User login
Pseudofolliculitis Barbae in the Military: Policy, Stigma, and Practical Solutions
Pseudofolliculitis Barbae in the Military: Policy, Stigma, and Practical Solutions
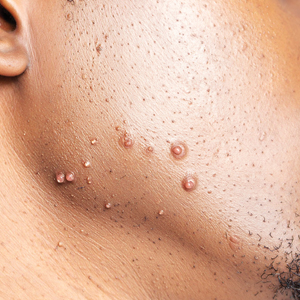
The impact of pseudofolliculitis barbae (PFB) on military service members and other uniformed professionals has been a topic of recent interest due to the announcement of the US Army’s new shaving rule in July 2025.1 The policy prohibits permanent shaving waivers, requires medical re-evaluation of shaving profiles within 90 days, and allows for administrative separation if a service member accumulates shaving exceptions totaling more than 12 months over a 24-month period.2 A common skin condition triggered or worsened by shaving, PFB causes painful bumps, pustules, and hyperpigmentation most often in the beard and cheek areas and negatively impacts quality of life. It disproportionately affects 45% to 83% of men in the United States, particularly those of African, Hispanic, or Middle Eastern descent.3,4 Genetic factors, particularly tightly coiled or coarse curly hair, can predispose individuals to PFB. The most successful treatment for PFB is to stop shaving, but this conflicts with military shaving standards and interferes with the use of protective equipment (eg, masks). Herein, we highlight the adverse impact of PFB on military career progression and provide context for clinicians who treat patients with PFB, especially as policies recently have shifted to allow nonmilitary clinicians to evaluate PFB in service members.5
Shaving Waivers and Advancement
Pseudofolliculitis barbae disproportionately prolongs the time to advancement of many service members, and those with PFB also are overburdened by policy changes related to shaving.6 In the US military, nearly 18% of the active-duty force is Black,7 a population that is more susceptible to PFB. Military personnel may request PFB-related accommodations, including medical shaving waivers that vary by branch. Through a formal documentation process, waivers allow service members to maintain facial hair up to one-quarter inch in length.5 Previously, waivers could be temporary (eg, up to 90 days) or permanent as subjectively determined based on clinician-documented disease severity. Almost 65% of US Air Force medical shaving waivers are held by Black men, and PFB is one of the most common reasons.6 Notably, the US Navy discontinued permanent shaving waivers in October 2019.8 A US Marine Corps policy issued in March 2025 now allows administrative separation of service members with PFB if symptoms do not improve after a 1-year medical shaving waiver due to “incompatibility with service.”9 This change reversed a 2022 policy that protected Marines from separation based on PFB.10 A Marine Corps spokesperson stated that this change aims to clarify how medical conditions can impact uniform compliance and standardize medical condition management while prioritizing compliance and duty readiness.1
Even in the absence of policy changes, obtaining a medical shaving waiver for PFB can be challenging. Service members may have little to no access to military dermatologists who specialize in management of PFB and experience long wait times for civilian network deferment. Service members seen in civilian clinics may have restricted treatment options due to limited insurance coverage for laser hair reduction, even in the most difficult-to-manage areas (eg, neck, jawline). Expanding access to military dermatologists, civilian dermatologists who are experienced with PFB and understand the impact and necessity of military waivers, and teledermatology services could help improve and streamline care. Other challenges include the subjective nature of documenting PFB disease severity, the need for validated assessment tools, a lack of standardized policies across military branches, and stigma. A standardized approach to documentation may reduce variability in how shaving waivers are evaluated across service branches, but at a minimum, clinicians should document the diagnosis, clinical findings, severity of PFB, and the treatment used. Having a waiver would help these service members focus on mastering critical skillsets and performing duties without the time pressures, angst, and expense dedicated to caring for and managing PFB.
Clinical and Policy Barriers
Unfortunately, service members with PFB or shaving waivers often face stigma that can hinder career advancement.6 In a recent analysis of 9339 US Air Force personnel, those with shaving waivers experienced longer times to promotion compared to those without waivers: in the waiver group, 94.47% were enlisted and 5.53% were officers; in the nonwaiver group, 72.11% were enlisted and 27.89% were officers (P=.0003).6 While delays in promotion were consistent across racial groups, most of the waiver holders identified as Black (64.8%), despite this demographic group representing only a small portion of the overall cohort (12.9%).6 Promotion delays may be linked to perceptions of unprofessionalism and exclusion from high-profile assignments, which notably require “the highest standards of military appearance and professional conduct.”11 The burden of career-limiting shaving policies falls disproportionately on military personnel with PFB who self-identify as Black. Perceptions about unprofessional appearance or job readiness often unintentionally introduce bias, unjustly restricting career advancement.6
Safety Equipment and Shaving Standards
Conditions that potentially affect the use of masks and chemical defense equipment extend beyond the military. Firefighters and law enforcement officers generally are required to maintain a clean-shaven face for proper fit of respirator masks; the standard is that no respirator fit test shall be conducted if hair—including stubble, beards, mustaches, or sideburns—grows between the skin and the facepiece sealing surface, and any apparel interfering with a proper seal must be altered or removed.12 This creates challenges for uniformed professionals with PFB who must manage their condition while adhering to safety requirements. Some endure long-term pain and scarring in order to comply, while others seek waivers to treat and prevent symptoms while also facing the stigma of doing so.13 One of the most effective treatments for PFB is to discontinue shaving,14 which may not be feasible for those in uniformed professions with strict grooming standards. Research on mask seal effectiveness in individuals with neatly trimmed beards or PFB remains limited.5 Studies evaluating mask fit across facial hair types and lengths are needed, along with the development of protective equipment that accommodates career-limiting conditions such as PFB, cystic acne, and acne keloidalis nuchae. This also may encourage development of equipment that does not induce such conditions (eg, mechanical acne from friction). These efforts would promote safety, scientific innovation for dermatologic follicular-based disorders, and overall quality of life for service members as well as increase their ability to serve without stigma. These developments also would positively impact other fields that require intermittent or full-time use of masks, including health care and some food service industries.
Final Thoughts
The disproportionate impact of PFB in the military highlights the need for improved access to treatment, culturally informed care, and policies that avoid penalizing service members with tightly coiled hair and a desire to serve. We discussed PFB management strategies, clinical features, and implications across various skin tones in a previous publication.14 It is important to consider insights from individuals with PFB who are serving in the military as well as the medical personnel who care for them. Ensuring or creating effective treatment options drives innovation, and evidence-based accommodation plans can help individuals in uniformed professions avoid choosing between PFB management and their career. Promoting awareness about the impact of PFB beyond the razor is key to reducing disparities and supporting excellence among those who serve and desire to continue to do so.
- Lawrence DF. Marines with skin condition affecting mostly black men could now be booted under new policy. Military.com. March 14, 2025. Accessed May 4, 2025. https://www.military.com/daily-news/2025/03/14/marines-can-now-be-kicked-out-skin-condition-affects-mostly-black-men.html
- Secretary of the Army. Army directive 2025-13 (facial hair grooming standards). Published July 7, 2025. Accessed September 19, 2025. https://lyster.tricare.mil/Portals/61/ARN44307-ARMY_DIR_2025-13-000.pdf
- Alexis A, Heath CR, Halder RM. Folliculitis keloidalis nuchae and pseudofolliculitis barbae: are prevention and effective treatment within reach? Dermatol Clin. 2014;32:183-191. doi:10.1016/j.det.2013.12.001
- Gray J, McMichael AJ. Pseudofolliculitis barbae: understanding the condition and the role of facial grooming. Int J Cosmet Sci. 2016;38:24-27. doi:10.1111/ics.12331
- Jung I, Lannan FM, Weiss A, et al. Treatment and current policies on pseudofolliculitis barbae in the US military. Cutis. 2023;112:299-302. doi:10.12788/cutis.0907
- Ritchie S, Park J, Banta J, et al. Shaving waivers in the United States Air Force and their impact on promotions of Black/African-American members. Mil Med. 2023;188:E242-E247. doi:10.1093/milmed/usab272
- Defense Manpower Data Center. Active-duty military personnel master file and reserve components common personnel data system. Military OneSource. September 2023. Accessed May 3, 2025. https://download.militaryonesource.mil/12038/MOS/Reports/2023-demographics-report.pdf
- Tshudy MT, Cho S. Pseudofolliculitis barbae in the US. Military, a review. Mil Med. 2021;186:E52-E57. doi:10.1093/milmed/usaa243
- US Marine Corps. Uniform and grooming standards for medical conditions (MARADMINS number: 124/25). Published March 13, 2025. Accessed September 19, 2025. https://www.marines.mil/News/Messages/Messages-Display/Article/4119098/uniform-and-grooming-standards-for-medical-conditions/
- US Marine Corps. Advance notification of change to MCO 6310.1C (Pseudofolliculitis Barbae), MCO 1900.16 CH2 (Marine Corps Retirement and Separation Manual), and MCO 1040.31 (Enlisted Retention and Career Development Program). Published January 21, 2022. Accessed September 19, 2025. https://www.marines.mil/News/Messages/Messages-Display/Article/2907104/advance-notification-of-change-to-mco-63101c-pseudofolliculitis-barbae-mco-1900/
- US Department of Defense. Special duty catalog (SPECAT). Published August 15, 2013. Accessed September 19, 2025. https://share.google/iuMrVMIASWx4EFLVN
- Occupational Safety and Health Administration. Appendix A to §1910.134—fit testing procedures (mandatory). Accessed September 19, 2025. https://www.osha.gov/laws-regs/regulations/standardnumber/1910/1910.134AppA
- Jiang YR. Reasonable accommodation and disparate impact: clean shave policy discrimination in today’s workplace. J Law Med Ethics. 2023;51:185-195. doi:10.1017/jme.2023.55
- Welch D, Usatine R, Heath C. Implications of PFB beyond the razor. Cutis. 2025;115:135-136. doi:10.12788/cutis.1194
The impact of pseudofolliculitis barbae (PFB) on military service members and other uniformed professionals has been a topic of recent interest due to the announcement of the US Army’s new shaving rule in July 2025.1 The policy prohibits permanent shaving waivers, requires medical re-evaluation of shaving profiles within 90 days, and allows for administrative separation if a service member accumulates shaving exceptions totaling more than 12 months over a 24-month period.2 A common skin condition triggered or worsened by shaving, PFB causes painful bumps, pustules, and hyperpigmentation most often in the beard and cheek areas and negatively impacts quality of life. It disproportionately affects 45% to 83% of men in the United States, particularly those of African, Hispanic, or Middle Eastern descent.3,4 Genetic factors, particularly tightly coiled or coarse curly hair, can predispose individuals to PFB. The most successful treatment for PFB is to stop shaving, but this conflicts with military shaving standards and interferes with the use of protective equipment (eg, masks). Herein, we highlight the adverse impact of PFB on military career progression and provide context for clinicians who treat patients with PFB, especially as policies recently have shifted to allow nonmilitary clinicians to evaluate PFB in service members.5
Shaving Waivers and Advancement
Pseudofolliculitis barbae disproportionately prolongs the time to advancement of many service members, and those with PFB also are overburdened by policy changes related to shaving.6 In the US military, nearly 18% of the active-duty force is Black,7 a population that is more susceptible to PFB. Military personnel may request PFB-related accommodations, including medical shaving waivers that vary by branch. Through a formal documentation process, waivers allow service members to maintain facial hair up to one-quarter inch in length.5 Previously, waivers could be temporary (eg, up to 90 days) or permanent as subjectively determined based on clinician-documented disease severity. Almost 65% of US Air Force medical shaving waivers are held by Black men, and PFB is one of the most common reasons.6 Notably, the US Navy discontinued permanent shaving waivers in October 2019.8 A US Marine Corps policy issued in March 2025 now allows administrative separation of service members with PFB if symptoms do not improve after a 1-year medical shaving waiver due to “incompatibility with service.”9 This change reversed a 2022 policy that protected Marines from separation based on PFB.10 A Marine Corps spokesperson stated that this change aims to clarify how medical conditions can impact uniform compliance and standardize medical condition management while prioritizing compliance and duty readiness.1
Even in the absence of policy changes, obtaining a medical shaving waiver for PFB can be challenging. Service members may have little to no access to military dermatologists who specialize in management of PFB and experience long wait times for civilian network deferment. Service members seen in civilian clinics may have restricted treatment options due to limited insurance coverage for laser hair reduction, even in the most difficult-to-manage areas (eg, neck, jawline). Expanding access to military dermatologists, civilian dermatologists who are experienced with PFB and understand the impact and necessity of military waivers, and teledermatology services could help improve and streamline care. Other challenges include the subjective nature of documenting PFB disease severity, the need for validated assessment tools, a lack of standardized policies across military branches, and stigma. A standardized approach to documentation may reduce variability in how shaving waivers are evaluated across service branches, but at a minimum, clinicians should document the diagnosis, clinical findings, severity of PFB, and the treatment used. Having a waiver would help these service members focus on mastering critical skillsets and performing duties without the time pressures, angst, and expense dedicated to caring for and managing PFB.
Clinical and Policy Barriers
Unfortunately, service members with PFB or shaving waivers often face stigma that can hinder career advancement.6 In a recent analysis of 9339 US Air Force personnel, those with shaving waivers experienced longer times to promotion compared to those without waivers: in the waiver group, 94.47% were enlisted and 5.53% were officers; in the nonwaiver group, 72.11% were enlisted and 27.89% were officers (P=.0003).6 While delays in promotion were consistent across racial groups, most of the waiver holders identified as Black (64.8%), despite this demographic group representing only a small portion of the overall cohort (12.9%).6 Promotion delays may be linked to perceptions of unprofessionalism and exclusion from high-profile assignments, which notably require “the highest standards of military appearance and professional conduct.”11 The burden of career-limiting shaving policies falls disproportionately on military personnel with PFB who self-identify as Black. Perceptions about unprofessional appearance or job readiness often unintentionally introduce bias, unjustly restricting career advancement.6
Safety Equipment and Shaving Standards
Conditions that potentially affect the use of masks and chemical defense equipment extend beyond the military. Firefighters and law enforcement officers generally are required to maintain a clean-shaven face for proper fit of respirator masks; the standard is that no respirator fit test shall be conducted if hair—including stubble, beards, mustaches, or sideburns—grows between the skin and the facepiece sealing surface, and any apparel interfering with a proper seal must be altered or removed.12 This creates challenges for uniformed professionals with PFB who must manage their condition while adhering to safety requirements. Some endure long-term pain and scarring in order to comply, while others seek waivers to treat and prevent symptoms while also facing the stigma of doing so.13 One of the most effective treatments for PFB is to discontinue shaving,14 which may not be feasible for those in uniformed professions with strict grooming standards. Research on mask seal effectiveness in individuals with neatly trimmed beards or PFB remains limited.5 Studies evaluating mask fit across facial hair types and lengths are needed, along with the development of protective equipment that accommodates career-limiting conditions such as PFB, cystic acne, and acne keloidalis nuchae. This also may encourage development of equipment that does not induce such conditions (eg, mechanical acne from friction). These efforts would promote safety, scientific innovation for dermatologic follicular-based disorders, and overall quality of life for service members as well as increase their ability to serve without stigma. These developments also would positively impact other fields that require intermittent or full-time use of masks, including health care and some food service industries.
Final Thoughts
The disproportionate impact of PFB in the military highlights the need for improved access to treatment, culturally informed care, and policies that avoid penalizing service members with tightly coiled hair and a desire to serve. We discussed PFB management strategies, clinical features, and implications across various skin tones in a previous publication.14 It is important to consider insights from individuals with PFB who are serving in the military as well as the medical personnel who care for them. Ensuring or creating effective treatment options drives innovation, and evidence-based accommodation plans can help individuals in uniformed professions avoid choosing between PFB management and their career. Promoting awareness about the impact of PFB beyond the razor is key to reducing disparities and supporting excellence among those who serve and desire to continue to do so.
The impact of pseudofolliculitis barbae (PFB) on military service members and other uniformed professionals has been a topic of recent interest due to the announcement of the US Army’s new shaving rule in July 2025.1 The policy prohibits permanent shaving waivers, requires medical re-evaluation of shaving profiles within 90 days, and allows for administrative separation if a service member accumulates shaving exceptions totaling more than 12 months over a 24-month period.2 A common skin condition triggered or worsened by shaving, PFB causes painful bumps, pustules, and hyperpigmentation most often in the beard and cheek areas and negatively impacts quality of life. It disproportionately affects 45% to 83% of men in the United States, particularly those of African, Hispanic, or Middle Eastern descent.3,4 Genetic factors, particularly tightly coiled or coarse curly hair, can predispose individuals to PFB. The most successful treatment for PFB is to stop shaving, but this conflicts with military shaving standards and interferes with the use of protective equipment (eg, masks). Herein, we highlight the adverse impact of PFB on military career progression and provide context for clinicians who treat patients with PFB, especially as policies recently have shifted to allow nonmilitary clinicians to evaluate PFB in service members.5
Shaving Waivers and Advancement
Pseudofolliculitis barbae disproportionately prolongs the time to advancement of many service members, and those with PFB also are overburdened by policy changes related to shaving.6 In the US military, nearly 18% of the active-duty force is Black,7 a population that is more susceptible to PFB. Military personnel may request PFB-related accommodations, including medical shaving waivers that vary by branch. Through a formal documentation process, waivers allow service members to maintain facial hair up to one-quarter inch in length.5 Previously, waivers could be temporary (eg, up to 90 days) or permanent as subjectively determined based on clinician-documented disease severity. Almost 65% of US Air Force medical shaving waivers are held by Black men, and PFB is one of the most common reasons.6 Notably, the US Navy discontinued permanent shaving waivers in October 2019.8 A US Marine Corps policy issued in March 2025 now allows administrative separation of service members with PFB if symptoms do not improve after a 1-year medical shaving waiver due to “incompatibility with service.”9 This change reversed a 2022 policy that protected Marines from separation based on PFB.10 A Marine Corps spokesperson stated that this change aims to clarify how medical conditions can impact uniform compliance and standardize medical condition management while prioritizing compliance and duty readiness.1
Even in the absence of policy changes, obtaining a medical shaving waiver for PFB can be challenging. Service members may have little to no access to military dermatologists who specialize in management of PFB and experience long wait times for civilian network deferment. Service members seen in civilian clinics may have restricted treatment options due to limited insurance coverage for laser hair reduction, even in the most difficult-to-manage areas (eg, neck, jawline). Expanding access to military dermatologists, civilian dermatologists who are experienced with PFB and understand the impact and necessity of military waivers, and teledermatology services could help improve and streamline care. Other challenges include the subjective nature of documenting PFB disease severity, the need for validated assessment tools, a lack of standardized policies across military branches, and stigma. A standardized approach to documentation may reduce variability in how shaving waivers are evaluated across service branches, but at a minimum, clinicians should document the diagnosis, clinical findings, severity of PFB, and the treatment used. Having a waiver would help these service members focus on mastering critical skillsets and performing duties without the time pressures, angst, and expense dedicated to caring for and managing PFB.
Clinical and Policy Barriers
Unfortunately, service members with PFB or shaving waivers often face stigma that can hinder career advancement.6 In a recent analysis of 9339 US Air Force personnel, those with shaving waivers experienced longer times to promotion compared to those without waivers: in the waiver group, 94.47% were enlisted and 5.53% were officers; in the nonwaiver group, 72.11% were enlisted and 27.89% were officers (P=.0003).6 While delays in promotion were consistent across racial groups, most of the waiver holders identified as Black (64.8%), despite this demographic group representing only a small portion of the overall cohort (12.9%).6 Promotion delays may be linked to perceptions of unprofessionalism and exclusion from high-profile assignments, which notably require “the highest standards of military appearance and professional conduct.”11 The burden of career-limiting shaving policies falls disproportionately on military personnel with PFB who self-identify as Black. Perceptions about unprofessional appearance or job readiness often unintentionally introduce bias, unjustly restricting career advancement.6
Safety Equipment and Shaving Standards
Conditions that potentially affect the use of masks and chemical defense equipment extend beyond the military. Firefighters and law enforcement officers generally are required to maintain a clean-shaven face for proper fit of respirator masks; the standard is that no respirator fit test shall be conducted if hair—including stubble, beards, mustaches, or sideburns—grows between the skin and the facepiece sealing surface, and any apparel interfering with a proper seal must be altered or removed.12 This creates challenges for uniformed professionals with PFB who must manage their condition while adhering to safety requirements. Some endure long-term pain and scarring in order to comply, while others seek waivers to treat and prevent symptoms while also facing the stigma of doing so.13 One of the most effective treatments for PFB is to discontinue shaving,14 which may not be feasible for those in uniformed professions with strict grooming standards. Research on mask seal effectiveness in individuals with neatly trimmed beards or PFB remains limited.5 Studies evaluating mask fit across facial hair types and lengths are needed, along with the development of protective equipment that accommodates career-limiting conditions such as PFB, cystic acne, and acne keloidalis nuchae. This also may encourage development of equipment that does not induce such conditions (eg, mechanical acne from friction). These efforts would promote safety, scientific innovation for dermatologic follicular-based disorders, and overall quality of life for service members as well as increase their ability to serve without stigma. These developments also would positively impact other fields that require intermittent or full-time use of masks, including health care and some food service industries.
Final Thoughts
The disproportionate impact of PFB in the military highlights the need for improved access to treatment, culturally informed care, and policies that avoid penalizing service members with tightly coiled hair and a desire to serve. We discussed PFB management strategies, clinical features, and implications across various skin tones in a previous publication.14 It is important to consider insights from individuals with PFB who are serving in the military as well as the medical personnel who care for them. Ensuring or creating effective treatment options drives innovation, and evidence-based accommodation plans can help individuals in uniformed professions avoid choosing between PFB management and their career. Promoting awareness about the impact of PFB beyond the razor is key to reducing disparities and supporting excellence among those who serve and desire to continue to do so.
- Lawrence DF. Marines with skin condition affecting mostly black men could now be booted under new policy. Military.com. March 14, 2025. Accessed May 4, 2025. https://www.military.com/daily-news/2025/03/14/marines-can-now-be-kicked-out-skin-condition-affects-mostly-black-men.html
- Secretary of the Army. Army directive 2025-13 (facial hair grooming standards). Published July 7, 2025. Accessed September 19, 2025. https://lyster.tricare.mil/Portals/61/ARN44307-ARMY_DIR_2025-13-000.pdf
- Alexis A, Heath CR, Halder RM. Folliculitis keloidalis nuchae and pseudofolliculitis barbae: are prevention and effective treatment within reach? Dermatol Clin. 2014;32:183-191. doi:10.1016/j.det.2013.12.001
- Gray J, McMichael AJ. Pseudofolliculitis barbae: understanding the condition and the role of facial grooming. Int J Cosmet Sci. 2016;38:24-27. doi:10.1111/ics.12331
- Jung I, Lannan FM, Weiss A, et al. Treatment and current policies on pseudofolliculitis barbae in the US military. Cutis. 2023;112:299-302. doi:10.12788/cutis.0907
- Ritchie S, Park J, Banta J, et al. Shaving waivers in the United States Air Force and their impact on promotions of Black/African-American members. Mil Med. 2023;188:E242-E247. doi:10.1093/milmed/usab272
- Defense Manpower Data Center. Active-duty military personnel master file and reserve components common personnel data system. Military OneSource. September 2023. Accessed May 3, 2025. https://download.militaryonesource.mil/12038/MOS/Reports/2023-demographics-report.pdf
- Tshudy MT, Cho S. Pseudofolliculitis barbae in the US. Military, a review. Mil Med. 2021;186:E52-E57. doi:10.1093/milmed/usaa243
- US Marine Corps. Uniform and grooming standards for medical conditions (MARADMINS number: 124/25). Published March 13, 2025. Accessed September 19, 2025. https://www.marines.mil/News/Messages/Messages-Display/Article/4119098/uniform-and-grooming-standards-for-medical-conditions/
- US Marine Corps. Advance notification of change to MCO 6310.1C (Pseudofolliculitis Barbae), MCO 1900.16 CH2 (Marine Corps Retirement and Separation Manual), and MCO 1040.31 (Enlisted Retention and Career Development Program). Published January 21, 2022. Accessed September 19, 2025. https://www.marines.mil/News/Messages/Messages-Display/Article/2907104/advance-notification-of-change-to-mco-63101c-pseudofolliculitis-barbae-mco-1900/
- US Department of Defense. Special duty catalog (SPECAT). Published August 15, 2013. Accessed September 19, 2025. https://share.google/iuMrVMIASWx4EFLVN
- Occupational Safety and Health Administration. Appendix A to §1910.134—fit testing procedures (mandatory). Accessed September 19, 2025. https://www.osha.gov/laws-regs/regulations/standardnumber/1910/1910.134AppA
- Jiang YR. Reasonable accommodation and disparate impact: clean shave policy discrimination in today’s workplace. J Law Med Ethics. 2023;51:185-195. doi:10.1017/jme.2023.55
- Welch D, Usatine R, Heath C. Implications of PFB beyond the razor. Cutis. 2025;115:135-136. doi:10.12788/cutis.1194
- Lawrence DF. Marines with skin condition affecting mostly black men could now be booted under new policy. Military.com. March 14, 2025. Accessed May 4, 2025. https://www.military.com/daily-news/2025/03/14/marines-can-now-be-kicked-out-skin-condition-affects-mostly-black-men.html
- Secretary of the Army. Army directive 2025-13 (facial hair grooming standards). Published July 7, 2025. Accessed September 19, 2025. https://lyster.tricare.mil/Portals/61/ARN44307-ARMY_DIR_2025-13-000.pdf
- Alexis A, Heath CR, Halder RM. Folliculitis keloidalis nuchae and pseudofolliculitis barbae: are prevention and effective treatment within reach? Dermatol Clin. 2014;32:183-191. doi:10.1016/j.det.2013.12.001
- Gray J, McMichael AJ. Pseudofolliculitis barbae: understanding the condition and the role of facial grooming. Int J Cosmet Sci. 2016;38:24-27. doi:10.1111/ics.12331
- Jung I, Lannan FM, Weiss A, et al. Treatment and current policies on pseudofolliculitis barbae in the US military. Cutis. 2023;112:299-302. doi:10.12788/cutis.0907
- Ritchie S, Park J, Banta J, et al. Shaving waivers in the United States Air Force and their impact on promotions of Black/African-American members. Mil Med. 2023;188:E242-E247. doi:10.1093/milmed/usab272
- Defense Manpower Data Center. Active-duty military personnel master file and reserve components common personnel data system. Military OneSource. September 2023. Accessed May 3, 2025. https://download.militaryonesource.mil/12038/MOS/Reports/2023-demographics-report.pdf
- Tshudy MT, Cho S. Pseudofolliculitis barbae in the US. Military, a review. Mil Med. 2021;186:E52-E57. doi:10.1093/milmed/usaa243
- US Marine Corps. Uniform and grooming standards for medical conditions (MARADMINS number: 124/25). Published March 13, 2025. Accessed September 19, 2025. https://www.marines.mil/News/Messages/Messages-Display/Article/4119098/uniform-and-grooming-standards-for-medical-conditions/
- US Marine Corps. Advance notification of change to MCO 6310.1C (Pseudofolliculitis Barbae), MCO 1900.16 CH2 (Marine Corps Retirement and Separation Manual), and MCO 1040.31 (Enlisted Retention and Career Development Program). Published January 21, 2022. Accessed September 19, 2025. https://www.marines.mil/News/Messages/Messages-Display/Article/2907104/advance-notification-of-change-to-mco-63101c-pseudofolliculitis-barbae-mco-1900/
- US Department of Defense. Special duty catalog (SPECAT). Published August 15, 2013. Accessed September 19, 2025. https://share.google/iuMrVMIASWx4EFLVN
- Occupational Safety and Health Administration. Appendix A to §1910.134—fit testing procedures (mandatory). Accessed September 19, 2025. https://www.osha.gov/laws-regs/regulations/standardnumber/1910/1910.134AppA
- Jiang YR. Reasonable accommodation and disparate impact: clean shave policy discrimination in today’s workplace. J Law Med Ethics. 2023;51:185-195. doi:10.1017/jme.2023.55
- Welch D, Usatine R, Heath C. Implications of PFB beyond the razor. Cutis. 2025;115:135-136. doi:10.12788/cutis.1194
Pseudofolliculitis Barbae in the Military: Policy, Stigma, and Practical Solutions
Pseudofolliculitis Barbae in the Military: Policy, Stigma, and Practical Solutions
Bridging the Funding Gap
Federal grants have supported cutting-edge research in scientific and biomedical fields since the mid-20th century, fueling public health breakthroughs and health innovations. This crucial support has been greatly diminished in recent months with deep cuts to federal research dollars.
As these acute policy changes continue to disrupt academic institutions and their investigators, introducing financial strain and operational uncertainty, the importance of research support from professional societies and foundations has become increasingly evident. Their targeted funding plays a critical role in sustaining biomedical research, which directly impacts clinical innovation and patient care. As one example, the AGA Research Foundation provides over $2 million annually to spur discoveries in gastroenterology and hepatology. This vital research support, awarded to 74 unique recipients (including 7 early-career Research Scholar Award recipients) in 2025, represents one of the most important investments that AGA makes in the future of gastroenterology and the patients we treat.
While foundation awards such as these cannot completely close the federal funding gap, they serve as an important lifeline both in supporting the core work of early career and established investigators in an uncertain funding environment and in funding high-risk, high-reward research that more conservative funders are often hesitant to invest in. – now more than ever, the funding it provides has tremendous impact.
In this issue of GI & Hepatology News, we update you on the FDA’s recent approval of semaglutide as a treatment for MASH with fibrosis and highlight a recent target trial emulation study that casts doubt on our traditional understanding regarding the link between common medications such as PPIs and NSAIDs and microscopic colitis in older adults. We also summarize newly-released, global guidelines for pregnancy and IBD, which deserve a careful read. In this month’s Member Spotlight, we feature Pascale White, MD, MBA, MS (Mount Sinai), a recent recipient of the AGA-Pfizer Beacon of Hope Award for Gender and Health Equity, who shares her inspirational work to improve colorectal cancer screening among underserved, high-risk patients in East Harlem. We hope you enjoy this and all the exciting content in our October issue.
Megan A. Adams, MD, JD, MSc
Editor in Chief
Federal grants have supported cutting-edge research in scientific and biomedical fields since the mid-20th century, fueling public health breakthroughs and health innovations. This crucial support has been greatly diminished in recent months with deep cuts to federal research dollars.
As these acute policy changes continue to disrupt academic institutions and their investigators, introducing financial strain and operational uncertainty, the importance of research support from professional societies and foundations has become increasingly evident. Their targeted funding plays a critical role in sustaining biomedical research, which directly impacts clinical innovation and patient care. As one example, the AGA Research Foundation provides over $2 million annually to spur discoveries in gastroenterology and hepatology. This vital research support, awarded to 74 unique recipients (including 7 early-career Research Scholar Award recipients) in 2025, represents one of the most important investments that AGA makes in the future of gastroenterology and the patients we treat.
While foundation awards such as these cannot completely close the federal funding gap, they serve as an important lifeline both in supporting the core work of early career and established investigators in an uncertain funding environment and in funding high-risk, high-reward research that more conservative funders are often hesitant to invest in. – now more than ever, the funding it provides has tremendous impact.
In this issue of GI & Hepatology News, we update you on the FDA’s recent approval of semaglutide as a treatment for MASH with fibrosis and highlight a recent target trial emulation study that casts doubt on our traditional understanding regarding the link between common medications such as PPIs and NSAIDs and microscopic colitis in older adults. We also summarize newly-released, global guidelines for pregnancy and IBD, which deserve a careful read. In this month’s Member Spotlight, we feature Pascale White, MD, MBA, MS (Mount Sinai), a recent recipient of the AGA-Pfizer Beacon of Hope Award for Gender and Health Equity, who shares her inspirational work to improve colorectal cancer screening among underserved, high-risk patients in East Harlem. We hope you enjoy this and all the exciting content in our October issue.
Megan A. Adams, MD, JD, MSc
Editor in Chief
Federal grants have supported cutting-edge research in scientific and biomedical fields since the mid-20th century, fueling public health breakthroughs and health innovations. This crucial support has been greatly diminished in recent months with deep cuts to federal research dollars.
As these acute policy changes continue to disrupt academic institutions and their investigators, introducing financial strain and operational uncertainty, the importance of research support from professional societies and foundations has become increasingly evident. Their targeted funding plays a critical role in sustaining biomedical research, which directly impacts clinical innovation and patient care. As one example, the AGA Research Foundation provides over $2 million annually to spur discoveries in gastroenterology and hepatology. This vital research support, awarded to 74 unique recipients (including 7 early-career Research Scholar Award recipients) in 2025, represents one of the most important investments that AGA makes in the future of gastroenterology and the patients we treat.
While foundation awards such as these cannot completely close the federal funding gap, they serve as an important lifeline both in supporting the core work of early career and established investigators in an uncertain funding environment and in funding high-risk, high-reward research that more conservative funders are often hesitant to invest in. – now more than ever, the funding it provides has tremendous impact.
In this issue of GI & Hepatology News, we update you on the FDA’s recent approval of semaglutide as a treatment for MASH with fibrosis and highlight a recent target trial emulation study that casts doubt on our traditional understanding regarding the link between common medications such as PPIs and NSAIDs and microscopic colitis in older adults. We also summarize newly-released, global guidelines for pregnancy and IBD, which deserve a careful read. In this month’s Member Spotlight, we feature Pascale White, MD, MBA, MS (Mount Sinai), a recent recipient of the AGA-Pfizer Beacon of Hope Award for Gender and Health Equity, who shares her inspirational work to improve colorectal cancer screening among underserved, high-risk patients in East Harlem. We hope you enjoy this and all the exciting content in our October issue.
Megan A. Adams, MD, JD, MSc
Editor in Chief
When The Giants and Those Who Stand on Their Shoulders Are Gone: The Loss of VA Institutional Memory
When The Giants and Those Who Stand on Their Shoulders Are Gone: The Loss of VA Institutional Memory
If I have seen further, it is by standing on the shoulders of giants.
Sir Isaac Newton (1642-1727) 1
Early in residency, I decided I only wanted to work at the US Department of Veterans Affairs (VA). It was a way to follow the example of service that my parents, an Army doctor and nurse, had set. I spent much of my residency, including all of my last year of training, at a VA medical center, hoping a vacancy would open in the psychiatry service. In those days, VA jobs were hard to come by; doctors spent their entire careers in the system, only retiring after decades of commitment to its unique mission. Finally, close to graduation, one of my favorite attending physicians left his post. After mountains of paperwork and running the human resources obstacle course with the usual stumbles, I arrived at my dream job as a VA psychiatrist.
So, it is with immense sadness and even shock that I read a recent ProPublica article reporting that from January to March 2025 almost 40% of the physicians who received employment offers from the Veterans Health Administration (VHA) declined the positions.2 Medical media rapidly picked up the story, likely further discouraging potential applicants.3
There have always been health care professionals (HCPs) who had zero interest in working for the VA. Medical students and residents often have a love/hate relationship with the VA, with some trainees not having the patience for the behemoth pace of the bureaucracy or finding the old-style physical environment and more relaxed pace antiquated and inefficient.
The reasons doctors are saying no to VA employment at 4 times the previous rate are different and more disturbing. According to ProPublica, VA officials in Texas reported in a June internal presentation that about 90 people had turned down job offers due to the “uncertainty of reorganization.”2 They reported that low morale was causing existing employees to recommend against working at the VA. My own anecdotal experience is similar: contrary to prior years, few residents, if any, are interested in working at the VA because of concerns about the stability of employment and the direction of its organizational culture.
It is fair to question the objectivity of the ProPublica report. However, the latest VA Office of the Inspector General (OIG) analysis of staffing had similar findings. “Despite the ability to make noncompetitive appointments for such occupations, VHA continues to experience severe occupational staffing shortages for these occupations that are fundamental to the delivery of health care.” The 4434 severe occupational shortage figures in fiscal year (FY) 2025 were 50% higher than in FY 2024.4 OIG reported that 57% of facilities noted severe occupational staffing shortages for psychology, making it the most frequently reported clinical shortage.
At this critical juncture, when new health care professional energy is not flowing into the VHA, there is an unprecedented drain of the lifeblood of any system—the departure of the bearers of institutional memory. Early and scheduled retirements, the deferred resignation program, and severance have decimated the ranks of senior HCPs, experienced leaders, and career clinicians. ProPublica noted the loss of 600 doctors and 1900 nurses at the VHA so far in 2025.2 Internal VA data from exit interviews suggest similar motivations. Many cited lack of trust and confidence in senior leaders and job stress/pressure.5
It should be noted the VA has an alternative and plausible explanation for the expected departure of 30,000 employees. They argue that the VHA was overstaffed and the increased workforce decreased the efficiency of service. Voluntary separation from employment, VA contends, has avoided the need for a far more disruptive reduction in force. VA leaders avow that downsizing has not adversely impacted its ability to deliver high-quality health care and benefits and they assert that a reduction in red tape will enable VA to provide easier access to care. VA Secretary Doug Collins has concluded that because of these difficult but necessary changes, “VA is headed in the right direction.”6
What is institutional memory, and why is it important? “The core of institutional memory is collective awareness and understanding of a collective set of facts, concepts, experiences, and know-how,” Bhugra and Ventriglio explain. “These are all held collectively at various levels in any given institution. Thus, collective memory or history can be utilized to build on what has gone before and how we take things forward.”7
The authors of this quote offer a modern twist on what Sir Isaac Newton described in more metaphorical language in the epigraph: to survive, and even more to thrive, an enterprise must have those who have accumulated technical knowledge and professional wisdom as well as those who assume responsibility for appropriating and adding to this storehouse of operational skill, expertise, unique cultural values, and ethical commitments. The VHA is losing its instructors and students of institutional memory which deals a serious blow to the stability and vitality of any learning health system.6 As Bhugra and Ventriglio put it, institutional memory identifies “what has worked in delivering the aims in the past and what has not, thereby ensuring the lessons learnt are remembered and passed on to the next generation.”7
Nearly every week, at all levels of the agency, I have encountered this exodus of builders and bearers of institutional memory. Those who have left did so for many of the same reasons cited by those who declined to come, leaving incalculable gaps at both ends of the career spectrum. Both the old and new are essential for organizational resilience: fresh ideas enable an institution to be agile in responding to challenges, while operational savvy ensures responses are ecologically aligned with the organizational mission.8
The dire shortage of HCPs—especially in mental health and primary care—has opened up unprecedented opportunities.9 Colleagues have noted that with only a little searching they found multiple lucrative positions. Once, HCPs picked the VA because they valued the commitment to public service and being part of a community of education and research more than fame or fortune. Having the best benefits packages in the industry only reinforced its value.
Even so, surpassing a genius such as Sir Isaac Newton, writing to a scientific competitor, Robert Hooke, recognized that progress and discovery in science and medicine are nigh well impossible without the collective achievements housed in institutional memory.1 It was inspiring teachers and attending physicians—Newton’s giants—who attracted the best and brightest in medicine and nursing, other HCPs, and research, to the VA, where they could participate in a transactive organizational learning process from their seniors, and then grow that fund of knowledge to improve patient care, educate their learners, and innovate. What will happen when there are no longer shoulders of giants or anyone to stand on them?
- Chen C. Mapping Scientific Frontiers: The Quest for Knowledge Visualization. Springer; 2013:135.
- Armstrong D, Umansky E, Coleman V. Veterans’ care at risk under Trump as hundreds of doctors and nurses reject working at VA hospitals. ProPublica. August 8, 2025. Accessed August 25, 2025. https://www.propublica.org/article/veterans-affairs-hospital-shortages-trump
- Kuchno K. VA physician job offers rejections up fourfold in 2025: report. Becker’s Hospital Review. August 12, 2025. Accessed August 26, 2025. https://www.beckershospitalreview.com/workforce/va-physician-job-offer-rejections-up-fourfold-in-2025-report/
- US Department of Veterans Affairs, Office of Inspector General. OIG determination of Veterans Health Administration’s severe occupational staffing shortages fiscal year 2025. August 12, 2025. Accessed August 25, 2025. https://www.vaoig.gov/reports/national-healthcare-review/oig-determination-veterans-health-administrations-severe-1
- US Department of Veterans Affairs. VA workforce dashboard. July 25, 2025. Accessed August 25, 2025. https://www.va.gov/EMPLOYEE/docs/workforce/VA-Workforce-Dashboard-Issue-27.pdf
- VA to reduce staff by nearly 30K by end of FY2025. News release. Veterans Affairs News. July 7, 2025. Accessed August 25, 2025. https://news.va.gov/press-room/va-to-reduce-staff-by-nearly-30k-by-end-of-fy2025/
- Bhugra D, Ventriglio A. Institutions, institutional memory, healthcare and research. Int J Soc Psychiatry. 2023;69(8):1843-1844. doi:10.1177/00207640231213905
- Jain A. Is organizational memory a useful capability? An analysis of its effects on productivity, absorptive capacity adaptation. In Argote L, Levine JM. The Oxford Handbook of Group and Organizational Learning. Oxford; 2020.
- Broder J. Ready to pick a specialty? These may have the brightest futures. Medscape. April 21, 2025. Accessed August 25, 2025. https://www.medscape.com/viewarticle/ready-pick-specialty-these-may-have-brightest-futures-2025a10009if
If I have seen further, it is by standing on the shoulders of giants.
Sir Isaac Newton (1642-1727) 1
Early in residency, I decided I only wanted to work at the US Department of Veterans Affairs (VA). It was a way to follow the example of service that my parents, an Army doctor and nurse, had set. I spent much of my residency, including all of my last year of training, at a VA medical center, hoping a vacancy would open in the psychiatry service. In those days, VA jobs were hard to come by; doctors spent their entire careers in the system, only retiring after decades of commitment to its unique mission. Finally, close to graduation, one of my favorite attending physicians left his post. After mountains of paperwork and running the human resources obstacle course with the usual stumbles, I arrived at my dream job as a VA psychiatrist.
So, it is with immense sadness and even shock that I read a recent ProPublica article reporting that from January to March 2025 almost 40% of the physicians who received employment offers from the Veterans Health Administration (VHA) declined the positions.2 Medical media rapidly picked up the story, likely further discouraging potential applicants.3
There have always been health care professionals (HCPs) who had zero interest in working for the VA. Medical students and residents often have a love/hate relationship with the VA, with some trainees not having the patience for the behemoth pace of the bureaucracy or finding the old-style physical environment and more relaxed pace antiquated and inefficient.
The reasons doctors are saying no to VA employment at 4 times the previous rate are different and more disturbing. According to ProPublica, VA officials in Texas reported in a June internal presentation that about 90 people had turned down job offers due to the “uncertainty of reorganization.”2 They reported that low morale was causing existing employees to recommend against working at the VA. My own anecdotal experience is similar: contrary to prior years, few residents, if any, are interested in working at the VA because of concerns about the stability of employment and the direction of its organizational culture.
It is fair to question the objectivity of the ProPublica report. However, the latest VA Office of the Inspector General (OIG) analysis of staffing had similar findings. “Despite the ability to make noncompetitive appointments for such occupations, VHA continues to experience severe occupational staffing shortages for these occupations that are fundamental to the delivery of health care.” The 4434 severe occupational shortage figures in fiscal year (FY) 2025 were 50% higher than in FY 2024.4 OIG reported that 57% of facilities noted severe occupational staffing shortages for psychology, making it the most frequently reported clinical shortage.
At this critical juncture, when new health care professional energy is not flowing into the VHA, there is an unprecedented drain of the lifeblood of any system—the departure of the bearers of institutional memory. Early and scheduled retirements, the deferred resignation program, and severance have decimated the ranks of senior HCPs, experienced leaders, and career clinicians. ProPublica noted the loss of 600 doctors and 1900 nurses at the VHA so far in 2025.2 Internal VA data from exit interviews suggest similar motivations. Many cited lack of trust and confidence in senior leaders and job stress/pressure.5
It should be noted the VA has an alternative and plausible explanation for the expected departure of 30,000 employees. They argue that the VHA was overstaffed and the increased workforce decreased the efficiency of service. Voluntary separation from employment, VA contends, has avoided the need for a far more disruptive reduction in force. VA leaders avow that downsizing has not adversely impacted its ability to deliver high-quality health care and benefits and they assert that a reduction in red tape will enable VA to provide easier access to care. VA Secretary Doug Collins has concluded that because of these difficult but necessary changes, “VA is headed in the right direction.”6
What is institutional memory, and why is it important? “The core of institutional memory is collective awareness and understanding of a collective set of facts, concepts, experiences, and know-how,” Bhugra and Ventriglio explain. “These are all held collectively at various levels in any given institution. Thus, collective memory or history can be utilized to build on what has gone before and how we take things forward.”7
The authors of this quote offer a modern twist on what Sir Isaac Newton described in more metaphorical language in the epigraph: to survive, and even more to thrive, an enterprise must have those who have accumulated technical knowledge and professional wisdom as well as those who assume responsibility for appropriating and adding to this storehouse of operational skill, expertise, unique cultural values, and ethical commitments. The VHA is losing its instructors and students of institutional memory which deals a serious blow to the stability and vitality of any learning health system.6 As Bhugra and Ventriglio put it, institutional memory identifies “what has worked in delivering the aims in the past and what has not, thereby ensuring the lessons learnt are remembered and passed on to the next generation.”7
Nearly every week, at all levels of the agency, I have encountered this exodus of builders and bearers of institutional memory. Those who have left did so for many of the same reasons cited by those who declined to come, leaving incalculable gaps at both ends of the career spectrum. Both the old and new are essential for organizational resilience: fresh ideas enable an institution to be agile in responding to challenges, while operational savvy ensures responses are ecologically aligned with the organizational mission.8
The dire shortage of HCPs—especially in mental health and primary care—has opened up unprecedented opportunities.9 Colleagues have noted that with only a little searching they found multiple lucrative positions. Once, HCPs picked the VA because they valued the commitment to public service and being part of a community of education and research more than fame or fortune. Having the best benefits packages in the industry only reinforced its value.
Even so, surpassing a genius such as Sir Isaac Newton, writing to a scientific competitor, Robert Hooke, recognized that progress and discovery in science and medicine are nigh well impossible without the collective achievements housed in institutional memory.1 It was inspiring teachers and attending physicians—Newton’s giants—who attracted the best and brightest in medicine and nursing, other HCPs, and research, to the VA, where they could participate in a transactive organizational learning process from their seniors, and then grow that fund of knowledge to improve patient care, educate their learners, and innovate. What will happen when there are no longer shoulders of giants or anyone to stand on them?
If I have seen further, it is by standing on the shoulders of giants.
Sir Isaac Newton (1642-1727) 1
Early in residency, I decided I only wanted to work at the US Department of Veterans Affairs (VA). It was a way to follow the example of service that my parents, an Army doctor and nurse, had set. I spent much of my residency, including all of my last year of training, at a VA medical center, hoping a vacancy would open in the psychiatry service. In those days, VA jobs were hard to come by; doctors spent their entire careers in the system, only retiring after decades of commitment to its unique mission. Finally, close to graduation, one of my favorite attending physicians left his post. After mountains of paperwork and running the human resources obstacle course with the usual stumbles, I arrived at my dream job as a VA psychiatrist.
So, it is with immense sadness and even shock that I read a recent ProPublica article reporting that from January to March 2025 almost 40% of the physicians who received employment offers from the Veterans Health Administration (VHA) declined the positions.2 Medical media rapidly picked up the story, likely further discouraging potential applicants.3
There have always been health care professionals (HCPs) who had zero interest in working for the VA. Medical students and residents often have a love/hate relationship with the VA, with some trainees not having the patience for the behemoth pace of the bureaucracy or finding the old-style physical environment and more relaxed pace antiquated and inefficient.
The reasons doctors are saying no to VA employment at 4 times the previous rate are different and more disturbing. According to ProPublica, VA officials in Texas reported in a June internal presentation that about 90 people had turned down job offers due to the “uncertainty of reorganization.”2 They reported that low morale was causing existing employees to recommend against working at the VA. My own anecdotal experience is similar: contrary to prior years, few residents, if any, are interested in working at the VA because of concerns about the stability of employment and the direction of its organizational culture.
It is fair to question the objectivity of the ProPublica report. However, the latest VA Office of the Inspector General (OIG) analysis of staffing had similar findings. “Despite the ability to make noncompetitive appointments for such occupations, VHA continues to experience severe occupational staffing shortages for these occupations that are fundamental to the delivery of health care.” The 4434 severe occupational shortage figures in fiscal year (FY) 2025 were 50% higher than in FY 2024.4 OIG reported that 57% of facilities noted severe occupational staffing shortages for psychology, making it the most frequently reported clinical shortage.
At this critical juncture, when new health care professional energy is not flowing into the VHA, there is an unprecedented drain of the lifeblood of any system—the departure of the bearers of institutional memory. Early and scheduled retirements, the deferred resignation program, and severance have decimated the ranks of senior HCPs, experienced leaders, and career clinicians. ProPublica noted the loss of 600 doctors and 1900 nurses at the VHA so far in 2025.2 Internal VA data from exit interviews suggest similar motivations. Many cited lack of trust and confidence in senior leaders and job stress/pressure.5
It should be noted the VA has an alternative and plausible explanation for the expected departure of 30,000 employees. They argue that the VHA was overstaffed and the increased workforce decreased the efficiency of service. Voluntary separation from employment, VA contends, has avoided the need for a far more disruptive reduction in force. VA leaders avow that downsizing has not adversely impacted its ability to deliver high-quality health care and benefits and they assert that a reduction in red tape will enable VA to provide easier access to care. VA Secretary Doug Collins has concluded that because of these difficult but necessary changes, “VA is headed in the right direction.”6
What is institutional memory, and why is it important? “The core of institutional memory is collective awareness and understanding of a collective set of facts, concepts, experiences, and know-how,” Bhugra and Ventriglio explain. “These are all held collectively at various levels in any given institution. Thus, collective memory or history can be utilized to build on what has gone before and how we take things forward.”7
The authors of this quote offer a modern twist on what Sir Isaac Newton described in more metaphorical language in the epigraph: to survive, and even more to thrive, an enterprise must have those who have accumulated technical knowledge and professional wisdom as well as those who assume responsibility for appropriating and adding to this storehouse of operational skill, expertise, unique cultural values, and ethical commitments. The VHA is losing its instructors and students of institutional memory which deals a serious blow to the stability and vitality of any learning health system.6 As Bhugra and Ventriglio put it, institutional memory identifies “what has worked in delivering the aims in the past and what has not, thereby ensuring the lessons learnt are remembered and passed on to the next generation.”7
Nearly every week, at all levels of the agency, I have encountered this exodus of builders and bearers of institutional memory. Those who have left did so for many of the same reasons cited by those who declined to come, leaving incalculable gaps at both ends of the career spectrum. Both the old and new are essential for organizational resilience: fresh ideas enable an institution to be agile in responding to challenges, while operational savvy ensures responses are ecologically aligned with the organizational mission.8
The dire shortage of HCPs—especially in mental health and primary care—has opened up unprecedented opportunities.9 Colleagues have noted that with only a little searching they found multiple lucrative positions. Once, HCPs picked the VA because they valued the commitment to public service and being part of a community of education and research more than fame or fortune. Having the best benefits packages in the industry only reinforced its value.
Even so, surpassing a genius such as Sir Isaac Newton, writing to a scientific competitor, Robert Hooke, recognized that progress and discovery in science and medicine are nigh well impossible without the collective achievements housed in institutional memory.1 It was inspiring teachers and attending physicians—Newton’s giants—who attracted the best and brightest in medicine and nursing, other HCPs, and research, to the VA, where they could participate in a transactive organizational learning process from their seniors, and then grow that fund of knowledge to improve patient care, educate their learners, and innovate. What will happen when there are no longer shoulders of giants or anyone to stand on them?
- Chen C. Mapping Scientific Frontiers: The Quest for Knowledge Visualization. Springer; 2013:135.
- Armstrong D, Umansky E, Coleman V. Veterans’ care at risk under Trump as hundreds of doctors and nurses reject working at VA hospitals. ProPublica. August 8, 2025. Accessed August 25, 2025. https://www.propublica.org/article/veterans-affairs-hospital-shortages-trump
- Kuchno K. VA physician job offers rejections up fourfold in 2025: report. Becker’s Hospital Review. August 12, 2025. Accessed August 26, 2025. https://www.beckershospitalreview.com/workforce/va-physician-job-offer-rejections-up-fourfold-in-2025-report/
- US Department of Veterans Affairs, Office of Inspector General. OIG determination of Veterans Health Administration’s severe occupational staffing shortages fiscal year 2025. August 12, 2025. Accessed August 25, 2025. https://www.vaoig.gov/reports/national-healthcare-review/oig-determination-veterans-health-administrations-severe-1
- US Department of Veterans Affairs. VA workforce dashboard. July 25, 2025. Accessed August 25, 2025. https://www.va.gov/EMPLOYEE/docs/workforce/VA-Workforce-Dashboard-Issue-27.pdf
- VA to reduce staff by nearly 30K by end of FY2025. News release. Veterans Affairs News. July 7, 2025. Accessed August 25, 2025. https://news.va.gov/press-room/va-to-reduce-staff-by-nearly-30k-by-end-of-fy2025/
- Bhugra D, Ventriglio A. Institutions, institutional memory, healthcare and research. Int J Soc Psychiatry. 2023;69(8):1843-1844. doi:10.1177/00207640231213905
- Jain A. Is organizational memory a useful capability? An analysis of its effects on productivity, absorptive capacity adaptation. In Argote L, Levine JM. The Oxford Handbook of Group and Organizational Learning. Oxford; 2020.
- Broder J. Ready to pick a specialty? These may have the brightest futures. Medscape. April 21, 2025. Accessed August 25, 2025. https://www.medscape.com/viewarticle/ready-pick-specialty-these-may-have-brightest-futures-2025a10009if
- Chen C. Mapping Scientific Frontiers: The Quest for Knowledge Visualization. Springer; 2013:135.
- Armstrong D, Umansky E, Coleman V. Veterans’ care at risk under Trump as hundreds of doctors and nurses reject working at VA hospitals. ProPublica. August 8, 2025. Accessed August 25, 2025. https://www.propublica.org/article/veterans-affairs-hospital-shortages-trump
- Kuchno K. VA physician job offers rejections up fourfold in 2025: report. Becker’s Hospital Review. August 12, 2025. Accessed August 26, 2025. https://www.beckershospitalreview.com/workforce/va-physician-job-offer-rejections-up-fourfold-in-2025-report/
- US Department of Veterans Affairs, Office of Inspector General. OIG determination of Veterans Health Administration’s severe occupational staffing shortages fiscal year 2025. August 12, 2025. Accessed August 25, 2025. https://www.vaoig.gov/reports/national-healthcare-review/oig-determination-veterans-health-administrations-severe-1
- US Department of Veterans Affairs. VA workforce dashboard. July 25, 2025. Accessed August 25, 2025. https://www.va.gov/EMPLOYEE/docs/workforce/VA-Workforce-Dashboard-Issue-27.pdf
- VA to reduce staff by nearly 30K by end of FY2025. News release. Veterans Affairs News. July 7, 2025. Accessed August 25, 2025. https://news.va.gov/press-room/va-to-reduce-staff-by-nearly-30k-by-end-of-fy2025/
- Bhugra D, Ventriglio A. Institutions, institutional memory, healthcare and research. Int J Soc Psychiatry. 2023;69(8):1843-1844. doi:10.1177/00207640231213905
- Jain A. Is organizational memory a useful capability? An analysis of its effects on productivity, absorptive capacity adaptation. In Argote L, Levine JM. The Oxford Handbook of Group and Organizational Learning. Oxford; 2020.
- Broder J. Ready to pick a specialty? These may have the brightest futures. Medscape. April 21, 2025. Accessed August 25, 2025. https://www.medscape.com/viewarticle/ready-pick-specialty-these-may-have-brightest-futures-2025a10009if
When The Giants and Those Who Stand on Their Shoulders Are Gone: The Loss of VA Institutional Memory
When The Giants and Those Who Stand on Their Shoulders Are Gone: The Loss of VA Institutional Memory
From Refractory to Responsive: The Expanding Therapeutic Landscape of Prurigo Nodularis
From Refractory to Responsive: The Expanding Therapeutic Landscape of Prurigo Nodularis
Prurigo nodularis (PN) is a chronic, severely pruritic neuroimmunologic skin disorder characterized by multiple firm hyperkeratotic nodules and intense pruritus, often leading to considerable impairment in quality of life and increased rates of depression and anxiety.1 It is considered difficult to treat due to its complex pathogenesis, the severity and chronicity of pruritus, and the limited efficacy of conventional therapies.2,3 The disease is driven by a self-perpetuating itch-scratch cycle, underpinned by dysregulation of both immune and neural pathways including type 2 (interleukin [IL] 4, IL-13, IL-31), Th17, and Th22 cytokines as well as neuropeptides and altered cutaneous nerve architecture.1,3 This results in persistent severe pruritus and nodular lesions that are highly refractory to standard treatments.1 Conventional therapies (eg, locally acting agents, phototherapy, and systemic immunomodulators and neuromodulators) have varied efficacy and notable adverse effect profiles.3 While the approval of targeted biologics has transformed the therapeutic landscape, several other treatment options also are being explored in clinical trials. Herein, we review all recently approved therapies as well as emerging treatments currently under investigation.
Dupilumab
Dupilumab, the first therapy for PN approved by the US Food and Drug Administration (FDA) in 2022—is a monoclonal antibody that inhibits signaling of IL-4 and IL-13, key drivers of type 2 inflammation implicated in PN pathogenesis.4,5 In 2 pivotal phase 3 randomized controlled trials (LIBERTY-PN PRIME and PRIME2),5 dupilumab demonstrated notable efficacy in adults with moderate to severe PN. A reduction of 4 points or more on the Worst Itch Numeric Rating Scale (WI-NRS) was achieved by 60.0% (45/75) of patients treated with dupilumab at week 24 compared with 18.4% (14/76) receiving placebo in the PRIME trial. In PRIME2, the same outcome was achieved by 37.2% (29/78) of patients receiving dupilumab at week 12 compared with 22.0% (18/82) of patients receiving placebo.5 Dupilumab also led to a greater proportion of patients achieving a substantial reduction in nodule count (≤5 nodules) and improved quality of life compared with placebo.5,6 The safety profile of dupilumab for treatment of PN was favorable and consistent with prior experience in atopic dermatitis; conjunctivitis was the most common adverse event.5,6
Nemolizumab
Nemolizumab, an IL-31 receptor A antagonist, is the most recent agent approved by the FDA for PN in 2024.7 In the OLYMPIA 1 and OLYMPIA 2 phase 3 trials,8 nemolizumab produced a clinically meaningful reduction in itch (defined as a ≥4-point improvement in the Peak Pruritus Numerical Rating Scale score) in 56.3% (103/183) of patients at week 16 compared with 20.9% (19/91) receiving placebo. Additionally, 37.7% (69/183) of patients receiving nemolizumab achieved clear or almost clear skin (Investigator’s Global Assessment score of 0 or 1 with a ≥2-point reduction) vs 11.0% with placebo (both P<.001). Benefits were observed as early as week 4, including rapid improvements in itch, sleep disturbance, and nodule count.8 Nemolizumab also improved quality of life and reduced symptoms of anxiety and depression. The safety profile was favorable, with headache and atopic dermatitis the most common adverse events; serious adverse events were infrequent and similar between groups.8
Abrocitinib
Abrocitinib, an oral selective Janus kinase 1 inhibitor, is an investigational therapy for PN and currently has not been approved by the FDA for this indication. In a phase 2 open-label trial, abrocitinib 200 mg daily for 12 weeks led to a 78.3% reduction in weekly Peak Pruritus Numerical Rating Scale scores in PN, with 80.0% (8/10) of patients achieving a clinically meaningful improvement of 4 points or higher. Nodule counts and quality of life also improved, with an onset of itch relief as early as week 2. The safety profile was favorable, with acneform eruptions the most common adverse event and no serious adverse events reported9; however, these results are based on small, nonrandomized studies and require confirmation in larger randomized controlled trials before abrocitinib can be considered a standard therapy for PN.
Cryosim-1
Transient receptor potential melastatin 8 (TRPM8) is a cold-sensing ion channel found in unmyelinated sensory neurons within the dorsal root and trigeminal ganglia.10 It is activated by cool temperatures (15-28 °C) and compounds such as menthol, leading to calcium influx and a cooling sensation. In a randomized, double-blind, vehicle-controlled trial, researchers investigated the efficacy of cryosim-1 (a synthetic TRPM8 agonist) in treating PN.10 Thirty patients were enrolled, with 18 (60.0%) receiving cryosim-1 and 12 (40.0%) receiving placebo over 8 weeks. By week 8, cryosim-1 significantly reduced itch severity (mean numerical rating scale score postapplication, 2.8 vs 4.3; P=.031) and improved sleep disturbances (2.2 vs 4.2; P=.031) compared to placebo. Patients reported higher satisfaction with itch relief, and no adverse effects were observed. The study concluded that cryosim-1 is a safe, effective topical therapy for PN, likely working by interrupting the itch-scratch cycle and potentially modulating inflammatory pathways involved in chronic itch.10
Nalbuphine
Nalbuphine is a κ opioid receptor agonist and μ opioid receptor antagonist that has been investigated for the treatment of PN.11 In a phase 2 randomized controlled trial, oral nalbuphine extended release (NAL-ER) 162 mg twice daily provided measurable antipruritic efficacy, with 44.4% (8/18) of patients achieving at least a 30% reduction in 7-day WI-NRS at week 10 compared with 36.4% (8/22) in the placebo group. Among those who completed the study, 66.7% (8/12) of patients receiving NAL-ER 162 mg achieved significant itch reduction vs 40% (8/20) receiving placebo (P=.03). At least a 50% reduction in WI-NRS was achieved by 33.3% (6/18) of patients receiving NAL-ER 162 mg twice daily. Extended open-label treatment was associated with further improvements in itch and lesion activity. Adverse events were mostly mild to moderate (eg, nausea, dizziness, headache, and fatigue) and occurred during dose titration. Physiologic opioid withdrawal symptoms were limited and resolved within a few days of discontinuing the medication.11
Final Thoughts
In conclusion, PN remains one of the most challenging chronic dermatologic conditions to manage and is driven by a complex interplay of neuroimmune mechanisms and resistance to many conventional therapies. The approval of dupilumab and nemolizumab has marked a pivotal shift in the therapeutic landscape, offering hope to patients who previously had limited options5,8; however, the burden of PN remains substantial, and many patients continue to experience relentless itch, poor sleep, and reduced quality of life.1 Emerging therapies such as TRPM8 agonists, Janus kinase inhibitors, and opioid modulators represent promising additions to the treatment options, targeting novel pathways beyond traditional immunosuppression.9-11
- Williams KA, Huang AH, Belzberg M, et al. Prurigo nodularis: pathogenesis and management. J Am Acad Dermatol. 2020;83:1567-1575. doi:10.1016/j.jaad.2020.04.182
- Gründel S, Pereira MP, Storck M, et al. Analysis of 325 patients with chronic nodular prurigo: clinics, burden of disease and course of treatment. Acta Derm Venereol. 2020;100:adv00269. doi:10.2340/00015555-3571
- Liao V, Cornman HL, Ma E, et al. Prurigo nodularis: new insights into pathogenesis and novel therapeutics. Br J Dermatol. 2024;190:798-810. doi:10.1093/bjd/ljae052
- Elmariah SB, Tao L, Valdes-Rodriguez R, et al. Individual article: management of prurigo nodularis. J Drugs Dermatol. 2023;22:SF365502s15-SF365502s22. doi:10.36849/JDD.SF365502
- Yosipovitch G, Mollanazar N, Ständer S, et al. Dupilumab in patients with prurigo nodularis: two randomized, double-blind, placebo-controlled phase 3 trials. Nat Med. 2023;29:1180-1190. doi:10.1038/s41591-023-02320-9
- Cao P, Xu W, Jiang S, et al. Dupilumab for the treatment of prurigo nodularis: a systematic review. Front Immunol. 2023;14:1092685. doi:10.3389/fimmu.2023.1092685
- Dagenet CB, Saadi C, Phillips MA, et al. Landscape of prurigo nodularis clinical trials. JAAD Rev. 2024;2:127-136. doi:10.1016/j.jdrv.2024.09.006
- Kwatra SG, Yosipovitch G, Legat FJ, et al. Phase 3 trial of nemolizumab in patients with prurigo nodularis. N Engl J Med. 2023;389:1579-1589. doi:10.1056/NEJMoa2301333
- Kwatra SG, Bordeaux ZA, Parthasarathy V, et al. Efficacy and safety of abrocitinib in prurigo nodularis and chronic pruritus of unknown origin: a nonrandomized controlled trial. JAMA Dermatol. 2024;160:717-724. doi:10.1001/jamadermatol.2024.1464
- Choi ME, Lee JH, Jung CJ, et al. A randomized, double-blinded, vehicle-controlled clinical trial of topical cryosim-1, a synthetic TRPM8 agonist, in prurigo nodularis. J Cosmet Dermatol. 2024;23:931-937. doi:10.1111/jocd.16079
- Weisshaar E, Szepietowski JC, Bernhard JD, et al. Efficacy and safety of oral nalbuphine extended release in prurigo nodularis: results of a phase 2 randomized controlled trial with an open-label extension phase. J Eur Acad Dermatol Venereol. 2022;36:453-461. doi:10.1111/jdv.17816
Prurigo nodularis (PN) is a chronic, severely pruritic neuroimmunologic skin disorder characterized by multiple firm hyperkeratotic nodules and intense pruritus, often leading to considerable impairment in quality of life and increased rates of depression and anxiety.1 It is considered difficult to treat due to its complex pathogenesis, the severity and chronicity of pruritus, and the limited efficacy of conventional therapies.2,3 The disease is driven by a self-perpetuating itch-scratch cycle, underpinned by dysregulation of both immune and neural pathways including type 2 (interleukin [IL] 4, IL-13, IL-31), Th17, and Th22 cytokines as well as neuropeptides and altered cutaneous nerve architecture.1,3 This results in persistent severe pruritus and nodular lesions that are highly refractory to standard treatments.1 Conventional therapies (eg, locally acting agents, phototherapy, and systemic immunomodulators and neuromodulators) have varied efficacy and notable adverse effect profiles.3 While the approval of targeted biologics has transformed the therapeutic landscape, several other treatment options also are being explored in clinical trials. Herein, we review all recently approved therapies as well as emerging treatments currently under investigation.
Dupilumab
Dupilumab, the first therapy for PN approved by the US Food and Drug Administration (FDA) in 2022—is a monoclonal antibody that inhibits signaling of IL-4 and IL-13, key drivers of type 2 inflammation implicated in PN pathogenesis.4,5 In 2 pivotal phase 3 randomized controlled trials (LIBERTY-PN PRIME and PRIME2),5 dupilumab demonstrated notable efficacy in adults with moderate to severe PN. A reduction of 4 points or more on the Worst Itch Numeric Rating Scale (WI-NRS) was achieved by 60.0% (45/75) of patients treated with dupilumab at week 24 compared with 18.4% (14/76) receiving placebo in the PRIME trial. In PRIME2, the same outcome was achieved by 37.2% (29/78) of patients receiving dupilumab at week 12 compared with 22.0% (18/82) of patients receiving placebo.5 Dupilumab also led to a greater proportion of patients achieving a substantial reduction in nodule count (≤5 nodules) and improved quality of life compared with placebo.5,6 The safety profile of dupilumab for treatment of PN was favorable and consistent with prior experience in atopic dermatitis; conjunctivitis was the most common adverse event.5,6
Nemolizumab
Nemolizumab, an IL-31 receptor A antagonist, is the most recent agent approved by the FDA for PN in 2024.7 In the OLYMPIA 1 and OLYMPIA 2 phase 3 trials,8 nemolizumab produced a clinically meaningful reduction in itch (defined as a ≥4-point improvement in the Peak Pruritus Numerical Rating Scale score) in 56.3% (103/183) of patients at week 16 compared with 20.9% (19/91) receiving placebo. Additionally, 37.7% (69/183) of patients receiving nemolizumab achieved clear or almost clear skin (Investigator’s Global Assessment score of 0 or 1 with a ≥2-point reduction) vs 11.0% with placebo (both P<.001). Benefits were observed as early as week 4, including rapid improvements in itch, sleep disturbance, and nodule count.8 Nemolizumab also improved quality of life and reduced symptoms of anxiety and depression. The safety profile was favorable, with headache and atopic dermatitis the most common adverse events; serious adverse events were infrequent and similar between groups.8
Abrocitinib
Abrocitinib, an oral selective Janus kinase 1 inhibitor, is an investigational therapy for PN and currently has not been approved by the FDA for this indication. In a phase 2 open-label trial, abrocitinib 200 mg daily for 12 weeks led to a 78.3% reduction in weekly Peak Pruritus Numerical Rating Scale scores in PN, with 80.0% (8/10) of patients achieving a clinically meaningful improvement of 4 points or higher. Nodule counts and quality of life also improved, with an onset of itch relief as early as week 2. The safety profile was favorable, with acneform eruptions the most common adverse event and no serious adverse events reported9; however, these results are based on small, nonrandomized studies and require confirmation in larger randomized controlled trials before abrocitinib can be considered a standard therapy for PN.
Cryosim-1
Transient receptor potential melastatin 8 (TRPM8) is a cold-sensing ion channel found in unmyelinated sensory neurons within the dorsal root and trigeminal ganglia.10 It is activated by cool temperatures (15-28 °C) and compounds such as menthol, leading to calcium influx and a cooling sensation. In a randomized, double-blind, vehicle-controlled trial, researchers investigated the efficacy of cryosim-1 (a synthetic TRPM8 agonist) in treating PN.10 Thirty patients were enrolled, with 18 (60.0%) receiving cryosim-1 and 12 (40.0%) receiving placebo over 8 weeks. By week 8, cryosim-1 significantly reduced itch severity (mean numerical rating scale score postapplication, 2.8 vs 4.3; P=.031) and improved sleep disturbances (2.2 vs 4.2; P=.031) compared to placebo. Patients reported higher satisfaction with itch relief, and no adverse effects were observed. The study concluded that cryosim-1 is a safe, effective topical therapy for PN, likely working by interrupting the itch-scratch cycle and potentially modulating inflammatory pathways involved in chronic itch.10
Nalbuphine
Nalbuphine is a κ opioid receptor agonist and μ opioid receptor antagonist that has been investigated for the treatment of PN.11 In a phase 2 randomized controlled trial, oral nalbuphine extended release (NAL-ER) 162 mg twice daily provided measurable antipruritic efficacy, with 44.4% (8/18) of patients achieving at least a 30% reduction in 7-day WI-NRS at week 10 compared with 36.4% (8/22) in the placebo group. Among those who completed the study, 66.7% (8/12) of patients receiving NAL-ER 162 mg achieved significant itch reduction vs 40% (8/20) receiving placebo (P=.03). At least a 50% reduction in WI-NRS was achieved by 33.3% (6/18) of patients receiving NAL-ER 162 mg twice daily. Extended open-label treatment was associated with further improvements in itch and lesion activity. Adverse events were mostly mild to moderate (eg, nausea, dizziness, headache, and fatigue) and occurred during dose titration. Physiologic opioid withdrawal symptoms were limited and resolved within a few days of discontinuing the medication.11
Final Thoughts
In conclusion, PN remains one of the most challenging chronic dermatologic conditions to manage and is driven by a complex interplay of neuroimmune mechanisms and resistance to many conventional therapies. The approval of dupilumab and nemolizumab has marked a pivotal shift in the therapeutic landscape, offering hope to patients who previously had limited options5,8; however, the burden of PN remains substantial, and many patients continue to experience relentless itch, poor sleep, and reduced quality of life.1 Emerging therapies such as TRPM8 agonists, Janus kinase inhibitors, and opioid modulators represent promising additions to the treatment options, targeting novel pathways beyond traditional immunosuppression.9-11
Prurigo nodularis (PN) is a chronic, severely pruritic neuroimmunologic skin disorder characterized by multiple firm hyperkeratotic nodules and intense pruritus, often leading to considerable impairment in quality of life and increased rates of depression and anxiety.1 It is considered difficult to treat due to its complex pathogenesis, the severity and chronicity of pruritus, and the limited efficacy of conventional therapies.2,3 The disease is driven by a self-perpetuating itch-scratch cycle, underpinned by dysregulation of both immune and neural pathways including type 2 (interleukin [IL] 4, IL-13, IL-31), Th17, and Th22 cytokines as well as neuropeptides and altered cutaneous nerve architecture.1,3 This results in persistent severe pruritus and nodular lesions that are highly refractory to standard treatments.1 Conventional therapies (eg, locally acting agents, phototherapy, and systemic immunomodulators and neuromodulators) have varied efficacy and notable adverse effect profiles.3 While the approval of targeted biologics has transformed the therapeutic landscape, several other treatment options also are being explored in clinical trials. Herein, we review all recently approved therapies as well as emerging treatments currently under investigation.
Dupilumab
Dupilumab, the first therapy for PN approved by the US Food and Drug Administration (FDA) in 2022—is a monoclonal antibody that inhibits signaling of IL-4 and IL-13, key drivers of type 2 inflammation implicated in PN pathogenesis.4,5 In 2 pivotal phase 3 randomized controlled trials (LIBERTY-PN PRIME and PRIME2),5 dupilumab demonstrated notable efficacy in adults with moderate to severe PN. A reduction of 4 points or more on the Worst Itch Numeric Rating Scale (WI-NRS) was achieved by 60.0% (45/75) of patients treated with dupilumab at week 24 compared with 18.4% (14/76) receiving placebo in the PRIME trial. In PRIME2, the same outcome was achieved by 37.2% (29/78) of patients receiving dupilumab at week 12 compared with 22.0% (18/82) of patients receiving placebo.5 Dupilumab also led to a greater proportion of patients achieving a substantial reduction in nodule count (≤5 nodules) and improved quality of life compared with placebo.5,6 The safety profile of dupilumab for treatment of PN was favorable and consistent with prior experience in atopic dermatitis; conjunctivitis was the most common adverse event.5,6
Nemolizumab
Nemolizumab, an IL-31 receptor A antagonist, is the most recent agent approved by the FDA for PN in 2024.7 In the OLYMPIA 1 and OLYMPIA 2 phase 3 trials,8 nemolizumab produced a clinically meaningful reduction in itch (defined as a ≥4-point improvement in the Peak Pruritus Numerical Rating Scale score) in 56.3% (103/183) of patients at week 16 compared with 20.9% (19/91) receiving placebo. Additionally, 37.7% (69/183) of patients receiving nemolizumab achieved clear or almost clear skin (Investigator’s Global Assessment score of 0 or 1 with a ≥2-point reduction) vs 11.0% with placebo (both P<.001). Benefits were observed as early as week 4, including rapid improvements in itch, sleep disturbance, and nodule count.8 Nemolizumab also improved quality of life and reduced symptoms of anxiety and depression. The safety profile was favorable, with headache and atopic dermatitis the most common adverse events; serious adverse events were infrequent and similar between groups.8
Abrocitinib
Abrocitinib, an oral selective Janus kinase 1 inhibitor, is an investigational therapy for PN and currently has not been approved by the FDA for this indication. In a phase 2 open-label trial, abrocitinib 200 mg daily for 12 weeks led to a 78.3% reduction in weekly Peak Pruritus Numerical Rating Scale scores in PN, with 80.0% (8/10) of patients achieving a clinically meaningful improvement of 4 points or higher. Nodule counts and quality of life also improved, with an onset of itch relief as early as week 2. The safety profile was favorable, with acneform eruptions the most common adverse event and no serious adverse events reported9; however, these results are based on small, nonrandomized studies and require confirmation in larger randomized controlled trials before abrocitinib can be considered a standard therapy for PN.
Cryosim-1
Transient receptor potential melastatin 8 (TRPM8) is a cold-sensing ion channel found in unmyelinated sensory neurons within the dorsal root and trigeminal ganglia.10 It is activated by cool temperatures (15-28 °C) and compounds such as menthol, leading to calcium influx and a cooling sensation. In a randomized, double-blind, vehicle-controlled trial, researchers investigated the efficacy of cryosim-1 (a synthetic TRPM8 agonist) in treating PN.10 Thirty patients were enrolled, with 18 (60.0%) receiving cryosim-1 and 12 (40.0%) receiving placebo over 8 weeks. By week 8, cryosim-1 significantly reduced itch severity (mean numerical rating scale score postapplication, 2.8 vs 4.3; P=.031) and improved sleep disturbances (2.2 vs 4.2; P=.031) compared to placebo. Patients reported higher satisfaction with itch relief, and no adverse effects were observed. The study concluded that cryosim-1 is a safe, effective topical therapy for PN, likely working by interrupting the itch-scratch cycle and potentially modulating inflammatory pathways involved in chronic itch.10
Nalbuphine
Nalbuphine is a κ opioid receptor agonist and μ opioid receptor antagonist that has been investigated for the treatment of PN.11 In a phase 2 randomized controlled trial, oral nalbuphine extended release (NAL-ER) 162 mg twice daily provided measurable antipruritic efficacy, with 44.4% (8/18) of patients achieving at least a 30% reduction in 7-day WI-NRS at week 10 compared with 36.4% (8/22) in the placebo group. Among those who completed the study, 66.7% (8/12) of patients receiving NAL-ER 162 mg achieved significant itch reduction vs 40% (8/20) receiving placebo (P=.03). At least a 50% reduction in WI-NRS was achieved by 33.3% (6/18) of patients receiving NAL-ER 162 mg twice daily. Extended open-label treatment was associated with further improvements in itch and lesion activity. Adverse events were mostly mild to moderate (eg, nausea, dizziness, headache, and fatigue) and occurred during dose titration. Physiologic opioid withdrawal symptoms were limited and resolved within a few days of discontinuing the medication.11
Final Thoughts
In conclusion, PN remains one of the most challenging chronic dermatologic conditions to manage and is driven by a complex interplay of neuroimmune mechanisms and resistance to many conventional therapies. The approval of dupilumab and nemolizumab has marked a pivotal shift in the therapeutic landscape, offering hope to patients who previously had limited options5,8; however, the burden of PN remains substantial, and many patients continue to experience relentless itch, poor sleep, and reduced quality of life.1 Emerging therapies such as TRPM8 agonists, Janus kinase inhibitors, and opioid modulators represent promising additions to the treatment options, targeting novel pathways beyond traditional immunosuppression.9-11
- Williams KA, Huang AH, Belzberg M, et al. Prurigo nodularis: pathogenesis and management. J Am Acad Dermatol. 2020;83:1567-1575. doi:10.1016/j.jaad.2020.04.182
- Gründel S, Pereira MP, Storck M, et al. Analysis of 325 patients with chronic nodular prurigo: clinics, burden of disease and course of treatment. Acta Derm Venereol. 2020;100:adv00269. doi:10.2340/00015555-3571
- Liao V, Cornman HL, Ma E, et al. Prurigo nodularis: new insights into pathogenesis and novel therapeutics. Br J Dermatol. 2024;190:798-810. doi:10.1093/bjd/ljae052
- Elmariah SB, Tao L, Valdes-Rodriguez R, et al. Individual article: management of prurigo nodularis. J Drugs Dermatol. 2023;22:SF365502s15-SF365502s22. doi:10.36849/JDD.SF365502
- Yosipovitch G, Mollanazar N, Ständer S, et al. Dupilumab in patients with prurigo nodularis: two randomized, double-blind, placebo-controlled phase 3 trials. Nat Med. 2023;29:1180-1190. doi:10.1038/s41591-023-02320-9
- Cao P, Xu W, Jiang S, et al. Dupilumab for the treatment of prurigo nodularis: a systematic review. Front Immunol. 2023;14:1092685. doi:10.3389/fimmu.2023.1092685
- Dagenet CB, Saadi C, Phillips MA, et al. Landscape of prurigo nodularis clinical trials. JAAD Rev. 2024;2:127-136. doi:10.1016/j.jdrv.2024.09.006
- Kwatra SG, Yosipovitch G, Legat FJ, et al. Phase 3 trial of nemolizumab in patients with prurigo nodularis. N Engl J Med. 2023;389:1579-1589. doi:10.1056/NEJMoa2301333
- Kwatra SG, Bordeaux ZA, Parthasarathy V, et al. Efficacy and safety of abrocitinib in prurigo nodularis and chronic pruritus of unknown origin: a nonrandomized controlled trial. JAMA Dermatol. 2024;160:717-724. doi:10.1001/jamadermatol.2024.1464
- Choi ME, Lee JH, Jung CJ, et al. A randomized, double-blinded, vehicle-controlled clinical trial of topical cryosim-1, a synthetic TRPM8 agonist, in prurigo nodularis. J Cosmet Dermatol. 2024;23:931-937. doi:10.1111/jocd.16079
- Weisshaar E, Szepietowski JC, Bernhard JD, et al. Efficacy and safety of oral nalbuphine extended release in prurigo nodularis: results of a phase 2 randomized controlled trial with an open-label extension phase. J Eur Acad Dermatol Venereol. 2022;36:453-461. doi:10.1111/jdv.17816
- Williams KA, Huang AH, Belzberg M, et al. Prurigo nodularis: pathogenesis and management. J Am Acad Dermatol. 2020;83:1567-1575. doi:10.1016/j.jaad.2020.04.182
- Gründel S, Pereira MP, Storck M, et al. Analysis of 325 patients with chronic nodular prurigo: clinics, burden of disease and course of treatment. Acta Derm Venereol. 2020;100:adv00269. doi:10.2340/00015555-3571
- Liao V, Cornman HL, Ma E, et al. Prurigo nodularis: new insights into pathogenesis and novel therapeutics. Br J Dermatol. 2024;190:798-810. doi:10.1093/bjd/ljae052
- Elmariah SB, Tao L, Valdes-Rodriguez R, et al. Individual article: management of prurigo nodularis. J Drugs Dermatol. 2023;22:SF365502s15-SF365502s22. doi:10.36849/JDD.SF365502
- Yosipovitch G, Mollanazar N, Ständer S, et al. Dupilumab in patients with prurigo nodularis: two randomized, double-blind, placebo-controlled phase 3 trials. Nat Med. 2023;29:1180-1190. doi:10.1038/s41591-023-02320-9
- Cao P, Xu W, Jiang S, et al. Dupilumab for the treatment of prurigo nodularis: a systematic review. Front Immunol. 2023;14:1092685. doi:10.3389/fimmu.2023.1092685
- Dagenet CB, Saadi C, Phillips MA, et al. Landscape of prurigo nodularis clinical trials. JAAD Rev. 2024;2:127-136. doi:10.1016/j.jdrv.2024.09.006
- Kwatra SG, Yosipovitch G, Legat FJ, et al. Phase 3 trial of nemolizumab in patients with prurigo nodularis. N Engl J Med. 2023;389:1579-1589. doi:10.1056/NEJMoa2301333
- Kwatra SG, Bordeaux ZA, Parthasarathy V, et al. Efficacy and safety of abrocitinib in prurigo nodularis and chronic pruritus of unknown origin: a nonrandomized controlled trial. JAMA Dermatol. 2024;160:717-724. doi:10.1001/jamadermatol.2024.1464
- Choi ME, Lee JH, Jung CJ, et al. A randomized, double-blinded, vehicle-controlled clinical trial of topical cryosim-1, a synthetic TRPM8 agonist, in prurigo nodularis. J Cosmet Dermatol. 2024;23:931-937. doi:10.1111/jocd.16079
- Weisshaar E, Szepietowski JC, Bernhard JD, et al. Efficacy and safety of oral nalbuphine extended release in prurigo nodularis: results of a phase 2 randomized controlled trial with an open-label extension phase. J Eur Acad Dermatol Venereol. 2022;36:453-461. doi:10.1111/jdv.17816
From Refractory to Responsive: The Expanding Therapeutic Landscape of Prurigo Nodularis
From Refractory to Responsive: The Expanding Therapeutic Landscape of Prurigo Nodularis
From Neglect to Novelty: Emerging Treatment Strategies in Papillary Renal Cell Carcinoma
This transcript has been edited for clarity.
Hi, I’m Doctor Monty Pal, and I’m a medical oncologist at City of Hope Comprehensive Cancer Center.
I run the kidney cancer program there, and it’s really been amazing to see the tidal wave of new therapies that we’ve developed for kidney cancer over the years.
What I will say is that most of the treatments that we have, the doublet therapies and adjuvant treatments, really pertain to the most dominant subset of kidney cancer, and that’s clear cell; that really represents about 75%-80% of all cases of kidney cancer.
Having said that, I’d like to focus on perhaps a less dominant subset of kidney cancer known as papillary. This represents about 15%-20% of cases.
To be fair, there are other rare subtypes and those certainly warrant focus as well: Chromophobe represents about 5% of all diseases; translocation, unclassified, a percentage point or 2.
But papillary really is a setting in which we can do — and as I’ll demonstrate, have done — clinical trials.
Now papillary kidney cancer is similar in terms of its demographics to clear cell renal cell carcinoma (RCC). There still tends to be a male predominance. It may have a slightly lower age of initial presentation, but otherwise there’s many commonalities.
I do think one of the areas where it differs, and this is critical, is in the context of biology: We think that papillary RCC is really driven by the MET proto-oncogene amongst other things. With that in mind, there have been a whole host of therapies directed at the MET proto-oncogene, and we’ll discuss that in just a moment.
I wanted to first talk about management of localized papillary kidney cancer. This management doesn’t differ significantly from what we would perhaps consider in the context of localized clear cell kidney cancer. For stage 1 through 3 disease, patients still receive surgery as the mainstay, and I would argue that, in the context of stage 4 disease, we should still really consider aggressive local definitive therapies if at all possible.
Having said that, the role of adjuvant therapy is a bit unclear in this context. I would suggest that in the context of adjuvant therapy for papillary kidney cancer, it’s a bit of a no go until we have greater data in this setting. Therapies like pembrolizumab and sunitinib really were only tested in the context of clear cell disease.
And with that background, I wanted to move into metastatic disease. For patients with stage 4 papillary kidney cancer, therapy may or may not resemble the treatments that we use for clear cell kidney cancer.
There have been trials in yesteryear with really creative names, ASPEN and ESPN for instance, that actually juxtaposed older therapies against one another. Sunitinib against everolimus, for instance, was common to both of those studies. And it really suggested perhaps that sunitinib was the preferred choice between those two targeted therapies. Sunitinib then became a bit of a standard when it came to randomized trials, and in fact, it still remains something that’s incorporated as a base regimen at phase 3 clinical trials.
I’ll show you that in the PAPMET clinical trial, which was a randomized phase 2 experience, we were able to compare sunitinib to cabozantinib, crizotinib, and savolitinib. The latter three drugs are all so-called MET inhibitors. And what’s quite interesting about this study is that cabozantinib, the dual VEGF/MET inhibitor, really is the one that seemed to win out.
When you look at median progression-free survival (PFS), in sunitinib in that study was around 6 months. When you look at cabozantinib, it was around 9 months.
Having said that, with cabozantinib, there was no overall survival advantage, and I will say that the PFS benefit is modest. So, we still need more in the way of clinical trials.
To that end, there’s a number of single arm studies supporting cabozantinib-based regimens, cabozantinib/atezolizumab and cabozantinib/nivolumab, with healthy response rates. For papillary kidney cancer, the response rate with those regimens is around 43%-47%.
In the context of lenvatinib and pembrolizumab, we actually see the response rate augmented to approximately 53%.
So, with those numbers in mind, I definitely think that doublet therapy is promising, but as we always tell our fellows in the clinic, randomization is really king.
So, we do have two phase 3 clinical trials, STELLAR-304, which is a study that I’m running, and the second study is known as SAMETA. Both evaluate papillary patients, but in very different ways.
STELLAR-304 includes patients with papillary, translocation, and unclassified kidney cancer and randomizes to sunitinib vs zanzalintinib, a novel TKI, with nivolumab.
In contrast, SAMETA takes the very interesting approach of actually selecting out patients with MET abnormalities and randomizing them to sunitinib vs savolitinib.
There are other approaches. For instance, the SUNNIFORECAST study recently assessed nivolumab and ipilimumab. There are randomized phase 2 studies looking at axitinib and pembrolizumab or perhaps axitinib and, sorry, cabozantinib with atezolizumab.
But I do think these phase 3 studies, SAMETA and STELLAR-304, are the ones that are really poised to change the landscape of therapy for papillary kidney cancer.
Sumanta K. Pal, MD, Professor, Department of Medical Oncology, City of Hope Comprehensive Cancer Center, Duarte, California, has disclosed the following relevant financial relationships: Received travel from: CRISPR; Ipsen. A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
Hi, I’m Doctor Monty Pal, and I’m a medical oncologist at City of Hope Comprehensive Cancer Center.
I run the kidney cancer program there, and it’s really been amazing to see the tidal wave of new therapies that we’ve developed for kidney cancer over the years.
What I will say is that most of the treatments that we have, the doublet therapies and adjuvant treatments, really pertain to the most dominant subset of kidney cancer, and that’s clear cell; that really represents about 75%-80% of all cases of kidney cancer.
Having said that, I’d like to focus on perhaps a less dominant subset of kidney cancer known as papillary. This represents about 15%-20% of cases.
To be fair, there are other rare subtypes and those certainly warrant focus as well: Chromophobe represents about 5% of all diseases; translocation, unclassified, a percentage point or 2.
But papillary really is a setting in which we can do — and as I’ll demonstrate, have done — clinical trials.
Now papillary kidney cancer is similar in terms of its demographics to clear cell renal cell carcinoma (RCC). There still tends to be a male predominance. It may have a slightly lower age of initial presentation, but otherwise there’s many commonalities.
I do think one of the areas where it differs, and this is critical, is in the context of biology: We think that papillary RCC is really driven by the MET proto-oncogene amongst other things. With that in mind, there have been a whole host of therapies directed at the MET proto-oncogene, and we’ll discuss that in just a moment.
I wanted to first talk about management of localized papillary kidney cancer. This management doesn’t differ significantly from what we would perhaps consider in the context of localized clear cell kidney cancer. For stage 1 through 3 disease, patients still receive surgery as the mainstay, and I would argue that, in the context of stage 4 disease, we should still really consider aggressive local definitive therapies if at all possible.
Having said that, the role of adjuvant therapy is a bit unclear in this context. I would suggest that in the context of adjuvant therapy for papillary kidney cancer, it’s a bit of a no go until we have greater data in this setting. Therapies like pembrolizumab and sunitinib really were only tested in the context of clear cell disease.
And with that background, I wanted to move into metastatic disease. For patients with stage 4 papillary kidney cancer, therapy may or may not resemble the treatments that we use for clear cell kidney cancer.
There have been trials in yesteryear with really creative names, ASPEN and ESPN for instance, that actually juxtaposed older therapies against one another. Sunitinib against everolimus, for instance, was common to both of those studies. And it really suggested perhaps that sunitinib was the preferred choice between those two targeted therapies. Sunitinib then became a bit of a standard when it came to randomized trials, and in fact, it still remains something that’s incorporated as a base regimen at phase 3 clinical trials.
I’ll show you that in the PAPMET clinical trial, which was a randomized phase 2 experience, we were able to compare sunitinib to cabozantinib, crizotinib, and savolitinib. The latter three drugs are all so-called MET inhibitors. And what’s quite interesting about this study is that cabozantinib, the dual VEGF/MET inhibitor, really is the one that seemed to win out.
When you look at median progression-free survival (PFS), in sunitinib in that study was around 6 months. When you look at cabozantinib, it was around 9 months.
Having said that, with cabozantinib, there was no overall survival advantage, and I will say that the PFS benefit is modest. So, we still need more in the way of clinical trials.
To that end, there’s a number of single arm studies supporting cabozantinib-based regimens, cabozantinib/atezolizumab and cabozantinib/nivolumab, with healthy response rates. For papillary kidney cancer, the response rate with those regimens is around 43%-47%.
In the context of lenvatinib and pembrolizumab, we actually see the response rate augmented to approximately 53%.
So, with those numbers in mind, I definitely think that doublet therapy is promising, but as we always tell our fellows in the clinic, randomization is really king.
So, we do have two phase 3 clinical trials, STELLAR-304, which is a study that I’m running, and the second study is known as SAMETA. Both evaluate papillary patients, but in very different ways.
STELLAR-304 includes patients with papillary, translocation, and unclassified kidney cancer and randomizes to sunitinib vs zanzalintinib, a novel TKI, with nivolumab.
In contrast, SAMETA takes the very interesting approach of actually selecting out patients with MET abnormalities and randomizing them to sunitinib vs savolitinib.
There are other approaches. For instance, the SUNNIFORECAST study recently assessed nivolumab and ipilimumab. There are randomized phase 2 studies looking at axitinib and pembrolizumab or perhaps axitinib and, sorry, cabozantinib with atezolizumab.
But I do think these phase 3 studies, SAMETA and STELLAR-304, are the ones that are really poised to change the landscape of therapy for papillary kidney cancer.
Sumanta K. Pal, MD, Professor, Department of Medical Oncology, City of Hope Comprehensive Cancer Center, Duarte, California, has disclosed the following relevant financial relationships: Received travel from: CRISPR; Ipsen. A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
Hi, I’m Doctor Monty Pal, and I’m a medical oncologist at City of Hope Comprehensive Cancer Center.
I run the kidney cancer program there, and it’s really been amazing to see the tidal wave of new therapies that we’ve developed for kidney cancer over the years.
What I will say is that most of the treatments that we have, the doublet therapies and adjuvant treatments, really pertain to the most dominant subset of kidney cancer, and that’s clear cell; that really represents about 75%-80% of all cases of kidney cancer.
Having said that, I’d like to focus on perhaps a less dominant subset of kidney cancer known as papillary. This represents about 15%-20% of cases.
To be fair, there are other rare subtypes and those certainly warrant focus as well: Chromophobe represents about 5% of all diseases; translocation, unclassified, a percentage point or 2.
But papillary really is a setting in which we can do — and as I’ll demonstrate, have done — clinical trials.
Now papillary kidney cancer is similar in terms of its demographics to clear cell renal cell carcinoma (RCC). There still tends to be a male predominance. It may have a slightly lower age of initial presentation, but otherwise there’s many commonalities.
I do think one of the areas where it differs, and this is critical, is in the context of biology: We think that papillary RCC is really driven by the MET proto-oncogene amongst other things. With that in mind, there have been a whole host of therapies directed at the MET proto-oncogene, and we’ll discuss that in just a moment.
I wanted to first talk about management of localized papillary kidney cancer. This management doesn’t differ significantly from what we would perhaps consider in the context of localized clear cell kidney cancer. For stage 1 through 3 disease, patients still receive surgery as the mainstay, and I would argue that, in the context of stage 4 disease, we should still really consider aggressive local definitive therapies if at all possible.
Having said that, the role of adjuvant therapy is a bit unclear in this context. I would suggest that in the context of adjuvant therapy for papillary kidney cancer, it’s a bit of a no go until we have greater data in this setting. Therapies like pembrolizumab and sunitinib really were only tested in the context of clear cell disease.
And with that background, I wanted to move into metastatic disease. For patients with stage 4 papillary kidney cancer, therapy may or may not resemble the treatments that we use for clear cell kidney cancer.
There have been trials in yesteryear with really creative names, ASPEN and ESPN for instance, that actually juxtaposed older therapies against one another. Sunitinib against everolimus, for instance, was common to both of those studies. And it really suggested perhaps that sunitinib was the preferred choice between those two targeted therapies. Sunitinib then became a bit of a standard when it came to randomized trials, and in fact, it still remains something that’s incorporated as a base regimen at phase 3 clinical trials.
I’ll show you that in the PAPMET clinical trial, which was a randomized phase 2 experience, we were able to compare sunitinib to cabozantinib, crizotinib, and savolitinib. The latter three drugs are all so-called MET inhibitors. And what’s quite interesting about this study is that cabozantinib, the dual VEGF/MET inhibitor, really is the one that seemed to win out.
When you look at median progression-free survival (PFS), in sunitinib in that study was around 6 months. When you look at cabozantinib, it was around 9 months.
Having said that, with cabozantinib, there was no overall survival advantage, and I will say that the PFS benefit is modest. So, we still need more in the way of clinical trials.
To that end, there’s a number of single arm studies supporting cabozantinib-based regimens, cabozantinib/atezolizumab and cabozantinib/nivolumab, with healthy response rates. For papillary kidney cancer, the response rate with those regimens is around 43%-47%.
In the context of lenvatinib and pembrolizumab, we actually see the response rate augmented to approximately 53%.
So, with those numbers in mind, I definitely think that doublet therapy is promising, but as we always tell our fellows in the clinic, randomization is really king.
So, we do have two phase 3 clinical trials, STELLAR-304, which is a study that I’m running, and the second study is known as SAMETA. Both evaluate papillary patients, but in very different ways.
STELLAR-304 includes patients with papillary, translocation, and unclassified kidney cancer and randomizes to sunitinib vs zanzalintinib, a novel TKI, with nivolumab.
In contrast, SAMETA takes the very interesting approach of actually selecting out patients with MET abnormalities and randomizing them to sunitinib vs savolitinib.
There are other approaches. For instance, the SUNNIFORECAST study recently assessed nivolumab and ipilimumab. There are randomized phase 2 studies looking at axitinib and pembrolizumab or perhaps axitinib and, sorry, cabozantinib with atezolizumab.
But I do think these phase 3 studies, SAMETA and STELLAR-304, are the ones that are really poised to change the landscape of therapy for papillary kidney cancer.
Sumanta K. Pal, MD, Professor, Department of Medical Oncology, City of Hope Comprehensive Cancer Center, Duarte, California, has disclosed the following relevant financial relationships: Received travel from: CRISPR; Ipsen. A version of this article appeared on Medscape.com.
Medical Liability for the Gastroenterologist
While nearly 75% of physicians in low-risk specialties and 99% of physicians in high-risk specialties may face a malpractice claim in their careers,1 malpractice is rarely discussed openly in medical school, residency, fellowship, or even amongst colleagues. Indeed, one study suggested that more than 10% of practicing gastroenterologists may face a malpractice claim,2 with gastroenterologists expected to spend 10-15% of their careers with an outstanding malpractice claim3 as cases may take 27-29 months to resolve on average.4
Believing that if a physician is sued, one must have done something “wrong” or that speaking about one’s experience may implicate a colleague, creates an intense stigma and isolation that only serves to aggravate the “second victim syndrome” (SVS) that is well documented in the surgical literature.2 Herein,
What is Malpractice? Why Do Physicians Get Sued?
Malpractice is defined as negligence on the part of a physician which causes physical or emotional damage to the patient. This requires a variety of legal issues to be evaluated (e.g. breach of duty between the physicians and patient, breach of standard of care), that often center around the question: would a “reasonable, careful, and prudent” doctor behave in the same manner in the same circumstance?
While some fields of medicine lend themselves better to algorithmic applications of highly evidence-based guidelines, many aspects of GI care and endoscopic practice are highly physician/patient-specific, dependent on local expertise, and based on low-quality evidence. As a result, an assessment of negligence may be quite subjective, depending on the expert retained by a plaintiff. Conflicting expert testimony on what professional custom is and whether practice deviates may hinge on technical details that may or may not be appreciated by a lay jury.
Plaintiffs must prove both that they have sustained an injury and that the injury (emotional or physical) was due to the physician’s negligence. While this may be straightforward in a “slip-and-fall” tort claim, medical malpractice claims usually involve sick patients with multiple comorbidities, where assigning causality to a single intervention/misinterpretation/missed opportunity is difficult to weigh against competing causes of adverse outcomes. Assessing a specific liability requires that the plaintiff prove this to a “more likely than not” standard which may be part of the reason why only 30% of cases are closed with indemnity payments, a figure that has not changed significantly in the past decade.4
While the perception amongst physicians is that tort legislation is ever increasing, data from the National Practitioner Data Bank (NPDB) demonstrates that the number of paid claims against physicians has decreased by 75% in the last 20 years.5 This may reflect a progressive improvement in the quality of care delivered or success of “tort reform” on the state level to limit damages and “nuisance” lawsuits. However, another more problematic possibility is that with the corporatization of medicine, an untold number of physicians may be removed from litigation as a named party, with their institution shielding them from reporting. While the number of cases may or may not be declining, the average indemnity payment appears to be rising to $330,000 on average,4 with one study suggesting a significant growth in paid claims in gastroenterology.6
Historically, studies of closed malpractice claims have demonstrated that 59% involved diagnostic errors involving a cancer diagnosis,7 though why this actually happens may be for a wide variety of reasons including errors in the development of a differential diagnosis, ordering of an appropriate diagnostic test, interpretation of the diagnostic test, or follow-up of an abnormality identified.
What are the Intended/Unintended Consequences of Litigation?
The objective of our tort system is to compensate patients for economic damages (medical costs and lost wages) and non-economic damages (pain and suffering), and to ideally deter negligent behavior of providers. Interestingly, data from the NPDB have suggested that approximately 1% of all physicians account for 32% of all paid claims, with the same study showing that among physicians with paid claims, 4% had at least 3.8
While certain fields are obviously more prone to litigation, the risk of additional claims on a physician with 3 prior claims was more than 3 times that of physicians with 1 lifetime claim. One would assume that the system was built to drive out a small proportion of “bad actors.” Indeed, similar data from the NPDB has demonstrated that the number of claims against physicians was associated both with their leaving the practice of medicine and relocating to smaller practice settings.9
Another frequent question is whether the threat of litigation drives “defensive medicine” (i.e. medical care that is not beneficial) or avoidance medicine (i.e. excluding high risk patients and procedures from ones’ practice). These behaviors have been well documented in physicians around the world,10 as well as several surveys of gastroenterologists specifically suggesting regular ordering of unnecessary imaging/endoscopy and referrals of patients to specialists that may not be necessary.11,12
However, does defensive medicine work: does spending more prevent you from being the target of a lawsuit? In an observational study in Florida from 2000-2009, researchers demonstrated that across specialties, greater average spending by physicians was associated with a reduced risk of incurring a malpractice claim. Indeed, the likelihood of a top quintile spending internist having a malpractice incident vs a bottom quintile spending internist was 0.3% vs 1.5%.13
Approximately 10.4-43.3% of physicians may experience SVS, experiencing trauma after an adverse patient event/medical error, manifesting with psychological trauma (shame, guilt, anxiety) and cognitive limitations (burnout, stress).2 Significant emotional consequences are common on the part of the physician and have well-documented stages to recovery,14 which if ignored may lead to long-term detrimental mental/emotional health of the physician and their future patients.
Specifically, in one study, 80.8% of physicians who had a closed malpractice claim reported significant emotional distress (regardless of the legal outcome), with frequent reports of mood symptoms that affected professional conduct.15 Recognizing these effects and implementing peer counseling and institutional support may help to expedite recovery and mitigate future adverse career outcomes.14
Anatomy/Timeline of a Liability Lawsuit
Medical malpractice cases are heard in state courts, in the jurisdictions where the care was provided. From the time an event occurs to when a jury verdict may be rendered may take 4-5 years or more depending on the local statute of limitations, discovery process, backlog of the local case docket, and specific circumstances of the case. The length of time is important to consider given the likelihood that a physician may advance in training or move practice locations during the course of litigation. Several common myths surrounding this process are summarized in the accompanying box, titled “Myths Surrounding Medical Liability Litigation.”
The plaintiff faces a statute of limitations to file a lawsuit that may range from 1-6 years depending on the state. The first indication that legal action may be pending will generally be a plaintiff’s formal request for medical records. After these records are reviewed, the plaintiff’s attorney will consult one or more experts (often credentialed in the same specialty) to assess if the case is viable and to ultimately form the basis of an affidavit of merit from a plaintiff expert.
Once the lawsuit is filed, the physician(s) named will be assigned an attorney by their employer/insurance company. A state medical board malpractice questionnaire will generally follow that will seek to independently evaluate the alleged malpractice with interrogatives to determine if censure is warranted. There is a formal response to the plaintiff’s petition by the defense and then the discovery phase begins where both sides depose the defendants/plaintiffs and retain medical experts that are favorable to their arguments.
In choosing potential “experts,” physicians must ensure that they are willing/able to be present for a potential trial, do not have any personal/professional/academic conflicts with the defendants, and are willing to provide compelling testimony to a jury. A pre-trial conference and trial date is set which may be >12 months away depending on the local docket. While the amount of time a trial may take is variable, it may be up to 5-7 days that the defendants are expected to be in court in addition to days where depositions are being taken.
During the discovery process, dismissal of the physician from the lawsuit is pursued. In addition, settlement negotiations generally proceed in parallel with discovery process and may result in a pre-trial/pre-verdict settlement. Once a verdict is reached, whether for the plaintiff or the defendant, the case may be appealed, and the trial preparation process may be repeated.
Conclusions
Awareness of the medical liability process is critical for trainees and attendings alike, given the high likelihood of litigation in a gastroenterologist’s career. Specific considerations like local tort law and malpractice coverage are important to be familiar. Ongoing health services research help to shape our understanding on the intended and unintended consequences of litigation on medicine, though detailed data on outcomes/settlements are limited by confidentiality agreements, which may hamper efforts to improve patient safety.
Dr. Das is associate professor of medicine in the Division of Gastroenterology at Washington University School of Medicine, St. Louis, Missouri. He has served as a consultant for Olympus, but has no other relevant conflicts.
References
1. Jena AB, et al. Malpractice Risk According to Physician Specialty. N Engl J Med. 2011 Aug. doi: 10.1056/NEJMsa1012370.
2. Chong RIH, et al. Scoping review of the second victim syndrome among surgeons: Understanding the impact, responses, and support systems. Am J Surg 2024 Mar. doi: 10.1016/j.amjsurg.2023.09.045.
3. Seabury S, et al. On Average, Physicians Spend Nearly 11 Percent Of Their 40-Year Careers With An Open, Unresolved Malpractice Claim. Health Aff Proj Hope. 2013 Jan. doi: 10.1377/hlthaff.2012.0967.
4. CRICO Strategies. Medical Malpractice in America: A 10-Year Asessment with Insights. 2018. Accessed Apr 28, 2025.
5. Studdert DM, Hall MA. Medical Malpractice Law — Doctrine and Dynamics. N Engl J Med 2022 Oct. doi: 10.1056/NEJMp2201675.
6. Schaffer AC, et al. Rates and Characteristics of Paid Malpractice Claims Among US Physicians by Specialty, 1992-2014. JAMA Intern Med. 2017 May. doi: 10.1001/jamainternmed.2017.0311.
7. Gandhi TK, et al. Missed and Delayed Diagnoses in the Ambulatory Setting: A Study of Closed Malpractice Claims. Ann Intern Med. 2006 Oct. doi: 10.7326/0003-4819-145-7-200610030-00006.
8. Studdert DM, et al. Prevalence and Characteristics of Physicians Prone to Malpractice Claims. N Engl J Med. 2016 Jan. doi: 10.1056/NEJMsa1506137.
9. Studdert DM, et al. Changes in Practice among Physicians with Malpractice Claims. N Engl J Med. 2019 Mar. doi: 10.1056/NEJMsa1809981.
10. Ries NM, Jansen J. Physicians’ views and experiences of defensive medicine: An international review of empirical research. Health Policy. 2021 May. doi: 10.1016/j.healthpol.2021.02.005.
11. Hiyama T, et al. Defensive medicine practices among gastroenterologists in Japan. World J Gastroenterol. 2006 Dec. doi: 10.3748/wjg.v12.i47.7671.
12. Elli L, et al. Defensive medicine practices among gastroenterologists in Lombardy: Between lawsuits and the economic crisis. Dig Liver Dis. 2013 Jun. doi: 10.1016/j.dld.2013.01.004.
13. Jena AB, et al. Physician spending and subsequent risk of malpractice claims: observational study. BMJ. 2015 Nov. doi: 10.1136/bmj.h5516.
14. Scott SD, et al. The natural history of recovery for the healthcare provider “second victim” after adverse patient events. BMJ Qual Saf. 2009 Oct. doi: 10.1136/qshc.2009.032870.
15. Gómez-Durán EL, et al. Physicians as second victims after a malpractice claim: An important issue in need of attention. J Healthc Qual Res. 2018 Oct. doi: 10.1016/j.jhqr.2018.06.002.
While nearly 75% of physicians in low-risk specialties and 99% of physicians in high-risk specialties may face a malpractice claim in their careers,1 malpractice is rarely discussed openly in medical school, residency, fellowship, or even amongst colleagues. Indeed, one study suggested that more than 10% of practicing gastroenterologists may face a malpractice claim,2 with gastroenterologists expected to spend 10-15% of their careers with an outstanding malpractice claim3 as cases may take 27-29 months to resolve on average.4
Believing that if a physician is sued, one must have done something “wrong” or that speaking about one’s experience may implicate a colleague, creates an intense stigma and isolation that only serves to aggravate the “second victim syndrome” (SVS) that is well documented in the surgical literature.2 Herein,
What is Malpractice? Why Do Physicians Get Sued?
Malpractice is defined as negligence on the part of a physician which causes physical or emotional damage to the patient. This requires a variety of legal issues to be evaluated (e.g. breach of duty between the physicians and patient, breach of standard of care), that often center around the question: would a “reasonable, careful, and prudent” doctor behave in the same manner in the same circumstance?
While some fields of medicine lend themselves better to algorithmic applications of highly evidence-based guidelines, many aspects of GI care and endoscopic practice are highly physician/patient-specific, dependent on local expertise, and based on low-quality evidence. As a result, an assessment of negligence may be quite subjective, depending on the expert retained by a plaintiff. Conflicting expert testimony on what professional custom is and whether practice deviates may hinge on technical details that may or may not be appreciated by a lay jury.
Plaintiffs must prove both that they have sustained an injury and that the injury (emotional or physical) was due to the physician’s negligence. While this may be straightforward in a “slip-and-fall” tort claim, medical malpractice claims usually involve sick patients with multiple comorbidities, where assigning causality to a single intervention/misinterpretation/missed opportunity is difficult to weigh against competing causes of adverse outcomes. Assessing a specific liability requires that the plaintiff prove this to a “more likely than not” standard which may be part of the reason why only 30% of cases are closed with indemnity payments, a figure that has not changed significantly in the past decade.4
While the perception amongst physicians is that tort legislation is ever increasing, data from the National Practitioner Data Bank (NPDB) demonstrates that the number of paid claims against physicians has decreased by 75% in the last 20 years.5 This may reflect a progressive improvement in the quality of care delivered or success of “tort reform” on the state level to limit damages and “nuisance” lawsuits. However, another more problematic possibility is that with the corporatization of medicine, an untold number of physicians may be removed from litigation as a named party, with their institution shielding them from reporting. While the number of cases may or may not be declining, the average indemnity payment appears to be rising to $330,000 on average,4 with one study suggesting a significant growth in paid claims in gastroenterology.6
Historically, studies of closed malpractice claims have demonstrated that 59% involved diagnostic errors involving a cancer diagnosis,7 though why this actually happens may be for a wide variety of reasons including errors in the development of a differential diagnosis, ordering of an appropriate diagnostic test, interpretation of the diagnostic test, or follow-up of an abnormality identified.
What are the Intended/Unintended Consequences of Litigation?
The objective of our tort system is to compensate patients for economic damages (medical costs and lost wages) and non-economic damages (pain and suffering), and to ideally deter negligent behavior of providers. Interestingly, data from the NPDB have suggested that approximately 1% of all physicians account for 32% of all paid claims, with the same study showing that among physicians with paid claims, 4% had at least 3.8
While certain fields are obviously more prone to litigation, the risk of additional claims on a physician with 3 prior claims was more than 3 times that of physicians with 1 lifetime claim. One would assume that the system was built to drive out a small proportion of “bad actors.” Indeed, similar data from the NPDB has demonstrated that the number of claims against physicians was associated both with their leaving the practice of medicine and relocating to smaller practice settings.9
Another frequent question is whether the threat of litigation drives “defensive medicine” (i.e. medical care that is not beneficial) or avoidance medicine (i.e. excluding high risk patients and procedures from ones’ practice). These behaviors have been well documented in physicians around the world,10 as well as several surveys of gastroenterologists specifically suggesting regular ordering of unnecessary imaging/endoscopy and referrals of patients to specialists that may not be necessary.11,12
However, does defensive medicine work: does spending more prevent you from being the target of a lawsuit? In an observational study in Florida from 2000-2009, researchers demonstrated that across specialties, greater average spending by physicians was associated with a reduced risk of incurring a malpractice claim. Indeed, the likelihood of a top quintile spending internist having a malpractice incident vs a bottom quintile spending internist was 0.3% vs 1.5%.13
Approximately 10.4-43.3% of physicians may experience SVS, experiencing trauma after an adverse patient event/medical error, manifesting with psychological trauma (shame, guilt, anxiety) and cognitive limitations (burnout, stress).2 Significant emotional consequences are common on the part of the physician and have well-documented stages to recovery,14 which if ignored may lead to long-term detrimental mental/emotional health of the physician and their future patients.
Specifically, in one study, 80.8% of physicians who had a closed malpractice claim reported significant emotional distress (regardless of the legal outcome), with frequent reports of mood symptoms that affected professional conduct.15 Recognizing these effects and implementing peer counseling and institutional support may help to expedite recovery and mitigate future adverse career outcomes.14
Anatomy/Timeline of a Liability Lawsuit
Medical malpractice cases are heard in state courts, in the jurisdictions where the care was provided. From the time an event occurs to when a jury verdict may be rendered may take 4-5 years or more depending on the local statute of limitations, discovery process, backlog of the local case docket, and specific circumstances of the case. The length of time is important to consider given the likelihood that a physician may advance in training or move practice locations during the course of litigation. Several common myths surrounding this process are summarized in the accompanying box, titled “Myths Surrounding Medical Liability Litigation.”
The plaintiff faces a statute of limitations to file a lawsuit that may range from 1-6 years depending on the state. The first indication that legal action may be pending will generally be a plaintiff’s formal request for medical records. After these records are reviewed, the plaintiff’s attorney will consult one or more experts (often credentialed in the same specialty) to assess if the case is viable and to ultimately form the basis of an affidavit of merit from a plaintiff expert.
Once the lawsuit is filed, the physician(s) named will be assigned an attorney by their employer/insurance company. A state medical board malpractice questionnaire will generally follow that will seek to independently evaluate the alleged malpractice with interrogatives to determine if censure is warranted. There is a formal response to the plaintiff’s petition by the defense and then the discovery phase begins where both sides depose the defendants/plaintiffs and retain medical experts that are favorable to their arguments.
In choosing potential “experts,” physicians must ensure that they are willing/able to be present for a potential trial, do not have any personal/professional/academic conflicts with the defendants, and are willing to provide compelling testimony to a jury. A pre-trial conference and trial date is set which may be >12 months away depending on the local docket. While the amount of time a trial may take is variable, it may be up to 5-7 days that the defendants are expected to be in court in addition to days where depositions are being taken.
During the discovery process, dismissal of the physician from the lawsuit is pursued. In addition, settlement negotiations generally proceed in parallel with discovery process and may result in a pre-trial/pre-verdict settlement. Once a verdict is reached, whether for the plaintiff or the defendant, the case may be appealed, and the trial preparation process may be repeated.
Conclusions
Awareness of the medical liability process is critical for trainees and attendings alike, given the high likelihood of litigation in a gastroenterologist’s career. Specific considerations like local tort law and malpractice coverage are important to be familiar. Ongoing health services research help to shape our understanding on the intended and unintended consequences of litigation on medicine, though detailed data on outcomes/settlements are limited by confidentiality agreements, which may hamper efforts to improve patient safety.
Dr. Das is associate professor of medicine in the Division of Gastroenterology at Washington University School of Medicine, St. Louis, Missouri. He has served as a consultant for Olympus, but has no other relevant conflicts.
References
1. Jena AB, et al. Malpractice Risk According to Physician Specialty. N Engl J Med. 2011 Aug. doi: 10.1056/NEJMsa1012370.
2. Chong RIH, et al. Scoping review of the second victim syndrome among surgeons: Understanding the impact, responses, and support systems. Am J Surg 2024 Mar. doi: 10.1016/j.amjsurg.2023.09.045.
3. Seabury S, et al. On Average, Physicians Spend Nearly 11 Percent Of Their 40-Year Careers With An Open, Unresolved Malpractice Claim. Health Aff Proj Hope. 2013 Jan. doi: 10.1377/hlthaff.2012.0967.
4. CRICO Strategies. Medical Malpractice in America: A 10-Year Asessment with Insights. 2018. Accessed Apr 28, 2025.
5. Studdert DM, Hall MA. Medical Malpractice Law — Doctrine and Dynamics. N Engl J Med 2022 Oct. doi: 10.1056/NEJMp2201675.
6. Schaffer AC, et al. Rates and Characteristics of Paid Malpractice Claims Among US Physicians by Specialty, 1992-2014. JAMA Intern Med. 2017 May. doi: 10.1001/jamainternmed.2017.0311.
7. Gandhi TK, et al. Missed and Delayed Diagnoses in the Ambulatory Setting: A Study of Closed Malpractice Claims. Ann Intern Med. 2006 Oct. doi: 10.7326/0003-4819-145-7-200610030-00006.
8. Studdert DM, et al. Prevalence and Characteristics of Physicians Prone to Malpractice Claims. N Engl J Med. 2016 Jan. doi: 10.1056/NEJMsa1506137.
9. Studdert DM, et al. Changes in Practice among Physicians with Malpractice Claims. N Engl J Med. 2019 Mar. doi: 10.1056/NEJMsa1809981.
10. Ries NM, Jansen J. Physicians’ views and experiences of defensive medicine: An international review of empirical research. Health Policy. 2021 May. doi: 10.1016/j.healthpol.2021.02.005.
11. Hiyama T, et al. Defensive medicine practices among gastroenterologists in Japan. World J Gastroenterol. 2006 Dec. doi: 10.3748/wjg.v12.i47.7671.
12. Elli L, et al. Defensive medicine practices among gastroenterologists in Lombardy: Between lawsuits and the economic crisis. Dig Liver Dis. 2013 Jun. doi: 10.1016/j.dld.2013.01.004.
13. Jena AB, et al. Physician spending and subsequent risk of malpractice claims: observational study. BMJ. 2015 Nov. doi: 10.1136/bmj.h5516.
14. Scott SD, et al. The natural history of recovery for the healthcare provider “second victim” after adverse patient events. BMJ Qual Saf. 2009 Oct. doi: 10.1136/qshc.2009.032870.
15. Gómez-Durán EL, et al. Physicians as second victims after a malpractice claim: An important issue in need of attention. J Healthc Qual Res. 2018 Oct. doi: 10.1016/j.jhqr.2018.06.002.
While nearly 75% of physicians in low-risk specialties and 99% of physicians in high-risk specialties may face a malpractice claim in their careers,1 malpractice is rarely discussed openly in medical school, residency, fellowship, or even amongst colleagues. Indeed, one study suggested that more than 10% of practicing gastroenterologists may face a malpractice claim,2 with gastroenterologists expected to spend 10-15% of their careers with an outstanding malpractice claim3 as cases may take 27-29 months to resolve on average.4
Believing that if a physician is sued, one must have done something “wrong” or that speaking about one’s experience may implicate a colleague, creates an intense stigma and isolation that only serves to aggravate the “second victim syndrome” (SVS) that is well documented in the surgical literature.2 Herein,
What is Malpractice? Why Do Physicians Get Sued?
Malpractice is defined as negligence on the part of a physician which causes physical or emotional damage to the patient. This requires a variety of legal issues to be evaluated (e.g. breach of duty between the physicians and patient, breach of standard of care), that often center around the question: would a “reasonable, careful, and prudent” doctor behave in the same manner in the same circumstance?
While some fields of medicine lend themselves better to algorithmic applications of highly evidence-based guidelines, many aspects of GI care and endoscopic practice are highly physician/patient-specific, dependent on local expertise, and based on low-quality evidence. As a result, an assessment of negligence may be quite subjective, depending on the expert retained by a plaintiff. Conflicting expert testimony on what professional custom is and whether practice deviates may hinge on technical details that may or may not be appreciated by a lay jury.
Plaintiffs must prove both that they have sustained an injury and that the injury (emotional or physical) was due to the physician’s negligence. While this may be straightforward in a “slip-and-fall” tort claim, medical malpractice claims usually involve sick patients with multiple comorbidities, where assigning causality to a single intervention/misinterpretation/missed opportunity is difficult to weigh against competing causes of adverse outcomes. Assessing a specific liability requires that the plaintiff prove this to a “more likely than not” standard which may be part of the reason why only 30% of cases are closed with indemnity payments, a figure that has not changed significantly in the past decade.4
While the perception amongst physicians is that tort legislation is ever increasing, data from the National Practitioner Data Bank (NPDB) demonstrates that the number of paid claims against physicians has decreased by 75% in the last 20 years.5 This may reflect a progressive improvement in the quality of care delivered or success of “tort reform” on the state level to limit damages and “nuisance” lawsuits. However, another more problematic possibility is that with the corporatization of medicine, an untold number of physicians may be removed from litigation as a named party, with their institution shielding them from reporting. While the number of cases may or may not be declining, the average indemnity payment appears to be rising to $330,000 on average,4 with one study suggesting a significant growth in paid claims in gastroenterology.6
Historically, studies of closed malpractice claims have demonstrated that 59% involved diagnostic errors involving a cancer diagnosis,7 though why this actually happens may be for a wide variety of reasons including errors in the development of a differential diagnosis, ordering of an appropriate diagnostic test, interpretation of the diagnostic test, or follow-up of an abnormality identified.
What are the Intended/Unintended Consequences of Litigation?
The objective of our tort system is to compensate patients for economic damages (medical costs and lost wages) and non-economic damages (pain and suffering), and to ideally deter negligent behavior of providers. Interestingly, data from the NPDB have suggested that approximately 1% of all physicians account for 32% of all paid claims, with the same study showing that among physicians with paid claims, 4% had at least 3.8
While certain fields are obviously more prone to litigation, the risk of additional claims on a physician with 3 prior claims was more than 3 times that of physicians with 1 lifetime claim. One would assume that the system was built to drive out a small proportion of “bad actors.” Indeed, similar data from the NPDB has demonstrated that the number of claims against physicians was associated both with their leaving the practice of medicine and relocating to smaller practice settings.9
Another frequent question is whether the threat of litigation drives “defensive medicine” (i.e. medical care that is not beneficial) or avoidance medicine (i.e. excluding high risk patients and procedures from ones’ practice). These behaviors have been well documented in physicians around the world,10 as well as several surveys of gastroenterologists specifically suggesting regular ordering of unnecessary imaging/endoscopy and referrals of patients to specialists that may not be necessary.11,12
However, does defensive medicine work: does spending more prevent you from being the target of a lawsuit? In an observational study in Florida from 2000-2009, researchers demonstrated that across specialties, greater average spending by physicians was associated with a reduced risk of incurring a malpractice claim. Indeed, the likelihood of a top quintile spending internist having a malpractice incident vs a bottom quintile spending internist was 0.3% vs 1.5%.13
Approximately 10.4-43.3% of physicians may experience SVS, experiencing trauma after an adverse patient event/medical error, manifesting with psychological trauma (shame, guilt, anxiety) and cognitive limitations (burnout, stress).2 Significant emotional consequences are common on the part of the physician and have well-documented stages to recovery,14 which if ignored may lead to long-term detrimental mental/emotional health of the physician and their future patients.
Specifically, in one study, 80.8% of physicians who had a closed malpractice claim reported significant emotional distress (regardless of the legal outcome), with frequent reports of mood symptoms that affected professional conduct.15 Recognizing these effects and implementing peer counseling and institutional support may help to expedite recovery and mitigate future adverse career outcomes.14
Anatomy/Timeline of a Liability Lawsuit
Medical malpractice cases are heard in state courts, in the jurisdictions where the care was provided. From the time an event occurs to when a jury verdict may be rendered may take 4-5 years or more depending on the local statute of limitations, discovery process, backlog of the local case docket, and specific circumstances of the case. The length of time is important to consider given the likelihood that a physician may advance in training or move practice locations during the course of litigation. Several common myths surrounding this process are summarized in the accompanying box, titled “Myths Surrounding Medical Liability Litigation.”
The plaintiff faces a statute of limitations to file a lawsuit that may range from 1-6 years depending on the state. The first indication that legal action may be pending will generally be a plaintiff’s formal request for medical records. After these records are reviewed, the plaintiff’s attorney will consult one or more experts (often credentialed in the same specialty) to assess if the case is viable and to ultimately form the basis of an affidavit of merit from a plaintiff expert.
Once the lawsuit is filed, the physician(s) named will be assigned an attorney by their employer/insurance company. A state medical board malpractice questionnaire will generally follow that will seek to independently evaluate the alleged malpractice with interrogatives to determine if censure is warranted. There is a formal response to the plaintiff’s petition by the defense and then the discovery phase begins where both sides depose the defendants/plaintiffs and retain medical experts that are favorable to their arguments.
In choosing potential “experts,” physicians must ensure that they are willing/able to be present for a potential trial, do not have any personal/professional/academic conflicts with the defendants, and are willing to provide compelling testimony to a jury. A pre-trial conference and trial date is set which may be >12 months away depending on the local docket. While the amount of time a trial may take is variable, it may be up to 5-7 days that the defendants are expected to be in court in addition to days where depositions are being taken.
During the discovery process, dismissal of the physician from the lawsuit is pursued. In addition, settlement negotiations generally proceed in parallel with discovery process and may result in a pre-trial/pre-verdict settlement. Once a verdict is reached, whether for the plaintiff or the defendant, the case may be appealed, and the trial preparation process may be repeated.
Conclusions
Awareness of the medical liability process is critical for trainees and attendings alike, given the high likelihood of litigation in a gastroenterologist’s career. Specific considerations like local tort law and malpractice coverage are important to be familiar. Ongoing health services research help to shape our understanding on the intended and unintended consequences of litigation on medicine, though detailed data on outcomes/settlements are limited by confidentiality agreements, which may hamper efforts to improve patient safety.
Dr. Das is associate professor of medicine in the Division of Gastroenterology at Washington University School of Medicine, St. Louis, Missouri. He has served as a consultant for Olympus, but has no other relevant conflicts.
References
1. Jena AB, et al. Malpractice Risk According to Physician Specialty. N Engl J Med. 2011 Aug. doi: 10.1056/NEJMsa1012370.
2. Chong RIH, et al. Scoping review of the second victim syndrome among surgeons: Understanding the impact, responses, and support systems. Am J Surg 2024 Mar. doi: 10.1016/j.amjsurg.2023.09.045.
3. Seabury S, et al. On Average, Physicians Spend Nearly 11 Percent Of Their 40-Year Careers With An Open, Unresolved Malpractice Claim. Health Aff Proj Hope. 2013 Jan. doi: 10.1377/hlthaff.2012.0967.
4. CRICO Strategies. Medical Malpractice in America: A 10-Year Asessment with Insights. 2018. Accessed Apr 28, 2025.
5. Studdert DM, Hall MA. Medical Malpractice Law — Doctrine and Dynamics. N Engl J Med 2022 Oct. doi: 10.1056/NEJMp2201675.
6. Schaffer AC, et al. Rates and Characteristics of Paid Malpractice Claims Among US Physicians by Specialty, 1992-2014. JAMA Intern Med. 2017 May. doi: 10.1001/jamainternmed.2017.0311.
7. Gandhi TK, et al. Missed and Delayed Diagnoses in the Ambulatory Setting: A Study of Closed Malpractice Claims. Ann Intern Med. 2006 Oct. doi: 10.7326/0003-4819-145-7-200610030-00006.
8. Studdert DM, et al. Prevalence and Characteristics of Physicians Prone to Malpractice Claims. N Engl J Med. 2016 Jan. doi: 10.1056/NEJMsa1506137.
9. Studdert DM, et al. Changes in Practice among Physicians with Malpractice Claims. N Engl J Med. 2019 Mar. doi: 10.1056/NEJMsa1809981.
10. Ries NM, Jansen J. Physicians’ views and experiences of defensive medicine: An international review of empirical research. Health Policy. 2021 May. doi: 10.1016/j.healthpol.2021.02.005.
11. Hiyama T, et al. Defensive medicine practices among gastroenterologists in Japan. World J Gastroenterol. 2006 Dec. doi: 10.3748/wjg.v12.i47.7671.
12. Elli L, et al. Defensive medicine practices among gastroenterologists in Lombardy: Between lawsuits and the economic crisis. Dig Liver Dis. 2013 Jun. doi: 10.1016/j.dld.2013.01.004.
13. Jena AB, et al. Physician spending and subsequent risk of malpractice claims: observational study. BMJ. 2015 Nov. doi: 10.1136/bmj.h5516.
14. Scott SD, et al. The natural history of recovery for the healthcare provider “second victim” after adverse patient events. BMJ Qual Saf. 2009 Oct. doi: 10.1136/qshc.2009.032870.
15. Gómez-Durán EL, et al. Physicians as second victims after a malpractice claim: An important issue in need of attention. J Healthc Qual Res. 2018 Oct. doi: 10.1016/j.jhqr.2018.06.002.
Remembering Why We Are In Medicine
Dear Friends,
There have been recent policy changes that may be affecting trainees and practicing physicians, whether directly impacting our current practices or influencing the decisions that shape our careers. During these challenging times, I am trying to remind myself more often of why I am in medicine – my patients. I will continue to advocate for my patients on Hill Days to affect change in policy. I will continue to provide the best care I can and fight for resources to do so. I will continue to adapt to the changing climate and do what is best for my practice so that I can deliver the care I think my patients need. By remembering why I am in medicine, I can fight for a future of medicine and science that is still bright.
In this issue’s “In Focus” article, Dr. Yasmin G. Hernandez-Barco and Dr. Motaz Ashkar review the diagnostic and treatment approaches to exocrine pancreatic insufficiency, including common symptoms, differential diagnoses, and the different pancreatic enzyme replacement therapies.
Medications for weight loss are becoming more widely available; however, the literature on what to do with these medications in gastrointestinal endoscopy is still lacking. Dr. Sitharthan Sekar and Dr. Nikiya Asamoah summarize the current data and available guidelines in our “Short Clinical Review.”
With another new academic year upon us, this issue’s “Early Career” section features Dr. Allon Kahn’s top tips for becoming an effective gastroenterology consultant. He describes the 5 principles that would improve patient care and relationships with referring providers.
In the “Finance/Legal” section, Dr. Koushik Das dissects what happens when a physician gets sued, including the basis of malpractice suits, consequences, and anticipated timeline.
If you are interested in contributing or have ideas for future TNG topics, please contact me (tjudy@wustl.edu) or Danielle Kiefer (dkiefer@gastro.org), Communications/Managing Editor of TNG.
Until next time, I leave you with a historical fun fact, because we would not be where we are now without appreciating where we were: the pancreas was first discovered by a Greek surgeon, Herophilus, in 336 BC, but its exocrine and endocrine functions were not described until the 1850s-1860s by D. Moyse in Paris and Paul Langerhans in Berlin, respectively.
Yours truly,
Judy A. Trieu, MD, MPH
Editor-in-Chief
Assistant Professor of Medicine
Interventional Endoscopy, Division of Gastroenterology
Washington University School of Medicine in St. Louis
Dear Friends,
There have been recent policy changes that may be affecting trainees and practicing physicians, whether directly impacting our current practices or influencing the decisions that shape our careers. During these challenging times, I am trying to remind myself more often of why I am in medicine – my patients. I will continue to advocate for my patients on Hill Days to affect change in policy. I will continue to provide the best care I can and fight for resources to do so. I will continue to adapt to the changing climate and do what is best for my practice so that I can deliver the care I think my patients need. By remembering why I am in medicine, I can fight for a future of medicine and science that is still bright.
In this issue’s “In Focus” article, Dr. Yasmin G. Hernandez-Barco and Dr. Motaz Ashkar review the diagnostic and treatment approaches to exocrine pancreatic insufficiency, including common symptoms, differential diagnoses, and the different pancreatic enzyme replacement therapies.
Medications for weight loss are becoming more widely available; however, the literature on what to do with these medications in gastrointestinal endoscopy is still lacking. Dr. Sitharthan Sekar and Dr. Nikiya Asamoah summarize the current data and available guidelines in our “Short Clinical Review.”
With another new academic year upon us, this issue’s “Early Career” section features Dr. Allon Kahn’s top tips for becoming an effective gastroenterology consultant. He describes the 5 principles that would improve patient care and relationships with referring providers.
In the “Finance/Legal” section, Dr. Koushik Das dissects what happens when a physician gets sued, including the basis of malpractice suits, consequences, and anticipated timeline.
If you are interested in contributing or have ideas for future TNG topics, please contact me (tjudy@wustl.edu) or Danielle Kiefer (dkiefer@gastro.org), Communications/Managing Editor of TNG.
Until next time, I leave you with a historical fun fact, because we would not be where we are now without appreciating where we were: the pancreas was first discovered by a Greek surgeon, Herophilus, in 336 BC, but its exocrine and endocrine functions were not described until the 1850s-1860s by D. Moyse in Paris and Paul Langerhans in Berlin, respectively.
Yours truly,
Judy A. Trieu, MD, MPH
Editor-in-Chief
Assistant Professor of Medicine
Interventional Endoscopy, Division of Gastroenterology
Washington University School of Medicine in St. Louis
Dear Friends,
There have been recent policy changes that may be affecting trainees and practicing physicians, whether directly impacting our current practices or influencing the decisions that shape our careers. During these challenging times, I am trying to remind myself more often of why I am in medicine – my patients. I will continue to advocate for my patients on Hill Days to affect change in policy. I will continue to provide the best care I can and fight for resources to do so. I will continue to adapt to the changing climate and do what is best for my practice so that I can deliver the care I think my patients need. By remembering why I am in medicine, I can fight for a future of medicine and science that is still bright.
In this issue’s “In Focus” article, Dr. Yasmin G. Hernandez-Barco and Dr. Motaz Ashkar review the diagnostic and treatment approaches to exocrine pancreatic insufficiency, including common symptoms, differential diagnoses, and the different pancreatic enzyme replacement therapies.
Medications for weight loss are becoming more widely available; however, the literature on what to do with these medications in gastrointestinal endoscopy is still lacking. Dr. Sitharthan Sekar and Dr. Nikiya Asamoah summarize the current data and available guidelines in our “Short Clinical Review.”
With another new academic year upon us, this issue’s “Early Career” section features Dr. Allon Kahn’s top tips for becoming an effective gastroenterology consultant. He describes the 5 principles that would improve patient care and relationships with referring providers.
In the “Finance/Legal” section, Dr. Koushik Das dissects what happens when a physician gets sued, including the basis of malpractice suits, consequences, and anticipated timeline.
If you are interested in contributing or have ideas for future TNG topics, please contact me (tjudy@wustl.edu) or Danielle Kiefer (dkiefer@gastro.org), Communications/Managing Editor of TNG.
Until next time, I leave you with a historical fun fact, because we would not be where we are now without appreciating where we were: the pancreas was first discovered by a Greek surgeon, Herophilus, in 336 BC, but its exocrine and endocrine functions were not described until the 1850s-1860s by D. Moyse in Paris and Paul Langerhans in Berlin, respectively.
Yours truly,
Judy A. Trieu, MD, MPH
Editor-in-Chief
Assistant Professor of Medicine
Interventional Endoscopy, Division of Gastroenterology
Washington University School of Medicine in St. Louis
Positioning Yourself For Success in Private Practice

In this video, Peter Naas, MD, of Gastroenterology Associates in Greenville, South Carolina, shares insights on how young physicians can best position themselves for a successful career in private practice gastroenterology.

In this video, Peter Naas, MD, of Gastroenterology Associates in Greenville, South Carolina, shares insights on how young physicians can best position themselves for a successful career in private practice gastroenterology.

In this video, Peter Naas, MD, of Gastroenterology Associates in Greenville, South Carolina, shares insights on how young physicians can best position themselves for a successful career in private practice gastroenterology.
A Voice for Those Caring for Veterans With Cancer
A Voice for Those Caring for Veterans With Cancer
At some point, most Americans will experience the anxiety associated with an organizational restructure or a corporate budget cut that leads to job loss. Self-assurances may follow by telling ourselves we will be fine, and we could even start a new position that (if we're lucky) will be better than our previous one. It can be devastating, but is not a life-or-death scenario.
Unless you care for veterans with cancer.
The recent workforce reductions across the US Department of Veterans of Affairs (VA) health care system, whether through voluntary retirements or forced layoffs, is a life-threatening crisis. Every position lost has the potential to directly impact whether a veteran receives the necessary care in their battle with cancer.
Veterans deserve every opportunity, treatment plan, and resource available to ensure their comfort and survival. They are entitled to the specialized, comprehensive, and thorough care they receive through the VA—care that cannot be duplicated in community health care. Because many of the health challenges they face are a direct result of serving our country, we owe it to them to provide the best care available from the most highly-trained and competent clinicians. This level of excellence cannot be achieved in a gutted or chaotic system.
Reducing or eliminating VA health care positions is a decision that demands careful examination. Like any organization, the VA experiences some measure of waste or inefficiency that should be eliminated. But that cannot be done swiftly or in large-scale action.
Consider these examples: the reduction of force resulting in the removal of those deemed to hold unnecessary administrative positions—such as continuing education or physician oversight—has a direct impact on a clinician's ability to provide the most current and precise care. Reduced research funding limits the VA's contribution to health care innovation. The loss of contract positions that appear superfluous on paper represent the staff who schedule appointments, chemotherapy or radiation therapy, and wrap-around services for veterans. Even reducing auxiliary services like laundry may seem like a cost-saving measure—until the hospital can't admit new patients due to lack of sanitized linens.
VA employees know that veterans need specialized care for their complex and unique challenges. That individualized care has led to the VA nearly eliminating disparity gaps experienced in traditional health care. The removal of support positions and opportunities in professional development demands coordination with less-prepared community-based health care; overpopulated work environments will have a lasting impact. Limiting the workforce will make it impossible to provide coordinated and exceptional care.
The Association of VA Hematology/Oncology (AVAHO) is a leader in professional development opportunities for those who care for veterans with cancer. As a nonprofit organization, AVAHO is also a voice for those working with veterans with cancer to ensure they receive the care they deserve. AVAHO is calling on its colleagues, veterans, and those committed to supporting veterans to voice their opposition to reducing critical staff, research, and resources within the VA.
We ask veterans to share stories describing the difference VA care makes. We ask clinicians—including those within the federal system—to explain how a system that is well-staffed, supported, and with ample resources can impact patient care. Americans must stand for the care our veterans have earned.
Most importantly, we call on policymakers to carefully consider the impact each position has on the outcome of excellent, well-coordinated, and state-of-the-art care. The lives of our veterans depend on it.
AVAHO is a 501(c)3 nonprofit organization dedicated to supporting and educating health care providers who serve veterans with cancer and hematological disorders. You can find out more and support their advocacy initiatives at www.avaho.org.
At some point, most Americans will experience the anxiety associated with an organizational restructure or a corporate budget cut that leads to job loss. Self-assurances may follow by telling ourselves we will be fine, and we could even start a new position that (if we're lucky) will be better than our previous one. It can be devastating, but is not a life-or-death scenario.
Unless you care for veterans with cancer.
The recent workforce reductions across the US Department of Veterans of Affairs (VA) health care system, whether through voluntary retirements or forced layoffs, is a life-threatening crisis. Every position lost has the potential to directly impact whether a veteran receives the necessary care in their battle with cancer.
Veterans deserve every opportunity, treatment plan, and resource available to ensure their comfort and survival. They are entitled to the specialized, comprehensive, and thorough care they receive through the VA—care that cannot be duplicated in community health care. Because many of the health challenges they face are a direct result of serving our country, we owe it to them to provide the best care available from the most highly-trained and competent clinicians. This level of excellence cannot be achieved in a gutted or chaotic system.
Reducing or eliminating VA health care positions is a decision that demands careful examination. Like any organization, the VA experiences some measure of waste or inefficiency that should be eliminated. But that cannot be done swiftly or in large-scale action.
Consider these examples: the reduction of force resulting in the removal of those deemed to hold unnecessary administrative positions—such as continuing education or physician oversight—has a direct impact on a clinician's ability to provide the most current and precise care. Reduced research funding limits the VA's contribution to health care innovation. The loss of contract positions that appear superfluous on paper represent the staff who schedule appointments, chemotherapy or radiation therapy, and wrap-around services for veterans. Even reducing auxiliary services like laundry may seem like a cost-saving measure—until the hospital can't admit new patients due to lack of sanitized linens.
VA employees know that veterans need specialized care for their complex and unique challenges. That individualized care has led to the VA nearly eliminating disparity gaps experienced in traditional health care. The removal of support positions and opportunities in professional development demands coordination with less-prepared community-based health care; overpopulated work environments will have a lasting impact. Limiting the workforce will make it impossible to provide coordinated and exceptional care.
The Association of VA Hematology/Oncology (AVAHO) is a leader in professional development opportunities for those who care for veterans with cancer. As a nonprofit organization, AVAHO is also a voice for those working with veterans with cancer to ensure they receive the care they deserve. AVAHO is calling on its colleagues, veterans, and those committed to supporting veterans to voice their opposition to reducing critical staff, research, and resources within the VA.
We ask veterans to share stories describing the difference VA care makes. We ask clinicians—including those within the federal system—to explain how a system that is well-staffed, supported, and with ample resources can impact patient care. Americans must stand for the care our veterans have earned.
Most importantly, we call on policymakers to carefully consider the impact each position has on the outcome of excellent, well-coordinated, and state-of-the-art care. The lives of our veterans depend on it.
AVAHO is a 501(c)3 nonprofit organization dedicated to supporting and educating health care providers who serve veterans with cancer and hematological disorders. You can find out more and support their advocacy initiatives at www.avaho.org.
At some point, most Americans will experience the anxiety associated with an organizational restructure or a corporate budget cut that leads to job loss. Self-assurances may follow by telling ourselves we will be fine, and we could even start a new position that (if we're lucky) will be better than our previous one. It can be devastating, but is not a life-or-death scenario.
Unless you care for veterans with cancer.
The recent workforce reductions across the US Department of Veterans of Affairs (VA) health care system, whether through voluntary retirements or forced layoffs, is a life-threatening crisis. Every position lost has the potential to directly impact whether a veteran receives the necessary care in their battle with cancer.
Veterans deserve every opportunity, treatment plan, and resource available to ensure their comfort and survival. They are entitled to the specialized, comprehensive, and thorough care they receive through the VA—care that cannot be duplicated in community health care. Because many of the health challenges they face are a direct result of serving our country, we owe it to them to provide the best care available from the most highly-trained and competent clinicians. This level of excellence cannot be achieved in a gutted or chaotic system.
Reducing or eliminating VA health care positions is a decision that demands careful examination. Like any organization, the VA experiences some measure of waste or inefficiency that should be eliminated. But that cannot be done swiftly or in large-scale action.
Consider these examples: the reduction of force resulting in the removal of those deemed to hold unnecessary administrative positions—such as continuing education or physician oversight—has a direct impact on a clinician's ability to provide the most current and precise care. Reduced research funding limits the VA's contribution to health care innovation. The loss of contract positions that appear superfluous on paper represent the staff who schedule appointments, chemotherapy or radiation therapy, and wrap-around services for veterans. Even reducing auxiliary services like laundry may seem like a cost-saving measure—until the hospital can't admit new patients due to lack of sanitized linens.
VA employees know that veterans need specialized care for their complex and unique challenges. That individualized care has led to the VA nearly eliminating disparity gaps experienced in traditional health care. The removal of support positions and opportunities in professional development demands coordination with less-prepared community-based health care; overpopulated work environments will have a lasting impact. Limiting the workforce will make it impossible to provide coordinated and exceptional care.
The Association of VA Hematology/Oncology (AVAHO) is a leader in professional development opportunities for those who care for veterans with cancer. As a nonprofit organization, AVAHO is also a voice for those working with veterans with cancer to ensure they receive the care they deserve. AVAHO is calling on its colleagues, veterans, and those committed to supporting veterans to voice their opposition to reducing critical staff, research, and resources within the VA.
We ask veterans to share stories describing the difference VA care makes. We ask clinicians—including those within the federal system—to explain how a system that is well-staffed, supported, and with ample resources can impact patient care. Americans must stand for the care our veterans have earned.
Most importantly, we call on policymakers to carefully consider the impact each position has on the outcome of excellent, well-coordinated, and state-of-the-art care. The lives of our veterans depend on it.
AVAHO is a 501(c)3 nonprofit organization dedicated to supporting and educating health care providers who serve veterans with cancer and hematological disorders. You can find out more and support their advocacy initiatives at www.avaho.org.
A Voice for Those Caring for Veterans With Cancer
A Voice for Those Caring for Veterans With Cancer
Evolving Standards of Practice: Esophageal Varices and Barrett’s Esophagus
Dear colleagues,
In the dynamic field of medicine, long-held practices are being reevaluated in light of new evidence and evolving standards of practice.
Dr. Anahita Rabiee discusses the importance of prioritizing non-selective beta blockers (NSBB) over endoscopic variceal ligation (EVL) in the primary prophylaxis of variceal bleeding in patients with compensated cirrhosis. Drawing on data from the PREDESCI trial and real-world experience, she argues that NSBB address the upstream driver—portal hypertension—more broadly and effectively than EVL. In a complementary piece, Dr. Tarek Sawas explores the nuanced landscape of screening and surveillance in Barrett’s esophagus. From how to manage irregular Z-lines, to rethinking the need for 1-year follow-up endoscopies and interpreting the implications of the BOSS trial, Dr. Sawas advocates for a more personalized, risk-based approach.
We hope these perspectives spark dialogue and reflection in your own practice. Join the conversation on X at @AGA_GIHN.
Gyanprakash A. Ketwaroo, MD, MSc, is associate professor of medicine, Yale University, New Haven, and chief of endoscopy at West Haven VA Medical Center, both in Connecticut. He is an associate editor for GI & Hepatology News.
Choose NSBBs, Not EVL, in Patients with Compensated Cirrhosis
BY ANAHITA RABIEE, MD, MHS
I strongly favor the use of non selective beta blockers (NSBBs) in patients with compensated cirrhosis, rather than endoscopy and esophageal variceal ligation (EVL) for primary prophylaxis.
Since the results of PREDESCI trial (β blockers to prevent decompensation of cirrhosis in patients with clinically significant portal hypertension (CSPH)) were published in 2019, there has been much debate on the role of screening endoscopy and EVL for primary prophylaxis. While many argue that a single randomized trial should not overturn long standing practice, several compelling reasons convince me to choose NSBBs, when possible.
Recent guidance from major liver societies now recommends NSBBs as first line therapy for CSPH. Yet, adoption in clinical practice remains inconsistent.
Here is why I believe NSBB represent a better solution:
Treating Upstream, Not Just a Local Treatment
NSBBs such as propranolol and nadolol decrease portal pressure by decreasing portal venous inflow through β1 and β2 adrenergic blockade. Carvedilol is often preferred given its additional α1 adrenergic blocking activity making it the most effective one in decreasing the portal pressure. Therefore, NSBBs address the upstream driver of decompensation by decreasing portal pressures.
EVL, in contrast, is a local fix that only prevents variceal bleeding. Ascites, not variceal bleeding, is the most common initial decompensating event and is associated with high mortality. Preventing all forms of decompensation is clearly preferable to preventing just one.
Broader Eligibility, More Patients Benefit
CSPH is defined as hepatic venous pressure gradient (HVPG)>10 mmHg, the threshold where increased portal venous inflow secondary to splanchnic vasodilation and hyperdynamic circulation drives the increase in portal hypertension. This threshold has been shown to strongly predict decompensation in patients with compensated disease.
While all patients with varices have CSPH, not all patients with CSPH have varices. They can be identified by other non invasive criteria such as cross sectional imaging showing collaterals, or liver stiffness and platelet thresholds that have been previously validated. By restricting intervention to those with large varices and offering only EVL, we miss the opportunity to intervene earlier and to a broader group that would benefit from this treatment.
Comprehensive Protection Without Repeated Endoscopies
Once on an appropriate NSBB dose, patients are protected against variceal bleeding (at least as effectively as EVL). This eliminates the need for repeated surveillance endoscopies to identify and treat large varices in otherwise compensated patients.
Better Tolerated and – In Many Cases – Overlaps With Existing Medication List!
While overtreatment is a concern, regular endoscopies every two years are also burdensome. Many patients already need beta blockers for cardiac conditions such as atrial fibrillation, ischemic heart disease or hypertension. Carvedilol, in particular, offers dual benefit for both hepatologists and cardiologists.
It is important to emphasize that these arguments apply to compensated cirrhosis. In decompensated disease, the approach changes. After a variceal bleed, both NSBBs and EVL are required for secondary prophylaxis. In patients with prior ascites but no variceal bleed, the benefit of NSBBs is less pronounced since decompensation has already occurred. In this setting, NSBBs can still be used selectively, but only if systolic blood pressure remains above 90 mmHg.
The evidence supporting NSBBs over EVL in compensated cirrhosis is not perfect, but few things in medicine are. Given current data, NSBBs should be the first line therapy in compensated cirrhosis with CSPH. Once a patient is on an appropriate and tolerated NSBB dose, routine endoscopic surveillance is unnecessary. Endoscopy should be reserved for those who cannot tolerate NSBBs, in whom EVL is then indicated if large varices are present.
Dr. Rabiee is based at the Yale School of Medicine, New Haven, Connecticut, and the Veterans Affairs Connecticut Healthcare System, West Haven, Connecticut. She has no disclosures in regard to this article.
Rethinking Screening and Surveillance in Barrett’s Esophagus: Navigating Controversies and Nuances
BY TAREK SAWAS, MD, MPH
Barrett’s esophagus (BE) is a precursor to esophageal adenocarcinoma (EAC). Despite our comprehensive guidelines, many of the day-to-day decisions still rely on clinical judgment and honest conversations with patients. This article explores common scenarios in which management decisions are nuanced and the right answer remains debatable.
Irregular Z-Line/Ultrashort Segment BE: Leave Or Watch It?
Few findings provoke more confusion than irregular Z-line or intestinal metaplasia (IM) < 1 cm at the gastroesophageal junction (GEJ). For years, we have debated whether these subtle changes represent a precursor to EAC or simply a benign variant. We have wrestled with how to handle these cases from whether we should take biopsies to how to perform surveillance.
The American College of Gastroenterology (ACG) guideline suggests that irregular Z-lines should not be routinely biopsied or surveyed. Similarly, the upcoming American Gastroenterology Association (AGA) surveillance guideline suggests against surveillance of IM<1 cm citing the low individual annual risk of progression to high-grade dysplasia (HGD) and EAC of 0.23% per year which is lower than that of non-dysplastic Barrett’s esophagus (NDBE). However, this is not the entire picture.
Despite the low per-patient risk, IM<1cm is highly prevalent with columnar mucosa observed in approximately 15% of patients undergoing upper endoscopy. This paradox is unsettling. While any one patient with IM<1 cm is unlikely to progress to EAC, the group accounts for a meaningful share of the EAC burden. Some experts have argued that this justifies routine biopsy and surveillance in all patients with visible columnar mucosa regardless of length. However, this approach risks overwhelming our surveillance infrastructure.
A recent decision modeling analysis suggested that at the lowest progression rates, either no surveillance or one-time endoscopy can be considered. Based on these data, I do not regularly biopsy ultrashort segments unless the mucosa appears suspicious. In those with IM<1 cm detected during a high-quality endoscopic exam, no follow-up is needed. However, if the exam is suboptimal, I perform a 1-time high-quality repeat exam. If there is no evidence of dysplasia then I do not pursue any further surveillance.
The One-Year Follow-Up Endoscopy: Is It Necessary?
Another controversy is the one-year follow-up endoscopy after an initial diagnosis of NDBE. Proponents of this approach cite the high proportion of post endoscopy esophageal neoplasia and cancer (PEEN/PEEC) detected in the first year after diagnosis (missed HGD/EAC). In fact, PEEN account for about a quarter of all HGD/EAC cases diagnosed during surveillance.
While this approach might mitigate PEEN/PEEC risk, it may not be necessary if the index endoscopy is high quality. To ensure high quality exams, several best practices have been proposed including:
- Use of high-definition white light endoscopy (HD-WLE) with chromoendoscopy (virtual or dye based)
- Appropriate inspection time (1 minute per cm of circumferential BE)
- Accurate documentation using the Prague criteria
- Adherence to the Seattle protocol with additional targeted biopsies
If the index endoscopy meets these quality metrics, I typically do not bring the patient back at one year. However, if the exam quality is in question, then I repeat it at one year to establish a reliable baseline and rule out prevalent neoplasia.
Surveillance In NDBE: After BOSS, Do We Rethink Everything?
The recently published BOSS trial (Barrett’s Oesophagus Surveillance Study) has reignited the debate over the value of endoscopic surveillance in NDBE. In this study, 3,453 patients with NDBE across the UK were randomized to either surveillance endoscopy every two years or endoscopy only as clinically indicated. After a median follow-up of 12.8 years, the trial found no significant difference in all-cause mortality between the two groups.
While these findings are important, they should be interpreted with caution. First, the primary endpoint, all-cause mortality, is not optimal for evaluating surveillance for EAC. Surveillance is not intended to reduce all-cause mortality but rather to reduce EAC–related mortality. Second, a substantial number of patients in the no surveillance group still underwent endoscopy at intervals that were not meaningfully different from those in the surveillance group. If both groups receive similar exposure to endoscopy, the comparison loses power. Lastly, the trial was underpowered due to overestimation of progression risk during its initial design. As we have since learned, the risk of progression of NDBE is lower than originally assumed.
So where do we stand now? For me, the BOSS trial does not negate the value of surveillance. it reminds us that a one-size-fits-all approach is inefficient, and our strategy must be risk based. For low-risk individuals, particularly older adults with short-segment NDBE, surveillance may offer little benefit. But in healthier, younger patients with longer segments or additional risk factors, surveillance remains an essential tool for early neoplasia detection.
When to Stop Surveillance
Perhaps the most under-discussed point is when to stop surveillance. Existing guidelines do not account for competing mortality risks unrelated to EAC or provide specific recommendations regarding cessation of surveillance. The desired benefits of surveillance likely diminish with advanced age and greater comorbidity because of lower life expectancy and ineligibility for definitive therapy for EAC.
A recent modeling study found that the optimal ages for last surveillance were 81, 80, 77, and 73 years for men with no, mild, moderate, and severe comorbidity respectively and 75, 73, 73, and 69 years for women. In my practice, I discuss surveillance cessation in patients older than 75 based on their comorbidities. If the risk of progression is outweighed by the risk of the procedure or by the reality of limited life expectancy, we should not hesitate to consider surveillance cessation.
In summary, high-quality endoscopic exam in appropriately selected patients remains the cornerstone of BE surveillance. A more personalized, risk-based approach is needed taking into account competing comorbidities. Emerging technology through risk stratification tools such as biomarkers and artificial intelligence may refine our approach and help address the current limitations.
Dr. Sawas is based at the University of Texas Southwestern, Dallas, Texas. He has no disclosures in regard to this article.
Dear colleagues,
In the dynamic field of medicine, long-held practices are being reevaluated in light of new evidence and evolving standards of practice.
Dr. Anahita Rabiee discusses the importance of prioritizing non-selective beta blockers (NSBB) over endoscopic variceal ligation (EVL) in the primary prophylaxis of variceal bleeding in patients with compensated cirrhosis. Drawing on data from the PREDESCI trial and real-world experience, she argues that NSBB address the upstream driver—portal hypertension—more broadly and effectively than EVL. In a complementary piece, Dr. Tarek Sawas explores the nuanced landscape of screening and surveillance in Barrett’s esophagus. From how to manage irregular Z-lines, to rethinking the need for 1-year follow-up endoscopies and interpreting the implications of the BOSS trial, Dr. Sawas advocates for a more personalized, risk-based approach.
We hope these perspectives spark dialogue and reflection in your own practice. Join the conversation on X at @AGA_GIHN.
Gyanprakash A. Ketwaroo, MD, MSc, is associate professor of medicine, Yale University, New Haven, and chief of endoscopy at West Haven VA Medical Center, both in Connecticut. He is an associate editor for GI & Hepatology News.
Choose NSBBs, Not EVL, in Patients with Compensated Cirrhosis
BY ANAHITA RABIEE, MD, MHS
I strongly favor the use of non selective beta blockers (NSBBs) in patients with compensated cirrhosis, rather than endoscopy and esophageal variceal ligation (EVL) for primary prophylaxis.
Since the results of PREDESCI trial (β blockers to prevent decompensation of cirrhosis in patients with clinically significant portal hypertension (CSPH)) were published in 2019, there has been much debate on the role of screening endoscopy and EVL for primary prophylaxis. While many argue that a single randomized trial should not overturn long standing practice, several compelling reasons convince me to choose NSBBs, when possible.
Recent guidance from major liver societies now recommends NSBBs as first line therapy for CSPH. Yet, adoption in clinical practice remains inconsistent.
Here is why I believe NSBB represent a better solution:
Treating Upstream, Not Just a Local Treatment
NSBBs such as propranolol and nadolol decrease portal pressure by decreasing portal venous inflow through β1 and β2 adrenergic blockade. Carvedilol is often preferred given its additional α1 adrenergic blocking activity making it the most effective one in decreasing the portal pressure. Therefore, NSBBs address the upstream driver of decompensation by decreasing portal pressures.
EVL, in contrast, is a local fix that only prevents variceal bleeding. Ascites, not variceal bleeding, is the most common initial decompensating event and is associated with high mortality. Preventing all forms of decompensation is clearly preferable to preventing just one.
Broader Eligibility, More Patients Benefit
CSPH is defined as hepatic venous pressure gradient (HVPG)>10 mmHg, the threshold where increased portal venous inflow secondary to splanchnic vasodilation and hyperdynamic circulation drives the increase in portal hypertension. This threshold has been shown to strongly predict decompensation in patients with compensated disease.
While all patients with varices have CSPH, not all patients with CSPH have varices. They can be identified by other non invasive criteria such as cross sectional imaging showing collaterals, or liver stiffness and platelet thresholds that have been previously validated. By restricting intervention to those with large varices and offering only EVL, we miss the opportunity to intervene earlier and to a broader group that would benefit from this treatment.
Comprehensive Protection Without Repeated Endoscopies
Once on an appropriate NSBB dose, patients are protected against variceal bleeding (at least as effectively as EVL). This eliminates the need for repeated surveillance endoscopies to identify and treat large varices in otherwise compensated patients.
Better Tolerated and – In Many Cases – Overlaps With Existing Medication List!
While overtreatment is a concern, regular endoscopies every two years are also burdensome. Many patients already need beta blockers for cardiac conditions such as atrial fibrillation, ischemic heart disease or hypertension. Carvedilol, in particular, offers dual benefit for both hepatologists and cardiologists.
It is important to emphasize that these arguments apply to compensated cirrhosis. In decompensated disease, the approach changes. After a variceal bleed, both NSBBs and EVL are required for secondary prophylaxis. In patients with prior ascites but no variceal bleed, the benefit of NSBBs is less pronounced since decompensation has already occurred. In this setting, NSBBs can still be used selectively, but only if systolic blood pressure remains above 90 mmHg.
The evidence supporting NSBBs over EVL in compensated cirrhosis is not perfect, but few things in medicine are. Given current data, NSBBs should be the first line therapy in compensated cirrhosis with CSPH. Once a patient is on an appropriate and tolerated NSBB dose, routine endoscopic surveillance is unnecessary. Endoscopy should be reserved for those who cannot tolerate NSBBs, in whom EVL is then indicated if large varices are present.
Dr. Rabiee is based at the Yale School of Medicine, New Haven, Connecticut, and the Veterans Affairs Connecticut Healthcare System, West Haven, Connecticut. She has no disclosures in regard to this article.
Rethinking Screening and Surveillance in Barrett’s Esophagus: Navigating Controversies and Nuances
BY TAREK SAWAS, MD, MPH
Barrett’s esophagus (BE) is a precursor to esophageal adenocarcinoma (EAC). Despite our comprehensive guidelines, many of the day-to-day decisions still rely on clinical judgment and honest conversations with patients. This article explores common scenarios in which management decisions are nuanced and the right answer remains debatable.
Irregular Z-Line/Ultrashort Segment BE: Leave Or Watch It?
Few findings provoke more confusion than irregular Z-line or intestinal metaplasia (IM) < 1 cm at the gastroesophageal junction (GEJ). For years, we have debated whether these subtle changes represent a precursor to EAC or simply a benign variant. We have wrestled with how to handle these cases from whether we should take biopsies to how to perform surveillance.
The American College of Gastroenterology (ACG) guideline suggests that irregular Z-lines should not be routinely biopsied or surveyed. Similarly, the upcoming American Gastroenterology Association (AGA) surveillance guideline suggests against surveillance of IM<1 cm citing the low individual annual risk of progression to high-grade dysplasia (HGD) and EAC of 0.23% per year which is lower than that of non-dysplastic Barrett’s esophagus (NDBE). However, this is not the entire picture.
Despite the low per-patient risk, IM<1cm is highly prevalent with columnar mucosa observed in approximately 15% of patients undergoing upper endoscopy. This paradox is unsettling. While any one patient with IM<1 cm is unlikely to progress to EAC, the group accounts for a meaningful share of the EAC burden. Some experts have argued that this justifies routine biopsy and surveillance in all patients with visible columnar mucosa regardless of length. However, this approach risks overwhelming our surveillance infrastructure.
A recent decision modeling analysis suggested that at the lowest progression rates, either no surveillance or one-time endoscopy can be considered. Based on these data, I do not regularly biopsy ultrashort segments unless the mucosa appears suspicious. In those with IM<1 cm detected during a high-quality endoscopic exam, no follow-up is needed. However, if the exam is suboptimal, I perform a 1-time high-quality repeat exam. If there is no evidence of dysplasia then I do not pursue any further surveillance.
The One-Year Follow-Up Endoscopy: Is It Necessary?
Another controversy is the one-year follow-up endoscopy after an initial diagnosis of NDBE. Proponents of this approach cite the high proportion of post endoscopy esophageal neoplasia and cancer (PEEN/PEEC) detected in the first year after diagnosis (missed HGD/EAC). In fact, PEEN account for about a quarter of all HGD/EAC cases diagnosed during surveillance.
While this approach might mitigate PEEN/PEEC risk, it may not be necessary if the index endoscopy is high quality. To ensure high quality exams, several best practices have been proposed including:
- Use of high-definition white light endoscopy (HD-WLE) with chromoendoscopy (virtual or dye based)
- Appropriate inspection time (1 minute per cm of circumferential BE)
- Accurate documentation using the Prague criteria
- Adherence to the Seattle protocol with additional targeted biopsies
If the index endoscopy meets these quality metrics, I typically do not bring the patient back at one year. However, if the exam quality is in question, then I repeat it at one year to establish a reliable baseline and rule out prevalent neoplasia.
Surveillance In NDBE: After BOSS, Do We Rethink Everything?
The recently published BOSS trial (Barrett’s Oesophagus Surveillance Study) has reignited the debate over the value of endoscopic surveillance in NDBE. In this study, 3,453 patients with NDBE across the UK were randomized to either surveillance endoscopy every two years or endoscopy only as clinically indicated. After a median follow-up of 12.8 years, the trial found no significant difference in all-cause mortality between the two groups.
While these findings are important, they should be interpreted with caution. First, the primary endpoint, all-cause mortality, is not optimal for evaluating surveillance for EAC. Surveillance is not intended to reduce all-cause mortality but rather to reduce EAC–related mortality. Second, a substantial number of patients in the no surveillance group still underwent endoscopy at intervals that were not meaningfully different from those in the surveillance group. If both groups receive similar exposure to endoscopy, the comparison loses power. Lastly, the trial was underpowered due to overestimation of progression risk during its initial design. As we have since learned, the risk of progression of NDBE is lower than originally assumed.
So where do we stand now? For me, the BOSS trial does not negate the value of surveillance. it reminds us that a one-size-fits-all approach is inefficient, and our strategy must be risk based. For low-risk individuals, particularly older adults with short-segment NDBE, surveillance may offer little benefit. But in healthier, younger patients with longer segments or additional risk factors, surveillance remains an essential tool for early neoplasia detection.
When to Stop Surveillance
Perhaps the most under-discussed point is when to stop surveillance. Existing guidelines do not account for competing mortality risks unrelated to EAC or provide specific recommendations regarding cessation of surveillance. The desired benefits of surveillance likely diminish with advanced age and greater comorbidity because of lower life expectancy and ineligibility for definitive therapy for EAC.
A recent modeling study found that the optimal ages for last surveillance were 81, 80, 77, and 73 years for men with no, mild, moderate, and severe comorbidity respectively and 75, 73, 73, and 69 years for women. In my practice, I discuss surveillance cessation in patients older than 75 based on their comorbidities. If the risk of progression is outweighed by the risk of the procedure or by the reality of limited life expectancy, we should not hesitate to consider surveillance cessation.
In summary, high-quality endoscopic exam in appropriately selected patients remains the cornerstone of BE surveillance. A more personalized, risk-based approach is needed taking into account competing comorbidities. Emerging technology through risk stratification tools such as biomarkers and artificial intelligence may refine our approach and help address the current limitations.
Dr. Sawas is based at the University of Texas Southwestern, Dallas, Texas. He has no disclosures in regard to this article.
Dear colleagues,
In the dynamic field of medicine, long-held practices are being reevaluated in light of new evidence and evolving standards of practice.
Dr. Anahita Rabiee discusses the importance of prioritizing non-selective beta blockers (NSBB) over endoscopic variceal ligation (EVL) in the primary prophylaxis of variceal bleeding in patients with compensated cirrhosis. Drawing on data from the PREDESCI trial and real-world experience, she argues that NSBB address the upstream driver—portal hypertension—more broadly and effectively than EVL. In a complementary piece, Dr. Tarek Sawas explores the nuanced landscape of screening and surveillance in Barrett’s esophagus. From how to manage irregular Z-lines, to rethinking the need for 1-year follow-up endoscopies and interpreting the implications of the BOSS trial, Dr. Sawas advocates for a more personalized, risk-based approach.
We hope these perspectives spark dialogue and reflection in your own practice. Join the conversation on X at @AGA_GIHN.
Gyanprakash A. Ketwaroo, MD, MSc, is associate professor of medicine, Yale University, New Haven, and chief of endoscopy at West Haven VA Medical Center, both in Connecticut. He is an associate editor for GI & Hepatology News.
Choose NSBBs, Not EVL, in Patients with Compensated Cirrhosis
BY ANAHITA RABIEE, MD, MHS
I strongly favor the use of non selective beta blockers (NSBBs) in patients with compensated cirrhosis, rather than endoscopy and esophageal variceal ligation (EVL) for primary prophylaxis.
Since the results of PREDESCI trial (β blockers to prevent decompensation of cirrhosis in patients with clinically significant portal hypertension (CSPH)) were published in 2019, there has been much debate on the role of screening endoscopy and EVL for primary prophylaxis. While many argue that a single randomized trial should not overturn long standing practice, several compelling reasons convince me to choose NSBBs, when possible.
Recent guidance from major liver societies now recommends NSBBs as first line therapy for CSPH. Yet, adoption in clinical practice remains inconsistent.
Here is why I believe NSBB represent a better solution:
Treating Upstream, Not Just a Local Treatment
NSBBs such as propranolol and nadolol decrease portal pressure by decreasing portal venous inflow through β1 and β2 adrenergic blockade. Carvedilol is often preferred given its additional α1 adrenergic blocking activity making it the most effective one in decreasing the portal pressure. Therefore, NSBBs address the upstream driver of decompensation by decreasing portal pressures.
EVL, in contrast, is a local fix that only prevents variceal bleeding. Ascites, not variceal bleeding, is the most common initial decompensating event and is associated with high mortality. Preventing all forms of decompensation is clearly preferable to preventing just one.
Broader Eligibility, More Patients Benefit
CSPH is defined as hepatic venous pressure gradient (HVPG)>10 mmHg, the threshold where increased portal venous inflow secondary to splanchnic vasodilation and hyperdynamic circulation drives the increase in portal hypertension. This threshold has been shown to strongly predict decompensation in patients with compensated disease.
While all patients with varices have CSPH, not all patients with CSPH have varices. They can be identified by other non invasive criteria such as cross sectional imaging showing collaterals, or liver stiffness and platelet thresholds that have been previously validated. By restricting intervention to those with large varices and offering only EVL, we miss the opportunity to intervene earlier and to a broader group that would benefit from this treatment.
Comprehensive Protection Without Repeated Endoscopies
Once on an appropriate NSBB dose, patients are protected against variceal bleeding (at least as effectively as EVL). This eliminates the need for repeated surveillance endoscopies to identify and treat large varices in otherwise compensated patients.
Better Tolerated and – In Many Cases – Overlaps With Existing Medication List!
While overtreatment is a concern, regular endoscopies every two years are also burdensome. Many patients already need beta blockers for cardiac conditions such as atrial fibrillation, ischemic heart disease or hypertension. Carvedilol, in particular, offers dual benefit for both hepatologists and cardiologists.
It is important to emphasize that these arguments apply to compensated cirrhosis. In decompensated disease, the approach changes. After a variceal bleed, both NSBBs and EVL are required for secondary prophylaxis. In patients with prior ascites but no variceal bleed, the benefit of NSBBs is less pronounced since decompensation has already occurred. In this setting, NSBBs can still be used selectively, but only if systolic blood pressure remains above 90 mmHg.
The evidence supporting NSBBs over EVL in compensated cirrhosis is not perfect, but few things in medicine are. Given current data, NSBBs should be the first line therapy in compensated cirrhosis with CSPH. Once a patient is on an appropriate and tolerated NSBB dose, routine endoscopic surveillance is unnecessary. Endoscopy should be reserved for those who cannot tolerate NSBBs, in whom EVL is then indicated if large varices are present.
Dr. Rabiee is based at the Yale School of Medicine, New Haven, Connecticut, and the Veterans Affairs Connecticut Healthcare System, West Haven, Connecticut. She has no disclosures in regard to this article.
Rethinking Screening and Surveillance in Barrett’s Esophagus: Navigating Controversies and Nuances
BY TAREK SAWAS, MD, MPH
Barrett’s esophagus (BE) is a precursor to esophageal adenocarcinoma (EAC). Despite our comprehensive guidelines, many of the day-to-day decisions still rely on clinical judgment and honest conversations with patients. This article explores common scenarios in which management decisions are nuanced and the right answer remains debatable.
Irregular Z-Line/Ultrashort Segment BE: Leave Or Watch It?
Few findings provoke more confusion than irregular Z-line or intestinal metaplasia (IM) < 1 cm at the gastroesophageal junction (GEJ). For years, we have debated whether these subtle changes represent a precursor to EAC or simply a benign variant. We have wrestled with how to handle these cases from whether we should take biopsies to how to perform surveillance.
The American College of Gastroenterology (ACG) guideline suggests that irregular Z-lines should not be routinely biopsied or surveyed. Similarly, the upcoming American Gastroenterology Association (AGA) surveillance guideline suggests against surveillance of IM<1 cm citing the low individual annual risk of progression to high-grade dysplasia (HGD) and EAC of 0.23% per year which is lower than that of non-dysplastic Barrett’s esophagus (NDBE). However, this is not the entire picture.
Despite the low per-patient risk, IM<1cm is highly prevalent with columnar mucosa observed in approximately 15% of patients undergoing upper endoscopy. This paradox is unsettling. While any one patient with IM<1 cm is unlikely to progress to EAC, the group accounts for a meaningful share of the EAC burden. Some experts have argued that this justifies routine biopsy and surveillance in all patients with visible columnar mucosa regardless of length. However, this approach risks overwhelming our surveillance infrastructure.
A recent decision modeling analysis suggested that at the lowest progression rates, either no surveillance or one-time endoscopy can be considered. Based on these data, I do not regularly biopsy ultrashort segments unless the mucosa appears suspicious. In those with IM<1 cm detected during a high-quality endoscopic exam, no follow-up is needed. However, if the exam is suboptimal, I perform a 1-time high-quality repeat exam. If there is no evidence of dysplasia then I do not pursue any further surveillance.
The One-Year Follow-Up Endoscopy: Is It Necessary?
Another controversy is the one-year follow-up endoscopy after an initial diagnosis of NDBE. Proponents of this approach cite the high proportion of post endoscopy esophageal neoplasia and cancer (PEEN/PEEC) detected in the first year after diagnosis (missed HGD/EAC). In fact, PEEN account for about a quarter of all HGD/EAC cases diagnosed during surveillance.
While this approach might mitigate PEEN/PEEC risk, it may not be necessary if the index endoscopy is high quality. To ensure high quality exams, several best practices have been proposed including:
- Use of high-definition white light endoscopy (HD-WLE) with chromoendoscopy (virtual or dye based)
- Appropriate inspection time (1 minute per cm of circumferential BE)
- Accurate documentation using the Prague criteria
- Adherence to the Seattle protocol with additional targeted biopsies
If the index endoscopy meets these quality metrics, I typically do not bring the patient back at one year. However, if the exam quality is in question, then I repeat it at one year to establish a reliable baseline and rule out prevalent neoplasia.
Surveillance In NDBE: After BOSS, Do We Rethink Everything?
The recently published BOSS trial (Barrett’s Oesophagus Surveillance Study) has reignited the debate over the value of endoscopic surveillance in NDBE. In this study, 3,453 patients with NDBE across the UK were randomized to either surveillance endoscopy every two years or endoscopy only as clinically indicated. After a median follow-up of 12.8 years, the trial found no significant difference in all-cause mortality between the two groups.
While these findings are important, they should be interpreted with caution. First, the primary endpoint, all-cause mortality, is not optimal for evaluating surveillance for EAC. Surveillance is not intended to reduce all-cause mortality but rather to reduce EAC–related mortality. Second, a substantial number of patients in the no surveillance group still underwent endoscopy at intervals that were not meaningfully different from those in the surveillance group. If both groups receive similar exposure to endoscopy, the comparison loses power. Lastly, the trial was underpowered due to overestimation of progression risk during its initial design. As we have since learned, the risk of progression of NDBE is lower than originally assumed.
So where do we stand now? For me, the BOSS trial does not negate the value of surveillance. it reminds us that a one-size-fits-all approach is inefficient, and our strategy must be risk based. For low-risk individuals, particularly older adults with short-segment NDBE, surveillance may offer little benefit. But in healthier, younger patients with longer segments or additional risk factors, surveillance remains an essential tool for early neoplasia detection.
When to Stop Surveillance
Perhaps the most under-discussed point is when to stop surveillance. Existing guidelines do not account for competing mortality risks unrelated to EAC or provide specific recommendations regarding cessation of surveillance. The desired benefits of surveillance likely diminish with advanced age and greater comorbidity because of lower life expectancy and ineligibility for definitive therapy for EAC.
A recent modeling study found that the optimal ages for last surveillance were 81, 80, 77, and 73 years for men with no, mild, moderate, and severe comorbidity respectively and 75, 73, 73, and 69 years for women. In my practice, I discuss surveillance cessation in patients older than 75 based on their comorbidities. If the risk of progression is outweighed by the risk of the procedure or by the reality of limited life expectancy, we should not hesitate to consider surveillance cessation.
In summary, high-quality endoscopic exam in appropriately selected patients remains the cornerstone of BE surveillance. A more personalized, risk-based approach is needed taking into account competing comorbidities. Emerging technology through risk stratification tools such as biomarkers and artificial intelligence may refine our approach and help address the current limitations.
Dr. Sawas is based at the University of Texas Southwestern, Dallas, Texas. He has no disclosures in regard to this article.