User login
Assessing Geographical Trends in End-of-Life Cancer Care Using CDC WONDER’s Place of Death Data
Background
19.8% of all deaths in the US in 2023 were due to cancer. Despite its prevalence, there is minimal literature analyzing geographical trends in end-of-life care in cancer patients. This study aims to assess the evolution of end-of-life preferences in cancer patients, particularly during the COVID-19 pandemic, and account for geographical disparities to optimize palliative care delivery.
Methods
The CDC WONDER database was used to collect data on place of death (home, hospice, medical facilities, nursing homes) in patients over 25 years old that died with malignant neoplasms (ICD 10: C00- C97) in the US from 2003-2023. Deaths were stratified by region and urbanization. Proportional mortality was calculated, and statistically significant trends in mortality over time were identified using Joinpoint regression.
Results
There were 13,654,631 total deaths from malignant neoplasms over the study period. Home (40.3%) was the most common place of death followed by medical facilities (30.4%), nursing homes (14.3%), and hospice (8.9%). In 2020, all places experienced a decreased in proportion except for home which rose 7.0% from 41.7% to 48.7%. The South had the highest hospice rates (11.3%); 5.0% greater than the next highest region (Northeast; 8.3%). The West had the highest home rates (47.1%); 6.2% greater than the next closest region (South; 40.9%). The Northeast had the highest medical facility rates (36.0%); 5.5% higher than the next highest region (South, 30.5%). Nonmetro areas (< 50,000 population) had the lowest hospice (4.9%) and highest nursing home rates (15.8%). They also saw a substantial jump (+15.4%) in home deaths from 2019-21. All urbanizations saw a drop in medical facility deaths in 2020 but all have since climbed to surpass their 2019 rates except for nonmetro areas which have dropped 7.3% from 2020-2023.
Conclusion
Hospice and home deaths have increased in frequency with home deaths spiking during the COVID-19 pandemic. Geographical disparities persist in end-of-life care, particularly in nonmetro areas. This highlights the need to increase education and access to palliative care. Further research should aim at why the rural populations have failed to revert to pre-COVID trends like the other urbanization groups.
Background
19.8% of all deaths in the US in 2023 were due to cancer. Despite its prevalence, there is minimal literature analyzing geographical trends in end-of-life care in cancer patients. This study aims to assess the evolution of end-of-life preferences in cancer patients, particularly during the COVID-19 pandemic, and account for geographical disparities to optimize palliative care delivery.
Methods
The CDC WONDER database was used to collect data on place of death (home, hospice, medical facilities, nursing homes) in patients over 25 years old that died with malignant neoplasms (ICD 10: C00- C97) in the US from 2003-2023. Deaths were stratified by region and urbanization. Proportional mortality was calculated, and statistically significant trends in mortality over time were identified using Joinpoint regression.
Results
There were 13,654,631 total deaths from malignant neoplasms over the study period. Home (40.3%) was the most common place of death followed by medical facilities (30.4%), nursing homes (14.3%), and hospice (8.9%). In 2020, all places experienced a decreased in proportion except for home which rose 7.0% from 41.7% to 48.7%. The South had the highest hospice rates (11.3%); 5.0% greater than the next highest region (Northeast; 8.3%). The West had the highest home rates (47.1%); 6.2% greater than the next closest region (South; 40.9%). The Northeast had the highest medical facility rates (36.0%); 5.5% higher than the next highest region (South, 30.5%). Nonmetro areas (< 50,000 population) had the lowest hospice (4.9%) and highest nursing home rates (15.8%). They also saw a substantial jump (+15.4%) in home deaths from 2019-21. All urbanizations saw a drop in medical facility deaths in 2020 but all have since climbed to surpass their 2019 rates except for nonmetro areas which have dropped 7.3% from 2020-2023.
Conclusion
Hospice and home deaths have increased in frequency with home deaths spiking during the COVID-19 pandemic. Geographical disparities persist in end-of-life care, particularly in nonmetro areas. This highlights the need to increase education and access to palliative care. Further research should aim at why the rural populations have failed to revert to pre-COVID trends like the other urbanization groups.
Background
19.8% of all deaths in the US in 2023 were due to cancer. Despite its prevalence, there is minimal literature analyzing geographical trends in end-of-life care in cancer patients. This study aims to assess the evolution of end-of-life preferences in cancer patients, particularly during the COVID-19 pandemic, and account for geographical disparities to optimize palliative care delivery.
Methods
The CDC WONDER database was used to collect data on place of death (home, hospice, medical facilities, nursing homes) in patients over 25 years old that died with malignant neoplasms (ICD 10: C00- C97) in the US from 2003-2023. Deaths were stratified by region and urbanization. Proportional mortality was calculated, and statistically significant trends in mortality over time were identified using Joinpoint regression.
Results
There were 13,654,631 total deaths from malignant neoplasms over the study period. Home (40.3%) was the most common place of death followed by medical facilities (30.4%), nursing homes (14.3%), and hospice (8.9%). In 2020, all places experienced a decreased in proportion except for home which rose 7.0% from 41.7% to 48.7%. The South had the highest hospice rates (11.3%); 5.0% greater than the next highest region (Northeast; 8.3%). The West had the highest home rates (47.1%); 6.2% greater than the next closest region (South; 40.9%). The Northeast had the highest medical facility rates (36.0%); 5.5% higher than the next highest region (South, 30.5%). Nonmetro areas (< 50,000 population) had the lowest hospice (4.9%) and highest nursing home rates (15.8%). They also saw a substantial jump (+15.4%) in home deaths from 2019-21. All urbanizations saw a drop in medical facility deaths in 2020 but all have since climbed to surpass their 2019 rates except for nonmetro areas which have dropped 7.3% from 2020-2023.
Conclusion
Hospice and home deaths have increased in frequency with home deaths spiking during the COVID-19 pandemic. Geographical disparities persist in end-of-life care, particularly in nonmetro areas. This highlights the need to increase education and access to palliative care. Further research should aim at why the rural populations have failed to revert to pre-COVID trends like the other urbanization groups.
Demographical Trends in End-of-Life Care in Malignant Neoplasms: A CDC Wonder Analysis Using Place of Death
Background
In 2024, it was estimated that 2,001,140 new cases of cancer were diagnosed in the United States with 611,720 people succumbing to the disease. There is scant literature analyzing how the place of death in cancer patients has evolved over time, particularly during the COVID-19 pandemic, and how it varies demographically. This study aims to analyze the evolution of end-of-life preferences in cancer patients and assess for racial or sexual disparities to optimize palliative care and ensure it aligns with the patient’s wishes.
Methods
The CDC Wonder database was used to collect data on place of death (home, hospice, medical facilities, nursing homes) in patients over 25 years old who died with malignant neoplasms (ICD-10: C00-C97) in the US from 2003-2023. Deaths were stratified by sex and race. Proportional mortality was calculated, and statistically significant temporal trends in mortality were identified using Joinpoint regression.
Results
From 2003 to 2023, there were 13,654,631 total deaths from malignant cancer. Home deaths were the most common (40.3%) followed by medical facilities (30.4%), nursing homes (14.3%), and hospice (8.9%). In 2020, all places experienced a decrease in proportion except for home which rose 7.1%. From 2003-2023, home (+4.0%) and hospice (+10.0%) rose in frequency while medical facility (-10.9%) and nursing home (-6.8%) declined. Females died in nursing homes at a greater proportion than males (15.8% vs. 13.1%) while males died in medical facilities more frequently (32.4% vs. 28.8%). Black patients were the least likely to die at home (33.1%), 5.9% less than the next lowest (Asian/ Pacific Islander; 39.0%), while Hispanic patients were most likely (46.9%); 5.7% more than the next highest (White, 41.7%). White patients were the least likely to die in medical facilities (28.4%) but were also most likely to die in nursing homes (15.3%).
Conclusions
Hospice and home deaths have increased in frequency with home deaths spiking during the COVID-19 pandemic. Disparities persist in end-of-life care across both sex and racial groups. This highlights the need to increase education and access to palliative care. Further research should elucidate cultural and racial discrepancies surrounding end-of-life treatment and preferences to provide context for these differences.
Background
In 2024, it was estimated that 2,001,140 new cases of cancer were diagnosed in the United States with 611,720 people succumbing to the disease. There is scant literature analyzing how the place of death in cancer patients has evolved over time, particularly during the COVID-19 pandemic, and how it varies demographically. This study aims to analyze the evolution of end-of-life preferences in cancer patients and assess for racial or sexual disparities to optimize palliative care and ensure it aligns with the patient’s wishes.
Methods
The CDC Wonder database was used to collect data on place of death (home, hospice, medical facilities, nursing homes) in patients over 25 years old who died with malignant neoplasms (ICD-10: C00-C97) in the US from 2003-2023. Deaths were stratified by sex and race. Proportional mortality was calculated, and statistically significant temporal trends in mortality were identified using Joinpoint regression.
Results
From 2003 to 2023, there were 13,654,631 total deaths from malignant cancer. Home deaths were the most common (40.3%) followed by medical facilities (30.4%), nursing homes (14.3%), and hospice (8.9%). In 2020, all places experienced a decrease in proportion except for home which rose 7.1%. From 2003-2023, home (+4.0%) and hospice (+10.0%) rose in frequency while medical facility (-10.9%) and nursing home (-6.8%) declined. Females died in nursing homes at a greater proportion than males (15.8% vs. 13.1%) while males died in medical facilities more frequently (32.4% vs. 28.8%). Black patients were the least likely to die at home (33.1%), 5.9% less than the next lowest (Asian/ Pacific Islander; 39.0%), while Hispanic patients were most likely (46.9%); 5.7% more than the next highest (White, 41.7%). White patients were the least likely to die in medical facilities (28.4%) but were also most likely to die in nursing homes (15.3%).
Conclusions
Hospice and home deaths have increased in frequency with home deaths spiking during the COVID-19 pandemic. Disparities persist in end-of-life care across both sex and racial groups. This highlights the need to increase education and access to palliative care. Further research should elucidate cultural and racial discrepancies surrounding end-of-life treatment and preferences to provide context for these differences.
Background
In 2024, it was estimated that 2,001,140 new cases of cancer were diagnosed in the United States with 611,720 people succumbing to the disease. There is scant literature analyzing how the place of death in cancer patients has evolved over time, particularly during the COVID-19 pandemic, and how it varies demographically. This study aims to analyze the evolution of end-of-life preferences in cancer patients and assess for racial or sexual disparities to optimize palliative care and ensure it aligns with the patient’s wishes.
Methods
The CDC Wonder database was used to collect data on place of death (home, hospice, medical facilities, nursing homes) in patients over 25 years old who died with malignant neoplasms (ICD-10: C00-C97) in the US from 2003-2023. Deaths were stratified by sex and race. Proportional mortality was calculated, and statistically significant temporal trends in mortality were identified using Joinpoint regression.
Results
From 2003 to 2023, there were 13,654,631 total deaths from malignant cancer. Home deaths were the most common (40.3%) followed by medical facilities (30.4%), nursing homes (14.3%), and hospice (8.9%). In 2020, all places experienced a decrease in proportion except for home which rose 7.1%. From 2003-2023, home (+4.0%) and hospice (+10.0%) rose in frequency while medical facility (-10.9%) and nursing home (-6.8%) declined. Females died in nursing homes at a greater proportion than males (15.8% vs. 13.1%) while males died in medical facilities more frequently (32.4% vs. 28.8%). Black patients were the least likely to die at home (33.1%), 5.9% less than the next lowest (Asian/ Pacific Islander; 39.0%), while Hispanic patients were most likely (46.9%); 5.7% more than the next highest (White, 41.7%). White patients were the least likely to die in medical facilities (28.4%) but were also most likely to die in nursing homes (15.3%).
Conclusions
Hospice and home deaths have increased in frequency with home deaths spiking during the COVID-19 pandemic. Disparities persist in end-of-life care across both sex and racial groups. This highlights the need to increase education and access to palliative care. Further research should elucidate cultural and racial discrepancies surrounding end-of-life treatment and preferences to provide context for these differences.
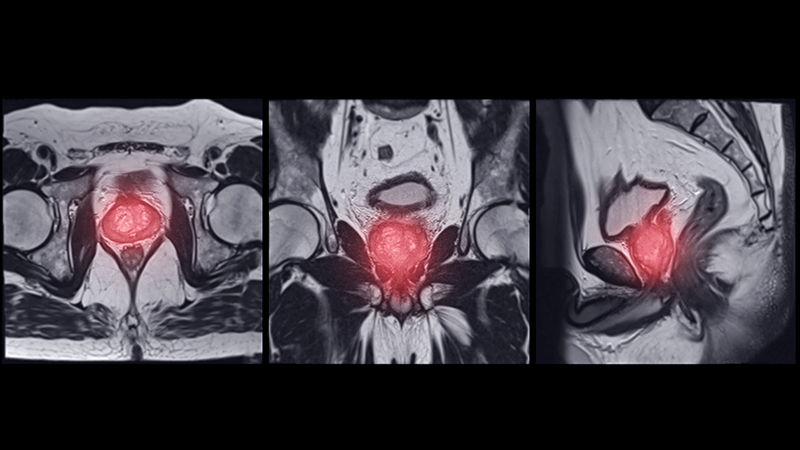
Findings from (ImPaCT): Improving Patients With Prostate Cancer’s Access to Germline Testing
Background
With the onset of precision oncology, findings from germline mutational analysis have been helpful in treating patients with cancer and aids in cancer prevention, early detection, and improved overall outcomes. Germline genetic testing is now part of the standard of care for certain types of patients with prostate cancer. There is a very limited body of work that investigated demographic, disease- related and social factors that may be influencing Veterans’ participation in germline genetic testing. This study helps to identify whether certain factors may be influencing decisions on participation in prostate germline testing among Veterans with prostate malignancy.
Methods
The study was conducted using retrospective chart review. Data was collected from the periods of August 1, 2022 to December 31, 2023 among Veterans with prostate cancer who met criteria for germline genetic testing. Demographic and clinical information were collected including age, race, extent of disease (high risk, very high-risk or metastatic disease), significant co-morbidities, educational level, family and personal history of cancer, travel time, germline genetic test findings, impact on treatment approaches, referral for genetic counseling, and whether Veterans agreed or declined germline genetic testing. Data was analyzed using descriptive statistics. A total of 180 charts were reviewed, with 171 meeting the criteria for inclusion. The mean age of the participants is 73, with the youngest being 55 and the oldest being 101 years old. Majority of the participants were African American (77%).
Results
Only about two percent of those who met the inclusion criteria declined to undergo testing with the one living the farthest away from the testing hospital residing 18 miles away. Those who declined testing ranged in age from 67 to 88, majority had high risk prostate cancer and no family history of malignancy, and had 0-1 serious co-morbidity. None of their educational informational was available for review.
Conclusions
Participation in germline genetic testing can be enhanced with adequate patient education and availability of accessible resources, even among patient populations that are not always well-represented in clinical research. The presence of multiple serious co-morbidities and distance from a testing facility do not seem to contribute to hesitancy in germline genetic testing participation.
Background
With the onset of precision oncology, findings from germline mutational analysis have been helpful in treating patients with cancer and aids in cancer prevention, early detection, and improved overall outcomes. Germline genetic testing is now part of the standard of care for certain types of patients with prostate cancer. There is a very limited body of work that investigated demographic, disease- related and social factors that may be influencing Veterans’ participation in germline genetic testing. This study helps to identify whether certain factors may be influencing decisions on participation in prostate germline testing among Veterans with prostate malignancy.
Methods
The study was conducted using retrospective chart review. Data was collected from the periods of August 1, 2022 to December 31, 2023 among Veterans with prostate cancer who met criteria for germline genetic testing. Demographic and clinical information were collected including age, race, extent of disease (high risk, very high-risk or metastatic disease), significant co-morbidities, educational level, family and personal history of cancer, travel time, germline genetic test findings, impact on treatment approaches, referral for genetic counseling, and whether Veterans agreed or declined germline genetic testing. Data was analyzed using descriptive statistics. A total of 180 charts were reviewed, with 171 meeting the criteria for inclusion. The mean age of the participants is 73, with the youngest being 55 and the oldest being 101 years old. Majority of the participants were African American (77%).
Results
Only about two percent of those who met the inclusion criteria declined to undergo testing with the one living the farthest away from the testing hospital residing 18 miles away. Those who declined testing ranged in age from 67 to 88, majority had high risk prostate cancer and no family history of malignancy, and had 0-1 serious co-morbidity. None of their educational informational was available for review.
Conclusions
Participation in germline genetic testing can be enhanced with adequate patient education and availability of accessible resources, even among patient populations that are not always well-represented in clinical research. The presence of multiple serious co-morbidities and distance from a testing facility do not seem to contribute to hesitancy in germline genetic testing participation.
Background
With the onset of precision oncology, findings from germline mutational analysis have been helpful in treating patients with cancer and aids in cancer prevention, early detection, and improved overall outcomes. Germline genetic testing is now part of the standard of care for certain types of patients with prostate cancer. There is a very limited body of work that investigated demographic, disease- related and social factors that may be influencing Veterans’ participation in germline genetic testing. This study helps to identify whether certain factors may be influencing decisions on participation in prostate germline testing among Veterans with prostate malignancy.
Methods
The study was conducted using retrospective chart review. Data was collected from the periods of August 1, 2022 to December 31, 2023 among Veterans with prostate cancer who met criteria for germline genetic testing. Demographic and clinical information were collected including age, race, extent of disease (high risk, very high-risk or metastatic disease), significant co-morbidities, educational level, family and personal history of cancer, travel time, germline genetic test findings, impact on treatment approaches, referral for genetic counseling, and whether Veterans agreed or declined germline genetic testing. Data was analyzed using descriptive statistics. A total of 180 charts were reviewed, with 171 meeting the criteria for inclusion. The mean age of the participants is 73, with the youngest being 55 and the oldest being 101 years old. Majority of the participants were African American (77%).
Results
Only about two percent of those who met the inclusion criteria declined to undergo testing with the one living the farthest away from the testing hospital residing 18 miles away. Those who declined testing ranged in age from 67 to 88, majority had high risk prostate cancer and no family history of malignancy, and had 0-1 serious co-morbidity. None of their educational informational was available for review.
Conclusions
Participation in germline genetic testing can be enhanced with adequate patient education and availability of accessible resources, even among patient populations that are not always well-represented in clinical research. The presence of multiple serious co-morbidities and distance from a testing facility do not seem to contribute to hesitancy in germline genetic testing participation.
E-Consults Bridge to Interdisciplinary Team Care for Rural Appalachian Veterans With Chronic Pain and Opioid Use Disorder
E-Consults Bridge to Interdisciplinary Team Care for Rural Appalachian Veterans With Chronic Pain and Opioid Use Disorder
Rural veterans are prescribed long-term opioid therapy for chronic pain at higher rates than urban veterans, increasing their risk of developing opioid use disorder (OUD).1,2 Veterans with co-occurring OUD and chronic pain have more severe health concerns, as well as higher rates of homelessness, psychoactive drug misuse, and mental health disorders, compared to veterans with either chronic pain or OUD alone.3 Interdisciplinary team (IDT) care is recommended for both chronic pain and OUD.4,5 Rural veterans with co-occurring chronic pain and OUD, however, are often unable to access IDTs due to long travel and wait times. As a result, these rural veterans often receive care from primary care practitioners (PCPs) who lack training in pain management and addiction and have low confidence in their ability to provide optimal treatment.6,7
In the Veterans Health Administration, electronic consultations (e-consults) provide support to PCPs by recommending evidence-based approaches such as buprenorphine for OUD and pain IDTs for chronic pain.5,8 However, research on the use of e-consults to connect to IDT care for co-occurring chronic pain and OUD are lacking, as well as studies on IDTs using innovative methods (eg, shared appointments) to overcome treatment barriers (eg, multiple appointments) for rural veterans at higher risk for co-occurring OUD and chronic pain.
This quality improvement study sought to determine the feasibility and impact of a pharmacy e-consult service that provided pain medication recommendations and subsequent referrals to RESTORE, a shared appointment program with an IDT, for assessment and treatment of chronic pain and OUD.
Methods
This retrospective chart review was approved as nonresearch by the Institutional Review Board Chair at the Salem Veterans Affairs Healthcare System (SVAHS), a low-complexity medical center in Virginia that primarily serves a rural and highly rural Central Appalachian veteran population.
This study included veterans whose clinicians placed a pain medication e-consult requesting recommendations for medication adjustments and/or a referral to RESTORE from January 1, 2022, through January 6, 2023. Requests for services that could not be provided through an e-consult were excluded (Figure 1). Veterans who had a pain medication e-consult were identified in the SVAHS electronic medical record (EMR). Data extracted from the EMR included demographics, referral source, reason for referral, RESTORE appointment attendance, OUD diagnosis made during the RESTORE initial evaluation, implementation of medication recommendations by the referrer within 6 months, engagement in ≥ 3 pain education classes, and a shared appointment with a pain IDT within 6 months. Data were entered into a REDCap database, and descriptive statistics summarized the results. Feasibility was assessed by use of the e-consult by PCPs, attendance at the RESTORE appointment, and OUD diagnosis by the RESTORE team.
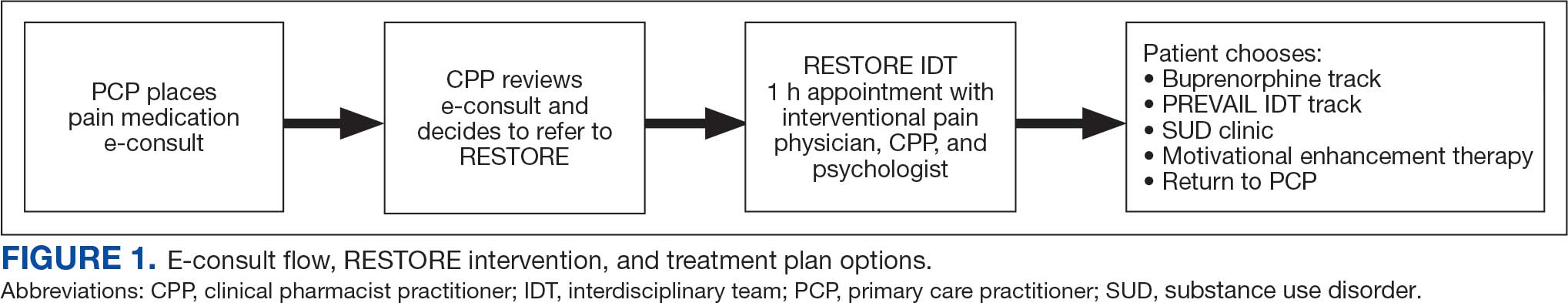
RESTORE Intervention
A pain medication e-consult was followed by referral to a shared appointment with the RESTORE IDT, with subsequent referrals to a pain IDT for chronic pain management if the veteran was amenable.
Pain medication e-consults in the EMR prompted a chart review by a clinical pharmacist practitioner (CPP). Recommendations for changes to medication regimens were documented in the EMR. At completion of the e-consult, the referring clinician received an automated view alert.
Veterans (and a support person, if preferred) were seen in a 60-minute, face-to-face shared appointment which included a psychologist, CPP, and pain physician. The psychologist conducted an OUD diagnostic interview, provided diagnostic feedback, and used motivational interviewing to provide psychoeducation on the biopsychosocial model of chronic pain, the IDT approach to chronic pain, and an overview of pain IDT care locally available. A CPP and physician then described medication options available to address OUD, if applicable. Together, the IDT and patient used shared decision making to determine a comprehensive treatment plan that may include a referral to the SVAHS PREVAIL Center for Chronic Pain IDT track (PREVAIL IDT track), a referral to substance use care in the case of polysubstance use, or medication initiation.9-11 If medication was prescribed, the patient was subsequently followed by the CPP through phone calls and face-to-face appointments at regularly scheduled intervals in coordination with the prescriber until they were stabilized. After stabilization, the prescription would be managed by their PCP (Figure 2). Veterans whose clinical condition changed significantly or worsened after returning to their PCP were invited to be reevaluated by the RESTORE team and restart care in that program. Individuals who were actively receiving RESTORE team care were discussed in a weekly care coordination meeting with all clinicians from both the PREVAIL and RESTORE teams.
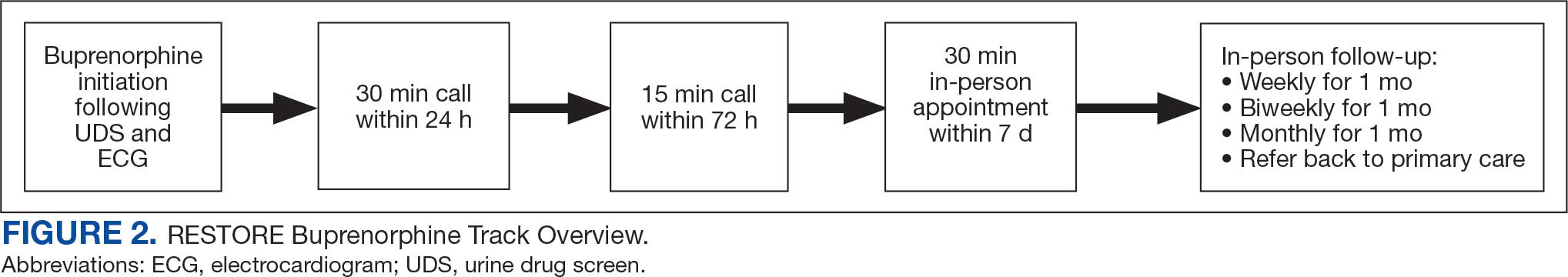
Program Metrics
Pain medication e-consults were placed for 77 patients; 7 were excluded as inappropriate referral requests. Seventy (83%) e-consults were placed by PCPs (Table). Fifty-seven referring PCPs (81%) implemented ≥ 1 medication recommendation and 41 (59%) implemented all recommendations within 6 months. CPPs referred 19 individuals to RESTORE due to concerns related to high risk. All attended the initial evaluation appointment with the RESTORE team, 17 (89%) agreed to be referred to PREVAIL IDT track for nonpharmacologic pain care, and 9 (53%) engaged with that care within 6 months. Of those who attended RESTORE, 7 patients (37%) initiated buprenorphine for OUD with 6 (86%) being prescribed buprenorphine for ≥ 6 months.

Discussion
Most e-consults placed at SVAHS, which primarily serves a rural veteran population in Central Appalachia, resulted in veterans engaging in evidence-based treatment for co-occurring chronic pain and OUD. The use of e-consults and subsequent shared appointments with an IDT appears to be feasible, as the service was most often used by PCPs who often feel unequipped to manage chronic pain.7 The attendance rate for the RESTORE appointments was notable given the typically poor follow-up for patients with OUD. It supports the feasibility of a shared appointment approach which may overcome frequent barriers to care in this vulnerable population (ie, time, transportation). By attending 1 appointment with all clinicians present as opposed to multiple appointments, veterans experience fewer barriers than attending multiple appointments. RESTORE continues to be offered as an active clinical service whose implementation is now supported by changes to SVAHS policies. Since this study was conducted, the number of patients seen weekly has doubled and will soon be tripled based on high demand from PCPs.
While this study was limited to 1 site, had a small sample size, and was limited in scope, its results suggest that future research is warranted. Future studies using a larger sample size utilizing both a randomized control trial design and qualitative methods are needed to answer critical questions such as the role of patient characteristics on treatment effectiveness and the impact of the RESTORE model on long-term OUD medication adherence, patients’ perceptions and satisfaction, barriers to implementation, PCP confidence in providing pain care, and use of evidence-based nonpharmacologic pain management services.12-14
Conclusions
The results of this quality-improvement project suggest that e-consults may facilitate referrals to and patient follow-through with evidence-based treatment for co-occurring chronic pain and OUD among veterans living in rural communities in Central Appalachia who tend to experience significant barriers to traditional care and may require an innovative approach to facilitate effective treatment.
- Lund BC, Ohl ME, Hadlandsmyth K, et al. Regional and rural-urban variation in opioid prescribing in the Veterans Health Administration. Mil Med. 2019;184(11-12):894-900. doi:10.1093/milmed/usz104
- Edlund MJ, Martin BC, Russo JE, et al. The role of opioid prescription in incident opioid abuse and dependence among individuals with chronic noncancer pain: the role of opioid prescription. Clin J Pain. 2014;30(7):557-564. doi:10.1097/AJP.0000000000000021
- MacLean RR, Sofuoglu M, Stefanovics E, et al. Opioid use disorder with chronic pain increases disease burden and service use. Psychol Serv. 2023;20(1):157-165. doi:10.1037/ser0000607
- US Department of Veterans Affairs, US Department of Defense. VA/DoD clinical practice guidelines: use of opioids in the management of chronic pain. Version 4.0. Updated May 2022. Accessed August 4, 2025. https://www.healthquality.va.gov/guidelines/Pain/cot/VADoDOpioidsCPG.pdf
- US Department of Veterans Affairs, US Department of Defense. VA/DoD clinical practice guideline for the diagnosis and treatment of low back pain: the diagnosis and treatment of low back pain. Version 3.0. Updated February 2022. Accessed August 4, 2025. https://www.healthquality.va.gov/guidelines/Pain/lbp/VADoDLBPCPGFinal508.pdf
- Shipton EE, Bate F, Garrick R, et al. Systematic review of pain medicine content, teaching, and assessment in medical school curricula internationally. Pain Ther. 2018;7(2):139-161. doi:10.1007/s40122-018-0103-z
- Jamison RN, Scanlan E, Matthews ML, et al. Attitudes of primary care practitioners in managing chronic pain patients prescribed opioids for pain: a prospective longitudinal controlled trial. Pain Med. 2016;17(1):99-113. doi:10.1111/pme.12871
- Miller DM, Harvey TL. Pharmacist pain e-consults that result in a therapy change. Fed Pract. 2015;32(7):14-19.
- Courtney RE, Schadegg MJ. Chronic, noncancer pain care in the Veterans Administration: current trends and future directions. Anesthesiol Clin. 2023;41(2):519-529. doi:10.1016/j.anclin.2023.02.004
- Courtney RE, Schadegg MJ, Bolton R, et al. Using a whole health approach to build biopsychosocial-spiritual personal health plans for veterans with chronic pain. Pain Manag Nurs. 2024;25(1):69-74. doi:10.1016/j.pmn.2023.09.010
- Darnall BD, Edwards KA, Courtney RE, et al. Innovative treatment formats, technologies, and clinician trainings that improve access to behavioral pain treatment for youth and adults. Front Pain Res. 2023;4. doi:10.3389/fpain.2023.1223172
- Lister JJ, Weaver A, Ellis JD, et al. A systematic review of rural-specific barriers to medication treatment for opioid use disorder in the United States. Am J Drug Alcohol Abuse. 2020;46:273-288. doi:10.1080/00952990.2019.1694536
- Bhatraju EP, Radick AC, Leroux BG, et al. Buprenorphine adherence and illicit opioid use among patients in treatment for opioid use disorder. Am J Drug Alcohol Abuse. 2023;49. doi:10.1080/00952990.2023.2220876
- Courtney RE, Halsey E, Patil T, Mastronardi KV, Browne HS, Darnall BD. Prescription opioid tapering practices and outcomes at a rural VA health care system. Pain Med. 2024;25:480-482. doi:10.1093/pm/pnae013
Rural veterans are prescribed long-term opioid therapy for chronic pain at higher rates than urban veterans, increasing their risk of developing opioid use disorder (OUD).1,2 Veterans with co-occurring OUD and chronic pain have more severe health concerns, as well as higher rates of homelessness, psychoactive drug misuse, and mental health disorders, compared to veterans with either chronic pain or OUD alone.3 Interdisciplinary team (IDT) care is recommended for both chronic pain and OUD.4,5 Rural veterans with co-occurring chronic pain and OUD, however, are often unable to access IDTs due to long travel and wait times. As a result, these rural veterans often receive care from primary care practitioners (PCPs) who lack training in pain management and addiction and have low confidence in their ability to provide optimal treatment.6,7
In the Veterans Health Administration, electronic consultations (e-consults) provide support to PCPs by recommending evidence-based approaches such as buprenorphine for OUD and pain IDTs for chronic pain.5,8 However, research on the use of e-consults to connect to IDT care for co-occurring chronic pain and OUD are lacking, as well as studies on IDTs using innovative methods (eg, shared appointments) to overcome treatment barriers (eg, multiple appointments) for rural veterans at higher risk for co-occurring OUD and chronic pain.
This quality improvement study sought to determine the feasibility and impact of a pharmacy e-consult service that provided pain medication recommendations and subsequent referrals to RESTORE, a shared appointment program with an IDT, for assessment and treatment of chronic pain and OUD.
Methods
This retrospective chart review was approved as nonresearch by the Institutional Review Board Chair at the Salem Veterans Affairs Healthcare System (SVAHS), a low-complexity medical center in Virginia that primarily serves a rural and highly rural Central Appalachian veteran population.
This study included veterans whose clinicians placed a pain medication e-consult requesting recommendations for medication adjustments and/or a referral to RESTORE from January 1, 2022, through January 6, 2023. Requests for services that could not be provided through an e-consult were excluded (Figure 1). Veterans who had a pain medication e-consult were identified in the SVAHS electronic medical record (EMR). Data extracted from the EMR included demographics, referral source, reason for referral, RESTORE appointment attendance, OUD diagnosis made during the RESTORE initial evaluation, implementation of medication recommendations by the referrer within 6 months, engagement in ≥ 3 pain education classes, and a shared appointment with a pain IDT within 6 months. Data were entered into a REDCap database, and descriptive statistics summarized the results. Feasibility was assessed by use of the e-consult by PCPs, attendance at the RESTORE appointment, and OUD diagnosis by the RESTORE team.

RESTORE Intervention
A pain medication e-consult was followed by referral to a shared appointment with the RESTORE IDT, with subsequent referrals to a pain IDT for chronic pain management if the veteran was amenable.
Pain medication e-consults in the EMR prompted a chart review by a clinical pharmacist practitioner (CPP). Recommendations for changes to medication regimens were documented in the EMR. At completion of the e-consult, the referring clinician received an automated view alert.
Veterans (and a support person, if preferred) were seen in a 60-minute, face-to-face shared appointment which included a psychologist, CPP, and pain physician. The psychologist conducted an OUD diagnostic interview, provided diagnostic feedback, and used motivational interviewing to provide psychoeducation on the biopsychosocial model of chronic pain, the IDT approach to chronic pain, and an overview of pain IDT care locally available. A CPP and physician then described medication options available to address OUD, if applicable. Together, the IDT and patient used shared decision making to determine a comprehensive treatment plan that may include a referral to the SVAHS PREVAIL Center for Chronic Pain IDT track (PREVAIL IDT track), a referral to substance use care in the case of polysubstance use, or medication initiation.9-11 If medication was prescribed, the patient was subsequently followed by the CPP through phone calls and face-to-face appointments at regularly scheduled intervals in coordination with the prescriber until they were stabilized. After stabilization, the prescription would be managed by their PCP (Figure 2). Veterans whose clinical condition changed significantly or worsened after returning to their PCP were invited to be reevaluated by the RESTORE team and restart care in that program. Individuals who were actively receiving RESTORE team care were discussed in a weekly care coordination meeting with all clinicians from both the PREVAIL and RESTORE teams.

Program Metrics
Pain medication e-consults were placed for 77 patients; 7 were excluded as inappropriate referral requests. Seventy (83%) e-consults were placed by PCPs (Table). Fifty-seven referring PCPs (81%) implemented ≥ 1 medication recommendation and 41 (59%) implemented all recommendations within 6 months. CPPs referred 19 individuals to RESTORE due to concerns related to high risk. All attended the initial evaluation appointment with the RESTORE team, 17 (89%) agreed to be referred to PREVAIL IDT track for nonpharmacologic pain care, and 9 (53%) engaged with that care within 6 months. Of those who attended RESTORE, 7 patients (37%) initiated buprenorphine for OUD with 6 (86%) being prescribed buprenorphine for ≥ 6 months.

Discussion
Most e-consults placed at SVAHS, which primarily serves a rural veteran population in Central Appalachia, resulted in veterans engaging in evidence-based treatment for co-occurring chronic pain and OUD. The use of e-consults and subsequent shared appointments with an IDT appears to be feasible, as the service was most often used by PCPs who often feel unequipped to manage chronic pain.7 The attendance rate for the RESTORE appointments was notable given the typically poor follow-up for patients with OUD. It supports the feasibility of a shared appointment approach which may overcome frequent barriers to care in this vulnerable population (ie, time, transportation). By attending 1 appointment with all clinicians present as opposed to multiple appointments, veterans experience fewer barriers than attending multiple appointments. RESTORE continues to be offered as an active clinical service whose implementation is now supported by changes to SVAHS policies. Since this study was conducted, the number of patients seen weekly has doubled and will soon be tripled based on high demand from PCPs.
While this study was limited to 1 site, had a small sample size, and was limited in scope, its results suggest that future research is warranted. Future studies using a larger sample size utilizing both a randomized control trial design and qualitative methods are needed to answer critical questions such as the role of patient characteristics on treatment effectiveness and the impact of the RESTORE model on long-term OUD medication adherence, patients’ perceptions and satisfaction, barriers to implementation, PCP confidence in providing pain care, and use of evidence-based nonpharmacologic pain management services.12-14
Conclusions
The results of this quality-improvement project suggest that e-consults may facilitate referrals to and patient follow-through with evidence-based treatment for co-occurring chronic pain and OUD among veterans living in rural communities in Central Appalachia who tend to experience significant barriers to traditional care and may require an innovative approach to facilitate effective treatment.
Rural veterans are prescribed long-term opioid therapy for chronic pain at higher rates than urban veterans, increasing their risk of developing opioid use disorder (OUD).1,2 Veterans with co-occurring OUD and chronic pain have more severe health concerns, as well as higher rates of homelessness, psychoactive drug misuse, and mental health disorders, compared to veterans with either chronic pain or OUD alone.3 Interdisciplinary team (IDT) care is recommended for both chronic pain and OUD.4,5 Rural veterans with co-occurring chronic pain and OUD, however, are often unable to access IDTs due to long travel and wait times. As a result, these rural veterans often receive care from primary care practitioners (PCPs) who lack training in pain management and addiction and have low confidence in their ability to provide optimal treatment.6,7
In the Veterans Health Administration, electronic consultations (e-consults) provide support to PCPs by recommending evidence-based approaches such as buprenorphine for OUD and pain IDTs for chronic pain.5,8 However, research on the use of e-consults to connect to IDT care for co-occurring chronic pain and OUD are lacking, as well as studies on IDTs using innovative methods (eg, shared appointments) to overcome treatment barriers (eg, multiple appointments) for rural veterans at higher risk for co-occurring OUD and chronic pain.
This quality improvement study sought to determine the feasibility and impact of a pharmacy e-consult service that provided pain medication recommendations and subsequent referrals to RESTORE, a shared appointment program with an IDT, for assessment and treatment of chronic pain and OUD.
Methods
This retrospective chart review was approved as nonresearch by the Institutional Review Board Chair at the Salem Veterans Affairs Healthcare System (SVAHS), a low-complexity medical center in Virginia that primarily serves a rural and highly rural Central Appalachian veteran population.
This study included veterans whose clinicians placed a pain medication e-consult requesting recommendations for medication adjustments and/or a referral to RESTORE from January 1, 2022, through January 6, 2023. Requests for services that could not be provided through an e-consult were excluded (Figure 1). Veterans who had a pain medication e-consult were identified in the SVAHS electronic medical record (EMR). Data extracted from the EMR included demographics, referral source, reason for referral, RESTORE appointment attendance, OUD diagnosis made during the RESTORE initial evaluation, implementation of medication recommendations by the referrer within 6 months, engagement in ≥ 3 pain education classes, and a shared appointment with a pain IDT within 6 months. Data were entered into a REDCap database, and descriptive statistics summarized the results. Feasibility was assessed by use of the e-consult by PCPs, attendance at the RESTORE appointment, and OUD diagnosis by the RESTORE team.

RESTORE Intervention
A pain medication e-consult was followed by referral to a shared appointment with the RESTORE IDT, with subsequent referrals to a pain IDT for chronic pain management if the veteran was amenable.
Pain medication e-consults in the EMR prompted a chart review by a clinical pharmacist practitioner (CPP). Recommendations for changes to medication regimens were documented in the EMR. At completion of the e-consult, the referring clinician received an automated view alert.
Veterans (and a support person, if preferred) were seen in a 60-minute, face-to-face shared appointment which included a psychologist, CPP, and pain physician. The psychologist conducted an OUD diagnostic interview, provided diagnostic feedback, and used motivational interviewing to provide psychoeducation on the biopsychosocial model of chronic pain, the IDT approach to chronic pain, and an overview of pain IDT care locally available. A CPP and physician then described medication options available to address OUD, if applicable. Together, the IDT and patient used shared decision making to determine a comprehensive treatment plan that may include a referral to the SVAHS PREVAIL Center for Chronic Pain IDT track (PREVAIL IDT track), a referral to substance use care in the case of polysubstance use, or medication initiation.9-11 If medication was prescribed, the patient was subsequently followed by the CPP through phone calls and face-to-face appointments at regularly scheduled intervals in coordination with the prescriber until they were stabilized. After stabilization, the prescription would be managed by their PCP (Figure 2). Veterans whose clinical condition changed significantly or worsened after returning to their PCP were invited to be reevaluated by the RESTORE team and restart care in that program. Individuals who were actively receiving RESTORE team care were discussed in a weekly care coordination meeting with all clinicians from both the PREVAIL and RESTORE teams.

Program Metrics
Pain medication e-consults were placed for 77 patients; 7 were excluded as inappropriate referral requests. Seventy (83%) e-consults were placed by PCPs (Table). Fifty-seven referring PCPs (81%) implemented ≥ 1 medication recommendation and 41 (59%) implemented all recommendations within 6 months. CPPs referred 19 individuals to RESTORE due to concerns related to high risk. All attended the initial evaluation appointment with the RESTORE team, 17 (89%) agreed to be referred to PREVAIL IDT track for nonpharmacologic pain care, and 9 (53%) engaged with that care within 6 months. Of those who attended RESTORE, 7 patients (37%) initiated buprenorphine for OUD with 6 (86%) being prescribed buprenorphine for ≥ 6 months.

Discussion
Most e-consults placed at SVAHS, which primarily serves a rural veteran population in Central Appalachia, resulted in veterans engaging in evidence-based treatment for co-occurring chronic pain and OUD. The use of e-consults and subsequent shared appointments with an IDT appears to be feasible, as the service was most often used by PCPs who often feel unequipped to manage chronic pain.7 The attendance rate for the RESTORE appointments was notable given the typically poor follow-up for patients with OUD. It supports the feasibility of a shared appointment approach which may overcome frequent barriers to care in this vulnerable population (ie, time, transportation). By attending 1 appointment with all clinicians present as opposed to multiple appointments, veterans experience fewer barriers than attending multiple appointments. RESTORE continues to be offered as an active clinical service whose implementation is now supported by changes to SVAHS policies. Since this study was conducted, the number of patients seen weekly has doubled and will soon be tripled based on high demand from PCPs.
While this study was limited to 1 site, had a small sample size, and was limited in scope, its results suggest that future research is warranted. Future studies using a larger sample size utilizing both a randomized control trial design and qualitative methods are needed to answer critical questions such as the role of patient characteristics on treatment effectiveness and the impact of the RESTORE model on long-term OUD medication adherence, patients’ perceptions and satisfaction, barriers to implementation, PCP confidence in providing pain care, and use of evidence-based nonpharmacologic pain management services.12-14
Conclusions
The results of this quality-improvement project suggest that e-consults may facilitate referrals to and patient follow-through with evidence-based treatment for co-occurring chronic pain and OUD among veterans living in rural communities in Central Appalachia who tend to experience significant barriers to traditional care and may require an innovative approach to facilitate effective treatment.
- Lund BC, Ohl ME, Hadlandsmyth K, et al. Regional and rural-urban variation in opioid prescribing in the Veterans Health Administration. Mil Med. 2019;184(11-12):894-900. doi:10.1093/milmed/usz104
- Edlund MJ, Martin BC, Russo JE, et al. The role of opioid prescription in incident opioid abuse and dependence among individuals with chronic noncancer pain: the role of opioid prescription. Clin J Pain. 2014;30(7):557-564. doi:10.1097/AJP.0000000000000021
- MacLean RR, Sofuoglu M, Stefanovics E, et al. Opioid use disorder with chronic pain increases disease burden and service use. Psychol Serv. 2023;20(1):157-165. doi:10.1037/ser0000607
- US Department of Veterans Affairs, US Department of Defense. VA/DoD clinical practice guidelines: use of opioids in the management of chronic pain. Version 4.0. Updated May 2022. Accessed August 4, 2025. https://www.healthquality.va.gov/guidelines/Pain/cot/VADoDOpioidsCPG.pdf
- US Department of Veterans Affairs, US Department of Defense. VA/DoD clinical practice guideline for the diagnosis and treatment of low back pain: the diagnosis and treatment of low back pain. Version 3.0. Updated February 2022. Accessed August 4, 2025. https://www.healthquality.va.gov/guidelines/Pain/lbp/VADoDLBPCPGFinal508.pdf
- Shipton EE, Bate F, Garrick R, et al. Systematic review of pain medicine content, teaching, and assessment in medical school curricula internationally. Pain Ther. 2018;7(2):139-161. doi:10.1007/s40122-018-0103-z
- Jamison RN, Scanlan E, Matthews ML, et al. Attitudes of primary care practitioners in managing chronic pain patients prescribed opioids for pain: a prospective longitudinal controlled trial. Pain Med. 2016;17(1):99-113. doi:10.1111/pme.12871
- Miller DM, Harvey TL. Pharmacist pain e-consults that result in a therapy change. Fed Pract. 2015;32(7):14-19.
- Courtney RE, Schadegg MJ. Chronic, noncancer pain care in the Veterans Administration: current trends and future directions. Anesthesiol Clin. 2023;41(2):519-529. doi:10.1016/j.anclin.2023.02.004
- Courtney RE, Schadegg MJ, Bolton R, et al. Using a whole health approach to build biopsychosocial-spiritual personal health plans for veterans with chronic pain. Pain Manag Nurs. 2024;25(1):69-74. doi:10.1016/j.pmn.2023.09.010
- Darnall BD, Edwards KA, Courtney RE, et al. Innovative treatment formats, technologies, and clinician trainings that improve access to behavioral pain treatment for youth and adults. Front Pain Res. 2023;4. doi:10.3389/fpain.2023.1223172
- Lister JJ, Weaver A, Ellis JD, et al. A systematic review of rural-specific barriers to medication treatment for opioid use disorder in the United States. Am J Drug Alcohol Abuse. 2020;46:273-288. doi:10.1080/00952990.2019.1694536
- Bhatraju EP, Radick AC, Leroux BG, et al. Buprenorphine adherence and illicit opioid use among patients in treatment for opioid use disorder. Am J Drug Alcohol Abuse. 2023;49. doi:10.1080/00952990.2023.2220876
- Courtney RE, Halsey E, Patil T, Mastronardi KV, Browne HS, Darnall BD. Prescription opioid tapering practices and outcomes at a rural VA health care system. Pain Med. 2024;25:480-482. doi:10.1093/pm/pnae013
- Lund BC, Ohl ME, Hadlandsmyth K, et al. Regional and rural-urban variation in opioid prescribing in the Veterans Health Administration. Mil Med. 2019;184(11-12):894-900. doi:10.1093/milmed/usz104
- Edlund MJ, Martin BC, Russo JE, et al. The role of opioid prescription in incident opioid abuse and dependence among individuals with chronic noncancer pain: the role of opioid prescription. Clin J Pain. 2014;30(7):557-564. doi:10.1097/AJP.0000000000000021
- MacLean RR, Sofuoglu M, Stefanovics E, et al. Opioid use disorder with chronic pain increases disease burden and service use. Psychol Serv. 2023;20(1):157-165. doi:10.1037/ser0000607
- US Department of Veterans Affairs, US Department of Defense. VA/DoD clinical practice guidelines: use of opioids in the management of chronic pain. Version 4.0. Updated May 2022. Accessed August 4, 2025. https://www.healthquality.va.gov/guidelines/Pain/cot/VADoDOpioidsCPG.pdf
- US Department of Veterans Affairs, US Department of Defense. VA/DoD clinical practice guideline for the diagnosis and treatment of low back pain: the diagnosis and treatment of low back pain. Version 3.0. Updated February 2022. Accessed August 4, 2025. https://www.healthquality.va.gov/guidelines/Pain/lbp/VADoDLBPCPGFinal508.pdf
- Shipton EE, Bate F, Garrick R, et al. Systematic review of pain medicine content, teaching, and assessment in medical school curricula internationally. Pain Ther. 2018;7(2):139-161. doi:10.1007/s40122-018-0103-z
- Jamison RN, Scanlan E, Matthews ML, et al. Attitudes of primary care practitioners in managing chronic pain patients prescribed opioids for pain: a prospective longitudinal controlled trial. Pain Med. 2016;17(1):99-113. doi:10.1111/pme.12871
- Miller DM, Harvey TL. Pharmacist pain e-consults that result in a therapy change. Fed Pract. 2015;32(7):14-19.
- Courtney RE, Schadegg MJ. Chronic, noncancer pain care in the Veterans Administration: current trends and future directions. Anesthesiol Clin. 2023;41(2):519-529. doi:10.1016/j.anclin.2023.02.004
- Courtney RE, Schadegg MJ, Bolton R, et al. Using a whole health approach to build biopsychosocial-spiritual personal health plans for veterans with chronic pain. Pain Manag Nurs. 2024;25(1):69-74. doi:10.1016/j.pmn.2023.09.010
- Darnall BD, Edwards KA, Courtney RE, et al. Innovative treatment formats, technologies, and clinician trainings that improve access to behavioral pain treatment for youth and adults. Front Pain Res. 2023;4. doi:10.3389/fpain.2023.1223172
- Lister JJ, Weaver A, Ellis JD, et al. A systematic review of rural-specific barriers to medication treatment for opioid use disorder in the United States. Am J Drug Alcohol Abuse. 2020;46:273-288. doi:10.1080/00952990.2019.1694536
- Bhatraju EP, Radick AC, Leroux BG, et al. Buprenorphine adherence and illicit opioid use among patients in treatment for opioid use disorder. Am J Drug Alcohol Abuse. 2023;49. doi:10.1080/00952990.2023.2220876
- Courtney RE, Halsey E, Patil T, Mastronardi KV, Browne HS, Darnall BD. Prescription opioid tapering practices and outcomes at a rural VA health care system. Pain Med. 2024;25:480-482. doi:10.1093/pm/pnae013
E-Consults Bridge to Interdisciplinary Team Care for Rural Appalachian Veterans With Chronic Pain and Opioid Use Disorder
E-Consults Bridge to Interdisciplinary Team Care for Rural Appalachian Veterans With Chronic Pain and Opioid Use Disorder
Unique Presentation of Postpartum Hypereosinophilic Syndrome With Atypical Features and Therapeutic Challenges
Unique Presentation of Postpartum Hypereosinophilic Syndrome With Atypical Features and Therapeutic Challenges
Hypereosinophilic syndrome (HES) is defined by marked, persistent absolute eosinophil count (AEC) > 1500 cells/μL on ≥ 2 peripheral smears separated by ≥ 1 month with evidence of accompanied end-organ damage, in the absence of other causes of eosinophilia such as malignancy, atopy, or parasitic infections.1-5 Hypereosinophilic infiltration can impact almost every organ system; however, the most profound complications in patients with HES are related to leukemias and cardiac manifestations of the disease.3,4 Although rare, the associated morbidity and mortality of HES are considerable, making prompt recognition and treatment essential. Management involves targeted therapy based on pathologic classification of HES and on decreasing associated inflammation, fibrosis, and end-organ damage.3,5-7
The patient in this case report met the diagnostic criteria for HES. However, this patient had several clinical and laboratory features that made it difficult to characterize a specific HES variant. Moreover, she had additional immunomodulating factors in the setting of pregnancy. This is the first documented case of HES of undetermined etiology diagnosed postpartum and managed in the setting of a new pregnancy.2,8
CASE PRESENTATION
A 32-year-old female active-duty military service member with allergic rhinitis and a history of childhood eczema was referred to allergy/immunology for evaluation of a new, progressive pruritic rash. Symptoms started 3 months after the birth of her first child, with a new diffuse erythematous skin rash sparing her palms, soles, and mucosal surfaces. Given her history of atopy, the rash was initially treated as severe atopic dermatitis with appropriate topical medications. The rash gradually worsened, with the development of intermittent facial swelling, night sweats, dyspnea, recurrent epigastric abdominal pain, and nausea with vomiting, resulting in decreased oral intake and weight loss.
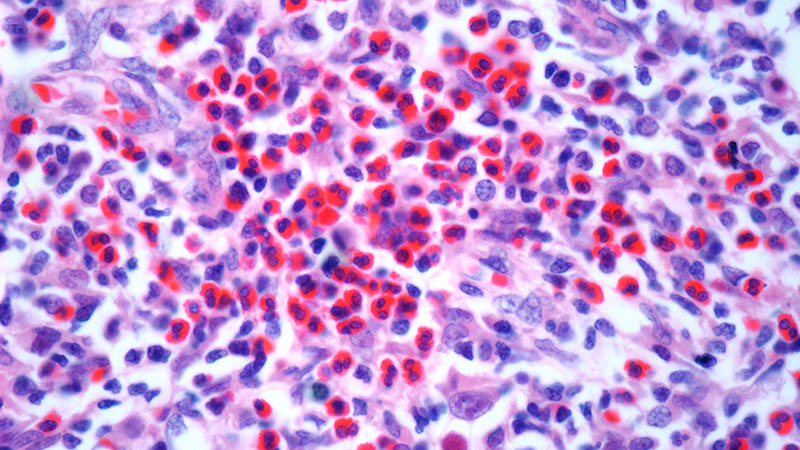
The patient was hospitalized and received an expedited multidisciplinary evaluation by dermatology, hematology/oncology, and gastroenterology. Her AEC of 4787 cells/μL peaked on admission and was markedly elevated from the 1070 cells/μL reported in the third trimester of her pregnancy. She was found to have mature eosinophilia on skin biopsy (Figure 1), endoscopic duodenal biopsy (Figure 2), peripheral blood smear (Figure 3), and bone marrow biopsy (Figure 4).




Radiographic imaging of the chest, abdomen, and pelvis revealed hepatomegaly without detectable neoplasm. There was no clinical evidence of cardiac involvement, and evaluation with electrocardiography and echocardiography did not indicate myocarditis. Extensive laboratory testing revealed no genetic mutations indicative of familial, myeloproliferative, or lymphocytic variants of HES.
The patient received topical emollients, omeprazole 40 mg daily, and ondansetron 8 mg 3 times daily as needed for symptom management, and was started on oral prednisone 40 mg daily with improvement in dyspnea, night sweats, and gastrointestinal complaints. During the patient's 6-day hospitalization and treatment, her AECs gradually decreased to 2110 cells/μL, and decreased to 1600 cells/μL over the course of a month, remaining in the hypereosinophilic range. The patient was discovered to be pregnant while symptoms were improving, resulting in stepwise discontinuation of oral steroids, but she reported continued improvement in symptoms.
DISCUSSION
Peripheral eosinophilia has a broad differential diagnoses, including HES, parasitic infections, atopic hypersensitivity diseases, eosinophilic lung diseases, eosinophilic gastrointestinal diseases, vasculitides such as eosinophilic granulomatosis with polyangiitis, genetic syndromes predisposing to eosinophilia, episodic angioedema with eosinophilia, and chronic metabolic disease with adrenal insufficiency.1-5 HES, although rare, is a disease process with potentially devastating associated morbidity and mortality if not promptly recognized and treated. HES is further delineated by hypereosinophilia with associated eosinophil-mediated organ damage or dysfunction.3-5
Clinical manifestations of HES can differ greatly depending on the HES variant and degree of organ involvement at the time of diagnosis and throughout the disease course. Patients with HES, as well as those with asymptomatic eosinophilia or hypereosinophilia, should be closely monitored for disease progression. In addition to trending peripheral AECs, clinicians should screen for symptoms of organ involvement and perform targeted evaluation of the suspected organs to promptly identify early signs of organ involvement and initiate treatment.1-4 Recommendations regarding screening intervals vary widely from monthly to annually, depending on a patient’s specific clinical picture.
HES has been subdivided into clinically relevant variants, including myeloproliferative (M-HES), T lymphocytic (L-HES), organ-restricted (or overlap) HES, familial HES, idiopathic HES, and specific syndromes with associated hypereosinophilia.3-5,9 Patients with M-HES have elevated circulating leukocyte precursors and clinical manifestations, including but not limited to hepatosplenomegaly, anemia, and thrombocytopenia. The most commonly associated genetic mutations include the FIP1L1-PDGFR-α fusion, BCR-ABL1, PDGFRA/B, JAK2, KIT, and FGFR1.3-6 L-HES usually has predominant skin and soft tissue involvement secondary to immunoglobulin E-mediated actions with clonal expansion of T cells (most commonly CD3-4+ or CD3+CD4-CD8-).3,5,6 Familial HES, a rare variant, follows an autosomal dominant inheritance pattern and is usually present at birth. It involves chromosome 5, which contains genes coding for cytokines that drive eosinophilic proliferation, including interleukin (IL)-3, IL-5, and granulocyte-macrophage colony-stimulating factor.5,9 Hypereosinophilia in the setting of end-organ damage restricted to a single organ is considered organ-restricted HES. There can be significant hepatic and gastrointestinal dysfunction, with or without malabsorption.
HES can also manifest with hematologic malignancy, restrictive obliterative cardiomyopathies, renal injury manifested by hematuria and electrolyte derangements, and neurologic complications including hemiparesis, dysarthria, and even coma.6 Endothelial damage due to eosinophil-driven inflammation can result in thrombus formation and increased risk of thromboembolic events in various organs.3 Idiopathic HES, otherwise known as HES of unknown etiology or significance, is a diagnosis of exclusion and constitutes a cohort of patients who do not fit into the other delineated categories.3-5 These patients often have multisystem involvement, making classification and treatment a challenge.5
The patient described in this case met the diagnostic criteria for HES, but her complicated clinical and laboratory features were challenging to characterize into a specific variant of HES. Organ-restricted HES was ruled out due to skin, marrow, and duodenal infiltration. She also had the potential for lung involvement based on her clinical symptoms, however no biopsy was obtained. Laboratory testing revealed no deletions or mutations indicative of familial, myeloproliferative, or lymphocytic variants. Her multisystem involvement without an underlying associated syndrome suggests idiopathic HES or HES of undetermined significance.1-5
Most patients with HES are diagnosed between the ages of 20 and 50 years.10 While HES has its peak incidence in the fourth decade of life, acute onset of new symptoms 3 months postpartum makes this an unusual presentation. In this unique case, it is important to highlight the role of the physiologic changes of pregnancy in inflammatory mediation. The physiologic changes that occur in pregnancy to ensure fetal tolerance can have profound implications for leukocyte count, AEC, and subsequent inflammatory responses. The phenomenon of inflammatory amelioration during pregnancy is well-documented, but there has only been 1 known published case report discussing decreasing HES symptoms during pregnancy with prepregnancy and postpartum hypereosinophilia.8 It is suggested that this amelioration is secondary to cortisol and progesterone shifts that occur in pregnancy. Physiologic increases in adrenocorticotropic hormone in pregnancy leads to subsequent secretion of endogenous steroids by the adrenal cortex. In turn, pregnancy can lead to leukocytosis and eosinopenia.8 Overall, pregnancy can have beneficial immunomodulating properties in the spectrum of hypereosinophilic syndromes. Even so, this patient with HES diagnosed postpartum remains at risk for the sequelae of hypereosinophilia, regardless of potential for AEC reduction during pregnancy. Therefore, treatment considerations need to be made with the safety of the maternal-fetal dyad as a priority.
Treatment
The treatment of symptomatic HES without acute life-threatening features or associated malignancy is generally determined by clinical variant.2-4 There is insufficient data to support initiation of treatment solely based on persistently elevated AEC. Patients with peripheral eosinophilia and hypereosinophilia should be monitored periodically with appropriate subspecialist evaluation for occult end-organ involvement, and targeted therapies should be deferred until an HES diagnosis.1-4 First-line therapy in most HES variants is systemic glucocorticoids.2,3,7 Since the disease course for this patient did not precisely match an HES variant, it was challenging to ascertain the optimal personalized treatment regimen. The approach to therapy was further complicated by newly identified pregnancy necessitating cessation of systemic glucocorticoids. In addition to glucocorticoids, hydroxyurea and interferon-α are among treatments historically used for HES, with tyrosine kinase inhibitors and monoclonal antibodies targeting IL-5 becoming more common.1-4 Although this patient may ultimately benefit from an IL-5 targeting biologic medication such as mepolizumab, safety in pregnancy is not well-studied and may require close clinical monitoring with treatment deferred until after delivery if possible.3,7,8,11
Military service members with frequent geographic relocation have additional barriers to timely diagnosis with often-limited access to subspecialty care depending on the duty station. While the patient was able to receive care at a large military medical center with many subspecialists, prompt recognition and timely referral to specialists would be even more critical at a smaller treatment facility. Depending on the severity and variant of HES, patients may warrant evaluation and treatment by hematology/oncology, cardiology, pulmonology, and immunology. Although HES can present in young children and older adults, this condition is most often diagnosed during the third and fourth decades of life, putting clinicians on the front line of hypereosinophilia identification and evaluation.10 Military physicians have the additional duty to not only think ahead in their diverse clinical settings to ensure proper care for patients, but also maintain a broad differential inclusive of more rare disease processes such as HES.
CONCLUSIONS
This case emphasizes how uncontrolled or untreated HES can lead to significant end-organ damage involving multiple systems and high morbidity. Prompt recognition of hypereosinophilia with potential HES can help expedite coordination of multidisciplinary care across multiple specialties to minimize delays in diagnosis and treatment. Doing so may minimize associated morbidity and mortality, especially in individuals located at more remote duty stations or deployed to austere environments.
- Cogan E, Roufosse F. Clinical management of the hypereosinophilic syndromes. Expert Rev Hematol. 2012;5:275-290. doi: 10.1586/ehm.12.14
- Klion A. Hypereosinophilic syndrome: approach to treatment in the era of precision medicine. Hematology Am Soc Hematol Educ Program. 2018;2018:326-331. doi:10.1182/asheducation-2018.1.326
- Shomali W, Gotlib J. World health organization-defined eosinophilic disorders: 2022 update on diagnosis, risk stratification, and management. Am J Hematol. 2022;97:129-148. doi:10.1002/ajh.26352
- Helbig G, Klion AD. Hypereosinophilic syndromes - an enigmatic group of disorders with an intriguing clinical spectrum and challenging treatment. Blood Rev. 2021;49:100809. doi:10.1016/j.blre.2021.100809
- Valent P, Klion AD, Horny HP, et al. Contemporary consensus proposal on criteria and classification of eosinophilic disorders and related syndromes. J Allergy Clin Immunol. 2012;130:607-612.e9. doi:10.1016/j.jaci.2012.02.019
- Roufosse FE, Goldman M, Cogan E. Hypereosinophilic syndromes. Orphanet J Rare Dis. 2007;2:37. doi:10.1186/1750-1172-2-37
- Pitlick MM, Li JT, Pongdee T. Current and emerging biologic therapies targeting eosinophilic disorders. World Allergy Organ J. 2022;15:100676. doi:10.1016/j.waojou.2022.10067
- Ault P, Cortes J, Lynn A, Keating M, Verstovsek S. Pregnancy in a patient with hypereosinophilic syndrome. Leuk Res. 2009;33:186-187. doi:10.1016/j.leukres.2008.05.013
- Rioux JD, Stone VA, Daly MJ, et al. Familial eosinophilia maps to the cytokine gene cluster on human chromosomal region 5q31-q33. Am J Hum Genet. 1998;63:1086-1094. doi:10.1086/302053
- Williams KW, Ware J, Abiodun A, et al. Hypereosinophilia in children and adults: a retrospective comparison. J Allergy Clin Immunol Pract. 2016;4:941-947.e1. doi:10.1016/j.jaip.2016.03.020
- Pane F, Lefevre G, Kwon N, et al. Characterization of disease flares and impact of mepolizumab in patients with hypereosinophilic syndrome. Front Immunol. 2022;13:935996. doi:10.3389/fimmu.2022.935996
Hypereosinophilic syndrome (HES) is defined by marked, persistent absolute eosinophil count (AEC) > 1500 cells/μL on ≥ 2 peripheral smears separated by ≥ 1 month with evidence of accompanied end-organ damage, in the absence of other causes of eosinophilia such as malignancy, atopy, or parasitic infections.1-5 Hypereosinophilic infiltration can impact almost every organ system; however, the most profound complications in patients with HES are related to leukemias and cardiac manifestations of the disease.3,4 Although rare, the associated morbidity and mortality of HES are considerable, making prompt recognition and treatment essential. Management involves targeted therapy based on pathologic classification of HES and on decreasing associated inflammation, fibrosis, and end-organ damage.3,5-7
The patient in this case report met the diagnostic criteria for HES. However, this patient had several clinical and laboratory features that made it difficult to characterize a specific HES variant. Moreover, she had additional immunomodulating factors in the setting of pregnancy. This is the first documented case of HES of undetermined etiology diagnosed postpartum and managed in the setting of a new pregnancy.2,8
CASE PRESENTATION
A 32-year-old female active-duty military service member with allergic rhinitis and a history of childhood eczema was referred to allergy/immunology for evaluation of a new, progressive pruritic rash. Symptoms started 3 months after the birth of her first child, with a new diffuse erythematous skin rash sparing her palms, soles, and mucosal surfaces. Given her history of atopy, the rash was initially treated as severe atopic dermatitis with appropriate topical medications. The rash gradually worsened, with the development of intermittent facial swelling, night sweats, dyspnea, recurrent epigastric abdominal pain, and nausea with vomiting, resulting in decreased oral intake and weight loss.
The patient was hospitalized and received an expedited multidisciplinary evaluation by dermatology, hematology/oncology, and gastroenterology. Her AEC of 4787 cells/μL peaked on admission and was markedly elevated from the 1070 cells/μL reported in the third trimester of her pregnancy. She was found to have mature eosinophilia on skin biopsy (Figure 1), endoscopic duodenal biopsy (Figure 2), peripheral blood smear (Figure 3), and bone marrow biopsy (Figure 4).




Radiographic imaging of the chest, abdomen, and pelvis revealed hepatomegaly without detectable neoplasm. There was no clinical evidence of cardiac involvement, and evaluation with electrocardiography and echocardiography did not indicate myocarditis. Extensive laboratory testing revealed no genetic mutations indicative of familial, myeloproliferative, or lymphocytic variants of HES.
The patient received topical emollients, omeprazole 40 mg daily, and ondansetron 8 mg 3 times daily as needed for symptom management, and was started on oral prednisone 40 mg daily with improvement in dyspnea, night sweats, and gastrointestinal complaints. During the patient's 6-day hospitalization and treatment, her AECs gradually decreased to 2110 cells/μL, and decreased to 1600 cells/μL over the course of a month, remaining in the hypereosinophilic range. The patient was discovered to be pregnant while symptoms were improving, resulting in stepwise discontinuation of oral steroids, but she reported continued improvement in symptoms.
DISCUSSION
Peripheral eosinophilia has a broad differential diagnoses, including HES, parasitic infections, atopic hypersensitivity diseases, eosinophilic lung diseases, eosinophilic gastrointestinal diseases, vasculitides such as eosinophilic granulomatosis with polyangiitis, genetic syndromes predisposing to eosinophilia, episodic angioedema with eosinophilia, and chronic metabolic disease with adrenal insufficiency.1-5 HES, although rare, is a disease process with potentially devastating associated morbidity and mortality if not promptly recognized and treated. HES is further delineated by hypereosinophilia with associated eosinophil-mediated organ damage or dysfunction.3-5
Clinical manifestations of HES can differ greatly depending on the HES variant and degree of organ involvement at the time of diagnosis and throughout the disease course. Patients with HES, as well as those with asymptomatic eosinophilia or hypereosinophilia, should be closely monitored for disease progression. In addition to trending peripheral AECs, clinicians should screen for symptoms of organ involvement and perform targeted evaluation of the suspected organs to promptly identify early signs of organ involvement and initiate treatment.1-4 Recommendations regarding screening intervals vary widely from monthly to annually, depending on a patient’s specific clinical picture.
HES has been subdivided into clinically relevant variants, including myeloproliferative (M-HES), T lymphocytic (L-HES), organ-restricted (or overlap) HES, familial HES, idiopathic HES, and specific syndromes with associated hypereosinophilia.3-5,9 Patients with M-HES have elevated circulating leukocyte precursors and clinical manifestations, including but not limited to hepatosplenomegaly, anemia, and thrombocytopenia. The most commonly associated genetic mutations include the FIP1L1-PDGFR-α fusion, BCR-ABL1, PDGFRA/B, JAK2, KIT, and FGFR1.3-6 L-HES usually has predominant skin and soft tissue involvement secondary to immunoglobulin E-mediated actions with clonal expansion of T cells (most commonly CD3-4+ or CD3+CD4-CD8-).3,5,6 Familial HES, a rare variant, follows an autosomal dominant inheritance pattern and is usually present at birth. It involves chromosome 5, which contains genes coding for cytokines that drive eosinophilic proliferation, including interleukin (IL)-3, IL-5, and granulocyte-macrophage colony-stimulating factor.5,9 Hypereosinophilia in the setting of end-organ damage restricted to a single organ is considered organ-restricted HES. There can be significant hepatic and gastrointestinal dysfunction, with or without malabsorption.
HES can also manifest with hematologic malignancy, restrictive obliterative cardiomyopathies, renal injury manifested by hematuria and electrolyte derangements, and neurologic complications including hemiparesis, dysarthria, and even coma.6 Endothelial damage due to eosinophil-driven inflammation can result in thrombus formation and increased risk of thromboembolic events in various organs.3 Idiopathic HES, otherwise known as HES of unknown etiology or significance, is a diagnosis of exclusion and constitutes a cohort of patients who do not fit into the other delineated categories.3-5 These patients often have multisystem involvement, making classification and treatment a challenge.5
The patient described in this case met the diagnostic criteria for HES, but her complicated clinical and laboratory features were challenging to characterize into a specific variant of HES. Organ-restricted HES was ruled out due to skin, marrow, and duodenal infiltration. She also had the potential for lung involvement based on her clinical symptoms, however no biopsy was obtained. Laboratory testing revealed no deletions or mutations indicative of familial, myeloproliferative, or lymphocytic variants. Her multisystem involvement without an underlying associated syndrome suggests idiopathic HES or HES of undetermined significance.1-5
Most patients with HES are diagnosed between the ages of 20 and 50 years.10 While HES has its peak incidence in the fourth decade of life, acute onset of new symptoms 3 months postpartum makes this an unusual presentation. In this unique case, it is important to highlight the role of the physiologic changes of pregnancy in inflammatory mediation. The physiologic changes that occur in pregnancy to ensure fetal tolerance can have profound implications for leukocyte count, AEC, and subsequent inflammatory responses. The phenomenon of inflammatory amelioration during pregnancy is well-documented, but there has only been 1 known published case report discussing decreasing HES symptoms during pregnancy with prepregnancy and postpartum hypereosinophilia.8 It is suggested that this amelioration is secondary to cortisol and progesterone shifts that occur in pregnancy. Physiologic increases in adrenocorticotropic hormone in pregnancy leads to subsequent secretion of endogenous steroids by the adrenal cortex. In turn, pregnancy can lead to leukocytosis and eosinopenia.8 Overall, pregnancy can have beneficial immunomodulating properties in the spectrum of hypereosinophilic syndromes. Even so, this patient with HES diagnosed postpartum remains at risk for the sequelae of hypereosinophilia, regardless of potential for AEC reduction during pregnancy. Therefore, treatment considerations need to be made with the safety of the maternal-fetal dyad as a priority.
Treatment
The treatment of symptomatic HES without acute life-threatening features or associated malignancy is generally determined by clinical variant.2-4 There is insufficient data to support initiation of treatment solely based on persistently elevated AEC. Patients with peripheral eosinophilia and hypereosinophilia should be monitored periodically with appropriate subspecialist evaluation for occult end-organ involvement, and targeted therapies should be deferred until an HES diagnosis.1-4 First-line therapy in most HES variants is systemic glucocorticoids.2,3,7 Since the disease course for this patient did not precisely match an HES variant, it was challenging to ascertain the optimal personalized treatment regimen. The approach to therapy was further complicated by newly identified pregnancy necessitating cessation of systemic glucocorticoids. In addition to glucocorticoids, hydroxyurea and interferon-α are among treatments historically used for HES, with tyrosine kinase inhibitors and monoclonal antibodies targeting IL-5 becoming more common.1-4 Although this patient may ultimately benefit from an IL-5 targeting biologic medication such as mepolizumab, safety in pregnancy is not well-studied and may require close clinical monitoring with treatment deferred until after delivery if possible.3,7,8,11
Military service members with frequent geographic relocation have additional barriers to timely diagnosis with often-limited access to subspecialty care depending on the duty station. While the patient was able to receive care at a large military medical center with many subspecialists, prompt recognition and timely referral to specialists would be even more critical at a smaller treatment facility. Depending on the severity and variant of HES, patients may warrant evaluation and treatment by hematology/oncology, cardiology, pulmonology, and immunology. Although HES can present in young children and older adults, this condition is most often diagnosed during the third and fourth decades of life, putting clinicians on the front line of hypereosinophilia identification and evaluation.10 Military physicians have the additional duty to not only think ahead in their diverse clinical settings to ensure proper care for patients, but also maintain a broad differential inclusive of more rare disease processes such as HES.
CONCLUSIONS
This case emphasizes how uncontrolled or untreated HES can lead to significant end-organ damage involving multiple systems and high morbidity. Prompt recognition of hypereosinophilia with potential HES can help expedite coordination of multidisciplinary care across multiple specialties to minimize delays in diagnosis and treatment. Doing so may minimize associated morbidity and mortality, especially in individuals located at more remote duty stations or deployed to austere environments.
Hypereosinophilic syndrome (HES) is defined by marked, persistent absolute eosinophil count (AEC) > 1500 cells/μL on ≥ 2 peripheral smears separated by ≥ 1 month with evidence of accompanied end-organ damage, in the absence of other causes of eosinophilia such as malignancy, atopy, or parasitic infections.1-5 Hypereosinophilic infiltration can impact almost every organ system; however, the most profound complications in patients with HES are related to leukemias and cardiac manifestations of the disease.3,4 Although rare, the associated morbidity and mortality of HES are considerable, making prompt recognition and treatment essential. Management involves targeted therapy based on pathologic classification of HES and on decreasing associated inflammation, fibrosis, and end-organ damage.3,5-7
The patient in this case report met the diagnostic criteria for HES. However, this patient had several clinical and laboratory features that made it difficult to characterize a specific HES variant. Moreover, she had additional immunomodulating factors in the setting of pregnancy. This is the first documented case of HES of undetermined etiology diagnosed postpartum and managed in the setting of a new pregnancy.2,8
CASE PRESENTATION
A 32-year-old female active-duty military service member with allergic rhinitis and a history of childhood eczema was referred to allergy/immunology for evaluation of a new, progressive pruritic rash. Symptoms started 3 months after the birth of her first child, with a new diffuse erythematous skin rash sparing her palms, soles, and mucosal surfaces. Given her history of atopy, the rash was initially treated as severe atopic dermatitis with appropriate topical medications. The rash gradually worsened, with the development of intermittent facial swelling, night sweats, dyspnea, recurrent epigastric abdominal pain, and nausea with vomiting, resulting in decreased oral intake and weight loss.
The patient was hospitalized and received an expedited multidisciplinary evaluation by dermatology, hematology/oncology, and gastroenterology. Her AEC of 4787 cells/μL peaked on admission and was markedly elevated from the 1070 cells/μL reported in the third trimester of her pregnancy. She was found to have mature eosinophilia on skin biopsy (Figure 1), endoscopic duodenal biopsy (Figure 2), peripheral blood smear (Figure 3), and bone marrow biopsy (Figure 4).




Radiographic imaging of the chest, abdomen, and pelvis revealed hepatomegaly without detectable neoplasm. There was no clinical evidence of cardiac involvement, and evaluation with electrocardiography and echocardiography did not indicate myocarditis. Extensive laboratory testing revealed no genetic mutations indicative of familial, myeloproliferative, or lymphocytic variants of HES.
The patient received topical emollients, omeprazole 40 mg daily, and ondansetron 8 mg 3 times daily as needed for symptom management, and was started on oral prednisone 40 mg daily with improvement in dyspnea, night sweats, and gastrointestinal complaints. During the patient's 6-day hospitalization and treatment, her AECs gradually decreased to 2110 cells/μL, and decreased to 1600 cells/μL over the course of a month, remaining in the hypereosinophilic range. The patient was discovered to be pregnant while symptoms were improving, resulting in stepwise discontinuation of oral steroids, but she reported continued improvement in symptoms.
DISCUSSION
Peripheral eosinophilia has a broad differential diagnoses, including HES, parasitic infections, atopic hypersensitivity diseases, eosinophilic lung diseases, eosinophilic gastrointestinal diseases, vasculitides such as eosinophilic granulomatosis with polyangiitis, genetic syndromes predisposing to eosinophilia, episodic angioedema with eosinophilia, and chronic metabolic disease with adrenal insufficiency.1-5 HES, although rare, is a disease process with potentially devastating associated morbidity and mortality if not promptly recognized and treated. HES is further delineated by hypereosinophilia with associated eosinophil-mediated organ damage or dysfunction.3-5
Clinical manifestations of HES can differ greatly depending on the HES variant and degree of organ involvement at the time of diagnosis and throughout the disease course. Patients with HES, as well as those with asymptomatic eosinophilia or hypereosinophilia, should be closely monitored for disease progression. In addition to trending peripheral AECs, clinicians should screen for symptoms of organ involvement and perform targeted evaluation of the suspected organs to promptly identify early signs of organ involvement and initiate treatment.1-4 Recommendations regarding screening intervals vary widely from monthly to annually, depending on a patient’s specific clinical picture.
HES has been subdivided into clinically relevant variants, including myeloproliferative (M-HES), T lymphocytic (L-HES), organ-restricted (or overlap) HES, familial HES, idiopathic HES, and specific syndromes with associated hypereosinophilia.3-5,9 Patients with M-HES have elevated circulating leukocyte precursors and clinical manifestations, including but not limited to hepatosplenomegaly, anemia, and thrombocytopenia. The most commonly associated genetic mutations include the FIP1L1-PDGFR-α fusion, BCR-ABL1, PDGFRA/B, JAK2, KIT, and FGFR1.3-6 L-HES usually has predominant skin and soft tissue involvement secondary to immunoglobulin E-mediated actions with clonal expansion of T cells (most commonly CD3-4+ or CD3+CD4-CD8-).3,5,6 Familial HES, a rare variant, follows an autosomal dominant inheritance pattern and is usually present at birth. It involves chromosome 5, which contains genes coding for cytokines that drive eosinophilic proliferation, including interleukin (IL)-3, IL-5, and granulocyte-macrophage colony-stimulating factor.5,9 Hypereosinophilia in the setting of end-organ damage restricted to a single organ is considered organ-restricted HES. There can be significant hepatic and gastrointestinal dysfunction, with or without malabsorption.
HES can also manifest with hematologic malignancy, restrictive obliterative cardiomyopathies, renal injury manifested by hematuria and electrolyte derangements, and neurologic complications including hemiparesis, dysarthria, and even coma.6 Endothelial damage due to eosinophil-driven inflammation can result in thrombus formation and increased risk of thromboembolic events in various organs.3 Idiopathic HES, otherwise known as HES of unknown etiology or significance, is a diagnosis of exclusion and constitutes a cohort of patients who do not fit into the other delineated categories.3-5 These patients often have multisystem involvement, making classification and treatment a challenge.5
The patient described in this case met the diagnostic criteria for HES, but her complicated clinical and laboratory features were challenging to characterize into a specific variant of HES. Organ-restricted HES was ruled out due to skin, marrow, and duodenal infiltration. She also had the potential for lung involvement based on her clinical symptoms, however no biopsy was obtained. Laboratory testing revealed no deletions or mutations indicative of familial, myeloproliferative, or lymphocytic variants. Her multisystem involvement without an underlying associated syndrome suggests idiopathic HES or HES of undetermined significance.1-5
Most patients with HES are diagnosed between the ages of 20 and 50 years.10 While HES has its peak incidence in the fourth decade of life, acute onset of new symptoms 3 months postpartum makes this an unusual presentation. In this unique case, it is important to highlight the role of the physiologic changes of pregnancy in inflammatory mediation. The physiologic changes that occur in pregnancy to ensure fetal tolerance can have profound implications for leukocyte count, AEC, and subsequent inflammatory responses. The phenomenon of inflammatory amelioration during pregnancy is well-documented, but there has only been 1 known published case report discussing decreasing HES symptoms during pregnancy with prepregnancy and postpartum hypereosinophilia.8 It is suggested that this amelioration is secondary to cortisol and progesterone shifts that occur in pregnancy. Physiologic increases in adrenocorticotropic hormone in pregnancy leads to subsequent secretion of endogenous steroids by the adrenal cortex. In turn, pregnancy can lead to leukocytosis and eosinopenia.8 Overall, pregnancy can have beneficial immunomodulating properties in the spectrum of hypereosinophilic syndromes. Even so, this patient with HES diagnosed postpartum remains at risk for the sequelae of hypereosinophilia, regardless of potential for AEC reduction during pregnancy. Therefore, treatment considerations need to be made with the safety of the maternal-fetal dyad as a priority.
Treatment
The treatment of symptomatic HES without acute life-threatening features or associated malignancy is generally determined by clinical variant.2-4 There is insufficient data to support initiation of treatment solely based on persistently elevated AEC. Patients with peripheral eosinophilia and hypereosinophilia should be monitored periodically with appropriate subspecialist evaluation for occult end-organ involvement, and targeted therapies should be deferred until an HES diagnosis.1-4 First-line therapy in most HES variants is systemic glucocorticoids.2,3,7 Since the disease course for this patient did not precisely match an HES variant, it was challenging to ascertain the optimal personalized treatment regimen. The approach to therapy was further complicated by newly identified pregnancy necessitating cessation of systemic glucocorticoids. In addition to glucocorticoids, hydroxyurea and interferon-α are among treatments historically used for HES, with tyrosine kinase inhibitors and monoclonal antibodies targeting IL-5 becoming more common.1-4 Although this patient may ultimately benefit from an IL-5 targeting biologic medication such as mepolizumab, safety in pregnancy is not well-studied and may require close clinical monitoring with treatment deferred until after delivery if possible.3,7,8,11
Military service members with frequent geographic relocation have additional barriers to timely diagnosis with often-limited access to subspecialty care depending on the duty station. While the patient was able to receive care at a large military medical center with many subspecialists, prompt recognition and timely referral to specialists would be even more critical at a smaller treatment facility. Depending on the severity and variant of HES, patients may warrant evaluation and treatment by hematology/oncology, cardiology, pulmonology, and immunology. Although HES can present in young children and older adults, this condition is most often diagnosed during the third and fourth decades of life, putting clinicians on the front line of hypereosinophilia identification and evaluation.10 Military physicians have the additional duty to not only think ahead in their diverse clinical settings to ensure proper care for patients, but also maintain a broad differential inclusive of more rare disease processes such as HES.
CONCLUSIONS
This case emphasizes how uncontrolled or untreated HES can lead to significant end-organ damage involving multiple systems and high morbidity. Prompt recognition of hypereosinophilia with potential HES can help expedite coordination of multidisciplinary care across multiple specialties to minimize delays in diagnosis and treatment. Doing so may minimize associated morbidity and mortality, especially in individuals located at more remote duty stations or deployed to austere environments.
- Cogan E, Roufosse F. Clinical management of the hypereosinophilic syndromes. Expert Rev Hematol. 2012;5:275-290. doi: 10.1586/ehm.12.14
- Klion A. Hypereosinophilic syndrome: approach to treatment in the era of precision medicine. Hematology Am Soc Hematol Educ Program. 2018;2018:326-331. doi:10.1182/asheducation-2018.1.326
- Shomali W, Gotlib J. World health organization-defined eosinophilic disorders: 2022 update on diagnosis, risk stratification, and management. Am J Hematol. 2022;97:129-148. doi:10.1002/ajh.26352
- Helbig G, Klion AD. Hypereosinophilic syndromes - an enigmatic group of disorders with an intriguing clinical spectrum and challenging treatment. Blood Rev. 2021;49:100809. doi:10.1016/j.blre.2021.100809
- Valent P, Klion AD, Horny HP, et al. Contemporary consensus proposal on criteria and classification of eosinophilic disorders and related syndromes. J Allergy Clin Immunol. 2012;130:607-612.e9. doi:10.1016/j.jaci.2012.02.019
- Roufosse FE, Goldman M, Cogan E. Hypereosinophilic syndromes. Orphanet J Rare Dis. 2007;2:37. doi:10.1186/1750-1172-2-37
- Pitlick MM, Li JT, Pongdee T. Current and emerging biologic therapies targeting eosinophilic disorders. World Allergy Organ J. 2022;15:100676. doi:10.1016/j.waojou.2022.10067
- Ault P, Cortes J, Lynn A, Keating M, Verstovsek S. Pregnancy in a patient with hypereosinophilic syndrome. Leuk Res. 2009;33:186-187. doi:10.1016/j.leukres.2008.05.013
- Rioux JD, Stone VA, Daly MJ, et al. Familial eosinophilia maps to the cytokine gene cluster on human chromosomal region 5q31-q33. Am J Hum Genet. 1998;63:1086-1094. doi:10.1086/302053
- Williams KW, Ware J, Abiodun A, et al. Hypereosinophilia in children and adults: a retrospective comparison. J Allergy Clin Immunol Pract. 2016;4:941-947.e1. doi:10.1016/j.jaip.2016.03.020
- Pane F, Lefevre G, Kwon N, et al. Characterization of disease flares and impact of mepolizumab in patients with hypereosinophilic syndrome. Front Immunol. 2022;13:935996. doi:10.3389/fimmu.2022.935996
- Cogan E, Roufosse F. Clinical management of the hypereosinophilic syndromes. Expert Rev Hematol. 2012;5:275-290. doi: 10.1586/ehm.12.14
- Klion A. Hypereosinophilic syndrome: approach to treatment in the era of precision medicine. Hematology Am Soc Hematol Educ Program. 2018;2018:326-331. doi:10.1182/asheducation-2018.1.326
- Shomali W, Gotlib J. World health organization-defined eosinophilic disorders: 2022 update on diagnosis, risk stratification, and management. Am J Hematol. 2022;97:129-148. doi:10.1002/ajh.26352
- Helbig G, Klion AD. Hypereosinophilic syndromes - an enigmatic group of disorders with an intriguing clinical spectrum and challenging treatment. Blood Rev. 2021;49:100809. doi:10.1016/j.blre.2021.100809
- Valent P, Klion AD, Horny HP, et al. Contemporary consensus proposal on criteria and classification of eosinophilic disorders and related syndromes. J Allergy Clin Immunol. 2012;130:607-612.e9. doi:10.1016/j.jaci.2012.02.019
- Roufosse FE, Goldman M, Cogan E. Hypereosinophilic syndromes. Orphanet J Rare Dis. 2007;2:37. doi:10.1186/1750-1172-2-37
- Pitlick MM, Li JT, Pongdee T. Current and emerging biologic therapies targeting eosinophilic disorders. World Allergy Organ J. 2022;15:100676. doi:10.1016/j.waojou.2022.10067
- Ault P, Cortes J, Lynn A, Keating M, Verstovsek S. Pregnancy in a patient with hypereosinophilic syndrome. Leuk Res. 2009;33:186-187. doi:10.1016/j.leukres.2008.05.013
- Rioux JD, Stone VA, Daly MJ, et al. Familial eosinophilia maps to the cytokine gene cluster on human chromosomal region 5q31-q33. Am J Hum Genet. 1998;63:1086-1094. doi:10.1086/302053
- Williams KW, Ware J, Abiodun A, et al. Hypereosinophilia in children and adults: a retrospective comparison. J Allergy Clin Immunol Pract. 2016;4:941-947.e1. doi:10.1016/j.jaip.2016.03.020
- Pane F, Lefevre G, Kwon N, et al. Characterization of disease flares and impact of mepolizumab in patients with hypereosinophilic syndrome. Front Immunol. 2022;13:935996. doi:10.3389/fimmu.2022.935996
Unique Presentation of Postpartum Hypereosinophilic Syndrome With Atypical Features and Therapeutic Challenges
Unique Presentation of Postpartum Hypereosinophilic Syndrome With Atypical Features and Therapeutic Challenges
Empowering Culture Change and Safety on the Journey to Zero Harm With Huddle Cards
Empowering Culture Change and Safety on the Journey to Zero Harm With Huddle Cards
Safety event reporting plays a vital role in fostering a culture of safety within a health care organization. The US Department of Veterans Affairs (VA) has shifted its focus from eradicating medical errors to minimizing or eliminating harm to patients.1 The National Center for Patient Safety’s objective is to prevent recurring errors by identifying and addressing systemic problems that may have been overlooked.2
Taking inspiration from industries known for high reliability, such as aviation and nuclear power, the Veterans Health Administration (VHA) patient safety program aims to identify and eliminate system vulnerabilities, such as medical errors. Learning from near misses, which occur more frequently than actual adverse events, is a crucial part of this process.3 By addressing these issues, the VHA can establish safer systems and encourage continuous identification of potential problems with proactive resolution.
All staff should participate actively in event reporting, which involves documenting and communicating details, outcomes, and relevant data about an event to understand what occurred, evaluate success, identify areas for improvement, and inform future decisions. This helps identify system weaknesses, create opportunities to standardize procedures and enhance patient care.
At the high complexity Central Texas Veterans Health Care System (CTVHCS), the fiscal year (FY) 2023 All Employee Survey (AES) found that staff members require additional education and awareness regarding the reporting of patient safety concerns.4 The survey highlighted areas such as lack of education on reporting, doubts about the effectiveness of reporting, confusion about the process after a report is made, and insufficient feedback.
BACKGROUND
To improve the culture of safety and address deficiencies noted in the AES, the CTVHCS patient safety (PS) and high reliability organization (HRO) teams partnered to develop a quality improvement initiative to increase staff understanding of safety event reporting and strengthen the safety culture. The PS and HRO teams developed an innovative education model that integrates Joint Patient Safety Reporting System (JPSR) education into huddles.
This initiative, called the JPSR Huddle Card Toolkit, sought to assess the impact of the toolkit on staff knowledge and behaviors related to patient safety event reporting. The toolkit consisted of educational materials encompassing 6 key areas: (1) reporting incidents; (2) close calls and near misses; (3) identification of root causes; (4) understanding the life cycle of a JPSR; (5) celebrating achievements; and (6) distinguishing between facts and fiction. Each JPSR huddle card included discussion points for the facilitator and was formatted on a 5 × 7-inch card (Figure 1). Topics were addressed during weekly safety huddles conducted in the pilot unit over a 6-week period. To evaluate its effectiveness, a pilot unit was selected and distributed an anonymous questionnaire paired with the JPSR huddle card toolkit to measure staff responses.

The pilot was conducted from November 2023 to January 2024. The participating pilot unit was a 10-bed critical care unit with 42 full-time employees. Nursing leadership, quality safety, and value personnel, and the Veterans Integrated Services Network (VISN) PS Team reviewed and approved the pilot.
Reporting of adverse events and near misses provides an opportunity to learn about latent systems errors.2 In 2018, the VHA began using the JPSR to standardize the capture and data management on medical errors and close calls across the Defense Health Administration (DHA) and VHA.1 The JPSR software is a joint application of the VHA and DHA. It improves the identification and documentation of patient safety-related events for VA medical centers, military hospitals and clinics, active-duty personnel, veterans and their families.
Event reporting is a key element in advancing high reliability and achieving zero preventable harm.1 Teams use these data to identify organizational patient safety trends and preempt common safety issues. All data are protected under 38 USC §5705 and 10 USC §1102.5 The JPSR single-source system standardizes the collection of core data points and increases collaboration between the DHA and VHA. This partnership increases insight into safety-related incidents, allowing for earlier detection and prevention of patient harm or injury incidents.
Numerous studies consistently commend huddles for their effectiveness in promoting teamwork and their positive impact on patient safety.6-8 Huddles facilitate connections between employees who may not typically interact, provide opportunities for discussions, and serve as a platform to encourage employees to voice their opinions. By fostering these interactions, huddles empower employees and create an environment for shared understanding, building trust, and promoting continuous learning.8
OBSERVATIONS
The JPSR huddle card initiative aimed to improve understanding of the JPSR process and promote knowledge and attitudes about patient safety and event reporting, while emphasizing shared responsibility. The goals focused on effective communication, respect for expertise, awareness of operational nuances, voicing concerns, and ensuring zero harm.
The facilitator initiated huddles by announcing their start to cultivate a constructive outcome.8 The JPSR huddle cards used a structured format designed to foster engagement and understanding of the topic. Each card begins with a factual statement or an open-ended question to gauge participants’ awareness or understanding. It then provides essential facts, principles, and relevant information to deepen knowledge. The card concludes with a discussion question, allowing facilitators to assess shared learning and encourage group reflection. This format promotes active participation and ensures that key concepts are both introduced and reinforced through dialogue.
The PS team standardized the format for all huddle cards, allowing 5 to 10 minutes for discussing training materials, receiving feedback, and concluding with a discussion question and call to action. Prior to each huddle, the facilitator would read a scripted remark that reviewed the objectives and ground rules for an effective huddle.
The PS and HRO teams promoted interactive discussions and welcomed ongoing feedback. Huddles provided a psychologically safe environment where individuals were encouraged to voice their thoughts and ideas.
Each weekly huddle card addressed a different patient safety topic. The Week 1 huddle card focuses on event reporting for safety improvement. The card outlines the purpose of JPSR as a tool to identify, manage, and analyze safety events to reduce preventable harm. The card emphasizes 3 core principles: (1) acknowledging mistakes, recognizing that errors happen; (2) no blame, no shame (encouraging a no-blame just culture to raise concerns); and (3) continuous improvement (committing to ongoing learning and prevention). It provides guidance on event details entry, advising staff to include facts in an SBAR (Situation, Background, Assessment, Response) format, avoid assumptions, and exclude personal identifiers. Tips include entering only relevant facts to help reviewers understand the incident. The card ends with discussion questions on reporting barriers and potential improvements in event reporting practices.
The Week 2 huddle card focuses on understanding and reporting near miss events, also known as close calls or good catches. A near miss is an incident where a potential hazard was identified and prevented before it reached the patient, avoiding harm due to timely intervention. The card emphasizes the importance of identifying these events to understand weaknesses and proactively reduce risks. Examples of near misses include discovering expired medication before use, catching a potential wrong-site surgery, and noticing incorrect medication dosages. Staff are encouraged to develop a mindset for anticipating and solving risks. The card ends with a discussion asking participants to share examples of near misses in their area.
The Week 3 huddle card covers root causes in preventing errors. The card highlights that errors in health care often stem from flawed processes rather than individual faults. By identifying root causes, systemic weaknesses can be addressed to reduce mistakes and build more error-tolerant and robust systems. All staff are advised to adopt a mindset of continuous improvement, error trapping behaviors and problem-solving. It concludes with discussion questions prompting reflection on assumptions and identifying weaknesses when something goes wrong.
The Week 4 huddle card covers the life of a JPSR, detailing that after entry JPSR events are viewed by the highest leadership levels at the morning report, and that lessons learned are distributed through frontline managers and chiefs in a monthly report to be shared with frontline staff. Additionally, JPSR trends are shared during monthly HRO safety forums. These practices promote a culture of safety through open communication and problem-solving. Staff and leaders are encouraged to prioritize safety daily. Discussion prompts ask team members if they had seen positive changes from JPSR reporting and what they would like leadership to communicate after investigations.
The Week 5 huddle card covers celebrating safety event reporting called Cue the Confetti. The VHA emphasizes recognizing staff who report safety events as part of their commitment to zero harm. By celebrating these contributions, the VHA fosters respect, joy, and satisfaction in the work. Staff are encouraged to nominate colleagues for recognition, reinforcing a supportive environment. Prompts invite teams to discuss how they celebrate JPSR reporting and how they’d like to enhance this culture of appreciation.
The Week 6 huddle card covers common misconceptions about JPSR. Key facts include that JPSRs are confidential, not for disciplinary action, and can be submitted by any staff member at any time. Only PS can view reporter identities for clarification purposes. The card concludes with prompts to ensure staff know how to access JPSR support and resources.
Measuring the impact on staff was essential to assess effectiveness and gather data for program improvement. To evaluate the impact of the huddle cards on the staff, the team provided a voluntary and anonymous 9 question survey (Figure 2). The survey was completed before the pilot began and again at the end of Week 6.
Questions 1 through 5 and 7 through 9 pertained to participants’ perceived knowledge and understanding of aspects of the JPSR. Perceived improvement among intensive care unit (ICU) participants ranged from 15% to 53%. There was a positive increase associated with every question with the top improvements: question 8, “How do you rate your understanding of how we share safety events for system and process improvement?” (53.4% increase); question 5, “How do you rate your understanding of what happens to a JPSR after it is entered?” (51.9% increase), and question 9, “How do you rate your understanding of the concepts of trust, psychological safety and a just culture?” (47.8% increase).
The survey analysis was not able to track individual changes. As a result, the findings reflect an overall change for the entire study group. Moreover, the questions assessed participants’ perceived knowledge rather than actual knowledge gained. It is important to note that there may be a significant gap between the actual knowledge gained and how participants perceive it. Additionally, improvement in knowledge and comprehension does not necessarily translate into behavior changes.
CONCLUSIONS
The use of JPSR huddle cards and direct engagement with staff during safety huddles yielded positive outcomes. On average, participants demonstrated higher scores in posttest questions compared to pretest questions. The posttest scores were consistently higher than the pretest scores, showing an average increase of around 2 standard deviations across all questions. This indicates an improvement in participants’ perceived knowledge and comprehension of the JPSR material.
During the pilot implementation of the huddle cards, there was a notable improvement in team member engagement. The structured format of the cards facilitated focused and meaningful discussions during safety huddles, encouraging open dialogue and fostering a culture of safety. Team members actively participated in identifying potential risks, sharing observations, and proposing actionable solutions, which reflected an enhanced sense of ownership regarding safety practices.
The support dialogue facilitated by the huddle cards highlighted the significance of mutual accountability and a collective commitment to achieving zero harm. This collaborative environment strengthened trust among team members and underscored the importance of shared vigilance in preventing adverse events. The pilot demonstrated the potential of huddle cards as an essential tool for enhancing team-based safety initiatives and promoting a culture of high reliability within the organization.
The total number of JPSR events in the ICU rose from 156 in FY 23 to 170 in FY 24. Adverse events increased from 19 to 31, while close calls saw a slight uptick from 137 to 139. Despite the overall rise in adverse events, a detailed analysis indicated that incidents of moderate harm decreased from 4 in FY 23 to 2 in FY 24. Furthermore, there was 1 reported case of death or severe harm in FY 23, which decreased to 0 in FY 24. This trend is consistent with the overarching objective of a high-reliability organization to achieve zero harm.
The next step is to expand this initiative across CTVHCS. This initiative aims to make this an annual education for all areas. The JPSR huddle card toolkit will be formatted by the media department for easy printing and retrieval. Leaders within units, clinics, and services will be empowered to facilitate the sessions in their safety huddles and reap the same outcomes as in the pilot. CTVHCS PS will monitor the effectiveness of this through ongoing CTVHCS patient safety rounding and future AES.
- Essen K, Villalobos C, Sculli GL, Steinbach L. Establishing a just culture: implications for the Veterans Health Administration journey to high reliability. Fed Pract. 2024;41:290-297. doi:10.12788/fp.0512
- Louis MY, Hussain LR, Dhanraj DN, et al. Improving patient safety event reporting among residents and teaching faculty. Ochsner J. 2016;16:73-80.
- Pimental CB, Snow AL, Carnes SL, et al. Huddles and their effectiveness at the frontlines of clinical care: a scoping review. J Gen Intern Med. 2021;36:2772-2783. doi:10.1007/s11606-021-06632-9
- National Academies of Sciences, Engineering, and Medicine. Appendix C: Nature of Veterans Health Administration Facilities Management (Engineering) Tasks and Staffing. Facilities Staffing Requirements for the Veterans Health Administration-Resource Planning and Methodology for the Future. National Academies Press. 2020:105-116. Accessed August 11, 2025. https://nap.nationalacademies.org/read/25454/chapter/11
- Woodier N, Burnett C, Moppett I. The value of learning from near misses to improve patient safety: a scoping review. J Patient Saf. 2023;19:42-47. doi:10.1097/pts.0000000000001078
- Ismail A, Khalid SNM. Patient safety culture and its determinants among healthcare professionals at a cluster hospital in Malaysia: a cross-sectional study. BMJ Open. 2022;12:e060546. doi:10.1136/bmjopen-2021-060546
- Ngo J, Lau D, Ploquin J, Receveur T, Stassen K, Del Castilho C. Improving incident reporting among physicians at south health campus hospital. BMJ Open Qual. 2022;11:e001945. doi:10.1136/bmjoq-2022-001945
- Oweidat I, Al-Mugheed K, Alsenany SA, et al. Awareness of reporting practices and barriers to incident reporting among nurses. BMC Nurs. 2023;22:231. doi:10.1186/s12912-023-01376-9
Safety event reporting plays a vital role in fostering a culture of safety within a health care organization. The US Department of Veterans Affairs (VA) has shifted its focus from eradicating medical errors to minimizing or eliminating harm to patients.1 The National Center for Patient Safety’s objective is to prevent recurring errors by identifying and addressing systemic problems that may have been overlooked.2
Taking inspiration from industries known for high reliability, such as aviation and nuclear power, the Veterans Health Administration (VHA) patient safety program aims to identify and eliminate system vulnerabilities, such as medical errors. Learning from near misses, which occur more frequently than actual adverse events, is a crucial part of this process.3 By addressing these issues, the VHA can establish safer systems and encourage continuous identification of potential problems with proactive resolution.
All staff should participate actively in event reporting, which involves documenting and communicating details, outcomes, and relevant data about an event to understand what occurred, evaluate success, identify areas for improvement, and inform future decisions. This helps identify system weaknesses, create opportunities to standardize procedures and enhance patient care.
At the high complexity Central Texas Veterans Health Care System (CTVHCS), the fiscal year (FY) 2023 All Employee Survey (AES) found that staff members require additional education and awareness regarding the reporting of patient safety concerns.4 The survey highlighted areas such as lack of education on reporting, doubts about the effectiveness of reporting, confusion about the process after a report is made, and insufficient feedback.
BACKGROUND
To improve the culture of safety and address deficiencies noted in the AES, the CTVHCS patient safety (PS) and high reliability organization (HRO) teams partnered to develop a quality improvement initiative to increase staff understanding of safety event reporting and strengthen the safety culture. The PS and HRO teams developed an innovative education model that integrates Joint Patient Safety Reporting System (JPSR) education into huddles.
This initiative, called the JPSR Huddle Card Toolkit, sought to assess the impact of the toolkit on staff knowledge and behaviors related to patient safety event reporting. The toolkit consisted of educational materials encompassing 6 key areas: (1) reporting incidents; (2) close calls and near misses; (3) identification of root causes; (4) understanding the life cycle of a JPSR; (5) celebrating achievements; and (6) distinguishing between facts and fiction. Each JPSR huddle card included discussion points for the facilitator and was formatted on a 5 × 7-inch card (Figure 1). Topics were addressed during weekly safety huddles conducted in the pilot unit over a 6-week period. To evaluate its effectiveness, a pilot unit was selected and distributed an anonymous questionnaire paired with the JPSR huddle card toolkit to measure staff responses.

The pilot was conducted from November 2023 to January 2024. The participating pilot unit was a 10-bed critical care unit with 42 full-time employees. Nursing leadership, quality safety, and value personnel, and the Veterans Integrated Services Network (VISN) PS Team reviewed and approved the pilot.
Reporting of adverse events and near misses provides an opportunity to learn about latent systems errors.2 In 2018, the VHA began using the JPSR to standardize the capture and data management on medical errors and close calls across the Defense Health Administration (DHA) and VHA.1 The JPSR software is a joint application of the VHA and DHA. It improves the identification and documentation of patient safety-related events for VA medical centers, military hospitals and clinics, active-duty personnel, veterans and their families.
Event reporting is a key element in advancing high reliability and achieving zero preventable harm.1 Teams use these data to identify organizational patient safety trends and preempt common safety issues. All data are protected under 38 USC §5705 and 10 USC §1102.5 The JPSR single-source system standardizes the collection of core data points and increases collaboration between the DHA and VHA. This partnership increases insight into safety-related incidents, allowing for earlier detection and prevention of patient harm or injury incidents.
Numerous studies consistently commend huddles for their effectiveness in promoting teamwork and their positive impact on patient safety.6-8 Huddles facilitate connections between employees who may not typically interact, provide opportunities for discussions, and serve as a platform to encourage employees to voice their opinions. By fostering these interactions, huddles empower employees and create an environment for shared understanding, building trust, and promoting continuous learning.8
OBSERVATIONS
The JPSR huddle card initiative aimed to improve understanding of the JPSR process and promote knowledge and attitudes about patient safety and event reporting, while emphasizing shared responsibility. The goals focused on effective communication, respect for expertise, awareness of operational nuances, voicing concerns, and ensuring zero harm.
The facilitator initiated huddles by announcing their start to cultivate a constructive outcome.8 The JPSR huddle cards used a structured format designed to foster engagement and understanding of the topic. Each card begins with a factual statement or an open-ended question to gauge participants’ awareness or understanding. It then provides essential facts, principles, and relevant information to deepen knowledge. The card concludes with a discussion question, allowing facilitators to assess shared learning and encourage group reflection. This format promotes active participation and ensures that key concepts are both introduced and reinforced through dialogue.
The PS team standardized the format for all huddle cards, allowing 5 to 10 minutes for discussing training materials, receiving feedback, and concluding with a discussion question and call to action. Prior to each huddle, the facilitator would read a scripted remark that reviewed the objectives and ground rules for an effective huddle.
The PS and HRO teams promoted interactive discussions and welcomed ongoing feedback. Huddles provided a psychologically safe environment where individuals were encouraged to voice their thoughts and ideas.
Each weekly huddle card addressed a different patient safety topic. The Week 1 huddle card focuses on event reporting for safety improvement. The card outlines the purpose of JPSR as a tool to identify, manage, and analyze safety events to reduce preventable harm. The card emphasizes 3 core principles: (1) acknowledging mistakes, recognizing that errors happen; (2) no blame, no shame (encouraging a no-blame just culture to raise concerns); and (3) continuous improvement (committing to ongoing learning and prevention). It provides guidance on event details entry, advising staff to include facts in an SBAR (Situation, Background, Assessment, Response) format, avoid assumptions, and exclude personal identifiers. Tips include entering only relevant facts to help reviewers understand the incident. The card ends with discussion questions on reporting barriers and potential improvements in event reporting practices.
The Week 2 huddle card focuses on understanding and reporting near miss events, also known as close calls or good catches. A near miss is an incident where a potential hazard was identified and prevented before it reached the patient, avoiding harm due to timely intervention. The card emphasizes the importance of identifying these events to understand weaknesses and proactively reduce risks. Examples of near misses include discovering expired medication before use, catching a potential wrong-site surgery, and noticing incorrect medication dosages. Staff are encouraged to develop a mindset for anticipating and solving risks. The card ends with a discussion asking participants to share examples of near misses in their area.
The Week 3 huddle card covers root causes in preventing errors. The card highlights that errors in health care often stem from flawed processes rather than individual faults. By identifying root causes, systemic weaknesses can be addressed to reduce mistakes and build more error-tolerant and robust systems. All staff are advised to adopt a mindset of continuous improvement, error trapping behaviors and problem-solving. It concludes with discussion questions prompting reflection on assumptions and identifying weaknesses when something goes wrong.
The Week 4 huddle card covers the life of a JPSR, detailing that after entry JPSR events are viewed by the highest leadership levels at the morning report, and that lessons learned are distributed through frontline managers and chiefs in a monthly report to be shared with frontline staff. Additionally, JPSR trends are shared during monthly HRO safety forums. These practices promote a culture of safety through open communication and problem-solving. Staff and leaders are encouraged to prioritize safety daily. Discussion prompts ask team members if they had seen positive changes from JPSR reporting and what they would like leadership to communicate after investigations.
The Week 5 huddle card covers celebrating safety event reporting called Cue the Confetti. The VHA emphasizes recognizing staff who report safety events as part of their commitment to zero harm. By celebrating these contributions, the VHA fosters respect, joy, and satisfaction in the work. Staff are encouraged to nominate colleagues for recognition, reinforcing a supportive environment. Prompts invite teams to discuss how they celebrate JPSR reporting and how they’d like to enhance this culture of appreciation.
The Week 6 huddle card covers common misconceptions about JPSR. Key facts include that JPSRs are confidential, not for disciplinary action, and can be submitted by any staff member at any time. Only PS can view reporter identities for clarification purposes. The card concludes with prompts to ensure staff know how to access JPSR support and resources.
Measuring the impact on staff was essential to assess effectiveness and gather data for program improvement. To evaluate the impact of the huddle cards on the staff, the team provided a voluntary and anonymous 9 question survey (Figure 2). The survey was completed before the pilot began and again at the end of Week 6.

Questions 1 through 5 and 7 through 9 pertained to participants’ perceived knowledge and understanding of aspects of the JPSR. Perceived improvement among intensive care unit (ICU) participants ranged from 15% to 53%. There was a positive increase associated with every question with the top improvements: question 8, “How do you rate your understanding of how we share safety events for system and process improvement?” (53.4% increase); question 5, “How do you rate your understanding of what happens to a JPSR after it is entered?” (51.9% increase), and question 9, “How do you rate your understanding of the concepts of trust, psychological safety and a just culture?” (47.8% increase).
The survey analysis was not able to track individual changes. As a result, the findings reflect an overall change for the entire study group. Moreover, the questions assessed participants’ perceived knowledge rather than actual knowledge gained. It is important to note that there may be a significant gap between the actual knowledge gained and how participants perceive it. Additionally, improvement in knowledge and comprehension does not necessarily translate into behavior changes.
CONCLUSIONS
The use of JPSR huddle cards and direct engagement with staff during safety huddles yielded positive outcomes. On average, participants demonstrated higher scores in posttest questions compared to pretest questions. The posttest scores were consistently higher than the pretest scores, showing an average increase of around 2 standard deviations across all questions. This indicates an improvement in participants’ perceived knowledge and comprehension of the JPSR material.
During the pilot implementation of the huddle cards, there was a notable improvement in team member engagement. The structured format of the cards facilitated focused and meaningful discussions during safety huddles, encouraging open dialogue and fostering a culture of safety. Team members actively participated in identifying potential risks, sharing observations, and proposing actionable solutions, which reflected an enhanced sense of ownership regarding safety practices.
The support dialogue facilitated by the huddle cards highlighted the significance of mutual accountability and a collective commitment to achieving zero harm. This collaborative environment strengthened trust among team members and underscored the importance of shared vigilance in preventing adverse events. The pilot demonstrated the potential of huddle cards as an essential tool for enhancing team-based safety initiatives and promoting a culture of high reliability within the organization.
The total number of JPSR events in the ICU rose from 156 in FY 23 to 170 in FY 24. Adverse events increased from 19 to 31, while close calls saw a slight uptick from 137 to 139. Despite the overall rise in adverse events, a detailed analysis indicated that incidents of moderate harm decreased from 4 in FY 23 to 2 in FY 24. Furthermore, there was 1 reported case of death or severe harm in FY 23, which decreased to 0 in FY 24. This trend is consistent with the overarching objective of a high-reliability organization to achieve zero harm.
The next step is to expand this initiative across CTVHCS. This initiative aims to make this an annual education for all areas. The JPSR huddle card toolkit will be formatted by the media department for easy printing and retrieval. Leaders within units, clinics, and services will be empowered to facilitate the sessions in their safety huddles and reap the same outcomes as in the pilot. CTVHCS PS will monitor the effectiveness of this through ongoing CTVHCS patient safety rounding and future AES.
Safety event reporting plays a vital role in fostering a culture of safety within a health care organization. The US Department of Veterans Affairs (VA) has shifted its focus from eradicating medical errors to minimizing or eliminating harm to patients.1 The National Center for Patient Safety’s objective is to prevent recurring errors by identifying and addressing systemic problems that may have been overlooked.2
Taking inspiration from industries known for high reliability, such as aviation and nuclear power, the Veterans Health Administration (VHA) patient safety program aims to identify and eliminate system vulnerabilities, such as medical errors. Learning from near misses, which occur more frequently than actual adverse events, is a crucial part of this process.3 By addressing these issues, the VHA can establish safer systems and encourage continuous identification of potential problems with proactive resolution.
All staff should participate actively in event reporting, which involves documenting and communicating details, outcomes, and relevant data about an event to understand what occurred, evaluate success, identify areas for improvement, and inform future decisions. This helps identify system weaknesses, create opportunities to standardize procedures and enhance patient care.
At the high complexity Central Texas Veterans Health Care System (CTVHCS), the fiscal year (FY) 2023 All Employee Survey (AES) found that staff members require additional education and awareness regarding the reporting of patient safety concerns.4 The survey highlighted areas such as lack of education on reporting, doubts about the effectiveness of reporting, confusion about the process after a report is made, and insufficient feedback.
BACKGROUND
To improve the culture of safety and address deficiencies noted in the AES, the CTVHCS patient safety (PS) and high reliability organization (HRO) teams partnered to develop a quality improvement initiative to increase staff understanding of safety event reporting and strengthen the safety culture. The PS and HRO teams developed an innovative education model that integrates Joint Patient Safety Reporting System (JPSR) education into huddles.
This initiative, called the JPSR Huddle Card Toolkit, sought to assess the impact of the toolkit on staff knowledge and behaviors related to patient safety event reporting. The toolkit consisted of educational materials encompassing 6 key areas: (1) reporting incidents; (2) close calls and near misses; (3) identification of root causes; (4) understanding the life cycle of a JPSR; (5) celebrating achievements; and (6) distinguishing between facts and fiction. Each JPSR huddle card included discussion points for the facilitator and was formatted on a 5 × 7-inch card (Figure 1). Topics were addressed during weekly safety huddles conducted in the pilot unit over a 6-week period. To evaluate its effectiveness, a pilot unit was selected and distributed an anonymous questionnaire paired with the JPSR huddle card toolkit to measure staff responses.

The pilot was conducted from November 2023 to January 2024. The participating pilot unit was a 10-bed critical care unit with 42 full-time employees. Nursing leadership, quality safety, and value personnel, and the Veterans Integrated Services Network (VISN) PS Team reviewed and approved the pilot.
Reporting of adverse events and near misses provides an opportunity to learn about latent systems errors.2 In 2018, the VHA began using the JPSR to standardize the capture and data management on medical errors and close calls across the Defense Health Administration (DHA) and VHA.1 The JPSR software is a joint application of the VHA and DHA. It improves the identification and documentation of patient safety-related events for VA medical centers, military hospitals and clinics, active-duty personnel, veterans and their families.
Event reporting is a key element in advancing high reliability and achieving zero preventable harm.1 Teams use these data to identify organizational patient safety trends and preempt common safety issues. All data are protected under 38 USC §5705 and 10 USC §1102.5 The JPSR single-source system standardizes the collection of core data points and increases collaboration between the DHA and VHA. This partnership increases insight into safety-related incidents, allowing for earlier detection and prevention of patient harm or injury incidents.
Numerous studies consistently commend huddles for their effectiveness in promoting teamwork and their positive impact on patient safety.6-8 Huddles facilitate connections between employees who may not typically interact, provide opportunities for discussions, and serve as a platform to encourage employees to voice their opinions. By fostering these interactions, huddles empower employees and create an environment for shared understanding, building trust, and promoting continuous learning.8
OBSERVATIONS
The JPSR huddle card initiative aimed to improve understanding of the JPSR process and promote knowledge and attitudes about patient safety and event reporting, while emphasizing shared responsibility. The goals focused on effective communication, respect for expertise, awareness of operational nuances, voicing concerns, and ensuring zero harm.
The facilitator initiated huddles by announcing their start to cultivate a constructive outcome.8 The JPSR huddle cards used a structured format designed to foster engagement and understanding of the topic. Each card begins with a factual statement or an open-ended question to gauge participants’ awareness or understanding. It then provides essential facts, principles, and relevant information to deepen knowledge. The card concludes with a discussion question, allowing facilitators to assess shared learning and encourage group reflection. This format promotes active participation and ensures that key concepts are both introduced and reinforced through dialogue.
The PS team standardized the format for all huddle cards, allowing 5 to 10 minutes for discussing training materials, receiving feedback, and concluding with a discussion question and call to action. Prior to each huddle, the facilitator would read a scripted remark that reviewed the objectives and ground rules for an effective huddle.
The PS and HRO teams promoted interactive discussions and welcomed ongoing feedback. Huddles provided a psychologically safe environment where individuals were encouraged to voice their thoughts and ideas.
Each weekly huddle card addressed a different patient safety topic. The Week 1 huddle card focuses on event reporting for safety improvement. The card outlines the purpose of JPSR as a tool to identify, manage, and analyze safety events to reduce preventable harm. The card emphasizes 3 core principles: (1) acknowledging mistakes, recognizing that errors happen; (2) no blame, no shame (encouraging a no-blame just culture to raise concerns); and (3) continuous improvement (committing to ongoing learning and prevention). It provides guidance on event details entry, advising staff to include facts in an SBAR (Situation, Background, Assessment, Response) format, avoid assumptions, and exclude personal identifiers. Tips include entering only relevant facts to help reviewers understand the incident. The card ends with discussion questions on reporting barriers and potential improvements in event reporting practices.
The Week 2 huddle card focuses on understanding and reporting near miss events, also known as close calls or good catches. A near miss is an incident where a potential hazard was identified and prevented before it reached the patient, avoiding harm due to timely intervention. The card emphasizes the importance of identifying these events to understand weaknesses and proactively reduce risks. Examples of near misses include discovering expired medication before use, catching a potential wrong-site surgery, and noticing incorrect medication dosages. Staff are encouraged to develop a mindset for anticipating and solving risks. The card ends with a discussion asking participants to share examples of near misses in their area.
The Week 3 huddle card covers root causes in preventing errors. The card highlights that errors in health care often stem from flawed processes rather than individual faults. By identifying root causes, systemic weaknesses can be addressed to reduce mistakes and build more error-tolerant and robust systems. All staff are advised to adopt a mindset of continuous improvement, error trapping behaviors and problem-solving. It concludes with discussion questions prompting reflection on assumptions and identifying weaknesses when something goes wrong.
The Week 4 huddle card covers the life of a JPSR, detailing that after entry JPSR events are viewed by the highest leadership levels at the morning report, and that lessons learned are distributed through frontline managers and chiefs in a monthly report to be shared with frontline staff. Additionally, JPSR trends are shared during monthly HRO safety forums. These practices promote a culture of safety through open communication and problem-solving. Staff and leaders are encouraged to prioritize safety daily. Discussion prompts ask team members if they had seen positive changes from JPSR reporting and what they would like leadership to communicate after investigations.
The Week 5 huddle card covers celebrating safety event reporting called Cue the Confetti. The VHA emphasizes recognizing staff who report safety events as part of their commitment to zero harm. By celebrating these contributions, the VHA fosters respect, joy, and satisfaction in the work. Staff are encouraged to nominate colleagues for recognition, reinforcing a supportive environment. Prompts invite teams to discuss how they celebrate JPSR reporting and how they’d like to enhance this culture of appreciation.
The Week 6 huddle card covers common misconceptions about JPSR. Key facts include that JPSRs are confidential, not for disciplinary action, and can be submitted by any staff member at any time. Only PS can view reporter identities for clarification purposes. The card concludes with prompts to ensure staff know how to access JPSR support and resources.
Measuring the impact on staff was essential to assess effectiveness and gather data for program improvement. To evaluate the impact of the huddle cards on the staff, the team provided a voluntary and anonymous 9 question survey (Figure 2). The survey was completed before the pilot began and again at the end of Week 6.

Questions 1 through 5 and 7 through 9 pertained to participants’ perceived knowledge and understanding of aspects of the JPSR. Perceived improvement among intensive care unit (ICU) participants ranged from 15% to 53%. There was a positive increase associated with every question with the top improvements: question 8, “How do you rate your understanding of how we share safety events for system and process improvement?” (53.4% increase); question 5, “How do you rate your understanding of what happens to a JPSR after it is entered?” (51.9% increase), and question 9, “How do you rate your understanding of the concepts of trust, psychological safety and a just culture?” (47.8% increase).
The survey analysis was not able to track individual changes. As a result, the findings reflect an overall change for the entire study group. Moreover, the questions assessed participants’ perceived knowledge rather than actual knowledge gained. It is important to note that there may be a significant gap between the actual knowledge gained and how participants perceive it. Additionally, improvement in knowledge and comprehension does not necessarily translate into behavior changes.
CONCLUSIONS
The use of JPSR huddle cards and direct engagement with staff during safety huddles yielded positive outcomes. On average, participants demonstrated higher scores in posttest questions compared to pretest questions. The posttest scores were consistently higher than the pretest scores, showing an average increase of around 2 standard deviations across all questions. This indicates an improvement in participants’ perceived knowledge and comprehension of the JPSR material.
During the pilot implementation of the huddle cards, there was a notable improvement in team member engagement. The structured format of the cards facilitated focused and meaningful discussions during safety huddles, encouraging open dialogue and fostering a culture of safety. Team members actively participated in identifying potential risks, sharing observations, and proposing actionable solutions, which reflected an enhanced sense of ownership regarding safety practices.
The support dialogue facilitated by the huddle cards highlighted the significance of mutual accountability and a collective commitment to achieving zero harm. This collaborative environment strengthened trust among team members and underscored the importance of shared vigilance in preventing adverse events. The pilot demonstrated the potential of huddle cards as an essential tool for enhancing team-based safety initiatives and promoting a culture of high reliability within the organization.
The total number of JPSR events in the ICU rose from 156 in FY 23 to 170 in FY 24. Adverse events increased from 19 to 31, while close calls saw a slight uptick from 137 to 139. Despite the overall rise in adverse events, a detailed analysis indicated that incidents of moderate harm decreased from 4 in FY 23 to 2 in FY 24. Furthermore, there was 1 reported case of death or severe harm in FY 23, which decreased to 0 in FY 24. This trend is consistent with the overarching objective of a high-reliability organization to achieve zero harm.
The next step is to expand this initiative across CTVHCS. This initiative aims to make this an annual education for all areas. The JPSR huddle card toolkit will be formatted by the media department for easy printing and retrieval. Leaders within units, clinics, and services will be empowered to facilitate the sessions in their safety huddles and reap the same outcomes as in the pilot. CTVHCS PS will monitor the effectiveness of this through ongoing CTVHCS patient safety rounding and future AES.
- Essen K, Villalobos C, Sculli GL, Steinbach L. Establishing a just culture: implications for the Veterans Health Administration journey to high reliability. Fed Pract. 2024;41:290-297. doi:10.12788/fp.0512
- Louis MY, Hussain LR, Dhanraj DN, et al. Improving patient safety event reporting among residents and teaching faculty. Ochsner J. 2016;16:73-80.
- Pimental CB, Snow AL, Carnes SL, et al. Huddles and their effectiveness at the frontlines of clinical care: a scoping review. J Gen Intern Med. 2021;36:2772-2783. doi:10.1007/s11606-021-06632-9
- National Academies of Sciences, Engineering, and Medicine. Appendix C: Nature of Veterans Health Administration Facilities Management (Engineering) Tasks and Staffing. Facilities Staffing Requirements for the Veterans Health Administration-Resource Planning and Methodology for the Future. National Academies Press. 2020:105-116. Accessed August 11, 2025. https://nap.nationalacademies.org/read/25454/chapter/11
- Woodier N, Burnett C, Moppett I. The value of learning from near misses to improve patient safety: a scoping review. J Patient Saf. 2023;19:42-47. doi:10.1097/pts.0000000000001078
- Ismail A, Khalid SNM. Patient safety culture and its determinants among healthcare professionals at a cluster hospital in Malaysia: a cross-sectional study. BMJ Open. 2022;12:e060546. doi:10.1136/bmjopen-2021-060546
- Ngo J, Lau D, Ploquin J, Receveur T, Stassen K, Del Castilho C. Improving incident reporting among physicians at south health campus hospital. BMJ Open Qual. 2022;11:e001945. doi:10.1136/bmjoq-2022-001945
- Oweidat I, Al-Mugheed K, Alsenany SA, et al. Awareness of reporting practices and barriers to incident reporting among nurses. BMC Nurs. 2023;22:231. doi:10.1186/s12912-023-01376-9
- Essen K, Villalobos C, Sculli GL, Steinbach L. Establishing a just culture: implications for the Veterans Health Administration journey to high reliability. Fed Pract. 2024;41:290-297. doi:10.12788/fp.0512
- Louis MY, Hussain LR, Dhanraj DN, et al. Improving patient safety event reporting among residents and teaching faculty. Ochsner J. 2016;16:73-80.
- Pimental CB, Snow AL, Carnes SL, et al. Huddles and their effectiveness at the frontlines of clinical care: a scoping review. J Gen Intern Med. 2021;36:2772-2783. doi:10.1007/s11606-021-06632-9
- National Academies of Sciences, Engineering, and Medicine. Appendix C: Nature of Veterans Health Administration Facilities Management (Engineering) Tasks and Staffing. Facilities Staffing Requirements for the Veterans Health Administration-Resource Planning and Methodology for the Future. National Academies Press. 2020:105-116. Accessed August 11, 2025. https://nap.nationalacademies.org/read/25454/chapter/11
- Woodier N, Burnett C, Moppett I. The value of learning from near misses to improve patient safety: a scoping review. J Patient Saf. 2023;19:42-47. doi:10.1097/pts.0000000000001078
- Ismail A, Khalid SNM. Patient safety culture and its determinants among healthcare professionals at a cluster hospital in Malaysia: a cross-sectional study. BMJ Open. 2022;12:e060546. doi:10.1136/bmjopen-2021-060546
- Ngo J, Lau D, Ploquin J, Receveur T, Stassen K, Del Castilho C. Improving incident reporting among physicians at south health campus hospital. BMJ Open Qual. 2022;11:e001945. doi:10.1136/bmjoq-2022-001945
- Oweidat I, Al-Mugheed K, Alsenany SA, et al. Awareness of reporting practices and barriers to incident reporting among nurses. BMC Nurs. 2023;22:231. doi:10.1186/s12912-023-01376-9
Empowering Culture Change and Safety on the Journey to Zero Harm With Huddle Cards
Empowering Culture Change and Safety on the Journey to Zero Harm With Huddle Cards
Advanced Imaging Techniques Use in Giant Cell Arteritis Diagnosis: The Experience at Walter Reed National Military Medical Center
Advanced Imaging Techniques Use in Giant Cell Arteritis Diagnosis: The Experience at Walter Reed National Military Medical Center
Giant cell arteritis (GCA), the most commonly diagnosed systemic vasculitis, is a large- and medium-vessel vasculitis that can lead to significant morbidity due to aneurysm formation or vascular occlusion if not diagnosed in a timely manner.1,2 Diagnosis is typically based on clinical history and inflammatory markers. Laboratory inflammatory markers may be normal in the early stages of GCA but can be abnormal due to other unrelated reasons leading to a false positive diagnosis.3 Delayed treatment may lead to visual loss, jaw or limb claudication, or ischemic stroke.2 Initial treatment typically includes high-dose steroids that can lead to significant adverse reactions such as hypothalamic-pituitary-adrenal axis dysfunction, metabolic syndrome, premature atherosclerosis, and increased risk of infection.4-6
The 1990 American College of Rheumatology (ACR) criteria for GCA are widely recognized (Table 1).7 The criteria focuses on clinical manifestations, including new onset headache, temporal artery tenderness, age ≥ 50 years, erythrocyte sedimentation rate (ESR) ≥ 50 mm/hr, and temporal artery biopsy with positive anatomical findings.8 When 3 of the 5 1990 ACR criteria are present, the sensitivity and specificity is estimated to be > 90% for GCA vs alternative vasculitides.7

Although the 1990 ACR criteria do not include imaging, modalities such as ultrasound, computed tomography angiography (CTA), 18F-FDG positron emission tomography (PET), and magnetic resonance imaging (MRI)/magnetic resonance angiography (MRA) may be used in GCA diagnosis.8-10 These imaging modalities have been added to the proposed ACR classification criteria for GCA.11 For this updated point system standard, age ≥ 50 years is a requirement and includes a positive temporal artery biopsy or temporal artery halo sign on ultrasound (+5 points), an ESR ≥ 50 mm/h or C-reactive protein (CRP) ≥ 10 mg/L (+3 points), or sudden visual loss (+3 points). Scalp tenderness, jaw or tongue claudication, new temporal headache, morning stiffness in shoulders or neck, temporal artery abnormality on vascular examination, bilateral axillary vessel involvement on imaging, and 18F-FDG PET activity throughout the aorta are scored +2 points each. With these new criteria, a cumulative score ≥ 6 is classified as GCA. Diagnostic accuracy is further improved with imaging: ultrasonography (sensitivity 55% and specificity 95%) and 18F-FDG PET (sensitivity 69% and specificity 92%), CTA (sensitivity 71% and specificity 86%), and MRI/MRA (sensitivity 73% and specificity 88%).12-15
In recent years, clinicians have reported increased glucose uptake in arteries observed on PET imaging that suggests GCA.9,10,16-20 18F-FDG accumulates in cells with high metabolic activity rates, such as areas of inflammation. In assessing temporal arteries or other involved vasculature (eg, axillary or great vessels) for GCA, this modality indicates increased glucose uptake in the lining of vessel walls. The inflammation of vessel walls can then be visualized with PET. 18F-FDG PET presents a noninvasive imaging technique for evaluating GCA but its use has been limited in the United States due to its high cost.
Methods
Approval for a retrospective chart review of patients evaluated for suspected GCA was obtained from the Walter Reed National Military Medical Center (WRNMMC) Institutional Review Board. The review included patients who underwent diagnostic procedures such as ultrasound, MRI, CT angiogram, and PET studies from 2016 through 2022. International Classification of Diseases codes used for case identification included: M31.6, M31.5, I77.6, I77.8, I77.89, I67.7, and I68.2. The Current Procedural Terminology code used for temporal artery biopsy is 37609.
Results
Seventy-eight charts were reviewed and 42 patients (54%) were diagnosed with GCA (Table 2). This study sample had a much higher proportion of African American subjects (31%) when compared with the civilian population, likely reflecting the higher representation of African Americans in the armed forces. Twenty-eight females (67%) were GCA positive. The most common presenting symptoms included 27 patients (64%) with headache, 17 (40%) with scalp tenderness, and 14 (33%) with jaw pain. The mean 1990 ACR score was 3.8 (range, 2-5). With respect to the score criteria: 41 patients (98%) were aged ≥ 50 years, 31 (74%) had new onset headache, and 31 (74%) had elevated ESR (Table 3). Acute ischemic optic neuropathy was documented in 4 patients (10%) with confirmed GCA. The mean ESR and CRP values at diagnosis were 66.2 mm/h (range, 7-122 mm/h) and 8.711 μg/mL (range, 0.054 – 92.690 μg/mL), respectively. Twenty-seven patients (64%) underwent biopsy: 24 (89%) were unilateral and 3 (11%) were bilateral (Table 4). Four patients with GCA (10%) were missing biopsy data. Nineteen patients with GCA (70%) had biopsies with pathologic findings consistent with GCA.
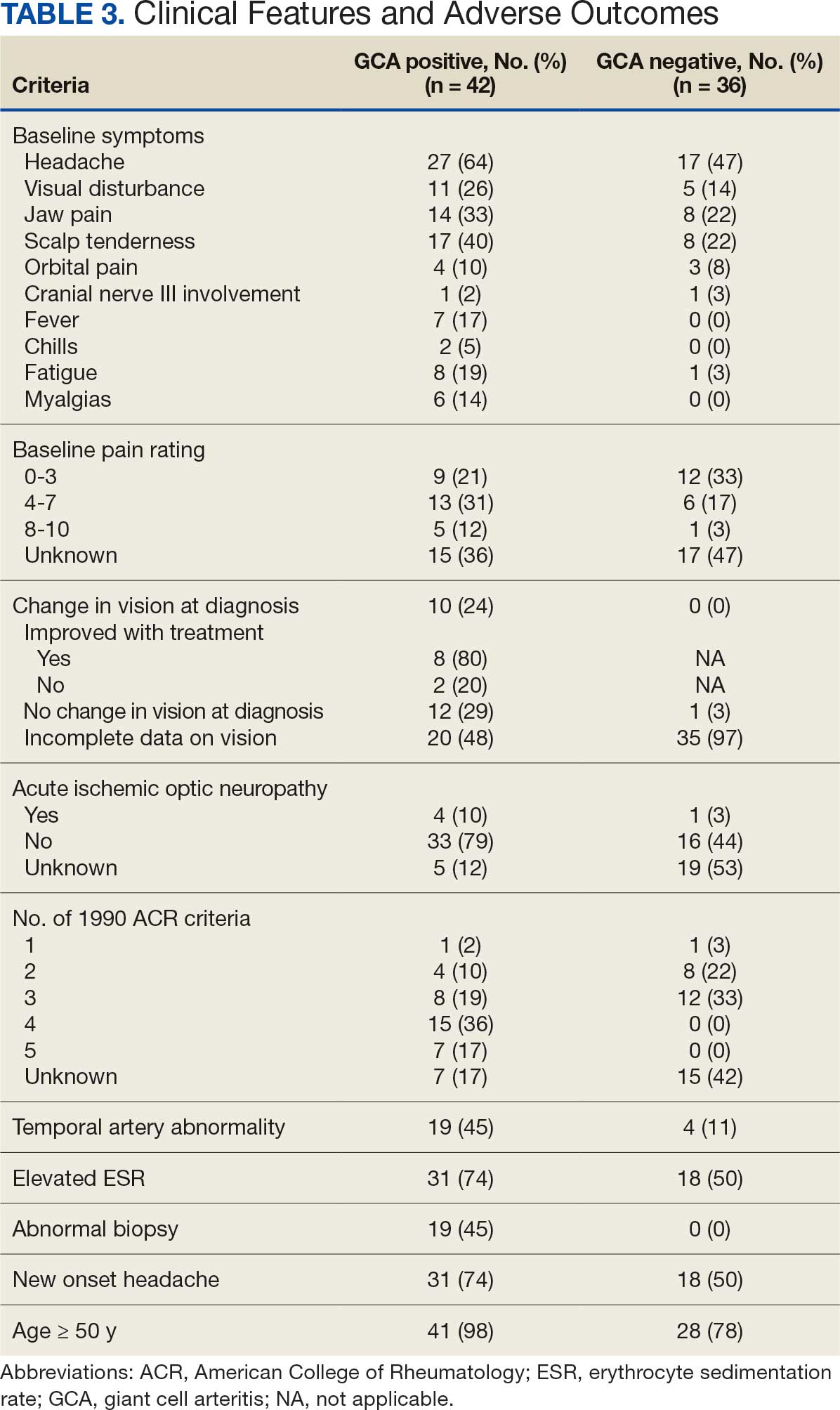
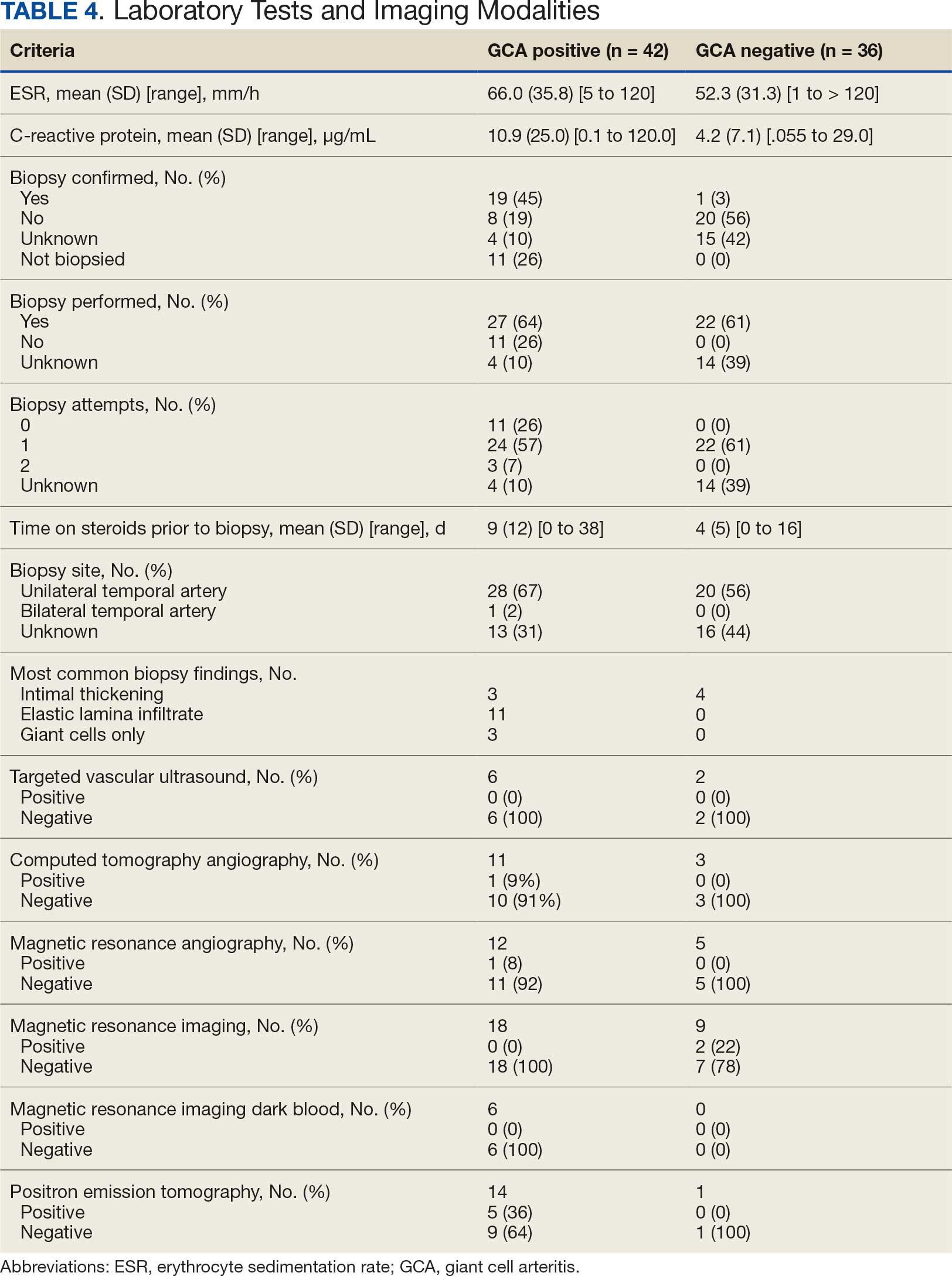
Twenty-five patients with GCA (60%) received ≥ 1 imaging modality. The most common imaging modality was MRI, which was used for 18 (43%) patients. Fourteen patients (33%) had 18F-FDG PET, 12 patients (29%) had MRA, and 11 patients (26%) had CTA. The small number of patients who underwent point-of-care ultrasound (POCUS), brain MRI, or dark blood MRI were negative for disease. Five patients who underwent 18F-FDG PET had findings consistent with GCA. One patient with GCA had CTA of the head and neck with radiographic findings supportive of GCA.
Discussion
The available evidence supports the use of additional screening tests to increase the temporal artery biopsy yield for GCA. Inflammatory laboratory markers demonstrate some sensitivity but are nonspecific for GCA. In this study, only 60% of patients with GCA underwent diagnostic imaging as part of the workup. There are multiple factors that may contribute to the underutilization of advanced imaging in the diagnosis of GCA, including outdated standardized diagnostic criteria, limited resources (direct access to modalities), and lack of clinician awareness of diagnostic testing options. In this retrospective review, 30 patients (71%) were diagnosed with GCA with a 1990 ACR GCA score ≤ 3. Of these 30 patients, 19 underwent confirmatory biopsy followed by prolonged courses of steroid therapy. In addition, only 25 patients underwent advanced imaging to increase diagnostic accuracy of the suspected syndrome.
A large meta-analysis demonstrated a sensitivity of 77.3% (95% CI, 71.8-81.9%) for temporal artery biopsy.21 The overall yield was 40% in the meta-analysis. Advanced noninvasive imaging represents an appropriate method of evaluating GCA.8-20 In our study, 18F-FDG PET demonstrated the highest sensitivity (36%) for the diagnosis of GCA. Ultrasonography is recommended as an initial screening tool to identify the noncompressible halo sign (a hypoechoic circumferential wall thickening due to edema) as a cost-effective and widely available technology.22 Other research has corroborated the beneficial use of ultrasonography in improving diagnostic accuracy by detecting the noncompressible halo sign in temporal arteries.22,23 GCA diagnostic performance has been significantly improved with the use of B-mode probes ≥ 15 MHz as well as proposals to incorporate a compression sign or interrogating the axillary vessels, showing a sensitivity of 54% to 77%.23,24
POCUS may reduce the risk of a false-negative biopsy and improve yield with more frequent utilization. However, ultrasonography may be limited by operator skills and visualization of the great vessels. The accuracy of ultrasonography is dependent on the experience and adeptness of the operator. Additional studies are needed to establish a systematic standard for POCUS training to ensure accurate interpretation and uniform interrogation procedure.24 Artificial intelligence (AI) may aid in interpreting results of POCUS and bridging the operator skill gap among operators.25,26 AI and machine learning techniques can assist in detecting the noncompressible halo sign and compression sign in temporal arteries and other affected vessels.
In comparing the WRNMMC patient population with other US civilian GCA cohorts, there are some differences and similarities. There was a high representation of African American patients in the study, which may reflect a greater severity of autoimmune disease expression in this population.27 We also observed a higher number of females and an association with polymyalgia rheumatica in the data, consistent with previous reports.28,29 The females in this study were primarily civilians and therefore more similar to the general population of individuals with GCA. In contrast, male patients were more likely to be active-duty service members or have prior service experience with increased exposure to novel environmental factors linked to increased risk of autoimmune disease. This includes an increased risk of Guillain-Barré syndrome and Graves disease among Vietnam veterans exposed to Agent Orange.30,31 Other studies have found that veterans with posttraumatic stress disorder are at increased risk for severe autoimmune diseases.32,33 As more women join the active-duty military, the impact of autoimmune disease in the military service population is expected to grow, requiring further research.
Conclusions
Early diagnosis and treatment of GCA are critical to preventing serious outcomes, such as visual loss, jaw or limb claudication, or ischemic stroke. The incidence of autoimmune disease is expected to rise in the armed forces and veteran populations due to exposure to novel environmental factors and the increasing representation of women in the military. The use of additional screening tools can aid in earlier diagnosis of GCA. The 2022 ACR classification criteria for GCA represent significant updates to the 1990 criteria, incorporating ancillary tests such as the temporal artery halo sign on ultrasound, bilateral axillary vessel screening on imaging, and 18F-FDG PET activity throughout the aorta. The updated criteria require further validation and supports the adoption of a multidisciplinary approach that includes ultrasonography, vascular MRI/CT, and 18F-FDG PET. Furthermore, AI may play a future key role in ultrasound interpretation and study interrogation procedure. Ultimately, ultrasonography is a noninvasive and promising technique for the early diagnosis of GCA. A target goal is to increase the yield of positive temporal artery biopsies to ≥ 70%.
- Jennette JC. Overview of the 2012 revised International Chapel Hill Consensus Conference nomenclature of vasculitides. Clin Exp Nephrol. 2013;17:603-606. doi:10.1007/s10157-013-0869-6
- Kale N, Eggenberger E. Diagnosis and management of giant cell arteritis: a review. Curr Opin Ophthalmol. 2010;21:417-422. doi:10.1097/ICU.0b013e32833eae8b
- Smetana GW, Shmerling RH. Does this patient have temporal arteritis? JAMA. 2002;287:92-101.
- Schäcke H, Döcke WD, Asadullah K. Mechanisms involved in the side effects of glucocorticoids. Pharmacol Ther. 2002;96:23-43. doi:10.1016/s0163-7258(02)00297-8
- Curtis JR, Patkar N, Xie A, et al. Risk of serious bacterial infections among rheumatoid arthritis patients exposed to tumor necrosis factor alpha antagonists. Arthritis Rheum. 2007;56:1125-1133. doi:10.1002/art.22504
- Hoes JN, van der Goes MC, van Raalte DH, et al. Glucose tolerance, insulin sensitivity and ß-cell function in patients with rheumatoid arthritis treated with or without low-to-medium dose glucocorticoids. Ann Rheum Dis. 2011;70:1887-1894. doi:10.1136/ard.2011.151464
- Hunder GG, Bloch DA, Michel BA, et al. The American College of Rheumatology 1990 criteria for the classification of giant cell arteritis. Arthritis Rheum. 1990;33:1122-1128. doi:10.1002/art.1780330810
- Dejaco C, Duftner C, Buttgereit F, Matteson EL, Dasgupta B. The spectrum of giant cell arteritis and polymyalgia rheumatica: revisiting the concept of the disease. Rheumatology (Oxford). 2017;56:506-515. doi:10.1093/rheumatology/kew273
- Slart RHJ, Nienhuis PH, Glaudemans AWJM, et al. Role of 18F-FDG PET/CT in large vessel vasculitis and polymyalgia rheumatica. J Nucl Med. 2023;64:515-521. doi:10.2967/jnumed.122.265016
- Shimol JB, Amital H, Lidar M, Domachevsky L, Shoenfeld Y, Davidson T. The utility of PET/CT in large vessel vasculitis. Sci Rep. 2020;10:17709. doi:10.1038/s41598-020-73818-2
- Ponte C, Grayson PC, Robson JC, et al. 2022 American College of Rheumatology/EULAR Classification Criteria for Giant Cell Arteritis. Arthritis Rheumatol. 2022;74:1881-1889. doi:10.1002/art.42325
- He J, Williamson L, Ng B, et al. The diagnostic accuracy of temporal artery ultrasound and temporal artery biopsy in giant cell arteritis: a single center Australian experience over 10 years. Int J Rheum Dis. 2022;25:447-453. doi:10.1111/1756-185X.14288
- Stellingwerff MD, Brouwer E, Lensen KDF, et al. Different scoring methods of FDG PET/CT in giant cell arteritis: need for standardization. Medicine (Baltimore). 2015;94:e1542. doi:10.1097/MD.0000000000001542
- Conway R, Smyth AE, Kavanagh RG, et al. Diagnostic utility of computed tomographic angiography in giant-cell arteritis. Stroke. 2018;49:2233-2236. doi:10.1161/STROKEAHA.118.021995
- Duftner C, Dejaco C, Sepriano A, et al. Imaging in diagnosis, outcome prediction and monitoring of large vessel vasculitis: a systematic literature review and meta-analysis informing the EULAR recommendations. RMD Open. 2018;4:e000612. doi:10.1136/rmdopen-2017-000612
- Rehak Z, Vasina J, Ptacek J, et al. PET/CT in giant cell arteritis: high 18F-FDG uptake in the temporal, occipital and vertebral arteries. Rev Esp Med Nucl Imagen Mol. 2016;35:398-401. doi:10.1016/j.remn.2016.03.007
- Salvarani C, Soriano A, Muratore F, et al. Is PET/CT essential in the diagnosis and follow-up of temporal arteritis? Autoimmun Rev. 2017;16:1125-1130. doi:10.1016/j.autrev.2017.09.007
- Brodmann M, Lipp RW, Passath A, et al. The role of 2-18F-fluoro-2-deoxy-D-glucose positron emission tomography in the diagnosis of giant cell arteritis of the temporal arteries. Rheumatology (Oxford). 2004;43:241-242. doi:10.1093/rheumatology/keh025
- Flaus A, Granjon D, Habouzit V, Gaultier JB, Prevot-Bitot N. Unusual and diffuse hypermetabolism in routine 18F-FDG PET/CT of the supra-aortic vessels in biopsy-positive giant cell arteritis. Clin Nucl Med. 2018;43:e336-e337. doi:10.1097/RLU.0000000000002198
- Berger CT, Sommer G, Aschwanden M, et al. The clinical benefit of imaging in the diagnosis and treatment of giant cell arteritis. Swiss Med Wkly. 2018;148:w14661. doi:10.4414/smw.2018.14661
- Rubenstein E, Maldini C, Gonzalez-Chiappe S, et al. Sensitivity of temporal artery biopsy in the diagnosis of giant cell arteritis: a systematic literature review and meta-analysis. Rheumatology (Oxford). 2020;59:1011-1020. doi:10.1093/rheumatology/kez385
- Tsivgoulis G, Heliopoulos I, Vadikolias K, et al. Teaching neuroimages: ultrasound findings in giant-cell arteritis. Neurology. 2010;75:e67-e68. doi:10.1212/WNL.0b013e3181f881e9
- Nakajima E, Moon FH, Canvas Jr N, et al. Accuracy of Doppler ultrasound in the diagnosis of giant cell arteritis: a systematic review and meta-analysis. Adv Rheumatol. 2023;63:5. doi:10.1186/s42358-023-00286-3
- Naumegni SR, Hoffmann C, Cornec D, et al. Temporal artery ultrasound to diagnose giant cell arteritis: a practical guide. Ultrasound Med Biol. 2021;47:201-213. doi:10.1016/j.ultrasmedbio.2020.10.004
- Kim YH. Artificial intelligence in medical ultrasonography: driving on an unpaved road. Ultrasonography. 2021;40:313-317. doi:10.14366/usg.21031
- Sultan LR, Mohamed MH, Andronikou S. ChatGPT-4: a breakthrough in ultrasound image analysis. Radiol Adv. 2024;1:umae006. doi:10.1093/radadv/umae006
- Cipriani VP, Klein S. Clinical characteristics of multiple sclerosis in African-Americans. Curr Neurol Neurosci Rep. 2019;19:87. doi:10.1007/s11910-019-1000-5
- Sturm A, Dechant C, Proft F, et al. Gender differences in giant cell arteritis: a case-control study. Clin Exp Rheumatol. 2016;34:S70-72.
- Li KJ, Semenov D, Turk M, et al. A meta-analysis of the epidemiology of giant cell arteritis across time and space. Arthritis Res Ther. 2021;23:82. doi:10.1186/s13075-021-02450-w
- Nelson L, Gormley R, Riddle MS, Tribble DR, Porter CK. The epidemiology of Guillain-Barré syndrome in U.S. military personnel: a case-control study. BMC Res Notes. 2009;2:171. doi:10.1186/1756-0500-2-171
- Spaulding SW. The possible roles of environmental factors and the aryl hydrocarbon receptor in the prevalence of thyroid diseases in Vietnam era veterans. Curr Opin Endocrinol Diabetes Obes. 2011;18:315-320.
- O’Donovan A, Cohen BE, Seal KH, et al. Elevated risk for autoimmune disorders in Iraq and Afghanistan veterans with posttraumatic stress disorder. Biol Psychiatry. 2015;77:365-374. doi:10.1016/j.biopsych.2014.06.015
- Bookwalter DB, Roenfeldt KA, LeardMann CA, Kong SY, Riddle MS, Rull RP. Posttraumatic stress disorder and risk of selected autoimmune diseases among US military personnel. BMC Psychiatry. 2020;20:23. doi:10.1186/s12888-020-2432-9
Giant cell arteritis (GCA), the most commonly diagnosed systemic vasculitis, is a large- and medium-vessel vasculitis that can lead to significant morbidity due to aneurysm formation or vascular occlusion if not diagnosed in a timely manner.1,2 Diagnosis is typically based on clinical history and inflammatory markers. Laboratory inflammatory markers may be normal in the early stages of GCA but can be abnormal due to other unrelated reasons leading to a false positive diagnosis.3 Delayed treatment may lead to visual loss, jaw or limb claudication, or ischemic stroke.2 Initial treatment typically includes high-dose steroids that can lead to significant adverse reactions such as hypothalamic-pituitary-adrenal axis dysfunction, metabolic syndrome, premature atherosclerosis, and increased risk of infection.4-6
The 1990 American College of Rheumatology (ACR) criteria for GCA are widely recognized (Table 1).7 The criteria focuses on clinical manifestations, including new onset headache, temporal artery tenderness, age ≥ 50 years, erythrocyte sedimentation rate (ESR) ≥ 50 mm/hr, and temporal artery biopsy with positive anatomical findings.8 When 3 of the 5 1990 ACR criteria are present, the sensitivity and specificity is estimated to be > 90% for GCA vs alternative vasculitides.7

Although the 1990 ACR criteria do not include imaging, modalities such as ultrasound, computed tomography angiography (CTA), 18F-FDG positron emission tomography (PET), and magnetic resonance imaging (MRI)/magnetic resonance angiography (MRA) may be used in GCA diagnosis.8-10 These imaging modalities have been added to the proposed ACR classification criteria for GCA.11 For this updated point system standard, age ≥ 50 years is a requirement and includes a positive temporal artery biopsy or temporal artery halo sign on ultrasound (+5 points), an ESR ≥ 50 mm/h or C-reactive protein (CRP) ≥ 10 mg/L (+3 points), or sudden visual loss (+3 points). Scalp tenderness, jaw or tongue claudication, new temporal headache, morning stiffness in shoulders or neck, temporal artery abnormality on vascular examination, bilateral axillary vessel involvement on imaging, and 18F-FDG PET activity throughout the aorta are scored +2 points each. With these new criteria, a cumulative score ≥ 6 is classified as GCA. Diagnostic accuracy is further improved with imaging: ultrasonography (sensitivity 55% and specificity 95%) and 18F-FDG PET (sensitivity 69% and specificity 92%), CTA (sensitivity 71% and specificity 86%), and MRI/MRA (sensitivity 73% and specificity 88%).12-15
In recent years, clinicians have reported increased glucose uptake in arteries observed on PET imaging that suggests GCA.9,10,16-20 18F-FDG accumulates in cells with high metabolic activity rates, such as areas of inflammation. In assessing temporal arteries or other involved vasculature (eg, axillary or great vessels) for GCA, this modality indicates increased glucose uptake in the lining of vessel walls. The inflammation of vessel walls can then be visualized with PET. 18F-FDG PET presents a noninvasive imaging technique for evaluating GCA but its use has been limited in the United States due to its high cost.
Methods
Approval for a retrospective chart review of patients evaluated for suspected GCA was obtained from the Walter Reed National Military Medical Center (WRNMMC) Institutional Review Board. The review included patients who underwent diagnostic procedures such as ultrasound, MRI, CT angiogram, and PET studies from 2016 through 2022. International Classification of Diseases codes used for case identification included: M31.6, M31.5, I77.6, I77.8, I77.89, I67.7, and I68.2. The Current Procedural Terminology code used for temporal artery biopsy is 37609.
Results
Seventy-eight charts were reviewed and 42 patients (54%) were diagnosed with GCA (Table 2). This study sample had a much higher proportion of African American subjects (31%) when compared with the civilian population, likely reflecting the higher representation of African Americans in the armed forces. Twenty-eight females (67%) were GCA positive. The most common presenting symptoms included 27 patients (64%) with headache, 17 (40%) with scalp tenderness, and 14 (33%) with jaw pain. The mean 1990 ACR score was 3.8 (range, 2-5). With respect to the score criteria: 41 patients (98%) were aged ≥ 50 years, 31 (74%) had new onset headache, and 31 (74%) had elevated ESR (Table 3). Acute ischemic optic neuropathy was documented in 4 patients (10%) with confirmed GCA. The mean ESR and CRP values at diagnosis were 66.2 mm/h (range, 7-122 mm/h) and 8.711 μg/mL (range, 0.054 – 92.690 μg/mL), respectively. Twenty-seven patients (64%) underwent biopsy: 24 (89%) were unilateral and 3 (11%) were bilateral (Table 4). Four patients with GCA (10%) were missing biopsy data. Nineteen patients with GCA (70%) had biopsies with pathologic findings consistent with GCA.



Twenty-five patients with GCA (60%) received ≥ 1 imaging modality. The most common imaging modality was MRI, which was used for 18 (43%) patients. Fourteen patients (33%) had 18F-FDG PET, 12 patients (29%) had MRA, and 11 patients (26%) had CTA. The small number of patients who underwent point-of-care ultrasound (POCUS), brain MRI, or dark blood MRI were negative for disease. Five patients who underwent 18F-FDG PET had findings consistent with GCA. One patient with GCA had CTA of the head and neck with radiographic findings supportive of GCA.
Discussion
The available evidence supports the use of additional screening tests to increase the temporal artery biopsy yield for GCA. Inflammatory laboratory markers demonstrate some sensitivity but are nonspecific for GCA. In this study, only 60% of patients with GCA underwent diagnostic imaging as part of the workup. There are multiple factors that may contribute to the underutilization of advanced imaging in the diagnosis of GCA, including outdated standardized diagnostic criteria, limited resources (direct access to modalities), and lack of clinician awareness of diagnostic testing options. In this retrospective review, 30 patients (71%) were diagnosed with GCA with a 1990 ACR GCA score ≤ 3. Of these 30 patients, 19 underwent confirmatory biopsy followed by prolonged courses of steroid therapy. In addition, only 25 patients underwent advanced imaging to increase diagnostic accuracy of the suspected syndrome.
A large meta-analysis demonstrated a sensitivity of 77.3% (95% CI, 71.8-81.9%) for temporal artery biopsy.21 The overall yield was 40% in the meta-analysis. Advanced noninvasive imaging represents an appropriate method of evaluating GCA.8-20 In our study, 18F-FDG PET demonstrated the highest sensitivity (36%) for the diagnosis of GCA. Ultrasonography is recommended as an initial screening tool to identify the noncompressible halo sign (a hypoechoic circumferential wall thickening due to edema) as a cost-effective and widely available technology.22 Other research has corroborated the beneficial use of ultrasonography in improving diagnostic accuracy by detecting the noncompressible halo sign in temporal arteries.22,23 GCA diagnostic performance has been significantly improved with the use of B-mode probes ≥ 15 MHz as well as proposals to incorporate a compression sign or interrogating the axillary vessels, showing a sensitivity of 54% to 77%.23,24
POCUS may reduce the risk of a false-negative biopsy and improve yield with more frequent utilization. However, ultrasonography may be limited by operator skills and visualization of the great vessels. The accuracy of ultrasonography is dependent on the experience and adeptness of the operator. Additional studies are needed to establish a systematic standard for POCUS training to ensure accurate interpretation and uniform interrogation procedure.24 Artificial intelligence (AI) may aid in interpreting results of POCUS and bridging the operator skill gap among operators.25,26 AI and machine learning techniques can assist in detecting the noncompressible halo sign and compression sign in temporal arteries and other affected vessels.
In comparing the WRNMMC patient population with other US civilian GCA cohorts, there are some differences and similarities. There was a high representation of African American patients in the study, which may reflect a greater severity of autoimmune disease expression in this population.27 We also observed a higher number of females and an association with polymyalgia rheumatica in the data, consistent with previous reports.28,29 The females in this study were primarily civilians and therefore more similar to the general population of individuals with GCA. In contrast, male patients were more likely to be active-duty service members or have prior service experience with increased exposure to novel environmental factors linked to increased risk of autoimmune disease. This includes an increased risk of Guillain-Barré syndrome and Graves disease among Vietnam veterans exposed to Agent Orange.30,31 Other studies have found that veterans with posttraumatic stress disorder are at increased risk for severe autoimmune diseases.32,33 As more women join the active-duty military, the impact of autoimmune disease in the military service population is expected to grow, requiring further research.
Conclusions
Early diagnosis and treatment of GCA are critical to preventing serious outcomes, such as visual loss, jaw or limb claudication, or ischemic stroke. The incidence of autoimmune disease is expected to rise in the armed forces and veteran populations due to exposure to novel environmental factors and the increasing representation of women in the military. The use of additional screening tools can aid in earlier diagnosis of GCA. The 2022 ACR classification criteria for GCA represent significant updates to the 1990 criteria, incorporating ancillary tests such as the temporal artery halo sign on ultrasound, bilateral axillary vessel screening on imaging, and 18F-FDG PET activity throughout the aorta. The updated criteria require further validation and supports the adoption of a multidisciplinary approach that includes ultrasonography, vascular MRI/CT, and 18F-FDG PET. Furthermore, AI may play a future key role in ultrasound interpretation and study interrogation procedure. Ultimately, ultrasonography is a noninvasive and promising technique for the early diagnosis of GCA. A target goal is to increase the yield of positive temporal artery biopsies to ≥ 70%.
Giant cell arteritis (GCA), the most commonly diagnosed systemic vasculitis, is a large- and medium-vessel vasculitis that can lead to significant morbidity due to aneurysm formation or vascular occlusion if not diagnosed in a timely manner.1,2 Diagnosis is typically based on clinical history and inflammatory markers. Laboratory inflammatory markers may be normal in the early stages of GCA but can be abnormal due to other unrelated reasons leading to a false positive diagnosis.3 Delayed treatment may lead to visual loss, jaw or limb claudication, or ischemic stroke.2 Initial treatment typically includes high-dose steroids that can lead to significant adverse reactions such as hypothalamic-pituitary-adrenal axis dysfunction, metabolic syndrome, premature atherosclerosis, and increased risk of infection.4-6
The 1990 American College of Rheumatology (ACR) criteria for GCA are widely recognized (Table 1).7 The criteria focuses on clinical manifestations, including new onset headache, temporal artery tenderness, age ≥ 50 years, erythrocyte sedimentation rate (ESR) ≥ 50 mm/hr, and temporal artery biopsy with positive anatomical findings.8 When 3 of the 5 1990 ACR criteria are present, the sensitivity and specificity is estimated to be > 90% for GCA vs alternative vasculitides.7

Although the 1990 ACR criteria do not include imaging, modalities such as ultrasound, computed tomography angiography (CTA), 18F-FDG positron emission tomography (PET), and magnetic resonance imaging (MRI)/magnetic resonance angiography (MRA) may be used in GCA diagnosis.8-10 These imaging modalities have been added to the proposed ACR classification criteria for GCA.11 For this updated point system standard, age ≥ 50 years is a requirement and includes a positive temporal artery biopsy or temporal artery halo sign on ultrasound (+5 points), an ESR ≥ 50 mm/h or C-reactive protein (CRP) ≥ 10 mg/L (+3 points), or sudden visual loss (+3 points). Scalp tenderness, jaw or tongue claudication, new temporal headache, morning stiffness in shoulders or neck, temporal artery abnormality on vascular examination, bilateral axillary vessel involvement on imaging, and 18F-FDG PET activity throughout the aorta are scored +2 points each. With these new criteria, a cumulative score ≥ 6 is classified as GCA. Diagnostic accuracy is further improved with imaging: ultrasonography (sensitivity 55% and specificity 95%) and 18F-FDG PET (sensitivity 69% and specificity 92%), CTA (sensitivity 71% and specificity 86%), and MRI/MRA (sensitivity 73% and specificity 88%).12-15
In recent years, clinicians have reported increased glucose uptake in arteries observed on PET imaging that suggests GCA.9,10,16-20 18F-FDG accumulates in cells with high metabolic activity rates, such as areas of inflammation. In assessing temporal arteries or other involved vasculature (eg, axillary or great vessels) for GCA, this modality indicates increased glucose uptake in the lining of vessel walls. The inflammation of vessel walls can then be visualized with PET. 18F-FDG PET presents a noninvasive imaging technique for evaluating GCA but its use has been limited in the United States due to its high cost.
Methods
Approval for a retrospective chart review of patients evaluated for suspected GCA was obtained from the Walter Reed National Military Medical Center (WRNMMC) Institutional Review Board. The review included patients who underwent diagnostic procedures such as ultrasound, MRI, CT angiogram, and PET studies from 2016 through 2022. International Classification of Diseases codes used for case identification included: M31.6, M31.5, I77.6, I77.8, I77.89, I67.7, and I68.2. The Current Procedural Terminology code used for temporal artery biopsy is 37609.
Results
Seventy-eight charts were reviewed and 42 patients (54%) were diagnosed with GCA (Table 2). This study sample had a much higher proportion of African American subjects (31%) when compared with the civilian population, likely reflecting the higher representation of African Americans in the armed forces. Twenty-eight females (67%) were GCA positive. The most common presenting symptoms included 27 patients (64%) with headache, 17 (40%) with scalp tenderness, and 14 (33%) with jaw pain. The mean 1990 ACR score was 3.8 (range, 2-5). With respect to the score criteria: 41 patients (98%) were aged ≥ 50 years, 31 (74%) had new onset headache, and 31 (74%) had elevated ESR (Table 3). Acute ischemic optic neuropathy was documented in 4 patients (10%) with confirmed GCA. The mean ESR and CRP values at diagnosis were 66.2 mm/h (range, 7-122 mm/h) and 8.711 μg/mL (range, 0.054 – 92.690 μg/mL), respectively. Twenty-seven patients (64%) underwent biopsy: 24 (89%) were unilateral and 3 (11%) were bilateral (Table 4). Four patients with GCA (10%) were missing biopsy data. Nineteen patients with GCA (70%) had biopsies with pathologic findings consistent with GCA.



Twenty-five patients with GCA (60%) received ≥ 1 imaging modality. The most common imaging modality was MRI, which was used for 18 (43%) patients. Fourteen patients (33%) had 18F-FDG PET, 12 patients (29%) had MRA, and 11 patients (26%) had CTA. The small number of patients who underwent point-of-care ultrasound (POCUS), brain MRI, or dark blood MRI were negative for disease. Five patients who underwent 18F-FDG PET had findings consistent with GCA. One patient with GCA had CTA of the head and neck with radiographic findings supportive of GCA.
Discussion
The available evidence supports the use of additional screening tests to increase the temporal artery biopsy yield for GCA. Inflammatory laboratory markers demonstrate some sensitivity but are nonspecific for GCA. In this study, only 60% of patients with GCA underwent diagnostic imaging as part of the workup. There are multiple factors that may contribute to the underutilization of advanced imaging in the diagnosis of GCA, including outdated standardized diagnostic criteria, limited resources (direct access to modalities), and lack of clinician awareness of diagnostic testing options. In this retrospective review, 30 patients (71%) were diagnosed with GCA with a 1990 ACR GCA score ≤ 3. Of these 30 patients, 19 underwent confirmatory biopsy followed by prolonged courses of steroid therapy. In addition, only 25 patients underwent advanced imaging to increase diagnostic accuracy of the suspected syndrome.
A large meta-analysis demonstrated a sensitivity of 77.3% (95% CI, 71.8-81.9%) for temporal artery biopsy.21 The overall yield was 40% in the meta-analysis. Advanced noninvasive imaging represents an appropriate method of evaluating GCA.8-20 In our study, 18F-FDG PET demonstrated the highest sensitivity (36%) for the diagnosis of GCA. Ultrasonography is recommended as an initial screening tool to identify the noncompressible halo sign (a hypoechoic circumferential wall thickening due to edema) as a cost-effective and widely available technology.22 Other research has corroborated the beneficial use of ultrasonography in improving diagnostic accuracy by detecting the noncompressible halo sign in temporal arteries.22,23 GCA diagnostic performance has been significantly improved with the use of B-mode probes ≥ 15 MHz as well as proposals to incorporate a compression sign or interrogating the axillary vessels, showing a sensitivity of 54% to 77%.23,24
POCUS may reduce the risk of a false-negative biopsy and improve yield with more frequent utilization. However, ultrasonography may be limited by operator skills and visualization of the great vessels. The accuracy of ultrasonography is dependent on the experience and adeptness of the operator. Additional studies are needed to establish a systematic standard for POCUS training to ensure accurate interpretation and uniform interrogation procedure.24 Artificial intelligence (AI) may aid in interpreting results of POCUS and bridging the operator skill gap among operators.25,26 AI and machine learning techniques can assist in detecting the noncompressible halo sign and compression sign in temporal arteries and other affected vessels.
In comparing the WRNMMC patient population with other US civilian GCA cohorts, there are some differences and similarities. There was a high representation of African American patients in the study, which may reflect a greater severity of autoimmune disease expression in this population.27 We also observed a higher number of females and an association with polymyalgia rheumatica in the data, consistent with previous reports.28,29 The females in this study were primarily civilians and therefore more similar to the general population of individuals with GCA. In contrast, male patients were more likely to be active-duty service members or have prior service experience with increased exposure to novel environmental factors linked to increased risk of autoimmune disease. This includes an increased risk of Guillain-Barré syndrome and Graves disease among Vietnam veterans exposed to Agent Orange.30,31 Other studies have found that veterans with posttraumatic stress disorder are at increased risk for severe autoimmune diseases.32,33 As more women join the active-duty military, the impact of autoimmune disease in the military service population is expected to grow, requiring further research.
Conclusions
Early diagnosis and treatment of GCA are critical to preventing serious outcomes, such as visual loss, jaw or limb claudication, or ischemic stroke. The incidence of autoimmune disease is expected to rise in the armed forces and veteran populations due to exposure to novel environmental factors and the increasing representation of women in the military. The use of additional screening tools can aid in earlier diagnosis of GCA. The 2022 ACR classification criteria for GCA represent significant updates to the 1990 criteria, incorporating ancillary tests such as the temporal artery halo sign on ultrasound, bilateral axillary vessel screening on imaging, and 18F-FDG PET activity throughout the aorta. The updated criteria require further validation and supports the adoption of a multidisciplinary approach that includes ultrasonography, vascular MRI/CT, and 18F-FDG PET. Furthermore, AI may play a future key role in ultrasound interpretation and study interrogation procedure. Ultimately, ultrasonography is a noninvasive and promising technique for the early diagnosis of GCA. A target goal is to increase the yield of positive temporal artery biopsies to ≥ 70%.
- Jennette JC. Overview of the 2012 revised International Chapel Hill Consensus Conference nomenclature of vasculitides. Clin Exp Nephrol. 2013;17:603-606. doi:10.1007/s10157-013-0869-6
- Kale N, Eggenberger E. Diagnosis and management of giant cell arteritis: a review. Curr Opin Ophthalmol. 2010;21:417-422. doi:10.1097/ICU.0b013e32833eae8b
- Smetana GW, Shmerling RH. Does this patient have temporal arteritis? JAMA. 2002;287:92-101.
- Schäcke H, Döcke WD, Asadullah K. Mechanisms involved in the side effects of glucocorticoids. Pharmacol Ther. 2002;96:23-43. doi:10.1016/s0163-7258(02)00297-8
- Curtis JR, Patkar N, Xie A, et al. Risk of serious bacterial infections among rheumatoid arthritis patients exposed to tumor necrosis factor alpha antagonists. Arthritis Rheum. 2007;56:1125-1133. doi:10.1002/art.22504
- Hoes JN, van der Goes MC, van Raalte DH, et al. Glucose tolerance, insulin sensitivity and ß-cell function in patients with rheumatoid arthritis treated with or without low-to-medium dose glucocorticoids. Ann Rheum Dis. 2011;70:1887-1894. doi:10.1136/ard.2011.151464
- Hunder GG, Bloch DA, Michel BA, et al. The American College of Rheumatology 1990 criteria for the classification of giant cell arteritis. Arthritis Rheum. 1990;33:1122-1128. doi:10.1002/art.1780330810
- Dejaco C, Duftner C, Buttgereit F, Matteson EL, Dasgupta B. The spectrum of giant cell arteritis and polymyalgia rheumatica: revisiting the concept of the disease. Rheumatology (Oxford). 2017;56:506-515. doi:10.1093/rheumatology/kew273
- Slart RHJ, Nienhuis PH, Glaudemans AWJM, et al. Role of 18F-FDG PET/CT in large vessel vasculitis and polymyalgia rheumatica. J Nucl Med. 2023;64:515-521. doi:10.2967/jnumed.122.265016
- Shimol JB, Amital H, Lidar M, Domachevsky L, Shoenfeld Y, Davidson T. The utility of PET/CT in large vessel vasculitis. Sci Rep. 2020;10:17709. doi:10.1038/s41598-020-73818-2
- Ponte C, Grayson PC, Robson JC, et al. 2022 American College of Rheumatology/EULAR Classification Criteria for Giant Cell Arteritis. Arthritis Rheumatol. 2022;74:1881-1889. doi:10.1002/art.42325
- He J, Williamson L, Ng B, et al. The diagnostic accuracy of temporal artery ultrasound and temporal artery biopsy in giant cell arteritis: a single center Australian experience over 10 years. Int J Rheum Dis. 2022;25:447-453. doi:10.1111/1756-185X.14288
- Stellingwerff MD, Brouwer E, Lensen KDF, et al. Different scoring methods of FDG PET/CT in giant cell arteritis: need for standardization. Medicine (Baltimore). 2015;94:e1542. doi:10.1097/MD.0000000000001542
- Conway R, Smyth AE, Kavanagh RG, et al. Diagnostic utility of computed tomographic angiography in giant-cell arteritis. Stroke. 2018;49:2233-2236. doi:10.1161/STROKEAHA.118.021995
- Duftner C, Dejaco C, Sepriano A, et al. Imaging in diagnosis, outcome prediction and monitoring of large vessel vasculitis: a systematic literature review and meta-analysis informing the EULAR recommendations. RMD Open. 2018;4:e000612. doi:10.1136/rmdopen-2017-000612
- Rehak Z, Vasina J, Ptacek J, et al. PET/CT in giant cell arteritis: high 18F-FDG uptake in the temporal, occipital and vertebral arteries. Rev Esp Med Nucl Imagen Mol. 2016;35:398-401. doi:10.1016/j.remn.2016.03.007
- Salvarani C, Soriano A, Muratore F, et al. Is PET/CT essential in the diagnosis and follow-up of temporal arteritis? Autoimmun Rev. 2017;16:1125-1130. doi:10.1016/j.autrev.2017.09.007
- Brodmann M, Lipp RW, Passath A, et al. The role of 2-18F-fluoro-2-deoxy-D-glucose positron emission tomography in the diagnosis of giant cell arteritis of the temporal arteries. Rheumatology (Oxford). 2004;43:241-242. doi:10.1093/rheumatology/keh025
- Flaus A, Granjon D, Habouzit V, Gaultier JB, Prevot-Bitot N. Unusual and diffuse hypermetabolism in routine 18F-FDG PET/CT of the supra-aortic vessels in biopsy-positive giant cell arteritis. Clin Nucl Med. 2018;43:e336-e337. doi:10.1097/RLU.0000000000002198
- Berger CT, Sommer G, Aschwanden M, et al. The clinical benefit of imaging in the diagnosis and treatment of giant cell arteritis. Swiss Med Wkly. 2018;148:w14661. doi:10.4414/smw.2018.14661
- Rubenstein E, Maldini C, Gonzalez-Chiappe S, et al. Sensitivity of temporal artery biopsy in the diagnosis of giant cell arteritis: a systematic literature review and meta-analysis. Rheumatology (Oxford). 2020;59:1011-1020. doi:10.1093/rheumatology/kez385
- Tsivgoulis G, Heliopoulos I, Vadikolias K, et al. Teaching neuroimages: ultrasound findings in giant-cell arteritis. Neurology. 2010;75:e67-e68. doi:10.1212/WNL.0b013e3181f881e9
- Nakajima E, Moon FH, Canvas Jr N, et al. Accuracy of Doppler ultrasound in the diagnosis of giant cell arteritis: a systematic review and meta-analysis. Adv Rheumatol. 2023;63:5. doi:10.1186/s42358-023-00286-3
- Naumegni SR, Hoffmann C, Cornec D, et al. Temporal artery ultrasound to diagnose giant cell arteritis: a practical guide. Ultrasound Med Biol. 2021;47:201-213. doi:10.1016/j.ultrasmedbio.2020.10.004
- Kim YH. Artificial intelligence in medical ultrasonography: driving on an unpaved road. Ultrasonography. 2021;40:313-317. doi:10.14366/usg.21031
- Sultan LR, Mohamed MH, Andronikou S. ChatGPT-4: a breakthrough in ultrasound image analysis. Radiol Adv. 2024;1:umae006. doi:10.1093/radadv/umae006
- Cipriani VP, Klein S. Clinical characteristics of multiple sclerosis in African-Americans. Curr Neurol Neurosci Rep. 2019;19:87. doi:10.1007/s11910-019-1000-5
- Sturm A, Dechant C, Proft F, et al. Gender differences in giant cell arteritis: a case-control study. Clin Exp Rheumatol. 2016;34:S70-72.
- Li KJ, Semenov D, Turk M, et al. A meta-analysis of the epidemiology of giant cell arteritis across time and space. Arthritis Res Ther. 2021;23:82. doi:10.1186/s13075-021-02450-w
- Nelson L, Gormley R, Riddle MS, Tribble DR, Porter CK. The epidemiology of Guillain-Barré syndrome in U.S. military personnel: a case-control study. BMC Res Notes. 2009;2:171. doi:10.1186/1756-0500-2-171
- Spaulding SW. The possible roles of environmental factors and the aryl hydrocarbon receptor in the prevalence of thyroid diseases in Vietnam era veterans. Curr Opin Endocrinol Diabetes Obes. 2011;18:315-320.
- O’Donovan A, Cohen BE, Seal KH, et al. Elevated risk for autoimmune disorders in Iraq and Afghanistan veterans with posttraumatic stress disorder. Biol Psychiatry. 2015;77:365-374. doi:10.1016/j.biopsych.2014.06.015
- Bookwalter DB, Roenfeldt KA, LeardMann CA, Kong SY, Riddle MS, Rull RP. Posttraumatic stress disorder and risk of selected autoimmune diseases among US military personnel. BMC Psychiatry. 2020;20:23. doi:10.1186/s12888-020-2432-9
- Jennette JC. Overview of the 2012 revised International Chapel Hill Consensus Conference nomenclature of vasculitides. Clin Exp Nephrol. 2013;17:603-606. doi:10.1007/s10157-013-0869-6
- Kale N, Eggenberger E. Diagnosis and management of giant cell arteritis: a review. Curr Opin Ophthalmol. 2010;21:417-422. doi:10.1097/ICU.0b013e32833eae8b
- Smetana GW, Shmerling RH. Does this patient have temporal arteritis? JAMA. 2002;287:92-101.
- Schäcke H, Döcke WD, Asadullah K. Mechanisms involved in the side effects of glucocorticoids. Pharmacol Ther. 2002;96:23-43. doi:10.1016/s0163-7258(02)00297-8
- Curtis JR, Patkar N, Xie A, et al. Risk of serious bacterial infections among rheumatoid arthritis patients exposed to tumor necrosis factor alpha antagonists. Arthritis Rheum. 2007;56:1125-1133. doi:10.1002/art.22504
- Hoes JN, van der Goes MC, van Raalte DH, et al. Glucose tolerance, insulin sensitivity and ß-cell function in patients with rheumatoid arthritis treated with or without low-to-medium dose glucocorticoids. Ann Rheum Dis. 2011;70:1887-1894. doi:10.1136/ard.2011.151464
- Hunder GG, Bloch DA, Michel BA, et al. The American College of Rheumatology 1990 criteria for the classification of giant cell arteritis. Arthritis Rheum. 1990;33:1122-1128. doi:10.1002/art.1780330810
- Dejaco C, Duftner C, Buttgereit F, Matteson EL, Dasgupta B. The spectrum of giant cell arteritis and polymyalgia rheumatica: revisiting the concept of the disease. Rheumatology (Oxford). 2017;56:506-515. doi:10.1093/rheumatology/kew273
- Slart RHJ, Nienhuis PH, Glaudemans AWJM, et al. Role of 18F-FDG PET/CT in large vessel vasculitis and polymyalgia rheumatica. J Nucl Med. 2023;64:515-521. doi:10.2967/jnumed.122.265016
- Shimol JB, Amital H, Lidar M, Domachevsky L, Shoenfeld Y, Davidson T. The utility of PET/CT in large vessel vasculitis. Sci Rep. 2020;10:17709. doi:10.1038/s41598-020-73818-2
- Ponte C, Grayson PC, Robson JC, et al. 2022 American College of Rheumatology/EULAR Classification Criteria for Giant Cell Arteritis. Arthritis Rheumatol. 2022;74:1881-1889. doi:10.1002/art.42325
- He J, Williamson L, Ng B, et al. The diagnostic accuracy of temporal artery ultrasound and temporal artery biopsy in giant cell arteritis: a single center Australian experience over 10 years. Int J Rheum Dis. 2022;25:447-453. doi:10.1111/1756-185X.14288
- Stellingwerff MD, Brouwer E, Lensen KDF, et al. Different scoring methods of FDG PET/CT in giant cell arteritis: need for standardization. Medicine (Baltimore). 2015;94:e1542. doi:10.1097/MD.0000000000001542
- Conway R, Smyth AE, Kavanagh RG, et al. Diagnostic utility of computed tomographic angiography in giant-cell arteritis. Stroke. 2018;49:2233-2236. doi:10.1161/STROKEAHA.118.021995
- Duftner C, Dejaco C, Sepriano A, et al. Imaging in diagnosis, outcome prediction and monitoring of large vessel vasculitis: a systematic literature review and meta-analysis informing the EULAR recommendations. RMD Open. 2018;4:e000612. doi:10.1136/rmdopen-2017-000612
- Rehak Z, Vasina J, Ptacek J, et al. PET/CT in giant cell arteritis: high 18F-FDG uptake in the temporal, occipital and vertebral arteries. Rev Esp Med Nucl Imagen Mol. 2016;35:398-401. doi:10.1016/j.remn.2016.03.007
- Salvarani C, Soriano A, Muratore F, et al. Is PET/CT essential in the diagnosis and follow-up of temporal arteritis? Autoimmun Rev. 2017;16:1125-1130. doi:10.1016/j.autrev.2017.09.007
- Brodmann M, Lipp RW, Passath A, et al. The role of 2-18F-fluoro-2-deoxy-D-glucose positron emission tomography in the diagnosis of giant cell arteritis of the temporal arteries. Rheumatology (Oxford). 2004;43:241-242. doi:10.1093/rheumatology/keh025
- Flaus A, Granjon D, Habouzit V, Gaultier JB, Prevot-Bitot N. Unusual and diffuse hypermetabolism in routine 18F-FDG PET/CT of the supra-aortic vessels in biopsy-positive giant cell arteritis. Clin Nucl Med. 2018;43:e336-e337. doi:10.1097/RLU.0000000000002198
- Berger CT, Sommer G, Aschwanden M, et al. The clinical benefit of imaging in the diagnosis and treatment of giant cell arteritis. Swiss Med Wkly. 2018;148:w14661. doi:10.4414/smw.2018.14661
- Rubenstein E, Maldini C, Gonzalez-Chiappe S, et al. Sensitivity of temporal artery biopsy in the diagnosis of giant cell arteritis: a systematic literature review and meta-analysis. Rheumatology (Oxford). 2020;59:1011-1020. doi:10.1093/rheumatology/kez385
- Tsivgoulis G, Heliopoulos I, Vadikolias K, et al. Teaching neuroimages: ultrasound findings in giant-cell arteritis. Neurology. 2010;75:e67-e68. doi:10.1212/WNL.0b013e3181f881e9
- Nakajima E, Moon FH, Canvas Jr N, et al. Accuracy of Doppler ultrasound in the diagnosis of giant cell arteritis: a systematic review and meta-analysis. Adv Rheumatol. 2023;63:5. doi:10.1186/s42358-023-00286-3
- Naumegni SR, Hoffmann C, Cornec D, et al. Temporal artery ultrasound to diagnose giant cell arteritis: a practical guide. Ultrasound Med Biol. 2021;47:201-213. doi:10.1016/j.ultrasmedbio.2020.10.004
- Kim YH. Artificial intelligence in medical ultrasonography: driving on an unpaved road. Ultrasonography. 2021;40:313-317. doi:10.14366/usg.21031
- Sultan LR, Mohamed MH, Andronikou S. ChatGPT-4: a breakthrough in ultrasound image analysis. Radiol Adv. 2024;1:umae006. doi:10.1093/radadv/umae006
- Cipriani VP, Klein S. Clinical characteristics of multiple sclerosis in African-Americans. Curr Neurol Neurosci Rep. 2019;19:87. doi:10.1007/s11910-019-1000-5
- Sturm A, Dechant C, Proft F, et al. Gender differences in giant cell arteritis: a case-control study. Clin Exp Rheumatol. 2016;34:S70-72.
- Li KJ, Semenov D, Turk M, et al. A meta-analysis of the epidemiology of giant cell arteritis across time and space. Arthritis Res Ther. 2021;23:82. doi:10.1186/s13075-021-02450-w
- Nelson L, Gormley R, Riddle MS, Tribble DR, Porter CK. The epidemiology of Guillain-Barré syndrome in U.S. military personnel: a case-control study. BMC Res Notes. 2009;2:171. doi:10.1186/1756-0500-2-171
- Spaulding SW. The possible roles of environmental factors and the aryl hydrocarbon receptor in the prevalence of thyroid diseases in Vietnam era veterans. Curr Opin Endocrinol Diabetes Obes. 2011;18:315-320.
- O’Donovan A, Cohen BE, Seal KH, et al. Elevated risk for autoimmune disorders in Iraq and Afghanistan veterans with posttraumatic stress disorder. Biol Psychiatry. 2015;77:365-374. doi:10.1016/j.biopsych.2014.06.015
- Bookwalter DB, Roenfeldt KA, LeardMann CA, Kong SY, Riddle MS, Rull RP. Posttraumatic stress disorder and risk of selected autoimmune diseases among US military personnel. BMC Psychiatry. 2020;20:23. doi:10.1186/s12888-020-2432-9
Advanced Imaging Techniques Use in Giant Cell Arteritis Diagnosis: The Experience at Walter Reed National Military Medical Center
Advanced Imaging Techniques Use in Giant Cell Arteritis Diagnosis: The Experience at Walter Reed National Military Medical Center
When The Giants and Those Who Stand on Their Shoulders Are Gone: The Loss of VA Institutional Memory
When The Giants and Those Who Stand on Their Shoulders Are Gone: The Loss of VA Institutional Memory
If I have seen further, it is by standing on the shoulders of giants.
Sir Isaac Newton (1642-1727) 1
Early in residency, I decided I only wanted to work at the US Department of Veterans Affairs (VA). It was a way to follow the example of service that my parents, an Army doctor and nurse, had set. I spent much of my residency, including all of my last year of training, at a VA medical center, hoping a vacancy would open in the psychiatry service. In those days, VA jobs were hard to come by; doctors spent their entire careers in the system, only retiring after decades of commitment to its unique mission. Finally, close to graduation, one of my favorite attending physicians left his post. After mountains of paperwork and running the human resources obstacle course with the usual stumbles, I arrived at my dream job as a VA psychiatrist.
So, it is with immense sadness and even shock that I read a recent ProPublica article reporting that from January to March 2025 almost 40% of the physicians who received employment offers from the Veterans Health Administration (VHA) declined the positions.2 Medical media rapidly picked up the story, likely further discouraging potential applicants.3
There have always been health care professionals (HCPs) who had zero interest in working for the VA. Medical students and residents often have a love/hate relationship with the VA, with some trainees not having the patience for the behemoth pace of the bureaucracy or finding the old-style physical environment and more relaxed pace antiquated and inefficient.
The reasons doctors are saying no to VA employment at 4 times the previous rate are different and more disturbing. According to ProPublica, VA officials in Texas reported in a June internal presentation that about 90 people had turned down job offers due to the “uncertainty of reorganization.”2 They reported that low morale was causing existing employees to recommend against working at the VA. My own anecdotal experience is similar: contrary to prior years, few residents, if any, are interested in working at the VA because of concerns about the stability of employment and the direction of its organizational culture.
It is fair to question the objectivity of the ProPublica report. However, the latest VA Office of the Inspector General (OIG) analysis of staffing had similar findings. “Despite the ability to make noncompetitive appointments for such occupations, VHA continues to experience severe occupational staffing shortages for these occupations that are fundamental to the delivery of health care.” The 4434 severe occupational shortage figures in fiscal year (FY) 2025 were 50% higher than in FY 2024.4 OIG reported that 57% of facilities noted severe occupational staffing shortages for psychology, making it the most frequently reported clinical shortage.
At this critical juncture, when new health care professional energy is not flowing into the VHA, there is an unprecedented drain of the lifeblood of any system—the departure of the bearers of institutional memory. Early and scheduled retirements, the deferred resignation program, and severance have decimated the ranks of senior HCPs, experienced leaders, and career clinicians. ProPublica noted the loss of 600 doctors and 1900 nurses at the VHA so far in 2025.2 Internal VA data from exit interviews suggest similar motivations. Many cited lack of trust and confidence in senior leaders and job stress/pressure.5
It should be noted the VA has an alternative and plausible explanation for the expected departure of 30,000 employees. They argue that the VHA was overstaffed and the increased workforce decreased the efficiency of service. Voluntary separation from employment, VA contends, has avoided the need for a far more disruptive reduction in force. VA leaders avow that downsizing has not adversely impacted its ability to deliver high-quality health care and benefits and they assert that a reduction in red tape will enable VA to provide easier access to care. VA Secretary Doug Collins has concluded that because of these difficult but necessary changes, “VA is headed in the right direction.”6
What is institutional memory, and why is it important? “The core of institutional memory is collective awareness and understanding of a collective set of facts, concepts, experiences, and know-how,” Bhugra and Ventriglio explain. “These are all held collectively at various levels in any given institution. Thus, collective memory or history can be utilized to build on what has gone before and how we take things forward.”7
The authors of this quote offer a modern twist on what Sir Isaac Newton described in more metaphorical language in the epigraph: to survive, and even more to thrive, an enterprise must have those who have accumulated technical knowledge and professional wisdom as well as those who assume responsibility for appropriating and adding to this storehouse of operational skill, expertise, unique cultural values, and ethical commitments. The VHA is losing its instructors and students of institutional memory which deals a serious blow to the stability and vitality of any learning health system.6 As Bhugra and Ventriglio put it, institutional memory identifies “what has worked in delivering the aims in the past and what has not, thereby ensuring the lessons learnt are remembered and passed on to the next generation.”7
Nearly every week, at all levels of the agency, I have encountered this exodus of builders and bearers of institutional memory. Those who have left did so for many of the same reasons cited by those who declined to come, leaving incalculable gaps at both ends of the career spectrum. Both the old and new are essential for organizational resilience: fresh ideas enable an institution to be agile in responding to challenges, while operational savvy ensures responses are ecologically aligned with the organizational mission.8
The dire shortage of HCPs—especially in mental health and primary care—has opened up unprecedented opportunities.9 Colleagues have noted that with only a little searching they found multiple lucrative positions. Once, HCPs picked the VA because they valued the commitment to public service and being part of a community of education and research more than fame or fortune. Having the best benefits packages in the industry only reinforced its value.
Even so, surpassing a genius such as Sir Isaac Newton, writing to a scientific competitor, Robert Hooke, recognized that progress and discovery in science and medicine are nigh well impossible without the collective achievements housed in institutional memory.1 It was inspiring teachers and attending physicians—Newton’s giants—who attracted the best and brightest in medicine and nursing, other HCPs, and research, to the VA, where they could participate in a transactive organizational learning process from their seniors, and then grow that fund of knowledge to improve patient care, educate their learners, and innovate. What will happen when there are no longer shoulders of giants or anyone to stand on them?
- Chen C. Mapping Scientific Frontiers: The Quest for Knowledge Visualization. Springer; 2013:135.
- Armstrong D, Umansky E, Coleman V. Veterans’ care at risk under Trump as hundreds of doctors and nurses reject working at VA hospitals. ProPublica. August 8, 2025. Accessed August 25, 2025. https://www.propublica.org/article/veterans-affairs-hospital-shortages-trump
- Kuchno K. VA physician job offers rejections up fourfold in 2025: report. Becker’s Hospital Review. August 12, 2025. Accessed August 26, 2025. https://www.beckershospitalreview.com/workforce/va-physician-job-offer-rejections-up-fourfold-in-2025-report/
- US Department of Veterans Affairs, Office of Inspector General. OIG determination of Veterans Health Administration’s severe occupational staffing shortages fiscal year 2025. August 12, 2025. Accessed August 25, 2025. https://www.vaoig.gov/reports/national-healthcare-review/oig-determination-veterans-health-administrations-severe-1
- US Department of Veterans Affairs. VA workforce dashboard. July 25, 2025. Accessed August 25, 2025. https://www.va.gov/EMPLOYEE/docs/workforce/VA-Workforce-Dashboard-Issue-27.pdf
- VA to reduce staff by nearly 30K by end of FY2025. News release. Veterans Affairs News. July 7, 2025. Accessed August 25, 2025. https://news.va.gov/press-room/va-to-reduce-staff-by-nearly-30k-by-end-of-fy2025/
- Bhugra D, Ventriglio A. Institutions, institutional memory, healthcare and research. Int J Soc Psychiatry. 2023;69(8):1843-1844. doi:10.1177/00207640231213905
- Jain A. Is organizational memory a useful capability? An analysis of its effects on productivity, absorptive capacity adaptation. In Argote L, Levine JM. The Oxford Handbook of Group and Organizational Learning. Oxford; 2020.
- Broder J. Ready to pick a specialty? These may have the brightest futures. Medscape. April 21, 2025. Accessed August 25, 2025. https://www.medscape.com/viewarticle/ready-pick-specialty-these-may-have-brightest-futures-2025a10009if
If I have seen further, it is by standing on the shoulders of giants.
Sir Isaac Newton (1642-1727) 1
Early in residency, I decided I only wanted to work at the US Department of Veterans Affairs (VA). It was a way to follow the example of service that my parents, an Army doctor and nurse, had set. I spent much of my residency, including all of my last year of training, at a VA medical center, hoping a vacancy would open in the psychiatry service. In those days, VA jobs were hard to come by; doctors spent their entire careers in the system, only retiring after decades of commitment to its unique mission. Finally, close to graduation, one of my favorite attending physicians left his post. After mountains of paperwork and running the human resources obstacle course with the usual stumbles, I arrived at my dream job as a VA psychiatrist.
So, it is with immense sadness and even shock that I read a recent ProPublica article reporting that from January to March 2025 almost 40% of the physicians who received employment offers from the Veterans Health Administration (VHA) declined the positions.2 Medical media rapidly picked up the story, likely further discouraging potential applicants.3
There have always been health care professionals (HCPs) who had zero interest in working for the VA. Medical students and residents often have a love/hate relationship with the VA, with some trainees not having the patience for the behemoth pace of the bureaucracy or finding the old-style physical environment and more relaxed pace antiquated and inefficient.
The reasons doctors are saying no to VA employment at 4 times the previous rate are different and more disturbing. According to ProPublica, VA officials in Texas reported in a June internal presentation that about 90 people had turned down job offers due to the “uncertainty of reorganization.”2 They reported that low morale was causing existing employees to recommend against working at the VA. My own anecdotal experience is similar: contrary to prior years, few residents, if any, are interested in working at the VA because of concerns about the stability of employment and the direction of its organizational culture.
It is fair to question the objectivity of the ProPublica report. However, the latest VA Office of the Inspector General (OIG) analysis of staffing had similar findings. “Despite the ability to make noncompetitive appointments for such occupations, VHA continues to experience severe occupational staffing shortages for these occupations that are fundamental to the delivery of health care.” The 4434 severe occupational shortage figures in fiscal year (FY) 2025 were 50% higher than in FY 2024.4 OIG reported that 57% of facilities noted severe occupational staffing shortages for psychology, making it the most frequently reported clinical shortage.
At this critical juncture, when new health care professional energy is not flowing into the VHA, there is an unprecedented drain of the lifeblood of any system—the departure of the bearers of institutional memory. Early and scheduled retirements, the deferred resignation program, and severance have decimated the ranks of senior HCPs, experienced leaders, and career clinicians. ProPublica noted the loss of 600 doctors and 1900 nurses at the VHA so far in 2025.2 Internal VA data from exit interviews suggest similar motivations. Many cited lack of trust and confidence in senior leaders and job stress/pressure.5
It should be noted the VA has an alternative and plausible explanation for the expected departure of 30,000 employees. They argue that the VHA was overstaffed and the increased workforce decreased the efficiency of service. Voluntary separation from employment, VA contends, has avoided the need for a far more disruptive reduction in force. VA leaders avow that downsizing has not adversely impacted its ability to deliver high-quality health care and benefits and they assert that a reduction in red tape will enable VA to provide easier access to care. VA Secretary Doug Collins has concluded that because of these difficult but necessary changes, “VA is headed in the right direction.”6
What is institutional memory, and why is it important? “The core of institutional memory is collective awareness and understanding of a collective set of facts, concepts, experiences, and know-how,” Bhugra and Ventriglio explain. “These are all held collectively at various levels in any given institution. Thus, collective memory or history can be utilized to build on what has gone before and how we take things forward.”7
The authors of this quote offer a modern twist on what Sir Isaac Newton described in more metaphorical language in the epigraph: to survive, and even more to thrive, an enterprise must have those who have accumulated technical knowledge and professional wisdom as well as those who assume responsibility for appropriating and adding to this storehouse of operational skill, expertise, unique cultural values, and ethical commitments. The VHA is losing its instructors and students of institutional memory which deals a serious blow to the stability and vitality of any learning health system.6 As Bhugra and Ventriglio put it, institutional memory identifies “what has worked in delivering the aims in the past and what has not, thereby ensuring the lessons learnt are remembered and passed on to the next generation.”7
Nearly every week, at all levels of the agency, I have encountered this exodus of builders and bearers of institutional memory. Those who have left did so for many of the same reasons cited by those who declined to come, leaving incalculable gaps at both ends of the career spectrum. Both the old and new are essential for organizational resilience: fresh ideas enable an institution to be agile in responding to challenges, while operational savvy ensures responses are ecologically aligned with the organizational mission.8
The dire shortage of HCPs—especially in mental health and primary care—has opened up unprecedented opportunities.9 Colleagues have noted that with only a little searching they found multiple lucrative positions. Once, HCPs picked the VA because they valued the commitment to public service and being part of a community of education and research more than fame or fortune. Having the best benefits packages in the industry only reinforced its value.
Even so, surpassing a genius such as Sir Isaac Newton, writing to a scientific competitor, Robert Hooke, recognized that progress and discovery in science and medicine are nigh well impossible without the collective achievements housed in institutional memory.1 It was inspiring teachers and attending physicians—Newton’s giants—who attracted the best and brightest in medicine and nursing, other HCPs, and research, to the VA, where they could participate in a transactive organizational learning process from their seniors, and then grow that fund of knowledge to improve patient care, educate their learners, and innovate. What will happen when there are no longer shoulders of giants or anyone to stand on them?
If I have seen further, it is by standing on the shoulders of giants.
Sir Isaac Newton (1642-1727) 1
Early in residency, I decided I only wanted to work at the US Department of Veterans Affairs (VA). It was a way to follow the example of service that my parents, an Army doctor and nurse, had set. I spent much of my residency, including all of my last year of training, at a VA medical center, hoping a vacancy would open in the psychiatry service. In those days, VA jobs were hard to come by; doctors spent their entire careers in the system, only retiring after decades of commitment to its unique mission. Finally, close to graduation, one of my favorite attending physicians left his post. After mountains of paperwork and running the human resources obstacle course with the usual stumbles, I arrived at my dream job as a VA psychiatrist.
So, it is with immense sadness and even shock that I read a recent ProPublica article reporting that from January to March 2025 almost 40% of the physicians who received employment offers from the Veterans Health Administration (VHA) declined the positions.2 Medical media rapidly picked up the story, likely further discouraging potential applicants.3
There have always been health care professionals (HCPs) who had zero interest in working for the VA. Medical students and residents often have a love/hate relationship with the VA, with some trainees not having the patience for the behemoth pace of the bureaucracy or finding the old-style physical environment and more relaxed pace antiquated and inefficient.
The reasons doctors are saying no to VA employment at 4 times the previous rate are different and more disturbing. According to ProPublica, VA officials in Texas reported in a June internal presentation that about 90 people had turned down job offers due to the “uncertainty of reorganization.”2 They reported that low morale was causing existing employees to recommend against working at the VA. My own anecdotal experience is similar: contrary to prior years, few residents, if any, are interested in working at the VA because of concerns about the stability of employment and the direction of its organizational culture.
It is fair to question the objectivity of the ProPublica report. However, the latest VA Office of the Inspector General (OIG) analysis of staffing had similar findings. “Despite the ability to make noncompetitive appointments for such occupations, VHA continues to experience severe occupational staffing shortages for these occupations that are fundamental to the delivery of health care.” The 4434 severe occupational shortage figures in fiscal year (FY) 2025 were 50% higher than in FY 2024.4 OIG reported that 57% of facilities noted severe occupational staffing shortages for psychology, making it the most frequently reported clinical shortage.
At this critical juncture, when new health care professional energy is not flowing into the VHA, there is an unprecedented drain of the lifeblood of any system—the departure of the bearers of institutional memory. Early and scheduled retirements, the deferred resignation program, and severance have decimated the ranks of senior HCPs, experienced leaders, and career clinicians. ProPublica noted the loss of 600 doctors and 1900 nurses at the VHA so far in 2025.2 Internal VA data from exit interviews suggest similar motivations. Many cited lack of trust and confidence in senior leaders and job stress/pressure.5
It should be noted the VA has an alternative and plausible explanation for the expected departure of 30,000 employees. They argue that the VHA was overstaffed and the increased workforce decreased the efficiency of service. Voluntary separation from employment, VA contends, has avoided the need for a far more disruptive reduction in force. VA leaders avow that downsizing has not adversely impacted its ability to deliver high-quality health care and benefits and they assert that a reduction in red tape will enable VA to provide easier access to care. VA Secretary Doug Collins has concluded that because of these difficult but necessary changes, “VA is headed in the right direction.”6
What is institutional memory, and why is it important? “The core of institutional memory is collective awareness and understanding of a collective set of facts, concepts, experiences, and know-how,” Bhugra and Ventriglio explain. “These are all held collectively at various levels in any given institution. Thus, collective memory or history can be utilized to build on what has gone before and how we take things forward.”7
The authors of this quote offer a modern twist on what Sir Isaac Newton described in more metaphorical language in the epigraph: to survive, and even more to thrive, an enterprise must have those who have accumulated technical knowledge and professional wisdom as well as those who assume responsibility for appropriating and adding to this storehouse of operational skill, expertise, unique cultural values, and ethical commitments. The VHA is losing its instructors and students of institutional memory which deals a serious blow to the stability and vitality of any learning health system.6 As Bhugra and Ventriglio put it, institutional memory identifies “what has worked in delivering the aims in the past and what has not, thereby ensuring the lessons learnt are remembered and passed on to the next generation.”7
Nearly every week, at all levels of the agency, I have encountered this exodus of builders and bearers of institutional memory. Those who have left did so for many of the same reasons cited by those who declined to come, leaving incalculable gaps at both ends of the career spectrum. Both the old and new are essential for organizational resilience: fresh ideas enable an institution to be agile in responding to challenges, while operational savvy ensures responses are ecologically aligned with the organizational mission.8
The dire shortage of HCPs—especially in mental health and primary care—has opened up unprecedented opportunities.9 Colleagues have noted that with only a little searching they found multiple lucrative positions. Once, HCPs picked the VA because they valued the commitment to public service and being part of a community of education and research more than fame or fortune. Having the best benefits packages in the industry only reinforced its value.
Even so, surpassing a genius such as Sir Isaac Newton, writing to a scientific competitor, Robert Hooke, recognized that progress and discovery in science and medicine are nigh well impossible without the collective achievements housed in institutional memory.1 It was inspiring teachers and attending physicians—Newton’s giants—who attracted the best and brightest in medicine and nursing, other HCPs, and research, to the VA, where they could participate in a transactive organizational learning process from their seniors, and then grow that fund of knowledge to improve patient care, educate their learners, and innovate. What will happen when there are no longer shoulders of giants or anyone to stand on them?
- Chen C. Mapping Scientific Frontiers: The Quest for Knowledge Visualization. Springer; 2013:135.
- Armstrong D, Umansky E, Coleman V. Veterans’ care at risk under Trump as hundreds of doctors and nurses reject working at VA hospitals. ProPublica. August 8, 2025. Accessed August 25, 2025. https://www.propublica.org/article/veterans-affairs-hospital-shortages-trump
- Kuchno K. VA physician job offers rejections up fourfold in 2025: report. Becker’s Hospital Review. August 12, 2025. Accessed August 26, 2025. https://www.beckershospitalreview.com/workforce/va-physician-job-offer-rejections-up-fourfold-in-2025-report/
- US Department of Veterans Affairs, Office of Inspector General. OIG determination of Veterans Health Administration’s severe occupational staffing shortages fiscal year 2025. August 12, 2025. Accessed August 25, 2025. https://www.vaoig.gov/reports/national-healthcare-review/oig-determination-veterans-health-administrations-severe-1
- US Department of Veterans Affairs. VA workforce dashboard. July 25, 2025. Accessed August 25, 2025. https://www.va.gov/EMPLOYEE/docs/workforce/VA-Workforce-Dashboard-Issue-27.pdf
- VA to reduce staff by nearly 30K by end of FY2025. News release. Veterans Affairs News. July 7, 2025. Accessed August 25, 2025. https://news.va.gov/press-room/va-to-reduce-staff-by-nearly-30k-by-end-of-fy2025/
- Bhugra D, Ventriglio A. Institutions, institutional memory, healthcare and research. Int J Soc Psychiatry. 2023;69(8):1843-1844. doi:10.1177/00207640231213905
- Jain A. Is organizational memory a useful capability? An analysis of its effects on productivity, absorptive capacity adaptation. In Argote L, Levine JM. The Oxford Handbook of Group and Organizational Learning. Oxford; 2020.
- Broder J. Ready to pick a specialty? These may have the brightest futures. Medscape. April 21, 2025. Accessed August 25, 2025. https://www.medscape.com/viewarticle/ready-pick-specialty-these-may-have-brightest-futures-2025a10009if
- Chen C. Mapping Scientific Frontiers: The Quest for Knowledge Visualization. Springer; 2013:135.
- Armstrong D, Umansky E, Coleman V. Veterans’ care at risk under Trump as hundreds of doctors and nurses reject working at VA hospitals. ProPublica. August 8, 2025. Accessed August 25, 2025. https://www.propublica.org/article/veterans-affairs-hospital-shortages-trump
- Kuchno K. VA physician job offers rejections up fourfold in 2025: report. Becker’s Hospital Review. August 12, 2025. Accessed August 26, 2025. https://www.beckershospitalreview.com/workforce/va-physician-job-offer-rejections-up-fourfold-in-2025-report/
- US Department of Veterans Affairs, Office of Inspector General. OIG determination of Veterans Health Administration’s severe occupational staffing shortages fiscal year 2025. August 12, 2025. Accessed August 25, 2025. https://www.vaoig.gov/reports/national-healthcare-review/oig-determination-veterans-health-administrations-severe-1
- US Department of Veterans Affairs. VA workforce dashboard. July 25, 2025. Accessed August 25, 2025. https://www.va.gov/EMPLOYEE/docs/workforce/VA-Workforce-Dashboard-Issue-27.pdf
- VA to reduce staff by nearly 30K by end of FY2025. News release. Veterans Affairs News. July 7, 2025. Accessed August 25, 2025. https://news.va.gov/press-room/va-to-reduce-staff-by-nearly-30k-by-end-of-fy2025/
- Bhugra D, Ventriglio A. Institutions, institutional memory, healthcare and research. Int J Soc Psychiatry. 2023;69(8):1843-1844. doi:10.1177/00207640231213905
- Jain A. Is organizational memory a useful capability? An analysis of its effects on productivity, absorptive capacity adaptation. In Argote L, Levine JM. The Oxford Handbook of Group and Organizational Learning. Oxford; 2020.
- Broder J. Ready to pick a specialty? These may have the brightest futures. Medscape. April 21, 2025. Accessed August 25, 2025. https://www.medscape.com/viewarticle/ready-pick-specialty-these-may-have-brightest-futures-2025a10009if
When The Giants and Those Who Stand on Their Shoulders Are Gone: The Loss of VA Institutional Memory
When The Giants and Those Who Stand on Their Shoulders Are Gone: The Loss of VA Institutional Memory
How Increasing Research Demands Threaten Equity in Dermatology Residency Selection and Strategies for Reform
How Increasing Research Demands Threaten Equity in Dermatology Residency Selection and Strategies for Reform
As one of the most competitive specialties in medicine, dermatology presents unique challenges for residency applicants, especially following the shift in United States Medical Licensing Examination (USMLE) Step 1 scoring to a pass/fail format.1,2 Historically, USMLE Step 1 served as a major screening metric for residency programs, with 90% of program directors in 2020 using USMLE Step 1 scores as a primary factor when deciding whether to invite applicants for interviews.1 However, the recent transition to pass/fail has made it much harder for program directors to objectively compare applicants, particularly in dermatology. In a 2020 survey, Patrinely Jr et al2 found that 77.2% of dermatology program directors agreed that this change would make it more difficult to assess candidates objectively. Consequently, research productivity has taken on greater importance as programs seek new ways to distinguish top applicants.1,2
In response to this increased emphasis on research, dermatology applicants have substantially boosted their scholarly output over the past several years. The 2022 and 2024 results from the National Residency Matching Program’s Charting Outcomes survey demonstrated a steady rise in research metrics among applicants across various specialties, with dermatology showing one of the largest increases.3,4 For instance, the average number of abstracts, presentations, and publications for matched allopathic dermatology applicants was 5.7 in 2007.5 This average increased to 20.9 in 20223 and to 27.7 in 2024,4 marking an astonishing 485% increase in 17 years. Interestingly, unmatched dermatology applicants had an average of 19.0 research products in 2024, which was similar to the average of successfully matched applicants just 2 years earlier.3,4
Engaging in research offers benefits beyond building a strong residency application. Specifically, it enhances critical thinking skills and provides hands-on experience in scientific inquiry.6 It allows students to explore dermatology topics of interest and address existing knowledge gaps within the specialty.6 Additionally, it creates opportunities to build meaningful relationships with experienced dermatologists who can guide and support students throughout their careers.7 Despite these benefits, the pursuit of research may be landscaped with obstacles, and the fervent race to obtain high research outputs may overshadow developmental advantages.8 These challenges and demands also could contribute to inequities in the residency selection process, particularly if barriers are influenced by socioeconomic and demographic disparities. As dermatology already ranks as the second least diverse specialty in medicine,9 research requirements that disproportionately disadvantage certain demographic groups risk further widening these concerning representation gaps rather than creating opportunities to address them.
Given these trends in research requirements and their potential impact on applicant success, understanding specific barriers to research engagement is essential for creating equitable opportunities in dermatology. In this study, we aimed to identify barriers to research engagement among dermatology applicants, analyze their relationship with demographic factors, assess their impact on specialty choice and research productivity, and provide actionable solutions to address these obstacles.
Methods
A cross-sectional survey was conducted targeting medical students applying to dermatology residency programs in the United States in the 2025 or 2026 match cycles as well as residents who applied to dermatology residency in the 2021 to 2024 match cycles. The 23-item survey was developed by adapting questions from several validated studies examining research barriers and experiences in medical education.6,7,10,11 Specifically, the survey included questions on demographics and background; research productivity; general research barriers; conference participation accessibility; mentorship access; and quality, career impact, and support needs. Socioeconomic background was measured via a single self-reported item asking participants to select the income class that best reflected their background growing up (low-income, lower-middle, upper-middle, or high-income); no income ranges were provided.
The survey was distributed electronically via Qualtrics between November 11, 2024, and December 30, 2024, through listserves of the Dermatology Interest Group Association (sent directly to medical students) and the Association of Professors of Dermatology (forwarded to residents by program directors). There was no way to determine the number of dermatology applicants and residents reached through either listserve. The surveys were reviewed and approved by the University of Alabama at Birmingham institutional review board (IRB-300013671).
Statistical analyses were conducted using RStudio (Posit, PBC; version 2024.12.0+467). Descriptive statistics characterized participant demographics and quantified barrier scores using frequencies and proportions. We performed regression analyses to examine relationships between demographic factors and barriers using linear regression; the relationship between barriers and research productivity correlation; and the prediction of specialty change consideration using logistic regression. For all analyses, barrier scores were rated on a scale of 0 to 3 (0=not a barrier, 1=minor barrier, 2=moderate barrier, 3=major barrier); R² values were reported to indicate strength of associations, and statistical significance was set at P<.05.
Results
Participant Demographics—A total of 136 participants completed the survey. Among the respondents, 12% identified as from a background of low-income class, 28% lower-middle class, 49% upper-middle class, and 11% high-income class. Additionally, 27% of respondents identified as underrepresented in medicine (URiM). Regarding debt levels (or expected debt levels) upon graduation from medical school, 32% reported no debt, 9% reported $1000 to $49,000 in debt, 5% reported $50,000 to $99,000 in debt, 15% reported $100,000 to $199,000 in debt, 22% reported $200,000 to $299,000 in debt, and 17% reported $300,000 in debt or higher. The majority of respondents (95%) were MD candidates, and the remaining 5% were DO candidates; additionally, 5% were participants in an MD/PhD program (eTable 1).
Respondents represented various stages of training: 13.2% and 16.2% were third- and fourth-year medical students, respectively, while 6.0%, 20.1%, 18.4%, and 22.8% were postgraduate year (PGY) 1, PGY-2, PGY-3, and PGY-4, respectively. A few respondents (2.9%) were participating in a research year or reapplying to dermatology residency (eTable 2).
Research Barriers and Productivity—Respondents were presented with a list of potential barriers and asked to rate each as not a barrier, a minor barrier, a moderate barrier, or a major barrier. The most common barriers (ie, those with >50% of respondents rating them as a moderate or major) included lack of time, limited access to research opportunities, not knowing how to begin research, and lack of mentorship or support. Lack of time and not knowing where to begin research were reported most frequently as major barriers, with 32% of participants identifying them as such. In contrast, barriers such as financial costs and personal obligations were less frequently rated as major barriers (10% and 4%, respectively), although they still were identified as obstacles by many respondents. Interestingly, most respondents (58%) indicated that institutional limitations were not a barrier, but a separate and sizeable proportion (25%) of respondents considered it to be a major barrier (eFigure 1).

The distributions for all research metrics were right-skewed. The total range was 0 to 45 (median, 6) for number of publications (excluding abstracts), 0 to 33 (median, 2) for published abstracts, 0 to 40 (median, 5) for poster publications, and 0 to 20 (median, 2) for oral presentations (eTable 3).
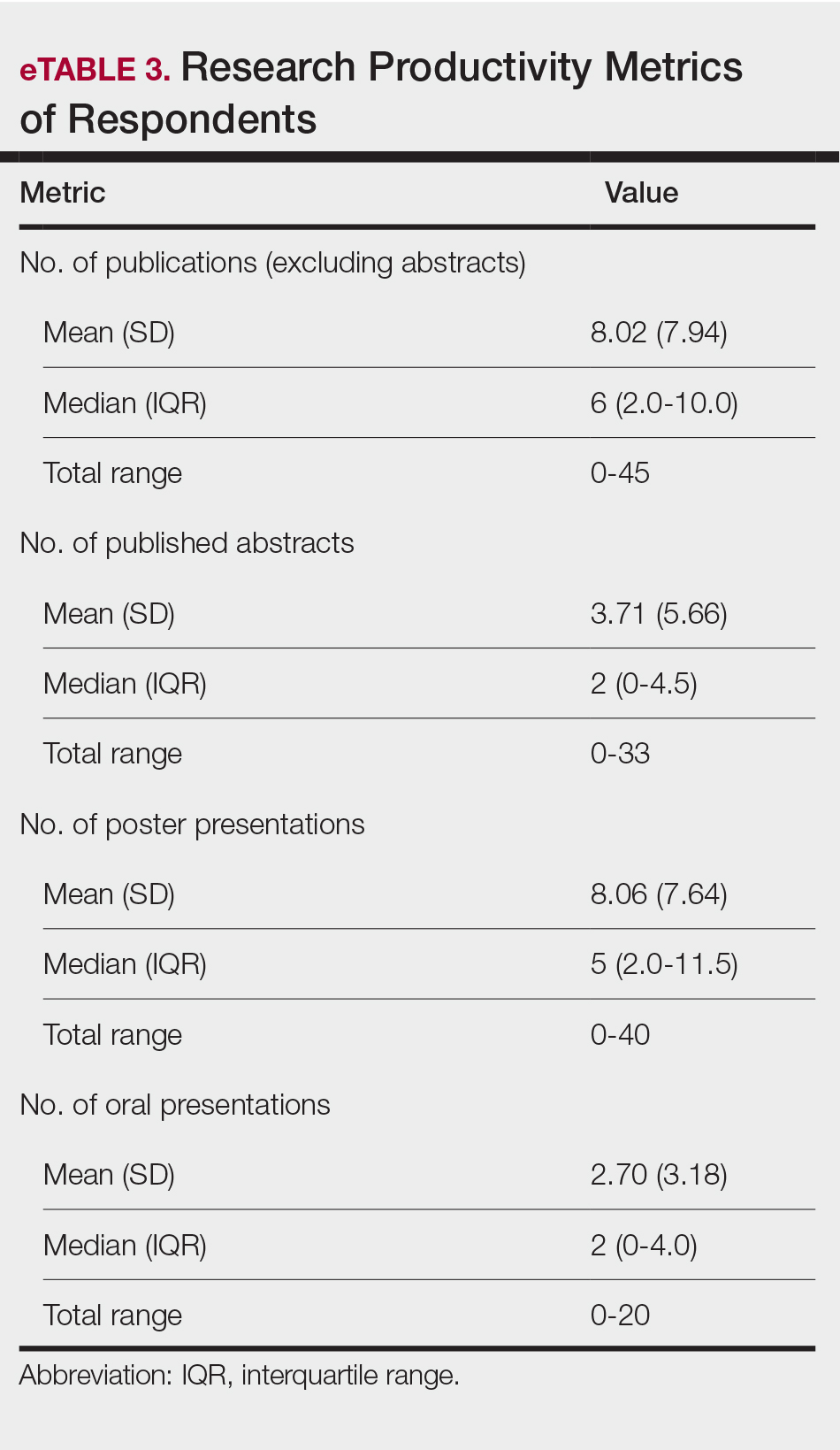
Regression Analysis—Linear regression analysis identified significant relationships between demographic variables (socioeconomic status [SES], URiM status, and debt level) and individual research barriers. The heatmap in eFigure 2 illustrates the strength of these relationships. Higher SES was predictive of lower reported financial barriers (R²=.2317; P<.0001) and lower reported institutional limitations (R²=.0884; P=.0006). A URiM status predicted higher reported financial barriers (R²=.1097; P<.0001) and institutional limitations (R²=.04537; P=.013). Also, higher debt level predicted increased financial barriers (R²=.2099; P<.0001), institutional limitations (R2=.1258; P<.0001), and lack of mentorship (R²=.06553; P=.003).
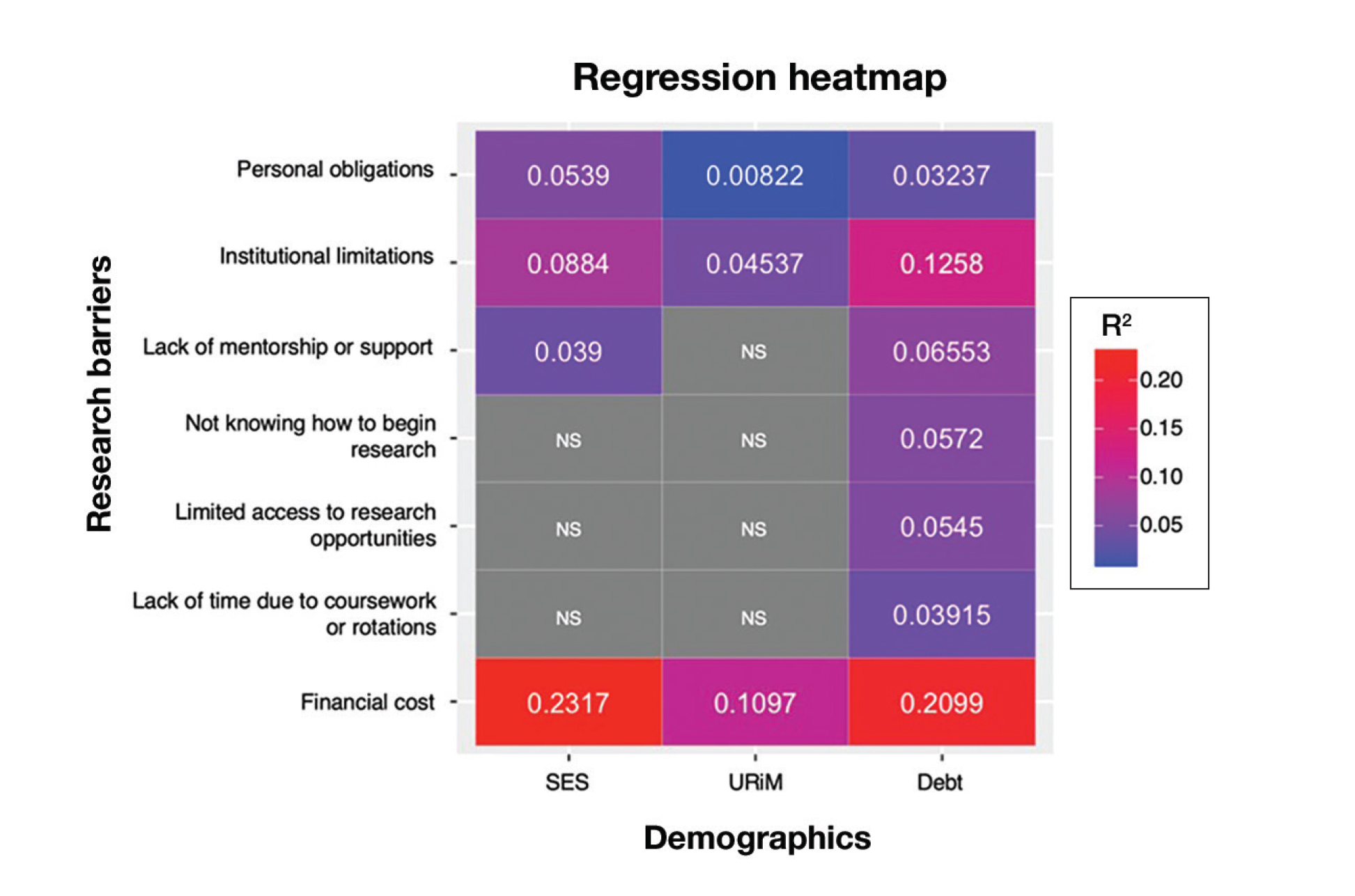
Next, the data were evaluated for correlative relationships between individual research barriers and research productivity metrics including number of publications, published abstracts and presentations (oral and poster) and total research output. While correlations were weak or nonsignificant between barriers and most research productivity metrics (published abstracts, oral and poster presentations, and total research output), the number of publications was significantly correlated with several research barriers, including limited access to research opportunities (P=.002), not knowing how to begin research (P=.025), lack of mentorship or support (P=.011), and institutional limitations (P=.042). Higher ratings for limited access to research opportunities, not knowing where to begin research, lack of mentorship or support, and institutional limitations all were negatively correlated with total number of publications (R2=−.27, –.19, −.22, and –.18, respectively)(eFigure 3).
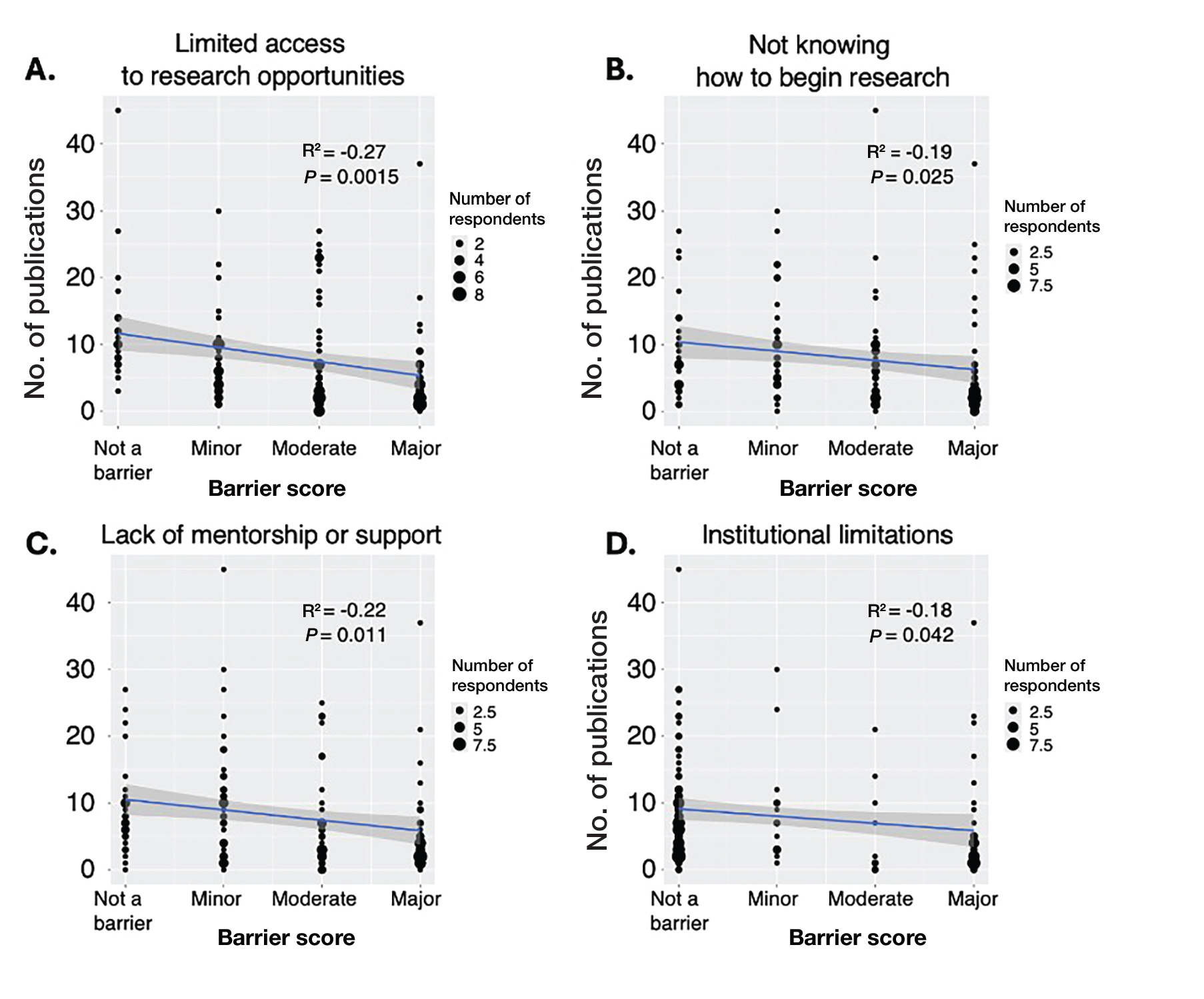
Logistic regression analysis examined the impact of research barriers on the likelihood of specialty change consideration. The results, presented in a forest plot, include odds ratios (ORs) and their corresponding 95% CIs and P values. Lack of time (P=.001) and not knowing where to begin research (P<.001) were the strongest predictors of specialty change consideration (OR, 6.3 and 4.7, respectively). Financial cost (P=.043), limited access to research opportunities (P=.006), and lack of mentorship or support (P=.001) also were significant predictors of specialty change consideration (OR, 2.2, 3.1, and 3.5, respectively). Institutional limitations and personal obligations did not predict specialty change consideration (eTable 4 and eFigure 4).
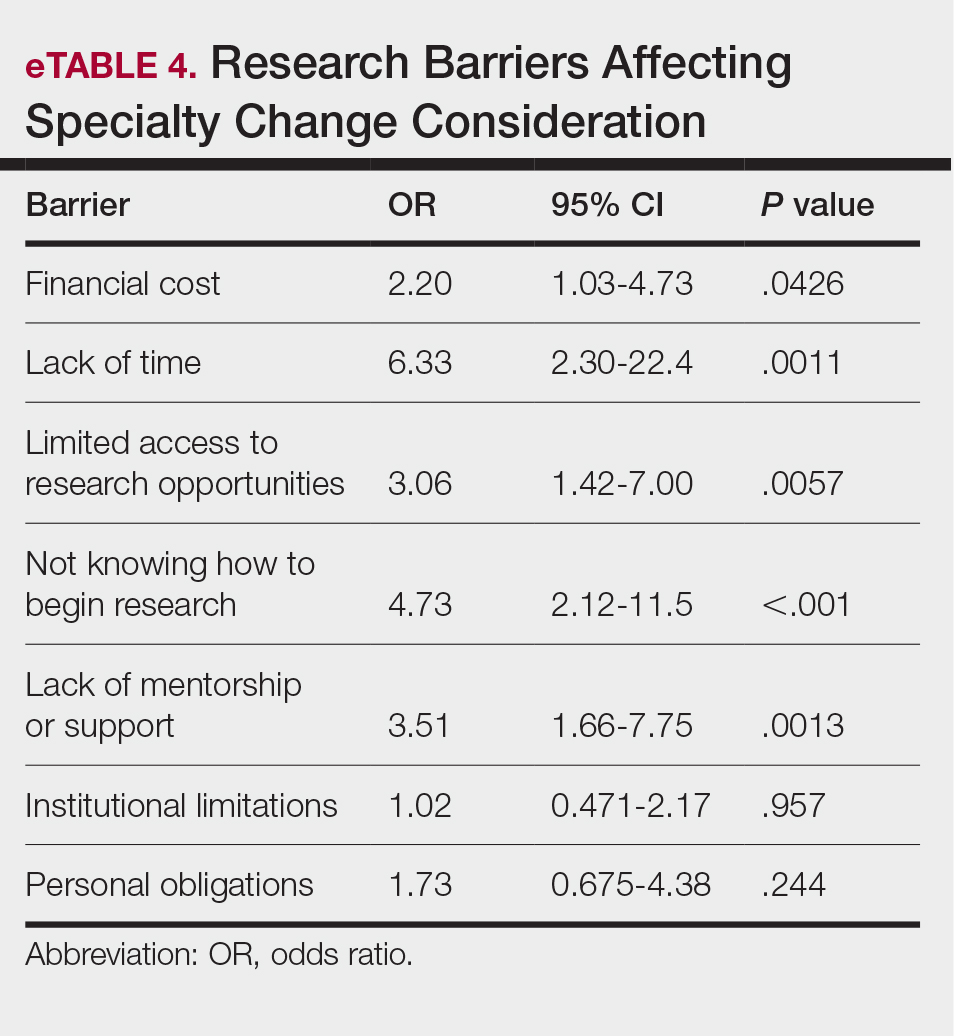
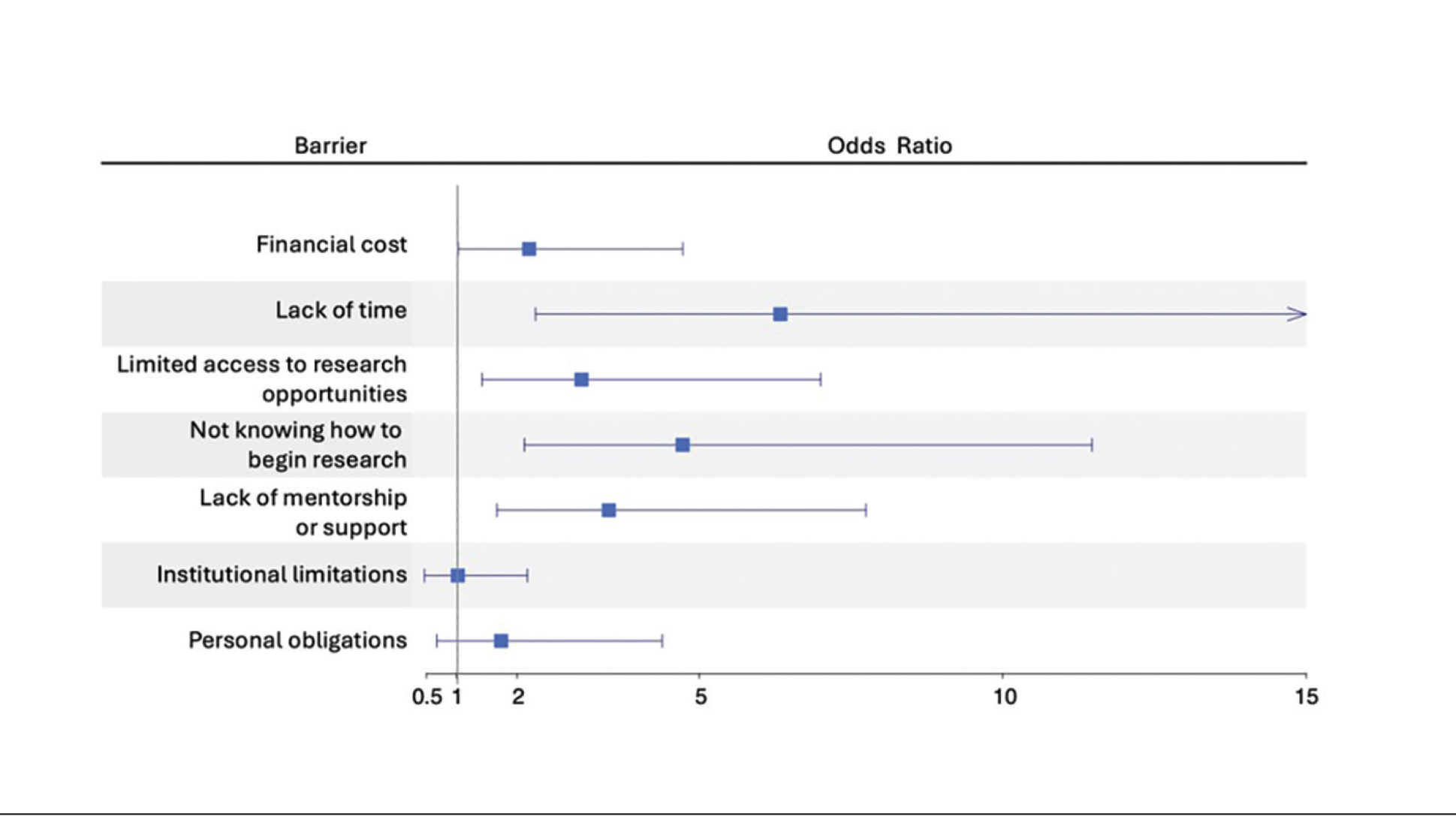
Mitigation Strategies—Mitigation strategies were ranked by respondents based on their perceived importance on a scale of 1 to 7 (1=most important, 7=least important)(eFigure 5). Respondents considered access to engaged mentors to be the most important mitigation strategy by far, with 95% ranking it in the top 3 (47% of respondents ranked it as the top most important mitigation strategy). Financial assistance was the mitigation strategy with the second highest number of respondents (28%) ranking it as the top strategy. Flexible scheduling during rotations, research training programs or discussions, and peer networking and research collaboration opportunities also were considered by respondents to be important mitigation strategies. Time management support/resources frequently was viewed as the least important mitigation strategy, with 38% of respondents ranking it last.
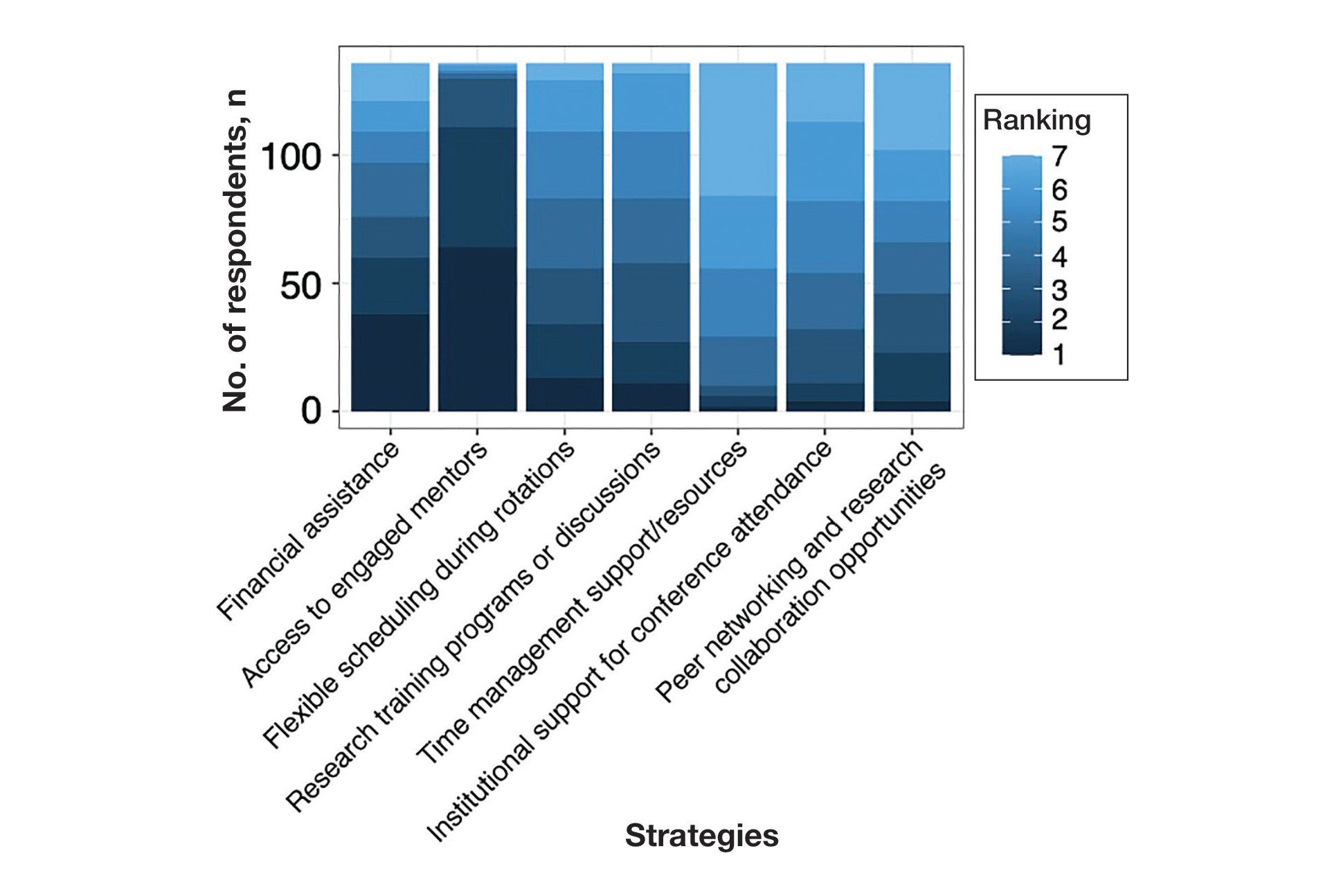
Comment
Our study revealed notable disparities in research barriers among dermatology applicants, with several demonstrating consistent patterns of association with SES, URiM status, and debt burden. Furthermore, the strong relationship between these barriers and decreased research productivity and specialty change consideration suggests that capable candidates may be deterred from pursuing dermatology due to surmountable obstacles rather than lack of interest or ability.
Impact of Demographic Factors on Research Barriers—All 7 general research barriers surveyed were correlated with distinct demographic predictors. Regression analyses indicated that the barrier of financial cost was significantly predicted by lower SES (R²=.2317; P<.001), URiM status (R²=.1097; P<.001), and higher debt levels (R²=.2099; P<.001)(eFigure 2). These findings are particularly concerning given the trend of dermatology applicants pursuing 1-year research fellowships, many of which are unpaid.12 In fact, Jacobson et al11 found that 71.7% (43/60) of dermatology applicants who pursued a year-long research fellowship experienced financial strain during their fellowship, with many requiring additional loans or drawing from personal savings despite already carrying substantial medical school debt of $200,000 or more. Our findings showcase how financial barriers to research disproportionately affect students from lower socioeconomic backgrounds, those who identify as URiM, and those with higher debt, creating systemic inequities in research access at a time when research productivity is increasingly vital for matching into dermatology. To address these financial barriers, institutions may consider establishing more funded research fellowships or expanding grant programs targeting students from economically disadvantaged and/or underrepresented backgrounds.
Institutional limitations (eg, the absence of a dermatology department) also was a notable barrier that was significantly predicted by lower SES (R²=.0884; P<.001) and URiM status (R²=.04537; P=.013)(eFigure 2). Students at institutions lacking dermatology programs face restricted access to mentorship and research opportunities,13 with our results demonstrating that these barriers disproportionately affect students from underresourced and minority groups. These limitations compound disparities in building competitive residency applications.14 The Women’s Dermatologic Society (WDS) has developed a model for addressing these institutional barriers through its summer research fellowship program for medical students who identify as URiM. By pairing students with WDS mentors who guide them through summer research projects, this initiative addresses access and mentorship gaps for students lacking dermatology departments at their home institution.15 The WDS program serves as a model for other organizations to adopt and expand, with particular attention to including students who identify as URiM as well as those from lower socioeconomic backgrounds.
Our results identified time constraints and lack of experience as notable research barriers. Higher debt levels significantly predicted both lack of time (R²=.03915; P=.021) and not knowing how to begin research (R²=.0572; P=.005)(eFigure 2). These statistical relationships may be explained by students with higher debt levels needing to prioritize paid work over unpaid research opportunities, limiting their engagement in research due to the scarcity of funded positions.12 The data further revealed that personal obligations, particularly family care responsibilities, were significantly predicted by both lower SES (R²=.0539; P=.008) and higher debt level (R²=.03237; P=.036)(eFigure 2). These findings demonstrate how students managing academic demands alongside financial and familial responsibilities may face compounded barriers to research engagement. To address these disparities, medical schools may consider implementing protected research time within their curricula; for example, the Emory University School of Medicine (Atlanta, Georgia) has implemented a Discovery Phase program that provides students with 5 months of protected faculty-mentored research time away from academic demands between their third and fourth years of medical school.16 Integrating similarly structured research periods across medical school curricula could help ensure equitable research opportunities for all students pursuing competitive specialties such as dermatology.8
Access to mentorship is a critical determinant of research engagement and productivity, as mentors provide valuable guidance on navigating research processes and professional development.17 Our analysis revealed that lack of mentorship was predicted by both lower SES (R²=.039; P=.023) and higher debt level (R²=.06553; P=.003)(eFigure 2). Several organizations have developed programs to address these mentorship gaps. The Skin of Color Society pairs medical students with skin of color experts while advancing its mission of increasing diversity in dermatology.18 Similarly, the American Academy of Dermatology founded a diversity mentorship program that connects students who identify as URiM with dermatologist mentors for summer research experiences.19 Notably, the Skin of Color Society’s program allows residents to serve as mentors for medical students. Involving residents and community dermatologists as potential dermatology mentors for medical students not only distributes mentorship demands more sustainably but also increases overall access to dermatology mentors. Our findings indicate that similar programs could be expanded to include more residents and community dermatologists as mentors and to target students from disadvantaged backgrounds, those facing financial constraints, and students who identify as URiM.
Impact of Research Barriers on Career Trajectories—Among survey participants, 35% reported considering changing their specialty choice due to research-related barriers. This substantial percentage likely stems from the escalating pressure to achieve increasingly high research output amidst a lack of sufficient support, time, or tools, as our results suggest. The specific barriers that most notably predicted specialty change consideration were lack of time and not knowing how to begin research (P=.001 and P<.001, respectively). Remarkably, our findings revealed that respondents who rated these as moderate or major barriers were 6.3 and 4.7 times more likely to consider changing their specialty choice, respectively. Respondents reporting financial cost (P=.043), limited access to research opportunities (P=.006), and lack of mentorship or support (P=.001) as at least moderate barriers also were 2.2 to 3.5 times more likely to consider a specialty change (eTable 4 and eFigure 4). Additionally, barriers such as limited access to research opportunities (R²=−.27; P=.002), lack of mentorship (R2=−.22; P=.011), not knowing how to begin research (R2=−.19; P=.025), and institutional limitations (R2=−.18; P=.042) all were associated with lower publication output according to our data (eFigure 3). These findings are especially concerning given current match statistics, where the trajectory of research productivity required for a successful dermatology match continues to rise sharply.3,4
Alarmingly, many of the barriers we identified—linked to both reduced research output and specialty change consideration—are associated with several demographic factors. Higher debt levels predicted greater likelihood of experiencing lack of time, insufficient mentorship, and uncertainty about initiating research, while lower SES was associated with lack of mentorship. These relationships suggest that structural barriers, rather than lack of interest or ability, may create cumulative disadvantages that deter capable candidates from pursuing dermatology or impact their success in the application process.
One potential solution to address the disproportionate emphasis on research quantity would be implementing caps on reportable research products in residency applications (eg, limiting applications to a certain number of publications, abstracts, and presentations). This change could shift applicant focus toward substantive scientific contributions rather than rapid output accumulation.8 The need for such caps was evident in our dataset, which revealed a stark contrast: some respondents reported 30 to 40 publications, while MD/PhD respondents—who dedicate 3 to 5 years to performing quality research—averaged only 7.4 publications. Implementing a research output ceiling could help alleviate barriers for applicants facing institutional and demographic disadvantages while simultaneously boosting the scientific rigor of dermatology research.8
Mitigation Strategies From Applicant Feedback—Our findings emphasize the multifaceted relationship between structural barriers and demographics in dermatology research engagement. While our statistical interpretations have outlined several potential interventions, the applicants’ perspectives on mitigation strategies offer qualitative insight. Although participants did not consistently mark financial cost and lack of mentorship as major barriers (eFigure 1), financial assistance and access to engaged mentors were among the highest-ranked mitigation strategies (eFigure 5), suggesting these resources may be fundamental to overcoming multiple structural challenges. To address these needs comprehensively, we propose a multilevel approach: at the institutional level, dermatology interest groups could establish centralized databases of research opportunities, mentorship programs, and funding sources. At the national level, dermatology organizations could consider expanding grant programs, developing virtual mentorship networks, and creating opportunities for external students through remote research projects or short-term research rotations. These interventions, informed by both our statistical analyses and applicant feedback, could help create more equitable access to research opportunities in dermatology.
Limitations
A major limitation of this study was that potential dermatology candidates who were deterred by barriers and later decided on a different specialty would not be captured in our data. As these candidates may have faced substantial barriers that caused them to choose a different path, their absence from the current data may indicate that the reported results underpredict the effect size of the true population. Another limitation is the absence of a control group, such as applicants to less competitive specialties, which would provide valuable context for whether the barriers identified are unique to dermatology.
Conclusion
Our study provides compelling evidence that research barriers in dermatology residency applications intersect with demographic factors to influence research engagement and career trajectories. Our findings suggest that without targeted intervention, increasing emphasis on research productivity may exacerbate existing disparities in dermatology. Moving forward, a coordinated effort among institutions, dermatology associations, and dermatology residency programs will be fundamental to ensure that research requirements enhance rather than impede the development of a diverse, qualified dermatology workforce.
- Ozair A, Bhat V, Detchou DKE. The US residency selection process after the United States Medical Licensing Examination Step 1 pass/fail change: overview for applicants and educators. JMIR Med Educ. 2023;9:E37069. doi:10.2196/37069
- Patrinely JR Jr, Zakria D, Drolet BC. USMLE Step 1 changes: dermatology program director perspectives and implications. Cutis. 2021;107:293-294. doi:10.12788/cutis.0277
- National Resident Matching Program. Charting outcomes in the match: US MD seniors, 2022. July 2022. Accessed February 14, 2024. https://www.nrmp.org/wp-content/uploads/2022/07/Charting-Outcomes-MD-Seniors-2022_Final.pdf
- National Resident Matching Program. Charting outcomes in the match: US MD seniors, 2024. August 2024. Accessed February 14, 2024. https://www.nrmp.org/match-data/2024/08/charting-outcomes-characteristics-of-u-s-md-seniors-who-matched-to-their-preferred-specialty-2024-main-residency-match/
- National Resident Matching Program. Charting outcomes in the match: characteristics of applicants who matched to their preferred specialty in the 2007 main residency match. July 2021. Accessed February 14, 2024. https://www.nrmp.org/wp-content/uploads/2021/07/chartingoutcomes2007.pdf
- Sanabria-de la Torre R, Quiñones-Vico MI, Ubago-Rodríguez A, et al. Medical students’ interest in research: changing trends during university training. Front Med. 2023;10. doi:10.3389/fmed.2023.1257574
- Alikhan A, Sivamani RK, Mutizwa MM, et al. Advice for medical students interested in dermatology: perspectives from fourth year students who matched. Dermatol Online J. 2009;15:7. doi:10.5070/D398p8q1m5
- Elliott B, Carmody JB. Publish or perish: the research arms race in residency selection. J Grad Med Educ. 2023;15:524-527. doi:10.4300/JGME-D-23-00262.1
- Akhiyat S, Cardwell L, Sokumbi O. Why dermatology is the second least diverse specialty in medicine: how did we get here? Clin Dermatol. 2020;38:310-315. doi:10.1016/j.clindermatol.2020.02.005
- Orebi HA, Shahin MR, Awad Allah MT, et al. Medical students’ perceptions, experiences, and barriers towards research implementation at the faculty of medicine, Tanta University. BMC Med Educ. 2023;23:902. doi:10.1186/s12909-023-04884-z
- Jacobsen A, Kabbur G, Freese RL, et al. Socioeconomic factors and financial burdens of research “gap years” for dermatology residency applicants. Int J Womens Dermatol. 2023;9:e099. doi:10.1097/JW9.0000000000000099
- Jung J, Stoff BK, Orenstein LAV. Unpaid research fellowships among dermatology residency applicants. J Am Acad Dermatol. 2022;87:1230-1231. doi:10.1016/j.jaad.2021.12.027
- Rehman R, Shareef SJ, Mohammad TF, et al. Applying to dermatology residency without a home program: advice to medical students in the COVID-19 pandemic and beyond. Clin Dermatol. 2022;40:513-515. doi:10.1016/j.clindermatol.2022.01.003
- Villa NM, Shi VY, Hsiao JL. An underrecognized barrier to the dermatology residency match: lack of a home program. Int J Womens Dermatol. 2021;7:512-513. doi:10.1016/j.ijwd.2021.02.011
- Sekyere NAN, Grimes PE, Roberts WE, et al. Turning the tide: how the Women’s Dermatologic Society leads in diversifying dermatology. Int J Womens Dermatol. 2020;7:135-136. doi:10.1016/j.ijwd.2020.12.012
- Emory School of Medicine. Four phases in four years. Accessed January 17, 2025. https://med.emory.edu/education/programs/md/curriculum/4phases/index.html
- Bhatnagar V, Diaz S, Bucur PA. The need for more mentorship in medical school. Cureus. 2020;12:E7984. doi:10.7759/cureus.7984
- Skin of Color Society. Mentorship. Accessed January 17, 2025. https://skinofcolorsociety.org/what-we-do/mentorship
- American Academy of Dermatology. Diversity Mentorship Program: information for medical students. Accessed January 17, 2025. https://www.aad.org/member/career/awards/diversity
As one of the most competitive specialties in medicine, dermatology presents unique challenges for residency applicants, especially following the shift in United States Medical Licensing Examination (USMLE) Step 1 scoring to a pass/fail format.1,2 Historically, USMLE Step 1 served as a major screening metric for residency programs, with 90% of program directors in 2020 using USMLE Step 1 scores as a primary factor when deciding whether to invite applicants for interviews.1 However, the recent transition to pass/fail has made it much harder for program directors to objectively compare applicants, particularly in dermatology. In a 2020 survey, Patrinely Jr et al2 found that 77.2% of dermatology program directors agreed that this change would make it more difficult to assess candidates objectively. Consequently, research productivity has taken on greater importance as programs seek new ways to distinguish top applicants.1,2
In response to this increased emphasis on research, dermatology applicants have substantially boosted their scholarly output over the past several years. The 2022 and 2024 results from the National Residency Matching Program’s Charting Outcomes survey demonstrated a steady rise in research metrics among applicants across various specialties, with dermatology showing one of the largest increases.3,4 For instance, the average number of abstracts, presentations, and publications for matched allopathic dermatology applicants was 5.7 in 2007.5 This average increased to 20.9 in 20223 and to 27.7 in 2024,4 marking an astonishing 485% increase in 17 years. Interestingly, unmatched dermatology applicants had an average of 19.0 research products in 2024, which was similar to the average of successfully matched applicants just 2 years earlier.3,4
Engaging in research offers benefits beyond building a strong residency application. Specifically, it enhances critical thinking skills and provides hands-on experience in scientific inquiry.6 It allows students to explore dermatology topics of interest and address existing knowledge gaps within the specialty.6 Additionally, it creates opportunities to build meaningful relationships with experienced dermatologists who can guide and support students throughout their careers.7 Despite these benefits, the pursuit of research may be landscaped with obstacles, and the fervent race to obtain high research outputs may overshadow developmental advantages.8 These challenges and demands also could contribute to inequities in the residency selection process, particularly if barriers are influenced by socioeconomic and demographic disparities. As dermatology already ranks as the second least diverse specialty in medicine,9 research requirements that disproportionately disadvantage certain demographic groups risk further widening these concerning representation gaps rather than creating opportunities to address them.
Given these trends in research requirements and their potential impact on applicant success, understanding specific barriers to research engagement is essential for creating equitable opportunities in dermatology. In this study, we aimed to identify barriers to research engagement among dermatology applicants, analyze their relationship with demographic factors, assess their impact on specialty choice and research productivity, and provide actionable solutions to address these obstacles.
Methods
A cross-sectional survey was conducted targeting medical students applying to dermatology residency programs in the United States in the 2025 or 2026 match cycles as well as residents who applied to dermatology residency in the 2021 to 2024 match cycles. The 23-item survey was developed by adapting questions from several validated studies examining research barriers and experiences in medical education.6,7,10,11 Specifically, the survey included questions on demographics and background; research productivity; general research barriers; conference participation accessibility; mentorship access; and quality, career impact, and support needs. Socioeconomic background was measured via a single self-reported item asking participants to select the income class that best reflected their background growing up (low-income, lower-middle, upper-middle, or high-income); no income ranges were provided.
The survey was distributed electronically via Qualtrics between November 11, 2024, and December 30, 2024, through listserves of the Dermatology Interest Group Association (sent directly to medical students) and the Association of Professors of Dermatology (forwarded to residents by program directors). There was no way to determine the number of dermatology applicants and residents reached through either listserve. The surveys were reviewed and approved by the University of Alabama at Birmingham institutional review board (IRB-300013671).
Statistical analyses were conducted using RStudio (Posit, PBC; version 2024.12.0+467). Descriptive statistics characterized participant demographics and quantified barrier scores using frequencies and proportions. We performed regression analyses to examine relationships between demographic factors and barriers using linear regression; the relationship between barriers and research productivity correlation; and the prediction of specialty change consideration using logistic regression. For all analyses, barrier scores were rated on a scale of 0 to 3 (0=not a barrier, 1=minor barrier, 2=moderate barrier, 3=major barrier); R² values were reported to indicate strength of associations, and statistical significance was set at P<.05.
Results
Participant Demographics—A total of 136 participants completed the survey. Among the respondents, 12% identified as from a background of low-income class, 28% lower-middle class, 49% upper-middle class, and 11% high-income class. Additionally, 27% of respondents identified as underrepresented in medicine (URiM). Regarding debt levels (or expected debt levels) upon graduation from medical school, 32% reported no debt, 9% reported $1000 to $49,000 in debt, 5% reported $50,000 to $99,000 in debt, 15% reported $100,000 to $199,000 in debt, 22% reported $200,000 to $299,000 in debt, and 17% reported $300,000 in debt or higher. The majority of respondents (95%) were MD candidates, and the remaining 5% were DO candidates; additionally, 5% were participants in an MD/PhD program (eTable 1).

Respondents represented various stages of training: 13.2% and 16.2% were third- and fourth-year medical students, respectively, while 6.0%, 20.1%, 18.4%, and 22.8% were postgraduate year (PGY) 1, PGY-2, PGY-3, and PGY-4, respectively. A few respondents (2.9%) were participating in a research year or reapplying to dermatology residency (eTable 2).

Research Barriers and Productivity—Respondents were presented with a list of potential barriers and asked to rate each as not a barrier, a minor barrier, a moderate barrier, or a major barrier. The most common barriers (ie, those with >50% of respondents rating them as a moderate or major) included lack of time, limited access to research opportunities, not knowing how to begin research, and lack of mentorship or support. Lack of time and not knowing where to begin research were reported most frequently as major barriers, with 32% of participants identifying them as such. In contrast, barriers such as financial costs and personal obligations were less frequently rated as major barriers (10% and 4%, respectively), although they still were identified as obstacles by many respondents. Interestingly, most respondents (58%) indicated that institutional limitations were not a barrier, but a separate and sizeable proportion (25%) of respondents considered it to be a major barrier (eFigure 1).

The distributions for all research metrics were right-skewed. The total range was 0 to 45 (median, 6) for number of publications (excluding abstracts), 0 to 33 (median, 2) for published abstracts, 0 to 40 (median, 5) for poster publications, and 0 to 20 (median, 2) for oral presentations (eTable 3).

Regression Analysis—Linear regression analysis identified significant relationships between demographic variables (socioeconomic status [SES], URiM status, and debt level) and individual research barriers. The heatmap in eFigure 2 illustrates the strength of these relationships. Higher SES was predictive of lower reported financial barriers (R²=.2317; P<.0001) and lower reported institutional limitations (R²=.0884; P=.0006). A URiM status predicted higher reported financial barriers (R²=.1097; P<.0001) and institutional limitations (R²=.04537; P=.013). Also, higher debt level predicted increased financial barriers (R²=.2099; P<.0001), institutional limitations (R2=.1258; P<.0001), and lack of mentorship (R²=.06553; P=.003).

Next, the data were evaluated for correlative relationships between individual research barriers and research productivity metrics including number of publications, published abstracts and presentations (oral and poster) and total research output. While correlations were weak or nonsignificant between barriers and most research productivity metrics (published abstracts, oral and poster presentations, and total research output), the number of publications was significantly correlated with several research barriers, including limited access to research opportunities (P=.002), not knowing how to begin research (P=.025), lack of mentorship or support (P=.011), and institutional limitations (P=.042). Higher ratings for limited access to research opportunities, not knowing where to begin research, lack of mentorship or support, and institutional limitations all were negatively correlated with total number of publications (R2=−.27, –.19, −.22, and –.18, respectively)(eFigure 3).

Logistic regression analysis examined the impact of research barriers on the likelihood of specialty change consideration. The results, presented in a forest plot, include odds ratios (ORs) and their corresponding 95% CIs and P values. Lack of time (P=.001) and not knowing where to begin research (P<.001) were the strongest predictors of specialty change consideration (OR, 6.3 and 4.7, respectively). Financial cost (P=.043), limited access to research opportunities (P=.006), and lack of mentorship or support (P=.001) also were significant predictors of specialty change consideration (OR, 2.2, 3.1, and 3.5, respectively). Institutional limitations and personal obligations did not predict specialty change consideration (eTable 4 and eFigure 4).


Mitigation Strategies—Mitigation strategies were ranked by respondents based on their perceived importance on a scale of 1 to 7 (1=most important, 7=least important)(eFigure 5). Respondents considered access to engaged mentors to be the most important mitigation strategy by far, with 95% ranking it in the top 3 (47% of respondents ranked it as the top most important mitigation strategy). Financial assistance was the mitigation strategy with the second highest number of respondents (28%) ranking it as the top strategy. Flexible scheduling during rotations, research training programs or discussions, and peer networking and research collaboration opportunities also were considered by respondents to be important mitigation strategies. Time management support/resources frequently was viewed as the least important mitigation strategy, with 38% of respondents ranking it last.

Comment
Our study revealed notable disparities in research barriers among dermatology applicants, with several demonstrating consistent patterns of association with SES, URiM status, and debt burden. Furthermore, the strong relationship between these barriers and decreased research productivity and specialty change consideration suggests that capable candidates may be deterred from pursuing dermatology due to surmountable obstacles rather than lack of interest or ability.
Impact of Demographic Factors on Research Barriers—All 7 general research barriers surveyed were correlated with distinct demographic predictors. Regression analyses indicated that the barrier of financial cost was significantly predicted by lower SES (R²=.2317; P<.001), URiM status (R²=.1097; P<.001), and higher debt levels (R²=.2099; P<.001)(eFigure 2). These findings are particularly concerning given the trend of dermatology applicants pursuing 1-year research fellowships, many of which are unpaid.12 In fact, Jacobson et al11 found that 71.7% (43/60) of dermatology applicants who pursued a year-long research fellowship experienced financial strain during their fellowship, with many requiring additional loans or drawing from personal savings despite already carrying substantial medical school debt of $200,000 or more. Our findings showcase how financial barriers to research disproportionately affect students from lower socioeconomic backgrounds, those who identify as URiM, and those with higher debt, creating systemic inequities in research access at a time when research productivity is increasingly vital for matching into dermatology. To address these financial barriers, institutions may consider establishing more funded research fellowships or expanding grant programs targeting students from economically disadvantaged and/or underrepresented backgrounds.
Institutional limitations (eg, the absence of a dermatology department) also was a notable barrier that was significantly predicted by lower SES (R²=.0884; P<.001) and URiM status (R²=.04537; P=.013)(eFigure 2). Students at institutions lacking dermatology programs face restricted access to mentorship and research opportunities,13 with our results demonstrating that these barriers disproportionately affect students from underresourced and minority groups. These limitations compound disparities in building competitive residency applications.14 The Women’s Dermatologic Society (WDS) has developed a model for addressing these institutional barriers through its summer research fellowship program for medical students who identify as URiM. By pairing students with WDS mentors who guide them through summer research projects, this initiative addresses access and mentorship gaps for students lacking dermatology departments at their home institution.15 The WDS program serves as a model for other organizations to adopt and expand, with particular attention to including students who identify as URiM as well as those from lower socioeconomic backgrounds.
Our results identified time constraints and lack of experience as notable research barriers. Higher debt levels significantly predicted both lack of time (R²=.03915; P=.021) and not knowing how to begin research (R²=.0572; P=.005)(eFigure 2). These statistical relationships may be explained by students with higher debt levels needing to prioritize paid work over unpaid research opportunities, limiting their engagement in research due to the scarcity of funded positions.12 The data further revealed that personal obligations, particularly family care responsibilities, were significantly predicted by both lower SES (R²=.0539; P=.008) and higher debt level (R²=.03237; P=.036)(eFigure 2). These findings demonstrate how students managing academic demands alongside financial and familial responsibilities may face compounded barriers to research engagement. To address these disparities, medical schools may consider implementing protected research time within their curricula; for example, the Emory University School of Medicine (Atlanta, Georgia) has implemented a Discovery Phase program that provides students with 5 months of protected faculty-mentored research time away from academic demands between their third and fourth years of medical school.16 Integrating similarly structured research periods across medical school curricula could help ensure equitable research opportunities for all students pursuing competitive specialties such as dermatology.8
Access to mentorship is a critical determinant of research engagement and productivity, as mentors provide valuable guidance on navigating research processes and professional development.17 Our analysis revealed that lack of mentorship was predicted by both lower SES (R²=.039; P=.023) and higher debt level (R²=.06553; P=.003)(eFigure 2). Several organizations have developed programs to address these mentorship gaps. The Skin of Color Society pairs medical students with skin of color experts while advancing its mission of increasing diversity in dermatology.18 Similarly, the American Academy of Dermatology founded a diversity mentorship program that connects students who identify as URiM with dermatologist mentors for summer research experiences.19 Notably, the Skin of Color Society’s program allows residents to serve as mentors for medical students. Involving residents and community dermatologists as potential dermatology mentors for medical students not only distributes mentorship demands more sustainably but also increases overall access to dermatology mentors. Our findings indicate that similar programs could be expanded to include more residents and community dermatologists as mentors and to target students from disadvantaged backgrounds, those facing financial constraints, and students who identify as URiM.
Impact of Research Barriers on Career Trajectories—Among survey participants, 35% reported considering changing their specialty choice due to research-related barriers. This substantial percentage likely stems from the escalating pressure to achieve increasingly high research output amidst a lack of sufficient support, time, or tools, as our results suggest. The specific barriers that most notably predicted specialty change consideration were lack of time and not knowing how to begin research (P=.001 and P<.001, respectively). Remarkably, our findings revealed that respondents who rated these as moderate or major barriers were 6.3 and 4.7 times more likely to consider changing their specialty choice, respectively. Respondents reporting financial cost (P=.043), limited access to research opportunities (P=.006), and lack of mentorship or support (P=.001) as at least moderate barriers also were 2.2 to 3.5 times more likely to consider a specialty change (eTable 4 and eFigure 4). Additionally, barriers such as limited access to research opportunities (R²=−.27; P=.002), lack of mentorship (R2=−.22; P=.011), not knowing how to begin research (R2=−.19; P=.025), and institutional limitations (R2=−.18; P=.042) all were associated with lower publication output according to our data (eFigure 3). These findings are especially concerning given current match statistics, where the trajectory of research productivity required for a successful dermatology match continues to rise sharply.3,4
Alarmingly, many of the barriers we identified—linked to both reduced research output and specialty change consideration—are associated with several demographic factors. Higher debt levels predicted greater likelihood of experiencing lack of time, insufficient mentorship, and uncertainty about initiating research, while lower SES was associated with lack of mentorship. These relationships suggest that structural barriers, rather than lack of interest or ability, may create cumulative disadvantages that deter capable candidates from pursuing dermatology or impact their success in the application process.
One potential solution to address the disproportionate emphasis on research quantity would be implementing caps on reportable research products in residency applications (eg, limiting applications to a certain number of publications, abstracts, and presentations). This change could shift applicant focus toward substantive scientific contributions rather than rapid output accumulation.8 The need for such caps was evident in our dataset, which revealed a stark contrast: some respondents reported 30 to 40 publications, while MD/PhD respondents—who dedicate 3 to 5 years to performing quality research—averaged only 7.4 publications. Implementing a research output ceiling could help alleviate barriers for applicants facing institutional and demographic disadvantages while simultaneously boosting the scientific rigor of dermatology research.8
Mitigation Strategies From Applicant Feedback—Our findings emphasize the multifaceted relationship between structural barriers and demographics in dermatology research engagement. While our statistical interpretations have outlined several potential interventions, the applicants’ perspectives on mitigation strategies offer qualitative insight. Although participants did not consistently mark financial cost and lack of mentorship as major barriers (eFigure 1), financial assistance and access to engaged mentors were among the highest-ranked mitigation strategies (eFigure 5), suggesting these resources may be fundamental to overcoming multiple structural challenges. To address these needs comprehensively, we propose a multilevel approach: at the institutional level, dermatology interest groups could establish centralized databases of research opportunities, mentorship programs, and funding sources. At the national level, dermatology organizations could consider expanding grant programs, developing virtual mentorship networks, and creating opportunities for external students through remote research projects or short-term research rotations. These interventions, informed by both our statistical analyses and applicant feedback, could help create more equitable access to research opportunities in dermatology.
Limitations
A major limitation of this study was that potential dermatology candidates who were deterred by barriers and later decided on a different specialty would not be captured in our data. As these candidates may have faced substantial barriers that caused them to choose a different path, their absence from the current data may indicate that the reported results underpredict the effect size of the true population. Another limitation is the absence of a control group, such as applicants to less competitive specialties, which would provide valuable context for whether the barriers identified are unique to dermatology.
Conclusion
Our study provides compelling evidence that research barriers in dermatology residency applications intersect with demographic factors to influence research engagement and career trajectories. Our findings suggest that without targeted intervention, increasing emphasis on research productivity may exacerbate existing disparities in dermatology. Moving forward, a coordinated effort among institutions, dermatology associations, and dermatology residency programs will be fundamental to ensure that research requirements enhance rather than impede the development of a diverse, qualified dermatology workforce.
As one of the most competitive specialties in medicine, dermatology presents unique challenges for residency applicants, especially following the shift in United States Medical Licensing Examination (USMLE) Step 1 scoring to a pass/fail format.1,2 Historically, USMLE Step 1 served as a major screening metric for residency programs, with 90% of program directors in 2020 using USMLE Step 1 scores as a primary factor when deciding whether to invite applicants for interviews.1 However, the recent transition to pass/fail has made it much harder for program directors to objectively compare applicants, particularly in dermatology. In a 2020 survey, Patrinely Jr et al2 found that 77.2% of dermatology program directors agreed that this change would make it more difficult to assess candidates objectively. Consequently, research productivity has taken on greater importance as programs seek new ways to distinguish top applicants.1,2
In response to this increased emphasis on research, dermatology applicants have substantially boosted their scholarly output over the past several years. The 2022 and 2024 results from the National Residency Matching Program’s Charting Outcomes survey demonstrated a steady rise in research metrics among applicants across various specialties, with dermatology showing one of the largest increases.3,4 For instance, the average number of abstracts, presentations, and publications for matched allopathic dermatology applicants was 5.7 in 2007.5 This average increased to 20.9 in 20223 and to 27.7 in 2024,4 marking an astonishing 485% increase in 17 years. Interestingly, unmatched dermatology applicants had an average of 19.0 research products in 2024, which was similar to the average of successfully matched applicants just 2 years earlier.3,4
Engaging in research offers benefits beyond building a strong residency application. Specifically, it enhances critical thinking skills and provides hands-on experience in scientific inquiry.6 It allows students to explore dermatology topics of interest and address existing knowledge gaps within the specialty.6 Additionally, it creates opportunities to build meaningful relationships with experienced dermatologists who can guide and support students throughout their careers.7 Despite these benefits, the pursuit of research may be landscaped with obstacles, and the fervent race to obtain high research outputs may overshadow developmental advantages.8 These challenges and demands also could contribute to inequities in the residency selection process, particularly if barriers are influenced by socioeconomic and demographic disparities. As dermatology already ranks as the second least diverse specialty in medicine,9 research requirements that disproportionately disadvantage certain demographic groups risk further widening these concerning representation gaps rather than creating opportunities to address them.
Given these trends in research requirements and their potential impact on applicant success, understanding specific barriers to research engagement is essential for creating equitable opportunities in dermatology. In this study, we aimed to identify barriers to research engagement among dermatology applicants, analyze their relationship with demographic factors, assess their impact on specialty choice and research productivity, and provide actionable solutions to address these obstacles.
Methods
A cross-sectional survey was conducted targeting medical students applying to dermatology residency programs in the United States in the 2025 or 2026 match cycles as well as residents who applied to dermatology residency in the 2021 to 2024 match cycles. The 23-item survey was developed by adapting questions from several validated studies examining research barriers and experiences in medical education.6,7,10,11 Specifically, the survey included questions on demographics and background; research productivity; general research barriers; conference participation accessibility; mentorship access; and quality, career impact, and support needs. Socioeconomic background was measured via a single self-reported item asking participants to select the income class that best reflected their background growing up (low-income, lower-middle, upper-middle, or high-income); no income ranges were provided.
The survey was distributed electronically via Qualtrics between November 11, 2024, and December 30, 2024, through listserves of the Dermatology Interest Group Association (sent directly to medical students) and the Association of Professors of Dermatology (forwarded to residents by program directors). There was no way to determine the number of dermatology applicants and residents reached through either listserve. The surveys were reviewed and approved by the University of Alabama at Birmingham institutional review board (IRB-300013671).
Statistical analyses were conducted using RStudio (Posit, PBC; version 2024.12.0+467). Descriptive statistics characterized participant demographics and quantified barrier scores using frequencies and proportions. We performed regression analyses to examine relationships between demographic factors and barriers using linear regression; the relationship between barriers and research productivity correlation; and the prediction of specialty change consideration using logistic regression. For all analyses, barrier scores were rated on a scale of 0 to 3 (0=not a barrier, 1=minor barrier, 2=moderate barrier, 3=major barrier); R² values were reported to indicate strength of associations, and statistical significance was set at P<.05.
Results
Participant Demographics—A total of 136 participants completed the survey. Among the respondents, 12% identified as from a background of low-income class, 28% lower-middle class, 49% upper-middle class, and 11% high-income class. Additionally, 27% of respondents identified as underrepresented in medicine (URiM). Regarding debt levels (or expected debt levels) upon graduation from medical school, 32% reported no debt, 9% reported $1000 to $49,000 in debt, 5% reported $50,000 to $99,000 in debt, 15% reported $100,000 to $199,000 in debt, 22% reported $200,000 to $299,000 in debt, and 17% reported $300,000 in debt or higher. The majority of respondents (95%) were MD candidates, and the remaining 5% were DO candidates; additionally, 5% were participants in an MD/PhD program (eTable 1).

Respondents represented various stages of training: 13.2% and 16.2% were third- and fourth-year medical students, respectively, while 6.0%, 20.1%, 18.4%, and 22.8% were postgraduate year (PGY) 1, PGY-2, PGY-3, and PGY-4, respectively. A few respondents (2.9%) were participating in a research year or reapplying to dermatology residency (eTable 2).

Research Barriers and Productivity—Respondents were presented with a list of potential barriers and asked to rate each as not a barrier, a minor barrier, a moderate barrier, or a major barrier. The most common barriers (ie, those with >50% of respondents rating them as a moderate or major) included lack of time, limited access to research opportunities, not knowing how to begin research, and lack of mentorship or support. Lack of time and not knowing where to begin research were reported most frequently as major barriers, with 32% of participants identifying them as such. In contrast, barriers such as financial costs and personal obligations were less frequently rated as major barriers (10% and 4%, respectively), although they still were identified as obstacles by many respondents. Interestingly, most respondents (58%) indicated that institutional limitations were not a barrier, but a separate and sizeable proportion (25%) of respondents considered it to be a major barrier (eFigure 1).

The distributions for all research metrics were right-skewed. The total range was 0 to 45 (median, 6) for number of publications (excluding abstracts), 0 to 33 (median, 2) for published abstracts, 0 to 40 (median, 5) for poster publications, and 0 to 20 (median, 2) for oral presentations (eTable 3).

Regression Analysis—Linear regression analysis identified significant relationships between demographic variables (socioeconomic status [SES], URiM status, and debt level) and individual research barriers. The heatmap in eFigure 2 illustrates the strength of these relationships. Higher SES was predictive of lower reported financial barriers (R²=.2317; P<.0001) and lower reported institutional limitations (R²=.0884; P=.0006). A URiM status predicted higher reported financial barriers (R²=.1097; P<.0001) and institutional limitations (R²=.04537; P=.013). Also, higher debt level predicted increased financial barriers (R²=.2099; P<.0001), institutional limitations (R2=.1258; P<.0001), and lack of mentorship (R²=.06553; P=.003).

Next, the data were evaluated for correlative relationships between individual research barriers and research productivity metrics including number of publications, published abstracts and presentations (oral and poster) and total research output. While correlations were weak or nonsignificant between barriers and most research productivity metrics (published abstracts, oral and poster presentations, and total research output), the number of publications was significantly correlated with several research barriers, including limited access to research opportunities (P=.002), not knowing how to begin research (P=.025), lack of mentorship or support (P=.011), and institutional limitations (P=.042). Higher ratings for limited access to research opportunities, not knowing where to begin research, lack of mentorship or support, and institutional limitations all were negatively correlated with total number of publications (R2=−.27, –.19, −.22, and –.18, respectively)(eFigure 3).

Logistic regression analysis examined the impact of research barriers on the likelihood of specialty change consideration. The results, presented in a forest plot, include odds ratios (ORs) and their corresponding 95% CIs and P values. Lack of time (P=.001) and not knowing where to begin research (P<.001) were the strongest predictors of specialty change consideration (OR, 6.3 and 4.7, respectively). Financial cost (P=.043), limited access to research opportunities (P=.006), and lack of mentorship or support (P=.001) also were significant predictors of specialty change consideration (OR, 2.2, 3.1, and 3.5, respectively). Institutional limitations and personal obligations did not predict specialty change consideration (eTable 4 and eFigure 4).


Mitigation Strategies—Mitigation strategies were ranked by respondents based on their perceived importance on a scale of 1 to 7 (1=most important, 7=least important)(eFigure 5). Respondents considered access to engaged mentors to be the most important mitigation strategy by far, with 95% ranking it in the top 3 (47% of respondents ranked it as the top most important mitigation strategy). Financial assistance was the mitigation strategy with the second highest number of respondents (28%) ranking it as the top strategy. Flexible scheduling during rotations, research training programs or discussions, and peer networking and research collaboration opportunities also were considered by respondents to be important mitigation strategies. Time management support/resources frequently was viewed as the least important mitigation strategy, with 38% of respondents ranking it last.

Comment
Our study revealed notable disparities in research barriers among dermatology applicants, with several demonstrating consistent patterns of association with SES, URiM status, and debt burden. Furthermore, the strong relationship between these barriers and decreased research productivity and specialty change consideration suggests that capable candidates may be deterred from pursuing dermatology due to surmountable obstacles rather than lack of interest or ability.
Impact of Demographic Factors on Research Barriers—All 7 general research barriers surveyed were correlated with distinct demographic predictors. Regression analyses indicated that the barrier of financial cost was significantly predicted by lower SES (R²=.2317; P<.001), URiM status (R²=.1097; P<.001), and higher debt levels (R²=.2099; P<.001)(eFigure 2). These findings are particularly concerning given the trend of dermatology applicants pursuing 1-year research fellowships, many of which are unpaid.12 In fact, Jacobson et al11 found that 71.7% (43/60) of dermatology applicants who pursued a year-long research fellowship experienced financial strain during their fellowship, with many requiring additional loans or drawing from personal savings despite already carrying substantial medical school debt of $200,000 or more. Our findings showcase how financial barriers to research disproportionately affect students from lower socioeconomic backgrounds, those who identify as URiM, and those with higher debt, creating systemic inequities in research access at a time when research productivity is increasingly vital for matching into dermatology. To address these financial barriers, institutions may consider establishing more funded research fellowships or expanding grant programs targeting students from economically disadvantaged and/or underrepresented backgrounds.
Institutional limitations (eg, the absence of a dermatology department) also was a notable barrier that was significantly predicted by lower SES (R²=.0884; P<.001) and URiM status (R²=.04537; P=.013)(eFigure 2). Students at institutions lacking dermatology programs face restricted access to mentorship and research opportunities,13 with our results demonstrating that these barriers disproportionately affect students from underresourced and minority groups. These limitations compound disparities in building competitive residency applications.14 The Women’s Dermatologic Society (WDS) has developed a model for addressing these institutional barriers through its summer research fellowship program for medical students who identify as URiM. By pairing students with WDS mentors who guide them through summer research projects, this initiative addresses access and mentorship gaps for students lacking dermatology departments at their home institution.15 The WDS program serves as a model for other organizations to adopt and expand, with particular attention to including students who identify as URiM as well as those from lower socioeconomic backgrounds.
Our results identified time constraints and lack of experience as notable research barriers. Higher debt levels significantly predicted both lack of time (R²=.03915; P=.021) and not knowing how to begin research (R²=.0572; P=.005)(eFigure 2). These statistical relationships may be explained by students with higher debt levels needing to prioritize paid work over unpaid research opportunities, limiting their engagement in research due to the scarcity of funded positions.12 The data further revealed that personal obligations, particularly family care responsibilities, were significantly predicted by both lower SES (R²=.0539; P=.008) and higher debt level (R²=.03237; P=.036)(eFigure 2). These findings demonstrate how students managing academic demands alongside financial and familial responsibilities may face compounded barriers to research engagement. To address these disparities, medical schools may consider implementing protected research time within their curricula; for example, the Emory University School of Medicine (Atlanta, Georgia) has implemented a Discovery Phase program that provides students with 5 months of protected faculty-mentored research time away from academic demands between their third and fourth years of medical school.16 Integrating similarly structured research periods across medical school curricula could help ensure equitable research opportunities for all students pursuing competitive specialties such as dermatology.8
Access to mentorship is a critical determinant of research engagement and productivity, as mentors provide valuable guidance on navigating research processes and professional development.17 Our analysis revealed that lack of mentorship was predicted by both lower SES (R²=.039; P=.023) and higher debt level (R²=.06553; P=.003)(eFigure 2). Several organizations have developed programs to address these mentorship gaps. The Skin of Color Society pairs medical students with skin of color experts while advancing its mission of increasing diversity in dermatology.18 Similarly, the American Academy of Dermatology founded a diversity mentorship program that connects students who identify as URiM with dermatologist mentors for summer research experiences.19 Notably, the Skin of Color Society’s program allows residents to serve as mentors for medical students. Involving residents and community dermatologists as potential dermatology mentors for medical students not only distributes mentorship demands more sustainably but also increases overall access to dermatology mentors. Our findings indicate that similar programs could be expanded to include more residents and community dermatologists as mentors and to target students from disadvantaged backgrounds, those facing financial constraints, and students who identify as URiM.
Impact of Research Barriers on Career Trajectories—Among survey participants, 35% reported considering changing their specialty choice due to research-related barriers. This substantial percentage likely stems from the escalating pressure to achieve increasingly high research output amidst a lack of sufficient support, time, or tools, as our results suggest. The specific barriers that most notably predicted specialty change consideration were lack of time and not knowing how to begin research (P=.001 and P<.001, respectively). Remarkably, our findings revealed that respondents who rated these as moderate or major barriers were 6.3 and 4.7 times more likely to consider changing their specialty choice, respectively. Respondents reporting financial cost (P=.043), limited access to research opportunities (P=.006), and lack of mentorship or support (P=.001) as at least moderate barriers also were 2.2 to 3.5 times more likely to consider a specialty change (eTable 4 and eFigure 4). Additionally, barriers such as limited access to research opportunities (R²=−.27; P=.002), lack of mentorship (R2=−.22; P=.011), not knowing how to begin research (R2=−.19; P=.025), and institutional limitations (R2=−.18; P=.042) all were associated with lower publication output according to our data (eFigure 3). These findings are especially concerning given current match statistics, where the trajectory of research productivity required for a successful dermatology match continues to rise sharply.3,4
Alarmingly, many of the barriers we identified—linked to both reduced research output and specialty change consideration—are associated with several demographic factors. Higher debt levels predicted greater likelihood of experiencing lack of time, insufficient mentorship, and uncertainty about initiating research, while lower SES was associated with lack of mentorship. These relationships suggest that structural barriers, rather than lack of interest or ability, may create cumulative disadvantages that deter capable candidates from pursuing dermatology or impact their success in the application process.
One potential solution to address the disproportionate emphasis on research quantity would be implementing caps on reportable research products in residency applications (eg, limiting applications to a certain number of publications, abstracts, and presentations). This change could shift applicant focus toward substantive scientific contributions rather than rapid output accumulation.8 The need for such caps was evident in our dataset, which revealed a stark contrast: some respondents reported 30 to 40 publications, while MD/PhD respondents—who dedicate 3 to 5 years to performing quality research—averaged only 7.4 publications. Implementing a research output ceiling could help alleviate barriers for applicants facing institutional and demographic disadvantages while simultaneously boosting the scientific rigor of dermatology research.8
Mitigation Strategies From Applicant Feedback—Our findings emphasize the multifaceted relationship between structural barriers and demographics in dermatology research engagement. While our statistical interpretations have outlined several potential interventions, the applicants’ perspectives on mitigation strategies offer qualitative insight. Although participants did not consistently mark financial cost and lack of mentorship as major barriers (eFigure 1), financial assistance and access to engaged mentors were among the highest-ranked mitigation strategies (eFigure 5), suggesting these resources may be fundamental to overcoming multiple structural challenges. To address these needs comprehensively, we propose a multilevel approach: at the institutional level, dermatology interest groups could establish centralized databases of research opportunities, mentorship programs, and funding sources. At the national level, dermatology organizations could consider expanding grant programs, developing virtual mentorship networks, and creating opportunities for external students through remote research projects or short-term research rotations. These interventions, informed by both our statistical analyses and applicant feedback, could help create more equitable access to research opportunities in dermatology.
Limitations
A major limitation of this study was that potential dermatology candidates who were deterred by barriers and later decided on a different specialty would not be captured in our data. As these candidates may have faced substantial barriers that caused them to choose a different path, their absence from the current data may indicate that the reported results underpredict the effect size of the true population. Another limitation is the absence of a control group, such as applicants to less competitive specialties, which would provide valuable context for whether the barriers identified are unique to dermatology.
Conclusion
Our study provides compelling evidence that research barriers in dermatology residency applications intersect with demographic factors to influence research engagement and career trajectories. Our findings suggest that without targeted intervention, increasing emphasis on research productivity may exacerbate existing disparities in dermatology. Moving forward, a coordinated effort among institutions, dermatology associations, and dermatology residency programs will be fundamental to ensure that research requirements enhance rather than impede the development of a diverse, qualified dermatology workforce.
- Ozair A, Bhat V, Detchou DKE. The US residency selection process after the United States Medical Licensing Examination Step 1 pass/fail change: overview for applicants and educators. JMIR Med Educ. 2023;9:E37069. doi:10.2196/37069
- Patrinely JR Jr, Zakria D, Drolet BC. USMLE Step 1 changes: dermatology program director perspectives and implications. Cutis. 2021;107:293-294. doi:10.12788/cutis.0277
- National Resident Matching Program. Charting outcomes in the match: US MD seniors, 2022. July 2022. Accessed February 14, 2024. https://www.nrmp.org/wp-content/uploads/2022/07/Charting-Outcomes-MD-Seniors-2022_Final.pdf
- National Resident Matching Program. Charting outcomes in the match: US MD seniors, 2024. August 2024. Accessed February 14, 2024. https://www.nrmp.org/match-data/2024/08/charting-outcomes-characteristics-of-u-s-md-seniors-who-matched-to-their-preferred-specialty-2024-main-residency-match/
- National Resident Matching Program. Charting outcomes in the match: characteristics of applicants who matched to their preferred specialty in the 2007 main residency match. July 2021. Accessed February 14, 2024. https://www.nrmp.org/wp-content/uploads/2021/07/chartingoutcomes2007.pdf
- Sanabria-de la Torre R, Quiñones-Vico MI, Ubago-Rodríguez A, et al. Medical students’ interest in research: changing trends during university training. Front Med. 2023;10. doi:10.3389/fmed.2023.1257574
- Alikhan A, Sivamani RK, Mutizwa MM, et al. Advice for medical students interested in dermatology: perspectives from fourth year students who matched. Dermatol Online J. 2009;15:7. doi:10.5070/D398p8q1m5
- Elliott B, Carmody JB. Publish or perish: the research arms race in residency selection. J Grad Med Educ. 2023;15:524-527. doi:10.4300/JGME-D-23-00262.1
- Akhiyat S, Cardwell L, Sokumbi O. Why dermatology is the second least diverse specialty in medicine: how did we get here? Clin Dermatol. 2020;38:310-315. doi:10.1016/j.clindermatol.2020.02.005
- Orebi HA, Shahin MR, Awad Allah MT, et al. Medical students’ perceptions, experiences, and barriers towards research implementation at the faculty of medicine, Tanta University. BMC Med Educ. 2023;23:902. doi:10.1186/s12909-023-04884-z
- Jacobsen A, Kabbur G, Freese RL, et al. Socioeconomic factors and financial burdens of research “gap years” for dermatology residency applicants. Int J Womens Dermatol. 2023;9:e099. doi:10.1097/JW9.0000000000000099
- Jung J, Stoff BK, Orenstein LAV. Unpaid research fellowships among dermatology residency applicants. J Am Acad Dermatol. 2022;87:1230-1231. doi:10.1016/j.jaad.2021.12.027
- Rehman R, Shareef SJ, Mohammad TF, et al. Applying to dermatology residency without a home program: advice to medical students in the COVID-19 pandemic and beyond. Clin Dermatol. 2022;40:513-515. doi:10.1016/j.clindermatol.2022.01.003
- Villa NM, Shi VY, Hsiao JL. An underrecognized barrier to the dermatology residency match: lack of a home program. Int J Womens Dermatol. 2021;7:512-513. doi:10.1016/j.ijwd.2021.02.011
- Sekyere NAN, Grimes PE, Roberts WE, et al. Turning the tide: how the Women’s Dermatologic Society leads in diversifying dermatology. Int J Womens Dermatol. 2020;7:135-136. doi:10.1016/j.ijwd.2020.12.012
- Emory School of Medicine. Four phases in four years. Accessed January 17, 2025. https://med.emory.edu/education/programs/md/curriculum/4phases/index.html
- Bhatnagar V, Diaz S, Bucur PA. The need for more mentorship in medical school. Cureus. 2020;12:E7984. doi:10.7759/cureus.7984
- Skin of Color Society. Mentorship. Accessed January 17, 2025. https://skinofcolorsociety.org/what-we-do/mentorship
- American Academy of Dermatology. Diversity Mentorship Program: information for medical students. Accessed January 17, 2025. https://www.aad.org/member/career/awards/diversity
- Ozair A, Bhat V, Detchou DKE. The US residency selection process after the United States Medical Licensing Examination Step 1 pass/fail change: overview for applicants and educators. JMIR Med Educ. 2023;9:E37069. doi:10.2196/37069
- Patrinely JR Jr, Zakria D, Drolet BC. USMLE Step 1 changes: dermatology program director perspectives and implications. Cutis. 2021;107:293-294. doi:10.12788/cutis.0277
- National Resident Matching Program. Charting outcomes in the match: US MD seniors, 2022. July 2022. Accessed February 14, 2024. https://www.nrmp.org/wp-content/uploads/2022/07/Charting-Outcomes-MD-Seniors-2022_Final.pdf
- National Resident Matching Program. Charting outcomes in the match: US MD seniors, 2024. August 2024. Accessed February 14, 2024. https://www.nrmp.org/match-data/2024/08/charting-outcomes-characteristics-of-u-s-md-seniors-who-matched-to-their-preferred-specialty-2024-main-residency-match/
- National Resident Matching Program. Charting outcomes in the match: characteristics of applicants who matched to their preferred specialty in the 2007 main residency match. July 2021. Accessed February 14, 2024. https://www.nrmp.org/wp-content/uploads/2021/07/chartingoutcomes2007.pdf
- Sanabria-de la Torre R, Quiñones-Vico MI, Ubago-Rodríguez A, et al. Medical students’ interest in research: changing trends during university training. Front Med. 2023;10. doi:10.3389/fmed.2023.1257574
- Alikhan A, Sivamani RK, Mutizwa MM, et al. Advice for medical students interested in dermatology: perspectives from fourth year students who matched. Dermatol Online J. 2009;15:7. doi:10.5070/D398p8q1m5
- Elliott B, Carmody JB. Publish or perish: the research arms race in residency selection. J Grad Med Educ. 2023;15:524-527. doi:10.4300/JGME-D-23-00262.1
- Akhiyat S, Cardwell L, Sokumbi O. Why dermatology is the second least diverse specialty in medicine: how did we get here? Clin Dermatol. 2020;38:310-315. doi:10.1016/j.clindermatol.2020.02.005
- Orebi HA, Shahin MR, Awad Allah MT, et al. Medical students’ perceptions, experiences, and barriers towards research implementation at the faculty of medicine, Tanta University. BMC Med Educ. 2023;23:902. doi:10.1186/s12909-023-04884-z
- Jacobsen A, Kabbur G, Freese RL, et al. Socioeconomic factors and financial burdens of research “gap years” for dermatology residency applicants. Int J Womens Dermatol. 2023;9:e099. doi:10.1097/JW9.0000000000000099
- Jung J, Stoff BK, Orenstein LAV. Unpaid research fellowships among dermatology residency applicants. J Am Acad Dermatol. 2022;87:1230-1231. doi:10.1016/j.jaad.2021.12.027
- Rehman R, Shareef SJ, Mohammad TF, et al. Applying to dermatology residency without a home program: advice to medical students in the COVID-19 pandemic and beyond. Clin Dermatol. 2022;40:513-515. doi:10.1016/j.clindermatol.2022.01.003
- Villa NM, Shi VY, Hsiao JL. An underrecognized barrier to the dermatology residency match: lack of a home program. Int J Womens Dermatol. 2021;7:512-513. doi:10.1016/j.ijwd.2021.02.011
- Sekyere NAN, Grimes PE, Roberts WE, et al. Turning the tide: how the Women’s Dermatologic Society leads in diversifying dermatology. Int J Womens Dermatol. 2020;7:135-136. doi:10.1016/j.ijwd.2020.12.012
- Emory School of Medicine. Four phases in four years. Accessed January 17, 2025. https://med.emory.edu/education/programs/md/curriculum/4phases/index.html
- Bhatnagar V, Diaz S, Bucur PA. The need for more mentorship in medical school. Cureus. 2020;12:E7984. doi:10.7759/cureus.7984
- Skin of Color Society. Mentorship. Accessed January 17, 2025. https://skinofcolorsociety.org/what-we-do/mentorship
- American Academy of Dermatology. Diversity Mentorship Program: information for medical students. Accessed January 17, 2025. https://www.aad.org/member/career/awards/diversity
How Increasing Research Demands Threaten Equity in Dermatology Residency Selection and Strategies for Reform
How Increasing Research Demands Threaten Equity in Dermatology Residency Selection and Strategies for Reform
Practice Points
- Dermatology programs should establish sustainable mentorship networks incorporating faculty, residents, and community dermatologists, as most applicants ranked access to engaged mentors as a top priority for overcoming research barriers.
- Protected research time and funding support for projects are critical, particularly since applicants reporting lack of time and financial barriers were more likely to consider changing their specialty choice.
- Programs should consider implementing caps on reportable research products in residency applications to shift emphasis from quantity to quality while helping address demographic disparities in research access.
Hyperpigmented Macules Caused by Burrowing Bugs (Cydnidae) May Mimic More Serious Conditions
Hyperpigmented Macules Caused by Burrowing Bugs (Cydnidae) May Mimic More Serious Conditions
Cydnidae is a family of small to medium-sized shield bugs with spiny legs that commonly are known as burrowing bugs (or burrower bugs). The family Cydnidae includes more than 100 genera and approximately 600 species worldwide.1 These insects are arthropods of the order Hemiptera (suborder: Heteroptera; superfamily: Pentatomoidae) and largely are concentrated in tropical and temperate regions. Approximately 145 species have been recorded in the Neotropical Region and have been included in the subfamilies Amnestinae, Cephalocteinae, and Sehirinae, in addition to Cydnidae.2 Burrowing bugs are ovoid in shape and 2 to 20 mm in length and morphologically are well adapted for burrowing. Their life span is 100 to 300 days. Being phytophagous, they burrow to feed on plants and roots. Adult burrowing bugs have wings and can fly. They have specialized glands located in either the abdomen (nymph) or thorax (adult) that secrete odorous chemicals for self-protection.3 The secretions contain hydrocarbonates that function as repellents and danger signals, can cause paralysis in prey, and act as a chemoattractant for mates.4-6 They also cause hyperpigmentation upon contact with the skin.
In this article, we present a series of cases from the same community to demonstrate the characteristic features of hyperpigmented macules caused by exposure to burrowing bugs. Dermatologists should be aware of this entity to prevent misdiagnosis and unnecessary investigations and treatment.
Case Series
A 36-year-old woman and 6 children (age range, 6-12 years) presented with a widespread, acute, brown-pigmented, macular eruption with lesions that increased in number over a 1-week period. All 7 patients resided in the same locality and were otherwise systemically healthy. Initially, the index case, a 7-year-old girl, was referred to our tertiary care center by a dermatologist with a provisional diagnosis of idiopathic macular eruptive pigmentation. The patient’s mother recalled noticing a tiny black insect on the child's scalp that left pigment on the skin when she crushed it between her fingers. The rest of the patients presented over the next few days: 3 of the children belonged to the same household as the index case, and there was history of all 6 children playing in the neighborhood park during late evening hours. The adult patient was the parent of one of the affected children. The lesions were associated with mild itching and tingling in 3 children but were asymptomatic in the other patients.
Clinical examination of the patients revealed multiple dark- to light-brown, discrete, irregularly shaped macules over the trunk, arms, and soles (eFigure 1). Dermoscopic examination of a pigmented macule showed an irregularly shaped, brownish, structureless area with accentuation of the pigment at skin creases and perieccrine pigmentation (eFigure 2). The pigmentation was unaffected by rubbing with alcohol or water. Clinicoepidemiologic parameters of the patients are summarized in the eTable.
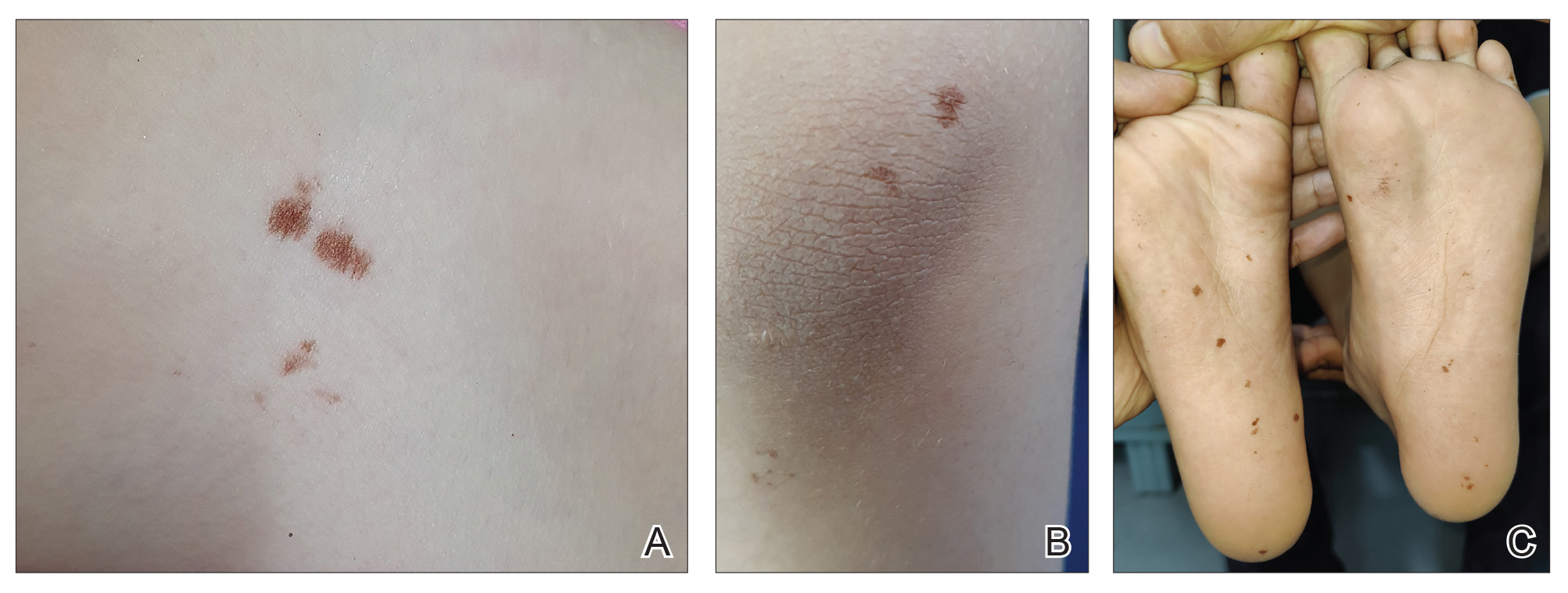
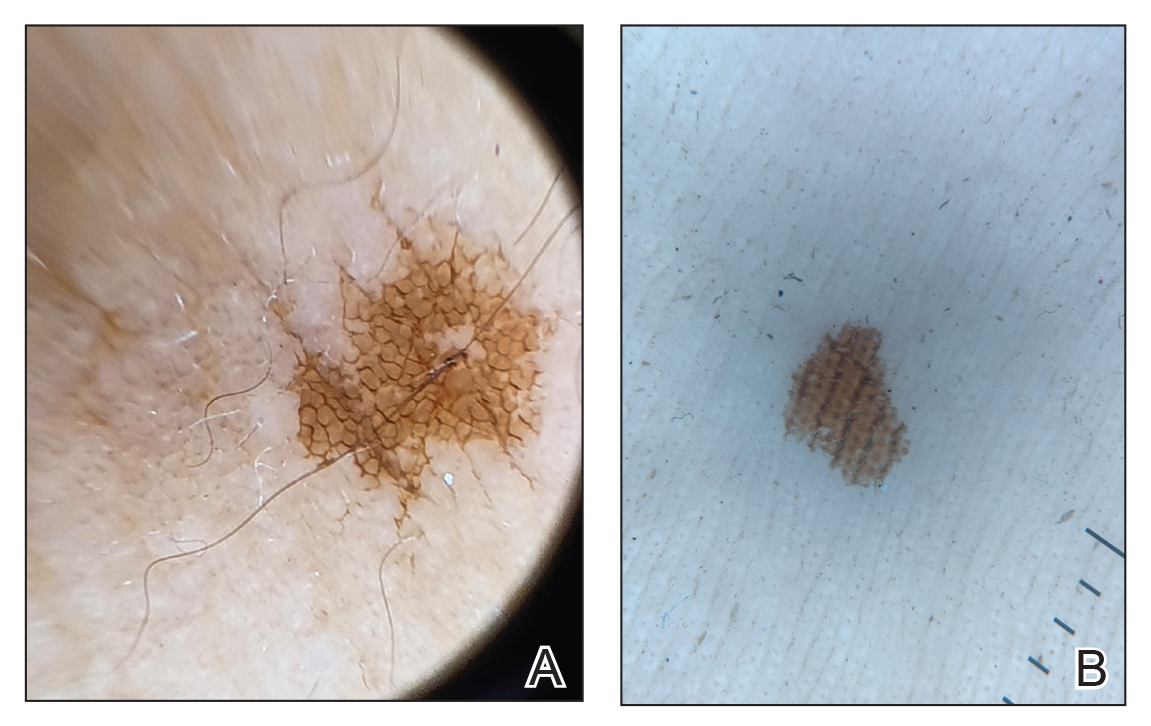
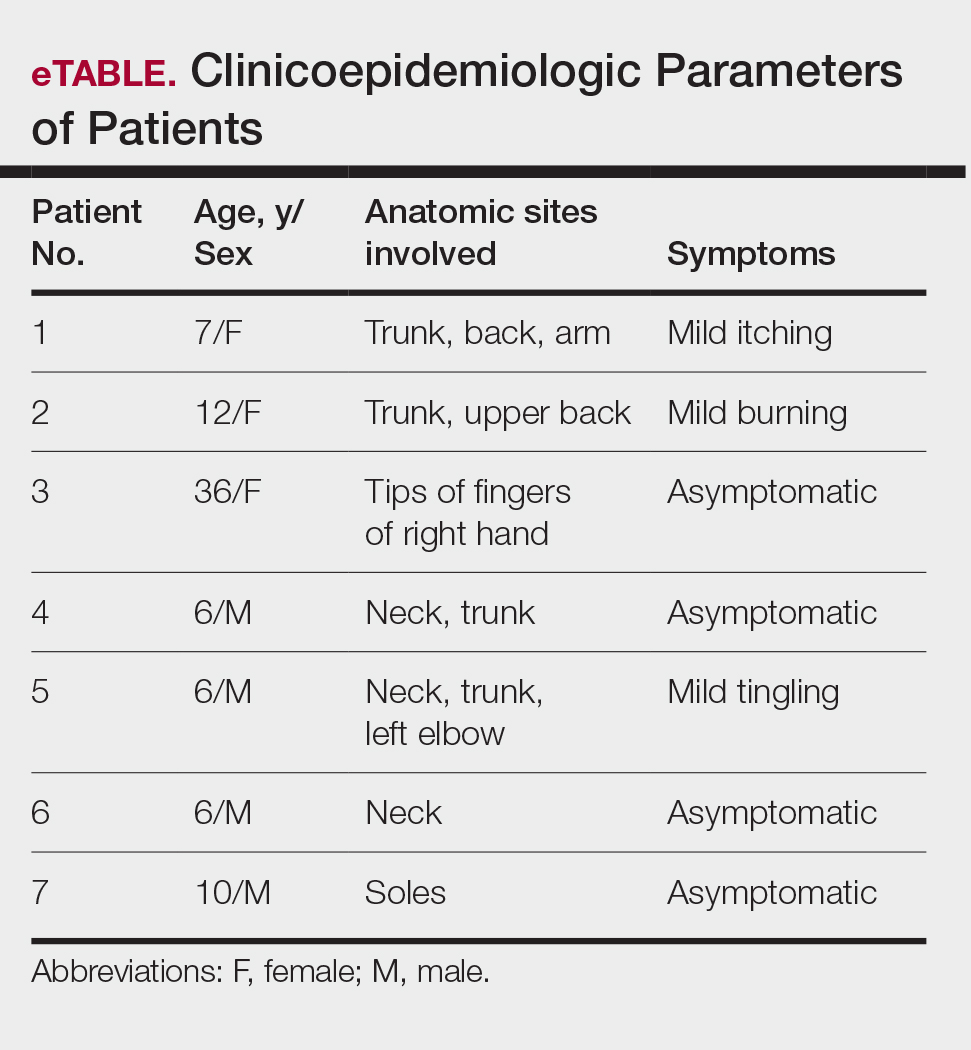
One of the children’s parents conducted a geological examination of the ground in the neighborhood park during evening hours and found tiny burrowing bugs (eFigure 3). When crushed between the fingers, these insects left a similar brownish hyperpigmentation on the skin. The parents were counseled on the nature of the eruption, and the patients were kept under observation for 2 weeks. On follow-up after 5 days, the lesions showed markedly decreased intensity of hyperpigmentation, and no new lesions were observed in any of the 7 patients.

Comment
Pentatomoidae insects generally are benign and harmless to humans. There have been isolated reports of erythematous plaques caused by Antiteuchus mixtus and Edessa maculate.7 Malhotra et al8 reported the first known series of cases with Cydnidae insect–induced hyperpigmented macules. The reported patients presented with asymptomatic, brown, hyperpigmented macules over exposed sites such as the feet, neck, and chest. All the cases occurred during the monsoon season in tropical and temperate regions of the world, and the patients were characteristically clustered in similar geographic areas. The causative insect was identified as Chilocoris assmuthi Breddin, 1904, belonging to the family Cydnidae. When it was crushed between the fingers, the skin became hyperpigmented, confirming the role of the secretions from the insect in the etiology.8
A second case was described by Sonthalia,9 who also described the dermoscopic features of hyperpigmented macules caused by burrowing bugs. The lesions showed a stuck-on, clustered appearance of ovoid and bizarre pigmented clods, globules, and granules.9 Although the lesions occur mainly over exposed sites, pigmented macules occurring over unusual sites such as the abdomen and back also have been reported in association with burrowing bugs.10 Characteristically, the lesions initially are faint and darken with time and usually fade within a week. They can be rubbed off with acetone but persist when washed with soap and water. The fleeting nature of the pigmentation also has led to the term transient pseudo-lentigines sign to describe hyperpigmentation caused by burrowing bugs.11
Soil and plants are burrowing bugs’ natural habitats, and the insects typically are seen in vegetation-rich, moist areas adjoining human dwellings (eg, parks, gardens), where clusters of cases can occur. These insects proliferate during the monsoon season in tropical and temperate areas, leading to more cases occurring during these months.
Compared to prior reports,8,9 a few of our patients had predominant trunk and neck involvement with an occasional tingling sensation or pruritus while the rest were asymptomatic. Dermoscopic features from our patients shared similar reported features of Cydnidae pigmentation.4,5 The accentuation of pigment over skin creases seen on dermoscopy was due to accumulation of Cydnidae secretion at these sites.
The differential diagnosis commonly includes idiopathic macular eruptive pigmentation, which is characterized by an asymptomatic progressive eruption of hyperpigmented macules over the trunk that persists from a few months up to 3 years. Other conditions in the differential include benign conditions such as acral benign melanocytic nevi, lentigines, pigmented purpuric dermatosis, and postinflammatory hyperpigmentation, as well as malignant conditions such as acral melanoma. Dermoscopy is a helpful, easy-to-use tool in differentiating these pigmentation disorders, obviating the need for an invasive investigation such as histopathologic analysis. Simultaneous involvement in a group of people living together or visiting the same place, abrupt onset, predominant involvement of the exposed sites, characteristic clinical and dermoscopic features, self-limiting course, and timing with the monsoon season should suggest a possibility of Cydnidae dermatitis/pigmentation, which can be confirmed by finding the causative bug in the affected locality.
Management
No specific treatment is required for the pigmentation caused by Cydnidae, as it is self-resolving. The macules can, however, be removed with acetone. Patients must be counseled regarding the benign and fleeting nature of this condition, as the abrupt onset may alarm them of a systemic disease. Affected patients should be advised against walking barefoot in areas where the insects can be found. Spraying insecticides in the affected locality also helps to reduce the presence of burrowing bugs.
- Hosokawa T, Kikuchi Y, Nikoh N, et al. Polyphyly of gut symbionts in stinkbugs of the family Cydnidae. Appl Environ Microbiol. 2012; 78:4758-4761.
- Schwertner CF, Nardi C. Burrower bugs (Cydnidae). In: Panizzi A, Grazia J, eds. True Bugs (Heteroptera) of the Neotropics. Entomology in Focus, vol 2. Springer; 2015.
- Lis JA. Burrower bugs of the Old World: a catalogue (Hemiptera: Heteroptera: Cydnidae). Genus (Wroclaw). 1999;10:165-249.
- Hayashi N, Yamamura Y, Ôhama S, et al. Defensive substances from stink bugs of Cydnidae. Experientia. 1976;32:418-419.
- Smith RM. The defensive secretion of the bugs Lampropharadifasciata, Adrisanumeensis, and Tectocorisdiophthalmus from Fiji. NZ J Zool. 1978;5:821-822.
- Krall BS, Zilkowski BW, Kight SL, et al. Chemistry and defensive efficacy of secretion of burrowing bugs. J Chem Ecol. 1997;23:1951-1962.
- Haddad V Jr, Cardoso J, Moraes R. Skin lesions caused by stink bugs (Insecta: Heteroptera: Pentatomidae): first report of dermatological injuries in humans. Wilderness Environ Med. 2002;13:48-50.
- Malhotra AK, Lis JA, Ramam M. Cydnidae (burrowing bug) pigmentation: a novel arthropod dermatosis. JAMA Dermatol. 2015;151:232-233.
- Sonthalia S. Dermoscopy of Cydnidae pigmentation: a novel disorder of pigmentation. Dermatol Pract Concept. 2019;9:228-229.
- Poojary S, Baddireddy K. Demystifying the stinking reddish brown stains through the dermoscope: Cydnidae pigmentation. Indian Dermatol Online J. 2019;10:757-758.
- Amrani A, Das A. Cydnidae pigmentation: unusual location on the abdomen and back. Br J Dermatol. 2021;184:E125.
Cydnidae is a family of small to medium-sized shield bugs with spiny legs that commonly are known as burrowing bugs (or burrower bugs). The family Cydnidae includes more than 100 genera and approximately 600 species worldwide.1 These insects are arthropods of the order Hemiptera (suborder: Heteroptera; superfamily: Pentatomoidae) and largely are concentrated in tropical and temperate regions. Approximately 145 species have been recorded in the Neotropical Region and have been included in the subfamilies Amnestinae, Cephalocteinae, and Sehirinae, in addition to Cydnidae.2 Burrowing bugs are ovoid in shape and 2 to 20 mm in length and morphologically are well adapted for burrowing. Their life span is 100 to 300 days. Being phytophagous, they burrow to feed on plants and roots. Adult burrowing bugs have wings and can fly. They have specialized glands located in either the abdomen (nymph) or thorax (adult) that secrete odorous chemicals for self-protection.3 The secretions contain hydrocarbonates that function as repellents and danger signals, can cause paralysis in prey, and act as a chemoattractant for mates.4-6 They also cause hyperpigmentation upon contact with the skin.
In this article, we present a series of cases from the same community to demonstrate the characteristic features of hyperpigmented macules caused by exposure to burrowing bugs. Dermatologists should be aware of this entity to prevent misdiagnosis and unnecessary investigations and treatment.
Case Series
A 36-year-old woman and 6 children (age range, 6-12 years) presented with a widespread, acute, brown-pigmented, macular eruption with lesions that increased in number over a 1-week period. All 7 patients resided in the same locality and were otherwise systemically healthy. Initially, the index case, a 7-year-old girl, was referred to our tertiary care center by a dermatologist with a provisional diagnosis of idiopathic macular eruptive pigmentation. The patient’s mother recalled noticing a tiny black insect on the child's scalp that left pigment on the skin when she crushed it between her fingers. The rest of the patients presented over the next few days: 3 of the children belonged to the same household as the index case, and there was history of all 6 children playing in the neighborhood park during late evening hours. The adult patient was the parent of one of the affected children. The lesions were associated with mild itching and tingling in 3 children but were asymptomatic in the other patients.
Clinical examination of the patients revealed multiple dark- to light-brown, discrete, irregularly shaped macules over the trunk, arms, and soles (eFigure 1). Dermoscopic examination of a pigmented macule showed an irregularly shaped, brownish, structureless area with accentuation of the pigment at skin creases and perieccrine pigmentation (eFigure 2). The pigmentation was unaffected by rubbing with alcohol or water. Clinicoepidemiologic parameters of the patients are summarized in the eTable.



One of the children’s parents conducted a geological examination of the ground in the neighborhood park during evening hours and found tiny burrowing bugs (eFigure 3). When crushed between the fingers, these insects left a similar brownish hyperpigmentation on the skin. The parents were counseled on the nature of the eruption, and the patients were kept under observation for 2 weeks. On follow-up after 5 days, the lesions showed markedly decreased intensity of hyperpigmentation, and no new lesions were observed in any of the 7 patients.

Comment
Pentatomoidae insects generally are benign and harmless to humans. There have been isolated reports of erythematous plaques caused by Antiteuchus mixtus and Edessa maculate.7 Malhotra et al8 reported the first known series of cases with Cydnidae insect–induced hyperpigmented macules. The reported patients presented with asymptomatic, brown, hyperpigmented macules over exposed sites such as the feet, neck, and chest. All the cases occurred during the monsoon season in tropical and temperate regions of the world, and the patients were characteristically clustered in similar geographic areas. The causative insect was identified as Chilocoris assmuthi Breddin, 1904, belonging to the family Cydnidae. When it was crushed between the fingers, the skin became hyperpigmented, confirming the role of the secretions from the insect in the etiology.8
A second case was described by Sonthalia,9 who also described the dermoscopic features of hyperpigmented macules caused by burrowing bugs. The lesions showed a stuck-on, clustered appearance of ovoid and bizarre pigmented clods, globules, and granules.9 Although the lesions occur mainly over exposed sites, pigmented macules occurring over unusual sites such as the abdomen and back also have been reported in association with burrowing bugs.10 Characteristically, the lesions initially are faint and darken with time and usually fade within a week. They can be rubbed off with acetone but persist when washed with soap and water. The fleeting nature of the pigmentation also has led to the term transient pseudo-lentigines sign to describe hyperpigmentation caused by burrowing bugs.11
Soil and plants are burrowing bugs’ natural habitats, and the insects typically are seen in vegetation-rich, moist areas adjoining human dwellings (eg, parks, gardens), where clusters of cases can occur. These insects proliferate during the monsoon season in tropical and temperate areas, leading to more cases occurring during these months.
Compared to prior reports,8,9 a few of our patients had predominant trunk and neck involvement with an occasional tingling sensation or pruritus while the rest were asymptomatic. Dermoscopic features from our patients shared similar reported features of Cydnidae pigmentation.4,5 The accentuation of pigment over skin creases seen on dermoscopy was due to accumulation of Cydnidae secretion at these sites.
The differential diagnosis commonly includes idiopathic macular eruptive pigmentation, which is characterized by an asymptomatic progressive eruption of hyperpigmented macules over the trunk that persists from a few months up to 3 years. Other conditions in the differential include benign conditions such as acral benign melanocytic nevi, lentigines, pigmented purpuric dermatosis, and postinflammatory hyperpigmentation, as well as malignant conditions such as acral melanoma. Dermoscopy is a helpful, easy-to-use tool in differentiating these pigmentation disorders, obviating the need for an invasive investigation such as histopathologic analysis. Simultaneous involvement in a group of people living together or visiting the same place, abrupt onset, predominant involvement of the exposed sites, characteristic clinical and dermoscopic features, self-limiting course, and timing with the monsoon season should suggest a possibility of Cydnidae dermatitis/pigmentation, which can be confirmed by finding the causative bug in the affected locality.
Management
No specific treatment is required for the pigmentation caused by Cydnidae, as it is self-resolving. The macules can, however, be removed with acetone. Patients must be counseled regarding the benign and fleeting nature of this condition, as the abrupt onset may alarm them of a systemic disease. Affected patients should be advised against walking barefoot in areas where the insects can be found. Spraying insecticides in the affected locality also helps to reduce the presence of burrowing bugs.
Cydnidae is a family of small to medium-sized shield bugs with spiny legs that commonly are known as burrowing bugs (or burrower bugs). The family Cydnidae includes more than 100 genera and approximately 600 species worldwide.1 These insects are arthropods of the order Hemiptera (suborder: Heteroptera; superfamily: Pentatomoidae) and largely are concentrated in tropical and temperate regions. Approximately 145 species have been recorded in the Neotropical Region and have been included in the subfamilies Amnestinae, Cephalocteinae, and Sehirinae, in addition to Cydnidae.2 Burrowing bugs are ovoid in shape and 2 to 20 mm in length and morphologically are well adapted for burrowing. Their life span is 100 to 300 days. Being phytophagous, they burrow to feed on plants and roots. Adult burrowing bugs have wings and can fly. They have specialized glands located in either the abdomen (nymph) or thorax (adult) that secrete odorous chemicals for self-protection.3 The secretions contain hydrocarbonates that function as repellents and danger signals, can cause paralysis in prey, and act as a chemoattractant for mates.4-6 They also cause hyperpigmentation upon contact with the skin.
In this article, we present a series of cases from the same community to demonstrate the characteristic features of hyperpigmented macules caused by exposure to burrowing bugs. Dermatologists should be aware of this entity to prevent misdiagnosis and unnecessary investigations and treatment.
Case Series
A 36-year-old woman and 6 children (age range, 6-12 years) presented with a widespread, acute, brown-pigmented, macular eruption with lesions that increased in number over a 1-week period. All 7 patients resided in the same locality and were otherwise systemically healthy. Initially, the index case, a 7-year-old girl, was referred to our tertiary care center by a dermatologist with a provisional diagnosis of idiopathic macular eruptive pigmentation. The patient’s mother recalled noticing a tiny black insect on the child's scalp that left pigment on the skin when she crushed it between her fingers. The rest of the patients presented over the next few days: 3 of the children belonged to the same household as the index case, and there was history of all 6 children playing in the neighborhood park during late evening hours. The adult patient was the parent of one of the affected children. The lesions were associated with mild itching and tingling in 3 children but were asymptomatic in the other patients.
Clinical examination of the patients revealed multiple dark- to light-brown, discrete, irregularly shaped macules over the trunk, arms, and soles (eFigure 1). Dermoscopic examination of a pigmented macule showed an irregularly shaped, brownish, structureless area with accentuation of the pigment at skin creases and perieccrine pigmentation (eFigure 2). The pigmentation was unaffected by rubbing with alcohol or water. Clinicoepidemiologic parameters of the patients are summarized in the eTable.



One of the children’s parents conducted a geological examination of the ground in the neighborhood park during evening hours and found tiny burrowing bugs (eFigure 3). When crushed between the fingers, these insects left a similar brownish hyperpigmentation on the skin. The parents were counseled on the nature of the eruption, and the patients were kept under observation for 2 weeks. On follow-up after 5 days, the lesions showed markedly decreased intensity of hyperpigmentation, and no new lesions were observed in any of the 7 patients.

Comment
Pentatomoidae insects generally are benign and harmless to humans. There have been isolated reports of erythematous plaques caused by Antiteuchus mixtus and Edessa maculate.7 Malhotra et al8 reported the first known series of cases with Cydnidae insect–induced hyperpigmented macules. The reported patients presented with asymptomatic, brown, hyperpigmented macules over exposed sites such as the feet, neck, and chest. All the cases occurred during the monsoon season in tropical and temperate regions of the world, and the patients were characteristically clustered in similar geographic areas. The causative insect was identified as Chilocoris assmuthi Breddin, 1904, belonging to the family Cydnidae. When it was crushed between the fingers, the skin became hyperpigmented, confirming the role of the secretions from the insect in the etiology.8
A second case was described by Sonthalia,9 who also described the dermoscopic features of hyperpigmented macules caused by burrowing bugs. The lesions showed a stuck-on, clustered appearance of ovoid and bizarre pigmented clods, globules, and granules.9 Although the lesions occur mainly over exposed sites, pigmented macules occurring over unusual sites such as the abdomen and back also have been reported in association with burrowing bugs.10 Characteristically, the lesions initially are faint and darken with time and usually fade within a week. They can be rubbed off with acetone but persist when washed with soap and water. The fleeting nature of the pigmentation also has led to the term transient pseudo-lentigines sign to describe hyperpigmentation caused by burrowing bugs.11
Soil and plants are burrowing bugs’ natural habitats, and the insects typically are seen in vegetation-rich, moist areas adjoining human dwellings (eg, parks, gardens), where clusters of cases can occur. These insects proliferate during the monsoon season in tropical and temperate areas, leading to more cases occurring during these months.
Compared to prior reports,8,9 a few of our patients had predominant trunk and neck involvement with an occasional tingling sensation or pruritus while the rest were asymptomatic. Dermoscopic features from our patients shared similar reported features of Cydnidae pigmentation.4,5 The accentuation of pigment over skin creases seen on dermoscopy was due to accumulation of Cydnidae secretion at these sites.
The differential diagnosis commonly includes idiopathic macular eruptive pigmentation, which is characterized by an asymptomatic progressive eruption of hyperpigmented macules over the trunk that persists from a few months up to 3 years. Other conditions in the differential include benign conditions such as acral benign melanocytic nevi, lentigines, pigmented purpuric dermatosis, and postinflammatory hyperpigmentation, as well as malignant conditions such as acral melanoma. Dermoscopy is a helpful, easy-to-use tool in differentiating these pigmentation disorders, obviating the need for an invasive investigation such as histopathologic analysis. Simultaneous involvement in a group of people living together or visiting the same place, abrupt onset, predominant involvement of the exposed sites, characteristic clinical and dermoscopic features, self-limiting course, and timing with the monsoon season should suggest a possibility of Cydnidae dermatitis/pigmentation, which can be confirmed by finding the causative bug in the affected locality.
Management
No specific treatment is required for the pigmentation caused by Cydnidae, as it is self-resolving. The macules can, however, be removed with acetone. Patients must be counseled regarding the benign and fleeting nature of this condition, as the abrupt onset may alarm them of a systemic disease. Affected patients should be advised against walking barefoot in areas where the insects can be found. Spraying insecticides in the affected locality also helps to reduce the presence of burrowing bugs.
- Hosokawa T, Kikuchi Y, Nikoh N, et al. Polyphyly of gut symbionts in stinkbugs of the family Cydnidae. Appl Environ Microbiol. 2012; 78:4758-4761.
- Schwertner CF, Nardi C. Burrower bugs (Cydnidae). In: Panizzi A, Grazia J, eds. True Bugs (Heteroptera) of the Neotropics. Entomology in Focus, vol 2. Springer; 2015.
- Lis JA. Burrower bugs of the Old World: a catalogue (Hemiptera: Heteroptera: Cydnidae). Genus (Wroclaw). 1999;10:165-249.
- Hayashi N, Yamamura Y, Ôhama S, et al. Defensive substances from stink bugs of Cydnidae. Experientia. 1976;32:418-419.
- Smith RM. The defensive secretion of the bugs Lampropharadifasciata, Adrisanumeensis, and Tectocorisdiophthalmus from Fiji. NZ J Zool. 1978;5:821-822.
- Krall BS, Zilkowski BW, Kight SL, et al. Chemistry and defensive efficacy of secretion of burrowing bugs. J Chem Ecol. 1997;23:1951-1962.
- Haddad V Jr, Cardoso J, Moraes R. Skin lesions caused by stink bugs (Insecta: Heteroptera: Pentatomidae): first report of dermatological injuries in humans. Wilderness Environ Med. 2002;13:48-50.
- Malhotra AK, Lis JA, Ramam M. Cydnidae (burrowing bug) pigmentation: a novel arthropod dermatosis. JAMA Dermatol. 2015;151:232-233.
- Sonthalia S. Dermoscopy of Cydnidae pigmentation: a novel disorder of pigmentation. Dermatol Pract Concept. 2019;9:228-229.
- Poojary S, Baddireddy K. Demystifying the stinking reddish brown stains through the dermoscope: Cydnidae pigmentation. Indian Dermatol Online J. 2019;10:757-758.
- Amrani A, Das A. Cydnidae pigmentation: unusual location on the abdomen and back. Br J Dermatol. 2021;184:E125.
- Hosokawa T, Kikuchi Y, Nikoh N, et al. Polyphyly of gut symbionts in stinkbugs of the family Cydnidae. Appl Environ Microbiol. 2012; 78:4758-4761.
- Schwertner CF, Nardi C. Burrower bugs (Cydnidae). In: Panizzi A, Grazia J, eds. True Bugs (Heteroptera) of the Neotropics. Entomology in Focus, vol 2. Springer; 2015.
- Lis JA. Burrower bugs of the Old World: a catalogue (Hemiptera: Heteroptera: Cydnidae). Genus (Wroclaw). 1999;10:165-249.
- Hayashi N, Yamamura Y, Ôhama S, et al. Defensive substances from stink bugs of Cydnidae. Experientia. 1976;32:418-419.
- Smith RM. The defensive secretion of the bugs Lampropharadifasciata, Adrisanumeensis, and Tectocorisdiophthalmus from Fiji. NZ J Zool. 1978;5:821-822.
- Krall BS, Zilkowski BW, Kight SL, et al. Chemistry and defensive efficacy of secretion of burrowing bugs. J Chem Ecol. 1997;23:1951-1962.
- Haddad V Jr, Cardoso J, Moraes R. Skin lesions caused by stink bugs (Insecta: Heteroptera: Pentatomidae): first report of dermatological injuries in humans. Wilderness Environ Med. 2002;13:48-50.
- Malhotra AK, Lis JA, Ramam M. Cydnidae (burrowing bug) pigmentation: a novel arthropod dermatosis. JAMA Dermatol. 2015;151:232-233.
- Sonthalia S. Dermoscopy of Cydnidae pigmentation: a novel disorder of pigmentation. Dermatol Pract Concept. 2019;9:228-229.
- Poojary S, Baddireddy K. Demystifying the stinking reddish brown stains through the dermoscope: Cydnidae pigmentation. Indian Dermatol Online J. 2019;10:757-758.
- Amrani A, Das A. Cydnidae pigmentation: unusual location on the abdomen and back. Br J Dermatol. 2021;184:E125.
Hyperpigmented Macules Caused by Burrowing Bugs (Cydnidae) May Mimic More Serious Conditions
Hyperpigmented Macules Caused by Burrowing Bugs (Cydnidae) May Mimic More Serious Conditions
Practice Points
- Burrowing bugs (Cydnidae) are phytophagous and burrow to feed on plants and roots. They are more numerous during the monsoon season in tropical and temperate regions.
- Secretions from burrowing bugs cause asymptomatic, hyperpigmented, irregularly shaped macules suggestive of an exogenous cause that commonly affect clusters of patients from the same geographic locality.
- The lesions are self-limiting and must be differentiated from close mimickers to ensure adequate and appropriate patient counseling.