User login
The Hospitalist Triage Role for Reducing Admission Delays: Impacts on Throughput, Quality, Interprofessional Practice, and Clinician Experience of Care
From the Division of Hospital Medicine, University of New Mexico Hospital, Albuquerque (Drs. Bartlett, Pizanis, Angeli, Lacy, and Rogers), Department of Emergency Medicine, University of New Mexico Hospital, Albuquerque (Dr. Scott), and University of New Mexico School of Medicine, Albuquerque (Ms. Baca).
ABSTRACT
Background: Emergency department (ED) crowding is associated with deleterious consequences for patient care and throughput. Admission delays worsen ED crowding. Time to admission (TTA)—the time between an ED admission request and internal medicine (IM) admission orders—can be shortened through implementation of a triage hospitalist role. Limited research is available highlighting the impact of triage hospitalists on throughput, care quality, interprofessional practice, and clinician experience of care.
Methods: A triage hospitalist role was piloted and implemented. Run charts were interpreted using accepted rules for deriving statistically significant conclusions. Statistical analysis was applied to interprofessional practice and clinician experience-of-care survey results.
Results: Following implementation, TTA decreased from 5 hours 19 minutes to 2 hours 8 minutes. Emergency department crowding increased from baseline. The reduction in TTA was associated with decreased time from ED arrival to IM admission request, no change in critical care transfers during the initial 24 hours, and increased admissions to inpatient status. Additionally, decreased TTA was associated with no change in referring hospital transfer rates and no change in hospital medicine length of stay. Interprofessional practice attitudes improved among ED clinicians but not IM clinicians. Clinician experience-of-care results were mixed.
Conclusion: A triage hospitalist role is an effective approach for mitigating admission delays, with no evident adverse clinical consequences. A triage hospitalist alone was incapable of resolving ED crowding issues without a complementary focus on downstream bottlenecks.
Keywords: triage hospitalist, admission delay, quality improvement.
Excess time to admission (TTA), defined as the time between an emergency department (ED) admission request and internal medicine (IM) admission orders, contributes to ED crowding, which is associated with deleterious impacts on patient care and throughput. Prior research has correlated ED crowding with an increase in length of stay (LOS)1-3 and total inpatient cost,1 as well as increased inpatient mortality, higher left-without-being-seen rates,4 delays in clinically meaningful care,5,6 and poor patient and clinician satisfaction.6,7 While various solutions have been proposed to alleviate ED crowding,8 excess TTA is one aspect that IM can directly address.
Like many institutions, ours is challenged by ED crowding. Time to admission is a known bottleneck. Underlying factors that contribute to excess TTA include varied admission request volumes in relation to fixed admitting capacity; learner-focused admitting processes; and unreliable strategies for determining whether patients are eligible for ED observation, transfer to an alternative facility, or admission to an alternative primary service.
To address excess TTA, we piloted then implemented a triage hospitalist role, envisioned as responsible for evaluating ED admission requests to IM, making timely determinations of admission appropriateness, and distributing patients to admitting teams. This intervention was selected because of its strengths, including the ability to standardize admission processes, improve the proximity of clinical decision-makers to patient care to reduce delays, and decrease hierarchical imbalances experienced by trainees, and also because the institution expressed a willingness to mitigate its primary weakness (ie, ongoing financial support for sustainability) should it prove successful.
Previously, a triage hospitalist has been defined as “a physician who assesses patients for admission, actively supporting the transition of the patient from the outpatient to the inpatient setting.”9 Velásquez et al surveyed 10 academic medical centers and identified significant heterogeneity in the roles and responsibilities of a triage hospitalist.9 Limited research addresses the impact of this role on throughput. One report described the volume and source of requests evaluated by a triage hospitalist and the frequency with which the triage hospitalists’ assessment of admission appropriateness aligned with that of the referring clinicians.10 No prior research is available demonstrating the impact of this role on care quality, interprofessional practice, or clinician experience of care. This article is intended to address these gaps in the literature.
Methods
Setting
The University of New Mexico Hospital has 537 beds and is the only level-1 trauma and academic medical center in the state. On average, approximately 8000 patients register to be seen in the ED per month. Roughly 600 are admitted to IM per month. This study coincided with the COVID-19 pandemic, with low patient volumes in April 2020, overcapacity census starting in May 2020, and markedly high patient volumes in May/June 2020 and November/December 2020. All authors participated in project development, implementation, and analysis.
Preintervention IM Admission Process
When requesting IM admission, ED clinicians (resident, advanced practice provider [APP], or attending) contacted the IM triage person (typically an IM resident physician) by phone or in person. The IM triage person would then assess whether the patient needed critical care consultation (a unique and separate admission pathway), was eligible for ED observation or transfer to an outside hospital, or was clinically appropriate for IM subacute and floor admission. Pending admissions were evaluated in order of severity of illness or based on wait time if severity of illness was equal. Transfers from the intensive care unit (ICU) and referring hospitals were prioritized. Between 7:00
Triage Hospitalist Pilot
Key changes made during the pilot included scheduling an IM attending to serve as triage hospitalist for all IM admission requests from the ED between 7:00
Measures for Triage Hospitalist Pilot
Data collected included request type (new vs overflow from night) and patient details (name, medical record number). Two time points were recorded: when the EDAR order was entered and when admission orders were entered. Process indicators, including whether the EDAR order was entered and the final triage decision (eg, discharge, IM), were recorded. General feedback was requested at the end of each shift.
Phased Implementation of Triage Hospitalist Role
Triage hospitalist role implementation was approved following the pilot, with additional salary support funded by the institution. A new performance measure (time from admission request to admission order, self-identified goal < 3 hours) was approved by all parties.
In January 2020, the role was scheduled from 7:00
In March 2020, to create a single communication pathway while simultaneously hardwiring our measurement strategy, the EDAR order was modified such that it would automatically prompt a 1-way communication to the triage hospitalist using the institution’s secure messaging software. The message included patient name, medical record number, location, ED attending, reason for admission, and consultation priority, as well as 2 questions prompting ED clinicians to reflect on the most common reasons for the triage hospitalist to recommend against IM admission (eligible for admission to other primary service, transfer to alternative hospital).
In July 2020, the triage hospitalist role was scheduled 24 hours a day, 7 days a week, to meet an institutional request. The schedule was divided into a daytime 7:00
Measures for Triage Hospitalist Role
The primary outcome measure was TTA, defined as the time between EDAR (operationalized using EDAR order timestamp) and IM admission decision (operationalized using inpatient bed request order timestamp). Additional outcome measures included the Centers for Medicare & Medicaid Services Electronic Clinical Quality Measure ED-2 (eCQM ED-2), defined as the median time from admit decision to departure from the ED for patients admitted to inpatient status.
Process measures included time between patient arrival to the ED (operationalized using ED registration timestamp) and EDAR and percentage of IM admissions with an EDAR order. Balancing measures included time between bed request order (referred to as the IM admission order) and subsequent admission orders. While the IM admission order prompts an inpatient clinical encounter and inpatient bed assignment, subsequent admission orders are necessary for clinical care. Additional balancing measures included ICU transfer rate within the first 24 hours, referring facility transfer frequency to IM (an indicator of access for patients at outside hospitals), average hospital medicine LOS (operationalized using ED registration timestamp to discharge timestamp), and admission status (inpatient vs observation).
An anonymous preintervention (December 2019) and postintervention (August 2020) survey focusing on interprofessional practice and clinician experience of care was used to obtain feedback from ED and IM attendings, APPs, and trainees. Emergency department clinicians were asked questions pertaining to their IM colleagues and vice versa. A Likert 5-point scale was used to respond.
Data Analysis
The preintervention period was June 1, 2019, to October 31, 2019; the pilot period was November 1, 2019, to December 31, 2019; the staged implementation period was January 1, 2020, to June 30, 2020; and the postintervention period was July 1, 2020, to December 31, 2020. Run charts for outcome, process, and balancing measures were interpreted using rules for deriving statistically significant conclusions.11 Statistical analysis using a t test assuming unequal variances with P < . 05 to indicate statistical significance was applied to experience-of-care results. The study was approved by the Institutional Review Board.
Results
Triage Hospitalist Pilot Time Period
Seventy-four entries were recorded, 56 (75.7%) reflecting new admission requests. Average time between EDAR order and IM admission order was 40 minutes. The EDAR order was entered into the EMR without prompting in 22 (29.7%) cases. In 56 (75.7%) cases, the final triage decision was IM admission. Other dispositions included 3 discharges, 4 transfers, 3 alternative primary service admissions, 1 ED observation, and 7 triage deferrals pending additional workup or stabilization.
Feedback substantiated several benefits, including improved coordination among IM, ED, and consultant clinicians, as well as early admission of seriously ill patients. Feedback also confirmed several expected challenges, including evidence of communication lapses, difficulty with transfer coordinator integration, difficulty hardwiring elements of the verbal and bedside handoff, and perceived high cognitive load for the triage hospitalist. Several unexpected issues included whether ED APPs can request admission independently and how reconsultation is expected to occur if admission is initially deferred.
Triage Hospitalist Implementation Time Period
Time to admission decreased from a baseline pre-pilot average of 5 hours 19 minutes (median, 4 hours 45 minutes) to a postintervention average of 2 hours 8 minutes, with a statistically significant downward shift post intervention (Figure 1).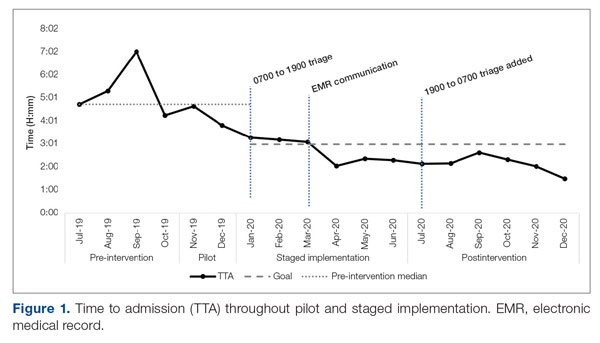
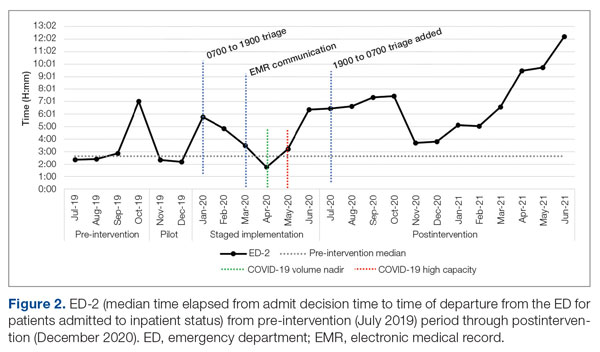
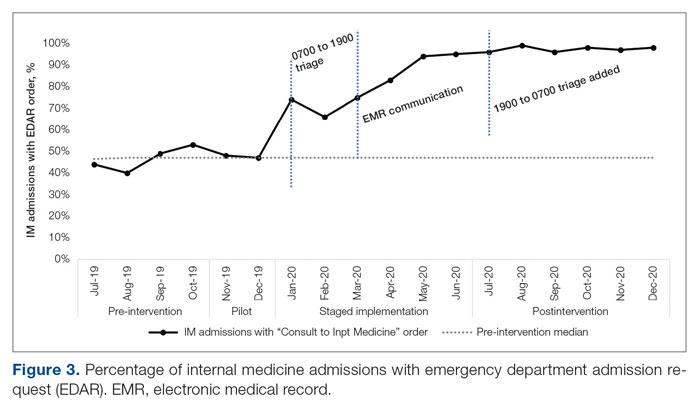
ED-2 increased from a baseline average of 3 hours 40 minutes (median, 2 hours 39 minutes), with a statistically significant upward shift starting in May 2020 (Figure 2). Time between patient arrival to the ED and EDAR order decreased from a baseline average of 8 hours 47 minutes (median, 8 hours 37 minutes) to a postintervention average of 5 hours 57 minutes, with a statistically significant downward shift post intervention. Percentage of IM admissions with an EDAR order increased from a baseline average of 47% (median, 47%) to 97%, with a statistically significant upward shift starting in January 2020 (Figure 3).
There was no change in observed average time between IM admission order and subsequent admission orders pre and post intervention (16 minutes vs 18 minutes). However, there was a statistically significant shift up to an average of 40 minutes from January through June 2020, which then resolved. The percentage of patients transferred to the ICU within 24 hours of admission to IM did not change (1.1% pre vs 1.4% post intervention). Frequency of patients transferred in from a referring facility also did not change (26/month vs 22/month). Average hospital medicine LOS did not change to a statistically significant degree (6.48 days vs 6.62 days). The percentage of inpatient admissions relative to short stays increased from a baseline of 74.0% (median, 73.6%) to a postintervention average of 82.4%, with a statistically significant shift upward starting March 2020.
Regarding interprofessional practice and clinician experience of care, 122 of 309 preintervention surveys (39.5% response rate) and 98 of 309 postintervention surveys (31.7% response rate) were completed. Pre- and postintervention responses were not linked.
Regarding interprofessional practice, EM residents and EM attendings experienced statistically significant improvements in all interprofessional practice domains (Table 1). Emergency medicine APPs experienced statistically significant improvements post intervention with “I am satisfied with the level of communication with IM hospitalist clinicians” and “Interactions
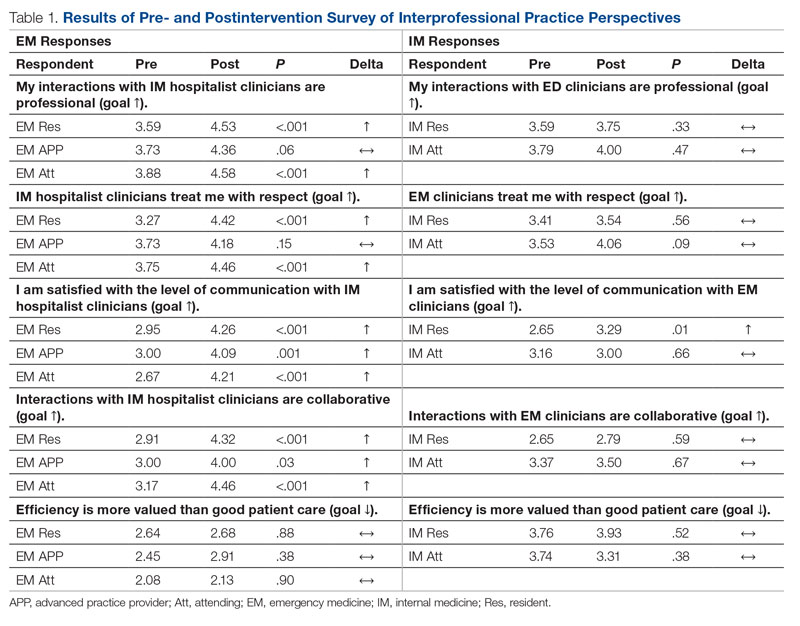
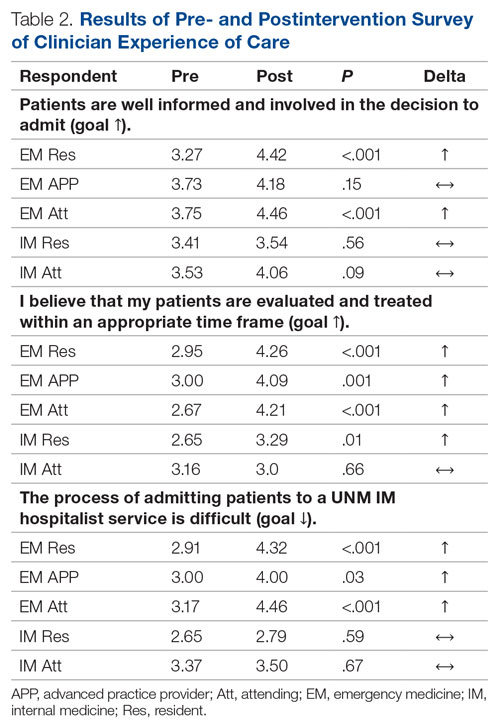
For clinician experience of care, EM residents (P < .001) and attendings (P < .001) experienced statistically significant improvements in “Patients are well informed and involved in the decision to admit,” whereas IM residents and attendings, as well as EM APPs, experienced nonstatistically significant improvements (Table 2). All groups except IM attendings experienced a statistically significant improvement (IM resident P = .011, EM resident P < .001, EM APP P = .001, EM attending P < .001) in “I believe that my patients are evaluated and treated within an appropriate time frame.” Internal medicine attendings felt that this indicator worsened to a nonstatistically significant degree. Post intervention, EM groups experienced a statistically significant worsening in “The process of admitting patients to a UNM IM hospitalist service is difficult,” while IM groups experienced a nonstatistically significant worsening.
Discussion
Implementation of the triage hospitalist role led to a significant reduction in average TTA, from 5 hours 19 minutes to 2 hours 8 minutes. Performance has been sustained at 1 hour 42 minutes on average over the past 6 months. The triage hospitalist was successful at reducing TTA because of their focus on evaluating new admission and transfer requests, deferring other admission responsibilities to on-call admitting teams. Early admission led to no increase in ICU transfers or hospitalist LOS. To ensure that earlier admission reflected improved timeliness of care and that new sources of delay were not being created, we measured the time between IM admission and subsequent admission orders. A statistically significant increase to 40 minutes from January through June 2020 was attributable to the hospitalist acclimating to their new role and the need to standardize workflow. This delay subsequently resolved. An additional benefit of the triage hospitalist was an increase in the proportion of inpatient admissions compared with short stays.
ED-2, an indicator of ED crowding, increased from 3 hours 40 minutes, with a statistically significant upward shift starting May 2020. Increasing ED-2 associated with the triage hospitalist role makes intuitive sense. Patients are admitted 2 hours 40 minutes earlier in their hospital course while downstream bottlenecks preventing patient movement to an inpatient bed remained unchanged. Unfortunately, the COVID-19 pandemic complicates interpretation of ED-2 because the measure reflects institutional capacity to match demand for inpatient beds. Fewer ED registrations and lower hospital medicine census (and resulting inpatient bed availability) in April 2020 during the first COVID-19 surge coincided with an ED-2 nadir of 1 hour 46 minutes. The statistically significant upward shift from May onward reflects ongoing and unprecedented patient volumes. It remains difficult to tease apart the presumed lesser contribution of the triage hospitalist role and presumed larger contribution of high patient volumes on ED-2 increases.
An important complementary change was linkage between the EDAR order and our secure messaging software, creating a single source of admission and transfer requests, prompting early ED clinician consideration of factors that could result in alternative disposition, and ensuring a sustainable data source for TTA. The order did not replace direct communication and included guidance for how triage hospitalists should connect with their ED colleagues. Percentage of IM admissions with the EDAR order increased to 97%. Fallouts are attributed to admissions from non-ED sources (eg, referring facility, endoscopy suite transfers). This communication strategy has been expanded as the primary mechanism of initiating consultation requests between IM and all consulting services.
This intervention was successful from the perspective of ED clinicians. Improvements can be attributed to the simplified admission process, timely patient assessment, a perception that patients are better informed of the decision to admit, and the ability to communicate with the triage hospitalist. Emergency medicine APPs may not have experienced similar improvements due to ongoing perceptions of a hierarchical imbalance. Unfortunately, the small but not statistically significant worsening perspective among ED clinicians that “efficiency is more valued than good patient care” and the statistically significant worsening perspective that “admitting patients to a UNM IM hospitalist service is difficult” may be due to the triage hospitalist responsibility for identifying the roughly 25% of patients who are safe for an alternative disposition.
Internal medicine clinicians experienced no significant changes in attitudes. Underlying causes are likely multifactorial and a focus of ongoing work. Internal medicine residents experienced statistically significant improvements for “I am satisfied with the level of communication with EM clinicians” and nonstatistically significant improvements for the other 3 domains, likely because the intervention enabled them to focus on clinical care rather than the administrative tasks and decision-making complexities inherent to the IM admission process. Internal medicine attendings reported a nonstatistically significant worsening in “I am satisfied with the level of communication with EM clinicians,” which is possibly attributable to challenges connecting with ED attendings after being notified that a new admission is pending. Unfortunately, bedside handoff was not hardwired and is done sporadically. Independent of the data, we believe that the triage hospitalist role has facilitated closer ED-IM relationships by aligning clinical priorities, standardizing processes, improving communication, and reducing sources of hierarchical imbalance and conflict. We expected IM attendings and residents to experience some degree of resolution of the perception that “efficiency is more valued than good patient care” because of the addition of a dedicated triage role. Our data also suggest that IM attendings are less likely to agree that “patients are evaluated and treated within an appropriate time frame.” Both concerns may be linked to the triage hospitalist facing multiple admission and transfer sources with variable arrival rates and variable patient complexity, resulting in high cognitive load and the perception that individual tasks are not completed to the best of their abilities.
To our knowledge, this is the first study assessing the impact of the triage hospitalist role on throughput, clinical care quality, interprofessional practice, and clinician experience of care. In the cross-sectional survey of 10 academic medical centers, 8 had defined triage roles filled by IM attendings, while the remainder had IM attendings supervising trainees.9 A complete picture of the prevalence and varying approaches of triage hospitalists models is unknown. Howell et al12 reported on an approach that reduced admission delays without a resulting increase in mortality or LOS. Our approach differed in several ways, with greater involvement of the triage hospitalist in determining a final admission decision, incorporation of EMR communication, and presence of existing throughput challenges preventing patients from moving seamlessly to an inpatient unit.
Conclusion
We believe this effort was successful for several reasons, including adherence to quality improvement best practices, such as engagement of stakeholders early on, the use of data to inform decision-making, the application of technology to hardwire process, and alignment with institutional priorities. Spread of this intervention will be limited by the financial investment required to start and maintain a triage hospitalist role. A primary limitation of this study is the confounding effect of the COVID-19 pandemic on our analysis. Next steps include identification of clinicians wishing to specialize in triage and expanding triage to include non-IM primary services. Additional research to optimize the triage hospitalist experience of care, as well as to measure improvements in patient-centered outcomes, is necessary.
Corresponding author: Christopher Bartlett, MD, MPH; MSC10 5550, 1 University of New Mexico, Albuquerque, NM 87131; CSBartlett@salud.unm.edu
Disclosures: None reported.
1. Huang Q, Thind A, Dreyer JF, et al. The impact of delays to admission from the emergency department on inpatient outcomes. BMC Emerg Med. 2010;10:16. doi:10.1186/1471-227X-10-16
2. Liew D, Liew D, Kennedy MP. Emergency department length of stay independently predicts excess inpatient length of stay. Med J Aust. 2003;179:524-526. doi:10.5694/j.1326-5377.2003.tb05676.x
3. Richardson DB. The access-block effect: relationship between delay to reaching an inpatient bed and inpatient length of stay. Med J Aust. 2002;177:492-495. doi:10.5694/j.1326-5377.2002.tb04917.x
4. Polevoi SK, Quinn JV, Kramer KR. Factors associated with patients who leave without being seen. Acad Emerg Med. 2005;12:232-236. doi:10.1197/j.aem.2004.10.029
5. Bernstein SL, Aronsky D, Duseja R, et al. The effect of emergency department crowding on clinically oriented outcomes. Acad Emerg Med. 2009;16:1-10. doi:10.1111/j.1553-2712.2008.00295.x
6. Vieth TL, Rhodes KV. The effect of crowding on access and quality in an academic ED. Am J Emerg Med. 2006;24:787-794. doi:10.1016/j.ajem.2006.03.026
7. Rondeau KV, Francescutti LH. Emergency department overcrowding: the impact of resource scarcity on physician job satisfaction. J Healthc Manag. 2005;50:327-340; discussion 341-342.
8. Emergency Department Crowding: High Impact Solutions. American College of Emergency Physicians. Emergency Medicine Practice Committee. 2016. Accessed March 31, 2023. https://www.acep.org/globalassets/sites/acep/media/crowding/empc_crowding-ip_092016.pdf
9. Velásquez ST, Wang ES, White AW, et al. Hospitalists as triagists: description of the triagist role across academic medical centers. J Hosp Med. 2020;15:87-90. doi:10.12788/jhm.3327
10. Amick A, Bann M. Characterizing the role of the “triagist”: reasons for triage discordance and impact on disposition. J Gen Intern Med. 2021;36:2177-2179. doi:10.1007/s11606-020-05887-y
11. Perla RJ, Provost LP, Murray SK. The run chart: a simple analytical tool for learning for variation in healthcare processes. BMJ Qual Saf. 2011;20:46-51. doi:10.1136/bmjqs.2009.037895
12. Howell EE, Bessman ES, Rubin HR. Hospitalists and an innovative emergency department admission process. J Gen Intern Med. 2004;19:266-268. doi:10.1111/j.1525-1497.2004.30431.x
From the Division of Hospital Medicine, University of New Mexico Hospital, Albuquerque (Drs. Bartlett, Pizanis, Angeli, Lacy, and Rogers), Department of Emergency Medicine, University of New Mexico Hospital, Albuquerque (Dr. Scott), and University of New Mexico School of Medicine, Albuquerque (Ms. Baca).
ABSTRACT
Background: Emergency department (ED) crowding is associated with deleterious consequences for patient care and throughput. Admission delays worsen ED crowding. Time to admission (TTA)—the time between an ED admission request and internal medicine (IM) admission orders—can be shortened through implementation of a triage hospitalist role. Limited research is available highlighting the impact of triage hospitalists on throughput, care quality, interprofessional practice, and clinician experience of care.
Methods: A triage hospitalist role was piloted and implemented. Run charts were interpreted using accepted rules for deriving statistically significant conclusions. Statistical analysis was applied to interprofessional practice and clinician experience-of-care survey results.
Results: Following implementation, TTA decreased from 5 hours 19 minutes to 2 hours 8 minutes. Emergency department crowding increased from baseline. The reduction in TTA was associated with decreased time from ED arrival to IM admission request, no change in critical care transfers during the initial 24 hours, and increased admissions to inpatient status. Additionally, decreased TTA was associated with no change in referring hospital transfer rates and no change in hospital medicine length of stay. Interprofessional practice attitudes improved among ED clinicians but not IM clinicians. Clinician experience-of-care results were mixed.
Conclusion: A triage hospitalist role is an effective approach for mitigating admission delays, with no evident adverse clinical consequences. A triage hospitalist alone was incapable of resolving ED crowding issues without a complementary focus on downstream bottlenecks.
Keywords: triage hospitalist, admission delay, quality improvement.
Excess time to admission (TTA), defined as the time between an emergency department (ED) admission request and internal medicine (IM) admission orders, contributes to ED crowding, which is associated with deleterious impacts on patient care and throughput. Prior research has correlated ED crowding with an increase in length of stay (LOS)1-3 and total inpatient cost,1 as well as increased inpatient mortality, higher left-without-being-seen rates,4 delays in clinically meaningful care,5,6 and poor patient and clinician satisfaction.6,7 While various solutions have been proposed to alleviate ED crowding,8 excess TTA is one aspect that IM can directly address.
Like many institutions, ours is challenged by ED crowding. Time to admission is a known bottleneck. Underlying factors that contribute to excess TTA include varied admission request volumes in relation to fixed admitting capacity; learner-focused admitting processes; and unreliable strategies for determining whether patients are eligible for ED observation, transfer to an alternative facility, or admission to an alternative primary service.
To address excess TTA, we piloted then implemented a triage hospitalist role, envisioned as responsible for evaluating ED admission requests to IM, making timely determinations of admission appropriateness, and distributing patients to admitting teams. This intervention was selected because of its strengths, including the ability to standardize admission processes, improve the proximity of clinical decision-makers to patient care to reduce delays, and decrease hierarchical imbalances experienced by trainees, and also because the institution expressed a willingness to mitigate its primary weakness (ie, ongoing financial support for sustainability) should it prove successful.
Previously, a triage hospitalist has been defined as “a physician who assesses patients for admission, actively supporting the transition of the patient from the outpatient to the inpatient setting.”9 Velásquez et al surveyed 10 academic medical centers and identified significant heterogeneity in the roles and responsibilities of a triage hospitalist.9 Limited research addresses the impact of this role on throughput. One report described the volume and source of requests evaluated by a triage hospitalist and the frequency with which the triage hospitalists’ assessment of admission appropriateness aligned with that of the referring clinicians.10 No prior research is available demonstrating the impact of this role on care quality, interprofessional practice, or clinician experience of care. This article is intended to address these gaps in the literature.
Methods
Setting
The University of New Mexico Hospital has 537 beds and is the only level-1 trauma and academic medical center in the state. On average, approximately 8000 patients register to be seen in the ED per month. Roughly 600 are admitted to IM per month. This study coincided with the COVID-19 pandemic, with low patient volumes in April 2020, overcapacity census starting in May 2020, and markedly high patient volumes in May/June 2020 and November/December 2020. All authors participated in project development, implementation, and analysis.
Preintervention IM Admission Process
When requesting IM admission, ED clinicians (resident, advanced practice provider [APP], or attending) contacted the IM triage person (typically an IM resident physician) by phone or in person. The IM triage person would then assess whether the patient needed critical care consultation (a unique and separate admission pathway), was eligible for ED observation or transfer to an outside hospital, or was clinically appropriate for IM subacute and floor admission. Pending admissions were evaluated in order of severity of illness or based on wait time if severity of illness was equal. Transfers from the intensive care unit (ICU) and referring hospitals were prioritized. Between 7:00
Triage Hospitalist Pilot
Key changes made during the pilot included scheduling an IM attending to serve as triage hospitalist for all IM admission requests from the ED between 7:00
Measures for Triage Hospitalist Pilot
Data collected included request type (new vs overflow from night) and patient details (name, medical record number). Two time points were recorded: when the EDAR order was entered and when admission orders were entered. Process indicators, including whether the EDAR order was entered and the final triage decision (eg, discharge, IM), were recorded. General feedback was requested at the end of each shift.
Phased Implementation of Triage Hospitalist Role
Triage hospitalist role implementation was approved following the pilot, with additional salary support funded by the institution. A new performance measure (time from admission request to admission order, self-identified goal < 3 hours) was approved by all parties.
In January 2020, the role was scheduled from 7:00
In March 2020, to create a single communication pathway while simultaneously hardwiring our measurement strategy, the EDAR order was modified such that it would automatically prompt a 1-way communication to the triage hospitalist using the institution’s secure messaging software. The message included patient name, medical record number, location, ED attending, reason for admission, and consultation priority, as well as 2 questions prompting ED clinicians to reflect on the most common reasons for the triage hospitalist to recommend against IM admission (eligible for admission to other primary service, transfer to alternative hospital).
In July 2020, the triage hospitalist role was scheduled 24 hours a day, 7 days a week, to meet an institutional request. The schedule was divided into a daytime 7:00
Measures for Triage Hospitalist Role
The primary outcome measure was TTA, defined as the time between EDAR (operationalized using EDAR order timestamp) and IM admission decision (operationalized using inpatient bed request order timestamp). Additional outcome measures included the Centers for Medicare & Medicaid Services Electronic Clinical Quality Measure ED-2 (eCQM ED-2), defined as the median time from admit decision to departure from the ED for patients admitted to inpatient status.
Process measures included time between patient arrival to the ED (operationalized using ED registration timestamp) and EDAR and percentage of IM admissions with an EDAR order. Balancing measures included time between bed request order (referred to as the IM admission order) and subsequent admission orders. While the IM admission order prompts an inpatient clinical encounter and inpatient bed assignment, subsequent admission orders are necessary for clinical care. Additional balancing measures included ICU transfer rate within the first 24 hours, referring facility transfer frequency to IM (an indicator of access for patients at outside hospitals), average hospital medicine LOS (operationalized using ED registration timestamp to discharge timestamp), and admission status (inpatient vs observation).
An anonymous preintervention (December 2019) and postintervention (August 2020) survey focusing on interprofessional practice and clinician experience of care was used to obtain feedback from ED and IM attendings, APPs, and trainees. Emergency department clinicians were asked questions pertaining to their IM colleagues and vice versa. A Likert 5-point scale was used to respond.
Data Analysis
The preintervention period was June 1, 2019, to October 31, 2019; the pilot period was November 1, 2019, to December 31, 2019; the staged implementation period was January 1, 2020, to June 30, 2020; and the postintervention period was July 1, 2020, to December 31, 2020. Run charts for outcome, process, and balancing measures were interpreted using rules for deriving statistically significant conclusions.11 Statistical analysis using a t test assuming unequal variances with P < . 05 to indicate statistical significance was applied to experience-of-care results. The study was approved by the Institutional Review Board.
Results
Triage Hospitalist Pilot Time Period
Seventy-four entries were recorded, 56 (75.7%) reflecting new admission requests. Average time between EDAR order and IM admission order was 40 minutes. The EDAR order was entered into the EMR without prompting in 22 (29.7%) cases. In 56 (75.7%) cases, the final triage decision was IM admission. Other dispositions included 3 discharges, 4 transfers, 3 alternative primary service admissions, 1 ED observation, and 7 triage deferrals pending additional workup or stabilization.
Feedback substantiated several benefits, including improved coordination among IM, ED, and consultant clinicians, as well as early admission of seriously ill patients. Feedback also confirmed several expected challenges, including evidence of communication lapses, difficulty with transfer coordinator integration, difficulty hardwiring elements of the verbal and bedside handoff, and perceived high cognitive load for the triage hospitalist. Several unexpected issues included whether ED APPs can request admission independently and how reconsultation is expected to occur if admission is initially deferred.
Triage Hospitalist Implementation Time Period
Time to admission decreased from a baseline pre-pilot average of 5 hours 19 minutes (median, 4 hours 45 minutes) to a postintervention average of 2 hours 8 minutes, with a statistically significant downward shift post intervention (Figure 1).
ED-2 increased from a baseline average of 3 hours 40 minutes (median, 2 hours 39 minutes), with a statistically significant upward shift starting in May 2020 (Figure 2). Time between patient arrival to the ED and EDAR order decreased from a baseline average of 8 hours 47 minutes (median, 8 hours 37 minutes) to a postintervention average of 5 hours 57 minutes, with a statistically significant downward shift post intervention. Percentage of IM admissions with an EDAR order increased from a baseline average of 47% (median, 47%) to 97%, with a statistically significant upward shift starting in January 2020 (Figure 3).

There was no change in observed average time between IM admission order and subsequent admission orders pre and post intervention (16 minutes vs 18 minutes). However, there was a statistically significant shift up to an average of 40 minutes from January through June 2020, which then resolved. The percentage of patients transferred to the ICU within 24 hours of admission to IM did not change (1.1% pre vs 1.4% post intervention). Frequency of patients transferred in from a referring facility also did not change (26/month vs 22/month). Average hospital medicine LOS did not change to a statistically significant degree (6.48 days vs 6.62 days). The percentage of inpatient admissions relative to short stays increased from a baseline of 74.0% (median, 73.6%) to a postintervention average of 82.4%, with a statistically significant shift upward starting March 2020.

Regarding interprofessional practice and clinician experience of care, 122 of 309 preintervention surveys (39.5% response rate) and 98 of 309 postintervention surveys (31.7% response rate) were completed. Pre- and postintervention responses were not linked.
Regarding interprofessional practice, EM residents and EM attendings experienced statistically significant improvements in all interprofessional practice domains (Table 1). Emergency medicine APPs experienced statistically significant improvements post intervention with “I am satisfied with the level of communication with IM hospitalist clinicians” and “Interactions

For clinician experience of care, EM residents (P < .001) and attendings (P < .001) experienced statistically significant improvements in “Patients are well informed and involved in the decision to admit,” whereas IM residents and attendings, as well as EM APPs, experienced nonstatistically significant improvements (Table 2). All groups except IM attendings experienced a statistically significant improvement (IM resident P = .011, EM resident P < .001, EM APP P = .001, EM attending P < .001) in “I believe that my patients are evaluated and treated within an appropriate time frame.” Internal medicine attendings felt that this indicator worsened to a nonstatistically significant degree. Post intervention, EM groups experienced a statistically significant worsening in “The process of admitting patients to a UNM IM hospitalist service is difficult,” while IM groups experienced a nonstatistically significant worsening.

Discussion
Implementation of the triage hospitalist role led to a significant reduction in average TTA, from 5 hours 19 minutes to 2 hours 8 minutes. Performance has been sustained at 1 hour 42 minutes on average over the past 6 months. The triage hospitalist was successful at reducing TTA because of their focus on evaluating new admission and transfer requests, deferring other admission responsibilities to on-call admitting teams. Early admission led to no increase in ICU transfers or hospitalist LOS. To ensure that earlier admission reflected improved timeliness of care and that new sources of delay were not being created, we measured the time between IM admission and subsequent admission orders. A statistically significant increase to 40 minutes from January through June 2020 was attributable to the hospitalist acclimating to their new role and the need to standardize workflow. This delay subsequently resolved. An additional benefit of the triage hospitalist was an increase in the proportion of inpatient admissions compared with short stays.
ED-2, an indicator of ED crowding, increased from 3 hours 40 minutes, with a statistically significant upward shift starting May 2020. Increasing ED-2 associated with the triage hospitalist role makes intuitive sense. Patients are admitted 2 hours 40 minutes earlier in their hospital course while downstream bottlenecks preventing patient movement to an inpatient bed remained unchanged. Unfortunately, the COVID-19 pandemic complicates interpretation of ED-2 because the measure reflects institutional capacity to match demand for inpatient beds. Fewer ED registrations and lower hospital medicine census (and resulting inpatient bed availability) in April 2020 during the first COVID-19 surge coincided with an ED-2 nadir of 1 hour 46 minutes. The statistically significant upward shift from May onward reflects ongoing and unprecedented patient volumes. It remains difficult to tease apart the presumed lesser contribution of the triage hospitalist role and presumed larger contribution of high patient volumes on ED-2 increases.
An important complementary change was linkage between the EDAR order and our secure messaging software, creating a single source of admission and transfer requests, prompting early ED clinician consideration of factors that could result in alternative disposition, and ensuring a sustainable data source for TTA. The order did not replace direct communication and included guidance for how triage hospitalists should connect with their ED colleagues. Percentage of IM admissions with the EDAR order increased to 97%. Fallouts are attributed to admissions from non-ED sources (eg, referring facility, endoscopy suite transfers). This communication strategy has been expanded as the primary mechanism of initiating consultation requests between IM and all consulting services.
This intervention was successful from the perspective of ED clinicians. Improvements can be attributed to the simplified admission process, timely patient assessment, a perception that patients are better informed of the decision to admit, and the ability to communicate with the triage hospitalist. Emergency medicine APPs may not have experienced similar improvements due to ongoing perceptions of a hierarchical imbalance. Unfortunately, the small but not statistically significant worsening perspective among ED clinicians that “efficiency is more valued than good patient care” and the statistically significant worsening perspective that “admitting patients to a UNM IM hospitalist service is difficult” may be due to the triage hospitalist responsibility for identifying the roughly 25% of patients who are safe for an alternative disposition.
Internal medicine clinicians experienced no significant changes in attitudes. Underlying causes are likely multifactorial and a focus of ongoing work. Internal medicine residents experienced statistically significant improvements for “I am satisfied with the level of communication with EM clinicians” and nonstatistically significant improvements for the other 3 domains, likely because the intervention enabled them to focus on clinical care rather than the administrative tasks and decision-making complexities inherent to the IM admission process. Internal medicine attendings reported a nonstatistically significant worsening in “I am satisfied with the level of communication with EM clinicians,” which is possibly attributable to challenges connecting with ED attendings after being notified that a new admission is pending. Unfortunately, bedside handoff was not hardwired and is done sporadically. Independent of the data, we believe that the triage hospitalist role has facilitated closer ED-IM relationships by aligning clinical priorities, standardizing processes, improving communication, and reducing sources of hierarchical imbalance and conflict. We expected IM attendings and residents to experience some degree of resolution of the perception that “efficiency is more valued than good patient care” because of the addition of a dedicated triage role. Our data also suggest that IM attendings are less likely to agree that “patients are evaluated and treated within an appropriate time frame.” Both concerns may be linked to the triage hospitalist facing multiple admission and transfer sources with variable arrival rates and variable patient complexity, resulting in high cognitive load and the perception that individual tasks are not completed to the best of their abilities.
To our knowledge, this is the first study assessing the impact of the triage hospitalist role on throughput, clinical care quality, interprofessional practice, and clinician experience of care. In the cross-sectional survey of 10 academic medical centers, 8 had defined triage roles filled by IM attendings, while the remainder had IM attendings supervising trainees.9 A complete picture of the prevalence and varying approaches of triage hospitalists models is unknown. Howell et al12 reported on an approach that reduced admission delays without a resulting increase in mortality or LOS. Our approach differed in several ways, with greater involvement of the triage hospitalist in determining a final admission decision, incorporation of EMR communication, and presence of existing throughput challenges preventing patients from moving seamlessly to an inpatient unit.
Conclusion
We believe this effort was successful for several reasons, including adherence to quality improvement best practices, such as engagement of stakeholders early on, the use of data to inform decision-making, the application of technology to hardwire process, and alignment with institutional priorities. Spread of this intervention will be limited by the financial investment required to start and maintain a triage hospitalist role. A primary limitation of this study is the confounding effect of the COVID-19 pandemic on our analysis. Next steps include identification of clinicians wishing to specialize in triage and expanding triage to include non-IM primary services. Additional research to optimize the triage hospitalist experience of care, as well as to measure improvements in patient-centered outcomes, is necessary.
Corresponding author: Christopher Bartlett, MD, MPH; MSC10 5550, 1 University of New Mexico, Albuquerque, NM 87131; CSBartlett@salud.unm.edu
Disclosures: None reported.
From the Division of Hospital Medicine, University of New Mexico Hospital, Albuquerque (Drs. Bartlett, Pizanis, Angeli, Lacy, and Rogers), Department of Emergency Medicine, University of New Mexico Hospital, Albuquerque (Dr. Scott), and University of New Mexico School of Medicine, Albuquerque (Ms. Baca).
ABSTRACT
Background: Emergency department (ED) crowding is associated with deleterious consequences for patient care and throughput. Admission delays worsen ED crowding. Time to admission (TTA)—the time between an ED admission request and internal medicine (IM) admission orders—can be shortened through implementation of a triage hospitalist role. Limited research is available highlighting the impact of triage hospitalists on throughput, care quality, interprofessional practice, and clinician experience of care.
Methods: A triage hospitalist role was piloted and implemented. Run charts were interpreted using accepted rules for deriving statistically significant conclusions. Statistical analysis was applied to interprofessional practice and clinician experience-of-care survey results.
Results: Following implementation, TTA decreased from 5 hours 19 minutes to 2 hours 8 minutes. Emergency department crowding increased from baseline. The reduction in TTA was associated with decreased time from ED arrival to IM admission request, no change in critical care transfers during the initial 24 hours, and increased admissions to inpatient status. Additionally, decreased TTA was associated with no change in referring hospital transfer rates and no change in hospital medicine length of stay. Interprofessional practice attitudes improved among ED clinicians but not IM clinicians. Clinician experience-of-care results were mixed.
Conclusion: A triage hospitalist role is an effective approach for mitigating admission delays, with no evident adverse clinical consequences. A triage hospitalist alone was incapable of resolving ED crowding issues without a complementary focus on downstream bottlenecks.
Keywords: triage hospitalist, admission delay, quality improvement.
Excess time to admission (TTA), defined as the time between an emergency department (ED) admission request and internal medicine (IM) admission orders, contributes to ED crowding, which is associated with deleterious impacts on patient care and throughput. Prior research has correlated ED crowding with an increase in length of stay (LOS)1-3 and total inpatient cost,1 as well as increased inpatient mortality, higher left-without-being-seen rates,4 delays in clinically meaningful care,5,6 and poor patient and clinician satisfaction.6,7 While various solutions have been proposed to alleviate ED crowding,8 excess TTA is one aspect that IM can directly address.
Like many institutions, ours is challenged by ED crowding. Time to admission is a known bottleneck. Underlying factors that contribute to excess TTA include varied admission request volumes in relation to fixed admitting capacity; learner-focused admitting processes; and unreliable strategies for determining whether patients are eligible for ED observation, transfer to an alternative facility, or admission to an alternative primary service.
To address excess TTA, we piloted then implemented a triage hospitalist role, envisioned as responsible for evaluating ED admission requests to IM, making timely determinations of admission appropriateness, and distributing patients to admitting teams. This intervention was selected because of its strengths, including the ability to standardize admission processes, improve the proximity of clinical decision-makers to patient care to reduce delays, and decrease hierarchical imbalances experienced by trainees, and also because the institution expressed a willingness to mitigate its primary weakness (ie, ongoing financial support for sustainability) should it prove successful.
Previously, a triage hospitalist has been defined as “a physician who assesses patients for admission, actively supporting the transition of the patient from the outpatient to the inpatient setting.”9 Velásquez et al surveyed 10 academic medical centers and identified significant heterogeneity in the roles and responsibilities of a triage hospitalist.9 Limited research addresses the impact of this role on throughput. One report described the volume and source of requests evaluated by a triage hospitalist and the frequency with which the triage hospitalists’ assessment of admission appropriateness aligned with that of the referring clinicians.10 No prior research is available demonstrating the impact of this role on care quality, interprofessional practice, or clinician experience of care. This article is intended to address these gaps in the literature.
Methods
Setting
The University of New Mexico Hospital has 537 beds and is the only level-1 trauma and academic medical center in the state. On average, approximately 8000 patients register to be seen in the ED per month. Roughly 600 are admitted to IM per month. This study coincided with the COVID-19 pandemic, with low patient volumes in April 2020, overcapacity census starting in May 2020, and markedly high patient volumes in May/June 2020 and November/December 2020. All authors participated in project development, implementation, and analysis.
Preintervention IM Admission Process
When requesting IM admission, ED clinicians (resident, advanced practice provider [APP], or attending) contacted the IM triage person (typically an IM resident physician) by phone or in person. The IM triage person would then assess whether the patient needed critical care consultation (a unique and separate admission pathway), was eligible for ED observation or transfer to an outside hospital, or was clinically appropriate for IM subacute and floor admission. Pending admissions were evaluated in order of severity of illness or based on wait time if severity of illness was equal. Transfers from the intensive care unit (ICU) and referring hospitals were prioritized. Between 7:00
Triage Hospitalist Pilot
Key changes made during the pilot included scheduling an IM attending to serve as triage hospitalist for all IM admission requests from the ED between 7:00
Measures for Triage Hospitalist Pilot
Data collected included request type (new vs overflow from night) and patient details (name, medical record number). Two time points were recorded: when the EDAR order was entered and when admission orders were entered. Process indicators, including whether the EDAR order was entered and the final triage decision (eg, discharge, IM), were recorded. General feedback was requested at the end of each shift.
Phased Implementation of Triage Hospitalist Role
Triage hospitalist role implementation was approved following the pilot, with additional salary support funded by the institution. A new performance measure (time from admission request to admission order, self-identified goal < 3 hours) was approved by all parties.
In January 2020, the role was scheduled from 7:00
In March 2020, to create a single communication pathway while simultaneously hardwiring our measurement strategy, the EDAR order was modified such that it would automatically prompt a 1-way communication to the triage hospitalist using the institution’s secure messaging software. The message included patient name, medical record number, location, ED attending, reason for admission, and consultation priority, as well as 2 questions prompting ED clinicians to reflect on the most common reasons for the triage hospitalist to recommend against IM admission (eligible for admission to other primary service, transfer to alternative hospital).
In July 2020, the triage hospitalist role was scheduled 24 hours a day, 7 days a week, to meet an institutional request. The schedule was divided into a daytime 7:00
Measures for Triage Hospitalist Role
The primary outcome measure was TTA, defined as the time between EDAR (operationalized using EDAR order timestamp) and IM admission decision (operationalized using inpatient bed request order timestamp). Additional outcome measures included the Centers for Medicare & Medicaid Services Electronic Clinical Quality Measure ED-2 (eCQM ED-2), defined as the median time from admit decision to departure from the ED for patients admitted to inpatient status.
Process measures included time between patient arrival to the ED (operationalized using ED registration timestamp) and EDAR and percentage of IM admissions with an EDAR order. Balancing measures included time between bed request order (referred to as the IM admission order) and subsequent admission orders. While the IM admission order prompts an inpatient clinical encounter and inpatient bed assignment, subsequent admission orders are necessary for clinical care. Additional balancing measures included ICU transfer rate within the first 24 hours, referring facility transfer frequency to IM (an indicator of access for patients at outside hospitals), average hospital medicine LOS (operationalized using ED registration timestamp to discharge timestamp), and admission status (inpatient vs observation).
An anonymous preintervention (December 2019) and postintervention (August 2020) survey focusing on interprofessional practice and clinician experience of care was used to obtain feedback from ED and IM attendings, APPs, and trainees. Emergency department clinicians were asked questions pertaining to their IM colleagues and vice versa. A Likert 5-point scale was used to respond.
Data Analysis
The preintervention period was June 1, 2019, to October 31, 2019; the pilot period was November 1, 2019, to December 31, 2019; the staged implementation period was January 1, 2020, to June 30, 2020; and the postintervention period was July 1, 2020, to December 31, 2020. Run charts for outcome, process, and balancing measures were interpreted using rules for deriving statistically significant conclusions.11 Statistical analysis using a t test assuming unequal variances with P < . 05 to indicate statistical significance was applied to experience-of-care results. The study was approved by the Institutional Review Board.
Results
Triage Hospitalist Pilot Time Period
Seventy-four entries were recorded, 56 (75.7%) reflecting new admission requests. Average time between EDAR order and IM admission order was 40 minutes. The EDAR order was entered into the EMR without prompting in 22 (29.7%) cases. In 56 (75.7%) cases, the final triage decision was IM admission. Other dispositions included 3 discharges, 4 transfers, 3 alternative primary service admissions, 1 ED observation, and 7 triage deferrals pending additional workup or stabilization.
Feedback substantiated several benefits, including improved coordination among IM, ED, and consultant clinicians, as well as early admission of seriously ill patients. Feedback also confirmed several expected challenges, including evidence of communication lapses, difficulty with transfer coordinator integration, difficulty hardwiring elements of the verbal and bedside handoff, and perceived high cognitive load for the triage hospitalist. Several unexpected issues included whether ED APPs can request admission independently and how reconsultation is expected to occur if admission is initially deferred.
Triage Hospitalist Implementation Time Period
Time to admission decreased from a baseline pre-pilot average of 5 hours 19 minutes (median, 4 hours 45 minutes) to a postintervention average of 2 hours 8 minutes, with a statistically significant downward shift post intervention (Figure 1).
ED-2 increased from a baseline average of 3 hours 40 minutes (median, 2 hours 39 minutes), with a statistically significant upward shift starting in May 2020 (Figure 2). Time between patient arrival to the ED and EDAR order decreased from a baseline average of 8 hours 47 minutes (median, 8 hours 37 minutes) to a postintervention average of 5 hours 57 minutes, with a statistically significant downward shift post intervention. Percentage of IM admissions with an EDAR order increased from a baseline average of 47% (median, 47%) to 97%, with a statistically significant upward shift starting in January 2020 (Figure 3).

There was no change in observed average time between IM admission order and subsequent admission orders pre and post intervention (16 minutes vs 18 minutes). However, there was a statistically significant shift up to an average of 40 minutes from January through June 2020, which then resolved. The percentage of patients transferred to the ICU within 24 hours of admission to IM did not change (1.1% pre vs 1.4% post intervention). Frequency of patients transferred in from a referring facility also did not change (26/month vs 22/month). Average hospital medicine LOS did not change to a statistically significant degree (6.48 days vs 6.62 days). The percentage of inpatient admissions relative to short stays increased from a baseline of 74.0% (median, 73.6%) to a postintervention average of 82.4%, with a statistically significant shift upward starting March 2020.

Regarding interprofessional practice and clinician experience of care, 122 of 309 preintervention surveys (39.5% response rate) and 98 of 309 postintervention surveys (31.7% response rate) were completed. Pre- and postintervention responses were not linked.
Regarding interprofessional practice, EM residents and EM attendings experienced statistically significant improvements in all interprofessional practice domains (Table 1). Emergency medicine APPs experienced statistically significant improvements post intervention with “I am satisfied with the level of communication with IM hospitalist clinicians” and “Interactions

For clinician experience of care, EM residents (P < .001) and attendings (P < .001) experienced statistically significant improvements in “Patients are well informed and involved in the decision to admit,” whereas IM residents and attendings, as well as EM APPs, experienced nonstatistically significant improvements (Table 2). All groups except IM attendings experienced a statistically significant improvement (IM resident P = .011, EM resident P < .001, EM APP P = .001, EM attending P < .001) in “I believe that my patients are evaluated and treated within an appropriate time frame.” Internal medicine attendings felt that this indicator worsened to a nonstatistically significant degree. Post intervention, EM groups experienced a statistically significant worsening in “The process of admitting patients to a UNM IM hospitalist service is difficult,” while IM groups experienced a nonstatistically significant worsening.

Discussion
Implementation of the triage hospitalist role led to a significant reduction in average TTA, from 5 hours 19 minutes to 2 hours 8 minutes. Performance has been sustained at 1 hour 42 minutes on average over the past 6 months. The triage hospitalist was successful at reducing TTA because of their focus on evaluating new admission and transfer requests, deferring other admission responsibilities to on-call admitting teams. Early admission led to no increase in ICU transfers or hospitalist LOS. To ensure that earlier admission reflected improved timeliness of care and that new sources of delay were not being created, we measured the time between IM admission and subsequent admission orders. A statistically significant increase to 40 minutes from January through June 2020 was attributable to the hospitalist acclimating to their new role and the need to standardize workflow. This delay subsequently resolved. An additional benefit of the triage hospitalist was an increase in the proportion of inpatient admissions compared with short stays.
ED-2, an indicator of ED crowding, increased from 3 hours 40 minutes, with a statistically significant upward shift starting May 2020. Increasing ED-2 associated with the triage hospitalist role makes intuitive sense. Patients are admitted 2 hours 40 minutes earlier in their hospital course while downstream bottlenecks preventing patient movement to an inpatient bed remained unchanged. Unfortunately, the COVID-19 pandemic complicates interpretation of ED-2 because the measure reflects institutional capacity to match demand for inpatient beds. Fewer ED registrations and lower hospital medicine census (and resulting inpatient bed availability) in April 2020 during the first COVID-19 surge coincided with an ED-2 nadir of 1 hour 46 minutes. The statistically significant upward shift from May onward reflects ongoing and unprecedented patient volumes. It remains difficult to tease apart the presumed lesser contribution of the triage hospitalist role and presumed larger contribution of high patient volumes on ED-2 increases.
An important complementary change was linkage between the EDAR order and our secure messaging software, creating a single source of admission and transfer requests, prompting early ED clinician consideration of factors that could result in alternative disposition, and ensuring a sustainable data source for TTA. The order did not replace direct communication and included guidance for how triage hospitalists should connect with their ED colleagues. Percentage of IM admissions with the EDAR order increased to 97%. Fallouts are attributed to admissions from non-ED sources (eg, referring facility, endoscopy suite transfers). This communication strategy has been expanded as the primary mechanism of initiating consultation requests between IM and all consulting services.
This intervention was successful from the perspective of ED clinicians. Improvements can be attributed to the simplified admission process, timely patient assessment, a perception that patients are better informed of the decision to admit, and the ability to communicate with the triage hospitalist. Emergency medicine APPs may not have experienced similar improvements due to ongoing perceptions of a hierarchical imbalance. Unfortunately, the small but not statistically significant worsening perspective among ED clinicians that “efficiency is more valued than good patient care” and the statistically significant worsening perspective that “admitting patients to a UNM IM hospitalist service is difficult” may be due to the triage hospitalist responsibility for identifying the roughly 25% of patients who are safe for an alternative disposition.
Internal medicine clinicians experienced no significant changes in attitudes. Underlying causes are likely multifactorial and a focus of ongoing work. Internal medicine residents experienced statistically significant improvements for “I am satisfied with the level of communication with EM clinicians” and nonstatistically significant improvements for the other 3 domains, likely because the intervention enabled them to focus on clinical care rather than the administrative tasks and decision-making complexities inherent to the IM admission process. Internal medicine attendings reported a nonstatistically significant worsening in “I am satisfied with the level of communication with EM clinicians,” which is possibly attributable to challenges connecting with ED attendings after being notified that a new admission is pending. Unfortunately, bedside handoff was not hardwired and is done sporadically. Independent of the data, we believe that the triage hospitalist role has facilitated closer ED-IM relationships by aligning clinical priorities, standardizing processes, improving communication, and reducing sources of hierarchical imbalance and conflict. We expected IM attendings and residents to experience some degree of resolution of the perception that “efficiency is more valued than good patient care” because of the addition of a dedicated triage role. Our data also suggest that IM attendings are less likely to agree that “patients are evaluated and treated within an appropriate time frame.” Both concerns may be linked to the triage hospitalist facing multiple admission and transfer sources with variable arrival rates and variable patient complexity, resulting in high cognitive load and the perception that individual tasks are not completed to the best of their abilities.
To our knowledge, this is the first study assessing the impact of the triage hospitalist role on throughput, clinical care quality, interprofessional practice, and clinician experience of care. In the cross-sectional survey of 10 academic medical centers, 8 had defined triage roles filled by IM attendings, while the remainder had IM attendings supervising trainees.9 A complete picture of the prevalence and varying approaches of triage hospitalists models is unknown. Howell et al12 reported on an approach that reduced admission delays without a resulting increase in mortality or LOS. Our approach differed in several ways, with greater involvement of the triage hospitalist in determining a final admission decision, incorporation of EMR communication, and presence of existing throughput challenges preventing patients from moving seamlessly to an inpatient unit.
Conclusion
We believe this effort was successful for several reasons, including adherence to quality improvement best practices, such as engagement of stakeholders early on, the use of data to inform decision-making, the application of technology to hardwire process, and alignment with institutional priorities. Spread of this intervention will be limited by the financial investment required to start and maintain a triage hospitalist role. A primary limitation of this study is the confounding effect of the COVID-19 pandemic on our analysis. Next steps include identification of clinicians wishing to specialize in triage and expanding triage to include non-IM primary services. Additional research to optimize the triage hospitalist experience of care, as well as to measure improvements in patient-centered outcomes, is necessary.
Corresponding author: Christopher Bartlett, MD, MPH; MSC10 5550, 1 University of New Mexico, Albuquerque, NM 87131; CSBartlett@salud.unm.edu
Disclosures: None reported.
1. Huang Q, Thind A, Dreyer JF, et al. The impact of delays to admission from the emergency department on inpatient outcomes. BMC Emerg Med. 2010;10:16. doi:10.1186/1471-227X-10-16
2. Liew D, Liew D, Kennedy MP. Emergency department length of stay independently predicts excess inpatient length of stay. Med J Aust. 2003;179:524-526. doi:10.5694/j.1326-5377.2003.tb05676.x
3. Richardson DB. The access-block effect: relationship between delay to reaching an inpatient bed and inpatient length of stay. Med J Aust. 2002;177:492-495. doi:10.5694/j.1326-5377.2002.tb04917.x
4. Polevoi SK, Quinn JV, Kramer KR. Factors associated with patients who leave without being seen. Acad Emerg Med. 2005;12:232-236. doi:10.1197/j.aem.2004.10.029
5. Bernstein SL, Aronsky D, Duseja R, et al. The effect of emergency department crowding on clinically oriented outcomes. Acad Emerg Med. 2009;16:1-10. doi:10.1111/j.1553-2712.2008.00295.x
6. Vieth TL, Rhodes KV. The effect of crowding on access and quality in an academic ED. Am J Emerg Med. 2006;24:787-794. doi:10.1016/j.ajem.2006.03.026
7. Rondeau KV, Francescutti LH. Emergency department overcrowding: the impact of resource scarcity on physician job satisfaction. J Healthc Manag. 2005;50:327-340; discussion 341-342.
8. Emergency Department Crowding: High Impact Solutions. American College of Emergency Physicians. Emergency Medicine Practice Committee. 2016. Accessed March 31, 2023. https://www.acep.org/globalassets/sites/acep/media/crowding/empc_crowding-ip_092016.pdf
9. Velásquez ST, Wang ES, White AW, et al. Hospitalists as triagists: description of the triagist role across academic medical centers. J Hosp Med. 2020;15:87-90. doi:10.12788/jhm.3327
10. Amick A, Bann M. Characterizing the role of the “triagist”: reasons for triage discordance and impact on disposition. J Gen Intern Med. 2021;36:2177-2179. doi:10.1007/s11606-020-05887-y
11. Perla RJ, Provost LP, Murray SK. The run chart: a simple analytical tool for learning for variation in healthcare processes. BMJ Qual Saf. 2011;20:46-51. doi:10.1136/bmjqs.2009.037895
12. Howell EE, Bessman ES, Rubin HR. Hospitalists and an innovative emergency department admission process. J Gen Intern Med. 2004;19:266-268. doi:10.1111/j.1525-1497.2004.30431.x
1. Huang Q, Thind A, Dreyer JF, et al. The impact of delays to admission from the emergency department on inpatient outcomes. BMC Emerg Med. 2010;10:16. doi:10.1186/1471-227X-10-16
2. Liew D, Liew D, Kennedy MP. Emergency department length of stay independently predicts excess inpatient length of stay. Med J Aust. 2003;179:524-526. doi:10.5694/j.1326-5377.2003.tb05676.x
3. Richardson DB. The access-block effect: relationship between delay to reaching an inpatient bed and inpatient length of stay. Med J Aust. 2002;177:492-495. doi:10.5694/j.1326-5377.2002.tb04917.x
4. Polevoi SK, Quinn JV, Kramer KR. Factors associated with patients who leave without being seen. Acad Emerg Med. 2005;12:232-236. doi:10.1197/j.aem.2004.10.029
5. Bernstein SL, Aronsky D, Duseja R, et al. The effect of emergency department crowding on clinically oriented outcomes. Acad Emerg Med. 2009;16:1-10. doi:10.1111/j.1553-2712.2008.00295.x
6. Vieth TL, Rhodes KV. The effect of crowding on access and quality in an academic ED. Am J Emerg Med. 2006;24:787-794. doi:10.1016/j.ajem.2006.03.026
7. Rondeau KV, Francescutti LH. Emergency department overcrowding: the impact of resource scarcity on physician job satisfaction. J Healthc Manag. 2005;50:327-340; discussion 341-342.
8. Emergency Department Crowding: High Impact Solutions. American College of Emergency Physicians. Emergency Medicine Practice Committee. 2016. Accessed March 31, 2023. https://www.acep.org/globalassets/sites/acep/media/crowding/empc_crowding-ip_092016.pdf
9. Velásquez ST, Wang ES, White AW, et al. Hospitalists as triagists: description of the triagist role across academic medical centers. J Hosp Med. 2020;15:87-90. doi:10.12788/jhm.3327
10. Amick A, Bann M. Characterizing the role of the “triagist”: reasons for triage discordance and impact on disposition. J Gen Intern Med. 2021;36:2177-2179. doi:10.1007/s11606-020-05887-y
11. Perla RJ, Provost LP, Murray SK. The run chart: a simple analytical tool for learning for variation in healthcare processes. BMJ Qual Saf. 2011;20:46-51. doi:10.1136/bmjqs.2009.037895
12. Howell EE, Bessman ES, Rubin HR. Hospitalists and an innovative emergency department admission process. J Gen Intern Med. 2004;19:266-268. doi:10.1111/j.1525-1497.2004.30431.x
Redesign of Health Care Systems to Reduce Diagnostic Errors: Leveraging Human Experience and Artificial Intelligence
From the Institute for Healthcare Improvement, Boston, MA (Dr. Abid); Continuous Quality Improvement and Patient Safety Department, Armed Forces Hospitals Taif Region, Taif, Saudi Arabia (Dr. Abid); Primary and Secondary Healthcare Department, Government of Punjab, Lahore, Pakistan (Dr. Ahmed); Infection Prevention and Control Department, Armed Forces Hospitals Taif Region, Taif, Saudi Arabia (Dr. Din); Internal Medicine Department, Greater Baltimore Medical Center, Baltimore, MD (Dr. Abid); Department of Anesthesiology and Critical Care, DeBakey Heart and Vascular Center, Houston Methodist Hospital, Houston, TX (Dr. Ratnani).
Diagnostic errors are defined by the National Academies of Sciences, Engineering, and Medicine (NASEM) as the failure to either establish an accurate and timely explanation of the patient’s health problem(s) or communicate that explanation to the patient.1 According to a report by the Institute of Medicine, diagnostic errors account for a substantial number of adverse events in health care, affecting an estimated 12 million Americans each year.1 Diagnostic errors are a common and serious issue in health care systems, with studies estimating that 5% to 15% of all diagnoses are incorrect.1 Such errors can result in unnecessary treatments, delays in necessary treatments, and harm to patients. The high prevalence of diagnostic errors in primary care has been identified as a global issue.2 While many factors contribute to diagnostic errors, the complex nature of health care systems, the limited processing capacity of human cognition, and deficiencies in interpersonal patient-clinician communication are primary contributors.3,4
Discussions around the redesign of health care systems to reduce diagnostic errors have been at the forefront of medical research for years.2,4 To decrease diagnostic errors in health care, a comprehensive strategy is necessary. This strategy should focus on utilizing both human experience (HX) in health care and artificial intelligence (AI) technologies to transform health care systems into proactive, patient-centered, and safer systems, specifically concerning diagnostic errors.1
Human Experience and Diagnostic Errors
The role of HX in health care cannot be overstated. The HX in health care integrates the sum of all interactions, every encounter among patients, families and care partners, and the health care workforce.5 Patients and their families have a unique perspective on their health care experiences that can provide valuable insight into potential diagnostic errors.6 The new definition of diagnostic errors introduced in the 2015 NASEM report emphasized the significance of effective communication during the diagnostic procedure.1 Engaging patients and their families in the diagnostic process can improve communication, improve diagnostic accuracy, and help to identify errors before they cause harm.7 However, many patients and families feel that they are not listened to or taken seriously by health care providers, and may not feel comfortable sharing information that they feel is important.8 To address this, health care systems can implement programs that encourage patients and families to be more engaged in the diagnostic process, such as shared decision-making, patient portals, and patient and family advisory councils.9 Health care systems must prioritize patient-centered care, teamwork, and communication. Patients and their families must be actively engaged in their care, and health care providers must be willing to work collaboratively and listen to patients’ concerns.6,10
Health care providers also bring their own valuable experiences and expertise to the diagnostic process, as they are often the ones on the front lines of patient care. However, health care providers may not always feel comfortable reporting errors or near misses, and may not have the time or resources to participate in quality improvement initiatives. To address this, health care systems can implement programs that encourage providers to report errors and near misses, such as anonymous reporting systems, just-culture initiatives, and peer review.11 Creating a culture of teamwork and collaboration among health care providers can improve the accuracy of diagnoses and reduce the risk of errors.12
A key factor in utilizing HX to reduce diagnostic errors is effective communication. Communication breakdowns among health care providers, patients, and their families are a common contributing factor resulting in diagnostic errors.2 Strategies to improve communication include using clear and concise language, involving patients and their families in the decision-making process, and utilizing electronic health records (EHRs) to ensure that all health care providers have access to relevant, accurate, and up-to-date patient information.4,13,14
Another important aspect of utilizing HX in health care to reduce diagnostic errors is the need to recognize and address cognitive biases that may influence diagnostic decisions.3 Cognitive biases are common in health care and can lead to errors in diagnosis. For example, confirmation bias, which is the tendency to look for information that confirms preexisting beliefs, can lead providers to overlook important diagnostic information.15 Biases such as anchoring bias, premature closure, and confirmation bias can lead to incorrect diagnoses and can be difficult to recognize and overcome. Addressing cognitive biases requires a commitment to self-reflection and self-awareness among health care providers as well as structured training of health care providers to improve their diagnostic reasoning skills and reduce the risk of cognitive errors.15 By implementing these strategies around HX in health care, health care systems can become more patient-centered and reduce the likelihood of diagnostic errors (Figure).
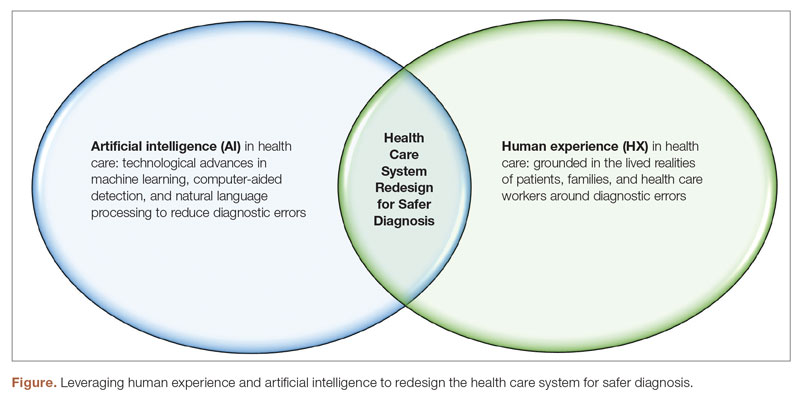
Artificial Intelligence and Diagnostic Errors
Artificial intelligence has the potential to significantly reduce diagnostic errors in health care (Figure), and its role in health care is rapidly expanding. AI technologies such as machine learning (ML) and natural language processing (NLP) have the potential to significantly reduce diagnostic errors by augmenting human cognition and improving access to relevant patient data.1,16 Machine learning algorithms can analyze large amounts of patient data sets to identify patterns and risk factors and predict patient outcomes, which can aid health care providers in making accurate diagnoses.17 Artificial intelligence can also help to address some of the communication breakdowns that contribute to diagnostic errors.18 Natural language processing can improve the accuracy of EHR documentation and reduce the associated clinician burden, making it easier for providers to access relevant patient information and communicate more effectively with each other.18
In health care, AI can be used to analyze medical images, laboratory results, genomic data, and EHRs to identify potential diagnoses and flag patients who may be at risk for diagnostic errors. One of the primary benefits of AI in health care is its ability to process large amounts of data quickly and accurately.19 This can be particularly valuable in diagnosing rare or complex conditions. Machine learning algorithms can analyze patient data to identify subtle patterns that may not be apparent to human providers.16 This can lead to earlier and more accurate diagnoses, which can reduce diagnostic errors and improve patient outcomes.17 One example of the application of AI in health care is the use of computer-aided detection (CAD) software to analyze medical images. This software can help radiologists detect abnormalities in medical images that may be missed by the human eye, such as early-stage breast cancer.20 Another example is the use of NLP and ML to analyze unstructured data in EHRs, such as physician notes, to identify potential diagnoses and flag patients who may be at risk for diagnostic errors.21 A recent study showed that using NLP on EHRs for screening and detecting individuals at risk for psychosis can considerably enhance the prognostic accuracy of psychosis risk calculators.22 This can help identify patients who require assessment and specialized care, facilitating earlier detection and potentially improving patient outcomes. On the same note, ML-based severe sepsis prediction algorithms have been shown to reduce the average length of stay and in-hospital mortality rate.23
However, there are also concerns about the use of AI in health care, including the potential for bias and the risk of overreliance on AI. Bias can occur when AI algorithms are trained on data that is not representative of the population being analyzed, leading to inaccurate or unfair results, hence, perpetuating and exacerbating existing biases in health care.24 Over-reliance on AI can occur when health care providers rely too heavily on AI algorithms and fail to consider other important information, such as the lived experience of patients, families, and health care providers. Addressing these concerns will require ongoing efforts to ensure that AI technologies are developed and implemented in an ethical and responsible manner.25
Conclusion
Reducing diagnostic errors is a critical goal for health care systems, and requires a comprehensive approach that utilizes both HX and AI technologies. Engaging patients and their families in the diagnostic process, promoting teamwork and collaboration among health care providers, addressing cognitive biases, and harnessing the power of AI can all contribute to more accurate diagnoses and better patient outcomes. By integrating the lived experience of patients, families, and health care providers with AI technologies, health care systems can be redesigned to become more proactive, safer, and patient-centered in identifying potential health problems and reducing the risk of diagnostic errors, ensuring that patients receive the care they need and deserve.
Corresponding author: Iqbal Ratnani, Department of Anesthesiology and Critical Care, DeBakey Heart and Vascular Center, Houston Methodist Hospital, 6565 Fannin St, Houston, TX 77030; IRatnani@houstonmethodist.org
Disclosures: None reported.
1. National Academy of Medicine. Improving Diagnosis in Health Care. Balogh EP, Miller BT, Ball JR, eds. National Academies Press; 2015. doi:10.17226/21794
2. Singh H, Schiff GD, Graber ML, et al. The global burden of diagnostic errors in primary care. BMJ Qual Saf. 2017;26(6):484-494. doi:10.1136/bmjqs-2016-005401
3. Croskerry P, Campbell SG, Petrie DA. The challenge of cognitive science for medical diagnosis. Cogn Res Princ Implic. 2023;8(1):13. doi:10.1186/s41235-022-00460-z
4. Dahm MR, Williams M, Crock C. ‘More than words’ - interpersonal communication, cogntive bias and diagnostic errors. Patient Educ Couns. 2022;105(1):252-256. doi:10.1016/j.pec.2021.05.012
5. Wolf JA, Niederhauser V, Marshburn D, LaVela SL. Reexamining “defining patient experience”: The human experience in Healthcare. Patient Experience J. 2021;8(1):16-29. doi:10.35680/2372-0247.1594
6. Sacco AY, Self QR, Worswick EL, et al. Patients’ perspectives of diagnostic error: A qualitative study. J Patient Saf. 2021;17(8):e1759-e1764. doi:10.1097/PTS.0000000000000642
7. Singh H, Graber ML. Improving diagnosis in health care—the next imperative for patient safety. N Engl J Med. 2015;373(26):2493-2495. doi:10.1056/NEJMp1512241
8. Austin E, LeRouge C, Hartzler AL, Segal C, Lavallee DC. Capturing the patient voice: implementing patient-reported outcomes across the health system. Qual Life Res. 2020;29(2):347-355. doi:10.1007/s11136-019-02320-8
9. Waddell A, Lennox A, Spassova G, Bragge P. Barriers and facilitators to shared decision-making in hospitals from policy to practice: a systematic review. Implement Sci. 2021;16(1):74. doi: 10.1186/s13012-021-01142-y
10. US Preventive Services Task Force. Collaboration and shared decision-making between patients and clinicians in preventive health care decisions and US Preventive Services Task Force Recommendations. JAMA. 2022;327(12):1171-1176. doi:10.1001/jama.2022.3267
11. Reporting patient safety events. Patient Safety Network. Published September 7, 2019. Accessed April 29, 2023. https://psnet.ahrq.gov/primer/reporting-patient-safety-events
12. McLaney E, Morassaei S, Hughes L, et al. A framework for interprofessional team collaboration in a hospital setting: Advancing team competencies and behaviours. Healthc Manage Forum. 2022;35(2):112-117. doi:10.1177/08404704211063584
13. Abid MH, Abid MM, Shahid R, et al. Patient and family engagement during challenging times: what works and what does not? Cureus. 2021;13(5):e14814. doi:10.7759/cureus.14814
14. Abimanyi-Ochom J, Bohingamu Mudiyanselage S, Catchpool M, et al. Strategies to reduce diagnostic errors: a systematic review. BMC Med Inform Decis Mak. 2019;19(1):174. doi:10.1186/s12911-019-0901-1
15. Watari T, Tokuda Y, Amano Y, et al. Cognitive bias and diagnostic errors among physicians in Japan: A self-reflection survey. Int J Environ Res Public Health. 2022;19(8):4645. doi:10.3390/ijerph19084645
16. Rajkomar A, Oren E, Chen K et al. Scalable and accurate deep learning with electronic health records. NPJ Digit Med. 2018;1:18. https://doi.org/10.1038/s41746-018-0029-1
17. Davenport T, Kalakota R. The potential for artificial intelligence in healthcare. Future Healthc J. 2019;6(2):94-98. doi:10.7861/futurehosp.6-2-94
18. Dymek C, Kim B, Melton GB, et al. Building the evidence-base to reduce electronic health record-related clinician burden. J Am Med Inform Assoc. 2021;28(5):1057-1061. doi:10.1093/jamia/ocaa238
19. Beam AL, Kohane IS. Big data and machine learning in health care. JAMA. 2018;319(13):1317-1318. doi:10.1001/jama.2017.18391
20. Lehman CD, Wellman RD, Buist DS, et al. Diagnostic accuracy of digital screening mammography with and without computer-aided detection. JAMA Intern Med. 2015;175(11):1828-1837. doi:10.1001/jamainternmed.2015.5231
21. Liao KP, Cai T, Savova GK, et al. Development of phenotype algorithms using electronic medical records and incorporating natural language processing. BMJ. 2015;350:h1885. doi:10.1136/bmj.h1885
22. Irving J, Patel R, Oliver D, et al. Using natural language processing on electronic health records to enhance detection and prediction of psychosis risk. Schizophr Bull. 2021;47(2):405-414. doi:10.1093/schbul/sbaa126. Erratum in: Schizophr Bull. 2021;47(2):575.
23. Shimabukuro DW, Barton CW, Feldman MD, et al. Effect of a machine learning-based severe sepsis prediction algorithm on patient survival and hospital length of stay: a randomised clinical trial. BMJ Open Respir Res. 2017;4(1):e000234. doi:10.1136/bmjresp-2017-000234
24. Obermeyer Z, Powers B, Vogeli C, Mullainathan S. Dissecting racial bias in an algorithm used to manage the health of populations. Science. 2019;366(6464):447-453. doi:10.1126/science.aax2342
25. Ibrahim SA, Pronovost PJ. Diagnostic errors, health disparities, and artificial intelligence: a combination for health or harm? JAMA Health Forum. 2021;2(9):e212430. doi:10.1001/jamahealthforum.2021.2430
From the Institute for Healthcare Improvement, Boston, MA (Dr. Abid); Continuous Quality Improvement and Patient Safety Department, Armed Forces Hospitals Taif Region, Taif, Saudi Arabia (Dr. Abid); Primary and Secondary Healthcare Department, Government of Punjab, Lahore, Pakistan (Dr. Ahmed); Infection Prevention and Control Department, Armed Forces Hospitals Taif Region, Taif, Saudi Arabia (Dr. Din); Internal Medicine Department, Greater Baltimore Medical Center, Baltimore, MD (Dr. Abid); Department of Anesthesiology and Critical Care, DeBakey Heart and Vascular Center, Houston Methodist Hospital, Houston, TX (Dr. Ratnani).
Diagnostic errors are defined by the National Academies of Sciences, Engineering, and Medicine (NASEM) as the failure to either establish an accurate and timely explanation of the patient’s health problem(s) or communicate that explanation to the patient.1 According to a report by the Institute of Medicine, diagnostic errors account for a substantial number of adverse events in health care, affecting an estimated 12 million Americans each year.1 Diagnostic errors are a common and serious issue in health care systems, with studies estimating that 5% to 15% of all diagnoses are incorrect.1 Such errors can result in unnecessary treatments, delays in necessary treatments, and harm to patients. The high prevalence of diagnostic errors in primary care has been identified as a global issue.2 While many factors contribute to diagnostic errors, the complex nature of health care systems, the limited processing capacity of human cognition, and deficiencies in interpersonal patient-clinician communication are primary contributors.3,4
Discussions around the redesign of health care systems to reduce diagnostic errors have been at the forefront of medical research for years.2,4 To decrease diagnostic errors in health care, a comprehensive strategy is necessary. This strategy should focus on utilizing both human experience (HX) in health care and artificial intelligence (AI) technologies to transform health care systems into proactive, patient-centered, and safer systems, specifically concerning diagnostic errors.1
Human Experience and Diagnostic Errors
The role of HX in health care cannot be overstated. The HX in health care integrates the sum of all interactions, every encounter among patients, families and care partners, and the health care workforce.5 Patients and their families have a unique perspective on their health care experiences that can provide valuable insight into potential diagnostic errors.6 The new definition of diagnostic errors introduced in the 2015 NASEM report emphasized the significance of effective communication during the diagnostic procedure.1 Engaging patients and their families in the diagnostic process can improve communication, improve diagnostic accuracy, and help to identify errors before they cause harm.7 However, many patients and families feel that they are not listened to or taken seriously by health care providers, and may not feel comfortable sharing information that they feel is important.8 To address this, health care systems can implement programs that encourage patients and families to be more engaged in the diagnostic process, such as shared decision-making, patient portals, and patient and family advisory councils.9 Health care systems must prioritize patient-centered care, teamwork, and communication. Patients and their families must be actively engaged in their care, and health care providers must be willing to work collaboratively and listen to patients’ concerns.6,10
Health care providers also bring their own valuable experiences and expertise to the diagnostic process, as they are often the ones on the front lines of patient care. However, health care providers may not always feel comfortable reporting errors or near misses, and may not have the time or resources to participate in quality improvement initiatives. To address this, health care systems can implement programs that encourage providers to report errors and near misses, such as anonymous reporting systems, just-culture initiatives, and peer review.11 Creating a culture of teamwork and collaboration among health care providers can improve the accuracy of diagnoses and reduce the risk of errors.12
A key factor in utilizing HX to reduce diagnostic errors is effective communication. Communication breakdowns among health care providers, patients, and their families are a common contributing factor resulting in diagnostic errors.2 Strategies to improve communication include using clear and concise language, involving patients and their families in the decision-making process, and utilizing electronic health records (EHRs) to ensure that all health care providers have access to relevant, accurate, and up-to-date patient information.4,13,14
Another important aspect of utilizing HX in health care to reduce diagnostic errors is the need to recognize and address cognitive biases that may influence diagnostic decisions.3 Cognitive biases are common in health care and can lead to errors in diagnosis. For example, confirmation bias, which is the tendency to look for information that confirms preexisting beliefs, can lead providers to overlook important diagnostic information.15 Biases such as anchoring bias, premature closure, and confirmation bias can lead to incorrect diagnoses and can be difficult to recognize and overcome. Addressing cognitive biases requires a commitment to self-reflection and self-awareness among health care providers as well as structured training of health care providers to improve their diagnostic reasoning skills and reduce the risk of cognitive errors.15 By implementing these strategies around HX in health care, health care systems can become more patient-centered and reduce the likelihood of diagnostic errors (Figure).

Artificial Intelligence and Diagnostic Errors
Artificial intelligence has the potential to significantly reduce diagnostic errors in health care (Figure), and its role in health care is rapidly expanding. AI technologies such as machine learning (ML) and natural language processing (NLP) have the potential to significantly reduce diagnostic errors by augmenting human cognition and improving access to relevant patient data.1,16 Machine learning algorithms can analyze large amounts of patient data sets to identify patterns and risk factors and predict patient outcomes, which can aid health care providers in making accurate diagnoses.17 Artificial intelligence can also help to address some of the communication breakdowns that contribute to diagnostic errors.18 Natural language processing can improve the accuracy of EHR documentation and reduce the associated clinician burden, making it easier for providers to access relevant patient information and communicate more effectively with each other.18
In health care, AI can be used to analyze medical images, laboratory results, genomic data, and EHRs to identify potential diagnoses and flag patients who may be at risk for diagnostic errors. One of the primary benefits of AI in health care is its ability to process large amounts of data quickly and accurately.19 This can be particularly valuable in diagnosing rare or complex conditions. Machine learning algorithms can analyze patient data to identify subtle patterns that may not be apparent to human providers.16 This can lead to earlier and more accurate diagnoses, which can reduce diagnostic errors and improve patient outcomes.17 One example of the application of AI in health care is the use of computer-aided detection (CAD) software to analyze medical images. This software can help radiologists detect abnormalities in medical images that may be missed by the human eye, such as early-stage breast cancer.20 Another example is the use of NLP and ML to analyze unstructured data in EHRs, such as physician notes, to identify potential diagnoses and flag patients who may be at risk for diagnostic errors.21 A recent study showed that using NLP on EHRs for screening and detecting individuals at risk for psychosis can considerably enhance the prognostic accuracy of psychosis risk calculators.22 This can help identify patients who require assessment and specialized care, facilitating earlier detection and potentially improving patient outcomes. On the same note, ML-based severe sepsis prediction algorithms have been shown to reduce the average length of stay and in-hospital mortality rate.23
However, there are also concerns about the use of AI in health care, including the potential for bias and the risk of overreliance on AI. Bias can occur when AI algorithms are trained on data that is not representative of the population being analyzed, leading to inaccurate or unfair results, hence, perpetuating and exacerbating existing biases in health care.24 Over-reliance on AI can occur when health care providers rely too heavily on AI algorithms and fail to consider other important information, such as the lived experience of patients, families, and health care providers. Addressing these concerns will require ongoing efforts to ensure that AI technologies are developed and implemented in an ethical and responsible manner.25
Conclusion
Reducing diagnostic errors is a critical goal for health care systems, and requires a comprehensive approach that utilizes both HX and AI technologies. Engaging patients and their families in the diagnostic process, promoting teamwork and collaboration among health care providers, addressing cognitive biases, and harnessing the power of AI can all contribute to more accurate diagnoses and better patient outcomes. By integrating the lived experience of patients, families, and health care providers with AI technologies, health care systems can be redesigned to become more proactive, safer, and patient-centered in identifying potential health problems and reducing the risk of diagnostic errors, ensuring that patients receive the care they need and deserve.
Corresponding author: Iqbal Ratnani, Department of Anesthesiology and Critical Care, DeBakey Heart and Vascular Center, Houston Methodist Hospital, 6565 Fannin St, Houston, TX 77030; IRatnani@houstonmethodist.org
Disclosures: None reported.
From the Institute for Healthcare Improvement, Boston, MA (Dr. Abid); Continuous Quality Improvement and Patient Safety Department, Armed Forces Hospitals Taif Region, Taif, Saudi Arabia (Dr. Abid); Primary and Secondary Healthcare Department, Government of Punjab, Lahore, Pakistan (Dr. Ahmed); Infection Prevention and Control Department, Armed Forces Hospitals Taif Region, Taif, Saudi Arabia (Dr. Din); Internal Medicine Department, Greater Baltimore Medical Center, Baltimore, MD (Dr. Abid); Department of Anesthesiology and Critical Care, DeBakey Heart and Vascular Center, Houston Methodist Hospital, Houston, TX (Dr. Ratnani).
Diagnostic errors are defined by the National Academies of Sciences, Engineering, and Medicine (NASEM) as the failure to either establish an accurate and timely explanation of the patient’s health problem(s) or communicate that explanation to the patient.1 According to a report by the Institute of Medicine, diagnostic errors account for a substantial number of adverse events in health care, affecting an estimated 12 million Americans each year.1 Diagnostic errors are a common and serious issue in health care systems, with studies estimating that 5% to 15% of all diagnoses are incorrect.1 Such errors can result in unnecessary treatments, delays in necessary treatments, and harm to patients. The high prevalence of diagnostic errors in primary care has been identified as a global issue.2 While many factors contribute to diagnostic errors, the complex nature of health care systems, the limited processing capacity of human cognition, and deficiencies in interpersonal patient-clinician communication are primary contributors.3,4
Discussions around the redesign of health care systems to reduce diagnostic errors have been at the forefront of medical research for years.2,4 To decrease diagnostic errors in health care, a comprehensive strategy is necessary. This strategy should focus on utilizing both human experience (HX) in health care and artificial intelligence (AI) technologies to transform health care systems into proactive, patient-centered, and safer systems, specifically concerning diagnostic errors.1
Human Experience and Diagnostic Errors
The role of HX in health care cannot be overstated. The HX in health care integrates the sum of all interactions, every encounter among patients, families and care partners, and the health care workforce.5 Patients and their families have a unique perspective on their health care experiences that can provide valuable insight into potential diagnostic errors.6 The new definition of diagnostic errors introduced in the 2015 NASEM report emphasized the significance of effective communication during the diagnostic procedure.1 Engaging patients and their families in the diagnostic process can improve communication, improve diagnostic accuracy, and help to identify errors before they cause harm.7 However, many patients and families feel that they are not listened to or taken seriously by health care providers, and may not feel comfortable sharing information that they feel is important.8 To address this, health care systems can implement programs that encourage patients and families to be more engaged in the diagnostic process, such as shared decision-making, patient portals, and patient and family advisory councils.9 Health care systems must prioritize patient-centered care, teamwork, and communication. Patients and their families must be actively engaged in their care, and health care providers must be willing to work collaboratively and listen to patients’ concerns.6,10
Health care providers also bring their own valuable experiences and expertise to the diagnostic process, as they are often the ones on the front lines of patient care. However, health care providers may not always feel comfortable reporting errors or near misses, and may not have the time or resources to participate in quality improvement initiatives. To address this, health care systems can implement programs that encourage providers to report errors and near misses, such as anonymous reporting systems, just-culture initiatives, and peer review.11 Creating a culture of teamwork and collaboration among health care providers can improve the accuracy of diagnoses and reduce the risk of errors.12
A key factor in utilizing HX to reduce diagnostic errors is effective communication. Communication breakdowns among health care providers, patients, and their families are a common contributing factor resulting in diagnostic errors.2 Strategies to improve communication include using clear and concise language, involving patients and their families in the decision-making process, and utilizing electronic health records (EHRs) to ensure that all health care providers have access to relevant, accurate, and up-to-date patient information.4,13,14
Another important aspect of utilizing HX in health care to reduce diagnostic errors is the need to recognize and address cognitive biases that may influence diagnostic decisions.3 Cognitive biases are common in health care and can lead to errors in diagnosis. For example, confirmation bias, which is the tendency to look for information that confirms preexisting beliefs, can lead providers to overlook important diagnostic information.15 Biases such as anchoring bias, premature closure, and confirmation bias can lead to incorrect diagnoses and can be difficult to recognize and overcome. Addressing cognitive biases requires a commitment to self-reflection and self-awareness among health care providers as well as structured training of health care providers to improve their diagnostic reasoning skills and reduce the risk of cognitive errors.15 By implementing these strategies around HX in health care, health care systems can become more patient-centered and reduce the likelihood of diagnostic errors (Figure).

Artificial Intelligence and Diagnostic Errors
Artificial intelligence has the potential to significantly reduce diagnostic errors in health care (Figure), and its role in health care is rapidly expanding. AI technologies such as machine learning (ML) and natural language processing (NLP) have the potential to significantly reduce diagnostic errors by augmenting human cognition and improving access to relevant patient data.1,16 Machine learning algorithms can analyze large amounts of patient data sets to identify patterns and risk factors and predict patient outcomes, which can aid health care providers in making accurate diagnoses.17 Artificial intelligence can also help to address some of the communication breakdowns that contribute to diagnostic errors.18 Natural language processing can improve the accuracy of EHR documentation and reduce the associated clinician burden, making it easier for providers to access relevant patient information and communicate more effectively with each other.18
In health care, AI can be used to analyze medical images, laboratory results, genomic data, and EHRs to identify potential diagnoses and flag patients who may be at risk for diagnostic errors. One of the primary benefits of AI in health care is its ability to process large amounts of data quickly and accurately.19 This can be particularly valuable in diagnosing rare or complex conditions. Machine learning algorithms can analyze patient data to identify subtle patterns that may not be apparent to human providers.16 This can lead to earlier and more accurate diagnoses, which can reduce diagnostic errors and improve patient outcomes.17 One example of the application of AI in health care is the use of computer-aided detection (CAD) software to analyze medical images. This software can help radiologists detect abnormalities in medical images that may be missed by the human eye, such as early-stage breast cancer.20 Another example is the use of NLP and ML to analyze unstructured data in EHRs, such as physician notes, to identify potential diagnoses and flag patients who may be at risk for diagnostic errors.21 A recent study showed that using NLP on EHRs for screening and detecting individuals at risk for psychosis can considerably enhance the prognostic accuracy of psychosis risk calculators.22 This can help identify patients who require assessment and specialized care, facilitating earlier detection and potentially improving patient outcomes. On the same note, ML-based severe sepsis prediction algorithms have been shown to reduce the average length of stay and in-hospital mortality rate.23
However, there are also concerns about the use of AI in health care, including the potential for bias and the risk of overreliance on AI. Bias can occur when AI algorithms are trained on data that is not representative of the population being analyzed, leading to inaccurate or unfair results, hence, perpetuating and exacerbating existing biases in health care.24 Over-reliance on AI can occur when health care providers rely too heavily on AI algorithms and fail to consider other important information, such as the lived experience of patients, families, and health care providers. Addressing these concerns will require ongoing efforts to ensure that AI technologies are developed and implemented in an ethical and responsible manner.25
Conclusion
Reducing diagnostic errors is a critical goal for health care systems, and requires a comprehensive approach that utilizes both HX and AI technologies. Engaging patients and their families in the diagnostic process, promoting teamwork and collaboration among health care providers, addressing cognitive biases, and harnessing the power of AI can all contribute to more accurate diagnoses and better patient outcomes. By integrating the lived experience of patients, families, and health care providers with AI technologies, health care systems can be redesigned to become more proactive, safer, and patient-centered in identifying potential health problems and reducing the risk of diagnostic errors, ensuring that patients receive the care they need and deserve.
Corresponding author: Iqbal Ratnani, Department of Anesthesiology and Critical Care, DeBakey Heart and Vascular Center, Houston Methodist Hospital, 6565 Fannin St, Houston, TX 77030; IRatnani@houstonmethodist.org
Disclosures: None reported.
1. National Academy of Medicine. Improving Diagnosis in Health Care. Balogh EP, Miller BT, Ball JR, eds. National Academies Press; 2015. doi:10.17226/21794
2. Singh H, Schiff GD, Graber ML, et al. The global burden of diagnostic errors in primary care. BMJ Qual Saf. 2017;26(6):484-494. doi:10.1136/bmjqs-2016-005401
3. Croskerry P, Campbell SG, Petrie DA. The challenge of cognitive science for medical diagnosis. Cogn Res Princ Implic. 2023;8(1):13. doi:10.1186/s41235-022-00460-z
4. Dahm MR, Williams M, Crock C. ‘More than words’ - interpersonal communication, cogntive bias and diagnostic errors. Patient Educ Couns. 2022;105(1):252-256. doi:10.1016/j.pec.2021.05.012
5. Wolf JA, Niederhauser V, Marshburn D, LaVela SL. Reexamining “defining patient experience”: The human experience in Healthcare. Patient Experience J. 2021;8(1):16-29. doi:10.35680/2372-0247.1594
6. Sacco AY, Self QR, Worswick EL, et al. Patients’ perspectives of diagnostic error: A qualitative study. J Patient Saf. 2021;17(8):e1759-e1764. doi:10.1097/PTS.0000000000000642
7. Singh H, Graber ML. Improving diagnosis in health care—the next imperative for patient safety. N Engl J Med. 2015;373(26):2493-2495. doi:10.1056/NEJMp1512241
8. Austin E, LeRouge C, Hartzler AL, Segal C, Lavallee DC. Capturing the patient voice: implementing patient-reported outcomes across the health system. Qual Life Res. 2020;29(2):347-355. doi:10.1007/s11136-019-02320-8
9. Waddell A, Lennox A, Spassova G, Bragge P. Barriers and facilitators to shared decision-making in hospitals from policy to practice: a systematic review. Implement Sci. 2021;16(1):74. doi: 10.1186/s13012-021-01142-y
10. US Preventive Services Task Force. Collaboration and shared decision-making between patients and clinicians in preventive health care decisions and US Preventive Services Task Force Recommendations. JAMA. 2022;327(12):1171-1176. doi:10.1001/jama.2022.3267
11. Reporting patient safety events. Patient Safety Network. Published September 7, 2019. Accessed April 29, 2023. https://psnet.ahrq.gov/primer/reporting-patient-safety-events
12. McLaney E, Morassaei S, Hughes L, et al. A framework for interprofessional team collaboration in a hospital setting: Advancing team competencies and behaviours. Healthc Manage Forum. 2022;35(2):112-117. doi:10.1177/08404704211063584
13. Abid MH, Abid MM, Shahid R, et al. Patient and family engagement during challenging times: what works and what does not? Cureus. 2021;13(5):e14814. doi:10.7759/cureus.14814
14. Abimanyi-Ochom J, Bohingamu Mudiyanselage S, Catchpool M, et al. Strategies to reduce diagnostic errors: a systematic review. BMC Med Inform Decis Mak. 2019;19(1):174. doi:10.1186/s12911-019-0901-1
15. Watari T, Tokuda Y, Amano Y, et al. Cognitive bias and diagnostic errors among physicians in Japan: A self-reflection survey. Int J Environ Res Public Health. 2022;19(8):4645. doi:10.3390/ijerph19084645
16. Rajkomar A, Oren E, Chen K et al. Scalable and accurate deep learning with electronic health records. NPJ Digit Med. 2018;1:18. https://doi.org/10.1038/s41746-018-0029-1
17. Davenport T, Kalakota R. The potential for artificial intelligence in healthcare. Future Healthc J. 2019;6(2):94-98. doi:10.7861/futurehosp.6-2-94
18. Dymek C, Kim B, Melton GB, et al. Building the evidence-base to reduce electronic health record-related clinician burden. J Am Med Inform Assoc. 2021;28(5):1057-1061. doi:10.1093/jamia/ocaa238
19. Beam AL, Kohane IS. Big data and machine learning in health care. JAMA. 2018;319(13):1317-1318. doi:10.1001/jama.2017.18391
20. Lehman CD, Wellman RD, Buist DS, et al. Diagnostic accuracy of digital screening mammography with and without computer-aided detection. JAMA Intern Med. 2015;175(11):1828-1837. doi:10.1001/jamainternmed.2015.5231
21. Liao KP, Cai T, Savova GK, et al. Development of phenotype algorithms using electronic medical records and incorporating natural language processing. BMJ. 2015;350:h1885. doi:10.1136/bmj.h1885
22. Irving J, Patel R, Oliver D, et al. Using natural language processing on electronic health records to enhance detection and prediction of psychosis risk. Schizophr Bull. 2021;47(2):405-414. doi:10.1093/schbul/sbaa126. Erratum in: Schizophr Bull. 2021;47(2):575.
23. Shimabukuro DW, Barton CW, Feldman MD, et al. Effect of a machine learning-based severe sepsis prediction algorithm on patient survival and hospital length of stay: a randomised clinical trial. BMJ Open Respir Res. 2017;4(1):e000234. doi:10.1136/bmjresp-2017-000234
24. Obermeyer Z, Powers B, Vogeli C, Mullainathan S. Dissecting racial bias in an algorithm used to manage the health of populations. Science. 2019;366(6464):447-453. doi:10.1126/science.aax2342
25. Ibrahim SA, Pronovost PJ. Diagnostic errors, health disparities, and artificial intelligence: a combination for health or harm? JAMA Health Forum. 2021;2(9):e212430. doi:10.1001/jamahealthforum.2021.2430
1. National Academy of Medicine. Improving Diagnosis in Health Care. Balogh EP, Miller BT, Ball JR, eds. National Academies Press; 2015. doi:10.17226/21794
2. Singh H, Schiff GD, Graber ML, et al. The global burden of diagnostic errors in primary care. BMJ Qual Saf. 2017;26(6):484-494. doi:10.1136/bmjqs-2016-005401
3. Croskerry P, Campbell SG, Petrie DA. The challenge of cognitive science for medical diagnosis. Cogn Res Princ Implic. 2023;8(1):13. doi:10.1186/s41235-022-00460-z
4. Dahm MR, Williams M, Crock C. ‘More than words’ - interpersonal communication, cogntive bias and diagnostic errors. Patient Educ Couns. 2022;105(1):252-256. doi:10.1016/j.pec.2021.05.012
5. Wolf JA, Niederhauser V, Marshburn D, LaVela SL. Reexamining “defining patient experience”: The human experience in Healthcare. Patient Experience J. 2021;8(1):16-29. doi:10.35680/2372-0247.1594
6. Sacco AY, Self QR, Worswick EL, et al. Patients’ perspectives of diagnostic error: A qualitative study. J Patient Saf. 2021;17(8):e1759-e1764. doi:10.1097/PTS.0000000000000642
7. Singh H, Graber ML. Improving diagnosis in health care—the next imperative for patient safety. N Engl J Med. 2015;373(26):2493-2495. doi:10.1056/NEJMp1512241
8. Austin E, LeRouge C, Hartzler AL, Segal C, Lavallee DC. Capturing the patient voice: implementing patient-reported outcomes across the health system. Qual Life Res. 2020;29(2):347-355. doi:10.1007/s11136-019-02320-8
9. Waddell A, Lennox A, Spassova G, Bragge P. Barriers and facilitators to shared decision-making in hospitals from policy to practice: a systematic review. Implement Sci. 2021;16(1):74. doi: 10.1186/s13012-021-01142-y
10. US Preventive Services Task Force. Collaboration and shared decision-making between patients and clinicians in preventive health care decisions and US Preventive Services Task Force Recommendations. JAMA. 2022;327(12):1171-1176. doi:10.1001/jama.2022.3267
11. Reporting patient safety events. Patient Safety Network. Published September 7, 2019. Accessed April 29, 2023. https://psnet.ahrq.gov/primer/reporting-patient-safety-events
12. McLaney E, Morassaei S, Hughes L, et al. A framework for interprofessional team collaboration in a hospital setting: Advancing team competencies and behaviours. Healthc Manage Forum. 2022;35(2):112-117. doi:10.1177/08404704211063584
13. Abid MH, Abid MM, Shahid R, et al. Patient and family engagement during challenging times: what works and what does not? Cureus. 2021;13(5):e14814. doi:10.7759/cureus.14814
14. Abimanyi-Ochom J, Bohingamu Mudiyanselage S, Catchpool M, et al. Strategies to reduce diagnostic errors: a systematic review. BMC Med Inform Decis Mak. 2019;19(1):174. doi:10.1186/s12911-019-0901-1
15. Watari T, Tokuda Y, Amano Y, et al. Cognitive bias and diagnostic errors among physicians in Japan: A self-reflection survey. Int J Environ Res Public Health. 2022;19(8):4645. doi:10.3390/ijerph19084645
16. Rajkomar A, Oren E, Chen K et al. Scalable and accurate deep learning with electronic health records. NPJ Digit Med. 2018;1:18. https://doi.org/10.1038/s41746-018-0029-1
17. Davenport T, Kalakota R. The potential for artificial intelligence in healthcare. Future Healthc J. 2019;6(2):94-98. doi:10.7861/futurehosp.6-2-94
18. Dymek C, Kim B, Melton GB, et al. Building the evidence-base to reduce electronic health record-related clinician burden. J Am Med Inform Assoc. 2021;28(5):1057-1061. doi:10.1093/jamia/ocaa238
19. Beam AL, Kohane IS. Big data and machine learning in health care. JAMA. 2018;319(13):1317-1318. doi:10.1001/jama.2017.18391
20. Lehman CD, Wellman RD, Buist DS, et al. Diagnostic accuracy of digital screening mammography with and without computer-aided detection. JAMA Intern Med. 2015;175(11):1828-1837. doi:10.1001/jamainternmed.2015.5231
21. Liao KP, Cai T, Savova GK, et al. Development of phenotype algorithms using electronic medical records and incorporating natural language processing. BMJ. 2015;350:h1885. doi:10.1136/bmj.h1885
22. Irving J, Patel R, Oliver D, et al. Using natural language processing on electronic health records to enhance detection and prediction of psychosis risk. Schizophr Bull. 2021;47(2):405-414. doi:10.1093/schbul/sbaa126. Erratum in: Schizophr Bull. 2021;47(2):575.
23. Shimabukuro DW, Barton CW, Feldman MD, et al. Effect of a machine learning-based severe sepsis prediction algorithm on patient survival and hospital length of stay: a randomised clinical trial. BMJ Open Respir Res. 2017;4(1):e000234. doi:10.1136/bmjresp-2017-000234
24. Obermeyer Z, Powers B, Vogeli C, Mullainathan S. Dissecting racial bias in an algorithm used to manage the health of populations. Science. 2019;366(6464):447-453. doi:10.1126/science.aax2342
25. Ibrahim SA, Pronovost PJ. Diagnostic errors, health disparities, and artificial intelligence: a combination for health or harm? JAMA Health Forum. 2021;2(9):e212430. doi:10.1001/jamahealthforum.2021.2430
Quality Improvement in Health Care: From Conceptual Frameworks and Definitions to Implementation
As the movement to improve quality in health care has evolved over the past several decades, organizations whose missions focus on supporting and promoting quality in health care have defined essential concepts, standards, and measures that comprise quality and that can be used to guide quality improvement (QI) work. The World Health Organization (WHO) defines quality in clinical care as safe, effective, and people-centered service.1 These 3 pillars of quality form the foundation of a quality system aiming to deliver health care in a timely, equitable, efficient, and integrated manner. The WHO estimates that 5.7 to 8.4 million deaths occur yearly in low- and middle-income countries due to poor quality care. Regarding safety, patient harm from unsafe care is estimated to be among the top 10 causes of death and disability worldwide.2 A health care QI plan involves identifying areas for improvement, setting measurable goals, implementing evidence-based strategies and interventions, monitoring progress toward achieving those goals, and continuously evaluating and adjusting the plan as needed to ensure sustained improvement over time. Such a plan can be implemented at various levels of health care organizations, from individual clinical units to entire hospitals or even regional health care systems.
The Institute of Medicine (IOM) identifies 5 domains of quality in health care: effectiveness, efficiency, equity, patient-centeredness, and safety.3 Effectiveness relies on providing care processes supported by scientific evidence and achieving desired outcomes in the IOM recommendations. The primary efficiency aim maximizes the quality of health care delivered or the benefits achieved for a given resource unit. Equity relates to providing health care of equal quality to all individuals, regardless of personal characteristics. Moreover, patient-centeredness relates to meeting patients’ needs and preferences and providing education and support. Safety relates to avoiding actual or potential harm. Timeliness relates to obtaining needed care while minimizing delays. Finally, the IOM defines health care quality as the systematic evaluation and provision of evidence-based and safe care characterized by a culture of continuous improvement, resulting in optimal health outcomes. Taking all these concepts into consideration, 4 key attributes have been identified as essential to the global definition of health care quality: effectiveness, safety, culture of continuous improvement, and desired outcomes. This conceptualization of health care quality encompasses the fundamental components and has the potential to enhance the delivery of care. This definition’s theoretical and practical implications provide a comprehensive and consistent understanding of the elements required to improve health care and maintain public trust.
Health care quality is a dynamic, ever-evolving construct that requires continuous assessment and evaluation to ensure the delivery of care meets the changing needs of society. The National Quality Forum’s National Voluntary Consensus Standards for health care provide measures, guidance, and recommendations on achieving effective outcomes through evidence-based practices.4 These standards establish criteria by which health care systems and providers can assess and improve their quality performance.
In the United States, in order to implement and disseminate best practices, the Centers for Medicare & Medicaid Services (CMS) developed Quality Payment Programs that offer incentives to health care providers to improve the quality of care delivery. This CMS program evaluates providers based on their performance in the Merit-Based Incentive Payment System performance categories.5 These include measures related to patient experience, cost, clinical quality, improvement activities, and the use of certified electronic health record technology. The scores that providers receive are used to determine their performance-based reimbursements under Medicare’s fee-for-service program.
The concept of health care quality is also applicable in other countries. In the United Kingdom, QI initiatives are led by the Department of Health and Social Care. The National Institute for Health and Care Excellence (NICE) produces guidelines on best practices to ensure that care delivery meets established safety and quality standards, reaching cost-effectiveness excellence.6 In Australia, the Australian Commission on Quality and Safety in Health Care is responsible for setting benchmarks for performance in health care systems through a clear, structured agenda.7 Ultimately, health care quality is a complex and multifaceted issue that requires a comprehensive approach to ensure the best outcomes for patients. With the implementation of measures such as the CMS Quality Payment Programs and NICE guidelines, health care organizations can take steps to ensure their systems of care delivery reflect evidence-based practices and demonstrate a commitment to providing high-quality care.
Implementing a health care QI plan that encompasses the 4 key attributes of health care quality—effectiveness, safety, culture of continuous improvement, and desired outcomes—requires collaboration among different departments and stakeholders and a data-driven approach to decision-making. Effective communication with patients and their families is critical to ensuring that their needs are being met and that they are active partners in their health care journey. While a health care QI plan is essential for delivering high-quality, safe patient care, it also helps health care organizations comply with regulatory requirements, meet accreditation standards, and stay competitive in the ever-evolving health care landscape.
Corresponding author: Ebrahim Barkoudah, MD, MPH; Ebrahim.Barkoudah@baystatehealth.org
1. World Health Organization. Quality of care. Accessed on May 17, 2023. www.who.int/health-topics/quality-of-care#tab=tab_1
2. World Health Organization. Patient safety. Accessed on May 17, 2023 www.who.int/news-room/fact-sheets/detail/patient-safety
3. Agency for Healthcare Research and Quality. Understanding quality measurement. Accessed on May 17, 2023. www.ahrq.gov/patient-safety/quality-resources/tools/chtoolbx/understand/index.html
4. Ferrell B, Connor SR, Cordes A, et al. The national agenda for quality palliative care: the National Consensus Project and the National Quality Forum. J Pain Symptom Manage. 2007;33(6):737-744. doi:10.1016/j.jpainsymman.2007.02.024
5. U.S Centers for Medicare & Medicaid Services. Quality payment program. Accessed on March 14, 2023 qpp.cms.gov/mips/overview
6. Claxton K, Martin S, Soares M, et al. Methods for the estimation of the National Institute for Health and Care Excellence cost-effectiveness threshold. Health Technol Assess. 2015;19(14):1-503, v-vi. doi: 10.3310/hta19140
7. Braithwaite J, Healy J, Dwan K. The Governance of Health Safety and Quality, Commonwealth of Australia. Accessed May 17, 2023. https://regnet.anu.edu.au/research/publications/3626/governance-health-safety-and-quality 2005
As the movement to improve quality in health care has evolved over the past several decades, organizations whose missions focus on supporting and promoting quality in health care have defined essential concepts, standards, and measures that comprise quality and that can be used to guide quality improvement (QI) work. The World Health Organization (WHO) defines quality in clinical care as safe, effective, and people-centered service.1 These 3 pillars of quality form the foundation of a quality system aiming to deliver health care in a timely, equitable, efficient, and integrated manner. The WHO estimates that 5.7 to 8.4 million deaths occur yearly in low- and middle-income countries due to poor quality care. Regarding safety, patient harm from unsafe care is estimated to be among the top 10 causes of death and disability worldwide.2 A health care QI plan involves identifying areas for improvement, setting measurable goals, implementing evidence-based strategies and interventions, monitoring progress toward achieving those goals, and continuously evaluating and adjusting the plan as needed to ensure sustained improvement over time. Such a plan can be implemented at various levels of health care organizations, from individual clinical units to entire hospitals or even regional health care systems.
The Institute of Medicine (IOM) identifies 5 domains of quality in health care: effectiveness, efficiency, equity, patient-centeredness, and safety.3 Effectiveness relies on providing care processes supported by scientific evidence and achieving desired outcomes in the IOM recommendations. The primary efficiency aim maximizes the quality of health care delivered or the benefits achieved for a given resource unit. Equity relates to providing health care of equal quality to all individuals, regardless of personal characteristics. Moreover, patient-centeredness relates to meeting patients’ needs and preferences and providing education and support. Safety relates to avoiding actual or potential harm. Timeliness relates to obtaining needed care while minimizing delays. Finally, the IOM defines health care quality as the systematic evaluation and provision of evidence-based and safe care characterized by a culture of continuous improvement, resulting in optimal health outcomes. Taking all these concepts into consideration, 4 key attributes have been identified as essential to the global definition of health care quality: effectiveness, safety, culture of continuous improvement, and desired outcomes. This conceptualization of health care quality encompasses the fundamental components and has the potential to enhance the delivery of care. This definition’s theoretical and practical implications provide a comprehensive and consistent understanding of the elements required to improve health care and maintain public trust.
Health care quality is a dynamic, ever-evolving construct that requires continuous assessment and evaluation to ensure the delivery of care meets the changing needs of society. The National Quality Forum’s National Voluntary Consensus Standards for health care provide measures, guidance, and recommendations on achieving effective outcomes through evidence-based practices.4 These standards establish criteria by which health care systems and providers can assess and improve their quality performance.
In the United States, in order to implement and disseminate best practices, the Centers for Medicare & Medicaid Services (CMS) developed Quality Payment Programs that offer incentives to health care providers to improve the quality of care delivery. This CMS program evaluates providers based on their performance in the Merit-Based Incentive Payment System performance categories.5 These include measures related to patient experience, cost, clinical quality, improvement activities, and the use of certified electronic health record technology. The scores that providers receive are used to determine their performance-based reimbursements under Medicare’s fee-for-service program.
The concept of health care quality is also applicable in other countries. In the United Kingdom, QI initiatives are led by the Department of Health and Social Care. The National Institute for Health and Care Excellence (NICE) produces guidelines on best practices to ensure that care delivery meets established safety and quality standards, reaching cost-effectiveness excellence.6 In Australia, the Australian Commission on Quality and Safety in Health Care is responsible for setting benchmarks for performance in health care systems through a clear, structured agenda.7 Ultimately, health care quality is a complex and multifaceted issue that requires a comprehensive approach to ensure the best outcomes for patients. With the implementation of measures such as the CMS Quality Payment Programs and NICE guidelines, health care organizations can take steps to ensure their systems of care delivery reflect evidence-based practices and demonstrate a commitment to providing high-quality care.
Implementing a health care QI plan that encompasses the 4 key attributes of health care quality—effectiveness, safety, culture of continuous improvement, and desired outcomes—requires collaboration among different departments and stakeholders and a data-driven approach to decision-making. Effective communication with patients and their families is critical to ensuring that their needs are being met and that they are active partners in their health care journey. While a health care QI plan is essential for delivering high-quality, safe patient care, it also helps health care organizations comply with regulatory requirements, meet accreditation standards, and stay competitive in the ever-evolving health care landscape.
Corresponding author: Ebrahim Barkoudah, MD, MPH; Ebrahim.Barkoudah@baystatehealth.org
As the movement to improve quality in health care has evolved over the past several decades, organizations whose missions focus on supporting and promoting quality in health care have defined essential concepts, standards, and measures that comprise quality and that can be used to guide quality improvement (QI) work. The World Health Organization (WHO) defines quality in clinical care as safe, effective, and people-centered service.1 These 3 pillars of quality form the foundation of a quality system aiming to deliver health care in a timely, equitable, efficient, and integrated manner. The WHO estimates that 5.7 to 8.4 million deaths occur yearly in low- and middle-income countries due to poor quality care. Regarding safety, patient harm from unsafe care is estimated to be among the top 10 causes of death and disability worldwide.2 A health care QI plan involves identifying areas for improvement, setting measurable goals, implementing evidence-based strategies and interventions, monitoring progress toward achieving those goals, and continuously evaluating and adjusting the plan as needed to ensure sustained improvement over time. Such a plan can be implemented at various levels of health care organizations, from individual clinical units to entire hospitals or even regional health care systems.
The Institute of Medicine (IOM) identifies 5 domains of quality in health care: effectiveness, efficiency, equity, patient-centeredness, and safety.3 Effectiveness relies on providing care processes supported by scientific evidence and achieving desired outcomes in the IOM recommendations. The primary efficiency aim maximizes the quality of health care delivered or the benefits achieved for a given resource unit. Equity relates to providing health care of equal quality to all individuals, regardless of personal characteristics. Moreover, patient-centeredness relates to meeting patients’ needs and preferences and providing education and support. Safety relates to avoiding actual or potential harm. Timeliness relates to obtaining needed care while minimizing delays. Finally, the IOM defines health care quality as the systematic evaluation and provision of evidence-based and safe care characterized by a culture of continuous improvement, resulting in optimal health outcomes. Taking all these concepts into consideration, 4 key attributes have been identified as essential to the global definition of health care quality: effectiveness, safety, culture of continuous improvement, and desired outcomes. This conceptualization of health care quality encompasses the fundamental components and has the potential to enhance the delivery of care. This definition’s theoretical and practical implications provide a comprehensive and consistent understanding of the elements required to improve health care and maintain public trust.
Health care quality is a dynamic, ever-evolving construct that requires continuous assessment and evaluation to ensure the delivery of care meets the changing needs of society. The National Quality Forum’s National Voluntary Consensus Standards for health care provide measures, guidance, and recommendations on achieving effective outcomes through evidence-based practices.4 These standards establish criteria by which health care systems and providers can assess and improve their quality performance.
In the United States, in order to implement and disseminate best practices, the Centers for Medicare & Medicaid Services (CMS) developed Quality Payment Programs that offer incentives to health care providers to improve the quality of care delivery. This CMS program evaluates providers based on their performance in the Merit-Based Incentive Payment System performance categories.5 These include measures related to patient experience, cost, clinical quality, improvement activities, and the use of certified electronic health record technology. The scores that providers receive are used to determine their performance-based reimbursements under Medicare’s fee-for-service program.
The concept of health care quality is also applicable in other countries. In the United Kingdom, QI initiatives are led by the Department of Health and Social Care. The National Institute for Health and Care Excellence (NICE) produces guidelines on best practices to ensure that care delivery meets established safety and quality standards, reaching cost-effectiveness excellence.6 In Australia, the Australian Commission on Quality and Safety in Health Care is responsible for setting benchmarks for performance in health care systems through a clear, structured agenda.7 Ultimately, health care quality is a complex and multifaceted issue that requires a comprehensive approach to ensure the best outcomes for patients. With the implementation of measures such as the CMS Quality Payment Programs and NICE guidelines, health care organizations can take steps to ensure their systems of care delivery reflect evidence-based practices and demonstrate a commitment to providing high-quality care.
Implementing a health care QI plan that encompasses the 4 key attributes of health care quality—effectiveness, safety, culture of continuous improvement, and desired outcomes—requires collaboration among different departments and stakeholders and a data-driven approach to decision-making. Effective communication with patients and their families is critical to ensuring that their needs are being met and that they are active partners in their health care journey. While a health care QI plan is essential for delivering high-quality, safe patient care, it also helps health care organizations comply with regulatory requirements, meet accreditation standards, and stay competitive in the ever-evolving health care landscape.
Corresponding author: Ebrahim Barkoudah, MD, MPH; Ebrahim.Barkoudah@baystatehealth.org
1. World Health Organization. Quality of care. Accessed on May 17, 2023. www.who.int/health-topics/quality-of-care#tab=tab_1
2. World Health Organization. Patient safety. Accessed on May 17, 2023 www.who.int/news-room/fact-sheets/detail/patient-safety
3. Agency for Healthcare Research and Quality. Understanding quality measurement. Accessed on May 17, 2023. www.ahrq.gov/patient-safety/quality-resources/tools/chtoolbx/understand/index.html
4. Ferrell B, Connor SR, Cordes A, et al. The national agenda for quality palliative care: the National Consensus Project and the National Quality Forum. J Pain Symptom Manage. 2007;33(6):737-744. doi:10.1016/j.jpainsymman.2007.02.024
5. U.S Centers for Medicare & Medicaid Services. Quality payment program. Accessed on March 14, 2023 qpp.cms.gov/mips/overview
6. Claxton K, Martin S, Soares M, et al. Methods for the estimation of the National Institute for Health and Care Excellence cost-effectiveness threshold. Health Technol Assess. 2015;19(14):1-503, v-vi. doi: 10.3310/hta19140
7. Braithwaite J, Healy J, Dwan K. The Governance of Health Safety and Quality, Commonwealth of Australia. Accessed May 17, 2023. https://regnet.anu.edu.au/research/publications/3626/governance-health-safety-and-quality 2005
1. World Health Organization. Quality of care. Accessed on May 17, 2023. www.who.int/health-topics/quality-of-care#tab=tab_1
2. World Health Organization. Patient safety. Accessed on May 17, 2023 www.who.int/news-room/fact-sheets/detail/patient-safety
3. Agency for Healthcare Research and Quality. Understanding quality measurement. Accessed on May 17, 2023. www.ahrq.gov/patient-safety/quality-resources/tools/chtoolbx/understand/index.html
4. Ferrell B, Connor SR, Cordes A, et al. The national agenda for quality palliative care: the National Consensus Project and the National Quality Forum. J Pain Symptom Manage. 2007;33(6):737-744. doi:10.1016/j.jpainsymman.2007.02.024
5. U.S Centers for Medicare & Medicaid Services. Quality payment program. Accessed on March 14, 2023 qpp.cms.gov/mips/overview
6. Claxton K, Martin S, Soares M, et al. Methods for the estimation of the National Institute for Health and Care Excellence cost-effectiveness threshold. Health Technol Assess. 2015;19(14):1-503, v-vi. doi: 10.3310/hta19140
7. Braithwaite J, Healy J, Dwan K. The Governance of Health Safety and Quality, Commonwealth of Australia. Accessed May 17, 2023. https://regnet.anu.edu.au/research/publications/3626/governance-health-safety-and-quality 2005
Differences in 30-Day Readmission Rates in Older Adults With Dementia
Study 1 Overview (Park et al)
Objective: To compare rates of adverse events and 30-day readmission among patients with dementia who undergo percutaneous coronary intervention (PCI) with those without dementia.
Design: This cohort study used a national database of hospital readmissions developed by the Agency for Healthcare Research and Quality.
Setting and participants: Data from State Inpatient Databases were used to derive this national readmissions database representing 80% of hospitals from 28 states that contribute data. The study included all individuals aged 18 years and older who were identified to have had a PCI procedure in the years 2017 and 2018. International Classification of Diseases, Tenth Revision (ICD-10) codes were used to identify PCI procedures, including drug-eluting stent placement, bare-metal stent placement, and balloon angioplasty, performed in patients who presented with myocardial infarction and unstable angina and those with stable ischemic heart disease. Patients were stratified into those with or without dementia, also defined using ICD-10 codes. A total of 755,406 index hospitalizations were included; 2.3% of the patients had dementia.
Main outcome measures: The primary study outcome was 30-day all-cause readmission, with the cause classified as cardiovascular or noncardiovascular. Secondary outcome measures examined were delirium, in-hospital mortality, cardiac arrest, blood transfusion, acute kidney injury, fall in hospital, length of hospital stay, and other adverse outcomes. Location at discharge was also examined. Other covariates included in the analysis were age, sex, comorbidities, hospital characteristics, primary payer, and median income. For analysis, a propensity score matching algorithm was applied to match patients with and without dementia. Kaplan-Meier curves were used to examine 30-day readmission rates, and a Cox proportional hazards model was used to calculate hazard ratios (HR) for those with and without dementia. For secondary outcomes, logistic regression models were used to calculate odds ratios (OR) of outcomes between those with and without dementia.
Main results: The average age of those with dementia was 78.8 years vs 64.9 years in those without dementia. Women made up 42.8% of those with dementia and 31.3% of those without dementia. Those with dementia also had higher rates of comorbidities, such as heart failure, renal failure, and depression. After propensity score matching, 17,309 and 17,187 patients with and without dementia, respectively, were included. Covariates were balanced between the 2 groups after matching. For the primary outcome, patients with dementia were more likely to be readmitted at 30 days (HR, 1.11; 95% CI, 1.05-1.18; P < .01) when compared to those without dementia. For other adverse outcomes, delirium was significantly more likely to occur for those with dementia (OR, 4.37; 95% CI, 3.69-5.16; P < .01). Patients with dementia were also more likely to die in hospital (OR, 1.15; 95% CI, 1.01-1.30; P = .03), have cardiac arrest (OR, 1.19; 95% CI, 1.01-1.39; P = .04), receive a blood transfusion (OR, 1.17; 95% CI, 1.00-1.36; P = .05), experience acute kidney injury (OR, 1.30; 95% CI, 1.21-1.39; P < .01), and fall in hospital (OR, 2.51; 95% CI, 2.06-3.07; P < .01). Hospital length of stay was higher for those with dementia, with a mean difference of 1.43 days. For discharge location, patients with dementia were more likely to be sent to a skilled nursing facility (30.1% vs 12.2%) and less likely to be discharged home.
Conclusion: Patients with dementia are more likely to experience adverse events, including delirium, mortality, kidney injury, and falls after PCI, and are more likely to be readmitted to the hospital in 30 days compared to those without dementia.
Study 2 Overview (Gilmore-Bykovskyi et al)
Objective: To examine the association between race and 30-day readmissions in Black and non-Hispanic White Medicare beneficiaries with dementia.
Design: This was a retrospective cohort study that used 100% Medicare fee-for service claims data from all hospitalizations between January 1, 2014, and November 30, 2014, for all enrollees with a dementia diagnosis. The claims data were linked to the patient, hospital stay, and hospital factors. Patients with dementia were identified using a validated algorithm that requires an inpatient, skilled nursing facility, home health, or Part B institutional or noninstitutional claim with a qualifying diagnostic code during a 3-year period. Persons enrolled in a health maintenance organization plan were excluded.
Main outcome measures: The primary outcome examined in this study was 30-day all-cause readmission. Self-reported race and ethnic identity was a baseline covariate. Persons who self-reported Black or non-Hispanic White race were included in the study; other categories of race and ethnicity were excluded because of prior evidence suggesting low accuracy of these categories in Medicare claims data. Other covariates included neighborhood disadvantage, measured using the Area Deprivation Index (ADI), and rurality; hospital-level and hospital stay–level characteristics such as for-profit status and number of annual discharges; and individual demographic characteristics and comorbidities. The ADI is constructed using variables of poverty, education, housing, and employment and is represented as a percentile ranking of level of disadvantage. Unadjusted and adjusted analyses of 30-day hospital readmission were conducted. Models using various levels of adjustment were constructed to examine the contributions of the identified covariates to the estimated association between 30-day readmission and race.
Main results: A total of 1,523,142 index hospital stays among 945,481 beneficiaries were included; 215,815 episodes were among Black beneficiaries and 1,307,327 episodes were among non-Hispanic White beneficiaries. Mean age was 81.5 years, and approximately 61% of beneficiaries were female. Black beneficiaries were younger but had higher rates of dual Medicare/Medicaid eligibility and disability; they were also more likely to reside in disadvantaged neighborhoods. Black beneficiaries had a 30-day readmission rate of 24.1% compared with 18.5% in non-Hispanic White beneficiaries (unadjusted OR, 1.37; 95% CI, 1.35-1.39). The differences in outcomes persisted after adjusting for geographic factors, social factors, hospital characteristics, hospital stay factors, demographics, and comorbidities, suggesting that unmeasured underlying racial disparities not included in this model accounted for the differences. The effects of certain variables, such as neighborhood, differed by race; for example, the protective effect of living in a less disadvantaged neighborhood was observed among White beneficiaries but not Black beneficiaries.
Conclusion: Racial and geographic disparities in 30-day readmission rates were observed among Medicare beneficiaries with dementia. Protective effects associated with neighborhood advantage may confer different levels of benefit for people of different race.
Commentary
Adults living with dementia are at higher risk of adverse outcomes across settings. In the first study, by Park et al, among adults who underwent a cardiac procedure (PCI), those with dementia were more likely to experience adverse events compared to those without dementia. These outcomes include increased rates of 30-day readmissions, delirium, cardiac arrest, and falls. These findings are consistent with other studies that found a similar association among patients who underwent other cardiac procedures, such as transcatheter aortic valve replacement.1 Because dementia is a strong predisposing factor for delirium, it is not surprising that delirium is observed across patients who underwent different procedures or hospitalization episodes.2 Because of the potential hazards for inpatients with dementia, hospitals have developed risk-reduction programs, such as those that promote recognition of dementia, and management strategies that reduce the risk of delirium.3 Delirium prevention may also impact other adverse outcomes, such as falls, discharge to institutional care, and readmissions.
Racial disparities in care outcomes have been documented across settings, including hospital4 and hospice care settings.5 In study 2, by Gilmore-Bykovskyi et al, the findings of higher rates of hospital readmission among Black patients when compared to non-Hispanic White patients were not surprising. The central finding of this study is that even when accounting for various levels of factors, including hospital-level, hospital stay–level, individual (demographics, comorbidities), and neighborhood characteristics (disadvantage), the observed disparity diminished but persisted, suggesting that while these various levels of factors contributed to the observed disparity, other unmeasured factors also contributed. Another key finding is that the effect of the various factors examined in this study may affect different subgroups in different ways, suggesting underlying factors, and thus potential solutions to reduce disparities in care outcomes, could differ among subgroups.
Applications for Clinical Practice and System Implementation
These 2 studies add to the literature on factors that can affect 30-day hospital readmission rates in patients with dementia. These data could allow for more robust discussions of what to anticipate when adults with dementia undergo specific procedures, and also further build the case that improvements in care, such as delirium prevention programs, could offer benefits. The observation about racial and ethnic disparities in care outcomes among patients with dementia highlights the continued need to better understand the drivers of these disparities so that hospital systems and policy makers can consider and test possible solutions. Future studies should further disentangle the relationships among the various levels of factors and observed disparities in outcomes, especially for this vulnerable population of adults living with dementia.
Practice Points
- Clinicians should be aware of the additional risks for poor outcomes that dementia confers.
- Awareness of this increased risk will inform discussions of risks and benefits for older adults considered for procedures.
–William W. Hung, MD, MPH
1. Park DY, Sana MK, Shoura S, et al. Readmission and in-hospital outcomes after transcatheter aortic valve replacement in patients with dementia. Cardiovasc Revasc Med. 2023;46:70-77. doi:10.1016/j.carrev.2022.08.016
2. McNicoll L, Pisani MA, Zhang Y, et al. Delirium in the intensive care unit: occurrence and clinical course in older patients. J Am Geriatr Soc. 2003;51(5):591-598. doi:10.1034/j.1600-0579.2003.00201.x
3. Weldingh NM, Mellingsæter MR, Hegna BW, et al. Impact of a dementia-friendly program on detection and management of patients with cognitive impairment and delirium in acute-care hospital units: a controlled clinical trial design. BMC Geriatr. 2022;22(1):266. doi:10.1186/s12877-022-02949-0
4. Hermosura AH, Noonan CJ, Fyfe-Johnson AL, et al. Hospital disparities between native Hawaiian and other pacific islanders and non-Hispanic whites with Alzheimer’s disease and related dementias. J Aging Health. 2020;32(10):1579-1590. doi:10.1177/0898264320945177
5. Zhang Y, Shao H, Zhang M, Li J. Healthcare utilization and mortality after hospice live discharge among Medicare patients with and without Alzheimer’s disease and related dementias. J Gen Intern Med. 2023 Jan 17. doi:10.1007/s11606-023-08031-8
Study 1 Overview (Park et al)
Objective: To compare rates of adverse events and 30-day readmission among patients with dementia who undergo percutaneous coronary intervention (PCI) with those without dementia.
Design: This cohort study used a national database of hospital readmissions developed by the Agency for Healthcare Research and Quality.
Setting and participants: Data from State Inpatient Databases were used to derive this national readmissions database representing 80% of hospitals from 28 states that contribute data. The study included all individuals aged 18 years and older who were identified to have had a PCI procedure in the years 2017 and 2018. International Classification of Diseases, Tenth Revision (ICD-10) codes were used to identify PCI procedures, including drug-eluting stent placement, bare-metal stent placement, and balloon angioplasty, performed in patients who presented with myocardial infarction and unstable angina and those with stable ischemic heart disease. Patients were stratified into those with or without dementia, also defined using ICD-10 codes. A total of 755,406 index hospitalizations were included; 2.3% of the patients had dementia.
Main outcome measures: The primary study outcome was 30-day all-cause readmission, with the cause classified as cardiovascular or noncardiovascular. Secondary outcome measures examined were delirium, in-hospital mortality, cardiac arrest, blood transfusion, acute kidney injury, fall in hospital, length of hospital stay, and other adverse outcomes. Location at discharge was also examined. Other covariates included in the analysis were age, sex, comorbidities, hospital characteristics, primary payer, and median income. For analysis, a propensity score matching algorithm was applied to match patients with and without dementia. Kaplan-Meier curves were used to examine 30-day readmission rates, and a Cox proportional hazards model was used to calculate hazard ratios (HR) for those with and without dementia. For secondary outcomes, logistic regression models were used to calculate odds ratios (OR) of outcomes between those with and without dementia.
Main results: The average age of those with dementia was 78.8 years vs 64.9 years in those without dementia. Women made up 42.8% of those with dementia and 31.3% of those without dementia. Those with dementia also had higher rates of comorbidities, such as heart failure, renal failure, and depression. After propensity score matching, 17,309 and 17,187 patients with and without dementia, respectively, were included. Covariates were balanced between the 2 groups after matching. For the primary outcome, patients with dementia were more likely to be readmitted at 30 days (HR, 1.11; 95% CI, 1.05-1.18; P < .01) when compared to those without dementia. For other adverse outcomes, delirium was significantly more likely to occur for those with dementia (OR, 4.37; 95% CI, 3.69-5.16; P < .01). Patients with dementia were also more likely to die in hospital (OR, 1.15; 95% CI, 1.01-1.30; P = .03), have cardiac arrest (OR, 1.19; 95% CI, 1.01-1.39; P = .04), receive a blood transfusion (OR, 1.17; 95% CI, 1.00-1.36; P = .05), experience acute kidney injury (OR, 1.30; 95% CI, 1.21-1.39; P < .01), and fall in hospital (OR, 2.51; 95% CI, 2.06-3.07; P < .01). Hospital length of stay was higher for those with dementia, with a mean difference of 1.43 days. For discharge location, patients with dementia were more likely to be sent to a skilled nursing facility (30.1% vs 12.2%) and less likely to be discharged home.
Conclusion: Patients with dementia are more likely to experience adverse events, including delirium, mortality, kidney injury, and falls after PCI, and are more likely to be readmitted to the hospital in 30 days compared to those without dementia.
Study 2 Overview (Gilmore-Bykovskyi et al)
Objective: To examine the association between race and 30-day readmissions in Black and non-Hispanic White Medicare beneficiaries with dementia.
Design: This was a retrospective cohort study that used 100% Medicare fee-for service claims data from all hospitalizations between January 1, 2014, and November 30, 2014, for all enrollees with a dementia diagnosis. The claims data were linked to the patient, hospital stay, and hospital factors. Patients with dementia were identified using a validated algorithm that requires an inpatient, skilled nursing facility, home health, or Part B institutional or noninstitutional claim with a qualifying diagnostic code during a 3-year period. Persons enrolled in a health maintenance organization plan were excluded.
Main outcome measures: The primary outcome examined in this study was 30-day all-cause readmission. Self-reported race and ethnic identity was a baseline covariate. Persons who self-reported Black or non-Hispanic White race were included in the study; other categories of race and ethnicity were excluded because of prior evidence suggesting low accuracy of these categories in Medicare claims data. Other covariates included neighborhood disadvantage, measured using the Area Deprivation Index (ADI), and rurality; hospital-level and hospital stay–level characteristics such as for-profit status and number of annual discharges; and individual demographic characteristics and comorbidities. The ADI is constructed using variables of poverty, education, housing, and employment and is represented as a percentile ranking of level of disadvantage. Unadjusted and adjusted analyses of 30-day hospital readmission were conducted. Models using various levels of adjustment were constructed to examine the contributions of the identified covariates to the estimated association between 30-day readmission and race.
Main results: A total of 1,523,142 index hospital stays among 945,481 beneficiaries were included; 215,815 episodes were among Black beneficiaries and 1,307,327 episodes were among non-Hispanic White beneficiaries. Mean age was 81.5 years, and approximately 61% of beneficiaries were female. Black beneficiaries were younger but had higher rates of dual Medicare/Medicaid eligibility and disability; they were also more likely to reside in disadvantaged neighborhoods. Black beneficiaries had a 30-day readmission rate of 24.1% compared with 18.5% in non-Hispanic White beneficiaries (unadjusted OR, 1.37; 95% CI, 1.35-1.39). The differences in outcomes persisted after adjusting for geographic factors, social factors, hospital characteristics, hospital stay factors, demographics, and comorbidities, suggesting that unmeasured underlying racial disparities not included in this model accounted for the differences. The effects of certain variables, such as neighborhood, differed by race; for example, the protective effect of living in a less disadvantaged neighborhood was observed among White beneficiaries but not Black beneficiaries.
Conclusion: Racial and geographic disparities in 30-day readmission rates were observed among Medicare beneficiaries with dementia. Protective effects associated with neighborhood advantage may confer different levels of benefit for people of different race.
Commentary
Adults living with dementia are at higher risk of adverse outcomes across settings. In the first study, by Park et al, among adults who underwent a cardiac procedure (PCI), those with dementia were more likely to experience adverse events compared to those without dementia. These outcomes include increased rates of 30-day readmissions, delirium, cardiac arrest, and falls. These findings are consistent with other studies that found a similar association among patients who underwent other cardiac procedures, such as transcatheter aortic valve replacement.1 Because dementia is a strong predisposing factor for delirium, it is not surprising that delirium is observed across patients who underwent different procedures or hospitalization episodes.2 Because of the potential hazards for inpatients with dementia, hospitals have developed risk-reduction programs, such as those that promote recognition of dementia, and management strategies that reduce the risk of delirium.3 Delirium prevention may also impact other adverse outcomes, such as falls, discharge to institutional care, and readmissions.
Racial disparities in care outcomes have been documented across settings, including hospital4 and hospice care settings.5 In study 2, by Gilmore-Bykovskyi et al, the findings of higher rates of hospital readmission among Black patients when compared to non-Hispanic White patients were not surprising. The central finding of this study is that even when accounting for various levels of factors, including hospital-level, hospital stay–level, individual (demographics, comorbidities), and neighborhood characteristics (disadvantage), the observed disparity diminished but persisted, suggesting that while these various levels of factors contributed to the observed disparity, other unmeasured factors also contributed. Another key finding is that the effect of the various factors examined in this study may affect different subgroups in different ways, suggesting underlying factors, and thus potential solutions to reduce disparities in care outcomes, could differ among subgroups.
Applications for Clinical Practice and System Implementation
These 2 studies add to the literature on factors that can affect 30-day hospital readmission rates in patients with dementia. These data could allow for more robust discussions of what to anticipate when adults with dementia undergo specific procedures, and also further build the case that improvements in care, such as delirium prevention programs, could offer benefits. The observation about racial and ethnic disparities in care outcomes among patients with dementia highlights the continued need to better understand the drivers of these disparities so that hospital systems and policy makers can consider and test possible solutions. Future studies should further disentangle the relationships among the various levels of factors and observed disparities in outcomes, especially for this vulnerable population of adults living with dementia.
Practice Points
- Clinicians should be aware of the additional risks for poor outcomes that dementia confers.
- Awareness of this increased risk will inform discussions of risks and benefits for older adults considered for procedures.
–William W. Hung, MD, MPH
Study 1 Overview (Park et al)
Objective: To compare rates of adverse events and 30-day readmission among patients with dementia who undergo percutaneous coronary intervention (PCI) with those without dementia.
Design: This cohort study used a national database of hospital readmissions developed by the Agency for Healthcare Research and Quality.
Setting and participants: Data from State Inpatient Databases were used to derive this national readmissions database representing 80% of hospitals from 28 states that contribute data. The study included all individuals aged 18 years and older who were identified to have had a PCI procedure in the years 2017 and 2018. International Classification of Diseases, Tenth Revision (ICD-10) codes were used to identify PCI procedures, including drug-eluting stent placement, bare-metal stent placement, and balloon angioplasty, performed in patients who presented with myocardial infarction and unstable angina and those with stable ischemic heart disease. Patients were stratified into those with or without dementia, also defined using ICD-10 codes. A total of 755,406 index hospitalizations were included; 2.3% of the patients had dementia.
Main outcome measures: The primary study outcome was 30-day all-cause readmission, with the cause classified as cardiovascular or noncardiovascular. Secondary outcome measures examined were delirium, in-hospital mortality, cardiac arrest, blood transfusion, acute kidney injury, fall in hospital, length of hospital stay, and other adverse outcomes. Location at discharge was also examined. Other covariates included in the analysis were age, sex, comorbidities, hospital characteristics, primary payer, and median income. For analysis, a propensity score matching algorithm was applied to match patients with and without dementia. Kaplan-Meier curves were used to examine 30-day readmission rates, and a Cox proportional hazards model was used to calculate hazard ratios (HR) for those with and without dementia. For secondary outcomes, logistic regression models were used to calculate odds ratios (OR) of outcomes between those with and without dementia.
Main results: The average age of those with dementia was 78.8 years vs 64.9 years in those without dementia. Women made up 42.8% of those with dementia and 31.3% of those without dementia. Those with dementia also had higher rates of comorbidities, such as heart failure, renal failure, and depression. After propensity score matching, 17,309 and 17,187 patients with and without dementia, respectively, were included. Covariates were balanced between the 2 groups after matching. For the primary outcome, patients with dementia were more likely to be readmitted at 30 days (HR, 1.11; 95% CI, 1.05-1.18; P < .01) when compared to those without dementia. For other adverse outcomes, delirium was significantly more likely to occur for those with dementia (OR, 4.37; 95% CI, 3.69-5.16; P < .01). Patients with dementia were also more likely to die in hospital (OR, 1.15; 95% CI, 1.01-1.30; P = .03), have cardiac arrest (OR, 1.19; 95% CI, 1.01-1.39; P = .04), receive a blood transfusion (OR, 1.17; 95% CI, 1.00-1.36; P = .05), experience acute kidney injury (OR, 1.30; 95% CI, 1.21-1.39; P < .01), and fall in hospital (OR, 2.51; 95% CI, 2.06-3.07; P < .01). Hospital length of stay was higher for those with dementia, with a mean difference of 1.43 days. For discharge location, patients with dementia were more likely to be sent to a skilled nursing facility (30.1% vs 12.2%) and less likely to be discharged home.
Conclusion: Patients with dementia are more likely to experience adverse events, including delirium, mortality, kidney injury, and falls after PCI, and are more likely to be readmitted to the hospital in 30 days compared to those without dementia.
Study 2 Overview (Gilmore-Bykovskyi et al)
Objective: To examine the association between race and 30-day readmissions in Black and non-Hispanic White Medicare beneficiaries with dementia.
Design: This was a retrospective cohort study that used 100% Medicare fee-for service claims data from all hospitalizations between January 1, 2014, and November 30, 2014, for all enrollees with a dementia diagnosis. The claims data were linked to the patient, hospital stay, and hospital factors. Patients with dementia were identified using a validated algorithm that requires an inpatient, skilled nursing facility, home health, or Part B institutional or noninstitutional claim with a qualifying diagnostic code during a 3-year period. Persons enrolled in a health maintenance organization plan were excluded.
Main outcome measures: The primary outcome examined in this study was 30-day all-cause readmission. Self-reported race and ethnic identity was a baseline covariate. Persons who self-reported Black or non-Hispanic White race were included in the study; other categories of race and ethnicity were excluded because of prior evidence suggesting low accuracy of these categories in Medicare claims data. Other covariates included neighborhood disadvantage, measured using the Area Deprivation Index (ADI), and rurality; hospital-level and hospital stay–level characteristics such as for-profit status and number of annual discharges; and individual demographic characteristics and comorbidities. The ADI is constructed using variables of poverty, education, housing, and employment and is represented as a percentile ranking of level of disadvantage. Unadjusted and adjusted analyses of 30-day hospital readmission were conducted. Models using various levels of adjustment were constructed to examine the contributions of the identified covariates to the estimated association between 30-day readmission and race.
Main results: A total of 1,523,142 index hospital stays among 945,481 beneficiaries were included; 215,815 episodes were among Black beneficiaries and 1,307,327 episodes were among non-Hispanic White beneficiaries. Mean age was 81.5 years, and approximately 61% of beneficiaries were female. Black beneficiaries were younger but had higher rates of dual Medicare/Medicaid eligibility and disability; they were also more likely to reside in disadvantaged neighborhoods. Black beneficiaries had a 30-day readmission rate of 24.1% compared with 18.5% in non-Hispanic White beneficiaries (unadjusted OR, 1.37; 95% CI, 1.35-1.39). The differences in outcomes persisted after adjusting for geographic factors, social factors, hospital characteristics, hospital stay factors, demographics, and comorbidities, suggesting that unmeasured underlying racial disparities not included in this model accounted for the differences. The effects of certain variables, such as neighborhood, differed by race; for example, the protective effect of living in a less disadvantaged neighborhood was observed among White beneficiaries but not Black beneficiaries.
Conclusion: Racial and geographic disparities in 30-day readmission rates were observed among Medicare beneficiaries with dementia. Protective effects associated with neighborhood advantage may confer different levels of benefit for people of different race.
Commentary
Adults living with dementia are at higher risk of adverse outcomes across settings. In the first study, by Park et al, among adults who underwent a cardiac procedure (PCI), those with dementia were more likely to experience adverse events compared to those without dementia. These outcomes include increased rates of 30-day readmissions, delirium, cardiac arrest, and falls. These findings are consistent with other studies that found a similar association among patients who underwent other cardiac procedures, such as transcatheter aortic valve replacement.1 Because dementia is a strong predisposing factor for delirium, it is not surprising that delirium is observed across patients who underwent different procedures or hospitalization episodes.2 Because of the potential hazards for inpatients with dementia, hospitals have developed risk-reduction programs, such as those that promote recognition of dementia, and management strategies that reduce the risk of delirium.3 Delirium prevention may also impact other adverse outcomes, such as falls, discharge to institutional care, and readmissions.
Racial disparities in care outcomes have been documented across settings, including hospital4 and hospice care settings.5 In study 2, by Gilmore-Bykovskyi et al, the findings of higher rates of hospital readmission among Black patients when compared to non-Hispanic White patients were not surprising. The central finding of this study is that even when accounting for various levels of factors, including hospital-level, hospital stay–level, individual (demographics, comorbidities), and neighborhood characteristics (disadvantage), the observed disparity diminished but persisted, suggesting that while these various levels of factors contributed to the observed disparity, other unmeasured factors also contributed. Another key finding is that the effect of the various factors examined in this study may affect different subgroups in different ways, suggesting underlying factors, and thus potential solutions to reduce disparities in care outcomes, could differ among subgroups.
Applications for Clinical Practice and System Implementation
These 2 studies add to the literature on factors that can affect 30-day hospital readmission rates in patients with dementia. These data could allow for more robust discussions of what to anticipate when adults with dementia undergo specific procedures, and also further build the case that improvements in care, such as delirium prevention programs, could offer benefits. The observation about racial and ethnic disparities in care outcomes among patients with dementia highlights the continued need to better understand the drivers of these disparities so that hospital systems and policy makers can consider and test possible solutions. Future studies should further disentangle the relationships among the various levels of factors and observed disparities in outcomes, especially for this vulnerable population of adults living with dementia.
Practice Points
- Clinicians should be aware of the additional risks for poor outcomes that dementia confers.
- Awareness of this increased risk will inform discussions of risks and benefits for older adults considered for procedures.
–William W. Hung, MD, MPH
1. Park DY, Sana MK, Shoura S, et al. Readmission and in-hospital outcomes after transcatheter aortic valve replacement in patients with dementia. Cardiovasc Revasc Med. 2023;46:70-77. doi:10.1016/j.carrev.2022.08.016
2. McNicoll L, Pisani MA, Zhang Y, et al. Delirium in the intensive care unit: occurrence and clinical course in older patients. J Am Geriatr Soc. 2003;51(5):591-598. doi:10.1034/j.1600-0579.2003.00201.x
3. Weldingh NM, Mellingsæter MR, Hegna BW, et al. Impact of a dementia-friendly program on detection and management of patients with cognitive impairment and delirium in acute-care hospital units: a controlled clinical trial design. BMC Geriatr. 2022;22(1):266. doi:10.1186/s12877-022-02949-0
4. Hermosura AH, Noonan CJ, Fyfe-Johnson AL, et al. Hospital disparities between native Hawaiian and other pacific islanders and non-Hispanic whites with Alzheimer’s disease and related dementias. J Aging Health. 2020;32(10):1579-1590. doi:10.1177/0898264320945177
5. Zhang Y, Shao H, Zhang M, Li J. Healthcare utilization and mortality after hospice live discharge among Medicare patients with and without Alzheimer’s disease and related dementias. J Gen Intern Med. 2023 Jan 17. doi:10.1007/s11606-023-08031-8
1. Park DY, Sana MK, Shoura S, et al. Readmission and in-hospital outcomes after transcatheter aortic valve replacement in patients with dementia. Cardiovasc Revasc Med. 2023;46:70-77. doi:10.1016/j.carrev.2022.08.016
2. McNicoll L, Pisani MA, Zhang Y, et al. Delirium in the intensive care unit: occurrence and clinical course in older patients. J Am Geriatr Soc. 2003;51(5):591-598. doi:10.1034/j.1600-0579.2003.00201.x
3. Weldingh NM, Mellingsæter MR, Hegna BW, et al. Impact of a dementia-friendly program on detection and management of patients with cognitive impairment and delirium in acute-care hospital units: a controlled clinical trial design. BMC Geriatr. 2022;22(1):266. doi:10.1186/s12877-022-02949-0
4. Hermosura AH, Noonan CJ, Fyfe-Johnson AL, et al. Hospital disparities between native Hawaiian and other pacific islanders and non-Hispanic whites with Alzheimer’s disease and related dementias. J Aging Health. 2020;32(10):1579-1590. doi:10.1177/0898264320945177
5. Zhang Y, Shao H, Zhang M, Li J. Healthcare utilization and mortality after hospice live discharge among Medicare patients with and without Alzheimer’s disease and related dementias. J Gen Intern Med. 2023 Jan 17. doi:10.1007/s11606-023-08031-8
Patient Safety in Transitions of Care: Addressing Discharge Communication Gaps and the Potential of the Teach-Back Method
Study 1 Overview (Trivedi et al)
Objective: This observational quality improvement study aimed to evaluate the discharge communication practices in internal medicine services at 2 urban academic teaching hospitals, specifically focusing on patient education and counseling in 6 key discharge communication domains.
Design: Observations were conducted over a 13-month period from September 2018 through October 2019, following the Standards for Quality Improvement Reporting Excellence (SQUIRE) guidelines.
Setting and participants: The study involved a total of 33 English- and Spanish-speaking patients purposefully selected from the “discharge before noon” list at 2 urban tertiary-care teaching hospitals. A total of 155 observation hours were accumulated, with an average observation time of 4.7 hours per patient on the day of discharge.
Main outcome measures: The study assessed 6 discharge communication domains: (1) the name and function of medication changes, (2) the purpose of postdischarge appointments, (3) disease self-management, (4) red flags or warning signs for complications, (5) teach-back techniques to confirm patient understanding, and (6) staff solicitation of patient questions or concerns.
Main results: The study found several gaps in discharge communication practices. Among the 29 patients with medication changes, 28% were not informed about the name and basic function of the changes, while 59% did not receive counseling on the purpose for the medication change. In terms of postdischarge appointments, 48% of patients were not told the purpose of these appointments. Moreover, 54% of patients did not receive counseling on self-management of their primary discharge diagnosis or other diagnoses, and 73% were not informed about symptom expectations or the expected course of their illness after leaving the hospital. Most patients (82%) were not counseled on red-flag signs and symptoms that should prompt immediate return to care.
Teach-back techniques, which are critical for ensuring patient understanding, were used in only 3% of cases, and 85% of patients were not asked by health care providers if there might be barriers to following the care plan. Less than half (42%) of the patients were asked if they had any questions, with most questions being logistical and often deferred to another team member or met with uncertainty. Of note, among the 33 patients, only 2 patients received extensive information that covered 5 or 6 out of 6 discharge communication domains.
The study found variable roles in who communicated what aspects of discharge education, with most domains being communicated in an ad hoc manner and no clear pattern of responsibility. However, 2 exceptions were observed: nurses were more likely to provide information about new or changed medications and follow-up appointments, and the only example of teach-back was conducted by an attending physician.
Conclusion: The study highlights a significant need for improved discharge techniques to enhance patient safety and quality of care upon leaving the hospital. Interventions should focus on increasing transparency in patient education and understanding, clarifying assumptions of roles among the interprofessional team, and implementing effective communication strategies and system redesigns that foster patient-centered discharge education. Also, the study revealed that some patients received more robust discharge education than others, indicating systemic inequality in the patient experience. Further studies are needed to explore the development and assessment of such interventions to ensure optimal patient outcomes and equal care following hospital discharge.
Study 2 Overview (Marks et al)
Objective: This study aimed to investigate the impact of a nurse-led discharge medication education program, Teaching Important Medication Effects (TIME), on patients’ new medication knowledge at discharge and 48 to 72 hours post discharge. The specific objectives were to identify patients’ priority learning needs, evaluate the influence of TIME on patients’ new medication knowledge before and after discharge, and assess the effect of TIME on patients’ experience and satisfaction with medication education.
Design: The study employed a longitudinal pretest/post-test, 2-group design involving 107 randomly selected medical-surgical patients from an academic hospital. Participants were interviewed before and within 72 hours after discharge following administration of medication instructions. Bivariate analyses were performed to assess demographic and outcome variable differences between groups.
Setting and participants: Conducted on a 24-bed medical-surgical unit at a large Magnet® hospital over 18 months (2018-2019), the study included patients with at least 1 new medication, aged 18 years or older, able to read and speak English or Spanish, admitted from home with a minimum 1 overnight stay, and planning to return home post discharge. Excluded were cognitively impaired patients, those assigned to a resource pool nurse without TIME training, and those having a research team member assigned. Participants were randomly selected from a computerized list of patients scheduled for discharge.
Main outcome measures: Primary outcome measures included patients’ new medication knowledge before and after discharge and patients’ experience and satisfaction with medication education.
Main results: The usual care (n = 52) and TIME (n = 55) patients had similar baseline demographic characteristics. The study revealed that almost all patients in both usual care and TIME groups were aware of their new medication and its purpose at discharge. However, differences were observed in medication side effect responses, with 72.5% of the usual-care group knowing side effects compared to 94.3% of the TIME group (P = .003). Additionally, 81.5% of the usual-care group understood the medication purpose compared to 100% of the TIME group (P = .02). During the 48- to 72-hour postdischarge calls, consistent responses were found from both groups regarding knowledge of new medication, medication name, and medication purpose. Similar to discharge results, differences in medication side effect responses were observed, with 75.8% of the usual care group correctly identifying at least 1 medication side effect compared to 93.9% of the TIME group (P = .04). TIME was associated with higher satisfaction with medication education compared to usual care (97% vs. 46.9%, P < .001).
Conclusion: The nurse-led discharge medication education program TIME effectively enhanced patients’ new medication knowledge at discharge and 48 to 72 hours after discharge. The program also significantly improved patients’ experience and satisfaction with medication education. These findings indicate that TIME is a valuable tool for augmenting patient education and medication adherence in a hospital setting. By incorporating the teach-back method, TIME offers a structured approach to educating patients about their medications at hospital discharge, leading to improved care transitions.
Commentary
Suboptimal communication between patients, caregivers, and providers upon hospital discharge is a major contributor to patients’ inadequate understanding of postdischarge care plans. This inadequate understanding leads to preventable harms, such as medication errors, adverse events, emergency room visits, and costly hospital readmissions.1 The issue is further exacerbated by a lack of clarity among health care team members’ respective roles in providing information that optimizes care transitions during the discharge communication process. Moreover, low health literacy, particularly prevalent among seniors, those from disadvantaged backgrouds, and those with lower education attainment or chronic illnesses, create additional barriers to effective discharge communication. A potential solution to this problem is the adoption of effective teaching strategies, specifically the teach-back method. This method employs techniques that ensure patients’ understanding and recall of new information regardless of health literacy, and places accountability on clinicians rather than patients. By closing communication gaps between clinicians and patients, the teach-back method can reduce hospital readmissions, hospital-acquired conditions, and mortality rates, while improving patient satisfaction with health care instructions and the overall hospital experience.2
Study 1, by Trivedi et al, and study 2, by Marks et al, aimed to identify and address problems related to poor communication between patients and health care team members at hospital discharge. Specifically, study 1 examined routine discharge communication practices to determine communication gaps, while study 2 evaluated a nurse-led teach-back intervention program designed to improve patients’ medication knowledge and satisfaction. These distinct objectives and designs reflected the unique ways each study approached the challenges associated with care transitions at the time of hospital discharge.
Study 1 used direct observation of patient-practitioner interactions to evaluate routine discharge communication practices in internal medicine services at 2 urban academic teaching hospitals. In the 33 patients observed, significant gaps in discharge communication practices were identified in the domains of medication changes, postdischarge appointments, disease self-management, and red flags or warning signs. Unsurprisingly, most of these domains were communicated in an ad hoc manner by members of the health care team without a clear pattern of responsibility in reference to patient discharge education, and teach-back was seldom used. These findings underscore the need for improved discharge techniques, effective communication strategies, and clarification of roles among the interprofessional team to enhance the safety, quality of care, and overall patient experience during hospital discharge.
Study 2 aimed to augment the hospital discharge communication process by implementing a nurse-led discharge medication education program (TIME), which targeted patients’ priority learning needs, new medication knowledge, and satisfaction with medication education. In the 107 patients assessed, this teach-back method enhanced patients’ new medication knowledge at discharge and 48 to 72 hours after discharge, as well as improved patients’ experience and satisfaction with medication education. These results suggest that a teach-back method such as the TIME program could be a solution to care transition problems identified in the Trivedi et al study by providing a structured approach to patient education and enhancing communication practices during the hospital discharge process. Thus, by implementing the TIME program, hospitals may improve patient outcomes, safety, and overall quality of care upon leaving the hospital.
Applications for Clinical Practice and System Implementation
Care transition at the time of hospital discharge is a particularly pivotal period in the care of vulnerable individuals. There is growing literature, including studies discussed in this review, to indicate that by focusing on improving patient-practitioner communication during the discharge process and using strategies such as the teach-back method, health care professionals can better prepare patients for self-management in the post-acute period and help them make informed decisions about their care. This emphasis on care-transition communication strategies may lead to a reduction in medication errors, adverse events, and hospital readmissions, ultimately improving patient outcomes and satisfaction. Barriers to system implementation of such strategies may include competing demands and responsibilities of busy practitioners as well as the inherent complexities associated with hospital discharge. Creative solutions, such as the utilization of telehealth and early transition-of-care visits, represent some potential approaches to counter these barriers.
While both studies illustrated barriers and facilitators of hospital discharge communication, each study had limitations that impacted their generalizability to real-world clinical practice. Limitations in study 1 included a small sample size, purposive sampling method, and a focus on planned discharges in a teaching hospital, which may introduce selection bias. The study’s findings may not be generalizable to unplanned discharges, patients who do not speak English or Spanish, or nonteaching hospitals. Additionally, the data were collected before the COVID-19 pandemic, which could have further impacted discharge education practices. The study also revealed that some patients received more robust discharge education than others, which indicated systemic inequality in the patient experience. Further research is required to address this discrepancy. Limitations in study 2 included a relatively small and homogeneous sample, with most participants being younger, non-Hispanic White, English-speaking, and well-educated. This lack of diversity may limit the generalizability of the findings. Furthermore, the study did not evaluate the patients’ knowledge of medication dosage and focused only on new medications. Future studies should examine the effect of teach-back on a broader range of self-management topics in preparation for discharge, while also including a more diverse population to account for factors related to social determinants of health. Taken together, further research is needed to address these limitations and ensure more generalizable results that can more broadly improve discharge education and care transitions that bridge acute and post-acute care.
Practice Points
- There is a significant need for improved discharge strategies to enhance patient safety and quality of care upon leaving the hospital.
- Teach-back method may offer a structured approach to educating patients about their medications at hospital discharge and improve care transitions.
–Yuka Shichijo, MD, and Fred Ko, MD, Mount Sinai Beth Israel Hospital, New York, NY
1. Snow V, Beck D, Budnitz T, Miller DC, Potter J, Wears RL, Weiss KB, Williams MV; American College of Physicians; Society of General Internal Medicine; Society of Hospital Medicine; American Geriatrics Society; American College of Emergency Physicians; Society of Academic Emergency Medicine. Transitions of care consensus policy statement American College of Physicians-Society of General Internal Medicine-Society of Hospital Medicine-American Geriatrics Society-American College of Emergency Physicians-Society of Academic Emergency Medicine. J Gen Intern Med. 2009;24(8):971-976. doi:10.1007/s11606-009-0969-x
2. Yen PH, Leasure AR. Use and effectiveness of the teach-back method in patient education and health outcomes. Fed. Pract. 2019;36(6):284-289.
Study 1 Overview (Trivedi et al)
Objective: This observational quality improvement study aimed to evaluate the discharge communication practices in internal medicine services at 2 urban academic teaching hospitals, specifically focusing on patient education and counseling in 6 key discharge communication domains.
Design: Observations were conducted over a 13-month period from September 2018 through October 2019, following the Standards for Quality Improvement Reporting Excellence (SQUIRE) guidelines.
Setting and participants: The study involved a total of 33 English- and Spanish-speaking patients purposefully selected from the “discharge before noon” list at 2 urban tertiary-care teaching hospitals. A total of 155 observation hours were accumulated, with an average observation time of 4.7 hours per patient on the day of discharge.
Main outcome measures: The study assessed 6 discharge communication domains: (1) the name and function of medication changes, (2) the purpose of postdischarge appointments, (3) disease self-management, (4) red flags or warning signs for complications, (5) teach-back techniques to confirm patient understanding, and (6) staff solicitation of patient questions or concerns.
Main results: The study found several gaps in discharge communication practices. Among the 29 patients with medication changes, 28% were not informed about the name and basic function of the changes, while 59% did not receive counseling on the purpose for the medication change. In terms of postdischarge appointments, 48% of patients were not told the purpose of these appointments. Moreover, 54% of patients did not receive counseling on self-management of their primary discharge diagnosis or other diagnoses, and 73% were not informed about symptom expectations or the expected course of their illness after leaving the hospital. Most patients (82%) were not counseled on red-flag signs and symptoms that should prompt immediate return to care.
Teach-back techniques, which are critical for ensuring patient understanding, were used in only 3% of cases, and 85% of patients were not asked by health care providers if there might be barriers to following the care plan. Less than half (42%) of the patients were asked if they had any questions, with most questions being logistical and often deferred to another team member or met with uncertainty. Of note, among the 33 patients, only 2 patients received extensive information that covered 5 or 6 out of 6 discharge communication domains.
The study found variable roles in who communicated what aspects of discharge education, with most domains being communicated in an ad hoc manner and no clear pattern of responsibility. However, 2 exceptions were observed: nurses were more likely to provide information about new or changed medications and follow-up appointments, and the only example of teach-back was conducted by an attending physician.
Conclusion: The study highlights a significant need for improved discharge techniques to enhance patient safety and quality of care upon leaving the hospital. Interventions should focus on increasing transparency in patient education and understanding, clarifying assumptions of roles among the interprofessional team, and implementing effective communication strategies and system redesigns that foster patient-centered discharge education. Also, the study revealed that some patients received more robust discharge education than others, indicating systemic inequality in the patient experience. Further studies are needed to explore the development and assessment of such interventions to ensure optimal patient outcomes and equal care following hospital discharge.
Study 2 Overview (Marks et al)
Objective: This study aimed to investigate the impact of a nurse-led discharge medication education program, Teaching Important Medication Effects (TIME), on patients’ new medication knowledge at discharge and 48 to 72 hours post discharge. The specific objectives were to identify patients’ priority learning needs, evaluate the influence of TIME on patients’ new medication knowledge before and after discharge, and assess the effect of TIME on patients’ experience and satisfaction with medication education.
Design: The study employed a longitudinal pretest/post-test, 2-group design involving 107 randomly selected medical-surgical patients from an academic hospital. Participants were interviewed before and within 72 hours after discharge following administration of medication instructions. Bivariate analyses were performed to assess demographic and outcome variable differences between groups.
Setting and participants: Conducted on a 24-bed medical-surgical unit at a large Magnet® hospital over 18 months (2018-2019), the study included patients with at least 1 new medication, aged 18 years or older, able to read and speak English or Spanish, admitted from home with a minimum 1 overnight stay, and planning to return home post discharge. Excluded were cognitively impaired patients, those assigned to a resource pool nurse without TIME training, and those having a research team member assigned. Participants were randomly selected from a computerized list of patients scheduled for discharge.
Main outcome measures: Primary outcome measures included patients’ new medication knowledge before and after discharge and patients’ experience and satisfaction with medication education.
Main results: The usual care (n = 52) and TIME (n = 55) patients had similar baseline demographic characteristics. The study revealed that almost all patients in both usual care and TIME groups were aware of their new medication and its purpose at discharge. However, differences were observed in medication side effect responses, with 72.5% of the usual-care group knowing side effects compared to 94.3% of the TIME group (P = .003). Additionally, 81.5% of the usual-care group understood the medication purpose compared to 100% of the TIME group (P = .02). During the 48- to 72-hour postdischarge calls, consistent responses were found from both groups regarding knowledge of new medication, medication name, and medication purpose. Similar to discharge results, differences in medication side effect responses were observed, with 75.8% of the usual care group correctly identifying at least 1 medication side effect compared to 93.9% of the TIME group (P = .04). TIME was associated with higher satisfaction with medication education compared to usual care (97% vs. 46.9%, P < .001).
Conclusion: The nurse-led discharge medication education program TIME effectively enhanced patients’ new medication knowledge at discharge and 48 to 72 hours after discharge. The program also significantly improved patients’ experience and satisfaction with medication education. These findings indicate that TIME is a valuable tool for augmenting patient education and medication adherence in a hospital setting. By incorporating the teach-back method, TIME offers a structured approach to educating patients about their medications at hospital discharge, leading to improved care transitions.
Commentary
Suboptimal communication between patients, caregivers, and providers upon hospital discharge is a major contributor to patients’ inadequate understanding of postdischarge care plans. This inadequate understanding leads to preventable harms, such as medication errors, adverse events, emergency room visits, and costly hospital readmissions.1 The issue is further exacerbated by a lack of clarity among health care team members’ respective roles in providing information that optimizes care transitions during the discharge communication process. Moreover, low health literacy, particularly prevalent among seniors, those from disadvantaged backgrouds, and those with lower education attainment or chronic illnesses, create additional barriers to effective discharge communication. A potential solution to this problem is the adoption of effective teaching strategies, specifically the teach-back method. This method employs techniques that ensure patients’ understanding and recall of new information regardless of health literacy, and places accountability on clinicians rather than patients. By closing communication gaps between clinicians and patients, the teach-back method can reduce hospital readmissions, hospital-acquired conditions, and mortality rates, while improving patient satisfaction with health care instructions and the overall hospital experience.2
Study 1, by Trivedi et al, and study 2, by Marks et al, aimed to identify and address problems related to poor communication between patients and health care team members at hospital discharge. Specifically, study 1 examined routine discharge communication practices to determine communication gaps, while study 2 evaluated a nurse-led teach-back intervention program designed to improve patients’ medication knowledge and satisfaction. These distinct objectives and designs reflected the unique ways each study approached the challenges associated with care transitions at the time of hospital discharge.
Study 1 used direct observation of patient-practitioner interactions to evaluate routine discharge communication practices in internal medicine services at 2 urban academic teaching hospitals. In the 33 patients observed, significant gaps in discharge communication practices were identified in the domains of medication changes, postdischarge appointments, disease self-management, and red flags or warning signs. Unsurprisingly, most of these domains were communicated in an ad hoc manner by members of the health care team without a clear pattern of responsibility in reference to patient discharge education, and teach-back was seldom used. These findings underscore the need for improved discharge techniques, effective communication strategies, and clarification of roles among the interprofessional team to enhance the safety, quality of care, and overall patient experience during hospital discharge.
Study 2 aimed to augment the hospital discharge communication process by implementing a nurse-led discharge medication education program (TIME), which targeted patients’ priority learning needs, new medication knowledge, and satisfaction with medication education. In the 107 patients assessed, this teach-back method enhanced patients’ new medication knowledge at discharge and 48 to 72 hours after discharge, as well as improved patients’ experience and satisfaction with medication education. These results suggest that a teach-back method such as the TIME program could be a solution to care transition problems identified in the Trivedi et al study by providing a structured approach to patient education and enhancing communication practices during the hospital discharge process. Thus, by implementing the TIME program, hospitals may improve patient outcomes, safety, and overall quality of care upon leaving the hospital.
Applications for Clinical Practice and System Implementation
Care transition at the time of hospital discharge is a particularly pivotal period in the care of vulnerable individuals. There is growing literature, including studies discussed in this review, to indicate that by focusing on improving patient-practitioner communication during the discharge process and using strategies such as the teach-back method, health care professionals can better prepare patients for self-management in the post-acute period and help them make informed decisions about their care. This emphasis on care-transition communication strategies may lead to a reduction in medication errors, adverse events, and hospital readmissions, ultimately improving patient outcomes and satisfaction. Barriers to system implementation of such strategies may include competing demands and responsibilities of busy practitioners as well as the inherent complexities associated with hospital discharge. Creative solutions, such as the utilization of telehealth and early transition-of-care visits, represent some potential approaches to counter these barriers.
While both studies illustrated barriers and facilitators of hospital discharge communication, each study had limitations that impacted their generalizability to real-world clinical practice. Limitations in study 1 included a small sample size, purposive sampling method, and a focus on planned discharges in a teaching hospital, which may introduce selection bias. The study’s findings may not be generalizable to unplanned discharges, patients who do not speak English or Spanish, or nonteaching hospitals. Additionally, the data were collected before the COVID-19 pandemic, which could have further impacted discharge education practices. The study also revealed that some patients received more robust discharge education than others, which indicated systemic inequality in the patient experience. Further research is required to address this discrepancy. Limitations in study 2 included a relatively small and homogeneous sample, with most participants being younger, non-Hispanic White, English-speaking, and well-educated. This lack of diversity may limit the generalizability of the findings. Furthermore, the study did not evaluate the patients’ knowledge of medication dosage and focused only on new medications. Future studies should examine the effect of teach-back on a broader range of self-management topics in preparation for discharge, while also including a more diverse population to account for factors related to social determinants of health. Taken together, further research is needed to address these limitations and ensure more generalizable results that can more broadly improve discharge education and care transitions that bridge acute and post-acute care.
Practice Points
- There is a significant need for improved discharge strategies to enhance patient safety and quality of care upon leaving the hospital.
- Teach-back method may offer a structured approach to educating patients about their medications at hospital discharge and improve care transitions.
–Yuka Shichijo, MD, and Fred Ko, MD, Mount Sinai Beth Israel Hospital, New York, NY
Study 1 Overview (Trivedi et al)
Objective: This observational quality improvement study aimed to evaluate the discharge communication practices in internal medicine services at 2 urban academic teaching hospitals, specifically focusing on patient education and counseling in 6 key discharge communication domains.
Design: Observations were conducted over a 13-month period from September 2018 through October 2019, following the Standards for Quality Improvement Reporting Excellence (SQUIRE) guidelines.
Setting and participants: The study involved a total of 33 English- and Spanish-speaking patients purposefully selected from the “discharge before noon” list at 2 urban tertiary-care teaching hospitals. A total of 155 observation hours were accumulated, with an average observation time of 4.7 hours per patient on the day of discharge.
Main outcome measures: The study assessed 6 discharge communication domains: (1) the name and function of medication changes, (2) the purpose of postdischarge appointments, (3) disease self-management, (4) red flags or warning signs for complications, (5) teach-back techniques to confirm patient understanding, and (6) staff solicitation of patient questions or concerns.
Main results: The study found several gaps in discharge communication practices. Among the 29 patients with medication changes, 28% were not informed about the name and basic function of the changes, while 59% did not receive counseling on the purpose for the medication change. In terms of postdischarge appointments, 48% of patients were not told the purpose of these appointments. Moreover, 54% of patients did not receive counseling on self-management of their primary discharge diagnosis or other diagnoses, and 73% were not informed about symptom expectations or the expected course of their illness after leaving the hospital. Most patients (82%) were not counseled on red-flag signs and symptoms that should prompt immediate return to care.
Teach-back techniques, which are critical for ensuring patient understanding, were used in only 3% of cases, and 85% of patients were not asked by health care providers if there might be barriers to following the care plan. Less than half (42%) of the patients were asked if they had any questions, with most questions being logistical and often deferred to another team member or met with uncertainty. Of note, among the 33 patients, only 2 patients received extensive information that covered 5 or 6 out of 6 discharge communication domains.
The study found variable roles in who communicated what aspects of discharge education, with most domains being communicated in an ad hoc manner and no clear pattern of responsibility. However, 2 exceptions were observed: nurses were more likely to provide information about new or changed medications and follow-up appointments, and the only example of teach-back was conducted by an attending physician.
Conclusion: The study highlights a significant need for improved discharge techniques to enhance patient safety and quality of care upon leaving the hospital. Interventions should focus on increasing transparency in patient education and understanding, clarifying assumptions of roles among the interprofessional team, and implementing effective communication strategies and system redesigns that foster patient-centered discharge education. Also, the study revealed that some patients received more robust discharge education than others, indicating systemic inequality in the patient experience. Further studies are needed to explore the development and assessment of such interventions to ensure optimal patient outcomes and equal care following hospital discharge.
Study 2 Overview (Marks et al)
Objective: This study aimed to investigate the impact of a nurse-led discharge medication education program, Teaching Important Medication Effects (TIME), on patients’ new medication knowledge at discharge and 48 to 72 hours post discharge. The specific objectives were to identify patients’ priority learning needs, evaluate the influence of TIME on patients’ new medication knowledge before and after discharge, and assess the effect of TIME on patients’ experience and satisfaction with medication education.
Design: The study employed a longitudinal pretest/post-test, 2-group design involving 107 randomly selected medical-surgical patients from an academic hospital. Participants were interviewed before and within 72 hours after discharge following administration of medication instructions. Bivariate analyses were performed to assess demographic and outcome variable differences between groups.
Setting and participants: Conducted on a 24-bed medical-surgical unit at a large Magnet® hospital over 18 months (2018-2019), the study included patients with at least 1 new medication, aged 18 years or older, able to read and speak English or Spanish, admitted from home with a minimum 1 overnight stay, and planning to return home post discharge. Excluded were cognitively impaired patients, those assigned to a resource pool nurse without TIME training, and those having a research team member assigned. Participants were randomly selected from a computerized list of patients scheduled for discharge.
Main outcome measures: Primary outcome measures included patients’ new medication knowledge before and after discharge and patients’ experience and satisfaction with medication education.
Main results: The usual care (n = 52) and TIME (n = 55) patients had similar baseline demographic characteristics. The study revealed that almost all patients in both usual care and TIME groups were aware of their new medication and its purpose at discharge. However, differences were observed in medication side effect responses, with 72.5% of the usual-care group knowing side effects compared to 94.3% of the TIME group (P = .003). Additionally, 81.5% of the usual-care group understood the medication purpose compared to 100% of the TIME group (P = .02). During the 48- to 72-hour postdischarge calls, consistent responses were found from both groups regarding knowledge of new medication, medication name, and medication purpose. Similar to discharge results, differences in medication side effect responses were observed, with 75.8% of the usual care group correctly identifying at least 1 medication side effect compared to 93.9% of the TIME group (P = .04). TIME was associated with higher satisfaction with medication education compared to usual care (97% vs. 46.9%, P < .001).
Conclusion: The nurse-led discharge medication education program TIME effectively enhanced patients’ new medication knowledge at discharge and 48 to 72 hours after discharge. The program also significantly improved patients’ experience and satisfaction with medication education. These findings indicate that TIME is a valuable tool for augmenting patient education and medication adherence in a hospital setting. By incorporating the teach-back method, TIME offers a structured approach to educating patients about their medications at hospital discharge, leading to improved care transitions.
Commentary
Suboptimal communication between patients, caregivers, and providers upon hospital discharge is a major contributor to patients’ inadequate understanding of postdischarge care plans. This inadequate understanding leads to preventable harms, such as medication errors, adverse events, emergency room visits, and costly hospital readmissions.1 The issue is further exacerbated by a lack of clarity among health care team members’ respective roles in providing information that optimizes care transitions during the discharge communication process. Moreover, low health literacy, particularly prevalent among seniors, those from disadvantaged backgrouds, and those with lower education attainment or chronic illnesses, create additional barriers to effective discharge communication. A potential solution to this problem is the adoption of effective teaching strategies, specifically the teach-back method. This method employs techniques that ensure patients’ understanding and recall of new information regardless of health literacy, and places accountability on clinicians rather than patients. By closing communication gaps between clinicians and patients, the teach-back method can reduce hospital readmissions, hospital-acquired conditions, and mortality rates, while improving patient satisfaction with health care instructions and the overall hospital experience.2
Study 1, by Trivedi et al, and study 2, by Marks et al, aimed to identify and address problems related to poor communication between patients and health care team members at hospital discharge. Specifically, study 1 examined routine discharge communication practices to determine communication gaps, while study 2 evaluated a nurse-led teach-back intervention program designed to improve patients’ medication knowledge and satisfaction. These distinct objectives and designs reflected the unique ways each study approached the challenges associated with care transitions at the time of hospital discharge.
Study 1 used direct observation of patient-practitioner interactions to evaluate routine discharge communication practices in internal medicine services at 2 urban academic teaching hospitals. In the 33 patients observed, significant gaps in discharge communication practices were identified in the domains of medication changes, postdischarge appointments, disease self-management, and red flags or warning signs. Unsurprisingly, most of these domains were communicated in an ad hoc manner by members of the health care team without a clear pattern of responsibility in reference to patient discharge education, and teach-back was seldom used. These findings underscore the need for improved discharge techniques, effective communication strategies, and clarification of roles among the interprofessional team to enhance the safety, quality of care, and overall patient experience during hospital discharge.
Study 2 aimed to augment the hospital discharge communication process by implementing a nurse-led discharge medication education program (TIME), which targeted patients’ priority learning needs, new medication knowledge, and satisfaction with medication education. In the 107 patients assessed, this teach-back method enhanced patients’ new medication knowledge at discharge and 48 to 72 hours after discharge, as well as improved patients’ experience and satisfaction with medication education. These results suggest that a teach-back method such as the TIME program could be a solution to care transition problems identified in the Trivedi et al study by providing a structured approach to patient education and enhancing communication practices during the hospital discharge process. Thus, by implementing the TIME program, hospitals may improve patient outcomes, safety, and overall quality of care upon leaving the hospital.
Applications for Clinical Practice and System Implementation
Care transition at the time of hospital discharge is a particularly pivotal period in the care of vulnerable individuals. There is growing literature, including studies discussed in this review, to indicate that by focusing on improving patient-practitioner communication during the discharge process and using strategies such as the teach-back method, health care professionals can better prepare patients for self-management in the post-acute period and help them make informed decisions about their care. This emphasis on care-transition communication strategies may lead to a reduction in medication errors, adverse events, and hospital readmissions, ultimately improving patient outcomes and satisfaction. Barriers to system implementation of such strategies may include competing demands and responsibilities of busy practitioners as well as the inherent complexities associated with hospital discharge. Creative solutions, such as the utilization of telehealth and early transition-of-care visits, represent some potential approaches to counter these barriers.
While both studies illustrated barriers and facilitators of hospital discharge communication, each study had limitations that impacted their generalizability to real-world clinical practice. Limitations in study 1 included a small sample size, purposive sampling method, and a focus on planned discharges in a teaching hospital, which may introduce selection bias. The study’s findings may not be generalizable to unplanned discharges, patients who do not speak English or Spanish, or nonteaching hospitals. Additionally, the data were collected before the COVID-19 pandemic, which could have further impacted discharge education practices. The study also revealed that some patients received more robust discharge education than others, which indicated systemic inequality in the patient experience. Further research is required to address this discrepancy. Limitations in study 2 included a relatively small and homogeneous sample, with most participants being younger, non-Hispanic White, English-speaking, and well-educated. This lack of diversity may limit the generalizability of the findings. Furthermore, the study did not evaluate the patients’ knowledge of medication dosage and focused only on new medications. Future studies should examine the effect of teach-back on a broader range of self-management topics in preparation for discharge, while also including a more diverse population to account for factors related to social determinants of health. Taken together, further research is needed to address these limitations and ensure more generalizable results that can more broadly improve discharge education and care transitions that bridge acute and post-acute care.
Practice Points
- There is a significant need for improved discharge strategies to enhance patient safety and quality of care upon leaving the hospital.
- Teach-back method may offer a structured approach to educating patients about their medications at hospital discharge and improve care transitions.
–Yuka Shichijo, MD, and Fred Ko, MD, Mount Sinai Beth Israel Hospital, New York, NY
1. Snow V, Beck D, Budnitz T, Miller DC, Potter J, Wears RL, Weiss KB, Williams MV; American College of Physicians; Society of General Internal Medicine; Society of Hospital Medicine; American Geriatrics Society; American College of Emergency Physicians; Society of Academic Emergency Medicine. Transitions of care consensus policy statement American College of Physicians-Society of General Internal Medicine-Society of Hospital Medicine-American Geriatrics Society-American College of Emergency Physicians-Society of Academic Emergency Medicine. J Gen Intern Med. 2009;24(8):971-976. doi:10.1007/s11606-009-0969-x
2. Yen PH, Leasure AR. Use and effectiveness of the teach-back method in patient education and health outcomes. Fed. Pract. 2019;36(6):284-289.
1. Snow V, Beck D, Budnitz T, Miller DC, Potter J, Wears RL, Weiss KB, Williams MV; American College of Physicians; Society of General Internal Medicine; Society of Hospital Medicine; American Geriatrics Society; American College of Emergency Physicians; Society of Academic Emergency Medicine. Transitions of care consensus policy statement American College of Physicians-Society of General Internal Medicine-Society of Hospital Medicine-American Geriatrics Society-American College of Emergency Physicians-Society of Academic Emergency Medicine. J Gen Intern Med. 2009;24(8):971-976. doi:10.1007/s11606-009-0969-x
2. Yen PH, Leasure AR. Use and effectiveness of the teach-back method in patient education and health outcomes. Fed. Pract. 2019;36(6):284-289.
Meet the JCOM Author with Dr. Barkoudah: A Multidisciplinary Team–Based Clinical Care Pathway for Infective Endocarditis
Meet the JCOM Author with Dr. Barkoudah: Leading for High Reliability During the COVID-19 Pandemic
Implementation of a Multidisciplinary Team–Based Clinical Care Pathway Is Associated With Increased Surgery Rates for Infective Endocarditis
From the University of Missouri School of Medicine, Columbia, MO (Haley Crosby); Department of Clinical Family and Community Medicine, University of Missouri, Columbia, MO (Dr. Pierce); and Department of Medicine, Divisions of Infectious Diseases and Pulmonary, Critical Care and Environmental Medicine, University of Missouri, Columbia, MO, and Divisions of Pulmonary and Critical Care Medicine and Infectious Diseases, University of Maryland Baltimore Washington Medical Center, Glen Burnie, MD (Dr. Regunath).
ABSTRACT
Objective: Multidisciplinary teams (MDTs) improve outcomes for patients with infective endocarditis (IE), but methods of implementation vary. In our academic medical center, we developed an MDT approach guided by a clinical care pathway and assessed outcomes of patients with IE.
Methods: We compared outcomes of patients with IE and indications for surgery between December 2018 and June 2020 with our prior published data for the period January to December 2016. MDT interventions involved recurring conferences with infectious diseases physicians in team meetings and promoting a clinical care pathway to guide providers on steps in management. Primary outcomes were surgery and in-hospital death.
Results: Prior to the intervention, 6 of 21 (28.6%) patients with indications for surgery underwent surgery or were transferred to higher centers for surgery, and 6 (28.6%) patients died. Post intervention, 17 of 31 (54.8%) patients underwent or were transferred for surgery, and 5 (16.1%) died. After adjusting for age and gender, the odds of surgery or transfer for surgery for patients in the postintervention period were 4.88 (95% CI, 1.20-19.79; P = .027) compared with the pre-intervention period. The odds ratio for death among patients in the postintervention period was 0.40 (95% CI, 0.09-1.69; P = .21).
Conclusion: An MDT team approach using a clinical pathway was associated with an increased number of surgeries performed for IE and may lower rates of in-hospital mortality.
Keywords: infective endocarditis, clinical pathway, quality improvement, multidisciplinary team, valve surgery.
Infective endocarditis (IE) is associated with significant morbidity and mortality.1 Rates of IE due to Staphylococcus aureus are increasing in the United States.2 Reported in-hospital mortality from IE ranges from 15% to 20%.3
Clinical pathways are defined as “structured, multidisciplinary plans of care used by health services to detail essential steps in the care of patients with a specific clinical problem.”12 In the modern era, these pathways are often developed and implemented via the electronic health record (EHR) system. Studies of clinical pathways generally demonstrate improvements in patient outcomes, quality of care, or resource utilization.13,14 Clinical pathways represent 1 possible approach to the implementation of a MDT in the care of patients with IE.15
In our earlier work, we used quality improvement principles in the design of an MDT approach to IE care at our institution.16 Despite having indications for surgery, 12 of 21 (57.1%) patients with IE did not undergo surgery, and we identified these missed opportunities for surgery as a leverage point for improvement of outcomes. With input from the various specialties and stakeholders, we developed a clinical pathway (algorithm) for the institutional management of IE that guides next steps in clinical care and their timelines, aiming to reduce by 50% (from 57.1% to 28.6%) the number of patients with IE who do not undergo surgery despite guideline indications for early surgical intervention. In this report, we describe the implementation of this clinical pathway as our MDT approach to the care of patients with IE at our institution.
Methods
The University of Missouri, Columbia, is a tertiary care academic health system with 5 hospitals and more than 60 clinic locations across central Missouri. In the spring of 2018, an MDT was developed, with support from administrative leaders, to improve the care of patients with IE at our institution. The work group prioritized one leverage point to improve IE outcomes, which was improving the number of surgeries performed on those IE patients who had guideline indications for surgery. A clinical pathway was developed around this leverage point (Figure 1). The pathway leveraged the 6 T’s (Table 1) to guide providers through the evaluation and management of IE.17 The pathway focused on improving adherence to standards of care and reduction in practice variation by defining indications for referrals and diagnostic interventions, helping to reduce delays in consultation and diagnosis. The pathway also clearly outlined the surgical indications and timing for patients with IE and provided the basis for decisions to proceed with surgery.

Starting in late 2018, in collaboration with cardiology and CTS teams, ID specialists socialized the clinical pathway to inpatient services that cared for patients with IE. Infectious diseases physicians also provided recurring conferences on the effectiveness of MDTs in IE management and participated in heart-valve team case discussions. Finally, in May 2019, an electronic version of the pathway was embedded in the EHR system using a Cerner PowerChart feature known as Care Pathways. The feature presents the user with algorithm questions in the EHR and provides recommendations, relevant orders, timelines, and other decision support in the clinical pathway. The feature is available to all providers in the health system.

To evaluate the effectiveness of our intervention, we recorded outcomes for patients with IE with surgical indications between December 2018 and June 2020 and compared them with our prior published data from January to December 2016. Cases of IE for the current study period were identified via retrospective chart review. Records from December 2018 to June 2020 were searched using International Statistical Classification of Diseases, Tenth Revision (ICD-10) discharge codes for IE (I33, I33.0, I33.9, I38, I39, M32.11). To select those patients with definitive IE and indications for surgery, the following criteria were applied: age ≥ 18 years; fulfilled modified Duke criteria for definite IE18; and met ≥ 1 American Heart Association (AHA)/Infection Diseases Society of America criteria for recommendation for surgery. Indications for surgery were ≥ 1 of the following: left-sided endocarditis caused by S aureus, fungal, or highly resistant organism; new heart block; annular or aortic abscess; persistent bacteremia or fever despite 5 days of appropriate antimicrobials; vegetation size ≥ 10 mm and evidence of embolic phenomena; recurrence of prosthetic valve infection; recurrent emboli and persistent vegetation despite antimicrobials; and increase in vegetation size despite antimicrobials.16
Age was treated as a categorical variable, using the age groups 18 to 44 years, 45 to 64 years, and 65 years and older. Gender was self-reported. Primary outcomes were surgery or transfer to a higher center for surgery and in-hospital death. Secondary outcomes included consults to teams involved in multidisciplinary care of patients with IE, including ID, cardiology, and CTS. Bivariate analyses were performed using Pearson χ2 tests. Odds ratios for surgery and death were calculated using a multivariate logistic regression model including age and gender covariates. Statistical significance was defined at α = 0.05, and statistical analysis was performed using Stata/IC v16.1 (StataCorp LLC). Our university institutional review board (IRB) reviewed the project (#2010858-QI) and determined that the project was quality-improvement activity, not human subject research, and therefore did not require additional IRB review.
Results
We identified 21 patients in the pre-intervention period and 31 patients in the postintervention period with definitive IE who had guideline indications for surgery. The postintervention cohort was older and had more male patients; this difference was not statistically significant. No differences were noted between the groups for race, gender, or intravenous (IV) drug use (Table 2). Chi-square tests of independence were performed to assess the relationship between age and our primary outcomes. There was a significant relationship between age and the likelihood of receiving or being transferred for surgery (59.3% vs 50% vs 7.7% for 18-44 y, 45-64 y, and ≥ 65 y, respectively; χ2 [2, N = 52] = 9.67; P = .008), but not between age and mortality (14.8% vs 25.0% vs 30.8% for 18-44 y, 45-64 y, and ≥ 65 y, respectively; χ2 = 1.48 [2, N = 52; P = .478]. The electronic version of the clinical pathway was activated and used in only 3 of the 31 patients in the postintervention period. Consultations to ID, cardiology, and CTS teams were compared between the study periods (Table 2). Although more consultations were seen in the postintervention period, differences were not statistically significant.

The unadjusted primary outcomes are shown in Table 2. More surgeries were performed per guideline indications, and fewer deaths were noted in the postintervention period than in the pre-intervention period, but the differences were not statistically significant (Table 2).
Because the postintervention period had more male patients and older patients, we evaluated the outcomes using a logistic regression model controlling for both age and gender. The odds of surgery or transfer for surgery for patients in the postintervention period were 4.88 (95% CI, 1.20-19.79; P = .027) as compared with the pre-intervention period, and the odds ratio for death among patients in the postintervention period compared with the pre-intervention period was 0.40 (95% CI, 0.09-1.69; P = .21) (Figure 2).

Discussion
In our study, patients with IE with guideline indications for surgery had significantly higher rates of surgery in the postintervention period than in the pre-intervention period. The implementation of an MDT, recurring educational sessions, and efforts to implement and familiarize team members with the clinical pathway approach are the most likely reasons for this change. The increased rates of surgery in the postintervention period were the likely proximate cause of the 60% reduction in in-hospital mortality. This improvement in mortality, while not statistically significant, is very likely to be clinically significant and helps reinforce the value of the MDT intervention used.
Our findings are consistent with existing and mounting literature on the use of MDTs to improve outcomes for patients with IE, including 2 studies that noted an increased rate of surgery for patients with indications.8,19 Several other studies in both Europe and North America have found significant decreases in mortality,6-11,20,21 rates of complications,9 time to diagnosis and treatment,11 and length of stay9,20 for patients with IE managed with an MDT strategy. Although current AHA guidelines for care of patients with IE do suggest an MDT approach, the strategy for this approach is not well established.22 Only 1 study that has implemented a new MDT protocol for care of IE has been conducted in the United States.8
While effective MDTs certainly improve outcomes in patients with IE, there are reported differences in implementation of such an approach. With the MDT model as the core, various implementations included regular case conferences,10,11,19,21,23 formation of a consulting team,6,8 or establishment of a new protocol or algorithm for care.8,9,20 Our approach used a clinical pathway as a basis for improved communication among consulting services, education of learning providers via regular case conferences, and implementation of an electronic clinical care pathway to guide them step by step. Our pathway followed the institutionally standardized algorithm (Figure 1), using what we called the 6 T’s approach (Table 1), that guides providers to evaluate critical cases in a fast track.17
To the best of our knowledge, ours is the first report of an MDT that used an electronic clinical care pathway embedded within the EHR. The electronic version of our clinical pathway went live for only the second half of the postintervention study period, which is the most likely reason for its limited utilization. It is also possible that educational efforts in the first half of the intervention period were sufficient to familiarize providers with the care pathway such that the electronic version was seldom needed. We are exploring other possible ways of improving electronic pathway utilization, such as improving the feature usability and further systemwide educational efforts.
Our study has other limitations. Quasi-experimental before-and-after comparisons are subject to confounding from concurrent interventions. We had a substantial change in cardiothoracic faculty soon after the commencement of our efforts to form the MDT, and thus cannot rule out differences related to their comfort level in considering or offering surgery. We also cannot rule out a Hawthorne effect, where knowledge of the ongoing quality-improvement project changed provider behavior, making surgery more likely. We did not evaluate rates of right- versus left-sided endocarditis, which have been linked to mortality.24 Our study also was performed across a single academic institution, which may limit its generalizability. Finally, our study may not have been adequately powered to detect differences in mortality due to implementation of the MDT approach.
Conclusion
Our work suggests that an MDT for IE can be successfully designed and implemented with a clinical pathway using quality-improvement tools in centers where subspecialty services are available. Our approach was associated with a higher rate of surgery among patients with guideline indications for surgery and may reduce in-hospital mortality. An electronic clinical care pathway embedded in the EHR is feasible and may have a role in MDT implementation.
These data were also accepted as a poster at IDWeek 2022, Washington, DC. The poster abstract is published in an online supplement of Open Forum Infectious Diseases as an abstract publication.
Corresponding author: Haley Crosby; hwc2pd@health.missouri.edu
Disclosures: None reported.
1. Baddour LM, Wilson WR, Bayer AS, et al. Infective endocarditis in adults: diagnosis, antimicrobial therapy, and management of complications: a scientific statement for healthcare professionals from the American Heart Association. Circulation. 2015;132(15):1435-1486. doi:10.1161/cir.0000000000000296
2. Federspiel JJ, Stearns SC, Peppercorn AF, et al. Increasing US rates of endocarditis with Staphylococcus aureus: 1999-2008. Arch Intern Med. 2012;172(4):363-365. doi:10.1001/archinternmed.2011.1027
3. Nishimura RA, Otto CM, Bonow RO, et al. 2014 AHA/ACC Guideline for the Management of Patients With Valvular Heart Disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129(23):e521-e643. doi:10.1161/cir.0000000000000031
4. Chambers J, Sandoe J, Ray S, et al. The infective endocarditis team: recommendations from an international working group. Heart. 2014;100(7):524-527. doi:10.1136/heartjnl-2013-304354
5. Habib G, Lancellotti P, Antunes MJ, et al. 2015 ESC Guidelines for the management of infective endocarditis: The Task Force for the Management of Infective Endocarditis of the European Society of Cardiology (ESC). Endorsed by: European Association for Cardio-Thoracic Surgery (EACTS), the European Association of Nuclear Medicine (EANM). Eur Heart J. 2015;36(44):3075-3128. doi:10.1093/eurheartj/ehv319
6. Chirillo F, Scotton P, Rocco F, et al. Impact of a multidisciplinary management strategy on the outcome of patients with native valve infective endocarditis. Am J Cardiol. 2013;112(8):1171-1176. doi:10.1016/j.amjcard.2013.05.060
7. Botelho-Nevers E, Thuny F, Casalta JP, et al. Dramatic reduction in infective endocarditis-related mortality with a management-based approach. Arch Intern Med. 2009;169(14):1290-1298. doi:10.1001/archinternmed.2009.192
8. El-Dalati S, Cronin D, Riddell IV J, et al. The clinical impact of implementation of a multidisciplinary endocarditis team. Ann Thorac Surg. 2022;113(1):118-124.
9. Carrasco-Chinchilla F, Sánchez-Espín G, Ruiz-Morales J, et al. Influence of a multidisciplinary alert strategy on mortality due to left-sided infective endocarditis. Rev Esp Cardiol (Engl Ed). 2014;67(5):380-386. doi:10.1016/j.rec.2013.09.010
10. Issa N, Dijos M, Greib C, et al. Impact of an endocarditis team in the management of 357 infective endocarditis [abstract]. Open Forum Infect Dis. 2016;3(suppl 1):S201. doi:10.1093/ofid/ofw172.825
11. Kaura A, Byrne J, Fife A, et al. Inception of the ‘endocarditis team’ is associated with improved survival in patients with infective endocarditis who are managed medically: findings from a before-and-after study. Open Heart. 2017;4(2):e000699. doi:10.1136/openhrt-2017-000699
12. Rotter T, Kinsman L, James E, et al. Clinical pathways: effects on professional practice, patient outcomes, length of stay and hospital costs. Cochrane Database Syst Rev. 2010;(3):Cd006632. doi:10.1002/14651858.CD006632.pub2
13. Neame MT, Chacko J, Surace AE, et al. A systematic review of the effects of implementing clinical pathways supported by health information technologies. J Am Med Inform Assoc. 2019;26(4):356-363. doi:10.1093/jamia/ocy176
14. Trimarchi L, Caruso R, Magon G, et al. Clinical pathways and patient-related outcomes in hospital-based settings: a systematic review and meta-analysis of randomized controlled trials. Acta Biomed. 2021;92(1):e2021093. doi:10.23750/abm.v92i1.10639
15. Gibbons EF, Huang G, Aldea G, et al. A multidisciplinary pathway for the diagnosis and treatment of infectious endocarditis. Crit Pathw Cardiol. 2020;19(4):187-194. doi:10.1097/hpc.0000000000000224
16. Regunath H, Vasudevan A, Vyas K, et al. A quality improvement initiative: developing a multi-disciplinary team for infective endocarditis. Mo Med. 2019;116(4):291-296.
17. Regunath H, Whitt SP. Multidisciplinary service delivery for the endocarditis patient. In: Infective Endocarditis: A Multidisciplinary Approach. 1st ed. Kilic A, ed. Academic Press; 2022.
18. Durack DT, Lukes AS, Bright DK. New criteria for diagnosis of infective endocarditis: utilization of specific echocardiographic findings. Duke Endocarditis Service. Am J Med. 1994;96(3):200-209. doi:10.1016/0002-9343(94)90143-0
19. Tan C, Hansen MS, Cohen G, et al. Case conferences for infective endocarditis: a quality improvement initiative. PLoS One. 2018;13(10):e0205528. doi:10.1371/journal.pone.0205528
20. Ruch Y, Mazzucotelli JP, Lefebvre F, et al. Impact of setting up an “endocarditis team” on the management of infective endocarditis. Open Forum Infect Dis. 2019;6(9):ofz308. doi:10.1093/ofid/ofz308
21. Camou F, Dijos M, Barandon L, et al. Management of infective endocarditis and multidisciplinary approach. Med Mal Infect. 2019;49(1):17-22. doi:10.1016/j.medmal.2018.06.007
22. Pettersson GB, Hussain ST. Current AATS guidelines on surgical treatment of infective endocarditis. Ann Cardiothorac Surg. 2019;8(6):630-644. doi:10.21037/acs.2019.10.05
23. Mestres CA, Paré JC, Miró JM. Organization and functioning of a multidisciplinary team for the diagnosis and treatment of infective endocarditis: a 30-year perspective (1985-2014). Rev Esp Cardiol (Engl Ed). 2015;68(5):363-368. doi:10.1016/j.rec.2014.10.006
24. Stavi V, Brandstaetter E, Sagy I, et al. Comparison of clinical characteristics and prognosis in patients with right- and left-sided infective endocarditis. Rambam Maimonides Med J. 2019;10(1):e00003. doi:10.5041/rmmj.10338
From the University of Missouri School of Medicine, Columbia, MO (Haley Crosby); Department of Clinical Family and Community Medicine, University of Missouri, Columbia, MO (Dr. Pierce); and Department of Medicine, Divisions of Infectious Diseases and Pulmonary, Critical Care and Environmental Medicine, University of Missouri, Columbia, MO, and Divisions of Pulmonary and Critical Care Medicine and Infectious Diseases, University of Maryland Baltimore Washington Medical Center, Glen Burnie, MD (Dr. Regunath).
ABSTRACT
Objective: Multidisciplinary teams (MDTs) improve outcomes for patients with infective endocarditis (IE), but methods of implementation vary. In our academic medical center, we developed an MDT approach guided by a clinical care pathway and assessed outcomes of patients with IE.
Methods: We compared outcomes of patients with IE and indications for surgery between December 2018 and June 2020 with our prior published data for the period January to December 2016. MDT interventions involved recurring conferences with infectious diseases physicians in team meetings and promoting a clinical care pathway to guide providers on steps in management. Primary outcomes were surgery and in-hospital death.
Results: Prior to the intervention, 6 of 21 (28.6%) patients with indications for surgery underwent surgery or were transferred to higher centers for surgery, and 6 (28.6%) patients died. Post intervention, 17 of 31 (54.8%) patients underwent or were transferred for surgery, and 5 (16.1%) died. After adjusting for age and gender, the odds of surgery or transfer for surgery for patients in the postintervention period were 4.88 (95% CI, 1.20-19.79; P = .027) compared with the pre-intervention period. The odds ratio for death among patients in the postintervention period was 0.40 (95% CI, 0.09-1.69; P = .21).
Conclusion: An MDT team approach using a clinical pathway was associated with an increased number of surgeries performed for IE and may lower rates of in-hospital mortality.
Keywords: infective endocarditis, clinical pathway, quality improvement, multidisciplinary team, valve surgery.
Infective endocarditis (IE) is associated with significant morbidity and mortality.1 Rates of IE due to Staphylococcus aureus are increasing in the United States.2 Reported in-hospital mortality from IE ranges from 15% to 20%.3
Clinical pathways are defined as “structured, multidisciplinary plans of care used by health services to detail essential steps in the care of patients with a specific clinical problem.”12 In the modern era, these pathways are often developed and implemented via the electronic health record (EHR) system. Studies of clinical pathways generally demonstrate improvements in patient outcomes, quality of care, or resource utilization.13,14 Clinical pathways represent 1 possible approach to the implementation of a MDT in the care of patients with IE.15
In our earlier work, we used quality improvement principles in the design of an MDT approach to IE care at our institution.16 Despite having indications for surgery, 12 of 21 (57.1%) patients with IE did not undergo surgery, and we identified these missed opportunities for surgery as a leverage point for improvement of outcomes. With input from the various specialties and stakeholders, we developed a clinical pathway (algorithm) for the institutional management of IE that guides next steps in clinical care and their timelines, aiming to reduce by 50% (from 57.1% to 28.6%) the number of patients with IE who do not undergo surgery despite guideline indications for early surgical intervention. In this report, we describe the implementation of this clinical pathway as our MDT approach to the care of patients with IE at our institution.
Methods
The University of Missouri, Columbia, is a tertiary care academic health system with 5 hospitals and more than 60 clinic locations across central Missouri. In the spring of 2018, an MDT was developed, with support from administrative leaders, to improve the care of patients with IE at our institution. The work group prioritized one leverage point to improve IE outcomes, which was improving the number of surgeries performed on those IE patients who had guideline indications for surgery. A clinical pathway was developed around this leverage point (Figure 1). The pathway leveraged the 6 T’s (Table 1) to guide providers through the evaluation and management of IE.17 The pathway focused on improving adherence to standards of care and reduction in practice variation by defining indications for referrals and diagnostic interventions, helping to reduce delays in consultation and diagnosis. The pathway also clearly outlined the surgical indications and timing for patients with IE and provided the basis for decisions to proceed with surgery.

Starting in late 2018, in collaboration with cardiology and CTS teams, ID specialists socialized the clinical pathway to inpatient services that cared for patients with IE. Infectious diseases physicians also provided recurring conferences on the effectiveness of MDTs in IE management and participated in heart-valve team case discussions. Finally, in May 2019, an electronic version of the pathway was embedded in the EHR system using a Cerner PowerChart feature known as Care Pathways. The feature presents the user with algorithm questions in the EHR and provides recommendations, relevant orders, timelines, and other decision support in the clinical pathway. The feature is available to all providers in the health system.

To evaluate the effectiveness of our intervention, we recorded outcomes for patients with IE with surgical indications between December 2018 and June 2020 and compared them with our prior published data from January to December 2016. Cases of IE for the current study period were identified via retrospective chart review. Records from December 2018 to June 2020 were searched using International Statistical Classification of Diseases, Tenth Revision (ICD-10) discharge codes for IE (I33, I33.0, I33.9, I38, I39, M32.11). To select those patients with definitive IE and indications for surgery, the following criteria were applied: age ≥ 18 years; fulfilled modified Duke criteria for definite IE18; and met ≥ 1 American Heart Association (AHA)/Infection Diseases Society of America criteria for recommendation for surgery. Indications for surgery were ≥ 1 of the following: left-sided endocarditis caused by S aureus, fungal, or highly resistant organism; new heart block; annular or aortic abscess; persistent bacteremia or fever despite 5 days of appropriate antimicrobials; vegetation size ≥ 10 mm and evidence of embolic phenomena; recurrence of prosthetic valve infection; recurrent emboli and persistent vegetation despite antimicrobials; and increase in vegetation size despite antimicrobials.16
Age was treated as a categorical variable, using the age groups 18 to 44 years, 45 to 64 years, and 65 years and older. Gender was self-reported. Primary outcomes were surgery or transfer to a higher center for surgery and in-hospital death. Secondary outcomes included consults to teams involved in multidisciplinary care of patients with IE, including ID, cardiology, and CTS. Bivariate analyses were performed using Pearson χ2 tests. Odds ratios for surgery and death were calculated using a multivariate logistic regression model including age and gender covariates. Statistical significance was defined at α = 0.05, and statistical analysis was performed using Stata/IC v16.1 (StataCorp LLC). Our university institutional review board (IRB) reviewed the project (#2010858-QI) and determined that the project was quality-improvement activity, not human subject research, and therefore did not require additional IRB review.
Results
We identified 21 patients in the pre-intervention period and 31 patients in the postintervention period with definitive IE who had guideline indications for surgery. The postintervention cohort was older and had more male patients; this difference was not statistically significant. No differences were noted between the groups for race, gender, or intravenous (IV) drug use (Table 2). Chi-square tests of independence were performed to assess the relationship between age and our primary outcomes. There was a significant relationship between age and the likelihood of receiving or being transferred for surgery (59.3% vs 50% vs 7.7% for 18-44 y, 45-64 y, and ≥ 65 y, respectively; χ2 [2, N = 52] = 9.67; P = .008), but not between age and mortality (14.8% vs 25.0% vs 30.8% for 18-44 y, 45-64 y, and ≥ 65 y, respectively; χ2 = 1.48 [2, N = 52; P = .478]. The electronic version of the clinical pathway was activated and used in only 3 of the 31 patients in the postintervention period. Consultations to ID, cardiology, and CTS teams were compared between the study periods (Table 2). Although more consultations were seen in the postintervention period, differences were not statistically significant.

The unadjusted primary outcomes are shown in Table 2. More surgeries were performed per guideline indications, and fewer deaths were noted in the postintervention period than in the pre-intervention period, but the differences were not statistically significant (Table 2).
Because the postintervention period had more male patients and older patients, we evaluated the outcomes using a logistic regression model controlling for both age and gender. The odds of surgery or transfer for surgery for patients in the postintervention period were 4.88 (95% CI, 1.20-19.79; P = .027) as compared with the pre-intervention period, and the odds ratio for death among patients in the postintervention period compared with the pre-intervention period was 0.40 (95% CI, 0.09-1.69; P = .21) (Figure 2).

Discussion
In our study, patients with IE with guideline indications for surgery had significantly higher rates of surgery in the postintervention period than in the pre-intervention period. The implementation of an MDT, recurring educational sessions, and efforts to implement and familiarize team members with the clinical pathway approach are the most likely reasons for this change. The increased rates of surgery in the postintervention period were the likely proximate cause of the 60% reduction in in-hospital mortality. This improvement in mortality, while not statistically significant, is very likely to be clinically significant and helps reinforce the value of the MDT intervention used.
Our findings are consistent with existing and mounting literature on the use of MDTs to improve outcomes for patients with IE, including 2 studies that noted an increased rate of surgery for patients with indications.8,19 Several other studies in both Europe and North America have found significant decreases in mortality,6-11,20,21 rates of complications,9 time to diagnosis and treatment,11 and length of stay9,20 for patients with IE managed with an MDT strategy. Although current AHA guidelines for care of patients with IE do suggest an MDT approach, the strategy for this approach is not well established.22 Only 1 study that has implemented a new MDT protocol for care of IE has been conducted in the United States.8
While effective MDTs certainly improve outcomes in patients with IE, there are reported differences in implementation of such an approach. With the MDT model as the core, various implementations included regular case conferences,10,11,19,21,23 formation of a consulting team,6,8 or establishment of a new protocol or algorithm for care.8,9,20 Our approach used a clinical pathway as a basis for improved communication among consulting services, education of learning providers via regular case conferences, and implementation of an electronic clinical care pathway to guide them step by step. Our pathway followed the institutionally standardized algorithm (Figure 1), using what we called the 6 T’s approach (Table 1), that guides providers to evaluate critical cases in a fast track.17
To the best of our knowledge, ours is the first report of an MDT that used an electronic clinical care pathway embedded within the EHR. The electronic version of our clinical pathway went live for only the second half of the postintervention study period, which is the most likely reason for its limited utilization. It is also possible that educational efforts in the first half of the intervention period were sufficient to familiarize providers with the care pathway such that the electronic version was seldom needed. We are exploring other possible ways of improving electronic pathway utilization, such as improving the feature usability and further systemwide educational efforts.
Our study has other limitations. Quasi-experimental before-and-after comparisons are subject to confounding from concurrent interventions. We had a substantial change in cardiothoracic faculty soon after the commencement of our efforts to form the MDT, and thus cannot rule out differences related to their comfort level in considering or offering surgery. We also cannot rule out a Hawthorne effect, where knowledge of the ongoing quality-improvement project changed provider behavior, making surgery more likely. We did not evaluate rates of right- versus left-sided endocarditis, which have been linked to mortality.24 Our study also was performed across a single academic institution, which may limit its generalizability. Finally, our study may not have been adequately powered to detect differences in mortality due to implementation of the MDT approach.
Conclusion
Our work suggests that an MDT for IE can be successfully designed and implemented with a clinical pathway using quality-improvement tools in centers where subspecialty services are available. Our approach was associated with a higher rate of surgery among patients with guideline indications for surgery and may reduce in-hospital mortality. An electronic clinical care pathway embedded in the EHR is feasible and may have a role in MDT implementation.
These data were also accepted as a poster at IDWeek 2022, Washington, DC. The poster abstract is published in an online supplement of Open Forum Infectious Diseases as an abstract publication.
Corresponding author: Haley Crosby; hwc2pd@health.missouri.edu
Disclosures: None reported.
From the University of Missouri School of Medicine, Columbia, MO (Haley Crosby); Department of Clinical Family and Community Medicine, University of Missouri, Columbia, MO (Dr. Pierce); and Department of Medicine, Divisions of Infectious Diseases and Pulmonary, Critical Care and Environmental Medicine, University of Missouri, Columbia, MO, and Divisions of Pulmonary and Critical Care Medicine and Infectious Diseases, University of Maryland Baltimore Washington Medical Center, Glen Burnie, MD (Dr. Regunath).
ABSTRACT
Objective: Multidisciplinary teams (MDTs) improve outcomes for patients with infective endocarditis (IE), but methods of implementation vary. In our academic medical center, we developed an MDT approach guided by a clinical care pathway and assessed outcomes of patients with IE.
Methods: We compared outcomes of patients with IE and indications for surgery between December 2018 and June 2020 with our prior published data for the period January to December 2016. MDT interventions involved recurring conferences with infectious diseases physicians in team meetings and promoting a clinical care pathway to guide providers on steps in management. Primary outcomes were surgery and in-hospital death.
Results: Prior to the intervention, 6 of 21 (28.6%) patients with indications for surgery underwent surgery or were transferred to higher centers for surgery, and 6 (28.6%) patients died. Post intervention, 17 of 31 (54.8%) patients underwent or were transferred for surgery, and 5 (16.1%) died. After adjusting for age and gender, the odds of surgery or transfer for surgery for patients in the postintervention period were 4.88 (95% CI, 1.20-19.79; P = .027) compared with the pre-intervention period. The odds ratio for death among patients in the postintervention period was 0.40 (95% CI, 0.09-1.69; P = .21).
Conclusion: An MDT team approach using a clinical pathway was associated with an increased number of surgeries performed for IE and may lower rates of in-hospital mortality.
Keywords: infective endocarditis, clinical pathway, quality improvement, multidisciplinary team, valve surgery.
Infective endocarditis (IE) is associated with significant morbidity and mortality.1 Rates of IE due to Staphylococcus aureus are increasing in the United States.2 Reported in-hospital mortality from IE ranges from 15% to 20%.3
Clinical pathways are defined as “structured, multidisciplinary plans of care used by health services to detail essential steps in the care of patients with a specific clinical problem.”12 In the modern era, these pathways are often developed and implemented via the electronic health record (EHR) system. Studies of clinical pathways generally demonstrate improvements in patient outcomes, quality of care, or resource utilization.13,14 Clinical pathways represent 1 possible approach to the implementation of a MDT in the care of patients with IE.15
In our earlier work, we used quality improvement principles in the design of an MDT approach to IE care at our institution.16 Despite having indications for surgery, 12 of 21 (57.1%) patients with IE did not undergo surgery, and we identified these missed opportunities for surgery as a leverage point for improvement of outcomes. With input from the various specialties and stakeholders, we developed a clinical pathway (algorithm) for the institutional management of IE that guides next steps in clinical care and their timelines, aiming to reduce by 50% (from 57.1% to 28.6%) the number of patients with IE who do not undergo surgery despite guideline indications for early surgical intervention. In this report, we describe the implementation of this clinical pathway as our MDT approach to the care of patients with IE at our institution.
Methods
The University of Missouri, Columbia, is a tertiary care academic health system with 5 hospitals and more than 60 clinic locations across central Missouri. In the spring of 2018, an MDT was developed, with support from administrative leaders, to improve the care of patients with IE at our institution. The work group prioritized one leverage point to improve IE outcomes, which was improving the number of surgeries performed on those IE patients who had guideline indications for surgery. A clinical pathway was developed around this leverage point (Figure 1). The pathway leveraged the 6 T’s (Table 1) to guide providers through the evaluation and management of IE.17 The pathway focused on improving adherence to standards of care and reduction in practice variation by defining indications for referrals and diagnostic interventions, helping to reduce delays in consultation and diagnosis. The pathway also clearly outlined the surgical indications and timing for patients with IE and provided the basis for decisions to proceed with surgery.

Starting in late 2018, in collaboration with cardiology and CTS teams, ID specialists socialized the clinical pathway to inpatient services that cared for patients with IE. Infectious diseases physicians also provided recurring conferences on the effectiveness of MDTs in IE management and participated in heart-valve team case discussions. Finally, in May 2019, an electronic version of the pathway was embedded in the EHR system using a Cerner PowerChart feature known as Care Pathways. The feature presents the user with algorithm questions in the EHR and provides recommendations, relevant orders, timelines, and other decision support in the clinical pathway. The feature is available to all providers in the health system.

To evaluate the effectiveness of our intervention, we recorded outcomes for patients with IE with surgical indications between December 2018 and June 2020 and compared them with our prior published data from January to December 2016. Cases of IE for the current study period were identified via retrospective chart review. Records from December 2018 to June 2020 were searched using International Statistical Classification of Diseases, Tenth Revision (ICD-10) discharge codes for IE (I33, I33.0, I33.9, I38, I39, M32.11). To select those patients with definitive IE and indications for surgery, the following criteria were applied: age ≥ 18 years; fulfilled modified Duke criteria for definite IE18; and met ≥ 1 American Heart Association (AHA)/Infection Diseases Society of America criteria for recommendation for surgery. Indications for surgery were ≥ 1 of the following: left-sided endocarditis caused by S aureus, fungal, or highly resistant organism; new heart block; annular or aortic abscess; persistent bacteremia or fever despite 5 days of appropriate antimicrobials; vegetation size ≥ 10 mm and evidence of embolic phenomena; recurrence of prosthetic valve infection; recurrent emboli and persistent vegetation despite antimicrobials; and increase in vegetation size despite antimicrobials.16
Age was treated as a categorical variable, using the age groups 18 to 44 years, 45 to 64 years, and 65 years and older. Gender was self-reported. Primary outcomes were surgery or transfer to a higher center for surgery and in-hospital death. Secondary outcomes included consults to teams involved in multidisciplinary care of patients with IE, including ID, cardiology, and CTS. Bivariate analyses were performed using Pearson χ2 tests. Odds ratios for surgery and death were calculated using a multivariate logistic regression model including age and gender covariates. Statistical significance was defined at α = 0.05, and statistical analysis was performed using Stata/IC v16.1 (StataCorp LLC). Our university institutional review board (IRB) reviewed the project (#2010858-QI) and determined that the project was quality-improvement activity, not human subject research, and therefore did not require additional IRB review.
Results
We identified 21 patients in the pre-intervention period and 31 patients in the postintervention period with definitive IE who had guideline indications for surgery. The postintervention cohort was older and had more male patients; this difference was not statistically significant. No differences were noted between the groups for race, gender, or intravenous (IV) drug use (Table 2). Chi-square tests of independence were performed to assess the relationship between age and our primary outcomes. There was a significant relationship between age and the likelihood of receiving or being transferred for surgery (59.3% vs 50% vs 7.7% for 18-44 y, 45-64 y, and ≥ 65 y, respectively; χ2 [2, N = 52] = 9.67; P = .008), but not between age and mortality (14.8% vs 25.0% vs 30.8% for 18-44 y, 45-64 y, and ≥ 65 y, respectively; χ2 = 1.48 [2, N = 52; P = .478]. The electronic version of the clinical pathway was activated and used in only 3 of the 31 patients in the postintervention period. Consultations to ID, cardiology, and CTS teams were compared between the study periods (Table 2). Although more consultations were seen in the postintervention period, differences were not statistically significant.

The unadjusted primary outcomes are shown in Table 2. More surgeries were performed per guideline indications, and fewer deaths were noted in the postintervention period than in the pre-intervention period, but the differences were not statistically significant (Table 2).
Because the postintervention period had more male patients and older patients, we evaluated the outcomes using a logistic regression model controlling for both age and gender. The odds of surgery or transfer for surgery for patients in the postintervention period were 4.88 (95% CI, 1.20-19.79; P = .027) as compared with the pre-intervention period, and the odds ratio for death among patients in the postintervention period compared with the pre-intervention period was 0.40 (95% CI, 0.09-1.69; P = .21) (Figure 2).

Discussion
In our study, patients with IE with guideline indications for surgery had significantly higher rates of surgery in the postintervention period than in the pre-intervention period. The implementation of an MDT, recurring educational sessions, and efforts to implement and familiarize team members with the clinical pathway approach are the most likely reasons for this change. The increased rates of surgery in the postintervention period were the likely proximate cause of the 60% reduction in in-hospital mortality. This improvement in mortality, while not statistically significant, is very likely to be clinically significant and helps reinforce the value of the MDT intervention used.
Our findings are consistent with existing and mounting literature on the use of MDTs to improve outcomes for patients with IE, including 2 studies that noted an increased rate of surgery for patients with indications.8,19 Several other studies in both Europe and North America have found significant decreases in mortality,6-11,20,21 rates of complications,9 time to diagnosis and treatment,11 and length of stay9,20 for patients with IE managed with an MDT strategy. Although current AHA guidelines for care of patients with IE do suggest an MDT approach, the strategy for this approach is not well established.22 Only 1 study that has implemented a new MDT protocol for care of IE has been conducted in the United States.8
While effective MDTs certainly improve outcomes in patients with IE, there are reported differences in implementation of such an approach. With the MDT model as the core, various implementations included regular case conferences,10,11,19,21,23 formation of a consulting team,6,8 or establishment of a new protocol or algorithm for care.8,9,20 Our approach used a clinical pathway as a basis for improved communication among consulting services, education of learning providers via regular case conferences, and implementation of an electronic clinical care pathway to guide them step by step. Our pathway followed the institutionally standardized algorithm (Figure 1), using what we called the 6 T’s approach (Table 1), that guides providers to evaluate critical cases in a fast track.17
To the best of our knowledge, ours is the first report of an MDT that used an electronic clinical care pathway embedded within the EHR. The electronic version of our clinical pathway went live for only the second half of the postintervention study period, which is the most likely reason for its limited utilization. It is also possible that educational efforts in the first half of the intervention period were sufficient to familiarize providers with the care pathway such that the electronic version was seldom needed. We are exploring other possible ways of improving electronic pathway utilization, such as improving the feature usability and further systemwide educational efforts.
Our study has other limitations. Quasi-experimental before-and-after comparisons are subject to confounding from concurrent interventions. We had a substantial change in cardiothoracic faculty soon after the commencement of our efforts to form the MDT, and thus cannot rule out differences related to their comfort level in considering or offering surgery. We also cannot rule out a Hawthorne effect, where knowledge of the ongoing quality-improvement project changed provider behavior, making surgery more likely. We did not evaluate rates of right- versus left-sided endocarditis, which have been linked to mortality.24 Our study also was performed across a single academic institution, which may limit its generalizability. Finally, our study may not have been adequately powered to detect differences in mortality due to implementation of the MDT approach.
Conclusion
Our work suggests that an MDT for IE can be successfully designed and implemented with a clinical pathway using quality-improvement tools in centers where subspecialty services are available. Our approach was associated with a higher rate of surgery among patients with guideline indications for surgery and may reduce in-hospital mortality. An electronic clinical care pathway embedded in the EHR is feasible and may have a role in MDT implementation.
These data were also accepted as a poster at IDWeek 2022, Washington, DC. The poster abstract is published in an online supplement of Open Forum Infectious Diseases as an abstract publication.
Corresponding author: Haley Crosby; hwc2pd@health.missouri.edu
Disclosures: None reported.
1. Baddour LM, Wilson WR, Bayer AS, et al. Infective endocarditis in adults: diagnosis, antimicrobial therapy, and management of complications: a scientific statement for healthcare professionals from the American Heart Association. Circulation. 2015;132(15):1435-1486. doi:10.1161/cir.0000000000000296
2. Federspiel JJ, Stearns SC, Peppercorn AF, et al. Increasing US rates of endocarditis with Staphylococcus aureus: 1999-2008. Arch Intern Med. 2012;172(4):363-365. doi:10.1001/archinternmed.2011.1027
3. Nishimura RA, Otto CM, Bonow RO, et al. 2014 AHA/ACC Guideline for the Management of Patients With Valvular Heart Disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129(23):e521-e643. doi:10.1161/cir.0000000000000031
4. Chambers J, Sandoe J, Ray S, et al. The infective endocarditis team: recommendations from an international working group. Heart. 2014;100(7):524-527. doi:10.1136/heartjnl-2013-304354
5. Habib G, Lancellotti P, Antunes MJ, et al. 2015 ESC Guidelines for the management of infective endocarditis: The Task Force for the Management of Infective Endocarditis of the European Society of Cardiology (ESC). Endorsed by: European Association for Cardio-Thoracic Surgery (EACTS), the European Association of Nuclear Medicine (EANM). Eur Heart J. 2015;36(44):3075-3128. doi:10.1093/eurheartj/ehv319
6. Chirillo F, Scotton P, Rocco F, et al. Impact of a multidisciplinary management strategy on the outcome of patients with native valve infective endocarditis. Am J Cardiol. 2013;112(8):1171-1176. doi:10.1016/j.amjcard.2013.05.060
7. Botelho-Nevers E, Thuny F, Casalta JP, et al. Dramatic reduction in infective endocarditis-related mortality with a management-based approach. Arch Intern Med. 2009;169(14):1290-1298. doi:10.1001/archinternmed.2009.192
8. El-Dalati S, Cronin D, Riddell IV J, et al. The clinical impact of implementation of a multidisciplinary endocarditis team. Ann Thorac Surg. 2022;113(1):118-124.
9. Carrasco-Chinchilla F, Sánchez-Espín G, Ruiz-Morales J, et al. Influence of a multidisciplinary alert strategy on mortality due to left-sided infective endocarditis. Rev Esp Cardiol (Engl Ed). 2014;67(5):380-386. doi:10.1016/j.rec.2013.09.010
10. Issa N, Dijos M, Greib C, et al. Impact of an endocarditis team in the management of 357 infective endocarditis [abstract]. Open Forum Infect Dis. 2016;3(suppl 1):S201. doi:10.1093/ofid/ofw172.825
11. Kaura A, Byrne J, Fife A, et al. Inception of the ‘endocarditis team’ is associated with improved survival in patients with infective endocarditis who are managed medically: findings from a before-and-after study. Open Heart. 2017;4(2):e000699. doi:10.1136/openhrt-2017-000699
12. Rotter T, Kinsman L, James E, et al. Clinical pathways: effects on professional practice, patient outcomes, length of stay and hospital costs. Cochrane Database Syst Rev. 2010;(3):Cd006632. doi:10.1002/14651858.CD006632.pub2
13. Neame MT, Chacko J, Surace AE, et al. A systematic review of the effects of implementing clinical pathways supported by health information technologies. J Am Med Inform Assoc. 2019;26(4):356-363. doi:10.1093/jamia/ocy176
14. Trimarchi L, Caruso R, Magon G, et al. Clinical pathways and patient-related outcomes in hospital-based settings: a systematic review and meta-analysis of randomized controlled trials. Acta Biomed. 2021;92(1):e2021093. doi:10.23750/abm.v92i1.10639
15. Gibbons EF, Huang G, Aldea G, et al. A multidisciplinary pathway for the diagnosis and treatment of infectious endocarditis. Crit Pathw Cardiol. 2020;19(4):187-194. doi:10.1097/hpc.0000000000000224
16. Regunath H, Vasudevan A, Vyas K, et al. A quality improvement initiative: developing a multi-disciplinary team for infective endocarditis. Mo Med. 2019;116(4):291-296.
17. Regunath H, Whitt SP. Multidisciplinary service delivery for the endocarditis patient. In: Infective Endocarditis: A Multidisciplinary Approach. 1st ed. Kilic A, ed. Academic Press; 2022.
18. Durack DT, Lukes AS, Bright DK. New criteria for diagnosis of infective endocarditis: utilization of specific echocardiographic findings. Duke Endocarditis Service. Am J Med. 1994;96(3):200-209. doi:10.1016/0002-9343(94)90143-0
19. Tan C, Hansen MS, Cohen G, et al. Case conferences for infective endocarditis: a quality improvement initiative. PLoS One. 2018;13(10):e0205528. doi:10.1371/journal.pone.0205528
20. Ruch Y, Mazzucotelli JP, Lefebvre F, et al. Impact of setting up an “endocarditis team” on the management of infective endocarditis. Open Forum Infect Dis. 2019;6(9):ofz308. doi:10.1093/ofid/ofz308
21. Camou F, Dijos M, Barandon L, et al. Management of infective endocarditis and multidisciplinary approach. Med Mal Infect. 2019;49(1):17-22. doi:10.1016/j.medmal.2018.06.007
22. Pettersson GB, Hussain ST. Current AATS guidelines on surgical treatment of infective endocarditis. Ann Cardiothorac Surg. 2019;8(6):630-644. doi:10.21037/acs.2019.10.05
23. Mestres CA, Paré JC, Miró JM. Organization and functioning of a multidisciplinary team for the diagnosis and treatment of infective endocarditis: a 30-year perspective (1985-2014). Rev Esp Cardiol (Engl Ed). 2015;68(5):363-368. doi:10.1016/j.rec.2014.10.006
24. Stavi V, Brandstaetter E, Sagy I, et al. Comparison of clinical characteristics and prognosis in patients with right- and left-sided infective endocarditis. Rambam Maimonides Med J. 2019;10(1):e00003. doi:10.5041/rmmj.10338
1. Baddour LM, Wilson WR, Bayer AS, et al. Infective endocarditis in adults: diagnosis, antimicrobial therapy, and management of complications: a scientific statement for healthcare professionals from the American Heart Association. Circulation. 2015;132(15):1435-1486. doi:10.1161/cir.0000000000000296
2. Federspiel JJ, Stearns SC, Peppercorn AF, et al. Increasing US rates of endocarditis with Staphylococcus aureus: 1999-2008. Arch Intern Med. 2012;172(4):363-365. doi:10.1001/archinternmed.2011.1027
3. Nishimura RA, Otto CM, Bonow RO, et al. 2014 AHA/ACC Guideline for the Management of Patients With Valvular Heart Disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129(23):e521-e643. doi:10.1161/cir.0000000000000031
4. Chambers J, Sandoe J, Ray S, et al. The infective endocarditis team: recommendations from an international working group. Heart. 2014;100(7):524-527. doi:10.1136/heartjnl-2013-304354
5. Habib G, Lancellotti P, Antunes MJ, et al. 2015 ESC Guidelines for the management of infective endocarditis: The Task Force for the Management of Infective Endocarditis of the European Society of Cardiology (ESC). Endorsed by: European Association for Cardio-Thoracic Surgery (EACTS), the European Association of Nuclear Medicine (EANM). Eur Heart J. 2015;36(44):3075-3128. doi:10.1093/eurheartj/ehv319
6. Chirillo F, Scotton P, Rocco F, et al. Impact of a multidisciplinary management strategy on the outcome of patients with native valve infective endocarditis. Am J Cardiol. 2013;112(8):1171-1176. doi:10.1016/j.amjcard.2013.05.060
7. Botelho-Nevers E, Thuny F, Casalta JP, et al. Dramatic reduction in infective endocarditis-related mortality with a management-based approach. Arch Intern Med. 2009;169(14):1290-1298. doi:10.1001/archinternmed.2009.192
8. El-Dalati S, Cronin D, Riddell IV J, et al. The clinical impact of implementation of a multidisciplinary endocarditis team. Ann Thorac Surg. 2022;113(1):118-124.
9. Carrasco-Chinchilla F, Sánchez-Espín G, Ruiz-Morales J, et al. Influence of a multidisciplinary alert strategy on mortality due to left-sided infective endocarditis. Rev Esp Cardiol (Engl Ed). 2014;67(5):380-386. doi:10.1016/j.rec.2013.09.010
10. Issa N, Dijos M, Greib C, et al. Impact of an endocarditis team in the management of 357 infective endocarditis [abstract]. Open Forum Infect Dis. 2016;3(suppl 1):S201. doi:10.1093/ofid/ofw172.825
11. Kaura A, Byrne J, Fife A, et al. Inception of the ‘endocarditis team’ is associated with improved survival in patients with infective endocarditis who are managed medically: findings from a before-and-after study. Open Heart. 2017;4(2):e000699. doi:10.1136/openhrt-2017-000699
12. Rotter T, Kinsman L, James E, et al. Clinical pathways: effects on professional practice, patient outcomes, length of stay and hospital costs. Cochrane Database Syst Rev. 2010;(3):Cd006632. doi:10.1002/14651858.CD006632.pub2
13. Neame MT, Chacko J, Surace AE, et al. A systematic review of the effects of implementing clinical pathways supported by health information technologies. J Am Med Inform Assoc. 2019;26(4):356-363. doi:10.1093/jamia/ocy176
14. Trimarchi L, Caruso R, Magon G, et al. Clinical pathways and patient-related outcomes in hospital-based settings: a systematic review and meta-analysis of randomized controlled trials. Acta Biomed. 2021;92(1):e2021093. doi:10.23750/abm.v92i1.10639
15. Gibbons EF, Huang G, Aldea G, et al. A multidisciplinary pathway for the diagnosis and treatment of infectious endocarditis. Crit Pathw Cardiol. 2020;19(4):187-194. doi:10.1097/hpc.0000000000000224
16. Regunath H, Vasudevan A, Vyas K, et al. A quality improvement initiative: developing a multi-disciplinary team for infective endocarditis. Mo Med. 2019;116(4):291-296.
17. Regunath H, Whitt SP. Multidisciplinary service delivery for the endocarditis patient. In: Infective Endocarditis: A Multidisciplinary Approach. 1st ed. Kilic A, ed. Academic Press; 2022.
18. Durack DT, Lukes AS, Bright DK. New criteria for diagnosis of infective endocarditis: utilization of specific echocardiographic findings. Duke Endocarditis Service. Am J Med. 1994;96(3):200-209. doi:10.1016/0002-9343(94)90143-0
19. Tan C, Hansen MS, Cohen G, et al. Case conferences for infective endocarditis: a quality improvement initiative. PLoS One. 2018;13(10):e0205528. doi:10.1371/journal.pone.0205528
20. Ruch Y, Mazzucotelli JP, Lefebvre F, et al. Impact of setting up an “endocarditis team” on the management of infective endocarditis. Open Forum Infect Dis. 2019;6(9):ofz308. doi:10.1093/ofid/ofz308
21. Camou F, Dijos M, Barandon L, et al. Management of infective endocarditis and multidisciplinary approach. Med Mal Infect. 2019;49(1):17-22. doi:10.1016/j.medmal.2018.06.007
22. Pettersson GB, Hussain ST. Current AATS guidelines on surgical treatment of infective endocarditis. Ann Cardiothorac Surg. 2019;8(6):630-644. doi:10.21037/acs.2019.10.05
23. Mestres CA, Paré JC, Miró JM. Organization and functioning of a multidisciplinary team for the diagnosis and treatment of infective endocarditis: a 30-year perspective (1985-2014). Rev Esp Cardiol (Engl Ed). 2015;68(5):363-368. doi:10.1016/j.rec.2014.10.006
24. Stavi V, Brandstaetter E, Sagy I, et al. Comparison of clinical characteristics and prognosis in patients with right- and left-sided infective endocarditis. Rambam Maimonides Med J. 2019;10(1):e00003. doi:10.5041/rmmj.10338
Leading for High Reliability During the COVID-19 Pandemic: A Pilot Quality Improvement Initiative to Identify Challenges Faced and Lessons Learned
From the U.S. Department of Veterans Affairs (all authors), and Cognosante, LLC, Falls Church, VA (Dr. Murray, Dr. Sawyer, and Jessica Fankhauser).
Abstract
Objective: The COVID-19 pandemic posed unprecedented leadership challenges to health care organizations worldwide, especially those on the journey to high reliability. The objective of this pilot quality improvement initiative was to describe the experiences of medical center leaders continuing along the journey to high reliability during the pandemic.
Methods: A convenience sample of Veterans Health Administration medical center directors at facilities that had initiated the journey to high reliability prior to or during the COVID-19 pandemic were asked to complete a confidential survey to explore the challenges experienced and lessons learned.
Results: Of the 35 potential participants, 15 completed the confidential web-based survey. Five major themes emerged from participants’ responses: (1) managing competing priorities, (2) staying committed, (3) adapting and overcoming, (4) prioritizing competing demands, and (5) maintaining momentum.
Conclusion: This pilot quality improvement initiative provides some insight into the challenges experienced and lessons learned during the COVID-19 pandemic to help inform health care leaders’ responses during crises they may encounter along the journey to becoming a high reliability organization.
Keywords: HRO, leadership, patient safety.
Health care leaders worldwide agree that the
Maintaining continuous progress toward advancing high reliability organization (HRO) principles and practices can be especially challenging during crises of unprecedented scale such as the pandemic. HROs must be continually focused on achieving safety, quality, and efficiency goals by attending to the 3 pillars of HRO: culture, leadership, and continuous process improvement. HROs promote a culture where all staff across the organization watch for and report any unsafe conditions before these conditions pose a greater risk in the workplace. Hospital leaders, from executives to frontline managers, must be cognizant of all systems and processes that have the potential to affect patient care.12 All of the principles of HROs must continue without fail to ensure patient safety; these principles include preoccupation with failure, anticipating unexpected risks, sensitivity to dynamic and ever-changing operations, avoiding oversimplifications of identified problems, fostering resilience across the organization, and deferring to those with the expertise to make the best decisions regardless of position, rank, or title.12,13 Given the demands faced by leaders during crises with unprecedented disruption to normal operating procedures, it can be especially difficult to identify systemic challenges and apply lessons learned in a timely manner. However, it is critical to identify such lessons in order to continuously improve and to increase preparedness for subsequent crises.13,14
Because of the COVID-19 pandemic’s unprecedented nature in recent history, a review of the literature produced little evidence exploring the challenges experienced and lessons learned by health care leaders, especially as it relates to implementing or sustaining HRO journeys during the COVID-19 pandemic. Related literature published to date consists of editorials on reliability, uncertainty, and the management of errors15; patient safety and high reliability preventive strategies16; and authentic leadership.17 Five viewpoints were published on HROs and maladaptive stress behaviors,18 mindful organizing and organizational reliability,19 the practical essence of HROs,20 embracing principles of HROs in crisis,8 and using observation and high reliability strategies when facing an unprecedented safety threat.21 Finally, the authors identified 2 studies that used a qualitative research approach to explore leadership functions within an HRO when managing crises22 and organizational change in response to the COVID-19 pandemic.23 Due to the paucity of available information, the authors undertook a pilot quality improvement (QI) initiative to address this knowledge gap.
The aim of this initiative was to gain a better understanding of the challenges experienced, lessons learned, and recommendations to be shared by VHA medical center directors (MCDs) of health care facilities that had initiated the journey to high reliability before or during the COVID-19 pandemic. The authors hope that this information will help health care leaders across both governmental and nongovernmental organizations, nationally and globally, to prepare for future pandemics, other unanticipated crises (eg, natural disasters, terrorist attacks), and major change initiatives (eg, electronic health record modernization) that may affect the delivery of safe, high-quality, and effective patient care. The initiative is described using the SQUIRE 2.0 guidelines.24,25
Methods
Survey
We used a qualitative approach and administered a confidential web-based survey, developed by the project team, to VHA MCDs at facilities that had initiated the journey to high reliability before or during the COVID-19 pandemic. The survey consisted of 8 participant characteristic questions (Table 1) and 4 open-ended questions. The open-ended questions were designed to encourage MCD participants to freely provide detailed descriptions of the challenges experienced, lessons learned, recommendations for other health care leaders, and any additional information they believed was relevant.26,27 Participants were asked to respond to the following items:
- Please describe any challenges you experienced while in the role of MCD at a facility that initiated implementation of HRO principles and practices prior to (February 2020) or during (March 2020–September 2021) the initial onset of the COVID-19 pandemic.
- What are some lessons that you learned when responding to the COVID-19 pandemic while on the journey to high reliability?
- What recommendations would you like to make to other health care leaders to enable them to respond effectively to crises while on the journey to high reliability?
- Please provide any additional information that would be of value.
An invitation to participate in this pilot QI initiative was sent via e-mail to 35 potential participants, who were all MCDs at Cohort 1 and Cohort 2 facilities. The invitation was sent on June 17, 2022, by a VHA senior High Reliability Enterprise Support government team member not directly involved with the initiative.
The invitation included the objective of the initiative, estimated time to complete the confidential web-based survey, time allotted for responses to be submitted, and a link to the survey should potential participants agree to participate. Potential participants were informed that their involvement was voluntary, based on their willingness to participate and available time to complete the survey. Finally, the invitation noted that any comments provided would remain confidential and nonattributional for the purpose of publishing and presenting. The inclusion criteria for participation were: (1) serving
Data Gathering and Analysis
To minimize bias and maintain neutrality at the organizational level, only non-VHA individuals working on the project were directly involved with participants’ data review and analysis. Participant characteristics were analyzed using descriptive statistics. Responses to the 4 open-ended questions were coded and analyzed by an experienced researcher and coauthor using NVivo 11 qualitative data analysis software.28 To ensure trustworthiness (credibility, transferability, dependability, and confirmability) in the data analysis procedure,29 inductive thematic analysis was also performed manually using the methodologies of Braun and Clarke (Table 2)30 and Erlingsson and Brysiewicz.31 The goal of inductive analysis is to allow themes to emerge from the data while minimizing preconceptions.32,33 Regular team meetings were held to discuss and review the progress of data collection and analysis. The authors agreed that the themes were representative of the participants’ responses.
Institutional review board (IRB) review and approval were not required, as this project was a pilot QI initiative. The intent of the initiative was to explore ways to improve the quality of care delivered in the participants’ local care settings and not to generalize the findings. Under these circumstances, formal IRB review and approval of a QI initiative are not required.34 Participation in this pilot QI initiative was voluntary, and participants could withdraw at any time without consequences. Completion of the survey indicated consent. Confidentiality was ensured at all times by avoiding both the use of facility names and the collection of participant identifiers. Unique numbers were assigned to each participant. All comments provided by survey participants remained confidential and nonattributional for the purpose of publishing and presenting.
Results
Of the 35 potential participants, 15 VHA MCDs (43%) completed the confidential web-based survey. Out of the 17 potential participants in Cohort 1, 6 (35%) completed the survey. With Cohort 2, 9 (50%) of the potential 18 participants responded. Although saturation was reached at 10 responses, the additional completed surveys were included in the analysis. Saturation can be achieved with a small number of participants (n = 9–17), particularly when the potential participants are relatively homogenous and project aims are narrowly defined.35 Most participants had more than 10 years of executive-level experience and most medical centers had been on the journey to high reliability for more than 12 months at the time of the pandemic (Table 3).
“There were too many competing priorities dealing with the pandemic and staffing crisis.” (Participant 8)
Other participants shared:
“We had our HRO mentor designated just as our first peak was descending on us. It was initially challenging to determine the proper pace of implementation when we clearly had other things going on. There was a real risk that people would say, ‘What, are you kidding?’ as we tried to roll this out.” (Participant 4)
“Prior to COVID, our main challenges were getting organized and operational rollout. During the pandemic, we had to shift our focus to COVID and the training aspects suffered. Also, many other priorities pulled us away from an HRO rollout focus.” (Participant 6)
“If you don’t need a highly reliable organization during a crisis, when do you need it? That was the message that we kicked off with. It was also VERY important to take things slowly. Education had to be done in bits, and we had a much more modest timeline than what would have been the norm for any initiative pre-COVID. The emphasis was on this being a long-term commitment, that we would be doing it the right way rather than rushing it, etc.” (Participant 4)
“Keeping HRO principles and a Just Culture on the forefront of our minds, we looked for opportunities to progress on our HRO journey, despite the challenges of the pandemic. Our monthly Town Halls became weekly events to share COVID updates and information with staff. We used the Town Halls to promote our HRO mission and to open communication lines with staff, designating 1 week each month as a ‘Safety Forum.’ The pandemic provided the springboard and backdrop for staff Safety Stories submissions, many of which were shared at our Town Halls and Safety Forums.” (Participant 7)
“We were able to utilize HRO principles in response to the COVID pandemic. Specifically standardized communication from the facility to VISN [Veterans Integrated Services Network] was initiated on a daily basis. This practice provided daily communication on key operational items and clinical items at the medical center, allowed timely feedback on actions being taken, as was instrumental in daily checks on staffing, COVID testing supplies, overall supply chain issues.” (Participant 9)
The recommendations provided by 10 participants (Cohort 1, n = 6; Cohort 2, n = 4) for other health care leaders experiencing a crisis during the journey to high reliability were insightful. The themes that frequently emerged from the responses to the survey were to adapt and overcome. Participants shared:
“Utilize the many tools you’re given, specifically your team. Try even the craziest ideas from frontline staff.” (Participant 1)
“Use your mentors for younger directors and, even if you think you know the answer, involve your staff. It makes them feel they have a voice and gives them ownership of the issues.” (Participant 5)
“Make sure that you have key leaders in place who are committed to HRO and can help the organization adjust.” (Participant 6)
“Take advantage of HRO Leader Coaching, which pairs MCDs with coaches who act as consultants for HRO leadership practices to ensure progress in reaching the next level in the journey to High Reliability.” (Participant 7)
“Meet regularly with the HRO Lead and team (more frequently during early stages of implementation) to provide support, eliminate barriers, and champion the HRO mission. It is important to include other members of the ELT [Executive Leadership Team] to ensure their involvement with the facility HRO strategic plan.” (Participant 7)
“Prioritize and understand that not everything is priority #1. Continue what you can with HRO, incorporate high reliability principles into the work being done during a crisis, but understand you may need to modify rollout schedules.” (Participant 8)
The theme of prioritizing competing demands emerged again from 5 participants (Cohort 1, n = 3; Cohort 2, n = 2) with question 3 describing recommendations for other leaders:
“Your first priority is to the crisis. Don’t get distracted by this or any other initiative. That was not a very popular message for the people pushing HRO, but it is the reality and the necessity. However, it IS possible to move forward with HRO (or other important initiatives) during crisis times, as long as you carefully consider what you are asking of people and don’t overload/overwhelm them. It is not your ego (or that of Central Office) that needs to be stoked. If the initiative truly has value, you need to be patient to see it done properly, rather than rushed/pushed/forced. Don’t kill it by being overeager and overwhelming your already overtaxed people. That said, keep moving forward. The key is pacing—and remember that your Type A hard-driving leader types (you know who you are) will certainly fail if they push it. Or even if they go at a normal pace that would be appropriate for noncrisis times.” (Participant 4)
“Prioritize and understand that not everything is priority #1. Continue what you can with HRO, incorporate high reliability principles into the work being done during a crisis, but understand you may need to modify rollout schedules.” (Participant 8)
“It was critical for us to always focus on the immediate workplace safety of staff (especially those on the frontlines of the pandemic response) when in the process of rolling out HRO initiatives.” (Participant 14)
Maintaining Momentum
“It seemed as though communication and education from VHA on HRO slowed down at the same time, which further slowed our progress. We are now trying to ramp our engagement up again.” (Participant 3)
“There can be synergy between crisis response and HRO implementation. As an example, one of the first steps we took was leadership rounding. That was necessary anyways for crisis management (raising the spirits on the front lines, so to speak). What we did was include scheduled time instead of (in addition to) ad hoc. And we got credit for taking an HRO step. I resisted whiteboards/visual management systems for a long time because (in my opinion) that would have been much too distracting during the crisis. Having waited for better times, I was able to move forward with that several months later and with good success.” (Participant 4)
Discussion
Health care leaders worldwide experienced an immense set of challenges because of the COVID-19 pandemic, which is a crisis of a magnitude with no parallel in modern times. Strong, adaptive leadership at all levels of health care systems was needed to effectively address the immense crisis at hand.36,37 Findings from this pilot QI initiative suggest that MCDs faced many new challenges, requiring them to perform unfamiliar tasks and manage numerous overlapping challenges (eg, staffing shortages and reassignments, safety concerns, changes to patient appointments, backlogs in essential services), all while also trying to continue with the journey to high reliability. Despite the challenges leaders faced, they recognized the need to manage competing priorities early and effectively. At times, the priority was to address the wide-ranging, urgent issues related to the pandemic. When the conditions improved, there was time to refocus efforts on important but longer-term activities related to the HRO journey. Other participants recognized that their commitment to HRO needed to remain a priority even during the periods of intense focus on COVID-19.
Some participants felt compelled to stay committed to the HRO journey despite numerous competing demands. They stayed committed to looking for opportunities to progress by implementing HRO principles and practices to achieve safety, quality, and efficiency goals. This dedication is noteworthy, especially in light of recently published research that demonstrates the vast number of patient safety issues that presented during the COVID-19 pandemic (eg, ineffective communication, poor teamwork, the absence of coordination)1 as well as perceptions that patient safety and quality of care had significantly declined as a result of the crisis.36,37
Participants also highlighted the need to be adaptive when responding to the complexity and unpredictability of the pandemic. Participants regularly sought ways to increase their knowledge, skills, and abilities by using the resources (eg, tools, experts) available to them. Research shows that in increasingly complex and ever-changing situation such as the COVID-19 pandemic, leaders must be adaptive with all levels of performance, especially when limited information is available.38,39
This is the first initiative of its kind to specifically explore the challenges experienced and lessons learned from health care leaders continuing along the journey to high reliability during the COVID-19 pandemic. Findings from this pilot QI initiative revealed that many participants recommended that leaders adapt and overcome challenges as much as possible when continuing with HRO during a crisis. These findings are echoed in the current literature suggesting that adaptive performance is a highly effective form of leadership during crises.38,40 Being able to effectively adapt during a crisis is essential for reducing further vulnerabilities across health care systems. In fact, this lesson is shared by many countries in response to the unprecedented global crisis.41A limitation of this pilot QI initiative is that the authors did not directly solicit responses from all VHA MCDs or from other health care executives (eg, Chief of Staff, Associate Director for Operations, Associate Director for Patient Care, and Nurse Executive). As such, our findings represent only a small segment of senior leadership perspectives from a large, integrated health care system. Individuals who did not respond to the survey may have had different experiences than those who did, and the authors excluded many MCDs who formally began their HRO journeys in 2022, well after the pandemic was underway. Similarly, the experiences of Veterans Affairs leaders may or may not be similar to that of other health care organizations. Although the goal of this initiative was to explore the participants’ experiences during the period of crisis, time and distance from the events at the height of the COVID-19 pandemic may have resulted in difficulty recalling information as well as making sense of the occurrence. This potential recall bias is a common occurrence in trying to explore past experiences, especially as they relate to crises. Finally, this pilot QI initiative did not explore personal challenges participants may have faced during this period of time (eg, burnout, personal or family illness), which may have also shaped their responses.
Conclusion
This initiative suggests that VHA MCDs often relied on HRO principles to guide and assist with their response to the COVID-19 pandemic, including managing periods of unprecedented crisis. The ability to adapt and prioritize was seen as an especially important lesson. Many MCDs continued their personal and organizational efforts toward high reliability even in periods of intense challenge because of the pandemic. These findings can help with future crises that may occur during an organization’s journey to high reliability. This pilot QI initiative’s findings warrant further investigation to explore the experiences of the broader range of health care leaders while responding to unplanned crises or even planned large-scale cultural change or technology modernization initiatives (eg, electronic health record modernization) to expand the state of the science of high reliability as well as inform policy and decision-making. Finally, another area for future study is examining how leadership responses vary across facilities, depending on factors such as leader roles, facility complexity level, resource availability, patient population characteristics, and organizational culture.
Acknowledgment: The authors express their sincere gratitude to the medical center directors who participated in this pilot study.
Corresponding author: John S. Murray, PhD, MPH, MSGH, RN, FAAN, 20 Chapel St., Unit A502, Brookline, MA 02446; JMurray325@aol.com
Disclosures: None reported.
1. Editors: Dying in a leadership vacuum. 9.4N Engl J Med. 2020;383(15):1479-1480. doi:10.1056/NEJMe2029812
2. Geerts JM, Kinnair D, Taheri P, et al. Guidance for health care leaders during the recovery stage of the COVID-19 pandemic: a consensus statement. JAMA Netw Open. 2021;4(7):1-16. doi:10.1001/jamanetworkopen.2021.20295
3. Boiral O, Brotherton M-C, Rivaud L, et al. Organizations’ management of the COVID-19 pandemic: a scoping review of business articles. Sustainability. 2021;13:1-20. doi:10.3390/su13073993
4. Razu SR, Yasmin T, Arif TB, et al. Challenges faced by healthcare professionals during the COVID-19 pandemic: a qualitative inquiry from Bangladesh. Front Public Health. 2021;9:1-13. doi:10.3389/fpubh.2021.647315
5. Lyng HB, Ree E, Wibe T, et al. Healthcare leaders’ use of innovative solutions to ensure resilience in healthcare during the Covid-19 pandemic: a qualitative study in Norwegian nursing homes and home care services. BMC Health Serv Res. 2021;21(1):1-11. doi:1186/s12913-021-06923-1
6. Freitas J. Queiroz A, Bortotti I, et al. Nurse leaders’ challenges fighting the COVID-19 pandemic: a qualitative study. Open J Nurs. 2021;11:267-280. doi:10.4236/ojn.2021.115024
7. McGuire AL, Aulisio MP, Davis FD, et al. Ethical challenges arising in the COVID-19 pandemic: an overview from the Association of Bioethics Program Directors (ABPD) Task Force. 9.4Am J Bioeth. 2020;20(7):15-27. doi:10.1080/15265161.2020.1764138
8. Turbow RM, Scibilia JP. Embracing principles of high reliability organizations can improve patient safety during pandemic. AAP News. January 19, 2021. Accessed March 1, 2023. https://publications.aap.org/aapnews/news/8975
9. Roberts BH, Damiano LA, Graham S, et al. A case study in fostering a learning culture in the context of Covid-19. American Association for Physician Leadership. June 24, 2021. Accessed March 1, 2023. https://www.physicianleaders.org/news/a-case-study-in-fostering-a-learning-culture-in-the-context-of-covid-19
10. U.S. Department of Veterans Affairs. Department of Veterans AffairsCOVID-19 National Summary. Veterans Affairs. Accessed December 4, 2022. https://www.accesstocare.va.gov/Healthcare/COVID19NationalSummary
11. U.S. Department of Veterans Affairs. VA fourth mission summary. Veterans Affairs. Accessed December 4, 2022. https://www.va.gov/health/coronavirus/statesupport.asp#:~:text=As%20part%20of%20the%20Fourth,the%20facilities%20we%20are%20supporting
12. Veazie S, Peterson K, Bourne D, et al. Implementing high-reliability organization principles into practice: a rapid evidence review. J Patient Saf. 2022;18(1):e320-e328. doi:10.1097/PTS.0000000000000768
13. Murray JS, Kelly S, Hanover C. Promoting psychological safety in healthcare organizations. 9.4Mil Med. 2022;187(7-8):808-810. doi:10.1093/milmed/usac041
14. Maison D, Jaworska D, Adamczyk D, et al. The challenges arising from the COVID-19 pandemic and the way people deal with them: a qualitative longitudinal study. PLoS One. 2021;16(10):1-17. doi:10.1371/journal.pone.0258133
15. Schulman PR. Reliability, uncertainty and the management of error: new perspectives in the COVID-19 era. J Contingencies Crisis Manag. 2022;30:92-101. doi:10.1111/1468-5973.12356
16. Adelman JS, Gandhi TK. COVID-19 and patient safety: time to tap into our investment in high reliability. J Patient Saf. 2021;17(4): 331-333. doi:10.1097/PTS.0000000000000843
17. Shingler-Nace A. COVID-19: when leadership calls. Nurs Lead. 2020;18(3):202-203. doi:10.1016/j.mnl.2020.03.017
18. Van Stralen D, Mercer TA. During pandemic COVID 19, the high reliability organization (HRO) identifies maladaptive stress behaviors: the stress-fear-threat cascade. Neonatol Tod. 2020;15(11):113-124. doi: 10.51362/neonatology.today/2020111511113124
19. Vogus TJ, Wilson AD, Randall K, et al. We’re all in this together: how COVID-19 revealed the coconstruction of mindful organising and organisational reliability. BMJ Qual Saf. 2022;31(3):230-233. doi:10.1136/bmjqs-2021-014068
20. Van Stralen D. Pragmatic high-reliability organization (HRO) during pandemic COVID-19. Neonatol Tod. 2020(4);15:109-117. doi:10.51362/neonatology.today/20208158109117
21. Thull-Freedman J, Mondoux S, Stang A, et al. Going to the COVID-19 Gemba: using observation and high reliability strategies to achieve safety in a time of crisis. CJEM. 2020;22(6):738-741. doi:10.1017/cem.2020.380
22. Sarihasan I, Dajnoki K, Oláh J, et al. The importance of the leadership functions of a high-reliability health care organization in managing the COVID-19 pandemic in Turkey. Econ Sociol. 2022;15:78-93. doi:10.14254/2071-789x.2022/15-1/5
23. Crain MA, Bush AL, Hayanga H, et al. Healthcare leadership in the COVID-19 pandemic: from innovative preparation to evolutionary transformation. J Health Leadersh. 2021;13:199-207. doi:10.2147/JHL.S319829
24. SQUIRE. Revised Standards for Quality Improvement Reporting Excellence (SQUIRE 2.0) SQUIRE; 2020. Accessed March 1, 2023. http://www.squire-statement.org/index.cfm?fuseaction=Page.ViewPage&pageId=471
25. Lounsbury O. How to write a quality improvement project. Patient Safety J. 2022;4(1):65-67. doi:10.33940/culture/2022.3.6
26. Bengtsson M. How to plan and perform a qualitative study using content analysis. Nurs Plus Open. 2016;2:8-14. doi:10.1016/j.npls.2016.01.001
27. Allen M. The Sage Encyclopedia of Communication Research Methods. (Vols. 1-4). SAGE Publications, Inc; 2017
28. Unlock insights with qualitative data analysis software. Lumivero. Accessed March 2, 2023. https://lumivero.com/products/nvivo/
29. Maher C, Hadfield M, Hutchings M, et al. Ensuring rigor in qualitative data analysis: a design research approach to coding combining NVivo with traditional material methods. Int J Qual Methods. 2018;17:1-13. doi:10.1177/1609406918786362
30. Braun V, Clarke V. Using thematic analysis in psychology. Qual Res Psychol. 2006;3:77-101. doi:10.1191/1478088706qp063oa
31. Erlingsson C, Brysiewicz P. A hands-on guide to doing content analysis. Afr J Emerg Med. 2017;7:93-99. doi:10.1016/j.afjem.2017.08.001
32. Vears DF, Gillam L. Inductive content analysis: a guide for beginning qualitative researchers. FoHPE. 2022;23:111-127. doi:10.11157/fohpe.v23i1.544
33. Nowell LS, Norris JM, White DE, et al. Thematic analysis: striving to meet the trustworthiness criteria. Int J Qual Methods. 2017;16:1-13. doi:10.1177/1609406917733847
34. Gautham KS, Pearlman S. Do quality improvement projects require IRB approval? J Perinatol. 2021;41:1209-1212. doi:10.1038/s41372-021-01038-1
35. Hennink M, Kaiser BN. Sample sizes for saturation in qualitative research: a systematic review of empirical tests. Soc Sci Med. 2022;292:1-10. doi:10.1016/j.socscimed.2021.114523
36. Balogun M, Dada FO, Oladimeji A, et al. Leading in a time of crisis: a qualitative study capturing experiences of health facility leaders during the early phases of the COVID-19 pandemic in Nigeria’s epicentre. Leadersh Health Serv (Bradf Engl). Published online May 12, 2022. doi:10.1108/lhs-02-2022-0017
37. Guttormson J, Calkins K, McAndrew N, et al. Critical care nurses’ experiences during the COVID-19 pandemic: a US national survey. Am J Crit Care. 2022;31:96-103. doi:10.4037/ajcc2022312
38. Bajaba A, Bajaba S, Algarni M, et al. Adaptive managers as emerging leaders during the COVID-19 crisis. Front Psychol. 2021;12:1-11. doi:10.3389/fpsyg.2021.661628
39. Ahern S, Loh E. Leadership during the COVID-19 pandemic: building and sustaining trust in times of uncertainty. BMJ Lead. 2021;59(4):266-269. doi.org/10.1136/leader-2020-000271
40. Cote R. Adaptive leadership approach with COVID 19 adaptive challenges. J Leadersh Account Ethics. 2022;19:34-44. doi:10.33423/jlae.v19i1.4992
41. Juvet TM, Corbaz-Kurth S, Roos P, et al. Adapting to the unexpected: problematic work situations and resilience strategies in healthcare institutions during the COVID-19 pandemic’s first wave. Saf Sci. 2021;139:1-9. doi:10.1016/j.ssci.2021.105277
From the U.S. Department of Veterans Affairs (all authors), and Cognosante, LLC, Falls Church, VA (Dr. Murray, Dr. Sawyer, and Jessica Fankhauser).
Abstract
Objective: The COVID-19 pandemic posed unprecedented leadership challenges to health care organizations worldwide, especially those on the journey to high reliability. The objective of this pilot quality improvement initiative was to describe the experiences of medical center leaders continuing along the journey to high reliability during the pandemic.
Methods: A convenience sample of Veterans Health Administration medical center directors at facilities that had initiated the journey to high reliability prior to or during the COVID-19 pandemic were asked to complete a confidential survey to explore the challenges experienced and lessons learned.
Results: Of the 35 potential participants, 15 completed the confidential web-based survey. Five major themes emerged from participants’ responses: (1) managing competing priorities, (2) staying committed, (3) adapting and overcoming, (4) prioritizing competing demands, and (5) maintaining momentum.
Conclusion: This pilot quality improvement initiative provides some insight into the challenges experienced and lessons learned during the COVID-19 pandemic to help inform health care leaders’ responses during crises they may encounter along the journey to becoming a high reliability organization.
Keywords: HRO, leadership, patient safety.
Health care leaders worldwide agree that the
Maintaining continuous progress toward advancing high reliability organization (HRO) principles and practices can be especially challenging during crises of unprecedented scale such as the pandemic. HROs must be continually focused on achieving safety, quality, and efficiency goals by attending to the 3 pillars of HRO: culture, leadership, and continuous process improvement. HROs promote a culture where all staff across the organization watch for and report any unsafe conditions before these conditions pose a greater risk in the workplace. Hospital leaders, from executives to frontline managers, must be cognizant of all systems and processes that have the potential to affect patient care.12 All of the principles of HROs must continue without fail to ensure patient safety; these principles include preoccupation with failure, anticipating unexpected risks, sensitivity to dynamic and ever-changing operations, avoiding oversimplifications of identified problems, fostering resilience across the organization, and deferring to those with the expertise to make the best decisions regardless of position, rank, or title.12,13 Given the demands faced by leaders during crises with unprecedented disruption to normal operating procedures, it can be especially difficult to identify systemic challenges and apply lessons learned in a timely manner. However, it is critical to identify such lessons in order to continuously improve and to increase preparedness for subsequent crises.13,14
Because of the COVID-19 pandemic’s unprecedented nature in recent history, a review of the literature produced little evidence exploring the challenges experienced and lessons learned by health care leaders, especially as it relates to implementing or sustaining HRO journeys during the COVID-19 pandemic. Related literature published to date consists of editorials on reliability, uncertainty, and the management of errors15; patient safety and high reliability preventive strategies16; and authentic leadership.17 Five viewpoints were published on HROs and maladaptive stress behaviors,18 mindful organizing and organizational reliability,19 the practical essence of HROs,20 embracing principles of HROs in crisis,8 and using observation and high reliability strategies when facing an unprecedented safety threat.21 Finally, the authors identified 2 studies that used a qualitative research approach to explore leadership functions within an HRO when managing crises22 and organizational change in response to the COVID-19 pandemic.23 Due to the paucity of available information, the authors undertook a pilot quality improvement (QI) initiative to address this knowledge gap.
The aim of this initiative was to gain a better understanding of the challenges experienced, lessons learned, and recommendations to be shared by VHA medical center directors (MCDs) of health care facilities that had initiated the journey to high reliability before or during the COVID-19 pandemic. The authors hope that this information will help health care leaders across both governmental and nongovernmental organizations, nationally and globally, to prepare for future pandemics, other unanticipated crises (eg, natural disasters, terrorist attacks), and major change initiatives (eg, electronic health record modernization) that may affect the delivery of safe, high-quality, and effective patient care. The initiative is described using the SQUIRE 2.0 guidelines.24,25
Methods
Survey
We used a qualitative approach and administered a confidential web-based survey, developed by the project team, to VHA MCDs at facilities that had initiated the journey to high reliability before or during the COVID-19 pandemic. The survey consisted of 8 participant characteristic questions (Table 1) and 4 open-ended questions. The open-ended questions were designed to encourage MCD participants to freely provide detailed descriptions of the challenges experienced, lessons learned, recommendations for other health care leaders, and any additional information they believed was relevant.26,27 Participants were asked to respond to the following items:
- Please describe any challenges you experienced while in the role of MCD at a facility that initiated implementation of HRO principles and practices prior to (February 2020) or during (March 2020–September 2021) the initial onset of the COVID-19 pandemic.
- What are some lessons that you learned when responding to the COVID-19 pandemic while on the journey to high reliability?
- What recommendations would you like to make to other health care leaders to enable them to respond effectively to crises while on the journey to high reliability?
- Please provide any additional information that would be of value.
An invitation to participate in this pilot QI initiative was sent via e-mail to 35 potential participants, who were all MCDs at Cohort 1 and Cohort 2 facilities. The invitation was sent on June 17, 2022, by a VHA senior High Reliability Enterprise Support government team member not directly involved with the initiative.
The invitation included the objective of the initiative, estimated time to complete the confidential web-based survey, time allotted for responses to be submitted, and a link to the survey should potential participants agree to participate. Potential participants were informed that their involvement was voluntary, based on their willingness to participate and available time to complete the survey. Finally, the invitation noted that any comments provided would remain confidential and nonattributional for the purpose of publishing and presenting. The inclusion criteria for participation were: (1) serving
Data Gathering and Analysis
To minimize bias and maintain neutrality at the organizational level, only non-VHA individuals working on the project were directly involved with participants’ data review and analysis. Participant characteristics were analyzed using descriptive statistics. Responses to the 4 open-ended questions were coded and analyzed by an experienced researcher and coauthor using NVivo 11 qualitative data analysis software.28 To ensure trustworthiness (credibility, transferability, dependability, and confirmability) in the data analysis procedure,29 inductive thematic analysis was also performed manually using the methodologies of Braun and Clarke (Table 2)30 and Erlingsson and Brysiewicz.31 The goal of inductive analysis is to allow themes to emerge from the data while minimizing preconceptions.32,33 Regular team meetings were held to discuss and review the progress of data collection and analysis. The authors agreed that the themes were representative of the participants’ responses.

Institutional review board (IRB) review and approval were not required, as this project was a pilot QI initiative. The intent of the initiative was to explore ways to improve the quality of care delivered in the participants’ local care settings and not to generalize the findings. Under these circumstances, formal IRB review and approval of a QI initiative are not required.34 Participation in this pilot QI initiative was voluntary, and participants could withdraw at any time without consequences. Completion of the survey indicated consent. Confidentiality was ensured at all times by avoiding both the use of facility names and the collection of participant identifiers. Unique numbers were assigned to each participant. All comments provided by survey participants remained confidential and nonattributional for the purpose of publishing and presenting.
Results
Of the 35 potential participants, 15 VHA MCDs (43%) completed the confidential web-based survey. Out of the 17 potential participants in Cohort 1, 6 (35%) completed the survey. With Cohort 2, 9 (50%) of the potential 18 participants responded. Although saturation was reached at 10 responses, the additional completed surveys were included in the analysis. Saturation can be achieved with a small number of participants (n = 9–17), particularly when the potential participants are relatively homogenous and project aims are narrowly defined.35 Most participants had more than 10 years of executive-level experience and most medical centers had been on the journey to high reliability for more than 12 months at the time of the pandemic (Table 3).

“There were too many competing priorities dealing with the pandemic and staffing crisis.” (Participant 8)
Other participants shared:
“We had our HRO mentor designated just as our first peak was descending on us. It was initially challenging to determine the proper pace of implementation when we clearly had other things going on. There was a real risk that people would say, ‘What, are you kidding?’ as we tried to roll this out.” (Participant 4)
“Prior to COVID, our main challenges were getting organized and operational rollout. During the pandemic, we had to shift our focus to COVID and the training aspects suffered. Also, many other priorities pulled us away from an HRO rollout focus.” (Participant 6)
“If you don’t need a highly reliable organization during a crisis, when do you need it? That was the message that we kicked off with. It was also VERY important to take things slowly. Education had to be done in bits, and we had a much more modest timeline than what would have been the norm for any initiative pre-COVID. The emphasis was on this being a long-term commitment, that we would be doing it the right way rather than rushing it, etc.” (Participant 4)
“Keeping HRO principles and a Just Culture on the forefront of our minds, we looked for opportunities to progress on our HRO journey, despite the challenges of the pandemic. Our monthly Town Halls became weekly events to share COVID updates and information with staff. We used the Town Halls to promote our HRO mission and to open communication lines with staff, designating 1 week each month as a ‘Safety Forum.’ The pandemic provided the springboard and backdrop for staff Safety Stories submissions, many of which were shared at our Town Halls and Safety Forums.” (Participant 7)
“We were able to utilize HRO principles in response to the COVID pandemic. Specifically standardized communication from the facility to VISN [Veterans Integrated Services Network] was initiated on a daily basis. This practice provided daily communication on key operational items and clinical items at the medical center, allowed timely feedback on actions being taken, as was instrumental in daily checks on staffing, COVID testing supplies, overall supply chain issues.” (Participant 9)
The recommendations provided by 10 participants (Cohort 1, n = 6; Cohort 2, n = 4) for other health care leaders experiencing a crisis during the journey to high reliability were insightful. The themes that frequently emerged from the responses to the survey were to adapt and overcome. Participants shared:
“Utilize the many tools you’re given, specifically your team. Try even the craziest ideas from frontline staff.” (Participant 1)
“Use your mentors for younger directors and, even if you think you know the answer, involve your staff. It makes them feel they have a voice and gives them ownership of the issues.” (Participant 5)
“Make sure that you have key leaders in place who are committed to HRO and can help the organization adjust.” (Participant 6)
“Take advantage of HRO Leader Coaching, which pairs MCDs with coaches who act as consultants for HRO leadership practices to ensure progress in reaching the next level in the journey to High Reliability.” (Participant 7)
“Meet regularly with the HRO Lead and team (more frequently during early stages of implementation) to provide support, eliminate barriers, and champion the HRO mission. It is important to include other members of the ELT [Executive Leadership Team] to ensure their involvement with the facility HRO strategic plan.” (Participant 7)
“Prioritize and understand that not everything is priority #1. Continue what you can with HRO, incorporate high reliability principles into the work being done during a crisis, but understand you may need to modify rollout schedules.” (Participant 8)
The theme of prioritizing competing demands emerged again from 5 participants (Cohort 1, n = 3; Cohort 2, n = 2) with question 3 describing recommendations for other leaders:
“Your first priority is to the crisis. Don’t get distracted by this or any other initiative. That was not a very popular message for the people pushing HRO, but it is the reality and the necessity. However, it IS possible to move forward with HRO (or other important initiatives) during crisis times, as long as you carefully consider what you are asking of people and don’t overload/overwhelm them. It is not your ego (or that of Central Office) that needs to be stoked. If the initiative truly has value, you need to be patient to see it done properly, rather than rushed/pushed/forced. Don’t kill it by being overeager and overwhelming your already overtaxed people. That said, keep moving forward. The key is pacing—and remember that your Type A hard-driving leader types (you know who you are) will certainly fail if they push it. Or even if they go at a normal pace that would be appropriate for noncrisis times.” (Participant 4)
“Prioritize and understand that not everything is priority #1. Continue what you can with HRO, incorporate high reliability principles into the work being done during a crisis, but understand you may need to modify rollout schedules.” (Participant 8)
“It was critical for us to always focus on the immediate workplace safety of staff (especially those on the frontlines of the pandemic response) when in the process of rolling out HRO initiatives.” (Participant 14)
Maintaining Momentum
“It seemed as though communication and education from VHA on HRO slowed down at the same time, which further slowed our progress. We are now trying to ramp our engagement up again.” (Participant 3)
“There can be synergy between crisis response and HRO implementation. As an example, one of the first steps we took was leadership rounding. That was necessary anyways for crisis management (raising the spirits on the front lines, so to speak). What we did was include scheduled time instead of (in addition to) ad hoc. And we got credit for taking an HRO step. I resisted whiteboards/visual management systems for a long time because (in my opinion) that would have been much too distracting during the crisis. Having waited for better times, I was able to move forward with that several months later and with good success.” (Participant 4)
Discussion
Health care leaders worldwide experienced an immense set of challenges because of the COVID-19 pandemic, which is a crisis of a magnitude with no parallel in modern times. Strong, adaptive leadership at all levels of health care systems was needed to effectively address the immense crisis at hand.36,37 Findings from this pilot QI initiative suggest that MCDs faced many new challenges, requiring them to perform unfamiliar tasks and manage numerous overlapping challenges (eg, staffing shortages and reassignments, safety concerns, changes to patient appointments, backlogs in essential services), all while also trying to continue with the journey to high reliability. Despite the challenges leaders faced, they recognized the need to manage competing priorities early and effectively. At times, the priority was to address the wide-ranging, urgent issues related to the pandemic. When the conditions improved, there was time to refocus efforts on important but longer-term activities related to the HRO journey. Other participants recognized that their commitment to HRO needed to remain a priority even during the periods of intense focus on COVID-19.
Some participants felt compelled to stay committed to the HRO journey despite numerous competing demands. They stayed committed to looking for opportunities to progress by implementing HRO principles and practices to achieve safety, quality, and efficiency goals. This dedication is noteworthy, especially in light of recently published research that demonstrates the vast number of patient safety issues that presented during the COVID-19 pandemic (eg, ineffective communication, poor teamwork, the absence of coordination)1 as well as perceptions that patient safety and quality of care had significantly declined as a result of the crisis.36,37
Participants also highlighted the need to be adaptive when responding to the complexity and unpredictability of the pandemic. Participants regularly sought ways to increase their knowledge, skills, and abilities by using the resources (eg, tools, experts) available to them. Research shows that in increasingly complex and ever-changing situation such as the COVID-19 pandemic, leaders must be adaptive with all levels of performance, especially when limited information is available.38,39
This is the first initiative of its kind to specifically explore the challenges experienced and lessons learned from health care leaders continuing along the journey to high reliability during the COVID-19 pandemic. Findings from this pilot QI initiative revealed that many participants recommended that leaders adapt and overcome challenges as much as possible when continuing with HRO during a crisis. These findings are echoed in the current literature suggesting that adaptive performance is a highly effective form of leadership during crises.38,40 Being able to effectively adapt during a crisis is essential for reducing further vulnerabilities across health care systems. In fact, this lesson is shared by many countries in response to the unprecedented global crisis.41A limitation of this pilot QI initiative is that the authors did not directly solicit responses from all VHA MCDs or from other health care executives (eg, Chief of Staff, Associate Director for Operations, Associate Director for Patient Care, and Nurse Executive). As such, our findings represent only a small segment of senior leadership perspectives from a large, integrated health care system. Individuals who did not respond to the survey may have had different experiences than those who did, and the authors excluded many MCDs who formally began their HRO journeys in 2022, well after the pandemic was underway. Similarly, the experiences of Veterans Affairs leaders may or may not be similar to that of other health care organizations. Although the goal of this initiative was to explore the participants’ experiences during the period of crisis, time and distance from the events at the height of the COVID-19 pandemic may have resulted in difficulty recalling information as well as making sense of the occurrence. This potential recall bias is a common occurrence in trying to explore past experiences, especially as they relate to crises. Finally, this pilot QI initiative did not explore personal challenges participants may have faced during this period of time (eg, burnout, personal or family illness), which may have also shaped their responses.
Conclusion
This initiative suggests that VHA MCDs often relied on HRO principles to guide and assist with their response to the COVID-19 pandemic, including managing periods of unprecedented crisis. The ability to adapt and prioritize was seen as an especially important lesson. Many MCDs continued their personal and organizational efforts toward high reliability even in periods of intense challenge because of the pandemic. These findings can help with future crises that may occur during an organization’s journey to high reliability. This pilot QI initiative’s findings warrant further investigation to explore the experiences of the broader range of health care leaders while responding to unplanned crises or even planned large-scale cultural change or technology modernization initiatives (eg, electronic health record modernization) to expand the state of the science of high reliability as well as inform policy and decision-making. Finally, another area for future study is examining how leadership responses vary across facilities, depending on factors such as leader roles, facility complexity level, resource availability, patient population characteristics, and organizational culture.
Acknowledgment: The authors express their sincere gratitude to the medical center directors who participated in this pilot study.
Corresponding author: John S. Murray, PhD, MPH, MSGH, RN, FAAN, 20 Chapel St., Unit A502, Brookline, MA 02446; JMurray325@aol.com
Disclosures: None reported.
From the U.S. Department of Veterans Affairs (all authors), and Cognosante, LLC, Falls Church, VA (Dr. Murray, Dr. Sawyer, and Jessica Fankhauser).
Abstract
Objective: The COVID-19 pandemic posed unprecedented leadership challenges to health care organizations worldwide, especially those on the journey to high reliability. The objective of this pilot quality improvement initiative was to describe the experiences of medical center leaders continuing along the journey to high reliability during the pandemic.
Methods: A convenience sample of Veterans Health Administration medical center directors at facilities that had initiated the journey to high reliability prior to or during the COVID-19 pandemic were asked to complete a confidential survey to explore the challenges experienced and lessons learned.
Results: Of the 35 potential participants, 15 completed the confidential web-based survey. Five major themes emerged from participants’ responses: (1) managing competing priorities, (2) staying committed, (3) adapting and overcoming, (4) prioritizing competing demands, and (5) maintaining momentum.
Conclusion: This pilot quality improvement initiative provides some insight into the challenges experienced and lessons learned during the COVID-19 pandemic to help inform health care leaders’ responses during crises they may encounter along the journey to becoming a high reliability organization.
Keywords: HRO, leadership, patient safety.
Health care leaders worldwide agree that the
Maintaining continuous progress toward advancing high reliability organization (HRO) principles and practices can be especially challenging during crises of unprecedented scale such as the pandemic. HROs must be continually focused on achieving safety, quality, and efficiency goals by attending to the 3 pillars of HRO: culture, leadership, and continuous process improvement. HROs promote a culture where all staff across the organization watch for and report any unsafe conditions before these conditions pose a greater risk in the workplace. Hospital leaders, from executives to frontline managers, must be cognizant of all systems and processes that have the potential to affect patient care.12 All of the principles of HROs must continue without fail to ensure patient safety; these principles include preoccupation with failure, anticipating unexpected risks, sensitivity to dynamic and ever-changing operations, avoiding oversimplifications of identified problems, fostering resilience across the organization, and deferring to those with the expertise to make the best decisions regardless of position, rank, or title.12,13 Given the demands faced by leaders during crises with unprecedented disruption to normal operating procedures, it can be especially difficult to identify systemic challenges and apply lessons learned in a timely manner. However, it is critical to identify such lessons in order to continuously improve and to increase preparedness for subsequent crises.13,14
Because of the COVID-19 pandemic’s unprecedented nature in recent history, a review of the literature produced little evidence exploring the challenges experienced and lessons learned by health care leaders, especially as it relates to implementing or sustaining HRO journeys during the COVID-19 pandemic. Related literature published to date consists of editorials on reliability, uncertainty, and the management of errors15; patient safety and high reliability preventive strategies16; and authentic leadership.17 Five viewpoints were published on HROs and maladaptive stress behaviors,18 mindful organizing and organizational reliability,19 the practical essence of HROs,20 embracing principles of HROs in crisis,8 and using observation and high reliability strategies when facing an unprecedented safety threat.21 Finally, the authors identified 2 studies that used a qualitative research approach to explore leadership functions within an HRO when managing crises22 and organizational change in response to the COVID-19 pandemic.23 Due to the paucity of available information, the authors undertook a pilot quality improvement (QI) initiative to address this knowledge gap.
The aim of this initiative was to gain a better understanding of the challenges experienced, lessons learned, and recommendations to be shared by VHA medical center directors (MCDs) of health care facilities that had initiated the journey to high reliability before or during the COVID-19 pandemic. The authors hope that this information will help health care leaders across both governmental and nongovernmental organizations, nationally and globally, to prepare for future pandemics, other unanticipated crises (eg, natural disasters, terrorist attacks), and major change initiatives (eg, electronic health record modernization) that may affect the delivery of safe, high-quality, and effective patient care. The initiative is described using the SQUIRE 2.0 guidelines.24,25
Methods
Survey
We used a qualitative approach and administered a confidential web-based survey, developed by the project team, to VHA MCDs at facilities that had initiated the journey to high reliability before or during the COVID-19 pandemic. The survey consisted of 8 participant characteristic questions (Table 1) and 4 open-ended questions. The open-ended questions were designed to encourage MCD participants to freely provide detailed descriptions of the challenges experienced, lessons learned, recommendations for other health care leaders, and any additional information they believed was relevant.26,27 Participants were asked to respond to the following items:
- Please describe any challenges you experienced while in the role of MCD at a facility that initiated implementation of HRO principles and practices prior to (February 2020) or during (March 2020–September 2021) the initial onset of the COVID-19 pandemic.
- What are some lessons that you learned when responding to the COVID-19 pandemic while on the journey to high reliability?
- What recommendations would you like to make to other health care leaders to enable them to respond effectively to crises while on the journey to high reliability?
- Please provide any additional information that would be of value.
An invitation to participate in this pilot QI initiative was sent via e-mail to 35 potential participants, who were all MCDs at Cohort 1 and Cohort 2 facilities. The invitation was sent on June 17, 2022, by a VHA senior High Reliability Enterprise Support government team member not directly involved with the initiative.
The invitation included the objective of the initiative, estimated time to complete the confidential web-based survey, time allotted for responses to be submitted, and a link to the survey should potential participants agree to participate. Potential participants were informed that their involvement was voluntary, based on their willingness to participate and available time to complete the survey. Finally, the invitation noted that any comments provided would remain confidential and nonattributional for the purpose of publishing and presenting. The inclusion criteria for participation were: (1) serving
Data Gathering and Analysis
To minimize bias and maintain neutrality at the organizational level, only non-VHA individuals working on the project were directly involved with participants’ data review and analysis. Participant characteristics were analyzed using descriptive statistics. Responses to the 4 open-ended questions were coded and analyzed by an experienced researcher and coauthor using NVivo 11 qualitative data analysis software.28 To ensure trustworthiness (credibility, transferability, dependability, and confirmability) in the data analysis procedure,29 inductive thematic analysis was also performed manually using the methodologies of Braun and Clarke (Table 2)30 and Erlingsson and Brysiewicz.31 The goal of inductive analysis is to allow themes to emerge from the data while minimizing preconceptions.32,33 Regular team meetings were held to discuss and review the progress of data collection and analysis. The authors agreed that the themes were representative of the participants’ responses.

Institutional review board (IRB) review and approval were not required, as this project was a pilot QI initiative. The intent of the initiative was to explore ways to improve the quality of care delivered in the participants’ local care settings and not to generalize the findings. Under these circumstances, formal IRB review and approval of a QI initiative are not required.34 Participation in this pilot QI initiative was voluntary, and participants could withdraw at any time without consequences. Completion of the survey indicated consent. Confidentiality was ensured at all times by avoiding both the use of facility names and the collection of participant identifiers. Unique numbers were assigned to each participant. All comments provided by survey participants remained confidential and nonattributional for the purpose of publishing and presenting.
Results
Of the 35 potential participants, 15 VHA MCDs (43%) completed the confidential web-based survey. Out of the 17 potential participants in Cohort 1, 6 (35%) completed the survey. With Cohort 2, 9 (50%) of the potential 18 participants responded. Although saturation was reached at 10 responses, the additional completed surveys were included in the analysis. Saturation can be achieved with a small number of participants (n = 9–17), particularly when the potential participants are relatively homogenous and project aims are narrowly defined.35 Most participants had more than 10 years of executive-level experience and most medical centers had been on the journey to high reliability for more than 12 months at the time of the pandemic (Table 3).

“There were too many competing priorities dealing with the pandemic and staffing crisis.” (Participant 8)
Other participants shared:
“We had our HRO mentor designated just as our first peak was descending on us. It was initially challenging to determine the proper pace of implementation when we clearly had other things going on. There was a real risk that people would say, ‘What, are you kidding?’ as we tried to roll this out.” (Participant 4)
“Prior to COVID, our main challenges were getting organized and operational rollout. During the pandemic, we had to shift our focus to COVID and the training aspects suffered. Also, many other priorities pulled us away from an HRO rollout focus.” (Participant 6)
“If you don’t need a highly reliable organization during a crisis, when do you need it? That was the message that we kicked off with. It was also VERY important to take things slowly. Education had to be done in bits, and we had a much more modest timeline than what would have been the norm for any initiative pre-COVID. The emphasis was on this being a long-term commitment, that we would be doing it the right way rather than rushing it, etc.” (Participant 4)
“Keeping HRO principles and a Just Culture on the forefront of our minds, we looked for opportunities to progress on our HRO journey, despite the challenges of the pandemic. Our monthly Town Halls became weekly events to share COVID updates and information with staff. We used the Town Halls to promote our HRO mission and to open communication lines with staff, designating 1 week each month as a ‘Safety Forum.’ The pandemic provided the springboard and backdrop for staff Safety Stories submissions, many of which were shared at our Town Halls and Safety Forums.” (Participant 7)
“We were able to utilize HRO principles in response to the COVID pandemic. Specifically standardized communication from the facility to VISN [Veterans Integrated Services Network] was initiated on a daily basis. This practice provided daily communication on key operational items and clinical items at the medical center, allowed timely feedback on actions being taken, as was instrumental in daily checks on staffing, COVID testing supplies, overall supply chain issues.” (Participant 9)
The recommendations provided by 10 participants (Cohort 1, n = 6; Cohort 2, n = 4) for other health care leaders experiencing a crisis during the journey to high reliability were insightful. The themes that frequently emerged from the responses to the survey were to adapt and overcome. Participants shared:
“Utilize the many tools you’re given, specifically your team. Try even the craziest ideas from frontline staff.” (Participant 1)
“Use your mentors for younger directors and, even if you think you know the answer, involve your staff. It makes them feel they have a voice and gives them ownership of the issues.” (Participant 5)
“Make sure that you have key leaders in place who are committed to HRO and can help the organization adjust.” (Participant 6)
“Take advantage of HRO Leader Coaching, which pairs MCDs with coaches who act as consultants for HRO leadership practices to ensure progress in reaching the next level in the journey to High Reliability.” (Participant 7)
“Meet regularly with the HRO Lead and team (more frequently during early stages of implementation) to provide support, eliminate barriers, and champion the HRO mission. It is important to include other members of the ELT [Executive Leadership Team] to ensure their involvement with the facility HRO strategic plan.” (Participant 7)
“Prioritize and understand that not everything is priority #1. Continue what you can with HRO, incorporate high reliability principles into the work being done during a crisis, but understand you may need to modify rollout schedules.” (Participant 8)
The theme of prioritizing competing demands emerged again from 5 participants (Cohort 1, n = 3; Cohort 2, n = 2) with question 3 describing recommendations for other leaders:
“Your first priority is to the crisis. Don’t get distracted by this or any other initiative. That was not a very popular message for the people pushing HRO, but it is the reality and the necessity. However, it IS possible to move forward with HRO (or other important initiatives) during crisis times, as long as you carefully consider what you are asking of people and don’t overload/overwhelm them. It is not your ego (or that of Central Office) that needs to be stoked. If the initiative truly has value, you need to be patient to see it done properly, rather than rushed/pushed/forced. Don’t kill it by being overeager and overwhelming your already overtaxed people. That said, keep moving forward. The key is pacing—and remember that your Type A hard-driving leader types (you know who you are) will certainly fail if they push it. Or even if they go at a normal pace that would be appropriate for noncrisis times.” (Participant 4)
“Prioritize and understand that not everything is priority #1. Continue what you can with HRO, incorporate high reliability principles into the work being done during a crisis, but understand you may need to modify rollout schedules.” (Participant 8)
“It was critical for us to always focus on the immediate workplace safety of staff (especially those on the frontlines of the pandemic response) when in the process of rolling out HRO initiatives.” (Participant 14)
Maintaining Momentum
“It seemed as though communication and education from VHA on HRO slowed down at the same time, which further slowed our progress. We are now trying to ramp our engagement up again.” (Participant 3)
“There can be synergy between crisis response and HRO implementation. As an example, one of the first steps we took was leadership rounding. That was necessary anyways for crisis management (raising the spirits on the front lines, so to speak). What we did was include scheduled time instead of (in addition to) ad hoc. And we got credit for taking an HRO step. I resisted whiteboards/visual management systems for a long time because (in my opinion) that would have been much too distracting during the crisis. Having waited for better times, I was able to move forward with that several months later and with good success.” (Participant 4)
Discussion
Health care leaders worldwide experienced an immense set of challenges because of the COVID-19 pandemic, which is a crisis of a magnitude with no parallel in modern times. Strong, adaptive leadership at all levels of health care systems was needed to effectively address the immense crisis at hand.36,37 Findings from this pilot QI initiative suggest that MCDs faced many new challenges, requiring them to perform unfamiliar tasks and manage numerous overlapping challenges (eg, staffing shortages and reassignments, safety concerns, changes to patient appointments, backlogs in essential services), all while also trying to continue with the journey to high reliability. Despite the challenges leaders faced, they recognized the need to manage competing priorities early and effectively. At times, the priority was to address the wide-ranging, urgent issues related to the pandemic. When the conditions improved, there was time to refocus efforts on important but longer-term activities related to the HRO journey. Other participants recognized that their commitment to HRO needed to remain a priority even during the periods of intense focus on COVID-19.
Some participants felt compelled to stay committed to the HRO journey despite numerous competing demands. They stayed committed to looking for opportunities to progress by implementing HRO principles and practices to achieve safety, quality, and efficiency goals. This dedication is noteworthy, especially in light of recently published research that demonstrates the vast number of patient safety issues that presented during the COVID-19 pandemic (eg, ineffective communication, poor teamwork, the absence of coordination)1 as well as perceptions that patient safety and quality of care had significantly declined as a result of the crisis.36,37
Participants also highlighted the need to be adaptive when responding to the complexity and unpredictability of the pandemic. Participants regularly sought ways to increase their knowledge, skills, and abilities by using the resources (eg, tools, experts) available to them. Research shows that in increasingly complex and ever-changing situation such as the COVID-19 pandemic, leaders must be adaptive with all levels of performance, especially when limited information is available.38,39
This is the first initiative of its kind to specifically explore the challenges experienced and lessons learned from health care leaders continuing along the journey to high reliability during the COVID-19 pandemic. Findings from this pilot QI initiative revealed that many participants recommended that leaders adapt and overcome challenges as much as possible when continuing with HRO during a crisis. These findings are echoed in the current literature suggesting that adaptive performance is a highly effective form of leadership during crises.38,40 Being able to effectively adapt during a crisis is essential for reducing further vulnerabilities across health care systems. In fact, this lesson is shared by many countries in response to the unprecedented global crisis.41A limitation of this pilot QI initiative is that the authors did not directly solicit responses from all VHA MCDs or from other health care executives (eg, Chief of Staff, Associate Director for Operations, Associate Director for Patient Care, and Nurse Executive). As such, our findings represent only a small segment of senior leadership perspectives from a large, integrated health care system. Individuals who did not respond to the survey may have had different experiences than those who did, and the authors excluded many MCDs who formally began their HRO journeys in 2022, well after the pandemic was underway. Similarly, the experiences of Veterans Affairs leaders may or may not be similar to that of other health care organizations. Although the goal of this initiative was to explore the participants’ experiences during the period of crisis, time and distance from the events at the height of the COVID-19 pandemic may have resulted in difficulty recalling information as well as making sense of the occurrence. This potential recall bias is a common occurrence in trying to explore past experiences, especially as they relate to crises. Finally, this pilot QI initiative did not explore personal challenges participants may have faced during this period of time (eg, burnout, personal or family illness), which may have also shaped their responses.
Conclusion
This initiative suggests that VHA MCDs often relied on HRO principles to guide and assist with their response to the COVID-19 pandemic, including managing periods of unprecedented crisis. The ability to adapt and prioritize was seen as an especially important lesson. Many MCDs continued their personal and organizational efforts toward high reliability even in periods of intense challenge because of the pandemic. These findings can help with future crises that may occur during an organization’s journey to high reliability. This pilot QI initiative’s findings warrant further investigation to explore the experiences of the broader range of health care leaders while responding to unplanned crises or even planned large-scale cultural change or technology modernization initiatives (eg, electronic health record modernization) to expand the state of the science of high reliability as well as inform policy and decision-making. Finally, another area for future study is examining how leadership responses vary across facilities, depending on factors such as leader roles, facility complexity level, resource availability, patient population characteristics, and organizational culture.
Acknowledgment: The authors express their sincere gratitude to the medical center directors who participated in this pilot study.
Corresponding author: John S. Murray, PhD, MPH, MSGH, RN, FAAN, 20 Chapel St., Unit A502, Brookline, MA 02446; JMurray325@aol.com
Disclosures: None reported.
1. Editors: Dying in a leadership vacuum. 9.4N Engl J Med. 2020;383(15):1479-1480. doi:10.1056/NEJMe2029812
2. Geerts JM, Kinnair D, Taheri P, et al. Guidance for health care leaders during the recovery stage of the COVID-19 pandemic: a consensus statement. JAMA Netw Open. 2021;4(7):1-16. doi:10.1001/jamanetworkopen.2021.20295
3. Boiral O, Brotherton M-C, Rivaud L, et al. Organizations’ management of the COVID-19 pandemic: a scoping review of business articles. Sustainability. 2021;13:1-20. doi:10.3390/su13073993
4. Razu SR, Yasmin T, Arif TB, et al. Challenges faced by healthcare professionals during the COVID-19 pandemic: a qualitative inquiry from Bangladesh. Front Public Health. 2021;9:1-13. doi:10.3389/fpubh.2021.647315
5. Lyng HB, Ree E, Wibe T, et al. Healthcare leaders’ use of innovative solutions to ensure resilience in healthcare during the Covid-19 pandemic: a qualitative study in Norwegian nursing homes and home care services. BMC Health Serv Res. 2021;21(1):1-11. doi:1186/s12913-021-06923-1
6. Freitas J. Queiroz A, Bortotti I, et al. Nurse leaders’ challenges fighting the COVID-19 pandemic: a qualitative study. Open J Nurs. 2021;11:267-280. doi:10.4236/ojn.2021.115024
7. McGuire AL, Aulisio MP, Davis FD, et al. Ethical challenges arising in the COVID-19 pandemic: an overview from the Association of Bioethics Program Directors (ABPD) Task Force. 9.4Am J Bioeth. 2020;20(7):15-27. doi:10.1080/15265161.2020.1764138
8. Turbow RM, Scibilia JP. Embracing principles of high reliability organizations can improve patient safety during pandemic. AAP News. January 19, 2021. Accessed March 1, 2023. https://publications.aap.org/aapnews/news/8975
9. Roberts BH, Damiano LA, Graham S, et al. A case study in fostering a learning culture in the context of Covid-19. American Association for Physician Leadership. June 24, 2021. Accessed March 1, 2023. https://www.physicianleaders.org/news/a-case-study-in-fostering-a-learning-culture-in-the-context-of-covid-19
10. U.S. Department of Veterans Affairs. Department of Veterans AffairsCOVID-19 National Summary. Veterans Affairs. Accessed December 4, 2022. https://www.accesstocare.va.gov/Healthcare/COVID19NationalSummary
11. U.S. Department of Veterans Affairs. VA fourth mission summary. Veterans Affairs. Accessed December 4, 2022. https://www.va.gov/health/coronavirus/statesupport.asp#:~:text=As%20part%20of%20the%20Fourth,the%20facilities%20we%20are%20supporting
12. Veazie S, Peterson K, Bourne D, et al. Implementing high-reliability organization principles into practice: a rapid evidence review. J Patient Saf. 2022;18(1):e320-e328. doi:10.1097/PTS.0000000000000768
13. Murray JS, Kelly S, Hanover C. Promoting psychological safety in healthcare organizations. 9.4Mil Med. 2022;187(7-8):808-810. doi:10.1093/milmed/usac041
14. Maison D, Jaworska D, Adamczyk D, et al. The challenges arising from the COVID-19 pandemic and the way people deal with them: a qualitative longitudinal study. PLoS One. 2021;16(10):1-17. doi:10.1371/journal.pone.0258133
15. Schulman PR. Reliability, uncertainty and the management of error: new perspectives in the COVID-19 era. J Contingencies Crisis Manag. 2022;30:92-101. doi:10.1111/1468-5973.12356
16. Adelman JS, Gandhi TK. COVID-19 and patient safety: time to tap into our investment in high reliability. J Patient Saf. 2021;17(4): 331-333. doi:10.1097/PTS.0000000000000843
17. Shingler-Nace A. COVID-19: when leadership calls. Nurs Lead. 2020;18(3):202-203. doi:10.1016/j.mnl.2020.03.017
18. Van Stralen D, Mercer TA. During pandemic COVID 19, the high reliability organization (HRO) identifies maladaptive stress behaviors: the stress-fear-threat cascade. Neonatol Tod. 2020;15(11):113-124. doi: 10.51362/neonatology.today/2020111511113124
19. Vogus TJ, Wilson AD, Randall K, et al. We’re all in this together: how COVID-19 revealed the coconstruction of mindful organising and organisational reliability. BMJ Qual Saf. 2022;31(3):230-233. doi:10.1136/bmjqs-2021-014068
20. Van Stralen D. Pragmatic high-reliability organization (HRO) during pandemic COVID-19. Neonatol Tod. 2020(4);15:109-117. doi:10.51362/neonatology.today/20208158109117
21. Thull-Freedman J, Mondoux S, Stang A, et al. Going to the COVID-19 Gemba: using observation and high reliability strategies to achieve safety in a time of crisis. CJEM. 2020;22(6):738-741. doi:10.1017/cem.2020.380
22. Sarihasan I, Dajnoki K, Oláh J, et al. The importance of the leadership functions of a high-reliability health care organization in managing the COVID-19 pandemic in Turkey. Econ Sociol. 2022;15:78-93. doi:10.14254/2071-789x.2022/15-1/5
23. Crain MA, Bush AL, Hayanga H, et al. Healthcare leadership in the COVID-19 pandemic: from innovative preparation to evolutionary transformation. J Health Leadersh. 2021;13:199-207. doi:10.2147/JHL.S319829
24. SQUIRE. Revised Standards for Quality Improvement Reporting Excellence (SQUIRE 2.0) SQUIRE; 2020. Accessed March 1, 2023. http://www.squire-statement.org/index.cfm?fuseaction=Page.ViewPage&pageId=471
25. Lounsbury O. How to write a quality improvement project. Patient Safety J. 2022;4(1):65-67. doi:10.33940/culture/2022.3.6
26. Bengtsson M. How to plan and perform a qualitative study using content analysis. Nurs Plus Open. 2016;2:8-14. doi:10.1016/j.npls.2016.01.001
27. Allen M. The Sage Encyclopedia of Communication Research Methods. (Vols. 1-4). SAGE Publications, Inc; 2017
28. Unlock insights with qualitative data analysis software. Lumivero. Accessed March 2, 2023. https://lumivero.com/products/nvivo/
29. Maher C, Hadfield M, Hutchings M, et al. Ensuring rigor in qualitative data analysis: a design research approach to coding combining NVivo with traditional material methods. Int J Qual Methods. 2018;17:1-13. doi:10.1177/1609406918786362
30. Braun V, Clarke V. Using thematic analysis in psychology. Qual Res Psychol. 2006;3:77-101. doi:10.1191/1478088706qp063oa
31. Erlingsson C, Brysiewicz P. A hands-on guide to doing content analysis. Afr J Emerg Med. 2017;7:93-99. doi:10.1016/j.afjem.2017.08.001
32. Vears DF, Gillam L. Inductive content analysis: a guide for beginning qualitative researchers. FoHPE. 2022;23:111-127. doi:10.11157/fohpe.v23i1.544
33. Nowell LS, Norris JM, White DE, et al. Thematic analysis: striving to meet the trustworthiness criteria. Int J Qual Methods. 2017;16:1-13. doi:10.1177/1609406917733847
34. Gautham KS, Pearlman S. Do quality improvement projects require IRB approval? J Perinatol. 2021;41:1209-1212. doi:10.1038/s41372-021-01038-1
35. Hennink M, Kaiser BN. Sample sizes for saturation in qualitative research: a systematic review of empirical tests. Soc Sci Med. 2022;292:1-10. doi:10.1016/j.socscimed.2021.114523
36. Balogun M, Dada FO, Oladimeji A, et al. Leading in a time of crisis: a qualitative study capturing experiences of health facility leaders during the early phases of the COVID-19 pandemic in Nigeria’s epicentre. Leadersh Health Serv (Bradf Engl). Published online May 12, 2022. doi:10.1108/lhs-02-2022-0017
37. Guttormson J, Calkins K, McAndrew N, et al. Critical care nurses’ experiences during the COVID-19 pandemic: a US national survey. Am J Crit Care. 2022;31:96-103. doi:10.4037/ajcc2022312
38. Bajaba A, Bajaba S, Algarni M, et al. Adaptive managers as emerging leaders during the COVID-19 crisis. Front Psychol. 2021;12:1-11. doi:10.3389/fpsyg.2021.661628
39. Ahern S, Loh E. Leadership during the COVID-19 pandemic: building and sustaining trust in times of uncertainty. BMJ Lead. 2021;59(4):266-269. doi.org/10.1136/leader-2020-000271
40. Cote R. Adaptive leadership approach with COVID 19 adaptive challenges. J Leadersh Account Ethics. 2022;19:34-44. doi:10.33423/jlae.v19i1.4992
41. Juvet TM, Corbaz-Kurth S, Roos P, et al. Adapting to the unexpected: problematic work situations and resilience strategies in healthcare institutions during the COVID-19 pandemic’s first wave. Saf Sci. 2021;139:1-9. doi:10.1016/j.ssci.2021.105277
1. Editors: Dying in a leadership vacuum. 9.4N Engl J Med. 2020;383(15):1479-1480. doi:10.1056/NEJMe2029812
2. Geerts JM, Kinnair D, Taheri P, et al. Guidance for health care leaders during the recovery stage of the COVID-19 pandemic: a consensus statement. JAMA Netw Open. 2021;4(7):1-16. doi:10.1001/jamanetworkopen.2021.20295
3. Boiral O, Brotherton M-C, Rivaud L, et al. Organizations’ management of the COVID-19 pandemic: a scoping review of business articles. Sustainability. 2021;13:1-20. doi:10.3390/su13073993
4. Razu SR, Yasmin T, Arif TB, et al. Challenges faced by healthcare professionals during the COVID-19 pandemic: a qualitative inquiry from Bangladesh. Front Public Health. 2021;9:1-13. doi:10.3389/fpubh.2021.647315
5. Lyng HB, Ree E, Wibe T, et al. Healthcare leaders’ use of innovative solutions to ensure resilience in healthcare during the Covid-19 pandemic: a qualitative study in Norwegian nursing homes and home care services. BMC Health Serv Res. 2021;21(1):1-11. doi:1186/s12913-021-06923-1
6. Freitas J. Queiroz A, Bortotti I, et al. Nurse leaders’ challenges fighting the COVID-19 pandemic: a qualitative study. Open J Nurs. 2021;11:267-280. doi:10.4236/ojn.2021.115024
7. McGuire AL, Aulisio MP, Davis FD, et al. Ethical challenges arising in the COVID-19 pandemic: an overview from the Association of Bioethics Program Directors (ABPD) Task Force. 9.4Am J Bioeth. 2020;20(7):15-27. doi:10.1080/15265161.2020.1764138
8. Turbow RM, Scibilia JP. Embracing principles of high reliability organizations can improve patient safety during pandemic. AAP News. January 19, 2021. Accessed March 1, 2023. https://publications.aap.org/aapnews/news/8975
9. Roberts BH, Damiano LA, Graham S, et al. A case study in fostering a learning culture in the context of Covid-19. American Association for Physician Leadership. June 24, 2021. Accessed March 1, 2023. https://www.physicianleaders.org/news/a-case-study-in-fostering-a-learning-culture-in-the-context-of-covid-19
10. U.S. Department of Veterans Affairs. Department of Veterans AffairsCOVID-19 National Summary. Veterans Affairs. Accessed December 4, 2022. https://www.accesstocare.va.gov/Healthcare/COVID19NationalSummary
11. U.S. Department of Veterans Affairs. VA fourth mission summary. Veterans Affairs. Accessed December 4, 2022. https://www.va.gov/health/coronavirus/statesupport.asp#:~:text=As%20part%20of%20the%20Fourth,the%20facilities%20we%20are%20supporting
12. Veazie S, Peterson K, Bourne D, et al. Implementing high-reliability organization principles into practice: a rapid evidence review. J Patient Saf. 2022;18(1):e320-e328. doi:10.1097/PTS.0000000000000768
13. Murray JS, Kelly S, Hanover C. Promoting psychological safety in healthcare organizations. 9.4Mil Med. 2022;187(7-8):808-810. doi:10.1093/milmed/usac041
14. Maison D, Jaworska D, Adamczyk D, et al. The challenges arising from the COVID-19 pandemic and the way people deal with them: a qualitative longitudinal study. PLoS One. 2021;16(10):1-17. doi:10.1371/journal.pone.0258133
15. Schulman PR. Reliability, uncertainty and the management of error: new perspectives in the COVID-19 era. J Contingencies Crisis Manag. 2022;30:92-101. doi:10.1111/1468-5973.12356
16. Adelman JS, Gandhi TK. COVID-19 and patient safety: time to tap into our investment in high reliability. J Patient Saf. 2021;17(4): 331-333. doi:10.1097/PTS.0000000000000843
17. Shingler-Nace A. COVID-19: when leadership calls. Nurs Lead. 2020;18(3):202-203. doi:10.1016/j.mnl.2020.03.017
18. Van Stralen D, Mercer TA. During pandemic COVID 19, the high reliability organization (HRO) identifies maladaptive stress behaviors: the stress-fear-threat cascade. Neonatol Tod. 2020;15(11):113-124. doi: 10.51362/neonatology.today/2020111511113124
19. Vogus TJ, Wilson AD, Randall K, et al. We’re all in this together: how COVID-19 revealed the coconstruction of mindful organising and organisational reliability. BMJ Qual Saf. 2022;31(3):230-233. doi:10.1136/bmjqs-2021-014068
20. Van Stralen D. Pragmatic high-reliability organization (HRO) during pandemic COVID-19. Neonatol Tod. 2020(4);15:109-117. doi:10.51362/neonatology.today/20208158109117
21. Thull-Freedman J, Mondoux S, Stang A, et al. Going to the COVID-19 Gemba: using observation and high reliability strategies to achieve safety in a time of crisis. CJEM. 2020;22(6):738-741. doi:10.1017/cem.2020.380
22. Sarihasan I, Dajnoki K, Oláh J, et al. The importance of the leadership functions of a high-reliability health care organization in managing the COVID-19 pandemic in Turkey. Econ Sociol. 2022;15:78-93. doi:10.14254/2071-789x.2022/15-1/5
23. Crain MA, Bush AL, Hayanga H, et al. Healthcare leadership in the COVID-19 pandemic: from innovative preparation to evolutionary transformation. J Health Leadersh. 2021;13:199-207. doi:10.2147/JHL.S319829
24. SQUIRE. Revised Standards for Quality Improvement Reporting Excellence (SQUIRE 2.0) SQUIRE; 2020. Accessed March 1, 2023. http://www.squire-statement.org/index.cfm?fuseaction=Page.ViewPage&pageId=471
25. Lounsbury O. How to write a quality improvement project. Patient Safety J. 2022;4(1):65-67. doi:10.33940/culture/2022.3.6
26. Bengtsson M. How to plan and perform a qualitative study using content analysis. Nurs Plus Open. 2016;2:8-14. doi:10.1016/j.npls.2016.01.001
27. Allen M. The Sage Encyclopedia of Communication Research Methods. (Vols. 1-4). SAGE Publications, Inc; 2017
28. Unlock insights with qualitative data analysis software. Lumivero. Accessed March 2, 2023. https://lumivero.com/products/nvivo/
29. Maher C, Hadfield M, Hutchings M, et al. Ensuring rigor in qualitative data analysis: a design research approach to coding combining NVivo with traditional material methods. Int J Qual Methods. 2018;17:1-13. doi:10.1177/1609406918786362
30. Braun V, Clarke V. Using thematic analysis in psychology. Qual Res Psychol. 2006;3:77-101. doi:10.1191/1478088706qp063oa
31. Erlingsson C, Brysiewicz P. A hands-on guide to doing content analysis. Afr J Emerg Med. 2017;7:93-99. doi:10.1016/j.afjem.2017.08.001
32. Vears DF, Gillam L. Inductive content analysis: a guide for beginning qualitative researchers. FoHPE. 2022;23:111-127. doi:10.11157/fohpe.v23i1.544
33. Nowell LS, Norris JM, White DE, et al. Thematic analysis: striving to meet the trustworthiness criteria. Int J Qual Methods. 2017;16:1-13. doi:10.1177/1609406917733847
34. Gautham KS, Pearlman S. Do quality improvement projects require IRB approval? J Perinatol. 2021;41:1209-1212. doi:10.1038/s41372-021-01038-1
35. Hennink M, Kaiser BN. Sample sizes for saturation in qualitative research: a systematic review of empirical tests. Soc Sci Med. 2022;292:1-10. doi:10.1016/j.socscimed.2021.114523
36. Balogun M, Dada FO, Oladimeji A, et al. Leading in a time of crisis: a qualitative study capturing experiences of health facility leaders during the early phases of the COVID-19 pandemic in Nigeria’s epicentre. Leadersh Health Serv (Bradf Engl). Published online May 12, 2022. doi:10.1108/lhs-02-2022-0017
37. Guttormson J, Calkins K, McAndrew N, et al. Critical care nurses’ experiences during the COVID-19 pandemic: a US national survey. Am J Crit Care. 2022;31:96-103. doi:10.4037/ajcc2022312
38. Bajaba A, Bajaba S, Algarni M, et al. Adaptive managers as emerging leaders during the COVID-19 crisis. Front Psychol. 2021;12:1-11. doi:10.3389/fpsyg.2021.661628
39. Ahern S, Loh E. Leadership during the COVID-19 pandemic: building and sustaining trust in times of uncertainty. BMJ Lead. 2021;59(4):266-269. doi.org/10.1136/leader-2020-000271
40. Cote R. Adaptive leadership approach with COVID 19 adaptive challenges. J Leadersh Account Ethics. 2022;19:34-44. doi:10.33423/jlae.v19i1.4992
41. Juvet TM, Corbaz-Kurth S, Roos P, et al. Adapting to the unexpected: problematic work situations and resilience strategies in healthcare institutions during the COVID-19 pandemic’s first wave. Saf Sci. 2021;139:1-9. doi:10.1016/j.ssci.2021.105277
JCOM: 30 Years of Advancing Quality Improvement and Innovation in Care Delivery
This year marks the publication of the 30th volume of the Journal of Clinical Outcomes Management (JCOM). As we celebrate JCOM’s 30th year, we look forward to the future and continuing the journey to inform quality improvement leaders and practitioners about advances in the field and share experiences. The path forward on this journey involves collaboration across stakeholders, the application of innovative improvement methods, and a commitment to achieving health equity. Health care quality improvement plans must prioritize patient-centered care, promote evidence-based practices and continuous learning, and establish clear metrics to measure progress and success. Furthermore, engagement with patients and communities must be at the forefront of any quality improvement plan, as their perspectives and experiences are essential to understanding and addressing the root causes of disparities in health care delivery. Additionally, effective communication and coordination among health care providers, administrators, policymakers, and other stakeholders are crucial to achieving sustainable improvements in health care quality.
JCOM’s mission is to serve as a platform for sharing knowledge, experiences, and best practices to improve patient outcomes and promote health equity. The vision encompasses a world where all individuals have access to high-quality, patient-centered health care that is free of disparities and achieves optimal health outcomes. JCOM’s strategy is to publish articles that showcase innovative quality improvement initiatives, share evidence-based practices and research findings, highlight successful collaborations, and provide practical guidance for health care professionals to implement quality improvement initiatives in their organizations.
We believe that by sharing these insights and experiences, we can accelerate progress toward achieving equitable and high-quality health care for all individuals and communities, regardless of their socioeconomic status, race/ethnicity, gender identity, or any other factor that may impact their access to care and health outcomes. We continue to welcome submissions from all health care professionals, researchers, and other stakeholders involved in quality improvement initiatives. Together, we can work toward a future where every individual has access to the highest quality of health care and experiences equitable health outcomes.
A comprehensive and collaborative approach to health care quality improvement, which is led by a peer review process and scientific publication of the progress, is a necessary part of ensuring that all patients receive high-quality care that is equitable and patient-centered. The future of health care quality will require further research and scholarly work in the areas of training and development, data infrastructure and analytics, as well as technology-enabled solutions that support continuous improvement and innovation. Health care organizations can build a culture of quality improvement that drives meaningful progress toward achieving health equity and improving health care delivery for all by sharing the output from their research.
Thank you for joining us in this mission to improve health care quality, promote optimal health care delivery methods, and create a world where health care is not only accessible, but also equitable and of the highest standards. Let us continue to work toward building a health care system that prioritizes patient-centered care. Together, we can make a difference and ensure that every individual receives the care they need and deserve.
Corresponding author: Ebrahim Barkoudah, MD, MPH; ebarkoudah@bwh.harvard.edu
This year marks the publication of the 30th volume of the Journal of Clinical Outcomes Management (JCOM). As we celebrate JCOM’s 30th year, we look forward to the future and continuing the journey to inform quality improvement leaders and practitioners about advances in the field and share experiences. The path forward on this journey involves collaboration across stakeholders, the application of innovative improvement methods, and a commitment to achieving health equity. Health care quality improvement plans must prioritize patient-centered care, promote evidence-based practices and continuous learning, and establish clear metrics to measure progress and success. Furthermore, engagement with patients and communities must be at the forefront of any quality improvement plan, as their perspectives and experiences are essential to understanding and addressing the root causes of disparities in health care delivery. Additionally, effective communication and coordination among health care providers, administrators, policymakers, and other stakeholders are crucial to achieving sustainable improvements in health care quality.
JCOM’s mission is to serve as a platform for sharing knowledge, experiences, and best practices to improve patient outcomes and promote health equity. The vision encompasses a world where all individuals have access to high-quality, patient-centered health care that is free of disparities and achieves optimal health outcomes. JCOM’s strategy is to publish articles that showcase innovative quality improvement initiatives, share evidence-based practices and research findings, highlight successful collaborations, and provide practical guidance for health care professionals to implement quality improvement initiatives in their organizations.
We believe that by sharing these insights and experiences, we can accelerate progress toward achieving equitable and high-quality health care for all individuals and communities, regardless of their socioeconomic status, race/ethnicity, gender identity, or any other factor that may impact their access to care and health outcomes. We continue to welcome submissions from all health care professionals, researchers, and other stakeholders involved in quality improvement initiatives. Together, we can work toward a future where every individual has access to the highest quality of health care and experiences equitable health outcomes.
A comprehensive and collaborative approach to health care quality improvement, which is led by a peer review process and scientific publication of the progress, is a necessary part of ensuring that all patients receive high-quality care that is equitable and patient-centered. The future of health care quality will require further research and scholarly work in the areas of training and development, data infrastructure and analytics, as well as technology-enabled solutions that support continuous improvement and innovation. Health care organizations can build a culture of quality improvement that drives meaningful progress toward achieving health equity and improving health care delivery for all by sharing the output from their research.
Thank you for joining us in this mission to improve health care quality, promote optimal health care delivery methods, and create a world where health care is not only accessible, but also equitable and of the highest standards. Let us continue to work toward building a health care system that prioritizes patient-centered care. Together, we can make a difference and ensure that every individual receives the care they need and deserve.
Corresponding author: Ebrahim Barkoudah, MD, MPH; ebarkoudah@bwh.harvard.edu
This year marks the publication of the 30th volume of the Journal of Clinical Outcomes Management (JCOM). As we celebrate JCOM’s 30th year, we look forward to the future and continuing the journey to inform quality improvement leaders and practitioners about advances in the field and share experiences. The path forward on this journey involves collaboration across stakeholders, the application of innovative improvement methods, and a commitment to achieving health equity. Health care quality improvement plans must prioritize patient-centered care, promote evidence-based practices and continuous learning, and establish clear metrics to measure progress and success. Furthermore, engagement with patients and communities must be at the forefront of any quality improvement plan, as their perspectives and experiences are essential to understanding and addressing the root causes of disparities in health care delivery. Additionally, effective communication and coordination among health care providers, administrators, policymakers, and other stakeholders are crucial to achieving sustainable improvements in health care quality.
JCOM’s mission is to serve as a platform for sharing knowledge, experiences, and best practices to improve patient outcomes and promote health equity. The vision encompasses a world where all individuals have access to high-quality, patient-centered health care that is free of disparities and achieves optimal health outcomes. JCOM’s strategy is to publish articles that showcase innovative quality improvement initiatives, share evidence-based practices and research findings, highlight successful collaborations, and provide practical guidance for health care professionals to implement quality improvement initiatives in their organizations.
We believe that by sharing these insights and experiences, we can accelerate progress toward achieving equitable and high-quality health care for all individuals and communities, regardless of their socioeconomic status, race/ethnicity, gender identity, or any other factor that may impact their access to care and health outcomes. We continue to welcome submissions from all health care professionals, researchers, and other stakeholders involved in quality improvement initiatives. Together, we can work toward a future where every individual has access to the highest quality of health care and experiences equitable health outcomes.
A comprehensive and collaborative approach to health care quality improvement, which is led by a peer review process and scientific publication of the progress, is a necessary part of ensuring that all patients receive high-quality care that is equitable and patient-centered. The future of health care quality will require further research and scholarly work in the areas of training and development, data infrastructure and analytics, as well as technology-enabled solutions that support continuous improvement and innovation. Health care organizations can build a culture of quality improvement that drives meaningful progress toward achieving health equity and improving health care delivery for all by sharing the output from their research.
Thank you for joining us in this mission to improve health care quality, promote optimal health care delivery methods, and create a world where health care is not only accessible, but also equitable and of the highest standards. Let us continue to work toward building a health care system that prioritizes patient-centered care. Together, we can make a difference and ensure that every individual receives the care they need and deserve.
Corresponding author: Ebrahim Barkoudah, MD, MPH; ebarkoudah@bwh.harvard.edu