User login
Obesity in Patients With RA Successfully Managed With Remote Diet, Exercise Intervention
TOPLINE:
A combination of remote, supervised aerobic training, resistance training, and a hypocaloric diet significantly improved cardiovascular risk factors in adults with rheumatoid arthritis (RA) and overweight or obesity.
METHODOLOGY:
- The researchers recruited 24 adults aged 60-80 years with RA who met criteria for overweight or obesity; participants were randomized to a Supervised Weight Loss and Exercise Training (SWET) or Counseling Health as Treatment (CHAT) program for 16 weeks.
- The SWET intervention included remote supervision of aerobic training of 150 minutes/week moderate-to-vigorous intensity, 2 days per week of resistance training, and a hypocaloric diet based on a weight loss goal of 7% of body weight. The CHAT patients served as controls and completed two lifestyle counseling sessions followed by monthly check-ins.
- The primary outcome was change in a composite measure of cardiovascular risk based on metabolic syndrome z-score (MSSc), a continuous weighted score of five metabolic syndrome components: Waist circumference, mean arterial blood pressure, fasting glucose, triglycerides, and high-density lipoprotein cholesterol.
TAKEAWAY:
- Both groups showed improvement in the primary outcome of MSSc, with absolute changes from baseline of −1.67 for the SWET group and −1.34 for the CHAT group (P < .01 for both).
- Participants in the SWET group showed significantly more improvement in secondary outcome measures of body weight, fat mass, and disease activity score in 28 joints based on C-reactive protein (DAS28-CRP), as well as greater improvement in patient-reported physical and mental health, physical function, and fatigue, than those in the CHAT group, but the CHAT group improved significantly compared with their baseline.
- The strongest specific effects for the different components of the intervention were those of aerobic training on physical function and fatigue, resistance training on DAS28-CRP, and weight loss on MSSc.
- Neither group experienced significant changes in lean mass, absolute peak V02, unilateral isometric knee extension, or bilateral grip strength.
IN PRACTICE:
“Findings from our study indicate, at a minimum, integrating even 2 hours of healthy lifestyle counseling may improve RA management, let alone demonstrate the substantial impact that can be provided by a comprehensive, remotely supervised lifestyle intervention,” the researchers wrote.
SOURCE:
The lead author on the study was Brian J. Andonian, MD, of Duke University, Durham, North Carolina. The study was published online in ACR Open Rheumatology.
LIMITATIONS:
The small sample size was a limitation of the study findings, as was the lack of blinding and high level of motivation in the CHAT group, who had greater improvements than expected in weight loss and increased physical activity; the study also was conducted during the COVID-19 pandemic, with potential physical and mental effects on participants who tested positive during the study period.
DISCLOSURES:
The study was supported by the US National Institute of Arthritis and Musculoskeletal and Skin Diseases and the Claude D. Pepper Older Americans Independence Center of the US National Institute on Aging.
A version of this article appeared on Medscape.com.
TOPLINE:
A combination of remote, supervised aerobic training, resistance training, and a hypocaloric diet significantly improved cardiovascular risk factors in adults with rheumatoid arthritis (RA) and overweight or obesity.
METHODOLOGY:
- The researchers recruited 24 adults aged 60-80 years with RA who met criteria for overweight or obesity; participants were randomized to a Supervised Weight Loss and Exercise Training (SWET) or Counseling Health as Treatment (CHAT) program for 16 weeks.
- The SWET intervention included remote supervision of aerobic training of 150 minutes/week moderate-to-vigorous intensity, 2 days per week of resistance training, and a hypocaloric diet based on a weight loss goal of 7% of body weight. The CHAT patients served as controls and completed two lifestyle counseling sessions followed by monthly check-ins.
- The primary outcome was change in a composite measure of cardiovascular risk based on metabolic syndrome z-score (MSSc), a continuous weighted score of five metabolic syndrome components: Waist circumference, mean arterial blood pressure, fasting glucose, triglycerides, and high-density lipoprotein cholesterol.
TAKEAWAY:
- Both groups showed improvement in the primary outcome of MSSc, with absolute changes from baseline of −1.67 for the SWET group and −1.34 for the CHAT group (P < .01 for both).
- Participants in the SWET group showed significantly more improvement in secondary outcome measures of body weight, fat mass, and disease activity score in 28 joints based on C-reactive protein (DAS28-CRP), as well as greater improvement in patient-reported physical and mental health, physical function, and fatigue, than those in the CHAT group, but the CHAT group improved significantly compared with their baseline.
- The strongest specific effects for the different components of the intervention were those of aerobic training on physical function and fatigue, resistance training on DAS28-CRP, and weight loss on MSSc.
- Neither group experienced significant changes in lean mass, absolute peak V02, unilateral isometric knee extension, or bilateral grip strength.
IN PRACTICE:
“Findings from our study indicate, at a minimum, integrating even 2 hours of healthy lifestyle counseling may improve RA management, let alone demonstrate the substantial impact that can be provided by a comprehensive, remotely supervised lifestyle intervention,” the researchers wrote.
SOURCE:
The lead author on the study was Brian J. Andonian, MD, of Duke University, Durham, North Carolina. The study was published online in ACR Open Rheumatology.
LIMITATIONS:
The small sample size was a limitation of the study findings, as was the lack of blinding and high level of motivation in the CHAT group, who had greater improvements than expected in weight loss and increased physical activity; the study also was conducted during the COVID-19 pandemic, with potential physical and mental effects on participants who tested positive during the study period.
DISCLOSURES:
The study was supported by the US National Institute of Arthritis and Musculoskeletal and Skin Diseases and the Claude D. Pepper Older Americans Independence Center of the US National Institute on Aging.
A version of this article appeared on Medscape.com.
TOPLINE:
A combination of remote, supervised aerobic training, resistance training, and a hypocaloric diet significantly improved cardiovascular risk factors in adults with rheumatoid arthritis (RA) and overweight or obesity.
METHODOLOGY:
- The researchers recruited 24 adults aged 60-80 years with RA who met criteria for overweight or obesity; participants were randomized to a Supervised Weight Loss and Exercise Training (SWET) or Counseling Health as Treatment (CHAT) program for 16 weeks.
- The SWET intervention included remote supervision of aerobic training of 150 minutes/week moderate-to-vigorous intensity, 2 days per week of resistance training, and a hypocaloric diet based on a weight loss goal of 7% of body weight. The CHAT patients served as controls and completed two lifestyle counseling sessions followed by monthly check-ins.
- The primary outcome was change in a composite measure of cardiovascular risk based on metabolic syndrome z-score (MSSc), a continuous weighted score of five metabolic syndrome components: Waist circumference, mean arterial blood pressure, fasting glucose, triglycerides, and high-density lipoprotein cholesterol.
TAKEAWAY:
- Both groups showed improvement in the primary outcome of MSSc, with absolute changes from baseline of −1.67 for the SWET group and −1.34 for the CHAT group (P < .01 for both).
- Participants in the SWET group showed significantly more improvement in secondary outcome measures of body weight, fat mass, and disease activity score in 28 joints based on C-reactive protein (DAS28-CRP), as well as greater improvement in patient-reported physical and mental health, physical function, and fatigue, than those in the CHAT group, but the CHAT group improved significantly compared with their baseline.
- The strongest specific effects for the different components of the intervention were those of aerobic training on physical function and fatigue, resistance training on DAS28-CRP, and weight loss on MSSc.
- Neither group experienced significant changes in lean mass, absolute peak V02, unilateral isometric knee extension, or bilateral grip strength.
IN PRACTICE:
“Findings from our study indicate, at a minimum, integrating even 2 hours of healthy lifestyle counseling may improve RA management, let alone demonstrate the substantial impact that can be provided by a comprehensive, remotely supervised lifestyle intervention,” the researchers wrote.
SOURCE:
The lead author on the study was Brian J. Andonian, MD, of Duke University, Durham, North Carolina. The study was published online in ACR Open Rheumatology.
LIMITATIONS:
The small sample size was a limitation of the study findings, as was the lack of blinding and high level of motivation in the CHAT group, who had greater improvements than expected in weight loss and increased physical activity; the study also was conducted during the COVID-19 pandemic, with potential physical and mental effects on participants who tested positive during the study period.
DISCLOSURES:
The study was supported by the US National Institute of Arthritis and Musculoskeletal and Skin Diseases and the Claude D. Pepper Older Americans Independence Center of the US National Institute on Aging.
A version of this article appeared on Medscape.com.
Testosterone Replacement Therapy and Prostate Cancer Risk
TOPLINE:
Testosterone replacement therapy in middle-aged and older men with hypogonadism does not increase the risk for high-grade or any prostate cancer, new data confirmed.
METHODOLOGY:
- Epidemiologic studies have shown inconsistent findings, and clinical trials have not examined prostate safety. As a result, guidelines generally advise against testosterone replacement therapy in men with a history of or increased risk for prostate cancer.
- The current placebo-controlled, double-blind, parallel-group randomized study included 5204 men, ages 45-80, who had two fasting testosterone concentrations < 300 ng/dL, one or more hypogonadal symptoms, and a history of cardiovascular disease or increased . Patients were randomly assigned 1:1 to receive either testosterone replacement therapy or placebo.
- The primary prostate safety endpoint was incident high-grade prostate cancer (Gleason score 4 + 3 or higher).
- Secondary endpoints included incidence of any prostate cancer, acute urinary retention, invasive procedure for , , and new pharmacologic treatment for lower urinary tract symptoms.
TAKEAWAY:
- The incidence of high-grade prostate cancer did not differ significantly between groups. Over a mean follow-up of 33 months, only 0.19% (5 of 2596 participants) in the testosterone replacement therapy group and 0.12% (3 of 2602) in the placebo group were diagnosed with high-grade disease (hazard ratio [HR], 1.62; P = .51).
- The rate of any prostate cancer also did not differ significantly between the testosterone vs placebo groups (0.46% vs 0.42%; HR, 1.07; P = .87).
- The rates of acute urinary retention (0.77% vs 0.61%; HR, 1.25; P = .50), invasive procedures for benign prostatic hyperplasia (0.89% vs 0.46%; HR, 1.91; P = .07), prostate biopsy (0.62% vs 0.54%; HR, 1.13; P = .74), or new treatment for lower urinary tract symptoms (3.89% vs 3.34%; HR, 1.16; P = .32) did not differ significantly between the testosterone vs placebo groups.
- Compared with placebo, testosterone therapy did increase prostate-specific antigen (PSA) levels, but the differences were small and did not increase after 12 months.
IN PRACTICE:
In a population of middle-aged and older men with hypogonadism, “the incidences of high-grade or any prostate cancer and other prostate events were low and did not differ significantly between testosterone- and placebo-treated men,” the authors concluded. “The study’s findings will facilitate a more informed appraisal of the potential prostate risks of testosterone replacement therapy.”
SOURCE:
This study, led by Shalender Bhasin, MB, BS, Brigham and Women’s Hospital, Harvard Medical School, Boston, Massachusetts, was published online in JAMA Network Open.
LIMITATIONS:
These study findings do not apply to men with known prostate cancer, higher PSA values, or those without confirmed hypogonadism. The study design did not include prostate imaging or other biomarker tests after PSA testing, which may have affected the decision to perform a biopsy. Also, the rates of treatment discontinuation and loss to follow-up were high.
DISCLOSURES:
This study was funded by a consortium of testosterone manufacturers led by AbbVie Inc., with additional financial support from Endo Pharmaceuticals, Acerus Pharmaceuticals Corporation, and Upsher-Smith Laboratories. Bhasin, Lincoff, and Khera reported receiving grants and consulting and personal fees from various sources. The remaining authors disclosed no conflicts of interest.
A version of this article appeared on Medscape.com.
TOPLINE:
Testosterone replacement therapy in middle-aged and older men with hypogonadism does not increase the risk for high-grade or any prostate cancer, new data confirmed.
METHODOLOGY:
- Epidemiologic studies have shown inconsistent findings, and clinical trials have not examined prostate safety. As a result, guidelines generally advise against testosterone replacement therapy in men with a history of or increased risk for prostate cancer.
- The current placebo-controlled, double-blind, parallel-group randomized study included 5204 men, ages 45-80, who had two fasting testosterone concentrations < 300 ng/dL, one or more hypogonadal symptoms, and a history of cardiovascular disease or increased . Patients were randomly assigned 1:1 to receive either testosterone replacement therapy or placebo.
- The primary prostate safety endpoint was incident high-grade prostate cancer (Gleason score 4 + 3 or higher).
- Secondary endpoints included incidence of any prostate cancer, acute urinary retention, invasive procedure for , , and new pharmacologic treatment for lower urinary tract symptoms.
TAKEAWAY:
- The incidence of high-grade prostate cancer did not differ significantly between groups. Over a mean follow-up of 33 months, only 0.19% (5 of 2596 participants) in the testosterone replacement therapy group and 0.12% (3 of 2602) in the placebo group were diagnosed with high-grade disease (hazard ratio [HR], 1.62; P = .51).
- The rate of any prostate cancer also did not differ significantly between the testosterone vs placebo groups (0.46% vs 0.42%; HR, 1.07; P = .87).
- The rates of acute urinary retention (0.77% vs 0.61%; HR, 1.25; P = .50), invasive procedures for benign prostatic hyperplasia (0.89% vs 0.46%; HR, 1.91; P = .07), prostate biopsy (0.62% vs 0.54%; HR, 1.13; P = .74), or new treatment for lower urinary tract symptoms (3.89% vs 3.34%; HR, 1.16; P = .32) did not differ significantly between the testosterone vs placebo groups.
- Compared with placebo, testosterone therapy did increase prostate-specific antigen (PSA) levels, but the differences were small and did not increase after 12 months.
IN PRACTICE:
In a population of middle-aged and older men with hypogonadism, “the incidences of high-grade or any prostate cancer and other prostate events were low and did not differ significantly between testosterone- and placebo-treated men,” the authors concluded. “The study’s findings will facilitate a more informed appraisal of the potential prostate risks of testosterone replacement therapy.”
SOURCE:
This study, led by Shalender Bhasin, MB, BS, Brigham and Women’s Hospital, Harvard Medical School, Boston, Massachusetts, was published online in JAMA Network Open.
LIMITATIONS:
These study findings do not apply to men with known prostate cancer, higher PSA values, or those without confirmed hypogonadism. The study design did not include prostate imaging or other biomarker tests after PSA testing, which may have affected the decision to perform a biopsy. Also, the rates of treatment discontinuation and loss to follow-up were high.
DISCLOSURES:
This study was funded by a consortium of testosterone manufacturers led by AbbVie Inc., with additional financial support from Endo Pharmaceuticals, Acerus Pharmaceuticals Corporation, and Upsher-Smith Laboratories. Bhasin, Lincoff, and Khera reported receiving grants and consulting and personal fees from various sources. The remaining authors disclosed no conflicts of interest.
A version of this article appeared on Medscape.com.
TOPLINE:
Testosterone replacement therapy in middle-aged and older men with hypogonadism does not increase the risk for high-grade or any prostate cancer, new data confirmed.
METHODOLOGY:
- Epidemiologic studies have shown inconsistent findings, and clinical trials have not examined prostate safety. As a result, guidelines generally advise against testosterone replacement therapy in men with a history of or increased risk for prostate cancer.
- The current placebo-controlled, double-blind, parallel-group randomized study included 5204 men, ages 45-80, who had two fasting testosterone concentrations < 300 ng/dL, one or more hypogonadal symptoms, and a history of cardiovascular disease or increased . Patients were randomly assigned 1:1 to receive either testosterone replacement therapy or placebo.
- The primary prostate safety endpoint was incident high-grade prostate cancer (Gleason score 4 + 3 or higher).
- Secondary endpoints included incidence of any prostate cancer, acute urinary retention, invasive procedure for , , and new pharmacologic treatment for lower urinary tract symptoms.
TAKEAWAY:
- The incidence of high-grade prostate cancer did not differ significantly between groups. Over a mean follow-up of 33 months, only 0.19% (5 of 2596 participants) in the testosterone replacement therapy group and 0.12% (3 of 2602) in the placebo group were diagnosed with high-grade disease (hazard ratio [HR], 1.62; P = .51).
- The rate of any prostate cancer also did not differ significantly between the testosterone vs placebo groups (0.46% vs 0.42%; HR, 1.07; P = .87).
- The rates of acute urinary retention (0.77% vs 0.61%; HR, 1.25; P = .50), invasive procedures for benign prostatic hyperplasia (0.89% vs 0.46%; HR, 1.91; P = .07), prostate biopsy (0.62% vs 0.54%; HR, 1.13; P = .74), or new treatment for lower urinary tract symptoms (3.89% vs 3.34%; HR, 1.16; P = .32) did not differ significantly between the testosterone vs placebo groups.
- Compared with placebo, testosterone therapy did increase prostate-specific antigen (PSA) levels, but the differences were small and did not increase after 12 months.
IN PRACTICE:
In a population of middle-aged and older men with hypogonadism, “the incidences of high-grade or any prostate cancer and other prostate events were low and did not differ significantly between testosterone- and placebo-treated men,” the authors concluded. “The study’s findings will facilitate a more informed appraisal of the potential prostate risks of testosterone replacement therapy.”
SOURCE:
This study, led by Shalender Bhasin, MB, BS, Brigham and Women’s Hospital, Harvard Medical School, Boston, Massachusetts, was published online in JAMA Network Open.
LIMITATIONS:
These study findings do not apply to men with known prostate cancer, higher PSA values, or those without confirmed hypogonadism. The study design did not include prostate imaging or other biomarker tests after PSA testing, which may have affected the decision to perform a biopsy. Also, the rates of treatment discontinuation and loss to follow-up were high.
DISCLOSURES:
This study was funded by a consortium of testosterone manufacturers led by AbbVie Inc., with additional financial support from Endo Pharmaceuticals, Acerus Pharmaceuticals Corporation, and Upsher-Smith Laboratories. Bhasin, Lincoff, and Khera reported receiving grants and consulting and personal fees from various sources. The remaining authors disclosed no conflicts of interest.
A version of this article appeared on Medscape.com.
Time Off Isn’t Really Off-Time for Most Physicians, Study Finds
About 20% of US physicians took less than 1 week of vacation in the previous year, a new study found. When doctors did go on vacation, 70% reported working on their days off to handle patient-related tasks.
, according to the cross-sectional study, which was published on January 12, 2024, in JAMA Network Open.“It’s important to provide physicians with adequate time to disconnect from work and recharge,” said study coauthor Tait Shanafelt, MD, chief wellness officer at Stanford Medicine, in an interview.
The study’s conclusion that most US physicians work on their days off “is a marker of inadequate staffing, suboptimal teamwork, and poorly designed coverage systems,” he added. “Simply allocating people a number of vacation days is not enough.”
According to Dr. Shanafelt, there’s been little research into vacation’s impact on physician well-being. However, it is clear that work overload and exhaustion are major problems among American physicians. “Inadequate time off may magnify these challenges.”
Research suggests that physicians suffer more burnout than other US workers even after adjusting for confounders, he said. Extensive evidence shows that burnout in physicians contributes to medical errors and erodes quality of care and patient satisfaction, he added.
For the new study, researchers mailed surveys to 3671 members of the American Medical Association from 2020 to 2021, and 1162 (31.7%) responded. Another 6348 (7.1%) responded to an email survey sent to 90,000 physicians. An analysis suggested the respondents were representative of all US practicing physicians.
Among 3024 respondents who responded to a subsurvey about vacations, about 40% took more than 15 days of vacation over the past year, about 40% took 6-15 days, and about 20% took 5 or fewer days.
Fewer than half of physicians said their electronic health record (EHR) inboxes were fully covered by others while they were away. About 70% said they worked while on vacation, with nearly 15% working an hour or more each day.
Emergency physicians were the least likely and anesthesiologists were the most likely to take at least 15 days of vacation per year, according to the study.
Women were more likely than men to work 30 or more minutes a day on vacation. Physicians aged 65 years and older were more likely to take 15 or more days of vacation per year than those under 35 years.
An adjusted analysis linked complete EHR inbox coverage to lower odds of taking time during vacation to work (odds ratio [OR], 0.68; 95% CI, 0.57-0.80).
“For many, difficulty finding clinical coverage, lack of EHR inbox coverage, and returning to an overwhelming backlog of EHR inbox work at were identified as barriers to taking vacation,” Dr. Shanafelt said.
Researchers linked lower rates of burnout to taking more than 3 weeks of vacation per year (OR, 0.59-0.66, depending on time spent; 95% CI, 0.40-0.98) vs none. They also linked less burnout to full EHR inbox coverage while on vacation (OR, 0.74; 95% CI, 0.63-0.88) and more burnout to spending 30 minutes or more on work while on a typical vacation day (OR, 1.58-1.97, depending on time spent; 95% CI, 1.22-2.77).
Study limitations include the low participation rate and lack of insight into causation. It’s not clear how burnout and less vacation time are related and whether one causes the other, Shanafelt said. “It is possible there are a number of interacting factors rather than a simple, linear relationship.”
In an interview, Lazar J. Greenfield, Jr., MD, PhD, professor and chairman of neurology at UConn Health, Farmington, Connecticut, said his department encourages clinicians to plan vacations well ahead of time, and “we make a real strong effort to make sure that people are fully covered and someone has their Epic inbox.”
Dr. Greenfield, who wasn’t involved in the new study, recommended that physicians plan active vacations, so they have less downtime to catch up on work matters. But he acknowledged that stepping away from emails can be difficult, especially when physicians fear pileups of work upon their return or don’t want to annoy patients with tardy responses.
“They have a hard time disengaging from their moral obligations to patients,” he said. “Another issue, particularly in my field of neurology, is that there’s a lot of subspecialties. Finding somebody with the exact subspecialty and expertise to cover a very specific patient population they treat can be really hard.”
The Stanford WellMD Center, Mayo Clinic Department of Medicine Program on Physician Well-being, and American Medical Association funded the study.
Dr. Shanafelt discloses coinventing the Well-Being Index and its derivatives with another study author; Mayo Clinic licensed the Well-Being Index and pays them royalties outside the submitted work. Dr. Shanafelt also reported support for grand rounds, lectures, and advising for healthcare organizations outside the submitted work. Other authors reported personal fees from Marvin Behavioral Health and grants from the National Institute of Nursing Research, National Science Foundation, and Med Ed Solutions.
Dr. Greenfield had no disclosures.
A version of this article appeared on Medscape.com.
About 20% of US physicians took less than 1 week of vacation in the previous year, a new study found. When doctors did go on vacation, 70% reported working on their days off to handle patient-related tasks.
, according to the cross-sectional study, which was published on January 12, 2024, in JAMA Network Open.“It’s important to provide physicians with adequate time to disconnect from work and recharge,” said study coauthor Tait Shanafelt, MD, chief wellness officer at Stanford Medicine, in an interview.
The study’s conclusion that most US physicians work on their days off “is a marker of inadequate staffing, suboptimal teamwork, and poorly designed coverage systems,” he added. “Simply allocating people a number of vacation days is not enough.”
According to Dr. Shanafelt, there’s been little research into vacation’s impact on physician well-being. However, it is clear that work overload and exhaustion are major problems among American physicians. “Inadequate time off may magnify these challenges.”
Research suggests that physicians suffer more burnout than other US workers even after adjusting for confounders, he said. Extensive evidence shows that burnout in physicians contributes to medical errors and erodes quality of care and patient satisfaction, he added.
For the new study, researchers mailed surveys to 3671 members of the American Medical Association from 2020 to 2021, and 1162 (31.7%) responded. Another 6348 (7.1%) responded to an email survey sent to 90,000 physicians. An analysis suggested the respondents were representative of all US practicing physicians.
Among 3024 respondents who responded to a subsurvey about vacations, about 40% took more than 15 days of vacation over the past year, about 40% took 6-15 days, and about 20% took 5 or fewer days.
Fewer than half of physicians said their electronic health record (EHR) inboxes were fully covered by others while they were away. About 70% said they worked while on vacation, with nearly 15% working an hour or more each day.
Emergency physicians were the least likely and anesthesiologists were the most likely to take at least 15 days of vacation per year, according to the study.
Women were more likely than men to work 30 or more minutes a day on vacation. Physicians aged 65 years and older were more likely to take 15 or more days of vacation per year than those under 35 years.
An adjusted analysis linked complete EHR inbox coverage to lower odds of taking time during vacation to work (odds ratio [OR], 0.68; 95% CI, 0.57-0.80).
“For many, difficulty finding clinical coverage, lack of EHR inbox coverage, and returning to an overwhelming backlog of EHR inbox work at were identified as barriers to taking vacation,” Dr. Shanafelt said.
Researchers linked lower rates of burnout to taking more than 3 weeks of vacation per year (OR, 0.59-0.66, depending on time spent; 95% CI, 0.40-0.98) vs none. They also linked less burnout to full EHR inbox coverage while on vacation (OR, 0.74; 95% CI, 0.63-0.88) and more burnout to spending 30 minutes or more on work while on a typical vacation day (OR, 1.58-1.97, depending on time spent; 95% CI, 1.22-2.77).
Study limitations include the low participation rate and lack of insight into causation. It’s not clear how burnout and less vacation time are related and whether one causes the other, Shanafelt said. “It is possible there are a number of interacting factors rather than a simple, linear relationship.”
In an interview, Lazar J. Greenfield, Jr., MD, PhD, professor and chairman of neurology at UConn Health, Farmington, Connecticut, said his department encourages clinicians to plan vacations well ahead of time, and “we make a real strong effort to make sure that people are fully covered and someone has their Epic inbox.”
Dr. Greenfield, who wasn’t involved in the new study, recommended that physicians plan active vacations, so they have less downtime to catch up on work matters. But he acknowledged that stepping away from emails can be difficult, especially when physicians fear pileups of work upon their return or don’t want to annoy patients with tardy responses.
“They have a hard time disengaging from their moral obligations to patients,” he said. “Another issue, particularly in my field of neurology, is that there’s a lot of subspecialties. Finding somebody with the exact subspecialty and expertise to cover a very specific patient population they treat can be really hard.”
The Stanford WellMD Center, Mayo Clinic Department of Medicine Program on Physician Well-being, and American Medical Association funded the study.
Dr. Shanafelt discloses coinventing the Well-Being Index and its derivatives with another study author; Mayo Clinic licensed the Well-Being Index and pays them royalties outside the submitted work. Dr. Shanafelt also reported support for grand rounds, lectures, and advising for healthcare organizations outside the submitted work. Other authors reported personal fees from Marvin Behavioral Health and grants from the National Institute of Nursing Research, National Science Foundation, and Med Ed Solutions.
Dr. Greenfield had no disclosures.
A version of this article appeared on Medscape.com.
About 20% of US physicians took less than 1 week of vacation in the previous year, a new study found. When doctors did go on vacation, 70% reported working on their days off to handle patient-related tasks.
, according to the cross-sectional study, which was published on January 12, 2024, in JAMA Network Open.“It’s important to provide physicians with adequate time to disconnect from work and recharge,” said study coauthor Tait Shanafelt, MD, chief wellness officer at Stanford Medicine, in an interview.
The study’s conclusion that most US physicians work on their days off “is a marker of inadequate staffing, suboptimal teamwork, and poorly designed coverage systems,” he added. “Simply allocating people a number of vacation days is not enough.”
According to Dr. Shanafelt, there’s been little research into vacation’s impact on physician well-being. However, it is clear that work overload and exhaustion are major problems among American physicians. “Inadequate time off may magnify these challenges.”
Research suggests that physicians suffer more burnout than other US workers even after adjusting for confounders, he said. Extensive evidence shows that burnout in physicians contributes to medical errors and erodes quality of care and patient satisfaction, he added.
For the new study, researchers mailed surveys to 3671 members of the American Medical Association from 2020 to 2021, and 1162 (31.7%) responded. Another 6348 (7.1%) responded to an email survey sent to 90,000 physicians. An analysis suggested the respondents were representative of all US practicing physicians.
Among 3024 respondents who responded to a subsurvey about vacations, about 40% took more than 15 days of vacation over the past year, about 40% took 6-15 days, and about 20% took 5 or fewer days.
Fewer than half of physicians said their electronic health record (EHR) inboxes were fully covered by others while they were away. About 70% said they worked while on vacation, with nearly 15% working an hour or more each day.
Emergency physicians were the least likely and anesthesiologists were the most likely to take at least 15 days of vacation per year, according to the study.
Women were more likely than men to work 30 or more minutes a day on vacation. Physicians aged 65 years and older were more likely to take 15 or more days of vacation per year than those under 35 years.
An adjusted analysis linked complete EHR inbox coverage to lower odds of taking time during vacation to work (odds ratio [OR], 0.68; 95% CI, 0.57-0.80).
“For many, difficulty finding clinical coverage, lack of EHR inbox coverage, and returning to an overwhelming backlog of EHR inbox work at were identified as barriers to taking vacation,” Dr. Shanafelt said.
Researchers linked lower rates of burnout to taking more than 3 weeks of vacation per year (OR, 0.59-0.66, depending on time spent; 95% CI, 0.40-0.98) vs none. They also linked less burnout to full EHR inbox coverage while on vacation (OR, 0.74; 95% CI, 0.63-0.88) and more burnout to spending 30 minutes or more on work while on a typical vacation day (OR, 1.58-1.97, depending on time spent; 95% CI, 1.22-2.77).
Study limitations include the low participation rate and lack of insight into causation. It’s not clear how burnout and less vacation time are related and whether one causes the other, Shanafelt said. “It is possible there are a number of interacting factors rather than a simple, linear relationship.”
In an interview, Lazar J. Greenfield, Jr., MD, PhD, professor and chairman of neurology at UConn Health, Farmington, Connecticut, said his department encourages clinicians to plan vacations well ahead of time, and “we make a real strong effort to make sure that people are fully covered and someone has their Epic inbox.”
Dr. Greenfield, who wasn’t involved in the new study, recommended that physicians plan active vacations, so they have less downtime to catch up on work matters. But he acknowledged that stepping away from emails can be difficult, especially when physicians fear pileups of work upon their return or don’t want to annoy patients with tardy responses.
“They have a hard time disengaging from their moral obligations to patients,” he said. “Another issue, particularly in my field of neurology, is that there’s a lot of subspecialties. Finding somebody with the exact subspecialty and expertise to cover a very specific patient population they treat can be really hard.”
The Stanford WellMD Center, Mayo Clinic Department of Medicine Program on Physician Well-being, and American Medical Association funded the study.
Dr. Shanafelt discloses coinventing the Well-Being Index and its derivatives with another study author; Mayo Clinic licensed the Well-Being Index and pays them royalties outside the submitted work. Dr. Shanafelt also reported support for grand rounds, lectures, and advising for healthcare organizations outside the submitted work. Other authors reported personal fees from Marvin Behavioral Health and grants from the National Institute of Nursing Research, National Science Foundation, and Med Ed Solutions.
Dr. Greenfield had no disclosures.
A version of this article appeared on Medscape.com.
FROM JAMA NETWORK OPEN
SUDs rates highest in head, neck, and gastric cancer survivors
.
The association between cancer and substance use is well known, but data on the prevalence of different substance use disorders (SUDs) in different types of cancer are limited, Katie F. Jones, PhD, of the VA Boston Healthcare System, and colleagues, wrote in their paper.
“Substance use and use disorders are on the rise in general and among older adults, who represent the majority of people diagnosed with cancer, and SUDs have significant potential to complicate cancer care and negatively impact cancer outcomes,” corresponding author Devon K. Check, PhD, of Duke University, Durham, N.C., said in an interview. “We thought it was important to understand whether SUDs are more common with certain types of cancer. We can use that information to guide resources toward populations where interventions to integrate SUD treatment and cancer treatment are most needed,” he said. “In addition, because different SUDs (opioid use disorder, alcohol use disorder) might complicate cancer treatment in different ways and necessitate different types of interventions, we thought it was important to understand the distribution of specific disorders,” he explained.
In the cross-sectional study published in JAMA Oncology, the researchers reviewed data from 6,101 adult cancer survivors who participated in the National Survey of Drug Use and Health (NSDUH) between 2015 and 2020.
The study population included survivors of solid tumor cancers. SUD was defined as meeting at least one of four criteria for substance abuse or at least 3 of 6 criteria for dependence based on the Diagnostic and Statistical Manual of Mental Disorders (Fourth Edition) criteria.
Overall, 3.83% of the participants met criteria for SUD. Survivors of head and neck cancers and survivors of gastric and esophageal cancers had the highest rates of SUDs (approximately 9%), followed by cervical cancer and melanoma survivors (approximately 6%).
Alcohol use disorder was the most common SUD both overall (2.8%) and among survivors of head and neck cancers, cervical cancers, and melanoma.
Cannabis use disorder was the most prevalent SUD among esophageal and gastric cancer survivors (approximately 9%).
The prevalence of SUDs overall and within the past year (active) was approximately 4%, but the prevalence of active SUDs was significantly higher for those with head and neck cancers and cervical cancer (18.73% and 15.70%, respectively). However, the distribution of specific SUDs was different in the newly diagnosed patients. Sedative use disorder took the top spot as the most common SUD for head and neck cancer survivors (9.81%), while alcohol use disorder was the most common SUD among cervical cancer survivors (10.49%).
Limitations and Implications
The findings were limited by several factors, including the nature of the study population and the data source, said Dr. Check.
“The average prevalence of SUD (or the prevalence across cancer types) was lower than we might have expected,” but the results make sense given the mainly older and female study population, he said. SUDs are less common among older adults compared with younger adults and among women compared with men, and the study’s data source (NSDUH) has been shown in other research to underestimate the prevalence of opioid use disorder, he added.
“Otherwise, the study findings were generally consistent with what we would expect,” Dr. Check said in an interview. “For example, alcohol use disorder is the most common SUD in the general U.S. population, and that was true for our study population of cancer survivors as well. In addition, SUD prevalence was higher in cancers such as cervical cancer and head and neck cancers that are causally linked to alcohol and/or tobacco use,” he said.
Integrated care is needed
“Among people diagnosed with certain types of cancers, including cervical and head and neck cancers, the estimated prevalence of SUD is similar to those [with] medical comorbidities such as diabetes and cardiopulmonary conditions,” said Dr. Check. “Within the field, there is an increasing emphasis on ensuring that people diagnosed with cancer have access to integrated care for their comorbid medical conditions. Similar efforts for people who concurrently manage cancer and SUD are largely absent but critically needed; these efforts should prioritize cancer populations where SUD prevalence is high,” he said.
Looking ahead, “We need to understand more about the specific challenges that arise at the intersection of cancer and SUD so we can design interventions and programs to better support both patients who concurrently manage cancer and SUD and the clinicians who care for them,” Dr. Check added.
Recognize risk factors
“It is very important to study overall substance use disorders in patients with cancer, because understanding the risks of developing these issues after treatment helps us develop approaches to best support these patients following their cancer therapies,” Henry S. Park, MD, a radiation oncologist at Yale University, New Haven, Connecticut, said in an interview.
The current study findings “are generally consistent with my experience and intuition, but it is still helpful to see the actual data,” said Dr. Park, who was not involved in the study. “This may be partially because of the baseline elevated risk of preexisting SUDs for certain patients from the higher-prevalence disease sites. However, it may also be related to the intense side effects that survivors of some types of cancers, such as head and neck cancer, gastroesophageal cancer, and cervical cancer, may experience soon after treatment, and even chronically long after treatment,” he said.
Individualize risk assessment
“Ultimately, clinicians should be aware that not all patients with cancer are the same, and that the majority do not necessarily develop SUDs,” Dr. Park said in an interview. “We should be careful to treat symptoms appropriately, and not withhold therapies purely because of an elevated risk of developing SUDs. However, there are some patients who are at higher risk of SUDs who will need extra support and care from physicians, advanced practice providers, nutritionists, social workers, psychologists, dietitians, and survivorship clinics, both in the short-term and long-term,” he emphasized.
As for additional research, “more work needs to be done on which particular patients within each disease subset are most likely to develop SUDs,” said Dr. Park. “Most importantly, once we identify our high-risk group as reliably as possible, we will have to study interventions that rely on supporting and partnering with patients to decrease the risk of developing SUDs as much as possible, while adequately treating residual symptoms and quality-of-life effects following cancer treatment,” he said.
The study received no outside funding. Dr. Check disclosed grants from Duke University during the study period and grants from the National Institutes of Health and AstraZeneca unrelated to the current study. Dr. Park had no financial conflicts to disclose.
.
The association between cancer and substance use is well known, but data on the prevalence of different substance use disorders (SUDs) in different types of cancer are limited, Katie F. Jones, PhD, of the VA Boston Healthcare System, and colleagues, wrote in their paper.
“Substance use and use disorders are on the rise in general and among older adults, who represent the majority of people diagnosed with cancer, and SUDs have significant potential to complicate cancer care and negatively impact cancer outcomes,” corresponding author Devon K. Check, PhD, of Duke University, Durham, N.C., said in an interview. “We thought it was important to understand whether SUDs are more common with certain types of cancer. We can use that information to guide resources toward populations where interventions to integrate SUD treatment and cancer treatment are most needed,” he said. “In addition, because different SUDs (opioid use disorder, alcohol use disorder) might complicate cancer treatment in different ways and necessitate different types of interventions, we thought it was important to understand the distribution of specific disorders,” he explained.
In the cross-sectional study published in JAMA Oncology, the researchers reviewed data from 6,101 adult cancer survivors who participated in the National Survey of Drug Use and Health (NSDUH) between 2015 and 2020.
The study population included survivors of solid tumor cancers. SUD was defined as meeting at least one of four criteria for substance abuse or at least 3 of 6 criteria for dependence based on the Diagnostic and Statistical Manual of Mental Disorders (Fourth Edition) criteria.
Overall, 3.83% of the participants met criteria for SUD. Survivors of head and neck cancers and survivors of gastric and esophageal cancers had the highest rates of SUDs (approximately 9%), followed by cervical cancer and melanoma survivors (approximately 6%).
Alcohol use disorder was the most common SUD both overall (2.8%) and among survivors of head and neck cancers, cervical cancers, and melanoma.
Cannabis use disorder was the most prevalent SUD among esophageal and gastric cancer survivors (approximately 9%).
The prevalence of SUDs overall and within the past year (active) was approximately 4%, but the prevalence of active SUDs was significantly higher for those with head and neck cancers and cervical cancer (18.73% and 15.70%, respectively). However, the distribution of specific SUDs was different in the newly diagnosed patients. Sedative use disorder took the top spot as the most common SUD for head and neck cancer survivors (9.81%), while alcohol use disorder was the most common SUD among cervical cancer survivors (10.49%).
Limitations and Implications
The findings were limited by several factors, including the nature of the study population and the data source, said Dr. Check.
“The average prevalence of SUD (or the prevalence across cancer types) was lower than we might have expected,” but the results make sense given the mainly older and female study population, he said. SUDs are less common among older adults compared with younger adults and among women compared with men, and the study’s data source (NSDUH) has been shown in other research to underestimate the prevalence of opioid use disorder, he added.
“Otherwise, the study findings were generally consistent with what we would expect,” Dr. Check said in an interview. “For example, alcohol use disorder is the most common SUD in the general U.S. population, and that was true for our study population of cancer survivors as well. In addition, SUD prevalence was higher in cancers such as cervical cancer and head and neck cancers that are causally linked to alcohol and/or tobacco use,” he said.
Integrated care is needed
“Among people diagnosed with certain types of cancers, including cervical and head and neck cancers, the estimated prevalence of SUD is similar to those [with] medical comorbidities such as diabetes and cardiopulmonary conditions,” said Dr. Check. “Within the field, there is an increasing emphasis on ensuring that people diagnosed with cancer have access to integrated care for their comorbid medical conditions. Similar efforts for people who concurrently manage cancer and SUD are largely absent but critically needed; these efforts should prioritize cancer populations where SUD prevalence is high,” he said.
Looking ahead, “We need to understand more about the specific challenges that arise at the intersection of cancer and SUD so we can design interventions and programs to better support both patients who concurrently manage cancer and SUD and the clinicians who care for them,” Dr. Check added.
Recognize risk factors
“It is very important to study overall substance use disorders in patients with cancer, because understanding the risks of developing these issues after treatment helps us develop approaches to best support these patients following their cancer therapies,” Henry S. Park, MD, a radiation oncologist at Yale University, New Haven, Connecticut, said in an interview.
The current study findings “are generally consistent with my experience and intuition, but it is still helpful to see the actual data,” said Dr. Park, who was not involved in the study. “This may be partially because of the baseline elevated risk of preexisting SUDs for certain patients from the higher-prevalence disease sites. However, it may also be related to the intense side effects that survivors of some types of cancers, such as head and neck cancer, gastroesophageal cancer, and cervical cancer, may experience soon after treatment, and even chronically long after treatment,” he said.
Individualize risk assessment
“Ultimately, clinicians should be aware that not all patients with cancer are the same, and that the majority do not necessarily develop SUDs,” Dr. Park said in an interview. “We should be careful to treat symptoms appropriately, and not withhold therapies purely because of an elevated risk of developing SUDs. However, there are some patients who are at higher risk of SUDs who will need extra support and care from physicians, advanced practice providers, nutritionists, social workers, psychologists, dietitians, and survivorship clinics, both in the short-term and long-term,” he emphasized.
As for additional research, “more work needs to be done on which particular patients within each disease subset are most likely to develop SUDs,” said Dr. Park. “Most importantly, once we identify our high-risk group as reliably as possible, we will have to study interventions that rely on supporting and partnering with patients to decrease the risk of developing SUDs as much as possible, while adequately treating residual symptoms and quality-of-life effects following cancer treatment,” he said.
The study received no outside funding. Dr. Check disclosed grants from Duke University during the study period and grants from the National Institutes of Health and AstraZeneca unrelated to the current study. Dr. Park had no financial conflicts to disclose.
.
The association between cancer and substance use is well known, but data on the prevalence of different substance use disorders (SUDs) in different types of cancer are limited, Katie F. Jones, PhD, of the VA Boston Healthcare System, and colleagues, wrote in their paper.
“Substance use and use disorders are on the rise in general and among older adults, who represent the majority of people diagnosed with cancer, and SUDs have significant potential to complicate cancer care and negatively impact cancer outcomes,” corresponding author Devon K. Check, PhD, of Duke University, Durham, N.C., said in an interview. “We thought it was important to understand whether SUDs are more common with certain types of cancer. We can use that information to guide resources toward populations where interventions to integrate SUD treatment and cancer treatment are most needed,” he said. “In addition, because different SUDs (opioid use disorder, alcohol use disorder) might complicate cancer treatment in different ways and necessitate different types of interventions, we thought it was important to understand the distribution of specific disorders,” he explained.
In the cross-sectional study published in JAMA Oncology, the researchers reviewed data from 6,101 adult cancer survivors who participated in the National Survey of Drug Use and Health (NSDUH) between 2015 and 2020.
The study population included survivors of solid tumor cancers. SUD was defined as meeting at least one of four criteria for substance abuse or at least 3 of 6 criteria for dependence based on the Diagnostic and Statistical Manual of Mental Disorders (Fourth Edition) criteria.
Overall, 3.83% of the participants met criteria for SUD. Survivors of head and neck cancers and survivors of gastric and esophageal cancers had the highest rates of SUDs (approximately 9%), followed by cervical cancer and melanoma survivors (approximately 6%).
Alcohol use disorder was the most common SUD both overall (2.8%) and among survivors of head and neck cancers, cervical cancers, and melanoma.
Cannabis use disorder was the most prevalent SUD among esophageal and gastric cancer survivors (approximately 9%).
The prevalence of SUDs overall and within the past year (active) was approximately 4%, but the prevalence of active SUDs was significantly higher for those with head and neck cancers and cervical cancer (18.73% and 15.70%, respectively). However, the distribution of specific SUDs was different in the newly diagnosed patients. Sedative use disorder took the top spot as the most common SUD for head and neck cancer survivors (9.81%), while alcohol use disorder was the most common SUD among cervical cancer survivors (10.49%).
Limitations and Implications
The findings were limited by several factors, including the nature of the study population and the data source, said Dr. Check.
“The average prevalence of SUD (or the prevalence across cancer types) was lower than we might have expected,” but the results make sense given the mainly older and female study population, he said. SUDs are less common among older adults compared with younger adults and among women compared with men, and the study’s data source (NSDUH) has been shown in other research to underestimate the prevalence of opioid use disorder, he added.
“Otherwise, the study findings were generally consistent with what we would expect,” Dr. Check said in an interview. “For example, alcohol use disorder is the most common SUD in the general U.S. population, and that was true for our study population of cancer survivors as well. In addition, SUD prevalence was higher in cancers such as cervical cancer and head and neck cancers that are causally linked to alcohol and/or tobacco use,” he said.
Integrated care is needed
“Among people diagnosed with certain types of cancers, including cervical and head and neck cancers, the estimated prevalence of SUD is similar to those [with] medical comorbidities such as diabetes and cardiopulmonary conditions,” said Dr. Check. “Within the field, there is an increasing emphasis on ensuring that people diagnosed with cancer have access to integrated care for their comorbid medical conditions. Similar efforts for people who concurrently manage cancer and SUD are largely absent but critically needed; these efforts should prioritize cancer populations where SUD prevalence is high,” he said.
Looking ahead, “We need to understand more about the specific challenges that arise at the intersection of cancer and SUD so we can design interventions and programs to better support both patients who concurrently manage cancer and SUD and the clinicians who care for them,” Dr. Check added.
Recognize risk factors
“It is very important to study overall substance use disorders in patients with cancer, because understanding the risks of developing these issues after treatment helps us develop approaches to best support these patients following their cancer therapies,” Henry S. Park, MD, a radiation oncologist at Yale University, New Haven, Connecticut, said in an interview.
The current study findings “are generally consistent with my experience and intuition, but it is still helpful to see the actual data,” said Dr. Park, who was not involved in the study. “This may be partially because of the baseline elevated risk of preexisting SUDs for certain patients from the higher-prevalence disease sites. However, it may also be related to the intense side effects that survivors of some types of cancers, such as head and neck cancer, gastroesophageal cancer, and cervical cancer, may experience soon after treatment, and even chronically long after treatment,” he said.
Individualize risk assessment
“Ultimately, clinicians should be aware that not all patients with cancer are the same, and that the majority do not necessarily develop SUDs,” Dr. Park said in an interview. “We should be careful to treat symptoms appropriately, and not withhold therapies purely because of an elevated risk of developing SUDs. However, there are some patients who are at higher risk of SUDs who will need extra support and care from physicians, advanced practice providers, nutritionists, social workers, psychologists, dietitians, and survivorship clinics, both in the short-term and long-term,” he emphasized.
As for additional research, “more work needs to be done on which particular patients within each disease subset are most likely to develop SUDs,” said Dr. Park. “Most importantly, once we identify our high-risk group as reliably as possible, we will have to study interventions that rely on supporting and partnering with patients to decrease the risk of developing SUDs as much as possible, while adequately treating residual symptoms and quality-of-life effects following cancer treatment,” he said.
The study received no outside funding. Dr. Check disclosed grants from Duke University during the study period and grants from the National Institutes of Health and AstraZeneca unrelated to the current study. Dr. Park had no financial conflicts to disclose.
FROM JAMA ONCOLOGY
Paget Disease of the Bone Progression Halted With Genetic Screening, Targeted Treatment
Prophylactic treatment with zoledronic acid (ZA) in individuals at high genetic risk for Paget disease of the bone (PDB) can prevent the development or progression of the condition, according to a new study. The authors argued that the positive results from the trial suggest that individuals with a familial history of PDB should undergo genetic screening.
“If it’s positive, you should be able to have a bone scan and take it from there,” senior author Stuart Ralston, MBChB, MD, professor of rheumatology at the University of Edinburgh (Scotland), said in an interview.
PDB is a chronic skeletal growth disorder that affects an estimated 1-3 million people in the United States and is most prevalent in individuals over 65 years old. Symptoms of the disease may not present until later stages when there is already skeletal damage that cannot be resolved by medications. Earlier intervention in individuals who have not yet shown signs of the condition could potentially halt disease progression, Dr. Ralston said.
Genetics plays a substantial role in PDB, especially pathogenic variants of the gene SQSTM1. An estimated 40%-50% of people with a familial history of PDB have these variants, according to the study, which are associated with earlier PDB onset and more severe disease.
However, it was unclear if early interventions in these higher-risk individuals may result in better health outcomes.
In this new study, published on December 20, 2023, in Annals of the Rheumatic Diseases, researchers recruited participants through family members already diagnosed with PDB who received treatment at outpatient clinics. Over 1400 individuals with PDB underwent genetic testing for pathogenic SQSTM1 variants. If they tested positive, their first-degree relatives — primarily children — were offered the same genetic test. In total, 350 relatives tested positive for these pathogenic SQSTM1 variants, and of these individuals, 222 agreed to participate in the trial.
At the beginning of the study, all participants received a radionuclide bone scan to screen for bone lesions. They also underwent testing for the bone resorption marker type I collagen C-terminal telopeptides (CTX) and the bone formation marker procollagen type I amino-terminal propeptide (P1NP).
Participants were then randomized to receive either a single intravenous infusion of 5 mg of ZA or placebo treatment. Researchers followed up with participants annually for a median of 84 months (7 years), and then baseline assessments were repeated.
A total of 90 individuals in the ZA treatment group and 90 individuals in the placebo group completed the trial.
Participants were, on average, 50 years old at the beginning of the study. In the ZA group, nine individuals had lesions detected in bone scans at baseline, compared with just one at the study’s end. In the placebo group, 12 individuals had detectable lesions at baseline, compared with 11 individuals at the study’s end.
While the proportion of individuals with lesions was similar between the two groups, there were about twice as many lesions overall in the placebo group, compared with the ZA group (29 vs 15), which researchers said was by chance. All but two lesions disappeared in the ZA group, compared with 26 lesions remaining in the placebo group (P < .0001).
“The bone scan reversal of abnormalities was amazing,” said Ralston, where eight of nine patients with lesions in the ZA group “had their bone scan evidence completely wiped out,” he said. “That’s a very powerful result.”
Both CTX and P1NP concentrations fell in the ZA group at 12 months and remained significantly lower than the placebo group throughout the study (P < .0001 for each).
Overall, the researchers reported that eight individuals in the placebo group and no individuals in the ZA group had a poor outcome, defined as new bone lesions or lesions that were unchanged or progressed (odds ratio, 0.08; P = .003). Two individuals in the placebo group developed lesions during the study, compared with none in the ZA group, but this difference was not statistically significant.
Importantly, there were no differences in adverse events between the two groups.
While only a small number of people in the study had legions — around 9% of participants — the effect of ZA is “dramatic,” Linda A. Russell, MD, director of the Osteoporosis and Metabolic Bone Health Center at the Hospital for Special Surgery in New York City, told this news organization.
While clinicians primarily diagnose PDB with X-rays or an alkaline phosphatase blood test, testing for SQSTM1 is a new way to understand if someone is at higher risk for the disease, she said.
“Now, it seems like [the test] is fairly easily available, so probably it’s something we can begin to incorporate into our armamentarium,” Dr. Russell said.
Individuals who test positive for pathogenic variants of SQSTM1 could then get a bone scan, while those who tested negative may not need any additional testing, she added.
Dr. Ralston and coauthors noted that the effect size shown in this study is similar to that of studies examining adjuvant bisphosphonate therapy for postmenopausal women with early breast cancer. That practice, they write, is now a part of the standard of care.
“We believe that a similar approach is now justified in people with a family history of PDB who test positive for SQSTM1 mutations,” they wrote.
However, it is not clear if all individuals with pathogenic SQSTM1 should receive ZA treatment or if treatment should be given to only those with bone lesions.
“Future research to gather the views of people with a family history of PDB will help to inform the most appropriate way forward,” the authors wrote.
The UK Medical Research Council and Arthritis Research UK funded the trial. Zoledronic acid and a placebo were supplied by Novartis. Dr. Ralston reported funding to his institution from Kyowa Kirin, UCB, the Paget’s Association, and the Royal Osteoporosis Society. Some coauthors reported financial relationships with pharmaceutical companies outside the trial. Dr. Russell had no relevant financial relationships.
A version of this article appeared on Medscape.com.
Prophylactic treatment with zoledronic acid (ZA) in individuals at high genetic risk for Paget disease of the bone (PDB) can prevent the development or progression of the condition, according to a new study. The authors argued that the positive results from the trial suggest that individuals with a familial history of PDB should undergo genetic screening.
“If it’s positive, you should be able to have a bone scan and take it from there,” senior author Stuart Ralston, MBChB, MD, professor of rheumatology at the University of Edinburgh (Scotland), said in an interview.
PDB is a chronic skeletal growth disorder that affects an estimated 1-3 million people in the United States and is most prevalent in individuals over 65 years old. Symptoms of the disease may not present until later stages when there is already skeletal damage that cannot be resolved by medications. Earlier intervention in individuals who have not yet shown signs of the condition could potentially halt disease progression, Dr. Ralston said.
Genetics plays a substantial role in PDB, especially pathogenic variants of the gene SQSTM1. An estimated 40%-50% of people with a familial history of PDB have these variants, according to the study, which are associated with earlier PDB onset and more severe disease.
However, it was unclear if early interventions in these higher-risk individuals may result in better health outcomes.
In this new study, published on December 20, 2023, in Annals of the Rheumatic Diseases, researchers recruited participants through family members already diagnosed with PDB who received treatment at outpatient clinics. Over 1400 individuals with PDB underwent genetic testing for pathogenic SQSTM1 variants. If they tested positive, their first-degree relatives — primarily children — were offered the same genetic test. In total, 350 relatives tested positive for these pathogenic SQSTM1 variants, and of these individuals, 222 agreed to participate in the trial.
At the beginning of the study, all participants received a radionuclide bone scan to screen for bone lesions. They also underwent testing for the bone resorption marker type I collagen C-terminal telopeptides (CTX) and the bone formation marker procollagen type I amino-terminal propeptide (P1NP).
Participants were then randomized to receive either a single intravenous infusion of 5 mg of ZA or placebo treatment. Researchers followed up with participants annually for a median of 84 months (7 years), and then baseline assessments were repeated.
A total of 90 individuals in the ZA treatment group and 90 individuals in the placebo group completed the trial.
Participants were, on average, 50 years old at the beginning of the study. In the ZA group, nine individuals had lesions detected in bone scans at baseline, compared with just one at the study’s end. In the placebo group, 12 individuals had detectable lesions at baseline, compared with 11 individuals at the study’s end.
While the proportion of individuals with lesions was similar between the two groups, there were about twice as many lesions overall in the placebo group, compared with the ZA group (29 vs 15), which researchers said was by chance. All but two lesions disappeared in the ZA group, compared with 26 lesions remaining in the placebo group (P < .0001).
“The bone scan reversal of abnormalities was amazing,” said Ralston, where eight of nine patients with lesions in the ZA group “had their bone scan evidence completely wiped out,” he said. “That’s a very powerful result.”
Both CTX and P1NP concentrations fell in the ZA group at 12 months and remained significantly lower than the placebo group throughout the study (P < .0001 for each).
Overall, the researchers reported that eight individuals in the placebo group and no individuals in the ZA group had a poor outcome, defined as new bone lesions or lesions that were unchanged or progressed (odds ratio, 0.08; P = .003). Two individuals in the placebo group developed lesions during the study, compared with none in the ZA group, but this difference was not statistically significant.
Importantly, there were no differences in adverse events between the two groups.
While only a small number of people in the study had legions — around 9% of participants — the effect of ZA is “dramatic,” Linda A. Russell, MD, director of the Osteoporosis and Metabolic Bone Health Center at the Hospital for Special Surgery in New York City, told this news organization.
While clinicians primarily diagnose PDB with X-rays or an alkaline phosphatase blood test, testing for SQSTM1 is a new way to understand if someone is at higher risk for the disease, she said.
“Now, it seems like [the test] is fairly easily available, so probably it’s something we can begin to incorporate into our armamentarium,” Dr. Russell said.
Individuals who test positive for pathogenic variants of SQSTM1 could then get a bone scan, while those who tested negative may not need any additional testing, she added.
Dr. Ralston and coauthors noted that the effect size shown in this study is similar to that of studies examining adjuvant bisphosphonate therapy for postmenopausal women with early breast cancer. That practice, they write, is now a part of the standard of care.
“We believe that a similar approach is now justified in people with a family history of PDB who test positive for SQSTM1 mutations,” they wrote.
However, it is not clear if all individuals with pathogenic SQSTM1 should receive ZA treatment or if treatment should be given to only those with bone lesions.
“Future research to gather the views of people with a family history of PDB will help to inform the most appropriate way forward,” the authors wrote.
The UK Medical Research Council and Arthritis Research UK funded the trial. Zoledronic acid and a placebo were supplied by Novartis. Dr. Ralston reported funding to his institution from Kyowa Kirin, UCB, the Paget’s Association, and the Royal Osteoporosis Society. Some coauthors reported financial relationships with pharmaceutical companies outside the trial. Dr. Russell had no relevant financial relationships.
A version of this article appeared on Medscape.com.
Prophylactic treatment with zoledronic acid (ZA) in individuals at high genetic risk for Paget disease of the bone (PDB) can prevent the development or progression of the condition, according to a new study. The authors argued that the positive results from the trial suggest that individuals with a familial history of PDB should undergo genetic screening.
“If it’s positive, you should be able to have a bone scan and take it from there,” senior author Stuart Ralston, MBChB, MD, professor of rheumatology at the University of Edinburgh (Scotland), said in an interview.
PDB is a chronic skeletal growth disorder that affects an estimated 1-3 million people in the United States and is most prevalent in individuals over 65 years old. Symptoms of the disease may not present until later stages when there is already skeletal damage that cannot be resolved by medications. Earlier intervention in individuals who have not yet shown signs of the condition could potentially halt disease progression, Dr. Ralston said.
Genetics plays a substantial role in PDB, especially pathogenic variants of the gene SQSTM1. An estimated 40%-50% of people with a familial history of PDB have these variants, according to the study, which are associated with earlier PDB onset and more severe disease.
However, it was unclear if early interventions in these higher-risk individuals may result in better health outcomes.
In this new study, published on December 20, 2023, in Annals of the Rheumatic Diseases, researchers recruited participants through family members already diagnosed with PDB who received treatment at outpatient clinics. Over 1400 individuals with PDB underwent genetic testing for pathogenic SQSTM1 variants. If they tested positive, their first-degree relatives — primarily children — were offered the same genetic test. In total, 350 relatives tested positive for these pathogenic SQSTM1 variants, and of these individuals, 222 agreed to participate in the trial.
At the beginning of the study, all participants received a radionuclide bone scan to screen for bone lesions. They also underwent testing for the bone resorption marker type I collagen C-terminal telopeptides (CTX) and the bone formation marker procollagen type I amino-terminal propeptide (P1NP).
Participants were then randomized to receive either a single intravenous infusion of 5 mg of ZA or placebo treatment. Researchers followed up with participants annually for a median of 84 months (7 years), and then baseline assessments were repeated.
A total of 90 individuals in the ZA treatment group and 90 individuals in the placebo group completed the trial.
Participants were, on average, 50 years old at the beginning of the study. In the ZA group, nine individuals had lesions detected in bone scans at baseline, compared with just one at the study’s end. In the placebo group, 12 individuals had detectable lesions at baseline, compared with 11 individuals at the study’s end.
While the proportion of individuals with lesions was similar between the two groups, there were about twice as many lesions overall in the placebo group, compared with the ZA group (29 vs 15), which researchers said was by chance. All but two lesions disappeared in the ZA group, compared with 26 lesions remaining in the placebo group (P < .0001).
“The bone scan reversal of abnormalities was amazing,” said Ralston, where eight of nine patients with lesions in the ZA group “had their bone scan evidence completely wiped out,” he said. “That’s a very powerful result.”
Both CTX and P1NP concentrations fell in the ZA group at 12 months and remained significantly lower than the placebo group throughout the study (P < .0001 for each).
Overall, the researchers reported that eight individuals in the placebo group and no individuals in the ZA group had a poor outcome, defined as new bone lesions or lesions that were unchanged or progressed (odds ratio, 0.08; P = .003). Two individuals in the placebo group developed lesions during the study, compared with none in the ZA group, but this difference was not statistically significant.
Importantly, there were no differences in adverse events between the two groups.
While only a small number of people in the study had legions — around 9% of participants — the effect of ZA is “dramatic,” Linda A. Russell, MD, director of the Osteoporosis and Metabolic Bone Health Center at the Hospital for Special Surgery in New York City, told this news organization.
While clinicians primarily diagnose PDB with X-rays or an alkaline phosphatase blood test, testing for SQSTM1 is a new way to understand if someone is at higher risk for the disease, she said.
“Now, it seems like [the test] is fairly easily available, so probably it’s something we can begin to incorporate into our armamentarium,” Dr. Russell said.
Individuals who test positive for pathogenic variants of SQSTM1 could then get a bone scan, while those who tested negative may not need any additional testing, she added.
Dr. Ralston and coauthors noted that the effect size shown in this study is similar to that of studies examining adjuvant bisphosphonate therapy for postmenopausal women with early breast cancer. That practice, they write, is now a part of the standard of care.
“We believe that a similar approach is now justified in people with a family history of PDB who test positive for SQSTM1 mutations,” they wrote.
However, it is not clear if all individuals with pathogenic SQSTM1 should receive ZA treatment or if treatment should be given to only those with bone lesions.
“Future research to gather the views of people with a family history of PDB will help to inform the most appropriate way forward,” the authors wrote.
The UK Medical Research Council and Arthritis Research UK funded the trial. Zoledronic acid and a placebo were supplied by Novartis. Dr. Ralston reported funding to his institution from Kyowa Kirin, UCB, the Paget’s Association, and the Royal Osteoporosis Society. Some coauthors reported financial relationships with pharmaceutical companies outside the trial. Dr. Russell had no relevant financial relationships.
A version of this article appeared on Medscape.com.
FROM ANNALS OF THE RHEUMATIC DISEASES
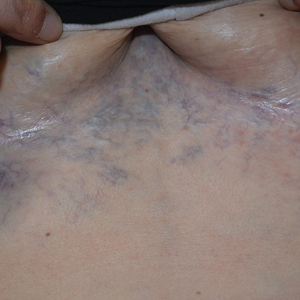
Ectatic Vessels on the Chest
The Diagnosis: Superior Vena Cava Syndrome
Computed tomography angiography of the chest confirmed a diagnosis of superior vena cava (SVC) syndrome due to external pressure of the indwelling catheter. Upon diagnosis, the left indwelling catheter was removed. Further testing to assess for a potential pulmonary embolism was negative. Resolution of the ectatic spider veins and patientreported intermittent facial swelling was achieved after catheter removal.
Superior vena cava syndrome occurs when the SVC is occluded due to extrinsic pressure or thrombosis. Although classically thought to be due to underlying bronchogenic carcinomas, all pathologies that cause compression of the SVC also can lead to vessel occlusion.1 Superior vena cava syndrome initially can be detected on physical examination. The most prominent skin finding includes diffusely dilated blood vessels on the central chest wall, which indicate the presence of collateral blood vessels.1 Imaging studies such as abdominal computed tomography can provide information on the etiology of the condition but are not required for diagnosis. Given the high correlation of SVC syndrome with underlying lung and mediastinal carcinomas, imaging was warranted in our patient. Imaging also can distinguish if the condition is due to external pressure or thrombosis.2 For SVC syndrome due to thrombosis, endovascular therapy is first-line management; however, mechanical thrombectomy may be preferred in patients with absolute contraindication to thrombolytic agents.3 In the setting of increased external pressure on the SVC, treatment includes the removal of the source of pressure.4
In a case series including 78 patients, ports and indwelling catheters accounted for 71% of benign SVC cases.5 Our patient’s SVC syndrome most likely was due to the indwelling catheter pressing on the SVC. The goal of treatment is to address the underlying cause—whether it be pressure or thrombosis. In the setting of increased external pressure, treatment includes removal of the source of pressure from the SVC.4
Other differential diagnoses to consider for newonset ectatic vessels on the chest wall include generalized essential telangiectasia, scleroderma, poikiloderma vasculare atrophicans, and caput medusae. Generalized essential telangiectasia is characterized by red or pink dilated capillary blood vessels in a branch or lacelike pattern predominantly on the lower limbs. The eruption primarily is asymptomatic, though tingling or numbness may be reported.6 The diagnosis can be made with a punch biopsy, with histopathology showing dilated vessels in the dermis.7
Scleroderma is a connective tissue fibrosis disorder with variable clinical presentations. The systemic sclerosis subset can be divided into localized systemic sclerosis and diffuse systemic sclerosis. Physical examination reveals cutaneous sclerosis in various areas of the body. Localized systemic sclerosis includes sclerosis of the fingers and face, while diffuse systemic sclerosis is notable for progression to the arms, legs, and trunk.8 In addition to sclerosis, diffuse telangiectases also can be observed. Systemic sclerosis is a clinical diagnosis based on physical examination and laboratory studies to identify antibodies such as antinuclear antibodies.
Poikiloderma vasculare atrophicans is a variant of cutaneous T-cell lymphoma. The initial presentation is characterized by plaques of hypopigmentation and hyperpigmentation with atrophy and telangiectases. The lesions may be asymptomatic or mildly pruritic and classically involve the trunk and flexural areas.9 The diagnosis is made with skin biopsy and immunohistochemical studies, with findings reflective of mycosis fungoides.
Caput medusae (palm tree sign) is a cardinal feature of portal hypertension characterized by grossly dilated and engorged periumbilical veins. To shunt blood from the portal venous system, cutaneous collateral veins between the umbilical veins and abdominal wall veins are used, resulting in the appearance of engorged veins in the anterior abdominal wall.10 The diagnosis can be made with abdominal ultrasonography showing the direction of blood flow through abdominal vessels.
- Drouin L, Pistorius MA, Lafforgue A, et al. Upper-extremity venous thrombosis: a retrospective study about 160 cases [in French]. Rev Med Interne. 2019;40:9-15.
- Richie E. Clinical pearl: diagnosing superior vena cava syndrome. Emergency Medicine News. 2017;39:22. doi:10.1097/01 .EEM.0000522220.37441.d2
- Azizi A, Shafi I, Shah N, et al. Superior vena cava syndrome. JACC Cardiovasc Interv. 2020;13:2896-2910. doi:10.1016/j.jcin.2020.08.038
- Dumantepe M, Tarhan A, Ozler A. Successful treatment of central venous catheter induced superior vena cava syndrome with ultrasound accelerated catheter-directed thrombolysis. Catheter Cardiovasc Interv. 2013;81:E269-E273.
- Rice TW, Rodriguez RM, Light RW. The superior vena cava syndrome: clinical characteristics and evolving etiology. Medicine (Baltimore) 2006;85:37-42. doi:10.1097/01.md.0000198474.99876.f0
- Long D, Marshman G. Generalized essential telangiectasia. Australas J Dermatol. 2004;45:67-69. doi:10.1111/j.1440-0960.2004.00033.x
- Braverman IM. Ultrastructure and organization of the cutaneous microvasculature in normal and pathologic states. J Invest Dermatol. 1989;93(2 suppl):2S-9S.
- Ferreli C, Gasparini G, Parodi A, et al. Cutaneous manifestations of scleroderma and scleroderma-like disorders: a comprehensive review. Clin Rev Allergy Immunol. 2017;53:306-336. doi:10.1007 /s12016-017-8625-4
- Bloom B, Marchbein S, Fischer M, et al. Poikilodermatous mycosis fungoides. Dermatol Online J. 2012;18:4.
- Sharma B, Raina S. Caput medusae. Indian J Med Res. 2015;141:494. doi:10.4103/0971-5916.159322
The Diagnosis: Superior Vena Cava Syndrome
Computed tomography angiography of the chest confirmed a diagnosis of superior vena cava (SVC) syndrome due to external pressure of the indwelling catheter. Upon diagnosis, the left indwelling catheter was removed. Further testing to assess for a potential pulmonary embolism was negative. Resolution of the ectatic spider veins and patientreported intermittent facial swelling was achieved after catheter removal.
Superior vena cava syndrome occurs when the SVC is occluded due to extrinsic pressure or thrombosis. Although classically thought to be due to underlying bronchogenic carcinomas, all pathologies that cause compression of the SVC also can lead to vessel occlusion.1 Superior vena cava syndrome initially can be detected on physical examination. The most prominent skin finding includes diffusely dilated blood vessels on the central chest wall, which indicate the presence of collateral blood vessels.1 Imaging studies such as abdominal computed tomography can provide information on the etiology of the condition but are not required for diagnosis. Given the high correlation of SVC syndrome with underlying lung and mediastinal carcinomas, imaging was warranted in our patient. Imaging also can distinguish if the condition is due to external pressure or thrombosis.2 For SVC syndrome due to thrombosis, endovascular therapy is first-line management; however, mechanical thrombectomy may be preferred in patients with absolute contraindication to thrombolytic agents.3 In the setting of increased external pressure on the SVC, treatment includes the removal of the source of pressure.4
In a case series including 78 patients, ports and indwelling catheters accounted for 71% of benign SVC cases.5 Our patient’s SVC syndrome most likely was due to the indwelling catheter pressing on the SVC. The goal of treatment is to address the underlying cause—whether it be pressure or thrombosis. In the setting of increased external pressure, treatment includes removal of the source of pressure from the SVC.4
Other differential diagnoses to consider for newonset ectatic vessels on the chest wall include generalized essential telangiectasia, scleroderma, poikiloderma vasculare atrophicans, and caput medusae. Generalized essential telangiectasia is characterized by red or pink dilated capillary blood vessels in a branch or lacelike pattern predominantly on the lower limbs. The eruption primarily is asymptomatic, though tingling or numbness may be reported.6 The diagnosis can be made with a punch biopsy, with histopathology showing dilated vessels in the dermis.7
Scleroderma is a connective tissue fibrosis disorder with variable clinical presentations. The systemic sclerosis subset can be divided into localized systemic sclerosis and diffuse systemic sclerosis. Physical examination reveals cutaneous sclerosis in various areas of the body. Localized systemic sclerosis includes sclerosis of the fingers and face, while diffuse systemic sclerosis is notable for progression to the arms, legs, and trunk.8 In addition to sclerosis, diffuse telangiectases also can be observed. Systemic sclerosis is a clinical diagnosis based on physical examination and laboratory studies to identify antibodies such as antinuclear antibodies.
Poikiloderma vasculare atrophicans is a variant of cutaneous T-cell lymphoma. The initial presentation is characterized by plaques of hypopigmentation and hyperpigmentation with atrophy and telangiectases. The lesions may be asymptomatic or mildly pruritic and classically involve the trunk and flexural areas.9 The diagnosis is made with skin biopsy and immunohistochemical studies, with findings reflective of mycosis fungoides.
Caput medusae (palm tree sign) is a cardinal feature of portal hypertension characterized by grossly dilated and engorged periumbilical veins. To shunt blood from the portal venous system, cutaneous collateral veins between the umbilical veins and abdominal wall veins are used, resulting in the appearance of engorged veins in the anterior abdominal wall.10 The diagnosis can be made with abdominal ultrasonography showing the direction of blood flow through abdominal vessels.
The Diagnosis: Superior Vena Cava Syndrome
Computed tomography angiography of the chest confirmed a diagnosis of superior vena cava (SVC) syndrome due to external pressure of the indwelling catheter. Upon diagnosis, the left indwelling catheter was removed. Further testing to assess for a potential pulmonary embolism was negative. Resolution of the ectatic spider veins and patientreported intermittent facial swelling was achieved after catheter removal.
Superior vena cava syndrome occurs when the SVC is occluded due to extrinsic pressure or thrombosis. Although classically thought to be due to underlying bronchogenic carcinomas, all pathologies that cause compression of the SVC also can lead to vessel occlusion.1 Superior vena cava syndrome initially can be detected on physical examination. The most prominent skin finding includes diffusely dilated blood vessels on the central chest wall, which indicate the presence of collateral blood vessels.1 Imaging studies such as abdominal computed tomography can provide information on the etiology of the condition but are not required for diagnosis. Given the high correlation of SVC syndrome with underlying lung and mediastinal carcinomas, imaging was warranted in our patient. Imaging also can distinguish if the condition is due to external pressure or thrombosis.2 For SVC syndrome due to thrombosis, endovascular therapy is first-line management; however, mechanical thrombectomy may be preferred in patients with absolute contraindication to thrombolytic agents.3 In the setting of increased external pressure on the SVC, treatment includes the removal of the source of pressure.4
In a case series including 78 patients, ports and indwelling catheters accounted for 71% of benign SVC cases.5 Our patient’s SVC syndrome most likely was due to the indwelling catheter pressing on the SVC. The goal of treatment is to address the underlying cause—whether it be pressure or thrombosis. In the setting of increased external pressure, treatment includes removal of the source of pressure from the SVC.4
Other differential diagnoses to consider for newonset ectatic vessels on the chest wall include generalized essential telangiectasia, scleroderma, poikiloderma vasculare atrophicans, and caput medusae. Generalized essential telangiectasia is characterized by red or pink dilated capillary blood vessels in a branch or lacelike pattern predominantly on the lower limbs. The eruption primarily is asymptomatic, though tingling or numbness may be reported.6 The diagnosis can be made with a punch biopsy, with histopathology showing dilated vessels in the dermis.7
Scleroderma is a connective tissue fibrosis disorder with variable clinical presentations. The systemic sclerosis subset can be divided into localized systemic sclerosis and diffuse systemic sclerosis. Physical examination reveals cutaneous sclerosis in various areas of the body. Localized systemic sclerosis includes sclerosis of the fingers and face, while diffuse systemic sclerosis is notable for progression to the arms, legs, and trunk.8 In addition to sclerosis, diffuse telangiectases also can be observed. Systemic sclerosis is a clinical diagnosis based on physical examination and laboratory studies to identify antibodies such as antinuclear antibodies.
Poikiloderma vasculare atrophicans is a variant of cutaneous T-cell lymphoma. The initial presentation is characterized by plaques of hypopigmentation and hyperpigmentation with atrophy and telangiectases. The lesions may be asymptomatic or mildly pruritic and classically involve the trunk and flexural areas.9 The diagnosis is made with skin biopsy and immunohistochemical studies, with findings reflective of mycosis fungoides.
Caput medusae (palm tree sign) is a cardinal feature of portal hypertension characterized by grossly dilated and engorged periumbilical veins. To shunt blood from the portal venous system, cutaneous collateral veins between the umbilical veins and abdominal wall veins are used, resulting in the appearance of engorged veins in the anterior abdominal wall.10 The diagnosis can be made with abdominal ultrasonography showing the direction of blood flow through abdominal vessels.
- Drouin L, Pistorius MA, Lafforgue A, et al. Upper-extremity venous thrombosis: a retrospective study about 160 cases [in French]. Rev Med Interne. 2019;40:9-15.
- Richie E. Clinical pearl: diagnosing superior vena cava syndrome. Emergency Medicine News. 2017;39:22. doi:10.1097/01 .EEM.0000522220.37441.d2
- Azizi A, Shafi I, Shah N, et al. Superior vena cava syndrome. JACC Cardiovasc Interv. 2020;13:2896-2910. doi:10.1016/j.jcin.2020.08.038
- Dumantepe M, Tarhan A, Ozler A. Successful treatment of central venous catheter induced superior vena cava syndrome with ultrasound accelerated catheter-directed thrombolysis. Catheter Cardiovasc Interv. 2013;81:E269-E273.
- Rice TW, Rodriguez RM, Light RW. The superior vena cava syndrome: clinical characteristics and evolving etiology. Medicine (Baltimore) 2006;85:37-42. doi:10.1097/01.md.0000198474.99876.f0
- Long D, Marshman G. Generalized essential telangiectasia. Australas J Dermatol. 2004;45:67-69. doi:10.1111/j.1440-0960.2004.00033.x
- Braverman IM. Ultrastructure and organization of the cutaneous microvasculature in normal and pathologic states. J Invest Dermatol. 1989;93(2 suppl):2S-9S.
- Ferreli C, Gasparini G, Parodi A, et al. Cutaneous manifestations of scleroderma and scleroderma-like disorders: a comprehensive review. Clin Rev Allergy Immunol. 2017;53:306-336. doi:10.1007 /s12016-017-8625-4
- Bloom B, Marchbein S, Fischer M, et al. Poikilodermatous mycosis fungoides. Dermatol Online J. 2012;18:4.
- Sharma B, Raina S. Caput medusae. Indian J Med Res. 2015;141:494. doi:10.4103/0971-5916.159322
- Drouin L, Pistorius MA, Lafforgue A, et al. Upper-extremity venous thrombosis: a retrospective study about 160 cases [in French]. Rev Med Interne. 2019;40:9-15.
- Richie E. Clinical pearl: diagnosing superior vena cava syndrome. Emergency Medicine News. 2017;39:22. doi:10.1097/01 .EEM.0000522220.37441.d2
- Azizi A, Shafi I, Shah N, et al. Superior vena cava syndrome. JACC Cardiovasc Interv. 2020;13:2896-2910. doi:10.1016/j.jcin.2020.08.038
- Dumantepe M, Tarhan A, Ozler A. Successful treatment of central venous catheter induced superior vena cava syndrome with ultrasound accelerated catheter-directed thrombolysis. Catheter Cardiovasc Interv. 2013;81:E269-E273.
- Rice TW, Rodriguez RM, Light RW. The superior vena cava syndrome: clinical characteristics and evolving etiology. Medicine (Baltimore) 2006;85:37-42. doi:10.1097/01.md.0000198474.99876.f0
- Long D, Marshman G. Generalized essential telangiectasia. Australas J Dermatol. 2004;45:67-69. doi:10.1111/j.1440-0960.2004.00033.x
- Braverman IM. Ultrastructure and organization of the cutaneous microvasculature in normal and pathologic states. J Invest Dermatol. 1989;93(2 suppl):2S-9S.
- Ferreli C, Gasparini G, Parodi A, et al. Cutaneous manifestations of scleroderma and scleroderma-like disorders: a comprehensive review. Clin Rev Allergy Immunol. 2017;53:306-336. doi:10.1007 /s12016-017-8625-4
- Bloom B, Marchbein S, Fischer M, et al. Poikilodermatous mycosis fungoides. Dermatol Online J. 2012;18:4.
- Sharma B, Raina S. Caput medusae. Indian J Med Res. 2015;141:494. doi:10.4103/0971-5916.159322
A 32-year-old woman presented to vascular surgery for evaluation of spider veins of 2 years’ duration that originated on the breasts but later spread to include the central chest, inframammary folds, and back. She reported associated pain and discomfort as well as intermittent facial swelling and tachycardia but denied pruritus and bleeding. The patient had a history of a kidney transplant 6 months prior, Langerhans cell histiocytosis, and Sjögren syndrome with a left indwelling catheter. Her current medications included systemic immunosuppressive agents. Physical examination revealed blue-purple ectatic vessels on the inframammary folds and central chest extending to the back. Erythema on the face, neck, and arms was not appreciated. No palpable cervical, supraclavicular, or axillary lymph nodes were noted.
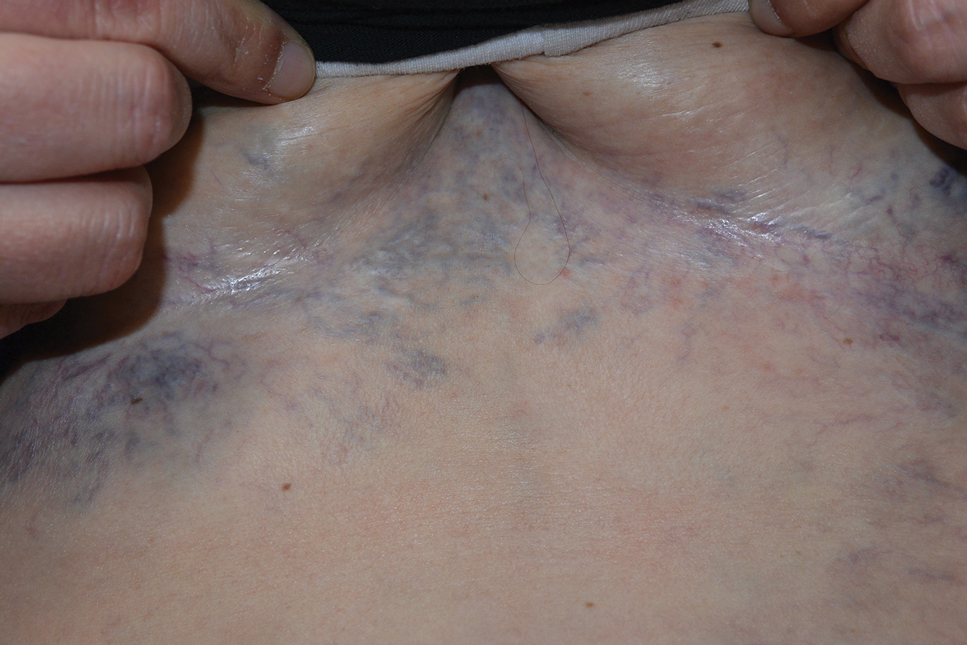
FDA Rejects GI Cancer Drug Over Manufacturing Issues
the company announced January 8.
The monoclonal antibody was under priority review as the first agent specifically for locally advanced unresectable or metastatic HER2-negative gastric or gastroesophageal junction adenocarcinoma that is claudin 18.2-positive. Overexpression of claudin 18.2 in gastric cancer cells is associated with tumor growth and progression.
The FDA, however, could not approve zolbetuximab by the planned decision date of January 12, 2024, because of “unresolved deficiencies following its pre-license inspection of a third-party manufacturing facility for zolbetuximab,” according to the company press release.
Astellas “is working closely with the FDA and the third-party manufacturer to establish a timeline to quickly resolve” the issues, the company said.
Astellas also clarified that the FDA isn’t asking for additional efficacy and safety data. In phase 3 testing, zolbetuximab improved median progression-free and overall survival by about 2-3 months over chemotherapy alone.
If zolbetuximab is approved, “pathologists will have to be facile with claudin 18.2 testing as a companion diagnostic before [it] can be used,” Mark Lewis, MD, a gastrointestinal oncologist at Intermountain Healthcare in Murray, Utah, told this news organization.
The agent is also under review in Japan, Europe, and China.
A version of this article appeared on Medscape.com.
the company announced January 8.
The monoclonal antibody was under priority review as the first agent specifically for locally advanced unresectable or metastatic HER2-negative gastric or gastroesophageal junction adenocarcinoma that is claudin 18.2-positive. Overexpression of claudin 18.2 in gastric cancer cells is associated with tumor growth and progression.
The FDA, however, could not approve zolbetuximab by the planned decision date of January 12, 2024, because of “unresolved deficiencies following its pre-license inspection of a third-party manufacturing facility for zolbetuximab,” according to the company press release.
Astellas “is working closely with the FDA and the third-party manufacturer to establish a timeline to quickly resolve” the issues, the company said.
Astellas also clarified that the FDA isn’t asking for additional efficacy and safety data. In phase 3 testing, zolbetuximab improved median progression-free and overall survival by about 2-3 months over chemotherapy alone.
If zolbetuximab is approved, “pathologists will have to be facile with claudin 18.2 testing as a companion diagnostic before [it] can be used,” Mark Lewis, MD, a gastrointestinal oncologist at Intermountain Healthcare in Murray, Utah, told this news organization.
The agent is also under review in Japan, Europe, and China.
A version of this article appeared on Medscape.com.
the company announced January 8.
The monoclonal antibody was under priority review as the first agent specifically for locally advanced unresectable or metastatic HER2-negative gastric or gastroesophageal junction adenocarcinoma that is claudin 18.2-positive. Overexpression of claudin 18.2 in gastric cancer cells is associated with tumor growth and progression.
The FDA, however, could not approve zolbetuximab by the planned decision date of January 12, 2024, because of “unresolved deficiencies following its pre-license inspection of a third-party manufacturing facility for zolbetuximab,” according to the company press release.
Astellas “is working closely with the FDA and the third-party manufacturer to establish a timeline to quickly resolve” the issues, the company said.
Astellas also clarified that the FDA isn’t asking for additional efficacy and safety data. In phase 3 testing, zolbetuximab improved median progression-free and overall survival by about 2-3 months over chemotherapy alone.
If zolbetuximab is approved, “pathologists will have to be facile with claudin 18.2 testing as a companion diagnostic before [it] can be used,” Mark Lewis, MD, a gastrointestinal oncologist at Intermountain Healthcare in Murray, Utah, told this news organization.
The agent is also under review in Japan, Europe, and China.
A version of this article appeared on Medscape.com.
Researchers Take Aim at Genetic Influence on Asthma and Allergy
The impact of maternal factors on allergy and asthma is the subject of new research in the wake of a grant from the National Institute of Allergy and Infectious Diseases to a team at Indiana University School of Medicine, according to a university press release.
Researchers led by Joan Cook-Mills, PhD, will examine the mechanisms behind the development of asthma, food allergies, and allergic diseases in children whose mothers had allergies.
“Research from the Cook-Mills lab revealed mothers with allergies have elevated levels of a specific lipid within the eicosanoid class of lipids, suggesting this lipid may have a potential influence on their offspring also developing allergies,” according to the press release.
A 5-year grant for $3.9 million was awarded to extend work by the Cook-Mills lab, and the research will focus on four areas, according to the university:
The potential impact of higher levels of lipid from mothers’ lungs may affect infants’ risk for allergy and whether this lipid is transmitted to infants during pregnancy or breastfeeding.
The potential impact of elevated levels of a specific eicosanoid in mothers with allergies promotes the creation of more dendritic cells by fetal bone marrow and how this might affect allergy risk for infants.
, potentially leading to altered lung bacteria, which can affect immune cell responses to allergies and asthma.
The potential impact of elevated eicosanoids on whether the altered lung microbiome “actively changes the production of this eicosanoid in the lungs of allergic mothers,” according to the press release.
“Allergies and asthma cause a significant burden of disease in our pediatric population, which is further complicated by limited therapies and interventions to combat these diseases, let alone prevent their development,” Anne C. Coates, MD, a pediatric pulmonologist at Maine Medical Center, Portland, said in an interview.
“The work by Cook-Mills and her colleagues will expand our understanding of the role maternal health may have on allergies and asthma and opportunities to mitigate it,” she said. The key implications of the research are the potential to facilitate the development of future clinical studies and trials that could yield novel targeted treatments for significant allergies, Dr. Coates told this news organization.
The research by Cook-Mills and her team had “the potential for the development of transformative approaches to allergy prevention and management, which could improve the health and quality of life for scores of individuals worldwide,” she said.
Dr. Coates had no financial conflicts to disclose but served on the Editorial Advisory Board of Chest Physician.
A version of this article appeared on Medscape.com.
The impact of maternal factors on allergy and asthma is the subject of new research in the wake of a grant from the National Institute of Allergy and Infectious Diseases to a team at Indiana University School of Medicine, according to a university press release.
Researchers led by Joan Cook-Mills, PhD, will examine the mechanisms behind the development of asthma, food allergies, and allergic diseases in children whose mothers had allergies.
“Research from the Cook-Mills lab revealed mothers with allergies have elevated levels of a specific lipid within the eicosanoid class of lipids, suggesting this lipid may have a potential influence on their offspring also developing allergies,” according to the press release.
A 5-year grant for $3.9 million was awarded to extend work by the Cook-Mills lab, and the research will focus on four areas, according to the university:
The potential impact of higher levels of lipid from mothers’ lungs may affect infants’ risk for allergy and whether this lipid is transmitted to infants during pregnancy or breastfeeding.
The potential impact of elevated levels of a specific eicosanoid in mothers with allergies promotes the creation of more dendritic cells by fetal bone marrow and how this might affect allergy risk for infants.
, potentially leading to altered lung bacteria, which can affect immune cell responses to allergies and asthma.
The potential impact of elevated eicosanoids on whether the altered lung microbiome “actively changes the production of this eicosanoid in the lungs of allergic mothers,” according to the press release.
“Allergies and asthma cause a significant burden of disease in our pediatric population, which is further complicated by limited therapies and interventions to combat these diseases, let alone prevent their development,” Anne C. Coates, MD, a pediatric pulmonologist at Maine Medical Center, Portland, said in an interview.
“The work by Cook-Mills and her colleagues will expand our understanding of the role maternal health may have on allergies and asthma and opportunities to mitigate it,” she said. The key implications of the research are the potential to facilitate the development of future clinical studies and trials that could yield novel targeted treatments for significant allergies, Dr. Coates told this news organization.
The research by Cook-Mills and her team had “the potential for the development of transformative approaches to allergy prevention and management, which could improve the health and quality of life for scores of individuals worldwide,” she said.
Dr. Coates had no financial conflicts to disclose but served on the Editorial Advisory Board of Chest Physician.
A version of this article appeared on Medscape.com.
The impact of maternal factors on allergy and asthma is the subject of new research in the wake of a grant from the National Institute of Allergy and Infectious Diseases to a team at Indiana University School of Medicine, according to a university press release.
Researchers led by Joan Cook-Mills, PhD, will examine the mechanisms behind the development of asthma, food allergies, and allergic diseases in children whose mothers had allergies.
“Research from the Cook-Mills lab revealed mothers with allergies have elevated levels of a specific lipid within the eicosanoid class of lipids, suggesting this lipid may have a potential influence on their offspring also developing allergies,” according to the press release.
A 5-year grant for $3.9 million was awarded to extend work by the Cook-Mills lab, and the research will focus on four areas, according to the university:
The potential impact of higher levels of lipid from mothers’ lungs may affect infants’ risk for allergy and whether this lipid is transmitted to infants during pregnancy or breastfeeding.
The potential impact of elevated levels of a specific eicosanoid in mothers with allergies promotes the creation of more dendritic cells by fetal bone marrow and how this might affect allergy risk for infants.
, potentially leading to altered lung bacteria, which can affect immune cell responses to allergies and asthma.
The potential impact of elevated eicosanoids on whether the altered lung microbiome “actively changes the production of this eicosanoid in the lungs of allergic mothers,” according to the press release.
“Allergies and asthma cause a significant burden of disease in our pediatric population, which is further complicated by limited therapies and interventions to combat these diseases, let alone prevent their development,” Anne C. Coates, MD, a pediatric pulmonologist at Maine Medical Center, Portland, said in an interview.
“The work by Cook-Mills and her colleagues will expand our understanding of the role maternal health may have on allergies and asthma and opportunities to mitigate it,” she said. The key implications of the research are the potential to facilitate the development of future clinical studies and trials that could yield novel targeted treatments for significant allergies, Dr. Coates told this news organization.
The research by Cook-Mills and her team had “the potential for the development of transformative approaches to allergy prevention and management, which could improve the health and quality of life for scores of individuals worldwide,” she said.
Dr. Coates had no financial conflicts to disclose but served on the Editorial Advisory Board of Chest Physician.
A version of this article appeared on Medscape.com.
Why GLP-1 Drugs Stop Working, and What to Do About It
There’s no question that glucagon-like peptide 1 (GLP-1) agonists represent a major advance in the treatment of obesity for patients with or without diabetes. In clinical trials, participants lost 15%-20% of their body weight, depending on the drug.
But studies also have shown that once people stop taking these drugs — either by choice, because of shortage, or lack of access — they regain most, if not all, the weight they lost.
Arguably more frustrating is the fact that those who continue on the drug eventually reach a plateau, at which point, the body seemingly stubbornly refuses to lose more weight. Essentially, it stabilizes at its set point, said Fatima Cody Stanford, MD, MPH, MPA, MBA, an obesity medicine physician at Massachusetts General Hospital and associate professor at Harvard Medical School in Boston.
‘Tug of War’
Every study of weight loss drugs done over the past 40 years or so shows a plateau, Dr. Stanford told this news organization. “If you look at the phentermine/topiramate studies, there’s a plateau. If you look at the bupropion/naltrexone studies, there’s a plateau. Or if we look at bariatric surgery, there’s a plateau. And it’s the same for the newer GLP-1 drugs.”
The reason? “It really depends on where the body gets to,” Dr. Stanford said. “The body knows what it needs to do to maintain itself, and the brain knows where it’s supposed to be. And when you lose weight and reach what you feel is a lower set point, the body resists.”
When the body goes below its set point, the hunger hormone ghrelin, which is housed in the brain, gets reactivated and gradually starts to reemerge, she explained. GLP-1, which is housed in the distal portion of the small intestine and in the colon, also starts to reemerge over time.
“It becomes kind of a tug of war” between the body and whatever weight loss strategy is being implemented, from drugs to surgery to lifestyle changes, Dr. Stanford said. “The patient will start to notice changes in how their body is responding. Usually, they’ll say they don’t feel like the treatment is working the same. But the treatment is working the same as it’s always been working — except their body is now acclimated to it.”
Anne L. Peters, MD, CDE, professor and clinical scholar, Keck School of Medicine of the University of Southern California, and director, agreed that in the simplest terms, a plateau occurs because “the body becomes more and more used to” the weight loss intervention.
However, when you lose weight, you lose both fat mass and lean body mass, and lean body mass is the metabolically active part of your body, explained Dr. Peters. “That’s what burns and basically makes up your basal metabolic rate.”
With weight loss, the metabolism slows down, she said. If patients need 2000 calories a day to survive at a certain weight and then lose 50 pounds, they may then need only a 1000 calories a day. “With any obesity treatment, you reach a point at which your metabolic rate and your daily caloric requirements become equal, and you stop losing weight, even though your daily caloric requirement is less than it was when your weight was higher.”
Managing the Plateau
Several strategies can be used to help patients break through a plateau. One is to try multiple weight loss agents with different targets — something often done in the real world, Dr. Stanford said. “You don’t see this in the studies, which are focused on just one drug, but many of our patients are on combination therapy. They’re on a GLP-1 drug plus phentermine/topiramate plus metformin, and more. They’re usually on three, four, five drugs, similar to what we would see with resistant hypertension.”
If a patient plateaus on a GLP-1 drug, Dr. Stanford might add phentermine. When the patient reaches a plateau on phentermine, she would switch again to another agent. “The goal is to use agents that treat different receptors in the brain,” she said. “You would never use two GLP-1 agonists; you would use the GLP-1, and then something that treats norepinephrine, for example.”
At the same time, Dr. Peters noted, “try to get them off the drugs that cause weight gain, like insulin and sulfonylurea agents.”
Tapering the GLP-1 dose can also help, Dr. Peters said. However, she added, “If I’m using a GLP-1 drug for type 2 diabetes, it’s different than if I’m using it just for weight loss. With type 2 diabetes, if you taper too much, the blood sugar and weight will go back up, so you need to reach a balance.”
Dr. Peters has successfully tapered patients from a 2-mg dose down to 1 mg. She has also changed the strategy for some — ie, the patient takes the drug every other week instead of every week. “I even have a patient or two who just take it once a month and that seems to be enough,” she said. “You want to help them be at the dose that maintains their weight and keeps them healthy with the least possible medication.”
Emphasizing lifestyle changes is also important, she said. Although resistance training won’t necessarily help with weight loss, “it’s critical to maintaining lean body mass. If people keep losing and regaining weight, they’re going to lose more and more lean body mass and gain the weight back primarily as fat mass. So, their exercise should include about half aerobic activity and half resistance training.”
Long-term Journey
Setting appropriate expectations is a key part of helping patients accept and deal with a plateau. “This is long-term, lifelong journey,” Dr. Stanford said. “We need to think about obesity as a complex, multifactorial chronic disease, like we think about hypertension or type 2 diabetes or hyperlipidemia.”
Furthermore, and in keeping with that perspective, emerging evidence is demonstrating that GLP-1 drugs also have important nonglycemic benefits that can be achieved and maintained, Dr. Peters said. “Obviously weight loss matters, and weight loss is good for you if you’re overweight or obese. But now we know that GLP-1 drugs have wonderful benefits for the heart as well as renal function.” These are reasons to continue the drugs even in the face of a plateau.
One of Dr. Peters’ patients, a physician with type 2 diabetes, had “fought with her weight her whole life. She’s been on one or another GLP-1 drug for more than 15 years, and while none seem to impact her weight, she’s gone from having relatively poorly controlled to now beautifully controlled diabetes,” Dr. Peters said. “Even if she hasn’t lost, she’s maintained her weight, a benefit since people tend to gain weight as they get older, and she hasn’t gained.”
Another patient was disabled, on oxygen, and had recurrent pulmonary embolisms. “She weighed 420 pounds, and I put her on semaglutide because she was too sick to be considered for bariatric surgery.” When that didn’t work, Dr. Peters switched her to tirzepatide, gradually increasing the dose; the patient lost 80 pounds, her emboli are gone, she can walk down the street, and went back to work.
“Part of why she could do that is that she started exercising,” Dr. Peters noted. “She felt so much better from the drug-related weight loss that she began to do things that help enhance weight loss. She became happier because she was no longer homebound.”
This points to another element that can help patients break through a plateau over time, Dr. Peters said — namely, behavioral health. “The more people lose weight, the more they feel better about themselves, and that may mean that they take better care of themselves. The psychological part of this journey is as important as anything else. Not everyone has the same response to these agents, and there are all sorts of issues behind why people are overweight that physicians can’t ignore.
“So, in addition to managing the drugs and lifestyle, it’s important to make sure that people access the behavioral health help they need, and that once they break through a plateau, they don’t develop an eating disorder or go to the opposite extreme and become too thin, which has happened with some of my patients,” she said. “We need to remember that we’re not just giving patients a miraculous weight loss. We’re helping them to be healthier, mentally as well as physically.”
Dr. Stanford disclosed that she had been a consultant for Calibrate, GoodRx, Pfizer, Eli Lilly, Boehringer Ingelheim, Gelesis, Vida Health, Life Force, Ilant Health, Melli Cell, and Novo Nordisk. Dr. Peters disclosed that she had been a consultant for Vertex, Medscape Medical News, and Lilly; received funding from Abbott and Insulet; and had stock options in Omada Health.
A version of this article appeared on Medscape.com.
There’s no question that glucagon-like peptide 1 (GLP-1) agonists represent a major advance in the treatment of obesity for patients with or without diabetes. In clinical trials, participants lost 15%-20% of their body weight, depending on the drug.
But studies also have shown that once people stop taking these drugs — either by choice, because of shortage, or lack of access — they regain most, if not all, the weight they lost.
Arguably more frustrating is the fact that those who continue on the drug eventually reach a plateau, at which point, the body seemingly stubbornly refuses to lose more weight. Essentially, it stabilizes at its set point, said Fatima Cody Stanford, MD, MPH, MPA, MBA, an obesity medicine physician at Massachusetts General Hospital and associate professor at Harvard Medical School in Boston.
‘Tug of War’
Every study of weight loss drugs done over the past 40 years or so shows a plateau, Dr. Stanford told this news organization. “If you look at the phentermine/topiramate studies, there’s a plateau. If you look at the bupropion/naltrexone studies, there’s a plateau. Or if we look at bariatric surgery, there’s a plateau. And it’s the same for the newer GLP-1 drugs.”
The reason? “It really depends on where the body gets to,” Dr. Stanford said. “The body knows what it needs to do to maintain itself, and the brain knows where it’s supposed to be. And when you lose weight and reach what you feel is a lower set point, the body resists.”
When the body goes below its set point, the hunger hormone ghrelin, which is housed in the brain, gets reactivated and gradually starts to reemerge, she explained. GLP-1, which is housed in the distal portion of the small intestine and in the colon, also starts to reemerge over time.
“It becomes kind of a tug of war” between the body and whatever weight loss strategy is being implemented, from drugs to surgery to lifestyle changes, Dr. Stanford said. “The patient will start to notice changes in how their body is responding. Usually, they’ll say they don’t feel like the treatment is working the same. But the treatment is working the same as it’s always been working — except their body is now acclimated to it.”
Anne L. Peters, MD, CDE, professor and clinical scholar, Keck School of Medicine of the University of Southern California, and director, agreed that in the simplest terms, a plateau occurs because “the body becomes more and more used to” the weight loss intervention.
However, when you lose weight, you lose both fat mass and lean body mass, and lean body mass is the metabolically active part of your body, explained Dr. Peters. “That’s what burns and basically makes up your basal metabolic rate.”
With weight loss, the metabolism slows down, she said. If patients need 2000 calories a day to survive at a certain weight and then lose 50 pounds, they may then need only a 1000 calories a day. “With any obesity treatment, you reach a point at which your metabolic rate and your daily caloric requirements become equal, and you stop losing weight, even though your daily caloric requirement is less than it was when your weight was higher.”
Managing the Plateau
Several strategies can be used to help patients break through a plateau. One is to try multiple weight loss agents with different targets — something often done in the real world, Dr. Stanford said. “You don’t see this in the studies, which are focused on just one drug, but many of our patients are on combination therapy. They’re on a GLP-1 drug plus phentermine/topiramate plus metformin, and more. They’re usually on three, four, five drugs, similar to what we would see with resistant hypertension.”
If a patient plateaus on a GLP-1 drug, Dr. Stanford might add phentermine. When the patient reaches a plateau on phentermine, she would switch again to another agent. “The goal is to use agents that treat different receptors in the brain,” she said. “You would never use two GLP-1 agonists; you would use the GLP-1, and then something that treats norepinephrine, for example.”
At the same time, Dr. Peters noted, “try to get them off the drugs that cause weight gain, like insulin and sulfonylurea agents.”
Tapering the GLP-1 dose can also help, Dr. Peters said. However, she added, “If I’m using a GLP-1 drug for type 2 diabetes, it’s different than if I’m using it just for weight loss. With type 2 diabetes, if you taper too much, the blood sugar and weight will go back up, so you need to reach a balance.”
Dr. Peters has successfully tapered patients from a 2-mg dose down to 1 mg. She has also changed the strategy for some — ie, the patient takes the drug every other week instead of every week. “I even have a patient or two who just take it once a month and that seems to be enough,” she said. “You want to help them be at the dose that maintains their weight and keeps them healthy with the least possible medication.”
Emphasizing lifestyle changes is also important, she said. Although resistance training won’t necessarily help with weight loss, “it’s critical to maintaining lean body mass. If people keep losing and regaining weight, they’re going to lose more and more lean body mass and gain the weight back primarily as fat mass. So, their exercise should include about half aerobic activity and half resistance training.”
Long-term Journey
Setting appropriate expectations is a key part of helping patients accept and deal with a plateau. “This is long-term, lifelong journey,” Dr. Stanford said. “We need to think about obesity as a complex, multifactorial chronic disease, like we think about hypertension or type 2 diabetes or hyperlipidemia.”
Furthermore, and in keeping with that perspective, emerging evidence is demonstrating that GLP-1 drugs also have important nonglycemic benefits that can be achieved and maintained, Dr. Peters said. “Obviously weight loss matters, and weight loss is good for you if you’re overweight or obese. But now we know that GLP-1 drugs have wonderful benefits for the heart as well as renal function.” These are reasons to continue the drugs even in the face of a plateau.
One of Dr. Peters’ patients, a physician with type 2 diabetes, had “fought with her weight her whole life. She’s been on one or another GLP-1 drug for more than 15 years, and while none seem to impact her weight, she’s gone from having relatively poorly controlled to now beautifully controlled diabetes,” Dr. Peters said. “Even if she hasn’t lost, she’s maintained her weight, a benefit since people tend to gain weight as they get older, and she hasn’t gained.”
Another patient was disabled, on oxygen, and had recurrent pulmonary embolisms. “She weighed 420 pounds, and I put her on semaglutide because she was too sick to be considered for bariatric surgery.” When that didn’t work, Dr. Peters switched her to tirzepatide, gradually increasing the dose; the patient lost 80 pounds, her emboli are gone, she can walk down the street, and went back to work.
“Part of why she could do that is that she started exercising,” Dr. Peters noted. “She felt so much better from the drug-related weight loss that she began to do things that help enhance weight loss. She became happier because she was no longer homebound.”
This points to another element that can help patients break through a plateau over time, Dr. Peters said — namely, behavioral health. “The more people lose weight, the more they feel better about themselves, and that may mean that they take better care of themselves. The psychological part of this journey is as important as anything else. Not everyone has the same response to these agents, and there are all sorts of issues behind why people are overweight that physicians can’t ignore.
“So, in addition to managing the drugs and lifestyle, it’s important to make sure that people access the behavioral health help they need, and that once they break through a plateau, they don’t develop an eating disorder or go to the opposite extreme and become too thin, which has happened with some of my patients,” she said. “We need to remember that we’re not just giving patients a miraculous weight loss. We’re helping them to be healthier, mentally as well as physically.”
Dr. Stanford disclosed that she had been a consultant for Calibrate, GoodRx, Pfizer, Eli Lilly, Boehringer Ingelheim, Gelesis, Vida Health, Life Force, Ilant Health, Melli Cell, and Novo Nordisk. Dr. Peters disclosed that she had been a consultant for Vertex, Medscape Medical News, and Lilly; received funding from Abbott and Insulet; and had stock options in Omada Health.
A version of this article appeared on Medscape.com.
There’s no question that glucagon-like peptide 1 (GLP-1) agonists represent a major advance in the treatment of obesity for patients with or without diabetes. In clinical trials, participants lost 15%-20% of their body weight, depending on the drug.
But studies also have shown that once people stop taking these drugs — either by choice, because of shortage, or lack of access — they regain most, if not all, the weight they lost.
Arguably more frustrating is the fact that those who continue on the drug eventually reach a plateau, at which point, the body seemingly stubbornly refuses to lose more weight. Essentially, it stabilizes at its set point, said Fatima Cody Stanford, MD, MPH, MPA, MBA, an obesity medicine physician at Massachusetts General Hospital and associate professor at Harvard Medical School in Boston.
‘Tug of War’
Every study of weight loss drugs done over the past 40 years or so shows a plateau, Dr. Stanford told this news organization. “If you look at the phentermine/topiramate studies, there’s a plateau. If you look at the bupropion/naltrexone studies, there’s a plateau. Or if we look at bariatric surgery, there’s a plateau. And it’s the same for the newer GLP-1 drugs.”
The reason? “It really depends on where the body gets to,” Dr. Stanford said. “The body knows what it needs to do to maintain itself, and the brain knows where it’s supposed to be. And when you lose weight and reach what you feel is a lower set point, the body resists.”
When the body goes below its set point, the hunger hormone ghrelin, which is housed in the brain, gets reactivated and gradually starts to reemerge, she explained. GLP-1, which is housed in the distal portion of the small intestine and in the colon, also starts to reemerge over time.
“It becomes kind of a tug of war” between the body and whatever weight loss strategy is being implemented, from drugs to surgery to lifestyle changes, Dr. Stanford said. “The patient will start to notice changes in how their body is responding. Usually, they’ll say they don’t feel like the treatment is working the same. But the treatment is working the same as it’s always been working — except their body is now acclimated to it.”
Anne L. Peters, MD, CDE, professor and clinical scholar, Keck School of Medicine of the University of Southern California, and director, agreed that in the simplest terms, a plateau occurs because “the body becomes more and more used to” the weight loss intervention.
However, when you lose weight, you lose both fat mass and lean body mass, and lean body mass is the metabolically active part of your body, explained Dr. Peters. “That’s what burns and basically makes up your basal metabolic rate.”
With weight loss, the metabolism slows down, she said. If patients need 2000 calories a day to survive at a certain weight and then lose 50 pounds, they may then need only a 1000 calories a day. “With any obesity treatment, you reach a point at which your metabolic rate and your daily caloric requirements become equal, and you stop losing weight, even though your daily caloric requirement is less than it was when your weight was higher.”
Managing the Plateau
Several strategies can be used to help patients break through a plateau. One is to try multiple weight loss agents with different targets — something often done in the real world, Dr. Stanford said. “You don’t see this in the studies, which are focused on just one drug, but many of our patients are on combination therapy. They’re on a GLP-1 drug plus phentermine/topiramate plus metformin, and more. They’re usually on three, four, five drugs, similar to what we would see with resistant hypertension.”
If a patient plateaus on a GLP-1 drug, Dr. Stanford might add phentermine. When the patient reaches a plateau on phentermine, she would switch again to another agent. “The goal is to use agents that treat different receptors in the brain,” she said. “You would never use two GLP-1 agonists; you would use the GLP-1, and then something that treats norepinephrine, for example.”
At the same time, Dr. Peters noted, “try to get them off the drugs that cause weight gain, like insulin and sulfonylurea agents.”
Tapering the GLP-1 dose can also help, Dr. Peters said. However, she added, “If I’m using a GLP-1 drug for type 2 diabetes, it’s different than if I’m using it just for weight loss. With type 2 diabetes, if you taper too much, the blood sugar and weight will go back up, so you need to reach a balance.”
Dr. Peters has successfully tapered patients from a 2-mg dose down to 1 mg. She has also changed the strategy for some — ie, the patient takes the drug every other week instead of every week. “I even have a patient or two who just take it once a month and that seems to be enough,” she said. “You want to help them be at the dose that maintains their weight and keeps them healthy with the least possible medication.”
Emphasizing lifestyle changes is also important, she said. Although resistance training won’t necessarily help with weight loss, “it’s critical to maintaining lean body mass. If people keep losing and regaining weight, they’re going to lose more and more lean body mass and gain the weight back primarily as fat mass. So, their exercise should include about half aerobic activity and half resistance training.”
Long-term Journey
Setting appropriate expectations is a key part of helping patients accept and deal with a plateau. “This is long-term, lifelong journey,” Dr. Stanford said. “We need to think about obesity as a complex, multifactorial chronic disease, like we think about hypertension or type 2 diabetes or hyperlipidemia.”
Furthermore, and in keeping with that perspective, emerging evidence is demonstrating that GLP-1 drugs also have important nonglycemic benefits that can be achieved and maintained, Dr. Peters said. “Obviously weight loss matters, and weight loss is good for you if you’re overweight or obese. But now we know that GLP-1 drugs have wonderful benefits for the heart as well as renal function.” These are reasons to continue the drugs even in the face of a plateau.
One of Dr. Peters’ patients, a physician with type 2 diabetes, had “fought with her weight her whole life. She’s been on one or another GLP-1 drug for more than 15 years, and while none seem to impact her weight, she’s gone from having relatively poorly controlled to now beautifully controlled diabetes,” Dr. Peters said. “Even if she hasn’t lost, she’s maintained her weight, a benefit since people tend to gain weight as they get older, and she hasn’t gained.”
Another patient was disabled, on oxygen, and had recurrent pulmonary embolisms. “She weighed 420 pounds, and I put her on semaglutide because she was too sick to be considered for bariatric surgery.” When that didn’t work, Dr. Peters switched her to tirzepatide, gradually increasing the dose; the patient lost 80 pounds, her emboli are gone, she can walk down the street, and went back to work.
“Part of why she could do that is that she started exercising,” Dr. Peters noted. “She felt so much better from the drug-related weight loss that she began to do things that help enhance weight loss. She became happier because she was no longer homebound.”
This points to another element that can help patients break through a plateau over time, Dr. Peters said — namely, behavioral health. “The more people lose weight, the more they feel better about themselves, and that may mean that they take better care of themselves. The psychological part of this journey is as important as anything else. Not everyone has the same response to these agents, and there are all sorts of issues behind why people are overweight that physicians can’t ignore.
“So, in addition to managing the drugs and lifestyle, it’s important to make sure that people access the behavioral health help they need, and that once they break through a plateau, they don’t develop an eating disorder or go to the opposite extreme and become too thin, which has happened with some of my patients,” she said. “We need to remember that we’re not just giving patients a miraculous weight loss. We’re helping them to be healthier, mentally as well as physically.”
Dr. Stanford disclosed that she had been a consultant for Calibrate, GoodRx, Pfizer, Eli Lilly, Boehringer Ingelheim, Gelesis, Vida Health, Life Force, Ilant Health, Melli Cell, and Novo Nordisk. Dr. Peters disclosed that she had been a consultant for Vertex, Medscape Medical News, and Lilly; received funding from Abbott and Insulet; and had stock options in Omada Health.
A version of this article appeared on Medscape.com.