User login
Resmetirom Reduces Liver Stiffness in MASH Cirrhosis
PHOENIX — according to the results of a new study.
As well as showing sustained reduction in liver stiffness on vibration-controlled transient elastography (VCTE) after 2 years of treatment with resmetirom, the study suggested that up to 35% of patients could “potentially reverse their cirrhosis,” said lead author Naim Alkhouri, MD, chief medical officer and director of the steatotic liver program at Arizona Liver Health in Phoenix.
Alkhouri presented data on patients with compensated cirrhosis from a 1-year open-label extension of the already-completed MAESTRO-NAFLD-1 study at American College of Gastroenterology (ACG) 2025 Annual Scientific Meeting.
The FDA approved resmetirom (Rezdiffra, Madrigal Pharmaceuticals) in 2024 for MASH and moderate-to-advanced liver fibrosis (consistent with stage F2 and F3 disease), to be used in conjunction with diet and exercise. The agency granted the once-daily, oral thyroid hormone receptor beta-selective agonist breakthrough therapy designation and priority review.
According to the American Liver Foundation, about 5% of adults in the US have MASH — one of the leading causes of liver transplantation in the country. There is currently no FDA-approved therapy for compensated cirrhosis caused by MASH, said Alkhouri. Patients with MASH cirrhosis with clinically significant portal hypertension (CSPH) experience major adverse liver outcomes.
In an analysis of 122 patients with Child Pugh A MASH cirrhosis who completed both a year in an open-label arm of MAESTRO-NAFLD-1 and a 1-year extension, 113 (93%) completed 2 years of treatment with resmetirom (80 mg). Of the 122 patients, only 114 received MRI proton density fat fraction (MRI-PDFF) testing — 93 (82%) had a baseline of > 5% indicating cirrhosis, while 21 (18%) had an MRI-PDFF of < 5%.
Patients were assessed for baseline portal hypertension (Baveno VII) with FibroScan VCTE and platelet count, which was confirmed using magnetic resonance elastography (MRE). Noninvasive biomarkers and imaging were analyzed at baseline and out to 2 years.
At baseline, 63% of patients were categorized as probable/definitive CSPH (Baveno VII). At 1 year of treatment with resmetirom, 20% of patients who were CSPH positive no longer met the criteria, and at 2 years this number had increased to 28%.
After 2 years of treatment, more than half of the patients had a sustained reduction in liver stiffness of more than 25%, as measured by VCTE; and 35% of patients with confirmed F4 at baseline (liver biopsy F4 and/or platelets < 140/MRE ≥ 5 with VCTE ≥ 15) had a conversion to F3.
Patients taking resmetirom also had significant improvements in MRI-PDFF and MRE at 2 years. Almost a third of those with a baseline MRI-PDFF > 5% improved, while 43% of those with a baseline of < 5% improved.
Although 113 patients had an adverse event — primarily gastrointestinal — the observed events were consistent with previous studies. Twenty-seven patients had a serious adverse event, but none were related to the study drug, said Alkhouri. The researchers reported that only 8% of patients discontinued the medication.
Changing the Treatment Landscape for MASH-Related Cirrhosis
When asked to comment by GI & Hepatology News, Hazem Ayesh, MD, an endocrinologist at Deaconess Health System, Evansville, Indiana, said that “reversal of cirrhosis from F4 to F3 and reduction of portal hypertension are quite surprising, since cirrhosis typically progresses slowly.”
Ayesh said it was notable that the researchers had used imaging to confirm both functional and hemodynamic improvements in liver architecture not just biochemical changes. Given the results, “clinicians may reasonably consider off-label use in selected compensated patients until more outcome data become available,” he said.
A phase 3 study is underway to examine those outcomes, MAESTRO-NASH OUTCOMES, with 845 patients with MASH cirrhosis, and should be completed in 2027.
“Resmetirom could change the treatment landscape for MASH-related cirrhosis,” said Ayesh, adding, “this drug offers a chance to target the disease process itself,” while other therapies focus on preventing complications.
“For patients without access to liver transplant, a therapy that can slow or reverse disease progression could be transformative,” he told GI & Hepatology News.
Alkhouri disclosed that he is a consultant and speaker for Madrigal Pharmaceuticals. Three coauthors are Madrigal employees and own stock options in the company. Two coauthors are Madrigal consultants and advisers. Ayesh reported no conflicts.
A version of this article appeared on Medscape.com.
PHOENIX — according to the results of a new study.
As well as showing sustained reduction in liver stiffness on vibration-controlled transient elastography (VCTE) after 2 years of treatment with resmetirom, the study suggested that up to 35% of patients could “potentially reverse their cirrhosis,” said lead author Naim Alkhouri, MD, chief medical officer and director of the steatotic liver program at Arizona Liver Health in Phoenix.
Alkhouri presented data on patients with compensated cirrhosis from a 1-year open-label extension of the already-completed MAESTRO-NAFLD-1 study at American College of Gastroenterology (ACG) 2025 Annual Scientific Meeting.
The FDA approved resmetirom (Rezdiffra, Madrigal Pharmaceuticals) in 2024 for MASH and moderate-to-advanced liver fibrosis (consistent with stage F2 and F3 disease), to be used in conjunction with diet and exercise. The agency granted the once-daily, oral thyroid hormone receptor beta-selective agonist breakthrough therapy designation and priority review.
According to the American Liver Foundation, about 5% of adults in the US have MASH — one of the leading causes of liver transplantation in the country. There is currently no FDA-approved therapy for compensated cirrhosis caused by MASH, said Alkhouri. Patients with MASH cirrhosis with clinically significant portal hypertension (CSPH) experience major adverse liver outcomes.
In an analysis of 122 patients with Child Pugh A MASH cirrhosis who completed both a year in an open-label arm of MAESTRO-NAFLD-1 and a 1-year extension, 113 (93%) completed 2 years of treatment with resmetirom (80 mg). Of the 122 patients, only 114 received MRI proton density fat fraction (MRI-PDFF) testing — 93 (82%) had a baseline of > 5% indicating cirrhosis, while 21 (18%) had an MRI-PDFF of < 5%.
Patients were assessed for baseline portal hypertension (Baveno VII) with FibroScan VCTE and platelet count, which was confirmed using magnetic resonance elastography (MRE). Noninvasive biomarkers and imaging were analyzed at baseline and out to 2 years.
At baseline, 63% of patients were categorized as probable/definitive CSPH (Baveno VII). At 1 year of treatment with resmetirom, 20% of patients who were CSPH positive no longer met the criteria, and at 2 years this number had increased to 28%.
After 2 years of treatment, more than half of the patients had a sustained reduction in liver stiffness of more than 25%, as measured by VCTE; and 35% of patients with confirmed F4 at baseline (liver biopsy F4 and/or platelets < 140/MRE ≥ 5 with VCTE ≥ 15) had a conversion to F3.
Patients taking resmetirom also had significant improvements in MRI-PDFF and MRE at 2 years. Almost a third of those with a baseline MRI-PDFF > 5% improved, while 43% of those with a baseline of < 5% improved.
Although 113 patients had an adverse event — primarily gastrointestinal — the observed events were consistent with previous studies. Twenty-seven patients had a serious adverse event, but none were related to the study drug, said Alkhouri. The researchers reported that only 8% of patients discontinued the medication.
Changing the Treatment Landscape for MASH-Related Cirrhosis
When asked to comment by GI & Hepatology News, Hazem Ayesh, MD, an endocrinologist at Deaconess Health System, Evansville, Indiana, said that “reversal of cirrhosis from F4 to F3 and reduction of portal hypertension are quite surprising, since cirrhosis typically progresses slowly.”
Ayesh said it was notable that the researchers had used imaging to confirm both functional and hemodynamic improvements in liver architecture not just biochemical changes. Given the results, “clinicians may reasonably consider off-label use in selected compensated patients until more outcome data become available,” he said.
A phase 3 study is underway to examine those outcomes, MAESTRO-NASH OUTCOMES, with 845 patients with MASH cirrhosis, and should be completed in 2027.
“Resmetirom could change the treatment landscape for MASH-related cirrhosis,” said Ayesh, adding, “this drug offers a chance to target the disease process itself,” while other therapies focus on preventing complications.
“For patients without access to liver transplant, a therapy that can slow or reverse disease progression could be transformative,” he told GI & Hepatology News.
Alkhouri disclosed that he is a consultant and speaker for Madrigal Pharmaceuticals. Three coauthors are Madrigal employees and own stock options in the company. Two coauthors are Madrigal consultants and advisers. Ayesh reported no conflicts.
A version of this article appeared on Medscape.com.
PHOENIX — according to the results of a new study.
As well as showing sustained reduction in liver stiffness on vibration-controlled transient elastography (VCTE) after 2 years of treatment with resmetirom, the study suggested that up to 35% of patients could “potentially reverse their cirrhosis,” said lead author Naim Alkhouri, MD, chief medical officer and director of the steatotic liver program at Arizona Liver Health in Phoenix.
Alkhouri presented data on patients with compensated cirrhosis from a 1-year open-label extension of the already-completed MAESTRO-NAFLD-1 study at American College of Gastroenterology (ACG) 2025 Annual Scientific Meeting.
The FDA approved resmetirom (Rezdiffra, Madrigal Pharmaceuticals) in 2024 for MASH and moderate-to-advanced liver fibrosis (consistent with stage F2 and F3 disease), to be used in conjunction with diet and exercise. The agency granted the once-daily, oral thyroid hormone receptor beta-selective agonist breakthrough therapy designation and priority review.
According to the American Liver Foundation, about 5% of adults in the US have MASH — one of the leading causes of liver transplantation in the country. There is currently no FDA-approved therapy for compensated cirrhosis caused by MASH, said Alkhouri. Patients with MASH cirrhosis with clinically significant portal hypertension (CSPH) experience major adverse liver outcomes.
In an analysis of 122 patients with Child Pugh A MASH cirrhosis who completed both a year in an open-label arm of MAESTRO-NAFLD-1 and a 1-year extension, 113 (93%) completed 2 years of treatment with resmetirom (80 mg). Of the 122 patients, only 114 received MRI proton density fat fraction (MRI-PDFF) testing — 93 (82%) had a baseline of > 5% indicating cirrhosis, while 21 (18%) had an MRI-PDFF of < 5%.
Patients were assessed for baseline portal hypertension (Baveno VII) with FibroScan VCTE and platelet count, which was confirmed using magnetic resonance elastography (MRE). Noninvasive biomarkers and imaging were analyzed at baseline and out to 2 years.
At baseline, 63% of patients were categorized as probable/definitive CSPH (Baveno VII). At 1 year of treatment with resmetirom, 20% of patients who were CSPH positive no longer met the criteria, and at 2 years this number had increased to 28%.
After 2 years of treatment, more than half of the patients had a sustained reduction in liver stiffness of more than 25%, as measured by VCTE; and 35% of patients with confirmed F4 at baseline (liver biopsy F4 and/or platelets < 140/MRE ≥ 5 with VCTE ≥ 15) had a conversion to F3.
Patients taking resmetirom also had significant improvements in MRI-PDFF and MRE at 2 years. Almost a third of those with a baseline MRI-PDFF > 5% improved, while 43% of those with a baseline of < 5% improved.
Although 113 patients had an adverse event — primarily gastrointestinal — the observed events were consistent with previous studies. Twenty-seven patients had a serious adverse event, but none were related to the study drug, said Alkhouri. The researchers reported that only 8% of patients discontinued the medication.
Changing the Treatment Landscape for MASH-Related Cirrhosis
When asked to comment by GI & Hepatology News, Hazem Ayesh, MD, an endocrinologist at Deaconess Health System, Evansville, Indiana, said that “reversal of cirrhosis from F4 to F3 and reduction of portal hypertension are quite surprising, since cirrhosis typically progresses slowly.”
Ayesh said it was notable that the researchers had used imaging to confirm both functional and hemodynamic improvements in liver architecture not just biochemical changes. Given the results, “clinicians may reasonably consider off-label use in selected compensated patients until more outcome data become available,” he said.
A phase 3 study is underway to examine those outcomes, MAESTRO-NASH OUTCOMES, with 845 patients with MASH cirrhosis, and should be completed in 2027.
“Resmetirom could change the treatment landscape for MASH-related cirrhosis,” said Ayesh, adding, “this drug offers a chance to target the disease process itself,” while other therapies focus on preventing complications.
“For patients without access to liver transplant, a therapy that can slow or reverse disease progression could be transformative,” he told GI & Hepatology News.
Alkhouri disclosed that he is a consultant and speaker for Madrigal Pharmaceuticals. Three coauthors are Madrigal employees and own stock options in the company. Two coauthors are Madrigal consultants and advisers. Ayesh reported no conflicts.
A version of this article appeared on Medscape.com.
FROM ACG 2025
The Litter Olympics: Addressing Individual Critical Tasks Lists Requirements in a Forward-Deployed Setting
The Litter Olympics: Addressing Individual Critical Tasks Lists Requirements in a Forward-Deployed Setting
Military medical personnel rely on individual critical tasks lists (ICTLs) to maintain proficiency in essential medical skills during deployments. However, sustaining these competencies in a low-casualty operational setting presents unique challenges. Traditional training methods, such as lectures or simulations outside operational contexts, may lack engagement and fail to replicate the stressors of real-world scenarios. Previous research has emphasized the importance of continuous medical readiness training in austere environments, highlighting the need for innovative approaches.1,2
The Litter Olympics was developed as an in-theater training exercise designed to enhance medical readiness, foster interdisciplinary teamwork, and incorporate physical exertion into skill maintenance. By requiring teams to carry a patient litter through multiple “events,” the exercise reinforced teamwork within a medical readiness-focused series inspired by an Olympic decathlon. This article discusses the feasibility, effectiveness, and potential impact of the Litter Olympics as a training tool for maintaining ICTLs in a deployed environment.
Program
The Litter Olympics were implemented at a Role 3 medical facility in Baghdad, Iraq, where teams composed of individuals from military occupational specialties (MOSs) and areas of concentration (AOCs) participated. Role 3 facilities provide specialty surgical and critical care capabilities, enabling a robust medical training environment.3 The event was designed to reflect the interdisciplinary nature of deployed medical teams and incorporated hands-on training stations covering critical medical skills such as traction splinting, spinal precautions, patient movement, hemorrhage control, airway management, and tactical evacuation procedures.
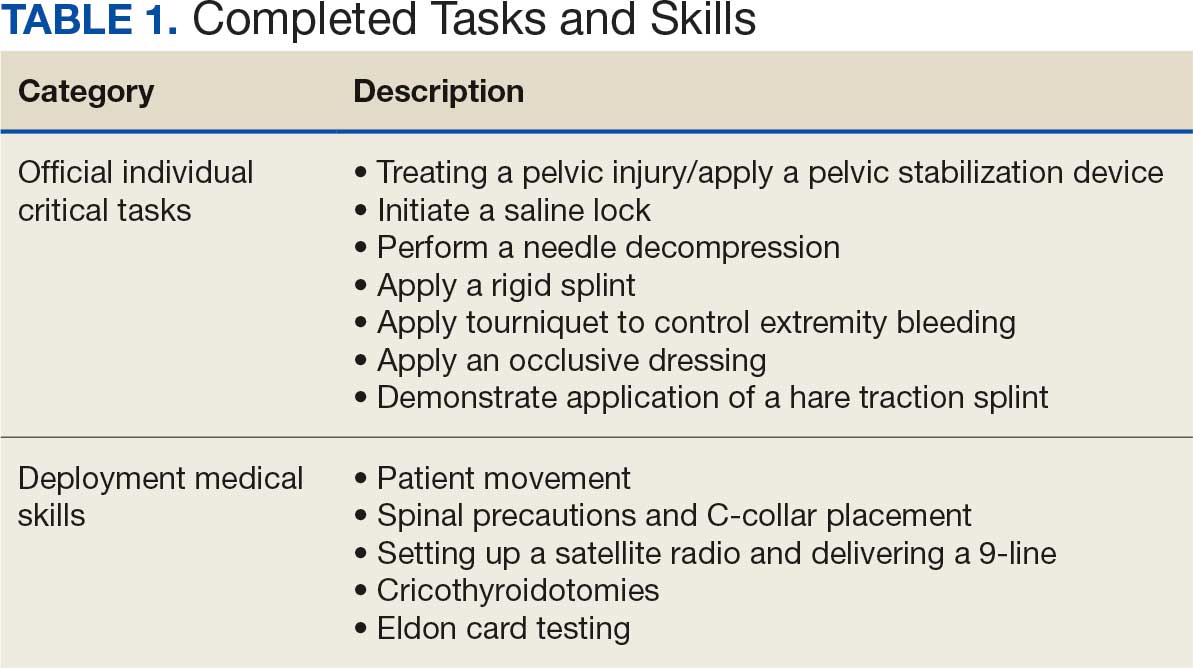
Tasks were selected based on their relevance to deployed medical care and their inclusion in ICTLs, ensuring alignment with mission-essential skills. Participants were evaluated on task completion, efficiency, and teamwork by experienced medical personnel. Postexercise surveys assessed skill improvement, confidence levels, and areas for refinement. Future studies should incorporate structured performance metrics, such as pre- and postevent evaluations, to quantify proficiency gains (Table 1).
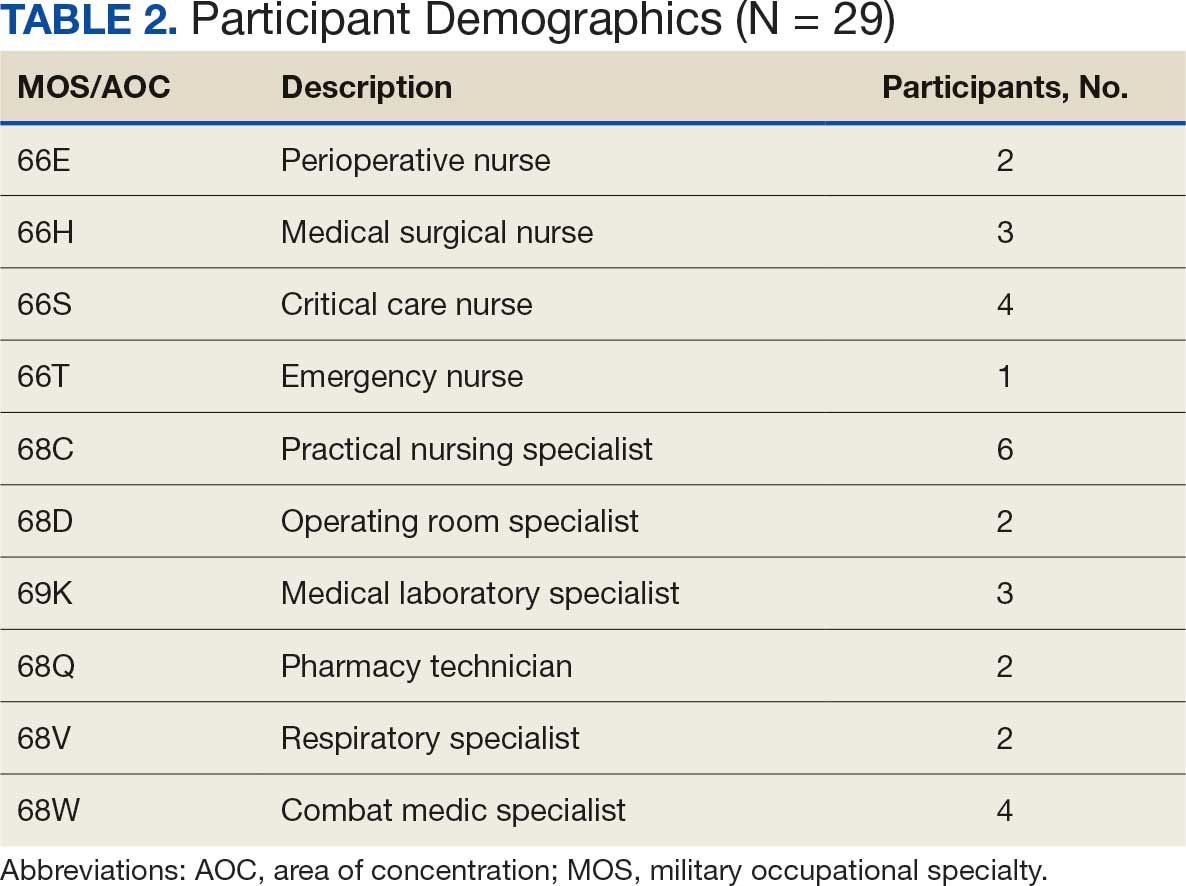
Five mixed MOS/AOC teams participated in the event, completing the exercise in an average time of 50 minutes (Table 2). Participants reported increased confidence in performing ICTs, particularly in patient movement, hemorrhage control, and airway management. The interdisciplinary nature of the teams facilitated peer teaching and cross-training, allowing individuals to better understand each other’s roles and responsibilities. This mirrors findings in previous studies on predeployment training that emphasize the importance of collaborative, hands-on learning.4 The physical aspect of the exercise was well received, as it simulated operational conditions and reinforced endurance in high-stress environments. Some tasks, such as cricothyroidotomy and satellite radio setup, required additional instruction, highlighting areas for improvement in future iterations.
Discussion
The Litter Olympics provide a dynamic alternative to traditional classroom instruction by integrating realistic, scenario-based training. However, several limitations were identified. The most significant was the lack of formalized outcome metrics. While qualitative feedback was overwhelmingly positive, no structured performance assessment tool, such as pre- and postevent skill evaluations, was used. Future studies should incorporate objective measures of competency to strengthen the evidence base for this training model. Additionally, participant feedback suggested that more structured debriefing sessions postexercise would enhance learning retention and provide actionable insights for future program modifications.
Another consideration is the scalability and adaptability of the exercise. While effective in a Role 3 setting, modifications may be required for smaller units or lower levels of care. Future iterations could adapt the format for Role 1 or 2 environments by reducing the number of stations while preserving the core training elements. Furthermore, the event relied on access to specialized personnel and equipment, which may not always be feasible in austere settings. Developing a streamlined version focusing on essential tasks could improve accessibility and sustainability across different operational environments.
Participants expressed a preference for this hands-on, competitive training model over traditional didactic instruction. However, further research should compare skill retention rates between the Litter Olympics and other training modalities to validate effectiveness. While peer teaching was a notable strength of the event, structured mentorship from senior medical personnel could further enhance skill acquisition and reinforce best practices.
Conclusions
The Litter Olympics present a reproducible, engaging, and effective method for sustaining medical readiness in a deployed Role 3 setting. By fostering interdisciplinary collaboration and incorporating physical and cognitive stressors, it enhances both individual and team preparedness. Future research should develop standardized, measurable outcome assessments, explore application in diverse deployment settings, and optimize scalability for broader military medical training programs. Standardized evaluation tools should be developed to quantify performance improvements, and the training model should be expanded to include lower levels of care and nonmedical personnel. Structured debriefing sessions would also provide valuable insight into lessons learned and potential refinements. By integrating these enhancements, the Litter Olympics can serve as a cornerstone for maintaining operational medical readiness in deployed environments.
- Suresh MR, Valdez-Delgado KK, Staudt AM, et al. An assessment of pre-deployment training for army nurses and medics. Mil Med. 2021;186:203-211. doi:10.1093/milmed/usaa291
- Mead KC, Tennent DJ, Stinner DJ. The importance of medical readiness training exercises: maintaining medical readiness in a low-volume combat casualty flow era. Mil Med. 2017;182:e1734-e1737. doi:10.7205/milmed-d-16-00335
- Brisebois R, Hennecke P, Kao R, et al. The Role 3 multinational medical nit at Kandahar airfield 2005–2010. Can J Surg. 2011;54:S124-S129. doi:10.1503/cjs.024811
- Huh J, Brockmeyer JR, Bertsch SR, et al. Conducting pre-deployment training in Honduras: the 240th forward resuscitative surgical team experience. Mil Med. 2021;187:e690-e695. doi:10.1093/milmed/usaa545
Military medical personnel rely on individual critical tasks lists (ICTLs) to maintain proficiency in essential medical skills during deployments. However, sustaining these competencies in a low-casualty operational setting presents unique challenges. Traditional training methods, such as lectures or simulations outside operational contexts, may lack engagement and fail to replicate the stressors of real-world scenarios. Previous research has emphasized the importance of continuous medical readiness training in austere environments, highlighting the need for innovative approaches.1,2
The Litter Olympics was developed as an in-theater training exercise designed to enhance medical readiness, foster interdisciplinary teamwork, and incorporate physical exertion into skill maintenance. By requiring teams to carry a patient litter through multiple “events,” the exercise reinforced teamwork within a medical readiness-focused series inspired by an Olympic decathlon. This article discusses the feasibility, effectiveness, and potential impact of the Litter Olympics as a training tool for maintaining ICTLs in a deployed environment.
Program
The Litter Olympics were implemented at a Role 3 medical facility in Baghdad, Iraq, where teams composed of individuals from military occupational specialties (MOSs) and areas of concentration (AOCs) participated. Role 3 facilities provide specialty surgical and critical care capabilities, enabling a robust medical training environment.3 The event was designed to reflect the interdisciplinary nature of deployed medical teams and incorporated hands-on training stations covering critical medical skills such as traction splinting, spinal precautions, patient movement, hemorrhage control, airway management, and tactical evacuation procedures.
Tasks were selected based on their relevance to deployed medical care and their inclusion in ICTLs, ensuring alignment with mission-essential skills. Participants were evaluated on task completion, efficiency, and teamwork by experienced medical personnel. Postexercise surveys assessed skill improvement, confidence levels, and areas for refinement. Future studies should incorporate structured performance metrics, such as pre- and postevent evaluations, to quantify proficiency gains (Table 1).

Five mixed MOS/AOC teams participated in the event, completing the exercise in an average time of 50 minutes (Table 2). Participants reported increased confidence in performing ICTs, particularly in patient movement, hemorrhage control, and airway management. The interdisciplinary nature of the teams facilitated peer teaching and cross-training, allowing individuals to better understand each other’s roles and responsibilities. This mirrors findings in previous studies on predeployment training that emphasize the importance of collaborative, hands-on learning.4 The physical aspect of the exercise was well received, as it simulated operational conditions and reinforced endurance in high-stress environments. Some tasks, such as cricothyroidotomy and satellite radio setup, required additional instruction, highlighting areas for improvement in future iterations.

Discussion
The Litter Olympics provide a dynamic alternative to traditional classroom instruction by integrating realistic, scenario-based training. However, several limitations were identified. The most significant was the lack of formalized outcome metrics. While qualitative feedback was overwhelmingly positive, no structured performance assessment tool, such as pre- and postevent skill evaluations, was used. Future studies should incorporate objective measures of competency to strengthen the evidence base for this training model. Additionally, participant feedback suggested that more structured debriefing sessions postexercise would enhance learning retention and provide actionable insights for future program modifications.
Another consideration is the scalability and adaptability of the exercise. While effective in a Role 3 setting, modifications may be required for smaller units or lower levels of care. Future iterations could adapt the format for Role 1 or 2 environments by reducing the number of stations while preserving the core training elements. Furthermore, the event relied on access to specialized personnel and equipment, which may not always be feasible in austere settings. Developing a streamlined version focusing on essential tasks could improve accessibility and sustainability across different operational environments.
Participants expressed a preference for this hands-on, competitive training model over traditional didactic instruction. However, further research should compare skill retention rates between the Litter Olympics and other training modalities to validate effectiveness. While peer teaching was a notable strength of the event, structured mentorship from senior medical personnel could further enhance skill acquisition and reinforce best practices.
Conclusions
The Litter Olympics present a reproducible, engaging, and effective method for sustaining medical readiness in a deployed Role 3 setting. By fostering interdisciplinary collaboration and incorporating physical and cognitive stressors, it enhances both individual and team preparedness. Future research should develop standardized, measurable outcome assessments, explore application in diverse deployment settings, and optimize scalability for broader military medical training programs. Standardized evaluation tools should be developed to quantify performance improvements, and the training model should be expanded to include lower levels of care and nonmedical personnel. Structured debriefing sessions would also provide valuable insight into lessons learned and potential refinements. By integrating these enhancements, the Litter Olympics can serve as a cornerstone for maintaining operational medical readiness in deployed environments.
Military medical personnel rely on individual critical tasks lists (ICTLs) to maintain proficiency in essential medical skills during deployments. However, sustaining these competencies in a low-casualty operational setting presents unique challenges. Traditional training methods, such as lectures or simulations outside operational contexts, may lack engagement and fail to replicate the stressors of real-world scenarios. Previous research has emphasized the importance of continuous medical readiness training in austere environments, highlighting the need for innovative approaches.1,2
The Litter Olympics was developed as an in-theater training exercise designed to enhance medical readiness, foster interdisciplinary teamwork, and incorporate physical exertion into skill maintenance. By requiring teams to carry a patient litter through multiple “events,” the exercise reinforced teamwork within a medical readiness-focused series inspired by an Olympic decathlon. This article discusses the feasibility, effectiveness, and potential impact of the Litter Olympics as a training tool for maintaining ICTLs in a deployed environment.
Program
The Litter Olympics were implemented at a Role 3 medical facility in Baghdad, Iraq, where teams composed of individuals from military occupational specialties (MOSs) and areas of concentration (AOCs) participated. Role 3 facilities provide specialty surgical and critical care capabilities, enabling a robust medical training environment.3 The event was designed to reflect the interdisciplinary nature of deployed medical teams and incorporated hands-on training stations covering critical medical skills such as traction splinting, spinal precautions, patient movement, hemorrhage control, airway management, and tactical evacuation procedures.
Tasks were selected based on their relevance to deployed medical care and their inclusion in ICTLs, ensuring alignment with mission-essential skills. Participants were evaluated on task completion, efficiency, and teamwork by experienced medical personnel. Postexercise surveys assessed skill improvement, confidence levels, and areas for refinement. Future studies should incorporate structured performance metrics, such as pre- and postevent evaluations, to quantify proficiency gains (Table 1).

Five mixed MOS/AOC teams participated in the event, completing the exercise in an average time of 50 minutes (Table 2). Participants reported increased confidence in performing ICTs, particularly in patient movement, hemorrhage control, and airway management. The interdisciplinary nature of the teams facilitated peer teaching and cross-training, allowing individuals to better understand each other’s roles and responsibilities. This mirrors findings in previous studies on predeployment training that emphasize the importance of collaborative, hands-on learning.4 The physical aspect of the exercise was well received, as it simulated operational conditions and reinforced endurance in high-stress environments. Some tasks, such as cricothyroidotomy and satellite radio setup, required additional instruction, highlighting areas for improvement in future iterations.

Discussion
The Litter Olympics provide a dynamic alternative to traditional classroom instruction by integrating realistic, scenario-based training. However, several limitations were identified. The most significant was the lack of formalized outcome metrics. While qualitative feedback was overwhelmingly positive, no structured performance assessment tool, such as pre- and postevent skill evaluations, was used. Future studies should incorporate objective measures of competency to strengthen the evidence base for this training model. Additionally, participant feedback suggested that more structured debriefing sessions postexercise would enhance learning retention and provide actionable insights for future program modifications.
Another consideration is the scalability and adaptability of the exercise. While effective in a Role 3 setting, modifications may be required for smaller units or lower levels of care. Future iterations could adapt the format for Role 1 or 2 environments by reducing the number of stations while preserving the core training elements. Furthermore, the event relied on access to specialized personnel and equipment, which may not always be feasible in austere settings. Developing a streamlined version focusing on essential tasks could improve accessibility and sustainability across different operational environments.
Participants expressed a preference for this hands-on, competitive training model over traditional didactic instruction. However, further research should compare skill retention rates between the Litter Olympics and other training modalities to validate effectiveness. While peer teaching was a notable strength of the event, structured mentorship from senior medical personnel could further enhance skill acquisition and reinforce best practices.
Conclusions
The Litter Olympics present a reproducible, engaging, and effective method for sustaining medical readiness in a deployed Role 3 setting. By fostering interdisciplinary collaboration and incorporating physical and cognitive stressors, it enhances both individual and team preparedness. Future research should develop standardized, measurable outcome assessments, explore application in diverse deployment settings, and optimize scalability for broader military medical training programs. Standardized evaluation tools should be developed to quantify performance improvements, and the training model should be expanded to include lower levels of care and nonmedical personnel. Structured debriefing sessions would also provide valuable insight into lessons learned and potential refinements. By integrating these enhancements, the Litter Olympics can serve as a cornerstone for maintaining operational medical readiness in deployed environments.
- Suresh MR, Valdez-Delgado KK, Staudt AM, et al. An assessment of pre-deployment training for army nurses and medics. Mil Med. 2021;186:203-211. doi:10.1093/milmed/usaa291
- Mead KC, Tennent DJ, Stinner DJ. The importance of medical readiness training exercises: maintaining medical readiness in a low-volume combat casualty flow era. Mil Med. 2017;182:e1734-e1737. doi:10.7205/milmed-d-16-00335
- Brisebois R, Hennecke P, Kao R, et al. The Role 3 multinational medical nit at Kandahar airfield 2005–2010. Can J Surg. 2011;54:S124-S129. doi:10.1503/cjs.024811
- Huh J, Brockmeyer JR, Bertsch SR, et al. Conducting pre-deployment training in Honduras: the 240th forward resuscitative surgical team experience. Mil Med. 2021;187:e690-e695. doi:10.1093/milmed/usaa545
- Suresh MR, Valdez-Delgado KK, Staudt AM, et al. An assessment of pre-deployment training for army nurses and medics. Mil Med. 2021;186:203-211. doi:10.1093/milmed/usaa291
- Mead KC, Tennent DJ, Stinner DJ. The importance of medical readiness training exercises: maintaining medical readiness in a low-volume combat casualty flow era. Mil Med. 2017;182:e1734-e1737. doi:10.7205/milmed-d-16-00335
- Brisebois R, Hennecke P, Kao R, et al. The Role 3 multinational medical nit at Kandahar airfield 2005–2010. Can J Surg. 2011;54:S124-S129. doi:10.1503/cjs.024811
- Huh J, Brockmeyer JR, Bertsch SR, et al. Conducting pre-deployment training in Honduras: the 240th forward resuscitative surgical team experience. Mil Med. 2021;187:e690-e695. doi:10.1093/milmed/usaa545
The Litter Olympics: Addressing Individual Critical Tasks Lists Requirements in a Forward-Deployed Setting
The Litter Olympics: Addressing Individual Critical Tasks Lists Requirements in a Forward-Deployed Setting
Patients With a Positive FIT Fail to Get Follow-Up Colonoscopies
PHOENIX — Patients with or without polyp removal in an index colonoscopy commonly receive follow-up surveillance with a fecal immunochemical test (FIT), yet many of these patients do not receive a recommended colonoscopy after a positive FIT.
“In this large US study, we found interval FITs are frequently performed in patients with and without prior polypectomy,” said first author Natalie J. Wilson, MD, of the University of Minnesota in Minneapolis, while presenting the findings at the American College of Gastroenterology (ACG) 2025 Annual Scientific Meeting.
“ and colorectal cancer, regardless of polypectomy history,” Wilson said.
Guideline recommendations stress the need for follow-up surveillance with a colonoscopy, particularly in patients who have had a prior polypectomy, because of the higher risk.
Reasons patients may instead turn to FIT may include cost or other factors, she said.
To determine just how often that happens, how having a previous polypectomy affects FIT results, and how adherent patients are to follow up if a FIT result is positive, Wilson and her colleagues evaluated data from nearly 4.8 million individuals in the Veterans Health Administration Corporate Data Warehouse who underwent colonoscopy between 2000 and 2024.
Of the patients, 10.9% were found to have subsequently received interval FIT within 10 years of the index colonoscopy, and of those patients, nearly half (49.9%) had received a polypectomy at the index colonoscopy.
The average time from the colonoscopy/polypectomy to the interval FIT was 5.9 years (5.6 years in the polypectomy group vs 6.2 years in the non-polypectomy group).
Among the FIT screenings, results were positive in 17.2% of post-polypectomy patients and 14.1% of patients with no prior polypectomy, indicating a history of polypectomy to be predictive of a positive interval FIT (odds ratio [OR], 1.12; P < .0001).
Notably, while a follow-up colonoscopy is considered essential following a positive FIT result — and having a previous polypectomy should add further urgency to the matter — the study showed only 50.4% of those who had an earlier polypectomy went on to receive the recommended follow-up colonoscopy after a positive follow-up FIT, and the rate was 49.3% among those who had not received a polypectomy (P = .001).
For those who did receive a follow-up colonoscopy after a positive FIT, the duration of time to receiving the colonoscopy was longer among those who had a prior polypectomy, at 2.9 months compared with 2.5 months in the non-polypectomy group (P < .001).
Colonoscopy results following a positive FIT showed higher rates of detections among patients who had prior polypectomies than among those with no prior polypectomy, including tubular adenomas (54.7% vs 45.8%), tubulovillous adenomas (5.6% vs 4.7%), adenomas with high-grade dysplasia (0.8% vs 0.7%), sessile serrated lesions (3.52% vs 2.4%), advanced colorectal neoplasia (9.2% vs 7.9%), and colorectal cancer (3.3% vs 3.0%).
However, a prior polypectomy was not independently predictive of colorectal cancer (OR, 0.96; P = .65) or advanced colorectal neoplasia (OR, 0.97; P = .57) in the post-colonoscopy interval FIT.
The findings underscore that “positive results carried a high risk of advanced neoplasia or cancer, irrespective of prior polypectomy history,” Wilson said.
Clinicians Must ‘Do a Better Job’
Commenting on the study, William D. Chey, MD, AGAF, chief of the Division of Gastroenterology & Hepatology at the University of Michigan in Ann Arbor, noted that the study “addresses one of the biggest challenges we face as a profession, which is making sure that patients who have a positive stool test get a colonoscopy.”
He noted that the low rate of just 50% of recipients of positive FITs going on to receive a colonoscopy is consistent with what is observed in other trials.
“Other data suggests that the rate might even be significantly higher — at 70%-80%, depending upon the population and the test,” Chey told Medscape Medical News.
Reasons for the failure to receive the follow-up testing range from income restrictions (due to the high cost of a colonoscopy, especially if not covered by insurance), education, speaking a foreign language, and other factors, he said.
The relatively high rates of colon cancers detected by FIT in the study, in those with and without a prior polypectomy, along with findings from other studies “should raise questions about whether there might be a role for FIT testing in addition to colonoscopy.” However, much stronger evidence would be needed, Chey noted.
In the meantime, a key issue is “how do we do a better job of making sure that individuals who have a positive FIT test get a colonoscopy,” he said.
“I think a lot of this is going to come down to how it’s done at the primary care level.”
Chey added that in that, and any other setting, “the main message that needs to get out to people who are undergoing stool-based screening is that the stool test is only the first part of the screening process, and if it’s positive, a follow-up colonoscopy must be performed.”
“Otherwise, the stool-based test is of no value.”
Wilson had no disclosures to report. Chey’s disclosures included consulting and/or other relationships with Ardelyx, Atmo, Biomerica, Commonwealth Diagnostics International, Corprata, Dieta, Evinature, Food Marble, Gemelli, Kiwi BioScience, Modify Health, Nestlé, Phathom, Redhill, Salix/Valeant, Takeda, and Vibrant.
A version of this article appeared on Medscape.com .
PHOENIX — Patients with or without polyp removal in an index colonoscopy commonly receive follow-up surveillance with a fecal immunochemical test (FIT), yet many of these patients do not receive a recommended colonoscopy after a positive FIT.
“In this large US study, we found interval FITs are frequently performed in patients with and without prior polypectomy,” said first author Natalie J. Wilson, MD, of the University of Minnesota in Minneapolis, while presenting the findings at the American College of Gastroenterology (ACG) 2025 Annual Scientific Meeting.
“ and colorectal cancer, regardless of polypectomy history,” Wilson said.
Guideline recommendations stress the need for follow-up surveillance with a colonoscopy, particularly in patients who have had a prior polypectomy, because of the higher risk.
Reasons patients may instead turn to FIT may include cost or other factors, she said.
To determine just how often that happens, how having a previous polypectomy affects FIT results, and how adherent patients are to follow up if a FIT result is positive, Wilson and her colleagues evaluated data from nearly 4.8 million individuals in the Veterans Health Administration Corporate Data Warehouse who underwent colonoscopy between 2000 and 2024.
Of the patients, 10.9% were found to have subsequently received interval FIT within 10 years of the index colonoscopy, and of those patients, nearly half (49.9%) had received a polypectomy at the index colonoscopy.
The average time from the colonoscopy/polypectomy to the interval FIT was 5.9 years (5.6 years in the polypectomy group vs 6.2 years in the non-polypectomy group).
Among the FIT screenings, results were positive in 17.2% of post-polypectomy patients and 14.1% of patients with no prior polypectomy, indicating a history of polypectomy to be predictive of a positive interval FIT (odds ratio [OR], 1.12; P < .0001).
Notably, while a follow-up colonoscopy is considered essential following a positive FIT result — and having a previous polypectomy should add further urgency to the matter — the study showed only 50.4% of those who had an earlier polypectomy went on to receive the recommended follow-up colonoscopy after a positive follow-up FIT, and the rate was 49.3% among those who had not received a polypectomy (P = .001).
For those who did receive a follow-up colonoscopy after a positive FIT, the duration of time to receiving the colonoscopy was longer among those who had a prior polypectomy, at 2.9 months compared with 2.5 months in the non-polypectomy group (P < .001).
Colonoscopy results following a positive FIT showed higher rates of detections among patients who had prior polypectomies than among those with no prior polypectomy, including tubular adenomas (54.7% vs 45.8%), tubulovillous adenomas (5.6% vs 4.7%), adenomas with high-grade dysplasia (0.8% vs 0.7%), sessile serrated lesions (3.52% vs 2.4%), advanced colorectal neoplasia (9.2% vs 7.9%), and colorectal cancer (3.3% vs 3.0%).
However, a prior polypectomy was not independently predictive of colorectal cancer (OR, 0.96; P = .65) or advanced colorectal neoplasia (OR, 0.97; P = .57) in the post-colonoscopy interval FIT.
The findings underscore that “positive results carried a high risk of advanced neoplasia or cancer, irrespective of prior polypectomy history,” Wilson said.
Clinicians Must ‘Do a Better Job’
Commenting on the study, William D. Chey, MD, AGAF, chief of the Division of Gastroenterology & Hepatology at the University of Michigan in Ann Arbor, noted that the study “addresses one of the biggest challenges we face as a profession, which is making sure that patients who have a positive stool test get a colonoscopy.”
He noted that the low rate of just 50% of recipients of positive FITs going on to receive a colonoscopy is consistent with what is observed in other trials.
“Other data suggests that the rate might even be significantly higher — at 70%-80%, depending upon the population and the test,” Chey told Medscape Medical News.
Reasons for the failure to receive the follow-up testing range from income restrictions (due to the high cost of a colonoscopy, especially if not covered by insurance), education, speaking a foreign language, and other factors, he said.
The relatively high rates of colon cancers detected by FIT in the study, in those with and without a prior polypectomy, along with findings from other studies “should raise questions about whether there might be a role for FIT testing in addition to colonoscopy.” However, much stronger evidence would be needed, Chey noted.
In the meantime, a key issue is “how do we do a better job of making sure that individuals who have a positive FIT test get a colonoscopy,” he said.
“I think a lot of this is going to come down to how it’s done at the primary care level.”
Chey added that in that, and any other setting, “the main message that needs to get out to people who are undergoing stool-based screening is that the stool test is only the first part of the screening process, and if it’s positive, a follow-up colonoscopy must be performed.”
“Otherwise, the stool-based test is of no value.”
Wilson had no disclosures to report. Chey’s disclosures included consulting and/or other relationships with Ardelyx, Atmo, Biomerica, Commonwealth Diagnostics International, Corprata, Dieta, Evinature, Food Marble, Gemelli, Kiwi BioScience, Modify Health, Nestlé, Phathom, Redhill, Salix/Valeant, Takeda, and Vibrant.
A version of this article appeared on Medscape.com .
PHOENIX — Patients with or without polyp removal in an index colonoscopy commonly receive follow-up surveillance with a fecal immunochemical test (FIT), yet many of these patients do not receive a recommended colonoscopy after a positive FIT.
“In this large US study, we found interval FITs are frequently performed in patients with and without prior polypectomy,” said first author Natalie J. Wilson, MD, of the University of Minnesota in Minneapolis, while presenting the findings at the American College of Gastroenterology (ACG) 2025 Annual Scientific Meeting.
“ and colorectal cancer, regardless of polypectomy history,” Wilson said.
Guideline recommendations stress the need for follow-up surveillance with a colonoscopy, particularly in patients who have had a prior polypectomy, because of the higher risk.
Reasons patients may instead turn to FIT may include cost or other factors, she said.
To determine just how often that happens, how having a previous polypectomy affects FIT results, and how adherent patients are to follow up if a FIT result is positive, Wilson and her colleagues evaluated data from nearly 4.8 million individuals in the Veterans Health Administration Corporate Data Warehouse who underwent colonoscopy between 2000 and 2024.
Of the patients, 10.9% were found to have subsequently received interval FIT within 10 years of the index colonoscopy, and of those patients, nearly half (49.9%) had received a polypectomy at the index colonoscopy.
The average time from the colonoscopy/polypectomy to the interval FIT was 5.9 years (5.6 years in the polypectomy group vs 6.2 years in the non-polypectomy group).
Among the FIT screenings, results were positive in 17.2% of post-polypectomy patients and 14.1% of patients with no prior polypectomy, indicating a history of polypectomy to be predictive of a positive interval FIT (odds ratio [OR], 1.12; P < .0001).
Notably, while a follow-up colonoscopy is considered essential following a positive FIT result — and having a previous polypectomy should add further urgency to the matter — the study showed only 50.4% of those who had an earlier polypectomy went on to receive the recommended follow-up colonoscopy after a positive follow-up FIT, and the rate was 49.3% among those who had not received a polypectomy (P = .001).
For those who did receive a follow-up colonoscopy after a positive FIT, the duration of time to receiving the colonoscopy was longer among those who had a prior polypectomy, at 2.9 months compared with 2.5 months in the non-polypectomy group (P < .001).
Colonoscopy results following a positive FIT showed higher rates of detections among patients who had prior polypectomies than among those with no prior polypectomy, including tubular adenomas (54.7% vs 45.8%), tubulovillous adenomas (5.6% vs 4.7%), adenomas with high-grade dysplasia (0.8% vs 0.7%), sessile serrated lesions (3.52% vs 2.4%), advanced colorectal neoplasia (9.2% vs 7.9%), and colorectal cancer (3.3% vs 3.0%).
However, a prior polypectomy was not independently predictive of colorectal cancer (OR, 0.96; P = .65) or advanced colorectal neoplasia (OR, 0.97; P = .57) in the post-colonoscopy interval FIT.
The findings underscore that “positive results carried a high risk of advanced neoplasia or cancer, irrespective of prior polypectomy history,” Wilson said.
Clinicians Must ‘Do a Better Job’
Commenting on the study, William D. Chey, MD, AGAF, chief of the Division of Gastroenterology & Hepatology at the University of Michigan in Ann Arbor, noted that the study “addresses one of the biggest challenges we face as a profession, which is making sure that patients who have a positive stool test get a colonoscopy.”
He noted that the low rate of just 50% of recipients of positive FITs going on to receive a colonoscopy is consistent with what is observed in other trials.
“Other data suggests that the rate might even be significantly higher — at 70%-80%, depending upon the population and the test,” Chey told Medscape Medical News.
Reasons for the failure to receive the follow-up testing range from income restrictions (due to the high cost of a colonoscopy, especially if not covered by insurance), education, speaking a foreign language, and other factors, he said.
The relatively high rates of colon cancers detected by FIT in the study, in those with and without a prior polypectomy, along with findings from other studies “should raise questions about whether there might be a role for FIT testing in addition to colonoscopy.” However, much stronger evidence would be needed, Chey noted.
In the meantime, a key issue is “how do we do a better job of making sure that individuals who have a positive FIT test get a colonoscopy,” he said.
“I think a lot of this is going to come down to how it’s done at the primary care level.”
Chey added that in that, and any other setting, “the main message that needs to get out to people who are undergoing stool-based screening is that the stool test is only the first part of the screening process, and if it’s positive, a follow-up colonoscopy must be performed.”
“Otherwise, the stool-based test is of no value.”
Wilson had no disclosures to report. Chey’s disclosures included consulting and/or other relationships with Ardelyx, Atmo, Biomerica, Commonwealth Diagnostics International, Corprata, Dieta, Evinature, Food Marble, Gemelli, Kiwi BioScience, Modify Health, Nestlé, Phathom, Redhill, Salix/Valeant, Takeda, and Vibrant.
A version of this article appeared on Medscape.com .
FROM ACG 2025
A True Community: The Vet-to-Vet Program for Chronic Pain
A True Community: The Vet-to-Vet Program for Chronic Pain
The Veterans Health Administration (VHA) has continued to advance its understanding and treatment of chronic pain. The VHA National Pain Management Strategy emphasizes the significance of the social context of pain while underscoring the importance of self-management.1 This established strategy ensures that all veterans have access to the appropriate pain care in the proper setting.2 VHA has instituted a stepped care model of pain management, delineating the domains of primary care, secondary consultative services, and tertiary care.3 This directive emphasized a biopsychosocial approach to pain management to prioritize the relationship between biological, psychological, and social factors that influence how veterans experience pain and should commensurately influence how it is managed.
The VHA Office of Patient-Centered Care and Cultural Transformation implemented the Whole Health System of Care as part of the Comprehensive Addiction and Recovery Act, which included a VHA directive to expand pain management.4,5 Reorientation within this system shifts from defining veterans as passive care recipients to viewing them as active partners in their own care and health. This partnership places additional emphasis on peer-led explorations of mission, aspiration, and purpose.6
Peer-led groups, also known as mutual aid, mutual support, and mutual help groups, have historically been successful for patients undergoing treatment for substance use disorders (eg, Alcoholics Anonymous).7 Mutual help groups have 3 defining characteristics. First, they are run by participants, not professionals, though the latter may have been integral in the founding of the groups. Second, participants share a similar problem (eg, disease state, experience, disposition). Finally, there is a reciprocal exchange of information and psychological support among participants.8,9 Mutual help groups that address chronic pain are rare but becoming more common.10-12 Emerging evidence suggests a positive relationship between peer support and improved well-being, self-efficacy, pain management, and pain self-management skills (eg, activity pacing).13-15
Storytelling as a tool for healing has a long history in indigenous and Western medical traditions.16-19 This includes the treatment of chronic disease, including pain.20,21 The use of storytelling in health care overlaps with the role it plays within many mutual help groups focused on chronic disease treatment.22 Storytelling allows an individual to share their experience with a disease, and take a more active role in their health, and facilitate stronger bonds with others.22 In effect, storytelling is not only important to group cohesion—it also plays a role in an individual’s healing.
Vet-to-Vet
The VHA Office of Rural Health funds Vet-to-Vet, a peer-to-peer program to address limited access to care for rural veterans with chronic pain. Similar to the VHA National Pain Management Strategy, Vet-to-Vet is grounded in the significance of the social context of pain and underscores the importance of self-management.1 The program combines pain care, mutual help, and storytelling to support veterans living with chronic pain. While the primary focus of Vet-to-Vet is rural veterans, the program serves any veteran experiencing chronic pain who is isolated from services, including home-bound urban veterans.
Following mutual help principles, Vet-to-Vet peer facilitators lead weekly online drop-in meetings. Meetings follow the general structure of reiterating group ground rules and sharing an individual pain story, followed by open discussions centered on well-being, chronic pain management, or any topic the group wishes to discuss. Meetings typically end with a mindfulness exercise. The organizational structure that supports Vet-to-Vet includes the implementation support team, site leads, Vet-to-Vet peer facilitators, and national partners (Figure 1).
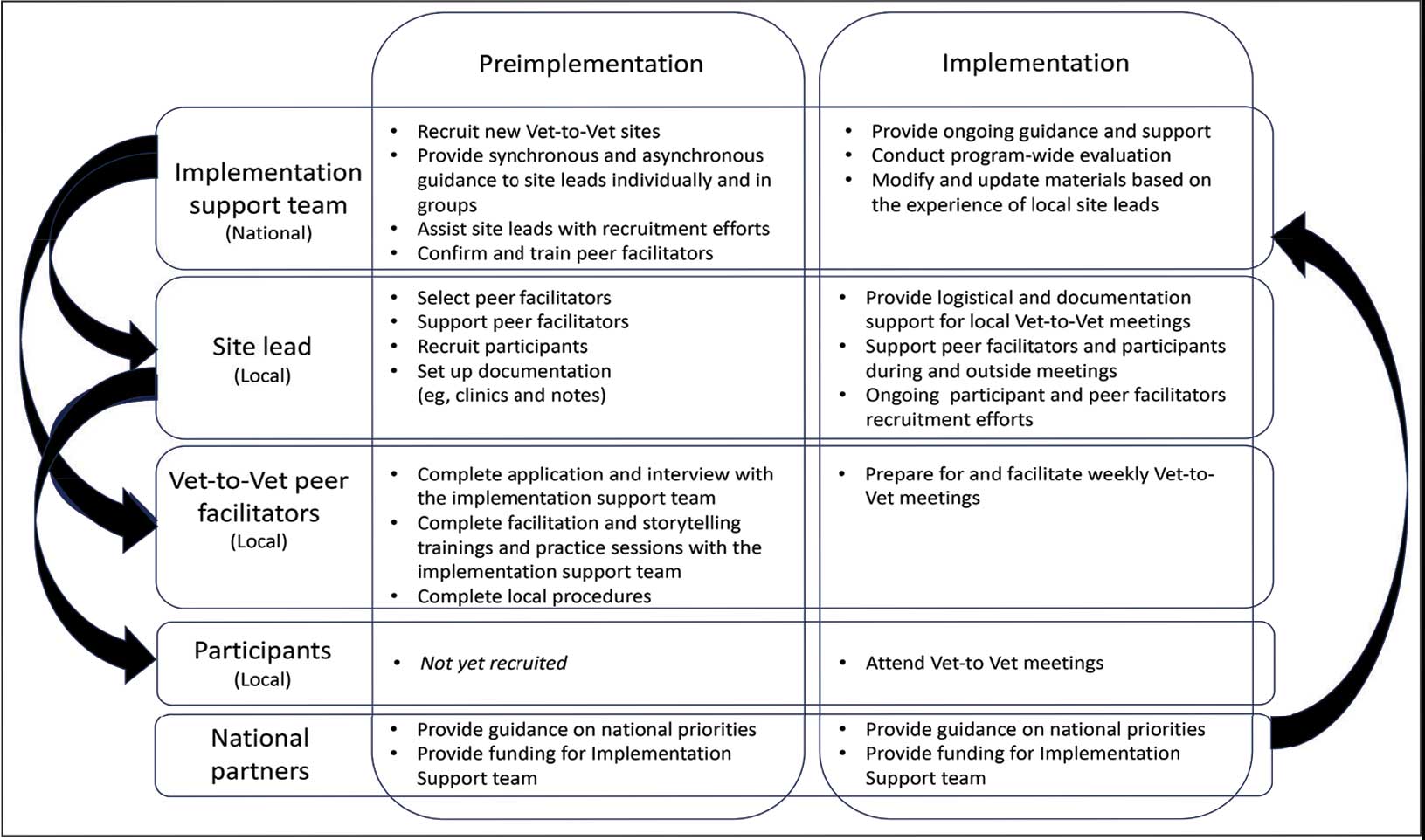
Implementation Support Team
The implementation support team consists of a principal investigator, coinvestigator, program manager, and program support specialist. The team provides facilitator training, monthly community practice sessions for Vet-to-Vet peer facilitators and site leads, and weekly office hours for site leads. The implementation support team also recruits new Vet-to-Vet sites; potential new locations ideally have an existing whole health program, leadership support, committed site and cosite leads, and ≥ 3 peer facilitator volunteers.
Site Leads
Most site and cosite leads are based in whole health or pain management teams and are whole health coaches or peer support specialists. The site lead is responsible for standing up the program and documenting encounters, recruiting and supporting peer facilitators and participants, and overseeing the meeting. During meetings, site leads generally leave their cameras off and only speak when called into the group; the peer facilitators lead the meetings. The implementation support team recommends that site leads dedicate ≥ 4 hours per week to Vet-to-Vet; 2 hours for weekly group meetings and 2 hours for documentation (ie, entering notes into the participants’ electronic health records) and supporting peer facilitators and participants. Cosite lead responsibilities vary by location, with some sites having 2 leads that equally share duties and others having a primary lead and a colead available if the site lead is unable to attend a meeting.
Vet-to-Vet Peer Facilitators
Peer facilitators are the core of the program. They lead meetings from start to finish. Like participants, they also experience chronic pain and are volunteers. The implementation support team encourages sites to establish volunteer peer facilitators, rather than assigning peer support specialists to facilitate meetings. Veterans are eager to connect and give back to their communities, and the Vet-to-Vet peer facilitator role is an opportunity for those unable to work to connect with peers and add meaning to their lives. Even if a VHA employee is a veteran who has chronic pain, they are not eligible to serve as this could create a service provider/service recipient dynamic that is not in the spirit of mutual help.
Vet-to-Vet peer facilitators attend a virtual 3-day training held by the implementation support team prior to starting. These training sessions are available on a quarterly basis and facilitated by the Vet-to-Vet program manager and 2 current peer facilitators. Training content includes established whole health facilitator training materials and program-specific storytelling training materials. Once trained, peer facilitators attend storytelling practice sessions and collaborate with their site leads during weekly meetings.
Participants
Vet-to-Vet participants find the program through direct outreach from site leads, word of mouth, and referrals. The only criteria to join are that the individual is a veteran who experiences chronic pain and is enrolled in the VHA (site leads can assist with enrollment if needed). Participants are not required to have a diagnosis or engage in any other health care. There is no commitment and no end date. Some participants only come once; others have attended for > 3 years. This approach is intended to embrace the idea that the need for support ebbs and flows.
National Partners
The VHA Office of Rural Health provides technical support. The Center for Development and Civic Engagement onboards peer facilitators as VHA volunteers. The Office of Patient-Centered Care and Cultural Transformation provides national guidance and site-level collaboration. The VHA Pain Management, Opioid Safety, and Prescription Drug Monitoring Program supports site recruitment. In addition to the VHA partners, 4 veteran evaluation consultants who have experience with chronic pain but do not participate in Vet-to-Vet meetings provide advice on evaluation activities, such as question development and communication strategies.
Evaluation
This evaluation shares preliminary results from a pilot evaluation of the Rocky Mountain Regional VA Medical Center (RMRVAMC) Vet-to-Vet group. It is intended for program improvement, was deemed nonresearch by the Colorado Multiple Institutional Review Board, and was structured using the RE-AIM (Reach, Effectiveness, Adoption, Implementation, and Maintenance) framework.23 This evaluation focused on capturing measures related to reach and effectiveness, while a forthcoming evaluation includes elements of adoption, implementation, and maintenance.
In 2022, 16 Vet-to-Vet peer facilitators and participants completed surveys and interviews to share their experience. Interviews were recorded, transcribed, and coded in ATLAS.ti. A priori codes were based on interview guide questions and emergent descriptive codes were used to identify specific topics which were categorized into RE-AIM domains, barriers, facilitators, what participants learned, how participants applied what they learned to their lives, and participant reported outcomes. This article contains high-level findings from the evaluation; more detailed results will be included in the ongoing evaluation.
Results
The RMRVAMC Vet-to-Vet group has met weekly since April 2022. Four Vet-to-Vet peer facilitators and 12 individuals participated in the pilot Vet-to-Vet group and evaluation. The mean age was 62 years, most were men, and half were married. Most participants lived in rural areas with a mean distance of 125 miles to the nearest VAMC. Many experienced multiple kinds of pain, with a mean 4.5 on a 10-point scale (bothered “a lot”). All participants reported that they experienced pain daily.
Participation in Vet-to-Vet meetings was high; 3 of 4 peer facilitators and 7 of 12 participants completed the first 6 months of the program. In interviews, participants described the positive impact of the program. They emphasized the importance of connecting with other veterans and helping one another, with one noting that opportunities to connect with other veterans “just drops off a lot” (peer facilitator 3) after leaving active duty.
Some participants and Vet-to-Vet peer facilitators outlined the content of the sessions (eg, learning about how pain impacts the body and one’s family relationships) and shared the skills they learned (eg, goal setting, self-advocacy) (Table). Most spoke about learning from one another and the power of sharing stories with one peer facilitator sharing how they felt that witnessing another participant’s story “really shifted how I was thinking about things and how I perceived people” (peer facilitator 1).

Participants reported several ways the program impacted their lives, such as learning that they could get help, how to get help, and how to overcome the mental aspects of chronic pain. One veteran shared profound health impacts and attributed the Vet-to-Vet program to having one of the best years of their life. Even those who did not attend many meetings spoke of it positively and stated that it should continue so others could try (Table).
From January 2022 to September 2025, > 80 veterans attended ≥ 1 meeting at RMRVAMC; 29 attended ≥ 1 meeting in the last quarter. There were > 1400 Vet-to-Vet encounters at RMRVAMC, with a mean (SD) of 14.2 (19.2) and a median of 4.5 encounters per participant. Half of the veterans attend ≥ 5 meetings, and one-third attended ≥ 10 meetings.
Since June 2023, 15 additional VHA facilities launched Vet-to-Vet programs. As of October 2025, > 350 veterans have participated in ≥ 1 Vet-to-Vet meeting, totaling > 4500 Vet-to-Vet encounters since the program’s inception (Figure 2).
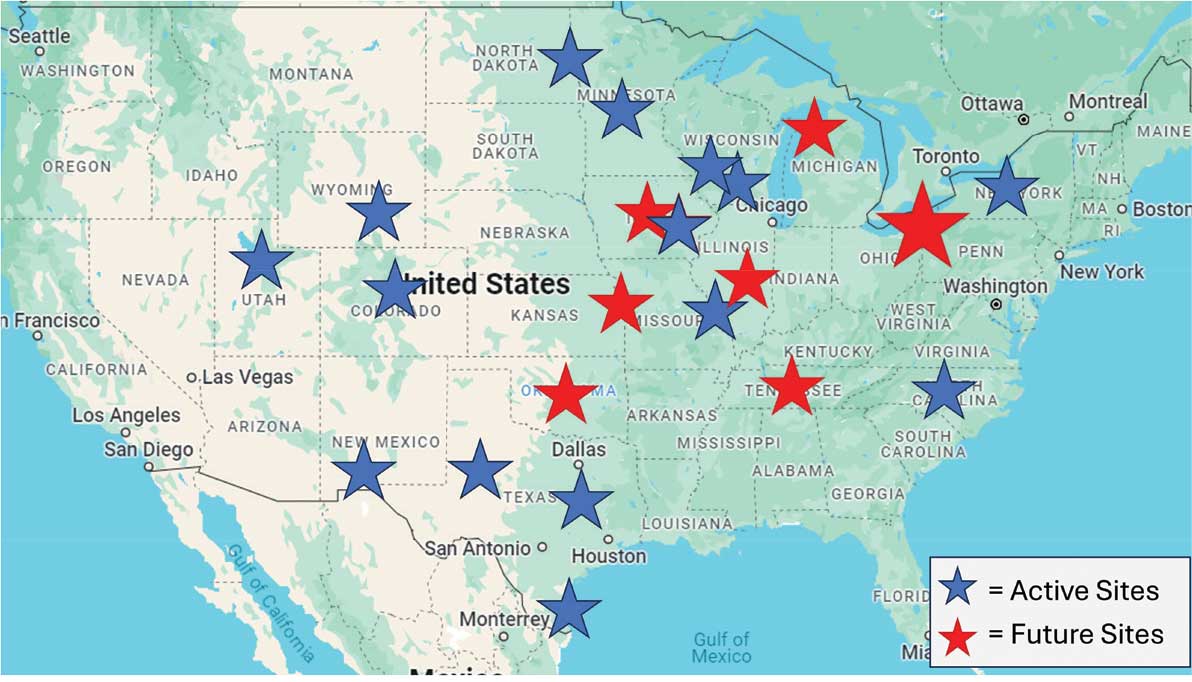
Challenges
The RMRVAMC site and cosite leads are part of the national implementation team and dedicate substantial time to developing the program: 40 and 10 hours per week, respectively. Site leads at new locations do not receive funding for Vet-to-Vet activities and are recommended to dedicate only 4 hours per week to the program. Formally embedding Vet-to-Vet into the site leads’ roles is critical for sustainment.
The Vet-to-Vet model has changed. The initial Vet-to-Vet cohort included the 6-week Taking Charge of My Life and Health curriculum prior to moving to the mutual help format.24 While this curriculum still informs peer facilitator training, it is not used in new groups. It has anecdotally been reported that this change was positive, but the impact of this adaptation is unknown.
This evaluation cohort was small (16 participants) and initial patient reported and administrative outcomes were inconclusive. However, most veterans who stopped participating in Vet-to-Vet spoke fondly of their experiences with the program.
CONCLUSIONS
Vet-to-Vet is a promising new initiative to support self-management and social connection in chronic pain care. The program employs a mutual help approach and storytelling to empower veterans living with chronic pain. The effectiveness of these strategies will be evaluated, which will inform its continued growth. The program's current goals focus on sustainment at existing sites and expansion to new sites to reach more rural veterans across the VA enterprise. While Vet-to-Vet is designed to serve those who experience chronic pain, a partnership with the Office of Whole Health has established goals to begin expanding this model to other chronic conditions in 2026.
- Kerns RD, Philip EJ, Lee AW, Rosenberger PH. Implementation of the Veterans Health Administration national pain management strategy. Transl Behav Med. 2011;1:635-643. doi:10.1007/s13142-011-0094-3
- Pain Management, Opioid Safety, and PDMP (PMOP). US Department of Veterans Affairs. Updated August 21, 2025. Accessed September 25, 2025. https://www.va.gov/PAINMANAGEMENT/Providers/IntegratedTeambasedPainCare.asp
- US Department of Veterans Affairs. VHA Directive 2009-053. October 28, 2009. Accessed September 25, 2025. https://www.va.gov/PAINMANAGEMENT/docs/VHA09PainDirective.pdf
- Comprehensive Addiction and Recovery Act of 2016, S524, 114th Cong (2015-2016). Pub L No. 114-198. July 22, 2016. Accessed September 25, 2025. https://www.congress.gov/bill/114th-congress/senate-bill/524
- Bokhour B, Hyde J, Zeliadt, Mohr D. Whole Health System of Care Evaluation. US Department of Veterans Affairs. February 18, 2020. Accessed September 25, 2025. https://www.va.gov/WHOLEHEALTH/docs/EPCC_WHSevaluation_FinalReport_508.pdf
- Gaudet T, Kligler B. Whole health in the whole system of the veterans administration: how will we know we have reached this future state? J Altern Complement Med. 2019;25:S7-S11. doi:10.1089/acm.2018.29061.gau
- Kelly JF, Yeterian JD. The role of mutual-help groups in extending the framework of treatment. Alcohol Res Health. 2011;33:350-355.
- Humphreys K. Self-help/mutual aid organizations: the view from Mars. Subst Use Misuse. 1997;32:2105-2109. doi:10.3109/10826089709035622
- Chinman M, Kloos B, O’Connell M, Davidson L. Service providers’ views of psychiatric mutual support groups. J Community Psychol. 2002;30:349-366. doi:10.1002/jcop.10010
- Shue SA, McGuire AB, Matthias MS. Facilitators and barriers to implementation of a peer support intervention for patients with chronic pain: a qualitative study. Pain Med. 2019;20:1311-1320. doi:10.1093/pm/pny229
- Pester BD, Tankha H, Caño A, et al. Facing pain together: a randomized controlled trial of the effects of Facebook support groups on adults with chronic pain. J Pain. 2022;23:2121-2134. doi:10.1016/j.jpain.2022.07.013
- Matthias MS, McGuire AB, Kukla M, Daggy J, Myers LJ, Bair MJ. A brief peer support intervention for veterans with chronic musculoskeletal pain: a pilot study of feasibility and effectiveness. Pain Med. 2015;16:81-87. doi:10.1111/pme.12571
- Finlay KA, Elander J. Reflecting the transition from pain management services to chronic pain support group attendance: an interpretative phenomenological analysis. Br J Health Psychol. 2016;21:660-676. doi:10.1111/bjhp.12194
- Finlay KA, Peacock S, Elander J. Developing successful social support: an interpretative phenomenological analysis of mechanisms and processes in a chronic pain support group. Psychol Health. 2018;33:846-871. doi:10.1080/08870446.2017.1421188
- Farr M, Brant H, Patel R, et al. Experiences of patient-led chronic pain peer support groups after pain management programs: a qualitative study. Pain Med. 2021;22:2884-2895. doi:10.1093/pm/pnab189
- Mehl-Madrona L. Narrative Medicine: The Use of History and Story in the Healing Process. Bear & Company; 2007.
- Fioretti C, Mazzocco K, Riva S, Oliveri S, Masiero M, Pravettoni G. Research studies on patients’ illness experience using the Narrative Medicine approach: a systematic review. BMJ Open. 2016;6:e011220. doi:10.1136/bmjopen-2016-011220
- Hall JM, Powell J. Understanding the person through narrative. Nurs Res Pract. 2011;2011:293837. doi:10.1155/2011/293837
- Ricks L, Kitchens S, Goodrich T, Hancock E. My story: the use of narrative therapy in individual and group counseling. J Creat Ment Health. 2014;9:99-110. doi:10.1080/15401383.2013.870947
- Hydén L-C. Illness and narrative. Sociol Health Illn. 1997;19:48-69. doi:10.1111/j.1467-9566.1997.tb00015.x
- Georgiadis E, Johnson MI. Incorporating personal narratives in positive psychology interventions to manage chronic pain. Front Pain Res (Lausanne). 2023;4:1253310. doi:10.3389/fpain.2023.1253310
- Gucciardi E, Jean-Pierre N, Karam G, Sidani S. Designing and delivering facilitated storytelling interventions for chronic disease self-management: a scoping review. BMC Health Serv Res. 2016;16:249. doi:10.1186/s12913-016-1474-7
- Glasgow RE, Vogt TM, Boles SM. Evaluating the public health impact of health promotion interventions: the RE-AIM framework. Am J Public Health. 1999;89:1322-1327. doi:10.2105/ajph.89.9.1322
- Abadi M, Richard B, Shamblen S, et al. Achieving whole health: a preliminary study of TCMLH, a group-based program promoting self-care and empowerment among veterans. Health Educ Behav. 2022;49:347-357. doi:10.1177/10901981211011043
The Veterans Health Administration (VHA) has continued to advance its understanding and treatment of chronic pain. The VHA National Pain Management Strategy emphasizes the significance of the social context of pain while underscoring the importance of self-management.1 This established strategy ensures that all veterans have access to the appropriate pain care in the proper setting.2 VHA has instituted a stepped care model of pain management, delineating the domains of primary care, secondary consultative services, and tertiary care.3 This directive emphasized a biopsychosocial approach to pain management to prioritize the relationship between biological, psychological, and social factors that influence how veterans experience pain and should commensurately influence how it is managed.
The VHA Office of Patient-Centered Care and Cultural Transformation implemented the Whole Health System of Care as part of the Comprehensive Addiction and Recovery Act, which included a VHA directive to expand pain management.4,5 Reorientation within this system shifts from defining veterans as passive care recipients to viewing them as active partners in their own care and health. This partnership places additional emphasis on peer-led explorations of mission, aspiration, and purpose.6
Peer-led groups, also known as mutual aid, mutual support, and mutual help groups, have historically been successful for patients undergoing treatment for substance use disorders (eg, Alcoholics Anonymous).7 Mutual help groups have 3 defining characteristics. First, they are run by participants, not professionals, though the latter may have been integral in the founding of the groups. Second, participants share a similar problem (eg, disease state, experience, disposition). Finally, there is a reciprocal exchange of information and psychological support among participants.8,9 Mutual help groups that address chronic pain are rare but becoming more common.10-12 Emerging evidence suggests a positive relationship between peer support and improved well-being, self-efficacy, pain management, and pain self-management skills (eg, activity pacing).13-15
Storytelling as a tool for healing has a long history in indigenous and Western medical traditions.16-19 This includes the treatment of chronic disease, including pain.20,21 The use of storytelling in health care overlaps with the role it plays within many mutual help groups focused on chronic disease treatment.22 Storytelling allows an individual to share their experience with a disease, and take a more active role in their health, and facilitate stronger bonds with others.22 In effect, storytelling is not only important to group cohesion—it also plays a role in an individual’s healing.
Vet-to-Vet
The VHA Office of Rural Health funds Vet-to-Vet, a peer-to-peer program to address limited access to care for rural veterans with chronic pain. Similar to the VHA National Pain Management Strategy, Vet-to-Vet is grounded in the significance of the social context of pain and underscores the importance of self-management.1 The program combines pain care, mutual help, and storytelling to support veterans living with chronic pain. While the primary focus of Vet-to-Vet is rural veterans, the program serves any veteran experiencing chronic pain who is isolated from services, including home-bound urban veterans.
Following mutual help principles, Vet-to-Vet peer facilitators lead weekly online drop-in meetings. Meetings follow the general structure of reiterating group ground rules and sharing an individual pain story, followed by open discussions centered on well-being, chronic pain management, or any topic the group wishes to discuss. Meetings typically end with a mindfulness exercise. The organizational structure that supports Vet-to-Vet includes the implementation support team, site leads, Vet-to-Vet peer facilitators, and national partners (Figure 1).

Implementation Support Team
The implementation support team consists of a principal investigator, coinvestigator, program manager, and program support specialist. The team provides facilitator training, monthly community practice sessions for Vet-to-Vet peer facilitators and site leads, and weekly office hours for site leads. The implementation support team also recruits new Vet-to-Vet sites; potential new locations ideally have an existing whole health program, leadership support, committed site and cosite leads, and ≥ 3 peer facilitator volunteers.
Site Leads
Most site and cosite leads are based in whole health or pain management teams and are whole health coaches or peer support specialists. The site lead is responsible for standing up the program and documenting encounters, recruiting and supporting peer facilitators and participants, and overseeing the meeting. During meetings, site leads generally leave their cameras off and only speak when called into the group; the peer facilitators lead the meetings. The implementation support team recommends that site leads dedicate ≥ 4 hours per week to Vet-to-Vet; 2 hours for weekly group meetings and 2 hours for documentation (ie, entering notes into the participants’ electronic health records) and supporting peer facilitators and participants. Cosite lead responsibilities vary by location, with some sites having 2 leads that equally share duties and others having a primary lead and a colead available if the site lead is unable to attend a meeting.
Vet-to-Vet Peer Facilitators
Peer facilitators are the core of the program. They lead meetings from start to finish. Like participants, they also experience chronic pain and are volunteers. The implementation support team encourages sites to establish volunteer peer facilitators, rather than assigning peer support specialists to facilitate meetings. Veterans are eager to connect and give back to their communities, and the Vet-to-Vet peer facilitator role is an opportunity for those unable to work to connect with peers and add meaning to their lives. Even if a VHA employee is a veteran who has chronic pain, they are not eligible to serve as this could create a service provider/service recipient dynamic that is not in the spirit of mutual help.
Vet-to-Vet peer facilitators attend a virtual 3-day training held by the implementation support team prior to starting. These training sessions are available on a quarterly basis and facilitated by the Vet-to-Vet program manager and 2 current peer facilitators. Training content includes established whole health facilitator training materials and program-specific storytelling training materials. Once trained, peer facilitators attend storytelling practice sessions and collaborate with their site leads during weekly meetings.
Participants
Vet-to-Vet participants find the program through direct outreach from site leads, word of mouth, and referrals. The only criteria to join are that the individual is a veteran who experiences chronic pain and is enrolled in the VHA (site leads can assist with enrollment if needed). Participants are not required to have a diagnosis or engage in any other health care. There is no commitment and no end date. Some participants only come once; others have attended for > 3 years. This approach is intended to embrace the idea that the need for support ebbs and flows.
National Partners
The VHA Office of Rural Health provides technical support. The Center for Development and Civic Engagement onboards peer facilitators as VHA volunteers. The Office of Patient-Centered Care and Cultural Transformation provides national guidance and site-level collaboration. The VHA Pain Management, Opioid Safety, and Prescription Drug Monitoring Program supports site recruitment. In addition to the VHA partners, 4 veteran evaluation consultants who have experience with chronic pain but do not participate in Vet-to-Vet meetings provide advice on evaluation activities, such as question development and communication strategies.
Evaluation
This evaluation shares preliminary results from a pilot evaluation of the Rocky Mountain Regional VA Medical Center (RMRVAMC) Vet-to-Vet group. It is intended for program improvement, was deemed nonresearch by the Colorado Multiple Institutional Review Board, and was structured using the RE-AIM (Reach, Effectiveness, Adoption, Implementation, and Maintenance) framework.23 This evaluation focused on capturing measures related to reach and effectiveness, while a forthcoming evaluation includes elements of adoption, implementation, and maintenance.
In 2022, 16 Vet-to-Vet peer facilitators and participants completed surveys and interviews to share their experience. Interviews were recorded, transcribed, and coded in ATLAS.ti. A priori codes were based on interview guide questions and emergent descriptive codes were used to identify specific topics which were categorized into RE-AIM domains, barriers, facilitators, what participants learned, how participants applied what they learned to their lives, and participant reported outcomes. This article contains high-level findings from the evaluation; more detailed results will be included in the ongoing evaluation.
Results
The RMRVAMC Vet-to-Vet group has met weekly since April 2022. Four Vet-to-Vet peer facilitators and 12 individuals participated in the pilot Vet-to-Vet group and evaluation. The mean age was 62 years, most were men, and half were married. Most participants lived in rural areas with a mean distance of 125 miles to the nearest VAMC. Many experienced multiple kinds of pain, with a mean 4.5 on a 10-point scale (bothered “a lot”). All participants reported that they experienced pain daily.
Participation in Vet-to-Vet meetings was high; 3 of 4 peer facilitators and 7 of 12 participants completed the first 6 months of the program. In interviews, participants described the positive impact of the program. They emphasized the importance of connecting with other veterans and helping one another, with one noting that opportunities to connect with other veterans “just drops off a lot” (peer facilitator 3) after leaving active duty.
Some participants and Vet-to-Vet peer facilitators outlined the content of the sessions (eg, learning about how pain impacts the body and one’s family relationships) and shared the skills they learned (eg, goal setting, self-advocacy) (Table). Most spoke about learning from one another and the power of sharing stories with one peer facilitator sharing how they felt that witnessing another participant’s story “really shifted how I was thinking about things and how I perceived people” (peer facilitator 1).

Participants reported several ways the program impacted their lives, such as learning that they could get help, how to get help, and how to overcome the mental aspects of chronic pain. One veteran shared profound health impacts and attributed the Vet-to-Vet program to having one of the best years of their life. Even those who did not attend many meetings spoke of it positively and stated that it should continue so others could try (Table).
From January 2022 to September 2025, > 80 veterans attended ≥ 1 meeting at RMRVAMC; 29 attended ≥ 1 meeting in the last quarter. There were > 1400 Vet-to-Vet encounters at RMRVAMC, with a mean (SD) of 14.2 (19.2) and a median of 4.5 encounters per participant. Half of the veterans attend ≥ 5 meetings, and one-third attended ≥ 10 meetings.
Since June 2023, 15 additional VHA facilities launched Vet-to-Vet programs. As of October 2025, > 350 veterans have participated in ≥ 1 Vet-to-Vet meeting, totaling > 4500 Vet-to-Vet encounters since the program’s inception (Figure 2).

Challenges
The RMRVAMC site and cosite leads are part of the national implementation team and dedicate substantial time to developing the program: 40 and 10 hours per week, respectively. Site leads at new locations do not receive funding for Vet-to-Vet activities and are recommended to dedicate only 4 hours per week to the program. Formally embedding Vet-to-Vet into the site leads’ roles is critical for sustainment.
The Vet-to-Vet model has changed. The initial Vet-to-Vet cohort included the 6-week Taking Charge of My Life and Health curriculum prior to moving to the mutual help format.24 While this curriculum still informs peer facilitator training, it is not used in new groups. It has anecdotally been reported that this change was positive, but the impact of this adaptation is unknown.
This evaluation cohort was small (16 participants) and initial patient reported and administrative outcomes were inconclusive. However, most veterans who stopped participating in Vet-to-Vet spoke fondly of their experiences with the program.
CONCLUSIONS
Vet-to-Vet is a promising new initiative to support self-management and social connection in chronic pain care. The program employs a mutual help approach and storytelling to empower veterans living with chronic pain. The effectiveness of these strategies will be evaluated, which will inform its continued growth. The program's current goals focus on sustainment at existing sites and expansion to new sites to reach more rural veterans across the VA enterprise. While Vet-to-Vet is designed to serve those who experience chronic pain, a partnership with the Office of Whole Health has established goals to begin expanding this model to other chronic conditions in 2026.
The Veterans Health Administration (VHA) has continued to advance its understanding and treatment of chronic pain. The VHA National Pain Management Strategy emphasizes the significance of the social context of pain while underscoring the importance of self-management.1 This established strategy ensures that all veterans have access to the appropriate pain care in the proper setting.2 VHA has instituted a stepped care model of pain management, delineating the domains of primary care, secondary consultative services, and tertiary care.3 This directive emphasized a biopsychosocial approach to pain management to prioritize the relationship between biological, psychological, and social factors that influence how veterans experience pain and should commensurately influence how it is managed.
The VHA Office of Patient-Centered Care and Cultural Transformation implemented the Whole Health System of Care as part of the Comprehensive Addiction and Recovery Act, which included a VHA directive to expand pain management.4,5 Reorientation within this system shifts from defining veterans as passive care recipients to viewing them as active partners in their own care and health. This partnership places additional emphasis on peer-led explorations of mission, aspiration, and purpose.6
Peer-led groups, also known as mutual aid, mutual support, and mutual help groups, have historically been successful for patients undergoing treatment for substance use disorders (eg, Alcoholics Anonymous).7 Mutual help groups have 3 defining characteristics. First, they are run by participants, not professionals, though the latter may have been integral in the founding of the groups. Second, participants share a similar problem (eg, disease state, experience, disposition). Finally, there is a reciprocal exchange of information and psychological support among participants.8,9 Mutual help groups that address chronic pain are rare but becoming more common.10-12 Emerging evidence suggests a positive relationship between peer support and improved well-being, self-efficacy, pain management, and pain self-management skills (eg, activity pacing).13-15
Storytelling as a tool for healing has a long history in indigenous and Western medical traditions.16-19 This includes the treatment of chronic disease, including pain.20,21 The use of storytelling in health care overlaps with the role it plays within many mutual help groups focused on chronic disease treatment.22 Storytelling allows an individual to share their experience with a disease, and take a more active role in their health, and facilitate stronger bonds with others.22 In effect, storytelling is not only important to group cohesion—it also plays a role in an individual’s healing.
Vet-to-Vet
The VHA Office of Rural Health funds Vet-to-Vet, a peer-to-peer program to address limited access to care for rural veterans with chronic pain. Similar to the VHA National Pain Management Strategy, Vet-to-Vet is grounded in the significance of the social context of pain and underscores the importance of self-management.1 The program combines pain care, mutual help, and storytelling to support veterans living with chronic pain. While the primary focus of Vet-to-Vet is rural veterans, the program serves any veteran experiencing chronic pain who is isolated from services, including home-bound urban veterans.
Following mutual help principles, Vet-to-Vet peer facilitators lead weekly online drop-in meetings. Meetings follow the general structure of reiterating group ground rules and sharing an individual pain story, followed by open discussions centered on well-being, chronic pain management, or any topic the group wishes to discuss. Meetings typically end with a mindfulness exercise. The organizational structure that supports Vet-to-Vet includes the implementation support team, site leads, Vet-to-Vet peer facilitators, and national partners (Figure 1).

Implementation Support Team
The implementation support team consists of a principal investigator, coinvestigator, program manager, and program support specialist. The team provides facilitator training, monthly community practice sessions for Vet-to-Vet peer facilitators and site leads, and weekly office hours for site leads. The implementation support team also recruits new Vet-to-Vet sites; potential new locations ideally have an existing whole health program, leadership support, committed site and cosite leads, and ≥ 3 peer facilitator volunteers.
Site Leads
Most site and cosite leads are based in whole health or pain management teams and are whole health coaches or peer support specialists. The site lead is responsible for standing up the program and documenting encounters, recruiting and supporting peer facilitators and participants, and overseeing the meeting. During meetings, site leads generally leave their cameras off and only speak when called into the group; the peer facilitators lead the meetings. The implementation support team recommends that site leads dedicate ≥ 4 hours per week to Vet-to-Vet; 2 hours for weekly group meetings and 2 hours for documentation (ie, entering notes into the participants’ electronic health records) and supporting peer facilitators and participants. Cosite lead responsibilities vary by location, with some sites having 2 leads that equally share duties and others having a primary lead and a colead available if the site lead is unable to attend a meeting.
Vet-to-Vet Peer Facilitators
Peer facilitators are the core of the program. They lead meetings from start to finish. Like participants, they also experience chronic pain and are volunteers. The implementation support team encourages sites to establish volunteer peer facilitators, rather than assigning peer support specialists to facilitate meetings. Veterans are eager to connect and give back to their communities, and the Vet-to-Vet peer facilitator role is an opportunity for those unable to work to connect with peers and add meaning to their lives. Even if a VHA employee is a veteran who has chronic pain, they are not eligible to serve as this could create a service provider/service recipient dynamic that is not in the spirit of mutual help.
Vet-to-Vet peer facilitators attend a virtual 3-day training held by the implementation support team prior to starting. These training sessions are available on a quarterly basis and facilitated by the Vet-to-Vet program manager and 2 current peer facilitators. Training content includes established whole health facilitator training materials and program-specific storytelling training materials. Once trained, peer facilitators attend storytelling practice sessions and collaborate with their site leads during weekly meetings.
Participants
Vet-to-Vet participants find the program through direct outreach from site leads, word of mouth, and referrals. The only criteria to join are that the individual is a veteran who experiences chronic pain and is enrolled in the VHA (site leads can assist with enrollment if needed). Participants are not required to have a diagnosis or engage in any other health care. There is no commitment and no end date. Some participants only come once; others have attended for > 3 years. This approach is intended to embrace the idea that the need for support ebbs and flows.
National Partners
The VHA Office of Rural Health provides technical support. The Center for Development and Civic Engagement onboards peer facilitators as VHA volunteers. The Office of Patient-Centered Care and Cultural Transformation provides national guidance and site-level collaboration. The VHA Pain Management, Opioid Safety, and Prescription Drug Monitoring Program supports site recruitment. In addition to the VHA partners, 4 veteran evaluation consultants who have experience with chronic pain but do not participate in Vet-to-Vet meetings provide advice on evaluation activities, such as question development and communication strategies.
Evaluation
This evaluation shares preliminary results from a pilot evaluation of the Rocky Mountain Regional VA Medical Center (RMRVAMC) Vet-to-Vet group. It is intended for program improvement, was deemed nonresearch by the Colorado Multiple Institutional Review Board, and was structured using the RE-AIM (Reach, Effectiveness, Adoption, Implementation, and Maintenance) framework.23 This evaluation focused on capturing measures related to reach and effectiveness, while a forthcoming evaluation includes elements of adoption, implementation, and maintenance.
In 2022, 16 Vet-to-Vet peer facilitators and participants completed surveys and interviews to share their experience. Interviews were recorded, transcribed, and coded in ATLAS.ti. A priori codes were based on interview guide questions and emergent descriptive codes were used to identify specific topics which were categorized into RE-AIM domains, barriers, facilitators, what participants learned, how participants applied what they learned to their lives, and participant reported outcomes. This article contains high-level findings from the evaluation; more detailed results will be included in the ongoing evaluation.
Results
The RMRVAMC Vet-to-Vet group has met weekly since April 2022. Four Vet-to-Vet peer facilitators and 12 individuals participated in the pilot Vet-to-Vet group and evaluation. The mean age was 62 years, most were men, and half were married. Most participants lived in rural areas with a mean distance of 125 miles to the nearest VAMC. Many experienced multiple kinds of pain, with a mean 4.5 on a 10-point scale (bothered “a lot”). All participants reported that they experienced pain daily.
Participation in Vet-to-Vet meetings was high; 3 of 4 peer facilitators and 7 of 12 participants completed the first 6 months of the program. In interviews, participants described the positive impact of the program. They emphasized the importance of connecting with other veterans and helping one another, with one noting that opportunities to connect with other veterans “just drops off a lot” (peer facilitator 3) after leaving active duty.
Some participants and Vet-to-Vet peer facilitators outlined the content of the sessions (eg, learning about how pain impacts the body and one’s family relationships) and shared the skills they learned (eg, goal setting, self-advocacy) (Table). Most spoke about learning from one another and the power of sharing stories with one peer facilitator sharing how they felt that witnessing another participant’s story “really shifted how I was thinking about things and how I perceived people” (peer facilitator 1).

Participants reported several ways the program impacted their lives, such as learning that they could get help, how to get help, and how to overcome the mental aspects of chronic pain. One veteran shared profound health impacts and attributed the Vet-to-Vet program to having one of the best years of their life. Even those who did not attend many meetings spoke of it positively and stated that it should continue so others could try (Table).
From January 2022 to September 2025, > 80 veterans attended ≥ 1 meeting at RMRVAMC; 29 attended ≥ 1 meeting in the last quarter. There were > 1400 Vet-to-Vet encounters at RMRVAMC, with a mean (SD) of 14.2 (19.2) and a median of 4.5 encounters per participant. Half of the veterans attend ≥ 5 meetings, and one-third attended ≥ 10 meetings.
Since June 2023, 15 additional VHA facilities launched Vet-to-Vet programs. As of October 2025, > 350 veterans have participated in ≥ 1 Vet-to-Vet meeting, totaling > 4500 Vet-to-Vet encounters since the program’s inception (Figure 2).

Challenges
The RMRVAMC site and cosite leads are part of the national implementation team and dedicate substantial time to developing the program: 40 and 10 hours per week, respectively. Site leads at new locations do not receive funding for Vet-to-Vet activities and are recommended to dedicate only 4 hours per week to the program. Formally embedding Vet-to-Vet into the site leads’ roles is critical for sustainment.
The Vet-to-Vet model has changed. The initial Vet-to-Vet cohort included the 6-week Taking Charge of My Life and Health curriculum prior to moving to the mutual help format.24 While this curriculum still informs peer facilitator training, it is not used in new groups. It has anecdotally been reported that this change was positive, but the impact of this adaptation is unknown.
This evaluation cohort was small (16 participants) and initial patient reported and administrative outcomes were inconclusive. However, most veterans who stopped participating in Vet-to-Vet spoke fondly of their experiences with the program.
CONCLUSIONS
Vet-to-Vet is a promising new initiative to support self-management and social connection in chronic pain care. The program employs a mutual help approach and storytelling to empower veterans living with chronic pain. The effectiveness of these strategies will be evaluated, which will inform its continued growth. The program's current goals focus on sustainment at existing sites and expansion to new sites to reach more rural veterans across the VA enterprise. While Vet-to-Vet is designed to serve those who experience chronic pain, a partnership with the Office of Whole Health has established goals to begin expanding this model to other chronic conditions in 2026.
- Kerns RD, Philip EJ, Lee AW, Rosenberger PH. Implementation of the Veterans Health Administration national pain management strategy. Transl Behav Med. 2011;1:635-643. doi:10.1007/s13142-011-0094-3
- Pain Management, Opioid Safety, and PDMP (PMOP). US Department of Veterans Affairs. Updated August 21, 2025. Accessed September 25, 2025. https://www.va.gov/PAINMANAGEMENT/Providers/IntegratedTeambasedPainCare.asp
- US Department of Veterans Affairs. VHA Directive 2009-053. October 28, 2009. Accessed September 25, 2025. https://www.va.gov/PAINMANAGEMENT/docs/VHA09PainDirective.pdf
- Comprehensive Addiction and Recovery Act of 2016, S524, 114th Cong (2015-2016). Pub L No. 114-198. July 22, 2016. Accessed September 25, 2025. https://www.congress.gov/bill/114th-congress/senate-bill/524
- Bokhour B, Hyde J, Zeliadt, Mohr D. Whole Health System of Care Evaluation. US Department of Veterans Affairs. February 18, 2020. Accessed September 25, 2025. https://www.va.gov/WHOLEHEALTH/docs/EPCC_WHSevaluation_FinalReport_508.pdf
- Gaudet T, Kligler B. Whole health in the whole system of the veterans administration: how will we know we have reached this future state? J Altern Complement Med. 2019;25:S7-S11. doi:10.1089/acm.2018.29061.gau
- Kelly JF, Yeterian JD. The role of mutual-help groups in extending the framework of treatment. Alcohol Res Health. 2011;33:350-355.
- Humphreys K. Self-help/mutual aid organizations: the view from Mars. Subst Use Misuse. 1997;32:2105-2109. doi:10.3109/10826089709035622
- Chinman M, Kloos B, O’Connell M, Davidson L. Service providers’ views of psychiatric mutual support groups. J Community Psychol. 2002;30:349-366. doi:10.1002/jcop.10010
- Shue SA, McGuire AB, Matthias MS. Facilitators and barriers to implementation of a peer support intervention for patients with chronic pain: a qualitative study. Pain Med. 2019;20:1311-1320. doi:10.1093/pm/pny229
- Pester BD, Tankha H, Caño A, et al. Facing pain together: a randomized controlled trial of the effects of Facebook support groups on adults with chronic pain. J Pain. 2022;23:2121-2134. doi:10.1016/j.jpain.2022.07.013
- Matthias MS, McGuire AB, Kukla M, Daggy J, Myers LJ, Bair MJ. A brief peer support intervention for veterans with chronic musculoskeletal pain: a pilot study of feasibility and effectiveness. Pain Med. 2015;16:81-87. doi:10.1111/pme.12571
- Finlay KA, Elander J. Reflecting the transition from pain management services to chronic pain support group attendance: an interpretative phenomenological analysis. Br J Health Psychol. 2016;21:660-676. doi:10.1111/bjhp.12194
- Finlay KA, Peacock S, Elander J. Developing successful social support: an interpretative phenomenological analysis of mechanisms and processes in a chronic pain support group. Psychol Health. 2018;33:846-871. doi:10.1080/08870446.2017.1421188
- Farr M, Brant H, Patel R, et al. Experiences of patient-led chronic pain peer support groups after pain management programs: a qualitative study. Pain Med. 2021;22:2884-2895. doi:10.1093/pm/pnab189
- Mehl-Madrona L. Narrative Medicine: The Use of History and Story in the Healing Process. Bear & Company; 2007.
- Fioretti C, Mazzocco K, Riva S, Oliveri S, Masiero M, Pravettoni G. Research studies on patients’ illness experience using the Narrative Medicine approach: a systematic review. BMJ Open. 2016;6:e011220. doi:10.1136/bmjopen-2016-011220
- Hall JM, Powell J. Understanding the person through narrative. Nurs Res Pract. 2011;2011:293837. doi:10.1155/2011/293837
- Ricks L, Kitchens S, Goodrich T, Hancock E. My story: the use of narrative therapy in individual and group counseling. J Creat Ment Health. 2014;9:99-110. doi:10.1080/15401383.2013.870947
- Hydén L-C. Illness and narrative. Sociol Health Illn. 1997;19:48-69. doi:10.1111/j.1467-9566.1997.tb00015.x
- Georgiadis E, Johnson MI. Incorporating personal narratives in positive psychology interventions to manage chronic pain. Front Pain Res (Lausanne). 2023;4:1253310. doi:10.3389/fpain.2023.1253310
- Gucciardi E, Jean-Pierre N, Karam G, Sidani S. Designing and delivering facilitated storytelling interventions for chronic disease self-management: a scoping review. BMC Health Serv Res. 2016;16:249. doi:10.1186/s12913-016-1474-7
- Glasgow RE, Vogt TM, Boles SM. Evaluating the public health impact of health promotion interventions: the RE-AIM framework. Am J Public Health. 1999;89:1322-1327. doi:10.2105/ajph.89.9.1322
- Abadi M, Richard B, Shamblen S, et al. Achieving whole health: a preliminary study of TCMLH, a group-based program promoting self-care and empowerment among veterans. Health Educ Behav. 2022;49:347-357. doi:10.1177/10901981211011043
- Kerns RD, Philip EJ, Lee AW, Rosenberger PH. Implementation of the Veterans Health Administration national pain management strategy. Transl Behav Med. 2011;1:635-643. doi:10.1007/s13142-011-0094-3
- Pain Management, Opioid Safety, and PDMP (PMOP). US Department of Veterans Affairs. Updated August 21, 2025. Accessed September 25, 2025. https://www.va.gov/PAINMANAGEMENT/Providers/IntegratedTeambasedPainCare.asp
- US Department of Veterans Affairs. VHA Directive 2009-053. October 28, 2009. Accessed September 25, 2025. https://www.va.gov/PAINMANAGEMENT/docs/VHA09PainDirective.pdf
- Comprehensive Addiction and Recovery Act of 2016, S524, 114th Cong (2015-2016). Pub L No. 114-198. July 22, 2016. Accessed September 25, 2025. https://www.congress.gov/bill/114th-congress/senate-bill/524
- Bokhour B, Hyde J, Zeliadt, Mohr D. Whole Health System of Care Evaluation. US Department of Veterans Affairs. February 18, 2020. Accessed September 25, 2025. https://www.va.gov/WHOLEHEALTH/docs/EPCC_WHSevaluation_FinalReport_508.pdf
- Gaudet T, Kligler B. Whole health in the whole system of the veterans administration: how will we know we have reached this future state? J Altern Complement Med. 2019;25:S7-S11. doi:10.1089/acm.2018.29061.gau
- Kelly JF, Yeterian JD. The role of mutual-help groups in extending the framework of treatment. Alcohol Res Health. 2011;33:350-355.
- Humphreys K. Self-help/mutual aid organizations: the view from Mars. Subst Use Misuse. 1997;32:2105-2109. doi:10.3109/10826089709035622
- Chinman M, Kloos B, O’Connell M, Davidson L. Service providers’ views of psychiatric mutual support groups. J Community Psychol. 2002;30:349-366. doi:10.1002/jcop.10010
- Shue SA, McGuire AB, Matthias MS. Facilitators and barriers to implementation of a peer support intervention for patients with chronic pain: a qualitative study. Pain Med. 2019;20:1311-1320. doi:10.1093/pm/pny229
- Pester BD, Tankha H, Caño A, et al. Facing pain together: a randomized controlled trial of the effects of Facebook support groups on adults with chronic pain. J Pain. 2022;23:2121-2134. doi:10.1016/j.jpain.2022.07.013
- Matthias MS, McGuire AB, Kukla M, Daggy J, Myers LJ, Bair MJ. A brief peer support intervention for veterans with chronic musculoskeletal pain: a pilot study of feasibility and effectiveness. Pain Med. 2015;16:81-87. doi:10.1111/pme.12571
- Finlay KA, Elander J. Reflecting the transition from pain management services to chronic pain support group attendance: an interpretative phenomenological analysis. Br J Health Psychol. 2016;21:660-676. doi:10.1111/bjhp.12194
- Finlay KA, Peacock S, Elander J. Developing successful social support: an interpretative phenomenological analysis of mechanisms and processes in a chronic pain support group. Psychol Health. 2018;33:846-871. doi:10.1080/08870446.2017.1421188
- Farr M, Brant H, Patel R, et al. Experiences of patient-led chronic pain peer support groups after pain management programs: a qualitative study. Pain Med. 2021;22:2884-2895. doi:10.1093/pm/pnab189
- Mehl-Madrona L. Narrative Medicine: The Use of History and Story in the Healing Process. Bear & Company; 2007.
- Fioretti C, Mazzocco K, Riva S, Oliveri S, Masiero M, Pravettoni G. Research studies on patients’ illness experience using the Narrative Medicine approach: a systematic review. BMJ Open. 2016;6:e011220. doi:10.1136/bmjopen-2016-011220
- Hall JM, Powell J. Understanding the person through narrative. Nurs Res Pract. 2011;2011:293837. doi:10.1155/2011/293837
- Ricks L, Kitchens S, Goodrich T, Hancock E. My story: the use of narrative therapy in individual and group counseling. J Creat Ment Health. 2014;9:99-110. doi:10.1080/15401383.2013.870947
- Hydén L-C. Illness and narrative. Sociol Health Illn. 1997;19:48-69. doi:10.1111/j.1467-9566.1997.tb00015.x
- Georgiadis E, Johnson MI. Incorporating personal narratives in positive psychology interventions to manage chronic pain. Front Pain Res (Lausanne). 2023;4:1253310. doi:10.3389/fpain.2023.1253310
- Gucciardi E, Jean-Pierre N, Karam G, Sidani S. Designing and delivering facilitated storytelling interventions for chronic disease self-management: a scoping review. BMC Health Serv Res. 2016;16:249. doi:10.1186/s12913-016-1474-7
- Glasgow RE, Vogt TM, Boles SM. Evaluating the public health impact of health promotion interventions: the RE-AIM framework. Am J Public Health. 1999;89:1322-1327. doi:10.2105/ajph.89.9.1322
- Abadi M, Richard B, Shamblen S, et al. Achieving whole health: a preliminary study of TCMLH, a group-based program promoting self-care and empowerment among veterans. Health Educ Behav. 2022;49:347-357. doi:10.1177/10901981211011043
A True Community: The Vet-to-Vet Program for Chronic Pain
A True Community: The Vet-to-Vet Program for Chronic Pain
Anticoagulation Stewardship Efforts Via Indication Reviews at a Veterans Affairs Health Care System
Anticoagulation Stewardship Efforts Via Indication Reviews at a Veterans Affairs Health Care System
Due to the underlying mechanism of atrial fibrillation (Afib), clots can form within the left atrial appendage. Clots that become dislodged may lead to ischemic stroke and possibly death. The 2023 guidelines for atrial fibrillation from the American College of Cardiology and American Heart Association recommend anticoagulation therapy for patients with an Afib diagnosis and a CHA2DS2-VASc (congestive heart failure, hypertension, age ≥ 75 years, diabetes, stroke/vascular disease, age 65 to 74 years, and female sex) score pertinent for ≥ 1 non–sex-related factor (score ≥ 2 for women; ≥ 1 for men) to prevent stroke-related complications. The CHA2DS2-VASc score is a 9-point scoring tool based on comorbidities and conditions that increase risk of stroke in patients with Afib. Each value correlates to an annualized stroke risk percentage that increases as the score increases.
In clinical practice, patients meeting these thresholds are indicated for anticoagulation and are considered for indefinite use unless ≥ 1 of the following conditions are present: bleeding risk outweighs the stroke prevention benefit, Afib is episodic (< 48 hours) or a nonpharmacologic intervention, such as a left atrial appendage occlusion (LAAO) device is present.1
In patients with a diagnosed venous thromboembolism (VTE), such as deep vein thrombosis or pulmonary embolism, anticoagulation is used to treat the current thrombosis and prevent embolization that can ultimately lead to death. The 2021 guideline for VTE from the American College of Chest Physicians identifies certain risk factors that increase risk for VTE and categorizes them as transient or persistent. Transient risk factors include hospitalization > 3 days, major trauma, surgery, cast immobilization, hormone therapy, pregnancy, or prolonged travel > 8 hours. Persistent risk factors include malignancy, thrombophilia, and certain medications.
The guideline recommends therapy durations based on event frequency, the presence and classification of provoking risk factors, and bleeding risk. As the risk of recurrent thrombosis and other potential complications is greatest in the first 3 to 6 months after a diagnosed event, at least 3 months anticoagulation therapy is recommended following VTE diagnosis. At the 3-month mark, all regimens are suggested to be re-evaluated and considered for extended treatment duration if the event was unprovoked, recurrent, secondary to a persistent risk factor, or low bleed risk.2Anticoagulation is an important guideline-recommended pharmacologic intervention for various disease states, although its use is not without risks. The Institute for Safe Medication Practices has classified oral anticoagulants as high-alert medications. This designation was made because anticoagulant medications have the potential to cause harm when used or omitted in error and lead to life-threatening bleed or thrombotic complications.3Anticoagulation stewardship ensures that anticoagulation therapy is appropriately initiated, maintained, and discontinued when indicated. Because of the potential for harm, anticoagulation stewardship is an important part of Afib and VTE management. Pharmacists can help verify and evaluate anticoagulation therapies. Research suggests that pharmacist-led anticoagulation stewardship efforts may play a role in ensuring safer patient outcomes.4The purpose of this quality improvement (QI) study was to implement pharmacist-led anticoagulation stewardship practices at Veterans Affairs Phoenix Health Care System (VAPHCS) to identify veterans with Afib not currently on anticoagulation, as well as to identify veterans with a history of VTE events who have completed a sufficient treatment duration.
Methods
Anticoagulation stewardship efforts were implemented in 2 cohorts of patients: those with Afib who may be indicated to initiate anticoagulation, and those with a history of VTE events who may be indicated to consider anticoagulation discontinuation. Patient records were reviewed using a standardized note template, and recommendations to either initiate or discontinue anticoagulation therapy were documented. The VAPHCS Research Service reviewed this study and determined that it was not research and was exempt from institutional review board review.
Atrial Fibrillation Cohort
A population health dashboard created by the Stroke Prevention in Atrial Fibrillation/Flutter Targeting the uNTreated: a focus on health care disparities (SPAFF-TNT-D) national VA study team was used to identify veterans at VAPHCS with a diagnosis of Afib without an active VA prescription for an anticoagulant. The dashboard filtered and produced data points from the medical record that correlated to the components of the CHA2DS2-VASc score. All veterans identified by the dashboard with scores of 7 or 8 were included. No patients had a score of 9. Comprehensive chart reviews of available VA and non–VA-provided care records were conducted by the investigators, and a standardized note template designed by the SPAFF-TNT-D team (eAppendix 1) was used to document findings within the electronic health record (EHR). If anticoagulation was deemed to be indicated, the assigned primary care practitioner (PCP) as listed in the EHR was alerted to the note by the investigators for further evaluation and consideration of prescribing anticoagulation.
Venous Thromboembolism Cohort
VAPHCS pharmacy informatics pulled data that included veterans with documented VTE and an active VA anticoagulant prescription between November 2022 and November 2023. Veterans were reviewed in chronological order based on when the anticoagulant prescription was written. All veterans were included until an equal number of charts were reviewed in both the Afib and VTE cohorts. Comprehensive chart review of available VA- and non–VA-provided care records was conducted by the investigators, and a standardized note template as designed by the investigators (eAppendix 2) was used to document findings within the EHR. If the duration of anticoagulation therapy was deemed sufficient, the assigned anticoagulation clinical pharmacist practitioner (CPP) was alerted to the note by the investigators for further evaluation and consideration of discontinuing anticoagulation.
EHR reviews were conducted in October and November 2023 and lasted about 10 to 20 minutes per patient. To evaluate completeness and accuracy of the documented findings within the EHR, both investigators reviewed and cosigned the completed note template and verified the correct PCP was alerted to the recommendation for appropriate continuity of care. Results were reviewed in March 2024.
Outcomes
Atrial fibrillation cohort. The primary outcome was the number of veterans with Afib who were recommended to start anticoagulation therapy. Additional outcomes evaluated included the number of interventions completed, action taken by PCPs in response to the provided recommendation, and reasons provided by the investigators for not recommending initiation of anticoagulation therapy in specific veteran cases.
Venous thromboembolism cohort. The primary outcome was the number of veterans with a history of VTE events recommended to discontinue anticoagulation therapy. Additional outcomes included number of interventions completed, action taken by the anticoagulation CPP in response to the provided recommendation, and reasons provided by the investigators for not recommending discontinuation of anticoagulation therapy in specific veteran cases.
Analysis
Sample size was determined by the inclusion criteria and was not designed to attain statistical power. Data embedded in the Afib cohort standardized note template, also known as health factors, were later used for data analysis. Recommendations in the VTE cohort were manually tracked and recorded by the investigators. Results for this study were analyzed using descriptive statistics.
Results
A total of 114 veterans were reviewed and included in this study: 57 in each cohort. Seven recommendations were made regarding anticoagulation initiation for patients with Afib and 7 were made for anticoagulation discontinuation for patients with VTE (Table 1).

In the Afib cohort, 1 veteran was successfully initiated on anticoagulation therapy and 1 veteran was deemed appropriate for initiation of anticoagulation but was not reachable. Of the 5 recommendations with no action taken, 4 PCPs acknowledged the alert with no further documentation, and 1 PCP deferred the decision to cardiology with no further documentation. In the VTE cohort, 3 veterans successfully discontinued anticoagulation therapy and 2 veterans were further evaluated by the anticoagulation CPP and deemed appropriate to continue therapy based on potential for malignancy. Of the 2 recommendations with no action taken, 1 anticoagulation CPP acknowledged the alert with no further documentation and 1 anticoagulation CPP suggested further evaluation by PCP with no further documentation.
In the Afib cohort, a nonpharmacologic approach was defined as documentation of a LAAO device. An inaccurate diagnosis was defined as an Afib diagnosis being used in a previous visit, although there was no further confirmation of diagnosis via chart review. Veterans classified as already being on anticoagulation had documentation of non–VA-written anticoagulant prescriptions or receiving a supply of anticoagulants from a facility such as a nursing home. Anticoagulation was defined as unfavorable if a documented risk/benefit conversation was found via EHR review. Anticoagulation was defined as not indicated if the Afib was documented as transient, episodic, or historical (Table 2).

In the VTE cohort, no recommendations for discontinuation were made for veterans indicated to continue anticoagulation due to a concurrent Afib diagnosis. Chronic or recurrent events were defined as documentation of multiple VTE events and associated dates in the EHR. Persistent risk factors included malignancy or medications contributing to hypercoagulable states. Thrombophilia was defined as having documentation of a diagnosis in the EHR. An unprovoked event was defined as VTE without any documented transient risk factors (eg, hospitalization, trauma, surgery, cast immobilization, hormone therapy, pregnancy, or prolonged travel). Anticoagulation had already been discontinued in 1 veteran after the data were collected but before chart review occurred (Table 3).

Discussion
Pharmacy-led indication reviews resulted in appropriate recommendations for anticoagulation use in veterans with Afib and a history of VTE events. Overall, 12.3% of chart reviews in each cohort resulted in a recommendation being made, which was similar to the rate found by Koolian et al.5 In that study, 10% of recommendations were related to initiation or interruption of anticoagulation. This recommendation category consisted of several subcategories, including “suggesting therapeutic anticoagulation when none is currently ordered” and “suggesting anticoagulation cessation if no longer indicated,” but specific numerical prevalence was not provided.5
Online dashboard use allowed for greater population health management and identification of veterans with Afib who were not on active anticoagulation, providing opportunities to prevent stroke-related complications. Wang et al completed a similarly designed study that included a population health tool to identify patients with Afib who were not on anticoagulation and implemented pharmacist-led chart review and facilitation of recommendations to the responsible clinician. This study reviewed 1727 patients and recommended initiation of anticoagulation therapy for 75 (4.3%).6 The current study had a higher percentage of patients with recommendations for changes despite its smaller size.
Evaluating the duration of therapy for anticoagulation in veterans with a history of VTE events provided an opportunity to reduce unnecessary exposure to anticoagulation and minimize bleeding risks. Using a chart review process and standardized note template enabled the documentation of pertinent information that could be readily reviewed by the PCP. This process is a step toward ensuring VAPHCS PCPs provide guideline-recommended care and actively prevent stroke and bleeding complications. Adoption of this process into the current VAPHCS Anticoagulation Clinic workflow for review of veterans with either Afib or VTE could lead to more EHRs being reviewed and recommendations made, ultimately improving patient outcomes.
Therapeutic interventions based on the recommendations were completed for 1 of 7 veterans (14%) and 3 of 7 veterans (43%) in the Afib and VTE cohorts, respectively. The prevalence of completed interventions in this anticoagulation stewardship study was higher than those in Wang et al, who found only 9% of their recommendations resulted in PCPs considering action related to anticoagulation, and only 4% were successfully initiated.6
In the Afib cohort, veterans identified by the dashboard with a CHA2DS2-VASc of 7 or 8 were prioritized for review. Reviewing these veterans ensured that patients with the highest stroke risk were sufficiently evaluated and started on anticoagulation as needed to reduce stroke-related complications. In contrast, because these veterans had higher CHA2DS2-VASc scores, they may have already been evaluated for anticoagulation in the past and had a documented rationale for not being placed on anticoagulation (LAAO device placement was the most common rationale). Focusing on veterans with a lower CHA2DS2-VASc score such as 1 for men or 2 for women could potentially include more opportunities for recommendations. Although stroke risk may be lower in this population compared with those with higher CHA2DS2-VASc scores, guideline-recommended anticoagulation use may be missed for these patients.
In the VTE cohort, veterans with an anticoagulant prescription written 12 months before data collection were prioritized for review. Reviewing these veterans ensured that anticoagulation therapy met guideline recommendations of at least 3 months, with potential for extended duration upon further evaluation by a provider at that time. Based on collected results, most veterans were already reevaluated and had documented reasons why anticoagulation was still indicated; concurrent Afib was most common followed by chronic or recurrent VTE. Reviewing veterans with more recent prescriptions just over the recommended 3-month duration could potentially include more opportunities for recommendations to be made. It is more likely that by 3 months another PCP had not already weighed in on the duration of therapy, and the anticoagulation CPP could ensure a thorough review is conducted with guideline-based recommendations.
Most published literature on anticoagulation stewardship efforts is focused on inpatient management and policy changes, or concentrate on attributes of therapy such as appropriate dosing and drug interactions. This study highlighted that gaps in care related to anticoagulation use and discontinuation are present in the VAPHCS population and can be appropriately addressed via pharmacist-led indication reviews. Future studies designed to focus on initiating anticoagulation where appropriate, and discontinuing where a sufficient treatment period has been completed, are warranted to minimize this gap in care and allow health systems to work toward process changes to ensure safe and optimized care is provided for the patients they serve.
Limitations
In the Afib cohort, 5 of 7 recommendations (71%) had no further action taken by the PCP, which may represent a barrier to care. In contrast, 2 of 7 recommendations (29%) had no further action in the VTE cohort. It is possible that the difference can be attributed to the anticoagulation CPP receiving VTE alerts and PCPs receiving Afib alerts. The anticoagulation CPP was familiar with this QI study and may have better understood the purpose of the chart review and the need to provide a timely response. PCPs may have been less likely to take action because they were unfamiliar with the anticoagulation stewardship initiative and standardized note template or overwhelmed by too many EHR alerts.
The lack of PCP response to a virtual alert or message also was observed by Wang et al, whereas Koolian et al reported higher intervention completion rates, with verbal recommendations being made to the responsible clinicians. To further ensure these pertinent recommendations for anticoagulation initiation in veterans with Afib are properly reviewed and evaluated, future research could include intentional follow-up with the PCP regarding the alert, PCP-specific education about the anticoagulation stewardship initiative and the role of the standardized note template, and collaboration with PCPs to identify alternative ways to relay recommendations in a way that would ensure the completion of appropriate and timely review.
Conclusions
This study identified gaps in care related to anticoagulation needs in the VAPHCS veteran population. Utilizing a standardized indication review process allows pharmacists to evaluate anticoagulant use for both appropriate indication and duration of therapy. Providing recommendations via chart review notes and alerting respective PCPs and CPPs results in veterans receiving safe and optimized care regarding their anticoagulation needs.
- Joglar JA, Chung MK, Armbruster AL, et al. 2023 ACC/AHA/ACCP/HRS guideline for the diagnosis and management of atrial fibrillation: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2024;149:e1-e156. doi:10.1161/CIR.0000000000001193
- Stevens SM, Woller SC, Kreuziger LB, et al. Antithrombotic therapy for VTE disease: second update of the CHEST guideline and expert panel report. Chest. 2021;160:e545-e608. doi:10.1016/j.chest.2021.07.055
- Institute for Safe Medication Practices (ISMP). List of high-alert medications in community/ambulatory care settings. ISMP. September 30, 2021. Accessed September 11, 2025. https://home.ecri.org/blogs/ismp-resources/high-alert-medications-in-community-ambulatory-care-settings
- Burnett AE, Barnes GD. A call to action for anticoagulation stewardship. Res Pract Thromb Haemost. 2022;6:e12757. doi:10.1002/rth2.12757
- Koolian M, Wiseman D, Mantzanis H, et al. Anticoagulation stewardship: descriptive analysis of a novel approach to appropriate anticoagulant prescription. Res Pract Thromb Haemost. 2022;6:e12758. doi:10.1002/rth2.12758
- Wang SV, Rogers JR, Jin Y, et al. Stepped-wedge randomised trial to evaluate population health intervention designed to increase appropriate anticoagulation in patients with atrial fibrillation. BMJ Qual Saf. 2019;28:835-842. doi:10.1136/bmjqs-2019-009367
Due to the underlying mechanism of atrial fibrillation (Afib), clots can form within the left atrial appendage. Clots that become dislodged may lead to ischemic stroke and possibly death. The 2023 guidelines for atrial fibrillation from the American College of Cardiology and American Heart Association recommend anticoagulation therapy for patients with an Afib diagnosis and a CHA2DS2-VASc (congestive heart failure, hypertension, age ≥ 75 years, diabetes, stroke/vascular disease, age 65 to 74 years, and female sex) score pertinent for ≥ 1 non–sex-related factor (score ≥ 2 for women; ≥ 1 for men) to prevent stroke-related complications. The CHA2DS2-VASc score is a 9-point scoring tool based on comorbidities and conditions that increase risk of stroke in patients with Afib. Each value correlates to an annualized stroke risk percentage that increases as the score increases.
In clinical practice, patients meeting these thresholds are indicated for anticoagulation and are considered for indefinite use unless ≥ 1 of the following conditions are present: bleeding risk outweighs the stroke prevention benefit, Afib is episodic (< 48 hours) or a nonpharmacologic intervention, such as a left atrial appendage occlusion (LAAO) device is present.1
In patients with a diagnosed venous thromboembolism (VTE), such as deep vein thrombosis or pulmonary embolism, anticoagulation is used to treat the current thrombosis and prevent embolization that can ultimately lead to death. The 2021 guideline for VTE from the American College of Chest Physicians identifies certain risk factors that increase risk for VTE and categorizes them as transient or persistent. Transient risk factors include hospitalization > 3 days, major trauma, surgery, cast immobilization, hormone therapy, pregnancy, or prolonged travel > 8 hours. Persistent risk factors include malignancy, thrombophilia, and certain medications.
The guideline recommends therapy durations based on event frequency, the presence and classification of provoking risk factors, and bleeding risk. As the risk of recurrent thrombosis and other potential complications is greatest in the first 3 to 6 months after a diagnosed event, at least 3 months anticoagulation therapy is recommended following VTE diagnosis. At the 3-month mark, all regimens are suggested to be re-evaluated and considered for extended treatment duration if the event was unprovoked, recurrent, secondary to a persistent risk factor, or low bleed risk.2Anticoagulation is an important guideline-recommended pharmacologic intervention for various disease states, although its use is not without risks. The Institute for Safe Medication Practices has classified oral anticoagulants as high-alert medications. This designation was made because anticoagulant medications have the potential to cause harm when used or omitted in error and lead to life-threatening bleed or thrombotic complications.3Anticoagulation stewardship ensures that anticoagulation therapy is appropriately initiated, maintained, and discontinued when indicated. Because of the potential for harm, anticoagulation stewardship is an important part of Afib and VTE management. Pharmacists can help verify and evaluate anticoagulation therapies. Research suggests that pharmacist-led anticoagulation stewardship efforts may play a role in ensuring safer patient outcomes.4The purpose of this quality improvement (QI) study was to implement pharmacist-led anticoagulation stewardship practices at Veterans Affairs Phoenix Health Care System (VAPHCS) to identify veterans with Afib not currently on anticoagulation, as well as to identify veterans with a history of VTE events who have completed a sufficient treatment duration.
Methods
Anticoagulation stewardship efforts were implemented in 2 cohorts of patients: those with Afib who may be indicated to initiate anticoagulation, and those with a history of VTE events who may be indicated to consider anticoagulation discontinuation. Patient records were reviewed using a standardized note template, and recommendations to either initiate or discontinue anticoagulation therapy were documented. The VAPHCS Research Service reviewed this study and determined that it was not research and was exempt from institutional review board review.
Atrial Fibrillation Cohort
A population health dashboard created by the Stroke Prevention in Atrial Fibrillation/Flutter Targeting the uNTreated: a focus on health care disparities (SPAFF-TNT-D) national VA study team was used to identify veterans at VAPHCS with a diagnosis of Afib without an active VA prescription for an anticoagulant. The dashboard filtered and produced data points from the medical record that correlated to the components of the CHA2DS2-VASc score. All veterans identified by the dashboard with scores of 7 or 8 were included. No patients had a score of 9. Comprehensive chart reviews of available VA and non–VA-provided care records were conducted by the investigators, and a standardized note template designed by the SPAFF-TNT-D team (eAppendix 1) was used to document findings within the electronic health record (EHR). If anticoagulation was deemed to be indicated, the assigned primary care practitioner (PCP) as listed in the EHR was alerted to the note by the investigators for further evaluation and consideration of prescribing anticoagulation.
Venous Thromboembolism Cohort
VAPHCS pharmacy informatics pulled data that included veterans with documented VTE and an active VA anticoagulant prescription between November 2022 and November 2023. Veterans were reviewed in chronological order based on when the anticoagulant prescription was written. All veterans were included until an equal number of charts were reviewed in both the Afib and VTE cohorts. Comprehensive chart review of available VA- and non–VA-provided care records was conducted by the investigators, and a standardized note template as designed by the investigators (eAppendix 2) was used to document findings within the EHR. If the duration of anticoagulation therapy was deemed sufficient, the assigned anticoagulation clinical pharmacist practitioner (CPP) was alerted to the note by the investigators for further evaluation and consideration of discontinuing anticoagulation.
EHR reviews were conducted in October and November 2023 and lasted about 10 to 20 minutes per patient. To evaluate completeness and accuracy of the documented findings within the EHR, both investigators reviewed and cosigned the completed note template and verified the correct PCP was alerted to the recommendation for appropriate continuity of care. Results were reviewed in March 2024.
Outcomes
Atrial fibrillation cohort. The primary outcome was the number of veterans with Afib who were recommended to start anticoagulation therapy. Additional outcomes evaluated included the number of interventions completed, action taken by PCPs in response to the provided recommendation, and reasons provided by the investigators for not recommending initiation of anticoagulation therapy in specific veteran cases.
Venous thromboembolism cohort. The primary outcome was the number of veterans with a history of VTE events recommended to discontinue anticoagulation therapy. Additional outcomes included number of interventions completed, action taken by the anticoagulation CPP in response to the provided recommendation, and reasons provided by the investigators for not recommending discontinuation of anticoagulation therapy in specific veteran cases.
Analysis
Sample size was determined by the inclusion criteria and was not designed to attain statistical power. Data embedded in the Afib cohort standardized note template, also known as health factors, were later used for data analysis. Recommendations in the VTE cohort were manually tracked and recorded by the investigators. Results for this study were analyzed using descriptive statistics.
Results
A total of 114 veterans were reviewed and included in this study: 57 in each cohort. Seven recommendations were made regarding anticoagulation initiation for patients with Afib and 7 were made for anticoagulation discontinuation for patients with VTE (Table 1).

In the Afib cohort, 1 veteran was successfully initiated on anticoagulation therapy and 1 veteran was deemed appropriate for initiation of anticoagulation but was not reachable. Of the 5 recommendations with no action taken, 4 PCPs acknowledged the alert with no further documentation, and 1 PCP deferred the decision to cardiology with no further documentation. In the VTE cohort, 3 veterans successfully discontinued anticoagulation therapy and 2 veterans were further evaluated by the anticoagulation CPP and deemed appropriate to continue therapy based on potential for malignancy. Of the 2 recommendations with no action taken, 1 anticoagulation CPP acknowledged the alert with no further documentation and 1 anticoagulation CPP suggested further evaluation by PCP with no further documentation.
In the Afib cohort, a nonpharmacologic approach was defined as documentation of a LAAO device. An inaccurate diagnosis was defined as an Afib diagnosis being used in a previous visit, although there was no further confirmation of diagnosis via chart review. Veterans classified as already being on anticoagulation had documentation of non–VA-written anticoagulant prescriptions or receiving a supply of anticoagulants from a facility such as a nursing home. Anticoagulation was defined as unfavorable if a documented risk/benefit conversation was found via EHR review. Anticoagulation was defined as not indicated if the Afib was documented as transient, episodic, or historical (Table 2).

In the VTE cohort, no recommendations for discontinuation were made for veterans indicated to continue anticoagulation due to a concurrent Afib diagnosis. Chronic or recurrent events were defined as documentation of multiple VTE events and associated dates in the EHR. Persistent risk factors included malignancy or medications contributing to hypercoagulable states. Thrombophilia was defined as having documentation of a diagnosis in the EHR. An unprovoked event was defined as VTE without any documented transient risk factors (eg, hospitalization, trauma, surgery, cast immobilization, hormone therapy, pregnancy, or prolonged travel). Anticoagulation had already been discontinued in 1 veteran after the data were collected but before chart review occurred (Table 3).

Discussion
Pharmacy-led indication reviews resulted in appropriate recommendations for anticoagulation use in veterans with Afib and a history of VTE events. Overall, 12.3% of chart reviews in each cohort resulted in a recommendation being made, which was similar to the rate found by Koolian et al.5 In that study, 10% of recommendations were related to initiation or interruption of anticoagulation. This recommendation category consisted of several subcategories, including “suggesting therapeutic anticoagulation when none is currently ordered” and “suggesting anticoagulation cessation if no longer indicated,” but specific numerical prevalence was not provided.5
Online dashboard use allowed for greater population health management and identification of veterans with Afib who were not on active anticoagulation, providing opportunities to prevent stroke-related complications. Wang et al completed a similarly designed study that included a population health tool to identify patients with Afib who were not on anticoagulation and implemented pharmacist-led chart review and facilitation of recommendations to the responsible clinician. This study reviewed 1727 patients and recommended initiation of anticoagulation therapy for 75 (4.3%).6 The current study had a higher percentage of patients with recommendations for changes despite its smaller size.
Evaluating the duration of therapy for anticoagulation in veterans with a history of VTE events provided an opportunity to reduce unnecessary exposure to anticoagulation and minimize bleeding risks. Using a chart review process and standardized note template enabled the documentation of pertinent information that could be readily reviewed by the PCP. This process is a step toward ensuring VAPHCS PCPs provide guideline-recommended care and actively prevent stroke and bleeding complications. Adoption of this process into the current VAPHCS Anticoagulation Clinic workflow for review of veterans with either Afib or VTE could lead to more EHRs being reviewed and recommendations made, ultimately improving patient outcomes.
Therapeutic interventions based on the recommendations were completed for 1 of 7 veterans (14%) and 3 of 7 veterans (43%) in the Afib and VTE cohorts, respectively. The prevalence of completed interventions in this anticoagulation stewardship study was higher than those in Wang et al, who found only 9% of their recommendations resulted in PCPs considering action related to anticoagulation, and only 4% were successfully initiated.6
In the Afib cohort, veterans identified by the dashboard with a CHA2DS2-VASc of 7 or 8 were prioritized for review. Reviewing these veterans ensured that patients with the highest stroke risk were sufficiently evaluated and started on anticoagulation as needed to reduce stroke-related complications. In contrast, because these veterans had higher CHA2DS2-VASc scores, they may have already been evaluated for anticoagulation in the past and had a documented rationale for not being placed on anticoagulation (LAAO device placement was the most common rationale). Focusing on veterans with a lower CHA2DS2-VASc score such as 1 for men or 2 for women could potentially include more opportunities for recommendations. Although stroke risk may be lower in this population compared with those with higher CHA2DS2-VASc scores, guideline-recommended anticoagulation use may be missed for these patients.
In the VTE cohort, veterans with an anticoagulant prescription written 12 months before data collection were prioritized for review. Reviewing these veterans ensured that anticoagulation therapy met guideline recommendations of at least 3 months, with potential for extended duration upon further evaluation by a provider at that time. Based on collected results, most veterans were already reevaluated and had documented reasons why anticoagulation was still indicated; concurrent Afib was most common followed by chronic or recurrent VTE. Reviewing veterans with more recent prescriptions just over the recommended 3-month duration could potentially include more opportunities for recommendations to be made. It is more likely that by 3 months another PCP had not already weighed in on the duration of therapy, and the anticoagulation CPP could ensure a thorough review is conducted with guideline-based recommendations.
Most published literature on anticoagulation stewardship efforts is focused on inpatient management and policy changes, or concentrate on attributes of therapy such as appropriate dosing and drug interactions. This study highlighted that gaps in care related to anticoagulation use and discontinuation are present in the VAPHCS population and can be appropriately addressed via pharmacist-led indication reviews. Future studies designed to focus on initiating anticoagulation where appropriate, and discontinuing where a sufficient treatment period has been completed, are warranted to minimize this gap in care and allow health systems to work toward process changes to ensure safe and optimized care is provided for the patients they serve.
Limitations
In the Afib cohort, 5 of 7 recommendations (71%) had no further action taken by the PCP, which may represent a barrier to care. In contrast, 2 of 7 recommendations (29%) had no further action in the VTE cohort. It is possible that the difference can be attributed to the anticoagulation CPP receiving VTE alerts and PCPs receiving Afib alerts. The anticoagulation CPP was familiar with this QI study and may have better understood the purpose of the chart review and the need to provide a timely response. PCPs may have been less likely to take action because they were unfamiliar with the anticoagulation stewardship initiative and standardized note template or overwhelmed by too many EHR alerts.
The lack of PCP response to a virtual alert or message also was observed by Wang et al, whereas Koolian et al reported higher intervention completion rates, with verbal recommendations being made to the responsible clinicians. To further ensure these pertinent recommendations for anticoagulation initiation in veterans with Afib are properly reviewed and evaluated, future research could include intentional follow-up with the PCP regarding the alert, PCP-specific education about the anticoagulation stewardship initiative and the role of the standardized note template, and collaboration with PCPs to identify alternative ways to relay recommendations in a way that would ensure the completion of appropriate and timely review.
Conclusions
This study identified gaps in care related to anticoagulation needs in the VAPHCS veteran population. Utilizing a standardized indication review process allows pharmacists to evaluate anticoagulant use for both appropriate indication and duration of therapy. Providing recommendations via chart review notes and alerting respective PCPs and CPPs results in veterans receiving safe and optimized care regarding their anticoagulation needs.
Due to the underlying mechanism of atrial fibrillation (Afib), clots can form within the left atrial appendage. Clots that become dislodged may lead to ischemic stroke and possibly death. The 2023 guidelines for atrial fibrillation from the American College of Cardiology and American Heart Association recommend anticoagulation therapy for patients with an Afib diagnosis and a CHA2DS2-VASc (congestive heart failure, hypertension, age ≥ 75 years, diabetes, stroke/vascular disease, age 65 to 74 years, and female sex) score pertinent for ≥ 1 non–sex-related factor (score ≥ 2 for women; ≥ 1 for men) to prevent stroke-related complications. The CHA2DS2-VASc score is a 9-point scoring tool based on comorbidities and conditions that increase risk of stroke in patients with Afib. Each value correlates to an annualized stroke risk percentage that increases as the score increases.
In clinical practice, patients meeting these thresholds are indicated for anticoagulation and are considered for indefinite use unless ≥ 1 of the following conditions are present: bleeding risk outweighs the stroke prevention benefit, Afib is episodic (< 48 hours) or a nonpharmacologic intervention, such as a left atrial appendage occlusion (LAAO) device is present.1
In patients with a diagnosed venous thromboembolism (VTE), such as deep vein thrombosis or pulmonary embolism, anticoagulation is used to treat the current thrombosis and prevent embolization that can ultimately lead to death. The 2021 guideline for VTE from the American College of Chest Physicians identifies certain risk factors that increase risk for VTE and categorizes them as transient or persistent. Transient risk factors include hospitalization > 3 days, major trauma, surgery, cast immobilization, hormone therapy, pregnancy, or prolonged travel > 8 hours. Persistent risk factors include malignancy, thrombophilia, and certain medications.
The guideline recommends therapy durations based on event frequency, the presence and classification of provoking risk factors, and bleeding risk. As the risk of recurrent thrombosis and other potential complications is greatest in the first 3 to 6 months after a diagnosed event, at least 3 months anticoagulation therapy is recommended following VTE diagnosis. At the 3-month mark, all regimens are suggested to be re-evaluated and considered for extended treatment duration if the event was unprovoked, recurrent, secondary to a persistent risk factor, or low bleed risk.2Anticoagulation is an important guideline-recommended pharmacologic intervention for various disease states, although its use is not without risks. The Institute for Safe Medication Practices has classified oral anticoagulants as high-alert medications. This designation was made because anticoagulant medications have the potential to cause harm when used or omitted in error and lead to life-threatening bleed or thrombotic complications.3Anticoagulation stewardship ensures that anticoagulation therapy is appropriately initiated, maintained, and discontinued when indicated. Because of the potential for harm, anticoagulation stewardship is an important part of Afib and VTE management. Pharmacists can help verify and evaluate anticoagulation therapies. Research suggests that pharmacist-led anticoagulation stewardship efforts may play a role in ensuring safer patient outcomes.4The purpose of this quality improvement (QI) study was to implement pharmacist-led anticoagulation stewardship practices at Veterans Affairs Phoenix Health Care System (VAPHCS) to identify veterans with Afib not currently on anticoagulation, as well as to identify veterans with a history of VTE events who have completed a sufficient treatment duration.
Methods
Anticoagulation stewardship efforts were implemented in 2 cohorts of patients: those with Afib who may be indicated to initiate anticoagulation, and those with a history of VTE events who may be indicated to consider anticoagulation discontinuation. Patient records were reviewed using a standardized note template, and recommendations to either initiate or discontinue anticoagulation therapy were documented. The VAPHCS Research Service reviewed this study and determined that it was not research and was exempt from institutional review board review.
Atrial Fibrillation Cohort
A population health dashboard created by the Stroke Prevention in Atrial Fibrillation/Flutter Targeting the uNTreated: a focus on health care disparities (SPAFF-TNT-D) national VA study team was used to identify veterans at VAPHCS with a diagnosis of Afib without an active VA prescription for an anticoagulant. The dashboard filtered and produced data points from the medical record that correlated to the components of the CHA2DS2-VASc score. All veterans identified by the dashboard with scores of 7 or 8 were included. No patients had a score of 9. Comprehensive chart reviews of available VA and non–VA-provided care records were conducted by the investigators, and a standardized note template designed by the SPAFF-TNT-D team (eAppendix 1) was used to document findings within the electronic health record (EHR). If anticoagulation was deemed to be indicated, the assigned primary care practitioner (PCP) as listed in the EHR was alerted to the note by the investigators for further evaluation and consideration of prescribing anticoagulation.
Venous Thromboembolism Cohort
VAPHCS pharmacy informatics pulled data that included veterans with documented VTE and an active VA anticoagulant prescription between November 2022 and November 2023. Veterans were reviewed in chronological order based on when the anticoagulant prescription was written. All veterans were included until an equal number of charts were reviewed in both the Afib and VTE cohorts. Comprehensive chart review of available VA- and non–VA-provided care records was conducted by the investigators, and a standardized note template as designed by the investigators (eAppendix 2) was used to document findings within the EHR. If the duration of anticoagulation therapy was deemed sufficient, the assigned anticoagulation clinical pharmacist practitioner (CPP) was alerted to the note by the investigators for further evaluation and consideration of discontinuing anticoagulation.
EHR reviews were conducted in October and November 2023 and lasted about 10 to 20 minutes per patient. To evaluate completeness and accuracy of the documented findings within the EHR, both investigators reviewed and cosigned the completed note template and verified the correct PCP was alerted to the recommendation for appropriate continuity of care. Results were reviewed in March 2024.
Outcomes
Atrial fibrillation cohort. The primary outcome was the number of veterans with Afib who were recommended to start anticoagulation therapy. Additional outcomes evaluated included the number of interventions completed, action taken by PCPs in response to the provided recommendation, and reasons provided by the investigators for not recommending initiation of anticoagulation therapy in specific veteran cases.
Venous thromboembolism cohort. The primary outcome was the number of veterans with a history of VTE events recommended to discontinue anticoagulation therapy. Additional outcomes included number of interventions completed, action taken by the anticoagulation CPP in response to the provided recommendation, and reasons provided by the investigators for not recommending discontinuation of anticoagulation therapy in specific veteran cases.
Analysis
Sample size was determined by the inclusion criteria and was not designed to attain statistical power. Data embedded in the Afib cohort standardized note template, also known as health factors, were later used for data analysis. Recommendations in the VTE cohort were manually tracked and recorded by the investigators. Results for this study were analyzed using descriptive statistics.
Results
A total of 114 veterans were reviewed and included in this study: 57 in each cohort. Seven recommendations were made regarding anticoagulation initiation for patients with Afib and 7 were made for anticoagulation discontinuation for patients with VTE (Table 1).

In the Afib cohort, 1 veteran was successfully initiated on anticoagulation therapy and 1 veteran was deemed appropriate for initiation of anticoagulation but was not reachable. Of the 5 recommendations with no action taken, 4 PCPs acknowledged the alert with no further documentation, and 1 PCP deferred the decision to cardiology with no further documentation. In the VTE cohort, 3 veterans successfully discontinued anticoagulation therapy and 2 veterans were further evaluated by the anticoagulation CPP and deemed appropriate to continue therapy based on potential for malignancy. Of the 2 recommendations with no action taken, 1 anticoagulation CPP acknowledged the alert with no further documentation and 1 anticoagulation CPP suggested further evaluation by PCP with no further documentation.
In the Afib cohort, a nonpharmacologic approach was defined as documentation of a LAAO device. An inaccurate diagnosis was defined as an Afib diagnosis being used in a previous visit, although there was no further confirmation of diagnosis via chart review. Veterans classified as already being on anticoagulation had documentation of non–VA-written anticoagulant prescriptions or receiving a supply of anticoagulants from a facility such as a nursing home. Anticoagulation was defined as unfavorable if a documented risk/benefit conversation was found via EHR review. Anticoagulation was defined as not indicated if the Afib was documented as transient, episodic, or historical (Table 2).

In the VTE cohort, no recommendations for discontinuation were made for veterans indicated to continue anticoagulation due to a concurrent Afib diagnosis. Chronic or recurrent events were defined as documentation of multiple VTE events and associated dates in the EHR. Persistent risk factors included malignancy or medications contributing to hypercoagulable states. Thrombophilia was defined as having documentation of a diagnosis in the EHR. An unprovoked event was defined as VTE without any documented transient risk factors (eg, hospitalization, trauma, surgery, cast immobilization, hormone therapy, pregnancy, or prolonged travel). Anticoagulation had already been discontinued in 1 veteran after the data were collected but before chart review occurred (Table 3).

Discussion
Pharmacy-led indication reviews resulted in appropriate recommendations for anticoagulation use in veterans with Afib and a history of VTE events. Overall, 12.3% of chart reviews in each cohort resulted in a recommendation being made, which was similar to the rate found by Koolian et al.5 In that study, 10% of recommendations were related to initiation or interruption of anticoagulation. This recommendation category consisted of several subcategories, including “suggesting therapeutic anticoagulation when none is currently ordered” and “suggesting anticoagulation cessation if no longer indicated,” but specific numerical prevalence was not provided.5
Online dashboard use allowed for greater population health management and identification of veterans with Afib who were not on active anticoagulation, providing opportunities to prevent stroke-related complications. Wang et al completed a similarly designed study that included a population health tool to identify patients with Afib who were not on anticoagulation and implemented pharmacist-led chart review and facilitation of recommendations to the responsible clinician. This study reviewed 1727 patients and recommended initiation of anticoagulation therapy for 75 (4.3%).6 The current study had a higher percentage of patients with recommendations for changes despite its smaller size.
Evaluating the duration of therapy for anticoagulation in veterans with a history of VTE events provided an opportunity to reduce unnecessary exposure to anticoagulation and minimize bleeding risks. Using a chart review process and standardized note template enabled the documentation of pertinent information that could be readily reviewed by the PCP. This process is a step toward ensuring VAPHCS PCPs provide guideline-recommended care and actively prevent stroke and bleeding complications. Adoption of this process into the current VAPHCS Anticoagulation Clinic workflow for review of veterans with either Afib or VTE could lead to more EHRs being reviewed and recommendations made, ultimately improving patient outcomes.
Therapeutic interventions based on the recommendations were completed for 1 of 7 veterans (14%) and 3 of 7 veterans (43%) in the Afib and VTE cohorts, respectively. The prevalence of completed interventions in this anticoagulation stewardship study was higher than those in Wang et al, who found only 9% of their recommendations resulted in PCPs considering action related to anticoagulation, and only 4% were successfully initiated.6
In the Afib cohort, veterans identified by the dashboard with a CHA2DS2-VASc of 7 or 8 were prioritized for review. Reviewing these veterans ensured that patients with the highest stroke risk were sufficiently evaluated and started on anticoagulation as needed to reduce stroke-related complications. In contrast, because these veterans had higher CHA2DS2-VASc scores, they may have already been evaluated for anticoagulation in the past and had a documented rationale for not being placed on anticoagulation (LAAO device placement was the most common rationale). Focusing on veterans with a lower CHA2DS2-VASc score such as 1 for men or 2 for women could potentially include more opportunities for recommendations. Although stroke risk may be lower in this population compared with those with higher CHA2DS2-VASc scores, guideline-recommended anticoagulation use may be missed for these patients.
In the VTE cohort, veterans with an anticoagulant prescription written 12 months before data collection were prioritized for review. Reviewing these veterans ensured that anticoagulation therapy met guideline recommendations of at least 3 months, with potential for extended duration upon further evaluation by a provider at that time. Based on collected results, most veterans were already reevaluated and had documented reasons why anticoagulation was still indicated; concurrent Afib was most common followed by chronic or recurrent VTE. Reviewing veterans with more recent prescriptions just over the recommended 3-month duration could potentially include more opportunities for recommendations to be made. It is more likely that by 3 months another PCP had not already weighed in on the duration of therapy, and the anticoagulation CPP could ensure a thorough review is conducted with guideline-based recommendations.
Most published literature on anticoagulation stewardship efforts is focused on inpatient management and policy changes, or concentrate on attributes of therapy such as appropriate dosing and drug interactions. This study highlighted that gaps in care related to anticoagulation use and discontinuation are present in the VAPHCS population and can be appropriately addressed via pharmacist-led indication reviews. Future studies designed to focus on initiating anticoagulation where appropriate, and discontinuing where a sufficient treatment period has been completed, are warranted to minimize this gap in care and allow health systems to work toward process changes to ensure safe and optimized care is provided for the patients they serve.
Limitations
In the Afib cohort, 5 of 7 recommendations (71%) had no further action taken by the PCP, which may represent a barrier to care. In contrast, 2 of 7 recommendations (29%) had no further action in the VTE cohort. It is possible that the difference can be attributed to the anticoagulation CPP receiving VTE alerts and PCPs receiving Afib alerts. The anticoagulation CPP was familiar with this QI study and may have better understood the purpose of the chart review and the need to provide a timely response. PCPs may have been less likely to take action because they were unfamiliar with the anticoagulation stewardship initiative and standardized note template or overwhelmed by too many EHR alerts.
The lack of PCP response to a virtual alert or message also was observed by Wang et al, whereas Koolian et al reported higher intervention completion rates, with verbal recommendations being made to the responsible clinicians. To further ensure these pertinent recommendations for anticoagulation initiation in veterans with Afib are properly reviewed and evaluated, future research could include intentional follow-up with the PCP regarding the alert, PCP-specific education about the anticoagulation stewardship initiative and the role of the standardized note template, and collaboration with PCPs to identify alternative ways to relay recommendations in a way that would ensure the completion of appropriate and timely review.
Conclusions
This study identified gaps in care related to anticoagulation needs in the VAPHCS veteran population. Utilizing a standardized indication review process allows pharmacists to evaluate anticoagulant use for both appropriate indication and duration of therapy. Providing recommendations via chart review notes and alerting respective PCPs and CPPs results in veterans receiving safe and optimized care regarding their anticoagulation needs.
- Joglar JA, Chung MK, Armbruster AL, et al. 2023 ACC/AHA/ACCP/HRS guideline for the diagnosis and management of atrial fibrillation: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2024;149:e1-e156. doi:10.1161/CIR.0000000000001193
- Stevens SM, Woller SC, Kreuziger LB, et al. Antithrombotic therapy for VTE disease: second update of the CHEST guideline and expert panel report. Chest. 2021;160:e545-e608. doi:10.1016/j.chest.2021.07.055
- Institute for Safe Medication Practices (ISMP). List of high-alert medications in community/ambulatory care settings. ISMP. September 30, 2021. Accessed September 11, 2025. https://home.ecri.org/blogs/ismp-resources/high-alert-medications-in-community-ambulatory-care-settings
- Burnett AE, Barnes GD. A call to action for anticoagulation stewardship. Res Pract Thromb Haemost. 2022;6:e12757. doi:10.1002/rth2.12757
- Koolian M, Wiseman D, Mantzanis H, et al. Anticoagulation stewardship: descriptive analysis of a novel approach to appropriate anticoagulant prescription. Res Pract Thromb Haemost. 2022;6:e12758. doi:10.1002/rth2.12758
- Wang SV, Rogers JR, Jin Y, et al. Stepped-wedge randomised trial to evaluate population health intervention designed to increase appropriate anticoagulation in patients with atrial fibrillation. BMJ Qual Saf. 2019;28:835-842. doi:10.1136/bmjqs-2019-009367
- Joglar JA, Chung MK, Armbruster AL, et al. 2023 ACC/AHA/ACCP/HRS guideline for the diagnosis and management of atrial fibrillation: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2024;149:e1-e156. doi:10.1161/CIR.0000000000001193
- Stevens SM, Woller SC, Kreuziger LB, et al. Antithrombotic therapy for VTE disease: second update of the CHEST guideline and expert panel report. Chest. 2021;160:e545-e608. doi:10.1016/j.chest.2021.07.055
- Institute for Safe Medication Practices (ISMP). List of high-alert medications in community/ambulatory care settings. ISMP. September 30, 2021. Accessed September 11, 2025. https://home.ecri.org/blogs/ismp-resources/high-alert-medications-in-community-ambulatory-care-settings
- Burnett AE, Barnes GD. A call to action for anticoagulation stewardship. Res Pract Thromb Haemost. 2022;6:e12757. doi:10.1002/rth2.12757
- Koolian M, Wiseman D, Mantzanis H, et al. Anticoagulation stewardship: descriptive analysis of a novel approach to appropriate anticoagulant prescription. Res Pract Thromb Haemost. 2022;6:e12758. doi:10.1002/rth2.12758
- Wang SV, Rogers JR, Jin Y, et al. Stepped-wedge randomised trial to evaluate population health intervention designed to increase appropriate anticoagulation in patients with atrial fibrillation. BMJ Qual Saf. 2019;28:835-842. doi:10.1136/bmjqs-2019-009367
Anticoagulation Stewardship Efforts Via Indication Reviews at a Veterans Affairs Health Care System
Anticoagulation Stewardship Efforts Via Indication Reviews at a Veterans Affairs Health Care System
Needle-Knife Fistulotomy is Safe During ERCP, Even for Trainees
, based on results of a randomized trial.
Across procedures conducted predominantly by trainees, safety outcomes were similar between NKF and standard cannulation, and all patients were successfully cannulated, suggesting this is a broadly accessible technique, reported lead author Aleksey Novikov, MD, of the University of Florida College of Medicine, Gainesville, and colleagues, reported.
Writing in Techniques and Innovations in Gastrointestinal Endoscopy, the investigators noted that standard cannulation fails in 5-20% of cases, which has led to development of various alternative techniques, including NKF. To perform the technique, the endoscopist makes a small incision in the intraduodenal biliary segment 3-6mm above the papillary orifice, with cephalad extension until bili-ary access is achieved.
To date, four prospective studies have evaluated NKF in the hands of expert advanced endoscopists.
“These studies showed that NKF is a safe and useful technique that significantly reduces the risk of PEP in the hands of expert advanced endoscopists,” the investigators wrote. ‘The suggestion that NKF should be restricted to expert advanced endoscopists likely limits widespread use.”
To determine whether NKF is a suitable technique for less experienced endoscopists, the investigators conducted the present single-center, prospective randomized controlled trial at Thomas Jefferson University Hospital in Philadelphia.
Adults undergoing ERCP for biliary indications were randomly assigned in a 1:1 ratio to undergo primary cannulation via NKF or standard cannulation. Patients with prior sphincterotomy, ampullectomy, or unfavorable anatomy were excluded.
A total of 186 patients were randomized, with 137 ultimately included in the per-protocol analysis after exclusions for anatomic factors. Most procedures (72.3%) were performed by advanced endoscopy trainees under direct supervision, 26 procedures (19.0%) were performed by attending endoscopists without substantive prior NKF experience, and 12 (8.8%) by an attending endoscopist with NKF expertise.
“It is important to note that the majority of procedures performed in the context of this study were performed by an advanced endoscopy trainee with no NKF experience or an attending advanced endoscopist with minimal NKF experience,” the investigators wrote.
All patients received prophylactic rectal indomethacin, and cannulation attempts were capped at 20 minutes before crossover to another technique was permitted.
The primary endpoint was incidence of post-ERCP pancreatitis. Secondary endpoints included successful biliary access, time to access, and rates of bleeding and perforation.
Post-ERCP pancreatitis occurred at similar rate across groups: 6 cases (8.2%) in the standard cannulation arm and 5 cases (7.8%) in the NKF arm (P = .93). Rates of bleeding and perforation were also similar for both techniques.
Within the initial 20-minute window, biliary access rates were comparable between groups, at 75.3% and 82.2% for standard cannulation and NKF, respectively (P = .89). Allowing additional attempts or crossover, overall success rose to 100% in both arms.
Mean time to access was longer with NKF, averaging 380 seconds compared with 268 seconds for standard cannulation (P less than .05).
“NKF was essentially equivalent to standard cannulation in many aspects,” the investigators wrote, calling the two techniques “complementary.”
They also suggested that the relative equivalence between techniques “carries more weight” after considering the low level of NKF experience among participating endoscopists.
“Overall, our data support teaching advanced endoscopy trainees NKF as a primary method of biliary access in patients with favorable anatomy,” the investigators concluded.
The investigators disclosed relationships with Medtronic, Boston Scientific, and Olympus.
, based on results of a randomized trial.
Across procedures conducted predominantly by trainees, safety outcomes were similar between NKF and standard cannulation, and all patients were successfully cannulated, suggesting this is a broadly accessible technique, reported lead author Aleksey Novikov, MD, of the University of Florida College of Medicine, Gainesville, and colleagues, reported.
Writing in Techniques and Innovations in Gastrointestinal Endoscopy, the investigators noted that standard cannulation fails in 5-20% of cases, which has led to development of various alternative techniques, including NKF. To perform the technique, the endoscopist makes a small incision in the intraduodenal biliary segment 3-6mm above the papillary orifice, with cephalad extension until bili-ary access is achieved.
To date, four prospective studies have evaluated NKF in the hands of expert advanced endoscopists.
“These studies showed that NKF is a safe and useful technique that significantly reduces the risk of PEP in the hands of expert advanced endoscopists,” the investigators wrote. ‘The suggestion that NKF should be restricted to expert advanced endoscopists likely limits widespread use.”
To determine whether NKF is a suitable technique for less experienced endoscopists, the investigators conducted the present single-center, prospective randomized controlled trial at Thomas Jefferson University Hospital in Philadelphia.
Adults undergoing ERCP for biliary indications were randomly assigned in a 1:1 ratio to undergo primary cannulation via NKF or standard cannulation. Patients with prior sphincterotomy, ampullectomy, or unfavorable anatomy were excluded.
A total of 186 patients were randomized, with 137 ultimately included in the per-protocol analysis after exclusions for anatomic factors. Most procedures (72.3%) were performed by advanced endoscopy trainees under direct supervision, 26 procedures (19.0%) were performed by attending endoscopists without substantive prior NKF experience, and 12 (8.8%) by an attending endoscopist with NKF expertise.
“It is important to note that the majority of procedures performed in the context of this study were performed by an advanced endoscopy trainee with no NKF experience or an attending advanced endoscopist with minimal NKF experience,” the investigators wrote.
All patients received prophylactic rectal indomethacin, and cannulation attempts were capped at 20 minutes before crossover to another technique was permitted.
The primary endpoint was incidence of post-ERCP pancreatitis. Secondary endpoints included successful biliary access, time to access, and rates of bleeding and perforation.
Post-ERCP pancreatitis occurred at similar rate across groups: 6 cases (8.2%) in the standard cannulation arm and 5 cases (7.8%) in the NKF arm (P = .93). Rates of bleeding and perforation were also similar for both techniques.
Within the initial 20-minute window, biliary access rates were comparable between groups, at 75.3% and 82.2% for standard cannulation and NKF, respectively (P = .89). Allowing additional attempts or crossover, overall success rose to 100% in both arms.
Mean time to access was longer with NKF, averaging 380 seconds compared with 268 seconds for standard cannulation (P less than .05).
“NKF was essentially equivalent to standard cannulation in many aspects,” the investigators wrote, calling the two techniques “complementary.”
They also suggested that the relative equivalence between techniques “carries more weight” after considering the low level of NKF experience among participating endoscopists.
“Overall, our data support teaching advanced endoscopy trainees NKF as a primary method of biliary access in patients with favorable anatomy,” the investigators concluded.
The investigators disclosed relationships with Medtronic, Boston Scientific, and Olympus.
, based on results of a randomized trial.
Across procedures conducted predominantly by trainees, safety outcomes were similar between NKF and standard cannulation, and all patients were successfully cannulated, suggesting this is a broadly accessible technique, reported lead author Aleksey Novikov, MD, of the University of Florida College of Medicine, Gainesville, and colleagues, reported.
Writing in Techniques and Innovations in Gastrointestinal Endoscopy, the investigators noted that standard cannulation fails in 5-20% of cases, which has led to development of various alternative techniques, including NKF. To perform the technique, the endoscopist makes a small incision in the intraduodenal biliary segment 3-6mm above the papillary orifice, with cephalad extension until bili-ary access is achieved.
To date, four prospective studies have evaluated NKF in the hands of expert advanced endoscopists.
“These studies showed that NKF is a safe and useful technique that significantly reduces the risk of PEP in the hands of expert advanced endoscopists,” the investigators wrote. ‘The suggestion that NKF should be restricted to expert advanced endoscopists likely limits widespread use.”
To determine whether NKF is a suitable technique for less experienced endoscopists, the investigators conducted the present single-center, prospective randomized controlled trial at Thomas Jefferson University Hospital in Philadelphia.
Adults undergoing ERCP for biliary indications were randomly assigned in a 1:1 ratio to undergo primary cannulation via NKF or standard cannulation. Patients with prior sphincterotomy, ampullectomy, or unfavorable anatomy were excluded.
A total of 186 patients were randomized, with 137 ultimately included in the per-protocol analysis after exclusions for anatomic factors. Most procedures (72.3%) were performed by advanced endoscopy trainees under direct supervision, 26 procedures (19.0%) were performed by attending endoscopists without substantive prior NKF experience, and 12 (8.8%) by an attending endoscopist with NKF expertise.
“It is important to note that the majority of procedures performed in the context of this study were performed by an advanced endoscopy trainee with no NKF experience or an attending advanced endoscopist with minimal NKF experience,” the investigators wrote.
All patients received prophylactic rectal indomethacin, and cannulation attempts were capped at 20 minutes before crossover to another technique was permitted.
The primary endpoint was incidence of post-ERCP pancreatitis. Secondary endpoints included successful biliary access, time to access, and rates of bleeding and perforation.
Post-ERCP pancreatitis occurred at similar rate across groups: 6 cases (8.2%) in the standard cannulation arm and 5 cases (7.8%) in the NKF arm (P = .93). Rates of bleeding and perforation were also similar for both techniques.
Within the initial 20-minute window, biliary access rates were comparable between groups, at 75.3% and 82.2% for standard cannulation and NKF, respectively (P = .89). Allowing additional attempts or crossover, overall success rose to 100% in both arms.
Mean time to access was longer with NKF, averaging 380 seconds compared with 268 seconds for standard cannulation (P less than .05).
“NKF was essentially equivalent to standard cannulation in many aspects,” the investigators wrote, calling the two techniques “complementary.”
They also suggested that the relative equivalence between techniques “carries more weight” after considering the low level of NKF experience among participating endoscopists.
“Overall, our data support teaching advanced endoscopy trainees NKF as a primary method of biliary access in patients with favorable anatomy,” the investigators concluded.
The investigators disclosed relationships with Medtronic, Boston Scientific, and Olympus.
FROM TECHNIQUES AND INNOVATIONS IN GASTROINTESTINAL ENDOSCOPY
Finding the Best Match for MASLD Management
, according to the authors of clinical reviews who offered guidance on the pros and cons of resmetirom and semaglutide.
MASLD has become one of the most common causes of chronic liver disease due to the increased prevalence of diabetes, obesity, and other metabolic disorders, Joanne Lin, DO, an internist in the Division of Gastroenterology and Hepatology at the University of California, San Francisco, and colleagues wrote, in a review published in the Journal of Clinical Gastroenterology.
Its complexity makes MASLD challenging to manage. Metabolic, genetic, and environmental factors are involved in the disease, so patients require multidisciplinary and individualized care, Lin told GI & Hepatology News.
Weight loss, dietary changes, and exercise had long been the only treatment approach clinicians could offer patients. But the approval of two drugs — the thyroid hormone receptor-beta agonist resmetirom and the GLP-1 receptor agonist (RA) semaglutide — for patients whose MASLD has advanced to metabolic dysfunction-associated steatohepatitis (MASH) gives physicians new options for patients with severe disease.
In the review, published online before the official approval of semaglutide, Lin and colleagues proposed an algorithm to guide clinicians in choosing a pharmacological therapy for MASLD. “Resmetirom should be primarily used to reverse fibrosis for patients with MASLD and F2-F3 stages, while GLP-1 RAs are beneficial in managing metabolic comorbidities and weight loss in patients with MASLD,” the researchers concluded.
GLP-1 Power and Potential
In August 2025, the FDA approved semaglutide for MASH and cited evidence from the ESSENCE trial in its decision.
The ESSENCE study, published in The New England Journal of Medicine, showed significantly higher rates of resolution of steatohepatitis without worsening of fibrosis and reduction in liver fibrosis without worsening steatohepatitis in patients with MASH and moderate or advanced liver fibrosis who received 2.4 mg of once-weekly semaglutide compared with patients who received placebo.
The most common adverse events reported with GLP-1 RAs are gastrointestinal-related, including nausea, diarrhea, vomiting, and constipation, and are mainly mild-to-moderate and dose dependent, Lin and colleagues noted in their review.
GLP-1s have some limitations, Lin said. “GLP-1s are great for weight loss and metabolic risk reduction, but studies are still ongoing to determine their effect on liver histology and reversing fibrosis/cirrhosis,” she said. Some patients seeking these medications also have trouble obtaining them because of their popularity for weight loss, she noted.
Resmetirom Shows Success
Resmetirom has demonstrated ability to target hepatocytes and increase the hepatic metabolism of lipids, Lin and colleagues wrote in their review.
Several trials have examined resmetirom as a treatment for MASH, notably the landmark MAESTRO-NASH study , a randomized, placebo-controlled trial of nearly 1000 adults with biopsy-confirmed MASH and stage F2 or F3 fibrosis, which was the basis for the FDA’s approval of the drug in 2024. In the study, 25.9% of the patients treated with 80 mg of resmetirom and 29.9% treated with 100 mg resmetirom achieved MASH resolution with no increase in fibrosis compared with 9.7% of patients treated with placebo. In addition, 24.2% of the patients in the 80-mg resmetirom group and 25.9% of those in the 100-mg resmetirom group achieved fibrosis improvement by at least one stage without worsening of MASLD activity scores compared with 14.2% of patients treated with placebo.
The most common reported side effects from resmetirom are diarrhea or constipation, nausea or vomiting, and abdominal pain.
“The limitations of resmetirom include the absence of validated predictors for individual patient response, and no societal guidelines are available to determine when to stop the medication if ineffective,” Lin told GI & Hepatology News. In addition, resmetirom is currently only recommended for a subset of patients with F2-F3 fibrosis, based on the existing trial, she said.
Other limitations include its high cost, which restricts access to the drug for some patients, and lack of long-term safety and efficacy data, Lin added.
Weighing the Options
Comparing the emerging agents in the context of MASLD/MASH is important to help clinicians understand how different patient populations respond and guide evidence-based treatment decisions, said Hazem Ayesh, MD, an endocrinologist at Deaconess Health System, Evansville, Indiana, in an interview.
“The choice of therapy should be individualized based on comorbidities,” said Ayesh, the lead author of a 2024 review published in Biomedicines that compared resmetirom, GLP-1 agonists, and fibroblast growth factor 21 analogs.
“For example, a GLP-1 receptor agonist may be more appropriate for patients with coexisting diabetes or obesity, while resmetirom may be better suited for patients with more advanced liver disease or minimal metabolic comorbidities,” he said.
GLP-1 RAs, such as semaglutide, offer benefits for diabetes, obesity, and metabolic dysfunction in patients with MASLD/MASH and may be more accessible and cost effective, Ayesh told GI & Hepatology News. However, some patients may experience gastrointestinal side effects or be unable to tolerate GLP-1 RAs, he noted.
By contrast, resmetirom may be preferable for patients with low BMI, advanced fibrosis, or an inability to tolerate GLP-1s, as resmetirom directly targets hepatic pathways involved in MASLD/MASH progression, Ayesh said.
Next Steps to Inform Practice
“More research is needed to validate noninvasive biomarkers to monitor response to these medications, determine predictors of efficacy, and evaluate the additive effects, safety, and drug-drug interactions of combination therapy,” Lin said.
Studies are needed to determine both medications’ effects on patients with advanced fibrosis/cirrhosis and special populations, such as individuals with advanced renal disease or posttransplant patients, she added. More studies are expected to inform clinical practice and proper guidelines for the treatment of MASLD, as has been the case with chronic diseases such as hypertension and diabetes, Lin said.
Long-term safety and efficacy data are critical, as most trials of the newly approved medications have had relatively short follow-up periods of approximately 1 year, Ayesh said. “We need real-world evidence and longitudinal studies spanning 3-5 years to confirm sustained efficacy and safety,” he said. Research on cost effectiveness and health-system impacts will be essential to guide policy and ensure equitable access to the medications, he added.
The study by Lin and colleagues received no outside funding. The researchers had no financial conflicts to disclose. Ayesh had no financial conflicts to disclose.
A version of this article appeared on Medscape.com.
, according to the authors of clinical reviews who offered guidance on the pros and cons of resmetirom and semaglutide.
MASLD has become one of the most common causes of chronic liver disease due to the increased prevalence of diabetes, obesity, and other metabolic disorders, Joanne Lin, DO, an internist in the Division of Gastroenterology and Hepatology at the University of California, San Francisco, and colleagues wrote, in a review published in the Journal of Clinical Gastroenterology.
Its complexity makes MASLD challenging to manage. Metabolic, genetic, and environmental factors are involved in the disease, so patients require multidisciplinary and individualized care, Lin told GI & Hepatology News.
Weight loss, dietary changes, and exercise had long been the only treatment approach clinicians could offer patients. But the approval of two drugs — the thyroid hormone receptor-beta agonist resmetirom and the GLP-1 receptor agonist (RA) semaglutide — for patients whose MASLD has advanced to metabolic dysfunction-associated steatohepatitis (MASH) gives physicians new options for patients with severe disease.
In the review, published online before the official approval of semaglutide, Lin and colleagues proposed an algorithm to guide clinicians in choosing a pharmacological therapy for MASLD. “Resmetirom should be primarily used to reverse fibrosis for patients with MASLD and F2-F3 stages, while GLP-1 RAs are beneficial in managing metabolic comorbidities and weight loss in patients with MASLD,” the researchers concluded.
GLP-1 Power and Potential
In August 2025, the FDA approved semaglutide for MASH and cited evidence from the ESSENCE trial in its decision.
The ESSENCE study, published in The New England Journal of Medicine, showed significantly higher rates of resolution of steatohepatitis without worsening of fibrosis and reduction in liver fibrosis without worsening steatohepatitis in patients with MASH and moderate or advanced liver fibrosis who received 2.4 mg of once-weekly semaglutide compared with patients who received placebo.
The most common adverse events reported with GLP-1 RAs are gastrointestinal-related, including nausea, diarrhea, vomiting, and constipation, and are mainly mild-to-moderate and dose dependent, Lin and colleagues noted in their review.
GLP-1s have some limitations, Lin said. “GLP-1s are great for weight loss and metabolic risk reduction, but studies are still ongoing to determine their effect on liver histology and reversing fibrosis/cirrhosis,” she said. Some patients seeking these medications also have trouble obtaining them because of their popularity for weight loss, she noted.
Resmetirom Shows Success
Resmetirom has demonstrated ability to target hepatocytes and increase the hepatic metabolism of lipids, Lin and colleagues wrote in their review.
Several trials have examined resmetirom as a treatment for MASH, notably the landmark MAESTRO-NASH study , a randomized, placebo-controlled trial of nearly 1000 adults with biopsy-confirmed MASH and stage F2 or F3 fibrosis, which was the basis for the FDA’s approval of the drug in 2024. In the study, 25.9% of the patients treated with 80 mg of resmetirom and 29.9% treated with 100 mg resmetirom achieved MASH resolution with no increase in fibrosis compared with 9.7% of patients treated with placebo. In addition, 24.2% of the patients in the 80-mg resmetirom group and 25.9% of those in the 100-mg resmetirom group achieved fibrosis improvement by at least one stage without worsening of MASLD activity scores compared with 14.2% of patients treated with placebo.
The most common reported side effects from resmetirom are diarrhea or constipation, nausea or vomiting, and abdominal pain.
“The limitations of resmetirom include the absence of validated predictors for individual patient response, and no societal guidelines are available to determine when to stop the medication if ineffective,” Lin told GI & Hepatology News. In addition, resmetirom is currently only recommended for a subset of patients with F2-F3 fibrosis, based on the existing trial, she said.
Other limitations include its high cost, which restricts access to the drug for some patients, and lack of long-term safety and efficacy data, Lin added.
Weighing the Options
Comparing the emerging agents in the context of MASLD/MASH is important to help clinicians understand how different patient populations respond and guide evidence-based treatment decisions, said Hazem Ayesh, MD, an endocrinologist at Deaconess Health System, Evansville, Indiana, in an interview.
“The choice of therapy should be individualized based on comorbidities,” said Ayesh, the lead author of a 2024 review published in Biomedicines that compared resmetirom, GLP-1 agonists, and fibroblast growth factor 21 analogs.
“For example, a GLP-1 receptor agonist may be more appropriate for patients with coexisting diabetes or obesity, while resmetirom may be better suited for patients with more advanced liver disease or minimal metabolic comorbidities,” he said.
GLP-1 RAs, such as semaglutide, offer benefits for diabetes, obesity, and metabolic dysfunction in patients with MASLD/MASH and may be more accessible and cost effective, Ayesh told GI & Hepatology News. However, some patients may experience gastrointestinal side effects or be unable to tolerate GLP-1 RAs, he noted.
By contrast, resmetirom may be preferable for patients with low BMI, advanced fibrosis, or an inability to tolerate GLP-1s, as resmetirom directly targets hepatic pathways involved in MASLD/MASH progression, Ayesh said.
Next Steps to Inform Practice
“More research is needed to validate noninvasive biomarkers to monitor response to these medications, determine predictors of efficacy, and evaluate the additive effects, safety, and drug-drug interactions of combination therapy,” Lin said.
Studies are needed to determine both medications’ effects on patients with advanced fibrosis/cirrhosis and special populations, such as individuals with advanced renal disease or posttransplant patients, she added. More studies are expected to inform clinical practice and proper guidelines for the treatment of MASLD, as has been the case with chronic diseases such as hypertension and diabetes, Lin said.
Long-term safety and efficacy data are critical, as most trials of the newly approved medications have had relatively short follow-up periods of approximately 1 year, Ayesh said. “We need real-world evidence and longitudinal studies spanning 3-5 years to confirm sustained efficacy and safety,” he said. Research on cost effectiveness and health-system impacts will be essential to guide policy and ensure equitable access to the medications, he added.
The study by Lin and colleagues received no outside funding. The researchers had no financial conflicts to disclose. Ayesh had no financial conflicts to disclose.
A version of this article appeared on Medscape.com.
, according to the authors of clinical reviews who offered guidance on the pros and cons of resmetirom and semaglutide.
MASLD has become one of the most common causes of chronic liver disease due to the increased prevalence of diabetes, obesity, and other metabolic disorders, Joanne Lin, DO, an internist in the Division of Gastroenterology and Hepatology at the University of California, San Francisco, and colleagues wrote, in a review published in the Journal of Clinical Gastroenterology.
Its complexity makes MASLD challenging to manage. Metabolic, genetic, and environmental factors are involved in the disease, so patients require multidisciplinary and individualized care, Lin told GI & Hepatology News.
Weight loss, dietary changes, and exercise had long been the only treatment approach clinicians could offer patients. But the approval of two drugs — the thyroid hormone receptor-beta agonist resmetirom and the GLP-1 receptor agonist (RA) semaglutide — for patients whose MASLD has advanced to metabolic dysfunction-associated steatohepatitis (MASH) gives physicians new options for patients with severe disease.
In the review, published online before the official approval of semaglutide, Lin and colleagues proposed an algorithm to guide clinicians in choosing a pharmacological therapy for MASLD. “Resmetirom should be primarily used to reverse fibrosis for patients with MASLD and F2-F3 stages, while GLP-1 RAs are beneficial in managing metabolic comorbidities and weight loss in patients with MASLD,” the researchers concluded.
GLP-1 Power and Potential
In August 2025, the FDA approved semaglutide for MASH and cited evidence from the ESSENCE trial in its decision.
The ESSENCE study, published in The New England Journal of Medicine, showed significantly higher rates of resolution of steatohepatitis without worsening of fibrosis and reduction in liver fibrosis without worsening steatohepatitis in patients with MASH and moderate or advanced liver fibrosis who received 2.4 mg of once-weekly semaglutide compared with patients who received placebo.
The most common adverse events reported with GLP-1 RAs are gastrointestinal-related, including nausea, diarrhea, vomiting, and constipation, and are mainly mild-to-moderate and dose dependent, Lin and colleagues noted in their review.
GLP-1s have some limitations, Lin said. “GLP-1s are great for weight loss and metabolic risk reduction, but studies are still ongoing to determine their effect on liver histology and reversing fibrosis/cirrhosis,” she said. Some patients seeking these medications also have trouble obtaining them because of their popularity for weight loss, she noted.
Resmetirom Shows Success
Resmetirom has demonstrated ability to target hepatocytes and increase the hepatic metabolism of lipids, Lin and colleagues wrote in their review.
Several trials have examined resmetirom as a treatment for MASH, notably the landmark MAESTRO-NASH study , a randomized, placebo-controlled trial of nearly 1000 adults with biopsy-confirmed MASH and stage F2 or F3 fibrosis, which was the basis for the FDA’s approval of the drug in 2024. In the study, 25.9% of the patients treated with 80 mg of resmetirom and 29.9% treated with 100 mg resmetirom achieved MASH resolution with no increase in fibrosis compared with 9.7% of patients treated with placebo. In addition, 24.2% of the patients in the 80-mg resmetirom group and 25.9% of those in the 100-mg resmetirom group achieved fibrosis improvement by at least one stage without worsening of MASLD activity scores compared with 14.2% of patients treated with placebo.
The most common reported side effects from resmetirom are diarrhea or constipation, nausea or vomiting, and abdominal pain.
“The limitations of resmetirom include the absence of validated predictors for individual patient response, and no societal guidelines are available to determine when to stop the medication if ineffective,” Lin told GI & Hepatology News. In addition, resmetirom is currently only recommended for a subset of patients with F2-F3 fibrosis, based on the existing trial, she said.
Other limitations include its high cost, which restricts access to the drug for some patients, and lack of long-term safety and efficacy data, Lin added.
Weighing the Options
Comparing the emerging agents in the context of MASLD/MASH is important to help clinicians understand how different patient populations respond and guide evidence-based treatment decisions, said Hazem Ayesh, MD, an endocrinologist at Deaconess Health System, Evansville, Indiana, in an interview.
“The choice of therapy should be individualized based on comorbidities,” said Ayesh, the lead author of a 2024 review published in Biomedicines that compared resmetirom, GLP-1 agonists, and fibroblast growth factor 21 analogs.
“For example, a GLP-1 receptor agonist may be more appropriate for patients with coexisting diabetes or obesity, while resmetirom may be better suited for patients with more advanced liver disease or minimal metabolic comorbidities,” he said.
GLP-1 RAs, such as semaglutide, offer benefits for diabetes, obesity, and metabolic dysfunction in patients with MASLD/MASH and may be more accessible and cost effective, Ayesh told GI & Hepatology News. However, some patients may experience gastrointestinal side effects or be unable to tolerate GLP-1 RAs, he noted.
By contrast, resmetirom may be preferable for patients with low BMI, advanced fibrosis, or an inability to tolerate GLP-1s, as resmetirom directly targets hepatic pathways involved in MASLD/MASH progression, Ayesh said.
Next Steps to Inform Practice
“More research is needed to validate noninvasive biomarkers to monitor response to these medications, determine predictors of efficacy, and evaluate the additive effects, safety, and drug-drug interactions of combination therapy,” Lin said.
Studies are needed to determine both medications’ effects on patients with advanced fibrosis/cirrhosis and special populations, such as individuals with advanced renal disease or posttransplant patients, she added. More studies are expected to inform clinical practice and proper guidelines for the treatment of MASLD, as has been the case with chronic diseases such as hypertension and diabetes, Lin said.
Long-term safety and efficacy data are critical, as most trials of the newly approved medications have had relatively short follow-up periods of approximately 1 year, Ayesh said. “We need real-world evidence and longitudinal studies spanning 3-5 years to confirm sustained efficacy and safety,” he said. Research on cost effectiveness and health-system impacts will be essential to guide policy and ensure equitable access to the medications, he added.
The study by Lin and colleagues received no outside funding. The researchers had no financial conflicts to disclose. Ayesh had no financial conflicts to disclose.
A version of this article appeared on Medscape.com.
Vaping Increases Peptic Ulcer Disease Risk
PHOENIX — , a cross-sectional study found.
The study also found increased risk of PUD among former users of e-cigarettes, reported Albert E. Ohrin, MBChB, MHS, of Ascension Saint Agnes Hospital, Baltimore, Maryland, who presented the study here at the American College of Gastroenterology (ACG) 2025 Annual Scientific Meeting.
While cigarette smoking is a known risk factor for PUD, there was little in the literature investigating whether vaping has a similar risk profile, said Ohrin, a first-year internal medicine resident. He told GI & Hepatology News he found e-cigarette users on Reddit discussing worsening PUD and decided to investigate further, especially since vaping is so popular among young people.
E-cigarettes are the most-used tobacco product among middle and high school students. The National Youth Tobacco Survey in the US reported that 1.6 million students (5.9%) vaped in 2024, a decline from 7.7% in 2023. And the number of adults using e-cigarettes is increasing, according to the US CDC. In 2023, 6.5% of adults over age 18 used e-cigarettes, up from 3.7% in 2020.
Ohrin and colleagues conducted a cross-sectional analysis of adults enrolled in the National Institutes of Health All of Us Research Program. Participants self-reported e-cigarette use. PUD was defined using validated electronic health record diagnosis codes.
Among the 371,398 participants, 29,373 (8%) reported using e-cigarettes, including 21,277 current users and 8096 former users. E-cigarette users were significantly younger (mean age 45.3 vs 59.3 years; P < .001), more likely to be female, and more likely to report lower education and income (P < .001).
Current e-cigarette users had 27% higher odds of PUD (adjusted odds ratio [aOR], 1.27; 95% CI, 1.12-1.45), compared to never-users. This was greater than the risk with traditional combustible cigarettes (aOR, 1.19) that was seen in the study.
Former e-cig users had 13% higher odds (aOR, 1.13; 95% CI, 1.04-1.24) compared to never-users, and any e-cigarette use was associated with higher odds of PUD (aOR, 1.17; 95% CI, 1.09-1.26) compared to never-use.
Use of non-steroidal anti-inflammatories (aOR, 2.15) and having gastroesophageal reflux disease (aOR, 4.45) presented the most significant PUD risk.
Ohrin said he and his colleagues were surprised to see that people who had stopped using e-cigarettes still had higher odds of PUD, although he pointed out that the researchers did not know the frequency of use or how long users had stopped.
“Now that we know there’s an association, we are going to do more studies on e-cigarettes” to see what the potential harms are, especially on the gastrointestinal system, he told GI & Hepatology News.
“One of the things we are looking to elicit is — is there a dose response?” he said, noting it would take a prospective trial to determine that effect.
‘Opens a Door’ to Looking at the GI System
Laura Crotty Alexander, MD, a professor of medicine and associate division chief of pulmonary, critical care, sleep medicine, and physiology at the University of California, San Diego, said she found the study novel and interesting.
“It’s the first I’ve heard of an association between e-cigarette vaping and peptic ulcer disease,” said Crotty Alexander, who has studied the health effects of e-cigarettes for a decade.
Previous studies have shown that nicotine itself can drive an increase in gastric acid production and decrease healing, which can contribute to PUD, Crotty Alexander told GI & Hepatology News. With combustible cigarettes, it is thought that “the larger drivers of that association are the other things in tobacco smoke, such as tar and carbon monoxide and a million other horrible chemicals,” she said.
Crotty Alexander and her colleagues have conducted studies in vitro and in mice that show that e-cigarette aerosols are irritants and cause oxidative stress, which can drive PUD.
While many studies have shown vaping impacts various organs, Ohrin’s study “opens a door” to start looking at the gastrointestinal system, she said.
The study is also a signal to clinicians to “take an accurate inhalant history,” which means asking about vaping, she added.
Ohrin and Crotty Alexander reported no conflicts.
A version of this article first appeared on Medscape.com.
PHOENIX — , a cross-sectional study found.
The study also found increased risk of PUD among former users of e-cigarettes, reported Albert E. Ohrin, MBChB, MHS, of Ascension Saint Agnes Hospital, Baltimore, Maryland, who presented the study here at the American College of Gastroenterology (ACG) 2025 Annual Scientific Meeting.
While cigarette smoking is a known risk factor for PUD, there was little in the literature investigating whether vaping has a similar risk profile, said Ohrin, a first-year internal medicine resident. He told GI & Hepatology News he found e-cigarette users on Reddit discussing worsening PUD and decided to investigate further, especially since vaping is so popular among young people.
E-cigarettes are the most-used tobacco product among middle and high school students. The National Youth Tobacco Survey in the US reported that 1.6 million students (5.9%) vaped in 2024, a decline from 7.7% in 2023. And the number of adults using e-cigarettes is increasing, according to the US CDC. In 2023, 6.5% of adults over age 18 used e-cigarettes, up from 3.7% in 2020.
Ohrin and colleagues conducted a cross-sectional analysis of adults enrolled in the National Institutes of Health All of Us Research Program. Participants self-reported e-cigarette use. PUD was defined using validated electronic health record diagnosis codes.
Among the 371,398 participants, 29,373 (8%) reported using e-cigarettes, including 21,277 current users and 8096 former users. E-cigarette users were significantly younger (mean age 45.3 vs 59.3 years; P < .001), more likely to be female, and more likely to report lower education and income (P < .001).
Current e-cigarette users had 27% higher odds of PUD (adjusted odds ratio [aOR], 1.27; 95% CI, 1.12-1.45), compared to never-users. This was greater than the risk with traditional combustible cigarettes (aOR, 1.19) that was seen in the study.
Former e-cig users had 13% higher odds (aOR, 1.13; 95% CI, 1.04-1.24) compared to never-users, and any e-cigarette use was associated with higher odds of PUD (aOR, 1.17; 95% CI, 1.09-1.26) compared to never-use.
Use of non-steroidal anti-inflammatories (aOR, 2.15) and having gastroesophageal reflux disease (aOR, 4.45) presented the most significant PUD risk.
Ohrin said he and his colleagues were surprised to see that people who had stopped using e-cigarettes still had higher odds of PUD, although he pointed out that the researchers did not know the frequency of use or how long users had stopped.
“Now that we know there’s an association, we are going to do more studies on e-cigarettes” to see what the potential harms are, especially on the gastrointestinal system, he told GI & Hepatology News.
“One of the things we are looking to elicit is — is there a dose response?” he said, noting it would take a prospective trial to determine that effect.
‘Opens a Door’ to Looking at the GI System
Laura Crotty Alexander, MD, a professor of medicine and associate division chief of pulmonary, critical care, sleep medicine, and physiology at the University of California, San Diego, said she found the study novel and interesting.
“It’s the first I’ve heard of an association between e-cigarette vaping and peptic ulcer disease,” said Crotty Alexander, who has studied the health effects of e-cigarettes for a decade.
Previous studies have shown that nicotine itself can drive an increase in gastric acid production and decrease healing, which can contribute to PUD, Crotty Alexander told GI & Hepatology News. With combustible cigarettes, it is thought that “the larger drivers of that association are the other things in tobacco smoke, such as tar and carbon monoxide and a million other horrible chemicals,” she said.
Crotty Alexander and her colleagues have conducted studies in vitro and in mice that show that e-cigarette aerosols are irritants and cause oxidative stress, which can drive PUD.
While many studies have shown vaping impacts various organs, Ohrin’s study “opens a door” to start looking at the gastrointestinal system, she said.
The study is also a signal to clinicians to “take an accurate inhalant history,” which means asking about vaping, she added.
Ohrin and Crotty Alexander reported no conflicts.
A version of this article first appeared on Medscape.com.
PHOENIX — , a cross-sectional study found.
The study also found increased risk of PUD among former users of e-cigarettes, reported Albert E. Ohrin, MBChB, MHS, of Ascension Saint Agnes Hospital, Baltimore, Maryland, who presented the study here at the American College of Gastroenterology (ACG) 2025 Annual Scientific Meeting.
While cigarette smoking is a known risk factor for PUD, there was little in the literature investigating whether vaping has a similar risk profile, said Ohrin, a first-year internal medicine resident. He told GI & Hepatology News he found e-cigarette users on Reddit discussing worsening PUD and decided to investigate further, especially since vaping is so popular among young people.
E-cigarettes are the most-used tobacco product among middle and high school students. The National Youth Tobacco Survey in the US reported that 1.6 million students (5.9%) vaped in 2024, a decline from 7.7% in 2023. And the number of adults using e-cigarettes is increasing, according to the US CDC. In 2023, 6.5% of adults over age 18 used e-cigarettes, up from 3.7% in 2020.
Ohrin and colleagues conducted a cross-sectional analysis of adults enrolled in the National Institutes of Health All of Us Research Program. Participants self-reported e-cigarette use. PUD was defined using validated electronic health record diagnosis codes.
Among the 371,398 participants, 29,373 (8%) reported using e-cigarettes, including 21,277 current users and 8096 former users. E-cigarette users were significantly younger (mean age 45.3 vs 59.3 years; P < .001), more likely to be female, and more likely to report lower education and income (P < .001).
Current e-cigarette users had 27% higher odds of PUD (adjusted odds ratio [aOR], 1.27; 95% CI, 1.12-1.45), compared to never-users. This was greater than the risk with traditional combustible cigarettes (aOR, 1.19) that was seen in the study.
Former e-cig users had 13% higher odds (aOR, 1.13; 95% CI, 1.04-1.24) compared to never-users, and any e-cigarette use was associated with higher odds of PUD (aOR, 1.17; 95% CI, 1.09-1.26) compared to never-use.
Use of non-steroidal anti-inflammatories (aOR, 2.15) and having gastroesophageal reflux disease (aOR, 4.45) presented the most significant PUD risk.
Ohrin said he and his colleagues were surprised to see that people who had stopped using e-cigarettes still had higher odds of PUD, although he pointed out that the researchers did not know the frequency of use or how long users had stopped.
“Now that we know there’s an association, we are going to do more studies on e-cigarettes” to see what the potential harms are, especially on the gastrointestinal system, he told GI & Hepatology News.
“One of the things we are looking to elicit is — is there a dose response?” he said, noting it would take a prospective trial to determine that effect.
‘Opens a Door’ to Looking at the GI System
Laura Crotty Alexander, MD, a professor of medicine and associate division chief of pulmonary, critical care, sleep medicine, and physiology at the University of California, San Diego, said she found the study novel and interesting.
“It’s the first I’ve heard of an association between e-cigarette vaping and peptic ulcer disease,” said Crotty Alexander, who has studied the health effects of e-cigarettes for a decade.
Previous studies have shown that nicotine itself can drive an increase in gastric acid production and decrease healing, which can contribute to PUD, Crotty Alexander told GI & Hepatology News. With combustible cigarettes, it is thought that “the larger drivers of that association are the other things in tobacco smoke, such as tar and carbon monoxide and a million other horrible chemicals,” she said.
Crotty Alexander and her colleagues have conducted studies in vitro and in mice that show that e-cigarette aerosols are irritants and cause oxidative stress, which can drive PUD.
While many studies have shown vaping impacts various organs, Ohrin’s study “opens a door” to start looking at the gastrointestinal system, she said.
The study is also a signal to clinicians to “take an accurate inhalant history,” which means asking about vaping, she added.
Ohrin and Crotty Alexander reported no conflicts.
A version of this article first appeared on Medscape.com.
FROM ACG 2025