User login
Considerations for the Use of Biologics in Pregnancy
Biologics have revolutionized dermatologic treatment, offering substantial relief from chronic and debilitating skin conditions such as psoriasis,
Biologics for Cutaneous Conditions
Biologics—tumor necrosis factor (TNF) α inhibitors; IL-17, IL-23, IL-12, and IL-36 inhibitors; and agents such as omalizumab and dupilumab—have shown remarkable efficacy in controlling severe or recalcitrant dermatologic conditions and typically are more effective than traditional systemic therapies.1 For instance, randomized clinical trials (RCTs) and real-world data have shown that patients with psoriasis can achieve considerable skin clearance with biologics, greatly enhancing QOL.2 Adalimumab and secukinumab, which have been approved for use in moderate to severe cases of hidradenitis suppurativa, reduce the frequency of painful nodules and abscesses, thereby decreasing pain and improving QOL. Dupilumab, an IL-4/13 receptor antagonist, has revolutionized the treatment of AD by drastically reducing itch and skin lesions and improving QOL.3 For chronic urticaria, the anti-IgE antibody omalizumab has effectively reduced the incidence of hives and itching, providing pronounced symptom relief when traditional antihistamines fail.4 Use of rituximab, an anti-CD20 monoclonal antibody, has led to remission in severe cases of pemphigus vulgaris and bullous pemphigoid.5
Impact of Untreated Cutaneous Conditions in Pregnancy
When treating patients who are pregnant, dermatologists must consider the health of both the expectant mother and the developing fetus. This dual focus complicates decision-making, particularly with the use of biologics. Untreated cutaneous conditions can profoundly impact a pregnant patient’s health and QOL as well as lead to pregnancy complications affecting the fetus, such as preterm birth or low birth weight. In some studies, moderate to severe psoriasis has been associated with increased risk for complications during pregnancy, including preeclampsia and intrauterine growth restriction.6 Although specific data on hidradenitis suppurativa are lacking, the highly inflammatory nature of the condition suggests similar adverse effects on pregnancy.7 Atopic dermatitis can be exacerbated during pregnancy due to a shift in the immune system to become more allergic dominant.8 Generalized pustular psoriasis manifests with widespread pustules, fever, and systemic inflammation, posing serious risks to both the mother and the fetus if left untreated9; in such a life-threatening scenario, the use of potent treatments such as spesolimab, an IL-36 receptor antagonist, may be warranted. Therefore, managing these conditions effectively is crucial not only for the mother’s health but also for fetal well-being.
Which Biologics Can Dermatologists Safely Prescribe?
Despite the benefits, many dermatologists are hesitant to prescribe biologics to pregnant patients due to the lack of understanding and definitive safety data.10,11 Although there are no RCTs that involve pregnant patients, current evidence suggests that several biologics are not teratogenic and do not cause fetal malformations. Extensive postexposure data support the safety of TNF-α inhibitors during pregnancy.12 Research has shown that children exposed to these agents in utero have normal development, infection rates, and vaccination outcomes comparable to nonexposed children. For example, a systematic review and meta-analysis found no significant increase in the risk for major congenital malformations, spontaneous abortions, or preterm births among patients exposed to anti–TNF-α agents during pregnancy.2 The Organization of Teratology Information Specialists Autoimmune Diseases in Pregnancy Project has provided valuable real-world data indicating that the use of TNF-α inhibitors in pregnancy, particularly during the first trimester, does not substantially elevate the risk for adverse outcomes.13 These findings have been corroborated by several other registry studies and RCTs, providing a robust safety profile for these agents during pregnancy.14
Similarly, postexposure data on IL-17 and IL-12/23 inhibitors indicate a favorable safety profile, though the sample sizes are smaller than those for anti–TNF-α agents.12,14 Studies of drugs such as secukinumab (IL-17 inhibitor), guselkumab (IL-23 inhibitor), or ustekinumab (IL-12/23 inhibitor) have shown no association with teratogenic effects or increased risk for miscarriage.14 However, agents such as spesolimab (IL-36 inhibitor) are relatively new, and ongoing studies are expected to provide more comprehensive safety data.15 Similarly, omalizumab and dupilumab have not been associated with increased risk for fetal malformations or adverse pregnancy outcomes. Omalizumab, indicated for chronic urticaria, has a good safety profile in pregnancy, with no significant increase in adverse outcomes reported in studies and registries.16 Dupilumab, used for AD, has demonstrated safety in pregnancy, with ongoing studies continuing to monitor outcomes.17
Conversely, rituximab (an anti-CD20 antibody for autoimmune bullous diseases) has shown evidence of adverse pregnancy outcomes, including fetal harm.18 Its use generally is discouraged unless deemed absolutely necessary, and no safer alternatives are available. Rituximab can cross the placenta, especially in the second and third trimesters, and has been associated with B-cell depletion in the fetus, leading to potential immunosuppression and increased risk for infections.5
Although the data on the safety of biologics in pregnancy are largely reassuring, it is essential to recognize that potential risks have not been ruled out entirely. There are extensive safety data for anti–TNF-α inhibitors, which provides a level of confidence; although newer agents such as IL-17 and IL-23 inhibitors have shown promising early results, further research is required to solidify their safety profiles during pregnancy.
Dermatologists must balance the risks and benefits of using biologics in pregnant patients. This decision-making process involves careful consideration of the severity of the mother’s condition, the potential risks to the fetus, and the availability of alternative treatments. For many severe dermatologic conditions, the benefits of biologics in controlling disease activity and improving QOL may outweigh the potential risks, especially when other treatments have failed or are not suitable.
Final Thoughts
The increasing use of biologics in dermatology has undoubtedly improved the management of severe skin conditions, substantially enhancing patients’ QOL. As more data become available and clinical guidelines evolve, health care providers will be better equipped to make informed decisions about the use of biologics, particularly in pregnant patients. Collaborative efforts between dermatologists, obstetricians, and researchers will help refine treatment guidelines and ensure that pregnant patients with severe dermatologic conditions receive the best possible care.
For now, although the current evidence supports the safety of many biologics during pregnancy,10,11 individualized care and informed decision-making remain paramount. Careful management and adherence to current guidelines make it possible to navigate the complexities of treating severe dermatologic conditions in pregnant patients, ensuring the best outcomes for both mother and child.
- Sehgal VN, Pandhi D, Khurana A. Biologics in dermatology: an integrated review. Indian J Dermatol. 2014; 59:425-441. doi:10.4103/0019-5154.139859
- Mahadevan U, Wolf DC, Dubinsky M, et al. Placental transfer of anti-tumor necrosis factor agents in pregnant patients with inflammatory bowel disease. Clin Gastroenterol Hepatol. 2013;11:286-292. doi:10.1016/j.cgh.2012.11.011
- Simpson EL, Bieber T, Guttman-Yassky E, et al. Two phase 3 trials of dupilumab versus placebo in atopic dermatitis. N Engl J Med. 2016;375:2335-2348.
- Saini SS, Bindslev-Jensen C, Maurer M, et al. Efficacy and safety of omalizumab in patients with chronic idiopathic/spontaneous urticaria who remain symptomatic on H1 antihistamines: a randomized, placebo-controlled study. J Invest Dermatol. 2015;135:67-75. doi:10.1038/jid.2014.306
- Mariette X, Forger F, Abraham B, et al. Lack of placental transfer of certolizumab pegol during pregnancy: results from CRIB, a prospective, postmarketing, pharmacokinetic study. Ann Rheum Dis. 2018;77:228-233. doi:10.1136/annrheumdis-2017-212196
- Yang Y-W, Chen C-S, Chen Y-H, et al. Psoriasis and pregnancy outcomes: a nationwide population-based study. J Am Acad Dermatol. 2011;64:71-77.
- Zouboulis CC, Del Marmol V, Mrowietz U, et al. Hidradenitis suppurativa/acne inversa: criteria for diagnosis, severity assessment, classification and disease evaluation. Dermatology. 2015;231:184-190.
- Balakirski G, Novak N. Atopic dermatitis and pregnancy. J Allergy Clin Immunol. 2022;149:1185-1194. doi:10.1016/j.jaci.2022.01.010
- Bachelez H, Choon S-E, Marrakchi S, et al. Inhibition of the interleukin-36 pathway for the treatment of generalized pustular psoriasis. N Engl J Med. 2019;380:981-983.
- McMullan P, Yaghi M, Truong TM, et al. Safety of dermatologic medications in pregnancy and lactation: an update—part I: pregnancy. J Am Acad Dermatol. Published online January 25, 2024. doi:10.1016/j.jaad.2023.10.072
- Yaghi M, McMullan P, Truong TM, et al. Safety of dermatologic medications in pregnancy and lactation: an update—part II: lactation. J Am Acad Dermatol. Published online January 25, 2024. doi:10.1016/j.jaad.2023.10.071
- Owczarek W, Walecka I, Lesiak A, et al. The use of biological drugs in psoriasis patients prior to pregnancy, during pregnancy and lactation: a review of current clinical guidelines. Postepy Dermatol Alergol. 2020;37:821-830. doi:10.5114/ada.2020.102089
- Organization of Teratology Information Services (OTIS) Autoimmune Diseases in Pregnancy Project. ClinicalTrials.gov identifier: NCT00116272. Updated October 6, 2023. Accessed August 29, 2024. https://clinicaltrials.gov/study/NCT00116272
- Sanchez-Garcia V, Hernandez-Quiles R, de-Miguel-Balsa E, et al. Exposure to biologic therapy before and during pregnancy in patients with psoriasis: systematic review and meta-analysis. J Eur Acad Dermatol Venereol. 2023;37:1971-1990. doi:10.1111/jdv.19238
- Silverberg JI, Boguniewicz M, Hanifin J, et al. Dupilumab treatment in adults with moderate-to-severe atopic dermatitis is efficacious regardless of age of disease onset: a post hoc analysis of two phase 3 clinical trials. Dermatol Ther (Heidelb). 2022;12:2731-2746. doi:10.1007/s13555-022-00822-x
- Levi-Schaffer F, Mankuta D. Omalizumab safety in pregnancy. J Allergy Clin Immunol. 2020;145:481-483. doi:10.1016/j.jaci.2019.11.018
- Thaci D, Simpson EL, Beck LA, et al. Efficacy and safety of dupilumab in adults with moderate-to-severe atopic dermatitis inadequately controlled by topical treatments: a randomised, placebo-controlled, dose-ranging phase 2b trial. Lancet. 2016;387:40-52.
- Chakravarty EF, Murray ER, Kelman A, et al. Pregnancy outcomes after maternal exposure to rituximab. Blood. 2011;117:1499-1506. doi:10.1182/blood-2010-07-295444
Biologics have revolutionized dermatologic treatment, offering substantial relief from chronic and debilitating skin conditions such as psoriasis,
Biologics for Cutaneous Conditions
Biologics—tumor necrosis factor (TNF) α inhibitors; IL-17, IL-23, IL-12, and IL-36 inhibitors; and agents such as omalizumab and dupilumab—have shown remarkable efficacy in controlling severe or recalcitrant dermatologic conditions and typically are more effective than traditional systemic therapies.1 For instance, randomized clinical trials (RCTs) and real-world data have shown that patients with psoriasis can achieve considerable skin clearance with biologics, greatly enhancing QOL.2 Adalimumab and secukinumab, which have been approved for use in moderate to severe cases of hidradenitis suppurativa, reduce the frequency of painful nodules and abscesses, thereby decreasing pain and improving QOL. Dupilumab, an IL-4/13 receptor antagonist, has revolutionized the treatment of AD by drastically reducing itch and skin lesions and improving QOL.3 For chronic urticaria, the anti-IgE antibody omalizumab has effectively reduced the incidence of hives and itching, providing pronounced symptom relief when traditional antihistamines fail.4 Use of rituximab, an anti-CD20 monoclonal antibody, has led to remission in severe cases of pemphigus vulgaris and bullous pemphigoid.5
Impact of Untreated Cutaneous Conditions in Pregnancy
When treating patients who are pregnant, dermatologists must consider the health of both the expectant mother and the developing fetus. This dual focus complicates decision-making, particularly with the use of biologics. Untreated cutaneous conditions can profoundly impact a pregnant patient’s health and QOL as well as lead to pregnancy complications affecting the fetus, such as preterm birth or low birth weight. In some studies, moderate to severe psoriasis has been associated with increased risk for complications during pregnancy, including preeclampsia and intrauterine growth restriction.6 Although specific data on hidradenitis suppurativa are lacking, the highly inflammatory nature of the condition suggests similar adverse effects on pregnancy.7 Atopic dermatitis can be exacerbated during pregnancy due to a shift in the immune system to become more allergic dominant.8 Generalized pustular psoriasis manifests with widespread pustules, fever, and systemic inflammation, posing serious risks to both the mother and the fetus if left untreated9; in such a life-threatening scenario, the use of potent treatments such as spesolimab, an IL-36 receptor antagonist, may be warranted. Therefore, managing these conditions effectively is crucial not only for the mother’s health but also for fetal well-being.
Which Biologics Can Dermatologists Safely Prescribe?
Despite the benefits, many dermatologists are hesitant to prescribe biologics to pregnant patients due to the lack of understanding and definitive safety data.10,11 Although there are no RCTs that involve pregnant patients, current evidence suggests that several biologics are not teratogenic and do not cause fetal malformations. Extensive postexposure data support the safety of TNF-α inhibitors during pregnancy.12 Research has shown that children exposed to these agents in utero have normal development, infection rates, and vaccination outcomes comparable to nonexposed children. For example, a systematic review and meta-analysis found no significant increase in the risk for major congenital malformations, spontaneous abortions, or preterm births among patients exposed to anti–TNF-α agents during pregnancy.2 The Organization of Teratology Information Specialists Autoimmune Diseases in Pregnancy Project has provided valuable real-world data indicating that the use of TNF-α inhibitors in pregnancy, particularly during the first trimester, does not substantially elevate the risk for adverse outcomes.13 These findings have been corroborated by several other registry studies and RCTs, providing a robust safety profile for these agents during pregnancy.14
Similarly, postexposure data on IL-17 and IL-12/23 inhibitors indicate a favorable safety profile, though the sample sizes are smaller than those for anti–TNF-α agents.12,14 Studies of drugs such as secukinumab (IL-17 inhibitor), guselkumab (IL-23 inhibitor), or ustekinumab (IL-12/23 inhibitor) have shown no association with teratogenic effects or increased risk for miscarriage.14 However, agents such as spesolimab (IL-36 inhibitor) are relatively new, and ongoing studies are expected to provide more comprehensive safety data.15 Similarly, omalizumab and dupilumab have not been associated with increased risk for fetal malformations or adverse pregnancy outcomes. Omalizumab, indicated for chronic urticaria, has a good safety profile in pregnancy, with no significant increase in adverse outcomes reported in studies and registries.16 Dupilumab, used for AD, has demonstrated safety in pregnancy, with ongoing studies continuing to monitor outcomes.17
Conversely, rituximab (an anti-CD20 antibody for autoimmune bullous diseases) has shown evidence of adverse pregnancy outcomes, including fetal harm.18 Its use generally is discouraged unless deemed absolutely necessary, and no safer alternatives are available. Rituximab can cross the placenta, especially in the second and third trimesters, and has been associated with B-cell depletion in the fetus, leading to potential immunosuppression and increased risk for infections.5
Although the data on the safety of biologics in pregnancy are largely reassuring, it is essential to recognize that potential risks have not been ruled out entirely. There are extensive safety data for anti–TNF-α inhibitors, which provides a level of confidence; although newer agents such as IL-17 and IL-23 inhibitors have shown promising early results, further research is required to solidify their safety profiles during pregnancy.
Dermatologists must balance the risks and benefits of using biologics in pregnant patients. This decision-making process involves careful consideration of the severity of the mother’s condition, the potential risks to the fetus, and the availability of alternative treatments. For many severe dermatologic conditions, the benefits of biologics in controlling disease activity and improving QOL may outweigh the potential risks, especially when other treatments have failed or are not suitable.
Final Thoughts
The increasing use of biologics in dermatology has undoubtedly improved the management of severe skin conditions, substantially enhancing patients’ QOL. As more data become available and clinical guidelines evolve, health care providers will be better equipped to make informed decisions about the use of biologics, particularly in pregnant patients. Collaborative efforts between dermatologists, obstetricians, and researchers will help refine treatment guidelines and ensure that pregnant patients with severe dermatologic conditions receive the best possible care.
For now, although the current evidence supports the safety of many biologics during pregnancy,10,11 individualized care and informed decision-making remain paramount. Careful management and adherence to current guidelines make it possible to navigate the complexities of treating severe dermatologic conditions in pregnant patients, ensuring the best outcomes for both mother and child.
Biologics have revolutionized dermatologic treatment, offering substantial relief from chronic and debilitating skin conditions such as psoriasis,
Biologics for Cutaneous Conditions
Biologics—tumor necrosis factor (TNF) α inhibitors; IL-17, IL-23, IL-12, and IL-36 inhibitors; and agents such as omalizumab and dupilumab—have shown remarkable efficacy in controlling severe or recalcitrant dermatologic conditions and typically are more effective than traditional systemic therapies.1 For instance, randomized clinical trials (RCTs) and real-world data have shown that patients with psoriasis can achieve considerable skin clearance with biologics, greatly enhancing QOL.2 Adalimumab and secukinumab, which have been approved for use in moderate to severe cases of hidradenitis suppurativa, reduce the frequency of painful nodules and abscesses, thereby decreasing pain and improving QOL. Dupilumab, an IL-4/13 receptor antagonist, has revolutionized the treatment of AD by drastically reducing itch and skin lesions and improving QOL.3 For chronic urticaria, the anti-IgE antibody omalizumab has effectively reduced the incidence of hives and itching, providing pronounced symptom relief when traditional antihistamines fail.4 Use of rituximab, an anti-CD20 monoclonal antibody, has led to remission in severe cases of pemphigus vulgaris and bullous pemphigoid.5
Impact of Untreated Cutaneous Conditions in Pregnancy
When treating patients who are pregnant, dermatologists must consider the health of both the expectant mother and the developing fetus. This dual focus complicates decision-making, particularly with the use of biologics. Untreated cutaneous conditions can profoundly impact a pregnant patient’s health and QOL as well as lead to pregnancy complications affecting the fetus, such as preterm birth or low birth weight. In some studies, moderate to severe psoriasis has been associated with increased risk for complications during pregnancy, including preeclampsia and intrauterine growth restriction.6 Although specific data on hidradenitis suppurativa are lacking, the highly inflammatory nature of the condition suggests similar adverse effects on pregnancy.7 Atopic dermatitis can be exacerbated during pregnancy due to a shift in the immune system to become more allergic dominant.8 Generalized pustular psoriasis manifests with widespread pustules, fever, and systemic inflammation, posing serious risks to both the mother and the fetus if left untreated9; in such a life-threatening scenario, the use of potent treatments such as spesolimab, an IL-36 receptor antagonist, may be warranted. Therefore, managing these conditions effectively is crucial not only for the mother’s health but also for fetal well-being.
Which Biologics Can Dermatologists Safely Prescribe?
Despite the benefits, many dermatologists are hesitant to prescribe biologics to pregnant patients due to the lack of understanding and definitive safety data.10,11 Although there are no RCTs that involve pregnant patients, current evidence suggests that several biologics are not teratogenic and do not cause fetal malformations. Extensive postexposure data support the safety of TNF-α inhibitors during pregnancy.12 Research has shown that children exposed to these agents in utero have normal development, infection rates, and vaccination outcomes comparable to nonexposed children. For example, a systematic review and meta-analysis found no significant increase in the risk for major congenital malformations, spontaneous abortions, or preterm births among patients exposed to anti–TNF-α agents during pregnancy.2 The Organization of Teratology Information Specialists Autoimmune Diseases in Pregnancy Project has provided valuable real-world data indicating that the use of TNF-α inhibitors in pregnancy, particularly during the first trimester, does not substantially elevate the risk for adverse outcomes.13 These findings have been corroborated by several other registry studies and RCTs, providing a robust safety profile for these agents during pregnancy.14
Similarly, postexposure data on IL-17 and IL-12/23 inhibitors indicate a favorable safety profile, though the sample sizes are smaller than those for anti–TNF-α agents.12,14 Studies of drugs such as secukinumab (IL-17 inhibitor), guselkumab (IL-23 inhibitor), or ustekinumab (IL-12/23 inhibitor) have shown no association with teratogenic effects or increased risk for miscarriage.14 However, agents such as spesolimab (IL-36 inhibitor) are relatively new, and ongoing studies are expected to provide more comprehensive safety data.15 Similarly, omalizumab and dupilumab have not been associated with increased risk for fetal malformations or adverse pregnancy outcomes. Omalizumab, indicated for chronic urticaria, has a good safety profile in pregnancy, with no significant increase in adverse outcomes reported in studies and registries.16 Dupilumab, used for AD, has demonstrated safety in pregnancy, with ongoing studies continuing to monitor outcomes.17
Conversely, rituximab (an anti-CD20 antibody for autoimmune bullous diseases) has shown evidence of adverse pregnancy outcomes, including fetal harm.18 Its use generally is discouraged unless deemed absolutely necessary, and no safer alternatives are available. Rituximab can cross the placenta, especially in the second and third trimesters, and has been associated with B-cell depletion in the fetus, leading to potential immunosuppression and increased risk for infections.5
Although the data on the safety of biologics in pregnancy are largely reassuring, it is essential to recognize that potential risks have not been ruled out entirely. There are extensive safety data for anti–TNF-α inhibitors, which provides a level of confidence; although newer agents such as IL-17 and IL-23 inhibitors have shown promising early results, further research is required to solidify their safety profiles during pregnancy.
Dermatologists must balance the risks and benefits of using biologics in pregnant patients. This decision-making process involves careful consideration of the severity of the mother’s condition, the potential risks to the fetus, and the availability of alternative treatments. For many severe dermatologic conditions, the benefits of biologics in controlling disease activity and improving QOL may outweigh the potential risks, especially when other treatments have failed or are not suitable.
Final Thoughts
The increasing use of biologics in dermatology has undoubtedly improved the management of severe skin conditions, substantially enhancing patients’ QOL. As more data become available and clinical guidelines evolve, health care providers will be better equipped to make informed decisions about the use of biologics, particularly in pregnant patients. Collaborative efforts between dermatologists, obstetricians, and researchers will help refine treatment guidelines and ensure that pregnant patients with severe dermatologic conditions receive the best possible care.
For now, although the current evidence supports the safety of many biologics during pregnancy,10,11 individualized care and informed decision-making remain paramount. Careful management and adherence to current guidelines make it possible to navigate the complexities of treating severe dermatologic conditions in pregnant patients, ensuring the best outcomes for both mother and child.
- Sehgal VN, Pandhi D, Khurana A. Biologics in dermatology: an integrated review. Indian J Dermatol. 2014; 59:425-441. doi:10.4103/0019-5154.139859
- Mahadevan U, Wolf DC, Dubinsky M, et al. Placental transfer of anti-tumor necrosis factor agents in pregnant patients with inflammatory bowel disease. Clin Gastroenterol Hepatol. 2013;11:286-292. doi:10.1016/j.cgh.2012.11.011
- Simpson EL, Bieber T, Guttman-Yassky E, et al. Two phase 3 trials of dupilumab versus placebo in atopic dermatitis. N Engl J Med. 2016;375:2335-2348.
- Saini SS, Bindslev-Jensen C, Maurer M, et al. Efficacy and safety of omalizumab in patients with chronic idiopathic/spontaneous urticaria who remain symptomatic on H1 antihistamines: a randomized, placebo-controlled study. J Invest Dermatol. 2015;135:67-75. doi:10.1038/jid.2014.306
- Mariette X, Forger F, Abraham B, et al. Lack of placental transfer of certolizumab pegol during pregnancy: results from CRIB, a prospective, postmarketing, pharmacokinetic study. Ann Rheum Dis. 2018;77:228-233. doi:10.1136/annrheumdis-2017-212196
- Yang Y-W, Chen C-S, Chen Y-H, et al. Psoriasis and pregnancy outcomes: a nationwide population-based study. J Am Acad Dermatol. 2011;64:71-77.
- Zouboulis CC, Del Marmol V, Mrowietz U, et al. Hidradenitis suppurativa/acne inversa: criteria for diagnosis, severity assessment, classification and disease evaluation. Dermatology. 2015;231:184-190.
- Balakirski G, Novak N. Atopic dermatitis and pregnancy. J Allergy Clin Immunol. 2022;149:1185-1194. doi:10.1016/j.jaci.2022.01.010
- Bachelez H, Choon S-E, Marrakchi S, et al. Inhibition of the interleukin-36 pathway for the treatment of generalized pustular psoriasis. N Engl J Med. 2019;380:981-983.
- McMullan P, Yaghi M, Truong TM, et al. Safety of dermatologic medications in pregnancy and lactation: an update—part I: pregnancy. J Am Acad Dermatol. Published online January 25, 2024. doi:10.1016/j.jaad.2023.10.072
- Yaghi M, McMullan P, Truong TM, et al. Safety of dermatologic medications in pregnancy and lactation: an update—part II: lactation. J Am Acad Dermatol. Published online January 25, 2024. doi:10.1016/j.jaad.2023.10.071
- Owczarek W, Walecka I, Lesiak A, et al. The use of biological drugs in psoriasis patients prior to pregnancy, during pregnancy and lactation: a review of current clinical guidelines. Postepy Dermatol Alergol. 2020;37:821-830. doi:10.5114/ada.2020.102089
- Organization of Teratology Information Services (OTIS) Autoimmune Diseases in Pregnancy Project. ClinicalTrials.gov identifier: NCT00116272. Updated October 6, 2023. Accessed August 29, 2024. https://clinicaltrials.gov/study/NCT00116272
- Sanchez-Garcia V, Hernandez-Quiles R, de-Miguel-Balsa E, et al. Exposure to biologic therapy before and during pregnancy in patients with psoriasis: systematic review and meta-analysis. J Eur Acad Dermatol Venereol. 2023;37:1971-1990. doi:10.1111/jdv.19238
- Silverberg JI, Boguniewicz M, Hanifin J, et al. Dupilumab treatment in adults with moderate-to-severe atopic dermatitis is efficacious regardless of age of disease onset: a post hoc analysis of two phase 3 clinical trials. Dermatol Ther (Heidelb). 2022;12:2731-2746. doi:10.1007/s13555-022-00822-x
- Levi-Schaffer F, Mankuta D. Omalizumab safety in pregnancy. J Allergy Clin Immunol. 2020;145:481-483. doi:10.1016/j.jaci.2019.11.018
- Thaci D, Simpson EL, Beck LA, et al. Efficacy and safety of dupilumab in adults with moderate-to-severe atopic dermatitis inadequately controlled by topical treatments: a randomised, placebo-controlled, dose-ranging phase 2b trial. Lancet. 2016;387:40-52.
- Chakravarty EF, Murray ER, Kelman A, et al. Pregnancy outcomes after maternal exposure to rituximab. Blood. 2011;117:1499-1506. doi:10.1182/blood-2010-07-295444
- Sehgal VN, Pandhi D, Khurana A. Biologics in dermatology: an integrated review. Indian J Dermatol. 2014; 59:425-441. doi:10.4103/0019-5154.139859
- Mahadevan U, Wolf DC, Dubinsky M, et al. Placental transfer of anti-tumor necrosis factor agents in pregnant patients with inflammatory bowel disease. Clin Gastroenterol Hepatol. 2013;11:286-292. doi:10.1016/j.cgh.2012.11.011
- Simpson EL, Bieber T, Guttman-Yassky E, et al. Two phase 3 trials of dupilumab versus placebo in atopic dermatitis. N Engl J Med. 2016;375:2335-2348.
- Saini SS, Bindslev-Jensen C, Maurer M, et al. Efficacy and safety of omalizumab in patients with chronic idiopathic/spontaneous urticaria who remain symptomatic on H1 antihistamines: a randomized, placebo-controlled study. J Invest Dermatol. 2015;135:67-75. doi:10.1038/jid.2014.306
- Mariette X, Forger F, Abraham B, et al. Lack of placental transfer of certolizumab pegol during pregnancy: results from CRIB, a prospective, postmarketing, pharmacokinetic study. Ann Rheum Dis. 2018;77:228-233. doi:10.1136/annrheumdis-2017-212196
- Yang Y-W, Chen C-S, Chen Y-H, et al. Psoriasis and pregnancy outcomes: a nationwide population-based study. J Am Acad Dermatol. 2011;64:71-77.
- Zouboulis CC, Del Marmol V, Mrowietz U, et al. Hidradenitis suppurativa/acne inversa: criteria for diagnosis, severity assessment, classification and disease evaluation. Dermatology. 2015;231:184-190.
- Balakirski G, Novak N. Atopic dermatitis and pregnancy. J Allergy Clin Immunol. 2022;149:1185-1194. doi:10.1016/j.jaci.2022.01.010
- Bachelez H, Choon S-E, Marrakchi S, et al. Inhibition of the interleukin-36 pathway for the treatment of generalized pustular psoriasis. N Engl J Med. 2019;380:981-983.
- McMullan P, Yaghi M, Truong TM, et al. Safety of dermatologic medications in pregnancy and lactation: an update—part I: pregnancy. J Am Acad Dermatol. Published online January 25, 2024. doi:10.1016/j.jaad.2023.10.072
- Yaghi M, McMullan P, Truong TM, et al. Safety of dermatologic medications in pregnancy and lactation: an update—part II: lactation. J Am Acad Dermatol. Published online January 25, 2024. doi:10.1016/j.jaad.2023.10.071
- Owczarek W, Walecka I, Lesiak A, et al. The use of biological drugs in psoriasis patients prior to pregnancy, during pregnancy and lactation: a review of current clinical guidelines. Postepy Dermatol Alergol. 2020;37:821-830. doi:10.5114/ada.2020.102089
- Organization of Teratology Information Services (OTIS) Autoimmune Diseases in Pregnancy Project. ClinicalTrials.gov identifier: NCT00116272. Updated October 6, 2023. Accessed August 29, 2024. https://clinicaltrials.gov/study/NCT00116272
- Sanchez-Garcia V, Hernandez-Quiles R, de-Miguel-Balsa E, et al. Exposure to biologic therapy before and during pregnancy in patients with psoriasis: systematic review and meta-analysis. J Eur Acad Dermatol Venereol. 2023;37:1971-1990. doi:10.1111/jdv.19238
- Silverberg JI, Boguniewicz M, Hanifin J, et al. Dupilumab treatment in adults with moderate-to-severe atopic dermatitis is efficacious regardless of age of disease onset: a post hoc analysis of two phase 3 clinical trials. Dermatol Ther (Heidelb). 2022;12:2731-2746. doi:10.1007/s13555-022-00822-x
- Levi-Schaffer F, Mankuta D. Omalizumab safety in pregnancy. J Allergy Clin Immunol. 2020;145:481-483. doi:10.1016/j.jaci.2019.11.018
- Thaci D, Simpson EL, Beck LA, et al. Efficacy and safety of dupilumab in adults with moderate-to-severe atopic dermatitis inadequately controlled by topical treatments: a randomised, placebo-controlled, dose-ranging phase 2b trial. Lancet. 2016;387:40-52.
- Chakravarty EF, Murray ER, Kelman A, et al. Pregnancy outcomes after maternal exposure to rituximab. Blood. 2011;117:1499-1506. doi:10.1182/blood-2010-07-295444
Treat-to-Target Outcomes With Tapinarof Cream 1% in Phase 3 Trials for Plaque Psoriasis
Psoriasis is a chronic inflammatory disease affecting approximately 8 million adults in the United States and 2% of the global population.1,2 Psoriasis causes pain, itching, and disfigurement and is associated with a physical, psychological, and economic burden that substantially affects health-related quality of life.3-5
Setting treatment goals and treating to target are evidence-based approaches that have been successfully applied to several chronic diseases to improve patient outcomes, including diabetes, hypertension, and rheumatoid arthritis.6-9 Treat-to-target strategies generally set low disease activity (or remission) as an overall goal and seek to achieve this using available therapeutic options as necessary. Introduced following the availability of biologics and targeted systemic therapies, treat-to-target strategies generally provide guidance on expectations of treatment but not specific treatments, as personalized treatment decisions depend on an assessment of individual patients and consider clinical and demographic features as well as preferences for available therapeutic options. If targets are not achieved in the assigned time span, adjustments can be made to the treatment approach in close consultation with the patient. If the target is reached, follow-up visits can be scheduled to ensure improvement is maintained or to establish if more aggressive goals could be selected.
Treat-to-target strategies for the management of psoriasis developed by the National Psoriasis Foundation (NPF) Medical Board include reducing the extent of psoriasis to 1% or lower total body surface area (BSA) after 3 months of treatment.10 Treatment targets endorsed by the European Academy of Dermatology and Venereology (EADV) in guidelines on the use of systemic therapies in psoriasis include achieving a 75% or greater reduction in Psoriasis Area and Severity Index (PASI) score within 3 to 4 months of treatment.11
In clinical practice, many patients do not achieve these treatment targets, and topical treatments alone generally are insufficient in achieving treatment goals for psoriasis.12,13 Moreover, conventional topical treatments (eg, topical corticosteroids) used by most patients with psoriasis regardless of disease severity are associated with adverse events that can limit their use. Most topical corticosteroids have US Food and Drug Administration label restrictions relating to sites of application, duration and extent of use, and frequency of administration.14,15
Tapinarof cream 1% (VTAMA [Dermavant Sciences, Inc]) is a first-in-class topical nonsteroidal aryl hydrocarbon receptor agonist that was approved by the US Food and Drug Administration for the treatment of plaque psoriasis in adults16 and is being studied for the treatment of plaque psoriasis in children 2 years and older as well as for atopic dermatitis in adults and children 2 years and older. In PSOARING 1 (ClinicalTrials .gov identifier NCT03956355) and PSOARING 2 (NCT03983980)—identical 12-week pivotal phase 3 trials—monotherapy with tapinarof cream 1% once daily (QD) demonstrated statistically significant efficacy vs vehicle cream and was well tolerated in adults with mild to severe plaque psoriasis (Supplementary Figure S1).17 Lebwohl et al17 reported that significantly higher PASI75 responses were observed at week 12 with tapinarof cream vs vehicle in PSOARING 1 and PSOARING 2 (36% and 48% vs 10% and 7%, respectively; both P<.0001). A significantly higher PASI90 response of 19% and 21% at week 12 also was observed with tapinarof cream vs 2% and 3% with vehicle in PSOARING 1 and PSOARING 2, respectively (P=.0005 and P<.0001).17
In PSOARING 3 (NCT04053387)—the long-term extension trial (Supplementary Figure S1)—efficacy continued to improve or was maintained beyond the two 12-week trials, with improvements in total BSA affected and PASI scores for up to 52 weeks.18 Tapinarof cream 1% QD demonstrated positive, rapid, and durable outcomes in PSOARING 3, including high rates of complete disease clearance (Physician Global Assessment [PGA] score=0 [clear])(40.9% [312/763]), durability of response on treatment with no evidence of tachyphylaxis, and a remittive effect of approximately 4 months when off therapy (defined as maintenance of a PGA score of 0 [clear] or 1 [almost clear] after first achieving a PGA score of 0).18
Herein, we report absolute treatment targets for patients with plaque psoriasis who received tapinarof cream 1% QD in the PSOARING trials that are at least as stringent as the corresponding NPF and EADV targets of achieving a total BSA affected of 1% or lower or a PASI75 response within 3 to 4 months, respectively.
METHODS
Study Design
The pooled efficacy analyses included all patients with a baseline PGA score of 2 or higher (mild or worse) before treatment with tapinarof cream 1% QD in the PSOARING trials. This included patients who received tapinarof cream 1% in PSOARING 1 and PSOARING 2 who may or may not have continued into PSOARING 3, as well as those who received the vehicle in PSOARING 1 and PSOARING 2 who enrolled in PSOARING 3 and had a PGA score of 2 or higher before receiving tapinarof cream 1%.
Trial Participants
Full methods, including inclusion and exclusion criteria, for the PSOARING trials have been previously reported.17,18 Patients were aged 18 to 75 years and had chronic plaque psoriasis that was stable for at least 6 months before randomization; 3% to 20% total BSA affected (excluding the scalp, palms, fingernails, toenails, and soles); and a PGA score of 2 (mild), 3 (moderate), or 4 (severe) at baseline.
The clinical trials were conducted in compliance with the guidelines for Good Clinical Practice and the Declaration of Helsinki. Approval was obtained from local ethics committees or institutional review boards at each center. All patients provided written informed consent.
Trial Treatment
In PSOARING 1 and PSOARING 2, patients were randomized (2:1) to receive tapinarof cream 1% or vehicle QD for 12 weeks. In PSOARING 3 (the long-term extension trial), patients received up to 40 weeks of open-label tapinarof, followed by 4 weeks of follow-up off treatment. Patients received intermittent or continuous treatment with tapinarof cream 1% in PSOARING 3 based on PGA score: those entering the trial with a PGA score of 1 or higher received tapinarof cream 1% until complete disease clearance was achieved (defined as a PGA score of 0 [clear]). Those entering PSOARING 3 with or achieving a PGA score of 0 (clear) discontinued treatment and were observed for the duration of maintenance of a PGA score of 0 (clear) or 1 (almost clear) while off therapy (the protocol-defined “duration of remittive effect”). If disease worsening (defined as a PGA score 2 or higher) occurred, tapinarof cream 1% was restarted and continued until a PGA score of 0 (clear) was achieved. This pattern of treatment, discontinuation on achieving a PGA score of 0 (clear), and retreatment on disease worsening continued until the end of the trial. As a result, patients in PSOARING 3 could receive tapinarof cream 1% continuously or intermittently for 40 weeks.
Outcome Measures and Statistical Analyses
The assessment of total BSA affected by plaque psoriasis is an estimate of the total extent of disease as a percentage of total skin area. In the PSOARING trials, the skin surface of one hand (palm and digits) was assumed to be approximately equivalent to 1% BSA. The total BSA affected by psoriasis was evaluated from 0% to 100%, with greater total BSA affected being an indication of more extensive disease. The BSA efficacy outcomes used in these analyses were based post hoc on the proportion of patients who achieved a 1% or lower or 0.5% or lower total BSA affected.
Psoriasis Area and Severity Index scores assess both the severity and extent of psoriasis. A PASI score lower than 5 often is considered indicative of mild psoriasis, a score of 5 to 10 indicates moderate disease, and a score higher than 10 indicates severe disease.19 The maximum PASI score is 72. The PASI efficacy outcomes used in these analyses were based post hoc on the proportion of patients who achieved an absolute total PASI score of 3 or lower, 2 or lower, and 1 or lower.
Efficacy analyses were based on pooled data for all patients in the PSOARING trials who had a PGA score of 2 to 4 (mild to severe) before treatment with tapinarof cream 1% in the intention-to-treat population using observed cases. Time-to-target analyses were based on Kaplan-Meier (KM) estimates using observed cases.
Safety analyses included the incidence and frequency of adverse events and were based on all patients who received tapinarof cream 1% in the PSOARING trials.
RESULTS
Baseline Patient Demographics and Disease Characteristics
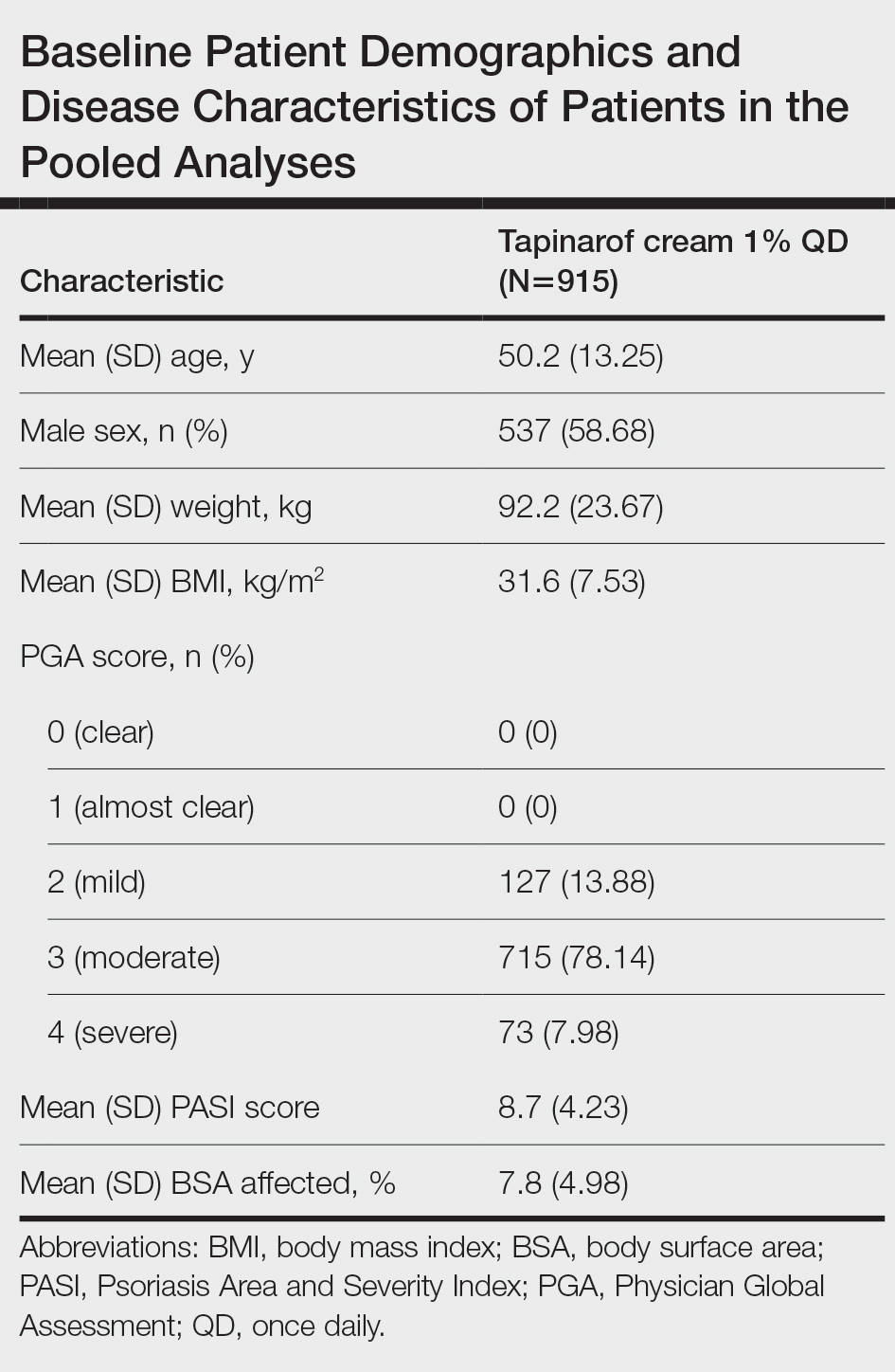
The pooled efficacy analyses included 915 eligible patients (Table). At baseline, the mean (SD) age was 50.2 (13.25) years, 58.7% were male, the mean (SD) weight was 92.2 (23.67) kg, and the mean (SD) body mass index was 31.6 (7.53) kg/m2. The percentage of patients with a PGA score of 2 (mild), 3 (moderate), or 4 (severe) was 13.9%, 78.1%, and 8.0%, respectively. The mean (SD) PASI score was 8.7 (4.23) and mean (SD) total BSA affected was 7.8% (4.98).
Efficacy
Achievement of BSA-Affected Targets—
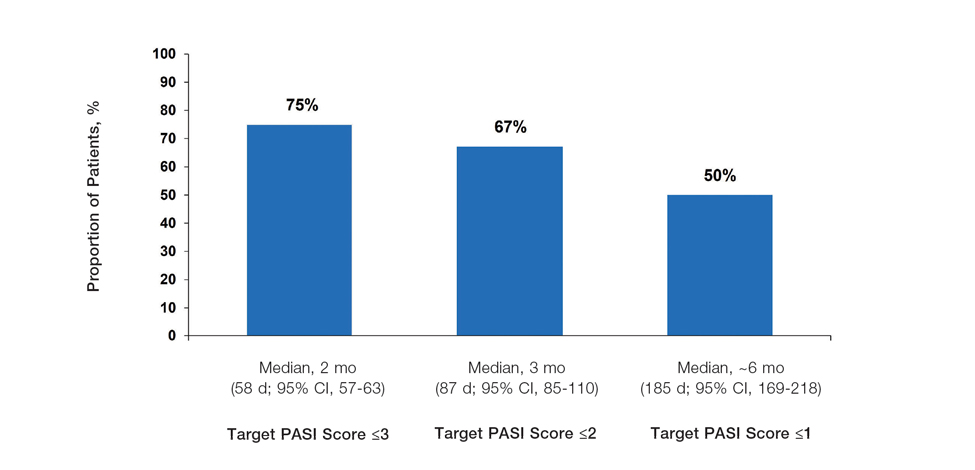
Achievement of Absolute PASI Targets—Across the total trial period (up to 52 weeks), an absolute total PASI score of 3 or lower was achieved by 75% of patients (686/915), with a median time to achieve this of 2 months (KM estimate: 58 days [95% CI, 57-63]); approximately 67% of patients (612/915) achieved a total PASI score of 2 or lower, with a median time to achieve of 3 months (KM estimate: 87 days [95% CI, 85-110])(Figure 2; Supplementary Figures S3a and S3b). A PASI score of 1 or lower was achieved by approximately 50% of patients (460/915), with a median time to achieve of approximately 6 months (KM estimate: 185 days [95% CI, 169-218])(Figure 2, Supplementary Figure S3c).
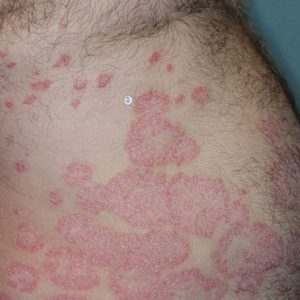
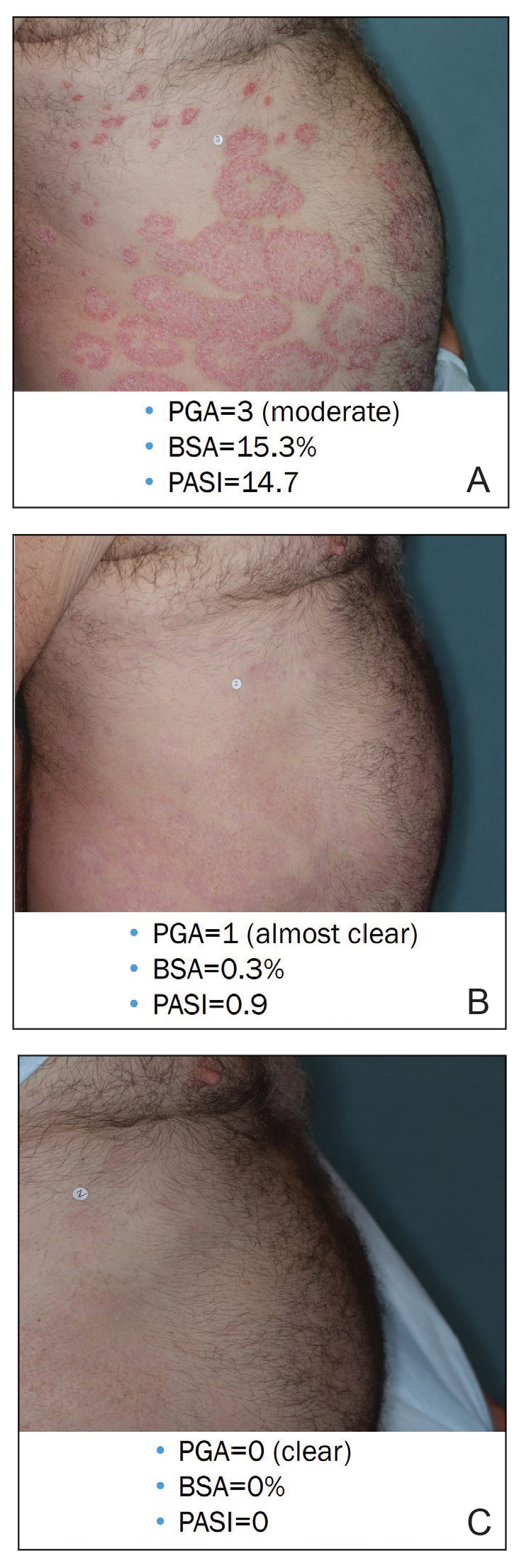
Illustrative Case—Case photography showing the clinical response in a 63-year-old man with moderate plaque psoriasis in PSOARING 2 is shown in Figure 3. After 12 weeks of treatment with tapinarof cream 1% QD, the patient achieved all primary and secondary efficacy end points. In addition to achieving the regulatory end point of a PGA score of 0 (clear) or 1 (almost clear) and a decrease from baseline of at least 2 points, achievement of 0% total BSA affected and a total PASI score of 0 at week 12 exceeded the NPF and EADV consensus treatment targets.10,11 Targets were achieved as early as week 4, with a total BSA affected of 0.5% or lower and a total PASI score of 1 or lower, illustrated by marked skin clearing and only faint residual erythema that completely resolved at week 12, with the absence of postinflammatory hyperpigmentation.
Safety
Safety data for the PSOARING trials have been previously reported.17,18 The most common treatment-emergent adverse events were folliculitis, contact dermatitis, upper respiratory tract infection, and nasopharyngitis. Treatment-emergent adverse events generally were mild or moderate in severity and did not lead to trial discontinuation.17,18
COMMENT
Treat-to-target management approaches aim to improve patient outcomes by striving to achieve optimal goals. The treat-to-target approach supports shared decision-making between clinicians and patients based on common expectations of what constitutes treatment success.
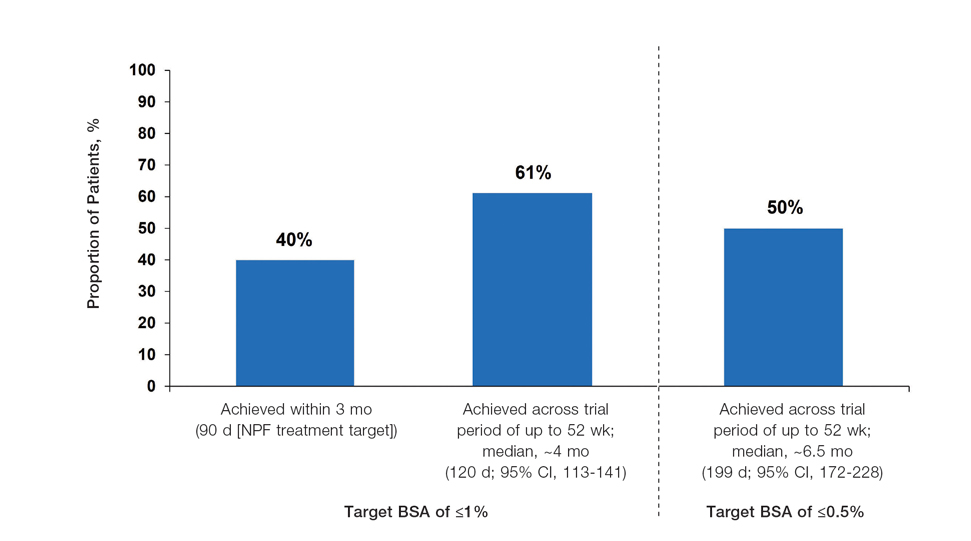
The findings of this analysis based on pooled data from a large cohort of patients demonstrate that a high proportion of patients can achieve or exceed recommended treatment targets with tapinarof cream 1% QD and maintain improvements long-term. The NPF-recommended treatment target of 1% or lower BSA affected within approximately 3 months (90 days) of treatment was achieved by 40% of tapinarof-treated patients. In addition, 1% or lower BSA affected at any time during the trials was achieved by 61% of patients (median, approximately 4 months). The analyses also indicated that PASI total scores of 3 or lower and 2 or lower were achieved by 75% and 67% of tapinarof-treated patients, respectively, within 2 to 3 months.
These findings support the previously reported efficacy of tapinarof cream, including high rates of complete disease clearance (40.9% [312/763]), durable response following treatment interruption, an off-therapy remittive effect of approximately 4 months, and good disease control on therapy with no evidence of tachyphylaxis.17,18
CONCLUSION
Taken together with previously reported tapinarof efficacy and safety results, our findings demonstrate that a high proportion of patients treated with tapinarof cream as monotherapy can achieve aggressive treatment targets set by both US and European guidelines developed for systemic and biologic therapies. Tapinarof cream 1% QD is an effective topical treatment option for patients with plaque psoriasis that has been approved without restrictions relating to severity or extent of disease treated, duration of use, or application sites, including application to sensitive and intertriginous skin.
Acknowledgments—Editorial and medical writing support under the guidance of the authors was provided by Melanie Govender, MSc (Med), ApotheCom (United Kingdom), and was funded by Dermavant Sciences, Inc, in accordance with Good Publication Practice (GPP) guidelines.
- Armstrong AW, Mehta MD, Schupp CW, et al. Psoriasis prevalence in adults in the United States. JAMA Dermatol. 2021;157:940-946.
- Parisi R, Iskandar IYK, Kontopantelis E, et al. National, regional, and worldwide epidemiology of psoriasis: systematic analysis and modelling study. BMJ. 2020;369:m1590.
- Pilon D, Teeple A, Zhdanava M, et al. The economic burden of psoriasis with high comorbidity among privately insured patients in the United States. J Med Econ. 2019;22:196-203.
- Singh S, Taylor C, Kornmehl H, et al. Psoriasis and suicidality: a systematic review and meta-analysis. J Am Acad Dermatol. 2017;77:425-440.e2.
- Feldman SR, Goffe B, Rice G, et al. The challenge of managing psoriasis: unmet medical needs and stakeholder perspectives. Am Health Drug Benefits. 2016;9:504-513.
- Ford JA, Solomon DH. Challenges in implementing treat-to-target strategies in rheumatology. Rheum Dis Clin North Am. 2019;45:101-112.
- Sitbon O, Galiè N. Treat-to-target strategies in pulmonary arterial hypertension: the importance of using multiple goals. Eur Respir Rev. 2010;19:272-278.
- Smolen JS, Aletaha D, Bijlsma JW, et al. Treating rheumatoid arthritis to target: recommendations of an international task force. Ann Rheum Dis. 2010;69:631-637.
- Wangnoo SK, Sethi B, Sahay RK, et al. Treat-to-target trials in diabetes. Indian J Endocrinol Metab. 2014;18:166-174.
- Armstrong AW, Siegel MP, Bagel J, et al. From the Medical Board of the National Psoriasis Foundation: treatment targets for plaque psoriasis. J Am Acad Dermatol. 2017;76:290-298.
- Pathirana D, Ormerod AD, Saiag P, et al. European S3-guidelines on the systemic treatment of psoriasis vulgaris. J Eur Acad Dermatol Venereol. 2009;23(Suppl 2):1-70.
- Strober BE, van der Walt JM, Armstrong AW, et al. Clinical goals and barriers to effective psoriasis care. Dermatol Ther (Heidelb). 2019; 9:5-18.
- Bagel J, Gold LS. Combining topical psoriasis treatment to enhance systemic and phototherapy: a review of the literature. J Drugs Dermatol. 2017;16:1209-1222.
- Elmets CA, Korman NJ, Prater EF, et al. Joint AAD-NPF Guidelines of care for the management and treatment of psoriasis with topical therapy and alternative medicine modalities for psoriasis severity measures. J Am Acad Dermatol. 2021;84:432-470.
- Stein Gold LF. Topical therapies for psoriasis: improving management strategies and patient adherence. Semin Cutan Med Surg. 2016;35 (2 Suppl 2):S36-S44; quiz S45.
- VTAMA® (tapinarof) cream. Prescribing information. Dermavant Sciences; 2022. Accessed September 13, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/215272s000lbl.pdf
- Lebwohl MG, Stein Gold L, Strober B, et al. Phase 3 trials of tapinarof cream for plaque psoriasis. N Engl J Med. 2021;385:2219-2229 and supplementary appendix.
- Strober B, Stein Gold L, Bissonnette R, et al. One-year safety and efficacy of tapinarof cream for the treatment of plaque psoriasis: results from the PSOARING 3 trial. J Am Acad Dermatol. 2022;87:800-806.
- Clinical Review Report: Guselkumab (Tremfya) [Internet]. Canadian Agency for Drugs and Technologies in Health; 2018. Accessed September 13, 2024. https://www.ncbi.nlm.nih.gov/books/NBK534047/pdf/Bookshelf_NBK534047.pdf
Psoriasis is a chronic inflammatory disease affecting approximately 8 million adults in the United States and 2% of the global population.1,2 Psoriasis causes pain, itching, and disfigurement and is associated with a physical, psychological, and economic burden that substantially affects health-related quality of life.3-5
Setting treatment goals and treating to target are evidence-based approaches that have been successfully applied to several chronic diseases to improve patient outcomes, including diabetes, hypertension, and rheumatoid arthritis.6-9 Treat-to-target strategies generally set low disease activity (or remission) as an overall goal and seek to achieve this using available therapeutic options as necessary. Introduced following the availability of biologics and targeted systemic therapies, treat-to-target strategies generally provide guidance on expectations of treatment but not specific treatments, as personalized treatment decisions depend on an assessment of individual patients and consider clinical and demographic features as well as preferences for available therapeutic options. If targets are not achieved in the assigned time span, adjustments can be made to the treatment approach in close consultation with the patient. If the target is reached, follow-up visits can be scheduled to ensure improvement is maintained or to establish if more aggressive goals could be selected.
Treat-to-target strategies for the management of psoriasis developed by the National Psoriasis Foundation (NPF) Medical Board include reducing the extent of psoriasis to 1% or lower total body surface area (BSA) after 3 months of treatment.10 Treatment targets endorsed by the European Academy of Dermatology and Venereology (EADV) in guidelines on the use of systemic therapies in psoriasis include achieving a 75% or greater reduction in Psoriasis Area and Severity Index (PASI) score within 3 to 4 months of treatment.11
In clinical practice, many patients do not achieve these treatment targets, and topical treatments alone generally are insufficient in achieving treatment goals for psoriasis.12,13 Moreover, conventional topical treatments (eg, topical corticosteroids) used by most patients with psoriasis regardless of disease severity are associated with adverse events that can limit their use. Most topical corticosteroids have US Food and Drug Administration label restrictions relating to sites of application, duration and extent of use, and frequency of administration.14,15
Tapinarof cream 1% (VTAMA [Dermavant Sciences, Inc]) is a first-in-class topical nonsteroidal aryl hydrocarbon receptor agonist that was approved by the US Food and Drug Administration for the treatment of plaque psoriasis in adults16 and is being studied for the treatment of plaque psoriasis in children 2 years and older as well as for atopic dermatitis in adults and children 2 years and older. In PSOARING 1 (ClinicalTrials .gov identifier NCT03956355) and PSOARING 2 (NCT03983980)—identical 12-week pivotal phase 3 trials—monotherapy with tapinarof cream 1% once daily (QD) demonstrated statistically significant efficacy vs vehicle cream and was well tolerated in adults with mild to severe plaque psoriasis (Supplementary Figure S1).17 Lebwohl et al17 reported that significantly higher PASI75 responses were observed at week 12 with tapinarof cream vs vehicle in PSOARING 1 and PSOARING 2 (36% and 48% vs 10% and 7%, respectively; both P<.0001). A significantly higher PASI90 response of 19% and 21% at week 12 also was observed with tapinarof cream vs 2% and 3% with vehicle in PSOARING 1 and PSOARING 2, respectively (P=.0005 and P<.0001).17
In PSOARING 3 (NCT04053387)—the long-term extension trial (Supplementary Figure S1)—efficacy continued to improve or was maintained beyond the two 12-week trials, with improvements in total BSA affected and PASI scores for up to 52 weeks.18 Tapinarof cream 1% QD demonstrated positive, rapid, and durable outcomes in PSOARING 3, including high rates of complete disease clearance (Physician Global Assessment [PGA] score=0 [clear])(40.9% [312/763]), durability of response on treatment with no evidence of tachyphylaxis, and a remittive effect of approximately 4 months when off therapy (defined as maintenance of a PGA score of 0 [clear] or 1 [almost clear] after first achieving a PGA score of 0).18
Herein, we report absolute treatment targets for patients with plaque psoriasis who received tapinarof cream 1% QD in the PSOARING trials that are at least as stringent as the corresponding NPF and EADV targets of achieving a total BSA affected of 1% or lower or a PASI75 response within 3 to 4 months, respectively.
METHODS
Study Design
The pooled efficacy analyses included all patients with a baseline PGA score of 2 or higher (mild or worse) before treatment with tapinarof cream 1% QD in the PSOARING trials. This included patients who received tapinarof cream 1% in PSOARING 1 and PSOARING 2 who may or may not have continued into PSOARING 3, as well as those who received the vehicle in PSOARING 1 and PSOARING 2 who enrolled in PSOARING 3 and had a PGA score of 2 or higher before receiving tapinarof cream 1%.
Trial Participants
Full methods, including inclusion and exclusion criteria, for the PSOARING trials have been previously reported.17,18 Patients were aged 18 to 75 years and had chronic plaque psoriasis that was stable for at least 6 months before randomization; 3% to 20% total BSA affected (excluding the scalp, palms, fingernails, toenails, and soles); and a PGA score of 2 (mild), 3 (moderate), or 4 (severe) at baseline.
The clinical trials were conducted in compliance with the guidelines for Good Clinical Practice and the Declaration of Helsinki. Approval was obtained from local ethics committees or institutional review boards at each center. All patients provided written informed consent.
Trial Treatment
In PSOARING 1 and PSOARING 2, patients were randomized (2:1) to receive tapinarof cream 1% or vehicle QD for 12 weeks. In PSOARING 3 (the long-term extension trial), patients received up to 40 weeks of open-label tapinarof, followed by 4 weeks of follow-up off treatment. Patients received intermittent or continuous treatment with tapinarof cream 1% in PSOARING 3 based on PGA score: those entering the trial with a PGA score of 1 or higher received tapinarof cream 1% until complete disease clearance was achieved (defined as a PGA score of 0 [clear]). Those entering PSOARING 3 with or achieving a PGA score of 0 (clear) discontinued treatment and were observed for the duration of maintenance of a PGA score of 0 (clear) or 1 (almost clear) while off therapy (the protocol-defined “duration of remittive effect”). If disease worsening (defined as a PGA score 2 or higher) occurred, tapinarof cream 1% was restarted and continued until a PGA score of 0 (clear) was achieved. This pattern of treatment, discontinuation on achieving a PGA score of 0 (clear), and retreatment on disease worsening continued until the end of the trial. As a result, patients in PSOARING 3 could receive tapinarof cream 1% continuously or intermittently for 40 weeks.
Outcome Measures and Statistical Analyses
The assessment of total BSA affected by plaque psoriasis is an estimate of the total extent of disease as a percentage of total skin area. In the PSOARING trials, the skin surface of one hand (palm and digits) was assumed to be approximately equivalent to 1% BSA. The total BSA affected by psoriasis was evaluated from 0% to 100%, with greater total BSA affected being an indication of more extensive disease. The BSA efficacy outcomes used in these analyses were based post hoc on the proportion of patients who achieved a 1% or lower or 0.5% or lower total BSA affected.
Psoriasis Area and Severity Index scores assess both the severity and extent of psoriasis. A PASI score lower than 5 often is considered indicative of mild psoriasis, a score of 5 to 10 indicates moderate disease, and a score higher than 10 indicates severe disease.19 The maximum PASI score is 72. The PASI efficacy outcomes used in these analyses were based post hoc on the proportion of patients who achieved an absolute total PASI score of 3 or lower, 2 or lower, and 1 or lower.
Efficacy analyses were based on pooled data for all patients in the PSOARING trials who had a PGA score of 2 to 4 (mild to severe) before treatment with tapinarof cream 1% in the intention-to-treat population using observed cases. Time-to-target analyses were based on Kaplan-Meier (KM) estimates using observed cases.
Safety analyses included the incidence and frequency of adverse events and were based on all patients who received tapinarof cream 1% in the PSOARING trials.
RESULTS
Baseline Patient Demographics and Disease Characteristics
The pooled efficacy analyses included 915 eligible patients (Table). At baseline, the mean (SD) age was 50.2 (13.25) years, 58.7% were male, the mean (SD) weight was 92.2 (23.67) kg, and the mean (SD) body mass index was 31.6 (7.53) kg/m2. The percentage of patients with a PGA score of 2 (mild), 3 (moderate), or 4 (severe) was 13.9%, 78.1%, and 8.0%, respectively. The mean (SD) PASI score was 8.7 (4.23) and mean (SD) total BSA affected was 7.8% (4.98).
Efficacy
Achievement of BSA-Affected Targets—



Achievement of Absolute PASI Targets—Across the total trial period (up to 52 weeks), an absolute total PASI score of 3 or lower was achieved by 75% of patients (686/915), with a median time to achieve this of 2 months (KM estimate: 58 days [95% CI, 57-63]); approximately 67% of patients (612/915) achieved a total PASI score of 2 or lower, with a median time to achieve of 3 months (KM estimate: 87 days [95% CI, 85-110])(Figure 2; Supplementary Figures S3a and S3b). A PASI score of 1 or lower was achieved by approximately 50% of patients (460/915), with a median time to achieve of approximately 6 months (KM estimate: 185 days [95% CI, 169-218])(Figure 2, Supplementary Figure S3c).
Illustrative Case—Case photography showing the clinical response in a 63-year-old man with moderate plaque psoriasis in PSOARING 2 is shown in Figure 3. After 12 weeks of treatment with tapinarof cream 1% QD, the patient achieved all primary and secondary efficacy end points. In addition to achieving the regulatory end point of a PGA score of 0 (clear) or 1 (almost clear) and a decrease from baseline of at least 2 points, achievement of 0% total BSA affected and a total PASI score of 0 at week 12 exceeded the NPF and EADV consensus treatment targets.10,11 Targets were achieved as early as week 4, with a total BSA affected of 0.5% or lower and a total PASI score of 1 or lower, illustrated by marked skin clearing and only faint residual erythema that completely resolved at week 12, with the absence of postinflammatory hyperpigmentation.
Safety
Safety data for the PSOARING trials have been previously reported.17,18 The most common treatment-emergent adverse events were folliculitis, contact dermatitis, upper respiratory tract infection, and nasopharyngitis. Treatment-emergent adverse events generally were mild or moderate in severity and did not lead to trial discontinuation.17,18

COMMENT
Treat-to-target management approaches aim to improve patient outcomes by striving to achieve optimal goals. The treat-to-target approach supports shared decision-making between clinicians and patients based on common expectations of what constitutes treatment success.
The findings of this analysis based on pooled data from a large cohort of patients demonstrate that a high proportion of patients can achieve or exceed recommended treatment targets with tapinarof cream 1% QD and maintain improvements long-term. The NPF-recommended treatment target of 1% or lower BSA affected within approximately 3 months (90 days) of treatment was achieved by 40% of tapinarof-treated patients. In addition, 1% or lower BSA affected at any time during the trials was achieved by 61% of patients (median, approximately 4 months). The analyses also indicated that PASI total scores of 3 or lower and 2 or lower were achieved by 75% and 67% of tapinarof-treated patients, respectively, within 2 to 3 months.
These findings support the previously reported efficacy of tapinarof cream, including high rates of complete disease clearance (40.9% [312/763]), durable response following treatment interruption, an off-therapy remittive effect of approximately 4 months, and good disease control on therapy with no evidence of tachyphylaxis.17,18
CONCLUSION
Taken together with previously reported tapinarof efficacy and safety results, our findings demonstrate that a high proportion of patients treated with tapinarof cream as monotherapy can achieve aggressive treatment targets set by both US and European guidelines developed for systemic and biologic therapies. Tapinarof cream 1% QD is an effective topical treatment option for patients with plaque psoriasis that has been approved without restrictions relating to severity or extent of disease treated, duration of use, or application sites, including application to sensitive and intertriginous skin.
Acknowledgments—Editorial and medical writing support under the guidance of the authors was provided by Melanie Govender, MSc (Med), ApotheCom (United Kingdom), and was funded by Dermavant Sciences, Inc, in accordance with Good Publication Practice (GPP) guidelines.
Psoriasis is a chronic inflammatory disease affecting approximately 8 million adults in the United States and 2% of the global population.1,2 Psoriasis causes pain, itching, and disfigurement and is associated with a physical, psychological, and economic burden that substantially affects health-related quality of life.3-5
Setting treatment goals and treating to target are evidence-based approaches that have been successfully applied to several chronic diseases to improve patient outcomes, including diabetes, hypertension, and rheumatoid arthritis.6-9 Treat-to-target strategies generally set low disease activity (or remission) as an overall goal and seek to achieve this using available therapeutic options as necessary. Introduced following the availability of biologics and targeted systemic therapies, treat-to-target strategies generally provide guidance on expectations of treatment but not specific treatments, as personalized treatment decisions depend on an assessment of individual patients and consider clinical and demographic features as well as preferences for available therapeutic options. If targets are not achieved in the assigned time span, adjustments can be made to the treatment approach in close consultation with the patient. If the target is reached, follow-up visits can be scheduled to ensure improvement is maintained or to establish if more aggressive goals could be selected.
Treat-to-target strategies for the management of psoriasis developed by the National Psoriasis Foundation (NPF) Medical Board include reducing the extent of psoriasis to 1% or lower total body surface area (BSA) after 3 months of treatment.10 Treatment targets endorsed by the European Academy of Dermatology and Venereology (EADV) in guidelines on the use of systemic therapies in psoriasis include achieving a 75% or greater reduction in Psoriasis Area and Severity Index (PASI) score within 3 to 4 months of treatment.11
In clinical practice, many patients do not achieve these treatment targets, and topical treatments alone generally are insufficient in achieving treatment goals for psoriasis.12,13 Moreover, conventional topical treatments (eg, topical corticosteroids) used by most patients with psoriasis regardless of disease severity are associated with adverse events that can limit their use. Most topical corticosteroids have US Food and Drug Administration label restrictions relating to sites of application, duration and extent of use, and frequency of administration.14,15
Tapinarof cream 1% (VTAMA [Dermavant Sciences, Inc]) is a first-in-class topical nonsteroidal aryl hydrocarbon receptor agonist that was approved by the US Food and Drug Administration for the treatment of plaque psoriasis in adults16 and is being studied for the treatment of plaque psoriasis in children 2 years and older as well as for atopic dermatitis in adults and children 2 years and older. In PSOARING 1 (ClinicalTrials .gov identifier NCT03956355) and PSOARING 2 (NCT03983980)—identical 12-week pivotal phase 3 trials—monotherapy with tapinarof cream 1% once daily (QD) demonstrated statistically significant efficacy vs vehicle cream and was well tolerated in adults with mild to severe plaque psoriasis (Supplementary Figure S1).17 Lebwohl et al17 reported that significantly higher PASI75 responses were observed at week 12 with tapinarof cream vs vehicle in PSOARING 1 and PSOARING 2 (36% and 48% vs 10% and 7%, respectively; both P<.0001). A significantly higher PASI90 response of 19% and 21% at week 12 also was observed with tapinarof cream vs 2% and 3% with vehicle in PSOARING 1 and PSOARING 2, respectively (P=.0005 and P<.0001).17
In PSOARING 3 (NCT04053387)—the long-term extension trial (Supplementary Figure S1)—efficacy continued to improve or was maintained beyond the two 12-week trials, with improvements in total BSA affected and PASI scores for up to 52 weeks.18 Tapinarof cream 1% QD demonstrated positive, rapid, and durable outcomes in PSOARING 3, including high rates of complete disease clearance (Physician Global Assessment [PGA] score=0 [clear])(40.9% [312/763]), durability of response on treatment with no evidence of tachyphylaxis, and a remittive effect of approximately 4 months when off therapy (defined as maintenance of a PGA score of 0 [clear] or 1 [almost clear] after first achieving a PGA score of 0).18
Herein, we report absolute treatment targets for patients with plaque psoriasis who received tapinarof cream 1% QD in the PSOARING trials that are at least as stringent as the corresponding NPF and EADV targets of achieving a total BSA affected of 1% or lower or a PASI75 response within 3 to 4 months, respectively.
METHODS
Study Design
The pooled efficacy analyses included all patients with a baseline PGA score of 2 or higher (mild or worse) before treatment with tapinarof cream 1% QD in the PSOARING trials. This included patients who received tapinarof cream 1% in PSOARING 1 and PSOARING 2 who may or may not have continued into PSOARING 3, as well as those who received the vehicle in PSOARING 1 and PSOARING 2 who enrolled in PSOARING 3 and had a PGA score of 2 or higher before receiving tapinarof cream 1%.
Trial Participants
Full methods, including inclusion and exclusion criteria, for the PSOARING trials have been previously reported.17,18 Patients were aged 18 to 75 years and had chronic plaque psoriasis that was stable for at least 6 months before randomization; 3% to 20% total BSA affected (excluding the scalp, palms, fingernails, toenails, and soles); and a PGA score of 2 (mild), 3 (moderate), or 4 (severe) at baseline.
The clinical trials were conducted in compliance with the guidelines for Good Clinical Practice and the Declaration of Helsinki. Approval was obtained from local ethics committees or institutional review boards at each center. All patients provided written informed consent.
Trial Treatment
In PSOARING 1 and PSOARING 2, patients were randomized (2:1) to receive tapinarof cream 1% or vehicle QD for 12 weeks. In PSOARING 3 (the long-term extension trial), patients received up to 40 weeks of open-label tapinarof, followed by 4 weeks of follow-up off treatment. Patients received intermittent or continuous treatment with tapinarof cream 1% in PSOARING 3 based on PGA score: those entering the trial with a PGA score of 1 or higher received tapinarof cream 1% until complete disease clearance was achieved (defined as a PGA score of 0 [clear]). Those entering PSOARING 3 with or achieving a PGA score of 0 (clear) discontinued treatment and were observed for the duration of maintenance of a PGA score of 0 (clear) or 1 (almost clear) while off therapy (the protocol-defined “duration of remittive effect”). If disease worsening (defined as a PGA score 2 or higher) occurred, tapinarof cream 1% was restarted and continued until a PGA score of 0 (clear) was achieved. This pattern of treatment, discontinuation on achieving a PGA score of 0 (clear), and retreatment on disease worsening continued until the end of the trial. As a result, patients in PSOARING 3 could receive tapinarof cream 1% continuously or intermittently for 40 weeks.
Outcome Measures and Statistical Analyses
The assessment of total BSA affected by plaque psoriasis is an estimate of the total extent of disease as a percentage of total skin area. In the PSOARING trials, the skin surface of one hand (palm and digits) was assumed to be approximately equivalent to 1% BSA. The total BSA affected by psoriasis was evaluated from 0% to 100%, with greater total BSA affected being an indication of more extensive disease. The BSA efficacy outcomes used in these analyses were based post hoc on the proportion of patients who achieved a 1% or lower or 0.5% or lower total BSA affected.
Psoriasis Area and Severity Index scores assess both the severity and extent of psoriasis. A PASI score lower than 5 often is considered indicative of mild psoriasis, a score of 5 to 10 indicates moderate disease, and a score higher than 10 indicates severe disease.19 The maximum PASI score is 72. The PASI efficacy outcomes used in these analyses were based post hoc on the proportion of patients who achieved an absolute total PASI score of 3 or lower, 2 or lower, and 1 or lower.
Efficacy analyses were based on pooled data for all patients in the PSOARING trials who had a PGA score of 2 to 4 (mild to severe) before treatment with tapinarof cream 1% in the intention-to-treat population using observed cases. Time-to-target analyses were based on Kaplan-Meier (KM) estimates using observed cases.
Safety analyses included the incidence and frequency of adverse events and were based on all patients who received tapinarof cream 1% in the PSOARING trials.
RESULTS
Baseline Patient Demographics and Disease Characteristics
The pooled efficacy analyses included 915 eligible patients (Table). At baseline, the mean (SD) age was 50.2 (13.25) years, 58.7% were male, the mean (SD) weight was 92.2 (23.67) kg, and the mean (SD) body mass index was 31.6 (7.53) kg/m2. The percentage of patients with a PGA score of 2 (mild), 3 (moderate), or 4 (severe) was 13.9%, 78.1%, and 8.0%, respectively. The mean (SD) PASI score was 8.7 (4.23) and mean (SD) total BSA affected was 7.8% (4.98).
Efficacy
Achievement of BSA-Affected Targets—



Achievement of Absolute PASI Targets—Across the total trial period (up to 52 weeks), an absolute total PASI score of 3 or lower was achieved by 75% of patients (686/915), with a median time to achieve this of 2 months (KM estimate: 58 days [95% CI, 57-63]); approximately 67% of patients (612/915) achieved a total PASI score of 2 or lower, with a median time to achieve of 3 months (KM estimate: 87 days [95% CI, 85-110])(Figure 2; Supplementary Figures S3a and S3b). A PASI score of 1 or lower was achieved by approximately 50% of patients (460/915), with a median time to achieve of approximately 6 months (KM estimate: 185 days [95% CI, 169-218])(Figure 2, Supplementary Figure S3c).
Illustrative Case—Case photography showing the clinical response in a 63-year-old man with moderate plaque psoriasis in PSOARING 2 is shown in Figure 3. After 12 weeks of treatment with tapinarof cream 1% QD, the patient achieved all primary and secondary efficacy end points. In addition to achieving the regulatory end point of a PGA score of 0 (clear) or 1 (almost clear) and a decrease from baseline of at least 2 points, achievement of 0% total BSA affected and a total PASI score of 0 at week 12 exceeded the NPF and EADV consensus treatment targets.10,11 Targets were achieved as early as week 4, with a total BSA affected of 0.5% or lower and a total PASI score of 1 or lower, illustrated by marked skin clearing and only faint residual erythema that completely resolved at week 12, with the absence of postinflammatory hyperpigmentation.
Safety
Safety data for the PSOARING trials have been previously reported.17,18 The most common treatment-emergent adverse events were folliculitis, contact dermatitis, upper respiratory tract infection, and nasopharyngitis. Treatment-emergent adverse events generally were mild or moderate in severity and did not lead to trial discontinuation.17,18

COMMENT
Treat-to-target management approaches aim to improve patient outcomes by striving to achieve optimal goals. The treat-to-target approach supports shared decision-making between clinicians and patients based on common expectations of what constitutes treatment success.
The findings of this analysis based on pooled data from a large cohort of patients demonstrate that a high proportion of patients can achieve or exceed recommended treatment targets with tapinarof cream 1% QD and maintain improvements long-term. The NPF-recommended treatment target of 1% or lower BSA affected within approximately 3 months (90 days) of treatment was achieved by 40% of tapinarof-treated patients. In addition, 1% or lower BSA affected at any time during the trials was achieved by 61% of patients (median, approximately 4 months). The analyses also indicated that PASI total scores of 3 or lower and 2 or lower were achieved by 75% and 67% of tapinarof-treated patients, respectively, within 2 to 3 months.
These findings support the previously reported efficacy of tapinarof cream, including high rates of complete disease clearance (40.9% [312/763]), durable response following treatment interruption, an off-therapy remittive effect of approximately 4 months, and good disease control on therapy with no evidence of tachyphylaxis.17,18
CONCLUSION
Taken together with previously reported tapinarof efficacy and safety results, our findings demonstrate that a high proportion of patients treated with tapinarof cream as monotherapy can achieve aggressive treatment targets set by both US and European guidelines developed for systemic and biologic therapies. Tapinarof cream 1% QD is an effective topical treatment option for patients with plaque psoriasis that has been approved without restrictions relating to severity or extent of disease treated, duration of use, or application sites, including application to sensitive and intertriginous skin.
Acknowledgments—Editorial and medical writing support under the guidance of the authors was provided by Melanie Govender, MSc (Med), ApotheCom (United Kingdom), and was funded by Dermavant Sciences, Inc, in accordance with Good Publication Practice (GPP) guidelines.
- Armstrong AW, Mehta MD, Schupp CW, et al. Psoriasis prevalence in adults in the United States. JAMA Dermatol. 2021;157:940-946.
- Parisi R, Iskandar IYK, Kontopantelis E, et al. National, regional, and worldwide epidemiology of psoriasis: systematic analysis and modelling study. BMJ. 2020;369:m1590.
- Pilon D, Teeple A, Zhdanava M, et al. The economic burden of psoriasis with high comorbidity among privately insured patients in the United States. J Med Econ. 2019;22:196-203.
- Singh S, Taylor C, Kornmehl H, et al. Psoriasis and suicidality: a systematic review and meta-analysis. J Am Acad Dermatol. 2017;77:425-440.e2.
- Feldman SR, Goffe B, Rice G, et al. The challenge of managing psoriasis: unmet medical needs and stakeholder perspectives. Am Health Drug Benefits. 2016;9:504-513.
- Ford JA, Solomon DH. Challenges in implementing treat-to-target strategies in rheumatology. Rheum Dis Clin North Am. 2019;45:101-112.
- Sitbon O, Galiè N. Treat-to-target strategies in pulmonary arterial hypertension: the importance of using multiple goals. Eur Respir Rev. 2010;19:272-278.
- Smolen JS, Aletaha D, Bijlsma JW, et al. Treating rheumatoid arthritis to target: recommendations of an international task force. Ann Rheum Dis. 2010;69:631-637.
- Wangnoo SK, Sethi B, Sahay RK, et al. Treat-to-target trials in diabetes. Indian J Endocrinol Metab. 2014;18:166-174.
- Armstrong AW, Siegel MP, Bagel J, et al. From the Medical Board of the National Psoriasis Foundation: treatment targets for plaque psoriasis. J Am Acad Dermatol. 2017;76:290-298.
- Pathirana D, Ormerod AD, Saiag P, et al. European S3-guidelines on the systemic treatment of psoriasis vulgaris. J Eur Acad Dermatol Venereol. 2009;23(Suppl 2):1-70.
- Strober BE, van der Walt JM, Armstrong AW, et al. Clinical goals and barriers to effective psoriasis care. Dermatol Ther (Heidelb). 2019; 9:5-18.
- Bagel J, Gold LS. Combining topical psoriasis treatment to enhance systemic and phototherapy: a review of the literature. J Drugs Dermatol. 2017;16:1209-1222.
- Elmets CA, Korman NJ, Prater EF, et al. Joint AAD-NPF Guidelines of care for the management and treatment of psoriasis with topical therapy and alternative medicine modalities for psoriasis severity measures. J Am Acad Dermatol. 2021;84:432-470.
- Stein Gold LF. Topical therapies for psoriasis: improving management strategies and patient adherence. Semin Cutan Med Surg. 2016;35 (2 Suppl 2):S36-S44; quiz S45.
- VTAMA® (tapinarof) cream. Prescribing information. Dermavant Sciences; 2022. Accessed September 13, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/215272s000lbl.pdf
- Lebwohl MG, Stein Gold L, Strober B, et al. Phase 3 trials of tapinarof cream for plaque psoriasis. N Engl J Med. 2021;385:2219-2229 and supplementary appendix.
- Strober B, Stein Gold L, Bissonnette R, et al. One-year safety and efficacy of tapinarof cream for the treatment of plaque psoriasis: results from the PSOARING 3 trial. J Am Acad Dermatol. 2022;87:800-806.
- Clinical Review Report: Guselkumab (Tremfya) [Internet]. Canadian Agency for Drugs and Technologies in Health; 2018. Accessed September 13, 2024. https://www.ncbi.nlm.nih.gov/books/NBK534047/pdf/Bookshelf_NBK534047.pdf
- Armstrong AW, Mehta MD, Schupp CW, et al. Psoriasis prevalence in adults in the United States. JAMA Dermatol. 2021;157:940-946.
- Parisi R, Iskandar IYK, Kontopantelis E, et al. National, regional, and worldwide epidemiology of psoriasis: systematic analysis and modelling study. BMJ. 2020;369:m1590.
- Pilon D, Teeple A, Zhdanava M, et al. The economic burden of psoriasis with high comorbidity among privately insured patients in the United States. J Med Econ. 2019;22:196-203.
- Singh S, Taylor C, Kornmehl H, et al. Psoriasis and suicidality: a systematic review and meta-analysis. J Am Acad Dermatol. 2017;77:425-440.e2.
- Feldman SR, Goffe B, Rice G, et al. The challenge of managing psoriasis: unmet medical needs and stakeholder perspectives. Am Health Drug Benefits. 2016;9:504-513.
- Ford JA, Solomon DH. Challenges in implementing treat-to-target strategies in rheumatology. Rheum Dis Clin North Am. 2019;45:101-112.
- Sitbon O, Galiè N. Treat-to-target strategies in pulmonary arterial hypertension: the importance of using multiple goals. Eur Respir Rev. 2010;19:272-278.
- Smolen JS, Aletaha D, Bijlsma JW, et al. Treating rheumatoid arthritis to target: recommendations of an international task force. Ann Rheum Dis. 2010;69:631-637.
- Wangnoo SK, Sethi B, Sahay RK, et al. Treat-to-target trials in diabetes. Indian J Endocrinol Metab. 2014;18:166-174.
- Armstrong AW, Siegel MP, Bagel J, et al. From the Medical Board of the National Psoriasis Foundation: treatment targets for plaque psoriasis. J Am Acad Dermatol. 2017;76:290-298.
- Pathirana D, Ormerod AD, Saiag P, et al. European S3-guidelines on the systemic treatment of psoriasis vulgaris. J Eur Acad Dermatol Venereol. 2009;23(Suppl 2):1-70.
- Strober BE, van der Walt JM, Armstrong AW, et al. Clinical goals and barriers to effective psoriasis care. Dermatol Ther (Heidelb). 2019; 9:5-18.
- Bagel J, Gold LS. Combining topical psoriasis treatment to enhance systemic and phototherapy: a review of the literature. J Drugs Dermatol. 2017;16:1209-1222.
- Elmets CA, Korman NJ, Prater EF, et al. Joint AAD-NPF Guidelines of care for the management and treatment of psoriasis with topical therapy and alternative medicine modalities for psoriasis severity measures. J Am Acad Dermatol. 2021;84:432-470.
- Stein Gold LF. Topical therapies for psoriasis: improving management strategies and patient adherence. Semin Cutan Med Surg. 2016;35 (2 Suppl 2):S36-S44; quiz S45.
- VTAMA® (tapinarof) cream. Prescribing information. Dermavant Sciences; 2022. Accessed September 13, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/215272s000lbl.pdf
- Lebwohl MG, Stein Gold L, Strober B, et al. Phase 3 trials of tapinarof cream for plaque psoriasis. N Engl J Med. 2021;385:2219-2229 and supplementary appendix.
- Strober B, Stein Gold L, Bissonnette R, et al. One-year safety and efficacy of tapinarof cream for the treatment of plaque psoriasis: results from the PSOARING 3 trial. J Am Acad Dermatol. 2022;87:800-806.
- Clinical Review Report: Guselkumab (Tremfya) [Internet]. Canadian Agency for Drugs and Technologies in Health; 2018. Accessed September 13, 2024. https://www.ncbi.nlm.nih.gov/books/NBK534047/pdf/Bookshelf_NBK534047.pdf
Practice Points
- In clinical practice, many patients with psoriasis do not achieve treatment targets set forth by the National Psoriasis Foundation and the European Academy of Dermatology and Venereology, and topical treatments alone generally are insufficient in achieving treatment goals for psoriasis.
- Tapinarof cream 1% is a nonsteroidal aryl hydrocarbon receptor agonist approved by the US Food and Drug Administration for the treatment of plaque psoriasis in adults; it also is being studied for the treatment of plaque psoriasis in children 2 years and older.
- Tapinarof cream 1% is an effective topical treatment option for patients with plaque psoriasis of any severity, with no limitations on treatment duration, total extent of use, or application sites, including intertriginous skin and sensitive areas.
Study Supports Efficacy of Home-Based Phototherapy for Psoriasis
TOPLINE:
study.
METHODOLOGY:
- The pragmatic, investigator-initiated, open-label, noninferiority, randomized trial compared the effectiveness of 12 weeks of treatment with narrow-band ultraviolet B phototherapy administered at home (n = 393) vs at the doctor’s office (n = 390).
- Overall, 783 patients with plaque or guttate psoriasis (mean age, 48 years; 48% women) were enrolled at 42 academic and private clinical dermatology practices in the United States from March 1, 2019, to December 4, 2023, and were followed up through June 2024. At baseline, the mean Physician Global Assessment (PGA) and the mean Dermatology Life Quality Index (DLQI) scores were 2.7 and 12.2, respectively.
- The two co-primary endpoints were a PGA score ≤ 1 indicating clear or almost clear skin and a DLQI score ≤ 5.
TAKEAWAY:
- At 12 weeks, a PGA score ≤ 1 was achieved in 32.8% of patients using home-based phototherapy and in 25.6% of those who received office-based phototherapy (P < .001).
- At 12 weeks, a DLQI score ≤ 5 was achieved in 52.4% and 33.6% of home- and office-treated patients, respectively (P < .001).
- Similar benefits were seen across all Fitzpatrick skin types.
- A higher percentage of patients were adherent to home-based (51.4%) vs office-based (15.9%) phototherapy (P < .001).
IN PRACTICE:
“These data support the use of home phototherapy as a first-line treatment option for psoriasis,” and “efforts are needed to make home and office phototherapy more available to patients,” said the study’s lead author.
SOURCE:
Joel M. Gelfand, MD, director of the Psoriasis and Phototherapy Treatment Center at the University of Pennsylvania, Philadelphia, presented the findings at the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis meeting during the annual meeting of the European Academy of Dermatology and Venereology, with simultaneous publication in JAMA Dermatology.
LIMITATIONS:
This was an open-label trial and because of its pragmatic design, outcome data were missing. The cost of the home-based phototherapy equipment used in the study was $6040.88, which was mostly covered by Medicare, but direct costs to patients may have varied depending on their insurance plan.
DISCLOSURES:
The Patient-Centered Outcomes Research Institute funded the study. Daavlin provided and shipped machines for home-based phototherapy to patients at no cost. Dr. Gelfand disclosed serving as a consultant for AbbVie, Artax, Bristol-Myers Squibb, Boehringer Ingelheim, Celldex, and other companies. The full list of author disclosures can be found in the published study.
A version of this article first appeared on Medscape.com.
TOPLINE:
study.
METHODOLOGY:
- The pragmatic, investigator-initiated, open-label, noninferiority, randomized trial compared the effectiveness of 12 weeks of treatment with narrow-band ultraviolet B phototherapy administered at home (n = 393) vs at the doctor’s office (n = 390).
- Overall, 783 patients with plaque or guttate psoriasis (mean age, 48 years; 48% women) were enrolled at 42 academic and private clinical dermatology practices in the United States from March 1, 2019, to December 4, 2023, and were followed up through June 2024. At baseline, the mean Physician Global Assessment (PGA) and the mean Dermatology Life Quality Index (DLQI) scores were 2.7 and 12.2, respectively.
- The two co-primary endpoints were a PGA score ≤ 1 indicating clear or almost clear skin and a DLQI score ≤ 5.
TAKEAWAY:
- At 12 weeks, a PGA score ≤ 1 was achieved in 32.8% of patients using home-based phototherapy and in 25.6% of those who received office-based phototherapy (P < .001).
- At 12 weeks, a DLQI score ≤ 5 was achieved in 52.4% and 33.6% of home- and office-treated patients, respectively (P < .001).
- Similar benefits were seen across all Fitzpatrick skin types.
- A higher percentage of patients were adherent to home-based (51.4%) vs office-based (15.9%) phototherapy (P < .001).
IN PRACTICE:
“These data support the use of home phototherapy as a first-line treatment option for psoriasis,” and “efforts are needed to make home and office phototherapy more available to patients,” said the study’s lead author.
SOURCE:
Joel M. Gelfand, MD, director of the Psoriasis and Phototherapy Treatment Center at the University of Pennsylvania, Philadelphia, presented the findings at the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis meeting during the annual meeting of the European Academy of Dermatology and Venereology, with simultaneous publication in JAMA Dermatology.
LIMITATIONS:
This was an open-label trial and because of its pragmatic design, outcome data were missing. The cost of the home-based phototherapy equipment used in the study was $6040.88, which was mostly covered by Medicare, but direct costs to patients may have varied depending on their insurance plan.
DISCLOSURES:
The Patient-Centered Outcomes Research Institute funded the study. Daavlin provided and shipped machines for home-based phototherapy to patients at no cost. Dr. Gelfand disclosed serving as a consultant for AbbVie, Artax, Bristol-Myers Squibb, Boehringer Ingelheim, Celldex, and other companies. The full list of author disclosures can be found in the published study.
A version of this article first appeared on Medscape.com.
TOPLINE:
study.
METHODOLOGY:
- The pragmatic, investigator-initiated, open-label, noninferiority, randomized trial compared the effectiveness of 12 weeks of treatment with narrow-band ultraviolet B phototherapy administered at home (n = 393) vs at the doctor’s office (n = 390).
- Overall, 783 patients with plaque or guttate psoriasis (mean age, 48 years; 48% women) were enrolled at 42 academic and private clinical dermatology practices in the United States from March 1, 2019, to December 4, 2023, and were followed up through June 2024. At baseline, the mean Physician Global Assessment (PGA) and the mean Dermatology Life Quality Index (DLQI) scores were 2.7 and 12.2, respectively.
- The two co-primary endpoints were a PGA score ≤ 1 indicating clear or almost clear skin and a DLQI score ≤ 5.
TAKEAWAY:
- At 12 weeks, a PGA score ≤ 1 was achieved in 32.8% of patients using home-based phototherapy and in 25.6% of those who received office-based phototherapy (P < .001).
- At 12 weeks, a DLQI score ≤ 5 was achieved in 52.4% and 33.6% of home- and office-treated patients, respectively (P < .001).
- Similar benefits were seen across all Fitzpatrick skin types.
- A higher percentage of patients were adherent to home-based (51.4%) vs office-based (15.9%) phototherapy (P < .001).
IN PRACTICE:
“These data support the use of home phototherapy as a first-line treatment option for psoriasis,” and “efforts are needed to make home and office phototherapy more available to patients,” said the study’s lead author.
SOURCE:
Joel M. Gelfand, MD, director of the Psoriasis and Phototherapy Treatment Center at the University of Pennsylvania, Philadelphia, presented the findings at the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis meeting during the annual meeting of the European Academy of Dermatology and Venereology, with simultaneous publication in JAMA Dermatology.
LIMITATIONS:
This was an open-label trial and because of its pragmatic design, outcome data were missing. The cost of the home-based phototherapy equipment used in the study was $6040.88, which was mostly covered by Medicare, but direct costs to patients may have varied depending on their insurance plan.
DISCLOSURES:
The Patient-Centered Outcomes Research Institute funded the study. Daavlin provided and shipped machines for home-based phototherapy to patients at no cost. Dr. Gelfand disclosed serving as a consultant for AbbVie, Artax, Bristol-Myers Squibb, Boehringer Ingelheim, Celldex, and other companies. The full list of author disclosures can be found in the published study.
A version of this article first appeared on Medscape.com.
Bimekizumab Gains FDA Approval for Psoriatic Arthritis, Axial Spondyloarthritis
The Food and Drug Administration has approved bimekizumab-bkzx (Bimzelx; UCB) for adult patients with active psoriatic arthritis (PsA), active nonradiographic axial spondyloarthritis (nr-axSpA) with objective signs of inflammation, and active ankylosing spondylitis (AS).
The drug, an interleukin (IL)–17A and IL-17F inhibitor, was first approved in October 2023 for treatment of moderate to severe plaque psoriasis in adults who are candidates for systemic therapy or phototherapy.
“In psoriatic arthritis and across the spectrum of axSpA, clinical study results and real-world experience outside the US have highlighted that Bimzelx can help patients achieve high thresholds of clinical response that are rapid in onset and sustained up to 2 years,” said Emmanuel Caeymaex, executive vice president, head of patient impact, and chief commercial officer of UCB in a press release.
The recommended dosage of bimekizumab for adult patients with active PsA, nr-axSpA, or AS is 160 mg by subcutaneous injection every 4 weeks. For patients with PsA and coexistent moderate to severe plaque psoriasis, the dosage is the same as for patients with plaque psoriasis. The dosing for plaque psoriasis is to administer 320 mg (two 160-mg injections) by subcutaneous injection at weeks 0, 4, 8, 12, and 16, then every 8 weeks thereafter. For patients weighing ≥ 120 kg, consider a dose of 320 mg every 4 weeks after week 16.
PsA Clinical Trials
The approval for PsA was based on data from two phase 3 clinical trials, including 852 participants naive to biologics (BE OPTIMAL) and 400 participants with inadequate response to treatment with one or two tumor necrosis factor (TNF) inhibitors (BE COMPLETE). Both studies met their primary endpoint, 50% improvement in American College of Rheumatology response criteria (ACR50) at 16 weeks, as well as ranked secondary endpoints. Secondary endpoints included minimal disease activity (MDA) and Psoriasis Area and Severity Index 100 (complete skin clearance) at week 16.
At 16 weeks:
- About 44% of both the biologic-naive (189 of 431) and TNF inhibitor–resistant (116 of 267) groups receiving bimekizumab achieved ACR50 response, compared with 10% (28 of 281) and 7% (9 of 133) receiving placebo, respectively.
- About 45% of all patients treated with bimekizumab achieved MDA.
- Nearly 60% of TNF inhibitor–resistant patients had complete skin clearance.
These responses generally were sustained for 1 year. The most common adverse reactions are upper respiratory tract infections, oral candidiasis, headache, diarrhea, and urinary tract infection.
NR-axSpA and AS Clinical Trials
The approval for active nr-axSpA and active AS was based on data from two clinical studies, BE MOBILE 1 (nr-axSpA) and BE MOBILE 2 (AS). Both studies met their primary endpoint, 40% improvement in Assessment of Spondyloarthritis International Society response criteria (ASAS40) at 16 weeks.
Key findings included:
- In nr-axSpA patients, 47.7% (61 of 128) receiving bimekizumab achieved ASAS40 at week 16, compared with 21.4% (27 of 126) receiving placebo.
- In AS patients, 44.8% (99 of 221) in the bimekizumab group achieved ASAS40 response at week 16 vs 22.5% (25 of 111) receiving placebo.
- At 1 year in both groups, 60% treated with bimekizumab achieved an Ankylosing Spondylitis Disease Activity Score < 2.1.
In nr-axSpA, the most common adverse reactions are upper respiratory tract infections, oral candidiasis, headache, diarrhea, cough, fatigue, musculoskeletal pain, myalgia, tonsillitis, increase in transaminase, and urinary tract infection. In AS, the most common adverse reactions are upper respiratory tract infections, oral candidiasis, headache, diarrhea, injection-site pain, rash, and vulvovaginal mycotic infection.
Bimekizumab was approved by the European Commission for the same rheumatologic indications in June 2023.
Bimekizumab is currently available to eligible patients in the United States, according to the press release.
A version of this article first appeared on Medscape.com.
The Food and Drug Administration has approved bimekizumab-bkzx (Bimzelx; UCB) for adult patients with active psoriatic arthritis (PsA), active nonradiographic axial spondyloarthritis (nr-axSpA) with objective signs of inflammation, and active ankylosing spondylitis (AS).
The drug, an interleukin (IL)–17A and IL-17F inhibitor, was first approved in October 2023 for treatment of moderate to severe plaque psoriasis in adults who are candidates for systemic therapy or phototherapy.
“In psoriatic arthritis and across the spectrum of axSpA, clinical study results and real-world experience outside the US have highlighted that Bimzelx can help patients achieve high thresholds of clinical response that are rapid in onset and sustained up to 2 years,” said Emmanuel Caeymaex, executive vice president, head of patient impact, and chief commercial officer of UCB in a press release.
The recommended dosage of bimekizumab for adult patients with active PsA, nr-axSpA, or AS is 160 mg by subcutaneous injection every 4 weeks. For patients with PsA and coexistent moderate to severe plaque psoriasis, the dosage is the same as for patients with plaque psoriasis. The dosing for plaque psoriasis is to administer 320 mg (two 160-mg injections) by subcutaneous injection at weeks 0, 4, 8, 12, and 16, then every 8 weeks thereafter. For patients weighing ≥ 120 kg, consider a dose of 320 mg every 4 weeks after week 16.
PsA Clinical Trials
The approval for PsA was based on data from two phase 3 clinical trials, including 852 participants naive to biologics (BE OPTIMAL) and 400 participants with inadequate response to treatment with one or two tumor necrosis factor (TNF) inhibitors (BE COMPLETE). Both studies met their primary endpoint, 50% improvement in American College of Rheumatology response criteria (ACR50) at 16 weeks, as well as ranked secondary endpoints. Secondary endpoints included minimal disease activity (MDA) and Psoriasis Area and Severity Index 100 (complete skin clearance) at week 16.
At 16 weeks:
- About 44% of both the biologic-naive (189 of 431) and TNF inhibitor–resistant (116 of 267) groups receiving bimekizumab achieved ACR50 response, compared with 10% (28 of 281) and 7% (9 of 133) receiving placebo, respectively.
- About 45% of all patients treated with bimekizumab achieved MDA.
- Nearly 60% of TNF inhibitor–resistant patients had complete skin clearance.
These responses generally were sustained for 1 year. The most common adverse reactions are upper respiratory tract infections, oral candidiasis, headache, diarrhea, and urinary tract infection.
NR-axSpA and AS Clinical Trials
The approval for active nr-axSpA and active AS was based on data from two clinical studies, BE MOBILE 1 (nr-axSpA) and BE MOBILE 2 (AS). Both studies met their primary endpoint, 40% improvement in Assessment of Spondyloarthritis International Society response criteria (ASAS40) at 16 weeks.
Key findings included:
- In nr-axSpA patients, 47.7% (61 of 128) receiving bimekizumab achieved ASAS40 at week 16, compared with 21.4% (27 of 126) receiving placebo.
- In AS patients, 44.8% (99 of 221) in the bimekizumab group achieved ASAS40 response at week 16 vs 22.5% (25 of 111) receiving placebo.
- At 1 year in both groups, 60% treated with bimekizumab achieved an Ankylosing Spondylitis Disease Activity Score < 2.1.
In nr-axSpA, the most common adverse reactions are upper respiratory tract infections, oral candidiasis, headache, diarrhea, cough, fatigue, musculoskeletal pain, myalgia, tonsillitis, increase in transaminase, and urinary tract infection. In AS, the most common adverse reactions are upper respiratory tract infections, oral candidiasis, headache, diarrhea, injection-site pain, rash, and vulvovaginal mycotic infection.
Bimekizumab was approved by the European Commission for the same rheumatologic indications in June 2023.
Bimekizumab is currently available to eligible patients in the United States, according to the press release.
A version of this article first appeared on Medscape.com.
The Food and Drug Administration has approved bimekizumab-bkzx (Bimzelx; UCB) for adult patients with active psoriatic arthritis (PsA), active nonradiographic axial spondyloarthritis (nr-axSpA) with objective signs of inflammation, and active ankylosing spondylitis (AS).
The drug, an interleukin (IL)–17A and IL-17F inhibitor, was first approved in October 2023 for treatment of moderate to severe plaque psoriasis in adults who are candidates for systemic therapy or phototherapy.
“In psoriatic arthritis and across the spectrum of axSpA, clinical study results and real-world experience outside the US have highlighted that Bimzelx can help patients achieve high thresholds of clinical response that are rapid in onset and sustained up to 2 years,” said Emmanuel Caeymaex, executive vice president, head of patient impact, and chief commercial officer of UCB in a press release.
The recommended dosage of bimekizumab for adult patients with active PsA, nr-axSpA, or AS is 160 mg by subcutaneous injection every 4 weeks. For patients with PsA and coexistent moderate to severe plaque psoriasis, the dosage is the same as for patients with plaque psoriasis. The dosing for plaque psoriasis is to administer 320 mg (two 160-mg injections) by subcutaneous injection at weeks 0, 4, 8, 12, and 16, then every 8 weeks thereafter. For patients weighing ≥ 120 kg, consider a dose of 320 mg every 4 weeks after week 16.
PsA Clinical Trials
The approval for PsA was based on data from two phase 3 clinical trials, including 852 participants naive to biologics (BE OPTIMAL) and 400 participants with inadequate response to treatment with one or two tumor necrosis factor (TNF) inhibitors (BE COMPLETE). Both studies met their primary endpoint, 50% improvement in American College of Rheumatology response criteria (ACR50) at 16 weeks, as well as ranked secondary endpoints. Secondary endpoints included minimal disease activity (MDA) and Psoriasis Area and Severity Index 100 (complete skin clearance) at week 16.
At 16 weeks:
- About 44% of both the biologic-naive (189 of 431) and TNF inhibitor–resistant (116 of 267) groups receiving bimekizumab achieved ACR50 response, compared with 10% (28 of 281) and 7% (9 of 133) receiving placebo, respectively.
- About 45% of all patients treated with bimekizumab achieved MDA.
- Nearly 60% of TNF inhibitor–resistant patients had complete skin clearance.
These responses generally were sustained for 1 year. The most common adverse reactions are upper respiratory tract infections, oral candidiasis, headache, diarrhea, and urinary tract infection.
NR-axSpA and AS Clinical Trials
The approval for active nr-axSpA and active AS was based on data from two clinical studies, BE MOBILE 1 (nr-axSpA) and BE MOBILE 2 (AS). Both studies met their primary endpoint, 40% improvement in Assessment of Spondyloarthritis International Society response criteria (ASAS40) at 16 weeks.
Key findings included:
- In nr-axSpA patients, 47.7% (61 of 128) receiving bimekizumab achieved ASAS40 at week 16, compared with 21.4% (27 of 126) receiving placebo.
- In AS patients, 44.8% (99 of 221) in the bimekizumab group achieved ASAS40 response at week 16 vs 22.5% (25 of 111) receiving placebo.
- At 1 year in both groups, 60% treated with bimekizumab achieved an Ankylosing Spondylitis Disease Activity Score < 2.1.
In nr-axSpA, the most common adverse reactions are upper respiratory tract infections, oral candidiasis, headache, diarrhea, cough, fatigue, musculoskeletal pain, myalgia, tonsillitis, increase in transaminase, and urinary tract infection. In AS, the most common adverse reactions are upper respiratory tract infections, oral candidiasis, headache, diarrhea, injection-site pain, rash, and vulvovaginal mycotic infection.
Bimekizumab was approved by the European Commission for the same rheumatologic indications in June 2023.
Bimekizumab is currently available to eligible patients in the United States, according to the press release.
A version of this article first appeared on Medscape.com.
Topical Tapinarof and Roflumilast for Psoriasis: Where Do they Fit In?
HUNTINGTON BEACH, CALIF. — The Food and Drug Administration and alternative medicine modalities for psoriasis severity measures were published in 2021, leaving some clinicians to wonder how these two newcomer drugs fit into their clinical practice.
At the annual meeting of the Pacific Dermatologic Association, Jashin J. Wu, MD, one of the authors of the guidelines and a voluntary associate professor of dermatology at the University of Miami, Coral Gables, Florida, proposed that tapinarof 1% cream and roflumilast 0.3% cream be considered first-line treatments for mild psoriasis. “The reason is because they’re very fast-acting, effective,” and result in a large improvement over steroids, Dr. Wu said. “You don’t have to worry about steroid atrophy, and it eliminates the need to use many different agents for different parts of the body necessarily, such as a weaker steroid for the face and sensitive areas. It also eliminates the need for patients to switch out steroids, such as 2 weeks on and 2 weeks off.”
Tapinarof 1% cream (Vtama) was approved in May 2022, for the topical treatment of plaque psoriasis in adults, and is under FDA review for treating atopic dermatitis (AD). “It’s once a day application, which is nice,” Dr. Wu said. “It is a first-in-class topical aryl hydrocarbon receptor agonist that can be used for the intertriginous areas. That’s where I find it helpful.”
Roflumilast 0.3% cream (Zoryve), a phosphodiesterase-4 inhibitor, was approved in July 2022 for the treatment of plaque psoriasis, including intertriginous areas, in patients aged 12 years and older. It was subsequently approved for treating plaque psoriasis in patients 6 years and older. (Roflumilast 0.15% cream is approved for mild to moderate AD in people aged 6 years or older, and roflumilast 0.3% topical foam is approved for seborrheic dermatitis in adults and children 9 years of age and older.)
The drug is contraindicated for use in patients with certain liver problems. “Patients are not going to be eating tubes of this drug, so I wouldn’t worry about that too much, but be aware if the pharmacist raises a concern about this,” Dr. Wu said.
Dr. Wu disclosed that he is or has been a consultant, investigator, or speaker for AbbVie, Almirall, Amgen, Arcutis, Aristea Therapeutics, Bausch Health, Boehringer Ingelheim, Bristol Myers Squibb, Codex Labs, Dermavant, DermTech, Dr. Reddy’s Laboratories, Eli Lilly, EPI Health, Galderma, Incyte, Janssen, LEO Pharma, Mindera, Novartis, Pfizer, Regeneron, Samsung Bioepis, Sanofi Genzyme, Solius, Sun Pharmaceuticals, UCB, and Zerigo Health.
A version of this article first appeared on Medscape.com.
HUNTINGTON BEACH, CALIF. — The Food and Drug Administration and alternative medicine modalities for psoriasis severity measures were published in 2021, leaving some clinicians to wonder how these two newcomer drugs fit into their clinical practice.
At the annual meeting of the Pacific Dermatologic Association, Jashin J. Wu, MD, one of the authors of the guidelines and a voluntary associate professor of dermatology at the University of Miami, Coral Gables, Florida, proposed that tapinarof 1% cream and roflumilast 0.3% cream be considered first-line treatments for mild psoriasis. “The reason is because they’re very fast-acting, effective,” and result in a large improvement over steroids, Dr. Wu said. “You don’t have to worry about steroid atrophy, and it eliminates the need to use many different agents for different parts of the body necessarily, such as a weaker steroid for the face and sensitive areas. It also eliminates the need for patients to switch out steroids, such as 2 weeks on and 2 weeks off.”
Tapinarof 1% cream (Vtama) was approved in May 2022, for the topical treatment of plaque psoriasis in adults, and is under FDA review for treating atopic dermatitis (AD). “It’s once a day application, which is nice,” Dr. Wu said. “It is a first-in-class topical aryl hydrocarbon receptor agonist that can be used for the intertriginous areas. That’s where I find it helpful.”
Roflumilast 0.3% cream (Zoryve), a phosphodiesterase-4 inhibitor, was approved in July 2022 for the treatment of plaque psoriasis, including intertriginous areas, in patients aged 12 years and older. It was subsequently approved for treating plaque psoriasis in patients 6 years and older. (Roflumilast 0.15% cream is approved for mild to moderate AD in people aged 6 years or older, and roflumilast 0.3% topical foam is approved for seborrheic dermatitis in adults and children 9 years of age and older.)
The drug is contraindicated for use in patients with certain liver problems. “Patients are not going to be eating tubes of this drug, so I wouldn’t worry about that too much, but be aware if the pharmacist raises a concern about this,” Dr. Wu said.
Dr. Wu disclosed that he is or has been a consultant, investigator, or speaker for AbbVie, Almirall, Amgen, Arcutis, Aristea Therapeutics, Bausch Health, Boehringer Ingelheim, Bristol Myers Squibb, Codex Labs, Dermavant, DermTech, Dr. Reddy’s Laboratories, Eli Lilly, EPI Health, Galderma, Incyte, Janssen, LEO Pharma, Mindera, Novartis, Pfizer, Regeneron, Samsung Bioepis, Sanofi Genzyme, Solius, Sun Pharmaceuticals, UCB, and Zerigo Health.
A version of this article first appeared on Medscape.com.
HUNTINGTON BEACH, CALIF. — The Food and Drug Administration and alternative medicine modalities for psoriasis severity measures were published in 2021, leaving some clinicians to wonder how these two newcomer drugs fit into their clinical practice.
At the annual meeting of the Pacific Dermatologic Association, Jashin J. Wu, MD, one of the authors of the guidelines and a voluntary associate professor of dermatology at the University of Miami, Coral Gables, Florida, proposed that tapinarof 1% cream and roflumilast 0.3% cream be considered first-line treatments for mild psoriasis. “The reason is because they’re very fast-acting, effective,” and result in a large improvement over steroids, Dr. Wu said. “You don’t have to worry about steroid atrophy, and it eliminates the need to use many different agents for different parts of the body necessarily, such as a weaker steroid for the face and sensitive areas. It also eliminates the need for patients to switch out steroids, such as 2 weeks on and 2 weeks off.”
Tapinarof 1% cream (Vtama) was approved in May 2022, for the topical treatment of plaque psoriasis in adults, and is under FDA review for treating atopic dermatitis (AD). “It’s once a day application, which is nice,” Dr. Wu said. “It is a first-in-class topical aryl hydrocarbon receptor agonist that can be used for the intertriginous areas. That’s where I find it helpful.”
Roflumilast 0.3% cream (Zoryve), a phosphodiesterase-4 inhibitor, was approved in July 2022 for the treatment of plaque psoriasis, including intertriginous areas, in patients aged 12 years and older. It was subsequently approved for treating plaque psoriasis in patients 6 years and older. (Roflumilast 0.15% cream is approved for mild to moderate AD in people aged 6 years or older, and roflumilast 0.3% topical foam is approved for seborrheic dermatitis in adults and children 9 years of age and older.)
The drug is contraindicated for use in patients with certain liver problems. “Patients are not going to be eating tubes of this drug, so I wouldn’t worry about that too much, but be aware if the pharmacist raises a concern about this,” Dr. Wu said.
Dr. Wu disclosed that he is or has been a consultant, investigator, or speaker for AbbVie, Almirall, Amgen, Arcutis, Aristea Therapeutics, Bausch Health, Boehringer Ingelheim, Bristol Myers Squibb, Codex Labs, Dermavant, DermTech, Dr. Reddy’s Laboratories, Eli Lilly, EPI Health, Galderma, Incyte, Janssen, LEO Pharma, Mindera, Novartis, Pfizer, Regeneron, Samsung Bioepis, Sanofi Genzyme, Solius, Sun Pharmaceuticals, UCB, and Zerigo Health.
A version of this article first appeared on Medscape.com.
FROM PDA 2024
Ustekinumab’s ‘Egregious’ Medicare Part B and D Pricing Differences Led to Federal Intervention
A US government report showed how a Medicare policy change made the drug ustekinumab (Stelara) for autoimmune diseases much more expensive, a finding that experts say illustrates the need for reforms created by the Inflation Reduction Act of 2022 (IRA).
The topline findings of an August report from the Department of Health and Human Services (HHS) about ustekinumab may seem somewhat surprising and a bit counterintuitive.
Ustekinumab costs spiked as Medicare pushed patients to get their supply through the Part D pharmacy program. The aim of Part D is to make medicines more affordable and accessible for patients. It runs on a model of insurers to negotiate deals for pharmaceuticals.
Earlier, many patients who needed ustekinumab had the drug covered by Medicare Part B. For many years, Medicare Part B has been largely a passive purchaser of medicines. Part B covers drugs administered by physicians. Its longtime model has been to add a premium of 6% to the reported average sales price to reimburse physicians who buy and administer the drug for patients.
But it was Part D, the Medicare program based on insurers’ negotiating clout, that saw a spike in ustekinumab costs after patients were shifted out of Part B coverage, where the cost of the medicine fell.
The average reported Part B cost for an ustekinumab injection slipped from $14,450 in 2016 to $12,912 by 2023, according to the report from HHS’ Office of Inspector General (OIG).
The Part D cost jumped in the same period. It rose by 84% from $17,717 in 2016 to $32,559 by 2023.
The IRA is intended to curb these kinds of increases in the future for drugs covered by Medicare, said Stacie B. Dusetzina, PhD, professor of health policy at Vanderbilt University School of Medicine, Nashville, Tennessee. The law demands companies pay rebates to Medicare if they increase drug prices faster than consumer inflation.
“That should at least help with some of this price growth that over time has seemed quite egregious,” Dr. Dusetzina told this news organization.
The IRA contains several provisions intended to curb rising drug costs for people enrolled in Medicare, including allowing the federal government to directly negotiate on some medicines.
Ustekinumab is one of the first 10 medicines that are subject to negotiations. Medicare will select as many as 15 additional drugs covered under Part D for negotiation in 2025, another 15 Part B and D drugs in 2026, and up to 20 drugs every year after that.
Earlier in August, the Centers for Medicare & Medicaid Services (CMS) announced the results of its first drug negotiations, with prices set to take effect in 2026. The Part D price for a 30-day supply of ustekinumab will be $4695 in 2026, a 66% reduction from the list price last year of $13,836.
Even at the negotiated price, ustekinumab’s cost will be high enough to trigger a new cap on out-of-pocket Part D spending, Dr. Dusetzina said.
Starting in 2025, Part D will have a cap of $2000 on individuals’ out-of-pocket costs, with annual adjustments in future years.
“It may not be better for someone who was filling this on Part B, who had a supplement [that covered their share of the ustekinumab cost], but it will be better for a lot of people that it’s covered under Part D,” Dr. Dusetzina said. “The good news is that at least from a beneficiary affordability standpoint, they’re going to have some price protection.”
OIG noted that the US Food and Drug Administration has approved three competing biosimilar versions of ustekinumab. These could also potentially work to lower costs.
‘A Complicated and Not Particularly Transparent Process’
OIG said it expects to release a report later this year with more detail about the decision that shifted ustekinumab coverage from Part B to Part D.
First cleared for US sales in 2009, ustekinumab is approved for psoriasis, psoriatic arthritis, Crohn’s disease, and ulcerative colitis. It can be given subcutaneously or intravenously.
Part B does not generally cover self-administered drugs. The infused version of ustekinumab has been covered under Medicare Part B since it reached the market.
“However, Part B coverage of the subcutaneous versions has been less straightforward,” OIG said in the report.
In 2020, Medicare administrative contractors — the units or affiliates of insurers that for decades have processed Part B claims for the traditional Medicare programs — determined that subcutaneous ustekinumab did not meet the criteria for coverage under Part B. Implementation of this change was delayed due to the COVID public health emergency but has since taken effect.
The shift in ustekinumab coverage to Part D eroded financial protections of many people on Medicare when Part B covered the drug.
Almost 9 in 10 people enrolled in Medicare Part B have supplemental insurance such as Medigap, employer coverage, or Medicaid to fully or partially cover their cost-sharing requirements, the OIG report said. That means Part B coverage shielded many patients from high ustekinumab costs.
In contrast, patients who self-administered the drug at home under Part D coverage paid an average of almost $6000 out of pocket if they did not receive any type of financial assistance, OIG said.
“From a financial standpoint, as long as you have Part B coinsurance, it would be much cheaper to get the drug in your doctor’s office than getting it through a pharmacy, unless you qualify for the low-income subsidy,” OIG Regional Inspector General David Tawes, who supervised the team that produced the report, told this news organization.
OIG has previously reported that post–point-of-sale rebates paid by manufacturers sometimes lower the costs incurred by Part D plans by a significant margin. But this was not the case with ustekinumab. Instead, OIG said the gap between initial and actual costs of ustekinumab was reduced by less than one third even with rebates. Rebate information is considered confidential.
“The whole negotiation structure is a complicated and not particularly transparent process,” Mr. Tawes said.
Backchannel Discounts, Top-Line Prices
The IRA is bringing some more transparency to the process through negotiations, said Mariana P. Socal, MD, associate professor at the Johns Hopkins Bloomberg School of Public Health in Baltimore. Patients who buy medicines that have been through the CMS negotiation process will be able to see if they are being charged correctly.
Dr. Socal noted that there’s something of a disconnect in discussions of Part D between how insurers and consumers view prices.
For Part D plans, the list prices represent the beginning of negotiations. They get rebates from drugmakers’ list prices for medicines, which insurers say work to lower premium costs.
“For plans, those prices are unrealistic. They are simply a sticker price. But for patients, for the Medicare beneficiaries, these prices are very real” because they are used to set copays, Dr. Socal said.
Dr. Dusetzina reported receiving funding from Arnold Ventures and the Commonwealth Fund for research related to drug pricing. Dr. Socal reported receiving funding from Arnold Ventures.
A version of this article first appeared on Medscape.com.
A US government report showed how a Medicare policy change made the drug ustekinumab (Stelara) for autoimmune diseases much more expensive, a finding that experts say illustrates the need for reforms created by the Inflation Reduction Act of 2022 (IRA).
The topline findings of an August report from the Department of Health and Human Services (HHS) about ustekinumab may seem somewhat surprising and a bit counterintuitive.
Ustekinumab costs spiked as Medicare pushed patients to get their supply through the Part D pharmacy program. The aim of Part D is to make medicines more affordable and accessible for patients. It runs on a model of insurers to negotiate deals for pharmaceuticals.
Earlier, many patients who needed ustekinumab had the drug covered by Medicare Part B. For many years, Medicare Part B has been largely a passive purchaser of medicines. Part B covers drugs administered by physicians. Its longtime model has been to add a premium of 6% to the reported average sales price to reimburse physicians who buy and administer the drug for patients.
But it was Part D, the Medicare program based on insurers’ negotiating clout, that saw a spike in ustekinumab costs after patients were shifted out of Part B coverage, where the cost of the medicine fell.
The average reported Part B cost for an ustekinumab injection slipped from $14,450 in 2016 to $12,912 by 2023, according to the report from HHS’ Office of Inspector General (OIG).
The Part D cost jumped in the same period. It rose by 84% from $17,717 in 2016 to $32,559 by 2023.
The IRA is intended to curb these kinds of increases in the future for drugs covered by Medicare, said Stacie B. Dusetzina, PhD, professor of health policy at Vanderbilt University School of Medicine, Nashville, Tennessee. The law demands companies pay rebates to Medicare if they increase drug prices faster than consumer inflation.
“That should at least help with some of this price growth that over time has seemed quite egregious,” Dr. Dusetzina told this news organization.
The IRA contains several provisions intended to curb rising drug costs for people enrolled in Medicare, including allowing the federal government to directly negotiate on some medicines.
Ustekinumab is one of the first 10 medicines that are subject to negotiations. Medicare will select as many as 15 additional drugs covered under Part D for negotiation in 2025, another 15 Part B and D drugs in 2026, and up to 20 drugs every year after that.
Earlier in August, the Centers for Medicare & Medicaid Services (CMS) announced the results of its first drug negotiations, with prices set to take effect in 2026. The Part D price for a 30-day supply of ustekinumab will be $4695 in 2026, a 66% reduction from the list price last year of $13,836.
Even at the negotiated price, ustekinumab’s cost will be high enough to trigger a new cap on out-of-pocket Part D spending, Dr. Dusetzina said.
Starting in 2025, Part D will have a cap of $2000 on individuals’ out-of-pocket costs, with annual adjustments in future years.
“It may not be better for someone who was filling this on Part B, who had a supplement [that covered their share of the ustekinumab cost], but it will be better for a lot of people that it’s covered under Part D,” Dr. Dusetzina said. “The good news is that at least from a beneficiary affordability standpoint, they’re going to have some price protection.”
OIG noted that the US Food and Drug Administration has approved three competing biosimilar versions of ustekinumab. These could also potentially work to lower costs.
‘A Complicated and Not Particularly Transparent Process’
OIG said it expects to release a report later this year with more detail about the decision that shifted ustekinumab coverage from Part B to Part D.
First cleared for US sales in 2009, ustekinumab is approved for psoriasis, psoriatic arthritis, Crohn’s disease, and ulcerative colitis. It can be given subcutaneously or intravenously.
Part B does not generally cover self-administered drugs. The infused version of ustekinumab has been covered under Medicare Part B since it reached the market.
“However, Part B coverage of the subcutaneous versions has been less straightforward,” OIG said in the report.
In 2020, Medicare administrative contractors — the units or affiliates of insurers that for decades have processed Part B claims for the traditional Medicare programs — determined that subcutaneous ustekinumab did not meet the criteria for coverage under Part B. Implementation of this change was delayed due to the COVID public health emergency but has since taken effect.
The shift in ustekinumab coverage to Part D eroded financial protections of many people on Medicare when Part B covered the drug.
Almost 9 in 10 people enrolled in Medicare Part B have supplemental insurance such as Medigap, employer coverage, or Medicaid to fully or partially cover their cost-sharing requirements, the OIG report said. That means Part B coverage shielded many patients from high ustekinumab costs.
In contrast, patients who self-administered the drug at home under Part D coverage paid an average of almost $6000 out of pocket if they did not receive any type of financial assistance, OIG said.
“From a financial standpoint, as long as you have Part B coinsurance, it would be much cheaper to get the drug in your doctor’s office than getting it through a pharmacy, unless you qualify for the low-income subsidy,” OIG Regional Inspector General David Tawes, who supervised the team that produced the report, told this news organization.
OIG has previously reported that post–point-of-sale rebates paid by manufacturers sometimes lower the costs incurred by Part D plans by a significant margin. But this was not the case with ustekinumab. Instead, OIG said the gap between initial and actual costs of ustekinumab was reduced by less than one third even with rebates. Rebate information is considered confidential.
“The whole negotiation structure is a complicated and not particularly transparent process,” Mr. Tawes said.
Backchannel Discounts, Top-Line Prices
The IRA is bringing some more transparency to the process through negotiations, said Mariana P. Socal, MD, associate professor at the Johns Hopkins Bloomberg School of Public Health in Baltimore. Patients who buy medicines that have been through the CMS negotiation process will be able to see if they are being charged correctly.
Dr. Socal noted that there’s something of a disconnect in discussions of Part D between how insurers and consumers view prices.
For Part D plans, the list prices represent the beginning of negotiations. They get rebates from drugmakers’ list prices for medicines, which insurers say work to lower premium costs.
“For plans, those prices are unrealistic. They are simply a sticker price. But for patients, for the Medicare beneficiaries, these prices are very real” because they are used to set copays, Dr. Socal said.
Dr. Dusetzina reported receiving funding from Arnold Ventures and the Commonwealth Fund for research related to drug pricing. Dr. Socal reported receiving funding from Arnold Ventures.
A version of this article first appeared on Medscape.com.
A US government report showed how a Medicare policy change made the drug ustekinumab (Stelara) for autoimmune diseases much more expensive, a finding that experts say illustrates the need for reforms created by the Inflation Reduction Act of 2022 (IRA).
The topline findings of an August report from the Department of Health and Human Services (HHS) about ustekinumab may seem somewhat surprising and a bit counterintuitive.
Ustekinumab costs spiked as Medicare pushed patients to get their supply through the Part D pharmacy program. The aim of Part D is to make medicines more affordable and accessible for patients. It runs on a model of insurers to negotiate deals for pharmaceuticals.
Earlier, many patients who needed ustekinumab had the drug covered by Medicare Part B. For many years, Medicare Part B has been largely a passive purchaser of medicines. Part B covers drugs administered by physicians. Its longtime model has been to add a premium of 6% to the reported average sales price to reimburse physicians who buy and administer the drug for patients.
But it was Part D, the Medicare program based on insurers’ negotiating clout, that saw a spike in ustekinumab costs after patients were shifted out of Part B coverage, where the cost of the medicine fell.
The average reported Part B cost for an ustekinumab injection slipped from $14,450 in 2016 to $12,912 by 2023, according to the report from HHS’ Office of Inspector General (OIG).
The Part D cost jumped in the same period. It rose by 84% from $17,717 in 2016 to $32,559 by 2023.
The IRA is intended to curb these kinds of increases in the future for drugs covered by Medicare, said Stacie B. Dusetzina, PhD, professor of health policy at Vanderbilt University School of Medicine, Nashville, Tennessee. The law demands companies pay rebates to Medicare if they increase drug prices faster than consumer inflation.
“That should at least help with some of this price growth that over time has seemed quite egregious,” Dr. Dusetzina told this news organization.
The IRA contains several provisions intended to curb rising drug costs for people enrolled in Medicare, including allowing the federal government to directly negotiate on some medicines.
Ustekinumab is one of the first 10 medicines that are subject to negotiations. Medicare will select as many as 15 additional drugs covered under Part D for negotiation in 2025, another 15 Part B and D drugs in 2026, and up to 20 drugs every year after that.
Earlier in August, the Centers for Medicare & Medicaid Services (CMS) announced the results of its first drug negotiations, with prices set to take effect in 2026. The Part D price for a 30-day supply of ustekinumab will be $4695 in 2026, a 66% reduction from the list price last year of $13,836.
Even at the negotiated price, ustekinumab’s cost will be high enough to trigger a new cap on out-of-pocket Part D spending, Dr. Dusetzina said.
Starting in 2025, Part D will have a cap of $2000 on individuals’ out-of-pocket costs, with annual adjustments in future years.
“It may not be better for someone who was filling this on Part B, who had a supplement [that covered their share of the ustekinumab cost], but it will be better for a lot of people that it’s covered under Part D,” Dr. Dusetzina said. “The good news is that at least from a beneficiary affordability standpoint, they’re going to have some price protection.”
OIG noted that the US Food and Drug Administration has approved three competing biosimilar versions of ustekinumab. These could also potentially work to lower costs.
‘A Complicated and Not Particularly Transparent Process’
OIG said it expects to release a report later this year with more detail about the decision that shifted ustekinumab coverage from Part B to Part D.
First cleared for US sales in 2009, ustekinumab is approved for psoriasis, psoriatic arthritis, Crohn’s disease, and ulcerative colitis. It can be given subcutaneously or intravenously.
Part B does not generally cover self-administered drugs. The infused version of ustekinumab has been covered under Medicare Part B since it reached the market.
“However, Part B coverage of the subcutaneous versions has been less straightforward,” OIG said in the report.
In 2020, Medicare administrative contractors — the units or affiliates of insurers that for decades have processed Part B claims for the traditional Medicare programs — determined that subcutaneous ustekinumab did not meet the criteria for coverage under Part B. Implementation of this change was delayed due to the COVID public health emergency but has since taken effect.
The shift in ustekinumab coverage to Part D eroded financial protections of many people on Medicare when Part B covered the drug.
Almost 9 in 10 people enrolled in Medicare Part B have supplemental insurance such as Medigap, employer coverage, or Medicaid to fully or partially cover their cost-sharing requirements, the OIG report said. That means Part B coverage shielded many patients from high ustekinumab costs.
In contrast, patients who self-administered the drug at home under Part D coverage paid an average of almost $6000 out of pocket if they did not receive any type of financial assistance, OIG said.
“From a financial standpoint, as long as you have Part B coinsurance, it would be much cheaper to get the drug in your doctor’s office than getting it through a pharmacy, unless you qualify for the low-income subsidy,” OIG Regional Inspector General David Tawes, who supervised the team that produced the report, told this news organization.
OIG has previously reported that post–point-of-sale rebates paid by manufacturers sometimes lower the costs incurred by Part D plans by a significant margin. But this was not the case with ustekinumab. Instead, OIG said the gap between initial and actual costs of ustekinumab was reduced by less than one third even with rebates. Rebate information is considered confidential.
“The whole negotiation structure is a complicated and not particularly transparent process,” Mr. Tawes said.
Backchannel Discounts, Top-Line Prices
The IRA is bringing some more transparency to the process through negotiations, said Mariana P. Socal, MD, associate professor at the Johns Hopkins Bloomberg School of Public Health in Baltimore. Patients who buy medicines that have been through the CMS negotiation process will be able to see if they are being charged correctly.
Dr. Socal noted that there’s something of a disconnect in discussions of Part D between how insurers and consumers view prices.
For Part D plans, the list prices represent the beginning of negotiations. They get rebates from drugmakers’ list prices for medicines, which insurers say work to lower premium costs.
“For plans, those prices are unrealistic. They are simply a sticker price. But for patients, for the Medicare beneficiaries, these prices are very real” because they are used to set copays, Dr. Socal said.
Dr. Dusetzina reported receiving funding from Arnold Ventures and the Commonwealth Fund for research related to drug pricing. Dr. Socal reported receiving funding from Arnold Ventures.
A version of this article first appeared on Medscape.com.
TYK2 Inhibitor Effective for Psoriasis in Phase 2 Study
TOPLINE:
METHODOLOGY:
- Researchers performed a phase 2b, randomized, double-blind trial to assess the efficacy, safety, and tolerability of different doses of zasocitinib in adults with moderate to severe psoriasis (mean age, 47 years; 32% women) at 47 centers in the United States and eight centers in Canada. Most (83%) were White, 7% were Black, and 8% were Asian.
- A total of 287 patients were randomly assigned to receive one of the four oral doses of zasocitinib (2 mg, 5 mg, 15 mg, or 30 mg, once daily) or a matched placebo for 12 weeks, followed by a 4-week safety monitoring period.
- The primary outcome was the proportion of patients achieving a ≥ 75% improvement in the Psoriasis Area and Severity Index score (PASI 75) from baseline at week 12.
TAKEAWAY:
- At week 12, PASI 75 was achieved by 18%, 44%, 68%, and 67% of patients receiving zasocitinib at doses of 2 mg, 5 mg, 15 mg, and 30 mg, respectively, vs 6% of patients receiving placebo.
- PASI 90 was achieved in 8%, 21%, 45%, and 46% of patients receiving zasocitinib at 2 mg, 5 mg, 15 mg, and 30 mg, respectively, and in no patients in the placebo group.
- At week 12, 10%, 27%, 49%, and 52% of patients receiving zasocitinib at 2 mg, 5 mg, 15 mg, and 30 mg, respectively, had no or mild disease (a score of 0 or 1) according to the Physician Global Assessment tool vs 4% in the placebo group.
- Treatment-emergent adverse events occurred in 53%-62% of patients in the zasocitinib groups compared with 44% in the placebo group. The most common were COVID-19, acne/acneiform dermatitis, and diarrhea. There were no reports of major adverse cardiovascular events, thromboembolic events, or opportunistic infections.
IN PRACTICE:
“Zasocitinib, an advanced, potent, and highly selective oral TYK2 inhibitor bioengineered to optimize target coverage and functional selectivity, achieved biologic-level efficacy with complete skin clearance observed after only a 12-week treatment period in up to one third of patients, with a low incidence of known tolerability issues and absence of serious toxic effects that are characteristic of [Janus kinase] 1-3 inhibition,” the authors wrote.
SOURCE:
The study was led by April W. Armstrong, MD, MPH, University of California, Los Angeles, and was published online on August 21, 2024, in JAMA Dermatology.
LIMITATIONS:
The study was limited by a relatively small sample size and a short duration. In addition, the inclusion of predominantly White patients may limit the generalizability of findings to a diverse population.
DISCLOSURES:
The study was funded by Nimbus Discovery, which includes Nimbus Therapeutics and Nimbus Lakshmi. Dr. Armstrong’s disclosures included receiving grants and/or personal fees from various pharmaceutical companies, including Nimbus Therapeutics and Nimbus. Three authors were employees of and reported holding equity, stocks, or shares in Nimbus. Several authors had disclosures related to pharmaceutical companies, including Nimbus.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- Researchers performed a phase 2b, randomized, double-blind trial to assess the efficacy, safety, and tolerability of different doses of zasocitinib in adults with moderate to severe psoriasis (mean age, 47 years; 32% women) at 47 centers in the United States and eight centers in Canada. Most (83%) were White, 7% were Black, and 8% were Asian.
- A total of 287 patients were randomly assigned to receive one of the four oral doses of zasocitinib (2 mg, 5 mg, 15 mg, or 30 mg, once daily) or a matched placebo for 12 weeks, followed by a 4-week safety monitoring period.
- The primary outcome was the proportion of patients achieving a ≥ 75% improvement in the Psoriasis Area and Severity Index score (PASI 75) from baseline at week 12.
TAKEAWAY:
- At week 12, PASI 75 was achieved by 18%, 44%, 68%, and 67% of patients receiving zasocitinib at doses of 2 mg, 5 mg, 15 mg, and 30 mg, respectively, vs 6% of patients receiving placebo.
- PASI 90 was achieved in 8%, 21%, 45%, and 46% of patients receiving zasocitinib at 2 mg, 5 mg, 15 mg, and 30 mg, respectively, and in no patients in the placebo group.
- At week 12, 10%, 27%, 49%, and 52% of patients receiving zasocitinib at 2 mg, 5 mg, 15 mg, and 30 mg, respectively, had no or mild disease (a score of 0 or 1) according to the Physician Global Assessment tool vs 4% in the placebo group.
- Treatment-emergent adverse events occurred in 53%-62% of patients in the zasocitinib groups compared with 44% in the placebo group. The most common were COVID-19, acne/acneiform dermatitis, and diarrhea. There were no reports of major adverse cardiovascular events, thromboembolic events, or opportunistic infections.
IN PRACTICE:
“Zasocitinib, an advanced, potent, and highly selective oral TYK2 inhibitor bioengineered to optimize target coverage and functional selectivity, achieved biologic-level efficacy with complete skin clearance observed after only a 12-week treatment period in up to one third of patients, with a low incidence of known tolerability issues and absence of serious toxic effects that are characteristic of [Janus kinase] 1-3 inhibition,” the authors wrote.
SOURCE:
The study was led by April W. Armstrong, MD, MPH, University of California, Los Angeles, and was published online on August 21, 2024, in JAMA Dermatology.
LIMITATIONS:
The study was limited by a relatively small sample size and a short duration. In addition, the inclusion of predominantly White patients may limit the generalizability of findings to a diverse population.
DISCLOSURES:
The study was funded by Nimbus Discovery, which includes Nimbus Therapeutics and Nimbus Lakshmi. Dr. Armstrong’s disclosures included receiving grants and/or personal fees from various pharmaceutical companies, including Nimbus Therapeutics and Nimbus. Three authors were employees of and reported holding equity, stocks, or shares in Nimbus. Several authors had disclosures related to pharmaceutical companies, including Nimbus.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- Researchers performed a phase 2b, randomized, double-blind trial to assess the efficacy, safety, and tolerability of different doses of zasocitinib in adults with moderate to severe psoriasis (mean age, 47 years; 32% women) at 47 centers in the United States and eight centers in Canada. Most (83%) were White, 7% were Black, and 8% were Asian.
- A total of 287 patients were randomly assigned to receive one of the four oral doses of zasocitinib (2 mg, 5 mg, 15 mg, or 30 mg, once daily) or a matched placebo for 12 weeks, followed by a 4-week safety monitoring period.
- The primary outcome was the proportion of patients achieving a ≥ 75% improvement in the Psoriasis Area and Severity Index score (PASI 75) from baseline at week 12.
TAKEAWAY:
- At week 12, PASI 75 was achieved by 18%, 44%, 68%, and 67% of patients receiving zasocitinib at doses of 2 mg, 5 mg, 15 mg, and 30 mg, respectively, vs 6% of patients receiving placebo.
- PASI 90 was achieved in 8%, 21%, 45%, and 46% of patients receiving zasocitinib at 2 mg, 5 mg, 15 mg, and 30 mg, respectively, and in no patients in the placebo group.
- At week 12, 10%, 27%, 49%, and 52% of patients receiving zasocitinib at 2 mg, 5 mg, 15 mg, and 30 mg, respectively, had no or mild disease (a score of 0 or 1) according to the Physician Global Assessment tool vs 4% in the placebo group.
- Treatment-emergent adverse events occurred in 53%-62% of patients in the zasocitinib groups compared with 44% in the placebo group. The most common were COVID-19, acne/acneiform dermatitis, and diarrhea. There were no reports of major adverse cardiovascular events, thromboembolic events, or opportunistic infections.
IN PRACTICE:
“Zasocitinib, an advanced, potent, and highly selective oral TYK2 inhibitor bioengineered to optimize target coverage and functional selectivity, achieved biologic-level efficacy with complete skin clearance observed after only a 12-week treatment period in up to one third of patients, with a low incidence of known tolerability issues and absence of serious toxic effects that are characteristic of [Janus kinase] 1-3 inhibition,” the authors wrote.
SOURCE:
The study was led by April W. Armstrong, MD, MPH, University of California, Los Angeles, and was published online on August 21, 2024, in JAMA Dermatology.
LIMITATIONS:
The study was limited by a relatively small sample size and a short duration. In addition, the inclusion of predominantly White patients may limit the generalizability of findings to a diverse population.
DISCLOSURES:
The study was funded by Nimbus Discovery, which includes Nimbus Therapeutics and Nimbus Lakshmi. Dr. Armstrong’s disclosures included receiving grants and/or personal fees from various pharmaceutical companies, including Nimbus Therapeutics and Nimbus. Three authors were employees of and reported holding equity, stocks, or shares in Nimbus. Several authors had disclosures related to pharmaceutical companies, including Nimbus.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
Patients With Immune-Mediated Inflammatory Diseases, Type 2 Diabetes Reap GLP-1 Receptor Agonist Benefits, Too
TOPLINE:
Compared with dipeptidyl peptidase 4 (DPP-4) inhibitors, glucagon-like peptide 1 receptor agonists (GLP-1 RAs) are associated with a lower risk for all-cause mortality and major adverse cardiovascular events (MACE) in patients with immune-mediated inflammatory diseases (IMIDs) and type 2 diabetes (T2D).
METHODOLOGY:
- GLP-1 RAs reduce the risk for all-cause mortality, cardiovascular mortality, and stroke in patients with diabetes. However, previous trials have excluded those with IMIDs, leaving a gap in understanding the cardioprotective effects of GLP-1 RAs in this population.
- Researchers conducted a population-based cohort study to assess if patients with an IMID derive greater benefits from GLP-1 RAs than DPP-4 inhibitors.
- They used administrative health data from British Columbia, Canada, to include 10,855 patients with IMIDs (rheumatoid arthritis, psoriatic disease, ankylosing spondylitis, inflammatory bowel disease, or systemic autoimmune rheumatic disease) and T2D who initiated either GLP-1 RA (n = 3570) or DPP-4 inhibitor (n = 7285).
- The mean follow-up was 1.46 and 1.88 years in the GLP-1 RA and DPP-4 inhibitor cohorts, respectively.
- The primary outcome was all-cause mortality, and the secondary outcome was MACE, including cardiovascular death, myocardial infarction, and ischemic stroke.
TAKEAWAY:
- The risk for all-cause mortality was 52% lower in patients who initiated GLP-1 RAs than in those who initiated DPP-4 inhibitors (weighted hazard ratio [HR], 0.48; 95% CI, 0.31-0.75).
- Additionally, patients initiating DPP-4 inhibitors.
- In the subgroup of patients with GLP-1 RAs had a significantly lower risk for MACE (weighted HR, 0.66; 95% CI, 0.50-0.88), particularly myocardial infarction (weighted HR, 0.62; 95% CI, 0.40-0.96), than those initiating rheumatoid arthritis and T2D, those who initiated GLP-1 RAs had a 55% lower risk for all-cause mortality and 61% lower risk for MACE than those who initiated DPP-4 inhibitors.
IN PRACTICE:
“This corresponds to nine fewer deaths and 11 fewer MACE per 1000 person-years, respectively, supporting the hypothesis that these agents have a cardioprotective effect in this high-risk population,” the authors wrote.
SOURCE:
This study was led by Derin Karacabeyli, MD, Division of Rheumatology, Department of Medicine, University of British Columbia, Vancouver, Canada, and was published online on August 8, 2024, in PLOS ONE.
LIMITATIONS:
The study’s dependence on administrative health data might have resulted in incomplete capture of comorbidities, particularly obesity. The mean follow-up period was relatively short, which might have limited the long-term applicability of these findings. The accuracy of the case definitions for IMIDs and T2D, according to International Classification of Diseases codes, could not be fully ascertained.
DISCLOSURES:
The study was supported by grants from the Canadian Institutes of Health Research. Two authors declared receiving research support, consulting fees, or participating in advisory boards outside the submitted work.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
Compared with dipeptidyl peptidase 4 (DPP-4) inhibitors, glucagon-like peptide 1 receptor agonists (GLP-1 RAs) are associated with a lower risk for all-cause mortality and major adverse cardiovascular events (MACE) in patients with immune-mediated inflammatory diseases (IMIDs) and type 2 diabetes (T2D).
METHODOLOGY:
- GLP-1 RAs reduce the risk for all-cause mortality, cardiovascular mortality, and stroke in patients with diabetes. However, previous trials have excluded those with IMIDs, leaving a gap in understanding the cardioprotective effects of GLP-1 RAs in this population.
- Researchers conducted a population-based cohort study to assess if patients with an IMID derive greater benefits from GLP-1 RAs than DPP-4 inhibitors.
- They used administrative health data from British Columbia, Canada, to include 10,855 patients with IMIDs (rheumatoid arthritis, psoriatic disease, ankylosing spondylitis, inflammatory bowel disease, or systemic autoimmune rheumatic disease) and T2D who initiated either GLP-1 RA (n = 3570) or DPP-4 inhibitor (n = 7285).
- The mean follow-up was 1.46 and 1.88 years in the GLP-1 RA and DPP-4 inhibitor cohorts, respectively.
- The primary outcome was all-cause mortality, and the secondary outcome was MACE, including cardiovascular death, myocardial infarction, and ischemic stroke.
TAKEAWAY:
- The risk for all-cause mortality was 52% lower in patients who initiated GLP-1 RAs than in those who initiated DPP-4 inhibitors (weighted hazard ratio [HR], 0.48; 95% CI, 0.31-0.75).
- Additionally, patients initiating DPP-4 inhibitors.
- In the subgroup of patients with GLP-1 RAs had a significantly lower risk for MACE (weighted HR, 0.66; 95% CI, 0.50-0.88), particularly myocardial infarction (weighted HR, 0.62; 95% CI, 0.40-0.96), than those initiating rheumatoid arthritis and T2D, those who initiated GLP-1 RAs had a 55% lower risk for all-cause mortality and 61% lower risk for MACE than those who initiated DPP-4 inhibitors.
IN PRACTICE:
“This corresponds to nine fewer deaths and 11 fewer MACE per 1000 person-years, respectively, supporting the hypothesis that these agents have a cardioprotective effect in this high-risk population,” the authors wrote.
SOURCE:
This study was led by Derin Karacabeyli, MD, Division of Rheumatology, Department of Medicine, University of British Columbia, Vancouver, Canada, and was published online on August 8, 2024, in PLOS ONE.
LIMITATIONS:
The study’s dependence on administrative health data might have resulted in incomplete capture of comorbidities, particularly obesity. The mean follow-up period was relatively short, which might have limited the long-term applicability of these findings. The accuracy of the case definitions for IMIDs and T2D, according to International Classification of Diseases codes, could not be fully ascertained.
DISCLOSURES:
The study was supported by grants from the Canadian Institutes of Health Research. Two authors declared receiving research support, consulting fees, or participating in advisory boards outside the submitted work.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
Compared with dipeptidyl peptidase 4 (DPP-4) inhibitors, glucagon-like peptide 1 receptor agonists (GLP-1 RAs) are associated with a lower risk for all-cause mortality and major adverse cardiovascular events (MACE) in patients with immune-mediated inflammatory diseases (IMIDs) and type 2 diabetes (T2D).
METHODOLOGY:
- GLP-1 RAs reduce the risk for all-cause mortality, cardiovascular mortality, and stroke in patients with diabetes. However, previous trials have excluded those with IMIDs, leaving a gap in understanding the cardioprotective effects of GLP-1 RAs in this population.
- Researchers conducted a population-based cohort study to assess if patients with an IMID derive greater benefits from GLP-1 RAs than DPP-4 inhibitors.
- They used administrative health data from British Columbia, Canada, to include 10,855 patients with IMIDs (rheumatoid arthritis, psoriatic disease, ankylosing spondylitis, inflammatory bowel disease, or systemic autoimmune rheumatic disease) and T2D who initiated either GLP-1 RA (n = 3570) or DPP-4 inhibitor (n = 7285).
- The mean follow-up was 1.46 and 1.88 years in the GLP-1 RA and DPP-4 inhibitor cohorts, respectively.
- The primary outcome was all-cause mortality, and the secondary outcome was MACE, including cardiovascular death, myocardial infarction, and ischemic stroke.
TAKEAWAY:
- The risk for all-cause mortality was 52% lower in patients who initiated GLP-1 RAs than in those who initiated DPP-4 inhibitors (weighted hazard ratio [HR], 0.48; 95% CI, 0.31-0.75).
- Additionally, patients initiating DPP-4 inhibitors.
- In the subgroup of patients with GLP-1 RAs had a significantly lower risk for MACE (weighted HR, 0.66; 95% CI, 0.50-0.88), particularly myocardial infarction (weighted HR, 0.62; 95% CI, 0.40-0.96), than those initiating rheumatoid arthritis and T2D, those who initiated GLP-1 RAs had a 55% lower risk for all-cause mortality and 61% lower risk for MACE than those who initiated DPP-4 inhibitors.
IN PRACTICE:
“This corresponds to nine fewer deaths and 11 fewer MACE per 1000 person-years, respectively, supporting the hypothesis that these agents have a cardioprotective effect in this high-risk population,” the authors wrote.
SOURCE:
This study was led by Derin Karacabeyli, MD, Division of Rheumatology, Department of Medicine, University of British Columbia, Vancouver, Canada, and was published online on August 8, 2024, in PLOS ONE.
LIMITATIONS:
The study’s dependence on administrative health data might have resulted in incomplete capture of comorbidities, particularly obesity. The mean follow-up period was relatively short, which might have limited the long-term applicability of these findings. The accuracy of the case definitions for IMIDs and T2D, according to International Classification of Diseases codes, could not be fully ascertained.
DISCLOSURES:
The study was supported by grants from the Canadian Institutes of Health Research. Two authors declared receiving research support, consulting fees, or participating in advisory boards outside the submitted work.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
Do You Have Patients With JAKne — JAK Inhibitor–Associated Acne? Here’s What to Know
Since the first Food and Drug Administration approval of a Janus kinase (JAK) inhibitor in 2011, the number of these medications available — and their treatment indications — have continued to grow. Prescribing physicians are familiar with the benefits and risks for these drugs, including higher risk for cardiac events and malignancy; however, one adverse effect may be overlooked, especially by specialties outside of dermatology: acne. Though less serious than some other side effects, JAK inhibitor–associated acne — JAKne, for short — can be a concern for patients.
“Your physical appearance and how you present yourself to the world is an important part of your self-confidence and living life on your own terms,” said Arash Mostaghimi, MD, the director of inpatient dermatology at Brigham and Women’s Hospital in Boston, Massachusetts. “I think letting people know about [JAKne] and then addressing it when it occurs should be a normal part of managing these medications.”
What Is JAKne?
JAKne generally looks like other kinds of acne, explained Janelle Nassim, MD, director of laser and cosmetic dermatology at the Indiana University School of Medicine, Indianapolis. “It can affect the same areas that typical acne affects, including the face, chest, back, neck, and upper shoulders.”
Though it appears like typical forms of acne, it is not clear what drives these skin eruptions in patients taking JAK inhibitors.
“We don’t understand the underlying pathophysiology,” Dr. Mostaghimi said. “It looks like acne, but we don’t know if the exact underlying inflammatory process is the same or if it’s different.”
In a 2023 systematic review of clinical studies, Dr. Mostaghimi and colleagues found that patients on any JAK inhibitor were nearly four times more likely to experience acne than patients who received placebo, but risk varied between medications. Patients taking JAK inhibitors for skin conditions had higher risk for acne than those given the medications for other indications. However, Dr. Mostaghimi thinks this finding is the result of selection bias.
Participants may not mention side effects like acne in trials for rheumatologic or gastrointestinal conditions, he said, unlike in trials for skin conditions. “Clinically, I’ve seen it in patients across every indication.”
Patients with a history of acne seem to be more likely to develop this side effect, though formal studies looking into risk factors are lacking. In Dr. Mostaghimi’s own clinical experience, JAKne is also more common in younger patients, but it can happen to anyone. “I’ve seen 70-year-olds develop acne — patients who’ve never had an issue their whole life — when they’re taking a JAK inhibitor.”
This issue also appears to be more common earlier in treatment, he added, and may improve over time as a patient continues with the medication.
How Do You Treat It?
“I think in other specialties, you will often feel awkward addressing skin conditions or pointing out acne,” Dr. Mostaghimi said. The most important steps are being aware of this potential side effect, and if you see it practice, to bring it up.
“Say: I’m noticing there’s some changes in your skin. Some patients on JAK inhibitors develop more acne. Have you noticed this? And if so, is this bothering you?”
Generally, JAKne is mild to moderate, explained Dr. Nassim, and if non-dermatologists are comfortable, they can prescribe a first-line topical regimen for patients. Dr. Mostaghimi recommends prescribing a clindamycin 1% lotion or gel. In addition, patients can use a benzoyl peroxide wash (4% or 10%) combined with a gentle retinoid, such as adapalene. (Both of these treatments are now available over the counter.)
In patients with scalp or hairline involvement, he often prescribes a ketoconazole 2% shampoo, which patients can use to wash their scalp, face, chest, and back in the shower.
If they aren’t responding to these initial treatments, then refer to a dermatologist for further assessment.
“Ultimately, referring to a dermatologist is the best course of action,” Dr. Nassim said. “I have had patients on JAK inhibitors who improved with topical acne treatments, and some that required more aggressive treatment with oral medications.”
Dr. Mostaghimi reported consulting fees from AbbVie, Concert Pharmaceuticals, Pfizer, and 3Derm Systems; research funding from Incyte, Aclaris Therapeutics, Eli Lilly, and Concert Pharmaceuticals; personal fees from Equillium, ASLAN Pharmaceuticals, ACOM, and Boehringer Ingelheim; and advisory board fees from Fig.1 Beauty, Eli Lilly, Pfizer, and Hims & Hers Health. Dr. Nassim had no relevant disclosures.
A version of this article first appeared on Medscape.com.
Since the first Food and Drug Administration approval of a Janus kinase (JAK) inhibitor in 2011, the number of these medications available — and their treatment indications — have continued to grow. Prescribing physicians are familiar with the benefits and risks for these drugs, including higher risk for cardiac events and malignancy; however, one adverse effect may be overlooked, especially by specialties outside of dermatology: acne. Though less serious than some other side effects, JAK inhibitor–associated acne — JAKne, for short — can be a concern for patients.
“Your physical appearance and how you present yourself to the world is an important part of your self-confidence and living life on your own terms,” said Arash Mostaghimi, MD, the director of inpatient dermatology at Brigham and Women’s Hospital in Boston, Massachusetts. “I think letting people know about [JAKne] and then addressing it when it occurs should be a normal part of managing these medications.”
What Is JAKne?
JAKne generally looks like other kinds of acne, explained Janelle Nassim, MD, director of laser and cosmetic dermatology at the Indiana University School of Medicine, Indianapolis. “It can affect the same areas that typical acne affects, including the face, chest, back, neck, and upper shoulders.”
Though it appears like typical forms of acne, it is not clear what drives these skin eruptions in patients taking JAK inhibitors.
“We don’t understand the underlying pathophysiology,” Dr. Mostaghimi said. “It looks like acne, but we don’t know if the exact underlying inflammatory process is the same or if it’s different.”
In a 2023 systematic review of clinical studies, Dr. Mostaghimi and colleagues found that patients on any JAK inhibitor were nearly four times more likely to experience acne than patients who received placebo, but risk varied between medications. Patients taking JAK inhibitors for skin conditions had higher risk for acne than those given the medications for other indications. However, Dr. Mostaghimi thinks this finding is the result of selection bias.
Participants may not mention side effects like acne in trials for rheumatologic or gastrointestinal conditions, he said, unlike in trials for skin conditions. “Clinically, I’ve seen it in patients across every indication.”
Patients with a history of acne seem to be more likely to develop this side effect, though formal studies looking into risk factors are lacking. In Dr. Mostaghimi’s own clinical experience, JAKne is also more common in younger patients, but it can happen to anyone. “I’ve seen 70-year-olds develop acne — patients who’ve never had an issue their whole life — when they’re taking a JAK inhibitor.”
This issue also appears to be more common earlier in treatment, he added, and may improve over time as a patient continues with the medication.
How Do You Treat It?
“I think in other specialties, you will often feel awkward addressing skin conditions or pointing out acne,” Dr. Mostaghimi said. The most important steps are being aware of this potential side effect, and if you see it practice, to bring it up.
“Say: I’m noticing there’s some changes in your skin. Some patients on JAK inhibitors develop more acne. Have you noticed this? And if so, is this bothering you?”
Generally, JAKne is mild to moderate, explained Dr. Nassim, and if non-dermatologists are comfortable, they can prescribe a first-line topical regimen for patients. Dr. Mostaghimi recommends prescribing a clindamycin 1% lotion or gel. In addition, patients can use a benzoyl peroxide wash (4% or 10%) combined with a gentle retinoid, such as adapalene. (Both of these treatments are now available over the counter.)
In patients with scalp or hairline involvement, he often prescribes a ketoconazole 2% shampoo, which patients can use to wash their scalp, face, chest, and back in the shower.
If they aren’t responding to these initial treatments, then refer to a dermatologist for further assessment.
“Ultimately, referring to a dermatologist is the best course of action,” Dr. Nassim said. “I have had patients on JAK inhibitors who improved with topical acne treatments, and some that required more aggressive treatment with oral medications.”
Dr. Mostaghimi reported consulting fees from AbbVie, Concert Pharmaceuticals, Pfizer, and 3Derm Systems; research funding from Incyte, Aclaris Therapeutics, Eli Lilly, and Concert Pharmaceuticals; personal fees from Equillium, ASLAN Pharmaceuticals, ACOM, and Boehringer Ingelheim; and advisory board fees from Fig.1 Beauty, Eli Lilly, Pfizer, and Hims & Hers Health. Dr. Nassim had no relevant disclosures.
A version of this article first appeared on Medscape.com.
Since the first Food and Drug Administration approval of a Janus kinase (JAK) inhibitor in 2011, the number of these medications available — and their treatment indications — have continued to grow. Prescribing physicians are familiar with the benefits and risks for these drugs, including higher risk for cardiac events and malignancy; however, one adverse effect may be overlooked, especially by specialties outside of dermatology: acne. Though less serious than some other side effects, JAK inhibitor–associated acne — JAKne, for short — can be a concern for patients.
“Your physical appearance and how you present yourself to the world is an important part of your self-confidence and living life on your own terms,” said Arash Mostaghimi, MD, the director of inpatient dermatology at Brigham and Women’s Hospital in Boston, Massachusetts. “I think letting people know about [JAKne] and then addressing it when it occurs should be a normal part of managing these medications.”
What Is JAKne?
JAKne generally looks like other kinds of acne, explained Janelle Nassim, MD, director of laser and cosmetic dermatology at the Indiana University School of Medicine, Indianapolis. “It can affect the same areas that typical acne affects, including the face, chest, back, neck, and upper shoulders.”
Though it appears like typical forms of acne, it is not clear what drives these skin eruptions in patients taking JAK inhibitors.
“We don’t understand the underlying pathophysiology,” Dr. Mostaghimi said. “It looks like acne, but we don’t know if the exact underlying inflammatory process is the same or if it’s different.”
In a 2023 systematic review of clinical studies, Dr. Mostaghimi and colleagues found that patients on any JAK inhibitor were nearly four times more likely to experience acne than patients who received placebo, but risk varied between medications. Patients taking JAK inhibitors for skin conditions had higher risk for acne than those given the medications for other indications. However, Dr. Mostaghimi thinks this finding is the result of selection bias.
Participants may not mention side effects like acne in trials for rheumatologic or gastrointestinal conditions, he said, unlike in trials for skin conditions. “Clinically, I’ve seen it in patients across every indication.”
Patients with a history of acne seem to be more likely to develop this side effect, though formal studies looking into risk factors are lacking. In Dr. Mostaghimi’s own clinical experience, JAKne is also more common in younger patients, but it can happen to anyone. “I’ve seen 70-year-olds develop acne — patients who’ve never had an issue their whole life — when they’re taking a JAK inhibitor.”
This issue also appears to be more common earlier in treatment, he added, and may improve over time as a patient continues with the medication.
How Do You Treat It?
“I think in other specialties, you will often feel awkward addressing skin conditions or pointing out acne,” Dr. Mostaghimi said. The most important steps are being aware of this potential side effect, and if you see it practice, to bring it up.
“Say: I’m noticing there’s some changes in your skin. Some patients on JAK inhibitors develop more acne. Have you noticed this? And if so, is this bothering you?”
Generally, JAKne is mild to moderate, explained Dr. Nassim, and if non-dermatologists are comfortable, they can prescribe a first-line topical regimen for patients. Dr. Mostaghimi recommends prescribing a clindamycin 1% lotion or gel. In addition, patients can use a benzoyl peroxide wash (4% or 10%) combined with a gentle retinoid, such as adapalene. (Both of these treatments are now available over the counter.)
In patients with scalp or hairline involvement, he often prescribes a ketoconazole 2% shampoo, which patients can use to wash their scalp, face, chest, and back in the shower.
If they aren’t responding to these initial treatments, then refer to a dermatologist for further assessment.
“Ultimately, referring to a dermatologist is the best course of action,” Dr. Nassim said. “I have had patients on JAK inhibitors who improved with topical acne treatments, and some that required more aggressive treatment with oral medications.”
Dr. Mostaghimi reported consulting fees from AbbVie, Concert Pharmaceuticals, Pfizer, and 3Derm Systems; research funding from Incyte, Aclaris Therapeutics, Eli Lilly, and Concert Pharmaceuticals; personal fees from Equillium, ASLAN Pharmaceuticals, ACOM, and Boehringer Ingelheim; and advisory board fees from Fig.1 Beauty, Eli Lilly, Pfizer, and Hims & Hers Health. Dr. Nassim had no relevant disclosures.
A version of this article first appeared on Medscape.com.
Chronic Back Pain in Patients With Psoriasis, Uveitis, or Colitis: How Often Is It Axial Spondyloarthritis?
TOPLINE:
Patients with psoriasis, uveitis, or colitis who present with undiagnosed chronic back pain should be referred to a rheumatologist for the assessment of axial spondyloarthritis (axSpA), with MRI being a more accurate diagnostic method than clinical features.
METHODOLOGY:
- Researchers assessed the prevalence of axSpA according to the extra-articular presentation and human leukocyte antigen B27 (HLA-B27) status in two Canadian cohorts (SASPIC 1 and 2).
- Overall, 363 adult patients aged ≤ 45 years with psoriasis, uveitis, or colitis who presented with chronic undiagnosed back and/or buttock pain lasting 3 months or more were included.
- Participants were referred to rheumatologists with expertise in axSpA for structured diagnostic evaluations, including history, physical exam, levels of C-reactive protein, HLA-B27 status, and imaging studies.
- An MRI of the sacroiliac joints was conducted in all patients in the SASPIC-2 cohort and in 62.3% of those in the SASPIC-1 cohort.
- The primary outcome was the proportion of patients diagnosed with axSpA after final global evaluation, and the secondary outcome was the impact of MRI on diagnosis and classification.
TAKEAWAY:
- AxSpA diagnoses were made in 46.7% with psoriasis, 61.6% with uveitis, and 46.8% with colitis in the SASPIC-1 cohort and in 23.5%, 57.9%, and 23.3%, respectively, in the SASPIC-2 cohort.
- Being positive for HLA-B27 was linked to the presence of axSpA in 56%-88% of those in both the cohorts.
- Musculoskeletal clinical features were not helpful in differentiating between patients with and without axSpA.
- In both the cohorts, the MRI of the sacroiliac joints was indicative of axSpA in a significantly greater number of patients with psoriasis, uveitis, or colitis who were diagnosed with axSpA than in those not diagnosed with axSpA (P < .05 for all).
IN PRACTICE:
“Our data supports the benefit of recent referral recommendations that advocate referral to a rheumatologist of patients with chronic back pain and extra-articular features related to axSpA,” the authors wrote.
SOURCE:
The study was led by Walter P. Maksymowych, MB ChB, University of Alberta, Edmonton, Alberta, Canada. It was published online in Arthritis & Rheumatology.
LIMITATIONS:
MRI readers had to rely on their own expertise to decide if an MRI was indeed positive and thus indicative of axSpA. This study included only patients with undiagnosed back pain, and a longer follow-up duration could have led to a higher number of patients being diagnosed with axial inflammation. In SASPIC-1, local rheumatologists conducted MRI evaluations of the spinal lesions only when necessary, while in SASPIC-2, MRI of only the sacroiliac joints was required.
DISCLOSURES:
SASPIC-1 was supported by AbbVie Canada and Janssen Canada, and SASPIC-2 was supported by AbbVie Canada. The authors disclosed receiving grants, consulting fees, speaking fees, and/or honoraria and having other ties with AbbVie and several other pharmaceutical companies.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
Patients with psoriasis, uveitis, or colitis who present with undiagnosed chronic back pain should be referred to a rheumatologist for the assessment of axial spondyloarthritis (axSpA), with MRI being a more accurate diagnostic method than clinical features.
METHODOLOGY:
- Researchers assessed the prevalence of axSpA according to the extra-articular presentation and human leukocyte antigen B27 (HLA-B27) status in two Canadian cohorts (SASPIC 1 and 2).
- Overall, 363 adult patients aged ≤ 45 years with psoriasis, uveitis, or colitis who presented with chronic undiagnosed back and/or buttock pain lasting 3 months or more were included.
- Participants were referred to rheumatologists with expertise in axSpA for structured diagnostic evaluations, including history, physical exam, levels of C-reactive protein, HLA-B27 status, and imaging studies.
- An MRI of the sacroiliac joints was conducted in all patients in the SASPIC-2 cohort and in 62.3% of those in the SASPIC-1 cohort.
- The primary outcome was the proportion of patients diagnosed with axSpA after final global evaluation, and the secondary outcome was the impact of MRI on diagnosis and classification.
TAKEAWAY:
- AxSpA diagnoses were made in 46.7% with psoriasis, 61.6% with uveitis, and 46.8% with colitis in the SASPIC-1 cohort and in 23.5%, 57.9%, and 23.3%, respectively, in the SASPIC-2 cohort.
- Being positive for HLA-B27 was linked to the presence of axSpA in 56%-88% of those in both the cohorts.
- Musculoskeletal clinical features were not helpful in differentiating between patients with and without axSpA.
- In both the cohorts, the MRI of the sacroiliac joints was indicative of axSpA in a significantly greater number of patients with psoriasis, uveitis, or colitis who were diagnosed with axSpA than in those not diagnosed with axSpA (P < .05 for all).
IN PRACTICE:
“Our data supports the benefit of recent referral recommendations that advocate referral to a rheumatologist of patients with chronic back pain and extra-articular features related to axSpA,” the authors wrote.
SOURCE:
The study was led by Walter P. Maksymowych, MB ChB, University of Alberta, Edmonton, Alberta, Canada. It was published online in Arthritis & Rheumatology.
LIMITATIONS:
MRI readers had to rely on their own expertise to decide if an MRI was indeed positive and thus indicative of axSpA. This study included only patients with undiagnosed back pain, and a longer follow-up duration could have led to a higher number of patients being diagnosed with axial inflammation. In SASPIC-1, local rheumatologists conducted MRI evaluations of the spinal lesions only when necessary, while in SASPIC-2, MRI of only the sacroiliac joints was required.
DISCLOSURES:
SASPIC-1 was supported by AbbVie Canada and Janssen Canada, and SASPIC-2 was supported by AbbVie Canada. The authors disclosed receiving grants, consulting fees, speaking fees, and/or honoraria and having other ties with AbbVie and several other pharmaceutical companies.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
Patients with psoriasis, uveitis, or colitis who present with undiagnosed chronic back pain should be referred to a rheumatologist for the assessment of axial spondyloarthritis (axSpA), with MRI being a more accurate diagnostic method than clinical features.
METHODOLOGY:
- Researchers assessed the prevalence of axSpA according to the extra-articular presentation and human leukocyte antigen B27 (HLA-B27) status in two Canadian cohorts (SASPIC 1 and 2).
- Overall, 363 adult patients aged ≤ 45 years with psoriasis, uveitis, or colitis who presented with chronic undiagnosed back and/or buttock pain lasting 3 months or more were included.
- Participants were referred to rheumatologists with expertise in axSpA for structured diagnostic evaluations, including history, physical exam, levels of C-reactive protein, HLA-B27 status, and imaging studies.
- An MRI of the sacroiliac joints was conducted in all patients in the SASPIC-2 cohort and in 62.3% of those in the SASPIC-1 cohort.
- The primary outcome was the proportion of patients diagnosed with axSpA after final global evaluation, and the secondary outcome was the impact of MRI on diagnosis and classification.
TAKEAWAY:
- AxSpA diagnoses were made in 46.7% with psoriasis, 61.6% with uveitis, and 46.8% with colitis in the SASPIC-1 cohort and in 23.5%, 57.9%, and 23.3%, respectively, in the SASPIC-2 cohort.
- Being positive for HLA-B27 was linked to the presence of axSpA in 56%-88% of those in both the cohorts.
- Musculoskeletal clinical features were not helpful in differentiating between patients with and without axSpA.
- In both the cohorts, the MRI of the sacroiliac joints was indicative of axSpA in a significantly greater number of patients with psoriasis, uveitis, or colitis who were diagnosed with axSpA than in those not diagnosed with axSpA (P < .05 for all).
IN PRACTICE:
“Our data supports the benefit of recent referral recommendations that advocate referral to a rheumatologist of patients with chronic back pain and extra-articular features related to axSpA,” the authors wrote.
SOURCE:
The study was led by Walter P. Maksymowych, MB ChB, University of Alberta, Edmonton, Alberta, Canada. It was published online in Arthritis & Rheumatology.
LIMITATIONS:
MRI readers had to rely on their own expertise to decide if an MRI was indeed positive and thus indicative of axSpA. This study included only patients with undiagnosed back pain, and a longer follow-up duration could have led to a higher number of patients being diagnosed with axial inflammation. In SASPIC-1, local rheumatologists conducted MRI evaluations of the spinal lesions only when necessary, while in SASPIC-2, MRI of only the sacroiliac joints was required.
DISCLOSURES:
SASPIC-1 was supported by AbbVie Canada and Janssen Canada, and SASPIC-2 was supported by AbbVie Canada. The authors disclosed receiving grants, consulting fees, speaking fees, and/or honoraria and having other ties with AbbVie and several other pharmaceutical companies.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.