User login
Are Oritavancin and Dalbavancin More Cost Effective for Outpatient Parenteral Antimicrobial Therapy at a Veterans Affairs Medical Center?
Are Oritavancin and Dalbavancin More Cost Effective for Outpatient Parenteral Antimicrobial Therapy at a Veterans Affairs Medical Center?
Oritavancin and dalbavancin are long acting lipoglycopeptides indicated for the treatment of acute bacterial skin and skin structure infections (ABSSSI).1,2 Largely due to their long half-lives, prolonged tissue concentrations at sites of infection, tolerability, and minimal requirement for therapeutic drug monitoring, these agents are attractive options in outpatient settings.3,4 A 1- or 2-dose treatment of oritavancin and dalbavancin may be sufficient for conditions traditionally treated with outpatient parenteral antimicrobial therapy (OPAT) via peripherally inserted central catheter (PICC).
Limited research supports the use of dalbavancin and oritavancin for bone and joint infections, infective endocarditis, and bloodstream infections (BSIs). However, the US Food and Drug Administration has approved an indication for the treatment of ABSSSI.3-9 Dosing for these off-label indications varies but typically consists of an initial intravenous (IV) dose (1000 mg, 1200 mg, or 1500 mg), with a subsequent dose 1 to 2 weeks later or administered once weekly.6-10
Due in part to the recent availability of oritavancin and dalbavancin relative to the publication of practice guidelines, their appropriate place in therapy continues to evolve based on emerging literature.11,12 One potential barrier of use for these medications is their cost. Based on the number of doses administered, the 2022 estimated total acquisition cost of therapy for oritavancin and dalbavancin was $1014 to $4397 and $3046 to $7150, respectively (eAppendix). Despite the high acquisition costs, these agents do not require the placement of an indwelling central line, can be administered in outpatient settings, and require minimal therapeutic dose monitoring compared to vancomycin.13-15 This medication use evaluation (MUE) compared the total cost of treatment with oritavancin and dalbavancin vs therapies traditionally used for OPAT or prolonged IV inpatient therapy.
METHODS
This retrospective MUE was conducted at the Boise Veterans Affairs Medical Center (BVAMC), a level 2 facility with an extensive rural catchment area. BVAMC provides many OPAT services, including medications, supplies, and dressing changes after initial clinic or inpatient education. Contracted vendors may also assist with at-home nursing care using supplies provided by the BVAMC. Cases were identified using an internal database of OPAT patients and those who received oritavancin or dalbavancin between September 1, 2017, and November 1, 2022. Patients aged ≥ 18 years who received ≥ 1 dose of oritavancin or dalbavancin for ABSSSI, osteomyelitis/joint infections, endocarditis, and BSI were included. Comparator treatments consisting of ≥ 1 week of vancomycin or daptomycin for ABSSSI, osteomyelitis/joint infections, endocarditis, and BSI were identified through review of OPAT and Infectious Diseases service consults during the same timeframe. Patients were excluded if any antibiotic was prescribed by a non- VA clinician, if medications were not provided by OPAT, or if chart review did not identify an ABSSSI, osteomyelitis/ joint infection, or BSI diagnosis.
Electronic medical record review was conducted using a standardized data collection form (eAppendix). Data collected included demographics, infectious diagnosis, treatment administered, administration procedures and related visits and treatment locations, outcomes including clinical failure, adverse events (AEs), and hospital readmission.
Clinical failure was defined as readmission or death due to worsening infection or readmission secondary to a documented potential AE to the evaluated antibiotics within 90 days after initiation. Clinical failures excluded readmissions not associated with infection including comorbidities or elective procedures. AEs included new onset renal failure (serum creatinine ≥ 0.5 mg/dL), neutropenia (neutrophils ≤ 500), thrombocytopenia (platelets < 100,000), eosinophilia (> 15% eosinophils), or creatine phosphokinase > 10 times the upper limit of normal, and Clostridioides difficile (C. difficile) infection. Line complications included thrombophlebitis, local inflammation, or infection requiring line replacement (eAppendix).
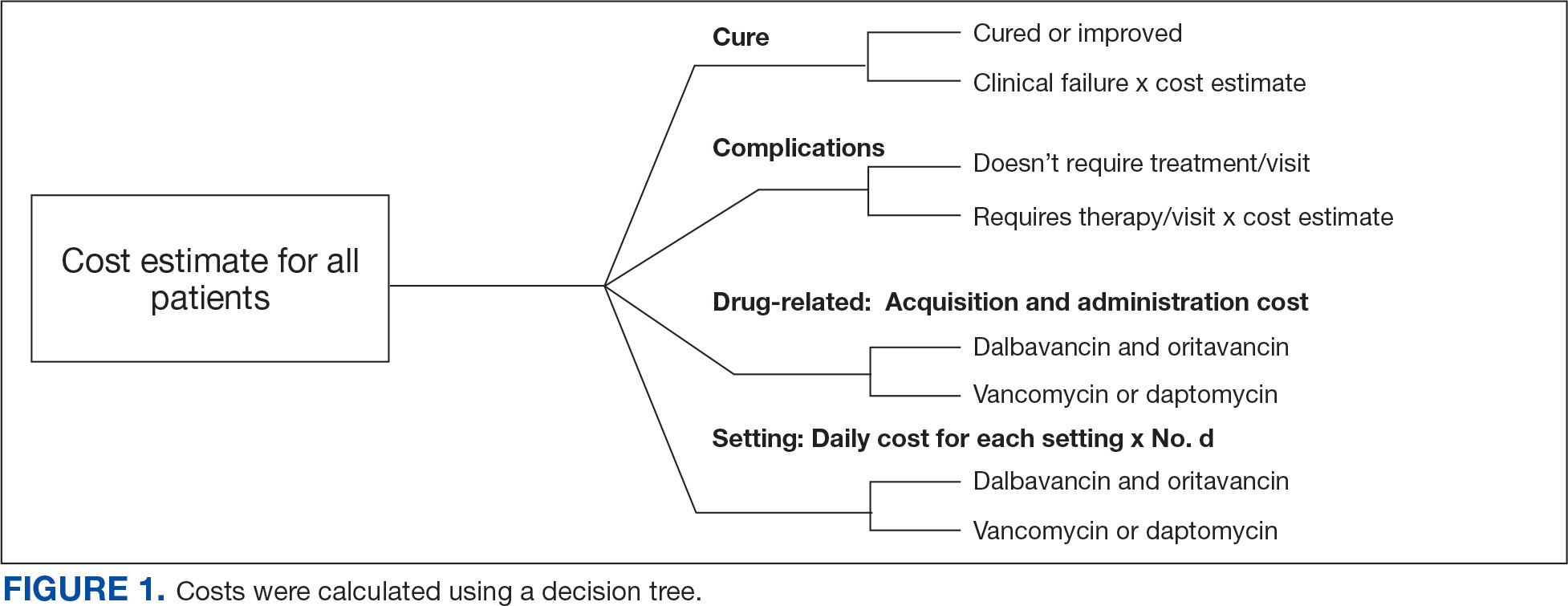
A cost-minimization approach was used to assess the total cost of treatment.16 Patients who received oritavancin or dalbavancin were matched with patients that received vancomycin and daptomycin for the same indication and about 1 month of initiation through the randomization function in Microsoft Excel. This accounted for changes in personnel, nonformulary drug approvals, cost, and changes in practice during the pandemic. Costs were calculated using a decision tree as a base model (Figure 1). In this model, each treatment dyad was assessed for the presence or absence of clinical failure, adverse event (medication and line complications), and treatment setting endpoints. Cost estimates were tabulated for each patient that received treatment using published VA data, literature, pharmacoeconomist guidance, or best faith effort based on workflow. 17-20 All cost estimates were based on 2022 figures or adjusted for inflation if obtained prior to 2022. Secondary endpoints of this analysis included estimated total cost of medication acquisition, administration supplies, laboratory monitoring, and human resources for OPAT visits or receiving home-health services.
This evaluation was classified by the BVAMC Medication Use Evaluation research determination subcommittee as a quality improvement project and was considered exempt from VA Human Subjects Research requirements based on the VA Policy Handbook guideline 1058.05.
RESULTS
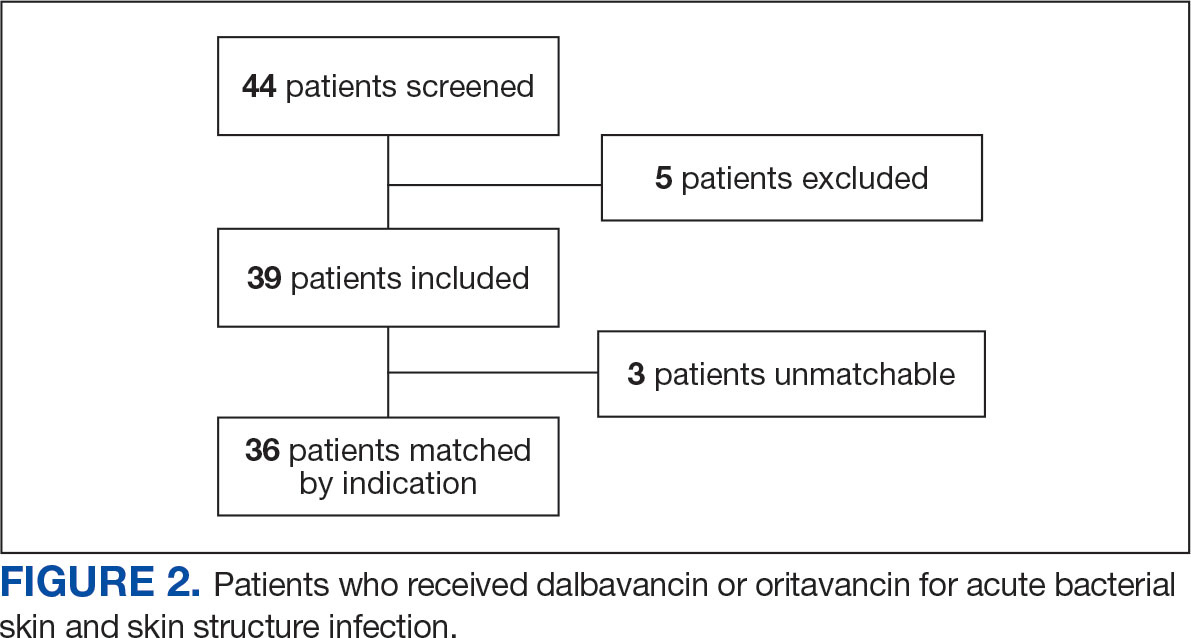
The study identified 44 patients who received dalbavancin or oritavancin between September 1, 2017, and October 31, 2022. Thirty-nine patients were included in the analysis: 24 received oritavancin and 15 received dalbavancin and were matched by indication to 10 patients who received vancomycin and 8 patients who received daptomycin. Three patients could not be matched by indication of ABSSSI (Figure 2). Most patients were male, aged > 65 years, and were treated for osteomyelitis (Table 1). No patients were treated for infective endocarditis. A myriad of concomitant antibiotics were used to treat patients and culture results indicated that most infections treated with oritavancin and dalbavancin were polymicrobial.
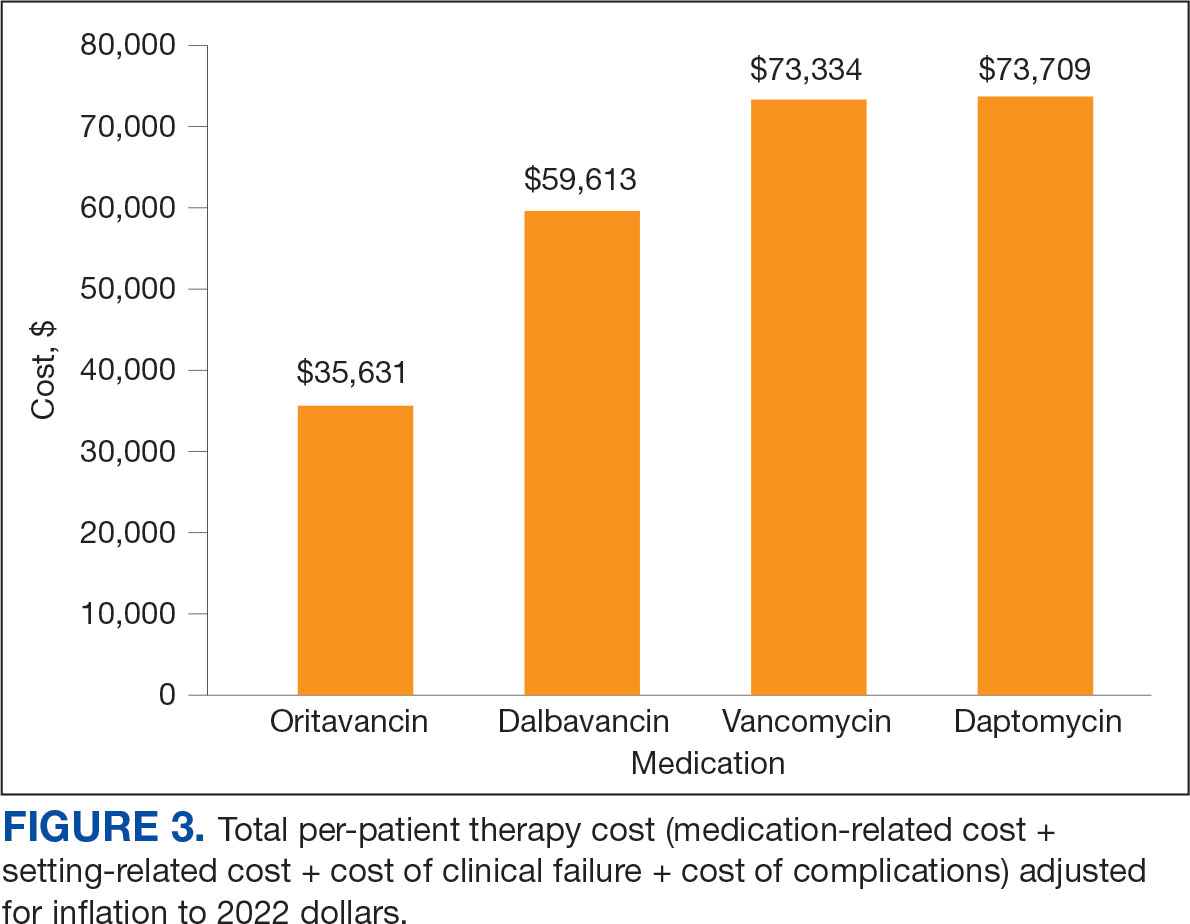
The mean total cost of therapy per patient receiving oritavancin, dalbavancin, vancomycin, and daptomycin was $35,630, $59,612, $73,333, and $73,708, respectively (Figure 3). When stratified by indication, 27 patients (69%) in the oritavancin/dalbavancin group were treated for osteomyelitis/ joint infections (16 oritavancin, 11 dalbavancin), 9 patients (23%) were treated for BSI (6 oritavancin, 3 dalbavancin), and 3 patients (8%) were treated for ABSSSI (2 oritavancin, 1 dalbavancin). The mean cost per patient for osteomyelitis/joint infections with oritavancin, dalbavancin, vancomycin, and daptomycin was $34,678, $54,224, $87,488, and $85,044, respectively. The mean cost per patient for BSI for oritavancin, dalbavancin, vancomycin, and daptomycin was $35,048, $75,349, $40,305, and $68,068, respectively. The mean cost per patient for ABSSSI for oritavancin and dalbavancin was $44,771 and $71,672.51.
Estimated total drug cost represents the cost of drug acquisition, administration supplies, laboratory monitoring, and human resources for OPAT visits or receiving home health services. The mean cost per patient of drug-related therapy for oritavancin, dalbavancin, vancomycin, and daptomycin was $2203, $5924, $3637, and $7146, respectively (Table 2).
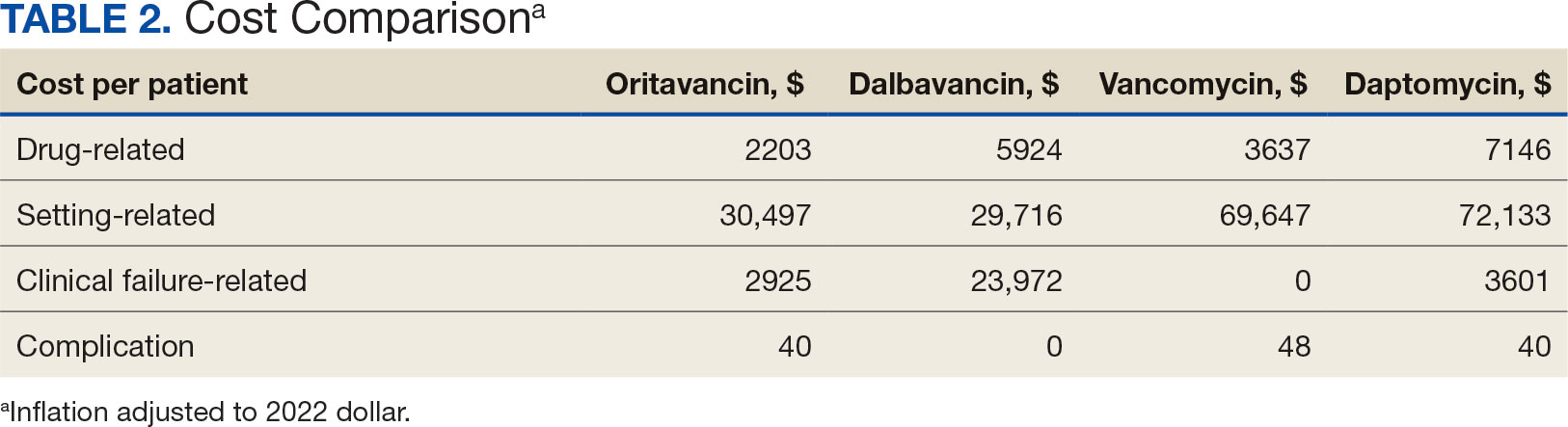
The mean cost per patient for osteomyelitis therapy for oritavancin, dalbavancin, vancomycin, and daptomycin was $2375, $6775, $4164, $8152, respectively. The mean cost of per patient for BSI treatment with oritavancin, dalbavancin, vancomycin, and daptomycin was $1737, $3475, $2409, and $1016, respectively. The mean cost per patient for oritavancin and dalbavancin for ABSSSI treatment, was $1553 and $3910, respectively.
Setting-related costs include expenses from inpatient admissions and postdischarge stays at community living centers (CLCs), skilled nursing facilities (SNFs), or rehabilitation facilities (RFs) for the duration of antimicrobial therapy. The mean setting-related therapy cost for osteomyelitis treatment with oritavancin, dalbavancin, vancomycin, and daptomycin was $27,852, $17,815, $83,324, and $72,856, respectively. The mean setting-related therapy cost per patient for BSI treatment with oritavancin, dalbavancin, vancomycin, and daptomycin was $33,310, $60,668, $37,734, and $67,074, respectively. The mean setting-related therapy cost per patient for ABSSSI treatment for oritavancin and dalbavancin was $43,218 and $67,762.00, respectively.
Six of 39 patients (15%) had clinical failure: 2 patients with oritavancin and 4 patients with dalbavancin. Four patients were readmitted for worsening infection and 2 for AEs. One patient (13%) in the daptomycin group had clinical failure due to readmission for worsening infection. There was no clinical failure with vancomycin. The costs associated with clinical failure per patient for oritavancin, dalbavancin, vancomycin, and daptomycin were $2925, $23,972, $0, and $3601, respectively (Table 3).
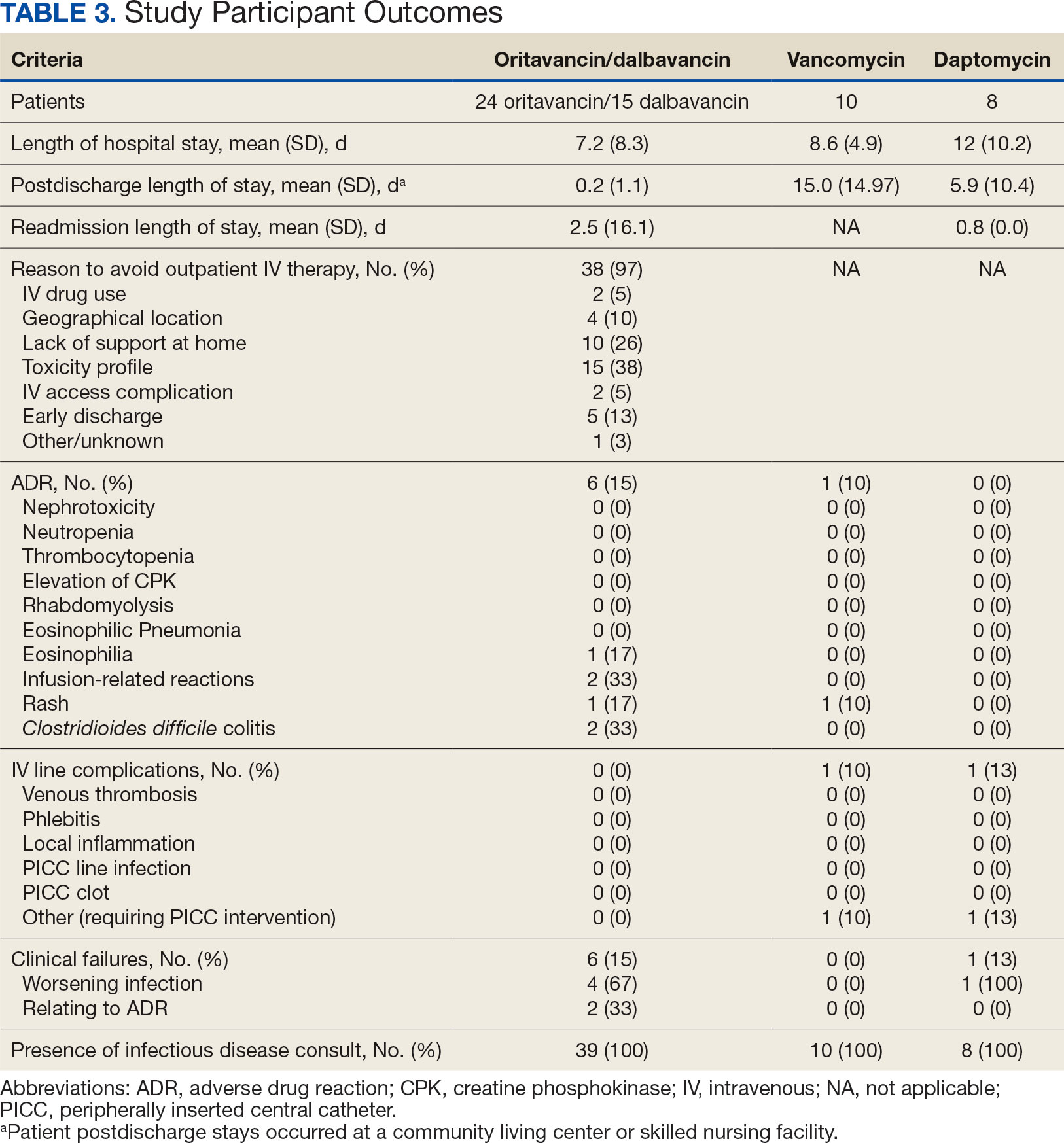
Thirty-eight patients (97%) who received oritavancin or dalbavancin had difficulty adhering to vancomycin or daptomycin OPAT. Oritavancin or dalbavancin was used in 10 patients (26%) who lacked support at home and 15 patients (38%) who had either a contraindication or previous failure with other antimicrobials, which were the most common explanations.
DISCUSSION
Long-acting lipoglycopeptides represent a potential alternative to home IV therapy that can avoid prolonged IV access with traditional OPAT. This offers significant advantages, allowing patients to be discharged from the hospital early, especially in rural areas with little OPAT infrastructure or those with logistic challenges. In this analysis, treatment with oritavancin for osteomyelitis, BSI, or ABSSSI, yielded an estimated cost savings of about $37,000 per patient, compared to treatment of matched indications with vancomycin and daptomycin. For every patient treated with dalbavancin for osteomyelitis, BSI, or ABSSSI, the cost savings was about $13,000 per patient, compared to treatment of matched indications for daptomycin and vancomycin. The estimated cost savings per patient for oritavancin was similar to previously published projections ($30,500 to $55,831).15
Cost savings were primarily driven by setting-related costs. The greatest contrast between the oritavancin and dalbavancin group compared to the vancomycin and daptomycin group was the length of stay in a postdischarge CLC, SNF, or RF setting. This analysis estimated that for every patient treated with oritavancin for osteomyelitis, the setting-related cost savings per patient was about $55,000 compared with vancomycin, and about $45,000 per patient compared with daptomycin. Furthermore, the estimated setting-related cost savings for osteomyelitis treatment with dalbavancin was about $65,000 compared with vancomycin and about $55,000 compared with daptomycin.
Clinical failure occurred with greater frequency in the oritavancin and dalbavancin groups (15%), compared with the vancomycin (0%) and daptomycin (13%) groups. Although the clinical failure rates in patients with osteomyelitis treated with oritavancin and dalbavancin compared with daptomycin were like those in previously published research (10%-30%), the rates of clinical failure for vancomycin in this analysis were lower than those in the oritavancin and dalbavancin group.8,21,22 The discrepancy in clinical failure rates between this analysis and previous research is likely due to selection bias. Based on the percentages of clinical failure found in the analysis, it is not surprising to note that the total clinical failure-related cost per patient was higher for oritavancin and dalbavancin compared to vancomycin, but similar between oritavancin and daptomycin.
This analysis also found that 15% of patients in the oritavancin and dalbavancin group experienced an AE compared to 10% of patients in the vancomycin group and none in the daptomycin group. In the oritavancin and dalbavancin group, the 2 most common AEs were infusion-related reactions and C. difficile colitis. Although infusion related reactions are easier to correspond to oritavancin and dalbavancin, it becomes difficult to definitively attribute the occurrence of C. difficile to these drugs as many patients were receiving concomitant antibiotics. Although not a primary or secondary objective, the rate of IV-line AEs were more prevalent in the vancomycin (10%), and daptomycin (13%) groups, compared to none in the oritavancin and dalbavancin group. This finding was expected; oritavancin and dalbavancin do not require a central IV line for administration.
Pharmacoeconomic literature continues to emerge with long-acting lipoglycopeptides. A 2024 Italian retrospective single-center analysis of 62 patients reported mean cost reductions > €3200 per patient (> $3400) given dalbavancin compared with the standard of care for ABSSSI or more deep-seeded infections such as osteomyelitis.23 A 2023 Spanish observational multicenter analysis of 124 patients with infective endocarditis demonstrated high efficacy, safety and cost-effectiveness with dalbavancin vs conventional treatments, with a mean savings of > €5548 per patient (> $6200).24 An analysis of the implementation of a dalbavancin order pathway for ABSSSI to avert inpatient admissions at 11 US emergency departments found a mean cost savings of $5133 per patient and $1211 per hospitalization day avoided, compared with inpatient usual care.25
Conversely, a multicenter, retrospective study of 209 patients in a community-based health care system failed to show a financial benefit for dalbavancin use when compared to standard of care for ABSSSI with higher readmission rates.26 Turco et al also reported increased cost results for 64 patients who received dalbavancin vs standard of care for ABSSSI.27 These discordant findings in ABSSSI studies may be impacted by the authors' patient selection choices and cost assumptions, especially with significantly cheaper oral alternatives. More data are needed to best identify the optimal therapeutic use for the long-acting lipoglycopeptides.
Limitations
The most significant limitation in this analysis was selection bias: 38 of 39 patients (97%) who received dalbavancin or oritavancin had a documented reason that described why OPAT therapy with traditional medications would not be optimal, including logistics, AEs, or clinical failures. Most patients treated with vancomycin and daptomycin were admitted into a SNF, RF, or CLC for the remainder of their treatment, allowing for closer monitoring and care compared to patients treated with oritavancin and dalbavancin, but at a greater cost. For patients sent to a community based SNF or RF, laboratory data were not available unless internally drawn or documented in the electronic medical record.
Additionally, not all cost data were available from VA sources; some were applied from literature, pharmacoeconomist, or best faith effort based on workflow. The cost data from third party contractors providing OPAT services to some BVAMC patients during the time frame of this analysis were not available. Due to its small sample size, outliers had the potential to affect averages reported and accuracy of the cost analysis. Emerging evidence suggests that daptomycin doses higher than the manufacturer-recommended regimen may be required for select indications, a factor that could affect cost, AEs, and efficacy outcomes.28 The acquisition cost of oritavancin and dalbavancin may vary by institution (ie, VA contract prices vs non- VA contract prices) and change over time. A current assessment of cost is needed to best visualize institutional benefit.
Finally, while the patient demographic of this MUE was highly representative of the demographic treated at the BVAMC (males aged >65 years), it may not be applicable to external patient populations. This analysis evaluated off-label indications for these medications. Consequently, this analysis would likely not be applicable to non-VA institution, as third-party payers (eg, insurance) are unlikely to cover medications for off-label indications.
CONCLUSIONS
This study found cost savings associated with the use of oritavancin and dalbavancin compared with vancomycin and daptomycin, particularly for the treatment of osteomyelitis. As safety and efficacy data continues to emerge, the use of long-acting lipoglycopeptides appears to be an increasingly attractive alternative option compared to traditional outpatient antimicrobial therapy, depending on the structure of the program. Larger, multicenter cost-effectiveness studies are needed to further establish the impact of these novel agents.
- Dalvance. Package insert. AbbVie Inc.; 2025.
- Orbactiv. Package insert. Melinta Therapeutics; 2022.
- Cooper CC, Stein GE, Mitra S, Abubaker A, Havlichek DH. Long-acting lipoglycopeptides for the treatment of bone and joint infections. Surg Infect (Larchmt). 2021;22(8):771- 779. doi:10.1089/sur.2020.413
- Simonetti O, Rizzetto G, Molinelli E, Cirioni O, Offidani A. Review: a safety profile of dalbavancin for on- and offlabel utilization. Ther Clin Risk Manag. 2021;17:223-232. doi:10.2147/TCRM.S271445
- Bloem A, Bax HI, Yusuf E, Verkaik NJ. New-generation antibiotics for treatment of gram-positive infections: a review with focus on endocarditis and osteomyelitis. J Clin Med. 2021;10(8):1743. doi:10.3390/jcm10081743
- Thomas G, Henao-Martínez AF, Franco-Paredes C, Chastain DB. Treatment of osteoarticular, cardiovascular, intravascular-catheter-related and other complicated infections with dalbavancin and oritavancin: a systematic review. Int J Antimicrob Agents. 2020;56(3):106069. doi:10.1016/j.ijantimicag.2020.106069
- Rappo U, Puttagunta S, Shevchenko V, et al. Dalbavancin for the treatment of osteomyelitis in adult patients: a randomized clinical trial of efficacy and safety. Open Forum Infect Dis. 2018;6(1):ofy331. doi:10.1093/ofid/ofy331
- Cain AR, Bremmer DN, Carr DR, et al. Effectiveness of dalbavancin compared with standard of care for the treatment of osteomyelitis: a real-world analysis. Open Forum Infect Dis. 2021;9(2):ofab589. doi:10.1093/ofid/ofab589
- Van Hise NW, Chundi V, Didwania V, et al. Treatment of acute osteomyelitis with once-weekly oritavancin: a two-year, multicenter, retrospective study. Drugs Real World Outcomes. 2020;7(Suppl 1):41-45. doi:10.1007/s40801-020-00195-7
- Cooper MM, Preslaski CR, Shihadeh KC, Hawkins KL, Jenkins TC. Multiple-dose dalbavancin regimens as the predominant treatment of deep-seated or endovascular infections: a scoping review. Open Forum Infect Dis. 2021;8(11):ofab486. doi:10.1093/ofid/ofab486
- Baddour LM, Wilson WR, Bayer AS, et al. Infective endocarditis in adults: diagnosis, antimicrobial therapy, and management of complications: a scientific statement for healthcare professionals from the American Heart Association. Circulation. 2015;132(15):1435-1486. doi:10.1161/CIR.0000000000000296
- Berbari EF, Kanj SS, Kowalski TJ, et al. 2015 Infectious Diseases Society of America (IDSA) Clinical Practice Guidelines for the Diagnosis and Treatment of Native Vertebral Osteomyelitis in Adults. Clin Infect Dis. 2015;61(6):e26-46. doi:10.1093/cid/civ482
- Arrieta-Loitegui M, Caro-Teller JM, Ortiz-Pérez S, López- Medrano F, San Juan-Garrido R, Ferrari-Piquero JM. Effectiveness, safety, and cost analysis of dalbavancin in clinical practice. Eur J Hosp Pharm. 2022;29(1):55-58. doi:10.1136/ejhpharm-2020-002315
- Pascale R, Maccaro A, Mikus E, et al. A retrospective multicentre study on dalbavancin effectiveness and cost-evaluation in sternotomic wound infection treatment: DALBA SWIT study. J Glob Antimicrob Resist. 2022;30:390-394. doi:10.1016/j.jgar.2022.07.018
- Antosz K, Al-Hasan MN, Lu ZK, et at. Clinical utility and cost effectiveness of long-acting lipoglycopeptides used in deep seated infections among patients with social and economic barriers to care. Pharmacy (Basel). 2021;10(1):1. doi:10.3390/pharmacy10010001
- Roberts MS. Economic aspects of evaluation. In: Friedman CP, Wyatt JC, eds. Evaluation Methods in Biomedical Informatics. 2nd ed. Springer; 2006:301-337.
- US Department of Veterans Affairs. HERC inpatient average cost data. Updated May 1, 2025. Accessed May 9, 2025. https://www.herc.research.va.gov/include/page.asp?id=inpatient
- US Department of Veterans Affairs. HERC Outpatient average cost dataset. Updated May 1, 2025. Accessed May 9, 2025. https://www.herc.research.va.gov/include/page.asp?id=outpatient
- Ektare V, Khachatryan A, Xue M, Dunne M, Johnson K, Stephens J. Assessing the economic value of avoiding hospital admissions by shifting the management of gram + acute bacterial skin and skin-structure infections to an outpatient care setting. J Med Econ. 2015;18(12):1092-1101. doi:10.3111/13696998.2015.1078339
- Ruh CA, Parameswaran GI, Wojciechowski AL, Mergenhagen KA. Outcomes and pharmacoeconomic analysis of a home intravenous antibiotic infusion program in veterans. Clin Ther. 2015;37(11):2527-2535. doi:10.1016/j.clinthera.2015.09.009
- Nakrani M, Yu D, Skikka M, et al. Comparison of vancomycin and daptomycin complications and interventions in outpatient parenteral antimicrobial therapy. Open Forum Infect Dis. 2020;7(Suppl 1):S361-S362. doi:10.1093/ofid/ofaa439.791
- Scoble PJ, Reilly J, Tilloston GS. Real-world use of oritavancin for the treatment of osteomyelitis. Drugs Real World Outcomes. 2020;7(Suppl 1):46-54. doi:10.1007/s40801-020-00194-8
- Segala D, Barbieri M, Di Nuzzo M, et al. Clinical, organizational, and pharmacoeconomic perspectives of dalbavancin vs standard of care in the infectious disease network. Glob Reg Health Technol Assess. 2024;11(Suppl 2):5-12. doi:10.33393/grhta.2024.3094
- Gómez A, et al. EN-DALBACEN 2.0 Cohort: real-life study of dalbavancin as sequential/consolidation therapy in patients with infective endocarditis due to Gram-positive cocci. Int J Antimicrob Agents. 2023;62(3):106918. doi:10.1016/j.ijantimicag.2023.106918
- LoVecchio F, McCarthy MW, Ye X, et al. Single intravenous dose dalbavancin pathway for the treatment of acute bacterial skin and skin structure infections: considerations for emergency department implementation and cost savings. J Emerg Med. 2024;67(2):e217-e229. doi:10.1016/j.jemermed.2024.03.003
- Gonzalez J, Andrade DC, Niu J. Cost-consequence analysis of single-dose dalbavancin versus standard of care for the treatment of acute bacterial skin and skin structure infections in a multisite healthcare system. Clin Infect Dis. 2021;73(7):e1436-e1442. doi:10.1093/cid/ciaa1732
- Turco NJ, Kane-Gill SL, Hernandez I, Oleksiuk LM, D’Amico F, Pickering AJ. A cost-minimization analysis of dalbavancin compared to conventional therapy for the outpatient treatment of acute bacterial skin and skin-structure infections. Expert Opin Pharmacother. 2018;19(4):319-325. doi:10.1080/14656566.2018.1442439
- Jones TW, Jun AH, Michal JL, Olney WJ. High-dose daptomycin and clinical applications. Ann Pharmacother. 2021;55(11):1363-1378. doi:10.1177/1060028021991943
Oritavancin and dalbavancin are long acting lipoglycopeptides indicated for the treatment of acute bacterial skin and skin structure infections (ABSSSI).1,2 Largely due to their long half-lives, prolonged tissue concentrations at sites of infection, tolerability, and minimal requirement for therapeutic drug monitoring, these agents are attractive options in outpatient settings.3,4 A 1- or 2-dose treatment of oritavancin and dalbavancin may be sufficient for conditions traditionally treated with outpatient parenteral antimicrobial therapy (OPAT) via peripherally inserted central catheter (PICC).
Limited research supports the use of dalbavancin and oritavancin for bone and joint infections, infective endocarditis, and bloodstream infections (BSIs). However, the US Food and Drug Administration has approved an indication for the treatment of ABSSSI.3-9 Dosing for these off-label indications varies but typically consists of an initial intravenous (IV) dose (1000 mg, 1200 mg, or 1500 mg), with a subsequent dose 1 to 2 weeks later or administered once weekly.6-10
Due in part to the recent availability of oritavancin and dalbavancin relative to the publication of practice guidelines, their appropriate place in therapy continues to evolve based on emerging literature.11,12 One potential barrier of use for these medications is their cost. Based on the number of doses administered, the 2022 estimated total acquisition cost of therapy for oritavancin and dalbavancin was $1014 to $4397 and $3046 to $7150, respectively (eAppendix). Despite the high acquisition costs, these agents do not require the placement of an indwelling central line, can be administered in outpatient settings, and require minimal therapeutic dose monitoring compared to vancomycin.13-15 This medication use evaluation (MUE) compared the total cost of treatment with oritavancin and dalbavancin vs therapies traditionally used for OPAT or prolonged IV inpatient therapy.
METHODS
This retrospective MUE was conducted at the Boise Veterans Affairs Medical Center (BVAMC), a level 2 facility with an extensive rural catchment area. BVAMC provides many OPAT services, including medications, supplies, and dressing changes after initial clinic or inpatient education. Contracted vendors may also assist with at-home nursing care using supplies provided by the BVAMC. Cases were identified using an internal database of OPAT patients and those who received oritavancin or dalbavancin between September 1, 2017, and November 1, 2022. Patients aged ≥ 18 years who received ≥ 1 dose of oritavancin or dalbavancin for ABSSSI, osteomyelitis/joint infections, endocarditis, and BSI were included. Comparator treatments consisting of ≥ 1 week of vancomycin or daptomycin for ABSSSI, osteomyelitis/joint infections, endocarditis, and BSI were identified through review of OPAT and Infectious Diseases service consults during the same timeframe. Patients were excluded if any antibiotic was prescribed by a non- VA clinician, if medications were not provided by OPAT, or if chart review did not identify an ABSSSI, osteomyelitis/ joint infection, or BSI diagnosis.
Electronic medical record review was conducted using a standardized data collection form (eAppendix). Data collected included demographics, infectious diagnosis, treatment administered, administration procedures and related visits and treatment locations, outcomes including clinical failure, adverse events (AEs), and hospital readmission.
Clinical failure was defined as readmission or death due to worsening infection or readmission secondary to a documented potential AE to the evaluated antibiotics within 90 days after initiation. Clinical failures excluded readmissions not associated with infection including comorbidities or elective procedures. AEs included new onset renal failure (serum creatinine ≥ 0.5 mg/dL), neutropenia (neutrophils ≤ 500), thrombocytopenia (platelets < 100,000), eosinophilia (> 15% eosinophils), or creatine phosphokinase > 10 times the upper limit of normal, and Clostridioides difficile (C. difficile) infection. Line complications included thrombophlebitis, local inflammation, or infection requiring line replacement (eAppendix).
A cost-minimization approach was used to assess the total cost of treatment.16 Patients who received oritavancin or dalbavancin were matched with patients that received vancomycin and daptomycin for the same indication and about 1 month of initiation through the randomization function in Microsoft Excel. This accounted for changes in personnel, nonformulary drug approvals, cost, and changes in practice during the pandemic. Costs were calculated using a decision tree as a base model (Figure 1). In this model, each treatment dyad was assessed for the presence or absence of clinical failure, adverse event (medication and line complications), and treatment setting endpoints. Cost estimates were tabulated for each patient that received treatment using published VA data, literature, pharmacoeconomist guidance, or best faith effort based on workflow. 17-20 All cost estimates were based on 2022 figures or adjusted for inflation if obtained prior to 2022. Secondary endpoints of this analysis included estimated total cost of medication acquisition, administration supplies, laboratory monitoring, and human resources for OPAT visits or receiving home-health services.

This evaluation was classified by the BVAMC Medication Use Evaluation research determination subcommittee as a quality improvement project and was considered exempt from VA Human Subjects Research requirements based on the VA Policy Handbook guideline 1058.05.
RESULTS
The study identified 44 patients who received dalbavancin or oritavancin between September 1, 2017, and October 31, 2022. Thirty-nine patients were included in the analysis: 24 received oritavancin and 15 received dalbavancin and were matched by indication to 10 patients who received vancomycin and 8 patients who received daptomycin. Three patients could not be matched by indication of ABSSSI (Figure 2). Most patients were male, aged > 65 years, and were treated for osteomyelitis (Table 1). No patients were treated for infective endocarditis. A myriad of concomitant antibiotics were used to treat patients and culture results indicated that most infections treated with oritavancin and dalbavancin were polymicrobial.


The mean total cost of therapy per patient receiving oritavancin, dalbavancin, vancomycin, and daptomycin was $35,630, $59,612, $73,333, and $73,708, respectively (Figure 3). When stratified by indication, 27 patients (69%) in the oritavancin/dalbavancin group were treated for osteomyelitis/ joint infections (16 oritavancin, 11 dalbavancin), 9 patients (23%) were treated for BSI (6 oritavancin, 3 dalbavancin), and 3 patients (8%) were treated for ABSSSI (2 oritavancin, 1 dalbavancin). The mean cost per patient for osteomyelitis/joint infections with oritavancin, dalbavancin, vancomycin, and daptomycin was $34,678, $54,224, $87,488, and $85,044, respectively. The mean cost per patient for BSI for oritavancin, dalbavancin, vancomycin, and daptomycin was $35,048, $75,349, $40,305, and $68,068, respectively. The mean cost per patient for ABSSSI for oritavancin and dalbavancin was $44,771 and $71,672.51.

Estimated total drug cost represents the cost of drug acquisition, administration supplies, laboratory monitoring, and human resources for OPAT visits or receiving home health services. The mean cost per patient of drug-related therapy for oritavancin, dalbavancin, vancomycin, and daptomycin was $2203, $5924, $3637, and $7146, respectively (Table 2).

The mean cost per patient for osteomyelitis therapy for oritavancin, dalbavancin, vancomycin, and daptomycin was $2375, $6775, $4164, $8152, respectively. The mean cost of per patient for BSI treatment with oritavancin, dalbavancin, vancomycin, and daptomycin was $1737, $3475, $2409, and $1016, respectively. The mean cost per patient for oritavancin and dalbavancin for ABSSSI treatment, was $1553 and $3910, respectively.
Setting-related costs include expenses from inpatient admissions and postdischarge stays at community living centers (CLCs), skilled nursing facilities (SNFs), or rehabilitation facilities (RFs) for the duration of antimicrobial therapy. The mean setting-related therapy cost for osteomyelitis treatment with oritavancin, dalbavancin, vancomycin, and daptomycin was $27,852, $17,815, $83,324, and $72,856, respectively. The mean setting-related therapy cost per patient for BSI treatment with oritavancin, dalbavancin, vancomycin, and daptomycin was $33,310, $60,668, $37,734, and $67,074, respectively. The mean setting-related therapy cost per patient for ABSSSI treatment for oritavancin and dalbavancin was $43,218 and $67,762.00, respectively.
Six of 39 patients (15%) had clinical failure: 2 patients with oritavancin and 4 patients with dalbavancin. Four patients were readmitted for worsening infection and 2 for AEs. One patient (13%) in the daptomycin group had clinical failure due to readmission for worsening infection. There was no clinical failure with vancomycin. The costs associated with clinical failure per patient for oritavancin, dalbavancin, vancomycin, and daptomycin were $2925, $23,972, $0, and $3601, respectively (Table 3).

Thirty-eight patients (97%) who received oritavancin or dalbavancin had difficulty adhering to vancomycin or daptomycin OPAT. Oritavancin or dalbavancin was used in 10 patients (26%) who lacked support at home and 15 patients (38%) who had either a contraindication or previous failure with other antimicrobials, which were the most common explanations.
DISCUSSION
Long-acting lipoglycopeptides represent a potential alternative to home IV therapy that can avoid prolonged IV access with traditional OPAT. This offers significant advantages, allowing patients to be discharged from the hospital early, especially in rural areas with little OPAT infrastructure or those with logistic challenges. In this analysis, treatment with oritavancin for osteomyelitis, BSI, or ABSSSI, yielded an estimated cost savings of about $37,000 per patient, compared to treatment of matched indications with vancomycin and daptomycin. For every patient treated with dalbavancin for osteomyelitis, BSI, or ABSSSI, the cost savings was about $13,000 per patient, compared to treatment of matched indications for daptomycin and vancomycin. The estimated cost savings per patient for oritavancin was similar to previously published projections ($30,500 to $55,831).15
Cost savings were primarily driven by setting-related costs. The greatest contrast between the oritavancin and dalbavancin group compared to the vancomycin and daptomycin group was the length of stay in a postdischarge CLC, SNF, or RF setting. This analysis estimated that for every patient treated with oritavancin for osteomyelitis, the setting-related cost savings per patient was about $55,000 compared with vancomycin, and about $45,000 per patient compared with daptomycin. Furthermore, the estimated setting-related cost savings for osteomyelitis treatment with dalbavancin was about $65,000 compared with vancomycin and about $55,000 compared with daptomycin.
Clinical failure occurred with greater frequency in the oritavancin and dalbavancin groups (15%), compared with the vancomycin (0%) and daptomycin (13%) groups. Although the clinical failure rates in patients with osteomyelitis treated with oritavancin and dalbavancin compared with daptomycin were like those in previously published research (10%-30%), the rates of clinical failure for vancomycin in this analysis were lower than those in the oritavancin and dalbavancin group.8,21,22 The discrepancy in clinical failure rates between this analysis and previous research is likely due to selection bias. Based on the percentages of clinical failure found in the analysis, it is not surprising to note that the total clinical failure-related cost per patient was higher for oritavancin and dalbavancin compared to vancomycin, but similar between oritavancin and daptomycin.
This analysis also found that 15% of patients in the oritavancin and dalbavancin group experienced an AE compared to 10% of patients in the vancomycin group and none in the daptomycin group. In the oritavancin and dalbavancin group, the 2 most common AEs were infusion-related reactions and C. difficile colitis. Although infusion related reactions are easier to correspond to oritavancin and dalbavancin, it becomes difficult to definitively attribute the occurrence of C. difficile to these drugs as many patients were receiving concomitant antibiotics. Although not a primary or secondary objective, the rate of IV-line AEs were more prevalent in the vancomycin (10%), and daptomycin (13%) groups, compared to none in the oritavancin and dalbavancin group. This finding was expected; oritavancin and dalbavancin do not require a central IV line for administration.
Pharmacoeconomic literature continues to emerge with long-acting lipoglycopeptides. A 2024 Italian retrospective single-center analysis of 62 patients reported mean cost reductions > €3200 per patient (> $3400) given dalbavancin compared with the standard of care for ABSSSI or more deep-seeded infections such as osteomyelitis.23 A 2023 Spanish observational multicenter analysis of 124 patients with infective endocarditis demonstrated high efficacy, safety and cost-effectiveness with dalbavancin vs conventional treatments, with a mean savings of > €5548 per patient (> $6200).24 An analysis of the implementation of a dalbavancin order pathway for ABSSSI to avert inpatient admissions at 11 US emergency departments found a mean cost savings of $5133 per patient and $1211 per hospitalization day avoided, compared with inpatient usual care.25
Conversely, a multicenter, retrospective study of 209 patients in a community-based health care system failed to show a financial benefit for dalbavancin use when compared to standard of care for ABSSSI with higher readmission rates.26 Turco et al also reported increased cost results for 64 patients who received dalbavancin vs standard of care for ABSSSI.27 These discordant findings in ABSSSI studies may be impacted by the authors' patient selection choices and cost assumptions, especially with significantly cheaper oral alternatives. More data are needed to best identify the optimal therapeutic use for the long-acting lipoglycopeptides.
Limitations
The most significant limitation in this analysis was selection bias: 38 of 39 patients (97%) who received dalbavancin or oritavancin had a documented reason that described why OPAT therapy with traditional medications would not be optimal, including logistics, AEs, or clinical failures. Most patients treated with vancomycin and daptomycin were admitted into a SNF, RF, or CLC for the remainder of their treatment, allowing for closer monitoring and care compared to patients treated with oritavancin and dalbavancin, but at a greater cost. For patients sent to a community based SNF or RF, laboratory data were not available unless internally drawn or documented in the electronic medical record.
Additionally, not all cost data were available from VA sources; some were applied from literature, pharmacoeconomist, or best faith effort based on workflow. The cost data from third party contractors providing OPAT services to some BVAMC patients during the time frame of this analysis were not available. Due to its small sample size, outliers had the potential to affect averages reported and accuracy of the cost analysis. Emerging evidence suggests that daptomycin doses higher than the manufacturer-recommended regimen may be required for select indications, a factor that could affect cost, AEs, and efficacy outcomes.28 The acquisition cost of oritavancin and dalbavancin may vary by institution (ie, VA contract prices vs non- VA contract prices) and change over time. A current assessment of cost is needed to best visualize institutional benefit.
Finally, while the patient demographic of this MUE was highly representative of the demographic treated at the BVAMC (males aged >65 years), it may not be applicable to external patient populations. This analysis evaluated off-label indications for these medications. Consequently, this analysis would likely not be applicable to non-VA institution, as third-party payers (eg, insurance) are unlikely to cover medications for off-label indications.
CONCLUSIONS
This study found cost savings associated with the use of oritavancin and dalbavancin compared with vancomycin and daptomycin, particularly for the treatment of osteomyelitis. As safety and efficacy data continues to emerge, the use of long-acting lipoglycopeptides appears to be an increasingly attractive alternative option compared to traditional outpatient antimicrobial therapy, depending on the structure of the program. Larger, multicenter cost-effectiveness studies are needed to further establish the impact of these novel agents.
Oritavancin and dalbavancin are long acting lipoglycopeptides indicated for the treatment of acute bacterial skin and skin structure infections (ABSSSI).1,2 Largely due to their long half-lives, prolonged tissue concentrations at sites of infection, tolerability, and minimal requirement for therapeutic drug monitoring, these agents are attractive options in outpatient settings.3,4 A 1- or 2-dose treatment of oritavancin and dalbavancin may be sufficient for conditions traditionally treated with outpatient parenteral antimicrobial therapy (OPAT) via peripherally inserted central catheter (PICC).
Limited research supports the use of dalbavancin and oritavancin for bone and joint infections, infective endocarditis, and bloodstream infections (BSIs). However, the US Food and Drug Administration has approved an indication for the treatment of ABSSSI.3-9 Dosing for these off-label indications varies but typically consists of an initial intravenous (IV) dose (1000 mg, 1200 mg, or 1500 mg), with a subsequent dose 1 to 2 weeks later or administered once weekly.6-10
Due in part to the recent availability of oritavancin and dalbavancin relative to the publication of practice guidelines, their appropriate place in therapy continues to evolve based on emerging literature.11,12 One potential barrier of use for these medications is their cost. Based on the number of doses administered, the 2022 estimated total acquisition cost of therapy for oritavancin and dalbavancin was $1014 to $4397 and $3046 to $7150, respectively (eAppendix). Despite the high acquisition costs, these agents do not require the placement of an indwelling central line, can be administered in outpatient settings, and require minimal therapeutic dose monitoring compared to vancomycin.13-15 This medication use evaluation (MUE) compared the total cost of treatment with oritavancin and dalbavancin vs therapies traditionally used for OPAT or prolonged IV inpatient therapy.
METHODS
This retrospective MUE was conducted at the Boise Veterans Affairs Medical Center (BVAMC), a level 2 facility with an extensive rural catchment area. BVAMC provides many OPAT services, including medications, supplies, and dressing changes after initial clinic or inpatient education. Contracted vendors may also assist with at-home nursing care using supplies provided by the BVAMC. Cases were identified using an internal database of OPAT patients and those who received oritavancin or dalbavancin between September 1, 2017, and November 1, 2022. Patients aged ≥ 18 years who received ≥ 1 dose of oritavancin or dalbavancin for ABSSSI, osteomyelitis/joint infections, endocarditis, and BSI were included. Comparator treatments consisting of ≥ 1 week of vancomycin or daptomycin for ABSSSI, osteomyelitis/joint infections, endocarditis, and BSI were identified through review of OPAT and Infectious Diseases service consults during the same timeframe. Patients were excluded if any antibiotic was prescribed by a non- VA clinician, if medications were not provided by OPAT, or if chart review did not identify an ABSSSI, osteomyelitis/ joint infection, or BSI diagnosis.
Electronic medical record review was conducted using a standardized data collection form (eAppendix). Data collected included demographics, infectious diagnosis, treatment administered, administration procedures and related visits and treatment locations, outcomes including clinical failure, adverse events (AEs), and hospital readmission.
Clinical failure was defined as readmission or death due to worsening infection or readmission secondary to a documented potential AE to the evaluated antibiotics within 90 days after initiation. Clinical failures excluded readmissions not associated with infection including comorbidities or elective procedures. AEs included new onset renal failure (serum creatinine ≥ 0.5 mg/dL), neutropenia (neutrophils ≤ 500), thrombocytopenia (platelets < 100,000), eosinophilia (> 15% eosinophils), or creatine phosphokinase > 10 times the upper limit of normal, and Clostridioides difficile (C. difficile) infection. Line complications included thrombophlebitis, local inflammation, or infection requiring line replacement (eAppendix).
A cost-minimization approach was used to assess the total cost of treatment.16 Patients who received oritavancin or dalbavancin were matched with patients that received vancomycin and daptomycin for the same indication and about 1 month of initiation through the randomization function in Microsoft Excel. This accounted for changes in personnel, nonformulary drug approvals, cost, and changes in practice during the pandemic. Costs were calculated using a decision tree as a base model (Figure 1). In this model, each treatment dyad was assessed for the presence or absence of clinical failure, adverse event (medication and line complications), and treatment setting endpoints. Cost estimates were tabulated for each patient that received treatment using published VA data, literature, pharmacoeconomist guidance, or best faith effort based on workflow. 17-20 All cost estimates were based on 2022 figures or adjusted for inflation if obtained prior to 2022. Secondary endpoints of this analysis included estimated total cost of medication acquisition, administration supplies, laboratory monitoring, and human resources for OPAT visits or receiving home-health services.

This evaluation was classified by the BVAMC Medication Use Evaluation research determination subcommittee as a quality improvement project and was considered exempt from VA Human Subjects Research requirements based on the VA Policy Handbook guideline 1058.05.
RESULTS
The study identified 44 patients who received dalbavancin or oritavancin between September 1, 2017, and October 31, 2022. Thirty-nine patients were included in the analysis: 24 received oritavancin and 15 received dalbavancin and were matched by indication to 10 patients who received vancomycin and 8 patients who received daptomycin. Three patients could not be matched by indication of ABSSSI (Figure 2). Most patients were male, aged > 65 years, and were treated for osteomyelitis (Table 1). No patients were treated for infective endocarditis. A myriad of concomitant antibiotics were used to treat patients and culture results indicated that most infections treated with oritavancin and dalbavancin were polymicrobial.


The mean total cost of therapy per patient receiving oritavancin, dalbavancin, vancomycin, and daptomycin was $35,630, $59,612, $73,333, and $73,708, respectively (Figure 3). When stratified by indication, 27 patients (69%) in the oritavancin/dalbavancin group were treated for osteomyelitis/ joint infections (16 oritavancin, 11 dalbavancin), 9 patients (23%) were treated for BSI (6 oritavancin, 3 dalbavancin), and 3 patients (8%) were treated for ABSSSI (2 oritavancin, 1 dalbavancin). The mean cost per patient for osteomyelitis/joint infections with oritavancin, dalbavancin, vancomycin, and daptomycin was $34,678, $54,224, $87,488, and $85,044, respectively. The mean cost per patient for BSI for oritavancin, dalbavancin, vancomycin, and daptomycin was $35,048, $75,349, $40,305, and $68,068, respectively. The mean cost per patient for ABSSSI for oritavancin and dalbavancin was $44,771 and $71,672.51.

Estimated total drug cost represents the cost of drug acquisition, administration supplies, laboratory monitoring, and human resources for OPAT visits or receiving home health services. The mean cost per patient of drug-related therapy for oritavancin, dalbavancin, vancomycin, and daptomycin was $2203, $5924, $3637, and $7146, respectively (Table 2).

The mean cost per patient for osteomyelitis therapy for oritavancin, dalbavancin, vancomycin, and daptomycin was $2375, $6775, $4164, $8152, respectively. The mean cost of per patient for BSI treatment with oritavancin, dalbavancin, vancomycin, and daptomycin was $1737, $3475, $2409, and $1016, respectively. The mean cost per patient for oritavancin and dalbavancin for ABSSSI treatment, was $1553 and $3910, respectively.
Setting-related costs include expenses from inpatient admissions and postdischarge stays at community living centers (CLCs), skilled nursing facilities (SNFs), or rehabilitation facilities (RFs) for the duration of antimicrobial therapy. The mean setting-related therapy cost for osteomyelitis treatment with oritavancin, dalbavancin, vancomycin, and daptomycin was $27,852, $17,815, $83,324, and $72,856, respectively. The mean setting-related therapy cost per patient for BSI treatment with oritavancin, dalbavancin, vancomycin, and daptomycin was $33,310, $60,668, $37,734, and $67,074, respectively. The mean setting-related therapy cost per patient for ABSSSI treatment for oritavancin and dalbavancin was $43,218 and $67,762.00, respectively.
Six of 39 patients (15%) had clinical failure: 2 patients with oritavancin and 4 patients with dalbavancin. Four patients were readmitted for worsening infection and 2 for AEs. One patient (13%) in the daptomycin group had clinical failure due to readmission for worsening infection. There was no clinical failure with vancomycin. The costs associated with clinical failure per patient for oritavancin, dalbavancin, vancomycin, and daptomycin were $2925, $23,972, $0, and $3601, respectively (Table 3).

Thirty-eight patients (97%) who received oritavancin or dalbavancin had difficulty adhering to vancomycin or daptomycin OPAT. Oritavancin or dalbavancin was used in 10 patients (26%) who lacked support at home and 15 patients (38%) who had either a contraindication or previous failure with other antimicrobials, which were the most common explanations.
DISCUSSION
Long-acting lipoglycopeptides represent a potential alternative to home IV therapy that can avoid prolonged IV access with traditional OPAT. This offers significant advantages, allowing patients to be discharged from the hospital early, especially in rural areas with little OPAT infrastructure or those with logistic challenges. In this analysis, treatment with oritavancin for osteomyelitis, BSI, or ABSSSI, yielded an estimated cost savings of about $37,000 per patient, compared to treatment of matched indications with vancomycin and daptomycin. For every patient treated with dalbavancin for osteomyelitis, BSI, or ABSSSI, the cost savings was about $13,000 per patient, compared to treatment of matched indications for daptomycin and vancomycin. The estimated cost savings per patient for oritavancin was similar to previously published projections ($30,500 to $55,831).15
Cost savings were primarily driven by setting-related costs. The greatest contrast between the oritavancin and dalbavancin group compared to the vancomycin and daptomycin group was the length of stay in a postdischarge CLC, SNF, or RF setting. This analysis estimated that for every patient treated with oritavancin for osteomyelitis, the setting-related cost savings per patient was about $55,000 compared with vancomycin, and about $45,000 per patient compared with daptomycin. Furthermore, the estimated setting-related cost savings for osteomyelitis treatment with dalbavancin was about $65,000 compared with vancomycin and about $55,000 compared with daptomycin.
Clinical failure occurred with greater frequency in the oritavancin and dalbavancin groups (15%), compared with the vancomycin (0%) and daptomycin (13%) groups. Although the clinical failure rates in patients with osteomyelitis treated with oritavancin and dalbavancin compared with daptomycin were like those in previously published research (10%-30%), the rates of clinical failure for vancomycin in this analysis were lower than those in the oritavancin and dalbavancin group.8,21,22 The discrepancy in clinical failure rates between this analysis and previous research is likely due to selection bias. Based on the percentages of clinical failure found in the analysis, it is not surprising to note that the total clinical failure-related cost per patient was higher for oritavancin and dalbavancin compared to vancomycin, but similar between oritavancin and daptomycin.
This analysis also found that 15% of patients in the oritavancin and dalbavancin group experienced an AE compared to 10% of patients in the vancomycin group and none in the daptomycin group. In the oritavancin and dalbavancin group, the 2 most common AEs were infusion-related reactions and C. difficile colitis. Although infusion related reactions are easier to correspond to oritavancin and dalbavancin, it becomes difficult to definitively attribute the occurrence of C. difficile to these drugs as many patients were receiving concomitant antibiotics. Although not a primary or secondary objective, the rate of IV-line AEs were more prevalent in the vancomycin (10%), and daptomycin (13%) groups, compared to none in the oritavancin and dalbavancin group. This finding was expected; oritavancin and dalbavancin do not require a central IV line for administration.
Pharmacoeconomic literature continues to emerge with long-acting lipoglycopeptides. A 2024 Italian retrospective single-center analysis of 62 patients reported mean cost reductions > €3200 per patient (> $3400) given dalbavancin compared with the standard of care for ABSSSI or more deep-seeded infections such as osteomyelitis.23 A 2023 Spanish observational multicenter analysis of 124 patients with infective endocarditis demonstrated high efficacy, safety and cost-effectiveness with dalbavancin vs conventional treatments, with a mean savings of > €5548 per patient (> $6200).24 An analysis of the implementation of a dalbavancin order pathway for ABSSSI to avert inpatient admissions at 11 US emergency departments found a mean cost savings of $5133 per patient and $1211 per hospitalization day avoided, compared with inpatient usual care.25
Conversely, a multicenter, retrospective study of 209 patients in a community-based health care system failed to show a financial benefit for dalbavancin use when compared to standard of care for ABSSSI with higher readmission rates.26 Turco et al also reported increased cost results for 64 patients who received dalbavancin vs standard of care for ABSSSI.27 These discordant findings in ABSSSI studies may be impacted by the authors' patient selection choices and cost assumptions, especially with significantly cheaper oral alternatives. More data are needed to best identify the optimal therapeutic use for the long-acting lipoglycopeptides.
Limitations
The most significant limitation in this analysis was selection bias: 38 of 39 patients (97%) who received dalbavancin or oritavancin had a documented reason that described why OPAT therapy with traditional medications would not be optimal, including logistics, AEs, or clinical failures. Most patients treated with vancomycin and daptomycin were admitted into a SNF, RF, or CLC for the remainder of their treatment, allowing for closer monitoring and care compared to patients treated with oritavancin and dalbavancin, but at a greater cost. For patients sent to a community based SNF or RF, laboratory data were not available unless internally drawn or documented in the electronic medical record.
Additionally, not all cost data were available from VA sources; some were applied from literature, pharmacoeconomist, or best faith effort based on workflow. The cost data from third party contractors providing OPAT services to some BVAMC patients during the time frame of this analysis were not available. Due to its small sample size, outliers had the potential to affect averages reported and accuracy of the cost analysis. Emerging evidence suggests that daptomycin doses higher than the manufacturer-recommended regimen may be required for select indications, a factor that could affect cost, AEs, and efficacy outcomes.28 The acquisition cost of oritavancin and dalbavancin may vary by institution (ie, VA contract prices vs non- VA contract prices) and change over time. A current assessment of cost is needed to best visualize institutional benefit.
Finally, while the patient demographic of this MUE was highly representative of the demographic treated at the BVAMC (males aged >65 years), it may not be applicable to external patient populations. This analysis evaluated off-label indications for these medications. Consequently, this analysis would likely not be applicable to non-VA institution, as third-party payers (eg, insurance) are unlikely to cover medications for off-label indications.
CONCLUSIONS
This study found cost savings associated with the use of oritavancin and dalbavancin compared with vancomycin and daptomycin, particularly for the treatment of osteomyelitis. As safety and efficacy data continues to emerge, the use of long-acting lipoglycopeptides appears to be an increasingly attractive alternative option compared to traditional outpatient antimicrobial therapy, depending on the structure of the program. Larger, multicenter cost-effectiveness studies are needed to further establish the impact of these novel agents.
- Dalvance. Package insert. AbbVie Inc.; 2025.
- Orbactiv. Package insert. Melinta Therapeutics; 2022.
- Cooper CC, Stein GE, Mitra S, Abubaker A, Havlichek DH. Long-acting lipoglycopeptides for the treatment of bone and joint infections. Surg Infect (Larchmt). 2021;22(8):771- 779. doi:10.1089/sur.2020.413
- Simonetti O, Rizzetto G, Molinelli E, Cirioni O, Offidani A. Review: a safety profile of dalbavancin for on- and offlabel utilization. Ther Clin Risk Manag. 2021;17:223-232. doi:10.2147/TCRM.S271445
- Bloem A, Bax HI, Yusuf E, Verkaik NJ. New-generation antibiotics for treatment of gram-positive infections: a review with focus on endocarditis and osteomyelitis. J Clin Med. 2021;10(8):1743. doi:10.3390/jcm10081743
- Thomas G, Henao-Martínez AF, Franco-Paredes C, Chastain DB. Treatment of osteoarticular, cardiovascular, intravascular-catheter-related and other complicated infections with dalbavancin and oritavancin: a systematic review. Int J Antimicrob Agents. 2020;56(3):106069. doi:10.1016/j.ijantimicag.2020.106069
- Rappo U, Puttagunta S, Shevchenko V, et al. Dalbavancin for the treatment of osteomyelitis in adult patients: a randomized clinical trial of efficacy and safety. Open Forum Infect Dis. 2018;6(1):ofy331. doi:10.1093/ofid/ofy331
- Cain AR, Bremmer DN, Carr DR, et al. Effectiveness of dalbavancin compared with standard of care for the treatment of osteomyelitis: a real-world analysis. Open Forum Infect Dis. 2021;9(2):ofab589. doi:10.1093/ofid/ofab589
- Van Hise NW, Chundi V, Didwania V, et al. Treatment of acute osteomyelitis with once-weekly oritavancin: a two-year, multicenter, retrospective study. Drugs Real World Outcomes. 2020;7(Suppl 1):41-45. doi:10.1007/s40801-020-00195-7
- Cooper MM, Preslaski CR, Shihadeh KC, Hawkins KL, Jenkins TC. Multiple-dose dalbavancin regimens as the predominant treatment of deep-seated or endovascular infections: a scoping review. Open Forum Infect Dis. 2021;8(11):ofab486. doi:10.1093/ofid/ofab486
- Baddour LM, Wilson WR, Bayer AS, et al. Infective endocarditis in adults: diagnosis, antimicrobial therapy, and management of complications: a scientific statement for healthcare professionals from the American Heart Association. Circulation. 2015;132(15):1435-1486. doi:10.1161/CIR.0000000000000296
- Berbari EF, Kanj SS, Kowalski TJ, et al. 2015 Infectious Diseases Society of America (IDSA) Clinical Practice Guidelines for the Diagnosis and Treatment of Native Vertebral Osteomyelitis in Adults. Clin Infect Dis. 2015;61(6):e26-46. doi:10.1093/cid/civ482
- Arrieta-Loitegui M, Caro-Teller JM, Ortiz-Pérez S, López- Medrano F, San Juan-Garrido R, Ferrari-Piquero JM. Effectiveness, safety, and cost analysis of dalbavancin in clinical practice. Eur J Hosp Pharm. 2022;29(1):55-58. doi:10.1136/ejhpharm-2020-002315
- Pascale R, Maccaro A, Mikus E, et al. A retrospective multicentre study on dalbavancin effectiveness and cost-evaluation in sternotomic wound infection treatment: DALBA SWIT study. J Glob Antimicrob Resist. 2022;30:390-394. doi:10.1016/j.jgar.2022.07.018
- Antosz K, Al-Hasan MN, Lu ZK, et at. Clinical utility and cost effectiveness of long-acting lipoglycopeptides used in deep seated infections among patients with social and economic barriers to care. Pharmacy (Basel). 2021;10(1):1. doi:10.3390/pharmacy10010001
- Roberts MS. Economic aspects of evaluation. In: Friedman CP, Wyatt JC, eds. Evaluation Methods in Biomedical Informatics. 2nd ed. Springer; 2006:301-337.
- US Department of Veterans Affairs. HERC inpatient average cost data. Updated May 1, 2025. Accessed May 9, 2025. https://www.herc.research.va.gov/include/page.asp?id=inpatient
- US Department of Veterans Affairs. HERC Outpatient average cost dataset. Updated May 1, 2025. Accessed May 9, 2025. https://www.herc.research.va.gov/include/page.asp?id=outpatient
- Ektare V, Khachatryan A, Xue M, Dunne M, Johnson K, Stephens J. Assessing the economic value of avoiding hospital admissions by shifting the management of gram + acute bacterial skin and skin-structure infections to an outpatient care setting. J Med Econ. 2015;18(12):1092-1101. doi:10.3111/13696998.2015.1078339
- Ruh CA, Parameswaran GI, Wojciechowski AL, Mergenhagen KA. Outcomes and pharmacoeconomic analysis of a home intravenous antibiotic infusion program in veterans. Clin Ther. 2015;37(11):2527-2535. doi:10.1016/j.clinthera.2015.09.009
- Nakrani M, Yu D, Skikka M, et al. Comparison of vancomycin and daptomycin complications and interventions in outpatient parenteral antimicrobial therapy. Open Forum Infect Dis. 2020;7(Suppl 1):S361-S362. doi:10.1093/ofid/ofaa439.791
- Scoble PJ, Reilly J, Tilloston GS. Real-world use of oritavancin for the treatment of osteomyelitis. Drugs Real World Outcomes. 2020;7(Suppl 1):46-54. doi:10.1007/s40801-020-00194-8
- Segala D, Barbieri M, Di Nuzzo M, et al. Clinical, organizational, and pharmacoeconomic perspectives of dalbavancin vs standard of care in the infectious disease network. Glob Reg Health Technol Assess. 2024;11(Suppl 2):5-12. doi:10.33393/grhta.2024.3094
- Gómez A, et al. EN-DALBACEN 2.0 Cohort: real-life study of dalbavancin as sequential/consolidation therapy in patients with infective endocarditis due to Gram-positive cocci. Int J Antimicrob Agents. 2023;62(3):106918. doi:10.1016/j.ijantimicag.2023.106918
- LoVecchio F, McCarthy MW, Ye X, et al. Single intravenous dose dalbavancin pathway for the treatment of acute bacterial skin and skin structure infections: considerations for emergency department implementation and cost savings. J Emerg Med. 2024;67(2):e217-e229. doi:10.1016/j.jemermed.2024.03.003
- Gonzalez J, Andrade DC, Niu J. Cost-consequence analysis of single-dose dalbavancin versus standard of care for the treatment of acute bacterial skin and skin structure infections in a multisite healthcare system. Clin Infect Dis. 2021;73(7):e1436-e1442. doi:10.1093/cid/ciaa1732
- Turco NJ, Kane-Gill SL, Hernandez I, Oleksiuk LM, D’Amico F, Pickering AJ. A cost-minimization analysis of dalbavancin compared to conventional therapy for the outpatient treatment of acute bacterial skin and skin-structure infections. Expert Opin Pharmacother. 2018;19(4):319-325. doi:10.1080/14656566.2018.1442439
- Jones TW, Jun AH, Michal JL, Olney WJ. High-dose daptomycin and clinical applications. Ann Pharmacother. 2021;55(11):1363-1378. doi:10.1177/1060028021991943
- Dalvance. Package insert. AbbVie Inc.; 2025.
- Orbactiv. Package insert. Melinta Therapeutics; 2022.
- Cooper CC, Stein GE, Mitra S, Abubaker A, Havlichek DH. Long-acting lipoglycopeptides for the treatment of bone and joint infections. Surg Infect (Larchmt). 2021;22(8):771- 779. doi:10.1089/sur.2020.413
- Simonetti O, Rizzetto G, Molinelli E, Cirioni O, Offidani A. Review: a safety profile of dalbavancin for on- and offlabel utilization. Ther Clin Risk Manag. 2021;17:223-232. doi:10.2147/TCRM.S271445
- Bloem A, Bax HI, Yusuf E, Verkaik NJ. New-generation antibiotics for treatment of gram-positive infections: a review with focus on endocarditis and osteomyelitis. J Clin Med. 2021;10(8):1743. doi:10.3390/jcm10081743
- Thomas G, Henao-Martínez AF, Franco-Paredes C, Chastain DB. Treatment of osteoarticular, cardiovascular, intravascular-catheter-related and other complicated infections with dalbavancin and oritavancin: a systematic review. Int J Antimicrob Agents. 2020;56(3):106069. doi:10.1016/j.ijantimicag.2020.106069
- Rappo U, Puttagunta S, Shevchenko V, et al. Dalbavancin for the treatment of osteomyelitis in adult patients: a randomized clinical trial of efficacy and safety. Open Forum Infect Dis. 2018;6(1):ofy331. doi:10.1093/ofid/ofy331
- Cain AR, Bremmer DN, Carr DR, et al. Effectiveness of dalbavancin compared with standard of care for the treatment of osteomyelitis: a real-world analysis. Open Forum Infect Dis. 2021;9(2):ofab589. doi:10.1093/ofid/ofab589
- Van Hise NW, Chundi V, Didwania V, et al. Treatment of acute osteomyelitis with once-weekly oritavancin: a two-year, multicenter, retrospective study. Drugs Real World Outcomes. 2020;7(Suppl 1):41-45. doi:10.1007/s40801-020-00195-7
- Cooper MM, Preslaski CR, Shihadeh KC, Hawkins KL, Jenkins TC. Multiple-dose dalbavancin regimens as the predominant treatment of deep-seated or endovascular infections: a scoping review. Open Forum Infect Dis. 2021;8(11):ofab486. doi:10.1093/ofid/ofab486
- Baddour LM, Wilson WR, Bayer AS, et al. Infective endocarditis in adults: diagnosis, antimicrobial therapy, and management of complications: a scientific statement for healthcare professionals from the American Heart Association. Circulation. 2015;132(15):1435-1486. doi:10.1161/CIR.0000000000000296
- Berbari EF, Kanj SS, Kowalski TJ, et al. 2015 Infectious Diseases Society of America (IDSA) Clinical Practice Guidelines for the Diagnosis and Treatment of Native Vertebral Osteomyelitis in Adults. Clin Infect Dis. 2015;61(6):e26-46. doi:10.1093/cid/civ482
- Arrieta-Loitegui M, Caro-Teller JM, Ortiz-Pérez S, López- Medrano F, San Juan-Garrido R, Ferrari-Piquero JM. Effectiveness, safety, and cost analysis of dalbavancin in clinical practice. Eur J Hosp Pharm. 2022;29(1):55-58. doi:10.1136/ejhpharm-2020-002315
- Pascale R, Maccaro A, Mikus E, et al. A retrospective multicentre study on dalbavancin effectiveness and cost-evaluation in sternotomic wound infection treatment: DALBA SWIT study. J Glob Antimicrob Resist. 2022;30:390-394. doi:10.1016/j.jgar.2022.07.018
- Antosz K, Al-Hasan MN, Lu ZK, et at. Clinical utility and cost effectiveness of long-acting lipoglycopeptides used in deep seated infections among patients with social and economic barriers to care. Pharmacy (Basel). 2021;10(1):1. doi:10.3390/pharmacy10010001
- Roberts MS. Economic aspects of evaluation. In: Friedman CP, Wyatt JC, eds. Evaluation Methods in Biomedical Informatics. 2nd ed. Springer; 2006:301-337.
- US Department of Veterans Affairs. HERC inpatient average cost data. Updated May 1, 2025. Accessed May 9, 2025. https://www.herc.research.va.gov/include/page.asp?id=inpatient
- US Department of Veterans Affairs. HERC Outpatient average cost dataset. Updated May 1, 2025. Accessed May 9, 2025. https://www.herc.research.va.gov/include/page.asp?id=outpatient
- Ektare V, Khachatryan A, Xue M, Dunne M, Johnson K, Stephens J. Assessing the economic value of avoiding hospital admissions by shifting the management of gram + acute bacterial skin and skin-structure infections to an outpatient care setting. J Med Econ. 2015;18(12):1092-1101. doi:10.3111/13696998.2015.1078339
- Ruh CA, Parameswaran GI, Wojciechowski AL, Mergenhagen KA. Outcomes and pharmacoeconomic analysis of a home intravenous antibiotic infusion program in veterans. Clin Ther. 2015;37(11):2527-2535. doi:10.1016/j.clinthera.2015.09.009
- Nakrani M, Yu D, Skikka M, et al. Comparison of vancomycin and daptomycin complications and interventions in outpatient parenteral antimicrobial therapy. Open Forum Infect Dis. 2020;7(Suppl 1):S361-S362. doi:10.1093/ofid/ofaa439.791
- Scoble PJ, Reilly J, Tilloston GS. Real-world use of oritavancin for the treatment of osteomyelitis. Drugs Real World Outcomes. 2020;7(Suppl 1):46-54. doi:10.1007/s40801-020-00194-8
- Segala D, Barbieri M, Di Nuzzo M, et al. Clinical, organizational, and pharmacoeconomic perspectives of dalbavancin vs standard of care in the infectious disease network. Glob Reg Health Technol Assess. 2024;11(Suppl 2):5-12. doi:10.33393/grhta.2024.3094
- Gómez A, et al. EN-DALBACEN 2.0 Cohort: real-life study of dalbavancin as sequential/consolidation therapy in patients with infective endocarditis due to Gram-positive cocci. Int J Antimicrob Agents. 2023;62(3):106918. doi:10.1016/j.ijantimicag.2023.106918
- LoVecchio F, McCarthy MW, Ye X, et al. Single intravenous dose dalbavancin pathway for the treatment of acute bacterial skin and skin structure infections: considerations for emergency department implementation and cost savings. J Emerg Med. 2024;67(2):e217-e229. doi:10.1016/j.jemermed.2024.03.003
- Gonzalez J, Andrade DC, Niu J. Cost-consequence analysis of single-dose dalbavancin versus standard of care for the treatment of acute bacterial skin and skin structure infections in a multisite healthcare system. Clin Infect Dis. 2021;73(7):e1436-e1442. doi:10.1093/cid/ciaa1732
- Turco NJ, Kane-Gill SL, Hernandez I, Oleksiuk LM, D’Amico F, Pickering AJ. A cost-minimization analysis of dalbavancin compared to conventional therapy for the outpatient treatment of acute bacterial skin and skin-structure infections. Expert Opin Pharmacother. 2018;19(4):319-325. doi:10.1080/14656566.2018.1442439
- Jones TW, Jun AH, Michal JL, Olney WJ. High-dose daptomycin and clinical applications. Ann Pharmacother. 2021;55(11):1363-1378. doi:10.1177/1060028021991943
Are Oritavancin and Dalbavancin More Cost Effective for Outpatient Parenteral Antimicrobial Therapy at a Veterans Affairs Medical Center?
Are Oritavancin and Dalbavancin More Cost Effective for Outpatient Parenteral Antimicrobial Therapy at a Veterans Affairs Medical Center?