User login
Painless Vulvar Nodule
The Diagnosis: Proximal-Type Epithelioid Sarcoma
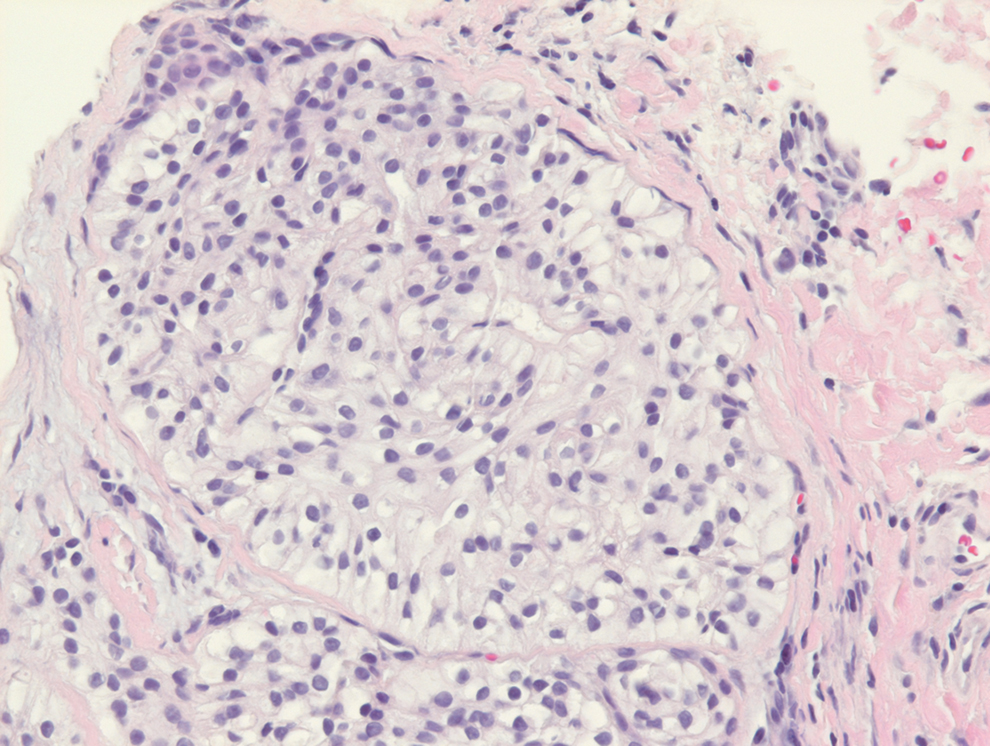
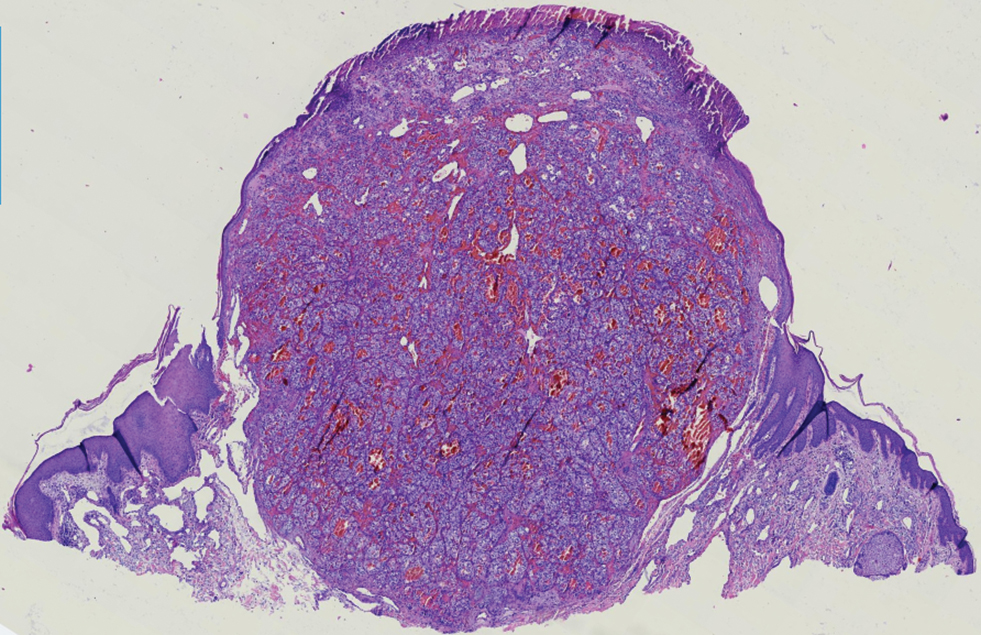
Proximal-type epithelioid sarcoma (PES) is a rare high-grade sarcoma of uncertain histogenesis that may present with a benign clinical appearance. Proximal-type epithelioid sarcoma peaks at 20 to 40 years of age and has a slight male predominance. This tumor exhibits aggressive behavior with both local recurrence and metastasis.1 The average overall survival is poor; however, tumor size less than 5 cm and complete excision with tumor-free margin status improves the prognosis.2 Proximal-type epithelioid sarcoma should not be confused with distal-type epithelioid sarcoma, which has a better prognosis and occurs in younger patients.1 Treatment of PES is wide excision, and chemotherapy trials with tazemetostat are ongoing.3
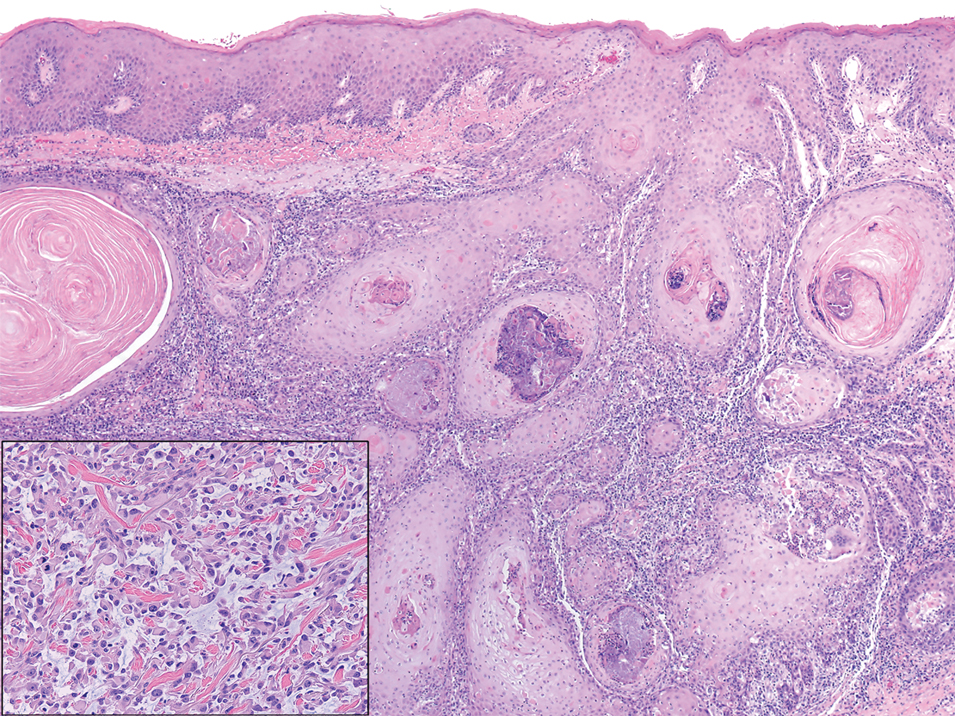
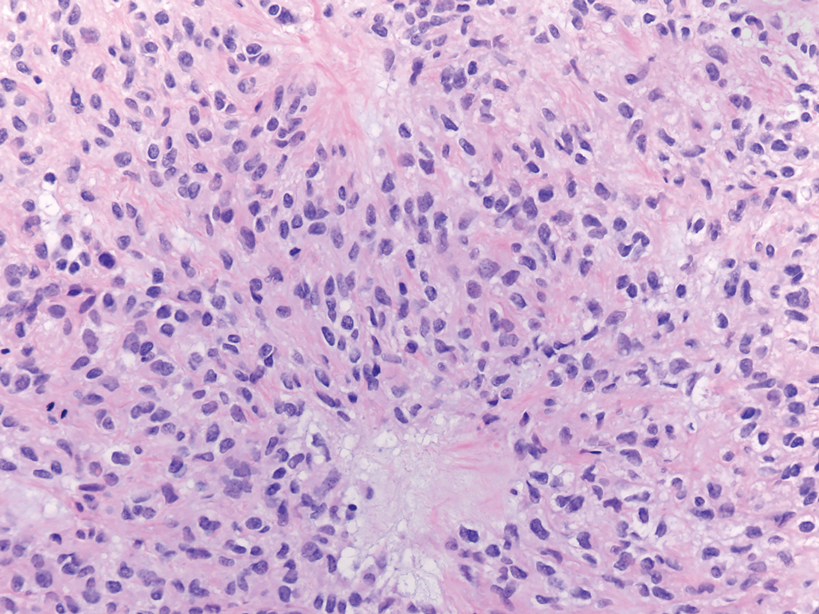
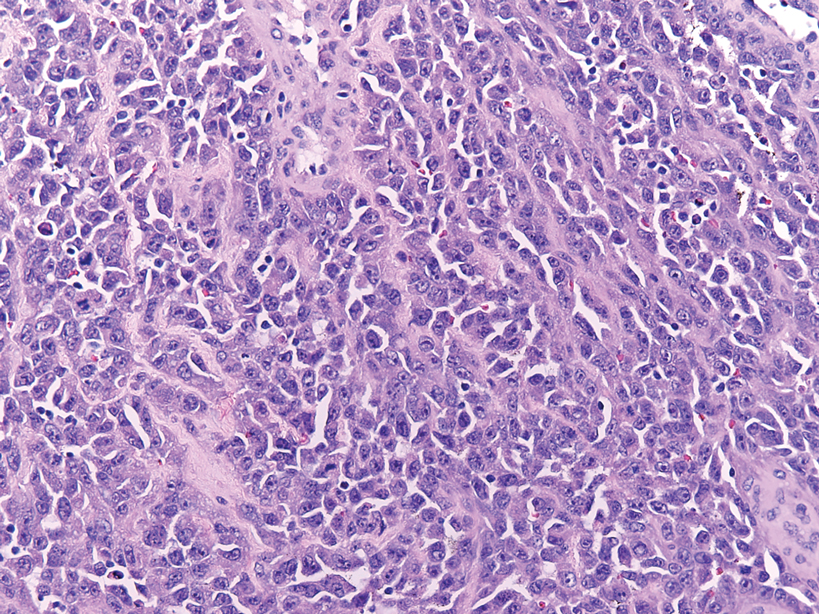
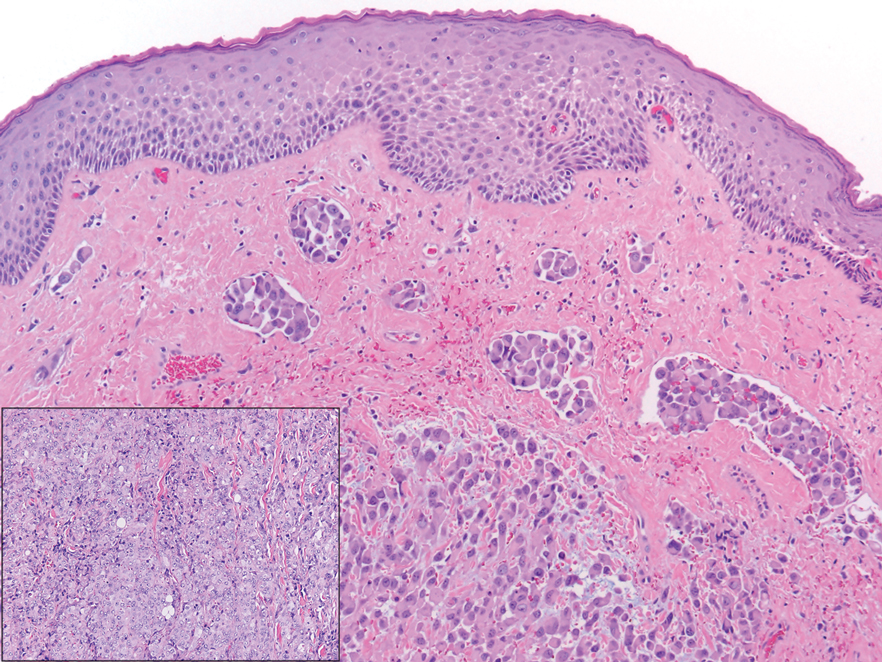
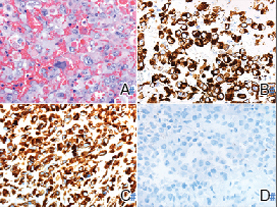
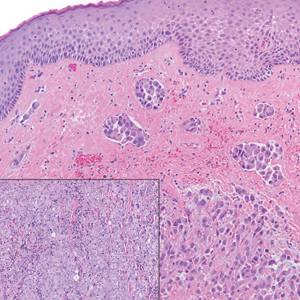
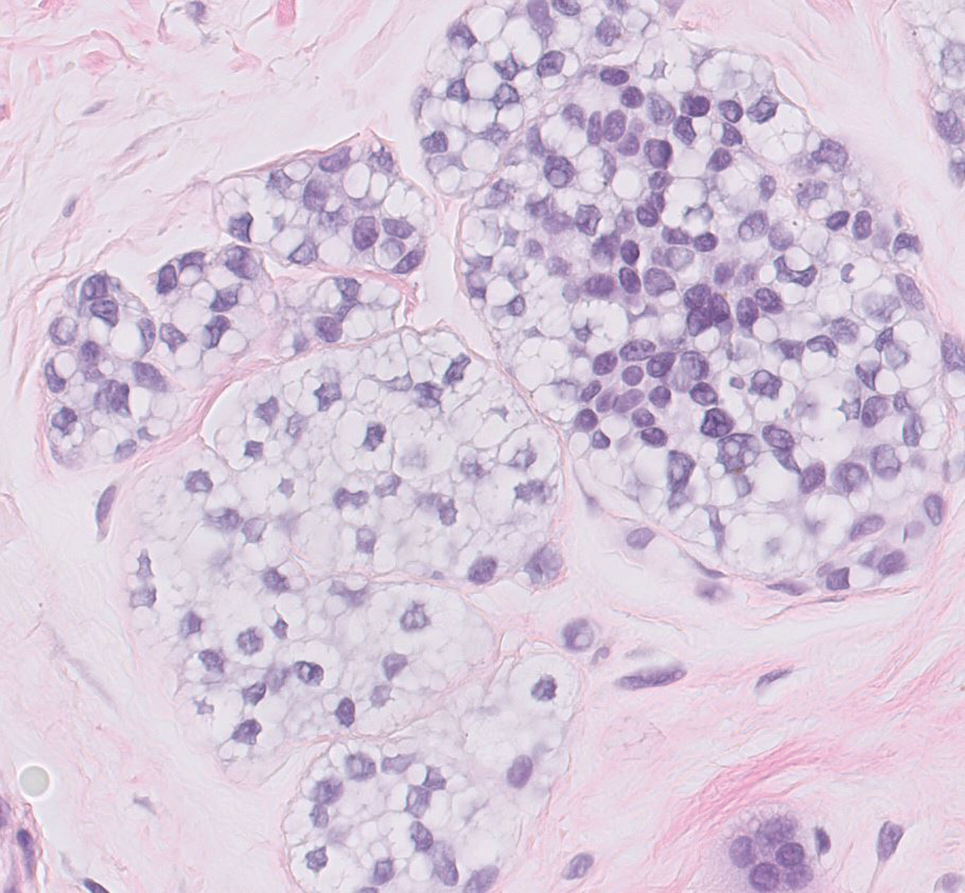
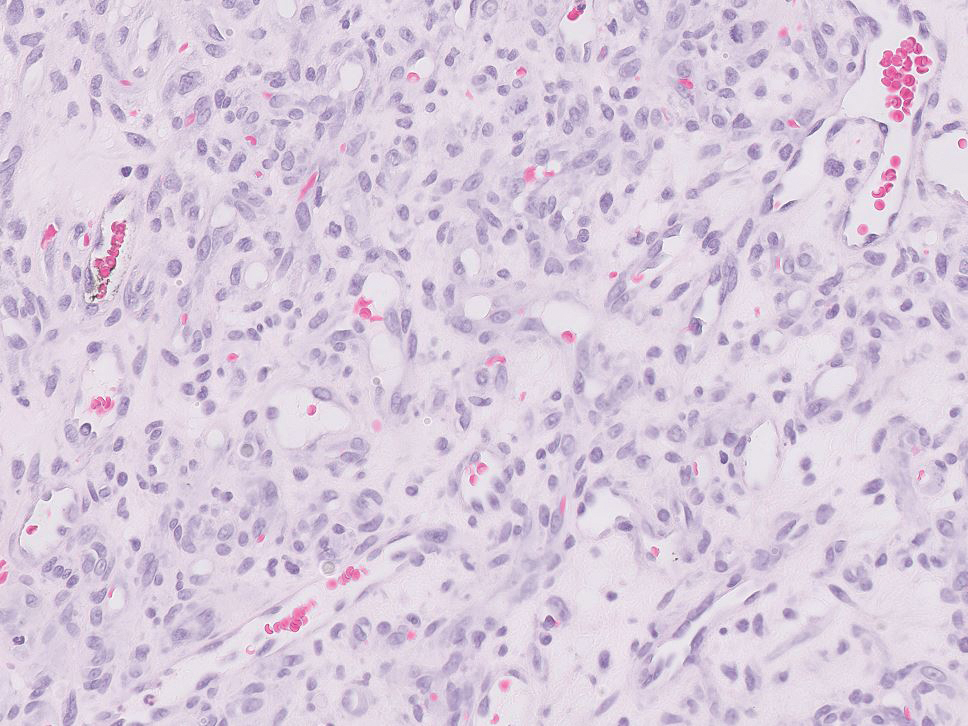
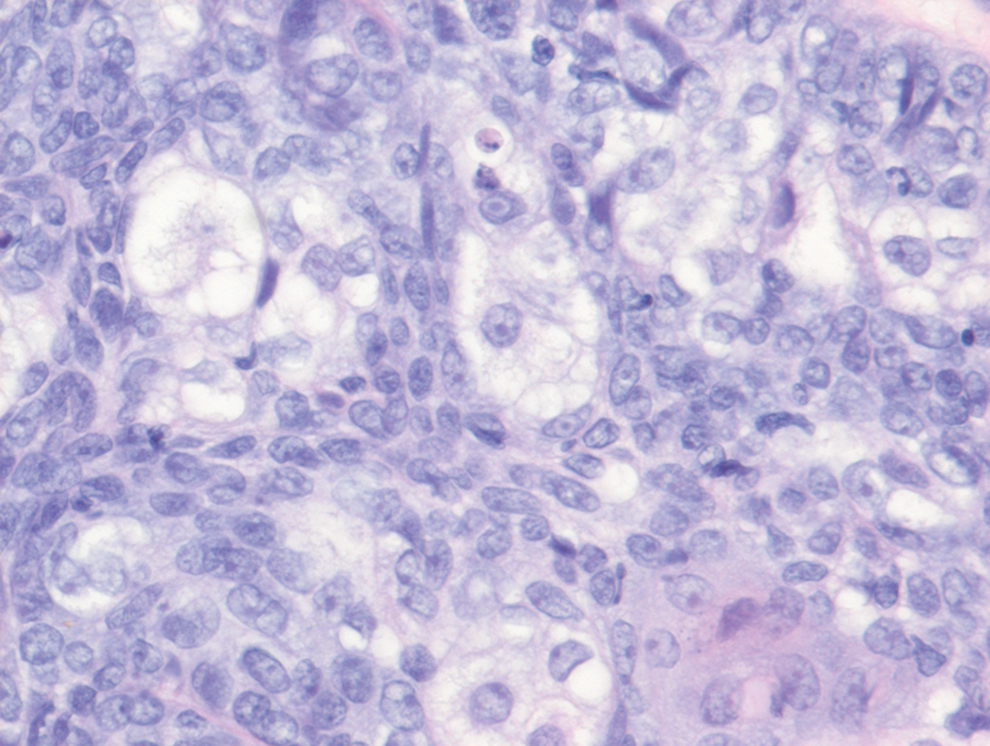
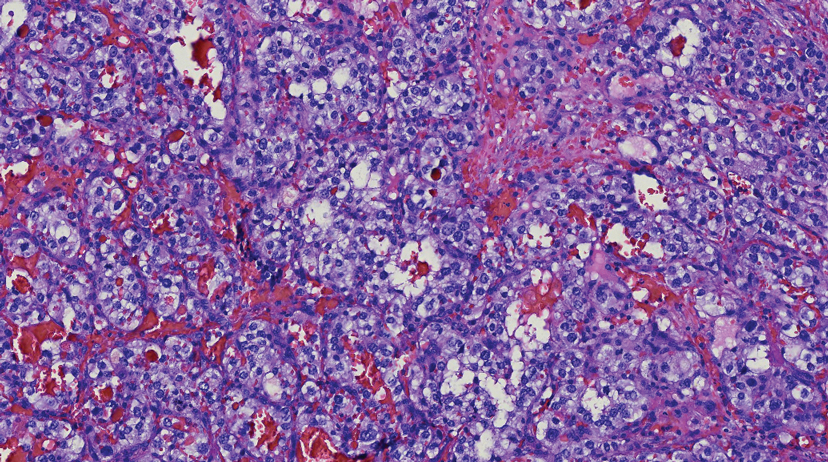
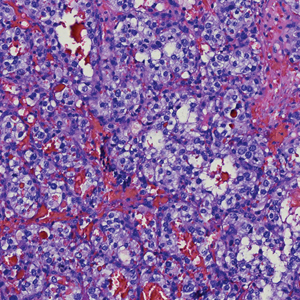
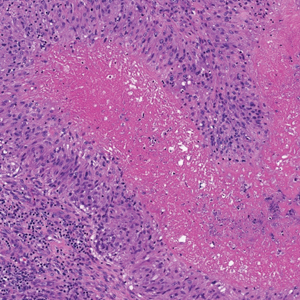
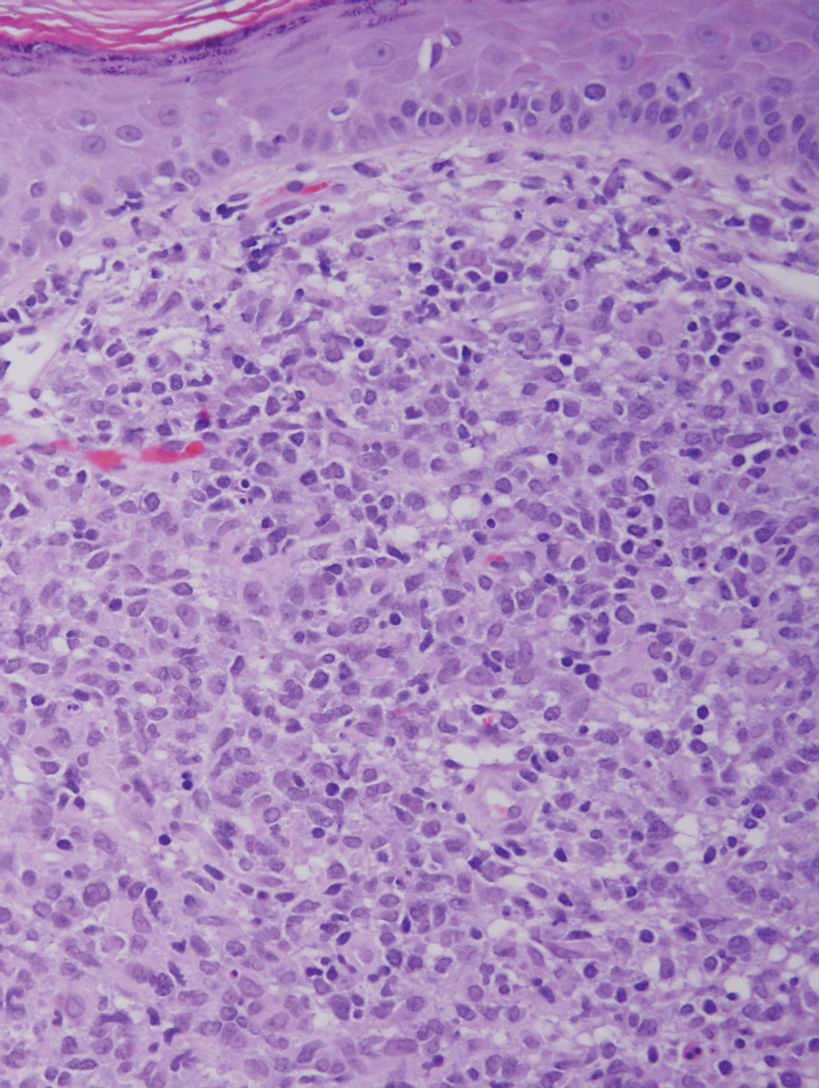
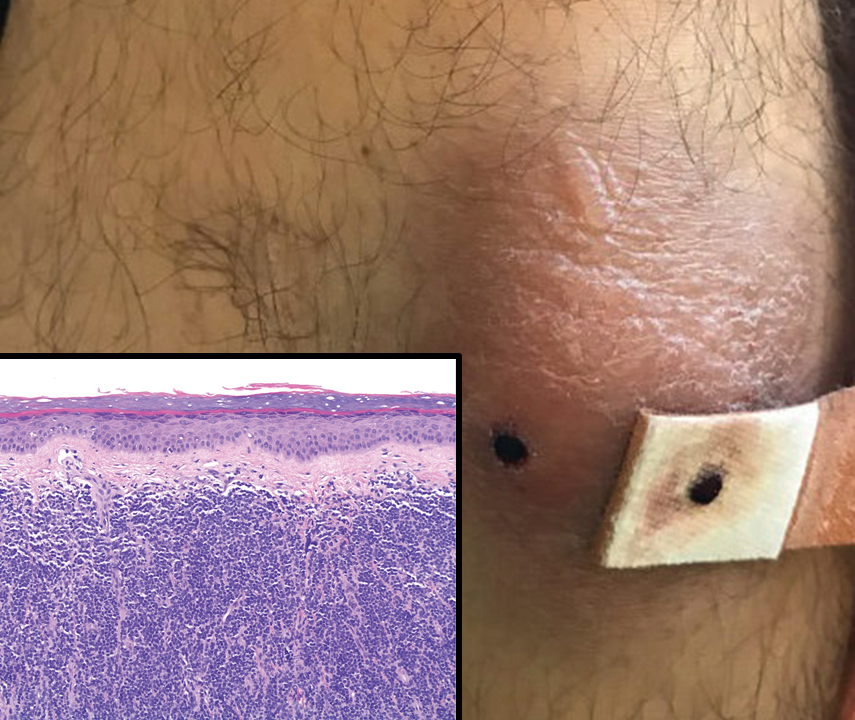
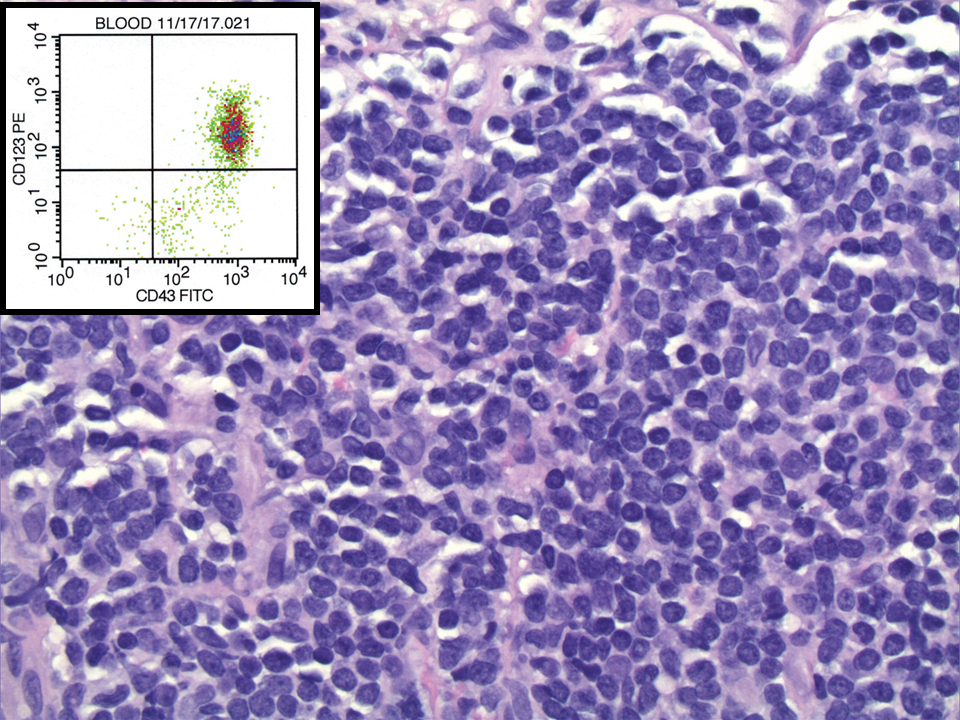
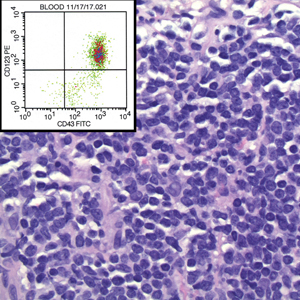
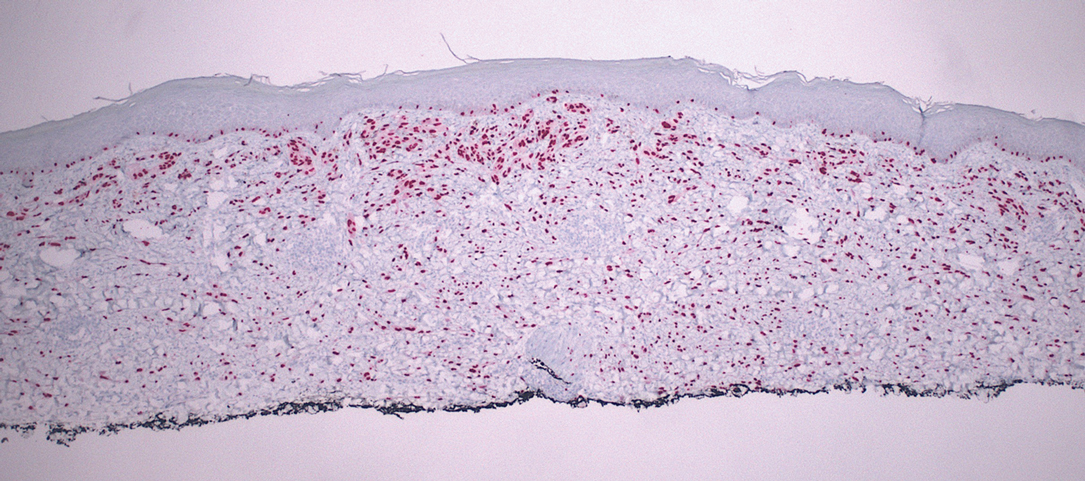
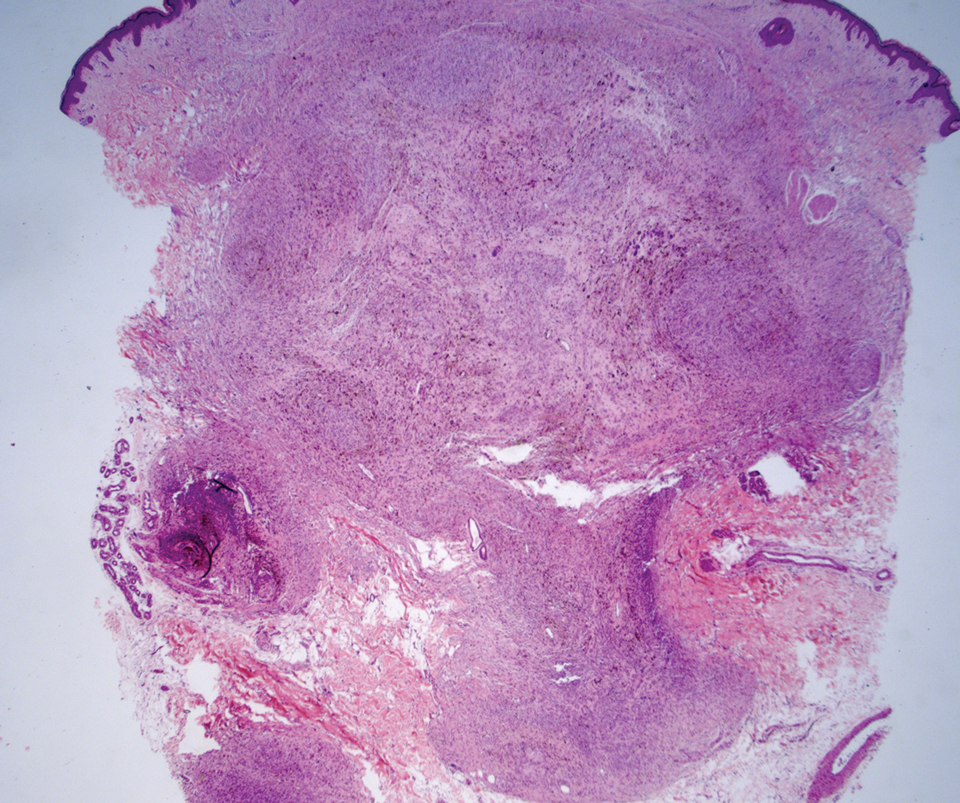
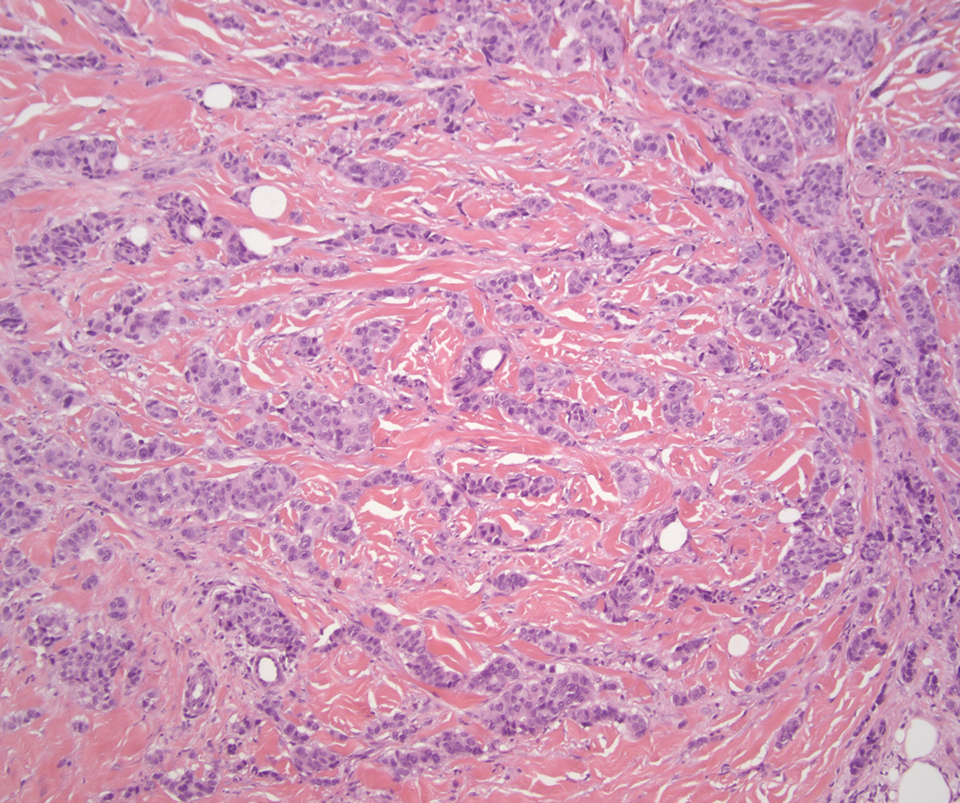
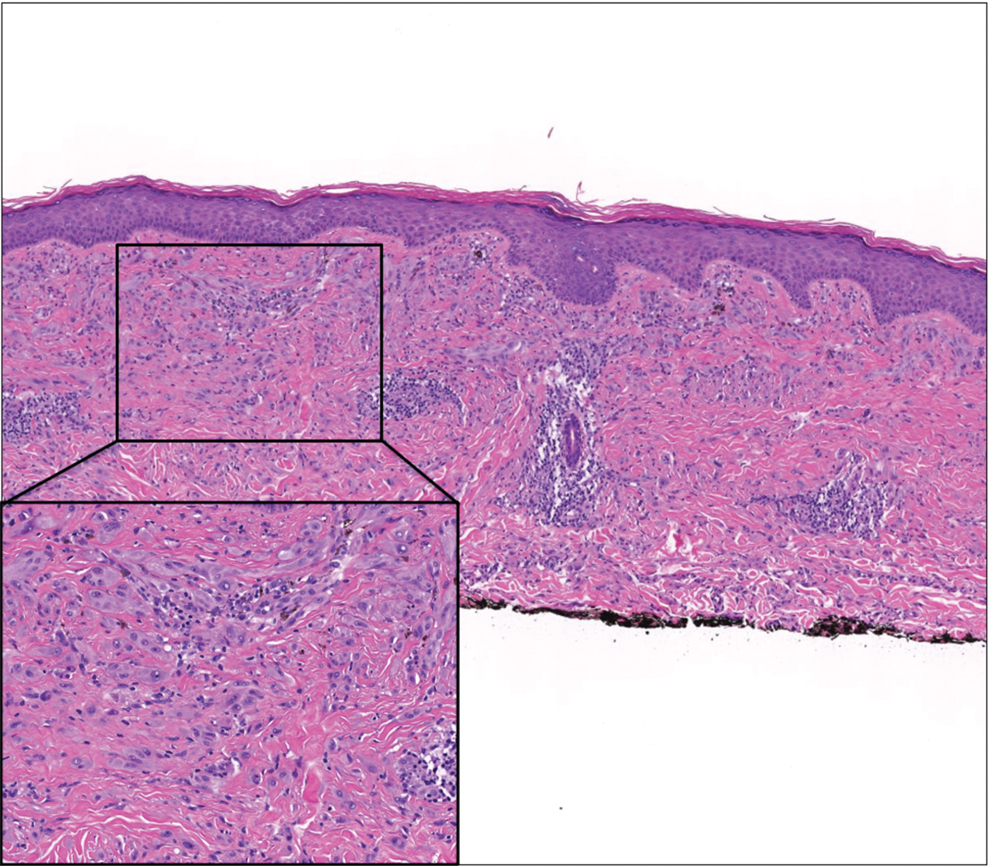
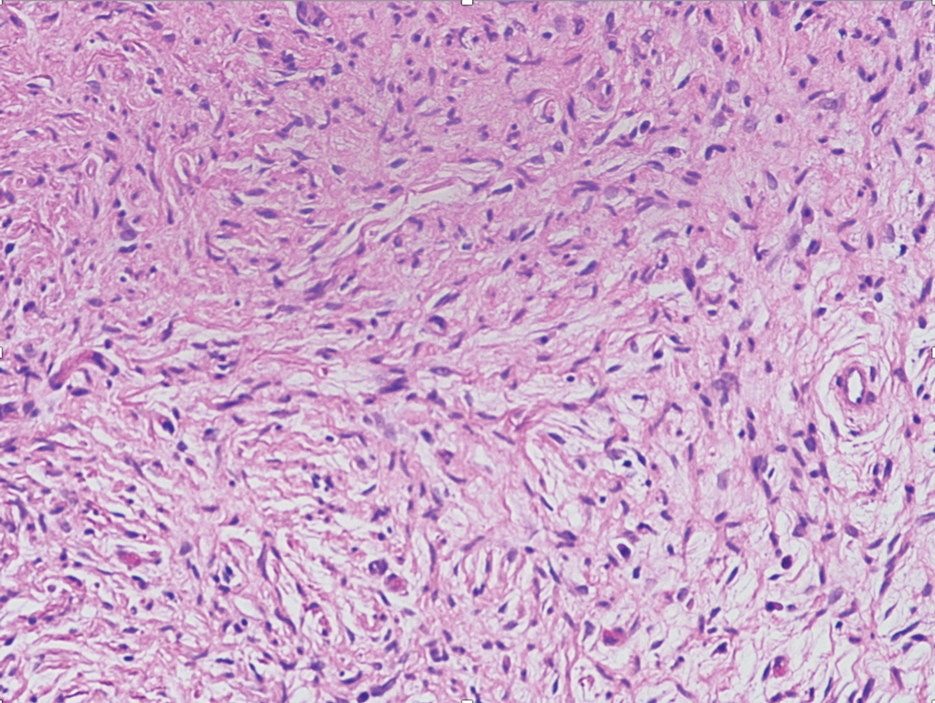
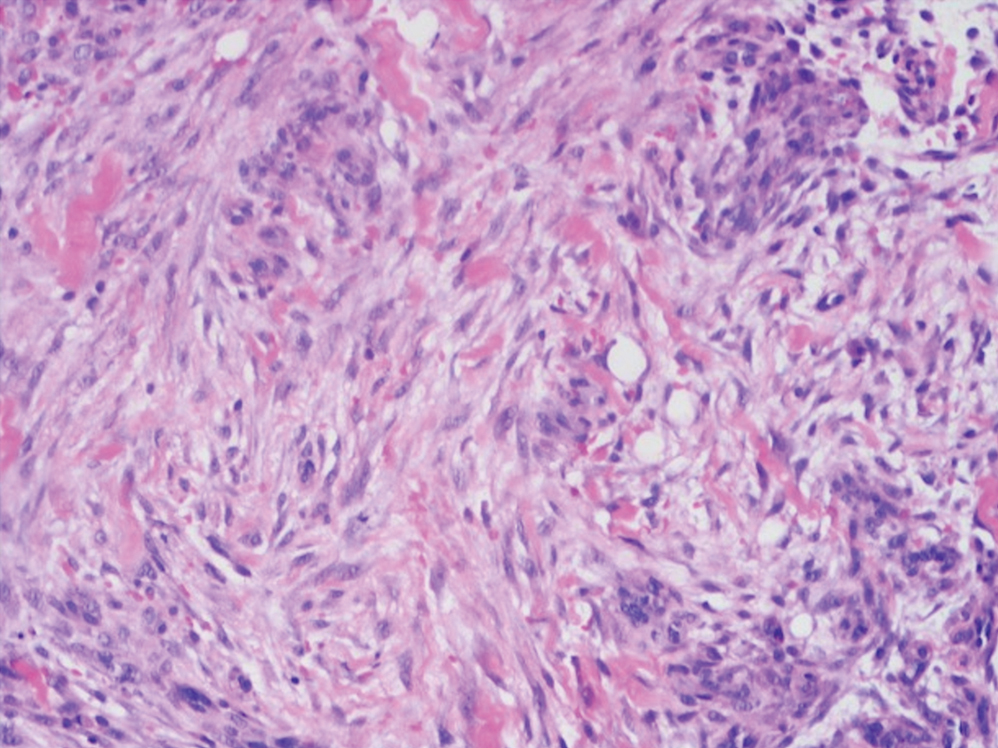
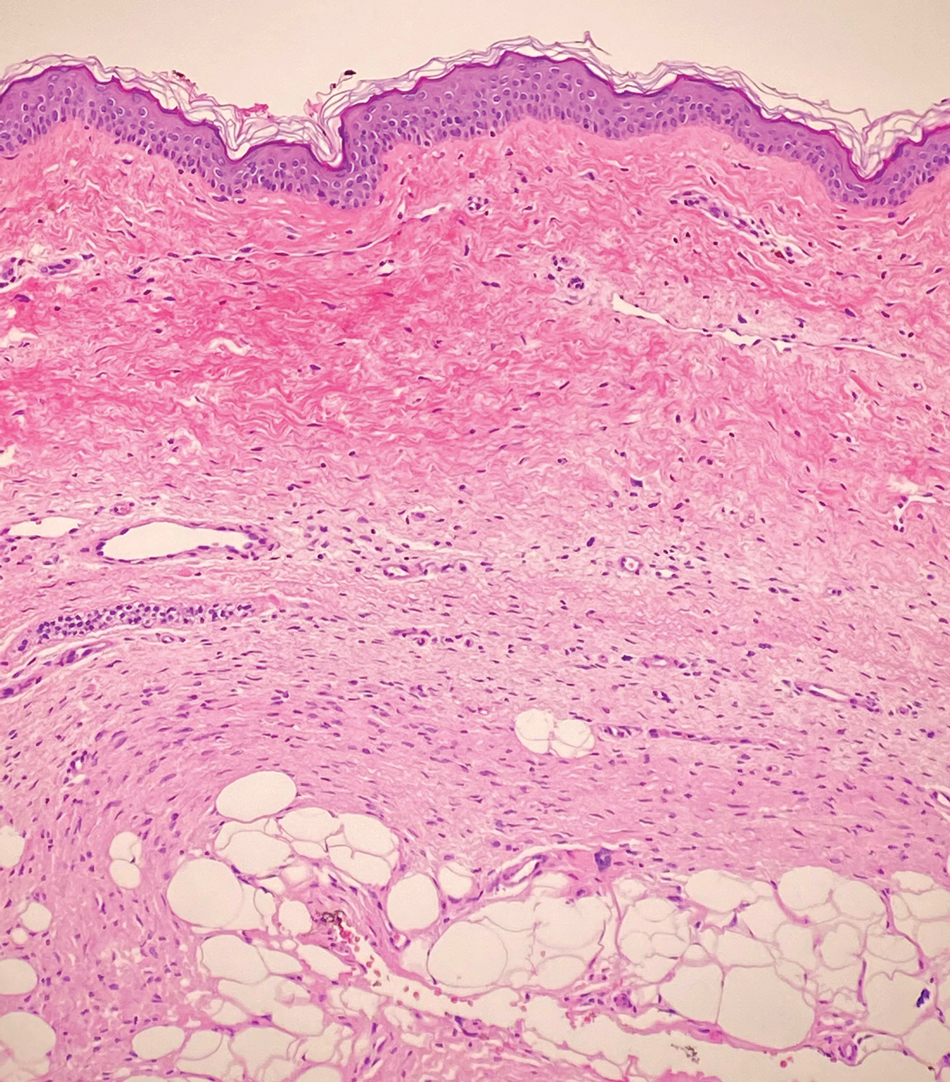
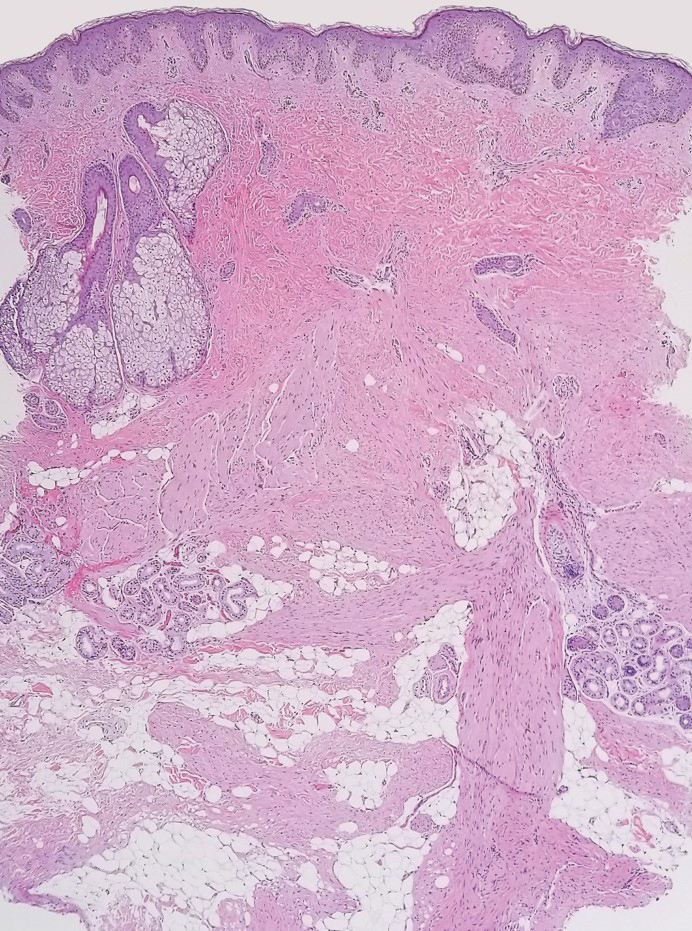
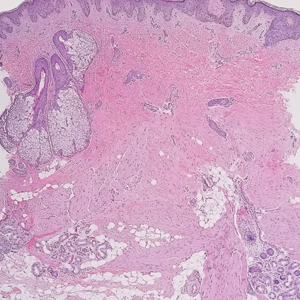
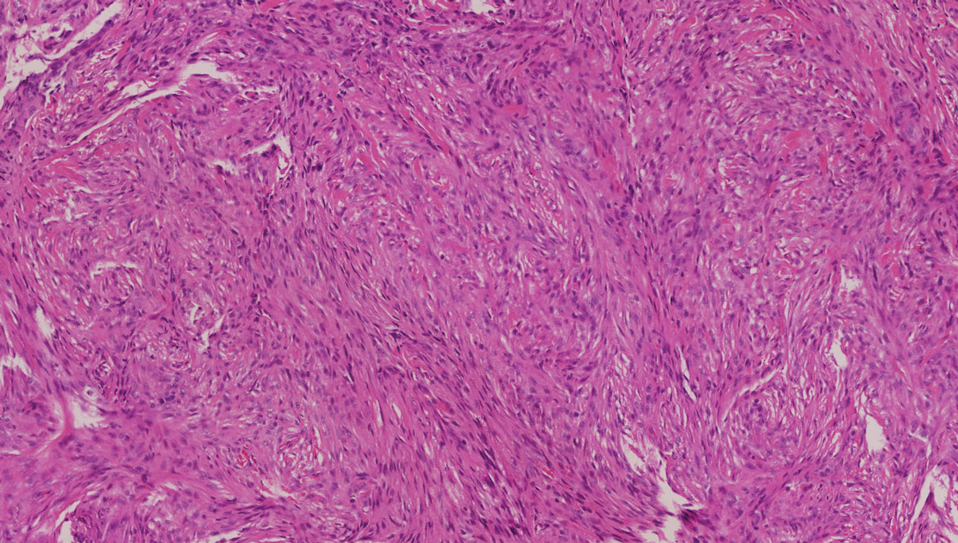
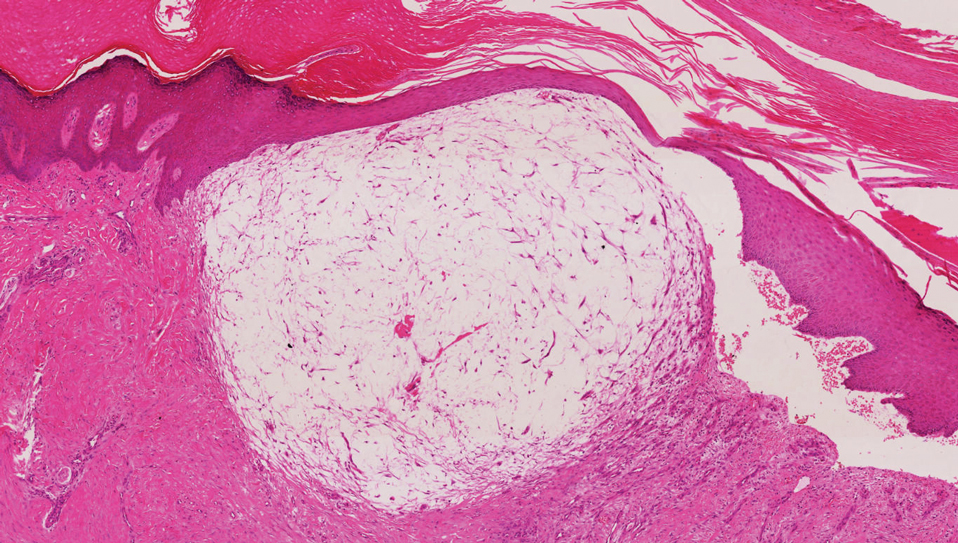
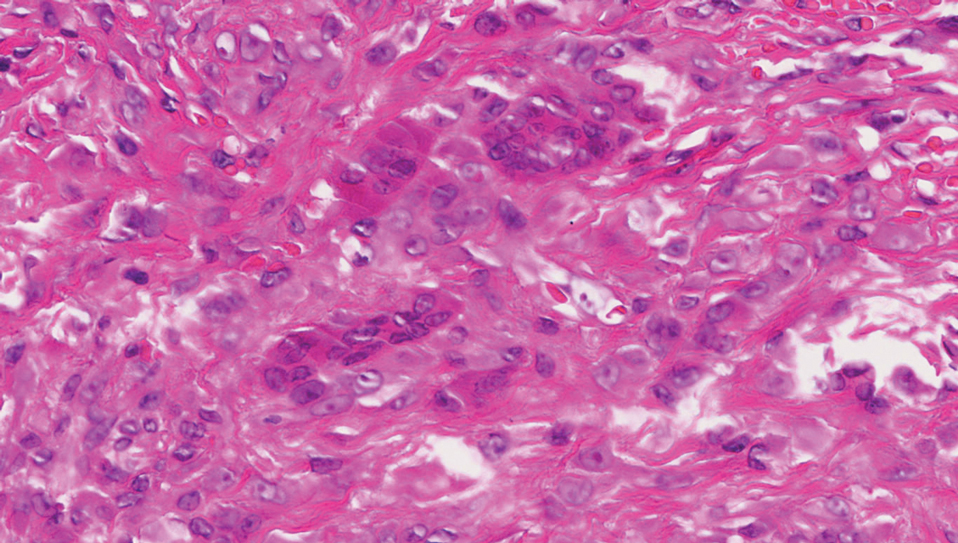
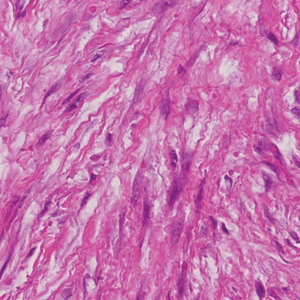
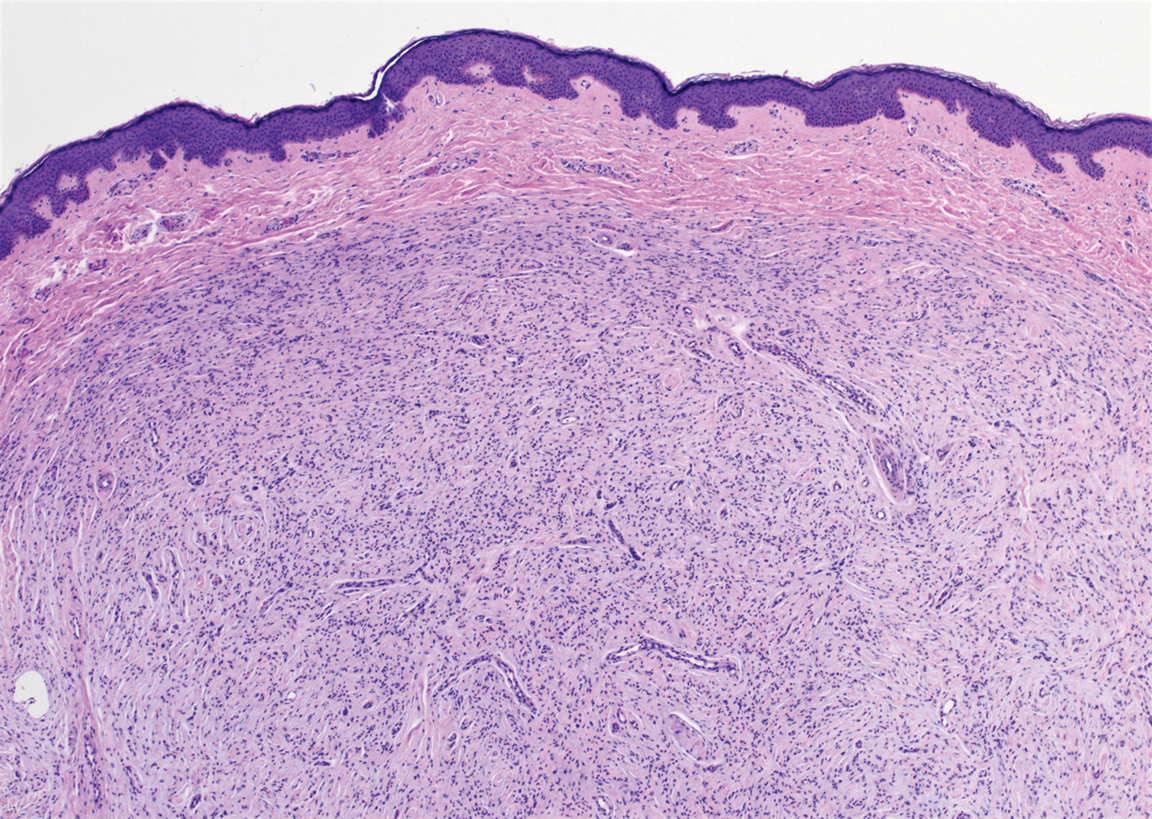
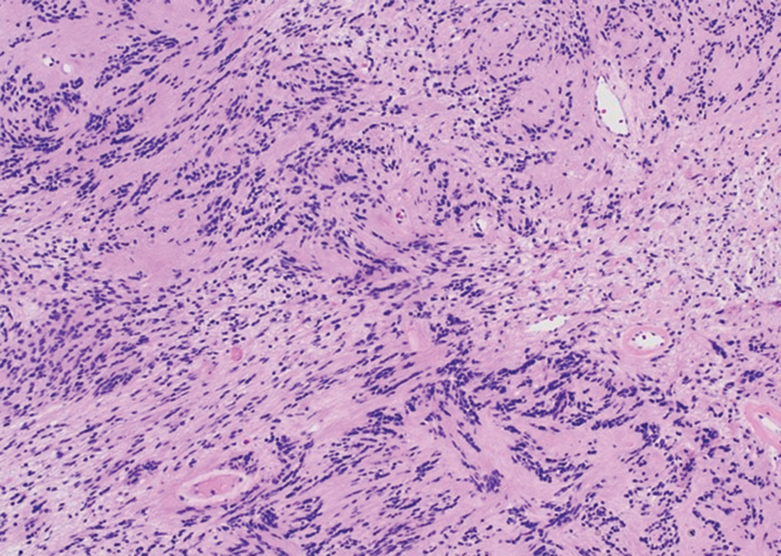
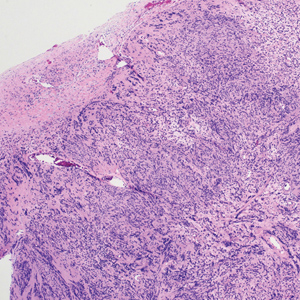
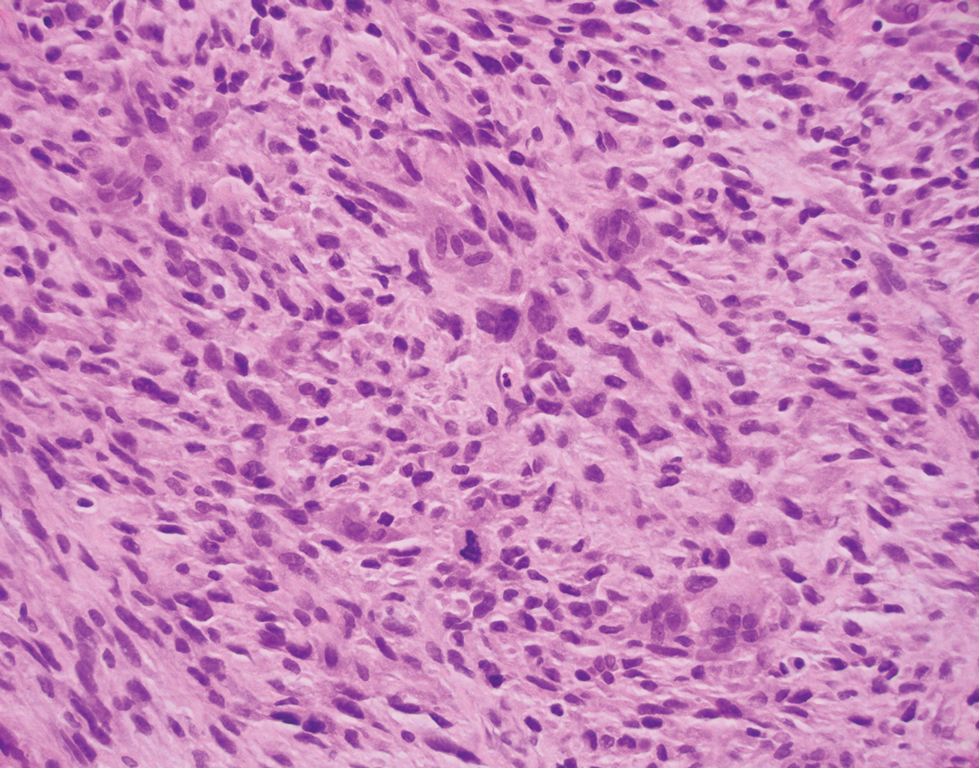
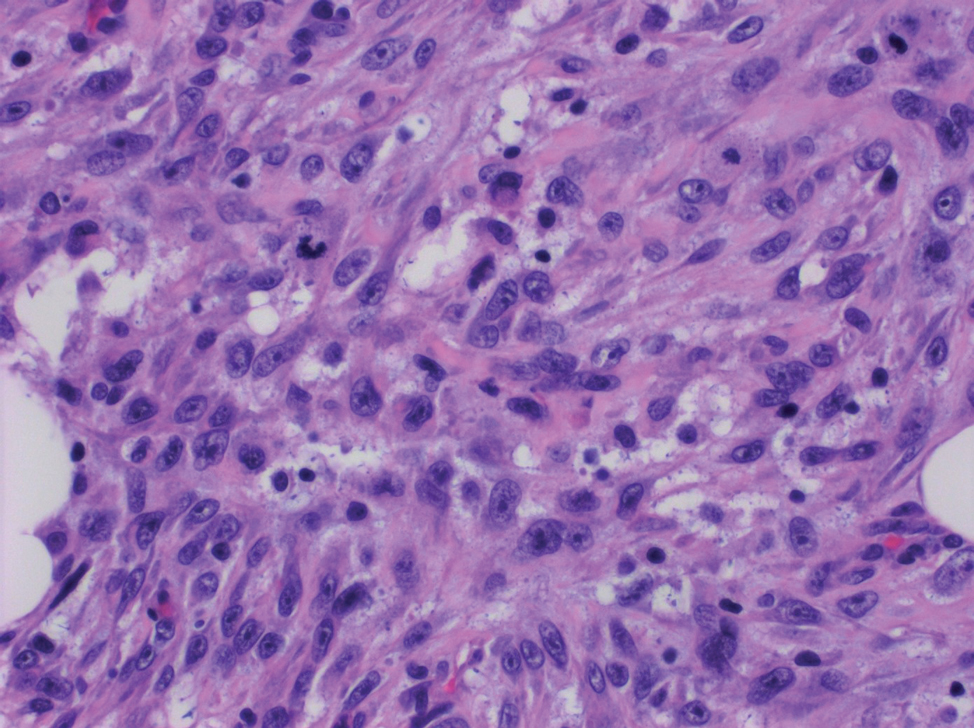
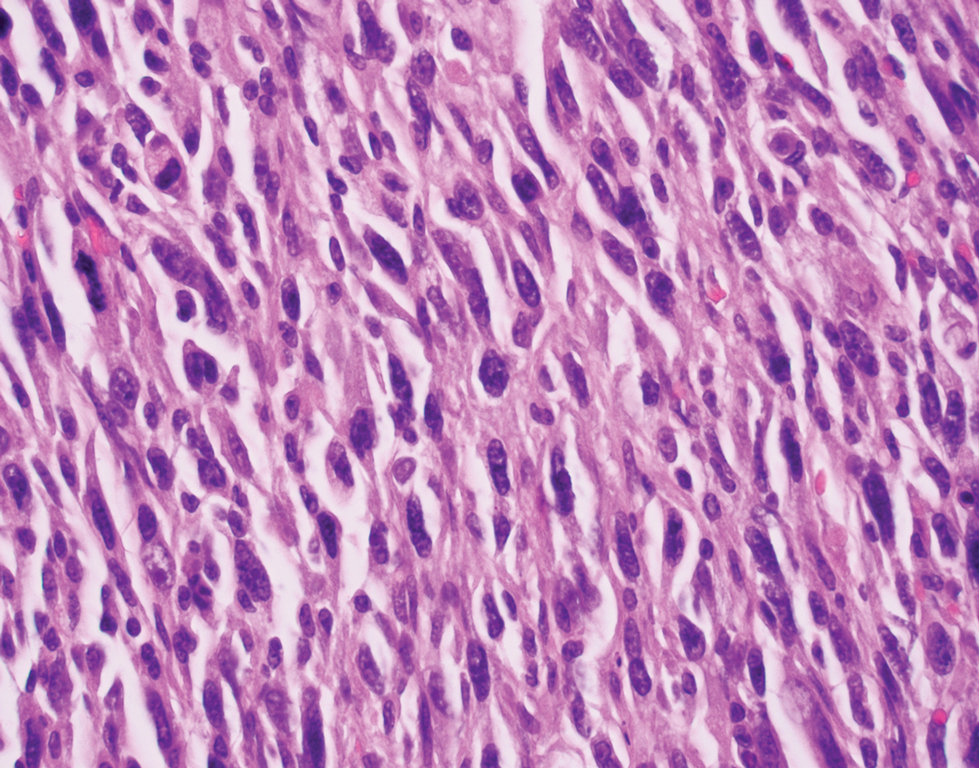
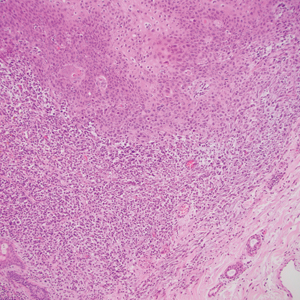
The deceptively banal clinical appearance of PES may delay appropriate diagnosis and treatment. Proximal-type epithelioid sarcoma often grows in sheets (quiz image [top] inset) and loose nests1 but also may take on a more corded appearance mimicking myoepithelial carcinoma. The cells themselves are plump dyscohesive epithelioid cells (quiz image [top]) with large nucleoli and eosinophilic cytoplasm or hyaline globules1 (quiz image [bottom]), but cells also may be focally spindled. Myxoid stroma, hemorrhage, and necrosis often are prominent features. Epithelioid sarcomas characteristically demonstrate positive immunostaining for both epithelial and mesenchymal markers (pan-cytokeratin and vimentin),1 with the majority having loss of expression of integrase interactor 1 (INI-1).2 Histology in this case was positive for cytokeratin monoclonal antibodies CAM5.2 and OSCAR, epithelial membrane antigen, and vimentin; it showed loss of INI-1 staining (quiz image [bottom]). Negative stains included S-100, p63, cytokeratins 7 and 20, CD34, CD31, ERG, glial fibrillary acidic protein, transducin-like enhancer of split 1, CD117, myogenin, synaptophysin, chromogranin, CD10, inhibin, CD99, and estrogen receptor.
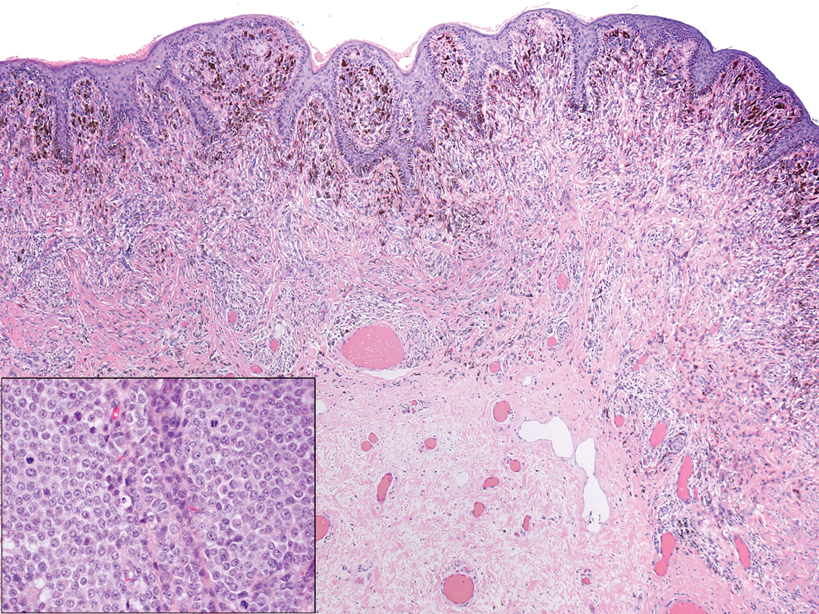
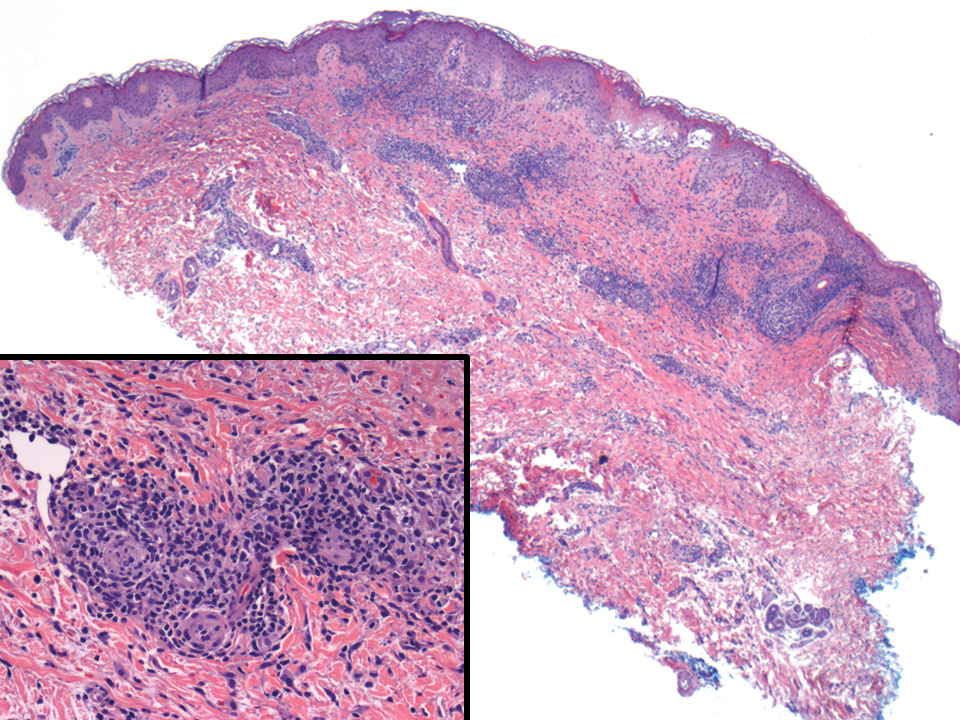
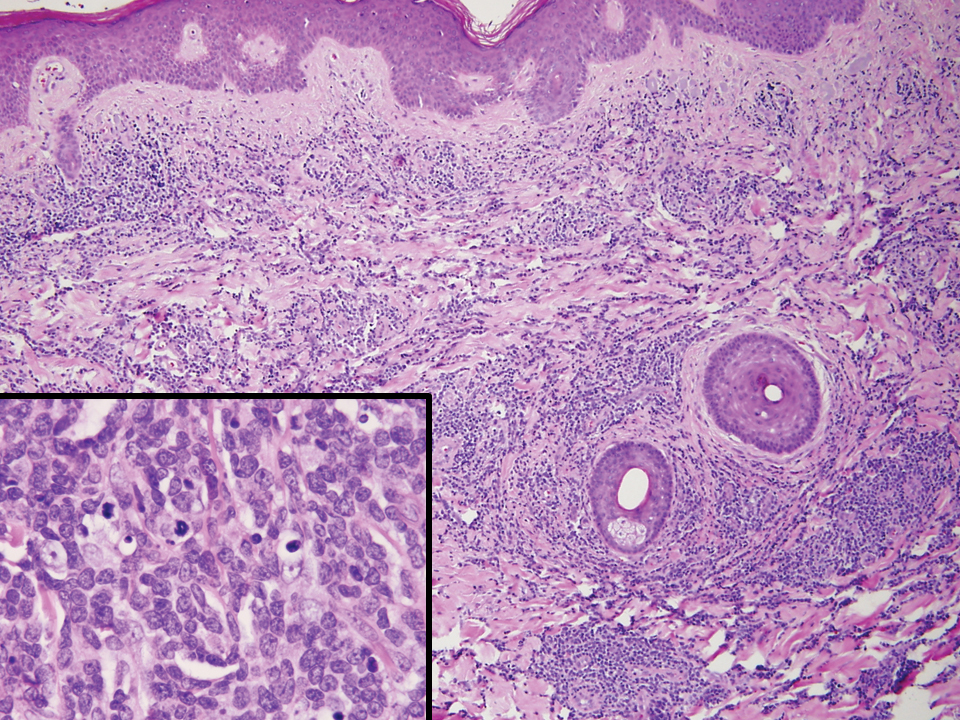
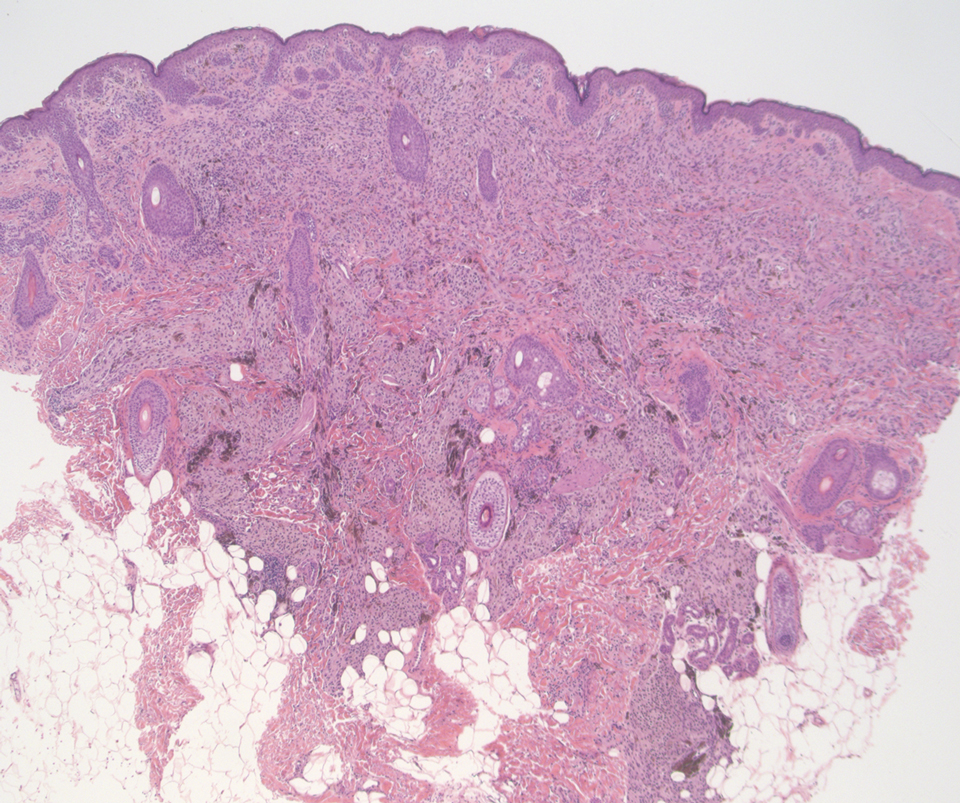
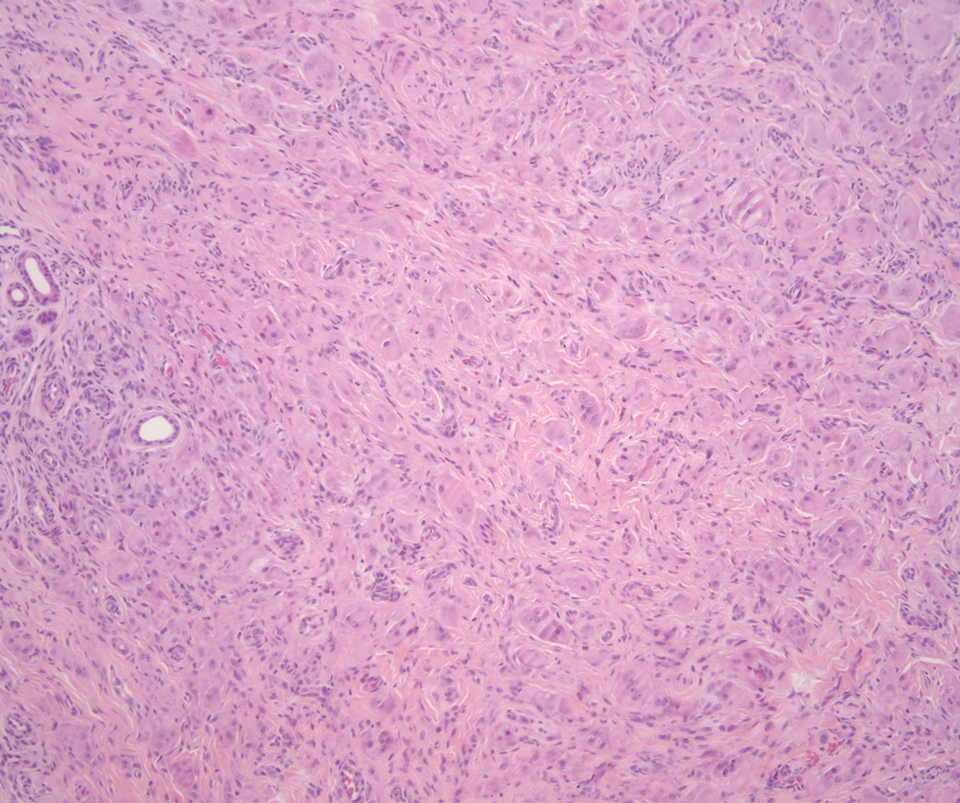
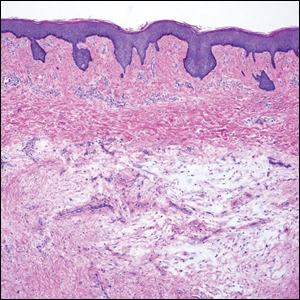
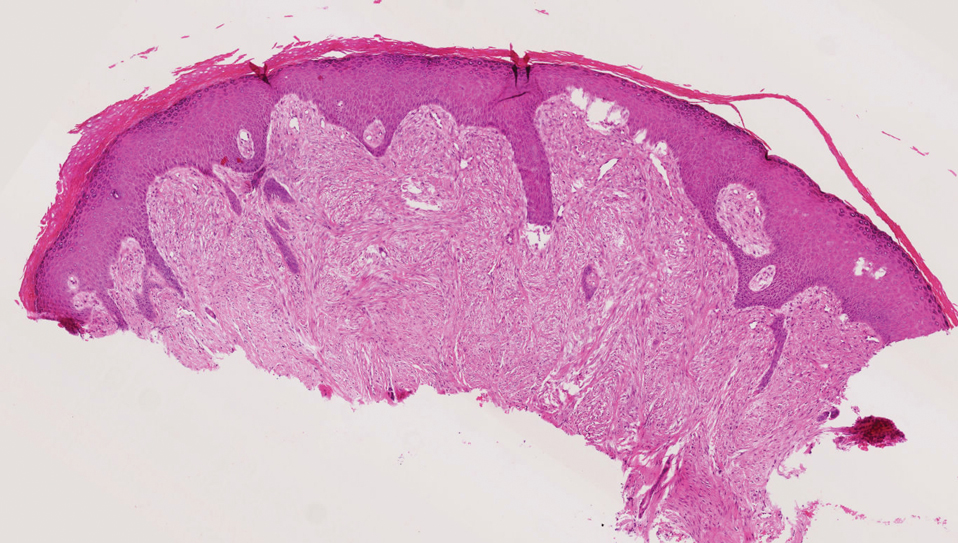
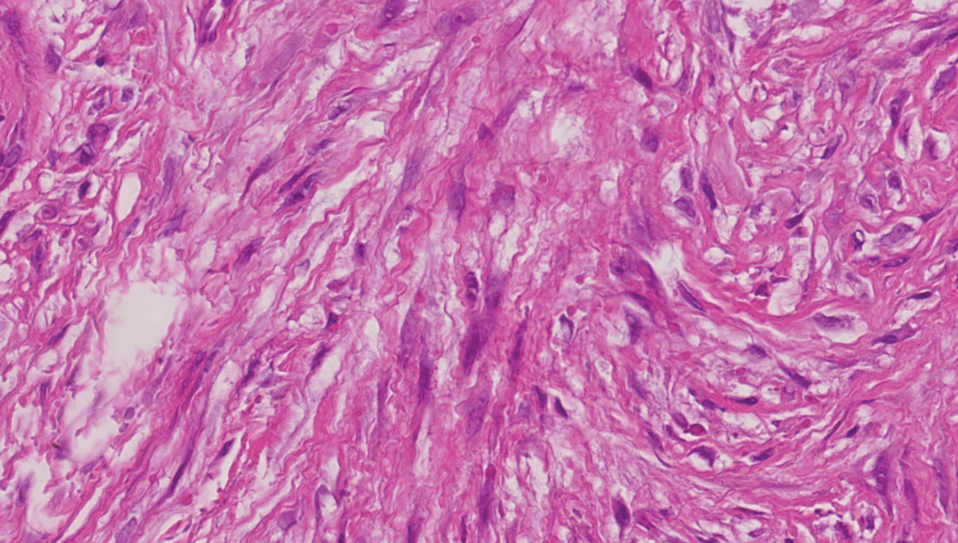
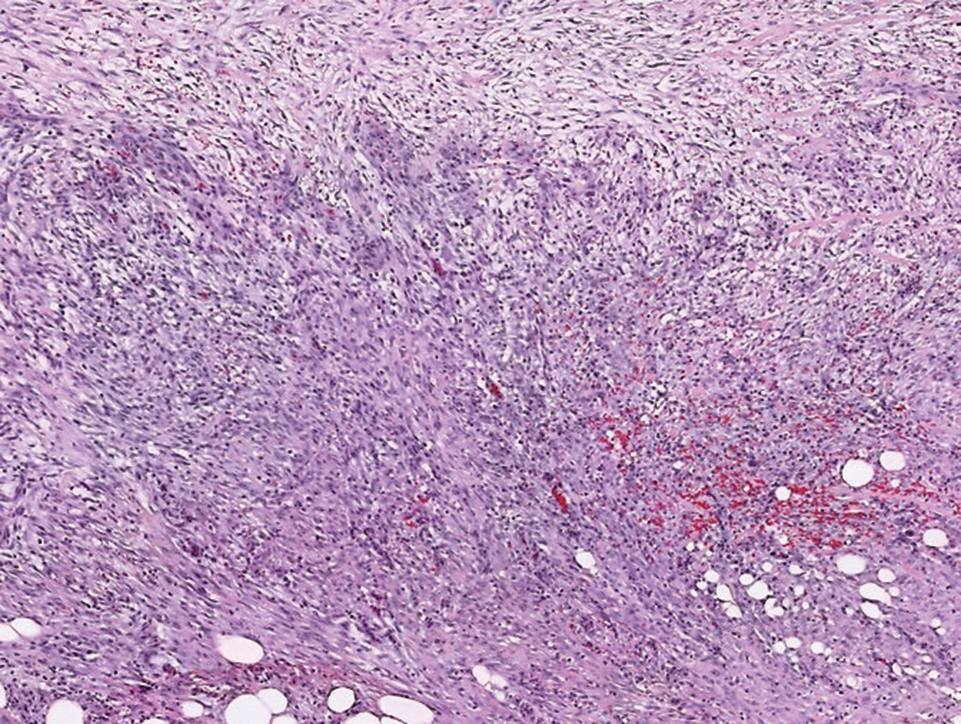
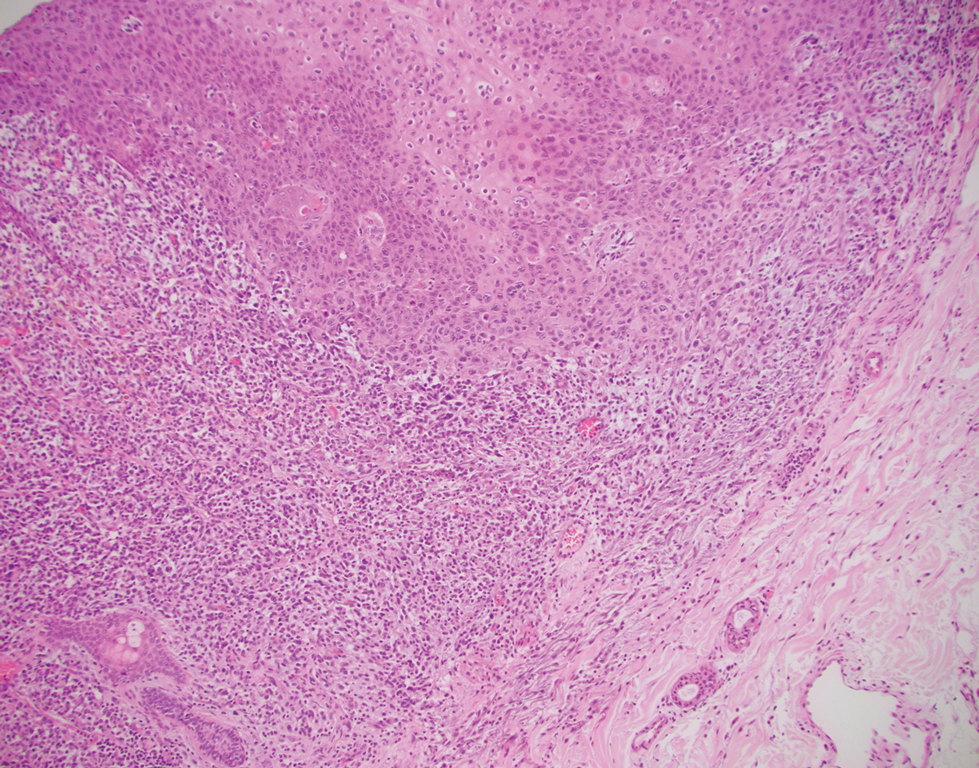
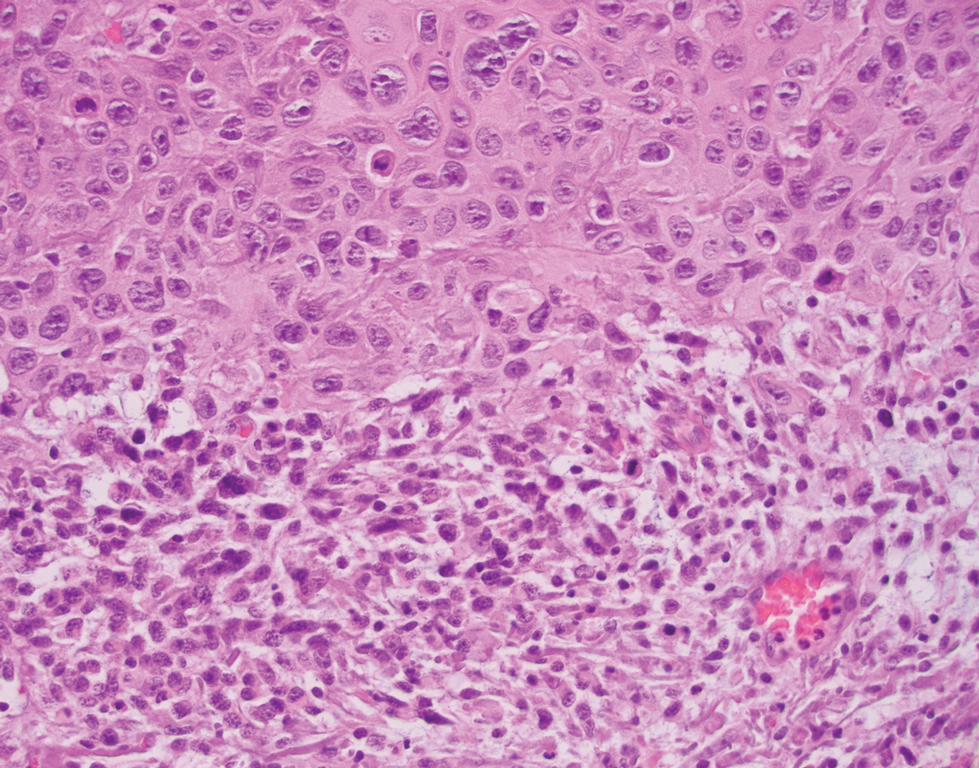
The differential diagnosis of PES includes poorly differentiated squamous cell carcinoma (Figure 1 [inset]), melanoma, myoepithelial carcinoma, and epithelioid angiosarcoma. Primary squamous cell carcinoma of the vulva presents as an endophytic or exophytic mass with raised borders. Vulvar cancer is uncommon among gynecologic malignancies, with squamous cell carcinoma being the most commonly encountered.4 Vulvar intraepithelial neoplasia (VIN) is increasing in incidence, while the occurrence of invasive squamous cell carcinoma remains stable.5 Human papillomavirus–related VIN (usual-type VIN) is less likely to progress to squamous cell carcinoma than differentiated VIN (d-VIN), a dysplasia that is unrelated to human papillomavirus that frequently harbors p53 mutations.4 The presence of histologic epidermal involvement can help distinguish squamous cell carcinoma from PES (Figure 1). As opposed to PES, metastatic squamous cell carcinoma is characterized by intercellular bridges and often at least focal keratinization (Figure 1). Squamous cell carcinoma demonstrates positivity with p63 and p40 immunohistochemical stains, while PES rarely stains for either.6
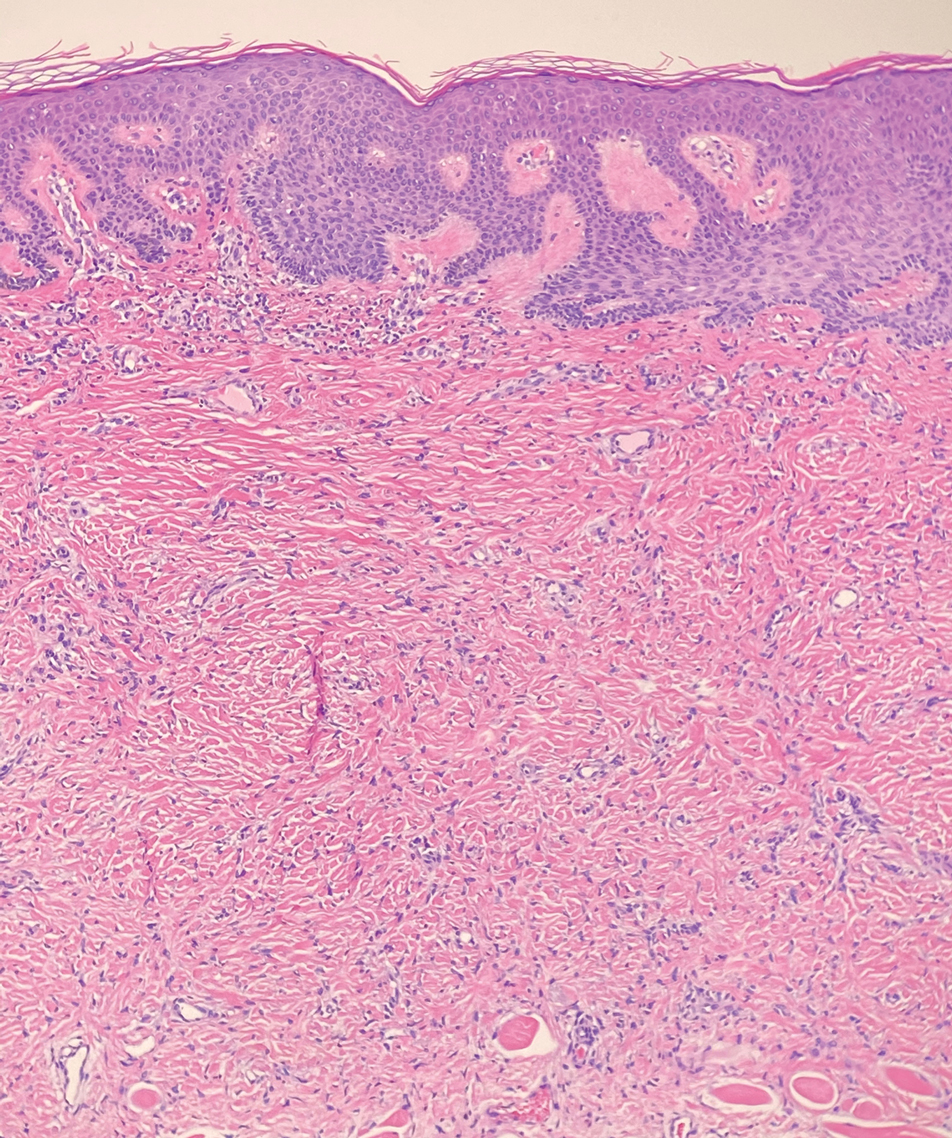
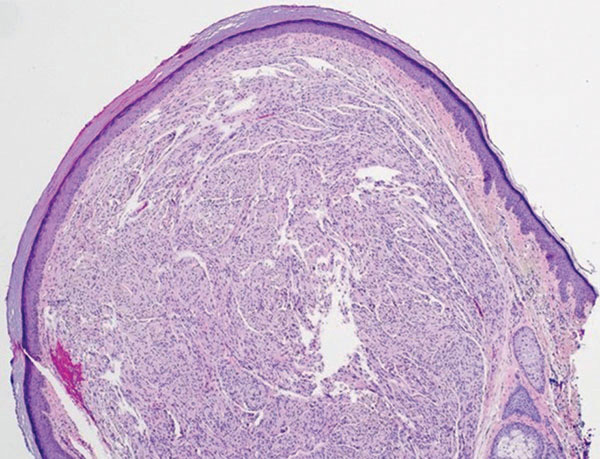
Melanoma is the second most common vulvar malignancy. Vulvar melanoma tends to occur in women of advanced age but has been reported in girls as young as 10 years old.7 There is some evidence that patients with lichen sclerosus may be at an increased risk for the development of vulvar melanoma.8 Compared to PES, primary vulvar melanoma usually demonstrates epidermal involvement as well as clinical findings of a pigmented lesion (Figure 2). A notable minority of vulvar melanomas are amelanotic.9 Melanoma may be distinguished from PES with a panel of melanocytic markers—human melanoma black 45, Melan-A, SRY-box transcription factor 10, S-100, and microphthalmia transcription factor—that rarely are expressed in the latter. Both PES and rhabdoid melanoma have eosinophilic and tinctorial cytoplasmic inclusions.10 Melanin pigment and more cohesive nests are helpful clues that may point to melanoma when present.
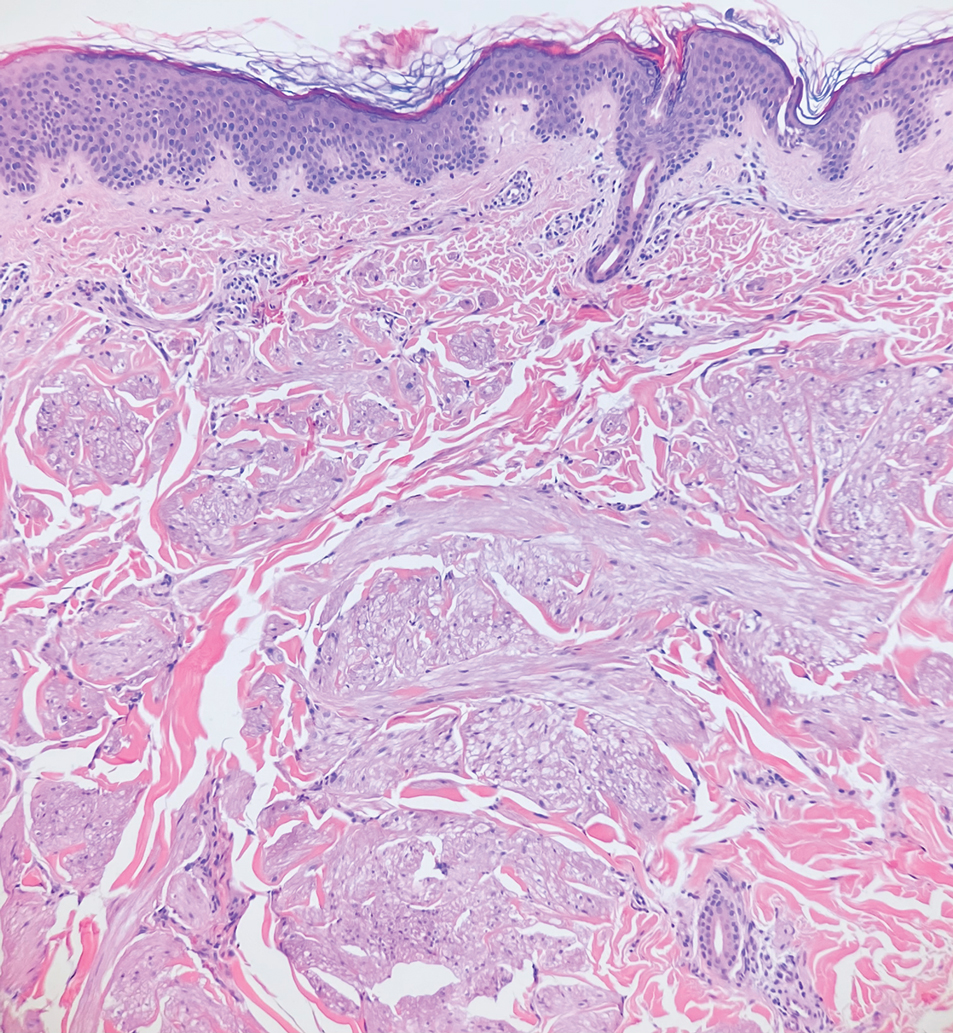
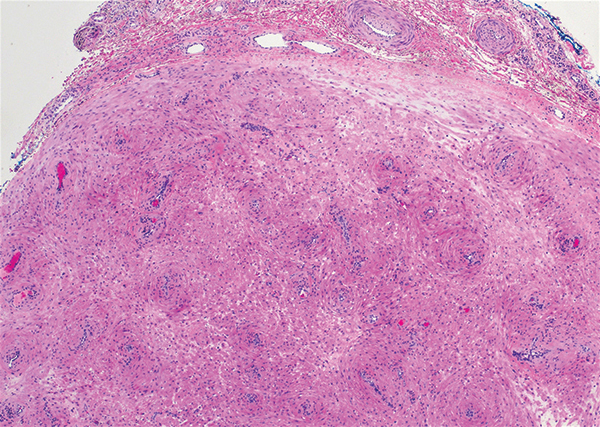
Myoepithelial carcinoma of the vulva is rare.11 Myoepithelial carcinoma of soft tissue is more aggressive than its benign counterpart, with up to a 50% metastasis rate.12 The presence of prominent corded or trabecular growth in a myxoid or hyaline background may point to the diagnosis (Figure 3). Similar to PES, myoepithelial carcinoma may lose expression of nuclear INI-1, while myoepithelial carcinoma is more likely to express S-100 and glial fibrillary acidic protein.13 Rearrangements of EWS RNA binding protein 1, EWSR1, have been found in half of myoepithelial neoplasms.12
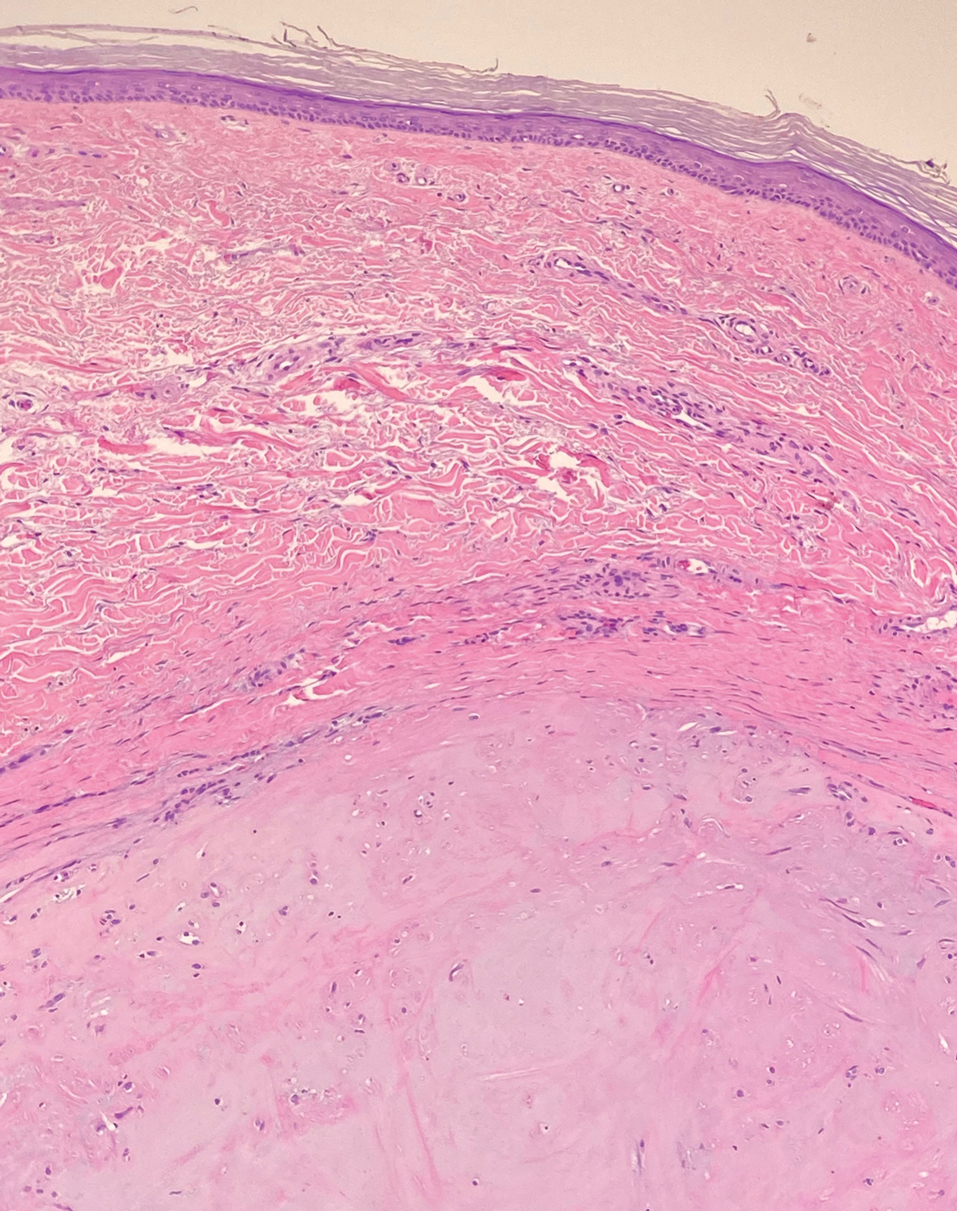
Angiosarcomas represent 5% of cutaneous sarcomas and rarely have been reported in the vulva, primarily occurring in the setting of long-standing lymphedema and radiation.14 Angiosarcoma more often occurs on the head and neck, breasts, or extremities. Additional risk factors for the development of angiosarcoma include toxin exposure (eg, polyvinyl chloride, thorium dioxide, arsenic), anabolic steroids, and filariasis, as well as genetic disorders (eg, neurofibromatosis type 1, BRCA gene mutations, Maffucci syndrome).15 Epithelioid angiosarcoma is an infiltrative tumor composed of irregular anastomosing vascular channels with extravasated erythrocytes (Figure 4). Solid growth and necrosis may be present in more aggressive tumors. The cells themselves are pleomorphic endothelial cells with vesicular chromatin and prominent nucleoli. Epithelioid angiosarcoma may resemble carcinoma and have focal keratin expression. However, the characteristic eosinophilic cytoplasm seen in PES should not be identified in epithelioid angiosarcoma. Unlike PES, epithelioid angiosarcoma is positive for CD31 and has retained expression for INI-1. Both angiosarcoma and proximal-type epithelioid sarcoma may express vascular markers CD34 and FLI-116; thus an expanded panel of immunohistochemical studies may be of utility.
- Guillou L, Wadden C, Coindre JM, et al. “Proximal-type” epithelioid sarcoma, a distinctive aggressive neoplasm showing rhabdoid features. clinicopathologic, immunohistochemical, and ultrastructural study of a series. Am J Surg Pathol. 1997;21:130-146.
- Hasegawa T, Matsuno Y, Shimoda T, et al. Proximal-type epithelioid sarcoma: a clinicopathological study of 20 cases. Mod Pathol. 2001;14:655-663.
- Czarnecka AM, Sobczuk P, Kostrzanowski M, et al. Epithelioid sarcoma—from genetics to clinical practice. Cancers. 2020:12:2112.
- Hoang LH, Park KJ, Soslow RA, et al. Squamous precursor lesions of the vulva: current classification and diagnostic challenges. Pathology. 2016;48:291-302.
- Allbritton J. Vulvar neoplasms, benign and malignant. Obstet Gynecol Clin North Am. 2017;44:339-352.
- Laskin WB, Miettinen M. Epithelioid sarcoma: new insights based on an extended immunohistochemical analysis. Arch Pathol Lab Med. 2003;127:1161-1168.
- Boer FL, Eikelder MLGT, Kapitejn EH, et al. Vulvar malignant melanoma: pathogenesis, clinical behavior and management: review of the literature. Cancer Treat Rev. 2019;73:91-103.
- Hieta N, Rintala SKM, Soderlund J, et al. Association of vulvar melanoma with lichen sclerosus. Acta Derm Venereol. 2019;99:339-340.
- Edwards L. Pigmented vulvar lesions. Dermatol Ther. 2010;23:449-457.
- Patterson JW, Hosler GA, Prenshaw KL, eds. Weedon's Skin Pathology. Elsevier Limited; 2021.
- Kyriazi MA, Carvounis EE, Kitsou M, et al. Myoepithelial carcinoma of the vulva mimicking Bartholin gland abscess in a pregnant woman: case report and review of literature. Int J Gynecol Pathol. 2010:29:501-504.
- Jo VY, Fletcher CD. Myoepithelial neoplasma of soft tissue: an updated review of the clinicopathological, immunophenotypic, and genetic features. Head Neck Pathol. 2015;9:32-38.
- Rekhi B, Sable M, Jambhekar NA. Histopathological, immunohistochemical and molecular spectrum of myoepithelial tumours of soft tissues. Virchows Arch. 2012;461:687-697.
- Yost S, Bradish J, Grossheim L, et al. Epithelioid angiosarcoma of the vulva: a case report. Gynecol Oncol Rep. 2017;21:91-93.
- Young RJ, Brown NJ, Reed MW, et al. Angiosarcoma. Lancet Oncol. 2010;11:983-991.
- Stockman DL, Hornick JL, Deavers MT, et al. ERG and FLI1 protein expression in epithelioid sarcoma. Mod Pathol. 2014;27:496-501.
The Diagnosis: Proximal-Type Epithelioid Sarcoma
Proximal-type epithelioid sarcoma (PES) is a rare high-grade sarcoma of uncertain histogenesis that may present with a benign clinical appearance. Proximal-type epithelioid sarcoma peaks at 20 to 40 years of age and has a slight male predominance. This tumor exhibits aggressive behavior with both local recurrence and metastasis.1 The average overall survival is poor; however, tumor size less than 5 cm and complete excision with tumor-free margin status improves the prognosis.2 Proximal-type epithelioid sarcoma should not be confused with distal-type epithelioid sarcoma, which has a better prognosis and occurs in younger patients.1 Treatment of PES is wide excision, and chemotherapy trials with tazemetostat are ongoing.3
The deceptively banal clinical appearance of PES may delay appropriate diagnosis and treatment. Proximal-type epithelioid sarcoma often grows in sheets (quiz image [top] inset) and loose nests1 but also may take on a more corded appearance mimicking myoepithelial carcinoma. The cells themselves are plump dyscohesive epithelioid cells (quiz image [top]) with large nucleoli and eosinophilic cytoplasm or hyaline globules1 (quiz image [bottom]), but cells also may be focally spindled. Myxoid stroma, hemorrhage, and necrosis often are prominent features. Epithelioid sarcomas characteristically demonstrate positive immunostaining for both epithelial and mesenchymal markers (pan-cytokeratin and vimentin),1 with the majority having loss of expression of integrase interactor 1 (INI-1).2 Histology in this case was positive for cytokeratin monoclonal antibodies CAM5.2 and OSCAR, epithelial membrane antigen, and vimentin; it showed loss of INI-1 staining (quiz image [bottom]). Negative stains included S-100, p63, cytokeratins 7 and 20, CD34, CD31, ERG, glial fibrillary acidic protein, transducin-like enhancer of split 1, CD117, myogenin, synaptophysin, chromogranin, CD10, inhibin, CD99, and estrogen receptor.
The differential diagnosis of PES includes poorly differentiated squamous cell carcinoma (Figure 1 [inset]), melanoma, myoepithelial carcinoma, and epithelioid angiosarcoma. Primary squamous cell carcinoma of the vulva presents as an endophytic or exophytic mass with raised borders. Vulvar cancer is uncommon among gynecologic malignancies, with squamous cell carcinoma being the most commonly encountered.4 Vulvar intraepithelial neoplasia (VIN) is increasing in incidence, while the occurrence of invasive squamous cell carcinoma remains stable.5 Human papillomavirus–related VIN (usual-type VIN) is less likely to progress to squamous cell carcinoma than differentiated VIN (d-VIN), a dysplasia that is unrelated to human papillomavirus that frequently harbors p53 mutations.4 The presence of histologic epidermal involvement can help distinguish squamous cell carcinoma from PES (Figure 1). As opposed to PES, metastatic squamous cell carcinoma is characterized by intercellular bridges and often at least focal keratinization (Figure 1). Squamous cell carcinoma demonstrates positivity with p63 and p40 immunohistochemical stains, while PES rarely stains for either.6
Melanoma is the second most common vulvar malignancy. Vulvar melanoma tends to occur in women of advanced age but has been reported in girls as young as 10 years old.7 There is some evidence that patients with lichen sclerosus may be at an increased risk for the development of vulvar melanoma.8 Compared to PES, primary vulvar melanoma usually demonstrates epidermal involvement as well as clinical findings of a pigmented lesion (Figure 2). A notable minority of vulvar melanomas are amelanotic.9 Melanoma may be distinguished from PES with a panel of melanocytic markers—human melanoma black 45, Melan-A, SRY-box transcription factor 10, S-100, and microphthalmia transcription factor—that rarely are expressed in the latter. Both PES and rhabdoid melanoma have eosinophilic and tinctorial cytoplasmic inclusions.10 Melanin pigment and more cohesive nests are helpful clues that may point to melanoma when present.
Myoepithelial carcinoma of the vulva is rare.11 Myoepithelial carcinoma of soft tissue is more aggressive than its benign counterpart, with up to a 50% metastasis rate.12 The presence of prominent corded or trabecular growth in a myxoid or hyaline background may point to the diagnosis (Figure 3). Similar to PES, myoepithelial carcinoma may lose expression of nuclear INI-1, while myoepithelial carcinoma is more likely to express S-100 and glial fibrillary acidic protein.13 Rearrangements of EWS RNA binding protein 1, EWSR1, have been found in half of myoepithelial neoplasms.12
Angiosarcomas represent 5% of cutaneous sarcomas and rarely have been reported in the vulva, primarily occurring in the setting of long-standing lymphedema and radiation.14 Angiosarcoma more often occurs on the head and neck, breasts, or extremities. Additional risk factors for the development of angiosarcoma include toxin exposure (eg, polyvinyl chloride, thorium dioxide, arsenic), anabolic steroids, and filariasis, as well as genetic disorders (eg, neurofibromatosis type 1, BRCA gene mutations, Maffucci syndrome).15 Epithelioid angiosarcoma is an infiltrative tumor composed of irregular anastomosing vascular channels with extravasated erythrocytes (Figure 4). Solid growth and necrosis may be present in more aggressive tumors. The cells themselves are pleomorphic endothelial cells with vesicular chromatin and prominent nucleoli. Epithelioid angiosarcoma may resemble carcinoma and have focal keratin expression. However, the characteristic eosinophilic cytoplasm seen in PES should not be identified in epithelioid angiosarcoma. Unlike PES, epithelioid angiosarcoma is positive for CD31 and has retained expression for INI-1. Both angiosarcoma and proximal-type epithelioid sarcoma may express vascular markers CD34 and FLI-116; thus an expanded panel of immunohistochemical studies may be of utility.
The Diagnosis: Proximal-Type Epithelioid Sarcoma
Proximal-type epithelioid sarcoma (PES) is a rare high-grade sarcoma of uncertain histogenesis that may present with a benign clinical appearance. Proximal-type epithelioid sarcoma peaks at 20 to 40 years of age and has a slight male predominance. This tumor exhibits aggressive behavior with both local recurrence and metastasis.1 The average overall survival is poor; however, tumor size less than 5 cm and complete excision with tumor-free margin status improves the prognosis.2 Proximal-type epithelioid sarcoma should not be confused with distal-type epithelioid sarcoma, which has a better prognosis and occurs in younger patients.1 Treatment of PES is wide excision, and chemotherapy trials with tazemetostat are ongoing.3
The deceptively banal clinical appearance of PES may delay appropriate diagnosis and treatment. Proximal-type epithelioid sarcoma often grows in sheets (quiz image [top] inset) and loose nests1 but also may take on a more corded appearance mimicking myoepithelial carcinoma. The cells themselves are plump dyscohesive epithelioid cells (quiz image [top]) with large nucleoli and eosinophilic cytoplasm or hyaline globules1 (quiz image [bottom]), but cells also may be focally spindled. Myxoid stroma, hemorrhage, and necrosis often are prominent features. Epithelioid sarcomas characteristically demonstrate positive immunostaining for both epithelial and mesenchymal markers (pan-cytokeratin and vimentin),1 with the majority having loss of expression of integrase interactor 1 (INI-1).2 Histology in this case was positive for cytokeratin monoclonal antibodies CAM5.2 and OSCAR, epithelial membrane antigen, and vimentin; it showed loss of INI-1 staining (quiz image [bottom]). Negative stains included S-100, p63, cytokeratins 7 and 20, CD34, CD31, ERG, glial fibrillary acidic protein, transducin-like enhancer of split 1, CD117, myogenin, synaptophysin, chromogranin, CD10, inhibin, CD99, and estrogen receptor.
The differential diagnosis of PES includes poorly differentiated squamous cell carcinoma (Figure 1 [inset]), melanoma, myoepithelial carcinoma, and epithelioid angiosarcoma. Primary squamous cell carcinoma of the vulva presents as an endophytic or exophytic mass with raised borders. Vulvar cancer is uncommon among gynecologic malignancies, with squamous cell carcinoma being the most commonly encountered.4 Vulvar intraepithelial neoplasia (VIN) is increasing in incidence, while the occurrence of invasive squamous cell carcinoma remains stable.5 Human papillomavirus–related VIN (usual-type VIN) is less likely to progress to squamous cell carcinoma than differentiated VIN (d-VIN), a dysplasia that is unrelated to human papillomavirus that frequently harbors p53 mutations.4 The presence of histologic epidermal involvement can help distinguish squamous cell carcinoma from PES (Figure 1). As opposed to PES, metastatic squamous cell carcinoma is characterized by intercellular bridges and often at least focal keratinization (Figure 1). Squamous cell carcinoma demonstrates positivity with p63 and p40 immunohistochemical stains, while PES rarely stains for either.6
Melanoma is the second most common vulvar malignancy. Vulvar melanoma tends to occur in women of advanced age but has been reported in girls as young as 10 years old.7 There is some evidence that patients with lichen sclerosus may be at an increased risk for the development of vulvar melanoma.8 Compared to PES, primary vulvar melanoma usually demonstrates epidermal involvement as well as clinical findings of a pigmented lesion (Figure 2). A notable minority of vulvar melanomas are amelanotic.9 Melanoma may be distinguished from PES with a panel of melanocytic markers—human melanoma black 45, Melan-A, SRY-box transcription factor 10, S-100, and microphthalmia transcription factor—that rarely are expressed in the latter. Both PES and rhabdoid melanoma have eosinophilic and tinctorial cytoplasmic inclusions.10 Melanin pigment and more cohesive nests are helpful clues that may point to melanoma when present.
Myoepithelial carcinoma of the vulva is rare.11 Myoepithelial carcinoma of soft tissue is more aggressive than its benign counterpart, with up to a 50% metastasis rate.12 The presence of prominent corded or trabecular growth in a myxoid or hyaline background may point to the diagnosis (Figure 3). Similar to PES, myoepithelial carcinoma may lose expression of nuclear INI-1, while myoepithelial carcinoma is more likely to express S-100 and glial fibrillary acidic protein.13 Rearrangements of EWS RNA binding protein 1, EWSR1, have been found in half of myoepithelial neoplasms.12
Angiosarcomas represent 5% of cutaneous sarcomas and rarely have been reported in the vulva, primarily occurring in the setting of long-standing lymphedema and radiation.14 Angiosarcoma more often occurs on the head and neck, breasts, or extremities. Additional risk factors for the development of angiosarcoma include toxin exposure (eg, polyvinyl chloride, thorium dioxide, arsenic), anabolic steroids, and filariasis, as well as genetic disorders (eg, neurofibromatosis type 1, BRCA gene mutations, Maffucci syndrome).15 Epithelioid angiosarcoma is an infiltrative tumor composed of irregular anastomosing vascular channels with extravasated erythrocytes (Figure 4). Solid growth and necrosis may be present in more aggressive tumors. The cells themselves are pleomorphic endothelial cells with vesicular chromatin and prominent nucleoli. Epithelioid angiosarcoma may resemble carcinoma and have focal keratin expression. However, the characteristic eosinophilic cytoplasm seen in PES should not be identified in epithelioid angiosarcoma. Unlike PES, epithelioid angiosarcoma is positive for CD31 and has retained expression for INI-1. Both angiosarcoma and proximal-type epithelioid sarcoma may express vascular markers CD34 and FLI-116; thus an expanded panel of immunohistochemical studies may be of utility.
- Guillou L, Wadden C, Coindre JM, et al. “Proximal-type” epithelioid sarcoma, a distinctive aggressive neoplasm showing rhabdoid features. clinicopathologic, immunohistochemical, and ultrastructural study of a series. Am J Surg Pathol. 1997;21:130-146.
- Hasegawa T, Matsuno Y, Shimoda T, et al. Proximal-type epithelioid sarcoma: a clinicopathological study of 20 cases. Mod Pathol. 2001;14:655-663.
- Czarnecka AM, Sobczuk P, Kostrzanowski M, et al. Epithelioid sarcoma—from genetics to clinical practice. Cancers. 2020:12:2112.
- Hoang LH, Park KJ, Soslow RA, et al. Squamous precursor lesions of the vulva: current classification and diagnostic challenges. Pathology. 2016;48:291-302.
- Allbritton J. Vulvar neoplasms, benign and malignant. Obstet Gynecol Clin North Am. 2017;44:339-352.
- Laskin WB, Miettinen M. Epithelioid sarcoma: new insights based on an extended immunohistochemical analysis. Arch Pathol Lab Med. 2003;127:1161-1168.
- Boer FL, Eikelder MLGT, Kapitejn EH, et al. Vulvar malignant melanoma: pathogenesis, clinical behavior and management: review of the literature. Cancer Treat Rev. 2019;73:91-103.
- Hieta N, Rintala SKM, Soderlund J, et al. Association of vulvar melanoma with lichen sclerosus. Acta Derm Venereol. 2019;99:339-340.
- Edwards L. Pigmented vulvar lesions. Dermatol Ther. 2010;23:449-457.
- Patterson JW, Hosler GA, Prenshaw KL, eds. Weedon's Skin Pathology. Elsevier Limited; 2021.
- Kyriazi MA, Carvounis EE, Kitsou M, et al. Myoepithelial carcinoma of the vulva mimicking Bartholin gland abscess in a pregnant woman: case report and review of literature. Int J Gynecol Pathol. 2010:29:501-504.
- Jo VY, Fletcher CD. Myoepithelial neoplasma of soft tissue: an updated review of the clinicopathological, immunophenotypic, and genetic features. Head Neck Pathol. 2015;9:32-38.
- Rekhi B, Sable M, Jambhekar NA. Histopathological, immunohistochemical and molecular spectrum of myoepithelial tumours of soft tissues. Virchows Arch. 2012;461:687-697.
- Yost S, Bradish J, Grossheim L, et al. Epithelioid angiosarcoma of the vulva: a case report. Gynecol Oncol Rep. 2017;21:91-93.
- Young RJ, Brown NJ, Reed MW, et al. Angiosarcoma. Lancet Oncol. 2010;11:983-991.
- Stockman DL, Hornick JL, Deavers MT, et al. ERG and FLI1 protein expression in epithelioid sarcoma. Mod Pathol. 2014;27:496-501.
- Guillou L, Wadden C, Coindre JM, et al. “Proximal-type” epithelioid sarcoma, a distinctive aggressive neoplasm showing rhabdoid features. clinicopathologic, immunohistochemical, and ultrastructural study of a series. Am J Surg Pathol. 1997;21:130-146.
- Hasegawa T, Matsuno Y, Shimoda T, et al. Proximal-type epithelioid sarcoma: a clinicopathological study of 20 cases. Mod Pathol. 2001;14:655-663.
- Czarnecka AM, Sobczuk P, Kostrzanowski M, et al. Epithelioid sarcoma—from genetics to clinical practice. Cancers. 2020:12:2112.
- Hoang LH, Park KJ, Soslow RA, et al. Squamous precursor lesions of the vulva: current classification and diagnostic challenges. Pathology. 2016;48:291-302.
- Allbritton J. Vulvar neoplasms, benign and malignant. Obstet Gynecol Clin North Am. 2017;44:339-352.
- Laskin WB, Miettinen M. Epithelioid sarcoma: new insights based on an extended immunohistochemical analysis. Arch Pathol Lab Med. 2003;127:1161-1168.
- Boer FL, Eikelder MLGT, Kapitejn EH, et al. Vulvar malignant melanoma: pathogenesis, clinical behavior and management: review of the literature. Cancer Treat Rev. 2019;73:91-103.
- Hieta N, Rintala SKM, Soderlund J, et al. Association of vulvar melanoma with lichen sclerosus. Acta Derm Venereol. 2019;99:339-340.
- Edwards L. Pigmented vulvar lesions. Dermatol Ther. 2010;23:449-457.
- Patterson JW, Hosler GA, Prenshaw KL, eds. Weedon's Skin Pathology. Elsevier Limited; 2021.
- Kyriazi MA, Carvounis EE, Kitsou M, et al. Myoepithelial carcinoma of the vulva mimicking Bartholin gland abscess in a pregnant woman: case report and review of literature. Int J Gynecol Pathol. 2010:29:501-504.
- Jo VY, Fletcher CD. Myoepithelial neoplasma of soft tissue: an updated review of the clinicopathological, immunophenotypic, and genetic features. Head Neck Pathol. 2015;9:32-38.
- Rekhi B, Sable M, Jambhekar NA. Histopathological, immunohistochemical and molecular spectrum of myoepithelial tumours of soft tissues. Virchows Arch. 2012;461:687-697.
- Yost S, Bradish J, Grossheim L, et al. Epithelioid angiosarcoma of the vulva: a case report. Gynecol Oncol Rep. 2017;21:91-93.
- Young RJ, Brown NJ, Reed MW, et al. Angiosarcoma. Lancet Oncol. 2010;11:983-991.
- Stockman DL, Hornick JL, Deavers MT, et al. ERG and FLI1 protein expression in epithelioid sarcoma. Mod Pathol. 2014;27:496-501.
A 45-year-old woman with no notable medical history presented with a small nodule in the left pubic region lateral to the left labia majora. The lesion grew to 8 cm over the course of several months, and she underwent a simple excision for what clinically appeared to be a cyst.
Bleeding Nodule on the Lip
The Diagnosis: Metastatic Clear Cell Renal Cell Carcinoma
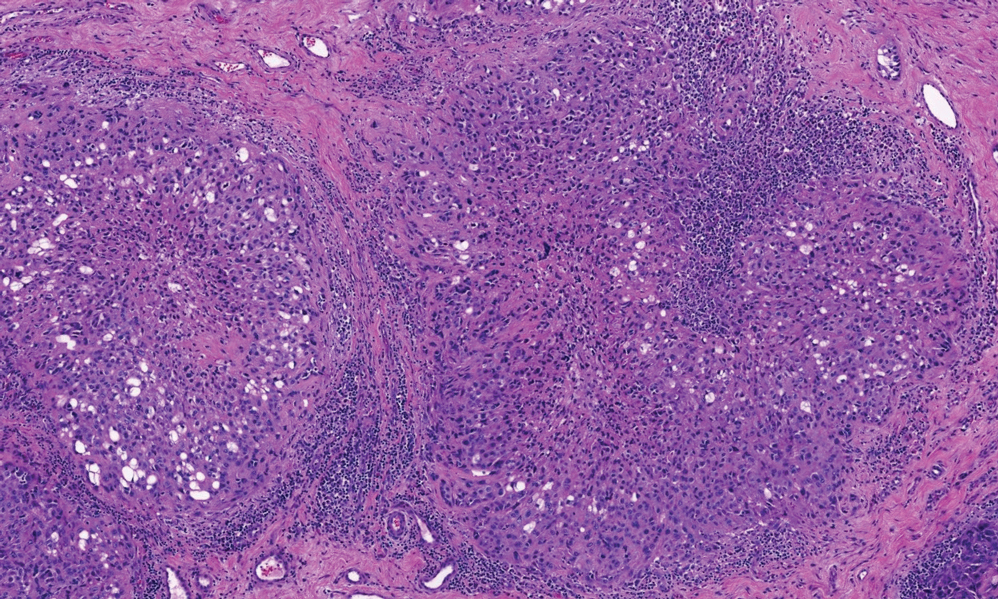
Renal cell carcinoma (RCC) is a common genitourinary system malignancy with incidence peaking between 50 and 70 years of age and a male predominance.1 The clear cell variant is the most common subtype of RCC, accounting for 70% to 75% of all cases. It is known to be a highly aggressive malignancy that frequently metastasizes to the lungs, lymphatics, bones, liver, and brain.2,3 Approximately 20% to 50% of patients with RCC eventually will develop metastasis after nephrectomy.4 Survival with metastatic RCC to any site typically is in the range of 10 to 22 months.5,6 Cutaneous metastases of RCC rarely have been reported in the literature (3%–6% of cases7) and most commonly are found on the scalp, followed by the chest or abdomen. 8 Cutaneous metastases generally are regarded as a late manifestation of the disease with a very poor prognosis. 9 It is unusual to identify cutaneous RCC metastasis without known RCC or other symptoms consistent with advanced RCC, such as hematuria or abdominal/flank pain. Renal cell carcinoma accounts for an estimated 6% to 7% of all cutaneous metastatic lesions.10 Cutaneous metastatic lesions of RCC often are solitary and grow rapidly, with the clinical appearance of an erythematous or violaceous, nodular, highly vascular, and often hemorrhagic growth.9,11,12
Following the histologic diagnosis of metastatic clear cell RCC, our patient was referred to medical oncology for further workup. Magnetic resonance imaging and a positron emission tomography scan demonstrated widespread disease with a 7-cm left renal mass, liver and lung metastases, and bilateral mediastinal lymphadenopathy. The patient was started on combination immunotherapy as a palliative treatment given the widespread disease.
Histologically, clear cell RCC is characterized by lipid and glycogen-rich cells with ample cytoplasm and a well-developed vascular network, which often is thin walled with a chicken wire–like architecture. Metastatic clear cell RCC tumor cells may form glandular, acinar, or papillary structures with variable lymphocytic inflammatory infiltrates and abundant capillary formation. Immunohistochemically, the tumor cells should demonstrate positivity for paired box gene 8, PAX8, and RCC marker antigen.13 Vimentin and carcinoembryonic antigen may be utilized to distinguish from hidradenoma as carcinoembryonic antigen will be positive in hidradenoma and vimentin will be negative.14 Renal cell carcinoma also has a common molecular signature of von Hippel-Lindau tumor suppressor gene inactivation as well as upregulation of hypoxia inducible factor and vascular endothelial growth factor.15
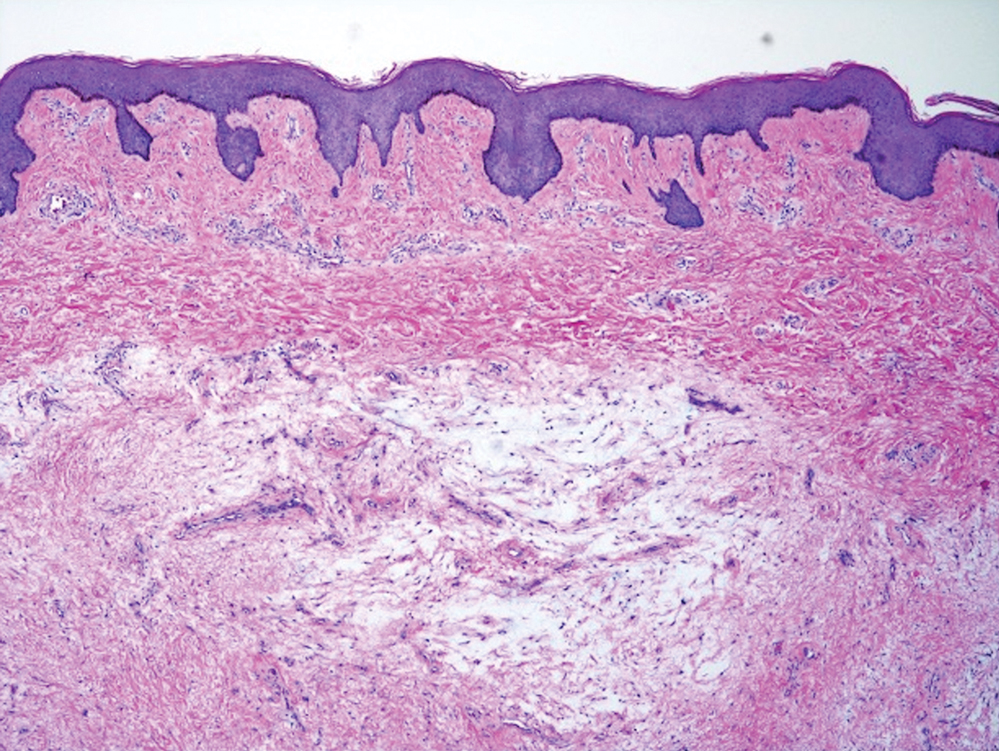
Balloon cell nevi often clinically present in young patients as bicolored nevi that sometimes are polypoid or verrucous in appearance with central yellow globules surrounded by a peripheral reticular pattern on dermoscopy. Histologically, balloon cell nevi are characterized by large cells with small, round, centrally located basophilic nuclei and clear foamy cytoplasm (Figure 1), which are thought to be formed by progressive vacuolization of melanocytes due to the enlargement and disintegration of melanosomes. This ballooning change reflects an seen in malignant melanoma, in which case nuclear pleomorphism, atypia, and increased mitotic activity also are observed. The prominent vascular network characteristic of RCC typically is not present.16
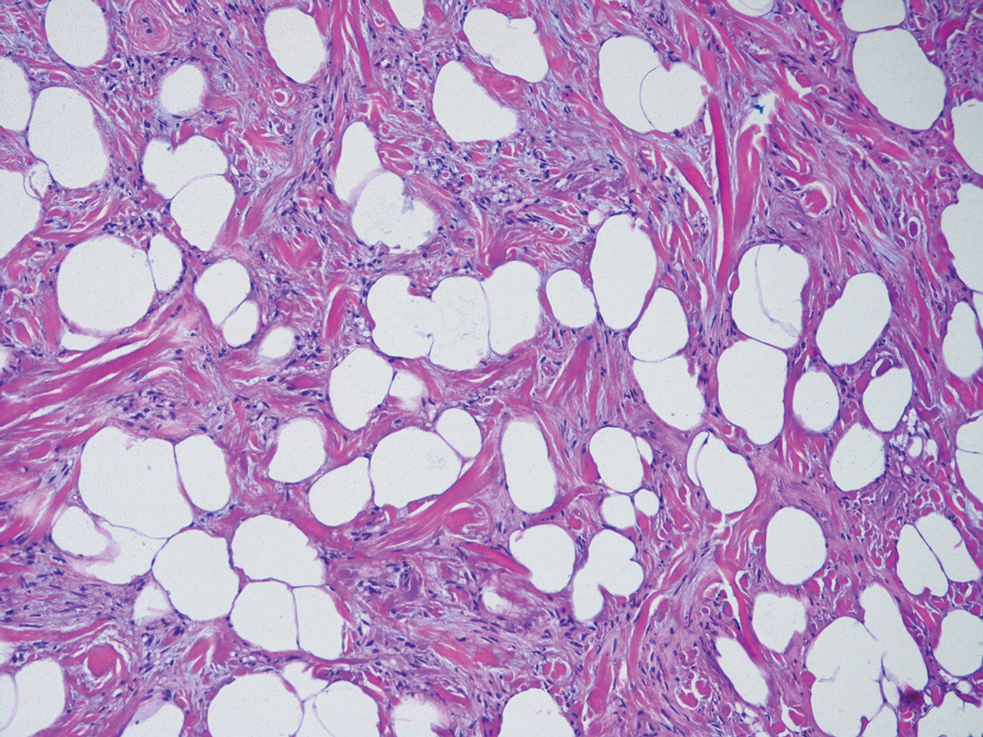
Clear cell hidradenomas are benign skin appendage tumors that often present as small, firm, solitary dermal nodules that may extend into the subcutaneous fat. They have a predilection for the head, face, and arms and demonstrate 2 predominant cell types, including a polyhedral cell with a rounded nucleus and slightly basophilic cytoplasm as well as a round cell with clear cytoplasm and bland nuclei (Figure 2). The latter cell type is less common, representing the predominant cell type in less than one-third of hidradenomas, and can present a diagnostic quandary based on histologic similarity to other clear cell neoplasms. The clear cells contain glycogen but no lipid. Ductlike structures often are present, and the intervening stroma varies from delicate vascularized cords of fibrous tissue to dense hyalinized collagen. Immunohistochemistry may be required for definitive diagnosis, and clear cell hidradenomas should react with monoclonal antibodies that label both eccrine and apocrine secretory elements, such as cytokeratins 6/18, 7, and 8/18.17
Pyogenic granulomas (also referred to as lobular capillary hemangiomas) are common and present clinically as rapidly growing, polypoid, red masses surrounded by a thickened epidermis that often are found on the fingers or lips. This entity is benign and often regresses spontaneously. Histologically, pyogenic granulomas are characterized by a lobular pattern of vascular proliferation associated with edema and inflammation resembling granulation tissue, with acanthosis and hyperkeratosis at the edges of the lesion (Figure 3).18
Sebaceous carcinoma is a locally aggressive malignant neoplasm arising from the cells of the sebaceous glands and occurring most commonly in the periorbital area. This neoplasm most often affects older adults, with a mean age at diagnosis of 63 to 77 years. It commonly presents as a solitary nodule with yellowish discoloration and madarosis, which is a key distinguishing feature to differentiate this entity from a chalazion or hordeolum. Histologically, sebaceous carcinoma is a dermal-based infiltrative, nodular tumor with varying degrees of clear cell changes—well-differentiated tumors show more clear cell change as compared to more poorly differentiated variants—along with basaloid or squamous features and abundant mitotic activity (Figure 4), which may be useful in distinguishing it from the other entities in the clear cell neoplasm differential.19-22
- Alves de Paula T, Lopes da Silva P, Sueth Berriel LG. Renal cell carcinoma with cutaneous metastasis: case report. J Bras Nefrol. 2010;32:213-215.
- Amaadour L, Atreche L, Azegrar M, et al. Cutaneous metastasis of renal cell carcinoma: a case report. J Cancer Ther. 2017;8:603-607.
- Weiss L, Harlos JP, Torhorst J, et al. Metastatic patterns of renal carcinoma: an analysis of 687 necropsies. J Cancer Res Clin Oncol. 1988;114:605-612.
- Flamigan RC, Campbell SC, Clark JI, et al. Metastatic renal cell carcinoma. Curr Treat Options Oncol. 2003;4:385-390.
- Motzer RJ, Bacik J, Schwarz LH, et al. Prognostic factors for survival in previously treated patients with metastatic renal cell carcinoma. J Clin Oncol. 2004;22:453-463.
- Heng DY, Xie W, Regan MM, et al. Prognostic factors for overall survival in patients with metastatic renal cell carcinoma treated with vascular endothelial growth factor–targeted agents: results from a large, multicenter study. J Clin Oncol. 2009;27:5694-5799.
- Smyth LG, Rowan GC, David MQ. Renal cell carcinoma presenting as an ominous metachronous scalp metastasis. Can Urol Assoc J. 2010;4:E64-E66.
- Dorairajan LN, Hemal AK, Aron M, et al. Cutaneous metastases in renal cell carcinoma. Urol Int. 1999;63:164-167.
- Koga S, Tsuda S, Nishikido M, et al. Renal cell carcinoma metastatic to the skin. Anticancer Res. 2000;20:1939-1940.
- Krathen RA, Orengo IF, Rosen T. Cutaneous metastasis: a metaanalysis of the data. South Med J. 2003;96:164-167.
- Amano Y, Ohni S, Ishige T, et al. A case of cutaneous metastasis from a clear cell renal cell carcinoma with an eosinophilic cell component to the submandibular region. J Nihon Univ Med Assoc. 2015;74:73-77.
- Arrabal-Polo MA, Arias-Santiago SA, Aneiros-Fernandez J, et al. Cutaneous metastases in renal cell carcinoma: a case report. Cases J. 2009;2:7948.
- Sangoi AR, Karamchandani J, Kim J, et al. The use of immunohistochemistry in the diagnosis of metastatic clear cell renal cell carcinoma: a review of PAX-8, PAX-2, hKIM-1, RCCma, and CD10. Adv Anat Pathol. 2010;17:377-393.
- Velez MJ, Thomas CL, Stratton J, et al. The utility of using immunohistochemistry in the differentiation of metastatic, cutaneous clear cell renal cell carcinoma and clear cell hidradenoma. J Cutan Pathol. 2017;44:612-615.
- Nezami BG, MacLennan G. Clear cell. PathologyOutlines website. Published April 20, 2021. Updated March 2, 2022. Accessed April 22, 2022. https://www.pathologyoutlines.com/topic/kidneytumormalignantrccclear.html
- Dhaille F, Courville P, Joly P, et al. Balloon cell nevus: histologic and dermoscopic features. J Am Acad Dermatol. 2015;72:E55-E56.
- Volmar KE, Cummings TJ, Wang WH, et al. Clear cell hidradenoma: a mimic of metastatic clear cell tumors. Arch Pathol Lab Med. 2005;129:E113-E116.
- Hale CS. Capillary/pyogenic granuloma. Pathology Outlines website. Published August 1, 2012. Updated March 10, 2022. Accessed April 20, 2022. https://www.pathologyoutlines.com/topic/skintumornonmelanocyticpyogenicgranuloma.html
- Zada S, Lee BA. Sebaceous carcinoma. Pathology Outlines website. Published August 11, 2021. Accessed April 20, 2022. https://www.pathologyoutlines.com/topic/skintumornonmelanocyticsebaceouscarcinoma.html
- Kahana A, Pribila, JT, Nelson CC, et al. Sebaceous cell carcinoma. In: Levin LA, Albert DM, eds. Ocular Disease: Mechanisms and Management. Elsevier; 2010:396-407.
- Wick MR. Cutaneous tumors and pseudotumors of the head and neck. In: Gnepp DR, ed. Diagnostic Surgical Pathology of the Head and Neck. 2nd ed. Saunders Elsevier; 2009:975-1068.
- Cassarino DS, Dadras SS, Lindberg MR, et al. Sebaceous carcinoma. In: Cassarino DS, Dadras SS, Lindberg MR, et al, eds. Diagnostic Pathology: Neoplastic Dermatopathology. 2nd ed. Elsevier; 2017:174-179.
The Diagnosis: Metastatic Clear Cell Renal Cell Carcinoma
Renal cell carcinoma (RCC) is a common genitourinary system malignancy with incidence peaking between 50 and 70 years of age and a male predominance.1 The clear cell variant is the most common subtype of RCC, accounting for 70% to 75% of all cases. It is known to be a highly aggressive malignancy that frequently metastasizes to the lungs, lymphatics, bones, liver, and brain.2,3 Approximately 20% to 50% of patients with RCC eventually will develop metastasis after nephrectomy.4 Survival with metastatic RCC to any site typically is in the range of 10 to 22 months.5,6 Cutaneous metastases of RCC rarely have been reported in the literature (3%–6% of cases7) and most commonly are found on the scalp, followed by the chest or abdomen. 8 Cutaneous metastases generally are regarded as a late manifestation of the disease with a very poor prognosis. 9 It is unusual to identify cutaneous RCC metastasis without known RCC or other symptoms consistent with advanced RCC, such as hematuria or abdominal/flank pain. Renal cell carcinoma accounts for an estimated 6% to 7% of all cutaneous metastatic lesions.10 Cutaneous metastatic lesions of RCC often are solitary and grow rapidly, with the clinical appearance of an erythematous or violaceous, nodular, highly vascular, and often hemorrhagic growth.9,11,12
Following the histologic diagnosis of metastatic clear cell RCC, our patient was referred to medical oncology for further workup. Magnetic resonance imaging and a positron emission tomography scan demonstrated widespread disease with a 7-cm left renal mass, liver and lung metastases, and bilateral mediastinal lymphadenopathy. The patient was started on combination immunotherapy as a palliative treatment given the widespread disease.
Histologically, clear cell RCC is characterized by lipid and glycogen-rich cells with ample cytoplasm and a well-developed vascular network, which often is thin walled with a chicken wire–like architecture. Metastatic clear cell RCC tumor cells may form glandular, acinar, or papillary structures with variable lymphocytic inflammatory infiltrates and abundant capillary formation. Immunohistochemically, the tumor cells should demonstrate positivity for paired box gene 8, PAX8, and RCC marker antigen.13 Vimentin and carcinoembryonic antigen may be utilized to distinguish from hidradenoma as carcinoembryonic antigen will be positive in hidradenoma and vimentin will be negative.14 Renal cell carcinoma also has a common molecular signature of von Hippel-Lindau tumor suppressor gene inactivation as well as upregulation of hypoxia inducible factor and vascular endothelial growth factor.15
Balloon cell nevi often clinically present in young patients as bicolored nevi that sometimes are polypoid or verrucous in appearance with central yellow globules surrounded by a peripheral reticular pattern on dermoscopy. Histologically, balloon cell nevi are characterized by large cells with small, round, centrally located basophilic nuclei and clear foamy cytoplasm (Figure 1), which are thought to be formed by progressive vacuolization of melanocytes due to the enlargement and disintegration of melanosomes. This ballooning change reflects an seen in malignant melanoma, in which case nuclear pleomorphism, atypia, and increased mitotic activity also are observed. The prominent vascular network characteristic of RCC typically is not present.16
Clear cell hidradenomas are benign skin appendage tumors that often present as small, firm, solitary dermal nodules that may extend into the subcutaneous fat. They have a predilection for the head, face, and arms and demonstrate 2 predominant cell types, including a polyhedral cell with a rounded nucleus and slightly basophilic cytoplasm as well as a round cell with clear cytoplasm and bland nuclei (Figure 2). The latter cell type is less common, representing the predominant cell type in less than one-third of hidradenomas, and can present a diagnostic quandary based on histologic similarity to other clear cell neoplasms. The clear cells contain glycogen but no lipid. Ductlike structures often are present, and the intervening stroma varies from delicate vascularized cords of fibrous tissue to dense hyalinized collagen. Immunohistochemistry may be required for definitive diagnosis, and clear cell hidradenomas should react with monoclonal antibodies that label both eccrine and apocrine secretory elements, such as cytokeratins 6/18, 7, and 8/18.17
Pyogenic granulomas (also referred to as lobular capillary hemangiomas) are common and present clinically as rapidly growing, polypoid, red masses surrounded by a thickened epidermis that often are found on the fingers or lips. This entity is benign and often regresses spontaneously. Histologically, pyogenic granulomas are characterized by a lobular pattern of vascular proliferation associated with edema and inflammation resembling granulation tissue, with acanthosis and hyperkeratosis at the edges of the lesion (Figure 3).18
Sebaceous carcinoma is a locally aggressive malignant neoplasm arising from the cells of the sebaceous glands and occurring most commonly in the periorbital area. This neoplasm most often affects older adults, with a mean age at diagnosis of 63 to 77 years. It commonly presents as a solitary nodule with yellowish discoloration and madarosis, which is a key distinguishing feature to differentiate this entity from a chalazion or hordeolum. Histologically, sebaceous carcinoma is a dermal-based infiltrative, nodular tumor with varying degrees of clear cell changes—well-differentiated tumors show more clear cell change as compared to more poorly differentiated variants—along with basaloid or squamous features and abundant mitotic activity (Figure 4), which may be useful in distinguishing it from the other entities in the clear cell neoplasm differential.19-22
The Diagnosis: Metastatic Clear Cell Renal Cell Carcinoma
Renal cell carcinoma (RCC) is a common genitourinary system malignancy with incidence peaking between 50 and 70 years of age and a male predominance.1 The clear cell variant is the most common subtype of RCC, accounting for 70% to 75% of all cases. It is known to be a highly aggressive malignancy that frequently metastasizes to the lungs, lymphatics, bones, liver, and brain.2,3 Approximately 20% to 50% of patients with RCC eventually will develop metastasis after nephrectomy.4 Survival with metastatic RCC to any site typically is in the range of 10 to 22 months.5,6 Cutaneous metastases of RCC rarely have been reported in the literature (3%–6% of cases7) and most commonly are found on the scalp, followed by the chest or abdomen. 8 Cutaneous metastases generally are regarded as a late manifestation of the disease with a very poor prognosis. 9 It is unusual to identify cutaneous RCC metastasis without known RCC or other symptoms consistent with advanced RCC, such as hematuria or abdominal/flank pain. Renal cell carcinoma accounts for an estimated 6% to 7% of all cutaneous metastatic lesions.10 Cutaneous metastatic lesions of RCC often are solitary and grow rapidly, with the clinical appearance of an erythematous or violaceous, nodular, highly vascular, and often hemorrhagic growth.9,11,12
Following the histologic diagnosis of metastatic clear cell RCC, our patient was referred to medical oncology for further workup. Magnetic resonance imaging and a positron emission tomography scan demonstrated widespread disease with a 7-cm left renal mass, liver and lung metastases, and bilateral mediastinal lymphadenopathy. The patient was started on combination immunotherapy as a palliative treatment given the widespread disease.
Histologically, clear cell RCC is characterized by lipid and glycogen-rich cells with ample cytoplasm and a well-developed vascular network, which often is thin walled with a chicken wire–like architecture. Metastatic clear cell RCC tumor cells may form glandular, acinar, or papillary structures with variable lymphocytic inflammatory infiltrates and abundant capillary formation. Immunohistochemically, the tumor cells should demonstrate positivity for paired box gene 8, PAX8, and RCC marker antigen.13 Vimentin and carcinoembryonic antigen may be utilized to distinguish from hidradenoma as carcinoembryonic antigen will be positive in hidradenoma and vimentin will be negative.14 Renal cell carcinoma also has a common molecular signature of von Hippel-Lindau tumor suppressor gene inactivation as well as upregulation of hypoxia inducible factor and vascular endothelial growth factor.15
Balloon cell nevi often clinically present in young patients as bicolored nevi that sometimes are polypoid or verrucous in appearance with central yellow globules surrounded by a peripheral reticular pattern on dermoscopy. Histologically, balloon cell nevi are characterized by large cells with small, round, centrally located basophilic nuclei and clear foamy cytoplasm (Figure 1), which are thought to be formed by progressive vacuolization of melanocytes due to the enlargement and disintegration of melanosomes. This ballooning change reflects an seen in malignant melanoma, in which case nuclear pleomorphism, atypia, and increased mitotic activity also are observed. The prominent vascular network characteristic of RCC typically is not present.16
Clear cell hidradenomas are benign skin appendage tumors that often present as small, firm, solitary dermal nodules that may extend into the subcutaneous fat. They have a predilection for the head, face, and arms and demonstrate 2 predominant cell types, including a polyhedral cell with a rounded nucleus and slightly basophilic cytoplasm as well as a round cell with clear cytoplasm and bland nuclei (Figure 2). The latter cell type is less common, representing the predominant cell type in less than one-third of hidradenomas, and can present a diagnostic quandary based on histologic similarity to other clear cell neoplasms. The clear cells contain glycogen but no lipid. Ductlike structures often are present, and the intervening stroma varies from delicate vascularized cords of fibrous tissue to dense hyalinized collagen. Immunohistochemistry may be required for definitive diagnosis, and clear cell hidradenomas should react with monoclonal antibodies that label both eccrine and apocrine secretory elements, such as cytokeratins 6/18, 7, and 8/18.17
Pyogenic granulomas (also referred to as lobular capillary hemangiomas) are common and present clinically as rapidly growing, polypoid, red masses surrounded by a thickened epidermis that often are found on the fingers or lips. This entity is benign and often regresses spontaneously. Histologically, pyogenic granulomas are characterized by a lobular pattern of vascular proliferation associated with edema and inflammation resembling granulation tissue, with acanthosis and hyperkeratosis at the edges of the lesion (Figure 3).18
Sebaceous carcinoma is a locally aggressive malignant neoplasm arising from the cells of the sebaceous glands and occurring most commonly in the periorbital area. This neoplasm most often affects older adults, with a mean age at diagnosis of 63 to 77 years. It commonly presents as a solitary nodule with yellowish discoloration and madarosis, which is a key distinguishing feature to differentiate this entity from a chalazion or hordeolum. Histologically, sebaceous carcinoma is a dermal-based infiltrative, nodular tumor with varying degrees of clear cell changes—well-differentiated tumors show more clear cell change as compared to more poorly differentiated variants—along with basaloid or squamous features and abundant mitotic activity (Figure 4), which may be useful in distinguishing it from the other entities in the clear cell neoplasm differential.19-22
- Alves de Paula T, Lopes da Silva P, Sueth Berriel LG. Renal cell carcinoma with cutaneous metastasis: case report. J Bras Nefrol. 2010;32:213-215.
- Amaadour L, Atreche L, Azegrar M, et al. Cutaneous metastasis of renal cell carcinoma: a case report. J Cancer Ther. 2017;8:603-607.
- Weiss L, Harlos JP, Torhorst J, et al. Metastatic patterns of renal carcinoma: an analysis of 687 necropsies. J Cancer Res Clin Oncol. 1988;114:605-612.
- Flamigan RC, Campbell SC, Clark JI, et al. Metastatic renal cell carcinoma. Curr Treat Options Oncol. 2003;4:385-390.
- Motzer RJ, Bacik J, Schwarz LH, et al. Prognostic factors for survival in previously treated patients with metastatic renal cell carcinoma. J Clin Oncol. 2004;22:453-463.
- Heng DY, Xie W, Regan MM, et al. Prognostic factors for overall survival in patients with metastatic renal cell carcinoma treated with vascular endothelial growth factor–targeted agents: results from a large, multicenter study. J Clin Oncol. 2009;27:5694-5799.
- Smyth LG, Rowan GC, David MQ. Renal cell carcinoma presenting as an ominous metachronous scalp metastasis. Can Urol Assoc J. 2010;4:E64-E66.
- Dorairajan LN, Hemal AK, Aron M, et al. Cutaneous metastases in renal cell carcinoma. Urol Int. 1999;63:164-167.
- Koga S, Tsuda S, Nishikido M, et al. Renal cell carcinoma metastatic to the skin. Anticancer Res. 2000;20:1939-1940.
- Krathen RA, Orengo IF, Rosen T. Cutaneous metastasis: a metaanalysis of the data. South Med J. 2003;96:164-167.
- Amano Y, Ohni S, Ishige T, et al. A case of cutaneous metastasis from a clear cell renal cell carcinoma with an eosinophilic cell component to the submandibular region. J Nihon Univ Med Assoc. 2015;74:73-77.
- Arrabal-Polo MA, Arias-Santiago SA, Aneiros-Fernandez J, et al. Cutaneous metastases in renal cell carcinoma: a case report. Cases J. 2009;2:7948.
- Sangoi AR, Karamchandani J, Kim J, et al. The use of immunohistochemistry in the diagnosis of metastatic clear cell renal cell carcinoma: a review of PAX-8, PAX-2, hKIM-1, RCCma, and CD10. Adv Anat Pathol. 2010;17:377-393.
- Velez MJ, Thomas CL, Stratton J, et al. The utility of using immunohistochemistry in the differentiation of metastatic, cutaneous clear cell renal cell carcinoma and clear cell hidradenoma. J Cutan Pathol. 2017;44:612-615.
- Nezami BG, MacLennan G. Clear cell. PathologyOutlines website. Published April 20, 2021. Updated March 2, 2022. Accessed April 22, 2022. https://www.pathologyoutlines.com/topic/kidneytumormalignantrccclear.html
- Dhaille F, Courville P, Joly P, et al. Balloon cell nevus: histologic and dermoscopic features. J Am Acad Dermatol. 2015;72:E55-E56.
- Volmar KE, Cummings TJ, Wang WH, et al. Clear cell hidradenoma: a mimic of metastatic clear cell tumors. Arch Pathol Lab Med. 2005;129:E113-E116.
- Hale CS. Capillary/pyogenic granuloma. Pathology Outlines website. Published August 1, 2012. Updated March 10, 2022. Accessed April 20, 2022. https://www.pathologyoutlines.com/topic/skintumornonmelanocyticpyogenicgranuloma.html
- Zada S, Lee BA. Sebaceous carcinoma. Pathology Outlines website. Published August 11, 2021. Accessed April 20, 2022. https://www.pathologyoutlines.com/topic/skintumornonmelanocyticsebaceouscarcinoma.html
- Kahana A, Pribila, JT, Nelson CC, et al. Sebaceous cell carcinoma. In: Levin LA, Albert DM, eds. Ocular Disease: Mechanisms and Management. Elsevier; 2010:396-407.
- Wick MR. Cutaneous tumors and pseudotumors of the head and neck. In: Gnepp DR, ed. Diagnostic Surgical Pathology of the Head and Neck. 2nd ed. Saunders Elsevier; 2009:975-1068.
- Cassarino DS, Dadras SS, Lindberg MR, et al. Sebaceous carcinoma. In: Cassarino DS, Dadras SS, Lindberg MR, et al, eds. Diagnostic Pathology: Neoplastic Dermatopathology. 2nd ed. Elsevier; 2017:174-179.
- Alves de Paula T, Lopes da Silva P, Sueth Berriel LG. Renal cell carcinoma with cutaneous metastasis: case report. J Bras Nefrol. 2010;32:213-215.
- Amaadour L, Atreche L, Azegrar M, et al. Cutaneous metastasis of renal cell carcinoma: a case report. J Cancer Ther. 2017;8:603-607.
- Weiss L, Harlos JP, Torhorst J, et al. Metastatic patterns of renal carcinoma: an analysis of 687 necropsies. J Cancer Res Clin Oncol. 1988;114:605-612.
- Flamigan RC, Campbell SC, Clark JI, et al. Metastatic renal cell carcinoma. Curr Treat Options Oncol. 2003;4:385-390.
- Motzer RJ, Bacik J, Schwarz LH, et al. Prognostic factors for survival in previously treated patients with metastatic renal cell carcinoma. J Clin Oncol. 2004;22:453-463.
- Heng DY, Xie W, Regan MM, et al. Prognostic factors for overall survival in patients with metastatic renal cell carcinoma treated with vascular endothelial growth factor–targeted agents: results from a large, multicenter study. J Clin Oncol. 2009;27:5694-5799.
- Smyth LG, Rowan GC, David MQ. Renal cell carcinoma presenting as an ominous metachronous scalp metastasis. Can Urol Assoc J. 2010;4:E64-E66.
- Dorairajan LN, Hemal AK, Aron M, et al. Cutaneous metastases in renal cell carcinoma. Urol Int. 1999;63:164-167.
- Koga S, Tsuda S, Nishikido M, et al. Renal cell carcinoma metastatic to the skin. Anticancer Res. 2000;20:1939-1940.
- Krathen RA, Orengo IF, Rosen T. Cutaneous metastasis: a metaanalysis of the data. South Med J. 2003;96:164-167.
- Amano Y, Ohni S, Ishige T, et al. A case of cutaneous metastasis from a clear cell renal cell carcinoma with an eosinophilic cell component to the submandibular region. J Nihon Univ Med Assoc. 2015;74:73-77.
- Arrabal-Polo MA, Arias-Santiago SA, Aneiros-Fernandez J, et al. Cutaneous metastases in renal cell carcinoma: a case report. Cases J. 2009;2:7948.
- Sangoi AR, Karamchandani J, Kim J, et al. The use of immunohistochemistry in the diagnosis of metastatic clear cell renal cell carcinoma: a review of PAX-8, PAX-2, hKIM-1, RCCma, and CD10. Adv Anat Pathol. 2010;17:377-393.
- Velez MJ, Thomas CL, Stratton J, et al. The utility of using immunohistochemistry in the differentiation of metastatic, cutaneous clear cell renal cell carcinoma and clear cell hidradenoma. J Cutan Pathol. 2017;44:612-615.
- Nezami BG, MacLennan G. Clear cell. PathologyOutlines website. Published April 20, 2021. Updated March 2, 2022. Accessed April 22, 2022. https://www.pathologyoutlines.com/topic/kidneytumormalignantrccclear.html
- Dhaille F, Courville P, Joly P, et al. Balloon cell nevus: histologic and dermoscopic features. J Am Acad Dermatol. 2015;72:E55-E56.
- Volmar KE, Cummings TJ, Wang WH, et al. Clear cell hidradenoma: a mimic of metastatic clear cell tumors. Arch Pathol Lab Med. 2005;129:E113-E116.
- Hale CS. Capillary/pyogenic granuloma. Pathology Outlines website. Published August 1, 2012. Updated March 10, 2022. Accessed April 20, 2022. https://www.pathologyoutlines.com/topic/skintumornonmelanocyticpyogenicgranuloma.html
- Zada S, Lee BA. Sebaceous carcinoma. Pathology Outlines website. Published August 11, 2021. Accessed April 20, 2022. https://www.pathologyoutlines.com/topic/skintumornonmelanocyticsebaceouscarcinoma.html
- Kahana A, Pribila, JT, Nelson CC, et al. Sebaceous cell carcinoma. In: Levin LA, Albert DM, eds. Ocular Disease: Mechanisms and Management. Elsevier; 2010:396-407.
- Wick MR. Cutaneous tumors and pseudotumors of the head and neck. In: Gnepp DR, ed. Diagnostic Surgical Pathology of the Head and Neck. 2nd ed. Saunders Elsevier; 2009:975-1068.
- Cassarino DS, Dadras SS, Lindberg MR, et al. Sebaceous carcinoma. In: Cassarino DS, Dadras SS, Lindberg MR, et al, eds. Diagnostic Pathology: Neoplastic Dermatopathology. 2nd ed. Elsevier; 2017:174-179.
A 71-year-old man with no notable medical history presented with a bleeding nodule on the right lower cutaneous lip of 9 weeks’ duration. The patient denied any systemic symptoms. A shave biopsy was performed.
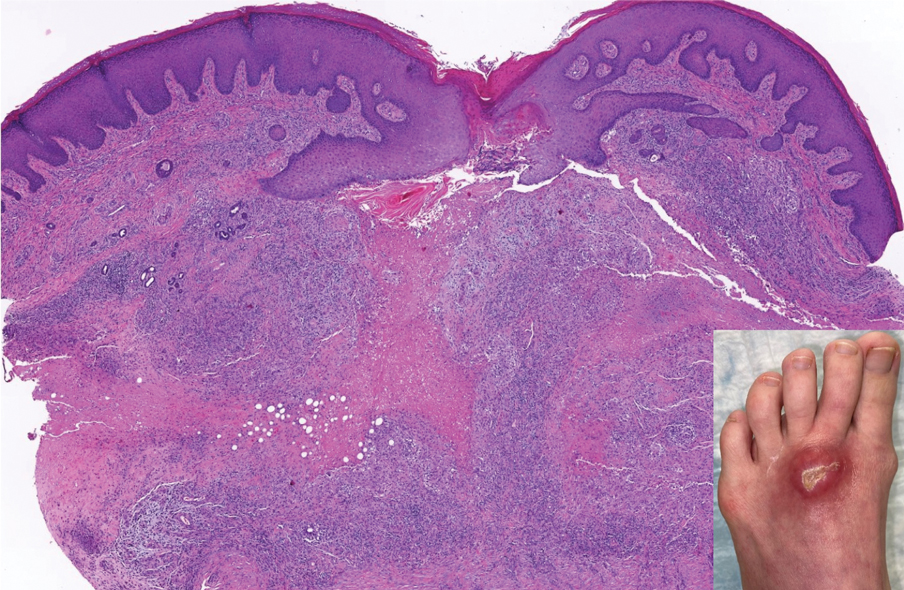
Ulcerating Nodule on the Foot
The Diagnosis: Perforating Rheumatoid Nodule
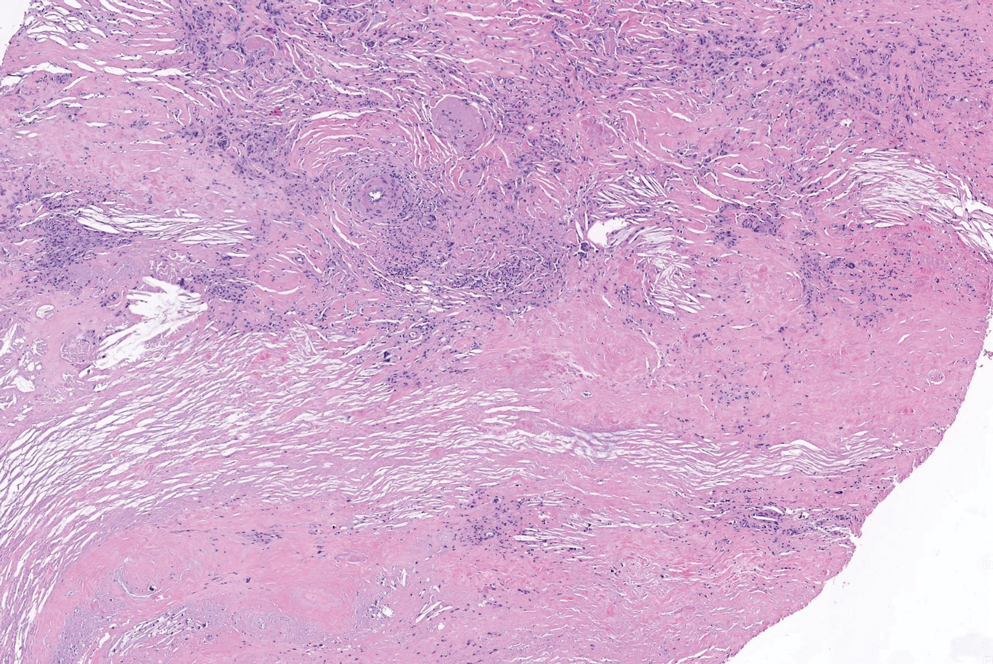
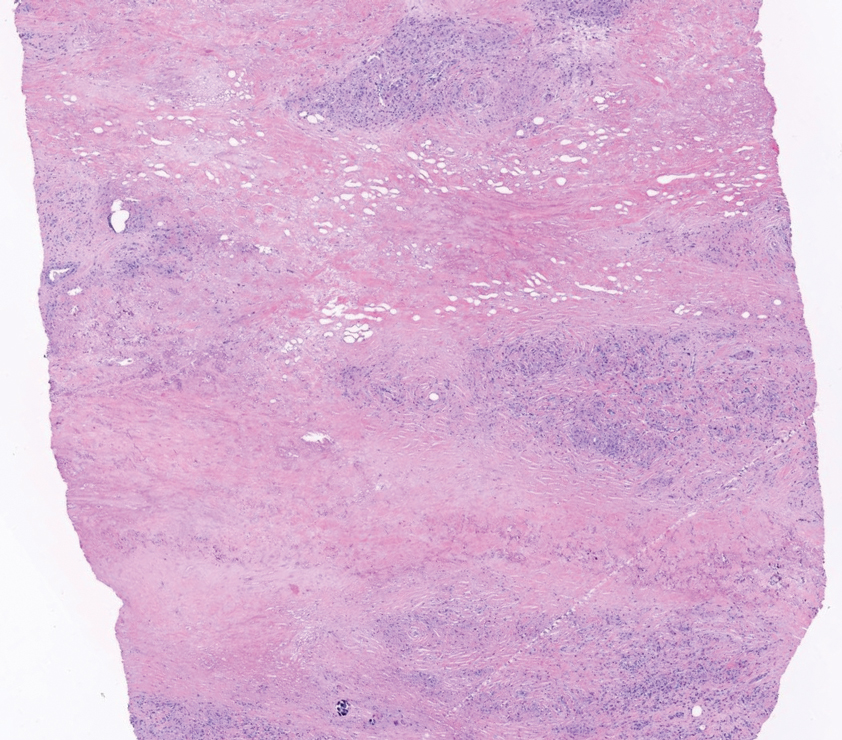
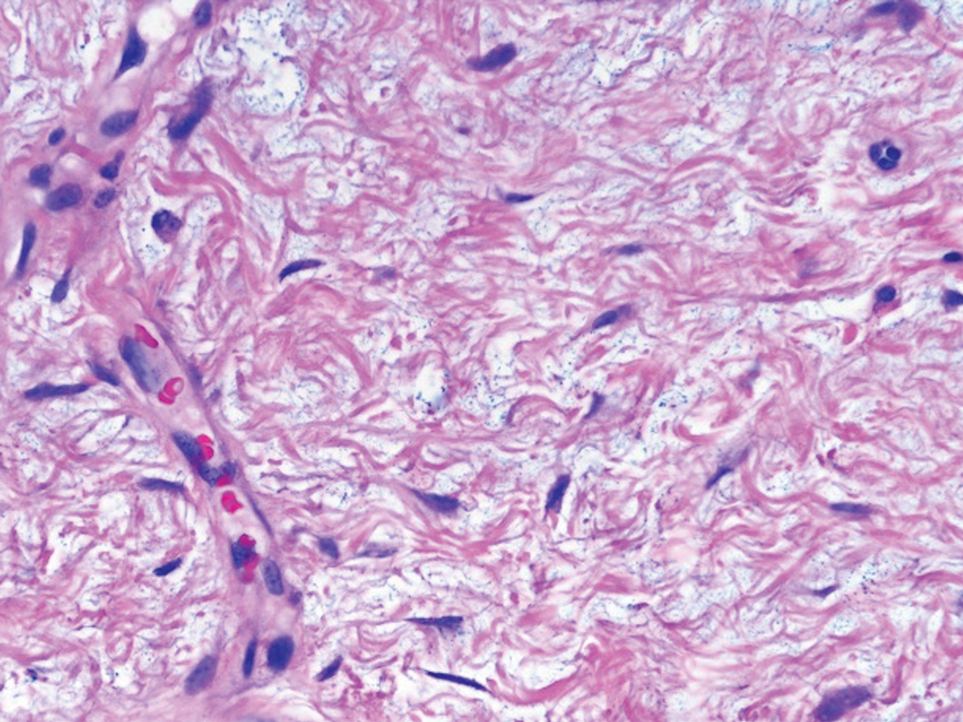
Perforating rheumatoid nodule (RN) is a variant of RN that demonstrates necrobiotic material extruding through the epidermis via the process of transepidermal elimination.1 The necrobiotic material contains fibrin and often harbors karyorrhectic debris. The pathogenesis of RN remains unclear; possible mechanisms include a small vessel vasculitis or mechanical trauma inciting a localized aggregation of inflammatory products and rheumatoid factor complexes. This induces macrophage activation, fibrin deposition, and necrosis.2 The majority of patients with RNs have detectable rheumatoid factor and anticyclic citrullinated protein in the blood.3 Rheumatoid nodules are the most common cutaneous manifestations of rheumatoid arthritis (RA) and will develop in 30% to 40% of RA patients.4,5 They typically are associated with advanced RA but may precede the onset of clinically severe RA in 5% to 10% of patients.5 Rheumatoid nodules generally range in size from 2 mm to 5 cm and are slightly more prevalent in men than in women. They present as firm painless masses typically on the extensor surfaces of the hands and olecranon process but can occur over any tendinous or ligamentlike structure.6,7 Perforating RNs are most common on areas subjected to pressure or repeated trauma, such as the sacrum.
The diagnosis usually is clinical; however, in cases of diagnostic uncertainty, RN can be distinguished by its histologic appearance. Rheumatoid nodules demonstrate granulomatous palisading necrobiosis with a central zone of highly eosinophilic fibrinoid necrobiosis surrounded by palisading mononuclear cells and an outer zone of granulation tissue. There may be a mixed chronic inflammatory infiltrate predominantly composed of lymphocytes and histiocytes in the background.
Rheumatoid nodules typically do not require treatment; however, perforation is known to increase the risk for infection, and surgical excision generally is indicated for prophylaxis against infection, though nodules may recur in the excision area.1,3,8 Alternatively, disease-modifying antirheumatic drugs and intralesional corticosteroids may effectively reduce the size of RNs. The differential diagnosis for perforating RNs includes epithelioid sarcoma, perforating granuloma annulare, necrobiotic xanthogranuloma, and necrobiosis lipoidica.
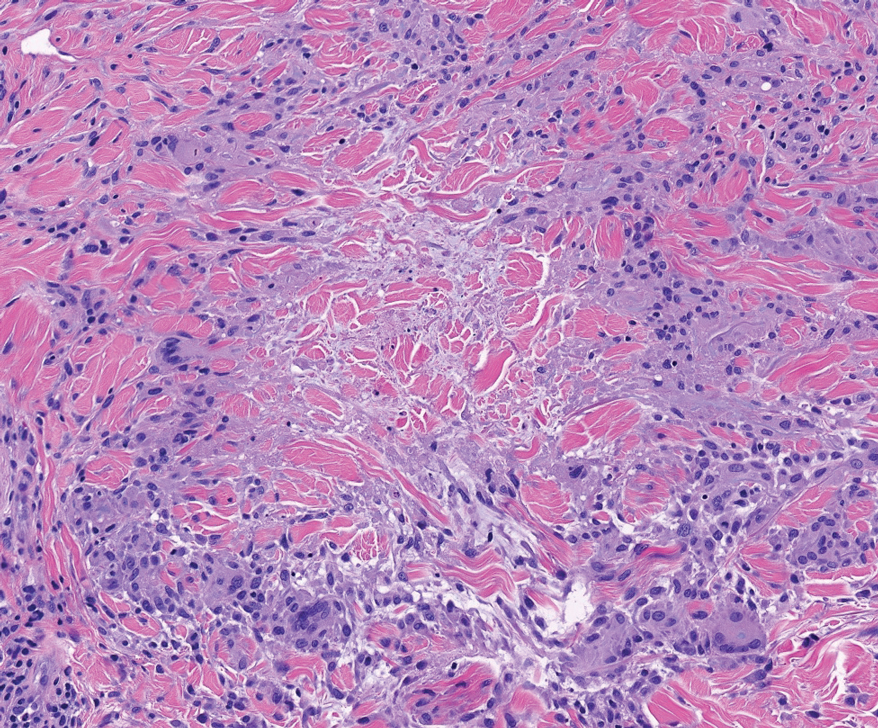
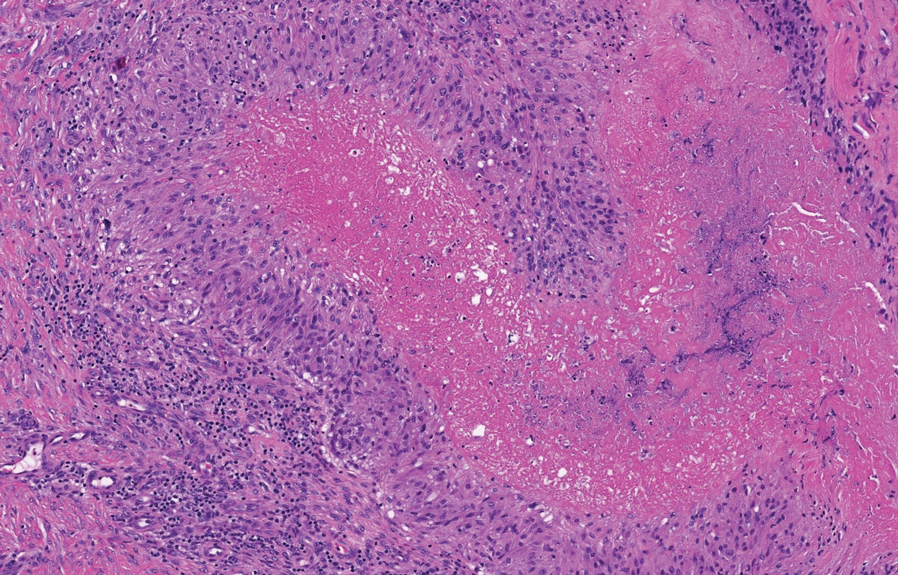
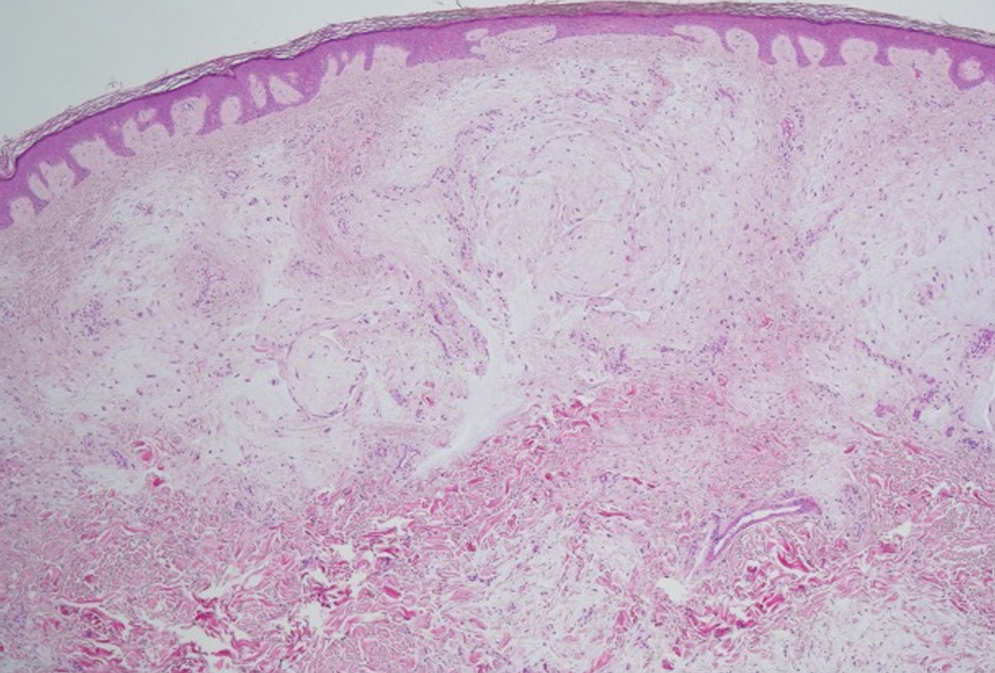
Epithelioid sarcoma is a malignant soft tissue tumor typically found on the upper extremities of adolescent or young adult males. They usually present as hard tender nodules that commonly ulcerate. Epithelioid sarcoma makes up less than 1% of soft tissue sarcomas.9 Although rare, they present a diagnostic pitfall, as the histology may mimic an inflammatory palisaded granulomatous dermatitis similar to RN and granuloma annulare, thus a high index of suspicion is required to not overlook this aggressive malignancy. Histology is typified by nodular aggregates of epithelioid cells with abundant eosinophilic cytoplasm and often with central zones of necrosis (Figure 1). Epithelioid sarcoma displays immunoreactivity to cytokeratin, CD34, and epithelial membrane antigen, but loss of integrase interactor 1 expression. Cytologic abnormalities such as pleomorphism and hyperchromatism can be helpful in distinguishing between epithelioid sarcoma and RN.
Perforating granuloma annulare is a rare subtype of granuloma annulare that presents with flesh- to red-colored papules that develop central crust or scale. Perforating granuloma annulare composes approximately 5% of granuloma annulare cases. Perforating granuloma annulare can develop on any region of the body but has an affinity for the extensor surfaces of the extremities. It most frequently occurs in young women and rarely presents as a single lesion.10 Granuloma annulare typically is not associated with joint pain, and thus it differs from most cases of RNs. Histologically, it presents with an inflammatory palisading granuloma. There may be overlying epidermal thinning or parakeratosis, which can progress to perforation and extrusion of necrobiotic material. In comparison with RN, perforating granuloma annulare displays mucin deposition in the necrobiotic zones in lieu of fibrin (Figure 2).10,11
Necrobiotic xanthogranuloma is a rare chronic form of non-Langerhans histiocytosis that characteristically presents with yellow or violaceous indurated plaques and nodules in a periorbital distribution. It often is associated with monoclonal gammopathy of IgG-κ. Lesions will ulcerate in 40% to 50% of patients.12 The mean age at presentation is in the sixth decade of life, and it is moderately predominant in females.13 Histopathology demonstrates palisading granulomatous formations with a lymphoplasmacytic infiltrate and zones of necrobiosis in the mid dermis extending into the panniculus. Characteristic histologic features that are variably present in necrobiotic xanthogranuloma but typically absent in RN include neutrophilic debris, cholesterol clefts, and Touton or foreign body giant cells (Figure 3).13
Necrobiosis lipoidica is a rare chronic granulomatous disease characterized by well-demarcated, atrophic, yellow-brown plaques on the pretibial surfaces. It typically presents in the third decade of life in women, and most cases are associated with diabetes mellitus types 1 or 2 or autoimmune conditions.14 Necrobiosis lipoidica begins as asymptomatic papules that enlarge progressively over months to years. They can become pruritic or painful and often develop ulceration. Histopathology shows horizontal zones of palisading histiocytes with intervening necrobiosis. An inflammatory infiltrate containing plasma cells also may be present (Figure 4).
- Horn RT Jr, Goette DK. Perforating rheumatoid nodule. Arch Dermatol. 1982;118:696-697.
- Tilstra JS, Lienesch DW. Rheumatoid nodules. Dermatol Clin. 2015;33:361-371. doi:10.1016/j.det.2015.03.004
- Kaye BR, Kaye RL, Bobrove A. Rheumatoid nodules. review of the spectrum of associated conditions and proposal of a new classification, with a report of four seronegative cases. Am J Med. 1984;76:279-292. doi:10.1016/0002-9343(84)90787-3
- Nyhäll-Wåhlin BM, Jacobsson LT, Petersson IF, et al; BARFOT study group. Smoking is a strong risk factor for rheumatoid nodules in early rheumatoid arthritis. Ann Rheum Dis. 2006;65:601-606. doi:10.1136/ard.2005.039172
- Turesson C, O’Fallon WM, Crowson CS, et al. Occurrence of extraarticular disease manifestations is associated with excess mortality in a community-based cohort of patients with rheumatoid arthritis. J Rheumatol. 2002;29:62-67.
- Bang S, Kim Y, Jang K, et al. Clinicopathologic features of rheumatoid nodules: a retrospective analysis. Clin Rheumatol. 2019;38:3041-3048. doi:10.1007/s10067-019-04668-1
- Chaganti S, Joshy S, Hariharan K, et al. Rheumatoid nodule presenting as Morton’s neuroma. J Orthop Traumatol. 2013;14:219-222. doi:10.1007/s10195-012-0215-x
- Sayah A, English JC 3rd. Rheumatoid arthritis: a review of the cutaneous manifestations. J Am Acad Dermatol. 2005;53:191-209; quiz 210-212. doi:10.1016/j.jaad.2004.07.023
- de Visscher SA, van Ginkel RJ, Wobbes T, et al. Epithelioid sarcoma: still an only surgically curable disease. Cancer. 2006;107:606-612. doi:10.1002/cncr.22037
- Penas PF, Jones-Caballero M, Fraga J, et al. Perforating granuloma annulare. Int J Dermatol. 1997;36:340-348. doi:10.1046 /j.1365-4362.1997.00047.x
- Gale M, Gilbert E, Blumenthal D. Isolated rheumatoid nodules: a diagnostic dilemma. Case Rep Med. 2015;2015:352352. doi:10.1155/2015/352352
- Wood AJ, Wagner MV, Abbott JJ, et al. Necrobiotic xanthogranuloma: a review of 17 cases with emphasis on clinical and pathologic correlation. Arch Dermatol. 2009;145:279-284. doi:10.1001 /archdermatol.2008.583
- Nelson CA, Zhong CS, Hashemi DA, et al. A multicenter crosssectional study and systematic review of necrobiotic xanthogranuloma with proposed diagnostic criteria. JAMA Dermatol. 2020;156:270-279. doi:10.1001/jamadermatol.2019.4221
- Sibbald C, Reid S, Alavi A. Necrobiosis lipoidica. Dermatol Clin. 2015;33:343-360. doi:10.1016/j.det.2015.03.003
The Diagnosis: Perforating Rheumatoid Nodule
Perforating rheumatoid nodule (RN) is a variant of RN that demonstrates necrobiotic material extruding through the epidermis via the process of transepidermal elimination.1 The necrobiotic material contains fibrin and often harbors karyorrhectic debris. The pathogenesis of RN remains unclear; possible mechanisms include a small vessel vasculitis or mechanical trauma inciting a localized aggregation of inflammatory products and rheumatoid factor complexes. This induces macrophage activation, fibrin deposition, and necrosis.2 The majority of patients with RNs have detectable rheumatoid factor and anticyclic citrullinated protein in the blood.3 Rheumatoid nodules are the most common cutaneous manifestations of rheumatoid arthritis (RA) and will develop in 30% to 40% of RA patients.4,5 They typically are associated with advanced RA but may precede the onset of clinically severe RA in 5% to 10% of patients.5 Rheumatoid nodules generally range in size from 2 mm to 5 cm and are slightly more prevalent in men than in women. They present as firm painless masses typically on the extensor surfaces of the hands and olecranon process but can occur over any tendinous or ligamentlike structure.6,7 Perforating RNs are most common on areas subjected to pressure or repeated trauma, such as the sacrum.
The diagnosis usually is clinical; however, in cases of diagnostic uncertainty, RN can be distinguished by its histologic appearance. Rheumatoid nodules demonstrate granulomatous palisading necrobiosis with a central zone of highly eosinophilic fibrinoid necrobiosis surrounded by palisading mononuclear cells and an outer zone of granulation tissue. There may be a mixed chronic inflammatory infiltrate predominantly composed of lymphocytes and histiocytes in the background.
Rheumatoid nodules typically do not require treatment; however, perforation is known to increase the risk for infection, and surgical excision generally is indicated for prophylaxis against infection, though nodules may recur in the excision area.1,3,8 Alternatively, disease-modifying antirheumatic drugs and intralesional corticosteroids may effectively reduce the size of RNs. The differential diagnosis for perforating RNs includes epithelioid sarcoma, perforating granuloma annulare, necrobiotic xanthogranuloma, and necrobiosis lipoidica.
Epithelioid sarcoma is a malignant soft tissue tumor typically found on the upper extremities of adolescent or young adult males. They usually present as hard tender nodules that commonly ulcerate. Epithelioid sarcoma makes up less than 1% of soft tissue sarcomas.9 Although rare, they present a diagnostic pitfall, as the histology may mimic an inflammatory palisaded granulomatous dermatitis similar to RN and granuloma annulare, thus a high index of suspicion is required to not overlook this aggressive malignancy. Histology is typified by nodular aggregates of epithelioid cells with abundant eosinophilic cytoplasm and often with central zones of necrosis (Figure 1). Epithelioid sarcoma displays immunoreactivity to cytokeratin, CD34, and epithelial membrane antigen, but loss of integrase interactor 1 expression. Cytologic abnormalities such as pleomorphism and hyperchromatism can be helpful in distinguishing between epithelioid sarcoma and RN.
Perforating granuloma annulare is a rare subtype of granuloma annulare that presents with flesh- to red-colored papules that develop central crust or scale. Perforating granuloma annulare composes approximately 5% of granuloma annulare cases. Perforating granuloma annulare can develop on any region of the body but has an affinity for the extensor surfaces of the extremities. It most frequently occurs in young women and rarely presents as a single lesion.10 Granuloma annulare typically is not associated with joint pain, and thus it differs from most cases of RNs. Histologically, it presents with an inflammatory palisading granuloma. There may be overlying epidermal thinning or parakeratosis, which can progress to perforation and extrusion of necrobiotic material. In comparison with RN, perforating granuloma annulare displays mucin deposition in the necrobiotic zones in lieu of fibrin (Figure 2).10,11
Necrobiotic xanthogranuloma is a rare chronic form of non-Langerhans histiocytosis that characteristically presents with yellow or violaceous indurated plaques and nodules in a periorbital distribution. It often is associated with monoclonal gammopathy of IgG-κ. Lesions will ulcerate in 40% to 50% of patients.12 The mean age at presentation is in the sixth decade of life, and it is moderately predominant in females.13 Histopathology demonstrates palisading granulomatous formations with a lymphoplasmacytic infiltrate and zones of necrobiosis in the mid dermis extending into the panniculus. Characteristic histologic features that are variably present in necrobiotic xanthogranuloma but typically absent in RN include neutrophilic debris, cholesterol clefts, and Touton or foreign body giant cells (Figure 3).13
Necrobiosis lipoidica is a rare chronic granulomatous disease characterized by well-demarcated, atrophic, yellow-brown plaques on the pretibial surfaces. It typically presents in the third decade of life in women, and most cases are associated with diabetes mellitus types 1 or 2 or autoimmune conditions.14 Necrobiosis lipoidica begins as asymptomatic papules that enlarge progressively over months to years. They can become pruritic or painful and often develop ulceration. Histopathology shows horizontal zones of palisading histiocytes with intervening necrobiosis. An inflammatory infiltrate containing plasma cells also may be present (Figure 4).
The Diagnosis: Perforating Rheumatoid Nodule
Perforating rheumatoid nodule (RN) is a variant of RN that demonstrates necrobiotic material extruding through the epidermis via the process of transepidermal elimination.1 The necrobiotic material contains fibrin and often harbors karyorrhectic debris. The pathogenesis of RN remains unclear; possible mechanisms include a small vessel vasculitis or mechanical trauma inciting a localized aggregation of inflammatory products and rheumatoid factor complexes. This induces macrophage activation, fibrin deposition, and necrosis.2 The majority of patients with RNs have detectable rheumatoid factor and anticyclic citrullinated protein in the blood.3 Rheumatoid nodules are the most common cutaneous manifestations of rheumatoid arthritis (RA) and will develop in 30% to 40% of RA patients.4,5 They typically are associated with advanced RA but may precede the onset of clinically severe RA in 5% to 10% of patients.5 Rheumatoid nodules generally range in size from 2 mm to 5 cm and are slightly more prevalent in men than in women. They present as firm painless masses typically on the extensor surfaces of the hands and olecranon process but can occur over any tendinous or ligamentlike structure.6,7 Perforating RNs are most common on areas subjected to pressure or repeated trauma, such as the sacrum.
The diagnosis usually is clinical; however, in cases of diagnostic uncertainty, RN can be distinguished by its histologic appearance. Rheumatoid nodules demonstrate granulomatous palisading necrobiosis with a central zone of highly eosinophilic fibrinoid necrobiosis surrounded by palisading mononuclear cells and an outer zone of granulation tissue. There may be a mixed chronic inflammatory infiltrate predominantly composed of lymphocytes and histiocytes in the background.
Rheumatoid nodules typically do not require treatment; however, perforation is known to increase the risk for infection, and surgical excision generally is indicated for prophylaxis against infection, though nodules may recur in the excision area.1,3,8 Alternatively, disease-modifying antirheumatic drugs and intralesional corticosteroids may effectively reduce the size of RNs. The differential diagnosis for perforating RNs includes epithelioid sarcoma, perforating granuloma annulare, necrobiotic xanthogranuloma, and necrobiosis lipoidica.
Epithelioid sarcoma is a malignant soft tissue tumor typically found on the upper extremities of adolescent or young adult males. They usually present as hard tender nodules that commonly ulcerate. Epithelioid sarcoma makes up less than 1% of soft tissue sarcomas.9 Although rare, they present a diagnostic pitfall, as the histology may mimic an inflammatory palisaded granulomatous dermatitis similar to RN and granuloma annulare, thus a high index of suspicion is required to not overlook this aggressive malignancy. Histology is typified by nodular aggregates of epithelioid cells with abundant eosinophilic cytoplasm and often with central zones of necrosis (Figure 1). Epithelioid sarcoma displays immunoreactivity to cytokeratin, CD34, and epithelial membrane antigen, but loss of integrase interactor 1 expression. Cytologic abnormalities such as pleomorphism and hyperchromatism can be helpful in distinguishing between epithelioid sarcoma and RN.
Perforating granuloma annulare is a rare subtype of granuloma annulare that presents with flesh- to red-colored papules that develop central crust or scale. Perforating granuloma annulare composes approximately 5% of granuloma annulare cases. Perforating granuloma annulare can develop on any region of the body but has an affinity for the extensor surfaces of the extremities. It most frequently occurs in young women and rarely presents as a single lesion.10 Granuloma annulare typically is not associated with joint pain, and thus it differs from most cases of RNs. Histologically, it presents with an inflammatory palisading granuloma. There may be overlying epidermal thinning or parakeratosis, which can progress to perforation and extrusion of necrobiotic material. In comparison with RN, perforating granuloma annulare displays mucin deposition in the necrobiotic zones in lieu of fibrin (Figure 2).10,11
Necrobiotic xanthogranuloma is a rare chronic form of non-Langerhans histiocytosis that characteristically presents with yellow or violaceous indurated plaques and nodules in a periorbital distribution. It often is associated with monoclonal gammopathy of IgG-κ. Lesions will ulcerate in 40% to 50% of patients.12 The mean age at presentation is in the sixth decade of life, and it is moderately predominant in females.13 Histopathology demonstrates palisading granulomatous formations with a lymphoplasmacytic infiltrate and zones of necrobiosis in the mid dermis extending into the panniculus. Characteristic histologic features that are variably present in necrobiotic xanthogranuloma but typically absent in RN include neutrophilic debris, cholesterol clefts, and Touton or foreign body giant cells (Figure 3).13
Necrobiosis lipoidica is a rare chronic granulomatous disease characterized by well-demarcated, atrophic, yellow-brown plaques on the pretibial surfaces. It typically presents in the third decade of life in women, and most cases are associated with diabetes mellitus types 1 or 2 or autoimmune conditions.14 Necrobiosis lipoidica begins as asymptomatic papules that enlarge progressively over months to years. They can become pruritic or painful and often develop ulceration. Histopathology shows horizontal zones of palisading histiocytes with intervening necrobiosis. An inflammatory infiltrate containing plasma cells also may be present (Figure 4).
- Horn RT Jr, Goette DK. Perforating rheumatoid nodule. Arch Dermatol. 1982;118:696-697.
- Tilstra JS, Lienesch DW. Rheumatoid nodules. Dermatol Clin. 2015;33:361-371. doi:10.1016/j.det.2015.03.004
- Kaye BR, Kaye RL, Bobrove A. Rheumatoid nodules. review of the spectrum of associated conditions and proposal of a new classification, with a report of four seronegative cases. Am J Med. 1984;76:279-292. doi:10.1016/0002-9343(84)90787-3
- Nyhäll-Wåhlin BM, Jacobsson LT, Petersson IF, et al; BARFOT study group. Smoking is a strong risk factor for rheumatoid nodules in early rheumatoid arthritis. Ann Rheum Dis. 2006;65:601-606. doi:10.1136/ard.2005.039172
- Turesson C, O’Fallon WM, Crowson CS, et al. Occurrence of extraarticular disease manifestations is associated with excess mortality in a community-based cohort of patients with rheumatoid arthritis. J Rheumatol. 2002;29:62-67.
- Bang S, Kim Y, Jang K, et al. Clinicopathologic features of rheumatoid nodules: a retrospective analysis. Clin Rheumatol. 2019;38:3041-3048. doi:10.1007/s10067-019-04668-1
- Chaganti S, Joshy S, Hariharan K, et al. Rheumatoid nodule presenting as Morton’s neuroma. J Orthop Traumatol. 2013;14:219-222. doi:10.1007/s10195-012-0215-x
- Sayah A, English JC 3rd. Rheumatoid arthritis: a review of the cutaneous manifestations. J Am Acad Dermatol. 2005;53:191-209; quiz 210-212. doi:10.1016/j.jaad.2004.07.023
- de Visscher SA, van Ginkel RJ, Wobbes T, et al. Epithelioid sarcoma: still an only surgically curable disease. Cancer. 2006;107:606-612. doi:10.1002/cncr.22037
- Penas PF, Jones-Caballero M, Fraga J, et al. Perforating granuloma annulare. Int J Dermatol. 1997;36:340-348. doi:10.1046 /j.1365-4362.1997.00047.x
- Gale M, Gilbert E, Blumenthal D. Isolated rheumatoid nodules: a diagnostic dilemma. Case Rep Med. 2015;2015:352352. doi:10.1155/2015/352352
- Wood AJ, Wagner MV, Abbott JJ, et al. Necrobiotic xanthogranuloma: a review of 17 cases with emphasis on clinical and pathologic correlation. Arch Dermatol. 2009;145:279-284. doi:10.1001 /archdermatol.2008.583
- Nelson CA, Zhong CS, Hashemi DA, et al. A multicenter crosssectional study and systematic review of necrobiotic xanthogranuloma with proposed diagnostic criteria. JAMA Dermatol. 2020;156:270-279. doi:10.1001/jamadermatol.2019.4221
- Sibbald C, Reid S, Alavi A. Necrobiosis lipoidica. Dermatol Clin. 2015;33:343-360. doi:10.1016/j.det.2015.03.003
- Horn RT Jr, Goette DK. Perforating rheumatoid nodule. Arch Dermatol. 1982;118:696-697.
- Tilstra JS, Lienesch DW. Rheumatoid nodules. Dermatol Clin. 2015;33:361-371. doi:10.1016/j.det.2015.03.004
- Kaye BR, Kaye RL, Bobrove A. Rheumatoid nodules. review of the spectrum of associated conditions and proposal of a new classification, with a report of four seronegative cases. Am J Med. 1984;76:279-292. doi:10.1016/0002-9343(84)90787-3
- Nyhäll-Wåhlin BM, Jacobsson LT, Petersson IF, et al; BARFOT study group. Smoking is a strong risk factor for rheumatoid nodules in early rheumatoid arthritis. Ann Rheum Dis. 2006;65:601-606. doi:10.1136/ard.2005.039172
- Turesson C, O’Fallon WM, Crowson CS, et al. Occurrence of extraarticular disease manifestations is associated with excess mortality in a community-based cohort of patients with rheumatoid arthritis. J Rheumatol. 2002;29:62-67.
- Bang S, Kim Y, Jang K, et al. Clinicopathologic features of rheumatoid nodules: a retrospective analysis. Clin Rheumatol. 2019;38:3041-3048. doi:10.1007/s10067-019-04668-1
- Chaganti S, Joshy S, Hariharan K, et al. Rheumatoid nodule presenting as Morton’s neuroma. J Orthop Traumatol. 2013;14:219-222. doi:10.1007/s10195-012-0215-x
- Sayah A, English JC 3rd. Rheumatoid arthritis: a review of the cutaneous manifestations. J Am Acad Dermatol. 2005;53:191-209; quiz 210-212. doi:10.1016/j.jaad.2004.07.023
- de Visscher SA, van Ginkel RJ, Wobbes T, et al. Epithelioid sarcoma: still an only surgically curable disease. Cancer. 2006;107:606-612. doi:10.1002/cncr.22037
- Penas PF, Jones-Caballero M, Fraga J, et al. Perforating granuloma annulare. Int J Dermatol. 1997;36:340-348. doi:10.1046 /j.1365-4362.1997.00047.x
- Gale M, Gilbert E, Blumenthal D. Isolated rheumatoid nodules: a diagnostic dilemma. Case Rep Med. 2015;2015:352352. doi:10.1155/2015/352352
- Wood AJ, Wagner MV, Abbott JJ, et al. Necrobiotic xanthogranuloma: a review of 17 cases with emphasis on clinical and pathologic correlation. Arch Dermatol. 2009;145:279-284. doi:10.1001 /archdermatol.2008.583
- Nelson CA, Zhong CS, Hashemi DA, et al. A multicenter crosssectional study and systematic review of necrobiotic xanthogranuloma with proposed diagnostic criteria. JAMA Dermatol. 2020;156:270-279. doi:10.1001/jamadermatol.2019.4221
- Sibbald C, Reid S, Alavi A. Necrobiosis lipoidica. Dermatol Clin. 2015;33:343-360. doi:10.1016/j.det.2015.03.003
A 59-year-old woman with a history of joint pain presented with a foot nodule that developed over the course of 2 years. Physical examination revealed a firm, mobile, mildly tender, 3-cm, deep red nodule on the dorsal aspect of the left foot (top [inset]) with an overlying central epidermal defect and thick keratinaceous debris. The remainder of the physical examination was unremarkable. Empiric treatments with oral antibiotics and intralesional corticosteroids were unsuccessful. Incisional biopsy was performed for histologic review, and tissue culture studies were negative.
Isolated Nodule and Generalized Lymphadenopathy
The Diagnosis: Blastic Plasmacytoid Dendritic Cell Neoplasm
A diagnosis of blastic plasmacytoid dendritic cell neoplasm (BPDCN) was rendered. Subsequent needle core biopsy of a left axillary lymph node as well as bone marrow aspiration and biopsy revealed a similar diffuse blastoid infiltrate with an identical immunophenotype to that in the skin biopsy from the pretibial mass and peripheral blood.
Previously known as blastic natural killer cell leukemia/lymphoma or agranular CD4+/CD56+ hematodermic neoplasm/tumor, BPDCN is a rare, clinically aggressive hematologic malignancy derived from the precursors of plasmacytoid dendritic cells. It often is diagnostically challenging, particularly when presenting at noncutaneous sites and in unusual (young) patient populations.1 It was included with other myeloid neoplasms in the 2008 World Health Organization classification; however, in the 2017 classification it was categorized as a separate entity. Blastic plasmacytoid dendritic cell neoplasm typically presents in the skin of elderly patients (age range at diagnosis, 61–67 years) with or without bone marrow involvement and systemic dissemination.1,2 The skin is the most common clinical site of disease in typical cases of BPDCN and often precedes bone marrow involvement. Thus, skin biopsy often is the key to making the diagnosis. Diagnosis of BPDCN may be delayed because of diagnostic pitfalls. Patients usually present with asymptomatic solitary or multiple lesions.3-5 Blastic plasmacytoid dendritic cell neoplasm can present as an isolated purplish nodule or bruiselike papule or more commonly as disseminated purplish nodules, papules, and macules. Isolated nodules are found on the head and lower limbs and can be more than 10 cm in diameter. Peripheral blood and bone marrow may be minimally involved at presentation but invariably become involved with the progression of disease. Cytopenia can occur at diagnosis and in a minority of severe cases indicates bone marrow failure.2-6
Skin involvement of BPDCN is thought to be secondary to the expression of skin migration molecules, such as cutaneous lymphocyte-associated antigen, one of the E-selectin ligands, which binds to E-selectin on high endothelial venules. In addition, the local dermal microenvironment of chemokines binding CXCR3, CXCR4, CCR6, or CCR7 present on neoplastic cells possibly leads to skin involvement. The full mechanism underlying the cutaneous tropism is still to be elucidated.4-7 Infiltration of the oral mucosa is seen in some patients, but it may be underreported. Mucosal disease typically appears similarly to cutaneous disease.
The cutaneous differential diagnosis for BPDCN depends on the clinical presentation, extent of disease spread, and thickness of infiltration. It includes common nonneoplastic diseases such as traumatic ecchymoses; purpuric disorders; extramedullary hematopoiesis; and soft-tissue neoplasms such as angiosarcoma, Kaposi sarcoma, neuroblastoma, and vascular metastases, as well as skin involvement by other hematologic neoplasms. An adequate incisional biopsy rather than a punch or shave biopsy is recommended for diagnosis. Dermatologists should alert the pathologist that BPDCN is in the clinical differential diagnosis when possible so that judicious use of appropriate immunophenotypic markers such as CD123, CD4, CD56, and T-cell leukemia/lymphoma protein 1 will avoid misdiagnosis of this aggressive condition, in addition to excluding acute myeloid leukemia, which also may express 3 of the above markers. However, most cases of acute myeloid leukemia lack terminal deoxynucleotidyl transferase (TdT) and express monocytic and other myeloid markers. Terminal deoxynucleotidyl transferase is positive in approximately one-third of cases of BPDCN, with expression in 10% to 80% of cells.1
It is important to include BPDCN in the differential diagnosis of immunophenotypically aberrant hematologic tumors. Diffuse large B-cell lymphoma, leg type, accounts for 4% of all primary cutaneous B-cell lymphomas.1 Compared with BPDCN, diffuse large B-cell lymphoma usually occurs in an older age group and is of B-cell lineage. Morphologically, these neoplasms are composed of a monotonous, diffuse, nonepidermotropic infiltrate of confluent sheets of centroblasts and immunoblasts (Figure 1). They may share immunohistochemical markers of CD79a, multiple myeloma 1, Bcl-2, and Bcl-6; however, they lack plasmacytoid dendritic cell (PDC)– associated antigens such as CD4, CD56, CD123, and T-cell leukemia/lymphoma protein 1.1
Adult T-cell leukemia/lymphoma is a neoplasm histologically composed of highly pleomorphic medium- to large-sized T cells with an irregular multilobated nuclear contour, so-called flower cells, in the peripheral blood. The nuclear chromatin is coarse and clumped with prominent nucleoli. Blastlike cells with dispersed chromatin are present in variable proportions. Most patients present with widespread lymph node and peripheral blood involvement. Skin is involved in more than half of patients with an epidermal as well as dermal pattern of infiltration (mainly perivascular)(Figure 2). Adult T-cell leukemia/lymphoma is endemic in several regions of the world, and the distribution is closely linked to the prevalence of human T-cell lymphotropic virus type 1 in the population. This neoplasm is of T-cell lineage and may share CD4 but not PDC-associated antigens with BPDCN.1
Cutaneous involvement by T-cell lymphoblastic leukemia/lymphoma (T-LBL) is a rare occurrence with a frequency of approximately 4.3%.8 T-cell lymphoblastic leukemia/lymphoma usually presents as multiple skin lesions throughout the body. Almost all cutaneous T-LBL cases are seen in association with bone marrow and/or mediastinal, lymph node, or extranodal involvement. Cutaneous T-LBLs present as a diffuse monomorphous infiltrate located in the entire dermis and subcutis without epidermotropism, composed of medium to large blasts with finely dispersed chromatin and relatively prominent nucleoli (Figure 3). Immunophenotyping studies show an immature T-cell immunophenotype, with expression of TdT (usually uniform), CD7, and cytoplasmic CD3 and an absence of PDC-associated antigens.8
Primary cutaneous γδ T-cell lymphoma (PCGDTL) is a neoplasm primarily involving the skin. Often rapidly fatal, PCGDTL has a broad clinical spectrum that may include indolent variants—subcutaneous, epidermotropic, and dermal. Patients typically present with nodular lesions that progress to ulceration and necrosis. Early lesions can be confused with erythema nodosum, mycosis fungoides, or infection. Histologically, they show variable epidermotropism as well as dermal and subcutaneous involvement by medium to large cells with coarse clumped chromatin (Figure 4). Large blastic cells with vesicular nuclei and prominent nucleoli are infrequent. In contrast to BPCDN, the neoplastic lymphocytes in dermal and subcutaneous PCGDTL typically are positive for T-cell intracellular antigen-1 and granzyme B with loss of CD4.9
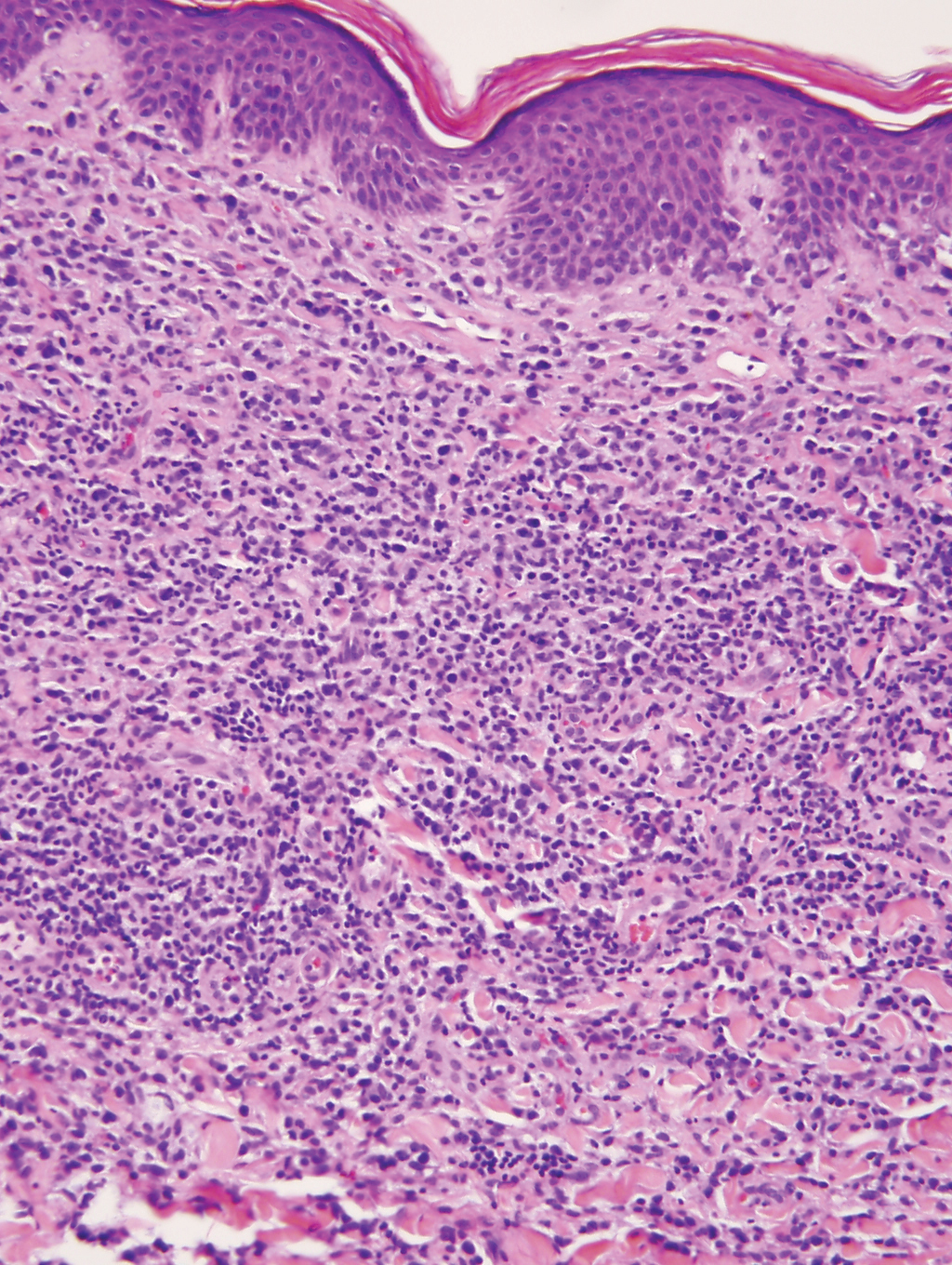
At the time of presentation, 27% to 87% of BPDCN patients will have bone marrow involvement, 22% to 28% will have blood involvement, and 6% to 41% will have lymph node involvement.1-4,6,7,10,11 The clinical course is aggressive, with a median survival of 10.0 to 19.8 months, irrespective of the initial pattern of disease.1 Most cases have shown initial response to multiagent chemotherapy, but relapses with subsequent resistance to drugs regularly have been observed. Age has an adverse impact of prognosis. Low TdT expression has been associated with shorter survival.1 Approximately 10% to 20% of cases of BPDCN are associated with or develop into chronic myelogenous leukemia, myelodysplastic syndrome, or acute myeloid leukemia.1,4 Pediatric patients have a greater 5-year overall survival rate than older patients, and overall survival worsens with increasing age. The extent of cutaneous involvement and presence of systemic involvement at initial presentation do not seem to be strong predictors of survival.1,2,5-7,10-12 In a retrospective analysis of 90 patients, Julia et al12 found that the type of skin disease did not predict survival. Specifically, the presence of nodular lesions and disseminated skin involvement were not adverse prognostic factors compared with macular lesions limited to 1 or 2 body areas.12
- Facchetti F, Petrella T, Pileri SA. Blastic plasmacytoid dendritic cells neoplasm. In: Swerdlow SH, Campo E, Harris NL, et al, eds. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. 4th ed. World Health Organization; 2017:174-177.
- Jegalian AG, Facchetti F, Jaffe ES. Plasmacytoid dendritic cells: physiologic roles and pathologic states. Adv Anat Pathol. 2009;16:392-404.
- Shi Y, Wang E. Blastic plasmacytoid dendritic cell neoplasm: a clinicopathologic review. Arch Pathol Lab Med. 2014;138:564-569.
- Khoury JD, Medeiros LJ, Manning JT, et al. CD56(+) TdT(+) blastic natural killer cell tumor of the skin: a primitive systemic malignancy related to myelomonocytic leukemia. Cancer. 2002;94:2401-2408.
- Kolerova A, Sergeeva I, Krinitsyna J, et al. Blastic plasmacytoid dendritic cell neoplasm: case report and literature overview. Indian J Dermatol. 2020;65:217-221.
- Hirner JP, O’Malley JT, LeBoeuf NR. Blastic plasmacytoid dendritic cell neoplasm: the dermatologist’s perspective. Hematol Oncol Clin North Am. 2020;34:501-509.
- Guiducii C, Tripodo C, Gong M, et al. Autoimmune skin inflammation is dependent on plasmacytoid dendritic cell activation by nucleic acids via TLR7 and TLR9. J Exp Med. 2010;207:2931-2942.
- Khurana S, Beltran M, Jiang L, et al. Primary cutaneous T-cell lymphoblastic lymphoma: case report and literature review. Case Rep Hematol. 2019;2019:3540487. doi:10.1155/2019/3540487
- Gladys TE, Helm MF, Anderson BE, et al. Rapid onset of widespread nodules and lymphadenopathy. Cutis. 2020;106:132, 153-155.
- Gregorio J, Meller S, Conrad C, et al. Plasmacytoid dendritic cells sense skin injury and promote wound healing through type I interferons. J Exp Med. 2010;207:2921-2930.
- Guru Murthy GS, Pemmaraju N, Attallah E. Epidemiology and survival of blastic plasmacytoid dendritic cell neoplasm. Leuk Res. 2018;73:21-23.
- Julia F, Petrella T, Beylot-Barry M, et al. Blastic plasmacytoid dendritic cell neoplasm: clinical features in 90 patients. Br J Dermatol. 2012;169:579-586.
The Diagnosis: Blastic Plasmacytoid Dendritic Cell Neoplasm
A diagnosis of blastic plasmacytoid dendritic cell neoplasm (BPDCN) was rendered. Subsequent needle core biopsy of a left axillary lymph node as well as bone marrow aspiration and biopsy revealed a similar diffuse blastoid infiltrate with an identical immunophenotype to that in the skin biopsy from the pretibial mass and peripheral blood.
Previously known as blastic natural killer cell leukemia/lymphoma or agranular CD4+/CD56+ hematodermic neoplasm/tumor, BPDCN is a rare, clinically aggressive hematologic malignancy derived from the precursors of plasmacytoid dendritic cells. It often is diagnostically challenging, particularly when presenting at noncutaneous sites and in unusual (young) patient populations.1 It was included with other myeloid neoplasms in the 2008 World Health Organization classification; however, in the 2017 classification it was categorized as a separate entity. Blastic plasmacytoid dendritic cell neoplasm typically presents in the skin of elderly patients (age range at diagnosis, 61–67 years) with or without bone marrow involvement and systemic dissemination.1,2 The skin is the most common clinical site of disease in typical cases of BPDCN and often precedes bone marrow involvement. Thus, skin biopsy often is the key to making the diagnosis. Diagnosis of BPDCN may be delayed because of diagnostic pitfalls. Patients usually present with asymptomatic solitary or multiple lesions.3-5 Blastic plasmacytoid dendritic cell neoplasm can present as an isolated purplish nodule or bruiselike papule or more commonly as disseminated purplish nodules, papules, and macules. Isolated nodules are found on the head and lower limbs and can be more than 10 cm in diameter. Peripheral blood and bone marrow may be minimally involved at presentation but invariably become involved with the progression of disease. Cytopenia can occur at diagnosis and in a minority of severe cases indicates bone marrow failure.2-6
Skin involvement of BPDCN is thought to be secondary to the expression of skin migration molecules, such as cutaneous lymphocyte-associated antigen, one of the E-selectin ligands, which binds to E-selectin on high endothelial venules. In addition, the local dermal microenvironment of chemokines binding CXCR3, CXCR4, CCR6, or CCR7 present on neoplastic cells possibly leads to skin involvement. The full mechanism underlying the cutaneous tropism is still to be elucidated.4-7 Infiltration of the oral mucosa is seen in some patients, but it may be underreported. Mucosal disease typically appears similarly to cutaneous disease.
The cutaneous differential diagnosis for BPDCN depends on the clinical presentation, extent of disease spread, and thickness of infiltration. It includes common nonneoplastic diseases such as traumatic ecchymoses; purpuric disorders; extramedullary hematopoiesis; and soft-tissue neoplasms such as angiosarcoma, Kaposi sarcoma, neuroblastoma, and vascular metastases, as well as skin involvement by other hematologic neoplasms. An adequate incisional biopsy rather than a punch or shave biopsy is recommended for diagnosis. Dermatologists should alert the pathologist that BPDCN is in the clinical differential diagnosis when possible so that judicious use of appropriate immunophenotypic markers such as CD123, CD4, CD56, and T-cell leukemia/lymphoma protein 1 will avoid misdiagnosis of this aggressive condition, in addition to excluding acute myeloid leukemia, which also may express 3 of the above markers. However, most cases of acute myeloid leukemia lack terminal deoxynucleotidyl transferase (TdT) and express monocytic and other myeloid markers. Terminal deoxynucleotidyl transferase is positive in approximately one-third of cases of BPDCN, with expression in 10% to 80% of cells.1
It is important to include BPDCN in the differential diagnosis of immunophenotypically aberrant hematologic tumors. Diffuse large B-cell lymphoma, leg type, accounts for 4% of all primary cutaneous B-cell lymphomas.1 Compared with BPDCN, diffuse large B-cell lymphoma usually occurs in an older age group and is of B-cell lineage. Morphologically, these neoplasms are composed of a monotonous, diffuse, nonepidermotropic infiltrate of confluent sheets of centroblasts and immunoblasts (Figure 1). They may share immunohistochemical markers of CD79a, multiple myeloma 1, Bcl-2, and Bcl-6; however, they lack plasmacytoid dendritic cell (PDC)– associated antigens such as CD4, CD56, CD123, and T-cell leukemia/lymphoma protein 1.1
Adult T-cell leukemia/lymphoma is a neoplasm histologically composed of highly pleomorphic medium- to large-sized T cells with an irregular multilobated nuclear contour, so-called flower cells, in the peripheral blood. The nuclear chromatin is coarse and clumped with prominent nucleoli. Blastlike cells with dispersed chromatin are present in variable proportions. Most patients present with widespread lymph node and peripheral blood involvement. Skin is involved in more than half of patients with an epidermal as well as dermal pattern of infiltration (mainly perivascular)(Figure 2). Adult T-cell leukemia/lymphoma is endemic in several regions of the world, and the distribution is closely linked to the prevalence of human T-cell lymphotropic virus type 1 in the population. This neoplasm is of T-cell lineage and may share CD4 but not PDC-associated antigens with BPDCN.1
Cutaneous involvement by T-cell lymphoblastic leukemia/lymphoma (T-LBL) is a rare occurrence with a frequency of approximately 4.3%.8 T-cell lymphoblastic leukemia/lymphoma usually presents as multiple skin lesions throughout the body. Almost all cutaneous T-LBL cases are seen in association with bone marrow and/or mediastinal, lymph node, or extranodal involvement. Cutaneous T-LBLs present as a diffuse monomorphous infiltrate located in the entire dermis and subcutis without epidermotropism, composed of medium to large blasts with finely dispersed chromatin and relatively prominent nucleoli (Figure 3). Immunophenotyping studies show an immature T-cell immunophenotype, with expression of TdT (usually uniform), CD7, and cytoplasmic CD3 and an absence of PDC-associated antigens.8
Primary cutaneous γδ T-cell lymphoma (PCGDTL) is a neoplasm primarily involving the skin. Often rapidly fatal, PCGDTL has a broad clinical spectrum that may include indolent variants—subcutaneous, epidermotropic, and dermal. Patients typically present with nodular lesions that progress to ulceration and necrosis. Early lesions can be confused with erythema nodosum, mycosis fungoides, or infection. Histologically, they show variable epidermotropism as well as dermal and subcutaneous involvement by medium to large cells with coarse clumped chromatin (Figure 4). Large blastic cells with vesicular nuclei and prominent nucleoli are infrequent. In contrast to BPCDN, the neoplastic lymphocytes in dermal and subcutaneous PCGDTL typically are positive for T-cell intracellular antigen-1 and granzyme B with loss of CD4.9

At the time of presentation, 27% to 87% of BPDCN patients will have bone marrow involvement, 22% to 28% will have blood involvement, and 6% to 41% will have lymph node involvement.1-4,6,7,10,11 The clinical course is aggressive, with a median survival of 10.0 to 19.8 months, irrespective of the initial pattern of disease.1 Most cases have shown initial response to multiagent chemotherapy, but relapses with subsequent resistance to drugs regularly have been observed. Age has an adverse impact of prognosis. Low TdT expression has been associated with shorter survival.1 Approximately 10% to 20% of cases of BPDCN are associated with or develop into chronic myelogenous leukemia, myelodysplastic syndrome, or acute myeloid leukemia.1,4 Pediatric patients have a greater 5-year overall survival rate than older patients, and overall survival worsens with increasing age. The extent of cutaneous involvement and presence of systemic involvement at initial presentation do not seem to be strong predictors of survival.1,2,5-7,10-12 In a retrospective analysis of 90 patients, Julia et al12 found that the type of skin disease did not predict survival. Specifically, the presence of nodular lesions and disseminated skin involvement were not adverse prognostic factors compared with macular lesions limited to 1 or 2 body areas.12
The Diagnosis: Blastic Plasmacytoid Dendritic Cell Neoplasm
A diagnosis of blastic plasmacytoid dendritic cell neoplasm (BPDCN) was rendered. Subsequent needle core biopsy of a left axillary lymph node as well as bone marrow aspiration and biopsy revealed a similar diffuse blastoid infiltrate with an identical immunophenotype to that in the skin biopsy from the pretibial mass and peripheral blood.
Previously known as blastic natural killer cell leukemia/lymphoma or agranular CD4+/CD56+ hematodermic neoplasm/tumor, BPDCN is a rare, clinically aggressive hematologic malignancy derived from the precursors of plasmacytoid dendritic cells. It often is diagnostically challenging, particularly when presenting at noncutaneous sites and in unusual (young) patient populations.1 It was included with other myeloid neoplasms in the 2008 World Health Organization classification; however, in the 2017 classification it was categorized as a separate entity. Blastic plasmacytoid dendritic cell neoplasm typically presents in the skin of elderly patients (age range at diagnosis, 61–67 years) with or without bone marrow involvement and systemic dissemination.1,2 The skin is the most common clinical site of disease in typical cases of BPDCN and often precedes bone marrow involvement. Thus, skin biopsy often is the key to making the diagnosis. Diagnosis of BPDCN may be delayed because of diagnostic pitfalls. Patients usually present with asymptomatic solitary or multiple lesions.3-5 Blastic plasmacytoid dendritic cell neoplasm can present as an isolated purplish nodule or bruiselike papule or more commonly as disseminated purplish nodules, papules, and macules. Isolated nodules are found on the head and lower limbs and can be more than 10 cm in diameter. Peripheral blood and bone marrow may be minimally involved at presentation but invariably become involved with the progression of disease. Cytopenia can occur at diagnosis and in a minority of severe cases indicates bone marrow failure.2-6
Skin involvement of BPDCN is thought to be secondary to the expression of skin migration molecules, such as cutaneous lymphocyte-associated antigen, one of the E-selectin ligands, which binds to E-selectin on high endothelial venules. In addition, the local dermal microenvironment of chemokines binding CXCR3, CXCR4, CCR6, or CCR7 present on neoplastic cells possibly leads to skin involvement. The full mechanism underlying the cutaneous tropism is still to be elucidated.4-7 Infiltration of the oral mucosa is seen in some patients, but it may be underreported. Mucosal disease typically appears similarly to cutaneous disease.
The cutaneous differential diagnosis for BPDCN depends on the clinical presentation, extent of disease spread, and thickness of infiltration. It includes common nonneoplastic diseases such as traumatic ecchymoses; purpuric disorders; extramedullary hematopoiesis; and soft-tissue neoplasms such as angiosarcoma, Kaposi sarcoma, neuroblastoma, and vascular metastases, as well as skin involvement by other hematologic neoplasms. An adequate incisional biopsy rather than a punch or shave biopsy is recommended for diagnosis. Dermatologists should alert the pathologist that BPDCN is in the clinical differential diagnosis when possible so that judicious use of appropriate immunophenotypic markers such as CD123, CD4, CD56, and T-cell leukemia/lymphoma protein 1 will avoid misdiagnosis of this aggressive condition, in addition to excluding acute myeloid leukemia, which also may express 3 of the above markers. However, most cases of acute myeloid leukemia lack terminal deoxynucleotidyl transferase (TdT) and express monocytic and other myeloid markers. Terminal deoxynucleotidyl transferase is positive in approximately one-third of cases of BPDCN, with expression in 10% to 80% of cells.1
It is important to include BPDCN in the differential diagnosis of immunophenotypically aberrant hematologic tumors. Diffuse large B-cell lymphoma, leg type, accounts for 4% of all primary cutaneous B-cell lymphomas.1 Compared with BPDCN, diffuse large B-cell lymphoma usually occurs in an older age group and is of B-cell lineage. Morphologically, these neoplasms are composed of a monotonous, diffuse, nonepidermotropic infiltrate of confluent sheets of centroblasts and immunoblasts (Figure 1). They may share immunohistochemical markers of CD79a, multiple myeloma 1, Bcl-2, and Bcl-6; however, they lack plasmacytoid dendritic cell (PDC)– associated antigens such as CD4, CD56, CD123, and T-cell leukemia/lymphoma protein 1.1
Adult T-cell leukemia/lymphoma is a neoplasm histologically composed of highly pleomorphic medium- to large-sized T cells with an irregular multilobated nuclear contour, so-called flower cells, in the peripheral blood. The nuclear chromatin is coarse and clumped with prominent nucleoli. Blastlike cells with dispersed chromatin are present in variable proportions. Most patients present with widespread lymph node and peripheral blood involvement. Skin is involved in more than half of patients with an epidermal as well as dermal pattern of infiltration (mainly perivascular)(Figure 2). Adult T-cell leukemia/lymphoma is endemic in several regions of the world, and the distribution is closely linked to the prevalence of human T-cell lymphotropic virus type 1 in the population. This neoplasm is of T-cell lineage and may share CD4 but not PDC-associated antigens with BPDCN.1
Cutaneous involvement by T-cell lymphoblastic leukemia/lymphoma (T-LBL) is a rare occurrence with a frequency of approximately 4.3%.8 T-cell lymphoblastic leukemia/lymphoma usually presents as multiple skin lesions throughout the body. Almost all cutaneous T-LBL cases are seen in association with bone marrow and/or mediastinal, lymph node, or extranodal involvement. Cutaneous T-LBLs present as a diffuse monomorphous infiltrate located in the entire dermis and subcutis without epidermotropism, composed of medium to large blasts with finely dispersed chromatin and relatively prominent nucleoli (Figure 3). Immunophenotyping studies show an immature T-cell immunophenotype, with expression of TdT (usually uniform), CD7, and cytoplasmic CD3 and an absence of PDC-associated antigens.8
Primary cutaneous γδ T-cell lymphoma (PCGDTL) is a neoplasm primarily involving the skin. Often rapidly fatal, PCGDTL has a broad clinical spectrum that may include indolent variants—subcutaneous, epidermotropic, and dermal. Patients typically present with nodular lesions that progress to ulceration and necrosis. Early lesions can be confused with erythema nodosum, mycosis fungoides, or infection. Histologically, they show variable epidermotropism as well as dermal and subcutaneous involvement by medium to large cells with coarse clumped chromatin (Figure 4). Large blastic cells with vesicular nuclei and prominent nucleoli are infrequent. In contrast to BPCDN, the neoplastic lymphocytes in dermal and subcutaneous PCGDTL typically are positive for T-cell intracellular antigen-1 and granzyme B with loss of CD4.9

At the time of presentation, 27% to 87% of BPDCN patients will have bone marrow involvement, 22% to 28% will have blood involvement, and 6% to 41% will have lymph node involvement.1-4,6,7,10,11 The clinical course is aggressive, with a median survival of 10.0 to 19.8 months, irrespective of the initial pattern of disease.1 Most cases have shown initial response to multiagent chemotherapy, but relapses with subsequent resistance to drugs regularly have been observed. Age has an adverse impact of prognosis. Low TdT expression has been associated with shorter survival.1 Approximately 10% to 20% of cases of BPDCN are associated with or develop into chronic myelogenous leukemia, myelodysplastic syndrome, or acute myeloid leukemia.1,4 Pediatric patients have a greater 5-year overall survival rate than older patients, and overall survival worsens with increasing age. The extent of cutaneous involvement and presence of systemic involvement at initial presentation do not seem to be strong predictors of survival.1,2,5-7,10-12 In a retrospective analysis of 90 patients, Julia et al12 found that the type of skin disease did not predict survival. Specifically, the presence of nodular lesions and disseminated skin involvement were not adverse prognostic factors compared with macular lesions limited to 1 or 2 body areas.12
- Facchetti F, Petrella T, Pileri SA. Blastic plasmacytoid dendritic cells neoplasm. In: Swerdlow SH, Campo E, Harris NL, et al, eds. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. 4th ed. World Health Organization; 2017:174-177.
- Jegalian AG, Facchetti F, Jaffe ES. Plasmacytoid dendritic cells: physiologic roles and pathologic states. Adv Anat Pathol. 2009;16:392-404.
- Shi Y, Wang E. Blastic plasmacytoid dendritic cell neoplasm: a clinicopathologic review. Arch Pathol Lab Med. 2014;138:564-569.
- Khoury JD, Medeiros LJ, Manning JT, et al. CD56(+) TdT(+) blastic natural killer cell tumor of the skin: a primitive systemic malignancy related to myelomonocytic leukemia. Cancer. 2002;94:2401-2408.
- Kolerova A, Sergeeva I, Krinitsyna J, et al. Blastic plasmacytoid dendritic cell neoplasm: case report and literature overview. Indian J Dermatol. 2020;65:217-221.
- Hirner JP, O’Malley JT, LeBoeuf NR. Blastic plasmacytoid dendritic cell neoplasm: the dermatologist’s perspective. Hematol Oncol Clin North Am. 2020;34:501-509.
- Guiducii C, Tripodo C, Gong M, et al. Autoimmune skin inflammation is dependent on plasmacytoid dendritic cell activation by nucleic acids via TLR7 and TLR9. J Exp Med. 2010;207:2931-2942.
- Khurana S, Beltran M, Jiang L, et al. Primary cutaneous T-cell lymphoblastic lymphoma: case report and literature review. Case Rep Hematol. 2019;2019:3540487. doi:10.1155/2019/3540487
- Gladys TE, Helm MF, Anderson BE, et al. Rapid onset of widespread nodules and lymphadenopathy. Cutis. 2020;106:132, 153-155.
- Gregorio J, Meller S, Conrad C, et al. Plasmacytoid dendritic cells sense skin injury and promote wound healing through type I interferons. J Exp Med. 2010;207:2921-2930.
- Guru Murthy GS, Pemmaraju N, Attallah E. Epidemiology and survival of blastic plasmacytoid dendritic cell neoplasm. Leuk Res. 2018;73:21-23.
- Julia F, Petrella T, Beylot-Barry M, et al. Blastic plasmacytoid dendritic cell neoplasm: clinical features in 90 patients. Br J Dermatol. 2012;169:579-586.
- Facchetti F, Petrella T, Pileri SA. Blastic plasmacytoid dendritic cells neoplasm. In: Swerdlow SH, Campo E, Harris NL, et al, eds. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. 4th ed. World Health Organization; 2017:174-177.
- Jegalian AG, Facchetti F, Jaffe ES. Plasmacytoid dendritic cells: physiologic roles and pathologic states. Adv Anat Pathol. 2009;16:392-404.
- Shi Y, Wang E. Blastic plasmacytoid dendritic cell neoplasm: a clinicopathologic review. Arch Pathol Lab Med. 2014;138:564-569.
- Khoury JD, Medeiros LJ, Manning JT, et al. CD56(+) TdT(+) blastic natural killer cell tumor of the skin: a primitive systemic malignancy related to myelomonocytic leukemia. Cancer. 2002;94:2401-2408.
- Kolerova A, Sergeeva I, Krinitsyna J, et al. Blastic plasmacytoid dendritic cell neoplasm: case report and literature overview. Indian J Dermatol. 2020;65:217-221.
- Hirner JP, O’Malley JT, LeBoeuf NR. Blastic plasmacytoid dendritic cell neoplasm: the dermatologist’s perspective. Hematol Oncol Clin North Am. 2020;34:501-509.
- Guiducii C, Tripodo C, Gong M, et al. Autoimmune skin inflammation is dependent on plasmacytoid dendritic cell activation by nucleic acids via TLR7 and TLR9. J Exp Med. 2010;207:2931-2942.
- Khurana S, Beltran M, Jiang L, et al. Primary cutaneous T-cell lymphoblastic lymphoma: case report and literature review. Case Rep Hematol. 2019;2019:3540487. doi:10.1155/2019/3540487
- Gladys TE, Helm MF, Anderson BE, et al. Rapid onset of widespread nodules and lymphadenopathy. Cutis. 2020;106:132, 153-155.
- Gregorio J, Meller S, Conrad C, et al. Plasmacytoid dendritic cells sense skin injury and promote wound healing through type I interferons. J Exp Med. 2010;207:2921-2930.
- Guru Murthy GS, Pemmaraju N, Attallah E. Epidemiology and survival of blastic plasmacytoid dendritic cell neoplasm. Leuk Res. 2018;73:21-23.
- Julia F, Petrella T, Beylot-Barry M, et al. Blastic plasmacytoid dendritic cell neoplasm: clinical features in 90 patients. Br J Dermatol. 2012;169:579-586.
A 23-year-old man presented with skin that bruised easily, pancytopenia, recent fatigue, fever, and loss of appetite, along with a nontender, brown-purple, left anterior pretibial mass of 2 years’ duration (top). Computed tomography showed diffuse lymphadenopathy involving the inguinal, mesenteric, retroperitoneal, mediastinal, and axillary regions. A biopsy of the mass showed a dense monomorphous infiltrate of medium-sized blastoid cells with small or inconspicuous nucleoli (bottom). The lesion diffusely involved the dermis and extended into the subcutaneous tissue but spared the epidermis. Flow cytometry immunophenotyping of peripheral blood neoplastic cells (bottom [inset]) showed high-level expression of CD123 together with expression of CD4, CD56, CD45RA, and CD43 but a lack of expression of any other myelomonocytic or lymphoid lineage–associated markers.
Light Brown and Pink Macule on the Upper Arm
The Diagnosis: Desmoplastic Spitz Nevus
Desmoplastic Spitz nevus is a rare variant of Spitz nevus that commonly presents as a red to brown papule on the head, neck, or extremities. It is pertinent to review the histologic features of this neoplasm, as it can be confused with other more sinister entities such as spitzoid melanoma. Histologically, there is a dermal infiltrate of melanocytes containing eosinophilic cytoplasm and vesicular nuclei. Junctional involvement is rare, and there should be no pagetoid spread.1 This entity features abundant stromal fibrosis formed by dense collagen bundles, low cellular density, and polygonal-shaped melanocytes, which helps to differentiate it from melanoma.2,3 In a retrospective study comparing the characteristics of desmoplastic Spitz nevi with desmoplastic melanoma, desmoplastic Spitz nevi histologically were more symmetric and circumscribed with greater melanocytic maturation and adnexal structure involvement.3 Although this entity demonstrates maturation from the superficial to the deep dermis, it also may feature deep dermal vascular proliferation.4 S-100 and SRY-related HMG box 10, SOX-10, are noted to be positive in desmoplastic Spitz nevi, which can help to differentiate it from nonmelanocytic entities (Figure 1).
Although spitzoid lesions can be ambiguous and difficult even for experts to classify, spitzoid melanoma tends to have a high Breslow thickness, high cell density, marked atypia, and an increased nucleus to cytoplasm ratio.5 Additionally, desmoplastic melanoma was found to more often display “melanocytic junctional nests associated with discohesive cells, variations in size and shape of the nests, lentiginous melanocytic proliferation, actinic elastosis, pagetoid spread, dermal mitosis, perineural involvement and brisk inflammatory infiltrate.”3 Given the challenge of histologically separating desmoplastic Spitz nevi from melanoma, immunostaining can be useful. For example, Hilliard et al6 used a p16 antibody to differentiate desmoplastic Spitz nevi from desmoplastic melanoma, finding that most desmoplastic melanomas (81.8%; n=11) were negative for p16, whereas all desmoplastic Spitz nevi were at least moderately positive. However, another study re-evaluated the utility of p16 in desmoplastic melanoma and found that 72.7% (16/22) were at least focally reactive for the immunostain.7 Thus, caution must be exercised when using p16.
PReferentially expressed Antigen in MElanoma (PRAME) is a newer nuclear immunohistochemical marker that tends to be positive in melanomas and negative in nevi. Desmoplastic Spitz nevi would be expected to be negative for PRAME, while desmoplastic melanoma may be positive; however, this marker seems to be less effective in desmoplastic melanoma than in most other subtypes of the malignancy. In one study, only 35% (n=20) of desmoplastic melanomas were positive for PRAME.8 Likewise, another study showed that some benign Spitz nevi may diffusely express PRAME.9 As such, PRAME should be used prudently.
For cases in which immunohistochemistry is equivocal, molecular testing may aid in differentiating Spitz nevi from melanoma. For example, comparative genomic hybridization has revealed an increased copy number of chromosome 11p in approximately 20% of Spitz nevi cases10; this finding is not seen in melanoma. Mutation analyses of HRas proto-oncogene, GTPase, HRAS; B-Raf proto-oncogene, serine/threonine kinase, BRAF; and NRAS proto-oncogene, GTPase, NRAS, also have shown some promise in distinguishing spitzoid lesions from melanoma, but these analyses may be oversimplified.11 Fluorescence in situ hybridization (FISH) is another diagnostic modality that has been studied to differentiate benign nevi from melanoma. One study challenged the utility of FISH, reporting 7 of 15 desmoplastic melanomas tested positive compared to 0 of 15 sclerotic melanocytic nevi.12 Thus, negative FISH cannot reliably rule out melanoma. Ultimately, a combination of immunostains along with FISH or another genetic study would prove to be most effective in ruling out melanoma in difficult cases. Even then, a dermatopathologist may be faced with a degree of uncertainty.
Cellular blue nevi predominantly affect adults younger than 40 years and commonly are seen on the buttocks.13 This benign neoplasm demonstrates areas that are distinctly sclerotic as well as those that are cellular in nature.14 This entity demonstrates a well-circumscribed dermal growth pattern with 2 main populations of cells. The sclerotic portion of the cellular blue nevus mimics that of the blue nevus in that it is noted superficially with irregular margins. The cellular aspect of the nevus features spindle cells contained within well-circumscribed nodules (Figure 2). Stromal melanophages are not uncommon, and some can be observed adjacent to nerve fibers. Although this blue nevus variant displays features of the common blue nevus, its melanocytes track along adnexal and neurovascular structures similar to the deep penetrating nevus and the desmoplastic Spitz nevus. However, these melanocytes are variable in morphology and can appear on a spectrum spanning from pale and lightly pigmented to clear.15
The breast is the most common site of origin of tumor metastasis to the skin. These cutaneous metastases can vary in both their clinical and histological presentations. For example, cutaneous metastatic breast adenocarcinoma often can present clinically as pink-violaceous papules and plaques on the breast or on other parts of the body. Histologically, it can demonstrate a varying degree of patterns such as collagen infiltration by single cells, cords, tubules, and sheets of atypical cells (Figure 3) that can be observed together in areas of mucin or can form glandular structures.16 Metastatic breast carcinoma is noted to be positive for gross cystic disease fluid protein-15, estrogen receptor, and cytokeratin 7, which can help differentiate this entity from other tumors of glandular origin.16 Although rare, primary melanoma of the breast has been reported in the literature.17,18 These malignant melanocytic lesions easily could be differentiated from other breast tumors such as adenocarcinoma using immunohistochemical staining patterns.
Deep penetrating nevi most often are observed clinically as blue, brown, or black papules or nodules on the head or neck.19 Histologically, this lesion features a wedge-shaped infiltrate of deep dermal melanocytes with oval nuclei. It commonly extends to the reticular dermis or further into the subcutis (Figure 4).20,21 This neoplasm frequently tracks along adnexal and neurovascular structures, resulting in a plexiform appearance.22 The adnexal involvement of deep penetrating nevi is a shared feature with desmoplastic Spitz nevi. The presence of any number of melanophages is characteristic of this lesion.23 Lastly, there is a well-documented association between β-catenin mutations and deep penetrating nevi.24 Multicentric reticulohistiocytosis (MRH) is a rare form of non-Langerhans cell histiocytosis that has the pathognomonic clinical finding of pink-red papules (coral beading) with a predilection for acral surfaces. Histology of affected skin reveals a dermal infiltrate of ground glass as well as eosinophilic histiocytes that most often stain positive for CD68 and human alveolar macrophage 56 but negative for S-100 and CD1a (Figure 5).25 Although MRH is rare, negative staining for S-100 could serve as a useful diagnostic clue to differentiate it from other entities that are positive for S-100, such as the desmoplastic Spitz nevus. Arthritis mutilans is a potential complication of MRH, but a reported association with an underlying malignancy is seen in approximately 25% of cases.26 Thus, the cutaneous, rheumatologic, and oncologic implications of this disease help to distinguish it from other differential diagnoses that may be considered.
- Luzar B, Bastian BC, North JP, et al. Melanocytic nevi. In: Calonje E, Brenn T, Lazar AJ, et al, eds. McKee’s Pathology of the Skin. 5th ed. Elsevier; 2020:1275-1280.
- Busam KJ, Gerami P. Spitz nevi. In: Busam KJ, Gerami P, Scolyer RA, eds. Pathology of Melanocytic Tumors. Elsevier; 2019:37-60.
- Nojavan H, Cribier B, Mehregan DR. Desmoplastic Spitz nevus: a histopathological review and comparison with desmoplastic melanoma [in French]. Ann Dermatol Venereol. 2009;136:689-695.
- Tomizawa K. Desmoplastic Spitz nevus showing vascular proliferation more prominently in the deep portion. Am J Dermatopathol. 2002;24:184-185.
- Requena C, Botella R, Nagore E, et al. Characteristics of spitzoid melanoma and clues for differential diagnosis with Spitz nevus. Am J Dermatopathol. 2012;34:478-486.
- Hilliard NJ, Krahl D, Sellheyer K. p16 expression differentiates between desmoplastic Spitz nevus and desmoplastic melanoma. J Cutan Pathol. 2009;36:753-759.
- Blokhin E, Pulitzer M, Busam KJ. Immunohistochemical expression of p16 in desmoplastic melanoma. J Cutan Pathol. 2013;40:796-800.
- Lezcano C, Jungbluth AA, Nehal KS, et al. PRAME expression in melanocytic tumors. Am J Surg Pathol. 2018;42:1456-1465.
- Raghavan SS, Wang JY, Kwok S, et al. PRAME expression in melanocytic proliferations with intermediate histopathologic or spitzoid features. J Cutan Pathol. 2020;47:1123-1131.
- Bauer J, Bastian BC. DNA copy number changes in the diagnosis of melanocytic tumors [in German]. Pathologe. 2007;28:464-473.
- Luo S, Sepehr A, Tsao H. Spitz nevi and other spitzoid lesions part I. background and diagnoses. J Am Acad Dermatol. 2011;65:1073-1084.
- Gerami P, Beilfuss B, Haghighat Z, et al. Fluorescence in situ hybridization as an ancillary method for the distinction of desmoplastic melanomas from sclerosing melanocytic nevi. J Cutan Pathol. 2011;38:329-334.
- Zembowicz A. Blue nevi and related tumors. Clin Lab Med. 2017; 37:401-415.
- Rodriguez HA, Ackerman LV. Cellular blue nevus. clinicopathologic study of forty-five cases. Cancer. 1968;21:393-405.
- Phadke PA, Zembowicz A. Blue nevi and related tumors. Clin Lab Med. 2011;31:345-358.
- Ko CJ. Metastatic tumors and simulators. In: Elston DM, Ferringer T, eds. Dermatopathology. 3rd ed. Elsevier Limited; 2019:496-504.
- Drueppel D, Schultheis B, Solass W, et al. Primary malignant melanoma of the breast: case report and review of the literature. Anticancer Res. 2015;35:1709-1713.
- Kurul S, Tas¸ F, Büyükbabani N, et al. Different manifestations of malignant melanoma in the breast: a report of 12 cases and a review of the literature. Jpn J Clin Oncol. 2005;35:202-206.
- Strazzula L, Senna MM, Yasuda M, et al. The deep penetrating nevus. J Am Acad Dermatol. 2014;71:1234-1240.
- Mehregan DA, Mehregan AH. Deep penetrating nevus. Arch Dermatol. 1993;129:328-331.
- Robson A, Morley-Quante M, Hempel H, et al. Deep penetrating naevus: clinicopathological study of 31 cases with further delineation of histological features allowing distinction from other pigmented benign melanocytic lesions and melanoma. Histopathology. 2003;43:529-537.
- Luzar B, Calonje E. Deep penetrating nevus: a review. Arch Pathol Lab Med. 2011;135:321-326.
- Cooper PH. Deep penetrating (plexiform spindle cell) nevus. a frequent participant in combined nevus. J Cutan Pathol. 1992;19:172-180.
- de la Fouchardière A, Caillot C, Jacquemus J, et al. β-Catenin nuclear expression discriminates deep penetrating nevi from other cutaneous melanocytic tumors. Virchows Arch. 2019;474:539-550.
- Gorman JD, Danning C, Schumacher HR, et al. Multicentric reticulohistiocytosis: case report with immunohistochemical analysis and literature review. Arthritis Rheum. 2000;43:930-938.
- Selmi C, Greenspan A, Huntley A, et al. Multicentric reticulohistiocytosis: a critical review. Curr Rheumatol Rep. 2015;17:511.
The Diagnosis: Desmoplastic Spitz Nevus
Desmoplastic Spitz nevus is a rare variant of Spitz nevus that commonly presents as a red to brown papule on the head, neck, or extremities. It is pertinent to review the histologic features of this neoplasm, as it can be confused with other more sinister entities such as spitzoid melanoma. Histologically, there is a dermal infiltrate of melanocytes containing eosinophilic cytoplasm and vesicular nuclei. Junctional involvement is rare, and there should be no pagetoid spread.1 This entity features abundant stromal fibrosis formed by dense collagen bundles, low cellular density, and polygonal-shaped melanocytes, which helps to differentiate it from melanoma.2,3 In a retrospective study comparing the characteristics of desmoplastic Spitz nevi with desmoplastic melanoma, desmoplastic Spitz nevi histologically were more symmetric and circumscribed with greater melanocytic maturation and adnexal structure involvement.3 Although this entity demonstrates maturation from the superficial to the deep dermis, it also may feature deep dermal vascular proliferation.4 S-100 and SRY-related HMG box 10, SOX-10, are noted to be positive in desmoplastic Spitz nevi, which can help to differentiate it from nonmelanocytic entities (Figure 1).
Although spitzoid lesions can be ambiguous and difficult even for experts to classify, spitzoid melanoma tends to have a high Breslow thickness, high cell density, marked atypia, and an increased nucleus to cytoplasm ratio.5 Additionally, desmoplastic melanoma was found to more often display “melanocytic junctional nests associated with discohesive cells, variations in size and shape of the nests, lentiginous melanocytic proliferation, actinic elastosis, pagetoid spread, dermal mitosis, perineural involvement and brisk inflammatory infiltrate.”3 Given the challenge of histologically separating desmoplastic Spitz nevi from melanoma, immunostaining can be useful. For example, Hilliard et al6 used a p16 antibody to differentiate desmoplastic Spitz nevi from desmoplastic melanoma, finding that most desmoplastic melanomas (81.8%; n=11) were negative for p16, whereas all desmoplastic Spitz nevi were at least moderately positive. However, another study re-evaluated the utility of p16 in desmoplastic melanoma and found that 72.7% (16/22) were at least focally reactive for the immunostain.7 Thus, caution must be exercised when using p16.
PReferentially expressed Antigen in MElanoma (PRAME) is a newer nuclear immunohistochemical marker that tends to be positive in melanomas and negative in nevi. Desmoplastic Spitz nevi would be expected to be negative for PRAME, while desmoplastic melanoma may be positive; however, this marker seems to be less effective in desmoplastic melanoma than in most other subtypes of the malignancy. In one study, only 35% (n=20) of desmoplastic melanomas were positive for PRAME.8 Likewise, another study showed that some benign Spitz nevi may diffusely express PRAME.9 As such, PRAME should be used prudently.
For cases in which immunohistochemistry is equivocal, molecular testing may aid in differentiating Spitz nevi from melanoma. For example, comparative genomic hybridization has revealed an increased copy number of chromosome 11p in approximately 20% of Spitz nevi cases10; this finding is not seen in melanoma. Mutation analyses of HRas proto-oncogene, GTPase, HRAS; B-Raf proto-oncogene, serine/threonine kinase, BRAF; and NRAS proto-oncogene, GTPase, NRAS, also have shown some promise in distinguishing spitzoid lesions from melanoma, but these analyses may be oversimplified.11 Fluorescence in situ hybridization (FISH) is another diagnostic modality that has been studied to differentiate benign nevi from melanoma. One study challenged the utility of FISH, reporting 7 of 15 desmoplastic melanomas tested positive compared to 0 of 15 sclerotic melanocytic nevi.12 Thus, negative FISH cannot reliably rule out melanoma. Ultimately, a combination of immunostains along with FISH or another genetic study would prove to be most effective in ruling out melanoma in difficult cases. Even then, a dermatopathologist may be faced with a degree of uncertainty.
Cellular blue nevi predominantly affect adults younger than 40 years and commonly are seen on the buttocks.13 This benign neoplasm demonstrates areas that are distinctly sclerotic as well as those that are cellular in nature.14 This entity demonstrates a well-circumscribed dermal growth pattern with 2 main populations of cells. The sclerotic portion of the cellular blue nevus mimics that of the blue nevus in that it is noted superficially with irregular margins. The cellular aspect of the nevus features spindle cells contained within well-circumscribed nodules (Figure 2). Stromal melanophages are not uncommon, and some can be observed adjacent to nerve fibers. Although this blue nevus variant displays features of the common blue nevus, its melanocytes track along adnexal and neurovascular structures similar to the deep penetrating nevus and the desmoplastic Spitz nevus. However, these melanocytes are variable in morphology and can appear on a spectrum spanning from pale and lightly pigmented to clear.15
The breast is the most common site of origin of tumor metastasis to the skin. These cutaneous metastases can vary in both their clinical and histological presentations. For example, cutaneous metastatic breast adenocarcinoma often can present clinically as pink-violaceous papules and plaques on the breast or on other parts of the body. Histologically, it can demonstrate a varying degree of patterns such as collagen infiltration by single cells, cords, tubules, and sheets of atypical cells (Figure 3) that can be observed together in areas of mucin or can form glandular structures.16 Metastatic breast carcinoma is noted to be positive for gross cystic disease fluid protein-15, estrogen receptor, and cytokeratin 7, which can help differentiate this entity from other tumors of glandular origin.16 Although rare, primary melanoma of the breast has been reported in the literature.17,18 These malignant melanocytic lesions easily could be differentiated from other breast tumors such as adenocarcinoma using immunohistochemical staining patterns.
Deep penetrating nevi most often are observed clinically as blue, brown, or black papules or nodules on the head or neck.19 Histologically, this lesion features a wedge-shaped infiltrate of deep dermal melanocytes with oval nuclei. It commonly extends to the reticular dermis or further into the subcutis (Figure 4).20,21 This neoplasm frequently tracks along adnexal and neurovascular structures, resulting in a plexiform appearance.22 The adnexal involvement of deep penetrating nevi is a shared feature with desmoplastic Spitz nevi. The presence of any number of melanophages is characteristic of this lesion.23 Lastly, there is a well-documented association between β-catenin mutations and deep penetrating nevi.24 Multicentric reticulohistiocytosis (MRH) is a rare form of non-Langerhans cell histiocytosis that has the pathognomonic clinical finding of pink-red papules (coral beading) with a predilection for acral surfaces. Histology of affected skin reveals a dermal infiltrate of ground glass as well as eosinophilic histiocytes that most often stain positive for CD68 and human alveolar macrophage 56 but negative for S-100 and CD1a (Figure 5).25 Although MRH is rare, negative staining for S-100 could serve as a useful diagnostic clue to differentiate it from other entities that are positive for S-100, such as the desmoplastic Spitz nevus. Arthritis mutilans is a potential complication of MRH, but a reported association with an underlying malignancy is seen in approximately 25% of cases.26 Thus, the cutaneous, rheumatologic, and oncologic implications of this disease help to distinguish it from other differential diagnoses that may be considered.
The Diagnosis: Desmoplastic Spitz Nevus
Desmoplastic Spitz nevus is a rare variant of Spitz nevus that commonly presents as a red to brown papule on the head, neck, or extremities. It is pertinent to review the histologic features of this neoplasm, as it can be confused with other more sinister entities such as spitzoid melanoma. Histologically, there is a dermal infiltrate of melanocytes containing eosinophilic cytoplasm and vesicular nuclei. Junctional involvement is rare, and there should be no pagetoid spread.1 This entity features abundant stromal fibrosis formed by dense collagen bundles, low cellular density, and polygonal-shaped melanocytes, which helps to differentiate it from melanoma.2,3 In a retrospective study comparing the characteristics of desmoplastic Spitz nevi with desmoplastic melanoma, desmoplastic Spitz nevi histologically were more symmetric and circumscribed with greater melanocytic maturation and adnexal structure involvement.3 Although this entity demonstrates maturation from the superficial to the deep dermis, it also may feature deep dermal vascular proliferation.4 S-100 and SRY-related HMG box 10, SOX-10, are noted to be positive in desmoplastic Spitz nevi, which can help to differentiate it from nonmelanocytic entities (Figure 1).
Although spitzoid lesions can be ambiguous and difficult even for experts to classify, spitzoid melanoma tends to have a high Breslow thickness, high cell density, marked atypia, and an increased nucleus to cytoplasm ratio.5 Additionally, desmoplastic melanoma was found to more often display “melanocytic junctional nests associated with discohesive cells, variations in size and shape of the nests, lentiginous melanocytic proliferation, actinic elastosis, pagetoid spread, dermal mitosis, perineural involvement and brisk inflammatory infiltrate.”3 Given the challenge of histologically separating desmoplastic Spitz nevi from melanoma, immunostaining can be useful. For example, Hilliard et al6 used a p16 antibody to differentiate desmoplastic Spitz nevi from desmoplastic melanoma, finding that most desmoplastic melanomas (81.8%; n=11) were negative for p16, whereas all desmoplastic Spitz nevi were at least moderately positive. However, another study re-evaluated the utility of p16 in desmoplastic melanoma and found that 72.7% (16/22) were at least focally reactive for the immunostain.7 Thus, caution must be exercised when using p16.
PReferentially expressed Antigen in MElanoma (PRAME) is a newer nuclear immunohistochemical marker that tends to be positive in melanomas and negative in nevi. Desmoplastic Spitz nevi would be expected to be negative for PRAME, while desmoplastic melanoma may be positive; however, this marker seems to be less effective in desmoplastic melanoma than in most other subtypes of the malignancy. In one study, only 35% (n=20) of desmoplastic melanomas were positive for PRAME.8 Likewise, another study showed that some benign Spitz nevi may diffusely express PRAME.9 As such, PRAME should be used prudently.
For cases in which immunohistochemistry is equivocal, molecular testing may aid in differentiating Spitz nevi from melanoma. For example, comparative genomic hybridization has revealed an increased copy number of chromosome 11p in approximately 20% of Spitz nevi cases10; this finding is not seen in melanoma. Mutation analyses of HRas proto-oncogene, GTPase, HRAS; B-Raf proto-oncogene, serine/threonine kinase, BRAF; and NRAS proto-oncogene, GTPase, NRAS, also have shown some promise in distinguishing spitzoid lesions from melanoma, but these analyses may be oversimplified.11 Fluorescence in situ hybridization (FISH) is another diagnostic modality that has been studied to differentiate benign nevi from melanoma. One study challenged the utility of FISH, reporting 7 of 15 desmoplastic melanomas tested positive compared to 0 of 15 sclerotic melanocytic nevi.12 Thus, negative FISH cannot reliably rule out melanoma. Ultimately, a combination of immunostains along with FISH or another genetic study would prove to be most effective in ruling out melanoma in difficult cases. Even then, a dermatopathologist may be faced with a degree of uncertainty.
Cellular blue nevi predominantly affect adults younger than 40 years and commonly are seen on the buttocks.13 This benign neoplasm demonstrates areas that are distinctly sclerotic as well as those that are cellular in nature.14 This entity demonstrates a well-circumscribed dermal growth pattern with 2 main populations of cells. The sclerotic portion of the cellular blue nevus mimics that of the blue nevus in that it is noted superficially with irregular margins. The cellular aspect of the nevus features spindle cells contained within well-circumscribed nodules (Figure 2). Stromal melanophages are not uncommon, and some can be observed adjacent to nerve fibers. Although this blue nevus variant displays features of the common blue nevus, its melanocytes track along adnexal and neurovascular structures similar to the deep penetrating nevus and the desmoplastic Spitz nevus. However, these melanocytes are variable in morphology and can appear on a spectrum spanning from pale and lightly pigmented to clear.15
The breast is the most common site of origin of tumor metastasis to the skin. These cutaneous metastases can vary in both their clinical and histological presentations. For example, cutaneous metastatic breast adenocarcinoma often can present clinically as pink-violaceous papules and plaques on the breast or on other parts of the body. Histologically, it can demonstrate a varying degree of patterns such as collagen infiltration by single cells, cords, tubules, and sheets of atypical cells (Figure 3) that can be observed together in areas of mucin or can form glandular structures.16 Metastatic breast carcinoma is noted to be positive for gross cystic disease fluid protein-15, estrogen receptor, and cytokeratin 7, which can help differentiate this entity from other tumors of glandular origin.16 Although rare, primary melanoma of the breast has been reported in the literature.17,18 These malignant melanocytic lesions easily could be differentiated from other breast tumors such as adenocarcinoma using immunohistochemical staining patterns.
Deep penetrating nevi most often are observed clinically as blue, brown, or black papules or nodules on the head or neck.19 Histologically, this lesion features a wedge-shaped infiltrate of deep dermal melanocytes with oval nuclei. It commonly extends to the reticular dermis or further into the subcutis (Figure 4).20,21 This neoplasm frequently tracks along adnexal and neurovascular structures, resulting in a plexiform appearance.22 The adnexal involvement of deep penetrating nevi is a shared feature with desmoplastic Spitz nevi. The presence of any number of melanophages is characteristic of this lesion.23 Lastly, there is a well-documented association between β-catenin mutations and deep penetrating nevi.24 Multicentric reticulohistiocytosis (MRH) is a rare form of non-Langerhans cell histiocytosis that has the pathognomonic clinical finding of pink-red papules (coral beading) with a predilection for acral surfaces. Histology of affected skin reveals a dermal infiltrate of ground glass as well as eosinophilic histiocytes that most often stain positive for CD68 and human alveolar macrophage 56 but negative for S-100 and CD1a (Figure 5).25 Although MRH is rare, negative staining for S-100 could serve as a useful diagnostic clue to differentiate it from other entities that are positive for S-100, such as the desmoplastic Spitz nevus. Arthritis mutilans is a potential complication of MRH, but a reported association with an underlying malignancy is seen in approximately 25% of cases.26 Thus, the cutaneous, rheumatologic, and oncologic implications of this disease help to distinguish it from other differential diagnoses that may be considered.
- Luzar B, Bastian BC, North JP, et al. Melanocytic nevi. In: Calonje E, Brenn T, Lazar AJ, et al, eds. McKee’s Pathology of the Skin. 5th ed. Elsevier; 2020:1275-1280.
- Busam KJ, Gerami P. Spitz nevi. In: Busam KJ, Gerami P, Scolyer RA, eds. Pathology of Melanocytic Tumors. Elsevier; 2019:37-60.
- Nojavan H, Cribier B, Mehregan DR. Desmoplastic Spitz nevus: a histopathological review and comparison with desmoplastic melanoma [in French]. Ann Dermatol Venereol. 2009;136:689-695.
- Tomizawa K. Desmoplastic Spitz nevus showing vascular proliferation more prominently in the deep portion. Am J Dermatopathol. 2002;24:184-185.
- Requena C, Botella R, Nagore E, et al. Characteristics of spitzoid melanoma and clues for differential diagnosis with Spitz nevus. Am J Dermatopathol. 2012;34:478-486.
- Hilliard NJ, Krahl D, Sellheyer K. p16 expression differentiates between desmoplastic Spitz nevus and desmoplastic melanoma. J Cutan Pathol. 2009;36:753-759.
- Blokhin E, Pulitzer M, Busam KJ. Immunohistochemical expression of p16 in desmoplastic melanoma. J Cutan Pathol. 2013;40:796-800.
- Lezcano C, Jungbluth AA, Nehal KS, et al. PRAME expression in melanocytic tumors. Am J Surg Pathol. 2018;42:1456-1465.
- Raghavan SS, Wang JY, Kwok S, et al. PRAME expression in melanocytic proliferations with intermediate histopathologic or spitzoid features. J Cutan Pathol. 2020;47:1123-1131.
- Bauer J, Bastian BC. DNA copy number changes in the diagnosis of melanocytic tumors [in German]. Pathologe. 2007;28:464-473.
- Luo S, Sepehr A, Tsao H. Spitz nevi and other spitzoid lesions part I. background and diagnoses. J Am Acad Dermatol. 2011;65:1073-1084.
- Gerami P, Beilfuss B, Haghighat Z, et al. Fluorescence in situ hybridization as an ancillary method for the distinction of desmoplastic melanomas from sclerosing melanocytic nevi. J Cutan Pathol. 2011;38:329-334.
- Zembowicz A. Blue nevi and related tumors. Clin Lab Med. 2017; 37:401-415.
- Rodriguez HA, Ackerman LV. Cellular blue nevus. clinicopathologic study of forty-five cases. Cancer. 1968;21:393-405.
- Phadke PA, Zembowicz A. Blue nevi and related tumors. Clin Lab Med. 2011;31:345-358.
- Ko CJ. Metastatic tumors and simulators. In: Elston DM, Ferringer T, eds. Dermatopathology. 3rd ed. Elsevier Limited; 2019:496-504.
- Drueppel D, Schultheis B, Solass W, et al. Primary malignant melanoma of the breast: case report and review of the literature. Anticancer Res. 2015;35:1709-1713.
- Kurul S, Tas¸ F, Büyükbabani N, et al. Different manifestations of malignant melanoma in the breast: a report of 12 cases and a review of the literature. Jpn J Clin Oncol. 2005;35:202-206.
- Strazzula L, Senna MM, Yasuda M, et al. The deep penetrating nevus. J Am Acad Dermatol. 2014;71:1234-1240.
- Mehregan DA, Mehregan AH. Deep penetrating nevus. Arch Dermatol. 1993;129:328-331.
- Robson A, Morley-Quante M, Hempel H, et al. Deep penetrating naevus: clinicopathological study of 31 cases with further delineation of histological features allowing distinction from other pigmented benign melanocytic lesions and melanoma. Histopathology. 2003;43:529-537.
- Luzar B, Calonje E. Deep penetrating nevus: a review. Arch Pathol Lab Med. 2011;135:321-326.
- Cooper PH. Deep penetrating (plexiform spindle cell) nevus. a frequent participant in combined nevus. J Cutan Pathol. 1992;19:172-180.
- de la Fouchardière A, Caillot C, Jacquemus J, et al. β-Catenin nuclear expression discriminates deep penetrating nevi from other cutaneous melanocytic tumors. Virchows Arch. 2019;474:539-550.
- Gorman JD, Danning C, Schumacher HR, et al. Multicentric reticulohistiocytosis: case report with immunohistochemical analysis and literature review. Arthritis Rheum. 2000;43:930-938.
- Selmi C, Greenspan A, Huntley A, et al. Multicentric reticulohistiocytosis: a critical review. Curr Rheumatol Rep. 2015;17:511.
- Luzar B, Bastian BC, North JP, et al. Melanocytic nevi. In: Calonje E, Brenn T, Lazar AJ, et al, eds. McKee’s Pathology of the Skin. 5th ed. Elsevier; 2020:1275-1280.
- Busam KJ, Gerami P. Spitz nevi. In: Busam KJ, Gerami P, Scolyer RA, eds. Pathology of Melanocytic Tumors. Elsevier; 2019:37-60.
- Nojavan H, Cribier B, Mehregan DR. Desmoplastic Spitz nevus: a histopathological review and comparison with desmoplastic melanoma [in French]. Ann Dermatol Venereol. 2009;136:689-695.
- Tomizawa K. Desmoplastic Spitz nevus showing vascular proliferation more prominently in the deep portion. Am J Dermatopathol. 2002;24:184-185.
- Requena C, Botella R, Nagore E, et al. Characteristics of spitzoid melanoma and clues for differential diagnosis with Spitz nevus. Am J Dermatopathol. 2012;34:478-486.
- Hilliard NJ, Krahl D, Sellheyer K. p16 expression differentiates between desmoplastic Spitz nevus and desmoplastic melanoma. J Cutan Pathol. 2009;36:753-759.
- Blokhin E, Pulitzer M, Busam KJ. Immunohistochemical expression of p16 in desmoplastic melanoma. J Cutan Pathol. 2013;40:796-800.
- Lezcano C, Jungbluth AA, Nehal KS, et al. PRAME expression in melanocytic tumors. Am J Surg Pathol. 2018;42:1456-1465.
- Raghavan SS, Wang JY, Kwok S, et al. PRAME expression in melanocytic proliferations with intermediate histopathologic or spitzoid features. J Cutan Pathol. 2020;47:1123-1131.
- Bauer J, Bastian BC. DNA copy number changes in the diagnosis of melanocytic tumors [in German]. Pathologe. 2007;28:464-473.
- Luo S, Sepehr A, Tsao H. Spitz nevi and other spitzoid lesions part I. background and diagnoses. J Am Acad Dermatol. 2011;65:1073-1084.
- Gerami P, Beilfuss B, Haghighat Z, et al. Fluorescence in situ hybridization as an ancillary method for the distinction of desmoplastic melanomas from sclerosing melanocytic nevi. J Cutan Pathol. 2011;38:329-334.
- Zembowicz A. Blue nevi and related tumors. Clin Lab Med. 2017; 37:401-415.
- Rodriguez HA, Ackerman LV. Cellular blue nevus. clinicopathologic study of forty-five cases. Cancer. 1968;21:393-405.
- Phadke PA, Zembowicz A. Blue nevi and related tumors. Clin Lab Med. 2011;31:345-358.
- Ko CJ. Metastatic tumors and simulators. In: Elston DM, Ferringer T, eds. Dermatopathology. 3rd ed. Elsevier Limited; 2019:496-504.
- Drueppel D, Schultheis B, Solass W, et al. Primary malignant melanoma of the breast: case report and review of the literature. Anticancer Res. 2015;35:1709-1713.
- Kurul S, Tas¸ F, Büyükbabani N, et al. Different manifestations of malignant melanoma in the breast: a report of 12 cases and a review of the literature. Jpn J Clin Oncol. 2005;35:202-206.
- Strazzula L, Senna MM, Yasuda M, et al. The deep penetrating nevus. J Am Acad Dermatol. 2014;71:1234-1240.
- Mehregan DA, Mehregan AH. Deep penetrating nevus. Arch Dermatol. 1993;129:328-331.
- Robson A, Morley-Quante M, Hempel H, et al. Deep penetrating naevus: clinicopathological study of 31 cases with further delineation of histological features allowing distinction from other pigmented benign melanocytic lesions and melanoma. Histopathology. 2003;43:529-537.
- Luzar B, Calonje E. Deep penetrating nevus: a review. Arch Pathol Lab Med. 2011;135:321-326.
- Cooper PH. Deep penetrating (plexiform spindle cell) nevus. a frequent participant in combined nevus. J Cutan Pathol. 1992;19:172-180.
- de la Fouchardière A, Caillot C, Jacquemus J, et al. β-Catenin nuclear expression discriminates deep penetrating nevi from other cutaneous melanocytic tumors. Virchows Arch. 2019;474:539-550.
- Gorman JD, Danning C, Schumacher HR, et al. Multicentric reticulohistiocytosis: case report with immunohistochemical analysis and literature review. Arthritis Rheum. 2000;43:930-938.
- Selmi C, Greenspan A, Huntley A, et al. Multicentric reticulohistiocytosis: a critical review. Curr Rheumatol Rep. 2015;17:511.
A 37-year-old woman with a history of fibrocystic breast disease and a family history of breast cancer presented with a light brown macule on the right upper arm of 10 years’ duration. The patient first noticed this macule 10 years prior; however, within the last 4 months she noticed a small amount of homogenous darkening and occasional pruritus. Physical examination revealed a 4.0-mm, light brown and pink macule on the right upper arm. Dermoscopy showed a homogenous pigment network with reticular lines and branched streaks centrally. No crystalline structures, milky red globules, or pseudopods were appreciated. A tangential shave biopsy was obtained and submitted for hematoxylin and eosin staining.
Enlarging Nodule on the Back
The Diagnosis: Cutaneous Myxoma
Microscopic analysis showed features of cutaneous myxoma (quiz images). The epidermis was essentially unremarkable. Stellate to spindle cells with bland nuclear chromatin were present in the dermis with abundant pools of myxoid stroma. Colloidal iron staining highlighted the markedly increased dermal mucin.
Cutaneous myxomas (also referred to as superficial angiomyxomas) are rare, well-demarcated tumors of the dermis and subcutis.1,2 They can present as solitary, fleshcolored nodules on the trunk, lower extremities, head, or neck, and they often measure between 1 and 5 cm.2,3 Histologically, cutaneous myxomas are hypocellular with some stellate fibroblasts, occasional epithelial structures, and an abundant myxoid stroma, with notable thinwalled small blood vessels.2,4 These lesions contain pools of mucin and are positive for mesenchymal mucin stains such as colloidal iron and Alcian blue.1 Moreover, perivascular neutrophils are a distinguishing characteristic of cutaneous myxomas.4
Multiple cutaneous myxomas should raise concern for Carney complex,1,5 a genodermatologic syndrome that arises due to a mutation in the protein kinase CAMP-dependent type I regulatory subunit alpha gene, PRKAR1A, on chromosome 2.1,5 Additional cutaneous manifestations include blue nevi, lentigines, and café-aulait macules.5 Carney complex also is known for endocrine overactivity and cardiac myxomas, which can cause serious embolic complications.1
Recommended management is complete excision with close follow-up, as these lesions may recur in up to one-third of cases. Although there is a potential for recurrence, metastases are uncommon.3 Even without recurrence in the presenting location, follow-up should include screening for manifestations of Carney complex.1,3
The clinical and histological differential for cutaneous myxoma may include nerve sheath myxoma or neurofibroma. A nerve sheath myxoma is a dermal tumor that manifests as a solitary, flesh-colored nodule, measuring less than 2 cm. These lesions commonly present on the head, neck, and upper body.6 Cutaneous myxomas can grow larger than 2 cm, but these two lesions have a great deal of overlap in their other features.3,6 Thus, histology can be used to distinguish them.
Nerve sheath myxomas are circumscribed nonencapsulated tumors of the dermis composed of multilobular aggregates of spindle to epithelioid cells in a mucinous matrix (Figure 1). Clefts often are present around the cell aggregates. Despite previously being termed myxoid neurothekeomas, nerve sheath myxomas are S-100 positive, whereas cellular neurothekeomas are S-100 negative and likely not of neural origin. Cutaneous myxomas, in contrast to nerve sheath myxomas, are S-100 negative. Nerve sheath myxomas are more cellular and lack the characteristic mucin pools compared with cutaneous myxomas.1,2,6 Neurofibromas frequently are flesh colored and pedunculated, as was the lesion in our patient, yet they are vastly different microscopically. The stroma of neurofibromas can vary, but cellularity typically is greater than a cutaneous myxoma and consists of increased numbers of bland spindle cells with wavy nuclei (Schwann cells) and fibrillar cytoplasm as well as mast cells and fibroblasts (Figure 2). Neurofibromas stain positively for S-100 and SOX-10 (Sry-related HMg-box 10).2,7 In addition to café-au-lait macules, axillary freckling, optic gliomas, and positive family history, neurofibromas are associated with neurofibromatosis type 1, which is linked to a defect in a tumor suppressor gene that codes for neurofibromin.7
Nodular fasciitis is a self-limited myofibroblastic neoplasm that contains fusion genes, with the most common being myosin-9–ubiquitin specific peptidase 6, MYH9-USP6, which leads to overexpression of USP6. Nodular fasciitis presents as a solitary, rapidly enlarging nodule affecting the subcutaneous tissue, muscles, or fascia.8,9 It usually presents in the third or fourth decades of life.8 The arms are the most common location in adults, while the most commonly affected site in children is the head or neck. Histopathology reveals a characteristic tissue culture pattern with a proliferation of plump spindle and stellate fibroblasts as well as myofibroblasts (Figure 3). Early lesions have haphazard spindle cells with a proliferation of small blood vessels and extravasated erythrocytes. Despite increased mitotic figures, cellular atypia is rare. The fibroblasts and myofibroblasts react positively for vimentin and muscle-specific actin.8 This lesion is highly cellular comparatively and notably lacks the perivascular neutrophils and epithelial structures that would be expected in a cutaneous myxoma.4,8
Spindle cell lipomas, solitary subcutaneous masses commonly presenting on the upper back in middle-aged men, also can mimic cutaneous myxomas.4 Histologically, these lesions may contain short bundles of spindle cells arranged in a school of fish–like pattern, mature adipocytes, or myxoid stroma and characteristic CD34 positivity (Figure 4). Spindle cell lipomas often will present with ropey collagen, which can easily distinguish them from cutaneous myxomas.4
- Lanjewar DN, Bhatia VO, Lanjewar SD, et al. Cutaneous myxoma: an important clue to Carney complex. Indian J Pathol Microbiol. 2014;57:460-462.
- Choi HJ, Kim YJ, Yim JH, et al. Unusual presentation of solitary cutaneous myxoma. J Eur Acad Dermatol Venereol. 2007;21:403-404. doi:10.1111/j.1468-3083.2006.01881.x
- Kura MM, Jindal SR. Solitary superficial acral angiomyxoma: an infrequently reported soft tissue tumor. Indian J Dermatol. 2014;59:1-3. doi:10.4103/0019-5154.139893
- Zou Y, Billings SD. Myxoid cutaneous tumors: a review. J Cutan Pathol. 2016;43:903-918.
- Sarfo A, Helm K, Flamm A. Cutaneous myxomas and a psammomatous melanotic schwannoma in a patient with Carney complex. J Cutan Pathol. 2019;46:93-96. doi:10.1111/cup.13385
- Gill P, Abi Daoud MS. Multiple cellular neurothekeomas in a middleaged woman including the lower extremity: a case report and review of the current literature. J Cutan Pathol. 2019;46:67-73. doi:10.1111/ cup.13366
- Ohgaki H, Kim Y, Steinbach JP. Nervous system tumors associated with familial tumor syndromes. Curr Opin Neurol. 2010;23:583-591. doi:10.1097/WCO.0b013e3283405b5f
- Luna A, Molinari L, Bollea Garlatti LA, et al. Nodular fasciitis, a forgotten entity. Int J Dermatol. 2019;58:190-193. doi:10.1111/ijd.14219
- Patel N, Chrisinger J, Demicco E, et al. USP6 activation in nodular fasciitis by promoter-swapping gene fusions. Mod Pathol. 2017; 30:1577-1588.
The Diagnosis: Cutaneous Myxoma
Microscopic analysis showed features of cutaneous myxoma (quiz images). The epidermis was essentially unremarkable. Stellate to spindle cells with bland nuclear chromatin were present in the dermis with abundant pools of myxoid stroma. Colloidal iron staining highlighted the markedly increased dermal mucin.
Cutaneous myxomas (also referred to as superficial angiomyxomas) are rare, well-demarcated tumors of the dermis and subcutis.1,2 They can present as solitary, fleshcolored nodules on the trunk, lower extremities, head, or neck, and they often measure between 1 and 5 cm.2,3 Histologically, cutaneous myxomas are hypocellular with some stellate fibroblasts, occasional epithelial structures, and an abundant myxoid stroma, with notable thinwalled small blood vessels.2,4 These lesions contain pools of mucin and are positive for mesenchymal mucin stains such as colloidal iron and Alcian blue.1 Moreover, perivascular neutrophils are a distinguishing characteristic of cutaneous myxomas.4
Multiple cutaneous myxomas should raise concern for Carney complex,1,5 a genodermatologic syndrome that arises due to a mutation in the protein kinase CAMP-dependent type I regulatory subunit alpha gene, PRKAR1A, on chromosome 2.1,5 Additional cutaneous manifestations include blue nevi, lentigines, and café-aulait macules.5 Carney complex also is known for endocrine overactivity and cardiac myxomas, which can cause serious embolic complications.1
Recommended management is complete excision with close follow-up, as these lesions may recur in up to one-third of cases. Although there is a potential for recurrence, metastases are uncommon.3 Even without recurrence in the presenting location, follow-up should include screening for manifestations of Carney complex.1,3
The clinical and histological differential for cutaneous myxoma may include nerve sheath myxoma or neurofibroma. A nerve sheath myxoma is a dermal tumor that manifests as a solitary, flesh-colored nodule, measuring less than 2 cm. These lesions commonly present on the head, neck, and upper body.6 Cutaneous myxomas can grow larger than 2 cm, but these two lesions have a great deal of overlap in their other features.3,6 Thus, histology can be used to distinguish them.
Nerve sheath myxomas are circumscribed nonencapsulated tumors of the dermis composed of multilobular aggregates of spindle to epithelioid cells in a mucinous matrix (Figure 1). Clefts often are present around the cell aggregates. Despite previously being termed myxoid neurothekeomas, nerve sheath myxomas are S-100 positive, whereas cellular neurothekeomas are S-100 negative and likely not of neural origin. Cutaneous myxomas, in contrast to nerve sheath myxomas, are S-100 negative. Nerve sheath myxomas are more cellular and lack the characteristic mucin pools compared with cutaneous myxomas.1,2,6 Neurofibromas frequently are flesh colored and pedunculated, as was the lesion in our patient, yet they are vastly different microscopically. The stroma of neurofibromas can vary, but cellularity typically is greater than a cutaneous myxoma and consists of increased numbers of bland spindle cells with wavy nuclei (Schwann cells) and fibrillar cytoplasm as well as mast cells and fibroblasts (Figure 2). Neurofibromas stain positively for S-100 and SOX-10 (Sry-related HMg-box 10).2,7 In addition to café-au-lait macules, axillary freckling, optic gliomas, and positive family history, neurofibromas are associated with neurofibromatosis type 1, which is linked to a defect in a tumor suppressor gene that codes for neurofibromin.7
Nodular fasciitis is a self-limited myofibroblastic neoplasm that contains fusion genes, with the most common being myosin-9–ubiquitin specific peptidase 6, MYH9-USP6, which leads to overexpression of USP6. Nodular fasciitis presents as a solitary, rapidly enlarging nodule affecting the subcutaneous tissue, muscles, or fascia.8,9 It usually presents in the third or fourth decades of life.8 The arms are the most common location in adults, while the most commonly affected site in children is the head or neck. Histopathology reveals a characteristic tissue culture pattern with a proliferation of plump spindle and stellate fibroblasts as well as myofibroblasts (Figure 3). Early lesions have haphazard spindle cells with a proliferation of small blood vessels and extravasated erythrocytes. Despite increased mitotic figures, cellular atypia is rare. The fibroblasts and myofibroblasts react positively for vimentin and muscle-specific actin.8 This lesion is highly cellular comparatively and notably lacks the perivascular neutrophils and epithelial structures that would be expected in a cutaneous myxoma.4,8
Spindle cell lipomas, solitary subcutaneous masses commonly presenting on the upper back in middle-aged men, also can mimic cutaneous myxomas.4 Histologically, these lesions may contain short bundles of spindle cells arranged in a school of fish–like pattern, mature adipocytes, or myxoid stroma and characteristic CD34 positivity (Figure 4). Spindle cell lipomas often will present with ropey collagen, which can easily distinguish them from cutaneous myxomas.4
The Diagnosis: Cutaneous Myxoma
Microscopic analysis showed features of cutaneous myxoma (quiz images). The epidermis was essentially unremarkable. Stellate to spindle cells with bland nuclear chromatin were present in the dermis with abundant pools of myxoid stroma. Colloidal iron staining highlighted the markedly increased dermal mucin.
Cutaneous myxomas (also referred to as superficial angiomyxomas) are rare, well-demarcated tumors of the dermis and subcutis.1,2 They can present as solitary, fleshcolored nodules on the trunk, lower extremities, head, or neck, and they often measure between 1 and 5 cm.2,3 Histologically, cutaneous myxomas are hypocellular with some stellate fibroblasts, occasional epithelial structures, and an abundant myxoid stroma, with notable thinwalled small blood vessels.2,4 These lesions contain pools of mucin and are positive for mesenchymal mucin stains such as colloidal iron and Alcian blue.1 Moreover, perivascular neutrophils are a distinguishing characteristic of cutaneous myxomas.4
Multiple cutaneous myxomas should raise concern for Carney complex,1,5 a genodermatologic syndrome that arises due to a mutation in the protein kinase CAMP-dependent type I regulatory subunit alpha gene, PRKAR1A, on chromosome 2.1,5 Additional cutaneous manifestations include blue nevi, lentigines, and café-aulait macules.5 Carney complex also is known for endocrine overactivity and cardiac myxomas, which can cause serious embolic complications.1
Recommended management is complete excision with close follow-up, as these lesions may recur in up to one-third of cases. Although there is a potential for recurrence, metastases are uncommon.3 Even without recurrence in the presenting location, follow-up should include screening for manifestations of Carney complex.1,3
The clinical and histological differential for cutaneous myxoma may include nerve sheath myxoma or neurofibroma. A nerve sheath myxoma is a dermal tumor that manifests as a solitary, flesh-colored nodule, measuring less than 2 cm. These lesions commonly present on the head, neck, and upper body.6 Cutaneous myxomas can grow larger than 2 cm, but these two lesions have a great deal of overlap in their other features.3,6 Thus, histology can be used to distinguish them.
Nerve sheath myxomas are circumscribed nonencapsulated tumors of the dermis composed of multilobular aggregates of spindle to epithelioid cells in a mucinous matrix (Figure 1). Clefts often are present around the cell aggregates. Despite previously being termed myxoid neurothekeomas, nerve sheath myxomas are S-100 positive, whereas cellular neurothekeomas are S-100 negative and likely not of neural origin. Cutaneous myxomas, in contrast to nerve sheath myxomas, are S-100 negative. Nerve sheath myxomas are more cellular and lack the characteristic mucin pools compared with cutaneous myxomas.1,2,6 Neurofibromas frequently are flesh colored and pedunculated, as was the lesion in our patient, yet they are vastly different microscopically. The stroma of neurofibromas can vary, but cellularity typically is greater than a cutaneous myxoma and consists of increased numbers of bland spindle cells with wavy nuclei (Schwann cells) and fibrillar cytoplasm as well as mast cells and fibroblasts (Figure 2). Neurofibromas stain positively for S-100 and SOX-10 (Sry-related HMg-box 10).2,7 In addition to café-au-lait macules, axillary freckling, optic gliomas, and positive family history, neurofibromas are associated with neurofibromatosis type 1, which is linked to a defect in a tumor suppressor gene that codes for neurofibromin.7
Nodular fasciitis is a self-limited myofibroblastic neoplasm that contains fusion genes, with the most common being myosin-9–ubiquitin specific peptidase 6, MYH9-USP6, which leads to overexpression of USP6. Nodular fasciitis presents as a solitary, rapidly enlarging nodule affecting the subcutaneous tissue, muscles, or fascia.8,9 It usually presents in the third or fourth decades of life.8 The arms are the most common location in adults, while the most commonly affected site in children is the head or neck. Histopathology reveals a characteristic tissue culture pattern with a proliferation of plump spindle and stellate fibroblasts as well as myofibroblasts (Figure 3). Early lesions have haphazard spindle cells with a proliferation of small blood vessels and extravasated erythrocytes. Despite increased mitotic figures, cellular atypia is rare. The fibroblasts and myofibroblasts react positively for vimentin and muscle-specific actin.8 This lesion is highly cellular comparatively and notably lacks the perivascular neutrophils and epithelial structures that would be expected in a cutaneous myxoma.4,8
Spindle cell lipomas, solitary subcutaneous masses commonly presenting on the upper back in middle-aged men, also can mimic cutaneous myxomas.4 Histologically, these lesions may contain short bundles of spindle cells arranged in a school of fish–like pattern, mature adipocytes, or myxoid stroma and characteristic CD34 positivity (Figure 4). Spindle cell lipomas often will present with ropey collagen, which can easily distinguish them from cutaneous myxomas.4
- Lanjewar DN, Bhatia VO, Lanjewar SD, et al. Cutaneous myxoma: an important clue to Carney complex. Indian J Pathol Microbiol. 2014;57:460-462.
- Choi HJ, Kim YJ, Yim JH, et al. Unusual presentation of solitary cutaneous myxoma. J Eur Acad Dermatol Venereol. 2007;21:403-404. doi:10.1111/j.1468-3083.2006.01881.x
- Kura MM, Jindal SR. Solitary superficial acral angiomyxoma: an infrequently reported soft tissue tumor. Indian J Dermatol. 2014;59:1-3. doi:10.4103/0019-5154.139893
- Zou Y, Billings SD. Myxoid cutaneous tumors: a review. J Cutan Pathol. 2016;43:903-918.
- Sarfo A, Helm K, Flamm A. Cutaneous myxomas and a psammomatous melanotic schwannoma in a patient with Carney complex. J Cutan Pathol. 2019;46:93-96. doi:10.1111/cup.13385
- Gill P, Abi Daoud MS. Multiple cellular neurothekeomas in a middleaged woman including the lower extremity: a case report and review of the current literature. J Cutan Pathol. 2019;46:67-73. doi:10.1111/ cup.13366
- Ohgaki H, Kim Y, Steinbach JP. Nervous system tumors associated with familial tumor syndromes. Curr Opin Neurol. 2010;23:583-591. doi:10.1097/WCO.0b013e3283405b5f
- Luna A, Molinari L, Bollea Garlatti LA, et al. Nodular fasciitis, a forgotten entity. Int J Dermatol. 2019;58:190-193. doi:10.1111/ijd.14219
- Patel N, Chrisinger J, Demicco E, et al. USP6 activation in nodular fasciitis by promoter-swapping gene fusions. Mod Pathol. 2017; 30:1577-1588.
- Lanjewar DN, Bhatia VO, Lanjewar SD, et al. Cutaneous myxoma: an important clue to Carney complex. Indian J Pathol Microbiol. 2014;57:460-462.
- Choi HJ, Kim YJ, Yim JH, et al. Unusual presentation of solitary cutaneous myxoma. J Eur Acad Dermatol Venereol. 2007;21:403-404. doi:10.1111/j.1468-3083.2006.01881.x
- Kura MM, Jindal SR. Solitary superficial acral angiomyxoma: an infrequently reported soft tissue tumor. Indian J Dermatol. 2014;59:1-3. doi:10.4103/0019-5154.139893
- Zou Y, Billings SD. Myxoid cutaneous tumors: a review. J Cutan Pathol. 2016;43:903-918.
- Sarfo A, Helm K, Flamm A. Cutaneous myxomas and a psammomatous melanotic schwannoma in a patient with Carney complex. J Cutan Pathol. 2019;46:93-96. doi:10.1111/cup.13385
- Gill P, Abi Daoud MS. Multiple cellular neurothekeomas in a middleaged woman including the lower extremity: a case report and review of the current literature. J Cutan Pathol. 2019;46:67-73. doi:10.1111/ cup.13366
- Ohgaki H, Kim Y, Steinbach JP. Nervous system tumors associated with familial tumor syndromes. Curr Opin Neurol. 2010;23:583-591. doi:10.1097/WCO.0b013e3283405b5f
- Luna A, Molinari L, Bollea Garlatti LA, et al. Nodular fasciitis, a forgotten entity. Int J Dermatol. 2019;58:190-193. doi:10.1111/ijd.14219
- Patel N, Chrisinger J, Demicco E, et al. USP6 activation in nodular fasciitis by promoter-swapping gene fusions. Mod Pathol. 2017; 30:1577-1588.
A 43-year-old man with an unremarkable medical history presented to our clinic with an enlarging painful nodule on the upper back that was present for years without bleeding or ulceration. He denied prior treatment or any similar lesions. Physical examination was notable for a 2×1.5-cm, pedunculated, flesh-colored nodule on the left upper back. A shave excision of the lesion was performed.
Tender Subcutaneous Nodule in a Prepubescent Boy
The Diagnosis: Dermatomyofibroma
Dermatomyofibroma is an uncommon, benign, cutaneous mesenchymal neoplasm composed of fibroblasts and myofibroblasts.1-3 This skin tumor was first described in 1991 by Hugel4 in the German literature as plaquelike fibromatosis. Pediatric dermatomyofibromas are exceedingly rare, with pediatric patients ranging in age from infants to teenagers.1
Clinically, dermatomyofibromas appear as long-standing, isolated, ill-demarcated, flesh-colored, slightly hyperpigmented or erythematous nodules or plaques that may be raised or indurated.1 Dermatomyofibromas may present with constant mild pain or pruritus, though in most cases the lesions are asymptomatic.1,3 The clinical presentation of dermatomyofibroma has a few distinct differences in children compared to adults. In adulthood, dermatomyofibroma has a strong female predominance and most commonly is located on the shoulder and adjacent upper body regions, including the axilla, neck, upper arm, and upper trunk.1-3 In childhood, the majority of dermatomyofibromas occur in young boys and usually are located on the neck with other upper body regions occurring less frequently.1,2 A shared characteristic includes the tendency for dermatomyofibromas to have an initial period of enlargement followed by stabilization or slow growth.1 Reported pediatric lesions have ranged in size from 4 to 60 mm with an average size of 14.9 mm (median, 12 mm).2
The diagnosis of dermatomyofibroma is based on histopathologic features in addition to clinical presentation. Histology from punch biopsy usually reveals a noninvasive dermal proliferation of bland, uniform, slender spindle cells oriented parallel to the overlying epidermis with increased and fragmented elastic fibers.1,3 Infiltration into the mid or deep dermis is common. The adnexal structures usually are spared; the stroma contains collagen and increased small blood vessels; and there typically is no inflammatory infiltrate, except for occasional scattered mast cells.2 Cytologically, the monomorphic spindleshaped tumor cells have an ill-defined, pale, eosinophilic cytoplasm and nuclei that are elongated with tapered edges.3 Dermatomyofibroma has a variable immunohistochemical profile, as it may stain focally positive for CD34 or smooth muscle actin, with occasional staining of factor XIIIa, desmin, calponin, or vimentin.1-3 Normal to increased levels of often fragmented elastic fibers is a helpful clue in distinguishing dermatomyofibroma from dermatofibroma, hypertrophic scar, dermatofibrosarcoma protuberans, and pilar leiomyoma, in which elastic fibers typically are reduced.3 Differential diagnoses of mesenchymal tumors in children include desmoid fibromatosis, connective tissue nevus, myofibromatosis, and smooth muscle hamartoma.1
A punch biopsy with clinical observation and followup is recommended for the management of lesions in cosmetically sensitive areas or in very young children who may not tolerate surgery. In symptomatic or cosmetically unappealing cases of dermatomyofibroma, simple surgical excision remains a viable treatment option. Recurrence is uncommon, even if only partially excised, and no instances of metastasis have been reported.1-5
Dermatomyofibromas may be mistaken for several other entities both benign and malignant. For example, the benign dermatofibroma is the second most common fibrohistiocytic tumor of the skin and presents as a firm, nontender, minimally elevated to dome-shaped papule that usually measures less than or equal to 1 cm in diameter with or without overlying skin changes.5,6 It primarily is seen in adults with a slight female predominance and favors the lower extremities.5 Patients usually are asymptomatic but often report a history of local trauma at the lesion site.6 Histologically, dermatofibroma is characterized by a nodular dermal proliferation of spindleshaped fibrous cells and histiocytes in a storiform pattern (Figure 1).6 Epidermal induction with acanthosis overlying the tumor often is found with occasional basilar hyperpigmentation.5 Dermatofibroma also characteristically has trapped collagen (“collagen balls”) seen at the periphery.5,6
Piloleiomyomas are benign smooth muscle tumors arising from arrector pili muscles that may be solitary or multiple.5 Clinically, they typically present as firm, reddish-brown to flesh-colored papules or nodules that develop more commonly in adulthood.5,7 Piloleiomyomas favor the extremities and trunk, particularly the shoulder, and can be associated with spontaneous or induced pain. Histologically, piloleiomyomas are well circumscribed and centered within the reticular dermis situated closely to hair follicles (Figure 2).5 They are composed of numerous interlacing fascicles or whorls of smooth muscle cells with abundant eosinophilic cytoplasm and blunt-ended, cigar-shaped nuclei.5,7
Solitary cutaneous myofibroma is a benign fibrous tumor found in adolescents and adults and is the counterpart to infantile myofibromatosis.8 Clinically, myofibromas typically present as painless, slow-growing, firm nodules with an occasional bluish hue. Histologically, solitary cutaneous myofibromas appear in a biphasic pattern, with hemangiopericytomatous components as well as spindle cells arranged in short bundles and fascicles resembling leiomyoma (Figure 3). The spindle cells also have abundant eosinophilic cytoplasm with short plump nuclei; the random, irregularly intersecting angles can be used to help differentiate myofibromas from smooth muscle lesions.8 Solitary cutaneous myofibroma is in the differential diagnosis for dermatomyofibroma because of their shared myofibroblastic nature.9
Dermatofibrosarcoma protuberans (DFSP) is an uncommon, locally invasive sarcoma with a high recurrence rate that favors young to middle-aged adults, with rare childhood onset reported.5,10,11 Clinically, DFSP typically presents as an asymptomatic, slow-growing, firm, flesh-colored, indurated plaque that develops into a violaceous to reddish-brown nodule.5 The atrophic variant of DFSP is characterized by a nonprotuberant lesion and can be especially difficult to distinguish from other entities such as dermatomyofibroma.11 The majority of DFSP lesions occur on the trunk, particularly in the shoulder or pelvic region.5 Histologically, early plaque lesions are comprised of monomorphic spindle cells arranged in long fascicles (parallel to the skin surface), infiltrating adnexal structures, and subcutaneous adipocytes in a multilayered honeycomb pattern; the spindle cells of late nodular lesions are arranged in short fascicles in a matted or storiform pattern (Figure 4).5,10 Early stages of DFSP as well as variations in childhood-onset DFSP can easily be misdiagnosed and incompletely excised.5
- Ma JE, Wieland CN, Tollefson MM. Dermatomyofibromas arising in children: report of two new cases and review of the literature. Pediatr Dermatol. 2017;34:347-351.
- Tardio JC, Azorin D, Hernandez-Nunez A, et al. Dermatomyofibromas presenting in pediatric patients: clinicopathologic characteristics and differential diagnosis. J Cutan Pathol. 2011;38:967-972.
- Mentzel T, Kutzner H. Dermatomyofibroma: clinicopathologic and immunohistochemical analysis of 56 cases and reappraisal of a rare and distinct cutaneous neoplasm. Am J Dermatopathol. 2009;31:44-49.
- Hugel H. Plaque-like dermal fibromatosis. Hautarzt. 1991;42:223-226.
- Bolognia JL, Jorizzo JL, Schaffer JV, eds. Dermatology. WB Saunders Co; 2012.
- Myers DJ, Fillman EP. Dermatofibroma. StatPearls [Internet]. StatPearls Publishing; 2020.
- Dilek N, Yuksel D, Sehitoglu I, et al. Cutaneous leiomyoma in a child: a case report. Oncol Lett. 2013;5:1163-1164.
- Roh HS, Paek JO, Yu HJ, et al. Solitary cutaneous myofibroma on the sole: an unusual localization. Ann Dermatol. 2012;24:220-222.
- Weedon D, Strutton G, Rubin AI, et al. Weedon’s Skin Pathology. Churchill Livingstone/Elsevier; 2010.
- Mendenhall WM, Zlotecki RA, Scarborough MT. Dermatofibrosarcoma protuberans. Cancer. 2004;101:2503-2508.
- Akay BN, Unlu E, Erdem C, et al. Dermatoscopic findings of atrophic dermatofibrosarcoma protuberans. Dermatol Pract Concept. 2015;5:71-73.
The Diagnosis: Dermatomyofibroma
Dermatomyofibroma is an uncommon, benign, cutaneous mesenchymal neoplasm composed of fibroblasts and myofibroblasts.1-3 This skin tumor was first described in 1991 by Hugel4 in the German literature as plaquelike fibromatosis. Pediatric dermatomyofibromas are exceedingly rare, with pediatric patients ranging in age from infants to teenagers.1
Clinically, dermatomyofibromas appear as long-standing, isolated, ill-demarcated, flesh-colored, slightly hyperpigmented or erythematous nodules or plaques that may be raised or indurated.1 Dermatomyofibromas may present with constant mild pain or pruritus, though in most cases the lesions are asymptomatic.1,3 The clinical presentation of dermatomyofibroma has a few distinct differences in children compared to adults. In adulthood, dermatomyofibroma has a strong female predominance and most commonly is located on the shoulder and adjacent upper body regions, including the axilla, neck, upper arm, and upper trunk.1-3 In childhood, the majority of dermatomyofibromas occur in young boys and usually are located on the neck with other upper body regions occurring less frequently.1,2 A shared characteristic includes the tendency for dermatomyofibromas to have an initial period of enlargement followed by stabilization or slow growth.1 Reported pediatric lesions have ranged in size from 4 to 60 mm with an average size of 14.9 mm (median, 12 mm).2
The diagnosis of dermatomyofibroma is based on histopathologic features in addition to clinical presentation. Histology from punch biopsy usually reveals a noninvasive dermal proliferation of bland, uniform, slender spindle cells oriented parallel to the overlying epidermis with increased and fragmented elastic fibers.1,3 Infiltration into the mid or deep dermis is common. The adnexal structures usually are spared; the stroma contains collagen and increased small blood vessels; and there typically is no inflammatory infiltrate, except for occasional scattered mast cells.2 Cytologically, the monomorphic spindleshaped tumor cells have an ill-defined, pale, eosinophilic cytoplasm and nuclei that are elongated with tapered edges.3 Dermatomyofibroma has a variable immunohistochemical profile, as it may stain focally positive for CD34 or smooth muscle actin, with occasional staining of factor XIIIa, desmin, calponin, or vimentin.1-3 Normal to increased levels of often fragmented elastic fibers is a helpful clue in distinguishing dermatomyofibroma from dermatofibroma, hypertrophic scar, dermatofibrosarcoma protuberans, and pilar leiomyoma, in which elastic fibers typically are reduced.3 Differential diagnoses of mesenchymal tumors in children include desmoid fibromatosis, connective tissue nevus, myofibromatosis, and smooth muscle hamartoma.1
A punch biopsy with clinical observation and followup is recommended for the management of lesions in cosmetically sensitive areas or in very young children who may not tolerate surgery. In symptomatic or cosmetically unappealing cases of dermatomyofibroma, simple surgical excision remains a viable treatment option. Recurrence is uncommon, even if only partially excised, and no instances of metastasis have been reported.1-5
Dermatomyofibromas may be mistaken for several other entities both benign and malignant. For example, the benign dermatofibroma is the second most common fibrohistiocytic tumor of the skin and presents as a firm, nontender, minimally elevated to dome-shaped papule that usually measures less than or equal to 1 cm in diameter with or without overlying skin changes.5,6 It primarily is seen in adults with a slight female predominance and favors the lower extremities.5 Patients usually are asymptomatic but often report a history of local trauma at the lesion site.6 Histologically, dermatofibroma is characterized by a nodular dermal proliferation of spindleshaped fibrous cells and histiocytes in a storiform pattern (Figure 1).6 Epidermal induction with acanthosis overlying the tumor often is found with occasional basilar hyperpigmentation.5 Dermatofibroma also characteristically has trapped collagen (“collagen balls”) seen at the periphery.5,6
Piloleiomyomas are benign smooth muscle tumors arising from arrector pili muscles that may be solitary or multiple.5 Clinically, they typically present as firm, reddish-brown to flesh-colored papules or nodules that develop more commonly in adulthood.5,7 Piloleiomyomas favor the extremities and trunk, particularly the shoulder, and can be associated with spontaneous or induced pain. Histologically, piloleiomyomas are well circumscribed and centered within the reticular dermis situated closely to hair follicles (Figure 2).5 They are composed of numerous interlacing fascicles or whorls of smooth muscle cells with abundant eosinophilic cytoplasm and blunt-ended, cigar-shaped nuclei.5,7
Solitary cutaneous myofibroma is a benign fibrous tumor found in adolescents and adults and is the counterpart to infantile myofibromatosis.8 Clinically, myofibromas typically present as painless, slow-growing, firm nodules with an occasional bluish hue. Histologically, solitary cutaneous myofibromas appear in a biphasic pattern, with hemangiopericytomatous components as well as spindle cells arranged in short bundles and fascicles resembling leiomyoma (Figure 3). The spindle cells also have abundant eosinophilic cytoplasm with short plump nuclei; the random, irregularly intersecting angles can be used to help differentiate myofibromas from smooth muscle lesions.8 Solitary cutaneous myofibroma is in the differential diagnosis for dermatomyofibroma because of their shared myofibroblastic nature.9
Dermatofibrosarcoma protuberans (DFSP) is an uncommon, locally invasive sarcoma with a high recurrence rate that favors young to middle-aged adults, with rare childhood onset reported.5,10,11 Clinically, DFSP typically presents as an asymptomatic, slow-growing, firm, flesh-colored, indurated plaque that develops into a violaceous to reddish-brown nodule.5 The atrophic variant of DFSP is characterized by a nonprotuberant lesion and can be especially difficult to distinguish from other entities such as dermatomyofibroma.11 The majority of DFSP lesions occur on the trunk, particularly in the shoulder or pelvic region.5 Histologically, early plaque lesions are comprised of monomorphic spindle cells arranged in long fascicles (parallel to the skin surface), infiltrating adnexal structures, and subcutaneous adipocytes in a multilayered honeycomb pattern; the spindle cells of late nodular lesions are arranged in short fascicles in a matted or storiform pattern (Figure 4).5,10 Early stages of DFSP as well as variations in childhood-onset DFSP can easily be misdiagnosed and incompletely excised.5
The Diagnosis: Dermatomyofibroma
Dermatomyofibroma is an uncommon, benign, cutaneous mesenchymal neoplasm composed of fibroblasts and myofibroblasts.1-3 This skin tumor was first described in 1991 by Hugel4 in the German literature as plaquelike fibromatosis. Pediatric dermatomyofibromas are exceedingly rare, with pediatric patients ranging in age from infants to teenagers.1
Clinically, dermatomyofibromas appear as long-standing, isolated, ill-demarcated, flesh-colored, slightly hyperpigmented or erythematous nodules or plaques that may be raised or indurated.1 Dermatomyofibromas may present with constant mild pain or pruritus, though in most cases the lesions are asymptomatic.1,3 The clinical presentation of dermatomyofibroma has a few distinct differences in children compared to adults. In adulthood, dermatomyofibroma has a strong female predominance and most commonly is located on the shoulder and adjacent upper body regions, including the axilla, neck, upper arm, and upper trunk.1-3 In childhood, the majority of dermatomyofibromas occur in young boys and usually are located on the neck with other upper body regions occurring less frequently.1,2 A shared characteristic includes the tendency for dermatomyofibromas to have an initial period of enlargement followed by stabilization or slow growth.1 Reported pediatric lesions have ranged in size from 4 to 60 mm with an average size of 14.9 mm (median, 12 mm).2
The diagnosis of dermatomyofibroma is based on histopathologic features in addition to clinical presentation. Histology from punch biopsy usually reveals a noninvasive dermal proliferation of bland, uniform, slender spindle cells oriented parallel to the overlying epidermis with increased and fragmented elastic fibers.1,3 Infiltration into the mid or deep dermis is common. The adnexal structures usually are spared; the stroma contains collagen and increased small blood vessels; and there typically is no inflammatory infiltrate, except for occasional scattered mast cells.2 Cytologically, the monomorphic spindleshaped tumor cells have an ill-defined, pale, eosinophilic cytoplasm and nuclei that are elongated with tapered edges.3 Dermatomyofibroma has a variable immunohistochemical profile, as it may stain focally positive for CD34 or smooth muscle actin, with occasional staining of factor XIIIa, desmin, calponin, or vimentin.1-3 Normal to increased levels of often fragmented elastic fibers is a helpful clue in distinguishing dermatomyofibroma from dermatofibroma, hypertrophic scar, dermatofibrosarcoma protuberans, and pilar leiomyoma, in which elastic fibers typically are reduced.3 Differential diagnoses of mesenchymal tumors in children include desmoid fibromatosis, connective tissue nevus, myofibromatosis, and smooth muscle hamartoma.1
A punch biopsy with clinical observation and followup is recommended for the management of lesions in cosmetically sensitive areas or in very young children who may not tolerate surgery. In symptomatic or cosmetically unappealing cases of dermatomyofibroma, simple surgical excision remains a viable treatment option. Recurrence is uncommon, even if only partially excised, and no instances of metastasis have been reported.1-5
Dermatomyofibromas may be mistaken for several other entities both benign and malignant. For example, the benign dermatofibroma is the second most common fibrohistiocytic tumor of the skin and presents as a firm, nontender, minimally elevated to dome-shaped papule that usually measures less than or equal to 1 cm in diameter with or without overlying skin changes.5,6 It primarily is seen in adults with a slight female predominance and favors the lower extremities.5 Patients usually are asymptomatic but often report a history of local trauma at the lesion site.6 Histologically, dermatofibroma is characterized by a nodular dermal proliferation of spindleshaped fibrous cells and histiocytes in a storiform pattern (Figure 1).6 Epidermal induction with acanthosis overlying the tumor often is found with occasional basilar hyperpigmentation.5 Dermatofibroma also characteristically has trapped collagen (“collagen balls”) seen at the periphery.5,6
Piloleiomyomas are benign smooth muscle tumors arising from arrector pili muscles that may be solitary or multiple.5 Clinically, they typically present as firm, reddish-brown to flesh-colored papules or nodules that develop more commonly in adulthood.5,7 Piloleiomyomas favor the extremities and trunk, particularly the shoulder, and can be associated with spontaneous or induced pain. Histologically, piloleiomyomas are well circumscribed and centered within the reticular dermis situated closely to hair follicles (Figure 2).5 They are composed of numerous interlacing fascicles or whorls of smooth muscle cells with abundant eosinophilic cytoplasm and blunt-ended, cigar-shaped nuclei.5,7
Solitary cutaneous myofibroma is a benign fibrous tumor found in adolescents and adults and is the counterpart to infantile myofibromatosis.8 Clinically, myofibromas typically present as painless, slow-growing, firm nodules with an occasional bluish hue. Histologically, solitary cutaneous myofibromas appear in a biphasic pattern, with hemangiopericytomatous components as well as spindle cells arranged in short bundles and fascicles resembling leiomyoma (Figure 3). The spindle cells also have abundant eosinophilic cytoplasm with short plump nuclei; the random, irregularly intersecting angles can be used to help differentiate myofibromas from smooth muscle lesions.8 Solitary cutaneous myofibroma is in the differential diagnosis for dermatomyofibroma because of their shared myofibroblastic nature.9
Dermatofibrosarcoma protuberans (DFSP) is an uncommon, locally invasive sarcoma with a high recurrence rate that favors young to middle-aged adults, with rare childhood onset reported.5,10,11 Clinically, DFSP typically presents as an asymptomatic, slow-growing, firm, flesh-colored, indurated plaque that develops into a violaceous to reddish-brown nodule.5 The atrophic variant of DFSP is characterized by a nonprotuberant lesion and can be especially difficult to distinguish from other entities such as dermatomyofibroma.11 The majority of DFSP lesions occur on the trunk, particularly in the shoulder or pelvic region.5 Histologically, early plaque lesions are comprised of monomorphic spindle cells arranged in long fascicles (parallel to the skin surface), infiltrating adnexal structures, and subcutaneous adipocytes in a multilayered honeycomb pattern; the spindle cells of late nodular lesions are arranged in short fascicles in a matted or storiform pattern (Figure 4).5,10 Early stages of DFSP as well as variations in childhood-onset DFSP can easily be misdiagnosed and incompletely excised.5
- Ma JE, Wieland CN, Tollefson MM. Dermatomyofibromas arising in children: report of two new cases and review of the literature. Pediatr Dermatol. 2017;34:347-351.
- Tardio JC, Azorin D, Hernandez-Nunez A, et al. Dermatomyofibromas presenting in pediatric patients: clinicopathologic characteristics and differential diagnosis. J Cutan Pathol. 2011;38:967-972.
- Mentzel T, Kutzner H. Dermatomyofibroma: clinicopathologic and immunohistochemical analysis of 56 cases and reappraisal of a rare and distinct cutaneous neoplasm. Am J Dermatopathol. 2009;31:44-49.
- Hugel H. Plaque-like dermal fibromatosis. Hautarzt. 1991;42:223-226.
- Bolognia JL, Jorizzo JL, Schaffer JV, eds. Dermatology. WB Saunders Co; 2012.
- Myers DJ, Fillman EP. Dermatofibroma. StatPearls [Internet]. StatPearls Publishing; 2020.
- Dilek N, Yuksel D, Sehitoglu I, et al. Cutaneous leiomyoma in a child: a case report. Oncol Lett. 2013;5:1163-1164.
- Roh HS, Paek JO, Yu HJ, et al. Solitary cutaneous myofibroma on the sole: an unusual localization. Ann Dermatol. 2012;24:220-222.
- Weedon D, Strutton G, Rubin AI, et al. Weedon’s Skin Pathology. Churchill Livingstone/Elsevier; 2010.
- Mendenhall WM, Zlotecki RA, Scarborough MT. Dermatofibrosarcoma protuberans. Cancer. 2004;101:2503-2508.
- Akay BN, Unlu E, Erdem C, et al. Dermatoscopic findings of atrophic dermatofibrosarcoma protuberans. Dermatol Pract Concept. 2015;5:71-73.
- Ma JE, Wieland CN, Tollefson MM. Dermatomyofibromas arising in children: report of two new cases and review of the literature. Pediatr Dermatol. 2017;34:347-351.
- Tardio JC, Azorin D, Hernandez-Nunez A, et al. Dermatomyofibromas presenting in pediatric patients: clinicopathologic characteristics and differential diagnosis. J Cutan Pathol. 2011;38:967-972.
- Mentzel T, Kutzner H. Dermatomyofibroma: clinicopathologic and immunohistochemical analysis of 56 cases and reappraisal of a rare and distinct cutaneous neoplasm. Am J Dermatopathol. 2009;31:44-49.
- Hugel H. Plaque-like dermal fibromatosis. Hautarzt. 1991;42:223-226.
- Bolognia JL, Jorizzo JL, Schaffer JV, eds. Dermatology. WB Saunders Co; 2012.
- Myers DJ, Fillman EP. Dermatofibroma. StatPearls [Internet]. StatPearls Publishing; 2020.
- Dilek N, Yuksel D, Sehitoglu I, et al. Cutaneous leiomyoma in a child: a case report. Oncol Lett. 2013;5:1163-1164.
- Roh HS, Paek JO, Yu HJ, et al. Solitary cutaneous myofibroma on the sole: an unusual localization. Ann Dermatol. 2012;24:220-222.
- Weedon D, Strutton G, Rubin AI, et al. Weedon’s Skin Pathology. Churchill Livingstone/Elsevier; 2010.
- Mendenhall WM, Zlotecki RA, Scarborough MT. Dermatofibrosarcoma protuberans. Cancer. 2004;101:2503-2508.
- Akay BN, Unlu E, Erdem C, et al. Dermatoscopic findings of atrophic dermatofibrosarcoma protuberans. Dermatol Pract Concept. 2015;5:71-73.
A 12-year-old boy with olive skin presented with a tender subcutaneous nodule on the back of 6 months’ duration. He reported the lesion initially grew rapidly with increasing pain for approximately 3 months with subsequent stabilization in size and modest resolution of his symptoms. Physical examination revealed a solitary, 15-mm, ill-defined, indurated, tender, subcutaneous nodule with subtle overlying hyperpigmentation on the left side of the upper back. Hematoxylin and eosin staining of a 4-mm punch biopsy revealed a nonencapsulated mass of monomorphic eosinophilic spindle cells organized into fascicles arranged predominantly parallel to the skin surface. The mass extended from the mid reticular dermis to the upper subcutis, sparing adnexal structures.
Firm Digital Papulonodules in an Infant
The Diagnosis: Infantile Digital Fibromatosis
Infantile digital fibromatosis (IDF) is a rare benign neoplasm of infancy prone to recurrence after resection but not to metastasis. It usually is limited to the fingers and toes.1 One-third of cases occur at birth. Most patients develop clinical symptoms within the first year of life, but presentation can occur in adolescents and adults. The exact etiology and pathogenesis of IDF remain unclear, but trauma is thought to be a trigger.
Physical examination reveals single or multiple smooth, round, pink papules or nodules confined to the sides and backs of the fingers, sparing the thumb and first toe.2,3 The nodules typically are firm, less than 2 cm in diameter, and often painless. Infantile digital fibromatosis exhibits an indolent progression followed by a rapid growth phase during several months, which may lead to functional impairment and joint deformities.4,5 Histopathology displays spindle cells with eosinophilic cytoplasmic inclusions that range from round to oval with uneven distribution, lack of refraction, and a large size difference (3–15 μm).6 The inclusions are deep red with Masson trichrome staining and can express smooth muscle actin and calponin. Tumor cells usually express vimentin, smooth muscle actin, calponin, and desmin but fail to express S-100 protein. The Ki67 proliferation index is 2% to 15%.6,7
Nonsurgical treatments for IDF include topical imiquimod, topical or intradermal injection of glucocorticoids, and intradermal injection of 5-fluorouracil. Complete resection should be reserved for cases with invasive growth that may lead to joint deformities, tendon or ligament involvement, digit or contracture deformity, and complications such as decreased joint mobility. Although there is a recurrence rate of up to 50% after excision, most lesions eventually will spontaneously regress and will leave no scar.8-10
The clinical and histopathologic differential diagnoses of IDF include other cutaneous diseases that occur in the digits. A dermatofibroma is a round, firm, fibrohistiocytic nodule that mainly occurs on the extensor limbs. Histopathology includes both fibrous and cellular types.11 Histologic analysis shows an ill-defined dermal proliferation of spindled fibroblasts with pale eosinophilic cytoplasm and bland fusiform nuclei growing in bands or fascicles that trap collagen fibers at the periphery (Figure 1). Generally, dermatofibromas have marked epidermal hyperplasia, which differs from IDF.
A digital myxoid cyst is characterized by a fleshcolored, hemispherical, and translucent cystic nodule that arises from the dorsum of the distal interphalangeal joint.12 It commonly is associated with injury and chronic pressure. Translucent viscous liquid may flow out when the cyst is punctured, a hallmark feature of this entity. Clinical variants of myxoid cyst include myxomatous and ganglion types. Histopathology reveals excessive mucin deposited in the dermis, and the surrounding collagen is compressed to form the pseudocyst (Figure 2).
A giant cell tumor of the tendon sheath presents with asymptomatic nodules or lumps. Lesions frequently are localized to the tendon sheath, especially on the fingers and wrists, with no malignant tendency or propensity for spontaneous regression.13 The local recurrence rate is as high as 45%, which is related to surgical resection insufficiency.14 Histopathologic examination shows lobulated tumor tissue surrounded by dense fibrosis. The tumor cells are histiocytic with scattered giant cells (Figure 3). The characteristic osteoclastlike giant cells have eosinophilic cytoplasm and irregularly arranged nuclei in varying numbers.
Keloids are connective tissue hyperplasias caused by skin injury. Histopathologically, keloids are characterized by nodules of thick hyalinized collagen bundles and whorled fibroblasts (Figure 4). No inclusions in the fibroblasts and a history of trauma can differentiate keloids from IDF.

- Marks E, Ewart M. Infantile digital fibroma: a rare fibromatosis. Arch Pathol Lab Med. 2016;140:1153‐1156.
- Botelho LF, Matsushigue T, Enokihara MM, et al. Case for diagnosis. An Bras Dermatol. 2012;87:493-494.
- Paloni G, Mattei I, Salmaso R, et al. Infantile digital fibromatosis. Arch Dis Child. 2013;98:308.
- Girgenti V, Restano L, Arcangeli F, et al. Infantile digital fibromatosis: a rare tumour of infancy. report of five cases. Australas J Dermatol. 2012;53:285-287.
- Eypper EH, Lee JC, Tarasen AJ, et al. An algorithmic approach to the management of infantile digital fibromatosis: review of literature and a case report. Eplasty. 2018;18:E19.
- Laskin WB, Miettinen M, Fetsch JF. Infantile digital fibroma /fibromatosis: a clinicopathologic and immunohistochemical study of 69 tumors from 57 patients with long-term follow-up. Am J Surg Pathol. 2009;33:1-13.
- Henderson H, Peng YJ, Salter DM. Anti-calponin 1 antibodies highlight intracytoplasmic inclusions of infantile digital fibromatosis. Histopathology. 2014,64:752-755.
- Campbell LB, Petrick MG. Mohs micrographic surgery for a problematic infantile digital fibroma. Dermatol Surg. 2007;33:385-387.
- Ochi H, Puhaindran ME, Tan KW. Firm digital papulonodules in a young boy. Int J Dermatol. 2019;58:91-92.
- Albertini JG, Welsch MJ, Conger LA, et al. Infantile digital fibroma treated with Mohs micrography surgery. Dermatol Surg. 2002;28:959-961.
- Alves JV, Matos DM, Barreiros HF, et al. Variants of dermatofibroma— a histopathological study. An Bras Dermatol. 2014;89:472-477.
- Meyers AL, Fallahi AKM. Digital Mucous Cyst. StatPearls Publishing; 2020.
- Zhao Q, Lu H. Giant cell tumor of tendon sheath in the wrist that damaged the extensor indicis proprius tendon: a case report and literature review. BMC Cancer. 2019;19:1057.
- DiGrazia S, Succi G, Fragetta F, et al. Giant cell tumor of tendon sheath: study of 64 cases and review of literature. G Chir. 2013;34:149-152.
The Diagnosis: Infantile Digital Fibromatosis
Infantile digital fibromatosis (IDF) is a rare benign neoplasm of infancy prone to recurrence after resection but not to metastasis. It usually is limited to the fingers and toes.1 One-third of cases occur at birth. Most patients develop clinical symptoms within the first year of life, but presentation can occur in adolescents and adults. The exact etiology and pathogenesis of IDF remain unclear, but trauma is thought to be a trigger.
Physical examination reveals single or multiple smooth, round, pink papules or nodules confined to the sides and backs of the fingers, sparing the thumb and first toe.2,3 The nodules typically are firm, less than 2 cm in diameter, and often painless. Infantile digital fibromatosis exhibits an indolent progression followed by a rapid growth phase during several months, which may lead to functional impairment and joint deformities.4,5 Histopathology displays spindle cells with eosinophilic cytoplasmic inclusions that range from round to oval with uneven distribution, lack of refraction, and a large size difference (3–15 μm).6 The inclusions are deep red with Masson trichrome staining and can express smooth muscle actin and calponin. Tumor cells usually express vimentin, smooth muscle actin, calponin, and desmin but fail to express S-100 protein. The Ki67 proliferation index is 2% to 15%.6,7
Nonsurgical treatments for IDF include topical imiquimod, topical or intradermal injection of glucocorticoids, and intradermal injection of 5-fluorouracil. Complete resection should be reserved for cases with invasive growth that may lead to joint deformities, tendon or ligament involvement, digit or contracture deformity, and complications such as decreased joint mobility. Although there is a recurrence rate of up to 50% after excision, most lesions eventually will spontaneously regress and will leave no scar.8-10
The clinical and histopathologic differential diagnoses of IDF include other cutaneous diseases that occur in the digits. A dermatofibroma is a round, firm, fibrohistiocytic nodule that mainly occurs on the extensor limbs. Histopathology includes both fibrous and cellular types.11 Histologic analysis shows an ill-defined dermal proliferation of spindled fibroblasts with pale eosinophilic cytoplasm and bland fusiform nuclei growing in bands or fascicles that trap collagen fibers at the periphery (Figure 1). Generally, dermatofibromas have marked epidermal hyperplasia, which differs from IDF.
A digital myxoid cyst is characterized by a fleshcolored, hemispherical, and translucent cystic nodule that arises from the dorsum of the distal interphalangeal joint.12 It commonly is associated with injury and chronic pressure. Translucent viscous liquid may flow out when the cyst is punctured, a hallmark feature of this entity. Clinical variants of myxoid cyst include myxomatous and ganglion types. Histopathology reveals excessive mucin deposited in the dermis, and the surrounding collagen is compressed to form the pseudocyst (Figure 2).
A giant cell tumor of the tendon sheath presents with asymptomatic nodules or lumps. Lesions frequently are localized to the tendon sheath, especially on the fingers and wrists, with no malignant tendency or propensity for spontaneous regression.13 The local recurrence rate is as high as 45%, which is related to surgical resection insufficiency.14 Histopathologic examination shows lobulated tumor tissue surrounded by dense fibrosis. The tumor cells are histiocytic with scattered giant cells (Figure 3). The characteristic osteoclastlike giant cells have eosinophilic cytoplasm and irregularly arranged nuclei in varying numbers.
Keloids are connective tissue hyperplasias caused by skin injury. Histopathologically, keloids are characterized by nodules of thick hyalinized collagen bundles and whorled fibroblasts (Figure 4). No inclusions in the fibroblasts and a history of trauma can differentiate keloids from IDF.

The Diagnosis: Infantile Digital Fibromatosis
Infantile digital fibromatosis (IDF) is a rare benign neoplasm of infancy prone to recurrence after resection but not to metastasis. It usually is limited to the fingers and toes.1 One-third of cases occur at birth. Most patients develop clinical symptoms within the first year of life, but presentation can occur in adolescents and adults. The exact etiology and pathogenesis of IDF remain unclear, but trauma is thought to be a trigger.
Physical examination reveals single or multiple smooth, round, pink papules or nodules confined to the sides and backs of the fingers, sparing the thumb and first toe.2,3 The nodules typically are firm, less than 2 cm in diameter, and often painless. Infantile digital fibromatosis exhibits an indolent progression followed by a rapid growth phase during several months, which may lead to functional impairment and joint deformities.4,5 Histopathology displays spindle cells with eosinophilic cytoplasmic inclusions that range from round to oval with uneven distribution, lack of refraction, and a large size difference (3–15 μm).6 The inclusions are deep red with Masson trichrome staining and can express smooth muscle actin and calponin. Tumor cells usually express vimentin, smooth muscle actin, calponin, and desmin but fail to express S-100 protein. The Ki67 proliferation index is 2% to 15%.6,7
Nonsurgical treatments for IDF include topical imiquimod, topical or intradermal injection of glucocorticoids, and intradermal injection of 5-fluorouracil. Complete resection should be reserved for cases with invasive growth that may lead to joint deformities, tendon or ligament involvement, digit or contracture deformity, and complications such as decreased joint mobility. Although there is a recurrence rate of up to 50% after excision, most lesions eventually will spontaneously regress and will leave no scar.8-10
The clinical and histopathologic differential diagnoses of IDF include other cutaneous diseases that occur in the digits. A dermatofibroma is a round, firm, fibrohistiocytic nodule that mainly occurs on the extensor limbs. Histopathology includes both fibrous and cellular types.11 Histologic analysis shows an ill-defined dermal proliferation of spindled fibroblasts with pale eosinophilic cytoplasm and bland fusiform nuclei growing in bands or fascicles that trap collagen fibers at the periphery (Figure 1). Generally, dermatofibromas have marked epidermal hyperplasia, which differs from IDF.
A digital myxoid cyst is characterized by a fleshcolored, hemispherical, and translucent cystic nodule that arises from the dorsum of the distal interphalangeal joint.12 It commonly is associated with injury and chronic pressure. Translucent viscous liquid may flow out when the cyst is punctured, a hallmark feature of this entity. Clinical variants of myxoid cyst include myxomatous and ganglion types. Histopathology reveals excessive mucin deposited in the dermis, and the surrounding collagen is compressed to form the pseudocyst (Figure 2).
A giant cell tumor of the tendon sheath presents with asymptomatic nodules or lumps. Lesions frequently are localized to the tendon sheath, especially on the fingers and wrists, with no malignant tendency or propensity for spontaneous regression.13 The local recurrence rate is as high as 45%, which is related to surgical resection insufficiency.14 Histopathologic examination shows lobulated tumor tissue surrounded by dense fibrosis. The tumor cells are histiocytic with scattered giant cells (Figure 3). The characteristic osteoclastlike giant cells have eosinophilic cytoplasm and irregularly arranged nuclei in varying numbers.
Keloids are connective tissue hyperplasias caused by skin injury. Histopathologically, keloids are characterized by nodules of thick hyalinized collagen bundles and whorled fibroblasts (Figure 4). No inclusions in the fibroblasts and a history of trauma can differentiate keloids from IDF.

- Marks E, Ewart M. Infantile digital fibroma: a rare fibromatosis. Arch Pathol Lab Med. 2016;140:1153‐1156.
- Botelho LF, Matsushigue T, Enokihara MM, et al. Case for diagnosis. An Bras Dermatol. 2012;87:493-494.
- Paloni G, Mattei I, Salmaso R, et al. Infantile digital fibromatosis. Arch Dis Child. 2013;98:308.
- Girgenti V, Restano L, Arcangeli F, et al. Infantile digital fibromatosis: a rare tumour of infancy. report of five cases. Australas J Dermatol. 2012;53:285-287.
- Eypper EH, Lee JC, Tarasen AJ, et al. An algorithmic approach to the management of infantile digital fibromatosis: review of literature and a case report. Eplasty. 2018;18:E19.
- Laskin WB, Miettinen M, Fetsch JF. Infantile digital fibroma /fibromatosis: a clinicopathologic and immunohistochemical study of 69 tumors from 57 patients with long-term follow-up. Am J Surg Pathol. 2009;33:1-13.
- Henderson H, Peng YJ, Salter DM. Anti-calponin 1 antibodies highlight intracytoplasmic inclusions of infantile digital fibromatosis. Histopathology. 2014,64:752-755.
- Campbell LB, Petrick MG. Mohs micrographic surgery for a problematic infantile digital fibroma. Dermatol Surg. 2007;33:385-387.
- Ochi H, Puhaindran ME, Tan KW. Firm digital papulonodules in a young boy. Int J Dermatol. 2019;58:91-92.
- Albertini JG, Welsch MJ, Conger LA, et al. Infantile digital fibroma treated with Mohs micrography surgery. Dermatol Surg. 2002;28:959-961.
- Alves JV, Matos DM, Barreiros HF, et al. Variants of dermatofibroma— a histopathological study. An Bras Dermatol. 2014;89:472-477.
- Meyers AL, Fallahi AKM. Digital Mucous Cyst. StatPearls Publishing; 2020.
- Zhao Q, Lu H. Giant cell tumor of tendon sheath in the wrist that damaged the extensor indicis proprius tendon: a case report and literature review. BMC Cancer. 2019;19:1057.
- DiGrazia S, Succi G, Fragetta F, et al. Giant cell tumor of tendon sheath: study of 64 cases and review of literature. G Chir. 2013;34:149-152.
- Marks E, Ewart M. Infantile digital fibroma: a rare fibromatosis. Arch Pathol Lab Med. 2016;140:1153‐1156.
- Botelho LF, Matsushigue T, Enokihara MM, et al. Case for diagnosis. An Bras Dermatol. 2012;87:493-494.
- Paloni G, Mattei I, Salmaso R, et al. Infantile digital fibromatosis. Arch Dis Child. 2013;98:308.
- Girgenti V, Restano L, Arcangeli F, et al. Infantile digital fibromatosis: a rare tumour of infancy. report of five cases. Australas J Dermatol. 2012;53:285-287.
- Eypper EH, Lee JC, Tarasen AJ, et al. An algorithmic approach to the management of infantile digital fibromatosis: review of literature and a case report. Eplasty. 2018;18:E19.
- Laskin WB, Miettinen M, Fetsch JF. Infantile digital fibroma /fibromatosis: a clinicopathologic and immunohistochemical study of 69 tumors from 57 patients with long-term follow-up. Am J Surg Pathol. 2009;33:1-13.
- Henderson H, Peng YJ, Salter DM. Anti-calponin 1 antibodies highlight intracytoplasmic inclusions of infantile digital fibromatosis. Histopathology. 2014,64:752-755.
- Campbell LB, Petrick MG. Mohs micrographic surgery for a problematic infantile digital fibroma. Dermatol Surg. 2007;33:385-387.
- Ochi H, Puhaindran ME, Tan KW. Firm digital papulonodules in a young boy. Int J Dermatol. 2019;58:91-92.
- Albertini JG, Welsch MJ, Conger LA, et al. Infantile digital fibroma treated with Mohs micrography surgery. Dermatol Surg. 2002;28:959-961.
- Alves JV, Matos DM, Barreiros HF, et al. Variants of dermatofibroma— a histopathological study. An Bras Dermatol. 2014;89:472-477.
- Meyers AL, Fallahi AKM. Digital Mucous Cyst. StatPearls Publishing; 2020.
- Zhao Q, Lu H. Giant cell tumor of tendon sheath in the wrist that damaged the extensor indicis proprius tendon: a case report and literature review. BMC Cancer. 2019;19:1057.
- DiGrazia S, Succi G, Fragetta F, et al. Giant cell tumor of tendon sheath: study of 64 cases and review of literature. G Chir. 2013;34:149-152.
A 3-month-old girl presented with papulonodules on the distal left ring finger. Initially the lesions were thought to be insect bites but became firm over the course of 3 weeks and then gradually increased in size over 2 months. Physical examination revealed a 0.5×0.5-cm firm nodule and a 0.2×0.3-cm firm papule on the radial aspect of the left ring finger over the distal interphalangeal joint. There was no deformity or dysfunction of the finger. Radiography showed soft tissue swelling without bony abnormalities. The lesions were excised; however, a new fleshy nodule reappeared 1 month postoperatively on the radial aspect of the left ring finger over the distal interphalangeal joint. The patient did not seem bothered by the lesions and was in good general health.
Soft Nodule on the Forearm
The Diagnosis: Schwannoma
Schwannoma, also known as neurilemmoma, is a benign encapsulated neoplasm of the peripheral nerve sheath that presents as a subcutaneous nodule.1 It also may present in the retroperitoneum, mediastinum, and viscera (eg, gastrointestinal tract, bone, upper respiratory tract, lymph nodes). It may occur as multiple lesions when associated with certain syndromes. It usually is an asymptomatic indolent tumor with neurologic symptoms, such as pain and tenderness, in the lesions that are deeper, larger, or closer in proximity to nearby structures.2,3
Histologically, a schwannoma is encapsulated by the perineurium of the nerve bundle from which it originates (quiz image [top]). The tumor consists of hypercellular (Antoni type A) and hypocellular (Antoni type B) areas. Antoni type A areas consist of tightly packed, spindleshaped cells with elongated wavy nuclei and indistinct cytoplasmic borders. These nuclei tend to align into parallel rows with intervening anuclear zones forming Verocay bodies (quiz image [bottom]).4 Verocay bodies are not seen in all schwannomas, and similar formations may be seen in other tumors as well. Solitary circumscribed neuromas also have Verocay bodies, whereas dermatofibromas and leiomyomas have Verocay-like bodies. Antoni type B areas have scattered spindled or ovoid cells in an edematous or myxoid matrix interspersed with inflammatory cells such as lymphocytes and histiocytes. Vessels with thick hyalinized walls are a helpful feature in diagnosis.2 Schwann cells of a schwannoma stain diffusely positive with S-100 protein. The capsule stains positively with epithelial membrane antigen due to the presence of perineurial cells.2
The morphologic variants of this entity include conventional (common, solitary), cellular, plexiform, ancient, melanotic, epithelioid, pseudoglandular, neuroblastomalike, and microcystic/reticular schwannomas. There are additional variants that are associated with genetic syndromes, such as multiple cutaneous plexiform schwannomas linked with neurofibromatosis type 2, psammomatous melanotic schwannoma presenting in Carney complex, schwannomatosis, and segmental schwannomatosis (a distinct form of neurofibromatosis characterized by multiple schwannomas localized to one limb). Either presentation may have alteration or deletion of the neurofibromatosis type 2 gene, NF2, on chromosome 22.2,5
Nodular fasciitis is a benign tumor of fibroblasts and myofibroblasts that usually arises in the subcutaneous tissues. It most commonly occurs in the upper extremities, trunk, head, and neck. It presents as a single, often painful, rapidly growing, subcutaneous nodule. Histologically, lesions mostly are well circumscribed yet unencapsulated, in contrast to schwannomas. They may be hypocellular or hypercellular and are composed of uniform spindle cells with a feathery or fascicular (tissue culture–like) appearance in a loose, myxoid to collagenous stroma. There may be foci of hemorrhage and conspicuous mitoses but not atypical figures (Figure 1). Immunohistochemically, the cells stain positively for smooth muscle actin and negatively for S-100 protein, which sets it apart from a schwannoma. Most cases contain fusion genes, with myosin heavy chain 9 ubiquitin-specific peptidase 6, MYH9-USP6, being the most common fusion product.6
Solitary circumscribed neuroma (palisaded encapsulated neuroma) is a benign, usually solitary dermal lesion. It most commonly occurs in middle-aged to elderly adults as a small (<1 cm), firm, flesh-colored to pink papule on the face (ie, cheeks, nose, nasolabial folds) and less commonly in the oral and acral regions and on the eyelids and penis. The lesion usually is unilobular; however, other growth patterns such as plexiform, multilobular, and fungating variants have been identified. Histologically, it is a well-circumscribed nodule with a thin capsule of perineurium that is composed of interlacing bundles of Schwann cells with a characteristic clefting artifact (Figure 2). Cells have wavy dark nuclei with scant cytoplasm that occasionally form palisades or Verocay bodies causing these lesions to be confused with schwannomas. Immunohistochemically, the Schwann cells stain positively with S-100 protein, and the perineurium stains positively with epithelial membrane antigen, Claudin-1, and Glut-1. Neurofilament protein stains axons throughout neuromas, whereas in schwannoma, the expression often is limited to entrapped axons at the periphery of the tumor.7
Angioleiomyoma is an uncommon, benign, smooth muscle neoplasm of the skin and subcutaneous tissue that originates from vascular smooth muscle. It most commonly presents in adult females aged 30 to 60 years, with a predilection for the lower limbs. These tumors typically are solitary, slow growing, and less than 2 cm in diameter and may be painful upon compression. Similar to schwannoma, angioleiomyoma is an encapsulated lesion composed of interlacing, uniform, smooth muscle bundles distributed around vessels (Figure 3). Smooth muscle cells have oval- or cigar-shaped nuclei with a small perinuclear vacuole of glycogen. Immunohistochemically, there is strong diffuse staining for smooth muscle actin and h-caldesmon. Recurrence after excision is rare.2,8
Neurofibroma is a common, mostly sporadic, benign tumor of nerve sheath origin. The solitary type may be localized (well circumscribed, unencapsulated) or diffuse. The presence of multiple, deep, and plexiform lesions is associated with neurofibromatosis type 1 (von Recklinghausen disease) that is caused by germline mutations in the NF1 gene. Histologically, the tumor is composed of Schwann cells, fibroblasts, perineurial cells, and nerve axons within an extracellular myxoid to collagenous matrix (Figure 4). The diffuse type is an ill-defined proliferation that entraps adnexal structures. The plexiform type is defined by multinodular serpentine fascicles. Immunohistochemically, the Schwann cells stain positive for S-100 protein and SOX10 (SRY-Box Transcription Factor 10). Epithelial membrane antigen stains admixed perineurial cells. Neurofilament protein highlights intratumoral axons, which generally are not found throughout schwannomas. Transformation to a malignant peripheral nerve sheath tumor occurs in up to 10% of patients with neurofibromatosis type 1, usually in plexiform neurofibromas, and is characterized by increased cellularity, atypia, mitotic activity, and necrosis.9
- Ritter SE, Elston DM. Cutaneous schwannoma of the foot. Cutis. 2001;67:127-129.
- Calonje E, Damaskou V, Lazar AJ. Connective tissue tumors. In: Calonje E, Brenn T, Lazar AJ, et al, eds. McKee’s Pathology of the Skin. 5th ed. Vol 2. Elsevier Saunders; 2020:1698-1894.
- Knight DM, Birch R, Pringle J. Benign solitary schwannomas: a review of 234 cases. J Bone Joint Surg Br. 2007;89:382-387.
- Lespi PJ, Smit R. Verocay body—prominent cutaneous leiomyoma. Am J Dermatopathol. 1999;21:110-111.
- Kurtkaya-Yapicier O, Scheithauer B, Woodruff JM. The pathobiologic spectrum of schwannomas. Histol Histopathol. 2003;18:925-934.
- Erickson-Johnson MR, Chou MM, Evers BR, et al. Nodular fasciitis: a novel model of transient neoplasia induced by MYH9-USP6 gene fusion. Lab Invest. 2011;91:1427-1433.
- Leblebici C, Savli TC, Yeni B, et al. Palisaded encapsulated (solitary circumscribed) neuroma: a review of 30 cases. Int J Surg Pathol. 2019;27:506-514.
- Yeung CM, Moore L, Lans J, et al. Angioleiomyoma of the hand: a case series and review of the literature. Arch Bone Jt Surg. 2020; 8:373-377.
- Skovronsky DM, Oberholtzer JC. Pathologic classification of peripheral nerve tumors. Neurosurg Clin North Am. 2004;15:157-166.
The Diagnosis: Schwannoma
Schwannoma, also known as neurilemmoma, is a benign encapsulated neoplasm of the peripheral nerve sheath that presents as a subcutaneous nodule.1 It also may present in the retroperitoneum, mediastinum, and viscera (eg, gastrointestinal tract, bone, upper respiratory tract, lymph nodes). It may occur as multiple lesions when associated with certain syndromes. It usually is an asymptomatic indolent tumor with neurologic symptoms, such as pain and tenderness, in the lesions that are deeper, larger, or closer in proximity to nearby structures.2,3
Histologically, a schwannoma is encapsulated by the perineurium of the nerve bundle from which it originates (quiz image [top]). The tumor consists of hypercellular (Antoni type A) and hypocellular (Antoni type B) areas. Antoni type A areas consist of tightly packed, spindleshaped cells with elongated wavy nuclei and indistinct cytoplasmic borders. These nuclei tend to align into parallel rows with intervening anuclear zones forming Verocay bodies (quiz image [bottom]).4 Verocay bodies are not seen in all schwannomas, and similar formations may be seen in other tumors as well. Solitary circumscribed neuromas also have Verocay bodies, whereas dermatofibromas and leiomyomas have Verocay-like bodies. Antoni type B areas have scattered spindled or ovoid cells in an edematous or myxoid matrix interspersed with inflammatory cells such as lymphocytes and histiocytes. Vessels with thick hyalinized walls are a helpful feature in diagnosis.2 Schwann cells of a schwannoma stain diffusely positive with S-100 protein. The capsule stains positively with epithelial membrane antigen due to the presence of perineurial cells.2
The morphologic variants of this entity include conventional (common, solitary), cellular, plexiform, ancient, melanotic, epithelioid, pseudoglandular, neuroblastomalike, and microcystic/reticular schwannomas. There are additional variants that are associated with genetic syndromes, such as multiple cutaneous plexiform schwannomas linked with neurofibromatosis type 2, psammomatous melanotic schwannoma presenting in Carney complex, schwannomatosis, and segmental schwannomatosis (a distinct form of neurofibromatosis characterized by multiple schwannomas localized to one limb). Either presentation may have alteration or deletion of the neurofibromatosis type 2 gene, NF2, on chromosome 22.2,5
Nodular fasciitis is a benign tumor of fibroblasts and myofibroblasts that usually arises in the subcutaneous tissues. It most commonly occurs in the upper extremities, trunk, head, and neck. It presents as a single, often painful, rapidly growing, subcutaneous nodule. Histologically, lesions mostly are well circumscribed yet unencapsulated, in contrast to schwannomas. They may be hypocellular or hypercellular and are composed of uniform spindle cells with a feathery or fascicular (tissue culture–like) appearance in a loose, myxoid to collagenous stroma. There may be foci of hemorrhage and conspicuous mitoses but not atypical figures (Figure 1). Immunohistochemically, the cells stain positively for smooth muscle actin and negatively for S-100 protein, which sets it apart from a schwannoma. Most cases contain fusion genes, with myosin heavy chain 9 ubiquitin-specific peptidase 6, MYH9-USP6, being the most common fusion product.6
Solitary circumscribed neuroma (palisaded encapsulated neuroma) is a benign, usually solitary dermal lesion. It most commonly occurs in middle-aged to elderly adults as a small (<1 cm), firm, flesh-colored to pink papule on the face (ie, cheeks, nose, nasolabial folds) and less commonly in the oral and acral regions and on the eyelids and penis. The lesion usually is unilobular; however, other growth patterns such as plexiform, multilobular, and fungating variants have been identified. Histologically, it is a well-circumscribed nodule with a thin capsule of perineurium that is composed of interlacing bundles of Schwann cells with a characteristic clefting artifact (Figure 2). Cells have wavy dark nuclei with scant cytoplasm that occasionally form palisades or Verocay bodies causing these lesions to be confused with schwannomas. Immunohistochemically, the Schwann cells stain positively with S-100 protein, and the perineurium stains positively with epithelial membrane antigen, Claudin-1, and Glut-1. Neurofilament protein stains axons throughout neuromas, whereas in schwannoma, the expression often is limited to entrapped axons at the periphery of the tumor.7
Angioleiomyoma is an uncommon, benign, smooth muscle neoplasm of the skin and subcutaneous tissue that originates from vascular smooth muscle. It most commonly presents in adult females aged 30 to 60 years, with a predilection for the lower limbs. These tumors typically are solitary, slow growing, and less than 2 cm in diameter and may be painful upon compression. Similar to schwannoma, angioleiomyoma is an encapsulated lesion composed of interlacing, uniform, smooth muscle bundles distributed around vessels (Figure 3). Smooth muscle cells have oval- or cigar-shaped nuclei with a small perinuclear vacuole of glycogen. Immunohistochemically, there is strong diffuse staining for smooth muscle actin and h-caldesmon. Recurrence after excision is rare.2,8
Neurofibroma is a common, mostly sporadic, benign tumor of nerve sheath origin. The solitary type may be localized (well circumscribed, unencapsulated) or diffuse. The presence of multiple, deep, and plexiform lesions is associated with neurofibromatosis type 1 (von Recklinghausen disease) that is caused by germline mutations in the NF1 gene. Histologically, the tumor is composed of Schwann cells, fibroblasts, perineurial cells, and nerve axons within an extracellular myxoid to collagenous matrix (Figure 4). The diffuse type is an ill-defined proliferation that entraps adnexal structures. The plexiform type is defined by multinodular serpentine fascicles. Immunohistochemically, the Schwann cells stain positive for S-100 protein and SOX10 (SRY-Box Transcription Factor 10). Epithelial membrane antigen stains admixed perineurial cells. Neurofilament protein highlights intratumoral axons, which generally are not found throughout schwannomas. Transformation to a malignant peripheral nerve sheath tumor occurs in up to 10% of patients with neurofibromatosis type 1, usually in plexiform neurofibromas, and is characterized by increased cellularity, atypia, mitotic activity, and necrosis.9
The Diagnosis: Schwannoma
Schwannoma, also known as neurilemmoma, is a benign encapsulated neoplasm of the peripheral nerve sheath that presents as a subcutaneous nodule.1 It also may present in the retroperitoneum, mediastinum, and viscera (eg, gastrointestinal tract, bone, upper respiratory tract, lymph nodes). It may occur as multiple lesions when associated with certain syndromes. It usually is an asymptomatic indolent tumor with neurologic symptoms, such as pain and tenderness, in the lesions that are deeper, larger, or closer in proximity to nearby structures.2,3
Histologically, a schwannoma is encapsulated by the perineurium of the nerve bundle from which it originates (quiz image [top]). The tumor consists of hypercellular (Antoni type A) and hypocellular (Antoni type B) areas. Antoni type A areas consist of tightly packed, spindleshaped cells with elongated wavy nuclei and indistinct cytoplasmic borders. These nuclei tend to align into parallel rows with intervening anuclear zones forming Verocay bodies (quiz image [bottom]).4 Verocay bodies are not seen in all schwannomas, and similar formations may be seen in other tumors as well. Solitary circumscribed neuromas also have Verocay bodies, whereas dermatofibromas and leiomyomas have Verocay-like bodies. Antoni type B areas have scattered spindled or ovoid cells in an edematous or myxoid matrix interspersed with inflammatory cells such as lymphocytes and histiocytes. Vessels with thick hyalinized walls are a helpful feature in diagnosis.2 Schwann cells of a schwannoma stain diffusely positive with S-100 protein. The capsule stains positively with epithelial membrane antigen due to the presence of perineurial cells.2
The morphologic variants of this entity include conventional (common, solitary), cellular, plexiform, ancient, melanotic, epithelioid, pseudoglandular, neuroblastomalike, and microcystic/reticular schwannomas. There are additional variants that are associated with genetic syndromes, such as multiple cutaneous plexiform schwannomas linked with neurofibromatosis type 2, psammomatous melanotic schwannoma presenting in Carney complex, schwannomatosis, and segmental schwannomatosis (a distinct form of neurofibromatosis characterized by multiple schwannomas localized to one limb). Either presentation may have alteration or deletion of the neurofibromatosis type 2 gene, NF2, on chromosome 22.2,5
Nodular fasciitis is a benign tumor of fibroblasts and myofibroblasts that usually arises in the subcutaneous tissues. It most commonly occurs in the upper extremities, trunk, head, and neck. It presents as a single, often painful, rapidly growing, subcutaneous nodule. Histologically, lesions mostly are well circumscribed yet unencapsulated, in contrast to schwannomas. They may be hypocellular or hypercellular and are composed of uniform spindle cells with a feathery or fascicular (tissue culture–like) appearance in a loose, myxoid to collagenous stroma. There may be foci of hemorrhage and conspicuous mitoses but not atypical figures (Figure 1). Immunohistochemically, the cells stain positively for smooth muscle actin and negatively for S-100 protein, which sets it apart from a schwannoma. Most cases contain fusion genes, with myosin heavy chain 9 ubiquitin-specific peptidase 6, MYH9-USP6, being the most common fusion product.6
Solitary circumscribed neuroma (palisaded encapsulated neuroma) is a benign, usually solitary dermal lesion. It most commonly occurs in middle-aged to elderly adults as a small (<1 cm), firm, flesh-colored to pink papule on the face (ie, cheeks, nose, nasolabial folds) and less commonly in the oral and acral regions and on the eyelids and penis. The lesion usually is unilobular; however, other growth patterns such as plexiform, multilobular, and fungating variants have been identified. Histologically, it is a well-circumscribed nodule with a thin capsule of perineurium that is composed of interlacing bundles of Schwann cells with a characteristic clefting artifact (Figure 2). Cells have wavy dark nuclei with scant cytoplasm that occasionally form palisades or Verocay bodies causing these lesions to be confused with schwannomas. Immunohistochemically, the Schwann cells stain positively with S-100 protein, and the perineurium stains positively with epithelial membrane antigen, Claudin-1, and Glut-1. Neurofilament protein stains axons throughout neuromas, whereas in schwannoma, the expression often is limited to entrapped axons at the periphery of the tumor.7
Angioleiomyoma is an uncommon, benign, smooth muscle neoplasm of the skin and subcutaneous tissue that originates from vascular smooth muscle. It most commonly presents in adult females aged 30 to 60 years, with a predilection for the lower limbs. These tumors typically are solitary, slow growing, and less than 2 cm in diameter and may be painful upon compression. Similar to schwannoma, angioleiomyoma is an encapsulated lesion composed of interlacing, uniform, smooth muscle bundles distributed around vessels (Figure 3). Smooth muscle cells have oval- or cigar-shaped nuclei with a small perinuclear vacuole of glycogen. Immunohistochemically, there is strong diffuse staining for smooth muscle actin and h-caldesmon. Recurrence after excision is rare.2,8
Neurofibroma is a common, mostly sporadic, benign tumor of nerve sheath origin. The solitary type may be localized (well circumscribed, unencapsulated) or diffuse. The presence of multiple, deep, and plexiform lesions is associated with neurofibromatosis type 1 (von Recklinghausen disease) that is caused by germline mutations in the NF1 gene. Histologically, the tumor is composed of Schwann cells, fibroblasts, perineurial cells, and nerve axons within an extracellular myxoid to collagenous matrix (Figure 4). The diffuse type is an ill-defined proliferation that entraps adnexal structures. The plexiform type is defined by multinodular serpentine fascicles. Immunohistochemically, the Schwann cells stain positive for S-100 protein and SOX10 (SRY-Box Transcription Factor 10). Epithelial membrane antigen stains admixed perineurial cells. Neurofilament protein highlights intratumoral axons, which generally are not found throughout schwannomas. Transformation to a malignant peripheral nerve sheath tumor occurs in up to 10% of patients with neurofibromatosis type 1, usually in plexiform neurofibromas, and is characterized by increased cellularity, atypia, mitotic activity, and necrosis.9
- Ritter SE, Elston DM. Cutaneous schwannoma of the foot. Cutis. 2001;67:127-129.
- Calonje E, Damaskou V, Lazar AJ. Connective tissue tumors. In: Calonje E, Brenn T, Lazar AJ, et al, eds. McKee’s Pathology of the Skin. 5th ed. Vol 2. Elsevier Saunders; 2020:1698-1894.
- Knight DM, Birch R, Pringle J. Benign solitary schwannomas: a review of 234 cases. J Bone Joint Surg Br. 2007;89:382-387.
- Lespi PJ, Smit R. Verocay body—prominent cutaneous leiomyoma. Am J Dermatopathol. 1999;21:110-111.
- Kurtkaya-Yapicier O, Scheithauer B, Woodruff JM. The pathobiologic spectrum of schwannomas. Histol Histopathol. 2003;18:925-934.
- Erickson-Johnson MR, Chou MM, Evers BR, et al. Nodular fasciitis: a novel model of transient neoplasia induced by MYH9-USP6 gene fusion. Lab Invest. 2011;91:1427-1433.
- Leblebici C, Savli TC, Yeni B, et al. Palisaded encapsulated (solitary circumscribed) neuroma: a review of 30 cases. Int J Surg Pathol. 2019;27:506-514.
- Yeung CM, Moore L, Lans J, et al. Angioleiomyoma of the hand: a case series and review of the literature. Arch Bone Jt Surg. 2020; 8:373-377.
- Skovronsky DM, Oberholtzer JC. Pathologic classification of peripheral nerve tumors. Neurosurg Clin North Am. 2004;15:157-166.
- Ritter SE, Elston DM. Cutaneous schwannoma of the foot. Cutis. 2001;67:127-129.
- Calonje E, Damaskou V, Lazar AJ. Connective tissue tumors. In: Calonje E, Brenn T, Lazar AJ, et al, eds. McKee’s Pathology of the Skin. 5th ed. Vol 2. Elsevier Saunders; 2020:1698-1894.
- Knight DM, Birch R, Pringle J. Benign solitary schwannomas: a review of 234 cases. J Bone Joint Surg Br. 2007;89:382-387.
- Lespi PJ, Smit R. Verocay body—prominent cutaneous leiomyoma. Am J Dermatopathol. 1999;21:110-111.
- Kurtkaya-Yapicier O, Scheithauer B, Woodruff JM. The pathobiologic spectrum of schwannomas. Histol Histopathol. 2003;18:925-934.
- Erickson-Johnson MR, Chou MM, Evers BR, et al. Nodular fasciitis: a novel model of transient neoplasia induced by MYH9-USP6 gene fusion. Lab Invest. 2011;91:1427-1433.
- Leblebici C, Savli TC, Yeni B, et al. Palisaded encapsulated (solitary circumscribed) neuroma: a review of 30 cases. Int J Surg Pathol. 2019;27:506-514.
- Yeung CM, Moore L, Lans J, et al. Angioleiomyoma of the hand: a case series and review of the literature. Arch Bone Jt Surg. 2020; 8:373-377.
- Skovronsky DM, Oberholtzer JC. Pathologic classification of peripheral nerve tumors. Neurosurg Clin North Am. 2004;15:157-166.
A 54-year-old woman presented with an enlarging mass on the right volar forearm. Physical examination revealed a 1-cm, soft, mobile, subcutaneous nodule. Excision revealed tan-pink, indurated, fibrous, nodular tissue.
Erythematous and Ulcerated Plaque on the Left Temple
The Diagnosis: Primary Cutaneous Carcinosarcoma
The immunohistochemical findings supported an epithelial component consistent with moderately differentiated squamous cell carcinoma (SCC) and a mesenchymal component with features consistent with a sarcoma. Consequently, the lesion was diagnosed as a primary cutaneous carcinosarcoma (PCCS).
Primary cutaneous carcinosarcoma is a rare biphasic neoplasm consisting of malignant epithelial (carcinoma) and mesenchymal (sarcoma) components.1 Primary cutaneous carcinosarcomas are uncommon, poorly understood, primary cutaneous tumors.2,3 Characteristic of this tumor, cytokeratins highlight the epithelial component while vimentin highlights the mesenchymal component.4 Histologically, the sarcomatous components of PCCS often are highly variable, with an absence of transitional areas within the epithelial component, which frequently resembles basal cell carcinoma and/ or SCC.5-7 Primary cutaneous carcinosarcoma favors areas of chronic UV radiation exposure, particularly on the head and neck. Most tumors present with a slowly growing, polypoid, flesh-colored to erythematous nodule due to the infiltrative mesenchymal component.7 Primary cutaneous carcinosarcoma primarily is diagnosed in elderly patients, with the majority of cases diagnosed in the eighth or ninth decades of life (range, 32–98 years).1,8 Men appear to be twice as likely to be diagnosed with a PCCS compared to women.1 Primary cutaneous carcinosarcomas are recognized as aggressive tumors with a high propensity to metastasize and recur locally, necessitating early diagnosis and treatment.4 Accurate diagnosis of PCCSs can be challenging due to the biphasic nature of the neoplasm as well as poor differentiation or unequal proportions of the epithelial and mesenchymal components.5 Additionally, overlapping diagnostic criteria coupled with vague demarcation between soft-tissue sarcomas and distinct carcinomas also may contribute to a delay in diagnosis.9 Treatment is achieved surgically by complete wide resection, with no evidence to support the use of adjuvant or neoadjuvant external beam radiation therapy. Due to the small number of reported cases, no treatment recommendations currently exist.1
Surgical management with wide local excision has been disappointing, with recurrence rates reported as high as 33%.6 Primary cutaneous carcinosarcoma has an estimated overall recurrence rate of 19% and a 5-year disease-free rate of 50%.10 Risk factors associated with poorer prognosis include tumors with adnexal subtype, age less than 65 years, rapid tumor growth, a tumor greater than 20 mm at presentation, and a long-standing tumor lasting up to 30 years.2,4 Although wide local excision and Mohs micrographic surgery (MMS) both have been utilized successfully, MMS has been shown to result in a cure rate of greater than 98%.6
Atypical fibroxanthoma (AFX) is a cutaneous tumor of fibrohistiocytic mesenchymal origin that typically manifests on sun-damaged skin in elderly individuals. Clinically, it presents as a rapidly growing neoplasm that often ulcerates and bleeds. These heterogenous neoplasms have several distinct characteristics, including dense cellularity with disorganized, large, pleomorphic, and atypical-appearing spindle-shaped cells arising in the upper layers of the dermis, often disseminating into the reticular dermis and occasionally into the subcutaneous fat (Figure 1). The neoplastic cells often exhibit hyperchromic and irregular nuclei, multinucleated giant cells, and atypical mitotic figures. In most cases, negative immunohistochemical staining with SOX-10, S-100, cytokeratins, desmin, and caldesmon will allow pathologists to differentiate between AFX and other common tumors on the differential diagnosis, such as SCC, melanoma, and leiomyosarcoma. CD10 and procollagen type 1 are positive antigenic markers in AFX, but they are not specific. The standard treatment of AFX includes wide local excision or MMS for superior margin control.11
Spindle cell SCC presents as a raised or exophytic nodule, often with spontaneous bleeding and central ulceration. It usually presents on sun-damaged skin or in individuals with a history of ionizing radiation. Histologically, it is characterized by atypical spindleshaped keratinocytes in the dermis existing as single cells or cohesive nests along with keratin pearls (Figure 2). The atypical spindle cells may comprise the entire tumor or only a small portion. The use of immunohistochemical markers often is required to establish a definitive diagnosis. Spindle cell SCC stains positively, albeit frequently focally, for p63, p40, and high-molecular-weight cytokeratins such as cytokeratin 5/6, while S-100 protein, SOX-10, MART-1/Melan-A, and muscle-specific actin stains typically are negative. Wide local excision or MMS is recommended for treatment of these lesions.12
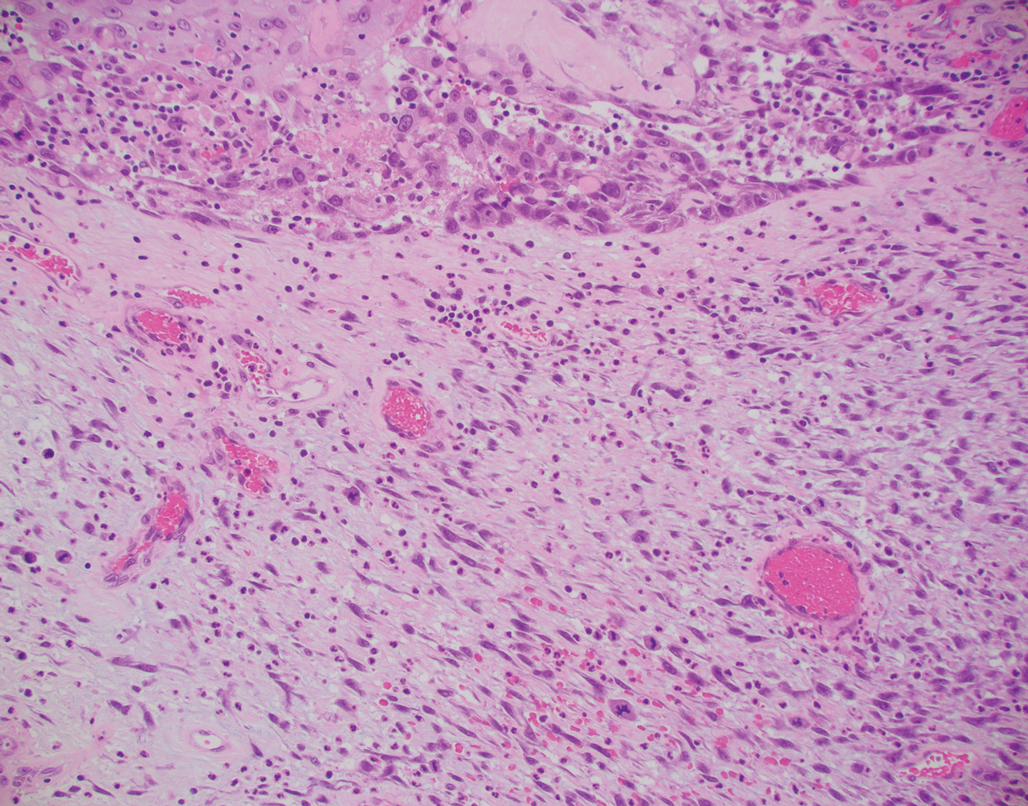
Primary cutaneous myoepithelial carcinomas are uncommon neoplasms of myoepithelial differentiation. Clinically, they often arise as soft nodular lesions on the head, neck, and lower extremities with a bimodal age distribution (50 years). Histologically cutaneous myoepithelial tumors are well-differentiated, dermal-based nodules without connection to the overlying epidermis (Figure 3). The myoepithelial cells can exhibit spindled, epithelioid, plasmacytoid, or clear cell morphologic features and show variability in cell growth patterns. One of the most common growth patterns is oval to round cells forming cords and chains in a chondromyxoid stroma. Most cases display an immunophenotyped co-expression of an epithelial cytokeratin and S-100 protein. Myoepithelial markers also may be present, including keratins, smooth muscle actin, calponin, glial fibrillary acidic protein, p63, and desmin. Surgical removal with wide local excision or MMS is essential.13
Leiomyosarcoma (LMS) is a tumor that originates from smooth muscle and rarely develops in the dermis.14 Pleomorphic LMS is a morphologic variant of LMS that has a low propensity to metastasize but commonly exhibits local recurrence.15 Leiomyosarcoma can present in any age group but most commonly manifests in individuals aged 50 to 70 years. Clinically, LMS presents as a firm solitary nodule with a smooth pink surface or a more exophytic tumor with a reddish or brown color on the extensor surface of the lower limbs; it is less common on the scalp and face.14 Histologically, most cases of pleomorphic LMS show small foci of fascicles consisting of smooth muscle tumor cells in addition to cellular pleomorphism (Figure 4).15 Many of these cells demonstrate a clear perinuclear vacuole that generally is appreciated in neoplastic smooth muscle cells.14 Pleomorphic LMS typically stains positively for at least one smooth muscle marker including desmin, h-caldesmon, muscle-specific actin, α-smooth muscle actin, or smooth muscle myosin in the leiomyosarcomatous fascicular areas.16 Complete surgical excision is the treatment of choice, and the best results are obtained with MMS.14
- Syme-Grant J, Syme-Grant NJ, Motta L, et al. Are primary cutaneous carcinosarcomas underdiagnosed? five cases and a review of the literature. J Plast Reconstr Aesthet Surg. 2006;59:1402-1408.
- Bourgeault E, Alain J, Gagne E. Primary cutaneous carcinosarcoma of the basal cell subtype should be treated as a high-risk basal cell carcinoma. J Cutan Med Surg. 2015;19:407-411.
- West L, Srivastava D. Cutaneous carcinosarcoma of the medial canthus discovered on Mohs debulk analysis. Dermatol Surg. 2019;45:1700-1702.
- Kwan JM, Satter EK. Carcinosarcoma: a primary cutaneous tumor with biphasic differentiation. Cutis. 2013;92:247-249.
- Suh KY, Lacouture M, Gerami P. p63 in primary cutaneous carcinosarcoma. Am J Dermatopathol. 2007;29:374‐377.
- Ruiz-Villaverde R, Aneiros-Fernandez J. Primary cutaneous carcinosarcoma: a cutaneous neoplasm with an exceptional presentation. Sultan Qaboos Univ Med J. 2018;18:E114-E115.
- Smart CN, Pucci RA, Binder SW, et al. Cutaneous carcinosarcoma with myoepithelial differentiation: immunohistochemical and cytogenetic analysis of a case presenting in an unusual location. Am J Dermatopathol. 2009;31:715‐717.
- Clark JJ, Bowen AR, Bowen GM, et al. Cutaneous carcinosarcoma: a series of six cases and a review of the literature. J Cutan Pathol. 2017;44:34‐44.
- Müller CS, Pföhler C, Schiekofer C, et al. Primary cutaneous carcinosarcomas: a morphological histogenetic concept revisited. Am J Dermatopathol. 2014;36:328‐339.
- Bellew S, Del Rosso JQ, Mobini N. Primary carcinosarcoma of the ear: case report and review of the literature. J Clin Aesthet Dermatol. 2009;2:33‐35.
- Hong SH, Hong SJ, Lee Y, et al. Primary cutaneous carcinosarcoma of the shoulder: case report with literature review. Dermatol Surg. 2013;39:338-340.
- Soleymani T, Aasi SZ, Novoa R, et al. Atypical fibroxanthoma and pleomorphic dermal sarcoma: updates on classification and management. Dermatol Clin. 2019;37:253-259.
- Parekh V, Seykora JT. Cutaneous squamous cell carcinoma. Clin Lab Med. 2017;37:503-525.
- Johnson GE, Stevens K, Morrison AO, et al. Cutaneous myoepithelial carcinoma with disseminated metastases. Cutis. 2017;99:E19-E26.
- Llombart B, Serra-Guillén C, Requena C, et al. Leiomyosarcoma and pleomorphic dermal sarcoma: guidelines for diagnosis and treatment. Actas Dermosifiliogr. 2019;110:4-11.
- Oda Y, Miyajima K, Kawaguchi K, et al. Pleomorphic leiomyosarcoma: clinicopathologic and immunohistochemical study with special emphasis on its distinction from ordinary leiomyosarcoma and malignant fibrous histiocytoma. Am J Surg Pathol. 2001;25:1030-1038.
The Diagnosis: Primary Cutaneous Carcinosarcoma
The immunohistochemical findings supported an epithelial component consistent with moderately differentiated squamous cell carcinoma (SCC) and a mesenchymal component with features consistent with a sarcoma. Consequently, the lesion was diagnosed as a primary cutaneous carcinosarcoma (PCCS).
Primary cutaneous carcinosarcoma is a rare biphasic neoplasm consisting of malignant epithelial (carcinoma) and mesenchymal (sarcoma) components.1 Primary cutaneous carcinosarcomas are uncommon, poorly understood, primary cutaneous tumors.2,3 Characteristic of this tumor, cytokeratins highlight the epithelial component while vimentin highlights the mesenchymal component.4 Histologically, the sarcomatous components of PCCS often are highly variable, with an absence of transitional areas within the epithelial component, which frequently resembles basal cell carcinoma and/ or SCC.5-7 Primary cutaneous carcinosarcoma favors areas of chronic UV radiation exposure, particularly on the head and neck. Most tumors present with a slowly growing, polypoid, flesh-colored to erythematous nodule due to the infiltrative mesenchymal component.7 Primary cutaneous carcinosarcoma primarily is diagnosed in elderly patients, with the majority of cases diagnosed in the eighth or ninth decades of life (range, 32–98 years).1,8 Men appear to be twice as likely to be diagnosed with a PCCS compared to women.1 Primary cutaneous carcinosarcomas are recognized as aggressive tumors with a high propensity to metastasize and recur locally, necessitating early diagnosis and treatment.4 Accurate diagnosis of PCCSs can be challenging due to the biphasic nature of the neoplasm as well as poor differentiation or unequal proportions of the epithelial and mesenchymal components.5 Additionally, overlapping diagnostic criteria coupled with vague demarcation between soft-tissue sarcomas and distinct carcinomas also may contribute to a delay in diagnosis.9 Treatment is achieved surgically by complete wide resection, with no evidence to support the use of adjuvant or neoadjuvant external beam radiation therapy. Due to the small number of reported cases, no treatment recommendations currently exist.1
Surgical management with wide local excision has been disappointing, with recurrence rates reported as high as 33%.6 Primary cutaneous carcinosarcoma has an estimated overall recurrence rate of 19% and a 5-year disease-free rate of 50%.10 Risk factors associated with poorer prognosis include tumors with adnexal subtype, age less than 65 years, rapid tumor growth, a tumor greater than 20 mm at presentation, and a long-standing tumor lasting up to 30 years.2,4 Although wide local excision and Mohs micrographic surgery (MMS) both have been utilized successfully, MMS has been shown to result in a cure rate of greater than 98%.6
Atypical fibroxanthoma (AFX) is a cutaneous tumor of fibrohistiocytic mesenchymal origin that typically manifests on sun-damaged skin in elderly individuals. Clinically, it presents as a rapidly growing neoplasm that often ulcerates and bleeds. These heterogenous neoplasms have several distinct characteristics, including dense cellularity with disorganized, large, pleomorphic, and atypical-appearing spindle-shaped cells arising in the upper layers of the dermis, often disseminating into the reticular dermis and occasionally into the subcutaneous fat (Figure 1). The neoplastic cells often exhibit hyperchromic and irregular nuclei, multinucleated giant cells, and atypical mitotic figures. In most cases, negative immunohistochemical staining with SOX-10, S-100, cytokeratins, desmin, and caldesmon will allow pathologists to differentiate between AFX and other common tumors on the differential diagnosis, such as SCC, melanoma, and leiomyosarcoma. CD10 and procollagen type 1 are positive antigenic markers in AFX, but they are not specific. The standard treatment of AFX includes wide local excision or MMS for superior margin control.11
Spindle cell SCC presents as a raised or exophytic nodule, often with spontaneous bleeding and central ulceration. It usually presents on sun-damaged skin or in individuals with a history of ionizing radiation. Histologically, it is characterized by atypical spindleshaped keratinocytes in the dermis existing as single cells or cohesive nests along with keratin pearls (Figure 2). The atypical spindle cells may comprise the entire tumor or only a small portion. The use of immunohistochemical markers often is required to establish a definitive diagnosis. Spindle cell SCC stains positively, albeit frequently focally, for p63, p40, and high-molecular-weight cytokeratins such as cytokeratin 5/6, while S-100 protein, SOX-10, MART-1/Melan-A, and muscle-specific actin stains typically are negative. Wide local excision or MMS is recommended for treatment of these lesions.12

Primary cutaneous myoepithelial carcinomas are uncommon neoplasms of myoepithelial differentiation. Clinically, they often arise as soft nodular lesions on the head, neck, and lower extremities with a bimodal age distribution (50 years). Histologically cutaneous myoepithelial tumors are well-differentiated, dermal-based nodules without connection to the overlying epidermis (Figure 3). The myoepithelial cells can exhibit spindled, epithelioid, plasmacytoid, or clear cell morphologic features and show variability in cell growth patterns. One of the most common growth patterns is oval to round cells forming cords and chains in a chondromyxoid stroma. Most cases display an immunophenotyped co-expression of an epithelial cytokeratin and S-100 protein. Myoepithelial markers also may be present, including keratins, smooth muscle actin, calponin, glial fibrillary acidic protein, p63, and desmin. Surgical removal with wide local excision or MMS is essential.13
Leiomyosarcoma (LMS) is a tumor that originates from smooth muscle and rarely develops in the dermis.14 Pleomorphic LMS is a morphologic variant of LMS that has a low propensity to metastasize but commonly exhibits local recurrence.15 Leiomyosarcoma can present in any age group but most commonly manifests in individuals aged 50 to 70 years. Clinically, LMS presents as a firm solitary nodule with a smooth pink surface or a more exophytic tumor with a reddish or brown color on the extensor surface of the lower limbs; it is less common on the scalp and face.14 Histologically, most cases of pleomorphic LMS show small foci of fascicles consisting of smooth muscle tumor cells in addition to cellular pleomorphism (Figure 4).15 Many of these cells demonstrate a clear perinuclear vacuole that generally is appreciated in neoplastic smooth muscle cells.14 Pleomorphic LMS typically stains positively for at least one smooth muscle marker including desmin, h-caldesmon, muscle-specific actin, α-smooth muscle actin, or smooth muscle myosin in the leiomyosarcomatous fascicular areas.16 Complete surgical excision is the treatment of choice, and the best results are obtained with MMS.14
The Diagnosis: Primary Cutaneous Carcinosarcoma
The immunohistochemical findings supported an epithelial component consistent with moderately differentiated squamous cell carcinoma (SCC) and a mesenchymal component with features consistent with a sarcoma. Consequently, the lesion was diagnosed as a primary cutaneous carcinosarcoma (PCCS).
Primary cutaneous carcinosarcoma is a rare biphasic neoplasm consisting of malignant epithelial (carcinoma) and mesenchymal (sarcoma) components.1 Primary cutaneous carcinosarcomas are uncommon, poorly understood, primary cutaneous tumors.2,3 Characteristic of this tumor, cytokeratins highlight the epithelial component while vimentin highlights the mesenchymal component.4 Histologically, the sarcomatous components of PCCS often are highly variable, with an absence of transitional areas within the epithelial component, which frequently resembles basal cell carcinoma and/ or SCC.5-7 Primary cutaneous carcinosarcoma favors areas of chronic UV radiation exposure, particularly on the head and neck. Most tumors present with a slowly growing, polypoid, flesh-colored to erythematous nodule due to the infiltrative mesenchymal component.7 Primary cutaneous carcinosarcoma primarily is diagnosed in elderly patients, with the majority of cases diagnosed in the eighth or ninth decades of life (range, 32–98 years).1,8 Men appear to be twice as likely to be diagnosed with a PCCS compared to women.1 Primary cutaneous carcinosarcomas are recognized as aggressive tumors with a high propensity to metastasize and recur locally, necessitating early diagnosis and treatment.4 Accurate diagnosis of PCCSs can be challenging due to the biphasic nature of the neoplasm as well as poor differentiation or unequal proportions of the epithelial and mesenchymal components.5 Additionally, overlapping diagnostic criteria coupled with vague demarcation between soft-tissue sarcomas and distinct carcinomas also may contribute to a delay in diagnosis.9 Treatment is achieved surgically by complete wide resection, with no evidence to support the use of adjuvant or neoadjuvant external beam radiation therapy. Due to the small number of reported cases, no treatment recommendations currently exist.1
Surgical management with wide local excision has been disappointing, with recurrence rates reported as high as 33%.6 Primary cutaneous carcinosarcoma has an estimated overall recurrence rate of 19% and a 5-year disease-free rate of 50%.10 Risk factors associated with poorer prognosis include tumors with adnexal subtype, age less than 65 years, rapid tumor growth, a tumor greater than 20 mm at presentation, and a long-standing tumor lasting up to 30 years.2,4 Although wide local excision and Mohs micrographic surgery (MMS) both have been utilized successfully, MMS has been shown to result in a cure rate of greater than 98%.6
Atypical fibroxanthoma (AFX) is a cutaneous tumor of fibrohistiocytic mesenchymal origin that typically manifests on sun-damaged skin in elderly individuals. Clinically, it presents as a rapidly growing neoplasm that often ulcerates and bleeds. These heterogenous neoplasms have several distinct characteristics, including dense cellularity with disorganized, large, pleomorphic, and atypical-appearing spindle-shaped cells arising in the upper layers of the dermis, often disseminating into the reticular dermis and occasionally into the subcutaneous fat (Figure 1). The neoplastic cells often exhibit hyperchromic and irregular nuclei, multinucleated giant cells, and atypical mitotic figures. In most cases, negative immunohistochemical staining with SOX-10, S-100, cytokeratins, desmin, and caldesmon will allow pathologists to differentiate between AFX and other common tumors on the differential diagnosis, such as SCC, melanoma, and leiomyosarcoma. CD10 and procollagen type 1 are positive antigenic markers in AFX, but they are not specific. The standard treatment of AFX includes wide local excision or MMS for superior margin control.11
Spindle cell SCC presents as a raised or exophytic nodule, often with spontaneous bleeding and central ulceration. It usually presents on sun-damaged skin or in individuals with a history of ionizing radiation. Histologically, it is characterized by atypical spindleshaped keratinocytes in the dermis existing as single cells or cohesive nests along with keratin pearls (Figure 2). The atypical spindle cells may comprise the entire tumor or only a small portion. The use of immunohistochemical markers often is required to establish a definitive diagnosis. Spindle cell SCC stains positively, albeit frequently focally, for p63, p40, and high-molecular-weight cytokeratins such as cytokeratin 5/6, while S-100 protein, SOX-10, MART-1/Melan-A, and muscle-specific actin stains typically are negative. Wide local excision or MMS is recommended for treatment of these lesions.12

Primary cutaneous myoepithelial carcinomas are uncommon neoplasms of myoepithelial differentiation. Clinically, they often arise as soft nodular lesions on the head, neck, and lower extremities with a bimodal age distribution (50 years). Histologically cutaneous myoepithelial tumors are well-differentiated, dermal-based nodules without connection to the overlying epidermis (Figure 3). The myoepithelial cells can exhibit spindled, epithelioid, plasmacytoid, or clear cell morphologic features and show variability in cell growth patterns. One of the most common growth patterns is oval to round cells forming cords and chains in a chondromyxoid stroma. Most cases display an immunophenotyped co-expression of an epithelial cytokeratin and S-100 protein. Myoepithelial markers also may be present, including keratins, smooth muscle actin, calponin, glial fibrillary acidic protein, p63, and desmin. Surgical removal with wide local excision or MMS is essential.13
Leiomyosarcoma (LMS) is a tumor that originates from smooth muscle and rarely develops in the dermis.14 Pleomorphic LMS is a morphologic variant of LMS that has a low propensity to metastasize but commonly exhibits local recurrence.15 Leiomyosarcoma can present in any age group but most commonly manifests in individuals aged 50 to 70 years. Clinically, LMS presents as a firm solitary nodule with a smooth pink surface or a more exophytic tumor with a reddish or brown color on the extensor surface of the lower limbs; it is less common on the scalp and face.14 Histologically, most cases of pleomorphic LMS show small foci of fascicles consisting of smooth muscle tumor cells in addition to cellular pleomorphism (Figure 4).15 Many of these cells demonstrate a clear perinuclear vacuole that generally is appreciated in neoplastic smooth muscle cells.14 Pleomorphic LMS typically stains positively for at least one smooth muscle marker including desmin, h-caldesmon, muscle-specific actin, α-smooth muscle actin, or smooth muscle myosin in the leiomyosarcomatous fascicular areas.16 Complete surgical excision is the treatment of choice, and the best results are obtained with MMS.14
- Syme-Grant J, Syme-Grant NJ, Motta L, et al. Are primary cutaneous carcinosarcomas underdiagnosed? five cases and a review of the literature. J Plast Reconstr Aesthet Surg. 2006;59:1402-1408.
- Bourgeault E, Alain J, Gagne E. Primary cutaneous carcinosarcoma of the basal cell subtype should be treated as a high-risk basal cell carcinoma. J Cutan Med Surg. 2015;19:407-411.
- West L, Srivastava D. Cutaneous carcinosarcoma of the medial canthus discovered on Mohs debulk analysis. Dermatol Surg. 2019;45:1700-1702.
- Kwan JM, Satter EK. Carcinosarcoma: a primary cutaneous tumor with biphasic differentiation. Cutis. 2013;92:247-249.
- Suh KY, Lacouture M, Gerami P. p63 in primary cutaneous carcinosarcoma. Am J Dermatopathol. 2007;29:374‐377.
- Ruiz-Villaverde R, Aneiros-Fernandez J. Primary cutaneous carcinosarcoma: a cutaneous neoplasm with an exceptional presentation. Sultan Qaboos Univ Med J. 2018;18:E114-E115.
- Smart CN, Pucci RA, Binder SW, et al. Cutaneous carcinosarcoma with myoepithelial differentiation: immunohistochemical and cytogenetic analysis of a case presenting in an unusual location. Am J Dermatopathol. 2009;31:715‐717.
- Clark JJ, Bowen AR, Bowen GM, et al. Cutaneous carcinosarcoma: a series of six cases and a review of the literature. J Cutan Pathol. 2017;44:34‐44.
- Müller CS, Pföhler C, Schiekofer C, et al. Primary cutaneous carcinosarcomas: a morphological histogenetic concept revisited. Am J Dermatopathol. 2014;36:328‐339.
- Bellew S, Del Rosso JQ, Mobini N. Primary carcinosarcoma of the ear: case report and review of the literature. J Clin Aesthet Dermatol. 2009;2:33‐35.
- Hong SH, Hong SJ, Lee Y, et al. Primary cutaneous carcinosarcoma of the shoulder: case report with literature review. Dermatol Surg. 2013;39:338-340.
- Soleymani T, Aasi SZ, Novoa R, et al. Atypical fibroxanthoma and pleomorphic dermal sarcoma: updates on classification and management. Dermatol Clin. 2019;37:253-259.
- Parekh V, Seykora JT. Cutaneous squamous cell carcinoma. Clin Lab Med. 2017;37:503-525.
- Johnson GE, Stevens K, Morrison AO, et al. Cutaneous myoepithelial carcinoma with disseminated metastases. Cutis. 2017;99:E19-E26.
- Llombart B, Serra-Guillén C, Requena C, et al. Leiomyosarcoma and pleomorphic dermal sarcoma: guidelines for diagnosis and treatment. Actas Dermosifiliogr. 2019;110:4-11.
- Oda Y, Miyajima K, Kawaguchi K, et al. Pleomorphic leiomyosarcoma: clinicopathologic and immunohistochemical study with special emphasis on its distinction from ordinary leiomyosarcoma and malignant fibrous histiocytoma. Am J Surg Pathol. 2001;25:1030-1038.
- Syme-Grant J, Syme-Grant NJ, Motta L, et al. Are primary cutaneous carcinosarcomas underdiagnosed? five cases and a review of the literature. J Plast Reconstr Aesthet Surg. 2006;59:1402-1408.
- Bourgeault E, Alain J, Gagne E. Primary cutaneous carcinosarcoma of the basal cell subtype should be treated as a high-risk basal cell carcinoma. J Cutan Med Surg. 2015;19:407-411.
- West L, Srivastava D. Cutaneous carcinosarcoma of the medial canthus discovered on Mohs debulk analysis. Dermatol Surg. 2019;45:1700-1702.
- Kwan JM, Satter EK. Carcinosarcoma: a primary cutaneous tumor with biphasic differentiation. Cutis. 2013;92:247-249.
- Suh KY, Lacouture M, Gerami P. p63 in primary cutaneous carcinosarcoma. Am J Dermatopathol. 2007;29:374‐377.
- Ruiz-Villaverde R, Aneiros-Fernandez J. Primary cutaneous carcinosarcoma: a cutaneous neoplasm with an exceptional presentation. Sultan Qaboos Univ Med J. 2018;18:E114-E115.
- Smart CN, Pucci RA, Binder SW, et al. Cutaneous carcinosarcoma with myoepithelial differentiation: immunohistochemical and cytogenetic analysis of a case presenting in an unusual location. Am J Dermatopathol. 2009;31:715‐717.
- Clark JJ, Bowen AR, Bowen GM, et al. Cutaneous carcinosarcoma: a series of six cases and a review of the literature. J Cutan Pathol. 2017;44:34‐44.
- Müller CS, Pföhler C, Schiekofer C, et al. Primary cutaneous carcinosarcomas: a morphological histogenetic concept revisited. Am J Dermatopathol. 2014;36:328‐339.
- Bellew S, Del Rosso JQ, Mobini N. Primary carcinosarcoma of the ear: case report and review of the literature. J Clin Aesthet Dermatol. 2009;2:33‐35.
- Hong SH, Hong SJ, Lee Y, et al. Primary cutaneous carcinosarcoma of the shoulder: case report with literature review. Dermatol Surg. 2013;39:338-340.
- Soleymani T, Aasi SZ, Novoa R, et al. Atypical fibroxanthoma and pleomorphic dermal sarcoma: updates on classification and management. Dermatol Clin. 2019;37:253-259.
- Parekh V, Seykora JT. Cutaneous squamous cell carcinoma. Clin Lab Med. 2017;37:503-525.
- Johnson GE, Stevens K, Morrison AO, et al. Cutaneous myoepithelial carcinoma with disseminated metastases. Cutis. 2017;99:E19-E26.
- Llombart B, Serra-Guillén C, Requena C, et al. Leiomyosarcoma and pleomorphic dermal sarcoma: guidelines for diagnosis and treatment. Actas Dermosifiliogr. 2019;110:4-11.
- Oda Y, Miyajima K, Kawaguchi K, et al. Pleomorphic leiomyosarcoma: clinicopathologic and immunohistochemical study with special emphasis on its distinction from ordinary leiomyosarcoma and malignant fibrous histiocytoma. Am J Surg Pathol. 2001;25:1030-1038.
A 72-year-old man with a history of nonmelanoma skin cancer and lung transplant maintained on stable doses of prednisone and tacrolimus presented with a 1.3×1.8-cm, slow-growing, well-demarcated, ulcerated, erythematous plaque with overlying serous crust on the left temple of 6 months’ duration. No cervical or axillary lymphadenopathy was appreciated on physical examination. A biopsy was performed followed by Mohs micrographic surgery. Microscopic examination of the debulking specimen revealed atypical spindle cells in the papillary and reticular dermis radiating from a central focus of a moderately differentiated squamous cell carcinoma. The squamous cells stained positive for cytokeratin 5/6, pankeratin, and p40, while the spindle cells stained positive only for vimentin.