 per CBC, the annual cost of universal postoperative hematocrit testing would be $40 million.11,15 If postoperative hematocrit testing were 70% consistent with our findings, the annual cost for hematocrit tests on 51% of the inpatient general surgeries would be approximately $20.4 million. A reduction in routine hematocrit testing to 25% of all inpatient general surgeries (vs our finding that 19% were deemed necessary) results in an annual savings of $30 million. This conservative estimate could be even higher since there were 4.4 hematocrit tests per patient; therefore, we have about $132 million in savings.
Assuming 181,384 elective VA inpatient general surgeries each year, costing $7.14 per CBC (the NMVAHCS cost), the VA could save $1.3 million annually. If postoperative HCT testing were 70% consistent with our findings, the annual cost for hematocrit tests on 50.4% of inpatient general surgery operations would be about $653,000. A reduction in routine hematocrit testing to 25% of all inpatient general surgeries (vs our 19%) results in annual VA savings of $330,000. This conservative estimate could be even higher since there were on average 4.4 hematocrit levels per patient; therefore, we estimate that annual savings for the VA of about $1.45 million.
Limitations
The retrospective chart review nature of this study may have led to selection bias. Only a small number of patients received a transfusion, which may have skewed the data. This study population comes from a single VA medical center; this patient population may not be reflective of other VA medical centers or the US population as a whole. Given that NMVAHCS does not perform hepatic, esophageal, pancreas, or transplant operations, the potential savings to both the US and the VA may be overestimated, but this could be studied in the future by VA medical centers that perform more complex operations.
Conclusions
This study found that over a 4-year period routine postoperative hematocrit tests for patients undergoing elective general surgery at a VA medical center were not necessary. General surgeons routinely order various pre- and postoperative laboratory tests despite their limited utility. Reduction in unneeded routine tests could result in notable savings to the VA without compromising quality of care.
Only general surgery patients undergoing operations that carry a high risk for needing a blood transfusion should have a routine postoperative hematocrit testing. In our study population, the chance of an elective colectomy, cholecystectomy, or hernia patient needing a transfusion was rare. This strategy could eliminate a considerable number of unnecessary blood tests and would potentially yield significant savings.
1. Kohli N, Mallipeddi PK, Neff JM, Sze EH, Roat TW. Routine hematocrit after elective gynecologic surgery. Obstet Gynecol. 2000;95(6 Pt 1):847-850. doi:10.1016/s0029-7844(00)00796-1
2. Olus A, Orhan, U, Murat A, et al. Do asymptomatic patients require routine hemoglobin testing following uneventful, unplanned cesarean sections? Arch Gynecol Obstet. 2010;281(2):195-199. doi:10.1007/s00404-009-1093-1
3. Wu XD, Zhu ZL, Xiao P, Liu JC, Wang JW, Huang W. Are routine postoperative laboratory tests necessary after primary total hip arthroplasty? J Arthroplasty. 2020;35(10):2892-2898. doi:10.1016/j.arth.2020.04.097
4. Kumar A, Srivastava U. Role of routine laboratory investigations in preoperative evaluation. J Anesthesiol Clin Pharmacol. 2011;27(2):174-179. doi:10.4103/0970-9185.81824
5. Aghajanian A, Grimes DA. Routine prothrombin time determination before elective gynecologic operations. Obstet Gynecol. 1991;78(5 Pt 1):837-839.
6. Ransom SB, McNeeley SG, Malone JM Jr. A cost-effectiveness evaluation of preoperative type-and-screen testing for vaginal hysterectomy. Am J Obstet Gynecol. 1996;175(5):1201-1203. doi:10.1016/s0002-9378(96)70028-5
7. Ransom SB, McNeeley SG, Hosseini RB. Cost-effectiveness of routine blood type and screen testing before elective laparoscopy. Obstet Gynecol. 1995;86(3):346-348. doi:10.1016/0029-7844(95)00187-V
8. Committee on Standards and Practice Parameters, Apfelbaum JL, Connis RT, et al. Practice advisory for preanesthesia evaluation: an updated report by the American Society of Anesthesiologists Task Force on Preanesthesia Evaluation. Anesthesiology. 2012;116(3):522-538. doi:10.1097/ALN.0b013e31823c1067
9. Weil IA, Seicean S, Neuhauser D, Schiltz NK, Seicean A. Use and utility of hemostatic screening in adults undergoing elective, non-cardiac surgery. PLoS One. 2015;10(12):e0139139. doi:10.1371/journal.pone.0139139
10. Wu WC, Schifftner TL, Henderson WG, et al. Preoperative hematocrit levels and postoperative outcomes in older patients undergoing non-cardiac surgery. JAMA. 2007;297(22):2481-2488. doi:10.1001/jama.297.22.2481
11. Healthcare Bluebook. Complete blood count (CBC) with differential. Accessed March 28, 2024. https://www.healthcarebluebook.com/page_ProcedureDetails.aspx?id=214&dataset=lab
12. Douketis JD, Spyropoulos AC, Murad MH, et al. Perioperative management of antithrombotic therapy: an American College of Chest Physicians Clinical Practice Guideline. Chest. 2022;162(5):e207-e243. doi:10.1016/j.chest.2022.07.025
13. Randall JA, Wagner KT, Brody F. Perioperative transfusions in veterans following noncardiac procedures. J Laparoendosc Adv Surg Tech A. 2023;33(10):923-931. doi:10.1089/lap. 2023.0307
14. Tartter PI, Barron DM. Unnecessary blood transfusions in elective colorectal cancer surgery. Transfusion. 1985;25(2):113-115. doi:10.1046/j.1537-2995.1985.25285169199.x
15. Steiner CA, Karaca Z, Moore BJ, Imshaug MC, Pickens G. Surgeries in hospital-based ambulatory surgery and hospital inpatient settings, 2014. Healthcare Cost and Utilization Project statistical brief #223. May 2017. Revised July 2020. Agency for Healthcare Research and Quality. Accessed February 26, 2024. https://hcup-us.ahrq.gov/reports/statbriefs/sb223-Ambulatory-Inpatient-Surgeries-2014.pdf
16. US Department of Veterans Affairs, National Surgery Office. Quarterly report: Q3 of fiscal year 2017. VISN operative complexity summary [Source not verified].
It is common practice to routinely measure postoperative hematocrit levels at US Department of Veterans Affairs (VA) hospitals for a wide range of elective general surgeries. While hematocrit measurement is a low-cost test, the high frequency with which these tests are performed may drastically increase overall costs.
Numerous studies have suggested that physicians overuse laboratory testing.1-10 Kohli and colleagues recommended that the routine practice of obtaining postoperative hematocrit tests following elective gynecologic surgery be abandoned.1 A similar recommendation was made by Olus and colleagues after studying uneventful, unplanned cesarean sections and by Wu and colleagues after investigating routine laboratory tests post total hip arthroplasty.2,3
To our knowledge, a study assessing routine postoperative hematocrit testing in elective general surgery has not yet been conducted. Many laboratory tests ordered in the perioperative period are not indicated, including complete blood count (CBC), electrolytes, and coagulation studies.4 Based on the results of these studies, we expected that the routine measurement of postoperative hematocrit levels after elective general surgeries at VA medical centers would not be cost effective. A PubMed search for articles published from 1990 to 2023 using the search terms “hematocrit,” “hemoglobin,” “general,” “surgery,” “routine,” and “cost” or “cost-effectiveness,” suggests that the clinical usefulness of postoperative hematocrit testing has not been well studied in the general surgery setting. The purpose of this study was to determine the clinical utility and associated cost of measuring routine postoperative hematocrit levels in order to generate a guide as to when the practice is warranted following common elective general surgery.
Although gynecologic textbooks may describe recommendations of routine hematocrit checking after elective gynecologic operations, one has difficulty finding the same recommendations in general surgery textbooks.1 However, it is common practice for surgical residents and attending surgeons to routinely order hematocrit on postoperative day-1 to ensure that the operation did not result in unsuspected anemia that then would need treatment (either with fluids or a blood transfusion). Many other surgeons rely on clinical factors such as tachycardia, oliguria, or hypotension to trigger a hematocrit (and other laboratory) tests. Our hypothesis is that the latter group has chosen the most cost-effective and prudent practice. One problem with checking the hematocrit routinely, as with any other screening test, is what to do with an abnormal result, assuming an asymptomatic patient? If the postoperative hematocrit is lower than expected given the estimated blood loss (EBL), what is one to do?
Methods
This retrospective case-control study conducted at the New Mexico VA Health Care System (NMVAHCS) in Albuquerque compared data for patients who received transfusion within 72 hours of elective surgeries vs patients who did not. Patients who underwent elective general surgery from January 2011 through December 2014 were included. An elective general surgery was defined as surgery performed following an outpatient preoperative anesthesia evaluation ≥ 30 days prior to operation. Patients who underwent emergency operations, and those with baseline anemia (preoperative hematocrit < 30%), and those transfused > 72 hours after their operation were excluded. The NMVAHCSInstitutional Review Board approved this study (No. 15-H184).
A detailed record review was conducted to collect data on demographics and other preoperative risk factors, including age, sex, body mass index (BMI), race and ethnicity, cardiac and pulmonary comorbidities, tobacco use, alcohol intake, diabetes, American Society of Anesthesiologists Physical Status Classification, metabolic equivalent of task, hematologic conditions, and renal disease.
For each procedure, we recorded the type of elective general surgery performed, the diagnosis/indication, pre- and postoperative hemoglobin/hematocrit, intraoperative EBL, length of operation, surgical wound class, length of hospital stay (LOS), intensive care unit (ICU) status, number of hematocrit tests, cardiovascular risk of operation (defined by anesthesia assessment), presence or absence of malignancy, preoperative platelet count, albumin level, preoperative prothrombin time/activated partial thromboplastin time (aPTT), international normalized ratio (INR), hemoglobin A1c, and incidence of transfusion. Signs and symptoms of anemia were recorded as present if the postoperative vital signs suggested low intravascular volume (pulse > 120 beats/minute, systolic blood pressure < 90 mm Hg, or vasoactive medication requirement [per anesthesia postoperative note]) or if the patient reported or exhibited symptoms of dizziness or fatigue or evidence of clinically apparent bleeding (ie, hematoma formation). Laboratory charges for hematocrit tests and CBC at the NMAVAHCS were used to assess cost.11
To stratify the transfusion risk, patients were distributed among 3 groups based on the following criteria: discharged home the same day as surgery; admitted but did not have postoperative hematocrit testing; and admitted and had postoperative hematocrit testing. We also stratified operations into low or high risk based on the risk for postoperative transfusion (Figure). Recognizing that the American College of Chest Physicians guidelines for perioperative management of antithrombotic therapy places bowel resection in a high-risk category, we designated a surgery as high risk when ≥ 2 patients in the transfusion group had that type of surgery over the 4 years of the study.12 Otherwise, the operations were deemed low risk.
Statistical Analysis
Numeric analysis used t tests and Binary and categorical variables used Fisher exact tests. P value ≤ .05 was considered statistically significant. SAS software was used for all statistical analyses.
Results
From 2011 through 2014, 1531 patients had elective general surgery at NMVAHCS. Twenty-two patients with preoperative anemia (hematocrit < 30%) and 1 patient who received a transfusion > 72 hours after the operation were excluded. Most elective operations (70%, n = 1075) were performed on an outpatient basis; none involved transfusion. Inguinal hernia repair was most common with 479 operations; 17 patients were treated inpatient of which 2 patients had routine postoperative hematocrit checks; (neither received transfusion). One patient with inguinal hernia surgery received transfusion without routine postoperative hematocrit monitoring.
Of 112 partial colon resections, 1 patient had a postoperative transfusion; and all but 3 received postoperative hematocrit monitoring. Nineteen patients undergoing partial colon resection had a clinical indication for postoperative hematocrit monitoring. None of the 5 patients with partial gastrectomy received a postoperative transfusion. Of 121 elective cholecystectomies, no patients had postoperative transfusion, whereas 34 had postoperative hematocrit monitoring; only 2 patients had a clinical reason for the hematocrit monitoring.
Of 430 elective inpatient operations, 12 received transfusions and 288 patients had ≥ 1 postoperative hematocrit test (67%). All hematocrit tests were requested by the attending surgeon, resident surgeon, or the surgical ICU team. Of the group that had postoperative hematocrit monitoring, there was an average of 4.4 postoperative hematocrit tests per patient (range, 1-44).
There were 12 transfusions for inpatients (2.8%), which is similar to the findings of a recent study of VA general surgery (2.3%).13 Five of the 12 patients received intraoperative transfusions while 7 were transfused within 72 hours postoperation. All but 1 patient receiving transfusion had EBL > 199 mL (range, 5-3000; mean, 950 mL; median, 500 mL) and/or signs or symptoms of anemia or other indications for measurement of the postoperative hematocrit. There were no statistically significant differences in patients’ age, sex, BMI, or race and ethnicity between groups receiving and not receiving transfusion (Table 1).
When comparing the transfusion vs the nontransfusion groups (after excluding those with clinical preoperative anemia) the risk factors for transfusion included: relatively low mean preoperative hematocrit (mean, 36.9% vs 42.7%, respectively; P = .003), low postoperative hematocrit (mean, 30.2% vs 37.1%, respectively; P < .001), high EBL (mean, 844 mL vs 109 mL, respectively; P = .005), large infusion of intraoperative fluids (mean, 4625 mL vs 2505 mL, respectively; P = .005), longer duration of operation (mean, 397 min vs 183 min, respectively; P < .001), and longer LOS (mean, 14.5 d vs 4.9 d, respectively; P < .001) (Table 2). Similarly, we found an increased risk for transfusion with high/intermediate cardiovascular risk (vs low), any wound not classified as clean, ICU stay, and postoperative symptoms of anemia.
We found no increased risk for transfusion with ethanol, tobacco, warfarin, or clopidogrel use; polycythemia; thrombocytopenia; preoperative INR; preoperative aPTT; preoperative albumin; Hemoglobin A1c; or diabetes mellitus; or for operations performed for malignancy. Ten patients in the ICU received transfusion (5.8%) compared with 2 patients (0.8%) not admitted to the ICU.
Operations were deemed high risk when ≥ 2 of patients having that operation received transfusions within 72 hours of their operation. There were 15 abdominoperineal resections; 3 of these received transfusions (20%). There were 7 total abdominal colectomies; 3 of these received transfusions (43%). We therefore had 22 high-risk operations, 6 of which were transfused (27%).
Discussion
Routine measurement of postoperative hematocrit levels after elective general surgery at NMVAHCS was not necessary. There were 12 transfusions for inpatients (2.8%), which is similar to the findings of a recent study of VA general surgery (2.3%).13 We found that routine postoperative hematocrit measurements to assess anemia had little or no effect on clinical decision-making or clinical outcomes.
According to our results, 88% of initial hematocrit tests after elective partial colectomies could have been eliminated; only 32 of 146 patients demonstrated a clinical reason for postoperative hematocrit testing. Similarly, 36 of 40 postcholecystectomy hematocrit tests (90%) could have been eliminated had the surgeons relied on clinical signs indicating possible postoperative anemia (none were transfused). Excluding patients with major intraoperative blood loss (> 300 mL), only 29 of 288 (10%) patients who had postoperative hematocrit tests had a clinical indication for a postoperative hematocrit test (ie, symptoms of anemia and/or active bleeding). One patient with inguinal hernia surgery who received transfusion was taking an anticoagulant and had a clinically indicated hematocrit test for a large hematoma that eventually required reoperation.
Our study found that routine hematocrit checks may actually increase the risk that a patient would receive an unnecessary transfusion. For instance, one elderly patient, after a right colectomy, had 6 hematocrit levels while on a heparin drip and received transfusion despite being asymptomatic. His lowest hematocrit level prior to transfusion was 23.7%. This patient had a total of 18 hematocrit tests. His EBL was 350 mL and his first postoperative HCT level was 33.1%. In another instance, a patient undergoing abdominoperineal resection had a transfusion on postoperative day 1, despite being hypertensive, with a hematocrit that ranged from 26% before transfusion to 31% after the transfusion. These 2 cases illustrate what has been shown in a recent study: A substantial number of patients with colorectal cancer receive unnecessary transfusions.14 On the other hand, one ileostomy closure patient had 33 hematocrit tests, yet his initial postoperative hematocrit was 37%, and he never received a transfusion. With low-risk surgeries, clinical judgment should dictate when a postoperative hematocrit level is needed. This strategy would have eliminated 206 unnecessary initial postoperative hematocrit tests (72%), could have decreased the number of unnecessary transfusions, and would have saved NMVAHCS about $1600 annually.
Abdominoperineal resections and total abdominal colectomies accounted for a high proportion of transfusions in our study. Inpatient elective operations can be risk stratified and have routine hematocrit tests ordered for patients at high risk. The probability of transfusion was greater in high-risk vs low-risk surgeries; 27% (6 of 22 patients) vs 2% (6 of 408 patients), respectively (P < .001). Since 14 of the 22 patients undergoing high-risk operation already had clinical reasons for a postoperative hematocrit test, we only need to add the remaining 8 patients with high-risk operations to the 74 who had a clinical reason for a hematocrit test and conclude that 82 of 430 patients (19%) had a clinical reason for a hematocrit test, either from signs or symptoms of blood loss or because they were in a high-risk group.
While our elective general surgery cases may not represent many general surgery programs in the US and VA health care systems, we can extrapolate cost savings using the same cost analyses outlined by Kohli and colleagues.1 Assuming 1.9 million elective inpatient general surgeries per year in the United States with an average cost of $21 per CBC, the annual cost of universal postoperative hematocrit testing would be $40 million.11,15 If postoperative hematocrit testing were 70% consistent with our findings, the annual cost for hematocrit tests on 51% of the inpatient general surgeries would be approximately $20.4 million. A reduction in routine hematocrit testing to 25% of all inpatient general surgeries (vs our finding that 19% were deemed necessary) results in an annual savings of $30 million. This conservative estimate could be even higher since there were 4.4 hematocrit tests per patient; therefore, we have about $132 million in savings.
Assuming 181,384 elective VA inpatient general surgeries each year, costing $7.14 per CBC (the NMVAHCS cost), the VA could save $1.3 million annually. If postoperative HCT testing were 70% consistent with our findings, the annual cost for hematocrit tests on 50.4% of inpatient general surgery operations would be about $653,000. A reduction in routine hematocrit testing to 25% of all inpatient general surgeries (vs our 19%) results in annual VA savings of $330,000. This conservative estimate could be even higher since there were on average 4.4 hematocrit levels per patient; therefore, we estimate that annual savings for the VA of about $1.45 million.
Limitations
The retrospective chart review nature of this study may have led to selection bias. Only a small number of patients received a transfusion, which may have skewed the data. This study population comes from a single VA medical center; this patient population may not be reflective of other VA medical centers or the US population as a whole. Given that NMVAHCS does not perform hepatic, esophageal, pancreas, or transplant operations, the potential savings to both the US and the VA may be overestimated, but this could be studied in the future by VA medical centers that perform more complex operations.
Conclusions
This study found that over a 4-year period routine postoperative hematocrit tests for patients undergoing elective general surgery at a VA medical center were not necessary. General surgeons routinely order various pre- and postoperative laboratory tests despite their limited utility. Reduction in unneeded routine tests could result in notable savings to the VA without compromising quality of care.
Only general surgery patients undergoing operations that carry a high risk for needing a blood transfusion should have a routine postoperative hematocrit testing. In our study population, the chance of an elective colectomy, cholecystectomy, or hernia patient needing a transfusion was rare. This strategy could eliminate a considerable number of unnecessary blood tests and would potentially yield significant savings.
It is common practice to routinely measure postoperative hematocrit levels at US Department of Veterans Affairs (VA) hospitals for a wide range of elective general surgeries. While hematocrit measurement is a low-cost test, the high frequency with which these tests are performed may drastically increase overall costs.
Numerous studies have suggested that physicians overuse laboratory testing.1-10 Kohli and colleagues recommended that the routine practice of obtaining postoperative hematocrit tests following elective gynecologic surgery be abandoned.1 A similar recommendation was made by Olus and colleagues after studying uneventful, unplanned cesarean sections and by Wu and colleagues after investigating routine laboratory tests post total hip arthroplasty.2,3
To our knowledge, a study assessing routine postoperative hematocrit testing in elective general surgery has not yet been conducted. Many laboratory tests ordered in the perioperative period are not indicated, including complete blood count (CBC), electrolytes, and coagulation studies.4 Based on the results of these studies, we expected that the routine measurement of postoperative hematocrit levels after elective general surgeries at VA medical centers would not be cost effective. A PubMed search for articles published from 1990 to 2023 using the search terms “hematocrit,” “hemoglobin,” “general,” “surgery,” “routine,” and “cost” or “cost-effectiveness,” suggests that the clinical usefulness of postoperative hematocrit testing has not been well studied in the general surgery setting. The purpose of this study was to determine the clinical utility and associated cost of measuring routine postoperative hematocrit levels in order to generate a guide as to when the practice is warranted following common elective general surgery.
Although gynecologic textbooks may describe recommendations of routine hematocrit checking after elective gynecologic operations, one has difficulty finding the same recommendations in general surgery textbooks.1 However, it is common practice for surgical residents and attending surgeons to routinely order hematocrit on postoperative day-1 to ensure that the operation did not result in unsuspected anemia that then would need treatment (either with fluids or a blood transfusion). Many other surgeons rely on clinical factors such as tachycardia, oliguria, or hypotension to trigger a hematocrit (and other laboratory) tests. Our hypothesis is that the latter group has chosen the most cost-effective and prudent practice. One problem with checking the hematocrit routinely, as with any other screening test, is what to do with an abnormal result, assuming an asymptomatic patient? If the postoperative hematocrit is lower than expected given the estimated blood loss (EBL), what is one to do?
Methods
This retrospective case-control study conducted at the New Mexico VA Health Care System (NMVAHCS) in Albuquerque compared data for patients who received transfusion within 72 hours of elective surgeries vs patients who did not. Patients who underwent elective general surgery from January 2011 through December 2014 were included. An elective general surgery was defined as surgery performed following an outpatient preoperative anesthesia evaluation ≥ 30 days prior to operation. Patients who underwent emergency operations, and those with baseline anemia (preoperative hematocrit < 30%), and those transfused > 72 hours after their operation were excluded. The NMVAHCSInstitutional Review Board approved this study (No. 15-H184).
A detailed record review was conducted to collect data on demographics and other preoperative risk factors, including age, sex, body mass index (BMI), race and ethnicity, cardiac and pulmonary comorbidities, tobacco use, alcohol intake, diabetes, American Society of Anesthesiologists Physical Status Classification, metabolic equivalent of task, hematologic conditions, and renal disease.
For each procedure, we recorded the type of elective general surgery performed, the diagnosis/indication, pre- and postoperative hemoglobin/hematocrit, intraoperative EBL, length of operation, surgical wound class, length of hospital stay (LOS), intensive care unit (ICU) status, number of hematocrit tests, cardiovascular risk of operation (defined by anesthesia assessment), presence or absence of malignancy, preoperative platelet count, albumin level, preoperative prothrombin time/activated partial thromboplastin time (aPTT), international normalized ratio (INR), hemoglobin A1c, and incidence of transfusion. Signs and symptoms of anemia were recorded as present if the postoperative vital signs suggested low intravascular volume (pulse > 120 beats/minute, systolic blood pressure < 90 mm Hg, or vasoactive medication requirement [per anesthesia postoperative note]) or if the patient reported or exhibited symptoms of dizziness or fatigue or evidence of clinically apparent bleeding (ie, hematoma formation). Laboratory charges for hematocrit tests and CBC at the NMAVAHCS were used to assess cost.11
To stratify the transfusion risk, patients were distributed among 3 groups based on the following criteria: discharged home the same day as surgery; admitted but did not have postoperative hematocrit testing; and admitted and had postoperative hematocrit testing. We also stratified operations into low or high risk based on the risk for postoperative transfusion (Figure). Recognizing that the American College of Chest Physicians guidelines for perioperative management of antithrombotic therapy places bowel resection in a high-risk category, we designated a surgery as high risk when ≥ 2 patients in the transfusion group had that type of surgery over the 4 years of the study.12 Otherwise, the operations were deemed low risk.
Statistical Analysis
Numeric analysis used t tests and Binary and categorical variables used Fisher exact tests. P value ≤ .05 was considered statistically significant. SAS software was used for all statistical analyses.
Results
From 2011 through 2014, 1531 patients had elective general surgery at NMVAHCS. Twenty-two patients with preoperative anemia (hematocrit < 30%) and 1 patient who received a transfusion > 72 hours after the operation were excluded. Most elective operations (70%, n = 1075) were performed on an outpatient basis; none involved transfusion. Inguinal hernia repair was most common with 479 operations; 17 patients were treated inpatient of which 2 patients had routine postoperative hematocrit checks; (neither received transfusion). One patient with inguinal hernia surgery received transfusion without routine postoperative hematocrit monitoring.
Of 112 partial colon resections, 1 patient had a postoperative transfusion; and all but 3 received postoperative hematocrit monitoring. Nineteen patients undergoing partial colon resection had a clinical indication for postoperative hematocrit monitoring. None of the 5 patients with partial gastrectomy received a postoperative transfusion. Of 121 elective cholecystectomies, no patients had postoperative transfusion, whereas 34 had postoperative hematocrit monitoring; only 2 patients had a clinical reason for the hematocrit monitoring.
Of 430 elective inpatient operations, 12 received transfusions and 288 patients had ≥ 1 postoperative hematocrit test (67%). All hematocrit tests were requested by the attending surgeon, resident surgeon, or the surgical ICU team. Of the group that had postoperative hematocrit monitoring, there was an average of 4.4 postoperative hematocrit tests per patient (range, 1-44).
There were 12 transfusions for inpatients (2.8%), which is similar to the findings of a recent study of VA general surgery (2.3%).13 Five of the 12 patients received intraoperative transfusions while 7 were transfused within 72 hours postoperation. All but 1 patient receiving transfusion had EBL > 199 mL (range, 5-3000; mean, 950 mL; median, 500 mL) and/or signs or symptoms of anemia or other indications for measurement of the postoperative hematocrit. There were no statistically significant differences in patients’ age, sex, BMI, or race and ethnicity between groups receiving and not receiving transfusion (Table 1).
When comparing the transfusion vs the nontransfusion groups (after excluding those with clinical preoperative anemia) the risk factors for transfusion included: relatively low mean preoperative hematocrit (mean, 36.9% vs 42.7%, respectively; P = .003), low postoperative hematocrit (mean, 30.2% vs 37.1%, respectively; P < .001), high EBL (mean, 844 mL vs 109 mL, respectively; P = .005), large infusion of intraoperative fluids (mean, 4625 mL vs 2505 mL, respectively; P = .005), longer duration of operation (mean, 397 min vs 183 min, respectively; P < .001), and longer LOS (mean, 14.5 d vs 4.9 d, respectively; P < .001) (Table 2). Similarly, we found an increased risk for transfusion with high/intermediate cardiovascular risk (vs low), any wound not classified as clean, ICU stay, and postoperative symptoms of anemia.
We found no increased risk for transfusion with ethanol, tobacco, warfarin, or clopidogrel use; polycythemia; thrombocytopenia; preoperative INR; preoperative aPTT; preoperative albumin; Hemoglobin A1c; or diabetes mellitus; or for operations performed for malignancy. Ten patients in the ICU received transfusion (5.8%) compared with 2 patients (0.8%) not admitted to the ICU.
Operations were deemed high risk when ≥ 2 of patients having that operation received transfusions within 72 hours of their operation. There were 15 abdominoperineal resections; 3 of these received transfusions (20%). There were 7 total abdominal colectomies; 3 of these received transfusions (43%). We therefore had 22 high-risk operations, 6 of which were transfused (27%).
Discussion
Routine measurement of postoperative hematocrit levels after elective general surgery at NMVAHCS was not necessary. There were 12 transfusions for inpatients (2.8%), which is similar to the findings of a recent study of VA general surgery (2.3%).13 We found that routine postoperative hematocrit measurements to assess anemia had little or no effect on clinical decision-making or clinical outcomes.
According to our results, 88% of initial hematocrit tests after elective partial colectomies could have been eliminated; only 32 of 146 patients demonstrated a clinical reason for postoperative hematocrit testing. Similarly, 36 of 40 postcholecystectomy hematocrit tests (90%) could have been eliminated had the surgeons relied on clinical signs indicating possible postoperative anemia (none were transfused). Excluding patients with major intraoperative blood loss (> 300 mL), only 29 of 288 (10%) patients who had postoperative hematocrit tests had a clinical indication for a postoperative hematocrit test (ie, symptoms of anemia and/or active bleeding). One patient with inguinal hernia surgery who received transfusion was taking an anticoagulant and had a clinically indicated hematocrit test for a large hematoma that eventually required reoperation.
Our study found that routine hematocrit checks may actually increase the risk that a patient would receive an unnecessary transfusion. For instance, one elderly patient, after a right colectomy, had 6 hematocrit levels while on a heparin drip and received transfusion despite being asymptomatic. His lowest hematocrit level prior to transfusion was 23.7%. This patient had a total of 18 hematocrit tests. His EBL was 350 mL and his first postoperative HCT level was 33.1%. In another instance, a patient undergoing abdominoperineal resection had a transfusion on postoperative day 1, despite being hypertensive, with a hematocrit that ranged from 26% before transfusion to 31% after the transfusion. These 2 cases illustrate what has been shown in a recent study: A substantial number of patients with colorectal cancer receive unnecessary transfusions.14 On the other hand, one ileostomy closure patient had 33 hematocrit tests, yet his initial postoperative hematocrit was 37%, and he never received a transfusion. With low-risk surgeries, clinical judgment should dictate when a postoperative hematocrit level is needed. This strategy would have eliminated 206 unnecessary initial postoperative hematocrit tests (72%), could have decreased the number of unnecessary transfusions, and would have saved NMVAHCS about $1600 annually.
Abdominoperineal resections and total abdominal colectomies accounted for a high proportion of transfusions in our study. Inpatient elective operations can be risk stratified and have routine hematocrit tests ordered for patients at high risk. The probability of transfusion was greater in high-risk vs low-risk surgeries; 27% (6 of 22 patients) vs 2% (6 of 408 patients), respectively (P < .001). Since 14 of the 22 patients undergoing high-risk operation already had clinical reasons for a postoperative hematocrit test, we only need to add the remaining 8 patients with high-risk operations to the 74 who had a clinical reason for a hematocrit test and conclude that 82 of 430 patients (19%) had a clinical reason for a hematocrit test, either from signs or symptoms of blood loss or because they were in a high-risk group.
While our elective general surgery cases may not represent many general surgery programs in the US and VA health care systems, we can extrapolate cost savings using the same cost analyses outlined by Kohli and colleagues.1 Assuming 1.9 million elective inpatient general surgeries per year in the United States with an average cost of $21 per CBC, the annual cost of universal postoperative hematocrit testing would be $40 million.11,15 If postoperative hematocrit testing were 70% consistent with our findings, the annual cost for hematocrit tests on 51% of the inpatient general surgeries would be approximately $20.4 million. A reduction in routine hematocrit testing to 25% of all inpatient general surgeries (vs our finding that 19% were deemed necessary) results in an annual savings of $30 million. This conservative estimate could be even higher since there were 4.4 hematocrit tests per patient; therefore, we have about $132 million in savings.
Assuming 181,384 elective VA inpatient general surgeries each year, costing $7.14 per CBC (the NMVAHCS cost), the VA could save $1.3 million annually. If postoperative HCT testing were 70% consistent with our findings, the annual cost for hematocrit tests on 50.4% of inpatient general surgery operations would be about $653,000. A reduction in routine hematocrit testing to 25% of all inpatient general surgeries (vs our 19%) results in annual VA savings of $330,000. This conservative estimate could be even higher since there were on average 4.4 hematocrit levels per patient; therefore, we estimate that annual savings for the VA of about $1.45 million.
Limitations
The retrospective chart review nature of this study may have led to selection bias. Only a small number of patients received a transfusion, which may have skewed the data. This study population comes from a single VA medical center; this patient population may not be reflective of other VA medical centers or the US population as a whole. Given that NMVAHCS does not perform hepatic, esophageal, pancreas, or transplant operations, the potential savings to both the US and the VA may be overestimated, but this could be studied in the future by VA medical centers that perform more complex operations.
Conclusions
This study found that over a 4-year period routine postoperative hematocrit tests for patients undergoing elective general surgery at a VA medical center were not necessary. General surgeons routinely order various pre- and postoperative laboratory tests despite their limited utility. Reduction in unneeded routine tests could result in notable savings to the VA without compromising quality of care.
Only general surgery patients undergoing operations that carry a high risk for needing a blood transfusion should have a routine postoperative hematocrit testing. In our study population, the chance of an elective colectomy, cholecystectomy, or hernia patient needing a transfusion was rare. This strategy could eliminate a considerable number of unnecessary blood tests and would potentially yield significant savings.
1. Kohli N, Mallipeddi PK, Neff JM, Sze EH, Roat TW. Routine hematocrit after elective gynecologic surgery. Obstet Gynecol. 2000;95(6 Pt 1):847-850. doi:10.1016/s0029-7844(00)00796-1
2. Olus A, Orhan, U, Murat A, et al. Do asymptomatic patients require routine hemoglobin testing following uneventful, unplanned cesarean sections? Arch Gynecol Obstet. 2010;281(2):195-199. doi:10.1007/s00404-009-1093-1
3. Wu XD, Zhu ZL, Xiao P, Liu JC, Wang JW, Huang W. Are routine postoperative laboratory tests necessary after primary total hip arthroplasty? J Arthroplasty. 2020;35(10):2892-2898. doi:10.1016/j.arth.2020.04.097
4. Kumar A, Srivastava U. Role of routine laboratory investigations in preoperative evaluation. J Anesthesiol Clin Pharmacol. 2011;27(2):174-179. doi:10.4103/0970-9185.81824
5. Aghajanian A, Grimes DA. Routine prothrombin time determination before elective gynecologic operations. Obstet Gynecol. 1991;78(5 Pt 1):837-839.
6. Ransom SB, McNeeley SG, Malone JM Jr. A cost-effectiveness evaluation of preoperative type-and-screen testing for vaginal hysterectomy. Am J Obstet Gynecol. 1996;175(5):1201-1203. doi:10.1016/s0002-9378(96)70028-5
7. Ransom SB, McNeeley SG, Hosseini RB. Cost-effectiveness of routine blood type and screen testing before elective laparoscopy. Obstet Gynecol. 1995;86(3):346-348. doi:10.1016/0029-7844(95)00187-V
8. Committee on Standards and Practice Parameters, Apfelbaum JL, Connis RT, et al. Practice advisory for preanesthesia evaluation: an updated report by the American Society of Anesthesiologists Task Force on Preanesthesia Evaluation. Anesthesiology. 2012;116(3):522-538. doi:10.1097/ALN.0b013e31823c1067
9. Weil IA, Seicean S, Neuhauser D, Schiltz NK, Seicean A. Use and utility of hemostatic screening in adults undergoing elective, non-cardiac surgery. PLoS One. 2015;10(12):e0139139. doi:10.1371/journal.pone.0139139
10. Wu WC, Schifftner TL, Henderson WG, et al. Preoperative hematocrit levels and postoperative outcomes in older patients undergoing non-cardiac surgery. JAMA. 2007;297(22):2481-2488. doi:10.1001/jama.297.22.2481
11. Healthcare Bluebook. Complete blood count (CBC) with differential. Accessed March 28, 2024. https://www.healthcarebluebook.com/page_ProcedureDetails.aspx?id=214&dataset=lab
12. Douketis JD, Spyropoulos AC, Murad MH, et al. Perioperative management of antithrombotic therapy: an American College of Chest Physicians Clinical Practice Guideline. Chest. 2022;162(5):e207-e243. doi:10.1016/j.chest.2022.07.025
13. Randall JA, Wagner KT, Brody F. Perioperative transfusions in veterans following noncardiac procedures. J Laparoendosc Adv Surg Tech A. 2023;33(10):923-931. doi:10.1089/lap. 2023.0307
14. Tartter PI, Barron DM. Unnecessary blood transfusions in elective colorectal cancer surgery. Transfusion. 1985;25(2):113-115. doi:10.1046/j.1537-2995.1985.25285169199.x
15. Steiner CA, Karaca Z, Moore BJ, Imshaug MC, Pickens G. Surgeries in hospital-based ambulatory surgery and hospital inpatient settings, 2014. Healthcare Cost and Utilization Project statistical brief #223. May 2017. Revised July 2020. Agency for Healthcare Research and Quality. Accessed February 26, 2024. https://hcup-us.ahrq.gov/reports/statbriefs/sb223-Ambulatory-Inpatient-Surgeries-2014.pdf
16. US Department of Veterans Affairs, National Surgery Office. Quarterly report: Q3 of fiscal year 2017. VISN operative complexity summary [Source not verified].
1. Kohli N, Mallipeddi PK, Neff JM, Sze EH, Roat TW. Routine hematocrit after elective gynecologic surgery. Obstet Gynecol. 2000;95(6 Pt 1):847-850. doi:10.1016/s0029-7844(00)00796-1
2. Olus A, Orhan, U, Murat A, et al. Do asymptomatic patients require routine hemoglobin testing following uneventful, unplanned cesarean sections? Arch Gynecol Obstet. 2010;281(2):195-199. doi:10.1007/s00404-009-1093-1
3. Wu XD, Zhu ZL, Xiao P, Liu JC, Wang JW, Huang W. Are routine postoperative laboratory tests necessary after primary total hip arthroplasty? J Arthroplasty. 2020;35(10):2892-2898. doi:10.1016/j.arth.2020.04.097
4. Kumar A, Srivastava U. Role of routine laboratory investigations in preoperative evaluation. J Anesthesiol Clin Pharmacol. 2011;27(2):174-179. doi:10.4103/0970-9185.81824
5. Aghajanian A, Grimes DA. Routine prothrombin time determination before elective gynecologic operations. Obstet Gynecol. 1991;78(5 Pt 1):837-839.
6. Ransom SB, McNeeley SG, Malone JM Jr. A cost-effectiveness evaluation of preoperative type-and-screen testing for vaginal hysterectomy. Am J Obstet Gynecol. 1996;175(5):1201-1203. doi:10.1016/s0002-9378(96)70028-5
7. Ransom SB, McNeeley SG, Hosseini RB. Cost-effectiveness of routine blood type and screen testing before elective laparoscopy. Obstet Gynecol. 1995;86(3):346-348. doi:10.1016/0029-7844(95)00187-V
8. Committee on Standards and Practice Parameters, Apfelbaum JL, Connis RT, et al. Practice advisory for preanesthesia evaluation: an updated report by the American Society of Anesthesiologists Task Force on Preanesthesia Evaluation. Anesthesiology. 2012;116(3):522-538. doi:10.1097/ALN.0b013e31823c1067
9. Weil IA, Seicean S, Neuhauser D, Schiltz NK, Seicean A. Use and utility of hemostatic screening in adults undergoing elective, non-cardiac surgery. PLoS One. 2015;10(12):e0139139. doi:10.1371/journal.pone.0139139
10. Wu WC, Schifftner TL, Henderson WG, et al. Preoperative hematocrit levels and postoperative outcomes in older patients undergoing non-cardiac surgery. JAMA. 2007;297(22):2481-2488. doi:10.1001/jama.297.22.2481
11. Healthcare Bluebook. Complete blood count (CBC) with differential. Accessed March 28, 2024. https://www.healthcarebluebook.com/page_ProcedureDetails.aspx?id=214&dataset=lab
12. Douketis JD, Spyropoulos AC, Murad MH, et al. Perioperative management of antithrombotic therapy: an American College of Chest Physicians Clinical Practice Guideline. Chest. 2022;162(5):e207-e243. doi:10.1016/j.chest.2022.07.025
13. Randall JA, Wagner KT, Brody F. Perioperative transfusions in veterans following noncardiac procedures. J Laparoendosc Adv Surg Tech A. 2023;33(10):923-931. doi:10.1089/lap. 2023.0307
14. Tartter PI, Barron DM. Unnecessary blood transfusions in elective colorectal cancer surgery. Transfusion. 1985;25(2):113-115. doi:10.1046/j.1537-2995.1985.25285169199.x
15. Steiner CA, Karaca Z, Moore BJ, Imshaug MC, Pickens G. Surgeries in hospital-based ambulatory surgery and hospital inpatient settings, 2014. Healthcare Cost and Utilization Project statistical brief #223. May 2017. Revised July 2020. Agency for Healthcare Research and Quality. Accessed February 26, 2024. https://hcup-us.ahrq.gov/reports/statbriefs/sb223-Ambulatory-Inpatient-Surgeries-2014.pdf
16. US Department of Veterans Affairs, National Surgery Office. Quarterly report: Q3 of fiscal year 2017. VISN operative complexity summary [Source not verified].
Graduate Medical Education Financing in the US Department of Veterans Affairs
The US Department of Veterans Affairs (VA) has partnered with academic medical centers and programs since 1946 to provide clinical training for physician residents. Ranking second in federal graduate medical education (GME) funding to the Centers for Medicare and Medicaid Services (CMS), the $850 million VA GME budget annually reimburses > 250 GME-sponsoring institutions (affiliates) of 8000 GME programs for the clinical training of 49,000 individual residents rotating through > 11,000 full-time equivalent (FTE) positions.1 The VA also distributes $1.6 billion to VA facilities to offset the costs of conducting health professions education (HPE) (eg, facility infrastructure, salary support for VA instructors and preceptors, education office administration, and instructional equipment).2 The VA financial and educational contributions account for payment of 11% of resident positions nationally and allow academic medical centers to be less reliant on CMS GME funding.3,4 The VA contributions also provide opportunities for GME expansion,1,5,6 educational innovations,5,7 interprofessional and team-based care,8,9 and quality and safety training.10,11 The Table provides a comparison of CMS and VA GME reimbursability based on activity.
GME financing is complex, particularly the formulaic approach used by CMS, the details of which are often obscured in federal regulations. Due to this complexity and the $16 billion CMS GME budget, academic publications have focused on CMS GME financing while not fully explaining the VA GME policies and processes.4,12-14 By comparison, the VA GME financing model is relatively straightforward and governed by different statues and VA regulations, yet sharing some of the same principles as CMS regulations. Given the challenges in CMS reimbursement to fully support the cost of resident education, as well as the educational opportunities at the VA, the VA designs its reimbursement model to assure that affiliates receive appropriate payments.4,12,15 To ensure the continued success of VA GME partnerships, knowledge of VA GME financing has become increasingly important for designated institutional officers (DIOs) and residency program directors, particularly in light of recent investigations into oversight of the VA’s reimbursement to academic affiliates.
VA AUTHORITY
While the VA’s primary mission is “to provide a complete hospital medical service for the medical care and treatment of veterans,” early VA leaders recognized the importance of affiliating with the nation’s academic institutions.19 In 1946, the VA Policy Memorandum Number 2 established a partnership between the VA and the academic medical community.20 Additional legislation authorized specific agreements with academic affiliates for the central administration of salary and benefits for residents rotating at VA facilities. This process, known as disbursement, is an alternative payroll mechanism whereby the VA reimburses the academic affiliate for resident salary and benefits and the affiliate acts as the disbursing agent, issuing paychecks to residents.21,22
Resident FUNDING
By policy, with rare exceptions, the VA does not sponsor residency programs due to the challenges of providing an appropriate patient mix of age, sex, and medical conditions to meet accreditation standards.4 Nearly all VA reimbursements are for residents in affiliate-sponsored programs, while just 1% pays for residents in legacy, VA-sponsored residency programs at 2 VA facilities. The VA budget for resident (including fellows) salary and benefits is managed by the VA Office of Academic Affiliations (OAA), the national VA office responsible for oversight, policy, and funding of VA HPE programs.
Resident Salaries and Benefits
VA funding of resident salary and benefits are analogous with CMS direct GME (DGME), which is designed to cover resident salary and benefits costs.4,14,23 CMS DGME payments depend on a hospital’s volume of CMS inpatients and are based on a statutory formula, which uses the hospital’s resident FTE positions, the per-resident amount, and Medicare’s share of inpatient beds (Medicare patient load) to determine payments.12 The per-resident amount is set by statute, varies geographically, and is calculated by dividing the hospital’s allowable costs of GME (percentage of CMS inpatient days) divided by the number of residents.12,24
By comparison, the VA GME payment reimburses for each FTE based on the salary and benefits rate set by the academic affiliate. Reimbursement is calculated based on resident time spent at the VA multiplied by a daily salary rate. The daily salary rate is determined by dividing the resident’s total compensation (salary and benefits) by the number of calendar days in an academic year. Resident time spent at the VA facility is determined by obtaining rotation schedules provided by the academic affiliate and verifying resident clinical and educational activity during scheduled rotations.
Indirect Medical Education Funding
In addition to resident salary and benefits, funds to offset the cost of conducting HPE are provided to VA facilities. These funds are intended to improve and maintain necessary infrastructure for all HPE programs not just GME, including education office administration needs, teaching costs (ie, a portion of VA preceptors salary), and instructional equipment.
The Veterans Equitable Resource Allocation (VERA) is a national budgeting process for VA medical facilities that funds facility operational needs such as staff salary and benefits, infrastructure, and equipment.2 The education portion of the VERA, the VERA Education Support Component (VESC), is not managed by the OAA, but rather is distributed through the VERA model to the general budget of VA facilities hosting HPE (Figure). VESC funding in the VA budget is based on labor mapping of physician time spent in education; other labor mapping categories include clinical care, research, and administration. VA facility VESC funding is calculated based on the number of paid health profession trainees (HPTs) from all professions, apportioned according to the number of FTEs for physician residents and VA-paid HPTs in other disciplines. In fiscal year 2024, VA facilities received $115,812 for each physician resident FTE position and $84,906 for each VA-paid, non-GME FTE position.
The VESC is like CMS's indirect GME funding, termed Indirect Medical Education (IME), an additional payment for each Medicare patient discharged reflecting teaching hospitals’ higher patient care costs relative to nonteaching hospitals. Described elsewhere, IME is calculated using a resident-to-bed ratio and a multiplier, which is set by statute.4,25 While IME can be used for reimbursement for some resident clinical and educational activities(eg, research), VA VESC funds cannot be used for such activities and are part of the general facility budget and appropriated per the discretion of the medical facility director.
ESTABLISHING GME PARTNERSHIPS
An affiliation agreement establishes the administrative and legal requirements for educational relationships with academic affiliates and includes standards for conducting HPE, responsibilities for accreditation standards, program leadership, faculty, resources, supervision, academic policies, and procedures. The VA uses standardized affiliation agreement templates that have been vetted with accrediting bodies and the VA Office of General Counsel.
A disbursement agreement authorizes the VA to reimburse affiliates for resident salary and benefits for VA clinical and educational activities. The disbursement agreement details the fiscal arrangements (eg, payment in advance vs arrears, salary, and benefit rates, leave) for the reimbursement payments. Veterans Health Administration (VHA) Directive 1400.05 provides the policy and procedures for calculating reimbursement for HPT educational activities.26
The VA facility designated education officer (DEO) oversees all HPE programs and coordinates the affiliation and disbursement agreement processes.27 The DEO, affiliate DIO, residency program director, and VA residency site director determine the physician resident FTE positions assigned to a VA facility based on educational objectives and availability of educational resources at the VA facility, such as patient care opportunities, faculty supervisors, space, and equipment. The VA facility requests for resident FTE positions are submitted to the OAA by the facility DEO.
Once GME FTE positions are approved by the OAA, VA facilities work with their academic affiliate to submit the physician resident salary and benefit rate. Affiliate DIOs attest to the accuracy of the salary rate schedule and the local DEO submits the budget request to the OAA. Upon approval, the funds are transferred to the VA facility each fiscal year, which begins October 1. DEOs report quarterly to the OAA both budget needs and excesses based on variations in the approved FTEs due to additional VA rotations, physician resident attrition, or reassignment.
Resident Position Allocation
VA GME financing provides flexibility through periodic needs assessments and expansion initiatives. In August and December, DEOs collaborate with an academic affiliate to submit reports to the OAA confirming their projected GME needs for the next academic year. Additional positions requests are reviewed by the OAA; funding depends on budget and the educational justification. The OAA periodically issues GME expansion requests for proposal, which typically arise from legislation to address specific VA workforce needs. The VA facility DEO and affiliate GME leaders collaborate to apply for additional positions. For example, a VA GME expansion under the Veterans Access, Choice, and Accountability Act of 2014 added 1500 GME positions in 8 years for critically needed specialties and in rural and underserved areas.5 The Maintaining Internal Systems and Strengthening Outside Networks (MISSION) Act of 2018 authorized a pilot program for VA to fund residents at non-VA facilities with priority for Indian Health Services, Tribes and Tribal Organizations, Federally Qualified Health Centers, and US Department of Defense facilities to provide access to veterans in underserved areas.6
The VA GME financing system has flexibility to meet local needs for additional resident positions and to address broader VA workforce gaps through targeted expansion. Generally, CMS does not fund positions to address workforce needs, place residents in specific geographic areas, or require the training of certain types of residents.4 However, the Consolidated Appropriations Act of 2021 has provided the opportunity to address rural workforce needs.28
Reimbursement
The VA provides reimbursement for clinical and educational activities performed in VA facilities for the benefit of veterans as well as research, didactics, meetings and conferences, annual and sick leave, and orientation. The VA also may provide reimbursement for educational activities that occur off VA grounds (eg, the VA proportional share of a residency program’s didactic sessions). The VA does not reimburse for affiliate clinical duties or administrative costs, although a national policy allows VA facilities to reimburse affiliates for some GME overhead costs.29
CMS similarly reimburses for residency training time spent in patient care activities as well as orientation activities, didactics, leave, and, in some cases, research.4,30,31 CMS makes payments to hospitals, which may include sponsoring institutions and Medicare-eligible participating training sites.4,30,31 For both the VA and CMS, residents may not be counted twice for reimbursement by 2 federal agencies; in other words, a resident may not count for > 1 FTE.4,30-32
GME Oversight
VA GME funding came under significant scrutiny. At a 2016 House Veterans Affairs Committee hearing, Representative Phil Roe, MD (R-Tennessee), noted that no process existed at many VA facilities for “determining trainee presence” and that many VA medical centers had “difficulty tracking resident rotations”16 A VA Office of the Inspector General investigation recommended that the VA implement policies and procedures to improve oversight to “ensure residents are fully participating in educational activities” and that the VA is “paying the correct amount” to the affiliate.17 A 2020 General Accountability Office report outlined unclear policy guidance, incomplete tracking of resident activities, and improper fiscal processes for reimbursement and reconciliation of affiliate invoices.18
In response, the OAA created an oversight and compliance unit, revised VHA Directive 1400.05 (the policy for disbursement), and improved resident tracking procedures.26 The standard operating procedure that accompanied VHA Directive 1400.05 provides detailed information for the DEO and VA facility staff for tracking resident clinical and educational activities. FTE counts are essential to both VA and CMS for accurate reimbursement. The eAppendix and the Table provide a guide to reimbursable activities in the VA for the calculation of reimbursement, with a comparison to CMS.33,34 The OAA in cooperation with other VA staff and officers periodically conducts audits to assess compliance with disbursement policy and affiliate reimbursement accuracy.
In the VA, resident activities are captured on the VA Educational Activity Record, a standardized spreadsheet to track activities and calculate reimbursement. Each VA facility hosting resident physicians manually records resident activity by the half-day. This process is labor intensive, involving both VA and affiliate staff to accurately reconcile payments. To address the workload demands, the OAA is developing an online tool that will automate aspects of the tracking process. Also, to ensure adequate staffing, the OAA is in the process of implementing an office optimization project, providing standardized position descriptions, an organizational chart, and staffing levels for DEO offices in VA facilities.
Conclusions
This report describes the key policies and principles of VA GME financing, highlighting the essential similarities and differences between VA and CMS. Neither the VA nor CMS regulations allow for reimbursement for > 1 FTE position per resident, a principle that underpins the assignment of resident rotations and federal funding for GME and are similar with respect to reimbursement for patient care activities, didactics, research, orientation, and scholarly activity. While reimbursable activities in the VA require physical presence and care of veteran patients, CMS also limits reimbursement to resident activities in the hospital and approved other settings if the hospital is paying for resident salary and benefits in these settings. The VA provides some flexibility for offsite activities including didactics and, in specific circumstances, remote care of veteran patients (eg, teleradiology).
The VA and CMS use different GME financing models. For example, the CMS calculations for resident FTEs are complex, whereas VA calculations reimburse the salary and benefits as set by the academic affiliate. The VA process accounts for local variation in salary rates, whereas the per-resident amount set by CMS varies regionally and does not fully account for differences in the cost of living.24 Because all patients in VA facilities are veterans, VA calculations for reimbursement do not involve ratios of beds like the CMS calculations to determine a proportional share of reimbursement. The VA GME expansion tends to be more directed to VA health workforce needs than CMS, specifying the types of programs and geographic locations to address these needs.
The VA regularly reevaluates how affiliates are reimbursed for VA resident activity, balancing compliance with VA policies and the workload for VA and its affiliates. The VA obtains input from key stakeholders including DEOs, DIOs, and professional organizations such as the Association of American Medical Colleges and the Accreditation Council for Graduate Medical Education.35,36
Looking ahead, the VA is developing an online tool to improve the accuracy of affiliate reimbursement. The VA will also implement a standardized staffing model, organizational structure, and position descriptions for DEO offices. These initiatives will help reduce the burden of tracking and verifying resident activity and continue to support the 77-year partnership between VA and its affiliated institutions.
1. Klink KA, Albanese AP, Bope ET, Sanders KM. Veterans Affairs graduate medical education expansion addresses US physician workforce needs. Acad Med. 2022;97(8):1144-1150. doi:10.1097/ACM.0000000000004545
2. Andrus CH, Johnson K, Pierce E, Romito PJ, Hartel P, Berrios‐Guccione S, Best W. Finance modeling in the delivery of medical care in tertiary‐care hospitals in the Department of Veterans Affairs. J Surg Res. 2001;96(2):152-157. doi:10.1006/jsre.1999.5728
3. Petrakis IL, Kozal M. Academic medical centers and the U.S. Department of Veterans Affairs: a 75-year partnership influences medical education, scientific discovery, and clinical care. Acad Med. 2022;97(8):1110-1113. doi:10.1097/ACM.0000000000004734
4. Heisler EJ, Mendez BH, Mitchell A, Panangala SV, Villagrana MA. Federal support for graduate medical education: an overview (R44376). Congressional Research Service report R44376; version 11. Updated December 27, 2018. Accessed March 2, 2024. https://crsreports.congress.gov/product/pdf/R/R44376/11
5. Chang BK, Brannen JL. The Veterans Access, Choice, and Accountability Act of 2014: examining graduate medical education enhancement in the Department of Veterans Affairs. Acad Med. 2015;90(9):1196-1198. doi:10.1097/ACM.0000000000000795
6. Albanese AP, Bope ET, Sanders KM, Bowman M. The VA MISSION Act of 2018: a potential game changer for rural GME expansion and veteran health care. J Rural Health. 2020;36(1):133-136. doi:10.1111/jrh.12360
7. Lypson ML, Roberts LW. Valuing the partnership between the Veterans Health Administration and academic medicine. Acad Med. 2022;97(8):1091-1093. doi:10.1097/ACM.0000000000004748
8. Harada ND, Traylor L, Rugen KW, et al. Interprofessional transformation of clinical education: the first six years of the Veterans Affairs Centers of Excellence in Primary Care Education. J Interprof Care. 2023;37(suppl 1):S86-S94. doi:10.1080/13561820.2018.1433642

9. Harada ND, Rajashekara S, Sansgiry S, et al. Developing interprofessional primary care teams: alumni evaluation of the Department of Veterans Affairs Centers of Excellence in Primary Care Education Program. J Med Educ Curric Dev. 2019;6:2382120519875455. doi:10.1177/2382120519875455
10. Splaine ME, Ogrinc G, Gilman SC, et al. The Department of Veterans Affairs National Quality Scholars Fellowship Program: experience from 10 years of training quality scholars. Acad Med. 2009;84(12):1741-1748. doi:10.1097/ACM.0b013e3181bfdcef
11. Watts BV, Paull DE, Williams LC, Neily J, Hemphill RR, Brannen JL. Department of Veterans Affairs chief resident in quality and patient safety program: a model to spread change. Am J Med Qual. 2016;31(6):598-600. doi:10.1177/1062860616643403
12. He K, Whang E, Kristo G. Graduate medical education funding mechanisms, challenges, and solutions: a narrative review. Am J Surg. 2021;221(1):65-71. doi:10.1016/j.amjsurg.2020.06.007
13. Villagrana M. Medicare graduate medical education payments: an overview. Congressional Research Service report IF10960. Updated September 29, 2022. Accessed March 2, 2024. https://crsreports.congress.gov/product/pdf/IF/IF10960
14. Committee on the Governance and Financing of Graduate Medical Education; Board on Health Care Services; Institute of Medicine. Graduate Medical Education That Meets the Nation’s Health Needs. Eden J, Berwick DM, Wilensky GR, eds. Washington, DC: National Academies Press; 2014. doi:10.17226/18754
15. Physician workforce: caps on Medicare-funded graduate medical education at teaching hospitals. Report to congressional requesters. GAO-21-391. May 21, 2021. Accessed March 1, 2024. https://www.gao.gov/assets/gao-21-391.pdf
16. VA and Academic Affiliates: Who Benefits? Hearing Before the Subcommittee on Oversight and Investigations of the Committee on Veterans’ Affairs, 114th Cong, 2nd Sess (2016). Accessed March 1, 2024. https://www.govinfo.gov/content/pkg/CHRG-115hhrg29685/html/CHRG-115hhrg29685.htm
17. US Department of Veterans Affairs, Office of Inspector General (OIG). Veterans Health Administration. Review of resident and part-time physician time and attendance at the Oklahoma City VA Health Care System. OIG report 17-00253-93. March 28, 2018. Accessed March 1, 2024. https://www.oversight.gov/sites/default/files/oig-reports/VAOIG-17-00253-93.pdf
18. VA health care: actions needed to improve oversight of graduate medical education reimbursement. Report to the ranking member, Committee on Veterans’ Affairs, House of Representatives. GAO-20-553. July 2020. Accessed March 1, 2024. https://www.gao.gov/assets/710/708275.pdf
19. Functions of Veterans Health Administration: in general, 38 USC §7301 (2022). Accessed March 1, 2024. https://www.govinfo.gov/content/pkg/USCODE-2022-title38/pdf/USCODE-2022-title38-partV-chap73-subchapI-sec7301.pdf
20. US Department of Veterans Affairs. Policy memorandum no. 2, policy in association of veterans’ hospitals with medical schools. January 30, 1946.
21. Veterans Health Care Expansion Act of 1973, Public Law 93-82. August 2, 1973. Accessed March 1, 2024. https://www.govinfo.gov/content/pkg/STATUTE-87/pdf/STATUTE-87-Pg179.pdf
22. Residencies and internships, 38 USC § 7406 (2022). Accessed March 1, 2024. https://www.govinfo.gov/content/pkg/USCODE-2022-title38/pdf/USCODE-2022-title38-partV-chap74-subchapI-sec7406.pdf
23. Direct graduate medical education (DGME). Centers for Medicaid and Medicare Services. Updated December 5, 2023. Accessed March 1, 2024. https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/AcuteInpatientPPS/DGME
24. Drezdzon MK, Cowley NJ, Sweeney DP, et al. Going for broke: the impact of cost of living on surgery resident stipend value. Ann Surg. 2023;278(6):1053-1059. doi:10.1097/SLA.0000000000005923
25. Special treatment: hospitals that incur indirect costs for graduate medical education programs, 42 CFR § 412.105 (2023). Accessed March 1, 2024. https://www.govinfo.gov/content/pkg/CFR-2023-title42-vol2/pdf/CFR-2023-title42-vol2-sec412-105.pdf
26. US Department of Veterans Affairs, Veterans Health Administration. VHA Directive 1400.05, Disbursement agreements for health professions trainees appointed under 38 U.S.C. § 7406. June 2, 2021. Accessed March 1, 2024. https://www.va.gov/vhapublications/ViewPublication.asp?pub_ID=9293
27. Harada ND, Sanders KM, Bowman MA. Health systems education leadership: learning from the VA designated education officer role. Fed Pract. 2022;39(6):266-273. doi:10.12788/fp.0278
28. Schleiter Hitchell K, Johnson L. CMS finalizes rules for distribution of 1000 new Medicare-funded residency positions and changes to rural training track programs. J Grad Med Educ. 2022;14(2):245-249. doi:10.4300/JGME-D-22-00193.1

29. US Department of Veterans Affairs, Veterans Health Administration. VHA Directive 1400.10, Educational cost contracts for health professions education. September 25, 2023. Accessed March 1, 2024. https://www.va.gov/VHAPUBLICATIONS/ViewPublication.asp?pub_ID=11480
30. Direct GME payments: general requirements, 42 CFR § 413.75 (2023). Accessed March 1, 2024. https://www.govinfo.gov/content/pkg/CFR-2023-title42-vol2/pdf/CFR-2023-title42-vol2-sec413-75.pdf
31. Direct GME payments: determination of the total number of FTE residents, 42 CFR § 413.78 (2023). Accessed March 1, 2024. https://www.govinfo.gov/content/pkg/CFR-2023-title42-vol2/pdf/CFR-2023-title42-vol2-sec413-78.pdf
32. US Department of Health and Human Services, Centers for Medicare and Medicaid Services. Medicare financial management manual, chapter 8. Contractor procedures for provider audits. Accessed March 1, 2024. https://www.cms.gov/regulations-and-guidance/guidance/manuals/downloads/fin106c08.pdf
33. US Department of Health and Human Services, Office of Inspector General. CMS did not always ensure hospitals complied with Medicare reimbursement requirements for graduate medical education. OIG report A-02-17-01017. November 2018. Accessed March 1, 2024. https://oig.hhs.gov/oas/reports/region2/21701017.pdf
34. US Department of Health and Human Services, Centers for Medicare and Medicaid Services. Interns and Residents Information System (IRIS) XML format. Publication 100-20. Transmittal 11418. Change request 12724. May 19, 2022. Accessed March 1, 2024. https://www.hhs.gov/guidance/sites/default/files/hhs-guidance-documents/R11418OTN.pdf
35. Birnbaum AD, Byrne J, on behalf of the VA Office of Academic Affiliations. VHA Updates: Disbursement Policy and Education Cost Contracts. Presented at: American Association of Medical Colleges Webinar; June 2021. Accessed March 1, 2024. https://vimeo.com/644415670
36. Byrne JM, on behalf of the VA Office of Academic Affiliations. Disbursement procedures update for AY 23-24. Accessed March 1, 2024. https://www.va.gov/oaa/Videos/AffiliatePresentationDisbursementandEARsAY23-24.pptx
The US Department of Veterans Affairs (VA) has partnered with academic medical centers and programs since 1946 to provide clinical training for physician residents. Ranking second in federal graduate medical education (GME) funding to the Centers for Medicare and Medicaid Services (CMS), the $850 million VA GME budget annually reimburses > 250 GME-sponsoring institutions (affiliates) of 8000 GME programs for the clinical training of 49,000 individual residents rotating through > 11,000 full-time equivalent (FTE) positions.1 The VA also distributes $1.6 billion to VA facilities to offset the costs of conducting health professions education (HPE) (eg, facility infrastructure, salary support for VA instructors and preceptors, education office administration, and instructional equipment).2 The VA financial and educational contributions account for payment of 11% of resident positions nationally and allow academic medical centers to be less reliant on CMS GME funding.3,4 The VA contributions also provide opportunities for GME expansion,1,5,6 educational innovations,5,7 interprofessional and team-based care,8,9 and quality and safety training.10,11 The Table provides a comparison of CMS and VA GME reimbursability based on activity.
GME financing is complex, particularly the formulaic approach used by CMS, the details of which are often obscured in federal regulations. Due to this complexity and the $16 billion CMS GME budget, academic publications have focused on CMS GME financing while not fully explaining the VA GME policies and processes.4,12-14 By comparison, the VA GME financing model is relatively straightforward and governed by different statues and VA regulations, yet sharing some of the same principles as CMS regulations. Given the challenges in CMS reimbursement to fully support the cost of resident education, as well as the educational opportunities at the VA, the VA designs its reimbursement model to assure that affiliates receive appropriate payments.4,12,15 To ensure the continued success of VA GME partnerships, knowledge of VA GME financing has become increasingly important for designated institutional officers (DIOs) and residency program directors, particularly in light of recent investigations into oversight of the VA’s reimbursement to academic affiliates.
VA AUTHORITY
While the VA’s primary mission is “to provide a complete hospital medical service for the medical care and treatment of veterans,” early VA leaders recognized the importance of affiliating with the nation’s academic institutions.19 In 1946, the VA Policy Memorandum Number 2 established a partnership between the VA and the academic medical community.20 Additional legislation authorized specific agreements with academic affiliates for the central administration of salary and benefits for residents rotating at VA facilities. This process, known as disbursement, is an alternative payroll mechanism whereby the VA reimburses the academic affiliate for resident salary and benefits and the affiliate acts as the disbursing agent, issuing paychecks to residents.21,22
Resident FUNDING
By policy, with rare exceptions, the VA does not sponsor residency programs due to the challenges of providing an appropriate patient mix of age, sex, and medical conditions to meet accreditation standards.4 Nearly all VA reimbursements are for residents in affiliate-sponsored programs, while just 1% pays for residents in legacy, VA-sponsored residency programs at 2 VA facilities. The VA budget for resident (including fellows) salary and benefits is managed by the VA Office of Academic Affiliations (OAA), the national VA office responsible for oversight, policy, and funding of VA HPE programs.
Resident Salaries and Benefits
VA funding of resident salary and benefits are analogous with CMS direct GME (DGME), which is designed to cover resident salary and benefits costs.4,14,23 CMS DGME payments depend on a hospital’s volume of CMS inpatients and are based on a statutory formula, which uses the hospital’s resident FTE positions, the per-resident amount, and Medicare’s share of inpatient beds (Medicare patient load) to determine payments.12 The per-resident amount is set by statute, varies geographically, and is calculated by dividing the hospital’s allowable costs of GME (percentage of CMS inpatient days) divided by the number of residents.12,24
By comparison, the VA GME payment reimburses for each FTE based on the salary and benefits rate set by the academic affiliate. Reimbursement is calculated based on resident time spent at the VA multiplied by a daily salary rate. The daily salary rate is determined by dividing the resident’s total compensation (salary and benefits) by the number of calendar days in an academic year. Resident time spent at the VA facility is determined by obtaining rotation schedules provided by the academic affiliate and verifying resident clinical and educational activity during scheduled rotations.
Indirect Medical Education Funding
In addition to resident salary and benefits, funds to offset the cost of conducting HPE are provided to VA facilities. These funds are intended to improve and maintain necessary infrastructure for all HPE programs not just GME, including education office administration needs, teaching costs (ie, a portion of VA preceptors salary), and instructional equipment.
The Veterans Equitable Resource Allocation (VERA) is a national budgeting process for VA medical facilities that funds facility operational needs such as staff salary and benefits, infrastructure, and equipment.2 The education portion of the VERA, the VERA Education Support Component (VESC), is not managed by the OAA, but rather is distributed through the VERA model to the general budget of VA facilities hosting HPE (Figure). VESC funding in the VA budget is based on labor mapping of physician time spent in education; other labor mapping categories include clinical care, research, and administration. VA facility VESC funding is calculated based on the number of paid health profession trainees (HPTs) from all professions, apportioned according to the number of FTEs for physician residents and VA-paid HPTs in other disciplines. In fiscal year 2024, VA facilities received $115,812 for each physician resident FTE position and $84,906 for each VA-paid, non-GME FTE position.
The VESC is like CMS's indirect GME funding, termed Indirect Medical Education (IME), an additional payment for each Medicare patient discharged reflecting teaching hospitals’ higher patient care costs relative to nonteaching hospitals. Described elsewhere, IME is calculated using a resident-to-bed ratio and a multiplier, which is set by statute.4,25 While IME can be used for reimbursement for some resident clinical and educational activities(eg, research), VA VESC funds cannot be used for such activities and are part of the general facility budget and appropriated per the discretion of the medical facility director.
ESTABLISHING GME PARTNERSHIPS
An affiliation agreement establishes the administrative and legal requirements for educational relationships with academic affiliates and includes standards for conducting HPE, responsibilities for accreditation standards, program leadership, faculty, resources, supervision, academic policies, and procedures. The VA uses standardized affiliation agreement templates that have been vetted with accrediting bodies and the VA Office of General Counsel.
A disbursement agreement authorizes the VA to reimburse affiliates for resident salary and benefits for VA clinical and educational activities. The disbursement agreement details the fiscal arrangements (eg, payment in advance vs arrears, salary, and benefit rates, leave) for the reimbursement payments. Veterans Health Administration (VHA) Directive 1400.05 provides the policy and procedures for calculating reimbursement for HPT educational activities.26
The VA facility designated education officer (DEO) oversees all HPE programs and coordinates the affiliation and disbursement agreement processes.27 The DEO, affiliate DIO, residency program director, and VA residency site director determine the physician resident FTE positions assigned to a VA facility based on educational objectives and availability of educational resources at the VA facility, such as patient care opportunities, faculty supervisors, space, and equipment. The VA facility requests for resident FTE positions are submitted to the OAA by the facility DEO.
Once GME FTE positions are approved by the OAA, VA facilities work with their academic affiliate to submit the physician resident salary and benefit rate. Affiliate DIOs attest to the accuracy of the salary rate schedule and the local DEO submits the budget request to the OAA. Upon approval, the funds are transferred to the VA facility each fiscal year, which begins October 1. DEOs report quarterly to the OAA both budget needs and excesses based on variations in the approved FTEs due to additional VA rotations, physician resident attrition, or reassignment.
Resident Position Allocation
VA GME financing provides flexibility through periodic needs assessments and expansion initiatives. In August and December, DEOs collaborate with an academic affiliate to submit reports to the OAA confirming their projected GME needs for the next academic year. Additional positions requests are reviewed by the OAA; funding depends on budget and the educational justification. The OAA periodically issues GME expansion requests for proposal, which typically arise from legislation to address specific VA workforce needs. The VA facility DEO and affiliate GME leaders collaborate to apply for additional positions. For example, a VA GME expansion under the Veterans Access, Choice, and Accountability Act of 2014 added 1500 GME positions in 8 years for critically needed specialties and in rural and underserved areas.5 The Maintaining Internal Systems and Strengthening Outside Networks (MISSION) Act of 2018 authorized a pilot program for VA to fund residents at non-VA facilities with priority for Indian Health Services, Tribes and Tribal Organizations, Federally Qualified Health Centers, and US Department of Defense facilities to provide access to veterans in underserved areas.6
The VA GME financing system has flexibility to meet local needs for additional resident positions and to address broader VA workforce gaps through targeted expansion. Generally, CMS does not fund positions to address workforce needs, place residents in specific geographic areas, or require the training of certain types of residents.4 However, the Consolidated Appropriations Act of 2021 has provided the opportunity to address rural workforce needs.28
Reimbursement
The VA provides reimbursement for clinical and educational activities performed in VA facilities for the benefit of veterans as well as research, didactics, meetings and conferences, annual and sick leave, and orientation. The VA also may provide reimbursement for educational activities that occur off VA grounds (eg, the VA proportional share of a residency program’s didactic sessions). The VA does not reimburse for affiliate clinical duties or administrative costs, although a national policy allows VA facilities to reimburse affiliates for some GME overhead costs.29
CMS similarly reimburses for residency training time spent in patient care activities as well as orientation activities, didactics, leave, and, in some cases, research.4,30,31 CMS makes payments to hospitals, which may include sponsoring institutions and Medicare-eligible participating training sites.4,30,31 For both the VA and CMS, residents may not be counted twice for reimbursement by 2 federal agencies; in other words, a resident may not count for > 1 FTE.4,30-32
GME Oversight
VA GME funding came under significant scrutiny. At a 2016 House Veterans Affairs Committee hearing, Representative Phil Roe, MD (R-Tennessee), noted that no process existed at many VA facilities for “determining trainee presence” and that many VA medical centers had “difficulty tracking resident rotations”16 A VA Office of the Inspector General investigation recommended that the VA implement policies and procedures to improve oversight to “ensure residents are fully participating in educational activities” and that the VA is “paying the correct amount” to the affiliate.17 A 2020 General Accountability Office report outlined unclear policy guidance, incomplete tracking of resident activities, and improper fiscal processes for reimbursement and reconciliation of affiliate invoices.18
In response, the OAA created an oversight and compliance unit, revised VHA Directive 1400.05 (the policy for disbursement), and improved resident tracking procedures.26 The standard operating procedure that accompanied VHA Directive 1400.05 provides detailed information for the DEO and VA facility staff for tracking resident clinical and educational activities. FTE counts are essential to both VA and CMS for accurate reimbursement. The eAppendix and the Table provide a guide to reimbursable activities in the VA for the calculation of reimbursement, with a comparison to CMS.33,34 The OAA in cooperation with other VA staff and officers periodically conducts audits to assess compliance with disbursement policy and affiliate reimbursement accuracy.
In the VA, resident activities are captured on the VA Educational Activity Record, a standardized spreadsheet to track activities and calculate reimbursement. Each VA facility hosting resident physicians manually records resident activity by the half-day. This process is labor intensive, involving both VA and affiliate staff to accurately reconcile payments. To address the workload demands, the OAA is developing an online tool that will automate aspects of the tracking process. Also, to ensure adequate staffing, the OAA is in the process of implementing an office optimization project, providing standardized position descriptions, an organizational chart, and staffing levels for DEO offices in VA facilities.
Conclusions
This report describes the key policies and principles of VA GME financing, highlighting the essential similarities and differences between VA and CMS. Neither the VA nor CMS regulations allow for reimbursement for > 1 FTE position per resident, a principle that underpins the assignment of resident rotations and federal funding for GME and are similar with respect to reimbursement for patient care activities, didactics, research, orientation, and scholarly activity. While reimbursable activities in the VA require physical presence and care of veteran patients, CMS also limits reimbursement to resident activities in the hospital and approved other settings if the hospital is paying for resident salary and benefits in these settings. The VA provides some flexibility for offsite activities including didactics and, in specific circumstances, remote care of veteran patients (eg, teleradiology).
The VA and CMS use different GME financing models. For example, the CMS calculations for resident FTEs are complex, whereas VA calculations reimburse the salary and benefits as set by the academic affiliate. The VA process accounts for local variation in salary rates, whereas the per-resident amount set by CMS varies regionally and does not fully account for differences in the cost of living.24 Because all patients in VA facilities are veterans, VA calculations for reimbursement do not involve ratios of beds like the CMS calculations to determine a proportional share of reimbursement. The VA GME expansion tends to be more directed to VA health workforce needs than CMS, specifying the types of programs and geographic locations to address these needs.
The VA regularly reevaluates how affiliates are reimbursed for VA resident activity, balancing compliance with VA policies and the workload for VA and its affiliates. The VA obtains input from key stakeholders including DEOs, DIOs, and professional organizations such as the Association of American Medical Colleges and the Accreditation Council for Graduate Medical Education.35,36
Looking ahead, the VA is developing an online tool to improve the accuracy of affiliate reimbursement. The VA will also implement a standardized staffing model, organizational structure, and position descriptions for DEO offices. These initiatives will help reduce the burden of tracking and verifying resident activity and continue to support the 77-year partnership between VA and its affiliated institutions.
The US Department of Veterans Affairs (VA) has partnered with academic medical centers and programs since 1946 to provide clinical training for physician residents. Ranking second in federal graduate medical education (GME) funding to the Centers for Medicare and Medicaid Services (CMS), the $850 million VA GME budget annually reimburses > 250 GME-sponsoring institutions (affiliates) of 8000 GME programs for the clinical training of 49,000 individual residents rotating through > 11,000 full-time equivalent (FTE) positions.1 The VA also distributes $1.6 billion to VA facilities to offset the costs of conducting health professions education (HPE) (eg, facility infrastructure, salary support for VA instructors and preceptors, education office administration, and instructional equipment).2 The VA financial and educational contributions account for payment of 11% of resident positions nationally and allow academic medical centers to be less reliant on CMS GME funding.3,4 The VA contributions also provide opportunities for GME expansion,1,5,6 educational innovations,5,7 interprofessional and team-based care,8,9 and quality and safety training.10,11 The Table provides a comparison of CMS and VA GME reimbursability based on activity.
GME financing is complex, particularly the formulaic approach used by CMS, the details of which are often obscured in federal regulations. Due to this complexity and the $16 billion CMS GME budget, academic publications have focused on CMS GME financing while not fully explaining the VA GME policies and processes.4,12-14 By comparison, the VA GME financing model is relatively straightforward and governed by different statues and VA regulations, yet sharing some of the same principles as CMS regulations. Given the challenges in CMS reimbursement to fully support the cost of resident education, as well as the educational opportunities at the VA, the VA designs its reimbursement model to assure that affiliates receive appropriate payments.4,12,15 To ensure the continued success of VA GME partnerships, knowledge of VA GME financing has become increasingly important for designated institutional officers (DIOs) and residency program directors, particularly in light of recent investigations into oversight of the VA’s reimbursement to academic affiliates.
VA AUTHORITY
While the VA’s primary mission is “to provide a complete hospital medical service for the medical care and treatment of veterans,” early VA leaders recognized the importance of affiliating with the nation’s academic institutions.19 In 1946, the VA Policy Memorandum Number 2 established a partnership between the VA and the academic medical community.20 Additional legislation authorized specific agreements with academic affiliates for the central administration of salary and benefits for residents rotating at VA facilities. This process, known as disbursement, is an alternative payroll mechanism whereby the VA reimburses the academic affiliate for resident salary and benefits and the affiliate acts as the disbursing agent, issuing paychecks to residents.21,22
Resident FUNDING
By policy, with rare exceptions, the VA does not sponsor residency programs due to the challenges of providing an appropriate patient mix of age, sex, and medical conditions to meet accreditation standards.4 Nearly all VA reimbursements are for residents in affiliate-sponsored programs, while just 1% pays for residents in legacy, VA-sponsored residency programs at 2 VA facilities. The VA budget for resident (including fellows) salary and benefits is managed by the VA Office of Academic Affiliations (OAA), the national VA office responsible for oversight, policy, and funding of VA HPE programs.
Resident Salaries and Benefits
VA funding of resident salary and benefits are analogous with CMS direct GME (DGME), which is designed to cover resident salary and benefits costs.4,14,23 CMS DGME payments depend on a hospital’s volume of CMS inpatients and are based on a statutory formula, which uses the hospital’s resident FTE positions, the per-resident amount, and Medicare’s share of inpatient beds (Medicare patient load) to determine payments.12 The per-resident amount is set by statute, varies geographically, and is calculated by dividing the hospital’s allowable costs of GME (percentage of CMS inpatient days) divided by the number of residents.12,24
By comparison, the VA GME payment reimburses for each FTE based on the salary and benefits rate set by the academic affiliate. Reimbursement is calculated based on resident time spent at the VA multiplied by a daily salary rate. The daily salary rate is determined by dividing the resident’s total compensation (salary and benefits) by the number of calendar days in an academic year. Resident time spent at the VA facility is determined by obtaining rotation schedules provided by the academic affiliate and verifying resident clinical and educational activity during scheduled rotations.
Indirect Medical Education Funding
In addition to resident salary and benefits, funds to offset the cost of conducting HPE are provided to VA facilities. These funds are intended to improve and maintain necessary infrastructure for all HPE programs not just GME, including education office administration needs, teaching costs (ie, a portion of VA preceptors salary), and instructional equipment.
The Veterans Equitable Resource Allocation (VERA) is a national budgeting process for VA medical facilities that funds facility operational needs such as staff salary and benefits, infrastructure, and equipment.2 The education portion of the VERA, the VERA Education Support Component (VESC), is not managed by the OAA, but rather is distributed through the VERA model to the general budget of VA facilities hosting HPE (Figure). VESC funding in the VA budget is based on labor mapping of physician time spent in education; other labor mapping categories include clinical care, research, and administration. VA facility VESC funding is calculated based on the number of paid health profession trainees (HPTs) from all professions, apportioned according to the number of FTEs for physician residents and VA-paid HPTs in other disciplines. In fiscal year 2024, VA facilities received $115,812 for each physician resident FTE position and $84,906 for each VA-paid, non-GME FTE position.
The VESC is like CMS's indirect GME funding, termed Indirect Medical Education (IME), an additional payment for each Medicare patient discharged reflecting teaching hospitals’ higher patient care costs relative to nonteaching hospitals. Described elsewhere, IME is calculated using a resident-to-bed ratio and a multiplier, which is set by statute.4,25 While IME can be used for reimbursement for some resident clinical and educational activities(eg, research), VA VESC funds cannot be used for such activities and are part of the general facility budget and appropriated per the discretion of the medical facility director.
ESTABLISHING GME PARTNERSHIPS
An affiliation agreement establishes the administrative and legal requirements for educational relationships with academic affiliates and includes standards for conducting HPE, responsibilities for accreditation standards, program leadership, faculty, resources, supervision, academic policies, and procedures. The VA uses standardized affiliation agreement templates that have been vetted with accrediting bodies and the VA Office of General Counsel.
A disbursement agreement authorizes the VA to reimburse affiliates for resident salary and benefits for VA clinical and educational activities. The disbursement agreement details the fiscal arrangements (eg, payment in advance vs arrears, salary, and benefit rates, leave) for the reimbursement payments. Veterans Health Administration (VHA) Directive 1400.05 provides the policy and procedures for calculating reimbursement for HPT educational activities.26
The VA facility designated education officer (DEO) oversees all HPE programs and coordinates the affiliation and disbursement agreement processes.27 The DEO, affiliate DIO, residency program director, and VA residency site director determine the physician resident FTE positions assigned to a VA facility based on educational objectives and availability of educational resources at the VA facility, such as patient care opportunities, faculty supervisors, space, and equipment. The VA facility requests for resident FTE positions are submitted to the OAA by the facility DEO.
Once GME FTE positions are approved by the OAA, VA facilities work with their academic affiliate to submit the physician resident salary and benefit rate. Affiliate DIOs attest to the accuracy of the salary rate schedule and the local DEO submits the budget request to the OAA. Upon approval, the funds are transferred to the VA facility each fiscal year, which begins October 1. DEOs report quarterly to the OAA both budget needs and excesses based on variations in the approved FTEs due to additional VA rotations, physician resident attrition, or reassignment.
Resident Position Allocation
VA GME financing provides flexibility through periodic needs assessments and expansion initiatives. In August and December, DEOs collaborate with an academic affiliate to submit reports to the OAA confirming their projected GME needs for the next academic year. Additional positions requests are reviewed by the OAA; funding depends on budget and the educational justification. The OAA periodically issues GME expansion requests for proposal, which typically arise from legislation to address specific VA workforce needs. The VA facility DEO and affiliate GME leaders collaborate to apply for additional positions. For example, a VA GME expansion under the Veterans Access, Choice, and Accountability Act of 2014 added 1500 GME positions in 8 years for critically needed specialties and in rural and underserved areas.5 The Maintaining Internal Systems and Strengthening Outside Networks (MISSION) Act of 2018 authorized a pilot program for VA to fund residents at non-VA facilities with priority for Indian Health Services, Tribes and Tribal Organizations, Federally Qualified Health Centers, and US Department of Defense facilities to provide access to veterans in underserved areas.6
The VA GME financing system has flexibility to meet local needs for additional resident positions and to address broader VA workforce gaps through targeted expansion. Generally, CMS does not fund positions to address workforce needs, place residents in specific geographic areas, or require the training of certain types of residents.4 However, the Consolidated Appropriations Act of 2021 has provided the opportunity to address rural workforce needs.28
Reimbursement
The VA provides reimbursement for clinical and educational activities performed in VA facilities for the benefit of veterans as well as research, didactics, meetings and conferences, annual and sick leave, and orientation. The VA also may provide reimbursement for educational activities that occur off VA grounds (eg, the VA proportional share of a residency program’s didactic sessions). The VA does not reimburse for affiliate clinical duties or administrative costs, although a national policy allows VA facilities to reimburse affiliates for some GME overhead costs.29
CMS similarly reimburses for residency training time spent in patient care activities as well as orientation activities, didactics, leave, and, in some cases, research.4,30,31 CMS makes payments to hospitals, which may include sponsoring institutions and Medicare-eligible participating training sites.4,30,31 For both the VA and CMS, residents may not be counted twice for reimbursement by 2 federal agencies; in other words, a resident may not count for > 1 FTE.4,30-32
GME Oversight
VA GME funding came under significant scrutiny. At a 2016 House Veterans Affairs Committee hearing, Representative Phil Roe, MD (R-Tennessee), noted that no process existed at many VA facilities for “determining trainee presence” and that many VA medical centers had “difficulty tracking resident rotations”16 A VA Office of the Inspector General investigation recommended that the VA implement policies and procedures to improve oversight to “ensure residents are fully participating in educational activities” and that the VA is “paying the correct amount” to the affiliate.17 A 2020 General Accountability Office report outlined unclear policy guidance, incomplete tracking of resident activities, and improper fiscal processes for reimbursement and reconciliation of affiliate invoices.18
In response, the OAA created an oversight and compliance unit, revised VHA Directive 1400.05 (the policy for disbursement), and improved resident tracking procedures.26 The standard operating procedure that accompanied VHA Directive 1400.05 provides detailed information for the DEO and VA facility staff for tracking resident clinical and educational activities. FTE counts are essential to both VA and CMS for accurate reimbursement. The eAppendix and the Table provide a guide to reimbursable activities in the VA for the calculation of reimbursement, with a comparison to CMS.33,34 The OAA in cooperation with other VA staff and officers periodically conducts audits to assess compliance with disbursement policy and affiliate reimbursement accuracy.
In the VA, resident activities are captured on the VA Educational Activity Record, a standardized spreadsheet to track activities and calculate reimbursement. Each VA facility hosting resident physicians manually records resident activity by the half-day. This process is labor intensive, involving both VA and affiliate staff to accurately reconcile payments. To address the workload demands, the OAA is developing an online tool that will automate aspects of the tracking process. Also, to ensure adequate staffing, the OAA is in the process of implementing an office optimization project, providing standardized position descriptions, an organizational chart, and staffing levels for DEO offices in VA facilities.
Conclusions
This report describes the key policies and principles of VA GME financing, highlighting the essential similarities and differences between VA and CMS. Neither the VA nor CMS regulations allow for reimbursement for > 1 FTE position per resident, a principle that underpins the assignment of resident rotations and federal funding for GME and are similar with respect to reimbursement for patient care activities, didactics, research, orientation, and scholarly activity. While reimbursable activities in the VA require physical presence and care of veteran patients, CMS also limits reimbursement to resident activities in the hospital and approved other settings if the hospital is paying for resident salary and benefits in these settings. The VA provides some flexibility for offsite activities including didactics and, in specific circumstances, remote care of veteran patients (eg, teleradiology).
The VA and CMS use different GME financing models. For example, the CMS calculations for resident FTEs are complex, whereas VA calculations reimburse the salary and benefits as set by the academic affiliate. The VA process accounts for local variation in salary rates, whereas the per-resident amount set by CMS varies regionally and does not fully account for differences in the cost of living.24 Because all patients in VA facilities are veterans, VA calculations for reimbursement do not involve ratios of beds like the CMS calculations to determine a proportional share of reimbursement. The VA GME expansion tends to be more directed to VA health workforce needs than CMS, specifying the types of programs and geographic locations to address these needs.
The VA regularly reevaluates how affiliates are reimbursed for VA resident activity, balancing compliance with VA policies and the workload for VA and its affiliates. The VA obtains input from key stakeholders including DEOs, DIOs, and professional organizations such as the Association of American Medical Colleges and the Accreditation Council for Graduate Medical Education.35,36
Looking ahead, the VA is developing an online tool to improve the accuracy of affiliate reimbursement. The VA will also implement a standardized staffing model, organizational structure, and position descriptions for DEO offices. These initiatives will help reduce the burden of tracking and verifying resident activity and continue to support the 77-year partnership between VA and its affiliated institutions.
1. Klink KA, Albanese AP, Bope ET, Sanders KM. Veterans Affairs graduate medical education expansion addresses US physician workforce needs. Acad Med. 2022;97(8):1144-1150. doi:10.1097/ACM.0000000000004545
2. Andrus CH, Johnson K, Pierce E, Romito PJ, Hartel P, Berrios‐Guccione S, Best W. Finance modeling in the delivery of medical care in tertiary‐care hospitals in the Department of Veterans Affairs. J Surg Res. 2001;96(2):152-157. doi:10.1006/jsre.1999.5728
3. Petrakis IL, Kozal M. Academic medical centers and the U.S. Department of Veterans Affairs: a 75-year partnership influences medical education, scientific discovery, and clinical care. Acad Med. 2022;97(8):1110-1113. doi:10.1097/ACM.0000000000004734
4. Heisler EJ, Mendez BH, Mitchell A, Panangala SV, Villagrana MA. Federal support for graduate medical education: an overview (R44376). Congressional Research Service report R44376; version 11. Updated December 27, 2018. Accessed March 2, 2024. https://crsreports.congress.gov/product/pdf/R/R44376/11
5. Chang BK, Brannen JL. The Veterans Access, Choice, and Accountability Act of 2014: examining graduate medical education enhancement in the Department of Veterans Affairs. Acad Med. 2015;90(9):1196-1198. doi:10.1097/ACM.0000000000000795
6. Albanese AP, Bope ET, Sanders KM, Bowman M. The VA MISSION Act of 2018: a potential game changer for rural GME expansion and veteran health care. J Rural Health. 2020;36(1):133-136. doi:10.1111/jrh.12360
7. Lypson ML, Roberts LW. Valuing the partnership between the Veterans Health Administration and academic medicine. Acad Med. 2022;97(8):1091-1093. doi:10.1097/ACM.0000000000004748
8. Harada ND, Traylor L, Rugen KW, et al. Interprofessional transformation of clinical education: the first six years of the Veterans Affairs Centers of Excellence in Primary Care Education. J Interprof Care. 2023;37(suppl 1):S86-S94. doi:10.1080/13561820.2018.1433642

9. Harada ND, Rajashekara S, Sansgiry S, et al. Developing interprofessional primary care teams: alumni evaluation of the Department of Veterans Affairs Centers of Excellence in Primary Care Education Program. J Med Educ Curric Dev. 2019;6:2382120519875455. doi:10.1177/2382120519875455
10. Splaine ME, Ogrinc G, Gilman SC, et al. The Department of Veterans Affairs National Quality Scholars Fellowship Program: experience from 10 years of training quality scholars. Acad Med. 2009;84(12):1741-1748. doi:10.1097/ACM.0b013e3181bfdcef
11. Watts BV, Paull DE, Williams LC, Neily J, Hemphill RR, Brannen JL. Department of Veterans Affairs chief resident in quality and patient safety program: a model to spread change. Am J Med Qual. 2016;31(6):598-600. doi:10.1177/1062860616643403
12. He K, Whang E, Kristo G. Graduate medical education funding mechanisms, challenges, and solutions: a narrative review. Am J Surg. 2021;221(1):65-71. doi:10.1016/j.amjsurg.2020.06.007
13. Villagrana M. Medicare graduate medical education payments: an overview. Congressional Research Service report IF10960. Updated September 29, 2022. Accessed March 2, 2024. https://crsreports.congress.gov/product/pdf/IF/IF10960
14. Committee on the Governance and Financing of Graduate Medical Education; Board on Health Care Services; Institute of Medicine. Graduate Medical Education That Meets the Nation’s Health Needs. Eden J, Berwick DM, Wilensky GR, eds. Washington, DC: National Academies Press; 2014. doi:10.17226/18754
15. Physician workforce: caps on Medicare-funded graduate medical education at teaching hospitals. Report to congressional requesters. GAO-21-391. May 21, 2021. Accessed March 1, 2024. https://www.gao.gov/assets/gao-21-391.pdf
16. VA and Academic Affiliates: Who Benefits? Hearing Before the Subcommittee on Oversight and Investigations of the Committee on Veterans’ Affairs, 114th Cong, 2nd Sess (2016). Accessed March 1, 2024. https://www.govinfo.gov/content/pkg/CHRG-115hhrg29685/html/CHRG-115hhrg29685.htm
17. US Department of Veterans Affairs, Office of Inspector General (OIG). Veterans Health Administration. Review of resident and part-time physician time and attendance at the Oklahoma City VA Health Care System. OIG report 17-00253-93. March 28, 2018. Accessed March 1, 2024. https://www.oversight.gov/sites/default/files/oig-reports/VAOIG-17-00253-93.pdf
18. VA health care: actions needed to improve oversight of graduate medical education reimbursement. Report to the ranking member, Committee on Veterans’ Affairs, House of Representatives. GAO-20-553. July 2020. Accessed March 1, 2024. https://www.gao.gov/assets/710/708275.pdf
19. Functions of Veterans Health Administration: in general, 38 USC §7301 (2022). Accessed March 1, 2024. https://www.govinfo.gov/content/pkg/USCODE-2022-title38/pdf/USCODE-2022-title38-partV-chap73-subchapI-sec7301.pdf
20. US Department of Veterans Affairs. Policy memorandum no. 2, policy in association of veterans’ hospitals with medical schools. January 30, 1946.
21. Veterans Health Care Expansion Act of 1973, Public Law 93-82. August 2, 1973. Accessed March 1, 2024. https://www.govinfo.gov/content/pkg/STATUTE-87/pdf/STATUTE-87-Pg179.pdf
22. Residencies and internships, 38 USC § 7406 (2022). Accessed March 1, 2024. https://www.govinfo.gov/content/pkg/USCODE-2022-title38/pdf/USCODE-2022-title38-partV-chap74-subchapI-sec7406.pdf
23. Direct graduate medical education (DGME). Centers for Medicaid and Medicare Services. Updated December 5, 2023. Accessed March 1, 2024. https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/AcuteInpatientPPS/DGME
24. Drezdzon MK, Cowley NJ, Sweeney DP, et al. Going for broke: the impact of cost of living on surgery resident stipend value. Ann Surg. 2023;278(6):1053-1059. doi:10.1097/SLA.0000000000005923
25. Special treatment: hospitals that incur indirect costs for graduate medical education programs, 42 CFR § 412.105 (2023). Accessed March 1, 2024. https://www.govinfo.gov/content/pkg/CFR-2023-title42-vol2/pdf/CFR-2023-title42-vol2-sec412-105.pdf
26. US Department of Veterans Affairs, Veterans Health Administration. VHA Directive 1400.05, Disbursement agreements for health professions trainees appointed under 38 U.S.C. § 7406. June 2, 2021. Accessed March 1, 2024. https://www.va.gov/vhapublications/ViewPublication.asp?pub_ID=9293
27. Harada ND, Sanders KM, Bowman MA. Health systems education leadership: learning from the VA designated education officer role. Fed Pract. 2022;39(6):266-273. doi:10.12788/fp.0278
28. Schleiter Hitchell K, Johnson L. CMS finalizes rules for distribution of 1000 new Medicare-funded residency positions and changes to rural training track programs. J Grad Med Educ. 2022;14(2):245-249. doi:10.4300/JGME-D-22-00193.1

29. US Department of Veterans Affairs, Veterans Health Administration. VHA Directive 1400.10, Educational cost contracts for health professions education. September 25, 2023. Accessed March 1, 2024. https://www.va.gov/VHAPUBLICATIONS/ViewPublication.asp?pub_ID=11480
30. Direct GME payments: general requirements, 42 CFR § 413.75 (2023). Accessed March 1, 2024. https://www.govinfo.gov/content/pkg/CFR-2023-title42-vol2/pdf/CFR-2023-title42-vol2-sec413-75.pdf
31. Direct GME payments: determination of the total number of FTE residents, 42 CFR § 413.78 (2023). Accessed March 1, 2024. https://www.govinfo.gov/content/pkg/CFR-2023-title42-vol2/pdf/CFR-2023-title42-vol2-sec413-78.pdf
32. US Department of Health and Human Services, Centers for Medicare and Medicaid Services. Medicare financial management manual, chapter 8. Contractor procedures for provider audits. Accessed March 1, 2024. https://www.cms.gov/regulations-and-guidance/guidance/manuals/downloads/fin106c08.pdf
33. US Department of Health and Human Services, Office of Inspector General. CMS did not always ensure hospitals complied with Medicare reimbursement requirements for graduate medical education. OIG report A-02-17-01017. November 2018. Accessed March 1, 2024. https://oig.hhs.gov/oas/reports/region2/21701017.pdf
34. US Department of Health and Human Services, Centers for Medicare and Medicaid Services. Interns and Residents Information System (IRIS) XML format. Publication 100-20. Transmittal 11418. Change request 12724. May 19, 2022. Accessed March 1, 2024. https://www.hhs.gov/guidance/sites/default/files/hhs-guidance-documents/R11418OTN.pdf
35. Birnbaum AD, Byrne J, on behalf of the VA Office of Academic Affiliations. VHA Updates: Disbursement Policy and Education Cost Contracts. Presented at: American Association of Medical Colleges Webinar; June 2021. Accessed March 1, 2024. https://vimeo.com/644415670
36. Byrne JM, on behalf of the VA Office of Academic Affiliations. Disbursement procedures update for AY 23-24. Accessed March 1, 2024. https://www.va.gov/oaa/Videos/AffiliatePresentationDisbursementandEARsAY23-24.pptx
1. Klink KA, Albanese AP, Bope ET, Sanders KM. Veterans Affairs graduate medical education expansion addresses US physician workforce needs. Acad Med. 2022;97(8):1144-1150. doi:10.1097/ACM.0000000000004545
2. Andrus CH, Johnson K, Pierce E, Romito PJ, Hartel P, Berrios‐Guccione S, Best W. Finance modeling in the delivery of medical care in tertiary‐care hospitals in the Department of Veterans Affairs. J Surg Res. 2001;96(2):152-157. doi:10.1006/jsre.1999.5728
3. Petrakis IL, Kozal M. Academic medical centers and the U.S. Department of Veterans Affairs: a 75-year partnership influences medical education, scientific discovery, and clinical care. Acad Med. 2022;97(8):1110-1113. doi:10.1097/ACM.0000000000004734
4. Heisler EJ, Mendez BH, Mitchell A, Panangala SV, Villagrana MA. Federal support for graduate medical education: an overview (R44376). Congressional Research Service report R44376; version 11. Updated December 27, 2018. Accessed March 2, 2024. https://crsreports.congress.gov/product/pdf/R/R44376/11
5. Chang BK, Brannen JL. The Veterans Access, Choice, and Accountability Act of 2014: examining graduate medical education enhancement in the Department of Veterans Affairs. Acad Med. 2015;90(9):1196-1198. doi:10.1097/ACM.0000000000000795
6. Albanese AP, Bope ET, Sanders KM, Bowman M. The VA MISSION Act of 2018: a potential game changer for rural GME expansion and veteran health care. J Rural Health. 2020;36(1):133-136. doi:10.1111/jrh.12360
7. Lypson ML, Roberts LW. Valuing the partnership between the Veterans Health Administration and academic medicine. Acad Med. 2022;97(8):1091-1093. doi:10.1097/ACM.0000000000004748
8. Harada ND, Traylor L, Rugen KW, et al. Interprofessional transformation of clinical education: the first six years of the Veterans Affairs Centers of Excellence in Primary Care Education. J Interprof Care. 2023;37(suppl 1):S86-S94. doi:10.1080/13561820.2018.1433642

9. Harada ND, Rajashekara S, Sansgiry S, et al. Developing interprofessional primary care teams: alumni evaluation of the Department of Veterans Affairs Centers of Excellence in Primary Care Education Program. J Med Educ Curric Dev. 2019;6:2382120519875455. doi:10.1177/2382120519875455
10. Splaine ME, Ogrinc G, Gilman SC, et al. The Department of Veterans Affairs National Quality Scholars Fellowship Program: experience from 10 years of training quality scholars. Acad Med. 2009;84(12):1741-1748. doi:10.1097/ACM.0b013e3181bfdcef
11. Watts BV, Paull DE, Williams LC, Neily J, Hemphill RR, Brannen JL. Department of Veterans Affairs chief resident in quality and patient safety program: a model to spread change. Am J Med Qual. 2016;31(6):598-600. doi:10.1177/1062860616643403
12. He K, Whang E, Kristo G. Graduate medical education funding mechanisms, challenges, and solutions: a narrative review. Am J Surg. 2021;221(1):65-71. doi:10.1016/j.amjsurg.2020.06.007
13. Villagrana M. Medicare graduate medical education payments: an overview. Congressional Research Service report IF10960. Updated September 29, 2022. Accessed March 2, 2024. https://crsreports.congress.gov/product/pdf/IF/IF10960
14. Committee on the Governance and Financing of Graduate Medical Education; Board on Health Care Services; Institute of Medicine. Graduate Medical Education That Meets the Nation’s Health Needs. Eden J, Berwick DM, Wilensky GR, eds. Washington, DC: National Academies Press; 2014. doi:10.17226/18754
15. Physician workforce: caps on Medicare-funded graduate medical education at teaching hospitals. Report to congressional requesters. GAO-21-391. May 21, 2021. Accessed March 1, 2024. https://www.gao.gov/assets/gao-21-391.pdf
16. VA and Academic Affiliates: Who Benefits? Hearing Before the Subcommittee on Oversight and Investigations of the Committee on Veterans’ Affairs, 114th Cong, 2nd Sess (2016). Accessed March 1, 2024. https://www.govinfo.gov/content/pkg/CHRG-115hhrg29685/html/CHRG-115hhrg29685.htm
17. US Department of Veterans Affairs, Office of Inspector General (OIG). Veterans Health Administration. Review of resident and part-time physician time and attendance at the Oklahoma City VA Health Care System. OIG report 17-00253-93. March 28, 2018. Accessed March 1, 2024. https://www.oversight.gov/sites/default/files/oig-reports/VAOIG-17-00253-93.pdf
18. VA health care: actions needed to improve oversight of graduate medical education reimbursement. Report to the ranking member, Committee on Veterans’ Affairs, House of Representatives. GAO-20-553. July 2020. Accessed March 1, 2024. https://www.gao.gov/assets/710/708275.pdf
19. Functions of Veterans Health Administration: in general, 38 USC §7301 (2022). Accessed March 1, 2024. https://www.govinfo.gov/content/pkg/USCODE-2022-title38/pdf/USCODE-2022-title38-partV-chap73-subchapI-sec7301.pdf
20. US Department of Veterans Affairs. Policy memorandum no. 2, policy in association of veterans’ hospitals with medical schools. January 30, 1946.
21. Veterans Health Care Expansion Act of 1973, Public Law 93-82. August 2, 1973. Accessed March 1, 2024. https://www.govinfo.gov/content/pkg/STATUTE-87/pdf/STATUTE-87-Pg179.pdf
22. Residencies and internships, 38 USC § 7406 (2022). Accessed March 1, 2024. https://www.govinfo.gov/content/pkg/USCODE-2022-title38/pdf/USCODE-2022-title38-partV-chap74-subchapI-sec7406.pdf
23. Direct graduate medical education (DGME). Centers for Medicaid and Medicare Services. Updated December 5, 2023. Accessed March 1, 2024. https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/AcuteInpatientPPS/DGME
24. Drezdzon MK, Cowley NJ, Sweeney DP, et al. Going for broke: the impact of cost of living on surgery resident stipend value. Ann Surg. 2023;278(6):1053-1059. doi:10.1097/SLA.0000000000005923
25. Special treatment: hospitals that incur indirect costs for graduate medical education programs, 42 CFR § 412.105 (2023). Accessed March 1, 2024. https://www.govinfo.gov/content/pkg/CFR-2023-title42-vol2/pdf/CFR-2023-title42-vol2-sec412-105.pdf
26. US Department of Veterans Affairs, Veterans Health Administration. VHA Directive 1400.05, Disbursement agreements for health professions trainees appointed under 38 U.S.C. § 7406. June 2, 2021. Accessed March 1, 2024. https://www.va.gov/vhapublications/ViewPublication.asp?pub_ID=9293
27. Harada ND, Sanders KM, Bowman MA. Health systems education leadership: learning from the VA designated education officer role. Fed Pract. 2022;39(6):266-273. doi:10.12788/fp.0278
28. Schleiter Hitchell K, Johnson L. CMS finalizes rules for distribution of 1000 new Medicare-funded residency positions and changes to rural training track programs. J Grad Med Educ. 2022;14(2):245-249. doi:10.4300/JGME-D-22-00193.1

29. US Department of Veterans Affairs, Veterans Health Administration. VHA Directive 1400.10, Educational cost contracts for health professions education. September 25, 2023. Accessed March 1, 2024. https://www.va.gov/VHAPUBLICATIONS/ViewPublication.asp?pub_ID=11480
30. Direct GME payments: general requirements, 42 CFR § 413.75 (2023). Accessed March 1, 2024. https://www.govinfo.gov/content/pkg/CFR-2023-title42-vol2/pdf/CFR-2023-title42-vol2-sec413-75.pdf
31. Direct GME payments: determination of the total number of FTE residents, 42 CFR § 413.78 (2023). Accessed March 1, 2024. https://www.govinfo.gov/content/pkg/CFR-2023-title42-vol2/pdf/CFR-2023-title42-vol2-sec413-78.pdf
32. US Department of Health and Human Services, Centers for Medicare and Medicaid Services. Medicare financial management manual, chapter 8. Contractor procedures for provider audits. Accessed March 1, 2024. https://www.cms.gov/regulations-and-guidance/guidance/manuals/downloads/fin106c08.pdf
33. US Department of Health and Human Services, Office of Inspector General. CMS did not always ensure hospitals complied with Medicare reimbursement requirements for graduate medical education. OIG report A-02-17-01017. November 2018. Accessed March 1, 2024. https://oig.hhs.gov/oas/reports/region2/21701017.pdf
34. US Department of Health and Human Services, Centers for Medicare and Medicaid Services. Interns and Residents Information System (IRIS) XML format. Publication 100-20. Transmittal 11418. Change request 12724. May 19, 2022. Accessed March 1, 2024. https://www.hhs.gov/guidance/sites/default/files/hhs-guidance-documents/R11418OTN.pdf
35. Birnbaum AD, Byrne J, on behalf of the VA Office of Academic Affiliations. VHA Updates: Disbursement Policy and Education Cost Contracts. Presented at: American Association of Medical Colleges Webinar; June 2021. Accessed March 1, 2024. https://vimeo.com/644415670
36. Byrne JM, on behalf of the VA Office of Academic Affiliations. Disbursement procedures update for AY 23-24. Accessed March 1, 2024. https://www.va.gov/oaa/Videos/AffiliatePresentationDisbursementandEARsAY23-24.pptx
Could EHR Pharmacy Errors Put Veterans at Risk?
Will the new US Department of Veterans Affairs (VA) pharmacy software be safe and effective? That was the topic when David Case, the VA Deputy Inspector General, spoke in the US House of Representatives Veterans Affairs Committee technology modernization subcommittee hearing on February 15.
Questions like that have dogged the project since 2018, when the VA began rolling out the Oracle Cerner electronic health record (EHR) system as the successor to ViSTA.
The Oracle system has been beset by one glitch after another since its arrival. And in that time, Case said, the VA Office of Inspector General (OIG) has been engaging with VA employees at sites in Washington, Oregon, Ohio, Illinois, and other locations where the modernization program has been piloted.
The most recent OIG investigation of pharmacy-related patient safety issues began with a review of an allegation of a prescription backlog at Columbus, Ohio, where the system went live on April 30, 2022. The OIG found that facility leaders took “timely and sustainable steps” to manage that issue. However, other unresolved patient safety issues came to light, such as medication inaccuracies, inaccurate medication data, and insufficient staffing. The OIG also found staff were creating “numerous work arounds” to provide patient care, and that the volume of staff educational materials for pharmacy-related functions was “overwhelming.”
Those problems were just the latest in a long queue. In May 2021, after the first VA deployment of the new EHR at the Mann-Grandstaff VA Medical Center in Spokane, Washington, a pharmacy patient safety team under the VA National Center for Patient Safety (NCPS) also had identified patient safety issues and “multiple” concerns regarding the system’s usability. For example, updates to a patient’s active medication list were not routinely reflected at the patient’s next appointment. Despite knowing about such challenges, Case noted in his report, VA leaders deployed the new EHR at 4 more VA medical centers.
Cerner/ViSTA Communication
One major cause of the current problems is the way the systems “talk” to each other. EHR information is communicated between VHA facilities through channels that include the Joint Longitudinal Viewer (JLV) and the Health Data Repository, which stores patient-specific clinical information from both the legacy and the new EHR systems. The JLV application allows clinicians to access a read only version of a patient’s EHR from both systems.
Every medication used in VHA has a VA Unique Identifier (VUID). When a patient is prescribed a medication at a new EHR site, that medication’s VUID is sent to the Health Data Repository. If that patient seeks care from a legacy health care practitioner (HCP), and that HCP enters a medication order, a software interface accesses the VUID from the Health Data Repository to verify that the medication being prescribed is safe and compatible with the medications and allergies previously documented in the patient’s record.
However, on March 31, 2023, staff from a ViSTA site found an incorrect medication order when prescribing a new medication to a patient who had received care and medications at a new EHR site. This in turn led to the discovery that an error in Oracle software coding had resulted in the “widespread transmission” of incorrect VUIDs from new EHR sites to legacy EHR sites, the OIG found. VA leaders and HCPs were notified of the potential clinical impact and were given specific instructions on how to mitigate the issue. They were asked to “please share widely.”
On top of that, days later, patient safety managers across the Veterans Health Administration (VHA) were told that drug-to-drug interactions, duplicate medication orders, and allergy checks were not functioning as expected, and they too were provided with remedial actions.
Oracle applied a successful software patch on in April 2023, to ensure accurate VUIDs were attached to all mail order pharmacy–processed prescriptions from that date forward. However, the OIG learned the incorrect VUIDs sent from new EHR sites and stored in the Health Data Repository from as far back as October 2020 had not been corrected. Case told the subcommittee that on November 29, 2023, the VHA Pharmacy Council reported withdrawing a request for Oracle to send corrected medication VUID data to the Health Data Repository, on the presumption that remaining inaccurate VUIDs would expire in early April 2024, and the data would be corrected at that time.
The OIG is concerned, Case said, that patient medication data remains inaccurate almost a year after VA learned of the issue. The mail order pharmacy-related data generated from approximately 120,000 patients served by new EHR sites are still incorrect. These patients face an ongoing risk of an adverse medication-related event if they receive care and medications from a VA medical center using the legacy EHR system.
The OIG also learned of other problems associated with transmission of medication and allergy information, which could have consequences such as:
- Patient medications being discontinued or stopped by new HCPs using Cerner that appear in ViSTA as active and current prescriptions;
- Allergy-warning messages not appearing when intended or inappropriately appearing for the wrong medication;
- Duplicate medication order checks not appearing when intended or inappropriately appearing for the wrong drug;
- Patient active medication lists having incomplete or inaccurate information, such as missing prescriptions, duplicate prescriptions, or incorrect medication order statuses.
The OIG warned VHA employees about the risks, although it wasn’t possible to determine who might actually be at risk. A VHA leader told the OIG that all patients who have been prescribed any medications or have medication allergies documented at a at a Cerner site are at risk. That could mean as many as 250,000 patients: As of September 2023, approximately 190,000 patients had a medication prescribed and 126,000 had an allergy documented at a new EHR site.
Case Example
Not surprisingly, “the OIG is not confident in [EHRM-Integration Office] leaders’ oversight and control of the new systems’ Health Data Repository interface programming,” Case said. He cited the case of a patient with posttraumatic stress disorder and traumatic brain injury with adrenal insufficiency. Four days prior to admission, a ViSTA site pharmacist used the EHR to perform a medication reconciliation for the patient. The data available did not include the patient’s most recent prednisone prescription, which had been ordered by an HCP at a facility using Cerner.
A nurse practitioner performed another reconciliation when the patient was admitted to the residential program, but the patient was unsure of all their medications. Because the most recent prednisone prescription was not visible in ViSTA, the prednisone appeared to have been completed at least 3 months prior to admission and was therefore not prescribed in the admission medication orders.
Five days into the residential program, the patient began exhibiting unusual behaviors associated with the lack of prednisone. The patient realized they needed more prednisone, but the nurse explained there was no prednisone on the patient’s medication list. Eventually, the patient found the active prednisone order on their personal cell phone and was transferred to a local emergency department for care.
Work Arounds
The VHA’s efforts to forestall or mitigate system errors have in some cases had a cascade effect. For example, HCPs must essentially back up what the automated software is intended to do, with “complex, time-consuming” multistep manual safety checks when prescribing new medications for patients previously cared for at a Cerner site. The OIG is concerned that this increased vigilance is “unsustainable” by pharmacists and frontline staff and could lead to burnout and medication-related patient safety events. After the new EHR launched, the OIG found, burnout symptoms for pharmacy staff increased. Nonetheless, Case told the committee, OIG staff “have observed [employees’] unwavering commitment to prioritizing the care of patients while mitigating implementation challenges.”
EHR-related workload burdens have necessitated other adjustments. Columbus, for instance, hired 9 full-time clinical pharmacists—a 62% staffing increase—to help reduce the backlog. Pharmacy leaders created approximately 29 additional work-arounds to support pharmacy staff and prevent delays. Facility pharmacy leaders also developed approximately 25 educational materials, such as tip sheets, reference guides, and job aids. The OIG’s concern—apart from the overwhelming amount of information for staff to implement—is that such prophylactic measures may in fact give rise to inconsistent practices, which increase risks to patient safety.
Committed to Working With the VA
Mike Sicilia, executive vice president of Oracle Corporation, told lawmakers in the hearing, “After the initial deployments, it became clear that the pharmacy system needed to be enhanced to better meet VA’s needs. To that end, in August 2022, shortly after Oracle completed its acquisition of Cerner, VA contracted with us for seven enhancements that overall would adapt the pharmacy system to a more bidirectional system between VA providers placing prescription orders and VA pharmacists fulfilling and dispensing them.” Those enhancements are all live for VA providers and pharmacists to use now, he said, except for one that is undergoing additional testing.
He added, “As with any healthcare technology system, there is a need for continuous improvements but that does not mean the system is not safe and effective in its current state. Oracle is committed to working with VA … throughout the reset period to identify workflows and other items that can be simplified or streamlined to improve the overall user and pharmacy experience.”
Standardizing workflows and ensuring training and communications to pharmacists about the latest updates will discourage use of work-arounds, Sicilia said, and “help with improving morale and satisfaction with the system.” During a visit in early February by VA and the Oracle team to the Lovell Federal Health Care Center in North Chicago, “feedback from pharmacists was positive about the training and readiness for using the new pharmacy system.”
The backlog, at least, may be resolved. Sicilia said on average more than 215,000 outpatient prescriptions are being filled each month. “The current live sites do not have a backlog in filling prescriptions. Recent data from this month show that three of the five live sites have zero prescriptions waiting to be processed that are older than seven days. The two other live sites have an average of two prescriptions older than seven days,” he said.
Although Oracle Health has since resolved some of the identified issues, the OIG is concerned that the new EHR will continue to be deployed at medical facilities despite “myriad” as-yet unresolved issues related to inaccurate medication ordering, reconciliation, and dispensing. The VHA has paused Cerner deployments multiple times.
“It is unclear whether identified problems are being adequately resolved before additional deployments,” Case said. “There is also the question of whether there is sufficient transparency and communication among EHRM-IO, VHA and facility leaders, VA leaders, and Oracle Health needed for quality control and critical coordination. Trust in VA is also dependent on patients being fully and quickly advised when issues affecting them are identified and addressed. As VA moves toward its deployment next month at a complex facility jointly operated with the Department of Defense, transparency, communication, and program management will be essential to getting it right. Failures in these areas risk cascading problems.”
Will the new US Department of Veterans Affairs (VA) pharmacy software be safe and effective? That was the topic when David Case, the VA Deputy Inspector General, spoke in the US House of Representatives Veterans Affairs Committee technology modernization subcommittee hearing on February 15.
Questions like that have dogged the project since 2018, when the VA began rolling out the Oracle Cerner electronic health record (EHR) system as the successor to ViSTA.
The Oracle system has been beset by one glitch after another since its arrival. And in that time, Case said, the VA Office of Inspector General (OIG) has been engaging with VA employees at sites in Washington, Oregon, Ohio, Illinois, and other locations where the modernization program has been piloted.
The most recent OIG investigation of pharmacy-related patient safety issues began with a review of an allegation of a prescription backlog at Columbus, Ohio, where the system went live on April 30, 2022. The OIG found that facility leaders took “timely and sustainable steps” to manage that issue. However, other unresolved patient safety issues came to light, such as medication inaccuracies, inaccurate medication data, and insufficient staffing. The OIG also found staff were creating “numerous work arounds” to provide patient care, and that the volume of staff educational materials for pharmacy-related functions was “overwhelming.”
Those problems were just the latest in a long queue. In May 2021, after the first VA deployment of the new EHR at the Mann-Grandstaff VA Medical Center in Spokane, Washington, a pharmacy patient safety team under the VA National Center for Patient Safety (NCPS) also had identified patient safety issues and “multiple” concerns regarding the system’s usability. For example, updates to a patient’s active medication list were not routinely reflected at the patient’s next appointment. Despite knowing about such challenges, Case noted in his report, VA leaders deployed the new EHR at 4 more VA medical centers.
Cerner/ViSTA Communication
One major cause of the current problems is the way the systems “talk” to each other. EHR information is communicated between VHA facilities through channels that include the Joint Longitudinal Viewer (JLV) and the Health Data Repository, which stores patient-specific clinical information from both the legacy and the new EHR systems. The JLV application allows clinicians to access a read only version of a patient’s EHR from both systems.
Every medication used in VHA has a VA Unique Identifier (VUID). When a patient is prescribed a medication at a new EHR site, that medication’s VUID is sent to the Health Data Repository. If that patient seeks care from a legacy health care practitioner (HCP), and that HCP enters a medication order, a software interface accesses the VUID from the Health Data Repository to verify that the medication being prescribed is safe and compatible with the medications and allergies previously documented in the patient’s record.
However, on March 31, 2023, staff from a ViSTA site found an incorrect medication order when prescribing a new medication to a patient who had received care and medications at a new EHR site. This in turn led to the discovery that an error in Oracle software coding had resulted in the “widespread transmission” of incorrect VUIDs from new EHR sites to legacy EHR sites, the OIG found. VA leaders and HCPs were notified of the potential clinical impact and were given specific instructions on how to mitigate the issue. They were asked to “please share widely.”
On top of that, days later, patient safety managers across the Veterans Health Administration (VHA) were told that drug-to-drug interactions, duplicate medication orders, and allergy checks were not functioning as expected, and they too were provided with remedial actions.
Oracle applied a successful software patch on in April 2023, to ensure accurate VUIDs were attached to all mail order pharmacy–processed prescriptions from that date forward. However, the OIG learned the incorrect VUIDs sent from new EHR sites and stored in the Health Data Repository from as far back as October 2020 had not been corrected. Case told the subcommittee that on November 29, 2023, the VHA Pharmacy Council reported withdrawing a request for Oracle to send corrected medication VUID data to the Health Data Repository, on the presumption that remaining inaccurate VUIDs would expire in early April 2024, and the data would be corrected at that time.
The OIG is concerned, Case said, that patient medication data remains inaccurate almost a year after VA learned of the issue. The mail order pharmacy-related data generated from approximately 120,000 patients served by new EHR sites are still incorrect. These patients face an ongoing risk of an adverse medication-related event if they receive care and medications from a VA medical center using the legacy EHR system.
The OIG also learned of other problems associated with transmission of medication and allergy information, which could have consequences such as:
- Patient medications being discontinued or stopped by new HCPs using Cerner that appear in ViSTA as active and current prescriptions;
- Allergy-warning messages not appearing when intended or inappropriately appearing for the wrong medication;
- Duplicate medication order checks not appearing when intended or inappropriately appearing for the wrong drug;
- Patient active medication lists having incomplete or inaccurate information, such as missing prescriptions, duplicate prescriptions, or incorrect medication order statuses.
The OIG warned VHA employees about the risks, although it wasn’t possible to determine who might actually be at risk. A VHA leader told the OIG that all patients who have been prescribed any medications or have medication allergies documented at a at a Cerner site are at risk. That could mean as many as 250,000 patients: As of September 2023, approximately 190,000 patients had a medication prescribed and 126,000 had an allergy documented at a new EHR site.
Case Example
Not surprisingly, “the OIG is not confident in [EHRM-Integration Office] leaders’ oversight and control of the new systems’ Health Data Repository interface programming,” Case said. He cited the case of a patient with posttraumatic stress disorder and traumatic brain injury with adrenal insufficiency. Four days prior to admission, a ViSTA site pharmacist used the EHR to perform a medication reconciliation for the patient. The data available did not include the patient’s most recent prednisone prescription, which had been ordered by an HCP at a facility using Cerner.
A nurse practitioner performed another reconciliation when the patient was admitted to the residential program, but the patient was unsure of all their medications. Because the most recent prednisone prescription was not visible in ViSTA, the prednisone appeared to have been completed at least 3 months prior to admission and was therefore not prescribed in the admission medication orders.
Five days into the residential program, the patient began exhibiting unusual behaviors associated with the lack of prednisone. The patient realized they needed more prednisone, but the nurse explained there was no prednisone on the patient’s medication list. Eventually, the patient found the active prednisone order on their personal cell phone and was transferred to a local emergency department for care.
Work Arounds
The VHA’s efforts to forestall or mitigate system errors have in some cases had a cascade effect. For example, HCPs must essentially back up what the automated software is intended to do, with “complex, time-consuming” multistep manual safety checks when prescribing new medications for patients previously cared for at a Cerner site. The OIG is concerned that this increased vigilance is “unsustainable” by pharmacists and frontline staff and could lead to burnout and medication-related patient safety events. After the new EHR launched, the OIG found, burnout symptoms for pharmacy staff increased. Nonetheless, Case told the committee, OIG staff “have observed [employees’] unwavering commitment to prioritizing the care of patients while mitigating implementation challenges.”
EHR-related workload burdens have necessitated other adjustments. Columbus, for instance, hired 9 full-time clinical pharmacists—a 62% staffing increase—to help reduce the backlog. Pharmacy leaders created approximately 29 additional work-arounds to support pharmacy staff and prevent delays. Facility pharmacy leaders also developed approximately 25 educational materials, such as tip sheets, reference guides, and job aids. The OIG’s concern—apart from the overwhelming amount of information for staff to implement—is that such prophylactic measures may in fact give rise to inconsistent practices, which increase risks to patient safety.
Committed to Working With the VA
Mike Sicilia, executive vice president of Oracle Corporation, told lawmakers in the hearing, “After the initial deployments, it became clear that the pharmacy system needed to be enhanced to better meet VA’s needs. To that end, in August 2022, shortly after Oracle completed its acquisition of Cerner, VA contracted with us for seven enhancements that overall would adapt the pharmacy system to a more bidirectional system between VA providers placing prescription orders and VA pharmacists fulfilling and dispensing them.” Those enhancements are all live for VA providers and pharmacists to use now, he said, except for one that is undergoing additional testing.
He added, “As with any healthcare technology system, there is a need for continuous improvements but that does not mean the system is not safe and effective in its current state. Oracle is committed to working with VA … throughout the reset period to identify workflows and other items that can be simplified or streamlined to improve the overall user and pharmacy experience.”
Standardizing workflows and ensuring training and communications to pharmacists about the latest updates will discourage use of work-arounds, Sicilia said, and “help with improving morale and satisfaction with the system.” During a visit in early February by VA and the Oracle team to the Lovell Federal Health Care Center in North Chicago, “feedback from pharmacists was positive about the training and readiness for using the new pharmacy system.”
The backlog, at least, may be resolved. Sicilia said on average more than 215,000 outpatient prescriptions are being filled each month. “The current live sites do not have a backlog in filling prescriptions. Recent data from this month show that three of the five live sites have zero prescriptions waiting to be processed that are older than seven days. The two other live sites have an average of two prescriptions older than seven days,” he said.
Although Oracle Health has since resolved some of the identified issues, the OIG is concerned that the new EHR will continue to be deployed at medical facilities despite “myriad” as-yet unresolved issues related to inaccurate medication ordering, reconciliation, and dispensing. The VHA has paused Cerner deployments multiple times.
“It is unclear whether identified problems are being adequately resolved before additional deployments,” Case said. “There is also the question of whether there is sufficient transparency and communication among EHRM-IO, VHA and facility leaders, VA leaders, and Oracle Health needed for quality control and critical coordination. Trust in VA is also dependent on patients being fully and quickly advised when issues affecting them are identified and addressed. As VA moves toward its deployment next month at a complex facility jointly operated with the Department of Defense, transparency, communication, and program management will be essential to getting it right. Failures in these areas risk cascading problems.”
Will the new US Department of Veterans Affairs (VA) pharmacy software be safe and effective? That was the topic when David Case, the VA Deputy Inspector General, spoke in the US House of Representatives Veterans Affairs Committee technology modernization subcommittee hearing on February 15.
Questions like that have dogged the project since 2018, when the VA began rolling out the Oracle Cerner electronic health record (EHR) system as the successor to ViSTA.
The Oracle system has been beset by one glitch after another since its arrival. And in that time, Case said, the VA Office of Inspector General (OIG) has been engaging with VA employees at sites in Washington, Oregon, Ohio, Illinois, and other locations where the modernization program has been piloted.
The most recent OIG investigation of pharmacy-related patient safety issues began with a review of an allegation of a prescription backlog at Columbus, Ohio, where the system went live on April 30, 2022. The OIG found that facility leaders took “timely and sustainable steps” to manage that issue. However, other unresolved patient safety issues came to light, such as medication inaccuracies, inaccurate medication data, and insufficient staffing. The OIG also found staff were creating “numerous work arounds” to provide patient care, and that the volume of staff educational materials for pharmacy-related functions was “overwhelming.”
Those problems were just the latest in a long queue. In May 2021, after the first VA deployment of the new EHR at the Mann-Grandstaff VA Medical Center in Spokane, Washington, a pharmacy patient safety team under the VA National Center for Patient Safety (NCPS) also had identified patient safety issues and “multiple” concerns regarding the system’s usability. For example, updates to a patient’s active medication list were not routinely reflected at the patient’s next appointment. Despite knowing about such challenges, Case noted in his report, VA leaders deployed the new EHR at 4 more VA medical centers.
Cerner/ViSTA Communication
One major cause of the current problems is the way the systems “talk” to each other. EHR information is communicated between VHA facilities through channels that include the Joint Longitudinal Viewer (JLV) and the Health Data Repository, which stores patient-specific clinical information from both the legacy and the new EHR systems. The JLV application allows clinicians to access a read only version of a patient’s EHR from both systems.
Every medication used in VHA has a VA Unique Identifier (VUID). When a patient is prescribed a medication at a new EHR site, that medication’s VUID is sent to the Health Data Repository. If that patient seeks care from a legacy health care practitioner (HCP), and that HCP enters a medication order, a software interface accesses the VUID from the Health Data Repository to verify that the medication being prescribed is safe and compatible with the medications and allergies previously documented in the patient’s record.
However, on March 31, 2023, staff from a ViSTA site found an incorrect medication order when prescribing a new medication to a patient who had received care and medications at a new EHR site. This in turn led to the discovery that an error in Oracle software coding had resulted in the “widespread transmission” of incorrect VUIDs from new EHR sites to legacy EHR sites, the OIG found. VA leaders and HCPs were notified of the potential clinical impact and were given specific instructions on how to mitigate the issue. They were asked to “please share widely.”
On top of that, days later, patient safety managers across the Veterans Health Administration (VHA) were told that drug-to-drug interactions, duplicate medication orders, and allergy checks were not functioning as expected, and they too were provided with remedial actions.
Oracle applied a successful software patch on in April 2023, to ensure accurate VUIDs were attached to all mail order pharmacy–processed prescriptions from that date forward. However, the OIG learned the incorrect VUIDs sent from new EHR sites and stored in the Health Data Repository from as far back as October 2020 had not been corrected. Case told the subcommittee that on November 29, 2023, the VHA Pharmacy Council reported withdrawing a request for Oracle to send corrected medication VUID data to the Health Data Repository, on the presumption that remaining inaccurate VUIDs would expire in early April 2024, and the data would be corrected at that time.
The OIG is concerned, Case said, that patient medication data remains inaccurate almost a year after VA learned of the issue. The mail order pharmacy-related data generated from approximately 120,000 patients served by new EHR sites are still incorrect. These patients face an ongoing risk of an adverse medication-related event if they receive care and medications from a VA medical center using the legacy EHR system.
The OIG also learned of other problems associated with transmission of medication and allergy information, which could have consequences such as:
- Patient medications being discontinued or stopped by new HCPs using Cerner that appear in ViSTA as active and current prescriptions;
- Allergy-warning messages not appearing when intended or inappropriately appearing for the wrong medication;
- Duplicate medication order checks not appearing when intended or inappropriately appearing for the wrong drug;
- Patient active medication lists having incomplete or inaccurate information, such as missing prescriptions, duplicate prescriptions, or incorrect medication order statuses.
The OIG warned VHA employees about the risks, although it wasn’t possible to determine who might actually be at risk. A VHA leader told the OIG that all patients who have been prescribed any medications or have medication allergies documented at a at a Cerner site are at risk. That could mean as many as 250,000 patients: As of September 2023, approximately 190,000 patients had a medication prescribed and 126,000 had an allergy documented at a new EHR site.
Case Example
Not surprisingly, “the OIG is not confident in [EHRM-Integration Office] leaders’ oversight and control of the new systems’ Health Data Repository interface programming,” Case said. He cited the case of a patient with posttraumatic stress disorder and traumatic brain injury with adrenal insufficiency. Four days prior to admission, a ViSTA site pharmacist used the EHR to perform a medication reconciliation for the patient. The data available did not include the patient’s most recent prednisone prescription, which had been ordered by an HCP at a facility using Cerner.
A nurse practitioner performed another reconciliation when the patient was admitted to the residential program, but the patient was unsure of all their medications. Because the most recent prednisone prescription was not visible in ViSTA, the prednisone appeared to have been completed at least 3 months prior to admission and was therefore not prescribed in the admission medication orders.
Five days into the residential program, the patient began exhibiting unusual behaviors associated with the lack of prednisone. The patient realized they needed more prednisone, but the nurse explained there was no prednisone on the patient’s medication list. Eventually, the patient found the active prednisone order on their personal cell phone and was transferred to a local emergency department for care.
Work Arounds
The VHA’s efforts to forestall or mitigate system errors have in some cases had a cascade effect. For example, HCPs must essentially back up what the automated software is intended to do, with “complex, time-consuming” multistep manual safety checks when prescribing new medications for patients previously cared for at a Cerner site. The OIG is concerned that this increased vigilance is “unsustainable” by pharmacists and frontline staff and could lead to burnout and medication-related patient safety events. After the new EHR launched, the OIG found, burnout symptoms for pharmacy staff increased. Nonetheless, Case told the committee, OIG staff “have observed [employees’] unwavering commitment to prioritizing the care of patients while mitigating implementation challenges.”
EHR-related workload burdens have necessitated other adjustments. Columbus, for instance, hired 9 full-time clinical pharmacists—a 62% staffing increase—to help reduce the backlog. Pharmacy leaders created approximately 29 additional work-arounds to support pharmacy staff and prevent delays. Facility pharmacy leaders also developed approximately 25 educational materials, such as tip sheets, reference guides, and job aids. The OIG’s concern—apart from the overwhelming amount of information for staff to implement—is that such prophylactic measures may in fact give rise to inconsistent practices, which increase risks to patient safety.
Committed to Working With the VA
Mike Sicilia, executive vice president of Oracle Corporation, told lawmakers in the hearing, “After the initial deployments, it became clear that the pharmacy system needed to be enhanced to better meet VA’s needs. To that end, in August 2022, shortly after Oracle completed its acquisition of Cerner, VA contracted with us for seven enhancements that overall would adapt the pharmacy system to a more bidirectional system between VA providers placing prescription orders and VA pharmacists fulfilling and dispensing them.” Those enhancements are all live for VA providers and pharmacists to use now, he said, except for one that is undergoing additional testing.
He added, “As with any healthcare technology system, there is a need for continuous improvements but that does not mean the system is not safe and effective in its current state. Oracle is committed to working with VA … throughout the reset period to identify workflows and other items that can be simplified or streamlined to improve the overall user and pharmacy experience.”
Standardizing workflows and ensuring training and communications to pharmacists about the latest updates will discourage use of work-arounds, Sicilia said, and “help with improving morale and satisfaction with the system.” During a visit in early February by VA and the Oracle team to the Lovell Federal Health Care Center in North Chicago, “feedback from pharmacists was positive about the training and readiness for using the new pharmacy system.”
The backlog, at least, may be resolved. Sicilia said on average more than 215,000 outpatient prescriptions are being filled each month. “The current live sites do not have a backlog in filling prescriptions. Recent data from this month show that three of the five live sites have zero prescriptions waiting to be processed that are older than seven days. The two other live sites have an average of two prescriptions older than seven days,” he said.
Although Oracle Health has since resolved some of the identified issues, the OIG is concerned that the new EHR will continue to be deployed at medical facilities despite “myriad” as-yet unresolved issues related to inaccurate medication ordering, reconciliation, and dispensing. The VHA has paused Cerner deployments multiple times.
“It is unclear whether identified problems are being adequately resolved before additional deployments,” Case said. “There is also the question of whether there is sufficient transparency and communication among EHRM-IO, VHA and facility leaders, VA leaders, and Oracle Health needed for quality control and critical coordination. Trust in VA is also dependent on patients being fully and quickly advised when issues affecting them are identified and addressed. As VA moves toward its deployment next month at a complex facility jointly operated with the Department of Defense, transparency, communication, and program management will be essential to getting it right. Failures in these areas risk cascading problems.”
The Impact of a Paracentesis Clinic on Internal Medicine Resident Procedural Competency
Competency in paracentesis is an important procedural skill for medical practitioners caring for patients with decompensated liver cirrhosis. Paracentesis is performed to drain ascitic fluid for both diagnosis and/or therapeutic purposes.1 While this procedure can be performed without the use of ultrasound, it is preferable to use ultrasound to identify an area of fluid that is away from dangerous anatomy including bowel loops, the liver, and spleen. After prepping the area, lidocaine is administered locally. A catheter is then inserted until fluid begins flowing freely. The catheter is connected to a suction canister or collection kit, and the patient is monitored until the flow ceases. Samples can be sent for analysis to determine the etiology of ascites, identify concerns for infection, and more.
Paracentesis is a very common procedure. Barsuk and colleagues noted that between 2010 and 2012, 97,577 procedures were performed across 120 academic medical centers and 290 affiliated hospitals.2 Patients undergo paracentesis in a variety of settings including the emergency department, inpatient hospitalizations, and clinics. Some patients may require only 1 paracentesis procedure while others may require it regularly.
Due to the rising need for paracentesis in the Central Texas Veterans Affairs Hospital (CTVAH) in Temple, a paracentesis clinic was started in February 2018. The goal of the paracentesis clinic was multifocal—to reduce hospital admissions, improve access to regularly scheduled procedures, decrease wait times, and increase patient satisfaction.3 Through the CTVAH affiliation with the Texas A&M internal medicine residency program, the paracentesis clinic started involving and training residents on this procedure. Up to 3 residents are on weekly rotation and can perform up to 6 paracentesis procedures in a week. The purpose of this article was to evaluate resident competency in paracentesis after completion of the paracentesis clinic.
Methods
The paracentesis clinic schedules up to 3 patients on Tuesdays and Thursdays between 8
A survey was sent via email to all categorical internal medicine residents across all 3 program years at the time of data collection. Competency for paracentesis sign-off was defined as completing and logging 5 procedures supervised by a competent physician who confirmed that all portions of the procedure were performed correctly. Residents were also asked to answer questions on a scale from 1 to 10, with 1 representing no confidence and 10 representing strong confidence to practice independently (Table).
We also evaluated the number of procedures performed by internal medicine residents 3 years before the clinic was started in 2015 up to the completion of 2022. The numbers were obtained by examining procedural log data for each year for all internal medicine residents.
Results
Thirty-three residents completed the survey: 10 first-year internal medicine residents (PGY1), 12 second-year residents (PGY2), and 11 third-year residents (PGY3). The mean participation was 4.8 paracentesis sessions per person for the duration of the study. The range of paracentesis procedures performed varied based on PGY year: PGY1s performed 1 to > 10 procedures, PGY2s performed 2 to > 10 procedures, and PGY3s performed 5 to > 10 procedures. Thirty-six percent of residents completed > 10 procedures in the paracentesis clinic; 82% of PGY3s had completed > 10 procedures by December of their third year. Twenty-six residents (79%) were credentialed to perform paracentesis procedures independently after performing > 5 procedures, and 7 residents were not yet cleared for procedural independence.
In the survey, residents rated their comfort with performing paracentesis procedures independently at a mean of 7.9. The mean comfort reported by PGY1s was 7.2, PGY2s was 7.3, and PGY3s was 9.3. Residents also rated their opinion on whether or not the paracentesis clinic adequately prepared them for paracentesis procedural independence; the mean was 8.9 across all residents.
The total number of procedures performed by residents at CTVAH also increased. Starting in 2015, 3 years before the clinic was started, 38 procedures were performed by internal medicine residents, followed by 72 procedures in 2016; 76 in 2017; 58 in 2018; 94 in 2019; 88 in 2020; 136 in 2021; and 188 in 2022.
Discussion
Paracentesis is a simple but invasive procedure to relieve ascites, often relieving patients’ symptoms, preventing hospital admission, and increasing patient satisfaction.4 The CTVAH does not have the capacity to perform outpatient paracentesis effectively in its emergency or radiology departments. Furthermore, the use of the emergency or radiology departments for routine paracentesis may not be feasible due to the acuity of care being provided, as these procedures can be time consuming and can draw away critical resources and time from patients that need emergent care. The paracentesis clinic was then formed to provide veterans access to the procedural care they need, while also preparing residents to ably and confidently perform the procedure independently.
Based on our study, most residents were cleared to independently perform paracentesis procedures across all 3 years, with 79% of residents having completed the required 5 supervised procedures to independently practice. A study assessing unsupervised practice standards showed that paracentesis skill declines as soon as 3 months after training. However, retraining was shown to potentially interrupt this skill decline.5 Studies have shown that procedure-driven electives or services significantly improved paracentesis certification rates and total logged procedures, with minimal funding or scheduling changes required.6 Our clinic showed a significant increase in the number of procedures logged starting with the minimum of 38 procedures in 2015 and ending with 188 procedures logged at the end of 2022.
By allowing residents to routinely return to the paracentesis clinic across all 3 years, residents were more likely to feel comfortable independently performing the procedure, with residents reporting a mean comfort score of 7.9. The spaced repetition and ability to work with the clinic during elective time allows regular opportunities to undergo supervised training in a controlled environment and created scheduled retraining opportunities. Future studies should evaluate residents prior to each paracentesis clinic to ascertain if skill decline is occurring at a slower rate.
The inpatient effect of the clinic is also multifocal. Pham and colleagues showed that integrating paracentesis into timely training can reduce paracentesis delay and delays in care.7 By increasing the volume of procedures each resident performs and creating a sense of confidence amongst residents, the clinic increases the number of residents able and willing to perform inpatient procedures, thus reducing the number of unnecessary consultations and hospital resources. One of the reasons the paracentesis clinic was started was to allow patients to have scheduled times to remove fluid from their abdomen, thus cutting down on emergency department procedures and unnecessary admissions. Additionally, the benefits of early paracentesis procedural performance by residents and internal medicine physicians have been demonstrated in the literature. A study by Gaetano and colleagues noted that patients undergoing early paracentesis had reduced mortality of 5.5% vs 7.5% in those undergoing late paracentesis.8 This study also showed the in-hospital mortality rate was decreased with paracentesis (6.3%) vs without paracentesis (8.9%).8 By offering residents a chance to participate in the clinic, we have shown that regular opportunities to perform paracentesis may increase the number of physicians capable of independently practicing, improve procedural competency, and improve patient access to this procedure.
Limitations
Our study was not free of bias and has potential weaknesses. The survey was sent to all current residents who have participated in the paracentesis clinic, but not every resident filled out the survey (55% of all residents across 3 years completed the survey, 68.7% who had done clinic that year completed the survey). There is a possibility that those not signed off avoided doing the survey, but we are unable to confirm this. The survey also depended on resident recall of the number of paracenteses completed or looking at their procedure log. It is possible that some procedures were not documented, changing the true number. Additionally, rating comfortability with procedures is subjective, which may also create a source of potential weakness. Future projects should include a baseline survey for residents, followed by a repeat survey a year later to show changes from baseline competency.
Conclusions
A dedicated paracentesis clinic with internal medicine resident involvement may increase resident paracentesis procedural independence, the number of procedures available and performed, and procedural comfort level.
1. Aponte EM, O’Rourke MC, Katta S. Paracentesis. StatPearls [internet]. September 5, 2022. Accessed December 11, 2023. https://www.ncbi.nlm.nih.gov/books/NBK435998
2. Barsuk JH, Feinglass J, Kozmic SE, Hohmann SF, Ganger D, Wayne DB. Specialties performing paracentesis procedures at university hospitals: implications for training and certification. J Hosp Med. 2014;9(3):162-168. doi:10.1002/jhm.2153
3. Cheng Y-W, Sandrasegaran K, Cheng K, et al. A dedicated paracentesis clinic decreases healthcare utilization for serial paracenteses in decompensated cirrhosis. Abdominal Radiology. 2017;43(8):2190-2197. doi:10.1007/s00261-017-1406-y
4. Wang J, Khan S, Wyer P, et al. The role of ultrasound-guided therapeutic paracentesis in an outpatient transitional care program: A case series. Am J Hospice Palliat Med. 2018;35(9):1256-1260. doi:10.1177/1049909118755378
5. Sall D, Warm EJ, Kinnear B, Kelleher M, Jandarov R, O’Toole J. See one, do one, forget one: early skill decay after paracentesis training. J Gen Int Med. 2020;36(5):1346-1351. doi:10.1007/s11606-020-06242-x
6. Berger M, Divilov V, Paredes H, Kesar V, Sun E. Improving resident paracentesis certification rates by using an innovative resident driven procedure service. Am J Gastroenterol. 2018;113(suppl). doi:10.14309/00000434-201810001-00980
7. Pham C, Xu A, Suaez MG. S1250 a pilot study to improve resident paracentesis training and reduce paracentesis delay in admitted patients with cirrhosis. Am J Gastroenterol. 2022;117(10S). doi:10.14309/01.ajg.0000861640.53682.93
8. Gaetano JN, Micic D, Aronsohn A, et al. The benefit of paracentesis on hospitalized adults with cirrhosis and ascites. J Gastroenterol Hepatol. 2016;31(5):1025-1030. doi:10.1111/jgh.13255
Competency in paracentesis is an important procedural skill for medical practitioners caring for patients with decompensated liver cirrhosis. Paracentesis is performed to drain ascitic fluid for both diagnosis and/or therapeutic purposes.1 While this procedure can be performed without the use of ultrasound, it is preferable to use ultrasound to identify an area of fluid that is away from dangerous anatomy including bowel loops, the liver, and spleen. After prepping the area, lidocaine is administered locally. A catheter is then inserted until fluid begins flowing freely. The catheter is connected to a suction canister or collection kit, and the patient is monitored until the flow ceases. Samples can be sent for analysis to determine the etiology of ascites, identify concerns for infection, and more.
Paracentesis is a very common procedure. Barsuk and colleagues noted that between 2010 and 2012, 97,577 procedures were performed across 120 academic medical centers and 290 affiliated hospitals.2 Patients undergo paracentesis in a variety of settings including the emergency department, inpatient hospitalizations, and clinics. Some patients may require only 1 paracentesis procedure while others may require it regularly.
Due to the rising need for paracentesis in the Central Texas Veterans Affairs Hospital (CTVAH) in Temple, a paracentesis clinic was started in February 2018. The goal of the paracentesis clinic was multifocal—to reduce hospital admissions, improve access to regularly scheduled procedures, decrease wait times, and increase patient satisfaction.3 Through the CTVAH affiliation with the Texas A&M internal medicine residency program, the paracentesis clinic started involving and training residents on this procedure. Up to 3 residents are on weekly rotation and can perform up to 6 paracentesis procedures in a week. The purpose of this article was to evaluate resident competency in paracentesis after completion of the paracentesis clinic.
Methods
The paracentesis clinic schedules up to 3 patients on Tuesdays and Thursdays between 8
A survey was sent via email to all categorical internal medicine residents across all 3 program years at the time of data collection. Competency for paracentesis sign-off was defined as completing and logging 5 procedures supervised by a competent physician who confirmed that all portions of the procedure were performed correctly. Residents were also asked to answer questions on a scale from 1 to 10, with 1 representing no confidence and 10 representing strong confidence to practice independently (Table).
We also evaluated the number of procedures performed by internal medicine residents 3 years before the clinic was started in 2015 up to the completion of 2022. The numbers were obtained by examining procedural log data for each year for all internal medicine residents.
Results
Thirty-three residents completed the survey: 10 first-year internal medicine residents (PGY1), 12 second-year residents (PGY2), and 11 third-year residents (PGY3). The mean participation was 4.8 paracentesis sessions per person for the duration of the study. The range of paracentesis procedures performed varied based on PGY year: PGY1s performed 1 to > 10 procedures, PGY2s performed 2 to > 10 procedures, and PGY3s performed 5 to > 10 procedures. Thirty-six percent of residents completed > 10 procedures in the paracentesis clinic; 82% of PGY3s had completed > 10 procedures by December of their third year. Twenty-six residents (79%) were credentialed to perform paracentesis procedures independently after performing > 5 procedures, and 7 residents were not yet cleared for procedural independence.
In the survey, residents rated their comfort with performing paracentesis procedures independently at a mean of 7.9. The mean comfort reported by PGY1s was 7.2, PGY2s was 7.3, and PGY3s was 9.3. Residents also rated their opinion on whether or not the paracentesis clinic adequately prepared them for paracentesis procedural independence; the mean was 8.9 across all residents.
The total number of procedures performed by residents at CTVAH also increased. Starting in 2015, 3 years before the clinic was started, 38 procedures were performed by internal medicine residents, followed by 72 procedures in 2016; 76 in 2017; 58 in 2018; 94 in 2019; 88 in 2020; 136 in 2021; and 188 in 2022.
Discussion
Paracentesis is a simple but invasive procedure to relieve ascites, often relieving patients’ symptoms, preventing hospital admission, and increasing patient satisfaction.4 The CTVAH does not have the capacity to perform outpatient paracentesis effectively in its emergency or radiology departments. Furthermore, the use of the emergency or radiology departments for routine paracentesis may not be feasible due to the acuity of care being provided, as these procedures can be time consuming and can draw away critical resources and time from patients that need emergent care. The paracentesis clinic was then formed to provide veterans access to the procedural care they need, while also preparing residents to ably and confidently perform the procedure independently.
Based on our study, most residents were cleared to independently perform paracentesis procedures across all 3 years, with 79% of residents having completed the required 5 supervised procedures to independently practice. A study assessing unsupervised practice standards showed that paracentesis skill declines as soon as 3 months after training. However, retraining was shown to potentially interrupt this skill decline.5 Studies have shown that procedure-driven electives or services significantly improved paracentesis certification rates and total logged procedures, with minimal funding or scheduling changes required.6 Our clinic showed a significant increase in the number of procedures logged starting with the minimum of 38 procedures in 2015 and ending with 188 procedures logged at the end of 2022.
By allowing residents to routinely return to the paracentesis clinic across all 3 years, residents were more likely to feel comfortable independently performing the procedure, with residents reporting a mean comfort score of 7.9. The spaced repetition and ability to work with the clinic during elective time allows regular opportunities to undergo supervised training in a controlled environment and created scheduled retraining opportunities. Future studies should evaluate residents prior to each paracentesis clinic to ascertain if skill decline is occurring at a slower rate.
The inpatient effect of the clinic is also multifocal. Pham and colleagues showed that integrating paracentesis into timely training can reduce paracentesis delay and delays in care.7 By increasing the volume of procedures each resident performs and creating a sense of confidence amongst residents, the clinic increases the number of residents able and willing to perform inpatient procedures, thus reducing the number of unnecessary consultations and hospital resources. One of the reasons the paracentesis clinic was started was to allow patients to have scheduled times to remove fluid from their abdomen, thus cutting down on emergency department procedures and unnecessary admissions. Additionally, the benefits of early paracentesis procedural performance by residents and internal medicine physicians have been demonstrated in the literature. A study by Gaetano and colleagues noted that patients undergoing early paracentesis had reduced mortality of 5.5% vs 7.5% in those undergoing late paracentesis.8 This study also showed the in-hospital mortality rate was decreased with paracentesis (6.3%) vs without paracentesis (8.9%).8 By offering residents a chance to participate in the clinic, we have shown that regular opportunities to perform paracentesis may increase the number of physicians capable of independently practicing, improve procedural competency, and improve patient access to this procedure.
Limitations
Our study was not free of bias and has potential weaknesses. The survey was sent to all current residents who have participated in the paracentesis clinic, but not every resident filled out the survey (55% of all residents across 3 years completed the survey, 68.7% who had done clinic that year completed the survey). There is a possibility that those not signed off avoided doing the survey, but we are unable to confirm this. The survey also depended on resident recall of the number of paracenteses completed or looking at their procedure log. It is possible that some procedures were not documented, changing the true number. Additionally, rating comfortability with procedures is subjective, which may also create a source of potential weakness. Future projects should include a baseline survey for residents, followed by a repeat survey a year later to show changes from baseline competency.
Conclusions
A dedicated paracentesis clinic with internal medicine resident involvement may increase resident paracentesis procedural independence, the number of procedures available and performed, and procedural comfort level.
Competency in paracentesis is an important procedural skill for medical practitioners caring for patients with decompensated liver cirrhosis. Paracentesis is performed to drain ascitic fluid for both diagnosis and/or therapeutic purposes.1 While this procedure can be performed without the use of ultrasound, it is preferable to use ultrasound to identify an area of fluid that is away from dangerous anatomy including bowel loops, the liver, and spleen. After prepping the area, lidocaine is administered locally. A catheter is then inserted until fluid begins flowing freely. The catheter is connected to a suction canister or collection kit, and the patient is monitored until the flow ceases. Samples can be sent for analysis to determine the etiology of ascites, identify concerns for infection, and more.
Paracentesis is a very common procedure. Barsuk and colleagues noted that between 2010 and 2012, 97,577 procedures were performed across 120 academic medical centers and 290 affiliated hospitals.2 Patients undergo paracentesis in a variety of settings including the emergency department, inpatient hospitalizations, and clinics. Some patients may require only 1 paracentesis procedure while others may require it regularly.
Due to the rising need for paracentesis in the Central Texas Veterans Affairs Hospital (CTVAH) in Temple, a paracentesis clinic was started in February 2018. The goal of the paracentesis clinic was multifocal—to reduce hospital admissions, improve access to regularly scheduled procedures, decrease wait times, and increase patient satisfaction.3 Through the CTVAH affiliation with the Texas A&M internal medicine residency program, the paracentesis clinic started involving and training residents on this procedure. Up to 3 residents are on weekly rotation and can perform up to 6 paracentesis procedures in a week. The purpose of this article was to evaluate resident competency in paracentesis after completion of the paracentesis clinic.
Methods
The paracentesis clinic schedules up to 3 patients on Tuesdays and Thursdays between 8
A survey was sent via email to all categorical internal medicine residents across all 3 program years at the time of data collection. Competency for paracentesis sign-off was defined as completing and logging 5 procedures supervised by a competent physician who confirmed that all portions of the procedure were performed correctly. Residents were also asked to answer questions on a scale from 1 to 10, with 1 representing no confidence and 10 representing strong confidence to practice independently (Table).
We also evaluated the number of procedures performed by internal medicine residents 3 years before the clinic was started in 2015 up to the completion of 2022. The numbers were obtained by examining procedural log data for each year for all internal medicine residents.
Results
Thirty-three residents completed the survey: 10 first-year internal medicine residents (PGY1), 12 second-year residents (PGY2), and 11 third-year residents (PGY3). The mean participation was 4.8 paracentesis sessions per person for the duration of the study. The range of paracentesis procedures performed varied based on PGY year: PGY1s performed 1 to > 10 procedures, PGY2s performed 2 to > 10 procedures, and PGY3s performed 5 to > 10 procedures. Thirty-six percent of residents completed > 10 procedures in the paracentesis clinic; 82% of PGY3s had completed > 10 procedures by December of their third year. Twenty-six residents (79%) were credentialed to perform paracentesis procedures independently after performing > 5 procedures, and 7 residents were not yet cleared for procedural independence.
In the survey, residents rated their comfort with performing paracentesis procedures independently at a mean of 7.9. The mean comfort reported by PGY1s was 7.2, PGY2s was 7.3, and PGY3s was 9.3. Residents also rated their opinion on whether or not the paracentesis clinic adequately prepared them for paracentesis procedural independence; the mean was 8.9 across all residents.
The total number of procedures performed by residents at CTVAH also increased. Starting in 2015, 3 years before the clinic was started, 38 procedures were performed by internal medicine residents, followed by 72 procedures in 2016; 76 in 2017; 58 in 2018; 94 in 2019; 88 in 2020; 136 in 2021; and 188 in 2022.
Discussion
Paracentesis is a simple but invasive procedure to relieve ascites, often relieving patients’ symptoms, preventing hospital admission, and increasing patient satisfaction.4 The CTVAH does not have the capacity to perform outpatient paracentesis effectively in its emergency or radiology departments. Furthermore, the use of the emergency or radiology departments for routine paracentesis may not be feasible due to the acuity of care being provided, as these procedures can be time consuming and can draw away critical resources and time from patients that need emergent care. The paracentesis clinic was then formed to provide veterans access to the procedural care they need, while also preparing residents to ably and confidently perform the procedure independently.
Based on our study, most residents were cleared to independently perform paracentesis procedures across all 3 years, with 79% of residents having completed the required 5 supervised procedures to independently practice. A study assessing unsupervised practice standards showed that paracentesis skill declines as soon as 3 months after training. However, retraining was shown to potentially interrupt this skill decline.5 Studies have shown that procedure-driven electives or services significantly improved paracentesis certification rates and total logged procedures, with minimal funding or scheduling changes required.6 Our clinic showed a significant increase in the number of procedures logged starting with the minimum of 38 procedures in 2015 and ending with 188 procedures logged at the end of 2022.
By allowing residents to routinely return to the paracentesis clinic across all 3 years, residents were more likely to feel comfortable independently performing the procedure, with residents reporting a mean comfort score of 7.9. The spaced repetition and ability to work with the clinic during elective time allows regular opportunities to undergo supervised training in a controlled environment and created scheduled retraining opportunities. Future studies should evaluate residents prior to each paracentesis clinic to ascertain if skill decline is occurring at a slower rate.
The inpatient effect of the clinic is also multifocal. Pham and colleagues showed that integrating paracentesis into timely training can reduce paracentesis delay and delays in care.7 By increasing the volume of procedures each resident performs and creating a sense of confidence amongst residents, the clinic increases the number of residents able and willing to perform inpatient procedures, thus reducing the number of unnecessary consultations and hospital resources. One of the reasons the paracentesis clinic was started was to allow patients to have scheduled times to remove fluid from their abdomen, thus cutting down on emergency department procedures and unnecessary admissions. Additionally, the benefits of early paracentesis procedural performance by residents and internal medicine physicians have been demonstrated in the literature. A study by Gaetano and colleagues noted that patients undergoing early paracentesis had reduced mortality of 5.5% vs 7.5% in those undergoing late paracentesis.8 This study also showed the in-hospital mortality rate was decreased with paracentesis (6.3%) vs without paracentesis (8.9%).8 By offering residents a chance to participate in the clinic, we have shown that regular opportunities to perform paracentesis may increase the number of physicians capable of independently practicing, improve procedural competency, and improve patient access to this procedure.
Limitations
Our study was not free of bias and has potential weaknesses. The survey was sent to all current residents who have participated in the paracentesis clinic, but not every resident filled out the survey (55% of all residents across 3 years completed the survey, 68.7% who had done clinic that year completed the survey). There is a possibility that those not signed off avoided doing the survey, but we are unable to confirm this. The survey also depended on resident recall of the number of paracenteses completed or looking at their procedure log. It is possible that some procedures were not documented, changing the true number. Additionally, rating comfortability with procedures is subjective, which may also create a source of potential weakness. Future projects should include a baseline survey for residents, followed by a repeat survey a year later to show changes from baseline competency.
Conclusions
A dedicated paracentesis clinic with internal medicine resident involvement may increase resident paracentesis procedural independence, the number of procedures available and performed, and procedural comfort level.
1. Aponte EM, O’Rourke MC, Katta S. Paracentesis. StatPearls [internet]. September 5, 2022. Accessed December 11, 2023. https://www.ncbi.nlm.nih.gov/books/NBK435998
2. Barsuk JH, Feinglass J, Kozmic SE, Hohmann SF, Ganger D, Wayne DB. Specialties performing paracentesis procedures at university hospitals: implications for training and certification. J Hosp Med. 2014;9(3):162-168. doi:10.1002/jhm.2153
3. Cheng Y-W, Sandrasegaran K, Cheng K, et al. A dedicated paracentesis clinic decreases healthcare utilization for serial paracenteses in decompensated cirrhosis. Abdominal Radiology. 2017;43(8):2190-2197. doi:10.1007/s00261-017-1406-y
4. Wang J, Khan S, Wyer P, et al. The role of ultrasound-guided therapeutic paracentesis in an outpatient transitional care program: A case series. Am J Hospice Palliat Med. 2018;35(9):1256-1260. doi:10.1177/1049909118755378
5. Sall D, Warm EJ, Kinnear B, Kelleher M, Jandarov R, O’Toole J. See one, do one, forget one: early skill decay after paracentesis training. J Gen Int Med. 2020;36(5):1346-1351. doi:10.1007/s11606-020-06242-x
6. Berger M, Divilov V, Paredes H, Kesar V, Sun E. Improving resident paracentesis certification rates by using an innovative resident driven procedure service. Am J Gastroenterol. 2018;113(suppl). doi:10.14309/00000434-201810001-00980
7. Pham C, Xu A, Suaez MG. S1250 a pilot study to improve resident paracentesis training and reduce paracentesis delay in admitted patients with cirrhosis. Am J Gastroenterol. 2022;117(10S). doi:10.14309/01.ajg.0000861640.53682.93
8. Gaetano JN, Micic D, Aronsohn A, et al. The benefit of paracentesis on hospitalized adults with cirrhosis and ascites. J Gastroenterol Hepatol. 2016;31(5):1025-1030. doi:10.1111/jgh.13255
1. Aponte EM, O’Rourke MC, Katta S. Paracentesis. StatPearls [internet]. September 5, 2022. Accessed December 11, 2023. https://www.ncbi.nlm.nih.gov/books/NBK435998
2. Barsuk JH, Feinglass J, Kozmic SE, Hohmann SF, Ganger D, Wayne DB. Specialties performing paracentesis procedures at university hospitals: implications for training and certification. J Hosp Med. 2014;9(3):162-168. doi:10.1002/jhm.2153
3. Cheng Y-W, Sandrasegaran K, Cheng K, et al. A dedicated paracentesis clinic decreases healthcare utilization for serial paracenteses in decompensated cirrhosis. Abdominal Radiology. 2017;43(8):2190-2197. doi:10.1007/s00261-017-1406-y
4. Wang J, Khan S, Wyer P, et al. The role of ultrasound-guided therapeutic paracentesis in an outpatient transitional care program: A case series. Am J Hospice Palliat Med. 2018;35(9):1256-1260. doi:10.1177/1049909118755378
5. Sall D, Warm EJ, Kinnear B, Kelleher M, Jandarov R, O’Toole J. See one, do one, forget one: early skill decay after paracentesis training. J Gen Int Med. 2020;36(5):1346-1351. doi:10.1007/s11606-020-06242-x
6. Berger M, Divilov V, Paredes H, Kesar V, Sun E. Improving resident paracentesis certification rates by using an innovative resident driven procedure service. Am J Gastroenterol. 2018;113(suppl). doi:10.14309/00000434-201810001-00980
7. Pham C, Xu A, Suaez MG. S1250 a pilot study to improve resident paracentesis training and reduce paracentesis delay in admitted patients with cirrhosis. Am J Gastroenterol. 2022;117(10S). doi:10.14309/01.ajg.0000861640.53682.93
8. Gaetano JN, Micic D, Aronsohn A, et al. The benefit of paracentesis on hospitalized adults with cirrhosis and ascites. J Gastroenterol Hepatol. 2016;31(5):1025-1030. doi:10.1111/jgh.13255
Leader Rounding for High Reliability and Improved Patient Safety
The hospital is altogether the most complex human organization ever devised. Peter Drucker 1
The ever-changing landscape of today’s increasingly complex health care system depends on implementing multifaceted, team-based methods of care delivery to provide safe, effective patient care.2 Critical to establishing and sustaining exceptionally safe, effective patient care is open, transparent communication among members of interprofessional teams with senior leaders.3 However, current evidence shows that poor communication among interprofessional health care teams and leadership is commonplace and a significant contributing factor to inefficiencies, medical errors, and poor outcomes.4 One strategy for improving communication is through the implementation of
We describe the importance of leader rounding for high reliability as an approach to improving patient safety. Based on a review of the literature, our experiences, and lessons learned, we offer recommendations for how health care organizations on the journey to high reliability can improve patient safety.
Rounding in health care is not new. In fact, rounding has been a strong principal practice globally for more than 2 decades.6 During this time, varied rounding approaches have emerged, oftentimes focused on areas of interest, such as patient care, environmental services, facilities management, and discharge planning.4,7 Variations also might involve the location of the rounds, such as a patient’s bedside, unit hallways, and conference rooms as well as the naming of rounds, such as interdisciplinary/multidisciplinary, teaching, and walkrounds.7-10
A different type of rounding that is characteristic of high reliability organizations (HROs) is leader rounding for high reliability. The Veterans Health Administration (VHA) formally launched its journey to becoming an
Leader rounding for high reliability includes regularly scheduled, structured visits, with interdisciplinary teams to discuss high reliability, safety, and improvement efforts. The specific aim of these particular rounds is for senior leaders to be visible where teams are located and learn from staff (especially those on the frontlines of care) about day-to-day challenges that may contribute to patient harm.12,13 Leader rounding for high reliability is also an important approach to improving leadership visibility across the organization, demonstrating a commitment to high reliability, and building trust and relationships with staff through open and honest dialogue. It is also an important approach to increasing leadership understanding of operational, clinical, nonclinical, patient experience issues, and concern related to safety.11 This opportunity enables leaders to provide and receive real-time feedback from staff.9,11 This experience also gives leaders an opportunity to reinforce
In preparation for implementing a leader rounding for high reliability process at the VABHS, we conducted an extensive literature review for peer-reviewed publications published between January 2015 and September 2022 regarding how other organizations implemented leader rounding. This search found a dearth of evidence as it specifically relates to leader rounding for high reliability. This motivated us to create a process for developing and implementing leader rounding for high reliability in pursuit of improving patient safety. With this objective in mind, we created and piloted a process in the fall of 2023. The first 3 months were focused on the medical center director rounding with other members of the executive leadership team to assess the feasibility and acceptability of the process. In December 2023, members of the executive leadership team began conducting leader rounding for high reliability separately. The following steps are based on the lessons we have gleaned from evolving evidence, our experiences, and developing and implementing an approach to leader rounding for high reliability.
ESTABLISH A PROCESS
Leader rounding for high reliability is performed by health care organization executive leadership, directors, managers, and supervisors. When properly conducted, increased levels of teamwork and more effective bidirectional communication take place, resulting in a united team motivated to improve patient safety.16,17 Important early steps for implementing leader rounding for high reliability include establishing a process and getting leadership buy-in. Purposeful attention to planning is critical as is understanding the organizational factors that might deter success. Establishing a process should consider facilitators and barriers to implementation, which can include high vs low leadership turnover, structured vs unstructured rounding, and time for rounding vs competing demands.18,19 We have learned that effective planning is important for ensuring that leadership teams are well prepared and ambitious about leader rounding for high reliability.
Leader rounding for high reliability involves brief 10-to-15-minute interactions with interdisciplinary teams, including frontline staff. For health care organizations beginning to implement this approach, having scripts or checklists accessible might be of help. If possible, the rounds should be scheduled in advance. This helps to avoid rounding in areas at their busiest times. When possible, leader rounding for high reliability should occur as planned. Canceling rounds sends the message that leader rounding for high reliability and the valuable interactions they support are a low priority. When conflicts arise, another leader should be sent to participate. Developing a list of questions in advance helps to underscore key messages to be shared as well as reinforce principles, practices, behaviors, and attitudes related to high reliability (Appendix 1).11
Finally, closing the loop is critical to the leader rounding process and to improve bidirectional communication. Closed-loop communication, following up on and/or closing out an area of discussion, not only promotes a shared understanding of information but has been found to improve patient safety.19 Effective leader rounding for high reliability includes summarizing issues and opportunities, deciding on a date for resolution for open action items, and identifying who is responsible for taking action. Senior leaders are not responsible for resolving all issues. If a team or manager of a work area can solve any issues identified, this should be encouraged and supported so accountability is maintained at the most appropriate level of the organization.
Instrumental to leader rounding for high reliability is establishing a cadence for when leaders will visit work areas.14 The most critical strategy, especially in times of change, is consistency in rounding.11 At the start of implementation, we decided on a biweekly cadence. Initially leaders visited areas of the organization within their respective reporting structure. Once this was established, leaders periodically round in areas outside their scope of responsibility. This affords leaders the opportunity to observe other areas of the organization. As noted, it is important for leaders to be flexible with the rounding process especially in areas where direct patient care is being provided.
Tracking
Developing a tracking tool also is important for an effective leader rounding process. This tool is used to document issues and concerns identified during the rounding process, assign accountability, track the status of items, and close the loop when completed. One of the most commonly reported hurdles to staff sharing information to promote a culture of safety is the lack of feedback on what actions were taken to address the concern or issue raised with leadership. Closed-loop communication is critical for keeping staff continually engaged in efforts to promote a culture of safety.20 We have found that a tracking tool helps to ensure that closed-loop communication takes place.
Various platforms can be used for tracking items and providing follow-up, including paper worksheets, spreadsheets, databases, or third party software (eg, SharePoint, TruthPoint Rounds, GetWell Rounds). The tracking tool should have a standardized approach for prioritizing issues.
The stoplight classification system uses color coding (Figure 3).21 Green represents a safe space where there are no or low safety risks and are easily addressed at the local level by the area manager with or without assistance from the leadership team rounding, such as staffing.22,23 The unit manager has control of the situation and a plan is actively being implemented. Yellow signifies that areas are at risk, but with increased vigilance, issues do not escalate to a crisis state.22,23 Yellow-coded issues require further investigation by the leadership team. The senior leader on the team designates a process as well as a person responsible for closing the loop with the area manager regarding the status of problem resolution. For example, if the unit manager mentioned previously needing help to find staff, the area manager would suggest or take steps to help the unit manager. The area manager is then responsible for updating the frontline staff. Red-coded issues are urgent, identifying a state of crisis or high risk. Red issues need to be immediately addressed but cannot be resolved during rounds. Senior leaders must evaluate and make decisions to mitigate the threat. A member of the leadership team is tasked with following up with the area manager, typically within 24 hours. A staffing crisis that requires executive leadership help with identifying additional resources would be coded red.
The area manager is responsible for closing the loop with frontline staff. As frontline staff became more comfortable with the process, we observed an upward trend in the number of reported issues. We are now starting to see a downward trend in concerns shared during rounding as managers and frontline staff feel empowered to address issues at the lowest level.
Measuring Impact
Measuring the impact is a critical step to determine the overall effectiveness of leader rounding for high reliability. It can be as simple as requesting candid feedback from frontline staff, supervisors, managers, and service chiefs. For example, 4 months into the implementation process, the VABHS administered a brief staff survey on the overall process, perceived benefits, and challenges experienced (Appendix 2). Potential measures include the counts of leaders rounding, total rounds, rounds cancelled, and staff members actively participating in rounds. Outcomes that can be measured include issues identified, addressed, elevated, and remaining open; number of extended workdays due to rounds; staff staying overtime; and delays in patient care activities.23 Other measures to consider are the effects of rounding on staff as well as patient/family satisfaction, increase in the number of errors and near-miss events reported per month in a health care organizations’ patient safety reporting system, and increased engagement of staff members in continuous process improvement activities. Since the inception of leader rounding for high reliability, the VABHS has seen a slight increase in the number of events entered in the patient safety reporting system. Other factors that may have contributed to this change, including encouragement of reporting at safety forums, and tiered safety huddles.
DISCUSSION
This initiative involved the development and implementation of a leader rounding for high reliability process at the VABHS with the overarching goal of ensuring efficient communication exists among members of the health care team for delivering safe, quality patient care. The initiative was well received by staff from senior leadership to frontline personnel and promoted significant interest in efforts to improve safety across the health care system.
The pilot phase permitted us to examine the feasibility and acceptability of the process to leadership as well as frontline staff. The insight gained and lessons learned through the implementation process helped us make revisions where needed and develop the tools to ensure success. In the second phase of implementation, which commenced in December 2023, each executive leadership team member began leader rounding for high reliability with their respective department service chiefs. Throughout this phase, feedback will be sought on the overall process, perceived benefits, and challenges experienced to make improvements or changes as needed. We also will continue to monitor the number of events entered in the patient safety reporting system. Future efforts will focus on developing a robust program of evaluation to explore the impact of the program on patient/family satisfaction as well as safety outcomes.
Limitations
Developing and implementing a process for leader rounding for high reliability was undertaken to support the VABHS and VHA journey to high reliability. Other health care organizations and integrated systems might identify different processes for improving patient safety and to support their journey to becoming an HRO.
CONCLUSIONS
The importance of leader rounding for high reliability to improve patient safety cannot be emphasized enough in a time where health care systems have become increasingly complex. Health care is a complex adaptive system that requires effective, bidirectional communication and collaboration among all disciplines. One of the most useful, evidence-based strategies for promoting this communication and collaboration to improve a culture of safety is leader rounding for high reliability.
1. Drucker PF. They’re not employees, they’re people. Accessed November 15, 2023. https://hbr.org/2002/02/theyre-not-employees-theyre-people
2. Adams HA, Feudale RM. Implementation of a structured rounding tool for interprofessional care team rounds to improve communication and collaboration in patient care. Pediatr Nurs. 2018;44(5):229-233, 246.
3. Witz I, Lucchese S, Valenzano TJ, et al: Perceptions on implementation of a new standardized reporting tool to support structured morning rounds: recommendations for interprofessional teams and healthcare leaders. J Med Radiat Sci. 2022;53(4):S85-S92. doi:10.1016/j.jmir.2022.06.006
4. Blakeney EA, Chu F, White AA, et al. A scoping review of new implementations of interprofessional bedside rounding models to improve teamwork, care, and outcomes in hospitals. J Interprof Care. 2021;10:1-16 [Online ahead of print.] doi:10.1080/13561820.2021.1980379
5. Agency for Research and Healthcare Quality. High reliability. Accessed December 4, 2023. https://psnet.ahrq.gov/primer/high-reliability
6. Hedenstrom M, Harrilson A, Heath M, Dyass S. “What’s old is new again”: innovative health care leader rounding—a strategy to foster connection. Nurse Lead. 2022;20(4):366-370.
7. Walton V, Hogden A, Long JC, Johnson JK, Greenfield D. How do interprofessional healthcare teams perceive the benefits and challenges of interdisciplinary ward rounds. J Multidiscip Healthc. 2019;12:1023-1032. doi:10.2147/JMDH.S226330
8. Walton V, Hogden A, Johnson J, Greenfield D. Ward rounds, participants, roles and perceptions: literature review. Int J Health Care Qual Assur. 2016;29(4):364-379. doi:10.1108/IJHCQA-04-2015-0053
9. Sexton JB, Adair KC, Leonard MW, et al. Providing feedback following leadership walkrounds is associated with better patient safety culture, higher employee engagement and lower burnout. BMJ Qual Saf. 2018;27(4):261-270. doi:10.1136/bmjqs-2016-006399
10. Sexton JB, Adair KC, Profit J, et al. Safety culture and workforce well-being associations with positive leadership walkrounds. Jt Comm J Qual Patient Saf. 2021;47(7):403-411. doi:10.1016/j.jcjq.2021.04.001
11. US Department of Veterans Affairs, Veterans Health Administration. Leader’s guide to foundational high reliability organization (HRO) practices. Accessed December 5, 2023. https://dvagov.sharepoint.com/sites/OHT-PMO/high-reliability/Pages/default.aspx
12. Zajac S, Woods A, Tannenbaum S, Salas E, Hollada CL. Overcoming challenges to teamwork in healthcare: a team effectiveness framework and evidence-based guidance. Front Commun. 2021;6:1-20. doi:10.3389/fcomm.2021.606445
13. Department of Veterans Affairs, Veterans Health Administration. VHA’s Vision for a High Reliability Organization. Accessed December 5, 2023. https://www.hsrd.research.va.gov/publications/forum/summer20/default.cfm?ForumMenu=summer20-1
14. Merchant NB, O’Neal J, Dealino-Perez C, Xiang J, Montoya A Jr, Murray JS. A high-reliability organization mindset. Am J Med Qual. 2022;37(6):504-510. doi:10.1097/JMQ.0000000000000086
15. Verhaegh KJ, Seller-Boersma A, Simons R, et al. An exploratory study of healthcare professionals’ perceptions of interprofessional communication and collaboration. J Interprof Care. 2017;31(3):397-400. doi:10.1080/13561820.2017.1289158
16. Winter M, Tjiong L. HCAHPS Series Part 2: Does purposeful leader rounding make a difference? Nurs Manag. 2015;46(2):26-32. doi:10.1097/01.NUMA.0000460034.25697.06
17. Beaird G, Baernholdt M, White KR. Perceptions of interdisciplinary rounding practices. J Clin Nurs. 2020;29(7-8):1141-1150. doi:10.1111/jocn.15161
18. Hendricks S, LaMothe VJ, Kara A. Facilitators and barriers for interprofessional rounding: a qualitative study. Clin Nurse Spec. 2017;31(4):219-228. doi:10.1097/NUR.0000000000000310
19. Diaz MCG, Dawson K. Impact of simulation-based closed-loop communication training on medical errors in a pediatric emergency department. Am J Med Qual. 2020;35(6):474-478. doi:10.1177/1062860620912480
20. Williams S, Fiumara K, Kachalia A, Desai S. Closing the loop with ambulatory staff on safety reports. Jt Comm J Qual Saf. 2020;46(1):44-50. doi:10.1016/j.jcjq.2019.09.009
21. Parbhoo A, Batte J. Traffic lights: putting a stop to unsafe patient transfers. BMJ Qual Improv Rep. 2015;4(1):u204799.w2079. doi:10.1136/bmjquality.u204799.w2079
22. Prineas S, Culwick M, Endlich Y. A proposed system for standardization of colour-coding stages of escalating criticality in clinical incidents. Curr Opin Anaesthesiol. 2021;34(6):752-760. doi:10.1097/ACO.0000000000001071.
23. Merchant NB, O’Neal J, Montoya A, Cox GR, Murray JS. Creating a process for the implementation of tiered huddles in a Veterans Affairs medical center. Mil Med. 2023;188(5-6):901-906. doi:10.1093/milmed/usac073
The hospital is altogether the most complex human organization ever devised. Peter Drucker 1
The ever-changing landscape of today’s increasingly complex health care system depends on implementing multifaceted, team-based methods of care delivery to provide safe, effective patient care.2 Critical to establishing and sustaining exceptionally safe, effective patient care is open, transparent communication among members of interprofessional teams with senior leaders.3 However, current evidence shows that poor communication among interprofessional health care teams and leadership is commonplace and a significant contributing factor to inefficiencies, medical errors, and poor outcomes.4 One strategy for improving communication is through the implementation of
We describe the importance of leader rounding for high reliability as an approach to improving patient safety. Based on a review of the literature, our experiences, and lessons learned, we offer recommendations for how health care organizations on the journey to high reliability can improve patient safety.
Rounding in health care is not new. In fact, rounding has been a strong principal practice globally for more than 2 decades.6 During this time, varied rounding approaches have emerged, oftentimes focused on areas of interest, such as patient care, environmental services, facilities management, and discharge planning.4,7 Variations also might involve the location of the rounds, such as a patient’s bedside, unit hallways, and conference rooms as well as the naming of rounds, such as interdisciplinary/multidisciplinary, teaching, and walkrounds.7-10
A different type of rounding that is characteristic of high reliability organizations (HROs) is leader rounding for high reliability. The Veterans Health Administration (VHA) formally launched its journey to becoming an
Leader rounding for high reliability includes regularly scheduled, structured visits, with interdisciplinary teams to discuss high reliability, safety, and improvement efforts. The specific aim of these particular rounds is for senior leaders to be visible where teams are located and learn from staff (especially those on the frontlines of care) about day-to-day challenges that may contribute to patient harm.12,13 Leader rounding for high reliability is also an important approach to improving leadership visibility across the organization, demonstrating a commitment to high reliability, and building trust and relationships with staff through open and honest dialogue. It is also an important approach to increasing leadership understanding of operational, clinical, nonclinical, patient experience issues, and concern related to safety.11 This opportunity enables leaders to provide and receive real-time feedback from staff.9,11 This experience also gives leaders an opportunity to reinforce
In preparation for implementing a leader rounding for high reliability process at the VABHS, we conducted an extensive literature review for peer-reviewed publications published between January 2015 and September 2022 regarding how other organizations implemented leader rounding. This search found a dearth of evidence as it specifically relates to leader rounding for high reliability. This motivated us to create a process for developing and implementing leader rounding for high reliability in pursuit of improving patient safety. With this objective in mind, we created and piloted a process in the fall of 2023. The first 3 months were focused on the medical center director rounding with other members of the executive leadership team to assess the feasibility and acceptability of the process. In December 2023, members of the executive leadership team began conducting leader rounding for high reliability separately. The following steps are based on the lessons we have gleaned from evolving evidence, our experiences, and developing and implementing an approach to leader rounding for high reliability.
ESTABLISH A PROCESS
Leader rounding for high reliability is performed by health care organization executive leadership, directors, managers, and supervisors. When properly conducted, increased levels of teamwork and more effective bidirectional communication take place, resulting in a united team motivated to improve patient safety.16,17 Important early steps for implementing leader rounding for high reliability include establishing a process and getting leadership buy-in. Purposeful attention to planning is critical as is understanding the organizational factors that might deter success. Establishing a process should consider facilitators and barriers to implementation, which can include high vs low leadership turnover, structured vs unstructured rounding, and time for rounding vs competing demands.18,19 We have learned that effective planning is important for ensuring that leadership teams are well prepared and ambitious about leader rounding for high reliability.
Leader rounding for high reliability involves brief 10-to-15-minute interactions with interdisciplinary teams, including frontline staff. For health care organizations beginning to implement this approach, having scripts or checklists accessible might be of help. If possible, the rounds should be scheduled in advance. This helps to avoid rounding in areas at their busiest times. When possible, leader rounding for high reliability should occur as planned. Canceling rounds sends the message that leader rounding for high reliability and the valuable interactions they support are a low priority. When conflicts arise, another leader should be sent to participate. Developing a list of questions in advance helps to underscore key messages to be shared as well as reinforce principles, practices, behaviors, and attitudes related to high reliability (Appendix 1).11
Finally, closing the loop is critical to the leader rounding process and to improve bidirectional communication. Closed-loop communication, following up on and/or closing out an area of discussion, not only promotes a shared understanding of information but has been found to improve patient safety.19 Effective leader rounding for high reliability includes summarizing issues and opportunities, deciding on a date for resolution for open action items, and identifying who is responsible for taking action. Senior leaders are not responsible for resolving all issues. If a team or manager of a work area can solve any issues identified, this should be encouraged and supported so accountability is maintained at the most appropriate level of the organization.
Instrumental to leader rounding for high reliability is establishing a cadence for when leaders will visit work areas.14 The most critical strategy, especially in times of change, is consistency in rounding.11 At the start of implementation, we decided on a biweekly cadence. Initially leaders visited areas of the organization within their respective reporting structure. Once this was established, leaders periodically round in areas outside their scope of responsibility. This affords leaders the opportunity to observe other areas of the organization. As noted, it is important for leaders to be flexible with the rounding process especially in areas where direct patient care is being provided.
Tracking
Developing a tracking tool also is important for an effective leader rounding process. This tool is used to document issues and concerns identified during the rounding process, assign accountability, track the status of items, and close the loop when completed. One of the most commonly reported hurdles to staff sharing information to promote a culture of safety is the lack of feedback on what actions were taken to address the concern or issue raised with leadership. Closed-loop communication is critical for keeping staff continually engaged in efforts to promote a culture of safety.20 We have found that a tracking tool helps to ensure that closed-loop communication takes place.
Various platforms can be used for tracking items and providing follow-up, including paper worksheets, spreadsheets, databases, or third party software (eg, SharePoint, TruthPoint Rounds, GetWell Rounds). The tracking tool should have a standardized approach for prioritizing issues.
The stoplight classification system uses color coding (Figure 3).21 Green represents a safe space where there are no or low safety risks and are easily addressed at the local level by the area manager with or without assistance from the leadership team rounding, such as staffing.22,23 The unit manager has control of the situation and a plan is actively being implemented. Yellow signifies that areas are at risk, but with increased vigilance, issues do not escalate to a crisis state.22,23 Yellow-coded issues require further investigation by the leadership team. The senior leader on the team designates a process as well as a person responsible for closing the loop with the area manager regarding the status of problem resolution. For example, if the unit manager mentioned previously needing help to find staff, the area manager would suggest or take steps to help the unit manager. The area manager is then responsible for updating the frontline staff. Red-coded issues are urgent, identifying a state of crisis or high risk. Red issues need to be immediately addressed but cannot be resolved during rounds. Senior leaders must evaluate and make decisions to mitigate the threat. A member of the leadership team is tasked with following up with the area manager, typically within 24 hours. A staffing crisis that requires executive leadership help with identifying additional resources would be coded red.
The area manager is responsible for closing the loop with frontline staff. As frontline staff became more comfortable with the process, we observed an upward trend in the number of reported issues. We are now starting to see a downward trend in concerns shared during rounding as managers and frontline staff feel empowered to address issues at the lowest level.
Measuring Impact
Measuring the impact is a critical step to determine the overall effectiveness of leader rounding for high reliability. It can be as simple as requesting candid feedback from frontline staff, supervisors, managers, and service chiefs. For example, 4 months into the implementation process, the VABHS administered a brief staff survey on the overall process, perceived benefits, and challenges experienced (Appendix 2). Potential measures include the counts of leaders rounding, total rounds, rounds cancelled, and staff members actively participating in rounds. Outcomes that can be measured include issues identified, addressed, elevated, and remaining open; number of extended workdays due to rounds; staff staying overtime; and delays in patient care activities.23 Other measures to consider are the effects of rounding on staff as well as patient/family satisfaction, increase in the number of errors and near-miss events reported per month in a health care organizations’ patient safety reporting system, and increased engagement of staff members in continuous process improvement activities. Since the inception of leader rounding for high reliability, the VABHS has seen a slight increase in the number of events entered in the patient safety reporting system. Other factors that may have contributed to this change, including encouragement of reporting at safety forums, and tiered safety huddles.
DISCUSSION
This initiative involved the development and implementation of a leader rounding for high reliability process at the VABHS with the overarching goal of ensuring efficient communication exists among members of the health care team for delivering safe, quality patient care. The initiative was well received by staff from senior leadership to frontline personnel and promoted significant interest in efforts to improve safety across the health care system.
The pilot phase permitted us to examine the feasibility and acceptability of the process to leadership as well as frontline staff. The insight gained and lessons learned through the implementation process helped us make revisions where needed and develop the tools to ensure success. In the second phase of implementation, which commenced in December 2023, each executive leadership team member began leader rounding for high reliability with their respective department service chiefs. Throughout this phase, feedback will be sought on the overall process, perceived benefits, and challenges experienced to make improvements or changes as needed. We also will continue to monitor the number of events entered in the patient safety reporting system. Future efforts will focus on developing a robust program of evaluation to explore the impact of the program on patient/family satisfaction as well as safety outcomes.
Limitations
Developing and implementing a process for leader rounding for high reliability was undertaken to support the VABHS and VHA journey to high reliability. Other health care organizations and integrated systems might identify different processes for improving patient safety and to support their journey to becoming an HRO.
CONCLUSIONS
The importance of leader rounding for high reliability to improve patient safety cannot be emphasized enough in a time where health care systems have become increasingly complex. Health care is a complex adaptive system that requires effective, bidirectional communication and collaboration among all disciplines. One of the most useful, evidence-based strategies for promoting this communication and collaboration to improve a culture of safety is leader rounding for high reliability.
The hospital is altogether the most complex human organization ever devised. Peter Drucker 1
The ever-changing landscape of today’s increasingly complex health care system depends on implementing multifaceted, team-based methods of care delivery to provide safe, effective patient care.2 Critical to establishing and sustaining exceptionally safe, effective patient care is open, transparent communication among members of interprofessional teams with senior leaders.3 However, current evidence shows that poor communication among interprofessional health care teams and leadership is commonplace and a significant contributing factor to inefficiencies, medical errors, and poor outcomes.4 One strategy for improving communication is through the implementation of
We describe the importance of leader rounding for high reliability as an approach to improving patient safety. Based on a review of the literature, our experiences, and lessons learned, we offer recommendations for how health care organizations on the journey to high reliability can improve patient safety.
Rounding in health care is not new. In fact, rounding has been a strong principal practice globally for more than 2 decades.6 During this time, varied rounding approaches have emerged, oftentimes focused on areas of interest, such as patient care, environmental services, facilities management, and discharge planning.4,7 Variations also might involve the location of the rounds, such as a patient’s bedside, unit hallways, and conference rooms as well as the naming of rounds, such as interdisciplinary/multidisciplinary, teaching, and walkrounds.7-10
A different type of rounding that is characteristic of high reliability organizations (HROs) is leader rounding for high reliability. The Veterans Health Administration (VHA) formally launched its journey to becoming an
Leader rounding for high reliability includes regularly scheduled, structured visits, with interdisciplinary teams to discuss high reliability, safety, and improvement efforts. The specific aim of these particular rounds is for senior leaders to be visible where teams are located and learn from staff (especially those on the frontlines of care) about day-to-day challenges that may contribute to patient harm.12,13 Leader rounding for high reliability is also an important approach to improving leadership visibility across the organization, demonstrating a commitment to high reliability, and building trust and relationships with staff through open and honest dialogue. It is also an important approach to increasing leadership understanding of operational, clinical, nonclinical, patient experience issues, and concern related to safety.11 This opportunity enables leaders to provide and receive real-time feedback from staff.9,11 This experience also gives leaders an opportunity to reinforce
In preparation for implementing a leader rounding for high reliability process at the VABHS, we conducted an extensive literature review for peer-reviewed publications published between January 2015 and September 2022 regarding how other organizations implemented leader rounding. This search found a dearth of evidence as it specifically relates to leader rounding for high reliability. This motivated us to create a process for developing and implementing leader rounding for high reliability in pursuit of improving patient safety. With this objective in mind, we created and piloted a process in the fall of 2023. The first 3 months were focused on the medical center director rounding with other members of the executive leadership team to assess the feasibility and acceptability of the process. In December 2023, members of the executive leadership team began conducting leader rounding for high reliability separately. The following steps are based on the lessons we have gleaned from evolving evidence, our experiences, and developing and implementing an approach to leader rounding for high reliability.
ESTABLISH A PROCESS
Leader rounding for high reliability is performed by health care organization executive leadership, directors, managers, and supervisors. When properly conducted, increased levels of teamwork and more effective bidirectional communication take place, resulting in a united team motivated to improve patient safety.16,17 Important early steps for implementing leader rounding for high reliability include establishing a process and getting leadership buy-in. Purposeful attention to planning is critical as is understanding the organizational factors that might deter success. Establishing a process should consider facilitators and barriers to implementation, which can include high vs low leadership turnover, structured vs unstructured rounding, and time for rounding vs competing demands.18,19 We have learned that effective planning is important for ensuring that leadership teams are well prepared and ambitious about leader rounding for high reliability.
Leader rounding for high reliability involves brief 10-to-15-minute interactions with interdisciplinary teams, including frontline staff. For health care organizations beginning to implement this approach, having scripts or checklists accessible might be of help. If possible, the rounds should be scheduled in advance. This helps to avoid rounding in areas at their busiest times. When possible, leader rounding for high reliability should occur as planned. Canceling rounds sends the message that leader rounding for high reliability and the valuable interactions they support are a low priority. When conflicts arise, another leader should be sent to participate. Developing a list of questions in advance helps to underscore key messages to be shared as well as reinforce principles, practices, behaviors, and attitudes related to high reliability (Appendix 1).11
Finally, closing the loop is critical to the leader rounding process and to improve bidirectional communication. Closed-loop communication, following up on and/or closing out an area of discussion, not only promotes a shared understanding of information but has been found to improve patient safety.19 Effective leader rounding for high reliability includes summarizing issues and opportunities, deciding on a date for resolution for open action items, and identifying who is responsible for taking action. Senior leaders are not responsible for resolving all issues. If a team or manager of a work area can solve any issues identified, this should be encouraged and supported so accountability is maintained at the most appropriate level of the organization.
Instrumental to leader rounding for high reliability is establishing a cadence for when leaders will visit work areas.14 The most critical strategy, especially in times of change, is consistency in rounding.11 At the start of implementation, we decided on a biweekly cadence. Initially leaders visited areas of the organization within their respective reporting structure. Once this was established, leaders periodically round in areas outside their scope of responsibility. This affords leaders the opportunity to observe other areas of the organization. As noted, it is important for leaders to be flexible with the rounding process especially in areas where direct patient care is being provided.
Tracking
Developing a tracking tool also is important for an effective leader rounding process. This tool is used to document issues and concerns identified during the rounding process, assign accountability, track the status of items, and close the loop when completed. One of the most commonly reported hurdles to staff sharing information to promote a culture of safety is the lack of feedback on what actions were taken to address the concern or issue raised with leadership. Closed-loop communication is critical for keeping staff continually engaged in efforts to promote a culture of safety.20 We have found that a tracking tool helps to ensure that closed-loop communication takes place.
Various platforms can be used for tracking items and providing follow-up, including paper worksheets, spreadsheets, databases, or third party software (eg, SharePoint, TruthPoint Rounds, GetWell Rounds). The tracking tool should have a standardized approach for prioritizing issues.
The stoplight classification system uses color coding (Figure 3).21 Green represents a safe space where there are no or low safety risks and are easily addressed at the local level by the area manager with or without assistance from the leadership team rounding, such as staffing.22,23 The unit manager has control of the situation and a plan is actively being implemented. Yellow signifies that areas are at risk, but with increased vigilance, issues do not escalate to a crisis state.22,23 Yellow-coded issues require further investigation by the leadership team. The senior leader on the team designates a process as well as a person responsible for closing the loop with the area manager regarding the status of problem resolution. For example, if the unit manager mentioned previously needing help to find staff, the area manager would suggest or take steps to help the unit manager. The area manager is then responsible for updating the frontline staff. Red-coded issues are urgent, identifying a state of crisis or high risk. Red issues need to be immediately addressed but cannot be resolved during rounds. Senior leaders must evaluate and make decisions to mitigate the threat. A member of the leadership team is tasked with following up with the area manager, typically within 24 hours. A staffing crisis that requires executive leadership help with identifying additional resources would be coded red.
The area manager is responsible for closing the loop with frontline staff. As frontline staff became more comfortable with the process, we observed an upward trend in the number of reported issues. We are now starting to see a downward trend in concerns shared during rounding as managers and frontline staff feel empowered to address issues at the lowest level.
Measuring Impact
Measuring the impact is a critical step to determine the overall effectiveness of leader rounding for high reliability. It can be as simple as requesting candid feedback from frontline staff, supervisors, managers, and service chiefs. For example, 4 months into the implementation process, the VABHS administered a brief staff survey on the overall process, perceived benefits, and challenges experienced (Appendix 2). Potential measures include the counts of leaders rounding, total rounds, rounds cancelled, and staff members actively participating in rounds. Outcomes that can be measured include issues identified, addressed, elevated, and remaining open; number of extended workdays due to rounds; staff staying overtime; and delays in patient care activities.23 Other measures to consider are the effects of rounding on staff as well as patient/family satisfaction, increase in the number of errors and near-miss events reported per month in a health care organizations’ patient safety reporting system, and increased engagement of staff members in continuous process improvement activities. Since the inception of leader rounding for high reliability, the VABHS has seen a slight increase in the number of events entered in the patient safety reporting system. Other factors that may have contributed to this change, including encouragement of reporting at safety forums, and tiered safety huddles.
DISCUSSION
This initiative involved the development and implementation of a leader rounding for high reliability process at the VABHS with the overarching goal of ensuring efficient communication exists among members of the health care team for delivering safe, quality patient care. The initiative was well received by staff from senior leadership to frontline personnel and promoted significant interest in efforts to improve safety across the health care system.
The pilot phase permitted us to examine the feasibility and acceptability of the process to leadership as well as frontline staff. The insight gained and lessons learned through the implementation process helped us make revisions where needed and develop the tools to ensure success. In the second phase of implementation, which commenced in December 2023, each executive leadership team member began leader rounding for high reliability with their respective department service chiefs. Throughout this phase, feedback will be sought on the overall process, perceived benefits, and challenges experienced to make improvements or changes as needed. We also will continue to monitor the number of events entered in the patient safety reporting system. Future efforts will focus on developing a robust program of evaluation to explore the impact of the program on patient/family satisfaction as well as safety outcomes.
Limitations
Developing and implementing a process for leader rounding for high reliability was undertaken to support the VABHS and VHA journey to high reliability. Other health care organizations and integrated systems might identify different processes for improving patient safety and to support their journey to becoming an HRO.
CONCLUSIONS
The importance of leader rounding for high reliability to improve patient safety cannot be emphasized enough in a time where health care systems have become increasingly complex. Health care is a complex adaptive system that requires effective, bidirectional communication and collaboration among all disciplines. One of the most useful, evidence-based strategies for promoting this communication and collaboration to improve a culture of safety is leader rounding for high reliability.
1. Drucker PF. They’re not employees, they’re people. Accessed November 15, 2023. https://hbr.org/2002/02/theyre-not-employees-theyre-people
2. Adams HA, Feudale RM. Implementation of a structured rounding tool for interprofessional care team rounds to improve communication and collaboration in patient care. Pediatr Nurs. 2018;44(5):229-233, 246.
3. Witz I, Lucchese S, Valenzano TJ, et al: Perceptions on implementation of a new standardized reporting tool to support structured morning rounds: recommendations for interprofessional teams and healthcare leaders. J Med Radiat Sci. 2022;53(4):S85-S92. doi:10.1016/j.jmir.2022.06.006
4. Blakeney EA, Chu F, White AA, et al. A scoping review of new implementations of interprofessional bedside rounding models to improve teamwork, care, and outcomes in hospitals. J Interprof Care. 2021;10:1-16 [Online ahead of print.] doi:10.1080/13561820.2021.1980379
5. Agency for Research and Healthcare Quality. High reliability. Accessed December 4, 2023. https://psnet.ahrq.gov/primer/high-reliability
6. Hedenstrom M, Harrilson A, Heath M, Dyass S. “What’s old is new again”: innovative health care leader rounding—a strategy to foster connection. Nurse Lead. 2022;20(4):366-370.
7. Walton V, Hogden A, Long JC, Johnson JK, Greenfield D. How do interprofessional healthcare teams perceive the benefits and challenges of interdisciplinary ward rounds. J Multidiscip Healthc. 2019;12:1023-1032. doi:10.2147/JMDH.S226330
8. Walton V, Hogden A, Johnson J, Greenfield D. Ward rounds, participants, roles and perceptions: literature review. Int J Health Care Qual Assur. 2016;29(4):364-379. doi:10.1108/IJHCQA-04-2015-0053
9. Sexton JB, Adair KC, Leonard MW, et al. Providing feedback following leadership walkrounds is associated with better patient safety culture, higher employee engagement and lower burnout. BMJ Qual Saf. 2018;27(4):261-270. doi:10.1136/bmjqs-2016-006399
10. Sexton JB, Adair KC, Profit J, et al. Safety culture and workforce well-being associations with positive leadership walkrounds. Jt Comm J Qual Patient Saf. 2021;47(7):403-411. doi:10.1016/j.jcjq.2021.04.001
11. US Department of Veterans Affairs, Veterans Health Administration. Leader’s guide to foundational high reliability organization (HRO) practices. Accessed December 5, 2023. https://dvagov.sharepoint.com/sites/OHT-PMO/high-reliability/Pages/default.aspx
12. Zajac S, Woods A, Tannenbaum S, Salas E, Hollada CL. Overcoming challenges to teamwork in healthcare: a team effectiveness framework and evidence-based guidance. Front Commun. 2021;6:1-20. doi:10.3389/fcomm.2021.606445
13. Department of Veterans Affairs, Veterans Health Administration. VHA’s Vision for a High Reliability Organization. Accessed December 5, 2023. https://www.hsrd.research.va.gov/publications/forum/summer20/default.cfm?ForumMenu=summer20-1
14. Merchant NB, O’Neal J, Dealino-Perez C, Xiang J, Montoya A Jr, Murray JS. A high-reliability organization mindset. Am J Med Qual. 2022;37(6):504-510. doi:10.1097/JMQ.0000000000000086
15. Verhaegh KJ, Seller-Boersma A, Simons R, et al. An exploratory study of healthcare professionals’ perceptions of interprofessional communication and collaboration. J Interprof Care. 2017;31(3):397-400. doi:10.1080/13561820.2017.1289158
16. Winter M, Tjiong L. HCAHPS Series Part 2: Does purposeful leader rounding make a difference? Nurs Manag. 2015;46(2):26-32. doi:10.1097/01.NUMA.0000460034.25697.06
17. Beaird G, Baernholdt M, White KR. Perceptions of interdisciplinary rounding practices. J Clin Nurs. 2020;29(7-8):1141-1150. doi:10.1111/jocn.15161
18. Hendricks S, LaMothe VJ, Kara A. Facilitators and barriers for interprofessional rounding: a qualitative study. Clin Nurse Spec. 2017;31(4):219-228. doi:10.1097/NUR.0000000000000310
19. Diaz MCG, Dawson K. Impact of simulation-based closed-loop communication training on medical errors in a pediatric emergency department. Am J Med Qual. 2020;35(6):474-478. doi:10.1177/1062860620912480
20. Williams S, Fiumara K, Kachalia A, Desai S. Closing the loop with ambulatory staff on safety reports. Jt Comm J Qual Saf. 2020;46(1):44-50. doi:10.1016/j.jcjq.2019.09.009
21. Parbhoo A, Batte J. Traffic lights: putting a stop to unsafe patient transfers. BMJ Qual Improv Rep. 2015;4(1):u204799.w2079. doi:10.1136/bmjquality.u204799.w2079
22. Prineas S, Culwick M, Endlich Y. A proposed system for standardization of colour-coding stages of escalating criticality in clinical incidents. Curr Opin Anaesthesiol. 2021;34(6):752-760. doi:10.1097/ACO.0000000000001071.
23. Merchant NB, O’Neal J, Montoya A, Cox GR, Murray JS. Creating a process for the implementation of tiered huddles in a Veterans Affairs medical center. Mil Med. 2023;188(5-6):901-906. doi:10.1093/milmed/usac073
1. Drucker PF. They’re not employees, they’re people. Accessed November 15, 2023. https://hbr.org/2002/02/theyre-not-employees-theyre-people
2. Adams HA, Feudale RM. Implementation of a structured rounding tool for interprofessional care team rounds to improve communication and collaboration in patient care. Pediatr Nurs. 2018;44(5):229-233, 246.
3. Witz I, Lucchese S, Valenzano TJ, et al: Perceptions on implementation of a new standardized reporting tool to support structured morning rounds: recommendations for interprofessional teams and healthcare leaders. J Med Radiat Sci. 2022;53(4):S85-S92. doi:10.1016/j.jmir.2022.06.006
4. Blakeney EA, Chu F, White AA, et al. A scoping review of new implementations of interprofessional bedside rounding models to improve teamwork, care, and outcomes in hospitals. J Interprof Care. 2021;10:1-16 [Online ahead of print.] doi:10.1080/13561820.2021.1980379
5. Agency for Research and Healthcare Quality. High reliability. Accessed December 4, 2023. https://psnet.ahrq.gov/primer/high-reliability
6. Hedenstrom M, Harrilson A, Heath M, Dyass S. “What’s old is new again”: innovative health care leader rounding—a strategy to foster connection. Nurse Lead. 2022;20(4):366-370.
7. Walton V, Hogden A, Long JC, Johnson JK, Greenfield D. How do interprofessional healthcare teams perceive the benefits and challenges of interdisciplinary ward rounds. J Multidiscip Healthc. 2019;12:1023-1032. doi:10.2147/JMDH.S226330
8. Walton V, Hogden A, Johnson J, Greenfield D. Ward rounds, participants, roles and perceptions: literature review. Int J Health Care Qual Assur. 2016;29(4):364-379. doi:10.1108/IJHCQA-04-2015-0053
9. Sexton JB, Adair KC, Leonard MW, et al. Providing feedback following leadership walkrounds is associated with better patient safety culture, higher employee engagement and lower burnout. BMJ Qual Saf. 2018;27(4):261-270. doi:10.1136/bmjqs-2016-006399
10. Sexton JB, Adair KC, Profit J, et al. Safety culture and workforce well-being associations with positive leadership walkrounds. Jt Comm J Qual Patient Saf. 2021;47(7):403-411. doi:10.1016/j.jcjq.2021.04.001
11. US Department of Veterans Affairs, Veterans Health Administration. Leader’s guide to foundational high reliability organization (HRO) practices. Accessed December 5, 2023. https://dvagov.sharepoint.com/sites/OHT-PMO/high-reliability/Pages/default.aspx
12. Zajac S, Woods A, Tannenbaum S, Salas E, Hollada CL. Overcoming challenges to teamwork in healthcare: a team effectiveness framework and evidence-based guidance. Front Commun. 2021;6:1-20. doi:10.3389/fcomm.2021.606445
13. Department of Veterans Affairs, Veterans Health Administration. VHA’s Vision for a High Reliability Organization. Accessed December 5, 2023. https://www.hsrd.research.va.gov/publications/forum/summer20/default.cfm?ForumMenu=summer20-1
14. Merchant NB, O’Neal J, Dealino-Perez C, Xiang J, Montoya A Jr, Murray JS. A high-reliability organization mindset. Am J Med Qual. 2022;37(6):504-510. doi:10.1097/JMQ.0000000000000086
15. Verhaegh KJ, Seller-Boersma A, Simons R, et al. An exploratory study of healthcare professionals’ perceptions of interprofessional communication and collaboration. J Interprof Care. 2017;31(3):397-400. doi:10.1080/13561820.2017.1289158
16. Winter M, Tjiong L. HCAHPS Series Part 2: Does purposeful leader rounding make a difference? Nurs Manag. 2015;46(2):26-32. doi:10.1097/01.NUMA.0000460034.25697.06
17. Beaird G, Baernholdt M, White KR. Perceptions of interdisciplinary rounding practices. J Clin Nurs. 2020;29(7-8):1141-1150. doi:10.1111/jocn.15161
18. Hendricks S, LaMothe VJ, Kara A. Facilitators and barriers for interprofessional rounding: a qualitative study. Clin Nurse Spec. 2017;31(4):219-228. doi:10.1097/NUR.0000000000000310
19. Diaz MCG, Dawson K. Impact of simulation-based closed-loop communication training on medical errors in a pediatric emergency department. Am J Med Qual. 2020;35(6):474-478. doi:10.1177/1062860620912480
20. Williams S, Fiumara K, Kachalia A, Desai S. Closing the loop with ambulatory staff on safety reports. Jt Comm J Qual Saf. 2020;46(1):44-50. doi:10.1016/j.jcjq.2019.09.009
21. Parbhoo A, Batte J. Traffic lights: putting a stop to unsafe patient transfers. BMJ Qual Improv Rep. 2015;4(1):u204799.w2079. doi:10.1136/bmjquality.u204799.w2079
22. Prineas S, Culwick M, Endlich Y. A proposed system for standardization of colour-coding stages of escalating criticality in clinical incidents. Curr Opin Anaesthesiol. 2021;34(6):752-760. doi:10.1097/ACO.0000000000001071.
23. Merchant NB, O’Neal J, Montoya A, Cox GR, Murray JS. Creating a process for the implementation of tiered huddles in a Veterans Affairs medical center. Mil Med. 2023;188(5-6):901-906. doi:10.1093/milmed/usac073
Nightmare on CIL Street: A Simulation Series to Increase Confidence and Skill in Responding to Clinical Emergencies
The Central Texas Veteran’s Health Care System (CTVHCS) in Temple, Texas, is a 189-bed teaching hospital. CTVHCS opened the Center for Innovation and Learning (CIL) in 2022. The CIL has about 279 m2 of simulation space that includes high- and low-fidelity simulation equipment and multiple laboratories, which can be used to simulate inpatient and outpatient settings. The CIL high-fidelity manikins and environment allow learners to be immersed in the simulation for maximum realism. Computer and video systems provide clear viewing of training, which allows for more in-depth debriefing and learning. CIL simulation training is used by CTVHCS staff, medical residents, and medical and physician assistant students.
The utility of technology in medical education is rapidly evolving. As noted in many studies, simulation creates an environment that can imitate real patients in the format of a lifelike manikin, anatomic regions stations, clinical tasks, and many real-life circumstances.1 Task trainers for procedure simulation have been widely used and studied. A 2020 study noted that simulation training is effective for developing procedural skills in surgery and prevents the decay of surgical skills.2
In reviewing health care education curriculums, we noted that most of the rapid response situations are learned through active patient experiences. Rapid responses are managed by the intensive care unit and primary care teams during the day but at night are run primarily by the postgraduate year 2 (PGY2) night resident and intern. Knowing these logistics and current studies, we decided to build a rapid response simulation curriculum to improve preparedness for PGY1 residents, medical students, and physician assistant students.
Curriculum Planning
Planning the simulation curriculum began with the CTVHCS internal medicine chief resident and registered nurse (RN) educator. CTVHCS data were reviewed to identify the 3 most common rapid response calls from the past 3 years; research on the most common systems affected by rapid responses also was evaluated.
A 2019 study by Lyons and colleagues evaluated 402,023 rapid response activations across 360 hospitals and found that respiratory scenarios made up 38% and cardiac scenarios made up 37%.3 In addition, the CTVHCS has limited support in stroke neurology. Therefore, the internal medicine chief resident and RN educator decided to run 3 evolving rapid response scenarios per session that included cardiac, respiratory, and neurological scenarios. Capabilities and limitations of different high-fidelity manikins were discussed to identify and use the most appropriate simulator for each situation. Objectives that met both general medicine and site-specific education were discussed, and the program was formulated.
Program Description
Nightmare on CIL Street is a simulation-based program designed for new internal medicine residents and students to encounter difficult situations (late at night, on call, or when resources are limited; ie, weekends/holidays) in a controlled simulation environment. During the simulation, learners will be unable to transfer the patient and no additional help is available. Each learner must determine a differential diagnosis and make appropriate medical interventions with only the assistance of a nurse. Scenarios are derived from common rapid response team calls and low-volume/high-impact situations where clinical decisions must be made quickly to ensure the best patient outcomes. High-fidelity manikins that have abilities to respond to questions, simulate breathing, reproduce pathological heart and breath sounds and more are used to create a realistic patient environment.
This program aligns with 2 national Veterans Health Administration priorities: (1) connect veterans to the soonest and best care; and (2) accelerate the Veterans Health Administration journey to be a high-reliability organization (sensitivity to operations, preoccupation with failure, commitment to resilience, and deference to expertise). Nightmare on CIL Street has 3 clinical episodes: 2 cardiac (A Tell-Tale Heart), respiratory (Don’t Breathe), and neurologic (Brain Scan). Additional clinical episodes will be added based on learner feedback and assessed need.
Each simulation event encompassed all 3 episodes that an individual or a team of 2 learners rotate through in a round-robin fashion. The overarching theme for each episode was a rapid response team call with minimal resources that the learner would have to provide care and stabilization. A literature search for rapid response team training programs found few results, but the literature assisted with providing a foundation for Nightmare on CIL Street.4,5 The goal was to completely envelop the learners in a nightmare scenario that required a solution.
After the safety brief and predata collection, learners received a phone call with minimal information about a patient in need of care. The learners responded to the requested area and provided treatment to the emergency over 25 minutes with the bedside nurse (who is an embedded participant). At the conclusion of the scenario, a physician subject matter expert who has been observing, provided a personalized 10-minute debriefing to the learner, which presented specific learning points and opportunities for the learner’s educational development. After the debriefing, learners returned to a conference room and awaited the next call. After all learners completed the 3 episodes, a group debriefing was conducted using the gather, analyze, summarize debriefing framework. The debriefing begins with an open-ended forum for learners to express their thoughts. Then, each scenario is discussed and broken down by key learning objectives. Starting with cardiac and ending with neurology, the logistics of the cases are discussed based on the trajectory of the learners during the scenarios. Each objective is discussed, and learners are allowed to ask questions before moving to the next scenario. After the debriefing, postevent data were gathered.
Objectives
The program objective was to educate residents and students on common rapid response scenarios. We devised each scenario as an evolving simulation where various interventions would improve or worsen vital signs and symptoms. Each scenario had an end goal: cardioversion (cardiac), intubation (respiratory), and transfer (neurologic). Objectives were tailored to the trainees present during the specific simulation (Table).
IMPLEMENTATION
The initial run of the simulation curriculum was implemented on February 22, 2023, and ended on May 17, 2023, with 5 events. Participants included internal medicine PGY1 residents, third-year medical students, and fourth-year physician assistant students. Internal medicine residents ran each scenario with a subject matter expert monitoring; the undergraduate medical trainees partnered with another student. Students were pulled from their ward rotations to attend the simulation, and residents were pulled from electives and wards. Each trainee was able to experience each planned scenario. They were then briefed, participated in each scenario, and ended with a debriefing, discussing each case in detail. Two subject matter experts were always available, and occasionally 4 were present to provide additional knowledge transfer to learners. These included board-certified physicians in internal medicine and pulmonary critical care. Most scenarios were conducted on Wednesday afternoon or Thursday.
The CIL provided 6 staff minimum for every event. The staff controlled the manikins and acted as embedded players for the learners to interact and work with at the bedside. Every embedded RN was provided the same script: They were a new nurse just off orientation and did not know what to do. In addition, they were instructed that no matter who the learner wanted to call/page, that person or service was not answering or unavailable. This forced learners to respond and treat the simulated patient on their own.
Survey Responses
To evaluate the effect of this program on medical education, we administered surveys to the trainees before and after the simulation (Appendix). All questions were evaluated on a 10-point Likert scale (1, minimal comfort; 10, maximum comfort). The postsurvey added an additional Likert scale question and an open-ended question.
Sixteen trainees underwent the simulation curriculum during the 2022 to 2023 academic year, 9 internal medicine PGY1 residents, 4 medical students, and 3 physician assistant students. Postsimulation surveys indicated a mean 2.2 point increase in comfort compared with the presimulation surveys across all questions and participants.
DISCUSSION
The simulation curriculum proved to be successful for all parties, including trainees, medical educators, and simulation staff. Trainees expressed gratitude for the teaching ability of the simulation and the challenge of confronting an evolving scenario. Students also stated that the simulation allowed them to identify knowledge weaknesses.
Medical technology is rapidly advancing. A study evaluating high-fidelity medical simulations between 1969 and 2003 found that they are educationally effective and complement other medical education modalities.6 It is also noted that care provided by junior physicians with a lack of prior exposure to emergencies and unusual clinical syndromes can lead to more adverse effects.7 Simulation curriculums can be used to educate junior physicians as well as trainees on a multitude of medical emergencies, teach systematic approaches to medical scenarios, and increase exposure to unfamiliar experiences.
The goals of this article are to share program details and encourage other training programs with similar capabilities to incorporate simulation into medical education. Using pre- and postsimulation surveys, there was a concrete improvement in the value obtained by participating in this simulation. The Nightmare on CIL Street learners experienced a mean 2.2 point improvement from presimulation survey to postsimulation survey. Some notable improvements were the feelings of preparedness for rapid response situations and developing a systematic approach. As the students who participated in our Nightmare on CIL Street simulation were early in training, we believe the improvement in preparation and developing a systematic approach can be key to their success in their practical environments.
From a site-specific standpoint, improvement in confidence working through cardiac, respiratory, and neurological emergencies will be very useful. The anesthesiology service intubates during respiratory failures and there is no stroke neurologist available at the CTVHCS hospital. Giving trainees experience in these conditions may allow them to better understand their role in coordination during these times and potentially improve patient outcomes. A follow-up questionnaire administered a year after this simulation may be useful in ascertaining the usefulness of the simulation and what items may have been approached differently. We encourage other institutions to build in aspects of their site-specific challenges to improve trainee awareness in approaches to critical scenarios.
Challenges
The greatest challenge for Nightmare on CIL Street was the ability to pull internal medicine residents from their clinical duties to participate in the simulation. As there are many moving parts to their clinical scheduling, residents do not always have sufficient coverage to participate in training. There were also instances where residents needed to cover for another resident preventing them from attending the simulation. In the future, this program will schedule residents months in advance and will have the simulation training built into their rotations.
Medical and physician assistant students were pulled from their ward rotations as well. They rotate on a 2-to-4-week basis and often had already experienced the simulation the week prior, leaving out students for the following week. With more longitudinal planning, students can be pulled on a rotating monthly basis to maximize their participation. Another challenge was deciding whether residents should partner or experience the simulation on their own. After some feedback, it was noted that residents preferred to experience the simulation on their own as this improves their learning value. With the limited resources available, only rotating 3 residents on a scenario limits the number of trainees who can be reached with the program. Running this program throughout an academic year can help to reach more trainees.
CONCLUSIONS
Educating trainees on rapid response scenarios by using a simulation curriculum provides many benefits. Our trainees reported improvement in addressing cardiac, respiratory, and neurological rapid response scenarios after experiencing the simulation. They felt better prepared and had developed a better systematic approach for the future.
Acknowledgments
The authors thank Pawan Sikka, MD, George Martinez, MD and Braden Anderson, MD for participating as physician experts and educating our students. We thank Naomi Devers; Dinetra Jones; Stephanie Garrett; Sara Holton; Evelina Bartnick; Tanelle Smith; Michael Lomax; Shaun Kelemen for their participation as nurses, assistants, and simulation technology experts.
1. Guze PA. Using technology to meet the challenges of medical education. Trans Am Clin Climatol Assoc. 2015;126:260-270.
2. Higgins M, Madan C, Patel R. Development and decay of procedural skills in surgery: a systematic review of the effectiveness of simulation-based medical education interventions. Surgeon. 2021;19(4):e67-e77. doi:10.1016/j.surge.2020.07.013
3. Lyons PG, Edelson DP, Carey KA, et al. Characteristics of rapid response calls in the United States: an analysis of the first 402,023 adult cases from the Get With the Guidelines Resuscitation-Medical Emergency Team registry. Crit Care Med. 2019;47(10):1283-1289. doi:10.1097/CCM.0000000000003912
4. McMurray L, Hall AK, Rich J, Merchant S, Chaplin T. The nightmares course: a longitudinal, multidisciplinary, simulation-based curriculum to train and assess resident competence in resuscitation. J Grad Med Educ. 2017;9(4):503-508. doi:10.4300/JGME-D-16-00462.1
5. Gilic F, Schultz K, Sempowski I, Blagojevic A. “Nightmares-Family Medicine” course is an effective acute care teaching tool for family medicine residents. Simul Healthc. 2019;14(3):157-162. doi:10.1097/SIH.0000000000000355
6. Issenberg SB, McGaghie WC, Petrusa ER, Lee Gordon D, Scalese RJ. Features and uses of high-fidelity medical simulations that lead to effective learning: a BEME systematic review. Med Teach. 2005;27(1):10-28. doi:10.1080/01421590500046924
7. Datta R, Upadhyay K, Jaideep C. Simulation and its role in medical education. Med J Armed Forces India. 2012;68(2):167-172. doi:10.1016/S0377-1237(12)60040-9
The Central Texas Veteran’s Health Care System (CTVHCS) in Temple, Texas, is a 189-bed teaching hospital. CTVHCS opened the Center for Innovation and Learning (CIL) in 2022. The CIL has about 279 m2 of simulation space that includes high- and low-fidelity simulation equipment and multiple laboratories, which can be used to simulate inpatient and outpatient settings. The CIL high-fidelity manikins and environment allow learners to be immersed in the simulation for maximum realism. Computer and video systems provide clear viewing of training, which allows for more in-depth debriefing and learning. CIL simulation training is used by CTVHCS staff, medical residents, and medical and physician assistant students.
The utility of technology in medical education is rapidly evolving. As noted in many studies, simulation creates an environment that can imitate real patients in the format of a lifelike manikin, anatomic regions stations, clinical tasks, and many real-life circumstances.1 Task trainers for procedure simulation have been widely used and studied. A 2020 study noted that simulation training is effective for developing procedural skills in surgery and prevents the decay of surgical skills.2
In reviewing health care education curriculums, we noted that most of the rapid response situations are learned through active patient experiences. Rapid responses are managed by the intensive care unit and primary care teams during the day but at night are run primarily by the postgraduate year 2 (PGY2) night resident and intern. Knowing these logistics and current studies, we decided to build a rapid response simulation curriculum to improve preparedness for PGY1 residents, medical students, and physician assistant students.
Curriculum Planning
Planning the simulation curriculum began with the CTVHCS internal medicine chief resident and registered nurse (RN) educator. CTVHCS data were reviewed to identify the 3 most common rapid response calls from the past 3 years; research on the most common systems affected by rapid responses also was evaluated.
A 2019 study by Lyons and colleagues evaluated 402,023 rapid response activations across 360 hospitals and found that respiratory scenarios made up 38% and cardiac scenarios made up 37%.3 In addition, the CTVHCS has limited support in stroke neurology. Therefore, the internal medicine chief resident and RN educator decided to run 3 evolving rapid response scenarios per session that included cardiac, respiratory, and neurological scenarios. Capabilities and limitations of different high-fidelity manikins were discussed to identify and use the most appropriate simulator for each situation. Objectives that met both general medicine and site-specific education were discussed, and the program was formulated.
Program Description
Nightmare on CIL Street is a simulation-based program designed for new internal medicine residents and students to encounter difficult situations (late at night, on call, or when resources are limited; ie, weekends/holidays) in a controlled simulation environment. During the simulation, learners will be unable to transfer the patient and no additional help is available. Each learner must determine a differential diagnosis and make appropriate medical interventions with only the assistance of a nurse. Scenarios are derived from common rapid response team calls and low-volume/high-impact situations where clinical decisions must be made quickly to ensure the best patient outcomes. High-fidelity manikins that have abilities to respond to questions, simulate breathing, reproduce pathological heart and breath sounds and more are used to create a realistic patient environment.
This program aligns with 2 national Veterans Health Administration priorities: (1) connect veterans to the soonest and best care; and (2) accelerate the Veterans Health Administration journey to be a high-reliability organization (sensitivity to operations, preoccupation with failure, commitment to resilience, and deference to expertise). Nightmare on CIL Street has 3 clinical episodes: 2 cardiac (A Tell-Tale Heart), respiratory (Don’t Breathe), and neurologic (Brain Scan). Additional clinical episodes will be added based on learner feedback and assessed need.
Each simulation event encompassed all 3 episodes that an individual or a team of 2 learners rotate through in a round-robin fashion. The overarching theme for each episode was a rapid response team call with minimal resources that the learner would have to provide care and stabilization. A literature search for rapid response team training programs found few results, but the literature assisted with providing a foundation for Nightmare on CIL Street.4,5 The goal was to completely envelop the learners in a nightmare scenario that required a solution.
After the safety brief and predata collection, learners received a phone call with minimal information about a patient in need of care. The learners responded to the requested area and provided treatment to the emergency over 25 minutes with the bedside nurse (who is an embedded participant). At the conclusion of the scenario, a physician subject matter expert who has been observing, provided a personalized 10-minute debriefing to the learner, which presented specific learning points and opportunities for the learner’s educational development. After the debriefing, learners returned to a conference room and awaited the next call. After all learners completed the 3 episodes, a group debriefing was conducted using the gather, analyze, summarize debriefing framework. The debriefing begins with an open-ended forum for learners to express their thoughts. Then, each scenario is discussed and broken down by key learning objectives. Starting with cardiac and ending with neurology, the logistics of the cases are discussed based on the trajectory of the learners during the scenarios. Each objective is discussed, and learners are allowed to ask questions before moving to the next scenario. After the debriefing, postevent data were gathered.
Objectives
The program objective was to educate residents and students on common rapid response scenarios. We devised each scenario as an evolving simulation where various interventions would improve or worsen vital signs and symptoms. Each scenario had an end goal: cardioversion (cardiac), intubation (respiratory), and transfer (neurologic). Objectives were tailored to the trainees present during the specific simulation (Table).
IMPLEMENTATION
The initial run of the simulation curriculum was implemented on February 22, 2023, and ended on May 17, 2023, with 5 events. Participants included internal medicine PGY1 residents, third-year medical students, and fourth-year physician assistant students. Internal medicine residents ran each scenario with a subject matter expert monitoring; the undergraduate medical trainees partnered with another student. Students were pulled from their ward rotations to attend the simulation, and residents were pulled from electives and wards. Each trainee was able to experience each planned scenario. They were then briefed, participated in each scenario, and ended with a debriefing, discussing each case in detail. Two subject matter experts were always available, and occasionally 4 were present to provide additional knowledge transfer to learners. These included board-certified physicians in internal medicine and pulmonary critical care. Most scenarios were conducted on Wednesday afternoon or Thursday.
The CIL provided 6 staff minimum for every event. The staff controlled the manikins and acted as embedded players for the learners to interact and work with at the bedside. Every embedded RN was provided the same script: They were a new nurse just off orientation and did not know what to do. In addition, they were instructed that no matter who the learner wanted to call/page, that person or service was not answering or unavailable. This forced learners to respond and treat the simulated patient on their own.
Survey Responses
To evaluate the effect of this program on medical education, we administered surveys to the trainees before and after the simulation (Appendix). All questions were evaluated on a 10-point Likert scale (1, minimal comfort; 10, maximum comfort). The postsurvey added an additional Likert scale question and an open-ended question.
Sixteen trainees underwent the simulation curriculum during the 2022 to 2023 academic year, 9 internal medicine PGY1 residents, 4 medical students, and 3 physician assistant students. Postsimulation surveys indicated a mean 2.2 point increase in comfort compared with the presimulation surveys across all questions and participants.
DISCUSSION
The simulation curriculum proved to be successful for all parties, including trainees, medical educators, and simulation staff. Trainees expressed gratitude for the teaching ability of the simulation and the challenge of confronting an evolving scenario. Students also stated that the simulation allowed them to identify knowledge weaknesses.
Medical technology is rapidly advancing. A study evaluating high-fidelity medical simulations between 1969 and 2003 found that they are educationally effective and complement other medical education modalities.6 It is also noted that care provided by junior physicians with a lack of prior exposure to emergencies and unusual clinical syndromes can lead to more adverse effects.7 Simulation curriculums can be used to educate junior physicians as well as trainees on a multitude of medical emergencies, teach systematic approaches to medical scenarios, and increase exposure to unfamiliar experiences.
The goals of this article are to share program details and encourage other training programs with similar capabilities to incorporate simulation into medical education. Using pre- and postsimulation surveys, there was a concrete improvement in the value obtained by participating in this simulation. The Nightmare on CIL Street learners experienced a mean 2.2 point improvement from presimulation survey to postsimulation survey. Some notable improvements were the feelings of preparedness for rapid response situations and developing a systematic approach. As the students who participated in our Nightmare on CIL Street simulation were early in training, we believe the improvement in preparation and developing a systematic approach can be key to their success in their practical environments.
From a site-specific standpoint, improvement in confidence working through cardiac, respiratory, and neurological emergencies will be very useful. The anesthesiology service intubates during respiratory failures and there is no stroke neurologist available at the CTVHCS hospital. Giving trainees experience in these conditions may allow them to better understand their role in coordination during these times and potentially improve patient outcomes. A follow-up questionnaire administered a year after this simulation may be useful in ascertaining the usefulness of the simulation and what items may have been approached differently. We encourage other institutions to build in aspects of their site-specific challenges to improve trainee awareness in approaches to critical scenarios.
Challenges
The greatest challenge for Nightmare on CIL Street was the ability to pull internal medicine residents from their clinical duties to participate in the simulation. As there are many moving parts to their clinical scheduling, residents do not always have sufficient coverage to participate in training. There were also instances where residents needed to cover for another resident preventing them from attending the simulation. In the future, this program will schedule residents months in advance and will have the simulation training built into their rotations.
Medical and physician assistant students were pulled from their ward rotations as well. They rotate on a 2-to-4-week basis and often had already experienced the simulation the week prior, leaving out students for the following week. With more longitudinal planning, students can be pulled on a rotating monthly basis to maximize their participation. Another challenge was deciding whether residents should partner or experience the simulation on their own. After some feedback, it was noted that residents preferred to experience the simulation on their own as this improves their learning value. With the limited resources available, only rotating 3 residents on a scenario limits the number of trainees who can be reached with the program. Running this program throughout an academic year can help to reach more trainees.
CONCLUSIONS
Educating trainees on rapid response scenarios by using a simulation curriculum provides many benefits. Our trainees reported improvement in addressing cardiac, respiratory, and neurological rapid response scenarios after experiencing the simulation. They felt better prepared and had developed a better systematic approach for the future.
Acknowledgments
The authors thank Pawan Sikka, MD, George Martinez, MD and Braden Anderson, MD for participating as physician experts and educating our students. We thank Naomi Devers; Dinetra Jones; Stephanie Garrett; Sara Holton; Evelina Bartnick; Tanelle Smith; Michael Lomax; Shaun Kelemen for their participation as nurses, assistants, and simulation technology experts.
The Central Texas Veteran’s Health Care System (CTVHCS) in Temple, Texas, is a 189-bed teaching hospital. CTVHCS opened the Center for Innovation and Learning (CIL) in 2022. The CIL has about 279 m2 of simulation space that includes high- and low-fidelity simulation equipment and multiple laboratories, which can be used to simulate inpatient and outpatient settings. The CIL high-fidelity manikins and environment allow learners to be immersed in the simulation for maximum realism. Computer and video systems provide clear viewing of training, which allows for more in-depth debriefing and learning. CIL simulation training is used by CTVHCS staff, medical residents, and medical and physician assistant students.
The utility of technology in medical education is rapidly evolving. As noted in many studies, simulation creates an environment that can imitate real patients in the format of a lifelike manikin, anatomic regions stations, clinical tasks, and many real-life circumstances.1 Task trainers for procedure simulation have been widely used and studied. A 2020 study noted that simulation training is effective for developing procedural skills in surgery and prevents the decay of surgical skills.2
In reviewing health care education curriculums, we noted that most of the rapid response situations are learned through active patient experiences. Rapid responses are managed by the intensive care unit and primary care teams during the day but at night are run primarily by the postgraduate year 2 (PGY2) night resident and intern. Knowing these logistics and current studies, we decided to build a rapid response simulation curriculum to improve preparedness for PGY1 residents, medical students, and physician assistant students.
Curriculum Planning
Planning the simulation curriculum began with the CTVHCS internal medicine chief resident and registered nurse (RN) educator. CTVHCS data were reviewed to identify the 3 most common rapid response calls from the past 3 years; research on the most common systems affected by rapid responses also was evaluated.
A 2019 study by Lyons and colleagues evaluated 402,023 rapid response activations across 360 hospitals and found that respiratory scenarios made up 38% and cardiac scenarios made up 37%.3 In addition, the CTVHCS has limited support in stroke neurology. Therefore, the internal medicine chief resident and RN educator decided to run 3 evolving rapid response scenarios per session that included cardiac, respiratory, and neurological scenarios. Capabilities and limitations of different high-fidelity manikins were discussed to identify and use the most appropriate simulator for each situation. Objectives that met both general medicine and site-specific education were discussed, and the program was formulated.
Program Description
Nightmare on CIL Street is a simulation-based program designed for new internal medicine residents and students to encounter difficult situations (late at night, on call, or when resources are limited; ie, weekends/holidays) in a controlled simulation environment. During the simulation, learners will be unable to transfer the patient and no additional help is available. Each learner must determine a differential diagnosis and make appropriate medical interventions with only the assistance of a nurse. Scenarios are derived from common rapid response team calls and low-volume/high-impact situations where clinical decisions must be made quickly to ensure the best patient outcomes. High-fidelity manikins that have abilities to respond to questions, simulate breathing, reproduce pathological heart and breath sounds and more are used to create a realistic patient environment.
This program aligns with 2 national Veterans Health Administration priorities: (1) connect veterans to the soonest and best care; and (2) accelerate the Veterans Health Administration journey to be a high-reliability organization (sensitivity to operations, preoccupation with failure, commitment to resilience, and deference to expertise). Nightmare on CIL Street has 3 clinical episodes: 2 cardiac (A Tell-Tale Heart), respiratory (Don’t Breathe), and neurologic (Brain Scan). Additional clinical episodes will be added based on learner feedback and assessed need.
Each simulation event encompassed all 3 episodes that an individual or a team of 2 learners rotate through in a round-robin fashion. The overarching theme for each episode was a rapid response team call with minimal resources that the learner would have to provide care and stabilization. A literature search for rapid response team training programs found few results, but the literature assisted with providing a foundation for Nightmare on CIL Street.4,5 The goal was to completely envelop the learners in a nightmare scenario that required a solution.
After the safety brief and predata collection, learners received a phone call with minimal information about a patient in need of care. The learners responded to the requested area and provided treatment to the emergency over 25 minutes with the bedside nurse (who is an embedded participant). At the conclusion of the scenario, a physician subject matter expert who has been observing, provided a personalized 10-minute debriefing to the learner, which presented specific learning points and opportunities for the learner’s educational development. After the debriefing, learners returned to a conference room and awaited the next call. After all learners completed the 3 episodes, a group debriefing was conducted using the gather, analyze, summarize debriefing framework. The debriefing begins with an open-ended forum for learners to express their thoughts. Then, each scenario is discussed and broken down by key learning objectives. Starting with cardiac and ending with neurology, the logistics of the cases are discussed based on the trajectory of the learners during the scenarios. Each objective is discussed, and learners are allowed to ask questions before moving to the next scenario. After the debriefing, postevent data were gathered.
Objectives
The program objective was to educate residents and students on common rapid response scenarios. We devised each scenario as an evolving simulation where various interventions would improve or worsen vital signs and symptoms. Each scenario had an end goal: cardioversion (cardiac), intubation (respiratory), and transfer (neurologic). Objectives were tailored to the trainees present during the specific simulation (Table).
IMPLEMENTATION
The initial run of the simulation curriculum was implemented on February 22, 2023, and ended on May 17, 2023, with 5 events. Participants included internal medicine PGY1 residents, third-year medical students, and fourth-year physician assistant students. Internal medicine residents ran each scenario with a subject matter expert monitoring; the undergraduate medical trainees partnered with another student. Students were pulled from their ward rotations to attend the simulation, and residents were pulled from electives and wards. Each trainee was able to experience each planned scenario. They were then briefed, participated in each scenario, and ended with a debriefing, discussing each case in detail. Two subject matter experts were always available, and occasionally 4 were present to provide additional knowledge transfer to learners. These included board-certified physicians in internal medicine and pulmonary critical care. Most scenarios were conducted on Wednesday afternoon or Thursday.
The CIL provided 6 staff minimum for every event. The staff controlled the manikins and acted as embedded players for the learners to interact and work with at the bedside. Every embedded RN was provided the same script: They were a new nurse just off orientation and did not know what to do. In addition, they were instructed that no matter who the learner wanted to call/page, that person or service was not answering or unavailable. This forced learners to respond and treat the simulated patient on their own.
Survey Responses
To evaluate the effect of this program on medical education, we administered surveys to the trainees before and after the simulation (Appendix). All questions were evaluated on a 10-point Likert scale (1, minimal comfort; 10, maximum comfort). The postsurvey added an additional Likert scale question and an open-ended question.
Sixteen trainees underwent the simulation curriculum during the 2022 to 2023 academic year, 9 internal medicine PGY1 residents, 4 medical students, and 3 physician assistant students. Postsimulation surveys indicated a mean 2.2 point increase in comfort compared with the presimulation surveys across all questions and participants.
DISCUSSION
The simulation curriculum proved to be successful for all parties, including trainees, medical educators, and simulation staff. Trainees expressed gratitude for the teaching ability of the simulation and the challenge of confronting an evolving scenario. Students also stated that the simulation allowed them to identify knowledge weaknesses.
Medical technology is rapidly advancing. A study evaluating high-fidelity medical simulations between 1969 and 2003 found that they are educationally effective and complement other medical education modalities.6 It is also noted that care provided by junior physicians with a lack of prior exposure to emergencies and unusual clinical syndromes can lead to more adverse effects.7 Simulation curriculums can be used to educate junior physicians as well as trainees on a multitude of medical emergencies, teach systematic approaches to medical scenarios, and increase exposure to unfamiliar experiences.
The goals of this article are to share program details and encourage other training programs with similar capabilities to incorporate simulation into medical education. Using pre- and postsimulation surveys, there was a concrete improvement in the value obtained by participating in this simulation. The Nightmare on CIL Street learners experienced a mean 2.2 point improvement from presimulation survey to postsimulation survey. Some notable improvements were the feelings of preparedness for rapid response situations and developing a systematic approach. As the students who participated in our Nightmare on CIL Street simulation were early in training, we believe the improvement in preparation and developing a systematic approach can be key to their success in their practical environments.
From a site-specific standpoint, improvement in confidence working through cardiac, respiratory, and neurological emergencies will be very useful. The anesthesiology service intubates during respiratory failures and there is no stroke neurologist available at the CTVHCS hospital. Giving trainees experience in these conditions may allow them to better understand their role in coordination during these times and potentially improve patient outcomes. A follow-up questionnaire administered a year after this simulation may be useful in ascertaining the usefulness of the simulation and what items may have been approached differently. We encourage other institutions to build in aspects of their site-specific challenges to improve trainee awareness in approaches to critical scenarios.
Challenges
The greatest challenge for Nightmare on CIL Street was the ability to pull internal medicine residents from their clinical duties to participate in the simulation. As there are many moving parts to their clinical scheduling, residents do not always have sufficient coverage to participate in training. There were also instances where residents needed to cover for another resident preventing them from attending the simulation. In the future, this program will schedule residents months in advance and will have the simulation training built into their rotations.
Medical and physician assistant students were pulled from their ward rotations as well. They rotate on a 2-to-4-week basis and often had already experienced the simulation the week prior, leaving out students for the following week. With more longitudinal planning, students can be pulled on a rotating monthly basis to maximize their participation. Another challenge was deciding whether residents should partner or experience the simulation on their own. After some feedback, it was noted that residents preferred to experience the simulation on their own as this improves their learning value. With the limited resources available, only rotating 3 residents on a scenario limits the number of trainees who can be reached with the program. Running this program throughout an academic year can help to reach more trainees.
CONCLUSIONS
Educating trainees on rapid response scenarios by using a simulation curriculum provides many benefits. Our trainees reported improvement in addressing cardiac, respiratory, and neurological rapid response scenarios after experiencing the simulation. They felt better prepared and had developed a better systematic approach for the future.
Acknowledgments
The authors thank Pawan Sikka, MD, George Martinez, MD and Braden Anderson, MD for participating as physician experts and educating our students. We thank Naomi Devers; Dinetra Jones; Stephanie Garrett; Sara Holton; Evelina Bartnick; Tanelle Smith; Michael Lomax; Shaun Kelemen for their participation as nurses, assistants, and simulation technology experts.
1. Guze PA. Using technology to meet the challenges of medical education. Trans Am Clin Climatol Assoc. 2015;126:260-270.
2. Higgins M, Madan C, Patel R. Development and decay of procedural skills in surgery: a systematic review of the effectiveness of simulation-based medical education interventions. Surgeon. 2021;19(4):e67-e77. doi:10.1016/j.surge.2020.07.013
3. Lyons PG, Edelson DP, Carey KA, et al. Characteristics of rapid response calls in the United States: an analysis of the first 402,023 adult cases from the Get With the Guidelines Resuscitation-Medical Emergency Team registry. Crit Care Med. 2019;47(10):1283-1289. doi:10.1097/CCM.0000000000003912
4. McMurray L, Hall AK, Rich J, Merchant S, Chaplin T. The nightmares course: a longitudinal, multidisciplinary, simulation-based curriculum to train and assess resident competence in resuscitation. J Grad Med Educ. 2017;9(4):503-508. doi:10.4300/JGME-D-16-00462.1
5. Gilic F, Schultz K, Sempowski I, Blagojevic A. “Nightmares-Family Medicine” course is an effective acute care teaching tool for family medicine residents. Simul Healthc. 2019;14(3):157-162. doi:10.1097/SIH.0000000000000355
6. Issenberg SB, McGaghie WC, Petrusa ER, Lee Gordon D, Scalese RJ. Features and uses of high-fidelity medical simulations that lead to effective learning: a BEME systematic review. Med Teach. 2005;27(1):10-28. doi:10.1080/01421590500046924
7. Datta R, Upadhyay K, Jaideep C. Simulation and its role in medical education. Med J Armed Forces India. 2012;68(2):167-172. doi:10.1016/S0377-1237(12)60040-9
1. Guze PA. Using technology to meet the challenges of medical education. Trans Am Clin Climatol Assoc. 2015;126:260-270.
2. Higgins M, Madan C, Patel R. Development and decay of procedural skills in surgery: a systematic review of the effectiveness of simulation-based medical education interventions. Surgeon. 2021;19(4):e67-e77. doi:10.1016/j.surge.2020.07.013
3. Lyons PG, Edelson DP, Carey KA, et al. Characteristics of rapid response calls in the United States: an analysis of the first 402,023 adult cases from the Get With the Guidelines Resuscitation-Medical Emergency Team registry. Crit Care Med. 2019;47(10):1283-1289. doi:10.1097/CCM.0000000000003912
4. McMurray L, Hall AK, Rich J, Merchant S, Chaplin T. The nightmares course: a longitudinal, multidisciplinary, simulation-based curriculum to train and assess resident competence in resuscitation. J Grad Med Educ. 2017;9(4):503-508. doi:10.4300/JGME-D-16-00462.1
5. Gilic F, Schultz K, Sempowski I, Blagojevic A. “Nightmares-Family Medicine” course is an effective acute care teaching tool for family medicine residents. Simul Healthc. 2019;14(3):157-162. doi:10.1097/SIH.0000000000000355
6. Issenberg SB, McGaghie WC, Petrusa ER, Lee Gordon D, Scalese RJ. Features and uses of high-fidelity medical simulations that lead to effective learning: a BEME systematic review. Med Teach. 2005;27(1):10-28. doi:10.1080/01421590500046924
7. Datta R, Upadhyay K, Jaideep C. Simulation and its role in medical education. Med J Armed Forces India. 2012;68(2):167-172. doi:10.1016/S0377-1237(12)60040-9
Elective Hand Surgery and Antithrombotic Use in Veterans
Patients planning plastic surgery traditionally were instructed to stop anticoagulants and antiplatelet medications during the perioperative period to avoid bleeding, which could result in flap loss, pain, skin necrosis, and blood transfusions. In the veteran patient population, anticoagulants are prescribed for the prevention of limb- and life-threatening embolic and thrombotic events.1-3 As of June 2021, > 332,000 veterans were prescribed direct oral anticoagulants.1
In 2015, the Malcom Randall Veterans Affairs Medical Center (MRVAMC) in Gainesville, Florida, Plastic Surgery Service began instructing patients planning elective hand surgery to continue their prescription anticoagulants and antiplatelets during the perioperative period. This decision was prompted by a patient who needed carpal tunnel release surgery and was prescribed coumadin for repeated thrombosis of his dialysis grafts. Hand surgery literature at the time suggested allowing patients to continue their anticoagulants and antiplatelets through the perioperative period to avoid life- and limb-threatening events and wide fluctuations in blood anticoagulant levels.4-6 The MRVAMC Plastic Surgery Service chose to accept the risk of perioperative bleeding after shared decision making with the patients rather than risk a cardiac stent obstruction, pulmonary embolism, or embolic stroke in the at-risk patients.
The objective of this study was to determine the postoperative bleeding complication rate over a 7.5-year period in the veteran patients who did not interrupt their prescription blood thinners. This would assist the MRVAMC Plastic Surgery Service with providing data-driven informed consent and determine whether this protocol should continue.
Methods
The North Florida/South Georgia Veterans Health System Research Committee and the University of Florida Institutional Review Board approved a retrospective chart review of elective hand cases performed by the MRVAMC Plastic Surgery Service from January 1, 2015, through June 30, 2022. Elective hand cases were identified based on the operation description and included nerve decompressions, tendon releases, trapeziectomy, small-joint fusion, neurectomy, elective amputations, and benign neoplasm removals (Table). Hand surgery included cubital tunnel releases (decompression of the ulnar nerve at the level of the elbow) because hand surgery fellowships, hand surgery training, and hand surgery practices traditionally include a high volume of cubital tunnel releases. We wanted this study to have real-world applications.
Patients’ histories and physicals were reviewed for prescription antithrombotics and for instructions not to interrupt these medications. Postoperative notes were reviewed for 30 days for evidence of postoperative bleeding complications.
The following prescription anticoagulants were included in the study: dabigatran, rivaroxaban, warfarin, edoxaban, and apixaban. In addition, the following prescription antiplatelets were included in the study:
Results
One hundred seventy-eight patients were identified for maintaining prescription blood thinners during their elective hand surgery. There was 1 major complication (0.6%) and 4 minor bleeding complications (2.2%). The major complication occurred when a patient had to return to surgery from the recovery room for emergent control of bleeding. The surgery was for an in situ cubital tunnel release. The patient, aged 48 years, was taking clopidogrel and aspirin and had a personal and family history of cardiovascular disease. The bleeding was controlled with bipolar cautery and Floseal, a topical haemostatic matrix made of bovine gelatin and human thrombin. The minor bleeding complications were treated in the clinic with compression, wound care, or expedited follow-up for reassurance. These included an in situ cubital tunnel release for a patient taking warfarin and aspirin, a digital inclusion cyst for a patient taking apixaban, an endoscopic carpal tunnel for a patient taking aspirin and clopidogrel, and an open carpal tunnel and ulnar tunnel release for a patient taking aspirin and clopidogrel. There were no thrombotic events during the study.
Discussion
Higher utilization of anticoagulation has been evidenced by a 30% increase in Medicare claims and a 277% increase in Medicaid anticoagulation claims between 2014 and 2019, driven by more prescriptions for direct oral anticoagulants such as apixaban and rivaroxaban.7 The MRVAMC Plastic Surgery Service began a protocol for managing perioperative anticoagulation in 2015 to avoid the risk of perioperative thrombotic events in veteran patients. Patients who choose elective hand surgery were instructed to continue their prescription blood thinners. Exceptions to this protocol were patients scheduled for a partial fasciectomy (for Dupuytren contracture) or cubital tunnel release with anterior ulnar nerve transposition. A hematoma would increase the risk for skin necrosis in the patients receiving a fasciectomy, resulting from the thin skin flaps and meticulous dissection to identify and protect the digital nerves. Worsening nerve dysfunction could result from hematoma compression and scarring in the ulnar nerve cases. If the risk of holding the blood thinner was felt to be unreasonably high, based on recommendations from the patients’ cardiologist or primary care doctor, we offered an in situ cubital tunnel release for the ulnar nerve patients.
Concerns regarding interrupting chronic anticoagulation involve the increased risk of thromboembolism and the theoretical risk of a rebound hypercoagulable effect.8 Patients prescribed warfarin have been found to unintentionally discontinue this medication after outpatient surgery at more than 1.5 times the rate of the general population.9
A systematic review of 9 published studies looking specifically at elective hand and wrist surgeries demonstrated no significant increase in perioperative bleeding risk with the continuation of anticoagulation and antiplatelet medications.10 Sardenberg and colleagues reviewed 7 studies in which 410 hand and wrist surgeries were performed in patients prescribed warfarin or aspirin and clopidogrel. These patients had a 0.7% serious complication rate, requiring surgical treatment only in patients having complex wrist surgeries (wrist arthrodesis with tenosynovectomy, resection of the distal ulna with tenosynovectomy and tendon transfer, and proximal row carpectomy).11 Bogunovic and colleagues compared 50 hand and wrist patients who were on uninterrupted warfarin with those who were not. They required patients to have an
These and our study are consistent with other disciplines, such as facial plastic surgery, dermatology, and ophthalmology, which do not support routine suspension of anticoagulants.13-16 A review of 30 cutaneous surgery studies involving > 14,000 patients recommended meticulous hemostasis over cessation of blood thinners.15 The University of Massachusetts Dermatology Clinic found a 40 times higher rate of bleeding complications in patients on clopidogrel and warfarin but still recommended continuation of these medications to avoid thrombotic events.16
Limitations
This study is a retrospective chart review and limited by what is already documented in the electronic health record. We can verify that the patients were given instructions to continue their medications up to the day of surgery but cannot be certain whether the instructions were followed. No control group was told to hold their anticoagulants for the same surgery. Once we decided on a protocol, we applied it to all patients. The study approval was for the specific time frame when the protocol was in place.
Our study was designed for elective hand cases because those surgeries can be anticipated, predicted, and patients can be given instructions during the preoperative appointments. We did incidentally find several nonelective hand cases (traumas, infections, and cancers) during the review of patients taking prescription blood thinners that had to be expedited to the operating room. Based on morbidity data during that time period, there were no additional postoperative hand surgery bleeding complications that had to return to the operating room. Future studies are indicated, but we believe our protocol can be applied to urgent and emergent hand surgeries as well as elective cases.
Conclusions
Our study supports continuing prescription anticoagulant and antiplatelet medications during the perioperative period for elective hand surgery. We found this is a safe practice in our veteran population with an acceptably low local bleeding complication rate.
Acknowledgments
This manuscript is the result of work supported with the resources and the use of facilities at the North Florida/South Georgia Veterans Health System in Gainesville, Florida.
1. Allen AL, Lucas J, Parra D, et al. Shifting the paradigm: a population health approach to the management of direct oral anticoagulants. J Am Heart Assoc. 2021;10(24):e022758. doi:10.1161/JAHA.121.022758
2. Buck J, Kaboli P, Gage BF, Cram P, Vaughan Sarrazin MS. Trends in antithrombotic therapy for atrial fibrillation: data from the Veterans Health Administration health system. Am Heart J. 2016;179:186-191. doi:10.1016/j.ahj.2016.03.029
3. Kinlay S, Young MM, Sherrod R, Gagnon DR. Long-term outcomes and duration of dual antiplatelet therapy after coronary intervention with second-generation drug-eluting stents: the Veterans Affairs Extended DAPT Study. J Am Heart Assoc. 2023;12(2):e027055.
4. Bogunovic L, Gelberman RH, Goldfarb CA, Boyer MI, Calfee RP. The impact of antiplatelet medication on hand and wrist surgery. J Hand Surg Am. 2013;38(6):1063-1070. doi:10.1016/j.jhsa.2013.03.034
5. Wallace DL, Latimer MD, Belcher HJ. Stopping warfarin therapy is unnecessary for hand surgery. J Hand Surg Br. 2004;29(3):203-205. doi:10.1016/j.jhsb.2003.12.008
6. Edmunds I, Avakian Z. Hand surgery on anticoagulated patients: a prospective study of 121 operations. Hand Surg. 2010;15(2):109-113. doi:10.1142/S021881041000468
7. Duvalyan A, Pandey A, Vaduganathan M, et al. Trends in anticoagulation prescription spending among Medicare Part D and Medicaid beneficiaries between 2014 and 2019. J Am Heart Assoc. 2021;10(24):e022644. doi:10.1161/JAHA.121.022644
8. Thakur NA, Czerwein JK, Butera JN, Palumbo MA. Perioperative management of chronic anticoagulation in orthopaedic surgery. J Am Acad Orthop Surg. 2010;18(12):729-738. doi:10.5435/00124635-201012000-00003
9. Bell C, Bajca J, Bierman A, Li P, Mamdani M, Urbach D. Potentially unintended discontinuation of long-term medication use after elective surgical procedures. Arch Int Med. 2003;166(22):2525-2531.
10. Stone MJ, Wilks DJ, Wade RG. Hand and wrist surgery on anticoagulants and antiplatelets: a systematic review and meta-analysis. J Plast Reconstr Aesthet Surg. 2020;73(8):1413-1423.
11. Sardenberg T, Deienno FS, Miranda RF, et al. Hand and wrist surgery without suspending warfarin or oral antiplatelet - systematic review. Rev Bras Ortop. 2017;52(4):390-395. doi:10.1016/j.rboe.2017.07.001
12. Bogunovic L, Gelberman RH, Goldfarb CA, Boyer MI, Calfee RP. The impact of uninterrupted warfarin on hand and wrist surgery. J Hand Surg Am. 2015;40(11):2133-2140. doi:10.1016/j.jhsa.2015.07.037
13. Kraft CT, Bellile E, Baker SR, Kim JC, Moyer JS. Anticoagulant complications in facial plastic and reconstructive surgery. JAMA Facial Plast Surg. 2015;17(2):103-107. doi:10.1001/jamafacial.2014.1147
14. He X, Chen AF, Nirwan RS, Sridhar J, Kuriyan AE. Perioperative management of anticoagulants in ocular surgeries. Int Ophthalmol Clin. 2020;60(3):3-15. doi:10.1097/IIO.0000000000000316
15. Isted A, Cooper L, Colville RJ. Bleeding on the cutting edge: a systematic review of anticoagulant and antiplatelet continuation in minor cutaneous surgery. J Plast Reconstr Aesthet Surg. 2018;71(4):455-467. doi:10.1016/j.bjps.2017.11.024
16. Bordeaux JS, Martires KJ, Goldberg D, Pattee SF, Fu P, Maloney ME. Prospective evaluation of dermatologic surgery complications including patients on multiple antiplatelet and anticoagulant medications. J Am Acad Dermatol. 2011;65(3):576-583. doi:10.1016/j.jaad.2011.02.012
Patients planning plastic surgery traditionally were instructed to stop anticoagulants and antiplatelet medications during the perioperative period to avoid bleeding, which could result in flap loss, pain, skin necrosis, and blood transfusions. In the veteran patient population, anticoagulants are prescribed for the prevention of limb- and life-threatening embolic and thrombotic events.1-3 As of June 2021, > 332,000 veterans were prescribed direct oral anticoagulants.1
In 2015, the Malcom Randall Veterans Affairs Medical Center (MRVAMC) in Gainesville, Florida, Plastic Surgery Service began instructing patients planning elective hand surgery to continue their prescription anticoagulants and antiplatelets during the perioperative period. This decision was prompted by a patient who needed carpal tunnel release surgery and was prescribed coumadin for repeated thrombosis of his dialysis grafts. Hand surgery literature at the time suggested allowing patients to continue their anticoagulants and antiplatelets through the perioperative period to avoid life- and limb-threatening events and wide fluctuations in blood anticoagulant levels.4-6 The MRVAMC Plastic Surgery Service chose to accept the risk of perioperative bleeding after shared decision making with the patients rather than risk a cardiac stent obstruction, pulmonary embolism, or embolic stroke in the at-risk patients.
The objective of this study was to determine the postoperative bleeding complication rate over a 7.5-year period in the veteran patients who did not interrupt their prescription blood thinners. This would assist the MRVAMC Plastic Surgery Service with providing data-driven informed consent and determine whether this protocol should continue.
Methods
The North Florida/South Georgia Veterans Health System Research Committee and the University of Florida Institutional Review Board approved a retrospective chart review of elective hand cases performed by the MRVAMC Plastic Surgery Service from January 1, 2015, through June 30, 2022. Elective hand cases were identified based on the operation description and included nerve decompressions, tendon releases, trapeziectomy, small-joint fusion, neurectomy, elective amputations, and benign neoplasm removals (Table). Hand surgery included cubital tunnel releases (decompression of the ulnar nerve at the level of the elbow) because hand surgery fellowships, hand surgery training, and hand surgery practices traditionally include a high volume of cubital tunnel releases. We wanted this study to have real-world applications.
Patients’ histories and physicals were reviewed for prescription antithrombotics and for instructions not to interrupt these medications. Postoperative notes were reviewed for 30 days for evidence of postoperative bleeding complications.
The following prescription anticoagulants were included in the study: dabigatran, rivaroxaban, warfarin, edoxaban, and apixaban. In addition, the following prescription antiplatelets were included in the study:
Results
One hundred seventy-eight patients were identified for maintaining prescription blood thinners during their elective hand surgery. There was 1 major complication (0.6%) and 4 minor bleeding complications (2.2%). The major complication occurred when a patient had to return to surgery from the recovery room for emergent control of bleeding. The surgery was for an in situ cubital tunnel release. The patient, aged 48 years, was taking clopidogrel and aspirin and had a personal and family history of cardiovascular disease. The bleeding was controlled with bipolar cautery and Floseal, a topical haemostatic matrix made of bovine gelatin and human thrombin. The minor bleeding complications were treated in the clinic with compression, wound care, or expedited follow-up for reassurance. These included an in situ cubital tunnel release for a patient taking warfarin and aspirin, a digital inclusion cyst for a patient taking apixaban, an endoscopic carpal tunnel for a patient taking aspirin and clopidogrel, and an open carpal tunnel and ulnar tunnel release for a patient taking aspirin and clopidogrel. There were no thrombotic events during the study.
Discussion
Higher utilization of anticoagulation has been evidenced by a 30% increase in Medicare claims and a 277% increase in Medicaid anticoagulation claims between 2014 and 2019, driven by more prescriptions for direct oral anticoagulants such as apixaban and rivaroxaban.7 The MRVAMC Plastic Surgery Service began a protocol for managing perioperative anticoagulation in 2015 to avoid the risk of perioperative thrombotic events in veteran patients. Patients who choose elective hand surgery were instructed to continue their prescription blood thinners. Exceptions to this protocol were patients scheduled for a partial fasciectomy (for Dupuytren contracture) or cubital tunnel release with anterior ulnar nerve transposition. A hematoma would increase the risk for skin necrosis in the patients receiving a fasciectomy, resulting from the thin skin flaps and meticulous dissection to identify and protect the digital nerves. Worsening nerve dysfunction could result from hematoma compression and scarring in the ulnar nerve cases. If the risk of holding the blood thinner was felt to be unreasonably high, based on recommendations from the patients’ cardiologist or primary care doctor, we offered an in situ cubital tunnel release for the ulnar nerve patients.
Concerns regarding interrupting chronic anticoagulation involve the increased risk of thromboembolism and the theoretical risk of a rebound hypercoagulable effect.8 Patients prescribed warfarin have been found to unintentionally discontinue this medication after outpatient surgery at more than 1.5 times the rate of the general population.9
A systematic review of 9 published studies looking specifically at elective hand and wrist surgeries demonstrated no significant increase in perioperative bleeding risk with the continuation of anticoagulation and antiplatelet medications.10 Sardenberg and colleagues reviewed 7 studies in which 410 hand and wrist surgeries were performed in patients prescribed warfarin or aspirin and clopidogrel. These patients had a 0.7% serious complication rate, requiring surgical treatment only in patients having complex wrist surgeries (wrist arthrodesis with tenosynovectomy, resection of the distal ulna with tenosynovectomy and tendon transfer, and proximal row carpectomy).11 Bogunovic and colleagues compared 50 hand and wrist patients who were on uninterrupted warfarin with those who were not. They required patients to have an
These and our study are consistent with other disciplines, such as facial plastic surgery, dermatology, and ophthalmology, which do not support routine suspension of anticoagulants.13-16 A review of 30 cutaneous surgery studies involving > 14,000 patients recommended meticulous hemostasis over cessation of blood thinners.15 The University of Massachusetts Dermatology Clinic found a 40 times higher rate of bleeding complications in patients on clopidogrel and warfarin but still recommended continuation of these medications to avoid thrombotic events.16
Limitations
This study is a retrospective chart review and limited by what is already documented in the electronic health record. We can verify that the patients were given instructions to continue their medications up to the day of surgery but cannot be certain whether the instructions were followed. No control group was told to hold their anticoagulants for the same surgery. Once we decided on a protocol, we applied it to all patients. The study approval was for the specific time frame when the protocol was in place.
Our study was designed for elective hand cases because those surgeries can be anticipated, predicted, and patients can be given instructions during the preoperative appointments. We did incidentally find several nonelective hand cases (traumas, infections, and cancers) during the review of patients taking prescription blood thinners that had to be expedited to the operating room. Based on morbidity data during that time period, there were no additional postoperative hand surgery bleeding complications that had to return to the operating room. Future studies are indicated, but we believe our protocol can be applied to urgent and emergent hand surgeries as well as elective cases.
Conclusions
Our study supports continuing prescription anticoagulant and antiplatelet medications during the perioperative period for elective hand surgery. We found this is a safe practice in our veteran population with an acceptably low local bleeding complication rate.
Acknowledgments
This manuscript is the result of work supported with the resources and the use of facilities at the North Florida/South Georgia Veterans Health System in Gainesville, Florida.
Patients planning plastic surgery traditionally were instructed to stop anticoagulants and antiplatelet medications during the perioperative period to avoid bleeding, which could result in flap loss, pain, skin necrosis, and blood transfusions. In the veteran patient population, anticoagulants are prescribed for the prevention of limb- and life-threatening embolic and thrombotic events.1-3 As of June 2021, > 332,000 veterans were prescribed direct oral anticoagulants.1
In 2015, the Malcom Randall Veterans Affairs Medical Center (MRVAMC) in Gainesville, Florida, Plastic Surgery Service began instructing patients planning elective hand surgery to continue their prescription anticoagulants and antiplatelets during the perioperative period. This decision was prompted by a patient who needed carpal tunnel release surgery and was prescribed coumadin for repeated thrombosis of his dialysis grafts. Hand surgery literature at the time suggested allowing patients to continue their anticoagulants and antiplatelets through the perioperative period to avoid life- and limb-threatening events and wide fluctuations in blood anticoagulant levels.4-6 The MRVAMC Plastic Surgery Service chose to accept the risk of perioperative bleeding after shared decision making with the patients rather than risk a cardiac stent obstruction, pulmonary embolism, or embolic stroke in the at-risk patients.
The objective of this study was to determine the postoperative bleeding complication rate over a 7.5-year period in the veteran patients who did not interrupt their prescription blood thinners. This would assist the MRVAMC Plastic Surgery Service with providing data-driven informed consent and determine whether this protocol should continue.
Methods
The North Florida/South Georgia Veterans Health System Research Committee and the University of Florida Institutional Review Board approved a retrospective chart review of elective hand cases performed by the MRVAMC Plastic Surgery Service from January 1, 2015, through June 30, 2022. Elective hand cases were identified based on the operation description and included nerve decompressions, tendon releases, trapeziectomy, small-joint fusion, neurectomy, elective amputations, and benign neoplasm removals (Table). Hand surgery included cubital tunnel releases (decompression of the ulnar nerve at the level of the elbow) because hand surgery fellowships, hand surgery training, and hand surgery practices traditionally include a high volume of cubital tunnel releases. We wanted this study to have real-world applications.
Patients’ histories and physicals were reviewed for prescription antithrombotics and for instructions not to interrupt these medications. Postoperative notes were reviewed for 30 days for evidence of postoperative bleeding complications.
The following prescription anticoagulants were included in the study: dabigatran, rivaroxaban, warfarin, edoxaban, and apixaban. In addition, the following prescription antiplatelets were included in the study:
Results
One hundred seventy-eight patients were identified for maintaining prescription blood thinners during their elective hand surgery. There was 1 major complication (0.6%) and 4 minor bleeding complications (2.2%). The major complication occurred when a patient had to return to surgery from the recovery room for emergent control of bleeding. The surgery was for an in situ cubital tunnel release. The patient, aged 48 years, was taking clopidogrel and aspirin and had a personal and family history of cardiovascular disease. The bleeding was controlled with bipolar cautery and Floseal, a topical haemostatic matrix made of bovine gelatin and human thrombin. The minor bleeding complications were treated in the clinic with compression, wound care, or expedited follow-up for reassurance. These included an in situ cubital tunnel release for a patient taking warfarin and aspirin, a digital inclusion cyst for a patient taking apixaban, an endoscopic carpal tunnel for a patient taking aspirin and clopidogrel, and an open carpal tunnel and ulnar tunnel release for a patient taking aspirin and clopidogrel. There were no thrombotic events during the study.
Discussion
Higher utilization of anticoagulation has been evidenced by a 30% increase in Medicare claims and a 277% increase in Medicaid anticoagulation claims between 2014 and 2019, driven by more prescriptions for direct oral anticoagulants such as apixaban and rivaroxaban.7 The MRVAMC Plastic Surgery Service began a protocol for managing perioperative anticoagulation in 2015 to avoid the risk of perioperative thrombotic events in veteran patients. Patients who choose elective hand surgery were instructed to continue their prescription blood thinners. Exceptions to this protocol were patients scheduled for a partial fasciectomy (for Dupuytren contracture) or cubital tunnel release with anterior ulnar nerve transposition. A hematoma would increase the risk for skin necrosis in the patients receiving a fasciectomy, resulting from the thin skin flaps and meticulous dissection to identify and protect the digital nerves. Worsening nerve dysfunction could result from hematoma compression and scarring in the ulnar nerve cases. If the risk of holding the blood thinner was felt to be unreasonably high, based on recommendations from the patients’ cardiologist or primary care doctor, we offered an in situ cubital tunnel release for the ulnar nerve patients.
Concerns regarding interrupting chronic anticoagulation involve the increased risk of thromboembolism and the theoretical risk of a rebound hypercoagulable effect.8 Patients prescribed warfarin have been found to unintentionally discontinue this medication after outpatient surgery at more than 1.5 times the rate of the general population.9
A systematic review of 9 published studies looking specifically at elective hand and wrist surgeries demonstrated no significant increase in perioperative bleeding risk with the continuation of anticoagulation and antiplatelet medications.10 Sardenberg and colleagues reviewed 7 studies in which 410 hand and wrist surgeries were performed in patients prescribed warfarin or aspirin and clopidogrel. These patients had a 0.7% serious complication rate, requiring surgical treatment only in patients having complex wrist surgeries (wrist arthrodesis with tenosynovectomy, resection of the distal ulna with tenosynovectomy and tendon transfer, and proximal row carpectomy).11 Bogunovic and colleagues compared 50 hand and wrist patients who were on uninterrupted warfarin with those who were not. They required patients to have an
These and our study are consistent with other disciplines, such as facial plastic surgery, dermatology, and ophthalmology, which do not support routine suspension of anticoagulants.13-16 A review of 30 cutaneous surgery studies involving > 14,000 patients recommended meticulous hemostasis over cessation of blood thinners.15 The University of Massachusetts Dermatology Clinic found a 40 times higher rate of bleeding complications in patients on clopidogrel and warfarin but still recommended continuation of these medications to avoid thrombotic events.16
Limitations
This study is a retrospective chart review and limited by what is already documented in the electronic health record. We can verify that the patients were given instructions to continue their medications up to the day of surgery but cannot be certain whether the instructions were followed. No control group was told to hold their anticoagulants for the same surgery. Once we decided on a protocol, we applied it to all patients. The study approval was for the specific time frame when the protocol was in place.
Our study was designed for elective hand cases because those surgeries can be anticipated, predicted, and patients can be given instructions during the preoperative appointments. We did incidentally find several nonelective hand cases (traumas, infections, and cancers) during the review of patients taking prescription blood thinners that had to be expedited to the operating room. Based on morbidity data during that time period, there were no additional postoperative hand surgery bleeding complications that had to return to the operating room. Future studies are indicated, but we believe our protocol can be applied to urgent and emergent hand surgeries as well as elective cases.
Conclusions
Our study supports continuing prescription anticoagulant and antiplatelet medications during the perioperative period for elective hand surgery. We found this is a safe practice in our veteran population with an acceptably low local bleeding complication rate.
Acknowledgments
This manuscript is the result of work supported with the resources and the use of facilities at the North Florida/South Georgia Veterans Health System in Gainesville, Florida.
1. Allen AL, Lucas J, Parra D, et al. Shifting the paradigm: a population health approach to the management of direct oral anticoagulants. J Am Heart Assoc. 2021;10(24):e022758. doi:10.1161/JAHA.121.022758
2. Buck J, Kaboli P, Gage BF, Cram P, Vaughan Sarrazin MS. Trends in antithrombotic therapy for atrial fibrillation: data from the Veterans Health Administration health system. Am Heart J. 2016;179:186-191. doi:10.1016/j.ahj.2016.03.029
3. Kinlay S, Young MM, Sherrod R, Gagnon DR. Long-term outcomes and duration of dual antiplatelet therapy after coronary intervention with second-generation drug-eluting stents: the Veterans Affairs Extended DAPT Study. J Am Heart Assoc. 2023;12(2):e027055.
4. Bogunovic L, Gelberman RH, Goldfarb CA, Boyer MI, Calfee RP. The impact of antiplatelet medication on hand and wrist surgery. J Hand Surg Am. 2013;38(6):1063-1070. doi:10.1016/j.jhsa.2013.03.034
5. Wallace DL, Latimer MD, Belcher HJ. Stopping warfarin therapy is unnecessary for hand surgery. J Hand Surg Br. 2004;29(3):203-205. doi:10.1016/j.jhsb.2003.12.008
6. Edmunds I, Avakian Z. Hand surgery on anticoagulated patients: a prospective study of 121 operations. Hand Surg. 2010;15(2):109-113. doi:10.1142/S021881041000468
7. Duvalyan A, Pandey A, Vaduganathan M, et al. Trends in anticoagulation prescription spending among Medicare Part D and Medicaid beneficiaries between 2014 and 2019. J Am Heart Assoc. 2021;10(24):e022644. doi:10.1161/JAHA.121.022644
8. Thakur NA, Czerwein JK, Butera JN, Palumbo MA. Perioperative management of chronic anticoagulation in orthopaedic surgery. J Am Acad Orthop Surg. 2010;18(12):729-738. doi:10.5435/00124635-201012000-00003
9. Bell C, Bajca J, Bierman A, Li P, Mamdani M, Urbach D. Potentially unintended discontinuation of long-term medication use after elective surgical procedures. Arch Int Med. 2003;166(22):2525-2531.
10. Stone MJ, Wilks DJ, Wade RG. Hand and wrist surgery on anticoagulants and antiplatelets: a systematic review and meta-analysis. J Plast Reconstr Aesthet Surg. 2020;73(8):1413-1423.
11. Sardenberg T, Deienno FS, Miranda RF, et al. Hand and wrist surgery without suspending warfarin or oral antiplatelet - systematic review. Rev Bras Ortop. 2017;52(4):390-395. doi:10.1016/j.rboe.2017.07.001
12. Bogunovic L, Gelberman RH, Goldfarb CA, Boyer MI, Calfee RP. The impact of uninterrupted warfarin on hand and wrist surgery. J Hand Surg Am. 2015;40(11):2133-2140. doi:10.1016/j.jhsa.2015.07.037
13. Kraft CT, Bellile E, Baker SR, Kim JC, Moyer JS. Anticoagulant complications in facial plastic and reconstructive surgery. JAMA Facial Plast Surg. 2015;17(2):103-107. doi:10.1001/jamafacial.2014.1147
14. He X, Chen AF, Nirwan RS, Sridhar J, Kuriyan AE. Perioperative management of anticoagulants in ocular surgeries. Int Ophthalmol Clin. 2020;60(3):3-15. doi:10.1097/IIO.0000000000000316
15. Isted A, Cooper L, Colville RJ. Bleeding on the cutting edge: a systematic review of anticoagulant and antiplatelet continuation in minor cutaneous surgery. J Plast Reconstr Aesthet Surg. 2018;71(4):455-467. doi:10.1016/j.bjps.2017.11.024
16. Bordeaux JS, Martires KJ, Goldberg D, Pattee SF, Fu P, Maloney ME. Prospective evaluation of dermatologic surgery complications including patients on multiple antiplatelet and anticoagulant medications. J Am Acad Dermatol. 2011;65(3):576-583. doi:10.1016/j.jaad.2011.02.012
1. Allen AL, Lucas J, Parra D, et al. Shifting the paradigm: a population health approach to the management of direct oral anticoagulants. J Am Heart Assoc. 2021;10(24):e022758. doi:10.1161/JAHA.121.022758
2. Buck J, Kaboli P, Gage BF, Cram P, Vaughan Sarrazin MS. Trends in antithrombotic therapy for atrial fibrillation: data from the Veterans Health Administration health system. Am Heart J. 2016;179:186-191. doi:10.1016/j.ahj.2016.03.029
3. Kinlay S, Young MM, Sherrod R, Gagnon DR. Long-term outcomes and duration of dual antiplatelet therapy after coronary intervention with second-generation drug-eluting stents: the Veterans Affairs Extended DAPT Study. J Am Heart Assoc. 2023;12(2):e027055.
4. Bogunovic L, Gelberman RH, Goldfarb CA, Boyer MI, Calfee RP. The impact of antiplatelet medication on hand and wrist surgery. J Hand Surg Am. 2013;38(6):1063-1070. doi:10.1016/j.jhsa.2013.03.034
5. Wallace DL, Latimer MD, Belcher HJ. Stopping warfarin therapy is unnecessary for hand surgery. J Hand Surg Br. 2004;29(3):203-205. doi:10.1016/j.jhsb.2003.12.008
6. Edmunds I, Avakian Z. Hand surgery on anticoagulated patients: a prospective study of 121 operations. Hand Surg. 2010;15(2):109-113. doi:10.1142/S021881041000468
7. Duvalyan A, Pandey A, Vaduganathan M, et al. Trends in anticoagulation prescription spending among Medicare Part D and Medicaid beneficiaries between 2014 and 2019. J Am Heart Assoc. 2021;10(24):e022644. doi:10.1161/JAHA.121.022644
8. Thakur NA, Czerwein JK, Butera JN, Palumbo MA. Perioperative management of chronic anticoagulation in orthopaedic surgery. J Am Acad Orthop Surg. 2010;18(12):729-738. doi:10.5435/00124635-201012000-00003
9. Bell C, Bajca J, Bierman A, Li P, Mamdani M, Urbach D. Potentially unintended discontinuation of long-term medication use after elective surgical procedures. Arch Int Med. 2003;166(22):2525-2531.
10. Stone MJ, Wilks DJ, Wade RG. Hand and wrist surgery on anticoagulants and antiplatelets: a systematic review and meta-analysis. J Plast Reconstr Aesthet Surg. 2020;73(8):1413-1423.
11. Sardenberg T, Deienno FS, Miranda RF, et al. Hand and wrist surgery without suspending warfarin or oral antiplatelet - systematic review. Rev Bras Ortop. 2017;52(4):390-395. doi:10.1016/j.rboe.2017.07.001
12. Bogunovic L, Gelberman RH, Goldfarb CA, Boyer MI, Calfee RP. The impact of uninterrupted warfarin on hand and wrist surgery. J Hand Surg Am. 2015;40(11):2133-2140. doi:10.1016/j.jhsa.2015.07.037
13. Kraft CT, Bellile E, Baker SR, Kim JC, Moyer JS. Anticoagulant complications in facial plastic and reconstructive surgery. JAMA Facial Plast Surg. 2015;17(2):103-107. doi:10.1001/jamafacial.2014.1147
14. He X, Chen AF, Nirwan RS, Sridhar J, Kuriyan AE. Perioperative management of anticoagulants in ocular surgeries. Int Ophthalmol Clin. 2020;60(3):3-15. doi:10.1097/IIO.0000000000000316
15. Isted A, Cooper L, Colville RJ. Bleeding on the cutting edge: a systematic review of anticoagulant and antiplatelet continuation in minor cutaneous surgery. J Plast Reconstr Aesthet Surg. 2018;71(4):455-467. doi:10.1016/j.bjps.2017.11.024
16. Bordeaux JS, Martires KJ, Goldberg D, Pattee SF, Fu P, Maloney ME. Prospective evaluation of dermatologic surgery complications including patients on multiple antiplatelet and anticoagulant medications. J Am Acad Dermatol. 2011;65(3):576-583. doi:10.1016/j.jaad.2011.02.012
A Case of Duodenocaval Fistula in the Setting of Respiratory Failure Initially Confused for Transfusion-Related Acute Lung Injury
A duodenocaval fistula (DCF) is seen when a connection exists between the duodenum and the inferior vena cava. It is a rare entity that is commonly missed and presents a diagnostic challenge due to its nonspecific presenting symptoms.1,2 Patients commonly present with gastrointestinal (GI) bleeding or sepsis. Here we present a case of a 37-year-old man who presented to the hospital for a workup related to melena but went into cardiac arrest prior to an esophagogastroduodenoscopy. Unfortunately, on autopsy, the patient was found to have a DCF. We highlight the diagnostic challenge associated with DCF and how in this case the presentation was confused by a diagnosis of possible transfusion-related acute lung injury (TRALI). To the best of our knowledge, this is also the first description of a case of DCF associated with food embolism to the lungs causing respiratory failure.
Case Presentation
A 37-year-old man with a history significant for bulimia presented to the hospital with a 3-day history of melena and reports of dizziness. The patient did not report being on any prescribed medications but noted that he took 4 aspirin daily to “calm his nerves.” The rest of the patient’s history was unremarkable aside from a reported history of induced emesis 3 to 4 times per week for an extended period up until 2 weeks before admission.
On admission, his vital signs demonstrated tachycardia and orthostatic hypotension. Pertinent findings on physical examination were skin pallor, a normal lung examination, mild epigastric tenderness, and guaiac-positive stools. He was alert and oriented to person, place, and time with no focal deficits. His admission laboratory tests were notable for a hemoglobin (Hb) level of 4.6 g/dL (reference range, 14-17.9), a white blood cell count of 13.5 K/cm (reference range, 4.5-11), an international normalized ratio of 1.21, a blood urea nitrogen of 61 mg/dL (reference range, 10-20), and a creatinine of 2.3 mg/dL (reference range, 0.8-1.4). The patient was placed on 2 L of oxygen via nasal cannula for comfort rather than true hypoxia. A chest X-ray on admission was negative with no signs of infiltrate, edema, or widened mediastinum. An abdominal X-ray was significant for a dilated stomach consistent with bulimia with no abdominal free air or signs of obstruction. The case was discussed with the gastroenterology service who felt that the patient needed to be more hemodynamically stable before pursuing endoscopic evaluation.
He was admitted to the intensive care unit and give a transfusion of 4 units of fresh frozen plasma and 2 packed red blood cells (PRBCs) without any issues. During the infusion of a third PRBC, he developed chills, tachycardia, and hypertension with accompanying respiratory distress characterized by wheezing, decreased breath sounds bilaterally, and a decrease in oxygen saturation to 70% on 2 L supplemental oxygen. He responded to treatment with meperidine, methylprednisolone sodium succinate, albuterol nebulizer, and acetaminophen. A new chest X-ray was read as “development of pulmonary edema vs bilateral pneumonitis.” A transfusion reaction was reported to the blood bank and a diagnosis of TRALI was considered. That evening, he completed a dose of platelets and another PRBC without difficulty after he was premedicated with meperidine, methylprednisolone sodium succinate, and acetaminophen. During the night, the patient spiked a temperature of 40.3 °C that was successfully treated with a cooling blanket and acetaminophen.
The following morning the patient was found to be tachypneic and tachycardic with his face mask off. His symptoms were corrected by replacing his face mask. He claimed he felt anxious about getting more transfusions and that he had breathing problems like this at home in the recent past. The patient requested an aspirin to calm his nerves. Over the course of the day, his Hb level dropped from 6.6 g/dL to 5.9 g/dL, and 2 washed leukopoor PRBCs were ordered.
The first unit was infused uneventfully, but after 125 cc of the second unit, the patient developed respiratory distress, rigors, and hypotension to 70/58 mm Hg despite premedication. He again was treated successfully with increased face mask support. A few rales were noted, but his fluid balance was even. A second transfusion reaction was filed with the blood bank and based on the 2 transfusion-associated events with no other clear explanation for his symptoms, the clinical team favored the TRALI diagnosis. However, the blood bank was suspicious this might not be TRALI as the previous night the patient had 2 episodes of respiratory distress with drops in oxygen saturation unassociated with transfusions. The patient was clinically stable for the remainder of the night.
Early the following morning the patient was scheduled for an esophagogastroduodenoscopy to evaluate for a source of his bleeding. At the beginning of the procedure, a unit of washed leukoreduced PRBCs was hung for a Hb level of 6.9 g/dL. No bleeding source was noted in the stomach, but as the endoscope was passed into the duodenum, and after an infusion of only 25 cc of RBCs, the patient became cyanotic and went into cardiac arrest. Despite advanced resuscitation efforts over 90 minutes, the patient could not be successfully resuscitated and died while in the endoscopy suite. A transfusion reaction workup was initiated but was unremarkable. The transfusion medicine staff was suspicious that something other than TRALI was the cause of the patient’s respiratory distress as he had respiratory distress remote to the transfusions and the unit was prepared correctly before administration. The patient’s family agreed to an autopsy.
Pathology
A full autopsy was performed 22 hours after the patient died. The lungs were congested and of increased weight: The right lung was 800 g, and the left was 750 g. The right lower lobe had a wedge-shaped infarction measuring 6 cm × 5 cm fed by a thrombosed vessel. Multiple small hemorrhagic wedge-shaped areas were noted in the left lung. An ulcer measuring 6 cm × 5 cm was noted just distal to the pylorus. At the base of this ulcer was a 1.5 cm × 0.5 cm tract that communicated with the inferior vena cava (Figure 1).
A postmortem blood culture was positive for Clostridium perfringens (C perfringens) and Candida albicans (C Albicans). Interestingly, one of the collected blood culture vials exploded en route to the laboratory, presumably due to the presence of many gas-forming C perfringens bacteria.
On microscopic examination of the autopsy samples, gram-positive rods were observed in the tissue of multiple organs, including the heart, lungs, liver, and kidneys (Figure 2).
Serology
Fourteen days after the patient’s death, both PRBC units infused during transfusion reactions were positive for granulocyte antibodies by immunofluorescence and agglutination techniques. Human leukocyte antigen antibody testing was also sent but was not found in either the donor or patient.
Discussion
Our case illustrates the unique and challenging diagnosis of DCF given the rarity of presentation and how quickly patients may clinically decompensate. After an extensive search of the medical literature, we were only able to identify about 40 previous cases of DCF, of which 37 were described in one review.1 DCF, although rare, should be considered at risk for forming in the following settings: migrating inferior vena cava filter, right nephrectomy and radiotherapy, duodenal peptic ulcer, abdominal trauma, and oncologic settings involving metastatic malignancy requiring radiation and/or surgical grafting of the inferior vena cava.1-4 When the diagnosis is considered, computed tomography (CT) is the best initial imaging modality as it allows for noninvasive evaluation of both the inferior vena cava and nonadjacent structures. A commonality of our case and those described in the literature is the diagnostic mystery and nonspecific symptoms patients present with, thus making CT an appropriate diagnostic modality. Endoscopy is useful for the further workup of GI bleeding and the diagnosis of peptic ulcer disease.5 In our case, given the patient’s autopsy findings and history of extensive nonsteroidal anti-inflammatory drug use, the duodenal peptic ulcer was likely the precipitating factor for his DCF.
The most challenging aspect in diagnosing DCF is that many times patients present with nonspecific symptoms, and given its rarity it is not something that is usually at the forefront of most differentials.2 This diagnostic difficulty may elucidate why there is such a relatively high mortality rate—nearly 40%—associated with DCF and why many times accurate diagnosis is not made until autopsy.1,3 The most common presenting manifestations are sepsis and/or GI bleeding; in less than half the cases described in the literature patients had both sepsis and GI bleeding. In our case, the patient had signs of melena but was not felt to be septic as his presenting signs were felt to be in the setting of blood loss and dehydration (given his history of bulimia), not an acute infectious source.
In retrospect, one of the more confounding aspects of this case is the clinical picture concerning for TRALI. The patient required supplemental oxygen throughout his hospitalization and decompensated while or after receiving a transfusion, thus having TRALI on the differential was not felt inappropriate at that time. However, this case also illustrates the power of an anchoring bias, and perhaps the clinical team anchored on the diagnosis of TRALI too quickly before considering other possible etiologies for the patient’s respiratory distress. TRALI can be one of the most challenging diagnoses to make in the field of transfusion medicine as there are no definitive diagnostic criteria.6 It is felt to be a clinical diagnosis of exclusion as there is no pathognomonic sign or diagnostic test to confirm it as the cause of the patient’s respiratory distress, though anti–human leukocyte antigen antibodies commonly are present.6,7 Considering how quickly the patient decompensated on day 2 of hospitalization and the presence of C perfringens bacteremia, which
Conclusions
Our investigation reports a case of a DCF in the setting of significant duodenal peptic ulcer disease. We highlight the diagnostic challenge that this commonly lethal etiology presents. We believe ours is the first case in which it was confused for TRALI and associated with food embolism to the lungs causing hypoxic respiratory failure. We want to highlight that DCF, though rare, should be considered for patients who present with GI bleeding and hypoxic respiratory failure.
1. Guillem PG, Binot D, Dupuy-Cuny J, et al. Duodenocaval fistula: a life-threatening condition of various origins. J Vasc Surg. 2001;33(3):643-645. doi:10.1067/mva.2001.111741
2. Ippolito D, Querques G, Drago SG, Bonaffini PA, Sironi S. Duodenocaval fistula in a patient with inferior vena cava leiomyosarcoma treated by surgical resection and caval polytetrafluoroethylene prosthesis. Case Rep Radiol. 2015;2015:1-5. doi:10.1155/2015/575961
3. Guo Y, Zhang YQ, Lin W. Radiological diagnosis of duodenocaval fistula: a case report and literature review. World J Gastroenterol. 2010;16(18):2314-2316. doi:10.3748/wjg.v16.i18.2314
4. Perera GB, Wilson SE, Barie PS, Butler JA. Duodenocaval fistula: A late complication of retroperitoneal irradiation and vena cava replacement. Ann Vasc Surg. 2004;18(1):52-58. doi:10.1007/s10016-003-0097-8
5. Addeo P, Rosso E, Oussoultzoglou E, Jaeck D, Pessaux P, Bachellier P. Inferior vena cava graft-enteric fistula after extended hepatectomy with caval replacement. J Vasc Surg. 2012;55(1):226-229. doi:10.1016/j.jvs.2011.05.118
6. Chapman CE, Stainsby D, Jones H, et al. Ten years of hemovigilance reports of transfusion-related acute lung injury in the United Kingdom and the impact of preferential use of male donor plasma. Transfusion. 2009;49(3):440-452. doi:10.1111/j.1537-2995.2008.01948.x
7. Fontaine MJ, Malone J, Mullins FM, Grumet FC. Diagnosis of transfusion-related acute lung injury: TRALI or not TRALI? Ann Clin Lab Sci. 2006;36(1):53-58.
8. Yang C-C, Hsu P-C, Chang H-J, Cheng C-W, Lee M-H. Clinical significance and outcomes of clostridium perfringens bacteremia—a 10-year experience at a tertiary care hospital. Int J Infect Dis. 2013;17(11):e9of55-e960. doi:10.1016/j.ijid.2013.03.001
A duodenocaval fistula (DCF) is seen when a connection exists between the duodenum and the inferior vena cava. It is a rare entity that is commonly missed and presents a diagnostic challenge due to its nonspecific presenting symptoms.1,2 Patients commonly present with gastrointestinal (GI) bleeding or sepsis. Here we present a case of a 37-year-old man who presented to the hospital for a workup related to melena but went into cardiac arrest prior to an esophagogastroduodenoscopy. Unfortunately, on autopsy, the patient was found to have a DCF. We highlight the diagnostic challenge associated with DCF and how in this case the presentation was confused by a diagnosis of possible transfusion-related acute lung injury (TRALI). To the best of our knowledge, this is also the first description of a case of DCF associated with food embolism to the lungs causing respiratory failure.
Case Presentation
A 37-year-old man with a history significant for bulimia presented to the hospital with a 3-day history of melena and reports of dizziness. The patient did not report being on any prescribed medications but noted that he took 4 aspirin daily to “calm his nerves.” The rest of the patient’s history was unremarkable aside from a reported history of induced emesis 3 to 4 times per week for an extended period up until 2 weeks before admission.
On admission, his vital signs demonstrated tachycardia and orthostatic hypotension. Pertinent findings on physical examination were skin pallor, a normal lung examination, mild epigastric tenderness, and guaiac-positive stools. He was alert and oriented to person, place, and time with no focal deficits. His admission laboratory tests were notable for a hemoglobin (Hb) level of 4.6 g/dL (reference range, 14-17.9), a white blood cell count of 13.5 K/cm (reference range, 4.5-11), an international normalized ratio of 1.21, a blood urea nitrogen of 61 mg/dL (reference range, 10-20), and a creatinine of 2.3 mg/dL (reference range, 0.8-1.4). The patient was placed on 2 L of oxygen via nasal cannula for comfort rather than true hypoxia. A chest X-ray on admission was negative with no signs of infiltrate, edema, or widened mediastinum. An abdominal X-ray was significant for a dilated stomach consistent with bulimia with no abdominal free air or signs of obstruction. The case was discussed with the gastroenterology service who felt that the patient needed to be more hemodynamically stable before pursuing endoscopic evaluation.
He was admitted to the intensive care unit and give a transfusion of 4 units of fresh frozen plasma and 2 packed red blood cells (PRBCs) without any issues. During the infusion of a third PRBC, he developed chills, tachycardia, and hypertension with accompanying respiratory distress characterized by wheezing, decreased breath sounds bilaterally, and a decrease in oxygen saturation to 70% on 2 L supplemental oxygen. He responded to treatment with meperidine, methylprednisolone sodium succinate, albuterol nebulizer, and acetaminophen. A new chest X-ray was read as “development of pulmonary edema vs bilateral pneumonitis.” A transfusion reaction was reported to the blood bank and a diagnosis of TRALI was considered. That evening, he completed a dose of platelets and another PRBC without difficulty after he was premedicated with meperidine, methylprednisolone sodium succinate, and acetaminophen. During the night, the patient spiked a temperature of 40.3 °C that was successfully treated with a cooling blanket and acetaminophen.
The following morning the patient was found to be tachypneic and tachycardic with his face mask off. His symptoms were corrected by replacing his face mask. He claimed he felt anxious about getting more transfusions and that he had breathing problems like this at home in the recent past. The patient requested an aspirin to calm his nerves. Over the course of the day, his Hb level dropped from 6.6 g/dL to 5.9 g/dL, and 2 washed leukopoor PRBCs were ordered.
The first unit was infused uneventfully, but after 125 cc of the second unit, the patient developed respiratory distress, rigors, and hypotension to 70/58 mm Hg despite premedication. He again was treated successfully with increased face mask support. A few rales were noted, but his fluid balance was even. A second transfusion reaction was filed with the blood bank and based on the 2 transfusion-associated events with no other clear explanation for his symptoms, the clinical team favored the TRALI diagnosis. However, the blood bank was suspicious this might not be TRALI as the previous night the patient had 2 episodes of respiratory distress with drops in oxygen saturation unassociated with transfusions. The patient was clinically stable for the remainder of the night.
Early the following morning the patient was scheduled for an esophagogastroduodenoscopy to evaluate for a source of his bleeding. At the beginning of the procedure, a unit of washed leukoreduced PRBCs was hung for a Hb level of 6.9 g/dL. No bleeding source was noted in the stomach, but as the endoscope was passed into the duodenum, and after an infusion of only 25 cc of RBCs, the patient became cyanotic and went into cardiac arrest. Despite advanced resuscitation efforts over 90 minutes, the patient could not be successfully resuscitated and died while in the endoscopy suite. A transfusion reaction workup was initiated but was unremarkable. The transfusion medicine staff was suspicious that something other than TRALI was the cause of the patient’s respiratory distress as he had respiratory distress remote to the transfusions and the unit was prepared correctly before administration. The patient’s family agreed to an autopsy.
Pathology
A full autopsy was performed 22 hours after the patient died. The lungs were congested and of increased weight: The right lung was 800 g, and the left was 750 g. The right lower lobe had a wedge-shaped infarction measuring 6 cm × 5 cm fed by a thrombosed vessel. Multiple small hemorrhagic wedge-shaped areas were noted in the left lung. An ulcer measuring 6 cm × 5 cm was noted just distal to the pylorus. At the base of this ulcer was a 1.5 cm × 0.5 cm tract that communicated with the inferior vena cava (Figure 1).
A postmortem blood culture was positive for Clostridium perfringens (C perfringens) and Candida albicans (C Albicans). Interestingly, one of the collected blood culture vials exploded en route to the laboratory, presumably due to the presence of many gas-forming C perfringens bacteria.
On microscopic examination of the autopsy samples, gram-positive rods were observed in the tissue of multiple organs, including the heart, lungs, liver, and kidneys (Figure 2).
Serology
Fourteen days after the patient’s death, both PRBC units infused during transfusion reactions were positive for granulocyte antibodies by immunofluorescence and agglutination techniques. Human leukocyte antigen antibody testing was also sent but was not found in either the donor or patient.
Discussion
Our case illustrates the unique and challenging diagnosis of DCF given the rarity of presentation and how quickly patients may clinically decompensate. After an extensive search of the medical literature, we were only able to identify about 40 previous cases of DCF, of which 37 were described in one review.1 DCF, although rare, should be considered at risk for forming in the following settings: migrating inferior vena cava filter, right nephrectomy and radiotherapy, duodenal peptic ulcer, abdominal trauma, and oncologic settings involving metastatic malignancy requiring radiation and/or surgical grafting of the inferior vena cava.1-4 When the diagnosis is considered, computed tomography (CT) is the best initial imaging modality as it allows for noninvasive evaluation of both the inferior vena cava and nonadjacent structures. A commonality of our case and those described in the literature is the diagnostic mystery and nonspecific symptoms patients present with, thus making CT an appropriate diagnostic modality. Endoscopy is useful for the further workup of GI bleeding and the diagnosis of peptic ulcer disease.5 In our case, given the patient’s autopsy findings and history of extensive nonsteroidal anti-inflammatory drug use, the duodenal peptic ulcer was likely the precipitating factor for his DCF.
The most challenging aspect in diagnosing DCF is that many times patients present with nonspecific symptoms, and given its rarity it is not something that is usually at the forefront of most differentials.2 This diagnostic difficulty may elucidate why there is such a relatively high mortality rate—nearly 40%—associated with DCF and why many times accurate diagnosis is not made until autopsy.1,3 The most common presenting manifestations are sepsis and/or GI bleeding; in less than half the cases described in the literature patients had both sepsis and GI bleeding. In our case, the patient had signs of melena but was not felt to be septic as his presenting signs were felt to be in the setting of blood loss and dehydration (given his history of bulimia), not an acute infectious source.
In retrospect, one of the more confounding aspects of this case is the clinical picture concerning for TRALI. The patient required supplemental oxygen throughout his hospitalization and decompensated while or after receiving a transfusion, thus having TRALI on the differential was not felt inappropriate at that time. However, this case also illustrates the power of an anchoring bias, and perhaps the clinical team anchored on the diagnosis of TRALI too quickly before considering other possible etiologies for the patient’s respiratory distress. TRALI can be one of the most challenging diagnoses to make in the field of transfusion medicine as there are no definitive diagnostic criteria.6 It is felt to be a clinical diagnosis of exclusion as there is no pathognomonic sign or diagnostic test to confirm it as the cause of the patient’s respiratory distress, though anti–human leukocyte antigen antibodies commonly are present.6,7 Considering how quickly the patient decompensated on day 2 of hospitalization and the presence of C perfringens bacteremia, which
Conclusions
Our investigation reports a case of a DCF in the setting of significant duodenal peptic ulcer disease. We highlight the diagnostic challenge that this commonly lethal etiology presents. We believe ours is the first case in which it was confused for TRALI and associated with food embolism to the lungs causing hypoxic respiratory failure. We want to highlight that DCF, though rare, should be considered for patients who present with GI bleeding and hypoxic respiratory failure.
A duodenocaval fistula (DCF) is seen when a connection exists between the duodenum and the inferior vena cava. It is a rare entity that is commonly missed and presents a diagnostic challenge due to its nonspecific presenting symptoms.1,2 Patients commonly present with gastrointestinal (GI) bleeding or sepsis. Here we present a case of a 37-year-old man who presented to the hospital for a workup related to melena but went into cardiac arrest prior to an esophagogastroduodenoscopy. Unfortunately, on autopsy, the patient was found to have a DCF. We highlight the diagnostic challenge associated with DCF and how in this case the presentation was confused by a diagnosis of possible transfusion-related acute lung injury (TRALI). To the best of our knowledge, this is also the first description of a case of DCF associated with food embolism to the lungs causing respiratory failure.
Case Presentation
A 37-year-old man with a history significant for bulimia presented to the hospital with a 3-day history of melena and reports of dizziness. The patient did not report being on any prescribed medications but noted that he took 4 aspirin daily to “calm his nerves.” The rest of the patient’s history was unremarkable aside from a reported history of induced emesis 3 to 4 times per week for an extended period up until 2 weeks before admission.
On admission, his vital signs demonstrated tachycardia and orthostatic hypotension. Pertinent findings on physical examination were skin pallor, a normal lung examination, mild epigastric tenderness, and guaiac-positive stools. He was alert and oriented to person, place, and time with no focal deficits. His admission laboratory tests were notable for a hemoglobin (Hb) level of 4.6 g/dL (reference range, 14-17.9), a white blood cell count of 13.5 K/cm (reference range, 4.5-11), an international normalized ratio of 1.21, a blood urea nitrogen of 61 mg/dL (reference range, 10-20), and a creatinine of 2.3 mg/dL (reference range, 0.8-1.4). The patient was placed on 2 L of oxygen via nasal cannula for comfort rather than true hypoxia. A chest X-ray on admission was negative with no signs of infiltrate, edema, or widened mediastinum. An abdominal X-ray was significant for a dilated stomach consistent with bulimia with no abdominal free air or signs of obstruction. The case was discussed with the gastroenterology service who felt that the patient needed to be more hemodynamically stable before pursuing endoscopic evaluation.
He was admitted to the intensive care unit and give a transfusion of 4 units of fresh frozen plasma and 2 packed red blood cells (PRBCs) without any issues. During the infusion of a third PRBC, he developed chills, tachycardia, and hypertension with accompanying respiratory distress characterized by wheezing, decreased breath sounds bilaterally, and a decrease in oxygen saturation to 70% on 2 L supplemental oxygen. He responded to treatment with meperidine, methylprednisolone sodium succinate, albuterol nebulizer, and acetaminophen. A new chest X-ray was read as “development of pulmonary edema vs bilateral pneumonitis.” A transfusion reaction was reported to the blood bank and a diagnosis of TRALI was considered. That evening, he completed a dose of platelets and another PRBC without difficulty after he was premedicated with meperidine, methylprednisolone sodium succinate, and acetaminophen. During the night, the patient spiked a temperature of 40.3 °C that was successfully treated with a cooling blanket and acetaminophen.
The following morning the patient was found to be tachypneic and tachycardic with his face mask off. His symptoms were corrected by replacing his face mask. He claimed he felt anxious about getting more transfusions and that he had breathing problems like this at home in the recent past. The patient requested an aspirin to calm his nerves. Over the course of the day, his Hb level dropped from 6.6 g/dL to 5.9 g/dL, and 2 washed leukopoor PRBCs were ordered.
The first unit was infused uneventfully, but after 125 cc of the second unit, the patient developed respiratory distress, rigors, and hypotension to 70/58 mm Hg despite premedication. He again was treated successfully with increased face mask support. A few rales were noted, but his fluid balance was even. A second transfusion reaction was filed with the blood bank and based on the 2 transfusion-associated events with no other clear explanation for his symptoms, the clinical team favored the TRALI diagnosis. However, the blood bank was suspicious this might not be TRALI as the previous night the patient had 2 episodes of respiratory distress with drops in oxygen saturation unassociated with transfusions. The patient was clinically stable for the remainder of the night.
Early the following morning the patient was scheduled for an esophagogastroduodenoscopy to evaluate for a source of his bleeding. At the beginning of the procedure, a unit of washed leukoreduced PRBCs was hung for a Hb level of 6.9 g/dL. No bleeding source was noted in the stomach, but as the endoscope was passed into the duodenum, and after an infusion of only 25 cc of RBCs, the patient became cyanotic and went into cardiac arrest. Despite advanced resuscitation efforts over 90 minutes, the patient could not be successfully resuscitated and died while in the endoscopy suite. A transfusion reaction workup was initiated but was unremarkable. The transfusion medicine staff was suspicious that something other than TRALI was the cause of the patient’s respiratory distress as he had respiratory distress remote to the transfusions and the unit was prepared correctly before administration. The patient’s family agreed to an autopsy.
Pathology
A full autopsy was performed 22 hours after the patient died. The lungs were congested and of increased weight: The right lung was 800 g, and the left was 750 g. The right lower lobe had a wedge-shaped infarction measuring 6 cm × 5 cm fed by a thrombosed vessel. Multiple small hemorrhagic wedge-shaped areas were noted in the left lung. An ulcer measuring 6 cm × 5 cm was noted just distal to the pylorus. At the base of this ulcer was a 1.5 cm × 0.5 cm tract that communicated with the inferior vena cava (Figure 1).
A postmortem blood culture was positive for Clostridium perfringens (C perfringens) and Candida albicans (C Albicans). Interestingly, one of the collected blood culture vials exploded en route to the laboratory, presumably due to the presence of many gas-forming C perfringens bacteria.
On microscopic examination of the autopsy samples, gram-positive rods were observed in the tissue of multiple organs, including the heart, lungs, liver, and kidneys (Figure 2).
Serology
Fourteen days after the patient’s death, both PRBC units infused during transfusion reactions were positive for granulocyte antibodies by immunofluorescence and agglutination techniques. Human leukocyte antigen antibody testing was also sent but was not found in either the donor or patient.
Discussion
Our case illustrates the unique and challenging diagnosis of DCF given the rarity of presentation and how quickly patients may clinically decompensate. After an extensive search of the medical literature, we were only able to identify about 40 previous cases of DCF, of which 37 were described in one review.1 DCF, although rare, should be considered at risk for forming in the following settings: migrating inferior vena cava filter, right nephrectomy and radiotherapy, duodenal peptic ulcer, abdominal trauma, and oncologic settings involving metastatic malignancy requiring radiation and/or surgical grafting of the inferior vena cava.1-4 When the diagnosis is considered, computed tomography (CT) is the best initial imaging modality as it allows for noninvasive evaluation of both the inferior vena cava and nonadjacent structures. A commonality of our case and those described in the literature is the diagnostic mystery and nonspecific symptoms patients present with, thus making CT an appropriate diagnostic modality. Endoscopy is useful for the further workup of GI bleeding and the diagnosis of peptic ulcer disease.5 In our case, given the patient’s autopsy findings and history of extensive nonsteroidal anti-inflammatory drug use, the duodenal peptic ulcer was likely the precipitating factor for his DCF.
The most challenging aspect in diagnosing DCF is that many times patients present with nonspecific symptoms, and given its rarity it is not something that is usually at the forefront of most differentials.2 This diagnostic difficulty may elucidate why there is such a relatively high mortality rate—nearly 40%—associated with DCF and why many times accurate diagnosis is not made until autopsy.1,3 The most common presenting manifestations are sepsis and/or GI bleeding; in less than half the cases described in the literature patients had both sepsis and GI bleeding. In our case, the patient had signs of melena but was not felt to be septic as his presenting signs were felt to be in the setting of blood loss and dehydration (given his history of bulimia), not an acute infectious source.
In retrospect, one of the more confounding aspects of this case is the clinical picture concerning for TRALI. The patient required supplemental oxygen throughout his hospitalization and decompensated while or after receiving a transfusion, thus having TRALI on the differential was not felt inappropriate at that time. However, this case also illustrates the power of an anchoring bias, and perhaps the clinical team anchored on the diagnosis of TRALI too quickly before considering other possible etiologies for the patient’s respiratory distress. TRALI can be one of the most challenging diagnoses to make in the field of transfusion medicine as there are no definitive diagnostic criteria.6 It is felt to be a clinical diagnosis of exclusion as there is no pathognomonic sign or diagnostic test to confirm it as the cause of the patient’s respiratory distress, though anti–human leukocyte antigen antibodies commonly are present.6,7 Considering how quickly the patient decompensated on day 2 of hospitalization and the presence of C perfringens bacteremia, which
Conclusions
Our investigation reports a case of a DCF in the setting of significant duodenal peptic ulcer disease. We highlight the diagnostic challenge that this commonly lethal etiology presents. We believe ours is the first case in which it was confused for TRALI and associated with food embolism to the lungs causing hypoxic respiratory failure. We want to highlight that DCF, though rare, should be considered for patients who present with GI bleeding and hypoxic respiratory failure.
1. Guillem PG, Binot D, Dupuy-Cuny J, et al. Duodenocaval fistula: a life-threatening condition of various origins. J Vasc Surg. 2001;33(3):643-645. doi:10.1067/mva.2001.111741
2. Ippolito D, Querques G, Drago SG, Bonaffini PA, Sironi S. Duodenocaval fistula in a patient with inferior vena cava leiomyosarcoma treated by surgical resection and caval polytetrafluoroethylene prosthesis. Case Rep Radiol. 2015;2015:1-5. doi:10.1155/2015/575961
3. Guo Y, Zhang YQ, Lin W. Radiological diagnosis of duodenocaval fistula: a case report and literature review. World J Gastroenterol. 2010;16(18):2314-2316. doi:10.3748/wjg.v16.i18.2314
4. Perera GB, Wilson SE, Barie PS, Butler JA. Duodenocaval fistula: A late complication of retroperitoneal irradiation and vena cava replacement. Ann Vasc Surg. 2004;18(1):52-58. doi:10.1007/s10016-003-0097-8
5. Addeo P, Rosso E, Oussoultzoglou E, Jaeck D, Pessaux P, Bachellier P. Inferior vena cava graft-enteric fistula after extended hepatectomy with caval replacement. J Vasc Surg. 2012;55(1):226-229. doi:10.1016/j.jvs.2011.05.118
6. Chapman CE, Stainsby D, Jones H, et al. Ten years of hemovigilance reports of transfusion-related acute lung injury in the United Kingdom and the impact of preferential use of male donor plasma. Transfusion. 2009;49(3):440-452. doi:10.1111/j.1537-2995.2008.01948.x
7. Fontaine MJ, Malone J, Mullins FM, Grumet FC. Diagnosis of transfusion-related acute lung injury: TRALI or not TRALI? Ann Clin Lab Sci. 2006;36(1):53-58.
8. Yang C-C, Hsu P-C, Chang H-J, Cheng C-W, Lee M-H. Clinical significance and outcomes of clostridium perfringens bacteremia—a 10-year experience at a tertiary care hospital. Int J Infect Dis. 2013;17(11):e9of55-e960. doi:10.1016/j.ijid.2013.03.001
1. Guillem PG, Binot D, Dupuy-Cuny J, et al. Duodenocaval fistula: a life-threatening condition of various origins. J Vasc Surg. 2001;33(3):643-645. doi:10.1067/mva.2001.111741
2. Ippolito D, Querques G, Drago SG, Bonaffini PA, Sironi S. Duodenocaval fistula in a patient with inferior vena cava leiomyosarcoma treated by surgical resection and caval polytetrafluoroethylene prosthesis. Case Rep Radiol. 2015;2015:1-5. doi:10.1155/2015/575961
3. Guo Y, Zhang YQ, Lin W. Radiological diagnosis of duodenocaval fistula: a case report and literature review. World J Gastroenterol. 2010;16(18):2314-2316. doi:10.3748/wjg.v16.i18.2314
4. Perera GB, Wilson SE, Barie PS, Butler JA. Duodenocaval fistula: A late complication of retroperitoneal irradiation and vena cava replacement. Ann Vasc Surg. 2004;18(1):52-58. doi:10.1007/s10016-003-0097-8
5. Addeo P, Rosso E, Oussoultzoglou E, Jaeck D, Pessaux P, Bachellier P. Inferior vena cava graft-enteric fistula after extended hepatectomy with caval replacement. J Vasc Surg. 2012;55(1):226-229. doi:10.1016/j.jvs.2011.05.118
6. Chapman CE, Stainsby D, Jones H, et al. Ten years of hemovigilance reports of transfusion-related acute lung injury in the United Kingdom and the impact of preferential use of male donor plasma. Transfusion. 2009;49(3):440-452. doi:10.1111/j.1537-2995.2008.01948.x
7. Fontaine MJ, Malone J, Mullins FM, Grumet FC. Diagnosis of transfusion-related acute lung injury: TRALI or not TRALI? Ann Clin Lab Sci. 2006;36(1):53-58.
8. Yang C-C, Hsu P-C, Chang H-J, Cheng C-W, Lee M-H. Clinical significance and outcomes of clostridium perfringens bacteremia—a 10-year experience at a tertiary care hospital. Int J Infect Dis. 2013;17(11):e9of55-e960. doi:10.1016/j.ijid.2013.03.001