User login
Most GI Service Chiefs Support POCUS Training, But Uptake Is Slow
, according to a national survey.
Low POCUS uptake may be explained by substantial barriers to implementation, including lack of trained instructors, necessary equipment, and support staff, lead author Keerthi Thallapureddy, MD, of the University of Texas Health San Antonio, and colleagues, reported.
“POCUS is being increasingly used by gastroenterologists due to its portability and real-time diagnostic ability,” the investigators wrote in Gastro Hep Advances, but “there is limited understanding of how gastroenterologists use POCUS.”
To learn more, the investigators conducted a nationwide survey of the VA healthcare system. Separate questionnaires were sent to chiefs of staff (n = 130) and GI service chiefs (n = 117), yielding response rates of 100% and 79%, respectively.
Respondents represented a wide distribution of geographic regions and institutional complexity levels, with 80% of GI groups based at high-complexity centers and 92% in urban locations. A minority (8%) reported the presence of a liver transplant program.
Data collection focused on the prevalence of POCUS use, types of clinical applications, institutional policies and training processes, and perceived or actual barriers to wider adoption. Barriers were sorted into three categories: training, equipment, and infrastructure.
Of the 93 GI service chiefs who participated in the survey, 44% reported that at least 1 gastroenterologist at their facility currently uses POCUS. Most common procedural uses were paracentesis (23%) and liver biopsy (13%), while ascites assessment (19%) and biliary visualization (7%) were the most common diagnostic uses.
Among the same respondents, 69% said they would support sending clinicians to a POCUS training course, and 37% said their teams had expressed an active interest in pursuing such training. Only 17% of facilities had a formal process in place to obtain POCUS training, and an equal proportion had implemented a facility-wide policy to guide its use.
Barriers to implementation were widespread and often multifactorial.
Most challenges related to training: 48% of sites reported a lack of trained providers, 28% cited insufficient funding for training, 24% noted a lack of training opportunities, and 14% reported difficulty securing travel funds.
Equipment limitations were also common, with 41% of sites lacking ultrasound machines and 27% lacking funding to purchase them.
Institutional infrastructure posed further hurdles. Nearly a quarter of GI chiefs (23%) reported lacking a clinician champion to lead implementation, while others cited a lack of support staff, simulation space, privileging criteria, image archiving capabilities, or standardized reporting forms.
“Our findings on current POCUS use, training, barriers, and infrastructure can guide expansion of POCUS use and training among GI groups,” Dr. Thallapureddy and colleagues wrote, noting that early efforts to expand access to GI-specific POCUS training are already underway.
They cited growing interest from national organizations such as the American Gastroenterological Association and the American Association for the Study of Liver Diseases, the latter of which piloted training workshops at the 2024 Liver Meeting. Similarly, the International Bowel Ultrasound Group now offers a 3-part certification program in intestinal ultrasound and is developing additional online and interactive modules to improve training accessibility.
The study was supported by the US Department of Veterans Affairs, Quality Enhancement Research Initiative Partnered Evaluation Initiative Grant, and the VA National Center for Patient Safety. The investigators reported no conflicts of interest.
, according to a national survey.
Low POCUS uptake may be explained by substantial barriers to implementation, including lack of trained instructors, necessary equipment, and support staff, lead author Keerthi Thallapureddy, MD, of the University of Texas Health San Antonio, and colleagues, reported.
“POCUS is being increasingly used by gastroenterologists due to its portability and real-time diagnostic ability,” the investigators wrote in Gastro Hep Advances, but “there is limited understanding of how gastroenterologists use POCUS.”
To learn more, the investigators conducted a nationwide survey of the VA healthcare system. Separate questionnaires were sent to chiefs of staff (n = 130) and GI service chiefs (n = 117), yielding response rates of 100% and 79%, respectively.
Respondents represented a wide distribution of geographic regions and institutional complexity levels, with 80% of GI groups based at high-complexity centers and 92% in urban locations. A minority (8%) reported the presence of a liver transplant program.
Data collection focused on the prevalence of POCUS use, types of clinical applications, institutional policies and training processes, and perceived or actual barriers to wider adoption. Barriers were sorted into three categories: training, equipment, and infrastructure.
Of the 93 GI service chiefs who participated in the survey, 44% reported that at least 1 gastroenterologist at their facility currently uses POCUS. Most common procedural uses were paracentesis (23%) and liver biopsy (13%), while ascites assessment (19%) and biliary visualization (7%) were the most common diagnostic uses.
Among the same respondents, 69% said they would support sending clinicians to a POCUS training course, and 37% said their teams had expressed an active interest in pursuing such training. Only 17% of facilities had a formal process in place to obtain POCUS training, and an equal proportion had implemented a facility-wide policy to guide its use.
Barriers to implementation were widespread and often multifactorial.
Most challenges related to training: 48% of sites reported a lack of trained providers, 28% cited insufficient funding for training, 24% noted a lack of training opportunities, and 14% reported difficulty securing travel funds.
Equipment limitations were also common, with 41% of sites lacking ultrasound machines and 27% lacking funding to purchase them.
Institutional infrastructure posed further hurdles. Nearly a quarter of GI chiefs (23%) reported lacking a clinician champion to lead implementation, while others cited a lack of support staff, simulation space, privileging criteria, image archiving capabilities, or standardized reporting forms.
“Our findings on current POCUS use, training, barriers, and infrastructure can guide expansion of POCUS use and training among GI groups,” Dr. Thallapureddy and colleagues wrote, noting that early efforts to expand access to GI-specific POCUS training are already underway.
They cited growing interest from national organizations such as the American Gastroenterological Association and the American Association for the Study of Liver Diseases, the latter of which piloted training workshops at the 2024 Liver Meeting. Similarly, the International Bowel Ultrasound Group now offers a 3-part certification program in intestinal ultrasound and is developing additional online and interactive modules to improve training accessibility.
The study was supported by the US Department of Veterans Affairs, Quality Enhancement Research Initiative Partnered Evaluation Initiative Grant, and the VA National Center for Patient Safety. The investigators reported no conflicts of interest.
, according to a national survey.
Low POCUS uptake may be explained by substantial barriers to implementation, including lack of trained instructors, necessary equipment, and support staff, lead author Keerthi Thallapureddy, MD, of the University of Texas Health San Antonio, and colleagues, reported.
“POCUS is being increasingly used by gastroenterologists due to its portability and real-time diagnostic ability,” the investigators wrote in Gastro Hep Advances, but “there is limited understanding of how gastroenterologists use POCUS.”
To learn more, the investigators conducted a nationwide survey of the VA healthcare system. Separate questionnaires were sent to chiefs of staff (n = 130) and GI service chiefs (n = 117), yielding response rates of 100% and 79%, respectively.
Respondents represented a wide distribution of geographic regions and institutional complexity levels, with 80% of GI groups based at high-complexity centers and 92% in urban locations. A minority (8%) reported the presence of a liver transplant program.
Data collection focused on the prevalence of POCUS use, types of clinical applications, institutional policies and training processes, and perceived or actual barriers to wider adoption. Barriers were sorted into three categories: training, equipment, and infrastructure.
Of the 93 GI service chiefs who participated in the survey, 44% reported that at least 1 gastroenterologist at their facility currently uses POCUS. Most common procedural uses were paracentesis (23%) and liver biopsy (13%), while ascites assessment (19%) and biliary visualization (7%) were the most common diagnostic uses.
Among the same respondents, 69% said they would support sending clinicians to a POCUS training course, and 37% said their teams had expressed an active interest in pursuing such training. Only 17% of facilities had a formal process in place to obtain POCUS training, and an equal proportion had implemented a facility-wide policy to guide its use.
Barriers to implementation were widespread and often multifactorial.
Most challenges related to training: 48% of sites reported a lack of trained providers, 28% cited insufficient funding for training, 24% noted a lack of training opportunities, and 14% reported difficulty securing travel funds.
Equipment limitations were also common, with 41% of sites lacking ultrasound machines and 27% lacking funding to purchase them.
Institutional infrastructure posed further hurdles. Nearly a quarter of GI chiefs (23%) reported lacking a clinician champion to lead implementation, while others cited a lack of support staff, simulation space, privileging criteria, image archiving capabilities, or standardized reporting forms.
“Our findings on current POCUS use, training, barriers, and infrastructure can guide expansion of POCUS use and training among GI groups,” Dr. Thallapureddy and colleagues wrote, noting that early efforts to expand access to GI-specific POCUS training are already underway.
They cited growing interest from national organizations such as the American Gastroenterological Association and the American Association for the Study of Liver Diseases, the latter of which piloted training workshops at the 2024 Liver Meeting. Similarly, the International Bowel Ultrasound Group now offers a 3-part certification program in intestinal ultrasound and is developing additional online and interactive modules to improve training accessibility.
The study was supported by the US Department of Veterans Affairs, Quality Enhancement Research Initiative Partnered Evaluation Initiative Grant, and the VA National Center for Patient Safety. The investigators reported no conflicts of interest.
FROM GASTRO HEP ADVANCES
Profound Hypoxemia in a Patient With Hypertriglyceridemia-Induced Pancreatitis
Profound Hypoxemia in a Patient With Hypertriglyceridemia-Induced Pancreatitis
Acute pancreatitis can be associated with multiorgan system failure, including respiratory failure, which has a high mortality rate. Acute respiratory distress syndrome (ARDS) is a known complication of severe, acute pancreatitis, and is fatal in up to 40% of cases. Mortality rates exceed 80% in patients with PaO2/FiO2 < 100 mm Hg.2 Although ARDS is typically associated with bilateral pulmonary infiltrates, severe hypoxemia in pancreatitis may not be visible in radiography in up to 50% of cases.1
Hypertriglyceridemia is the third-most common cause of acute pancreatitis, with an incidence of 2% to 10% among patients diagnosed with acute pancreatitis.3.4 Elevated serum triglycerides have been proposed to trigger acute pancreatitis by increasing plasma viscosity, which leads to ischemia and inflammation of the pancreas.4 In severe cases of hypertriglyceridemia-induced acute pancreatitis, plasmapheresis is used to rapidly reduce serum chylomicron and triglyceride levels.3
This case report discusses a patient with acute pancreatitis whose hypoxemia coincided with the severity of hypertriglyceridemia, but without radiographic evidence of pulmonary infiltrates or other known pulmonary causes.
Case Presentation
A 60-year-old male presented to the emergency department with several hours of diffuse abdominal pain, nausea, and vomiting. The patient reported that his symptoms began after eating fried chicken. He reported no dyspnea, fever, chills, or other symptoms. His medical history included type 2 diabetes (hemoglobin A1c, 11.1%), Hashimoto hypothyroidism, severe obstructive sleep apnea not on continuous positive airway pressure (apnea-hypoxia index, 59/h), and obesity (body mass index, 52). Initial vital signs were afebrile, heart rate of 90 beats/min, and oxygen saturation (SpO2) of 85% on 6L oxygen via nasal cannula. He was admitted to the intensive care unit and quickly maximized on high flow nasal cannula, ultimately requiring endotracheal intubation and mechanical ventilation.
Initial laboratory studies were remarkable for serum sodium of 120 mmol/L (reference range, 136-146 mmol/L), creatinine of 1.65 mg/dL (reference range, 0.52-1.28 mg/dL), anion gap of 18 mEq/L (reference range, 3-11 mEq/L), lipase level of 1115 U/L (reference range, 11-82 U/L), glucose level of 334 mg/dL (reference range, 70-110 mg/dL), white blood count of 13.1 K/uL (reference range, 4.5-11.0 K/uL), lactate level of 3.8 mmol/L (reference range, 0.5-2.2 mmol/L), triglyceride level of 1605 mg/dL (reference range, 40-160 mg/dL), cholesterol level of 565 mg/dL (reference range, < 200 mg/dL), aminotransferase of 21 U/L (reference range, 13-36 U/L), alanine aminotransferase of < 3 U/L (reference range, 7-45 U/L), and total bilirubin level of 1.6 mg/dL (reference range, 0.2-1 mg/dL).
The patient had an initial arterial blood gas pH of 7.26, partial pressure of CO2 and O2 of 64.1 mm Hg and 74.1 mm Hg, respectively, on volume control with a tidal volume of 500 mL, positive end-expiratory pressure of 10 cm H2O, respiratory rate of 26 breaths/min, and FiO2 was 100%, which yielded a PaO2/FiO2 of 74 mm Hg. The patient was maintained in steep reverse-Trendelenburg position with moderate improvement in his SpO2.
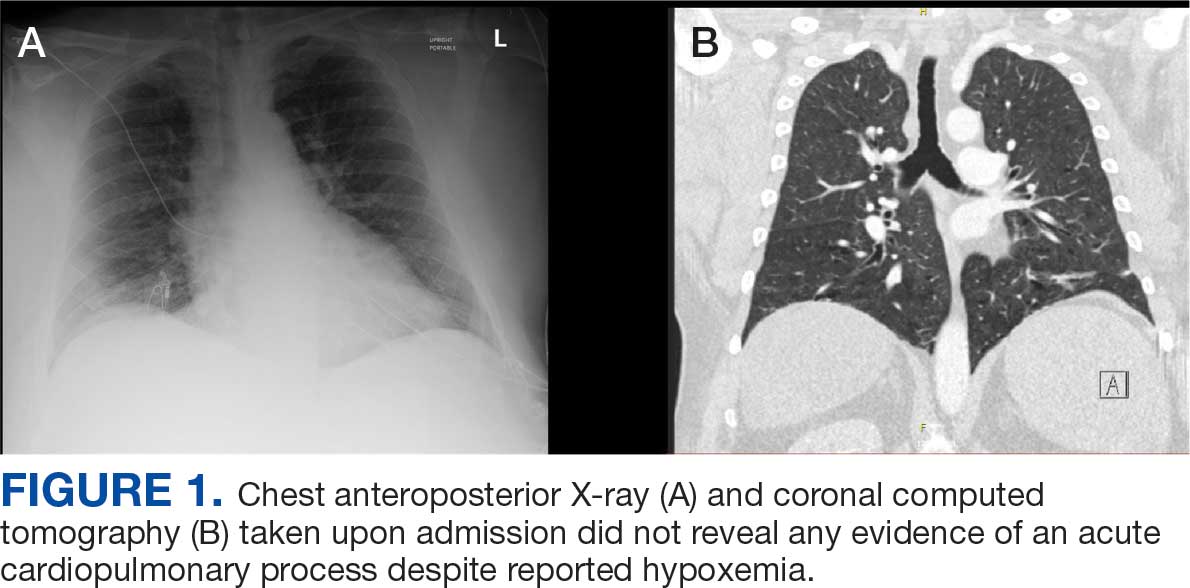
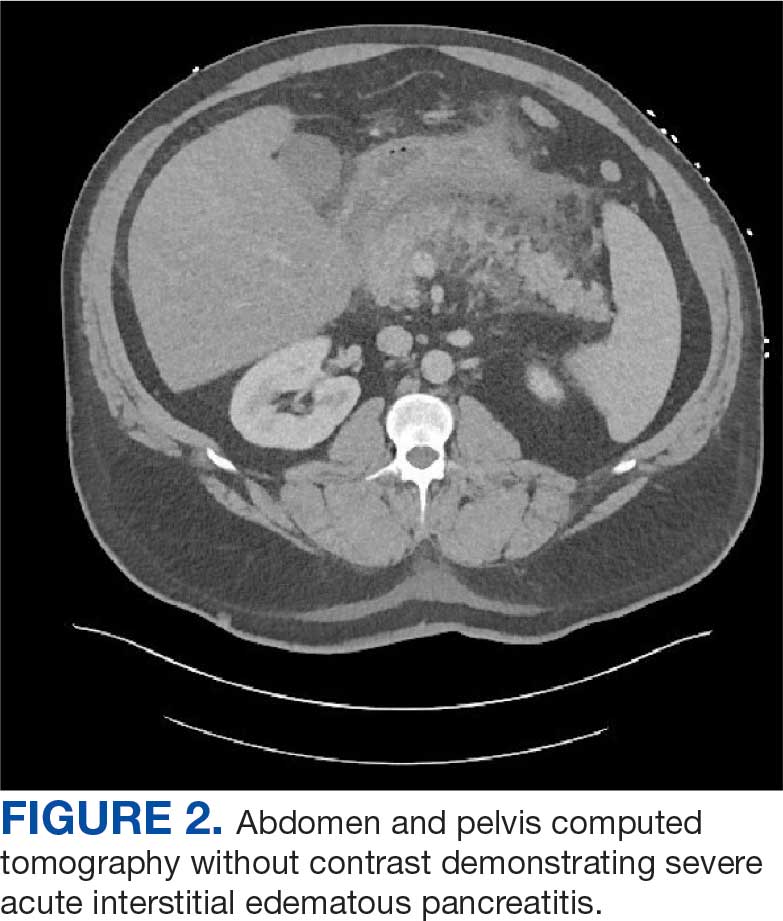
Chest X-ray and computed tomography angiogram did not reveal pleural effusions, pulmonary infiltrates, or pulmonary embolism (Figure 1). Computed tomography of the abdomen and pelvis demonstrated severe acute interstitial edematous pancreatitis with no evidence of pancreatic necrosis or evidence of gallstones (Figure 2). A transthoracic echocardiogram with bubble was negative for intracardiac right to left shunting.
The leading diagnosis was ARDS secondary to acute pancreatitis with hypoxemia exacerbated by morbid obesity and untreated obstructive sleep apnea leading to hypoventilation.
Treatment
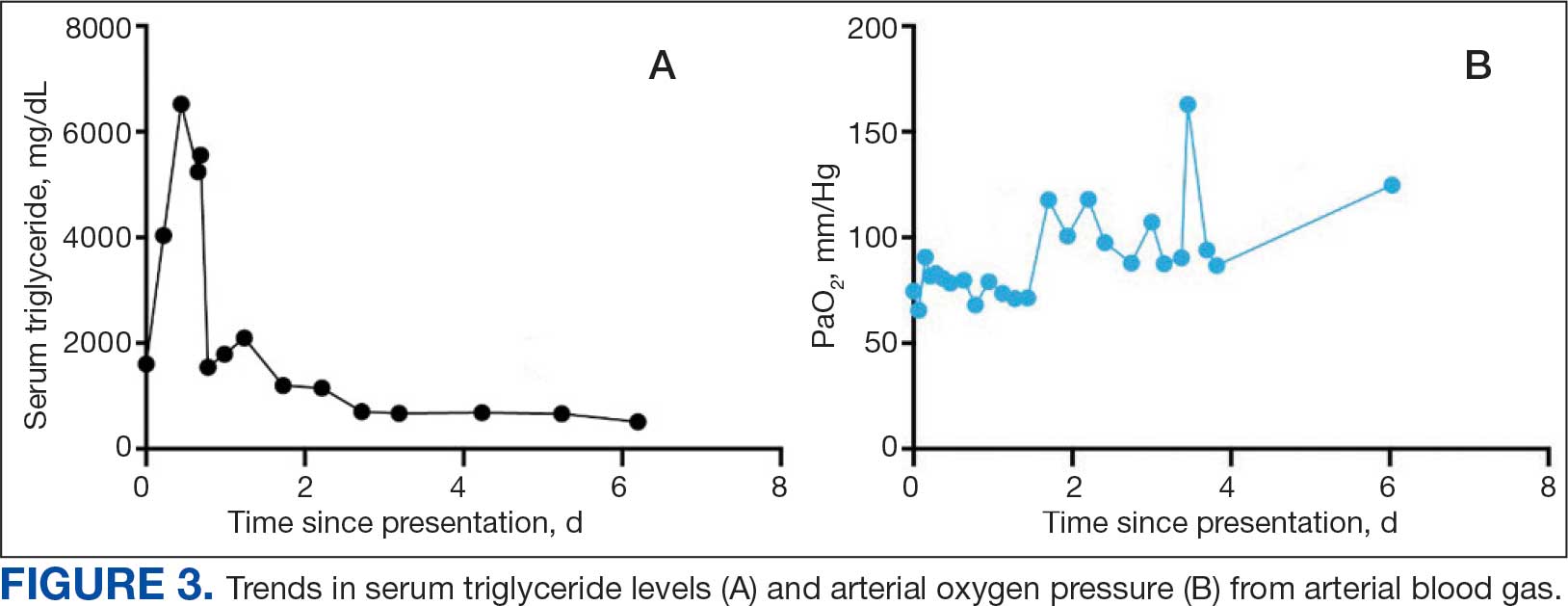
The patient was intubated and restricted to nothing by mouth and provided fluid resuscitation with crystalloids. On hospital day 1, he remained intubated and on mechanical ventilation, started on plasmapheresis and continued insulin infusion for severe hypertriglyceridemia. The patient’s PaO2/FiO2 ratio remained persistently < 100 mm Hg despite maximal ventilatory support. After 3 sessions of plasmapheresis, the serum triglyceride levels and oxygen requirements improved (Figure 3).
Due to prolonged intubation, the patient ultimately required a tracheostomy. By hospital day 48, the patient was successfully weaned off mechanical ventilation. His tracheostomy was decannulated uneventfully on hospital day 55 and the stoma was closed. The patient was discharged to a skilled nursing home for rehabilitation and received intensive physical therapy for deconditioning from prolonged hospitalization.
Discussion
Respiratory insufficiency is a common and potentially lethal complication observed in one-third of patients with acute pancreatitis.1 Radiographic evidence of pleural effusions, atelectasis and pulmonary infiltrates are often present. Acute lung injury (ALI) and ARDS are the most severe pulmonary complications of acute pancreatitis.5 It has been proposed that ALI and ARDS are driven by a hyperinflammatory state, which has multiple downstream effects. Pulmonary parenchymal and vascular damage has been associated with activated proinflammatory cytokines, trypsin, phospholipase A, and free fatty acids (Figure 4).1
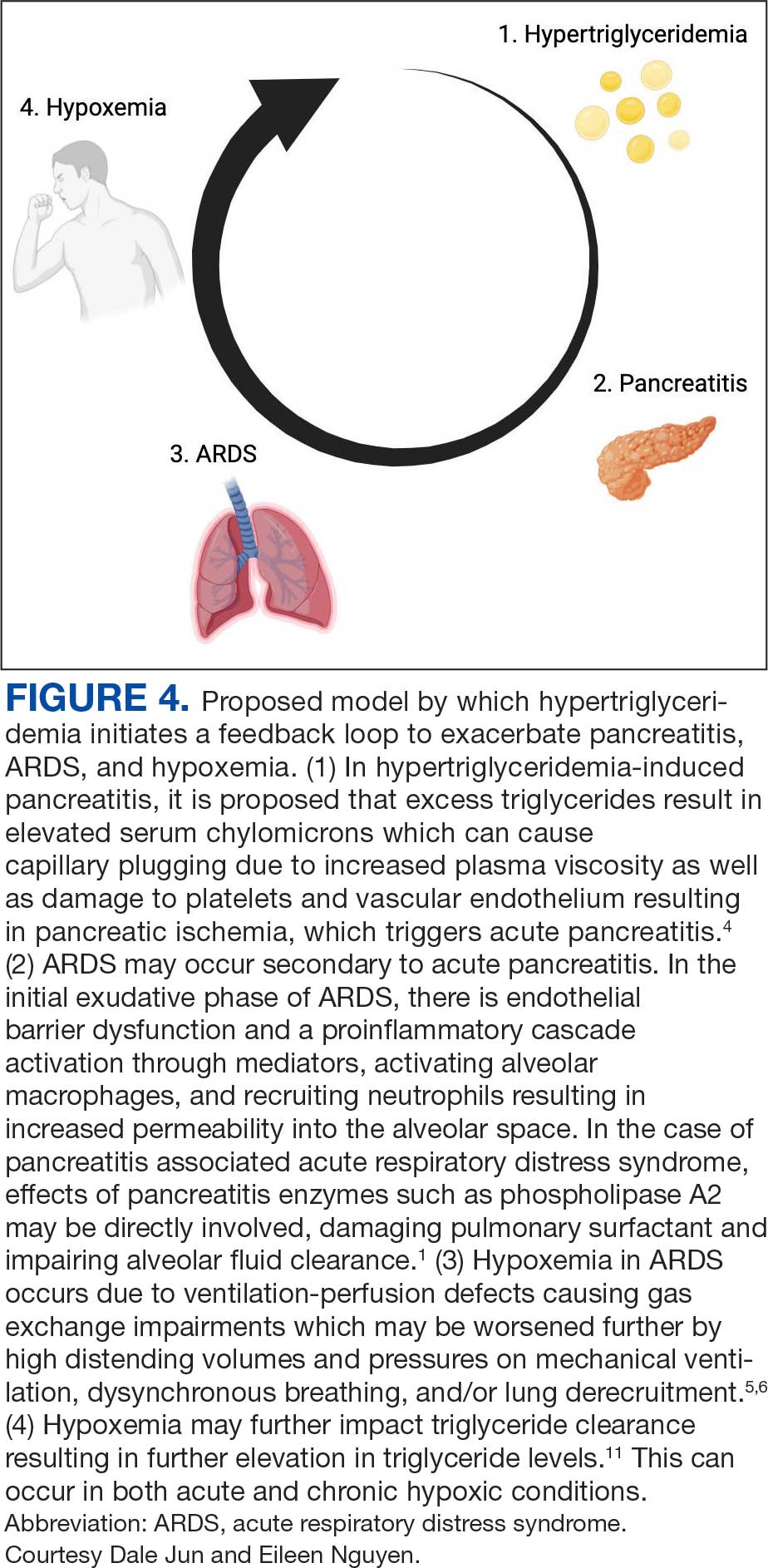
Hypoxemia secondary to acute pancreatitis may occur without initial radiographic findings and has been observed in up to half of patients.1 Hypoxemia in ARDS occurs due to ventilation-perfusion defects causing gas exchange impairments which may be worsened further by high distending volumes and pressures on mechanical ventilation, dyssynchronous breathing, and/or lung derecruitment.6 Patients who require intubation for pancreatitis-associated ALI or ARDS eventually exhibit imaging findings consistent with their disease.1 The patient in this case exhibited severe hypoxemia for several days despite persistently negative radiographic studies. His history of obstructive sleep apnea and a body mass index of 52 may have contributed to respiratory failure; however, assessment of other contributors to the acute and profound hypoxemia yielded largely unremarkable results. The patient did not have a history or evidence of heart failure and his hypoxemia did not improve with diuresis. He tested positive for COVID-19 on admission and was briefly treated with remdesivir and dexamethasone, but it was determined that the test was likely a false positive due to negative subsequent tests and elevated cycle thresholds (> 40). A concomitant COVID-19 infection likely did not contribute to his symptoms.
Ventilation-perfusion mismatch is a well-recognized complication of pancreatitis, which results in right-to-left shunting.5 While we considered whether an intracardiac shunt may have contributed to the patient’s hypoxemia, a transthoracic echocardiogram with bubble contrast was negative.
The patient had a peak serum triglyceride of > 6000 mg/dl, which meets the criteria for very severe hypertriglyceridemia.7 As observed in prior reports, the extent of the hypertriglyceridemia in this patient resulted in pronounced lipemic blood, which was appreciable by the eye and necessitated several rounds of centrifugation to analyze the laboratory studies.8 In this case, plasmapheresis was used to rapidly treat the hypertriglyceridemia, thereby reducing inflammation and further damage to the pancreas.9
It is possible the patient’s hypertriglyceridemia may have been associated with his hypoxemia. His hypoxemia was most pronounced approximately 24 hours postadmission, which coincided with the peak of the hypertriglyceridemia. It remains unclear whether the severity of triglyceride elevation could accurately predict the severity of respiratory insufficiency. Hypoxemia is thought to modulate triglyceride metabolism through stimulation of intracellular lipolysis, upregulation of very low-density lipoproteins production in the liver, and inhibition of triglyceride-rich lipoprotein metabolism.10 Evidence from rodent studies supports the idea that acute hypoxemia increases triglycerides, and the degree of hypoxemia correlates with the elevated triglyceride levels.11 However, this has not been consistently observed in humans and may vary by prandial state.12,13 Thus, dysfunction of lipid metabolism may be a relevant clinical indicator of hypoxemia; further work is needed to elucidate this association.
Patient Perspective
The patient continues to undergo extensive rehabilitation following his prolonged illness and hospitalization. He expressed gratitude for the care received. However, he has limited and distorted recollection of the events during his hospitalization and stated that it felt “like an extraterrestrial state.”
Conclusions
This report describes a case of marked hypoxemia in the setting of acute pancreatitis. Pulmonary insufficiency in acute pancreatitis is commonly associated with imaging findings such as atelectasis, pleural effusions, and pulmonary infiltrates; however, up to half of cases initially lack any radiographic findings. Plasmapheresis is an effective treatment for hypertriglyceridemia-induced pancreatitis to both directly reduce circulating triglycerides and inflammation. Plasmapheresis also represents a promising therapy for the prevention of further episodes of pancreatitis in patients with recurrent pancreatitis. We propose a feedback mechanism through which pancreatitis induces severe hypoxemia, which may modulate lipid metabolism and severe hypertriglyceridemia correlates with respiratory failure.
- Zhou M-T, Chen C-S, Chen B-C, Zhang Q-Y, Andersson R. Acute lung injury and ARDS in acute pancreatitis: mechanisms and potential intervention. World J Gastroenterol. 2010;16(17):2094-2099. doi:10.3748/wjg.v16.i17.2094
- Peek GJ, White S, Scott AD, et al. Severe acute respiratory distress syndrome secondary to acute pancreatitis successfully treated with extracorporeal membrane oxygenation in three patients. Ann Surg. 1998;227(4):572-574. doi:10.1097/00000658-199804000-00020
- Searles GE, Ooi TC. Underrecognition of chylomicronemia as a cause of acute pancreatitis. Can Med Assoc J. 1992;147(12):1806-1808.
- de Pretis N, Amodio A, Frulloni L. Hypertriglyceridemic pancreatitis: Epidemiology, pathophysiology and clinical management. United European Gastroenterol J. 2018;6(5):649-655. doi:10.1177/2050640618755002
- Ranson JH, Turner JW, Roses DF, et al. Respiratory compli cations in acute pancreatitis. Ann Surg. 1974;179(5):557-566. doi:10.1097/00000658-197405000-00006 6. Swenson KE, Swenson ER. Pathophysiology of acute respiratory distress syndrome and COVID-19 lung injury. Crit Care Clin. 2021;37(4):749-776. doi:10.1016/j.ccc.2021.05.003
- Swenson KE, Swenson ER. Pathophysiology of acute respiratory distress syndrome and COVID- 19 lung injury. Crit Care Clin. 2021;37(4):749-776. doi:10.1016/j.ccc.2021.05.003
- Berglund L, Brunzell JD, Goldberg AC, et al. Evaluation and treatment of hypertriglyceridemia: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2012;97(9):2969-2989. doi:10.1210/jc.2011-3213
- Ahern BJ, Yi HJ, Somma CL. Hypertriglyceridemia-induced pancreatitis and a lipemic blood sample: a case report and brief clinical review. J Emerg Nurs. 2022;48(4):455-459. doi:10.1016/j.jen.2022.02.001
- Garg R, Rustagi T. Management of hypertriglyceridemia induced acute pancreatitis. Biomed Res Int. 2018;2018:4721357. doi:10.1155/2018/4721357
- Morin R, Goulet N, Mauger J-F, Imbeault P. Physiological responses to hypoxia on triglyceride levels. Front Physiol. 2021;12:730935. doi:10.3389/fphys.2021.730935
- Jun JC, Shin M-K, Yao Q, et al. Acute hypoxia induces hypertriglyceridemia by decreasing plasma triglyceride clearance in mice. Am J Physiol Endocrinol Metab. 2012;303(3):E377-88. doi:10.1152/ajpendo.00641.2011
- Mahat B, Chassé É, Lindon C, Mauger J-F, Imbeault P. No effect of acute normobaric hypoxia on plasma triglyceride levels in fasting healthy men. Appl Physiol Nutr Metab. 2018;43(7):727-732. doi:10.1139/apnm-2017-0505
- Mauger J-F, Chassé É, Mahat B, Lindon C, Bordenave N, Imbeault P. The effect of acute continuous hypoxia on triglyceride levels in constantly fed healthy men. Front Physiol. 2019;10:752. doi:10.3389/fphys.2019.00752
Acute pancreatitis can be associated with multiorgan system failure, including respiratory failure, which has a high mortality rate. Acute respiratory distress syndrome (ARDS) is a known complication of severe, acute pancreatitis, and is fatal in up to 40% of cases. Mortality rates exceed 80% in patients with PaO2/FiO2 < 100 mm Hg.2 Although ARDS is typically associated with bilateral pulmonary infiltrates, severe hypoxemia in pancreatitis may not be visible in radiography in up to 50% of cases.1
Hypertriglyceridemia is the third-most common cause of acute pancreatitis, with an incidence of 2% to 10% among patients diagnosed with acute pancreatitis.3.4 Elevated serum triglycerides have been proposed to trigger acute pancreatitis by increasing plasma viscosity, which leads to ischemia and inflammation of the pancreas.4 In severe cases of hypertriglyceridemia-induced acute pancreatitis, plasmapheresis is used to rapidly reduce serum chylomicron and triglyceride levels.3
This case report discusses a patient with acute pancreatitis whose hypoxemia coincided with the severity of hypertriglyceridemia, but without radiographic evidence of pulmonary infiltrates or other known pulmonary causes.
Case Presentation
A 60-year-old male presented to the emergency department with several hours of diffuse abdominal pain, nausea, and vomiting. The patient reported that his symptoms began after eating fried chicken. He reported no dyspnea, fever, chills, or other symptoms. His medical history included type 2 diabetes (hemoglobin A1c, 11.1%), Hashimoto hypothyroidism, severe obstructive sleep apnea not on continuous positive airway pressure (apnea-hypoxia index, 59/h), and obesity (body mass index, 52). Initial vital signs were afebrile, heart rate of 90 beats/min, and oxygen saturation (SpO2) of 85% on 6L oxygen via nasal cannula. He was admitted to the intensive care unit and quickly maximized on high flow nasal cannula, ultimately requiring endotracheal intubation and mechanical ventilation.
Initial laboratory studies were remarkable for serum sodium of 120 mmol/L (reference range, 136-146 mmol/L), creatinine of 1.65 mg/dL (reference range, 0.52-1.28 mg/dL), anion gap of 18 mEq/L (reference range, 3-11 mEq/L), lipase level of 1115 U/L (reference range, 11-82 U/L), glucose level of 334 mg/dL (reference range, 70-110 mg/dL), white blood count of 13.1 K/uL (reference range, 4.5-11.0 K/uL), lactate level of 3.8 mmol/L (reference range, 0.5-2.2 mmol/L), triglyceride level of 1605 mg/dL (reference range, 40-160 mg/dL), cholesterol level of 565 mg/dL (reference range, < 200 mg/dL), aminotransferase of 21 U/L (reference range, 13-36 U/L), alanine aminotransferase of < 3 U/L (reference range, 7-45 U/L), and total bilirubin level of 1.6 mg/dL (reference range, 0.2-1 mg/dL).
The patient had an initial arterial blood gas pH of 7.26, partial pressure of CO2 and O2 of 64.1 mm Hg and 74.1 mm Hg, respectively, on volume control with a tidal volume of 500 mL, positive end-expiratory pressure of 10 cm H2O, respiratory rate of 26 breaths/min, and FiO2 was 100%, which yielded a PaO2/FiO2 of 74 mm Hg. The patient was maintained in steep reverse-Trendelenburg position with moderate improvement in his SpO2.
Chest X-ray and computed tomography angiogram did not reveal pleural effusions, pulmonary infiltrates, or pulmonary embolism (Figure 1). Computed tomography of the abdomen and pelvis demonstrated severe acute interstitial edematous pancreatitis with no evidence of pancreatic necrosis or evidence of gallstones (Figure 2). A transthoracic echocardiogram with bubble was negative for intracardiac right to left shunting.


The leading diagnosis was ARDS secondary to acute pancreatitis with hypoxemia exacerbated by morbid obesity and untreated obstructive sleep apnea leading to hypoventilation.
Treatment
The patient was intubated and restricted to nothing by mouth and provided fluid resuscitation with crystalloids. On hospital day 1, he remained intubated and on mechanical ventilation, started on plasmapheresis and continued insulin infusion for severe hypertriglyceridemia. The patient’s PaO2/FiO2 ratio remained persistently < 100 mm Hg despite maximal ventilatory support. After 3 sessions of plasmapheresis, the serum triglyceride levels and oxygen requirements improved (Figure 3).

Due to prolonged intubation, the patient ultimately required a tracheostomy. By hospital day 48, the patient was successfully weaned off mechanical ventilation. His tracheostomy was decannulated uneventfully on hospital day 55 and the stoma was closed. The patient was discharged to a skilled nursing home for rehabilitation and received intensive physical therapy for deconditioning from prolonged hospitalization.
Discussion
Respiratory insufficiency is a common and potentially lethal complication observed in one-third of patients with acute pancreatitis.1 Radiographic evidence of pleural effusions, atelectasis and pulmonary infiltrates are often present. Acute lung injury (ALI) and ARDS are the most severe pulmonary complications of acute pancreatitis.5 It has been proposed that ALI and ARDS are driven by a hyperinflammatory state, which has multiple downstream effects. Pulmonary parenchymal and vascular damage has been associated with activated proinflammatory cytokines, trypsin, phospholipase A, and free fatty acids (Figure 4).1

Hypoxemia secondary to acute pancreatitis may occur without initial radiographic findings and has been observed in up to half of patients.1 Hypoxemia in ARDS occurs due to ventilation-perfusion defects causing gas exchange impairments which may be worsened further by high distending volumes and pressures on mechanical ventilation, dyssynchronous breathing, and/or lung derecruitment.6 Patients who require intubation for pancreatitis-associated ALI or ARDS eventually exhibit imaging findings consistent with their disease.1 The patient in this case exhibited severe hypoxemia for several days despite persistently negative radiographic studies. His history of obstructive sleep apnea and a body mass index of 52 may have contributed to respiratory failure; however, assessment of other contributors to the acute and profound hypoxemia yielded largely unremarkable results. The patient did not have a history or evidence of heart failure and his hypoxemia did not improve with diuresis. He tested positive for COVID-19 on admission and was briefly treated with remdesivir and dexamethasone, but it was determined that the test was likely a false positive due to negative subsequent tests and elevated cycle thresholds (> 40). A concomitant COVID-19 infection likely did not contribute to his symptoms.
Ventilation-perfusion mismatch is a well-recognized complication of pancreatitis, which results in right-to-left shunting.5 While we considered whether an intracardiac shunt may have contributed to the patient’s hypoxemia, a transthoracic echocardiogram with bubble contrast was negative.
The patient had a peak serum triglyceride of > 6000 mg/dl, which meets the criteria for very severe hypertriglyceridemia.7 As observed in prior reports, the extent of the hypertriglyceridemia in this patient resulted in pronounced lipemic blood, which was appreciable by the eye and necessitated several rounds of centrifugation to analyze the laboratory studies.8 In this case, plasmapheresis was used to rapidly treat the hypertriglyceridemia, thereby reducing inflammation and further damage to the pancreas.9
It is possible the patient’s hypertriglyceridemia may have been associated with his hypoxemia. His hypoxemia was most pronounced approximately 24 hours postadmission, which coincided with the peak of the hypertriglyceridemia. It remains unclear whether the severity of triglyceride elevation could accurately predict the severity of respiratory insufficiency. Hypoxemia is thought to modulate triglyceride metabolism through stimulation of intracellular lipolysis, upregulation of very low-density lipoproteins production in the liver, and inhibition of triglyceride-rich lipoprotein metabolism.10 Evidence from rodent studies supports the idea that acute hypoxemia increases triglycerides, and the degree of hypoxemia correlates with the elevated triglyceride levels.11 However, this has not been consistently observed in humans and may vary by prandial state.12,13 Thus, dysfunction of lipid metabolism may be a relevant clinical indicator of hypoxemia; further work is needed to elucidate this association.
Patient Perspective
The patient continues to undergo extensive rehabilitation following his prolonged illness and hospitalization. He expressed gratitude for the care received. However, he has limited and distorted recollection of the events during his hospitalization and stated that it felt “like an extraterrestrial state.”
Conclusions
This report describes a case of marked hypoxemia in the setting of acute pancreatitis. Pulmonary insufficiency in acute pancreatitis is commonly associated with imaging findings such as atelectasis, pleural effusions, and pulmonary infiltrates; however, up to half of cases initially lack any radiographic findings. Plasmapheresis is an effective treatment for hypertriglyceridemia-induced pancreatitis to both directly reduce circulating triglycerides and inflammation. Plasmapheresis also represents a promising therapy for the prevention of further episodes of pancreatitis in patients with recurrent pancreatitis. We propose a feedback mechanism through which pancreatitis induces severe hypoxemia, which may modulate lipid metabolism and severe hypertriglyceridemia correlates with respiratory failure.
Acute pancreatitis can be associated with multiorgan system failure, including respiratory failure, which has a high mortality rate. Acute respiratory distress syndrome (ARDS) is a known complication of severe, acute pancreatitis, and is fatal in up to 40% of cases. Mortality rates exceed 80% in patients with PaO2/FiO2 < 100 mm Hg.2 Although ARDS is typically associated with bilateral pulmonary infiltrates, severe hypoxemia in pancreatitis may not be visible in radiography in up to 50% of cases.1
Hypertriglyceridemia is the third-most common cause of acute pancreatitis, with an incidence of 2% to 10% among patients diagnosed with acute pancreatitis.3.4 Elevated serum triglycerides have been proposed to trigger acute pancreatitis by increasing plasma viscosity, which leads to ischemia and inflammation of the pancreas.4 In severe cases of hypertriglyceridemia-induced acute pancreatitis, plasmapheresis is used to rapidly reduce serum chylomicron and triglyceride levels.3
This case report discusses a patient with acute pancreatitis whose hypoxemia coincided with the severity of hypertriglyceridemia, but without radiographic evidence of pulmonary infiltrates or other known pulmonary causes.
Case Presentation
A 60-year-old male presented to the emergency department with several hours of diffuse abdominal pain, nausea, and vomiting. The patient reported that his symptoms began after eating fried chicken. He reported no dyspnea, fever, chills, or other symptoms. His medical history included type 2 diabetes (hemoglobin A1c, 11.1%), Hashimoto hypothyroidism, severe obstructive sleep apnea not on continuous positive airway pressure (apnea-hypoxia index, 59/h), and obesity (body mass index, 52). Initial vital signs were afebrile, heart rate of 90 beats/min, and oxygen saturation (SpO2) of 85% on 6L oxygen via nasal cannula. He was admitted to the intensive care unit and quickly maximized on high flow nasal cannula, ultimately requiring endotracheal intubation and mechanical ventilation.
Initial laboratory studies were remarkable for serum sodium of 120 mmol/L (reference range, 136-146 mmol/L), creatinine of 1.65 mg/dL (reference range, 0.52-1.28 mg/dL), anion gap of 18 mEq/L (reference range, 3-11 mEq/L), lipase level of 1115 U/L (reference range, 11-82 U/L), glucose level of 334 mg/dL (reference range, 70-110 mg/dL), white blood count of 13.1 K/uL (reference range, 4.5-11.0 K/uL), lactate level of 3.8 mmol/L (reference range, 0.5-2.2 mmol/L), triglyceride level of 1605 mg/dL (reference range, 40-160 mg/dL), cholesterol level of 565 mg/dL (reference range, < 200 mg/dL), aminotransferase of 21 U/L (reference range, 13-36 U/L), alanine aminotransferase of < 3 U/L (reference range, 7-45 U/L), and total bilirubin level of 1.6 mg/dL (reference range, 0.2-1 mg/dL).
The patient had an initial arterial blood gas pH of 7.26, partial pressure of CO2 and O2 of 64.1 mm Hg and 74.1 mm Hg, respectively, on volume control with a tidal volume of 500 mL, positive end-expiratory pressure of 10 cm H2O, respiratory rate of 26 breaths/min, and FiO2 was 100%, which yielded a PaO2/FiO2 of 74 mm Hg. The patient was maintained in steep reverse-Trendelenburg position with moderate improvement in his SpO2.
Chest X-ray and computed tomography angiogram did not reveal pleural effusions, pulmonary infiltrates, or pulmonary embolism (Figure 1). Computed tomography of the abdomen and pelvis demonstrated severe acute interstitial edematous pancreatitis with no evidence of pancreatic necrosis or evidence of gallstones (Figure 2). A transthoracic echocardiogram with bubble was negative for intracardiac right to left shunting.


The leading diagnosis was ARDS secondary to acute pancreatitis with hypoxemia exacerbated by morbid obesity and untreated obstructive sleep apnea leading to hypoventilation.
Treatment
The patient was intubated and restricted to nothing by mouth and provided fluid resuscitation with crystalloids. On hospital day 1, he remained intubated and on mechanical ventilation, started on plasmapheresis and continued insulin infusion for severe hypertriglyceridemia. The patient’s PaO2/FiO2 ratio remained persistently < 100 mm Hg despite maximal ventilatory support. After 3 sessions of plasmapheresis, the serum triglyceride levels and oxygen requirements improved (Figure 3).

Due to prolonged intubation, the patient ultimately required a tracheostomy. By hospital day 48, the patient was successfully weaned off mechanical ventilation. His tracheostomy was decannulated uneventfully on hospital day 55 and the stoma was closed. The patient was discharged to a skilled nursing home for rehabilitation and received intensive physical therapy for deconditioning from prolonged hospitalization.
Discussion
Respiratory insufficiency is a common and potentially lethal complication observed in one-third of patients with acute pancreatitis.1 Radiographic evidence of pleural effusions, atelectasis and pulmonary infiltrates are often present. Acute lung injury (ALI) and ARDS are the most severe pulmonary complications of acute pancreatitis.5 It has been proposed that ALI and ARDS are driven by a hyperinflammatory state, which has multiple downstream effects. Pulmonary parenchymal and vascular damage has been associated with activated proinflammatory cytokines, trypsin, phospholipase A, and free fatty acids (Figure 4).1

Hypoxemia secondary to acute pancreatitis may occur without initial radiographic findings and has been observed in up to half of patients.1 Hypoxemia in ARDS occurs due to ventilation-perfusion defects causing gas exchange impairments which may be worsened further by high distending volumes and pressures on mechanical ventilation, dyssynchronous breathing, and/or lung derecruitment.6 Patients who require intubation for pancreatitis-associated ALI or ARDS eventually exhibit imaging findings consistent with their disease.1 The patient in this case exhibited severe hypoxemia for several days despite persistently negative radiographic studies. His history of obstructive sleep apnea and a body mass index of 52 may have contributed to respiratory failure; however, assessment of other contributors to the acute and profound hypoxemia yielded largely unremarkable results. The patient did not have a history or evidence of heart failure and his hypoxemia did not improve with diuresis. He tested positive for COVID-19 on admission and was briefly treated with remdesivir and dexamethasone, but it was determined that the test was likely a false positive due to negative subsequent tests and elevated cycle thresholds (> 40). A concomitant COVID-19 infection likely did not contribute to his symptoms.
Ventilation-perfusion mismatch is a well-recognized complication of pancreatitis, which results in right-to-left shunting.5 While we considered whether an intracardiac shunt may have contributed to the patient’s hypoxemia, a transthoracic echocardiogram with bubble contrast was negative.
The patient had a peak serum triglyceride of > 6000 mg/dl, which meets the criteria for very severe hypertriglyceridemia.7 As observed in prior reports, the extent of the hypertriglyceridemia in this patient resulted in pronounced lipemic blood, which was appreciable by the eye and necessitated several rounds of centrifugation to analyze the laboratory studies.8 In this case, plasmapheresis was used to rapidly treat the hypertriglyceridemia, thereby reducing inflammation and further damage to the pancreas.9
It is possible the patient’s hypertriglyceridemia may have been associated with his hypoxemia. His hypoxemia was most pronounced approximately 24 hours postadmission, which coincided with the peak of the hypertriglyceridemia. It remains unclear whether the severity of triglyceride elevation could accurately predict the severity of respiratory insufficiency. Hypoxemia is thought to modulate triglyceride metabolism through stimulation of intracellular lipolysis, upregulation of very low-density lipoproteins production in the liver, and inhibition of triglyceride-rich lipoprotein metabolism.10 Evidence from rodent studies supports the idea that acute hypoxemia increases triglycerides, and the degree of hypoxemia correlates with the elevated triglyceride levels.11 However, this has not been consistently observed in humans and may vary by prandial state.12,13 Thus, dysfunction of lipid metabolism may be a relevant clinical indicator of hypoxemia; further work is needed to elucidate this association.
Patient Perspective
The patient continues to undergo extensive rehabilitation following his prolonged illness and hospitalization. He expressed gratitude for the care received. However, he has limited and distorted recollection of the events during his hospitalization and stated that it felt “like an extraterrestrial state.”
Conclusions
This report describes a case of marked hypoxemia in the setting of acute pancreatitis. Pulmonary insufficiency in acute pancreatitis is commonly associated with imaging findings such as atelectasis, pleural effusions, and pulmonary infiltrates; however, up to half of cases initially lack any radiographic findings. Plasmapheresis is an effective treatment for hypertriglyceridemia-induced pancreatitis to both directly reduce circulating triglycerides and inflammation. Plasmapheresis also represents a promising therapy for the prevention of further episodes of pancreatitis in patients with recurrent pancreatitis. We propose a feedback mechanism through which pancreatitis induces severe hypoxemia, which may modulate lipid metabolism and severe hypertriglyceridemia correlates with respiratory failure.
- Zhou M-T, Chen C-S, Chen B-C, Zhang Q-Y, Andersson R. Acute lung injury and ARDS in acute pancreatitis: mechanisms and potential intervention. World J Gastroenterol. 2010;16(17):2094-2099. doi:10.3748/wjg.v16.i17.2094
- Peek GJ, White S, Scott AD, et al. Severe acute respiratory distress syndrome secondary to acute pancreatitis successfully treated with extracorporeal membrane oxygenation in three patients. Ann Surg. 1998;227(4):572-574. doi:10.1097/00000658-199804000-00020
- Searles GE, Ooi TC. Underrecognition of chylomicronemia as a cause of acute pancreatitis. Can Med Assoc J. 1992;147(12):1806-1808.
- de Pretis N, Amodio A, Frulloni L. Hypertriglyceridemic pancreatitis: Epidemiology, pathophysiology and clinical management. United European Gastroenterol J. 2018;6(5):649-655. doi:10.1177/2050640618755002
- Ranson JH, Turner JW, Roses DF, et al. Respiratory compli cations in acute pancreatitis. Ann Surg. 1974;179(5):557-566. doi:10.1097/00000658-197405000-00006 6. Swenson KE, Swenson ER. Pathophysiology of acute respiratory distress syndrome and COVID-19 lung injury. Crit Care Clin. 2021;37(4):749-776. doi:10.1016/j.ccc.2021.05.003
- Swenson KE, Swenson ER. Pathophysiology of acute respiratory distress syndrome and COVID- 19 lung injury. Crit Care Clin. 2021;37(4):749-776. doi:10.1016/j.ccc.2021.05.003
- Berglund L, Brunzell JD, Goldberg AC, et al. Evaluation and treatment of hypertriglyceridemia: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2012;97(9):2969-2989. doi:10.1210/jc.2011-3213
- Ahern BJ, Yi HJ, Somma CL. Hypertriglyceridemia-induced pancreatitis and a lipemic blood sample: a case report and brief clinical review. J Emerg Nurs. 2022;48(4):455-459. doi:10.1016/j.jen.2022.02.001
- Garg R, Rustagi T. Management of hypertriglyceridemia induced acute pancreatitis. Biomed Res Int. 2018;2018:4721357. doi:10.1155/2018/4721357
- Morin R, Goulet N, Mauger J-F, Imbeault P. Physiological responses to hypoxia on triglyceride levels. Front Physiol. 2021;12:730935. doi:10.3389/fphys.2021.730935
- Jun JC, Shin M-K, Yao Q, et al. Acute hypoxia induces hypertriglyceridemia by decreasing plasma triglyceride clearance in mice. Am J Physiol Endocrinol Metab. 2012;303(3):E377-88. doi:10.1152/ajpendo.00641.2011
- Mahat B, Chassé É, Lindon C, Mauger J-F, Imbeault P. No effect of acute normobaric hypoxia on plasma triglyceride levels in fasting healthy men. Appl Physiol Nutr Metab. 2018;43(7):727-732. doi:10.1139/apnm-2017-0505
- Mauger J-F, Chassé É, Mahat B, Lindon C, Bordenave N, Imbeault P. The effect of acute continuous hypoxia on triglyceride levels in constantly fed healthy men. Front Physiol. 2019;10:752. doi:10.3389/fphys.2019.00752
- Zhou M-T, Chen C-S, Chen B-C, Zhang Q-Y, Andersson R. Acute lung injury and ARDS in acute pancreatitis: mechanisms and potential intervention. World J Gastroenterol. 2010;16(17):2094-2099. doi:10.3748/wjg.v16.i17.2094
- Peek GJ, White S, Scott AD, et al. Severe acute respiratory distress syndrome secondary to acute pancreatitis successfully treated with extracorporeal membrane oxygenation in three patients. Ann Surg. 1998;227(4):572-574. doi:10.1097/00000658-199804000-00020
- Searles GE, Ooi TC. Underrecognition of chylomicronemia as a cause of acute pancreatitis. Can Med Assoc J. 1992;147(12):1806-1808.
- de Pretis N, Amodio A, Frulloni L. Hypertriglyceridemic pancreatitis: Epidemiology, pathophysiology and clinical management. United European Gastroenterol J. 2018;6(5):649-655. doi:10.1177/2050640618755002
- Ranson JH, Turner JW, Roses DF, et al. Respiratory compli cations in acute pancreatitis. Ann Surg. 1974;179(5):557-566. doi:10.1097/00000658-197405000-00006 6. Swenson KE, Swenson ER. Pathophysiology of acute respiratory distress syndrome and COVID-19 lung injury. Crit Care Clin. 2021;37(4):749-776. doi:10.1016/j.ccc.2021.05.003
- Swenson KE, Swenson ER. Pathophysiology of acute respiratory distress syndrome and COVID- 19 lung injury. Crit Care Clin. 2021;37(4):749-776. doi:10.1016/j.ccc.2021.05.003
- Berglund L, Brunzell JD, Goldberg AC, et al. Evaluation and treatment of hypertriglyceridemia: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2012;97(9):2969-2989. doi:10.1210/jc.2011-3213
- Ahern BJ, Yi HJ, Somma CL. Hypertriglyceridemia-induced pancreatitis and a lipemic blood sample: a case report and brief clinical review. J Emerg Nurs. 2022;48(4):455-459. doi:10.1016/j.jen.2022.02.001
- Garg R, Rustagi T. Management of hypertriglyceridemia induced acute pancreatitis. Biomed Res Int. 2018;2018:4721357. doi:10.1155/2018/4721357
- Morin R, Goulet N, Mauger J-F, Imbeault P. Physiological responses to hypoxia on triglyceride levels. Front Physiol. 2021;12:730935. doi:10.3389/fphys.2021.730935
- Jun JC, Shin M-K, Yao Q, et al. Acute hypoxia induces hypertriglyceridemia by decreasing plasma triglyceride clearance in mice. Am J Physiol Endocrinol Metab. 2012;303(3):E377-88. doi:10.1152/ajpendo.00641.2011
- Mahat B, Chassé É, Lindon C, Mauger J-F, Imbeault P. No effect of acute normobaric hypoxia on plasma triglyceride levels in fasting healthy men. Appl Physiol Nutr Metab. 2018;43(7):727-732. doi:10.1139/apnm-2017-0505
- Mauger J-F, Chassé É, Mahat B, Lindon C, Bordenave N, Imbeault P. The effect of acute continuous hypoxia on triglyceride levels in constantly fed healthy men. Front Physiol. 2019;10:752. doi:10.3389/fphys.2019.00752
Profound Hypoxemia in a Patient With Hypertriglyceridemia-Induced Pancreatitis
Profound Hypoxemia in a Patient With Hypertriglyceridemia-Induced Pancreatitis
An Approach to Exocrine Pancreatic Insufficiency: Considerations in Diagnosis and Treatment
Exocrine pancreatic insufficiency (EPI) is a recognized condition in patients with underlying pancreatic disease. However, it is a disease state that requires a meticulous approach to diagnose, as misdiagnosis can lead to inappropriate testing and unnecessary treatment.
EPI has been defined as “a near total decline in the quantity and/or activity of endogenous pancreatic enzymes to a level that is inadequate to maintain normal digestive capacity leading to steatorrhea.”1 It can lead to complications including malnutrition, micronutrient deficiencies, metabolic bone disease and have significant impact on quality of life. In this article,
EPI Diagnosis
EPI results from ineffective or insufficient pancreatic digestive enzyme secretion. In 2021, a group of experts from the American Gastroenterological Association (AGA) and PancreasFest met and proposed a new mechanistic definition of EPI. This suggests that EPI is the failure of sufficient pancreatic enzymes to effectively reach the intestine in order to allow for optimal digestion of ingested nutrients, leading to downstream macronutrient and micronutrient deficiencies with symptoms of maldigestion including post-prandial abdominal pain, bloating, steatorrhea, loose stools, or weight loss.2
A more pragmatic definition by Khan, et al in 2022 utilized a staging system to distinguish exocrine pancreatic dysfunction (EDP) from EPI. As such EPD occurs when there is a decline in pancreatic function without impaired digestive capacity, while EPI requires digestive capacity impairment leading to objective steatorrhea (coefficient of fat absorption <93 %).3Differential Diagnosis: There are many factors that can impact normal digestion. In approaching EPI, symptoms are often the most common reason to test for disease state in the appropriate clinical context. There can be pancreatic causes of EPI and non-pancreatic (secondary) causes of EPI (see Figure 1), though the latter can be challenging to detect.
The most common parenchymal etiologies for EPI include chronic pancreatitis, recurrent acute pancreatitis, cystic fibrosis, pancreatic cancer or prior pancreatic resections. Non-pancreatic conditions that impact synchronous mixing of endogenous pancreatic enzymes with meals (i.e., Roux-en-Y gastric bypass, short bowel syndrome, delayed gastric emptying), mucosal barriers causing decrease endogenous pancreatic stimulation despite intact parenchyma, such as celiac disease, foregut Crohn’s disease, intraluminal inactivation of pancreatic enzymes (Zollinger-Ellison syndrome), and bile salts de-conjugation with small intestinal bacterial overgrowth (SIBO) can predispose to EPI.4-6 The true prevalence of EPI is difficult to ascertain due to a variety of factors including challenges in diagnosis and misdiagnosis.
Some of the major challenges in the diagnosis and treatment of EPI is that the symptoms of EPI overlap with many other GI conditions including celiac disease, diabetes mellitus, SIBO, irritable bowel syndrome (IBS), bile acid diarrhea, and other functional GI syndromes. These non-pancreatic conditions can also be associated with falsely low FE-1. Hence, ordering FE-1 should be employed with caution when the pretest probability is low. Patients with EPI will generally have a significant response to pancreatic enzyme replacement therapy (PERT) if it is adequately dosed and a lack of response should prompt consideration of an alternative diagnosis. A framework to factors which contribute to EPI is outlined in Figure 2.
Symptoms Screening and Signs: Pancreatic enzymes output estimation is the most reliable indicator for pancreatic digestive capacity. However, EPI diagnosis requires a combination of symptoms screening, stool-based (indirect pancreatic function) testing or direct pancreatic function testing (PFT).
Although symptoms might not correlate with objective disease state, in screening for symptoms of steatorrhea or maldigestion, it is important to ask specific questions regarding bloating, abdominal pain, stool frequency, consistency, and quality. Screening questions should be specific and include question such as, “Is there oil in the toilet bowl or is the stool greasy/shiny?”, “Is the stool sticky and difficult to flush or wipe?”, “Is there malodorous flatus?” If patients screen positive for EPI symptoms and there is a high pre-test probability of EPI such as the presence of severe chronic pancreatitis or significant pancreatic resection (> 90% loss of pancreatic parenchyma), then cautious trial of PERT and assessment for treatment response can be considered without additional stool-based testing. However, this practice end points are unclear and mainly based on subjective response.
Patients with EPI are at increased risk for malnutrition and micronutrient deficiencies. While not required for the diagnosis, low levels of fat-soluble vitamins (vitamin AEDK) or other minerals (zinc, selenium, magnesium, phosphorus) can suggest issues with malabsorption. Once the diagnosis of EPI is made, micronutrient screening should occur annually.
Stool Based Testing: The gold standard clinical test for steatorrhea is measuring coefficient of fat absorption (CFA). With a normal range of 93% fat absorption, the test is performed on a 72-hours fecal fat collection kit. To ensure accurate results, a patient must adhere to a diet with a minimum of 100 grams of fat per day in the three days leading up to the test and during the duration of the test. Patients must also abstain from taking PERT during the duration of the test. This can be incredibly challenging for someone with underlying steatorrhea but can reliably distinguish between EPD and EPI.
A more commonly used stool test is fecal elastase (FE-1). While easier to perform, the test often results in many false positives and false negatives. FE-1 is an ELISA based test, which measures the concentration of the specific isoform CELA3 (chymotrypsin-like elastase family) in the stool sample. The test must be run on a solid stool sample as soft or liquid stool will dilute down elastase concentration falsely. One test advantage is that a patient can continue PERT if needed. FE-1 test measures the concentration of patients’ elastase and PERT is porcine derived. As such, there is no interaction between porcine lipase and human elastase in stool. FE-1 sensitivity and specificity are high for severe disease (<100 mcg/g) if the test is performed properly on patients with a high pretest probability. However, the sensitivity and specificity are poor in mild to moderate pancreatic disease and in the absence of known pancreatic disease.7
Our suggested approach to utilizing FE-1 test is to reserve it for patients with known severe chronic pancreatitis or prior pancreatic surgery in patients with symptoms. In patients without pancreatic disease who are at low risk of EPI, a positive FE-1 can lead to misdiagnosis, further diagnostic testing, and unnecessary treatment. Currently, there is no stool-based test that is accurate, reproducible, and reliable.
Direct Pancreatic Function testing: Secretin stimulated PFT is highly reliable in measuring ductal function with bicarbonate concentration. However, it cannot reliably estimate acinar function as both do not decline at the same rate, unless in severe pancreatic disease. A much more robust test should include cholecystokinin analog to measure pancreatic enzymes concentration. This test involves endoscopy, administration of secretin, and/or a cholecystokinin analog, and subsequent measurement of bicarbonate and digestive enzymes in the pancreatic juice. This test is not routinely offered as it is invasive, cumbersome, and difficult to repeat for reassessment of pancreatic function over time.8
Treatment
The primary goal of treatment is to improve symptoms and nutritional status of the patient. EPI treatment consists of PERT and nutritional counseling. In the United States, there are multiple FDA approved PERT preparations, which include Creon, Zenpep, Pancreaze, Pertzye, Viokase, and Relizorb. While dosing is dependent on lipase concentration, all PERT (aside from Relizorb) preparations have a combination of lipase, proteases, and amylase. All but Viokace and Relizorb are enteric-coated formulations.9
In patients with an inadequate response to enteric coated PERT, non-enteric coated PERT can be added as it may provide a more immediate effect than enteric coated formulations, specially if concern about rapid gut transit with inadequate mixing is raised. If a non-enteric formations is used, acid suppression should be added to prevent inactivation of the PERT. Relizorb is a cartridge system which delivers lipase directly to tube feeds. This cartridge is only utilized in patients receiving enteral nutrition and allows for treatment of EPI even when patients are unable to tolerate oral feeding.
PERT dosing is intended to at least compensate for 10% of the physiologically secreted amount of endogenous lipase after a normal meal (approximately 30,000 IU). Hence, dosing is primarily weight-based. In symptomatic adults, PERT dose of 500-1000 units/kg/meal and half of the amount with snacks is appropriate. Although higher doses of 1500-2000 units/kg/meal may be needed when there is significant steatorrhea, weight loss, or micronutrient deficiencies, PERT doses exceeding 2500 units/kg/meal are not recommended and warrant further investigation.10
Proper counseling is important to ensure compliance with pancreatin preparations. PERT will generally be effective in improving steatorrhea, weight loss, bowel movement frequency, and reversal of nutritional deficiencies, but it does not reliably help symptoms of bloating or abdominal pain. If a patient’s steatorrhea does not respond to PERT, then alternative diagnoses such as SIBO, or diarrhea-predominant IBS should be considered.
PERT must be taken with meals. There are studies that support split dosing as a more effective way of absorbing fat.11 If PERT is ineffective or minimally effective, review of appropriate dosing and timing of PERT to a meal is recommended. Addition of acid suppression may be required to improve treatment efficacy, especially in patients with abnormal intestinal motility or prior pancreatic surgery as PERT is effective at a pH of 4.5. Cost, pill burden, and persistence of certain symptoms may impact adherence to PERT and thus pre-treatment counseling and close follow-up after initiation is important. This aids in assessment of patients’ response to therapy, ensure appropriate PERT administration, and identifying any barriers to therapy adherence.
Nutritional management of EPI consists of an assessment of nutritional status, diet, and lifestyle. An important component of nutritional management is the assessment of micronutrient deficiencies. Patients with a confirmed diagnosis of EPI should be screened for the following micronutrients annually: Vitamins (A, E, D, K, B12), folate, zinc, selenium, magnesium, and iron. Patients with chronic pancreatitis and EPI should also be screened for metabolic bone disease once every two years and for diabetes mellitus annually.4, 12
Conclusion
EPI is a challenging diagnosis as many symptoms overlap with other GI conditions. Pancreas exocrine function is rich with significant reserve to allow for proper digestive capacity, yet EPI occurs when an individual’s pancreatic digestive enzymes are insufficient to meet their nutritional needs. In patients with high likelihood of having EPI, such as those with pre-existing pancreatic disease, diagnosing EPI combines clinical evidence based on subjective symptoms and stool-based testing to support a disease state.
Appropriate dosing and timing of PERT is critical to improve nutritional outcomes and improve certain symptoms of EPI. Failure of PERT requires evaluating for proper dosing/timing, and consideration of additional or alternative diagnosis. EPI morbidity can lead to significant impact on patients’ quality of life, but with counseling, proper PERT use, nutritional consequences can be mediated, and quality of life can improve.
Dr. Hernandez-Barco is based in the Division of Gastroenterology, Massachusetts General Hospital, Boston, Massachusetts. Dr. Ashkar is based in the Division of Gastroenterology and Hepatology, Mayo Clinic, Rochester, Minnesota. Dr. Hernandez-Barco disclosed consulting for AMGEN and served as a scientific advisor for Nestle Health Science. She had project-related funding support or conflicts of interest to disclose. Dr. Ashkar disclosed consulting for AMGEN. He had no project-related funding support or conflicts of interest to disclose.
References
1. Othman MO, et al. Introduction and practical approach to exocrine pancreatic insufficiency for the practicing clinician. Int J Clin Pract. 2018 Feb. doi: 10.1111/ijcp.13066.
2. Whitcomb DC, et al. AGA-PancreasFest Joint Symposium on Exocrine Pancreatitic Insufficiency. Gastro Hep Adv. 2022 Nov. doi: 10.1016/j.gastha.2022.11.008.
3. Khan A, et al. Staging Exocrine Pancreatic Dysfunction. Pancreatology. 2022 Jan. doi: 10.1016/j.pan.2021.11.005.
4. Whitcomb DC, et al. AGA Clinical Practice Update on the Epidemiology, Evaluation, and Management of Exocrine Pancreatitis insufficiency: Expert Review. Gastroenterology. 2023 Nov. doi: 10.1053/j.gastro.2023.07.007.
5. Kunovský L, et al. Causes of Exocrine Pancreatic Insufficiency Other than Chronic Pancreatitis. J Clin Med. 2021 Dec. doi: 10.3390/jcm10245779.
6. Singh VK, et al. Less common etiologies of exocrine pancreatic insufficiency. World J Gastroenterol. 2017 Oct. doi: 10.3748/wjg.v23.i39.7059.
7. Lankisch PG, et al. Faecal elastase 1: not helpful in diagnosing chronic pancreatitis associated with mid to moderate exocrine pancreatic insufficiency. Gut. 1998 Apr. doi: 10.1136/gut.42.4.551.
8. Gardner TB, et al. ACG Clinical Guideline: Chronic Pancreatitis. Am J Gastroenterol. 2020 Mar. doi: 10.14309/ajg.0000000000000535.
9. Lewis DM, et al. Exocrine Pancreatic Insufficiency Dosing Guidelines for Pancreatic Enzyme Replacement Therapy Vary Widely Across Disease Types. Dig Dis Sci. 2024 Feb. doi: 10.1007/s10620-023-08184-w.
10. Borowitz DS, et al. Use of pancreatic enzyme supplements for patients with cystic fibrosis in the context of fibrosing colonopathy. Consensus Committee. J Pediatr. 1995 Nov. doi: 10.1016/s0022-3476(95)70153-2.
11. Domínguez-Muñoz JE, et al. Effect of the Administration Schedule on the therapeutic efficacy of oral pancreatic enzyme supplements in patients with exocrine pancreatic insufficiency: a randomized, three-way crossover study. Aliment Pharmacol Ther. 2005 Apr. doi: 10.1111/j.1365-2036.2005.02390.x.
12. Hart PA and Conwell DL. Chronic Pancreatitis: Managing a Difficult Disease. Am J Gastroenterol. 2020 Jan. doi: 10.14309/ajg.0000000000000421.
Exocrine pancreatic insufficiency (EPI) is a recognized condition in patients with underlying pancreatic disease. However, it is a disease state that requires a meticulous approach to diagnose, as misdiagnosis can lead to inappropriate testing and unnecessary treatment.
EPI has been defined as “a near total decline in the quantity and/or activity of endogenous pancreatic enzymes to a level that is inadequate to maintain normal digestive capacity leading to steatorrhea.”1 It can lead to complications including malnutrition, micronutrient deficiencies, metabolic bone disease and have significant impact on quality of life. In this article,
EPI Diagnosis
EPI results from ineffective or insufficient pancreatic digestive enzyme secretion. In 2021, a group of experts from the American Gastroenterological Association (AGA) and PancreasFest met and proposed a new mechanistic definition of EPI. This suggests that EPI is the failure of sufficient pancreatic enzymes to effectively reach the intestine in order to allow for optimal digestion of ingested nutrients, leading to downstream macronutrient and micronutrient deficiencies with symptoms of maldigestion including post-prandial abdominal pain, bloating, steatorrhea, loose stools, or weight loss.2
A more pragmatic definition by Khan, et al in 2022 utilized a staging system to distinguish exocrine pancreatic dysfunction (EDP) from EPI. As such EPD occurs when there is a decline in pancreatic function without impaired digestive capacity, while EPI requires digestive capacity impairment leading to objective steatorrhea (coefficient of fat absorption <93 %).3Differential Diagnosis: There are many factors that can impact normal digestion. In approaching EPI, symptoms are often the most common reason to test for disease state in the appropriate clinical context. There can be pancreatic causes of EPI and non-pancreatic (secondary) causes of EPI (see Figure 1), though the latter can be challenging to detect.
The most common parenchymal etiologies for EPI include chronic pancreatitis, recurrent acute pancreatitis, cystic fibrosis, pancreatic cancer or prior pancreatic resections. Non-pancreatic conditions that impact synchronous mixing of endogenous pancreatic enzymes with meals (i.e., Roux-en-Y gastric bypass, short bowel syndrome, delayed gastric emptying), mucosal barriers causing decrease endogenous pancreatic stimulation despite intact parenchyma, such as celiac disease, foregut Crohn’s disease, intraluminal inactivation of pancreatic enzymes (Zollinger-Ellison syndrome), and bile salts de-conjugation with small intestinal bacterial overgrowth (SIBO) can predispose to EPI.4-6 The true prevalence of EPI is difficult to ascertain due to a variety of factors including challenges in diagnosis and misdiagnosis.
Some of the major challenges in the diagnosis and treatment of EPI is that the symptoms of EPI overlap with many other GI conditions including celiac disease, diabetes mellitus, SIBO, irritable bowel syndrome (IBS), bile acid diarrhea, and other functional GI syndromes. These non-pancreatic conditions can also be associated with falsely low FE-1. Hence, ordering FE-1 should be employed with caution when the pretest probability is low. Patients with EPI will generally have a significant response to pancreatic enzyme replacement therapy (PERT) if it is adequately dosed and a lack of response should prompt consideration of an alternative diagnosis. A framework to factors which contribute to EPI is outlined in Figure 2.
Symptoms Screening and Signs: Pancreatic enzymes output estimation is the most reliable indicator for pancreatic digestive capacity. However, EPI diagnosis requires a combination of symptoms screening, stool-based (indirect pancreatic function) testing or direct pancreatic function testing (PFT).
Although symptoms might not correlate with objective disease state, in screening for symptoms of steatorrhea or maldigestion, it is important to ask specific questions regarding bloating, abdominal pain, stool frequency, consistency, and quality. Screening questions should be specific and include question such as, “Is there oil in the toilet bowl or is the stool greasy/shiny?”, “Is the stool sticky and difficult to flush or wipe?”, “Is there malodorous flatus?” If patients screen positive for EPI symptoms and there is a high pre-test probability of EPI such as the presence of severe chronic pancreatitis or significant pancreatic resection (> 90% loss of pancreatic parenchyma), then cautious trial of PERT and assessment for treatment response can be considered without additional stool-based testing. However, this practice end points are unclear and mainly based on subjective response.
Patients with EPI are at increased risk for malnutrition and micronutrient deficiencies. While not required for the diagnosis, low levels of fat-soluble vitamins (vitamin AEDK) or other minerals (zinc, selenium, magnesium, phosphorus) can suggest issues with malabsorption. Once the diagnosis of EPI is made, micronutrient screening should occur annually.
Stool Based Testing: The gold standard clinical test for steatorrhea is measuring coefficient of fat absorption (CFA). With a normal range of 93% fat absorption, the test is performed on a 72-hours fecal fat collection kit. To ensure accurate results, a patient must adhere to a diet with a minimum of 100 grams of fat per day in the three days leading up to the test and during the duration of the test. Patients must also abstain from taking PERT during the duration of the test. This can be incredibly challenging for someone with underlying steatorrhea but can reliably distinguish between EPD and EPI.
A more commonly used stool test is fecal elastase (FE-1). While easier to perform, the test often results in many false positives and false negatives. FE-1 is an ELISA based test, which measures the concentration of the specific isoform CELA3 (chymotrypsin-like elastase family) in the stool sample. The test must be run on a solid stool sample as soft or liquid stool will dilute down elastase concentration falsely. One test advantage is that a patient can continue PERT if needed. FE-1 test measures the concentration of patients’ elastase and PERT is porcine derived. As such, there is no interaction between porcine lipase and human elastase in stool. FE-1 sensitivity and specificity are high for severe disease (<100 mcg/g) if the test is performed properly on patients with a high pretest probability. However, the sensitivity and specificity are poor in mild to moderate pancreatic disease and in the absence of known pancreatic disease.7
Our suggested approach to utilizing FE-1 test is to reserve it for patients with known severe chronic pancreatitis or prior pancreatic surgery in patients with symptoms. In patients without pancreatic disease who are at low risk of EPI, a positive FE-1 can lead to misdiagnosis, further diagnostic testing, and unnecessary treatment. Currently, there is no stool-based test that is accurate, reproducible, and reliable.
Direct Pancreatic Function testing: Secretin stimulated PFT is highly reliable in measuring ductal function with bicarbonate concentration. However, it cannot reliably estimate acinar function as both do not decline at the same rate, unless in severe pancreatic disease. A much more robust test should include cholecystokinin analog to measure pancreatic enzymes concentration. This test involves endoscopy, administration of secretin, and/or a cholecystokinin analog, and subsequent measurement of bicarbonate and digestive enzymes in the pancreatic juice. This test is not routinely offered as it is invasive, cumbersome, and difficult to repeat for reassessment of pancreatic function over time.8
Treatment
The primary goal of treatment is to improve symptoms and nutritional status of the patient. EPI treatment consists of PERT and nutritional counseling. In the United States, there are multiple FDA approved PERT preparations, which include Creon, Zenpep, Pancreaze, Pertzye, Viokase, and Relizorb. While dosing is dependent on lipase concentration, all PERT (aside from Relizorb) preparations have a combination of lipase, proteases, and amylase. All but Viokace and Relizorb are enteric-coated formulations.9
In patients with an inadequate response to enteric coated PERT, non-enteric coated PERT can be added as it may provide a more immediate effect than enteric coated formulations, specially if concern about rapid gut transit with inadequate mixing is raised. If a non-enteric formations is used, acid suppression should be added to prevent inactivation of the PERT. Relizorb is a cartridge system which delivers lipase directly to tube feeds. This cartridge is only utilized in patients receiving enteral nutrition and allows for treatment of EPI even when patients are unable to tolerate oral feeding.
PERT dosing is intended to at least compensate for 10% of the physiologically secreted amount of endogenous lipase after a normal meal (approximately 30,000 IU). Hence, dosing is primarily weight-based. In symptomatic adults, PERT dose of 500-1000 units/kg/meal and half of the amount with snacks is appropriate. Although higher doses of 1500-2000 units/kg/meal may be needed when there is significant steatorrhea, weight loss, or micronutrient deficiencies, PERT doses exceeding 2500 units/kg/meal are not recommended and warrant further investigation.10
Proper counseling is important to ensure compliance with pancreatin preparations. PERT will generally be effective in improving steatorrhea, weight loss, bowel movement frequency, and reversal of nutritional deficiencies, but it does not reliably help symptoms of bloating or abdominal pain. If a patient’s steatorrhea does not respond to PERT, then alternative diagnoses such as SIBO, or diarrhea-predominant IBS should be considered.
PERT must be taken with meals. There are studies that support split dosing as a more effective way of absorbing fat.11 If PERT is ineffective or minimally effective, review of appropriate dosing and timing of PERT to a meal is recommended. Addition of acid suppression may be required to improve treatment efficacy, especially in patients with abnormal intestinal motility or prior pancreatic surgery as PERT is effective at a pH of 4.5. Cost, pill burden, and persistence of certain symptoms may impact adherence to PERT and thus pre-treatment counseling and close follow-up after initiation is important. This aids in assessment of patients’ response to therapy, ensure appropriate PERT administration, and identifying any barriers to therapy adherence.
Nutritional management of EPI consists of an assessment of nutritional status, diet, and lifestyle. An important component of nutritional management is the assessment of micronutrient deficiencies. Patients with a confirmed diagnosis of EPI should be screened for the following micronutrients annually: Vitamins (A, E, D, K, B12), folate, zinc, selenium, magnesium, and iron. Patients with chronic pancreatitis and EPI should also be screened for metabolic bone disease once every two years and for diabetes mellitus annually.4, 12
Conclusion
EPI is a challenging diagnosis as many symptoms overlap with other GI conditions. Pancreas exocrine function is rich with significant reserve to allow for proper digestive capacity, yet EPI occurs when an individual’s pancreatic digestive enzymes are insufficient to meet their nutritional needs. In patients with high likelihood of having EPI, such as those with pre-existing pancreatic disease, diagnosing EPI combines clinical evidence based on subjective symptoms and stool-based testing to support a disease state.
Appropriate dosing and timing of PERT is critical to improve nutritional outcomes and improve certain symptoms of EPI. Failure of PERT requires evaluating for proper dosing/timing, and consideration of additional or alternative diagnosis. EPI morbidity can lead to significant impact on patients’ quality of life, but with counseling, proper PERT use, nutritional consequences can be mediated, and quality of life can improve.
Dr. Hernandez-Barco is based in the Division of Gastroenterology, Massachusetts General Hospital, Boston, Massachusetts. Dr. Ashkar is based in the Division of Gastroenterology and Hepatology, Mayo Clinic, Rochester, Minnesota. Dr. Hernandez-Barco disclosed consulting for AMGEN and served as a scientific advisor for Nestle Health Science. She had project-related funding support or conflicts of interest to disclose. Dr. Ashkar disclosed consulting for AMGEN. He had no project-related funding support or conflicts of interest to disclose.
References
1. Othman MO, et al. Introduction and practical approach to exocrine pancreatic insufficiency for the practicing clinician. Int J Clin Pract. 2018 Feb. doi: 10.1111/ijcp.13066.
2. Whitcomb DC, et al. AGA-PancreasFest Joint Symposium on Exocrine Pancreatitic Insufficiency. Gastro Hep Adv. 2022 Nov. doi: 10.1016/j.gastha.2022.11.008.
3. Khan A, et al. Staging Exocrine Pancreatic Dysfunction. Pancreatology. 2022 Jan. doi: 10.1016/j.pan.2021.11.005.
4. Whitcomb DC, et al. AGA Clinical Practice Update on the Epidemiology, Evaluation, and Management of Exocrine Pancreatitis insufficiency: Expert Review. Gastroenterology. 2023 Nov. doi: 10.1053/j.gastro.2023.07.007.
5. Kunovský L, et al. Causes of Exocrine Pancreatic Insufficiency Other than Chronic Pancreatitis. J Clin Med. 2021 Dec. doi: 10.3390/jcm10245779.
6. Singh VK, et al. Less common etiologies of exocrine pancreatic insufficiency. World J Gastroenterol. 2017 Oct. doi: 10.3748/wjg.v23.i39.7059.
7. Lankisch PG, et al. Faecal elastase 1: not helpful in diagnosing chronic pancreatitis associated with mid to moderate exocrine pancreatic insufficiency. Gut. 1998 Apr. doi: 10.1136/gut.42.4.551.
8. Gardner TB, et al. ACG Clinical Guideline: Chronic Pancreatitis. Am J Gastroenterol. 2020 Mar. doi: 10.14309/ajg.0000000000000535.
9. Lewis DM, et al. Exocrine Pancreatic Insufficiency Dosing Guidelines for Pancreatic Enzyme Replacement Therapy Vary Widely Across Disease Types. Dig Dis Sci. 2024 Feb. doi: 10.1007/s10620-023-08184-w.
10. Borowitz DS, et al. Use of pancreatic enzyme supplements for patients with cystic fibrosis in the context of fibrosing colonopathy. Consensus Committee. J Pediatr. 1995 Nov. doi: 10.1016/s0022-3476(95)70153-2.
11. Domínguez-Muñoz JE, et al. Effect of the Administration Schedule on the therapeutic efficacy of oral pancreatic enzyme supplements in patients with exocrine pancreatic insufficiency: a randomized, three-way crossover study. Aliment Pharmacol Ther. 2005 Apr. doi: 10.1111/j.1365-2036.2005.02390.x.
12. Hart PA and Conwell DL. Chronic Pancreatitis: Managing a Difficult Disease. Am J Gastroenterol. 2020 Jan. doi: 10.14309/ajg.0000000000000421.
Exocrine pancreatic insufficiency (EPI) is a recognized condition in patients with underlying pancreatic disease. However, it is a disease state that requires a meticulous approach to diagnose, as misdiagnosis can lead to inappropriate testing and unnecessary treatment.
EPI has been defined as “a near total decline in the quantity and/or activity of endogenous pancreatic enzymes to a level that is inadequate to maintain normal digestive capacity leading to steatorrhea.”1 It can lead to complications including malnutrition, micronutrient deficiencies, metabolic bone disease and have significant impact on quality of life. In this article,
EPI Diagnosis
EPI results from ineffective or insufficient pancreatic digestive enzyme secretion. In 2021, a group of experts from the American Gastroenterological Association (AGA) and PancreasFest met and proposed a new mechanistic definition of EPI. This suggests that EPI is the failure of sufficient pancreatic enzymes to effectively reach the intestine in order to allow for optimal digestion of ingested nutrients, leading to downstream macronutrient and micronutrient deficiencies with symptoms of maldigestion including post-prandial abdominal pain, bloating, steatorrhea, loose stools, or weight loss.2
A more pragmatic definition by Khan, et al in 2022 utilized a staging system to distinguish exocrine pancreatic dysfunction (EDP) from EPI. As such EPD occurs when there is a decline in pancreatic function without impaired digestive capacity, while EPI requires digestive capacity impairment leading to objective steatorrhea (coefficient of fat absorption <93 %).3Differential Diagnosis: There are many factors that can impact normal digestion. In approaching EPI, symptoms are often the most common reason to test for disease state in the appropriate clinical context. There can be pancreatic causes of EPI and non-pancreatic (secondary) causes of EPI (see Figure 1), though the latter can be challenging to detect.
The most common parenchymal etiologies for EPI include chronic pancreatitis, recurrent acute pancreatitis, cystic fibrosis, pancreatic cancer or prior pancreatic resections. Non-pancreatic conditions that impact synchronous mixing of endogenous pancreatic enzymes with meals (i.e., Roux-en-Y gastric bypass, short bowel syndrome, delayed gastric emptying), mucosal barriers causing decrease endogenous pancreatic stimulation despite intact parenchyma, such as celiac disease, foregut Crohn’s disease, intraluminal inactivation of pancreatic enzymes (Zollinger-Ellison syndrome), and bile salts de-conjugation with small intestinal bacterial overgrowth (SIBO) can predispose to EPI.4-6 The true prevalence of EPI is difficult to ascertain due to a variety of factors including challenges in diagnosis and misdiagnosis.
Some of the major challenges in the diagnosis and treatment of EPI is that the symptoms of EPI overlap with many other GI conditions including celiac disease, diabetes mellitus, SIBO, irritable bowel syndrome (IBS), bile acid diarrhea, and other functional GI syndromes. These non-pancreatic conditions can also be associated with falsely low FE-1. Hence, ordering FE-1 should be employed with caution when the pretest probability is low. Patients with EPI will generally have a significant response to pancreatic enzyme replacement therapy (PERT) if it is adequately dosed and a lack of response should prompt consideration of an alternative diagnosis. A framework to factors which contribute to EPI is outlined in Figure 2.
Symptoms Screening and Signs: Pancreatic enzymes output estimation is the most reliable indicator for pancreatic digestive capacity. However, EPI diagnosis requires a combination of symptoms screening, stool-based (indirect pancreatic function) testing or direct pancreatic function testing (PFT).
Although symptoms might not correlate with objective disease state, in screening for symptoms of steatorrhea or maldigestion, it is important to ask specific questions regarding bloating, abdominal pain, stool frequency, consistency, and quality. Screening questions should be specific and include question such as, “Is there oil in the toilet bowl or is the stool greasy/shiny?”, “Is the stool sticky and difficult to flush or wipe?”, “Is there malodorous flatus?” If patients screen positive for EPI symptoms and there is a high pre-test probability of EPI such as the presence of severe chronic pancreatitis or significant pancreatic resection (> 90% loss of pancreatic parenchyma), then cautious trial of PERT and assessment for treatment response can be considered without additional stool-based testing. However, this practice end points are unclear and mainly based on subjective response.
Patients with EPI are at increased risk for malnutrition and micronutrient deficiencies. While not required for the diagnosis, low levels of fat-soluble vitamins (vitamin AEDK) or other minerals (zinc, selenium, magnesium, phosphorus) can suggest issues with malabsorption. Once the diagnosis of EPI is made, micronutrient screening should occur annually.
Stool Based Testing: The gold standard clinical test for steatorrhea is measuring coefficient of fat absorption (CFA). With a normal range of 93% fat absorption, the test is performed on a 72-hours fecal fat collection kit. To ensure accurate results, a patient must adhere to a diet with a minimum of 100 grams of fat per day in the three days leading up to the test and during the duration of the test. Patients must also abstain from taking PERT during the duration of the test. This can be incredibly challenging for someone with underlying steatorrhea but can reliably distinguish between EPD and EPI.
A more commonly used stool test is fecal elastase (FE-1). While easier to perform, the test often results in many false positives and false negatives. FE-1 is an ELISA based test, which measures the concentration of the specific isoform CELA3 (chymotrypsin-like elastase family) in the stool sample. The test must be run on a solid stool sample as soft or liquid stool will dilute down elastase concentration falsely. One test advantage is that a patient can continue PERT if needed. FE-1 test measures the concentration of patients’ elastase and PERT is porcine derived. As such, there is no interaction between porcine lipase and human elastase in stool. FE-1 sensitivity and specificity are high for severe disease (<100 mcg/g) if the test is performed properly on patients with a high pretest probability. However, the sensitivity and specificity are poor in mild to moderate pancreatic disease and in the absence of known pancreatic disease.7
Our suggested approach to utilizing FE-1 test is to reserve it for patients with known severe chronic pancreatitis or prior pancreatic surgery in patients with symptoms. In patients without pancreatic disease who are at low risk of EPI, a positive FE-1 can lead to misdiagnosis, further diagnostic testing, and unnecessary treatment. Currently, there is no stool-based test that is accurate, reproducible, and reliable.
Direct Pancreatic Function testing: Secretin stimulated PFT is highly reliable in measuring ductal function with bicarbonate concentration. However, it cannot reliably estimate acinar function as both do not decline at the same rate, unless in severe pancreatic disease. A much more robust test should include cholecystokinin analog to measure pancreatic enzymes concentration. This test involves endoscopy, administration of secretin, and/or a cholecystokinin analog, and subsequent measurement of bicarbonate and digestive enzymes in the pancreatic juice. This test is not routinely offered as it is invasive, cumbersome, and difficult to repeat for reassessment of pancreatic function over time.8
Treatment
The primary goal of treatment is to improve symptoms and nutritional status of the patient. EPI treatment consists of PERT and nutritional counseling. In the United States, there are multiple FDA approved PERT preparations, which include Creon, Zenpep, Pancreaze, Pertzye, Viokase, and Relizorb. While dosing is dependent on lipase concentration, all PERT (aside from Relizorb) preparations have a combination of lipase, proteases, and amylase. All but Viokace and Relizorb are enteric-coated formulations.9
In patients with an inadequate response to enteric coated PERT, non-enteric coated PERT can be added as it may provide a more immediate effect than enteric coated formulations, specially if concern about rapid gut transit with inadequate mixing is raised. If a non-enteric formations is used, acid suppression should be added to prevent inactivation of the PERT. Relizorb is a cartridge system which delivers lipase directly to tube feeds. This cartridge is only utilized in patients receiving enteral nutrition and allows for treatment of EPI even when patients are unable to tolerate oral feeding.
PERT dosing is intended to at least compensate for 10% of the physiologically secreted amount of endogenous lipase after a normal meal (approximately 30,000 IU). Hence, dosing is primarily weight-based. In symptomatic adults, PERT dose of 500-1000 units/kg/meal and half of the amount with snacks is appropriate. Although higher doses of 1500-2000 units/kg/meal may be needed when there is significant steatorrhea, weight loss, or micronutrient deficiencies, PERT doses exceeding 2500 units/kg/meal are not recommended and warrant further investigation.10
Proper counseling is important to ensure compliance with pancreatin preparations. PERT will generally be effective in improving steatorrhea, weight loss, bowel movement frequency, and reversal of nutritional deficiencies, but it does not reliably help symptoms of bloating or abdominal pain. If a patient’s steatorrhea does not respond to PERT, then alternative diagnoses such as SIBO, or diarrhea-predominant IBS should be considered.
PERT must be taken with meals. There are studies that support split dosing as a more effective way of absorbing fat.11 If PERT is ineffective or minimally effective, review of appropriate dosing and timing of PERT to a meal is recommended. Addition of acid suppression may be required to improve treatment efficacy, especially in patients with abnormal intestinal motility or prior pancreatic surgery as PERT is effective at a pH of 4.5. Cost, pill burden, and persistence of certain symptoms may impact adherence to PERT and thus pre-treatment counseling and close follow-up after initiation is important. This aids in assessment of patients’ response to therapy, ensure appropriate PERT administration, and identifying any barriers to therapy adherence.
Nutritional management of EPI consists of an assessment of nutritional status, diet, and lifestyle. An important component of nutritional management is the assessment of micronutrient deficiencies. Patients with a confirmed diagnosis of EPI should be screened for the following micronutrients annually: Vitamins (A, E, D, K, B12), folate, zinc, selenium, magnesium, and iron. Patients with chronic pancreatitis and EPI should also be screened for metabolic bone disease once every two years and for diabetes mellitus annually.4, 12
Conclusion
EPI is a challenging diagnosis as many symptoms overlap with other GI conditions. Pancreas exocrine function is rich with significant reserve to allow for proper digestive capacity, yet EPI occurs when an individual’s pancreatic digestive enzymes are insufficient to meet their nutritional needs. In patients with high likelihood of having EPI, such as those with pre-existing pancreatic disease, diagnosing EPI combines clinical evidence based on subjective symptoms and stool-based testing to support a disease state.
Appropriate dosing and timing of PERT is critical to improve nutritional outcomes and improve certain symptoms of EPI. Failure of PERT requires evaluating for proper dosing/timing, and consideration of additional or alternative diagnosis. EPI morbidity can lead to significant impact on patients’ quality of life, but with counseling, proper PERT use, nutritional consequences can be mediated, and quality of life can improve.
Dr. Hernandez-Barco is based in the Division of Gastroenterology, Massachusetts General Hospital, Boston, Massachusetts. Dr. Ashkar is based in the Division of Gastroenterology and Hepatology, Mayo Clinic, Rochester, Minnesota. Dr. Hernandez-Barco disclosed consulting for AMGEN and served as a scientific advisor for Nestle Health Science. She had project-related funding support or conflicts of interest to disclose. Dr. Ashkar disclosed consulting for AMGEN. He had no project-related funding support or conflicts of interest to disclose.
References
1. Othman MO, et al. Introduction and practical approach to exocrine pancreatic insufficiency for the practicing clinician. Int J Clin Pract. 2018 Feb. doi: 10.1111/ijcp.13066.
2. Whitcomb DC, et al. AGA-PancreasFest Joint Symposium on Exocrine Pancreatitic Insufficiency. Gastro Hep Adv. 2022 Nov. doi: 10.1016/j.gastha.2022.11.008.
3. Khan A, et al. Staging Exocrine Pancreatic Dysfunction. Pancreatology. 2022 Jan. doi: 10.1016/j.pan.2021.11.005.
4. Whitcomb DC, et al. AGA Clinical Practice Update on the Epidemiology, Evaluation, and Management of Exocrine Pancreatitis insufficiency: Expert Review. Gastroenterology. 2023 Nov. doi: 10.1053/j.gastro.2023.07.007.
5. Kunovský L, et al. Causes of Exocrine Pancreatic Insufficiency Other than Chronic Pancreatitis. J Clin Med. 2021 Dec. doi: 10.3390/jcm10245779.
6. Singh VK, et al. Less common etiologies of exocrine pancreatic insufficiency. World J Gastroenterol. 2017 Oct. doi: 10.3748/wjg.v23.i39.7059.
7. Lankisch PG, et al. Faecal elastase 1: not helpful in diagnosing chronic pancreatitis associated with mid to moderate exocrine pancreatic insufficiency. Gut. 1998 Apr. doi: 10.1136/gut.42.4.551.
8. Gardner TB, et al. ACG Clinical Guideline: Chronic Pancreatitis. Am J Gastroenterol. 2020 Mar. doi: 10.14309/ajg.0000000000000535.
9. Lewis DM, et al. Exocrine Pancreatic Insufficiency Dosing Guidelines for Pancreatic Enzyme Replacement Therapy Vary Widely Across Disease Types. Dig Dis Sci. 2024 Feb. doi: 10.1007/s10620-023-08184-w.
10. Borowitz DS, et al. Use of pancreatic enzyme supplements for patients with cystic fibrosis in the context of fibrosing colonopathy. Consensus Committee. J Pediatr. 1995 Nov. doi: 10.1016/s0022-3476(95)70153-2.
11. Domínguez-Muñoz JE, et al. Effect of the Administration Schedule on the therapeutic efficacy of oral pancreatic enzyme supplements in patients with exocrine pancreatic insufficiency: a randomized, three-way crossover study. Aliment Pharmacol Ther. 2005 Apr. doi: 10.1111/j.1365-2036.2005.02390.x.
12. Hart PA and Conwell DL. Chronic Pancreatitis: Managing a Difficult Disease. Am J Gastroenterol. 2020 Jan. doi: 10.14309/ajg.0000000000000421.
Journal Highlights: January-April 2025
Esophagus/Motility
Carlson DA, et al. A Standardized Approach to Performing and Interpreting Functional Lumen Imaging Probe Panometry for Esophageal Motility Disorders: The Dallas Consensus. Gastroenterology. 2025 Feb. doi: 10.1053/j.gastro.2025.01.234.
Parkman HP, et al; NIDDK Gastroparesis Clinical Research Consortium. Characterization of Patients with Symptoms of Gastroparesis Having Frequent Emergency Department Visits and Hospitalizations. Clin Gastroenterol Hepatol. 2025 Apr. doi: 10.1016/j.cgh.2025.01.033.
Dellon ES, et al. Long-term Safety and Efficacy of Budesonide Oral Suspension for Eosinophilic Esophagitis: A 4-Year, Phase 3, Open-Label Study. Clin Gastroenterol Hepatol. 2025 Feb. doi: 10.1016/j.cgh.2024.12.024.
Small Bowel
Hård Af Segerstad EM, et al; TEDDY Study Group. Early Dietary Fiber Intake Reduces Celiac Disease Risk in Genetically Prone Children: Insights From the TEDDY Study. Gastroenterology. 2025 Feb. doi: 10.1053/j.gastro.2025.01.241.
Colon
Shaukat A, et al. AGA Clinical Practice Update on Current Role of Blood Tests for Colorectal Cancer Screening: Commentary. Clin Gastroenterol Hepatol. 2025 Apr. doi: 10.1016/j.cgh.2025.04.003.
Bergman D, et al. Cholecystectomy is a Risk Factor for Microscopic Colitis: A Nationwide Population-based Matched Case Control Study. Clin Gastroenterol Hepatol. 2025 Mar. doi: 10.1016/j.cgh.2024.12.032.
Inflammatory Bowel Disease
Ben-Horin S, et al; Israeli IBD Research Nucleus (IIRN). Capsule Endoscopy-Guided Proactive Treat-to-Target Versus Continued Standard Care in Patients With Quiescent Crohn’s Disease: A Randomized Controlled Trial. Gastroenterology. 2025 Mar. doi: 10.1053/j.gastro.2025.02.031.
Pancreas
Guilabert L, et al; ERICA Consortium. Impact of Fluid Therapy in the Emergency Department in Acute Pancreatitis: a posthoc analysis of the WATERFALL Trial. Clin Gastroenterol Hepatol. 2025 Apr. doi: 10.1016/j.cgh.2025.01.038.
Hepatology
Rhee H, et al. Noncontrast Magnetic Resonance Imaging vs Ultrasonography for Hepatocellular Carcinoma Surveillance: A Randomized, Single-Center Trial. Gastroenterology. 2025 Jan. doi: 10.1053/j.gastro.2024.12.035.
Kronsten VT, et al. Hepatic Encephalopathy: When Lactulose and Rifaximin Are Not Working. Gastroenterology. 2025 Jan. doi: 10.1053/j.gastro.2025.01.010.
Edelson JC, et al. Accuracy and Safety of Endoscopic Ultrasound–Guided Liver Biopsy in Patients with Metabolic Dysfunction–Associated Liver Disease. Tech Innov Gastrointest Endosc. 2025 Apr. doi: 10.1016/j.tige.2025.250918.
Miscellaneous
Martin J, et al. Practical and Impactful Tips for Private Industry Collaborations with Gastroenterology Practices. Clin Gastroenterol Hepatol. 2025 Mar. doi: 10.1016/j.cgh.2025.01.021.
Tejada, Natalia et al. Glucagon-like Peptide-1 Receptor Agonists Are Not Associated With Increased Incidence of Pneumonia After Endoscopic Procedures. Tech Innov Gastrointest Endosc. 2025 Apr. doi: 10.1016/j.tige.2025.250925.
Lazaridis KN, et al. Microplastics and Nanoplastics and the Digestive System. Gastro Hep Adv. 2025 May. doi: 10.1016/j.gastha.2025.100694.
Dr. Trieu is assistant professor of medicine, interventional endoscopy, in the Division of Gastroenterology at Washington University in St. Louis School of Medicine, Missouri.
Esophagus/Motility
Carlson DA, et al. A Standardized Approach to Performing and Interpreting Functional Lumen Imaging Probe Panometry for Esophageal Motility Disorders: The Dallas Consensus. Gastroenterology. 2025 Feb. doi: 10.1053/j.gastro.2025.01.234.
Parkman HP, et al; NIDDK Gastroparesis Clinical Research Consortium. Characterization of Patients with Symptoms of Gastroparesis Having Frequent Emergency Department Visits and Hospitalizations. Clin Gastroenterol Hepatol. 2025 Apr. doi: 10.1016/j.cgh.2025.01.033.
Dellon ES, et al. Long-term Safety and Efficacy of Budesonide Oral Suspension for Eosinophilic Esophagitis: A 4-Year, Phase 3, Open-Label Study. Clin Gastroenterol Hepatol. 2025 Feb. doi: 10.1016/j.cgh.2024.12.024.
Small Bowel
Hård Af Segerstad EM, et al; TEDDY Study Group. Early Dietary Fiber Intake Reduces Celiac Disease Risk in Genetically Prone Children: Insights From the TEDDY Study. Gastroenterology. 2025 Feb. doi: 10.1053/j.gastro.2025.01.241.
Colon
Shaukat A, et al. AGA Clinical Practice Update on Current Role of Blood Tests for Colorectal Cancer Screening: Commentary. Clin Gastroenterol Hepatol. 2025 Apr. doi: 10.1016/j.cgh.2025.04.003.
Bergman D, et al. Cholecystectomy is a Risk Factor for Microscopic Colitis: A Nationwide Population-based Matched Case Control Study. Clin Gastroenterol Hepatol. 2025 Mar. doi: 10.1016/j.cgh.2024.12.032.
Inflammatory Bowel Disease
Ben-Horin S, et al; Israeli IBD Research Nucleus (IIRN). Capsule Endoscopy-Guided Proactive Treat-to-Target Versus Continued Standard Care in Patients With Quiescent Crohn’s Disease: A Randomized Controlled Trial. Gastroenterology. 2025 Mar. doi: 10.1053/j.gastro.2025.02.031.
Pancreas
Guilabert L, et al; ERICA Consortium. Impact of Fluid Therapy in the Emergency Department in Acute Pancreatitis: a posthoc analysis of the WATERFALL Trial. Clin Gastroenterol Hepatol. 2025 Apr. doi: 10.1016/j.cgh.2025.01.038.
Hepatology
Rhee H, et al. Noncontrast Magnetic Resonance Imaging vs Ultrasonography for Hepatocellular Carcinoma Surveillance: A Randomized, Single-Center Trial. Gastroenterology. 2025 Jan. doi: 10.1053/j.gastro.2024.12.035.
Kronsten VT, et al. Hepatic Encephalopathy: When Lactulose and Rifaximin Are Not Working. Gastroenterology. 2025 Jan. doi: 10.1053/j.gastro.2025.01.010.
Edelson JC, et al. Accuracy and Safety of Endoscopic Ultrasound–Guided Liver Biopsy in Patients with Metabolic Dysfunction–Associated Liver Disease. Tech Innov Gastrointest Endosc. 2025 Apr. doi: 10.1016/j.tige.2025.250918.
Miscellaneous
Martin J, et al. Practical and Impactful Tips for Private Industry Collaborations with Gastroenterology Practices. Clin Gastroenterol Hepatol. 2025 Mar. doi: 10.1016/j.cgh.2025.01.021.
Tejada, Natalia et al. Glucagon-like Peptide-1 Receptor Agonists Are Not Associated With Increased Incidence of Pneumonia After Endoscopic Procedures. Tech Innov Gastrointest Endosc. 2025 Apr. doi: 10.1016/j.tige.2025.250925.
Lazaridis KN, et al. Microplastics and Nanoplastics and the Digestive System. Gastro Hep Adv. 2025 May. doi: 10.1016/j.gastha.2025.100694.
Dr. Trieu is assistant professor of medicine, interventional endoscopy, in the Division of Gastroenterology at Washington University in St. Louis School of Medicine, Missouri.
Esophagus/Motility
Carlson DA, et al. A Standardized Approach to Performing and Interpreting Functional Lumen Imaging Probe Panometry for Esophageal Motility Disorders: The Dallas Consensus. Gastroenterology. 2025 Feb. doi: 10.1053/j.gastro.2025.01.234.
Parkman HP, et al; NIDDK Gastroparesis Clinical Research Consortium. Characterization of Patients with Symptoms of Gastroparesis Having Frequent Emergency Department Visits and Hospitalizations. Clin Gastroenterol Hepatol. 2025 Apr. doi: 10.1016/j.cgh.2025.01.033.
Dellon ES, et al. Long-term Safety and Efficacy of Budesonide Oral Suspension for Eosinophilic Esophagitis: A 4-Year, Phase 3, Open-Label Study. Clin Gastroenterol Hepatol. 2025 Feb. doi: 10.1016/j.cgh.2024.12.024.
Small Bowel
Hård Af Segerstad EM, et al; TEDDY Study Group. Early Dietary Fiber Intake Reduces Celiac Disease Risk in Genetically Prone Children: Insights From the TEDDY Study. Gastroenterology. 2025 Feb. doi: 10.1053/j.gastro.2025.01.241.
Colon
Shaukat A, et al. AGA Clinical Practice Update on Current Role of Blood Tests for Colorectal Cancer Screening: Commentary. Clin Gastroenterol Hepatol. 2025 Apr. doi: 10.1016/j.cgh.2025.04.003.
Bergman D, et al. Cholecystectomy is a Risk Factor for Microscopic Colitis: A Nationwide Population-based Matched Case Control Study. Clin Gastroenterol Hepatol. 2025 Mar. doi: 10.1016/j.cgh.2024.12.032.
Inflammatory Bowel Disease
Ben-Horin S, et al; Israeli IBD Research Nucleus (IIRN). Capsule Endoscopy-Guided Proactive Treat-to-Target Versus Continued Standard Care in Patients With Quiescent Crohn’s Disease: A Randomized Controlled Trial. Gastroenterology. 2025 Mar. doi: 10.1053/j.gastro.2025.02.031.
Pancreas
Guilabert L, et al; ERICA Consortium. Impact of Fluid Therapy in the Emergency Department in Acute Pancreatitis: a posthoc analysis of the WATERFALL Trial. Clin Gastroenterol Hepatol. 2025 Apr. doi: 10.1016/j.cgh.2025.01.038.
Hepatology
Rhee H, et al. Noncontrast Magnetic Resonance Imaging vs Ultrasonography for Hepatocellular Carcinoma Surveillance: A Randomized, Single-Center Trial. Gastroenterology. 2025 Jan. doi: 10.1053/j.gastro.2024.12.035.
Kronsten VT, et al. Hepatic Encephalopathy: When Lactulose and Rifaximin Are Not Working. Gastroenterology. 2025 Jan. doi: 10.1053/j.gastro.2025.01.010.
Edelson JC, et al. Accuracy and Safety of Endoscopic Ultrasound–Guided Liver Biopsy in Patients with Metabolic Dysfunction–Associated Liver Disease. Tech Innov Gastrointest Endosc. 2025 Apr. doi: 10.1016/j.tige.2025.250918.
Miscellaneous
Martin J, et al. Practical and Impactful Tips for Private Industry Collaborations with Gastroenterology Practices. Clin Gastroenterol Hepatol. 2025 Mar. doi: 10.1016/j.cgh.2025.01.021.
Tejada, Natalia et al. Glucagon-like Peptide-1 Receptor Agonists Are Not Associated With Increased Incidence of Pneumonia After Endoscopic Procedures. Tech Innov Gastrointest Endosc. 2025 Apr. doi: 10.1016/j.tige.2025.250925.
Lazaridis KN, et al. Microplastics and Nanoplastics and the Digestive System. Gastro Hep Adv. 2025 May. doi: 10.1016/j.gastha.2025.100694.
Dr. Trieu is assistant professor of medicine, interventional endoscopy, in the Division of Gastroenterology at Washington University in St. Louis School of Medicine, Missouri.
Papilla Sphincterotomy Shows No Risk Reduction in Pancreas Divisum
SAN DIEGO — , suggesting that patients can be spared the intervention, which can carry risks of its own.
“This is a topic that has been debated for decades,” said first author Gregory A. Coté, MD, AGAF, Division Head, professor of medicine, Division of Gastroenterology & Hepatology, Oregon Health & Science University, in Portland, Oregon.
“Many doctors believe the procedure helps and offer it because we have limited options to help our patients, whereas others believe the procedure is harmful and doesn’t help,” he explained in a press briefing for the late-breaking study, presented at Digestive Disease Week (DDW) 2025.
The study’s findings supported the latter argument.
“Patients who underwent ERCP with sphincterotomy were just as likely as those who did not have this procedure to develop acute pancreatitis again,” Coté reported.
While clinical guidelines currently recommend ERCP as treatment for pancreas divisum, “these guidelines are likely to change based on this study,” he said.
Pancreas divisum, occurring in about 7%-10% of people, is an anatomic variation that can represent an obstructive risk factor for acute recurrent pancreatitis.
The common use of ERCP with minor papilla endoscopic sphincterotomy to treat the condition is based on prior retrospective studies showing that in patients who did develop acute pancreatitis, up to 70% with the treatment never developed acute pancreatitis again. However, there have been no studies comparing the use of the treatment with a control group.
Coté and colleagues conducted the multicenter SHARP trial, in which 148 patients with pancreas divisum were enrolled between September 2018 and August 2024 and randomized to receive either ERCP with minor papilla endoscopic sphincterotomy (n = 75) or a sham treatment (n = 73).
The patients, who had a median age of 51 years, had a median of 3 acute pancreatitis episodes prior to randomization.
With a median follow-up of 33.5 months (range, 6-48 months), 34.7% of patients in the ERCP arm experienced an acute pancreatitis incident compared with 43.8% in the sham arm, for a hazard ratio of 0.83 after adjusting for duct size and the number of episodes, which was not a statistically significant difference (P = .27).
A subgroup analysis further showed no indication of a treatment effect based on factors including age, diabetes status, sex, alcohol or tobacco use, or other factors.
“Compared with a sham ERCP group, we found that minor papillotomy did not reduce the risk of acute pancreatitis, incident chronic pancreatitis, endocrine pancreatic insufficiency or diabetes, or pancreas-related pain events,” Coté said.
The findings are particularly important because the treatment itself is associated with some risks, he added.
“Ironically, the problem with this procedure is that it can cause acute pancreatitis in 10%-20% of patients and may instigate other issues later,” such as the development of scarring of the pancreas related to incisions in the procedure.
“No one wants to offer an expensive procedure that has its own risks if it doesn’t help,” Coté said.
Based on the findings, “pancreas divisum anatomy should no longer be considered an indication for ERCP, even for idiopathic acute pancreatitis,” he concluded.
A version of this article appeared on Medscape.com.
SAN DIEGO — , suggesting that patients can be spared the intervention, which can carry risks of its own.
“This is a topic that has been debated for decades,” said first author Gregory A. Coté, MD, AGAF, Division Head, professor of medicine, Division of Gastroenterology & Hepatology, Oregon Health & Science University, in Portland, Oregon.
“Many doctors believe the procedure helps and offer it because we have limited options to help our patients, whereas others believe the procedure is harmful and doesn’t help,” he explained in a press briefing for the late-breaking study, presented at Digestive Disease Week (DDW) 2025.
The study’s findings supported the latter argument.
“Patients who underwent ERCP with sphincterotomy were just as likely as those who did not have this procedure to develop acute pancreatitis again,” Coté reported.
While clinical guidelines currently recommend ERCP as treatment for pancreas divisum, “these guidelines are likely to change based on this study,” he said.
Pancreas divisum, occurring in about 7%-10% of people, is an anatomic variation that can represent an obstructive risk factor for acute recurrent pancreatitis.
The common use of ERCP with minor papilla endoscopic sphincterotomy to treat the condition is based on prior retrospective studies showing that in patients who did develop acute pancreatitis, up to 70% with the treatment never developed acute pancreatitis again. However, there have been no studies comparing the use of the treatment with a control group.
Coté and colleagues conducted the multicenter SHARP trial, in which 148 patients with pancreas divisum were enrolled between September 2018 and August 2024 and randomized to receive either ERCP with minor papilla endoscopic sphincterotomy (n = 75) or a sham treatment (n = 73).
The patients, who had a median age of 51 years, had a median of 3 acute pancreatitis episodes prior to randomization.
With a median follow-up of 33.5 months (range, 6-48 months), 34.7% of patients in the ERCP arm experienced an acute pancreatitis incident compared with 43.8% in the sham arm, for a hazard ratio of 0.83 after adjusting for duct size and the number of episodes, which was not a statistically significant difference (P = .27).
A subgroup analysis further showed no indication of a treatment effect based on factors including age, diabetes status, sex, alcohol or tobacco use, or other factors.
“Compared with a sham ERCP group, we found that minor papillotomy did not reduce the risk of acute pancreatitis, incident chronic pancreatitis, endocrine pancreatic insufficiency or diabetes, or pancreas-related pain events,” Coté said.
The findings are particularly important because the treatment itself is associated with some risks, he added.
“Ironically, the problem with this procedure is that it can cause acute pancreatitis in 10%-20% of patients and may instigate other issues later,” such as the development of scarring of the pancreas related to incisions in the procedure.
“No one wants to offer an expensive procedure that has its own risks if it doesn’t help,” Coté said.
Based on the findings, “pancreas divisum anatomy should no longer be considered an indication for ERCP, even for idiopathic acute pancreatitis,” he concluded.
A version of this article appeared on Medscape.com.
SAN DIEGO — , suggesting that patients can be spared the intervention, which can carry risks of its own.
“This is a topic that has been debated for decades,” said first author Gregory A. Coté, MD, AGAF, Division Head, professor of medicine, Division of Gastroenterology & Hepatology, Oregon Health & Science University, in Portland, Oregon.
“Many doctors believe the procedure helps and offer it because we have limited options to help our patients, whereas others believe the procedure is harmful and doesn’t help,” he explained in a press briefing for the late-breaking study, presented at Digestive Disease Week (DDW) 2025.
The study’s findings supported the latter argument.
“Patients who underwent ERCP with sphincterotomy were just as likely as those who did not have this procedure to develop acute pancreatitis again,” Coté reported.
While clinical guidelines currently recommend ERCP as treatment for pancreas divisum, “these guidelines are likely to change based on this study,” he said.
Pancreas divisum, occurring in about 7%-10% of people, is an anatomic variation that can represent an obstructive risk factor for acute recurrent pancreatitis.
The common use of ERCP with minor papilla endoscopic sphincterotomy to treat the condition is based on prior retrospective studies showing that in patients who did develop acute pancreatitis, up to 70% with the treatment never developed acute pancreatitis again. However, there have been no studies comparing the use of the treatment with a control group.
Coté and colleagues conducted the multicenter SHARP trial, in which 148 patients with pancreas divisum were enrolled between September 2018 and August 2024 and randomized to receive either ERCP with minor papilla endoscopic sphincterotomy (n = 75) or a sham treatment (n = 73).
The patients, who had a median age of 51 years, had a median of 3 acute pancreatitis episodes prior to randomization.
With a median follow-up of 33.5 months (range, 6-48 months), 34.7% of patients in the ERCP arm experienced an acute pancreatitis incident compared with 43.8% in the sham arm, for a hazard ratio of 0.83 after adjusting for duct size and the number of episodes, which was not a statistically significant difference (P = .27).
A subgroup analysis further showed no indication of a treatment effect based on factors including age, diabetes status, sex, alcohol or tobacco use, or other factors.
“Compared with a sham ERCP group, we found that minor papillotomy did not reduce the risk of acute pancreatitis, incident chronic pancreatitis, endocrine pancreatic insufficiency or diabetes, or pancreas-related pain events,” Coté said.
The findings are particularly important because the treatment itself is associated with some risks, he added.
“Ironically, the problem with this procedure is that it can cause acute pancreatitis in 10%-20% of patients and may instigate other issues later,” such as the development of scarring of the pancreas related to incisions in the procedure.
“No one wants to offer an expensive procedure that has its own risks if it doesn’t help,” Coté said.
Based on the findings, “pancreas divisum anatomy should no longer be considered an indication for ERCP, even for idiopathic acute pancreatitis,” he concluded.
A version of this article appeared on Medscape.com.
FROM DDW 2025
Autoimmune Pancreatitis: What’s Really Behind Those Symptoms
“Defined about 30 years ago, autoimmune pancreatitis [AIP] remains a diagnostic challenge,” said Vinciane Rebours, MD, PhD, professor and head of the Pancreatology and Digestive Oncology Department, Beaujon Hospital in Clichy, France. She spoke at the Francophone Days of Hepatology, Gastroenterology, and Digestive Oncology 2025, held in Paris. The challenge lies in the fact that AIP includes two distinct clinical entities, neither of which is truly autoimmune. However, much remains unknown, including its natural history, cancer risk, and optimal treatment strategies. However, some aspects are now better understood.
Autoimmune Pancreatitis
These forms differ in their histological characteristics. Type 1 exhibits lymphoplasmacytic infiltration, extensive fibrosis, and IgG4-positive plasma cells. Type 2 presents with granulocytic lesions similar to those in Crohn’s disease.
Type 1 AIP typically affects men aged 50 years or older and is often associated with jaundice, pseudotumor formation, diabetes, and exocrine pancreatic insufficiency. “It is a systemic disease where lymphoplasmacytic infiltration can affect multiple organs, with the pancreas and lymph nodes most commonly involved,” said Rebours.
A definitive diagnosis of type 1 AIP requires three criteria: Organ involvement, serum IgG4 levels more than twice the normal level, and histological abnormalities on biopsy. If one of these criteria is missing, the diagnosis is considered probable or possible.
Diagnosing type 1 AIP is challenging because it can affect multiple organs, often with few symptoms, leading to significant clinical variability. Type 2 AIP, in contrast, generally affects younger individuals, with no gender preference. It is pathophysiologically distinct and is linked to IBD in 87% of cases. Diagnosis relies on clinical criteria, imaging abnormalities (parenchymal or ductal changes identifiable on scans), response to corticosteroids in symptomatic patients, and the presence of IBD. The absence of IgG4 can also aid in the diagnosis. However, gathering all these elements can be difficult.
Evolving Treatment
Symptomatic patients and those at risk for organ failure, particularly lung and kidney failure, are eligible for induction treatment. This involves the administration of full-dose corticosteroids for 4 weeks, followed by a tapering regimen. Remission was achieved in 99% of type 1 and 92% of type 2 cases. Corticosteroids can also be used as a “trial treatment” to assess corticosteroid sensitivity in patients with type 2 AIP.
The risk for recurrence (in case of nonresponse or recurrence before 12 months posttreatment) is higher in type 1 (one third of cases) than in type 2 (15%). In such cases, immunomodulators, primarily rituximab, are recommended for type 1 AIP. Rituximab can also be used as an induction treatment, either alone or in combination, or as maintenance therapy. Alternatives include mycophenolate mofetil or inebilizumab, which showed an 87% reduction in relapse risk according to data published in 2024.
Maintenance treatment for type 2 AIP is not yet fully standardized. The disease is often managed in a manner similar to that of IBD treatment. Rebours cautioned, “Management cannot stop at the pancreas; it is essential to detect all other paucisymptomatic manifestations through comprehensive annual imaging and biannual biological and functional screenings.”
Monitoring IgG4
Monitoring IgG4 levels is important for therapeutic follow-up but is not the “holy grail” for diagnosis, Rebours acknowledged. For instance, 20% of IgG4-RD cases have normal IgG4 levels, 20% of pancreatic cancers show elevated IgG4 levels, and some patients achieve clinical remission despite persistently abnormal IgG4 levels. Without strong suspicion of type 1 AIP, measuring IgG4 levels is “zero cost-effective.”
This disease, which is associated with the risk for underlying cancer, requires extensive imaging (CT, MRI, and endoscopic ultrasound) to differentiate between AIP and cancer. This step is essential to avoid unnecessary surgery on organs affected by IgG4-RD or for treating cancer with corticosteroids.
A version of this article appeared on Medscape.com.
“Defined about 30 years ago, autoimmune pancreatitis [AIP] remains a diagnostic challenge,” said Vinciane Rebours, MD, PhD, professor and head of the Pancreatology and Digestive Oncology Department, Beaujon Hospital in Clichy, France. She spoke at the Francophone Days of Hepatology, Gastroenterology, and Digestive Oncology 2025, held in Paris. The challenge lies in the fact that AIP includes two distinct clinical entities, neither of which is truly autoimmune. However, much remains unknown, including its natural history, cancer risk, and optimal treatment strategies. However, some aspects are now better understood.
Autoimmune Pancreatitis
These forms differ in their histological characteristics. Type 1 exhibits lymphoplasmacytic infiltration, extensive fibrosis, and IgG4-positive plasma cells. Type 2 presents with granulocytic lesions similar to those in Crohn’s disease.
Type 1 AIP typically affects men aged 50 years or older and is often associated with jaundice, pseudotumor formation, diabetes, and exocrine pancreatic insufficiency. “It is a systemic disease where lymphoplasmacytic infiltration can affect multiple organs, with the pancreas and lymph nodes most commonly involved,” said Rebours.
A definitive diagnosis of type 1 AIP requires three criteria: Organ involvement, serum IgG4 levels more than twice the normal level, and histological abnormalities on biopsy. If one of these criteria is missing, the diagnosis is considered probable or possible.
Diagnosing type 1 AIP is challenging because it can affect multiple organs, often with few symptoms, leading to significant clinical variability. Type 2 AIP, in contrast, generally affects younger individuals, with no gender preference. It is pathophysiologically distinct and is linked to IBD in 87% of cases. Diagnosis relies on clinical criteria, imaging abnormalities (parenchymal or ductal changes identifiable on scans), response to corticosteroids in symptomatic patients, and the presence of IBD. The absence of IgG4 can also aid in the diagnosis. However, gathering all these elements can be difficult.
Evolving Treatment
Symptomatic patients and those at risk for organ failure, particularly lung and kidney failure, are eligible for induction treatment. This involves the administration of full-dose corticosteroids for 4 weeks, followed by a tapering regimen. Remission was achieved in 99% of type 1 and 92% of type 2 cases. Corticosteroids can also be used as a “trial treatment” to assess corticosteroid sensitivity in patients with type 2 AIP.
The risk for recurrence (in case of nonresponse or recurrence before 12 months posttreatment) is higher in type 1 (one third of cases) than in type 2 (15%). In such cases, immunomodulators, primarily rituximab, are recommended for type 1 AIP. Rituximab can also be used as an induction treatment, either alone or in combination, or as maintenance therapy. Alternatives include mycophenolate mofetil or inebilizumab, which showed an 87% reduction in relapse risk according to data published in 2024.
Maintenance treatment for type 2 AIP is not yet fully standardized. The disease is often managed in a manner similar to that of IBD treatment. Rebours cautioned, “Management cannot stop at the pancreas; it is essential to detect all other paucisymptomatic manifestations through comprehensive annual imaging and biannual biological and functional screenings.”
Monitoring IgG4
Monitoring IgG4 levels is important for therapeutic follow-up but is not the “holy grail” for diagnosis, Rebours acknowledged. For instance, 20% of IgG4-RD cases have normal IgG4 levels, 20% of pancreatic cancers show elevated IgG4 levels, and some patients achieve clinical remission despite persistently abnormal IgG4 levels. Without strong suspicion of type 1 AIP, measuring IgG4 levels is “zero cost-effective.”
This disease, which is associated with the risk for underlying cancer, requires extensive imaging (CT, MRI, and endoscopic ultrasound) to differentiate between AIP and cancer. This step is essential to avoid unnecessary surgery on organs affected by IgG4-RD or for treating cancer with corticosteroids.
A version of this article appeared on Medscape.com.
“Defined about 30 years ago, autoimmune pancreatitis [AIP] remains a diagnostic challenge,” said Vinciane Rebours, MD, PhD, professor and head of the Pancreatology and Digestive Oncology Department, Beaujon Hospital in Clichy, France. She spoke at the Francophone Days of Hepatology, Gastroenterology, and Digestive Oncology 2025, held in Paris. The challenge lies in the fact that AIP includes two distinct clinical entities, neither of which is truly autoimmune. However, much remains unknown, including its natural history, cancer risk, and optimal treatment strategies. However, some aspects are now better understood.
Autoimmune Pancreatitis
These forms differ in their histological characteristics. Type 1 exhibits lymphoplasmacytic infiltration, extensive fibrosis, and IgG4-positive plasma cells. Type 2 presents with granulocytic lesions similar to those in Crohn’s disease.
Type 1 AIP typically affects men aged 50 years or older and is often associated with jaundice, pseudotumor formation, diabetes, and exocrine pancreatic insufficiency. “It is a systemic disease where lymphoplasmacytic infiltration can affect multiple organs, with the pancreas and lymph nodes most commonly involved,” said Rebours.
A definitive diagnosis of type 1 AIP requires three criteria: Organ involvement, serum IgG4 levels more than twice the normal level, and histological abnormalities on biopsy. If one of these criteria is missing, the diagnosis is considered probable or possible.
Diagnosing type 1 AIP is challenging because it can affect multiple organs, often with few symptoms, leading to significant clinical variability. Type 2 AIP, in contrast, generally affects younger individuals, with no gender preference. It is pathophysiologically distinct and is linked to IBD in 87% of cases. Diagnosis relies on clinical criteria, imaging abnormalities (parenchymal or ductal changes identifiable on scans), response to corticosteroids in symptomatic patients, and the presence of IBD. The absence of IgG4 can also aid in the diagnosis. However, gathering all these elements can be difficult.
Evolving Treatment
Symptomatic patients and those at risk for organ failure, particularly lung and kidney failure, are eligible for induction treatment. This involves the administration of full-dose corticosteroids for 4 weeks, followed by a tapering regimen. Remission was achieved in 99% of type 1 and 92% of type 2 cases. Corticosteroids can also be used as a “trial treatment” to assess corticosteroid sensitivity in patients with type 2 AIP.
The risk for recurrence (in case of nonresponse or recurrence before 12 months posttreatment) is higher in type 1 (one third of cases) than in type 2 (15%). In such cases, immunomodulators, primarily rituximab, are recommended for type 1 AIP. Rituximab can also be used as an induction treatment, either alone or in combination, or as maintenance therapy. Alternatives include mycophenolate mofetil or inebilizumab, which showed an 87% reduction in relapse risk according to data published in 2024.
Maintenance treatment for type 2 AIP is not yet fully standardized. The disease is often managed in a manner similar to that of IBD treatment. Rebours cautioned, “Management cannot stop at the pancreas; it is essential to detect all other paucisymptomatic manifestations through comprehensive annual imaging and biannual biological and functional screenings.”
Monitoring IgG4
Monitoring IgG4 levels is important for therapeutic follow-up but is not the “holy grail” for diagnosis, Rebours acknowledged. For instance, 20% of IgG4-RD cases have normal IgG4 levels, 20% of pancreatic cancers show elevated IgG4 levels, and some patients achieve clinical remission despite persistently abnormal IgG4 levels. Without strong suspicion of type 1 AIP, measuring IgG4 levels is “zero cost-effective.”
This disease, which is associated with the risk for underlying cancer, requires extensive imaging (CT, MRI, and endoscopic ultrasound) to differentiate between AIP and cancer. This step is essential to avoid unnecessary surgery on organs affected by IgG4-RD or for treating cancer with corticosteroids.
A version of this article appeared on Medscape.com.
Salvage Rendezvous Technique on Par With Precut Sphincterotomy for Tough Biliary Access
, new data suggest.
Selective deep cannulation of the common bile duct remains the key rate-limiting step in successful endoscopic retrograde cholangiopancreatography (ERCP), especially in benign biliary disease.
In cases of difficult cannulation, the traditional fallback has been precut sphincterotomy. Recently, EUS-RV has emerged as an alternative. However, head-to-head comparisons of these salvage techniques in homogeneous patient populations have been lacking, until now.
A team led by Arup Choudhury, MD, DM, with Department of Gastroenterology, Postgraduate Institute of Medical Education and Research, Chandigarh, India, compared the two salvage techniques in a single-center, randomized controlled trial of 100 patients with benign biliary disease and difficult bile duct cannulation.
There were 50 patients in each group. When one technique failed, patients were crossed over to the other technique.
The technical success rate for achieving deep biliary, the primary outcome measure, was similar with EUS-RV and precut sphincterotomy (92% and 90%, respectively; P = 1.00; relative risk [RR], 1.02), the authors reported in Annals of Internal Medicine.
Median procedure time was also comparable (10.1 minutes with EUS-RV and 9.75 minutes with precut sphincterotomy). As expected, radiation exposure was notably higher in the EUS-RV group (median, 200.2 vs 67.8 mGy).
There was no difference in overall complication rates (12% and 10%, respectively; RR, 1.20).
Five patients in each group (10%) developed post-ERCP pancreatitis (PEP); one patient in the EUS-RV had moderately severe pancreatitis, whereas the rest had mild pancreatitis.
In an exploratory analysis of the subcohort of 72 patients who did not have 1 or more inadvertent pancreatic duct cannulation, two (5.6%) patients in the precut sphincterotomy group had PEP, whereas none of the patients in the EUS-RV had PEP (RR, 0.21). The investigators caution that a larger, multicenter, randomized controlled trial would be required to evaluate the “probable benefit” of lower PEP in the EUS-RV approach.
None of the patients had bleeding or perforation, but two (4%) patients in the EUS-RV group had an infection after the intervention. One required repeated ERCP due to post procedure cholangitis, whereas the other developed lower respiratory tract infection with transient acute lung injury and sputum showing gram-negative organism. None of the patients required surgical intervention.
“Interestingly,” said the investigators, on crossover from one salvage technique to the other, all of the cases could be successfully cannulated, suggesting the two salvage techniques are “complementary to each other and can help achieve successful cannulation in all cases when used in any sequence.”
Summing up, they said it appears from this head-to-head comparison that both EUS-RV and precut sphincterotomy can be considered effective salvage techniques in expert centers with similar safety and success profiles.
Limitations included the single-center design with both procedures performed by expert operators. EUS-RV entailed additional cost of needle and use of a separate scope, and a cost-efficacy analysis was not done.
This study had no specific funding. Disclosures for the authors are available with the original article.
A version of this article appeared on Medscape.com.
, new data suggest.
Selective deep cannulation of the common bile duct remains the key rate-limiting step in successful endoscopic retrograde cholangiopancreatography (ERCP), especially in benign biliary disease.
In cases of difficult cannulation, the traditional fallback has been precut sphincterotomy. Recently, EUS-RV has emerged as an alternative. However, head-to-head comparisons of these salvage techniques in homogeneous patient populations have been lacking, until now.
A team led by Arup Choudhury, MD, DM, with Department of Gastroenterology, Postgraduate Institute of Medical Education and Research, Chandigarh, India, compared the two salvage techniques in a single-center, randomized controlled trial of 100 patients with benign biliary disease and difficult bile duct cannulation.
There were 50 patients in each group. When one technique failed, patients were crossed over to the other technique.
The technical success rate for achieving deep biliary, the primary outcome measure, was similar with EUS-RV and precut sphincterotomy (92% and 90%, respectively; P = 1.00; relative risk [RR], 1.02), the authors reported in Annals of Internal Medicine.
Median procedure time was also comparable (10.1 minutes with EUS-RV and 9.75 minutes with precut sphincterotomy). As expected, radiation exposure was notably higher in the EUS-RV group (median, 200.2 vs 67.8 mGy).
There was no difference in overall complication rates (12% and 10%, respectively; RR, 1.20).
Five patients in each group (10%) developed post-ERCP pancreatitis (PEP); one patient in the EUS-RV had moderately severe pancreatitis, whereas the rest had mild pancreatitis.
In an exploratory analysis of the subcohort of 72 patients who did not have 1 or more inadvertent pancreatic duct cannulation, two (5.6%) patients in the precut sphincterotomy group had PEP, whereas none of the patients in the EUS-RV had PEP (RR, 0.21). The investigators caution that a larger, multicenter, randomized controlled trial would be required to evaluate the “probable benefit” of lower PEP in the EUS-RV approach.
None of the patients had bleeding or perforation, but two (4%) patients in the EUS-RV group had an infection after the intervention. One required repeated ERCP due to post procedure cholangitis, whereas the other developed lower respiratory tract infection with transient acute lung injury and sputum showing gram-negative organism. None of the patients required surgical intervention.
“Interestingly,” said the investigators, on crossover from one salvage technique to the other, all of the cases could be successfully cannulated, suggesting the two salvage techniques are “complementary to each other and can help achieve successful cannulation in all cases when used in any sequence.”
Summing up, they said it appears from this head-to-head comparison that both EUS-RV and precut sphincterotomy can be considered effective salvage techniques in expert centers with similar safety and success profiles.
Limitations included the single-center design with both procedures performed by expert operators. EUS-RV entailed additional cost of needle and use of a separate scope, and a cost-efficacy analysis was not done.
This study had no specific funding. Disclosures for the authors are available with the original article.
A version of this article appeared on Medscape.com.
, new data suggest.
Selective deep cannulation of the common bile duct remains the key rate-limiting step in successful endoscopic retrograde cholangiopancreatography (ERCP), especially in benign biliary disease.
In cases of difficult cannulation, the traditional fallback has been precut sphincterotomy. Recently, EUS-RV has emerged as an alternative. However, head-to-head comparisons of these salvage techniques in homogeneous patient populations have been lacking, until now.
A team led by Arup Choudhury, MD, DM, with Department of Gastroenterology, Postgraduate Institute of Medical Education and Research, Chandigarh, India, compared the two salvage techniques in a single-center, randomized controlled trial of 100 patients with benign biliary disease and difficult bile duct cannulation.
There were 50 patients in each group. When one technique failed, patients were crossed over to the other technique.
The technical success rate for achieving deep biliary, the primary outcome measure, was similar with EUS-RV and precut sphincterotomy (92% and 90%, respectively; P = 1.00; relative risk [RR], 1.02), the authors reported in Annals of Internal Medicine.
Median procedure time was also comparable (10.1 minutes with EUS-RV and 9.75 minutes with precut sphincterotomy). As expected, radiation exposure was notably higher in the EUS-RV group (median, 200.2 vs 67.8 mGy).
There was no difference in overall complication rates (12% and 10%, respectively; RR, 1.20).
Five patients in each group (10%) developed post-ERCP pancreatitis (PEP); one patient in the EUS-RV had moderately severe pancreatitis, whereas the rest had mild pancreatitis.
In an exploratory analysis of the subcohort of 72 patients who did not have 1 or more inadvertent pancreatic duct cannulation, two (5.6%) patients in the precut sphincterotomy group had PEP, whereas none of the patients in the EUS-RV had PEP (RR, 0.21). The investigators caution that a larger, multicenter, randomized controlled trial would be required to evaluate the “probable benefit” of lower PEP in the EUS-RV approach.
None of the patients had bleeding or perforation, but two (4%) patients in the EUS-RV group had an infection after the intervention. One required repeated ERCP due to post procedure cholangitis, whereas the other developed lower respiratory tract infection with transient acute lung injury and sputum showing gram-negative organism. None of the patients required surgical intervention.
“Interestingly,” said the investigators, on crossover from one salvage technique to the other, all of the cases could be successfully cannulated, suggesting the two salvage techniques are “complementary to each other and can help achieve successful cannulation in all cases when used in any sequence.”
Summing up, they said it appears from this head-to-head comparison that both EUS-RV and precut sphincterotomy can be considered effective salvage techniques in expert centers with similar safety and success profiles.
Limitations included the single-center design with both procedures performed by expert operators. EUS-RV entailed additional cost of needle and use of a separate scope, and a cost-efficacy analysis was not done.
This study had no specific funding. Disclosures for the authors are available with the original article.
A version of this article appeared on Medscape.com.
A Common Pancreatic Condition That Few Have Heard Of
— a disorder experienced by roughly one fifth of the world’s population. Although it is more common than type 2 diabetes, pancreatitis, and pancreatic cancer combined, it has remained relatively obscure.
By contrast, fatty liver — once called nonalcoholic fatty liver disease and recently renamed metabolic dysfunction–associated steatotic liver disease (MASLD) — is well-known.
“When it comes to diseases of the liver and pancreas, the liver is the big brother that has gotten all the attention, while the pancreas is the neglected little stepbrother that’s not sufficiently profiled in most medical textbooks and gets very little attention,” Max Petrov, MD, MPH, PhD, professor of pancreatology, University of Auckland, New Zealand, said in an interview. “The phenomenon of fatty pancreas has been observed for decades, but it is underappreciated and underrecognized.”
As early as 1926, fat depositions were identified during autopsies, but the condition remained relatively unknown, Mohammad Bilal, MD, associate professor of medicine-gastroenterology, University of Colorado Anschutz Medical Campus, Aurora, said in an interview. “Fortunately, FPD has recently been receiving more focus.”
Generally, healthy individuals have small amounts of fat in their pancreas. IPFD is defined as “the diffuse presence of fat in the pancreas, measured on a continuous scale,” and FPD refers to IPFD above the upper limit of normal. While there is no clear consensus as to what the normal range is, studies suggest it’s a pancreatic fat content ranging from 1.8% to 10.4%.
FPD’s “most important implication is that it can be a precursor for more challenging and burdensome diseases of the pancreas,” Petrov said.
Fatty changes in the pancreas affect both its endocrine and exocrine systems. FPD is associated with type 2 diabetes, the most common disease of the endocrine pancreas, as well as pancreatitis and pancreatic cancer, the most common diseases of the exocrine pancreas. It’s also implicated in the development of carotid atherosclerosis, pancreatic fistula following surgery, and exocrine pancreatic insufficiency (EPI).
A ‘Pandora’s Box’
Up to half of people with fatty pancreas are lean. The condition isn’t merely caused by an overflow of fat from the liver into the pancreas in people who consume more calories than they burn, Petrov said. Neither robust postmortem nor biopsy studies have found a statistically significant association between fatty deposition in the pancreas and liver fat.
Compared with the way people accumulate liver fat, the development of FPD is more complex, Petrov said.
“Hepatic fat is a relatively simple process: Lipid droplets accumulate in the hepatocytes; but, in the pancreas, there are several ways by which fat may accumulate,” he said.
One relates to the location of the pancreas within visceral, retroperitoneal fat, Petrov said. That fat can migrate and build up between pancreatic lobules.
Fat also can accumulate inside the lobes. This process can involve a buildup of fat droplets in acinar and stellate cells on the exocrine side and in the islets of Langerhans on the endocrine side. Additionally, when functional pancreatic cells die, particularly acinar cells, adult stem cells may replace them with adipocytes. Transformation of acinar cells into fat cells — a process called acinar-to-adipocyte transdifferentiation — also may be a way fat accumulates inside the lobes, Petrov said.
The accumulation of fat is a response to a wide array of insults to the pancreas over time. For example, obesity and metabolic syndrome lead to the accumulation of adipocytes and fat infiltration, whereas alcohol abuse and viral infections may lead to the death of acinar cells, which produce digestive enzymes.
Ultimately, the negative changes produced by excess fat in the pancreas are the origin of all common noninherited pancreatic diseases, bringing them under one umbrella, Petrov maintained. He dubbed this hypothesis PANcreatic Diseases Originating from intRapancreatic fAt (PANDORA).
The type of cells involved has implications for which disease may arise. For example, fat infiltration in stellate cells may promote pancreatic cancer, whereas its accumulation in the islets of Langerhans, which produce insulin and glucagon, is associated with type 2 diabetes.
The PANDORA hypothesis has eight foundational principles:
- Fatty pancreas is a key driver of pancreatic diseases in most people.
- Inflammation within the pancreatic microenvironment results from overwhelming lipotoxicity fueled by fatty pancreas.
- Aberrant communication between acinar cells involving lipid droplets drives acute pancreatitis.
- The pancreas responds to lipotoxicity with fibrosis and calcification — the hallmarks of chronic pancreatitis.
- Fat deposition affects signaling between stellate cells and other components of the microenvironment in ways that raise the risk for pancreatic cancer.
- The development of diabetes of the exocrine pancreas and EPI is affected by the presence of fatty pancreas.
- The higher risk for pancreatic disease in older adults is influenced by fatty pancreas.
- The multipronged nature of intrapancreatic fat deposition accounts for the common development of one pancreatic disease after another.
The idea that all common pancreatic diseases are the result of pathways emanating from FPD could “explain the bidirectional relationship between diabetes and pancreatitis or pancreatic cancer,” Petrov said.
Risk Factors, Symptoms, and Diagnosis
A variety of risk factors are involved in the accumulation of fat that may lead to pancreatic diseases, including aging, cholelithiasis, dyslipidemia, drugs/toxins (eg, steroids), genetic predisposition, iron overload, diet (eg, fatty foods, ultraprocessed foods), heavy alcohol use, overweight/obesity, pancreatic duct obstruction, tobacco use, viral infection (eg, hepatitis B, COVID-19), severe malnutrition, prediabetes, and dysglycemia.
Petrov described FPD as a “silent disease” that’s often asymptomatic, with its presence emerging as an incidental finding during abdominal ultrasonography for other reasons. However, patients may sometimes experience stomach pain or nausea if they have concurrent diseases of the pancreas, he said.
There are no currently available lab tests that can definitively detect the presence of FPD. Rather, the gold standard for a noninvasive diagnosis of FPD is MRI, with CT as the second-best choice, Petrov said.
In countries where advanced imaging is not available, a low-cost alternative might be a simple abdominal ultrasound, but it is not definitive, he said. “It’s operator-dependent and can be subjective.”
Some risk factors, such as derangements of glucose and lipid metabolism, especially in the presence of heavy alcohol use and a high-fat diet, can “be detected on lab tests,” Petrov said. “This, in combination with the abdominal ultrasound, might suggest the patients will benefit from deeper investigation, including MRI.”
Because the exocrine pancreas helps with digestion of fatty food, intralobular fatty deposits or replacement of pancreatic exocrine cells with adipose cells can lead to steatorrhea, Bilal said.
“Fat within the stool or oily diarrhea is a clue to the presence of FPD,” Bilal said.
Although this symptom isn’t unique to FPD and is found in other types of pancreatic conditions, its presence suggests that further investigation for FPD is warranted, he added.
Common-Sense Treatment Approaches
At present, there are no US Food and Drug Administration–approved treatments for FPD, Petrov said.
“What might be recommended is something along the lines of treatment of MASLD — appropriate diet and physical activity,” he said. Petrov hopes that as the disease entity garners more research attention, more clinical drug trials will be initiated, and new medications are found and approved.
Petrov suggested that there could be a “theoretical rationale” for the use of glucagon-like peptide 1 receptor agonists (GLP-1 RAs) as a treatment, given their effectiveness in multiple conditions, including MASLD, but no human trials have robustly shown specific benefits of these drugs for FPD.
Petrov added that, to date, 12 classes of drugs have been investigated for reducing IPFD: biguanides, sulfonylureas, GLP-1 RAs, thiazolidinediones, dipeptidyl peptidase–4 (DPP-4) inhibitors, sodium-glucose cotransporter 2 inhibitors, statins, fibrates, pancreatic lipase inhibitors, angiotensin II receptor blockers, somatostatin receptor agonists, and antioxidants.
Of these, most have shown promise in preclinical animal models. But only thiazolidinediones, GLP-1 RAs, DPP-4 inhibitors, and somatostatin receptor agonists have been investigated in randomized controlled trials in humans. The findings have been inconsistent, with the active treatment often not achieving statistically significant improvements.
“At this stage of our knowledge, we can’t recommend a specific pharmacotherapy,” Petrov said. But we can suggest dietary changes, such as saturated fat reduction, alcohol reduction, smoking cessation, reduction in consumption of ultraprocessed food, physical exercise, and addressing obesity and other drivers of metabolic disease.
Bilal, who is also a spokesperson for AGA, suggested that pancreatic enzyme replacement therapy, often used to treat pancreatic EPI, may treat some symptoms of FPD such as diarrhea.
Bariatric surgery has shown promise for FPD, in that it can decrease the patient’s body mass and potentially reduce the fat in the pancreas as well as it can improve metabolic diseases and hyperlipidemia. One study showed that it significantly decreased IPFD, fatty acid uptake, and blood flow, and these improvements were associated with more favorable glucose homeostasis and beta-cell function.
However, bariatric surgery is only appropriate for certain patients; is associated with potentially adverse sequelae including malnutrition, anemia, and digestive tract stenosis; and is currently not indicated for FPD.
Bilal advises clinicians to “keep an eye on FPD” if it’s detected incidentally and to screen patients more carefully for MASLD, metabolic disease, and diabetes.
“Although there are no consensus guidelines and recommendations for managing FPD at present, these common-sense approaches will benefit the patient’s overall health and hopefully will have a beneficial impact on pancreatic health as well,” he said.
Petrov reported no relevant financial relationships. Bilal reported being a consultant for Boston Scientific, Steris Endoscopy, and Cook Medical.
A version of this article first appeared on Medscape.com.
— a disorder experienced by roughly one fifth of the world’s population. Although it is more common than type 2 diabetes, pancreatitis, and pancreatic cancer combined, it has remained relatively obscure.
By contrast, fatty liver — once called nonalcoholic fatty liver disease and recently renamed metabolic dysfunction–associated steatotic liver disease (MASLD) — is well-known.
“When it comes to diseases of the liver and pancreas, the liver is the big brother that has gotten all the attention, while the pancreas is the neglected little stepbrother that’s not sufficiently profiled in most medical textbooks and gets very little attention,” Max Petrov, MD, MPH, PhD, professor of pancreatology, University of Auckland, New Zealand, said in an interview. “The phenomenon of fatty pancreas has been observed for decades, but it is underappreciated and underrecognized.”
As early as 1926, fat depositions were identified during autopsies, but the condition remained relatively unknown, Mohammad Bilal, MD, associate professor of medicine-gastroenterology, University of Colorado Anschutz Medical Campus, Aurora, said in an interview. “Fortunately, FPD has recently been receiving more focus.”
Generally, healthy individuals have small amounts of fat in their pancreas. IPFD is defined as “the diffuse presence of fat in the pancreas, measured on a continuous scale,” and FPD refers to IPFD above the upper limit of normal. While there is no clear consensus as to what the normal range is, studies suggest it’s a pancreatic fat content ranging from 1.8% to 10.4%.
FPD’s “most important implication is that it can be a precursor for more challenging and burdensome diseases of the pancreas,” Petrov said.
Fatty changes in the pancreas affect both its endocrine and exocrine systems. FPD is associated with type 2 diabetes, the most common disease of the endocrine pancreas, as well as pancreatitis and pancreatic cancer, the most common diseases of the exocrine pancreas. It’s also implicated in the development of carotid atherosclerosis, pancreatic fistula following surgery, and exocrine pancreatic insufficiency (EPI).
A ‘Pandora’s Box’
Up to half of people with fatty pancreas are lean. The condition isn’t merely caused by an overflow of fat from the liver into the pancreas in people who consume more calories than they burn, Petrov said. Neither robust postmortem nor biopsy studies have found a statistically significant association between fatty deposition in the pancreas and liver fat.
Compared with the way people accumulate liver fat, the development of FPD is more complex, Petrov said.
“Hepatic fat is a relatively simple process: Lipid droplets accumulate in the hepatocytes; but, in the pancreas, there are several ways by which fat may accumulate,” he said.
One relates to the location of the pancreas within visceral, retroperitoneal fat, Petrov said. That fat can migrate and build up between pancreatic lobules.
Fat also can accumulate inside the lobes. This process can involve a buildup of fat droplets in acinar and stellate cells on the exocrine side and in the islets of Langerhans on the endocrine side. Additionally, when functional pancreatic cells die, particularly acinar cells, adult stem cells may replace them with adipocytes. Transformation of acinar cells into fat cells — a process called acinar-to-adipocyte transdifferentiation — also may be a way fat accumulates inside the lobes, Petrov said.
The accumulation of fat is a response to a wide array of insults to the pancreas over time. For example, obesity and metabolic syndrome lead to the accumulation of adipocytes and fat infiltration, whereas alcohol abuse and viral infections may lead to the death of acinar cells, which produce digestive enzymes.
Ultimately, the negative changes produced by excess fat in the pancreas are the origin of all common noninherited pancreatic diseases, bringing them under one umbrella, Petrov maintained. He dubbed this hypothesis PANcreatic Diseases Originating from intRapancreatic fAt (PANDORA).
The type of cells involved has implications for which disease may arise. For example, fat infiltration in stellate cells may promote pancreatic cancer, whereas its accumulation in the islets of Langerhans, which produce insulin and glucagon, is associated with type 2 diabetes.
The PANDORA hypothesis has eight foundational principles:
- Fatty pancreas is a key driver of pancreatic diseases in most people.
- Inflammation within the pancreatic microenvironment results from overwhelming lipotoxicity fueled by fatty pancreas.
- Aberrant communication between acinar cells involving lipid droplets drives acute pancreatitis.
- The pancreas responds to lipotoxicity with fibrosis and calcification — the hallmarks of chronic pancreatitis.
- Fat deposition affects signaling between stellate cells and other components of the microenvironment in ways that raise the risk for pancreatic cancer.
- The development of diabetes of the exocrine pancreas and EPI is affected by the presence of fatty pancreas.
- The higher risk for pancreatic disease in older adults is influenced by fatty pancreas.
- The multipronged nature of intrapancreatic fat deposition accounts for the common development of one pancreatic disease after another.
The idea that all common pancreatic diseases are the result of pathways emanating from FPD could “explain the bidirectional relationship between diabetes and pancreatitis or pancreatic cancer,” Petrov said.
Risk Factors, Symptoms, and Diagnosis
A variety of risk factors are involved in the accumulation of fat that may lead to pancreatic diseases, including aging, cholelithiasis, dyslipidemia, drugs/toxins (eg, steroids), genetic predisposition, iron overload, diet (eg, fatty foods, ultraprocessed foods), heavy alcohol use, overweight/obesity, pancreatic duct obstruction, tobacco use, viral infection (eg, hepatitis B, COVID-19), severe malnutrition, prediabetes, and dysglycemia.
Petrov described FPD as a “silent disease” that’s often asymptomatic, with its presence emerging as an incidental finding during abdominal ultrasonography for other reasons. However, patients may sometimes experience stomach pain or nausea if they have concurrent diseases of the pancreas, he said.
There are no currently available lab tests that can definitively detect the presence of FPD. Rather, the gold standard for a noninvasive diagnosis of FPD is MRI, with CT as the second-best choice, Petrov said.
In countries where advanced imaging is not available, a low-cost alternative might be a simple abdominal ultrasound, but it is not definitive, he said. “It’s operator-dependent and can be subjective.”
Some risk factors, such as derangements of glucose and lipid metabolism, especially in the presence of heavy alcohol use and a high-fat diet, can “be detected on lab tests,” Petrov said. “This, in combination with the abdominal ultrasound, might suggest the patients will benefit from deeper investigation, including MRI.”
Because the exocrine pancreas helps with digestion of fatty food, intralobular fatty deposits or replacement of pancreatic exocrine cells with adipose cells can lead to steatorrhea, Bilal said.
“Fat within the stool or oily diarrhea is a clue to the presence of FPD,” Bilal said.
Although this symptom isn’t unique to FPD and is found in other types of pancreatic conditions, its presence suggests that further investigation for FPD is warranted, he added.
Common-Sense Treatment Approaches
At present, there are no US Food and Drug Administration–approved treatments for FPD, Petrov said.
“What might be recommended is something along the lines of treatment of MASLD — appropriate diet and physical activity,” he said. Petrov hopes that as the disease entity garners more research attention, more clinical drug trials will be initiated, and new medications are found and approved.
Petrov suggested that there could be a “theoretical rationale” for the use of glucagon-like peptide 1 receptor agonists (GLP-1 RAs) as a treatment, given their effectiveness in multiple conditions, including MASLD, but no human trials have robustly shown specific benefits of these drugs for FPD.
Petrov added that, to date, 12 classes of drugs have been investigated for reducing IPFD: biguanides, sulfonylureas, GLP-1 RAs, thiazolidinediones, dipeptidyl peptidase–4 (DPP-4) inhibitors, sodium-glucose cotransporter 2 inhibitors, statins, fibrates, pancreatic lipase inhibitors, angiotensin II receptor blockers, somatostatin receptor agonists, and antioxidants.
Of these, most have shown promise in preclinical animal models. But only thiazolidinediones, GLP-1 RAs, DPP-4 inhibitors, and somatostatin receptor agonists have been investigated in randomized controlled trials in humans. The findings have been inconsistent, with the active treatment often not achieving statistically significant improvements.
“At this stage of our knowledge, we can’t recommend a specific pharmacotherapy,” Petrov said. But we can suggest dietary changes, such as saturated fat reduction, alcohol reduction, smoking cessation, reduction in consumption of ultraprocessed food, physical exercise, and addressing obesity and other drivers of metabolic disease.
Bilal, who is also a spokesperson for AGA, suggested that pancreatic enzyme replacement therapy, often used to treat pancreatic EPI, may treat some symptoms of FPD such as diarrhea.
Bariatric surgery has shown promise for FPD, in that it can decrease the patient’s body mass and potentially reduce the fat in the pancreas as well as it can improve metabolic diseases and hyperlipidemia. One study showed that it significantly decreased IPFD, fatty acid uptake, and blood flow, and these improvements were associated with more favorable glucose homeostasis and beta-cell function.
However, bariatric surgery is only appropriate for certain patients; is associated with potentially adverse sequelae including malnutrition, anemia, and digestive tract stenosis; and is currently not indicated for FPD.
Bilal advises clinicians to “keep an eye on FPD” if it’s detected incidentally and to screen patients more carefully for MASLD, metabolic disease, and diabetes.
“Although there are no consensus guidelines and recommendations for managing FPD at present, these common-sense approaches will benefit the patient’s overall health and hopefully will have a beneficial impact on pancreatic health as well,” he said.
Petrov reported no relevant financial relationships. Bilal reported being a consultant for Boston Scientific, Steris Endoscopy, and Cook Medical.
A version of this article first appeared on Medscape.com.
— a disorder experienced by roughly one fifth of the world’s population. Although it is more common than type 2 diabetes, pancreatitis, and pancreatic cancer combined, it has remained relatively obscure.
By contrast, fatty liver — once called nonalcoholic fatty liver disease and recently renamed metabolic dysfunction–associated steatotic liver disease (MASLD) — is well-known.
“When it comes to diseases of the liver and pancreas, the liver is the big brother that has gotten all the attention, while the pancreas is the neglected little stepbrother that’s not sufficiently profiled in most medical textbooks and gets very little attention,” Max Petrov, MD, MPH, PhD, professor of pancreatology, University of Auckland, New Zealand, said in an interview. “The phenomenon of fatty pancreas has been observed for decades, but it is underappreciated and underrecognized.”
As early as 1926, fat depositions were identified during autopsies, but the condition remained relatively unknown, Mohammad Bilal, MD, associate professor of medicine-gastroenterology, University of Colorado Anschutz Medical Campus, Aurora, said in an interview. “Fortunately, FPD has recently been receiving more focus.”
Generally, healthy individuals have small amounts of fat in their pancreas. IPFD is defined as “the diffuse presence of fat in the pancreas, measured on a continuous scale,” and FPD refers to IPFD above the upper limit of normal. While there is no clear consensus as to what the normal range is, studies suggest it’s a pancreatic fat content ranging from 1.8% to 10.4%.
FPD’s “most important implication is that it can be a precursor for more challenging and burdensome diseases of the pancreas,” Petrov said.
Fatty changes in the pancreas affect both its endocrine and exocrine systems. FPD is associated with type 2 diabetes, the most common disease of the endocrine pancreas, as well as pancreatitis and pancreatic cancer, the most common diseases of the exocrine pancreas. It’s also implicated in the development of carotid atherosclerosis, pancreatic fistula following surgery, and exocrine pancreatic insufficiency (EPI).
A ‘Pandora’s Box’
Up to half of people with fatty pancreas are lean. The condition isn’t merely caused by an overflow of fat from the liver into the pancreas in people who consume more calories than they burn, Petrov said. Neither robust postmortem nor biopsy studies have found a statistically significant association between fatty deposition in the pancreas and liver fat.
Compared with the way people accumulate liver fat, the development of FPD is more complex, Petrov said.
“Hepatic fat is a relatively simple process: Lipid droplets accumulate in the hepatocytes; but, in the pancreas, there are several ways by which fat may accumulate,” he said.
One relates to the location of the pancreas within visceral, retroperitoneal fat, Petrov said. That fat can migrate and build up between pancreatic lobules.
Fat also can accumulate inside the lobes. This process can involve a buildup of fat droplets in acinar and stellate cells on the exocrine side and in the islets of Langerhans on the endocrine side. Additionally, when functional pancreatic cells die, particularly acinar cells, adult stem cells may replace them with adipocytes. Transformation of acinar cells into fat cells — a process called acinar-to-adipocyte transdifferentiation — also may be a way fat accumulates inside the lobes, Petrov said.
The accumulation of fat is a response to a wide array of insults to the pancreas over time. For example, obesity and metabolic syndrome lead to the accumulation of adipocytes and fat infiltration, whereas alcohol abuse and viral infections may lead to the death of acinar cells, which produce digestive enzymes.
Ultimately, the negative changes produced by excess fat in the pancreas are the origin of all common noninherited pancreatic diseases, bringing them under one umbrella, Petrov maintained. He dubbed this hypothesis PANcreatic Diseases Originating from intRapancreatic fAt (PANDORA).
The type of cells involved has implications for which disease may arise. For example, fat infiltration in stellate cells may promote pancreatic cancer, whereas its accumulation in the islets of Langerhans, which produce insulin and glucagon, is associated with type 2 diabetes.
The PANDORA hypothesis has eight foundational principles:
- Fatty pancreas is a key driver of pancreatic diseases in most people.
- Inflammation within the pancreatic microenvironment results from overwhelming lipotoxicity fueled by fatty pancreas.
- Aberrant communication between acinar cells involving lipid droplets drives acute pancreatitis.
- The pancreas responds to lipotoxicity with fibrosis and calcification — the hallmarks of chronic pancreatitis.
- Fat deposition affects signaling between stellate cells and other components of the microenvironment in ways that raise the risk for pancreatic cancer.
- The development of diabetes of the exocrine pancreas and EPI is affected by the presence of fatty pancreas.
- The higher risk for pancreatic disease in older adults is influenced by fatty pancreas.
- The multipronged nature of intrapancreatic fat deposition accounts for the common development of one pancreatic disease after another.
The idea that all common pancreatic diseases are the result of pathways emanating from FPD could “explain the bidirectional relationship between diabetes and pancreatitis or pancreatic cancer,” Petrov said.
Risk Factors, Symptoms, and Diagnosis
A variety of risk factors are involved in the accumulation of fat that may lead to pancreatic diseases, including aging, cholelithiasis, dyslipidemia, drugs/toxins (eg, steroids), genetic predisposition, iron overload, diet (eg, fatty foods, ultraprocessed foods), heavy alcohol use, overweight/obesity, pancreatic duct obstruction, tobacco use, viral infection (eg, hepatitis B, COVID-19), severe malnutrition, prediabetes, and dysglycemia.
Petrov described FPD as a “silent disease” that’s often asymptomatic, with its presence emerging as an incidental finding during abdominal ultrasonography for other reasons. However, patients may sometimes experience stomach pain or nausea if they have concurrent diseases of the pancreas, he said.
There are no currently available lab tests that can definitively detect the presence of FPD. Rather, the gold standard for a noninvasive diagnosis of FPD is MRI, with CT as the second-best choice, Petrov said.
In countries where advanced imaging is not available, a low-cost alternative might be a simple abdominal ultrasound, but it is not definitive, he said. “It’s operator-dependent and can be subjective.”
Some risk factors, such as derangements of glucose and lipid metabolism, especially in the presence of heavy alcohol use and a high-fat diet, can “be detected on lab tests,” Petrov said. “This, in combination with the abdominal ultrasound, might suggest the patients will benefit from deeper investigation, including MRI.”
Because the exocrine pancreas helps with digestion of fatty food, intralobular fatty deposits or replacement of pancreatic exocrine cells with adipose cells can lead to steatorrhea, Bilal said.
“Fat within the stool or oily diarrhea is a clue to the presence of FPD,” Bilal said.
Although this symptom isn’t unique to FPD and is found in other types of pancreatic conditions, its presence suggests that further investigation for FPD is warranted, he added.
Common-Sense Treatment Approaches
At present, there are no US Food and Drug Administration–approved treatments for FPD, Petrov said.
“What might be recommended is something along the lines of treatment of MASLD — appropriate diet and physical activity,” he said. Petrov hopes that as the disease entity garners more research attention, more clinical drug trials will be initiated, and new medications are found and approved.
Petrov suggested that there could be a “theoretical rationale” for the use of glucagon-like peptide 1 receptor agonists (GLP-1 RAs) as a treatment, given their effectiveness in multiple conditions, including MASLD, but no human trials have robustly shown specific benefits of these drugs for FPD.
Petrov added that, to date, 12 classes of drugs have been investigated for reducing IPFD: biguanides, sulfonylureas, GLP-1 RAs, thiazolidinediones, dipeptidyl peptidase–4 (DPP-4) inhibitors, sodium-glucose cotransporter 2 inhibitors, statins, fibrates, pancreatic lipase inhibitors, angiotensin II receptor blockers, somatostatin receptor agonists, and antioxidants.
Of these, most have shown promise in preclinical animal models. But only thiazolidinediones, GLP-1 RAs, DPP-4 inhibitors, and somatostatin receptor agonists have been investigated in randomized controlled trials in humans. The findings have been inconsistent, with the active treatment often not achieving statistically significant improvements.
“At this stage of our knowledge, we can’t recommend a specific pharmacotherapy,” Petrov said. But we can suggest dietary changes, such as saturated fat reduction, alcohol reduction, smoking cessation, reduction in consumption of ultraprocessed food, physical exercise, and addressing obesity and other drivers of metabolic disease.
Bilal, who is also a spokesperson for AGA, suggested that pancreatic enzyme replacement therapy, often used to treat pancreatic EPI, may treat some symptoms of FPD such as diarrhea.
Bariatric surgery has shown promise for FPD, in that it can decrease the patient’s body mass and potentially reduce the fat in the pancreas as well as it can improve metabolic diseases and hyperlipidemia. One study showed that it significantly decreased IPFD, fatty acid uptake, and blood flow, and these improvements were associated with more favorable glucose homeostasis and beta-cell function.
However, bariatric surgery is only appropriate for certain patients; is associated with potentially adverse sequelae including malnutrition, anemia, and digestive tract stenosis; and is currently not indicated for FPD.
Bilal advises clinicians to “keep an eye on FPD” if it’s detected incidentally and to screen patients more carefully for MASLD, metabolic disease, and diabetes.
“Although there are no consensus guidelines and recommendations for managing FPD at present, these common-sense approaches will benefit the patient’s overall health and hopefully will have a beneficial impact on pancreatic health as well,” he said.
Petrov reported no relevant financial relationships. Bilal reported being a consultant for Boston Scientific, Steris Endoscopy, and Cook Medical.
A version of this article first appeared on Medscape.com.
Two Cystic Duct Stents Appear Better Than One
according to a retrospective multicenter study.
These findings suggest that endoscopists should prioritize dual stent placement when feasible, and consider adding a second stent in patients who previously received a single stent, James D. Haddad, MD, of the University of Texas Southwestern, Dallas, and colleagues reported.
The American Gastroenterological Association (AGA) has recognized the role of endoscopic drainage in managing acute cholecystitis in high-risk patients, but specific guidance on optimal technique and follow-up remains unclear, the investigators wrote in Techniques and Innovations in Gastrointestinal Endoscopy.
“Despite accumulating data and increased interest in this technique, clear guidance on the ideal strategy for ETGBD is lacking,” Dr. Haddad and colleagues wrote. “For example, the optimal size, number, and follow-up of cystic duct stents for patients undergoing ETGBD has not been well established.”
To address this knowledge gap, the investigators analyzed data from 75 patients at five academic medical centers who had undergone ETGBD between June 2013 and October 2022. Patients were divided into two groups based on whether they received one or two cystic duct stents.
The primary outcome was clinical success, defined as symptom resolution without requiring another drainage procedure. Secondary outcomes included technical success (defined as successful stent placement), along with rates of adverse events and unplanned reinterventions.
Out of the 75 patients, 59 received a single stent, while 16 received dual stents. The median follow-up time was 407 days overall, with a longer follow-up in the single-stent group (433 days), compared with the double-stent group (118 days).
Clinical success was reported in 81.3% of cases, which technical success was achieved in 88.2% of cases.
Patients who received two stents had significantly lower rates of unplanned reintervention, compared with those who received a single stent (0% vs 25.4%; P = .02). The median time to unplanned reintervention in the single-stent group was 210 days.
Use of a 7 French stent was strongly associated with placement of two stents (odd ratio [OR], 15.5; P = .01). Similarly, patients with a prior percutaneous cholecystostomy tube were significantly more likely to have two stents placed (OR, 10.8; P = .001).
Adverse event rates were uncommon and not statistically different between groups, with an overall rate of 6.7%. Post-endoscopic retrograde cholangiopancreatography pancreatitis was the most common adverse event, occurring in two patients in the single-stent group and one patient in the double-stent group. There were no reported cases of cystic duct or gallbladder perforation.
“In conclusion,” the investigators wrote, “ETGBD with dual transpapillary gallbladder stenting is associated with a lower rate of unplanned reinterventions, compared with that with single stenting, and has a low rate of adverse events. Endoscopists performing ETGBD should consider planned exchange of solitary transpapillary gallbladder stents or interval ERCP for reattempted placement of a second stent if placement of two stents is not possible at the index ERCP.”
The investigators disclosed relationships with Boston Scientific, Motus GI, and ConMed.
according to a retrospective multicenter study.
These findings suggest that endoscopists should prioritize dual stent placement when feasible, and consider adding a second stent in patients who previously received a single stent, James D. Haddad, MD, of the University of Texas Southwestern, Dallas, and colleagues reported.
The American Gastroenterological Association (AGA) has recognized the role of endoscopic drainage in managing acute cholecystitis in high-risk patients, but specific guidance on optimal technique and follow-up remains unclear, the investigators wrote in Techniques and Innovations in Gastrointestinal Endoscopy.
“Despite accumulating data and increased interest in this technique, clear guidance on the ideal strategy for ETGBD is lacking,” Dr. Haddad and colleagues wrote. “For example, the optimal size, number, and follow-up of cystic duct stents for patients undergoing ETGBD has not been well established.”
To address this knowledge gap, the investigators analyzed data from 75 patients at five academic medical centers who had undergone ETGBD between June 2013 and October 2022. Patients were divided into two groups based on whether they received one or two cystic duct stents.
The primary outcome was clinical success, defined as symptom resolution without requiring another drainage procedure. Secondary outcomes included technical success (defined as successful stent placement), along with rates of adverse events and unplanned reinterventions.
Out of the 75 patients, 59 received a single stent, while 16 received dual stents. The median follow-up time was 407 days overall, with a longer follow-up in the single-stent group (433 days), compared with the double-stent group (118 days).
Clinical success was reported in 81.3% of cases, which technical success was achieved in 88.2% of cases.
Patients who received two stents had significantly lower rates of unplanned reintervention, compared with those who received a single stent (0% vs 25.4%; P = .02). The median time to unplanned reintervention in the single-stent group was 210 days.
Use of a 7 French stent was strongly associated with placement of two stents (odd ratio [OR], 15.5; P = .01). Similarly, patients with a prior percutaneous cholecystostomy tube were significantly more likely to have two stents placed (OR, 10.8; P = .001).
Adverse event rates were uncommon and not statistically different between groups, with an overall rate of 6.7%. Post-endoscopic retrograde cholangiopancreatography pancreatitis was the most common adverse event, occurring in two patients in the single-stent group and one patient in the double-stent group. There were no reported cases of cystic duct or gallbladder perforation.
“In conclusion,” the investigators wrote, “ETGBD with dual transpapillary gallbladder stenting is associated with a lower rate of unplanned reinterventions, compared with that with single stenting, and has a low rate of adverse events. Endoscopists performing ETGBD should consider planned exchange of solitary transpapillary gallbladder stents or interval ERCP for reattempted placement of a second stent if placement of two stents is not possible at the index ERCP.”
The investigators disclosed relationships with Boston Scientific, Motus GI, and ConMed.
according to a retrospective multicenter study.
These findings suggest that endoscopists should prioritize dual stent placement when feasible, and consider adding a second stent in patients who previously received a single stent, James D. Haddad, MD, of the University of Texas Southwestern, Dallas, and colleagues reported.
The American Gastroenterological Association (AGA) has recognized the role of endoscopic drainage in managing acute cholecystitis in high-risk patients, but specific guidance on optimal technique and follow-up remains unclear, the investigators wrote in Techniques and Innovations in Gastrointestinal Endoscopy.
“Despite accumulating data and increased interest in this technique, clear guidance on the ideal strategy for ETGBD is lacking,” Dr. Haddad and colleagues wrote. “For example, the optimal size, number, and follow-up of cystic duct stents for patients undergoing ETGBD has not been well established.”
To address this knowledge gap, the investigators analyzed data from 75 patients at five academic medical centers who had undergone ETGBD between June 2013 and October 2022. Patients were divided into two groups based on whether they received one or two cystic duct stents.
The primary outcome was clinical success, defined as symptom resolution without requiring another drainage procedure. Secondary outcomes included technical success (defined as successful stent placement), along with rates of adverse events and unplanned reinterventions.
Out of the 75 patients, 59 received a single stent, while 16 received dual stents. The median follow-up time was 407 days overall, with a longer follow-up in the single-stent group (433 days), compared with the double-stent group (118 days).
Clinical success was reported in 81.3% of cases, which technical success was achieved in 88.2% of cases.
Patients who received two stents had significantly lower rates of unplanned reintervention, compared with those who received a single stent (0% vs 25.4%; P = .02). The median time to unplanned reintervention in the single-stent group was 210 days.
Use of a 7 French stent was strongly associated with placement of two stents (odd ratio [OR], 15.5; P = .01). Similarly, patients with a prior percutaneous cholecystostomy tube were significantly more likely to have two stents placed (OR, 10.8; P = .001).
Adverse event rates were uncommon and not statistically different between groups, with an overall rate of 6.7%. Post-endoscopic retrograde cholangiopancreatography pancreatitis was the most common adverse event, occurring in two patients in the single-stent group and one patient in the double-stent group. There were no reported cases of cystic duct or gallbladder perforation.
“In conclusion,” the investigators wrote, “ETGBD with dual transpapillary gallbladder stenting is associated with a lower rate of unplanned reinterventions, compared with that with single stenting, and has a low rate of adverse events. Endoscopists performing ETGBD should consider planned exchange of solitary transpapillary gallbladder stents or interval ERCP for reattempted placement of a second stent if placement of two stents is not possible at the index ERCP.”
The investigators disclosed relationships with Boston Scientific, Motus GI, and ConMed.
FROM TECHNIQUES AND INNOVATIONS IN GASTROINTESTINAL ENDOSCOPY
Identifying Pancreatic Cancer Remains Elusive: Here’s Why
Now, a growing body of evidence indicates that this deadly cancer has been steadily on the rise, particularly in younger individuals who may not even realize they are at risk.
A recent survey, for instance, found that 33% of 1000 respondents younger than 50 years believe that only older adults are at risk for pancreatic cancer, and more than half said they wouldn’t even recognize the early signs and symptoms, which include unexplained weight loss, fatigue, jaundice, abdominal pain that radiates to the back, nausea, and vomiting.
These survey findings allude to a bigger challenge: Identifying the disease remains elusive against a backdrop of these increasing rates and nonspecific risks and symptoms.
Currently, only about 15% of pancreatic cancers are caught at a localized, resectable stage, when 5-year survival rates are highest at 44%. But most are found later, after symptoms arise, and at this point, the 5-year survival odds plummet —16% for regional disease, 3% for distant, and 1% for stage IV.
“This disease is too often a silent killer, with no symptoms until it has progressed to less treatable stages,” said survey coauthor Zobeida Cruz-Monserrate, PhD, in the division of gastroenterology, hepatology and nutrition at Ohio State University Medical Center, Columbus.
Rising Rates
Since 2001, rates of pancreatic cancer have steadily increased by about 1% annually, and this increase appears greater among younger individuals, especially women.
A recent study in Gastroenterology, for instance, found that, while overall rates of pancreatic cancer among people aged 15-34 years remained low (0.3% in women and 0.2% in men) between 2001 and 2018, the average annual percent change in this age group was considerably higher than that for older individuals — 6.45% for women and 2.97% for men compared with 1.11% for women aged 55 years and 1.17% for men aged 55 years. Another recent analysis, published in Annals of Internal Medicine, reported similar increased rates in men and women aged 15-39 years between 2011 and 2019.
Although more than 90% of cases do occur in those 55 years or older, “we’re now seeing this disease in people who are in their 40s much more regularly,” Cruz-Monserrate said. “This is a concerning trend — and more research is needed to learn why.”
But it’s early days. Studies so far indicate that early onset pancreatic cancer tends to be even more aggressive, but the “underlying reason is not yet clear,” researcher wrote in a 2025 review.
Some evidence indicates younger individuals may have distinct molecular characteristics, whereas other research shows younger and older patients have similar genetic profiles. Younger patients may also be more likely to smoke, drink more, and delay seeking medical attention as well as experience delays in being diagnosed by physicians, the authors explained.
Catching It Early
Given the rising rates, early detection is especially important.
There are some known genetic and medical risk factors for pancreatic cancer. About 10% of these cancers are linked to heredity risk or genetic markers, including BRCA1 and BRCA2 or Lynch syndrome. People with chronic pancreatitis, type 2 diabetes, obesity, or with a family history of pancreatic cancer face an elevated risk.
Lifestyle factors can play a role as well. Alcohol consumption, a poor diet that includes red or processed meat, and smoking increase people’s risk for pancreatic cancer. In fact, smoking leads to a twofold higher risk, compared with not smoking.
However, uncovering pancreatic cancer from these factors alone can be like “finding a needle in a haystack,” said Srinivas Gaddam, MD, head of the pancreatic cancer screening and early detection program at Cedars-Sinai Medical Center in Los Angeles.
One strategy to help detect the disease earlier would be to screen more.
The latest guidance from the American Cancer Society suggests that people with a genetic predisposition or a family history of pancreatic cancer could benefit from annual surveillance with endoscopic ultrasound or MRI.
But the US Preventive Services Task Force currently recommends against routine screening of average-risk asymptomatic adults (JAMA. 2019;322[5]:438-444). The task force found no evidence that screening for pancreatic cancer improves disease-specific morbidity or mortality or all-cause mortality.
“The absolute incidence in younger people is far too small to make screening beneficial,” explained The Lancet Gastroenterology & Hepatology editors in a 2023 editorial.
In fact, more screening could lead to overdiagnosis, a concern reinforced by the recent study in Annals of Internal Medicine. That analysis found that much of the observed increase in early-onset pancreatic cancer stemmed from the detection of more small, early-stage endocrine cancer, rather than pancreatic adenocarcinoma, whereas mortality from the disease remained stable over the study period.
Recent findings do “suggest the potential for overdiagnosis and overtreatment, particularly in cases of indolent pancreatic neuroendocrine tumors,” Gaddam said.
Gaddam has observed an increase in both adenocarcinoma and neuroendocrine tumors in the clinic and in his research, especially in women younger than 50 years, but he noted these early onset diagnoses do remain rare.
Staying Vigilant
As the understanding of pancreatic cancer risks and symptoms evolves, ensuring that patients, especially younger individuals, recognize the warning signs, without causing alarm, remains a challenge.
The disease “presents more advanced in younger patients, but symptoms are so nonspecific,” said Randall Brand, MD, AGAF, director of the gastrointestinal malignancy early detection, diagnosis, and prevention program at the University of Pittsburgh Medical Center, Pennsylvania. Given that, “I am not sure how to best highlight a communication approach that would not cause undue stress to the patient and our healthcare resources.”
Gaddam agreed that it’s tough to pinpoint or communicate straightforward risks or symptoms to the general public without potentially leading to unnecessary screening.
At a minimum, however, clinicians can share more general risk-mitigating strategies with their patients.
Communicating such strategies may be especially important for younger patients, given that the recent survey found almost 40% of younger adults believe there’s nothing they can do to change their risk for pancreatic cancer.
However, Cruz-Monserrate explained, adults of all ages can lower their risks through regular exercise, limited alcohol and tobacco use, and a healthy diet with less red meat or processed meat.
Ultimately, for clinicians, given how difficult it is now to identify pancreatic cancer early, we have to “follow their good clinical judgment when alarming features, such as weight loss or nuances of pancreatic pain arise, and then get good imaging,” Gaddam said.
Cruz-Monserrate, Brand, and Gaddam reported no relevant disclosures.
A version of this article appeared on Medscape.com.
Now, a growing body of evidence indicates that this deadly cancer has been steadily on the rise, particularly in younger individuals who may not even realize they are at risk.
A recent survey, for instance, found that 33% of 1000 respondents younger than 50 years believe that only older adults are at risk for pancreatic cancer, and more than half said they wouldn’t even recognize the early signs and symptoms, which include unexplained weight loss, fatigue, jaundice, abdominal pain that radiates to the back, nausea, and vomiting.
These survey findings allude to a bigger challenge: Identifying the disease remains elusive against a backdrop of these increasing rates and nonspecific risks and symptoms.
Currently, only about 15% of pancreatic cancers are caught at a localized, resectable stage, when 5-year survival rates are highest at 44%. But most are found later, after symptoms arise, and at this point, the 5-year survival odds plummet —16% for regional disease, 3% for distant, and 1% for stage IV.
“This disease is too often a silent killer, with no symptoms until it has progressed to less treatable stages,” said survey coauthor Zobeida Cruz-Monserrate, PhD, in the division of gastroenterology, hepatology and nutrition at Ohio State University Medical Center, Columbus.
Rising Rates
Since 2001, rates of pancreatic cancer have steadily increased by about 1% annually, and this increase appears greater among younger individuals, especially women.
A recent study in Gastroenterology, for instance, found that, while overall rates of pancreatic cancer among people aged 15-34 years remained low (0.3% in women and 0.2% in men) between 2001 and 2018, the average annual percent change in this age group was considerably higher than that for older individuals — 6.45% for women and 2.97% for men compared with 1.11% for women aged 55 years and 1.17% for men aged 55 years. Another recent analysis, published in Annals of Internal Medicine, reported similar increased rates in men and women aged 15-39 years between 2011 and 2019.
Although more than 90% of cases do occur in those 55 years or older, “we’re now seeing this disease in people who are in their 40s much more regularly,” Cruz-Monserrate said. “This is a concerning trend — and more research is needed to learn why.”
But it’s early days. Studies so far indicate that early onset pancreatic cancer tends to be even more aggressive, but the “underlying reason is not yet clear,” researcher wrote in a 2025 review.
Some evidence indicates younger individuals may have distinct molecular characteristics, whereas other research shows younger and older patients have similar genetic profiles. Younger patients may also be more likely to smoke, drink more, and delay seeking medical attention as well as experience delays in being diagnosed by physicians, the authors explained.
Catching It Early
Given the rising rates, early detection is especially important.
There are some known genetic and medical risk factors for pancreatic cancer. About 10% of these cancers are linked to heredity risk or genetic markers, including BRCA1 and BRCA2 or Lynch syndrome. People with chronic pancreatitis, type 2 diabetes, obesity, or with a family history of pancreatic cancer face an elevated risk.
Lifestyle factors can play a role as well. Alcohol consumption, a poor diet that includes red or processed meat, and smoking increase people’s risk for pancreatic cancer. In fact, smoking leads to a twofold higher risk, compared with not smoking.
However, uncovering pancreatic cancer from these factors alone can be like “finding a needle in a haystack,” said Srinivas Gaddam, MD, head of the pancreatic cancer screening and early detection program at Cedars-Sinai Medical Center in Los Angeles.
One strategy to help detect the disease earlier would be to screen more.
The latest guidance from the American Cancer Society suggests that people with a genetic predisposition or a family history of pancreatic cancer could benefit from annual surveillance with endoscopic ultrasound or MRI.
But the US Preventive Services Task Force currently recommends against routine screening of average-risk asymptomatic adults (JAMA. 2019;322[5]:438-444). The task force found no evidence that screening for pancreatic cancer improves disease-specific morbidity or mortality or all-cause mortality.
“The absolute incidence in younger people is far too small to make screening beneficial,” explained The Lancet Gastroenterology & Hepatology editors in a 2023 editorial.
In fact, more screening could lead to overdiagnosis, a concern reinforced by the recent study in Annals of Internal Medicine. That analysis found that much of the observed increase in early-onset pancreatic cancer stemmed from the detection of more small, early-stage endocrine cancer, rather than pancreatic adenocarcinoma, whereas mortality from the disease remained stable over the study period.
Recent findings do “suggest the potential for overdiagnosis and overtreatment, particularly in cases of indolent pancreatic neuroendocrine tumors,” Gaddam said.
Gaddam has observed an increase in both adenocarcinoma and neuroendocrine tumors in the clinic and in his research, especially in women younger than 50 years, but he noted these early onset diagnoses do remain rare.
Staying Vigilant
As the understanding of pancreatic cancer risks and symptoms evolves, ensuring that patients, especially younger individuals, recognize the warning signs, without causing alarm, remains a challenge.
The disease “presents more advanced in younger patients, but symptoms are so nonspecific,” said Randall Brand, MD, AGAF, director of the gastrointestinal malignancy early detection, diagnosis, and prevention program at the University of Pittsburgh Medical Center, Pennsylvania. Given that, “I am not sure how to best highlight a communication approach that would not cause undue stress to the patient and our healthcare resources.”
Gaddam agreed that it’s tough to pinpoint or communicate straightforward risks or symptoms to the general public without potentially leading to unnecessary screening.
At a minimum, however, clinicians can share more general risk-mitigating strategies with their patients.
Communicating such strategies may be especially important for younger patients, given that the recent survey found almost 40% of younger adults believe there’s nothing they can do to change their risk for pancreatic cancer.
However, Cruz-Monserrate explained, adults of all ages can lower their risks through regular exercise, limited alcohol and tobacco use, and a healthy diet with less red meat or processed meat.
Ultimately, for clinicians, given how difficult it is now to identify pancreatic cancer early, we have to “follow their good clinical judgment when alarming features, such as weight loss or nuances of pancreatic pain arise, and then get good imaging,” Gaddam said.
Cruz-Monserrate, Brand, and Gaddam reported no relevant disclosures.
A version of this article appeared on Medscape.com.
Now, a growing body of evidence indicates that this deadly cancer has been steadily on the rise, particularly in younger individuals who may not even realize they are at risk.
A recent survey, for instance, found that 33% of 1000 respondents younger than 50 years believe that only older adults are at risk for pancreatic cancer, and more than half said they wouldn’t even recognize the early signs and symptoms, which include unexplained weight loss, fatigue, jaundice, abdominal pain that radiates to the back, nausea, and vomiting.
These survey findings allude to a bigger challenge: Identifying the disease remains elusive against a backdrop of these increasing rates and nonspecific risks and symptoms.
Currently, only about 15% of pancreatic cancers are caught at a localized, resectable stage, when 5-year survival rates are highest at 44%. But most are found later, after symptoms arise, and at this point, the 5-year survival odds plummet —16% for regional disease, 3% for distant, and 1% for stage IV.
“This disease is too often a silent killer, with no symptoms until it has progressed to less treatable stages,” said survey coauthor Zobeida Cruz-Monserrate, PhD, in the division of gastroenterology, hepatology and nutrition at Ohio State University Medical Center, Columbus.
Rising Rates
Since 2001, rates of pancreatic cancer have steadily increased by about 1% annually, and this increase appears greater among younger individuals, especially women.
A recent study in Gastroenterology, for instance, found that, while overall rates of pancreatic cancer among people aged 15-34 years remained low (0.3% in women and 0.2% in men) between 2001 and 2018, the average annual percent change in this age group was considerably higher than that for older individuals — 6.45% for women and 2.97% for men compared with 1.11% for women aged 55 years and 1.17% for men aged 55 years. Another recent analysis, published in Annals of Internal Medicine, reported similar increased rates in men and women aged 15-39 years between 2011 and 2019.
Although more than 90% of cases do occur in those 55 years or older, “we’re now seeing this disease in people who are in their 40s much more regularly,” Cruz-Monserrate said. “This is a concerning trend — and more research is needed to learn why.”
But it’s early days. Studies so far indicate that early onset pancreatic cancer tends to be even more aggressive, but the “underlying reason is not yet clear,” researcher wrote in a 2025 review.
Some evidence indicates younger individuals may have distinct molecular characteristics, whereas other research shows younger and older patients have similar genetic profiles. Younger patients may also be more likely to smoke, drink more, and delay seeking medical attention as well as experience delays in being diagnosed by physicians, the authors explained.
Catching It Early
Given the rising rates, early detection is especially important.
There are some known genetic and medical risk factors for pancreatic cancer. About 10% of these cancers are linked to heredity risk or genetic markers, including BRCA1 and BRCA2 or Lynch syndrome. People with chronic pancreatitis, type 2 diabetes, obesity, or with a family history of pancreatic cancer face an elevated risk.
Lifestyle factors can play a role as well. Alcohol consumption, a poor diet that includes red or processed meat, and smoking increase people’s risk for pancreatic cancer. In fact, smoking leads to a twofold higher risk, compared with not smoking.
However, uncovering pancreatic cancer from these factors alone can be like “finding a needle in a haystack,” said Srinivas Gaddam, MD, head of the pancreatic cancer screening and early detection program at Cedars-Sinai Medical Center in Los Angeles.
One strategy to help detect the disease earlier would be to screen more.
The latest guidance from the American Cancer Society suggests that people with a genetic predisposition or a family history of pancreatic cancer could benefit from annual surveillance with endoscopic ultrasound or MRI.
But the US Preventive Services Task Force currently recommends against routine screening of average-risk asymptomatic adults (JAMA. 2019;322[5]:438-444). The task force found no evidence that screening for pancreatic cancer improves disease-specific morbidity or mortality or all-cause mortality.
“The absolute incidence in younger people is far too small to make screening beneficial,” explained The Lancet Gastroenterology & Hepatology editors in a 2023 editorial.
In fact, more screening could lead to overdiagnosis, a concern reinforced by the recent study in Annals of Internal Medicine. That analysis found that much of the observed increase in early-onset pancreatic cancer stemmed from the detection of more small, early-stage endocrine cancer, rather than pancreatic adenocarcinoma, whereas mortality from the disease remained stable over the study period.
Recent findings do “suggest the potential for overdiagnosis and overtreatment, particularly in cases of indolent pancreatic neuroendocrine tumors,” Gaddam said.
Gaddam has observed an increase in both adenocarcinoma and neuroendocrine tumors in the clinic and in his research, especially in women younger than 50 years, but he noted these early onset diagnoses do remain rare.
Staying Vigilant
As the understanding of pancreatic cancer risks and symptoms evolves, ensuring that patients, especially younger individuals, recognize the warning signs, without causing alarm, remains a challenge.
The disease “presents more advanced in younger patients, but symptoms are so nonspecific,” said Randall Brand, MD, AGAF, director of the gastrointestinal malignancy early detection, diagnosis, and prevention program at the University of Pittsburgh Medical Center, Pennsylvania. Given that, “I am not sure how to best highlight a communication approach that would not cause undue stress to the patient and our healthcare resources.”
Gaddam agreed that it’s tough to pinpoint or communicate straightforward risks or symptoms to the general public without potentially leading to unnecessary screening.
At a minimum, however, clinicians can share more general risk-mitigating strategies with their patients.
Communicating such strategies may be especially important for younger patients, given that the recent survey found almost 40% of younger adults believe there’s nothing they can do to change their risk for pancreatic cancer.
However, Cruz-Monserrate explained, adults of all ages can lower their risks through regular exercise, limited alcohol and tobacco use, and a healthy diet with less red meat or processed meat.
Ultimately, for clinicians, given how difficult it is now to identify pancreatic cancer early, we have to “follow their good clinical judgment when alarming features, such as weight loss or nuances of pancreatic pain arise, and then get good imaging,” Gaddam said.
Cruz-Monserrate, Brand, and Gaddam reported no relevant disclosures.
A version of this article appeared on Medscape.com.