User login
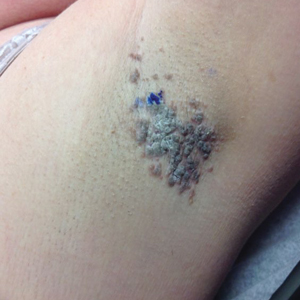
Hyperpigmentation on the Head and Neck
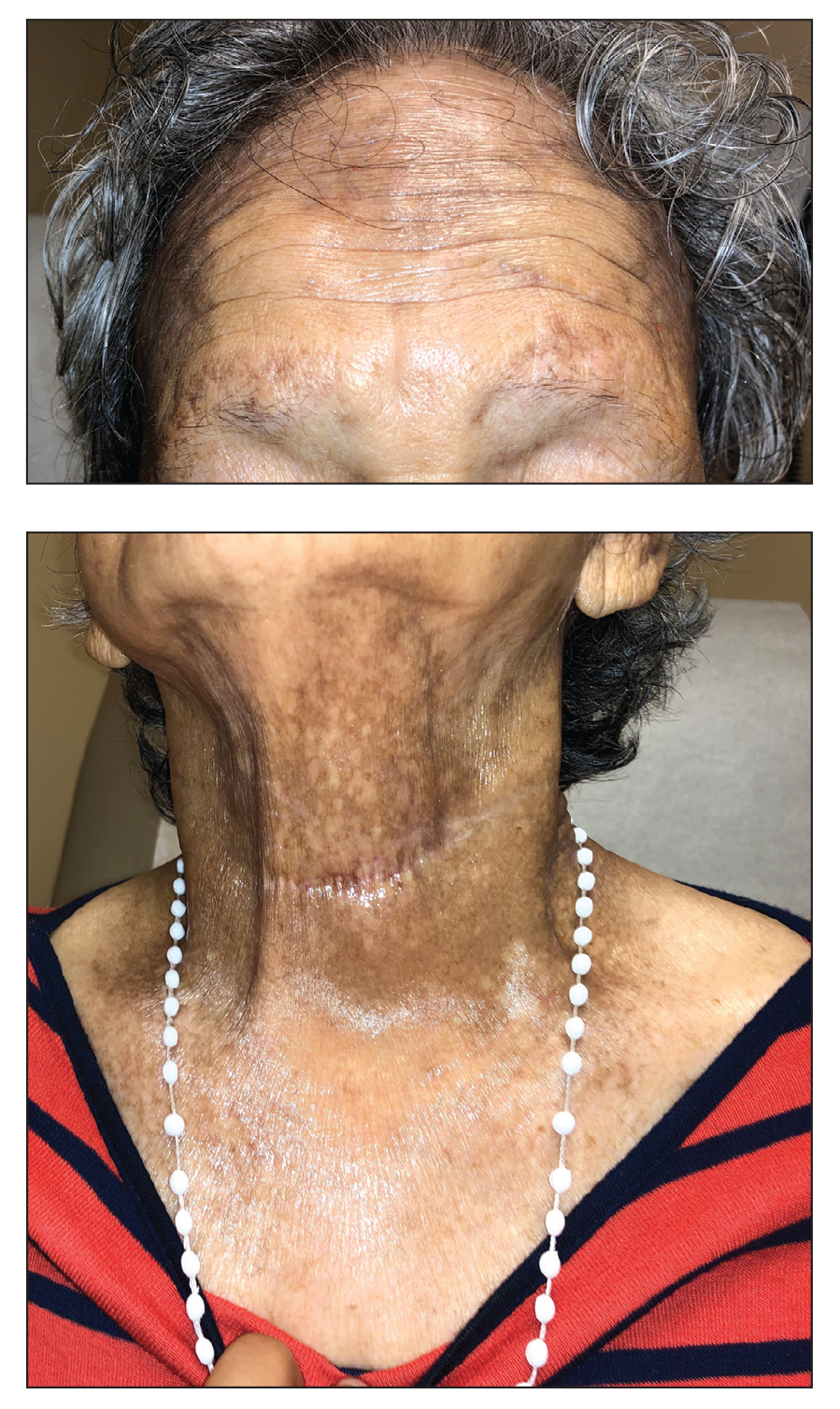
The Diagnosis: Frontal Fibrosing Alopecia Overlapping With Lichen Planus Pigmentosus
Microscopic examination revealed focal dermal pigmentation, papillary fibrosis, and epidermal atrophy. These clinical and histologic findings indicated a diagnosis of fully developed lichen planus pigmentosus (LPP) overlapping with frontal fibrosing alopecia (FFA). Other cases have demonstrated an association between LPP and FFA.1,2
Lichen planus pigmentosus is considered an uncommon variant of lichen planus, as it has similar histopathologic findings and occasional coexistence.3,4 It is characterized by hyperpigmented macules primarily located in sun-exposed and flexural areas of the skin. First described in India,5 this disease has a predilection for darker skin (Fitzpatrick skin types III-V),6,7 and it has been reported in other racial and ethnic groups including Latin Americans, Middle Eastern populations, Japanese, and Koreans.4,8 Typically, lesions initially appear as ill-defined, blue-grey, round to oval macules that coalesce into hyperpigmented patches. Involvement most commonly begins at the forehead and temples, which are affected in nearly all patients. Infrequently, LPP can be generalized or affect the oral mucosa; involvement of the palms, soles, and nails does not occur. Patients may be asymptomatic, but some experience mild pruritus and burning. The disease course is chronic and insidious, with new lesions appearing over time and old lesions progressively darkening and expanding.6,7,9
Although the pathogenesis of LPP is unknown, several exposures have been implicated, such as amla oil, mustard oil, henna, hair dye, and environmental pollutants.7 Because lesions characteristically occur in sun-exposed areas, UV light also may be involved. In addition, studies have suggested that LPP is associated with endocrinopathies such as diabetes mellitus and dyslipidemias, as in our patient, as well as autoimmune conditions such as vitiligo and systemic lupus erythematosus.10,11
Histopathologic findings are characterized by vacuolar degeneration of the basal layer in the epidermis as well as perivascular lymphohistiocytic infiltration and the presence of melanophages in the dermis.3,9 Lichen planus pigmentosus is difficult to treat, as no consistently effective modality has been established. Topical tacrolimus, topical corticosteroids, oral retinoids, lasers, and sun protection have been implemented with underwhelming results.12
Frontal fibrosing alopecia is a variant of lichen planopilaris that predominantly affects postmenopausal women and presents with frontotemporal hair loss in a bandlike distribution.5,13 Both terminal and vellus hairs are affected. Involvement of multiple hair-bearing sites of the skin have been reported, including the entire scalp, eyebrows, and eyelashes. Affected areas may display hypopigmentation and be accompanied by pruritus and trichodynia.14,15 The pathogenesis currently is under investigation, with studies demonstrating autoimmune, genetic, and possibly even endocrine predispositions.16-18 Biopsies of lesions are indistinguishable from lichen planopilaris, which shows follicular lymphocytic infiltration, perifollicular fibrosis, interface dermatitis of the follicular infundibulum and isthmus, and vertical fibrous tracks.5 Patients with FFA have demonstrated variable responses to treatments, with one study showing improvement with oral finasteride or dutasteride.14 Topical and intralesional corticosteroids have yielded suboptimal effects. Other modalities include hydroxychloroquine and mycophenolate mofetil.15,19
Co-occurrence of LPP and FFA primarily is seen in postmenopausal women with darker skin,14,15 as in our patient, though premenopausal cases have been reported. Lichen planus pigmentosus may serve as a harbinger in most patients.1,2 In a similar fashion, our patient presented with hyperpigmented macular lesions prior to the onset of frontotemporal hair loss.
Our patient was started on finasteride 2.5 mg daily, minoxidil foam 5%, clobetasol solution 0.05%, triamcinolone ointment 0.1%, and hydrocortisone ointment 2.5%. She was instructed to commence treatment and follow up in 6 months.
The differential diagnosis includes dermatologic conditions that mimic both LPP and FFA. Postinflammatory hyperpigmentation and fixed drug reaction were unlikely based on the patient's history. The lesions of ashy dermatosis are characteristically gray erythematous macules on the trunk and limbs. Riehl melanosis is a rare pigmented contact dermatitis that is associated with a history of repeated contact with sensitizing allergens. Although Hori nevus is characterized by small, blue-gray or brown macules on the face, lesions predominantly occur on the bony prominences of the cheeks. Melasma also presents with dark to gray macules that affect the face and less commonly the neck, as in our patient.2
Early discoid lupus erythematosus presents with round erythematous plaques with overlying scale extending into the hair follicles. In pseudopalade of Brocq, an idiopathic cicatricial alopecia, lesions typically are flesh colored. Biopsy also shows epidermal atrophy with additional dermal sclerosis and fibrosis. Folliculitis decalvans is a scarring form of alopecia associated with erythema and pustules, findings that were not present in our patient. Keratosis follicularis spinulosa decalvans is a rare, X-linked inherited ichthyosis manifesting as scarring alopecia with follicular depressions and papules on the scalp in younger males. Photophobia and other manifestations may be present. Alopecia mucinosa is a nonscarring alopecia with grouped follicular erythematous patches or plaques. Mucin sometimes can be squeezed from affected areas, and histopathologic examination shows mucin accumulation.4
- Dlova NC. Frontal fibrosing alopecia and lichen planus pigmentosus: is there a link? Br J Dermatol. 2013;168:439-442.
- Pirmez R, Duque-Estrada B, Donati A, et al. Clinical and dermoscopic features of lichen planus pigmentosus in 37 patients with frontal fibrosing alopecia. Br J Dermatol. 2016;175:1387-1390.
- Rieder E, Kaplan J, Kamino H, et al. Lichen planus pigmentosus. Dermatol Online J. 2013;19:20713.
- Kashima A, Tajiri A, Yamashita A, et al. Two Japanese cases of lichen planus pigmentosus-inversus. Int J Dermatol. 2007;46:740-742.
- Bhutani L, Bedi T, Pandhi R. Lichen planus pigmentosus. Dermatologica. 1974;149:43-50.
- Ross EK, Tan E, Shapiro J. Update on primary cicatricial alopecias. J Am Acad Dermatol. 2005;53:1-37.
- Kanwa AJ, Dogra S, Handa S, et al. A study of 124 Indian patients with lichen planus pigmentosus. Clin Exp Dermatol. 2003;28:481-485.
- Al-Mutairi N, El-Khalawany M. Clinicopathological characteristics of lichen planus pigmentosus and its response to tacrolimus ointment: an open label, non-randomized, prospective study. J Eur Acad Dermatol Venereol. 2010;24:535-540.
- Vega ME, Waxtein L, Arenas R, et al. Ashy dermatosis and lichen planus pigmentosus: a clinicopathologic study of 31 cases. Int J Dermatol. 1992;31:90-94.
- Robles-Méndez JC, Rizo-Frías P, Herz-Ruelas ME, et al. Lichen planus pigmentosus and its variants: review and update. Int J Dermatol. 2018;57:505-514.
- Torres J, Guadalupe A, Reyes E, et al. Lichen planus pigmentosus in patients with endocrinopathies and hepatitis C. J Am Acad Dermatol. 2013;68:AB139.
- Kim JE, Won CH, Chang S, et al. Linear lichen planus pigmentosus of the forehead treated by neodymium:yttrium-aluminum-garnet laser and topical tacrolimus. J Dermatol. 2012;39:189-191.
- Kossard S. Postmenopausal frontal fibrosing alopecia: scarring alopecia in a pattern distribution. Arch Dermatol. 1994;130:770-774.
- Vano-Galvan S, Molina-Ruiz AM, Serrano-Falcon C, et al. Frontal fibrosing alopecia: a multicenter review of 355 patients. J Am Acad Dermatol. 2014;70:670-678.
- MacDonald A, Clark C, Holmes S. Frontal fibrosing alopecia: a review of 60 cases. J Am Acad Dermatol. 2012;67:955-961.
- Harries MJ, Meyer K, Chaudhry I, et al. Lichen planopilaris is characterized by immune privilege collapse of the hair follicle's epithelial stem cell niche. J Pathol. 2013;231:236-247.
- Karnik P, Tekeste Z, McCormick TS, et al. Hair follicle stem cell-specific PPARgamma deletion causes scarring alopecia. J Invest Dermatol. 2009;129:1243-1257.
- Rodriguez-Bayona B, Ruchaud S, Rodriguez C, et al. Autoantibodies against the chromosomal passenger protein INCENP found in a patient with Graham Little-Piccardi-Lassueur syndrome. J Autoimmune Dis. 2007;4:1.
- Rácz E, Gho C, Moorman PW, et al. Treatment of frontal fibrosing alopecia and lichen planopilaris: a systematic review. J Eur Acad Dermatol Venereol. 2013;27:1461-1470.
The Diagnosis: Frontal Fibrosing Alopecia Overlapping With Lichen Planus Pigmentosus
Microscopic examination revealed focal dermal pigmentation, papillary fibrosis, and epidermal atrophy. These clinical and histologic findings indicated a diagnosis of fully developed lichen planus pigmentosus (LPP) overlapping with frontal fibrosing alopecia (FFA). Other cases have demonstrated an association between LPP and FFA.1,2
Lichen planus pigmentosus is considered an uncommon variant of lichen planus, as it has similar histopathologic findings and occasional coexistence.3,4 It is characterized by hyperpigmented macules primarily located in sun-exposed and flexural areas of the skin. First described in India,5 this disease has a predilection for darker skin (Fitzpatrick skin types III-V),6,7 and it has been reported in other racial and ethnic groups including Latin Americans, Middle Eastern populations, Japanese, and Koreans.4,8 Typically, lesions initially appear as ill-defined, blue-grey, round to oval macules that coalesce into hyperpigmented patches. Involvement most commonly begins at the forehead and temples, which are affected in nearly all patients. Infrequently, LPP can be generalized or affect the oral mucosa; involvement of the palms, soles, and nails does not occur. Patients may be asymptomatic, but some experience mild pruritus and burning. The disease course is chronic and insidious, with new lesions appearing over time and old lesions progressively darkening and expanding.6,7,9
Although the pathogenesis of LPP is unknown, several exposures have been implicated, such as amla oil, mustard oil, henna, hair dye, and environmental pollutants.7 Because lesions characteristically occur in sun-exposed areas, UV light also may be involved. In addition, studies have suggested that LPP is associated with endocrinopathies such as diabetes mellitus and dyslipidemias, as in our patient, as well as autoimmune conditions such as vitiligo and systemic lupus erythematosus.10,11
Histopathologic findings are characterized by vacuolar degeneration of the basal layer in the epidermis as well as perivascular lymphohistiocytic infiltration and the presence of melanophages in the dermis.3,9 Lichen planus pigmentosus is difficult to treat, as no consistently effective modality has been established. Topical tacrolimus, topical corticosteroids, oral retinoids, lasers, and sun protection have been implemented with underwhelming results.12
Frontal fibrosing alopecia is a variant of lichen planopilaris that predominantly affects postmenopausal women and presents with frontotemporal hair loss in a bandlike distribution.5,13 Both terminal and vellus hairs are affected. Involvement of multiple hair-bearing sites of the skin have been reported, including the entire scalp, eyebrows, and eyelashes. Affected areas may display hypopigmentation and be accompanied by pruritus and trichodynia.14,15 The pathogenesis currently is under investigation, with studies demonstrating autoimmune, genetic, and possibly even endocrine predispositions.16-18 Biopsies of lesions are indistinguishable from lichen planopilaris, which shows follicular lymphocytic infiltration, perifollicular fibrosis, interface dermatitis of the follicular infundibulum and isthmus, and vertical fibrous tracks.5 Patients with FFA have demonstrated variable responses to treatments, with one study showing improvement with oral finasteride or dutasteride.14 Topical and intralesional corticosteroids have yielded suboptimal effects. Other modalities include hydroxychloroquine and mycophenolate mofetil.15,19
Co-occurrence of LPP and FFA primarily is seen in postmenopausal women with darker skin,14,15 as in our patient, though premenopausal cases have been reported. Lichen planus pigmentosus may serve as a harbinger in most patients.1,2 In a similar fashion, our patient presented with hyperpigmented macular lesions prior to the onset of frontotemporal hair loss.
Our patient was started on finasteride 2.5 mg daily, minoxidil foam 5%, clobetasol solution 0.05%, triamcinolone ointment 0.1%, and hydrocortisone ointment 2.5%. She was instructed to commence treatment and follow up in 6 months.
The differential diagnosis includes dermatologic conditions that mimic both LPP and FFA. Postinflammatory hyperpigmentation and fixed drug reaction were unlikely based on the patient's history. The lesions of ashy dermatosis are characteristically gray erythematous macules on the trunk and limbs. Riehl melanosis is a rare pigmented contact dermatitis that is associated with a history of repeated contact with sensitizing allergens. Although Hori nevus is characterized by small, blue-gray or brown macules on the face, lesions predominantly occur on the bony prominences of the cheeks. Melasma also presents with dark to gray macules that affect the face and less commonly the neck, as in our patient.2
Early discoid lupus erythematosus presents with round erythematous plaques with overlying scale extending into the hair follicles. In pseudopalade of Brocq, an idiopathic cicatricial alopecia, lesions typically are flesh colored. Biopsy also shows epidermal atrophy with additional dermal sclerosis and fibrosis. Folliculitis decalvans is a scarring form of alopecia associated with erythema and pustules, findings that were not present in our patient. Keratosis follicularis spinulosa decalvans is a rare, X-linked inherited ichthyosis manifesting as scarring alopecia with follicular depressions and papules on the scalp in younger males. Photophobia and other manifestations may be present. Alopecia mucinosa is a nonscarring alopecia with grouped follicular erythematous patches or plaques. Mucin sometimes can be squeezed from affected areas, and histopathologic examination shows mucin accumulation.4
The Diagnosis: Frontal Fibrosing Alopecia Overlapping With Lichen Planus Pigmentosus
Microscopic examination revealed focal dermal pigmentation, papillary fibrosis, and epidermal atrophy. These clinical and histologic findings indicated a diagnosis of fully developed lichen planus pigmentosus (LPP) overlapping with frontal fibrosing alopecia (FFA). Other cases have demonstrated an association between LPP and FFA.1,2
Lichen planus pigmentosus is considered an uncommon variant of lichen planus, as it has similar histopathologic findings and occasional coexistence.3,4 It is characterized by hyperpigmented macules primarily located in sun-exposed and flexural areas of the skin. First described in India,5 this disease has a predilection for darker skin (Fitzpatrick skin types III-V),6,7 and it has been reported in other racial and ethnic groups including Latin Americans, Middle Eastern populations, Japanese, and Koreans.4,8 Typically, lesions initially appear as ill-defined, blue-grey, round to oval macules that coalesce into hyperpigmented patches. Involvement most commonly begins at the forehead and temples, which are affected in nearly all patients. Infrequently, LPP can be generalized or affect the oral mucosa; involvement of the palms, soles, and nails does not occur. Patients may be asymptomatic, but some experience mild pruritus and burning. The disease course is chronic and insidious, with new lesions appearing over time and old lesions progressively darkening and expanding.6,7,9
Although the pathogenesis of LPP is unknown, several exposures have been implicated, such as amla oil, mustard oil, henna, hair dye, and environmental pollutants.7 Because lesions characteristically occur in sun-exposed areas, UV light also may be involved. In addition, studies have suggested that LPP is associated with endocrinopathies such as diabetes mellitus and dyslipidemias, as in our patient, as well as autoimmune conditions such as vitiligo and systemic lupus erythematosus.10,11
Histopathologic findings are characterized by vacuolar degeneration of the basal layer in the epidermis as well as perivascular lymphohistiocytic infiltration and the presence of melanophages in the dermis.3,9 Lichen planus pigmentosus is difficult to treat, as no consistently effective modality has been established. Topical tacrolimus, topical corticosteroids, oral retinoids, lasers, and sun protection have been implemented with underwhelming results.12
Frontal fibrosing alopecia is a variant of lichen planopilaris that predominantly affects postmenopausal women and presents with frontotemporal hair loss in a bandlike distribution.5,13 Both terminal and vellus hairs are affected. Involvement of multiple hair-bearing sites of the skin have been reported, including the entire scalp, eyebrows, and eyelashes. Affected areas may display hypopigmentation and be accompanied by pruritus and trichodynia.14,15 The pathogenesis currently is under investigation, with studies demonstrating autoimmune, genetic, and possibly even endocrine predispositions.16-18 Biopsies of lesions are indistinguishable from lichen planopilaris, which shows follicular lymphocytic infiltration, perifollicular fibrosis, interface dermatitis of the follicular infundibulum and isthmus, and vertical fibrous tracks.5 Patients with FFA have demonstrated variable responses to treatments, with one study showing improvement with oral finasteride or dutasteride.14 Topical and intralesional corticosteroids have yielded suboptimal effects. Other modalities include hydroxychloroquine and mycophenolate mofetil.15,19
Co-occurrence of LPP and FFA primarily is seen in postmenopausal women with darker skin,14,15 as in our patient, though premenopausal cases have been reported. Lichen planus pigmentosus may serve as a harbinger in most patients.1,2 In a similar fashion, our patient presented with hyperpigmented macular lesions prior to the onset of frontotemporal hair loss.
Our patient was started on finasteride 2.5 mg daily, minoxidil foam 5%, clobetasol solution 0.05%, triamcinolone ointment 0.1%, and hydrocortisone ointment 2.5%. She was instructed to commence treatment and follow up in 6 months.
The differential diagnosis includes dermatologic conditions that mimic both LPP and FFA. Postinflammatory hyperpigmentation and fixed drug reaction were unlikely based on the patient's history. The lesions of ashy dermatosis are characteristically gray erythematous macules on the trunk and limbs. Riehl melanosis is a rare pigmented contact dermatitis that is associated with a history of repeated contact with sensitizing allergens. Although Hori nevus is characterized by small, blue-gray or brown macules on the face, lesions predominantly occur on the bony prominences of the cheeks. Melasma also presents with dark to gray macules that affect the face and less commonly the neck, as in our patient.2
Early discoid lupus erythematosus presents with round erythematous plaques with overlying scale extending into the hair follicles. In pseudopalade of Brocq, an idiopathic cicatricial alopecia, lesions typically are flesh colored. Biopsy also shows epidermal atrophy with additional dermal sclerosis and fibrosis. Folliculitis decalvans is a scarring form of alopecia associated with erythema and pustules, findings that were not present in our patient. Keratosis follicularis spinulosa decalvans is a rare, X-linked inherited ichthyosis manifesting as scarring alopecia with follicular depressions and papules on the scalp in younger males. Photophobia and other manifestations may be present. Alopecia mucinosa is a nonscarring alopecia with grouped follicular erythematous patches or plaques. Mucin sometimes can be squeezed from affected areas, and histopathologic examination shows mucin accumulation.4
- Dlova NC. Frontal fibrosing alopecia and lichen planus pigmentosus: is there a link? Br J Dermatol. 2013;168:439-442.
- Pirmez R, Duque-Estrada B, Donati A, et al. Clinical and dermoscopic features of lichen planus pigmentosus in 37 patients with frontal fibrosing alopecia. Br J Dermatol. 2016;175:1387-1390.
- Rieder E, Kaplan J, Kamino H, et al. Lichen planus pigmentosus. Dermatol Online J. 2013;19:20713.
- Kashima A, Tajiri A, Yamashita A, et al. Two Japanese cases of lichen planus pigmentosus-inversus. Int J Dermatol. 2007;46:740-742.
- Bhutani L, Bedi T, Pandhi R. Lichen planus pigmentosus. Dermatologica. 1974;149:43-50.
- Ross EK, Tan E, Shapiro J. Update on primary cicatricial alopecias. J Am Acad Dermatol. 2005;53:1-37.
- Kanwa AJ, Dogra S, Handa S, et al. A study of 124 Indian patients with lichen planus pigmentosus. Clin Exp Dermatol. 2003;28:481-485.
- Al-Mutairi N, El-Khalawany M. Clinicopathological characteristics of lichen planus pigmentosus and its response to tacrolimus ointment: an open label, non-randomized, prospective study. J Eur Acad Dermatol Venereol. 2010;24:535-540.
- Vega ME, Waxtein L, Arenas R, et al. Ashy dermatosis and lichen planus pigmentosus: a clinicopathologic study of 31 cases. Int J Dermatol. 1992;31:90-94.
- Robles-Méndez JC, Rizo-Frías P, Herz-Ruelas ME, et al. Lichen planus pigmentosus and its variants: review and update. Int J Dermatol. 2018;57:505-514.
- Torres J, Guadalupe A, Reyes E, et al. Lichen planus pigmentosus in patients with endocrinopathies and hepatitis C. J Am Acad Dermatol. 2013;68:AB139.
- Kim JE, Won CH, Chang S, et al. Linear lichen planus pigmentosus of the forehead treated by neodymium:yttrium-aluminum-garnet laser and topical tacrolimus. J Dermatol. 2012;39:189-191.
- Kossard S. Postmenopausal frontal fibrosing alopecia: scarring alopecia in a pattern distribution. Arch Dermatol. 1994;130:770-774.
- Vano-Galvan S, Molina-Ruiz AM, Serrano-Falcon C, et al. Frontal fibrosing alopecia: a multicenter review of 355 patients. J Am Acad Dermatol. 2014;70:670-678.
- MacDonald A, Clark C, Holmes S. Frontal fibrosing alopecia: a review of 60 cases. J Am Acad Dermatol. 2012;67:955-961.
- Harries MJ, Meyer K, Chaudhry I, et al. Lichen planopilaris is characterized by immune privilege collapse of the hair follicle's epithelial stem cell niche. J Pathol. 2013;231:236-247.
- Karnik P, Tekeste Z, McCormick TS, et al. Hair follicle stem cell-specific PPARgamma deletion causes scarring alopecia. J Invest Dermatol. 2009;129:1243-1257.
- Rodriguez-Bayona B, Ruchaud S, Rodriguez C, et al. Autoantibodies against the chromosomal passenger protein INCENP found in a patient with Graham Little-Piccardi-Lassueur syndrome. J Autoimmune Dis. 2007;4:1.
- Rácz E, Gho C, Moorman PW, et al. Treatment of frontal fibrosing alopecia and lichen planopilaris: a systematic review. J Eur Acad Dermatol Venereol. 2013;27:1461-1470.
- Dlova NC. Frontal fibrosing alopecia and lichen planus pigmentosus: is there a link? Br J Dermatol. 2013;168:439-442.
- Pirmez R, Duque-Estrada B, Donati A, et al. Clinical and dermoscopic features of lichen planus pigmentosus in 37 patients with frontal fibrosing alopecia. Br J Dermatol. 2016;175:1387-1390.
- Rieder E, Kaplan J, Kamino H, et al. Lichen planus pigmentosus. Dermatol Online J. 2013;19:20713.
- Kashima A, Tajiri A, Yamashita A, et al. Two Japanese cases of lichen planus pigmentosus-inversus. Int J Dermatol. 2007;46:740-742.
- Bhutani L, Bedi T, Pandhi R. Lichen planus pigmentosus. Dermatologica. 1974;149:43-50.
- Ross EK, Tan E, Shapiro J. Update on primary cicatricial alopecias. J Am Acad Dermatol. 2005;53:1-37.
- Kanwa AJ, Dogra S, Handa S, et al. A study of 124 Indian patients with lichen planus pigmentosus. Clin Exp Dermatol. 2003;28:481-485.
- Al-Mutairi N, El-Khalawany M. Clinicopathological characteristics of lichen planus pigmentosus and its response to tacrolimus ointment: an open label, non-randomized, prospective study. J Eur Acad Dermatol Venereol. 2010;24:535-540.
- Vega ME, Waxtein L, Arenas R, et al. Ashy dermatosis and lichen planus pigmentosus: a clinicopathologic study of 31 cases. Int J Dermatol. 1992;31:90-94.
- Robles-Méndez JC, Rizo-Frías P, Herz-Ruelas ME, et al. Lichen planus pigmentosus and its variants: review and update. Int J Dermatol. 2018;57:505-514.
- Torres J, Guadalupe A, Reyes E, et al. Lichen planus pigmentosus in patients with endocrinopathies and hepatitis C. J Am Acad Dermatol. 2013;68:AB139.
- Kim JE, Won CH, Chang S, et al. Linear lichen planus pigmentosus of the forehead treated by neodymium:yttrium-aluminum-garnet laser and topical tacrolimus. J Dermatol. 2012;39:189-191.
- Kossard S. Postmenopausal frontal fibrosing alopecia: scarring alopecia in a pattern distribution. Arch Dermatol. 1994;130:770-774.
- Vano-Galvan S, Molina-Ruiz AM, Serrano-Falcon C, et al. Frontal fibrosing alopecia: a multicenter review of 355 patients. J Am Acad Dermatol. 2014;70:670-678.
- MacDonald A, Clark C, Holmes S. Frontal fibrosing alopecia: a review of 60 cases. J Am Acad Dermatol. 2012;67:955-961.
- Harries MJ, Meyer K, Chaudhry I, et al. Lichen planopilaris is characterized by immune privilege collapse of the hair follicle's epithelial stem cell niche. J Pathol. 2013;231:236-247.
- Karnik P, Tekeste Z, McCormick TS, et al. Hair follicle stem cell-specific PPARgamma deletion causes scarring alopecia. J Invest Dermatol. 2009;129:1243-1257.
- Rodriguez-Bayona B, Ruchaud S, Rodriguez C, et al. Autoantibodies against the chromosomal passenger protein INCENP found in a patient with Graham Little-Piccardi-Lassueur syndrome. J Autoimmune Dis. 2007;4:1.
- Rácz E, Gho C, Moorman PW, et al. Treatment of frontal fibrosing alopecia and lichen planopilaris: a systematic review. J Eur Acad Dermatol Venereol. 2013;27:1461-1470.
A 78-year-old Asian woman presented to the dermatology clinic with progressively worsening dark spots on the forehead and neck of 3 months’ duration. She noted mild pruritis and hair loss involving the eyebrows and anterior scalp. Her medical history was notable for type 2 diabetes mellitus. She denied any new medical conditions or medications and had no prior history of similar symptoms. Physical examination showed hyperpigmented brown macules and patches on the forehead (top) and anterior neck (bottom) with sparing of the posterior neck and lower face. Alopecia with areas of perifollicular erythema and hyperpigmentation with reduced follicular openings were present on the eyebrows and anterior forehead. Two punch biopsies of head and neck lesions were performed.
Vitiligo patients share their experiences, frustrations with treatment options with FDA
Patients with vitiligo have faced significant impacts psychosocially and in many cases, profound losses of identity – and they’ve had only minimal success with treatment, according to participants who spoke at and provided input at a public meeting on patient-focused drug development for the disease.
The virtual meeting, held in March, was part of the Food and Drug Administration’s Patient-Focused Drug Development (PFDD) initiative, which began in 2012 and aims to provide a systematic way for patients’ experiences, needs and priorities to be “captured and meaningfully incorporated” into drug development and evaluation.
Seemal Desai, MD, of the department of dermatology at the University of Texas, Dallas, who attended the meeting as an observer, said in a later interview that while “all skin diseases have a psychosocial component … vitiligo is a really unique one, because it really relates to the patient’s own identity.
“What I heard loud and clear from the FDA [leaders who ran and attended the meeting] is recognition that patients are suffering. They needed to hear about the emotional devastation of the disease and how it is a medical condition,” Dr. Desai said.
The meeting was the “first-ever vitiligo meeting at the FDA” and was a “historic moment for the vitiligo community,” he added.
The pigmentation disorder affects 1% of the world’s population. Nearly 50% have an onset before age 20, and onset before age 12 is common, Brenda Carr, MD, medical officer with the FDA’s Division of Dermatology and Dentistry in the Center for Drug Evaluation and Research, said in an introductory overview.
The only FDA-approved treatment for vitiligo is monobenzone cream, but this is indicated for final depigmentation in extensive vitiligo and is no longer marketed. Treatment options include corticosteroids, calcineurin inhibitors, vitamin D analogues, phototherapy, surgical treatments (tissue grafts and cellular grafts), and camouflage (make-up, tattoos, self-tanning products), Dr. Carr said.
Patients participated in one of two panels – one about the health effects and daily impacts of vitiligo and the other about treatments – or submitted input electronically. All patients were invited to answer poll questions and open-ended queries, including questions about how they would assess new treatments.
Several panel members who are Black shared series of photos that showed the evolution of defined white patches into widespread, generalized depigmentation. One man with skin of color who lives in the Netherlands said he has had vitiligo since the age of 12, but that when he became older, over a 4-year period, he was “transformed from a man of Indonesian roots to a totally white man.”
Experiencing only minimal benefit from treatment and the short-term effectiveness of treatments were the top two answers to a poll question asking participants about the most burdensome impacts of the medical products and interventions they have used. Difficulty in accessing treatment, concern about serious risks of treatment, and uncertainty about long-term effects of treatment were other frequently chosen answers.
Patients described the onerous nature of phototherapy (treatments repeated several times a week over long periods) and other treatments, and several described feeling that some physicians did not take the condition seriously or fully know of treatment options.
In her closing remarks, Kendall Marcus, MD, director of the Division of Dermatology and Dentistry at the FDA, acknowledged the input. “Some of you have had difficulty having your disease taken seriously by physicians who view it as a cosmetic condition and are reluctant to treat because they believe your expectations will not be met, that it will be an exercise in frustration,” she said.
Regarding the impacts of treatments that have been utilized, “some of the treatments make it impossible to do other activities such as work or care for yourself in other ways,” Dr. Marcus said. “Certainly that’s not the kind of treatment … that anybody wants to have.”
Dr. Desai, who utilizes an array of oral and topical treatments and phototherapies in his practice, said he was surprised and disheartened to hear the level of concern about side effects of treatment. Most of those who expressed concerns alluded to phototherapy. “I think light treatments are very safe and effective,” he said in the interview. “I might equate [such concerns] to the older PUVA [psoralen plus UVA ultraviolet light] therapy, but not so much the newer therapies.”
The FDA participants probed patients for their perspective on a meaningful level of repigmentation and an acceptable level of risk for any new hypothetical treatment. Specifically, they asked whether patients would use a new topical cream approved for vitiligo if the cream needed to be applied once a day, would have up to 50% efficacy in some people, and would have common side effects of redness and irritation at the application site, mild acne, and burning, as well as several rarer but more serious side effects.
Only 36% answered yes; 24% said no, and 40% answered maybe. Some patients said during the meeting that they had accepted their condition and were not pursuing any treatment. Others said they were very interested in treatment but only if the level of repigmentation were significantly higher than 50%. Some described their fear that positive treatment effects would be short term only.
Meri Izrail Kohen, who lives in France and has lost half of her skin’s pigmentation, said that treatment efficacy is “not only about how much recovery of pigment it allows, but how long the recovery will last.” Some treatments will work for some patients, she said, “but even in these cases when we stop the treatment, it will come back somehow.”
Lee Thomas, a TV anchor in Detroit, and a reporter and author of the book “Turning White,” described how he tried “every treatment he could afford” but stopped trying 10 years ago. A treatment in Germany “gave me 80% of my pigment back, but it has gone again,” he said. “I would love to have my face back again. I was born a Black child, and I’d like to die a Black man.”
Patients also spoke of their skin burning easily outdoors; skin sensitivity, itchiness, and burning with the spread of disease; treatment expenses and not being able to afford treatment; and worsening of their vitiligo with the stress of the pandemic. Parents expressed having fear that their children would develop vitiligo and experience bullying, isolation, or other emotional or psychosocial impacts that they had experienced; one described having an almost-paralyzing anxiety when he saw patchy white spots on his 20-month-old daughter (it was not diagnosed as vitiligo).
Calls for further advancement with home phototherapy – which Dr. Desai said is a growing market but not yet adequately covered by insurance plans – were also made, as were pleas for research on the root causes of the disease.
Patients clearly indicated “that they need more efficacious treatments, and more comprehensive treatments,” said Dr. Desai, who chairs the advisory committee of the Global Vitiligo Foundation. “It’s disappointing to me that patients come in with a not fully optimistic viewpoint, with a lot of anxiety and angst that treatments are not going to work. … But the Agency needs to hear that. This means that there haven’t been good treatments and we need more.”
The FDA will accept public comments until May 10, 2021, at which time comments will be compiled into a summary report. FDA officials assured patients that the report would be visible and circulated not only within the FDA but among drug companies, researchers, and other product developers.
Patients with vitiligo have faced significant impacts psychosocially and in many cases, profound losses of identity – and they’ve had only minimal success with treatment, according to participants who spoke at and provided input at a public meeting on patient-focused drug development for the disease.
The virtual meeting, held in March, was part of the Food and Drug Administration’s Patient-Focused Drug Development (PFDD) initiative, which began in 2012 and aims to provide a systematic way for patients’ experiences, needs and priorities to be “captured and meaningfully incorporated” into drug development and evaluation.
Seemal Desai, MD, of the department of dermatology at the University of Texas, Dallas, who attended the meeting as an observer, said in a later interview that while “all skin diseases have a psychosocial component … vitiligo is a really unique one, because it really relates to the patient’s own identity.
“What I heard loud and clear from the FDA [leaders who ran and attended the meeting] is recognition that patients are suffering. They needed to hear about the emotional devastation of the disease and how it is a medical condition,” Dr. Desai said.
The meeting was the “first-ever vitiligo meeting at the FDA” and was a “historic moment for the vitiligo community,” he added.
The pigmentation disorder affects 1% of the world’s population. Nearly 50% have an onset before age 20, and onset before age 12 is common, Brenda Carr, MD, medical officer with the FDA’s Division of Dermatology and Dentistry in the Center for Drug Evaluation and Research, said in an introductory overview.
The only FDA-approved treatment for vitiligo is monobenzone cream, but this is indicated for final depigmentation in extensive vitiligo and is no longer marketed. Treatment options include corticosteroids, calcineurin inhibitors, vitamin D analogues, phototherapy, surgical treatments (tissue grafts and cellular grafts), and camouflage (make-up, tattoos, self-tanning products), Dr. Carr said.
Patients participated in one of two panels – one about the health effects and daily impacts of vitiligo and the other about treatments – or submitted input electronically. All patients were invited to answer poll questions and open-ended queries, including questions about how they would assess new treatments.
Several panel members who are Black shared series of photos that showed the evolution of defined white patches into widespread, generalized depigmentation. One man with skin of color who lives in the Netherlands said he has had vitiligo since the age of 12, but that when he became older, over a 4-year period, he was “transformed from a man of Indonesian roots to a totally white man.”
Experiencing only minimal benefit from treatment and the short-term effectiveness of treatments were the top two answers to a poll question asking participants about the most burdensome impacts of the medical products and interventions they have used. Difficulty in accessing treatment, concern about serious risks of treatment, and uncertainty about long-term effects of treatment were other frequently chosen answers.
Patients described the onerous nature of phototherapy (treatments repeated several times a week over long periods) and other treatments, and several described feeling that some physicians did not take the condition seriously or fully know of treatment options.
In her closing remarks, Kendall Marcus, MD, director of the Division of Dermatology and Dentistry at the FDA, acknowledged the input. “Some of you have had difficulty having your disease taken seriously by physicians who view it as a cosmetic condition and are reluctant to treat because they believe your expectations will not be met, that it will be an exercise in frustration,” she said.
Regarding the impacts of treatments that have been utilized, “some of the treatments make it impossible to do other activities such as work or care for yourself in other ways,” Dr. Marcus said. “Certainly that’s not the kind of treatment … that anybody wants to have.”
Dr. Desai, who utilizes an array of oral and topical treatments and phototherapies in his practice, said he was surprised and disheartened to hear the level of concern about side effects of treatment. Most of those who expressed concerns alluded to phototherapy. “I think light treatments are very safe and effective,” he said in the interview. “I might equate [such concerns] to the older PUVA [psoralen plus UVA ultraviolet light] therapy, but not so much the newer therapies.”
The FDA participants probed patients for their perspective on a meaningful level of repigmentation and an acceptable level of risk for any new hypothetical treatment. Specifically, they asked whether patients would use a new topical cream approved for vitiligo if the cream needed to be applied once a day, would have up to 50% efficacy in some people, and would have common side effects of redness and irritation at the application site, mild acne, and burning, as well as several rarer but more serious side effects.
Only 36% answered yes; 24% said no, and 40% answered maybe. Some patients said during the meeting that they had accepted their condition and were not pursuing any treatment. Others said they were very interested in treatment but only if the level of repigmentation were significantly higher than 50%. Some described their fear that positive treatment effects would be short term only.
Meri Izrail Kohen, who lives in France and has lost half of her skin’s pigmentation, said that treatment efficacy is “not only about how much recovery of pigment it allows, but how long the recovery will last.” Some treatments will work for some patients, she said, “but even in these cases when we stop the treatment, it will come back somehow.”
Lee Thomas, a TV anchor in Detroit, and a reporter and author of the book “Turning White,” described how he tried “every treatment he could afford” but stopped trying 10 years ago. A treatment in Germany “gave me 80% of my pigment back, but it has gone again,” he said. “I would love to have my face back again. I was born a Black child, and I’d like to die a Black man.”
Patients also spoke of their skin burning easily outdoors; skin sensitivity, itchiness, and burning with the spread of disease; treatment expenses and not being able to afford treatment; and worsening of their vitiligo with the stress of the pandemic. Parents expressed having fear that their children would develop vitiligo and experience bullying, isolation, or other emotional or psychosocial impacts that they had experienced; one described having an almost-paralyzing anxiety when he saw patchy white spots on his 20-month-old daughter (it was not diagnosed as vitiligo).
Calls for further advancement with home phototherapy – which Dr. Desai said is a growing market but not yet adequately covered by insurance plans – were also made, as were pleas for research on the root causes of the disease.
Patients clearly indicated “that they need more efficacious treatments, and more comprehensive treatments,” said Dr. Desai, who chairs the advisory committee of the Global Vitiligo Foundation. “It’s disappointing to me that patients come in with a not fully optimistic viewpoint, with a lot of anxiety and angst that treatments are not going to work. … But the Agency needs to hear that. This means that there haven’t been good treatments and we need more.”
The FDA will accept public comments until May 10, 2021, at which time comments will be compiled into a summary report. FDA officials assured patients that the report would be visible and circulated not only within the FDA but among drug companies, researchers, and other product developers.
Patients with vitiligo have faced significant impacts psychosocially and in many cases, profound losses of identity – and they’ve had only minimal success with treatment, according to participants who spoke at and provided input at a public meeting on patient-focused drug development for the disease.
The virtual meeting, held in March, was part of the Food and Drug Administration’s Patient-Focused Drug Development (PFDD) initiative, which began in 2012 and aims to provide a systematic way for patients’ experiences, needs and priorities to be “captured and meaningfully incorporated” into drug development and evaluation.
Seemal Desai, MD, of the department of dermatology at the University of Texas, Dallas, who attended the meeting as an observer, said in a later interview that while “all skin diseases have a psychosocial component … vitiligo is a really unique one, because it really relates to the patient’s own identity.
“What I heard loud and clear from the FDA [leaders who ran and attended the meeting] is recognition that patients are suffering. They needed to hear about the emotional devastation of the disease and how it is a medical condition,” Dr. Desai said.
The meeting was the “first-ever vitiligo meeting at the FDA” and was a “historic moment for the vitiligo community,” he added.
The pigmentation disorder affects 1% of the world’s population. Nearly 50% have an onset before age 20, and onset before age 12 is common, Brenda Carr, MD, medical officer with the FDA’s Division of Dermatology and Dentistry in the Center for Drug Evaluation and Research, said in an introductory overview.
The only FDA-approved treatment for vitiligo is monobenzone cream, but this is indicated for final depigmentation in extensive vitiligo and is no longer marketed. Treatment options include corticosteroids, calcineurin inhibitors, vitamin D analogues, phototherapy, surgical treatments (tissue grafts and cellular grafts), and camouflage (make-up, tattoos, self-tanning products), Dr. Carr said.
Patients participated in one of two panels – one about the health effects and daily impacts of vitiligo and the other about treatments – or submitted input electronically. All patients were invited to answer poll questions and open-ended queries, including questions about how they would assess new treatments.
Several panel members who are Black shared series of photos that showed the evolution of defined white patches into widespread, generalized depigmentation. One man with skin of color who lives in the Netherlands said he has had vitiligo since the age of 12, but that when he became older, over a 4-year period, he was “transformed from a man of Indonesian roots to a totally white man.”
Experiencing only minimal benefit from treatment and the short-term effectiveness of treatments were the top two answers to a poll question asking participants about the most burdensome impacts of the medical products and interventions they have used. Difficulty in accessing treatment, concern about serious risks of treatment, and uncertainty about long-term effects of treatment were other frequently chosen answers.
Patients described the onerous nature of phototherapy (treatments repeated several times a week over long periods) and other treatments, and several described feeling that some physicians did not take the condition seriously or fully know of treatment options.
In her closing remarks, Kendall Marcus, MD, director of the Division of Dermatology and Dentistry at the FDA, acknowledged the input. “Some of you have had difficulty having your disease taken seriously by physicians who view it as a cosmetic condition and are reluctant to treat because they believe your expectations will not be met, that it will be an exercise in frustration,” she said.
Regarding the impacts of treatments that have been utilized, “some of the treatments make it impossible to do other activities such as work or care for yourself in other ways,” Dr. Marcus said. “Certainly that’s not the kind of treatment … that anybody wants to have.”
Dr. Desai, who utilizes an array of oral and topical treatments and phototherapies in his practice, said he was surprised and disheartened to hear the level of concern about side effects of treatment. Most of those who expressed concerns alluded to phototherapy. “I think light treatments are very safe and effective,” he said in the interview. “I might equate [such concerns] to the older PUVA [psoralen plus UVA ultraviolet light] therapy, but not so much the newer therapies.”
The FDA participants probed patients for their perspective on a meaningful level of repigmentation and an acceptable level of risk for any new hypothetical treatment. Specifically, they asked whether patients would use a new topical cream approved for vitiligo if the cream needed to be applied once a day, would have up to 50% efficacy in some people, and would have common side effects of redness and irritation at the application site, mild acne, and burning, as well as several rarer but more serious side effects.
Only 36% answered yes; 24% said no, and 40% answered maybe. Some patients said during the meeting that they had accepted their condition and were not pursuing any treatment. Others said they were very interested in treatment but only if the level of repigmentation were significantly higher than 50%. Some described their fear that positive treatment effects would be short term only.
Meri Izrail Kohen, who lives in France and has lost half of her skin’s pigmentation, said that treatment efficacy is “not only about how much recovery of pigment it allows, but how long the recovery will last.” Some treatments will work for some patients, she said, “but even in these cases when we stop the treatment, it will come back somehow.”
Lee Thomas, a TV anchor in Detroit, and a reporter and author of the book “Turning White,” described how he tried “every treatment he could afford” but stopped trying 10 years ago. A treatment in Germany “gave me 80% of my pigment back, but it has gone again,” he said. “I would love to have my face back again. I was born a Black child, and I’d like to die a Black man.”
Patients also spoke of their skin burning easily outdoors; skin sensitivity, itchiness, and burning with the spread of disease; treatment expenses and not being able to afford treatment; and worsening of their vitiligo with the stress of the pandemic. Parents expressed having fear that their children would develop vitiligo and experience bullying, isolation, or other emotional or psychosocial impacts that they had experienced; one described having an almost-paralyzing anxiety when he saw patchy white spots on his 20-month-old daughter (it was not diagnosed as vitiligo).
Calls for further advancement with home phototherapy – which Dr. Desai said is a growing market but not yet adequately covered by insurance plans – were also made, as were pleas for research on the root causes of the disease.
Patients clearly indicated “that they need more efficacious treatments, and more comprehensive treatments,” said Dr. Desai, who chairs the advisory committee of the Global Vitiligo Foundation. “It’s disappointing to me that patients come in with a not fully optimistic viewpoint, with a lot of anxiety and angst that treatments are not going to work. … But the Agency needs to hear that. This means that there haven’t been good treatments and we need more.”
The FDA will accept public comments until May 10, 2021, at which time comments will be compiled into a summary report. FDA officials assured patients that the report would be visible and circulated not only within the FDA but among drug companies, researchers, and other product developers.
FROM AN FDA PATIENT-FOCUSED DRUG DEVELOPMENT MEETING
Infantile hemangiomas: Accurate diagnosis is crucial
The first rule about infantile hemangiomas: Make sure they’re actually infantile hemangiomas, a pediatric dermatologist urged colleagues. Then watch patients closely, refer to specialists when appropriate, and consider propranolol in complicated or high-risk cases, Andrea L. Zaenglein, MD, said at MedscapeLive’s Women’s & Pediatric Dermatology Seminar.
“In my career as a pediatric dermatologist, propranolol has been a life changer for us more than any other medicine,” said Dr. Zaenglein, professor of dermatology and pediatric dermatology, Penn State University, Hershey.
Before the point where propranolol is prescribed, confirm the diagnosis and use the correct terminology, she advised. It’s still appropriate to use the International Society for the Study of Vascular Anomalies (ISSVA) vascular lesion classification system released in 1982. “For most people, it serves the purpose well,” she said. Another option is an updated and more complex classification system from 2015.
Dr. Zaenglein highlighted two studies – one published in 2011 and the other published in 2020 – that revealed high levels of misclassification of vascular malformations in research reports. The earlier study found that 21% of patients with misclassified lesions were mistreated, compared with none of those who were classified using ISSVA terminology.
“I cannot stress [proper classification] enough when you’re dealing with babies and children with vascular lesions. If not sure, be vague. Say ‘a vascular tumor’ or a ‘vascular malformation.’ But only reserve ‘infantile hemangioma’ for that very diagnosis,” she said.
As Dr. Zaenglein noted, infantile hemangiomas affect 5%-10% of 1-year-olds, of whom 20% have multiple lesions. They’re more common in females by a 3-to-1 margin, and also seen more in premature infants, and in cases of multiple births, higher maternal age, and low birth weight.
The pathogenesis of these lesions is unclear, she said, although there are hints about genetic components and tissue hypoxia, among other possible causes. “Importantly, you get 80% of the growth by 3-4 months of age. Then it’ll slow in its growth and kind of slowly go away over time, but it’s not linear regression. It’s more that you get more improvement up front, usually until about 5, and then you can get some continued gradual evolution up until about 7 or 10 years of age.”
Complications can include ulceration, infection and – in rare cases – hemorrhage and high-output cardiac failure, she said. “Knowing which ones are at high risk for complications is important, and also there are systemic associations that we have to be mindful of. We also want to think about aesthetic outcomes as well when we talk about management of infantile hemangiomas.”
High-risk infantile hemangiomas include those with the following features:
- Extensive facial involvement. Dr. Zaenglein highlighted a case of a 2-year-old baby with a large, bulky hemangioma that distorted facial features around the eye. “This would be a medical emergency” requiring immediate evaluation and treatment, she said.
- Periocular involvement. Refer to ophthalmology, she recommended. “Even smaller hemangiomas can cause refractive errors or amblyopia, and oftentimes need to be treated with either systemic or topical therapy depending on the size and extent,” she said.
- PHACE syndrome (Posterior fossa malformations, hemangiomas, arterial anomalies, coarctation of the aorta and cardiac defects, eye abnormalities). “Propranolol has been safely used in PHACE, but every patient is different,” she said. “You need to make sure to do a good risk assessment before starting because if they have narrowed blood flow or limited blood flow, there is a question of whether there is potential risk for stroke if you drop a baby’s blood pressure. Make sure that the vasculature is evaluated before started on propranolol. Also, there are recent reports of risk of long-term risk of stroke with PHACE syndrome as patients are getting into their adulthood.”
- Beard distribution. Be aware of possible airway involvement that can be revealed by biphasic stridor. In those cases, immediate treatment – perhaps even with tracheostomy – is needed to avoid mortality, she said.
- Multiple sites: Patients with five or more hemangiomas may have liver involvement, she said, and should undergo hepatic evaluation. Consider evaluating if this is suspected, even if the number of hemangiomas is under five, she said.
- Perineal/lumbosacral involvement: A third of these cases are associated with spinal dysraphism. Refer to neurosurgery, she recommended.
Dr. Zaenglein highlighted a report on the use of propranolol published in 2008 and noted that clinical practice guidelines for managing infantile hemangiomas published in 2019 are also helpful.
Flat hemangiomas, meanwhile, can benefit from timolol maleate 0.5% solution or gel-forming solution – 1 drop twice daily or 2 drops once daily, she said. This treatment should be avoided in thick hemangiomas, she said.
MedscapeLive and this news organization are owned by the same parent company. Dr. Zaenglein disclosed consulting fees (Dermata, Cassiopea, and Regeneron), and fees for contracted research support (Incyte).
The first rule about infantile hemangiomas: Make sure they’re actually infantile hemangiomas, a pediatric dermatologist urged colleagues. Then watch patients closely, refer to specialists when appropriate, and consider propranolol in complicated or high-risk cases, Andrea L. Zaenglein, MD, said at MedscapeLive’s Women’s & Pediatric Dermatology Seminar.
“In my career as a pediatric dermatologist, propranolol has been a life changer for us more than any other medicine,” said Dr. Zaenglein, professor of dermatology and pediatric dermatology, Penn State University, Hershey.
Before the point where propranolol is prescribed, confirm the diagnosis and use the correct terminology, she advised. It’s still appropriate to use the International Society for the Study of Vascular Anomalies (ISSVA) vascular lesion classification system released in 1982. “For most people, it serves the purpose well,” she said. Another option is an updated and more complex classification system from 2015.
Dr. Zaenglein highlighted two studies – one published in 2011 and the other published in 2020 – that revealed high levels of misclassification of vascular malformations in research reports. The earlier study found that 21% of patients with misclassified lesions were mistreated, compared with none of those who were classified using ISSVA terminology.
“I cannot stress [proper classification] enough when you’re dealing with babies and children with vascular lesions. If not sure, be vague. Say ‘a vascular tumor’ or a ‘vascular malformation.’ But only reserve ‘infantile hemangioma’ for that very diagnosis,” she said.
As Dr. Zaenglein noted, infantile hemangiomas affect 5%-10% of 1-year-olds, of whom 20% have multiple lesions. They’re more common in females by a 3-to-1 margin, and also seen more in premature infants, and in cases of multiple births, higher maternal age, and low birth weight.
The pathogenesis of these lesions is unclear, she said, although there are hints about genetic components and tissue hypoxia, among other possible causes. “Importantly, you get 80% of the growth by 3-4 months of age. Then it’ll slow in its growth and kind of slowly go away over time, but it’s not linear regression. It’s more that you get more improvement up front, usually until about 5, and then you can get some continued gradual evolution up until about 7 or 10 years of age.”
Complications can include ulceration, infection and – in rare cases – hemorrhage and high-output cardiac failure, she said. “Knowing which ones are at high risk for complications is important, and also there are systemic associations that we have to be mindful of. We also want to think about aesthetic outcomes as well when we talk about management of infantile hemangiomas.”
High-risk infantile hemangiomas include those with the following features:
- Extensive facial involvement. Dr. Zaenglein highlighted a case of a 2-year-old baby with a large, bulky hemangioma that distorted facial features around the eye. “This would be a medical emergency” requiring immediate evaluation and treatment, she said.
- Periocular involvement. Refer to ophthalmology, she recommended. “Even smaller hemangiomas can cause refractive errors or amblyopia, and oftentimes need to be treated with either systemic or topical therapy depending on the size and extent,” she said.
- PHACE syndrome (Posterior fossa malformations, hemangiomas, arterial anomalies, coarctation of the aorta and cardiac defects, eye abnormalities). “Propranolol has been safely used in PHACE, but every patient is different,” she said. “You need to make sure to do a good risk assessment before starting because if they have narrowed blood flow or limited blood flow, there is a question of whether there is potential risk for stroke if you drop a baby’s blood pressure. Make sure that the vasculature is evaluated before started on propranolol. Also, there are recent reports of risk of long-term risk of stroke with PHACE syndrome as patients are getting into their adulthood.”
- Beard distribution. Be aware of possible airway involvement that can be revealed by biphasic stridor. In those cases, immediate treatment – perhaps even with tracheostomy – is needed to avoid mortality, she said.
- Multiple sites: Patients with five or more hemangiomas may have liver involvement, she said, and should undergo hepatic evaluation. Consider evaluating if this is suspected, even if the number of hemangiomas is under five, she said.
- Perineal/lumbosacral involvement: A third of these cases are associated with spinal dysraphism. Refer to neurosurgery, she recommended.
Dr. Zaenglein highlighted a report on the use of propranolol published in 2008 and noted that clinical practice guidelines for managing infantile hemangiomas published in 2019 are also helpful.
Flat hemangiomas, meanwhile, can benefit from timolol maleate 0.5% solution or gel-forming solution – 1 drop twice daily or 2 drops once daily, she said. This treatment should be avoided in thick hemangiomas, she said.
MedscapeLive and this news organization are owned by the same parent company. Dr. Zaenglein disclosed consulting fees (Dermata, Cassiopea, and Regeneron), and fees for contracted research support (Incyte).
The first rule about infantile hemangiomas: Make sure they’re actually infantile hemangiomas, a pediatric dermatologist urged colleagues. Then watch patients closely, refer to specialists when appropriate, and consider propranolol in complicated or high-risk cases, Andrea L. Zaenglein, MD, said at MedscapeLive’s Women’s & Pediatric Dermatology Seminar.
“In my career as a pediatric dermatologist, propranolol has been a life changer for us more than any other medicine,” said Dr. Zaenglein, professor of dermatology and pediatric dermatology, Penn State University, Hershey.
Before the point where propranolol is prescribed, confirm the diagnosis and use the correct terminology, she advised. It’s still appropriate to use the International Society for the Study of Vascular Anomalies (ISSVA) vascular lesion classification system released in 1982. “For most people, it serves the purpose well,” she said. Another option is an updated and more complex classification system from 2015.
Dr. Zaenglein highlighted two studies – one published in 2011 and the other published in 2020 – that revealed high levels of misclassification of vascular malformations in research reports. The earlier study found that 21% of patients with misclassified lesions were mistreated, compared with none of those who were classified using ISSVA terminology.
“I cannot stress [proper classification] enough when you’re dealing with babies and children with vascular lesions. If not sure, be vague. Say ‘a vascular tumor’ or a ‘vascular malformation.’ But only reserve ‘infantile hemangioma’ for that very diagnosis,” she said.
As Dr. Zaenglein noted, infantile hemangiomas affect 5%-10% of 1-year-olds, of whom 20% have multiple lesions. They’re more common in females by a 3-to-1 margin, and also seen more in premature infants, and in cases of multiple births, higher maternal age, and low birth weight.
The pathogenesis of these lesions is unclear, she said, although there are hints about genetic components and tissue hypoxia, among other possible causes. “Importantly, you get 80% of the growth by 3-4 months of age. Then it’ll slow in its growth and kind of slowly go away over time, but it’s not linear regression. It’s more that you get more improvement up front, usually until about 5, and then you can get some continued gradual evolution up until about 7 or 10 years of age.”
Complications can include ulceration, infection and – in rare cases – hemorrhage and high-output cardiac failure, she said. “Knowing which ones are at high risk for complications is important, and also there are systemic associations that we have to be mindful of. We also want to think about aesthetic outcomes as well when we talk about management of infantile hemangiomas.”
High-risk infantile hemangiomas include those with the following features:
- Extensive facial involvement. Dr. Zaenglein highlighted a case of a 2-year-old baby with a large, bulky hemangioma that distorted facial features around the eye. “This would be a medical emergency” requiring immediate evaluation and treatment, she said.
- Periocular involvement. Refer to ophthalmology, she recommended. “Even smaller hemangiomas can cause refractive errors or amblyopia, and oftentimes need to be treated with either systemic or topical therapy depending on the size and extent,” she said.
- PHACE syndrome (Posterior fossa malformations, hemangiomas, arterial anomalies, coarctation of the aorta and cardiac defects, eye abnormalities). “Propranolol has been safely used in PHACE, but every patient is different,” she said. “You need to make sure to do a good risk assessment before starting because if they have narrowed blood flow or limited blood flow, there is a question of whether there is potential risk for stroke if you drop a baby’s blood pressure. Make sure that the vasculature is evaluated before started on propranolol. Also, there are recent reports of risk of long-term risk of stroke with PHACE syndrome as patients are getting into their adulthood.”
- Beard distribution. Be aware of possible airway involvement that can be revealed by biphasic stridor. In those cases, immediate treatment – perhaps even with tracheostomy – is needed to avoid mortality, she said.
- Multiple sites: Patients with five or more hemangiomas may have liver involvement, she said, and should undergo hepatic evaluation. Consider evaluating if this is suspected, even if the number of hemangiomas is under five, she said.
- Perineal/lumbosacral involvement: A third of these cases are associated with spinal dysraphism. Refer to neurosurgery, she recommended.
Dr. Zaenglein highlighted a report on the use of propranolol published in 2008 and noted that clinical practice guidelines for managing infantile hemangiomas published in 2019 are also helpful.
Flat hemangiomas, meanwhile, can benefit from timolol maleate 0.5% solution or gel-forming solution – 1 drop twice daily or 2 drops once daily, she said. This treatment should be avoided in thick hemangiomas, she said.
MedscapeLive and this news organization are owned by the same parent company. Dr. Zaenglein disclosed consulting fees (Dermata, Cassiopea, and Regeneron), and fees for contracted research support (Incyte).
FROM MEDSCAPELIVE WOMEN’S & PEDIATRIC DERMATOLOGY SEMINAR
Topical tranexamic acid for melasma
By addressing the vascular component of melasma, off-label use of oral tranexamic acid has been a beneficial adjunct for this difficult-to-treat condition. For on-label use treating menorrhagia (the oral form) and short-term prophylaxis of bleeding in hemophilia patients undergoing dental procedures – (the injectable form), tranexamic acid acts as an antifibrinolytic.
By inhibiting plasminogen activation, according to a 2018 review article “tranexamic acid mitigates UV radiation–induced melanogenesis and neovascularization,” both exhibited in the clinical manifestations of melasma.1 In addition to inhibiting fibrinolysis, tranexamic acid has direct effects on UV-induced pigmentation, “via its inhibitory effects on UV light–induced plasminogen activator on keratinocytes and [subsequent] plasmin activity,” the article states. “Plasminogen activator induces tyrosinase activity, resulting in increased melanin synthesis. The presence of plasmin [which dissolves clots by degrading fibrin] results in increased production of both arachidonic acid and fibroblast growth factor, which stimulate melanogenesis and neovascularization, respectively.”
With oral use, the risk of clot formation, especially in those who have a history of blood clots, clotting disorders (such as factor V Leiden), smoking, or other hypercoagulability risks should be weighed.
Topical tranexamic acid used locally mitigates systemic risk, and according to published studies, has been found to be efficacious for hemostasis in knee and hip arthroplasty surgery and for epistaxis. However, clinical outcomes with the topical treatment have largely not been on par with regards to efficacy for melasma when compared with oral tranexamic acid.
. Topical tranexamic acid, in my experience, when applied immediately after fractional 1927-nm diode laser treatment, not only has been noted by patients to feel soothing, but anecdotally has been found to improve pigmentation.
Moreover, there are now several peer-reviewed studies showing some benefit for treating pigmentation from photodamage or melasma with laser-assisted delivery of topical tranexamic acid. Treatment of these conditions may also benefit from nonablative 1927-nm laser alone.
In one recently published study, 10 female melasma patients, Fitzpatrick skin types II-IV, underwent five full-face low-energy, low-density (power 4-5 W, fluence 2-8 mJ, 2-8 passes) 1927-nm fractional thulium fiber laser treatment.2 Topical tranexamic acid was applied immediately after laser treatment and continued twice daily for 7 days. Seven patients completed the study. Based on the Global Aesthetics Improvement Scale (GAIS) ratings, all seven patients noted improvement at day 180, at which time six of the patients were considered to have improved from baseline, according to the investigator GAIS ratings. Using the Melasma Area Severity Index (MASI) score, the greatest degree of improvement was seen at day 90; there were three recurrences of melasma with worsening of the MASI score between day 90 and day 180.
In a split-face, double-blind, randomized controlled study, 46 patients with Fitzpatrick skin types III-V, with recalcitrant melasma received four weekly treatments of full-face fractional 1927-nm thulium laser; topical tranexamic acid was applied to one side of the face and normal saline applied to the other side under occlusion, immediately after treatment.3 At 3 months, significant improvements from baseline were seen with Melanin Index (MI) and modified MASI (mMASI) scores for the sides treated with tranexamic acid and the control side, with no statistically significant differences between the two. However, at month 6, among the 29 patients available for follow-up, significant differences in MI and mMASI scores from baseline were still evident, with the exception of MI scores on the control sides.
No adverse events from using topical tranexamic acid with laser were noted in either study. Split-face randomized control studies with use of topical tranexamic acid after fractional 1927-nm diode laser in comparison to fractional 1927-nm thulium laser would be notable in this vascular and heat-sensitive condition as well.
Dr. Wesley and Dr. Talakoub are cocontributors to this column. Dr. Wesley practices dermatology in Beverly Hills, Calif. Dr. Talakoub is in private practice in McLean, Va. This month’s column is by Dr. Wesley. Write to them at dermnews@mdedge.com. They had no relevant disclosures.
References
1. Sheu SL. Cutis. 2018 Feb;101(2):E7-E8.
2. Wang, JV et al. J Cosmet Dermatol. 2021 Jan;20(1):105-9.
3. Wanitphakdeedecha R. et al. Lasers Med Sci. 2020 Dec;35(9):2015-21.
By addressing the vascular component of melasma, off-label use of oral tranexamic acid has been a beneficial adjunct for this difficult-to-treat condition. For on-label use treating menorrhagia (the oral form) and short-term prophylaxis of bleeding in hemophilia patients undergoing dental procedures – (the injectable form), tranexamic acid acts as an antifibrinolytic.
By inhibiting plasminogen activation, according to a 2018 review article “tranexamic acid mitigates UV radiation–induced melanogenesis and neovascularization,” both exhibited in the clinical manifestations of melasma.1 In addition to inhibiting fibrinolysis, tranexamic acid has direct effects on UV-induced pigmentation, “via its inhibitory effects on UV light–induced plasminogen activator on keratinocytes and [subsequent] plasmin activity,” the article states. “Plasminogen activator induces tyrosinase activity, resulting in increased melanin synthesis. The presence of plasmin [which dissolves clots by degrading fibrin] results in increased production of both arachidonic acid and fibroblast growth factor, which stimulate melanogenesis and neovascularization, respectively.”
With oral use, the risk of clot formation, especially in those who have a history of blood clots, clotting disorders (such as factor V Leiden), smoking, or other hypercoagulability risks should be weighed.
Topical tranexamic acid used locally mitigates systemic risk, and according to published studies, has been found to be efficacious for hemostasis in knee and hip arthroplasty surgery and for epistaxis. However, clinical outcomes with the topical treatment have largely not been on par with regards to efficacy for melasma when compared with oral tranexamic acid.
. Topical tranexamic acid, in my experience, when applied immediately after fractional 1927-nm diode laser treatment, not only has been noted by patients to feel soothing, but anecdotally has been found to improve pigmentation.
Moreover, there are now several peer-reviewed studies showing some benefit for treating pigmentation from photodamage or melasma with laser-assisted delivery of topical tranexamic acid. Treatment of these conditions may also benefit from nonablative 1927-nm laser alone.
In one recently published study, 10 female melasma patients, Fitzpatrick skin types II-IV, underwent five full-face low-energy, low-density (power 4-5 W, fluence 2-8 mJ, 2-8 passes) 1927-nm fractional thulium fiber laser treatment.2 Topical tranexamic acid was applied immediately after laser treatment and continued twice daily for 7 days. Seven patients completed the study. Based on the Global Aesthetics Improvement Scale (GAIS) ratings, all seven patients noted improvement at day 180, at which time six of the patients were considered to have improved from baseline, according to the investigator GAIS ratings. Using the Melasma Area Severity Index (MASI) score, the greatest degree of improvement was seen at day 90; there were three recurrences of melasma with worsening of the MASI score between day 90 and day 180.
In a split-face, double-blind, randomized controlled study, 46 patients with Fitzpatrick skin types III-V, with recalcitrant melasma received four weekly treatments of full-face fractional 1927-nm thulium laser; topical tranexamic acid was applied to one side of the face and normal saline applied to the other side under occlusion, immediately after treatment.3 At 3 months, significant improvements from baseline were seen with Melanin Index (MI) and modified MASI (mMASI) scores for the sides treated with tranexamic acid and the control side, with no statistically significant differences between the two. However, at month 6, among the 29 patients available for follow-up, significant differences in MI and mMASI scores from baseline were still evident, with the exception of MI scores on the control sides.
No adverse events from using topical tranexamic acid with laser were noted in either study. Split-face randomized control studies with use of topical tranexamic acid after fractional 1927-nm diode laser in comparison to fractional 1927-nm thulium laser would be notable in this vascular and heat-sensitive condition as well.
Dr. Wesley and Dr. Talakoub are cocontributors to this column. Dr. Wesley practices dermatology in Beverly Hills, Calif. Dr. Talakoub is in private practice in McLean, Va. This month’s column is by Dr. Wesley. Write to them at dermnews@mdedge.com. They had no relevant disclosures.
References
1. Sheu SL. Cutis. 2018 Feb;101(2):E7-E8.
2. Wang, JV et al. J Cosmet Dermatol. 2021 Jan;20(1):105-9.
3. Wanitphakdeedecha R. et al. Lasers Med Sci. 2020 Dec;35(9):2015-21.
By addressing the vascular component of melasma, off-label use of oral tranexamic acid has been a beneficial adjunct for this difficult-to-treat condition. For on-label use treating menorrhagia (the oral form) and short-term prophylaxis of bleeding in hemophilia patients undergoing dental procedures – (the injectable form), tranexamic acid acts as an antifibrinolytic.
By inhibiting plasminogen activation, according to a 2018 review article “tranexamic acid mitigates UV radiation–induced melanogenesis and neovascularization,” both exhibited in the clinical manifestations of melasma.1 In addition to inhibiting fibrinolysis, tranexamic acid has direct effects on UV-induced pigmentation, “via its inhibitory effects on UV light–induced plasminogen activator on keratinocytes and [subsequent] plasmin activity,” the article states. “Plasminogen activator induces tyrosinase activity, resulting in increased melanin synthesis. The presence of plasmin [which dissolves clots by degrading fibrin] results in increased production of both arachidonic acid and fibroblast growth factor, which stimulate melanogenesis and neovascularization, respectively.”
With oral use, the risk of clot formation, especially in those who have a history of blood clots, clotting disorders (such as factor V Leiden), smoking, or other hypercoagulability risks should be weighed.
Topical tranexamic acid used locally mitigates systemic risk, and according to published studies, has been found to be efficacious for hemostasis in knee and hip arthroplasty surgery and for epistaxis. However, clinical outcomes with the topical treatment have largely not been on par with regards to efficacy for melasma when compared with oral tranexamic acid.
. Topical tranexamic acid, in my experience, when applied immediately after fractional 1927-nm diode laser treatment, not only has been noted by patients to feel soothing, but anecdotally has been found to improve pigmentation.
Moreover, there are now several peer-reviewed studies showing some benefit for treating pigmentation from photodamage or melasma with laser-assisted delivery of topical tranexamic acid. Treatment of these conditions may also benefit from nonablative 1927-nm laser alone.
In one recently published study, 10 female melasma patients, Fitzpatrick skin types II-IV, underwent five full-face low-energy, low-density (power 4-5 W, fluence 2-8 mJ, 2-8 passes) 1927-nm fractional thulium fiber laser treatment.2 Topical tranexamic acid was applied immediately after laser treatment and continued twice daily for 7 days. Seven patients completed the study. Based on the Global Aesthetics Improvement Scale (GAIS) ratings, all seven patients noted improvement at day 180, at which time six of the patients were considered to have improved from baseline, according to the investigator GAIS ratings. Using the Melasma Area Severity Index (MASI) score, the greatest degree of improvement was seen at day 90; there were three recurrences of melasma with worsening of the MASI score between day 90 and day 180.
In a split-face, double-blind, randomized controlled study, 46 patients with Fitzpatrick skin types III-V, with recalcitrant melasma received four weekly treatments of full-face fractional 1927-nm thulium laser; topical tranexamic acid was applied to one side of the face and normal saline applied to the other side under occlusion, immediately after treatment.3 At 3 months, significant improvements from baseline were seen with Melanin Index (MI) and modified MASI (mMASI) scores for the sides treated with tranexamic acid and the control side, with no statistically significant differences between the two. However, at month 6, among the 29 patients available for follow-up, significant differences in MI and mMASI scores from baseline were still evident, with the exception of MI scores on the control sides.
No adverse events from using topical tranexamic acid with laser were noted in either study. Split-face randomized control studies with use of topical tranexamic acid after fractional 1927-nm diode laser in comparison to fractional 1927-nm thulium laser would be notable in this vascular and heat-sensitive condition as well.
Dr. Wesley and Dr. Talakoub are cocontributors to this column. Dr. Wesley practices dermatology in Beverly Hills, Calif. Dr. Talakoub is in private practice in McLean, Va. This month’s column is by Dr. Wesley. Write to them at dermnews@mdedge.com. They had no relevant disclosures.
References
1. Sheu SL. Cutis. 2018 Feb;101(2):E7-E8.
2. Wang, JV et al. J Cosmet Dermatol. 2021 Jan;20(1):105-9.
3. Wanitphakdeedecha R. et al. Lasers Med Sci. 2020 Dec;35(9):2015-21.
Vitiligo treatment options abound but consider patient goals
, according to Seemal Desai, MD, of the University of Texas, Dallas.
“We have topical steroids. We have vitamin D analogs, calcineurin inhibitors, and depigmentation therapy. We also have systemic therapy, phototherapy, surgical treatment, and even psychological therapy, Dr. Desai said in a presentation at MedscapeLive’s virtual Women’s & Pediatric Dermatology Seminar.
Head and neck vitiligo, which “tends to respond very nicely to treatment,” is one of the affected areas “where we have an important obligation to make sure our patients are effectively and aggressively treated,” he said.
According to Dr. Desai, there are three kinds of vitiligo. Active/unstable vitiligo is marked by depigmentation spreading across 1%-2% of body surface area per month, the size of about one to two palms. Refractory vitiligo responds poorly to therapy with less than 25% of affected areas experiencing repigmentation. And the third type is chronic vitiligo. “The majority of patients we see are in this phase, where depigmentation is present for at least 1 year with no history of spontaneous repigmentation.”
Before turning to therapy, he said, make sure to understand what the patient wants. “Are they even interested in being treated? I’ve had some patients with vitiligo, it’s only on their chest, and they’re always covered. They don’t even want anything. Then I have other patients who only want their face and hands treated because those are the only parts of their body that are exposed.”
To stabilize vitiligo, Dr. Desai recommends treating patients with “mini-pulse” oral therapy with systemic steroids. “I prescribe 4 milligrams of dexamethasone to be taken 2 consecutive days per week, such as Saturdays and Sundays. I usually halve the dose in children aged less than 16 years of age, so they’d be taking 2 milligrams.” Make sure, he said, to counsel patients on side effects.
He also recommends antioxidants, particularly polypodium leucotomos, “which has been shown in studies to increase the rates of head and neck repigmentation when combined with narrowband UVB.” He recommends 240 milligrams or higher, 2 or 3 times a day. He adds that alpha lipoic acid – in combination with vitamin C, vitamin E, and phototherapy – has also been shown to be effective in inducing repigmentation, especially on the head and neck.
As for newer drugs, Dr. Desai said afamelanotide, an analogue of alpha melanocyte-stimulating hormone combined with phototherapy, has shown promise. (It was approved in 2019 to increase pain free light exposure in adults with a history of phototoxic reactions related to erythropoietic protoporphyria.) Like other medications he mentioned, it isn’t FDA approved for treating vitiligo.
On another front, “Janus kinase inhibitors are our new frontier in treating vitiligo,” he said. “Tofacitinib can be dosed as an off-label usage in vitiligo in doses of 5 milligrams every other day, up to 5 milligrams daily. It’s half of the dose of rheumatoid arthritis, which is 5 milligrams b.i.d. You can actually start to see repigmentation as soon as 2 months, and then improvement up to 5 months.”
The drug requires laboratory monitoring and is expensive, he said, and JAK inhibitor side effects must be discussed with all patients.
Topical JAK inhibitors – tofacitinib 2% cream and ruxolitinib 1.5% cream – are also being evaluated as treatment for vitiligo. “I find that ruxolitinib works a little bit better, and the early bit of vitiligo data has shown that it tends to have more of a robust pigmentation response compared to tofacitinib,” said Dr. Desai, who gets these drugs compounded for topical use.
Dr. Desai added that he prefers to combine JAK inhibitors with phototherapy when possible.
For resistant vitiligo, he said, “lasers can help, especially Q-switched ruby and Q-switched Alexandrite laser. Q-switched Nd:Yag is very popular in Asia.”
In the big picture, he said, patients can benefit greatly from treatment. “Just think about the psychological improvement a patient would get by not having to get stares when walking in a mall and not having to deal with vitiligo lesions all over their cheek and neck.”
Dr. Desai disclosed performing clinical trials and/or consulting for numerous companies, including Pfizer, Allergan, AbbVie, and Dr. Reddy’s, among others. MedscapeLive and this news organization are owned by the same parent company.
, according to Seemal Desai, MD, of the University of Texas, Dallas.
“We have topical steroids. We have vitamin D analogs, calcineurin inhibitors, and depigmentation therapy. We also have systemic therapy, phototherapy, surgical treatment, and even psychological therapy, Dr. Desai said in a presentation at MedscapeLive’s virtual Women’s & Pediatric Dermatology Seminar.
Head and neck vitiligo, which “tends to respond very nicely to treatment,” is one of the affected areas “where we have an important obligation to make sure our patients are effectively and aggressively treated,” he said.
According to Dr. Desai, there are three kinds of vitiligo. Active/unstable vitiligo is marked by depigmentation spreading across 1%-2% of body surface area per month, the size of about one to two palms. Refractory vitiligo responds poorly to therapy with less than 25% of affected areas experiencing repigmentation. And the third type is chronic vitiligo. “The majority of patients we see are in this phase, where depigmentation is present for at least 1 year with no history of spontaneous repigmentation.”
Before turning to therapy, he said, make sure to understand what the patient wants. “Are they even interested in being treated? I’ve had some patients with vitiligo, it’s only on their chest, and they’re always covered. They don’t even want anything. Then I have other patients who only want their face and hands treated because those are the only parts of their body that are exposed.”
To stabilize vitiligo, Dr. Desai recommends treating patients with “mini-pulse” oral therapy with systemic steroids. “I prescribe 4 milligrams of dexamethasone to be taken 2 consecutive days per week, such as Saturdays and Sundays. I usually halve the dose in children aged less than 16 years of age, so they’d be taking 2 milligrams.” Make sure, he said, to counsel patients on side effects.
He also recommends antioxidants, particularly polypodium leucotomos, “which has been shown in studies to increase the rates of head and neck repigmentation when combined with narrowband UVB.” He recommends 240 milligrams or higher, 2 or 3 times a day. He adds that alpha lipoic acid – in combination with vitamin C, vitamin E, and phototherapy – has also been shown to be effective in inducing repigmentation, especially on the head and neck.
As for newer drugs, Dr. Desai said afamelanotide, an analogue of alpha melanocyte-stimulating hormone combined with phototherapy, has shown promise. (It was approved in 2019 to increase pain free light exposure in adults with a history of phototoxic reactions related to erythropoietic protoporphyria.) Like other medications he mentioned, it isn’t FDA approved for treating vitiligo.
On another front, “Janus kinase inhibitors are our new frontier in treating vitiligo,” he said. “Tofacitinib can be dosed as an off-label usage in vitiligo in doses of 5 milligrams every other day, up to 5 milligrams daily. It’s half of the dose of rheumatoid arthritis, which is 5 milligrams b.i.d. You can actually start to see repigmentation as soon as 2 months, and then improvement up to 5 months.”
The drug requires laboratory monitoring and is expensive, he said, and JAK inhibitor side effects must be discussed with all patients.
Topical JAK inhibitors – tofacitinib 2% cream and ruxolitinib 1.5% cream – are also being evaluated as treatment for vitiligo. “I find that ruxolitinib works a little bit better, and the early bit of vitiligo data has shown that it tends to have more of a robust pigmentation response compared to tofacitinib,” said Dr. Desai, who gets these drugs compounded for topical use.
Dr. Desai added that he prefers to combine JAK inhibitors with phototherapy when possible.
For resistant vitiligo, he said, “lasers can help, especially Q-switched ruby and Q-switched Alexandrite laser. Q-switched Nd:Yag is very popular in Asia.”
In the big picture, he said, patients can benefit greatly from treatment. “Just think about the psychological improvement a patient would get by not having to get stares when walking in a mall and not having to deal with vitiligo lesions all over their cheek and neck.”
Dr. Desai disclosed performing clinical trials and/or consulting for numerous companies, including Pfizer, Allergan, AbbVie, and Dr. Reddy’s, among others. MedscapeLive and this news organization are owned by the same parent company.
, according to Seemal Desai, MD, of the University of Texas, Dallas.
“We have topical steroids. We have vitamin D analogs, calcineurin inhibitors, and depigmentation therapy. We also have systemic therapy, phototherapy, surgical treatment, and even psychological therapy, Dr. Desai said in a presentation at MedscapeLive’s virtual Women’s & Pediatric Dermatology Seminar.
Head and neck vitiligo, which “tends to respond very nicely to treatment,” is one of the affected areas “where we have an important obligation to make sure our patients are effectively and aggressively treated,” he said.
According to Dr. Desai, there are three kinds of vitiligo. Active/unstable vitiligo is marked by depigmentation spreading across 1%-2% of body surface area per month, the size of about one to two palms. Refractory vitiligo responds poorly to therapy with less than 25% of affected areas experiencing repigmentation. And the third type is chronic vitiligo. “The majority of patients we see are in this phase, where depigmentation is present for at least 1 year with no history of spontaneous repigmentation.”
Before turning to therapy, he said, make sure to understand what the patient wants. “Are they even interested in being treated? I’ve had some patients with vitiligo, it’s only on their chest, and they’re always covered. They don’t even want anything. Then I have other patients who only want their face and hands treated because those are the only parts of their body that are exposed.”
To stabilize vitiligo, Dr. Desai recommends treating patients with “mini-pulse” oral therapy with systemic steroids. “I prescribe 4 milligrams of dexamethasone to be taken 2 consecutive days per week, such as Saturdays and Sundays. I usually halve the dose in children aged less than 16 years of age, so they’d be taking 2 milligrams.” Make sure, he said, to counsel patients on side effects.
He also recommends antioxidants, particularly polypodium leucotomos, “which has been shown in studies to increase the rates of head and neck repigmentation when combined with narrowband UVB.” He recommends 240 milligrams or higher, 2 or 3 times a day. He adds that alpha lipoic acid – in combination with vitamin C, vitamin E, and phototherapy – has also been shown to be effective in inducing repigmentation, especially on the head and neck.
As for newer drugs, Dr. Desai said afamelanotide, an analogue of alpha melanocyte-stimulating hormone combined with phototherapy, has shown promise. (It was approved in 2019 to increase pain free light exposure in adults with a history of phototoxic reactions related to erythropoietic protoporphyria.) Like other medications he mentioned, it isn’t FDA approved for treating vitiligo.
On another front, “Janus kinase inhibitors are our new frontier in treating vitiligo,” he said. “Tofacitinib can be dosed as an off-label usage in vitiligo in doses of 5 milligrams every other day, up to 5 milligrams daily. It’s half of the dose of rheumatoid arthritis, which is 5 milligrams b.i.d. You can actually start to see repigmentation as soon as 2 months, and then improvement up to 5 months.”
The drug requires laboratory monitoring and is expensive, he said, and JAK inhibitor side effects must be discussed with all patients.
Topical JAK inhibitors – tofacitinib 2% cream and ruxolitinib 1.5% cream – are also being evaluated as treatment for vitiligo. “I find that ruxolitinib works a little bit better, and the early bit of vitiligo data has shown that it tends to have more of a robust pigmentation response compared to tofacitinib,” said Dr. Desai, who gets these drugs compounded for topical use.
Dr. Desai added that he prefers to combine JAK inhibitors with phototherapy when possible.
For resistant vitiligo, he said, “lasers can help, especially Q-switched ruby and Q-switched Alexandrite laser. Q-switched Nd:Yag is very popular in Asia.”
In the big picture, he said, patients can benefit greatly from treatment. “Just think about the psychological improvement a patient would get by not having to get stares when walking in a mall and not having to deal with vitiligo lesions all over their cheek and neck.”
Dr. Desai disclosed performing clinical trials and/or consulting for numerous companies, including Pfizer, Allergan, AbbVie, and Dr. Reddy’s, among others. MedscapeLive and this news organization are owned by the same parent company.
FROM MEDSCAPELIVE WOMEN’S & PEDIATRIC DERMATOLOGY SEMINAR
Combination approach to melasma treatment yields best results
When establishing a treatment plan for patients with melasma, counseling them about realistic expectations is key.
“It’s important that they understand that this is a chronic condition, so it does require long-term maintenance therapy,” Arisa E. Ortiz, MD, said at the virtual annual Masters of Aesthetics Symposium. “We can improve melasma, but it’s difficult to cure melasma.”
While hydroquinone and other bleaching agents are typical treatment mainstays, chemical peels with glycolic acid, trichloroacetic acid, and salicylic acid can benefit some individuals. “For chemical peels, I really like glycolic acid peels because there is no downtime; it peels at the microscopic level,” said Dr. Ortiz, who is director of laser and cosmetic dermatology at the University of California, San Diego. “This is something they may need to repeat monthly, and having a week of peeling may be difficult to go through every month.”
Other common melasma treatments include lasers, intense pulsed light (IPL), and oral medications. “I personally am not impressed with microdermabrasion for melasma, so I don’t use that very much,” she said. “With laser treatment, you want to make sure you’re using low-energy lasers so that it doesn’t exacerbate or make them relapse or rebound.”
While hydroquinone is a mainstay of therapy, “you can’t use it chronically because of the risk of ochronosis (permanent darkening), so you do need to take drug holidays,” Dr. Ortiz said. “During those drug holidays, you want to make sure patients have a nonhydroquinone bleaching agent so that they don’t flare.” Options include lignin peroxidase, oligopeptide, Lytera, Melaplex, 4-n-butylresorcinol, Cysteamine cream, tranexamic acid, and oral antioxidants.
In a study sponsored by SkinMedica, investigators conducted a randomized, double-blind, half-face study in females with moderate to severe facial hyperpigmentation to assess the efficacy and tolerability of three new skin brightener formulations containing SMA-432, a prostaglandin E2 inhibitor, compared with topical 4% hydroquinone (J Drugs Dermatol 2012 Dec;11[12]:1478-82). They found that the nonhydroquinone skin formulations were better tolerated and were just as effective as 4% hydroquinone.
In a separate unpublished study of 22 females, investigators assessed the efficacy of the U.SK Advanced Defense Booster, which contains ferulic acid, maslinic acid, peptides, and olive leaf extract. They observed that 98% of patients saw improvement after 28 days of treatment.
When it comes to using lasers for melasma treatment, low-energy devices provide the best outcomes. “I prefer using something like the 1927-nm fractional diode lasers at 3.75% density, really low densities because there’s less risk for rebound,” Dr. Ortiz said. “They also enhance skin permeability for the use of topicals.”
In an observational study of 27 female patients with refractory melasma, Arielle Kauvar, MD, director of New York Laser & Skin Care, combined microdermabrasion with the Q-switched Nd:YAG (Lasers in Surgery and Medicine 2012; 44:117-24). “The settings she used were very low fluence, so there was no clinical endpoint or no whitening,” Dr. Ortiz said. Specifically, she used a laser at 1.6-2 J/cm2 with a 5- or 6-mm spot size immediately following microdermabrasion for 4 weeks. “She got a good improvement using a skin care regimen of sunscreen, hydroquinone, and tretinoin or vitamin C,” she said. “Remission lasted at least 6 months.”
In a study presented at the 2019 annual meeting of the America Society for Laser Medicine and Surgery, Dr. Ortiz and Tanya Greywal, MD, of the University of California, San Diego, used three passes of the 10764-nm Nd:YAG laser to treat 10 subjects with melasma skin types 2-5. The device has a 650-microsecond pulse duration, a 6-mm spot size, and an energy mode of 11-14 J/cm3. “There was no downtime with these patients, and they saw a mean improvement of 26%-50% as early as 3 weeks,” she said. “Patients did require multiple treatments to see adequate resolution, but no anesthesia or numbing cream was required. This is a good option for patients who need chronic maintenance treatment.”
Topicals also play a key role following the laser treatment of melasma. Dr. Ortiz characterized clobetasol as “kind of like the magic ointment.” She uses one application immediately post procedure “whenever I’m worried about a patient having postinflammatory hyperpigmentation or if I don’t want melasma patients to rebound. It can help reduce swelling and inflammation to decrease the risk of postinflammatory hyperpigmentation.”
Researchers have discovered that there is a vascular component to melasma. Paul M. Friedman, MD, of the Dermatology and Laser Surgery Center, Houston, and his colleagues used spectrocolorimetry to detect an underlying prominent vascular component in 11 patients with melasma (Lasers Surg Med 2017 Jan;49[1]:20-6). They determined that melasma lesions exhibiting subtle or subclinical telangiectatic erythema may be improved by combined vascular-targeted laser therapy together with fractional low-powered diode laser therapy. “A parallel improvement in telangiectatic erythema suggests a relationship between the underlying vasculature and hyperpigmentation,” said Dr. Ortiz, who was not affiliated with the study. “So, patients who have a vascular component to their melasma actually can get improved efficacy.”
Another strategy for melasma patients involves oral treatment with Polypodium leucotomos extract (PLE), a fern from the Polypodiaceae family with antioxidant properties that has been shown to be photoprotective against UVA and UVB radiation. “I like to think of it as an internal sunscreen,” Dr. Ortiz said. “It does not replace your external sunscreen, but it adds extra protection. It has been shown to significantly reduce the severity of sunburn and decrease the risk of UV radiation–induced skin cancer, as well as prevent skin aging.” The purported mechanism of action includes decreasing UV-mediated oxidative damage to DNA, enhancing the activity of endogenous antioxidant systems, increasing the minimal erythema dose, blocking UV radiation–induced cyclooxygenase-2 expression, reducing UV-induced immune suppression, and promoting p53 suppressor gene expression.
In a pilot placebo-controlled study of melasma patients on their normal regimen of hydroquinone and sunscreen, 40 Asian patients with melasma were randomized to receive either oral PLE supplementation or placebo for 12 weeks (J Clin Aesthet Dermatol 2018 Mar;11[3]:14-9). They found that PLE significantly improved and accelerated the outcome reached with hydroquinone and sunscreen from the first month of treatment, compared with placebo.
Dr. Ortiz next discussed the role of oral tranexamic acid, an antifibrinolytic, procoagulant agent that is approved by the Food and Drug Administration for the treatment of menorrhagia and for prevention of hemorrhage in patients with hemophilia undergoing tooth extractions. “It is a synthetic lysine derivative that inhibits plasminogen activation by blocking lysine-binding sites on the plasminogen molecule, and it’s a game changer for melasma treatment,” she said. “One of the side effects is that it inhibits melanogenesis and neovascularization. It’s been effective for melasma, but its use is limited by the risk for thromboembolism. It’s a slight increased risk, something patients should be aware of, but not something that should scare us away from prescribing it.”
In a study of 561 patients with melasma, 90% improved after a median treatment duration of 4 months, and only 7% had side effects (J Am Acad Dermatol 2016;75:385-92). The most common side effects were abdominal bloating and pain. One patient developed a DVT during treatment, but that person was found to have a protein S deficiency.
The daily dosing of tranexamic acid for menorrhagia is 3,900 mg daily, while the dose for melasma has ranged from 500 mg-1,500 mg per day, Dr. Ortiz said. It’s available as a 650-mg pill in the United States. “I prescribe 325 mg twice a day, but studies have shown that 650 mg once a day is just as effective,” she said.
Prior to prescribing tranexamic acid, Dr. Ortiz does not order labs, but she performs an extensive history of present illness. She does not prescribe it in patients with an increased risk of clotting, including people who smoke and those who take oral contraceptives or are on hormone supplementation. Use is also contraindicated in people with a current malignancy, those with a history of stroke or DVT, and those who have any clotting disorder.
She concluded her presentation by noting that she favors a combination approach to treating melasma patients that starts with a broad spectrum sunscreen and PLE. “For bleaching, I like to use 12% hydroquinone with 6% kojic acid in VersaBase,” she said. “Once I get them in better control, then I switch them to 4% hydroquinone for maintenance. I use glycolic peels, low-energy lasers, and tranexamic acid if the melasma is severe, and they have no contraindications. A combination approach really achieves the best results, and counseling is key.”
Dr. Ortiz disclosed having financial relationships with numerous pharmaceutical and device companies. She is also cochair of MOA.
When establishing a treatment plan for patients with melasma, counseling them about realistic expectations is key.
“It’s important that they understand that this is a chronic condition, so it does require long-term maintenance therapy,” Arisa E. Ortiz, MD, said at the virtual annual Masters of Aesthetics Symposium. “We can improve melasma, but it’s difficult to cure melasma.”
While hydroquinone and other bleaching agents are typical treatment mainstays, chemical peels with glycolic acid, trichloroacetic acid, and salicylic acid can benefit some individuals. “For chemical peels, I really like glycolic acid peels because there is no downtime; it peels at the microscopic level,” said Dr. Ortiz, who is director of laser and cosmetic dermatology at the University of California, San Diego. “This is something they may need to repeat monthly, and having a week of peeling may be difficult to go through every month.”
Other common melasma treatments include lasers, intense pulsed light (IPL), and oral medications. “I personally am not impressed with microdermabrasion for melasma, so I don’t use that very much,” she said. “With laser treatment, you want to make sure you’re using low-energy lasers so that it doesn’t exacerbate or make them relapse or rebound.”
While hydroquinone is a mainstay of therapy, “you can’t use it chronically because of the risk of ochronosis (permanent darkening), so you do need to take drug holidays,” Dr. Ortiz said. “During those drug holidays, you want to make sure patients have a nonhydroquinone bleaching agent so that they don’t flare.” Options include lignin peroxidase, oligopeptide, Lytera, Melaplex, 4-n-butylresorcinol, Cysteamine cream, tranexamic acid, and oral antioxidants.
In a study sponsored by SkinMedica, investigators conducted a randomized, double-blind, half-face study in females with moderate to severe facial hyperpigmentation to assess the efficacy and tolerability of three new skin brightener formulations containing SMA-432, a prostaglandin E2 inhibitor, compared with topical 4% hydroquinone (J Drugs Dermatol 2012 Dec;11[12]:1478-82). They found that the nonhydroquinone skin formulations were better tolerated and were just as effective as 4% hydroquinone.
In a separate unpublished study of 22 females, investigators assessed the efficacy of the U.SK Advanced Defense Booster, which contains ferulic acid, maslinic acid, peptides, and olive leaf extract. They observed that 98% of patients saw improvement after 28 days of treatment.
When it comes to using lasers for melasma treatment, low-energy devices provide the best outcomes. “I prefer using something like the 1927-nm fractional diode lasers at 3.75% density, really low densities because there’s less risk for rebound,” Dr. Ortiz said. “They also enhance skin permeability for the use of topicals.”
In an observational study of 27 female patients with refractory melasma, Arielle Kauvar, MD, director of New York Laser & Skin Care, combined microdermabrasion with the Q-switched Nd:YAG (Lasers in Surgery and Medicine 2012; 44:117-24). “The settings she used were very low fluence, so there was no clinical endpoint or no whitening,” Dr. Ortiz said. Specifically, she used a laser at 1.6-2 J/cm2 with a 5- or 6-mm spot size immediately following microdermabrasion for 4 weeks. “She got a good improvement using a skin care regimen of sunscreen, hydroquinone, and tretinoin or vitamin C,” she said. “Remission lasted at least 6 months.”
In a study presented at the 2019 annual meeting of the America Society for Laser Medicine and Surgery, Dr. Ortiz and Tanya Greywal, MD, of the University of California, San Diego, used three passes of the 10764-nm Nd:YAG laser to treat 10 subjects with melasma skin types 2-5. The device has a 650-microsecond pulse duration, a 6-mm spot size, and an energy mode of 11-14 J/cm3. “There was no downtime with these patients, and they saw a mean improvement of 26%-50% as early as 3 weeks,” she said. “Patients did require multiple treatments to see adequate resolution, but no anesthesia or numbing cream was required. This is a good option for patients who need chronic maintenance treatment.”
Topicals also play a key role following the laser treatment of melasma. Dr. Ortiz characterized clobetasol as “kind of like the magic ointment.” She uses one application immediately post procedure “whenever I’m worried about a patient having postinflammatory hyperpigmentation or if I don’t want melasma patients to rebound. It can help reduce swelling and inflammation to decrease the risk of postinflammatory hyperpigmentation.”
Researchers have discovered that there is a vascular component to melasma. Paul M. Friedman, MD, of the Dermatology and Laser Surgery Center, Houston, and his colleagues used spectrocolorimetry to detect an underlying prominent vascular component in 11 patients with melasma (Lasers Surg Med 2017 Jan;49[1]:20-6). They determined that melasma lesions exhibiting subtle or subclinical telangiectatic erythema may be improved by combined vascular-targeted laser therapy together with fractional low-powered diode laser therapy. “A parallel improvement in telangiectatic erythema suggests a relationship between the underlying vasculature and hyperpigmentation,” said Dr. Ortiz, who was not affiliated with the study. “So, patients who have a vascular component to their melasma actually can get improved efficacy.”
Another strategy for melasma patients involves oral treatment with Polypodium leucotomos extract (PLE), a fern from the Polypodiaceae family with antioxidant properties that has been shown to be photoprotective against UVA and UVB radiation. “I like to think of it as an internal sunscreen,” Dr. Ortiz said. “It does not replace your external sunscreen, but it adds extra protection. It has been shown to significantly reduce the severity of sunburn and decrease the risk of UV radiation–induced skin cancer, as well as prevent skin aging.” The purported mechanism of action includes decreasing UV-mediated oxidative damage to DNA, enhancing the activity of endogenous antioxidant systems, increasing the minimal erythema dose, blocking UV radiation–induced cyclooxygenase-2 expression, reducing UV-induced immune suppression, and promoting p53 suppressor gene expression.
In a pilot placebo-controlled study of melasma patients on their normal regimen of hydroquinone and sunscreen, 40 Asian patients with melasma were randomized to receive either oral PLE supplementation or placebo for 12 weeks (J Clin Aesthet Dermatol 2018 Mar;11[3]:14-9). They found that PLE significantly improved and accelerated the outcome reached with hydroquinone and sunscreen from the first month of treatment, compared with placebo.
Dr. Ortiz next discussed the role of oral tranexamic acid, an antifibrinolytic, procoagulant agent that is approved by the Food and Drug Administration for the treatment of menorrhagia and for prevention of hemorrhage in patients with hemophilia undergoing tooth extractions. “It is a synthetic lysine derivative that inhibits plasminogen activation by blocking lysine-binding sites on the plasminogen molecule, and it’s a game changer for melasma treatment,” she said. “One of the side effects is that it inhibits melanogenesis and neovascularization. It’s been effective for melasma, but its use is limited by the risk for thromboembolism. It’s a slight increased risk, something patients should be aware of, but not something that should scare us away from prescribing it.”
In a study of 561 patients with melasma, 90% improved after a median treatment duration of 4 months, and only 7% had side effects (J Am Acad Dermatol 2016;75:385-92). The most common side effects were abdominal bloating and pain. One patient developed a DVT during treatment, but that person was found to have a protein S deficiency.
The daily dosing of tranexamic acid for menorrhagia is 3,900 mg daily, while the dose for melasma has ranged from 500 mg-1,500 mg per day, Dr. Ortiz said. It’s available as a 650-mg pill in the United States. “I prescribe 325 mg twice a day, but studies have shown that 650 mg once a day is just as effective,” she said.
Prior to prescribing tranexamic acid, Dr. Ortiz does not order labs, but she performs an extensive history of present illness. She does not prescribe it in patients with an increased risk of clotting, including people who smoke and those who take oral contraceptives or are on hormone supplementation. Use is also contraindicated in people with a current malignancy, those with a history of stroke or DVT, and those who have any clotting disorder.
She concluded her presentation by noting that she favors a combination approach to treating melasma patients that starts with a broad spectrum sunscreen and PLE. “For bleaching, I like to use 12% hydroquinone with 6% kojic acid in VersaBase,” she said. “Once I get them in better control, then I switch them to 4% hydroquinone for maintenance. I use glycolic peels, low-energy lasers, and tranexamic acid if the melasma is severe, and they have no contraindications. A combination approach really achieves the best results, and counseling is key.”
Dr. Ortiz disclosed having financial relationships with numerous pharmaceutical and device companies. She is also cochair of MOA.
When establishing a treatment plan for patients with melasma, counseling them about realistic expectations is key.
“It’s important that they understand that this is a chronic condition, so it does require long-term maintenance therapy,” Arisa E. Ortiz, MD, said at the virtual annual Masters of Aesthetics Symposium. “We can improve melasma, but it’s difficult to cure melasma.”
While hydroquinone and other bleaching agents are typical treatment mainstays, chemical peels with glycolic acid, trichloroacetic acid, and salicylic acid can benefit some individuals. “For chemical peels, I really like glycolic acid peels because there is no downtime; it peels at the microscopic level,” said Dr. Ortiz, who is director of laser and cosmetic dermatology at the University of California, San Diego. “This is something they may need to repeat monthly, and having a week of peeling may be difficult to go through every month.”
Other common melasma treatments include lasers, intense pulsed light (IPL), and oral medications. “I personally am not impressed with microdermabrasion for melasma, so I don’t use that very much,” she said. “With laser treatment, you want to make sure you’re using low-energy lasers so that it doesn’t exacerbate or make them relapse or rebound.”
While hydroquinone is a mainstay of therapy, “you can’t use it chronically because of the risk of ochronosis (permanent darkening), so you do need to take drug holidays,” Dr. Ortiz said. “During those drug holidays, you want to make sure patients have a nonhydroquinone bleaching agent so that they don’t flare.” Options include lignin peroxidase, oligopeptide, Lytera, Melaplex, 4-n-butylresorcinol, Cysteamine cream, tranexamic acid, and oral antioxidants.
In a study sponsored by SkinMedica, investigators conducted a randomized, double-blind, half-face study in females with moderate to severe facial hyperpigmentation to assess the efficacy and tolerability of three new skin brightener formulations containing SMA-432, a prostaglandin E2 inhibitor, compared with topical 4% hydroquinone (J Drugs Dermatol 2012 Dec;11[12]:1478-82). They found that the nonhydroquinone skin formulations were better tolerated and were just as effective as 4% hydroquinone.
In a separate unpublished study of 22 females, investigators assessed the efficacy of the U.SK Advanced Defense Booster, which contains ferulic acid, maslinic acid, peptides, and olive leaf extract. They observed that 98% of patients saw improvement after 28 days of treatment.
When it comes to using lasers for melasma treatment, low-energy devices provide the best outcomes. “I prefer using something like the 1927-nm fractional diode lasers at 3.75% density, really low densities because there’s less risk for rebound,” Dr. Ortiz said. “They also enhance skin permeability for the use of topicals.”
In an observational study of 27 female patients with refractory melasma, Arielle Kauvar, MD, director of New York Laser & Skin Care, combined microdermabrasion with the Q-switched Nd:YAG (Lasers in Surgery and Medicine 2012; 44:117-24). “The settings she used were very low fluence, so there was no clinical endpoint or no whitening,” Dr. Ortiz said. Specifically, she used a laser at 1.6-2 J/cm2 with a 5- or 6-mm spot size immediately following microdermabrasion for 4 weeks. “She got a good improvement using a skin care regimen of sunscreen, hydroquinone, and tretinoin or vitamin C,” she said. “Remission lasted at least 6 months.”
In a study presented at the 2019 annual meeting of the America Society for Laser Medicine and Surgery, Dr. Ortiz and Tanya Greywal, MD, of the University of California, San Diego, used three passes of the 10764-nm Nd:YAG laser to treat 10 subjects with melasma skin types 2-5. The device has a 650-microsecond pulse duration, a 6-mm spot size, and an energy mode of 11-14 J/cm3. “There was no downtime with these patients, and they saw a mean improvement of 26%-50% as early as 3 weeks,” she said. “Patients did require multiple treatments to see adequate resolution, but no anesthesia or numbing cream was required. This is a good option for patients who need chronic maintenance treatment.”
Topicals also play a key role following the laser treatment of melasma. Dr. Ortiz characterized clobetasol as “kind of like the magic ointment.” She uses one application immediately post procedure “whenever I’m worried about a patient having postinflammatory hyperpigmentation or if I don’t want melasma patients to rebound. It can help reduce swelling and inflammation to decrease the risk of postinflammatory hyperpigmentation.”
Researchers have discovered that there is a vascular component to melasma. Paul M. Friedman, MD, of the Dermatology and Laser Surgery Center, Houston, and his colleagues used spectrocolorimetry to detect an underlying prominent vascular component in 11 patients with melasma (Lasers Surg Med 2017 Jan;49[1]:20-6). They determined that melasma lesions exhibiting subtle or subclinical telangiectatic erythema may be improved by combined vascular-targeted laser therapy together with fractional low-powered diode laser therapy. “A parallel improvement in telangiectatic erythema suggests a relationship between the underlying vasculature and hyperpigmentation,” said Dr. Ortiz, who was not affiliated with the study. “So, patients who have a vascular component to their melasma actually can get improved efficacy.”
Another strategy for melasma patients involves oral treatment with Polypodium leucotomos extract (PLE), a fern from the Polypodiaceae family with antioxidant properties that has been shown to be photoprotective against UVA and UVB radiation. “I like to think of it as an internal sunscreen,” Dr. Ortiz said. “It does not replace your external sunscreen, but it adds extra protection. It has been shown to significantly reduce the severity of sunburn and decrease the risk of UV radiation–induced skin cancer, as well as prevent skin aging.” The purported mechanism of action includes decreasing UV-mediated oxidative damage to DNA, enhancing the activity of endogenous antioxidant systems, increasing the minimal erythema dose, blocking UV radiation–induced cyclooxygenase-2 expression, reducing UV-induced immune suppression, and promoting p53 suppressor gene expression.
In a pilot placebo-controlled study of melasma patients on their normal regimen of hydroquinone and sunscreen, 40 Asian patients with melasma were randomized to receive either oral PLE supplementation or placebo for 12 weeks (J Clin Aesthet Dermatol 2018 Mar;11[3]:14-9). They found that PLE significantly improved and accelerated the outcome reached with hydroquinone and sunscreen from the first month of treatment, compared with placebo.
Dr. Ortiz next discussed the role of oral tranexamic acid, an antifibrinolytic, procoagulant agent that is approved by the Food and Drug Administration for the treatment of menorrhagia and for prevention of hemorrhage in patients with hemophilia undergoing tooth extractions. “It is a synthetic lysine derivative that inhibits plasminogen activation by blocking lysine-binding sites on the plasminogen molecule, and it’s a game changer for melasma treatment,” she said. “One of the side effects is that it inhibits melanogenesis and neovascularization. It’s been effective for melasma, but its use is limited by the risk for thromboembolism. It’s a slight increased risk, something patients should be aware of, but not something that should scare us away from prescribing it.”
In a study of 561 patients with melasma, 90% improved after a median treatment duration of 4 months, and only 7% had side effects (J Am Acad Dermatol 2016;75:385-92). The most common side effects were abdominal bloating and pain. One patient developed a DVT during treatment, but that person was found to have a protein S deficiency.
The daily dosing of tranexamic acid for menorrhagia is 3,900 mg daily, while the dose for melasma has ranged from 500 mg-1,500 mg per day, Dr. Ortiz said. It’s available as a 650-mg pill in the United States. “I prescribe 325 mg twice a day, but studies have shown that 650 mg once a day is just as effective,” she said.
Prior to prescribing tranexamic acid, Dr. Ortiz does not order labs, but she performs an extensive history of present illness. She does not prescribe it in patients with an increased risk of clotting, including people who smoke and those who take oral contraceptives or are on hormone supplementation. Use is also contraindicated in people with a current malignancy, those with a history of stroke or DVT, and those who have any clotting disorder.
She concluded her presentation by noting that she favors a combination approach to treating melasma patients that starts with a broad spectrum sunscreen and PLE. “For bleaching, I like to use 12% hydroquinone with 6% kojic acid in VersaBase,” she said. “Once I get them in better control, then I switch them to 4% hydroquinone for maintenance. I use glycolic peels, low-energy lasers, and tranexamic acid if the melasma is severe, and they have no contraindications. A combination approach really achieves the best results, and counseling is key.”
Dr. Ortiz disclosed having financial relationships with numerous pharmaceutical and device companies. She is also cochair of MOA.
EXPERT ANALYSIS FROM MOA 2020
Bullae and Hyperpigmented Patches on the Legs
The Diagnosis: Lichen Planus Pemphigoides
A skin biopsy from the right thigh demonstrated subepidermal blisters containing neutrophils (Figure 1). Direct immunofluorescence revealed linear basement membrane zone staining with C3 and trace staining with IgG (Figure 2), supporting a diagnosis of lichen planus pemphigoides (LPP). Oral prednisone—starting at 60 mg daily and tapered to 40 mg for a week, 20 mg for a week, then 10 mg for a month—along with triamcinolone ointment 0.1% to affected areas led to improvement. Hydrochlorothiazide and UV light therapy were discontinued. Doxycycline 100 mg twice daily and nicotinamide 500 mg twice daily prescribed as adjunctive therapy also led to improvement. The patient achieved remission with doxycycline and was doing well without prednisone; however, he experienced a flare of his disease about 6 months later and was started on mycophenolate mofetil 1 g twice daily after clearance from his gastroenterologist, given his history of hepatitis B. He has been doing well since starting mycophenolate mofetil.
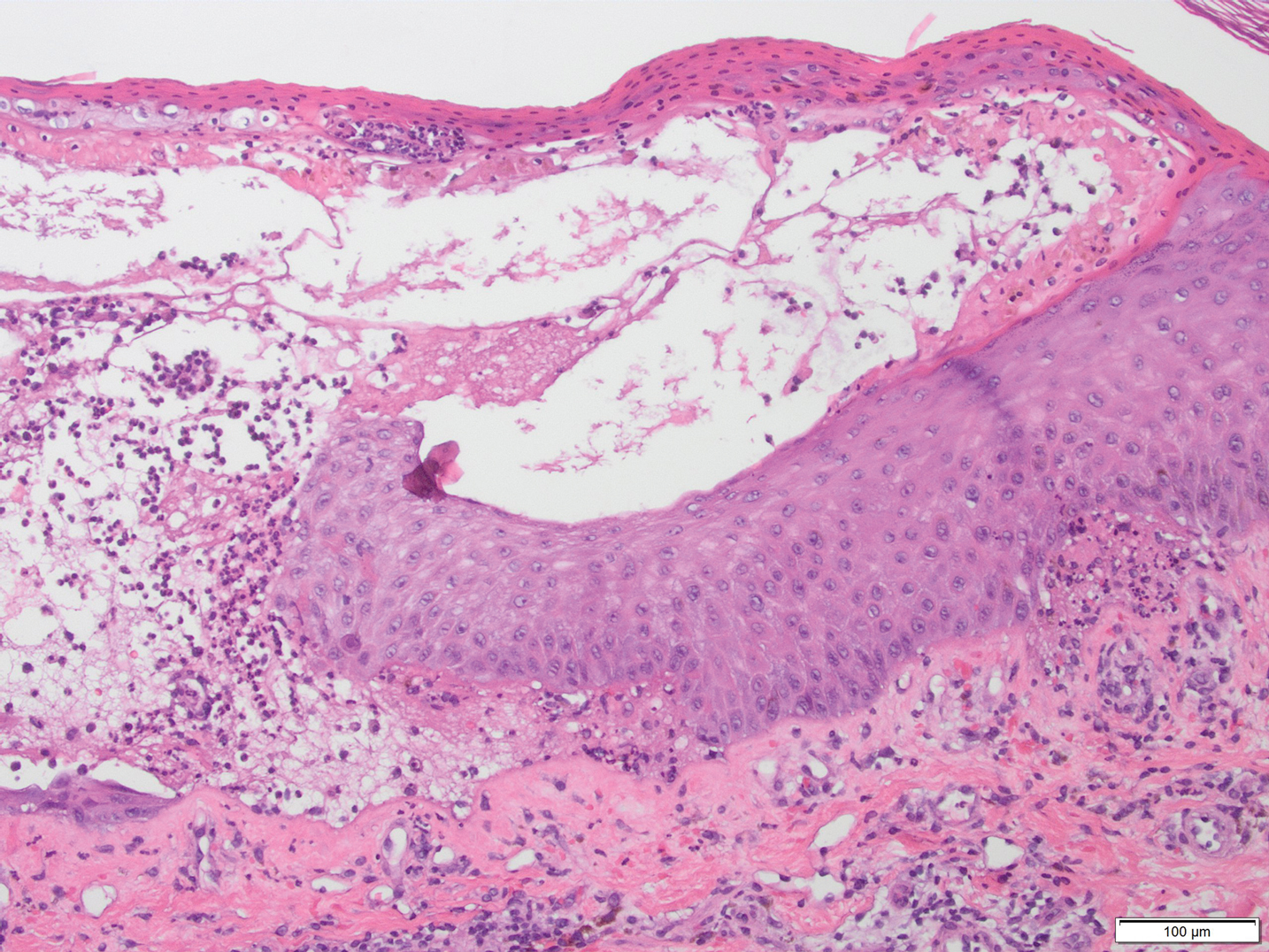
Lichen planus pemphigoides is a rare autoimmune bullous dermatosis with features of both lichen planus and bullous pemphigoid.1 Violaceous papules and tense bullae may be superimposed or arise independently. The chest, abdomen, back, and upper and lower extremities typically are involved.2 Oral mucosal involvement with white reticular streaks or erosions and nail involvement have been reported.2 Histopathologic and immunologic findings establish the diagnosis. Lichen planus pemphigoides is associated with subepidermal bullae and linear deposits of IgG and C3 on the basement membrane zone.1 Autoantibodies to bullous pemphigoid (BP) antigens BP180 and BP230 are associated with LPP.3 The pathogenesis of LPP remains unclear, but there are associations with chronic diseases, medications, and certain therapies.1,4-6 Several case reports have linked LPP to chronic viral hepatitis infections, as well as malignant tumors of the skin, mucosa, and gastrointestinal tract.2 Lichen planus pemphigoides has been reported in a patient on entecavir for hepatitis B as well as in a patient treated for hepatitis C with interferon and ribavirin.1 Lichen planus pemphigoides has been described in patients treated with the angiotensin-converting enzyme inhibitors enalapril, captopril, and ramipril.4,5,7 UV phototherapy also has been associated with the development of LPP.6 Hydrochlorothiazide previously has been reported as a cause of drug-induced lichen planus.8 A PubMed search of articles indexed for MEDLINE using the terms lichen planus pemphigoides and hydrochlorothiazide revealed no reports of hydrochlorothiazide-induced LPP.
Lichen planus pemphigoides demonstrates overlap with other blistering dermatoses, such as BP, bullous lupus erythematosus, and bullous lichen planus. Although histologically and immunologically similar to BP, LPP can be differentiated clinically by the presence of violaceous papules or plaques typical of lichen planus.9 Bullous pemphigoid is more common in individuals older than 70 years, whereas LPP tends to occur in middle-aged adults.2 Bullous lupus erythematosus usually is associated with manifestations of systemic lupus erythematosus and autoantibodies to collagen type VII.10 Salt-split skin studies demonstrate immunofluorescence on the dermal side of the split. Individuals affected by bullous lupus erythematosus typically have a history of photosensitivity.10 Blisters in LPP may form de novo from unaffected skin, whereas the bullae in bullous lichen planus are limited to existing lichenoid papules.9 The autoantibodies typical of LPP are absent in bullous lichen planus. Lichen planus actinicus is a variant of lichen planus that presents with annular, dyschromic, or violaceous plaques in a photodistributed pattern without bullous lesions.9
Lichen planus pemphigoides most commonly is treated with systemic corticosteroids. Topical steroids, dapsone, erythromycin, tetracycline and nicotinamide, azathioprine, and mycophenolate mofetil have been reported as adjuncts to systemic steroid therapy.2,11 Most reports describe treatment success with resolution of blistering lesions.
- Jang SH, Yun SJ, Lee SC, et al. Lichen planus pemphigoides associated with chronic hepatitis B virus infection. Clin Exp Dermatol. 2015;40:868-871.
- Zaraa I, Mahfoudh A, Sellami MK, et al. Lichen planus pemphigoides: four new cases and a review of the literature. Int J Dermatol. 2013;52:406-412.
- Harting MS, Hsu S. Lichen planus pemphigoides: a case report and review of the literature. Dermatol Online J. 2006;12:10.
- Onprasert W, Chanprapaph K. Lichen planus pemphigoides induced by enalapril: a case report and a review of literature. Case Rep Dermatol. 2017;9:217-224.
- Ben Salem C, Chengeul L, Ghariani N, et al. Captopril-induced lichen planus pemphigoides. Pharmacoepidemiol Drug Saf. 2008;17:722-724.
- Kuramoto N, Kishimoto S, Shibagaki R, et al. PUVA-induced lichen planus pemphigoides. Br J Dermatol. 2000;142:509-512.
- Zhu YI, Fitzpatrick JE, Kornfield BW. Lichen planus pemphigoides associated with Ramipril. Int J Dermatol. 2006;45:1453-1455.
- Sin B, Miller M, Chew E. Hydrochlorothiazide induced lichen planus in the emergency department. J Pharm Pract. 2017;30:266-269.
- Weston G, Payette M. Update on lichen planus and its clinical variants. Int J Women Dermatol. 2015;1:140-149.
- Contestable JJ, Edhegard KD, Meyerle JH. Bullous systemic lupus erythematosus: a review and update to diagnosis and treatment. Am J Clin Dermatol. 2014;15:517-524.
- Fivenson DP, Kimbrough TL. Lichen planus pemphigoides: combination therapy with tetracycline and nicotinamide. J Am Acad Dermatol. 1997;36:638-640.
The Diagnosis: Lichen Planus Pemphigoides
A skin biopsy from the right thigh demonstrated subepidermal blisters containing neutrophils (Figure 1). Direct immunofluorescence revealed linear basement membrane zone staining with C3 and trace staining with IgG (Figure 2), supporting a diagnosis of lichen planus pemphigoides (LPP). Oral prednisone—starting at 60 mg daily and tapered to 40 mg for a week, 20 mg for a week, then 10 mg for a month—along with triamcinolone ointment 0.1% to affected areas led to improvement. Hydrochlorothiazide and UV light therapy were discontinued. Doxycycline 100 mg twice daily and nicotinamide 500 mg twice daily prescribed as adjunctive therapy also led to improvement. The patient achieved remission with doxycycline and was doing well without prednisone; however, he experienced a flare of his disease about 6 months later and was started on mycophenolate mofetil 1 g twice daily after clearance from his gastroenterologist, given his history of hepatitis B. He has been doing well since starting mycophenolate mofetil.

Lichen planus pemphigoides is a rare autoimmune bullous dermatosis with features of both lichen planus and bullous pemphigoid.1 Violaceous papules and tense bullae may be superimposed or arise independently. The chest, abdomen, back, and upper and lower extremities typically are involved.2 Oral mucosal involvement with white reticular streaks or erosions and nail involvement have been reported.2 Histopathologic and immunologic findings establish the diagnosis. Lichen planus pemphigoides is associated with subepidermal bullae and linear deposits of IgG and C3 on the basement membrane zone.1 Autoantibodies to bullous pemphigoid (BP) antigens BP180 and BP230 are associated with LPP.3 The pathogenesis of LPP remains unclear, but there are associations with chronic diseases, medications, and certain therapies.1,4-6 Several case reports have linked LPP to chronic viral hepatitis infections, as well as malignant tumors of the skin, mucosa, and gastrointestinal tract.2 Lichen planus pemphigoides has been reported in a patient on entecavir for hepatitis B as well as in a patient treated for hepatitis C with interferon and ribavirin.1 Lichen planus pemphigoides has been described in patients treated with the angiotensin-converting enzyme inhibitors enalapril, captopril, and ramipril.4,5,7 UV phototherapy also has been associated with the development of LPP.6 Hydrochlorothiazide previously has been reported as a cause of drug-induced lichen planus.8 A PubMed search of articles indexed for MEDLINE using the terms lichen planus pemphigoides and hydrochlorothiazide revealed no reports of hydrochlorothiazide-induced LPP.

Lichen planus pemphigoides demonstrates overlap with other blistering dermatoses, such as BP, bullous lupus erythematosus, and bullous lichen planus. Although histologically and immunologically similar to BP, LPP can be differentiated clinically by the presence of violaceous papules or plaques typical of lichen planus.9 Bullous pemphigoid is more common in individuals older than 70 years, whereas LPP tends to occur in middle-aged adults.2 Bullous lupus erythematosus usually is associated with manifestations of systemic lupus erythematosus and autoantibodies to collagen type VII.10 Salt-split skin studies demonstrate immunofluorescence on the dermal side of the split. Individuals affected by bullous lupus erythematosus typically have a history of photosensitivity.10 Blisters in LPP may form de novo from unaffected skin, whereas the bullae in bullous lichen planus are limited to existing lichenoid papules.9 The autoantibodies typical of LPP are absent in bullous lichen planus. Lichen planus actinicus is a variant of lichen planus that presents with annular, dyschromic, or violaceous plaques in a photodistributed pattern without bullous lesions.9
Lichen planus pemphigoides most commonly is treated with systemic corticosteroids. Topical steroids, dapsone, erythromycin, tetracycline and nicotinamide, azathioprine, and mycophenolate mofetil have been reported as adjuncts to systemic steroid therapy.2,11 Most reports describe treatment success with resolution of blistering lesions.
The Diagnosis: Lichen Planus Pemphigoides
A skin biopsy from the right thigh demonstrated subepidermal blisters containing neutrophils (Figure 1). Direct immunofluorescence revealed linear basement membrane zone staining with C3 and trace staining with IgG (Figure 2), supporting a diagnosis of lichen planus pemphigoides (LPP). Oral prednisone—starting at 60 mg daily and tapered to 40 mg for a week, 20 mg for a week, then 10 mg for a month—along with triamcinolone ointment 0.1% to affected areas led to improvement. Hydrochlorothiazide and UV light therapy were discontinued. Doxycycline 100 mg twice daily and nicotinamide 500 mg twice daily prescribed as adjunctive therapy also led to improvement. The patient achieved remission with doxycycline and was doing well without prednisone; however, he experienced a flare of his disease about 6 months later and was started on mycophenolate mofetil 1 g twice daily after clearance from his gastroenterologist, given his history of hepatitis B. He has been doing well since starting mycophenolate mofetil.

Lichen planus pemphigoides is a rare autoimmune bullous dermatosis with features of both lichen planus and bullous pemphigoid.1 Violaceous papules and tense bullae may be superimposed or arise independently. The chest, abdomen, back, and upper and lower extremities typically are involved.2 Oral mucosal involvement with white reticular streaks or erosions and nail involvement have been reported.2 Histopathologic and immunologic findings establish the diagnosis. Lichen planus pemphigoides is associated with subepidermal bullae and linear deposits of IgG and C3 on the basement membrane zone.1 Autoantibodies to bullous pemphigoid (BP) antigens BP180 and BP230 are associated with LPP.3 The pathogenesis of LPP remains unclear, but there are associations with chronic diseases, medications, and certain therapies.1,4-6 Several case reports have linked LPP to chronic viral hepatitis infections, as well as malignant tumors of the skin, mucosa, and gastrointestinal tract.2 Lichen planus pemphigoides has been reported in a patient on entecavir for hepatitis B as well as in a patient treated for hepatitis C with interferon and ribavirin.1 Lichen planus pemphigoides has been described in patients treated with the angiotensin-converting enzyme inhibitors enalapril, captopril, and ramipril.4,5,7 UV phototherapy also has been associated with the development of LPP.6 Hydrochlorothiazide previously has been reported as a cause of drug-induced lichen planus.8 A PubMed search of articles indexed for MEDLINE using the terms lichen planus pemphigoides and hydrochlorothiazide revealed no reports of hydrochlorothiazide-induced LPP.

Lichen planus pemphigoides demonstrates overlap with other blistering dermatoses, such as BP, bullous lupus erythematosus, and bullous lichen planus. Although histologically and immunologically similar to BP, LPP can be differentiated clinically by the presence of violaceous papules or plaques typical of lichen planus.9 Bullous pemphigoid is more common in individuals older than 70 years, whereas LPP tends to occur in middle-aged adults.2 Bullous lupus erythematosus usually is associated with manifestations of systemic lupus erythematosus and autoantibodies to collagen type VII.10 Salt-split skin studies demonstrate immunofluorescence on the dermal side of the split. Individuals affected by bullous lupus erythematosus typically have a history of photosensitivity.10 Blisters in LPP may form de novo from unaffected skin, whereas the bullae in bullous lichen planus are limited to existing lichenoid papules.9 The autoantibodies typical of LPP are absent in bullous lichen planus. Lichen planus actinicus is a variant of lichen planus that presents with annular, dyschromic, or violaceous plaques in a photodistributed pattern without bullous lesions.9
Lichen planus pemphigoides most commonly is treated with systemic corticosteroids. Topical steroids, dapsone, erythromycin, tetracycline and nicotinamide, azathioprine, and mycophenolate mofetil have been reported as adjuncts to systemic steroid therapy.2,11 Most reports describe treatment success with resolution of blistering lesions.
- Jang SH, Yun SJ, Lee SC, et al. Lichen planus pemphigoides associated with chronic hepatitis B virus infection. Clin Exp Dermatol. 2015;40:868-871.
- Zaraa I, Mahfoudh A, Sellami MK, et al. Lichen planus pemphigoides: four new cases and a review of the literature. Int J Dermatol. 2013;52:406-412.
- Harting MS, Hsu S. Lichen planus pemphigoides: a case report and review of the literature. Dermatol Online J. 2006;12:10.
- Onprasert W, Chanprapaph K. Lichen planus pemphigoides induced by enalapril: a case report and a review of literature. Case Rep Dermatol. 2017;9:217-224.
- Ben Salem C, Chengeul L, Ghariani N, et al. Captopril-induced lichen planus pemphigoides. Pharmacoepidemiol Drug Saf. 2008;17:722-724.
- Kuramoto N, Kishimoto S, Shibagaki R, et al. PUVA-induced lichen planus pemphigoides. Br J Dermatol. 2000;142:509-512.
- Zhu YI, Fitzpatrick JE, Kornfield BW. Lichen planus pemphigoides associated with Ramipril. Int J Dermatol. 2006;45:1453-1455.
- Sin B, Miller M, Chew E. Hydrochlorothiazide induced lichen planus in the emergency department. J Pharm Pract. 2017;30:266-269.
- Weston G, Payette M. Update on lichen planus and its clinical variants. Int J Women Dermatol. 2015;1:140-149.
- Contestable JJ, Edhegard KD, Meyerle JH. Bullous systemic lupus erythematosus: a review and update to diagnosis and treatment. Am J Clin Dermatol. 2014;15:517-524.
- Fivenson DP, Kimbrough TL. Lichen planus pemphigoides: combination therapy with tetracycline and nicotinamide. J Am Acad Dermatol. 1997;36:638-640.
- Jang SH, Yun SJ, Lee SC, et al. Lichen planus pemphigoides associated with chronic hepatitis B virus infection. Clin Exp Dermatol. 2015;40:868-871.
- Zaraa I, Mahfoudh A, Sellami MK, et al. Lichen planus pemphigoides: four new cases and a review of the literature. Int J Dermatol. 2013;52:406-412.
- Harting MS, Hsu S. Lichen planus pemphigoides: a case report and review of the literature. Dermatol Online J. 2006;12:10.
- Onprasert W, Chanprapaph K. Lichen planus pemphigoides induced by enalapril: a case report and a review of literature. Case Rep Dermatol. 2017;9:217-224.
- Ben Salem C, Chengeul L, Ghariani N, et al. Captopril-induced lichen planus pemphigoides. Pharmacoepidemiol Drug Saf. 2008;17:722-724.
- Kuramoto N, Kishimoto S, Shibagaki R, et al. PUVA-induced lichen planus pemphigoides. Br J Dermatol. 2000;142:509-512.
- Zhu YI, Fitzpatrick JE, Kornfield BW. Lichen planus pemphigoides associated with Ramipril. Int J Dermatol. 2006;45:1453-1455.
- Sin B, Miller M, Chew E. Hydrochlorothiazide induced lichen planus in the emergency department. J Pharm Pract. 2017;30:266-269.
- Weston G, Payette M. Update on lichen planus and its clinical variants. Int J Women Dermatol. 2015;1:140-149.
- Contestable JJ, Edhegard KD, Meyerle JH. Bullous systemic lupus erythematosus: a review and update to diagnosis and treatment. Am J Clin Dermatol. 2014;15:517-524.
- Fivenson DP, Kimbrough TL. Lichen planus pemphigoides: combination therapy with tetracycline and nicotinamide. J Am Acad Dermatol. 1997;36:638-640.
A 50-year-old man presented with a pruritic bullous dermatosis on the lower legs, arms, and back of 1 month’s duration. He had an 8-year history of lichen planus, and the lesions recently had worsened despite the addition of UVB phototherapy. His medical history was remarkable for hepatitis B treated with entecavir and the addition of hydrochlorothiazide for essential hypertension 2 weeks prior to the dramatic worsening of the rash. Physical examination revealed multiple bullae on the lower legs associated with violaceous and hyperpigmented papules and patches. He also had violaceous papules on the lower back and eroded lesions on the oral mucosa. Shave biopsies were obtained from the right thigh and mid back, and histopathologic analysis was performed for both routine histology and direct immunofluorescence.
Hyperpigmentation of the Tongue
The Diagnosis: Addison Disease in the Context of Polyglandular Autoimmune Syndrome Type 2
The patient’s hormone levels as well as distinct clinical features led to a diagnosis of Addison disease in the context of polyglandular autoimmune syndrome type 2 (PAS-2). Approximately 50% of PAS-2 cases are familiar, and different modes of inheritance—autosomal recessive, autosomal dominant, and polygenic—have been reported. Women are affected up to 3 times more often than men.1,2 The age of onset ranges from infancy to late adulthood, with most cases occurring in early adulthood. Primary adrenal insufficiency (Addison disease) is the principal manifestation of PAS-2. It appears in approximately 50% of patients, occurring simultaneously with autoimmune thyroid disease or diabetes mellitus in 20% of patients and following them in 30% of patients.1,2 Autoimmune thyroid diseases such as chronic autoimmune thyroiditis and occasionally Graves disease as well as type 1 diabetes mellitus also are common. Polyglandular autoimmune syndrome type 2 with primary adrenal insufficiency and autoimmune thyroid disease was formerly referred to as Schmidt syndrome.3 It must be differentiated from polyglandular autoimmune syndrome type 1, a rare condition that also is referred to as autoimmune polyendocrinopathycandidiasis-ectodermal dystrophy syndrome.1,3 As with any other cause of adrenal insufficiency, the treatment involves hormone replacement therapy up to normal levels and then tapering according to stress levels (ie, surgery or infections that require a dose increase). Our patient was diagnosed according to hormone levels and clinical features and was started on 30 mg daily of hydrocortisone and 50 μg daily of levothyroxine. No improvement in her condition was noted after 6 months of treatment. The patient is still under yearly follow-up, and the mucous hyperpigmentation faded approximately 6 months after hormonal homeostasis was achieved.
Peutz-Jeghers syndrome is inherited in an autosomal-dominant fashion. It is characterized by multiple hamartomatous polyps in the gastrointestinal tract, mucocutaneous pigmentation, and an increased risk for gastrointestinal and nongastrointestinal cancer. Mucocutaneous pigmented macules most commonly occur on the lips and perioral region, buccal mucosa, and the palms and soles. However, mucocutaneous pigmentation usually occurs during the first 1 to 2 years of life, increases in size and number over the ensuing years, and usually fades after puberty.4
Laugier-Hunziker syndrome is an acquired benign disorder presenting in adults with lentigines on the lips and buccal mucosa. It frequently is accompaniedby longitudinal melanonychia, macular pigmentation of the genitals, and involvement of the palms and soles. The diagnosis of Laugier-Hunziker syndrome is one of exclusion and is made after ruling out other causes of oral and labial hyperpigmentation, including physiologic pigmentation seen in darker-skinned individuals as well as inherited diseases associated with lentiginosis, requiring complete physical examination, endoscopy, and colonscopy.5
A wide variety of drugs and chemicals can lead to diffuse cutaneous hyperpigmentation. Increased production of melanin and/or the deposition of drug complexes or metals in the dermis is responsible for the skin discoloration. Drugs that most often cause hyperpigmentation on mucosal surfaces are hydroxychloroquine, minocycline, nicotine, silver, and some chemotherapy agents. The hyperpigmentation usually resolves with discontinuation of the offending agent, but the course may be prolonged over months to years.6
Changes in the skin and subcutaneous tissue occur in patients with Cushing syndrome. Hyperpigmentation is induced by increased secretion of adrenocorticotropic hormone, not cortisol, and occurs most often in patients with the ectopic adrenocorticotropic hormone syndrome. Hyperpigmentation may be generalized but is more intense in areas exposed to light (eg, face, neck, dorsal aspects of the hands) or to chronic mild trauma, friction, or pressure (eg, elbows, knees, spine, knuckles). Patchy pigmentation may occur on the inner surface of the lips and the buccal mucosa along the line of dental occlusion. Acanthosis nigricans also can be present in the axillae and around the neck.7
- Ferre EM, Rose SR, Rosenzweig SD, et al. Redefined clinical features and diagnostic criteria in autoimmune polyendocrinopathycandidiasis-ectodermal dystrophy. JCI Insight. 2016;1:E88782.
- Orlova EM, Sozaeva LS, Kareva MA, et al. Expanding the phenotypic and genotypic landscape of autoimmune polyendocrine syndrome type 1. J Clin Endocrinol Metab. 2017;102:3546-3556.
- Ahonen P, Myllärniemi S, Sipilä I, et al. Clinical variation of autoimmune polyendocrinopathy-candidiasis-ectodermal dystrophy (APECED) in a series of 68 patients. N Engl J Med. 1990;322:1829-1836.
- Utsunomiya J, Gocho H, Miyanaga T, et al. Peutz-Jeghers syndrome: its natural course and management. Johns Hopkins Med J. 1975;136:71-82.
- Nayak RS, Kotrashetti VS, Hosmani JV. Laugier-Hunziker syndrome. J Oral Maxillofac Pathol. 2012;16:245-250.
- Krause W. Drug-induced hyperpigmentation: a systematic review. J Dtsch Dermatol Ges. 2013;11:644-651.
- Newell-Price J, Trainer P, Besser M, et al. The diagnosis and differential diagnosis of Cushing’s syndrome and pseudo-Cushing’s states. Endocr Rev. 1998;19:647-672.
The Diagnosis: Addison Disease in the Context of Polyglandular Autoimmune Syndrome Type 2
The patient’s hormone levels as well as distinct clinical features led to a diagnosis of Addison disease in the context of polyglandular autoimmune syndrome type 2 (PAS-2). Approximately 50% of PAS-2 cases are familiar, and different modes of inheritance—autosomal recessive, autosomal dominant, and polygenic—have been reported. Women are affected up to 3 times more often than men.1,2 The age of onset ranges from infancy to late adulthood, with most cases occurring in early adulthood. Primary adrenal insufficiency (Addison disease) is the principal manifestation of PAS-2. It appears in approximately 50% of patients, occurring simultaneously with autoimmune thyroid disease or diabetes mellitus in 20% of patients and following them in 30% of patients.1,2 Autoimmune thyroid diseases such as chronic autoimmune thyroiditis and occasionally Graves disease as well as type 1 diabetes mellitus also are common. Polyglandular autoimmune syndrome type 2 with primary adrenal insufficiency and autoimmune thyroid disease was formerly referred to as Schmidt syndrome.3 It must be differentiated from polyglandular autoimmune syndrome type 1, a rare condition that also is referred to as autoimmune polyendocrinopathycandidiasis-ectodermal dystrophy syndrome.1,3 As with any other cause of adrenal insufficiency, the treatment involves hormone replacement therapy up to normal levels and then tapering according to stress levels (ie, surgery or infections that require a dose increase). Our patient was diagnosed according to hormone levels and clinical features and was started on 30 mg daily of hydrocortisone and 50 μg daily of levothyroxine. No improvement in her condition was noted after 6 months of treatment. The patient is still under yearly follow-up, and the mucous hyperpigmentation faded approximately 6 months after hormonal homeostasis was achieved.
Peutz-Jeghers syndrome is inherited in an autosomal-dominant fashion. It is characterized by multiple hamartomatous polyps in the gastrointestinal tract, mucocutaneous pigmentation, and an increased risk for gastrointestinal and nongastrointestinal cancer. Mucocutaneous pigmented macules most commonly occur on the lips and perioral region, buccal mucosa, and the palms and soles. However, mucocutaneous pigmentation usually occurs during the first 1 to 2 years of life, increases in size and number over the ensuing years, and usually fades after puberty.4
Laugier-Hunziker syndrome is an acquired benign disorder presenting in adults with lentigines on the lips and buccal mucosa. It frequently is accompaniedby longitudinal melanonychia, macular pigmentation of the genitals, and involvement of the palms and soles. The diagnosis of Laugier-Hunziker syndrome is one of exclusion and is made after ruling out other causes of oral and labial hyperpigmentation, including physiologic pigmentation seen in darker-skinned individuals as well as inherited diseases associated with lentiginosis, requiring complete physical examination, endoscopy, and colonscopy.5
A wide variety of drugs and chemicals can lead to diffuse cutaneous hyperpigmentation. Increased production of melanin and/or the deposition of drug complexes or metals in the dermis is responsible for the skin discoloration. Drugs that most often cause hyperpigmentation on mucosal surfaces are hydroxychloroquine, minocycline, nicotine, silver, and some chemotherapy agents. The hyperpigmentation usually resolves with discontinuation of the offending agent, but the course may be prolonged over months to years.6
Changes in the skin and subcutaneous tissue occur in patients with Cushing syndrome. Hyperpigmentation is induced by increased secretion of adrenocorticotropic hormone, not cortisol, and occurs most often in patients with the ectopic adrenocorticotropic hormone syndrome. Hyperpigmentation may be generalized but is more intense in areas exposed to light (eg, face, neck, dorsal aspects of the hands) or to chronic mild trauma, friction, or pressure (eg, elbows, knees, spine, knuckles). Patchy pigmentation may occur on the inner surface of the lips and the buccal mucosa along the line of dental occlusion. Acanthosis nigricans also can be present in the axillae and around the neck.7
The Diagnosis: Addison Disease in the Context of Polyglandular Autoimmune Syndrome Type 2
The patient’s hormone levels as well as distinct clinical features led to a diagnosis of Addison disease in the context of polyglandular autoimmune syndrome type 2 (PAS-2). Approximately 50% of PAS-2 cases are familiar, and different modes of inheritance—autosomal recessive, autosomal dominant, and polygenic—have been reported. Women are affected up to 3 times more often than men.1,2 The age of onset ranges from infancy to late adulthood, with most cases occurring in early adulthood. Primary adrenal insufficiency (Addison disease) is the principal manifestation of PAS-2. It appears in approximately 50% of patients, occurring simultaneously with autoimmune thyroid disease or diabetes mellitus in 20% of patients and following them in 30% of patients.1,2 Autoimmune thyroid diseases such as chronic autoimmune thyroiditis and occasionally Graves disease as well as type 1 diabetes mellitus also are common. Polyglandular autoimmune syndrome type 2 with primary adrenal insufficiency and autoimmune thyroid disease was formerly referred to as Schmidt syndrome.3 It must be differentiated from polyglandular autoimmune syndrome type 1, a rare condition that also is referred to as autoimmune polyendocrinopathycandidiasis-ectodermal dystrophy syndrome.1,3 As with any other cause of adrenal insufficiency, the treatment involves hormone replacement therapy up to normal levels and then tapering according to stress levels (ie, surgery or infections that require a dose increase). Our patient was diagnosed according to hormone levels and clinical features and was started on 30 mg daily of hydrocortisone and 50 μg daily of levothyroxine. No improvement in her condition was noted after 6 months of treatment. The patient is still under yearly follow-up, and the mucous hyperpigmentation faded approximately 6 months after hormonal homeostasis was achieved.
Peutz-Jeghers syndrome is inherited in an autosomal-dominant fashion. It is characterized by multiple hamartomatous polyps in the gastrointestinal tract, mucocutaneous pigmentation, and an increased risk for gastrointestinal and nongastrointestinal cancer. Mucocutaneous pigmented macules most commonly occur on the lips and perioral region, buccal mucosa, and the palms and soles. However, mucocutaneous pigmentation usually occurs during the first 1 to 2 years of life, increases in size and number over the ensuing years, and usually fades after puberty.4
Laugier-Hunziker syndrome is an acquired benign disorder presenting in adults with lentigines on the lips and buccal mucosa. It frequently is accompaniedby longitudinal melanonychia, macular pigmentation of the genitals, and involvement of the palms and soles. The diagnosis of Laugier-Hunziker syndrome is one of exclusion and is made after ruling out other causes of oral and labial hyperpigmentation, including physiologic pigmentation seen in darker-skinned individuals as well as inherited diseases associated with lentiginosis, requiring complete physical examination, endoscopy, and colonscopy.5
A wide variety of drugs and chemicals can lead to diffuse cutaneous hyperpigmentation. Increased production of melanin and/or the deposition of drug complexes or metals in the dermis is responsible for the skin discoloration. Drugs that most often cause hyperpigmentation on mucosal surfaces are hydroxychloroquine, minocycline, nicotine, silver, and some chemotherapy agents. The hyperpigmentation usually resolves with discontinuation of the offending agent, but the course may be prolonged over months to years.6
Changes in the skin and subcutaneous tissue occur in patients with Cushing syndrome. Hyperpigmentation is induced by increased secretion of adrenocorticotropic hormone, not cortisol, and occurs most often in patients with the ectopic adrenocorticotropic hormone syndrome. Hyperpigmentation may be generalized but is more intense in areas exposed to light (eg, face, neck, dorsal aspects of the hands) or to chronic mild trauma, friction, or pressure (eg, elbows, knees, spine, knuckles). Patchy pigmentation may occur on the inner surface of the lips and the buccal mucosa along the line of dental occlusion. Acanthosis nigricans also can be present in the axillae and around the neck.7
- Ferre EM, Rose SR, Rosenzweig SD, et al. Redefined clinical features and diagnostic criteria in autoimmune polyendocrinopathycandidiasis-ectodermal dystrophy. JCI Insight. 2016;1:E88782.
- Orlova EM, Sozaeva LS, Kareva MA, et al. Expanding the phenotypic and genotypic landscape of autoimmune polyendocrine syndrome type 1. J Clin Endocrinol Metab. 2017;102:3546-3556.
- Ahonen P, Myllärniemi S, Sipilä I, et al. Clinical variation of autoimmune polyendocrinopathy-candidiasis-ectodermal dystrophy (APECED) in a series of 68 patients. N Engl J Med. 1990;322:1829-1836.
- Utsunomiya J, Gocho H, Miyanaga T, et al. Peutz-Jeghers syndrome: its natural course and management. Johns Hopkins Med J. 1975;136:71-82.
- Nayak RS, Kotrashetti VS, Hosmani JV. Laugier-Hunziker syndrome. J Oral Maxillofac Pathol. 2012;16:245-250.
- Krause W. Drug-induced hyperpigmentation: a systematic review. J Dtsch Dermatol Ges. 2013;11:644-651.
- Newell-Price J, Trainer P, Besser M, et al. The diagnosis and differential diagnosis of Cushing’s syndrome and pseudo-Cushing’s states. Endocr Rev. 1998;19:647-672.
- Ferre EM, Rose SR, Rosenzweig SD, et al. Redefined clinical features and diagnostic criteria in autoimmune polyendocrinopathycandidiasis-ectodermal dystrophy. JCI Insight. 2016;1:E88782.
- Orlova EM, Sozaeva LS, Kareva MA, et al. Expanding the phenotypic and genotypic landscape of autoimmune polyendocrine syndrome type 1. J Clin Endocrinol Metab. 2017;102:3546-3556.
- Ahonen P, Myllärniemi S, Sipilä I, et al. Clinical variation of autoimmune polyendocrinopathy-candidiasis-ectodermal dystrophy (APECED) in a series of 68 patients. N Engl J Med. 1990;322:1829-1836.
- Utsunomiya J, Gocho H, Miyanaga T, et al. Peutz-Jeghers syndrome: its natural course and management. Johns Hopkins Med J. 1975;136:71-82.
- Nayak RS, Kotrashetti VS, Hosmani JV. Laugier-Hunziker syndrome. J Oral Maxillofac Pathol. 2012;16:245-250.
- Krause W. Drug-induced hyperpigmentation: a systematic review. J Dtsch Dermatol Ges. 2013;11:644-651.
- Newell-Price J, Trainer P, Besser M, et al. The diagnosis and differential diagnosis of Cushing’s syndrome and pseudo-Cushing’s states. Endocr Rev. 1998;19:647-672.
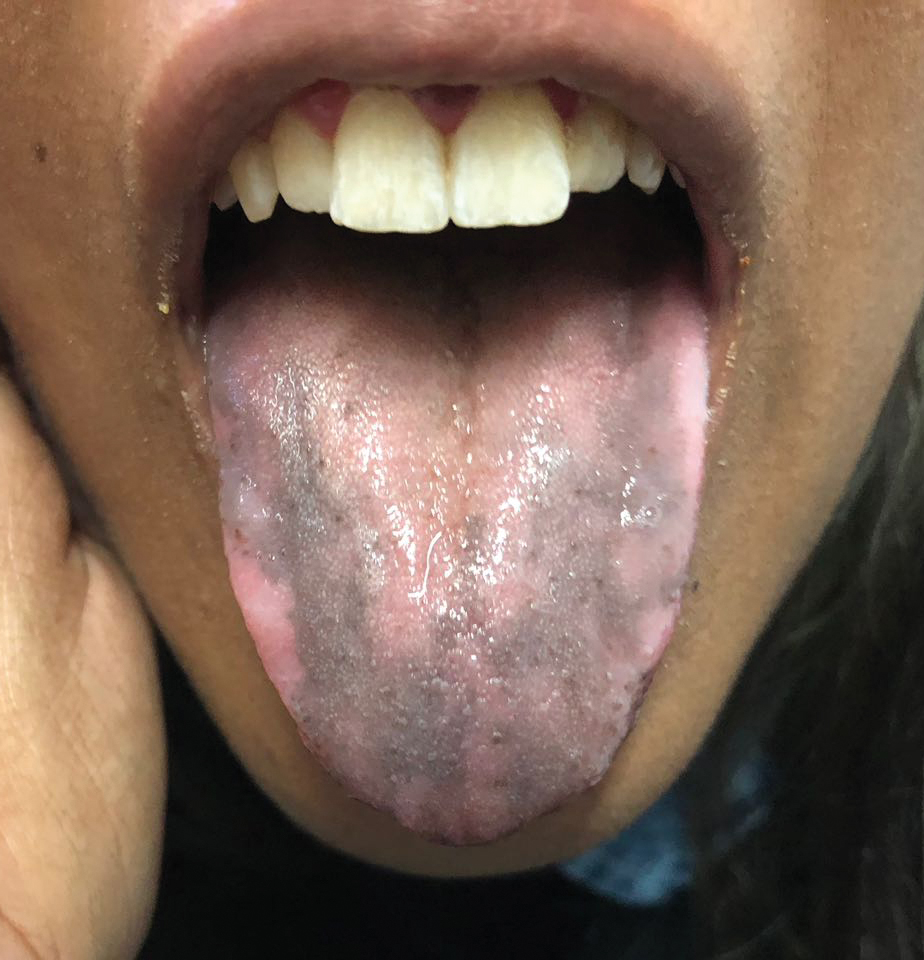
An otherwise healthy 17-year-old adolescent girl from Spain presented with hyperpigmentation on the tongue of several weeks’ duration. She denied licking graphite pencils or pens. Physical examination revealed pigmentation in the palmar creases and a slight generalized tan. The patient denied sun exposure. Neither melanonychia nor genital hyperpigmented lesions were noted. Blood tests showed overt hypothyroidism.
Granular Parakeratosis
To the Editor:
A 46-year-old overweight woman presented with a rash in the axillae of 2 months’ duration. She did not report any additional symptoms such as pruritus or pain. She reported changing her deodorant recently from Secret Original to Secret Clinical Strength (both Procter & Gamble). Her medical history was remarkable for asthma and gastroesophageal reflux disease. Clinical examination revealed erythematous-brown, stuccolike, hyperkeratotic papules coalescing into plaques in recently shaved axillae, affecting the left axilla more than the right axilla (Figure 1). The clinical differential diagnosis included granular parakeratosis, intertrigo, Hailey-Hailey disease, Darier disease, pemphigus vegetans, confluent and reticulated papillomatosis, acanthosis nigricans, seborrheic keratoses, and irritant or allergic contact dermatitis. A punch biopsy revealed a marked compact parakeratotic horn with retention of keratohyalin granules (Figure 2). The subjacent epidermis showed some acanthosis and spongiosis with mild chronic inflammation of the dermal rim. Based on histopathology, granular parakeratosis was diagnosed.
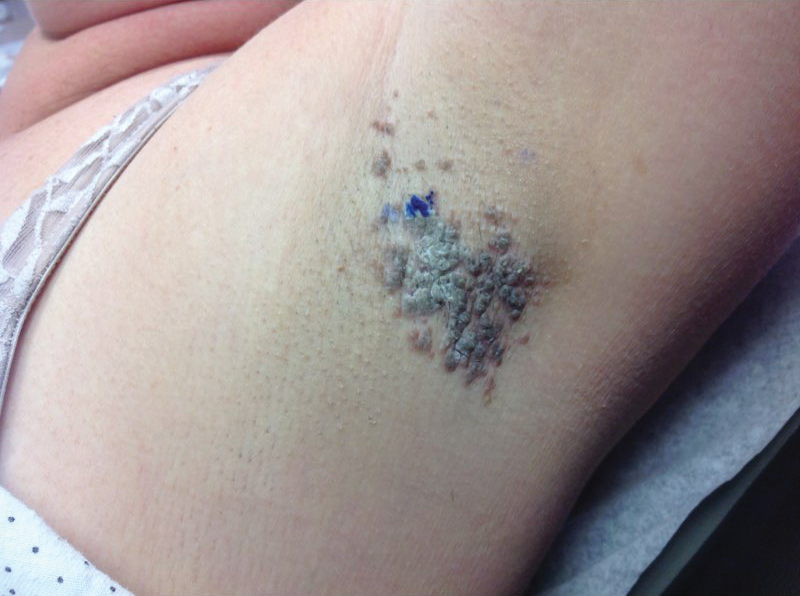
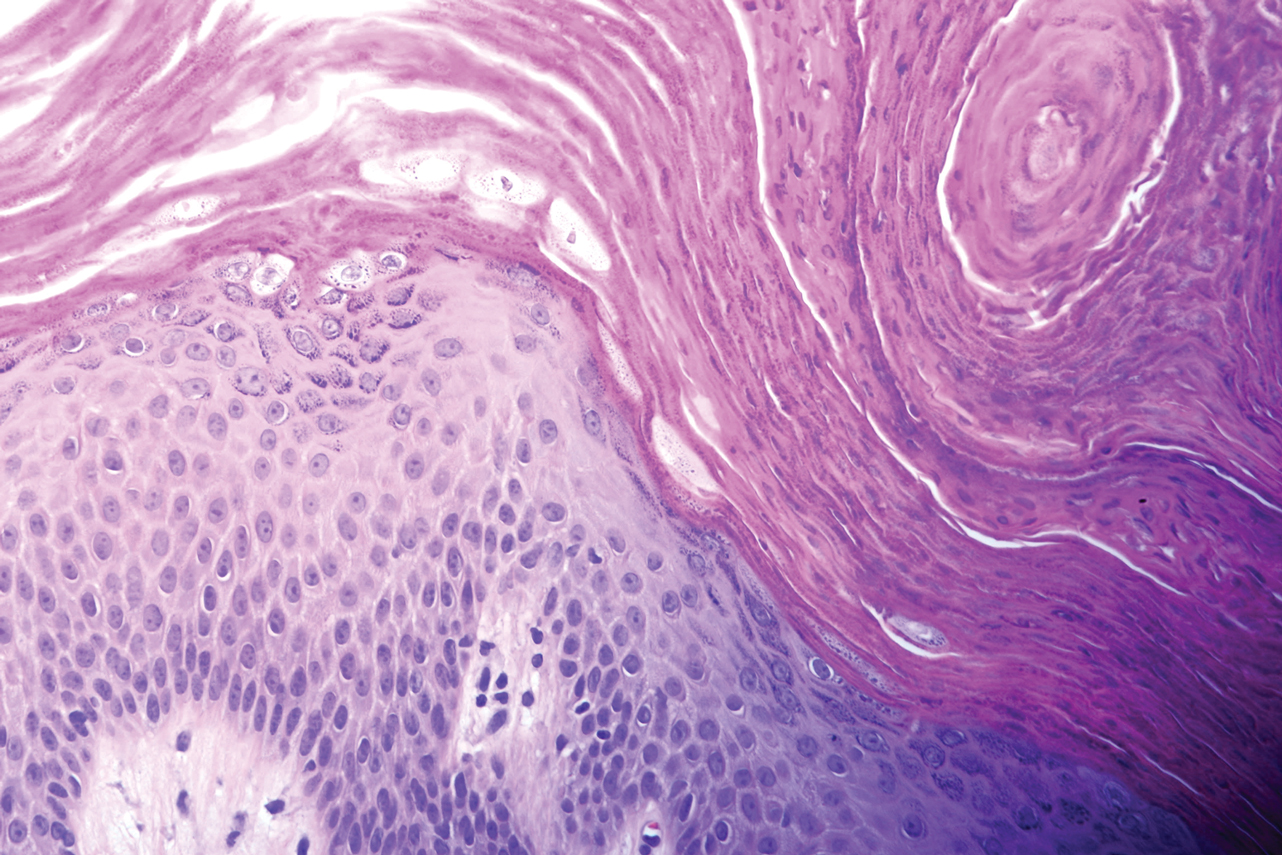
At a subsequent visit 2 weeks later, we prescribed glycolic acid lotion 10% applied to the axillae twice daily, plus tretinoin gel 0.05% applied to the axillae each evening. She reported clearing after 1 week of therapy. She also had changed her deodorant from Secret Clinical Strength back to the usual Secret Original. The patient discontinued topical treatment after clearing of the lesions. Three weeks later, clinical examination revealed postinflammatory hyperpigmentation in the axillae, and the prior lesions had resolved (Figure 3).
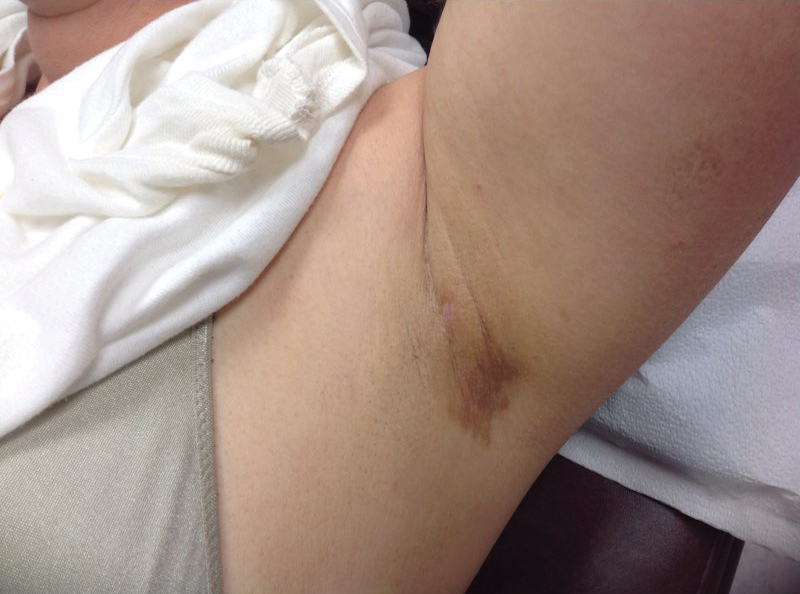
Granular parakeratosis is an unusual condition most commonly presenting in middle-aged women in the axillae, with a clinical presentation of erythematous to brownish hyperkeratotic papules coalescing into plaques. Although few cases have been reported, granular parakeratosis likely is more common than has been reported. There have been reports involving the scalp, cheeks, abdomen, thighs, and other intertriginous areas including inguinal folds and the submammary region.1-4 There also is an infantile form related to diapers and zinc oxide paste.5 Although uncommon, granular parakeratosis can occur as a single papule or plaque and is termed granular parakeratotic acanthoma.6 Lesions may persist for months, spontaneously resolve and recur, and occasionally evolve into fissures and erosions due to irritation. Pruritus is a common concern. Histology of granular parakeratosis reveals hyperkeratosis with eosinophilic staining, compact parakeratosis with retention of basophilic keratohyalin granules, and vascular proliferation and ectasia.5
The cause is unknown but possibly related to irritation from rubbing, occlusion, sweating, or deodorants.5,7 Cases indicate a link to obesity. Hypotheses as to the etiology include the disruption of cornification. Normally, filaggrin maintains the keratohyaline granules in the stratum corneum during cornification. Therefore, the retention of keratohyaline granules in granular parakeratosis may be due to a defect in processing profilaggrin to filaggrin, which has been proposed based on ultrastructural and immunohistochemical studies.8
The differential diagnosis includes granular parakeratosis, intertrigo (caused by seborrheic dermatitis, candidiasis, inverse psoriasis, or erythrasma), Hailey-Hailey disease, Darier disease, pemphigus vegetans, confluent and reticulated papillomatosis, and irritant or allergic contact dermatitis. The papules may resemble seborrheic keratoses, while the plaques can be mistaken for acanthosis nigricans.
Therapeutic success has been reported with topical corticosteroids, vitamin D analogues, topical or oral retinoids, ammonium lactate, calcineurin inhibitors, topical or oral antifungals, cryotherapy, and botulinum toxin injections.3,9-11 In addition, parakeratosis has decreased in biopsies from psoriatic patients after acitretin, methotrexate, and phototherapy, which may be alternative treatments for unusually difficult or recalcitrant cases of granular parakeratosis. To minimize side effects and resolve the papules quickly, we combined 2 synergistic agents—glycolic acid and tretinoin—each with different mechanisms of action, and we observed excellent clinical response.
Granular parakeratosis is possibly related to a combination of topical products that potentiate irritation, rubbing, and occlusion of sweat. Multiple treatment modalities likely contribute to clearing, the most important being removal of any triggering topical products. Our patient’s change in deodorant may have been the inciting factor for the disease. Withdrawal of the Secret Clinical Strength deodorant prompted clearing, though topical retinoid and glycolic acid acted as facilitating therapies for timely results. A thorough history, as highlighted by this case, may help pinpoint etiologic factors. By identifying a seemingly innocuous change in hygienic routine, we were able to minimize the need for ongoing therapy.
- Graham R. Intertriginous granular parakeratosis: a case report and review of the literature. J Am Acad Dermatol. 2011;64:AB45-AB45.
- Compton AK, Jackson JM. Isotretinoin as a treatment for axillary granular parakeratosis. Cutis. 2007;80:55-56.
- Channual J, Fife DJ, Wu JJ. Axillary granular parakeratosis. Cutis. 2013;92;61, 65-66.
- Streams S, Gottwald L, Zaher A, et al. Granular parakeratosis of the scalp: a case report. J Am Acad Dermatol. 2007;56:AB81-AB81.
- James WD, Berger T, Elston D. Andrews’ Diseases of the Skin. 12th ed. Philadelphia, PA: Elsevier, Inc; 2015.
- Resnik KS, Kantor GR, DiLeonardo M. Granular parakeratotic acanthoma. Am J Dermatopathol. 2005;27:393-396.
- Naylor E, Wartman D, Telang G, et al. Granular parakeratosis secondary to postsurgical occlusion. J Am Acad Dermatol. 2008;58:AB126.
- Bolognia JL, Jorizzo JL, Schaffer JV. Dermatology. 3rd ed. Philadelphia, PA: Elsevier, Inc; 2012.
- Baum B, Skopit S. Granular parakeratosis treatment with tacrolimus 0.1% ointment: a case presentation and discussion. J Am Osteo Coll Dermatol. 2013;26:40-41.
- Brown SK, Heilman ER. Granular parakeratosis: resolution with topical tretinoin. J Am Acad Dermatol. 2002;47:S279-S280.
- Webster CG, Resnik KS, Webster GF. Axillary granular parakeratosis: response to isotretinoin. J Am Acad Dermatol. 1997;37:789790.
To the Editor:
A 46-year-old overweight woman presented with a rash in the axillae of 2 months’ duration. She did not report any additional symptoms such as pruritus or pain. She reported changing her deodorant recently from Secret Original to Secret Clinical Strength (both Procter & Gamble). Her medical history was remarkable for asthma and gastroesophageal reflux disease. Clinical examination revealed erythematous-brown, stuccolike, hyperkeratotic papules coalescing into plaques in recently shaved axillae, affecting the left axilla more than the right axilla (Figure 1). The clinical differential diagnosis included granular parakeratosis, intertrigo, Hailey-Hailey disease, Darier disease, pemphigus vegetans, confluent and reticulated papillomatosis, acanthosis nigricans, seborrheic keratoses, and irritant or allergic contact dermatitis. A punch biopsy revealed a marked compact parakeratotic horn with retention of keratohyalin granules (Figure 2). The subjacent epidermis showed some acanthosis and spongiosis with mild chronic inflammation of the dermal rim. Based on histopathology, granular parakeratosis was diagnosed.


At a subsequent visit 2 weeks later, we prescribed glycolic acid lotion 10% applied to the axillae twice daily, plus tretinoin gel 0.05% applied to the axillae each evening. She reported clearing after 1 week of therapy. She also had changed her deodorant from Secret Clinical Strength back to the usual Secret Original. The patient discontinued topical treatment after clearing of the lesions. Three weeks later, clinical examination revealed postinflammatory hyperpigmentation in the axillae, and the prior lesions had resolved (Figure 3).

Granular parakeratosis is an unusual condition most commonly presenting in middle-aged women in the axillae, with a clinical presentation of erythematous to brownish hyperkeratotic papules coalescing into plaques. Although few cases have been reported, granular parakeratosis likely is more common than has been reported. There have been reports involving the scalp, cheeks, abdomen, thighs, and other intertriginous areas including inguinal folds and the submammary region.1-4 There also is an infantile form related to diapers and zinc oxide paste.5 Although uncommon, granular parakeratosis can occur as a single papule or plaque and is termed granular parakeratotic acanthoma.6 Lesions may persist for months, spontaneously resolve and recur, and occasionally evolve into fissures and erosions due to irritation. Pruritus is a common concern. Histology of granular parakeratosis reveals hyperkeratosis with eosinophilic staining, compact parakeratosis with retention of basophilic keratohyalin granules, and vascular proliferation and ectasia.5
The cause is unknown but possibly related to irritation from rubbing, occlusion, sweating, or deodorants.5,7 Cases indicate a link to obesity. Hypotheses as to the etiology include the disruption of cornification. Normally, filaggrin maintains the keratohyaline granules in the stratum corneum during cornification. Therefore, the retention of keratohyaline granules in granular parakeratosis may be due to a defect in processing profilaggrin to filaggrin, which has been proposed based on ultrastructural and immunohistochemical studies.8
The differential diagnosis includes granular parakeratosis, intertrigo (caused by seborrheic dermatitis, candidiasis, inverse psoriasis, or erythrasma), Hailey-Hailey disease, Darier disease, pemphigus vegetans, confluent and reticulated papillomatosis, and irritant or allergic contact dermatitis. The papules may resemble seborrheic keratoses, while the plaques can be mistaken for acanthosis nigricans.
Therapeutic success has been reported with topical corticosteroids, vitamin D analogues, topical or oral retinoids, ammonium lactate, calcineurin inhibitors, topical or oral antifungals, cryotherapy, and botulinum toxin injections.3,9-11 In addition, parakeratosis has decreased in biopsies from psoriatic patients after acitretin, methotrexate, and phototherapy, which may be alternative treatments for unusually difficult or recalcitrant cases of granular parakeratosis. To minimize side effects and resolve the papules quickly, we combined 2 synergistic agents—glycolic acid and tretinoin—each with different mechanisms of action, and we observed excellent clinical response.
Granular parakeratosis is possibly related to a combination of topical products that potentiate irritation, rubbing, and occlusion of sweat. Multiple treatment modalities likely contribute to clearing, the most important being removal of any triggering topical products. Our patient’s change in deodorant may have been the inciting factor for the disease. Withdrawal of the Secret Clinical Strength deodorant prompted clearing, though topical retinoid and glycolic acid acted as facilitating therapies for timely results. A thorough history, as highlighted by this case, may help pinpoint etiologic factors. By identifying a seemingly innocuous change in hygienic routine, we were able to minimize the need for ongoing therapy.
To the Editor:
A 46-year-old overweight woman presented with a rash in the axillae of 2 months’ duration. She did not report any additional symptoms such as pruritus or pain. She reported changing her deodorant recently from Secret Original to Secret Clinical Strength (both Procter & Gamble). Her medical history was remarkable for asthma and gastroesophageal reflux disease. Clinical examination revealed erythematous-brown, stuccolike, hyperkeratotic papules coalescing into plaques in recently shaved axillae, affecting the left axilla more than the right axilla (Figure 1). The clinical differential diagnosis included granular parakeratosis, intertrigo, Hailey-Hailey disease, Darier disease, pemphigus vegetans, confluent and reticulated papillomatosis, acanthosis nigricans, seborrheic keratoses, and irritant or allergic contact dermatitis. A punch biopsy revealed a marked compact parakeratotic horn with retention of keratohyalin granules (Figure 2). The subjacent epidermis showed some acanthosis and spongiosis with mild chronic inflammation of the dermal rim. Based on histopathology, granular parakeratosis was diagnosed.


At a subsequent visit 2 weeks later, we prescribed glycolic acid lotion 10% applied to the axillae twice daily, plus tretinoin gel 0.05% applied to the axillae each evening. She reported clearing after 1 week of therapy. She also had changed her deodorant from Secret Clinical Strength back to the usual Secret Original. The patient discontinued topical treatment after clearing of the lesions. Three weeks later, clinical examination revealed postinflammatory hyperpigmentation in the axillae, and the prior lesions had resolved (Figure 3).

Granular parakeratosis is an unusual condition most commonly presenting in middle-aged women in the axillae, with a clinical presentation of erythematous to brownish hyperkeratotic papules coalescing into plaques. Although few cases have been reported, granular parakeratosis likely is more common than has been reported. There have been reports involving the scalp, cheeks, abdomen, thighs, and other intertriginous areas including inguinal folds and the submammary region.1-4 There also is an infantile form related to diapers and zinc oxide paste.5 Although uncommon, granular parakeratosis can occur as a single papule or plaque and is termed granular parakeratotic acanthoma.6 Lesions may persist for months, spontaneously resolve and recur, and occasionally evolve into fissures and erosions due to irritation. Pruritus is a common concern. Histology of granular parakeratosis reveals hyperkeratosis with eosinophilic staining, compact parakeratosis with retention of basophilic keratohyalin granules, and vascular proliferation and ectasia.5
The cause is unknown but possibly related to irritation from rubbing, occlusion, sweating, or deodorants.5,7 Cases indicate a link to obesity. Hypotheses as to the etiology include the disruption of cornification. Normally, filaggrin maintains the keratohyaline granules in the stratum corneum during cornification. Therefore, the retention of keratohyaline granules in granular parakeratosis may be due to a defect in processing profilaggrin to filaggrin, which has been proposed based on ultrastructural and immunohistochemical studies.8
The differential diagnosis includes granular parakeratosis, intertrigo (caused by seborrheic dermatitis, candidiasis, inverse psoriasis, or erythrasma), Hailey-Hailey disease, Darier disease, pemphigus vegetans, confluent and reticulated papillomatosis, and irritant or allergic contact dermatitis. The papules may resemble seborrheic keratoses, while the plaques can be mistaken for acanthosis nigricans.
Therapeutic success has been reported with topical corticosteroids, vitamin D analogues, topical or oral retinoids, ammonium lactate, calcineurin inhibitors, topical or oral antifungals, cryotherapy, and botulinum toxin injections.3,9-11 In addition, parakeratosis has decreased in biopsies from psoriatic patients after acitretin, methotrexate, and phototherapy, which may be alternative treatments for unusually difficult or recalcitrant cases of granular parakeratosis. To minimize side effects and resolve the papules quickly, we combined 2 synergistic agents—glycolic acid and tretinoin—each with different mechanisms of action, and we observed excellent clinical response.
Granular parakeratosis is possibly related to a combination of topical products that potentiate irritation, rubbing, and occlusion of sweat. Multiple treatment modalities likely contribute to clearing, the most important being removal of any triggering topical products. Our patient’s change in deodorant may have been the inciting factor for the disease. Withdrawal of the Secret Clinical Strength deodorant prompted clearing, though topical retinoid and glycolic acid acted as facilitating therapies for timely results. A thorough history, as highlighted by this case, may help pinpoint etiologic factors. By identifying a seemingly innocuous change in hygienic routine, we were able to minimize the need for ongoing therapy.
- Graham R. Intertriginous granular parakeratosis: a case report and review of the literature. J Am Acad Dermatol. 2011;64:AB45-AB45.
- Compton AK, Jackson JM. Isotretinoin as a treatment for axillary granular parakeratosis. Cutis. 2007;80:55-56.
- Channual J, Fife DJ, Wu JJ. Axillary granular parakeratosis. Cutis. 2013;92;61, 65-66.
- Streams S, Gottwald L, Zaher A, et al. Granular parakeratosis of the scalp: a case report. J Am Acad Dermatol. 2007;56:AB81-AB81.
- James WD, Berger T, Elston D. Andrews’ Diseases of the Skin. 12th ed. Philadelphia, PA: Elsevier, Inc; 2015.
- Resnik KS, Kantor GR, DiLeonardo M. Granular parakeratotic acanthoma. Am J Dermatopathol. 2005;27:393-396.
- Naylor E, Wartman D, Telang G, et al. Granular parakeratosis secondary to postsurgical occlusion. J Am Acad Dermatol. 2008;58:AB126.
- Bolognia JL, Jorizzo JL, Schaffer JV. Dermatology. 3rd ed. Philadelphia, PA: Elsevier, Inc; 2012.
- Baum B, Skopit S. Granular parakeratosis treatment with tacrolimus 0.1% ointment: a case presentation and discussion. J Am Osteo Coll Dermatol. 2013;26:40-41.
- Brown SK, Heilman ER. Granular parakeratosis: resolution with topical tretinoin. J Am Acad Dermatol. 2002;47:S279-S280.
- Webster CG, Resnik KS, Webster GF. Axillary granular parakeratosis: response to isotretinoin. J Am Acad Dermatol. 1997;37:789790.
- Graham R. Intertriginous granular parakeratosis: a case report and review of the literature. J Am Acad Dermatol. 2011;64:AB45-AB45.
- Compton AK, Jackson JM. Isotretinoin as a treatment for axillary granular parakeratosis. Cutis. 2007;80:55-56.
- Channual J, Fife DJ, Wu JJ. Axillary granular parakeratosis. Cutis. 2013;92;61, 65-66.
- Streams S, Gottwald L, Zaher A, et al. Granular parakeratosis of the scalp: a case report. J Am Acad Dermatol. 2007;56:AB81-AB81.
- James WD, Berger T, Elston D. Andrews’ Diseases of the Skin. 12th ed. Philadelphia, PA: Elsevier, Inc; 2015.
- Resnik KS, Kantor GR, DiLeonardo M. Granular parakeratotic acanthoma. Am J Dermatopathol. 2005;27:393-396.
- Naylor E, Wartman D, Telang G, et al. Granular parakeratosis secondary to postsurgical occlusion. J Am Acad Dermatol. 2008;58:AB126.
- Bolognia JL, Jorizzo JL, Schaffer JV. Dermatology. 3rd ed. Philadelphia, PA: Elsevier, Inc; 2012.
- Baum B, Skopit S. Granular parakeratosis treatment with tacrolimus 0.1% ointment: a case presentation and discussion. J Am Osteo Coll Dermatol. 2013;26:40-41.
- Brown SK, Heilman ER. Granular parakeratosis: resolution with topical tretinoin. J Am Acad Dermatol. 2002;47:S279-S280.
- Webster CG, Resnik KS, Webster GF. Axillary granular parakeratosis: response to isotretinoin. J Am Acad Dermatol. 1997;37:789790.
Practice Points
- Granular parakeratosis most commonly presents in middle-aged women in the axillae.
- The cause is unknown but possibly related to irritation from rubbing, occlusion, sweating, or deodorants.
- Multiple treatment modalities likely contribute to clearing, the most important being removal of any triggering topical products.
Microneedling plus 10% TCA peels bests CO2 laser alone for infraorbital dark circles
In a study of patients with mild to moderate infraorbital dark circles, treatment with carbon dioxide laser resurfacing did not produce a significant improvement in infraorbital hyperpigmentation. However, the combination of microneedling and 10% trichloroacetic acid peels did.
The finding comes from what is believed to be the first head-to-head comparison of the two procedures for infraorbital dark circles, which are a common cosmetic concern with increased age.
During a late-breaking abstract session at the virtual annual meeting of the American Academy of Dermatology, lead study author Banu Farabi, MD, said that dark circles seen in the periorbital area are defined as bilateral, round homogeneous pigmented macules whose etiology is thought to be multifactorial. Available treatments include bleaching creams, topical retinoids, chemical peels, lasers, autologous fat transplantation, injectable fillers, and blepharoplasty.
“Microneedling has been recently suggested as an effective and efficient method for reducing infraorbital dark circles,” Dr. Farabi said. “This technique is based on creating microchannels that can stimulate the production of subcutaneous collagen and elastin. It also enhances the revascularization and fibroblast activity, which increases the skin thickness and gives a shiny appearance to the skin. The fractional CO2 has also been introduced as an effective procedure to remove infraorbital dark circles. However, there are some potential complications with that therapy.”
For the current study, Dr. Farabi, of the department of dermatology at Ankara (Turkey) University, and Mohamad Goldust, MD, of University Hospital Basel (Switzerland), They used the handheld Automatic Microneedle Therapy System-Handhold from MCure. After creating microchannels, the investigators topically applied 10% trichloroacetic acid peels to each infraorbital area and waited for 5 minutes.
In the carbon dioxide laser group, a Lutronic CO2 laser was used with a pulse energy of 10 J/cm2, a 100-microsecond pulse rate, 30 W of power, and a pulse width of 4 mm. The treatment outcome was assessed with the patient’s satisfaction and the physician’s judgment, which were no response, partial response, and complete response. Patients in both study groups were followed up for blinded-investigator assessment of infraorbital hyperpigmentation, adverse events, and improvement, compared with baseline.
The mean age of patients was 40 years, with a range between 27 and 58 years. About one-third of patients in each group had Fitzpatrick skin types II, III, and IV, respectively. In the blinded investigator assessment, the laser-resurfacing procedure did not demonstrate a significant improvement in infraorbital hyperpigmentation at day 90 (P = .24). However, the combination of microneedling and 10% trichloroacetic acid peels significantly improved infraorbital hyperpigmentation by day 90, with improvement maintained through day 180 (P = .012 and .002, respectively).
Adverse events were mild and temporary in both groups. In the laser-resurfacing group, 7 of the patients (22.5%) developed transient infraorbital hyperpigmentation postoperatively that lasted 4 weeks. In the combination treatment group, 18 patients (58%) developed transient erythema that lasted for up to 1 week.
“We suggest using microneedling plus 10% [trichloroacetic acid] as a cost-effective and efficient method for reducing infraorbital dark circles,” Dr. Farabi concluded.
The researchers reported having no financial disclosures.
In a study of patients with mild to moderate infraorbital dark circles, treatment with carbon dioxide laser resurfacing did not produce a significant improvement in infraorbital hyperpigmentation. However, the combination of microneedling and 10% trichloroacetic acid peels did.
The finding comes from what is believed to be the first head-to-head comparison of the two procedures for infraorbital dark circles, which are a common cosmetic concern with increased age.
During a late-breaking abstract session at the virtual annual meeting of the American Academy of Dermatology, lead study author Banu Farabi, MD, said that dark circles seen in the periorbital area are defined as bilateral, round homogeneous pigmented macules whose etiology is thought to be multifactorial. Available treatments include bleaching creams, topical retinoids, chemical peels, lasers, autologous fat transplantation, injectable fillers, and blepharoplasty.
“Microneedling has been recently suggested as an effective and efficient method for reducing infraorbital dark circles,” Dr. Farabi said. “This technique is based on creating microchannels that can stimulate the production of subcutaneous collagen and elastin. It also enhances the revascularization and fibroblast activity, which increases the skin thickness and gives a shiny appearance to the skin. The fractional CO2 has also been introduced as an effective procedure to remove infraorbital dark circles. However, there are some potential complications with that therapy.”
For the current study, Dr. Farabi, of the department of dermatology at Ankara (Turkey) University, and Mohamad Goldust, MD, of University Hospital Basel (Switzerland), They used the handheld Automatic Microneedle Therapy System-Handhold from MCure. After creating microchannels, the investigators topically applied 10% trichloroacetic acid peels to each infraorbital area and waited for 5 minutes.
In the carbon dioxide laser group, a Lutronic CO2 laser was used with a pulse energy of 10 J/cm2, a 100-microsecond pulse rate, 30 W of power, and a pulse width of 4 mm. The treatment outcome was assessed with the patient’s satisfaction and the physician’s judgment, which were no response, partial response, and complete response. Patients in both study groups were followed up for blinded-investigator assessment of infraorbital hyperpigmentation, adverse events, and improvement, compared with baseline.
The mean age of patients was 40 years, with a range between 27 and 58 years. About one-third of patients in each group had Fitzpatrick skin types II, III, and IV, respectively. In the blinded investigator assessment, the laser-resurfacing procedure did not demonstrate a significant improvement in infraorbital hyperpigmentation at day 90 (P = .24). However, the combination of microneedling and 10% trichloroacetic acid peels significantly improved infraorbital hyperpigmentation by day 90, with improvement maintained through day 180 (P = .012 and .002, respectively).
Adverse events were mild and temporary in both groups. In the laser-resurfacing group, 7 of the patients (22.5%) developed transient infraorbital hyperpigmentation postoperatively that lasted 4 weeks. In the combination treatment group, 18 patients (58%) developed transient erythema that lasted for up to 1 week.
“We suggest using microneedling plus 10% [trichloroacetic acid] as a cost-effective and efficient method for reducing infraorbital dark circles,” Dr. Farabi concluded.
The researchers reported having no financial disclosures.
In a study of patients with mild to moderate infraorbital dark circles, treatment with carbon dioxide laser resurfacing did not produce a significant improvement in infraorbital hyperpigmentation. However, the combination of microneedling and 10% trichloroacetic acid peels did.
The finding comes from what is believed to be the first head-to-head comparison of the two procedures for infraorbital dark circles, which are a common cosmetic concern with increased age.
During a late-breaking abstract session at the virtual annual meeting of the American Academy of Dermatology, lead study author Banu Farabi, MD, said that dark circles seen in the periorbital area are defined as bilateral, round homogeneous pigmented macules whose etiology is thought to be multifactorial. Available treatments include bleaching creams, topical retinoids, chemical peels, lasers, autologous fat transplantation, injectable fillers, and blepharoplasty.
“Microneedling has been recently suggested as an effective and efficient method for reducing infraorbital dark circles,” Dr. Farabi said. “This technique is based on creating microchannels that can stimulate the production of subcutaneous collagen and elastin. It also enhances the revascularization and fibroblast activity, which increases the skin thickness and gives a shiny appearance to the skin. The fractional CO2 has also been introduced as an effective procedure to remove infraorbital dark circles. However, there are some potential complications with that therapy.”
For the current study, Dr. Farabi, of the department of dermatology at Ankara (Turkey) University, and Mohamad Goldust, MD, of University Hospital Basel (Switzerland), They used the handheld Automatic Microneedle Therapy System-Handhold from MCure. After creating microchannels, the investigators topically applied 10% trichloroacetic acid peels to each infraorbital area and waited for 5 minutes.
In the carbon dioxide laser group, a Lutronic CO2 laser was used with a pulse energy of 10 J/cm2, a 100-microsecond pulse rate, 30 W of power, and a pulse width of 4 mm. The treatment outcome was assessed with the patient’s satisfaction and the physician’s judgment, which were no response, partial response, and complete response. Patients in both study groups were followed up for blinded-investigator assessment of infraorbital hyperpigmentation, adverse events, and improvement, compared with baseline.
The mean age of patients was 40 years, with a range between 27 and 58 years. About one-third of patients in each group had Fitzpatrick skin types II, III, and IV, respectively. In the blinded investigator assessment, the laser-resurfacing procedure did not demonstrate a significant improvement in infraorbital hyperpigmentation at day 90 (P = .24). However, the combination of microneedling and 10% trichloroacetic acid peels significantly improved infraorbital hyperpigmentation by day 90, with improvement maintained through day 180 (P = .012 and .002, respectively).
Adverse events were mild and temporary in both groups. In the laser-resurfacing group, 7 of the patients (22.5%) developed transient infraorbital hyperpigmentation postoperatively that lasted 4 weeks. In the combination treatment group, 18 patients (58%) developed transient erythema that lasted for up to 1 week.
“We suggest using microneedling plus 10% [trichloroacetic acid] as a cost-effective and efficient method for reducing infraorbital dark circles,” Dr. Farabi concluded.
The researchers reported having no financial disclosures.
FROM AAD 20