User login
For MD-IQ use only
Infinite Learning
Dear Friends,
This issue of The New Gastroenterologist marks my first year completed as faculty. It has been both the best year and the HARDEST year. I celebrated many successes, felt intellectually and emotionally drained by difficult and complicated cases, and learned that there is so much more I still do not know. But that’s the beauty of our field — we are constantly learning to be better physicians for our patients. To trainees and my fellow gastroenterologists in practice, never stop asking questions!
In this issue’s “In Focus,” Dr. Rajan Singh and Dr. Baharak Moshiree describe a practical approach to patients with bloating by evaluating and investigating the pathophysiology and etiology of bloating, such as food intolerances, visceral hypersensitivity, pelvic floor dysfunction, abdominophrenic dyssynergia, gut dysmotility, and small intestinal bacterial overgrowth, as well as treatment management. In the “Short Clinical Review” section, Dr. Ahmad Bazarbashi and his colleagues review when to refer complex polyps to an advanced endoscopist and the different techniques of advanced tissue resection, including endoscopic mucosal resection, endoscopic submucosal dissection, submucosal tunneling endoscopic resection, and full thickness resection.
Locum practices have become more popular among gastroenterologists. Dr. Catherine Bartholomew is a retired professor of medicine who was chief of gastroenterology at an academic institution, and is now working as a GI locum after retirement. She details what a locum tenens is, the role of the company, being an independent contractor, and the benefits.
Navigating and negotiating maternity and paternity leave may be challenging in private practice. Dr. Marybeth Spanarkel gives her opinion on the nuances of maternity/paternity leave in private practices, what it may mean financially, and things to inquire of the practice if planning to have children.
As we move from joining non-traditional practices and navigating family planning with private practices, Dr. Vasu Appalaneni shares her experiences with financial planning for retirement. She describes ways to financially plan a retirement, but also to consider aspects that affect financial well-being during retirement, including healthcare coverage, lifestyle and traveling, legal and estate, professional development, and emotional and social support.
If you are interested in contributing or have ideas for future TNG topics, please contact me (tjudy@wustl.edu) or Danielle Kiefer (dkiefer@gastro.org), communications/managing editor of TNG.
Until next time, I leave you with a historical fun fact because we would not be where we are not without appreciating where we were: The first colonic polypectomy using an electrosurgical snare was performed by Dr. Hiromi Shinya at Beth Israel Medical Center in New York City, in 1969.
Yours truly,
Judy A. Trieu, MD, MPH
Editor-in-Chief
Interventional Endoscopy, Division of Gastroenterology
Washington University in St. Louis
Dear Friends,
This issue of The New Gastroenterologist marks my first year completed as faculty. It has been both the best year and the HARDEST year. I celebrated many successes, felt intellectually and emotionally drained by difficult and complicated cases, and learned that there is so much more I still do not know. But that’s the beauty of our field — we are constantly learning to be better physicians for our patients. To trainees and my fellow gastroenterologists in practice, never stop asking questions!
In this issue’s “In Focus,” Dr. Rajan Singh and Dr. Baharak Moshiree describe a practical approach to patients with bloating by evaluating and investigating the pathophysiology and etiology of bloating, such as food intolerances, visceral hypersensitivity, pelvic floor dysfunction, abdominophrenic dyssynergia, gut dysmotility, and small intestinal bacterial overgrowth, as well as treatment management. In the “Short Clinical Review” section, Dr. Ahmad Bazarbashi and his colleagues review when to refer complex polyps to an advanced endoscopist and the different techniques of advanced tissue resection, including endoscopic mucosal resection, endoscopic submucosal dissection, submucosal tunneling endoscopic resection, and full thickness resection.
Locum practices have become more popular among gastroenterologists. Dr. Catherine Bartholomew is a retired professor of medicine who was chief of gastroenterology at an academic institution, and is now working as a GI locum after retirement. She details what a locum tenens is, the role of the company, being an independent contractor, and the benefits.
Navigating and negotiating maternity and paternity leave may be challenging in private practice. Dr. Marybeth Spanarkel gives her opinion on the nuances of maternity/paternity leave in private practices, what it may mean financially, and things to inquire of the practice if planning to have children.
As we move from joining non-traditional practices and navigating family planning with private practices, Dr. Vasu Appalaneni shares her experiences with financial planning for retirement. She describes ways to financially plan a retirement, but also to consider aspects that affect financial well-being during retirement, including healthcare coverage, lifestyle and traveling, legal and estate, professional development, and emotional and social support.
If you are interested in contributing or have ideas for future TNG topics, please contact me (tjudy@wustl.edu) or Danielle Kiefer (dkiefer@gastro.org), communications/managing editor of TNG.
Until next time, I leave you with a historical fun fact because we would not be where we are not without appreciating where we were: The first colonic polypectomy using an electrosurgical snare was performed by Dr. Hiromi Shinya at Beth Israel Medical Center in New York City, in 1969.
Yours truly,
Judy A. Trieu, MD, MPH
Editor-in-Chief
Interventional Endoscopy, Division of Gastroenterology
Washington University in St. Louis
Dear Friends,
This issue of The New Gastroenterologist marks my first year completed as faculty. It has been both the best year and the HARDEST year. I celebrated many successes, felt intellectually and emotionally drained by difficult and complicated cases, and learned that there is so much more I still do not know. But that’s the beauty of our field — we are constantly learning to be better physicians for our patients. To trainees and my fellow gastroenterologists in practice, never stop asking questions!
In this issue’s “In Focus,” Dr. Rajan Singh and Dr. Baharak Moshiree describe a practical approach to patients with bloating by evaluating and investigating the pathophysiology and etiology of bloating, such as food intolerances, visceral hypersensitivity, pelvic floor dysfunction, abdominophrenic dyssynergia, gut dysmotility, and small intestinal bacterial overgrowth, as well as treatment management. In the “Short Clinical Review” section, Dr. Ahmad Bazarbashi and his colleagues review when to refer complex polyps to an advanced endoscopist and the different techniques of advanced tissue resection, including endoscopic mucosal resection, endoscopic submucosal dissection, submucosal tunneling endoscopic resection, and full thickness resection.
Locum practices have become more popular among gastroenterologists. Dr. Catherine Bartholomew is a retired professor of medicine who was chief of gastroenterology at an academic institution, and is now working as a GI locum after retirement. She details what a locum tenens is, the role of the company, being an independent contractor, and the benefits.
Navigating and negotiating maternity and paternity leave may be challenging in private practice. Dr. Marybeth Spanarkel gives her opinion on the nuances of maternity/paternity leave in private practices, what it may mean financially, and things to inquire of the practice if planning to have children.
As we move from joining non-traditional practices and navigating family planning with private practices, Dr. Vasu Appalaneni shares her experiences with financial planning for retirement. She describes ways to financially plan a retirement, but also to consider aspects that affect financial well-being during retirement, including healthcare coverage, lifestyle and traveling, legal and estate, professional development, and emotional and social support.
If you are interested in contributing or have ideas for future TNG topics, please contact me (tjudy@wustl.edu) or Danielle Kiefer (dkiefer@gastro.org), communications/managing editor of TNG.
Until next time, I leave you with a historical fun fact because we would not be where we are not without appreciating where we were: The first colonic polypectomy using an electrosurgical snare was performed by Dr. Hiromi Shinya at Beth Israel Medical Center in New York City, in 1969.
Yours truly,
Judy A. Trieu, MD, MPH
Editor-in-Chief
Interventional Endoscopy, Division of Gastroenterology
Washington University in St. Louis
Study Finds Gout Drug Effective for Aphthous Ulcers in Children
“Complex aphthous stomatitis in children is typically treated with topical supportive care, which is often not effective,” one of the study investigators, Ananya Shah, a third-year medical student at the University of Rochester School of Medicine & Dentistry, Rochester, New York, told this news organization following the Society for Pediatric Dermatology annual meeting, where the study was presented during a poster session. “There is limited research on CAS and its treatment in children. Colchicine is often used for treatment of CAS in adults, but its use in children has not been studied.”
Ms. Shah, in collaboration with Hilary Kunkel, MD, Nessa Aghazadeh, MD, and Megha Tollefson, MD, of the Department of Dermatology, Mayo Clinic, Rochester, Minnesota, retrospectively reviewed the charts of 20 children diagnosed with CAS who were treated with colchicine, an anti-inflammatory drug often used to treat gout, at the clinic between 2000 and 2023. Treatment responses were defined as no response, partial response, and complete response. Half of the patients were girls, and their median age at presentation was 5 years.
Ulcers were most commonly located in the buccal mucosa (80%), followed by the gingiva (50%), the mucosal lip (50%), and the palate (40%). Nearly all patients (95%) reported that the CAS caused difficulties with eating or drinking. Other effects on their quality of life included weight loss (35%), bleeding (30%), and difficulty brushing teeth (25%). “I was surprised by how much CAS impacts pediatric patients’ quality of life,” Ms. Shah said. “Almost all of the patients experienced trouble with basic activities of daily living, including eating and drinking. In addition, CAS negatively impacted mental health and led to missed school for patients.”
The researchers had follow-up data on responses to colchicine for 14 of the 20 patients. Of these, 12 (86%) had symptom improvement, 5 (36%) had a complete response, 8 (57%) had a partial response, and 1 (7%) did not respond. Nine patients (64%) experienced side effects. Of these, six had diarrhea, two had nausea, and one had constipation.
“Colchicine should be considered as a treatment in pediatric patients who have refractory complex aphthous stomatitis as it is generally well tolerated with minimal side effects,” Ms. Shah said. She acknowledged certain limitations of the study, including its single-center, retrospective design.
The researchers reported having no relevant disclosures.
A version of this article first appeared on Medscape.com.
“Complex aphthous stomatitis in children is typically treated with topical supportive care, which is often not effective,” one of the study investigators, Ananya Shah, a third-year medical student at the University of Rochester School of Medicine & Dentistry, Rochester, New York, told this news organization following the Society for Pediatric Dermatology annual meeting, where the study was presented during a poster session. “There is limited research on CAS and its treatment in children. Colchicine is often used for treatment of CAS in adults, but its use in children has not been studied.”
Ms. Shah, in collaboration with Hilary Kunkel, MD, Nessa Aghazadeh, MD, and Megha Tollefson, MD, of the Department of Dermatology, Mayo Clinic, Rochester, Minnesota, retrospectively reviewed the charts of 20 children diagnosed with CAS who were treated with colchicine, an anti-inflammatory drug often used to treat gout, at the clinic between 2000 and 2023. Treatment responses were defined as no response, partial response, and complete response. Half of the patients were girls, and their median age at presentation was 5 years.
Ulcers were most commonly located in the buccal mucosa (80%), followed by the gingiva (50%), the mucosal lip (50%), and the palate (40%). Nearly all patients (95%) reported that the CAS caused difficulties with eating or drinking. Other effects on their quality of life included weight loss (35%), bleeding (30%), and difficulty brushing teeth (25%). “I was surprised by how much CAS impacts pediatric patients’ quality of life,” Ms. Shah said. “Almost all of the patients experienced trouble with basic activities of daily living, including eating and drinking. In addition, CAS negatively impacted mental health and led to missed school for patients.”
The researchers had follow-up data on responses to colchicine for 14 of the 20 patients. Of these, 12 (86%) had symptom improvement, 5 (36%) had a complete response, 8 (57%) had a partial response, and 1 (7%) did not respond. Nine patients (64%) experienced side effects. Of these, six had diarrhea, two had nausea, and one had constipation.
“Colchicine should be considered as a treatment in pediatric patients who have refractory complex aphthous stomatitis as it is generally well tolerated with minimal side effects,” Ms. Shah said. She acknowledged certain limitations of the study, including its single-center, retrospective design.
The researchers reported having no relevant disclosures.
A version of this article first appeared on Medscape.com.
“Complex aphthous stomatitis in children is typically treated with topical supportive care, which is often not effective,” one of the study investigators, Ananya Shah, a third-year medical student at the University of Rochester School of Medicine & Dentistry, Rochester, New York, told this news organization following the Society for Pediatric Dermatology annual meeting, where the study was presented during a poster session. “There is limited research on CAS and its treatment in children. Colchicine is often used for treatment of CAS in adults, but its use in children has not been studied.”
Ms. Shah, in collaboration with Hilary Kunkel, MD, Nessa Aghazadeh, MD, and Megha Tollefson, MD, of the Department of Dermatology, Mayo Clinic, Rochester, Minnesota, retrospectively reviewed the charts of 20 children diagnosed with CAS who were treated with colchicine, an anti-inflammatory drug often used to treat gout, at the clinic between 2000 and 2023. Treatment responses were defined as no response, partial response, and complete response. Half of the patients were girls, and their median age at presentation was 5 years.
Ulcers were most commonly located in the buccal mucosa (80%), followed by the gingiva (50%), the mucosal lip (50%), and the palate (40%). Nearly all patients (95%) reported that the CAS caused difficulties with eating or drinking. Other effects on their quality of life included weight loss (35%), bleeding (30%), and difficulty brushing teeth (25%). “I was surprised by how much CAS impacts pediatric patients’ quality of life,” Ms. Shah said. “Almost all of the patients experienced trouble with basic activities of daily living, including eating and drinking. In addition, CAS negatively impacted mental health and led to missed school for patients.”
The researchers had follow-up data on responses to colchicine for 14 of the 20 patients. Of these, 12 (86%) had symptom improvement, 5 (36%) had a complete response, 8 (57%) had a partial response, and 1 (7%) did not respond. Nine patients (64%) experienced side effects. Of these, six had diarrhea, two had nausea, and one had constipation.
“Colchicine should be considered as a treatment in pediatric patients who have refractory complex aphthous stomatitis as it is generally well tolerated with minimal side effects,” Ms. Shah said. She acknowledged certain limitations of the study, including its single-center, retrospective design.
The researchers reported having no relevant disclosures.
A version of this article first appeared on Medscape.com.
FROM SPD 2024
Pilot Study Finds Experimental CBD Cream Decreases UVA Skin Damage
, results from a small prospective pilot study showed.
“This study hopefully reinvigorates interest in the utilization of whether it be plant-based, human-derived, or synthetic cannabinoids in the management of dermatologic disease,” one of the study investigators, Adam Friedman, MD, professor and chair of dermatology at George Washington University, Washington, DC, told this news organization. The study was published in the Journal of the American Academy of Dermatology.
For the prospective, single-center, pilot trial, which is believed to be the first of its kind, 19 volunteers aged 22-65 with Fitzpatrick skin types I-III applied either a nano-encapsulated CBD cream or a vehicle cream to blind spots on the skin of the buttocks twice daily for 14 days. Next, researchers applied a minimal erythema dose of UV radiation to the treated skin areas for 30 minutes. After 24 hours, they visually inspected the treated areas to clinically compare the erythema. They also performed five 4-mm punch biopsies from UVA- and non-UVA–exposed treatment sites on each buttock, as well as from an untreated control site that was at least 5 cm away from the treated left buttock.
At 24 hours, 21% of study participants showed less redness on CBD-treated skin compared with control-treated skin, while histology showed that CBD-treated skin demonstrated reduced UVA-induced epidermal hyperplasia compared with control-treated skin (a mean 11.3% change from baseline vs 28.7%, respectively; P = .01). In other findings, application of CBD cream reduced DNA damage and DNA mutations associated with UVA-induced skin aging/damage and ultimately skin cancer.
In addition, the CBD-treated skin samples had a reduction in the UVA-associated increase in the premutagenic marker 8-oxoguanine DNA glycosylase 1 and a reduction of two major UVA-induced mitochondrial DNA deletions associated with skin photoaging.
The research, Dr. Friedman noted, “took a village of collaborators and almost 3 years to pull together,” including collaborating with his long-standing mentor, Brian Berman, MD, PhD, professor emeritus of dermatology and dermatologic surgery at the University of Miami, Coral Gables, Florida, and a study coauthor. The study “demonstrated that purposeful delivery of CBD using an established nanoparticle platform ... can have a quantifiable impact on preventing the expected DNA damage and cellular injury one should see from UVA exposure,” said Dr. Friedman, who codeveloped the nanoparticle platform with his father, Joel M. Friedman, MD, PhD, professor of microbiology and immunology at Albert Einstein College of Medicine, New York City.
“Never before has a dermatologic study on topical cannabinoids dove so deeply into the biological impact of this natural ingredient to highlight its potential, here, as a mitigation strategy for unprotected exposure to prevent the downstream sequelae of UV radiation,” Dr. Friedman said.
In the paper, he and his coauthors acknowledged certain limitations of their study, including its small sample size and the single-center design.
Dr. Friedman disclosed that he coinvented the nanoparticle technology used in the trial. Dr. Berman is a consultant at MINO Labs, which funded the study. The remaining authors had no disclosures. The study was done in collaboration with the Center for Clinical and Cosmetic Research in Aventura, Florida.
A version of this article first appeared on Medscape.com.
, results from a small prospective pilot study showed.
“This study hopefully reinvigorates interest in the utilization of whether it be plant-based, human-derived, or synthetic cannabinoids in the management of dermatologic disease,” one of the study investigators, Adam Friedman, MD, professor and chair of dermatology at George Washington University, Washington, DC, told this news organization. The study was published in the Journal of the American Academy of Dermatology.
For the prospective, single-center, pilot trial, which is believed to be the first of its kind, 19 volunteers aged 22-65 with Fitzpatrick skin types I-III applied either a nano-encapsulated CBD cream or a vehicle cream to blind spots on the skin of the buttocks twice daily for 14 days. Next, researchers applied a minimal erythema dose of UV radiation to the treated skin areas for 30 minutes. After 24 hours, they visually inspected the treated areas to clinically compare the erythema. They also performed five 4-mm punch biopsies from UVA- and non-UVA–exposed treatment sites on each buttock, as well as from an untreated control site that was at least 5 cm away from the treated left buttock.
At 24 hours, 21% of study participants showed less redness on CBD-treated skin compared with control-treated skin, while histology showed that CBD-treated skin demonstrated reduced UVA-induced epidermal hyperplasia compared with control-treated skin (a mean 11.3% change from baseline vs 28.7%, respectively; P = .01). In other findings, application of CBD cream reduced DNA damage and DNA mutations associated with UVA-induced skin aging/damage and ultimately skin cancer.
In addition, the CBD-treated skin samples had a reduction in the UVA-associated increase in the premutagenic marker 8-oxoguanine DNA glycosylase 1 and a reduction of two major UVA-induced mitochondrial DNA deletions associated with skin photoaging.
The research, Dr. Friedman noted, “took a village of collaborators and almost 3 years to pull together,” including collaborating with his long-standing mentor, Brian Berman, MD, PhD, professor emeritus of dermatology and dermatologic surgery at the University of Miami, Coral Gables, Florida, and a study coauthor. The study “demonstrated that purposeful delivery of CBD using an established nanoparticle platform ... can have a quantifiable impact on preventing the expected DNA damage and cellular injury one should see from UVA exposure,” said Dr. Friedman, who codeveloped the nanoparticle platform with his father, Joel M. Friedman, MD, PhD, professor of microbiology and immunology at Albert Einstein College of Medicine, New York City.
“Never before has a dermatologic study on topical cannabinoids dove so deeply into the biological impact of this natural ingredient to highlight its potential, here, as a mitigation strategy for unprotected exposure to prevent the downstream sequelae of UV radiation,” Dr. Friedman said.
In the paper, he and his coauthors acknowledged certain limitations of their study, including its small sample size and the single-center design.
Dr. Friedman disclosed that he coinvented the nanoparticle technology used in the trial. Dr. Berman is a consultant at MINO Labs, which funded the study. The remaining authors had no disclosures. The study was done in collaboration with the Center for Clinical and Cosmetic Research in Aventura, Florida.
A version of this article first appeared on Medscape.com.
, results from a small prospective pilot study showed.
“This study hopefully reinvigorates interest in the utilization of whether it be plant-based, human-derived, or synthetic cannabinoids in the management of dermatologic disease,” one of the study investigators, Adam Friedman, MD, professor and chair of dermatology at George Washington University, Washington, DC, told this news organization. The study was published in the Journal of the American Academy of Dermatology.
For the prospective, single-center, pilot trial, which is believed to be the first of its kind, 19 volunteers aged 22-65 with Fitzpatrick skin types I-III applied either a nano-encapsulated CBD cream or a vehicle cream to blind spots on the skin of the buttocks twice daily for 14 days. Next, researchers applied a minimal erythema dose of UV radiation to the treated skin areas for 30 minutes. After 24 hours, they visually inspected the treated areas to clinically compare the erythema. They also performed five 4-mm punch biopsies from UVA- and non-UVA–exposed treatment sites on each buttock, as well as from an untreated control site that was at least 5 cm away from the treated left buttock.
At 24 hours, 21% of study participants showed less redness on CBD-treated skin compared with control-treated skin, while histology showed that CBD-treated skin demonstrated reduced UVA-induced epidermal hyperplasia compared with control-treated skin (a mean 11.3% change from baseline vs 28.7%, respectively; P = .01). In other findings, application of CBD cream reduced DNA damage and DNA mutations associated with UVA-induced skin aging/damage and ultimately skin cancer.
In addition, the CBD-treated skin samples had a reduction in the UVA-associated increase in the premutagenic marker 8-oxoguanine DNA glycosylase 1 and a reduction of two major UVA-induced mitochondrial DNA deletions associated with skin photoaging.
The research, Dr. Friedman noted, “took a village of collaborators and almost 3 years to pull together,” including collaborating with his long-standing mentor, Brian Berman, MD, PhD, professor emeritus of dermatology and dermatologic surgery at the University of Miami, Coral Gables, Florida, and a study coauthor. The study “demonstrated that purposeful delivery of CBD using an established nanoparticle platform ... can have a quantifiable impact on preventing the expected DNA damage and cellular injury one should see from UVA exposure,” said Dr. Friedman, who codeveloped the nanoparticle platform with his father, Joel M. Friedman, MD, PhD, professor of microbiology and immunology at Albert Einstein College of Medicine, New York City.
“Never before has a dermatologic study on topical cannabinoids dove so deeply into the biological impact of this natural ingredient to highlight its potential, here, as a mitigation strategy for unprotected exposure to prevent the downstream sequelae of UV radiation,” Dr. Friedman said.
In the paper, he and his coauthors acknowledged certain limitations of their study, including its small sample size and the single-center design.
Dr. Friedman disclosed that he coinvented the nanoparticle technology used in the trial. Dr. Berman is a consultant at MINO Labs, which funded the study. The remaining authors had no disclosures. The study was done in collaboration with the Center for Clinical and Cosmetic Research in Aventura, Florida.
A version of this article first appeared on Medscape.com.
FROM THE JOURNAL OF THE AMERICAN ACADEMY OF DERMATOLOGY
Risk of MACE Comparable Among Biologic Classes for Psoriasis, PsA
TOPLINE:
a database analysis finds.
METHODOLOGY:
- Data from the TriNetX health records database included 32,758 patients treated with TNF inhibitors (TNFi, 62.9%), interleukin-17 inhibitors (IL-17i, 15.4%), IL-23i (10.7%), and IL-12i/IL-23i (10.7%).
- The researchers calculated time-dependent risk for MACE using multinomial Cox proportional hazard ratios. The reference was TNFi exposure.
- Subset analyses compared MACE in patients with and without existing cardiovascular disease.
TAKEAWAY:
- Compared with TNFi use, there was no difference in the incidence of MACE events in the IL-17i, IL-23i, or IL-12i/IL-23i group.
- There were also no significant differences between biologic groups in the incidence of congestive heart failure, myocardial infarction, or cerebral vascular accident/stroke.
IN PRACTICE:
Despite some concern about increased risk for MACE with TNFi use, this study suggests no special risk for patients with psoriasis or PsA associated with TNFi vs other biologics. “Given our results, as it pertains to MACE, prescribers shouldn’t favor any one biologic class over another,” said lead investigator Shikha Singla, MD, medical director of the Psoriatic Arthritis Program at Medical College of Wisconsin in Milwaukee, Wisconsin.
SOURCE:
Bonit Gill, MD, a second-year fellow at Medical College of Wisconsin, presented the study as a poster at the annual meeting of the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis.
LIMITATIONS:
The study’s retrospective nature makes it impossible to prove causation and the patients included in the study were from Wisconsin, which may limit generalizability.
DISCLOSURES:
Dr. Gill had no relevant financial disclosures. Other study authors participated in trials or consulted for AbbVie, AstraZeneca, Novartis, Eli Lilly, Janssen, and UCB.
A version of this article first appeared on Medscape.com.
TOPLINE:
a database analysis finds.
METHODOLOGY:
- Data from the TriNetX health records database included 32,758 patients treated with TNF inhibitors (TNFi, 62.9%), interleukin-17 inhibitors (IL-17i, 15.4%), IL-23i (10.7%), and IL-12i/IL-23i (10.7%).
- The researchers calculated time-dependent risk for MACE using multinomial Cox proportional hazard ratios. The reference was TNFi exposure.
- Subset analyses compared MACE in patients with and without existing cardiovascular disease.
TAKEAWAY:
- Compared with TNFi use, there was no difference in the incidence of MACE events in the IL-17i, IL-23i, or IL-12i/IL-23i group.
- There were also no significant differences between biologic groups in the incidence of congestive heart failure, myocardial infarction, or cerebral vascular accident/stroke.
IN PRACTICE:
Despite some concern about increased risk for MACE with TNFi use, this study suggests no special risk for patients with psoriasis or PsA associated with TNFi vs other biologics. “Given our results, as it pertains to MACE, prescribers shouldn’t favor any one biologic class over another,” said lead investigator Shikha Singla, MD, medical director of the Psoriatic Arthritis Program at Medical College of Wisconsin in Milwaukee, Wisconsin.
SOURCE:
Bonit Gill, MD, a second-year fellow at Medical College of Wisconsin, presented the study as a poster at the annual meeting of the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis.
LIMITATIONS:
The study’s retrospective nature makes it impossible to prove causation and the patients included in the study were from Wisconsin, which may limit generalizability.
DISCLOSURES:
Dr. Gill had no relevant financial disclosures. Other study authors participated in trials or consulted for AbbVie, AstraZeneca, Novartis, Eli Lilly, Janssen, and UCB.
A version of this article first appeared on Medscape.com.
TOPLINE:
a database analysis finds.
METHODOLOGY:
- Data from the TriNetX health records database included 32,758 patients treated with TNF inhibitors (TNFi, 62.9%), interleukin-17 inhibitors (IL-17i, 15.4%), IL-23i (10.7%), and IL-12i/IL-23i (10.7%).
- The researchers calculated time-dependent risk for MACE using multinomial Cox proportional hazard ratios. The reference was TNFi exposure.
- Subset analyses compared MACE in patients with and without existing cardiovascular disease.
TAKEAWAY:
- Compared with TNFi use, there was no difference in the incidence of MACE events in the IL-17i, IL-23i, or IL-12i/IL-23i group.
- There were also no significant differences between biologic groups in the incidence of congestive heart failure, myocardial infarction, or cerebral vascular accident/stroke.
IN PRACTICE:
Despite some concern about increased risk for MACE with TNFi use, this study suggests no special risk for patients with psoriasis or PsA associated with TNFi vs other biologics. “Given our results, as it pertains to MACE, prescribers shouldn’t favor any one biologic class over another,” said lead investigator Shikha Singla, MD, medical director of the Psoriatic Arthritis Program at Medical College of Wisconsin in Milwaukee, Wisconsin.
SOURCE:
Bonit Gill, MD, a second-year fellow at Medical College of Wisconsin, presented the study as a poster at the annual meeting of the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis.
LIMITATIONS:
The study’s retrospective nature makes it impossible to prove causation and the patients included in the study were from Wisconsin, which may limit generalizability.
DISCLOSURES:
Dr. Gill had no relevant financial disclosures. Other study authors participated in trials or consulted for AbbVie, AstraZeneca, Novartis, Eli Lilly, Janssen, and UCB.
A version of this article first appeared on Medscape.com.
Study Links Newer Shingles Vaccine to Delayed Dementia Diagnosis
The study builds on previous observations of a reduction in dementia risk with the older live shingles vaccine and reports a delay in dementia diagnosis of 164 days with the newer recombinant version, compared with the live vaccine.
“Given the prevalence of dementia, a delay of 164 days in diagnosis would not be a trivial effect at the public health level. It’s a big enough effect that if there is a causality it feels meaningful,” said senior author Paul Harrison, DM, FRCPsych, professor of psychiatry at the University of Oxford, Oxford, England.
But Dr. Harrison stressed that the study had not proven that the shingles vaccine reduced dementia risk.
“The design of the study allows us to do away with many of the confounding effects we usually see in observational studies, but this is still an observational study, and as such it cannot prove a definite causal effect,” he said.
The study was published online on July 25 in Nature Medicine.
‘Natural Experiment’
Given the risk for deleterious consequences of shingles, vaccination is now recommended for older adults in many countries. The previously used live shingles vaccine (Zostavax) is being replaced in most countries with the new recombinant shingles vaccine (Shingrix), which is more effective at preventing shingles infection.
The current study made use of a “natural experiment” in the United States, which switched over from use of the live vaccine to the recombinant vaccine in October 2017.
Researchers used electronic heath records to compare the incidence of a dementia diagnosis in individuals who received the live shingles vaccine prior to October 2017 with those who received the recombinant version after the United States made the switch.
They also used propensity score matching to further control for confounding factors, comparing 103,837 individuals who received a first dose of the live shingles vaccine between October 2014 and September 2017 with the same number of matched people who received the recombinant vaccine between November 2017 and October 2020.
Results showed that within the 6 years after vaccination, the recombinant vaccine was associated with a delay in the diagnosis of dementia, compared with the live vaccine. Specifically, receiving the recombinant vaccine was associated with a 17% increase in diagnosis-free time, translating to 164 additional days lived without a diagnosis of dementia in those subsequently affected.
As an additional control, the researchers also found significantly lower risks for dementia in individuals receiving the new recombinant shingles vaccine vs two other vaccines commonly used in older people: influenza and tetanus/diphtheria/pertussis vaccines, with increases in diagnosis-free time of 14%-27%.
Reduced Risk or Delayed Diagnosis?
Speaking at a Science Media Centre press conference on the study, lead author Maxime Taquet, PhD, FRCPsych, clinical lecturer in psychiatry at the University of Oxford, noted that the total number of dementia cases were similar in the two shingles vaccine groups by the end of the 6-year follow-up period but there was a difference in the time at which they received a diagnosis of dementia.
“The study suggests that rather than actually reducing dementia risk, the recombinant vaccine delays the onset of dementia compared to the live vaccine in patients who go on to develop the condition,” he explained.
But when comparing the recombinant vaccine with the influenza and tetanus/diphtheria/pertussis vaccines there was a clear reduction in dementia risk itself, Dr. Taquet reported.
“It might well be that the live vaccine has a potential effect on the risk of dementia itself and therefore the recombinant vaccine only shows a delay in dementia compared to the live vaccine, but both of them might decrease the overall risk of dementia,” he suggested.
But the researchers cautioned that this study could not prove causality.
“While the two groups were very carefully matched in terms of factors that might influence the development of dementia, we still have to be cautious before assuming that the vaccine is indeed causally reducing the risk of onset of dementia,” Dr. Harrison warned.
The researchers say the results would need to be confirmed in a randomized trial, which may have to be conducted in a slightly younger age group, as currently shingles vaccine is recommended for all older individuals in the United Kingdom.
Vaccine recommendations vary from country to country, Dr. Harrison added. In the United States, the Centers for Disease Control and Prevention recommends the recombinant shingles vaccine for all adults aged 50 years or older.
In the meantime, it would be interesting to see whether further observational studies in other countries find similar results as this US study, Dr. Harrison said.
Mechanism Uncertain
Speculating on a possible mechanism behind the findings, Dr. Harrison suggested two plausible explanations.
“First, it is thought that the herpes virus could be one of many factors that could promote dementia, so a vaccine that stops reactivation of this virus might therefore be delaying that process,” he noted.
The other possibility is that adjuvants included in the recombinant vaccine to stimulate the immune system might have played a role.
“We don’t have any data on the mechanism, and thus study did not address that, so further studies are needed to look into this,” Dr. Harrison said.
Stronger Effect in Women
Another intriguing finding is that the association with the recombinant vaccine and delayed dementia diagnosis seemed to be stronger in women vs men.
In the original study of the live shingles vaccine, a protective effect against dementia was shown only in women.
In the current study, the delay in dementia diagnosis was seen in both sexes but was stronger in women, showing a 22% increased time without dementia in women versus a 13% increased time in men with the recombinant versus the live vaccine.
As expected, the recombinant vaccine was associated with a lower risk for shingles disease vs the live vaccine (2.5% versus 3.5%), but women did not have a better response than men did in this respect.
“The better protection against shingles with the recombinant vaccine was similar in men and women, an observation that might be one reason to question the possible mechanism behind the dementia effect being better suppression of the herpes zoster virus by the recombinant vaccine,” Dr. Harrison commented.
Though these findings are not likely to lead to any immediate changes in policy regarding the shingles vaccine, Dr. Harrison said it would be interesting to see whether uptake of the vaccine increased after this study.
He estimated that, currently in the United Kingdom, about 60% of older adults choose to have the shingles vaccine. A 2020 study in the United States found that only about one-third of US adults over 60 had received the vaccine.
“It will be interesting to see if that figure increases after these data are publicized, but I am not recommending that people have the vaccine specifically to lower their risk of dementia because of the caveats about the study that we have discussed,” he commented.
Outside Experts Positive
Outside experts, providing comment to the Science Media Centre, welcomed the new research.
“ The study is very well-conducted and adds to previous data indicating that vaccination against shingles is associated with lower dementia risk. More research is needed in future to determine why this vaccine is associated with lower dementia risk,” said Tara Spires-Jones, FMedSci, president of the British Neuroscience Association.
The high number of patients in the study and the adjustments for potential confounders are also strong points, noted Andrew Doig, PhD, professor of biochemistry, University of Manchester, Manchester, England.
“This is a significant result, comparable in effectiveness to the recent antibody drugs for Alzheimer’s disease,” Dr. Doig said. “Administering the recombinant shingles vaccine could well be a simple and cheap way to lower the risk of Alzheimer’s disease.”
Dr. Doig noted that a link between herpes zoster infection and the onset of dementia has been suspected for some time, and a trial of the antiviral drug valacyclovir against Alzheimer’s disease is currently underway.
In regard to the shingles vaccine, he said a placebo-controlled trial would be needed to prove causality.
“We also need to see how many years the effect might last and whether we should vaccinate people at a younger age. We know that the path to Alzheimer’s can start decades before any symptoms are apparent, so the vaccine might be even more effective if given to people in their 40s or 50s,” he said.
Dr. Harrison and Dr. Taquet reported no disclosures. Dr. Doig is a founder, director, and consultant for PharmaKure, which works on Alzheimer’s drugs and diagnostics. Other commentators declared no disclosures.
A version of this article first appeared on Medscape.com.
The study builds on previous observations of a reduction in dementia risk with the older live shingles vaccine and reports a delay in dementia diagnosis of 164 days with the newer recombinant version, compared with the live vaccine.
“Given the prevalence of dementia, a delay of 164 days in diagnosis would not be a trivial effect at the public health level. It’s a big enough effect that if there is a causality it feels meaningful,” said senior author Paul Harrison, DM, FRCPsych, professor of psychiatry at the University of Oxford, Oxford, England.
But Dr. Harrison stressed that the study had not proven that the shingles vaccine reduced dementia risk.
“The design of the study allows us to do away with many of the confounding effects we usually see in observational studies, but this is still an observational study, and as such it cannot prove a definite causal effect,” he said.
The study was published online on July 25 in Nature Medicine.
‘Natural Experiment’
Given the risk for deleterious consequences of shingles, vaccination is now recommended for older adults in many countries. The previously used live shingles vaccine (Zostavax) is being replaced in most countries with the new recombinant shingles vaccine (Shingrix), which is more effective at preventing shingles infection.
The current study made use of a “natural experiment” in the United States, which switched over from use of the live vaccine to the recombinant vaccine in October 2017.
Researchers used electronic heath records to compare the incidence of a dementia diagnosis in individuals who received the live shingles vaccine prior to October 2017 with those who received the recombinant version after the United States made the switch.
They also used propensity score matching to further control for confounding factors, comparing 103,837 individuals who received a first dose of the live shingles vaccine between October 2014 and September 2017 with the same number of matched people who received the recombinant vaccine between November 2017 and October 2020.
Results showed that within the 6 years after vaccination, the recombinant vaccine was associated with a delay in the diagnosis of dementia, compared with the live vaccine. Specifically, receiving the recombinant vaccine was associated with a 17% increase in diagnosis-free time, translating to 164 additional days lived without a diagnosis of dementia in those subsequently affected.
As an additional control, the researchers also found significantly lower risks for dementia in individuals receiving the new recombinant shingles vaccine vs two other vaccines commonly used in older people: influenza and tetanus/diphtheria/pertussis vaccines, with increases in diagnosis-free time of 14%-27%.
Reduced Risk or Delayed Diagnosis?
Speaking at a Science Media Centre press conference on the study, lead author Maxime Taquet, PhD, FRCPsych, clinical lecturer in psychiatry at the University of Oxford, noted that the total number of dementia cases were similar in the two shingles vaccine groups by the end of the 6-year follow-up period but there was a difference in the time at which they received a diagnosis of dementia.
“The study suggests that rather than actually reducing dementia risk, the recombinant vaccine delays the onset of dementia compared to the live vaccine in patients who go on to develop the condition,” he explained.
But when comparing the recombinant vaccine with the influenza and tetanus/diphtheria/pertussis vaccines there was a clear reduction in dementia risk itself, Dr. Taquet reported.
“It might well be that the live vaccine has a potential effect on the risk of dementia itself and therefore the recombinant vaccine only shows a delay in dementia compared to the live vaccine, but both of them might decrease the overall risk of dementia,” he suggested.
But the researchers cautioned that this study could not prove causality.
“While the two groups were very carefully matched in terms of factors that might influence the development of dementia, we still have to be cautious before assuming that the vaccine is indeed causally reducing the risk of onset of dementia,” Dr. Harrison warned.
The researchers say the results would need to be confirmed in a randomized trial, which may have to be conducted in a slightly younger age group, as currently shingles vaccine is recommended for all older individuals in the United Kingdom.
Vaccine recommendations vary from country to country, Dr. Harrison added. In the United States, the Centers for Disease Control and Prevention recommends the recombinant shingles vaccine for all adults aged 50 years or older.
In the meantime, it would be interesting to see whether further observational studies in other countries find similar results as this US study, Dr. Harrison said.
Mechanism Uncertain
Speculating on a possible mechanism behind the findings, Dr. Harrison suggested two plausible explanations.
“First, it is thought that the herpes virus could be one of many factors that could promote dementia, so a vaccine that stops reactivation of this virus might therefore be delaying that process,” he noted.
The other possibility is that adjuvants included in the recombinant vaccine to stimulate the immune system might have played a role.
“We don’t have any data on the mechanism, and thus study did not address that, so further studies are needed to look into this,” Dr. Harrison said.
Stronger Effect in Women
Another intriguing finding is that the association with the recombinant vaccine and delayed dementia diagnosis seemed to be stronger in women vs men.
In the original study of the live shingles vaccine, a protective effect against dementia was shown only in women.
In the current study, the delay in dementia diagnosis was seen in both sexes but was stronger in women, showing a 22% increased time without dementia in women versus a 13% increased time in men with the recombinant versus the live vaccine.
As expected, the recombinant vaccine was associated with a lower risk for shingles disease vs the live vaccine (2.5% versus 3.5%), but women did not have a better response than men did in this respect.
“The better protection against shingles with the recombinant vaccine was similar in men and women, an observation that might be one reason to question the possible mechanism behind the dementia effect being better suppression of the herpes zoster virus by the recombinant vaccine,” Dr. Harrison commented.
Though these findings are not likely to lead to any immediate changes in policy regarding the shingles vaccine, Dr. Harrison said it would be interesting to see whether uptake of the vaccine increased after this study.
He estimated that, currently in the United Kingdom, about 60% of older adults choose to have the shingles vaccine. A 2020 study in the United States found that only about one-third of US adults over 60 had received the vaccine.
“It will be interesting to see if that figure increases after these data are publicized, but I am not recommending that people have the vaccine specifically to lower their risk of dementia because of the caveats about the study that we have discussed,” he commented.
Outside Experts Positive
Outside experts, providing comment to the Science Media Centre, welcomed the new research.
“ The study is very well-conducted and adds to previous data indicating that vaccination against shingles is associated with lower dementia risk. More research is needed in future to determine why this vaccine is associated with lower dementia risk,” said Tara Spires-Jones, FMedSci, president of the British Neuroscience Association.
The high number of patients in the study and the adjustments for potential confounders are also strong points, noted Andrew Doig, PhD, professor of biochemistry, University of Manchester, Manchester, England.
“This is a significant result, comparable in effectiveness to the recent antibody drugs for Alzheimer’s disease,” Dr. Doig said. “Administering the recombinant shingles vaccine could well be a simple and cheap way to lower the risk of Alzheimer’s disease.”
Dr. Doig noted that a link between herpes zoster infection and the onset of dementia has been suspected for some time, and a trial of the antiviral drug valacyclovir against Alzheimer’s disease is currently underway.
In regard to the shingles vaccine, he said a placebo-controlled trial would be needed to prove causality.
“We also need to see how many years the effect might last and whether we should vaccinate people at a younger age. We know that the path to Alzheimer’s can start decades before any symptoms are apparent, so the vaccine might be even more effective if given to people in their 40s or 50s,” he said.
Dr. Harrison and Dr. Taquet reported no disclosures. Dr. Doig is a founder, director, and consultant for PharmaKure, which works on Alzheimer’s drugs and diagnostics. Other commentators declared no disclosures.
A version of this article first appeared on Medscape.com.
The study builds on previous observations of a reduction in dementia risk with the older live shingles vaccine and reports a delay in dementia diagnosis of 164 days with the newer recombinant version, compared with the live vaccine.
“Given the prevalence of dementia, a delay of 164 days in diagnosis would not be a trivial effect at the public health level. It’s a big enough effect that if there is a causality it feels meaningful,” said senior author Paul Harrison, DM, FRCPsych, professor of psychiatry at the University of Oxford, Oxford, England.
But Dr. Harrison stressed that the study had not proven that the shingles vaccine reduced dementia risk.
“The design of the study allows us to do away with many of the confounding effects we usually see in observational studies, but this is still an observational study, and as such it cannot prove a definite causal effect,” he said.
The study was published online on July 25 in Nature Medicine.
‘Natural Experiment’
Given the risk for deleterious consequences of shingles, vaccination is now recommended for older adults in many countries. The previously used live shingles vaccine (Zostavax) is being replaced in most countries with the new recombinant shingles vaccine (Shingrix), which is more effective at preventing shingles infection.
The current study made use of a “natural experiment” in the United States, which switched over from use of the live vaccine to the recombinant vaccine in October 2017.
Researchers used electronic heath records to compare the incidence of a dementia diagnosis in individuals who received the live shingles vaccine prior to October 2017 with those who received the recombinant version after the United States made the switch.
They also used propensity score matching to further control for confounding factors, comparing 103,837 individuals who received a first dose of the live shingles vaccine between October 2014 and September 2017 with the same number of matched people who received the recombinant vaccine between November 2017 and October 2020.
Results showed that within the 6 years after vaccination, the recombinant vaccine was associated with a delay in the diagnosis of dementia, compared with the live vaccine. Specifically, receiving the recombinant vaccine was associated with a 17% increase in diagnosis-free time, translating to 164 additional days lived without a diagnosis of dementia in those subsequently affected.
As an additional control, the researchers also found significantly lower risks for dementia in individuals receiving the new recombinant shingles vaccine vs two other vaccines commonly used in older people: influenza and tetanus/diphtheria/pertussis vaccines, with increases in diagnosis-free time of 14%-27%.
Reduced Risk or Delayed Diagnosis?
Speaking at a Science Media Centre press conference on the study, lead author Maxime Taquet, PhD, FRCPsych, clinical lecturer in psychiatry at the University of Oxford, noted that the total number of dementia cases were similar in the two shingles vaccine groups by the end of the 6-year follow-up period but there was a difference in the time at which they received a diagnosis of dementia.
“The study suggests that rather than actually reducing dementia risk, the recombinant vaccine delays the onset of dementia compared to the live vaccine in patients who go on to develop the condition,” he explained.
But when comparing the recombinant vaccine with the influenza and tetanus/diphtheria/pertussis vaccines there was a clear reduction in dementia risk itself, Dr. Taquet reported.
“It might well be that the live vaccine has a potential effect on the risk of dementia itself and therefore the recombinant vaccine only shows a delay in dementia compared to the live vaccine, but both of them might decrease the overall risk of dementia,” he suggested.
But the researchers cautioned that this study could not prove causality.
“While the two groups were very carefully matched in terms of factors that might influence the development of dementia, we still have to be cautious before assuming that the vaccine is indeed causally reducing the risk of onset of dementia,” Dr. Harrison warned.
The researchers say the results would need to be confirmed in a randomized trial, which may have to be conducted in a slightly younger age group, as currently shingles vaccine is recommended for all older individuals in the United Kingdom.
Vaccine recommendations vary from country to country, Dr. Harrison added. In the United States, the Centers for Disease Control and Prevention recommends the recombinant shingles vaccine for all adults aged 50 years or older.
In the meantime, it would be interesting to see whether further observational studies in other countries find similar results as this US study, Dr. Harrison said.
Mechanism Uncertain
Speculating on a possible mechanism behind the findings, Dr. Harrison suggested two plausible explanations.
“First, it is thought that the herpes virus could be one of many factors that could promote dementia, so a vaccine that stops reactivation of this virus might therefore be delaying that process,” he noted.
The other possibility is that adjuvants included in the recombinant vaccine to stimulate the immune system might have played a role.
“We don’t have any data on the mechanism, and thus study did not address that, so further studies are needed to look into this,” Dr. Harrison said.
Stronger Effect in Women
Another intriguing finding is that the association with the recombinant vaccine and delayed dementia diagnosis seemed to be stronger in women vs men.
In the original study of the live shingles vaccine, a protective effect against dementia was shown only in women.
In the current study, the delay in dementia diagnosis was seen in both sexes but was stronger in women, showing a 22% increased time without dementia in women versus a 13% increased time in men with the recombinant versus the live vaccine.
As expected, the recombinant vaccine was associated with a lower risk for shingles disease vs the live vaccine (2.5% versus 3.5%), but women did not have a better response than men did in this respect.
“The better protection against shingles with the recombinant vaccine was similar in men and women, an observation that might be one reason to question the possible mechanism behind the dementia effect being better suppression of the herpes zoster virus by the recombinant vaccine,” Dr. Harrison commented.
Though these findings are not likely to lead to any immediate changes in policy regarding the shingles vaccine, Dr. Harrison said it would be interesting to see whether uptake of the vaccine increased after this study.
He estimated that, currently in the United Kingdom, about 60% of older adults choose to have the shingles vaccine. A 2020 study in the United States found that only about one-third of US adults over 60 had received the vaccine.
“It will be interesting to see if that figure increases after these data are publicized, but I am not recommending that people have the vaccine specifically to lower their risk of dementia because of the caveats about the study that we have discussed,” he commented.
Outside Experts Positive
Outside experts, providing comment to the Science Media Centre, welcomed the new research.
“ The study is very well-conducted and adds to previous data indicating that vaccination against shingles is associated with lower dementia risk. More research is needed in future to determine why this vaccine is associated with lower dementia risk,” said Tara Spires-Jones, FMedSci, president of the British Neuroscience Association.
The high number of patients in the study and the adjustments for potential confounders are also strong points, noted Andrew Doig, PhD, professor of biochemistry, University of Manchester, Manchester, England.
“This is a significant result, comparable in effectiveness to the recent antibody drugs for Alzheimer’s disease,” Dr. Doig said. “Administering the recombinant shingles vaccine could well be a simple and cheap way to lower the risk of Alzheimer’s disease.”
Dr. Doig noted that a link between herpes zoster infection and the onset of dementia has been suspected for some time, and a trial of the antiviral drug valacyclovir against Alzheimer’s disease is currently underway.
In regard to the shingles vaccine, he said a placebo-controlled trial would be needed to prove causality.
“We also need to see how many years the effect might last and whether we should vaccinate people at a younger age. We know that the path to Alzheimer’s can start decades before any symptoms are apparent, so the vaccine might be even more effective if given to people in their 40s or 50s,” he said.
Dr. Harrison and Dr. Taquet reported no disclosures. Dr. Doig is a founder, director, and consultant for PharmaKure, which works on Alzheimer’s drugs and diagnostics. Other commentators declared no disclosures.
A version of this article first appeared on Medscape.com.
FROM NATURE MEDICINE
Acquired Factor VIII Deficiency Presenting as Compartment Syndrome
Compartment syndrome occurs when the interstitial tissue pressures within a confined space are elevated to a level at which the arterial perfusion is diminished. Multiple etiologies exist and can be extrinsic (a cast that is too tight or prolonged compression on a limb), iatrogenic (aggressive resuscitation, drug infiltration, arterial puncture, or a spontaneous bleed from anticoagulation), and traumatic (fracture, snake envenomation, circumferential burn, or electrocution). If the compartments are not released, irreversible changes happen to the cells, including nerve and muscle death.1 Definitive management of this emergency requires prompt fasciotomy to decompress the compartment(s).1-3
Case Presentation
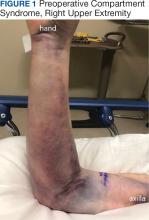
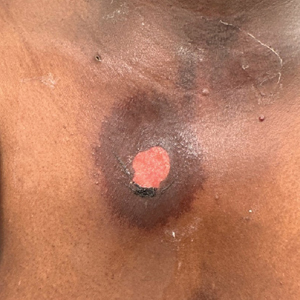
A 76-year-old right-handed woman with a history of chronic obstructive pulmonary disease, hypertension, and hyperlipidemia presented to the emergency department with 2 days of extensive right upper extremity ecchymosis and severe pain that was localized to her forearm (Figure 1). She was taking low-dose aspirin (81 mg/d) for left subclavian stenosis and over-the-counter ginkgo biloba. Leading up to the presentation, the patient was able to perform routine household chores, including yard work, cleaning, and taking care of her cats. Wrist and elbow X-rays were negative for a fracture. An upper extremity ultrasound found no venous occlusion. A computed tomography (CT) angiogram of her arm and chest found diffuse edema around the right elbow and forearm without pulmonary or right upper extremity emboli, fractures, hematoma, abscess, or air in the tissues.
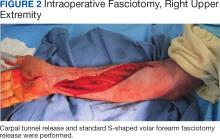
The plastic surgery service was consulted. The patient was found to have a very tense forearm and pain to passive digital extension. The 2-point discrimination and pulses were intact. The patient was diagnosed with compartment syndrome based on the examination alone and gave consent for an emergent forearm and hand fasciotomy. A carpal tunnel release and a standard S-shaped volar forearm fasciotomy release were performed, which provided immediate decompression (Figure 2). The rest of the hand and extremity were soft. Edematous, healthy flexor muscle belly was identified without a hematoma. Most of the forearm wound was left open because the skin could not be reapproximated. Oxidized regenerated cellulose (Surgicel) was placed around the wound edges and the muscle was covered with a nonadherent dressing. Hemoglobin on admission was 12.9 g/dL(reference range, 12 to 16 g/dL). Kidney function was within normal limits. The rest of the complete blood count was unremarkable. Postoperative hemoglobin was 8.6 g/dL. Over the next several days, the patient's skin edges and muscle bellies continued to slowly bleed, and her hemoglobin fell to 5.6 g/dL by postoperative Day 2. The bleeding was managed with topical oxidized regenerated cellulose, thrombin spray, a hemostatic dressing made with kaolin (QuikClot), and a transfusion of 2 units of packed red blood cells.
A hematology consultation was requested. The patient was noted to have an elevated partial thromboplastin time (PTT) since admission measuring between 39.9 to 61.7 seconds (reference range, 26.2 to 37.2 seconds) and a normal prothrombin time test with an international normalized ratio. A PTT measured 17 months prior to admission was within the normal range. She reported no personal or family history of bleeding disorders. Until recently, she had never had easy bruisability. She reported no history of heavy menses or epistaxis. The patient had no children and had never been pregnant. She had tolerated an exploratory laparotomy 40 years prior to admission without bleeding complications and had never required blood transfusions before. A PTT 1:1 mixing study revealed incomplete correction. Subsequent workup included factor VIII (FVIII) activity, factor IX activity, factor XI activity, von Willebrand factor antigen, ristocetin cofactor assay, and von Willebrand factor multimers. FVIII activity was severely reduced at 7.8% (reference, > 54%) with a positive Bethesda assay of 300 to 400 Bodansky units (BU), indicating a strong FVIII inhibitor was present and establishing a diagnosis of acquired hemophilia A. Further workup for secondary causes of acquired hemophilia A including abdominal and pelvic CT, serum protein electrophoresis, and serum free light chains, were negative. She was started on prednisone 1 mg/kg daily and rituximab 375 mg/m2. Her hemoglobin stabilized, and she required no further blood transfusions.
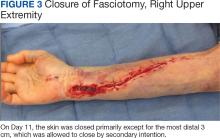
The patient underwent wound closure on postoperative Day 11. At the time of the second surgery, there was still no improvement in her FVIII levels or PTT; therefore, 70 mcg/kg of recombinant coagulation-activated FVII was given just before surgery with no bleeding complications. The skin was closed primarily except for the most distal 3 cm (Figure 3). Due to concerns regarding further bleeding with skin graft, the remaining wound was allowed to close by secondary intention. As a precaution, the wound was covered with oxidized regenerated cellulose and thrombin spray. The patient continued to progress postoperatively without bleeding complications or a need for additional transfusions. She was seen by the hand therapist before and after the second surgery to help with edema management and joint mobility. She completed 4 weekly doses of 375 mg/m² rituximab and prednisone was tapered by 10 mg weekly.
Three weeks after starting treatment, her PTT normalized, and her FVIII increased to 33.7%. The Bethesda assay remained high at 198 BU, although it was lower than at admission. She was discharged home with dressing changes and monthly follow-up appointments. The wounds were fully closed at her 3-month appointment when she proudly demonstrated full digital extension and flexion into her palm.
Discussion
Forearm compartment syndrome is most often caused by fractures—distal radius in adults and supracondylar in children.2 This case initially presented as a diagnostic puzzle to the emergency department due to the patient’s lucid review of several days of nontraumatic injury.
The clinical hallmarks of compartment syndrome are the 5 Ps: pain, pallor, paresthesia, paralysis, and pulselessness. Patients will describe the pain as out of proportion to the nature of the injury; the compartments will be tense and swollen, they will have pain to passive muscle stretch, and sensation will progressively diminish. Distal pulses are the last to go, and permanent tissue damage can still occur when pulses are present.1
Compartment Syndrome
Compartment syndrome is generally a clinical diagnosis; however, in patients who are sedated or uncooperative, or if the clinical findings are equivocal, the examination can be supplemented with intercompartmental pressures using an arterial line transducer system.2 In general, a tissue pressure of 30 mm Hg or a 20- to 30-mm Hg difference between the diastolic and compartment pressures are indications for fasciotomy.1 The hand is treated with an open carpal tunnel release, interosseous muscle release through 2 dorsal hand incisions, and thenar and hypothenar muscle release. The forearm is treated through a curved volar incision that usually decompresses the dorsal compartment, as it did in our patient. If pressures are still high in the forearm, a longitudinal dorsal incision over the mobile wad is necessary. Wounds can be closed primarily days later, left open to close by secondary intention, or reconstructed with skin grafts.2 In our patient, compartment syndrome was isolated to her forearm and the carpal tunnel release was performed prophylactically since it did not add significant time or morbidity to the surgery.
Nontraumatic upper extremity compartment syndrome is rare. A 2021 review of acute nontraumatic upper extremity compartment syndrome found a bleeding disorder as the etiology in 3 cases published in the literature between 1993 and 2016.4 One of these cases was secondary to a known diagnosis of hemophilia A in a teenager.5 Ogrodnik and colleagues described a spontaneous hand hematoma secondary to previously undiagnosed acquired hemophilia A and Waldenström macroglobulinemia.4 Ilyas and colleagues described a spontaneous hematoma in the forearm dorsal compartment in a 67-year-old woman, which presented as compartment syndrome and elevated PTT and led to a diagnosis of acquired FVIII inhibitor. The authors recommended prompt hematology consultation to coordinate treatment once this diagnosis issuspected.6 Compartment syndrome also has been found to develop slowly over weeks in patients with acquired FVIII deficiency, suggesting a high index of suspicion and frequent examinations are needed when patients with known acquired hemophilia A present with a painful extremity.7
Nontraumatic compartment syndrome in the lower extremity in patients with previously undiagnosed acquired hemophilia A has also been described in the literature.8-11 Case reports describe the delay in diagnosis as the patients were originally seen by clinicians for lower extremity pain and swelling within days of presenting to the emergency room with compartment syndrome. Persistent bleeding and abnormal laboratory results prompted further tests and examinations.8,9,11 This underscores the need to be suspicious of this unusual pathology without a history of trauma.
Acquired Hemophilia A
Acquired hemophilia A is an autoimmune disease most often found in older individuals, with a mean age of approximately 70 years.12 It is caused by the spontaneous production of neutralizing immunoglobin autoantibodies that target endogenous FVIII. Many cases are idiopathic; however, up to 50% of cases are associated with underlying autoimmunity, malignancy (especially lymphoproliferative disorders), or pregnancy. It often presents as bleeding that is subcutaneous or in the gastrointestinal system, muscle, retroperitoneal space, or genitourinary system. Unlike congenital hemophilia A, joint bleeding is rare.13
The diagnosis is suspected with an isolated elevated PTT in the absence of other coagulation abnormalities. A 1:1 mixing study will typically show incomplete correction, which suggests the presence of an inhibitor. FVIII activity is reduced, and the FVIII inhibitor is confirmed with the Bethesda assay. Clinically active bleeding is treated with bypassing agents such as recombinant coagulation-activated FVII, activated prothrombin complex concentrates such as anti-inhibitor coagulant complex (FEIBA), or recombinant porcine FVIII.12,14 Not all patients require hemostatic treatment, but close monitoring, education, recognition, and immediate treatment, if needed, are indicated.13 Immunosuppressive therapy (corticosteroids, rituximab, and/or cyclophosphamide) is prescribed to eradicate the antibodies and induce remission.12
Conclusions
An older woman without a preceding trauma was diagnosed with an unusual case of acute compartment syndrome in the forearm. No hematoma was found, but muscle and skin bleeding plus an elevated PTT prompted a hematology workup, and, ultimately, the diagnosis of FVIII inhibitor secondary to acquired hemophilia A.
While a nontraumatic cause of compartment syndrome is rare, it should be considered in differential diagnosis for clinicians who see hand and upper extremity emergencies. An isolated elevated PTT in a patient with a bleed should raise suspicions and trigger immediate further evaluation. Once suspected, multidisciplinary treatment is indicated for immediate and long-term successful outcomes.
Acknowledgments
This manuscript is the result of work supported withresources and the use of facilities at the North Florida/South Georgia Veterans Health System, Gainesville, Florida.
1. Leversedge FJ, Moore TJ, Peterson BC, Seiler JG 3rd. Compartment syndrome of the upper extremity. J Hand Surg Am. 2011;36:544-559. doi:10.1016/j.jhsa.2010.12.008
2. Kalyani BS, Fisher BE, Roberts CS, Giannoudis PV. Compartment syndrome of the forearm: a systematic review. J Hand Surg Am. 2011;36:535-543. doi:10.1016/j.jhsa.2010.12.007
3. Steadman W, Wu R, Hamilton AT, Richardson MD, Wall CJ. Review article: a comprehensive review of unusual causes of acute limb compartment syndrome. Emerg Med Australas. 2022;34:871-876. doi:10.1111/1742-6723.14098
4. Ogrodnik J, Oliver JD, Cani D, Boczar D, Huayllani MT, Restrepo DJ, et al. Clinical case of acute non-traumatic hand compartment syndrome and systematic review for the upper extremity. Hand (N Y). 2021;16:285-291. doi:10.1177/1558944719856106
5. Kim J, Zelken J, Sacks JM. Case report. Spontaneous forearm compartment syndrome in a boy with hemophilia a: a therapeutic dilemma. Eplasty. 2013:13:e16.
6. Ilyas AM, Wisbeck JM, Shaffer GW, Thoder JJ. Upper extremity compartment syndrome secondary to acquired factor VIII inhibitor. A case report. J Bone Joint Surg Am. 2005;87:1606-1608. doi:10.2106/JBJS.C.01720
7. Adeclat GJ, Hayes M, Amick M, Kahan J, Halim A. Acute forearm compartment syndrome in the setting of acquired hemophilia A. Case Reports Plast Surg Hand Surg. 2022;9:140-144. doi:10.1080/23320885.2022.2071274
8. Abudaqqa RY, Arun KP, Mas AJA, Abushaaban FA. Acute atraumatic compartment syndrome of the thigh due to acquired coagulopathy disorder: a case report in known healthy patient. J Orthop Case Rep. 2021;11:59-62. doi:10.13107/jocr.2021.v11.i08.2366
9. Alidoost M, Conte GA, Chaudry R, Nahum K, Marchesani D. A unique presentation of spontaneous compartment syndrome due to acquired hemophilia A and associated malignancy: case report and literature review. World J Oncol. 2020;11:72-75. doi:10.14740/wjon1260
10. Jentzsch T, Brand-Staufer B, Schäfer FP, Wanner GA, Simmen H-P. Illustrated operative management of spontaneous bleeding and compartment syndrome of the lower extremity in a patient with acquired hemophilia A: a case report. J Med Case Rep. 2014;8:132. doi:10.1186/1752-1947-8-132
11. Pham TV, Sorenson CA, Nable JV. Acquired factor VIII deficiency presenting with compartment syndrome. Am J Emerg Med. 2014;32:195.e1-2. doi:10.1016/j.ajem.2013.09.022
12. Tiede A, Zieger B, Lisman T. Acquired bleeding disorders. Haemophilia. 2022;28(suppl 4):68-76. doi:10.1111/hae.14548
13. Kruse-Jarres R, Kempton CL, Baudo F, Collins PW, Knoebl P, Leissinger CA, et al. Acquired hemophilia A: updated review of evidence and treatment guidance. Am J Hematol. 2017;92:695-705. doi:10.1002/ajh.24777
14. Ilkhchoui Y, Koshkin E, Windsor JJ, Petersen TR, Charles M, Pack JD. Perioperative management of acquired hemophilia A: a case report and review of literature. Anesth Pain Med. 2013;4:e11906. doi:10.5812/aapm.11906
Compartment syndrome occurs when the interstitial tissue pressures within a confined space are elevated to a level at which the arterial perfusion is diminished. Multiple etiologies exist and can be extrinsic (a cast that is too tight or prolonged compression on a limb), iatrogenic (aggressive resuscitation, drug infiltration, arterial puncture, or a spontaneous bleed from anticoagulation), and traumatic (fracture, snake envenomation, circumferential burn, or electrocution). If the compartments are not released, irreversible changes happen to the cells, including nerve and muscle death.1 Definitive management of this emergency requires prompt fasciotomy to decompress the compartment(s).1-3
Case Presentation
A 76-year-old right-handed woman with a history of chronic obstructive pulmonary disease, hypertension, and hyperlipidemia presented to the emergency department with 2 days of extensive right upper extremity ecchymosis and severe pain that was localized to her forearm (Figure 1). She was taking low-dose aspirin (81 mg/d) for left subclavian stenosis and over-the-counter ginkgo biloba. Leading up to the presentation, the patient was able to perform routine household chores, including yard work, cleaning, and taking care of her cats. Wrist and elbow X-rays were negative for a fracture. An upper extremity ultrasound found no venous occlusion. A computed tomography (CT) angiogram of her arm and chest found diffuse edema around the right elbow and forearm without pulmonary or right upper extremity emboli, fractures, hematoma, abscess, or air in the tissues.
The plastic surgery service was consulted. The patient was found to have a very tense forearm and pain to passive digital extension. The 2-point discrimination and pulses were intact. The patient was diagnosed with compartment syndrome based on the examination alone and gave consent for an emergent forearm and hand fasciotomy. A carpal tunnel release and a standard S-shaped volar forearm fasciotomy release were performed, which provided immediate decompression (Figure 2). The rest of the hand and extremity were soft. Edematous, healthy flexor muscle belly was identified without a hematoma. Most of the forearm wound was left open because the skin could not be reapproximated. Oxidized regenerated cellulose (Surgicel) was placed around the wound edges and the muscle was covered with a nonadherent dressing. Hemoglobin on admission was 12.9 g/dL(reference range, 12 to 16 g/dL). Kidney function was within normal limits. The rest of the complete blood count was unremarkable. Postoperative hemoglobin was 8.6 g/dL. Over the next several days, the patient's skin edges and muscle bellies continued to slowly bleed, and her hemoglobin fell to 5.6 g/dL by postoperative Day 2. The bleeding was managed with topical oxidized regenerated cellulose, thrombin spray, a hemostatic dressing made with kaolin (QuikClot), and a transfusion of 2 units of packed red blood cells.
A hematology consultation was requested. The patient was noted to have an elevated partial thromboplastin time (PTT) since admission measuring between 39.9 to 61.7 seconds (reference range, 26.2 to 37.2 seconds) and a normal prothrombin time test with an international normalized ratio. A PTT measured 17 months prior to admission was within the normal range. She reported no personal or family history of bleeding disorders. Until recently, she had never had easy bruisability. She reported no history of heavy menses or epistaxis. The patient had no children and had never been pregnant. She had tolerated an exploratory laparotomy 40 years prior to admission without bleeding complications and had never required blood transfusions before. A PTT 1:1 mixing study revealed incomplete correction. Subsequent workup included factor VIII (FVIII) activity, factor IX activity, factor XI activity, von Willebrand factor antigen, ristocetin cofactor assay, and von Willebrand factor multimers. FVIII activity was severely reduced at 7.8% (reference, > 54%) with a positive Bethesda assay of 300 to 400 Bodansky units (BU), indicating a strong FVIII inhibitor was present and establishing a diagnosis of acquired hemophilia A. Further workup for secondary causes of acquired hemophilia A including abdominal and pelvic CT, serum protein electrophoresis, and serum free light chains, were negative. She was started on prednisone 1 mg/kg daily and rituximab 375 mg/m2. Her hemoglobin stabilized, and she required no further blood transfusions.
The patient underwent wound closure on postoperative Day 11. At the time of the second surgery, there was still no improvement in her FVIII levels or PTT; therefore, 70 mcg/kg of recombinant coagulation-activated FVII was given just before surgery with no bleeding complications. The skin was closed primarily except for the most distal 3 cm (Figure 3). Due to concerns regarding further bleeding with skin graft, the remaining wound was allowed to close by secondary intention. As a precaution, the wound was covered with oxidized regenerated cellulose and thrombin spray. The patient continued to progress postoperatively without bleeding complications or a need for additional transfusions. She was seen by the hand therapist before and after the second surgery to help with edema management and joint mobility. She completed 4 weekly doses of 375 mg/m² rituximab and prednisone was tapered by 10 mg weekly.
Three weeks after starting treatment, her PTT normalized, and her FVIII increased to 33.7%. The Bethesda assay remained high at 198 BU, although it was lower than at admission. She was discharged home with dressing changes and monthly follow-up appointments. The wounds were fully closed at her 3-month appointment when she proudly demonstrated full digital extension and flexion into her palm.
Discussion
Forearm compartment syndrome is most often caused by fractures—distal radius in adults and supracondylar in children.2 This case initially presented as a diagnostic puzzle to the emergency department due to the patient’s lucid review of several days of nontraumatic injury.
The clinical hallmarks of compartment syndrome are the 5 Ps: pain, pallor, paresthesia, paralysis, and pulselessness. Patients will describe the pain as out of proportion to the nature of the injury; the compartments will be tense and swollen, they will have pain to passive muscle stretch, and sensation will progressively diminish. Distal pulses are the last to go, and permanent tissue damage can still occur when pulses are present.1
Compartment Syndrome
Compartment syndrome is generally a clinical diagnosis; however, in patients who are sedated or uncooperative, or if the clinical findings are equivocal, the examination can be supplemented with intercompartmental pressures using an arterial line transducer system.2 In general, a tissue pressure of 30 mm Hg or a 20- to 30-mm Hg difference between the diastolic and compartment pressures are indications for fasciotomy.1 The hand is treated with an open carpal tunnel release, interosseous muscle release through 2 dorsal hand incisions, and thenar and hypothenar muscle release. The forearm is treated through a curved volar incision that usually decompresses the dorsal compartment, as it did in our patient. If pressures are still high in the forearm, a longitudinal dorsal incision over the mobile wad is necessary. Wounds can be closed primarily days later, left open to close by secondary intention, or reconstructed with skin grafts.2 In our patient, compartment syndrome was isolated to her forearm and the carpal tunnel release was performed prophylactically since it did not add significant time or morbidity to the surgery.
Nontraumatic upper extremity compartment syndrome is rare. A 2021 review of acute nontraumatic upper extremity compartment syndrome found a bleeding disorder as the etiology in 3 cases published in the literature between 1993 and 2016.4 One of these cases was secondary to a known diagnosis of hemophilia A in a teenager.5 Ogrodnik and colleagues described a spontaneous hand hematoma secondary to previously undiagnosed acquired hemophilia A and Waldenström macroglobulinemia.4 Ilyas and colleagues described a spontaneous hematoma in the forearm dorsal compartment in a 67-year-old woman, which presented as compartment syndrome and elevated PTT and led to a diagnosis of acquired FVIII inhibitor. The authors recommended prompt hematology consultation to coordinate treatment once this diagnosis issuspected.6 Compartment syndrome also has been found to develop slowly over weeks in patients with acquired FVIII deficiency, suggesting a high index of suspicion and frequent examinations are needed when patients with known acquired hemophilia A present with a painful extremity.7
Nontraumatic compartment syndrome in the lower extremity in patients with previously undiagnosed acquired hemophilia A has also been described in the literature.8-11 Case reports describe the delay in diagnosis as the patients were originally seen by clinicians for lower extremity pain and swelling within days of presenting to the emergency room with compartment syndrome. Persistent bleeding and abnormal laboratory results prompted further tests and examinations.8,9,11 This underscores the need to be suspicious of this unusual pathology without a history of trauma.
Acquired Hemophilia A
Acquired hemophilia A is an autoimmune disease most often found in older individuals, with a mean age of approximately 70 years.12 It is caused by the spontaneous production of neutralizing immunoglobin autoantibodies that target endogenous FVIII. Many cases are idiopathic; however, up to 50% of cases are associated with underlying autoimmunity, malignancy (especially lymphoproliferative disorders), or pregnancy. It often presents as bleeding that is subcutaneous or in the gastrointestinal system, muscle, retroperitoneal space, or genitourinary system. Unlike congenital hemophilia A, joint bleeding is rare.13
The diagnosis is suspected with an isolated elevated PTT in the absence of other coagulation abnormalities. A 1:1 mixing study will typically show incomplete correction, which suggests the presence of an inhibitor. FVIII activity is reduced, and the FVIII inhibitor is confirmed with the Bethesda assay. Clinically active bleeding is treated with bypassing agents such as recombinant coagulation-activated FVII, activated prothrombin complex concentrates such as anti-inhibitor coagulant complex (FEIBA), or recombinant porcine FVIII.12,14 Not all patients require hemostatic treatment, but close monitoring, education, recognition, and immediate treatment, if needed, are indicated.13 Immunosuppressive therapy (corticosteroids, rituximab, and/or cyclophosphamide) is prescribed to eradicate the antibodies and induce remission.12
Conclusions
An older woman without a preceding trauma was diagnosed with an unusual case of acute compartment syndrome in the forearm. No hematoma was found, but muscle and skin bleeding plus an elevated PTT prompted a hematology workup, and, ultimately, the diagnosis of FVIII inhibitor secondary to acquired hemophilia A.
While a nontraumatic cause of compartment syndrome is rare, it should be considered in differential diagnosis for clinicians who see hand and upper extremity emergencies. An isolated elevated PTT in a patient with a bleed should raise suspicions and trigger immediate further evaluation. Once suspected, multidisciplinary treatment is indicated for immediate and long-term successful outcomes.
Acknowledgments
This manuscript is the result of work supported withresources and the use of facilities at the North Florida/South Georgia Veterans Health System, Gainesville, Florida.
Compartment syndrome occurs when the interstitial tissue pressures within a confined space are elevated to a level at which the arterial perfusion is diminished. Multiple etiologies exist and can be extrinsic (a cast that is too tight or prolonged compression on a limb), iatrogenic (aggressive resuscitation, drug infiltration, arterial puncture, or a spontaneous bleed from anticoagulation), and traumatic (fracture, snake envenomation, circumferential burn, or electrocution). If the compartments are not released, irreversible changes happen to the cells, including nerve and muscle death.1 Definitive management of this emergency requires prompt fasciotomy to decompress the compartment(s).1-3
Case Presentation
A 76-year-old right-handed woman with a history of chronic obstructive pulmonary disease, hypertension, and hyperlipidemia presented to the emergency department with 2 days of extensive right upper extremity ecchymosis and severe pain that was localized to her forearm (Figure 1). She was taking low-dose aspirin (81 mg/d) for left subclavian stenosis and over-the-counter ginkgo biloba. Leading up to the presentation, the patient was able to perform routine household chores, including yard work, cleaning, and taking care of her cats. Wrist and elbow X-rays were negative for a fracture. An upper extremity ultrasound found no venous occlusion. A computed tomography (CT) angiogram of her arm and chest found diffuse edema around the right elbow and forearm without pulmonary or right upper extremity emboli, fractures, hematoma, abscess, or air in the tissues.
The plastic surgery service was consulted. The patient was found to have a very tense forearm and pain to passive digital extension. The 2-point discrimination and pulses were intact. The patient was diagnosed with compartment syndrome based on the examination alone and gave consent for an emergent forearm and hand fasciotomy. A carpal tunnel release and a standard S-shaped volar forearm fasciotomy release were performed, which provided immediate decompression (Figure 2). The rest of the hand and extremity were soft. Edematous, healthy flexor muscle belly was identified without a hematoma. Most of the forearm wound was left open because the skin could not be reapproximated. Oxidized regenerated cellulose (Surgicel) was placed around the wound edges and the muscle was covered with a nonadherent dressing. Hemoglobin on admission was 12.9 g/dL(reference range, 12 to 16 g/dL). Kidney function was within normal limits. The rest of the complete blood count was unremarkable. Postoperative hemoglobin was 8.6 g/dL. Over the next several days, the patient's skin edges and muscle bellies continued to slowly bleed, and her hemoglobin fell to 5.6 g/dL by postoperative Day 2. The bleeding was managed with topical oxidized regenerated cellulose, thrombin spray, a hemostatic dressing made with kaolin (QuikClot), and a transfusion of 2 units of packed red blood cells.
A hematology consultation was requested. The patient was noted to have an elevated partial thromboplastin time (PTT) since admission measuring between 39.9 to 61.7 seconds (reference range, 26.2 to 37.2 seconds) and a normal prothrombin time test with an international normalized ratio. A PTT measured 17 months prior to admission was within the normal range. She reported no personal or family history of bleeding disorders. Until recently, she had never had easy bruisability. She reported no history of heavy menses or epistaxis. The patient had no children and had never been pregnant. She had tolerated an exploratory laparotomy 40 years prior to admission without bleeding complications and had never required blood transfusions before. A PTT 1:1 mixing study revealed incomplete correction. Subsequent workup included factor VIII (FVIII) activity, factor IX activity, factor XI activity, von Willebrand factor antigen, ristocetin cofactor assay, and von Willebrand factor multimers. FVIII activity was severely reduced at 7.8% (reference, > 54%) with a positive Bethesda assay of 300 to 400 Bodansky units (BU), indicating a strong FVIII inhibitor was present and establishing a diagnosis of acquired hemophilia A. Further workup for secondary causes of acquired hemophilia A including abdominal and pelvic CT, serum protein electrophoresis, and serum free light chains, were negative. She was started on prednisone 1 mg/kg daily and rituximab 375 mg/m2. Her hemoglobin stabilized, and she required no further blood transfusions.
The patient underwent wound closure on postoperative Day 11. At the time of the second surgery, there was still no improvement in her FVIII levels or PTT; therefore, 70 mcg/kg of recombinant coagulation-activated FVII was given just before surgery with no bleeding complications. The skin was closed primarily except for the most distal 3 cm (Figure 3). Due to concerns regarding further bleeding with skin graft, the remaining wound was allowed to close by secondary intention. As a precaution, the wound was covered with oxidized regenerated cellulose and thrombin spray. The patient continued to progress postoperatively without bleeding complications or a need for additional transfusions. She was seen by the hand therapist before and after the second surgery to help with edema management and joint mobility. She completed 4 weekly doses of 375 mg/m² rituximab and prednisone was tapered by 10 mg weekly.
Three weeks after starting treatment, her PTT normalized, and her FVIII increased to 33.7%. The Bethesda assay remained high at 198 BU, although it was lower than at admission. She was discharged home with dressing changes and monthly follow-up appointments. The wounds were fully closed at her 3-month appointment when she proudly demonstrated full digital extension and flexion into her palm.
Discussion
Forearm compartment syndrome is most often caused by fractures—distal radius in adults and supracondylar in children.2 This case initially presented as a diagnostic puzzle to the emergency department due to the patient’s lucid review of several days of nontraumatic injury.
The clinical hallmarks of compartment syndrome are the 5 Ps: pain, pallor, paresthesia, paralysis, and pulselessness. Patients will describe the pain as out of proportion to the nature of the injury; the compartments will be tense and swollen, they will have pain to passive muscle stretch, and sensation will progressively diminish. Distal pulses are the last to go, and permanent tissue damage can still occur when pulses are present.1
Compartment Syndrome
Compartment syndrome is generally a clinical diagnosis; however, in patients who are sedated or uncooperative, or if the clinical findings are equivocal, the examination can be supplemented with intercompartmental pressures using an arterial line transducer system.2 In general, a tissue pressure of 30 mm Hg or a 20- to 30-mm Hg difference between the diastolic and compartment pressures are indications for fasciotomy.1 The hand is treated with an open carpal tunnel release, interosseous muscle release through 2 dorsal hand incisions, and thenar and hypothenar muscle release. The forearm is treated through a curved volar incision that usually decompresses the dorsal compartment, as it did in our patient. If pressures are still high in the forearm, a longitudinal dorsal incision over the mobile wad is necessary. Wounds can be closed primarily days later, left open to close by secondary intention, or reconstructed with skin grafts.2 In our patient, compartment syndrome was isolated to her forearm and the carpal tunnel release was performed prophylactically since it did not add significant time or morbidity to the surgery.
Nontraumatic upper extremity compartment syndrome is rare. A 2021 review of acute nontraumatic upper extremity compartment syndrome found a bleeding disorder as the etiology in 3 cases published in the literature between 1993 and 2016.4 One of these cases was secondary to a known diagnosis of hemophilia A in a teenager.5 Ogrodnik and colleagues described a spontaneous hand hematoma secondary to previously undiagnosed acquired hemophilia A and Waldenström macroglobulinemia.4 Ilyas and colleagues described a spontaneous hematoma in the forearm dorsal compartment in a 67-year-old woman, which presented as compartment syndrome and elevated PTT and led to a diagnosis of acquired FVIII inhibitor. The authors recommended prompt hematology consultation to coordinate treatment once this diagnosis issuspected.6 Compartment syndrome also has been found to develop slowly over weeks in patients with acquired FVIII deficiency, suggesting a high index of suspicion and frequent examinations are needed when patients with known acquired hemophilia A present with a painful extremity.7
Nontraumatic compartment syndrome in the lower extremity in patients with previously undiagnosed acquired hemophilia A has also been described in the literature.8-11 Case reports describe the delay in diagnosis as the patients were originally seen by clinicians for lower extremity pain and swelling within days of presenting to the emergency room with compartment syndrome. Persistent bleeding and abnormal laboratory results prompted further tests and examinations.8,9,11 This underscores the need to be suspicious of this unusual pathology without a history of trauma.
Acquired Hemophilia A
Acquired hemophilia A is an autoimmune disease most often found in older individuals, with a mean age of approximately 70 years.12 It is caused by the spontaneous production of neutralizing immunoglobin autoantibodies that target endogenous FVIII. Many cases are idiopathic; however, up to 50% of cases are associated with underlying autoimmunity, malignancy (especially lymphoproliferative disorders), or pregnancy. It often presents as bleeding that is subcutaneous or in the gastrointestinal system, muscle, retroperitoneal space, or genitourinary system. Unlike congenital hemophilia A, joint bleeding is rare.13
The diagnosis is suspected with an isolated elevated PTT in the absence of other coagulation abnormalities. A 1:1 mixing study will typically show incomplete correction, which suggests the presence of an inhibitor. FVIII activity is reduced, and the FVIII inhibitor is confirmed with the Bethesda assay. Clinically active bleeding is treated with bypassing agents such as recombinant coagulation-activated FVII, activated prothrombin complex concentrates such as anti-inhibitor coagulant complex (FEIBA), or recombinant porcine FVIII.12,14 Not all patients require hemostatic treatment, but close monitoring, education, recognition, and immediate treatment, if needed, are indicated.13 Immunosuppressive therapy (corticosteroids, rituximab, and/or cyclophosphamide) is prescribed to eradicate the antibodies and induce remission.12
Conclusions
An older woman without a preceding trauma was diagnosed with an unusual case of acute compartment syndrome in the forearm. No hematoma was found, but muscle and skin bleeding plus an elevated PTT prompted a hematology workup, and, ultimately, the diagnosis of FVIII inhibitor secondary to acquired hemophilia A.
While a nontraumatic cause of compartment syndrome is rare, it should be considered in differential diagnosis for clinicians who see hand and upper extremity emergencies. An isolated elevated PTT in a patient with a bleed should raise suspicions and trigger immediate further evaluation. Once suspected, multidisciplinary treatment is indicated for immediate and long-term successful outcomes.
Acknowledgments
This manuscript is the result of work supported withresources and the use of facilities at the North Florida/South Georgia Veterans Health System, Gainesville, Florida.
1. Leversedge FJ, Moore TJ, Peterson BC, Seiler JG 3rd. Compartment syndrome of the upper extremity. J Hand Surg Am. 2011;36:544-559. doi:10.1016/j.jhsa.2010.12.008
2. Kalyani BS, Fisher BE, Roberts CS, Giannoudis PV. Compartment syndrome of the forearm: a systematic review. J Hand Surg Am. 2011;36:535-543. doi:10.1016/j.jhsa.2010.12.007
3. Steadman W, Wu R, Hamilton AT, Richardson MD, Wall CJ. Review article: a comprehensive review of unusual causes of acute limb compartment syndrome. Emerg Med Australas. 2022;34:871-876. doi:10.1111/1742-6723.14098
4. Ogrodnik J, Oliver JD, Cani D, Boczar D, Huayllani MT, Restrepo DJ, et al. Clinical case of acute non-traumatic hand compartment syndrome and systematic review for the upper extremity. Hand (N Y). 2021;16:285-291. doi:10.1177/1558944719856106
5. Kim J, Zelken J, Sacks JM. Case report. Spontaneous forearm compartment syndrome in a boy with hemophilia a: a therapeutic dilemma. Eplasty. 2013:13:e16.
6. Ilyas AM, Wisbeck JM, Shaffer GW, Thoder JJ. Upper extremity compartment syndrome secondary to acquired factor VIII inhibitor. A case report. J Bone Joint Surg Am. 2005;87:1606-1608. doi:10.2106/JBJS.C.01720
7. Adeclat GJ, Hayes M, Amick M, Kahan J, Halim A. Acute forearm compartment syndrome in the setting of acquired hemophilia A. Case Reports Plast Surg Hand Surg. 2022;9:140-144. doi:10.1080/23320885.2022.2071274
8. Abudaqqa RY, Arun KP, Mas AJA, Abushaaban FA. Acute atraumatic compartment syndrome of the thigh due to acquired coagulopathy disorder: a case report in known healthy patient. J Orthop Case Rep. 2021;11:59-62. doi:10.13107/jocr.2021.v11.i08.2366
9. Alidoost M, Conte GA, Chaudry R, Nahum K, Marchesani D. A unique presentation of spontaneous compartment syndrome due to acquired hemophilia A and associated malignancy: case report and literature review. World J Oncol. 2020;11:72-75. doi:10.14740/wjon1260
10. Jentzsch T, Brand-Staufer B, Schäfer FP, Wanner GA, Simmen H-P. Illustrated operative management of spontaneous bleeding and compartment syndrome of the lower extremity in a patient with acquired hemophilia A: a case report. J Med Case Rep. 2014;8:132. doi:10.1186/1752-1947-8-132
11. Pham TV, Sorenson CA, Nable JV. Acquired factor VIII deficiency presenting with compartment syndrome. Am J Emerg Med. 2014;32:195.e1-2. doi:10.1016/j.ajem.2013.09.022
12. Tiede A, Zieger B, Lisman T. Acquired bleeding disorders. Haemophilia. 2022;28(suppl 4):68-76. doi:10.1111/hae.14548
13. Kruse-Jarres R, Kempton CL, Baudo F, Collins PW, Knoebl P, Leissinger CA, et al. Acquired hemophilia A: updated review of evidence and treatment guidance. Am J Hematol. 2017;92:695-705. doi:10.1002/ajh.24777
14. Ilkhchoui Y, Koshkin E, Windsor JJ, Petersen TR, Charles M, Pack JD. Perioperative management of acquired hemophilia A: a case report and review of literature. Anesth Pain Med. 2013;4:e11906. doi:10.5812/aapm.11906
1. Leversedge FJ, Moore TJ, Peterson BC, Seiler JG 3rd. Compartment syndrome of the upper extremity. J Hand Surg Am. 2011;36:544-559. doi:10.1016/j.jhsa.2010.12.008
2. Kalyani BS, Fisher BE, Roberts CS, Giannoudis PV. Compartment syndrome of the forearm: a systematic review. J Hand Surg Am. 2011;36:535-543. doi:10.1016/j.jhsa.2010.12.007
3. Steadman W, Wu R, Hamilton AT, Richardson MD, Wall CJ. Review article: a comprehensive review of unusual causes of acute limb compartment syndrome. Emerg Med Australas. 2022;34:871-876. doi:10.1111/1742-6723.14098
4. Ogrodnik J, Oliver JD, Cani D, Boczar D, Huayllani MT, Restrepo DJ, et al. Clinical case of acute non-traumatic hand compartment syndrome and systematic review for the upper extremity. Hand (N Y). 2021;16:285-291. doi:10.1177/1558944719856106
5. Kim J, Zelken J, Sacks JM. Case report. Spontaneous forearm compartment syndrome in a boy with hemophilia a: a therapeutic dilemma. Eplasty. 2013:13:e16.
6. Ilyas AM, Wisbeck JM, Shaffer GW, Thoder JJ. Upper extremity compartment syndrome secondary to acquired factor VIII inhibitor. A case report. J Bone Joint Surg Am. 2005;87:1606-1608. doi:10.2106/JBJS.C.01720
7. Adeclat GJ, Hayes M, Amick M, Kahan J, Halim A. Acute forearm compartment syndrome in the setting of acquired hemophilia A. Case Reports Plast Surg Hand Surg. 2022;9:140-144. doi:10.1080/23320885.2022.2071274
8. Abudaqqa RY, Arun KP, Mas AJA, Abushaaban FA. Acute atraumatic compartment syndrome of the thigh due to acquired coagulopathy disorder: a case report in known healthy patient. J Orthop Case Rep. 2021;11:59-62. doi:10.13107/jocr.2021.v11.i08.2366
9. Alidoost M, Conte GA, Chaudry R, Nahum K, Marchesani D. A unique presentation of spontaneous compartment syndrome due to acquired hemophilia A and associated malignancy: case report and literature review. World J Oncol. 2020;11:72-75. doi:10.14740/wjon1260
10. Jentzsch T, Brand-Staufer B, Schäfer FP, Wanner GA, Simmen H-P. Illustrated operative management of spontaneous bleeding and compartment syndrome of the lower extremity in a patient with acquired hemophilia A: a case report. J Med Case Rep. 2014;8:132. doi:10.1186/1752-1947-8-132
11. Pham TV, Sorenson CA, Nable JV. Acquired factor VIII deficiency presenting with compartment syndrome. Am J Emerg Med. 2014;32:195.e1-2. doi:10.1016/j.ajem.2013.09.022
12. Tiede A, Zieger B, Lisman T. Acquired bleeding disorders. Haemophilia. 2022;28(suppl 4):68-76. doi:10.1111/hae.14548
13. Kruse-Jarres R, Kempton CL, Baudo F, Collins PW, Knoebl P, Leissinger CA, et al. Acquired hemophilia A: updated review of evidence and treatment guidance. Am J Hematol. 2017;92:695-705. doi:10.1002/ajh.24777
14. Ilkhchoui Y, Koshkin E, Windsor JJ, Petersen TR, Charles M, Pack JD. Perioperative management of acquired hemophilia A: a case report and review of literature. Anesth Pain Med. 2013;4:e11906. doi:10.5812/aapm.11906
Prognostication in Hospice Care: Challenges, Opportunities, and the Importance of Functional Status
Predicting life expectancy and providing an end-of-life diagnosis in hospice and palliative care is a challenge for most clinicians. Lack of training, limited communication skills, and relationships with patients are all contributing factors. These skills can improve with the use of functional scoring tools in conjunction with the patient’s comorbidities and physical/psychological symptoms. The Palliative Performance Scale (PPS), Karnofsky Performance Scale (KPS), and Eastern Cooperative Oncology Group Performance Status Scale (ECOG) are commonly used functional scoring tools.
The PPS measures 5 functional dimensions including ambulation, activity level, ability to administer self-care, oral intake, and level of consciousness.1 It has been shown to be valid for a broad range of palliative care patients, including those with advanced cancer or life-threatening noncancer diagnoses in hospitals or hospice care.2 The scale, measured in 10% increments, runs from 100% (completely functional) to 0% (dead). A PPS ≤ 70% helps meet hospice eligibility criteria.
The KPS evaluates functional impairment and helps with prognostication. Developed in 1948, it evaluates a patient’s functional ability to tolerate chemotherapy, specifically in lung cancer,and has since been validated to predict mortality across older adults and in chronic disease populations.3,4 The KPS is also measured in 10% increments ranging from 100% (completely functional without assistance) to 0% (dead). A KPS ≤ 70% assists with hospice eligibility criteria (Table 1).5
Developed in 1974, the ECOG has been identified as one of the most important functional status tools in adult cancer care.6 It describes a cancer patient’s functional ability, evaluating their ability to care for oneself and participate in daily activities.7 The ECOG is a 6-point scale; patients can receive scores ranging from 0 (fully active) to 5 (dead). An ECOG score of 4 (sometimes 3) is generally supportive of meeting hospice eligibility (Table 2).6
CASE Presentation
An 80-year-old patient was admitted to the hospice service at the Veterans Affairs Puget Sound Health Care System (VAPSHCS) community living center (CLC) in Tacoma, Washington, from a community-based acute care hospital. His medical history included prostate cancer with metastasis to his pelvis and type 2 diabetes mellitus, which was stable with treatment with oral medication. Six weeks earlier the patient reported a severe frontal headache that was not responding to over-the-counter analgesics. After 2 days with these symptoms, including a ground-level fall without injuries, he presented to the VAPSHCS emergency department (ED) where a complete neurological examination, including magnetic resonance imaging, revealed a left frontoparietal brain lesion that was 4.2 cm × 3.4 cm × 4.2 cm.
The patient experienced a seizure during his ED evaluation and was admitted for treatment. He underwent a craniotomy where most, but not all the lesions were successfully removed. Postoperatively, the patient exhibited right-sided neglect, gait instability, emotional lability, and cognitive communication disorder. The patient completed 15 of 20 planned radiation treatments but declined further radiation or chemotherapy. The patient decided to halt radiation treatments after being informed by the oncology service that the treatments would likely only add 1 to 2 months to his overall survival, which was < 6 months. The patient elected to focus his goals of care on comfort, dignity, and respect at the end of life and accepted recommendations to be placed into end-of-life hospice care. He was then transferred to the VAPSHCS CLC in Tacoma, Washington, for hospice care.
Upon admission, the patient weighed 94 kg, his vital signs were within reference range, and he reported no pain or headaches. His initial laboratory results revealed a 13.2 g/dL hemoglobin, 3.6 g/dL serum albumin, and a 5.5% hemoglobin A1c, all of which fall into a normal reference range. He had a reported ECOG score of 3 and a KPS score of 50% by the transferring medical team. The patient’s medications included scheduled dexamethasone, metformin, senna, levetiracetam, and as-needed midazolam nasal spray for breakthrough seizures. He also had as-needed acetaminophen for pain. He was alert, oriented ×3, and fully ambulatory but continuously used a 4-wheeled walker for safety and gait instability.
After the patient’s first night, the hospice team met with him to discuss his understanding of his health issues. The patient appeared to have low health literacy but told the team, “I know I am dying.” He had completed written advance directives and a Portable Order for Life-Sustaining Treatment indicating that life-sustaining treatments, including cardiopulmonary resuscitation, supplemental mechanical feeding, or intubation, were not to be used to keep him alive.
At his first 90-day recertification, the patient had gained 8 kg and laboratory results revealed a 14.6 g/dL hemoglobin, 3.8 g/dL serum albumin, and a 6.1% hemoglobin A1c. His ECOG score remained at 3, but his KPS score had increased to 60%. The patient exhibited no new neurologic symptoms or seizures and reported no headaches but had 2 ground-level falls without injury. On both occasions the patient chose not to use his walker to go to the bathroom because it was “too far from my bed.” Per VA policy, after discussions with the hospice team, he was recertified for 90 more days of hospice care. At the end of 6 months in CLC, the patient’s weight remained stable, as did his complete blood count and comprehensive medical panel. He had 1 additional noninjurious ground-level fall and again reported no pain and no use of as-needed acetaminophen. His only medical complication was testing positive for COVID-19, but he remained asymptomatic. The patient was graduated from hospice care and referred to a nearby non-VA adult family home in the community after 180 days. At that time his ECOG score was 2 and his KPS score had increased to 70%.
DISCUSSION
Primary brain tumors account for about 2% of all malignant neoplasms in adults. About half of them represent gliomas. Glioblastoma multiforme derived from neuroepithelial cells is the most frequent and deadly primary malignant central nervous system tumor in adults.8 About 50% of patients with glioblastomas are aged ≥ 65 years at diagnosis.9 A retrospective study of Centers for Medicare and Medicaid Services claims data paired with the Surveillance, Epidemiology, and End Results database indicated a median survival of 4 months for patients with glioblastoma multiforme aged > 65 years, including all treatment modalities.10 Surgical resection combined with radiation and chemotherapy offers the best prognosis for the preservation of neurologic function.11 However, comorbidities, adverse drug effects, and the potential for postoperative complications pose significant risks, especially for older patients. Ultimately, goals of care conversations and advance directives play a very important role in evaluating benefits vs risks with this malignancy.
Our patient was aged 80 years and had previously been diagnosed with metastatic prostate malignancy. His goals of care focused on spending time with his friends, leaving his room to eat in the facility dining area, and continuing his daily walks. He remained clear that he did not want his care team to institute life-sustaining treatments to be kept alive and felt the information regarding the risks vs benefits of accepting chemotherapy was not aligned with his goals of care. Over the 6 months that he received hospice care, he gained weight, improved his hemoglobin and serum albumin levels, and ambulated with the use of a 4-wheeled walker. As the patient exhibited no functional decline or new comorbidities and his functional status improved, the clinical staff felt he no longer needed hospice services. The patient had an ECOG score of 2 and a KPS score of 70% at his hospice graduation.
Medical prognostication is one of the biggest challenges clinicians face. Clinicians are generally “over prognosticators,” and their thoughts tend to be based on the patient relationship, overall experiences in health care, and desire to treat and cure patients.12 In hospice we are asked to define the usual, normal, or expected course of a disease, but what does that mean? Although metastatic malignancies usually have a predictable course in comparison to diagnoses such as dementia, chronic obstructive pulmonary disease, or congestive heart failure, the challenges to improve prognostic ability andpredict disease course continue.13-15 Focusing on functional status, goals of care, and comorbidities are keys to helping with prognosis. Given the challenge, we find the PPS, KPS, and ECOG scales important tools.
When prognosticating, we attempt to define quantity and quality of life (which our patients must define independently or from the voice of their surrogate) and their ability to perform daily activities. Quality of life in patients with glioblastoma is progressively and significantly impacted due to the emergence of debilitating neurologic symptoms arising from infiltrative tumor growth into functionally intact brain tissue that restricts and disrupts normal day-to-day activities. However, functional status plays a significant role in helping the hospice team improve its overall prognosis.
Conclusions
This case study illustrates the difficulty that comes with prognostication(s) despite a patient's severely morbid disease, history of metastatic prostate cancer, and advanced age. Although a diagnosis may be concerning, documenting a patient’s status using functional scales prior to hospice admission and during the recertification process is helpful in prognostication. Doing so will allow health care professionals to have an accepted medical standard to use regardless how distinct the patient's diagnosis. The expression, “as the disease does not read the textbook,” may serve as a helpful reminder in talking with patients and their families. This is important as most patient’s clinical disease courses are different and having the opportunity to use performance status scales may help improve prognostic skills.
1. Cleary TA. The Palliative Performance Scale (PPSv2) Version 2. In: Downing GM, ed. Medical Care of the Dying. 4th ed. Victoria Hospice Society, Learning Centre for Palliative Care; 2006:120.
2. Palliative Performance Scale. ePrognosis, University of California San Francisco. Accessed June 14, 2024. https://eprognosis.ucsf.edu/pps.php
3. Karnofsky DA, Burchenal JH. The Clinical Evaluation of Chemotherapeutic Agents in Cancer. In: MacLeod CM, ed. Evaluation of Chemotherapeutic Agents. Columbia University Press; 1949:191-205.
4. Khalid MA, Achakzai IK, Ahmed Khan S, et al. The use of Karnofsky Performance Status (KPS) as a predictor of 3 month post discharge mortality in cirrhotic patients. Gastroenterol Hepatol Bed Bench. 2018;11(4):301-305.
5. Karnofsky Performance Scale. US Dept of Veterans Affairs. Accessed June 14, 2024. https://www.hiv.va.gov/provider/tools/karnofsky-performance-scale.asp
6. Mischel A-M, Rosielle DA. Eastern Cooperative Oncology Group Performance Status. Palliative Care Network of Wisconsin. December 10, 2021. Accessed June 14, 2024. https://www.mypcnow.org/fast-fact/eastern-cooperative-oncology-group-performance-status/
7. Oken MM, Creech RH, Tormey DC, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol. 1982;5(6):649-655.
8. Nizamutdinov D, Stock EM, Dandashi JA, et al. Prognostication of survival outcomes in patients diagnosed with glioblastoma. World Neurosurg. 2018;109:e67-e74. doi:10.1016/j.wneu.2017.09.104
9. Kita D Ciernik IFVaccarella S Age as a predictive factor in glioblastomas: population-based study. Neuroepidemiology. 2009;33(1):17-22. doi:10.1159/000210017
10. Jordan JT, Gerstner ER, Batchelor TT, Cahill DP, Plotkin SR. Glioblastoma care in the elderly. Cancer. 2016;122(2):189-197. doi:10.1002/cnr.29742
11. Brown, NF, Ottaviani D, Tazare J, et al. Survival outcomes and prognostic factors in glioblastoma. Cancers (Basel). 2022;14(13):3161. doi:10.3390/cancers14133161
12. Christalakis NA. Death Foretold: Prophecy and Prognosis in Medical Care. University of Chicago Press; 2000.
13. Weissman DE. Determining Prognosis in Advanced Cancer. Palliative Care Network of Wisconsin. January 28, 2019. Accessed June 14, 2014. https://www.mypcnow.org/fast-fact/determining-prognosis-in-advanced-cancer/
14. Childers JW, Arnold R, Curtis JR. Prognosis in End-Stage COPD. Palliative Care Network of Wisconsin. February 11, 2019. Accessed June 14, 2024. https://www.mypcnow.org/fast-fact/prognosis-in-end-stage-copd/
15. Reisfield GM, Wilson GR. Prognostication in Heart Failure. Palliative Care Network of Wisconsin. February 11, 2019. Accessed June 14, 2024. https://www.mypcnow.org/fast-fact/prognostication-in-heart-failure/
Predicting life expectancy and providing an end-of-life diagnosis in hospice and palliative care is a challenge for most clinicians. Lack of training, limited communication skills, and relationships with patients are all contributing factors. These skills can improve with the use of functional scoring tools in conjunction with the patient’s comorbidities and physical/psychological symptoms. The Palliative Performance Scale (PPS), Karnofsky Performance Scale (KPS), and Eastern Cooperative Oncology Group Performance Status Scale (ECOG) are commonly used functional scoring tools.
The PPS measures 5 functional dimensions including ambulation, activity level, ability to administer self-care, oral intake, and level of consciousness.1 It has been shown to be valid for a broad range of palliative care patients, including those with advanced cancer or life-threatening noncancer diagnoses in hospitals or hospice care.2 The scale, measured in 10% increments, runs from 100% (completely functional) to 0% (dead). A PPS ≤ 70% helps meet hospice eligibility criteria.
The KPS evaluates functional impairment and helps with prognostication. Developed in 1948, it evaluates a patient’s functional ability to tolerate chemotherapy, specifically in lung cancer,and has since been validated to predict mortality across older adults and in chronic disease populations.3,4 The KPS is also measured in 10% increments ranging from 100% (completely functional without assistance) to 0% (dead). A KPS ≤ 70% assists with hospice eligibility criteria (Table 1).5
Developed in 1974, the ECOG has been identified as one of the most important functional status tools in adult cancer care.6 It describes a cancer patient’s functional ability, evaluating their ability to care for oneself and participate in daily activities.7 The ECOG is a 6-point scale; patients can receive scores ranging from 0 (fully active) to 5 (dead). An ECOG score of 4 (sometimes 3) is generally supportive of meeting hospice eligibility (Table 2).6
CASE Presentation
An 80-year-old patient was admitted to the hospice service at the Veterans Affairs Puget Sound Health Care System (VAPSHCS) community living center (CLC) in Tacoma, Washington, from a community-based acute care hospital. His medical history included prostate cancer with metastasis to his pelvis and type 2 diabetes mellitus, which was stable with treatment with oral medication. Six weeks earlier the patient reported a severe frontal headache that was not responding to over-the-counter analgesics. After 2 days with these symptoms, including a ground-level fall without injuries, he presented to the VAPSHCS emergency department (ED) where a complete neurological examination, including magnetic resonance imaging, revealed a left frontoparietal brain lesion that was 4.2 cm × 3.4 cm × 4.2 cm.
The patient experienced a seizure during his ED evaluation and was admitted for treatment. He underwent a craniotomy where most, but not all the lesions were successfully removed. Postoperatively, the patient exhibited right-sided neglect, gait instability, emotional lability, and cognitive communication disorder. The patient completed 15 of 20 planned radiation treatments but declined further radiation or chemotherapy. The patient decided to halt radiation treatments after being informed by the oncology service that the treatments would likely only add 1 to 2 months to his overall survival, which was < 6 months. The patient elected to focus his goals of care on comfort, dignity, and respect at the end of life and accepted recommendations to be placed into end-of-life hospice care. He was then transferred to the VAPSHCS CLC in Tacoma, Washington, for hospice care.
Upon admission, the patient weighed 94 kg, his vital signs were within reference range, and he reported no pain or headaches. His initial laboratory results revealed a 13.2 g/dL hemoglobin, 3.6 g/dL serum albumin, and a 5.5% hemoglobin A1c, all of which fall into a normal reference range. He had a reported ECOG score of 3 and a KPS score of 50% by the transferring medical team. The patient’s medications included scheduled dexamethasone, metformin, senna, levetiracetam, and as-needed midazolam nasal spray for breakthrough seizures. He also had as-needed acetaminophen for pain. He was alert, oriented ×3, and fully ambulatory but continuously used a 4-wheeled walker for safety and gait instability.
After the patient’s first night, the hospice team met with him to discuss his understanding of his health issues. The patient appeared to have low health literacy but told the team, “I know I am dying.” He had completed written advance directives and a Portable Order for Life-Sustaining Treatment indicating that life-sustaining treatments, including cardiopulmonary resuscitation, supplemental mechanical feeding, or intubation, were not to be used to keep him alive.
At his first 90-day recertification, the patient had gained 8 kg and laboratory results revealed a 14.6 g/dL hemoglobin, 3.8 g/dL serum albumin, and a 6.1% hemoglobin A1c. His ECOG score remained at 3, but his KPS score had increased to 60%. The patient exhibited no new neurologic symptoms or seizures and reported no headaches but had 2 ground-level falls without injury. On both occasions the patient chose not to use his walker to go to the bathroom because it was “too far from my bed.” Per VA policy, after discussions with the hospice team, he was recertified for 90 more days of hospice care. At the end of 6 months in CLC, the patient’s weight remained stable, as did his complete blood count and comprehensive medical panel. He had 1 additional noninjurious ground-level fall and again reported no pain and no use of as-needed acetaminophen. His only medical complication was testing positive for COVID-19, but he remained asymptomatic. The patient was graduated from hospice care and referred to a nearby non-VA adult family home in the community after 180 days. At that time his ECOG score was 2 and his KPS score had increased to 70%.
DISCUSSION
Primary brain tumors account for about 2% of all malignant neoplasms in adults. About half of them represent gliomas. Glioblastoma multiforme derived from neuroepithelial cells is the most frequent and deadly primary malignant central nervous system tumor in adults.8 About 50% of patients with glioblastomas are aged ≥ 65 years at diagnosis.9 A retrospective study of Centers for Medicare and Medicaid Services claims data paired with the Surveillance, Epidemiology, and End Results database indicated a median survival of 4 months for patients with glioblastoma multiforme aged > 65 years, including all treatment modalities.10 Surgical resection combined with radiation and chemotherapy offers the best prognosis for the preservation of neurologic function.11 However, comorbidities, adverse drug effects, and the potential for postoperative complications pose significant risks, especially for older patients. Ultimately, goals of care conversations and advance directives play a very important role in evaluating benefits vs risks with this malignancy.
Our patient was aged 80 years and had previously been diagnosed with metastatic prostate malignancy. His goals of care focused on spending time with his friends, leaving his room to eat in the facility dining area, and continuing his daily walks. He remained clear that he did not want his care team to institute life-sustaining treatments to be kept alive and felt the information regarding the risks vs benefits of accepting chemotherapy was not aligned with his goals of care. Over the 6 months that he received hospice care, he gained weight, improved his hemoglobin and serum albumin levels, and ambulated with the use of a 4-wheeled walker. As the patient exhibited no functional decline or new comorbidities and his functional status improved, the clinical staff felt he no longer needed hospice services. The patient had an ECOG score of 2 and a KPS score of 70% at his hospice graduation.
Medical prognostication is one of the biggest challenges clinicians face. Clinicians are generally “over prognosticators,” and their thoughts tend to be based on the patient relationship, overall experiences in health care, and desire to treat and cure patients.12 In hospice we are asked to define the usual, normal, or expected course of a disease, but what does that mean? Although metastatic malignancies usually have a predictable course in comparison to diagnoses such as dementia, chronic obstructive pulmonary disease, or congestive heart failure, the challenges to improve prognostic ability andpredict disease course continue.13-15 Focusing on functional status, goals of care, and comorbidities are keys to helping with prognosis. Given the challenge, we find the PPS, KPS, and ECOG scales important tools.
When prognosticating, we attempt to define quantity and quality of life (which our patients must define independently or from the voice of their surrogate) and their ability to perform daily activities. Quality of life in patients with glioblastoma is progressively and significantly impacted due to the emergence of debilitating neurologic symptoms arising from infiltrative tumor growth into functionally intact brain tissue that restricts and disrupts normal day-to-day activities. However, functional status plays a significant role in helping the hospice team improve its overall prognosis.
Conclusions
This case study illustrates the difficulty that comes with prognostication(s) despite a patient's severely morbid disease, history of metastatic prostate cancer, and advanced age. Although a diagnosis may be concerning, documenting a patient’s status using functional scales prior to hospice admission and during the recertification process is helpful in prognostication. Doing so will allow health care professionals to have an accepted medical standard to use regardless how distinct the patient's diagnosis. The expression, “as the disease does not read the textbook,” may serve as a helpful reminder in talking with patients and their families. This is important as most patient’s clinical disease courses are different and having the opportunity to use performance status scales may help improve prognostic skills.
Predicting life expectancy and providing an end-of-life diagnosis in hospice and palliative care is a challenge for most clinicians. Lack of training, limited communication skills, and relationships with patients are all contributing factors. These skills can improve with the use of functional scoring tools in conjunction with the patient’s comorbidities and physical/psychological symptoms. The Palliative Performance Scale (PPS), Karnofsky Performance Scale (KPS), and Eastern Cooperative Oncology Group Performance Status Scale (ECOG) are commonly used functional scoring tools.
The PPS measures 5 functional dimensions including ambulation, activity level, ability to administer self-care, oral intake, and level of consciousness.1 It has been shown to be valid for a broad range of palliative care patients, including those with advanced cancer or life-threatening noncancer diagnoses in hospitals or hospice care.2 The scale, measured in 10% increments, runs from 100% (completely functional) to 0% (dead). A PPS ≤ 70% helps meet hospice eligibility criteria.
The KPS evaluates functional impairment and helps with prognostication. Developed in 1948, it evaluates a patient’s functional ability to tolerate chemotherapy, specifically in lung cancer,and has since been validated to predict mortality across older adults and in chronic disease populations.3,4 The KPS is also measured in 10% increments ranging from 100% (completely functional without assistance) to 0% (dead). A KPS ≤ 70% assists with hospice eligibility criteria (Table 1).5
Developed in 1974, the ECOG has been identified as one of the most important functional status tools in adult cancer care.6 It describes a cancer patient’s functional ability, evaluating their ability to care for oneself and participate in daily activities.7 The ECOG is a 6-point scale; patients can receive scores ranging from 0 (fully active) to 5 (dead). An ECOG score of 4 (sometimes 3) is generally supportive of meeting hospice eligibility (Table 2).6
CASE Presentation
An 80-year-old patient was admitted to the hospice service at the Veterans Affairs Puget Sound Health Care System (VAPSHCS) community living center (CLC) in Tacoma, Washington, from a community-based acute care hospital. His medical history included prostate cancer with metastasis to his pelvis and type 2 diabetes mellitus, which was stable with treatment with oral medication. Six weeks earlier the patient reported a severe frontal headache that was not responding to over-the-counter analgesics. After 2 days with these symptoms, including a ground-level fall without injuries, he presented to the VAPSHCS emergency department (ED) where a complete neurological examination, including magnetic resonance imaging, revealed a left frontoparietal brain lesion that was 4.2 cm × 3.4 cm × 4.2 cm.
The patient experienced a seizure during his ED evaluation and was admitted for treatment. He underwent a craniotomy where most, but not all the lesions were successfully removed. Postoperatively, the patient exhibited right-sided neglect, gait instability, emotional lability, and cognitive communication disorder. The patient completed 15 of 20 planned radiation treatments but declined further radiation or chemotherapy. The patient decided to halt radiation treatments after being informed by the oncology service that the treatments would likely only add 1 to 2 months to his overall survival, which was < 6 months. The patient elected to focus his goals of care on comfort, dignity, and respect at the end of life and accepted recommendations to be placed into end-of-life hospice care. He was then transferred to the VAPSHCS CLC in Tacoma, Washington, for hospice care.
Upon admission, the patient weighed 94 kg, his vital signs were within reference range, and he reported no pain or headaches. His initial laboratory results revealed a 13.2 g/dL hemoglobin, 3.6 g/dL serum albumin, and a 5.5% hemoglobin A1c, all of which fall into a normal reference range. He had a reported ECOG score of 3 and a KPS score of 50% by the transferring medical team. The patient’s medications included scheduled dexamethasone, metformin, senna, levetiracetam, and as-needed midazolam nasal spray for breakthrough seizures. He also had as-needed acetaminophen for pain. He was alert, oriented ×3, and fully ambulatory but continuously used a 4-wheeled walker for safety and gait instability.
After the patient’s first night, the hospice team met with him to discuss his understanding of his health issues. The patient appeared to have low health literacy but told the team, “I know I am dying.” He had completed written advance directives and a Portable Order for Life-Sustaining Treatment indicating that life-sustaining treatments, including cardiopulmonary resuscitation, supplemental mechanical feeding, or intubation, were not to be used to keep him alive.
At his first 90-day recertification, the patient had gained 8 kg and laboratory results revealed a 14.6 g/dL hemoglobin, 3.8 g/dL serum albumin, and a 6.1% hemoglobin A1c. His ECOG score remained at 3, but his KPS score had increased to 60%. The patient exhibited no new neurologic symptoms or seizures and reported no headaches but had 2 ground-level falls without injury. On both occasions the patient chose not to use his walker to go to the bathroom because it was “too far from my bed.” Per VA policy, after discussions with the hospice team, he was recertified for 90 more days of hospice care. At the end of 6 months in CLC, the patient’s weight remained stable, as did his complete blood count and comprehensive medical panel. He had 1 additional noninjurious ground-level fall and again reported no pain and no use of as-needed acetaminophen. His only medical complication was testing positive for COVID-19, but he remained asymptomatic. The patient was graduated from hospice care and referred to a nearby non-VA adult family home in the community after 180 days. At that time his ECOG score was 2 and his KPS score had increased to 70%.
DISCUSSION
Primary brain tumors account for about 2% of all malignant neoplasms in adults. About half of them represent gliomas. Glioblastoma multiforme derived from neuroepithelial cells is the most frequent and deadly primary malignant central nervous system tumor in adults.8 About 50% of patients with glioblastomas are aged ≥ 65 years at diagnosis.9 A retrospective study of Centers for Medicare and Medicaid Services claims data paired with the Surveillance, Epidemiology, and End Results database indicated a median survival of 4 months for patients with glioblastoma multiforme aged > 65 years, including all treatment modalities.10 Surgical resection combined with radiation and chemotherapy offers the best prognosis for the preservation of neurologic function.11 However, comorbidities, adverse drug effects, and the potential for postoperative complications pose significant risks, especially for older patients. Ultimately, goals of care conversations and advance directives play a very important role in evaluating benefits vs risks with this malignancy.
Our patient was aged 80 years and had previously been diagnosed with metastatic prostate malignancy. His goals of care focused on spending time with his friends, leaving his room to eat in the facility dining area, and continuing his daily walks. He remained clear that he did not want his care team to institute life-sustaining treatments to be kept alive and felt the information regarding the risks vs benefits of accepting chemotherapy was not aligned with his goals of care. Over the 6 months that he received hospice care, he gained weight, improved his hemoglobin and serum albumin levels, and ambulated with the use of a 4-wheeled walker. As the patient exhibited no functional decline or new comorbidities and his functional status improved, the clinical staff felt he no longer needed hospice services. The patient had an ECOG score of 2 and a KPS score of 70% at his hospice graduation.
Medical prognostication is one of the biggest challenges clinicians face. Clinicians are generally “over prognosticators,” and their thoughts tend to be based on the patient relationship, overall experiences in health care, and desire to treat and cure patients.12 In hospice we are asked to define the usual, normal, or expected course of a disease, but what does that mean? Although metastatic malignancies usually have a predictable course in comparison to diagnoses such as dementia, chronic obstructive pulmonary disease, or congestive heart failure, the challenges to improve prognostic ability andpredict disease course continue.13-15 Focusing on functional status, goals of care, and comorbidities are keys to helping with prognosis. Given the challenge, we find the PPS, KPS, and ECOG scales important tools.
When prognosticating, we attempt to define quantity and quality of life (which our patients must define independently or from the voice of their surrogate) and their ability to perform daily activities. Quality of life in patients with glioblastoma is progressively and significantly impacted due to the emergence of debilitating neurologic symptoms arising from infiltrative tumor growth into functionally intact brain tissue that restricts and disrupts normal day-to-day activities. However, functional status plays a significant role in helping the hospice team improve its overall prognosis.
Conclusions
This case study illustrates the difficulty that comes with prognostication(s) despite a patient's severely morbid disease, history of metastatic prostate cancer, and advanced age. Although a diagnosis may be concerning, documenting a patient’s status using functional scales prior to hospice admission and during the recertification process is helpful in prognostication. Doing so will allow health care professionals to have an accepted medical standard to use regardless how distinct the patient's diagnosis. The expression, “as the disease does not read the textbook,” may serve as a helpful reminder in talking with patients and their families. This is important as most patient’s clinical disease courses are different and having the opportunity to use performance status scales may help improve prognostic skills.
1. Cleary TA. The Palliative Performance Scale (PPSv2) Version 2. In: Downing GM, ed. Medical Care of the Dying. 4th ed. Victoria Hospice Society, Learning Centre for Palliative Care; 2006:120.
2. Palliative Performance Scale. ePrognosis, University of California San Francisco. Accessed June 14, 2024. https://eprognosis.ucsf.edu/pps.php
3. Karnofsky DA, Burchenal JH. The Clinical Evaluation of Chemotherapeutic Agents in Cancer. In: MacLeod CM, ed. Evaluation of Chemotherapeutic Agents. Columbia University Press; 1949:191-205.
4. Khalid MA, Achakzai IK, Ahmed Khan S, et al. The use of Karnofsky Performance Status (KPS) as a predictor of 3 month post discharge mortality in cirrhotic patients. Gastroenterol Hepatol Bed Bench. 2018;11(4):301-305.
5. Karnofsky Performance Scale. US Dept of Veterans Affairs. Accessed June 14, 2024. https://www.hiv.va.gov/provider/tools/karnofsky-performance-scale.asp
6. Mischel A-M, Rosielle DA. Eastern Cooperative Oncology Group Performance Status. Palliative Care Network of Wisconsin. December 10, 2021. Accessed June 14, 2024. https://www.mypcnow.org/fast-fact/eastern-cooperative-oncology-group-performance-status/
7. Oken MM, Creech RH, Tormey DC, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol. 1982;5(6):649-655.
8. Nizamutdinov D, Stock EM, Dandashi JA, et al. Prognostication of survival outcomes in patients diagnosed with glioblastoma. World Neurosurg. 2018;109:e67-e74. doi:10.1016/j.wneu.2017.09.104
9. Kita D Ciernik IFVaccarella S Age as a predictive factor in glioblastomas: population-based study. Neuroepidemiology. 2009;33(1):17-22. doi:10.1159/000210017
10. Jordan JT, Gerstner ER, Batchelor TT, Cahill DP, Plotkin SR. Glioblastoma care in the elderly. Cancer. 2016;122(2):189-197. doi:10.1002/cnr.29742
11. Brown, NF, Ottaviani D, Tazare J, et al. Survival outcomes and prognostic factors in glioblastoma. Cancers (Basel). 2022;14(13):3161. doi:10.3390/cancers14133161
12. Christalakis NA. Death Foretold: Prophecy and Prognosis in Medical Care. University of Chicago Press; 2000.
13. Weissman DE. Determining Prognosis in Advanced Cancer. Palliative Care Network of Wisconsin. January 28, 2019. Accessed June 14, 2014. https://www.mypcnow.org/fast-fact/determining-prognosis-in-advanced-cancer/
14. Childers JW, Arnold R, Curtis JR. Prognosis in End-Stage COPD. Palliative Care Network of Wisconsin. February 11, 2019. Accessed June 14, 2024. https://www.mypcnow.org/fast-fact/prognosis-in-end-stage-copd/
15. Reisfield GM, Wilson GR. Prognostication in Heart Failure. Palliative Care Network of Wisconsin. February 11, 2019. Accessed June 14, 2024. https://www.mypcnow.org/fast-fact/prognostication-in-heart-failure/
1. Cleary TA. The Palliative Performance Scale (PPSv2) Version 2. In: Downing GM, ed. Medical Care of the Dying. 4th ed. Victoria Hospice Society, Learning Centre for Palliative Care; 2006:120.
2. Palliative Performance Scale. ePrognosis, University of California San Francisco. Accessed June 14, 2024. https://eprognosis.ucsf.edu/pps.php
3. Karnofsky DA, Burchenal JH. The Clinical Evaluation of Chemotherapeutic Agents in Cancer. In: MacLeod CM, ed. Evaluation of Chemotherapeutic Agents. Columbia University Press; 1949:191-205.
4. Khalid MA, Achakzai IK, Ahmed Khan S, et al. The use of Karnofsky Performance Status (KPS) as a predictor of 3 month post discharge mortality in cirrhotic patients. Gastroenterol Hepatol Bed Bench. 2018;11(4):301-305.
5. Karnofsky Performance Scale. US Dept of Veterans Affairs. Accessed June 14, 2024. https://www.hiv.va.gov/provider/tools/karnofsky-performance-scale.asp
6. Mischel A-M, Rosielle DA. Eastern Cooperative Oncology Group Performance Status. Palliative Care Network of Wisconsin. December 10, 2021. Accessed June 14, 2024. https://www.mypcnow.org/fast-fact/eastern-cooperative-oncology-group-performance-status/
7. Oken MM, Creech RH, Tormey DC, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol. 1982;5(6):649-655.
8. Nizamutdinov D, Stock EM, Dandashi JA, et al. Prognostication of survival outcomes in patients diagnosed with glioblastoma. World Neurosurg. 2018;109:e67-e74. doi:10.1016/j.wneu.2017.09.104
9. Kita D Ciernik IFVaccarella S Age as a predictive factor in glioblastomas: population-based study. Neuroepidemiology. 2009;33(1):17-22. doi:10.1159/000210017
10. Jordan JT, Gerstner ER, Batchelor TT, Cahill DP, Plotkin SR. Glioblastoma care in the elderly. Cancer. 2016;122(2):189-197. doi:10.1002/cnr.29742
11. Brown, NF, Ottaviani D, Tazare J, et al. Survival outcomes and prognostic factors in glioblastoma. Cancers (Basel). 2022;14(13):3161. doi:10.3390/cancers14133161
12. Christalakis NA. Death Foretold: Prophecy and Prognosis in Medical Care. University of Chicago Press; 2000.
13. Weissman DE. Determining Prognosis in Advanced Cancer. Palliative Care Network of Wisconsin. January 28, 2019. Accessed June 14, 2014. https://www.mypcnow.org/fast-fact/determining-prognosis-in-advanced-cancer/
14. Childers JW, Arnold R, Curtis JR. Prognosis in End-Stage COPD. Palliative Care Network of Wisconsin. February 11, 2019. Accessed June 14, 2024. https://www.mypcnow.org/fast-fact/prognosis-in-end-stage-copd/
15. Reisfield GM, Wilson GR. Prognostication in Heart Failure. Palliative Care Network of Wisconsin. February 11, 2019. Accessed June 14, 2024. https://www.mypcnow.org/fast-fact/prognostication-in-heart-failure/
Impact of VA Hematology/Oncology Clinical Pharmacy Practitioners in the Review of Community Prescriptions for Specialty Medications
The value of a hematology/oncology clinical pharmacy practitioner (CPP) has been validated in several studies documenting their positive impact on patient outcomes, supportive care management, laboratory monitoring, medication error identification, and drug expenditure.1-6 With> 200 oncology-related US Food and Drug Administration approval notifications published from 2020 to 2023, it is no surprise that national trends in oncology drug clinic expenditures increased from $39.9 billion in 2020 to $44.1 billion in 2021.7,8 With the rapidly changing treatment landscape, new drug approvals, and risk of polypharmacy, oral anticancer agents carry a high risk for medication errors.4 Additional challenges include complex dosing regimens and instructions, adherence issues, drug interactions, adjustments for organ dysfunction, and extensive adverse effect (AE) profiles.
Because of the niche and complexity of oral anticancer agents, trained CPPs havehematology/oncology education and expertise that pharmacists without specialized training lack. A survey of 243 nonspecialized community pharmacists that assessed their knowledge of oral anticancer therapies revealed that only about half of the knowledge questions were answered correctly, illustrating an education gap among these pharmacists.9 The Hematology/Oncology Pharmacist Association's suggests that best practices for managing oral oncology therapy should include comprehensive medication review by an oncology-trained pharmacist for each prescription.10
The US Department of Veterans Affairs (VA) community care network, which was established by the MISSION Act, allows covered access for eligible veterans in the local community outside of the VA network. Unfortunately, this dual-system use of health care could increase the risk of poorly coordinated care and has been associated with the risk of inappropriate prescribing.11,12 It is unclear how many private practices enrolled in the community care program have access to oncology-trained pharmacists. Specialized pharmaceutical reviews of oral anticancer medication prescriptions from these practices are vital for veteran care. This study evaluates the clinical and financial interventions of hematology/oncology CPPs review of specialty hematology/oncology prescriptions from community care health care practitioners (HCPs) at the Veterans Affairs North Texas Health Care System (VANTHCS) in Dallas.
METHODS
This study is a retrospective review of Computerized Patient Record System (CPRS) records of patients at VANTHCS from January 1, 2015, to June 30, 2023. Patients included were aged ≥ 18 years, enrolled in the VA community care program, received a specialty hematology/oncology medication that was dispensed through VA pharmacies or VA-contracted pharmacies, and had an hematology/oncology CPP medication review documented in CPRS. The primary aim of this study was to assess the number and types of clinical interventions performed. A clinical intervention was defined as a documented communication attempt with a community care HCP or direct communication with a patient to address a specific medication-related issue noted during CPP review.
Review of specialty hematology/oncology medications by a hematology/oncology CPP included evaluation of therapy indication, such as whether the prescription meets clinical guidelines, VA criteria for use, or other clinical literature as judged appropriate by the CPP. In some cases, the CPP requested that the community care HCP prescribe a more cost-effective or formulary-preferred agent. Each prescription was reviewed for dosage and formulation appropriateness, drug interactions with available medication lists, baseline laboratory test completion, and recommended supportive care medicines. At times, patient counseling is completed as part of the clinical review. When necessary, CPPs could discuss patient cases with a VA-employed oncologist for further oversight regarding appropriateness and safety. Secondary outcomes included the number of interventions accepted or denied by the prescriber provider and cost savings.
Data collected included the type of malignancy, hematology/oncology specialty medication requested, number and type of interventions sent to the community care prescriber, number of interventions accepted or denied by the community care prescriber, and whether the CPP conducted patient counseling or dispensed or denied the product. Cost savings were calculated for medications that were denied or changed to a formulary preferred or cost-effective agent using pricing data from the National Acquisition Center Contract Catalog or Federal Supply Schedule Service as of April 2024.
RESULTS
A total of 221 hematology/oncology prescriptions met inclusion criteria. Among patients receiving these prescriptions, the median age was 70 years and 91% were male. The most common malignancies included 31 instances of multiple myeloma (14%), 26 for chronic lymphocytic leukemia (12%), 24 for prostate cancer (11%), 23 for glioblastoma/brain cancer (10%), 18 for renal cell carcinoma (8%), 17 for colorectal cancer (8%), and 15 for acute myeloid leukemia (7%). Clinical interventions by the hematology/oncology CPP were completed for 82 (37%) of the 221 prescriptions. One clinical intervention was communicated directly to the patient, and attempts were made to communicate with the community care HCP for the remaining 81 prescriptions. The CPP documented 97 clinical interventions for the 82 prescriptions (Table 1). The most commonly documented clinical interventions included: 25 for managing/preventing a drug interaction (26%), 24 for dose adjustment request (25%), 13 for prescription denial (13%), and 11 for requesting the use of a preferred or more cost-effective product (11%). Of note, 16 patients (7%) received counseling from the hematology/oncology CPP. Ten patients (5%) received counseling alone with no other intervention and did not meet the definition of a clinical intervention.
The most frequent prescriptions requiring intervention included 8 for enzalutamide, 7 for venetoclax, 6 for ibrutinib, and 5 each for lenalidomide, cabozantinib, and temozolomide. Among the 97 interventions, 68 were approved (70%), 15 received no response (16%), and 14 were denied by the community care HCP (14%). Despite obtaining no response or intervention denial from the community care HCP, hematology/oncology CPPs could approve these prescriptions if clinically appropriate, and their reasoning was documented. Table 2 further describes the types of interventions that were denied or obtained no response by the community care practitioner. Among the prescriptions denied by the hematology/oncology CPP, 11 were rejected for off-label indications and/or did not have support through primary literature, national guidelines, or VA criteria for use. Only 2 prescriptions were denied for safety concerns.
These documented clinical interventions had financial implications. For drugs with available cost data, requesting the use of a preferred/cost-effective product led to estimated savings of at least $263,536 over the study period with some ongoing cost savings. Prescription denials led to further estimated savings of $186,275 per month, although this is limited by the lack of known costs of alternative therapies the community care physicians chose.
DISCUSSION
More than one-third of prescriptions required clinical interventions, and 70% of these interventions were accepted by the community care prescriber, demonstrating the CPP’s essential role. Results indicate that most CPP clinical interventions involved clarifying and correcting doses, managing pertinent drug interactions, and ensuring appropriate use of medications according to clinical and national VA guidelines. Other studies have examined the impact of CPPs on patient care and cancer treatment.5,6 The randomized, multicenter AMBORA trial found that clinical pharmacist support reduced severe AEs and medication errors related to oral anticancer agents.5 The per-patient mean number of medication errors found by pharmacist review was 1.7 (range, 0 to 9), with most medication errors noted at the prescribing stage.5 Suzuki and colleagues analyzed data from 35,062 chemotherapy regimens and found that 53.1% of the chemotherapy prescriptions were modified because of pharmacist interventions.6 The most common reason for prescription modifications was prescription error.
Most of the clinical interventions in this study were accepted by community HCPs, indicating that these prescribers are receptive to hematology/oncology CPP input. Among those with no response, most were in relation to recommendations regarding drug interactions. In most of these cases, the drug interaction was not clinically concerning enough to require a response before the CPP approved the prescription. Therefore, it is unknown whether the outside HCP implemented the clinical recommendations. The most common types of clinical interventions the community care HCP declined were dose adjustment requests or requests to switch to a more cost-effective/formulary-preferred agent. In these cases, the prescriber’s preference was documented and, if clinically appropriate, approved by the CPP.
Although the financial implications of CPP clinical interventions were only marginally evaluated in this review, results suggest that cost savings by requests to switch to a cost-effective/formulary preferred agent or prescription denials are substantial. Because of changes in prescription costs over time, it is possible that savings from CPP intervention were greater than calculations using current Federal Supply Schedule Service pricing. The total impact of CPP prescription interventions on reducing or preventing hospitalizations or AEs is not known from this review, but other data suggest that cost savings may benefit the system.13,14
Limitations
This study's retrospective design is a limitation because practice patterns at the VANTHCS involving multiple hematology/oncology CPPs review of community care prescriptions might have evolved over time. The total financial implications of CPP interventions cannot fully be elucidated. The cost of alternative therapies used for patients who received a prescription denial is not factored into this review.
Conclusions
VANTHCS CPPs played an essential role in reviewing anticancer medication prescriptions from community care prescribers. In this study, CPP clinical interventions were completed for more than one-third of the prescriptions and the community-based HCP approved most of these interventions. These changes also resulted in financial benefits.
These findings add to the body of literature emphasizing the need for hematology/oncology-trained CPPs to review anticancer prescriptions and treatment plans. Our review could be used to justify CPP involvement in community care specialty medication review at VA facilities that do not currently have CPP involvement.
1. Shah NN, Casella E, Capozzi D, et al. Improving the safety of oral chemotherapy at an academic medical center. J Oncol Pract. 2016;12(1):e71-e76. doi:10.1200/JOP.2015.007260
2. Gatwood J, Gatwood K, Gabre E, Alexander M. Impact of clinical pharmacists in outpatient oncology practices: a review. Am J Health Syst Pharm. 2017;74(19):1549-1557. doi:10.2146/ajhp160475
3. Lankford C, Dura J, Tran A, et al. Effect of clinical pharmacist interventions on cost in an integrated health system specialty pharmacy. J Manag Care Spec Pharm. 2021;27(3):379-384. doi:10.18553/jmcp.2021.27.3.379
4. Schlichtig K, Dürr P, Dörje F, Fromm MF. Medication errors during treatment with new oral anticancer agents: consequences for clinical practice based on the AMBORA Study. Clin Pharmacol Ther. 2021;110(4):1075-1086. doi:10.1002/cpt.2338
5. Dürr P, Schlichtig K, Kelz C, et al. The randomized AMBORA Trial: impact of pharmacological/pharmaceutical care on medication safety and patient-reported outcomes during treatment with new oral anticancer agents. J Clin Oncol. 2021;39(18):1983-1994. doi:10.1200/JCO.20.03088
6. Suzuki S, Chan A, Nomura H, Johnson PE, Endo K, Saito S. Chemotherapy regimen checks performed by pharmacists contribute to safe administration of chemotherapy. J Oncol Pharm Pract. 2017;23(1):18-25. doi:10.1177/1078155215614998
7. Tichy EM, Hoffman JM, Suda KJ, et al. National trends in prescription drug expenditures and projections for 2022. Am J Health Syst Pharm. 2022;79(14):1158-1172. doi:10.1093/ajhp/zxac102
8. US Food and Drug Administration. Oncology (cancer)/hematologic malignancies approval notifications. 2023.
9. O’Bryant CL, Crandell BC. Community pharmacists’ knowledge of and attitudes toward oral chemotherapy. J Am Pharm Assoc (2003). 2008;48(5):632-639. doi:10.1331/JAPhA.2008.07082
10. Mackler E, Segal EM, Muluneh B, Jeffers K, Carmichael J. 2018 hematology/oncology pharmacist association best practices for the management of oral oncolytic therapy: pharmacy practice standard. J Oncol Pract. 2019;15(4):e346-e355. doi:10.1200/JOP.18.00581
11. Thorpe JM, Thorpe CT, Schleiden L, et al. Association between dual use of Department of Veterans Affairs and Medicare part D drug benefits and potentially unsafe prescribing. JAMA Intern Med. 2019;179(11):1584-1586. doi:10.1001/jamainternmed.2019.2788
12. Thorpe JM, Thorpe CT, Gellad WF, et al. Dual health care system use and high-risk prescribing in patients with dementia: a national cohort study. Ann Intern Med. 2017;166(3):157-163. doi:10.7326/M16-0551
13. Chen P-Z, Wu C-C, Huang C-F. Clinical and economic impact of clinical pharmacist intervention in a hematology unit. J Oncol Pharm Pract. 2020;26(4):866-872. doi:10.1177/1078155219875806
14. Dalton K, Byrne S. Role of the pharmacist in reducing healthcare costs: current insights. Integr Pharm Res Pract. 2017;6:37-46. doi:10.2147/IPRP.S108047
The value of a hematology/oncology clinical pharmacy practitioner (CPP) has been validated in several studies documenting their positive impact on patient outcomes, supportive care management, laboratory monitoring, medication error identification, and drug expenditure.1-6 With> 200 oncology-related US Food and Drug Administration approval notifications published from 2020 to 2023, it is no surprise that national trends in oncology drug clinic expenditures increased from $39.9 billion in 2020 to $44.1 billion in 2021.7,8 With the rapidly changing treatment landscape, new drug approvals, and risk of polypharmacy, oral anticancer agents carry a high risk for medication errors.4 Additional challenges include complex dosing regimens and instructions, adherence issues, drug interactions, adjustments for organ dysfunction, and extensive adverse effect (AE) profiles.
Because of the niche and complexity of oral anticancer agents, trained CPPs havehematology/oncology education and expertise that pharmacists without specialized training lack. A survey of 243 nonspecialized community pharmacists that assessed their knowledge of oral anticancer therapies revealed that only about half of the knowledge questions were answered correctly, illustrating an education gap among these pharmacists.9 The Hematology/Oncology Pharmacist Association's suggests that best practices for managing oral oncology therapy should include comprehensive medication review by an oncology-trained pharmacist for each prescription.10
The US Department of Veterans Affairs (VA) community care network, which was established by the MISSION Act, allows covered access for eligible veterans in the local community outside of the VA network. Unfortunately, this dual-system use of health care could increase the risk of poorly coordinated care and has been associated with the risk of inappropriate prescribing.11,12 It is unclear how many private practices enrolled in the community care program have access to oncology-trained pharmacists. Specialized pharmaceutical reviews of oral anticancer medication prescriptions from these practices are vital for veteran care. This study evaluates the clinical and financial interventions of hematology/oncology CPPs review of specialty hematology/oncology prescriptions from community care health care practitioners (HCPs) at the Veterans Affairs North Texas Health Care System (VANTHCS) in Dallas.
METHODS
This study is a retrospective review of Computerized Patient Record System (CPRS) records of patients at VANTHCS from January 1, 2015, to June 30, 2023. Patients included were aged ≥ 18 years, enrolled in the VA community care program, received a specialty hematology/oncology medication that was dispensed through VA pharmacies or VA-contracted pharmacies, and had an hematology/oncology CPP medication review documented in CPRS. The primary aim of this study was to assess the number and types of clinical interventions performed. A clinical intervention was defined as a documented communication attempt with a community care HCP or direct communication with a patient to address a specific medication-related issue noted during CPP review.
Review of specialty hematology/oncology medications by a hematology/oncology CPP included evaluation of therapy indication, such as whether the prescription meets clinical guidelines, VA criteria for use, or other clinical literature as judged appropriate by the CPP. In some cases, the CPP requested that the community care HCP prescribe a more cost-effective or formulary-preferred agent. Each prescription was reviewed for dosage and formulation appropriateness, drug interactions with available medication lists, baseline laboratory test completion, and recommended supportive care medicines. At times, patient counseling is completed as part of the clinical review. When necessary, CPPs could discuss patient cases with a VA-employed oncologist for further oversight regarding appropriateness and safety. Secondary outcomes included the number of interventions accepted or denied by the prescriber provider and cost savings.
Data collected included the type of malignancy, hematology/oncology specialty medication requested, number and type of interventions sent to the community care prescriber, number of interventions accepted or denied by the community care prescriber, and whether the CPP conducted patient counseling or dispensed or denied the product. Cost savings were calculated for medications that were denied or changed to a formulary preferred or cost-effective agent using pricing data from the National Acquisition Center Contract Catalog or Federal Supply Schedule Service as of April 2024.
RESULTS
A total of 221 hematology/oncology prescriptions met inclusion criteria. Among patients receiving these prescriptions, the median age was 70 years and 91% were male. The most common malignancies included 31 instances of multiple myeloma (14%), 26 for chronic lymphocytic leukemia (12%), 24 for prostate cancer (11%), 23 for glioblastoma/brain cancer (10%), 18 for renal cell carcinoma (8%), 17 for colorectal cancer (8%), and 15 for acute myeloid leukemia (7%). Clinical interventions by the hematology/oncology CPP were completed for 82 (37%) of the 221 prescriptions. One clinical intervention was communicated directly to the patient, and attempts were made to communicate with the community care HCP for the remaining 81 prescriptions. The CPP documented 97 clinical interventions for the 82 prescriptions (Table 1). The most commonly documented clinical interventions included: 25 for managing/preventing a drug interaction (26%), 24 for dose adjustment request (25%), 13 for prescription denial (13%), and 11 for requesting the use of a preferred or more cost-effective product (11%). Of note, 16 patients (7%) received counseling from the hematology/oncology CPP. Ten patients (5%) received counseling alone with no other intervention and did not meet the definition of a clinical intervention.
The most frequent prescriptions requiring intervention included 8 for enzalutamide, 7 for venetoclax, 6 for ibrutinib, and 5 each for lenalidomide, cabozantinib, and temozolomide. Among the 97 interventions, 68 were approved (70%), 15 received no response (16%), and 14 were denied by the community care HCP (14%). Despite obtaining no response or intervention denial from the community care HCP, hematology/oncology CPPs could approve these prescriptions if clinically appropriate, and their reasoning was documented. Table 2 further describes the types of interventions that were denied or obtained no response by the community care practitioner. Among the prescriptions denied by the hematology/oncology CPP, 11 were rejected for off-label indications and/or did not have support through primary literature, national guidelines, or VA criteria for use. Only 2 prescriptions were denied for safety concerns.
These documented clinical interventions had financial implications. For drugs with available cost data, requesting the use of a preferred/cost-effective product led to estimated savings of at least $263,536 over the study period with some ongoing cost savings. Prescription denials led to further estimated savings of $186,275 per month, although this is limited by the lack of known costs of alternative therapies the community care physicians chose.
DISCUSSION
More than one-third of prescriptions required clinical interventions, and 70% of these interventions were accepted by the community care prescriber, demonstrating the CPP’s essential role. Results indicate that most CPP clinical interventions involved clarifying and correcting doses, managing pertinent drug interactions, and ensuring appropriate use of medications according to clinical and national VA guidelines. Other studies have examined the impact of CPPs on patient care and cancer treatment.5,6 The randomized, multicenter AMBORA trial found that clinical pharmacist support reduced severe AEs and medication errors related to oral anticancer agents.5 The per-patient mean number of medication errors found by pharmacist review was 1.7 (range, 0 to 9), with most medication errors noted at the prescribing stage.5 Suzuki and colleagues analyzed data from 35,062 chemotherapy regimens and found that 53.1% of the chemotherapy prescriptions were modified because of pharmacist interventions.6 The most common reason for prescription modifications was prescription error.
Most of the clinical interventions in this study were accepted by community HCPs, indicating that these prescribers are receptive to hematology/oncology CPP input. Among those with no response, most were in relation to recommendations regarding drug interactions. In most of these cases, the drug interaction was not clinically concerning enough to require a response before the CPP approved the prescription. Therefore, it is unknown whether the outside HCP implemented the clinical recommendations. The most common types of clinical interventions the community care HCP declined were dose adjustment requests or requests to switch to a more cost-effective/formulary-preferred agent. In these cases, the prescriber’s preference was documented and, if clinically appropriate, approved by the CPP.
Although the financial implications of CPP clinical interventions were only marginally evaluated in this review, results suggest that cost savings by requests to switch to a cost-effective/formulary preferred agent or prescription denials are substantial. Because of changes in prescription costs over time, it is possible that savings from CPP intervention were greater than calculations using current Federal Supply Schedule Service pricing. The total impact of CPP prescription interventions on reducing or preventing hospitalizations or AEs is not known from this review, but other data suggest that cost savings may benefit the system.13,14
Limitations
This study's retrospective design is a limitation because practice patterns at the VANTHCS involving multiple hematology/oncology CPPs review of community care prescriptions might have evolved over time. The total financial implications of CPP interventions cannot fully be elucidated. The cost of alternative therapies used for patients who received a prescription denial is not factored into this review.
Conclusions
VANTHCS CPPs played an essential role in reviewing anticancer medication prescriptions from community care prescribers. In this study, CPP clinical interventions were completed for more than one-third of the prescriptions and the community-based HCP approved most of these interventions. These changes also resulted in financial benefits.
These findings add to the body of literature emphasizing the need for hematology/oncology-trained CPPs to review anticancer prescriptions and treatment plans. Our review could be used to justify CPP involvement in community care specialty medication review at VA facilities that do not currently have CPP involvement.
The value of a hematology/oncology clinical pharmacy practitioner (CPP) has been validated in several studies documenting their positive impact on patient outcomes, supportive care management, laboratory monitoring, medication error identification, and drug expenditure.1-6 With> 200 oncology-related US Food and Drug Administration approval notifications published from 2020 to 2023, it is no surprise that national trends in oncology drug clinic expenditures increased from $39.9 billion in 2020 to $44.1 billion in 2021.7,8 With the rapidly changing treatment landscape, new drug approvals, and risk of polypharmacy, oral anticancer agents carry a high risk for medication errors.4 Additional challenges include complex dosing regimens and instructions, adherence issues, drug interactions, adjustments for organ dysfunction, and extensive adverse effect (AE) profiles.
Because of the niche and complexity of oral anticancer agents, trained CPPs havehematology/oncology education and expertise that pharmacists without specialized training lack. A survey of 243 nonspecialized community pharmacists that assessed their knowledge of oral anticancer therapies revealed that only about half of the knowledge questions were answered correctly, illustrating an education gap among these pharmacists.9 The Hematology/Oncology Pharmacist Association's suggests that best practices for managing oral oncology therapy should include comprehensive medication review by an oncology-trained pharmacist for each prescription.10
The US Department of Veterans Affairs (VA) community care network, which was established by the MISSION Act, allows covered access for eligible veterans in the local community outside of the VA network. Unfortunately, this dual-system use of health care could increase the risk of poorly coordinated care and has been associated with the risk of inappropriate prescribing.11,12 It is unclear how many private practices enrolled in the community care program have access to oncology-trained pharmacists. Specialized pharmaceutical reviews of oral anticancer medication prescriptions from these practices are vital for veteran care. This study evaluates the clinical and financial interventions of hematology/oncology CPPs review of specialty hematology/oncology prescriptions from community care health care practitioners (HCPs) at the Veterans Affairs North Texas Health Care System (VANTHCS) in Dallas.
METHODS
This study is a retrospective review of Computerized Patient Record System (CPRS) records of patients at VANTHCS from January 1, 2015, to June 30, 2023. Patients included were aged ≥ 18 years, enrolled in the VA community care program, received a specialty hematology/oncology medication that was dispensed through VA pharmacies or VA-contracted pharmacies, and had an hematology/oncology CPP medication review documented in CPRS. The primary aim of this study was to assess the number and types of clinical interventions performed. A clinical intervention was defined as a documented communication attempt with a community care HCP or direct communication with a patient to address a specific medication-related issue noted during CPP review.
Review of specialty hematology/oncology medications by a hematology/oncology CPP included evaluation of therapy indication, such as whether the prescription meets clinical guidelines, VA criteria for use, or other clinical literature as judged appropriate by the CPP. In some cases, the CPP requested that the community care HCP prescribe a more cost-effective or formulary-preferred agent. Each prescription was reviewed for dosage and formulation appropriateness, drug interactions with available medication lists, baseline laboratory test completion, and recommended supportive care medicines. At times, patient counseling is completed as part of the clinical review. When necessary, CPPs could discuss patient cases with a VA-employed oncologist for further oversight regarding appropriateness and safety. Secondary outcomes included the number of interventions accepted or denied by the prescriber provider and cost savings.
Data collected included the type of malignancy, hematology/oncology specialty medication requested, number and type of interventions sent to the community care prescriber, number of interventions accepted or denied by the community care prescriber, and whether the CPP conducted patient counseling or dispensed or denied the product. Cost savings were calculated for medications that were denied or changed to a formulary preferred or cost-effective agent using pricing data from the National Acquisition Center Contract Catalog or Federal Supply Schedule Service as of April 2024.
RESULTS
A total of 221 hematology/oncology prescriptions met inclusion criteria. Among patients receiving these prescriptions, the median age was 70 years and 91% were male. The most common malignancies included 31 instances of multiple myeloma (14%), 26 for chronic lymphocytic leukemia (12%), 24 for prostate cancer (11%), 23 for glioblastoma/brain cancer (10%), 18 for renal cell carcinoma (8%), 17 for colorectal cancer (8%), and 15 for acute myeloid leukemia (7%). Clinical interventions by the hematology/oncology CPP were completed for 82 (37%) of the 221 prescriptions. One clinical intervention was communicated directly to the patient, and attempts were made to communicate with the community care HCP for the remaining 81 prescriptions. The CPP documented 97 clinical interventions for the 82 prescriptions (Table 1). The most commonly documented clinical interventions included: 25 for managing/preventing a drug interaction (26%), 24 for dose adjustment request (25%), 13 for prescription denial (13%), and 11 for requesting the use of a preferred or more cost-effective product (11%). Of note, 16 patients (7%) received counseling from the hematology/oncology CPP. Ten patients (5%) received counseling alone with no other intervention and did not meet the definition of a clinical intervention.
The most frequent prescriptions requiring intervention included 8 for enzalutamide, 7 for venetoclax, 6 for ibrutinib, and 5 each for lenalidomide, cabozantinib, and temozolomide. Among the 97 interventions, 68 were approved (70%), 15 received no response (16%), and 14 were denied by the community care HCP (14%). Despite obtaining no response or intervention denial from the community care HCP, hematology/oncology CPPs could approve these prescriptions if clinically appropriate, and their reasoning was documented. Table 2 further describes the types of interventions that were denied or obtained no response by the community care practitioner. Among the prescriptions denied by the hematology/oncology CPP, 11 were rejected for off-label indications and/or did not have support through primary literature, national guidelines, or VA criteria for use. Only 2 prescriptions were denied for safety concerns.
These documented clinical interventions had financial implications. For drugs with available cost data, requesting the use of a preferred/cost-effective product led to estimated savings of at least $263,536 over the study period with some ongoing cost savings. Prescription denials led to further estimated savings of $186,275 per month, although this is limited by the lack of known costs of alternative therapies the community care physicians chose.
DISCUSSION
More than one-third of prescriptions required clinical interventions, and 70% of these interventions were accepted by the community care prescriber, demonstrating the CPP’s essential role. Results indicate that most CPP clinical interventions involved clarifying and correcting doses, managing pertinent drug interactions, and ensuring appropriate use of medications according to clinical and national VA guidelines. Other studies have examined the impact of CPPs on patient care and cancer treatment.5,6 The randomized, multicenter AMBORA trial found that clinical pharmacist support reduced severe AEs and medication errors related to oral anticancer agents.5 The per-patient mean number of medication errors found by pharmacist review was 1.7 (range, 0 to 9), with most medication errors noted at the prescribing stage.5 Suzuki and colleagues analyzed data from 35,062 chemotherapy regimens and found that 53.1% of the chemotherapy prescriptions were modified because of pharmacist interventions.6 The most common reason for prescription modifications was prescription error.
Most of the clinical interventions in this study were accepted by community HCPs, indicating that these prescribers are receptive to hematology/oncology CPP input. Among those with no response, most were in relation to recommendations regarding drug interactions. In most of these cases, the drug interaction was not clinically concerning enough to require a response before the CPP approved the prescription. Therefore, it is unknown whether the outside HCP implemented the clinical recommendations. The most common types of clinical interventions the community care HCP declined were dose adjustment requests or requests to switch to a more cost-effective/formulary-preferred agent. In these cases, the prescriber’s preference was documented and, if clinically appropriate, approved by the CPP.
Although the financial implications of CPP clinical interventions were only marginally evaluated in this review, results suggest that cost savings by requests to switch to a cost-effective/formulary preferred agent or prescription denials are substantial. Because of changes in prescription costs over time, it is possible that savings from CPP intervention were greater than calculations using current Federal Supply Schedule Service pricing. The total impact of CPP prescription interventions on reducing or preventing hospitalizations or AEs is not known from this review, but other data suggest that cost savings may benefit the system.13,14
Limitations
This study's retrospective design is a limitation because practice patterns at the VANTHCS involving multiple hematology/oncology CPPs review of community care prescriptions might have evolved over time. The total financial implications of CPP interventions cannot fully be elucidated. The cost of alternative therapies used for patients who received a prescription denial is not factored into this review.
Conclusions
VANTHCS CPPs played an essential role in reviewing anticancer medication prescriptions from community care prescribers. In this study, CPP clinical interventions were completed for more than one-third of the prescriptions and the community-based HCP approved most of these interventions. These changes also resulted in financial benefits.
These findings add to the body of literature emphasizing the need for hematology/oncology-trained CPPs to review anticancer prescriptions and treatment plans. Our review could be used to justify CPP involvement in community care specialty medication review at VA facilities that do not currently have CPP involvement.
1. Shah NN, Casella E, Capozzi D, et al. Improving the safety of oral chemotherapy at an academic medical center. J Oncol Pract. 2016;12(1):e71-e76. doi:10.1200/JOP.2015.007260
2. Gatwood J, Gatwood K, Gabre E, Alexander M. Impact of clinical pharmacists in outpatient oncology practices: a review. Am J Health Syst Pharm. 2017;74(19):1549-1557. doi:10.2146/ajhp160475
3. Lankford C, Dura J, Tran A, et al. Effect of clinical pharmacist interventions on cost in an integrated health system specialty pharmacy. J Manag Care Spec Pharm. 2021;27(3):379-384. doi:10.18553/jmcp.2021.27.3.379
4. Schlichtig K, Dürr P, Dörje F, Fromm MF. Medication errors during treatment with new oral anticancer agents: consequences for clinical practice based on the AMBORA Study. Clin Pharmacol Ther. 2021;110(4):1075-1086. doi:10.1002/cpt.2338
5. Dürr P, Schlichtig K, Kelz C, et al. The randomized AMBORA Trial: impact of pharmacological/pharmaceutical care on medication safety and patient-reported outcomes during treatment with new oral anticancer agents. J Clin Oncol. 2021;39(18):1983-1994. doi:10.1200/JCO.20.03088
6. Suzuki S, Chan A, Nomura H, Johnson PE, Endo K, Saito S. Chemotherapy regimen checks performed by pharmacists contribute to safe administration of chemotherapy. J Oncol Pharm Pract. 2017;23(1):18-25. doi:10.1177/1078155215614998
7. Tichy EM, Hoffman JM, Suda KJ, et al. National trends in prescription drug expenditures and projections for 2022. Am J Health Syst Pharm. 2022;79(14):1158-1172. doi:10.1093/ajhp/zxac102
8. US Food and Drug Administration. Oncology (cancer)/hematologic malignancies approval notifications. 2023.
9. O’Bryant CL, Crandell BC. Community pharmacists’ knowledge of and attitudes toward oral chemotherapy. J Am Pharm Assoc (2003). 2008;48(5):632-639. doi:10.1331/JAPhA.2008.07082
10. Mackler E, Segal EM, Muluneh B, Jeffers K, Carmichael J. 2018 hematology/oncology pharmacist association best practices for the management of oral oncolytic therapy: pharmacy practice standard. J Oncol Pract. 2019;15(4):e346-e355. doi:10.1200/JOP.18.00581
11. Thorpe JM, Thorpe CT, Schleiden L, et al. Association between dual use of Department of Veterans Affairs and Medicare part D drug benefits and potentially unsafe prescribing. JAMA Intern Med. 2019;179(11):1584-1586. doi:10.1001/jamainternmed.2019.2788
12. Thorpe JM, Thorpe CT, Gellad WF, et al. Dual health care system use and high-risk prescribing in patients with dementia: a national cohort study. Ann Intern Med. 2017;166(3):157-163. doi:10.7326/M16-0551
13. Chen P-Z, Wu C-C, Huang C-F. Clinical and economic impact of clinical pharmacist intervention in a hematology unit. J Oncol Pharm Pract. 2020;26(4):866-872. doi:10.1177/1078155219875806
14. Dalton K, Byrne S. Role of the pharmacist in reducing healthcare costs: current insights. Integr Pharm Res Pract. 2017;6:37-46. doi:10.2147/IPRP.S108047
1. Shah NN, Casella E, Capozzi D, et al. Improving the safety of oral chemotherapy at an academic medical center. J Oncol Pract. 2016;12(1):e71-e76. doi:10.1200/JOP.2015.007260
2. Gatwood J, Gatwood K, Gabre E, Alexander M. Impact of clinical pharmacists in outpatient oncology practices: a review. Am J Health Syst Pharm. 2017;74(19):1549-1557. doi:10.2146/ajhp160475
3. Lankford C, Dura J, Tran A, et al. Effect of clinical pharmacist interventions on cost in an integrated health system specialty pharmacy. J Manag Care Spec Pharm. 2021;27(3):379-384. doi:10.18553/jmcp.2021.27.3.379
4. Schlichtig K, Dürr P, Dörje F, Fromm MF. Medication errors during treatment with new oral anticancer agents: consequences for clinical practice based on the AMBORA Study. Clin Pharmacol Ther. 2021;110(4):1075-1086. doi:10.1002/cpt.2338
5. Dürr P, Schlichtig K, Kelz C, et al. The randomized AMBORA Trial: impact of pharmacological/pharmaceutical care on medication safety and patient-reported outcomes during treatment with new oral anticancer agents. J Clin Oncol. 2021;39(18):1983-1994. doi:10.1200/JCO.20.03088
6. Suzuki S, Chan A, Nomura H, Johnson PE, Endo K, Saito S. Chemotherapy regimen checks performed by pharmacists contribute to safe administration of chemotherapy. J Oncol Pharm Pract. 2017;23(1):18-25. doi:10.1177/1078155215614998
7. Tichy EM, Hoffman JM, Suda KJ, et al. National trends in prescription drug expenditures and projections for 2022. Am J Health Syst Pharm. 2022;79(14):1158-1172. doi:10.1093/ajhp/zxac102
8. US Food and Drug Administration. Oncology (cancer)/hematologic malignancies approval notifications. 2023.
9. O’Bryant CL, Crandell BC. Community pharmacists’ knowledge of and attitudes toward oral chemotherapy. J Am Pharm Assoc (2003). 2008;48(5):632-639. doi:10.1331/JAPhA.2008.07082
10. Mackler E, Segal EM, Muluneh B, Jeffers K, Carmichael J. 2018 hematology/oncology pharmacist association best practices for the management of oral oncolytic therapy: pharmacy practice standard. J Oncol Pract. 2019;15(4):e346-e355. doi:10.1200/JOP.18.00581
11. Thorpe JM, Thorpe CT, Schleiden L, et al. Association between dual use of Department of Veterans Affairs and Medicare part D drug benefits and potentially unsafe prescribing. JAMA Intern Med. 2019;179(11):1584-1586. doi:10.1001/jamainternmed.2019.2788
12. Thorpe JM, Thorpe CT, Gellad WF, et al. Dual health care system use and high-risk prescribing in patients with dementia: a national cohort study. Ann Intern Med. 2017;166(3):157-163. doi:10.7326/M16-0551
13. Chen P-Z, Wu C-C, Huang C-F. Clinical and economic impact of clinical pharmacist intervention in a hematology unit. J Oncol Pharm Pract. 2020;26(4):866-872. doi:10.1177/1078155219875806
14. Dalton K, Byrne S. Role of the pharmacist in reducing healthcare costs: current insights. Integr Pharm Res Pract. 2017;6:37-46. doi:10.2147/IPRP.S108047
Several Skin Conditions More Likely in Children With Obesity
TORONTO — results of new research show.
The retrospective cohort study found markedly higher rates of skin infections, atopic dermatitis (AD), and acanthosis nigricans among children with overweight, compared with children with average weight.
“Many conditions associated with obesity are strong predictors of cardiovascular mortality as these children age, so doctors can play a key role in advocating for weight loss strategies in this population,” lead study author Samantha Epstein, third-year medical student at Case Western Reserve University, Cleveland, Ohio, said in an interview. The findings were presented at the annual meeting of the Society for Pediatric Dermatology.
Previous research has linked obesity, a chronic inflammatory condition, to psoriasis, AD, hidradenitis suppurativa (HS), acne vulgaris, infections, and rosacea in adults. However, there’s scant research exploring the connection between obesity and cutaneous conditions in children.
According to the Cleveland Clinic, childhood obesity is defined as a body mass index, which is weight in kg divided by the square of height in m2, at or above the 95th percentile for age and sex in children aged 2 years or older.
For the study, Ms. Epstein and coauthor Sonal D. Shah, MD, associate professor, Department of Dermatology, Case Western Reserve University, and a board-certified pediatric dermatologist accessed a large national research database and used diagnostic codes to identify over 1 million children (mean age, 8.5 years). Most (about 44%) were White; about one-quarter were Black. The groups were propensity matched, so there were about equal numbers of youngsters with and without obesity and of boys and girls.
They collected data on AD, HS, rosacea, psoriasis, and acanthosis nigricans (a thickened purplish discoloration typically found in body folds around the armpits, groin, and neck). They also gathered information on comorbidities.
Acanthosis nigricans, which is linked to metabolic syndrome, type 2 diabetes, and insulin resistance , was more prevalent among children with obesity (20,885 cases in the with-obesity group and 336 in the without-obesity group, for a relative risk [RR] of 62.16 and an odds ratio [OR] of 64.38).
Skin and subcutaneous tissue infections were also more common among those with obesity (14,795 cases) vs 4720 cases among those without obesity (RR, 3.14; OR, 3.2). As for AD, there were 11,892 cases in the with-obesity group and 2983 in the without-obesity group (RR, 3.99; OR, 4.06). There were 1166 cases of psoriasis among those with obesity and 408 among those without obesity (RR, 2.86; OR, 2.88).
HS (587 cases in the with-obesity group and 70 in the without-obesity group; RR, 8.39; OR, 8.39) and rosacea (351 in the with-obesity group and 138 in the without-obesity group; RR, 2.54; OR, 2.55) were the least common skin conditions.
Higher Comorbidity Rates
Compared with their average-weight counterparts, the children with obesity had higher rates of comorbidities, including type 2 diabetes. Ms. Epstein noted that children with diabetes and obesity had increased risks for every skin condition except for infections of the skin and subcutaneous tissue when compared with children without obesity.
Such infections were the most common skin conditions among children without obesity. “This was expected just due to the fact that children are outside, they’re playing in the grass and the dirt, and they get infected,” said Ms. Epstein. Still, these infections were three times more common in youngsters with obesity.
Although acanthosis nigricans is “highly correlated” with type 2 diabetes, “not as many children as we would expect in this population have developed type 2 diabetes,” said Ms. Epstein. This might make some sense, though, because these children are still quite young. “When dermatologists recognize this skin condition, they can advocate for weight loss management to try to prevent it.”
Other conditions seen more often in the overweight children with overweight included: hypertension, hyperlipidemia, obstructive sleep apnea, polycystic ovarian syndrome, attention-deficit/hyperactivity disorder, major depressive disorder, depressive episodes, and anxiety (all P < .001).
Commenting on the results, Sonia Havele, MD, a pediatrician and dermatology resident at Children’s Mercy Hospital, Kansas City, Missouri, said in an interview that the study reflects trends that she and her colleagues see in clinic: There are more common skin conditions in their patients with obesity.
She agreed that it offers an opening for education. “The results of this study highlight the opportunity we have as pediatric dermatologists to provide additional counseling on obesity and offer referrals to our colleagues in endocrinology, gastroenterology, and nutrition if needed.”
No conflicts of interest were reported.
TORONTO — results of new research show.
The retrospective cohort study found markedly higher rates of skin infections, atopic dermatitis (AD), and acanthosis nigricans among children with overweight, compared with children with average weight.
“Many conditions associated with obesity are strong predictors of cardiovascular mortality as these children age, so doctors can play a key role in advocating for weight loss strategies in this population,” lead study author Samantha Epstein, third-year medical student at Case Western Reserve University, Cleveland, Ohio, said in an interview. The findings were presented at the annual meeting of the Society for Pediatric Dermatology.
Previous research has linked obesity, a chronic inflammatory condition, to psoriasis, AD, hidradenitis suppurativa (HS), acne vulgaris, infections, and rosacea in adults. However, there’s scant research exploring the connection between obesity and cutaneous conditions in children.
According to the Cleveland Clinic, childhood obesity is defined as a body mass index, which is weight in kg divided by the square of height in m2, at or above the 95th percentile for age and sex in children aged 2 years or older.
For the study, Ms. Epstein and coauthor Sonal D. Shah, MD, associate professor, Department of Dermatology, Case Western Reserve University, and a board-certified pediatric dermatologist accessed a large national research database and used diagnostic codes to identify over 1 million children (mean age, 8.5 years). Most (about 44%) were White; about one-quarter were Black. The groups were propensity matched, so there were about equal numbers of youngsters with and without obesity and of boys and girls.
They collected data on AD, HS, rosacea, psoriasis, and acanthosis nigricans (a thickened purplish discoloration typically found in body folds around the armpits, groin, and neck). They also gathered information on comorbidities.
Acanthosis nigricans, which is linked to metabolic syndrome, type 2 diabetes, and insulin resistance , was more prevalent among children with obesity (20,885 cases in the with-obesity group and 336 in the without-obesity group, for a relative risk [RR] of 62.16 and an odds ratio [OR] of 64.38).
Skin and subcutaneous tissue infections were also more common among those with obesity (14,795 cases) vs 4720 cases among those without obesity (RR, 3.14; OR, 3.2). As for AD, there were 11,892 cases in the with-obesity group and 2983 in the without-obesity group (RR, 3.99; OR, 4.06). There were 1166 cases of psoriasis among those with obesity and 408 among those without obesity (RR, 2.86; OR, 2.88).
HS (587 cases in the with-obesity group and 70 in the without-obesity group; RR, 8.39; OR, 8.39) and rosacea (351 in the with-obesity group and 138 in the without-obesity group; RR, 2.54; OR, 2.55) were the least common skin conditions.
Higher Comorbidity Rates
Compared with their average-weight counterparts, the children with obesity had higher rates of comorbidities, including type 2 diabetes. Ms. Epstein noted that children with diabetes and obesity had increased risks for every skin condition except for infections of the skin and subcutaneous tissue when compared with children without obesity.
Such infections were the most common skin conditions among children without obesity. “This was expected just due to the fact that children are outside, they’re playing in the grass and the dirt, and they get infected,” said Ms. Epstein. Still, these infections were three times more common in youngsters with obesity.
Although acanthosis nigricans is “highly correlated” with type 2 diabetes, “not as many children as we would expect in this population have developed type 2 diabetes,” said Ms. Epstein. This might make some sense, though, because these children are still quite young. “When dermatologists recognize this skin condition, they can advocate for weight loss management to try to prevent it.”
Other conditions seen more often in the overweight children with overweight included: hypertension, hyperlipidemia, obstructive sleep apnea, polycystic ovarian syndrome, attention-deficit/hyperactivity disorder, major depressive disorder, depressive episodes, and anxiety (all P < .001).
Commenting on the results, Sonia Havele, MD, a pediatrician and dermatology resident at Children’s Mercy Hospital, Kansas City, Missouri, said in an interview that the study reflects trends that she and her colleagues see in clinic: There are more common skin conditions in their patients with obesity.
She agreed that it offers an opening for education. “The results of this study highlight the opportunity we have as pediatric dermatologists to provide additional counseling on obesity and offer referrals to our colleagues in endocrinology, gastroenterology, and nutrition if needed.”
No conflicts of interest were reported.
TORONTO — results of new research show.
The retrospective cohort study found markedly higher rates of skin infections, atopic dermatitis (AD), and acanthosis nigricans among children with overweight, compared with children with average weight.
“Many conditions associated with obesity are strong predictors of cardiovascular mortality as these children age, so doctors can play a key role in advocating for weight loss strategies in this population,” lead study author Samantha Epstein, third-year medical student at Case Western Reserve University, Cleveland, Ohio, said in an interview. The findings were presented at the annual meeting of the Society for Pediatric Dermatology.
Previous research has linked obesity, a chronic inflammatory condition, to psoriasis, AD, hidradenitis suppurativa (HS), acne vulgaris, infections, and rosacea in adults. However, there’s scant research exploring the connection between obesity and cutaneous conditions in children.
According to the Cleveland Clinic, childhood obesity is defined as a body mass index, which is weight in kg divided by the square of height in m2, at or above the 95th percentile for age and sex in children aged 2 years or older.
For the study, Ms. Epstein and coauthor Sonal D. Shah, MD, associate professor, Department of Dermatology, Case Western Reserve University, and a board-certified pediatric dermatologist accessed a large national research database and used diagnostic codes to identify over 1 million children (mean age, 8.5 years). Most (about 44%) were White; about one-quarter were Black. The groups were propensity matched, so there were about equal numbers of youngsters with and without obesity and of boys and girls.
They collected data on AD, HS, rosacea, psoriasis, and acanthosis nigricans (a thickened purplish discoloration typically found in body folds around the armpits, groin, and neck). They also gathered information on comorbidities.
Acanthosis nigricans, which is linked to metabolic syndrome, type 2 diabetes, and insulin resistance , was more prevalent among children with obesity (20,885 cases in the with-obesity group and 336 in the without-obesity group, for a relative risk [RR] of 62.16 and an odds ratio [OR] of 64.38).
Skin and subcutaneous tissue infections were also more common among those with obesity (14,795 cases) vs 4720 cases among those without obesity (RR, 3.14; OR, 3.2). As for AD, there were 11,892 cases in the with-obesity group and 2983 in the without-obesity group (RR, 3.99; OR, 4.06). There were 1166 cases of psoriasis among those with obesity and 408 among those without obesity (RR, 2.86; OR, 2.88).
HS (587 cases in the with-obesity group and 70 in the without-obesity group; RR, 8.39; OR, 8.39) and rosacea (351 in the with-obesity group and 138 in the without-obesity group; RR, 2.54; OR, 2.55) were the least common skin conditions.
Higher Comorbidity Rates
Compared with their average-weight counterparts, the children with obesity had higher rates of comorbidities, including type 2 diabetes. Ms. Epstein noted that children with diabetes and obesity had increased risks for every skin condition except for infections of the skin and subcutaneous tissue when compared with children without obesity.
Such infections were the most common skin conditions among children without obesity. “This was expected just due to the fact that children are outside, they’re playing in the grass and the dirt, and they get infected,” said Ms. Epstein. Still, these infections were three times more common in youngsters with obesity.
Although acanthosis nigricans is “highly correlated” with type 2 diabetes, “not as many children as we would expect in this population have developed type 2 diabetes,” said Ms. Epstein. This might make some sense, though, because these children are still quite young. “When dermatologists recognize this skin condition, they can advocate for weight loss management to try to prevent it.”
Other conditions seen more often in the overweight children with overweight included: hypertension, hyperlipidemia, obstructive sleep apnea, polycystic ovarian syndrome, attention-deficit/hyperactivity disorder, major depressive disorder, depressive episodes, and anxiety (all P < .001).
Commenting on the results, Sonia Havele, MD, a pediatrician and dermatology resident at Children’s Mercy Hospital, Kansas City, Missouri, said in an interview that the study reflects trends that she and her colleagues see in clinic: There are more common skin conditions in their patients with obesity.
She agreed that it offers an opening for education. “The results of this study highlight the opportunity we have as pediatric dermatologists to provide additional counseling on obesity and offer referrals to our colleagues in endocrinology, gastroenterology, and nutrition if needed.”
No conflicts of interest were reported.
FROM SPD 2024
Generalized Fixed Drug Eruptions Require Urgent Care: A Case Series
Recognizing cutaneous drug eruptions is important for treatment and prevention of recurrence. Fixed drug eruptions (FDEs) typically are harmless but can have major negative cosmetic consequences for patients. In its more severe forms, patients are at risk for widespread epithelial necrosis with accompanying complications. We report 1 patient with generalized FDE and 2 with generalized bullous FDE. We also discuss the recognition and treatment of the condition. Two patients previously had been diagnosed with systemic lupus erythematosus (SLE).
Case Series
Patient 1—A 60-year-old woman presented to dermatology with a rash on the trunk and groin folds of 4 days’ duration. She had a history of SLE and cutaneous lupus treated with hydroxychloroquine 200 mg twice daily and topical corticosteroids. She had started sulfamethoxazole-trimethoprim for a urinary tract infection with a rash appearing 1 day later. She reported burning skin pain with progression to blisters that “sloughed” off. She denied any known history of allergy to sulfa drugs. Prior to evaluation by dermatology, she visited an urgent care facility and was prescribed hydroxyzine and intramuscular corticosteroids. At presentation to dermatology 3 days after taking sulfamethoxazole-trimethoprim, she had annular flaccid bullae and superficial erosions with dusky borders on the right posterior thigh, right side of the chest, left inframammary fold, and right inguinal fold (Figure 1). She had no ocular, oral, or vaginal erosions. A diagnosis of generalized bullous FDE was favored over erythema multiforme or Stevens-Johnson syndrome (SJS)/toxic epidermal necrolysis (TEN). Shave biopsies from lesions on the right posterior thigh and right inguinal fold demonstrated interface dermatitis with epidermal necrosis, pigment incontinence, and numerous eosinophils. Direct immunofluorescence of the perilesional skin was negative for immunoprotein deposition. These findings were consistent with the clinical impression of generalized bullous FDE. Prior to receiving the histopathology report, the patient was initiated on a regimen of cyclosporine 5 mg/kg/d in the setting of normal renal function and followed until the eruption resolved completely. Cyclosporine was tapered at 2 weeks and discontinued at 3 weeks.
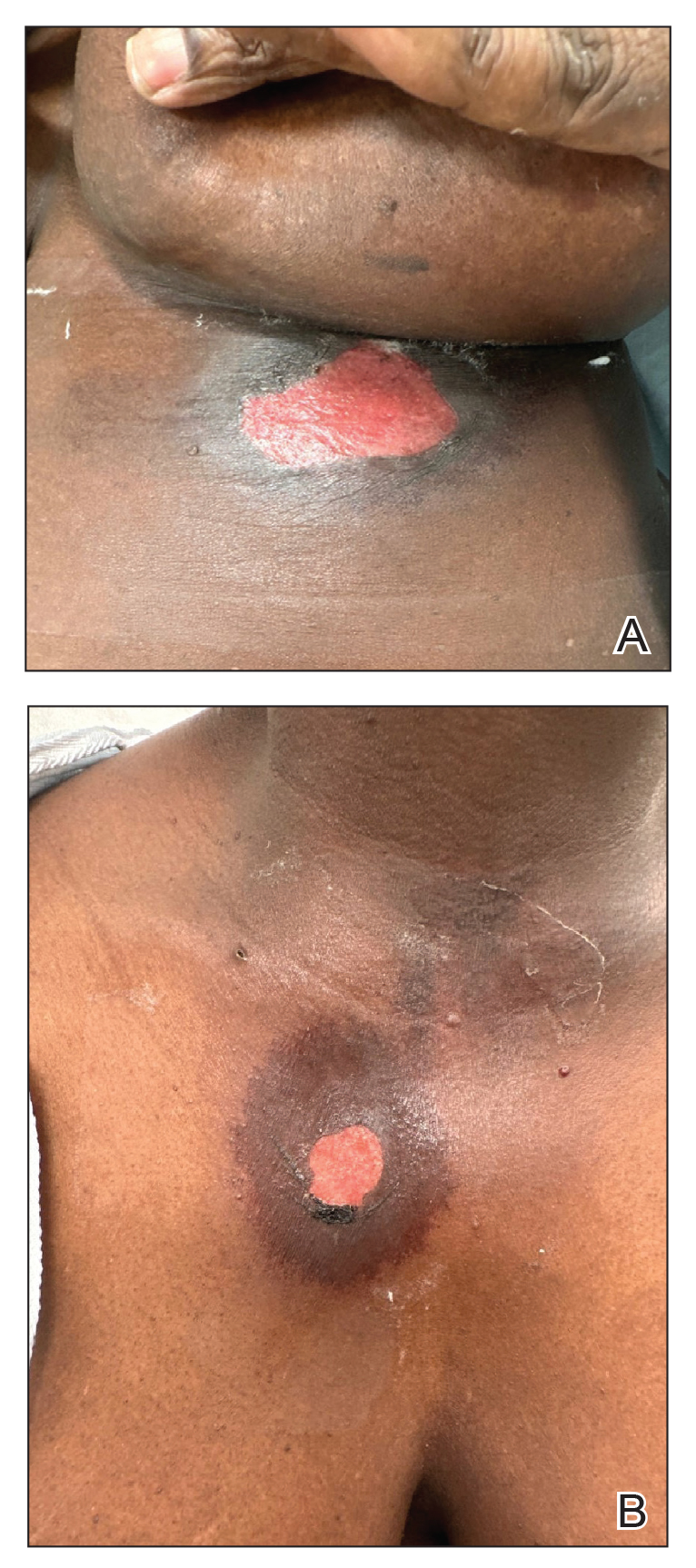
Patient 2—A 32-year-old woman presented for follow-up management of discoid lupus erythematosus. She had a history of systemic and cutaneous lupus, juvenile rheumatoid arthritis, and mixed connective tissue disease managed with prednisone, hydroxychloroquine, azathioprine, and belimumab. Physical examination revealed scarring alopecia with dyspigmentation and active inflammation consistent with uncontrolled cutaneous lupus. However, she also had oval-shaped hyperpigmented patches over the left breast, clavicle, and anterior chest consistent with a generalized FDE (Figure 2). The patient did not recall a history of similar lesions and could not identify a possible trigger. She was counseled on possible culprits and advised to avoid unnecessary medications. She had an unremarkable clinical course; therefore, no further intervention was necessary.
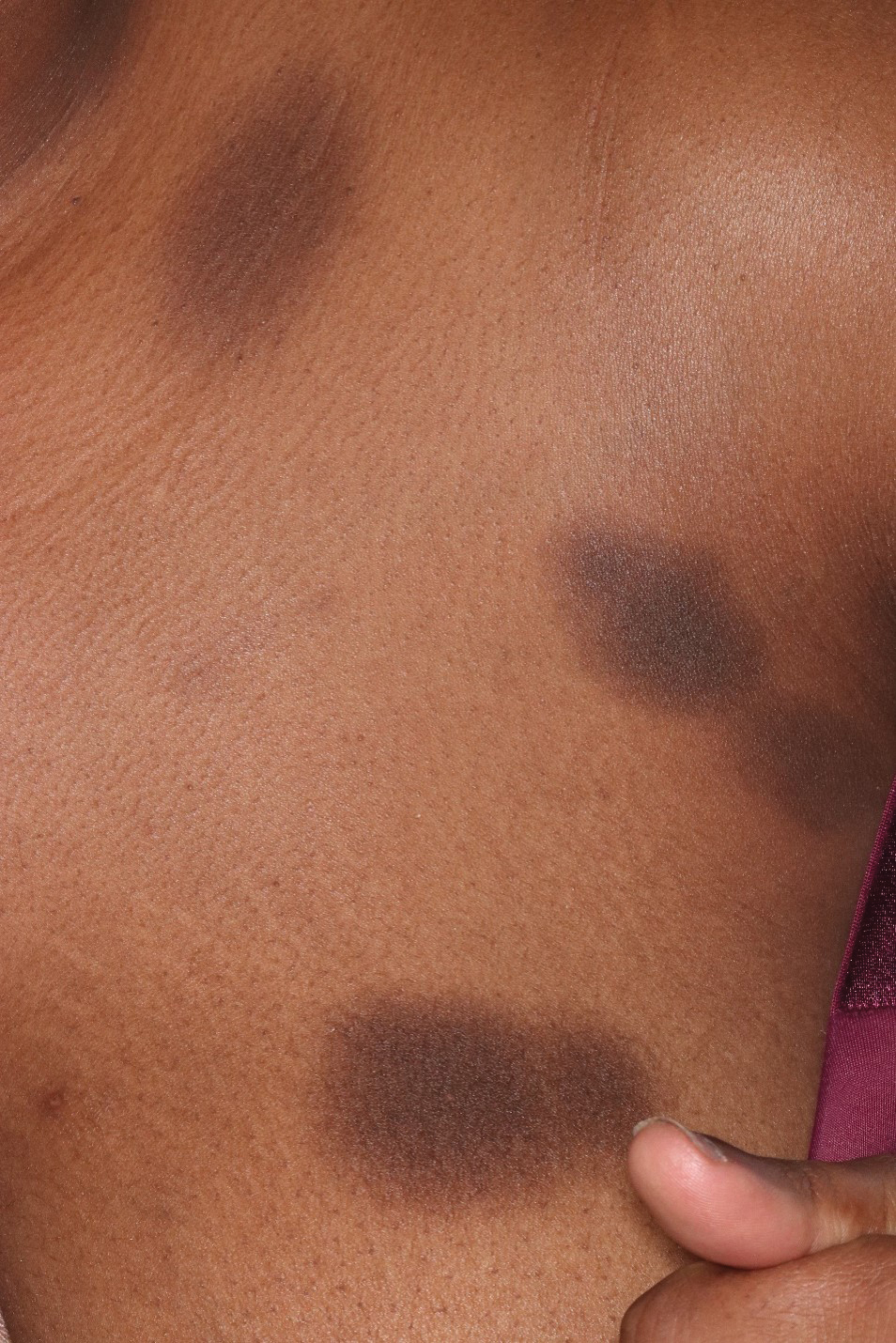
Patient 3—A 33-year-old man presented to the emergency department with a painful rash on the chest and back of 2 days’ duration that began 1 hour after taking naproxen (dosage unknown) for back pain. He had no notable medical history. The patient stated that the rash had slowly worsened and started to develop blisters. He visited an urgent care facility 1 day prior to the current presentation and was started on a 5-day course of prednisone 40 mg daily; the first 2 doses did not help. He denied any mucosal involvement apart from a tender lesion on the penis. He reported a history of an allergic reaction to penicillin. Physical examination revealed extensive dusky violaceous annular plaques with erythematous borders across the anterior and posterior trunk (Figure 3). Multiple flaccid bullae developed within these plaques, involving 15% of the body surface area. He was diagnosed with generalized bullous FDE based on the clinical history and histopathology. He was admitted to the burn intensive care unit and treated with cyclosporine 3 mg/kg/d with subsequent resolution of the eruption.
Comment
Presentation of FDEs—A fixed drug eruption manifests with 1 or more well-demarcated, red or violaceous, annular patches that resolve with postinflammatory hyperpigmentation; it occasionally may manifest with bullae. Initial eruptions may occur up to 2 weeks following medication exposure, but recurrent eruptions usually happen within minutes to hours later. They often are in the same location as prior lesions. A fixed drug eruption can be solitary, scattered, or generalized; a generalized FDE typically demonstrates multiple bilateral lesions that may itch, burn, or cause no symptoms. Patients can experience an FDE at any age, though the median age is reported as 35 to 60 years of age.1 A fixed drug eruption usually occurs after ingestion of oral medications, though there have been a few reports with iodinated contrast.2 Well-known culprits include antibiotics (eg, sulfamethoxazole-trimethoprim, tetracyclines, penicillins/cephalosporins, quinolones, dapsone), nonsteroidal anti-inflammatory drugs, acetaminophen (eg, paracetamol), barbiturates, antimalarials, and anticonvulsants. It also can occur with vaccines or with certain foods (fixed food eruption).3,4 Clinicians may try an oral drug challenge to identify the cause of an FDE, but in patients with a history of a generalized FDE, the risk for developing an increasingly severe reaction with repeated exposure to the medication is too high.5
Histopathology—Patch testing at the site of prior eruption with suspected drug culprits may be useful.6 Histopathology of FDE typically demonstrates vacuolar changes at the dermoepidermal junction with a lichenoid lymphocytic infiltrate. Early lesions often show a predominance of eosinophils. Subepidermal clefting is a feature of the bullous variant. In an active lesion, there are large numbers of CD8+ T lymphocytes expressing natural killer cell–associated molecules.7 The pathologic mechanism is not well understood, though it has been hypothesized that memory CD8+ cells are maintained in specific regions of the epidermis by IL-15 produced in the microenvironment and are activated upon rechallenge.7Considerations in Generalized Bullous FDE—Generalized FDE is defined in the literature as an FDE with involvement of 3 of 6 body areas: head, neck, trunk, upper limbs, lower limbs, and genital area. It may cover more or less than 10% of the body surface area.8-10 Although an isolated FDE frequently is asymptomatic and may not be cause for alarm, recurring drug eruptions increase the risk for development of generalized bullous FDE. Generalized bullous FDE is a rare subset. It is frequently misdiagnosed, and data on its incidence are uncertain.11 Of note, several pathologies causing bullous lesions may be in the differential diagnosis, including bullous pemphigoid; pemphigus vulgaris; bullous SLE; or bullae from cutaneous lupus, staphylococcal scalded skin syndrome, erythema multiforme, or SJS/TEN.12 When matched for body surface area involvement with SJS/TEN, generalized bullous FDE shares nearly identical mortality rates10; therefore, these patients should be treated with the same level of urgency and admitted to a critical care or burn unit, as they are at serious risk for infection and other complications.13
Clinical history and presentation along with histopathologic findings help to narrow down the differential diagnosis. Clinically, generalized bullous FDE does not affect the surrounding skin and manifests sooner after drug exposure (1–24 hours) with less mucosal involvement than SJS/TEN.9 Additionally, SJS/TEN patients frequently have generalized malaise and/or fever, while generalized bullous FDE patients do not. Finally, patients with generalized bullous FDE may report a history of a cutaneous eruption similar in morphology or in the same location.
Histopathologically, generalized bullous FDE may be similar to FDE with the addition of a subepidermal blister. Generalized bullous FDE patients have greater eosinophil infiltration and dermal melanophages than patients with SJS/TEN.9 Cellular infiltrates in generalized bullous FDE include more dermal CD41 cells, such as Foxp31 regulatory T cells; fewer intraepidermal CD561 cells; and fewer intraepidermal cells with granulysin.9 Occasionally, generalized bullous FDE causes full-thickness necrosis. In those cases, generalized bullous FDE cannot reliably be distinguished from other conditions with epidermal necrolysis on histopathology.13
FDE Diagnostics—A cytotoxin produced by
Management—Avoidance of the inciting drug often is sufficient for patients with an FDE, as demonstrated in patient 2 in our case series. Clinicians also should counsel patients on avoidance of potential cross-reacting drugs. Symptomatic treatment for itch or pain is appropriate and may include antihistamines or topical steroids. Nonsteroidal anti-inflammatory drugs may exacerbate or be causative of FDE. For generalized bullous FDE, cyclosporine is favored in the literature15,16 and was used to successfully treat both patients 1 and 3 in our case series. A short course of systemic corticosteroids or intravenous immunoglobulin also may be considered. Mild cases of generalized bullous FDE may be treated with close outpatient follow-up (patient 1), while severe cases require inpatient or even critical care monitoring with aggressive medical management to prevent the progression of skin desquamation (patient 3). Patients with severe oral lesions may require inpatient support for fluid maintenance.
Lupus History—Two patients in our case series had a history of lupus. Lupus itself can cause primary bullous lesions. Similar to FDE, bullous SLE can involve sun-exposed and nonexposed areas of the skin as well as the mucous membranes with a predilection for the lower vermilion lip.17 In bullous SLE, tense subepidermal blisters with a neutrophil-rich infiltrate form due to circulating antibodies to type VII collagen. These blisters have an erythematous or urticated base, most commonly on the face, upper trunk, and proximal extremities.18 In both SLE with skin manifestations and lupus limited to the skin, bullae may form due to extensive vacuolar degeneration. Similar to TEN, they can form rapidly in a widespread distribution.17 However, there is limited mucosal involvement, no clear drug association, and a better prognosis. Bullae caused by lupus will frequently demonstrate deposition of immunoproteins IgG, IgM, IgA, and complement component 3 at the basement membrane zone in perilesional skin on direct immunofluorescence. However, negative direct immunofluorescence does not rule out lupus.12 At the same time, patients with lupus frequently have comorbidities requiring multiple medications; the need for these medications may predispose patients to higher rates of cutaneous drug eruptions.19 To our knowledge, there is no known association between FDE and lupus.
Conclusion
Patients with acute eruptions following the initiation of a new prescription or over-the-counter medication require urgent evaluation. Generalized bullous FDE requires timely diagnosis and intervention. Patients with lupus have an increased risk for cutaneous drug eruptions due to polypharmacy. Further investigation is necessary to determine if there is a pathophysiologic mechanism responsible for the development of FDE in lupus patients.
- Anderson HJ, Lee JB. A review of fixed drug eruption with a special focus on generalized bullous fixed drug eruption. Medicina (Kaunas). 2021;57:925.
- Gavin M, Sharp L, Walker K, et al. Contrast-induced generalized bullous fixed drug eruption resembling Stevens-Johnson syndrome. Proc (Bayl Univ Med Cent). 2019;32:601-602.
- Kabir S, Feit EJ, Heilman ER. Generalized fixed drug eruption following Pfizer-BioNtech COVID-19 vaccination. Clin Case Rep. 2022;10:E6684.
- Choi S, Kim SH, Hwang JH, et al. Rapidly progressing generalized bullous fixed drug eruption after the first dose of COVID-19 messenger RNA vaccination. J Dermatol. 2023;50:1190-1193.
- Mahboob A, Haroon TS. Drugs causing fixed eruptions: a study of 450 cases. Int J Dermatol. 1998;37:833-838.
- Shiohara T. Fixed drug eruption: pathogenesis and diagnostic tests. Curr Opin Allergy Clin Immunol. 2009;9:316-321.
- Mizukawa Y, Yamazaki Y, Shiohara T. In vivo dynamics of intraepidermal CD8+ T cells and CD4+ T cells during the evolution of fixed drug eruption. Br J Dermatol. 2008;158:1230-1238.
- Lee CH, Chen YC, Cho YT, et al. Fixed-drug eruption: a retrospective study in a single referral center in northern Taiwan. Dermatologica Sinica. 2012;30:11-15.
- Cho YT, Lin JW, Chen YC, et al. Generalized bullous fixed drug eruption is distinct from Stevens-Johnson syndrome/toxic epidermal necrolysis by immunohistopathological features. J Am Acad Dermatol. 2014;70:539-548.
- Lipowicz S, Sekula P, Ingen-Housz-Oro S, et al. Prognosis of generalized bullous fixed drug eruption: comparison with Stevens-Johnson syndrome and toxic epidermal necrolysis. Br J Dermatol. 2013;168:726-732.
- Patel S, John AM, Handler MZ, et al. Fixed drug eruptions: an update, emphasizing the potentially lethal generalized bullous fixed drug eruption. Am J Clin Dermatol. 2020;21:393-399.
- Ranario JS, Smith JL. Bullous lesions in a patient with systemic lupus erythematosus. J Clin Aesthet Dermatol. 2014;7:44-49.
- Perron E, Viarnaud A, Marciano L, et al. Clinical and histological features of fixed drug eruption: a single-centre series of 73 cases with comparison between bullous and non-bullous forms. Eur J Dermatol. 2021;31:372-380.
- Chen CB, Kuo KL, Wang CW, et al. Detecting lesional granulysin levels for rapid diagnosis of cytotoxic T lymphocyte-mediated bullous skin disorders. J Allergy Clin Immunol Pract. 2021;9:1327-1337.e3.
- Beniwal R, Gupta LK, Khare AK, et al. Cyclosporine in generalized bullous-fixed drug eruption. Indian J Dermatol. 2018;63:432-433.
- Vargas Mora P, García S, Valenzuela F, et al. Generalized bullous fixed drug eruption successfully treated with cyclosporine. Dermatol Ther. 2020;33:E13492.
- Montagnon CM, Tolkachjov SN, Murrell DF, et al. Subepithelial autoimmune blistering dermatoses: clinical features and diagnosis. J Am Acad Dermatol. 2021;85:1-14.
- Sebaratnam DF, Murrell DF. Bullous systemic lupus erythematosus. Dermatol Clin. 2011;29:649-653.
- Zonzits E, Aberer W, Tappeiner G. Drug eruptions from mesna. After cyclophosphamide treatment of patients with systemic lupus erythematosus and dermatomyositis. Arch Dermatol. 1992;128:80-82.
Recognizing cutaneous drug eruptions is important for treatment and prevention of recurrence. Fixed drug eruptions (FDEs) typically are harmless but can have major negative cosmetic consequences for patients. In its more severe forms, patients are at risk for widespread epithelial necrosis with accompanying complications. We report 1 patient with generalized FDE and 2 with generalized bullous FDE. We also discuss the recognition and treatment of the condition. Two patients previously had been diagnosed with systemic lupus erythematosus (SLE).
Case Series
Patient 1—A 60-year-old woman presented to dermatology with a rash on the trunk and groin folds of 4 days’ duration. She had a history of SLE and cutaneous lupus treated with hydroxychloroquine 200 mg twice daily and topical corticosteroids. She had started sulfamethoxazole-trimethoprim for a urinary tract infection with a rash appearing 1 day later. She reported burning skin pain with progression to blisters that “sloughed” off. She denied any known history of allergy to sulfa drugs. Prior to evaluation by dermatology, she visited an urgent care facility and was prescribed hydroxyzine and intramuscular corticosteroids. At presentation to dermatology 3 days after taking sulfamethoxazole-trimethoprim, she had annular flaccid bullae and superficial erosions with dusky borders on the right posterior thigh, right side of the chest, left inframammary fold, and right inguinal fold (Figure 1). She had no ocular, oral, or vaginal erosions. A diagnosis of generalized bullous FDE was favored over erythema multiforme or Stevens-Johnson syndrome (SJS)/toxic epidermal necrolysis (TEN). Shave biopsies from lesions on the right posterior thigh and right inguinal fold demonstrated interface dermatitis with epidermal necrosis, pigment incontinence, and numerous eosinophils. Direct immunofluorescence of the perilesional skin was negative for immunoprotein deposition. These findings were consistent with the clinical impression of generalized bullous FDE. Prior to receiving the histopathology report, the patient was initiated on a regimen of cyclosporine 5 mg/kg/d in the setting of normal renal function and followed until the eruption resolved completely. Cyclosporine was tapered at 2 weeks and discontinued at 3 weeks.

Patient 2—A 32-year-old woman presented for follow-up management of discoid lupus erythematosus. She had a history of systemic and cutaneous lupus, juvenile rheumatoid arthritis, and mixed connective tissue disease managed with prednisone, hydroxychloroquine, azathioprine, and belimumab. Physical examination revealed scarring alopecia with dyspigmentation and active inflammation consistent with uncontrolled cutaneous lupus. However, she also had oval-shaped hyperpigmented patches over the left breast, clavicle, and anterior chest consistent with a generalized FDE (Figure 2). The patient did not recall a history of similar lesions and could not identify a possible trigger. She was counseled on possible culprits and advised to avoid unnecessary medications. She had an unremarkable clinical course; therefore, no further intervention was necessary.

Patient 3—A 33-year-old man presented to the emergency department with a painful rash on the chest and back of 2 days’ duration that began 1 hour after taking naproxen (dosage unknown) for back pain. He had no notable medical history. The patient stated that the rash had slowly worsened and started to develop blisters. He visited an urgent care facility 1 day prior to the current presentation and was started on a 5-day course of prednisone 40 mg daily; the first 2 doses did not help. He denied any mucosal involvement apart from a tender lesion on the penis. He reported a history of an allergic reaction to penicillin. Physical examination revealed extensive dusky violaceous annular plaques with erythematous borders across the anterior and posterior trunk (Figure 3). Multiple flaccid bullae developed within these plaques, involving 15% of the body surface area. He was diagnosed with generalized bullous FDE based on the clinical history and histopathology. He was admitted to the burn intensive care unit and treated with cyclosporine 3 mg/kg/d with subsequent resolution of the eruption.

Comment
Presentation of FDEs—A fixed drug eruption manifests with 1 or more well-demarcated, red or violaceous, annular patches that resolve with postinflammatory hyperpigmentation; it occasionally may manifest with bullae. Initial eruptions may occur up to 2 weeks following medication exposure, but recurrent eruptions usually happen within minutes to hours later. They often are in the same location as prior lesions. A fixed drug eruption can be solitary, scattered, or generalized; a generalized FDE typically demonstrates multiple bilateral lesions that may itch, burn, or cause no symptoms. Patients can experience an FDE at any age, though the median age is reported as 35 to 60 years of age.1 A fixed drug eruption usually occurs after ingestion of oral medications, though there have been a few reports with iodinated contrast.2 Well-known culprits include antibiotics (eg, sulfamethoxazole-trimethoprim, tetracyclines, penicillins/cephalosporins, quinolones, dapsone), nonsteroidal anti-inflammatory drugs, acetaminophen (eg, paracetamol), barbiturates, antimalarials, and anticonvulsants. It also can occur with vaccines or with certain foods (fixed food eruption).3,4 Clinicians may try an oral drug challenge to identify the cause of an FDE, but in patients with a history of a generalized FDE, the risk for developing an increasingly severe reaction with repeated exposure to the medication is too high.5
Histopathology—Patch testing at the site of prior eruption with suspected drug culprits may be useful.6 Histopathology of FDE typically demonstrates vacuolar changes at the dermoepidermal junction with a lichenoid lymphocytic infiltrate. Early lesions often show a predominance of eosinophils. Subepidermal clefting is a feature of the bullous variant. In an active lesion, there are large numbers of CD8+ T lymphocytes expressing natural killer cell–associated molecules.7 The pathologic mechanism is not well understood, though it has been hypothesized that memory CD8+ cells are maintained in specific regions of the epidermis by IL-15 produced in the microenvironment and are activated upon rechallenge.7Considerations in Generalized Bullous FDE—Generalized FDE is defined in the literature as an FDE with involvement of 3 of 6 body areas: head, neck, trunk, upper limbs, lower limbs, and genital area. It may cover more or less than 10% of the body surface area.8-10 Although an isolated FDE frequently is asymptomatic and may not be cause for alarm, recurring drug eruptions increase the risk for development of generalized bullous FDE. Generalized bullous FDE is a rare subset. It is frequently misdiagnosed, and data on its incidence are uncertain.11 Of note, several pathologies causing bullous lesions may be in the differential diagnosis, including bullous pemphigoid; pemphigus vulgaris; bullous SLE; or bullae from cutaneous lupus, staphylococcal scalded skin syndrome, erythema multiforme, or SJS/TEN.12 When matched for body surface area involvement with SJS/TEN, generalized bullous FDE shares nearly identical mortality rates10; therefore, these patients should be treated with the same level of urgency and admitted to a critical care or burn unit, as they are at serious risk for infection and other complications.13
Clinical history and presentation along with histopathologic findings help to narrow down the differential diagnosis. Clinically, generalized bullous FDE does not affect the surrounding skin and manifests sooner after drug exposure (1–24 hours) with less mucosal involvement than SJS/TEN.9 Additionally, SJS/TEN patients frequently have generalized malaise and/or fever, while generalized bullous FDE patients do not. Finally, patients with generalized bullous FDE may report a history of a cutaneous eruption similar in morphology or in the same location.
Histopathologically, generalized bullous FDE may be similar to FDE with the addition of a subepidermal blister. Generalized bullous FDE patients have greater eosinophil infiltration and dermal melanophages than patients with SJS/TEN.9 Cellular infiltrates in generalized bullous FDE include more dermal CD41 cells, such as Foxp31 regulatory T cells; fewer intraepidermal CD561 cells; and fewer intraepidermal cells with granulysin.9 Occasionally, generalized bullous FDE causes full-thickness necrosis. In those cases, generalized bullous FDE cannot reliably be distinguished from other conditions with epidermal necrolysis on histopathology.13
FDE Diagnostics—A cytotoxin produced by
Management—Avoidance of the inciting drug often is sufficient for patients with an FDE, as demonstrated in patient 2 in our case series. Clinicians also should counsel patients on avoidance of potential cross-reacting drugs. Symptomatic treatment for itch or pain is appropriate and may include antihistamines or topical steroids. Nonsteroidal anti-inflammatory drugs may exacerbate or be causative of FDE. For generalized bullous FDE, cyclosporine is favored in the literature15,16 and was used to successfully treat both patients 1 and 3 in our case series. A short course of systemic corticosteroids or intravenous immunoglobulin also may be considered. Mild cases of generalized bullous FDE may be treated with close outpatient follow-up (patient 1), while severe cases require inpatient or even critical care monitoring with aggressive medical management to prevent the progression of skin desquamation (patient 3). Patients with severe oral lesions may require inpatient support for fluid maintenance.
Lupus History—Two patients in our case series had a history of lupus. Lupus itself can cause primary bullous lesions. Similar to FDE, bullous SLE can involve sun-exposed and nonexposed areas of the skin as well as the mucous membranes with a predilection for the lower vermilion lip.17 In bullous SLE, tense subepidermal blisters with a neutrophil-rich infiltrate form due to circulating antibodies to type VII collagen. These blisters have an erythematous or urticated base, most commonly on the face, upper trunk, and proximal extremities.18 In both SLE with skin manifestations and lupus limited to the skin, bullae may form due to extensive vacuolar degeneration. Similar to TEN, they can form rapidly in a widespread distribution.17 However, there is limited mucosal involvement, no clear drug association, and a better prognosis. Bullae caused by lupus will frequently demonstrate deposition of immunoproteins IgG, IgM, IgA, and complement component 3 at the basement membrane zone in perilesional skin on direct immunofluorescence. However, negative direct immunofluorescence does not rule out lupus.12 At the same time, patients with lupus frequently have comorbidities requiring multiple medications; the need for these medications may predispose patients to higher rates of cutaneous drug eruptions.19 To our knowledge, there is no known association between FDE and lupus.
Conclusion
Patients with acute eruptions following the initiation of a new prescription or over-the-counter medication require urgent evaluation. Generalized bullous FDE requires timely diagnosis and intervention. Patients with lupus have an increased risk for cutaneous drug eruptions due to polypharmacy. Further investigation is necessary to determine if there is a pathophysiologic mechanism responsible for the development of FDE in lupus patients.
Recognizing cutaneous drug eruptions is important for treatment and prevention of recurrence. Fixed drug eruptions (FDEs) typically are harmless but can have major negative cosmetic consequences for patients. In its more severe forms, patients are at risk for widespread epithelial necrosis with accompanying complications. We report 1 patient with generalized FDE and 2 with generalized bullous FDE. We also discuss the recognition and treatment of the condition. Two patients previously had been diagnosed with systemic lupus erythematosus (SLE).
Case Series
Patient 1—A 60-year-old woman presented to dermatology with a rash on the trunk and groin folds of 4 days’ duration. She had a history of SLE and cutaneous lupus treated with hydroxychloroquine 200 mg twice daily and topical corticosteroids. She had started sulfamethoxazole-trimethoprim for a urinary tract infection with a rash appearing 1 day later. She reported burning skin pain with progression to blisters that “sloughed” off. She denied any known history of allergy to sulfa drugs. Prior to evaluation by dermatology, she visited an urgent care facility and was prescribed hydroxyzine and intramuscular corticosteroids. At presentation to dermatology 3 days after taking sulfamethoxazole-trimethoprim, she had annular flaccid bullae and superficial erosions with dusky borders on the right posterior thigh, right side of the chest, left inframammary fold, and right inguinal fold (Figure 1). She had no ocular, oral, or vaginal erosions. A diagnosis of generalized bullous FDE was favored over erythema multiforme or Stevens-Johnson syndrome (SJS)/toxic epidermal necrolysis (TEN). Shave biopsies from lesions on the right posterior thigh and right inguinal fold demonstrated interface dermatitis with epidermal necrosis, pigment incontinence, and numerous eosinophils. Direct immunofluorescence of the perilesional skin was negative for immunoprotein deposition. These findings were consistent with the clinical impression of generalized bullous FDE. Prior to receiving the histopathology report, the patient was initiated on a regimen of cyclosporine 5 mg/kg/d in the setting of normal renal function and followed until the eruption resolved completely. Cyclosporine was tapered at 2 weeks and discontinued at 3 weeks.

Patient 2—A 32-year-old woman presented for follow-up management of discoid lupus erythematosus. She had a history of systemic and cutaneous lupus, juvenile rheumatoid arthritis, and mixed connective tissue disease managed with prednisone, hydroxychloroquine, azathioprine, and belimumab. Physical examination revealed scarring alopecia with dyspigmentation and active inflammation consistent with uncontrolled cutaneous lupus. However, she also had oval-shaped hyperpigmented patches over the left breast, clavicle, and anterior chest consistent with a generalized FDE (Figure 2). The patient did not recall a history of similar lesions and could not identify a possible trigger. She was counseled on possible culprits and advised to avoid unnecessary medications. She had an unremarkable clinical course; therefore, no further intervention was necessary.

Patient 3—A 33-year-old man presented to the emergency department with a painful rash on the chest and back of 2 days’ duration that began 1 hour after taking naproxen (dosage unknown) for back pain. He had no notable medical history. The patient stated that the rash had slowly worsened and started to develop blisters. He visited an urgent care facility 1 day prior to the current presentation and was started on a 5-day course of prednisone 40 mg daily; the first 2 doses did not help. He denied any mucosal involvement apart from a tender lesion on the penis. He reported a history of an allergic reaction to penicillin. Physical examination revealed extensive dusky violaceous annular plaques with erythematous borders across the anterior and posterior trunk (Figure 3). Multiple flaccid bullae developed within these plaques, involving 15% of the body surface area. He was diagnosed with generalized bullous FDE based on the clinical history and histopathology. He was admitted to the burn intensive care unit and treated with cyclosporine 3 mg/kg/d with subsequent resolution of the eruption.

Comment
Presentation of FDEs—A fixed drug eruption manifests with 1 or more well-demarcated, red or violaceous, annular patches that resolve with postinflammatory hyperpigmentation; it occasionally may manifest with bullae. Initial eruptions may occur up to 2 weeks following medication exposure, but recurrent eruptions usually happen within minutes to hours later. They often are in the same location as prior lesions. A fixed drug eruption can be solitary, scattered, or generalized; a generalized FDE typically demonstrates multiple bilateral lesions that may itch, burn, or cause no symptoms. Patients can experience an FDE at any age, though the median age is reported as 35 to 60 years of age.1 A fixed drug eruption usually occurs after ingestion of oral medications, though there have been a few reports with iodinated contrast.2 Well-known culprits include antibiotics (eg, sulfamethoxazole-trimethoprim, tetracyclines, penicillins/cephalosporins, quinolones, dapsone), nonsteroidal anti-inflammatory drugs, acetaminophen (eg, paracetamol), barbiturates, antimalarials, and anticonvulsants. It also can occur with vaccines or with certain foods (fixed food eruption).3,4 Clinicians may try an oral drug challenge to identify the cause of an FDE, but in patients with a history of a generalized FDE, the risk for developing an increasingly severe reaction with repeated exposure to the medication is too high.5
Histopathology—Patch testing at the site of prior eruption with suspected drug culprits may be useful.6 Histopathology of FDE typically demonstrates vacuolar changes at the dermoepidermal junction with a lichenoid lymphocytic infiltrate. Early lesions often show a predominance of eosinophils. Subepidermal clefting is a feature of the bullous variant. In an active lesion, there are large numbers of CD8+ T lymphocytes expressing natural killer cell–associated molecules.7 The pathologic mechanism is not well understood, though it has been hypothesized that memory CD8+ cells are maintained in specific regions of the epidermis by IL-15 produced in the microenvironment and are activated upon rechallenge.7Considerations in Generalized Bullous FDE—Generalized FDE is defined in the literature as an FDE with involvement of 3 of 6 body areas: head, neck, trunk, upper limbs, lower limbs, and genital area. It may cover more or less than 10% of the body surface area.8-10 Although an isolated FDE frequently is asymptomatic and may not be cause for alarm, recurring drug eruptions increase the risk for development of generalized bullous FDE. Generalized bullous FDE is a rare subset. It is frequently misdiagnosed, and data on its incidence are uncertain.11 Of note, several pathologies causing bullous lesions may be in the differential diagnosis, including bullous pemphigoid; pemphigus vulgaris; bullous SLE; or bullae from cutaneous lupus, staphylococcal scalded skin syndrome, erythema multiforme, or SJS/TEN.12 When matched for body surface area involvement with SJS/TEN, generalized bullous FDE shares nearly identical mortality rates10; therefore, these patients should be treated with the same level of urgency and admitted to a critical care or burn unit, as they are at serious risk for infection and other complications.13
Clinical history and presentation along with histopathologic findings help to narrow down the differential diagnosis. Clinically, generalized bullous FDE does not affect the surrounding skin and manifests sooner after drug exposure (1–24 hours) with less mucosal involvement than SJS/TEN.9 Additionally, SJS/TEN patients frequently have generalized malaise and/or fever, while generalized bullous FDE patients do not. Finally, patients with generalized bullous FDE may report a history of a cutaneous eruption similar in morphology or in the same location.
Histopathologically, generalized bullous FDE may be similar to FDE with the addition of a subepidermal blister. Generalized bullous FDE patients have greater eosinophil infiltration and dermal melanophages than patients with SJS/TEN.9 Cellular infiltrates in generalized bullous FDE include more dermal CD41 cells, such as Foxp31 regulatory T cells; fewer intraepidermal CD561 cells; and fewer intraepidermal cells with granulysin.9 Occasionally, generalized bullous FDE causes full-thickness necrosis. In those cases, generalized bullous FDE cannot reliably be distinguished from other conditions with epidermal necrolysis on histopathology.13
FDE Diagnostics—A cytotoxin produced by
Management—Avoidance of the inciting drug often is sufficient for patients with an FDE, as demonstrated in patient 2 in our case series. Clinicians also should counsel patients on avoidance of potential cross-reacting drugs. Symptomatic treatment for itch or pain is appropriate and may include antihistamines or topical steroids. Nonsteroidal anti-inflammatory drugs may exacerbate or be causative of FDE. For generalized bullous FDE, cyclosporine is favored in the literature15,16 and was used to successfully treat both patients 1 and 3 in our case series. A short course of systemic corticosteroids or intravenous immunoglobulin also may be considered. Mild cases of generalized bullous FDE may be treated with close outpatient follow-up (patient 1), while severe cases require inpatient or even critical care monitoring with aggressive medical management to prevent the progression of skin desquamation (patient 3). Patients with severe oral lesions may require inpatient support for fluid maintenance.
Lupus History—Two patients in our case series had a history of lupus. Lupus itself can cause primary bullous lesions. Similar to FDE, bullous SLE can involve sun-exposed and nonexposed areas of the skin as well as the mucous membranes with a predilection for the lower vermilion lip.17 In bullous SLE, tense subepidermal blisters with a neutrophil-rich infiltrate form due to circulating antibodies to type VII collagen. These blisters have an erythematous or urticated base, most commonly on the face, upper trunk, and proximal extremities.18 In both SLE with skin manifestations and lupus limited to the skin, bullae may form due to extensive vacuolar degeneration. Similar to TEN, they can form rapidly in a widespread distribution.17 However, there is limited mucosal involvement, no clear drug association, and a better prognosis. Bullae caused by lupus will frequently demonstrate deposition of immunoproteins IgG, IgM, IgA, and complement component 3 at the basement membrane zone in perilesional skin on direct immunofluorescence. However, negative direct immunofluorescence does not rule out lupus.12 At the same time, patients with lupus frequently have comorbidities requiring multiple medications; the need for these medications may predispose patients to higher rates of cutaneous drug eruptions.19 To our knowledge, there is no known association between FDE and lupus.
Conclusion
Patients with acute eruptions following the initiation of a new prescription or over-the-counter medication require urgent evaluation. Generalized bullous FDE requires timely diagnosis and intervention. Patients with lupus have an increased risk for cutaneous drug eruptions due to polypharmacy. Further investigation is necessary to determine if there is a pathophysiologic mechanism responsible for the development of FDE in lupus patients.
- Anderson HJ, Lee JB. A review of fixed drug eruption with a special focus on generalized bullous fixed drug eruption. Medicina (Kaunas). 2021;57:925.
- Gavin M, Sharp L, Walker K, et al. Contrast-induced generalized bullous fixed drug eruption resembling Stevens-Johnson syndrome. Proc (Bayl Univ Med Cent). 2019;32:601-602.
- Kabir S, Feit EJ, Heilman ER. Generalized fixed drug eruption following Pfizer-BioNtech COVID-19 vaccination. Clin Case Rep. 2022;10:E6684.
- Choi S, Kim SH, Hwang JH, et al. Rapidly progressing generalized bullous fixed drug eruption after the first dose of COVID-19 messenger RNA vaccination. J Dermatol. 2023;50:1190-1193.
- Mahboob A, Haroon TS. Drugs causing fixed eruptions: a study of 450 cases. Int J Dermatol. 1998;37:833-838.
- Shiohara T. Fixed drug eruption: pathogenesis and diagnostic tests. Curr Opin Allergy Clin Immunol. 2009;9:316-321.
- Mizukawa Y, Yamazaki Y, Shiohara T. In vivo dynamics of intraepidermal CD8+ T cells and CD4+ T cells during the evolution of fixed drug eruption. Br J Dermatol. 2008;158:1230-1238.
- Lee CH, Chen YC, Cho YT, et al. Fixed-drug eruption: a retrospective study in a single referral center in northern Taiwan. Dermatologica Sinica. 2012;30:11-15.
- Cho YT, Lin JW, Chen YC, et al. Generalized bullous fixed drug eruption is distinct from Stevens-Johnson syndrome/toxic epidermal necrolysis by immunohistopathological features. J Am Acad Dermatol. 2014;70:539-548.
- Lipowicz S, Sekula P, Ingen-Housz-Oro S, et al. Prognosis of generalized bullous fixed drug eruption: comparison with Stevens-Johnson syndrome and toxic epidermal necrolysis. Br J Dermatol. 2013;168:726-732.
- Patel S, John AM, Handler MZ, et al. Fixed drug eruptions: an update, emphasizing the potentially lethal generalized bullous fixed drug eruption. Am J Clin Dermatol. 2020;21:393-399.
- Ranario JS, Smith JL. Bullous lesions in a patient with systemic lupus erythematosus. J Clin Aesthet Dermatol. 2014;7:44-49.
- Perron E, Viarnaud A, Marciano L, et al. Clinical and histological features of fixed drug eruption: a single-centre series of 73 cases with comparison between bullous and non-bullous forms. Eur J Dermatol. 2021;31:372-380.
- Chen CB, Kuo KL, Wang CW, et al. Detecting lesional granulysin levels for rapid diagnosis of cytotoxic T lymphocyte-mediated bullous skin disorders. J Allergy Clin Immunol Pract. 2021;9:1327-1337.e3.
- Beniwal R, Gupta LK, Khare AK, et al. Cyclosporine in generalized bullous-fixed drug eruption. Indian J Dermatol. 2018;63:432-433.
- Vargas Mora P, García S, Valenzuela F, et al. Generalized bullous fixed drug eruption successfully treated with cyclosporine. Dermatol Ther. 2020;33:E13492.
- Montagnon CM, Tolkachjov SN, Murrell DF, et al. Subepithelial autoimmune blistering dermatoses: clinical features and diagnosis. J Am Acad Dermatol. 2021;85:1-14.
- Sebaratnam DF, Murrell DF. Bullous systemic lupus erythematosus. Dermatol Clin. 2011;29:649-653.
- Zonzits E, Aberer W, Tappeiner G. Drug eruptions from mesna. After cyclophosphamide treatment of patients with systemic lupus erythematosus and dermatomyositis. Arch Dermatol. 1992;128:80-82.
- Anderson HJ, Lee JB. A review of fixed drug eruption with a special focus on generalized bullous fixed drug eruption. Medicina (Kaunas). 2021;57:925.
- Gavin M, Sharp L, Walker K, et al. Contrast-induced generalized bullous fixed drug eruption resembling Stevens-Johnson syndrome. Proc (Bayl Univ Med Cent). 2019;32:601-602.
- Kabir S, Feit EJ, Heilman ER. Generalized fixed drug eruption following Pfizer-BioNtech COVID-19 vaccination. Clin Case Rep. 2022;10:E6684.
- Choi S, Kim SH, Hwang JH, et al. Rapidly progressing generalized bullous fixed drug eruption after the first dose of COVID-19 messenger RNA vaccination. J Dermatol. 2023;50:1190-1193.
- Mahboob A, Haroon TS. Drugs causing fixed eruptions: a study of 450 cases. Int J Dermatol. 1998;37:833-838.
- Shiohara T. Fixed drug eruption: pathogenesis and diagnostic tests. Curr Opin Allergy Clin Immunol. 2009;9:316-321.
- Mizukawa Y, Yamazaki Y, Shiohara T. In vivo dynamics of intraepidermal CD8+ T cells and CD4+ T cells during the evolution of fixed drug eruption. Br J Dermatol. 2008;158:1230-1238.
- Lee CH, Chen YC, Cho YT, et al. Fixed-drug eruption: a retrospective study in a single referral center in northern Taiwan. Dermatologica Sinica. 2012;30:11-15.
- Cho YT, Lin JW, Chen YC, et al. Generalized bullous fixed drug eruption is distinct from Stevens-Johnson syndrome/toxic epidermal necrolysis by immunohistopathological features. J Am Acad Dermatol. 2014;70:539-548.
- Lipowicz S, Sekula P, Ingen-Housz-Oro S, et al. Prognosis of generalized bullous fixed drug eruption: comparison with Stevens-Johnson syndrome and toxic epidermal necrolysis. Br J Dermatol. 2013;168:726-732.
- Patel S, John AM, Handler MZ, et al. Fixed drug eruptions: an update, emphasizing the potentially lethal generalized bullous fixed drug eruption. Am J Clin Dermatol. 2020;21:393-399.
- Ranario JS, Smith JL. Bullous lesions in a patient with systemic lupus erythematosus. J Clin Aesthet Dermatol. 2014;7:44-49.
- Perron E, Viarnaud A, Marciano L, et al. Clinical and histological features of fixed drug eruption: a single-centre series of 73 cases with comparison between bullous and non-bullous forms. Eur J Dermatol. 2021;31:372-380.
- Chen CB, Kuo KL, Wang CW, et al. Detecting lesional granulysin levels for rapid diagnosis of cytotoxic T lymphocyte-mediated bullous skin disorders. J Allergy Clin Immunol Pract. 2021;9:1327-1337.e3.
- Beniwal R, Gupta LK, Khare AK, et al. Cyclosporine in generalized bullous-fixed drug eruption. Indian J Dermatol. 2018;63:432-433.
- Vargas Mora P, García S, Valenzuela F, et al. Generalized bullous fixed drug eruption successfully treated with cyclosporine. Dermatol Ther. 2020;33:E13492.
- Montagnon CM, Tolkachjov SN, Murrell DF, et al. Subepithelial autoimmune blistering dermatoses: clinical features and diagnosis. J Am Acad Dermatol. 2021;85:1-14.
- Sebaratnam DF, Murrell DF. Bullous systemic lupus erythematosus. Dermatol Clin. 2011;29:649-653.
- Zonzits E, Aberer W, Tappeiner G. Drug eruptions from mesna. After cyclophosphamide treatment of patients with systemic lupus erythematosus and dermatomyositis. Arch Dermatol. 1992;128:80-82.
Practice Points
- Although localized fixed drug eruption (FDE) is a relatively benign diagnosis, generalized bullous FDE requires urgent management and may necessitate intensive burn care.
- Patients with lupus are at increased risk for drug eruptions due to polypharmacy, and there is a wide differential for bullous eruptions in these patients.