User login
Meta-analysis presents relative effect estimates for systemic immunomodulatory treatments for atopic dermatitis
Key clinical point: In patients with atopic dermatitis (AD), once-daily 200 mg abrocitinib or 30 mg upadacitinib more effectively improved Eczema Area and Severity Index (EASI) scores than dupilumab, whereas improvement with baricitinib and tralokinumab were comparable to dupilumab.
Major finding: Compared with dupilumab, up to 16 weeks of treatment with 200 mg abrocitinib (mean difference [MD] 2.2; 95% credible interval [CrI] 0.2-4.0) or 30 mg upadacitinib (MD 2.7; 95% CrI 0.6-4.7) led to greater reduction in EASI scores, whereas 600 mg tralokinumab followed by 300 mg every 2 weeks (MD −3.5; 95% CrI −5.8 to −1.3) and 2 mg baricitinib daily (MD −5.2; 95% CrI −7.5 to −2.9) and 4 mg baricitinib daily (MD −3.2; 95% CrI −5.7 to −0.8) were associated with a lesser reduction.
Study details: Findings are from a meta-analysis of 60 trials including 16,579 children and adults with moderate-to-severe AD.
Disclosures: This work was supported by the UK National Institute for Health Research Career Development Fellowship. The authors declared serving as consultants, co-principal/chief investigators, or employees or receiving compensation, research grants, fees, and funding from several sources.
Source: Drucker AM et al. Systemic immunomodulatory treatments for atopic dermatitis: Update of a living systematic review and network meta-analysis. JAMA Dermatol. 2022 (Mar 16). Doi: 10.1001/jamadermatol.2022.0455
Key clinical point: In patients with atopic dermatitis (AD), once-daily 200 mg abrocitinib or 30 mg upadacitinib more effectively improved Eczema Area and Severity Index (EASI) scores than dupilumab, whereas improvement with baricitinib and tralokinumab were comparable to dupilumab.
Major finding: Compared with dupilumab, up to 16 weeks of treatment with 200 mg abrocitinib (mean difference [MD] 2.2; 95% credible interval [CrI] 0.2-4.0) or 30 mg upadacitinib (MD 2.7; 95% CrI 0.6-4.7) led to greater reduction in EASI scores, whereas 600 mg tralokinumab followed by 300 mg every 2 weeks (MD −3.5; 95% CrI −5.8 to −1.3) and 2 mg baricitinib daily (MD −5.2; 95% CrI −7.5 to −2.9) and 4 mg baricitinib daily (MD −3.2; 95% CrI −5.7 to −0.8) were associated with a lesser reduction.
Study details: Findings are from a meta-analysis of 60 trials including 16,579 children and adults with moderate-to-severe AD.
Disclosures: This work was supported by the UK National Institute for Health Research Career Development Fellowship. The authors declared serving as consultants, co-principal/chief investigators, or employees or receiving compensation, research grants, fees, and funding from several sources.
Source: Drucker AM et al. Systemic immunomodulatory treatments for atopic dermatitis: Update of a living systematic review and network meta-analysis. JAMA Dermatol. 2022 (Mar 16). Doi: 10.1001/jamadermatol.2022.0455
Key clinical point: In patients with atopic dermatitis (AD), once-daily 200 mg abrocitinib or 30 mg upadacitinib more effectively improved Eczema Area and Severity Index (EASI) scores than dupilumab, whereas improvement with baricitinib and tralokinumab were comparable to dupilumab.
Major finding: Compared with dupilumab, up to 16 weeks of treatment with 200 mg abrocitinib (mean difference [MD] 2.2; 95% credible interval [CrI] 0.2-4.0) or 30 mg upadacitinib (MD 2.7; 95% CrI 0.6-4.7) led to greater reduction in EASI scores, whereas 600 mg tralokinumab followed by 300 mg every 2 weeks (MD −3.5; 95% CrI −5.8 to −1.3) and 2 mg baricitinib daily (MD −5.2; 95% CrI −7.5 to −2.9) and 4 mg baricitinib daily (MD −3.2; 95% CrI −5.7 to −0.8) were associated with a lesser reduction.
Study details: Findings are from a meta-analysis of 60 trials including 16,579 children and adults with moderate-to-severe AD.
Disclosures: This work was supported by the UK National Institute for Health Research Career Development Fellowship. The authors declared serving as consultants, co-principal/chief investigators, or employees or receiving compensation, research grants, fees, and funding from several sources.
Source: Drucker AM et al. Systemic immunomodulatory treatments for atopic dermatitis: Update of a living systematic review and network meta-analysis. JAMA Dermatol. 2022 (Mar 16). Doi: 10.1001/jamadermatol.2022.0455
The benefits of conducting clinical research in private practice
Most people believe that, if you want to conduct clinical research, the best path is going into academic medicine. However, for physicians who want both the benefits of practicing in the community setting and a career in research,
Our practices, Capital Digestive Care in Silver Spring, Md., and the PACT-Gastroenterology Center in Hamden, Conn, have been conducting clinical trials for many years on serious diseases such as inflammatory bowel disease, gastroparesis, and most recently, recurrent infection of Clostridioides difficile. We both also worked on the National Institutes of Health–sponsored Anal Cancer/HSIL Outcomes Research (ANCHOR) study.
Academic setting vs. private practice
Research in our practices is similar to the academic setting with regards to how studies are conducted and structured since everyone involved in the study follows the same protocol. The benefit of being in a community setting is that you have a wide range of patients that you are seeing every day.
Getting involved in research is not for everyone, but for those who do get involved, the decision is a rewarding one that can make a significant difference in patients’ lives. Offering new therapeutics for disease states is a powerful tool for a provider, and it is exciting and rewarding to engage in the research considering new mechanisms of action and new approaches to treating diseases.
Finding a better treatment for C. difficile
For example, C. difficile is common in older people who’ve received antibiotics for other infections, especially residents of long-term care facilities. These residents have frequent antibiotic exposure and are already vulnerable to infection because of advanced age, multiple comorbid conditions, and communal living conditions. Once a case of C. difficile is diagnosed in a nursing home, it can spread through contaminated equipment, environments, or hands.
The treatment for C. difficile is to control the bacteria with antibiotics, but spores remain, so after a few days in certain people the spores germinate, and the C. difficile returns: a recurrence. It used to be that, after a second reoccurrence, you would send the patient for a fecal transplant, which was a scarce resource and a challenging process.
To perform a fecal transplant, you would need a spouse or a family member to provide a stool sample. After their stool was tested, the family member would need to process their stool in a blender with saline and draw it up in syringes. Once you had the material, the patient would need to go through a full colonoscopy to infuse the material into the colon. Of course, increased restrictions and safety precautions from the COVID-19 pandemic have made fecal transplants even more complex.
Given all these challenges, conducting research considering microbiota-based live biotherapeutics, the term the Food and Drug Administration uses for pharmaceutically produced forms of fecal microbiota transplantation, is very appealing. There are several different formulations that have come through clinical trials recently including RBX-2660, SER-109, and CP101.
SER-109 is an orally taken treatment produced by Seres Therapeutics. Once patients with acute recurrence of C. difficile are treated with standard antibiotics, they are given a course of four SER-109 capsules for 3 days. The results of the SER-109 study were published recently in the New England Journal of Medicine. This is the first phase 3 clinical trial published on a microbiota-based live biotherapeutic treatment, and the results were exciting, showing a clear efficacy benefit for SER-109.
In the case of C. difficile, we understand the deficiency that SER-109 replaces. SER-109 changes the microbiome within the colon so that the environment becomes less hospitable to C. difficile, which helps to better resist recurrence. With this therapy, we are replenishing the good bacteria, which helps to keep C. difficile from regerminating.
The therapy showed excellent results through the significant difference in rates of recurrence seen in patients with recurrent C. difficile infection following 8 weeks of follow-up. This is exciting because we believe the future of therapeutics for many diseases might involve this type of manipulation of the microbiota, and this is the first to show such an impact with this class of therapeutic.
Joining a practice that conducts clinical research
Within private practice settings, the opportunity to participate in clinical trials usually involves somewhat less bureaucracy and a more patient-centric approach. Private practitioners can also be selective in their research, and we only participate in a handful of selected trials that fit with the expertise of the providers in our practice.
We find the people best suited for involvement in pharmaceutical trials are those providers who want to participate in the scientific process and who see specialized patient populations with the diseases treated by the therapies being studied. In our experience, the young practitioner who enjoyed conducting research in fellowship, who attends national conferences, who keeps track of cutting-edge therapeutics within gastroenterology, and who is highly motivated will be successful in providing this service to their patients.
If you’re an early-career physician who would like to explore clinical research in private practice, there are a several things to look for when considering joining a practice.
Make sure the group has a support infrastructure and a clear compensation model for physicians who want to conduct research. Another important consideration is the kind of support staff the practice provides to manage clinical trials. Does the practice have physician and physician assistant subinvestigators and certified clinical research coordinators? It would be smart to research what kind of capabilities the practice has and inquire about what kind of commitment they have in terms of supporting research efforts.
If the practice you’re thinking of joining has a well-supported research program, you’ll soon be on the way to studying innovative treatments for a wide range of diseases affecting our communities, such as Crohn’s disease, ulcerative colitis, eosinophilic esophagitis, celiac disease, and many others. Many practices also participate in trials assessing new technologies in endoscopy, such as capsule endoscopy of the colon.
It’s incredibly important for community practices to engage in studies and actively recruit younger physicians to participate in their research programs. It changes the character of the practice by bringing a certain level of scholarly activity that benefits the patients we serve, the field of gastroenterology, and medicine as a whole.
Dr. Feuerstadt is a practicing gastroenterologist at the PACT-Gastroenterology Center and is affiliated with Yale New Haven Hospital. Dr. Korman is codirector of Chevy Chase Clinical Research at Capital Digestive Care and a principal investigator in the Seres Therapeutics phase 3 ECOSPOR III study evaluating SER-109. Dr. Feuerstadt disclosed relationships with SERES Therapeutics, Ferring Rebiotix, Finch Therapeutics, and Merck. Dr. Korman disclosed a relationship with SERES Therapeutics.
Most people believe that, if you want to conduct clinical research, the best path is going into academic medicine. However, for physicians who want both the benefits of practicing in the community setting and a career in research,
Our practices, Capital Digestive Care in Silver Spring, Md., and the PACT-Gastroenterology Center in Hamden, Conn, have been conducting clinical trials for many years on serious diseases such as inflammatory bowel disease, gastroparesis, and most recently, recurrent infection of Clostridioides difficile. We both also worked on the National Institutes of Health–sponsored Anal Cancer/HSIL Outcomes Research (ANCHOR) study.
Academic setting vs. private practice
Research in our practices is similar to the academic setting with regards to how studies are conducted and structured since everyone involved in the study follows the same protocol. The benefit of being in a community setting is that you have a wide range of patients that you are seeing every day.
Getting involved in research is not for everyone, but for those who do get involved, the decision is a rewarding one that can make a significant difference in patients’ lives. Offering new therapeutics for disease states is a powerful tool for a provider, and it is exciting and rewarding to engage in the research considering new mechanisms of action and new approaches to treating diseases.
Finding a better treatment for C. difficile
For example, C. difficile is common in older people who’ve received antibiotics for other infections, especially residents of long-term care facilities. These residents have frequent antibiotic exposure and are already vulnerable to infection because of advanced age, multiple comorbid conditions, and communal living conditions. Once a case of C. difficile is diagnosed in a nursing home, it can spread through contaminated equipment, environments, or hands.
The treatment for C. difficile is to control the bacteria with antibiotics, but spores remain, so after a few days in certain people the spores germinate, and the C. difficile returns: a recurrence. It used to be that, after a second reoccurrence, you would send the patient for a fecal transplant, which was a scarce resource and a challenging process.
To perform a fecal transplant, you would need a spouse or a family member to provide a stool sample. After their stool was tested, the family member would need to process their stool in a blender with saline and draw it up in syringes. Once you had the material, the patient would need to go through a full colonoscopy to infuse the material into the colon. Of course, increased restrictions and safety precautions from the COVID-19 pandemic have made fecal transplants even more complex.
Given all these challenges, conducting research considering microbiota-based live biotherapeutics, the term the Food and Drug Administration uses for pharmaceutically produced forms of fecal microbiota transplantation, is very appealing. There are several different formulations that have come through clinical trials recently including RBX-2660, SER-109, and CP101.
SER-109 is an orally taken treatment produced by Seres Therapeutics. Once patients with acute recurrence of C. difficile are treated with standard antibiotics, they are given a course of four SER-109 capsules for 3 days. The results of the SER-109 study were published recently in the New England Journal of Medicine. This is the first phase 3 clinical trial published on a microbiota-based live biotherapeutic treatment, and the results were exciting, showing a clear efficacy benefit for SER-109.
In the case of C. difficile, we understand the deficiency that SER-109 replaces. SER-109 changes the microbiome within the colon so that the environment becomes less hospitable to C. difficile, which helps to better resist recurrence. With this therapy, we are replenishing the good bacteria, which helps to keep C. difficile from regerminating.
The therapy showed excellent results through the significant difference in rates of recurrence seen in patients with recurrent C. difficile infection following 8 weeks of follow-up. This is exciting because we believe the future of therapeutics for many diseases might involve this type of manipulation of the microbiota, and this is the first to show such an impact with this class of therapeutic.
Joining a practice that conducts clinical research
Within private practice settings, the opportunity to participate in clinical trials usually involves somewhat less bureaucracy and a more patient-centric approach. Private practitioners can also be selective in their research, and we only participate in a handful of selected trials that fit with the expertise of the providers in our practice.
We find the people best suited for involvement in pharmaceutical trials are those providers who want to participate in the scientific process and who see specialized patient populations with the diseases treated by the therapies being studied. In our experience, the young practitioner who enjoyed conducting research in fellowship, who attends national conferences, who keeps track of cutting-edge therapeutics within gastroenterology, and who is highly motivated will be successful in providing this service to their patients.
If you’re an early-career physician who would like to explore clinical research in private practice, there are a several things to look for when considering joining a practice.
Make sure the group has a support infrastructure and a clear compensation model for physicians who want to conduct research. Another important consideration is the kind of support staff the practice provides to manage clinical trials. Does the practice have physician and physician assistant subinvestigators and certified clinical research coordinators? It would be smart to research what kind of capabilities the practice has and inquire about what kind of commitment they have in terms of supporting research efforts.
If the practice you’re thinking of joining has a well-supported research program, you’ll soon be on the way to studying innovative treatments for a wide range of diseases affecting our communities, such as Crohn’s disease, ulcerative colitis, eosinophilic esophagitis, celiac disease, and many others. Many practices also participate in trials assessing new technologies in endoscopy, such as capsule endoscopy of the colon.
It’s incredibly important for community practices to engage in studies and actively recruit younger physicians to participate in their research programs. It changes the character of the practice by bringing a certain level of scholarly activity that benefits the patients we serve, the field of gastroenterology, and medicine as a whole.
Dr. Feuerstadt is a practicing gastroenterologist at the PACT-Gastroenterology Center and is affiliated with Yale New Haven Hospital. Dr. Korman is codirector of Chevy Chase Clinical Research at Capital Digestive Care and a principal investigator in the Seres Therapeutics phase 3 ECOSPOR III study evaluating SER-109. Dr. Feuerstadt disclosed relationships with SERES Therapeutics, Ferring Rebiotix, Finch Therapeutics, and Merck. Dr. Korman disclosed a relationship with SERES Therapeutics.
Most people believe that, if you want to conduct clinical research, the best path is going into academic medicine. However, for physicians who want both the benefits of practicing in the community setting and a career in research,
Our practices, Capital Digestive Care in Silver Spring, Md., and the PACT-Gastroenterology Center in Hamden, Conn, have been conducting clinical trials for many years on serious diseases such as inflammatory bowel disease, gastroparesis, and most recently, recurrent infection of Clostridioides difficile. We both also worked on the National Institutes of Health–sponsored Anal Cancer/HSIL Outcomes Research (ANCHOR) study.
Academic setting vs. private practice
Research in our practices is similar to the academic setting with regards to how studies are conducted and structured since everyone involved in the study follows the same protocol. The benefit of being in a community setting is that you have a wide range of patients that you are seeing every day.
Getting involved in research is not for everyone, but for those who do get involved, the decision is a rewarding one that can make a significant difference in patients’ lives. Offering new therapeutics for disease states is a powerful tool for a provider, and it is exciting and rewarding to engage in the research considering new mechanisms of action and new approaches to treating diseases.
Finding a better treatment for C. difficile
For example, C. difficile is common in older people who’ve received antibiotics for other infections, especially residents of long-term care facilities. These residents have frequent antibiotic exposure and are already vulnerable to infection because of advanced age, multiple comorbid conditions, and communal living conditions. Once a case of C. difficile is diagnosed in a nursing home, it can spread through contaminated equipment, environments, or hands.
The treatment for C. difficile is to control the bacteria with antibiotics, but spores remain, so after a few days in certain people the spores germinate, and the C. difficile returns: a recurrence. It used to be that, after a second reoccurrence, you would send the patient for a fecal transplant, which was a scarce resource and a challenging process.
To perform a fecal transplant, you would need a spouse or a family member to provide a stool sample. After their stool was tested, the family member would need to process their stool in a blender with saline and draw it up in syringes. Once you had the material, the patient would need to go through a full colonoscopy to infuse the material into the colon. Of course, increased restrictions and safety precautions from the COVID-19 pandemic have made fecal transplants even more complex.
Given all these challenges, conducting research considering microbiota-based live biotherapeutics, the term the Food and Drug Administration uses for pharmaceutically produced forms of fecal microbiota transplantation, is very appealing. There are several different formulations that have come through clinical trials recently including RBX-2660, SER-109, and CP101.
SER-109 is an orally taken treatment produced by Seres Therapeutics. Once patients with acute recurrence of C. difficile are treated with standard antibiotics, they are given a course of four SER-109 capsules for 3 days. The results of the SER-109 study were published recently in the New England Journal of Medicine. This is the first phase 3 clinical trial published on a microbiota-based live biotherapeutic treatment, and the results were exciting, showing a clear efficacy benefit for SER-109.
In the case of C. difficile, we understand the deficiency that SER-109 replaces. SER-109 changes the microbiome within the colon so that the environment becomes less hospitable to C. difficile, which helps to better resist recurrence. With this therapy, we are replenishing the good bacteria, which helps to keep C. difficile from regerminating.
The therapy showed excellent results through the significant difference in rates of recurrence seen in patients with recurrent C. difficile infection following 8 weeks of follow-up. This is exciting because we believe the future of therapeutics for many diseases might involve this type of manipulation of the microbiota, and this is the first to show such an impact with this class of therapeutic.
Joining a practice that conducts clinical research
Within private practice settings, the opportunity to participate in clinical trials usually involves somewhat less bureaucracy and a more patient-centric approach. Private practitioners can also be selective in their research, and we only participate in a handful of selected trials that fit with the expertise of the providers in our practice.
We find the people best suited for involvement in pharmaceutical trials are those providers who want to participate in the scientific process and who see specialized patient populations with the diseases treated by the therapies being studied. In our experience, the young practitioner who enjoyed conducting research in fellowship, who attends national conferences, who keeps track of cutting-edge therapeutics within gastroenterology, and who is highly motivated will be successful in providing this service to their patients.
If you’re an early-career physician who would like to explore clinical research in private practice, there are a several things to look for when considering joining a practice.
Make sure the group has a support infrastructure and a clear compensation model for physicians who want to conduct research. Another important consideration is the kind of support staff the practice provides to manage clinical trials. Does the practice have physician and physician assistant subinvestigators and certified clinical research coordinators? It would be smart to research what kind of capabilities the practice has and inquire about what kind of commitment they have in terms of supporting research efforts.
If the practice you’re thinking of joining has a well-supported research program, you’ll soon be on the way to studying innovative treatments for a wide range of diseases affecting our communities, such as Crohn’s disease, ulcerative colitis, eosinophilic esophagitis, celiac disease, and many others. Many practices also participate in trials assessing new technologies in endoscopy, such as capsule endoscopy of the colon.
It’s incredibly important for community practices to engage in studies and actively recruit younger physicians to participate in their research programs. It changes the character of the practice by bringing a certain level of scholarly activity that benefits the patients we serve, the field of gastroenterology, and medicine as a whole.
Dr. Feuerstadt is a practicing gastroenterologist at the PACT-Gastroenterology Center and is affiliated with Yale New Haven Hospital. Dr. Korman is codirector of Chevy Chase Clinical Research at Capital Digestive Care and a principal investigator in the Seres Therapeutics phase 3 ECOSPOR III study evaluating SER-109. Dr. Feuerstadt disclosed relationships with SERES Therapeutics, Ferring Rebiotix, Finch Therapeutics, and Merck. Dr. Korman disclosed a relationship with SERES Therapeutics.
My path: Challenges and decisions along the way
It took me a little while to get started on this assignment. What would be most useful to young gastroenterologists embarking on their careers? When I asked around, I heard that many of you wanted me to describe challenges and decision points. The following list is vaguely chronological, surely noncomprehensive, and meant to serve as a starting point.
1. To stay in basic science or return to patient care
My start in science was rocky. I had come to the United States for a post-doc after medical school in Germany. I had never pipetted before. It was the early days of array technologies, and the lab was very technical and basic. We made our own arrays and our own analytics, and none of my experiments worked. So, I spent 1 year feeling like I made no progress – but in hindsight I appreciate the tremendous growth in these formative years honing inquiry and persistence, as well as building resilience. I added a third year as some results were finally emerging; however, the bedside started to feel very far away. I could not ignore the tug back to the patient care, and after contemplating a PhD program, I decided to apply for residency in a physician-scientist pathway. Given the streamlined training that allowed for science and clinical education in an organized fashion, I also decided to stay in the U.S. This of course had vast personal consequences, which I did not fully appreciate at the time.
Residency was another time of immense growth, I was the only “foreign medical graduate” and had a lot to catch up on, but I enjoyed my amazing peers and the hands-on learning.
Pearl: Follow your passion. Not what makes most sense or what someone wants for you or what you could achieve given your past work. Do what will get you up in the morning and add a bounce to your step.
2. To go for big impact or climate
At UCSD at the time, there was a culture of impactful mega-labs, up 30 post-docs, often with many working on the same project with the ones finishing first garnering the publication. This created a “go big or go home” (literally) atmosphere. As part of the PSTP program, I was supported by the GI T32 and, being “free labor,” had a pretty wide array of labs to choose from. To the program director’s surprise, I settled on a fairly junior investigator, who was a fellow gastroenterologist and took a personal interest in my career. When making that decision, I prioritized climate over outcome. I remember thinking to myself that how I spent my time was just as important as the potential outcome of the time spent. Through my years in Dr. John Carethers’ lab, I gained insight into his administrative and leadership roles which added another dimension to our mentorship relationship. These years were fun and productive, and our mentorship grew into a friendship.
Pearl: Look for the right people to work with. Particularly who you work for. Everything else is secondary as the right people will set the tone and most influence your day-to-day experience, which is the foundation of your success.
3. To cultivate a life outside of academia
When I turned 30, I remember driving down Interstate 5 in San Diego and taking stock. Yes, I loved clinical work, I felt valued, and was in a stimulating supportive environment. Yet, I was so immersed that everything else seemed to take a back seat. I made the conscious decision on that drive to prioritize life outside of academia. It is not like I did not have one, I just decided to set an intention so it would not get away from me. I continue to make a conscious effort to be present for my husband, my kids, my family – to take time and spend it together without work bleeding into it. And since this is a goal in and of itself, there is no conflict! Through less travel and no more late nights or weekends, your nonacademic life will flourish.
Pearl: Deliberately prioritize your family and hobbies in the long run. Make key decisions with that in mind.
4. To grow your own program or lead others
When we moved to Chicago for my husband’s residency (he went to medical school as his third career at age 35), I was very excited to build my own comprehensive GI cancer genetics program at Northwestern. It was a little scary but also fun to now run my own lab and try to connect the clinical community around hereditary GI cancers. The program was moving along nicely when I received a generic letter asking for applications to become division chief at the neighboring University of Illinois. The letter concluded with an enticing “Chicago is a vibrant city,” so clearly it was meant for a broad audience. I was not sure what to do and again took stock. Did I want to continue to increase the impact of my own work – clearly there was a lot more ground to cover. Or, did I want to be part of making further-reaching decisions? I had been approached by fellows who wanted to be recruited, and I had ideas for programs and thoughts around processes. While my input was valued, I was not the ultimate decision maker. I decided that I either focus on one or the other and so applied for the position and then took the leap.
Pearl: There are many forks and they will present when you do not expect them. Assess and consider. Also know yourself – not everything that is attainable is desirable.
5. To have greater influence or stay with what you know
Becoming a division chief was transformative. Learning to integrate the needs of various and sometimes conflicting stakeholders, running an operation but also thinking strategically and mission-based – I was drinking from a firehose. How to measure success as a leader? I was fortunate to enter the division at a turbulent time where much rebuilding was needed and it was easy to implement and see change.
Pearl: Again know yourself – not everything that is attainable is desirable. But also – take risks. What is the worst that can happen? Growth may not be attained by waiting.
6. To be spread too thin or close doors
As you develop your focus and expertise while implementing No. 3, you will run out of hours in the day. This means you will need to become more and more efficient, as in delegating (and letting go) where you can and doing fewer nonessential tasks. However, you want to think hard about closing doors completely. I have been careful to hone and keep my endoscopy skills as well as my scientific output. To leave the doors ajar, I have tried to find ways to be very deliberate with my involvement and also understand that at some point it may make sense to close a door.
Pearl: Do not try to do everything well, you will risk doing everything poorly. Work on “good enough” for tasks that can be very involved. Think hard before permanently leaving something behind as you may lose flexibility down the road.
7. To enjoy fruits of labor or continue to grow
A question I get asked often is regarding the ideal time to move. In my mind, there is no perfect time. It depends on your satisfaction with your current position (see No. 2), your personal situation (see No. 3), and what you want at that juncture (see No. 5). At some point, one may want to stay awhile and enjoy. Or continue to change and grow – both have their merits and there is no right or wrong.
Pearl: When contemplating next steps, go back to your passion and priorities. Has anything shifted? Are your goals being met? Are you enjoying yourself? Advice can be helpful but also confusing. Remember, no one knows you like you do.
8. To show tangible results or build out relationships
Over time, as you become more and more efficient, you simultaneously need to spend more time fostering relationships. This feels strange at first as it is the opposite of a fast-paced to-do list and the “results” appear elusive. Build in time for relating – with peers, superiors, fellows, members of your lab.
Pearl: Form relationships early and often. Take care of them (No. 3) and include relationship building into your workstream – I promise it will make your path more successful and satisfying.
I hope this list shows that there are many forks and no one right way. Advice is helpful and subjective. No path is the same, and it truly is yours to shape. Be thoughtful and enjoy – your journey will be amazing and full of surprises.
Dr. Jung is professor and chair, and the Robert G. Petersdorf Endowed Chair in Medicine, in the department of medicine at the University of Washington, Seattle. She is on Twitter @barbarahjung. She has no conflicts of interest.
It took me a little while to get started on this assignment. What would be most useful to young gastroenterologists embarking on their careers? When I asked around, I heard that many of you wanted me to describe challenges and decision points. The following list is vaguely chronological, surely noncomprehensive, and meant to serve as a starting point.
1. To stay in basic science or return to patient care
My start in science was rocky. I had come to the United States for a post-doc after medical school in Germany. I had never pipetted before. It was the early days of array technologies, and the lab was very technical and basic. We made our own arrays and our own analytics, and none of my experiments worked. So, I spent 1 year feeling like I made no progress – but in hindsight I appreciate the tremendous growth in these formative years honing inquiry and persistence, as well as building resilience. I added a third year as some results were finally emerging; however, the bedside started to feel very far away. I could not ignore the tug back to the patient care, and after contemplating a PhD program, I decided to apply for residency in a physician-scientist pathway. Given the streamlined training that allowed for science and clinical education in an organized fashion, I also decided to stay in the U.S. This of course had vast personal consequences, which I did not fully appreciate at the time.
Residency was another time of immense growth, I was the only “foreign medical graduate” and had a lot to catch up on, but I enjoyed my amazing peers and the hands-on learning.
Pearl: Follow your passion. Not what makes most sense or what someone wants for you or what you could achieve given your past work. Do what will get you up in the morning and add a bounce to your step.
2. To go for big impact or climate
At UCSD at the time, there was a culture of impactful mega-labs, up 30 post-docs, often with many working on the same project with the ones finishing first garnering the publication. This created a “go big or go home” (literally) atmosphere. As part of the PSTP program, I was supported by the GI T32 and, being “free labor,” had a pretty wide array of labs to choose from. To the program director’s surprise, I settled on a fairly junior investigator, who was a fellow gastroenterologist and took a personal interest in my career. When making that decision, I prioritized climate over outcome. I remember thinking to myself that how I spent my time was just as important as the potential outcome of the time spent. Through my years in Dr. John Carethers’ lab, I gained insight into his administrative and leadership roles which added another dimension to our mentorship relationship. These years were fun and productive, and our mentorship grew into a friendship.
Pearl: Look for the right people to work with. Particularly who you work for. Everything else is secondary as the right people will set the tone and most influence your day-to-day experience, which is the foundation of your success.
3. To cultivate a life outside of academia
When I turned 30, I remember driving down Interstate 5 in San Diego and taking stock. Yes, I loved clinical work, I felt valued, and was in a stimulating supportive environment. Yet, I was so immersed that everything else seemed to take a back seat. I made the conscious decision on that drive to prioritize life outside of academia. It is not like I did not have one, I just decided to set an intention so it would not get away from me. I continue to make a conscious effort to be present for my husband, my kids, my family – to take time and spend it together without work bleeding into it. And since this is a goal in and of itself, there is no conflict! Through less travel and no more late nights or weekends, your nonacademic life will flourish.
Pearl: Deliberately prioritize your family and hobbies in the long run. Make key decisions with that in mind.
4. To grow your own program or lead others
When we moved to Chicago for my husband’s residency (he went to medical school as his third career at age 35), I was very excited to build my own comprehensive GI cancer genetics program at Northwestern. It was a little scary but also fun to now run my own lab and try to connect the clinical community around hereditary GI cancers. The program was moving along nicely when I received a generic letter asking for applications to become division chief at the neighboring University of Illinois. The letter concluded with an enticing “Chicago is a vibrant city,” so clearly it was meant for a broad audience. I was not sure what to do and again took stock. Did I want to continue to increase the impact of my own work – clearly there was a lot more ground to cover. Or, did I want to be part of making further-reaching decisions? I had been approached by fellows who wanted to be recruited, and I had ideas for programs and thoughts around processes. While my input was valued, I was not the ultimate decision maker. I decided that I either focus on one or the other and so applied for the position and then took the leap.
Pearl: There are many forks and they will present when you do not expect them. Assess and consider. Also know yourself – not everything that is attainable is desirable.
5. To have greater influence or stay with what you know
Becoming a division chief was transformative. Learning to integrate the needs of various and sometimes conflicting stakeholders, running an operation but also thinking strategically and mission-based – I was drinking from a firehose. How to measure success as a leader? I was fortunate to enter the division at a turbulent time where much rebuilding was needed and it was easy to implement and see change.
Pearl: Again know yourself – not everything that is attainable is desirable. But also – take risks. What is the worst that can happen? Growth may not be attained by waiting.
6. To be spread too thin or close doors
As you develop your focus and expertise while implementing No. 3, you will run out of hours in the day. This means you will need to become more and more efficient, as in delegating (and letting go) where you can and doing fewer nonessential tasks. However, you want to think hard about closing doors completely. I have been careful to hone and keep my endoscopy skills as well as my scientific output. To leave the doors ajar, I have tried to find ways to be very deliberate with my involvement and also understand that at some point it may make sense to close a door.
Pearl: Do not try to do everything well, you will risk doing everything poorly. Work on “good enough” for tasks that can be very involved. Think hard before permanently leaving something behind as you may lose flexibility down the road.
7. To enjoy fruits of labor or continue to grow
A question I get asked often is regarding the ideal time to move. In my mind, there is no perfect time. It depends on your satisfaction with your current position (see No. 2), your personal situation (see No. 3), and what you want at that juncture (see No. 5). At some point, one may want to stay awhile and enjoy. Or continue to change and grow – both have their merits and there is no right or wrong.
Pearl: When contemplating next steps, go back to your passion and priorities. Has anything shifted? Are your goals being met? Are you enjoying yourself? Advice can be helpful but also confusing. Remember, no one knows you like you do.
8. To show tangible results or build out relationships
Over time, as you become more and more efficient, you simultaneously need to spend more time fostering relationships. This feels strange at first as it is the opposite of a fast-paced to-do list and the “results” appear elusive. Build in time for relating – with peers, superiors, fellows, members of your lab.
Pearl: Form relationships early and often. Take care of them (No. 3) and include relationship building into your workstream – I promise it will make your path more successful and satisfying.
I hope this list shows that there are many forks and no one right way. Advice is helpful and subjective. No path is the same, and it truly is yours to shape. Be thoughtful and enjoy – your journey will be amazing and full of surprises.
Dr. Jung is professor and chair, and the Robert G. Petersdorf Endowed Chair in Medicine, in the department of medicine at the University of Washington, Seattle. She is on Twitter @barbarahjung. She has no conflicts of interest.
It took me a little while to get started on this assignment. What would be most useful to young gastroenterologists embarking on their careers? When I asked around, I heard that many of you wanted me to describe challenges and decision points. The following list is vaguely chronological, surely noncomprehensive, and meant to serve as a starting point.
1. To stay in basic science or return to patient care
My start in science was rocky. I had come to the United States for a post-doc after medical school in Germany. I had never pipetted before. It was the early days of array technologies, and the lab was very technical and basic. We made our own arrays and our own analytics, and none of my experiments worked. So, I spent 1 year feeling like I made no progress – but in hindsight I appreciate the tremendous growth in these formative years honing inquiry and persistence, as well as building resilience. I added a third year as some results were finally emerging; however, the bedside started to feel very far away. I could not ignore the tug back to the patient care, and after contemplating a PhD program, I decided to apply for residency in a physician-scientist pathway. Given the streamlined training that allowed for science and clinical education in an organized fashion, I also decided to stay in the U.S. This of course had vast personal consequences, which I did not fully appreciate at the time.
Residency was another time of immense growth, I was the only “foreign medical graduate” and had a lot to catch up on, but I enjoyed my amazing peers and the hands-on learning.
Pearl: Follow your passion. Not what makes most sense or what someone wants for you or what you could achieve given your past work. Do what will get you up in the morning and add a bounce to your step.
2. To go for big impact or climate
At UCSD at the time, there was a culture of impactful mega-labs, up 30 post-docs, often with many working on the same project with the ones finishing first garnering the publication. This created a “go big or go home” (literally) atmosphere. As part of the PSTP program, I was supported by the GI T32 and, being “free labor,” had a pretty wide array of labs to choose from. To the program director’s surprise, I settled on a fairly junior investigator, who was a fellow gastroenterologist and took a personal interest in my career. When making that decision, I prioritized climate over outcome. I remember thinking to myself that how I spent my time was just as important as the potential outcome of the time spent. Through my years in Dr. John Carethers’ lab, I gained insight into his administrative and leadership roles which added another dimension to our mentorship relationship. These years were fun and productive, and our mentorship grew into a friendship.
Pearl: Look for the right people to work with. Particularly who you work for. Everything else is secondary as the right people will set the tone and most influence your day-to-day experience, which is the foundation of your success.
3. To cultivate a life outside of academia
When I turned 30, I remember driving down Interstate 5 in San Diego and taking stock. Yes, I loved clinical work, I felt valued, and was in a stimulating supportive environment. Yet, I was so immersed that everything else seemed to take a back seat. I made the conscious decision on that drive to prioritize life outside of academia. It is not like I did not have one, I just decided to set an intention so it would not get away from me. I continue to make a conscious effort to be present for my husband, my kids, my family – to take time and spend it together without work bleeding into it. And since this is a goal in and of itself, there is no conflict! Through less travel and no more late nights or weekends, your nonacademic life will flourish.
Pearl: Deliberately prioritize your family and hobbies in the long run. Make key decisions with that in mind.
4. To grow your own program or lead others
When we moved to Chicago for my husband’s residency (he went to medical school as his third career at age 35), I was very excited to build my own comprehensive GI cancer genetics program at Northwestern. It was a little scary but also fun to now run my own lab and try to connect the clinical community around hereditary GI cancers. The program was moving along nicely when I received a generic letter asking for applications to become division chief at the neighboring University of Illinois. The letter concluded with an enticing “Chicago is a vibrant city,” so clearly it was meant for a broad audience. I was not sure what to do and again took stock. Did I want to continue to increase the impact of my own work – clearly there was a lot more ground to cover. Or, did I want to be part of making further-reaching decisions? I had been approached by fellows who wanted to be recruited, and I had ideas for programs and thoughts around processes. While my input was valued, I was not the ultimate decision maker. I decided that I either focus on one or the other and so applied for the position and then took the leap.
Pearl: There are many forks and they will present when you do not expect them. Assess and consider. Also know yourself – not everything that is attainable is desirable.
5. To have greater influence or stay with what you know
Becoming a division chief was transformative. Learning to integrate the needs of various and sometimes conflicting stakeholders, running an operation but also thinking strategically and mission-based – I was drinking from a firehose. How to measure success as a leader? I was fortunate to enter the division at a turbulent time where much rebuilding was needed and it was easy to implement and see change.
Pearl: Again know yourself – not everything that is attainable is desirable. But also – take risks. What is the worst that can happen? Growth may not be attained by waiting.
6. To be spread too thin or close doors
As you develop your focus and expertise while implementing No. 3, you will run out of hours in the day. This means you will need to become more and more efficient, as in delegating (and letting go) where you can and doing fewer nonessential tasks. However, you want to think hard about closing doors completely. I have been careful to hone and keep my endoscopy skills as well as my scientific output. To leave the doors ajar, I have tried to find ways to be very deliberate with my involvement and also understand that at some point it may make sense to close a door.
Pearl: Do not try to do everything well, you will risk doing everything poorly. Work on “good enough” for tasks that can be very involved. Think hard before permanently leaving something behind as you may lose flexibility down the road.
7. To enjoy fruits of labor or continue to grow
A question I get asked often is regarding the ideal time to move. In my mind, there is no perfect time. It depends on your satisfaction with your current position (see No. 2), your personal situation (see No. 3), and what you want at that juncture (see No. 5). At some point, one may want to stay awhile and enjoy. Or continue to change and grow – both have their merits and there is no right or wrong.
Pearl: When contemplating next steps, go back to your passion and priorities. Has anything shifted? Are your goals being met? Are you enjoying yourself? Advice can be helpful but also confusing. Remember, no one knows you like you do.
8. To show tangible results or build out relationships
Over time, as you become more and more efficient, you simultaneously need to spend more time fostering relationships. This feels strange at first as it is the opposite of a fast-paced to-do list and the “results” appear elusive. Build in time for relating – with peers, superiors, fellows, members of your lab.
Pearl: Form relationships early and often. Take care of them (No. 3) and include relationship building into your workstream – I promise it will make your path more successful and satisfying.
I hope this list shows that there are many forks and no one right way. Advice is helpful and subjective. No path is the same, and it truly is yours to shape. Be thoughtful and enjoy – your journey will be amazing and full of surprises.
Dr. Jung is professor and chair, and the Robert G. Petersdorf Endowed Chair in Medicine, in the department of medicine at the University of Washington, Seattle. She is on Twitter @barbarahjung. She has no conflicts of interest.
High antipsychotic switch rates suggest ‘suboptimal’ prescribing for first-episode psychosis
In a large-scale, real-world analysis of U.K. prescribing patterns, researchers found more than two-thirds of patients who received antipsychotics for FEP switched medication, and almost half switched drugs three times.
Although this is “one of the largest real-world studies examining antipsychotic treatment strategies,” it reflects findings from previous, smaller studies showing “antipsychotic switching in first episode psychosis is high,” said study investigator Aimee Brinn, Institute of Psychiatry, Psychology & Neuroscience at King’s College London.
This may reflect reports of poor efficacy and suggests that first-line prescribing is “suboptimal,” Ms. Brinn noted. In addition, olanzapine remains the most popular antipsychotic for prescribing despite recent guidelines indicating it is “not ideal ... due to its dangerous metabolic side effects,” she added.
The findings were presented at the Congress of the Schizophrenia International Research Society (SIRS) 2022.
Real-world data
The response to, and tolerability of, antipsychotics differs between patients with FEP; and prescribing patterns “reflect clinician and patient-led decisionmaking,” Ms. Brinn told meeting attendees.
Since randomized controlled trials “do not necessarily reflect prescribing practice in real-world clinical settings,” the researchers gathered data from a large mental health care electronic health record dataset.
The investigators examined records from the South London and Maudsley NHS Foundation Trust (SLaM), which has a catchment area of 1.2 million individuals across four boroughs of London. The group sees approximately 37,500 active patients per week.
The team used the Clinical Interactive Record Search tool to extract data on 2,309 adults with FEP who received care from a SLaM early intervention in psychosis service between April 1, 2008, and March 31, 2019.
They found that 12 different antipsychotics were prescribed as first-line treatment. The most common were olanzapine (43.9%), risperidone (24.7%), and aripiprazole (19.9%).
Results showed that over 81,969.5 person-years of follow-up, at a minimum of 24 months per patient, 68.8% had an antipsychotic switch. The most common first treatment switch, in 17.9% of patients, was from olanzapine to aripiprazole.
Of patients who switched to aripiprazole, 48.4% stayed on the drug, 26% switched back to olanzapine, and 25.6% received other treatment. Overall, 44.7% of patients switched medication at least three times.
Among patients with FEP who did not switch, 42.2% were prescribed olanzapine, 26.2% risperidone, 23.3% aripiprazole, 5.6% quetiapine, and 2.7% amisulpride.
During the post-presentation discussion, Ms. Brinn was asked whether the high rate of first-line olanzapine prescribing could be because patients started treatment as inpatients and were then switched once they were moved to community care.
“We found that a lot of patients would be prescribed olanzapine for around 7 days at the start of their prescription and then switch,” Ms. Brinn said, adding it is “likely” they started as inpatients. The investigators are currently examining the differences between inpatient and outpatient prescriptions to verify whether this is indeed the case, she added.
‘Pulling out the big guns too fast?’
Commenting on the findings, Thomas W. Sedlak, MD, PhD, Johns Hopkins University School of Medicine, Baltimore, said the study raises a “number of questions.”
Both olanzapine and risperidone “tend to have higher treatment effect improvements than aripiprazole, so it’s curious that a switch to aripiprazole was common,” said Dr. Sedlak, who was not involved with the research.
“Are we pulling out the ‘big guns’ too fast, or inappropriately, especially as olanzapine and risperidone carry greater risk of weight gain?” he asked. In addition, “now that olanzapine is available with samidorphan to mitigate weight gain, will that shape future patterns, if it can be paid for?”
Dr. Sedlak noted it was unclear why olanzapine was chosen so often as first-line treatment in the study and agreed it is “possible that hospitalized patients had been prescribed a ‘stronger’ medication like olanzapine compared to never-hospitalized patients.”
He also underlined that it is “not clear if patients in this FEP program are representative of all FEP patients.”
“For instance, if the program is well known to inpatient hospital social workers, then the program might be disproportionately filled with patients who have had more severe symptoms,” Dr. Sedlak said.
The study was supported by Janssen-Cilag. The investigators and Dr. Sedlak have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
In a large-scale, real-world analysis of U.K. prescribing patterns, researchers found more than two-thirds of patients who received antipsychotics for FEP switched medication, and almost half switched drugs three times.
Although this is “one of the largest real-world studies examining antipsychotic treatment strategies,” it reflects findings from previous, smaller studies showing “antipsychotic switching in first episode psychosis is high,” said study investigator Aimee Brinn, Institute of Psychiatry, Psychology & Neuroscience at King’s College London.
This may reflect reports of poor efficacy and suggests that first-line prescribing is “suboptimal,” Ms. Brinn noted. In addition, olanzapine remains the most popular antipsychotic for prescribing despite recent guidelines indicating it is “not ideal ... due to its dangerous metabolic side effects,” she added.
The findings were presented at the Congress of the Schizophrenia International Research Society (SIRS) 2022.
Real-world data
The response to, and tolerability of, antipsychotics differs between patients with FEP; and prescribing patterns “reflect clinician and patient-led decisionmaking,” Ms. Brinn told meeting attendees.
Since randomized controlled trials “do not necessarily reflect prescribing practice in real-world clinical settings,” the researchers gathered data from a large mental health care electronic health record dataset.
The investigators examined records from the South London and Maudsley NHS Foundation Trust (SLaM), which has a catchment area of 1.2 million individuals across four boroughs of London. The group sees approximately 37,500 active patients per week.
The team used the Clinical Interactive Record Search tool to extract data on 2,309 adults with FEP who received care from a SLaM early intervention in psychosis service between April 1, 2008, and March 31, 2019.
They found that 12 different antipsychotics were prescribed as first-line treatment. The most common were olanzapine (43.9%), risperidone (24.7%), and aripiprazole (19.9%).
Results showed that over 81,969.5 person-years of follow-up, at a minimum of 24 months per patient, 68.8% had an antipsychotic switch. The most common first treatment switch, in 17.9% of patients, was from olanzapine to aripiprazole.
Of patients who switched to aripiprazole, 48.4% stayed on the drug, 26% switched back to olanzapine, and 25.6% received other treatment. Overall, 44.7% of patients switched medication at least three times.
Among patients with FEP who did not switch, 42.2% were prescribed olanzapine, 26.2% risperidone, 23.3% aripiprazole, 5.6% quetiapine, and 2.7% amisulpride.
During the post-presentation discussion, Ms. Brinn was asked whether the high rate of first-line olanzapine prescribing could be because patients started treatment as inpatients and were then switched once they were moved to community care.
“We found that a lot of patients would be prescribed olanzapine for around 7 days at the start of their prescription and then switch,” Ms. Brinn said, adding it is “likely” they started as inpatients. The investigators are currently examining the differences between inpatient and outpatient prescriptions to verify whether this is indeed the case, she added.
‘Pulling out the big guns too fast?’
Commenting on the findings, Thomas W. Sedlak, MD, PhD, Johns Hopkins University School of Medicine, Baltimore, said the study raises a “number of questions.”
Both olanzapine and risperidone “tend to have higher treatment effect improvements than aripiprazole, so it’s curious that a switch to aripiprazole was common,” said Dr. Sedlak, who was not involved with the research.
“Are we pulling out the ‘big guns’ too fast, or inappropriately, especially as olanzapine and risperidone carry greater risk of weight gain?” he asked. In addition, “now that olanzapine is available with samidorphan to mitigate weight gain, will that shape future patterns, if it can be paid for?”
Dr. Sedlak noted it was unclear why olanzapine was chosen so often as first-line treatment in the study and agreed it is “possible that hospitalized patients had been prescribed a ‘stronger’ medication like olanzapine compared to never-hospitalized patients.”
He also underlined that it is “not clear if patients in this FEP program are representative of all FEP patients.”
“For instance, if the program is well known to inpatient hospital social workers, then the program might be disproportionately filled with patients who have had more severe symptoms,” Dr. Sedlak said.
The study was supported by Janssen-Cilag. The investigators and Dr. Sedlak have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
In a large-scale, real-world analysis of U.K. prescribing patterns, researchers found more than two-thirds of patients who received antipsychotics for FEP switched medication, and almost half switched drugs three times.
Although this is “one of the largest real-world studies examining antipsychotic treatment strategies,” it reflects findings from previous, smaller studies showing “antipsychotic switching in first episode psychosis is high,” said study investigator Aimee Brinn, Institute of Psychiatry, Psychology & Neuroscience at King’s College London.
This may reflect reports of poor efficacy and suggests that first-line prescribing is “suboptimal,” Ms. Brinn noted. In addition, olanzapine remains the most popular antipsychotic for prescribing despite recent guidelines indicating it is “not ideal ... due to its dangerous metabolic side effects,” she added.
The findings were presented at the Congress of the Schizophrenia International Research Society (SIRS) 2022.
Real-world data
The response to, and tolerability of, antipsychotics differs between patients with FEP; and prescribing patterns “reflect clinician and patient-led decisionmaking,” Ms. Brinn told meeting attendees.
Since randomized controlled trials “do not necessarily reflect prescribing practice in real-world clinical settings,” the researchers gathered data from a large mental health care electronic health record dataset.
The investigators examined records from the South London and Maudsley NHS Foundation Trust (SLaM), which has a catchment area of 1.2 million individuals across four boroughs of London. The group sees approximately 37,500 active patients per week.
The team used the Clinical Interactive Record Search tool to extract data on 2,309 adults with FEP who received care from a SLaM early intervention in psychosis service between April 1, 2008, and March 31, 2019.
They found that 12 different antipsychotics were prescribed as first-line treatment. The most common were olanzapine (43.9%), risperidone (24.7%), and aripiprazole (19.9%).
Results showed that over 81,969.5 person-years of follow-up, at a minimum of 24 months per patient, 68.8% had an antipsychotic switch. The most common first treatment switch, in 17.9% of patients, was from olanzapine to aripiprazole.
Of patients who switched to aripiprazole, 48.4% stayed on the drug, 26% switched back to olanzapine, and 25.6% received other treatment. Overall, 44.7% of patients switched medication at least three times.
Among patients with FEP who did not switch, 42.2% were prescribed olanzapine, 26.2% risperidone, 23.3% aripiprazole, 5.6% quetiapine, and 2.7% amisulpride.
During the post-presentation discussion, Ms. Brinn was asked whether the high rate of first-line olanzapine prescribing could be because patients started treatment as inpatients and were then switched once they were moved to community care.
“We found that a lot of patients would be prescribed olanzapine for around 7 days at the start of their prescription and then switch,” Ms. Brinn said, adding it is “likely” they started as inpatients. The investigators are currently examining the differences between inpatient and outpatient prescriptions to verify whether this is indeed the case, she added.
‘Pulling out the big guns too fast?’
Commenting on the findings, Thomas W. Sedlak, MD, PhD, Johns Hopkins University School of Medicine, Baltimore, said the study raises a “number of questions.”
Both olanzapine and risperidone “tend to have higher treatment effect improvements than aripiprazole, so it’s curious that a switch to aripiprazole was common,” said Dr. Sedlak, who was not involved with the research.
“Are we pulling out the ‘big guns’ too fast, or inappropriately, especially as olanzapine and risperidone carry greater risk of weight gain?” he asked. In addition, “now that olanzapine is available with samidorphan to mitigate weight gain, will that shape future patterns, if it can be paid for?”
Dr. Sedlak noted it was unclear why olanzapine was chosen so often as first-line treatment in the study and agreed it is “possible that hospitalized patients had been prescribed a ‘stronger’ medication like olanzapine compared to never-hospitalized patients.”
He also underlined that it is “not clear if patients in this FEP program are representative of all FEP patients.”
“For instance, if the program is well known to inpatient hospital social workers, then the program might be disproportionately filled with patients who have had more severe symptoms,” Dr. Sedlak said.
The study was supported by Janssen-Cilag. The investigators and Dr. Sedlak have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM SIRS 2022
More antibodies with longer intervals between COVID vaccine doses
An overall ninefold increase in COVID-19 antibody levels can be seen with a longer interval between first and second doses of the Pfizer/BioNTech (BNT162b2) vaccine in people without prior infection, according to data from the U.K. government’s SIREN (SARS-CoV-2 Immunity and Reinfection Evaluation) study.
This interval-dependent antibody level varied by age, with those aged 45-54 years showing an 11-fold increase with a longer dosing interval (greater than 10 weeks vs. 2-4 weeks). People younger than age 25 years showed a 13-fold increase with the longer interval, but participant numbers were low in this sub-group.
Overall antibody levels in infection-naive participants were 1,268.72 Binding Antibody Units (BAU)/mL (1,043.25-1,542.91) in those with a 2-4–week interval, compared with 11,479.73 BAU/mL (10,742.78-12,267.24) (P < .0001), in those with at least a 10-week interval between doses.
The work is the latest analysis from SIREN, which measured antibody levels in the blood from nearly 6,000 health care workers from across the United Kingdom. Study lead Ashley Otter, PhD, technical lead for SIREN serology at the UK Health Security Agency (UKHSA), will present the work on Tuesday at the 2022 European Congress of Clinical Microbiology & Infectious Diseases (ECCMID), Lisbon.
In an interview, Dr. Otter noted that, “it is important to remember that antibody levels are only one aspect of the immune response, and in our recent vaccine effectiveness analysis, we found that dosing intervals did not affect protection against infection.”
The study, which appeared in the March issue of the New England Journal of Medicine, also found that after the second dose of vaccine, there was about a 2.5–fold difference in antibody levels between those who had prior infection of 16.052 (14.071-18.312) BAU/mL, compared with 7.050 (6.634-7.491) BAU/mL in infection-naive individuals (P < .0001).
Following the first dose only, antibody levels were up to 10 times higher in participants who were previously infected, compared with infection-naive individuals. This effect lasted up to 8 months and then began to plateau.
Natural infection increased antibody levels
Dr. Otter remarked that, “COVID-19 antibody levels are high in those people who were previously naturally infected and vaccinated, highlighting that vaccination provides an additional benefit to these individuals.”
This news organization asked Charlotte Thålin, PhD, an immunologist from the Karolinska Institute, Stockholm, to comment on the study. Dr. Thålin studies a cohort similar to SIREN, called the Swedish COMMUNITY health care worker cohort. “The new data from the SIREN emphasizes the importance of the number of antigenic exposures and the time interval between them, whether it be exposure through vaccination or exposure through infection.”
“We see similar data in our Swedish COMMUNITY health care worker cohort,” Dr. Thålin continued, “where infection prior to vaccination yields a more than twofold enhancement in antibodies, neutralizing breadth, and T cell responses, and an even larger increase with a longer time interval between infection and vaccination.”
However, she cautioned that they now see a high rate of Omicron vaccine breakthrough infections, and this is also true in people with previous infection and three vaccine doses.
“As we approach a second booster – a fourth vaccine dose – we need to consider that many individuals will have had up to five to six antigen exposures within a short period of time, sometimes within a year,” she pointed out. “This is a whole new scenario, with a lot of different combinations of vaccine and infection-induced immunity. We do not yet know the impact of these frequent immune exposures, and we now need to monitor immune responses following Omicron and booster doses closely.”
SIREN originally aimed to understand how much protection people got after developing a primary infection and why they might become reinfected with COVID-19. Following the rollout of the United Kingdom’s vaccination program, the protective effects of vaccination against COVID-19 were investigated, as well as why some people still become ill after being vaccinated, Dr. Otter explained.
In this latest analysis, Dr. Otter and colleagues assessed anti-spike binding antibodies in serum samples from a total of 5,871 health care workers, with 3,989 after one dose (at least 21 days) and 1,882 after two doses (at least 14 days).
Most participants were women (82.3%), of White ethnicity (87%), and came from across the United Kingdom.
Participants were also categorized into those who had evidence of natural COVID-19 infection (confirmed by a PCR test or assumed because of their antibody profile) or those who were infection-naive. Almost all (> 99%) of those who were infection-naive seroconverted after vaccination.
The primary outcome was anti-spike antibody levels assessed according to dose, previous infection, dosing interval, age, ethnicity, and comorbidities, including immunosuppressive disease such as immune system cancers, rheumatologic disease, chronic respiratory diseases, diabetes, obesity, and chronic neurologic disease.
In the infection-naive group, the mean antibody (anti-S titer) was 75.48 BAU/mL after the first vaccine dose, and this rose to 7,049.76 BAU/mL after the second dose.
The much higher antibody titer with the second dose in infection-naive individuals “is what gives you the most protection, as your antibody titers are at their peak. They then start to gradually wane from this peak,” said Dr. Otter.
In the post-infection group, antibody titers also rose (2,111.08 BAU/mL after first dose and 16,052.39 BAU/mL after second dose), although less so than in the infection-naive group, because of the additional exposure of infection, added Dr. Otter.
Antibody levels also varied according to time elapsed between natural infection and dose 1 of vaccination. With a 3-month interval, antibody levels were 1,970.83 (1,506.01-2,579.1) BAU/mL, compared with 13,759.31 (8,097.78-23,379.09) BAU/mL after a 9-month interval. Antibody levels after one dose in those previously infected are higher than the infection-naive because “previous infection, then vaccination, is likely explained by T-cell expansion upon a boost with a second antigen exposure, and then a maturing memory B-cell response that has been demonstrated up to 6 months,” explained Dr. Otter.
Timing of fourth dose
By March of this year, 86.2% of the U.K. population aged over 12 years had received at least two doses, but with rises in disease prevalence and the spread of variants of concern, further work is ongoing to understand the waning of the immune response, level of protection, and why some individuals develop COVID-19 even when double-vaccinated.
This news organization asked Susanna Dunachie, BMChB, professor of infectious diseases, University of Oxford, U.K., what the interval findings might mean for the timing of the fourth dose of vaccine across the U.K. population.
In the United Kingdom, fourth doses are being given to people who are 75 years and older, residents in care homes for older people, and those with weakened immune systems. “To make decisions about fourth doses for healthy people, we need to see how quickly antibody and T-cell responses drop,” said Ms. Dunachie, who is part of the large SIREN study team but was not involved in the analysis led by Dr. Otter. “Current research suggests that the T-cell response may be better maintained than the antibody response, and less affected by variants like Omicron.”
She explained the balance between antibody and T-cell responses to vaccination. “It is likely that antibodies that neutralize the virus are important for preventing any infection at all, and these unfortunately do fall in time, but T-cell responses are better sustained and help keep people out of [the] hospital,” she said.
Ms. Dunachie added that it was necessary to wait and observe what happens next with SARS-CoV-2 evolution, as well as wait for longer follow-up after the third dose in healthy people. “On current evidence, my estimate is we postpone decisions on fourth doses in healthy people to late summer/autumn.”
A version of this article first appeared on Medscape.com.
An overall ninefold increase in COVID-19 antibody levels can be seen with a longer interval between first and second doses of the Pfizer/BioNTech (BNT162b2) vaccine in people without prior infection, according to data from the U.K. government’s SIREN (SARS-CoV-2 Immunity and Reinfection Evaluation) study.
This interval-dependent antibody level varied by age, with those aged 45-54 years showing an 11-fold increase with a longer dosing interval (greater than 10 weeks vs. 2-4 weeks). People younger than age 25 years showed a 13-fold increase with the longer interval, but participant numbers were low in this sub-group.
Overall antibody levels in infection-naive participants were 1,268.72 Binding Antibody Units (BAU)/mL (1,043.25-1,542.91) in those with a 2-4–week interval, compared with 11,479.73 BAU/mL (10,742.78-12,267.24) (P < .0001), in those with at least a 10-week interval between doses.
The work is the latest analysis from SIREN, which measured antibody levels in the blood from nearly 6,000 health care workers from across the United Kingdom. Study lead Ashley Otter, PhD, technical lead for SIREN serology at the UK Health Security Agency (UKHSA), will present the work on Tuesday at the 2022 European Congress of Clinical Microbiology & Infectious Diseases (ECCMID), Lisbon.
In an interview, Dr. Otter noted that, “it is important to remember that antibody levels are only one aspect of the immune response, and in our recent vaccine effectiveness analysis, we found that dosing intervals did not affect protection against infection.”
The study, which appeared in the March issue of the New England Journal of Medicine, also found that after the second dose of vaccine, there was about a 2.5–fold difference in antibody levels between those who had prior infection of 16.052 (14.071-18.312) BAU/mL, compared with 7.050 (6.634-7.491) BAU/mL in infection-naive individuals (P < .0001).
Following the first dose only, antibody levels were up to 10 times higher in participants who were previously infected, compared with infection-naive individuals. This effect lasted up to 8 months and then began to plateau.
Natural infection increased antibody levels
Dr. Otter remarked that, “COVID-19 antibody levels are high in those people who were previously naturally infected and vaccinated, highlighting that vaccination provides an additional benefit to these individuals.”
This news organization asked Charlotte Thålin, PhD, an immunologist from the Karolinska Institute, Stockholm, to comment on the study. Dr. Thålin studies a cohort similar to SIREN, called the Swedish COMMUNITY health care worker cohort. “The new data from the SIREN emphasizes the importance of the number of antigenic exposures and the time interval between them, whether it be exposure through vaccination or exposure through infection.”
“We see similar data in our Swedish COMMUNITY health care worker cohort,” Dr. Thålin continued, “where infection prior to vaccination yields a more than twofold enhancement in antibodies, neutralizing breadth, and T cell responses, and an even larger increase with a longer time interval between infection and vaccination.”
However, she cautioned that they now see a high rate of Omicron vaccine breakthrough infections, and this is also true in people with previous infection and three vaccine doses.
“As we approach a second booster – a fourth vaccine dose – we need to consider that many individuals will have had up to five to six antigen exposures within a short period of time, sometimes within a year,” she pointed out. “This is a whole new scenario, with a lot of different combinations of vaccine and infection-induced immunity. We do not yet know the impact of these frequent immune exposures, and we now need to monitor immune responses following Omicron and booster doses closely.”
SIREN originally aimed to understand how much protection people got after developing a primary infection and why they might become reinfected with COVID-19. Following the rollout of the United Kingdom’s vaccination program, the protective effects of vaccination against COVID-19 were investigated, as well as why some people still become ill after being vaccinated, Dr. Otter explained.
In this latest analysis, Dr. Otter and colleagues assessed anti-spike binding antibodies in serum samples from a total of 5,871 health care workers, with 3,989 after one dose (at least 21 days) and 1,882 after two doses (at least 14 days).
Most participants were women (82.3%), of White ethnicity (87%), and came from across the United Kingdom.
Participants were also categorized into those who had evidence of natural COVID-19 infection (confirmed by a PCR test or assumed because of their antibody profile) or those who were infection-naive. Almost all (> 99%) of those who were infection-naive seroconverted after vaccination.
The primary outcome was anti-spike antibody levels assessed according to dose, previous infection, dosing interval, age, ethnicity, and comorbidities, including immunosuppressive disease such as immune system cancers, rheumatologic disease, chronic respiratory diseases, diabetes, obesity, and chronic neurologic disease.
In the infection-naive group, the mean antibody (anti-S titer) was 75.48 BAU/mL after the first vaccine dose, and this rose to 7,049.76 BAU/mL after the second dose.
The much higher antibody titer with the second dose in infection-naive individuals “is what gives you the most protection, as your antibody titers are at their peak. They then start to gradually wane from this peak,” said Dr. Otter.
In the post-infection group, antibody titers also rose (2,111.08 BAU/mL after first dose and 16,052.39 BAU/mL after second dose), although less so than in the infection-naive group, because of the additional exposure of infection, added Dr. Otter.
Antibody levels also varied according to time elapsed between natural infection and dose 1 of vaccination. With a 3-month interval, antibody levels were 1,970.83 (1,506.01-2,579.1) BAU/mL, compared with 13,759.31 (8,097.78-23,379.09) BAU/mL after a 9-month interval. Antibody levels after one dose in those previously infected are higher than the infection-naive because “previous infection, then vaccination, is likely explained by T-cell expansion upon a boost with a second antigen exposure, and then a maturing memory B-cell response that has been demonstrated up to 6 months,” explained Dr. Otter.
Timing of fourth dose
By March of this year, 86.2% of the U.K. population aged over 12 years had received at least two doses, but with rises in disease prevalence and the spread of variants of concern, further work is ongoing to understand the waning of the immune response, level of protection, and why some individuals develop COVID-19 even when double-vaccinated.
This news organization asked Susanna Dunachie, BMChB, professor of infectious diseases, University of Oxford, U.K., what the interval findings might mean for the timing of the fourth dose of vaccine across the U.K. population.
In the United Kingdom, fourth doses are being given to people who are 75 years and older, residents in care homes for older people, and those with weakened immune systems. “To make decisions about fourth doses for healthy people, we need to see how quickly antibody and T-cell responses drop,” said Ms. Dunachie, who is part of the large SIREN study team but was not involved in the analysis led by Dr. Otter. “Current research suggests that the T-cell response may be better maintained than the antibody response, and less affected by variants like Omicron.”
She explained the balance between antibody and T-cell responses to vaccination. “It is likely that antibodies that neutralize the virus are important for preventing any infection at all, and these unfortunately do fall in time, but T-cell responses are better sustained and help keep people out of [the] hospital,” she said.
Ms. Dunachie added that it was necessary to wait and observe what happens next with SARS-CoV-2 evolution, as well as wait for longer follow-up after the third dose in healthy people. “On current evidence, my estimate is we postpone decisions on fourth doses in healthy people to late summer/autumn.”
A version of this article first appeared on Medscape.com.
An overall ninefold increase in COVID-19 antibody levels can be seen with a longer interval between first and second doses of the Pfizer/BioNTech (BNT162b2) vaccine in people without prior infection, according to data from the U.K. government’s SIREN (SARS-CoV-2 Immunity and Reinfection Evaluation) study.
This interval-dependent antibody level varied by age, with those aged 45-54 years showing an 11-fold increase with a longer dosing interval (greater than 10 weeks vs. 2-4 weeks). People younger than age 25 years showed a 13-fold increase with the longer interval, but participant numbers were low in this sub-group.
Overall antibody levels in infection-naive participants were 1,268.72 Binding Antibody Units (BAU)/mL (1,043.25-1,542.91) in those with a 2-4–week interval, compared with 11,479.73 BAU/mL (10,742.78-12,267.24) (P < .0001), in those with at least a 10-week interval between doses.
The work is the latest analysis from SIREN, which measured antibody levels in the blood from nearly 6,000 health care workers from across the United Kingdom. Study lead Ashley Otter, PhD, technical lead for SIREN serology at the UK Health Security Agency (UKHSA), will present the work on Tuesday at the 2022 European Congress of Clinical Microbiology & Infectious Diseases (ECCMID), Lisbon.
In an interview, Dr. Otter noted that, “it is important to remember that antibody levels are only one aspect of the immune response, and in our recent vaccine effectiveness analysis, we found that dosing intervals did not affect protection against infection.”
The study, which appeared in the March issue of the New England Journal of Medicine, also found that after the second dose of vaccine, there was about a 2.5–fold difference in antibody levels between those who had prior infection of 16.052 (14.071-18.312) BAU/mL, compared with 7.050 (6.634-7.491) BAU/mL in infection-naive individuals (P < .0001).
Following the first dose only, antibody levels were up to 10 times higher in participants who were previously infected, compared with infection-naive individuals. This effect lasted up to 8 months and then began to plateau.
Natural infection increased antibody levels
Dr. Otter remarked that, “COVID-19 antibody levels are high in those people who were previously naturally infected and vaccinated, highlighting that vaccination provides an additional benefit to these individuals.”
This news organization asked Charlotte Thålin, PhD, an immunologist from the Karolinska Institute, Stockholm, to comment on the study. Dr. Thålin studies a cohort similar to SIREN, called the Swedish COMMUNITY health care worker cohort. “The new data from the SIREN emphasizes the importance of the number of antigenic exposures and the time interval between them, whether it be exposure through vaccination or exposure through infection.”
“We see similar data in our Swedish COMMUNITY health care worker cohort,” Dr. Thålin continued, “where infection prior to vaccination yields a more than twofold enhancement in antibodies, neutralizing breadth, and T cell responses, and an even larger increase with a longer time interval between infection and vaccination.”
However, she cautioned that they now see a high rate of Omicron vaccine breakthrough infections, and this is also true in people with previous infection and three vaccine doses.
“As we approach a second booster – a fourth vaccine dose – we need to consider that many individuals will have had up to five to six antigen exposures within a short period of time, sometimes within a year,” she pointed out. “This is a whole new scenario, with a lot of different combinations of vaccine and infection-induced immunity. We do not yet know the impact of these frequent immune exposures, and we now need to monitor immune responses following Omicron and booster doses closely.”
SIREN originally aimed to understand how much protection people got after developing a primary infection and why they might become reinfected with COVID-19. Following the rollout of the United Kingdom’s vaccination program, the protective effects of vaccination against COVID-19 were investigated, as well as why some people still become ill after being vaccinated, Dr. Otter explained.
In this latest analysis, Dr. Otter and colleagues assessed anti-spike binding antibodies in serum samples from a total of 5,871 health care workers, with 3,989 after one dose (at least 21 days) and 1,882 after two doses (at least 14 days).
Most participants were women (82.3%), of White ethnicity (87%), and came from across the United Kingdom.
Participants were also categorized into those who had evidence of natural COVID-19 infection (confirmed by a PCR test or assumed because of their antibody profile) or those who were infection-naive. Almost all (> 99%) of those who were infection-naive seroconverted after vaccination.
The primary outcome was anti-spike antibody levels assessed according to dose, previous infection, dosing interval, age, ethnicity, and comorbidities, including immunosuppressive disease such as immune system cancers, rheumatologic disease, chronic respiratory diseases, diabetes, obesity, and chronic neurologic disease.
In the infection-naive group, the mean antibody (anti-S titer) was 75.48 BAU/mL after the first vaccine dose, and this rose to 7,049.76 BAU/mL after the second dose.
The much higher antibody titer with the second dose in infection-naive individuals “is what gives you the most protection, as your antibody titers are at their peak. They then start to gradually wane from this peak,” said Dr. Otter.
In the post-infection group, antibody titers also rose (2,111.08 BAU/mL after first dose and 16,052.39 BAU/mL after second dose), although less so than in the infection-naive group, because of the additional exposure of infection, added Dr. Otter.
Antibody levels also varied according to time elapsed between natural infection and dose 1 of vaccination. With a 3-month interval, antibody levels were 1,970.83 (1,506.01-2,579.1) BAU/mL, compared with 13,759.31 (8,097.78-23,379.09) BAU/mL after a 9-month interval. Antibody levels after one dose in those previously infected are higher than the infection-naive because “previous infection, then vaccination, is likely explained by T-cell expansion upon a boost with a second antigen exposure, and then a maturing memory B-cell response that has been demonstrated up to 6 months,” explained Dr. Otter.
Timing of fourth dose
By March of this year, 86.2% of the U.K. population aged over 12 years had received at least two doses, but with rises in disease prevalence and the spread of variants of concern, further work is ongoing to understand the waning of the immune response, level of protection, and why some individuals develop COVID-19 even when double-vaccinated.
This news organization asked Susanna Dunachie, BMChB, professor of infectious diseases, University of Oxford, U.K., what the interval findings might mean for the timing of the fourth dose of vaccine across the U.K. population.
In the United Kingdom, fourth doses are being given to people who are 75 years and older, residents in care homes for older people, and those with weakened immune systems. “To make decisions about fourth doses for healthy people, we need to see how quickly antibody and T-cell responses drop,” said Ms. Dunachie, who is part of the large SIREN study team but was not involved in the analysis led by Dr. Otter. “Current research suggests that the T-cell response may be better maintained than the antibody response, and less affected by variants like Omicron.”
She explained the balance between antibody and T-cell responses to vaccination. “It is likely that antibodies that neutralize the virus are important for preventing any infection at all, and these unfortunately do fall in time, but T-cell responses are better sustained and help keep people out of [the] hospital,” she said.
Ms. Dunachie added that it was necessary to wait and observe what happens next with SARS-CoV-2 evolution, as well as wait for longer follow-up after the third dose in healthy people. “On current evidence, my estimate is we postpone decisions on fourth doses in healthy people to late summer/autumn.”
A version of this article first appeared on Medscape.com.
Long-term efficacy, safety data for ixekizumab in pediatric psoriasis reported
with the interleukin (IL)-17 inhibitor, investigators reported.
In addition, findings of a substudy, which evaluated randomized withdrawal of treatment after 60 weeks, suggest patients were able to regain benefit after not being treated for a period.
Ixekizumab (Taltz) was approved by the U.S. Food and Drug Administration for treating pediatric psoriasis in March 2020 for patients aged 6 years and older with moderate to severe plaque psoriasis who are candidates for systemic therapy or phototherapy.
The trial (IXORA-PEDS) involved 171 patients aged 6-17 years (mean age, 13.5 years; 99 females and 72 males), who were randomly assigned to receive ixekizumab via subcutaneous administration every 4 weeks (115) or placebo for 12 weeks (56). Thereafter, 166 patients continued in an open-label maintenance period in which they were treated every 4 weeks for 12-60 weeks. This was followed by an extension period of up to 108 weeks, which was completed by 139 patients (83.7%). At baseline, the patients’ Psoriasis Area and Severity Index (PASI) score was 12 or higher, the static Physician’s Global Assessment (sPGA) score was 3 or higher, and 10% or more of body surface area was affected.
In the study, at 12 weeks, treatment with ixekizumab was superior to placebo, with sustained responses through 48 weeks. In the follow-up phase, primary and secondary endpoints were sustained through week 108, with patients achieving or maintaining PASI 75 (91.7%), PASI 90 (79%), PASI 100 (55.1%), sPGA 0 or 1 (78.3%), and sPGA 0 (52.4%). Significant improvements in itch were seen at 12 weeks and were sustained with “meaningful improvements in itch for 78.5% of these patients at week 108,” the investigators report.
Among the patients who received ixekizumab, clearance rates in areas that are difficult to treat increased from week 12 to week 108 among those affected. During this time, clearance of nail psoriasis increased from 22.8% to 68.1%, clearance of palmoplantar psoriasis increased from 46.2% to 90%, clearance of scalp psoriasis increased from 70.7% to 76.2%, and clearance of genital psoriasis increased from 83.3% to 87.5%.
No new safety findings during weeks 48-108 of the trial were reported, including no new cases of inflammatory bowel disease (IBD) or Candida infections. The results were reported in JAMA Dermatology.
“Safety is really what we think of most when we are talking about pediatric patients, especially since they may be on these for decades and ... since they most commonly start these therapies in adolescence,” said Amy Paller, MD, the study’s lead author, in an interview. “To be able to take this out 108 weeks, 2 years, is starting to get to a point where we are getting more comfortable with safety. Clearly, no new signals arose.” Dr. Paller is chair of the department of dermatology and professor of dermatology and pediatrics, Northwestern University, Chicago.
One of the biggest concerns with using IL-17 inhibitors such as ixekizumab to manage psoriasis is the development of IBD, said Dr. Paller. She noted that four cases of IBD were reported before the extension phase of the trial but that no new IBD cases were reported after week 48.
“We would not start this as a treatment of choice in someone with Crohn’s disease, or perhaps we would think twice about using it in someone with a strong family history [of Crohn’s disease],” said Dr. Paller, who is also the director of the Skin Biology and Diseases Resource-Based Center at Northwestern. “Otherwise, it does not make me concerned about its use.”
Commenting on the study, Kelly M. Cordoro, MD, professor of dermatology and pediatrics at the University of California, San Francisco, said that the trial’s results provide additional evidence regarding the optimal management of pediatric psoriasis.
“The landscape has shifted toward involving more pediatric patients in clinical trials, thereby providing dermatologists with data to select safe and effective therapies to manage children with psoriasis,” Dr. Cordoro said in an interview. “We have data showing that children with psoriasis have been undertreated, likely because of concerns about safety. The more evidence available from trials such as this, the more likely children are to receive necessary treatment.”
The efficacy data from the study on difficult-to-treat areas of psoriasis, in addition to improvements in BSA and PASI measures, are significant for clinicians deciding on a therapy for patients with psoriasis concentrated in specific body sites. “It was very valuable that the efficacy data was provided by site, such as scalp, palmoplantar, nails, and genital psoriasis, as these are low-BSA but high-impact areas for patients,” said Dr. Cordoro.
The trial data on Crohn’s disease buttress her decision to continue to refrain from initiating ixekizumab in a child with IBD or who is at high risk for IBD. “I was happy to see that there was not a signal for Candida infection,” she added.
Interestingly, in the substudy in the European population, in which there was a double-blind, randomized withdrawal period, fewer patients who were reassigned to receive ixekizumab experienced relapse, compared with those who were reassigned to receive placebo. A total of 90.9% of patients who received placebo experienced relapse, compared with 17.6% of patients treated with ixekizumab. The median time to relapse in the placebo group was 149 days.
“There are data in the adult population that suggest intermittent treatment does allow for recapture of clinical response,” said Dr. Cordoro. “While it is not a large enough dataset to know definitively, this substudy of patients suggests the possibility of intermittent treatment and the ability to regain control [of psoriasis] after a period off drug.”
The study was funded by Eli Lilly. Dr. Paller is an investigator and consultant for Eli Lilly. Several other authors have received grants, personal fees, and/or were a consultant to Eli Lilly, and two authors are Eli Lilly employees. Dr. Cordoro reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
with the interleukin (IL)-17 inhibitor, investigators reported.
In addition, findings of a substudy, which evaluated randomized withdrawal of treatment after 60 weeks, suggest patients were able to regain benefit after not being treated for a period.
Ixekizumab (Taltz) was approved by the U.S. Food and Drug Administration for treating pediatric psoriasis in March 2020 for patients aged 6 years and older with moderate to severe plaque psoriasis who are candidates for systemic therapy or phototherapy.
The trial (IXORA-PEDS) involved 171 patients aged 6-17 years (mean age, 13.5 years; 99 females and 72 males), who were randomly assigned to receive ixekizumab via subcutaneous administration every 4 weeks (115) or placebo for 12 weeks (56). Thereafter, 166 patients continued in an open-label maintenance period in which they were treated every 4 weeks for 12-60 weeks. This was followed by an extension period of up to 108 weeks, which was completed by 139 patients (83.7%). At baseline, the patients’ Psoriasis Area and Severity Index (PASI) score was 12 or higher, the static Physician’s Global Assessment (sPGA) score was 3 or higher, and 10% or more of body surface area was affected.
In the study, at 12 weeks, treatment with ixekizumab was superior to placebo, with sustained responses through 48 weeks. In the follow-up phase, primary and secondary endpoints were sustained through week 108, with patients achieving or maintaining PASI 75 (91.7%), PASI 90 (79%), PASI 100 (55.1%), sPGA 0 or 1 (78.3%), and sPGA 0 (52.4%). Significant improvements in itch were seen at 12 weeks and were sustained with “meaningful improvements in itch for 78.5% of these patients at week 108,” the investigators report.
Among the patients who received ixekizumab, clearance rates in areas that are difficult to treat increased from week 12 to week 108 among those affected. During this time, clearance of nail psoriasis increased from 22.8% to 68.1%, clearance of palmoplantar psoriasis increased from 46.2% to 90%, clearance of scalp psoriasis increased from 70.7% to 76.2%, and clearance of genital psoriasis increased from 83.3% to 87.5%.
No new safety findings during weeks 48-108 of the trial were reported, including no new cases of inflammatory bowel disease (IBD) or Candida infections. The results were reported in JAMA Dermatology.
“Safety is really what we think of most when we are talking about pediatric patients, especially since they may be on these for decades and ... since they most commonly start these therapies in adolescence,” said Amy Paller, MD, the study’s lead author, in an interview. “To be able to take this out 108 weeks, 2 years, is starting to get to a point where we are getting more comfortable with safety. Clearly, no new signals arose.” Dr. Paller is chair of the department of dermatology and professor of dermatology and pediatrics, Northwestern University, Chicago.
One of the biggest concerns with using IL-17 inhibitors such as ixekizumab to manage psoriasis is the development of IBD, said Dr. Paller. She noted that four cases of IBD were reported before the extension phase of the trial but that no new IBD cases were reported after week 48.
“We would not start this as a treatment of choice in someone with Crohn’s disease, or perhaps we would think twice about using it in someone with a strong family history [of Crohn’s disease],” said Dr. Paller, who is also the director of the Skin Biology and Diseases Resource-Based Center at Northwestern. “Otherwise, it does not make me concerned about its use.”
Commenting on the study, Kelly M. Cordoro, MD, professor of dermatology and pediatrics at the University of California, San Francisco, said that the trial’s results provide additional evidence regarding the optimal management of pediatric psoriasis.
“The landscape has shifted toward involving more pediatric patients in clinical trials, thereby providing dermatologists with data to select safe and effective therapies to manage children with psoriasis,” Dr. Cordoro said in an interview. “We have data showing that children with psoriasis have been undertreated, likely because of concerns about safety. The more evidence available from trials such as this, the more likely children are to receive necessary treatment.”
The efficacy data from the study on difficult-to-treat areas of psoriasis, in addition to improvements in BSA and PASI measures, are significant for clinicians deciding on a therapy for patients with psoriasis concentrated in specific body sites. “It was very valuable that the efficacy data was provided by site, such as scalp, palmoplantar, nails, and genital psoriasis, as these are low-BSA but high-impact areas for patients,” said Dr. Cordoro.
The trial data on Crohn’s disease buttress her decision to continue to refrain from initiating ixekizumab in a child with IBD or who is at high risk for IBD. “I was happy to see that there was not a signal for Candida infection,” she added.
Interestingly, in the substudy in the European population, in which there was a double-blind, randomized withdrawal period, fewer patients who were reassigned to receive ixekizumab experienced relapse, compared with those who were reassigned to receive placebo. A total of 90.9% of patients who received placebo experienced relapse, compared with 17.6% of patients treated with ixekizumab. The median time to relapse in the placebo group was 149 days.
“There are data in the adult population that suggest intermittent treatment does allow for recapture of clinical response,” said Dr. Cordoro. “While it is not a large enough dataset to know definitively, this substudy of patients suggests the possibility of intermittent treatment and the ability to regain control [of psoriasis] after a period off drug.”
The study was funded by Eli Lilly. Dr. Paller is an investigator and consultant for Eli Lilly. Several other authors have received grants, personal fees, and/or were a consultant to Eli Lilly, and two authors are Eli Lilly employees. Dr. Cordoro reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
with the interleukin (IL)-17 inhibitor, investigators reported.
In addition, findings of a substudy, which evaluated randomized withdrawal of treatment after 60 weeks, suggest patients were able to regain benefit after not being treated for a period.
Ixekizumab (Taltz) was approved by the U.S. Food and Drug Administration for treating pediatric psoriasis in March 2020 for patients aged 6 years and older with moderate to severe plaque psoriasis who are candidates for systemic therapy or phototherapy.
The trial (IXORA-PEDS) involved 171 patients aged 6-17 years (mean age, 13.5 years; 99 females and 72 males), who were randomly assigned to receive ixekizumab via subcutaneous administration every 4 weeks (115) or placebo for 12 weeks (56). Thereafter, 166 patients continued in an open-label maintenance period in which they were treated every 4 weeks for 12-60 weeks. This was followed by an extension period of up to 108 weeks, which was completed by 139 patients (83.7%). At baseline, the patients’ Psoriasis Area and Severity Index (PASI) score was 12 or higher, the static Physician’s Global Assessment (sPGA) score was 3 or higher, and 10% or more of body surface area was affected.
In the study, at 12 weeks, treatment with ixekizumab was superior to placebo, with sustained responses through 48 weeks. In the follow-up phase, primary and secondary endpoints were sustained through week 108, with patients achieving or maintaining PASI 75 (91.7%), PASI 90 (79%), PASI 100 (55.1%), sPGA 0 or 1 (78.3%), and sPGA 0 (52.4%). Significant improvements in itch were seen at 12 weeks and were sustained with “meaningful improvements in itch for 78.5% of these patients at week 108,” the investigators report.
Among the patients who received ixekizumab, clearance rates in areas that are difficult to treat increased from week 12 to week 108 among those affected. During this time, clearance of nail psoriasis increased from 22.8% to 68.1%, clearance of palmoplantar psoriasis increased from 46.2% to 90%, clearance of scalp psoriasis increased from 70.7% to 76.2%, and clearance of genital psoriasis increased from 83.3% to 87.5%.
No new safety findings during weeks 48-108 of the trial were reported, including no new cases of inflammatory bowel disease (IBD) or Candida infections. The results were reported in JAMA Dermatology.
“Safety is really what we think of most when we are talking about pediatric patients, especially since they may be on these for decades and ... since they most commonly start these therapies in adolescence,” said Amy Paller, MD, the study’s lead author, in an interview. “To be able to take this out 108 weeks, 2 years, is starting to get to a point where we are getting more comfortable with safety. Clearly, no new signals arose.” Dr. Paller is chair of the department of dermatology and professor of dermatology and pediatrics, Northwestern University, Chicago.
One of the biggest concerns with using IL-17 inhibitors such as ixekizumab to manage psoriasis is the development of IBD, said Dr. Paller. She noted that four cases of IBD were reported before the extension phase of the trial but that no new IBD cases were reported after week 48.
“We would not start this as a treatment of choice in someone with Crohn’s disease, or perhaps we would think twice about using it in someone with a strong family history [of Crohn’s disease],” said Dr. Paller, who is also the director of the Skin Biology and Diseases Resource-Based Center at Northwestern. “Otherwise, it does not make me concerned about its use.”
Commenting on the study, Kelly M. Cordoro, MD, professor of dermatology and pediatrics at the University of California, San Francisco, said that the trial’s results provide additional evidence regarding the optimal management of pediatric psoriasis.
“The landscape has shifted toward involving more pediatric patients in clinical trials, thereby providing dermatologists with data to select safe and effective therapies to manage children with psoriasis,” Dr. Cordoro said in an interview. “We have data showing that children with psoriasis have been undertreated, likely because of concerns about safety. The more evidence available from trials such as this, the more likely children are to receive necessary treatment.”
The efficacy data from the study on difficult-to-treat areas of psoriasis, in addition to improvements in BSA and PASI measures, are significant for clinicians deciding on a therapy for patients with psoriasis concentrated in specific body sites. “It was very valuable that the efficacy data was provided by site, such as scalp, palmoplantar, nails, and genital psoriasis, as these are low-BSA but high-impact areas for patients,” said Dr. Cordoro.
The trial data on Crohn’s disease buttress her decision to continue to refrain from initiating ixekizumab in a child with IBD or who is at high risk for IBD. “I was happy to see that there was not a signal for Candida infection,” she added.
Interestingly, in the substudy in the European population, in which there was a double-blind, randomized withdrawal period, fewer patients who were reassigned to receive ixekizumab experienced relapse, compared with those who were reassigned to receive placebo. A total of 90.9% of patients who received placebo experienced relapse, compared with 17.6% of patients treated with ixekizumab. The median time to relapse in the placebo group was 149 days.
“There are data in the adult population that suggest intermittent treatment does allow for recapture of clinical response,” said Dr. Cordoro. “While it is not a large enough dataset to know definitively, this substudy of patients suggests the possibility of intermittent treatment and the ability to regain control [of psoriasis] after a period off drug.”
The study was funded by Eli Lilly. Dr. Paller is an investigator and consultant for Eli Lilly. Several other authors have received grants, personal fees, and/or were a consultant to Eli Lilly, and two authors are Eli Lilly employees. Dr. Cordoro reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Indications and techniques for multifetal pregnancy reduction
Multifetal pregnancy reduction (MPR) was developed in the 1980s in the wake of significant increases in the incidence of triplets and other higher-order multiples emanating from assisted reproductive technologies (ART). It was offered to reduce fetal number and improve outcomes for remaining fetuses by reducing rates of preterm delivery, fetal growth restriction, and other adverse perinatal outcomes, as well as maternal complications such as preeclampsia and postpartum hemorrhage.
In recent years, improvements in ART – mainly changes in ovulation induction practices and limitations in the number of embryos implanted to two at most – have reversed the increase in higher-order multiples. However, with intrauterine insemination, higher-order multiples still occur, and even without any reproductive assistance, the reality is that multiple pregnancies – particularly twins – continue to exist. In 2018, twins comprised about 3% of births in the United States.1
Twin pregnancies have a significantly higher risk than singleton gestations of preterm birth, maternal complications, and neonatal morbidity and mortality. The pregnancies are complicated more often by preterm premature rupture of membranes, fetal growth restriction, and hypertensive disorders of pregnancy.
Monochorionic diamniotic twin pregnancies face additional, unique risks of twin-to-twin transfusion syndrome, twin reversed arterial perfusion sequence, and twin-anemia polycythemia sequence. These pregnancies account for about 20% of all twin gestations, and decades of experience with ART have shown us that monochorionic diamniotic gestations occur at a higher rate after in-vitro fertilization.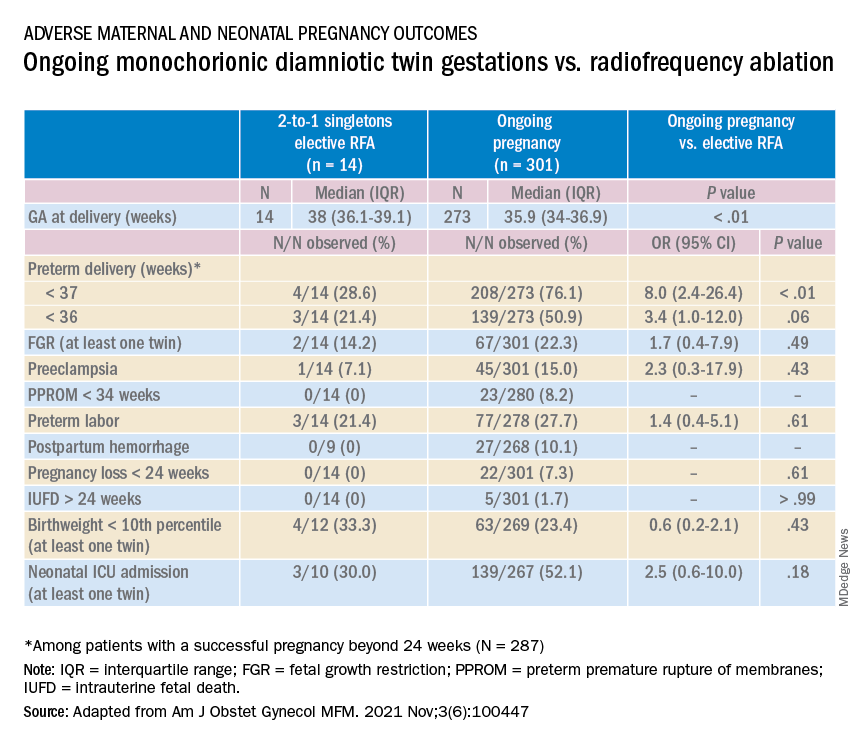
Although advances have improved the outcomes of multiple births, risks remain and elective MPR is still very relevant for twin gestations. Patients routinely receive counseling about the risks of twin gestations, but they often are not made aware of the option of elective fetal reduction.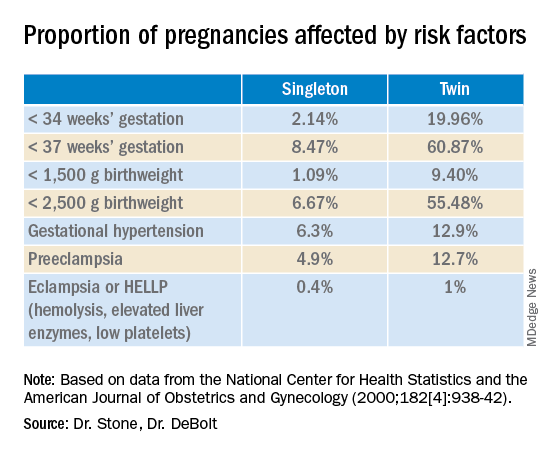
We have offered elective reduction (of nonanomalous fetuses) to a singleton for almost 30 years and have published several reports documenting that MPR in dichorionic diamniotic pregnancies reduces the risk of preterm delivery and other complications without increasing the risk of pregnancy loss.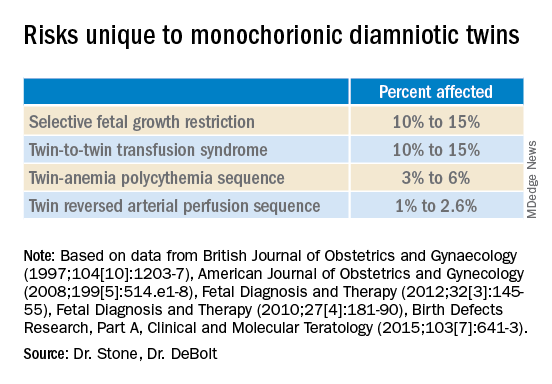
Most recently, we also published data comparing the outcomes of patients with monochorionic diamniotic gestations who underwent elective MPR by radiofrequency ablation (RFA) vs. those with ongoing monochorionic diamniotic gestations.2 While the numbers were small, the data show significantly lower rates of preterm birth without an increased risk of pregnancy loss.
Experience with dichorionic diamniotic twins, genetic testing
Our most recent review3 of outcomes in dichorionic diamniotic gestations covered 855 patients, 29% of whom underwent planned elective MPR at less than 15 weeks, and 71% of whom had ongoing twin gestations. Those with ongoing twin gestations had adjusted odds ratios of preterm delivery at less than 37 weeks and less than 34 weeks of 5.62 and 2.22, respectively (adjustments controlled for maternal characteristics such as maternal age, BMI, use of chorionic villus sampling [CVS], and history of preterm birth).
Ongoing twin pregnancies were also more likely to have preeclampsia (AOR, 3.33), preterm premature rupture of membranes (3.86), and low birthweight (under the 5th and 10th percentiles). There were no significant differences in the rate of unintended pregnancy loss (2.4% vs. 2.3%), and rates for total pregnancy loss at less than 24 weeks and less than 20 weeks were similar.
An important issue in the consideration of MPR is that prenatal diagnosis of chromosomal abnormalities is very safe in twins. Multiple gestations are at greater risk of chromosomal abnormalities, so performing MPR selectively – if a chromosomally abnormal fetus is present – is desirable for many parents.
A recent meta-analysis and systematic review of studies reporting fetal loss following amniocentesis or CVS in twin pregnancies found an exceedingly low risk of loss. Procedure-related fetal loss (the primary outcome) was lower than previously reported, and the rate of fetal loss before 24 weeks gestation or within 4 weeks after the procedure (secondary outcomes), did not differ from the background risk in twin pregnancies not undergoing invasive prenatal testing.4
Our data have shown no significant differences in pregnancy loss between patients who underwent CVS prior to MPR and those who did not. Looking specifically at reduction to a singleton gestation, patients who underwent CVS prior to MPR had a fourfold reduction in loss.5 Therefore, we counsel patients that CVS provides useful information – especially now with the common use of chromosomal microarray – at almost negligible risk.
MPR for monochorionic diamniotic twins
Most of the literature on MPR from twin to singleton gestations reports on intrathoracic potassium chloride injection used in dichorionic diamniotic twins.
MPR in monochorionic diamniotic twins is reserved in the United States for monochorionic pregnancies in which there are severe fetal anomalies, severe growth restriction, or other significant complications. It is performed in such cases around 20 weeks gestation. However, given the significant risks of monochorionic twin pregnancies, we also have been offering MPR electively and earlier in pregnancy. While many modalities of intrafetal cord occlusion exist, RFA at the cord insertion site into the fetal abdomen is our preferred technique.
In our retrospective review of 315 monochorionic diamniotic twin gestations, the 14 patients who had RFA electively had no pregnancy losses and a significantly lower rate of preterm birth at less than 37-weeks gestation, compared with 301 ongoing monochorionic diamniotic twin pregnancies (29% vs. 76%).5 Reduction with RFA, performed at a mean gestational age of 15 weeks, also eliminated the risks unique to monochorionic twins, such as twin-to-twin transfusion syndrome and twin-anemia polycythemia sequence. (Of the ongoing twin gestations, 12% required medically indicated RFA, fetoscopic laser ablation, and/or amnioreduction; 4% had unintended loss of one fetus; and 4% had unintended loss of both fetuses before 24 weeks’ gestation. Fewer than 70% of the ongoing twin gestations had none of the significant adverse outcomes unique to monochorionic twins.)
Interestingly, there were still a couple of cases of fetal growth restriction in patients who underwent elective MPR – a rate higher than that seen in singleton gestations – most likely because of the early timing of the procedure.
Our numbers of MPRs in this review were small, but the data offer at least preliminary evidence that planned elective RFA before 17 weeks gestation may be offered to patients who do not want to assume the risks of monochorionic diamniotic twin pregnancies.
Counseling in twin pregnancies
We perform thorough, early assessments of fetal anatomy in our twin pregnancies, and we undertake thorough medical and obstetrical histories to uncover birth complications or medical conditions that would increase risks of preeclampsia, preterm birth, fetal growth restriction, and other complications.
Because monochorionic gestations are at particularly high risk for heart defects, we also routinely perform fetal echocardiography in these pregnancies.
Genetic testing is offered to all twin pregnancies, and as mentioned above, we especially counsel those considering MPR that such testing provides useful information.
Patients are made aware of the option of MPR and receive nondirective counseling. It is the patient’s choice. We recognize that elective termination is a controversial procedure, but we believe that the option of MPR should be available to patients who want to improve outcomes for their pregnancy.
When anomalies are discovered and selective termination is chosen, we usually try to perform MPR as early as possible. After 16 weeks, we’ve found, the rate of pregnancy loss increases slightly.
Dr. Stone is the Ellen and Howard C. Katz Chairman’s Chair, and Dr. DeBolt is a clinical fellow in maternal-fetal medicine, in the Raquel and Jaime Gilinski Department of Obstetrics, Gynecology, and Reproductive Science at the Icahn School of Medicine at Mount Sinai, New York.
References
1. Martin JA and Osterman MJK. National Center of Health Statistics. NCHS Data Brief, 2019;no 351.
2. Manasa GR et al. Am J Obstet Gynecol MFM 2021;3:100447.
3. Vieira LA et al. Am J. Obstet Gynecol. 2019;221:253.e1-8.
4. Di Mascio et al. Ultrasound Obstet Gynecol 2020 Nov;56(5):647-55.
5. Ferrara L et al. Am J Obstet Gynecol. 2008 Oct;199(4):408.e1-4.
Multifetal pregnancy reduction (MPR) was developed in the 1980s in the wake of significant increases in the incidence of triplets and other higher-order multiples emanating from assisted reproductive technologies (ART). It was offered to reduce fetal number and improve outcomes for remaining fetuses by reducing rates of preterm delivery, fetal growth restriction, and other adverse perinatal outcomes, as well as maternal complications such as preeclampsia and postpartum hemorrhage.
In recent years, improvements in ART – mainly changes in ovulation induction practices and limitations in the number of embryos implanted to two at most – have reversed the increase in higher-order multiples. However, with intrauterine insemination, higher-order multiples still occur, and even without any reproductive assistance, the reality is that multiple pregnancies – particularly twins – continue to exist. In 2018, twins comprised about 3% of births in the United States.1
Twin pregnancies have a significantly higher risk than singleton gestations of preterm birth, maternal complications, and neonatal morbidity and mortality. The pregnancies are complicated more often by preterm premature rupture of membranes, fetal growth restriction, and hypertensive disorders of pregnancy.
Monochorionic diamniotic twin pregnancies face additional, unique risks of twin-to-twin transfusion syndrome, twin reversed arterial perfusion sequence, and twin-anemia polycythemia sequence. These pregnancies account for about 20% of all twin gestations, and decades of experience with ART have shown us that monochorionic diamniotic gestations occur at a higher rate after in-vitro fertilization.
Although advances have improved the outcomes of multiple births, risks remain and elective MPR is still very relevant for twin gestations. Patients routinely receive counseling about the risks of twin gestations, but they often are not made aware of the option of elective fetal reduction.
We have offered elective reduction (of nonanomalous fetuses) to a singleton for almost 30 years and have published several reports documenting that MPR in dichorionic diamniotic pregnancies reduces the risk of preterm delivery and other complications without increasing the risk of pregnancy loss.
Most recently, we also published data comparing the outcomes of patients with monochorionic diamniotic gestations who underwent elective MPR by radiofrequency ablation (RFA) vs. those with ongoing monochorionic diamniotic gestations.2 While the numbers were small, the data show significantly lower rates of preterm birth without an increased risk of pregnancy loss.
Experience with dichorionic diamniotic twins, genetic testing
Our most recent review3 of outcomes in dichorionic diamniotic gestations covered 855 patients, 29% of whom underwent planned elective MPR at less than 15 weeks, and 71% of whom had ongoing twin gestations. Those with ongoing twin gestations had adjusted odds ratios of preterm delivery at less than 37 weeks and less than 34 weeks of 5.62 and 2.22, respectively (adjustments controlled for maternal characteristics such as maternal age, BMI, use of chorionic villus sampling [CVS], and history of preterm birth).
Ongoing twin pregnancies were also more likely to have preeclampsia (AOR, 3.33), preterm premature rupture of membranes (3.86), and low birthweight (under the 5th and 10th percentiles). There were no significant differences in the rate of unintended pregnancy loss (2.4% vs. 2.3%), and rates for total pregnancy loss at less than 24 weeks and less than 20 weeks were similar.
An important issue in the consideration of MPR is that prenatal diagnosis of chromosomal abnormalities is very safe in twins. Multiple gestations are at greater risk of chromosomal abnormalities, so performing MPR selectively – if a chromosomally abnormal fetus is present – is desirable for many parents.
A recent meta-analysis and systematic review of studies reporting fetal loss following amniocentesis or CVS in twin pregnancies found an exceedingly low risk of loss. Procedure-related fetal loss (the primary outcome) was lower than previously reported, and the rate of fetal loss before 24 weeks gestation or within 4 weeks after the procedure (secondary outcomes), did not differ from the background risk in twin pregnancies not undergoing invasive prenatal testing.4
Our data have shown no significant differences in pregnancy loss between patients who underwent CVS prior to MPR and those who did not. Looking specifically at reduction to a singleton gestation, patients who underwent CVS prior to MPR had a fourfold reduction in loss.5 Therefore, we counsel patients that CVS provides useful information – especially now with the common use of chromosomal microarray – at almost negligible risk.
MPR for monochorionic diamniotic twins
Most of the literature on MPR from twin to singleton gestations reports on intrathoracic potassium chloride injection used in dichorionic diamniotic twins.
MPR in monochorionic diamniotic twins is reserved in the United States for monochorionic pregnancies in which there are severe fetal anomalies, severe growth restriction, or other significant complications. It is performed in such cases around 20 weeks gestation. However, given the significant risks of monochorionic twin pregnancies, we also have been offering MPR electively and earlier in pregnancy. While many modalities of intrafetal cord occlusion exist, RFA at the cord insertion site into the fetal abdomen is our preferred technique.
In our retrospective review of 315 monochorionic diamniotic twin gestations, the 14 patients who had RFA electively had no pregnancy losses and a significantly lower rate of preterm birth at less than 37-weeks gestation, compared with 301 ongoing monochorionic diamniotic twin pregnancies (29% vs. 76%).5 Reduction with RFA, performed at a mean gestational age of 15 weeks, also eliminated the risks unique to monochorionic twins, such as twin-to-twin transfusion syndrome and twin-anemia polycythemia sequence. (Of the ongoing twin gestations, 12% required medically indicated RFA, fetoscopic laser ablation, and/or amnioreduction; 4% had unintended loss of one fetus; and 4% had unintended loss of both fetuses before 24 weeks’ gestation. Fewer than 70% of the ongoing twin gestations had none of the significant adverse outcomes unique to monochorionic twins.)
Interestingly, there were still a couple of cases of fetal growth restriction in patients who underwent elective MPR – a rate higher than that seen in singleton gestations – most likely because of the early timing of the procedure.
Our numbers of MPRs in this review were small, but the data offer at least preliminary evidence that planned elective RFA before 17 weeks gestation may be offered to patients who do not want to assume the risks of monochorionic diamniotic twin pregnancies.
Counseling in twin pregnancies
We perform thorough, early assessments of fetal anatomy in our twin pregnancies, and we undertake thorough medical and obstetrical histories to uncover birth complications or medical conditions that would increase risks of preeclampsia, preterm birth, fetal growth restriction, and other complications.
Because monochorionic gestations are at particularly high risk for heart defects, we also routinely perform fetal echocardiography in these pregnancies.
Genetic testing is offered to all twin pregnancies, and as mentioned above, we especially counsel those considering MPR that such testing provides useful information.
Patients are made aware of the option of MPR and receive nondirective counseling. It is the patient’s choice. We recognize that elective termination is a controversial procedure, but we believe that the option of MPR should be available to patients who want to improve outcomes for their pregnancy.
When anomalies are discovered and selective termination is chosen, we usually try to perform MPR as early as possible. After 16 weeks, we’ve found, the rate of pregnancy loss increases slightly.
Dr. Stone is the Ellen and Howard C. Katz Chairman’s Chair, and Dr. DeBolt is a clinical fellow in maternal-fetal medicine, in the Raquel and Jaime Gilinski Department of Obstetrics, Gynecology, and Reproductive Science at the Icahn School of Medicine at Mount Sinai, New York.
References
1. Martin JA and Osterman MJK. National Center of Health Statistics. NCHS Data Brief, 2019;no 351.
2. Manasa GR et al. Am J Obstet Gynecol MFM 2021;3:100447.
3. Vieira LA et al. Am J. Obstet Gynecol. 2019;221:253.e1-8.
4. Di Mascio et al. Ultrasound Obstet Gynecol 2020 Nov;56(5):647-55.
5. Ferrara L et al. Am J Obstet Gynecol. 2008 Oct;199(4):408.e1-4.
Multifetal pregnancy reduction (MPR) was developed in the 1980s in the wake of significant increases in the incidence of triplets and other higher-order multiples emanating from assisted reproductive technologies (ART). It was offered to reduce fetal number and improve outcomes for remaining fetuses by reducing rates of preterm delivery, fetal growth restriction, and other adverse perinatal outcomes, as well as maternal complications such as preeclampsia and postpartum hemorrhage.
In recent years, improvements in ART – mainly changes in ovulation induction practices and limitations in the number of embryos implanted to two at most – have reversed the increase in higher-order multiples. However, with intrauterine insemination, higher-order multiples still occur, and even without any reproductive assistance, the reality is that multiple pregnancies – particularly twins – continue to exist. In 2018, twins comprised about 3% of births in the United States.1
Twin pregnancies have a significantly higher risk than singleton gestations of preterm birth, maternal complications, and neonatal morbidity and mortality. The pregnancies are complicated more often by preterm premature rupture of membranes, fetal growth restriction, and hypertensive disorders of pregnancy.
Monochorionic diamniotic twin pregnancies face additional, unique risks of twin-to-twin transfusion syndrome, twin reversed arterial perfusion sequence, and twin-anemia polycythemia sequence. These pregnancies account for about 20% of all twin gestations, and decades of experience with ART have shown us that monochorionic diamniotic gestations occur at a higher rate after in-vitro fertilization.
Although advances have improved the outcomes of multiple births, risks remain and elective MPR is still very relevant for twin gestations. Patients routinely receive counseling about the risks of twin gestations, but they often are not made aware of the option of elective fetal reduction.
We have offered elective reduction (of nonanomalous fetuses) to a singleton for almost 30 years and have published several reports documenting that MPR in dichorionic diamniotic pregnancies reduces the risk of preterm delivery and other complications without increasing the risk of pregnancy loss.
Most recently, we also published data comparing the outcomes of patients with monochorionic diamniotic gestations who underwent elective MPR by radiofrequency ablation (RFA) vs. those with ongoing monochorionic diamniotic gestations.2 While the numbers were small, the data show significantly lower rates of preterm birth without an increased risk of pregnancy loss.
Experience with dichorionic diamniotic twins, genetic testing
Our most recent review3 of outcomes in dichorionic diamniotic gestations covered 855 patients, 29% of whom underwent planned elective MPR at less than 15 weeks, and 71% of whom had ongoing twin gestations. Those with ongoing twin gestations had adjusted odds ratios of preterm delivery at less than 37 weeks and less than 34 weeks of 5.62 and 2.22, respectively (adjustments controlled for maternal characteristics such as maternal age, BMI, use of chorionic villus sampling [CVS], and history of preterm birth).
Ongoing twin pregnancies were also more likely to have preeclampsia (AOR, 3.33), preterm premature rupture of membranes (3.86), and low birthweight (under the 5th and 10th percentiles). There were no significant differences in the rate of unintended pregnancy loss (2.4% vs. 2.3%), and rates for total pregnancy loss at less than 24 weeks and less than 20 weeks were similar.
An important issue in the consideration of MPR is that prenatal diagnosis of chromosomal abnormalities is very safe in twins. Multiple gestations are at greater risk of chromosomal abnormalities, so performing MPR selectively – if a chromosomally abnormal fetus is present – is desirable for many parents.
A recent meta-analysis and systematic review of studies reporting fetal loss following amniocentesis or CVS in twin pregnancies found an exceedingly low risk of loss. Procedure-related fetal loss (the primary outcome) was lower than previously reported, and the rate of fetal loss before 24 weeks gestation or within 4 weeks after the procedure (secondary outcomes), did not differ from the background risk in twin pregnancies not undergoing invasive prenatal testing.4
Our data have shown no significant differences in pregnancy loss between patients who underwent CVS prior to MPR and those who did not. Looking specifically at reduction to a singleton gestation, patients who underwent CVS prior to MPR had a fourfold reduction in loss.5 Therefore, we counsel patients that CVS provides useful information – especially now with the common use of chromosomal microarray – at almost negligible risk.
MPR for monochorionic diamniotic twins
Most of the literature on MPR from twin to singleton gestations reports on intrathoracic potassium chloride injection used in dichorionic diamniotic twins.
MPR in monochorionic diamniotic twins is reserved in the United States for monochorionic pregnancies in which there are severe fetal anomalies, severe growth restriction, or other significant complications. It is performed in such cases around 20 weeks gestation. However, given the significant risks of monochorionic twin pregnancies, we also have been offering MPR electively and earlier in pregnancy. While many modalities of intrafetal cord occlusion exist, RFA at the cord insertion site into the fetal abdomen is our preferred technique.
In our retrospective review of 315 monochorionic diamniotic twin gestations, the 14 patients who had RFA electively had no pregnancy losses and a significantly lower rate of preterm birth at less than 37-weeks gestation, compared with 301 ongoing monochorionic diamniotic twin pregnancies (29% vs. 76%).5 Reduction with RFA, performed at a mean gestational age of 15 weeks, also eliminated the risks unique to monochorionic twins, such as twin-to-twin transfusion syndrome and twin-anemia polycythemia sequence. (Of the ongoing twin gestations, 12% required medically indicated RFA, fetoscopic laser ablation, and/or amnioreduction; 4% had unintended loss of one fetus; and 4% had unintended loss of both fetuses before 24 weeks’ gestation. Fewer than 70% of the ongoing twin gestations had none of the significant adverse outcomes unique to monochorionic twins.)
Interestingly, there were still a couple of cases of fetal growth restriction in patients who underwent elective MPR – a rate higher than that seen in singleton gestations – most likely because of the early timing of the procedure.
Our numbers of MPRs in this review were small, but the data offer at least preliminary evidence that planned elective RFA before 17 weeks gestation may be offered to patients who do not want to assume the risks of monochorionic diamniotic twin pregnancies.
Counseling in twin pregnancies
We perform thorough, early assessments of fetal anatomy in our twin pregnancies, and we undertake thorough medical and obstetrical histories to uncover birth complications or medical conditions that would increase risks of preeclampsia, preterm birth, fetal growth restriction, and other complications.
Because monochorionic gestations are at particularly high risk for heart defects, we also routinely perform fetal echocardiography in these pregnancies.
Genetic testing is offered to all twin pregnancies, and as mentioned above, we especially counsel those considering MPR that such testing provides useful information.
Patients are made aware of the option of MPR and receive nondirective counseling. It is the patient’s choice. We recognize that elective termination is a controversial procedure, but we believe that the option of MPR should be available to patients who want to improve outcomes for their pregnancy.
When anomalies are discovered and selective termination is chosen, we usually try to perform MPR as early as possible. After 16 weeks, we’ve found, the rate of pregnancy loss increases slightly.
Dr. Stone is the Ellen and Howard C. Katz Chairman’s Chair, and Dr. DeBolt is a clinical fellow in maternal-fetal medicine, in the Raquel and Jaime Gilinski Department of Obstetrics, Gynecology, and Reproductive Science at the Icahn School of Medicine at Mount Sinai, New York.
References
1. Martin JA and Osterman MJK. National Center of Health Statistics. NCHS Data Brief, 2019;no 351.
2. Manasa GR et al. Am J Obstet Gynecol MFM 2021;3:100447.
3. Vieira LA et al. Am J. Obstet Gynecol. 2019;221:253.e1-8.
4. Di Mascio et al. Ultrasound Obstet Gynecol 2020 Nov;56(5):647-55.
5. Ferrara L et al. Am J Obstet Gynecol. 2008 Oct;199(4):408.e1-4.
Managing maternal mortality with multifetal pregnancy reduction
For over 2 years, the world has reeled from the COVID-19 pandemic. Life has changed dramatically, priorities have been re-examined, and the collective approach to health care has shifted tremendously. While concerns regarding coronavirus and its variants are warranted, another “pandemic” is ravaging the world and has yet to be fully addressed: pregnancy-related maternal mortality.
The rate of pregnancy-related deaths in the United States is unconscionable. Compared with other developed nations – such as Germany, the United Kingdom, and Canada – we lag far behind. Data published in 2020 showed that the rate of maternal deaths per 100,000 live births in the United States was 17.4, more than double that of France (8.7 deaths per 100,000 live births),1 the country with the next-highest rate. Americans like being first – first to invent the light bulb, first to perform a successful solid organ xenotransplantation, first to go to the moon – but holding “first place” in maternal mortality is not something we should wish to maintain.
Ob.gyns. have long raised the alarm regarding the exceedingly high rates of pregnancy-related deaths in the United States. While there have been many advances in antenatal care to reduce these severe adverse events – improvements in surveillance and data reporting, maternal-focused telemedicine services, multidisciplinary care team models, and numerous research initiatives by federal and nonprofit organizations2 – the recent wave of legislation restricting reproductive choice may also have the unintended consequence of further increasing the rate of pregnancy-related maternal morbidity and mortality.3
While we have an obligation to provide our maternal and fetal patients with the best possible care, under some circumstances, that care may require prioritizing the mother’s health above all else.
To discuss the judicious use of multifetal pregnancy reduction, we have invited Dr. Joanne Stone, The Ellen and Howard C. Katz Chairman’s Chair, and Dr. Chelsea DeBolt, clinical fellow in maternal-fetal medicine, both in the Raquel and Jaime Gilinski Department of Obstetrics, Gynecology, and Reproductive Science at the Icahn School of Medicine at Mount Sinai.
Dr. Reece, who specializes in maternal-fetal medicine, is executive vice president for medical affairs at the University of Maryland, Baltimore, as well as the John Z. and Akiko K. Bowers Distinguished Professor and dean of the school of medicine. He is the medical editor of this column. He said he had no relevant financial disclosures. Contact him at obnews@mdedge.com.
References
1. Tikkanen R et al. The Commonwealth Fund. Nov 2020. doi: 10.26099/411v-9255
2. Ahn R et al. Ann Intern Med. 2020;173(11 Suppl):S3-10. doi: 10.7326/M19-3258.
3. Pabayo R et al. Int J Environ Res Public Health. 2020;17(11):3773. doi: 10.3390/ijerph17113773.
For over 2 years, the world has reeled from the COVID-19 pandemic. Life has changed dramatically, priorities have been re-examined, and the collective approach to health care has shifted tremendously. While concerns regarding coronavirus and its variants are warranted, another “pandemic” is ravaging the world and has yet to be fully addressed: pregnancy-related maternal mortality.
The rate of pregnancy-related deaths in the United States is unconscionable. Compared with other developed nations – such as Germany, the United Kingdom, and Canada – we lag far behind. Data published in 2020 showed that the rate of maternal deaths per 100,000 live births in the United States was 17.4, more than double that of France (8.7 deaths per 100,000 live births),1 the country with the next-highest rate. Americans like being first – first to invent the light bulb, first to perform a successful solid organ xenotransplantation, first to go to the moon – but holding “first place” in maternal mortality is not something we should wish to maintain.
Ob.gyns. have long raised the alarm regarding the exceedingly high rates of pregnancy-related deaths in the United States. While there have been many advances in antenatal care to reduce these severe adverse events – improvements in surveillance and data reporting, maternal-focused telemedicine services, multidisciplinary care team models, and numerous research initiatives by federal and nonprofit organizations2 – the recent wave of legislation restricting reproductive choice may also have the unintended consequence of further increasing the rate of pregnancy-related maternal morbidity and mortality.3
While we have an obligation to provide our maternal and fetal patients with the best possible care, under some circumstances, that care may require prioritizing the mother’s health above all else.
To discuss the judicious use of multifetal pregnancy reduction, we have invited Dr. Joanne Stone, The Ellen and Howard C. Katz Chairman’s Chair, and Dr. Chelsea DeBolt, clinical fellow in maternal-fetal medicine, both in the Raquel and Jaime Gilinski Department of Obstetrics, Gynecology, and Reproductive Science at the Icahn School of Medicine at Mount Sinai.
Dr. Reece, who specializes in maternal-fetal medicine, is executive vice president for medical affairs at the University of Maryland, Baltimore, as well as the John Z. and Akiko K. Bowers Distinguished Professor and dean of the school of medicine. He is the medical editor of this column. He said he had no relevant financial disclosures. Contact him at obnews@mdedge.com.
References
1. Tikkanen R et al. The Commonwealth Fund. Nov 2020. doi: 10.26099/411v-9255
2. Ahn R et al. Ann Intern Med. 2020;173(11 Suppl):S3-10. doi: 10.7326/M19-3258.
3. Pabayo R et al. Int J Environ Res Public Health. 2020;17(11):3773. doi: 10.3390/ijerph17113773.
For over 2 years, the world has reeled from the COVID-19 pandemic. Life has changed dramatically, priorities have been re-examined, and the collective approach to health care has shifted tremendously. While concerns regarding coronavirus and its variants are warranted, another “pandemic” is ravaging the world and has yet to be fully addressed: pregnancy-related maternal mortality.
The rate of pregnancy-related deaths in the United States is unconscionable. Compared with other developed nations – such as Germany, the United Kingdom, and Canada – we lag far behind. Data published in 2020 showed that the rate of maternal deaths per 100,000 live births in the United States was 17.4, more than double that of France (8.7 deaths per 100,000 live births),1 the country with the next-highest rate. Americans like being first – first to invent the light bulb, first to perform a successful solid organ xenotransplantation, first to go to the moon – but holding “first place” in maternal mortality is not something we should wish to maintain.
Ob.gyns. have long raised the alarm regarding the exceedingly high rates of pregnancy-related deaths in the United States. While there have been many advances in antenatal care to reduce these severe adverse events – improvements in surveillance and data reporting, maternal-focused telemedicine services, multidisciplinary care team models, and numerous research initiatives by federal and nonprofit organizations2 – the recent wave of legislation restricting reproductive choice may also have the unintended consequence of further increasing the rate of pregnancy-related maternal morbidity and mortality.3
While we have an obligation to provide our maternal and fetal patients with the best possible care, under some circumstances, that care may require prioritizing the mother’s health above all else.
To discuss the judicious use of multifetal pregnancy reduction, we have invited Dr. Joanne Stone, The Ellen and Howard C. Katz Chairman’s Chair, and Dr. Chelsea DeBolt, clinical fellow in maternal-fetal medicine, both in the Raquel and Jaime Gilinski Department of Obstetrics, Gynecology, and Reproductive Science at the Icahn School of Medicine at Mount Sinai.
Dr. Reece, who specializes in maternal-fetal medicine, is executive vice president for medical affairs at the University of Maryland, Baltimore, as well as the John Z. and Akiko K. Bowers Distinguished Professor and dean of the school of medicine. He is the medical editor of this column. He said he had no relevant financial disclosures. Contact him at obnews@mdedge.com.
References
1. Tikkanen R et al. The Commonwealth Fund. Nov 2020. doi: 10.26099/411v-9255
2. Ahn R et al. Ann Intern Med. 2020;173(11 Suppl):S3-10. doi: 10.7326/M19-3258.
3. Pabayo R et al. Int J Environ Res Public Health. 2020;17(11):3773. doi: 10.3390/ijerph17113773.
New combination med for severe mental illness tied to less weight gain
, new research suggests. However, at least one expert says the weight difference between the two drugs is of “questionable clinical benefit.”
Last year, the Food and Drug Administration approved the drug for the treatment of adults with schizophrenia or bipolar I disorder, as a maintenance monotherapy or as either monotherapy or an adjunct to lithium or valproate for acute manic or mixed episodes.
In the ENLIGHTEN-Early trial, researchers examined weight-gain profiles of more than 400 patients with early schizophrenia, schizophreniform disorder, or bipolar I disorder.
Results showed those given combination treatment gained just over half the amount of weight as those given monotherapy. They were also 36% less likely to gain at least 10% of their body weight during the 12-week treatment period.
They indicate that the weight-mitigating effects shown with olanzapine plus samidorphan are “consistent, regardless of the stage of illness,” Dr. Kahn added.
He presented the findings at the annual congress of the Schizophrenia International Research Society.
Potential benefit
“Early intervention with antipsychotic treatment is critical in shaping the course of treatment and the disease trajectory,” coinvestigator Christine Graham, PhD, with Alkermes, which manufactures the drug, told this news organization.
Olanzapine is a “highly effective antipsychotic, but it’s really avoided a lot in this population,” Dr. Graham said. Therefore, patients “could really stand to benefit” from a combination that delivers the same amount of antipsychotic effect, but “reduces the propensity” for clinically significant weight gain, she added.
Dr. Kahn noted in his meeting presentation that antipsychotics are the “cornerstone” of the treatment of serious mental illness, but that “many are associated with concerning weight gain and cardiometabolic effects.”
While olanzapine is an effective medication, it has “one of the highest weight gain” profiles of the available antipsychotics and patients early on in their illness are “especially vulnerable,” Dr. Kahn said.
Previous studies have shown the combination of olanzapine plus samidorphan is similarly effective as olanzapine, but is associated with less weight gain.
To determine its impact in recent-onset illness, the current researchers screened patients with schizophrenia, schizophreniform disorder, or bipolar I disorder. The patients were aged 16-39 years and had an initial onset of active phase symptoms less than 4 years previously. They had less than 24 weeks’ cumulative lifetime exposure to antipsychotics.
Participants were randomly assigned to receive olanzapine plus samidorphan or olanzapine alone for 12 weeks, and then followed up for safety assessment for a further 4 weeks.
A total of 426 patients were recruited and 76.5% completed the study. The mean age was 25.8 years, 66.2% were men, 66.4% were White, and 28.2% were Black.
The mean body mass index at baseline was 23.69 kg/m2. The most common diagnosis among the participants was schizophrenia (62.9%) followed by bipolar I disorder (21.6%).
Less weight gain
Results of the 12-week study showed a significant difference in percent change in body weight from baseline between the two treatment groups, with a gain of 4.91% for the olanzapine plus samidorphan group vs. 6.77% for the olanzapine-alone group (between-group difference, 1.87%; P = .012).
Dr. Kahn noted this equates to an average weight gain of 2.8 kg (6.2 pounds) with olanzapine plus samidorphan and a gain of about 5 kg (11pounds) with olanzapine.
“It’s not a huge difference, but it’s certainly a significant one,” he said. “I also think it’s clinically important and significant.”
The reduction in weight gain compared with olanzapine was even maintained in patients assigned to olanzapine plus samidorphan who dropped out and did not complete the study, Dr. Kahn reported. “No one really had a weight gain,” he said.
In contrast, patients in the olanzapine groups who dropped out of the study had weight gain larger than their counterparts who stayed in it.
Further analysis showed the proportion of patients who gained 10% or more of their body weight by week 12 was 21.9% for those receiving olanzapine plus samidorphan vs. 30.4% for those receiving just olanzapine (odds ratio, 0.64; P = .075).
As expected, the improvement in Clinical Global Impression–Severity scale scores was almost identical between the olanzapine + samidorphan and olanzapine-only groups.
For safety, Dr. Kahn said the adverse event rates were “very, very similar” between the two treatment arms, which was a pattern that was repeated for serious AEs. This led him to note that “nothing out of the ordinary” was observed.
Clinical impact 'questionable'
Commenting on the study, Laura LaChance, MD, a psychiatrist at St. Mary’s Hospital Centre, McGill University, Montreal, said the actual amount of weight loss shown in the study “is of questionable clinical significance.”
On the other hand, Dr. LaChance said she has achieved “better results with metformin, which has a great safety profile and is cheap and widely available.
“Cost is always a concern in patients with psychotic disorders,” she concluded.
The study was funded by Alkermes. Dr. Kahn reported having relationships with Alkermes, Angelini, Janssen, Sunovion, Otsuka, Merck, Minerva Neuroscience, Roche, and Teva. Dr. Graham is an employee of Alkermes.
A version of this article first appeared on Medscape.com.
, new research suggests. However, at least one expert says the weight difference between the two drugs is of “questionable clinical benefit.”
Last year, the Food and Drug Administration approved the drug for the treatment of adults with schizophrenia or bipolar I disorder, as a maintenance monotherapy or as either monotherapy or an adjunct to lithium or valproate for acute manic or mixed episodes.
In the ENLIGHTEN-Early trial, researchers examined weight-gain profiles of more than 400 patients with early schizophrenia, schizophreniform disorder, or bipolar I disorder.
Results showed those given combination treatment gained just over half the amount of weight as those given monotherapy. They were also 36% less likely to gain at least 10% of their body weight during the 12-week treatment period.
They indicate that the weight-mitigating effects shown with olanzapine plus samidorphan are “consistent, regardless of the stage of illness,” Dr. Kahn added.
He presented the findings at the annual congress of the Schizophrenia International Research Society.
Potential benefit
“Early intervention with antipsychotic treatment is critical in shaping the course of treatment and the disease trajectory,” coinvestigator Christine Graham, PhD, with Alkermes, which manufactures the drug, told this news organization.
Olanzapine is a “highly effective antipsychotic, but it’s really avoided a lot in this population,” Dr. Graham said. Therefore, patients “could really stand to benefit” from a combination that delivers the same amount of antipsychotic effect, but “reduces the propensity” for clinically significant weight gain, she added.
Dr. Kahn noted in his meeting presentation that antipsychotics are the “cornerstone” of the treatment of serious mental illness, but that “many are associated with concerning weight gain and cardiometabolic effects.”
While olanzapine is an effective medication, it has “one of the highest weight gain” profiles of the available antipsychotics and patients early on in their illness are “especially vulnerable,” Dr. Kahn said.
Previous studies have shown the combination of olanzapine plus samidorphan is similarly effective as olanzapine, but is associated with less weight gain.
To determine its impact in recent-onset illness, the current researchers screened patients with schizophrenia, schizophreniform disorder, or bipolar I disorder. The patients were aged 16-39 years and had an initial onset of active phase symptoms less than 4 years previously. They had less than 24 weeks’ cumulative lifetime exposure to antipsychotics.
Participants were randomly assigned to receive olanzapine plus samidorphan or olanzapine alone for 12 weeks, and then followed up for safety assessment for a further 4 weeks.
A total of 426 patients were recruited and 76.5% completed the study. The mean age was 25.8 years, 66.2% were men, 66.4% were White, and 28.2% were Black.
The mean body mass index at baseline was 23.69 kg/m2. The most common diagnosis among the participants was schizophrenia (62.9%) followed by bipolar I disorder (21.6%).
Less weight gain
Results of the 12-week study showed a significant difference in percent change in body weight from baseline between the two treatment groups, with a gain of 4.91% for the olanzapine plus samidorphan group vs. 6.77% for the olanzapine-alone group (between-group difference, 1.87%; P = .012).
Dr. Kahn noted this equates to an average weight gain of 2.8 kg (6.2 pounds) with olanzapine plus samidorphan and a gain of about 5 kg (11pounds) with olanzapine.
“It’s not a huge difference, but it’s certainly a significant one,” he said. “I also think it’s clinically important and significant.”
The reduction in weight gain compared with olanzapine was even maintained in patients assigned to olanzapine plus samidorphan who dropped out and did not complete the study, Dr. Kahn reported. “No one really had a weight gain,” he said.
In contrast, patients in the olanzapine groups who dropped out of the study had weight gain larger than their counterparts who stayed in it.
Further analysis showed the proportion of patients who gained 10% or more of their body weight by week 12 was 21.9% for those receiving olanzapine plus samidorphan vs. 30.4% for those receiving just olanzapine (odds ratio, 0.64; P = .075).
As expected, the improvement in Clinical Global Impression–Severity scale scores was almost identical between the olanzapine + samidorphan and olanzapine-only groups.
For safety, Dr. Kahn said the adverse event rates were “very, very similar” between the two treatment arms, which was a pattern that was repeated for serious AEs. This led him to note that “nothing out of the ordinary” was observed.
Clinical impact 'questionable'
Commenting on the study, Laura LaChance, MD, a psychiatrist at St. Mary’s Hospital Centre, McGill University, Montreal, said the actual amount of weight loss shown in the study “is of questionable clinical significance.”
On the other hand, Dr. LaChance said she has achieved “better results with metformin, which has a great safety profile and is cheap and widely available.
“Cost is always a concern in patients with psychotic disorders,” she concluded.
The study was funded by Alkermes. Dr. Kahn reported having relationships with Alkermes, Angelini, Janssen, Sunovion, Otsuka, Merck, Minerva Neuroscience, Roche, and Teva. Dr. Graham is an employee of Alkermes.
A version of this article first appeared on Medscape.com.
, new research suggests. However, at least one expert says the weight difference between the two drugs is of “questionable clinical benefit.”
Last year, the Food and Drug Administration approved the drug for the treatment of adults with schizophrenia or bipolar I disorder, as a maintenance monotherapy or as either monotherapy or an adjunct to lithium or valproate for acute manic or mixed episodes.
In the ENLIGHTEN-Early trial, researchers examined weight-gain profiles of more than 400 patients with early schizophrenia, schizophreniform disorder, or bipolar I disorder.
Results showed those given combination treatment gained just over half the amount of weight as those given monotherapy. They were also 36% less likely to gain at least 10% of their body weight during the 12-week treatment period.
They indicate that the weight-mitigating effects shown with olanzapine plus samidorphan are “consistent, regardless of the stage of illness,” Dr. Kahn added.
He presented the findings at the annual congress of the Schizophrenia International Research Society.
Potential benefit
“Early intervention with antipsychotic treatment is critical in shaping the course of treatment and the disease trajectory,” coinvestigator Christine Graham, PhD, with Alkermes, which manufactures the drug, told this news organization.
Olanzapine is a “highly effective antipsychotic, but it’s really avoided a lot in this population,” Dr. Graham said. Therefore, patients “could really stand to benefit” from a combination that delivers the same amount of antipsychotic effect, but “reduces the propensity” for clinically significant weight gain, she added.
Dr. Kahn noted in his meeting presentation that antipsychotics are the “cornerstone” of the treatment of serious mental illness, but that “many are associated with concerning weight gain and cardiometabolic effects.”
While olanzapine is an effective medication, it has “one of the highest weight gain” profiles of the available antipsychotics and patients early on in their illness are “especially vulnerable,” Dr. Kahn said.
Previous studies have shown the combination of olanzapine plus samidorphan is similarly effective as olanzapine, but is associated with less weight gain.
To determine its impact in recent-onset illness, the current researchers screened patients with schizophrenia, schizophreniform disorder, or bipolar I disorder. The patients were aged 16-39 years and had an initial onset of active phase symptoms less than 4 years previously. They had less than 24 weeks’ cumulative lifetime exposure to antipsychotics.
Participants were randomly assigned to receive olanzapine plus samidorphan or olanzapine alone for 12 weeks, and then followed up for safety assessment for a further 4 weeks.
A total of 426 patients were recruited and 76.5% completed the study. The mean age was 25.8 years, 66.2% were men, 66.4% were White, and 28.2% were Black.
The mean body mass index at baseline was 23.69 kg/m2. The most common diagnosis among the participants was schizophrenia (62.9%) followed by bipolar I disorder (21.6%).
Less weight gain
Results of the 12-week study showed a significant difference in percent change in body weight from baseline between the two treatment groups, with a gain of 4.91% for the olanzapine plus samidorphan group vs. 6.77% for the olanzapine-alone group (between-group difference, 1.87%; P = .012).
Dr. Kahn noted this equates to an average weight gain of 2.8 kg (6.2 pounds) with olanzapine plus samidorphan and a gain of about 5 kg (11pounds) with olanzapine.
“It’s not a huge difference, but it’s certainly a significant one,” he said. “I also think it’s clinically important and significant.”
The reduction in weight gain compared with olanzapine was even maintained in patients assigned to olanzapine plus samidorphan who dropped out and did not complete the study, Dr. Kahn reported. “No one really had a weight gain,” he said.
In contrast, patients in the olanzapine groups who dropped out of the study had weight gain larger than their counterparts who stayed in it.
Further analysis showed the proportion of patients who gained 10% or more of their body weight by week 12 was 21.9% for those receiving olanzapine plus samidorphan vs. 30.4% for those receiving just olanzapine (odds ratio, 0.64; P = .075).
As expected, the improvement in Clinical Global Impression–Severity scale scores was almost identical between the olanzapine + samidorphan and olanzapine-only groups.
For safety, Dr. Kahn said the adverse event rates were “very, very similar” between the two treatment arms, which was a pattern that was repeated for serious AEs. This led him to note that “nothing out of the ordinary” was observed.
Clinical impact 'questionable'
Commenting on the study, Laura LaChance, MD, a psychiatrist at St. Mary’s Hospital Centre, McGill University, Montreal, said the actual amount of weight loss shown in the study “is of questionable clinical significance.”
On the other hand, Dr. LaChance said she has achieved “better results with metformin, which has a great safety profile and is cheap and widely available.
“Cost is always a concern in patients with psychotic disorders,” she concluded.
The study was funded by Alkermes. Dr. Kahn reported having relationships with Alkermes, Angelini, Janssen, Sunovion, Otsuka, Merck, Minerva Neuroscience, Roche, and Teva. Dr. Graham is an employee of Alkermes.
A version of this article first appeared on Medscape.com.
FROM SIRS 2022
How effective are sterilization procedures? Study raises questions
Women opt for sterilization for a variety of reasons, but the goal is the same: to avoid getting pregnant.
But a head-to-head study of two forms of female sterilization has found surprisingly high rates of failure with the procedures.
The study compared the effectiveness of hysteroscopic sterilization, a nonincisional procedure, and minimally invasive laparoscopic sterilization. Although both methods prevented pregnancy in the vast majority of women, each was associated with more than a 6% failure rate 5 years after the procedure.
That figure is “much higher than expected,” said Aileen Gariepy, MD, MPH, the director of complex family planning at Weill Cornell Medicine, New York, who led the study.
The American College of Obstetricians and Gynecologists reported that the chance of pregnancy after sterilization is less than 1%, Dr. Gariepy said. “Women and pregnancy-capable people considering sterilization should be informed that, after the procedure, they have at least a 6% – not 1% – chance of pregnancy in the next 5 years.”
The study was published in Fertility and Sterility.
For laparoscopic sterilization, surgeons close or sever the fallopian tubes to prevent eggs from reaching the uterus and becoming fertilized.
Hysteroscopic sterilization involves the implantation of small, flexible metal coils into each fallopian tube, a process that produces inflammation and scarring that in turn prevents pregnancy. This method, called Essure and formerly marketed by Bayer, received approval by the Food and Drug Administration in 2002. But the agency received thousands of reports of adverse events with Essure, prompting regulators in 2016 to add a boxed warning to the product label about the risk for adverse events, including perforation, migration of the coils, allergic reactions, and pain.
Bayer pulled Essure from the market in 2019, citing decreased sales of the product. Some women did not have the device removed, however, and questions remain about its effectiveness, according to the researchers.
In the new study, Dr. Gariepy and colleagues examined Medicaid claims for 5906 hysteroscopic and 23,965 laparoscopic sterilizations performed in California between 2008 and 2014. They excluded sterilizations that were performed immediately after delivery, which involve a different approach.
The average age of the women in the study was 33 years.
The study found that, 5 years after the sterilization procedure, 6% of women in either group had become pregnant.
Despite the surprising new data, Chailee Moss, MD, an assistant professor of gynecology and obstetrics at Johns Hopkins University Medical Center, Baltimore, said she did not think the study would significantly affect the way she counsels her patients.
The main reason, she said, is that the study relied on an analysis of medical claims, which “is likely inferior to careful review of individual patient records or prospective collection of clinical data.” Home pregnancy tests may easily be excluded from such data and that patients can undergo ultrasound and termination procedures that would likely not be included in the data the researchers analyzed.
Dr. Moss added that the study was limited to California and that the researchers could not determine pregnancy rates for women who moved out of the state and thus received pregnancy care elsewhere. Nor did the authors account for the use of assistive reproductive technology, which can facilitate pregnancy after sterilization despite the success of the original procedure.
Dr. Gariepy, however, said the study may in fact have undercounted pregnancies and that the failure rates might be even higher than 6%, noting that California is “one of the largest, most populous and most diverse states” in terms of race, ethnicity, and other factors, making the new findings highly generalizable.
“I agree that study results should be confirmed by new nationwide study to determine risk of pregnancy after different sterilization methods,” she said. “Nevertheless, this retrospective cohort study delivers a strong signal that doctors and patients need to know about.”
Dr. Gariepy is on the board of directors of the Society of Family Planning. Dr. Moss has received research funding from Merck.
A version of this article first appeared on Medscape.com.
Women opt for sterilization for a variety of reasons, but the goal is the same: to avoid getting pregnant.
But a head-to-head study of two forms of female sterilization has found surprisingly high rates of failure with the procedures.
The study compared the effectiveness of hysteroscopic sterilization, a nonincisional procedure, and minimally invasive laparoscopic sterilization. Although both methods prevented pregnancy in the vast majority of women, each was associated with more than a 6% failure rate 5 years after the procedure.
That figure is “much higher than expected,” said Aileen Gariepy, MD, MPH, the director of complex family planning at Weill Cornell Medicine, New York, who led the study.
The American College of Obstetricians and Gynecologists reported that the chance of pregnancy after sterilization is less than 1%, Dr. Gariepy said. “Women and pregnancy-capable people considering sterilization should be informed that, after the procedure, they have at least a 6% – not 1% – chance of pregnancy in the next 5 years.”
The study was published in Fertility and Sterility.
For laparoscopic sterilization, surgeons close or sever the fallopian tubes to prevent eggs from reaching the uterus and becoming fertilized.
Hysteroscopic sterilization involves the implantation of small, flexible metal coils into each fallopian tube, a process that produces inflammation and scarring that in turn prevents pregnancy. This method, called Essure and formerly marketed by Bayer, received approval by the Food and Drug Administration in 2002. But the agency received thousands of reports of adverse events with Essure, prompting regulators in 2016 to add a boxed warning to the product label about the risk for adverse events, including perforation, migration of the coils, allergic reactions, and pain.
Bayer pulled Essure from the market in 2019, citing decreased sales of the product. Some women did not have the device removed, however, and questions remain about its effectiveness, according to the researchers.
In the new study, Dr. Gariepy and colleagues examined Medicaid claims for 5906 hysteroscopic and 23,965 laparoscopic sterilizations performed in California between 2008 and 2014. They excluded sterilizations that were performed immediately after delivery, which involve a different approach.
The average age of the women in the study was 33 years.
The study found that, 5 years after the sterilization procedure, 6% of women in either group had become pregnant.
Despite the surprising new data, Chailee Moss, MD, an assistant professor of gynecology and obstetrics at Johns Hopkins University Medical Center, Baltimore, said she did not think the study would significantly affect the way she counsels her patients.
The main reason, she said, is that the study relied on an analysis of medical claims, which “is likely inferior to careful review of individual patient records or prospective collection of clinical data.” Home pregnancy tests may easily be excluded from such data and that patients can undergo ultrasound and termination procedures that would likely not be included in the data the researchers analyzed.
Dr. Moss added that the study was limited to California and that the researchers could not determine pregnancy rates for women who moved out of the state and thus received pregnancy care elsewhere. Nor did the authors account for the use of assistive reproductive technology, which can facilitate pregnancy after sterilization despite the success of the original procedure.
Dr. Gariepy, however, said the study may in fact have undercounted pregnancies and that the failure rates might be even higher than 6%, noting that California is “one of the largest, most populous and most diverse states” in terms of race, ethnicity, and other factors, making the new findings highly generalizable.
“I agree that study results should be confirmed by new nationwide study to determine risk of pregnancy after different sterilization methods,” she said. “Nevertheless, this retrospective cohort study delivers a strong signal that doctors and patients need to know about.”
Dr. Gariepy is on the board of directors of the Society of Family Planning. Dr. Moss has received research funding from Merck.
A version of this article first appeared on Medscape.com.
Women opt for sterilization for a variety of reasons, but the goal is the same: to avoid getting pregnant.
But a head-to-head study of two forms of female sterilization has found surprisingly high rates of failure with the procedures.
The study compared the effectiveness of hysteroscopic sterilization, a nonincisional procedure, and minimally invasive laparoscopic sterilization. Although both methods prevented pregnancy in the vast majority of women, each was associated with more than a 6% failure rate 5 years after the procedure.
That figure is “much higher than expected,” said Aileen Gariepy, MD, MPH, the director of complex family planning at Weill Cornell Medicine, New York, who led the study.
The American College of Obstetricians and Gynecologists reported that the chance of pregnancy after sterilization is less than 1%, Dr. Gariepy said. “Women and pregnancy-capable people considering sterilization should be informed that, after the procedure, they have at least a 6% – not 1% – chance of pregnancy in the next 5 years.”
The study was published in Fertility and Sterility.
For laparoscopic sterilization, surgeons close or sever the fallopian tubes to prevent eggs from reaching the uterus and becoming fertilized.
Hysteroscopic sterilization involves the implantation of small, flexible metal coils into each fallopian tube, a process that produces inflammation and scarring that in turn prevents pregnancy. This method, called Essure and formerly marketed by Bayer, received approval by the Food and Drug Administration in 2002. But the agency received thousands of reports of adverse events with Essure, prompting regulators in 2016 to add a boxed warning to the product label about the risk for adverse events, including perforation, migration of the coils, allergic reactions, and pain.
Bayer pulled Essure from the market in 2019, citing decreased sales of the product. Some women did not have the device removed, however, and questions remain about its effectiveness, according to the researchers.
In the new study, Dr. Gariepy and colleagues examined Medicaid claims for 5906 hysteroscopic and 23,965 laparoscopic sterilizations performed in California between 2008 and 2014. They excluded sterilizations that were performed immediately after delivery, which involve a different approach.
The average age of the women in the study was 33 years.
The study found that, 5 years after the sterilization procedure, 6% of women in either group had become pregnant.
Despite the surprising new data, Chailee Moss, MD, an assistant professor of gynecology and obstetrics at Johns Hopkins University Medical Center, Baltimore, said she did not think the study would significantly affect the way she counsels her patients.
The main reason, she said, is that the study relied on an analysis of medical claims, which “is likely inferior to careful review of individual patient records or prospective collection of clinical data.” Home pregnancy tests may easily be excluded from such data and that patients can undergo ultrasound and termination procedures that would likely not be included in the data the researchers analyzed.
Dr. Moss added that the study was limited to California and that the researchers could not determine pregnancy rates for women who moved out of the state and thus received pregnancy care elsewhere. Nor did the authors account for the use of assistive reproductive technology, which can facilitate pregnancy after sterilization despite the success of the original procedure.
Dr. Gariepy, however, said the study may in fact have undercounted pregnancies and that the failure rates might be even higher than 6%, noting that California is “one of the largest, most populous and most diverse states” in terms of race, ethnicity, and other factors, making the new findings highly generalizable.
“I agree that study results should be confirmed by new nationwide study to determine risk of pregnancy after different sterilization methods,” she said. “Nevertheless, this retrospective cohort study delivers a strong signal that doctors and patients need to know about.”
Dr. Gariepy is on the board of directors of the Society of Family Planning. Dr. Moss has received research funding from Merck.
A version of this article first appeared on Medscape.com.
FROM FERTILITY AND STERILITY