User login
Lucas Franki is an associate editor for MDedge News, and has been with the company since 2014. He has a BA in English from Penn State University and is an Eagle Scout.
Disease burden in OA worse than RA 6 months post presentation
Patients with osteoarthritis (OA) have RAPID3 scores at their initial visit (16.0) similar to patients with rheumatoid arthritis (RA) and either prior use of disease-modifying antirheumatic drugs (DMARDs) or no exposure to DMARDs (15.6 and 15.5, respectively). After 6 months of treatment, the RAPID3 (Routine Assessment of Patient Index Data 3) score fell by just 1.7 points for patients with OA, compared with 5.7 points in RA patients naive to DMARDs and 4.3 points in those with prior DMARD exposure. These findings were published March 20 in Arthritis & Rheumatology (doi: 10.1002/art.40869).
We reported this story at the 2018 World Congress on Osteoarthritis before it was published in the journal. Read the story at the link above.
Patients with osteoarthritis (OA) have RAPID3 scores at their initial visit (16.0) similar to patients with rheumatoid arthritis (RA) and either prior use of disease-modifying antirheumatic drugs (DMARDs) or no exposure to DMARDs (15.6 and 15.5, respectively). After 6 months of treatment, the RAPID3 (Routine Assessment of Patient Index Data 3) score fell by just 1.7 points for patients with OA, compared with 5.7 points in RA patients naive to DMARDs and 4.3 points in those with prior DMARD exposure. These findings were published March 20 in Arthritis & Rheumatology (doi: 10.1002/art.40869).
We reported this story at the 2018 World Congress on Osteoarthritis before it was published in the journal. Read the story at the link above.
Patients with osteoarthritis (OA) have RAPID3 scores at their initial visit (16.0) similar to patients with rheumatoid arthritis (RA) and either prior use of disease-modifying antirheumatic drugs (DMARDs) or no exposure to DMARDs (15.6 and 15.5, respectively). After 6 months of treatment, the RAPID3 (Routine Assessment of Patient Index Data 3) score fell by just 1.7 points for patients with OA, compared with 5.7 points in RA patients naive to DMARDs and 4.3 points in those with prior DMARD exposure. These findings were published March 20 in Arthritis & Rheumatology (doi: 10.1002/art.40869).
We reported this story at the 2018 World Congress on Osteoarthritis before it was published in the journal. Read the story at the link above.
FROM ARTHRITIS & RHEUMATOLOGY
FDA approves atezolizumab for first-line ES-SCLC treatment
The Food and Drug Administration has approved atezolizumab (Tecentriq), in combination with carboplatin and etoposide, for the first-line treatment of adults with extensive-stage small cell lung cancer (ES-SCLC).
Approval was based on results from the phase 3 IMpower133 study, in which 403 treatment-naive patients with ES-SCLC received atezolizumab at 1,200 mg with carboplatin at 5 mg/mL per minute on day 1 and etoposide 100 mg/m2 on days 1, 2, and 3 of a 21-day cycle for four cycles, followed by atezolizumab at 1,200 mg once every 3 weeks until disease progression or unacceptable toxicity; or received placebo with the same dosage of carboplatin and etoposide for a similar duration.
Overall survival was significantly better in patients who received atezolizumab, compared with placebo (12.3 vs. 10.3 months; hazard ratio, 0.70; 95% confidence interval, 0.54-0.91; P = .0069), as was progression-free survival (5.2 vs. 4.3 months; HR, 0.77; 95% CI, 0.62-0.96; P = .017).
The most common adverse events associated with atezolizumab in the study were fatigue/asthenia, nausea, alopecia, constipation, and decreased appetite.
According to the FDA, the recommended dose is 1,200 mg IV over 60 minutes every 3 weeks. When administered on the same day as chemotherapy, atezolizumab should be given first. If the first infusion is tolerated, all subsequent infusions can be delivered over 30 minutes.
Find the full press release on the FDA website.
The Food and Drug Administration has approved atezolizumab (Tecentriq), in combination with carboplatin and etoposide, for the first-line treatment of adults with extensive-stage small cell lung cancer (ES-SCLC).
Approval was based on results from the phase 3 IMpower133 study, in which 403 treatment-naive patients with ES-SCLC received atezolizumab at 1,200 mg with carboplatin at 5 mg/mL per minute on day 1 and etoposide 100 mg/m2 on days 1, 2, and 3 of a 21-day cycle for four cycles, followed by atezolizumab at 1,200 mg once every 3 weeks until disease progression or unacceptable toxicity; or received placebo with the same dosage of carboplatin and etoposide for a similar duration.
Overall survival was significantly better in patients who received atezolizumab, compared with placebo (12.3 vs. 10.3 months; hazard ratio, 0.70; 95% confidence interval, 0.54-0.91; P = .0069), as was progression-free survival (5.2 vs. 4.3 months; HR, 0.77; 95% CI, 0.62-0.96; P = .017).
The most common adverse events associated with atezolizumab in the study were fatigue/asthenia, nausea, alopecia, constipation, and decreased appetite.
According to the FDA, the recommended dose is 1,200 mg IV over 60 minutes every 3 weeks. When administered on the same day as chemotherapy, atezolizumab should be given first. If the first infusion is tolerated, all subsequent infusions can be delivered over 30 minutes.
Find the full press release on the FDA website.
The Food and Drug Administration has approved atezolizumab (Tecentriq), in combination with carboplatin and etoposide, for the first-line treatment of adults with extensive-stage small cell lung cancer (ES-SCLC).
Approval was based on results from the phase 3 IMpower133 study, in which 403 treatment-naive patients with ES-SCLC received atezolizumab at 1,200 mg with carboplatin at 5 mg/mL per minute on day 1 and etoposide 100 mg/m2 on days 1, 2, and 3 of a 21-day cycle for four cycles, followed by atezolizumab at 1,200 mg once every 3 weeks until disease progression or unacceptable toxicity; or received placebo with the same dosage of carboplatin and etoposide for a similar duration.
Overall survival was significantly better in patients who received atezolizumab, compared with placebo (12.3 vs. 10.3 months; hazard ratio, 0.70; 95% confidence interval, 0.54-0.91; P = .0069), as was progression-free survival (5.2 vs. 4.3 months; HR, 0.77; 95% CI, 0.62-0.96; P = .017).
The most common adverse events associated with atezolizumab in the study were fatigue/asthenia, nausea, alopecia, constipation, and decreased appetite.
According to the FDA, the recommended dose is 1,200 mg IV over 60 minutes every 3 weeks. When administered on the same day as chemotherapy, atezolizumab should be given first. If the first infusion is tolerated, all subsequent infusions can be delivered over 30 minutes.
Find the full press release on the FDA website.
Match Day 2019: Dermatology steps up growth after slow 2018
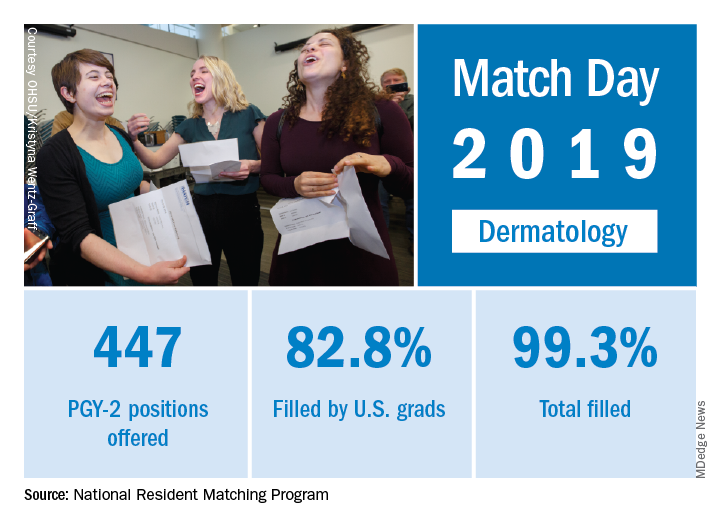
Available dermatology PGY-2 slots rose by 4.9% from 426 in 2018 to 447 in 2019, while slots filled grew by 5.5% from 420 to 443, for an overall fill rate of 99.3%. In addition, the fill rate for U.S. graduates grew for the first time since 2015, rising from 81.7% to 82.8%.
An overall total of 2,756 PGY-2 slots were offered, 97.2% of which were filled; 67.5% were filled by U.S. graduates, the NRMP said in its 2019 Main Residency Match report.
The 2019 Match set a record for most positions offered (35,185; up 6.1%), most positions filled (up 4.8%), most PGY-1 positions offered (32,194; up 6.5%), and total applicants (38,376; up 3.4%).
“The results of the Match are closely watched because they can be predictors of future physician workforce supply. There also is significant interest in the competitiveness of specialties, as measured by the percentage of positions filled overall and the percentage filled by senior students in U.S. allopathic medical schools,” NRMP officials said in a statement.

Available dermatology PGY-2 slots rose by 4.9% from 426 in 2018 to 447 in 2019, while slots filled grew by 5.5% from 420 to 443, for an overall fill rate of 99.3%. In addition, the fill rate for U.S. graduates grew for the first time since 2015, rising from 81.7% to 82.8%.
An overall total of 2,756 PGY-2 slots were offered, 97.2% of which were filled; 67.5% were filled by U.S. graduates, the NRMP said in its 2019 Main Residency Match report.
The 2019 Match set a record for most positions offered (35,185; up 6.1%), most positions filled (up 4.8%), most PGY-1 positions offered (32,194; up 6.5%), and total applicants (38,376; up 3.4%).
“The results of the Match are closely watched because they can be predictors of future physician workforce supply. There also is significant interest in the competitiveness of specialties, as measured by the percentage of positions filled overall and the percentage filled by senior students in U.S. allopathic medical schools,” NRMP officials said in a statement.

Available dermatology PGY-2 slots rose by 4.9% from 426 in 2018 to 447 in 2019, while slots filled grew by 5.5% from 420 to 443, for an overall fill rate of 99.3%. In addition, the fill rate for U.S. graduates grew for the first time since 2015, rising from 81.7% to 82.8%.
An overall total of 2,756 PGY-2 slots were offered, 97.2% of which were filled; 67.5% were filled by U.S. graduates, the NRMP said in its 2019 Main Residency Match report.
The 2019 Match set a record for most positions offered (35,185; up 6.1%), most positions filled (up 4.8%), most PGY-1 positions offered (32,194; up 6.5%), and total applicants (38,376; up 3.4%).
“The results of the Match are closely watched because they can be predictors of future physician workforce supply. There also is significant interest in the competitiveness of specialties, as measured by the percentage of positions filled overall and the percentage filled by senior students in U.S. allopathic medical schools,” NRMP officials said in a statement.
Match Day 2019: Psychiatry sees double-digit growth
Match Day 2019 was another record breaker, and psychiatry helped play a big role by offering nearly 200 more residency slots and matches than in 2018, according to the National Resident Matching Program (NRMP).

There were a total of 1,740 PGY-1 psychiatry slots offered in 2019, up from 1,556 in 2018, for an increase of 11.8%. The 184-position increase was the fourth-largest overall increase among all measured specialties, behind only internal medicine, family medicine, and emergency medicine.
in a press release. However, while the fill rate increased slightly to 99%, leaving only 20 slots unfilled, the fill rate by U.S. graduates dropped from 63.1% to 60.6%.
Overall, a total of 32,194 PGY-1 slots were offered, a new record, and 94.9% were filled, with U.S. graduates filling 55.2%, the NRMP said in its 2019 Main Residency Match report.
The 2019 Match set a record for most positions offered (35,185; up 6.1%), most positions filled (33,426; up 4.8%), most PGY-1 positions offered (32,194; up 6.5%), and total applicants (38,376; up 3.4%).
“The results of the Match are closely watched because they can be predictors of future physician workforce supply. There also is significant interest in the competitiveness of specialties, as measured by the percentage of positions filled overall and the percentage filled by senior students in U.S. allopathic medical schools,” the NRMP said.
Match Day 2019 was another record breaker, and psychiatry helped play a big role by offering nearly 200 more residency slots and matches than in 2018, according to the National Resident Matching Program (NRMP).

There were a total of 1,740 PGY-1 psychiatry slots offered in 2019, up from 1,556 in 2018, for an increase of 11.8%. The 184-position increase was the fourth-largest overall increase among all measured specialties, behind only internal medicine, family medicine, and emergency medicine.
in a press release. However, while the fill rate increased slightly to 99%, leaving only 20 slots unfilled, the fill rate by U.S. graduates dropped from 63.1% to 60.6%.
Overall, a total of 32,194 PGY-1 slots were offered, a new record, and 94.9% were filled, with U.S. graduates filling 55.2%, the NRMP said in its 2019 Main Residency Match report.
The 2019 Match set a record for most positions offered (35,185; up 6.1%), most positions filled (33,426; up 4.8%), most PGY-1 positions offered (32,194; up 6.5%), and total applicants (38,376; up 3.4%).
“The results of the Match are closely watched because they can be predictors of future physician workforce supply. There also is significant interest in the competitiveness of specialties, as measured by the percentage of positions filled overall and the percentage filled by senior students in U.S. allopathic medical schools,” the NRMP said.
Match Day 2019 was another record breaker, and psychiatry helped play a big role by offering nearly 200 more residency slots and matches than in 2018, according to the National Resident Matching Program (NRMP).

There were a total of 1,740 PGY-1 psychiatry slots offered in 2019, up from 1,556 in 2018, for an increase of 11.8%. The 184-position increase was the fourth-largest overall increase among all measured specialties, behind only internal medicine, family medicine, and emergency medicine.
in a press release. However, while the fill rate increased slightly to 99%, leaving only 20 slots unfilled, the fill rate by U.S. graduates dropped from 63.1% to 60.6%.
Overall, a total of 32,194 PGY-1 slots were offered, a new record, and 94.9% were filled, with U.S. graduates filling 55.2%, the NRMP said in its 2019 Main Residency Match report.
The 2019 Match set a record for most positions offered (35,185; up 6.1%), most positions filled (33,426; up 4.8%), most PGY-1 positions offered (32,194; up 6.5%), and total applicants (38,376; up 3.4%).
“The results of the Match are closely watched because they can be predictors of future physician workforce supply. There also is significant interest in the competitiveness of specialties, as measured by the percentage of positions filled overall and the percentage filled by senior students in U.S. allopathic medical schools,” the NRMP said.
Friendly gut bugs, MCI-battling mushrooms, and remembering to forget
Friend or foe, how do we know?
That’s the question immune cells ask all the time, especially about gut bacteria. A study published March 7 seeks to explain how immune systems can distinguish between happy-go-lucky gut microbes and deadly pathogens. Turns out, the friendly microbes simply high five!
Well, not really. But they do have a hook-like arm, called a holdfast, which latches onto the gut lining. The holdfast is lined with vesicles that carry antigens into the gut. While antigens normally cause immune cells to attack, something about these antigens are telling T cells to hold their fire.
The authors of the study hypothesized that the packaging of the antigens – the vesicles – might be the reason for the friendliness between microbes and T cells. It’s like the immune system expects a cannonball, but is pleasantly surprised by an Amazon Prime package full of goodies showing up on their doorstep instead. Yay for presents!
Don’t skimp on the ’shrooms
While you’re piling onions onto your plate to reduce cancer and cheese for your heart, make sure you add mushrooms for extra brain power. Researchers conducting a 6-year study in Singapore observed cognitive decline in 600 Chinese people aged at least 60 years, and they found that those who eat more than two portions of cooked mushrooms per week have up to 50% reduced odds of mild cognitive impairment.
Researchers here at the LOTME Lab have harnessed the power of these food studies to determine that a Philly cheesesteak with mushrooms and onions is the healthiest meal out there. Chow down!
As far as we know, all the mushrooms were standard edible fungi, and none were magic mushrooms (although, that might help, too; try that on your own time). Researchers believe that the compound ergothioneine, an antioxidant and anti-inflammatory that cannot be synthesized by humans, might be reason for the reduced risk of mild cognitive impairment Maybe it’s time to add a cup of cooked shiitake mushrooms to your morning routine.
Fuggedaboutit!
We all have unwanted memories that we’d rather forget about. An embarrassing incident, a painful experience – everyone has moments they’d rather not think about. So, the question is: How do you get rid of these bad memories?
The obvious solution is to stop thinking about it. But if you’re a regular reader of Livin’ on the MDedge, you can probably guess that the answer isn’t that simple.
And, in fact, it isn’t! A group of researchers at the University of Texas at Austin, has performed a study on intentional forgetting, and they found that the best way to forget something is ... to think about it. Study subjects were shown a series of images and told to either remember or forget those images while their ventral temporal cortex was monitored for activity. Not only were participants successfully able to forget images by thinking about it, but activity in the brain was higher when forgetting than while remembering.
Obviously, this research would be helpful for anyone dealing with trauma, and we hope doctors who have to treat such patients keep it in mind. Just don’t think about it too much, or you’ll forget about it.
The Golden Lobbyist
If you need health care in your neighborhood
Who you gonna call? Jack Nicklaus!
You need 20 mill to make it good
Who you gonna call? Jack Nicklaus!
Health care in general didn’t do very well in President Trump’s 2020 budget proposal; Medicare, Medicaid, and the National Cancer Institute were all targeted for cuts. But it did include one particular $20-million initiative for a mobile children’s hospital.
Politico reports that the nation’s golfer in chief “personally directed the Department of Health and Human Services to earmark the funds” after playing a couple of rounds with the Golden Bear himself, Jack Nicklaus. The mobile unit would be part of the Nicklaus Children’s Hospital in Miami. The golf legend turned lobbyist also had meetings off the course with HHS Secretary Alex Azar and then-OMB Director Mick Mulvaney.
Are health care ideas running through your head?
Who you gonna call? Jack Nicklaus!
He’ll golf with the prez, and get your bread
Who you gonna call? Jack Nicklaus!
He ain’t afraid of no tweets

Friend or foe, how do we know?
That’s the question immune cells ask all the time, especially about gut bacteria. A study published March 7 seeks to explain how immune systems can distinguish between happy-go-lucky gut microbes and deadly pathogens. Turns out, the friendly microbes simply high five!
Well, not really. But they do have a hook-like arm, called a holdfast, which latches onto the gut lining. The holdfast is lined with vesicles that carry antigens into the gut. While antigens normally cause immune cells to attack, something about these antigens are telling T cells to hold their fire.
The authors of the study hypothesized that the packaging of the antigens – the vesicles – might be the reason for the friendliness between microbes and T cells. It’s like the immune system expects a cannonball, but is pleasantly surprised by an Amazon Prime package full of goodies showing up on their doorstep instead. Yay for presents!
Don’t skimp on the ’shrooms
While you’re piling onions onto your plate to reduce cancer and cheese for your heart, make sure you add mushrooms for extra brain power. Researchers conducting a 6-year study in Singapore observed cognitive decline in 600 Chinese people aged at least 60 years, and they found that those who eat more than two portions of cooked mushrooms per week have up to 50% reduced odds of mild cognitive impairment.
Researchers here at the LOTME Lab have harnessed the power of these food studies to determine that a Philly cheesesteak with mushrooms and onions is the healthiest meal out there. Chow down!
As far as we know, all the mushrooms were standard edible fungi, and none were magic mushrooms (although, that might help, too; try that on your own time). Researchers believe that the compound ergothioneine, an antioxidant and anti-inflammatory that cannot be synthesized by humans, might be reason for the reduced risk of mild cognitive impairment Maybe it’s time to add a cup of cooked shiitake mushrooms to your morning routine.
Fuggedaboutit!
We all have unwanted memories that we’d rather forget about. An embarrassing incident, a painful experience – everyone has moments they’d rather not think about. So, the question is: How do you get rid of these bad memories?
The obvious solution is to stop thinking about it. But if you’re a regular reader of Livin’ on the MDedge, you can probably guess that the answer isn’t that simple.
And, in fact, it isn’t! A group of researchers at the University of Texas at Austin, has performed a study on intentional forgetting, and they found that the best way to forget something is ... to think about it. Study subjects were shown a series of images and told to either remember or forget those images while their ventral temporal cortex was monitored for activity. Not only were participants successfully able to forget images by thinking about it, but activity in the brain was higher when forgetting than while remembering.
Obviously, this research would be helpful for anyone dealing with trauma, and we hope doctors who have to treat such patients keep it in mind. Just don’t think about it too much, or you’ll forget about it.
The Golden Lobbyist
If you need health care in your neighborhood
Who you gonna call? Jack Nicklaus!
You need 20 mill to make it good
Who you gonna call? Jack Nicklaus!
Health care in general didn’t do very well in President Trump’s 2020 budget proposal; Medicare, Medicaid, and the National Cancer Institute were all targeted for cuts. But it did include one particular $20-million initiative for a mobile children’s hospital.
Politico reports that the nation’s golfer in chief “personally directed the Department of Health and Human Services to earmark the funds” after playing a couple of rounds with the Golden Bear himself, Jack Nicklaus. The mobile unit would be part of the Nicklaus Children’s Hospital in Miami. The golf legend turned lobbyist also had meetings off the course with HHS Secretary Alex Azar and then-OMB Director Mick Mulvaney.
Are health care ideas running through your head?
Who you gonna call? Jack Nicklaus!
He’ll golf with the prez, and get your bread
Who you gonna call? Jack Nicklaus!
He ain’t afraid of no tweets

Friend or foe, how do we know?
That’s the question immune cells ask all the time, especially about gut bacteria. A study published March 7 seeks to explain how immune systems can distinguish between happy-go-lucky gut microbes and deadly pathogens. Turns out, the friendly microbes simply high five!
Well, not really. But they do have a hook-like arm, called a holdfast, which latches onto the gut lining. The holdfast is lined with vesicles that carry antigens into the gut. While antigens normally cause immune cells to attack, something about these antigens are telling T cells to hold their fire.
The authors of the study hypothesized that the packaging of the antigens – the vesicles – might be the reason for the friendliness between microbes and T cells. It’s like the immune system expects a cannonball, but is pleasantly surprised by an Amazon Prime package full of goodies showing up on their doorstep instead. Yay for presents!
Don’t skimp on the ’shrooms
While you’re piling onions onto your plate to reduce cancer and cheese for your heart, make sure you add mushrooms for extra brain power. Researchers conducting a 6-year study in Singapore observed cognitive decline in 600 Chinese people aged at least 60 years, and they found that those who eat more than two portions of cooked mushrooms per week have up to 50% reduced odds of mild cognitive impairment.
Researchers here at the LOTME Lab have harnessed the power of these food studies to determine that a Philly cheesesteak with mushrooms and onions is the healthiest meal out there. Chow down!
As far as we know, all the mushrooms were standard edible fungi, and none were magic mushrooms (although, that might help, too; try that on your own time). Researchers believe that the compound ergothioneine, an antioxidant and anti-inflammatory that cannot be synthesized by humans, might be reason for the reduced risk of mild cognitive impairment Maybe it’s time to add a cup of cooked shiitake mushrooms to your morning routine.
Fuggedaboutit!
We all have unwanted memories that we’d rather forget about. An embarrassing incident, a painful experience – everyone has moments they’d rather not think about. So, the question is: How do you get rid of these bad memories?
The obvious solution is to stop thinking about it. But if you’re a regular reader of Livin’ on the MDedge, you can probably guess that the answer isn’t that simple.
And, in fact, it isn’t! A group of researchers at the University of Texas at Austin, has performed a study on intentional forgetting, and they found that the best way to forget something is ... to think about it. Study subjects were shown a series of images and told to either remember or forget those images while their ventral temporal cortex was monitored for activity. Not only were participants successfully able to forget images by thinking about it, but activity in the brain was higher when forgetting than while remembering.
Obviously, this research would be helpful for anyone dealing with trauma, and we hope doctors who have to treat such patients keep it in mind. Just don’t think about it too much, or you’ll forget about it.
The Golden Lobbyist
If you need health care in your neighborhood
Who you gonna call? Jack Nicklaus!
You need 20 mill to make it good
Who you gonna call? Jack Nicklaus!
Health care in general didn’t do very well in President Trump’s 2020 budget proposal; Medicare, Medicaid, and the National Cancer Institute were all targeted for cuts. But it did include one particular $20-million initiative for a mobile children’s hospital.
Politico reports that the nation’s golfer in chief “personally directed the Department of Health and Human Services to earmark the funds” after playing a couple of rounds with the Golden Bear himself, Jack Nicklaus. The mobile unit would be part of the Nicklaus Children’s Hospital in Miami. The golf legend turned lobbyist also had meetings off the course with HHS Secretary Alex Azar and then-OMB Director Mick Mulvaney.
Are health care ideas running through your head?
Who you gonna call? Jack Nicklaus!
He’ll golf with the prez, and get your bread
Who you gonna call? Jack Nicklaus!
He ain’t afraid of no tweets

ACC to offer new certification for transcatheter valve repair, replacement
The American College of Cardiology announced at its ACC Quality Summit in New Orleans that it will offer a Transcatheter Valve Certification to assist hospitals that perform transcatheter valve repair and replacement.
The certification is an external review process that will allow hospitals to meet standards for multidisciplinary teams, formalized training, shared decision making, and registry performance. During the certification process, hospitals will learn best practices for implementing evidence-based medicine in the care of individual patients and identify quality improvement opportunities.
The Transcatheter Valve Certification will be launched in mid-2019. To earn the certification, hospitals must already participate in an established national clinical database.
“. This certification incorporates recent guidelines and expert consensus statements regarding the care of patients requiring transcatheter valve therapies,” Phillip D. Levy, MD, chair of the ACC accreditation management board, said in a press release.
Find the full press release on the ACC website.
The American College of Cardiology announced at its ACC Quality Summit in New Orleans that it will offer a Transcatheter Valve Certification to assist hospitals that perform transcatheter valve repair and replacement.
The certification is an external review process that will allow hospitals to meet standards for multidisciplinary teams, formalized training, shared decision making, and registry performance. During the certification process, hospitals will learn best practices for implementing evidence-based medicine in the care of individual patients and identify quality improvement opportunities.
The Transcatheter Valve Certification will be launched in mid-2019. To earn the certification, hospitals must already participate in an established national clinical database.
“. This certification incorporates recent guidelines and expert consensus statements regarding the care of patients requiring transcatheter valve therapies,” Phillip D. Levy, MD, chair of the ACC accreditation management board, said in a press release.
Find the full press release on the ACC website.
The American College of Cardiology announced at its ACC Quality Summit in New Orleans that it will offer a Transcatheter Valve Certification to assist hospitals that perform transcatheter valve repair and replacement.
The certification is an external review process that will allow hospitals to meet standards for multidisciplinary teams, formalized training, shared decision making, and registry performance. During the certification process, hospitals will learn best practices for implementing evidence-based medicine in the care of individual patients and identify quality improvement opportunities.
The Transcatheter Valve Certification will be launched in mid-2019. To earn the certification, hospitals must already participate in an established national clinical database.
“. This certification incorporates recent guidelines and expert consensus statements regarding the care of patients requiring transcatheter valve therapies,” Phillip D. Levy, MD, chair of the ACC accreditation management board, said in a press release.
Find the full press release on the ACC website.
FDA approves new valsartan generic
In response to a medication shortage, the Food and Drug Administration has approved a new generic of valsartan (Diovan), produced by Alkem Laboratories, for the treatment of high blood pressure and heart failure, the regulatory agency announced in a statement.
The FDA conducted an investigation into generic angiotensin II receptor blocker (ARB) products following reports of N-nitrosodimethylamine impurities being found in a separate valsartan product in the summer of 2018. Since that time, nitrosamine impurities have been detected in multiple ARBs, and as of March 1, 2019, hundreds of lots of ARBs produced by several companies have been recalled. The FDA has implemented new rules to prevent further contamination, but the ongoing recalls have caused a significant shortage.
“[To] address the public health consequences of these shortages, we’ve prioritized the review of generic applications for these valsartan products,” FDA commissioner Scott Gottlieb, MD, said in the statement.
For the new generic’s approval, the FDA assessed Alkem Laboratories’ manufacturing process and ensured that the company used proper testing methods to rule out the presence of nitrosamine impurities.
“We hope that today’s approval of this new generic will help reduce the valsartan shortage, and we remain committed to implementing measures to prevent the formation of these impurities during drug manufacturing processes for existing and future products,” Dr. Gottlieb said.
In response to a medication shortage, the Food and Drug Administration has approved a new generic of valsartan (Diovan), produced by Alkem Laboratories, for the treatment of high blood pressure and heart failure, the regulatory agency announced in a statement.
The FDA conducted an investigation into generic angiotensin II receptor blocker (ARB) products following reports of N-nitrosodimethylamine impurities being found in a separate valsartan product in the summer of 2018. Since that time, nitrosamine impurities have been detected in multiple ARBs, and as of March 1, 2019, hundreds of lots of ARBs produced by several companies have been recalled. The FDA has implemented new rules to prevent further contamination, but the ongoing recalls have caused a significant shortage.
“[To] address the public health consequences of these shortages, we’ve prioritized the review of generic applications for these valsartan products,” FDA commissioner Scott Gottlieb, MD, said in the statement.
For the new generic’s approval, the FDA assessed Alkem Laboratories’ manufacturing process and ensured that the company used proper testing methods to rule out the presence of nitrosamine impurities.
“We hope that today’s approval of this new generic will help reduce the valsartan shortage, and we remain committed to implementing measures to prevent the formation of these impurities during drug manufacturing processes for existing and future products,” Dr. Gottlieb said.
In response to a medication shortage, the Food and Drug Administration has approved a new generic of valsartan (Diovan), produced by Alkem Laboratories, for the treatment of high blood pressure and heart failure, the regulatory agency announced in a statement.
The FDA conducted an investigation into generic angiotensin II receptor blocker (ARB) products following reports of N-nitrosodimethylamine impurities being found in a separate valsartan product in the summer of 2018. Since that time, nitrosamine impurities have been detected in multiple ARBs, and as of March 1, 2019, hundreds of lots of ARBs produced by several companies have been recalled. The FDA has implemented new rules to prevent further contamination, but the ongoing recalls have caused a significant shortage.
“[To] address the public health consequences of these shortages, we’ve prioritized the review of generic applications for these valsartan products,” FDA commissioner Scott Gottlieb, MD, said in the statement.
For the new generic’s approval, the FDA assessed Alkem Laboratories’ manufacturing process and ensured that the company used proper testing methods to rule out the presence of nitrosamine impurities.
“We hope that today’s approval of this new generic will help reduce the valsartan shortage, and we remain committed to implementing measures to prevent the formation of these impurities during drug manufacturing processes for existing and future products,” Dr. Gottlieb said.
FDA approves another trastuzumab biosimilar for HER2-positive breast cancer, gastric cancer
The Food and Drug Administration has approved Trazimera (trastuzumab-qyyp), a biosimilar of Herceptin (trastuzumab), for the treatment of HER2-positive breast cancer and HER2-positive metastatic gastric or gastroesophageal junction adenocarcinoma.
FDA approval was based on a review of a comprehensive data package, which included results from the REFLECTIONS B327-02 trial. In this trial, Trazimera was found to have clinical equivalence with trastuzumab in the first-line treatment setting in patients with HER2-positive metastatic breast cancer.
The most common adverse events associated with Trazimera in patients with breast cancer include fever, nausea, vomiting, infusion reactions, diarrhea, infections, increased cough, headache, fatigue, shortness of breath, rash, low white and red blood cell counts, and muscle pain. For patients with metastatic adenocarcinoma, the most common adverse events include low white and red blood cell counts; diarrhea; fatigue; swelling of the mouth lining, mucous membranes, nose, or throat; weight loss; upper respiratory tract infections; fever; low platelet counts; and change in taste.
“Approximately 15-30% of breast cancers and 10-30% of gastric cancers are HER2-positive, which is associated with aggressive disease and poor prognoses for patients. With the availability of biosimilars like Trazimera in the U.S., oncologists will have additional treatment options to choose from, which may help provide patients with greater access to the medicines they need,” Mark Pegram, MD, director of the breast oncology program at the Stanford Women’s Cancer Center at Stanford (Calif.) University, said in the press release.
Find the full press release on the Pfizer website.
The Food and Drug Administration has approved Trazimera (trastuzumab-qyyp), a biosimilar of Herceptin (trastuzumab), for the treatment of HER2-positive breast cancer and HER2-positive metastatic gastric or gastroesophageal junction adenocarcinoma.
FDA approval was based on a review of a comprehensive data package, which included results from the REFLECTIONS B327-02 trial. In this trial, Trazimera was found to have clinical equivalence with trastuzumab in the first-line treatment setting in patients with HER2-positive metastatic breast cancer.
The most common adverse events associated with Trazimera in patients with breast cancer include fever, nausea, vomiting, infusion reactions, diarrhea, infections, increased cough, headache, fatigue, shortness of breath, rash, low white and red blood cell counts, and muscle pain. For patients with metastatic adenocarcinoma, the most common adverse events include low white and red blood cell counts; diarrhea; fatigue; swelling of the mouth lining, mucous membranes, nose, or throat; weight loss; upper respiratory tract infections; fever; low platelet counts; and change in taste.
“Approximately 15-30% of breast cancers and 10-30% of gastric cancers are HER2-positive, which is associated with aggressive disease and poor prognoses for patients. With the availability of biosimilars like Trazimera in the U.S., oncologists will have additional treatment options to choose from, which may help provide patients with greater access to the medicines they need,” Mark Pegram, MD, director of the breast oncology program at the Stanford Women’s Cancer Center at Stanford (Calif.) University, said in the press release.
Find the full press release on the Pfizer website.
The Food and Drug Administration has approved Trazimera (trastuzumab-qyyp), a biosimilar of Herceptin (trastuzumab), for the treatment of HER2-positive breast cancer and HER2-positive metastatic gastric or gastroesophageal junction adenocarcinoma.
FDA approval was based on a review of a comprehensive data package, which included results from the REFLECTIONS B327-02 trial. In this trial, Trazimera was found to have clinical equivalence with trastuzumab in the first-line treatment setting in patients with HER2-positive metastatic breast cancer.
The most common adverse events associated with Trazimera in patients with breast cancer include fever, nausea, vomiting, infusion reactions, diarrhea, infections, increased cough, headache, fatigue, shortness of breath, rash, low white and red blood cell counts, and muscle pain. For patients with metastatic adenocarcinoma, the most common adverse events include low white and red blood cell counts; diarrhea; fatigue; swelling of the mouth lining, mucous membranes, nose, or throat; weight loss; upper respiratory tract infections; fever; low platelet counts; and change in taste.
“Approximately 15-30% of breast cancers and 10-30% of gastric cancers are HER2-positive, which is associated with aggressive disease and poor prognoses for patients. With the availability of biosimilars like Trazimera in the U.S., oncologists will have additional treatment options to choose from, which may help provide patients with greater access to the medicines they need,” Mark Pegram, MD, director of the breast oncology program at the Stanford Women’s Cancer Center at Stanford (Calif.) University, said in the press release.
Find the full press release on the Pfizer website.
Dupilumab to undergo FDA Priority Review for CRSwNP treatment
The Food and Drug Administration will conduct a Priority Review on the supplemental Biologics License Application (sBLA) for dupilumab (Dupixent) as an add-on treatment for adults with inadequately controlled severe chronic rhinosinusitis with nasal polyps (CRSwNP).
CRSwNP is a chronic disease of the upper airway in which patients can experience severe nasal obstruction with breathing difficulties, nasal discharge, reduction or loss of sense of smell and taste, and facial pain or pressure. There are currently no FDA-approved treatments for the disease, Regeneron said in the press release.
The sBLA is based on results from a pair of phase 3 trials in which patients with CRSwNP received either dupilumab plus a standard-of-care corticosteroid nasal spray or the standard-of-care spray alone. In results presented at the 2019 annual meeting of the American Academy of Allergy, Asthma, and Immunology, dupilumab plus the spray improved nasal polyp size, nasal congestion severity, chronic sinus disease, sense of smell, and comorbid asthma outcomes while reducing the need for corticosteroid use and nasal/sinus surgery.
Dupilumab is currently approved in the United States to treat moderate to severe atopic dermatitis in adults whose disease is poorly controlled with topical agents and as a maintenance treatment in combination with other asthma medications in patients aged 12 years and older whose disease is not controlled with their current prescription. The most common adverse events include injection-site reactions, oropharyngeal pain, and cold sores.
The target action date for the FDA decision is June 26, 2019, Regeneron said.
Find the full press release on the Regeneron website.
The Food and Drug Administration will conduct a Priority Review on the supplemental Biologics License Application (sBLA) for dupilumab (Dupixent) as an add-on treatment for adults with inadequately controlled severe chronic rhinosinusitis with nasal polyps (CRSwNP).
CRSwNP is a chronic disease of the upper airway in which patients can experience severe nasal obstruction with breathing difficulties, nasal discharge, reduction or loss of sense of smell and taste, and facial pain or pressure. There are currently no FDA-approved treatments for the disease, Regeneron said in the press release.
The sBLA is based on results from a pair of phase 3 trials in which patients with CRSwNP received either dupilumab plus a standard-of-care corticosteroid nasal spray or the standard-of-care spray alone. In results presented at the 2019 annual meeting of the American Academy of Allergy, Asthma, and Immunology, dupilumab plus the spray improved nasal polyp size, nasal congestion severity, chronic sinus disease, sense of smell, and comorbid asthma outcomes while reducing the need for corticosteroid use and nasal/sinus surgery.
Dupilumab is currently approved in the United States to treat moderate to severe atopic dermatitis in adults whose disease is poorly controlled with topical agents and as a maintenance treatment in combination with other asthma medications in patients aged 12 years and older whose disease is not controlled with their current prescription. The most common adverse events include injection-site reactions, oropharyngeal pain, and cold sores.
The target action date for the FDA decision is June 26, 2019, Regeneron said.
Find the full press release on the Regeneron website.
The Food and Drug Administration will conduct a Priority Review on the supplemental Biologics License Application (sBLA) for dupilumab (Dupixent) as an add-on treatment for adults with inadequately controlled severe chronic rhinosinusitis with nasal polyps (CRSwNP).
CRSwNP is a chronic disease of the upper airway in which patients can experience severe nasal obstruction with breathing difficulties, nasal discharge, reduction or loss of sense of smell and taste, and facial pain or pressure. There are currently no FDA-approved treatments for the disease, Regeneron said in the press release.
The sBLA is based on results from a pair of phase 3 trials in which patients with CRSwNP received either dupilumab plus a standard-of-care corticosteroid nasal spray or the standard-of-care spray alone. In results presented at the 2019 annual meeting of the American Academy of Allergy, Asthma, and Immunology, dupilumab plus the spray improved nasal polyp size, nasal congestion severity, chronic sinus disease, sense of smell, and comorbid asthma outcomes while reducing the need for corticosteroid use and nasal/sinus surgery.
Dupilumab is currently approved in the United States to treat moderate to severe atopic dermatitis in adults whose disease is poorly controlled with topical agents and as a maintenance treatment in combination with other asthma medications in patients aged 12 years and older whose disease is not controlled with their current prescription. The most common adverse events include injection-site reactions, oropharyngeal pain, and cold sores.
The target action date for the FDA decision is June 26, 2019, Regeneron said.
Find the full press release on the Regeneron website.
FDA approves patient-controlled injector for guselkumab
The Food and Drug Administration has in adults, the manufacturer announced.
FDA approval is based on results from the phase 3, multicenter, randomized ORION trial, according to a press release issued by Janssen. In a Self-Injection Assessment Questionnaire, patients who received the One-Press injection rated their satisfaction with self-injection a mean score of 9.18 (0 indicated least satisfaction, 10 indicated highest satisfaction) and rated ease of use at 9.24 (10 indicated “very easy”).
In addition, 81% of patients who received One-Press achieved a Investigator’s Global Assessment score of 0 or 1, and 76% achieved a Psoriasis Area Severity Index (PASI) 90 response after 16 weeks; no patients who received the placebo achieved either.
The most common adverse event during the ORION study was injection-site reaction; the most common adverse events associated with guselkumab, an interleukin-23 blocker, include upper respiratory infections, headache, injection-site reactions, joint pain, diarrhea, gastroenteritis, fungal skin infections, and herpes simplex infections.
The Food and Drug Administration has in adults, the manufacturer announced.
FDA approval is based on results from the phase 3, multicenter, randomized ORION trial, according to a press release issued by Janssen. In a Self-Injection Assessment Questionnaire, patients who received the One-Press injection rated their satisfaction with self-injection a mean score of 9.18 (0 indicated least satisfaction, 10 indicated highest satisfaction) and rated ease of use at 9.24 (10 indicated “very easy”).
In addition, 81% of patients who received One-Press achieved a Investigator’s Global Assessment score of 0 or 1, and 76% achieved a Psoriasis Area Severity Index (PASI) 90 response after 16 weeks; no patients who received the placebo achieved either.
The most common adverse event during the ORION study was injection-site reaction; the most common adverse events associated with guselkumab, an interleukin-23 blocker, include upper respiratory infections, headache, injection-site reactions, joint pain, diarrhea, gastroenteritis, fungal skin infections, and herpes simplex infections.
The Food and Drug Administration has in adults, the manufacturer announced.
FDA approval is based on results from the phase 3, multicenter, randomized ORION trial, according to a press release issued by Janssen. In a Self-Injection Assessment Questionnaire, patients who received the One-Press injection rated their satisfaction with self-injection a mean score of 9.18 (0 indicated least satisfaction, 10 indicated highest satisfaction) and rated ease of use at 9.24 (10 indicated “very easy”).
In addition, 81% of patients who received One-Press achieved a Investigator’s Global Assessment score of 0 or 1, and 76% achieved a Psoriasis Area Severity Index (PASI) 90 response after 16 weeks; no patients who received the placebo achieved either.
The most common adverse event during the ORION study was injection-site reaction; the most common adverse events associated with guselkumab, an interleukin-23 blocker, include upper respiratory infections, headache, injection-site reactions, joint pain, diarrhea, gastroenteritis, fungal skin infections, and herpes simplex infections.