User login
Association Between Psoriasis and Obesity Among US Adults in the 2009-2014 National Health and Nutrition Examination Survey
To the Editor:
Psoriasis is an immune-mediated dermatologic condition that is associated with various comorbidities, including obesity.1 The underlying pathophysiology of psoriasis has been extensively studied, and recent research has discussed the role of obesity in IL-17 secretion.2 The relationship between being overweight/obese and having psoriasis has been documented in the literature.1,2 However, this association in a recent population is lacking. We sought to investigate the association between psoriasis and obesity utilizing a representative US population of adults—the 2009-2014 National Health and Nutrition Examination Survey (NHANES) data,3 which contains the most recent psoriasis data.
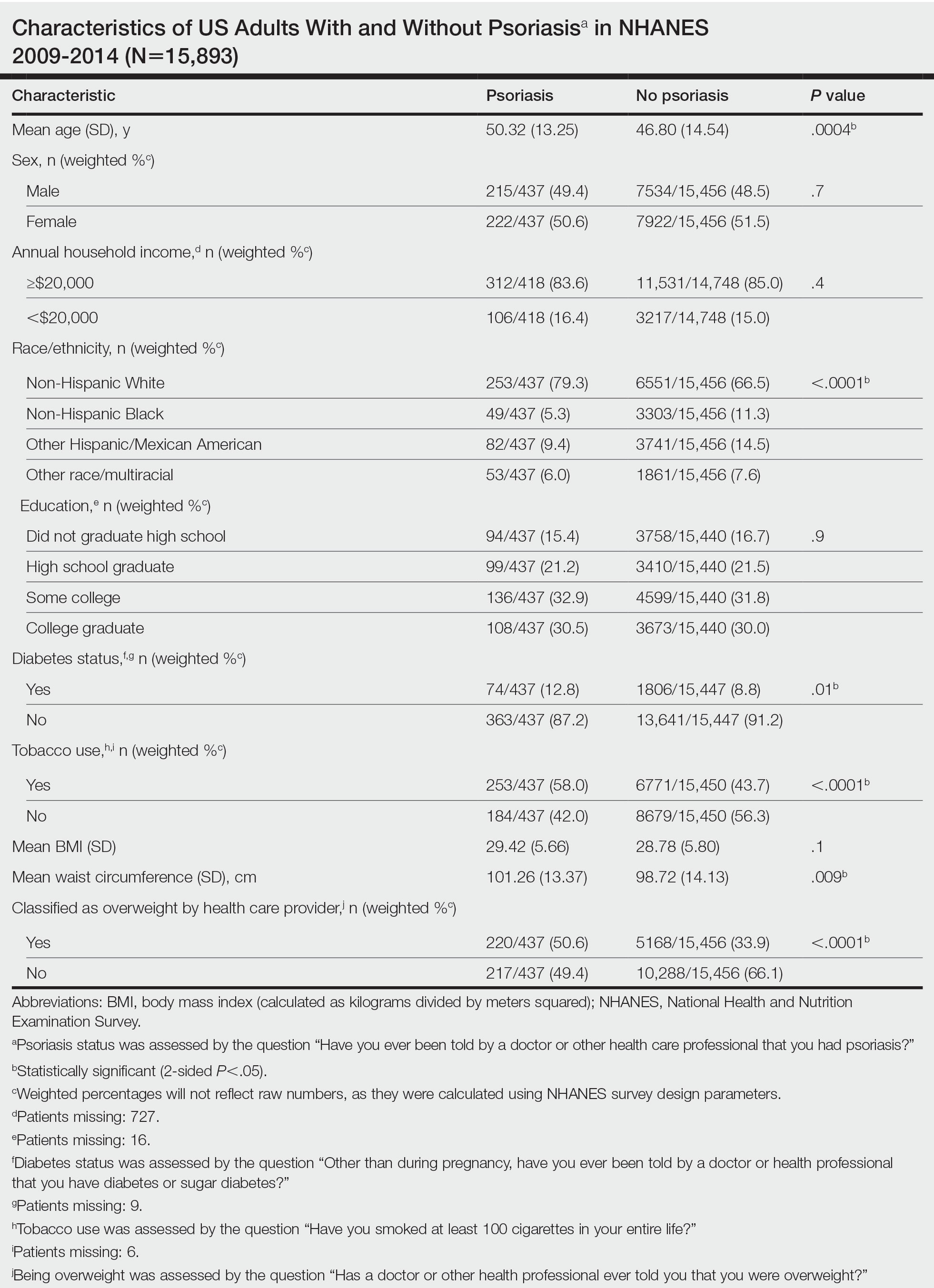
We conducted a population-based, cross-sectional study focused on patients 20 years and older with psoriasis from the 2009-2014 NHANES database. Three 2-year cycles of NHANES data were combined to create our 2009 to 2014 dataset. In the Table, numerous variables including age, sex, household income, race/ethnicity, education, diabetes status, tobacco use, body mass index (BMI), waist circumference, and being called overweight by a health care provider were analyzed using χ2 or t test analyses to evaluate for differences among those with and without psoriasis. Diabetes status was assessed by the question “Other than during pregnancy, have you ever been told by a doctor or health professional that you have diabetes or sugar diabetes?” Tobacco use was assessed by the question “Have you smoked at least 100 cigarettes in your entire life?” Psoriasis status was assessed by a self-reported response to the question “Have you ever been told by a doctor or other health care professional that you had psoriasis?” Three different outcome variables were used to determine if patients were overweight or obese: BMI, waist circumference, and response to the question “Has a doctor or other health professional ever told you that you were overweight?” Obesity was defined as having a BMI of 30 or higher or waist circumference of 102 cm or more in males and 88 cm or more in females.4 Being overweight was defined as having a BMI of 25 to 29.99 or response of Yes to “Has a doctor or other health professional ever told you that you were overweight?”
Initially, there were 17,547 participants 20 years and older from 2009 to 2014, but 1654 participants were excluded because of missing data for obesity or psoriasis; therefore, 15,893 patients were included in our analysis. Multivariable logistic regressions were utilized to examine the association between psoriasis and being overweight/obese (eTable). Additionally, the models were adjusted based on age, sex, household income, race/ethnicity, diabetes status, and tobacco use. All data processing and analysis were performed in Stata/MP 17 (StataCorp LLC). P<.05 was considered statistically significant.
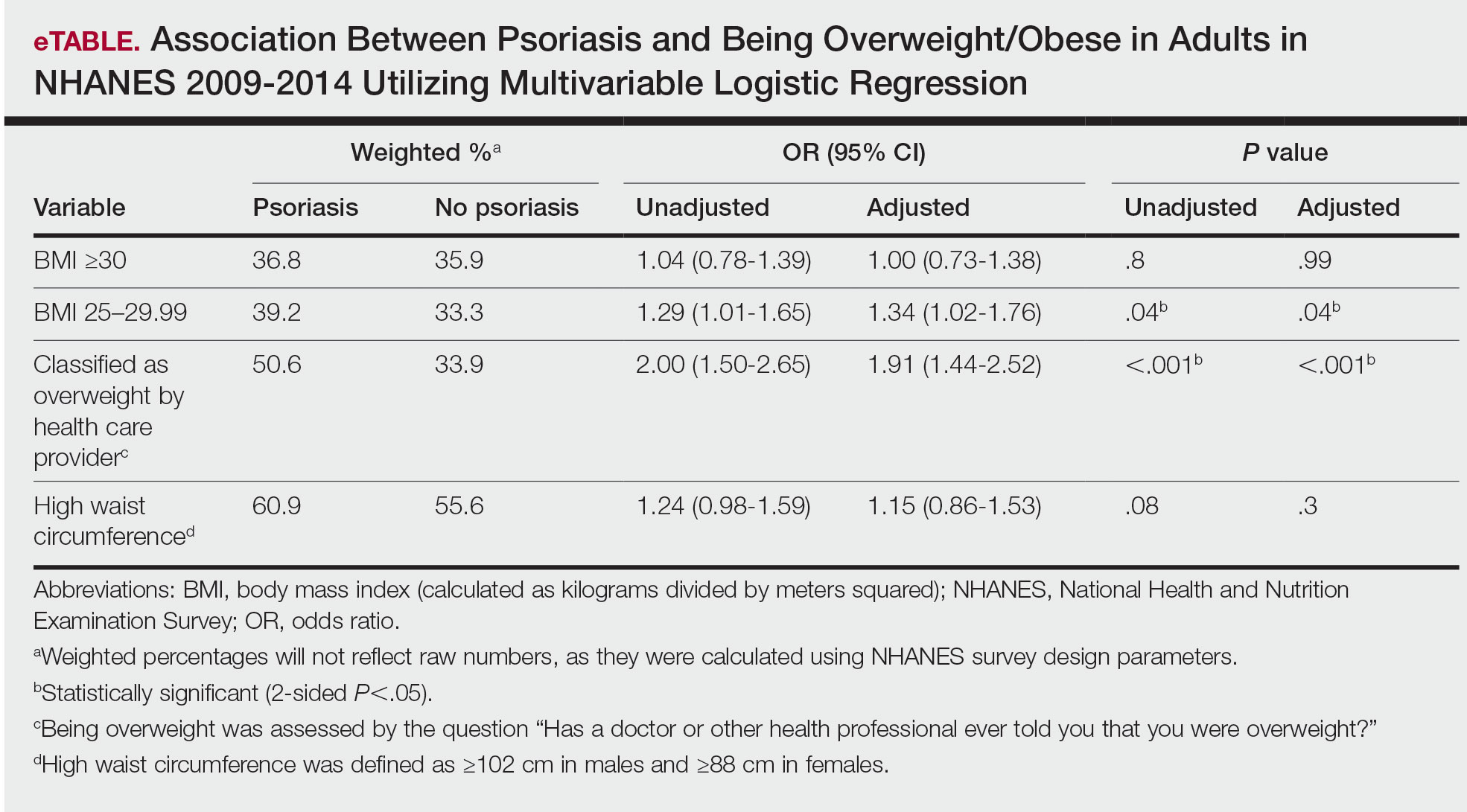
The Table shows characteristics of US adults with and without psoriasis in NHANES 2009-2014. We found that the variables of interest evaluating body weight that were significantly different on analysis between patients with and without psoriasis included waist circumference—patients with psoriasis had a significantly higher waist circumference (P=.009)—and being told by a health care provider that they are overweight (P<.0001), which supports the findings by Love et al,5 who reported abdominal obesity was the most common feature of metabolic syndrome exhibited among patients with psoriasis.
Multivariable logistic regression analysis (eTable) revealed that there was a significant association between psoriasis and BMI of 25 to 29.99 (adjusted odds ratio [AOR], 1.34; 95% CI, 1.02-1.76; P=.04) and being told by a health care provider that they are overweight (AOR, 1.91; 95% CI, 1.44-2.52; P<.001). After adjusting for confounding variables, there was no significant association between psoriasis and a BMI of 30 or higher (AOR, 1.00; 95% CI, 0.73-1.38; P=.99) or a waist circumference of 102 cm or more in males and 88 cm or more in females (AOR, 1.15; 95% CI, 0.86-1.53; P=.3).
Our findings suggest that a few variables indicative of being overweight or obese are associated with psoriasis. This relationship most likely is due to increased adipokine, including resistin, levels in overweight individuals, resulting in a proinflammatory state.6 It has been suggested that BMI alone is not a definitive marker for measuring fat storage levels in individuals. People can have a normal or slightly elevated BMI but possess excessive adiposity, resulting in chronic inflammation.6 Therefore, our findings of a significant association between psoriasis and being told by a health care provider that they are overweight might be a stronger measurement for possessing excessive fat, as this is likely due to clinical judgment rather than BMI measurement.
Moreover, it should be noted that the potential reason for the lack of association between BMI of 30 or higher and psoriasis in our analysis may be a result of BMI serving as a poor measurement for adiposity. Additionally, Armstrong and colleagues7 discussed that the association between BMI and psoriasis was stronger for patients with moderate to severe psoriasis. Our study consisted of NHANES data for self-reported psoriasis diagnoses without a psoriasis severity index, making it difficult to extrapolate which individuals had mild or moderate to severe psoriasis, which may have contributed to our finding of no association between BMI of 30 or higher and psoriasis.
The self-reported nature of the survey questions and lack of questions regarding psoriasis severity serve as limitations to the study. Both obesity and psoriasis can have various systemic consequences, such as cardiovascular disease, due to the development of an inflammatory state.8 Future studies may explore other body measurements that indicate being overweight or obese and the potential synergistic relationship of obesity and psoriasis severity, optimizing the development of effective treatment plans.
- Jensen P, Skov L. Psoriasis and obesity. Dermatology. 2016;232:633-639.
- Xu C, Ji J, Su T, et al. The association of psoriasis and obesity: focusing on IL-17A-related immunological mechanisms. Int J Dermatol Venereol. 2021;4:116-121.
- National Center for Health Statistics. NHANES questionnaires, datasets, and related documentation. Centers for Disease Control and Prevention website. Accessed June 22, 2023. https://wwwn.cdc.govnchs/nhanes/Default.aspx
- Ross R, Neeland IJ, Yamashita S, et al. Waist circumference as a vital sign in clinical practice: a Consensus Statement from the IAS and ICCR Working Group on Visceral Obesity. Nat Rev Endocrinol. 2020;16:177-189.
- Love TJ, Qureshi AA, Karlson EW, et al. Prevalence of the metabolic syndrome in psoriasis: results from the National Health and Nutrition Examination Survey, 2003-2006. Arch Dermatol. 2011;147:419-424.
- Paroutoglou K, Papadavid E, Christodoulatos GS, et al. Deciphering the association between psoriasis and obesity: current evidence and treatment considerations. Curr Obes Rep. 2020;9:165-178.
- Armstrong AW, Harskamp CT, Armstrong EJ. The association between psoriasis and obesity: a systematic review and meta-analysis of observational studies. Nutr Diabetes. 2012;2:E54.
- Hamminga EA, van der Lely AJ, Neumann HAM, et al. Chronic inflammation in psoriasis and obesity: implications for therapy. Med Hypotheses. 2006;67:768-773.
To the Editor:
Psoriasis is an immune-mediated dermatologic condition that is associated with various comorbidities, including obesity.1 The underlying pathophysiology of psoriasis has been extensively studied, and recent research has discussed the role of obesity in IL-17 secretion.2 The relationship between being overweight/obese and having psoriasis has been documented in the literature.1,2 However, this association in a recent population is lacking. We sought to investigate the association between psoriasis and obesity utilizing a representative US population of adults—the 2009-2014 National Health and Nutrition Examination Survey (NHANES) data,3 which contains the most recent psoriasis data.
We conducted a population-based, cross-sectional study focused on patients 20 years and older with psoriasis from the 2009-2014 NHANES database. Three 2-year cycles of NHANES data were combined to create our 2009 to 2014 dataset. In the Table, numerous variables including age, sex, household income, race/ethnicity, education, diabetes status, tobacco use, body mass index (BMI), waist circumference, and being called overweight by a health care provider were analyzed using χ2 or t test analyses to evaluate for differences among those with and without psoriasis. Diabetes status was assessed by the question “Other than during pregnancy, have you ever been told by a doctor or health professional that you have diabetes or sugar diabetes?” Tobacco use was assessed by the question “Have you smoked at least 100 cigarettes in your entire life?” Psoriasis status was assessed by a self-reported response to the question “Have you ever been told by a doctor or other health care professional that you had psoriasis?” Three different outcome variables were used to determine if patients were overweight or obese: BMI, waist circumference, and response to the question “Has a doctor or other health professional ever told you that you were overweight?” Obesity was defined as having a BMI of 30 or higher or waist circumference of 102 cm or more in males and 88 cm or more in females.4 Being overweight was defined as having a BMI of 25 to 29.99 or response of Yes to “Has a doctor or other health professional ever told you that you were overweight?”

Initially, there were 17,547 participants 20 years and older from 2009 to 2014, but 1654 participants were excluded because of missing data for obesity or psoriasis; therefore, 15,893 patients were included in our analysis. Multivariable logistic regressions were utilized to examine the association between psoriasis and being overweight/obese (eTable). Additionally, the models were adjusted based on age, sex, household income, race/ethnicity, diabetes status, and tobacco use. All data processing and analysis were performed in Stata/MP 17 (StataCorp LLC). P<.05 was considered statistically significant.

The Table shows characteristics of US adults with and without psoriasis in NHANES 2009-2014. We found that the variables of interest evaluating body weight that were significantly different on analysis between patients with and without psoriasis included waist circumference—patients with psoriasis had a significantly higher waist circumference (P=.009)—and being told by a health care provider that they are overweight (P<.0001), which supports the findings by Love et al,5 who reported abdominal obesity was the most common feature of metabolic syndrome exhibited among patients with psoriasis.
Multivariable logistic regression analysis (eTable) revealed that there was a significant association between psoriasis and BMI of 25 to 29.99 (adjusted odds ratio [AOR], 1.34; 95% CI, 1.02-1.76; P=.04) and being told by a health care provider that they are overweight (AOR, 1.91; 95% CI, 1.44-2.52; P<.001). After adjusting for confounding variables, there was no significant association between psoriasis and a BMI of 30 or higher (AOR, 1.00; 95% CI, 0.73-1.38; P=.99) or a waist circumference of 102 cm or more in males and 88 cm or more in females (AOR, 1.15; 95% CI, 0.86-1.53; P=.3).
Our findings suggest that a few variables indicative of being overweight or obese are associated with psoriasis. This relationship most likely is due to increased adipokine, including resistin, levels in overweight individuals, resulting in a proinflammatory state.6 It has been suggested that BMI alone is not a definitive marker for measuring fat storage levels in individuals. People can have a normal or slightly elevated BMI but possess excessive adiposity, resulting in chronic inflammation.6 Therefore, our findings of a significant association between psoriasis and being told by a health care provider that they are overweight might be a stronger measurement for possessing excessive fat, as this is likely due to clinical judgment rather than BMI measurement.
Moreover, it should be noted that the potential reason for the lack of association between BMI of 30 or higher and psoriasis in our analysis may be a result of BMI serving as a poor measurement for adiposity. Additionally, Armstrong and colleagues7 discussed that the association between BMI and psoriasis was stronger for patients with moderate to severe psoriasis. Our study consisted of NHANES data for self-reported psoriasis diagnoses without a psoriasis severity index, making it difficult to extrapolate which individuals had mild or moderate to severe psoriasis, which may have contributed to our finding of no association between BMI of 30 or higher and psoriasis.
The self-reported nature of the survey questions and lack of questions regarding psoriasis severity serve as limitations to the study. Both obesity and psoriasis can have various systemic consequences, such as cardiovascular disease, due to the development of an inflammatory state.8 Future studies may explore other body measurements that indicate being overweight or obese and the potential synergistic relationship of obesity and psoriasis severity, optimizing the development of effective treatment plans.
To the Editor:
Psoriasis is an immune-mediated dermatologic condition that is associated with various comorbidities, including obesity.1 The underlying pathophysiology of psoriasis has been extensively studied, and recent research has discussed the role of obesity in IL-17 secretion.2 The relationship between being overweight/obese and having psoriasis has been documented in the literature.1,2 However, this association in a recent population is lacking. We sought to investigate the association between psoriasis and obesity utilizing a representative US population of adults—the 2009-2014 National Health and Nutrition Examination Survey (NHANES) data,3 which contains the most recent psoriasis data.
We conducted a population-based, cross-sectional study focused on patients 20 years and older with psoriasis from the 2009-2014 NHANES database. Three 2-year cycles of NHANES data were combined to create our 2009 to 2014 dataset. In the Table, numerous variables including age, sex, household income, race/ethnicity, education, diabetes status, tobacco use, body mass index (BMI), waist circumference, and being called overweight by a health care provider were analyzed using χ2 or t test analyses to evaluate for differences among those with and without psoriasis. Diabetes status was assessed by the question “Other than during pregnancy, have you ever been told by a doctor or health professional that you have diabetes or sugar diabetes?” Tobacco use was assessed by the question “Have you smoked at least 100 cigarettes in your entire life?” Psoriasis status was assessed by a self-reported response to the question “Have you ever been told by a doctor or other health care professional that you had psoriasis?” Three different outcome variables were used to determine if patients were overweight or obese: BMI, waist circumference, and response to the question “Has a doctor or other health professional ever told you that you were overweight?” Obesity was defined as having a BMI of 30 or higher or waist circumference of 102 cm or more in males and 88 cm or more in females.4 Being overweight was defined as having a BMI of 25 to 29.99 or response of Yes to “Has a doctor or other health professional ever told you that you were overweight?”

Initially, there were 17,547 participants 20 years and older from 2009 to 2014, but 1654 participants were excluded because of missing data for obesity or psoriasis; therefore, 15,893 patients were included in our analysis. Multivariable logistic regressions were utilized to examine the association between psoriasis and being overweight/obese (eTable). Additionally, the models were adjusted based on age, sex, household income, race/ethnicity, diabetes status, and tobacco use. All data processing and analysis were performed in Stata/MP 17 (StataCorp LLC). P<.05 was considered statistically significant.

The Table shows characteristics of US adults with and without psoriasis in NHANES 2009-2014. We found that the variables of interest evaluating body weight that were significantly different on analysis between patients with and without psoriasis included waist circumference—patients with psoriasis had a significantly higher waist circumference (P=.009)—and being told by a health care provider that they are overweight (P<.0001), which supports the findings by Love et al,5 who reported abdominal obesity was the most common feature of metabolic syndrome exhibited among patients with psoriasis.
Multivariable logistic regression analysis (eTable) revealed that there was a significant association between psoriasis and BMI of 25 to 29.99 (adjusted odds ratio [AOR], 1.34; 95% CI, 1.02-1.76; P=.04) and being told by a health care provider that they are overweight (AOR, 1.91; 95% CI, 1.44-2.52; P<.001). After adjusting for confounding variables, there was no significant association between psoriasis and a BMI of 30 or higher (AOR, 1.00; 95% CI, 0.73-1.38; P=.99) or a waist circumference of 102 cm or more in males and 88 cm or more in females (AOR, 1.15; 95% CI, 0.86-1.53; P=.3).
Our findings suggest that a few variables indicative of being overweight or obese are associated with psoriasis. This relationship most likely is due to increased adipokine, including resistin, levels in overweight individuals, resulting in a proinflammatory state.6 It has been suggested that BMI alone is not a definitive marker for measuring fat storage levels in individuals. People can have a normal or slightly elevated BMI but possess excessive adiposity, resulting in chronic inflammation.6 Therefore, our findings of a significant association between psoriasis and being told by a health care provider that they are overweight might be a stronger measurement for possessing excessive fat, as this is likely due to clinical judgment rather than BMI measurement.
Moreover, it should be noted that the potential reason for the lack of association between BMI of 30 or higher and psoriasis in our analysis may be a result of BMI serving as a poor measurement for adiposity. Additionally, Armstrong and colleagues7 discussed that the association between BMI and psoriasis was stronger for patients with moderate to severe psoriasis. Our study consisted of NHANES data for self-reported psoriasis diagnoses without a psoriasis severity index, making it difficult to extrapolate which individuals had mild or moderate to severe psoriasis, which may have contributed to our finding of no association between BMI of 30 or higher and psoriasis.
The self-reported nature of the survey questions and lack of questions regarding psoriasis severity serve as limitations to the study. Both obesity and psoriasis can have various systemic consequences, such as cardiovascular disease, due to the development of an inflammatory state.8 Future studies may explore other body measurements that indicate being overweight or obese and the potential synergistic relationship of obesity and psoriasis severity, optimizing the development of effective treatment plans.
- Jensen P, Skov L. Psoriasis and obesity. Dermatology. 2016;232:633-639.
- Xu C, Ji J, Su T, et al. The association of psoriasis and obesity: focusing on IL-17A-related immunological mechanisms. Int J Dermatol Venereol. 2021;4:116-121.
- National Center for Health Statistics. NHANES questionnaires, datasets, and related documentation. Centers for Disease Control and Prevention website. Accessed June 22, 2023. https://wwwn.cdc.govnchs/nhanes/Default.aspx
- Ross R, Neeland IJ, Yamashita S, et al. Waist circumference as a vital sign in clinical practice: a Consensus Statement from the IAS and ICCR Working Group on Visceral Obesity. Nat Rev Endocrinol. 2020;16:177-189.
- Love TJ, Qureshi AA, Karlson EW, et al. Prevalence of the metabolic syndrome in psoriasis: results from the National Health and Nutrition Examination Survey, 2003-2006. Arch Dermatol. 2011;147:419-424.
- Paroutoglou K, Papadavid E, Christodoulatos GS, et al. Deciphering the association between psoriasis and obesity: current evidence and treatment considerations. Curr Obes Rep. 2020;9:165-178.
- Armstrong AW, Harskamp CT, Armstrong EJ. The association between psoriasis and obesity: a systematic review and meta-analysis of observational studies. Nutr Diabetes. 2012;2:E54.
- Hamminga EA, van der Lely AJ, Neumann HAM, et al. Chronic inflammation in psoriasis and obesity: implications for therapy. Med Hypotheses. 2006;67:768-773.
- Jensen P, Skov L. Psoriasis and obesity. Dermatology. 2016;232:633-639.
- Xu C, Ji J, Su T, et al. The association of psoriasis and obesity: focusing on IL-17A-related immunological mechanisms. Int J Dermatol Venereol. 2021;4:116-121.
- National Center for Health Statistics. NHANES questionnaires, datasets, and related documentation. Centers for Disease Control and Prevention website. Accessed June 22, 2023. https://wwwn.cdc.govnchs/nhanes/Default.aspx
- Ross R, Neeland IJ, Yamashita S, et al. Waist circumference as a vital sign in clinical practice: a Consensus Statement from the IAS and ICCR Working Group on Visceral Obesity. Nat Rev Endocrinol. 2020;16:177-189.
- Love TJ, Qureshi AA, Karlson EW, et al. Prevalence of the metabolic syndrome in psoriasis: results from the National Health and Nutrition Examination Survey, 2003-2006. Arch Dermatol. 2011;147:419-424.
- Paroutoglou K, Papadavid E, Christodoulatos GS, et al. Deciphering the association between psoriasis and obesity: current evidence and treatment considerations. Curr Obes Rep. 2020;9:165-178.
- Armstrong AW, Harskamp CT, Armstrong EJ. The association between psoriasis and obesity: a systematic review and meta-analysis of observational studies. Nutr Diabetes. 2012;2:E54.
- Hamminga EA, van der Lely AJ, Neumann HAM, et al. Chronic inflammation in psoriasis and obesity: implications for therapy. Med Hypotheses. 2006;67:768-773.
Practice Points
- There are many comorbidities that are associated with psoriasis, making it crucial to evaluate for these diseases in patients with psoriasis.
- Obesity may be a contributing factor to psoriasis development due to the role of IL-17 secretion.
Adverse Effects of the COVID-19 Vaccine in Patients With Psoriasis
To the Editor:
Because the SARS-CoV-2 virus is constantly changing, routine vaccination to prevent COVID-19 infection is recommended. The messenger RNA (mRNA) vaccines from Pfizer-BioNTech and Moderna as well as the Ad26.COV2.S (Johnson & Johnson) and NVX-CoV2373 (Novavax) vaccines are the most commonly used COVID-19 vaccines in the United States. Adverse effects following vaccination against SARS-CoV-2 are well documented; recent studies report a small incidence of adverse effects in the general population, with most being minor (eg, headache, fever, muscle pain).1,2 Interestingly, reports of exacerbation of psoriasis and new-onset psoriasis following COVID-19 vaccination suggest a potential association.3,4 However, the literature investigating the vaccine adverse effect profile in this demographic is scarce. We examined the incidence of adverse effects from SARS-CoV-2 vaccines in patients with psoriasis.
This retrospective cohort study used the COVID-19 Research Database (https://covid19researchdatabase.org/) to examine the adverse effects following the first and second doses of the mRNA vaccines in patients with and without psoriasis. The sample size for the Ad26.COV2.S vaccine was too small to analyze.
Claims were evaluated from August to October 2021 for 2 diagnoses of psoriasis prior to January 1, 2020, using the International Classification of Diseases, Tenth Revision (ICD-10) code L40.9 to increase the positive predictive value and ensure that the diagnosis preceded the COVID-19 pandemic. Patients younger than 18 years and those who did not receive 2 doses of a SARS-CoV-2 vaccine were excluded. Controls who did not have a diagnosis of psoriasis were matched for age, sex, and hypertension at a 4:1 ratio. Hypertension represented the most common comorbidity that could feasibly be controlled for in this study population. Other comorbidities recorded included obesity, type 2 diabetes mellitus, congestive heart failure, asthma, chronic obstructive pulmonary disease, chronic ischemic heart disease, rhinitis, and chronic kidney disease.
Common adverse effects as long as 30 days after vaccination were identified using ICD-10 codes. Adverse effects of interest were anaphylactic reaction, initial encounter of adverse effect of viral vaccines, fever, allergic urticaria, weakness, altered mental status, malaise, allergic reaction, chest pain, symptoms involving circulatory or respiratory systems, localized rash, axillary lymphadenopathy, infection, and myocarditis.5 Poisson regression was performed using Stata 17 analytical software.
We identified 4273 patients with psoriasis and 17,092 controls who received mRNA COVID-19 vaccines (Table). Adjusted odds ratios (aORs) for doses 1 and 2 were calculated for each vaccine (eTable). Adverse effects with sufficient data to generate an aOR included weakness, altered mental status, malaise, chest pain, and symptoms involving the circulatory or respiratory system. The aORs for allergic urticaria and initial encounter of adverse effect of viral vaccines were only calculated for the Moderna mRNA vaccine due to low sample size.

This study demonstrated that patients with psoriasis do not appear to have a significantly increased risk of adverse effects from mRNA SARS-CoV-2 vaccines. Although the ORs in this study were not significant, most recorded adverse effects demonstrated an aOR less than 1, suggesting that there might be a lower risk of certain adverse effects in psoriasis patients. This could be explained by the immunomodulatory effects of certain systemic psoriasis treatments that might influence the adverse effect presentation.

The study is limited by the lack of treatment data, small sample size, and the fact that it did not assess flares or worsening of psoriasis with the vaccines. Underreporting of adverse effects by patients and underdiagnosis of adverse effects secondary to SARS-CoV-2 vaccines due to its novel nature, incompletely understood consequences, and limited ICD-10 codes associated with adverse effects all contributed to the small sample size.
Our findings suggest that the risk for immediate adverse effects from the mRNA SARS-CoV-2 vaccines is not increased among psoriasis patients. However, the impact of immunomodulatory agents on vaccine efficacy and expected adverse effects should be investigated. As more individuals receive the COVID-19 vaccine, the adverse effect profile in patients with psoriasis is an important area of investigation.
- Singh A, Khillan R, Mishra Y, et al. The safety profile of COVID-19 vaccinations in the United States. Am J Infect Control. 2022;50:15-19. doi: 10.1016/j.ajic.2021.10.015
- Beatty AL, Peyser ND, Butcher XE, et al. Analysis of COVID-19 vaccine type and adverse effects following vaccination. JAMA Netw Open. 2021;4:e2140364. doi:10.1001/jamanetworkopen.2021.40364
- Bellinato F, Maurelli M, Gisondi P, et al. Cutaneous adverse reactions associated with SARS-CoV-2 vaccines. J Clin Med. 2021;10:5344. doi:10.3390/jcm10225344
- Elamin S, Hinds F, Tolland J. De novo generalized pustular psoriasis following Oxford-AstraZeneca COVID-19 vaccine. Clin Exp Dermatol. 2022;47:153-155. doi:10.1111/ced.14895
- Remer EE. Coding COVID-19 vaccination. ICD10monitor. Published March 2, 2021. Updated October 18, 2022. Accessed January 17, 2023. https://icd10monitor.medlearn.com/coding-covid-19-vaccination/
To the Editor:
Because the SARS-CoV-2 virus is constantly changing, routine vaccination to prevent COVID-19 infection is recommended. The messenger RNA (mRNA) vaccines from Pfizer-BioNTech and Moderna as well as the Ad26.COV2.S (Johnson & Johnson) and NVX-CoV2373 (Novavax) vaccines are the most commonly used COVID-19 vaccines in the United States. Adverse effects following vaccination against SARS-CoV-2 are well documented; recent studies report a small incidence of adverse effects in the general population, with most being minor (eg, headache, fever, muscle pain).1,2 Interestingly, reports of exacerbation of psoriasis and new-onset psoriasis following COVID-19 vaccination suggest a potential association.3,4 However, the literature investigating the vaccine adverse effect profile in this demographic is scarce. We examined the incidence of adverse effects from SARS-CoV-2 vaccines in patients with psoriasis.
This retrospective cohort study used the COVID-19 Research Database (https://covid19researchdatabase.org/) to examine the adverse effects following the first and second doses of the mRNA vaccines in patients with and without psoriasis. The sample size for the Ad26.COV2.S vaccine was too small to analyze.
Claims were evaluated from August to October 2021 for 2 diagnoses of psoriasis prior to January 1, 2020, using the International Classification of Diseases, Tenth Revision (ICD-10) code L40.9 to increase the positive predictive value and ensure that the diagnosis preceded the COVID-19 pandemic. Patients younger than 18 years and those who did not receive 2 doses of a SARS-CoV-2 vaccine were excluded. Controls who did not have a diagnosis of psoriasis were matched for age, sex, and hypertension at a 4:1 ratio. Hypertension represented the most common comorbidity that could feasibly be controlled for in this study population. Other comorbidities recorded included obesity, type 2 diabetes mellitus, congestive heart failure, asthma, chronic obstructive pulmonary disease, chronic ischemic heart disease, rhinitis, and chronic kidney disease.
Common adverse effects as long as 30 days after vaccination were identified using ICD-10 codes. Adverse effects of interest were anaphylactic reaction, initial encounter of adverse effect of viral vaccines, fever, allergic urticaria, weakness, altered mental status, malaise, allergic reaction, chest pain, symptoms involving circulatory or respiratory systems, localized rash, axillary lymphadenopathy, infection, and myocarditis.5 Poisson regression was performed using Stata 17 analytical software.
We identified 4273 patients with psoriasis and 17,092 controls who received mRNA COVID-19 vaccines (Table). Adjusted odds ratios (aORs) for doses 1 and 2 were calculated for each vaccine (eTable). Adverse effects with sufficient data to generate an aOR included weakness, altered mental status, malaise, chest pain, and symptoms involving the circulatory or respiratory system. The aORs for allergic urticaria and initial encounter of adverse effect of viral vaccines were only calculated for the Moderna mRNA vaccine due to low sample size.

This study demonstrated that patients with psoriasis do not appear to have a significantly increased risk of adverse effects from mRNA SARS-CoV-2 vaccines. Although the ORs in this study were not significant, most recorded adverse effects demonstrated an aOR less than 1, suggesting that there might be a lower risk of certain adverse effects in psoriasis patients. This could be explained by the immunomodulatory effects of certain systemic psoriasis treatments that might influence the adverse effect presentation.

The study is limited by the lack of treatment data, small sample size, and the fact that it did not assess flares or worsening of psoriasis with the vaccines. Underreporting of adverse effects by patients and underdiagnosis of adverse effects secondary to SARS-CoV-2 vaccines due to its novel nature, incompletely understood consequences, and limited ICD-10 codes associated with adverse effects all contributed to the small sample size.
Our findings suggest that the risk for immediate adverse effects from the mRNA SARS-CoV-2 vaccines is not increased among psoriasis patients. However, the impact of immunomodulatory agents on vaccine efficacy and expected adverse effects should be investigated. As more individuals receive the COVID-19 vaccine, the adverse effect profile in patients with psoriasis is an important area of investigation.
To the Editor:
Because the SARS-CoV-2 virus is constantly changing, routine vaccination to prevent COVID-19 infection is recommended. The messenger RNA (mRNA) vaccines from Pfizer-BioNTech and Moderna as well as the Ad26.COV2.S (Johnson & Johnson) and NVX-CoV2373 (Novavax) vaccines are the most commonly used COVID-19 vaccines in the United States. Adverse effects following vaccination against SARS-CoV-2 are well documented; recent studies report a small incidence of adverse effects in the general population, with most being minor (eg, headache, fever, muscle pain).1,2 Interestingly, reports of exacerbation of psoriasis and new-onset psoriasis following COVID-19 vaccination suggest a potential association.3,4 However, the literature investigating the vaccine adverse effect profile in this demographic is scarce. We examined the incidence of adverse effects from SARS-CoV-2 vaccines in patients with psoriasis.
This retrospective cohort study used the COVID-19 Research Database (https://covid19researchdatabase.org/) to examine the adverse effects following the first and second doses of the mRNA vaccines in patients with and without psoriasis. The sample size for the Ad26.COV2.S vaccine was too small to analyze.
Claims were evaluated from August to October 2021 for 2 diagnoses of psoriasis prior to January 1, 2020, using the International Classification of Diseases, Tenth Revision (ICD-10) code L40.9 to increase the positive predictive value and ensure that the diagnosis preceded the COVID-19 pandemic. Patients younger than 18 years and those who did not receive 2 doses of a SARS-CoV-2 vaccine were excluded. Controls who did not have a diagnosis of psoriasis were matched for age, sex, and hypertension at a 4:1 ratio. Hypertension represented the most common comorbidity that could feasibly be controlled for in this study population. Other comorbidities recorded included obesity, type 2 diabetes mellitus, congestive heart failure, asthma, chronic obstructive pulmonary disease, chronic ischemic heart disease, rhinitis, and chronic kidney disease.
Common adverse effects as long as 30 days after vaccination were identified using ICD-10 codes. Adverse effects of interest were anaphylactic reaction, initial encounter of adverse effect of viral vaccines, fever, allergic urticaria, weakness, altered mental status, malaise, allergic reaction, chest pain, symptoms involving circulatory or respiratory systems, localized rash, axillary lymphadenopathy, infection, and myocarditis.5 Poisson regression was performed using Stata 17 analytical software.
We identified 4273 patients with psoriasis and 17,092 controls who received mRNA COVID-19 vaccines (Table). Adjusted odds ratios (aORs) for doses 1 and 2 were calculated for each vaccine (eTable). Adverse effects with sufficient data to generate an aOR included weakness, altered mental status, malaise, chest pain, and symptoms involving the circulatory or respiratory system. The aORs for allergic urticaria and initial encounter of adverse effect of viral vaccines were only calculated for the Moderna mRNA vaccine due to low sample size.

This study demonstrated that patients with psoriasis do not appear to have a significantly increased risk of adverse effects from mRNA SARS-CoV-2 vaccines. Although the ORs in this study were not significant, most recorded adverse effects demonstrated an aOR less than 1, suggesting that there might be a lower risk of certain adverse effects in psoriasis patients. This could be explained by the immunomodulatory effects of certain systemic psoriasis treatments that might influence the adverse effect presentation.

The study is limited by the lack of treatment data, small sample size, and the fact that it did not assess flares or worsening of psoriasis with the vaccines. Underreporting of adverse effects by patients and underdiagnosis of adverse effects secondary to SARS-CoV-2 vaccines due to its novel nature, incompletely understood consequences, and limited ICD-10 codes associated with adverse effects all contributed to the small sample size.
Our findings suggest that the risk for immediate adverse effects from the mRNA SARS-CoV-2 vaccines is not increased among psoriasis patients. However, the impact of immunomodulatory agents on vaccine efficacy and expected adverse effects should be investigated. As more individuals receive the COVID-19 vaccine, the adverse effect profile in patients with psoriasis is an important area of investigation.
- Singh A, Khillan R, Mishra Y, et al. The safety profile of COVID-19 vaccinations in the United States. Am J Infect Control. 2022;50:15-19. doi: 10.1016/j.ajic.2021.10.015
- Beatty AL, Peyser ND, Butcher XE, et al. Analysis of COVID-19 vaccine type and adverse effects following vaccination. JAMA Netw Open. 2021;4:e2140364. doi:10.1001/jamanetworkopen.2021.40364
- Bellinato F, Maurelli M, Gisondi P, et al. Cutaneous adverse reactions associated with SARS-CoV-2 vaccines. J Clin Med. 2021;10:5344. doi:10.3390/jcm10225344
- Elamin S, Hinds F, Tolland J. De novo generalized pustular psoriasis following Oxford-AstraZeneca COVID-19 vaccine. Clin Exp Dermatol. 2022;47:153-155. doi:10.1111/ced.14895
- Remer EE. Coding COVID-19 vaccination. ICD10monitor. Published March 2, 2021. Updated October 18, 2022. Accessed January 17, 2023. https://icd10monitor.medlearn.com/coding-covid-19-vaccination/
- Singh A, Khillan R, Mishra Y, et al. The safety profile of COVID-19 vaccinations in the United States. Am J Infect Control. 2022;50:15-19. doi: 10.1016/j.ajic.2021.10.015
- Beatty AL, Peyser ND, Butcher XE, et al. Analysis of COVID-19 vaccine type and adverse effects following vaccination. JAMA Netw Open. 2021;4:e2140364. doi:10.1001/jamanetworkopen.2021.40364
- Bellinato F, Maurelli M, Gisondi P, et al. Cutaneous adverse reactions associated with SARS-CoV-2 vaccines. J Clin Med. 2021;10:5344. doi:10.3390/jcm10225344
- Elamin S, Hinds F, Tolland J. De novo generalized pustular psoriasis following Oxford-AstraZeneca COVID-19 vaccine. Clin Exp Dermatol. 2022;47:153-155. doi:10.1111/ced.14895
- Remer EE. Coding COVID-19 vaccination. ICD10monitor. Published March 2, 2021. Updated October 18, 2022. Accessed January 17, 2023. https://icd10monitor.medlearn.com/coding-covid-19-vaccination/
PRACTICE POINTS
- Patients who have psoriasis do not appear to have an increased incidence of adverse effects from messenger RNA COVID-19 vaccines.
- Clinicians can safely recommend COVID-19 vaccines to patients who have psoriasis.
New Treatments for Psoriasis: An Update on a Therapeutic Frontier
The landscape of psoriasis treatments has undergone rapid change within the last decade, and the dizzying speed of drug development has not slowed, with 4 notable entries into the psoriasis treatment armamentarium within the last year: tapinarof, roflumilast, deucravacitinib, and spesolimab. Several others are in late-stage development, and these therapies represent new mechanisms, pathways, and delivery systems that will meaningfully broaden the spectrum of treatment choices for our patients. However, it can be quite difficult to keep track of all of the medication options. This review aims to present the mechanisms and data on both newly available therapeutics for psoriasis and products in the pipeline that may have a major impact on our treatment paradigm for psoriasis in the near future.
Topical Treatments
Tapinarof—Tapinarof is a topical aryl hydrocarbon receptor (AhR)–modulating agent derived from a secondary metabolite produced by a bacterial symbiont of entomopathogenic nematodes.1 Tapinarof binds and activates AhR, inducing a signaling cascade that suppresses the expression of helper T cells TH17 and TH22, upregulates skin barrier protein expression, and reduces epidermal oxidative stress.2 This is a familiar mechanism, as AhR agonism is one of the pathways modulated by coal tar. Tapinarof’s overall effects on immune function, skin barrier integrity, and antioxidant activity show great promise for the treatment of plaque psoriasis.
Two phase 3 trials (N=1025) evaluated the efficacy and safety of once-daily tapinarof cream 1% for plaque psoriasis.3 A physician global assessment (PGA) score of 0/1 occurred in 35.4% to 40.2% of patients in the tapinarof group and in 6.0% of patients in the vehicle group. At week 12, 36.1% to 47.6% of patients treated with daily applications of tapinarof cream achieved a 75% reduction in their baseline psoriasis area and severity index (PASI 75) score compared with 6.9% to 10.2% in the vehicle group.3 In a long-term extension study, a substantial remittive effect of at least 4 months off tapinarof therapy was observed in patients who achieved complete clearance (PGA=0).4 Use of tapinarof cream was associated with folliculitis in up to 23.5% of patients.3,4
Roflumilast—
Topical roflumilast is a selective, highly potent PDE-4 inhibitor with greater affinity for PDE-4 compared to crisaborole and apremilast.8 Two phase 3 trials (N=881) evaluated the efficacy and safety profile of roflumilast cream for plaque psoriasis, with a particular interest in its use for intertriginous areas.9 At week 8, 37.5% to 42.4% of roflumilast-treated patients achieved investigator global assessment (IGA) success compared with 6.1% to 6.9% of vehicle-treated patients. Intertriginous IGA success was observed in 68.1% to 71.2% of patients treated with roflumilast cream compared with 13.8% to 18.5% of vehicle-treated patients. At 8-week follow-up, 39.0% to 41.6% of roflumilast-treated patients achieved PASI 75 vs 5.3% to 7.6% of patients in the vehicle group. Few stinging, burning, or application-site reactions were reported with roflumilast, along with rare instances of gastrointestinal AEs (<4%).9
Oral Therapy
Deucravacitinib—Tyrosine kinase 2 (TYK2) mediates the intracellular signaling of the TH17 and TH1 inflammatory cytokines IL-12/IL-23 and type I interferons, respectively, the former of which are critical in the development of psoriasis via the Janus kinase (JAK) signal transducer and activator of transcription pathway.10 Deucravacitinib is an oral selective TYK2 allosteric inhibitor that binds to the regulatory domain of the enzyme rather than the active catalytic domain, where other TYK2 and JAK1, JAK2, and JAK3 inhibitors bind.11 This unique inhibitory mechanism accounts for the high functional selectivity of deucravacitinib for TYK2 vs the closely related JAK1, JAK2, and JAK3 kinases, thus avoiding the pitfall of prior JAK inhibitors that were associated with major AEs, including an increased risk for serious infections, malignancies, and thrombosis.12 The selective suppression of the inflammatory TYK2 pathway has the potential to shift future therapeutic targets to a narrower range of receptors that may contribute to favorable benefit-risk profiles.
Two phase 3 trials (N=1686) compared the efficacy and safety of deucravacitinib vs placebo and apremilast in adults with moderate to severe plaque psoriasis.13,14 At week 16, 53.0% to 58.4% of deucravacitinib-treated patients achieved PASI 75 compared with 35.1% to 39.8% of apremilast-treated patients. At 16-week follow-up, static PGA response was observed in 49.5% to 53.6% of patients in the deucravacitinib group and 32.1% to 33.9% of the apremilast group. The most frequent AEs associated with deucravacitinib therapy were nasopharyngitis and upper respiratory tract infection, whereas headache, diarrhea, and nausea were more common with apremilast. Treatment with deucravacitinib caused no meaningful changes in laboratory parameters, which are known to change with JAK1, JAK2, and JAK3 inhibitors.13,14 A long-term extension study demonstrated that deucravacitinib had persistent efficacy and consistent safety for up to 2 years.15
Other TYK2 Inhibitors in the Pipeline
Novel oral allosteric TYK2 inhibitors—VTX958 and NDI-034858—and the competitive TYK2 inhibitor PF-06826647 are being developed. Theoretically, these new allosteric inhibitors possess unique structural properties to provide greater TYK2 suppression while bypassing JAK1, JAK2, and JAK3 pathways that may contribute to improved efficacy and safety profiles compared with other TYK2 inhibitors such as deucravacitinib. The results of a phase 1b trial (ClinicalTrials.gov Identifier NCT04999839) showed a dose-dependent reduction of disease severity associated with NDI-034858 treatment for patients with moderate to severe plaque psoriasis, albeit in only 26 patients. At week 4, PASI 50 was achieved in 13%, 57%, and 40% of patients in the 5-, 10-, and 30-mg groups, respectively, compared with 0% in the placebo group.16 In a phase 2 trial of 179 patients, 46.5% and 33.0% of patients treated with 400 and 200 mg of PF-06826647, respectively, achieved PASI 90 at week 16. Conversely, dose-dependent laboratory abnormalities were observed with PF-06826647, including anemia, neutropenia, and increases in creatine phosphokinase.17 At high concentrations, PF-06826647 may disrupt JAK signaling pathways involved in hematopoiesis and renal functions owing to its mode of action as a competitive inhibitor. Overall, these agents are much farther from market, and long-term studies with larger diverse patient cohorts are required to adequately assess the efficacy and safety data of these novel oral TYK2 inhibitors for patients with psoriasis.
EDP1815—EDP1815 is an oral preparation of a single strain of Prevotella histicola being developed for the treatment of inflammatory diseases, including psoriasis. EDP1815 interacts with host intestinal immune cells through the small intestinal axis (SINTAX) to suppress systemic inflammation across the TH1, TH2, and TH17 pathways. Therapy triggers broad immunomodulatory effects without causing systemic absorption, colonic colonization, or modification of the gut microbiome.18 In a phase 2 study (NCT04603027), the primary end point analysis, mean percentage change in PASI between treatment and placebo, demonstrated that at week 16, EDP1815 was superior to placebo with 80% to 90% probability across each cohort. At week 16, 25% to 32% of patients across the 3 cohorts treated with EDP1815 achieved PASI 50 compared with 12% of patients receiving placebo. Gastrointestinal AEs were comparable between treatment and placebo groups. These results suggest that SINTAX-targeted therapies may provide efficacious and safe immunomodulatory effects for patients with mild to moderate psoriasis, who often have limited treatment options. Although improvements may be mild, SINTAX-targeted therapies can be seen as a particularly attractive adjunctive treatment for patients with severe psoriasis taking other medications or as part of a treatment approach for a patient with milder psoriasis.
Biologics
Bimekizumab—Bimekizumab is a monoclonal IgG1 antibody that selectively inhibits IL-17A and IL-17F. Although IL-17A is a more potent cytokine, IL-17F may be more highly expressed in psoriatic lesional skin and independently contribute to the activation of proinflammatory signaling pathways implicated in the pathophysiology of psoriasis.19 Evidence suggests that dual inhibition of IL-17A and IL-17F may provide more complete suppression of inflammation and improved clinical responses than IL-17A inhibition alone.20
Prior bimekizumab phase 3 clinical studies have shown both rapid and durable clinical improvements in skin clearance compared with placebo.21 Three phase 3 trials—BE VIVID (N=567),22 BE SURE (N=478),23 and BE RADIANT (N=743)24—assessed the efficacy and safety of bimekizumab vs the IL-12/IL-23 inhibitor ustekinumab, the tumor necrosis factor inhibitor adalimumab, and the selective IL-17A inhibitor secukinumab, respectively. At week 4, significantly more patients treated with bimekizumab (71%–77%) achieved PASI 75 than patients treated with ustekinumab (15%; P<.0001), adalimumab (31.4%; P<.001), or secukinumab (47.3%; P<.001).22-24 After 16 weeks of treatment, PASI 90 was achieved by 85% to 86.2%, 50%, and 47.2% of patients treated with bimekizumab, ustekinumab, and adalimumab, respectively.22,23 At week 16, PASI 100 was observed in 59% to 61.7%, 21%, 23.9%, and 48.9% of patients treated with bimekizumab, ustekinumab, adalimumab, and secukinumab, respectively. An IGA response (score of 0/1) at week 16 was achieved by 84% to 85.5%, 53%, 57.2%, and 78.6% of patients receiving bimekizumab, ustekinumab, adalimumab, and secukinumab, respectively.22-24
The most common AEs in bimekizumab-treated patients were nasopharyngitis, oral candidiasis, and upper respiratory tract infection.22-24 The dual inhibition of IL-17A and IL-17F suppresses host defenses against Candida at the oral mucosa, increasing the incidence of bimekizumab-associated oral candidiasis.25 Despite the increased risk of Candida infections, these data suggest that inhibition of both IL-17A and IL-17F with bimekizumab may provide faster and greater clinical benefit for patients with moderate to severe plaque psoriasis than inhibition of IL-17A alone and other biologic therapies, as the PASI 100 clearance rates across the multiple comparator trials and the placebo-controlled pivotal trial are consistently the highest among any biologic for the treatment of psoriasis.
Spesolimab—The IL-36 pathway and IL-36 receptor genes have been linked to the pathogenesis of generalized pustular psoriasis.26 In a phase 2 trial, 19 of 35 patients (54%) receiving an intravenous dose of spesolimab, an IL-36 receptor inhibitor, had a generalized pustular psoriasis PGA pustulation subscore of 0 (no visible pustules) at the end of week 1 vs 6% of patients in the placebo group.27 A generalized pustular psoriasis PGA total score of 0 or 1 was observed in 43% (15/35) of spesolimab-treated patients compared with 11% (2/18) of patients in the placebo group. The most common AEs in patients treated with spesolimab were minor infections.27 Two open-label phase 3 trials—NCT05200247 and NCT05239039—are underway to determine the long-term efficacy and safety of spesolimab in patients with generalized pustular psoriasis.
Conclusion
Although we have seen a renaissance in psoriasis therapies with the advent of biologics in the last 20 years, recent evidence shows that more innovation is underway. Just in the last year, 2 new mechanisms for treating psoriasis topically without steroids have come to fruition, and there have not been truly novel mechanisms for treating psoriasis topically since approvals for tazarotene and calcipotriene in the 1990s. An entirely new class—TYK2 inhibitors—was developed and landed in psoriasis first, greatly improving the efficacy measures attained with oral medications in general. Finally, an orphan diagnosis got its due with an ambitiously designed study looking at a previously unheard-of 1-week end point, but it comes for one of the few true dermatologic emergencies we encounter, generalized pustular psoriasis. We are fortunate to have so many meaningful new treatments available to us, and it is invigorating to see that even more efficacious biologics and treatments are coming, along with novel concepts such as a treatment affecting the microbiome. Now, we just need to make sure that our patients have the access they deserve to the wide array of available treatments.
- Bissonnette R, Stein Gold L, Rubenstein DS, et al. Tapinarof in the treatment of psoriasis: a review of the unique mechanism of action of a novel therapeutic aryl hydrocarbon receptor-modulating agent. J Am Acad Dermatol. 2021;84:1059-1067.
- Smith SH, Jayawickreme C, Rickard DJ, et al. Tapinarof is a natural AhR agonist that resolves skin inflammation in mice and humans. J Invest Dermatol. 2017;137:2110-2119.
- Lebwohl MG, Stein Gold L, Strober B, et al. Phase 3 trials of tapinarof cream for plaque psoriasis. N Engl J Med. 2021;385:2219-2229.
- Strober B, Stein Gold L, Bissonnette R, et al. One-year safety and efficacy of tapinarof cream for the treatment of plaque psoriasis: results from the PSOARING 3 trial. J Am Acad Dermatol. 2022;87:800-806.
- Card GL, England BP, Suzuki Y, et al. Structural basis for the activity of drugs that inhibit phosphodiesterases. Structure. 2004;12:2233-2247.
- Milakovic M, Gooderham MJ. Phosphodiesterase-4 inhibition in psoriasis. Psoriasis (Auckl). 2021;11:21-29.
- Papp K, Reich K, Leonardi CL, et al. Apremilast, an oral phosphodiesterase 4 (PDE4) inhibitor, in patients with moderate to severe plaque psoriasis: results of a phase III, randomized, controlled trial (Efficacy and Safety Trial Evaluating the Effects of Apremilast in Psoriasis [ESTEEM] 1). J Am Acad Dermatol. 2015;73:37-49.
- Dong C, Virtucio C, Zemska O, et al. Treatment of skin inflammation with benzoxaborole phosphodiesterase inhibitors: selectivity, cellular activity, and effect on cytokines associated with skin inflammation and skin architecture changes. J Pharmacol Exp Ther. 2016;358:413-422.
- Lebwohl MG, Kircik LH, Moore AY, et al. Effect of roflumilast cream vs vehicle cream on chronic plaque psoriasis: the DERMIS-1 and DERMIS-2 randomized clinical trials. JAMA. 2022;328:1073-1084.
- Nogueira M, Puig L, Torres T. JAK inhibitors for treatment of psoriasis: focus on selective tyk2 inhibitors. Drugs. 2020;80:341-352.
- Wrobleski ST, Moslin R, Lin S, et al. Highly selective inhibition of tyrosine kinase 2 (TYK2) for the treatment of autoimmune diseases: discovery of the allosteric inhibitor BMS-986165. J Med Chem. 2019;62:8973-8995.
- Chimalakonda A, Burke J, Cheng L, et al. Selectivity profile of the tyrosine kinase 2 inhibitor deucravacitinib compared with janus kinase 1/2/3 inhibitors. Dermatol Ther (Heidelb). 2021;11:1763-1776.
- Strober B, Thaçi D, Sofen H, et al. Deucravacitinib versus placebo and apremilast in moderate to severe plaque psoriasis: efficacy and safety results from the 52-week, randomized, double-blinded, phase 3 Program for Evaluation of TYK2 inhibitor psoriasis second trial. J Am Acad Dermatol. 2023;88:40-51.
- Armstrong AW, Gooderham M, Warren RB, et al. Deucravacitinib versus placebo and apremilast in moderate to severe plaque psoriasis: efficacy and safety results from the 52-week, randomized, double-blinded, placebo-controlled phase 3 POETYK PSO-1 trial. J Am Acad Dermatol. 2023;88:29-39.
- Warren RB, Sofen H, Imafuku S, et al. POS1046 deucravacitinib long-term efficacy and safety in plaque psoriasis: 2-year results from the phase 3 POETYK PSO program [abstract]. Ann Rheum Dis. 2022;81(suppl 1):841.
- McElwee JJ, Garcet S, Li X, et al. Analysis of histologic, molecular and clinical improvement in moderate-to-severe psoriasis: results from a Phase 1b trial of the novel allosteric TYK2 inhibitor NDI-034858. Poster presented at: American Academy of Dermatology Annual Meeting; March 25, 2022; Boston, MA.
- Tehlirian C, Singh RSP, Pradhan V, et al. Oral tyrosine kinase 2 inhibitor PF-06826647 demonstrates efficacy and an acceptable safety profile in participants with moderate-to-severe plaque psoriasis in a phase 2b, randomized, double-blind, placebo-controlled study. J Am Acad Dermatol. 2022;87:333-342.
- Hilliard-Barth K, Cormack T, Ramani K, et al. Immune mechanisms of the systemic effects of EDP1815: an orally delivered, gut-restricted microbial drug candidate for the treatment of inflammatory diseases. Poster presented at: Society for Mucosal Immunology Virtual Congress; July 20-22, 2021, Cambridge, MA.
- Glatt S, Baeten D, Baker T, et al. Dual IL-17A and IL-17F neutralisation by bimekizumab in psoriatic arthritis: evidence from preclinical experiments and a randomised placebo-controlled clinical trial that IL-17F contributes to human chronic tissue inflammation. Ann Rheum Dis. 2018;77:523-532.
- Adams R, Maroof A, Baker T, et al. Bimekizumab, a novel humanized IgG1 antibody that neutralizes both IL-17A and IL-17F. Front Immunol. 2020;11:1894.
- Gordon KB, Foley P, Krueger JG, et al. Bimekizumab efficacy and safety in moderate to severe plaque psoriasis (BE READY): a multicentre, double-blind, placebo-controlled, randomised withdrawal phase 3 trial. Lancet. 2021;397:475-486.
- Reich K, Papp KA, Blauvelt A, et al. Bimekizumab versus ustekinumab for the treatment of moderate to severe plaque psoriasis (BE VIVID): efficacy and safety from a 52-week, multicentre, double-blind, active comparator and placebo controlled phase 3 trial. Lancet. 2021;397:487-498.
- Warren RB, Blauvelt A, Bagel J, et al. Bimekizumab versus adalimumab in plaque psoriasis. N Engl J Med. 2021;385:130-141.
- Reich K, Warren RB, Lebwohl M, et al. Bimekizumab versus secukinumab in plaque psoriasis. N Engl J Med. 2021;385:142-152.
- Blauvelt A, Lebwohl MG, Bissonnette R. IL-23/IL-17A dysfunction phenotypes inform possible clinical effects from anti-IL-17A therapies. J Invest Dermatol. 2015;135:1946-1953.
- Marrakchi S, Guigue P, Renshaw BR, et al. Interleukin-36-receptor antagonist deficiency and generalized pustular psoriasis. N Engl J Med. 2011;365:620-628.
- Bachelez H, Choon SE, Marrakchi S, et al. Trial of spesolimab for generalized pustular psoriasis. N Engl J Med. 2021;385:2431-2440.
The landscape of psoriasis treatments has undergone rapid change within the last decade, and the dizzying speed of drug development has not slowed, with 4 notable entries into the psoriasis treatment armamentarium within the last year: tapinarof, roflumilast, deucravacitinib, and spesolimab. Several others are in late-stage development, and these therapies represent new mechanisms, pathways, and delivery systems that will meaningfully broaden the spectrum of treatment choices for our patients. However, it can be quite difficult to keep track of all of the medication options. This review aims to present the mechanisms and data on both newly available therapeutics for psoriasis and products in the pipeline that may have a major impact on our treatment paradigm for psoriasis in the near future.
Topical Treatments
Tapinarof—Tapinarof is a topical aryl hydrocarbon receptor (AhR)–modulating agent derived from a secondary metabolite produced by a bacterial symbiont of entomopathogenic nematodes.1 Tapinarof binds and activates AhR, inducing a signaling cascade that suppresses the expression of helper T cells TH17 and TH22, upregulates skin barrier protein expression, and reduces epidermal oxidative stress.2 This is a familiar mechanism, as AhR agonism is one of the pathways modulated by coal tar. Tapinarof’s overall effects on immune function, skin barrier integrity, and antioxidant activity show great promise for the treatment of plaque psoriasis.
Two phase 3 trials (N=1025) evaluated the efficacy and safety of once-daily tapinarof cream 1% for plaque psoriasis.3 A physician global assessment (PGA) score of 0/1 occurred in 35.4% to 40.2% of patients in the tapinarof group and in 6.0% of patients in the vehicle group. At week 12, 36.1% to 47.6% of patients treated with daily applications of tapinarof cream achieved a 75% reduction in their baseline psoriasis area and severity index (PASI 75) score compared with 6.9% to 10.2% in the vehicle group.3 In a long-term extension study, a substantial remittive effect of at least 4 months off tapinarof therapy was observed in patients who achieved complete clearance (PGA=0).4 Use of tapinarof cream was associated with folliculitis in up to 23.5% of patients.3,4
Roflumilast—
Topical roflumilast is a selective, highly potent PDE-4 inhibitor with greater affinity for PDE-4 compared to crisaborole and apremilast.8 Two phase 3 trials (N=881) evaluated the efficacy and safety profile of roflumilast cream for plaque psoriasis, with a particular interest in its use for intertriginous areas.9 At week 8, 37.5% to 42.4% of roflumilast-treated patients achieved investigator global assessment (IGA) success compared with 6.1% to 6.9% of vehicle-treated patients. Intertriginous IGA success was observed in 68.1% to 71.2% of patients treated with roflumilast cream compared with 13.8% to 18.5% of vehicle-treated patients. At 8-week follow-up, 39.0% to 41.6% of roflumilast-treated patients achieved PASI 75 vs 5.3% to 7.6% of patients in the vehicle group. Few stinging, burning, or application-site reactions were reported with roflumilast, along with rare instances of gastrointestinal AEs (<4%).9
Oral Therapy
Deucravacitinib—Tyrosine kinase 2 (TYK2) mediates the intracellular signaling of the TH17 and TH1 inflammatory cytokines IL-12/IL-23 and type I interferons, respectively, the former of which are critical in the development of psoriasis via the Janus kinase (JAK) signal transducer and activator of transcription pathway.10 Deucravacitinib is an oral selective TYK2 allosteric inhibitor that binds to the regulatory domain of the enzyme rather than the active catalytic domain, where other TYK2 and JAK1, JAK2, and JAK3 inhibitors bind.11 This unique inhibitory mechanism accounts for the high functional selectivity of deucravacitinib for TYK2 vs the closely related JAK1, JAK2, and JAK3 kinases, thus avoiding the pitfall of prior JAK inhibitors that were associated with major AEs, including an increased risk for serious infections, malignancies, and thrombosis.12 The selective suppression of the inflammatory TYK2 pathway has the potential to shift future therapeutic targets to a narrower range of receptors that may contribute to favorable benefit-risk profiles.
Two phase 3 trials (N=1686) compared the efficacy and safety of deucravacitinib vs placebo and apremilast in adults with moderate to severe plaque psoriasis.13,14 At week 16, 53.0% to 58.4% of deucravacitinib-treated patients achieved PASI 75 compared with 35.1% to 39.8% of apremilast-treated patients. At 16-week follow-up, static PGA response was observed in 49.5% to 53.6% of patients in the deucravacitinib group and 32.1% to 33.9% of the apremilast group. The most frequent AEs associated with deucravacitinib therapy were nasopharyngitis and upper respiratory tract infection, whereas headache, diarrhea, and nausea were more common with apremilast. Treatment with deucravacitinib caused no meaningful changes in laboratory parameters, which are known to change with JAK1, JAK2, and JAK3 inhibitors.13,14 A long-term extension study demonstrated that deucravacitinib had persistent efficacy and consistent safety for up to 2 years.15
Other TYK2 Inhibitors in the Pipeline
Novel oral allosteric TYK2 inhibitors—VTX958 and NDI-034858—and the competitive TYK2 inhibitor PF-06826647 are being developed. Theoretically, these new allosteric inhibitors possess unique structural properties to provide greater TYK2 suppression while bypassing JAK1, JAK2, and JAK3 pathways that may contribute to improved efficacy and safety profiles compared with other TYK2 inhibitors such as deucravacitinib. The results of a phase 1b trial (ClinicalTrials.gov Identifier NCT04999839) showed a dose-dependent reduction of disease severity associated with NDI-034858 treatment for patients with moderate to severe plaque psoriasis, albeit in only 26 patients. At week 4, PASI 50 was achieved in 13%, 57%, and 40% of patients in the 5-, 10-, and 30-mg groups, respectively, compared with 0% in the placebo group.16 In a phase 2 trial of 179 patients, 46.5% and 33.0% of patients treated with 400 and 200 mg of PF-06826647, respectively, achieved PASI 90 at week 16. Conversely, dose-dependent laboratory abnormalities were observed with PF-06826647, including anemia, neutropenia, and increases in creatine phosphokinase.17 At high concentrations, PF-06826647 may disrupt JAK signaling pathways involved in hematopoiesis and renal functions owing to its mode of action as a competitive inhibitor. Overall, these agents are much farther from market, and long-term studies with larger diverse patient cohorts are required to adequately assess the efficacy and safety data of these novel oral TYK2 inhibitors for patients with psoriasis.
EDP1815—EDP1815 is an oral preparation of a single strain of Prevotella histicola being developed for the treatment of inflammatory diseases, including psoriasis. EDP1815 interacts with host intestinal immune cells through the small intestinal axis (SINTAX) to suppress systemic inflammation across the TH1, TH2, and TH17 pathways. Therapy triggers broad immunomodulatory effects without causing systemic absorption, colonic colonization, or modification of the gut microbiome.18 In a phase 2 study (NCT04603027), the primary end point analysis, mean percentage change in PASI between treatment and placebo, demonstrated that at week 16, EDP1815 was superior to placebo with 80% to 90% probability across each cohort. At week 16, 25% to 32% of patients across the 3 cohorts treated with EDP1815 achieved PASI 50 compared with 12% of patients receiving placebo. Gastrointestinal AEs were comparable between treatment and placebo groups. These results suggest that SINTAX-targeted therapies may provide efficacious and safe immunomodulatory effects for patients with mild to moderate psoriasis, who often have limited treatment options. Although improvements may be mild, SINTAX-targeted therapies can be seen as a particularly attractive adjunctive treatment for patients with severe psoriasis taking other medications or as part of a treatment approach for a patient with milder psoriasis.
Biologics
Bimekizumab—Bimekizumab is a monoclonal IgG1 antibody that selectively inhibits IL-17A and IL-17F. Although IL-17A is a more potent cytokine, IL-17F may be more highly expressed in psoriatic lesional skin and independently contribute to the activation of proinflammatory signaling pathways implicated in the pathophysiology of psoriasis.19 Evidence suggests that dual inhibition of IL-17A and IL-17F may provide more complete suppression of inflammation and improved clinical responses than IL-17A inhibition alone.20
Prior bimekizumab phase 3 clinical studies have shown both rapid and durable clinical improvements in skin clearance compared with placebo.21 Three phase 3 trials—BE VIVID (N=567),22 BE SURE (N=478),23 and BE RADIANT (N=743)24—assessed the efficacy and safety of bimekizumab vs the IL-12/IL-23 inhibitor ustekinumab, the tumor necrosis factor inhibitor adalimumab, and the selective IL-17A inhibitor secukinumab, respectively. At week 4, significantly more patients treated with bimekizumab (71%–77%) achieved PASI 75 than patients treated with ustekinumab (15%; P<.0001), adalimumab (31.4%; P<.001), or secukinumab (47.3%; P<.001).22-24 After 16 weeks of treatment, PASI 90 was achieved by 85% to 86.2%, 50%, and 47.2% of patients treated with bimekizumab, ustekinumab, and adalimumab, respectively.22,23 At week 16, PASI 100 was observed in 59% to 61.7%, 21%, 23.9%, and 48.9% of patients treated with bimekizumab, ustekinumab, adalimumab, and secukinumab, respectively. An IGA response (score of 0/1) at week 16 was achieved by 84% to 85.5%, 53%, 57.2%, and 78.6% of patients receiving bimekizumab, ustekinumab, adalimumab, and secukinumab, respectively.22-24
The most common AEs in bimekizumab-treated patients were nasopharyngitis, oral candidiasis, and upper respiratory tract infection.22-24 The dual inhibition of IL-17A and IL-17F suppresses host defenses against Candida at the oral mucosa, increasing the incidence of bimekizumab-associated oral candidiasis.25 Despite the increased risk of Candida infections, these data suggest that inhibition of both IL-17A and IL-17F with bimekizumab may provide faster and greater clinical benefit for patients with moderate to severe plaque psoriasis than inhibition of IL-17A alone and other biologic therapies, as the PASI 100 clearance rates across the multiple comparator trials and the placebo-controlled pivotal trial are consistently the highest among any biologic for the treatment of psoriasis.
Spesolimab—The IL-36 pathway and IL-36 receptor genes have been linked to the pathogenesis of generalized pustular psoriasis.26 In a phase 2 trial, 19 of 35 patients (54%) receiving an intravenous dose of spesolimab, an IL-36 receptor inhibitor, had a generalized pustular psoriasis PGA pustulation subscore of 0 (no visible pustules) at the end of week 1 vs 6% of patients in the placebo group.27 A generalized pustular psoriasis PGA total score of 0 or 1 was observed in 43% (15/35) of spesolimab-treated patients compared with 11% (2/18) of patients in the placebo group. The most common AEs in patients treated with spesolimab were minor infections.27 Two open-label phase 3 trials—NCT05200247 and NCT05239039—are underway to determine the long-term efficacy and safety of spesolimab in patients with generalized pustular psoriasis.
Conclusion
Although we have seen a renaissance in psoriasis therapies with the advent of biologics in the last 20 years, recent evidence shows that more innovation is underway. Just in the last year, 2 new mechanisms for treating psoriasis topically without steroids have come to fruition, and there have not been truly novel mechanisms for treating psoriasis topically since approvals for tazarotene and calcipotriene in the 1990s. An entirely new class—TYK2 inhibitors—was developed and landed in psoriasis first, greatly improving the efficacy measures attained with oral medications in general. Finally, an orphan diagnosis got its due with an ambitiously designed study looking at a previously unheard-of 1-week end point, but it comes for one of the few true dermatologic emergencies we encounter, generalized pustular psoriasis. We are fortunate to have so many meaningful new treatments available to us, and it is invigorating to see that even more efficacious biologics and treatments are coming, along with novel concepts such as a treatment affecting the microbiome. Now, we just need to make sure that our patients have the access they deserve to the wide array of available treatments.
The landscape of psoriasis treatments has undergone rapid change within the last decade, and the dizzying speed of drug development has not slowed, with 4 notable entries into the psoriasis treatment armamentarium within the last year: tapinarof, roflumilast, deucravacitinib, and spesolimab. Several others are in late-stage development, and these therapies represent new mechanisms, pathways, and delivery systems that will meaningfully broaden the spectrum of treatment choices for our patients. However, it can be quite difficult to keep track of all of the medication options. This review aims to present the mechanisms and data on both newly available therapeutics for psoriasis and products in the pipeline that may have a major impact on our treatment paradigm for psoriasis in the near future.
Topical Treatments
Tapinarof—Tapinarof is a topical aryl hydrocarbon receptor (AhR)–modulating agent derived from a secondary metabolite produced by a bacterial symbiont of entomopathogenic nematodes.1 Tapinarof binds and activates AhR, inducing a signaling cascade that suppresses the expression of helper T cells TH17 and TH22, upregulates skin barrier protein expression, and reduces epidermal oxidative stress.2 This is a familiar mechanism, as AhR agonism is one of the pathways modulated by coal tar. Tapinarof’s overall effects on immune function, skin barrier integrity, and antioxidant activity show great promise for the treatment of plaque psoriasis.
Two phase 3 trials (N=1025) evaluated the efficacy and safety of once-daily tapinarof cream 1% for plaque psoriasis.3 A physician global assessment (PGA) score of 0/1 occurred in 35.4% to 40.2% of patients in the tapinarof group and in 6.0% of patients in the vehicle group. At week 12, 36.1% to 47.6% of patients treated with daily applications of tapinarof cream achieved a 75% reduction in their baseline psoriasis area and severity index (PASI 75) score compared with 6.9% to 10.2% in the vehicle group.3 In a long-term extension study, a substantial remittive effect of at least 4 months off tapinarof therapy was observed in patients who achieved complete clearance (PGA=0).4 Use of tapinarof cream was associated with folliculitis in up to 23.5% of patients.3,4
Roflumilast—
Topical roflumilast is a selective, highly potent PDE-4 inhibitor with greater affinity for PDE-4 compared to crisaborole and apremilast.8 Two phase 3 trials (N=881) evaluated the efficacy and safety profile of roflumilast cream for plaque psoriasis, with a particular interest in its use for intertriginous areas.9 At week 8, 37.5% to 42.4% of roflumilast-treated patients achieved investigator global assessment (IGA) success compared with 6.1% to 6.9% of vehicle-treated patients. Intertriginous IGA success was observed in 68.1% to 71.2% of patients treated with roflumilast cream compared with 13.8% to 18.5% of vehicle-treated patients. At 8-week follow-up, 39.0% to 41.6% of roflumilast-treated patients achieved PASI 75 vs 5.3% to 7.6% of patients in the vehicle group. Few stinging, burning, or application-site reactions were reported with roflumilast, along with rare instances of gastrointestinal AEs (<4%).9
Oral Therapy
Deucravacitinib—Tyrosine kinase 2 (TYK2) mediates the intracellular signaling of the TH17 and TH1 inflammatory cytokines IL-12/IL-23 and type I interferons, respectively, the former of which are critical in the development of psoriasis via the Janus kinase (JAK) signal transducer and activator of transcription pathway.10 Deucravacitinib is an oral selective TYK2 allosteric inhibitor that binds to the regulatory domain of the enzyme rather than the active catalytic domain, where other TYK2 and JAK1, JAK2, and JAK3 inhibitors bind.11 This unique inhibitory mechanism accounts for the high functional selectivity of deucravacitinib for TYK2 vs the closely related JAK1, JAK2, and JAK3 kinases, thus avoiding the pitfall of prior JAK inhibitors that were associated with major AEs, including an increased risk for serious infections, malignancies, and thrombosis.12 The selective suppression of the inflammatory TYK2 pathway has the potential to shift future therapeutic targets to a narrower range of receptors that may contribute to favorable benefit-risk profiles.
Two phase 3 trials (N=1686) compared the efficacy and safety of deucravacitinib vs placebo and apremilast in adults with moderate to severe plaque psoriasis.13,14 At week 16, 53.0% to 58.4% of deucravacitinib-treated patients achieved PASI 75 compared with 35.1% to 39.8% of apremilast-treated patients. At 16-week follow-up, static PGA response was observed in 49.5% to 53.6% of patients in the deucravacitinib group and 32.1% to 33.9% of the apremilast group. The most frequent AEs associated with deucravacitinib therapy were nasopharyngitis and upper respiratory tract infection, whereas headache, diarrhea, and nausea were more common with apremilast. Treatment with deucravacitinib caused no meaningful changes in laboratory parameters, which are known to change with JAK1, JAK2, and JAK3 inhibitors.13,14 A long-term extension study demonstrated that deucravacitinib had persistent efficacy and consistent safety for up to 2 years.15
Other TYK2 Inhibitors in the Pipeline
Novel oral allosteric TYK2 inhibitors—VTX958 and NDI-034858—and the competitive TYK2 inhibitor PF-06826647 are being developed. Theoretically, these new allosteric inhibitors possess unique structural properties to provide greater TYK2 suppression while bypassing JAK1, JAK2, and JAK3 pathways that may contribute to improved efficacy and safety profiles compared with other TYK2 inhibitors such as deucravacitinib. The results of a phase 1b trial (ClinicalTrials.gov Identifier NCT04999839) showed a dose-dependent reduction of disease severity associated with NDI-034858 treatment for patients with moderate to severe plaque psoriasis, albeit in only 26 patients. At week 4, PASI 50 was achieved in 13%, 57%, and 40% of patients in the 5-, 10-, and 30-mg groups, respectively, compared with 0% in the placebo group.16 In a phase 2 trial of 179 patients, 46.5% and 33.0% of patients treated with 400 and 200 mg of PF-06826647, respectively, achieved PASI 90 at week 16. Conversely, dose-dependent laboratory abnormalities were observed with PF-06826647, including anemia, neutropenia, and increases in creatine phosphokinase.17 At high concentrations, PF-06826647 may disrupt JAK signaling pathways involved in hematopoiesis and renal functions owing to its mode of action as a competitive inhibitor. Overall, these agents are much farther from market, and long-term studies with larger diverse patient cohorts are required to adequately assess the efficacy and safety data of these novel oral TYK2 inhibitors for patients with psoriasis.
EDP1815—EDP1815 is an oral preparation of a single strain of Prevotella histicola being developed for the treatment of inflammatory diseases, including psoriasis. EDP1815 interacts with host intestinal immune cells through the small intestinal axis (SINTAX) to suppress systemic inflammation across the TH1, TH2, and TH17 pathways. Therapy triggers broad immunomodulatory effects without causing systemic absorption, colonic colonization, or modification of the gut microbiome.18 In a phase 2 study (NCT04603027), the primary end point analysis, mean percentage change in PASI between treatment and placebo, demonstrated that at week 16, EDP1815 was superior to placebo with 80% to 90% probability across each cohort. At week 16, 25% to 32% of patients across the 3 cohorts treated with EDP1815 achieved PASI 50 compared with 12% of patients receiving placebo. Gastrointestinal AEs were comparable between treatment and placebo groups. These results suggest that SINTAX-targeted therapies may provide efficacious and safe immunomodulatory effects for patients with mild to moderate psoriasis, who often have limited treatment options. Although improvements may be mild, SINTAX-targeted therapies can be seen as a particularly attractive adjunctive treatment for patients with severe psoriasis taking other medications or as part of a treatment approach for a patient with milder psoriasis.
Biologics
Bimekizumab—Bimekizumab is a monoclonal IgG1 antibody that selectively inhibits IL-17A and IL-17F. Although IL-17A is a more potent cytokine, IL-17F may be more highly expressed in psoriatic lesional skin and independently contribute to the activation of proinflammatory signaling pathways implicated in the pathophysiology of psoriasis.19 Evidence suggests that dual inhibition of IL-17A and IL-17F may provide more complete suppression of inflammation and improved clinical responses than IL-17A inhibition alone.20
Prior bimekizumab phase 3 clinical studies have shown both rapid and durable clinical improvements in skin clearance compared with placebo.21 Three phase 3 trials—BE VIVID (N=567),22 BE SURE (N=478),23 and BE RADIANT (N=743)24—assessed the efficacy and safety of bimekizumab vs the IL-12/IL-23 inhibitor ustekinumab, the tumor necrosis factor inhibitor adalimumab, and the selective IL-17A inhibitor secukinumab, respectively. At week 4, significantly more patients treated with bimekizumab (71%–77%) achieved PASI 75 than patients treated with ustekinumab (15%; P<.0001), adalimumab (31.4%; P<.001), or secukinumab (47.3%; P<.001).22-24 After 16 weeks of treatment, PASI 90 was achieved by 85% to 86.2%, 50%, and 47.2% of patients treated with bimekizumab, ustekinumab, and adalimumab, respectively.22,23 At week 16, PASI 100 was observed in 59% to 61.7%, 21%, 23.9%, and 48.9% of patients treated with bimekizumab, ustekinumab, adalimumab, and secukinumab, respectively. An IGA response (score of 0/1) at week 16 was achieved by 84% to 85.5%, 53%, 57.2%, and 78.6% of patients receiving bimekizumab, ustekinumab, adalimumab, and secukinumab, respectively.22-24
The most common AEs in bimekizumab-treated patients were nasopharyngitis, oral candidiasis, and upper respiratory tract infection.22-24 The dual inhibition of IL-17A and IL-17F suppresses host defenses against Candida at the oral mucosa, increasing the incidence of bimekizumab-associated oral candidiasis.25 Despite the increased risk of Candida infections, these data suggest that inhibition of both IL-17A and IL-17F with bimekizumab may provide faster and greater clinical benefit for patients with moderate to severe plaque psoriasis than inhibition of IL-17A alone and other biologic therapies, as the PASI 100 clearance rates across the multiple comparator trials and the placebo-controlled pivotal trial are consistently the highest among any biologic for the treatment of psoriasis.
Spesolimab—The IL-36 pathway and IL-36 receptor genes have been linked to the pathogenesis of generalized pustular psoriasis.26 In a phase 2 trial, 19 of 35 patients (54%) receiving an intravenous dose of spesolimab, an IL-36 receptor inhibitor, had a generalized pustular psoriasis PGA pustulation subscore of 0 (no visible pustules) at the end of week 1 vs 6% of patients in the placebo group.27 A generalized pustular psoriasis PGA total score of 0 or 1 was observed in 43% (15/35) of spesolimab-treated patients compared with 11% (2/18) of patients in the placebo group. The most common AEs in patients treated with spesolimab were minor infections.27 Two open-label phase 3 trials—NCT05200247 and NCT05239039—are underway to determine the long-term efficacy and safety of spesolimab in patients with generalized pustular psoriasis.
Conclusion
Although we have seen a renaissance in psoriasis therapies with the advent of biologics in the last 20 years, recent evidence shows that more innovation is underway. Just in the last year, 2 new mechanisms for treating psoriasis topically without steroids have come to fruition, and there have not been truly novel mechanisms for treating psoriasis topically since approvals for tazarotene and calcipotriene in the 1990s. An entirely new class—TYK2 inhibitors—was developed and landed in psoriasis first, greatly improving the efficacy measures attained with oral medications in general. Finally, an orphan diagnosis got its due with an ambitiously designed study looking at a previously unheard-of 1-week end point, but it comes for one of the few true dermatologic emergencies we encounter, generalized pustular psoriasis. We are fortunate to have so many meaningful new treatments available to us, and it is invigorating to see that even more efficacious biologics and treatments are coming, along with novel concepts such as a treatment affecting the microbiome. Now, we just need to make sure that our patients have the access they deserve to the wide array of available treatments.
- Bissonnette R, Stein Gold L, Rubenstein DS, et al. Tapinarof in the treatment of psoriasis: a review of the unique mechanism of action of a novel therapeutic aryl hydrocarbon receptor-modulating agent. J Am Acad Dermatol. 2021;84:1059-1067.
- Smith SH, Jayawickreme C, Rickard DJ, et al. Tapinarof is a natural AhR agonist that resolves skin inflammation in mice and humans. J Invest Dermatol. 2017;137:2110-2119.
- Lebwohl MG, Stein Gold L, Strober B, et al. Phase 3 trials of tapinarof cream for plaque psoriasis. N Engl J Med. 2021;385:2219-2229.
- Strober B, Stein Gold L, Bissonnette R, et al. One-year safety and efficacy of tapinarof cream for the treatment of plaque psoriasis: results from the PSOARING 3 trial. J Am Acad Dermatol. 2022;87:800-806.
- Card GL, England BP, Suzuki Y, et al. Structural basis for the activity of drugs that inhibit phosphodiesterases. Structure. 2004;12:2233-2247.
- Milakovic M, Gooderham MJ. Phosphodiesterase-4 inhibition in psoriasis. Psoriasis (Auckl). 2021;11:21-29.
- Papp K, Reich K, Leonardi CL, et al. Apremilast, an oral phosphodiesterase 4 (PDE4) inhibitor, in patients with moderate to severe plaque psoriasis: results of a phase III, randomized, controlled trial (Efficacy and Safety Trial Evaluating the Effects of Apremilast in Psoriasis [ESTEEM] 1). J Am Acad Dermatol. 2015;73:37-49.
- Dong C, Virtucio C, Zemska O, et al. Treatment of skin inflammation with benzoxaborole phosphodiesterase inhibitors: selectivity, cellular activity, and effect on cytokines associated with skin inflammation and skin architecture changes. J Pharmacol Exp Ther. 2016;358:413-422.
- Lebwohl MG, Kircik LH, Moore AY, et al. Effect of roflumilast cream vs vehicle cream on chronic plaque psoriasis: the DERMIS-1 and DERMIS-2 randomized clinical trials. JAMA. 2022;328:1073-1084.
- Nogueira M, Puig L, Torres T. JAK inhibitors for treatment of psoriasis: focus on selective tyk2 inhibitors. Drugs. 2020;80:341-352.
- Wrobleski ST, Moslin R, Lin S, et al. Highly selective inhibition of tyrosine kinase 2 (TYK2) for the treatment of autoimmune diseases: discovery of the allosteric inhibitor BMS-986165. J Med Chem. 2019;62:8973-8995.
- Chimalakonda A, Burke J, Cheng L, et al. Selectivity profile of the tyrosine kinase 2 inhibitor deucravacitinib compared with janus kinase 1/2/3 inhibitors. Dermatol Ther (Heidelb). 2021;11:1763-1776.
- Strober B, Thaçi D, Sofen H, et al. Deucravacitinib versus placebo and apremilast in moderate to severe plaque psoriasis: efficacy and safety results from the 52-week, randomized, double-blinded, phase 3 Program for Evaluation of TYK2 inhibitor psoriasis second trial. J Am Acad Dermatol. 2023;88:40-51.
- Armstrong AW, Gooderham M, Warren RB, et al. Deucravacitinib versus placebo and apremilast in moderate to severe plaque psoriasis: efficacy and safety results from the 52-week, randomized, double-blinded, placebo-controlled phase 3 POETYK PSO-1 trial. J Am Acad Dermatol. 2023;88:29-39.
- Warren RB, Sofen H, Imafuku S, et al. POS1046 deucravacitinib long-term efficacy and safety in plaque psoriasis: 2-year results from the phase 3 POETYK PSO program [abstract]. Ann Rheum Dis. 2022;81(suppl 1):841.
- McElwee JJ, Garcet S, Li X, et al. Analysis of histologic, molecular and clinical improvement in moderate-to-severe psoriasis: results from a Phase 1b trial of the novel allosteric TYK2 inhibitor NDI-034858. Poster presented at: American Academy of Dermatology Annual Meeting; March 25, 2022; Boston, MA.
- Tehlirian C, Singh RSP, Pradhan V, et al. Oral tyrosine kinase 2 inhibitor PF-06826647 demonstrates efficacy and an acceptable safety profile in participants with moderate-to-severe plaque psoriasis in a phase 2b, randomized, double-blind, placebo-controlled study. J Am Acad Dermatol. 2022;87:333-342.
- Hilliard-Barth K, Cormack T, Ramani K, et al. Immune mechanisms of the systemic effects of EDP1815: an orally delivered, gut-restricted microbial drug candidate for the treatment of inflammatory diseases. Poster presented at: Society for Mucosal Immunology Virtual Congress; July 20-22, 2021, Cambridge, MA.
- Glatt S, Baeten D, Baker T, et al. Dual IL-17A and IL-17F neutralisation by bimekizumab in psoriatic arthritis: evidence from preclinical experiments and a randomised placebo-controlled clinical trial that IL-17F contributes to human chronic tissue inflammation. Ann Rheum Dis. 2018;77:523-532.
- Adams R, Maroof A, Baker T, et al. Bimekizumab, a novel humanized IgG1 antibody that neutralizes both IL-17A and IL-17F. Front Immunol. 2020;11:1894.
- Gordon KB, Foley P, Krueger JG, et al. Bimekizumab efficacy and safety in moderate to severe plaque psoriasis (BE READY): a multicentre, double-blind, placebo-controlled, randomised withdrawal phase 3 trial. Lancet. 2021;397:475-486.
- Reich K, Papp KA, Blauvelt A, et al. Bimekizumab versus ustekinumab for the treatment of moderate to severe plaque psoriasis (BE VIVID): efficacy and safety from a 52-week, multicentre, double-blind, active comparator and placebo controlled phase 3 trial. Lancet. 2021;397:487-498.
- Warren RB, Blauvelt A, Bagel J, et al. Bimekizumab versus adalimumab in plaque psoriasis. N Engl J Med. 2021;385:130-141.
- Reich K, Warren RB, Lebwohl M, et al. Bimekizumab versus secukinumab in plaque psoriasis. N Engl J Med. 2021;385:142-152.
- Blauvelt A, Lebwohl MG, Bissonnette R. IL-23/IL-17A dysfunction phenotypes inform possible clinical effects from anti-IL-17A therapies. J Invest Dermatol. 2015;135:1946-1953.
- Marrakchi S, Guigue P, Renshaw BR, et al. Interleukin-36-receptor antagonist deficiency and generalized pustular psoriasis. N Engl J Med. 2011;365:620-628.
- Bachelez H, Choon SE, Marrakchi S, et al. Trial of spesolimab for generalized pustular psoriasis. N Engl J Med. 2021;385:2431-2440.
- Bissonnette R, Stein Gold L, Rubenstein DS, et al. Tapinarof in the treatment of psoriasis: a review of the unique mechanism of action of a novel therapeutic aryl hydrocarbon receptor-modulating agent. J Am Acad Dermatol. 2021;84:1059-1067.
- Smith SH, Jayawickreme C, Rickard DJ, et al. Tapinarof is a natural AhR agonist that resolves skin inflammation in mice and humans. J Invest Dermatol. 2017;137:2110-2119.
- Lebwohl MG, Stein Gold L, Strober B, et al. Phase 3 trials of tapinarof cream for plaque psoriasis. N Engl J Med. 2021;385:2219-2229.
- Strober B, Stein Gold L, Bissonnette R, et al. One-year safety and efficacy of tapinarof cream for the treatment of plaque psoriasis: results from the PSOARING 3 trial. J Am Acad Dermatol. 2022;87:800-806.
- Card GL, England BP, Suzuki Y, et al. Structural basis for the activity of drugs that inhibit phosphodiesterases. Structure. 2004;12:2233-2247.
- Milakovic M, Gooderham MJ. Phosphodiesterase-4 inhibition in psoriasis. Psoriasis (Auckl). 2021;11:21-29.
- Papp K, Reich K, Leonardi CL, et al. Apremilast, an oral phosphodiesterase 4 (PDE4) inhibitor, in patients with moderate to severe plaque psoriasis: results of a phase III, randomized, controlled trial (Efficacy and Safety Trial Evaluating the Effects of Apremilast in Psoriasis [ESTEEM] 1). J Am Acad Dermatol. 2015;73:37-49.
- Dong C, Virtucio C, Zemska O, et al. Treatment of skin inflammation with benzoxaborole phosphodiesterase inhibitors: selectivity, cellular activity, and effect on cytokines associated with skin inflammation and skin architecture changes. J Pharmacol Exp Ther. 2016;358:413-422.
- Lebwohl MG, Kircik LH, Moore AY, et al. Effect of roflumilast cream vs vehicle cream on chronic plaque psoriasis: the DERMIS-1 and DERMIS-2 randomized clinical trials. JAMA. 2022;328:1073-1084.
- Nogueira M, Puig L, Torres T. JAK inhibitors for treatment of psoriasis: focus on selective tyk2 inhibitors. Drugs. 2020;80:341-352.
- Wrobleski ST, Moslin R, Lin S, et al. Highly selective inhibition of tyrosine kinase 2 (TYK2) for the treatment of autoimmune diseases: discovery of the allosteric inhibitor BMS-986165. J Med Chem. 2019;62:8973-8995.
- Chimalakonda A, Burke J, Cheng L, et al. Selectivity profile of the tyrosine kinase 2 inhibitor deucravacitinib compared with janus kinase 1/2/3 inhibitors. Dermatol Ther (Heidelb). 2021;11:1763-1776.
- Strober B, Thaçi D, Sofen H, et al. Deucravacitinib versus placebo and apremilast in moderate to severe plaque psoriasis: efficacy and safety results from the 52-week, randomized, double-blinded, phase 3 Program for Evaluation of TYK2 inhibitor psoriasis second trial. J Am Acad Dermatol. 2023;88:40-51.
- Armstrong AW, Gooderham M, Warren RB, et al. Deucravacitinib versus placebo and apremilast in moderate to severe plaque psoriasis: efficacy and safety results from the 52-week, randomized, double-blinded, placebo-controlled phase 3 POETYK PSO-1 trial. J Am Acad Dermatol. 2023;88:29-39.
- Warren RB, Sofen H, Imafuku S, et al. POS1046 deucravacitinib long-term efficacy and safety in plaque psoriasis: 2-year results from the phase 3 POETYK PSO program [abstract]. Ann Rheum Dis. 2022;81(suppl 1):841.
- McElwee JJ, Garcet S, Li X, et al. Analysis of histologic, molecular and clinical improvement in moderate-to-severe psoriasis: results from a Phase 1b trial of the novel allosteric TYK2 inhibitor NDI-034858. Poster presented at: American Academy of Dermatology Annual Meeting; March 25, 2022; Boston, MA.
- Tehlirian C, Singh RSP, Pradhan V, et al. Oral tyrosine kinase 2 inhibitor PF-06826647 demonstrates efficacy and an acceptable safety profile in participants with moderate-to-severe plaque psoriasis in a phase 2b, randomized, double-blind, placebo-controlled study. J Am Acad Dermatol. 2022;87:333-342.
- Hilliard-Barth K, Cormack T, Ramani K, et al. Immune mechanisms of the systemic effects of EDP1815: an orally delivered, gut-restricted microbial drug candidate for the treatment of inflammatory diseases. Poster presented at: Society for Mucosal Immunology Virtual Congress; July 20-22, 2021, Cambridge, MA.
- Glatt S, Baeten D, Baker T, et al. Dual IL-17A and IL-17F neutralisation by bimekizumab in psoriatic arthritis: evidence from preclinical experiments and a randomised placebo-controlled clinical trial that IL-17F contributes to human chronic tissue inflammation. Ann Rheum Dis. 2018;77:523-532.
- Adams R, Maroof A, Baker T, et al. Bimekizumab, a novel humanized IgG1 antibody that neutralizes both IL-17A and IL-17F. Front Immunol. 2020;11:1894.
- Gordon KB, Foley P, Krueger JG, et al. Bimekizumab efficacy and safety in moderate to severe plaque psoriasis (BE READY): a multicentre, double-blind, placebo-controlled, randomised withdrawal phase 3 trial. Lancet. 2021;397:475-486.
- Reich K, Papp KA, Blauvelt A, et al. Bimekizumab versus ustekinumab for the treatment of moderate to severe plaque psoriasis (BE VIVID): efficacy and safety from a 52-week, multicentre, double-blind, active comparator and placebo controlled phase 3 trial. Lancet. 2021;397:487-498.
- Warren RB, Blauvelt A, Bagel J, et al. Bimekizumab versus adalimumab in plaque psoriasis. N Engl J Med. 2021;385:130-141.
- Reich K, Warren RB, Lebwohl M, et al. Bimekizumab versus secukinumab in plaque psoriasis. N Engl J Med. 2021;385:142-152.
- Blauvelt A, Lebwohl MG, Bissonnette R. IL-23/IL-17A dysfunction phenotypes inform possible clinical effects from anti-IL-17A therapies. J Invest Dermatol. 2015;135:1946-1953.
- Marrakchi S, Guigue P, Renshaw BR, et al. Interleukin-36-receptor antagonist deficiency and generalized pustular psoriasis. N Engl J Med. 2011;365:620-628.
- Bachelez H, Choon SE, Marrakchi S, et al. Trial of spesolimab for generalized pustular psoriasis. N Engl J Med. 2021;385:2431-2440.
PRACTICE POINTS
- Roflumilast, a phosphodiesterase 4 inhibitor, and tapinarof, an aryl hydrocarbon receptor–modulating agent, are 2 novel nonsteroidal topical treatments safe for regular long-term use on all affected areas of the skin in adult patients with plaque psoriasis.
- Deucravacitinib is an oral selective tyrosine kinase 2 allosteric inhibitor that has demonstrated a favorable safety profile and greater levels of efficacy than other available oral medications for plaque psoriasis.
- The dual inhibition of IL-17A and IL-17F with bimekizumab provides faster responses and greater clinical benefits for patients with moderate to severe plaque psoriasis than inhibition of IL-17A alone, achieving higher levels of efficacy than has been reported with any other biologic therapy.
- Spesolimab, an IL-36 receptor inhibitor, is an effective, US Food and Drug Administration–approved treatment for patients with generalized pustular psoriasis.
Insights From the 2020-2021 Dermatology Residency Match
To the Editor:
Data from the program director survey of the National Resident Matching Program offer key insights into the 2021 dermatology application process.1,2 Examination of data from the 2020 (N=12) and 2021 (N=17) program director survey regarding interviewing applicants revealed that specialty-specific letters of recommendation (LORs), personal prior knowledge of an applicant, and personal statement increased in importance by 17%, 7.4%, and 17%, respectively, whereas away rotations within the department decreased in importance by 44.9% (Table).1,2 Interestingly, for ranking applicants, programs decreased their emphasis on specialty-specific LORs by 25.8% and away rotations within the department by 22.7% and increased emphasis on personal statements by 14.7% and personal prior knowledge of an applicant by 0.8% from 2020 to 2021 (Table).1,2 These findings align with the prior recommendation to limit away rotations; data are contradictory—when comparing factors for interviewing as compared to ranking applicants—for specialty-specific LORs.
We further compared data from the otolaryngology cycle, which implemented preference signaling by which an applicant can signal their interest in a particular residency program in the 2021 Match, to data from dermatology with no preference signaling. A 90% probability of matching is estimated to require approximately 8 or 9 interviews for dermatology or 12 interviews for otolaryngology for MD senior students in 2020.4 In prior dermatology application cycles, the most highly qualified candidates constituted 7% to 21% of all applicants but were estimated to receive half of all interviews, causing a maldistribution of interviews.5,6
For the 2021 otolaryngology match, the Society of University Otolaryngologists implemented a novel preference signaling system that allowed candidates to show interest in programs by sending 5 preferences, or tokens.7 Recent data reports from the otolaryngology cycle demonstrated at least a 2-fold increase in the rate of receiving an interview invitation for signaled programs compared to the closest nonsignaled program if applicants were provided an additional token.7 Regarding overall applicant competitiveness (ie, dividing participants into quartiles based on their competitiveness), the highest increase in the overall rate of interview invitations (3.5 [total invitations/total applications]) was demonstrated for fourth-quartile (ie, “lowest quartile”) applicants compared with the increase in the overall rate of interview invitations seen in other quartiles (first quartile, an increase of 2.3; second quartile, an increase of 2.6; and third quartile, an increase of 2.4).7 We look forward to seeing the impact of preference signaling on the results of the 2022 dermatology cycle.
Despite changes in the interviewing process to accommodate COVID-19 pandemic safety recommendations, the overall dermatology postgraduate year (PGY) 2 fill rate remained unchanged from 2018 (98.6%) to 2021 (98.7%). Zero PGY-1 positions and 5 PGY-2 positions were unfilled in the 2021 Main Residency Match compared to 1 unfilled PGY-1 position and 4 unfilled PGY-2 positions in 2018.8 The coordinated interview invitation release, holistic review of applications, increased number of rankings, and virtual interviews might have helped offset potential obstacles imparted by inability to complete away rotations, inability to obtain LORs, and conducting interviews virtually.5
A limitation of our analysis is the low response rate of program directors to National Resident Matching Program surveys.
These strategies—holistic application review and coordinated interview release—may be considered in future cycles given their convenience and negligible impact on the dermatology match rate. For example, virtual interviews relieve the financial and time burdens of in-person interviews—approximately $10,000 for each US senior applicant—thus potentially allowing for a more equitable matching process.3 Inversely, in-person interviews allow participants to effectively network and form more meaningful connections while obtaining a better understanding of facilities and surrounding locales. As such, the medical community should continue to come to a consensus on the optimal format to host interviews.
- Results of the 2021 NRMP Program Director Survey. National Resident Matching Program. August 2021. Accessed December 6, 2021. https://www.nrmp.org/wp-content/uploads/2021/11/2021-PD-Survey-Report-for-WWW.pdf
- Results of the 2020 NRMP Program Director Survey. National Resident Matching Program. August 2020. Accessed December 6, 2021. https://www.nrmp.org/wp-content/uploads/2022/01/2020-PD-Survey.pdf
- Rojek NW, Shinkai K, Fett N. Dermatology faculty and residents’ perspectives on the dermatology residency application process: a nationwide survey. J Am Acad Dermatol. 2018;79:157-159. doi:10.1016/j.jaad.2018.01.00
- Charting Outcomes in the Match: Senior Students of U.S. MD Medical Schools. National Resident Matching Program. July 2020. Accessed December 6, 2021. https://www.nrmp.org/wp-content/uploads/2021/08/Charting-Outcomes-in-the-Match-2020_MD-Senior_final.pdf
- Thatiparthi A, Martin A, Liu J, et al. Preliminary outcomes of 2020-2021 dermatology residency application cycle and adverse effects of COVID-19. J Am Acad Dermatol. 2021;84:e263-e264. doi:10.1016/j.jaad.2021.03.034
- Hammoud MM, Standiford T, Carmody JB. Potential implications of COVID-19 for the 2020-2021 residency application cycle. JAMA. 2020;324:29-30. doi:10.1001/jama.2020.8911
- Interview offer rate with/without ENTSignaling. Society of University Otolaryngologists. Updated July 19, 2022. Accessed December 12, 2022. https://opdo-hns.org/mpage/signaling-updates
- Results and Data: 2021 Main Residency Match. National Resident Matching Program. May 2021. Accessed December 6, 2021. https://www.nrmp.org/wp-content/uploads/2021/08/MRM-Results_and-Data_2021.pdf
To the Editor:
Data from the program director survey of the National Resident Matching Program offer key insights into the 2021 dermatology application process.1,2 Examination of data from the 2020 (N=12) and 2021 (N=17) program director survey regarding interviewing applicants revealed that specialty-specific letters of recommendation (LORs), personal prior knowledge of an applicant, and personal statement increased in importance by 17%, 7.4%, and 17%, respectively, whereas away rotations within the department decreased in importance by 44.9% (Table).1,2 Interestingly, for ranking applicants, programs decreased their emphasis on specialty-specific LORs by 25.8% and away rotations within the department by 22.7% and increased emphasis on personal statements by 14.7% and personal prior knowledge of an applicant by 0.8% from 2020 to 2021 (Table).1,2 These findings align with the prior recommendation to limit away rotations; data are contradictory—when comparing factors for interviewing as compared to ranking applicants—for specialty-specific LORs.

We further compared data from the otolaryngology cycle, which implemented preference signaling by which an applicant can signal their interest in a particular residency program in the 2021 Match, to data from dermatology with no preference signaling. A 90% probability of matching is estimated to require approximately 8 or 9 interviews for dermatology or 12 interviews for otolaryngology for MD senior students in 2020.4 In prior dermatology application cycles, the most highly qualified candidates constituted 7% to 21% of all applicants but were estimated to receive half of all interviews, causing a maldistribution of interviews.5,6
For the 2021 otolaryngology match, the Society of University Otolaryngologists implemented a novel preference signaling system that allowed candidates to show interest in programs by sending 5 preferences, or tokens.7 Recent data reports from the otolaryngology cycle demonstrated at least a 2-fold increase in the rate of receiving an interview invitation for signaled programs compared to the closest nonsignaled program if applicants were provided an additional token.7 Regarding overall applicant competitiveness (ie, dividing participants into quartiles based on their competitiveness), the highest increase in the overall rate of interview invitations (3.5 [total invitations/total applications]) was demonstrated for fourth-quartile (ie, “lowest quartile”) applicants compared with the increase in the overall rate of interview invitations seen in other quartiles (first quartile, an increase of 2.3; second quartile, an increase of 2.6; and third quartile, an increase of 2.4).7 We look forward to seeing the impact of preference signaling on the results of the 2022 dermatology cycle.
Despite changes in the interviewing process to accommodate COVID-19 pandemic safety recommendations, the overall dermatology postgraduate year (PGY) 2 fill rate remained unchanged from 2018 (98.6%) to 2021 (98.7%). Zero PGY-1 positions and 5 PGY-2 positions were unfilled in the 2021 Main Residency Match compared to 1 unfilled PGY-1 position and 4 unfilled PGY-2 positions in 2018.8 The coordinated interview invitation release, holistic review of applications, increased number of rankings, and virtual interviews might have helped offset potential obstacles imparted by inability to complete away rotations, inability to obtain LORs, and conducting interviews virtually.5
A limitation of our analysis is the low response rate of program directors to National Resident Matching Program surveys.
These strategies—holistic application review and coordinated interview release—may be considered in future cycles given their convenience and negligible impact on the dermatology match rate. For example, virtual interviews relieve the financial and time burdens of in-person interviews—approximately $10,000 for each US senior applicant—thus potentially allowing for a more equitable matching process.3 Inversely, in-person interviews allow participants to effectively network and form more meaningful connections while obtaining a better understanding of facilities and surrounding locales. As such, the medical community should continue to come to a consensus on the optimal format to host interviews.
To the Editor:
Data from the program director survey of the National Resident Matching Program offer key insights into the 2021 dermatology application process.1,2 Examination of data from the 2020 (N=12) and 2021 (N=17) program director survey regarding interviewing applicants revealed that specialty-specific letters of recommendation (LORs), personal prior knowledge of an applicant, and personal statement increased in importance by 17%, 7.4%, and 17%, respectively, whereas away rotations within the department decreased in importance by 44.9% (Table).1,2 Interestingly, for ranking applicants, programs decreased their emphasis on specialty-specific LORs by 25.8% and away rotations within the department by 22.7% and increased emphasis on personal statements by 14.7% and personal prior knowledge of an applicant by 0.8% from 2020 to 2021 (Table).1,2 These findings align with the prior recommendation to limit away rotations; data are contradictory—when comparing factors for interviewing as compared to ranking applicants—for specialty-specific LORs.

We further compared data from the otolaryngology cycle, which implemented preference signaling by which an applicant can signal their interest in a particular residency program in the 2021 Match, to data from dermatology with no preference signaling. A 90% probability of matching is estimated to require approximately 8 or 9 interviews for dermatology or 12 interviews for otolaryngology for MD senior students in 2020.4 In prior dermatology application cycles, the most highly qualified candidates constituted 7% to 21% of all applicants but were estimated to receive half of all interviews, causing a maldistribution of interviews.5,6
For the 2021 otolaryngology match, the Society of University Otolaryngologists implemented a novel preference signaling system that allowed candidates to show interest in programs by sending 5 preferences, or tokens.7 Recent data reports from the otolaryngology cycle demonstrated at least a 2-fold increase in the rate of receiving an interview invitation for signaled programs compared to the closest nonsignaled program if applicants were provided an additional token.7 Regarding overall applicant competitiveness (ie, dividing participants into quartiles based on their competitiveness), the highest increase in the overall rate of interview invitations (3.5 [total invitations/total applications]) was demonstrated for fourth-quartile (ie, “lowest quartile”) applicants compared with the increase in the overall rate of interview invitations seen in other quartiles (first quartile, an increase of 2.3; second quartile, an increase of 2.6; and third quartile, an increase of 2.4).7 We look forward to seeing the impact of preference signaling on the results of the 2022 dermatology cycle.
Despite changes in the interviewing process to accommodate COVID-19 pandemic safety recommendations, the overall dermatology postgraduate year (PGY) 2 fill rate remained unchanged from 2018 (98.6%) to 2021 (98.7%). Zero PGY-1 positions and 5 PGY-2 positions were unfilled in the 2021 Main Residency Match compared to 1 unfilled PGY-1 position and 4 unfilled PGY-2 positions in 2018.8 The coordinated interview invitation release, holistic review of applications, increased number of rankings, and virtual interviews might have helped offset potential obstacles imparted by inability to complete away rotations, inability to obtain LORs, and conducting interviews virtually.5
A limitation of our analysis is the low response rate of program directors to National Resident Matching Program surveys.
These strategies—holistic application review and coordinated interview release—may be considered in future cycles given their convenience and negligible impact on the dermatology match rate. For example, virtual interviews relieve the financial and time burdens of in-person interviews—approximately $10,000 for each US senior applicant—thus potentially allowing for a more equitable matching process.3 Inversely, in-person interviews allow participants to effectively network and form more meaningful connections while obtaining a better understanding of facilities and surrounding locales. As such, the medical community should continue to come to a consensus on the optimal format to host interviews.
- Results of the 2021 NRMP Program Director Survey. National Resident Matching Program. August 2021. Accessed December 6, 2021. https://www.nrmp.org/wp-content/uploads/2021/11/2021-PD-Survey-Report-for-WWW.pdf
- Results of the 2020 NRMP Program Director Survey. National Resident Matching Program. August 2020. Accessed December 6, 2021. https://www.nrmp.org/wp-content/uploads/2022/01/2020-PD-Survey.pdf
- Rojek NW, Shinkai K, Fett N. Dermatology faculty and residents’ perspectives on the dermatology residency application process: a nationwide survey. J Am Acad Dermatol. 2018;79:157-159. doi:10.1016/j.jaad.2018.01.00
- Charting Outcomes in the Match: Senior Students of U.S. MD Medical Schools. National Resident Matching Program. July 2020. Accessed December 6, 2021. https://www.nrmp.org/wp-content/uploads/2021/08/Charting-Outcomes-in-the-Match-2020_MD-Senior_final.pdf
- Thatiparthi A, Martin A, Liu J, et al. Preliminary outcomes of 2020-2021 dermatology residency application cycle and adverse effects of COVID-19. J Am Acad Dermatol. 2021;84:e263-e264. doi:10.1016/j.jaad.2021.03.034
- Hammoud MM, Standiford T, Carmody JB. Potential implications of COVID-19 for the 2020-2021 residency application cycle. JAMA. 2020;324:29-30. doi:10.1001/jama.2020.8911
- Interview offer rate with/without ENTSignaling. Society of University Otolaryngologists. Updated July 19, 2022. Accessed December 12, 2022. https://opdo-hns.org/mpage/signaling-updates
- Results and Data: 2021 Main Residency Match. National Resident Matching Program. May 2021. Accessed December 6, 2021. https://www.nrmp.org/wp-content/uploads/2021/08/MRM-Results_and-Data_2021.pdf
- Results of the 2021 NRMP Program Director Survey. National Resident Matching Program. August 2021. Accessed December 6, 2021. https://www.nrmp.org/wp-content/uploads/2021/11/2021-PD-Survey-Report-for-WWW.pdf
- Results of the 2020 NRMP Program Director Survey. National Resident Matching Program. August 2020. Accessed December 6, 2021. https://www.nrmp.org/wp-content/uploads/2022/01/2020-PD-Survey.pdf
- Rojek NW, Shinkai K, Fett N. Dermatology faculty and residents’ perspectives on the dermatology residency application process: a nationwide survey. J Am Acad Dermatol. 2018;79:157-159. doi:10.1016/j.jaad.2018.01.00
- Charting Outcomes in the Match: Senior Students of U.S. MD Medical Schools. National Resident Matching Program. July 2020. Accessed December 6, 2021. https://www.nrmp.org/wp-content/uploads/2021/08/Charting-Outcomes-in-the-Match-2020_MD-Senior_final.pdf
- Thatiparthi A, Martin A, Liu J, et al. Preliminary outcomes of 2020-2021 dermatology residency application cycle and adverse effects of COVID-19. J Am Acad Dermatol. 2021;84:e263-e264. doi:10.1016/j.jaad.2021.03.034
- Hammoud MM, Standiford T, Carmody JB. Potential implications of COVID-19 for the 2020-2021 residency application cycle. JAMA. 2020;324:29-30. doi:10.1001/jama.2020.8911
- Interview offer rate with/without ENTSignaling. Society of University Otolaryngologists. Updated July 19, 2022. Accessed December 12, 2022. https://opdo-hns.org/mpage/signaling-updates
- Results and Data: 2021 Main Residency Match. National Resident Matching Program. May 2021. Accessed December 6, 2021. https://www.nrmp.org/wp-content/uploads/2021/08/MRM-Results_and-Data_2021.pdf
PRACTICE POINTS
- Although there have been numerous changes to the dermatology interview process due to the COVID-19 pandemic, the overall fill rate for postgraduate year 2 positions remained unchanged from 2018 (prepandemic) to 2021 (postpandemic).
- Strategies to accommodate new safety recommendations for interviews may reduce the financial burden (approximately $10,000 for each senior applicant) and time constraints on applicants. These strategies should be considered for implementation in future cycles.
Medicare Part D Prescription Claims for Brodalumab: Analysis of Annual Trends for 2017-2019
To the Editor:
Brodalumab, a monoclonal antibody targeting IL-17RA, was approved by the US Food and Drug Administration (FDA) in 2017 for the treatment of moderate to severe chronic plaque psoriasis. The drug is the only biologic agent available for the treatment of psoriasis for which a psoriasis area severity index score of 100 is a primary end point.1,2 Brodalumab is associated with an FDA boxed warning due to an increased risk for suicidal ideation and behavior (SIB), including completed suicides, during clinical trials.
We sought to characterize national utilization of this effective yet underutilized drug among Medicare beneficiaries by surveying the Medicare Part D Prescriber dataset.3 We tabulated brodalumab utilization statistics and characteristics of high-volume prescribers who had 11 or more annual claims for brodalumab.
Despite its associated boxed warning, the number of Medicare D claims for brodalumab increased by 1756 from 2017 to 2019, surpassing $7 million in costs by 2019. The number of beneficiaries also increased from 11 to 292—a 415.2% annual increase in beneficiaries for whom brodalumab was prescribed (Table 1).
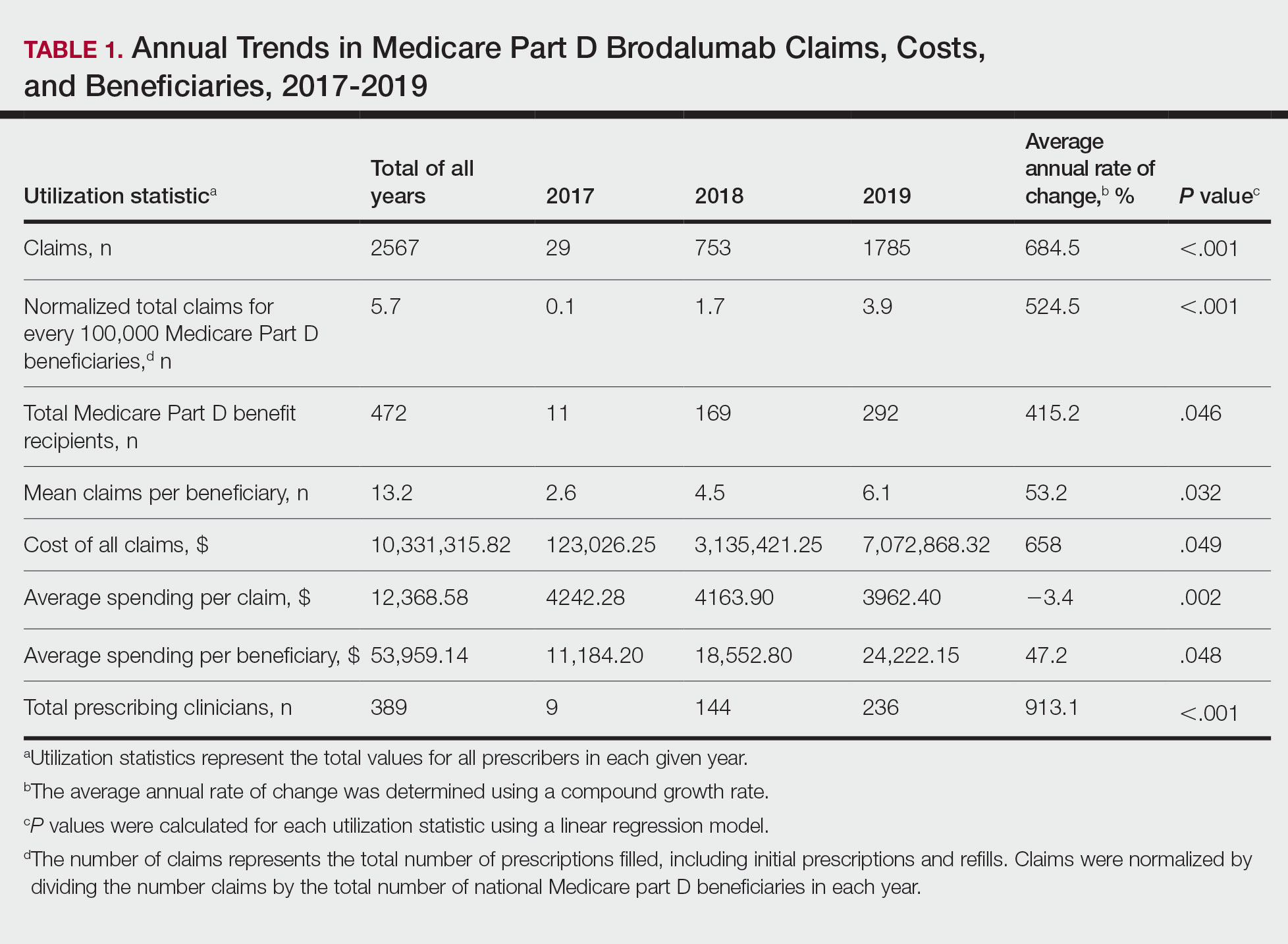
In addition, states in the West and South had the highest utilization rates of brodalumab in 2019. There also was an increasing trend toward high-volume prescribers of brodalumab, with private practice clinicians constituting the majority (Table 2).
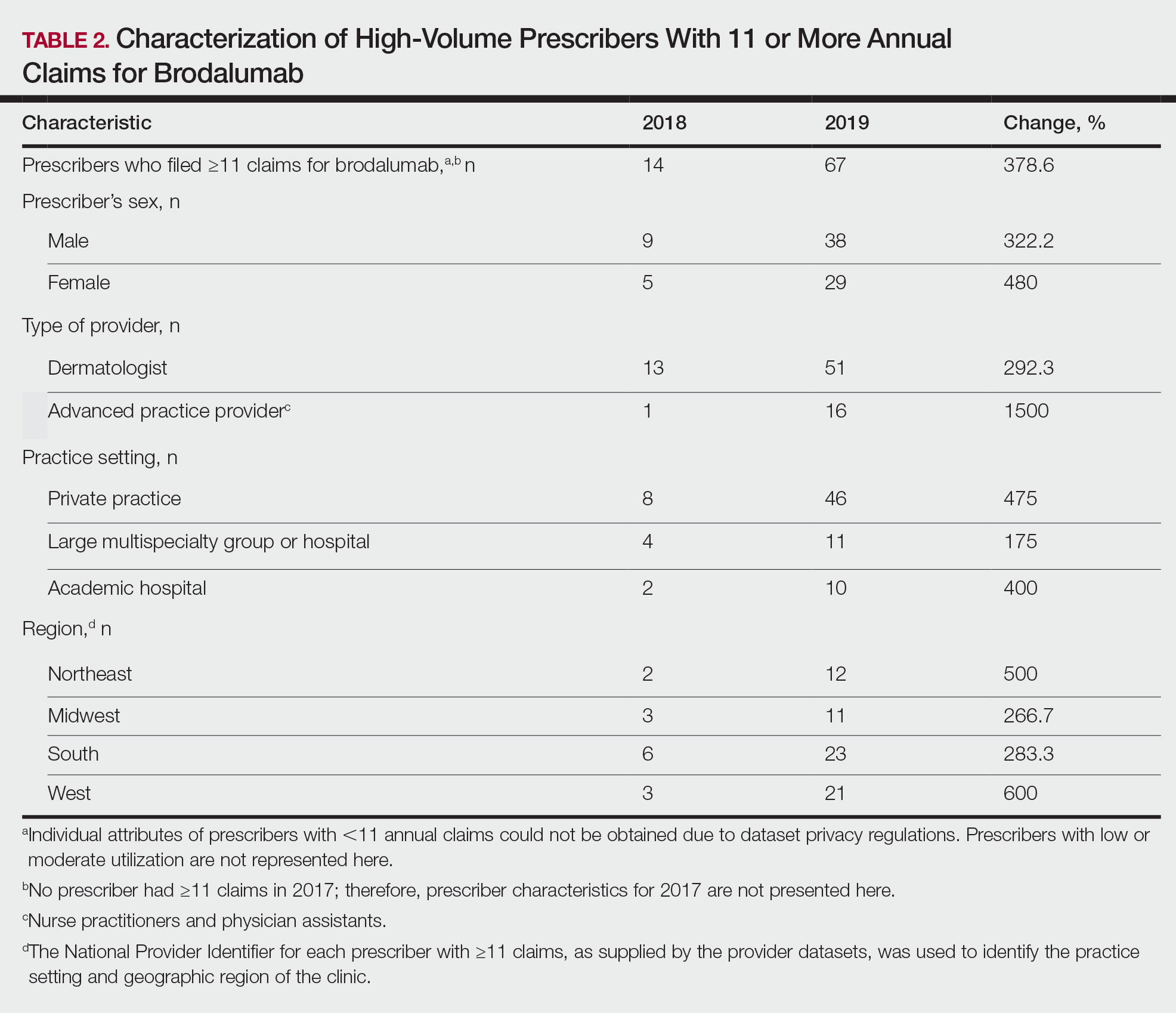
There was a substantial increase in advanced practice providers including nurse practitioners and physician assistants who were brodalumab prescribers. Although this trend might promote greater access to brodalumab, it is vital to ensure that advanced practice providers receive targeted training to properly understand the complexities of treatment with brodalumab.
Although the utilization of brodalumab has increased since 2017 (P<.001), it is still underutilized compared to the other IL-17 inhibitors secukinumab and ixekizumab. Secukinumab was FDA approved for the treatment of moderate to severe plaque psoriasis in 2015, followed by ixekizumab in 2016.4
According to the Medicare Part D database, both secukinumab and ixekizumab had a higher number of total claims and prescribers compared to brodalumab in the years of their debut.3 In 2015, there were 3593 claims for and 862 prescribers of secukinumab; in 2016, there were 1731 claims for and 681 prescribers of ixekizumab. In contrast, there were only 29 claims for and 11 prescribers of brodalumab in 2017, the year that the drug was approved by the FDA. During the same 3-year period, secukinumab and ixekizumab had a substantially greater number of claims—totals of 176,823 and 55,289, respectively—than brodalumab. The higher number of claims for secukinumab and ixekizumab compared to brodalumab may reflect clinicians’ increasing confidence in prescribing those drugs, given their long-term safety and efficacy. In addition, secukinumab and ixekizumab do not require completion of a Risk Evaluation and Mitigation Strategy (REMS) program, which makes them more readily prescribable.3
Overall, most experts agree that there is no increase in the risk for suicide associated with brodalumab compared to the general population. A 2-year pharmacovigilance report on brodalumab supports the safety of this drug.5 All participants who completed suicide during the clinical trials harbored an underlying psychiatric disorder or stressor(s).6
Although causation between brodalumab and SIB has not been demonstrated, it remains imperative that prescribers diligently assess patients’ risk of SIB and subsequently their access to appropriate psychiatric services as a precaution, if necessary. This is particularly important for private practice prescribers, who constitute the majority of Medicare D brodalumab claims, because they must ensure collaboration with a multidisciplinary team involving mental health providers. Lastly, considering that the highest number of brodalumab Medicare D claims were in western and southern states, it is critical to note that those 2 regions also harbor comparatively fewer mental health facilities that accept Medicare than other regions of the country.7 Prescribers in western and southern states must be mindful of mental health coverage limitations when treating psoriasis patients with brodalumab.
The increase in the number of claims, beneficiaries, and prescribers of brodalumab during its first 3 years of availability might be attributed to its efficacy and safety. On the other hand, the boxed warning and REMS associated with brodalumab might have led to underutilization of this drug compared to other IL-17 inhibitors.
Our analysis is limited by its representative restriction to Medicare patients. There also are limited data on brodalumab given its novelty. Individual attributes of prescribers with fewer than 11 annual claims for brodalumab could not be obtained because of dataset regulations; however, aggregated utilization statistics provide an indication of brodalumab prescribing patterns among all providers. Furthermore, during this analysis, data on the Medicare D database were limited to 2013 through 2020. Studies are needed to determine prescribing patterns of brodalumab since this study period.
- Foulkes AC, Warren RB. Brodalumab in psoriasis: evidence to date and clinical potential. Drugs Context. 2019;8:212570. doi:10.7573/dic.212570
- Beck KM, Koo J. Brodalumab for the treatment of plaque psoriasis: up-to-date. Expert Opin Biol Ther. 2019;19:287-292. doi:10.1080/14712598.2019.1579794
- Centers for Medicare & Medicaid Services. Medicare Part D Prescribers. Updated July 27, 2022. Accessed September 23, 2022. https://data.cms.gov/provider-summary-by-type-of-service/medicare-part-d-prescribers/medicare-part-d-prescribers-by-provider
- Drugs. US Food and Drug Administration website. Accessed September 23, 2022. https://www.fda.gov/drugs
- Lebwohl M, Leonardi C, Wu JJ, et al. Two-year US pharmacovigilance report on brodalumab. Dermatol Ther (Heidelb). 2021;11:173-180. doi:10.1007/s13555-020-00472-x
- Lebwohl MG, Papp KA, Marangell LB, et al. Psychiatric adverse events during treatment with brodalumab: analysis of psoriasis clinical trials. J Am Acad Dermatol. 2018;78:81-89.e5. doi:10.1016/j.jaad.2017.08.024
- Substance Abuse and Mental Health Services Administration. National Mental Health Services Survey (N-MHSS): 2019, Data On Mental Health Treatment Facilities. Rockville, MD: Substance Abuse and Mental Health Services Administration; August 13, 2020. Accessed September 21, 2022. https://www.samhsa.gov/data/report/national-mental-health-services-survey-n-mhss-2019-data-mental-health-treatment-facilities
To the Editor:
Brodalumab, a monoclonal antibody targeting IL-17RA, was approved by the US Food and Drug Administration (FDA) in 2017 for the treatment of moderate to severe chronic plaque psoriasis. The drug is the only biologic agent available for the treatment of psoriasis for which a psoriasis area severity index score of 100 is a primary end point.1,2 Brodalumab is associated with an FDA boxed warning due to an increased risk for suicidal ideation and behavior (SIB), including completed suicides, during clinical trials.
We sought to characterize national utilization of this effective yet underutilized drug among Medicare beneficiaries by surveying the Medicare Part D Prescriber dataset.3 We tabulated brodalumab utilization statistics and characteristics of high-volume prescribers who had 11 or more annual claims for brodalumab.
Despite its associated boxed warning, the number of Medicare D claims for brodalumab increased by 1756 from 2017 to 2019, surpassing $7 million in costs by 2019. The number of beneficiaries also increased from 11 to 292—a 415.2% annual increase in beneficiaries for whom brodalumab was prescribed (Table 1).

In addition, states in the West and South had the highest utilization rates of brodalumab in 2019. There also was an increasing trend toward high-volume prescribers of brodalumab, with private practice clinicians constituting the majority (Table 2).

There was a substantial increase in advanced practice providers including nurse practitioners and physician assistants who were brodalumab prescribers. Although this trend might promote greater access to brodalumab, it is vital to ensure that advanced practice providers receive targeted training to properly understand the complexities of treatment with brodalumab.
Although the utilization of brodalumab has increased since 2017 (P<.001), it is still underutilized compared to the other IL-17 inhibitors secukinumab and ixekizumab. Secukinumab was FDA approved for the treatment of moderate to severe plaque psoriasis in 2015, followed by ixekizumab in 2016.4
According to the Medicare Part D database, both secukinumab and ixekizumab had a higher number of total claims and prescribers compared to brodalumab in the years of their debut.3 In 2015, there were 3593 claims for and 862 prescribers of secukinumab; in 2016, there were 1731 claims for and 681 prescribers of ixekizumab. In contrast, there were only 29 claims for and 11 prescribers of brodalumab in 2017, the year that the drug was approved by the FDA. During the same 3-year period, secukinumab and ixekizumab had a substantially greater number of claims—totals of 176,823 and 55,289, respectively—than brodalumab. The higher number of claims for secukinumab and ixekizumab compared to brodalumab may reflect clinicians’ increasing confidence in prescribing those drugs, given their long-term safety and efficacy. In addition, secukinumab and ixekizumab do not require completion of a Risk Evaluation and Mitigation Strategy (REMS) program, which makes them more readily prescribable.3
Overall, most experts agree that there is no increase in the risk for suicide associated with brodalumab compared to the general population. A 2-year pharmacovigilance report on brodalumab supports the safety of this drug.5 All participants who completed suicide during the clinical trials harbored an underlying psychiatric disorder or stressor(s).6
Although causation between brodalumab and SIB has not been demonstrated, it remains imperative that prescribers diligently assess patients’ risk of SIB and subsequently their access to appropriate psychiatric services as a precaution, if necessary. This is particularly important for private practice prescribers, who constitute the majority of Medicare D brodalumab claims, because they must ensure collaboration with a multidisciplinary team involving mental health providers. Lastly, considering that the highest number of brodalumab Medicare D claims were in western and southern states, it is critical to note that those 2 regions also harbor comparatively fewer mental health facilities that accept Medicare than other regions of the country.7 Prescribers in western and southern states must be mindful of mental health coverage limitations when treating psoriasis patients with brodalumab.
The increase in the number of claims, beneficiaries, and prescribers of brodalumab during its first 3 years of availability might be attributed to its efficacy and safety. On the other hand, the boxed warning and REMS associated with brodalumab might have led to underutilization of this drug compared to other IL-17 inhibitors.
Our analysis is limited by its representative restriction to Medicare patients. There also are limited data on brodalumab given its novelty. Individual attributes of prescribers with fewer than 11 annual claims for brodalumab could not be obtained because of dataset regulations; however, aggregated utilization statistics provide an indication of brodalumab prescribing patterns among all providers. Furthermore, during this analysis, data on the Medicare D database were limited to 2013 through 2020. Studies are needed to determine prescribing patterns of brodalumab since this study period.
To the Editor:
Brodalumab, a monoclonal antibody targeting IL-17RA, was approved by the US Food and Drug Administration (FDA) in 2017 for the treatment of moderate to severe chronic plaque psoriasis. The drug is the only biologic agent available for the treatment of psoriasis for which a psoriasis area severity index score of 100 is a primary end point.1,2 Brodalumab is associated with an FDA boxed warning due to an increased risk for suicidal ideation and behavior (SIB), including completed suicides, during clinical trials.
We sought to characterize national utilization of this effective yet underutilized drug among Medicare beneficiaries by surveying the Medicare Part D Prescriber dataset.3 We tabulated brodalumab utilization statistics and characteristics of high-volume prescribers who had 11 or more annual claims for brodalumab.
Despite its associated boxed warning, the number of Medicare D claims for brodalumab increased by 1756 from 2017 to 2019, surpassing $7 million in costs by 2019. The number of beneficiaries also increased from 11 to 292—a 415.2% annual increase in beneficiaries for whom brodalumab was prescribed (Table 1).

In addition, states in the West and South had the highest utilization rates of brodalumab in 2019. There also was an increasing trend toward high-volume prescribers of brodalumab, with private practice clinicians constituting the majority (Table 2).

There was a substantial increase in advanced practice providers including nurse practitioners and physician assistants who were brodalumab prescribers. Although this trend might promote greater access to brodalumab, it is vital to ensure that advanced practice providers receive targeted training to properly understand the complexities of treatment with brodalumab.
Although the utilization of brodalumab has increased since 2017 (P<.001), it is still underutilized compared to the other IL-17 inhibitors secukinumab and ixekizumab. Secukinumab was FDA approved for the treatment of moderate to severe plaque psoriasis in 2015, followed by ixekizumab in 2016.4
According to the Medicare Part D database, both secukinumab and ixekizumab had a higher number of total claims and prescribers compared to brodalumab in the years of their debut.3 In 2015, there were 3593 claims for and 862 prescribers of secukinumab; in 2016, there were 1731 claims for and 681 prescribers of ixekizumab. In contrast, there were only 29 claims for and 11 prescribers of brodalumab in 2017, the year that the drug was approved by the FDA. During the same 3-year period, secukinumab and ixekizumab had a substantially greater number of claims—totals of 176,823 and 55,289, respectively—than brodalumab. The higher number of claims for secukinumab and ixekizumab compared to brodalumab may reflect clinicians’ increasing confidence in prescribing those drugs, given their long-term safety and efficacy. In addition, secukinumab and ixekizumab do not require completion of a Risk Evaluation and Mitigation Strategy (REMS) program, which makes them more readily prescribable.3
Overall, most experts agree that there is no increase in the risk for suicide associated with brodalumab compared to the general population. A 2-year pharmacovigilance report on brodalumab supports the safety of this drug.5 All participants who completed suicide during the clinical trials harbored an underlying psychiatric disorder or stressor(s).6
Although causation between brodalumab and SIB has not been demonstrated, it remains imperative that prescribers diligently assess patients’ risk of SIB and subsequently their access to appropriate psychiatric services as a precaution, if necessary. This is particularly important for private practice prescribers, who constitute the majority of Medicare D brodalumab claims, because they must ensure collaboration with a multidisciplinary team involving mental health providers. Lastly, considering that the highest number of brodalumab Medicare D claims were in western and southern states, it is critical to note that those 2 regions also harbor comparatively fewer mental health facilities that accept Medicare than other regions of the country.7 Prescribers in western and southern states must be mindful of mental health coverage limitations when treating psoriasis patients with brodalumab.
The increase in the number of claims, beneficiaries, and prescribers of brodalumab during its first 3 years of availability might be attributed to its efficacy and safety. On the other hand, the boxed warning and REMS associated with brodalumab might have led to underutilization of this drug compared to other IL-17 inhibitors.
Our analysis is limited by its representative restriction to Medicare patients. There also are limited data on brodalumab given its novelty. Individual attributes of prescribers with fewer than 11 annual claims for brodalumab could not be obtained because of dataset regulations; however, aggregated utilization statistics provide an indication of brodalumab prescribing patterns among all providers. Furthermore, during this analysis, data on the Medicare D database were limited to 2013 through 2020. Studies are needed to determine prescribing patterns of brodalumab since this study period.
- Foulkes AC, Warren RB. Brodalumab in psoriasis: evidence to date and clinical potential. Drugs Context. 2019;8:212570. doi:10.7573/dic.212570
- Beck KM, Koo J. Brodalumab for the treatment of plaque psoriasis: up-to-date. Expert Opin Biol Ther. 2019;19:287-292. doi:10.1080/14712598.2019.1579794
- Centers for Medicare & Medicaid Services. Medicare Part D Prescribers. Updated July 27, 2022. Accessed September 23, 2022. https://data.cms.gov/provider-summary-by-type-of-service/medicare-part-d-prescribers/medicare-part-d-prescribers-by-provider
- Drugs. US Food and Drug Administration website. Accessed September 23, 2022. https://www.fda.gov/drugs
- Lebwohl M, Leonardi C, Wu JJ, et al. Two-year US pharmacovigilance report on brodalumab. Dermatol Ther (Heidelb). 2021;11:173-180. doi:10.1007/s13555-020-00472-x
- Lebwohl MG, Papp KA, Marangell LB, et al. Psychiatric adverse events during treatment with brodalumab: analysis of psoriasis clinical trials. J Am Acad Dermatol. 2018;78:81-89.e5. doi:10.1016/j.jaad.2017.08.024
- Substance Abuse and Mental Health Services Administration. National Mental Health Services Survey (N-MHSS): 2019, Data On Mental Health Treatment Facilities. Rockville, MD: Substance Abuse and Mental Health Services Administration; August 13, 2020. Accessed September 21, 2022. https://www.samhsa.gov/data/report/national-mental-health-services-survey-n-mhss-2019-data-mental-health-treatment-facilities
- Foulkes AC, Warren RB. Brodalumab in psoriasis: evidence to date and clinical potential. Drugs Context. 2019;8:212570. doi:10.7573/dic.212570
- Beck KM, Koo J. Brodalumab for the treatment of plaque psoriasis: up-to-date. Expert Opin Biol Ther. 2019;19:287-292. doi:10.1080/14712598.2019.1579794
- Centers for Medicare & Medicaid Services. Medicare Part D Prescribers. Updated July 27, 2022. Accessed September 23, 2022. https://data.cms.gov/provider-summary-by-type-of-service/medicare-part-d-prescribers/medicare-part-d-prescribers-by-provider
- Drugs. US Food and Drug Administration website. Accessed September 23, 2022. https://www.fda.gov/drugs
- Lebwohl M, Leonardi C, Wu JJ, et al. Two-year US pharmacovigilance report on brodalumab. Dermatol Ther (Heidelb). 2021;11:173-180. doi:10.1007/s13555-020-00472-x
- Lebwohl MG, Papp KA, Marangell LB, et al. Psychiatric adverse events during treatment with brodalumab: analysis of psoriasis clinical trials. J Am Acad Dermatol. 2018;78:81-89.e5. doi:10.1016/j.jaad.2017.08.024
- Substance Abuse and Mental Health Services Administration. National Mental Health Services Survey (N-MHSS): 2019, Data On Mental Health Treatment Facilities. Rockville, MD: Substance Abuse and Mental Health Services Administration; August 13, 2020. Accessed September 21, 2022. https://www.samhsa.gov/data/report/national-mental-health-services-survey-n-mhss-2019-data-mental-health-treatment-facilities
Practice Points
- Brodalumab is associated with a boxed warning due to increased suicidal ideation and behavior (SIB), including completed suicides, during clinical trials.
- Brodalumab is underutilized compared to the other US Food and Drug Administration–approved IL-17 inhibitors used to treat psoriasis.
- Most experts agree that there is no increased risk for suicide associated with brodalumab. However, it remains imperative that prescribers assess patients’ risk of SIB and subsequently their access to appropriate psychiatric services prior to initiating and during treatment with brodalumab.
Generalized Pustular Psoriasis: A Review of the Pathophysiology, Clinical Manifestations, Diagnosis, and Treatment
Acute generalized pustular psoriasis (GPP) is a rare severe variant of psoriasis characterized by the sudden widespread eruption of sterile pustules.1,2 The cutaneous manifestations of GPP also may be accompanied by signs of systemic inflammation, including fever, malaise, and leukocytosis.2 Complications are common and may be life-threatening, especially in older patients with comorbid diseases.3 Generalized pustular psoriasis most commonly occurs in patients with a preceding history of psoriasis, but it also may occur de novo.4 Generalized pustular psoriasis is associated with notable morbidity and mortality, and relapses are common.3,4 Many triggers of GPP have been identified, including initiation and withdrawal of various medications, infections, pregnancy, and other conditions.5,6 Although GPP most often occurs in adults, it also may arise in children and infants.3 In pregnancy, GPP is referred to as impetigo herpetiformis, despite having no etiologic ties with either herpes simplex virus or staphylococcal or streptococcal infection. Impetigo herpetiformis is considered one of the most dangerous dermatoses of pregnancy because of high rates of associated maternal and fetal morbidity.6,7
Acute GPP has proven to be a challenging disease to treat due to the rarity and relapsing-remitting nature of the disease; additionally, there are relatively few randomized controlled trials investigating the efficacy and safety of treatments for GPP. This review summarizes the features of GPP, including the pathophysiology of the disease, clinical and histological manifestations, and recommendations for management based on a PubMed search of articles indexed for MEDLINE using MeSH terms pertaining to the disease, including generalized pustular psoriasis, impetigo herpetiformis, and von Zumbusch psoriasis.
Pathophysiology
The pathophysiology of GPP is only partially understood, but it is thought to have a distinct pattern of immune activation compared with plaque psoriasis.8 Although there is a considerable amount of overlap and cross-talk among cytokine pathways, GPP generally is driven by innate immunity and unrestrained IL-36 cytokine activity. In contrast, adaptive immune responses—namely the tumor necrosis factor (TNF) α, IL-23, IL-17, and IL-22 axes—underlie plaque psoriasis.8-10
Proinflammatory IL-36 cytokines α, β, and γ, which are all part of the IL-1 superfamily, bind to the IL-36 receptor (IL-36R) to recruit and activate immune cells via various mediators, including IL-1β; IL-8; and chemokines CXCL1, CXCL2, and CXCL8.3 The IL-36 receptor antagonist (IL-36ra) acts to inhibit this inflammatory cascade.3,8 Microarray analyses of skin biopsy samples have shown that overexpression of IL-17A, TNF-α, IL-1, and IL-36 are seen in both GPP and plaque psoriasis lesions, but GPP lesions had higher expression of IL-1β, IL-36α, and IL-36γ and elevated neutrophil chemokines—CXCL1, CXCL2, and CXCL8—compared with plaque psoriasis lesions.8
Gene Mutations Associated With GPP
There are 3 gene mutations that have been associated with pustular variants of psoriasis, though these mutations account for a minority of cases of GPP.4 Genetic screenings are not routinely indicated in patients with GPP, but they may be warranted in severe cases when a familial pattern of inheritance is suspected.4
IL36RN—The gene IL36RN codes the anti-inflammatory IL-36ra. Loss-of-function mutations in IL36RN lead to impairment of IL-36ra and consequently hyperactivity of the proinflammatory responses triggered by IL-36.3 Homozygous and heterozygous mutations in IL36RN have been observed in both familial and sporadic cases of GPP.11-13 Subsequent retrospective analyses have identified the presence of IL36RN mutations in patients with GPP with frequencies ranging from 23% to 37%.14-17IL36RN mutations are thought to be more common in patients without concomitant plaque psoriasis and have been associated with severe disease and early disease onset.15
CARD14—A gain-of-function mutation in CARD14 results in overactivation of the proinflammatory nuclear factor κB pathway and has been implicated in cases of GPP with concurrent psoriasis vulgaris. Interestingly, this may suggest distinct etiologies underlying GPP de novo and GPP in patients with a history of psoriasis.18,19
AP1S3—A loss-of-function mutation in AP1S3 results in abnormal endosomal trafficking and autophagy as well as increased expression of IL-36α.20,21
Clinical Presentation and Diagnosis Cutaneous Manifestations of GPP
Generalized pustular psoriasis is characterized by the onset of widespread 2- to 3-mm sterile pustules on erythematous skin or within psoriasiform plaques4 (Figure). In patients with skin of color, the erythema may appear less obvious or perhaps slightly violaceous compared to White skin. Pustules may coalesce to form “lakes” of pus.5 Cutaneous symptoms include pain, burning, and pruritus. Associated mucosal findings may include cheilitis, geographic tongue, conjunctivitis, and uveitis.4
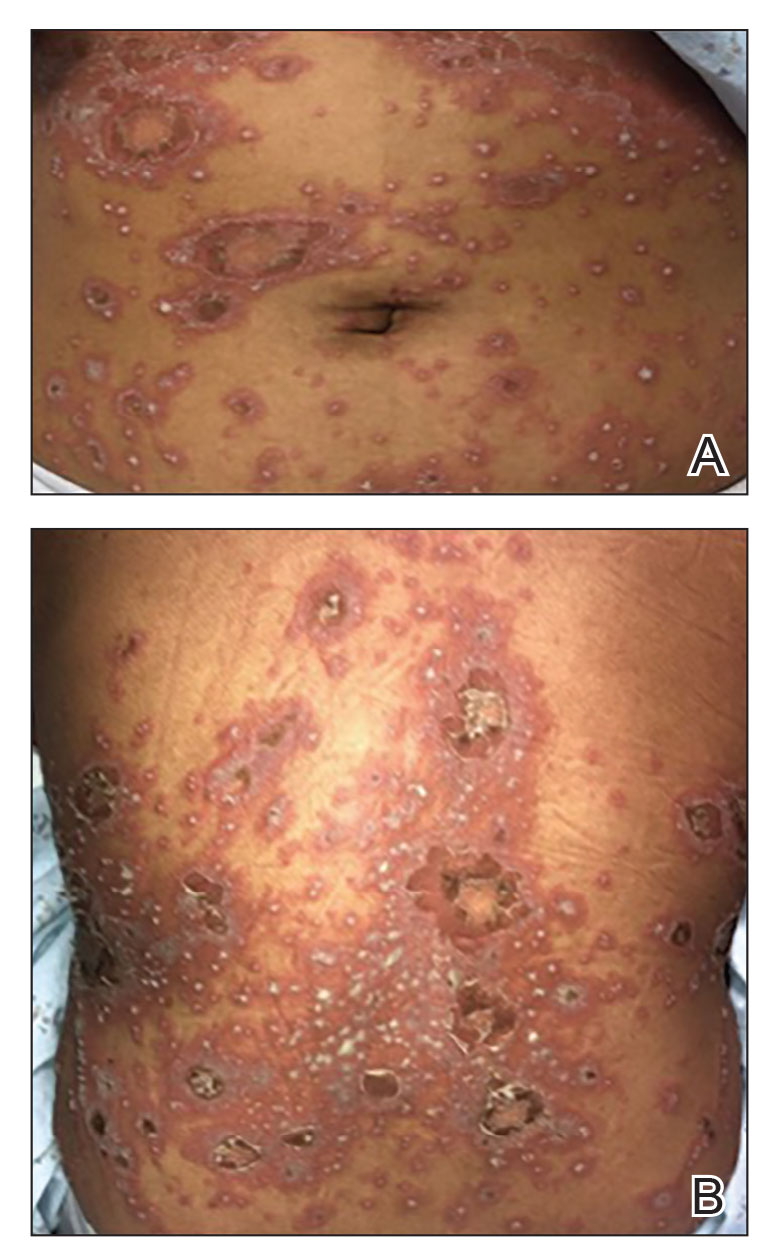
The severity of symptoms can vary greatly among patients as well as between flares within the same patient.2,3 Four distinct patterns of GPP have been described. The von Zumbusch pattern is characterized by a rapid, generalized, painful, erythematous and pustular eruption accompanied by fever and asthenia. The pustules usually resolve after several days with extensive scaling. The annular pattern is characterized by annular, erythematous, scaly lesions with pustules present centrifugally. The lesions enlarge by centrifugal expansion over a period of hours to days, while healing occurs centrally. The exanthematic type is an acute eruption of small pustules that abruptly appear and disappear within a few days, usually from infection or medication initiation. Sometimes pustules appear within or at the edge of existing psoriatic plaques in a localized pattern—the fourth pattern—often following the exposure to irritants (eg, tars, anthralin).5
Impetigo Herpetiformis—Impetigo herpetiformis is a form of GPP associated with pregnancy. It generally presents early in the third trimester with symmetric erythematous plaques in flexural and intertriginous areas with pustules present at lesion margins. Lesions expand centrifugally, with pustulation present at the advancing edge.6,7 Patients often are acutely ill with fever, delirium, vomiting, and tetany. Mucous membranes, including the tongue, mouth, and esophagus, also may be involved. The eruption typically resolves after delivery, though it often recurs with subsequent pregnancies, with the morbidity risk rising with each successive pregnancy.7
Systemic and Extracutaneous Manifestations of GPP
Although the severity of GPP is highly variable, skin manifestations often are accompanied by systemic manifestations of inflammation, including fever and malaise. Common laboratory abnormalities include leukocytosis with peripheral neutrophilia, a high serum C-reactive protein level, hypocalcemia, and hypoalbuminemia.22 Abnormal liver enzymes often are present and result from neutrophilic cholangitis, with alternating strictures and dilations of biliary ducts observed on magnetic resonance imaging.23 Additional laboratory abnormalities are provided in Table 2. Other extracutaneous findings associated with GPP include arthralgia, edema, and characteristic psoriatic nail changes.4 Fatal complications include acute respiratory distress syndrome, renal dysfunction, cardiovascular shock, and sepsis.24,25
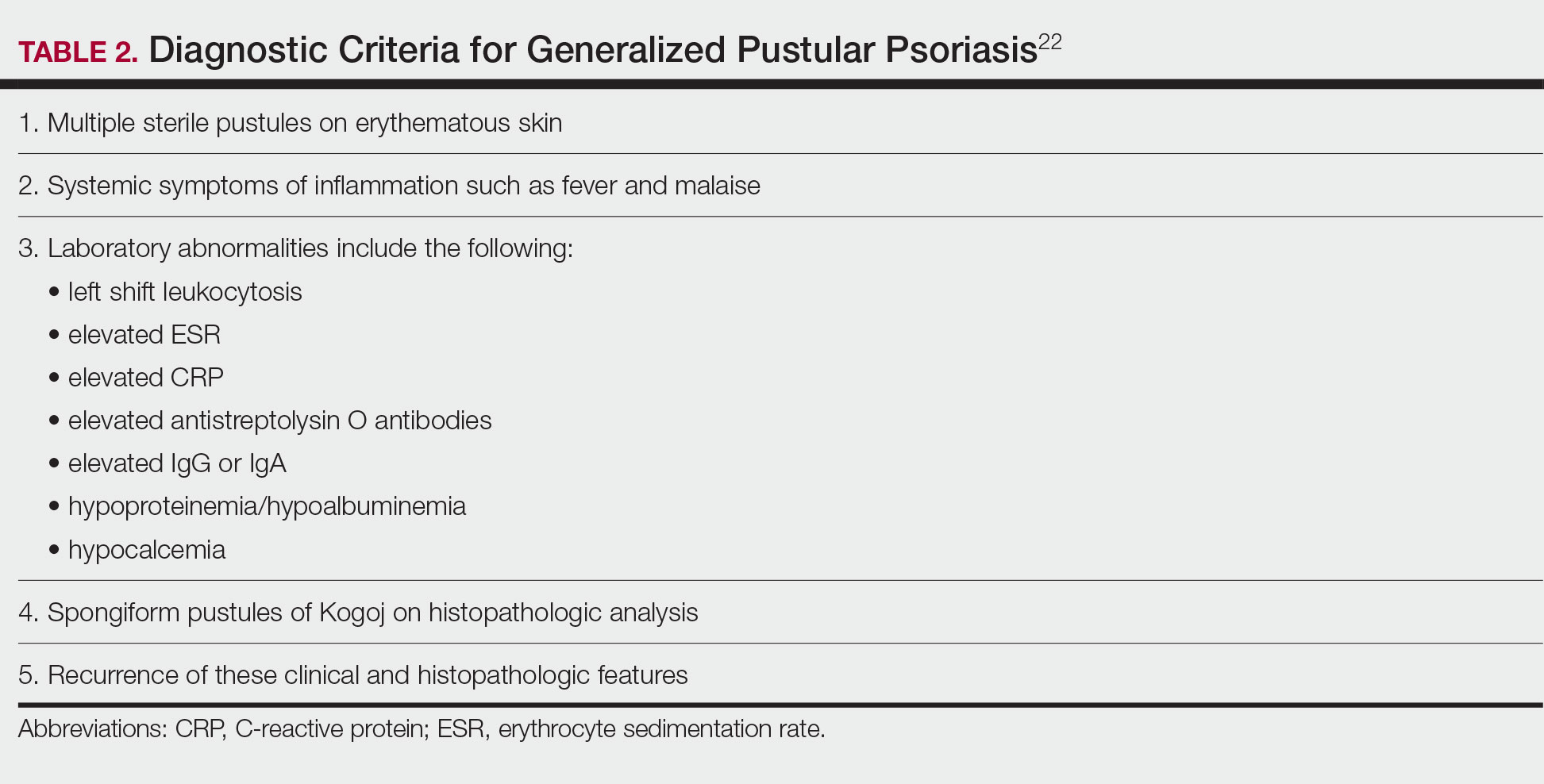
Histologic Features
Given the potential for the skin manifestations of GPP to mimic other disorders, a skin biopsy is warranted to confirm the diagnosis. Generalized pustular psoriasis is histologically characterized by the presence of subcorneal macropustules (ie, spongiform pustules of Kogoj) formed by neutrophil infiltration into the spongelike network of the epidermis.6 Otherwise, the architecture of the epithelium in GPP is similar to that seen with plaque psoriasis, with parakeratosis, acanthosis, rete-ridge elongation, diminished stratum granulosum, and thinning of the suprapapillary epidermis, though the inflammatory cell infiltrate and edema are markedly more severe in GPP than plaque psoriasis.3,4
Differential Diagnosis
There are many other cutaneous pustular diagnoses that must be ruled out when evaluating a patient with GPP (Table 1).26 Acute generalized exanthematous pustulosis (AGEP) is a common mimicker of GPP that is differentiated histologically by the presence of eosinophils and necrotic keratinocytes.4 In addition to its distinct histopathologic findings, AGEP is classically associated with recent initiation of certain medications, most commonly penicillins, macrolides, quinolones, sulfonamides, terbinafine, and diltiazem.27 In contrast, GPP more commonly is related to withdrawal of corticosteroids as well as initiation of some biologic medications, including anti-TNF agents.3 Generalized pustular psoriasis should be suspected over AGEP in patients with a personal or family history of psoriasis, though GPP may arise in patients with or without a history of psoriasis. Acute generalized exanthematous pustulosis usually is more abrupt in both onset and resolution compared with GPP, with clearance of pustules within a few days to weeks following cessation of the triggering factor.4
Other pustular variants of psoriasis (eg, palmoplantar pustular psoriasis, acrodermatitis continua of Hallopeau) are differentiated from GPP by their chronicity and localization to palmoplantar and/or ungual surfaces.5 Other differential diagnoses are listed in Table 1.
Diagnostic Criteria for GPP
Diagnostic criteria have been proposed for GPP (Table 2), including (1) the presence of sterile pustules, (2) systemic signs of inflammation, (3) laboratory abnormalities, (4) histopathologic confirmation of spongiform pustules of Kogoj, and (5) recurrence of symptoms.22 To definitively diagnose GPP, all 5 criteria must be met. To rule out mimickers, it may be worthwhile to perform Gram staining, potassium hydroxide preparation, in vitro cultures, and/or immunofluorescence testing.6
Treatment
Given the high potential for mortality associated with GPP, the most essential component of management is to ensure adequate supportive care. Any temperature, fluid, or electrolyte imbalances should be corrected as they arise. Secondary infections also must be identified and treated, if present, to reduce the risk for fatal complications, including systemic infection and sepsis. Precautions must be taken to ensure that serious end-organ damage, including hepatic, renal, and respiratory dysfunction, is avoided.
Adjunctive topical intervention often is initiated with bland emollients, corticosteroids, calcineurin inhibitors, and/or vitamin D derivatives to help soothe skin symptoms, but treatment with systemic therapies usually is warranted to achieve symptom control.2,25 Importantly, there are no systemic or topical agents that have specifically been approved for the treatment of GPP in Europe or the United States.3 Given the absence of universally accepted treatment guidelines, therapeutic agents for GPP usually are selected based on clinical experience while also taking the extent of involvement and disease severity into consideration.3
Treatment Recommendations for Adults
Oral Systemic Agents—Treatment guidelines set forth by the National Psoriasis Foundation (NPF) in 2012 proposed that first-line therapies for GPP should be acitretin, cyclosporine, methotrexate, and infliximab.28 However, since those guidelines were established, many new biologic therapies have been approved for the treatment of psoriasis and often are considered in the treatment of psoriasis subtypes, including GPP.29 Although retinoids previously were considered to be a preferred first-line therapy, they are associated with a high incidence of adverse effects and must be used with caution in women of childbearing age.6 Oral acitretin at a dosage of 0.75 to 1.0 mg/kg/d has been shown to result in clinical improvement within 1 to 2 weeks, and a maintenance dosage of 0.125 to 0.25 mg/kg/d is required for several months to prevent recurrence.30 Methotrexate—5.0 to 15.0 mg/wk, or perhaps higher in patients with refractory disease, increased by 2.5-mg intervals until symptoms improve—is recommended by the NPF in patients who are unresponsive or cannot tolerate retinoids, though close monitoring for hematologic abnormalities is required. Cyclosporine 2.5 to 5.0 mg/kg/d is considered an alternative to methotrexate and retinoids; it has a faster onset of action, with improvement reported as early as 2 weeks after initiation of therapy.1,28 Although cyclosporine may be effective in the acute phase, especially in severe cases of GPP, long-term use of cyclosporine is not recommended because of the potential for renal dysfunction and hypertension.31
Biologic Agents—More recent evidence has accumulated supporting the efficacy of anti-TNF agents in the treatment of GPP, suggesting the positioning of these agents as first line. A number of case series have shown dramatic and rapid improvement of GPP with intravenous infliximab 3 to 5 mg/kg, with results observed hours to days after the first infusion.32-37 Thus, infliximab is recommended as first-line treatment in severe acute cases, though its efficacy as a maintenance therapy has not been sufficiently investigated.6 Case reports and case series document the safety and efficacy of adalimumab 40 to 80 mg every 1 to 2 weeks38,39 and etanercept 25 to 50 mg twice weekly40-42 in patients with recalcitrantGPP. Therefore, these anti-TNF agents may be considered in patients who are nonresponsive to treatment with infliximab.
Rarely, there have been reports of paradoxical induction of GPP with the use of some anti-TNF agents,43-45 which may be due to a cytokine imbalance characterized by unopposed IFN-α activation.6 In patients with a history of GPP after initiation of a biologic, treatment with agents from within the offending class should be avoided.
The IL-17A monoclonal antibodies secukinumab, ixekizumab, and brodalumab have been shown in open-label phase 3 studies to result in disease remission at 12 weeks.46-48 Treatment with guselkumab, an IL-23 monoclonal antibody, also has demonstrated efficacy in patients with GPP.49 Ustekinumab, an IL-12/23 inhibitor, in combination with acitretin also has been shown to be successful in achieving disease remission after a few weeks of treatment.50
More recent case reports have shown the efficacy of IL-1 inhibitors including gevokizumab, canakinumab, and anakinra in achieving GPP clearance, though more prospective studies are needed to evaluate their efficacy.51-53 Given the etiologic association between IL-1 disinhibition and GPP, future investigations of these therapies as well as those that target the IL-36 pathway may prove to be particularly interesting.
Phototherapy and Combination Therapies—Phototherapy may be considered as maintenance therapy after disease control is achieved, though it is not considered appropriate for acute cases.28 Combination therapies with a biologic plus a nonbiologic systemic agent or alternating among various biologics may allow physicians to maximize benefits and minimize adverse effects in the long term, though there is insufficient evidence to suggest any specific combination treatment algorithm for GPP.28
Treatment Recommendations for Pediatric Patients
Based on a small number of case series and case reports, the first-line treatment strategy for children with GPP is similar to adults. Given the notable adverse events of most oral systemic agents, biologic therapies may emerge as first-line therapy in the pediatric population as more evidence accumulates.28
Treatment Recommendations for Pregnant Patients
Systemic corticosteroids are widely considered to be the first-line treatments for the management of impetigo herpetiformis.7 Low-dose prednisone (15–30 mg/d) usually is effective, but severe cases may require increasing the dosage to 60 mg/d.6 Given the potential for rebound flares upon withdrawal of systemic corticosteroids, these agents must be gradually tapered after the resolution of symptoms.
Certolizumab pegol also is an attractive option in pregnant patients with impetigo herpetiformis because of its favorable safety profile and negligible mother-to-infant transfer through the placenta or breast milk. It has been shown to be effective in treating GPP and impetigo herpetiformis during pregnancy in recently published case reports.54,55 In refractory cases, other TNF-α inhibitors (eg, adalimumab, infliximab, etanercept) or cyclosporine may be considered. However, cautious medical monitoring is warranted, as little is known about the potential adverse effects of these agents to the mother and fetus.28,56 Data from transplant recipients along with several case reports indicate that cyclosporine is not associated with an increased risk for adverse effects during pregnancy at a dose of 2 to 3 mg/kg.57-59 Both methotrexate and retinoids are known teratogens and are therefore contraindicated in pregnant patients.56
If pustules do not resolve in the postpartum period, patients should be treated with standard GPP therapies. However, long-term and population studies are lacking regarding the potential for infant exposure to systemic agents in breast milk. Therefore, the NPF recommends avoiding breastfeeding while taking systemic medications, if possible.56
Limitations of Treatment Recommendations
The ability to generate an evidence-based treatment strategy for GPP is limited by a lack of high-quality studies investigating the efficacy and safety of treatments in patients with GPP due to the rarity and relapsing-remitting nature of the disease, which makes randomized controlled trials difficult to conduct. The quality of the available research is further limited by the lack of validated outcome measures to specifically assess improvements in patients with GPP, such that results are difficult to synthesize and compare among studies.31
Conclusion
Although limited, the available research suggests that treatment with various biologics, especially infliximab, is effective in achieving rapid clearance in patients with GPP. In general, biologics may be the most appropriate treatment option in patients with GPP given their relatively favorable safety profiles. Other oral systemic agents, including acitretin, cyclosporine, and methotrexate, have limited evidence to support their use in the acute phase, but their safety profiles often limit their utility in the long-term. Emerging evidence regarding the association of GPP with IL36RN mutations suggests a unique role for agents targeting the IL-36 or IL-1 pathways, though this has yet to be thoroughly investigated.
- Benjegerdes KE, Hyde K, Kivelevitch D, et al. Pustular psoriasis: pathophysiology and current treatment perspectives. Psoriasis (Auckl). 2016;6:131‐144.
- Bachelez H. Pustular psoriasis and related pustular skin diseases. Br J Dermatol. 2018;178:614‐618.
- Gooderham MJ, Van Voorhees AS, Lebwohl MG. An update on generalized pustular psoriasis. Expert Rev Clin Immunol. 2019;15:907‐919.
- Ly K, Beck KM, Smith MP, et al. Diagnosis and screening of patients with generalized pustular psoriasis. Psoriasis (Auckl). 2019;9:37‐42.
- van de Kerkhof PCM, Nestle FO. Psoriasis. In: Bolognia JL, Jorizzo JJ, Schaffer JV, eds. Dermatology. 3rd ed. Elsevier; 2012:138-160.
- Hoegler KM, John AM, Handler MZ, et al. Generalized pustular psoriasis: a review and update on treatment. J Eur Acad Dermatol Venereol. 2018;32:1645‐1651.
- Oumeish OY, Parish JL. Impetigo herpetiformis. Clin Dermatol. 2006;24:101‐104.
- Johnston A, Xing X, Wolterink L, et al. IL-1 and IL-36 are dominant cytokines in generalized pustular psoriasis. J Allergy Clin Immunol. 2017;140:109-120.
- Furue K, Yamamura K, Tsuji G, et al. Highlighting interleukin-36 signalling in plaque psoriasis and pustular psoriasis. Acta Derm Venereol. 2018;98:5-13.
- Ogawa E, Sato Y, Minagawa A, et al. Pathogenesis of psoriasis and development of treatment. J Dermatol. 2018;45:264-272.
- Marrakchi S, Guigue P, Renshaw BR, et al. Interleukin-36-receptor antagonist deficiency and generalized pustular psoriasis. N Engl J Med. 2011;365:620-628.
- Onoufriadis A, Simpson MA, Pink AE, et al. Mutations in IL36RN/IL1F5 are associated with the severe episodic inflammatory skin disease known as generalized pustular psoriasis. Am J Hum Genet. 2011;89:432-437.
- Setta-Kaffetzi N, Navarini AA, Patel VM, et al. Rare pathogenic variants in IL36RN underlie a spectrum of psoriasis-associated pustular phenotypes. J Invest Dermatol. 2013;133:1366-1369.
- Sugiura K, Takemoto A, Yamaguchi M, et al. The majority of generalized pustular psoriasis without psoriasis vulgaris is caused by deficiency of interleukin-36 receptor antagonist. J Invest Dermatol. 2013;133:2514-2521.
- Hussain S, Berki DM, Choon SE, et al. IL36RN mutations define a severe autoinflammatory phenotype of generalized pustular psoriasis. J Allergy Clin Immunol. 2015;135:1067-1070.e9.
- Körber A, Mossner R, Renner R, et al. Mutations in IL36RN in patients with generalized pustular psoriasis. J Invest Dermatol. 2013;133:2634-2637.
- Twelves S, Mostafa A, Dand N, et al. Clinical and genetic differences between pustular psoriasis subtypes. J Allergy Clin Immunol. 2019;143:1021-1026.
- Sugiura K. The genetic background of generalized pustular psoriasis: IL36RN mutations and CARD14 gain-of-function variants. J Dermatol Sci. 2014;74:187-192
- Wang Y, Cheng R, Lu Z, et al. Clinical profiles of pediatric patients with GPP alone and with different IL36RN genotypes. J Dermatol Sci. 2017;85:235-240.
- Setta-Kaffetzi N, Simpson MA, Navarini AA, et al. AP1S3 mutations are associated with pustular psoriasis and impaired Toll-like receptor 3 trafficking. Am J Hum Genet. 2014;94:790-797.
- Mahil SK, Twelves S, Farkas K, et al. AP1S3 mutations cause skin autoinflammation by disrupting keratinocyte autophagy and upregulating IL-36 production. J Invest Dermatol. 2016;136:2251-2259.
- Umezawa Y, Ozawa A, Kawasima T, et al. Therapeutic guidelines for the treatment of generalized pustular psoriasis (GPP) based on a proposed classification of disease severity. Arch Dermatol Res. 2003;295(suppl 1):S43-S54.
- Viguier M, Allez M, Zagdanski AM, et al. High frequency of cholestasis in generalized pustular psoriasis: evidence for neutrophilic involvement of the biliary tract. Hepatology. 2004;40:452-458.
- Ryan TJ, Baker H. The prognosis of generalized pustular psoriasis. Br J Dermatol. 1971;85:407-411.
- Kalb RE. Pustular psoriasis: management. In: Ofori AO, Duffin KC, eds. UpToDate. UpToDate; 2014. Accessed July 20, 2022. https://www.uptodate.com/contents/pustular-psoriasis-management/print
- Naik HB, Cowen EW. Autoinflammatory pustular neutrophilic diseases. Dermatol Clin. 2013;31:405-425.
- Sidoroff A, Dunant A, Viboud C, et al. Risk factors for acute generalized exanthematous pustulosis (AGEP)—results of a multinational case-control study (EuroSCAR). Br J Dermatol. 2007;157:989-996.
- Robinson A, Van Voorhees AS, Hsu S, et al. Treatment of pustular psoriasis: from the Medical Board of the National Psoriasis Foundation. J Am Acad Dermatol. 2012;67:279‐288.
- Menter A, Strober BE, Kaplan DH, et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with biologics. J Am Acad Dermatol. 2019;80:1029-1072.
- Mengesha YM, Bennett ML. Pustular skin disorders: diagnosis and treatment. Am J Clin Dermatol 2002;3:389-400.
- Zhou LL, Georgakopoulos JR, Ighani A, et al. Systemic monotherapy treatments for generalized pustular psoriasis: a systematic review. J Cutan Med Surg. 2018;22:591‐601.
- Elewski BE. Infliximab for the treatment of severe pustular psoriasis. J Am Acad Dermatol. 2002;47:796-797.
- Kim HS, You HS, Cho HH, et al. Two cases of generalized pustular psoriasis: successful treatment with infliximab. Ann Dermatol. 2014;26:787-788.
- Trent JT, Kerdel FA. Successful treatment of Von Zumbusch pustular psoriasis with infliximab. J Cutan Med Surg. 2004;8:224-228.
- Poulalhon N, Begon E, Lebbé C, et al. A follow-up study in 28 patients treated with infliximab for severe recalcitrant psoriasis: evidence for efficacy and high incidence of biological autoimmunity. Br J Dermatol. 2007;156:329-336.
- Routhouska S, Sheth PB, Korman NJ. Long-term management of generalized pustular psoriasis with infliximab: case series. J Cutan Med Surg. 2008;12:184-188.
- Lisby S, Gniadecki R. Infliximab (Remicade) for acute, severe pustular and erythrodermic psoriasis. Acta Derm Venereol. 2004;84:247-248.
- Zangrilli A, Papoutsaki M, Talamonti M, et al. Long-term efficacy of adalimumab in generalized pustular psoriasis. J Dermatol Treat. 2008;19:185-187.
- Matsumoto A, Komine M, Karakawa M, et al. Adalimumab administration after infliximab therapy is a successful treatment strategy for generalized pustular psoriasis. J Dermatol. 2017;44:202-204.
- Kamarashev J, Lor P, Forster A, et al. Generalized pustular psoriasis induced by cyclosporin in a withdrawal responding to the tumour necrosis factor alpha inhibitor etanercept. Dermatology. 2002;205:213-216.
- Esposito M, Mazzotta A, Casciello C, et al. Etanercept at different dosages in the treatment of generalized pustular psoriasis: a case series. Dermatology. 2008;216:355-360.
- Lo Schiavo A, Brancaccio G, Puca RV, et al. Etanercept in the treatment of generalized annular pustular psoriasis. Ann Dermatol. 2012;24:233-234.
- Goiriz R, Daudén E, Pérez-Gala S, et al. Flare and change of psoriasis morphology during the course of treatment with tumor necrosis factor blockers. Clin Exp Dermatol. 2006;32:176-179.
- Collamer AN, Battafarano DF. Psoriatic skin lesions induced by tumor necrosis factor antagonist therapy: clinical features and possible immunopathogenesis. Semin Arthritis Rheum. 2010;40:233-240.
- Almutairi D, Sheasgreen C, Weizman A, et al. Generalized pustular psoriasis induced by infliximab in a patient with inflammatory bowel disease. J Cutan Med Surg. 2018;1:507-510.
- Imafuku S, Honma M, Okubo Y, et al. Efficacy and safety of secukinumab in patients with generalized pustular psoriasis: a 52-week analysis from phase III open-label multicenter Japanese study. J Dermatol. 2016;43:1011-1017
- Saeki H, Nakagawa H, Ishii T, et al. Efficacy and safety of open-label ixekizumab treatment in Japanese patients with moderate-to-severe plaque psoriasis, erythrodermic psoriasis, and generalized pustular psoriasis. J Eur Acad Dermatol Venereol. 2015;29:1148-1155.
- Yamasaki K, Nakagawa H, Kubo Y, et al. Efficacy and safety of brodalumab in patients with generalized pustular psoriasis and psoriatic erythroderma: results from a 52-week, open-label study. Br J Dermatol. 2017;176:741-751.
- Sano S, Kubo H, Morishima H, et al. Guselkumab, a human interleukin-23 monoclonal antibody in Japanese patients with generalized pustular psoriasis and erythrodermic psoriasis: efficacy and safety analyses of a 52-week, phase 3, multicenter, open-label study. J Dermatol. 2018;45:529‐539.
- Arakawa A, Ruzicka T, Prinz JC. Therapeutic efficacy of interleukin 12/interleukin 23 blockade in generalized pustular psoriasis regardless of IL36RN mutation status. JAMA Dermatol. 2016;152:825-828.
- Mansouri B, Richards L, Menter A. Treatment of two patients with generalized pustular psoriasis with the interleukin-1beta inhibitor gevokizumab. Br J Dermatol. 2015;173:239-241.
- Skendros P, Papagoras C, Lefaki I, et al. Successful response in a case of severe pustular psoriasis after interleukin-1 beta inhibition. Br J Dermatol. 2017;176:212-215.
- Viguier M, Guigue P, Pagès C, et al. Successful treatment of generalized pustular psoriasis with the interleukin-1-receptor antagonist Anakinra: lack of correlation with IL1RN mutations. Ann Intern Med. 2010;153:66-67.
- Fukushima H, Iwata Y, Arima M, et al. Efficacy and safety of treatment with anti-tumor necrosis factor‐α drugs for severe impetigo herpetiformis. J Dermatol. 2021;48:207-210.
- Mizutani Y, Mizutani YH, Matsuyama K, et al. Generalized pustular psoriasis in pregnancy, successfully treated with certolizumab pegol. J Dermatol. 2021;47:e262-e263.
- Bae YS, Van Voorhees AS, Hsu S, et al. Review of treatment options for psoriasis in pregnant or lactating women: from the Medical Board of the National Psoriasis Foundation. J Am Acad Dermatol. 2012;67:459‐477.
- Finch TM, Tan CY. Pustular psoriasis exacerbated by pregnancy and controlled by cyclosporin A. Br J Dermatol. 2000;142:582-584.
- Gaughan WJ, Moritz MJ, Radomski JS, et al. National Transplantation Pregnancy Registry: report on outcomes of cyclosporine-treated female kidney transplant recipients with an interval from transplantation to pregnancy of greater than five years. Am J Kidney Dis. 1996;28:266-269.
- Kura MM, Surjushe AU. Generalized pustular psoriasis of pregnancy treated with oral cyclosporin. Indian J Dermatol Venereol Leprol. 2006;72:458-459.
Acute generalized pustular psoriasis (GPP) is a rare severe variant of psoriasis characterized by the sudden widespread eruption of sterile pustules.1,2 The cutaneous manifestations of GPP also may be accompanied by signs of systemic inflammation, including fever, malaise, and leukocytosis.2 Complications are common and may be life-threatening, especially in older patients with comorbid diseases.3 Generalized pustular psoriasis most commonly occurs in patients with a preceding history of psoriasis, but it also may occur de novo.4 Generalized pustular psoriasis is associated with notable morbidity and mortality, and relapses are common.3,4 Many triggers of GPP have been identified, including initiation and withdrawal of various medications, infections, pregnancy, and other conditions.5,6 Although GPP most often occurs in adults, it also may arise in children and infants.3 In pregnancy, GPP is referred to as impetigo herpetiformis, despite having no etiologic ties with either herpes simplex virus or staphylococcal or streptococcal infection. Impetigo herpetiformis is considered one of the most dangerous dermatoses of pregnancy because of high rates of associated maternal and fetal morbidity.6,7
Acute GPP has proven to be a challenging disease to treat due to the rarity and relapsing-remitting nature of the disease; additionally, there are relatively few randomized controlled trials investigating the efficacy and safety of treatments for GPP. This review summarizes the features of GPP, including the pathophysiology of the disease, clinical and histological manifestations, and recommendations for management based on a PubMed search of articles indexed for MEDLINE using MeSH terms pertaining to the disease, including generalized pustular psoriasis, impetigo herpetiformis, and von Zumbusch psoriasis.
Pathophysiology
The pathophysiology of GPP is only partially understood, but it is thought to have a distinct pattern of immune activation compared with plaque psoriasis.8 Although there is a considerable amount of overlap and cross-talk among cytokine pathways, GPP generally is driven by innate immunity and unrestrained IL-36 cytokine activity. In contrast, adaptive immune responses—namely the tumor necrosis factor (TNF) α, IL-23, IL-17, and IL-22 axes—underlie plaque psoriasis.8-10
Proinflammatory IL-36 cytokines α, β, and γ, which are all part of the IL-1 superfamily, bind to the IL-36 receptor (IL-36R) to recruit and activate immune cells via various mediators, including IL-1β; IL-8; and chemokines CXCL1, CXCL2, and CXCL8.3 The IL-36 receptor antagonist (IL-36ra) acts to inhibit this inflammatory cascade.3,8 Microarray analyses of skin biopsy samples have shown that overexpression of IL-17A, TNF-α, IL-1, and IL-36 are seen in both GPP and plaque psoriasis lesions, but GPP lesions had higher expression of IL-1β, IL-36α, and IL-36γ and elevated neutrophil chemokines—CXCL1, CXCL2, and CXCL8—compared with plaque psoriasis lesions.8
Gene Mutations Associated With GPP
There are 3 gene mutations that have been associated with pustular variants of psoriasis, though these mutations account for a minority of cases of GPP.4 Genetic screenings are not routinely indicated in patients with GPP, but they may be warranted in severe cases when a familial pattern of inheritance is suspected.4
IL36RN—The gene IL36RN codes the anti-inflammatory IL-36ra. Loss-of-function mutations in IL36RN lead to impairment of IL-36ra and consequently hyperactivity of the proinflammatory responses triggered by IL-36.3 Homozygous and heterozygous mutations in IL36RN have been observed in both familial and sporadic cases of GPP.11-13 Subsequent retrospective analyses have identified the presence of IL36RN mutations in patients with GPP with frequencies ranging from 23% to 37%.14-17IL36RN mutations are thought to be more common in patients without concomitant plaque psoriasis and have been associated with severe disease and early disease onset.15
CARD14—A gain-of-function mutation in CARD14 results in overactivation of the proinflammatory nuclear factor κB pathway and has been implicated in cases of GPP with concurrent psoriasis vulgaris. Interestingly, this may suggest distinct etiologies underlying GPP de novo and GPP in patients with a history of psoriasis.18,19
AP1S3—A loss-of-function mutation in AP1S3 results in abnormal endosomal trafficking and autophagy as well as increased expression of IL-36α.20,21
Clinical Presentation and Diagnosis Cutaneous Manifestations of GPP
Generalized pustular psoriasis is characterized by the onset of widespread 2- to 3-mm sterile pustules on erythematous skin or within psoriasiform plaques4 (Figure). In patients with skin of color, the erythema may appear less obvious or perhaps slightly violaceous compared to White skin. Pustules may coalesce to form “lakes” of pus.5 Cutaneous symptoms include pain, burning, and pruritus. Associated mucosal findings may include cheilitis, geographic tongue, conjunctivitis, and uveitis.4

The severity of symptoms can vary greatly among patients as well as between flares within the same patient.2,3 Four distinct patterns of GPP have been described. The von Zumbusch pattern is characterized by a rapid, generalized, painful, erythematous and pustular eruption accompanied by fever and asthenia. The pustules usually resolve after several days with extensive scaling. The annular pattern is characterized by annular, erythematous, scaly lesions with pustules present centrifugally. The lesions enlarge by centrifugal expansion over a period of hours to days, while healing occurs centrally. The exanthematic type is an acute eruption of small pustules that abruptly appear and disappear within a few days, usually from infection or medication initiation. Sometimes pustules appear within or at the edge of existing psoriatic plaques in a localized pattern—the fourth pattern—often following the exposure to irritants (eg, tars, anthralin).5
Impetigo Herpetiformis—Impetigo herpetiformis is a form of GPP associated with pregnancy. It generally presents early in the third trimester with symmetric erythematous plaques in flexural and intertriginous areas with pustules present at lesion margins. Lesions expand centrifugally, with pustulation present at the advancing edge.6,7 Patients often are acutely ill with fever, delirium, vomiting, and tetany. Mucous membranes, including the tongue, mouth, and esophagus, also may be involved. The eruption typically resolves after delivery, though it often recurs with subsequent pregnancies, with the morbidity risk rising with each successive pregnancy.7
Systemic and Extracutaneous Manifestations of GPP
Although the severity of GPP is highly variable, skin manifestations often are accompanied by systemic manifestations of inflammation, including fever and malaise. Common laboratory abnormalities include leukocytosis with peripheral neutrophilia, a high serum C-reactive protein level, hypocalcemia, and hypoalbuminemia.22 Abnormal liver enzymes often are present and result from neutrophilic cholangitis, with alternating strictures and dilations of biliary ducts observed on magnetic resonance imaging.23 Additional laboratory abnormalities are provided in Table 2. Other extracutaneous findings associated with GPP include arthralgia, edema, and characteristic psoriatic nail changes.4 Fatal complications include acute respiratory distress syndrome, renal dysfunction, cardiovascular shock, and sepsis.24,25

Histologic Features
Given the potential for the skin manifestations of GPP to mimic other disorders, a skin biopsy is warranted to confirm the diagnosis. Generalized pustular psoriasis is histologically characterized by the presence of subcorneal macropustules (ie, spongiform pustules of Kogoj) formed by neutrophil infiltration into the spongelike network of the epidermis.6 Otherwise, the architecture of the epithelium in GPP is similar to that seen with plaque psoriasis, with parakeratosis, acanthosis, rete-ridge elongation, diminished stratum granulosum, and thinning of the suprapapillary epidermis, though the inflammatory cell infiltrate and edema are markedly more severe in GPP than plaque psoriasis.3,4
Differential Diagnosis
There are many other cutaneous pustular diagnoses that must be ruled out when evaluating a patient with GPP (Table 1).26 Acute generalized exanthematous pustulosis (AGEP) is a common mimicker of GPP that is differentiated histologically by the presence of eosinophils and necrotic keratinocytes.4 In addition to its distinct histopathologic findings, AGEP is classically associated with recent initiation of certain medications, most commonly penicillins, macrolides, quinolones, sulfonamides, terbinafine, and diltiazem.27 In contrast, GPP more commonly is related to withdrawal of corticosteroids as well as initiation of some biologic medications, including anti-TNF agents.3 Generalized pustular psoriasis should be suspected over AGEP in patients with a personal or family history of psoriasis, though GPP may arise in patients with or without a history of psoriasis. Acute generalized exanthematous pustulosis usually is more abrupt in both onset and resolution compared with GPP, with clearance of pustules within a few days to weeks following cessation of the triggering factor.4

Other pustular variants of psoriasis (eg, palmoplantar pustular psoriasis, acrodermatitis continua of Hallopeau) are differentiated from GPP by their chronicity and localization to palmoplantar and/or ungual surfaces.5 Other differential diagnoses are listed in Table 1.
Diagnostic Criteria for GPP
Diagnostic criteria have been proposed for GPP (Table 2), including (1) the presence of sterile pustules, (2) systemic signs of inflammation, (3) laboratory abnormalities, (4) histopathologic confirmation of spongiform pustules of Kogoj, and (5) recurrence of symptoms.22 To definitively diagnose GPP, all 5 criteria must be met. To rule out mimickers, it may be worthwhile to perform Gram staining, potassium hydroxide preparation, in vitro cultures, and/or immunofluorescence testing.6
Treatment
Given the high potential for mortality associated with GPP, the most essential component of management is to ensure adequate supportive care. Any temperature, fluid, or electrolyte imbalances should be corrected as they arise. Secondary infections also must be identified and treated, if present, to reduce the risk for fatal complications, including systemic infection and sepsis. Precautions must be taken to ensure that serious end-organ damage, including hepatic, renal, and respiratory dysfunction, is avoided.
Adjunctive topical intervention often is initiated with bland emollients, corticosteroids, calcineurin inhibitors, and/or vitamin D derivatives to help soothe skin symptoms, but treatment with systemic therapies usually is warranted to achieve symptom control.2,25 Importantly, there are no systemic or topical agents that have specifically been approved for the treatment of GPP in Europe or the United States.3 Given the absence of universally accepted treatment guidelines, therapeutic agents for GPP usually are selected based on clinical experience while also taking the extent of involvement and disease severity into consideration.3
Treatment Recommendations for Adults
Oral Systemic Agents—Treatment guidelines set forth by the National Psoriasis Foundation (NPF) in 2012 proposed that first-line therapies for GPP should be acitretin, cyclosporine, methotrexate, and infliximab.28 However, since those guidelines were established, many new biologic therapies have been approved for the treatment of psoriasis and often are considered in the treatment of psoriasis subtypes, including GPP.29 Although retinoids previously were considered to be a preferred first-line therapy, they are associated with a high incidence of adverse effects and must be used with caution in women of childbearing age.6 Oral acitretin at a dosage of 0.75 to 1.0 mg/kg/d has been shown to result in clinical improvement within 1 to 2 weeks, and a maintenance dosage of 0.125 to 0.25 mg/kg/d is required for several months to prevent recurrence.30 Methotrexate—5.0 to 15.0 mg/wk, or perhaps higher in patients with refractory disease, increased by 2.5-mg intervals until symptoms improve—is recommended by the NPF in patients who are unresponsive or cannot tolerate retinoids, though close monitoring for hematologic abnormalities is required. Cyclosporine 2.5 to 5.0 mg/kg/d is considered an alternative to methotrexate and retinoids; it has a faster onset of action, with improvement reported as early as 2 weeks after initiation of therapy.1,28 Although cyclosporine may be effective in the acute phase, especially in severe cases of GPP, long-term use of cyclosporine is not recommended because of the potential for renal dysfunction and hypertension.31
Biologic Agents—More recent evidence has accumulated supporting the efficacy of anti-TNF agents in the treatment of GPP, suggesting the positioning of these agents as first line. A number of case series have shown dramatic and rapid improvement of GPP with intravenous infliximab 3 to 5 mg/kg, with results observed hours to days after the first infusion.32-37 Thus, infliximab is recommended as first-line treatment in severe acute cases, though its efficacy as a maintenance therapy has not been sufficiently investigated.6 Case reports and case series document the safety and efficacy of adalimumab 40 to 80 mg every 1 to 2 weeks38,39 and etanercept 25 to 50 mg twice weekly40-42 in patients with recalcitrantGPP. Therefore, these anti-TNF agents may be considered in patients who are nonresponsive to treatment with infliximab.
Rarely, there have been reports of paradoxical induction of GPP with the use of some anti-TNF agents,43-45 which may be due to a cytokine imbalance characterized by unopposed IFN-α activation.6 In patients with a history of GPP after initiation of a biologic, treatment with agents from within the offending class should be avoided.
The IL-17A monoclonal antibodies secukinumab, ixekizumab, and brodalumab have been shown in open-label phase 3 studies to result in disease remission at 12 weeks.46-48 Treatment with guselkumab, an IL-23 monoclonal antibody, also has demonstrated efficacy in patients with GPP.49 Ustekinumab, an IL-12/23 inhibitor, in combination with acitretin also has been shown to be successful in achieving disease remission after a few weeks of treatment.50
More recent case reports have shown the efficacy of IL-1 inhibitors including gevokizumab, canakinumab, and anakinra in achieving GPP clearance, though more prospective studies are needed to evaluate their efficacy.51-53 Given the etiologic association between IL-1 disinhibition and GPP, future investigations of these therapies as well as those that target the IL-36 pathway may prove to be particularly interesting.
Phototherapy and Combination Therapies—Phototherapy may be considered as maintenance therapy after disease control is achieved, though it is not considered appropriate for acute cases.28 Combination therapies with a biologic plus a nonbiologic systemic agent or alternating among various biologics may allow physicians to maximize benefits and minimize adverse effects in the long term, though there is insufficient evidence to suggest any specific combination treatment algorithm for GPP.28
Treatment Recommendations for Pediatric Patients
Based on a small number of case series and case reports, the first-line treatment strategy for children with GPP is similar to adults. Given the notable adverse events of most oral systemic agents, biologic therapies may emerge as first-line therapy in the pediatric population as more evidence accumulates.28
Treatment Recommendations for Pregnant Patients
Systemic corticosteroids are widely considered to be the first-line treatments for the management of impetigo herpetiformis.7 Low-dose prednisone (15–30 mg/d) usually is effective, but severe cases may require increasing the dosage to 60 mg/d.6 Given the potential for rebound flares upon withdrawal of systemic corticosteroids, these agents must be gradually tapered after the resolution of symptoms.
Certolizumab pegol also is an attractive option in pregnant patients with impetigo herpetiformis because of its favorable safety profile and negligible mother-to-infant transfer through the placenta or breast milk. It has been shown to be effective in treating GPP and impetigo herpetiformis during pregnancy in recently published case reports.54,55 In refractory cases, other TNF-α inhibitors (eg, adalimumab, infliximab, etanercept) or cyclosporine may be considered. However, cautious medical monitoring is warranted, as little is known about the potential adverse effects of these agents to the mother and fetus.28,56 Data from transplant recipients along with several case reports indicate that cyclosporine is not associated with an increased risk for adverse effects during pregnancy at a dose of 2 to 3 mg/kg.57-59 Both methotrexate and retinoids are known teratogens and are therefore contraindicated in pregnant patients.56
If pustules do not resolve in the postpartum period, patients should be treated with standard GPP therapies. However, long-term and population studies are lacking regarding the potential for infant exposure to systemic agents in breast milk. Therefore, the NPF recommends avoiding breastfeeding while taking systemic medications, if possible.56
Limitations of Treatment Recommendations
The ability to generate an evidence-based treatment strategy for GPP is limited by a lack of high-quality studies investigating the efficacy and safety of treatments in patients with GPP due to the rarity and relapsing-remitting nature of the disease, which makes randomized controlled trials difficult to conduct. The quality of the available research is further limited by the lack of validated outcome measures to specifically assess improvements in patients with GPP, such that results are difficult to synthesize and compare among studies.31
Conclusion
Although limited, the available research suggests that treatment with various biologics, especially infliximab, is effective in achieving rapid clearance in patients with GPP. In general, biologics may be the most appropriate treatment option in patients with GPP given their relatively favorable safety profiles. Other oral systemic agents, including acitretin, cyclosporine, and methotrexate, have limited evidence to support their use in the acute phase, but their safety profiles often limit their utility in the long-term. Emerging evidence regarding the association of GPP with IL36RN mutations suggests a unique role for agents targeting the IL-36 or IL-1 pathways, though this has yet to be thoroughly investigated.
Acute generalized pustular psoriasis (GPP) is a rare severe variant of psoriasis characterized by the sudden widespread eruption of sterile pustules.1,2 The cutaneous manifestations of GPP also may be accompanied by signs of systemic inflammation, including fever, malaise, and leukocytosis.2 Complications are common and may be life-threatening, especially in older patients with comorbid diseases.3 Generalized pustular psoriasis most commonly occurs in patients with a preceding history of psoriasis, but it also may occur de novo.4 Generalized pustular psoriasis is associated with notable morbidity and mortality, and relapses are common.3,4 Many triggers of GPP have been identified, including initiation and withdrawal of various medications, infections, pregnancy, and other conditions.5,6 Although GPP most often occurs in adults, it also may arise in children and infants.3 In pregnancy, GPP is referred to as impetigo herpetiformis, despite having no etiologic ties with either herpes simplex virus or staphylococcal or streptococcal infection. Impetigo herpetiformis is considered one of the most dangerous dermatoses of pregnancy because of high rates of associated maternal and fetal morbidity.6,7
Acute GPP has proven to be a challenging disease to treat due to the rarity and relapsing-remitting nature of the disease; additionally, there are relatively few randomized controlled trials investigating the efficacy and safety of treatments for GPP. This review summarizes the features of GPP, including the pathophysiology of the disease, clinical and histological manifestations, and recommendations for management based on a PubMed search of articles indexed for MEDLINE using MeSH terms pertaining to the disease, including generalized pustular psoriasis, impetigo herpetiformis, and von Zumbusch psoriasis.
Pathophysiology
The pathophysiology of GPP is only partially understood, but it is thought to have a distinct pattern of immune activation compared with plaque psoriasis.8 Although there is a considerable amount of overlap and cross-talk among cytokine pathways, GPP generally is driven by innate immunity and unrestrained IL-36 cytokine activity. In contrast, adaptive immune responses—namely the tumor necrosis factor (TNF) α, IL-23, IL-17, and IL-22 axes—underlie plaque psoriasis.8-10
Proinflammatory IL-36 cytokines α, β, and γ, which are all part of the IL-1 superfamily, bind to the IL-36 receptor (IL-36R) to recruit and activate immune cells via various mediators, including IL-1β; IL-8; and chemokines CXCL1, CXCL2, and CXCL8.3 The IL-36 receptor antagonist (IL-36ra) acts to inhibit this inflammatory cascade.3,8 Microarray analyses of skin biopsy samples have shown that overexpression of IL-17A, TNF-α, IL-1, and IL-36 are seen in both GPP and plaque psoriasis lesions, but GPP lesions had higher expression of IL-1β, IL-36α, and IL-36γ and elevated neutrophil chemokines—CXCL1, CXCL2, and CXCL8—compared with plaque psoriasis lesions.8
Gene Mutations Associated With GPP
There are 3 gene mutations that have been associated with pustular variants of psoriasis, though these mutations account for a minority of cases of GPP.4 Genetic screenings are not routinely indicated in patients with GPP, but they may be warranted in severe cases when a familial pattern of inheritance is suspected.4
IL36RN—The gene IL36RN codes the anti-inflammatory IL-36ra. Loss-of-function mutations in IL36RN lead to impairment of IL-36ra and consequently hyperactivity of the proinflammatory responses triggered by IL-36.3 Homozygous and heterozygous mutations in IL36RN have been observed in both familial and sporadic cases of GPP.11-13 Subsequent retrospective analyses have identified the presence of IL36RN mutations in patients with GPP with frequencies ranging from 23% to 37%.14-17IL36RN mutations are thought to be more common in patients without concomitant plaque psoriasis and have been associated with severe disease and early disease onset.15
CARD14—A gain-of-function mutation in CARD14 results in overactivation of the proinflammatory nuclear factor κB pathway and has been implicated in cases of GPP with concurrent psoriasis vulgaris. Interestingly, this may suggest distinct etiologies underlying GPP de novo and GPP in patients with a history of psoriasis.18,19
AP1S3—A loss-of-function mutation in AP1S3 results in abnormal endosomal trafficking and autophagy as well as increased expression of IL-36α.20,21
Clinical Presentation and Diagnosis Cutaneous Manifestations of GPP
Generalized pustular psoriasis is characterized by the onset of widespread 2- to 3-mm sterile pustules on erythematous skin or within psoriasiform plaques4 (Figure). In patients with skin of color, the erythema may appear less obvious or perhaps slightly violaceous compared to White skin. Pustules may coalesce to form “lakes” of pus.5 Cutaneous symptoms include pain, burning, and pruritus. Associated mucosal findings may include cheilitis, geographic tongue, conjunctivitis, and uveitis.4

The severity of symptoms can vary greatly among patients as well as between flares within the same patient.2,3 Four distinct patterns of GPP have been described. The von Zumbusch pattern is characterized by a rapid, generalized, painful, erythematous and pustular eruption accompanied by fever and asthenia. The pustules usually resolve after several days with extensive scaling. The annular pattern is characterized by annular, erythematous, scaly lesions with pustules present centrifugally. The lesions enlarge by centrifugal expansion over a period of hours to days, while healing occurs centrally. The exanthematic type is an acute eruption of small pustules that abruptly appear and disappear within a few days, usually from infection or medication initiation. Sometimes pustules appear within or at the edge of existing psoriatic plaques in a localized pattern—the fourth pattern—often following the exposure to irritants (eg, tars, anthralin).5
Impetigo Herpetiformis—Impetigo herpetiformis is a form of GPP associated with pregnancy. It generally presents early in the third trimester with symmetric erythematous plaques in flexural and intertriginous areas with pustules present at lesion margins. Lesions expand centrifugally, with pustulation present at the advancing edge.6,7 Patients often are acutely ill with fever, delirium, vomiting, and tetany. Mucous membranes, including the tongue, mouth, and esophagus, also may be involved. The eruption typically resolves after delivery, though it often recurs with subsequent pregnancies, with the morbidity risk rising with each successive pregnancy.7
Systemic and Extracutaneous Manifestations of GPP
Although the severity of GPP is highly variable, skin manifestations often are accompanied by systemic manifestations of inflammation, including fever and malaise. Common laboratory abnormalities include leukocytosis with peripheral neutrophilia, a high serum C-reactive protein level, hypocalcemia, and hypoalbuminemia.22 Abnormal liver enzymes often are present and result from neutrophilic cholangitis, with alternating strictures and dilations of biliary ducts observed on magnetic resonance imaging.23 Additional laboratory abnormalities are provided in Table 2. Other extracutaneous findings associated with GPP include arthralgia, edema, and characteristic psoriatic nail changes.4 Fatal complications include acute respiratory distress syndrome, renal dysfunction, cardiovascular shock, and sepsis.24,25

Histologic Features
Given the potential for the skin manifestations of GPP to mimic other disorders, a skin biopsy is warranted to confirm the diagnosis. Generalized pustular psoriasis is histologically characterized by the presence of subcorneal macropustules (ie, spongiform pustules of Kogoj) formed by neutrophil infiltration into the spongelike network of the epidermis.6 Otherwise, the architecture of the epithelium in GPP is similar to that seen with plaque psoriasis, with parakeratosis, acanthosis, rete-ridge elongation, diminished stratum granulosum, and thinning of the suprapapillary epidermis, though the inflammatory cell infiltrate and edema are markedly more severe in GPP than plaque psoriasis.3,4
Differential Diagnosis
There are many other cutaneous pustular diagnoses that must be ruled out when evaluating a patient with GPP (Table 1).26 Acute generalized exanthematous pustulosis (AGEP) is a common mimicker of GPP that is differentiated histologically by the presence of eosinophils and necrotic keratinocytes.4 In addition to its distinct histopathologic findings, AGEP is classically associated with recent initiation of certain medications, most commonly penicillins, macrolides, quinolones, sulfonamides, terbinafine, and diltiazem.27 In contrast, GPP more commonly is related to withdrawal of corticosteroids as well as initiation of some biologic medications, including anti-TNF agents.3 Generalized pustular psoriasis should be suspected over AGEP in patients with a personal or family history of psoriasis, though GPP may arise in patients with or without a history of psoriasis. Acute generalized exanthematous pustulosis usually is more abrupt in both onset and resolution compared with GPP, with clearance of pustules within a few days to weeks following cessation of the triggering factor.4

Other pustular variants of psoriasis (eg, palmoplantar pustular psoriasis, acrodermatitis continua of Hallopeau) are differentiated from GPP by their chronicity and localization to palmoplantar and/or ungual surfaces.5 Other differential diagnoses are listed in Table 1.
Diagnostic Criteria for GPP
Diagnostic criteria have been proposed for GPP (Table 2), including (1) the presence of sterile pustules, (2) systemic signs of inflammation, (3) laboratory abnormalities, (4) histopathologic confirmation of spongiform pustules of Kogoj, and (5) recurrence of symptoms.22 To definitively diagnose GPP, all 5 criteria must be met. To rule out mimickers, it may be worthwhile to perform Gram staining, potassium hydroxide preparation, in vitro cultures, and/or immunofluorescence testing.6
Treatment
Given the high potential for mortality associated with GPP, the most essential component of management is to ensure adequate supportive care. Any temperature, fluid, or electrolyte imbalances should be corrected as they arise. Secondary infections also must be identified and treated, if present, to reduce the risk for fatal complications, including systemic infection and sepsis. Precautions must be taken to ensure that serious end-organ damage, including hepatic, renal, and respiratory dysfunction, is avoided.
Adjunctive topical intervention often is initiated with bland emollients, corticosteroids, calcineurin inhibitors, and/or vitamin D derivatives to help soothe skin symptoms, but treatment with systemic therapies usually is warranted to achieve symptom control.2,25 Importantly, there are no systemic or topical agents that have specifically been approved for the treatment of GPP in Europe or the United States.3 Given the absence of universally accepted treatment guidelines, therapeutic agents for GPP usually are selected based on clinical experience while also taking the extent of involvement and disease severity into consideration.3
Treatment Recommendations for Adults
Oral Systemic Agents—Treatment guidelines set forth by the National Psoriasis Foundation (NPF) in 2012 proposed that first-line therapies for GPP should be acitretin, cyclosporine, methotrexate, and infliximab.28 However, since those guidelines were established, many new biologic therapies have been approved for the treatment of psoriasis and often are considered in the treatment of psoriasis subtypes, including GPP.29 Although retinoids previously were considered to be a preferred first-line therapy, they are associated with a high incidence of adverse effects and must be used with caution in women of childbearing age.6 Oral acitretin at a dosage of 0.75 to 1.0 mg/kg/d has been shown to result in clinical improvement within 1 to 2 weeks, and a maintenance dosage of 0.125 to 0.25 mg/kg/d is required for several months to prevent recurrence.30 Methotrexate—5.0 to 15.0 mg/wk, or perhaps higher in patients with refractory disease, increased by 2.5-mg intervals until symptoms improve—is recommended by the NPF in patients who are unresponsive or cannot tolerate retinoids, though close monitoring for hematologic abnormalities is required. Cyclosporine 2.5 to 5.0 mg/kg/d is considered an alternative to methotrexate and retinoids; it has a faster onset of action, with improvement reported as early as 2 weeks after initiation of therapy.1,28 Although cyclosporine may be effective in the acute phase, especially in severe cases of GPP, long-term use of cyclosporine is not recommended because of the potential for renal dysfunction and hypertension.31
Biologic Agents—More recent evidence has accumulated supporting the efficacy of anti-TNF agents in the treatment of GPP, suggesting the positioning of these agents as first line. A number of case series have shown dramatic and rapid improvement of GPP with intravenous infliximab 3 to 5 mg/kg, with results observed hours to days after the first infusion.32-37 Thus, infliximab is recommended as first-line treatment in severe acute cases, though its efficacy as a maintenance therapy has not been sufficiently investigated.6 Case reports and case series document the safety and efficacy of adalimumab 40 to 80 mg every 1 to 2 weeks38,39 and etanercept 25 to 50 mg twice weekly40-42 in patients with recalcitrantGPP. Therefore, these anti-TNF agents may be considered in patients who are nonresponsive to treatment with infliximab.
Rarely, there have been reports of paradoxical induction of GPP with the use of some anti-TNF agents,43-45 which may be due to a cytokine imbalance characterized by unopposed IFN-α activation.6 In patients with a history of GPP after initiation of a biologic, treatment with agents from within the offending class should be avoided.
The IL-17A monoclonal antibodies secukinumab, ixekizumab, and brodalumab have been shown in open-label phase 3 studies to result in disease remission at 12 weeks.46-48 Treatment with guselkumab, an IL-23 monoclonal antibody, also has demonstrated efficacy in patients with GPP.49 Ustekinumab, an IL-12/23 inhibitor, in combination with acitretin also has been shown to be successful in achieving disease remission after a few weeks of treatment.50
More recent case reports have shown the efficacy of IL-1 inhibitors including gevokizumab, canakinumab, and anakinra in achieving GPP clearance, though more prospective studies are needed to evaluate their efficacy.51-53 Given the etiologic association between IL-1 disinhibition and GPP, future investigations of these therapies as well as those that target the IL-36 pathway may prove to be particularly interesting.
Phototherapy and Combination Therapies—Phototherapy may be considered as maintenance therapy after disease control is achieved, though it is not considered appropriate for acute cases.28 Combination therapies with a biologic plus a nonbiologic systemic agent or alternating among various biologics may allow physicians to maximize benefits and minimize adverse effects in the long term, though there is insufficient evidence to suggest any specific combination treatment algorithm for GPP.28
Treatment Recommendations for Pediatric Patients
Based on a small number of case series and case reports, the first-line treatment strategy for children with GPP is similar to adults. Given the notable adverse events of most oral systemic agents, biologic therapies may emerge as first-line therapy in the pediatric population as more evidence accumulates.28
Treatment Recommendations for Pregnant Patients
Systemic corticosteroids are widely considered to be the first-line treatments for the management of impetigo herpetiformis.7 Low-dose prednisone (15–30 mg/d) usually is effective, but severe cases may require increasing the dosage to 60 mg/d.6 Given the potential for rebound flares upon withdrawal of systemic corticosteroids, these agents must be gradually tapered after the resolution of symptoms.
Certolizumab pegol also is an attractive option in pregnant patients with impetigo herpetiformis because of its favorable safety profile and negligible mother-to-infant transfer through the placenta or breast milk. It has been shown to be effective in treating GPP and impetigo herpetiformis during pregnancy in recently published case reports.54,55 In refractory cases, other TNF-α inhibitors (eg, adalimumab, infliximab, etanercept) or cyclosporine may be considered. However, cautious medical monitoring is warranted, as little is known about the potential adverse effects of these agents to the mother and fetus.28,56 Data from transplant recipients along with several case reports indicate that cyclosporine is not associated with an increased risk for adverse effects during pregnancy at a dose of 2 to 3 mg/kg.57-59 Both methotrexate and retinoids are known teratogens and are therefore contraindicated in pregnant patients.56
If pustules do not resolve in the postpartum period, patients should be treated with standard GPP therapies. However, long-term and population studies are lacking regarding the potential for infant exposure to systemic agents in breast milk. Therefore, the NPF recommends avoiding breastfeeding while taking systemic medications, if possible.56
Limitations of Treatment Recommendations
The ability to generate an evidence-based treatment strategy for GPP is limited by a lack of high-quality studies investigating the efficacy and safety of treatments in patients with GPP due to the rarity and relapsing-remitting nature of the disease, which makes randomized controlled trials difficult to conduct. The quality of the available research is further limited by the lack of validated outcome measures to specifically assess improvements in patients with GPP, such that results are difficult to synthesize and compare among studies.31
Conclusion
Although limited, the available research suggests that treatment with various biologics, especially infliximab, is effective in achieving rapid clearance in patients with GPP. In general, biologics may be the most appropriate treatment option in patients with GPP given their relatively favorable safety profiles. Other oral systemic agents, including acitretin, cyclosporine, and methotrexate, have limited evidence to support their use in the acute phase, but their safety profiles often limit their utility in the long-term. Emerging evidence regarding the association of GPP with IL36RN mutations suggests a unique role for agents targeting the IL-36 or IL-1 pathways, though this has yet to be thoroughly investigated.
- Benjegerdes KE, Hyde K, Kivelevitch D, et al. Pustular psoriasis: pathophysiology and current treatment perspectives. Psoriasis (Auckl). 2016;6:131‐144.
- Bachelez H. Pustular psoriasis and related pustular skin diseases. Br J Dermatol. 2018;178:614‐618.
- Gooderham MJ, Van Voorhees AS, Lebwohl MG. An update on generalized pustular psoriasis. Expert Rev Clin Immunol. 2019;15:907‐919.
- Ly K, Beck KM, Smith MP, et al. Diagnosis and screening of patients with generalized pustular psoriasis. Psoriasis (Auckl). 2019;9:37‐42.
- van de Kerkhof PCM, Nestle FO. Psoriasis. In: Bolognia JL, Jorizzo JJ, Schaffer JV, eds. Dermatology. 3rd ed. Elsevier; 2012:138-160.
- Hoegler KM, John AM, Handler MZ, et al. Generalized pustular psoriasis: a review and update on treatment. J Eur Acad Dermatol Venereol. 2018;32:1645‐1651.
- Oumeish OY, Parish JL. Impetigo herpetiformis. Clin Dermatol. 2006;24:101‐104.
- Johnston A, Xing X, Wolterink L, et al. IL-1 and IL-36 are dominant cytokines in generalized pustular psoriasis. J Allergy Clin Immunol. 2017;140:109-120.
- Furue K, Yamamura K, Tsuji G, et al. Highlighting interleukin-36 signalling in plaque psoriasis and pustular psoriasis. Acta Derm Venereol. 2018;98:5-13.
- Ogawa E, Sato Y, Minagawa A, et al. Pathogenesis of psoriasis and development of treatment. J Dermatol. 2018;45:264-272.
- Marrakchi S, Guigue P, Renshaw BR, et al. Interleukin-36-receptor antagonist deficiency and generalized pustular psoriasis. N Engl J Med. 2011;365:620-628.
- Onoufriadis A, Simpson MA, Pink AE, et al. Mutations in IL36RN/IL1F5 are associated with the severe episodic inflammatory skin disease known as generalized pustular psoriasis. Am J Hum Genet. 2011;89:432-437.
- Setta-Kaffetzi N, Navarini AA, Patel VM, et al. Rare pathogenic variants in IL36RN underlie a spectrum of psoriasis-associated pustular phenotypes. J Invest Dermatol. 2013;133:1366-1369.
- Sugiura K, Takemoto A, Yamaguchi M, et al. The majority of generalized pustular psoriasis without psoriasis vulgaris is caused by deficiency of interleukin-36 receptor antagonist. J Invest Dermatol. 2013;133:2514-2521.
- Hussain S, Berki DM, Choon SE, et al. IL36RN mutations define a severe autoinflammatory phenotype of generalized pustular psoriasis. J Allergy Clin Immunol. 2015;135:1067-1070.e9.
- Körber A, Mossner R, Renner R, et al. Mutations in IL36RN in patients with generalized pustular psoriasis. J Invest Dermatol. 2013;133:2634-2637.
- Twelves S, Mostafa A, Dand N, et al. Clinical and genetic differences between pustular psoriasis subtypes. J Allergy Clin Immunol. 2019;143:1021-1026.
- Sugiura K. The genetic background of generalized pustular psoriasis: IL36RN mutations and CARD14 gain-of-function variants. J Dermatol Sci. 2014;74:187-192
- Wang Y, Cheng R, Lu Z, et al. Clinical profiles of pediatric patients with GPP alone and with different IL36RN genotypes. J Dermatol Sci. 2017;85:235-240.
- Setta-Kaffetzi N, Simpson MA, Navarini AA, et al. AP1S3 mutations are associated with pustular psoriasis and impaired Toll-like receptor 3 trafficking. Am J Hum Genet. 2014;94:790-797.
- Mahil SK, Twelves S, Farkas K, et al. AP1S3 mutations cause skin autoinflammation by disrupting keratinocyte autophagy and upregulating IL-36 production. J Invest Dermatol. 2016;136:2251-2259.
- Umezawa Y, Ozawa A, Kawasima T, et al. Therapeutic guidelines for the treatment of generalized pustular psoriasis (GPP) based on a proposed classification of disease severity. Arch Dermatol Res. 2003;295(suppl 1):S43-S54.
- Viguier M, Allez M, Zagdanski AM, et al. High frequency of cholestasis in generalized pustular psoriasis: evidence for neutrophilic involvement of the biliary tract. Hepatology. 2004;40:452-458.
- Ryan TJ, Baker H. The prognosis of generalized pustular psoriasis. Br J Dermatol. 1971;85:407-411.
- Kalb RE. Pustular psoriasis: management. In: Ofori AO, Duffin KC, eds. UpToDate. UpToDate; 2014. Accessed July 20, 2022. https://www.uptodate.com/contents/pustular-psoriasis-management/print
- Naik HB, Cowen EW. Autoinflammatory pustular neutrophilic diseases. Dermatol Clin. 2013;31:405-425.
- Sidoroff A, Dunant A, Viboud C, et al. Risk factors for acute generalized exanthematous pustulosis (AGEP)—results of a multinational case-control study (EuroSCAR). Br J Dermatol. 2007;157:989-996.
- Robinson A, Van Voorhees AS, Hsu S, et al. Treatment of pustular psoriasis: from the Medical Board of the National Psoriasis Foundation. J Am Acad Dermatol. 2012;67:279‐288.
- Menter A, Strober BE, Kaplan DH, et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with biologics. J Am Acad Dermatol. 2019;80:1029-1072.
- Mengesha YM, Bennett ML. Pustular skin disorders: diagnosis and treatment. Am J Clin Dermatol 2002;3:389-400.
- Zhou LL, Georgakopoulos JR, Ighani A, et al. Systemic monotherapy treatments for generalized pustular psoriasis: a systematic review. J Cutan Med Surg. 2018;22:591‐601.
- Elewski BE. Infliximab for the treatment of severe pustular psoriasis. J Am Acad Dermatol. 2002;47:796-797.
- Kim HS, You HS, Cho HH, et al. Two cases of generalized pustular psoriasis: successful treatment with infliximab. Ann Dermatol. 2014;26:787-788.
- Trent JT, Kerdel FA. Successful treatment of Von Zumbusch pustular psoriasis with infliximab. J Cutan Med Surg. 2004;8:224-228.
- Poulalhon N, Begon E, Lebbé C, et al. A follow-up study in 28 patients treated with infliximab for severe recalcitrant psoriasis: evidence for efficacy and high incidence of biological autoimmunity. Br J Dermatol. 2007;156:329-336.
- Routhouska S, Sheth PB, Korman NJ. Long-term management of generalized pustular psoriasis with infliximab: case series. J Cutan Med Surg. 2008;12:184-188.
- Lisby S, Gniadecki R. Infliximab (Remicade) for acute, severe pustular and erythrodermic psoriasis. Acta Derm Venereol. 2004;84:247-248.
- Zangrilli A, Papoutsaki M, Talamonti M, et al. Long-term efficacy of adalimumab in generalized pustular psoriasis. J Dermatol Treat. 2008;19:185-187.
- Matsumoto A, Komine M, Karakawa M, et al. Adalimumab administration after infliximab therapy is a successful treatment strategy for generalized pustular psoriasis. J Dermatol. 2017;44:202-204.
- Kamarashev J, Lor P, Forster A, et al. Generalized pustular psoriasis induced by cyclosporin in a withdrawal responding to the tumour necrosis factor alpha inhibitor etanercept. Dermatology. 2002;205:213-216.
- Esposito M, Mazzotta A, Casciello C, et al. Etanercept at different dosages in the treatment of generalized pustular psoriasis: a case series. Dermatology. 2008;216:355-360.
- Lo Schiavo A, Brancaccio G, Puca RV, et al. Etanercept in the treatment of generalized annular pustular psoriasis. Ann Dermatol. 2012;24:233-234.
- Goiriz R, Daudén E, Pérez-Gala S, et al. Flare and change of psoriasis morphology during the course of treatment with tumor necrosis factor blockers. Clin Exp Dermatol. 2006;32:176-179.
- Collamer AN, Battafarano DF. Psoriatic skin lesions induced by tumor necrosis factor antagonist therapy: clinical features and possible immunopathogenesis. Semin Arthritis Rheum. 2010;40:233-240.
- Almutairi D, Sheasgreen C, Weizman A, et al. Generalized pustular psoriasis induced by infliximab in a patient with inflammatory bowel disease. J Cutan Med Surg. 2018;1:507-510.
- Imafuku S, Honma M, Okubo Y, et al. Efficacy and safety of secukinumab in patients with generalized pustular psoriasis: a 52-week analysis from phase III open-label multicenter Japanese study. J Dermatol. 2016;43:1011-1017
- Saeki H, Nakagawa H, Ishii T, et al. Efficacy and safety of open-label ixekizumab treatment in Japanese patients with moderate-to-severe plaque psoriasis, erythrodermic psoriasis, and generalized pustular psoriasis. J Eur Acad Dermatol Venereol. 2015;29:1148-1155.
- Yamasaki K, Nakagawa H, Kubo Y, et al. Efficacy and safety of brodalumab in patients with generalized pustular psoriasis and psoriatic erythroderma: results from a 52-week, open-label study. Br J Dermatol. 2017;176:741-751.
- Sano S, Kubo H, Morishima H, et al. Guselkumab, a human interleukin-23 monoclonal antibody in Japanese patients with generalized pustular psoriasis and erythrodermic psoriasis: efficacy and safety analyses of a 52-week, phase 3, multicenter, open-label study. J Dermatol. 2018;45:529‐539.
- Arakawa A, Ruzicka T, Prinz JC. Therapeutic efficacy of interleukin 12/interleukin 23 blockade in generalized pustular psoriasis regardless of IL36RN mutation status. JAMA Dermatol. 2016;152:825-828.
- Mansouri B, Richards L, Menter A. Treatment of two patients with generalized pustular psoriasis with the interleukin-1beta inhibitor gevokizumab. Br J Dermatol. 2015;173:239-241.
- Skendros P, Papagoras C, Lefaki I, et al. Successful response in a case of severe pustular psoriasis after interleukin-1 beta inhibition. Br J Dermatol. 2017;176:212-215.
- Viguier M, Guigue P, Pagès C, et al. Successful treatment of generalized pustular psoriasis with the interleukin-1-receptor antagonist Anakinra: lack of correlation with IL1RN mutations. Ann Intern Med. 2010;153:66-67.
- Fukushima H, Iwata Y, Arima M, et al. Efficacy and safety of treatment with anti-tumor necrosis factor‐α drugs for severe impetigo herpetiformis. J Dermatol. 2021;48:207-210.
- Mizutani Y, Mizutani YH, Matsuyama K, et al. Generalized pustular psoriasis in pregnancy, successfully treated with certolizumab pegol. J Dermatol. 2021;47:e262-e263.
- Bae YS, Van Voorhees AS, Hsu S, et al. Review of treatment options for psoriasis in pregnant or lactating women: from the Medical Board of the National Psoriasis Foundation. J Am Acad Dermatol. 2012;67:459‐477.
- Finch TM, Tan CY. Pustular psoriasis exacerbated by pregnancy and controlled by cyclosporin A. Br J Dermatol. 2000;142:582-584.
- Gaughan WJ, Moritz MJ, Radomski JS, et al. National Transplantation Pregnancy Registry: report on outcomes of cyclosporine-treated female kidney transplant recipients with an interval from transplantation to pregnancy of greater than five years. Am J Kidney Dis. 1996;28:266-269.
- Kura MM, Surjushe AU. Generalized pustular psoriasis of pregnancy treated with oral cyclosporin. Indian J Dermatol Venereol Leprol. 2006;72:458-459.
- Benjegerdes KE, Hyde K, Kivelevitch D, et al. Pustular psoriasis: pathophysiology and current treatment perspectives. Psoriasis (Auckl). 2016;6:131‐144.
- Bachelez H. Pustular psoriasis and related pustular skin diseases. Br J Dermatol. 2018;178:614‐618.
- Gooderham MJ, Van Voorhees AS, Lebwohl MG. An update on generalized pustular psoriasis. Expert Rev Clin Immunol. 2019;15:907‐919.
- Ly K, Beck KM, Smith MP, et al. Diagnosis and screening of patients with generalized pustular psoriasis. Psoriasis (Auckl). 2019;9:37‐42.
- van de Kerkhof PCM, Nestle FO. Psoriasis. In: Bolognia JL, Jorizzo JJ, Schaffer JV, eds. Dermatology. 3rd ed. Elsevier; 2012:138-160.
- Hoegler KM, John AM, Handler MZ, et al. Generalized pustular psoriasis: a review and update on treatment. J Eur Acad Dermatol Venereol. 2018;32:1645‐1651.
- Oumeish OY, Parish JL. Impetigo herpetiformis. Clin Dermatol. 2006;24:101‐104.
- Johnston A, Xing X, Wolterink L, et al. IL-1 and IL-36 are dominant cytokines in generalized pustular psoriasis. J Allergy Clin Immunol. 2017;140:109-120.
- Furue K, Yamamura K, Tsuji G, et al. Highlighting interleukin-36 signalling in plaque psoriasis and pustular psoriasis. Acta Derm Venereol. 2018;98:5-13.
- Ogawa E, Sato Y, Minagawa A, et al. Pathogenesis of psoriasis and development of treatment. J Dermatol. 2018;45:264-272.
- Marrakchi S, Guigue P, Renshaw BR, et al. Interleukin-36-receptor antagonist deficiency and generalized pustular psoriasis. N Engl J Med. 2011;365:620-628.
- Onoufriadis A, Simpson MA, Pink AE, et al. Mutations in IL36RN/IL1F5 are associated with the severe episodic inflammatory skin disease known as generalized pustular psoriasis. Am J Hum Genet. 2011;89:432-437.
- Setta-Kaffetzi N, Navarini AA, Patel VM, et al. Rare pathogenic variants in IL36RN underlie a spectrum of psoriasis-associated pustular phenotypes. J Invest Dermatol. 2013;133:1366-1369.
- Sugiura K, Takemoto A, Yamaguchi M, et al. The majority of generalized pustular psoriasis without psoriasis vulgaris is caused by deficiency of interleukin-36 receptor antagonist. J Invest Dermatol. 2013;133:2514-2521.
- Hussain S, Berki DM, Choon SE, et al. IL36RN mutations define a severe autoinflammatory phenotype of generalized pustular psoriasis. J Allergy Clin Immunol. 2015;135:1067-1070.e9.
- Körber A, Mossner R, Renner R, et al. Mutations in IL36RN in patients with generalized pustular psoriasis. J Invest Dermatol. 2013;133:2634-2637.
- Twelves S, Mostafa A, Dand N, et al. Clinical and genetic differences between pustular psoriasis subtypes. J Allergy Clin Immunol. 2019;143:1021-1026.
- Sugiura K. The genetic background of generalized pustular psoriasis: IL36RN mutations and CARD14 gain-of-function variants. J Dermatol Sci. 2014;74:187-192
- Wang Y, Cheng R, Lu Z, et al. Clinical profiles of pediatric patients with GPP alone and with different IL36RN genotypes. J Dermatol Sci. 2017;85:235-240.
- Setta-Kaffetzi N, Simpson MA, Navarini AA, et al. AP1S3 mutations are associated with pustular psoriasis and impaired Toll-like receptor 3 trafficking. Am J Hum Genet. 2014;94:790-797.
- Mahil SK, Twelves S, Farkas K, et al. AP1S3 mutations cause skin autoinflammation by disrupting keratinocyte autophagy and upregulating IL-36 production. J Invest Dermatol. 2016;136:2251-2259.
- Umezawa Y, Ozawa A, Kawasima T, et al. Therapeutic guidelines for the treatment of generalized pustular psoriasis (GPP) based on a proposed classification of disease severity. Arch Dermatol Res. 2003;295(suppl 1):S43-S54.
- Viguier M, Allez M, Zagdanski AM, et al. High frequency of cholestasis in generalized pustular psoriasis: evidence for neutrophilic involvement of the biliary tract. Hepatology. 2004;40:452-458.
- Ryan TJ, Baker H. The prognosis of generalized pustular psoriasis. Br J Dermatol. 1971;85:407-411.
- Kalb RE. Pustular psoriasis: management. In: Ofori AO, Duffin KC, eds. UpToDate. UpToDate; 2014. Accessed July 20, 2022. https://www.uptodate.com/contents/pustular-psoriasis-management/print
- Naik HB, Cowen EW. Autoinflammatory pustular neutrophilic diseases. Dermatol Clin. 2013;31:405-425.
- Sidoroff A, Dunant A, Viboud C, et al. Risk factors for acute generalized exanthematous pustulosis (AGEP)—results of a multinational case-control study (EuroSCAR). Br J Dermatol. 2007;157:989-996.
- Robinson A, Van Voorhees AS, Hsu S, et al. Treatment of pustular psoriasis: from the Medical Board of the National Psoriasis Foundation. J Am Acad Dermatol. 2012;67:279‐288.
- Menter A, Strober BE, Kaplan DH, et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with biologics. J Am Acad Dermatol. 2019;80:1029-1072.
- Mengesha YM, Bennett ML. Pustular skin disorders: diagnosis and treatment. Am J Clin Dermatol 2002;3:389-400.
- Zhou LL, Georgakopoulos JR, Ighani A, et al. Systemic monotherapy treatments for generalized pustular psoriasis: a systematic review. J Cutan Med Surg. 2018;22:591‐601.
- Elewski BE. Infliximab for the treatment of severe pustular psoriasis. J Am Acad Dermatol. 2002;47:796-797.
- Kim HS, You HS, Cho HH, et al. Two cases of generalized pustular psoriasis: successful treatment with infliximab. Ann Dermatol. 2014;26:787-788.
- Trent JT, Kerdel FA. Successful treatment of Von Zumbusch pustular psoriasis with infliximab. J Cutan Med Surg. 2004;8:224-228.
- Poulalhon N, Begon E, Lebbé C, et al. A follow-up study in 28 patients treated with infliximab for severe recalcitrant psoriasis: evidence for efficacy and high incidence of biological autoimmunity. Br J Dermatol. 2007;156:329-336.
- Routhouska S, Sheth PB, Korman NJ. Long-term management of generalized pustular psoriasis with infliximab: case series. J Cutan Med Surg. 2008;12:184-188.
- Lisby S, Gniadecki R. Infliximab (Remicade) for acute, severe pustular and erythrodermic psoriasis. Acta Derm Venereol. 2004;84:247-248.
- Zangrilli A, Papoutsaki M, Talamonti M, et al. Long-term efficacy of adalimumab in generalized pustular psoriasis. J Dermatol Treat. 2008;19:185-187.
- Matsumoto A, Komine M, Karakawa M, et al. Adalimumab administration after infliximab therapy is a successful treatment strategy for generalized pustular psoriasis. J Dermatol. 2017;44:202-204.
- Kamarashev J, Lor P, Forster A, et al. Generalized pustular psoriasis induced by cyclosporin in a withdrawal responding to the tumour necrosis factor alpha inhibitor etanercept. Dermatology. 2002;205:213-216.
- Esposito M, Mazzotta A, Casciello C, et al. Etanercept at different dosages in the treatment of generalized pustular psoriasis: a case series. Dermatology. 2008;216:355-360.
- Lo Schiavo A, Brancaccio G, Puca RV, et al. Etanercept in the treatment of generalized annular pustular psoriasis. Ann Dermatol. 2012;24:233-234.
- Goiriz R, Daudén E, Pérez-Gala S, et al. Flare and change of psoriasis morphology during the course of treatment with tumor necrosis factor blockers. Clin Exp Dermatol. 2006;32:176-179.
- Collamer AN, Battafarano DF. Psoriatic skin lesions induced by tumor necrosis factor antagonist therapy: clinical features and possible immunopathogenesis. Semin Arthritis Rheum. 2010;40:233-240.
- Almutairi D, Sheasgreen C, Weizman A, et al. Generalized pustular psoriasis induced by infliximab in a patient with inflammatory bowel disease. J Cutan Med Surg. 2018;1:507-510.
- Imafuku S, Honma M, Okubo Y, et al. Efficacy and safety of secukinumab in patients with generalized pustular psoriasis: a 52-week analysis from phase III open-label multicenter Japanese study. J Dermatol. 2016;43:1011-1017
- Saeki H, Nakagawa H, Ishii T, et al. Efficacy and safety of open-label ixekizumab treatment in Japanese patients with moderate-to-severe plaque psoriasis, erythrodermic psoriasis, and generalized pustular psoriasis. J Eur Acad Dermatol Venereol. 2015;29:1148-1155.
- Yamasaki K, Nakagawa H, Kubo Y, et al. Efficacy and safety of brodalumab in patients with generalized pustular psoriasis and psoriatic erythroderma: results from a 52-week, open-label study. Br J Dermatol. 2017;176:741-751.
- Sano S, Kubo H, Morishima H, et al. Guselkumab, a human interleukin-23 monoclonal antibody in Japanese patients with generalized pustular psoriasis and erythrodermic psoriasis: efficacy and safety analyses of a 52-week, phase 3, multicenter, open-label study. J Dermatol. 2018;45:529‐539.
- Arakawa A, Ruzicka T, Prinz JC. Therapeutic efficacy of interleukin 12/interleukin 23 blockade in generalized pustular psoriasis regardless of IL36RN mutation status. JAMA Dermatol. 2016;152:825-828.
- Mansouri B, Richards L, Menter A. Treatment of two patients with generalized pustular psoriasis with the interleukin-1beta inhibitor gevokizumab. Br J Dermatol. 2015;173:239-241.
- Skendros P, Papagoras C, Lefaki I, et al. Successful response in a case of severe pustular psoriasis after interleukin-1 beta inhibition. Br J Dermatol. 2017;176:212-215.
- Viguier M, Guigue P, Pagès C, et al. Successful treatment of generalized pustular psoriasis with the interleukin-1-receptor antagonist Anakinra: lack of correlation with IL1RN mutations. Ann Intern Med. 2010;153:66-67.
- Fukushima H, Iwata Y, Arima M, et al. Efficacy and safety of treatment with anti-tumor necrosis factor‐α drugs for severe impetigo herpetiformis. J Dermatol. 2021;48:207-210.
- Mizutani Y, Mizutani YH, Matsuyama K, et al. Generalized pustular psoriasis in pregnancy, successfully treated with certolizumab pegol. J Dermatol. 2021;47:e262-e263.
- Bae YS, Van Voorhees AS, Hsu S, et al. Review of treatment options for psoriasis in pregnant or lactating women: from the Medical Board of the National Psoriasis Foundation. J Am Acad Dermatol. 2012;67:459‐477.
- Finch TM, Tan CY. Pustular psoriasis exacerbated by pregnancy and controlled by cyclosporin A. Br J Dermatol. 2000;142:582-584.
- Gaughan WJ, Moritz MJ, Radomski JS, et al. National Transplantation Pregnancy Registry: report on outcomes of cyclosporine-treated female kidney transplant recipients with an interval from transplantation to pregnancy of greater than five years. Am J Kidney Dis. 1996;28:266-269.
- Kura MM, Surjushe AU. Generalized pustular psoriasis of pregnancy treated with oral cyclosporin. Indian J Dermatol Venereol Leprol. 2006;72:458-459.
Practice Points
- Generalized pustular psoriasis (GPP) is a rare severe variant of psoriasis that is characterized by the abrupt widespread onset of small pustules.
- Although no treatments have specifically been approved for GPP, various biologics, especially infliximab, may be effective in achieving rapid clearance in patients with GPP. Other oral systemic agents including acitretin, cyclosporine, and methotrexate also have been shown to be effective.
Management of Psoriasis With Topicals: Applying the 2020 AAD-NPF Guidelines of Care to Clinical Practice
Psoriasis is a chronic inflammatory skin disease characterized by erythematous scaly plaques that can invoke substantial pain, pruritus, and quality-of-life disturbance in patients. Topical therapies are the most commonly used medications for treating psoriasis, with one study (N = 128,308) showing that more than 85% of patients with psoriasis were managed solely with topical medications. 1 For patients with mild to moderate psoriasis, topical agents alone may be able to control disease completely. For those with more severe disease, topical agents are used adjunctively with systemic or biologic agents to optimize disease control in localized areas.
The American Academy of Dermatology (AAD) and National Psoriasis Foundation (NPF) published guidelines in 2020 for managing psoriasis with topical agents in adults.2 This review presents the most up-to-date clinical recommendations for topical agent use in adult patients with psoriasis and elaborates on each drug’s pharmacologic and safety profile. Specifically, evidence-based treatment recommendations for topical steroids, calcineurin inhibitors (CNIs), vitamin D analogues, retinoids (tazarotene), emollients, keratolytics (salicylic acid), anthracenes (anthralin), and keratoplastics (coal tar) will be addressed (Table 1). Recommendations for combination therapy with other treatment modalities including UVB light therapy, biologics, and systemic nonbiologic agents also will be discussed.


Selecting a Topical Agent Based on Disease Localization
When treating patients with psoriasis with topical therapies, clinicians should take into consideration drug potency, as it determines how effective a treatment will be in penetrating the skin barrier. Plaque characteristics, such as distribution (localized vs widespread), anatomical localization (flexural, scalp, palms/soles/nails), size (large vs small), and thickness (thick vs thin), not only influence treatment effectiveness but also the incidence of drug-related adverse events. Furthermore, preferred topical therapies are tailored to each patient based on disease characteristics and activity. Coal tar and anthralin have been used less frequently than other topical therapies for psoriasis because of their undesirable side-effect profiles (Table 1).3
Face and Intertriginous Regions—The face and intertriginous areas are sensitive because skin tends to be thin in these regions. Emollients are recommended for disease in these locations given their safety and flexibility in use for most areas. Conversely, anthralin should be avoided on the face, intertriginous areas, and even highly visible locations because of the potential for skin staining. Low-potency corticosteroids also have utility in psoriasis distributed on the face and intertriginous regions. Additionally, application of steroids around the eyes should be cautioned because topical steroids can induce ocular complications such as glaucoma and cataracts in rare circumstances.4
Off-label use of CNIs for psoriasis on the face and intertriginous areas also is effective. Currently, there is a level B recommendation for off-label use of 0.1% tacrolimus for up to 8 weeks for inverse psoriasis or psoriasis on the face. Off-label use of pimecrolimus for 4 to 8 weeks also can be considered for inverse psoriasis. Combination therapy consisting of hydrocortisone with calcipotriol ointment is another effective regimen.5 One study also suggested that use of crisaborole for 4 to 8 weeks in intertriginous psoriasis can be effective and well tolerated.6
Scalp—The vehicle of medication administration is especially important in hair-bearing areas such as the scalp, as these areas are challenging for medication application and patient adherence. Thus, patient preferences for the vehicle must be considered. Several studies have been conducted to assess preference for various vehicles in scalp psoriasis. A foam or solution may be preferable to ointments, gels, or creams.7 Gels may be preferred over ointments.8 There is a level A recommendation supporting the use of class 1 to 7 topical steroids for a minimum of 4 weeks as initial and maintenance treatment of scalp psoriasis. The highest level of evidence (level A) also supports the use of calcipotriol foam or combination therapy of calcipotriol–betamethasone dipropionate gel for 4 to 12 weeks as treatment of mild to moderate scalp psoriasis.
Nails—Several options for topical medications have been recommended for the treatment of nail psoriasis. Currently, there is a level B recommendation for the use of tazarotene for the treatment of nail psoriasis. Another effective regimen is combination therapy with vitamin D analogues and betamethasone dipropionate.9 Topical steroid use for nail psoriasis should be limited to 12 weeks because of the risk for bone atrophy with chronic steroid use.
Palmoplantar—The palms and soles have a thicker epidermal layer than other areas of the body. As a result, class 1 corticosteroids can be used for palmoplantar psoriasis for more than 4 weeks with vigilant monitoring for adverse effects such as skin atrophy, tachyphylaxis, or tinea infection. Tazarotene also has been shown to be helpful in treating palmoplantar psoriasis.
Resistant Disease—Intralesional steroids are beneficial treatment options for recalcitrant psoriasis in glabrous areas, as well as for palmoplantar, nail, and scalp psoriasis. Up to 10 mg/mL of triamcinolone acetonide used every 3 to 4 weeks is an effective regimen.10Pregnancy/Breastfeeding—Women of childbearing potential have additional safety precautions that should be considered during medication selection. Emollients have been shown to be safe during pregnancy and lactation. Currently, there is little known about CNI use during pregnancy. During lactation, CNIs can be used by breastfeeding mothers in most areas, excluding the breasts. Evaluation of the safety of anthralin and vitamin D analogues during pregnancy and lactation have not been studied. For these agents, dermatologists need to use their clinical judgment to weigh the risks and benefits of medication, particularly in patients requiring occlusion, higher medication doses, or treatment over a large surface area. Salicylic acid should be used with caution in pregnant and breastfeeding mothers because it is a pregnancy category C drug. Lower-potency corticosteroids may be used with caution during pregnancy and breastfeeding. More potent corticosteroids and coal tar, however, should be avoided. Similarly, tazarotene use is contraindicated in pregnancy. According to the US Food and Drug Administration labels for all forms of topical tazarotene, a pregnancy test must be obtained 2 weeks prior to tazarotene treatment initiation in women of childbearing potential because of the risk for serious fetal malformations and toxicity.
Recommendations, Risks, and Benefits of Topical Therapy for the Management of Psoriasis
Topical Corticosteroids—Topical corticosteroids (TCs) are widely used for inflammatory skin conditions and are available in a variety of strengths (Table 2). They are thought to exert their action by regulating the gene transcription of proinflammatory mediators. For psoriasis, steroids are recommended for 2 to 4 weeks, depending on disease severity. Although potent and superpotent steroids are more effective than mild- to moderate-strength TCs, use of lower-potency TCs may be warranted depending on disease distribution and localization.11 For treatment of psoriasis with no involvement of the intertriginous areas, use of class 1 to 5 TCs for up to 4 weeks is recommended.

For moderate to severe psoriasis with 20% or less body surface area (BSA) affected, combination therapy consisting of mometasone and salicylic acid has been shown to be more effective than mometasone alone.12,13 There currently is a level A recommendation for the use of combination therapy with class 1 TCs and etanercept for 12 weeks in patients with moderate to severe psoriasis who require both systemic and topical therapies for disease control. Similarly, combination therapy with infliximab and high-potency TCs has a level B recommendation to enhance efficacy for the treatment of moderate to severe psoriasis.14 High-quality studies on the use of TCs with anti–IL-12/IL-23, anti–IL-23, and anti–IL-17 currently are unavailable, but the combination is not expected to be unsafe.14,15 Combination therapy of betamethasone dipropionate ointment and low-dose cyclosporine is an alternative regimen with a level B recommendation.
The most common adverse effects with use of TCs are skin thinning and atrophy, telangiectasia, and striae (Table 1). With clinical improvement of disease, it is recommended that clinicians taper TCs to prevent rebound effect. To decrease TC-related adverse effects, clinicians should use combination therapy with steroid-sparing agents for disease maintenance, transition to lower-potency corticosteroids, or use intermittent steroid therapy. Systemic effects of TC use include hypothalamic-pituitary-adrenal axis suppression, Cushing syndrome, and osteonecrosis of the femoral head.16-18 These systemic effects with TC use are rare unless treatment is for disease involving greater than 20% BSA or occlusion for more than 4 weeks.
Calcineurin Inhibitors—Calcineurin inhibitors inhibit calcineurin phosphorylation and T-cell activation, subsequently decreasing the expression of proinflammatory cytokines. Currently, they are not approved by the US Food and Drug Administration to treat psoriasis but have demonstrated efficacy in randomized control trials (RCTs) for facial and intertriginous psoriasis. In RCTs, 71% of patients using pimecrolimus cream 0.1% twice daily for 8 weeks achieved an investigator global assessment score of clear (0) or almost clear (1) compared with 21% of placebo-treated patients (N=57).19 Other trials have shown that 65% of patients receiving tacrolimus ointment 0.1% for 8 weeks achieved an investigator global assessment score of 0 or 1 compared with 31% of placebo-treated patients (N=167).20 Because of their efficacy in RCTs, CNIs commonly are used off label to treat psoriasis.
The most common adverse effects with CNI use are burning, pruritus, and flushing with alcohol ingestion (Table 1). Additionally, CNIs have a black box warning that use may increase the risk for malignancy, but this risk has not been demonstrated with topical use in humans.21Vitamin D Analogues—The class of vitamin D analogues—calcipotriol/calcipotriene and calcitriol—frequently are used to treat psoriasis. Vitamin D analogues exert their beneficial effects by inhibiting keratinocyte proliferation and enhancing keratinocyte differentiation. They also are ideal for long-term use (up to 52 weeks) in mild to moderate psoriasis and can be used in combination with class 2 and 3 TCs. There is a level A recommendation that supports the use of combination therapy with calcipotriol and TCs for the treatment of mild to moderate psoriasis.
For severe psoriasis, many studies have investigated the efficacy of combination therapy with vitamin D analogues and systemic treatments. Combination therapy with calcipotriol and methotrexate or calcipotriol and acitretin are effective treatment regimens with level A recommendations. Calcipotriol–betamethasone dipropionate ointment in combination with low-dose cyclosporine is an alternative option with a level B recommendation. Because vitamin D analogues are inactivated by UVA and UVB radiation, clinicians should advise their patients to use vitamin D analogues after receiving UVB phototherapy.22
Common adverse effects of vitamin D analogues include burning, pruritus, erythema, and dryness (Table 1). Hypercalcemia and parathyroid hormone suppression are extremely rare unless treatment occurs over a large surface area (>30% BSA) or the patient has concurrent renal disease or impairments in calcium metabolism.
Tazarotene—Tazarotene is a topical retinoid that acts by decreasing keratinocyte proliferation, facilitating keratinocyte differentiation, and inhibiting inflammation. Patients with mild to moderate psoriasis are recommended to receive tazarotene treatment for 8 to 12 weeks. In several RCTs, tazarotene gel 0.1% and tazarotene cream 0.1% and 0.05% achieved treatment success in treating plaque psoriasis.23,24
For increased efficacy, clinicians can recommend combination therapy with tazarotene and a TC. Combination therapy with tazarotene and a mid- or high-potency TC for 8 to 16 weeks has been shown to be more effective than treatment with tazarotene alone.25 Thus, there is a level A recommendation for use of this combination to treat mild to moderate psoriasis. Agents used in combination therapy work synergistically to decrease the length of treatment and increase the duration of remission. The frequency of adverse effects, such as irritation from tazarotene and skin atrophy from TCs, also are reduced.26 Combination therapy with tazarotene and narrowband UVB (NB-UVB) is another effective option that requires less UV radiation than NB-UVB alone because of the synergistic effects of both treatment modalities.27 Clinicians should counsel patients on the adverse effects of tazarotene, which include local irritation, burning, pruritus, and erythema (Table 1).
Emollients—Emollients are nonmedicated moisturizers that decrease the amount of transepidermal water loss. There is a level B recommendation for use of emollients and TCs in combination for 4 to 8 weeks to treat psoriasis. In fact, combination therapy with mometasone and emollients has demonstrated greater improvement in symptoms of palmoplantar psoriasis (ie, erythema, desquamation, infiltration, BSA involvement) than mometasone alone.28 Emollients are safe options that can be used on all areas of the body and during pregnancy and lactation. Although adverse effects of emollients are rare, clinicians should counsel patients on the risk for contact dermatitis if specific allergies to ingredients/fragrances exist (Table 1).
Salicylic Acid—Salicylic acid is a topical keratolytic that can be used to treat psoriatic plaques. Use of salicylic acid for 8 to 16 weeks has been shown to be effective for mild to moderate psoriasis. Combination therapy of salicylic acid and TCs in patients with 20% or less BSA affected is a safe and effective option with a level B recommendation. Combination therapy with salicylic acid and calcipotriene, however, should be avoided because calcipotriene is inactivated by salicylic acid. It also is recommended that salicylic acid application follow phototherapy when both treatment modalities are used in combination.29,30 Clinicians should be cautious about using salicylic acid in patients with renal or hepatic disease because of the increased risk for salicylate toxicity (Table 1).
Anthralin—Anthralin is a synthetic hydrocarbon derivative that has been shown to reduce inflammation and normalize keratinocyte proliferation through an unknown mechanism. It is recommended that patients with mild to moderate psoriasis receive anthralin treatment for 8 to 12 weeks, with a maximum application time of 2 hours per day. Combination therapy of excimer laser and anthralin has been shown to be more effective in treating psoriasis than anthralin alone.31 Therefore, clinicians have the option of including excimer laser therapy for additional disease control. Anthralin should be avoided on the face, flexural regions, and highly visible areas because of potential skin staining (Table 1). Other adverse effects include application-site burning and erythema.
Coal Tar—Coal tar is a heterogenous mixture of aromatic hydrocarbons that is an effective treatment of psoriasis because of its inherent anti-inflammatory and keratoplastic properties. There is high-quality evidence supporting a level A recommendation for coal tar use in mild to moderate psoriasis. Combination therapy with NB-UVB and coal tar (also known as Goeckerman therapy) is a recommended treatment option with a quicker onset of action and improved outcomes compared with NB-UVB therapy alone.32,33 Adverse events of coal tar include application-site irritation, folliculitis, contact dermatitis, phototoxicity, and skin pigmentation (Table 1).
Conclusion
Topical medications are versatile treatment options that can be utilized as monotherapy or adjunct therapy for mild to severe psoriasis. Benefits of topical agents include minimal required monitoring, few contraindications, and direct localized effect on plaques. Therefore, side effects with topical agent use rarely are systemic. Medication interactions are less of a concern with topical therapies; thus, they have better safety profiles compared with systemic therapies. This clinical review summarizes the recently published evidence-based guidelines from the AAD and NPF on the use of topical agents in psoriasis and may be a useful guiding framework for clinicians in their everyday practice.
- Murage MJ, Kern DM, Chang L, et al. Treatment patterns among patients with psoriasis using a large national payer database in the United States: a retrospective study. J Med Econ. 2018:1-9.
- Elmets CA, Korman NJ, Prater EF, et al. Joint AAD-NPF Guidelines of care for the management and treatment of psoriasis with topical therapy and alternative medicine modalities for psoriasis severity measures. J Am Acad Dermatol. 2021;84:432-470.
- Svendsen MT, Jeyabalan J, Andersen KE, et al. Worldwide utilization of topical remedies in treatment of psoriasis: a systematic review. J Dermatolog Treat. 2017;28:374-383.
- Day A, Abramson AK, Patel M, et al. The spectrum of oculocutaneous disease: part II. neoplastic and drug-related causes of oculocutaneous disease. J Am Acad Dermatol. 2014;70:821.e821-819.
- Choi JW, Choi JW, Kwon IH, et al. High-concentration (20 μg g-¹) tacalcitol ointment in the treatment of facial psoriasis: an 8-week open-label clinical trial. Br J Dermatol. 2010;162:1359-1364.
- Hashim PW, Chima M, Kim HJ, et al. Crisaborole 2% ointment for the treatment of intertriginous, anogenital, and facial psoriasis: a double-blind, randomized, vehicle-controlled trial. J Am Acad Dermatol. 2020;82:360-365.
- Housman TS, Mellen BG, Rapp SR, et al. Patients with psoriasis prefer solution and foam vehicles: a quantitative assessment of vehicle preference. Cutis. 2002;70:327-332.
- Iversen L, Jakobsen HB. Patient preferences for topical psoriasis treatments are diverse and difficult to predict. Dermatol Ther. 2016;6:273-285.
- Clobex Package insert. Galderma Laboratories, LP; 2012.
- Kenalog-10 Injection. Package insert. Bristol-Myers Squibb Company; 2018.
- Mason J, Mason AR, Cork MJ. Topical preparations for the treatment of psoriasis: a systematic review. Br J Dermatol. 2002;146:351-364.
- Koo J, Cuffie CA, Tanner DJ, et al. Mometasone furoate 0.1%-salicylic acid 5% ointment versus mometasone furoate 0.1% ointment in the treatment of moderate-to-severe psoriasis: a multicenter study. Clin Ther. 1998;20:283-291.
- Tiplica GS, Salavastru CM. Mometasone furoate 0.1% and salicylic acid 5% vs. mometasone furoate 0.1% as sequential local therapy in psoriasis vulgaris. J Eur Acad Dermatol Venereol. 2009;23:905-912.
- Menter A, Strober BE, Kaplan DH, et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with biologics. J Am Acad Dermatol. 2019;80:1029-1072.
- Strober BE, Bissonnette R, Fiorentino D, et al. Comparative effectiveness of biologic agents for the treatment of psoriasis in a real-world setting: results from a large, prospective, observational study (Psoriasis Longitudinal Assessment and Registry [PSOLAR]). J Am Acad Dermatol. 2016;74:851-861.e854.
- Castela E, Archier E, Devaux S, et al. Topical corticosteroids in plaque psoriasis: a systematic review of risk of adrenal axis suppression and skin atrophy. J Eur Acad Dermatol Venereol. 2012;26(suppl 3):47-51.
- Takahashi H, Tsuji H, Honma M, et al. Femoral head osteonecrosis after long-term topical corticosteroid treatment in a psoriasis patient. J Dermatol. 2012;39:887-888.
- el Maghraoui A, Tabache F, Bezza A, et al. Femoral head osteonecrosis after topical corticosteroid therapy. Clin Exp Rheumatol. 2001;19:233.
- Gribetz C, Ling M, Lebwohl M, et al. Pimecrolimus cream 1% in the treatment of intertriginous psoriasis: a double-blind, randomized study. J Am Acad Dermatol. 2004;51:731-738.
- Lebwohl M, Freeman AK, Chapman MS, et al. Tacrolimus ointment is effective for facial and intertriginous psoriasis. J Am Acad Dermatol. 2004;51:723-730.
- Paller AS, Fölster-Holst R, Chen SC, et al. No evidence of increased cancer incidence in children using topical tacrolimus for atopic dermatitis. J Am Acad Dermatol. 2020;83:375-381.
- Elmets CA, Lim HW, Stoff B, et al. Joint American Academy of Dermatology-National Psoriasis Foundation guidelines of care for the management and treatment of psoriasis with phototherapy. J Am Acad Dermatol. 2019;81:775-804.
- Lebwohl M, Ast E, Callen JP, et al. Once-daily tazarotene gel versus twice-daily fluocinonide cream in the treatment of plaque psoriasis. J Am Acad Dermatol. 1998;38:705-711.
- Weinstein GD, Koo JY, Krueger GG, et al. Tazarotene cream in the treatment of psoriasis: two multicenter, double-blind, randomized, vehicle-controlled studies of the safety and efficacy of tazarotene creams 0.05% and 0.1% applied once daily for 12 weeks. J Am Acad Dermatol. 2003;48:760-767.
- Lebwohl M, Lombardi K, Tan MH. Duration of improvement in psoriasis after treatment with tazarotene 0.1% gel plus clobetasol propionate 0.05% ointment: comparison of maintenance treatments. Int J Dermatol. 2001;40:64-66.
- Sugarman JL, Weiss J, Tanghetti EA, et al. Safety and efficacy of a fixed combination halobetasol and tazarotene lotion in the treatment of moderate-to-severe plaque psoriasis: a pooled analysis of two phase 3 studies. J Drugs Dermatol. 2018;17:855-861.
- Koo JY, Lowe NJ, Lew-Kaya DA, et al. Tazarotene plus UVB phototherapy in the treatment of psoriasis. J Am Acad Dermatol. 2000;43:821-828.
- Cassano N, Mantegazza R, Battaglini S, et al. Adjuvant role of a new emollient cream in patients with palmar and/or plantar psoriasis: a pilot randomized open-label study. G Ital Dermatol Venereol. 2010;145:789-792.
- Kristensen B, Kristensen O. Topical salicylic acid interferes with UVB therapy for psoriasis. Acta Derm Venereol. 1991;71:37-40.
- Menter A, Korman NJ, Elmets CA, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis. section 3. guidelines of care for the management and treatment of psoriasis with topical therapies. J Am Acad Dermatol. 2009;60:643-659.
- Rogalski C, Grunewald S, Schetschorke M, et al. Treatment of plaque-type psoriasis with the 308 nm excimer laser in combination with dithranol or calcipotriol. Int J Hyperthermia. 2012;28:184-190.
- Bagel J. LCD plus NB-UVB reduces time to improvement of psoriasis vs. NB-UVB alone. J Drugs Dermatol. 2009;8:351-357.
- Abdallah MA, El-Khateeb EA, Abdel-Rahman SH. The influence of psoriatic plaques pretreatment with crude coal tar vs. petrolatum on the efficacy of narrow-band ultraviolet B: a half-vs.-half intra-individual double-blinded comparative study. Photodermatol Photoimmunol Photomed. 2011;27:226-230.
Psoriasis is a chronic inflammatory skin disease characterized by erythematous scaly plaques that can invoke substantial pain, pruritus, and quality-of-life disturbance in patients. Topical therapies are the most commonly used medications for treating psoriasis, with one study (N = 128,308) showing that more than 85% of patients with psoriasis were managed solely with topical medications. 1 For patients with mild to moderate psoriasis, topical agents alone may be able to control disease completely. For those with more severe disease, topical agents are used adjunctively with systemic or biologic agents to optimize disease control in localized areas.
The American Academy of Dermatology (AAD) and National Psoriasis Foundation (NPF) published guidelines in 2020 for managing psoriasis with topical agents in adults.2 This review presents the most up-to-date clinical recommendations for topical agent use in adult patients with psoriasis and elaborates on each drug’s pharmacologic and safety profile. Specifically, evidence-based treatment recommendations for topical steroids, calcineurin inhibitors (CNIs), vitamin D analogues, retinoids (tazarotene), emollients, keratolytics (salicylic acid), anthracenes (anthralin), and keratoplastics (coal tar) will be addressed (Table 1). Recommendations for combination therapy with other treatment modalities including UVB light therapy, biologics, and systemic nonbiologic agents also will be discussed.


Selecting a Topical Agent Based on Disease Localization
When treating patients with psoriasis with topical therapies, clinicians should take into consideration drug potency, as it determines how effective a treatment will be in penetrating the skin barrier. Plaque characteristics, such as distribution (localized vs widespread), anatomical localization (flexural, scalp, palms/soles/nails), size (large vs small), and thickness (thick vs thin), not only influence treatment effectiveness but also the incidence of drug-related adverse events. Furthermore, preferred topical therapies are tailored to each patient based on disease characteristics and activity. Coal tar and anthralin have been used less frequently than other topical therapies for psoriasis because of their undesirable side-effect profiles (Table 1).3
Face and Intertriginous Regions—The face and intertriginous areas are sensitive because skin tends to be thin in these regions. Emollients are recommended for disease in these locations given their safety and flexibility in use for most areas. Conversely, anthralin should be avoided on the face, intertriginous areas, and even highly visible locations because of the potential for skin staining. Low-potency corticosteroids also have utility in psoriasis distributed on the face and intertriginous regions. Additionally, application of steroids around the eyes should be cautioned because topical steroids can induce ocular complications such as glaucoma and cataracts in rare circumstances.4
Off-label use of CNIs for psoriasis on the face and intertriginous areas also is effective. Currently, there is a level B recommendation for off-label use of 0.1% tacrolimus for up to 8 weeks for inverse psoriasis or psoriasis on the face. Off-label use of pimecrolimus for 4 to 8 weeks also can be considered for inverse psoriasis. Combination therapy consisting of hydrocortisone with calcipotriol ointment is another effective regimen.5 One study also suggested that use of crisaborole for 4 to 8 weeks in intertriginous psoriasis can be effective and well tolerated.6
Scalp—The vehicle of medication administration is especially important in hair-bearing areas such as the scalp, as these areas are challenging for medication application and patient adherence. Thus, patient preferences for the vehicle must be considered. Several studies have been conducted to assess preference for various vehicles in scalp psoriasis. A foam or solution may be preferable to ointments, gels, or creams.7 Gels may be preferred over ointments.8 There is a level A recommendation supporting the use of class 1 to 7 topical steroids for a minimum of 4 weeks as initial and maintenance treatment of scalp psoriasis. The highest level of evidence (level A) also supports the use of calcipotriol foam or combination therapy of calcipotriol–betamethasone dipropionate gel for 4 to 12 weeks as treatment of mild to moderate scalp psoriasis.
Nails—Several options for topical medications have been recommended for the treatment of nail psoriasis. Currently, there is a level B recommendation for the use of tazarotene for the treatment of nail psoriasis. Another effective regimen is combination therapy with vitamin D analogues and betamethasone dipropionate.9 Topical steroid use for nail psoriasis should be limited to 12 weeks because of the risk for bone atrophy with chronic steroid use.
Palmoplantar—The palms and soles have a thicker epidermal layer than other areas of the body. As a result, class 1 corticosteroids can be used for palmoplantar psoriasis for more than 4 weeks with vigilant monitoring for adverse effects such as skin atrophy, tachyphylaxis, or tinea infection. Tazarotene also has been shown to be helpful in treating palmoplantar psoriasis.
Resistant Disease—Intralesional steroids are beneficial treatment options for recalcitrant psoriasis in glabrous areas, as well as for palmoplantar, nail, and scalp psoriasis. Up to 10 mg/mL of triamcinolone acetonide used every 3 to 4 weeks is an effective regimen.10Pregnancy/Breastfeeding—Women of childbearing potential have additional safety precautions that should be considered during medication selection. Emollients have been shown to be safe during pregnancy and lactation. Currently, there is little known about CNI use during pregnancy. During lactation, CNIs can be used by breastfeeding mothers in most areas, excluding the breasts. Evaluation of the safety of anthralin and vitamin D analogues during pregnancy and lactation have not been studied. For these agents, dermatologists need to use their clinical judgment to weigh the risks and benefits of medication, particularly in patients requiring occlusion, higher medication doses, or treatment over a large surface area. Salicylic acid should be used with caution in pregnant and breastfeeding mothers because it is a pregnancy category C drug. Lower-potency corticosteroids may be used with caution during pregnancy and breastfeeding. More potent corticosteroids and coal tar, however, should be avoided. Similarly, tazarotene use is contraindicated in pregnancy. According to the US Food and Drug Administration labels for all forms of topical tazarotene, a pregnancy test must be obtained 2 weeks prior to tazarotene treatment initiation in women of childbearing potential because of the risk for serious fetal malformations and toxicity.
Recommendations, Risks, and Benefits of Topical Therapy for the Management of Psoriasis
Topical Corticosteroids—Topical corticosteroids (TCs) are widely used for inflammatory skin conditions and are available in a variety of strengths (Table 2). They are thought to exert their action by regulating the gene transcription of proinflammatory mediators. For psoriasis, steroids are recommended for 2 to 4 weeks, depending on disease severity. Although potent and superpotent steroids are more effective than mild- to moderate-strength TCs, use of lower-potency TCs may be warranted depending on disease distribution and localization.11 For treatment of psoriasis with no involvement of the intertriginous areas, use of class 1 to 5 TCs for up to 4 weeks is recommended.

For moderate to severe psoriasis with 20% or less body surface area (BSA) affected, combination therapy consisting of mometasone and salicylic acid has been shown to be more effective than mometasone alone.12,13 There currently is a level A recommendation for the use of combination therapy with class 1 TCs and etanercept for 12 weeks in patients with moderate to severe psoriasis who require both systemic and topical therapies for disease control. Similarly, combination therapy with infliximab and high-potency TCs has a level B recommendation to enhance efficacy for the treatment of moderate to severe psoriasis.14 High-quality studies on the use of TCs with anti–IL-12/IL-23, anti–IL-23, and anti–IL-17 currently are unavailable, but the combination is not expected to be unsafe.14,15 Combination therapy of betamethasone dipropionate ointment and low-dose cyclosporine is an alternative regimen with a level B recommendation.
The most common adverse effects with use of TCs are skin thinning and atrophy, telangiectasia, and striae (Table 1). With clinical improvement of disease, it is recommended that clinicians taper TCs to prevent rebound effect. To decrease TC-related adverse effects, clinicians should use combination therapy with steroid-sparing agents for disease maintenance, transition to lower-potency corticosteroids, or use intermittent steroid therapy. Systemic effects of TC use include hypothalamic-pituitary-adrenal axis suppression, Cushing syndrome, and osteonecrosis of the femoral head.16-18 These systemic effects with TC use are rare unless treatment is for disease involving greater than 20% BSA or occlusion for more than 4 weeks.
Calcineurin Inhibitors—Calcineurin inhibitors inhibit calcineurin phosphorylation and T-cell activation, subsequently decreasing the expression of proinflammatory cytokines. Currently, they are not approved by the US Food and Drug Administration to treat psoriasis but have demonstrated efficacy in randomized control trials (RCTs) for facial and intertriginous psoriasis. In RCTs, 71% of patients using pimecrolimus cream 0.1% twice daily for 8 weeks achieved an investigator global assessment score of clear (0) or almost clear (1) compared with 21% of placebo-treated patients (N=57).19 Other trials have shown that 65% of patients receiving tacrolimus ointment 0.1% for 8 weeks achieved an investigator global assessment score of 0 or 1 compared with 31% of placebo-treated patients (N=167).20 Because of their efficacy in RCTs, CNIs commonly are used off label to treat psoriasis.
The most common adverse effects with CNI use are burning, pruritus, and flushing with alcohol ingestion (Table 1). Additionally, CNIs have a black box warning that use may increase the risk for malignancy, but this risk has not been demonstrated with topical use in humans.21Vitamin D Analogues—The class of vitamin D analogues—calcipotriol/calcipotriene and calcitriol—frequently are used to treat psoriasis. Vitamin D analogues exert their beneficial effects by inhibiting keratinocyte proliferation and enhancing keratinocyte differentiation. They also are ideal for long-term use (up to 52 weeks) in mild to moderate psoriasis and can be used in combination with class 2 and 3 TCs. There is a level A recommendation that supports the use of combination therapy with calcipotriol and TCs for the treatment of mild to moderate psoriasis.
For severe psoriasis, many studies have investigated the efficacy of combination therapy with vitamin D analogues and systemic treatments. Combination therapy with calcipotriol and methotrexate or calcipotriol and acitretin are effective treatment regimens with level A recommendations. Calcipotriol–betamethasone dipropionate ointment in combination with low-dose cyclosporine is an alternative option with a level B recommendation. Because vitamin D analogues are inactivated by UVA and UVB radiation, clinicians should advise their patients to use vitamin D analogues after receiving UVB phototherapy.22
Common adverse effects of vitamin D analogues include burning, pruritus, erythema, and dryness (Table 1). Hypercalcemia and parathyroid hormone suppression are extremely rare unless treatment occurs over a large surface area (>30% BSA) or the patient has concurrent renal disease or impairments in calcium metabolism.
Tazarotene—Tazarotene is a topical retinoid that acts by decreasing keratinocyte proliferation, facilitating keratinocyte differentiation, and inhibiting inflammation. Patients with mild to moderate psoriasis are recommended to receive tazarotene treatment for 8 to 12 weeks. In several RCTs, tazarotene gel 0.1% and tazarotene cream 0.1% and 0.05% achieved treatment success in treating plaque psoriasis.23,24
For increased efficacy, clinicians can recommend combination therapy with tazarotene and a TC. Combination therapy with tazarotene and a mid- or high-potency TC for 8 to 16 weeks has been shown to be more effective than treatment with tazarotene alone.25 Thus, there is a level A recommendation for use of this combination to treat mild to moderate psoriasis. Agents used in combination therapy work synergistically to decrease the length of treatment and increase the duration of remission. The frequency of adverse effects, such as irritation from tazarotene and skin atrophy from TCs, also are reduced.26 Combination therapy with tazarotene and narrowband UVB (NB-UVB) is another effective option that requires less UV radiation than NB-UVB alone because of the synergistic effects of both treatment modalities.27 Clinicians should counsel patients on the adverse effects of tazarotene, which include local irritation, burning, pruritus, and erythema (Table 1).
Emollients—Emollients are nonmedicated moisturizers that decrease the amount of transepidermal water loss. There is a level B recommendation for use of emollients and TCs in combination for 4 to 8 weeks to treat psoriasis. In fact, combination therapy with mometasone and emollients has demonstrated greater improvement in symptoms of palmoplantar psoriasis (ie, erythema, desquamation, infiltration, BSA involvement) than mometasone alone.28 Emollients are safe options that can be used on all areas of the body and during pregnancy and lactation. Although adverse effects of emollients are rare, clinicians should counsel patients on the risk for contact dermatitis if specific allergies to ingredients/fragrances exist (Table 1).
Salicylic Acid—Salicylic acid is a topical keratolytic that can be used to treat psoriatic plaques. Use of salicylic acid for 8 to 16 weeks has been shown to be effective for mild to moderate psoriasis. Combination therapy of salicylic acid and TCs in patients with 20% or less BSA affected is a safe and effective option with a level B recommendation. Combination therapy with salicylic acid and calcipotriene, however, should be avoided because calcipotriene is inactivated by salicylic acid. It also is recommended that salicylic acid application follow phototherapy when both treatment modalities are used in combination.29,30 Clinicians should be cautious about using salicylic acid in patients with renal or hepatic disease because of the increased risk for salicylate toxicity (Table 1).
Anthralin—Anthralin is a synthetic hydrocarbon derivative that has been shown to reduce inflammation and normalize keratinocyte proliferation through an unknown mechanism. It is recommended that patients with mild to moderate psoriasis receive anthralin treatment for 8 to 12 weeks, with a maximum application time of 2 hours per day. Combination therapy of excimer laser and anthralin has been shown to be more effective in treating psoriasis than anthralin alone.31 Therefore, clinicians have the option of including excimer laser therapy for additional disease control. Anthralin should be avoided on the face, flexural regions, and highly visible areas because of potential skin staining (Table 1). Other adverse effects include application-site burning and erythema.
Coal Tar—Coal tar is a heterogenous mixture of aromatic hydrocarbons that is an effective treatment of psoriasis because of its inherent anti-inflammatory and keratoplastic properties. There is high-quality evidence supporting a level A recommendation for coal tar use in mild to moderate psoriasis. Combination therapy with NB-UVB and coal tar (also known as Goeckerman therapy) is a recommended treatment option with a quicker onset of action and improved outcomes compared with NB-UVB therapy alone.32,33 Adverse events of coal tar include application-site irritation, folliculitis, contact dermatitis, phototoxicity, and skin pigmentation (Table 1).
Conclusion
Topical medications are versatile treatment options that can be utilized as monotherapy or adjunct therapy for mild to severe psoriasis. Benefits of topical agents include minimal required monitoring, few contraindications, and direct localized effect on plaques. Therefore, side effects with topical agent use rarely are systemic. Medication interactions are less of a concern with topical therapies; thus, they have better safety profiles compared with systemic therapies. This clinical review summarizes the recently published evidence-based guidelines from the AAD and NPF on the use of topical agents in psoriasis and may be a useful guiding framework for clinicians in their everyday practice.
Psoriasis is a chronic inflammatory skin disease characterized by erythematous scaly plaques that can invoke substantial pain, pruritus, and quality-of-life disturbance in patients. Topical therapies are the most commonly used medications for treating psoriasis, with one study (N = 128,308) showing that more than 85% of patients with psoriasis were managed solely with topical medications. 1 For patients with mild to moderate psoriasis, topical agents alone may be able to control disease completely. For those with more severe disease, topical agents are used adjunctively with systemic or biologic agents to optimize disease control in localized areas.
The American Academy of Dermatology (AAD) and National Psoriasis Foundation (NPF) published guidelines in 2020 for managing psoriasis with topical agents in adults.2 This review presents the most up-to-date clinical recommendations for topical agent use in adult patients with psoriasis and elaborates on each drug’s pharmacologic and safety profile. Specifically, evidence-based treatment recommendations for topical steroids, calcineurin inhibitors (CNIs), vitamin D analogues, retinoids (tazarotene), emollients, keratolytics (salicylic acid), anthracenes (anthralin), and keratoplastics (coal tar) will be addressed (Table 1). Recommendations for combination therapy with other treatment modalities including UVB light therapy, biologics, and systemic nonbiologic agents also will be discussed.


Selecting a Topical Agent Based on Disease Localization
When treating patients with psoriasis with topical therapies, clinicians should take into consideration drug potency, as it determines how effective a treatment will be in penetrating the skin barrier. Plaque characteristics, such as distribution (localized vs widespread), anatomical localization (flexural, scalp, palms/soles/nails), size (large vs small), and thickness (thick vs thin), not only influence treatment effectiveness but also the incidence of drug-related adverse events. Furthermore, preferred topical therapies are tailored to each patient based on disease characteristics and activity. Coal tar and anthralin have been used less frequently than other topical therapies for psoriasis because of their undesirable side-effect profiles (Table 1).3
Face and Intertriginous Regions—The face and intertriginous areas are sensitive because skin tends to be thin in these regions. Emollients are recommended for disease in these locations given their safety and flexibility in use for most areas. Conversely, anthralin should be avoided on the face, intertriginous areas, and even highly visible locations because of the potential for skin staining. Low-potency corticosteroids also have utility in psoriasis distributed on the face and intertriginous regions. Additionally, application of steroids around the eyes should be cautioned because topical steroids can induce ocular complications such as glaucoma and cataracts in rare circumstances.4
Off-label use of CNIs for psoriasis on the face and intertriginous areas also is effective. Currently, there is a level B recommendation for off-label use of 0.1% tacrolimus for up to 8 weeks for inverse psoriasis or psoriasis on the face. Off-label use of pimecrolimus for 4 to 8 weeks also can be considered for inverse psoriasis. Combination therapy consisting of hydrocortisone with calcipotriol ointment is another effective regimen.5 One study also suggested that use of crisaborole for 4 to 8 weeks in intertriginous psoriasis can be effective and well tolerated.6
Scalp—The vehicle of medication administration is especially important in hair-bearing areas such as the scalp, as these areas are challenging for medication application and patient adherence. Thus, patient preferences for the vehicle must be considered. Several studies have been conducted to assess preference for various vehicles in scalp psoriasis. A foam or solution may be preferable to ointments, gels, or creams.7 Gels may be preferred over ointments.8 There is a level A recommendation supporting the use of class 1 to 7 topical steroids for a minimum of 4 weeks as initial and maintenance treatment of scalp psoriasis. The highest level of evidence (level A) also supports the use of calcipotriol foam or combination therapy of calcipotriol–betamethasone dipropionate gel for 4 to 12 weeks as treatment of mild to moderate scalp psoriasis.
Nails—Several options for topical medications have been recommended for the treatment of nail psoriasis. Currently, there is a level B recommendation for the use of tazarotene for the treatment of nail psoriasis. Another effective regimen is combination therapy with vitamin D analogues and betamethasone dipropionate.9 Topical steroid use for nail psoriasis should be limited to 12 weeks because of the risk for bone atrophy with chronic steroid use.
Palmoplantar—The palms and soles have a thicker epidermal layer than other areas of the body. As a result, class 1 corticosteroids can be used for palmoplantar psoriasis for more than 4 weeks with vigilant monitoring for adverse effects such as skin atrophy, tachyphylaxis, or tinea infection. Tazarotene also has been shown to be helpful in treating palmoplantar psoriasis.
Resistant Disease—Intralesional steroids are beneficial treatment options for recalcitrant psoriasis in glabrous areas, as well as for palmoplantar, nail, and scalp psoriasis. Up to 10 mg/mL of triamcinolone acetonide used every 3 to 4 weeks is an effective regimen.10Pregnancy/Breastfeeding—Women of childbearing potential have additional safety precautions that should be considered during medication selection. Emollients have been shown to be safe during pregnancy and lactation. Currently, there is little known about CNI use during pregnancy. During lactation, CNIs can be used by breastfeeding mothers in most areas, excluding the breasts. Evaluation of the safety of anthralin and vitamin D analogues during pregnancy and lactation have not been studied. For these agents, dermatologists need to use their clinical judgment to weigh the risks and benefits of medication, particularly in patients requiring occlusion, higher medication doses, or treatment over a large surface area. Salicylic acid should be used with caution in pregnant and breastfeeding mothers because it is a pregnancy category C drug. Lower-potency corticosteroids may be used with caution during pregnancy and breastfeeding. More potent corticosteroids and coal tar, however, should be avoided. Similarly, tazarotene use is contraindicated in pregnancy. According to the US Food and Drug Administration labels for all forms of topical tazarotene, a pregnancy test must be obtained 2 weeks prior to tazarotene treatment initiation in women of childbearing potential because of the risk for serious fetal malformations and toxicity.
Recommendations, Risks, and Benefits of Topical Therapy for the Management of Psoriasis
Topical Corticosteroids—Topical corticosteroids (TCs) are widely used for inflammatory skin conditions and are available in a variety of strengths (Table 2). They are thought to exert their action by regulating the gene transcription of proinflammatory mediators. For psoriasis, steroids are recommended for 2 to 4 weeks, depending on disease severity. Although potent and superpotent steroids are more effective than mild- to moderate-strength TCs, use of lower-potency TCs may be warranted depending on disease distribution and localization.11 For treatment of psoriasis with no involvement of the intertriginous areas, use of class 1 to 5 TCs for up to 4 weeks is recommended.

For moderate to severe psoriasis with 20% or less body surface area (BSA) affected, combination therapy consisting of mometasone and salicylic acid has been shown to be more effective than mometasone alone.12,13 There currently is a level A recommendation for the use of combination therapy with class 1 TCs and etanercept for 12 weeks in patients with moderate to severe psoriasis who require both systemic and topical therapies for disease control. Similarly, combination therapy with infliximab and high-potency TCs has a level B recommendation to enhance efficacy for the treatment of moderate to severe psoriasis.14 High-quality studies on the use of TCs with anti–IL-12/IL-23, anti–IL-23, and anti–IL-17 currently are unavailable, but the combination is not expected to be unsafe.14,15 Combination therapy of betamethasone dipropionate ointment and low-dose cyclosporine is an alternative regimen with a level B recommendation.
The most common adverse effects with use of TCs are skin thinning and atrophy, telangiectasia, and striae (Table 1). With clinical improvement of disease, it is recommended that clinicians taper TCs to prevent rebound effect. To decrease TC-related adverse effects, clinicians should use combination therapy with steroid-sparing agents for disease maintenance, transition to lower-potency corticosteroids, or use intermittent steroid therapy. Systemic effects of TC use include hypothalamic-pituitary-adrenal axis suppression, Cushing syndrome, and osteonecrosis of the femoral head.16-18 These systemic effects with TC use are rare unless treatment is for disease involving greater than 20% BSA or occlusion for more than 4 weeks.
Calcineurin Inhibitors—Calcineurin inhibitors inhibit calcineurin phosphorylation and T-cell activation, subsequently decreasing the expression of proinflammatory cytokines. Currently, they are not approved by the US Food and Drug Administration to treat psoriasis but have demonstrated efficacy in randomized control trials (RCTs) for facial and intertriginous psoriasis. In RCTs, 71% of patients using pimecrolimus cream 0.1% twice daily for 8 weeks achieved an investigator global assessment score of clear (0) or almost clear (1) compared with 21% of placebo-treated patients (N=57).19 Other trials have shown that 65% of patients receiving tacrolimus ointment 0.1% for 8 weeks achieved an investigator global assessment score of 0 or 1 compared with 31% of placebo-treated patients (N=167).20 Because of their efficacy in RCTs, CNIs commonly are used off label to treat psoriasis.
The most common adverse effects with CNI use are burning, pruritus, and flushing with alcohol ingestion (Table 1). Additionally, CNIs have a black box warning that use may increase the risk for malignancy, but this risk has not been demonstrated with topical use in humans.21Vitamin D Analogues—The class of vitamin D analogues—calcipotriol/calcipotriene and calcitriol—frequently are used to treat psoriasis. Vitamin D analogues exert their beneficial effects by inhibiting keratinocyte proliferation and enhancing keratinocyte differentiation. They also are ideal for long-term use (up to 52 weeks) in mild to moderate psoriasis and can be used in combination with class 2 and 3 TCs. There is a level A recommendation that supports the use of combination therapy with calcipotriol and TCs for the treatment of mild to moderate psoriasis.
For severe psoriasis, many studies have investigated the efficacy of combination therapy with vitamin D analogues and systemic treatments. Combination therapy with calcipotriol and methotrexate or calcipotriol and acitretin are effective treatment regimens with level A recommendations. Calcipotriol–betamethasone dipropionate ointment in combination with low-dose cyclosporine is an alternative option with a level B recommendation. Because vitamin D analogues are inactivated by UVA and UVB radiation, clinicians should advise their patients to use vitamin D analogues after receiving UVB phototherapy.22
Common adverse effects of vitamin D analogues include burning, pruritus, erythema, and dryness (Table 1). Hypercalcemia and parathyroid hormone suppression are extremely rare unless treatment occurs over a large surface area (>30% BSA) or the patient has concurrent renal disease or impairments in calcium metabolism.
Tazarotene—Tazarotene is a topical retinoid that acts by decreasing keratinocyte proliferation, facilitating keratinocyte differentiation, and inhibiting inflammation. Patients with mild to moderate psoriasis are recommended to receive tazarotene treatment for 8 to 12 weeks. In several RCTs, tazarotene gel 0.1% and tazarotene cream 0.1% and 0.05% achieved treatment success in treating plaque psoriasis.23,24
For increased efficacy, clinicians can recommend combination therapy with tazarotene and a TC. Combination therapy with tazarotene and a mid- or high-potency TC for 8 to 16 weeks has been shown to be more effective than treatment with tazarotene alone.25 Thus, there is a level A recommendation for use of this combination to treat mild to moderate psoriasis. Agents used in combination therapy work synergistically to decrease the length of treatment and increase the duration of remission. The frequency of adverse effects, such as irritation from tazarotene and skin atrophy from TCs, also are reduced.26 Combination therapy with tazarotene and narrowband UVB (NB-UVB) is another effective option that requires less UV radiation than NB-UVB alone because of the synergistic effects of both treatment modalities.27 Clinicians should counsel patients on the adverse effects of tazarotene, which include local irritation, burning, pruritus, and erythema (Table 1).
Emollients—Emollients are nonmedicated moisturizers that decrease the amount of transepidermal water loss. There is a level B recommendation for use of emollients and TCs in combination for 4 to 8 weeks to treat psoriasis. In fact, combination therapy with mometasone and emollients has demonstrated greater improvement in symptoms of palmoplantar psoriasis (ie, erythema, desquamation, infiltration, BSA involvement) than mometasone alone.28 Emollients are safe options that can be used on all areas of the body and during pregnancy and lactation. Although adverse effects of emollients are rare, clinicians should counsel patients on the risk for contact dermatitis if specific allergies to ingredients/fragrances exist (Table 1).
Salicylic Acid—Salicylic acid is a topical keratolytic that can be used to treat psoriatic plaques. Use of salicylic acid for 8 to 16 weeks has been shown to be effective for mild to moderate psoriasis. Combination therapy of salicylic acid and TCs in patients with 20% or less BSA affected is a safe and effective option with a level B recommendation. Combination therapy with salicylic acid and calcipotriene, however, should be avoided because calcipotriene is inactivated by salicylic acid. It also is recommended that salicylic acid application follow phototherapy when both treatment modalities are used in combination.29,30 Clinicians should be cautious about using salicylic acid in patients with renal or hepatic disease because of the increased risk for salicylate toxicity (Table 1).
Anthralin—Anthralin is a synthetic hydrocarbon derivative that has been shown to reduce inflammation and normalize keratinocyte proliferation through an unknown mechanism. It is recommended that patients with mild to moderate psoriasis receive anthralin treatment for 8 to 12 weeks, with a maximum application time of 2 hours per day. Combination therapy of excimer laser and anthralin has been shown to be more effective in treating psoriasis than anthralin alone.31 Therefore, clinicians have the option of including excimer laser therapy for additional disease control. Anthralin should be avoided on the face, flexural regions, and highly visible areas because of potential skin staining (Table 1). Other adverse effects include application-site burning and erythema.
Coal Tar—Coal tar is a heterogenous mixture of aromatic hydrocarbons that is an effective treatment of psoriasis because of its inherent anti-inflammatory and keratoplastic properties. There is high-quality evidence supporting a level A recommendation for coal tar use in mild to moderate psoriasis. Combination therapy with NB-UVB and coal tar (also known as Goeckerman therapy) is a recommended treatment option with a quicker onset of action and improved outcomes compared with NB-UVB therapy alone.32,33 Adverse events of coal tar include application-site irritation, folliculitis, contact dermatitis, phototoxicity, and skin pigmentation (Table 1).
Conclusion
Topical medications are versatile treatment options that can be utilized as monotherapy or adjunct therapy for mild to severe psoriasis. Benefits of topical agents include minimal required monitoring, few contraindications, and direct localized effect on plaques. Therefore, side effects with topical agent use rarely are systemic. Medication interactions are less of a concern with topical therapies; thus, they have better safety profiles compared with systemic therapies. This clinical review summarizes the recently published evidence-based guidelines from the AAD and NPF on the use of topical agents in psoriasis and may be a useful guiding framework for clinicians in their everyday practice.
- Murage MJ, Kern DM, Chang L, et al. Treatment patterns among patients with psoriasis using a large national payer database in the United States: a retrospective study. J Med Econ. 2018:1-9.
- Elmets CA, Korman NJ, Prater EF, et al. Joint AAD-NPF Guidelines of care for the management and treatment of psoriasis with topical therapy and alternative medicine modalities for psoriasis severity measures. J Am Acad Dermatol. 2021;84:432-470.
- Svendsen MT, Jeyabalan J, Andersen KE, et al. Worldwide utilization of topical remedies in treatment of psoriasis: a systematic review. J Dermatolog Treat. 2017;28:374-383.
- Day A, Abramson AK, Patel M, et al. The spectrum of oculocutaneous disease: part II. neoplastic and drug-related causes of oculocutaneous disease. J Am Acad Dermatol. 2014;70:821.e821-819.
- Choi JW, Choi JW, Kwon IH, et al. High-concentration (20 μg g-¹) tacalcitol ointment in the treatment of facial psoriasis: an 8-week open-label clinical trial. Br J Dermatol. 2010;162:1359-1364.
- Hashim PW, Chima M, Kim HJ, et al. Crisaborole 2% ointment for the treatment of intertriginous, anogenital, and facial psoriasis: a double-blind, randomized, vehicle-controlled trial. J Am Acad Dermatol. 2020;82:360-365.
- Housman TS, Mellen BG, Rapp SR, et al. Patients with psoriasis prefer solution and foam vehicles: a quantitative assessment of vehicle preference. Cutis. 2002;70:327-332.
- Iversen L, Jakobsen HB. Patient preferences for topical psoriasis treatments are diverse and difficult to predict. Dermatol Ther. 2016;6:273-285.
- Clobex Package insert. Galderma Laboratories, LP; 2012.
- Kenalog-10 Injection. Package insert. Bristol-Myers Squibb Company; 2018.
- Mason J, Mason AR, Cork MJ. Topical preparations for the treatment of psoriasis: a systematic review. Br J Dermatol. 2002;146:351-364.
- Koo J, Cuffie CA, Tanner DJ, et al. Mometasone furoate 0.1%-salicylic acid 5% ointment versus mometasone furoate 0.1% ointment in the treatment of moderate-to-severe psoriasis: a multicenter study. Clin Ther. 1998;20:283-291.
- Tiplica GS, Salavastru CM. Mometasone furoate 0.1% and salicylic acid 5% vs. mometasone furoate 0.1% as sequential local therapy in psoriasis vulgaris. J Eur Acad Dermatol Venereol. 2009;23:905-912.
- Menter A, Strober BE, Kaplan DH, et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with biologics. J Am Acad Dermatol. 2019;80:1029-1072.
- Strober BE, Bissonnette R, Fiorentino D, et al. Comparative effectiveness of biologic agents for the treatment of psoriasis in a real-world setting: results from a large, prospective, observational study (Psoriasis Longitudinal Assessment and Registry [PSOLAR]). J Am Acad Dermatol. 2016;74:851-861.e854.
- Castela E, Archier E, Devaux S, et al. Topical corticosteroids in plaque psoriasis: a systematic review of risk of adrenal axis suppression and skin atrophy. J Eur Acad Dermatol Venereol. 2012;26(suppl 3):47-51.
- Takahashi H, Tsuji H, Honma M, et al. Femoral head osteonecrosis after long-term topical corticosteroid treatment in a psoriasis patient. J Dermatol. 2012;39:887-888.
- el Maghraoui A, Tabache F, Bezza A, et al. Femoral head osteonecrosis after topical corticosteroid therapy. Clin Exp Rheumatol. 2001;19:233.
- Gribetz C, Ling M, Lebwohl M, et al. Pimecrolimus cream 1% in the treatment of intertriginous psoriasis: a double-blind, randomized study. J Am Acad Dermatol. 2004;51:731-738.
- Lebwohl M, Freeman AK, Chapman MS, et al. Tacrolimus ointment is effective for facial and intertriginous psoriasis. J Am Acad Dermatol. 2004;51:723-730.
- Paller AS, Fölster-Holst R, Chen SC, et al. No evidence of increased cancer incidence in children using topical tacrolimus for atopic dermatitis. J Am Acad Dermatol. 2020;83:375-381.
- Elmets CA, Lim HW, Stoff B, et al. Joint American Academy of Dermatology-National Psoriasis Foundation guidelines of care for the management and treatment of psoriasis with phototherapy. J Am Acad Dermatol. 2019;81:775-804.
- Lebwohl M, Ast E, Callen JP, et al. Once-daily tazarotene gel versus twice-daily fluocinonide cream in the treatment of plaque psoriasis. J Am Acad Dermatol. 1998;38:705-711.
- Weinstein GD, Koo JY, Krueger GG, et al. Tazarotene cream in the treatment of psoriasis: two multicenter, double-blind, randomized, vehicle-controlled studies of the safety and efficacy of tazarotene creams 0.05% and 0.1% applied once daily for 12 weeks. J Am Acad Dermatol. 2003;48:760-767.
- Lebwohl M, Lombardi K, Tan MH. Duration of improvement in psoriasis after treatment with tazarotene 0.1% gel plus clobetasol propionate 0.05% ointment: comparison of maintenance treatments. Int J Dermatol. 2001;40:64-66.
- Sugarman JL, Weiss J, Tanghetti EA, et al. Safety and efficacy of a fixed combination halobetasol and tazarotene lotion in the treatment of moderate-to-severe plaque psoriasis: a pooled analysis of two phase 3 studies. J Drugs Dermatol. 2018;17:855-861.
- Koo JY, Lowe NJ, Lew-Kaya DA, et al. Tazarotene plus UVB phototherapy in the treatment of psoriasis. J Am Acad Dermatol. 2000;43:821-828.
- Cassano N, Mantegazza R, Battaglini S, et al. Adjuvant role of a new emollient cream in patients with palmar and/or plantar psoriasis: a pilot randomized open-label study. G Ital Dermatol Venereol. 2010;145:789-792.
- Kristensen B, Kristensen O. Topical salicylic acid interferes with UVB therapy for psoriasis. Acta Derm Venereol. 1991;71:37-40.
- Menter A, Korman NJ, Elmets CA, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis. section 3. guidelines of care for the management and treatment of psoriasis with topical therapies. J Am Acad Dermatol. 2009;60:643-659.
- Rogalski C, Grunewald S, Schetschorke M, et al. Treatment of plaque-type psoriasis with the 308 nm excimer laser in combination with dithranol or calcipotriol. Int J Hyperthermia. 2012;28:184-190.
- Bagel J. LCD plus NB-UVB reduces time to improvement of psoriasis vs. NB-UVB alone. J Drugs Dermatol. 2009;8:351-357.
- Abdallah MA, El-Khateeb EA, Abdel-Rahman SH. The influence of psoriatic plaques pretreatment with crude coal tar vs. petrolatum on the efficacy of narrow-band ultraviolet B: a half-vs.-half intra-individual double-blinded comparative study. Photodermatol Photoimmunol Photomed. 2011;27:226-230.
- Murage MJ, Kern DM, Chang L, et al. Treatment patterns among patients with psoriasis using a large national payer database in the United States: a retrospective study. J Med Econ. 2018:1-9.
- Elmets CA, Korman NJ, Prater EF, et al. Joint AAD-NPF Guidelines of care for the management and treatment of psoriasis with topical therapy and alternative medicine modalities for psoriasis severity measures. J Am Acad Dermatol. 2021;84:432-470.
- Svendsen MT, Jeyabalan J, Andersen KE, et al. Worldwide utilization of topical remedies in treatment of psoriasis: a systematic review. J Dermatolog Treat. 2017;28:374-383.
- Day A, Abramson AK, Patel M, et al. The spectrum of oculocutaneous disease: part II. neoplastic and drug-related causes of oculocutaneous disease. J Am Acad Dermatol. 2014;70:821.e821-819.
- Choi JW, Choi JW, Kwon IH, et al. High-concentration (20 μg g-¹) tacalcitol ointment in the treatment of facial psoriasis: an 8-week open-label clinical trial. Br J Dermatol. 2010;162:1359-1364.
- Hashim PW, Chima M, Kim HJ, et al. Crisaborole 2% ointment for the treatment of intertriginous, anogenital, and facial psoriasis: a double-blind, randomized, vehicle-controlled trial. J Am Acad Dermatol. 2020;82:360-365.
- Housman TS, Mellen BG, Rapp SR, et al. Patients with psoriasis prefer solution and foam vehicles: a quantitative assessment of vehicle preference. Cutis. 2002;70:327-332.
- Iversen L, Jakobsen HB. Patient preferences for topical psoriasis treatments are diverse and difficult to predict. Dermatol Ther. 2016;6:273-285.
- Clobex Package insert. Galderma Laboratories, LP; 2012.
- Kenalog-10 Injection. Package insert. Bristol-Myers Squibb Company; 2018.
- Mason J, Mason AR, Cork MJ. Topical preparations for the treatment of psoriasis: a systematic review. Br J Dermatol. 2002;146:351-364.
- Koo J, Cuffie CA, Tanner DJ, et al. Mometasone furoate 0.1%-salicylic acid 5% ointment versus mometasone furoate 0.1% ointment in the treatment of moderate-to-severe psoriasis: a multicenter study. Clin Ther. 1998;20:283-291.
- Tiplica GS, Salavastru CM. Mometasone furoate 0.1% and salicylic acid 5% vs. mometasone furoate 0.1% as sequential local therapy in psoriasis vulgaris. J Eur Acad Dermatol Venereol. 2009;23:905-912.
- Menter A, Strober BE, Kaplan DH, et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with biologics. J Am Acad Dermatol. 2019;80:1029-1072.
- Strober BE, Bissonnette R, Fiorentino D, et al. Comparative effectiveness of biologic agents for the treatment of psoriasis in a real-world setting: results from a large, prospective, observational study (Psoriasis Longitudinal Assessment and Registry [PSOLAR]). J Am Acad Dermatol. 2016;74:851-861.e854.
- Castela E, Archier E, Devaux S, et al. Topical corticosteroids in plaque psoriasis: a systematic review of risk of adrenal axis suppression and skin atrophy. J Eur Acad Dermatol Venereol. 2012;26(suppl 3):47-51.
- Takahashi H, Tsuji H, Honma M, et al. Femoral head osteonecrosis after long-term topical corticosteroid treatment in a psoriasis patient. J Dermatol. 2012;39:887-888.
- el Maghraoui A, Tabache F, Bezza A, et al. Femoral head osteonecrosis after topical corticosteroid therapy. Clin Exp Rheumatol. 2001;19:233.
- Gribetz C, Ling M, Lebwohl M, et al. Pimecrolimus cream 1% in the treatment of intertriginous psoriasis: a double-blind, randomized study. J Am Acad Dermatol. 2004;51:731-738.
- Lebwohl M, Freeman AK, Chapman MS, et al. Tacrolimus ointment is effective for facial and intertriginous psoriasis. J Am Acad Dermatol. 2004;51:723-730.
- Paller AS, Fölster-Holst R, Chen SC, et al. No evidence of increased cancer incidence in children using topical tacrolimus for atopic dermatitis. J Am Acad Dermatol. 2020;83:375-381.
- Elmets CA, Lim HW, Stoff B, et al. Joint American Academy of Dermatology-National Psoriasis Foundation guidelines of care for the management and treatment of psoriasis with phototherapy. J Am Acad Dermatol. 2019;81:775-804.
- Lebwohl M, Ast E, Callen JP, et al. Once-daily tazarotene gel versus twice-daily fluocinonide cream in the treatment of plaque psoriasis. J Am Acad Dermatol. 1998;38:705-711.
- Weinstein GD, Koo JY, Krueger GG, et al. Tazarotene cream in the treatment of psoriasis: two multicenter, double-blind, randomized, vehicle-controlled studies of the safety and efficacy of tazarotene creams 0.05% and 0.1% applied once daily for 12 weeks. J Am Acad Dermatol. 2003;48:760-767.
- Lebwohl M, Lombardi K, Tan MH. Duration of improvement in psoriasis after treatment with tazarotene 0.1% gel plus clobetasol propionate 0.05% ointment: comparison of maintenance treatments. Int J Dermatol. 2001;40:64-66.
- Sugarman JL, Weiss J, Tanghetti EA, et al. Safety and efficacy of a fixed combination halobetasol and tazarotene lotion in the treatment of moderate-to-severe plaque psoriasis: a pooled analysis of two phase 3 studies. J Drugs Dermatol. 2018;17:855-861.
- Koo JY, Lowe NJ, Lew-Kaya DA, et al. Tazarotene plus UVB phototherapy in the treatment of psoriasis. J Am Acad Dermatol. 2000;43:821-828.
- Cassano N, Mantegazza R, Battaglini S, et al. Adjuvant role of a new emollient cream in patients with palmar and/or plantar psoriasis: a pilot randomized open-label study. G Ital Dermatol Venereol. 2010;145:789-792.
- Kristensen B, Kristensen O. Topical salicylic acid interferes with UVB therapy for psoriasis. Acta Derm Venereol. 1991;71:37-40.
- Menter A, Korman NJ, Elmets CA, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis. section 3. guidelines of care for the management and treatment of psoriasis with topical therapies. J Am Acad Dermatol. 2009;60:643-659.
- Rogalski C, Grunewald S, Schetschorke M, et al. Treatment of plaque-type psoriasis with the 308 nm excimer laser in combination with dithranol or calcipotriol. Int J Hyperthermia. 2012;28:184-190.
- Bagel J. LCD plus NB-UVB reduces time to improvement of psoriasis vs. NB-UVB alone. J Drugs Dermatol. 2009;8:351-357.
- Abdallah MA, El-Khateeb EA, Abdel-Rahman SH. The influence of psoriatic plaques pretreatment with crude coal tar vs. petrolatum on the efficacy of narrow-band ultraviolet B: a half-vs.-half intra-individual double-blinded comparative study. Photodermatol Photoimmunol Photomed. 2011;27:226-230.
Practice Points
- Topical medications collectively represent the most common form of psoriasis treatment. Depending on disease severity and distribution, topical agents can be used as monotherapy or adjunct therapy, offering the benefit of localized treatment without systemic side effects.
- Dermatologists should base the selection of an appropriate topical medication on factors including adverse effects, potency, vehicle, and anatomic localization of disease.
An Update on JAK Inhibitors in Skin Disease
Atopic dermatitis (AD) is a chronic inflammatory skin disorder affecting 7% of adults and 13% of children in the United States.1,2 Atopic dermatitis is characterized by pruritus, dry skin, and pain, all of which can negatively impact quality of life and put patients at higher risk for psychiatric comorbidities such as anxiety and depression.3 The pathogenesis of AD is multifactorial, involving genetics, epidermal barrier dysfunction, and immune dysregulation. Overactivation of helper T cell (TH2) pathway cytokines, including IL-4, IL-13, and IL-31, is thought to propagate both inflammation and pruritus, which are central to AD. The JAK-STAT signaling pathway plays a pivotal role in the immune system dysregulation and exaggeration of TH2 cell response, making JAK-STAT inhibitors (or JAK inhibitors) strong theoretical candidates for the treatment of AD.4 In humans, the Janus kinases are composed of 4 different members—JAK1, JAK2, JAK3, and tyrosine kinase 2—all of which can be targeted by JAK inhibitors.5
JAK inhibitors such as tofacitinib have already been approved by the US Food and Drug Administration (FDA) to treat various inflammatory conditions, including rheumatoid arthritis, ulcerative colitis, and psoriatic arthritis; other JAK inhibitors such as baricitinib are only approved for patients with rheumatoid arthritis.6,7 The success of these small molecule inhibitors in these immune-mediated conditions make them attractive candidates for the treatment of AD. Several JAK inhibitors are in phase 2 and phase 3 clinical trials as oral therapies (moderate to severe AD) or as topical treatments (mild to moderate AD). Currently, ruxolitinib (RUX) is the only topical JAK inhibitor that is FDA approved for the treatment of AD in the United States.8 In this editorial, we focus on recent trials of JAK inhibitors tested in patients with AD, including topical RUX, as well as oral abrocitinib, upadacitinib, and baricitinib.
Topical RUX in AD
Ruxolitinib is a topical JAK1/2 small molecule inhibitor approved by the FDA for the treatment of AD in 2021. In a randomized trial by Kim et al9 in 2020, all tested regimens of RUX demonstrated significant improvement in eczema area and severity index (EASI) scores vs vehicle; notably, RUX cream 1.5% applied twice daily achieved the greatest mean percentage change in baseline EASI score vs vehicle at 4 weeks (76.1% vs 15.5%; P<.0001). Ruxolitinib cream was well tolerated through week 8 of the trial, and all adverse events (AEs) were mild to moderate in severity and comparable to those in the vehicle group.9
Topical JAK inhibitors appear to be effective for mild to moderate AD and have had an acceptable safety profile in clinical trials thus far. Although topical corticosteroids and calcineurin inhibitors can have great clinical benefit in AD, they are recommended for short-term use given side effects such as thinning of the skin, burning, or telangiectasia formation.10,11 The hope is that topical JAK inhibitors may be an alternative to standard topical treatments for AD, as they can be used for longer periods due to a safer side-effect profile.
Oral JAK Inhibitors in AD
Several oral JAK inhibitors are undergoing investigation for the systemic treatment of moderate to severe AD. Abrocitinib is an oral JAK1 inhibitor that has demonstrated efficacy in several phase 3 trials in patients with moderate to severe AD. In a 2021 trial, patients were randomized in a 2:2:2:1 ratio to receive abrocitinib 200 mg daily, abrocitinib 100 mg daily, subcutaneous dupilumab 300 mg every other week, or placebo, respectively.12 Patients in both abrocitinib groups showed significant improvement in AD vs placebo, and EASI-75 response was achieved in 70.3%, 58.7%, 58.1%, and 27.1% of patients, respectively (P<.001 for both abrocitinib doses vs placebo). Adverse events occurred more frequently in the abrocitinib 200-mg group vs placebo. Nausea, acne, nasopharyngitis, and headache were the most frequently reported AEs with abrocitinib.12 Another phase 3 trial by Silverberg et al13 (N=391) had similar treatment results, with 38.1% of participants receiving abrocitinib 200 mg and 28.4% of participants receiving abrocitinib 100 mg achieving investigator global assessment scores of 0 (clear) or 1 (almost clear) vs 9.1% of participants receiving placebo (P<.001). Abrocitinib was well tolerated in this trial with few serious AEs (ie, herpangina [0.6%], pneumonia [0.6%]).13 In both trials, there were rare instances of laboratory values indicating thrombocytopenia with the 200-mg dose (0.9%12 and 3.2%13) without any clinical manifestations. Although a decrease in platelets was observed, no thrombocytopenia occurred in the abrocitinib 100-mg group in the latter trial.13
Baricitinib is another oral inhibitor of JAK1 and JAK2 with potential for the treatment of AD. One randomized trial (N=329) demonstrated its efficacy in combination with a topical corticosteroid (TCS). At 16 weeks, a higher number of participants treated with baricitinib and TCS achieved investigator global assessment scores of 0 (clear) or 1 (almost clear) compared to those who received placebo and TCS (31% with baricitinib 4 mg + TCS, 24% with baricitinib 2 mg + TCS, and 15% with placebo + TCS).14 Similarly, in BREEZE-AD5,another phase 3 trial (N=440), baricitinib monotherapy demonstrated a higher rate of treatment success vs placebo.15 Specifically, 13% of patients treated with baricitinib 1 mg and 30% of those treated with baricitinib 2 mg achieved 75% or greater reduction in EASI scores compared to 8% in the placebo group. The most common AEs associated with baricitinib were nasopharyngitis and headache. Adverse events occurred with similar frequency across both experimental and control groups.15 Reich et al14 demonstrated a higher overall rate of AEs—most commonly nasopharyngitis, upper respiratory tract infections, and folliculitis—in baricitinib-treated patients; however, serious AEs occurred with similar frequency across all groups, including the control group.
The selective JAK1 inhibitor upadacitinib also is undergoing testing in treating moderate to severe AD. In one trial, 167 patients were randomized to once daily oral upadacitinib 7.5 mg, 15 mg, or 30 mg or placebo.16 All doses of upadacitinib demonstrated considerably higher percentage improvements from baseline in EASI scores compared to placebo at 16 weeks with a clear dose-response relationship (39%, 62%, and 74% vs 23%, respectively). In this trial, there were no dose-limiting safety events. Serious AEs were infrequent, occurring in 4.8%, 2.4%, and 0% of upadacitinib groups vs 2.5% for placebo. The serious AEs observed with upadacitinib were 1 case of appendicitis, lower jaw pericoronitis in a patient with a history of repeated tooth infections, and an exacerbation of AD.16
Tofacitinib, another JAK inhibitor, has been shown to increase the risk for blood clots and death in a large trial in the treatment of rheumatoid arthritis. Following this study, the FDA is requiring black box warnings for tofacitinib and also for the 2 JAK inhibitors baricitinib and upadacitinib regarding the risks for heart-related events, cancer, blood clots, and death. Given that these medications share a similar mechanism of action to tofacitinib, they may have similar risks, though they have not yet been fully evaluated in large safety trials.17
With more recent investigation into novel therapeutics for AD, oral JAK inhibitors may play an important role in the future to treat patients with moderate to severe AD with inadequate response or contraindications to other systemic therapies. In trials thus far, oral JAK inhibitors have exhibited acceptable safety profiles and have demonstrated treatment success in AD. More randomized, controlled, phase 3 studies with larger patient populations are required to confirm their potential as effective treatments and elucidate their long-term safety.
Deucravacitinib in Psoriasis
Deucravacitinib is a first-in-class, oral, selective TYK2 inhibitor currently undergoing testing for the treatment of psoriasis. A randomized phase 2 trial (N=267) found that deucravacitinib was more effective than placebo in treating chronic plaque psoriasis at doses of 3 to 12 mg daily.18 The percentage of participants with a 75% or greater reduction from baseline in the psoriasis area and severity index score was 7% with placebo, 9% with deucravacitinib 3 mg every other day (P=.49 vs placebo), 39% with 3 mg once daily (P<.001 vs placebo), 69% with 3 mg twice daily (P<.001 vs placebo), 67% with 6 mg twice daily (P<.001 vs placebo), and 75% with 12 mg once daily (P<.001 vs placebo). The most commonly reported AEs were nasopharyngitis, headache, diarrhea, nausea, and upper respiratory tract infection. Adverse events occurred in 51% of participants in the control group and in 55% to 80% of those in the experimental groups. Additionally, there was 1 reported case of melanoma (stage 0) 96 days after the start of treatment in a patient in the 3-mg once-daily group. Serious AEs occurred in only 0% to 2% of participants who received deucravacitinib.18
Two phase 3 trials—POETYK PSO-1 and POETYK PSO-2 (N=1686)—found deucravacitinib to be notably more effective than both placebo and apremilast in treating psoriasis.19 Among participants receiving deucravacitinib 6 mg daily, 58.7% and 53.6% in the 2 respective trials achieved psoriasis area and severity index 75 response vs 12.7% and 9.4% receiving placebo and 35.1% and 40.2% receiving apremilast. Overall, the treatment was well tolerated, with a low rate of discontinuation of deucravacitinib due to AEs (2.4% of patients on deucravacitinib compared to 3.8% on placebo and 5.2% on apremilast). The most frequently observed AEs with deucravacitinib were nasopharyngitis and upper respiratory tract infection. The full results of these trials are expected to be published soon.19,20
Final Thoughts
Overall, JAK inhibitors are a novel class of therapeutics that may have further success in the treatment of other dermatologic conditions that negatively affect patients’ quality of life and productivity. We should look forward to additional successful trials with these promising medications.
- Chiesa Fuxench ZC, Block JK, Boguniewicz M, et al. Atopic dermatitis in America study: a cross-sectional study examining the prevalence and disease burden of atopic dermatitis in the US adult population. J Invest Dermatol. 2019;139:583-590.
- Silverberg JI , Simpson EL. Associations of childhood eczema severity: a US population-based study. Dermatitis. 2014;25:107-114.
- Schonmann Y, Mansfield KE, Hayes JF, et al. Atopic eczema in adulthood and risk of depression and anxiety: a population-based cohort study. J Allergy Clin Immunol Pract. 2020;8:248-257.e16.
- Bao L, Zhang H, Chan LS. The involvement of the JAK-STAT signaling pathway in chronic inflammatory skin disease atopic dermatitis. JAKSTAT. 2013;2:e24137.
- Villarino AV, Kanno Y, O’Shea JJ. Mechanisms and consequences of Jak-STAT signaling in the immune system. Nat Immunol. 2017;18:374-384.
- Xeljanz FDA approval history. Drugs.com website. Updated December 14, 2021. Accessed February 16, 2022. https://www.drugs.com/history/xeljanz.html
- Mullard A. FDA approves Eli Lilly’s baricitinib. Nat Rev Drug Discov. 2018;17:460.
- FDA approves Opzelura. Drugs.com website. Published September 2021. Accessed February 16, 2022. https://www.drugs.com/newdrugs/fda-approves-opzelura-ruxolitinib-cream-atopic-dermatitis-ad-5666.html
- Kim BS, Sun K, Papp K, et al. Effects of ruxolitinib cream on pruritus and quality of life in atopic dermatitis: results from a phase 2, randomized, dose-ranging, vehicle- and active-controlled study.J Am Acad Dermatol. 2020;82:1305-1313.
- Eichenfield LF, Tom WL, Berger TG, et al. Guidelines of care for the management of atopic dermatitis: section 2, management and treatment of atopic dermatitis with topical therapies. J Am Acad Dermatol. 2014;71:116-132.
- Wollenberg A, Barbarot S, Bieber T, et al. Consensus-based European guidelines for treatment of atopic eczema (atopic dermatitis) in adults and children: part I. J Eur Acad Dermatol Venereol. 2018;32:657-682.
- Bieber T, Simpson EL, Silverberg JI, et al. Abrocitinib versus placebo or dupilumab for atopic dermatitis. N Engl J Med. 2021;384:1101-1112.
- Silverberg JI, Simpson EL, Thyssen JP, et al. Efficacy and safety of abrocitinib in patients with moderate-to-severe atopic dermatitis: a randomized clinical trial. JAMA Dermatol. 2020;156:863-873.
- Reich K, Kabashima K, Peris K, et al. Efficacy and safety of baricitinib combined with topical corticosteroids for treatment of moderate to severe atopic dermatitis: a randomized clinical trial. JAMA Dermatol. 2020;156:1333-1343.
- Simpson EL, Forman S, Silverberg JI, et al. Baricitinib in patients with moderate-to-severe atopic dermatitis: results from a randomized monotherapy phase 3 trial in the United States and Canada (BREEZE-AD5). J Am Acad Dermatol. 2021;85:62-70.
- Guttman-Yassky E, Thaçi D, Pangan AL, et al. Upadacitinib in adults with moderate to severe atopic dermatitis: 16-week results from a randomized, placebo-controlled trial. J Allergy Clin Immunol. 2020;145:877-884.
- US Food and Drug Administration. FDA requires warnings about increased risk of serious heart-related events, cancer, blood clots, and death for JAK inhibitors that treat certain chronic inflammatory conditions. Published September 1, 2022. Accessed February 16, 2022. https://www.fda.gov/drugs/drug-safety-and-availability/fda-requires-warnings-about-increased-risk-serious-heart-related-events-cancer-blood-clots-and-death
- Papp K, Gordon K, Thaçi D, et al. Phase 2 trial of selective tyrosine kinase 2 inhibition in psoriasis. N Engl J Med. 2018;379:1313-1321.
- Bristol Myers Squibb presents positive data from two pivotal phase 3 psoriasis studies demonstrating superiority of deucravacitinib compared to placebo and Otezla® (apremilast). Press release. Bristol Meyers Squibb. April 23, 2021. Accessed February 16, 2022. https://news.bms.com/news/details/2021/Bristol-Myers-Squibb-Presents-Positive-Data-from-Two-Pivotal-Phase-3-Psoriasis-Studies-Demonstrating-Superiority-of-Deucravacitinib-Compared-to-Placebo-and-Otezla-apremilast/default.aspx
- Armstrong A, Gooderham M, Warren R, et al. Efficacy and safety of deucravacitinib, an oral, selective tyrosine kinase 2 (TYK2) inhibitor, compared with placebo and apremilast in moderate to severe plaque psoriasis: results from the POETYK PSO-1 study [abstract]. Abstract presented at: 2021 American Academy of Dermatology annual meeting; April 23-25, 2021; San Francisco, California.
Atopic dermatitis (AD) is a chronic inflammatory skin disorder affecting 7% of adults and 13% of children in the United States.1,2 Atopic dermatitis is characterized by pruritus, dry skin, and pain, all of which can negatively impact quality of life and put patients at higher risk for psychiatric comorbidities such as anxiety and depression.3 The pathogenesis of AD is multifactorial, involving genetics, epidermal barrier dysfunction, and immune dysregulation. Overactivation of helper T cell (TH2) pathway cytokines, including IL-4, IL-13, and IL-31, is thought to propagate both inflammation and pruritus, which are central to AD. The JAK-STAT signaling pathway plays a pivotal role in the immune system dysregulation and exaggeration of TH2 cell response, making JAK-STAT inhibitors (or JAK inhibitors) strong theoretical candidates for the treatment of AD.4 In humans, the Janus kinases are composed of 4 different members—JAK1, JAK2, JAK3, and tyrosine kinase 2—all of which can be targeted by JAK inhibitors.5
JAK inhibitors such as tofacitinib have already been approved by the US Food and Drug Administration (FDA) to treat various inflammatory conditions, including rheumatoid arthritis, ulcerative colitis, and psoriatic arthritis; other JAK inhibitors such as baricitinib are only approved for patients with rheumatoid arthritis.6,7 The success of these small molecule inhibitors in these immune-mediated conditions make them attractive candidates for the treatment of AD. Several JAK inhibitors are in phase 2 and phase 3 clinical trials as oral therapies (moderate to severe AD) or as topical treatments (mild to moderate AD). Currently, ruxolitinib (RUX) is the only topical JAK inhibitor that is FDA approved for the treatment of AD in the United States.8 In this editorial, we focus on recent trials of JAK inhibitors tested in patients with AD, including topical RUX, as well as oral abrocitinib, upadacitinib, and baricitinib.
Topical RUX in AD
Ruxolitinib is a topical JAK1/2 small molecule inhibitor approved by the FDA for the treatment of AD in 2021. In a randomized trial by Kim et al9 in 2020, all tested regimens of RUX demonstrated significant improvement in eczema area and severity index (EASI) scores vs vehicle; notably, RUX cream 1.5% applied twice daily achieved the greatest mean percentage change in baseline EASI score vs vehicle at 4 weeks (76.1% vs 15.5%; P<.0001). Ruxolitinib cream was well tolerated through week 8 of the trial, and all adverse events (AEs) were mild to moderate in severity and comparable to those in the vehicle group.9
Topical JAK inhibitors appear to be effective for mild to moderate AD and have had an acceptable safety profile in clinical trials thus far. Although topical corticosteroids and calcineurin inhibitors can have great clinical benefit in AD, they are recommended for short-term use given side effects such as thinning of the skin, burning, or telangiectasia formation.10,11 The hope is that topical JAK inhibitors may be an alternative to standard topical treatments for AD, as they can be used for longer periods due to a safer side-effect profile.
Oral JAK Inhibitors in AD
Several oral JAK inhibitors are undergoing investigation for the systemic treatment of moderate to severe AD. Abrocitinib is an oral JAK1 inhibitor that has demonstrated efficacy in several phase 3 trials in patients with moderate to severe AD. In a 2021 trial, patients were randomized in a 2:2:2:1 ratio to receive abrocitinib 200 mg daily, abrocitinib 100 mg daily, subcutaneous dupilumab 300 mg every other week, or placebo, respectively.12 Patients in both abrocitinib groups showed significant improvement in AD vs placebo, and EASI-75 response was achieved in 70.3%, 58.7%, 58.1%, and 27.1% of patients, respectively (P<.001 for both abrocitinib doses vs placebo). Adverse events occurred more frequently in the abrocitinib 200-mg group vs placebo. Nausea, acne, nasopharyngitis, and headache were the most frequently reported AEs with abrocitinib.12 Another phase 3 trial by Silverberg et al13 (N=391) had similar treatment results, with 38.1% of participants receiving abrocitinib 200 mg and 28.4% of participants receiving abrocitinib 100 mg achieving investigator global assessment scores of 0 (clear) or 1 (almost clear) vs 9.1% of participants receiving placebo (P<.001). Abrocitinib was well tolerated in this trial with few serious AEs (ie, herpangina [0.6%], pneumonia [0.6%]).13 In both trials, there were rare instances of laboratory values indicating thrombocytopenia with the 200-mg dose (0.9%12 and 3.2%13) without any clinical manifestations. Although a decrease in platelets was observed, no thrombocytopenia occurred in the abrocitinib 100-mg group in the latter trial.13
Baricitinib is another oral inhibitor of JAK1 and JAK2 with potential for the treatment of AD. One randomized trial (N=329) demonstrated its efficacy in combination with a topical corticosteroid (TCS). At 16 weeks, a higher number of participants treated with baricitinib and TCS achieved investigator global assessment scores of 0 (clear) or 1 (almost clear) compared to those who received placebo and TCS (31% with baricitinib 4 mg + TCS, 24% with baricitinib 2 mg + TCS, and 15% with placebo + TCS).14 Similarly, in BREEZE-AD5,another phase 3 trial (N=440), baricitinib monotherapy demonstrated a higher rate of treatment success vs placebo.15 Specifically, 13% of patients treated with baricitinib 1 mg and 30% of those treated with baricitinib 2 mg achieved 75% or greater reduction in EASI scores compared to 8% in the placebo group. The most common AEs associated with baricitinib were nasopharyngitis and headache. Adverse events occurred with similar frequency across both experimental and control groups.15 Reich et al14 demonstrated a higher overall rate of AEs—most commonly nasopharyngitis, upper respiratory tract infections, and folliculitis—in baricitinib-treated patients; however, serious AEs occurred with similar frequency across all groups, including the control group.
The selective JAK1 inhibitor upadacitinib also is undergoing testing in treating moderate to severe AD. In one trial, 167 patients were randomized to once daily oral upadacitinib 7.5 mg, 15 mg, or 30 mg or placebo.16 All doses of upadacitinib demonstrated considerably higher percentage improvements from baseline in EASI scores compared to placebo at 16 weeks with a clear dose-response relationship (39%, 62%, and 74% vs 23%, respectively). In this trial, there were no dose-limiting safety events. Serious AEs were infrequent, occurring in 4.8%, 2.4%, and 0% of upadacitinib groups vs 2.5% for placebo. The serious AEs observed with upadacitinib were 1 case of appendicitis, lower jaw pericoronitis in a patient with a history of repeated tooth infections, and an exacerbation of AD.16
Tofacitinib, another JAK inhibitor, has been shown to increase the risk for blood clots and death in a large trial in the treatment of rheumatoid arthritis. Following this study, the FDA is requiring black box warnings for tofacitinib and also for the 2 JAK inhibitors baricitinib and upadacitinib regarding the risks for heart-related events, cancer, blood clots, and death. Given that these medications share a similar mechanism of action to tofacitinib, they may have similar risks, though they have not yet been fully evaluated in large safety trials.17
With more recent investigation into novel therapeutics for AD, oral JAK inhibitors may play an important role in the future to treat patients with moderate to severe AD with inadequate response or contraindications to other systemic therapies. In trials thus far, oral JAK inhibitors have exhibited acceptable safety profiles and have demonstrated treatment success in AD. More randomized, controlled, phase 3 studies with larger patient populations are required to confirm their potential as effective treatments and elucidate their long-term safety.
Deucravacitinib in Psoriasis
Deucravacitinib is a first-in-class, oral, selective TYK2 inhibitor currently undergoing testing for the treatment of psoriasis. A randomized phase 2 trial (N=267) found that deucravacitinib was more effective than placebo in treating chronic plaque psoriasis at doses of 3 to 12 mg daily.18 The percentage of participants with a 75% or greater reduction from baseline in the psoriasis area and severity index score was 7% with placebo, 9% with deucravacitinib 3 mg every other day (P=.49 vs placebo), 39% with 3 mg once daily (P<.001 vs placebo), 69% with 3 mg twice daily (P<.001 vs placebo), 67% with 6 mg twice daily (P<.001 vs placebo), and 75% with 12 mg once daily (P<.001 vs placebo). The most commonly reported AEs were nasopharyngitis, headache, diarrhea, nausea, and upper respiratory tract infection. Adverse events occurred in 51% of participants in the control group and in 55% to 80% of those in the experimental groups. Additionally, there was 1 reported case of melanoma (stage 0) 96 days after the start of treatment in a patient in the 3-mg once-daily group. Serious AEs occurred in only 0% to 2% of participants who received deucravacitinib.18
Two phase 3 trials—POETYK PSO-1 and POETYK PSO-2 (N=1686)—found deucravacitinib to be notably more effective than both placebo and apremilast in treating psoriasis.19 Among participants receiving deucravacitinib 6 mg daily, 58.7% and 53.6% in the 2 respective trials achieved psoriasis area and severity index 75 response vs 12.7% and 9.4% receiving placebo and 35.1% and 40.2% receiving apremilast. Overall, the treatment was well tolerated, with a low rate of discontinuation of deucravacitinib due to AEs (2.4% of patients on deucravacitinib compared to 3.8% on placebo and 5.2% on apremilast). The most frequently observed AEs with deucravacitinib were nasopharyngitis and upper respiratory tract infection. The full results of these trials are expected to be published soon.19,20
Final Thoughts
Overall, JAK inhibitors are a novel class of therapeutics that may have further success in the treatment of other dermatologic conditions that negatively affect patients’ quality of life and productivity. We should look forward to additional successful trials with these promising medications.
Atopic dermatitis (AD) is a chronic inflammatory skin disorder affecting 7% of adults and 13% of children in the United States.1,2 Atopic dermatitis is characterized by pruritus, dry skin, and pain, all of which can negatively impact quality of life and put patients at higher risk for psychiatric comorbidities such as anxiety and depression.3 The pathogenesis of AD is multifactorial, involving genetics, epidermal barrier dysfunction, and immune dysregulation. Overactivation of helper T cell (TH2) pathway cytokines, including IL-4, IL-13, and IL-31, is thought to propagate both inflammation and pruritus, which are central to AD. The JAK-STAT signaling pathway plays a pivotal role in the immune system dysregulation and exaggeration of TH2 cell response, making JAK-STAT inhibitors (or JAK inhibitors) strong theoretical candidates for the treatment of AD.4 In humans, the Janus kinases are composed of 4 different members—JAK1, JAK2, JAK3, and tyrosine kinase 2—all of which can be targeted by JAK inhibitors.5
JAK inhibitors such as tofacitinib have already been approved by the US Food and Drug Administration (FDA) to treat various inflammatory conditions, including rheumatoid arthritis, ulcerative colitis, and psoriatic arthritis; other JAK inhibitors such as baricitinib are only approved for patients with rheumatoid arthritis.6,7 The success of these small molecule inhibitors in these immune-mediated conditions make them attractive candidates for the treatment of AD. Several JAK inhibitors are in phase 2 and phase 3 clinical trials as oral therapies (moderate to severe AD) or as topical treatments (mild to moderate AD). Currently, ruxolitinib (RUX) is the only topical JAK inhibitor that is FDA approved for the treatment of AD in the United States.8 In this editorial, we focus on recent trials of JAK inhibitors tested in patients with AD, including topical RUX, as well as oral abrocitinib, upadacitinib, and baricitinib.
Topical RUX in AD
Ruxolitinib is a topical JAK1/2 small molecule inhibitor approved by the FDA for the treatment of AD in 2021. In a randomized trial by Kim et al9 in 2020, all tested regimens of RUX demonstrated significant improvement in eczema area and severity index (EASI) scores vs vehicle; notably, RUX cream 1.5% applied twice daily achieved the greatest mean percentage change in baseline EASI score vs vehicle at 4 weeks (76.1% vs 15.5%; P<.0001). Ruxolitinib cream was well tolerated through week 8 of the trial, and all adverse events (AEs) were mild to moderate in severity and comparable to those in the vehicle group.9
Topical JAK inhibitors appear to be effective for mild to moderate AD and have had an acceptable safety profile in clinical trials thus far. Although topical corticosteroids and calcineurin inhibitors can have great clinical benefit in AD, they are recommended for short-term use given side effects such as thinning of the skin, burning, or telangiectasia formation.10,11 The hope is that topical JAK inhibitors may be an alternative to standard topical treatments for AD, as they can be used for longer periods due to a safer side-effect profile.
Oral JAK Inhibitors in AD
Several oral JAK inhibitors are undergoing investigation for the systemic treatment of moderate to severe AD. Abrocitinib is an oral JAK1 inhibitor that has demonstrated efficacy in several phase 3 trials in patients with moderate to severe AD. In a 2021 trial, patients were randomized in a 2:2:2:1 ratio to receive abrocitinib 200 mg daily, abrocitinib 100 mg daily, subcutaneous dupilumab 300 mg every other week, or placebo, respectively.12 Patients in both abrocitinib groups showed significant improvement in AD vs placebo, and EASI-75 response was achieved in 70.3%, 58.7%, 58.1%, and 27.1% of patients, respectively (P<.001 for both abrocitinib doses vs placebo). Adverse events occurred more frequently in the abrocitinib 200-mg group vs placebo. Nausea, acne, nasopharyngitis, and headache were the most frequently reported AEs with abrocitinib.12 Another phase 3 trial by Silverberg et al13 (N=391) had similar treatment results, with 38.1% of participants receiving abrocitinib 200 mg and 28.4% of participants receiving abrocitinib 100 mg achieving investigator global assessment scores of 0 (clear) or 1 (almost clear) vs 9.1% of participants receiving placebo (P<.001). Abrocitinib was well tolerated in this trial with few serious AEs (ie, herpangina [0.6%], pneumonia [0.6%]).13 In both trials, there were rare instances of laboratory values indicating thrombocytopenia with the 200-mg dose (0.9%12 and 3.2%13) without any clinical manifestations. Although a decrease in platelets was observed, no thrombocytopenia occurred in the abrocitinib 100-mg group in the latter trial.13
Baricitinib is another oral inhibitor of JAK1 and JAK2 with potential for the treatment of AD. One randomized trial (N=329) demonstrated its efficacy in combination with a topical corticosteroid (TCS). At 16 weeks, a higher number of participants treated with baricitinib and TCS achieved investigator global assessment scores of 0 (clear) or 1 (almost clear) compared to those who received placebo and TCS (31% with baricitinib 4 mg + TCS, 24% with baricitinib 2 mg + TCS, and 15% with placebo + TCS).14 Similarly, in BREEZE-AD5,another phase 3 trial (N=440), baricitinib monotherapy demonstrated a higher rate of treatment success vs placebo.15 Specifically, 13% of patients treated with baricitinib 1 mg and 30% of those treated with baricitinib 2 mg achieved 75% or greater reduction in EASI scores compared to 8% in the placebo group. The most common AEs associated with baricitinib were nasopharyngitis and headache. Adverse events occurred with similar frequency across both experimental and control groups.15 Reich et al14 demonstrated a higher overall rate of AEs—most commonly nasopharyngitis, upper respiratory tract infections, and folliculitis—in baricitinib-treated patients; however, serious AEs occurred with similar frequency across all groups, including the control group.
The selective JAK1 inhibitor upadacitinib also is undergoing testing in treating moderate to severe AD. In one trial, 167 patients were randomized to once daily oral upadacitinib 7.5 mg, 15 mg, or 30 mg or placebo.16 All doses of upadacitinib demonstrated considerably higher percentage improvements from baseline in EASI scores compared to placebo at 16 weeks with a clear dose-response relationship (39%, 62%, and 74% vs 23%, respectively). In this trial, there were no dose-limiting safety events. Serious AEs were infrequent, occurring in 4.8%, 2.4%, and 0% of upadacitinib groups vs 2.5% for placebo. The serious AEs observed with upadacitinib were 1 case of appendicitis, lower jaw pericoronitis in a patient with a history of repeated tooth infections, and an exacerbation of AD.16
Tofacitinib, another JAK inhibitor, has been shown to increase the risk for blood clots and death in a large trial in the treatment of rheumatoid arthritis. Following this study, the FDA is requiring black box warnings for tofacitinib and also for the 2 JAK inhibitors baricitinib and upadacitinib regarding the risks for heart-related events, cancer, blood clots, and death. Given that these medications share a similar mechanism of action to tofacitinib, they may have similar risks, though they have not yet been fully evaluated in large safety trials.17
With more recent investigation into novel therapeutics for AD, oral JAK inhibitors may play an important role in the future to treat patients with moderate to severe AD with inadequate response or contraindications to other systemic therapies. In trials thus far, oral JAK inhibitors have exhibited acceptable safety profiles and have demonstrated treatment success in AD. More randomized, controlled, phase 3 studies with larger patient populations are required to confirm their potential as effective treatments and elucidate their long-term safety.
Deucravacitinib in Psoriasis
Deucravacitinib is a first-in-class, oral, selective TYK2 inhibitor currently undergoing testing for the treatment of psoriasis. A randomized phase 2 trial (N=267) found that deucravacitinib was more effective than placebo in treating chronic plaque psoriasis at doses of 3 to 12 mg daily.18 The percentage of participants with a 75% or greater reduction from baseline in the psoriasis area and severity index score was 7% with placebo, 9% with deucravacitinib 3 mg every other day (P=.49 vs placebo), 39% with 3 mg once daily (P<.001 vs placebo), 69% with 3 mg twice daily (P<.001 vs placebo), 67% with 6 mg twice daily (P<.001 vs placebo), and 75% with 12 mg once daily (P<.001 vs placebo). The most commonly reported AEs were nasopharyngitis, headache, diarrhea, nausea, and upper respiratory tract infection. Adverse events occurred in 51% of participants in the control group and in 55% to 80% of those in the experimental groups. Additionally, there was 1 reported case of melanoma (stage 0) 96 days after the start of treatment in a patient in the 3-mg once-daily group. Serious AEs occurred in only 0% to 2% of participants who received deucravacitinib.18
Two phase 3 trials—POETYK PSO-1 and POETYK PSO-2 (N=1686)—found deucravacitinib to be notably more effective than both placebo and apremilast in treating psoriasis.19 Among participants receiving deucravacitinib 6 mg daily, 58.7% and 53.6% in the 2 respective trials achieved psoriasis area and severity index 75 response vs 12.7% and 9.4% receiving placebo and 35.1% and 40.2% receiving apremilast. Overall, the treatment was well tolerated, with a low rate of discontinuation of deucravacitinib due to AEs (2.4% of patients on deucravacitinib compared to 3.8% on placebo and 5.2% on apremilast). The most frequently observed AEs with deucravacitinib were nasopharyngitis and upper respiratory tract infection. The full results of these trials are expected to be published soon.19,20
Final Thoughts
Overall, JAK inhibitors are a novel class of therapeutics that may have further success in the treatment of other dermatologic conditions that negatively affect patients’ quality of life and productivity. We should look forward to additional successful trials with these promising medications.
- Chiesa Fuxench ZC, Block JK, Boguniewicz M, et al. Atopic dermatitis in America study: a cross-sectional study examining the prevalence and disease burden of atopic dermatitis in the US adult population. J Invest Dermatol. 2019;139:583-590.
- Silverberg JI , Simpson EL. Associations of childhood eczema severity: a US population-based study. Dermatitis. 2014;25:107-114.
- Schonmann Y, Mansfield KE, Hayes JF, et al. Atopic eczema in adulthood and risk of depression and anxiety: a population-based cohort study. J Allergy Clin Immunol Pract. 2020;8:248-257.e16.
- Bao L, Zhang H, Chan LS. The involvement of the JAK-STAT signaling pathway in chronic inflammatory skin disease atopic dermatitis. JAKSTAT. 2013;2:e24137.
- Villarino AV, Kanno Y, O’Shea JJ. Mechanisms and consequences of Jak-STAT signaling in the immune system. Nat Immunol. 2017;18:374-384.
- Xeljanz FDA approval history. Drugs.com website. Updated December 14, 2021. Accessed February 16, 2022. https://www.drugs.com/history/xeljanz.html
- Mullard A. FDA approves Eli Lilly’s baricitinib. Nat Rev Drug Discov. 2018;17:460.
- FDA approves Opzelura. Drugs.com website. Published September 2021. Accessed February 16, 2022. https://www.drugs.com/newdrugs/fda-approves-opzelura-ruxolitinib-cream-atopic-dermatitis-ad-5666.html
- Kim BS, Sun K, Papp K, et al. Effects of ruxolitinib cream on pruritus and quality of life in atopic dermatitis: results from a phase 2, randomized, dose-ranging, vehicle- and active-controlled study.J Am Acad Dermatol. 2020;82:1305-1313.
- Eichenfield LF, Tom WL, Berger TG, et al. Guidelines of care for the management of atopic dermatitis: section 2, management and treatment of atopic dermatitis with topical therapies. J Am Acad Dermatol. 2014;71:116-132.
- Wollenberg A, Barbarot S, Bieber T, et al. Consensus-based European guidelines for treatment of atopic eczema (atopic dermatitis) in adults and children: part I. J Eur Acad Dermatol Venereol. 2018;32:657-682.
- Bieber T, Simpson EL, Silverberg JI, et al. Abrocitinib versus placebo or dupilumab for atopic dermatitis. N Engl J Med. 2021;384:1101-1112.
- Silverberg JI, Simpson EL, Thyssen JP, et al. Efficacy and safety of abrocitinib in patients with moderate-to-severe atopic dermatitis: a randomized clinical trial. JAMA Dermatol. 2020;156:863-873.
- Reich K, Kabashima K, Peris K, et al. Efficacy and safety of baricitinib combined with topical corticosteroids for treatment of moderate to severe atopic dermatitis: a randomized clinical trial. JAMA Dermatol. 2020;156:1333-1343.
- Simpson EL, Forman S, Silverberg JI, et al. Baricitinib in patients with moderate-to-severe atopic dermatitis: results from a randomized monotherapy phase 3 trial in the United States and Canada (BREEZE-AD5). J Am Acad Dermatol. 2021;85:62-70.
- Guttman-Yassky E, Thaçi D, Pangan AL, et al. Upadacitinib in adults with moderate to severe atopic dermatitis: 16-week results from a randomized, placebo-controlled trial. J Allergy Clin Immunol. 2020;145:877-884.
- US Food and Drug Administration. FDA requires warnings about increased risk of serious heart-related events, cancer, blood clots, and death for JAK inhibitors that treat certain chronic inflammatory conditions. Published September 1, 2022. Accessed February 16, 2022. https://www.fda.gov/drugs/drug-safety-and-availability/fda-requires-warnings-about-increased-risk-serious-heart-related-events-cancer-blood-clots-and-death
- Papp K, Gordon K, Thaçi D, et al. Phase 2 trial of selective tyrosine kinase 2 inhibition in psoriasis. N Engl J Med. 2018;379:1313-1321.
- Bristol Myers Squibb presents positive data from two pivotal phase 3 psoriasis studies demonstrating superiority of deucravacitinib compared to placebo and Otezla® (apremilast). Press release. Bristol Meyers Squibb. April 23, 2021. Accessed February 16, 2022. https://news.bms.com/news/details/2021/Bristol-Myers-Squibb-Presents-Positive-Data-from-Two-Pivotal-Phase-3-Psoriasis-Studies-Demonstrating-Superiority-of-Deucravacitinib-Compared-to-Placebo-and-Otezla-apremilast/default.aspx
- Armstrong A, Gooderham M, Warren R, et al. Efficacy and safety of deucravacitinib, an oral, selective tyrosine kinase 2 (TYK2) inhibitor, compared with placebo and apremilast in moderate to severe plaque psoriasis: results from the POETYK PSO-1 study [abstract]. Abstract presented at: 2021 American Academy of Dermatology annual meeting; April 23-25, 2021; San Francisco, California.
- Chiesa Fuxench ZC, Block JK, Boguniewicz M, et al. Atopic dermatitis in America study: a cross-sectional study examining the prevalence and disease burden of atopic dermatitis in the US adult population. J Invest Dermatol. 2019;139:583-590.
- Silverberg JI , Simpson EL. Associations of childhood eczema severity: a US population-based study. Dermatitis. 2014;25:107-114.
- Schonmann Y, Mansfield KE, Hayes JF, et al. Atopic eczema in adulthood and risk of depression and anxiety: a population-based cohort study. J Allergy Clin Immunol Pract. 2020;8:248-257.e16.
- Bao L, Zhang H, Chan LS. The involvement of the JAK-STAT signaling pathway in chronic inflammatory skin disease atopic dermatitis. JAKSTAT. 2013;2:e24137.
- Villarino AV, Kanno Y, O’Shea JJ. Mechanisms and consequences of Jak-STAT signaling in the immune system. Nat Immunol. 2017;18:374-384.
- Xeljanz FDA approval history. Drugs.com website. Updated December 14, 2021. Accessed February 16, 2022. https://www.drugs.com/history/xeljanz.html
- Mullard A. FDA approves Eli Lilly’s baricitinib. Nat Rev Drug Discov. 2018;17:460.
- FDA approves Opzelura. Drugs.com website. Published September 2021. Accessed February 16, 2022. https://www.drugs.com/newdrugs/fda-approves-opzelura-ruxolitinib-cream-atopic-dermatitis-ad-5666.html
- Kim BS, Sun K, Papp K, et al. Effects of ruxolitinib cream on pruritus and quality of life in atopic dermatitis: results from a phase 2, randomized, dose-ranging, vehicle- and active-controlled study.J Am Acad Dermatol. 2020;82:1305-1313.
- Eichenfield LF, Tom WL, Berger TG, et al. Guidelines of care for the management of atopic dermatitis: section 2, management and treatment of atopic dermatitis with topical therapies. J Am Acad Dermatol. 2014;71:116-132.
- Wollenberg A, Barbarot S, Bieber T, et al. Consensus-based European guidelines for treatment of atopic eczema (atopic dermatitis) in adults and children: part I. J Eur Acad Dermatol Venereol. 2018;32:657-682.
- Bieber T, Simpson EL, Silverberg JI, et al. Abrocitinib versus placebo or dupilumab for atopic dermatitis. N Engl J Med. 2021;384:1101-1112.
- Silverberg JI, Simpson EL, Thyssen JP, et al. Efficacy and safety of abrocitinib in patients with moderate-to-severe atopic dermatitis: a randomized clinical trial. JAMA Dermatol. 2020;156:863-873.
- Reich K, Kabashima K, Peris K, et al. Efficacy and safety of baricitinib combined with topical corticosteroids for treatment of moderate to severe atopic dermatitis: a randomized clinical trial. JAMA Dermatol. 2020;156:1333-1343.
- Simpson EL, Forman S, Silverberg JI, et al. Baricitinib in patients with moderate-to-severe atopic dermatitis: results from a randomized monotherapy phase 3 trial in the United States and Canada (BREEZE-AD5). J Am Acad Dermatol. 2021;85:62-70.
- Guttman-Yassky E, Thaçi D, Pangan AL, et al. Upadacitinib in adults with moderate to severe atopic dermatitis: 16-week results from a randomized, placebo-controlled trial. J Allergy Clin Immunol. 2020;145:877-884.
- US Food and Drug Administration. FDA requires warnings about increased risk of serious heart-related events, cancer, blood clots, and death for JAK inhibitors that treat certain chronic inflammatory conditions. Published September 1, 2022. Accessed February 16, 2022. https://www.fda.gov/drugs/drug-safety-and-availability/fda-requires-warnings-about-increased-risk-serious-heart-related-events-cancer-blood-clots-and-death
- Papp K, Gordon K, Thaçi D, et al. Phase 2 trial of selective tyrosine kinase 2 inhibition in psoriasis. N Engl J Med. 2018;379:1313-1321.
- Bristol Myers Squibb presents positive data from two pivotal phase 3 psoriasis studies demonstrating superiority of deucravacitinib compared to placebo and Otezla® (apremilast). Press release. Bristol Meyers Squibb. April 23, 2021. Accessed February 16, 2022. https://news.bms.com/news/details/2021/Bristol-Myers-Squibb-Presents-Positive-Data-from-Two-Pivotal-Phase-3-Psoriasis-Studies-Demonstrating-Superiority-of-Deucravacitinib-Compared-to-Placebo-and-Otezla-apremilast/default.aspx
- Armstrong A, Gooderham M, Warren R, et al. Efficacy and safety of deucravacitinib, an oral, selective tyrosine kinase 2 (TYK2) inhibitor, compared with placebo and apremilast in moderate to severe plaque psoriasis: results from the POETYK PSO-1 study [abstract]. Abstract presented at: 2021 American Academy of Dermatology annual meeting; April 23-25, 2021; San Francisco, California.
Atopic Dermatitis Oral Therapies: What Are Patients Learning on YouTube?
To the Editor:
Oral immunosuppressive therapies are prescribed for moderate to severe atopic dermatitis. Patients often consult YouTube to make informed decisions about these therapies. In the United States, most health-related online searches are initiated through a search engine, which frequently leads to social media sites such as YouTube. Recent studies have examined the reasons why users turn to the Internet for health-related information, indicating that users typically seek specific information regarding health concerns.1,2 Furthermore, social media platforms such as YouTube are a popular means of sharing health information with the public.3-5 Currently, YouTube has more than 1 billion registered users, and 30 million health-related videos are watched each day.6 Almost one-third of US consumers use YouTube, Facebook, and Twitter to obtain medical information.7 YouTube is a versatile tool because of its video-discovery mechanisms such as a keyword-based search engine, video-recommendation system, highlight feature for videos on home pages, and the capacity to embed YouTube videos on various web pages.8 Searchers use videos that are short, fast paced, emotion evoking, from credible sources, recently uploaded, and relevant to the searcher for aiding in health decisions.9 Furthermore, studies have demonstrated YouTube’s capacity to support a change in attitude and increase users’ knowledge. In fact, YouTube had higher impact on recall, attitudes, and behaviors when compared with written materials on other social media platforms, such as Facebook and Twitter.9 We conducted a cross-sectional study to examine the quality of YouTube videos on oral therapies for atopic dermatitis, such as cyclosporine, methotrexate, azathioprine, and mycophenolate mofetil.
On April 23, 2020, we performed 8 searches using a private browser with default filters on YouTube (Figure). Injectables were not included in the analysis, as the YouTube experience on dupilumab previously has been investigated.10 The top 40 videos from each search were screened by 3 researchers. Duplicates, non–English-language videos, and videos that did not discuss atopic dermatitis or oral therapies were excluded, resulting in 73 videos included in this analysis. Testimonials generated by patients made up 39 of 73 (53.4%) videos. Health care professionals created 23 of 73 (31.5%) videos, and educators with financial interest created 11 of 73 (15.1%) videos. The dates of production for the videos spanned from 2008 to 2020.
The major topics addressed in the videos were symptomatic changes (63 [68.8% of all topics discussed]), adverse effects (52 [67.5%]), and quality-of-life changes (37 [48.1%]). Of the videos included, the majority (42/73 [57.5%]) contained a neutral tone about the medication, citing advantages and disadvantages with therapy, while 22 of 73 (30.1%) had an encouraging tone, and 9 of 73 (12.3%) had a discouraging tone. Regarding videos with positive tones, there were 17 videos on cyclosporine, 9 on azathioprine, 7 on methotrexate, 4 on oral steroids, and 2 on mycophenolate mofetil. Regarding videos with negative tones, there were 4 on cyclosporine, 3 on azathioprine, 2 on methotrexate, and 2 on mycophenolate mofetil.
Of the videos made with financial interest, the majority (28/34 [77.8%]) were more suitable for informing health care providers rather than patients, containing jargon as well as complex information on clinical trials, dosing, and mechanisms of action. From the videos discussing clinical recommendations, there were 9 of 73 (12.3%) Grade A recommendations (eg, citing evidence-based information and clinical trials) and 64 of 73 (87.7%) Grade B recommendations (eg, anecdotal information on patient experience). Thirty-seven of 73 (50.7%) videos were evidence based, and 36 of 73 (49.3%) were non–evidence based. Six videos were patient-oriented news broadcasts.
Patient-generated testimonials had the most views (mean, 9238.4) and highest interaction ratio (the sum of likes, dislikes, and comments divided by the number of views)(mean, 0.027), while health care provider–generated videos had fewer views (mean, 9218.7) and a lower interaction ratio (mean, 0.011). Financial-based videos had 4233.4 views on average, with an average interaction ratio of 0.014. Based on these results, biased, patient-generated content comprised greater than 50% of YouTube videos about oral therapies for atopic dermatitis and was quite likely to be engaged with by users. Thus, these patient testimonials have great potential to affect decision-making.
The high number of patient-generated videos about oral therapies was consistent with prior studies of YouTube videos about therapies for numerous conditions.11-13 Dermatologists should consider utilizing YouTube for providing evidence-based, patient-oriented information about novel therapeutics. They may consider collaborating with patients to assist with their creation of YouTube videos and directing patients to credible resources by the American Academy of Dermatology and Canadian Dermatology Association for decision-making.
Importantly, this analysis is limited by its lack of quality-assessment tools for video-based resources such as JAMA score and DISCERN score.14,15 However, these metrics have limited ability to evaluate audiovisual elements, indicating the need for novel tools to score their validity.
- Fox S, Duggan M. Health online 2013. January 15, 2013. Accessed August 15, 2021. https://www.pewresearch.org/internet/2013/01/15/health-online-2013/
- Ní Ríordáin R, McCreary C. Dental patients’ use of the Internet. Br Dent J. 2009;207:583-586, 575.
- Fergie G, Hilton S, Hunt K. Young adults’ experiences of seeking online information about diabetes and mental health in the age of social media. Health Expect. 2016;19:1324-1335.
- Antheunis ML, Tates K, Nieboer TE. Patients’ and health professionals’ use of social media in health care: motives, barriers and expectations. Patient Educ Couns. 2013;92:426-431.
- McGregor F, Somner JE, Bourne RR, et al. Social media use by patients with glaucoma: what can we learn? Ophthalmic Physiol Opt. 2014;34:46-52.
- YouTube Statistics—15 Amazing Stats for 2015. Published April 30, 2015. Accessed August 27, 2021. YouTube.com/watch?v=9ZLBSPzY7GQ
- Health Research Institute. Social media “likes” healthcare: from marketing to social business. April 2012. Accessed August 15, 2021. https://www.pwc.com/us/en/health-industries/health-research-institute/publications/pdf/health-care-social-media-report.pdf
- Zhou R, Khemmarat S, Gao L, et al. How YouTube videos are discovered and its impact on videos views. Multimed Tools Appl. 2016;75:6035-6058.
- Haslam K, Doucette H, Hachey S, et al. YouTube videos as health decision aids for the public: an integrative review. Can J Dent Hyg. 2019;53:53-66.
- Pithadia D, Reynolds K, Lee E, et al. Dupilumab for atopic dermatitis: what are patients learning on YouTube [published online ahead of print April 16,2020]? J Dermatolog Treat. doi: 10.1080/09546634.2020.1755418
- Tolu S, Yurdakul OV, Basaran B, et al. English-language videos on YouTube as a source of information on self-administer subcutaneous anti-tumour necrosis factor agent injections. Rheumatol Int. 2018;38:1285-1292.
- Reynolds KA, Pithadia DJ, Lee EB, et al. A cross-sectional study of YouTube videos about psoriasis biologics. Int J Dermatol. 2019;58:E61-E62.
- Kocyigit BF, Akaltun MS. Does YouTube provide high quality information? assessment of secukinumab videos. Rheumatol Int. 2019;39:1263-1268.
- Qi J, Trang T, Doong J, et al. Misinformation is prevalent in psoriasis-related YouTube videos. Dermatol Online J. 2016;22:13030/qt7qc9z2m5
- Gokcen HB, Gumussuyu G. A quality analysis of disc herniation videos on YouTube. World Neurosurg. 2019;124:E799-E804.
To the Editor:
Oral immunosuppressive therapies are prescribed for moderate to severe atopic dermatitis. Patients often consult YouTube to make informed decisions about these therapies. In the United States, most health-related online searches are initiated through a search engine, which frequently leads to social media sites such as YouTube. Recent studies have examined the reasons why users turn to the Internet for health-related information, indicating that users typically seek specific information regarding health concerns.1,2 Furthermore, social media platforms such as YouTube are a popular means of sharing health information with the public.3-5 Currently, YouTube has more than 1 billion registered users, and 30 million health-related videos are watched each day.6 Almost one-third of US consumers use YouTube, Facebook, and Twitter to obtain medical information.7 YouTube is a versatile tool because of its video-discovery mechanisms such as a keyword-based search engine, video-recommendation system, highlight feature for videos on home pages, and the capacity to embed YouTube videos on various web pages.8 Searchers use videos that are short, fast paced, emotion evoking, from credible sources, recently uploaded, and relevant to the searcher for aiding in health decisions.9 Furthermore, studies have demonstrated YouTube’s capacity to support a change in attitude and increase users’ knowledge. In fact, YouTube had higher impact on recall, attitudes, and behaviors when compared with written materials on other social media platforms, such as Facebook and Twitter.9 We conducted a cross-sectional study to examine the quality of YouTube videos on oral therapies for atopic dermatitis, such as cyclosporine, methotrexate, azathioprine, and mycophenolate mofetil.
On April 23, 2020, we performed 8 searches using a private browser with default filters on YouTube (Figure). Injectables were not included in the analysis, as the YouTube experience on dupilumab previously has been investigated.10 The top 40 videos from each search were screened by 3 researchers. Duplicates, non–English-language videos, and videos that did not discuss atopic dermatitis or oral therapies were excluded, resulting in 73 videos included in this analysis. Testimonials generated by patients made up 39 of 73 (53.4%) videos. Health care professionals created 23 of 73 (31.5%) videos, and educators with financial interest created 11 of 73 (15.1%) videos. The dates of production for the videos spanned from 2008 to 2020.

The major topics addressed in the videos were symptomatic changes (63 [68.8% of all topics discussed]), adverse effects (52 [67.5%]), and quality-of-life changes (37 [48.1%]). Of the videos included, the majority (42/73 [57.5%]) contained a neutral tone about the medication, citing advantages and disadvantages with therapy, while 22 of 73 (30.1%) had an encouraging tone, and 9 of 73 (12.3%) had a discouraging tone. Regarding videos with positive tones, there were 17 videos on cyclosporine, 9 on azathioprine, 7 on methotrexate, 4 on oral steroids, and 2 on mycophenolate mofetil. Regarding videos with negative tones, there were 4 on cyclosporine, 3 on azathioprine, 2 on methotrexate, and 2 on mycophenolate mofetil.
Of the videos made with financial interest, the majority (28/34 [77.8%]) were more suitable for informing health care providers rather than patients, containing jargon as well as complex information on clinical trials, dosing, and mechanisms of action. From the videos discussing clinical recommendations, there were 9 of 73 (12.3%) Grade A recommendations (eg, citing evidence-based information and clinical trials) and 64 of 73 (87.7%) Grade B recommendations (eg, anecdotal information on patient experience). Thirty-seven of 73 (50.7%) videos were evidence based, and 36 of 73 (49.3%) were non–evidence based. Six videos were patient-oriented news broadcasts.
Patient-generated testimonials had the most views (mean, 9238.4) and highest interaction ratio (the sum of likes, dislikes, and comments divided by the number of views)(mean, 0.027), while health care provider–generated videos had fewer views (mean, 9218.7) and a lower interaction ratio (mean, 0.011). Financial-based videos had 4233.4 views on average, with an average interaction ratio of 0.014. Based on these results, biased, patient-generated content comprised greater than 50% of YouTube videos about oral therapies for atopic dermatitis and was quite likely to be engaged with by users. Thus, these patient testimonials have great potential to affect decision-making.
The high number of patient-generated videos about oral therapies was consistent with prior studies of YouTube videos about therapies for numerous conditions.11-13 Dermatologists should consider utilizing YouTube for providing evidence-based, patient-oriented information about novel therapeutics. They may consider collaborating with patients to assist with their creation of YouTube videos and directing patients to credible resources by the American Academy of Dermatology and Canadian Dermatology Association for decision-making.
Importantly, this analysis is limited by its lack of quality-assessment tools for video-based resources such as JAMA score and DISCERN score.14,15 However, these metrics have limited ability to evaluate audiovisual elements, indicating the need for novel tools to score their validity.
To the Editor:
Oral immunosuppressive therapies are prescribed for moderate to severe atopic dermatitis. Patients often consult YouTube to make informed decisions about these therapies. In the United States, most health-related online searches are initiated through a search engine, which frequently leads to social media sites such as YouTube. Recent studies have examined the reasons why users turn to the Internet for health-related information, indicating that users typically seek specific information regarding health concerns.1,2 Furthermore, social media platforms such as YouTube are a popular means of sharing health information with the public.3-5 Currently, YouTube has more than 1 billion registered users, and 30 million health-related videos are watched each day.6 Almost one-third of US consumers use YouTube, Facebook, and Twitter to obtain medical information.7 YouTube is a versatile tool because of its video-discovery mechanisms such as a keyword-based search engine, video-recommendation system, highlight feature for videos on home pages, and the capacity to embed YouTube videos on various web pages.8 Searchers use videos that are short, fast paced, emotion evoking, from credible sources, recently uploaded, and relevant to the searcher for aiding in health decisions.9 Furthermore, studies have demonstrated YouTube’s capacity to support a change in attitude and increase users’ knowledge. In fact, YouTube had higher impact on recall, attitudes, and behaviors when compared with written materials on other social media platforms, such as Facebook and Twitter.9 We conducted a cross-sectional study to examine the quality of YouTube videos on oral therapies for atopic dermatitis, such as cyclosporine, methotrexate, azathioprine, and mycophenolate mofetil.
On April 23, 2020, we performed 8 searches using a private browser with default filters on YouTube (Figure). Injectables were not included in the analysis, as the YouTube experience on dupilumab previously has been investigated.10 The top 40 videos from each search were screened by 3 researchers. Duplicates, non–English-language videos, and videos that did not discuss atopic dermatitis or oral therapies were excluded, resulting in 73 videos included in this analysis. Testimonials generated by patients made up 39 of 73 (53.4%) videos. Health care professionals created 23 of 73 (31.5%) videos, and educators with financial interest created 11 of 73 (15.1%) videos. The dates of production for the videos spanned from 2008 to 2020.

The major topics addressed in the videos were symptomatic changes (63 [68.8% of all topics discussed]), adverse effects (52 [67.5%]), and quality-of-life changes (37 [48.1%]). Of the videos included, the majority (42/73 [57.5%]) contained a neutral tone about the medication, citing advantages and disadvantages with therapy, while 22 of 73 (30.1%) had an encouraging tone, and 9 of 73 (12.3%) had a discouraging tone. Regarding videos with positive tones, there were 17 videos on cyclosporine, 9 on azathioprine, 7 on methotrexate, 4 on oral steroids, and 2 on mycophenolate mofetil. Regarding videos with negative tones, there were 4 on cyclosporine, 3 on azathioprine, 2 on methotrexate, and 2 on mycophenolate mofetil.
Of the videos made with financial interest, the majority (28/34 [77.8%]) were more suitable for informing health care providers rather than patients, containing jargon as well as complex information on clinical trials, dosing, and mechanisms of action. From the videos discussing clinical recommendations, there were 9 of 73 (12.3%) Grade A recommendations (eg, citing evidence-based information and clinical trials) and 64 of 73 (87.7%) Grade B recommendations (eg, anecdotal information on patient experience). Thirty-seven of 73 (50.7%) videos were evidence based, and 36 of 73 (49.3%) were non–evidence based. Six videos were patient-oriented news broadcasts.
Patient-generated testimonials had the most views (mean, 9238.4) and highest interaction ratio (the sum of likes, dislikes, and comments divided by the number of views)(mean, 0.027), while health care provider–generated videos had fewer views (mean, 9218.7) and a lower interaction ratio (mean, 0.011). Financial-based videos had 4233.4 views on average, with an average interaction ratio of 0.014. Based on these results, biased, patient-generated content comprised greater than 50% of YouTube videos about oral therapies for atopic dermatitis and was quite likely to be engaged with by users. Thus, these patient testimonials have great potential to affect decision-making.
The high number of patient-generated videos about oral therapies was consistent with prior studies of YouTube videos about therapies for numerous conditions.11-13 Dermatologists should consider utilizing YouTube for providing evidence-based, patient-oriented information about novel therapeutics. They may consider collaborating with patients to assist with their creation of YouTube videos and directing patients to credible resources by the American Academy of Dermatology and Canadian Dermatology Association for decision-making.
Importantly, this analysis is limited by its lack of quality-assessment tools for video-based resources such as JAMA score and DISCERN score.14,15 However, these metrics have limited ability to evaluate audiovisual elements, indicating the need for novel tools to score their validity.
- Fox S, Duggan M. Health online 2013. January 15, 2013. Accessed August 15, 2021. https://www.pewresearch.org/internet/2013/01/15/health-online-2013/
- Ní Ríordáin R, McCreary C. Dental patients’ use of the Internet. Br Dent J. 2009;207:583-586, 575.
- Fergie G, Hilton S, Hunt K. Young adults’ experiences of seeking online information about diabetes and mental health in the age of social media. Health Expect. 2016;19:1324-1335.
- Antheunis ML, Tates K, Nieboer TE. Patients’ and health professionals’ use of social media in health care: motives, barriers and expectations. Patient Educ Couns. 2013;92:426-431.
- McGregor F, Somner JE, Bourne RR, et al. Social media use by patients with glaucoma: what can we learn? Ophthalmic Physiol Opt. 2014;34:46-52.
- YouTube Statistics—15 Amazing Stats for 2015. Published April 30, 2015. Accessed August 27, 2021. YouTube.com/watch?v=9ZLBSPzY7GQ
- Health Research Institute. Social media “likes” healthcare: from marketing to social business. April 2012. Accessed August 15, 2021. https://www.pwc.com/us/en/health-industries/health-research-institute/publications/pdf/health-care-social-media-report.pdf
- Zhou R, Khemmarat S, Gao L, et al. How YouTube videos are discovered and its impact on videos views. Multimed Tools Appl. 2016;75:6035-6058.
- Haslam K, Doucette H, Hachey S, et al. YouTube videos as health decision aids for the public: an integrative review. Can J Dent Hyg. 2019;53:53-66.
- Pithadia D, Reynolds K, Lee E, et al. Dupilumab for atopic dermatitis: what are patients learning on YouTube [published online ahead of print April 16,2020]? J Dermatolog Treat. doi: 10.1080/09546634.2020.1755418
- Tolu S, Yurdakul OV, Basaran B, et al. English-language videos on YouTube as a source of information on self-administer subcutaneous anti-tumour necrosis factor agent injections. Rheumatol Int. 2018;38:1285-1292.
- Reynolds KA, Pithadia DJ, Lee EB, et al. A cross-sectional study of YouTube videos about psoriasis biologics. Int J Dermatol. 2019;58:E61-E62.
- Kocyigit BF, Akaltun MS. Does YouTube provide high quality information? assessment of secukinumab videos. Rheumatol Int. 2019;39:1263-1268.
- Qi J, Trang T, Doong J, et al. Misinformation is prevalent in psoriasis-related YouTube videos. Dermatol Online J. 2016;22:13030/qt7qc9z2m5
- Gokcen HB, Gumussuyu G. A quality analysis of disc herniation videos on YouTube. World Neurosurg. 2019;124:E799-E804.
- Fox S, Duggan M. Health online 2013. January 15, 2013. Accessed August 15, 2021. https://www.pewresearch.org/internet/2013/01/15/health-online-2013/
- Ní Ríordáin R, McCreary C. Dental patients’ use of the Internet. Br Dent J. 2009;207:583-586, 575.
- Fergie G, Hilton S, Hunt K. Young adults’ experiences of seeking online information about diabetes and mental health in the age of social media. Health Expect. 2016;19:1324-1335.
- Antheunis ML, Tates K, Nieboer TE. Patients’ and health professionals’ use of social media in health care: motives, barriers and expectations. Patient Educ Couns. 2013;92:426-431.
- McGregor F, Somner JE, Bourne RR, et al. Social media use by patients with glaucoma: what can we learn? Ophthalmic Physiol Opt. 2014;34:46-52.
- YouTube Statistics—15 Amazing Stats for 2015. Published April 30, 2015. Accessed August 27, 2021. YouTube.com/watch?v=9ZLBSPzY7GQ
- Health Research Institute. Social media “likes” healthcare: from marketing to social business. April 2012. Accessed August 15, 2021. https://www.pwc.com/us/en/health-industries/health-research-institute/publications/pdf/health-care-social-media-report.pdf
- Zhou R, Khemmarat S, Gao L, et al. How YouTube videos are discovered and its impact on videos views. Multimed Tools Appl. 2016;75:6035-6058.
- Haslam K, Doucette H, Hachey S, et al. YouTube videos as health decision aids for the public: an integrative review. Can J Dent Hyg. 2019;53:53-66.
- Pithadia D, Reynolds K, Lee E, et al. Dupilumab for atopic dermatitis: what are patients learning on YouTube [published online ahead of print April 16,2020]? J Dermatolog Treat. doi: 10.1080/09546634.2020.1755418
- Tolu S, Yurdakul OV, Basaran B, et al. English-language videos on YouTube as a source of information on self-administer subcutaneous anti-tumour necrosis factor agent injections. Rheumatol Int. 2018;38:1285-1292.
- Reynolds KA, Pithadia DJ, Lee EB, et al. A cross-sectional study of YouTube videos about psoriasis biologics. Int J Dermatol. 2019;58:E61-E62.
- Kocyigit BF, Akaltun MS. Does YouTube provide high quality information? assessment of secukinumab videos. Rheumatol Int. 2019;39:1263-1268.
- Qi J, Trang T, Doong J, et al. Misinformation is prevalent in psoriasis-related YouTube videos. Dermatol Online J. 2016;22:13030/qt7qc9z2m5
- Gokcen HB, Gumussuyu G. A quality analysis of disc herniation videos on YouTube. World Neurosurg. 2019;124:E799-E804.
Practice Points
- Patient-based YouTube videos comprised the majority of videos on oral therapies for atopic dermatitis, with the greatest views and interaction ratio.
- Most YouTube videos on this topic contained a neutral tone and Grade B recommendations, thus meriting production of more evidence-based videos in collaboration with patients on the YouTube platform.
Atopic Dermatitis Topical Therapies: Study of YouTube Videos as a Source of Patient Information
To the Editor:
Atopic dermatitis (eczema) affects approximately 20% of children worldwide.1 In atopic dermatitis management, patient education is crucial for optimal outcomes.2 The COVID-19 pandemic has impacted patient-physician interactions. To ensure safety of patients and physicians, visits may have been canceled, postponed, or conducted virtually, leaving less time for discussion and questions.3 As a consequence, patients may seek information about atopic dermatitis from alternative sources, including YouTube videos. We performed a cross-sectional study to analyze YouTube videos about topical treatments for atopic dermatitis.
During the week of July 16, 2020, we performed 4 private browser YouTube searches with default filters using the following terms: eczema topicals, eczema topical treatments, atopic dermatitis topicals, and atopic dermatitis topical treatments. For video selection, we defined topical treatments as topical corticosteroids, topical calcineurin inhibitors, crisaborole, emollients, wet wraps, and any prospective treatment topically administered. For each of the 4 searches, 2 researchers (A.M. and A.T.) independently examined the top 75 videos, yielding a total of 300 videos. Of them, 98 videos were duplicates, 19 videos were not about atopic dermatitis, and 91 videos were not about topical treatments, leaving a total of 92 videos for analysis (Figure 1).
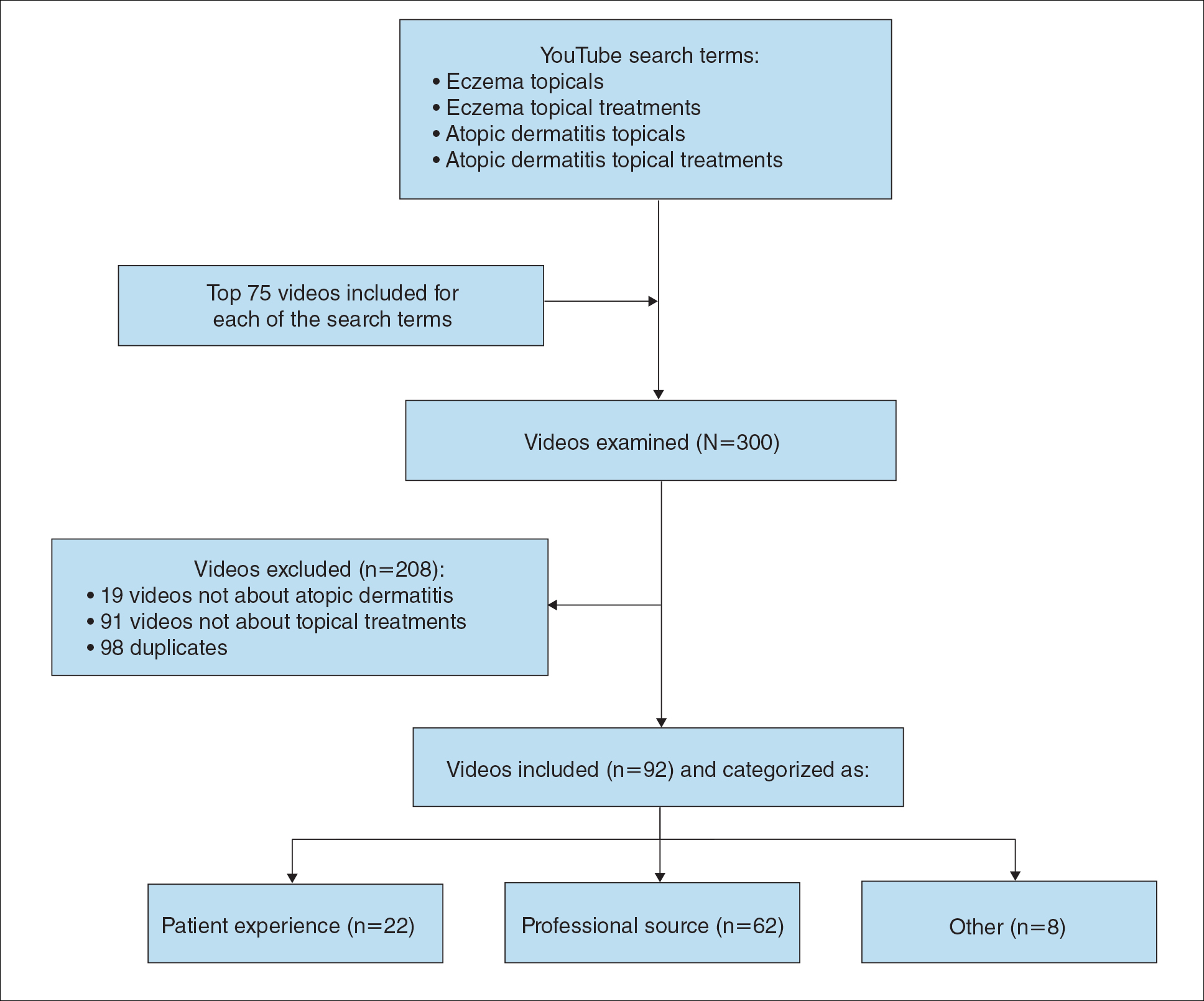
For the 92 included videos, the length; upload year; number of views, likes, dislikes, and comments; interaction ratio (IR)(the sum of likes, dislikes, and comments divided by the number of views); and video content were determined. The videos were placed into mutually exclusive categories as follows: (1) patient experience, defined as a video about patient perspective; (2) professional source, defined as a video featuring a physician, physician extender, pharmacist, or scientist, or produced by a formal organization; or (3) other. The DISCERN Instrument was used for grading the reliability and quality of the 92 included videos. This instrument consists of 16 questions with the responses rated on a scale of 1 to 5.4 For analysis of DISCERN scores, patient experience and other videos were grouped together as nonprofessional source videos. A 2-sample t-test was used to compare DISCERN scores between professional source and nonprofessional source videos.
Most videos were uploaded in 2017 (n=19), 2018 (n=23), and 2019 (n=25), but 20 were uploaded in 2012-2016 and 5 were uploaded in 2020. The 92 videos had a mean length of 8 minutes and 35 seconds (range, 30 seconds to 62 minutes and 23 seconds).
Patient experience videos accounted for 23.9% (n=22) of videos. These videos discussed topical steroid withdrawal (TSW)(n=16), instructions for making emollients (n=2), and treatment successes (n=4). Professional source videos represented 67.4% (n=62) of videos. Of them, 40.3% (n=25) were physician oriented, defined as having extensive medical terminology or qualifying for continuing medical education credit. Three (4.8%) of the professional source videos were sponsored by a drug company. Other constituted the remaining 8.7% (n=8) of videos. Patient experience videos had more views (median views [interquartile range], 6865 [10,307]) and higher engagement (median IR [interquartile range], 0.038 [0.022]) than professional source videos (views: median views [interquartile range], 1052.5 [10,610.5]; engagement: median IR [interquartile range], 0.006 [0.008]).
Although less popular, professional source videos had a significantly higher DISCERN overall quality rating score (question 16) compared to those categorized as nonprofessional source (3.92 vs 1.53; P<.001). In contrast, nonprofessional source videos scored significantly higher on the quality-of-life question (question 13) compared to professional source videos (3.90 vs 2.56; P<.001)(eTable). (Three professional source videos were removed from YouTube before DISCERN scores could be assigned.)
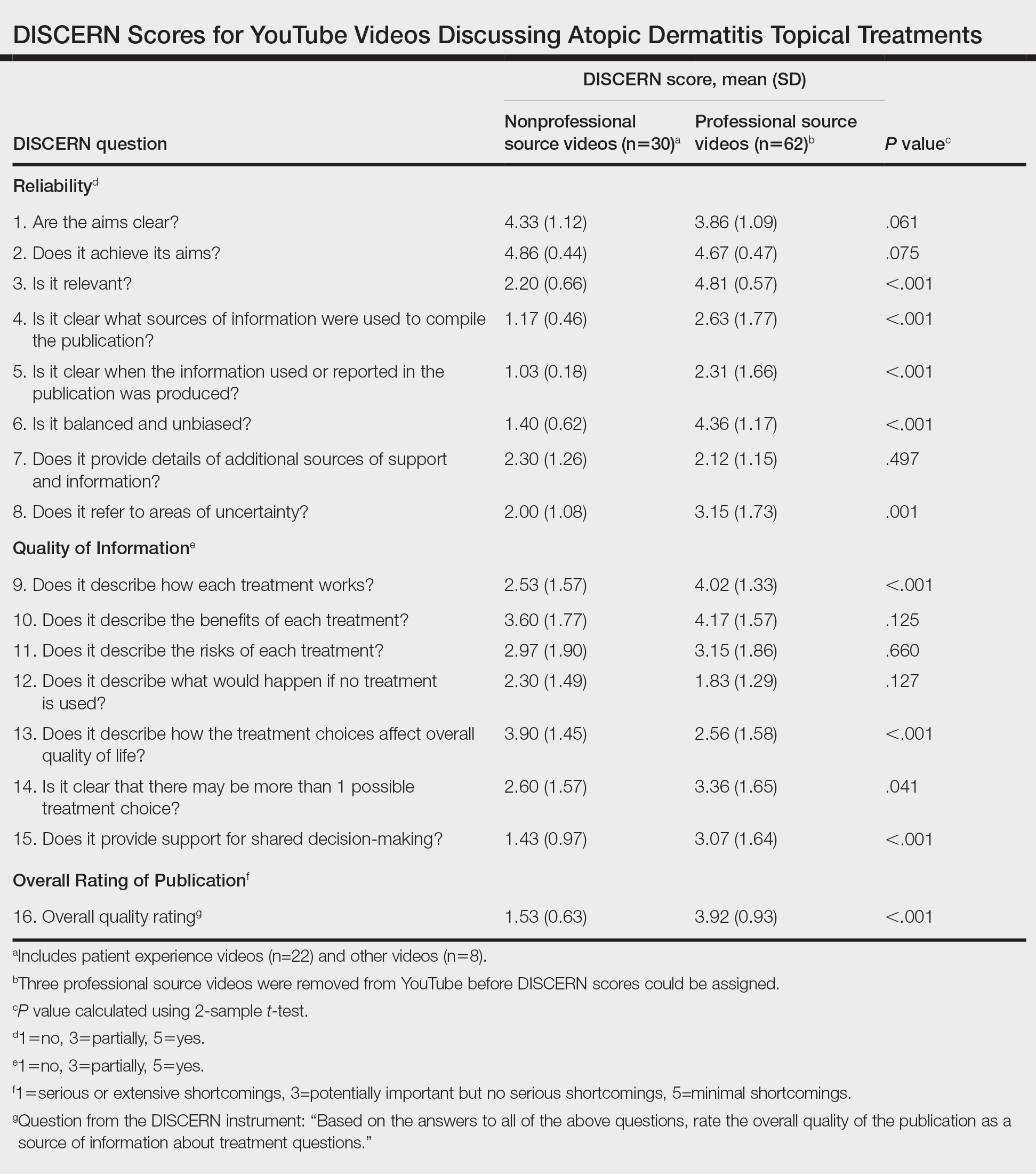
Notably, 20.7% (n=19) of the 92 videos discussed TSW, and most of them were patient experiences (n=16). Other categories included topical steroids excluding TSW (n=11), steroid phobia (n=2), topical calcineurin inhibitors (n=2), crisaborole (n=6), news broadcast (n=7), wet wraps (n=5), product advertisement (n=7), and research (n=11)(Figure 2). Interestingly, there were no videos focusing on the calcineurin inhibitor black box warning.

Similar to prior studies, our results indicate preference for patient-generated videos over videos produced by or including a professional source.5 Additionally, only 3 of 19 videos about TSW were from a professional source, increasing the potential for patient misconceptions about topical corticosteroids. Future studies should examine the educational impact of patient-generated videos as well as features that make the patient experience videos more desirable for viewing.
- Mueller SM, Hongler VNS, Jungo P, et al. Fiction, falsehoods, and few facts: cross-sectional study on the content-related quality of atopic eczema-related videos on YouTube. J Med Internet Res. 2020;22:e15599. doi:10.2196/15599
- Torres T, Ferreira EO, Gonçalo M, et al. Update on atopic dermatitis. Acta Med Port. 2019;32:606-613. doi:10.20344/amp.11963
- Vogler SA, Lightner AL. Rethinking how we care for our patients in a time of social distancing during the COVID-19 pandemic. Br J Surg. 2020;107:937-939. doi:10.1002/bjs.11636
- The DISCERN Instrument. discern online. Accessed January 22, 2021. http://www.discern.org.uk/discern_instrument.php
- Pithadia DJ, Reynolds KA, Lee EB, et al. Dupilumab for atopic dermatitis: what are patients learning on YouTube? [published online April 16, 2020]. J Dermatolog Treat. doi:10.1080/09546634.2020.1755418
To the Editor:
Atopic dermatitis (eczema) affects approximately 20% of children worldwide.1 In atopic dermatitis management, patient education is crucial for optimal outcomes.2 The COVID-19 pandemic has impacted patient-physician interactions. To ensure safety of patients and physicians, visits may have been canceled, postponed, or conducted virtually, leaving less time for discussion and questions.3 As a consequence, patients may seek information about atopic dermatitis from alternative sources, including YouTube videos. We performed a cross-sectional study to analyze YouTube videos about topical treatments for atopic dermatitis.
During the week of July 16, 2020, we performed 4 private browser YouTube searches with default filters using the following terms: eczema topicals, eczema topical treatments, atopic dermatitis topicals, and atopic dermatitis topical treatments. For video selection, we defined topical treatments as topical corticosteroids, topical calcineurin inhibitors, crisaborole, emollients, wet wraps, and any prospective treatment topically administered. For each of the 4 searches, 2 researchers (A.M. and A.T.) independently examined the top 75 videos, yielding a total of 300 videos. Of them, 98 videos were duplicates, 19 videos were not about atopic dermatitis, and 91 videos were not about topical treatments, leaving a total of 92 videos for analysis (Figure 1).

For the 92 included videos, the length; upload year; number of views, likes, dislikes, and comments; interaction ratio (IR)(the sum of likes, dislikes, and comments divided by the number of views); and video content were determined. The videos were placed into mutually exclusive categories as follows: (1) patient experience, defined as a video about patient perspective; (2) professional source, defined as a video featuring a physician, physician extender, pharmacist, or scientist, or produced by a formal organization; or (3) other. The DISCERN Instrument was used for grading the reliability and quality of the 92 included videos. This instrument consists of 16 questions with the responses rated on a scale of 1 to 5.4 For analysis of DISCERN scores, patient experience and other videos were grouped together as nonprofessional source videos. A 2-sample t-test was used to compare DISCERN scores between professional source and nonprofessional source videos.
Most videos were uploaded in 2017 (n=19), 2018 (n=23), and 2019 (n=25), but 20 were uploaded in 2012-2016 and 5 were uploaded in 2020. The 92 videos had a mean length of 8 minutes and 35 seconds (range, 30 seconds to 62 minutes and 23 seconds).
Patient experience videos accounted for 23.9% (n=22) of videos. These videos discussed topical steroid withdrawal (TSW)(n=16), instructions for making emollients (n=2), and treatment successes (n=4). Professional source videos represented 67.4% (n=62) of videos. Of them, 40.3% (n=25) were physician oriented, defined as having extensive medical terminology or qualifying for continuing medical education credit. Three (4.8%) of the professional source videos were sponsored by a drug company. Other constituted the remaining 8.7% (n=8) of videos. Patient experience videos had more views (median views [interquartile range], 6865 [10,307]) and higher engagement (median IR [interquartile range], 0.038 [0.022]) than professional source videos (views: median views [interquartile range], 1052.5 [10,610.5]; engagement: median IR [interquartile range], 0.006 [0.008]).
Although less popular, professional source videos had a significantly higher DISCERN overall quality rating score (question 16) compared to those categorized as nonprofessional source (3.92 vs 1.53; P<.001). In contrast, nonprofessional source videos scored significantly higher on the quality-of-life question (question 13) compared to professional source videos (3.90 vs 2.56; P<.001)(eTable). (Three professional source videos were removed from YouTube before DISCERN scores could be assigned.)

Notably, 20.7% (n=19) of the 92 videos discussed TSW, and most of them were patient experiences (n=16). Other categories included topical steroids excluding TSW (n=11), steroid phobia (n=2), topical calcineurin inhibitors (n=2), crisaborole (n=6), news broadcast (n=7), wet wraps (n=5), product advertisement (n=7), and research (n=11)(Figure 2). Interestingly, there were no videos focusing on the calcineurin inhibitor black box warning.

Similar to prior studies, our results indicate preference for patient-generated videos over videos produced by or including a professional source.5 Additionally, only 3 of 19 videos about TSW were from a professional source, increasing the potential for patient misconceptions about topical corticosteroids. Future studies should examine the educational impact of patient-generated videos as well as features that make the patient experience videos more desirable for viewing.
To the Editor:
Atopic dermatitis (eczema) affects approximately 20% of children worldwide.1 In atopic dermatitis management, patient education is crucial for optimal outcomes.2 The COVID-19 pandemic has impacted patient-physician interactions. To ensure safety of patients and physicians, visits may have been canceled, postponed, or conducted virtually, leaving less time for discussion and questions.3 As a consequence, patients may seek information about atopic dermatitis from alternative sources, including YouTube videos. We performed a cross-sectional study to analyze YouTube videos about topical treatments for atopic dermatitis.
During the week of July 16, 2020, we performed 4 private browser YouTube searches with default filters using the following terms: eczema topicals, eczema topical treatments, atopic dermatitis topicals, and atopic dermatitis topical treatments. For video selection, we defined topical treatments as topical corticosteroids, topical calcineurin inhibitors, crisaborole, emollients, wet wraps, and any prospective treatment topically administered. For each of the 4 searches, 2 researchers (A.M. and A.T.) independently examined the top 75 videos, yielding a total of 300 videos. Of them, 98 videos were duplicates, 19 videos were not about atopic dermatitis, and 91 videos were not about topical treatments, leaving a total of 92 videos for analysis (Figure 1).

For the 92 included videos, the length; upload year; number of views, likes, dislikes, and comments; interaction ratio (IR)(the sum of likes, dislikes, and comments divided by the number of views); and video content were determined. The videos were placed into mutually exclusive categories as follows: (1) patient experience, defined as a video about patient perspective; (2) professional source, defined as a video featuring a physician, physician extender, pharmacist, or scientist, or produced by a formal organization; or (3) other. The DISCERN Instrument was used for grading the reliability and quality of the 92 included videos. This instrument consists of 16 questions with the responses rated on a scale of 1 to 5.4 For analysis of DISCERN scores, patient experience and other videos were grouped together as nonprofessional source videos. A 2-sample t-test was used to compare DISCERN scores between professional source and nonprofessional source videos.
Most videos were uploaded in 2017 (n=19), 2018 (n=23), and 2019 (n=25), but 20 were uploaded in 2012-2016 and 5 were uploaded in 2020. The 92 videos had a mean length of 8 minutes and 35 seconds (range, 30 seconds to 62 minutes and 23 seconds).
Patient experience videos accounted for 23.9% (n=22) of videos. These videos discussed topical steroid withdrawal (TSW)(n=16), instructions for making emollients (n=2), and treatment successes (n=4). Professional source videos represented 67.4% (n=62) of videos. Of them, 40.3% (n=25) were physician oriented, defined as having extensive medical terminology or qualifying for continuing medical education credit. Three (4.8%) of the professional source videos were sponsored by a drug company. Other constituted the remaining 8.7% (n=8) of videos. Patient experience videos had more views (median views [interquartile range], 6865 [10,307]) and higher engagement (median IR [interquartile range], 0.038 [0.022]) than professional source videos (views: median views [interquartile range], 1052.5 [10,610.5]; engagement: median IR [interquartile range], 0.006 [0.008]).
Although less popular, professional source videos had a significantly higher DISCERN overall quality rating score (question 16) compared to those categorized as nonprofessional source (3.92 vs 1.53; P<.001). In contrast, nonprofessional source videos scored significantly higher on the quality-of-life question (question 13) compared to professional source videos (3.90 vs 2.56; P<.001)(eTable). (Three professional source videos were removed from YouTube before DISCERN scores could be assigned.)

Notably, 20.7% (n=19) of the 92 videos discussed TSW, and most of them were patient experiences (n=16). Other categories included topical steroids excluding TSW (n=11), steroid phobia (n=2), topical calcineurin inhibitors (n=2), crisaborole (n=6), news broadcast (n=7), wet wraps (n=5), product advertisement (n=7), and research (n=11)(Figure 2). Interestingly, there were no videos focusing on the calcineurin inhibitor black box warning.

Similar to prior studies, our results indicate preference for patient-generated videos over videos produced by or including a professional source.5 Additionally, only 3 of 19 videos about TSW were from a professional source, increasing the potential for patient misconceptions about topical corticosteroids. Future studies should examine the educational impact of patient-generated videos as well as features that make the patient experience videos more desirable for viewing.
- Mueller SM, Hongler VNS, Jungo P, et al. Fiction, falsehoods, and few facts: cross-sectional study on the content-related quality of atopic eczema-related videos on YouTube. J Med Internet Res. 2020;22:e15599. doi:10.2196/15599
- Torres T, Ferreira EO, Gonçalo M, et al. Update on atopic dermatitis. Acta Med Port. 2019;32:606-613. doi:10.20344/amp.11963
- Vogler SA, Lightner AL. Rethinking how we care for our patients in a time of social distancing during the COVID-19 pandemic. Br J Surg. 2020;107:937-939. doi:10.1002/bjs.11636
- The DISCERN Instrument. discern online. Accessed January 22, 2021. http://www.discern.org.uk/discern_instrument.php
- Pithadia DJ, Reynolds KA, Lee EB, et al. Dupilumab for atopic dermatitis: what are patients learning on YouTube? [published online April 16, 2020]. J Dermatolog Treat. doi:10.1080/09546634.2020.1755418
- Mueller SM, Hongler VNS, Jungo P, et al. Fiction, falsehoods, and few facts: cross-sectional study on the content-related quality of atopic eczema-related videos on YouTube. J Med Internet Res. 2020;22:e15599. doi:10.2196/15599
- Torres T, Ferreira EO, Gonçalo M, et al. Update on atopic dermatitis. Acta Med Port. 2019;32:606-613. doi:10.20344/amp.11963
- Vogler SA, Lightner AL. Rethinking how we care for our patients in a time of social distancing during the COVID-19 pandemic. Br J Surg. 2020;107:937-939. doi:10.1002/bjs.11636
- The DISCERN Instrument. discern online. Accessed January 22, 2021. http://www.discern.org.uk/discern_instrument.php
- Pithadia DJ, Reynolds KA, Lee EB, et al. Dupilumab for atopic dermatitis: what are patients learning on YouTube? [published online April 16, 2020]. J Dermatolog Treat. doi:10.1080/09546634.2020.1755418
Practice Points
- YouTube is a readily accessible resource for educating patients about topical treatments for atopic dermatitis.
- Although professional source videos comprised a larger percentage of the videos included within our study, patient experience videos had a higher number of views and engagement.
- Twenty-one percent (19/92) of the videos examined in our study discussed topical steroid withdrawal, and the majority of them were patient experience videos.