User login
Can Iron Supplementation Protect Against Celiac Disease?
TOPLINE:
Genetically lower iron levels were associated with an increased risk for celiac disease, pointing to a potential opportunity to prevent the disease, new data suggested.
METHODOLOGY:
- To investigate, researchers conducted a Mendelian randomization study examining the relationship between single nucleotide polymorphisms (SNPs) associated with iron status and the presence of celiac disease.
- SNPs were drawn from a meta-analysis of three genome-wide association studies. Their association with celiac disease was assessed using data from 336,638 White UK Biobank participants, including 1855 with celiac disease.
TAKEAWAY:
- Four SNPs were strongly and independently associated with systemic iron status: rs1800562 and rs1799945 in the hemochromatosis gene, rs855791 in the transmembrane protease serine 6 gene, and rs57659670 predicted to affect the Dual Oxidase 2 gene. None were associated with known celiac disease risk factors.
- Higher iron status was negatively associated with celiac disease risk (odds ratio per 1 SD increase in serum iron: 0.65).
- No single SNP appeared to drive the association in sensitivity analyses.
- By relying on SNPs associated with iron status, and not on iron status itself, this Mendelian randomization analysis suggests a causal effect of iron deficiency on subsequent celiac disease development.
IN PRACTICE:
“These findings suggest that iron supplementation in select individuals may provide a potential protective effect against celiac disease development,” the authors wrote.
SOURCE:
The study, with first author Isabel A. Hujoel, MD, a gastroenterologist with University of Washington, Seattle, was published online on January 4, 2024, in BMJ Open Gastroenterology.
LIMITATIONS:
Researchers used a PheCode to identify celiac disease cases, which could lead to misclassification. Mendelian randomization provides some protection against biases, such as reverse causation, but is not completely invulnerable.
DISCLOSURES:
The study had no specific funding. The authors declared no conflicts of interest.
A version of this article appeared on Medscape.com.
TOPLINE:
Genetically lower iron levels were associated with an increased risk for celiac disease, pointing to a potential opportunity to prevent the disease, new data suggested.
METHODOLOGY:
- To investigate, researchers conducted a Mendelian randomization study examining the relationship between single nucleotide polymorphisms (SNPs) associated with iron status and the presence of celiac disease.
- SNPs were drawn from a meta-analysis of three genome-wide association studies. Their association with celiac disease was assessed using data from 336,638 White UK Biobank participants, including 1855 with celiac disease.
TAKEAWAY:
- Four SNPs were strongly and independently associated with systemic iron status: rs1800562 and rs1799945 in the hemochromatosis gene, rs855791 in the transmembrane protease serine 6 gene, and rs57659670 predicted to affect the Dual Oxidase 2 gene. None were associated with known celiac disease risk factors.
- Higher iron status was negatively associated with celiac disease risk (odds ratio per 1 SD increase in serum iron: 0.65).
- No single SNP appeared to drive the association in sensitivity analyses.
- By relying on SNPs associated with iron status, and not on iron status itself, this Mendelian randomization analysis suggests a causal effect of iron deficiency on subsequent celiac disease development.
IN PRACTICE:
“These findings suggest that iron supplementation in select individuals may provide a potential protective effect against celiac disease development,” the authors wrote.
SOURCE:
The study, with first author Isabel A. Hujoel, MD, a gastroenterologist with University of Washington, Seattle, was published online on January 4, 2024, in BMJ Open Gastroenterology.
LIMITATIONS:
Researchers used a PheCode to identify celiac disease cases, which could lead to misclassification. Mendelian randomization provides some protection against biases, such as reverse causation, but is not completely invulnerable.
DISCLOSURES:
The study had no specific funding. The authors declared no conflicts of interest.
A version of this article appeared on Medscape.com.
TOPLINE:
Genetically lower iron levels were associated with an increased risk for celiac disease, pointing to a potential opportunity to prevent the disease, new data suggested.
METHODOLOGY:
- To investigate, researchers conducted a Mendelian randomization study examining the relationship between single nucleotide polymorphisms (SNPs) associated with iron status and the presence of celiac disease.
- SNPs were drawn from a meta-analysis of three genome-wide association studies. Their association with celiac disease was assessed using data from 336,638 White UK Biobank participants, including 1855 with celiac disease.
TAKEAWAY:
- Four SNPs were strongly and independently associated with systemic iron status: rs1800562 and rs1799945 in the hemochromatosis gene, rs855791 in the transmembrane protease serine 6 gene, and rs57659670 predicted to affect the Dual Oxidase 2 gene. None were associated with known celiac disease risk factors.
- Higher iron status was negatively associated with celiac disease risk (odds ratio per 1 SD increase in serum iron: 0.65).
- No single SNP appeared to drive the association in sensitivity analyses.
- By relying on SNPs associated with iron status, and not on iron status itself, this Mendelian randomization analysis suggests a causal effect of iron deficiency on subsequent celiac disease development.
IN PRACTICE:
“These findings suggest that iron supplementation in select individuals may provide a potential protective effect against celiac disease development,” the authors wrote.
SOURCE:
The study, with first author Isabel A. Hujoel, MD, a gastroenterologist with University of Washington, Seattle, was published online on January 4, 2024, in BMJ Open Gastroenterology.
LIMITATIONS:
Researchers used a PheCode to identify celiac disease cases, which could lead to misclassification. Mendelian randomization provides some protection against biases, such as reverse causation, but is not completely invulnerable.
DISCLOSURES:
The study had no specific funding. The authors declared no conflicts of interest.
A version of this article appeared on Medscape.com.
What Do Results from Acoustic Subcision for Cellulite Look Like at One Year?
.
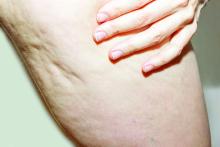
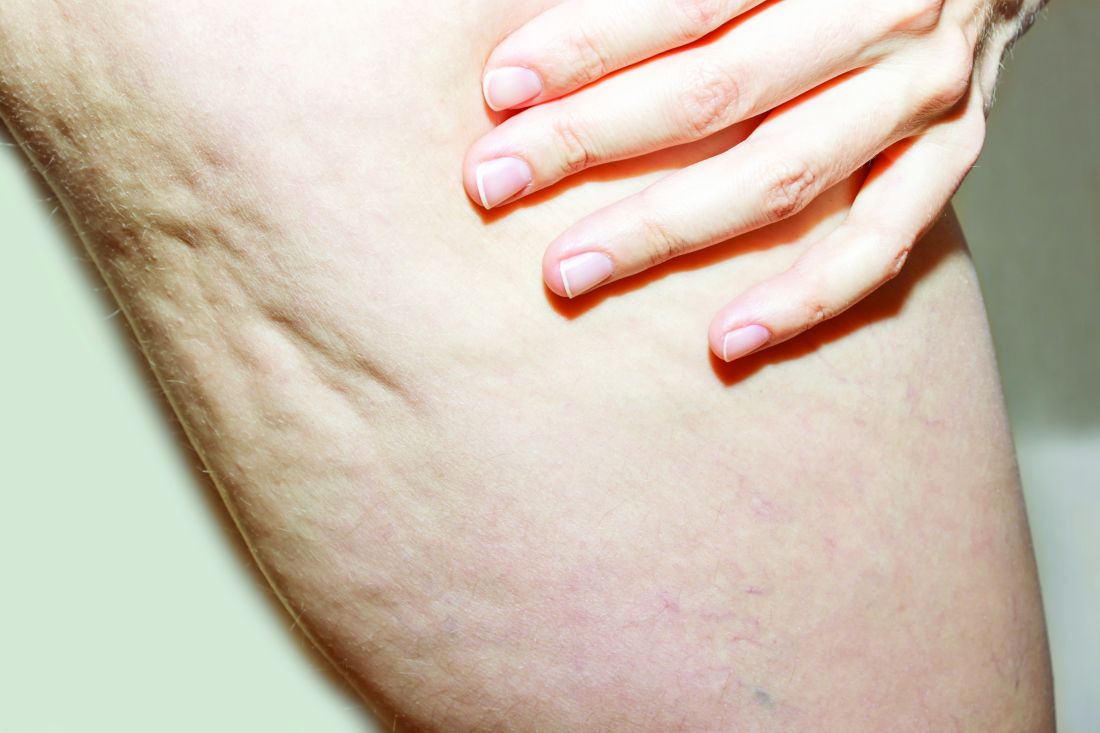
The findings build on results from a 12-week study of the device, marketed as Resonic. In that trial of 56 women with moderate to severe cellulite, a single treatment provided a roughly 1.01-point reduction in the five-point Cellulite Severity Scale (CSS) at 12 weeks, which corresponds to a roughly 29.5% reduction in cellulite from baseline.
The device, which is indicated for long-term improvement in the appearance of cellulite, emits rapid acoustic pulses and shock waves at 50 Hz that are transmitted through the skin. The device “induces physical shearing of fibrous septa through rapid acoustic pulses,” investigators led by Elizabeth Tanzi, MD, who practices cosmetic dermatology in Chevy Chase, Md., wrote in the follow-up study, which was published in Dermatologic Surgery in February “In contrast to current treatment options, the device requires no anesthesia or downtime and was well-tolerated based on an average pain score of 2.4 (on a scale 0–10) during treatment” in the 12-week study, they noted.
To evaluate the long-term efficacy of the acoustic subcision device, Dr. Tanzi and her coauthors at four centers prospectively followed 42 patients who participated in the 12-week trial. The study involved four visits: screening, a single treatment visit, and a follow-up visit 12 weeks after treatment and another after 52 weeks. Because of lockdowns and other reasons related to the COVID-19 pandemic, several participants were unable to make it to follow-up visits and had follow-up visits beyond the 52-week time-point, the authors explained.
Blinded board-certified dermatologists assessed efficacy by correctly identifying post-treatment photographs, from the visit after 52 weeks, and using a 6-point simplified CSS. They also assessed safety and collected data on participant satisfaction. The mean age of the women was 45.5 years, and their mean BMI was 23.9 kg/m2. The blinded reviewers correctly identified post-treatment photographs at the visit after 52 weeks at a rate of 95.2%.
In addition, 70.4% of the study participants had at least a 1-point change in their CSS score from baseline. Overall, their mean reduction in CSS score from baseline was 1.09 at the visit after 52 weeks, and a mean 34.1% reduction in cellulite at that visit, the authors reported.
In other findings, 41 of the 42 study participants (97.6%) rated their cellulite improvement as good and 33 (78.6%) agreed that the treatment was relatively pain free. Immediately following treatment, 85.7% reported an expected adverse event attributable to the device or treatment, which included mild to moderate erythema (76.7%), mild contusion/bruise (5.3%), mild pain (1.7%) and mild heat (1.7%). All adverse events resolved without intervention.
The study authors acknowledged certain limitations of the study, including the lack of a control group and the inability to differentiate effectiveness of the treatment on the buttocks versus the thighs.
“Cellulite is a common complaint among those presenting to cosmetic dermatology clinics, and prior treatment options have been somewhat disappointing in terms of invasiveness, side effects, or lack of improvement,” said Patricia M. Richey, MD, director of Mohs surgery at Boston Medical Center, who also conducts research for the Wellman Center for Photomedicine and the Dermatology Laser and Cosmetic Center at Massachusetts General Hospital, Boston.
Acoustic subcision “would potentially be a very attractive and unparalleled option given tolerability and sustained clinical improvement after only one treatment,” she told this news organization. “I agree with the authors that a possible limitation is the lack of comparison between response in different body areas,” namely, the buttocks versus the thighs, she said. “This information would be helpful to set patient expectations, and I suspect future studies will address this.”
Also asked to comment on the study, Pooja Sodha, MD, director of the Center for Laser and Cosmetic Dermatology at George Washington University, Washington, said in an interview that while the results were modest after a single treatment, “there is room for further experimentation to see how modifications of settings, treatment numbers, treatment intervals, and location-specific treatment regimens based on tissue depth and tissue band size/dimple size may enhance results.”
She added that cost of treatment and correlation with clinical improvement “will become a more real-world matter when it comes to bringing this more broadly to the clinic settings.”
Soliton sponsored the trial prior to its acquisition by AbbVie. Dr. Tanzi reported having no relevant financial disclosures. Four coauthors reported being employees, consultants, or advisory board members, or having stock options in AbbVie. Dr. Richey and Dr. Sodha were not involved with the study and reported having no disclosures.
.
The findings build on results from a 12-week study of the device, marketed as Resonic. In that trial of 56 women with moderate to severe cellulite, a single treatment provided a roughly 1.01-point reduction in the five-point Cellulite Severity Scale (CSS) at 12 weeks, which corresponds to a roughly 29.5% reduction in cellulite from baseline.
The device, which is indicated for long-term improvement in the appearance of cellulite, emits rapid acoustic pulses and shock waves at 50 Hz that are transmitted through the skin. The device “induces physical shearing of fibrous septa through rapid acoustic pulses,” investigators led by Elizabeth Tanzi, MD, who practices cosmetic dermatology in Chevy Chase, Md., wrote in the follow-up study, which was published in Dermatologic Surgery in February “In contrast to current treatment options, the device requires no anesthesia or downtime and was well-tolerated based on an average pain score of 2.4 (on a scale 0–10) during treatment” in the 12-week study, they noted.
To evaluate the long-term efficacy of the acoustic subcision device, Dr. Tanzi and her coauthors at four centers prospectively followed 42 patients who participated in the 12-week trial. The study involved four visits: screening, a single treatment visit, and a follow-up visit 12 weeks after treatment and another after 52 weeks. Because of lockdowns and other reasons related to the COVID-19 pandemic, several participants were unable to make it to follow-up visits and had follow-up visits beyond the 52-week time-point, the authors explained.
Blinded board-certified dermatologists assessed efficacy by correctly identifying post-treatment photographs, from the visit after 52 weeks, and using a 6-point simplified CSS. They also assessed safety and collected data on participant satisfaction. The mean age of the women was 45.5 years, and their mean BMI was 23.9 kg/m2. The blinded reviewers correctly identified post-treatment photographs at the visit after 52 weeks at a rate of 95.2%.
In addition, 70.4% of the study participants had at least a 1-point change in their CSS score from baseline. Overall, their mean reduction in CSS score from baseline was 1.09 at the visit after 52 weeks, and a mean 34.1% reduction in cellulite at that visit, the authors reported.
In other findings, 41 of the 42 study participants (97.6%) rated their cellulite improvement as good and 33 (78.6%) agreed that the treatment was relatively pain free. Immediately following treatment, 85.7% reported an expected adverse event attributable to the device or treatment, which included mild to moderate erythema (76.7%), mild contusion/bruise (5.3%), mild pain (1.7%) and mild heat (1.7%). All adverse events resolved without intervention.
The study authors acknowledged certain limitations of the study, including the lack of a control group and the inability to differentiate effectiveness of the treatment on the buttocks versus the thighs.
“Cellulite is a common complaint among those presenting to cosmetic dermatology clinics, and prior treatment options have been somewhat disappointing in terms of invasiveness, side effects, or lack of improvement,” said Patricia M. Richey, MD, director of Mohs surgery at Boston Medical Center, who also conducts research for the Wellman Center for Photomedicine and the Dermatology Laser and Cosmetic Center at Massachusetts General Hospital, Boston.
Acoustic subcision “would potentially be a very attractive and unparalleled option given tolerability and sustained clinical improvement after only one treatment,” she told this news organization. “I agree with the authors that a possible limitation is the lack of comparison between response in different body areas,” namely, the buttocks versus the thighs, she said. “This information would be helpful to set patient expectations, and I suspect future studies will address this.”
Also asked to comment on the study, Pooja Sodha, MD, director of the Center for Laser and Cosmetic Dermatology at George Washington University, Washington, said in an interview that while the results were modest after a single treatment, “there is room for further experimentation to see how modifications of settings, treatment numbers, treatment intervals, and location-specific treatment regimens based on tissue depth and tissue band size/dimple size may enhance results.”
She added that cost of treatment and correlation with clinical improvement “will become a more real-world matter when it comes to bringing this more broadly to the clinic settings.”
Soliton sponsored the trial prior to its acquisition by AbbVie. Dr. Tanzi reported having no relevant financial disclosures. Four coauthors reported being employees, consultants, or advisory board members, or having stock options in AbbVie. Dr. Richey and Dr. Sodha were not involved with the study and reported having no disclosures.
.
The findings build on results from a 12-week study of the device, marketed as Resonic. In that trial of 56 women with moderate to severe cellulite, a single treatment provided a roughly 1.01-point reduction in the five-point Cellulite Severity Scale (CSS) at 12 weeks, which corresponds to a roughly 29.5% reduction in cellulite from baseline.
The device, which is indicated for long-term improvement in the appearance of cellulite, emits rapid acoustic pulses and shock waves at 50 Hz that are transmitted through the skin. The device “induces physical shearing of fibrous septa through rapid acoustic pulses,” investigators led by Elizabeth Tanzi, MD, who practices cosmetic dermatology in Chevy Chase, Md., wrote in the follow-up study, which was published in Dermatologic Surgery in February “In contrast to current treatment options, the device requires no anesthesia or downtime and was well-tolerated based on an average pain score of 2.4 (on a scale 0–10) during treatment” in the 12-week study, they noted.
To evaluate the long-term efficacy of the acoustic subcision device, Dr. Tanzi and her coauthors at four centers prospectively followed 42 patients who participated in the 12-week trial. The study involved four visits: screening, a single treatment visit, and a follow-up visit 12 weeks after treatment and another after 52 weeks. Because of lockdowns and other reasons related to the COVID-19 pandemic, several participants were unable to make it to follow-up visits and had follow-up visits beyond the 52-week time-point, the authors explained.
Blinded board-certified dermatologists assessed efficacy by correctly identifying post-treatment photographs, from the visit after 52 weeks, and using a 6-point simplified CSS. They also assessed safety and collected data on participant satisfaction. The mean age of the women was 45.5 years, and their mean BMI was 23.9 kg/m2. The blinded reviewers correctly identified post-treatment photographs at the visit after 52 weeks at a rate of 95.2%.
In addition, 70.4% of the study participants had at least a 1-point change in their CSS score from baseline. Overall, their mean reduction in CSS score from baseline was 1.09 at the visit after 52 weeks, and a mean 34.1% reduction in cellulite at that visit, the authors reported.
In other findings, 41 of the 42 study participants (97.6%) rated their cellulite improvement as good and 33 (78.6%) agreed that the treatment was relatively pain free. Immediately following treatment, 85.7% reported an expected adverse event attributable to the device or treatment, which included mild to moderate erythema (76.7%), mild contusion/bruise (5.3%), mild pain (1.7%) and mild heat (1.7%). All adverse events resolved without intervention.
The study authors acknowledged certain limitations of the study, including the lack of a control group and the inability to differentiate effectiveness of the treatment on the buttocks versus the thighs.
“Cellulite is a common complaint among those presenting to cosmetic dermatology clinics, and prior treatment options have been somewhat disappointing in terms of invasiveness, side effects, or lack of improvement,” said Patricia M. Richey, MD, director of Mohs surgery at Boston Medical Center, who also conducts research for the Wellman Center for Photomedicine and the Dermatology Laser and Cosmetic Center at Massachusetts General Hospital, Boston.
Acoustic subcision “would potentially be a very attractive and unparalleled option given tolerability and sustained clinical improvement after only one treatment,” she told this news organization. “I agree with the authors that a possible limitation is the lack of comparison between response in different body areas,” namely, the buttocks versus the thighs, she said. “This information would be helpful to set patient expectations, and I suspect future studies will address this.”
Also asked to comment on the study, Pooja Sodha, MD, director of the Center for Laser and Cosmetic Dermatology at George Washington University, Washington, said in an interview that while the results were modest after a single treatment, “there is room for further experimentation to see how modifications of settings, treatment numbers, treatment intervals, and location-specific treatment regimens based on tissue depth and tissue band size/dimple size may enhance results.”
She added that cost of treatment and correlation with clinical improvement “will become a more real-world matter when it comes to bringing this more broadly to the clinic settings.”
Soliton sponsored the trial prior to its acquisition by AbbVie. Dr. Tanzi reported having no relevant financial disclosures. Four coauthors reported being employees, consultants, or advisory board members, or having stock options in AbbVie. Dr. Richey and Dr. Sodha were not involved with the study and reported having no disclosures.
FROM DERMATOLOGIC SURGERY
Migraine Variants
Mood Interventions May Reduce IBD Inflammation
, according to a new study.
“IBD is a distressing condition, and current medication that reduces inflammation is expensive and can have side effects,” said Natasha Seaton, first author and a PhD student at the Institute of Psychiatry, Psychology and Neuroscience (IoPPN) at King’s College London.
“Our study showed that interventions that treat mental health reduce levels of inflammation in the body,” she said. “This indicates that mood interventions could be a valuable tool in our approach to help those with IBD.”
The study was published online in eBioMedicine.
Analyzing Mood Interventions
Ms. Seaton and colleagues conducted a systematic review and meta-analysis of randomized controlled trials in adults with IBD that measured inflammatory biomarker levels and tested a mood intervention, including those aimed at reducing depression, anxiety, stress, or distress or improving emotional well-being.
Looking at data from 28 randomized controlled trials with 1789 participants, the research team evaluated whether mood interventions affected IBD inflammation, particularly IBD indicators such as C-reactive protein and fecal calprotectin, and other general inflammatory biomarkers.
The researchers found mood interventions significantly reduced levels of inflammatory biomarkers, compared with controls, corresponding to an 18% reduction in inflammatory biomarkers.
Psychological therapies had the best outcomes related to IBD inflammation, compared with antidepressants or exercise. These therapies included cognitive behavioral therapy, acceptance and commitment therapy, and mindfulness-based stress reduction.
Individual analyses of IBD-specific inflammatory markers found small but statistically significant reductions in C-reactive protein and fecal calprotectin after a mood intervention. This could mean mood treatments have positive effects on both inflammation and disease-specific biomarkers, the authors wrote.
In addition, interventions that had a larger positive effect on mood had a greater effect in reducing inflammatory biomarkers. This suggests that a better mood could reduce IBD inflammation, they noted.
“We know stress-related feelings can increase inflammation, and the findings suggest that by improving mood, we can reduce this type of inflammation,” said Valeria Mondelli, MD, PhD, clinical professor of psychoneuroimmunology at King’s IoPPN.
“This adds to the growing body of research demonstrating the role of inflammation in mental health and suggests that interventions working to improve mood could also have direct physical effects on levels of inflammation,” she said. “However, more research is needed to understand exact mechanisms in IBD.”
Cost Benefit
Many IBD interventions and medications can be expensive for patients, have significant negative side effects, and have a lower long-term treatment response, the authors noted. Mood interventions, whether psychological therapy or medication, could potentially reduce costs and improve both mood and inflammation.
Previous studies have indicated that psychosocial factors, as well as mood disorders such as anxiety and depression, affect IBD symptom severity and progression, the authors wrote. However, researchers still need to understand the mechanisms behind this connection, including gut-brain dynamics.
Future research should focus on interventions that have been effective at improving mood in patients with IBD, assess inflammation and disease activity at numerous timepoints, and include potential variables related to illness self-management, the authors wrote.
In addition, implementation of mood interventions for IBD management may require better continuity of care and healthcare integration.
“Integrated mental health support, alongside pharmacological treatments, may offer a more holistic approach to IBD care, potentially leading to reduced disease and healthcare costs,” said Rona Moss-Morris, PhD, senior author and professor of psychology at King’s IoPPN.
Medications taken to reduce inflammation can be costly compared with psychological therapies, she said. “Given this, including psychological interventions, such as cost-effective digital interventions, within IBD management might reduce the need for anti-inflammatory medication, resulting in an overall cost benefit.”
The study was funded by the Medical Research Council (MRC) and National Institute for Health and Care Research Maudsley Biomedical Research Centre, which is hosted by South London and Maudsley NHS Foundation Trust in partnership with King’s College London. Ms. Seaton was funded by an MRC Doctoral Training Partnership. No other interests were declared.
A version of this article appeared on Medscape.com.
, according to a new study.
“IBD is a distressing condition, and current medication that reduces inflammation is expensive and can have side effects,” said Natasha Seaton, first author and a PhD student at the Institute of Psychiatry, Psychology and Neuroscience (IoPPN) at King’s College London.
“Our study showed that interventions that treat mental health reduce levels of inflammation in the body,” she said. “This indicates that mood interventions could be a valuable tool in our approach to help those with IBD.”
The study was published online in eBioMedicine.
Analyzing Mood Interventions
Ms. Seaton and colleagues conducted a systematic review and meta-analysis of randomized controlled trials in adults with IBD that measured inflammatory biomarker levels and tested a mood intervention, including those aimed at reducing depression, anxiety, stress, or distress or improving emotional well-being.
Looking at data from 28 randomized controlled trials with 1789 participants, the research team evaluated whether mood interventions affected IBD inflammation, particularly IBD indicators such as C-reactive protein and fecal calprotectin, and other general inflammatory biomarkers.
The researchers found mood interventions significantly reduced levels of inflammatory biomarkers, compared with controls, corresponding to an 18% reduction in inflammatory biomarkers.
Psychological therapies had the best outcomes related to IBD inflammation, compared with antidepressants or exercise. These therapies included cognitive behavioral therapy, acceptance and commitment therapy, and mindfulness-based stress reduction.
Individual analyses of IBD-specific inflammatory markers found small but statistically significant reductions in C-reactive protein and fecal calprotectin after a mood intervention. This could mean mood treatments have positive effects on both inflammation and disease-specific biomarkers, the authors wrote.
In addition, interventions that had a larger positive effect on mood had a greater effect in reducing inflammatory biomarkers. This suggests that a better mood could reduce IBD inflammation, they noted.
“We know stress-related feelings can increase inflammation, and the findings suggest that by improving mood, we can reduce this type of inflammation,” said Valeria Mondelli, MD, PhD, clinical professor of psychoneuroimmunology at King’s IoPPN.
“This adds to the growing body of research demonstrating the role of inflammation in mental health and suggests that interventions working to improve mood could also have direct physical effects on levels of inflammation,” she said. “However, more research is needed to understand exact mechanisms in IBD.”
Cost Benefit
Many IBD interventions and medications can be expensive for patients, have significant negative side effects, and have a lower long-term treatment response, the authors noted. Mood interventions, whether psychological therapy or medication, could potentially reduce costs and improve both mood and inflammation.
Previous studies have indicated that psychosocial factors, as well as mood disorders such as anxiety and depression, affect IBD symptom severity and progression, the authors wrote. However, researchers still need to understand the mechanisms behind this connection, including gut-brain dynamics.
Future research should focus on interventions that have been effective at improving mood in patients with IBD, assess inflammation and disease activity at numerous timepoints, and include potential variables related to illness self-management, the authors wrote.
In addition, implementation of mood interventions for IBD management may require better continuity of care and healthcare integration.
“Integrated mental health support, alongside pharmacological treatments, may offer a more holistic approach to IBD care, potentially leading to reduced disease and healthcare costs,” said Rona Moss-Morris, PhD, senior author and professor of psychology at King’s IoPPN.
Medications taken to reduce inflammation can be costly compared with psychological therapies, she said. “Given this, including psychological interventions, such as cost-effective digital interventions, within IBD management might reduce the need for anti-inflammatory medication, resulting in an overall cost benefit.”
The study was funded by the Medical Research Council (MRC) and National Institute for Health and Care Research Maudsley Biomedical Research Centre, which is hosted by South London and Maudsley NHS Foundation Trust in partnership with King’s College London. Ms. Seaton was funded by an MRC Doctoral Training Partnership. No other interests were declared.
A version of this article appeared on Medscape.com.
, according to a new study.
“IBD is a distressing condition, and current medication that reduces inflammation is expensive and can have side effects,” said Natasha Seaton, first author and a PhD student at the Institute of Psychiatry, Psychology and Neuroscience (IoPPN) at King’s College London.
“Our study showed that interventions that treat mental health reduce levels of inflammation in the body,” she said. “This indicates that mood interventions could be a valuable tool in our approach to help those with IBD.”
The study was published online in eBioMedicine.
Analyzing Mood Interventions
Ms. Seaton and colleagues conducted a systematic review and meta-analysis of randomized controlled trials in adults with IBD that measured inflammatory biomarker levels and tested a mood intervention, including those aimed at reducing depression, anxiety, stress, or distress or improving emotional well-being.
Looking at data from 28 randomized controlled trials with 1789 participants, the research team evaluated whether mood interventions affected IBD inflammation, particularly IBD indicators such as C-reactive protein and fecal calprotectin, and other general inflammatory biomarkers.
The researchers found mood interventions significantly reduced levels of inflammatory biomarkers, compared with controls, corresponding to an 18% reduction in inflammatory biomarkers.
Psychological therapies had the best outcomes related to IBD inflammation, compared with antidepressants or exercise. These therapies included cognitive behavioral therapy, acceptance and commitment therapy, and mindfulness-based stress reduction.
Individual analyses of IBD-specific inflammatory markers found small but statistically significant reductions in C-reactive protein and fecal calprotectin after a mood intervention. This could mean mood treatments have positive effects on both inflammation and disease-specific biomarkers, the authors wrote.
In addition, interventions that had a larger positive effect on mood had a greater effect in reducing inflammatory biomarkers. This suggests that a better mood could reduce IBD inflammation, they noted.
“We know stress-related feelings can increase inflammation, and the findings suggest that by improving mood, we can reduce this type of inflammation,” said Valeria Mondelli, MD, PhD, clinical professor of psychoneuroimmunology at King’s IoPPN.
“This adds to the growing body of research demonstrating the role of inflammation in mental health and suggests that interventions working to improve mood could also have direct physical effects on levels of inflammation,” she said. “However, more research is needed to understand exact mechanisms in IBD.”
Cost Benefit
Many IBD interventions and medications can be expensive for patients, have significant negative side effects, and have a lower long-term treatment response, the authors noted. Mood interventions, whether psychological therapy or medication, could potentially reduce costs and improve both mood and inflammation.
Previous studies have indicated that psychosocial factors, as well as mood disorders such as anxiety and depression, affect IBD symptom severity and progression, the authors wrote. However, researchers still need to understand the mechanisms behind this connection, including gut-brain dynamics.
Future research should focus on interventions that have been effective at improving mood in patients with IBD, assess inflammation and disease activity at numerous timepoints, and include potential variables related to illness self-management, the authors wrote.
In addition, implementation of mood interventions for IBD management may require better continuity of care and healthcare integration.
“Integrated mental health support, alongside pharmacological treatments, may offer a more holistic approach to IBD care, potentially leading to reduced disease and healthcare costs,” said Rona Moss-Morris, PhD, senior author and professor of psychology at King’s IoPPN.
Medications taken to reduce inflammation can be costly compared with psychological therapies, she said. “Given this, including psychological interventions, such as cost-effective digital interventions, within IBD management might reduce the need for anti-inflammatory medication, resulting in an overall cost benefit.”
The study was funded by the Medical Research Council (MRC) and National Institute for Health and Care Research Maudsley Biomedical Research Centre, which is hosted by South London and Maudsley NHS Foundation Trust in partnership with King’s College London. Ms. Seaton was funded by an MRC Doctoral Training Partnership. No other interests were declared.
A version of this article appeared on Medscape.com.
Nonepidemic Kaposi Sarcoma: A Case of a Rare Epidemiologic Subtype
To the Editor:
Kaposi sarcoma (KS) is a rare angioproliferative disorder associated with human herpesvirus 8 (HHV-8) infection.1 There are 4 main recognized epidemiologic forms of KS: classic, endemic, epidemic, and iatrogenic (Table). Nonepidemic KS is a recently described rare fifth type of KS that occurs in a subset of patients who do not fit the other classifications—HIV-negative patients without detectable cellular or humoral immune deficiency. This subset has been described as clinically similar to classic KS with limited disease but occurring in younger men.2,3 We describe a case of nonepidemic KS in a Middle Eastern heterosexual immunocompetent man.

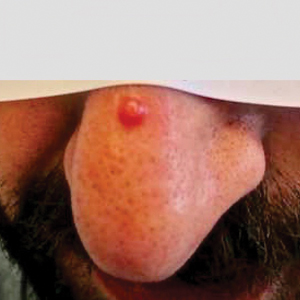
A 30-year-old man presented for evaluation of a growth on the nose of 3 months’ duration. The patient reported being otherwise healthy and was not taking long-term medications. He denied a history of malignancy, organ transplant, or immunosuppressive therapy. He was born in Syria and lived in Thailand for several years prior to moving to the United States. HIV testing 6 months prior to presentation was negative. He denied fever, chills, lymphadenopathy, shortness of breath, hemoptysis, melena, hematochezia, and intravenous drug use.

Physical examination revealed a solitary shiny, 7-mm, pink-red papule on the nasal dorsum (Figure 1). No other skin or mucosal lesions were identified. There was no cervical, axillary, or inguinal lymphadenopathy. A laboratory workup consisting of serum immunoglobulins and serum protein electrophoresis was unremarkable. Tests for HIV-1 and HIV-2 as well as human T-lymphotropic virus 1 and 2 were negative. The CD4 and CD8 counts were within reference range. Histopathology of a shave biopsy revealed a dermal spindle cell proliferation arranged in short intersecting fascicles and admixed with plasma cells and occasional mitotic figures. Immunohistochemistry showed that the spindle cells stained positive for CD34, CD31, and HHV-8 (Figure 2). The lesion resolved after treatment with cryotherapy. Repeat HIV testing 3 months later was negative. No recurrence or new lesions were identified at 3-month follow-up.

Similar to the other subtypes of KS, the nonepidemic form is dependent on HHV-8 infection, which is more commonly transmitted via saliva and sexual contact.3,4 After infecting endothelial cells, HHV-8 is believed to activate the mammalian target of rapamycin and nuclear factor κB pathways, resulting in aberrant cellular differentiation and neoangiogenesis through upregulation of vascular endothelial growth factor and basic fibroblast growth factor.2,4 Similar to what is seen with other herpesviruses, HHV-8 infection typically is lifelong due to the virus’s ability to establish latency within human B cells and endothelial cells as well as undergo sporadic bouts of lytic reactivation during its life cycle.4
Nonepidemic KS resembles other variants clinically, manifesting as erythematous or violaceous, painless, nonblanchable macules, papules, and nodules.1 Early lesions often are asymptomatic and can manifest as pigmented macules or small papules that vary from pale pink to vivid purple. Nodules also can occur and be exophytic and ulcerated with bleeding.1 Secondary lymphoproliferative disorders including Castleman disease and lymphoma have been reported.2,5
In contrast to other types of KS in which pulmonary or gastrointestinal tract lesions can develop with hemoptysis or hematochezia, mucocutaneous and visceral lesions rarely are reported in nonepidemic KS.3 Lymphedema, a feature associated with endemic KS, is notably absent in nonepidemic KS.1,3
The differential diagnosis applicable to all KS subtypes includes other vascular lesions such as angiomatosis and angiosarcoma. Histopathologic analysis is critical to differentiate KS from these conditions; visual diagnosis alone has only an 80% positive predictive value for KS.4 The histopathologic presentation of KS is a vascular proliferation in the dermis accompanied by an increased number of vessels without an endothelial cell lining.4 Spindle cell proliferation also is a common feature and is considered to be the KS tumor cell. Immunostaining for HHV-8 antigen as well as for CD31 and CD34 can be used to confirm the diagnosis.4
The management and prognosis of KS depends on the epidemiologic subtype. Classic and nonepidemic KS generally are indolent with a good prognosis. Periodic follow-up is recommended because of an increased risk for secondary malignancy such as lymphoma. The treatment of epidemic KS is highly active antiretroviral therapy. Similarly, reduction of immunosuppression is warranted for iatrogenic KS. For all types, cutaneous lesions can be treated with local excision, cryosurgery, radiation, chemotherapy, intralesional vincristine, or a topical agent such as imiquimod or alitretinoin.6
- Hinojosa T, Lewis DJ, Liu M, et al. Nonepidemic Kaposi sarcoma: a recently proposed category. J Am Acad Dermatol. 2017;3:441-443. doi: 10.1016/j.jdcr.2017.04.012
- Heymann WR. Nonepidemic Kaposi sarcoma: the fifth dimension. Dermatology World Insights and Inquiries. Published October 16, 2019. Accessed January 30, 2024. https://www.aad.org/dw/dw-insights-and-inquiries/2019-archive/october/nonepidemic-kaposi-sarcoma
- Vangipuram R, Tyring SK. Epidemiology of Kaposi sarcoma: review and description of the nonepidemic variant. Int J Dermatol. 2019;58:538-542. doi: 10.1111/ijd.14080
- Cesarman E, Damania B, Krown SE, et al. Kaposi sarcoma. Nat Rev Dis Primers. 2019;5:9. doi:10.1038/s41572-019-0060-9
- Vecerek N, Truong A, Turner R, et al. Nonepidemic Kaposi’s sarcoma: an underrecognized subtype in HIV-negative patients. J Am Acad Dermatol. 2019;81(suppl 1):AB247. doi:10.1016/j.jaad.2019.09.1096
- Schneider JW, Dittmer DP. Diagnosis and treatment of Kaposi sarcoma. Am J Clin Dermatol. 2017;18:529-539. doi:10.1007/s40257-017-0270-4
To the Editor:
Kaposi sarcoma (KS) is a rare angioproliferative disorder associated with human herpesvirus 8 (HHV-8) infection.1 There are 4 main recognized epidemiologic forms of KS: classic, endemic, epidemic, and iatrogenic (Table). Nonepidemic KS is a recently described rare fifth type of KS that occurs in a subset of patients who do not fit the other classifications—HIV-negative patients without detectable cellular or humoral immune deficiency. This subset has been described as clinically similar to classic KS with limited disease but occurring in younger men.2,3 We describe a case of nonepidemic KS in a Middle Eastern heterosexual immunocompetent man.

A 30-year-old man presented for evaluation of a growth on the nose of 3 months’ duration. The patient reported being otherwise healthy and was not taking long-term medications. He denied a history of malignancy, organ transplant, or immunosuppressive therapy. He was born in Syria and lived in Thailand for several years prior to moving to the United States. HIV testing 6 months prior to presentation was negative. He denied fever, chills, lymphadenopathy, shortness of breath, hemoptysis, melena, hematochezia, and intravenous drug use.

Physical examination revealed a solitary shiny, 7-mm, pink-red papule on the nasal dorsum (Figure 1). No other skin or mucosal lesions were identified. There was no cervical, axillary, or inguinal lymphadenopathy. A laboratory workup consisting of serum immunoglobulins and serum protein electrophoresis was unremarkable. Tests for HIV-1 and HIV-2 as well as human T-lymphotropic virus 1 and 2 were negative. The CD4 and CD8 counts were within reference range. Histopathology of a shave biopsy revealed a dermal spindle cell proliferation arranged in short intersecting fascicles and admixed with plasma cells and occasional mitotic figures. Immunohistochemistry showed that the spindle cells stained positive for CD34, CD31, and HHV-8 (Figure 2). The lesion resolved after treatment with cryotherapy. Repeat HIV testing 3 months later was negative. No recurrence or new lesions were identified at 3-month follow-up.

Similar to the other subtypes of KS, the nonepidemic form is dependent on HHV-8 infection, which is more commonly transmitted via saliva and sexual contact.3,4 After infecting endothelial cells, HHV-8 is believed to activate the mammalian target of rapamycin and nuclear factor κB pathways, resulting in aberrant cellular differentiation and neoangiogenesis through upregulation of vascular endothelial growth factor and basic fibroblast growth factor.2,4 Similar to what is seen with other herpesviruses, HHV-8 infection typically is lifelong due to the virus’s ability to establish latency within human B cells and endothelial cells as well as undergo sporadic bouts of lytic reactivation during its life cycle.4
Nonepidemic KS resembles other variants clinically, manifesting as erythematous or violaceous, painless, nonblanchable macules, papules, and nodules.1 Early lesions often are asymptomatic and can manifest as pigmented macules or small papules that vary from pale pink to vivid purple. Nodules also can occur and be exophytic and ulcerated with bleeding.1 Secondary lymphoproliferative disorders including Castleman disease and lymphoma have been reported.2,5
In contrast to other types of KS in which pulmonary or gastrointestinal tract lesions can develop with hemoptysis or hematochezia, mucocutaneous and visceral lesions rarely are reported in nonepidemic KS.3 Lymphedema, a feature associated with endemic KS, is notably absent in nonepidemic KS.1,3
The differential diagnosis applicable to all KS subtypes includes other vascular lesions such as angiomatosis and angiosarcoma. Histopathologic analysis is critical to differentiate KS from these conditions; visual diagnosis alone has only an 80% positive predictive value for KS.4 The histopathologic presentation of KS is a vascular proliferation in the dermis accompanied by an increased number of vessels without an endothelial cell lining.4 Spindle cell proliferation also is a common feature and is considered to be the KS tumor cell. Immunostaining for HHV-8 antigen as well as for CD31 and CD34 can be used to confirm the diagnosis.4
The management and prognosis of KS depends on the epidemiologic subtype. Classic and nonepidemic KS generally are indolent with a good prognosis. Periodic follow-up is recommended because of an increased risk for secondary malignancy such as lymphoma. The treatment of epidemic KS is highly active antiretroviral therapy. Similarly, reduction of immunosuppression is warranted for iatrogenic KS. For all types, cutaneous lesions can be treated with local excision, cryosurgery, radiation, chemotherapy, intralesional vincristine, or a topical agent such as imiquimod or alitretinoin.6
To the Editor:
Kaposi sarcoma (KS) is a rare angioproliferative disorder associated with human herpesvirus 8 (HHV-8) infection.1 There are 4 main recognized epidemiologic forms of KS: classic, endemic, epidemic, and iatrogenic (Table). Nonepidemic KS is a recently described rare fifth type of KS that occurs in a subset of patients who do not fit the other classifications—HIV-negative patients without detectable cellular or humoral immune deficiency. This subset has been described as clinically similar to classic KS with limited disease but occurring in younger men.2,3 We describe a case of nonepidemic KS in a Middle Eastern heterosexual immunocompetent man.

A 30-year-old man presented for evaluation of a growth on the nose of 3 months’ duration. The patient reported being otherwise healthy and was not taking long-term medications. He denied a history of malignancy, organ transplant, or immunosuppressive therapy. He was born in Syria and lived in Thailand for several years prior to moving to the United States. HIV testing 6 months prior to presentation was negative. He denied fever, chills, lymphadenopathy, shortness of breath, hemoptysis, melena, hematochezia, and intravenous drug use.

Physical examination revealed a solitary shiny, 7-mm, pink-red papule on the nasal dorsum (Figure 1). No other skin or mucosal lesions were identified. There was no cervical, axillary, or inguinal lymphadenopathy. A laboratory workup consisting of serum immunoglobulins and serum protein electrophoresis was unremarkable. Tests for HIV-1 and HIV-2 as well as human T-lymphotropic virus 1 and 2 were negative. The CD4 and CD8 counts were within reference range. Histopathology of a shave biopsy revealed a dermal spindle cell proliferation arranged in short intersecting fascicles and admixed with plasma cells and occasional mitotic figures. Immunohistochemistry showed that the spindle cells stained positive for CD34, CD31, and HHV-8 (Figure 2). The lesion resolved after treatment with cryotherapy. Repeat HIV testing 3 months later was negative. No recurrence or new lesions were identified at 3-month follow-up.

Similar to the other subtypes of KS, the nonepidemic form is dependent on HHV-8 infection, which is more commonly transmitted via saliva and sexual contact.3,4 After infecting endothelial cells, HHV-8 is believed to activate the mammalian target of rapamycin and nuclear factor κB pathways, resulting in aberrant cellular differentiation and neoangiogenesis through upregulation of vascular endothelial growth factor and basic fibroblast growth factor.2,4 Similar to what is seen with other herpesviruses, HHV-8 infection typically is lifelong due to the virus’s ability to establish latency within human B cells and endothelial cells as well as undergo sporadic bouts of lytic reactivation during its life cycle.4
Nonepidemic KS resembles other variants clinically, manifesting as erythematous or violaceous, painless, nonblanchable macules, papules, and nodules.1 Early lesions often are asymptomatic and can manifest as pigmented macules or small papules that vary from pale pink to vivid purple. Nodules also can occur and be exophytic and ulcerated with bleeding.1 Secondary lymphoproliferative disorders including Castleman disease and lymphoma have been reported.2,5
In contrast to other types of KS in which pulmonary or gastrointestinal tract lesions can develop with hemoptysis or hematochezia, mucocutaneous and visceral lesions rarely are reported in nonepidemic KS.3 Lymphedema, a feature associated with endemic KS, is notably absent in nonepidemic KS.1,3
The differential diagnosis applicable to all KS subtypes includes other vascular lesions such as angiomatosis and angiosarcoma. Histopathologic analysis is critical to differentiate KS from these conditions; visual diagnosis alone has only an 80% positive predictive value for KS.4 The histopathologic presentation of KS is a vascular proliferation in the dermis accompanied by an increased number of vessels without an endothelial cell lining.4 Spindle cell proliferation also is a common feature and is considered to be the KS tumor cell. Immunostaining for HHV-8 antigen as well as for CD31 and CD34 can be used to confirm the diagnosis.4
The management and prognosis of KS depends on the epidemiologic subtype. Classic and nonepidemic KS generally are indolent with a good prognosis. Periodic follow-up is recommended because of an increased risk for secondary malignancy such as lymphoma. The treatment of epidemic KS is highly active antiretroviral therapy. Similarly, reduction of immunosuppression is warranted for iatrogenic KS. For all types, cutaneous lesions can be treated with local excision, cryosurgery, radiation, chemotherapy, intralesional vincristine, or a topical agent such as imiquimod or alitretinoin.6
- Hinojosa T, Lewis DJ, Liu M, et al. Nonepidemic Kaposi sarcoma: a recently proposed category. J Am Acad Dermatol. 2017;3:441-443. doi: 10.1016/j.jdcr.2017.04.012
- Heymann WR. Nonepidemic Kaposi sarcoma: the fifth dimension. Dermatology World Insights and Inquiries. Published October 16, 2019. Accessed January 30, 2024. https://www.aad.org/dw/dw-insights-and-inquiries/2019-archive/october/nonepidemic-kaposi-sarcoma
- Vangipuram R, Tyring SK. Epidemiology of Kaposi sarcoma: review and description of the nonepidemic variant. Int J Dermatol. 2019;58:538-542. doi: 10.1111/ijd.14080
- Cesarman E, Damania B, Krown SE, et al. Kaposi sarcoma. Nat Rev Dis Primers. 2019;5:9. doi:10.1038/s41572-019-0060-9
- Vecerek N, Truong A, Turner R, et al. Nonepidemic Kaposi’s sarcoma: an underrecognized subtype in HIV-negative patients. J Am Acad Dermatol. 2019;81(suppl 1):AB247. doi:10.1016/j.jaad.2019.09.1096
- Schneider JW, Dittmer DP. Diagnosis and treatment of Kaposi sarcoma. Am J Clin Dermatol. 2017;18:529-539. doi:10.1007/s40257-017-0270-4
- Hinojosa T, Lewis DJ, Liu M, et al. Nonepidemic Kaposi sarcoma: a recently proposed category. J Am Acad Dermatol. 2017;3:441-443. doi: 10.1016/j.jdcr.2017.04.012
- Heymann WR. Nonepidemic Kaposi sarcoma: the fifth dimension. Dermatology World Insights and Inquiries. Published October 16, 2019. Accessed January 30, 2024. https://www.aad.org/dw/dw-insights-and-inquiries/2019-archive/october/nonepidemic-kaposi-sarcoma
- Vangipuram R, Tyring SK. Epidemiology of Kaposi sarcoma: review and description of the nonepidemic variant. Int J Dermatol. 2019;58:538-542. doi: 10.1111/ijd.14080
- Cesarman E, Damania B, Krown SE, et al. Kaposi sarcoma. Nat Rev Dis Primers. 2019;5:9. doi:10.1038/s41572-019-0060-9
- Vecerek N, Truong A, Turner R, et al. Nonepidemic Kaposi’s sarcoma: an underrecognized subtype in HIV-negative patients. J Am Acad Dermatol. 2019;81(suppl 1):AB247. doi:10.1016/j.jaad.2019.09.1096
- Schneider JW, Dittmer DP. Diagnosis and treatment of Kaposi sarcoma. Am J Clin Dermatol. 2017;18:529-539. doi:10.1007/s40257-017-0270-4
Practice Points
- Nonepidemic Kaposi sarcoma (KS) is a recently described fifth subtype of the disease that typically occurs in younger men who are HIV-negative without detectable cellular or humoral immune deficiency.
- The cutaneous manifestations of nonepidemic KS are similar to those of classic KS, except that disease extent is limited and the prognosis is favorable in nonepidemic KS.
- Dermatologists should consider KS when a patient presents with clinically representative findings, even in the absence of typical risk factors such as immunosuppression.
FDA OKs First Oral Agent for Eosinophilic Esophagitis
Budesonide oral suspension is a corticosteroid indicated for 12 weeks of treatment of EoE in adults and children as young as 11 years.
It will be available in 2-mg/10-mL single-dose stick packs by the end of February.
“Developed specifically for EoE, Eohilia’s novel formulation of budesonide confers thixotropic properties — flowing more freely when shaken and returning to a more viscous state when swallowed,” the company said in a news release.
“Various formulations of corticosteroids have been used in the past to manage EoE, but in an off-label capacity and using multiple delivery options. With Eohilia, it’s gratifying to now have an FDA-approved treatment specifically formulated for a consistent dose delivery with demonstrated ability to address esophageal inflammation and EoE dysphagia symptoms,” Ikuo Hirano, MD, professor of medicine and director of the Esophageal Center at Northwestern University Feinberg School of Medicine, Chicago, said in the release.
Supporting Data
The FDA approved budesonide oral suspension for EoE based on efficacy and safety data from two multicenter, randomized, double-blind, parallel-group, placebo-controlled 12-week studies.
In study 1, significantly more patients receiving active treatment achieved histologic remission (53.1% vs 1% with placebo). The same was true in study 2, with 38% of patients receiving active treatment achieving histologic remission compared with 2.4% of those receiving placebo.
The absolute change from baseline in the patient-reported Dysphagia Symptom Questionnaire combined score was -10.2 with budesonide vs -6.5 with placebo in Study 1 and -14.5 vs -5.9 in Study 2.
During the last 2 weeks of treatment, more patients receiving budesonide oral suspension experienced no dysphagia or only experienced dysphagia that “got better or cleared up on its own” compared with those receiving placebo, the company said.
The most common adverse reactions seen in the clinical trials of budesonide oral suspension for EoE included respiratory tract infection (13%), gastrointestinal mucosal candidiasis (8%), headache (5%), gastroenteritis (3%), throat irritation (3%), adrenal suppression (2%), and erosive esophagitis (2%).
Complete prescribing information is available on the FDA website.
A version of this article appeared on Medscape.com.
Budesonide oral suspension is a corticosteroid indicated for 12 weeks of treatment of EoE in adults and children as young as 11 years.
It will be available in 2-mg/10-mL single-dose stick packs by the end of February.
“Developed specifically for EoE, Eohilia’s novel formulation of budesonide confers thixotropic properties — flowing more freely when shaken and returning to a more viscous state when swallowed,” the company said in a news release.
“Various formulations of corticosteroids have been used in the past to manage EoE, but in an off-label capacity and using multiple delivery options. With Eohilia, it’s gratifying to now have an FDA-approved treatment specifically formulated for a consistent dose delivery with demonstrated ability to address esophageal inflammation and EoE dysphagia symptoms,” Ikuo Hirano, MD, professor of medicine and director of the Esophageal Center at Northwestern University Feinberg School of Medicine, Chicago, said in the release.
Supporting Data
The FDA approved budesonide oral suspension for EoE based on efficacy and safety data from two multicenter, randomized, double-blind, parallel-group, placebo-controlled 12-week studies.
In study 1, significantly more patients receiving active treatment achieved histologic remission (53.1% vs 1% with placebo). The same was true in study 2, with 38% of patients receiving active treatment achieving histologic remission compared with 2.4% of those receiving placebo.
The absolute change from baseline in the patient-reported Dysphagia Symptom Questionnaire combined score was -10.2 with budesonide vs -6.5 with placebo in Study 1 and -14.5 vs -5.9 in Study 2.
During the last 2 weeks of treatment, more patients receiving budesonide oral suspension experienced no dysphagia or only experienced dysphagia that “got better or cleared up on its own” compared with those receiving placebo, the company said.
The most common adverse reactions seen in the clinical trials of budesonide oral suspension for EoE included respiratory tract infection (13%), gastrointestinal mucosal candidiasis (8%), headache (5%), gastroenteritis (3%), throat irritation (3%), adrenal suppression (2%), and erosive esophagitis (2%).
Complete prescribing information is available on the FDA website.
A version of this article appeared on Medscape.com.
Budesonide oral suspension is a corticosteroid indicated for 12 weeks of treatment of EoE in adults and children as young as 11 years.
It will be available in 2-mg/10-mL single-dose stick packs by the end of February.
“Developed specifically for EoE, Eohilia’s novel formulation of budesonide confers thixotropic properties — flowing more freely when shaken and returning to a more viscous state when swallowed,” the company said in a news release.
“Various formulations of corticosteroids have been used in the past to manage EoE, but in an off-label capacity and using multiple delivery options. With Eohilia, it’s gratifying to now have an FDA-approved treatment specifically formulated for a consistent dose delivery with demonstrated ability to address esophageal inflammation and EoE dysphagia symptoms,” Ikuo Hirano, MD, professor of medicine and director of the Esophageal Center at Northwestern University Feinberg School of Medicine, Chicago, said in the release.
Supporting Data
The FDA approved budesonide oral suspension for EoE based on efficacy and safety data from two multicenter, randomized, double-blind, parallel-group, placebo-controlled 12-week studies.
In study 1, significantly more patients receiving active treatment achieved histologic remission (53.1% vs 1% with placebo). The same was true in study 2, with 38% of patients receiving active treatment achieving histologic remission compared with 2.4% of those receiving placebo.
The absolute change from baseline in the patient-reported Dysphagia Symptom Questionnaire combined score was -10.2 with budesonide vs -6.5 with placebo in Study 1 and -14.5 vs -5.9 in Study 2.
During the last 2 weeks of treatment, more patients receiving budesonide oral suspension experienced no dysphagia or only experienced dysphagia that “got better or cleared up on its own” compared with those receiving placebo, the company said.
The most common adverse reactions seen in the clinical trials of budesonide oral suspension for EoE included respiratory tract infection (13%), gastrointestinal mucosal candidiasis (8%), headache (5%), gastroenteritis (3%), throat irritation (3%), adrenal suppression (2%), and erosive esophagitis (2%).
Complete prescribing information is available on the FDA website.
A version of this article appeared on Medscape.com.
Painful Retiform Purpura in a Peritoneal Dialysis Patient
The Diagnosis: Calcific Uremic Arteriolopathy
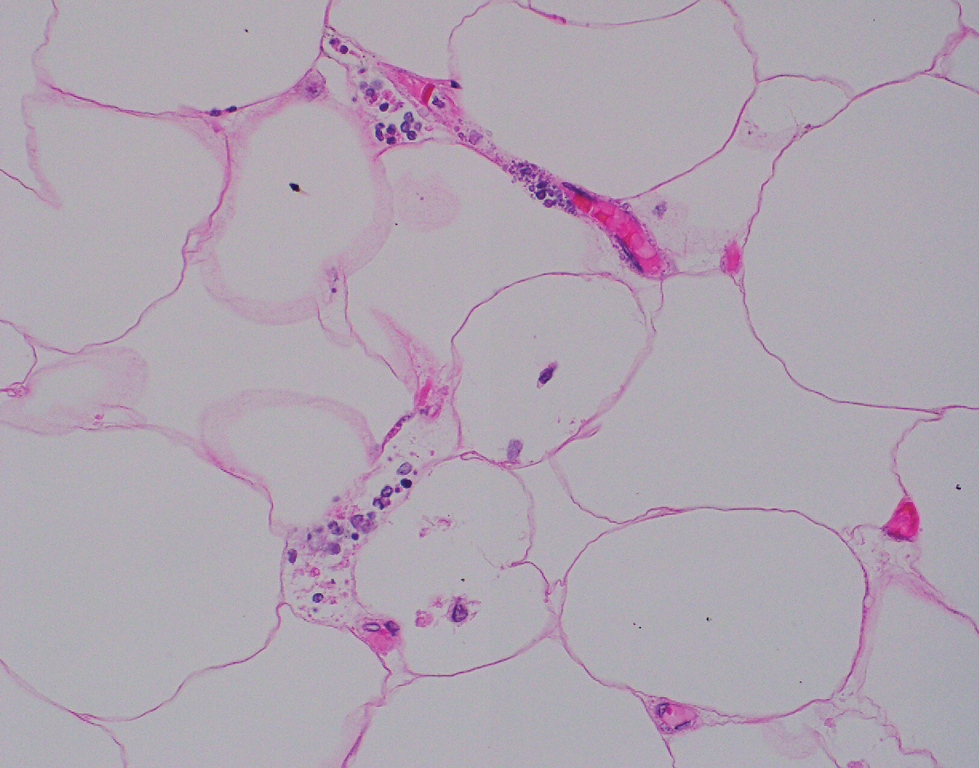
Computed tomography of the abdomen and pelvis with contrast revealed a right complex renal cyst with peripheral calcification; computed tomography of the head without contrast revealed atherosclerotic changes with calcification of the intracranial arteries, vertebral basilar arteries, and bilateral branches of the ophthalmic artery. Histopathology revealed occlusive vasculopathy with epidermal ischemic changes as well as dermal and subcutaneous vascular congestion and small thrombi. Within the subcutis, there were tiny stippled calcium deposits within very small vascular lumina (Figure). The combination of clinical and histological findings was highly suggestive of calcific uremic arteriolopathy, and the patient was transitioned to hemodialysis against a low-calcium bath to avoid hypercalcemia. Unfortunately, she developed complications related to sepsis and experienced worsening mentation. After a discussion with palliative care, the patient was transitioned to comfort measures and discharged home on hospice 1 week after the biopsy at her family’s request.
Calcific uremic arteriolopathy (also known as calciphylaxis) is a rare, life-threatening syndrome of widespread vascular calcification leading to microvascular occlusion within the dermis and subcutaneous tissues.1 Clinically, it typically manifests as severely painful, purpuric skin lesions that evolve through phases of blistering, ulceration, and ultimately visible skin necrosis.2 The pain likely is a consequence of ischemia and nociceptive activation and often may precede any visibly apparent skin lesions.3 Risk factors associated with the development of this condition include female sex; history of diabetes mellitus, obesity, rapid weight loss, or end-stage renal disease; abnormalities in calcium and phosphorus homeostasis; and vitamin K deficiency.1,3 It is more prevalent in patients on peritoneal dialysis compared to hemodialysis.4
Calciphylaxis is diagnosed with combined clinical and histopathological evidence. Laboratory test abnormalities are not specific for disease; therefore, skin biopsy is the standard confirmatory test, though its practice is contentious due to the risk for nonhealing ulceration and increasing risk for infection.1 Findings suggestive of disease include focal to diffuse calcification (intravascular, extravascular, or perieccrine), superficial fat calcium deposition, mid panniculus calcium deposition, mid panniculus vascular thrombi, and focal to diffuse angioplasia.5 The hallmark feature is diffuse calcification of small capillaries in adipose tissue.6
The mortality rate associated with this disease is high—a 6-month mortality rate of 27% to 43% has been reported from the time of diagnosis7-9—which often is related to subsequent superimposed infections patients acquire from necrotic skin tissue.2 The disease also carries high morbidity, with patients experiencing frequent hospitalizations related to pain, infections, and nonhealing wounds.6 There is no standard treatment, and trials have been limited to small sample sizes. A multidisciplinary treatment approach is essential to maximize outcomes, which includes wound care, risk factor modification, analgesia, and symptomatic management strategies.1,2,6
Some pharmacologic agents have received noteworthy attention in treating calciphylaxis, including sodium thiosulfate (STS), bisphosphonates, and vitamin K supplementation.1 The strongest evidence supporting the use of STS comes from 2 trials involving 53 and 27 dialysis patients, with complete remission in 14 (26%) and 14 (52%) patients, respectively.10,11 However, these trials did not include control groups to compare outcomes, and mortality rates were similarly high among partial responders and nonresponders compared with patients not treated with STS. A 2018 systematic review failed to assess the efficacy of STS alone for the treatment of calciphylaxis but suggested there may be a future role for it, with 251 of 358 patients (70.1%) responding to therapy.12
Erythema ab igne is a cutaneous reaction related to long-term heat exposure, often from electronic devices such as laptops, heating pads, space heaters, or hot-water bottles.13,14 Clinically, this rash appears as an erythematous, purpuric, or hyperpigmented reticular dermatosis that is below the clinical threshold to define a thermal burn.13 Lesions often are seen on the anterior thighs or across the abdomen.15 There usually are no long-term clinical sequelae; however, rare malignant transformation has been documented in cases of atrophy or nonhealing ulceration.16 Treatment is supportive with removal of the offending agent, but hyperpigmentation may persist for months to years.14
Livedo reticularis is a cutaneous pattern of mottled violaceous or hyperpigmented changes that often signifies underlying vascular dermal changes.17 It can be seen in various pathologic states, including vasculitis, autoimmune disease, connective tissue disease, neurologic disease, infection, or malignancy, or it can be drug induced.18 There are no pathognomonic microscopic changes, as the histology will drastically differ based on the etiology. Workup can be extensive; cues to the underlying pathology should be sought based on the patient’s history and concurrent presenting symptoms. Livedo reticularis is the most common dermatologic finding in patients with antiphospholipid syndrome, and workup should include antiphospholipid antibodies (eg, lupus anticoagulant, anticardiolipin, anti–beta-2-glycoproteins) as well as lupus testing (eg, antinuclear antibodies, anti– double-stranded DNA).19 Treatment is targeted at the underlying disease process.
Cryoglobulinemia is a disease characterized by abnormal serum immunoglobulins that precipitate at cold temperatures and is further subcategorized by the type of complexes that are deposited.20 Type I represents purely monoclonal cryoglobulins, type III purely polyclonal, and type II a mixed picture. Clinical manifestations arise from excessive deposition of these proteins in the skin, joints, peripheral vasculature, and kidneys leading to purpuric skin lesions, chronic ulceration, arthralgia, and glomerulonephritis. Cutaneous findings may include erythematous to purpuric macular or papular changes with or without the presence of ulceration, infarction, or hemorrhagic crusting.21 Systemic disease often underlies a diagnosis, and further investigation for hepatitis C virus, connective tissue disease, and hematologic malignancies should be considered.20 Treatment is targeted at underlying systemic disease, such as antiviral treatment for hepatitis or chemotherapeutic regimens for hematologic disease.22
Polyarteritis nodosa is a systemic necrotizing vasculitis that typically involves small- to medium-sized arteries. Cutaneous manifestations often include subcutaneous nodules, livedo reticularis, and ulcerations most found on the lower extremities.23 Systemic symptoms including fever, myalgia, arthralgia, and neuropathy often are present. Characteristic histopathology findings include inflammation and destruction of medium-sized arteries at the junctional zone of the dermis and subcutis along with microaneurysms along the vessels.24 Treatment is based on the severity of disease, with localized cutaneous disease often being controlled with topical steroids and anti-inflammatory agents, while more widespread disease requires immunosuppression with systemic steroids, hydroxychloroquine, azathioprine, methotrexate, mycophenolate mofetil, or intravenous immunoglobulins.23
- Nigwekar SU, Thadhani R, Brandenburg VM. Calciphylaxis. N Engl J Med. 2018;378:1704-1714. doi:10.1056/NEJMra1505292
- Nigwekar SU, Kroshinsky D, Nazarian RM, et al. Calciphylaxis: risk factors, diagnosis, and treatment. Am J Kidney Dis. 2015;66:133-146. doi:10.1053/j.ajkd.2015.01.034
- Chang JJ. Calciphylaxis: diagnosis, pathogenesis, and treatment. Adv Skin Wound Care. 2019;32:205-215. doi:10.1097/01 .ASW.0000554443.14002.13
- Zhang Y, Corapi KM, Luongo M, et al. Calciphylaxis in peritoneal dialysis patients: a single center cohort study. Int J Nephrol Renovasc Dis. 2016;9:235-241. doi:10.2147/ijnrd.S115701
- Chen TY, Lehman JS, Gibson LE, et al. Histopathology of calciphylaxis: cohort study with clinical correlations. Am J Dermatopathol. 2017;39:795-802. doi:10.1097/DAD.0000000000000824
- Kodumudi V, Jeha GM, Mydlo N, et al. Management of cutaneous calciphylaxis. Adv Ther. 2020;37:4797-4807. doi:10.1007 /s12325-020-01504-w
- Nigwekar SU, Zhao S, Wenger J, et al. A nationally representative study of calcific uremic arteriolopathy risk factors. J Am Soc Nephrol. 2016;27:3421-3429. doi:10.1681/asn.2015091065
- McCarthy JT, El-Azhary RA, Patzelt MT, et al. Survival, risk factors, and effect of treatment in 101 patients with calciphylaxis. Mayo Clin Proc. 2016;91:1384-1394. doi:10.1016/j.mayocp.2016.06.025
- Fine A, Zacharias J. Calciphylaxis is usually non-ulcerating: risk factors, outcome and therapy. Kidney Int. 2002;61:2210-2217. doi:10.1046/j.1523-1755.2002.00375.x
- Nigwekar SU, Brunelli SM, Meade D, et al. Sodium thiosulfate therapy for calcific uremic arteriolopathy. Clin J Am Soc Nephrol. 2013;8:1162-1170. doi:10.2215/cjn.09880912
- Zitt E, König M, Vychytil A, et al. Use of sodium thiosulphate in a multi-interventional setting for the treatment of calciphylaxis in dialysis patients. Nephrol Dial Transplant. 2013;28:1232-1240. doi:10.1093/ndt/gfs548
- Peng T, Zhuo L, Wang Y, et al. Systematic review of sodium thiosulfate in treating calciphylaxis in chronic kidney disease patients. Nephrology (Carlton). 2018;23:669-675. doi:10.1111/nep.13081
- Miller K, Hunt R, Chu J, et al. Erythema ab igne. Dermatol Online J. 2011;17:28.
- Kettelhut EA, Traylor J, Sathe NC, et al. Erythema ab igne. StatPearls. StatPearls Publishing; 2022.
- Knöpfel N, Weibel L. Erythema Ab Igne. JAMA Dermatol. 2021;157: 106. doi:10.1001/jamadermatol.2020.3995
- Sigmon JR, Cantrell J, Teague D, et al. Poorly differentiated carcinoma arising in the setting of erythema ab igne. Am J Dermatopathol. 2013;35:676-678. doi:10.1097/DAD.0b013e3182871648
- Rose AE, Sagger V, Boyd KP, et al. Livedo reticularis. Dermatol Online J. 2013;19:20705.
- Sajjan VV, Lunge S, Swamy MB, et al. Livedo reticularis: a review of the literature. Indian Dermatol Online J. 2015;6:315-321. doi:10.4103/2229-5178.164493
- Uthman IW, Khamashta MA. Livedo racemosa: a striking dermatological sign for the antiphospholipid syndrome. J Rheumatol. 2006;33:2379-2382.
- Desbois AC, Cacoub P, Saadoun D. Cryoglobulinemia: an update in 2019. Joint Bone Spine. 2019;86:707-713. doi:10.1016/j .jbspin.2019.01.016
- Cohen SJ, Pittelkow MR, Su WP. Cutaneous manifestations of cryoglobulinemia: clinical and histopathologic study of seventy-two patients. J Am Acad Dermatol. 1991;25(1, pt 1):21-27. doi:10.1016 /0190-9622(91)70168-2
- Takada S, Shimizu T, Hadano Y, et al. Cryoglobulinemia (review). Mol Med Rep. 2012;6:3-8. doi:10.3892/mmr.2012.861
- Turska M, Parada-Turska J. Cutaneous polyarteritis nodosa. Wiad Lek. 2018;71(1, pt 1):73-77.
- De Virgilio A, Greco A, Magliulo G, et al. Polyarteritis nodosa: a contemporary overview. Autoimmun Rev. 2016;15:564-570. doi:10.1016/j.autrev.2016.02.015
The Diagnosis: Calcific Uremic Arteriolopathy
Computed tomography of the abdomen and pelvis with contrast revealed a right complex renal cyst with peripheral calcification; computed tomography of the head without contrast revealed atherosclerotic changes with calcification of the intracranial arteries, vertebral basilar arteries, and bilateral branches of the ophthalmic artery. Histopathology revealed occlusive vasculopathy with epidermal ischemic changes as well as dermal and subcutaneous vascular congestion and small thrombi. Within the subcutis, there were tiny stippled calcium deposits within very small vascular lumina (Figure). The combination of clinical and histological findings was highly suggestive of calcific uremic arteriolopathy, and the patient was transitioned to hemodialysis against a low-calcium bath to avoid hypercalcemia. Unfortunately, she developed complications related to sepsis and experienced worsening mentation. After a discussion with palliative care, the patient was transitioned to comfort measures and discharged home on hospice 1 week after the biopsy at her family’s request.

Calcific uremic arteriolopathy (also known as calciphylaxis) is a rare, life-threatening syndrome of widespread vascular calcification leading to microvascular occlusion within the dermis and subcutaneous tissues.1 Clinically, it typically manifests as severely painful, purpuric skin lesions that evolve through phases of blistering, ulceration, and ultimately visible skin necrosis.2 The pain likely is a consequence of ischemia and nociceptive activation and often may precede any visibly apparent skin lesions.3 Risk factors associated with the development of this condition include female sex; history of diabetes mellitus, obesity, rapid weight loss, or end-stage renal disease; abnormalities in calcium and phosphorus homeostasis; and vitamin K deficiency.1,3 It is more prevalent in patients on peritoneal dialysis compared to hemodialysis.4
Calciphylaxis is diagnosed with combined clinical and histopathological evidence. Laboratory test abnormalities are not specific for disease; therefore, skin biopsy is the standard confirmatory test, though its practice is contentious due to the risk for nonhealing ulceration and increasing risk for infection.1 Findings suggestive of disease include focal to diffuse calcification (intravascular, extravascular, or perieccrine), superficial fat calcium deposition, mid panniculus calcium deposition, mid panniculus vascular thrombi, and focal to diffuse angioplasia.5 The hallmark feature is diffuse calcification of small capillaries in adipose tissue.6
The mortality rate associated with this disease is high—a 6-month mortality rate of 27% to 43% has been reported from the time of diagnosis7-9—which often is related to subsequent superimposed infections patients acquire from necrotic skin tissue.2 The disease also carries high morbidity, with patients experiencing frequent hospitalizations related to pain, infections, and nonhealing wounds.6 There is no standard treatment, and trials have been limited to small sample sizes. A multidisciplinary treatment approach is essential to maximize outcomes, which includes wound care, risk factor modification, analgesia, and symptomatic management strategies.1,2,6
Some pharmacologic agents have received noteworthy attention in treating calciphylaxis, including sodium thiosulfate (STS), bisphosphonates, and vitamin K supplementation.1 The strongest evidence supporting the use of STS comes from 2 trials involving 53 and 27 dialysis patients, with complete remission in 14 (26%) and 14 (52%) patients, respectively.10,11 However, these trials did not include control groups to compare outcomes, and mortality rates were similarly high among partial responders and nonresponders compared with patients not treated with STS. A 2018 systematic review failed to assess the efficacy of STS alone for the treatment of calciphylaxis but suggested there may be a future role for it, with 251 of 358 patients (70.1%) responding to therapy.12
Erythema ab igne is a cutaneous reaction related to long-term heat exposure, often from electronic devices such as laptops, heating pads, space heaters, or hot-water bottles.13,14 Clinically, this rash appears as an erythematous, purpuric, or hyperpigmented reticular dermatosis that is below the clinical threshold to define a thermal burn.13 Lesions often are seen on the anterior thighs or across the abdomen.15 There usually are no long-term clinical sequelae; however, rare malignant transformation has been documented in cases of atrophy or nonhealing ulceration.16 Treatment is supportive with removal of the offending agent, but hyperpigmentation may persist for months to years.14
Livedo reticularis is a cutaneous pattern of mottled violaceous or hyperpigmented changes that often signifies underlying vascular dermal changes.17 It can be seen in various pathologic states, including vasculitis, autoimmune disease, connective tissue disease, neurologic disease, infection, or malignancy, or it can be drug induced.18 There are no pathognomonic microscopic changes, as the histology will drastically differ based on the etiology. Workup can be extensive; cues to the underlying pathology should be sought based on the patient’s history and concurrent presenting symptoms. Livedo reticularis is the most common dermatologic finding in patients with antiphospholipid syndrome, and workup should include antiphospholipid antibodies (eg, lupus anticoagulant, anticardiolipin, anti–beta-2-glycoproteins) as well as lupus testing (eg, antinuclear antibodies, anti– double-stranded DNA).19 Treatment is targeted at the underlying disease process.
Cryoglobulinemia is a disease characterized by abnormal serum immunoglobulins that precipitate at cold temperatures and is further subcategorized by the type of complexes that are deposited.20 Type I represents purely monoclonal cryoglobulins, type III purely polyclonal, and type II a mixed picture. Clinical manifestations arise from excessive deposition of these proteins in the skin, joints, peripheral vasculature, and kidneys leading to purpuric skin lesions, chronic ulceration, arthralgia, and glomerulonephritis. Cutaneous findings may include erythematous to purpuric macular or papular changes with or without the presence of ulceration, infarction, or hemorrhagic crusting.21 Systemic disease often underlies a diagnosis, and further investigation for hepatitis C virus, connective tissue disease, and hematologic malignancies should be considered.20 Treatment is targeted at underlying systemic disease, such as antiviral treatment for hepatitis or chemotherapeutic regimens for hematologic disease.22
Polyarteritis nodosa is a systemic necrotizing vasculitis that typically involves small- to medium-sized arteries. Cutaneous manifestations often include subcutaneous nodules, livedo reticularis, and ulcerations most found on the lower extremities.23 Systemic symptoms including fever, myalgia, arthralgia, and neuropathy often are present. Characteristic histopathology findings include inflammation and destruction of medium-sized arteries at the junctional zone of the dermis and subcutis along with microaneurysms along the vessels.24 Treatment is based on the severity of disease, with localized cutaneous disease often being controlled with topical steroids and anti-inflammatory agents, while more widespread disease requires immunosuppression with systemic steroids, hydroxychloroquine, azathioprine, methotrexate, mycophenolate mofetil, or intravenous immunoglobulins.23
The Diagnosis: Calcific Uremic Arteriolopathy
Computed tomography of the abdomen and pelvis with contrast revealed a right complex renal cyst with peripheral calcification; computed tomography of the head without contrast revealed atherosclerotic changes with calcification of the intracranial arteries, vertebral basilar arteries, and bilateral branches of the ophthalmic artery. Histopathology revealed occlusive vasculopathy with epidermal ischemic changes as well as dermal and subcutaneous vascular congestion and small thrombi. Within the subcutis, there were tiny stippled calcium deposits within very small vascular lumina (Figure). The combination of clinical and histological findings was highly suggestive of calcific uremic arteriolopathy, and the patient was transitioned to hemodialysis against a low-calcium bath to avoid hypercalcemia. Unfortunately, she developed complications related to sepsis and experienced worsening mentation. After a discussion with palliative care, the patient was transitioned to comfort measures and discharged home on hospice 1 week after the biopsy at her family’s request.

Calcific uremic arteriolopathy (also known as calciphylaxis) is a rare, life-threatening syndrome of widespread vascular calcification leading to microvascular occlusion within the dermis and subcutaneous tissues.1 Clinically, it typically manifests as severely painful, purpuric skin lesions that evolve through phases of blistering, ulceration, and ultimately visible skin necrosis.2 The pain likely is a consequence of ischemia and nociceptive activation and often may precede any visibly apparent skin lesions.3 Risk factors associated with the development of this condition include female sex; history of diabetes mellitus, obesity, rapid weight loss, or end-stage renal disease; abnormalities in calcium and phosphorus homeostasis; and vitamin K deficiency.1,3 It is more prevalent in patients on peritoneal dialysis compared to hemodialysis.4
Calciphylaxis is diagnosed with combined clinical and histopathological evidence. Laboratory test abnormalities are not specific for disease; therefore, skin biopsy is the standard confirmatory test, though its practice is contentious due to the risk for nonhealing ulceration and increasing risk for infection.1 Findings suggestive of disease include focal to diffuse calcification (intravascular, extravascular, or perieccrine), superficial fat calcium deposition, mid panniculus calcium deposition, mid panniculus vascular thrombi, and focal to diffuse angioplasia.5 The hallmark feature is diffuse calcification of small capillaries in adipose tissue.6
The mortality rate associated with this disease is high—a 6-month mortality rate of 27% to 43% has been reported from the time of diagnosis7-9—which often is related to subsequent superimposed infections patients acquire from necrotic skin tissue.2 The disease also carries high morbidity, with patients experiencing frequent hospitalizations related to pain, infections, and nonhealing wounds.6 There is no standard treatment, and trials have been limited to small sample sizes. A multidisciplinary treatment approach is essential to maximize outcomes, which includes wound care, risk factor modification, analgesia, and symptomatic management strategies.1,2,6
Some pharmacologic agents have received noteworthy attention in treating calciphylaxis, including sodium thiosulfate (STS), bisphosphonates, and vitamin K supplementation.1 The strongest evidence supporting the use of STS comes from 2 trials involving 53 and 27 dialysis patients, with complete remission in 14 (26%) and 14 (52%) patients, respectively.10,11 However, these trials did not include control groups to compare outcomes, and mortality rates were similarly high among partial responders and nonresponders compared with patients not treated with STS. A 2018 systematic review failed to assess the efficacy of STS alone for the treatment of calciphylaxis but suggested there may be a future role for it, with 251 of 358 patients (70.1%) responding to therapy.12
Erythema ab igne is a cutaneous reaction related to long-term heat exposure, often from electronic devices such as laptops, heating pads, space heaters, or hot-water bottles.13,14 Clinically, this rash appears as an erythematous, purpuric, or hyperpigmented reticular dermatosis that is below the clinical threshold to define a thermal burn.13 Lesions often are seen on the anterior thighs or across the abdomen.15 There usually are no long-term clinical sequelae; however, rare malignant transformation has been documented in cases of atrophy or nonhealing ulceration.16 Treatment is supportive with removal of the offending agent, but hyperpigmentation may persist for months to years.14
Livedo reticularis is a cutaneous pattern of mottled violaceous or hyperpigmented changes that often signifies underlying vascular dermal changes.17 It can be seen in various pathologic states, including vasculitis, autoimmune disease, connective tissue disease, neurologic disease, infection, or malignancy, or it can be drug induced.18 There are no pathognomonic microscopic changes, as the histology will drastically differ based on the etiology. Workup can be extensive; cues to the underlying pathology should be sought based on the patient’s history and concurrent presenting symptoms. Livedo reticularis is the most common dermatologic finding in patients with antiphospholipid syndrome, and workup should include antiphospholipid antibodies (eg, lupus anticoagulant, anticardiolipin, anti–beta-2-glycoproteins) as well as lupus testing (eg, antinuclear antibodies, anti– double-stranded DNA).19 Treatment is targeted at the underlying disease process.
Cryoglobulinemia is a disease characterized by abnormal serum immunoglobulins that precipitate at cold temperatures and is further subcategorized by the type of complexes that are deposited.20 Type I represents purely monoclonal cryoglobulins, type III purely polyclonal, and type II a mixed picture. Clinical manifestations arise from excessive deposition of these proteins in the skin, joints, peripheral vasculature, and kidneys leading to purpuric skin lesions, chronic ulceration, arthralgia, and glomerulonephritis. Cutaneous findings may include erythematous to purpuric macular or papular changes with or without the presence of ulceration, infarction, or hemorrhagic crusting.21 Systemic disease often underlies a diagnosis, and further investigation for hepatitis C virus, connective tissue disease, and hematologic malignancies should be considered.20 Treatment is targeted at underlying systemic disease, such as antiviral treatment for hepatitis or chemotherapeutic regimens for hematologic disease.22
Polyarteritis nodosa is a systemic necrotizing vasculitis that typically involves small- to medium-sized arteries. Cutaneous manifestations often include subcutaneous nodules, livedo reticularis, and ulcerations most found on the lower extremities.23 Systemic symptoms including fever, myalgia, arthralgia, and neuropathy often are present. Characteristic histopathology findings include inflammation and destruction of medium-sized arteries at the junctional zone of the dermis and subcutis along with microaneurysms along the vessels.24 Treatment is based on the severity of disease, with localized cutaneous disease often being controlled with topical steroids and anti-inflammatory agents, while more widespread disease requires immunosuppression with systemic steroids, hydroxychloroquine, azathioprine, methotrexate, mycophenolate mofetil, or intravenous immunoglobulins.23
- Nigwekar SU, Thadhani R, Brandenburg VM. Calciphylaxis. N Engl J Med. 2018;378:1704-1714. doi:10.1056/NEJMra1505292
- Nigwekar SU, Kroshinsky D, Nazarian RM, et al. Calciphylaxis: risk factors, diagnosis, and treatment. Am J Kidney Dis. 2015;66:133-146. doi:10.1053/j.ajkd.2015.01.034
- Chang JJ. Calciphylaxis: diagnosis, pathogenesis, and treatment. Adv Skin Wound Care. 2019;32:205-215. doi:10.1097/01 .ASW.0000554443.14002.13
- Zhang Y, Corapi KM, Luongo M, et al. Calciphylaxis in peritoneal dialysis patients: a single center cohort study. Int J Nephrol Renovasc Dis. 2016;9:235-241. doi:10.2147/ijnrd.S115701
- Chen TY, Lehman JS, Gibson LE, et al. Histopathology of calciphylaxis: cohort study with clinical correlations. Am J Dermatopathol. 2017;39:795-802. doi:10.1097/DAD.0000000000000824
- Kodumudi V, Jeha GM, Mydlo N, et al. Management of cutaneous calciphylaxis. Adv Ther. 2020;37:4797-4807. doi:10.1007 /s12325-020-01504-w
- Nigwekar SU, Zhao S, Wenger J, et al. A nationally representative study of calcific uremic arteriolopathy risk factors. J Am Soc Nephrol. 2016;27:3421-3429. doi:10.1681/asn.2015091065
- McCarthy JT, El-Azhary RA, Patzelt MT, et al. Survival, risk factors, and effect of treatment in 101 patients with calciphylaxis. Mayo Clin Proc. 2016;91:1384-1394. doi:10.1016/j.mayocp.2016.06.025
- Fine A, Zacharias J. Calciphylaxis is usually non-ulcerating: risk factors, outcome and therapy. Kidney Int. 2002;61:2210-2217. doi:10.1046/j.1523-1755.2002.00375.x
- Nigwekar SU, Brunelli SM, Meade D, et al. Sodium thiosulfate therapy for calcific uremic arteriolopathy. Clin J Am Soc Nephrol. 2013;8:1162-1170. doi:10.2215/cjn.09880912
- Zitt E, König M, Vychytil A, et al. Use of sodium thiosulphate in a multi-interventional setting for the treatment of calciphylaxis in dialysis patients. Nephrol Dial Transplant. 2013;28:1232-1240. doi:10.1093/ndt/gfs548
- Peng T, Zhuo L, Wang Y, et al. Systematic review of sodium thiosulfate in treating calciphylaxis in chronic kidney disease patients. Nephrology (Carlton). 2018;23:669-675. doi:10.1111/nep.13081
- Miller K, Hunt R, Chu J, et al. Erythema ab igne. Dermatol Online J. 2011;17:28.
- Kettelhut EA, Traylor J, Sathe NC, et al. Erythema ab igne. StatPearls. StatPearls Publishing; 2022.
- Knöpfel N, Weibel L. Erythema Ab Igne. JAMA Dermatol. 2021;157: 106. doi:10.1001/jamadermatol.2020.3995
- Sigmon JR, Cantrell J, Teague D, et al. Poorly differentiated carcinoma arising in the setting of erythema ab igne. Am J Dermatopathol. 2013;35:676-678. doi:10.1097/DAD.0b013e3182871648
- Rose AE, Sagger V, Boyd KP, et al. Livedo reticularis. Dermatol Online J. 2013;19:20705.
- Sajjan VV, Lunge S, Swamy MB, et al. Livedo reticularis: a review of the literature. Indian Dermatol Online J. 2015;6:315-321. doi:10.4103/2229-5178.164493
- Uthman IW, Khamashta MA. Livedo racemosa: a striking dermatological sign for the antiphospholipid syndrome. J Rheumatol. 2006;33:2379-2382.
- Desbois AC, Cacoub P, Saadoun D. Cryoglobulinemia: an update in 2019. Joint Bone Spine. 2019;86:707-713. doi:10.1016/j .jbspin.2019.01.016
- Cohen SJ, Pittelkow MR, Su WP. Cutaneous manifestations of cryoglobulinemia: clinical and histopathologic study of seventy-two patients. J Am Acad Dermatol. 1991;25(1, pt 1):21-27. doi:10.1016 /0190-9622(91)70168-2
- Takada S, Shimizu T, Hadano Y, et al. Cryoglobulinemia (review). Mol Med Rep. 2012;6:3-8. doi:10.3892/mmr.2012.861
- Turska M, Parada-Turska J. Cutaneous polyarteritis nodosa. Wiad Lek. 2018;71(1, pt 1):73-77.
- De Virgilio A, Greco A, Magliulo G, et al. Polyarteritis nodosa: a contemporary overview. Autoimmun Rev. 2016;15:564-570. doi:10.1016/j.autrev.2016.02.015
- Nigwekar SU, Thadhani R, Brandenburg VM. Calciphylaxis. N Engl J Med. 2018;378:1704-1714. doi:10.1056/NEJMra1505292
- Nigwekar SU, Kroshinsky D, Nazarian RM, et al. Calciphylaxis: risk factors, diagnosis, and treatment. Am J Kidney Dis. 2015;66:133-146. doi:10.1053/j.ajkd.2015.01.034
- Chang JJ. Calciphylaxis: diagnosis, pathogenesis, and treatment. Adv Skin Wound Care. 2019;32:205-215. doi:10.1097/01 .ASW.0000554443.14002.13
- Zhang Y, Corapi KM, Luongo M, et al. Calciphylaxis in peritoneal dialysis patients: a single center cohort study. Int J Nephrol Renovasc Dis. 2016;9:235-241. doi:10.2147/ijnrd.S115701
- Chen TY, Lehman JS, Gibson LE, et al. Histopathology of calciphylaxis: cohort study with clinical correlations. Am J Dermatopathol. 2017;39:795-802. doi:10.1097/DAD.0000000000000824
- Kodumudi V, Jeha GM, Mydlo N, et al. Management of cutaneous calciphylaxis. Adv Ther. 2020;37:4797-4807. doi:10.1007 /s12325-020-01504-w
- Nigwekar SU, Zhao S, Wenger J, et al. A nationally representative study of calcific uremic arteriolopathy risk factors. J Am Soc Nephrol. 2016;27:3421-3429. doi:10.1681/asn.2015091065
- McCarthy JT, El-Azhary RA, Patzelt MT, et al. Survival, risk factors, and effect of treatment in 101 patients with calciphylaxis. Mayo Clin Proc. 2016;91:1384-1394. doi:10.1016/j.mayocp.2016.06.025
- Fine A, Zacharias J. Calciphylaxis is usually non-ulcerating: risk factors, outcome and therapy. Kidney Int. 2002;61:2210-2217. doi:10.1046/j.1523-1755.2002.00375.x
- Nigwekar SU, Brunelli SM, Meade D, et al. Sodium thiosulfate therapy for calcific uremic arteriolopathy. Clin J Am Soc Nephrol. 2013;8:1162-1170. doi:10.2215/cjn.09880912
- Zitt E, König M, Vychytil A, et al. Use of sodium thiosulphate in a multi-interventional setting for the treatment of calciphylaxis in dialysis patients. Nephrol Dial Transplant. 2013;28:1232-1240. doi:10.1093/ndt/gfs548
- Peng T, Zhuo L, Wang Y, et al. Systematic review of sodium thiosulfate in treating calciphylaxis in chronic kidney disease patients. Nephrology (Carlton). 2018;23:669-675. doi:10.1111/nep.13081
- Miller K, Hunt R, Chu J, et al. Erythema ab igne. Dermatol Online J. 2011;17:28.
- Kettelhut EA, Traylor J, Sathe NC, et al. Erythema ab igne. StatPearls. StatPearls Publishing; 2022.
- Knöpfel N, Weibel L. Erythema Ab Igne. JAMA Dermatol. 2021;157: 106. doi:10.1001/jamadermatol.2020.3995
- Sigmon JR, Cantrell J, Teague D, et al. Poorly differentiated carcinoma arising in the setting of erythema ab igne. Am J Dermatopathol. 2013;35:676-678. doi:10.1097/DAD.0b013e3182871648
- Rose AE, Sagger V, Boyd KP, et al. Livedo reticularis. Dermatol Online J. 2013;19:20705.
- Sajjan VV, Lunge S, Swamy MB, et al. Livedo reticularis: a review of the literature. Indian Dermatol Online J. 2015;6:315-321. doi:10.4103/2229-5178.164493
- Uthman IW, Khamashta MA. Livedo racemosa: a striking dermatological sign for the antiphospholipid syndrome. J Rheumatol. 2006;33:2379-2382.
- Desbois AC, Cacoub P, Saadoun D. Cryoglobulinemia: an update in 2019. Joint Bone Spine. 2019;86:707-713. doi:10.1016/j .jbspin.2019.01.016
- Cohen SJ, Pittelkow MR, Su WP. Cutaneous manifestations of cryoglobulinemia: clinical and histopathologic study of seventy-two patients. J Am Acad Dermatol. 1991;25(1, pt 1):21-27. doi:10.1016 /0190-9622(91)70168-2
- Takada S, Shimizu T, Hadano Y, et al. Cryoglobulinemia (review). Mol Med Rep. 2012;6:3-8. doi:10.3892/mmr.2012.861
- Turska M, Parada-Turska J. Cutaneous polyarteritis nodosa. Wiad Lek. 2018;71(1, pt 1):73-77.
- De Virgilio A, Greco A, Magliulo G, et al. Polyarteritis nodosa: a contemporary overview. Autoimmun Rev. 2016;15:564-570. doi:10.1016/j.autrev.2016.02.015
A 72-year-old woman presented to the emergency department with concerns of confusion and lethargy during a session of peritoneal dialysis, which she had been receiving for the last 2 years for end-stage renal disease. She had a history of type 2 diabetes mellitus, diabetic retinopathy, hypertension, coronary artery disease, and peripheral vascular disease preceding a recent right below-knee amputation. A review of systems was positive for a rash on the thighs of several weeks’ duration that was preceded by several days of burning pain in the same distribution. Physical examination revealed retiform purpura with irregular contours and interspersed white stellate patterns scattered across the superomedial thighs, right lower back, and left lower abdomen. An initial laboratory workup revealed an elevated creatinine level of 5.03 mg/dL (reference range, 0.6–1.1 mg/dL; baseline level, 3.0 mg/dL) and mild leukocytosis (12.5 cells/mm3 [reference range, 4.5–11.0 cells/mm3]). Dermatology was consulted, and a 4-mm punch biopsy was obtained from the left medial thigh. Nephrology, infectious disease, and wound care consultations also were placed.
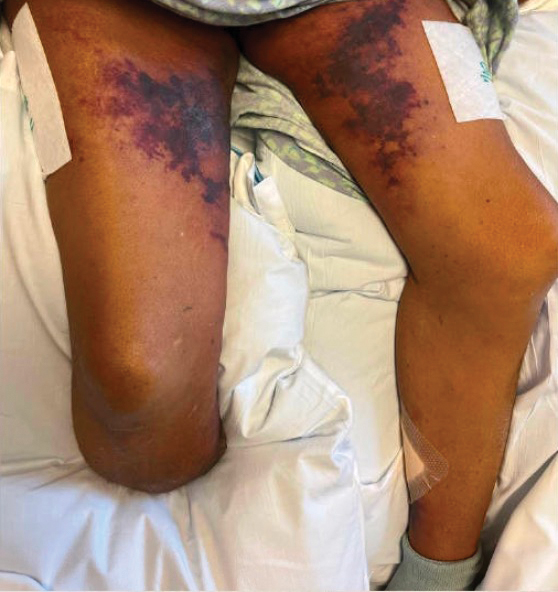
Prednisolone May Improve MOH Withdrawal
, an observational study out of South Korea has found.
The study, a post-hoc analysis of the RELEASE multicenter observational cohort study of MOH patients in South Korea, found that patients who took prednisolone as a bridge therapy in the early phase of withdrawal from headache medications, or detoxification, had statistically significant higher rates of MOH reversal at 3 months after enrollment than those who did not, 73.8% versus 57.8% (P = .034)
The reversal trend also was noted at 1 month after treatment, the study authors, led by Mi Ji Lee, MD, PhD, an assistant professor at Seoul National University Hospital, Seoul, South Korea, wrote. “Although an observational study cannot draw a definitive conclusion, our study supports the use of prednisolone for the treatment of MOH in a real-world setting,” Dr. Lee and colleagues wrote.
Study methods
The study was a post hoc analysis of the RELEASE study, which stands for Registry for Load and Management of Medication Overuse Headache. RELEASE is a multicenter observational cohort study that has been ongoing in South Korea since April 2020. The post hoc analysis included 309 patients, 59 of whom received prednisolone at a varying dose of 10-40 mg a day, with a varying course of 5-14 days. About 74% of patients (228 of 309) completed the 3-month follow-up period, including 41 in the prednisolone group.
The study used three different forms of medication withdrawal before the patients started prednisolone therapy: abrupt discontinuation; gradual discontinuation concurrent with starting prednisolone; and no withdrawal.
Because of the observational nature of the RELEASE study, participating physicians prescribed prednisolone at their own discretion. The study authors noted prednisolone use was neither randomized nor controlled, which they acknowledged as a limitation.
Dr. Lee and colleagues also acknowledged that newer calcitonin gene–related peptide (CGRP) receptor antagonists may not require detoxification to reverse MOH, but that those therapies are not always available for a variety of reasons, such as reimbursement restrictions, regional distribution issues, and financial issues.
The study also evaluated a number of secondary outcomes. For example, 72% of prednisolone patients achieved MOH reversal 1 month after starting treatment versus 54.9% of the nonprednisolone patients. (P = .33). Prednisolone users also had greater reductions in acute medication days (AMD) at 1 month and scores on headache impact test-6 (HIT-6) at 6 months.
Dr. Lee and colleagues noted that the concept of detoxification, or discontinuing medication overuse, as a treatment for MOH has been controversial due to a lack of high-quality evidence to support the approach. “Nevertheless,” they wrote, “several experts still put withdrawal of medication overuse as an important step of MOH treatment in clinical practice despite limited evidence.”
Commentary
Alan Rapoport, MD, a clinical professor of neurology at the David Geffen School of Medicine at University of California, Los Angeles, noted a number of limitations with the study. “It wasn’t a unified population of patients,” he said, “which makes it a little harder to say this medicine worked — worked on whom?” The lack of a treatment regimen — the varied dosing and treatment durations, along with the different withdrawal approaches — are further limitations, Dr. Rapoport said.
Nonetheless, the study is an important addition to the evidence on how to manage medication withdrawal in MOH, said Dr. Rapoport, a past president of the International Headache Society and founder and director emeritus of the New England Center for Headache in Stamford, Connecticut, who has a keen interest in MOH research.
“I think this shows to some extent, although it doesn’t prove it because it’s a whole mixture of patients who were all treated differently by different doctors, but when you put them all together the patients who took steroids did better than the patients who did not,” he said. “The study authors did the best they could with the information they had.”
He termed the study “well-done by well-known authors in South Korea.” As medications such as CGRP receptor antagonists and monoclonal antibodies that target CGRP and its receptors become more available, MOH patients “may not need actual detoxification or steroids in their treatment,” Dr. Rapoport said.
Dr. Lee and co-authors have no disclosures. Dr. Rapoport is editor-in-chief of Neurology Reviews. He disclosed relationships with AbbVie, Biohaven, Cala Health, Dr. Reddy’s, Pfizer, Satsuma, Teva Pharmaceutical Industries, and Theranica.
, an observational study out of South Korea has found.
The study, a post-hoc analysis of the RELEASE multicenter observational cohort study of MOH patients in South Korea, found that patients who took prednisolone as a bridge therapy in the early phase of withdrawal from headache medications, or detoxification, had statistically significant higher rates of MOH reversal at 3 months after enrollment than those who did not, 73.8% versus 57.8% (P = .034)
The reversal trend also was noted at 1 month after treatment, the study authors, led by Mi Ji Lee, MD, PhD, an assistant professor at Seoul National University Hospital, Seoul, South Korea, wrote. “Although an observational study cannot draw a definitive conclusion, our study supports the use of prednisolone for the treatment of MOH in a real-world setting,” Dr. Lee and colleagues wrote.
Study methods
The study was a post hoc analysis of the RELEASE study, which stands for Registry for Load and Management of Medication Overuse Headache. RELEASE is a multicenter observational cohort study that has been ongoing in South Korea since April 2020. The post hoc analysis included 309 patients, 59 of whom received prednisolone at a varying dose of 10-40 mg a day, with a varying course of 5-14 days. About 74% of patients (228 of 309) completed the 3-month follow-up period, including 41 in the prednisolone group.
The study used three different forms of medication withdrawal before the patients started prednisolone therapy: abrupt discontinuation; gradual discontinuation concurrent with starting prednisolone; and no withdrawal.
Because of the observational nature of the RELEASE study, participating physicians prescribed prednisolone at their own discretion. The study authors noted prednisolone use was neither randomized nor controlled, which they acknowledged as a limitation.
Dr. Lee and colleagues also acknowledged that newer calcitonin gene–related peptide (CGRP) receptor antagonists may not require detoxification to reverse MOH, but that those therapies are not always available for a variety of reasons, such as reimbursement restrictions, regional distribution issues, and financial issues.
The study also evaluated a number of secondary outcomes. For example, 72% of prednisolone patients achieved MOH reversal 1 month after starting treatment versus 54.9% of the nonprednisolone patients. (P = .33). Prednisolone users also had greater reductions in acute medication days (AMD) at 1 month and scores on headache impact test-6 (HIT-6) at 6 months.
Dr. Lee and colleagues noted that the concept of detoxification, or discontinuing medication overuse, as a treatment for MOH has been controversial due to a lack of high-quality evidence to support the approach. “Nevertheless,” they wrote, “several experts still put withdrawal of medication overuse as an important step of MOH treatment in clinical practice despite limited evidence.”
Commentary
Alan Rapoport, MD, a clinical professor of neurology at the David Geffen School of Medicine at University of California, Los Angeles, noted a number of limitations with the study. “It wasn’t a unified population of patients,” he said, “which makes it a little harder to say this medicine worked — worked on whom?” The lack of a treatment regimen — the varied dosing and treatment durations, along with the different withdrawal approaches — are further limitations, Dr. Rapoport said.
Nonetheless, the study is an important addition to the evidence on how to manage medication withdrawal in MOH, said Dr. Rapoport, a past president of the International Headache Society and founder and director emeritus of the New England Center for Headache in Stamford, Connecticut, who has a keen interest in MOH research.
“I think this shows to some extent, although it doesn’t prove it because it’s a whole mixture of patients who were all treated differently by different doctors, but when you put them all together the patients who took steroids did better than the patients who did not,” he said. “The study authors did the best they could with the information they had.”
He termed the study “well-done by well-known authors in South Korea.” As medications such as CGRP receptor antagonists and monoclonal antibodies that target CGRP and its receptors become more available, MOH patients “may not need actual detoxification or steroids in their treatment,” Dr. Rapoport said.
Dr. Lee and co-authors have no disclosures. Dr. Rapoport is editor-in-chief of Neurology Reviews. He disclosed relationships with AbbVie, Biohaven, Cala Health, Dr. Reddy’s, Pfizer, Satsuma, Teva Pharmaceutical Industries, and Theranica.
, an observational study out of South Korea has found.
The study, a post-hoc analysis of the RELEASE multicenter observational cohort study of MOH patients in South Korea, found that patients who took prednisolone as a bridge therapy in the early phase of withdrawal from headache medications, or detoxification, had statistically significant higher rates of MOH reversal at 3 months after enrollment than those who did not, 73.8% versus 57.8% (P = .034)
The reversal trend also was noted at 1 month after treatment, the study authors, led by Mi Ji Lee, MD, PhD, an assistant professor at Seoul National University Hospital, Seoul, South Korea, wrote. “Although an observational study cannot draw a definitive conclusion, our study supports the use of prednisolone for the treatment of MOH in a real-world setting,” Dr. Lee and colleagues wrote.
Study methods
The study was a post hoc analysis of the RELEASE study, which stands for Registry for Load and Management of Medication Overuse Headache. RELEASE is a multicenter observational cohort study that has been ongoing in South Korea since April 2020. The post hoc analysis included 309 patients, 59 of whom received prednisolone at a varying dose of 10-40 mg a day, with a varying course of 5-14 days. About 74% of patients (228 of 309) completed the 3-month follow-up period, including 41 in the prednisolone group.
The study used three different forms of medication withdrawal before the patients started prednisolone therapy: abrupt discontinuation; gradual discontinuation concurrent with starting prednisolone; and no withdrawal.
Because of the observational nature of the RELEASE study, participating physicians prescribed prednisolone at their own discretion. The study authors noted prednisolone use was neither randomized nor controlled, which they acknowledged as a limitation.
Dr. Lee and colleagues also acknowledged that newer calcitonin gene–related peptide (CGRP) receptor antagonists may not require detoxification to reverse MOH, but that those therapies are not always available for a variety of reasons, such as reimbursement restrictions, regional distribution issues, and financial issues.
The study also evaluated a number of secondary outcomes. For example, 72% of prednisolone patients achieved MOH reversal 1 month after starting treatment versus 54.9% of the nonprednisolone patients. (P = .33). Prednisolone users also had greater reductions in acute medication days (AMD) at 1 month and scores on headache impact test-6 (HIT-6) at 6 months.
Dr. Lee and colleagues noted that the concept of detoxification, or discontinuing medication overuse, as a treatment for MOH has been controversial due to a lack of high-quality evidence to support the approach. “Nevertheless,” they wrote, “several experts still put withdrawal of medication overuse as an important step of MOH treatment in clinical practice despite limited evidence.”
Commentary
Alan Rapoport, MD, a clinical professor of neurology at the David Geffen School of Medicine at University of California, Los Angeles, noted a number of limitations with the study. “It wasn’t a unified population of patients,” he said, “which makes it a little harder to say this medicine worked — worked on whom?” The lack of a treatment regimen — the varied dosing and treatment durations, along with the different withdrawal approaches — are further limitations, Dr. Rapoport said.
Nonetheless, the study is an important addition to the evidence on how to manage medication withdrawal in MOH, said Dr. Rapoport, a past president of the International Headache Society and founder and director emeritus of the New England Center for Headache in Stamford, Connecticut, who has a keen interest in MOH research.
“I think this shows to some extent, although it doesn’t prove it because it’s a whole mixture of patients who were all treated differently by different doctors, but when you put them all together the patients who took steroids did better than the patients who did not,” he said. “The study authors did the best they could with the information they had.”
He termed the study “well-done by well-known authors in South Korea.” As medications such as CGRP receptor antagonists and monoclonal antibodies that target CGRP and its receptors become more available, MOH patients “may not need actual detoxification or steroids in their treatment,” Dr. Rapoport said.
Dr. Lee and co-authors have no disclosures. Dr. Rapoport is editor-in-chief of Neurology Reviews. He disclosed relationships with AbbVie, Biohaven, Cala Health, Dr. Reddy’s, Pfizer, Satsuma, Teva Pharmaceutical Industries, and Theranica.
FROM HEADACHE
OTC Topical Scar Products May Contain Allergens, Study Finds
TOPLINE:
METHODOLOGY:
- OTC topical scar treatments have the potential to cause an allergic reaction, but the prevalence of North American Contact Dermatitis Group (NACDG) core allergens in these products is unclear.
- Researchers used the word scar in a query of Amazon.com and four other retail websites to identify topical scar products for consumers and noted the list of ingredients.
- The investigators also surveyed the American Contact Dermatitis Society’s Contact Allergen Management Program (CAMP), a resource that helps patients with allergies find personal care products that are safe to use, for pertinent products.
TAKEAWAY:
- The search query identified 156 products. Of these, 119 (76.2%) were gels, creams, or oils and 37 (23.7%) were sheets, strips, or tape.
- Of the 125 products that had a list of ingredients, 69 (55.2%) contained at least one NACDG allergen and 45 (36%) contained more than one.
- The top six most common allergens listed in the ingredients were fragrance (16.8%), phenoxyethanol (16.8%), parabens (14.4%), panthenol (12.8%), sodium benzoate (9.60%), and ethylhexylglycerin (8%).
- Analysis of CAMP revealed that the program only had five unique scar products in its list, suggesting that CAMP might not be a reliable source of scar product information for patients with known allergies to pertinent NACDG allergens.
IN PRACTICE:
“Patients can consider trying a ‘use test’ on the inner forearm before applying to the surgical site,” the authors wrote. “It may reveal they are sensitive or sensitized by a product.
SOURCE:
First author Meera Kattapuram, MD, of the Department of Internal Medicine at Mount Sinai Hospital, New York, led the study, published in the February issue of Dermatologic Surgery.
LIMITATIONS:
Limitations include the selection of five retailers and the top 100 products from each website and the potential for ingredient list inaccuracies.
DISCLOSURES:
The authors reported having no financial conflicts of interest. The research was supported by a grant from the National Institutes of Health/National Cancer Institute.
A version of this article appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- OTC topical scar treatments have the potential to cause an allergic reaction, but the prevalence of North American Contact Dermatitis Group (NACDG) core allergens in these products is unclear.
- Researchers used the word scar in a query of Amazon.com and four other retail websites to identify topical scar products for consumers and noted the list of ingredients.
- The investigators also surveyed the American Contact Dermatitis Society’s Contact Allergen Management Program (CAMP), a resource that helps patients with allergies find personal care products that are safe to use, for pertinent products.
TAKEAWAY:
- The search query identified 156 products. Of these, 119 (76.2%) were gels, creams, or oils and 37 (23.7%) were sheets, strips, or tape.
- Of the 125 products that had a list of ingredients, 69 (55.2%) contained at least one NACDG allergen and 45 (36%) contained more than one.
- The top six most common allergens listed in the ingredients were fragrance (16.8%), phenoxyethanol (16.8%), parabens (14.4%), panthenol (12.8%), sodium benzoate (9.60%), and ethylhexylglycerin (8%).
- Analysis of CAMP revealed that the program only had five unique scar products in its list, suggesting that CAMP might not be a reliable source of scar product information for patients with known allergies to pertinent NACDG allergens.
IN PRACTICE:
“Patients can consider trying a ‘use test’ on the inner forearm before applying to the surgical site,” the authors wrote. “It may reveal they are sensitive or sensitized by a product.
SOURCE:
First author Meera Kattapuram, MD, of the Department of Internal Medicine at Mount Sinai Hospital, New York, led the study, published in the February issue of Dermatologic Surgery.
LIMITATIONS:
Limitations include the selection of five retailers and the top 100 products from each website and the potential for ingredient list inaccuracies.
DISCLOSURES:
The authors reported having no financial conflicts of interest. The research was supported by a grant from the National Institutes of Health/National Cancer Institute.
A version of this article appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- OTC topical scar treatments have the potential to cause an allergic reaction, but the prevalence of North American Contact Dermatitis Group (NACDG) core allergens in these products is unclear.
- Researchers used the word scar in a query of Amazon.com and four other retail websites to identify topical scar products for consumers and noted the list of ingredients.
- The investigators also surveyed the American Contact Dermatitis Society’s Contact Allergen Management Program (CAMP), a resource that helps patients with allergies find personal care products that are safe to use, for pertinent products.
TAKEAWAY:
- The search query identified 156 products. Of these, 119 (76.2%) were gels, creams, or oils and 37 (23.7%) were sheets, strips, or tape.
- Of the 125 products that had a list of ingredients, 69 (55.2%) contained at least one NACDG allergen and 45 (36%) contained more than one.
- The top six most common allergens listed in the ingredients were fragrance (16.8%), phenoxyethanol (16.8%), parabens (14.4%), panthenol (12.8%), sodium benzoate (9.60%), and ethylhexylglycerin (8%).
- Analysis of CAMP revealed that the program only had five unique scar products in its list, suggesting that CAMP might not be a reliable source of scar product information for patients with known allergies to pertinent NACDG allergens.
IN PRACTICE:
“Patients can consider trying a ‘use test’ on the inner forearm before applying to the surgical site,” the authors wrote. “It may reveal they are sensitive or sensitized by a product.
SOURCE:
First author Meera Kattapuram, MD, of the Department of Internal Medicine at Mount Sinai Hospital, New York, led the study, published in the February issue of Dermatologic Surgery.
LIMITATIONS:
Limitations include the selection of five retailers and the top 100 products from each website and the potential for ingredient list inaccuracies.
DISCLOSURES:
The authors reported having no financial conflicts of interest. The research was supported by a grant from the National Institutes of Health/National Cancer Institute.
A version of this article appeared on Medscape.com.
High Rate of Dementia Among Attendees in Adult Day Service Centers
About one-quarter of all adult day services center (ADSC) participants have dementia, and the prevalence of dementia in ADSCs that specialize in the disorder is more than 40%, a new US National Health Statistics Report revealed.
ADSCs are a growing sector of the US home- and community-based long-term care delivery system, providing daytime services to adults with disabilities who often have multiple chronic conditions, including various types of dementia, according to report authors Priyanka Singha, MPH, and colleagues at the US Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Health Statistics in Bethesda, Maryland.
Dementia often leads to the transition to receiving long-term care services, such as nursing home care. Delaying institutionalization is a primary goal of ADSCs, so they also try to meet the needs of a growing population of community-dwelling adults with dementia.
Survey responses from 1800 ADSCs across the United States showed that overall, 42.2% of participants had dementia in ADSCs specializing in dementia care, while 22.7% of participants in nonspecialized ADSCs also had dementia.
Dementia was more prevalent in the Midwest and West, where nearly one half of participants in specialized centers had dementia.
Nevertheless, the overall prevalence of dementia in ADSCs was similar across US regions, with a slightly lower percentage in the West.
Positive Outcomes
The new report used data from the ADSC component of the 2020 National Post-acute and Long-term Care Study collected from January 2020 through mid-July 2021. About 1800 ADSCs from a census of 5500 ADSCs were included and weighted to be nationally representative.
The authors compared dementia prevalence among participants in ADSCs that provide specialized care for dementia with other ADSCs by census region, metropolitan statistical area (MSA) status, chain affiliation, and ownership type.
MSA is a core urban area population of 50,000 or more. ADSCs that specialize in dementia care have specially trained staff, activities, and facilities. They offer social activities, including art and music therapy, dementia-appropriate games, and group exercises, as well as respite care for unpaid caregivers. The survey found that 14% of ADSCs reported specializing in dementia.
The investigators also found that the percentage of ADSC participants with dementia, regardless of center specialization, was higher in the Midwest (32.1%), Northeast (28.5%), and South (24.5%) than in the West (21.1%).
The percentage of participants with dementia in specialized centers was higher in the Midwest (49.5%) and West (48.8%) than in the Northeast (31.9%) and in nonchain centers (50.5%) than in chain-affiliated centers (30.4%).
In addition, the percentage of participants with dementia, regardless of specialization, was higher in nonchain ADSCs (25%) than in chain-affiliated centers (20.1%). In addition, the percentage of participants with dementia in nonspecialized centers was higher in nonchain centers (25%) than in chain-affiliated centers (20.1%).
Finally, the research revealed that the percentage of participants with dementia, regardless of specialization, was higher in nonprofit ADSCs (28.7%) than for-profit centers (21%).
“These findings indicate that ADSCs in MSAs, nonprofit organizations, and nonchain centers provide services to a higher proportion of participants with dementia, particularly among centers that specialize in dementia care,” the investigators wrote.
Whereas “caregivers manage prescription medications, help with activities of daily living, and offer nutritional diets, exercise, and social engagement, ADSCs play a role in providing this type of care for people with dementia while also offering respite for their unpaid caregivers,” they noted.
Overall, they concluded that ADSCs provide positive outcomes for both family caregivers and people with dementia.
They noted that the study’s limitations include the use of cross-sectional data, which cannot show effectiveness for participants receiving care in specialized centers or be used to analyze relationships between other participant-level sociodemographic or health characteristics and specialized dementia care.
A version of this article appeared on Medscape.com.
About one-quarter of all adult day services center (ADSC) participants have dementia, and the prevalence of dementia in ADSCs that specialize in the disorder is more than 40%, a new US National Health Statistics Report revealed.
ADSCs are a growing sector of the US home- and community-based long-term care delivery system, providing daytime services to adults with disabilities who often have multiple chronic conditions, including various types of dementia, according to report authors Priyanka Singha, MPH, and colleagues at the US Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Health Statistics in Bethesda, Maryland.
Dementia often leads to the transition to receiving long-term care services, such as nursing home care. Delaying institutionalization is a primary goal of ADSCs, so they also try to meet the needs of a growing population of community-dwelling adults with dementia.
Survey responses from 1800 ADSCs across the United States showed that overall, 42.2% of participants had dementia in ADSCs specializing in dementia care, while 22.7% of participants in nonspecialized ADSCs also had dementia.
Dementia was more prevalent in the Midwest and West, where nearly one half of participants in specialized centers had dementia.
Nevertheless, the overall prevalence of dementia in ADSCs was similar across US regions, with a slightly lower percentage in the West.
Positive Outcomes
The new report used data from the ADSC component of the 2020 National Post-acute and Long-term Care Study collected from January 2020 through mid-July 2021. About 1800 ADSCs from a census of 5500 ADSCs were included and weighted to be nationally representative.
The authors compared dementia prevalence among participants in ADSCs that provide specialized care for dementia with other ADSCs by census region, metropolitan statistical area (MSA) status, chain affiliation, and ownership type.
MSA is a core urban area population of 50,000 or more. ADSCs that specialize in dementia care have specially trained staff, activities, and facilities. They offer social activities, including art and music therapy, dementia-appropriate games, and group exercises, as well as respite care for unpaid caregivers. The survey found that 14% of ADSCs reported specializing in dementia.
The investigators also found that the percentage of ADSC participants with dementia, regardless of center specialization, was higher in the Midwest (32.1%), Northeast (28.5%), and South (24.5%) than in the West (21.1%).
The percentage of participants with dementia in specialized centers was higher in the Midwest (49.5%) and West (48.8%) than in the Northeast (31.9%) and in nonchain centers (50.5%) than in chain-affiliated centers (30.4%).
In addition, the percentage of participants with dementia, regardless of specialization, was higher in nonchain ADSCs (25%) than in chain-affiliated centers (20.1%). In addition, the percentage of participants with dementia in nonspecialized centers was higher in nonchain centers (25%) than in chain-affiliated centers (20.1%).
Finally, the research revealed that the percentage of participants with dementia, regardless of specialization, was higher in nonprofit ADSCs (28.7%) than for-profit centers (21%).
“These findings indicate that ADSCs in MSAs, nonprofit organizations, and nonchain centers provide services to a higher proportion of participants with dementia, particularly among centers that specialize in dementia care,” the investigators wrote.
Whereas “caregivers manage prescription medications, help with activities of daily living, and offer nutritional diets, exercise, and social engagement, ADSCs play a role in providing this type of care for people with dementia while also offering respite for their unpaid caregivers,” they noted.
Overall, they concluded that ADSCs provide positive outcomes for both family caregivers and people with dementia.
They noted that the study’s limitations include the use of cross-sectional data, which cannot show effectiveness for participants receiving care in specialized centers or be used to analyze relationships between other participant-level sociodemographic or health characteristics and specialized dementia care.
A version of this article appeared on Medscape.com.
About one-quarter of all adult day services center (ADSC) participants have dementia, and the prevalence of dementia in ADSCs that specialize in the disorder is more than 40%, a new US National Health Statistics Report revealed.
ADSCs are a growing sector of the US home- and community-based long-term care delivery system, providing daytime services to adults with disabilities who often have multiple chronic conditions, including various types of dementia, according to report authors Priyanka Singha, MPH, and colleagues at the US Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Health Statistics in Bethesda, Maryland.
Dementia often leads to the transition to receiving long-term care services, such as nursing home care. Delaying institutionalization is a primary goal of ADSCs, so they also try to meet the needs of a growing population of community-dwelling adults with dementia.
Survey responses from 1800 ADSCs across the United States showed that overall, 42.2% of participants had dementia in ADSCs specializing in dementia care, while 22.7% of participants in nonspecialized ADSCs also had dementia.
Dementia was more prevalent in the Midwest and West, where nearly one half of participants in specialized centers had dementia.
Nevertheless, the overall prevalence of dementia in ADSCs was similar across US regions, with a slightly lower percentage in the West.
Positive Outcomes
The new report used data from the ADSC component of the 2020 National Post-acute and Long-term Care Study collected from January 2020 through mid-July 2021. About 1800 ADSCs from a census of 5500 ADSCs were included and weighted to be nationally representative.
The authors compared dementia prevalence among participants in ADSCs that provide specialized care for dementia with other ADSCs by census region, metropolitan statistical area (MSA) status, chain affiliation, and ownership type.
MSA is a core urban area population of 50,000 or more. ADSCs that specialize in dementia care have specially trained staff, activities, and facilities. They offer social activities, including art and music therapy, dementia-appropriate games, and group exercises, as well as respite care for unpaid caregivers. The survey found that 14% of ADSCs reported specializing in dementia.
The investigators also found that the percentage of ADSC participants with dementia, regardless of center specialization, was higher in the Midwest (32.1%), Northeast (28.5%), and South (24.5%) than in the West (21.1%).
The percentage of participants with dementia in specialized centers was higher in the Midwest (49.5%) and West (48.8%) than in the Northeast (31.9%) and in nonchain centers (50.5%) than in chain-affiliated centers (30.4%).
In addition, the percentage of participants with dementia, regardless of specialization, was higher in nonchain ADSCs (25%) than in chain-affiliated centers (20.1%). In addition, the percentage of participants with dementia in nonspecialized centers was higher in nonchain centers (25%) than in chain-affiliated centers (20.1%).
Finally, the research revealed that the percentage of participants with dementia, regardless of specialization, was higher in nonprofit ADSCs (28.7%) than for-profit centers (21%).
“These findings indicate that ADSCs in MSAs, nonprofit organizations, and nonchain centers provide services to a higher proportion of participants with dementia, particularly among centers that specialize in dementia care,” the investigators wrote.
Whereas “caregivers manage prescription medications, help with activities of daily living, and offer nutritional diets, exercise, and social engagement, ADSCs play a role in providing this type of care for people with dementia while also offering respite for their unpaid caregivers,” they noted.
Overall, they concluded that ADSCs provide positive outcomes for both family caregivers and people with dementia.
They noted that the study’s limitations include the use of cross-sectional data, which cannot show effectiveness for participants receiving care in specialized centers or be used to analyze relationships between other participant-level sociodemographic or health characteristics and specialized dementia care.
A version of this article appeared on Medscape.com.