User login
Prostate Cancer in Seniors Part 1: Epidemiology, Pathology, and Screening
Prostate cancer is the most common cancer and the second leading cause of cancer deaths in men. The incidence of prostate cancer continues to rise. Roughly 220,800 men were expected to be newly diagnosed with prostate cancer in 2015.1 As the population ages and overall life expectancy increases, prostate cancer is likely to become a growing health care burden, especially because prostate cancer is primarily a disease of elderly males.
There have been no specific practice guidelines for managing prostate cancer in older adults, and the current management of older patients with prostate cancer is often suboptimal. Fortunately, the International Society of Geriatric Oncology recently assembled a multidisciplinary prostate cancer working group, which has begun offering guidelines on evidence-based treatments of prostate cancer in the geriatric population.
This article (part 1 of 2) provides a brief overview of prostate cancer epidemiology, pathology and screening in senior patients. The second part (to be published in August 2015) will focus on treatment.
Epidemiology
Currently more than 2 million men are estimated to have prostate cancer in the U.S. About 18% to 20% of U.S. males have a lifetime risk of developing prostate
cancer. Prostate cancer is mainly a disease of seniors aged between 60 and 70 years—the median age of prostate cancer at diagnosis is about 65 to 68 years. About 65% of new prostate cancers are diagnosed in males aged 65 years and 25% in males > aged 75 years.2 Most older patients with prostate cancer do not die of prostate cancer.
As the life expectancy of the general population increases, the risk of developing prostate cancer among seniors is also expected to proportionally rise. Historically, the cancer-specific mortality rate of prostate cancer in patients aged > 70 years was only 29% if managed either with active surveillance or hormonal manipulation.
Prevalence of Incidental Prostate Cancer
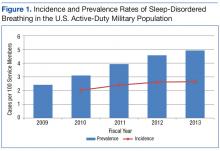
There is an abrupt age-dependent increase of prostate cancer incidence from the 5th decade of life on. Furthermore, there is a 1 in 3 chance of incidental prostate cancer in men aged between 60 to 69 years and a 46% prevalence in men aged > 70 years. Yin and colleagues found that 12% of patients in their study group harbored incidental, preclinical prostate cancer.3-5 The increasing prostate cancer incidence showed a strong and clear correlation with advancing age (Figure 1).
The lifetime probability of being diagnosed with prostate cancer also increases significantly with age.6,7 Patients with a life expectancy of < 5 years are unlikely
to benefit from cancer screening and may be more likely to experience complications and potential treatment-related harm as a result of screening. Therefore, estimating the patient’s residual life expectancy is a critical factor in the decision-making process for patients with prostate cancer. Life expectancy can differ, depending on various factors besides age, such as health, functional status, and medical comorbidities. The estimated age-related life expectancy for seniors has gradually increased over the previous 5 decades.8
Risk Factors
There are several risk factors for prostate cancer: age, race, and ethnicity; genetic factors; environmental and socioeconomic status; dietary status; and others. However, these factors may play only a limited role in the risk of prostate cancer, and a cautious approach and careful interpretation are required for their application in clinical practice.9,10
- Age. There is a sudden and dramatic increase in the prevalence of prostate cancer with advancing age. Prostate cancer is rarely diagnosed in men aged < 40 years, but thereafter, the incidence of prostate cancer climbs steadily.11 Surprisingly, subclinical microscopic prostate cancer was found at autopsy (death from unrelated causes) in a majority of senior males in their eighth decade of life.3
- Race/ethnicity. Epidemiologic studies in the U.S. found the highest incidence of prostate cancer in African American men (incidence rate of 235 per 100,000 African American vs 150 per 100,000 white men). Also, African American men tended to present with higher grades and stages of prostate cancer. There were much lower incidence rates of prostate cancer in Asian Americans and Pacific Islanders, Hispanics, and American Indian and Alaska Natives (90 per 100,000, 126 per 100,000, and 78 per 100,000, respectively).9,10
- Diet. According to researchers, the western diet may be an important risk factors for prostate cancer. However, the actual relationship between obesity and prostate cancer is somewhat unclear, and any correlation is at present highly controversial. Some investigators have postulated that obesity can contribute to the development of prostate cancer; other studies have clearly established that obese patients, once diagnosed with prostate cancer, have inferior outcomes irrespective of the treatment modality used. Other studies, however, have suggested that certain hormonal profiles related to obesity may be protective against the development of prostate cancer.12,13
Pathologic Evaluation
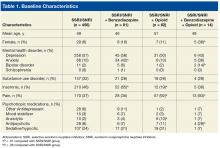
The original Gleason Grading System was devised based on the careful analysis of the cellular pattern of tumor architecture, using a 5-point scale: Tumor cells similar to normal-appearing prostate tissue were designated Gleason 1, 2, and 3; whereas cells/glands appearing abnormal were designated Gleason 4 and 5. The total Gleason score is the sum of the 2 most representative patterns, applied to both prostatectomy and needle biopsy specimens. The main differences from the original Gleason system, proposed by the 2005 International Society of Urological Pathology Modified Gleason System, are summarized in Table 1.
Early Detection and Screening
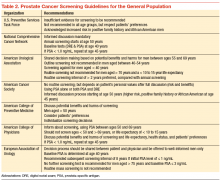
Although prostate cancer screening with prostate-specific antigen (PSA) detects many prostate cancer cases, concerns surrounding universal screening include the potential for overdiagnosis and overtreatment, along with the real possibility for adverse effects and complications from treatment. In addition, the recommendations for prostate cancer screening are not consistent among the various national health organizations. The American Cancer Society (ACS) recommends having an informed discussion between the health care provider and patient about the possible benefits and harms of screening. The discussion should not be initiated in men aged < 50 years (or aged < 45 years in men with high-risk features), and there is no need for screening in men with a life expectancy of < 10 years.
Prostate cancer screening may detect cancers that would not have become clinically significant. This is even more likely to be true when life expectancy decreases. Informed screening decisions in senior adults should be made according to the individual’s values and preferences in addition to the estimated outcomes and possible harms as a result of screening. The National Comprehensive Cancer Network offers similar recommendation to the ACS Screening Guidelines (Table 2).
Screening Recommendations for Seniors
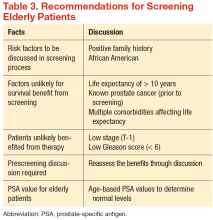
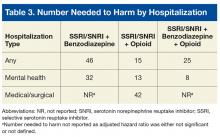
There have been no generally recognized guidelines on prostate cancer screening for seniors, although recently, Konety and colleagues published “The Iowa Prostate Cancer Consensus” for elderly prostate cancer patients (Table 3).17 The consensus includes:
- More prostate cancers are detected at an earlier stage, but many of them would never become clinically apparent in most patients’ life times
- A reduced mortality (either overall or disease specific) from screening is not proven during the course of 10-year follow-up
- Harms related to diagnostic and therapeutic procedures develop early and remain for an extended period, causing a negative impact on quality of life
- The small benefits of screening leading up to a prostatectomy are seen only after 12 years of follow-up and may be limited to a certain population group of
patients who are aged < 65 years - Current recommendations discourage the routine screening of seniors with short life expectancies (< 10 years) and depend on existing comorbidities and disease group risk
Conclusion
Prostate cancer is the most common cancer in American men and the second most common cause of cancer death. Prostate cancer is almost twice as common among African Americans vs whites, and much less common in Asian Americans and Pacific Islanders, Hispanics, American Indian and Alaska Natives. Prostate cancer is generally a cancer of older seniors, and nearly 80% of seniors are estimated to harbor subclinical prostate cancer by their eighth decade of life.8 Prostate cancer screening is not universally recommended, and major professional associations support an informed, evidence-based, shared decision-making process between medical professionals and patients. This decision should include the careful consideration of patients’ life expectancy and existing medical comorbidities, always weighing the potential benefits against the possible screening and treatment-related harms.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
Click here to read the digital edition.
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin.2015;65(1):5-29.
2. Fitzpatrick JM. Management of localized prostate cancer in senior adults: the crucial role of comorbidity. BJU Int. 2008;101(suppl 2):16-22.
3. Yin M, Bastacky S, Chandran U, Becich MJ, Dhir R. Prevalence of incidental prostate cancer in the general population: a study of healthy organ donors. J Urol. 2008;179(3):892-895.
4. Soos G, Tsakiris I, Szanto J, Turzo C, Haas PG, Dezso B.. The prevalence of prostate carcinoma and its precursor in Hungary: an autopsy study. Euro Urol. 2005;48(5):739-744.
5. Sánchez-Chapado M, Olmedilla G, Cabeza M, Donat E, Ruiz A. Prevalence of prostate cancer and prostatic intraepithelial neoplasia in Caucasian Mediterranean males: an autopsy study. Prostate. 2003;54(3):238-247.
6. Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008;58(2):71-96.
7. Sun L, Caire AA, Robertson CN, et al. Men older than 70 years have higher risk prostate cancer and poorer survival in the early and late prostate specific antigen eras. J Urol. 2009;182(5):2242-2248.
8. Haas GP, Sakr WA. Epidemiology of prostate cancer. CA Cancer J Clin. 1997;47(5):273-287.
9. Miocinovic R. Epidemiology and risk factors. In: Klein EA, Jones JP, eds. Management of Prostate Cancer. 3rd ed. Totowa, NJ: Humana Press; 2013:1-11.
10. Crawford ED. Epidemiology of prostate cancer. Urology. 2003;62(6 suppl 1):3-12.
11. Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60(5):277-300.
12. Giovannucci E, Rimm EB, Stampfer MJ, Colditz GA, Willett WC. Height, body weight, and risk of prostate cancer. Cancer Epidemiol Biomarkers Prev. 1997;6(8):557-563.
13. Gann PH, Hennekens CH, Ma J, Longcope C, Stampfer MJ. Prospective
study of sex hormone levels and risk of prostate cancer. J Natl Cancer Inst. 1996;88(16):1118-1126.
14. Epstein JI. An update of the Gleason grading system. J Urol. 2010;183(2):433-440.
15. Egevad L, Mazzucchelli R, Montironi R. Implications of the International Society of Urological Pathology modified Gleason grading system. Arch Pathol Lab Med. 2012;136(4):426-434.
16. Epstein JI, Allsbrook WC Jr, Amin MB, Egevad LL; ISUP Grading Committee. The 2005 International Society of Urological Pathology (ISUP) Consensus Conference on Gleason Grading of Prostate Carcinoma. Am J Surg Pathol. 2005;29(9): 1228-1242.
17. Konety BR, Sharp VJ, Raut H, Williams RD. Screening and management of prostate cancer in elderly men: the Iowa Prostate Cancer Consensus. Urology. 2008;71(3):511-514.
Prostate cancer is the most common cancer and the second leading cause of cancer deaths in men. The incidence of prostate cancer continues to rise. Roughly 220,800 men were expected to be newly diagnosed with prostate cancer in 2015.1 As the population ages and overall life expectancy increases, prostate cancer is likely to become a growing health care burden, especially because prostate cancer is primarily a disease of elderly males.
There have been no specific practice guidelines for managing prostate cancer in older adults, and the current management of older patients with prostate cancer is often suboptimal. Fortunately, the International Society of Geriatric Oncology recently assembled a multidisciplinary prostate cancer working group, which has begun offering guidelines on evidence-based treatments of prostate cancer in the geriatric population.
This article (part 1 of 2) provides a brief overview of prostate cancer epidemiology, pathology and screening in senior patients. The second part (to be published in August 2015) will focus on treatment.
Epidemiology
Currently more than 2 million men are estimated to have prostate cancer in the U.S. About 18% to 20% of U.S. males have a lifetime risk of developing prostate
cancer. Prostate cancer is mainly a disease of seniors aged between 60 and 70 years—the median age of prostate cancer at diagnosis is about 65 to 68 years. About 65% of new prostate cancers are diagnosed in males aged 65 years and 25% in males > aged 75 years.2 Most older patients with prostate cancer do not die of prostate cancer.
As the life expectancy of the general population increases, the risk of developing prostate cancer among seniors is also expected to proportionally rise. Historically, the cancer-specific mortality rate of prostate cancer in patients aged > 70 years was only 29% if managed either with active surveillance or hormonal manipulation.
Prevalence of Incidental Prostate Cancer
There is an abrupt age-dependent increase of prostate cancer incidence from the 5th decade of life on. Furthermore, there is a 1 in 3 chance of incidental prostate cancer in men aged between 60 to 69 years and a 46% prevalence in men aged > 70 years. Yin and colleagues found that 12% of patients in their study group harbored incidental, preclinical prostate cancer.3-5 The increasing prostate cancer incidence showed a strong and clear correlation with advancing age (Figure 1).
The lifetime probability of being diagnosed with prostate cancer also increases significantly with age.6,7 Patients with a life expectancy of < 5 years are unlikely
to benefit from cancer screening and may be more likely to experience complications and potential treatment-related harm as a result of screening. Therefore, estimating the patient’s residual life expectancy is a critical factor in the decision-making process for patients with prostate cancer. Life expectancy can differ, depending on various factors besides age, such as health, functional status, and medical comorbidities. The estimated age-related life expectancy for seniors has gradually increased over the previous 5 decades.8
Risk Factors
There are several risk factors for prostate cancer: age, race, and ethnicity; genetic factors; environmental and socioeconomic status; dietary status; and others. However, these factors may play only a limited role in the risk of prostate cancer, and a cautious approach and careful interpretation are required for their application in clinical practice.9,10
- Age. There is a sudden and dramatic increase in the prevalence of prostate cancer with advancing age. Prostate cancer is rarely diagnosed in men aged < 40 years, but thereafter, the incidence of prostate cancer climbs steadily.11 Surprisingly, subclinical microscopic prostate cancer was found at autopsy (death from unrelated causes) in a majority of senior males in their eighth decade of life.3
- Race/ethnicity. Epidemiologic studies in the U.S. found the highest incidence of prostate cancer in African American men (incidence rate of 235 per 100,000 African American vs 150 per 100,000 white men). Also, African American men tended to present with higher grades and stages of prostate cancer. There were much lower incidence rates of prostate cancer in Asian Americans and Pacific Islanders, Hispanics, and American Indian and Alaska Natives (90 per 100,000, 126 per 100,000, and 78 per 100,000, respectively).9,10
- Diet. According to researchers, the western diet may be an important risk factors for prostate cancer. However, the actual relationship between obesity and prostate cancer is somewhat unclear, and any correlation is at present highly controversial. Some investigators have postulated that obesity can contribute to the development of prostate cancer; other studies have clearly established that obese patients, once diagnosed with prostate cancer, have inferior outcomes irrespective of the treatment modality used. Other studies, however, have suggested that certain hormonal profiles related to obesity may be protective against the development of prostate cancer.12,13
Pathologic Evaluation
The original Gleason Grading System was devised based on the careful analysis of the cellular pattern of tumor architecture, using a 5-point scale: Tumor cells similar to normal-appearing prostate tissue were designated Gleason 1, 2, and 3; whereas cells/glands appearing abnormal were designated Gleason 4 and 5. The total Gleason score is the sum of the 2 most representative patterns, applied to both prostatectomy and needle biopsy specimens. The main differences from the original Gleason system, proposed by the 2005 International Society of Urological Pathology Modified Gleason System, are summarized in Table 1.
Early Detection and Screening
Although prostate cancer screening with prostate-specific antigen (PSA) detects many prostate cancer cases, concerns surrounding universal screening include the potential for overdiagnosis and overtreatment, along with the real possibility for adverse effects and complications from treatment. In addition, the recommendations for prostate cancer screening are not consistent among the various national health organizations. The American Cancer Society (ACS) recommends having an informed discussion between the health care provider and patient about the possible benefits and harms of screening. The discussion should not be initiated in men aged < 50 years (or aged < 45 years in men with high-risk features), and there is no need for screening in men with a life expectancy of < 10 years.
Prostate cancer screening may detect cancers that would not have become clinically significant. This is even more likely to be true when life expectancy decreases. Informed screening decisions in senior adults should be made according to the individual’s values and preferences in addition to the estimated outcomes and possible harms as a result of screening. The National Comprehensive Cancer Network offers similar recommendation to the ACS Screening Guidelines (Table 2).
Screening Recommendations for Seniors
There have been no generally recognized guidelines on prostate cancer screening for seniors, although recently, Konety and colleagues published “The Iowa Prostate Cancer Consensus” for elderly prostate cancer patients (Table 3).17 The consensus includes:
- More prostate cancers are detected at an earlier stage, but many of them would never become clinically apparent in most patients’ life times
- A reduced mortality (either overall or disease specific) from screening is not proven during the course of 10-year follow-up
- Harms related to diagnostic and therapeutic procedures develop early and remain for an extended period, causing a negative impact on quality of life
- The small benefits of screening leading up to a prostatectomy are seen only after 12 years of follow-up and may be limited to a certain population group of
patients who are aged < 65 years - Current recommendations discourage the routine screening of seniors with short life expectancies (< 10 years) and depend on existing comorbidities and disease group risk
Conclusion
Prostate cancer is the most common cancer in American men and the second most common cause of cancer death. Prostate cancer is almost twice as common among African Americans vs whites, and much less common in Asian Americans and Pacific Islanders, Hispanics, American Indian and Alaska Natives. Prostate cancer is generally a cancer of older seniors, and nearly 80% of seniors are estimated to harbor subclinical prostate cancer by their eighth decade of life.8 Prostate cancer screening is not universally recommended, and major professional associations support an informed, evidence-based, shared decision-making process between medical professionals and patients. This decision should include the careful consideration of patients’ life expectancy and existing medical comorbidities, always weighing the potential benefits against the possible screening and treatment-related harms.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
Click here to read the digital edition.
Prostate cancer is the most common cancer and the second leading cause of cancer deaths in men. The incidence of prostate cancer continues to rise. Roughly 220,800 men were expected to be newly diagnosed with prostate cancer in 2015.1 As the population ages and overall life expectancy increases, prostate cancer is likely to become a growing health care burden, especially because prostate cancer is primarily a disease of elderly males.
There have been no specific practice guidelines for managing prostate cancer in older adults, and the current management of older patients with prostate cancer is often suboptimal. Fortunately, the International Society of Geriatric Oncology recently assembled a multidisciplinary prostate cancer working group, which has begun offering guidelines on evidence-based treatments of prostate cancer in the geriatric population.
This article (part 1 of 2) provides a brief overview of prostate cancer epidemiology, pathology and screening in senior patients. The second part (to be published in August 2015) will focus on treatment.
Epidemiology
Currently more than 2 million men are estimated to have prostate cancer in the U.S. About 18% to 20% of U.S. males have a lifetime risk of developing prostate
cancer. Prostate cancer is mainly a disease of seniors aged between 60 and 70 years—the median age of prostate cancer at diagnosis is about 65 to 68 years. About 65% of new prostate cancers are diagnosed in males aged 65 years and 25% in males > aged 75 years.2 Most older patients with prostate cancer do not die of prostate cancer.
As the life expectancy of the general population increases, the risk of developing prostate cancer among seniors is also expected to proportionally rise. Historically, the cancer-specific mortality rate of prostate cancer in patients aged > 70 years was only 29% if managed either with active surveillance or hormonal manipulation.
Prevalence of Incidental Prostate Cancer
There is an abrupt age-dependent increase of prostate cancer incidence from the 5th decade of life on. Furthermore, there is a 1 in 3 chance of incidental prostate cancer in men aged between 60 to 69 years and a 46% prevalence in men aged > 70 years. Yin and colleagues found that 12% of patients in their study group harbored incidental, preclinical prostate cancer.3-5 The increasing prostate cancer incidence showed a strong and clear correlation with advancing age (Figure 1).
The lifetime probability of being diagnosed with prostate cancer also increases significantly with age.6,7 Patients with a life expectancy of < 5 years are unlikely
to benefit from cancer screening and may be more likely to experience complications and potential treatment-related harm as a result of screening. Therefore, estimating the patient’s residual life expectancy is a critical factor in the decision-making process for patients with prostate cancer. Life expectancy can differ, depending on various factors besides age, such as health, functional status, and medical comorbidities. The estimated age-related life expectancy for seniors has gradually increased over the previous 5 decades.8
Risk Factors
There are several risk factors for prostate cancer: age, race, and ethnicity; genetic factors; environmental and socioeconomic status; dietary status; and others. However, these factors may play only a limited role in the risk of prostate cancer, and a cautious approach and careful interpretation are required for their application in clinical practice.9,10
- Age. There is a sudden and dramatic increase in the prevalence of prostate cancer with advancing age. Prostate cancer is rarely diagnosed in men aged < 40 years, but thereafter, the incidence of prostate cancer climbs steadily.11 Surprisingly, subclinical microscopic prostate cancer was found at autopsy (death from unrelated causes) in a majority of senior males in their eighth decade of life.3
- Race/ethnicity. Epidemiologic studies in the U.S. found the highest incidence of prostate cancer in African American men (incidence rate of 235 per 100,000 African American vs 150 per 100,000 white men). Also, African American men tended to present with higher grades and stages of prostate cancer. There were much lower incidence rates of prostate cancer in Asian Americans and Pacific Islanders, Hispanics, and American Indian and Alaska Natives (90 per 100,000, 126 per 100,000, and 78 per 100,000, respectively).9,10
- Diet. According to researchers, the western diet may be an important risk factors for prostate cancer. However, the actual relationship between obesity and prostate cancer is somewhat unclear, and any correlation is at present highly controversial. Some investigators have postulated that obesity can contribute to the development of prostate cancer; other studies have clearly established that obese patients, once diagnosed with prostate cancer, have inferior outcomes irrespective of the treatment modality used. Other studies, however, have suggested that certain hormonal profiles related to obesity may be protective against the development of prostate cancer.12,13
Pathologic Evaluation
The original Gleason Grading System was devised based on the careful analysis of the cellular pattern of tumor architecture, using a 5-point scale: Tumor cells similar to normal-appearing prostate tissue were designated Gleason 1, 2, and 3; whereas cells/glands appearing abnormal were designated Gleason 4 and 5. The total Gleason score is the sum of the 2 most representative patterns, applied to both prostatectomy and needle biopsy specimens. The main differences from the original Gleason system, proposed by the 2005 International Society of Urological Pathology Modified Gleason System, are summarized in Table 1.
Early Detection and Screening
Although prostate cancer screening with prostate-specific antigen (PSA) detects many prostate cancer cases, concerns surrounding universal screening include the potential for overdiagnosis and overtreatment, along with the real possibility for adverse effects and complications from treatment. In addition, the recommendations for prostate cancer screening are not consistent among the various national health organizations. The American Cancer Society (ACS) recommends having an informed discussion between the health care provider and patient about the possible benefits and harms of screening. The discussion should not be initiated in men aged < 50 years (or aged < 45 years in men with high-risk features), and there is no need for screening in men with a life expectancy of < 10 years.
Prostate cancer screening may detect cancers that would not have become clinically significant. This is even more likely to be true when life expectancy decreases. Informed screening decisions in senior adults should be made according to the individual’s values and preferences in addition to the estimated outcomes and possible harms as a result of screening. The National Comprehensive Cancer Network offers similar recommendation to the ACS Screening Guidelines (Table 2).
Screening Recommendations for Seniors
There have been no generally recognized guidelines on prostate cancer screening for seniors, although recently, Konety and colleagues published “The Iowa Prostate Cancer Consensus” for elderly prostate cancer patients (Table 3).17 The consensus includes:
- More prostate cancers are detected at an earlier stage, but many of them would never become clinically apparent in most patients’ life times
- A reduced mortality (either overall or disease specific) from screening is not proven during the course of 10-year follow-up
- Harms related to diagnostic and therapeutic procedures develop early and remain for an extended period, causing a negative impact on quality of life
- The small benefits of screening leading up to a prostatectomy are seen only after 12 years of follow-up and may be limited to a certain population group of
patients who are aged < 65 years - Current recommendations discourage the routine screening of seniors with short life expectancies (< 10 years) and depend on existing comorbidities and disease group risk
Conclusion
Prostate cancer is the most common cancer in American men and the second most common cause of cancer death. Prostate cancer is almost twice as common among African Americans vs whites, and much less common in Asian Americans and Pacific Islanders, Hispanics, American Indian and Alaska Natives. Prostate cancer is generally a cancer of older seniors, and nearly 80% of seniors are estimated to harbor subclinical prostate cancer by their eighth decade of life.8 Prostate cancer screening is not universally recommended, and major professional associations support an informed, evidence-based, shared decision-making process between medical professionals and patients. This decision should include the careful consideration of patients’ life expectancy and existing medical comorbidities, always weighing the potential benefits against the possible screening and treatment-related harms.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
Click here to read the digital edition.
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin.2015;65(1):5-29.
2. Fitzpatrick JM. Management of localized prostate cancer in senior adults: the crucial role of comorbidity. BJU Int. 2008;101(suppl 2):16-22.
3. Yin M, Bastacky S, Chandran U, Becich MJ, Dhir R. Prevalence of incidental prostate cancer in the general population: a study of healthy organ donors. J Urol. 2008;179(3):892-895.
4. Soos G, Tsakiris I, Szanto J, Turzo C, Haas PG, Dezso B.. The prevalence of prostate carcinoma and its precursor in Hungary: an autopsy study. Euro Urol. 2005;48(5):739-744.
5. Sánchez-Chapado M, Olmedilla G, Cabeza M, Donat E, Ruiz A. Prevalence of prostate cancer and prostatic intraepithelial neoplasia in Caucasian Mediterranean males: an autopsy study. Prostate. 2003;54(3):238-247.
6. Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008;58(2):71-96.
7. Sun L, Caire AA, Robertson CN, et al. Men older than 70 years have higher risk prostate cancer and poorer survival in the early and late prostate specific antigen eras. J Urol. 2009;182(5):2242-2248.
8. Haas GP, Sakr WA. Epidemiology of prostate cancer. CA Cancer J Clin. 1997;47(5):273-287.
9. Miocinovic R. Epidemiology and risk factors. In: Klein EA, Jones JP, eds. Management of Prostate Cancer. 3rd ed. Totowa, NJ: Humana Press; 2013:1-11.
10. Crawford ED. Epidemiology of prostate cancer. Urology. 2003;62(6 suppl 1):3-12.
11. Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60(5):277-300.
12. Giovannucci E, Rimm EB, Stampfer MJ, Colditz GA, Willett WC. Height, body weight, and risk of prostate cancer. Cancer Epidemiol Biomarkers Prev. 1997;6(8):557-563.
13. Gann PH, Hennekens CH, Ma J, Longcope C, Stampfer MJ. Prospective
study of sex hormone levels and risk of prostate cancer. J Natl Cancer Inst. 1996;88(16):1118-1126.
14. Epstein JI. An update of the Gleason grading system. J Urol. 2010;183(2):433-440.
15. Egevad L, Mazzucchelli R, Montironi R. Implications of the International Society of Urological Pathology modified Gleason grading system. Arch Pathol Lab Med. 2012;136(4):426-434.
16. Epstein JI, Allsbrook WC Jr, Amin MB, Egevad LL; ISUP Grading Committee. The 2005 International Society of Urological Pathology (ISUP) Consensus Conference on Gleason Grading of Prostate Carcinoma. Am J Surg Pathol. 2005;29(9): 1228-1242.
17. Konety BR, Sharp VJ, Raut H, Williams RD. Screening and management of prostate cancer in elderly men: the Iowa Prostate Cancer Consensus. Urology. 2008;71(3):511-514.
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin.2015;65(1):5-29.
2. Fitzpatrick JM. Management of localized prostate cancer in senior adults: the crucial role of comorbidity. BJU Int. 2008;101(suppl 2):16-22.
3. Yin M, Bastacky S, Chandran U, Becich MJ, Dhir R. Prevalence of incidental prostate cancer in the general population: a study of healthy organ donors. J Urol. 2008;179(3):892-895.
4. Soos G, Tsakiris I, Szanto J, Turzo C, Haas PG, Dezso B.. The prevalence of prostate carcinoma and its precursor in Hungary: an autopsy study. Euro Urol. 2005;48(5):739-744.
5. Sánchez-Chapado M, Olmedilla G, Cabeza M, Donat E, Ruiz A. Prevalence of prostate cancer and prostatic intraepithelial neoplasia in Caucasian Mediterranean males: an autopsy study. Prostate. 2003;54(3):238-247.
6. Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008;58(2):71-96.
7. Sun L, Caire AA, Robertson CN, et al. Men older than 70 years have higher risk prostate cancer and poorer survival in the early and late prostate specific antigen eras. J Urol. 2009;182(5):2242-2248.
8. Haas GP, Sakr WA. Epidemiology of prostate cancer. CA Cancer J Clin. 1997;47(5):273-287.
9. Miocinovic R. Epidemiology and risk factors. In: Klein EA, Jones JP, eds. Management of Prostate Cancer. 3rd ed. Totowa, NJ: Humana Press; 2013:1-11.
10. Crawford ED. Epidemiology of prostate cancer. Urology. 2003;62(6 suppl 1):3-12.
11. Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60(5):277-300.
12. Giovannucci E, Rimm EB, Stampfer MJ, Colditz GA, Willett WC. Height, body weight, and risk of prostate cancer. Cancer Epidemiol Biomarkers Prev. 1997;6(8):557-563.
13. Gann PH, Hennekens CH, Ma J, Longcope C, Stampfer MJ. Prospective
study of sex hormone levels and risk of prostate cancer. J Natl Cancer Inst. 1996;88(16):1118-1126.
14. Epstein JI. An update of the Gleason grading system. J Urol. 2010;183(2):433-440.
15. Egevad L, Mazzucchelli R, Montironi R. Implications of the International Society of Urological Pathology modified Gleason grading system. Arch Pathol Lab Med. 2012;136(4):426-434.
16. Epstein JI, Allsbrook WC Jr, Amin MB, Egevad LL; ISUP Grading Committee. The 2005 International Society of Urological Pathology (ISUP) Consensus Conference on Gleason Grading of Prostate Carcinoma. Am J Surg Pathol. 2005;29(9): 1228-1242.
17. Konety BR, Sharp VJ, Raut H, Williams RD. Screening and management of prostate cancer in elderly men: the Iowa Prostate Cancer Consensus. Urology. 2008;71(3):511-514.
Multidisciplinary Management of a Patient With Multiple Sclerosis: Part 2. Nurses’ Perspective
Nurses are important members of the VHA, which employs more nurses than does any other system—89,000. Nursing care is patient-centered, whole person, and multidisciplinary. Nurses enhance access to care through alternative communication strategies, such as telemedicine.1,2 Besides clinical care, nurses focus on health promotion, disease prevention, health education, attentiveness, and counseling.2 Nurses working in the Multiple Sclerosis Centers of Excellence (MSCoEs) adopt the precepts of the Patient Aligned Care Team of the VA. Also, nurses who care for patients with multiple sclerosis (MS) establish, maintain, and sustain care that is culturally sensitive and wellness focused and incorporates family and community resources with the goal of living well with MS.3
Establishing Care
Educating patients and their families about MS, its symptoms, and self-management skills at the time of diagnosis is paramount. That said, nurses are less concerned with MS immune pathology and white matter lesion count and are more concerned with helping patients maintain hope and optimism.4 Patients abilities to adapt to chronic disease, manage symptoms and drug adverse events (AEs), and participate fully in life are essential after receiving a diagnosis of MS. Establishing care for patients with MS should be focused on relationship building, open communication, sharing information, and building trust. Developing partnerships becomes the goal, and ongoing assessment builds the case for the continuum of care.4,5
William's Story
As William walked into the nurse’s office, careful to allow one finger to linger on the wall and backs of furniture, the nurse recognized a young man heavy with a new MS diagnosis. William was tentative, bristly, and trying to maintain his balance. The nurse noted William’s too-big clothes, part army fatigues, part athletic wear. William stated he was there only because his primary care provider wanted him to get a diagnosis for his balance problems. Developing a trusting relationship was paramount in caring for William.
William boasted of his athletic prowess and strength. Indeed, William was strong with full power in all extremities. He recounted that poor balance kept him from competing successfully in an athletic event requiring that he walk across a plank over electrified water. As the nurse listened, she recognized William’s reticence to accept his MS diagnosis. The nurse understood that previous history could affect his ability to accept a diagnosis and the treatment plan at this time. The nurse’s role was to help William navigate the VA health care system and access its resources. William was encouraged to participate in My HealtheVet (https://www.myhealth.va.gov) in order to exchange secure messages with the nurse. His messages revealed a lonely, angry man, distant from family and friends.
William acknowledged problems with balance and thinking. He quit a job he had held for 5 years as a financial advisor for fear that coworkers would discover he could no longer think clearly. He also quit his position as a Boy Scout leader for fear people would discover his poor balance. He stopped interacting with his family; he felt badgered by their probing questions about his health. William suspected that his family was gleeful he was no longer the smartest and wealthiest sibling. The nurse and William together defined and developed mutual goals, including management of his primary concerns of balance and thinking.
On William’s next visit, he sported a T-shirt that read, “Fight the Bully.” William explained that MS was the bully, and he would never give up the fight. The nurse suggested that the best fighting strategy was disease-modifying therapy (DMT). The nurse reinforced the information given to him about DMTs by the neurologist and reviewed the current research on treatments. The nurse offered strategies for remembering to take medication, sent secure messages, and phoned frequently to assess and encourage William and to announce availability if needed.
Continuing Care
The nurse role in the continuum of care is to assess patient self-management skills and provide, when needed, interventions to restore self-management to the highest level.4 Although William told the neurologist he was not having any difficulty injecting the DMT, William told the nurse he stopped the DMT. “This drug is not helping my balance, I fall all the time,” he told the nurse.
William reported that his family said not to believe the doctors at the VA. “They are giving you experimental medication—be careful—don’t take their drugs.” Finally, William admitted that he hated injecting himself and had painful injection-site reactions. The nurse recognized an opportunity for teaching and reinforced realistic expectations of the DMT, which do not improve MS or its symptoms but may reduce the rate of relapse, slow disease progression, and limit white matter lesions seen on magnetic resonance imaging.
The nurse invited family members to come to the clinic, and William’s brother and sister attended a group education event. They both had many questions about MS. William was very quiet, as it became evident that his family wanted information and to help. The family was not the hindrance to care as William previously described. The nurse helped William reframe his attitude toward the role of his family in his care.
William talked about something he learned in an MS chat room about natural therapies. The nurse provided evidence-based and reliable information, including the MSCoE website (http://www.va.gov/ms), containing information for professionals as well as patients with MS.6
William refused to go back to an injectable DMT. The nurse therefore discussed several oral medication options; however, William wanted to “fight” MS with alternative therapies. He said that exercise, a plant-based diet, and magnet therapy were all he needed. Those choices provided the opportunity to discuss complementary and alternative medicine (CAM) and use the latest American Academy of Neurology guidelines on CAM.7 The nurse encouraged and validated William’s desire to treat his MS with diet and exercise but focused the conversation on evidence-based therapies. He ultimately decided to initiate an oral DMT.
William had an opportunity to participate in the VAsponsored Winter Sports clinic. His roommate at the games, John, was also a veteran with MS, and William developed a relationship with John. John was taking an oral DMT to manage his disease. William returned from the games and requested the same DMT that John used.
The nurse recognized the importance of peer-to-peer influence and helped William feel in control of his MS. He was grieving lost abilities. Continuing care meant boosting William’s self-esteem, enhancing coping, allaying misconceptions and false beliefs, reframing life events, decreasing feelings of chronic sorrow, and offering hope.4
Sustaining Care
The goal of the nurse in sustaining MS care is focused on maintaining well-being, coordinating referrals, identifying community resources, and advocating for comprehensive care.3 Nurses continually reformulate the patient’s primary and long-term goals of care. They exercise their role as advocates, helping fulfill patient needs while maintaining good stewardship of resources. Nurses sustain the therapeutic relationship over time, providing caring throughout the MS disease trajectory.
As the disease progresses, nurses are vigilant to both prevention of complications and management. In this regard, the MS Assessment Tool is a useful portal to document dynamic changes in disability and therapy and track AEs. Infection, pain due to immobility, wounds, difficulty with respiration and swallowing, and neurogenic bowel and bladder are initially assessed and managed while preserving the patient’s physical, emotional, and spiritual values. Last, nurses cultivate relationships with other providers for personalized referrals, ensuring continuity and efficiency of care.4
The nurse and William discussed his greatest difficulties, which were primarily social. Without a job or income, William relied on savings to pay his mortgage, car loan, utilities, and other bills. William admitted that he had very little money to buy food as his savings dwindled. The nurse connected William with both VA social workers and a veterans service organization (VSO) and brokered a relationship for William with a community organization, the local chapter of the National Multiple Sclerosis Society (NMSS).
Benefits and Assistance
The NMSS was able to offer some limited financial assistance. William enrolled in a support group for newly diagnosed patients sponsored by NMSS and joined a class for people with MS and balance difficulties. The VSO helped William with his application for service connection for his disability. Multiple sclerosis is considered service connected if neurologic symptoms leading to a diagnosis are established during the military career or within 7 years of service discharge.6 William’s first symptom occurred 5 years after his army discharge. The social worker helped William apply for Social Security Disability Insurance (SSDI). The nurse also wrote letters, and he was subsequently approved for both SSDI and VA service connection for his MS. As a result, William accessed vocational rehabilitation services through the Veterans Benefits Agency.
Consults to prosthetics and rehabilitation services are essential for optimizing patient safety and energy. The opportunities for William in the VA health care system exceeded many private sector plans in that all medically necessary durable medical equipment is available without charge. William was able to focus on managing his MS without worries about food, shelter, or health care.
The neurology outpatient clinic brings the nurse, neurologist, physiatrist, neuropsychologist, social worker, dietitian, urology, occupational and physical therapists, wound care nurse, and prosthetics representative together in one place. Patients can access the care of each discipline during a single clinic visit. Quality of care, cost savings in travel, and patient satisfaction are clear rewards. When William encountered difficulty driving to the VA
for clinic appointments, he was referred to the Driving Program at selected sites and evaluated for assistive technology in his vehicle or an adaptive vehicle.
If driving can’t be maintained, care can be sustained through clinical video telemedicine, use of VA travel services, and home care. Should his family need to provide care, respite options also are available through the VA to maintain care at home for as long as possible. If his level of disability increases and his home is not accessible, William will be eligible for a Home Improvement and Structural Alterations grant to maintain safe access and egress and an Adaptive Housing Grant for an accessible home. Should residential living be needed, this option is also provided to eligible patients.
Conclusion
Veterans with MS served by the MSCoE network have access to a knowledgeable clinical team. Nurses have the skills to build enduring relationships. The nurse can instill confidence, empowering patients to take control of MS self-management. Nurses have the unique ability to establish, maintain, and sustain care for the person diagnosed with MS throughout the disease trajectory. Most important, nurses in specialty care clinics realize the VA mission—to offer access to efficient, quality care.
1. U.S. Department of Veterans Affairs, Office of Nursing Services. VA Nursing Service Fact Sheet. VA nursing service: Excellence in patient-driven care. U.S. Department of Veterans Affairs Website. http://www.va.gov/nursing/docs/about/vansgfacts.doc. Accessed February 23, 2015.
2. Budzi D, Lurie S, Singh K, Hooker R. Veterans’ perceptions of care by nurse practitioners, physician assistants, and physicians: A comparison from satisfaction surveys. J Am Acad Nurse Pract. 2010;22(3):170-176.
3. Costello K, Halper J. Advanced Practice Nursing in Multiple Sclerosis. Advanced Skills, Advancing Responsibilities. 3rd ed. International Organization of Multiple Sclerosis Nurses Website. http://iomsn.org/images/pdf/APN_Monograph_3rdEd.pdf. 2010. Accessed February 22, 2015.
4. New York City Coalition of Multiple Sclerosis Nurses. The Dynamic Multiple Sclerosis Nurse: Challenges, Expanding Role and Future Directions. International Organization of MS Nurses Website. http://www.iomsn.org/images/pdf/Article_Advanced_NYCMSNurse.pdf. Accessed February 22, 2015.
5. Halper J, Harris C. Nursing Practice in Multiple Sclerosis. A Core Curriculum. 3rd ed. New York, NY: Springer Publishing Company; 2012.
6. U.S. Department of Veterans Affairs. Multiple sclerosis centers of excellence. What are my VA benefits for multiple sclerosis. U.S. Department of Veterans Affairs Website. http://www.va.gov/MS/Veterans/benefits/What_Are_My_VA_Benefits_for_Multiple_Sclerosis.asp. Updated June 2013. Accessed February 22, 2015.
7. Yadav V, Bever C Jr, Bowen J, et al. Summary of evidence-based guideline: Complementary and alternative medicine in multiple sclerosis: Report of the guideline development subcommittee of the American Academy of Neurology. Neurology. 2014;82(12):1083-1092.
Nurses are important members of the VHA, which employs more nurses than does any other system—89,000. Nursing care is patient-centered, whole person, and multidisciplinary. Nurses enhance access to care through alternative communication strategies, such as telemedicine.1,2 Besides clinical care, nurses focus on health promotion, disease prevention, health education, attentiveness, and counseling.2 Nurses working in the Multiple Sclerosis Centers of Excellence (MSCoEs) adopt the precepts of the Patient Aligned Care Team of the VA. Also, nurses who care for patients with multiple sclerosis (MS) establish, maintain, and sustain care that is culturally sensitive and wellness focused and incorporates family and community resources with the goal of living well with MS.3
Establishing Care
Educating patients and their families about MS, its symptoms, and self-management skills at the time of diagnosis is paramount. That said, nurses are less concerned with MS immune pathology and white matter lesion count and are more concerned with helping patients maintain hope and optimism.4 Patients abilities to adapt to chronic disease, manage symptoms and drug adverse events (AEs), and participate fully in life are essential after receiving a diagnosis of MS. Establishing care for patients with MS should be focused on relationship building, open communication, sharing information, and building trust. Developing partnerships becomes the goal, and ongoing assessment builds the case for the continuum of care.4,5
William's Story
As William walked into the nurse’s office, careful to allow one finger to linger on the wall and backs of furniture, the nurse recognized a young man heavy with a new MS diagnosis. William was tentative, bristly, and trying to maintain his balance. The nurse noted William’s too-big clothes, part army fatigues, part athletic wear. William stated he was there only because his primary care provider wanted him to get a diagnosis for his balance problems. Developing a trusting relationship was paramount in caring for William.
William boasted of his athletic prowess and strength. Indeed, William was strong with full power in all extremities. He recounted that poor balance kept him from competing successfully in an athletic event requiring that he walk across a plank over electrified water. As the nurse listened, she recognized William’s reticence to accept his MS diagnosis. The nurse understood that previous history could affect his ability to accept a diagnosis and the treatment plan at this time. The nurse’s role was to help William navigate the VA health care system and access its resources. William was encouraged to participate in My HealtheVet (https://www.myhealth.va.gov) in order to exchange secure messages with the nurse. His messages revealed a lonely, angry man, distant from family and friends.
William acknowledged problems with balance and thinking. He quit a job he had held for 5 years as a financial advisor for fear that coworkers would discover he could no longer think clearly. He also quit his position as a Boy Scout leader for fear people would discover his poor balance. He stopped interacting with his family; he felt badgered by their probing questions about his health. William suspected that his family was gleeful he was no longer the smartest and wealthiest sibling. The nurse and William together defined and developed mutual goals, including management of his primary concerns of balance and thinking.
On William’s next visit, he sported a T-shirt that read, “Fight the Bully.” William explained that MS was the bully, and he would never give up the fight. The nurse suggested that the best fighting strategy was disease-modifying therapy (DMT). The nurse reinforced the information given to him about DMTs by the neurologist and reviewed the current research on treatments. The nurse offered strategies for remembering to take medication, sent secure messages, and phoned frequently to assess and encourage William and to announce availability if needed.
Continuing Care
The nurse role in the continuum of care is to assess patient self-management skills and provide, when needed, interventions to restore self-management to the highest level.4 Although William told the neurologist he was not having any difficulty injecting the DMT, William told the nurse he stopped the DMT. “This drug is not helping my balance, I fall all the time,” he told the nurse.
William reported that his family said not to believe the doctors at the VA. “They are giving you experimental medication—be careful—don’t take their drugs.” Finally, William admitted that he hated injecting himself and had painful injection-site reactions. The nurse recognized an opportunity for teaching and reinforced realistic expectations of the DMT, which do not improve MS or its symptoms but may reduce the rate of relapse, slow disease progression, and limit white matter lesions seen on magnetic resonance imaging.
The nurse invited family members to come to the clinic, and William’s brother and sister attended a group education event. They both had many questions about MS. William was very quiet, as it became evident that his family wanted information and to help. The family was not the hindrance to care as William previously described. The nurse helped William reframe his attitude toward the role of his family in his care.
William talked about something he learned in an MS chat room about natural therapies. The nurse provided evidence-based and reliable information, including the MSCoE website (http://www.va.gov/ms), containing information for professionals as well as patients with MS.6
William refused to go back to an injectable DMT. The nurse therefore discussed several oral medication options; however, William wanted to “fight” MS with alternative therapies. He said that exercise, a plant-based diet, and magnet therapy were all he needed. Those choices provided the opportunity to discuss complementary and alternative medicine (CAM) and use the latest American Academy of Neurology guidelines on CAM.7 The nurse encouraged and validated William’s desire to treat his MS with diet and exercise but focused the conversation on evidence-based therapies. He ultimately decided to initiate an oral DMT.
William had an opportunity to participate in the VAsponsored Winter Sports clinic. His roommate at the games, John, was also a veteran with MS, and William developed a relationship with John. John was taking an oral DMT to manage his disease. William returned from the games and requested the same DMT that John used.
The nurse recognized the importance of peer-to-peer influence and helped William feel in control of his MS. He was grieving lost abilities. Continuing care meant boosting William’s self-esteem, enhancing coping, allaying misconceptions and false beliefs, reframing life events, decreasing feelings of chronic sorrow, and offering hope.4
Sustaining Care
The goal of the nurse in sustaining MS care is focused on maintaining well-being, coordinating referrals, identifying community resources, and advocating for comprehensive care.3 Nurses continually reformulate the patient’s primary and long-term goals of care. They exercise their role as advocates, helping fulfill patient needs while maintaining good stewardship of resources. Nurses sustain the therapeutic relationship over time, providing caring throughout the MS disease trajectory.
As the disease progresses, nurses are vigilant to both prevention of complications and management. In this regard, the MS Assessment Tool is a useful portal to document dynamic changes in disability and therapy and track AEs. Infection, pain due to immobility, wounds, difficulty with respiration and swallowing, and neurogenic bowel and bladder are initially assessed and managed while preserving the patient’s physical, emotional, and spiritual values. Last, nurses cultivate relationships with other providers for personalized referrals, ensuring continuity and efficiency of care.4
The nurse and William discussed his greatest difficulties, which were primarily social. Without a job or income, William relied on savings to pay his mortgage, car loan, utilities, and other bills. William admitted that he had very little money to buy food as his savings dwindled. The nurse connected William with both VA social workers and a veterans service organization (VSO) and brokered a relationship for William with a community organization, the local chapter of the National Multiple Sclerosis Society (NMSS).
Benefits and Assistance
The NMSS was able to offer some limited financial assistance. William enrolled in a support group for newly diagnosed patients sponsored by NMSS and joined a class for people with MS and balance difficulties. The VSO helped William with his application for service connection for his disability. Multiple sclerosis is considered service connected if neurologic symptoms leading to a diagnosis are established during the military career or within 7 years of service discharge.6 William’s first symptom occurred 5 years after his army discharge. The social worker helped William apply for Social Security Disability Insurance (SSDI). The nurse also wrote letters, and he was subsequently approved for both SSDI and VA service connection for his MS. As a result, William accessed vocational rehabilitation services through the Veterans Benefits Agency.
Consults to prosthetics and rehabilitation services are essential for optimizing patient safety and energy. The opportunities for William in the VA health care system exceeded many private sector plans in that all medically necessary durable medical equipment is available without charge. William was able to focus on managing his MS without worries about food, shelter, or health care.
The neurology outpatient clinic brings the nurse, neurologist, physiatrist, neuropsychologist, social worker, dietitian, urology, occupational and physical therapists, wound care nurse, and prosthetics representative together in one place. Patients can access the care of each discipline during a single clinic visit. Quality of care, cost savings in travel, and patient satisfaction are clear rewards. When William encountered difficulty driving to the VA
for clinic appointments, he was referred to the Driving Program at selected sites and evaluated for assistive technology in his vehicle or an adaptive vehicle.
If driving can’t be maintained, care can be sustained through clinical video telemedicine, use of VA travel services, and home care. Should his family need to provide care, respite options also are available through the VA to maintain care at home for as long as possible. If his level of disability increases and his home is not accessible, William will be eligible for a Home Improvement and Structural Alterations grant to maintain safe access and egress and an Adaptive Housing Grant for an accessible home. Should residential living be needed, this option is also provided to eligible patients.
Conclusion
Veterans with MS served by the MSCoE network have access to a knowledgeable clinical team. Nurses have the skills to build enduring relationships. The nurse can instill confidence, empowering patients to take control of MS self-management. Nurses have the unique ability to establish, maintain, and sustain care for the person diagnosed with MS throughout the disease trajectory. Most important, nurses in specialty care clinics realize the VA mission—to offer access to efficient, quality care.
Nurses are important members of the VHA, which employs more nurses than does any other system—89,000. Nursing care is patient-centered, whole person, and multidisciplinary. Nurses enhance access to care through alternative communication strategies, such as telemedicine.1,2 Besides clinical care, nurses focus on health promotion, disease prevention, health education, attentiveness, and counseling.2 Nurses working in the Multiple Sclerosis Centers of Excellence (MSCoEs) adopt the precepts of the Patient Aligned Care Team of the VA. Also, nurses who care for patients with multiple sclerosis (MS) establish, maintain, and sustain care that is culturally sensitive and wellness focused and incorporates family and community resources with the goal of living well with MS.3
Establishing Care
Educating patients and their families about MS, its symptoms, and self-management skills at the time of diagnosis is paramount. That said, nurses are less concerned with MS immune pathology and white matter lesion count and are more concerned with helping patients maintain hope and optimism.4 Patients abilities to adapt to chronic disease, manage symptoms and drug adverse events (AEs), and participate fully in life are essential after receiving a diagnosis of MS. Establishing care for patients with MS should be focused on relationship building, open communication, sharing information, and building trust. Developing partnerships becomes the goal, and ongoing assessment builds the case for the continuum of care.4,5
William's Story
As William walked into the nurse’s office, careful to allow one finger to linger on the wall and backs of furniture, the nurse recognized a young man heavy with a new MS diagnosis. William was tentative, bristly, and trying to maintain his balance. The nurse noted William’s too-big clothes, part army fatigues, part athletic wear. William stated he was there only because his primary care provider wanted him to get a diagnosis for his balance problems. Developing a trusting relationship was paramount in caring for William.
William boasted of his athletic prowess and strength. Indeed, William was strong with full power in all extremities. He recounted that poor balance kept him from competing successfully in an athletic event requiring that he walk across a plank over electrified water. As the nurse listened, she recognized William’s reticence to accept his MS diagnosis. The nurse understood that previous history could affect his ability to accept a diagnosis and the treatment plan at this time. The nurse’s role was to help William navigate the VA health care system and access its resources. William was encouraged to participate in My HealtheVet (https://www.myhealth.va.gov) in order to exchange secure messages with the nurse. His messages revealed a lonely, angry man, distant from family and friends.
William acknowledged problems with balance and thinking. He quit a job he had held for 5 years as a financial advisor for fear that coworkers would discover he could no longer think clearly. He also quit his position as a Boy Scout leader for fear people would discover his poor balance. He stopped interacting with his family; he felt badgered by their probing questions about his health. William suspected that his family was gleeful he was no longer the smartest and wealthiest sibling. The nurse and William together defined and developed mutual goals, including management of his primary concerns of balance and thinking.
On William’s next visit, he sported a T-shirt that read, “Fight the Bully.” William explained that MS was the bully, and he would never give up the fight. The nurse suggested that the best fighting strategy was disease-modifying therapy (DMT). The nurse reinforced the information given to him about DMTs by the neurologist and reviewed the current research on treatments. The nurse offered strategies for remembering to take medication, sent secure messages, and phoned frequently to assess and encourage William and to announce availability if needed.
Continuing Care
The nurse role in the continuum of care is to assess patient self-management skills and provide, when needed, interventions to restore self-management to the highest level.4 Although William told the neurologist he was not having any difficulty injecting the DMT, William told the nurse he stopped the DMT. “This drug is not helping my balance, I fall all the time,” he told the nurse.
William reported that his family said not to believe the doctors at the VA. “They are giving you experimental medication—be careful—don’t take their drugs.” Finally, William admitted that he hated injecting himself and had painful injection-site reactions. The nurse recognized an opportunity for teaching and reinforced realistic expectations of the DMT, which do not improve MS or its symptoms but may reduce the rate of relapse, slow disease progression, and limit white matter lesions seen on magnetic resonance imaging.
The nurse invited family members to come to the clinic, and William’s brother and sister attended a group education event. They both had many questions about MS. William was very quiet, as it became evident that his family wanted information and to help. The family was not the hindrance to care as William previously described. The nurse helped William reframe his attitude toward the role of his family in his care.
William talked about something he learned in an MS chat room about natural therapies. The nurse provided evidence-based and reliable information, including the MSCoE website (http://www.va.gov/ms), containing information for professionals as well as patients with MS.6
William refused to go back to an injectable DMT. The nurse therefore discussed several oral medication options; however, William wanted to “fight” MS with alternative therapies. He said that exercise, a plant-based diet, and magnet therapy were all he needed. Those choices provided the opportunity to discuss complementary and alternative medicine (CAM) and use the latest American Academy of Neurology guidelines on CAM.7 The nurse encouraged and validated William’s desire to treat his MS with diet and exercise but focused the conversation on evidence-based therapies. He ultimately decided to initiate an oral DMT.
William had an opportunity to participate in the VAsponsored Winter Sports clinic. His roommate at the games, John, was also a veteran with MS, and William developed a relationship with John. John was taking an oral DMT to manage his disease. William returned from the games and requested the same DMT that John used.
The nurse recognized the importance of peer-to-peer influence and helped William feel in control of his MS. He was grieving lost abilities. Continuing care meant boosting William’s self-esteem, enhancing coping, allaying misconceptions and false beliefs, reframing life events, decreasing feelings of chronic sorrow, and offering hope.4
Sustaining Care
The goal of the nurse in sustaining MS care is focused on maintaining well-being, coordinating referrals, identifying community resources, and advocating for comprehensive care.3 Nurses continually reformulate the patient’s primary and long-term goals of care. They exercise their role as advocates, helping fulfill patient needs while maintaining good stewardship of resources. Nurses sustain the therapeutic relationship over time, providing caring throughout the MS disease trajectory.
As the disease progresses, nurses are vigilant to both prevention of complications and management. In this regard, the MS Assessment Tool is a useful portal to document dynamic changes in disability and therapy and track AEs. Infection, pain due to immobility, wounds, difficulty with respiration and swallowing, and neurogenic bowel and bladder are initially assessed and managed while preserving the patient’s physical, emotional, and spiritual values. Last, nurses cultivate relationships with other providers for personalized referrals, ensuring continuity and efficiency of care.4
The nurse and William discussed his greatest difficulties, which were primarily social. Without a job or income, William relied on savings to pay his mortgage, car loan, utilities, and other bills. William admitted that he had very little money to buy food as his savings dwindled. The nurse connected William with both VA social workers and a veterans service organization (VSO) and brokered a relationship for William with a community organization, the local chapter of the National Multiple Sclerosis Society (NMSS).
Benefits and Assistance
The NMSS was able to offer some limited financial assistance. William enrolled in a support group for newly diagnosed patients sponsored by NMSS and joined a class for people with MS and balance difficulties. The VSO helped William with his application for service connection for his disability. Multiple sclerosis is considered service connected if neurologic symptoms leading to a diagnosis are established during the military career or within 7 years of service discharge.6 William’s first symptom occurred 5 years after his army discharge. The social worker helped William apply for Social Security Disability Insurance (SSDI). The nurse also wrote letters, and he was subsequently approved for both SSDI and VA service connection for his MS. As a result, William accessed vocational rehabilitation services through the Veterans Benefits Agency.
Consults to prosthetics and rehabilitation services are essential for optimizing patient safety and energy. The opportunities for William in the VA health care system exceeded many private sector plans in that all medically necessary durable medical equipment is available without charge. William was able to focus on managing his MS without worries about food, shelter, or health care.
The neurology outpatient clinic brings the nurse, neurologist, physiatrist, neuropsychologist, social worker, dietitian, urology, occupational and physical therapists, wound care nurse, and prosthetics representative together in one place. Patients can access the care of each discipline during a single clinic visit. Quality of care, cost savings in travel, and patient satisfaction are clear rewards. When William encountered difficulty driving to the VA
for clinic appointments, he was referred to the Driving Program at selected sites and evaluated for assistive technology in his vehicle or an adaptive vehicle.
If driving can’t be maintained, care can be sustained through clinical video telemedicine, use of VA travel services, and home care. Should his family need to provide care, respite options also are available through the VA to maintain care at home for as long as possible. If his level of disability increases and his home is not accessible, William will be eligible for a Home Improvement and Structural Alterations grant to maintain safe access and egress and an Adaptive Housing Grant for an accessible home. Should residential living be needed, this option is also provided to eligible patients.
Conclusion
Veterans with MS served by the MSCoE network have access to a knowledgeable clinical team. Nurses have the skills to build enduring relationships. The nurse can instill confidence, empowering patients to take control of MS self-management. Nurses have the unique ability to establish, maintain, and sustain care for the person diagnosed with MS throughout the disease trajectory. Most important, nurses in specialty care clinics realize the VA mission—to offer access to efficient, quality care.
1. U.S. Department of Veterans Affairs, Office of Nursing Services. VA Nursing Service Fact Sheet. VA nursing service: Excellence in patient-driven care. U.S. Department of Veterans Affairs Website. http://www.va.gov/nursing/docs/about/vansgfacts.doc. Accessed February 23, 2015.
2. Budzi D, Lurie S, Singh K, Hooker R. Veterans’ perceptions of care by nurse practitioners, physician assistants, and physicians: A comparison from satisfaction surveys. J Am Acad Nurse Pract. 2010;22(3):170-176.
3. Costello K, Halper J. Advanced Practice Nursing in Multiple Sclerosis. Advanced Skills, Advancing Responsibilities. 3rd ed. International Organization of Multiple Sclerosis Nurses Website. http://iomsn.org/images/pdf/APN_Monograph_3rdEd.pdf. 2010. Accessed February 22, 2015.
4. New York City Coalition of Multiple Sclerosis Nurses. The Dynamic Multiple Sclerosis Nurse: Challenges, Expanding Role and Future Directions. International Organization of MS Nurses Website. http://www.iomsn.org/images/pdf/Article_Advanced_NYCMSNurse.pdf. Accessed February 22, 2015.
5. Halper J, Harris C. Nursing Practice in Multiple Sclerosis. A Core Curriculum. 3rd ed. New York, NY: Springer Publishing Company; 2012.
6. U.S. Department of Veterans Affairs. Multiple sclerosis centers of excellence. What are my VA benefits for multiple sclerosis. U.S. Department of Veterans Affairs Website. http://www.va.gov/MS/Veterans/benefits/What_Are_My_VA_Benefits_for_Multiple_Sclerosis.asp. Updated June 2013. Accessed February 22, 2015.
7. Yadav V, Bever C Jr, Bowen J, et al. Summary of evidence-based guideline: Complementary and alternative medicine in multiple sclerosis: Report of the guideline development subcommittee of the American Academy of Neurology. Neurology. 2014;82(12):1083-1092.
1. U.S. Department of Veterans Affairs, Office of Nursing Services. VA Nursing Service Fact Sheet. VA nursing service: Excellence in patient-driven care. U.S. Department of Veterans Affairs Website. http://www.va.gov/nursing/docs/about/vansgfacts.doc. Accessed February 23, 2015.
2. Budzi D, Lurie S, Singh K, Hooker R. Veterans’ perceptions of care by nurse practitioners, physician assistants, and physicians: A comparison from satisfaction surveys. J Am Acad Nurse Pract. 2010;22(3):170-176.
3. Costello K, Halper J. Advanced Practice Nursing in Multiple Sclerosis. Advanced Skills, Advancing Responsibilities. 3rd ed. International Organization of Multiple Sclerosis Nurses Website. http://iomsn.org/images/pdf/APN_Monograph_3rdEd.pdf. 2010. Accessed February 22, 2015.
4. New York City Coalition of Multiple Sclerosis Nurses. The Dynamic Multiple Sclerosis Nurse: Challenges, Expanding Role and Future Directions. International Organization of MS Nurses Website. http://www.iomsn.org/images/pdf/Article_Advanced_NYCMSNurse.pdf. Accessed February 22, 2015.
5. Halper J, Harris C. Nursing Practice in Multiple Sclerosis. A Core Curriculum. 3rd ed. New York, NY: Springer Publishing Company; 2012.
6. U.S. Department of Veterans Affairs. Multiple sclerosis centers of excellence. What are my VA benefits for multiple sclerosis. U.S. Department of Veterans Affairs Website. http://www.va.gov/MS/Veterans/benefits/What_Are_My_VA_Benefits_for_Multiple_Sclerosis.asp. Updated June 2013. Accessed February 22, 2015.
7. Yadav V, Bever C Jr, Bowen J, et al. Summary of evidence-based guideline: Complementary and alternative medicine in multiple sclerosis: Report of the guideline development subcommittee of the American Academy of Neurology. Neurology. 2014;82(12):1083-1092.
Multidisciplinary Management of a Patient With Multiple Sclerosis: Part 1. Neurologists’ and Physiatrists’ Perspectives
Multiple sclerosis (MS) is the most common progressive neurologic disease of young adults, affecting 350,000 to 400,000 people in the U.S.1 The disease most commonly presents with intermittent relapses and evolves to a progressive form. Common symptoms include weakness, sensory loss, vision disturbances, ataxia, bladder dysfunction, cognitive deficits, and fatigue. A thoughtful multidisciplinary approach is essential for patients with MS who live with an unpredictable disease, numerous secondary symptoms, and the fear of debilitating progression. The goal is to maintain good quality of life (QOL) for patients with MS.
This article responds to the issues presented by a young patient recently released from active-duty military service and illustrate the power of a team
approach to managing the care of patients with MS. The 3 sections are written from the perspectives of (1) neurologists and physiatrists; (2) nurse practitioners; and (3) psychologists and also represent contributions of each discipline toward the goal of maintaining QOL for patients with MS. Although these health care specialists are highlighted, many more were involved in the care of this patient and are not included due to space constraints.
Case Presentation
William is a 31-year-old African American man who began experiencing headaches, occasional imbalance, periods of confusion, and mental fogginess following discharge from active duty 5 years ago. William had been deployed to Afghanistan and was exposed to at least 1 improvised explosive device blast while there. He did not disclose to army physicians a 24-hour loss of vision in his right eye while driving a tactical vehicle in Kandahar, Afghanistan. The patient thought his vision changes were caused by stress and worried that he would be removed from patrol duties if he reported the problem. At the time of his discharge, William was diagnosed with mild traumatic brain injury from blast exposure.
After discharge, William sought care in the private sector but felt providers assumed all his symptoms were the result of depression or posttraumatic stress disorder. He began noticing problems at his new job: missing deadlines, forgetting conversations, and having difficulty making decisions. William’s gait became clumsy, and he occasionally tripped and ran into walls. He worried that his supervisor and colleagues would discover his problems, so he quit his job. William knew something was wrong and wanted a provider who would understand him. He decided to take advantage of the VA health care system and its promise of care for Operation Enduring Freedom/Operation Iraqi Freedom veterans. He had an initial evaluation with his primary care Patient Aligned Care Team (PACT), which noted deficits on his neurologic examination and referred him to the neurology clinic affiliated with the MS Centers of Excellence (MSCoE).2
William’s neurologic examination was significant for psychomotor slowing, memory loss, cerebellar ataxia, and lower extremity spasticity. Magnetic resonance imaging (MRI) of the brain and spinal cord were obtained as recommended by the Consortium of MS Centers guidelines.3 The MRI showed 12 high-frequency T2 lesions in the periventricular regions bilaterally and cerebellum. One lesion was gadolinium enhanced. Two additional high T2 lesions were noted in the upper cervical spinal cord. After ruling out mimics, William was diagnosed with relapsing remitting MS, based on McDonald Criteria.4 His Expanded Disability Status score was 3.0, and deficits were documented in the pyramidal, cerebellar, and cerebral functional systems.5 William’s history and symptoms, blood, and cerebrospinal fluid studies were consistent with the typical pattern of relapses and remissions with neurologic symptoms separated in space and time.
The neurologist gave William some context to the diagnosis. He noted the changing epidemiology of the disease and that African Americans are considered a highrisk group for rapid progression of MS symptoms.6-8 A progress note and MS Assessment Tool were completed in the Computerized Patient Record System (CPRS)—an annual requirement recommended by the Multiple Sclerosis System of Care Procedures.9
Symptom Management
All FDA-approved MS disease-modifying therapies (DMT) are available within the VA MSCoE network. After a discussion of risks and benefits with William, the neurologist recommended 2 options for therapy. One option was more aggressive and had the greatest efficacy in a randomized controlled trial, yet a higher risk for adverse events (AEs). The other option had modest efficacy and favorable long-term safety data. After further discussion with his neurologist, William indicated he wanted to avoid risks and preferred medication with a long track record for success and safety. He selected an injectable DMT.
William returned to the clinic 3 months later and reported no new or worsening neurologic symptoms and stated that he was “fine.” However, in a review of his MS symptoms, the neurologist discovered gait ataxia, bladder urgency, and constipation. On further questioning, William admitted to experiencing excessive fatigue, an inability to go to or stay asleep, and difficulty in finishing tasks. William also indicated that he had forgotten to take
his DMT as prescribed, had a fear of injecting himself, and had painful injection site reactions. The neurologist referred him for a bladder ultrasound and for urinalysis and urine cultures, as well as for more extensive cognitive testing with a neuropsychologist and for gait assessment with the physiatrist.
William reported 2 recent falls to the physiatrist, both occurring while walking on his lawn to get the mail, neither resulting in significant injury. He reported using walls and furniture to maintain balance at home. Although he continued to insist that he was fine, further questioning revealed that he at times avoided leaving home due to a fear of falling.
A focused physical examination consisting of an assessment of lower limb muscular strength, coordination, sensation, and spasticity helped identify issues that were causing his impaired gait. Additionally, the impact of changes in vision, cognition, and fatigue were explored, because they also contributed to falls and impacted use of mobility assistive devices.
William had normal strength except for mild weakness in his left ankle dorsiflexors, intact sensation, impaired heel-to-shin performance, and minimal resistance of his hip adductors. A wide base of support, short stride length, and a slow cadence characterized his gait. His average of two 25-foot walk tests was 15 seconds, and by the end of his second trial, he had mild foot drop.
Impairments in gait are typically multifactorial, so treatment plans often consider multiple issues. Because William was able to use a wide base of support to maintain his balance and quality of gait, it did not seem that spasticity was impairing his function. Therefore, an oral spasticity agent was not indicated, although a stretching program was started to prevent potential future complications related to spasticity.
An ankle-foot orthosis could address William’s foot drop. An assistive device, such as a cane or walker, could also help minimize falls associated with ataxia. It is important to screen patients for psychological impacts of loss of function and psychological barriers to the use of assistive devices; referral to a rehabilitation psychologist may be warranted. Last, William’s walking speed was slightly impaired. When speed of walking is a concern, patients might benefit from a trial of dalfampridine once walking safety has been optimized.
MS Care Models
Because MS is a dynamic disease, producing multifocal neurologic deficits and disability, a wide range of health care specialists are required to assist in MS care throughout the life of the patient. The neurologist is typically the principal caregiver, but referrals to rehabilitation specialists, psychologists, ophthalmologists, urologists, speech pathologists, wound specialists, and social workers are common. Multiple sclerosis advocacy groups within the U.S. frequently promote multidisciplinary care. Yet few attempts have been made to define multidisciplinary MS care models or to test their effectiveness. Like other chronic conditions, coordination and continuity of care for patients with MS are often suboptimal.
About 30,000 patients with MS use the VA health care system. Treatment takes place largely in outpatient clinics. Patients with MS require more visits per person than do all but a handful of other patient populations. Multiple sclerosis therapy includes complex and expensive pharmacologic agents as well as multidisciplinary medical and rehabilitation services and assistive technology. In 2003, after surveys showed wide and unexplained variations across the VA in the care of patients with MS, 2 MSCoE coordinating centers were established to improve access to MS specialty care, develop national standards of care, and implement those standards through a network of regional MS programs.
With input from the MSCoE and a network of more than 70 VA MS programs, the VA Central Office released a handbook for MS care in December 2009. The VA handbook Multiple Sclerosis System of Care Procedures is the first MS health care policy directive that has been created, outlining a comprehensive plan of care for patients with MS.9 The handbook describes the diagnostic and therapeutic health care services that are required by patients with MS, including primary care, MS specialty care, rehabilitation, palliative care, respite care, home care, long-term care, mental health care, social work services, telehealth services, and access to disease-modifying and symptomatic pharmacologic therapies.
According to the MS handbook, every patient with MS should receive an annual evaluation in which the care plan is reviewed with a provider who is knowledgeable about MS. The provider then completes the required MS Assessment Tool within the CPRS. The annual visit and MS Assessment Tool help to identify patients with MS, track medication AEs, and populate the VA national MS surveillance registry. Ideally, this evaluation takes place in
a face-to-face visit with a MS subspecialist. However, this requirement also could be satisfied by a visit with another provider knowledgeable about MS or through a video or telephone telehealth interview.
The location of care should be dictated by the needs of the patient and be as convenient as possible. To support this, a regional hub-and-spoke network has been outlined. Each VISN supports at least 1 MS regional program (hub site). The MS regional program team consists of a physician with MS expertise, a nurse, a social worker, and access to specialty care services. These teams lead and coordinate integrated MS care at the local medical center and assist in the care of patients with MS at remote facilities within the VISN.
Facilities without a MS regional program are designated as spoke sites that have a designated MS care coordinator to assist with care at that facility. Spoke sites work with the regional hub to deliver MS care to the local population. Consultations from the closest MS regional center may be provided by telephone, telehealth, or a live visit. The level of MS care for regional centers is influenced by the patient’s MS stage and the location. Different MS care models that incorporate more consultative and telehealth approaches may need to be developed for patients with MS in rural areas with limited access to specialists.
1. Anderson DW, Ellenberg JH, Leventhan CM, Reingold SC, Rodriguez M, Silberberg DH. Revised estimate of the prevalence of multiple sclerosis in the United States. Ann Neurol. 1992;31(3):333-336.
2. Klein S. The Veterans Health Administration: Implementing patient-centered medical homes in the nation’s largest integrated delivery system. Commonwealth Fund Website. http://www.commonwealthfund.org/publications/case-studies/2011/sep/va-medical-homes. Updated September 2011. Accessed February 27, 2015.
3. Simon JH, Li D, Traboulsee A, et al. Standardized MR imaging protocol for multiple sclerosis: Consortium of MS Centers consensus guidelines. AJNR Am J Neuroradiol. 2006;27(2):455-461.
4. Polman CH, Reingold SC, Banwell B, et al. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol. 2011;69(2):292-302.
5. Kurtzke JF. Rating neurological impairment in multiple sclerosis: An expanded disability status scale (EDSS). Neurology. 1983;33(11):1444-1452.
6. Wallin MT, Culpepper WJ, Coffman P, et al; Veterans Affairs Multiple Sclerosis Centres of Excellence Epidemiology Group. The Gulf War era multiple sclerosis cohort: Age and incidence rates by race, sex and service. Brain. 2012;135(pt 6):1780-1785.
7. Langer-Gould A, Brara SM, Beaber BE, Zhang JL. Incidence of multiple sclerosis in
multiple racial and ethnic groups. Neurology. 2013;80(19):1734-1739.
8. Cree BA, Khan O, Bourdette D, et al. Clinical characteristics of African Americans vs Caucasian Americans with multiple sclerosis. Neurology. 2004;63(11):2039-2045.
9. U.S. Department of Veterans Affairs, Veterans Health Administration. Multiple Sclerosis System of Care Procedures. Washington, DC: Department of Veterans Affairs; 2009.
Multiple sclerosis (MS) is the most common progressive neurologic disease of young adults, affecting 350,000 to 400,000 people in the U.S.1 The disease most commonly presents with intermittent relapses and evolves to a progressive form. Common symptoms include weakness, sensory loss, vision disturbances, ataxia, bladder dysfunction, cognitive deficits, and fatigue. A thoughtful multidisciplinary approach is essential for patients with MS who live with an unpredictable disease, numerous secondary symptoms, and the fear of debilitating progression. The goal is to maintain good quality of life (QOL) for patients with MS.
This article responds to the issues presented by a young patient recently released from active-duty military service and illustrate the power of a team
approach to managing the care of patients with MS. The 3 sections are written from the perspectives of (1) neurologists and physiatrists; (2) nurse practitioners; and (3) psychologists and also represent contributions of each discipline toward the goal of maintaining QOL for patients with MS. Although these health care specialists are highlighted, many more were involved in the care of this patient and are not included due to space constraints.
Case Presentation
William is a 31-year-old African American man who began experiencing headaches, occasional imbalance, periods of confusion, and mental fogginess following discharge from active duty 5 years ago. William had been deployed to Afghanistan and was exposed to at least 1 improvised explosive device blast while there. He did not disclose to army physicians a 24-hour loss of vision in his right eye while driving a tactical vehicle in Kandahar, Afghanistan. The patient thought his vision changes were caused by stress and worried that he would be removed from patrol duties if he reported the problem. At the time of his discharge, William was diagnosed with mild traumatic brain injury from blast exposure.
After discharge, William sought care in the private sector but felt providers assumed all his symptoms were the result of depression or posttraumatic stress disorder. He began noticing problems at his new job: missing deadlines, forgetting conversations, and having difficulty making decisions. William’s gait became clumsy, and he occasionally tripped and ran into walls. He worried that his supervisor and colleagues would discover his problems, so he quit his job. William knew something was wrong and wanted a provider who would understand him. He decided to take advantage of the VA health care system and its promise of care for Operation Enduring Freedom/Operation Iraqi Freedom veterans. He had an initial evaluation with his primary care Patient Aligned Care Team (PACT), which noted deficits on his neurologic examination and referred him to the neurology clinic affiliated with the MS Centers of Excellence (MSCoE).2
William’s neurologic examination was significant for psychomotor slowing, memory loss, cerebellar ataxia, and lower extremity spasticity. Magnetic resonance imaging (MRI) of the brain and spinal cord were obtained as recommended by the Consortium of MS Centers guidelines.3 The MRI showed 12 high-frequency T2 lesions in the periventricular regions bilaterally and cerebellum. One lesion was gadolinium enhanced. Two additional high T2 lesions were noted in the upper cervical spinal cord. After ruling out mimics, William was diagnosed with relapsing remitting MS, based on McDonald Criteria.4 His Expanded Disability Status score was 3.0, and deficits were documented in the pyramidal, cerebellar, and cerebral functional systems.5 William’s history and symptoms, blood, and cerebrospinal fluid studies were consistent with the typical pattern of relapses and remissions with neurologic symptoms separated in space and time.
The neurologist gave William some context to the diagnosis. He noted the changing epidemiology of the disease and that African Americans are considered a highrisk group for rapid progression of MS symptoms.6-8 A progress note and MS Assessment Tool were completed in the Computerized Patient Record System (CPRS)—an annual requirement recommended by the Multiple Sclerosis System of Care Procedures.9
Symptom Management
All FDA-approved MS disease-modifying therapies (DMT) are available within the VA MSCoE network. After a discussion of risks and benefits with William, the neurologist recommended 2 options for therapy. One option was more aggressive and had the greatest efficacy in a randomized controlled trial, yet a higher risk for adverse events (AEs). The other option had modest efficacy and favorable long-term safety data. After further discussion with his neurologist, William indicated he wanted to avoid risks and preferred medication with a long track record for success and safety. He selected an injectable DMT.
William returned to the clinic 3 months later and reported no new or worsening neurologic symptoms and stated that he was “fine.” However, in a review of his MS symptoms, the neurologist discovered gait ataxia, bladder urgency, and constipation. On further questioning, William admitted to experiencing excessive fatigue, an inability to go to or stay asleep, and difficulty in finishing tasks. William also indicated that he had forgotten to take
his DMT as prescribed, had a fear of injecting himself, and had painful injection site reactions. The neurologist referred him for a bladder ultrasound and for urinalysis and urine cultures, as well as for more extensive cognitive testing with a neuropsychologist and for gait assessment with the physiatrist.
William reported 2 recent falls to the physiatrist, both occurring while walking on his lawn to get the mail, neither resulting in significant injury. He reported using walls and furniture to maintain balance at home. Although he continued to insist that he was fine, further questioning revealed that he at times avoided leaving home due to a fear of falling.
A focused physical examination consisting of an assessment of lower limb muscular strength, coordination, sensation, and spasticity helped identify issues that were causing his impaired gait. Additionally, the impact of changes in vision, cognition, and fatigue were explored, because they also contributed to falls and impacted use of mobility assistive devices.
William had normal strength except for mild weakness in his left ankle dorsiflexors, intact sensation, impaired heel-to-shin performance, and minimal resistance of his hip adductors. A wide base of support, short stride length, and a slow cadence characterized his gait. His average of two 25-foot walk tests was 15 seconds, and by the end of his second trial, he had mild foot drop.
Impairments in gait are typically multifactorial, so treatment plans often consider multiple issues. Because William was able to use a wide base of support to maintain his balance and quality of gait, it did not seem that spasticity was impairing his function. Therefore, an oral spasticity agent was not indicated, although a stretching program was started to prevent potential future complications related to spasticity.
An ankle-foot orthosis could address William’s foot drop. An assistive device, such as a cane or walker, could also help minimize falls associated with ataxia. It is important to screen patients for psychological impacts of loss of function and psychological barriers to the use of assistive devices; referral to a rehabilitation psychologist may be warranted. Last, William’s walking speed was slightly impaired. When speed of walking is a concern, patients might benefit from a trial of dalfampridine once walking safety has been optimized.
MS Care Models
Because MS is a dynamic disease, producing multifocal neurologic deficits and disability, a wide range of health care specialists are required to assist in MS care throughout the life of the patient. The neurologist is typically the principal caregiver, but referrals to rehabilitation specialists, psychologists, ophthalmologists, urologists, speech pathologists, wound specialists, and social workers are common. Multiple sclerosis advocacy groups within the U.S. frequently promote multidisciplinary care. Yet few attempts have been made to define multidisciplinary MS care models or to test their effectiveness. Like other chronic conditions, coordination and continuity of care for patients with MS are often suboptimal.
About 30,000 patients with MS use the VA health care system. Treatment takes place largely in outpatient clinics. Patients with MS require more visits per person than do all but a handful of other patient populations. Multiple sclerosis therapy includes complex and expensive pharmacologic agents as well as multidisciplinary medical and rehabilitation services and assistive technology. In 2003, after surveys showed wide and unexplained variations across the VA in the care of patients with MS, 2 MSCoE coordinating centers were established to improve access to MS specialty care, develop national standards of care, and implement those standards through a network of regional MS programs.
With input from the MSCoE and a network of more than 70 VA MS programs, the VA Central Office released a handbook for MS care in December 2009. The VA handbook Multiple Sclerosis System of Care Procedures is the first MS health care policy directive that has been created, outlining a comprehensive plan of care for patients with MS.9 The handbook describes the diagnostic and therapeutic health care services that are required by patients with MS, including primary care, MS specialty care, rehabilitation, palliative care, respite care, home care, long-term care, mental health care, social work services, telehealth services, and access to disease-modifying and symptomatic pharmacologic therapies.
According to the MS handbook, every patient with MS should receive an annual evaluation in which the care plan is reviewed with a provider who is knowledgeable about MS. The provider then completes the required MS Assessment Tool within the CPRS. The annual visit and MS Assessment Tool help to identify patients with MS, track medication AEs, and populate the VA national MS surveillance registry. Ideally, this evaluation takes place in
a face-to-face visit with a MS subspecialist. However, this requirement also could be satisfied by a visit with another provider knowledgeable about MS or through a video or telephone telehealth interview.
The location of care should be dictated by the needs of the patient and be as convenient as possible. To support this, a regional hub-and-spoke network has been outlined. Each VISN supports at least 1 MS regional program (hub site). The MS regional program team consists of a physician with MS expertise, a nurse, a social worker, and access to specialty care services. These teams lead and coordinate integrated MS care at the local medical center and assist in the care of patients with MS at remote facilities within the VISN.
Facilities without a MS regional program are designated as spoke sites that have a designated MS care coordinator to assist with care at that facility. Spoke sites work with the regional hub to deliver MS care to the local population. Consultations from the closest MS regional center may be provided by telephone, telehealth, or a live visit. The level of MS care for regional centers is influenced by the patient’s MS stage and the location. Different MS care models that incorporate more consultative and telehealth approaches may need to be developed for patients with MS in rural areas with limited access to specialists.
Multiple sclerosis (MS) is the most common progressive neurologic disease of young adults, affecting 350,000 to 400,000 people in the U.S.1 The disease most commonly presents with intermittent relapses and evolves to a progressive form. Common symptoms include weakness, sensory loss, vision disturbances, ataxia, bladder dysfunction, cognitive deficits, and fatigue. A thoughtful multidisciplinary approach is essential for patients with MS who live with an unpredictable disease, numerous secondary symptoms, and the fear of debilitating progression. The goal is to maintain good quality of life (QOL) for patients with MS.
This article responds to the issues presented by a young patient recently released from active-duty military service and illustrate the power of a team
approach to managing the care of patients with MS. The 3 sections are written from the perspectives of (1) neurologists and physiatrists; (2) nurse practitioners; and (3) psychologists and also represent contributions of each discipline toward the goal of maintaining QOL for patients with MS. Although these health care specialists are highlighted, many more were involved in the care of this patient and are not included due to space constraints.
Case Presentation
William is a 31-year-old African American man who began experiencing headaches, occasional imbalance, periods of confusion, and mental fogginess following discharge from active duty 5 years ago. William had been deployed to Afghanistan and was exposed to at least 1 improvised explosive device blast while there. He did not disclose to army physicians a 24-hour loss of vision in his right eye while driving a tactical vehicle in Kandahar, Afghanistan. The patient thought his vision changes were caused by stress and worried that he would be removed from patrol duties if he reported the problem. At the time of his discharge, William was diagnosed with mild traumatic brain injury from blast exposure.
After discharge, William sought care in the private sector but felt providers assumed all his symptoms were the result of depression or posttraumatic stress disorder. He began noticing problems at his new job: missing deadlines, forgetting conversations, and having difficulty making decisions. William’s gait became clumsy, and he occasionally tripped and ran into walls. He worried that his supervisor and colleagues would discover his problems, so he quit his job. William knew something was wrong and wanted a provider who would understand him. He decided to take advantage of the VA health care system and its promise of care for Operation Enduring Freedom/Operation Iraqi Freedom veterans. He had an initial evaluation with his primary care Patient Aligned Care Team (PACT), which noted deficits on his neurologic examination and referred him to the neurology clinic affiliated with the MS Centers of Excellence (MSCoE).2
William’s neurologic examination was significant for psychomotor slowing, memory loss, cerebellar ataxia, and lower extremity spasticity. Magnetic resonance imaging (MRI) of the brain and spinal cord were obtained as recommended by the Consortium of MS Centers guidelines.3 The MRI showed 12 high-frequency T2 lesions in the periventricular regions bilaterally and cerebellum. One lesion was gadolinium enhanced. Two additional high T2 lesions were noted in the upper cervical spinal cord. After ruling out mimics, William was diagnosed with relapsing remitting MS, based on McDonald Criteria.4 His Expanded Disability Status score was 3.0, and deficits were documented in the pyramidal, cerebellar, and cerebral functional systems.5 William’s history and symptoms, blood, and cerebrospinal fluid studies were consistent with the typical pattern of relapses and remissions with neurologic symptoms separated in space and time.
The neurologist gave William some context to the diagnosis. He noted the changing epidemiology of the disease and that African Americans are considered a highrisk group for rapid progression of MS symptoms.6-8 A progress note and MS Assessment Tool were completed in the Computerized Patient Record System (CPRS)—an annual requirement recommended by the Multiple Sclerosis System of Care Procedures.9
Symptom Management
All FDA-approved MS disease-modifying therapies (DMT) are available within the VA MSCoE network. After a discussion of risks and benefits with William, the neurologist recommended 2 options for therapy. One option was more aggressive and had the greatest efficacy in a randomized controlled trial, yet a higher risk for adverse events (AEs). The other option had modest efficacy and favorable long-term safety data. After further discussion with his neurologist, William indicated he wanted to avoid risks and preferred medication with a long track record for success and safety. He selected an injectable DMT.
William returned to the clinic 3 months later and reported no new or worsening neurologic symptoms and stated that he was “fine.” However, in a review of his MS symptoms, the neurologist discovered gait ataxia, bladder urgency, and constipation. On further questioning, William admitted to experiencing excessive fatigue, an inability to go to or stay asleep, and difficulty in finishing tasks. William also indicated that he had forgotten to take
his DMT as prescribed, had a fear of injecting himself, and had painful injection site reactions. The neurologist referred him for a bladder ultrasound and for urinalysis and urine cultures, as well as for more extensive cognitive testing with a neuropsychologist and for gait assessment with the physiatrist.
William reported 2 recent falls to the physiatrist, both occurring while walking on his lawn to get the mail, neither resulting in significant injury. He reported using walls and furniture to maintain balance at home. Although he continued to insist that he was fine, further questioning revealed that he at times avoided leaving home due to a fear of falling.
A focused physical examination consisting of an assessment of lower limb muscular strength, coordination, sensation, and spasticity helped identify issues that were causing his impaired gait. Additionally, the impact of changes in vision, cognition, and fatigue were explored, because they also contributed to falls and impacted use of mobility assistive devices.
William had normal strength except for mild weakness in his left ankle dorsiflexors, intact sensation, impaired heel-to-shin performance, and minimal resistance of his hip adductors. A wide base of support, short stride length, and a slow cadence characterized his gait. His average of two 25-foot walk tests was 15 seconds, and by the end of his second trial, he had mild foot drop.
Impairments in gait are typically multifactorial, so treatment plans often consider multiple issues. Because William was able to use a wide base of support to maintain his balance and quality of gait, it did not seem that spasticity was impairing his function. Therefore, an oral spasticity agent was not indicated, although a stretching program was started to prevent potential future complications related to spasticity.
An ankle-foot orthosis could address William’s foot drop. An assistive device, such as a cane or walker, could also help minimize falls associated with ataxia. It is important to screen patients for psychological impacts of loss of function and psychological barriers to the use of assistive devices; referral to a rehabilitation psychologist may be warranted. Last, William’s walking speed was slightly impaired. When speed of walking is a concern, patients might benefit from a trial of dalfampridine once walking safety has been optimized.
MS Care Models
Because MS is a dynamic disease, producing multifocal neurologic deficits and disability, a wide range of health care specialists are required to assist in MS care throughout the life of the patient. The neurologist is typically the principal caregiver, but referrals to rehabilitation specialists, psychologists, ophthalmologists, urologists, speech pathologists, wound specialists, and social workers are common. Multiple sclerosis advocacy groups within the U.S. frequently promote multidisciplinary care. Yet few attempts have been made to define multidisciplinary MS care models or to test their effectiveness. Like other chronic conditions, coordination and continuity of care for patients with MS are often suboptimal.
About 30,000 patients with MS use the VA health care system. Treatment takes place largely in outpatient clinics. Patients with MS require more visits per person than do all but a handful of other patient populations. Multiple sclerosis therapy includes complex and expensive pharmacologic agents as well as multidisciplinary medical and rehabilitation services and assistive technology. In 2003, after surveys showed wide and unexplained variations across the VA in the care of patients with MS, 2 MSCoE coordinating centers were established to improve access to MS specialty care, develop national standards of care, and implement those standards through a network of regional MS programs.
With input from the MSCoE and a network of more than 70 VA MS programs, the VA Central Office released a handbook for MS care in December 2009. The VA handbook Multiple Sclerosis System of Care Procedures is the first MS health care policy directive that has been created, outlining a comprehensive plan of care for patients with MS.9 The handbook describes the diagnostic and therapeutic health care services that are required by patients with MS, including primary care, MS specialty care, rehabilitation, palliative care, respite care, home care, long-term care, mental health care, social work services, telehealth services, and access to disease-modifying and symptomatic pharmacologic therapies.
According to the MS handbook, every patient with MS should receive an annual evaluation in which the care plan is reviewed with a provider who is knowledgeable about MS. The provider then completes the required MS Assessment Tool within the CPRS. The annual visit and MS Assessment Tool help to identify patients with MS, track medication AEs, and populate the VA national MS surveillance registry. Ideally, this evaluation takes place in
a face-to-face visit with a MS subspecialist. However, this requirement also could be satisfied by a visit with another provider knowledgeable about MS or through a video or telephone telehealth interview.
The location of care should be dictated by the needs of the patient and be as convenient as possible. To support this, a regional hub-and-spoke network has been outlined. Each VISN supports at least 1 MS regional program (hub site). The MS regional program team consists of a physician with MS expertise, a nurse, a social worker, and access to specialty care services. These teams lead and coordinate integrated MS care at the local medical center and assist in the care of patients with MS at remote facilities within the VISN.
Facilities without a MS regional program are designated as spoke sites that have a designated MS care coordinator to assist with care at that facility. Spoke sites work with the regional hub to deliver MS care to the local population. Consultations from the closest MS regional center may be provided by telephone, telehealth, or a live visit. The level of MS care for regional centers is influenced by the patient’s MS stage and the location. Different MS care models that incorporate more consultative and telehealth approaches may need to be developed for patients with MS in rural areas with limited access to specialists.
1. Anderson DW, Ellenberg JH, Leventhan CM, Reingold SC, Rodriguez M, Silberberg DH. Revised estimate of the prevalence of multiple sclerosis in the United States. Ann Neurol. 1992;31(3):333-336.
2. Klein S. The Veterans Health Administration: Implementing patient-centered medical homes in the nation’s largest integrated delivery system. Commonwealth Fund Website. http://www.commonwealthfund.org/publications/case-studies/2011/sep/va-medical-homes. Updated September 2011. Accessed February 27, 2015.
3. Simon JH, Li D, Traboulsee A, et al. Standardized MR imaging protocol for multiple sclerosis: Consortium of MS Centers consensus guidelines. AJNR Am J Neuroradiol. 2006;27(2):455-461.
4. Polman CH, Reingold SC, Banwell B, et al. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol. 2011;69(2):292-302.
5. Kurtzke JF. Rating neurological impairment in multiple sclerosis: An expanded disability status scale (EDSS). Neurology. 1983;33(11):1444-1452.
6. Wallin MT, Culpepper WJ, Coffman P, et al; Veterans Affairs Multiple Sclerosis Centres of Excellence Epidemiology Group. The Gulf War era multiple sclerosis cohort: Age and incidence rates by race, sex and service. Brain. 2012;135(pt 6):1780-1785.
7. Langer-Gould A, Brara SM, Beaber BE, Zhang JL. Incidence of multiple sclerosis in
multiple racial and ethnic groups. Neurology. 2013;80(19):1734-1739.
8. Cree BA, Khan O, Bourdette D, et al. Clinical characteristics of African Americans vs Caucasian Americans with multiple sclerosis. Neurology. 2004;63(11):2039-2045.
9. U.S. Department of Veterans Affairs, Veterans Health Administration. Multiple Sclerosis System of Care Procedures. Washington, DC: Department of Veterans Affairs; 2009.
1. Anderson DW, Ellenberg JH, Leventhan CM, Reingold SC, Rodriguez M, Silberberg DH. Revised estimate of the prevalence of multiple sclerosis in the United States. Ann Neurol. 1992;31(3):333-336.
2. Klein S. The Veterans Health Administration: Implementing patient-centered medical homes in the nation’s largest integrated delivery system. Commonwealth Fund Website. http://www.commonwealthfund.org/publications/case-studies/2011/sep/va-medical-homes. Updated September 2011. Accessed February 27, 2015.
3. Simon JH, Li D, Traboulsee A, et al. Standardized MR imaging protocol for multiple sclerosis: Consortium of MS Centers consensus guidelines. AJNR Am J Neuroradiol. 2006;27(2):455-461.
4. Polman CH, Reingold SC, Banwell B, et al. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol. 2011;69(2):292-302.
5. Kurtzke JF. Rating neurological impairment in multiple sclerosis: An expanded disability status scale (EDSS). Neurology. 1983;33(11):1444-1452.
6. Wallin MT, Culpepper WJ, Coffman P, et al; Veterans Affairs Multiple Sclerosis Centres of Excellence Epidemiology Group. The Gulf War era multiple sclerosis cohort: Age and incidence rates by race, sex and service. Brain. 2012;135(pt 6):1780-1785.
7. Langer-Gould A, Brara SM, Beaber BE, Zhang JL. Incidence of multiple sclerosis in
multiple racial and ethnic groups. Neurology. 2013;80(19):1734-1739.
8. Cree BA, Khan O, Bourdette D, et al. Clinical characteristics of African Americans vs Caucasian Americans with multiple sclerosis. Neurology. 2004;63(11):2039-2045.
9. U.S. Department of Veterans Affairs, Veterans Health Administration. Multiple Sclerosis System of Care Procedures. Washington, DC: Department of Veterans Affairs; 2009.
Pretreatment Imaging May Help Prevent Hodgkin Lymphoma Recurrence
Advances in radiation treatment have led to better targeting, minimizing the dose to healthy tissue. For patients with Hodgkin lymphoma (HL), pretreatment scanning with positron emission tomography and computed tomography (PET/CT) has become the gold standard, say researchers from University of Florida, in determining the extent of HL. Because HL may recur at the site of the original cancer, the scans are important to accurately capture the scope of the disease. Moreover, the researchers say pretreatment PET/CT may reduce disease progression.
Related: Study Points to Risk Factors for Lymphoma
In their study of 37 patients with stage I or II HL, 31 had PET/CT before chemotherapy. Two of the remaining 6 had PET/CT done within 5 days after chemotherapy was started. Median follow-up was 46 months.
The 4-year rate of relapse-free survival was 92%. Patients who did not receive pretreatment PET/CT were more likely to have a relapse (67%). Of 4 recurrences, 3 were within 12 months of follow-up; 1 developed 5 years after treatment.
Among the 6 patients who did not have a baseline PET/CT scan, all 3 recurrences were in lymph node regions outside of, but adjacent to, the radiation field. None of the 6 experienced an in-field treatment failure.
Related: Development and Implementation of a Veterans’ Cancer Survivorship Program
Long-term survivors of HL are vulnerable to late adverse effects, the researchers note, and that fact is “the impetus behind efforts to reduce radiation exposure to organs at risk.” They cite studies that have found that PET/CT scans, compared with using only pretreatment contrast-enhanced CT scans, can alter the staging in 10% to 30% of patients with HL. Their study, the researchers add, helps support the National Comprehensive Cancer Network guidelines that advise prechemotherapy PET/CT imaging in staging all HL patients. Not doing complete staging, the researchers say, puts patients at “unnecessary, and in some instances preventable, risk for recurrence.”
Source:
Figura N, Flampouri S, Mendenhall NP, et al. Adv Radiat Oncol. 2017;1-16.
Advances in radiation treatment have led to better targeting, minimizing the dose to healthy tissue. For patients with Hodgkin lymphoma (HL), pretreatment scanning with positron emission tomography and computed tomography (PET/CT) has become the gold standard, say researchers from University of Florida, in determining the extent of HL. Because HL may recur at the site of the original cancer, the scans are important to accurately capture the scope of the disease. Moreover, the researchers say pretreatment PET/CT may reduce disease progression.
Related: Study Points to Risk Factors for Lymphoma
In their study of 37 patients with stage I or II HL, 31 had PET/CT before chemotherapy. Two of the remaining 6 had PET/CT done within 5 days after chemotherapy was started. Median follow-up was 46 months.
The 4-year rate of relapse-free survival was 92%. Patients who did not receive pretreatment PET/CT were more likely to have a relapse (67%). Of 4 recurrences, 3 were within 12 months of follow-up; 1 developed 5 years after treatment.
Among the 6 patients who did not have a baseline PET/CT scan, all 3 recurrences were in lymph node regions outside of, but adjacent to, the radiation field. None of the 6 experienced an in-field treatment failure.
Related: Development and Implementation of a Veterans’ Cancer Survivorship Program
Long-term survivors of HL are vulnerable to late adverse effects, the researchers note, and that fact is “the impetus behind efforts to reduce radiation exposure to organs at risk.” They cite studies that have found that PET/CT scans, compared with using only pretreatment contrast-enhanced CT scans, can alter the staging in 10% to 30% of patients with HL. Their study, the researchers add, helps support the National Comprehensive Cancer Network guidelines that advise prechemotherapy PET/CT imaging in staging all HL patients. Not doing complete staging, the researchers say, puts patients at “unnecessary, and in some instances preventable, risk for recurrence.”
Source:
Figura N, Flampouri S, Mendenhall NP, et al. Adv Radiat Oncol. 2017;1-16.
Advances in radiation treatment have led to better targeting, minimizing the dose to healthy tissue. For patients with Hodgkin lymphoma (HL), pretreatment scanning with positron emission tomography and computed tomography (PET/CT) has become the gold standard, say researchers from University of Florida, in determining the extent of HL. Because HL may recur at the site of the original cancer, the scans are important to accurately capture the scope of the disease. Moreover, the researchers say pretreatment PET/CT may reduce disease progression.
Related: Study Points to Risk Factors for Lymphoma
In their study of 37 patients with stage I or II HL, 31 had PET/CT before chemotherapy. Two of the remaining 6 had PET/CT done within 5 days after chemotherapy was started. Median follow-up was 46 months.
The 4-year rate of relapse-free survival was 92%. Patients who did not receive pretreatment PET/CT were more likely to have a relapse (67%). Of 4 recurrences, 3 were within 12 months of follow-up; 1 developed 5 years after treatment.
Among the 6 patients who did not have a baseline PET/CT scan, all 3 recurrences were in lymph node regions outside of, but adjacent to, the radiation field. None of the 6 experienced an in-field treatment failure.
Related: Development and Implementation of a Veterans’ Cancer Survivorship Program
Long-term survivors of HL are vulnerable to late adverse effects, the researchers note, and that fact is “the impetus behind efforts to reduce radiation exposure to organs at risk.” They cite studies that have found that PET/CT scans, compared with using only pretreatment contrast-enhanced CT scans, can alter the staging in 10% to 30% of patients with HL. Their study, the researchers add, helps support the National Comprehensive Cancer Network guidelines that advise prechemotherapy PET/CT imaging in staging all HL patients. Not doing complete staging, the researchers say, puts patients at “unnecessary, and in some instances preventable, risk for recurrence.”
Source:
Figura N, Flampouri S, Mendenhall NP, et al. Adv Radiat Oncol. 2017;1-16.
Engagement Along the HIV Care Continuum and the Potential Role of Mental Health and Substance Use Disorders
The VHA is the largest single provider of human immunodeficiency virus (HIV) care in the U.S., with over 26,000 HIV-positive veterans in care in 2013.1 HIV care in the VHA is delivered across 152 hospitals and nearly 1,400 health care facilities, including
community-based outpatient clinics, skilled nursing facilities, veteran centers, rehabilitation centers, hospices, and domiciliaries in the U.S. and its territories.2
The structure of HIV care in the VHA has been described in more detail elsewhere and, for context, is briefly highlighted here.3 Many veterans with HIV infection receive both HIV care and primary care through an infectious disease or HIV specialist, whereas others receive HIV care and primary care in separate clinics. Every medical center has a designated HIV lead clinician who serves as the point of contact for the VHA National HIV Program. This network facilitates rapid dissemination of policy changes and clinical updates to HIV providers nationally, as well communication to the National HIV Program about barriers and challenges to providing high-quality care at the local level. The National HIV Program monitors and evaluates comprehensive HIV care programming, which includes increasing HIV testing, availability of pre-exposure prophylaxis, and access to and quality of comorbidity care (for more information on VHA HIV care, please visit: http://www.hiv.va.gov).
Comorbidity care is a particular focus of the National HIV Program. The majority of veterans with HIV in VHA care are now in late middle-age; in 2011, 66% were aged between 50 and 69 years.4 As the population of veterans with HIV grows older, age-related chronic medical comorbidities (eg, diabetes mellitus, cardiovascular disease) and mental health considerations (eg, cognitive impairment, depression, substance use) become increasingly critical areas of clinical focus at the individual and the population level.
The HIV care continuum provides a useful model to conceptualize critical components of providing high-quality comprehensive HIV clinical care, including diagnosis, linkage to care, retention in care, prescription of antiretroviral therapy (ART), and viral suppression. Achieving virologic suppression is the primary goal of HIV care. It decreases morbidity and mortality for the HIV-infected individual and decreases the risk of transmission to others.5
This continuum of care is used increasingly by federal agencies and other health care systems to assess engagement at each critical juncture. The Centers for Disease Control and Prevention has estimated that, due to significant drop-offs between each step of the continuum, only 25% of the 1.1 million people living with HIV in the U. S. are virally suppressed (Figure 1).6,7
Mental Health and Substance Use
Although individual facilities within VHA may perform better across the care continuum compared with national samples, as a health care system, significant dropoffs remain at each stage, particularly in the transition from linkage to care to retention in care. The barriers to engagement in care at various steps along this continuum are not well understood, although mental health and substance use disorder (MH/SUD) comorbidities likely play an important role.
Mental health and substance use disorder comorbidities impact over half of veterans with HIV infection in VHA care. In 2012, 55% of veterans with HIV infection in care had ever been diagnosed with depression, 31% with anxiety, 16% with posttraumatic stress disorder (PTSD), and 62% with any mental illness (Table).11 Substance use is also quite prevalent in veterans with HIV infection, with 34% reporting a history of alcohol abuse, 28% a history of stimulant use, and 16% a history of cannabis use in 2012; nearly 50% reported tobacco use.
Compared with that of the general population, MH/SUDs are much higher among people living with HIV/acquired immune deficiency syndrome (AIDS) and higher still among veterans living with HIV/AIDS.11-13 Mental health conditions such as depression, anxiety and PTSD as well as substance use have been linked not only to increases in HIV risk behavior, but also to more rapid progression of HIV and increases in AIDSdefining illnesses and death.14-16 The high burden of MH/SUD among veterans with HIV is further complicated by the fact that this is an aging population. The risk of HIV-associated neurocognitive disorders (HAND) as well as non–HIV-related neurocognitive disorders increases with age.17,18 These risks may be higher for those with comorbid MH/SUD, and this interaction may increase patients’ vulnerability to HAND.12,19,20
The HIV care continuum is characterized by engagement at each step. Psychiatric and substance use problems represent potential engagementlimiting comorbidities. Several studies highlight the importance of addressing MH/SUDs that have adverse effects on adherence, linkage to and retention in care, and care utilization patterns.13,18,21-24 HIV-related stigma may also play a significant role in disengagement in care, adherence, patient satisfaction with care, and follow-up.25-28
Overall, integrated HIV care has been found to positively impact hospital utilization, quality of life and functioning, adherence, patient satisfaction, and process outcomes.29 Integrated HIV care is a specific intervention that could positively impact the HIV care continuum, particularly in managing depression and substance use.28,30,31 In the VHA, integrated HIV care is associated with increased access to mental health services and increased viral suppression compared with nonintegrated HIV clinics in VHA.32,33
A qualitative study of care integration found that in VHA HIV clinics that were integrated, patients reported less stigma and more positive relationships with their providers.34 The VHA Office of Public Health, in collaboration with the Office of Academic Affiliations, has supported the expansion of a postdoctoral fellowship in clinical psychology with a specific focus on integrated HIV and hepatitis C clinical care in an effort to increase the availability of mental health providers specifically trained to address MH/SUDs in the HIV-infected population. This national fellowship program has been described in detail elsewhere.11
Conclusion
The HIV care continuum is a useful model to describe the cascade of HIV care and opportunities for points of engagement. Although VHA may be performing higher along the care continuum than national samples, there is still much work to be done to better understand the barriers to engagement and interventions that will boost each step so that more veterans with HIV infection achieve viral suppression.
Increasing access to mental health and substance use services, particularly through integrated care models, as well as addressing issues of HIV-related stigma, may positively impact engagement in HIV clinical care. Given the use of electronic medical records, the availability of MH/SUD treatment, and the increasing emphasis on integrated HIV care at multiple facilities across the system, the VHA is well positioned to address gaps in care, with a particular focus on the role that MH/SUDs have in HIV care continuum drop-offs.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combination s—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
1. Office of Public Health/Population Health. National HIV Registry Reports: 2013. Personal Communication October 1, 2014.
2. Veterans Health Administration. Where do I get the care I need? VA website. http://www.va.gov/health/findcare.asp. Accessed October 3, 2014.
3. Maier M, Chartier M. Department of Veterans Affairs: HIV program, policies, and infrastructure. HIV Specialist. 2014;6(2):19-25.
4. Center for Quality Management in Public Health. The State of Care for Veterans with HIV/AIDS: 2011 Summary Report. Washington, DC: U.S. Department of Veterans Affairs, 2012.
5. Cohen MS, Chen YQ, McCauley M, et al; HPTN 052 Study Team. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med. 2011;365(6):493-505.
6. HIV/AIDS Care Continuum. AIDS.gov Website. http://aids.gov/federal-resources/policies/care-continuum. Updated December 18, 2013. Accessed October 6, 2014.
7. National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention. Key graphics from CDC analysis showing proportion of people engaged in each of the five main stages of HIV care. Centers for Disease Control and Prevention Website. http://www.cdc.gov/nchhstp/newsroom/2012/Continuum-of-Care-Graphics.html. Updated December 27, 2013. Accessed November 18, 2014.
8. Mangal JP, Rimland D, Marconi VC. The continuum of HIV care in a Veterans’ Affairs clinic. AIDS Res Hum Retroviruses. 2014;30(5):409-415.
9. Gardner EM, McLees MP, Steiner JF, Del Rio C, Burman WJ. The spectrum of engagement in HIV care and its relevance to test-and-treat strategies for prevention of HIV infection. Clin Infect Dis. 2011;52(6):793-800.
10. Hall HI, Frazier EL, Rhodes, P, et al. Differences in human immunodeficiency virus care and treatment among subpopulations in the United States. JAMA Intern Med. 2013;173(14):1337-1344.
11. Chartier M, Blais RK, Steinberg T, et al. A psychology postdoctoral fellowship program in integrated HIV and hepatitis C clinical care: Rationale, progress, and future directions. Training Educ Professional Psychol. doi: 10.1037/tep0000038.
12. Hinkin CH, Castellon SA, Atkinson JH, Goodkin K. Neuropsychiatric aspects of HIV infection among older adults. J Clin Epidemiol. 2001;54(suppl 1):S44-S52.
13. Klinkenberg WD, Sacks S; HIV/AIDS Treatment Adherence, Health Outcomes and Cost Study Group. Mental disorders and drug abuse in persons living with HIV/AIDS. AIDS Care. 2004;16(suppl 1):S22-S42.
14. Carrico AW, Riley ED, Johnson MO, et al. Psychiatric risk factors for HIV disease progression: The role of inconsistent patterns of antiretroviral therapy utilization. J Acquir Immune Defic Syndr. 2011;56(2):146-150.
15. Leserman J. HIV disease progression: Depression, stress, and possible mechanisms. Biol Psychiatry. 2003;54(3):295-306.
16. Nurutdinova D, Abdallah AB, Bradford S, O’Leary CC, Cottler LB. Risk factors associated with hepatitis C among female substance users enrolled in communitybased HIV prevention studies. BMC Res Notes. 2011;4:126.
17. Heaton RK, Clifford DB, Franklin DR Jr, et al; CHARTER Group. HIVassociated neurocognitive disorders persist in the era of potent antiretroviral therapy: CHARTER Study. Neurology. 2010;75(23):2087-2096.
18. Simoni JM, Amico KR, Smith L, Nelson K. Antiretroviral adherence interventions: Translating research findings to the real world clinic. Curr HIV/AIDS Rep. 2010;7(1):44-51.
19. Becker JT, Lopez OL, Dew MA, Aizenstein HJ. Prevalence of cognitive disorders differs as a function of age in HIV virus infection. AIDS. 2004;18(suppl 1):S11-S18.
20. Valcour VG, Shikuma CM, Watters MR, Sacktor NC. Cognitive impairment in
older HIV-1-seropositive individuals: Prevalence and potential mechanisms. AIDS.
2004;18(suppl 1):S79-S86.
21. Carrico AW, Bangsberg DR, Weiser SD, Chartier M, Dilworth SE, Riley ED. Psychiatric correlates of HAART utilization and viral load among HIV-positive impoverished persons. AIDS. 2011;25(8):1113-1118.
22. Chartier M, Carrico AW, Weiser SD, Kushel MB, Riley ED. Specific psychiatric correlates of acute care utilization among unstably housed HIV-positive adults. AIDS Care. 2012;24(12):1514-1518.
23. Palmer NB, Salcedo J, Miller AL, Winiarski M, Arno P. Psychiatric and social barriers to HIV medication adherence in a triply diagnosed methadone population. AIDS Patient Care STDS. 2003;17(12):635-644
24. Sternhell PS, Corr MJ. Psychiatric morbidity and adherence to antiretroviral medication
in patients with HIV/AIDS. Aust N Z J Psychiatry. 2002;36(4):528-533.
25. Chesney MA, Smith AW. Critical delays in HIV testing and care: The potential role of stigma. Am Behav Sci. 1999;42(7):1162-1174.
26. Li L, Comulada WS, Wu Z, Ding Y, Zhu W. Providers’ HIV-related avoidance attitude and patient satisfaction. Health Expect. 2013;16(1):105-112.
27. Kinsler JJ, Wong MD, Sayles JN, Davis C, Cunningham WE. The effect of perceived
stigma from a health care provider on access to care among a low-income
HIV-positive population. AIDS Patient Care STDS. 2007;21(8):584-592.
28. Treisman GJ. Impact of depression on ART adherence and retention in care. HIV
Specialist. 2014;6(2):26-29.
29. Willenbring ML. Integrating care for patients with infectious, psychiatric, and substance use disorders: Concepts and approaches. AIDS. 2005;19(suppl 3): S227–S237.
30. Farber EW. Psychological aspects of HIV. HIV Specialist. 2013;6(4):10-13.
31. Durvasula R, Miller T. Management of substance abuse in HIV care. HIV Specialist. 2013;6(4):26-29.
32. Chartier M, Carmody T, Lamipris H. HIV psychology: Mental health integration and co-location. Poster presented at: The American Conference for the Treatment of HIV; May 20-23, 2010; Denver, CO. Poster SPC-6.
33. Hoang T, Goetz MB, Yano EM, et al. The impact of integrated HIV care on patient health outcomes. Med Care. 2009;47(5):560-567.
34. Fix GM, Asch SM, Saifu HN, Fletcher MD, Gifford AL, Bokhour BG. Delivering PACT-principled care: Are specialty care patients being left behind? J Gen Intern Med. 2014;29(suppl 2):S695-S702.
The VHA is the largest single provider of human immunodeficiency virus (HIV) care in the U.S., with over 26,000 HIV-positive veterans in care in 2013.1 HIV care in the VHA is delivered across 152 hospitals and nearly 1,400 health care facilities, including
community-based outpatient clinics, skilled nursing facilities, veteran centers, rehabilitation centers, hospices, and domiciliaries in the U.S. and its territories.2
The structure of HIV care in the VHA has been described in more detail elsewhere and, for context, is briefly highlighted here.3 Many veterans with HIV infection receive both HIV care and primary care through an infectious disease or HIV specialist, whereas others receive HIV care and primary care in separate clinics. Every medical center has a designated HIV lead clinician who serves as the point of contact for the VHA National HIV Program. This network facilitates rapid dissemination of policy changes and clinical updates to HIV providers nationally, as well communication to the National HIV Program about barriers and challenges to providing high-quality care at the local level. The National HIV Program monitors and evaluates comprehensive HIV care programming, which includes increasing HIV testing, availability of pre-exposure prophylaxis, and access to and quality of comorbidity care (for more information on VHA HIV care, please visit: http://www.hiv.va.gov).
Comorbidity care is a particular focus of the National HIV Program. The majority of veterans with HIV in VHA care are now in late middle-age; in 2011, 66% were aged between 50 and 69 years.4 As the population of veterans with HIV grows older, age-related chronic medical comorbidities (eg, diabetes mellitus, cardiovascular disease) and mental health considerations (eg, cognitive impairment, depression, substance use) become increasingly critical areas of clinical focus at the individual and the population level.
The HIV care continuum provides a useful model to conceptualize critical components of providing high-quality comprehensive HIV clinical care, including diagnosis, linkage to care, retention in care, prescription of antiretroviral therapy (ART), and viral suppression. Achieving virologic suppression is the primary goal of HIV care. It decreases morbidity and mortality for the HIV-infected individual and decreases the risk of transmission to others.5
This continuum of care is used increasingly by federal agencies and other health care systems to assess engagement at each critical juncture. The Centers for Disease Control and Prevention has estimated that, due to significant drop-offs between each step of the continuum, only 25% of the 1.1 million people living with HIV in the U. S. are virally suppressed (Figure 1).6,7
Mental Health and Substance Use
Although individual facilities within VHA may perform better across the care continuum compared with national samples, as a health care system, significant dropoffs remain at each stage, particularly in the transition from linkage to care to retention in care. The barriers to engagement in care at various steps along this continuum are not well understood, although mental health and substance use disorder (MH/SUD) comorbidities likely play an important role.
Mental health and substance use disorder comorbidities impact over half of veterans with HIV infection in VHA care. In 2012, 55% of veterans with HIV infection in care had ever been diagnosed with depression, 31% with anxiety, 16% with posttraumatic stress disorder (PTSD), and 62% with any mental illness (Table).11 Substance use is also quite prevalent in veterans with HIV infection, with 34% reporting a history of alcohol abuse, 28% a history of stimulant use, and 16% a history of cannabis use in 2012; nearly 50% reported tobacco use.
Compared with that of the general population, MH/SUDs are much higher among people living with HIV/acquired immune deficiency syndrome (AIDS) and higher still among veterans living with HIV/AIDS.11-13 Mental health conditions such as depression, anxiety and PTSD as well as substance use have been linked not only to increases in HIV risk behavior, but also to more rapid progression of HIV and increases in AIDSdefining illnesses and death.14-16 The high burden of MH/SUD among veterans with HIV is further complicated by the fact that this is an aging population. The risk of HIV-associated neurocognitive disorders (HAND) as well as non–HIV-related neurocognitive disorders increases with age.17,18 These risks may be higher for those with comorbid MH/SUD, and this interaction may increase patients’ vulnerability to HAND.12,19,20
The HIV care continuum is characterized by engagement at each step. Psychiatric and substance use problems represent potential engagementlimiting comorbidities. Several studies highlight the importance of addressing MH/SUDs that have adverse effects on adherence, linkage to and retention in care, and care utilization patterns.13,18,21-24 HIV-related stigma may also play a significant role in disengagement in care, adherence, patient satisfaction with care, and follow-up.25-28
Overall, integrated HIV care has been found to positively impact hospital utilization, quality of life and functioning, adherence, patient satisfaction, and process outcomes.29 Integrated HIV care is a specific intervention that could positively impact the HIV care continuum, particularly in managing depression and substance use.28,30,31 In the VHA, integrated HIV care is associated with increased access to mental health services and increased viral suppression compared with nonintegrated HIV clinics in VHA.32,33
A qualitative study of care integration found that in VHA HIV clinics that were integrated, patients reported less stigma and more positive relationships with their providers.34 The VHA Office of Public Health, in collaboration with the Office of Academic Affiliations, has supported the expansion of a postdoctoral fellowship in clinical psychology with a specific focus on integrated HIV and hepatitis C clinical care in an effort to increase the availability of mental health providers specifically trained to address MH/SUDs in the HIV-infected population. This national fellowship program has been described in detail elsewhere.11
Conclusion
The HIV care continuum is a useful model to describe the cascade of HIV care and opportunities for points of engagement. Although VHA may be performing higher along the care continuum than national samples, there is still much work to be done to better understand the barriers to engagement and interventions that will boost each step so that more veterans with HIV infection achieve viral suppression.
Increasing access to mental health and substance use services, particularly through integrated care models, as well as addressing issues of HIV-related stigma, may positively impact engagement in HIV clinical care. Given the use of electronic medical records, the availability of MH/SUD treatment, and the increasing emphasis on integrated HIV care at multiple facilities across the system, the VHA is well positioned to address gaps in care, with a particular focus on the role that MH/SUDs have in HIV care continuum drop-offs.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combination s—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
The VHA is the largest single provider of human immunodeficiency virus (HIV) care in the U.S., with over 26,000 HIV-positive veterans in care in 2013.1 HIV care in the VHA is delivered across 152 hospitals and nearly 1,400 health care facilities, including
community-based outpatient clinics, skilled nursing facilities, veteran centers, rehabilitation centers, hospices, and domiciliaries in the U.S. and its territories.2
The structure of HIV care in the VHA has been described in more detail elsewhere and, for context, is briefly highlighted here.3 Many veterans with HIV infection receive both HIV care and primary care through an infectious disease or HIV specialist, whereas others receive HIV care and primary care in separate clinics. Every medical center has a designated HIV lead clinician who serves as the point of contact for the VHA National HIV Program. This network facilitates rapid dissemination of policy changes and clinical updates to HIV providers nationally, as well communication to the National HIV Program about barriers and challenges to providing high-quality care at the local level. The National HIV Program monitors and evaluates comprehensive HIV care programming, which includes increasing HIV testing, availability of pre-exposure prophylaxis, and access to and quality of comorbidity care (for more information on VHA HIV care, please visit: http://www.hiv.va.gov).
Comorbidity care is a particular focus of the National HIV Program. The majority of veterans with HIV in VHA care are now in late middle-age; in 2011, 66% were aged between 50 and 69 years.4 As the population of veterans with HIV grows older, age-related chronic medical comorbidities (eg, diabetes mellitus, cardiovascular disease) and mental health considerations (eg, cognitive impairment, depression, substance use) become increasingly critical areas of clinical focus at the individual and the population level.
The HIV care continuum provides a useful model to conceptualize critical components of providing high-quality comprehensive HIV clinical care, including diagnosis, linkage to care, retention in care, prescription of antiretroviral therapy (ART), and viral suppression. Achieving virologic suppression is the primary goal of HIV care. It decreases morbidity and mortality for the HIV-infected individual and decreases the risk of transmission to others.5
This continuum of care is used increasingly by federal agencies and other health care systems to assess engagement at each critical juncture. The Centers for Disease Control and Prevention has estimated that, due to significant drop-offs between each step of the continuum, only 25% of the 1.1 million people living with HIV in the U. S. are virally suppressed (Figure 1).6,7
Mental Health and Substance Use
Although individual facilities within VHA may perform better across the care continuum compared with national samples, as a health care system, significant dropoffs remain at each stage, particularly in the transition from linkage to care to retention in care. The barriers to engagement in care at various steps along this continuum are not well understood, although mental health and substance use disorder (MH/SUD) comorbidities likely play an important role.
Mental health and substance use disorder comorbidities impact over half of veterans with HIV infection in VHA care. In 2012, 55% of veterans with HIV infection in care had ever been diagnosed with depression, 31% with anxiety, 16% with posttraumatic stress disorder (PTSD), and 62% with any mental illness (Table).11 Substance use is also quite prevalent in veterans with HIV infection, with 34% reporting a history of alcohol abuse, 28% a history of stimulant use, and 16% a history of cannabis use in 2012; nearly 50% reported tobacco use.
Compared with that of the general population, MH/SUDs are much higher among people living with HIV/acquired immune deficiency syndrome (AIDS) and higher still among veterans living with HIV/AIDS.11-13 Mental health conditions such as depression, anxiety and PTSD as well as substance use have been linked not only to increases in HIV risk behavior, but also to more rapid progression of HIV and increases in AIDSdefining illnesses and death.14-16 The high burden of MH/SUD among veterans with HIV is further complicated by the fact that this is an aging population. The risk of HIV-associated neurocognitive disorders (HAND) as well as non–HIV-related neurocognitive disorders increases with age.17,18 These risks may be higher for those with comorbid MH/SUD, and this interaction may increase patients’ vulnerability to HAND.12,19,20
The HIV care continuum is characterized by engagement at each step. Psychiatric and substance use problems represent potential engagementlimiting comorbidities. Several studies highlight the importance of addressing MH/SUDs that have adverse effects on adherence, linkage to and retention in care, and care utilization patterns.13,18,21-24 HIV-related stigma may also play a significant role in disengagement in care, adherence, patient satisfaction with care, and follow-up.25-28
Overall, integrated HIV care has been found to positively impact hospital utilization, quality of life and functioning, adherence, patient satisfaction, and process outcomes.29 Integrated HIV care is a specific intervention that could positively impact the HIV care continuum, particularly in managing depression and substance use.28,30,31 In the VHA, integrated HIV care is associated with increased access to mental health services and increased viral suppression compared with nonintegrated HIV clinics in VHA.32,33
A qualitative study of care integration found that in VHA HIV clinics that were integrated, patients reported less stigma and more positive relationships with their providers.34 The VHA Office of Public Health, in collaboration with the Office of Academic Affiliations, has supported the expansion of a postdoctoral fellowship in clinical psychology with a specific focus on integrated HIV and hepatitis C clinical care in an effort to increase the availability of mental health providers specifically trained to address MH/SUDs in the HIV-infected population. This national fellowship program has been described in detail elsewhere.11
Conclusion
The HIV care continuum is a useful model to describe the cascade of HIV care and opportunities for points of engagement. Although VHA may be performing higher along the care continuum than national samples, there is still much work to be done to better understand the barriers to engagement and interventions that will boost each step so that more veterans with HIV infection achieve viral suppression.
Increasing access to mental health and substance use services, particularly through integrated care models, as well as addressing issues of HIV-related stigma, may positively impact engagement in HIV clinical care. Given the use of electronic medical records, the availability of MH/SUD treatment, and the increasing emphasis on integrated HIV care at multiple facilities across the system, the VHA is well positioned to address gaps in care, with a particular focus on the role that MH/SUDs have in HIV care continuum drop-offs.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combination s—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
1. Office of Public Health/Population Health. National HIV Registry Reports: 2013. Personal Communication October 1, 2014.
2. Veterans Health Administration. Where do I get the care I need? VA website. http://www.va.gov/health/findcare.asp. Accessed October 3, 2014.
3. Maier M, Chartier M. Department of Veterans Affairs: HIV program, policies, and infrastructure. HIV Specialist. 2014;6(2):19-25.
4. Center for Quality Management in Public Health. The State of Care for Veterans with HIV/AIDS: 2011 Summary Report. Washington, DC: U.S. Department of Veterans Affairs, 2012.
5. Cohen MS, Chen YQ, McCauley M, et al; HPTN 052 Study Team. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med. 2011;365(6):493-505.
6. HIV/AIDS Care Continuum. AIDS.gov Website. http://aids.gov/federal-resources/policies/care-continuum. Updated December 18, 2013. Accessed October 6, 2014.
7. National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention. Key graphics from CDC analysis showing proportion of people engaged in each of the five main stages of HIV care. Centers for Disease Control and Prevention Website. http://www.cdc.gov/nchhstp/newsroom/2012/Continuum-of-Care-Graphics.html. Updated December 27, 2013. Accessed November 18, 2014.
8. Mangal JP, Rimland D, Marconi VC. The continuum of HIV care in a Veterans’ Affairs clinic. AIDS Res Hum Retroviruses. 2014;30(5):409-415.
9. Gardner EM, McLees MP, Steiner JF, Del Rio C, Burman WJ. The spectrum of engagement in HIV care and its relevance to test-and-treat strategies for prevention of HIV infection. Clin Infect Dis. 2011;52(6):793-800.
10. Hall HI, Frazier EL, Rhodes, P, et al. Differences in human immunodeficiency virus care and treatment among subpopulations in the United States. JAMA Intern Med. 2013;173(14):1337-1344.
11. Chartier M, Blais RK, Steinberg T, et al. A psychology postdoctoral fellowship program in integrated HIV and hepatitis C clinical care: Rationale, progress, and future directions. Training Educ Professional Psychol. doi: 10.1037/tep0000038.
12. Hinkin CH, Castellon SA, Atkinson JH, Goodkin K. Neuropsychiatric aspects of HIV infection among older adults. J Clin Epidemiol. 2001;54(suppl 1):S44-S52.
13. Klinkenberg WD, Sacks S; HIV/AIDS Treatment Adherence, Health Outcomes and Cost Study Group. Mental disorders and drug abuse in persons living with HIV/AIDS. AIDS Care. 2004;16(suppl 1):S22-S42.
14. Carrico AW, Riley ED, Johnson MO, et al. Psychiatric risk factors for HIV disease progression: The role of inconsistent patterns of antiretroviral therapy utilization. J Acquir Immune Defic Syndr. 2011;56(2):146-150.
15. Leserman J. HIV disease progression: Depression, stress, and possible mechanisms. Biol Psychiatry. 2003;54(3):295-306.
16. Nurutdinova D, Abdallah AB, Bradford S, O’Leary CC, Cottler LB. Risk factors associated with hepatitis C among female substance users enrolled in communitybased HIV prevention studies. BMC Res Notes. 2011;4:126.
17. Heaton RK, Clifford DB, Franklin DR Jr, et al; CHARTER Group. HIVassociated neurocognitive disorders persist in the era of potent antiretroviral therapy: CHARTER Study. Neurology. 2010;75(23):2087-2096.
18. Simoni JM, Amico KR, Smith L, Nelson K. Antiretroviral adherence interventions: Translating research findings to the real world clinic. Curr HIV/AIDS Rep. 2010;7(1):44-51.
19. Becker JT, Lopez OL, Dew MA, Aizenstein HJ. Prevalence of cognitive disorders differs as a function of age in HIV virus infection. AIDS. 2004;18(suppl 1):S11-S18.
20. Valcour VG, Shikuma CM, Watters MR, Sacktor NC. Cognitive impairment in
older HIV-1-seropositive individuals: Prevalence and potential mechanisms. AIDS.
2004;18(suppl 1):S79-S86.
21. Carrico AW, Bangsberg DR, Weiser SD, Chartier M, Dilworth SE, Riley ED. Psychiatric correlates of HAART utilization and viral load among HIV-positive impoverished persons. AIDS. 2011;25(8):1113-1118.
22. Chartier M, Carrico AW, Weiser SD, Kushel MB, Riley ED. Specific psychiatric correlates of acute care utilization among unstably housed HIV-positive adults. AIDS Care. 2012;24(12):1514-1518.
23. Palmer NB, Salcedo J, Miller AL, Winiarski M, Arno P. Psychiatric and social barriers to HIV medication adherence in a triply diagnosed methadone population. AIDS Patient Care STDS. 2003;17(12):635-644
24. Sternhell PS, Corr MJ. Psychiatric morbidity and adherence to antiretroviral medication
in patients with HIV/AIDS. Aust N Z J Psychiatry. 2002;36(4):528-533.
25. Chesney MA, Smith AW. Critical delays in HIV testing and care: The potential role of stigma. Am Behav Sci. 1999;42(7):1162-1174.
26. Li L, Comulada WS, Wu Z, Ding Y, Zhu W. Providers’ HIV-related avoidance attitude and patient satisfaction. Health Expect. 2013;16(1):105-112.
27. Kinsler JJ, Wong MD, Sayles JN, Davis C, Cunningham WE. The effect of perceived
stigma from a health care provider on access to care among a low-income
HIV-positive population. AIDS Patient Care STDS. 2007;21(8):584-592.
28. Treisman GJ. Impact of depression on ART adherence and retention in care. HIV
Specialist. 2014;6(2):26-29.
29. Willenbring ML. Integrating care for patients with infectious, psychiatric, and substance use disorders: Concepts and approaches. AIDS. 2005;19(suppl 3): S227–S237.
30. Farber EW. Psychological aspects of HIV. HIV Specialist. 2013;6(4):10-13.
31. Durvasula R, Miller T. Management of substance abuse in HIV care. HIV Specialist. 2013;6(4):26-29.
32. Chartier M, Carmody T, Lamipris H. HIV psychology: Mental health integration and co-location. Poster presented at: The American Conference for the Treatment of HIV; May 20-23, 2010; Denver, CO. Poster SPC-6.
33. Hoang T, Goetz MB, Yano EM, et al. The impact of integrated HIV care on patient health outcomes. Med Care. 2009;47(5):560-567.
34. Fix GM, Asch SM, Saifu HN, Fletcher MD, Gifford AL, Bokhour BG. Delivering PACT-principled care: Are specialty care patients being left behind? J Gen Intern Med. 2014;29(suppl 2):S695-S702.
1. Office of Public Health/Population Health. National HIV Registry Reports: 2013. Personal Communication October 1, 2014.
2. Veterans Health Administration. Where do I get the care I need? VA website. http://www.va.gov/health/findcare.asp. Accessed October 3, 2014.
3. Maier M, Chartier M. Department of Veterans Affairs: HIV program, policies, and infrastructure. HIV Specialist. 2014;6(2):19-25.
4. Center for Quality Management in Public Health. The State of Care for Veterans with HIV/AIDS: 2011 Summary Report. Washington, DC: U.S. Department of Veterans Affairs, 2012.
5. Cohen MS, Chen YQ, McCauley M, et al; HPTN 052 Study Team. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med. 2011;365(6):493-505.
6. HIV/AIDS Care Continuum. AIDS.gov Website. http://aids.gov/federal-resources/policies/care-continuum. Updated December 18, 2013. Accessed October 6, 2014.
7. National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention. Key graphics from CDC analysis showing proportion of people engaged in each of the five main stages of HIV care. Centers for Disease Control and Prevention Website. http://www.cdc.gov/nchhstp/newsroom/2012/Continuum-of-Care-Graphics.html. Updated December 27, 2013. Accessed November 18, 2014.
8. Mangal JP, Rimland D, Marconi VC. The continuum of HIV care in a Veterans’ Affairs clinic. AIDS Res Hum Retroviruses. 2014;30(5):409-415.
9. Gardner EM, McLees MP, Steiner JF, Del Rio C, Burman WJ. The spectrum of engagement in HIV care and its relevance to test-and-treat strategies for prevention of HIV infection. Clin Infect Dis. 2011;52(6):793-800.
10. Hall HI, Frazier EL, Rhodes, P, et al. Differences in human immunodeficiency virus care and treatment among subpopulations in the United States. JAMA Intern Med. 2013;173(14):1337-1344.
11. Chartier M, Blais RK, Steinberg T, et al. A psychology postdoctoral fellowship program in integrated HIV and hepatitis C clinical care: Rationale, progress, and future directions. Training Educ Professional Psychol. doi: 10.1037/tep0000038.
12. Hinkin CH, Castellon SA, Atkinson JH, Goodkin K. Neuropsychiatric aspects of HIV infection among older adults. J Clin Epidemiol. 2001;54(suppl 1):S44-S52.
13. Klinkenberg WD, Sacks S; HIV/AIDS Treatment Adherence, Health Outcomes and Cost Study Group. Mental disorders and drug abuse in persons living with HIV/AIDS. AIDS Care. 2004;16(suppl 1):S22-S42.
14. Carrico AW, Riley ED, Johnson MO, et al. Psychiatric risk factors for HIV disease progression: The role of inconsistent patterns of antiretroviral therapy utilization. J Acquir Immune Defic Syndr. 2011;56(2):146-150.
15. Leserman J. HIV disease progression: Depression, stress, and possible mechanisms. Biol Psychiatry. 2003;54(3):295-306.
16. Nurutdinova D, Abdallah AB, Bradford S, O’Leary CC, Cottler LB. Risk factors associated with hepatitis C among female substance users enrolled in communitybased HIV prevention studies. BMC Res Notes. 2011;4:126.
17. Heaton RK, Clifford DB, Franklin DR Jr, et al; CHARTER Group. HIVassociated neurocognitive disorders persist in the era of potent antiretroviral therapy: CHARTER Study. Neurology. 2010;75(23):2087-2096.
18. Simoni JM, Amico KR, Smith L, Nelson K. Antiretroviral adherence interventions: Translating research findings to the real world clinic. Curr HIV/AIDS Rep. 2010;7(1):44-51.
19. Becker JT, Lopez OL, Dew MA, Aizenstein HJ. Prevalence of cognitive disorders differs as a function of age in HIV virus infection. AIDS. 2004;18(suppl 1):S11-S18.
20. Valcour VG, Shikuma CM, Watters MR, Sacktor NC. Cognitive impairment in
older HIV-1-seropositive individuals: Prevalence and potential mechanisms. AIDS.
2004;18(suppl 1):S79-S86.
21. Carrico AW, Bangsberg DR, Weiser SD, Chartier M, Dilworth SE, Riley ED. Psychiatric correlates of HAART utilization and viral load among HIV-positive impoverished persons. AIDS. 2011;25(8):1113-1118.
22. Chartier M, Carrico AW, Weiser SD, Kushel MB, Riley ED. Specific psychiatric correlates of acute care utilization among unstably housed HIV-positive adults. AIDS Care. 2012;24(12):1514-1518.
23. Palmer NB, Salcedo J, Miller AL, Winiarski M, Arno P. Psychiatric and social barriers to HIV medication adherence in a triply diagnosed methadone population. AIDS Patient Care STDS. 2003;17(12):635-644
24. Sternhell PS, Corr MJ. Psychiatric morbidity and adherence to antiretroviral medication
in patients with HIV/AIDS. Aust N Z J Psychiatry. 2002;36(4):528-533.
25. Chesney MA, Smith AW. Critical delays in HIV testing and care: The potential role of stigma. Am Behav Sci. 1999;42(7):1162-1174.
26. Li L, Comulada WS, Wu Z, Ding Y, Zhu W. Providers’ HIV-related avoidance attitude and patient satisfaction. Health Expect. 2013;16(1):105-112.
27. Kinsler JJ, Wong MD, Sayles JN, Davis C, Cunningham WE. The effect of perceived
stigma from a health care provider on access to care among a low-income
HIV-positive population. AIDS Patient Care STDS. 2007;21(8):584-592.
28. Treisman GJ. Impact of depression on ART adherence and retention in care. HIV
Specialist. 2014;6(2):26-29.
29. Willenbring ML. Integrating care for patients with infectious, psychiatric, and substance use disorders: Concepts and approaches. AIDS. 2005;19(suppl 3): S227–S237.
30. Farber EW. Psychological aspects of HIV. HIV Specialist. 2013;6(4):10-13.
31. Durvasula R, Miller T. Management of substance abuse in HIV care. HIV Specialist. 2013;6(4):26-29.
32. Chartier M, Carmody T, Lamipris H. HIV psychology: Mental health integration and co-location. Poster presented at: The American Conference for the Treatment of HIV; May 20-23, 2010; Denver, CO. Poster SPC-6.
33. Hoang T, Goetz MB, Yano EM, et al. The impact of integrated HIV care on patient health outcomes. Med Care. 2009;47(5):560-567.
34. Fix GM, Asch SM, Saifu HN, Fletcher MD, Gifford AL, Bokhour BG. Delivering PACT-principled care: Are specialty care patients being left behind? J Gen Intern Med. 2014;29(suppl 2):S695-S702.
Patient-Centered HIV Treatment Options: Practical Considerations
The one pill daily revolution in human immunodeficiency virus (HIV) management continues with the recent FDA approval of Triumeq, a combination tablet with an integrase inhibitor backbone containing dolutegravir, abacavir, and lamivudine. The first all-in-one protease inhibitor (PI) option is on the horizon and might receive approval before this article is published. The Department of Health and Human Services (DHHS) recommends 7 regimens for any treatment-naive patient with HIV, with 3 additional options if the patient has a baseline plasma RNA viral load (VL) < 100,000 copies/mL (Table 1).1 With 10 first-line options and 4 once daily pill regimens, HIV treatment is poised to enter the realm of managed care.
Guidelines recommend treatment of all patients with HIV, both to slow disease progression, and to reduce the risk of HIV transmission.1 Regimens should be individual ized based on vi rologi c efficacy, toxicity, pill burden, dosing frequency, drug interaction potential, drug resistance testing results, comorbid conditions, and cost. Therapy should only be deferred on a case-by-case basis. Patients considering therapy must be willing and able to commit to taking daily medications and understand the risks and benefits of therapy. Each antiretroviral (ARV) class, and each individual drug, has its own set of advantages and disadvantages. This article will review considerations when choosing a regimen, provide a brief overview of the first-line treatment options, and finally touch on cost considerations.
Choosing An Appropriate Regimen
Choosing an antiviral regimen has 2 basic steps. First, a provider must determine what choices are medically appropriate. An appropriate regimen has 3 active agents with acceptable performance and no contraindications. An ARV regimen generally consists of 2 nucleoside reverse transcriptase inhibitors (NRTIs) plus 1 drug from 1 of the following classes: non-nucleoside reverse transcriptase inhibitor (NNRTI); PI boosted with ritonavir or cobicistat; or integrase strand transfer inhibitor (INSTI). The next step is helping the patient determine which regimen will suit him or her best considering dosing, food requirements, and potential adverse effects.
Genotype, Viral Load, and CD4 Testing at Baseline
A primary consideration is, “Will the regimen work?” This question can best be answered by the genotype, plasma RNA VL, and CD4 count. Baseline genotypes should be obtained for all patients and used to eliminate any inferior drug-resistant treatment options. Viral loads > 100,000 copies/mL eliminate 3 treatment options due to inferiority (inability to fully suppress the HIV virus) in patients with high VL (Table 1). Specifically, abacavir is not recommended (unless given with dolutegravir) with VL > 100,000 copies/mL. Rilpivirine is also not recommended for patients with VL > 100,000 copies/mL or CD4 < 200 cells/mm3.
Creatinine Clearance
Many NRTIs require dosage adjustment when creatinine clearance (CrCl) falls below 50 mL/min. Cobicistat is a novel pharmacokinetic enhancer or boosting agent and is only recommended for baseline CrCl > 70 mL/min.2
HLA-B 5701
If a patient has a positive HLA-B 5701 screening test, abacavir is strictly contraindicated due to the propensity for abacavir hypersensitivity, a potentially life-threatening reaction.
Allergies
Darunavir has a sulfonamide moiety and should be avoided in patients with a sulfa allergy.
Drug Interactions
Drug interactions should be a consideration for all patients with an extensive review of their current medications. Pharmaco-enhancers or “boosting agents,” such as ritonavir or cobicistat, are used to increase drug levels of HIV medications. However, since this is accomplished by inhibition of CYP3A there is great potential for interaction with other medications; opiates, amphetamines, sildenafil, most lipid-lowering agents, and numerous other drugs have significant interactions with ritonavir and cobicistat. Looking at current medications may not be enough; some consideration should be made for the potential of adding medications. For example, if a patient has a seizure disorder and is not well controlled on levetiracetam, consideration should be made if there could be a potential need for phenytoin or other agent that commonly interacts with some HIV medications.
Adverse Events
Adverse events (AEs) are a large consideration when choosing ARV therapy (ART) and can either have medical implications or be patient-specific. Some agents have severe AEs like worsening depression and suicidal ideation. Others are less severe and a particular patient may prefer a risk for gastrointestinal (GI) AEs rather than insomnia.
Adherence
Predicting adherence is difficult; however, certain factors may predispose a patient to being less adherent, including active substance abuse, depression or other mood disorder, unstable housing, and inconsistent access to food. Some regimens are more forgiving and require a lower level of adherence and are less likely to have resistance mutations with failure. Both ritonavir-boosted PIs, as well as dolutegravir, seem to have the best outcomes with lower levels of adherence. Tailoring the dosing and food requirements to a patient’s lifestyle can also improve adherence.
Childbearing Age
Pregnancy and potential for pregnancy must be considered in all female patients. Although pregnancy is beyond the scope of this discussion, the perinatal guidelines should be considered in women of childbearing age.3
Custom A Regimen
Convenience is a major consideration when selecting a regimen. Comparator studies done to demonstrate regimen efficacy using intention-to-treat structures highlight this point. Many patients express that a single tablet is their most important priority. Other important considerations for regimen selection are food requirements or restrictions, and whether there are drug coadministration concerns (eg, divalent cations in antacids or vitamins). These will be discussed more with each
individual ARV agent.
Now that regimen considerations have been reviewed from both provider and patient perspectives, we will focus on the individual classes of ARVs to make selection of a regimen more tailored to patient needs.
The NNRTI Class
The NNRTI class has 2 first-line “recommended” agents, efavirenz and rilpivirine, which are both conveniently available as “one-pill-a-day” coformulated regimens with tenofovir and emtricitabine.1 While the 2 NNRTIs are in the same class, they each have unique properties that would lead a clinician to choose one versus the other.
Efavirenz is the only NNRTI recommended to be paired with abacavir and lamivudine (coformulated as Epzicom) first-line nucleosides. However, it is only recommended to be used in patients with VL < 100,000 copies/mL.
Efavirenz is one of the most studied and widely used ARV. Efavirenz/emtricitabine/tenofovir (coformulated as Atripla) was the first once daily regimen and has shown noninferiority or superiority to most ARV regimens. However, AEs are the most common reason for discontinuation. Efavirenz is well known for its CNS AEs, including vivid dreams, somnolence, impaired concentration, headaches, and depression. These AEs typically diminish or abate after several weeks of therapy. However, certain CNS effects may be long term, including depression or suicidal thoughts.
The recommendation of taking efavirenz regimens at bedtime on an empty stomach to reduce CNS AEs is not ideal for every patient. Efavirenz can also have additive CNS AEs with alcohol and potentially lead to blackouts. It can also lead to false positives for marijuana and benzodiazepines on drug screenings. The FDA classifies efavirenz as Pregnancy Category D, so it should be avoided in women of childbearing age.4 However, if pregnancy isdetected while someone is taking efavirenz there is no need to discontinue the agent since the neural tube has formed by that time.3
Rilpivirine is better tolerated than efavirenz, with fewer and less severe CNS AEs. Rilpivirine/emtricitabine/tenofovir (coformulated as Complera) has the convenience of being 1 pill once daily; however, it is not appropriate for all patients. It should be avoided in patients with a pretreatment VL > 100,000 copies/mL or CD4 < 200 cells/mm3. Patients with values beyond these thresholds were more likely to experience early virologic failure in the first months of therapy compared to efavirenz/emtricitabine/tenofovir.5 Because it is a CYP3A4 substrate, rilpivirine has important drug-drug interactions. Proton pump inhibitors are contraindicated, and H2 receptor antagonists, like ranitidine or famotidine, require specific separation intervals, as do antacids and divalent cations. Another item of importance is that it should be administered with a meal.
The NNRTI class is plagued by its low genetic barrier to resistance, with a single mutation that may confer resistance to nearly all NNRTIs. Estimated transmitted HIV drug resistance at baseline in men who have sex with men can be 8% to 10%.6 Transmitted resistance is a concern, a baseline genotype can determine if a patient is at a higher risk for virologic failure with NNRTIs.
Protease Inhibitors
Protease inhibitors have gone from the main stay of HIV treatment to being a less desirable first-line treatment option. Protease inhibitors have the highest pill burden and drug interactions of the first-line agents; they must be taken with food, and as of October 2014 there are no coformulated tablets (other than lopinavir/ritonavir which is not first-line). New combination tablets utilizing cobicistat as a boosting agent may resuscitate the use of the PIs. The coformulations under investigation are atazanavir/cobicistat, darunavir/cobicistat, and darunavir/cobicistat/emtricitabine/tenofovir alafenamide. These combinations will decrease the pill burden for PIs to 1 to 2 pill(s) a day instead of 3 pills a day. Although 1 to 3 pills taken once daily does not sound like a big difference, given a choice, the majority of patients choose the least number of pills.
How Does Cobicistat Compare With Ritonavir?
Cobicistat has no HIV activity and may be better tolerated than ritonavir. Effects on total cholesterol and triglycerides are similar between the 2 boosting agents.7 Cobicistat causes a modest, rapid increase in serum creatinine (SrCr) through inhibition of tubular secretion without impact on the glomerular filtration rate. The coformulated product of cobicistat/elvitegravir is not recommended to be initiated in patients with CrCl < 70 mL/min at baseline.2 It is anticipated that cobicistat-boosted PI regimens will have a similar limitation. The elevation of SrCr from cobicistat makes it difficult to monitor for tenofovir-related renal dysfunction since SrCr is no longer a reliable surrogate marker for renal function.7 Cobicistat and ritonavir inhibit CYP3A4 and have almost identical drug interactions. However, only ritonavir interacts with methadone.
The first-line PIs are better tolerated than other PIs. Gastrointestinal AEs are still common. Scleral icterus and moderate to severe jaundice occur in 5% to 9% of patients taking atazanavir due to inhibition of the indirect metabolism of bilirubin.8 As a sulfa-related drug, darunavir is generally avoided if the patient has a sulfa allergy, but can be used with caution.9 Another limitation for PIs is that both options are required to be taken with food.
Protease inhibitors will always have a place in treatment-experienced patients with drug resistance, but may have a unique role in patients with baseline M184V mutations. A retrospective analysis showed that patients with a M184V mutation alone achieved equivalent viral suppression if the patient was on 3 fully active HIV ARVs or on a boosted PI, lamivudine or emtricitabine, and 1 additional NRTI.10 Patients with only a M184V mutation can still achieve full suppression without adding an additional ARV agent or using 3 HIV drug classes, preserving future treatment options. Boosted-PI regimens have less drug resistance at failure than NNRTI-based regimens. Less than 5% of new infections have baseline (pretreatment) drug resistance to PIs.6
Boosted PIs have a long clinical history and are still appropriate for many treatment-naive and experienced patients. Reduced pill burden with cobicistat formulation may revive utilization when the pill burden decreases from 3 tablets per day to 1 or 2.
Integrase Strand Inhibitors
INSTI-based regimens have had low discontinuation rates overall in clinical trials, and are great options for a majority of patients. There are 3 INSTIs, all of which are on the list of preferred agents according to the DHHS guidelines. INSTIs may be used with any baseline VL or CD4 counts, without concern for potency. Each of the 3 agents has clear benefits and disadvantages when choosing among them.
Raltegravir has no food requirements and has the least amount of drug interactions of nearly all ART regimens since it is not processed via cytochrome P450 enzymes, but it is dosed twice daily. When paired with emtricitabine/tenofovir, it is also the preferred regimen for occupational postexposure prophylaxis.
Dolut egr avi r i s onc e da i ly, ha s no food requirements, and also avoids drug interactions due to cytochrome P450 enzymes. However, this ARV agent does inhibit the renal organic cation transporter, so some clinically relevant interactions exist. Dolutegravir also has the benefit of being paired with either emtricitabine/tenofovir or abacavir/lamivudine, both of which are preferred DHHS regimens. As mentioned previously, dolutegravir/abacavir/lamivudine are now coformulated
and are an additional all-in-one option. Abacavir has conflicting data regarding increased risk for myocardial infarction; this risk should be considered in light of other risk factors for the patient including age, diabetes, etc.11
Elvitegravir is unique as an INSTI. Elvitegravir/cobicistat/emtricitabine/tenofovir must be administered with food and since it utilizes cobicistat as a pharmaco-enhancer to boost elvitegravir’s levels, there are a significant number of potential drug interactions. Cobicistat-containing regimens must not be prescribed for patients with a baseline CrCl <70 mL/min.2
Both dolutegravir and raltegravir can be taken with or without food. However, if the patient is taking a divalent cation (magnesium, iron, or calcium) then the medications must be separated, causing the patient to take medications multiple times per day, or taken with food (and lose the benefit of with or without food).
Cost and the New Era of ARV Generics
Previously, the majority of HIV regimens were similarly priced and had little impact on prescribing practices. Several ARV are now available as generics. In the next 2 years, generics may have major impact on prescribing practice as first-line options (darunavir, atazanavir, and efavirenz) will be available as generics (Table 2).12
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Author Disclaimer
The views expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the U.S. Department of Health and Human Services, the Indian Health Services, or the U.S. Government. Please review complete prescribing information for specific drugs or drug combinations—including but not limited to indications, contraindications, warnings, adverse effects, and drug interactions- before administering pharmacologic therapy to patients.
Journal Disclaimer
The opinions expressed herein are those of the author and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combination—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
1. HHS Panel on Antiretroviral Guidelines for Adults and Adolescents—A Working Group of the Office of AIDS Research Advisory Council (OARAC). Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. http://aidsinfo.nih.gov/contentfile/lvguidelines/AdultandAdolescentGL.pdf. Updated November 13, 2014. Accessed December 1, 2014.
2. Stribild [package insert]. Foster City, CA: Gilead Sciences, Inc; 2012.
3. HHS Panel on Treatment of HIV-Infected Pregnant Women and Prevention of Perinatal Transmission—A Working Group of the Office of AIDS Research Advisory Council (OARAC). Recommendations for use of antiretroviral drugs in pregnant HIV-1-infected women for maternal health and interventions to reduce perinatal HIV transmission in the United States. http://aidsinfo.nih.gov/contentfiles/lvguidelines/PerinatalGL.pdf. Updated March 28, 2014. Accessed October 27, 2014.
4. Atripla [package insert] Princeton, NJ: Bristol-Myers Squibb; Foster City, CA: Gilead Sciences, Inc; 2013.
5. Cohen CJ, Molina JM, Cassetti I, et al. Week 96 efficacy and safety of rilpivirine in treatment-naive, HIV-1 patients in two Phase III randomized trials. AIDS. 2013;27(6):939-950.
6. Bañez Ocfemia MC, Saduvala N, Oster AM, et al. Transmitted HIV-1 drug resistance among men who have sex with men, 11 US jurisdictions, 2008-2011. Presented at: 21st Conference on Retroviruses and Opportunistic Infections; March 3-6, 2014; Boston, MA. Abstract 579.
7. Gallant JE, Koenig E, Andrade-Villaneuva J, et al. Cobicistat versus ritonavir as a pharmacoenhancer of atazanavir plus emtricitabine/tenofovir disoproxil fumarate in treatment-naive HIV type 1-infected patients: Week 48 results. J Infect Dis. 2013;208(1):32-39.
8. Reyatoz [package insert]. Princeton, NJ: Bristol-Myers Squibb Company; 2014.
9. Prezista [package insert]. Titusville, NJ: Janssen Pharmaceuicals, Inc; 2013.
10. Hull M, Moore D, Harris M, et al. A lamivudine (3TC)-based backbone in conjunction with a boosted protease inhibitor (PI) is sufficient to achieve virologic suppression in the presence of M184V mutations. Presented at: 49th Interscience Conference on Antimicrobial Agents and Chemotherapy; September 12-15, 2009; San Francisco, CA. Abstract H-916.
11. Triumeq [package insert]. Research Triangle Park, NC: ViiV Healthcare; 2014.
12. Maxmen A. Generic HIV drugs will widen US treatment net. Nature.
2012;488(7411):267.
The one pill daily revolution in human immunodeficiency virus (HIV) management continues with the recent FDA approval of Triumeq, a combination tablet with an integrase inhibitor backbone containing dolutegravir, abacavir, and lamivudine. The first all-in-one protease inhibitor (PI) option is on the horizon and might receive approval before this article is published. The Department of Health and Human Services (DHHS) recommends 7 regimens for any treatment-naive patient with HIV, with 3 additional options if the patient has a baseline plasma RNA viral load (VL) < 100,000 copies/mL (Table 1).1 With 10 first-line options and 4 once daily pill regimens, HIV treatment is poised to enter the realm of managed care.
Guidelines recommend treatment of all patients with HIV, both to slow disease progression, and to reduce the risk of HIV transmission.1 Regimens should be individual ized based on vi rologi c efficacy, toxicity, pill burden, dosing frequency, drug interaction potential, drug resistance testing results, comorbid conditions, and cost. Therapy should only be deferred on a case-by-case basis. Patients considering therapy must be willing and able to commit to taking daily medications and understand the risks and benefits of therapy. Each antiretroviral (ARV) class, and each individual drug, has its own set of advantages and disadvantages. This article will review considerations when choosing a regimen, provide a brief overview of the first-line treatment options, and finally touch on cost considerations.
Choosing An Appropriate Regimen
Choosing an antiviral regimen has 2 basic steps. First, a provider must determine what choices are medically appropriate. An appropriate regimen has 3 active agents with acceptable performance and no contraindications. An ARV regimen generally consists of 2 nucleoside reverse transcriptase inhibitors (NRTIs) plus 1 drug from 1 of the following classes: non-nucleoside reverse transcriptase inhibitor (NNRTI); PI boosted with ritonavir or cobicistat; or integrase strand transfer inhibitor (INSTI). The next step is helping the patient determine which regimen will suit him or her best considering dosing, food requirements, and potential adverse effects.
Genotype, Viral Load, and CD4 Testing at Baseline
A primary consideration is, “Will the regimen work?” This question can best be answered by the genotype, plasma RNA VL, and CD4 count. Baseline genotypes should be obtained for all patients and used to eliminate any inferior drug-resistant treatment options. Viral loads > 100,000 copies/mL eliminate 3 treatment options due to inferiority (inability to fully suppress the HIV virus) in patients with high VL (Table 1). Specifically, abacavir is not recommended (unless given with dolutegravir) with VL > 100,000 copies/mL. Rilpivirine is also not recommended for patients with VL > 100,000 copies/mL or CD4 < 200 cells/mm3.
Creatinine Clearance
Many NRTIs require dosage adjustment when creatinine clearance (CrCl) falls below 50 mL/min. Cobicistat is a novel pharmacokinetic enhancer or boosting agent and is only recommended for baseline CrCl > 70 mL/min.2
HLA-B 5701
If a patient has a positive HLA-B 5701 screening test, abacavir is strictly contraindicated due to the propensity for abacavir hypersensitivity, a potentially life-threatening reaction.
Allergies
Darunavir has a sulfonamide moiety and should be avoided in patients with a sulfa allergy.
Drug Interactions
Drug interactions should be a consideration for all patients with an extensive review of their current medications. Pharmaco-enhancers or “boosting agents,” such as ritonavir or cobicistat, are used to increase drug levels of HIV medications. However, since this is accomplished by inhibition of CYP3A there is great potential for interaction with other medications; opiates, amphetamines, sildenafil, most lipid-lowering agents, and numerous other drugs have significant interactions with ritonavir and cobicistat. Looking at current medications may not be enough; some consideration should be made for the potential of adding medications. For example, if a patient has a seizure disorder and is not well controlled on levetiracetam, consideration should be made if there could be a potential need for phenytoin or other agent that commonly interacts with some HIV medications.
Adverse Events
Adverse events (AEs) are a large consideration when choosing ARV therapy (ART) and can either have medical implications or be patient-specific. Some agents have severe AEs like worsening depression and suicidal ideation. Others are less severe and a particular patient may prefer a risk for gastrointestinal (GI) AEs rather than insomnia.
Adherence
Predicting adherence is difficult; however, certain factors may predispose a patient to being less adherent, including active substance abuse, depression or other mood disorder, unstable housing, and inconsistent access to food. Some regimens are more forgiving and require a lower level of adherence and are less likely to have resistance mutations with failure. Both ritonavir-boosted PIs, as well as dolutegravir, seem to have the best outcomes with lower levels of adherence. Tailoring the dosing and food requirements to a patient’s lifestyle can also improve adherence.
Childbearing Age
Pregnancy and potential for pregnancy must be considered in all female patients. Although pregnancy is beyond the scope of this discussion, the perinatal guidelines should be considered in women of childbearing age.3
Custom A Regimen
Convenience is a major consideration when selecting a regimen. Comparator studies done to demonstrate regimen efficacy using intention-to-treat structures highlight this point. Many patients express that a single tablet is their most important priority. Other important considerations for regimen selection are food requirements or restrictions, and whether there are drug coadministration concerns (eg, divalent cations in antacids or vitamins). These will be discussed more with each
individual ARV agent.
Now that regimen considerations have been reviewed from both provider and patient perspectives, we will focus on the individual classes of ARVs to make selection of a regimen more tailored to patient needs.
The NNRTI Class
The NNRTI class has 2 first-line “recommended” agents, efavirenz and rilpivirine, which are both conveniently available as “one-pill-a-day” coformulated regimens with tenofovir and emtricitabine.1 While the 2 NNRTIs are in the same class, they each have unique properties that would lead a clinician to choose one versus the other.
Efavirenz is the only NNRTI recommended to be paired with abacavir and lamivudine (coformulated as Epzicom) first-line nucleosides. However, it is only recommended to be used in patients with VL < 100,000 copies/mL.
Efavirenz is one of the most studied and widely used ARV. Efavirenz/emtricitabine/tenofovir (coformulated as Atripla) was the first once daily regimen and has shown noninferiority or superiority to most ARV regimens. However, AEs are the most common reason for discontinuation. Efavirenz is well known for its CNS AEs, including vivid dreams, somnolence, impaired concentration, headaches, and depression. These AEs typically diminish or abate after several weeks of therapy. However, certain CNS effects may be long term, including depression or suicidal thoughts.
The recommendation of taking efavirenz regimens at bedtime on an empty stomach to reduce CNS AEs is not ideal for every patient. Efavirenz can also have additive CNS AEs with alcohol and potentially lead to blackouts. It can also lead to false positives for marijuana and benzodiazepines on drug screenings. The FDA classifies efavirenz as Pregnancy Category D, so it should be avoided in women of childbearing age.4 However, if pregnancy isdetected while someone is taking efavirenz there is no need to discontinue the agent since the neural tube has formed by that time.3
Rilpivirine is better tolerated than efavirenz, with fewer and less severe CNS AEs. Rilpivirine/emtricitabine/tenofovir (coformulated as Complera) has the convenience of being 1 pill once daily; however, it is not appropriate for all patients. It should be avoided in patients with a pretreatment VL > 100,000 copies/mL or CD4 < 200 cells/mm3. Patients with values beyond these thresholds were more likely to experience early virologic failure in the first months of therapy compared to efavirenz/emtricitabine/tenofovir.5 Because it is a CYP3A4 substrate, rilpivirine has important drug-drug interactions. Proton pump inhibitors are contraindicated, and H2 receptor antagonists, like ranitidine or famotidine, require specific separation intervals, as do antacids and divalent cations. Another item of importance is that it should be administered with a meal.
The NNRTI class is plagued by its low genetic barrier to resistance, with a single mutation that may confer resistance to nearly all NNRTIs. Estimated transmitted HIV drug resistance at baseline in men who have sex with men can be 8% to 10%.6 Transmitted resistance is a concern, a baseline genotype can determine if a patient is at a higher risk for virologic failure with NNRTIs.
Protease Inhibitors
Protease inhibitors have gone from the main stay of HIV treatment to being a less desirable first-line treatment option. Protease inhibitors have the highest pill burden and drug interactions of the first-line agents; they must be taken with food, and as of October 2014 there are no coformulated tablets (other than lopinavir/ritonavir which is not first-line). New combination tablets utilizing cobicistat as a boosting agent may resuscitate the use of the PIs. The coformulations under investigation are atazanavir/cobicistat, darunavir/cobicistat, and darunavir/cobicistat/emtricitabine/tenofovir alafenamide. These combinations will decrease the pill burden for PIs to 1 to 2 pill(s) a day instead of 3 pills a day. Although 1 to 3 pills taken once daily does not sound like a big difference, given a choice, the majority of patients choose the least number of pills.
How Does Cobicistat Compare With Ritonavir?
Cobicistat has no HIV activity and may be better tolerated than ritonavir. Effects on total cholesterol and triglycerides are similar between the 2 boosting agents.7 Cobicistat causes a modest, rapid increase in serum creatinine (SrCr) through inhibition of tubular secretion without impact on the glomerular filtration rate. The coformulated product of cobicistat/elvitegravir is not recommended to be initiated in patients with CrCl < 70 mL/min at baseline.2 It is anticipated that cobicistat-boosted PI regimens will have a similar limitation. The elevation of SrCr from cobicistat makes it difficult to monitor for tenofovir-related renal dysfunction since SrCr is no longer a reliable surrogate marker for renal function.7 Cobicistat and ritonavir inhibit CYP3A4 and have almost identical drug interactions. However, only ritonavir interacts with methadone.
The first-line PIs are better tolerated than other PIs. Gastrointestinal AEs are still common. Scleral icterus and moderate to severe jaundice occur in 5% to 9% of patients taking atazanavir due to inhibition of the indirect metabolism of bilirubin.8 As a sulfa-related drug, darunavir is generally avoided if the patient has a sulfa allergy, but can be used with caution.9 Another limitation for PIs is that both options are required to be taken with food.
Protease inhibitors will always have a place in treatment-experienced patients with drug resistance, but may have a unique role in patients with baseline M184V mutations. A retrospective analysis showed that patients with a M184V mutation alone achieved equivalent viral suppression if the patient was on 3 fully active HIV ARVs or on a boosted PI, lamivudine or emtricitabine, and 1 additional NRTI.10 Patients with only a M184V mutation can still achieve full suppression without adding an additional ARV agent or using 3 HIV drug classes, preserving future treatment options. Boosted-PI regimens have less drug resistance at failure than NNRTI-based regimens. Less than 5% of new infections have baseline (pretreatment) drug resistance to PIs.6
Boosted PIs have a long clinical history and are still appropriate for many treatment-naive and experienced patients. Reduced pill burden with cobicistat formulation may revive utilization when the pill burden decreases from 3 tablets per day to 1 or 2.
Integrase Strand Inhibitors
INSTI-based regimens have had low discontinuation rates overall in clinical trials, and are great options for a majority of patients. There are 3 INSTIs, all of which are on the list of preferred agents according to the DHHS guidelines. INSTIs may be used with any baseline VL or CD4 counts, without concern for potency. Each of the 3 agents has clear benefits and disadvantages when choosing among them.
Raltegravir has no food requirements and has the least amount of drug interactions of nearly all ART regimens since it is not processed via cytochrome P450 enzymes, but it is dosed twice daily. When paired with emtricitabine/tenofovir, it is also the preferred regimen for occupational postexposure prophylaxis.
Dolut egr avi r i s onc e da i ly, ha s no food requirements, and also avoids drug interactions due to cytochrome P450 enzymes. However, this ARV agent does inhibit the renal organic cation transporter, so some clinically relevant interactions exist. Dolutegravir also has the benefit of being paired with either emtricitabine/tenofovir or abacavir/lamivudine, both of which are preferred DHHS regimens. As mentioned previously, dolutegravir/abacavir/lamivudine are now coformulated
and are an additional all-in-one option. Abacavir has conflicting data regarding increased risk for myocardial infarction; this risk should be considered in light of other risk factors for the patient including age, diabetes, etc.11
Elvitegravir is unique as an INSTI. Elvitegravir/cobicistat/emtricitabine/tenofovir must be administered with food and since it utilizes cobicistat as a pharmaco-enhancer to boost elvitegravir’s levels, there are a significant number of potential drug interactions. Cobicistat-containing regimens must not be prescribed for patients with a baseline CrCl <70 mL/min.2
Both dolutegravir and raltegravir can be taken with or without food. However, if the patient is taking a divalent cation (magnesium, iron, or calcium) then the medications must be separated, causing the patient to take medications multiple times per day, or taken with food (and lose the benefit of with or without food).
Cost and the New Era of ARV Generics
Previously, the majority of HIV regimens were similarly priced and had little impact on prescribing practices. Several ARV are now available as generics. In the next 2 years, generics may have major impact on prescribing practice as first-line options (darunavir, atazanavir, and efavirenz) will be available as generics (Table 2).12
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Author Disclaimer
The views expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the U.S. Department of Health and Human Services, the Indian Health Services, or the U.S. Government. Please review complete prescribing information for specific drugs or drug combinations—including but not limited to indications, contraindications, warnings, adverse effects, and drug interactions- before administering pharmacologic therapy to patients.
Journal Disclaimer
The opinions expressed herein are those of the author and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combination—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
The one pill daily revolution in human immunodeficiency virus (HIV) management continues with the recent FDA approval of Triumeq, a combination tablet with an integrase inhibitor backbone containing dolutegravir, abacavir, and lamivudine. The first all-in-one protease inhibitor (PI) option is on the horizon and might receive approval before this article is published. The Department of Health and Human Services (DHHS) recommends 7 regimens for any treatment-naive patient with HIV, with 3 additional options if the patient has a baseline plasma RNA viral load (VL) < 100,000 copies/mL (Table 1).1 With 10 first-line options and 4 once daily pill regimens, HIV treatment is poised to enter the realm of managed care.
Guidelines recommend treatment of all patients with HIV, both to slow disease progression, and to reduce the risk of HIV transmission.1 Regimens should be individual ized based on vi rologi c efficacy, toxicity, pill burden, dosing frequency, drug interaction potential, drug resistance testing results, comorbid conditions, and cost. Therapy should only be deferred on a case-by-case basis. Patients considering therapy must be willing and able to commit to taking daily medications and understand the risks and benefits of therapy. Each antiretroviral (ARV) class, and each individual drug, has its own set of advantages and disadvantages. This article will review considerations when choosing a regimen, provide a brief overview of the first-line treatment options, and finally touch on cost considerations.
Choosing An Appropriate Regimen
Choosing an antiviral regimen has 2 basic steps. First, a provider must determine what choices are medically appropriate. An appropriate regimen has 3 active agents with acceptable performance and no contraindications. An ARV regimen generally consists of 2 nucleoside reverse transcriptase inhibitors (NRTIs) plus 1 drug from 1 of the following classes: non-nucleoside reverse transcriptase inhibitor (NNRTI); PI boosted with ritonavir or cobicistat; or integrase strand transfer inhibitor (INSTI). The next step is helping the patient determine which regimen will suit him or her best considering dosing, food requirements, and potential adverse effects.
Genotype, Viral Load, and CD4 Testing at Baseline
A primary consideration is, “Will the regimen work?” This question can best be answered by the genotype, plasma RNA VL, and CD4 count. Baseline genotypes should be obtained for all patients and used to eliminate any inferior drug-resistant treatment options. Viral loads > 100,000 copies/mL eliminate 3 treatment options due to inferiority (inability to fully suppress the HIV virus) in patients with high VL (Table 1). Specifically, abacavir is not recommended (unless given with dolutegravir) with VL > 100,000 copies/mL. Rilpivirine is also not recommended for patients with VL > 100,000 copies/mL or CD4 < 200 cells/mm3.
Creatinine Clearance
Many NRTIs require dosage adjustment when creatinine clearance (CrCl) falls below 50 mL/min. Cobicistat is a novel pharmacokinetic enhancer or boosting agent and is only recommended for baseline CrCl > 70 mL/min.2
HLA-B 5701
If a patient has a positive HLA-B 5701 screening test, abacavir is strictly contraindicated due to the propensity for abacavir hypersensitivity, a potentially life-threatening reaction.
Allergies
Darunavir has a sulfonamide moiety and should be avoided in patients with a sulfa allergy.
Drug Interactions
Drug interactions should be a consideration for all patients with an extensive review of their current medications. Pharmaco-enhancers or “boosting agents,” such as ritonavir or cobicistat, are used to increase drug levels of HIV medications. However, since this is accomplished by inhibition of CYP3A there is great potential for interaction with other medications; opiates, amphetamines, sildenafil, most lipid-lowering agents, and numerous other drugs have significant interactions with ritonavir and cobicistat. Looking at current medications may not be enough; some consideration should be made for the potential of adding medications. For example, if a patient has a seizure disorder and is not well controlled on levetiracetam, consideration should be made if there could be a potential need for phenytoin or other agent that commonly interacts with some HIV medications.
Adverse Events
Adverse events (AEs) are a large consideration when choosing ARV therapy (ART) and can either have medical implications or be patient-specific. Some agents have severe AEs like worsening depression and suicidal ideation. Others are less severe and a particular patient may prefer a risk for gastrointestinal (GI) AEs rather than insomnia.
Adherence
Predicting adherence is difficult; however, certain factors may predispose a patient to being less adherent, including active substance abuse, depression or other mood disorder, unstable housing, and inconsistent access to food. Some regimens are more forgiving and require a lower level of adherence and are less likely to have resistance mutations with failure. Both ritonavir-boosted PIs, as well as dolutegravir, seem to have the best outcomes with lower levels of adherence. Tailoring the dosing and food requirements to a patient’s lifestyle can also improve adherence.
Childbearing Age
Pregnancy and potential for pregnancy must be considered in all female patients. Although pregnancy is beyond the scope of this discussion, the perinatal guidelines should be considered in women of childbearing age.3
Custom A Regimen
Convenience is a major consideration when selecting a regimen. Comparator studies done to demonstrate regimen efficacy using intention-to-treat structures highlight this point. Many patients express that a single tablet is their most important priority. Other important considerations for regimen selection are food requirements or restrictions, and whether there are drug coadministration concerns (eg, divalent cations in antacids or vitamins). These will be discussed more with each
individual ARV agent.
Now that regimen considerations have been reviewed from both provider and patient perspectives, we will focus on the individual classes of ARVs to make selection of a regimen more tailored to patient needs.
The NNRTI Class
The NNRTI class has 2 first-line “recommended” agents, efavirenz and rilpivirine, which are both conveniently available as “one-pill-a-day” coformulated regimens with tenofovir and emtricitabine.1 While the 2 NNRTIs are in the same class, they each have unique properties that would lead a clinician to choose one versus the other.
Efavirenz is the only NNRTI recommended to be paired with abacavir and lamivudine (coformulated as Epzicom) first-line nucleosides. However, it is only recommended to be used in patients with VL < 100,000 copies/mL.
Efavirenz is one of the most studied and widely used ARV. Efavirenz/emtricitabine/tenofovir (coformulated as Atripla) was the first once daily regimen and has shown noninferiority or superiority to most ARV regimens. However, AEs are the most common reason for discontinuation. Efavirenz is well known for its CNS AEs, including vivid dreams, somnolence, impaired concentration, headaches, and depression. These AEs typically diminish or abate after several weeks of therapy. However, certain CNS effects may be long term, including depression or suicidal thoughts.
The recommendation of taking efavirenz regimens at bedtime on an empty stomach to reduce CNS AEs is not ideal for every patient. Efavirenz can also have additive CNS AEs with alcohol and potentially lead to blackouts. It can also lead to false positives for marijuana and benzodiazepines on drug screenings. The FDA classifies efavirenz as Pregnancy Category D, so it should be avoided in women of childbearing age.4 However, if pregnancy isdetected while someone is taking efavirenz there is no need to discontinue the agent since the neural tube has formed by that time.3
Rilpivirine is better tolerated than efavirenz, with fewer and less severe CNS AEs. Rilpivirine/emtricitabine/tenofovir (coformulated as Complera) has the convenience of being 1 pill once daily; however, it is not appropriate for all patients. It should be avoided in patients with a pretreatment VL > 100,000 copies/mL or CD4 < 200 cells/mm3. Patients with values beyond these thresholds were more likely to experience early virologic failure in the first months of therapy compared to efavirenz/emtricitabine/tenofovir.5 Because it is a CYP3A4 substrate, rilpivirine has important drug-drug interactions. Proton pump inhibitors are contraindicated, and H2 receptor antagonists, like ranitidine or famotidine, require specific separation intervals, as do antacids and divalent cations. Another item of importance is that it should be administered with a meal.
The NNRTI class is plagued by its low genetic barrier to resistance, with a single mutation that may confer resistance to nearly all NNRTIs. Estimated transmitted HIV drug resistance at baseline in men who have sex with men can be 8% to 10%.6 Transmitted resistance is a concern, a baseline genotype can determine if a patient is at a higher risk for virologic failure with NNRTIs.
Protease Inhibitors
Protease inhibitors have gone from the main stay of HIV treatment to being a less desirable first-line treatment option. Protease inhibitors have the highest pill burden and drug interactions of the first-line agents; they must be taken with food, and as of October 2014 there are no coformulated tablets (other than lopinavir/ritonavir which is not first-line). New combination tablets utilizing cobicistat as a boosting agent may resuscitate the use of the PIs. The coformulations under investigation are atazanavir/cobicistat, darunavir/cobicistat, and darunavir/cobicistat/emtricitabine/tenofovir alafenamide. These combinations will decrease the pill burden for PIs to 1 to 2 pill(s) a day instead of 3 pills a day. Although 1 to 3 pills taken once daily does not sound like a big difference, given a choice, the majority of patients choose the least number of pills.
How Does Cobicistat Compare With Ritonavir?
Cobicistat has no HIV activity and may be better tolerated than ritonavir. Effects on total cholesterol and triglycerides are similar between the 2 boosting agents.7 Cobicistat causes a modest, rapid increase in serum creatinine (SrCr) through inhibition of tubular secretion without impact on the glomerular filtration rate. The coformulated product of cobicistat/elvitegravir is not recommended to be initiated in patients with CrCl < 70 mL/min at baseline.2 It is anticipated that cobicistat-boosted PI regimens will have a similar limitation. The elevation of SrCr from cobicistat makes it difficult to monitor for tenofovir-related renal dysfunction since SrCr is no longer a reliable surrogate marker for renal function.7 Cobicistat and ritonavir inhibit CYP3A4 and have almost identical drug interactions. However, only ritonavir interacts with methadone.
The first-line PIs are better tolerated than other PIs. Gastrointestinal AEs are still common. Scleral icterus and moderate to severe jaundice occur in 5% to 9% of patients taking atazanavir due to inhibition of the indirect metabolism of bilirubin.8 As a sulfa-related drug, darunavir is generally avoided if the patient has a sulfa allergy, but can be used with caution.9 Another limitation for PIs is that both options are required to be taken with food.
Protease inhibitors will always have a place in treatment-experienced patients with drug resistance, but may have a unique role in patients with baseline M184V mutations. A retrospective analysis showed that patients with a M184V mutation alone achieved equivalent viral suppression if the patient was on 3 fully active HIV ARVs or on a boosted PI, lamivudine or emtricitabine, and 1 additional NRTI.10 Patients with only a M184V mutation can still achieve full suppression without adding an additional ARV agent or using 3 HIV drug classes, preserving future treatment options. Boosted-PI regimens have less drug resistance at failure than NNRTI-based regimens. Less than 5% of new infections have baseline (pretreatment) drug resistance to PIs.6
Boosted PIs have a long clinical history and are still appropriate for many treatment-naive and experienced patients. Reduced pill burden with cobicistat formulation may revive utilization when the pill burden decreases from 3 tablets per day to 1 or 2.
Integrase Strand Inhibitors
INSTI-based regimens have had low discontinuation rates overall in clinical trials, and are great options for a majority of patients. There are 3 INSTIs, all of which are on the list of preferred agents according to the DHHS guidelines. INSTIs may be used with any baseline VL or CD4 counts, without concern for potency. Each of the 3 agents has clear benefits and disadvantages when choosing among them.
Raltegravir has no food requirements and has the least amount of drug interactions of nearly all ART regimens since it is not processed via cytochrome P450 enzymes, but it is dosed twice daily. When paired with emtricitabine/tenofovir, it is also the preferred regimen for occupational postexposure prophylaxis.
Dolut egr avi r i s onc e da i ly, ha s no food requirements, and also avoids drug interactions due to cytochrome P450 enzymes. However, this ARV agent does inhibit the renal organic cation transporter, so some clinically relevant interactions exist. Dolutegravir also has the benefit of being paired with either emtricitabine/tenofovir or abacavir/lamivudine, both of which are preferred DHHS regimens. As mentioned previously, dolutegravir/abacavir/lamivudine are now coformulated
and are an additional all-in-one option. Abacavir has conflicting data regarding increased risk for myocardial infarction; this risk should be considered in light of other risk factors for the patient including age, diabetes, etc.11
Elvitegravir is unique as an INSTI. Elvitegravir/cobicistat/emtricitabine/tenofovir must be administered with food and since it utilizes cobicistat as a pharmaco-enhancer to boost elvitegravir’s levels, there are a significant number of potential drug interactions. Cobicistat-containing regimens must not be prescribed for patients with a baseline CrCl <70 mL/min.2
Both dolutegravir and raltegravir can be taken with or without food. However, if the patient is taking a divalent cation (magnesium, iron, or calcium) then the medications must be separated, causing the patient to take medications multiple times per day, or taken with food (and lose the benefit of with or without food).
Cost and the New Era of ARV Generics
Previously, the majority of HIV regimens were similarly priced and had little impact on prescribing practices. Several ARV are now available as generics. In the next 2 years, generics may have major impact on prescribing practice as first-line options (darunavir, atazanavir, and efavirenz) will be available as generics (Table 2).12
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Author Disclaimer
The views expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the U.S. Department of Health and Human Services, the Indian Health Services, or the U.S. Government. Please review complete prescribing information for specific drugs or drug combinations—including but not limited to indications, contraindications, warnings, adverse effects, and drug interactions- before administering pharmacologic therapy to patients.
Journal Disclaimer
The opinions expressed herein are those of the author and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combination—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
1. HHS Panel on Antiretroviral Guidelines for Adults and Adolescents—A Working Group of the Office of AIDS Research Advisory Council (OARAC). Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. http://aidsinfo.nih.gov/contentfile/lvguidelines/AdultandAdolescentGL.pdf. Updated November 13, 2014. Accessed December 1, 2014.
2. Stribild [package insert]. Foster City, CA: Gilead Sciences, Inc; 2012.
3. HHS Panel on Treatment of HIV-Infected Pregnant Women and Prevention of Perinatal Transmission—A Working Group of the Office of AIDS Research Advisory Council (OARAC). Recommendations for use of antiretroviral drugs in pregnant HIV-1-infected women for maternal health and interventions to reduce perinatal HIV transmission in the United States. http://aidsinfo.nih.gov/contentfiles/lvguidelines/PerinatalGL.pdf. Updated March 28, 2014. Accessed October 27, 2014.
4. Atripla [package insert] Princeton, NJ: Bristol-Myers Squibb; Foster City, CA: Gilead Sciences, Inc; 2013.
5. Cohen CJ, Molina JM, Cassetti I, et al. Week 96 efficacy and safety of rilpivirine in treatment-naive, HIV-1 patients in two Phase III randomized trials. AIDS. 2013;27(6):939-950.
6. Bañez Ocfemia MC, Saduvala N, Oster AM, et al. Transmitted HIV-1 drug resistance among men who have sex with men, 11 US jurisdictions, 2008-2011. Presented at: 21st Conference on Retroviruses and Opportunistic Infections; March 3-6, 2014; Boston, MA. Abstract 579.
7. Gallant JE, Koenig E, Andrade-Villaneuva J, et al. Cobicistat versus ritonavir as a pharmacoenhancer of atazanavir plus emtricitabine/tenofovir disoproxil fumarate in treatment-naive HIV type 1-infected patients: Week 48 results. J Infect Dis. 2013;208(1):32-39.
8. Reyatoz [package insert]. Princeton, NJ: Bristol-Myers Squibb Company; 2014.
9. Prezista [package insert]. Titusville, NJ: Janssen Pharmaceuicals, Inc; 2013.
10. Hull M, Moore D, Harris M, et al. A lamivudine (3TC)-based backbone in conjunction with a boosted protease inhibitor (PI) is sufficient to achieve virologic suppression in the presence of M184V mutations. Presented at: 49th Interscience Conference on Antimicrobial Agents and Chemotherapy; September 12-15, 2009; San Francisco, CA. Abstract H-916.
11. Triumeq [package insert]. Research Triangle Park, NC: ViiV Healthcare; 2014.
12. Maxmen A. Generic HIV drugs will widen US treatment net. Nature.
2012;488(7411):267.
1. HHS Panel on Antiretroviral Guidelines for Adults and Adolescents—A Working Group of the Office of AIDS Research Advisory Council (OARAC). Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. http://aidsinfo.nih.gov/contentfile/lvguidelines/AdultandAdolescentGL.pdf. Updated November 13, 2014. Accessed December 1, 2014.
2. Stribild [package insert]. Foster City, CA: Gilead Sciences, Inc; 2012.
3. HHS Panel on Treatment of HIV-Infected Pregnant Women and Prevention of Perinatal Transmission—A Working Group of the Office of AIDS Research Advisory Council (OARAC). Recommendations for use of antiretroviral drugs in pregnant HIV-1-infected women for maternal health and interventions to reduce perinatal HIV transmission in the United States. http://aidsinfo.nih.gov/contentfiles/lvguidelines/PerinatalGL.pdf. Updated March 28, 2014. Accessed October 27, 2014.
4. Atripla [package insert] Princeton, NJ: Bristol-Myers Squibb; Foster City, CA: Gilead Sciences, Inc; 2013.
5. Cohen CJ, Molina JM, Cassetti I, et al. Week 96 efficacy and safety of rilpivirine in treatment-naive, HIV-1 patients in two Phase III randomized trials. AIDS. 2013;27(6):939-950.
6. Bañez Ocfemia MC, Saduvala N, Oster AM, et al. Transmitted HIV-1 drug resistance among men who have sex with men, 11 US jurisdictions, 2008-2011. Presented at: 21st Conference on Retroviruses and Opportunistic Infections; March 3-6, 2014; Boston, MA. Abstract 579.
7. Gallant JE, Koenig E, Andrade-Villaneuva J, et al. Cobicistat versus ritonavir as a pharmacoenhancer of atazanavir plus emtricitabine/tenofovir disoproxil fumarate in treatment-naive HIV type 1-infected patients: Week 48 results. J Infect Dis. 2013;208(1):32-39.
8. Reyatoz [package insert]. Princeton, NJ: Bristol-Myers Squibb Company; 2014.
9. Prezista [package insert]. Titusville, NJ: Janssen Pharmaceuicals, Inc; 2013.
10. Hull M, Moore D, Harris M, et al. A lamivudine (3TC)-based backbone in conjunction with a boosted protease inhibitor (PI) is sufficient to achieve virologic suppression in the presence of M184V mutations. Presented at: 49th Interscience Conference on Antimicrobial Agents and Chemotherapy; September 12-15, 2009; San Francisco, CA. Abstract H-916.
11. Triumeq [package insert]. Research Triangle Park, NC: ViiV Healthcare; 2014.
12. Maxmen A. Generic HIV drugs will widen US treatment net. Nature.
2012;488(7411):267.
Improving Caregiver Knowledge of Support Resources
In 2012, 1.3 million veterans who were wounded in action had a severe service-connected disability—nearly triple the number in 2001 (482,000).1 Given the increased number of wounded veterans, the need for caregivers also increased.
The act of caring for an individual with a chronic disability can be a daunting task for the caregiver. However, what is not so commonly recognized is the need for caregiver awareness of the available support resources. Caregivers who do not receive necessary support experience physical and emotional consequences that interfere with their ability to care for veterans with disabilities. Therefore, there is a significant need to provide adequate support for the caregiver to maintain optimum care of the veteran.2
Increased caregiver strain among family caregivers of veterans with long-term disabilities and their lack of knowledge of support resources is a clinical concern. A comprehensive review of the literature provided evidence that access and use of caregiver support resources improved caregiver quality of life.
The purpose of this project was to provide an educational intervention of caregiver resources that were available at the Durham VA Health Care System in North Carolina and in the surrounding community. The desired outcomes included (a) increasing the caregiver’s knowledge of resources available at the VA and within the community to decrease caregiver burden; and (b) assisting the caregiver in determining the best resources for the caregiver and patient. This project was deemed to be a quality improvement project and did not require institutional review board (IRB) approval.
Background
The term strain is used to describe the burden, trouble, or burnout that a caregiver encounters when caring for a person with a long-term illness or disability.3 Caregivers of veterans remain in their role longer and have a heavier burden of care than that of all other caregivers: 65% are in a high-burden caregiving situation compared with 31% nationally.4 The consequence of providing care without assistance has all the features of chronic stress.2 Moreover, the decline of the caregiver’s health can significantly compromise the ability to provide care.3
Empirical observations of the negative health effects of caregiving noted over the past 2 decades have helped convince policymakers that supporting caregivers is an important public health issue.2 To this end, Congress mandated legislation that required the VA to provide a support program for veteran caregivers. In May 2010, President Obama signed the Caregivers and Veterans Omnibus Health Services Act of 2010 into law.1
Supporting Literature
The VA caregiver resource program offers a variety of support resources.1 A better understanding of caregiver needs is necessary to provide the right support resources, improve the health and well-being of caregivers, and make decisions regarding individual caregiving situations.5 For example, respite care offers temporary or periodic relief from caregiving, allowing caregivers to attend to personal tasks, such as shopping, running errands, relaxing, and socializing. This service can increase the physical and mental well-being of the caregiver.6 Studies show that early use of support services is paramount in order for caregivers to receive the greatest positive impact.5
Chen and colleaguesconducted a study of 164 caregivers. The study showed that caregivers who received assistance with accessing the correct support resource exhibited considerably higher satisfaction with the services they received.7 Determining which support therapy was best for the caregiver and the patient for whom they were caring was seen as the initial step. Providing a tool that supplies all the information caregivers need as well as assisting them with accessing services more efficiently is beneficial.
The National Alliance for Caregiving conducted a study to evaluate the needs of caregivers of veterans of various conflicts.4 In the study, caregivers reported that a resource guide would be beneficial. Some of the services they wanted to include in the directory were VA disability benefits, respite care, home health care, hospice services, assisted living, rehabilitation therapies, caregiver support group information, and community resources.
Based on the literature, the author believed that better knowledge of support resources was needed for caregivers. The literature included detailed descriptions of how knowledge of support resources improved caregiver’s well-being by increasing his or her ability to cope with stress related to providing care. However, the literature could have provided a more elaborate discussion on this topic. That was the only weakness identified in each of the studies. Nonetheless, it was clearly noted that resource knowledge yielded a positive effect.
Methods
The project took place at the Durham VA Health Care System and was implemented from August 2015 to October 2015. The participants targeted were caregivers of veterans with disabilities who were considered the veteran’s primary caregiver. Participation was voluntary.
During the veteran’s clinic appointment, the caregiver was given an implied consent letter, pre- and postquestionnaire forms, and a caregiver resource manual. The manual included information on caregiver support resources at the Durham VA Health Care System and in the community (eg, adult day care centers; home-based primary care, hospice care, skilled care, and telehealth; homemaker and home health aide programs; respite care). Other information was provided, such as the Caregiver Support Program application process, contact names, numbers, and helpful websites. Before reading the manual, participants completed the prequestionnaire form and returned it the day of the veteran’s visit. After reading the manual, the caregiver was instructed to complete the postquestionnaire form.
The project coordinator (PC) collaborated with the Veteran Health Education coordinator in developing the caregiver resource manual and questionnaires to ensure that the material met the requirements set forth by the educational program within the Durham VA Health Care System. The PC also collaborated with the Caregiver Support Program subject experts, the chief and acting assistant chief of social work when formulating the contents of the manual and questionnaires. The questionnaires were used to assess the effectiveness of the manual.
The 3 questions on the prequestionnaire and 3 questions on the postquestionnaire were geared to measure the caregiver’s knowledge. There also were 4 questions on the postquestionnaire that were used to address manual revisions.
On the prequestionnaire form, the following questions were asked: (1) If you needed to find caregiver support resources, how much knowledge do you have finding the resources that fit your needs as well as the veteran’s needs? (2) Rate how aware you are with knowing what caregiver support resources are available at the VA and within the community; and (3) Would knowing which caregiver support resources to choose from at the VA and within the community decrease your stress level and give you “peace of mind?”
The same questions were asked postintervention, and the participants were asked to rate their knowledge after reviewing the manual. The participant’s responses on the questionnaires were measured using a 5-point Likert scale.
Participant Demographics
Demographic information was obtained from the cover letter distributed to each participant. The demographic information included age, gender, relationship to the veteran, and number of years to date in the current caregiving role. Participants eligible for inclusion in this project were primary caregivers of veterans with disabilities from all eras of conflict, aged ≥ 18 years.
Fifteen caregivers participated by returning the cover letter containing the implied consent, reading the manual, and completing the pre- and postquestionnaires. There was a wide age range of caregivers who participated, from 29 to 77 years. Of those who responded, there also was a wide range in time in their current caregiving role, ranging from 1 to 41 years. The mean number of years in the current caregiving role was 7 years.
Of the 15 participants, most were female spouses. There were no husbands who participated. The relative’s category included a cousin, a son, and a daughter. The “other” category included a son-in-law and a fiancé.
Data Analysis
Both outcomes were measured using the responses from questions 1 through 3 with the use of running a descriptive statistical analysis. In addition, a t test was used to determine statistical significance, set at α level < .05 of knowledge increase from pre- to postintervention data. Based on the facility, educational benchmarks were set at 80% with the 80% equal to 4 on the Likert scale. Therefore, 80% was the identified benchmark for this project. The goal was that > 70% of the participants would score 80% or better on the postquestionnaire.
Results
Both outcomes were met: (1) increasing the caregiver’s knowledge regarding resources available at the VA and within the community to decrease caregiver burden; and (2) assisting the caregiver in deciding which caregiver resources located in the manual were the right fit for the caregiver and the veteran for whom they were caring. The percentage of participants who scored 80% or better on the prequestionnaire was 54% (n = 8). The postquestionnaire outcomes were considered an improvement based on caregiver’s knowledge of support resources as well as whether the information in the manual decreased their stress level and gave them peace of mind. The intended outcome for the postquestionnaire was that > 70% of the participants would score ≥ 80% after the intervention. This goal was met as final results revealed 73% (n = 11) of the participants scored > 80% on the postquestionnaire.
The postresults supported that caregivers’ knowledge increased, they had peace of mind, and stress levels were decreased with the use of an educational intervention, a comprehensive Caregiver Resource Manual. The postquestionnaire revealed that all of the participants found the Caregiver Resource Manual easy to navigate, and 93% of participants found the Caregiver Resource Manual useful. Out of 15 participants, 8 provided comments. Seven provided positive comments, reporting that the information in the manual was interesting, the manual was simple/easy to read, and the outside resources listed were helpful.
The participant who provided a negative comment was one of the caregivers who did not meet the benchmark of 80% on the pre- or postquestionnaire. The participant was a 33-year-old wife of a veteran with disabilities who had been in the current caregiving role for 9 years. This participant reported that the Caregiver Resource Manual was not geared to younger caregivers, so she would not benefit from using the manual. This caregiver also was the only participant who reported that the Caregiver Resource Manual neither gave her peace of mind nor decreased her stress level.
Comments or suggestions would have been helpful from the other 8 individuals. Because it was not written in the IRB proposal to contact the participants other than to follow-up with telephone calls regarding unreturned questionnaires, no further contact was made with the participants.
Discussion
The preliminary success of this project suggests that there is a significant need for an educational conduit to ensure sufficient caregiver knowledge. Interprofessional collaborative efforts along with using information systems/technology to deliver the Caregiver Resource Manual electronically are important future consideration for improvement of the overall outcomes for a wide range of caregivers, veterans, and health care providers. Health care policy changes on the organizational level, systems level, and national level could further support caregivers of disabled veterans by enabling easy access to caregiver resources as a mandated practice.
Limitations
Limitations centered on the recruitment process. There were a total of 15 caregivers who participated. Although the participation did not meet the PC’s expectation, the final number of participants was adequate in obtaining data regarding evaluating the impact of this project.
Conclusion
The results of this project provided evidence that the Caregiver Resource Manual was effective. Caregivers gained a sense of knowledge and empowerment regarding available resources within the VA and the community. Providing the caregiver with peace of mind and improving the overall health and well-being of the caregiver and veteran were essential.
Moreover, just as the veterans who fought for freedom were equipped with full body armor to help protect them from the potential negative consequences of combat, caregivers who care for these brave soldiers are now equipped with a resource tool and a “full armor of knowledge” to care for their loved ones…our nation’s heroes…our veterans.
1. VA Health Care. Actions needed to address higher-than-expected demand for the family caregiver program. http://www.gao.gov/assets/670/665928.pdf. Published September 18, 2014. Accessed January 9, 2017.
2. Schulz R, Sherwood PR. Physical and mental health effects of family caregiving. Am J Nurs. 2008;108(suppl 9):23-27.
3. Centers for Disease Control; Kimberly-Clark Corporation. Assuring healthy caregivers, a public health approach to translating research into practice: the RE-AIM framework. https://www.cdc.gov /aging/pdf/caregiving_monograph.pdf. Published 2008. Accessed January 9, 2017.
4. National Alliance for Caregiving. Caregivers of veterans—serving on the home front report of study findings. http://www.caregiving.org/data/2010 _Caregivers_of_Veterans_FULLREPORT_WEB _FINAL.pdf. Published 2010. Accessed January 9, 2017.
5. Whittier S, Coon D, Aaker J. Caregiving support interventions. http://cssr.berkeley.edu/pdfs/famcare_04.pdf. Updated 2001. Accessed January 9, 2017.
6. Alzheimer’s Association. Respite care. http://www .alz.org/care/alzheimers-dementia-caregiver-respite.asp. Updated 2016. Accessed January 9, 2017.
7. Chen YM, Hedrick SC, Young HM. A pilot evaluation of the family caregiver support program. Eval Program Plann. 2010;33(2):113-119.
In 2012, 1.3 million veterans who were wounded in action had a severe service-connected disability—nearly triple the number in 2001 (482,000).1 Given the increased number of wounded veterans, the need for caregivers also increased.
The act of caring for an individual with a chronic disability can be a daunting task for the caregiver. However, what is not so commonly recognized is the need for caregiver awareness of the available support resources. Caregivers who do not receive necessary support experience physical and emotional consequences that interfere with their ability to care for veterans with disabilities. Therefore, there is a significant need to provide adequate support for the caregiver to maintain optimum care of the veteran.2
Increased caregiver strain among family caregivers of veterans with long-term disabilities and their lack of knowledge of support resources is a clinical concern. A comprehensive review of the literature provided evidence that access and use of caregiver support resources improved caregiver quality of life.
The purpose of this project was to provide an educational intervention of caregiver resources that were available at the Durham VA Health Care System in North Carolina and in the surrounding community. The desired outcomes included (a) increasing the caregiver’s knowledge of resources available at the VA and within the community to decrease caregiver burden; and (b) assisting the caregiver in determining the best resources for the caregiver and patient. This project was deemed to be a quality improvement project and did not require institutional review board (IRB) approval.
Background
The term strain is used to describe the burden, trouble, or burnout that a caregiver encounters when caring for a person with a long-term illness or disability.3 Caregivers of veterans remain in their role longer and have a heavier burden of care than that of all other caregivers: 65% are in a high-burden caregiving situation compared with 31% nationally.4 The consequence of providing care without assistance has all the features of chronic stress.2 Moreover, the decline of the caregiver’s health can significantly compromise the ability to provide care.3
Empirical observations of the negative health effects of caregiving noted over the past 2 decades have helped convince policymakers that supporting caregivers is an important public health issue.2 To this end, Congress mandated legislation that required the VA to provide a support program for veteran caregivers. In May 2010, President Obama signed the Caregivers and Veterans Omnibus Health Services Act of 2010 into law.1
Supporting Literature
The VA caregiver resource program offers a variety of support resources.1 A better understanding of caregiver needs is necessary to provide the right support resources, improve the health and well-being of caregivers, and make decisions regarding individual caregiving situations.5 For example, respite care offers temporary or periodic relief from caregiving, allowing caregivers to attend to personal tasks, such as shopping, running errands, relaxing, and socializing. This service can increase the physical and mental well-being of the caregiver.6 Studies show that early use of support services is paramount in order for caregivers to receive the greatest positive impact.5
Chen and colleaguesconducted a study of 164 caregivers. The study showed that caregivers who received assistance with accessing the correct support resource exhibited considerably higher satisfaction with the services they received.7 Determining which support therapy was best for the caregiver and the patient for whom they were caring was seen as the initial step. Providing a tool that supplies all the information caregivers need as well as assisting them with accessing services more efficiently is beneficial.
The National Alliance for Caregiving conducted a study to evaluate the needs of caregivers of veterans of various conflicts.4 In the study, caregivers reported that a resource guide would be beneficial. Some of the services they wanted to include in the directory were VA disability benefits, respite care, home health care, hospice services, assisted living, rehabilitation therapies, caregiver support group information, and community resources.
Based on the literature, the author believed that better knowledge of support resources was needed for caregivers. The literature included detailed descriptions of how knowledge of support resources improved caregiver’s well-being by increasing his or her ability to cope with stress related to providing care. However, the literature could have provided a more elaborate discussion on this topic. That was the only weakness identified in each of the studies. Nonetheless, it was clearly noted that resource knowledge yielded a positive effect.
Methods
The project took place at the Durham VA Health Care System and was implemented from August 2015 to October 2015. The participants targeted were caregivers of veterans with disabilities who were considered the veteran’s primary caregiver. Participation was voluntary.
During the veteran’s clinic appointment, the caregiver was given an implied consent letter, pre- and postquestionnaire forms, and a caregiver resource manual. The manual included information on caregiver support resources at the Durham VA Health Care System and in the community (eg, adult day care centers; home-based primary care, hospice care, skilled care, and telehealth; homemaker and home health aide programs; respite care). Other information was provided, such as the Caregiver Support Program application process, contact names, numbers, and helpful websites. Before reading the manual, participants completed the prequestionnaire form and returned it the day of the veteran’s visit. After reading the manual, the caregiver was instructed to complete the postquestionnaire form.
The project coordinator (PC) collaborated with the Veteran Health Education coordinator in developing the caregiver resource manual and questionnaires to ensure that the material met the requirements set forth by the educational program within the Durham VA Health Care System. The PC also collaborated with the Caregiver Support Program subject experts, the chief and acting assistant chief of social work when formulating the contents of the manual and questionnaires. The questionnaires were used to assess the effectiveness of the manual.
The 3 questions on the prequestionnaire and 3 questions on the postquestionnaire were geared to measure the caregiver’s knowledge. There also were 4 questions on the postquestionnaire that were used to address manual revisions.
On the prequestionnaire form, the following questions were asked: (1) If you needed to find caregiver support resources, how much knowledge do you have finding the resources that fit your needs as well as the veteran’s needs? (2) Rate how aware you are with knowing what caregiver support resources are available at the VA and within the community; and (3) Would knowing which caregiver support resources to choose from at the VA and within the community decrease your stress level and give you “peace of mind?”
The same questions were asked postintervention, and the participants were asked to rate their knowledge after reviewing the manual. The participant’s responses on the questionnaires were measured using a 5-point Likert scale.
Participant Demographics
Demographic information was obtained from the cover letter distributed to each participant. The demographic information included age, gender, relationship to the veteran, and number of years to date in the current caregiving role. Participants eligible for inclusion in this project were primary caregivers of veterans with disabilities from all eras of conflict, aged ≥ 18 years.
Fifteen caregivers participated by returning the cover letter containing the implied consent, reading the manual, and completing the pre- and postquestionnaires. There was a wide age range of caregivers who participated, from 29 to 77 years. Of those who responded, there also was a wide range in time in their current caregiving role, ranging from 1 to 41 years. The mean number of years in the current caregiving role was 7 years.
Of the 15 participants, most were female spouses. There were no husbands who participated. The relative’s category included a cousin, a son, and a daughter. The “other” category included a son-in-law and a fiancé.
Data Analysis
Both outcomes were measured using the responses from questions 1 through 3 with the use of running a descriptive statistical analysis. In addition, a t test was used to determine statistical significance, set at α level < .05 of knowledge increase from pre- to postintervention data. Based on the facility, educational benchmarks were set at 80% with the 80% equal to 4 on the Likert scale. Therefore, 80% was the identified benchmark for this project. The goal was that > 70% of the participants would score 80% or better on the postquestionnaire.
Results
Both outcomes were met: (1) increasing the caregiver’s knowledge regarding resources available at the VA and within the community to decrease caregiver burden; and (2) assisting the caregiver in deciding which caregiver resources located in the manual were the right fit for the caregiver and the veteran for whom they were caring. The percentage of participants who scored 80% or better on the prequestionnaire was 54% (n = 8). The postquestionnaire outcomes were considered an improvement based on caregiver’s knowledge of support resources as well as whether the information in the manual decreased their stress level and gave them peace of mind. The intended outcome for the postquestionnaire was that > 70% of the participants would score ≥ 80% after the intervention. This goal was met as final results revealed 73% (n = 11) of the participants scored > 80% on the postquestionnaire.
The postresults supported that caregivers’ knowledge increased, they had peace of mind, and stress levels were decreased with the use of an educational intervention, a comprehensive Caregiver Resource Manual. The postquestionnaire revealed that all of the participants found the Caregiver Resource Manual easy to navigate, and 93% of participants found the Caregiver Resource Manual useful. Out of 15 participants, 8 provided comments. Seven provided positive comments, reporting that the information in the manual was interesting, the manual was simple/easy to read, and the outside resources listed were helpful.
The participant who provided a negative comment was one of the caregivers who did not meet the benchmark of 80% on the pre- or postquestionnaire. The participant was a 33-year-old wife of a veteran with disabilities who had been in the current caregiving role for 9 years. This participant reported that the Caregiver Resource Manual was not geared to younger caregivers, so she would not benefit from using the manual. This caregiver also was the only participant who reported that the Caregiver Resource Manual neither gave her peace of mind nor decreased her stress level.
Comments or suggestions would have been helpful from the other 8 individuals. Because it was not written in the IRB proposal to contact the participants other than to follow-up with telephone calls regarding unreturned questionnaires, no further contact was made with the participants.
Discussion
The preliminary success of this project suggests that there is a significant need for an educational conduit to ensure sufficient caregiver knowledge. Interprofessional collaborative efforts along with using information systems/technology to deliver the Caregiver Resource Manual electronically are important future consideration for improvement of the overall outcomes for a wide range of caregivers, veterans, and health care providers. Health care policy changes on the organizational level, systems level, and national level could further support caregivers of disabled veterans by enabling easy access to caregiver resources as a mandated practice.
Limitations
Limitations centered on the recruitment process. There were a total of 15 caregivers who participated. Although the participation did not meet the PC’s expectation, the final number of participants was adequate in obtaining data regarding evaluating the impact of this project.
Conclusion
The results of this project provided evidence that the Caregiver Resource Manual was effective. Caregivers gained a sense of knowledge and empowerment regarding available resources within the VA and the community. Providing the caregiver with peace of mind and improving the overall health and well-being of the caregiver and veteran were essential.
Moreover, just as the veterans who fought for freedom were equipped with full body armor to help protect them from the potential negative consequences of combat, caregivers who care for these brave soldiers are now equipped with a resource tool and a “full armor of knowledge” to care for their loved ones…our nation’s heroes…our veterans.
In 2012, 1.3 million veterans who were wounded in action had a severe service-connected disability—nearly triple the number in 2001 (482,000).1 Given the increased number of wounded veterans, the need for caregivers also increased.
The act of caring for an individual with a chronic disability can be a daunting task for the caregiver. However, what is not so commonly recognized is the need for caregiver awareness of the available support resources. Caregivers who do not receive necessary support experience physical and emotional consequences that interfere with their ability to care for veterans with disabilities. Therefore, there is a significant need to provide adequate support for the caregiver to maintain optimum care of the veteran.2
Increased caregiver strain among family caregivers of veterans with long-term disabilities and their lack of knowledge of support resources is a clinical concern. A comprehensive review of the literature provided evidence that access and use of caregiver support resources improved caregiver quality of life.
The purpose of this project was to provide an educational intervention of caregiver resources that were available at the Durham VA Health Care System in North Carolina and in the surrounding community. The desired outcomes included (a) increasing the caregiver’s knowledge of resources available at the VA and within the community to decrease caregiver burden; and (b) assisting the caregiver in determining the best resources for the caregiver and patient. This project was deemed to be a quality improvement project and did not require institutional review board (IRB) approval.
Background
The term strain is used to describe the burden, trouble, or burnout that a caregiver encounters when caring for a person with a long-term illness or disability.3 Caregivers of veterans remain in their role longer and have a heavier burden of care than that of all other caregivers: 65% are in a high-burden caregiving situation compared with 31% nationally.4 The consequence of providing care without assistance has all the features of chronic stress.2 Moreover, the decline of the caregiver’s health can significantly compromise the ability to provide care.3
Empirical observations of the negative health effects of caregiving noted over the past 2 decades have helped convince policymakers that supporting caregivers is an important public health issue.2 To this end, Congress mandated legislation that required the VA to provide a support program for veteran caregivers. In May 2010, President Obama signed the Caregivers and Veterans Omnibus Health Services Act of 2010 into law.1
Supporting Literature
The VA caregiver resource program offers a variety of support resources.1 A better understanding of caregiver needs is necessary to provide the right support resources, improve the health and well-being of caregivers, and make decisions regarding individual caregiving situations.5 For example, respite care offers temporary or periodic relief from caregiving, allowing caregivers to attend to personal tasks, such as shopping, running errands, relaxing, and socializing. This service can increase the physical and mental well-being of the caregiver.6 Studies show that early use of support services is paramount in order for caregivers to receive the greatest positive impact.5
Chen and colleaguesconducted a study of 164 caregivers. The study showed that caregivers who received assistance with accessing the correct support resource exhibited considerably higher satisfaction with the services they received.7 Determining which support therapy was best for the caregiver and the patient for whom they were caring was seen as the initial step. Providing a tool that supplies all the information caregivers need as well as assisting them with accessing services more efficiently is beneficial.
The National Alliance for Caregiving conducted a study to evaluate the needs of caregivers of veterans of various conflicts.4 In the study, caregivers reported that a resource guide would be beneficial. Some of the services they wanted to include in the directory were VA disability benefits, respite care, home health care, hospice services, assisted living, rehabilitation therapies, caregiver support group information, and community resources.
Based on the literature, the author believed that better knowledge of support resources was needed for caregivers. The literature included detailed descriptions of how knowledge of support resources improved caregiver’s well-being by increasing his or her ability to cope with stress related to providing care. However, the literature could have provided a more elaborate discussion on this topic. That was the only weakness identified in each of the studies. Nonetheless, it was clearly noted that resource knowledge yielded a positive effect.
Methods
The project took place at the Durham VA Health Care System and was implemented from August 2015 to October 2015. The participants targeted were caregivers of veterans with disabilities who were considered the veteran’s primary caregiver. Participation was voluntary.
During the veteran’s clinic appointment, the caregiver was given an implied consent letter, pre- and postquestionnaire forms, and a caregiver resource manual. The manual included information on caregiver support resources at the Durham VA Health Care System and in the community (eg, adult day care centers; home-based primary care, hospice care, skilled care, and telehealth; homemaker and home health aide programs; respite care). Other information was provided, such as the Caregiver Support Program application process, contact names, numbers, and helpful websites. Before reading the manual, participants completed the prequestionnaire form and returned it the day of the veteran’s visit. After reading the manual, the caregiver was instructed to complete the postquestionnaire form.
The project coordinator (PC) collaborated with the Veteran Health Education coordinator in developing the caregiver resource manual and questionnaires to ensure that the material met the requirements set forth by the educational program within the Durham VA Health Care System. The PC also collaborated with the Caregiver Support Program subject experts, the chief and acting assistant chief of social work when formulating the contents of the manual and questionnaires. The questionnaires were used to assess the effectiveness of the manual.
The 3 questions on the prequestionnaire and 3 questions on the postquestionnaire were geared to measure the caregiver’s knowledge. There also were 4 questions on the postquestionnaire that were used to address manual revisions.
On the prequestionnaire form, the following questions were asked: (1) If you needed to find caregiver support resources, how much knowledge do you have finding the resources that fit your needs as well as the veteran’s needs? (2) Rate how aware you are with knowing what caregiver support resources are available at the VA and within the community; and (3) Would knowing which caregiver support resources to choose from at the VA and within the community decrease your stress level and give you “peace of mind?”
The same questions were asked postintervention, and the participants were asked to rate their knowledge after reviewing the manual. The participant’s responses on the questionnaires were measured using a 5-point Likert scale.
Participant Demographics
Demographic information was obtained from the cover letter distributed to each participant. The demographic information included age, gender, relationship to the veteran, and number of years to date in the current caregiving role. Participants eligible for inclusion in this project were primary caregivers of veterans with disabilities from all eras of conflict, aged ≥ 18 years.
Fifteen caregivers participated by returning the cover letter containing the implied consent, reading the manual, and completing the pre- and postquestionnaires. There was a wide age range of caregivers who participated, from 29 to 77 years. Of those who responded, there also was a wide range in time in their current caregiving role, ranging from 1 to 41 years. The mean number of years in the current caregiving role was 7 years.
Of the 15 participants, most were female spouses. There were no husbands who participated. The relative’s category included a cousin, a son, and a daughter. The “other” category included a son-in-law and a fiancé.
Data Analysis
Both outcomes were measured using the responses from questions 1 through 3 with the use of running a descriptive statistical analysis. In addition, a t test was used to determine statistical significance, set at α level < .05 of knowledge increase from pre- to postintervention data. Based on the facility, educational benchmarks were set at 80% with the 80% equal to 4 on the Likert scale. Therefore, 80% was the identified benchmark for this project. The goal was that > 70% of the participants would score 80% or better on the postquestionnaire.
Results
Both outcomes were met: (1) increasing the caregiver’s knowledge regarding resources available at the VA and within the community to decrease caregiver burden; and (2) assisting the caregiver in deciding which caregiver resources located in the manual were the right fit for the caregiver and the veteran for whom they were caring. The percentage of participants who scored 80% or better on the prequestionnaire was 54% (n = 8). The postquestionnaire outcomes were considered an improvement based on caregiver’s knowledge of support resources as well as whether the information in the manual decreased their stress level and gave them peace of mind. The intended outcome for the postquestionnaire was that > 70% of the participants would score ≥ 80% after the intervention. This goal was met as final results revealed 73% (n = 11) of the participants scored > 80% on the postquestionnaire.
The postresults supported that caregivers’ knowledge increased, they had peace of mind, and stress levels were decreased with the use of an educational intervention, a comprehensive Caregiver Resource Manual. The postquestionnaire revealed that all of the participants found the Caregiver Resource Manual easy to navigate, and 93% of participants found the Caregiver Resource Manual useful. Out of 15 participants, 8 provided comments. Seven provided positive comments, reporting that the information in the manual was interesting, the manual was simple/easy to read, and the outside resources listed were helpful.
The participant who provided a negative comment was one of the caregivers who did not meet the benchmark of 80% on the pre- or postquestionnaire. The participant was a 33-year-old wife of a veteran with disabilities who had been in the current caregiving role for 9 years. This participant reported that the Caregiver Resource Manual was not geared to younger caregivers, so she would not benefit from using the manual. This caregiver also was the only participant who reported that the Caregiver Resource Manual neither gave her peace of mind nor decreased her stress level.
Comments or suggestions would have been helpful from the other 8 individuals. Because it was not written in the IRB proposal to contact the participants other than to follow-up with telephone calls regarding unreturned questionnaires, no further contact was made with the participants.
Discussion
The preliminary success of this project suggests that there is a significant need for an educational conduit to ensure sufficient caregiver knowledge. Interprofessional collaborative efforts along with using information systems/technology to deliver the Caregiver Resource Manual electronically are important future consideration for improvement of the overall outcomes for a wide range of caregivers, veterans, and health care providers. Health care policy changes on the organizational level, systems level, and national level could further support caregivers of disabled veterans by enabling easy access to caregiver resources as a mandated practice.
Limitations
Limitations centered on the recruitment process. There were a total of 15 caregivers who participated. Although the participation did not meet the PC’s expectation, the final number of participants was adequate in obtaining data regarding evaluating the impact of this project.
Conclusion
The results of this project provided evidence that the Caregiver Resource Manual was effective. Caregivers gained a sense of knowledge and empowerment regarding available resources within the VA and the community. Providing the caregiver with peace of mind and improving the overall health and well-being of the caregiver and veteran were essential.
Moreover, just as the veterans who fought for freedom were equipped with full body armor to help protect them from the potential negative consequences of combat, caregivers who care for these brave soldiers are now equipped with a resource tool and a “full armor of knowledge” to care for their loved ones…our nation’s heroes…our veterans.
1. VA Health Care. Actions needed to address higher-than-expected demand for the family caregiver program. http://www.gao.gov/assets/670/665928.pdf. Published September 18, 2014. Accessed January 9, 2017.
2. Schulz R, Sherwood PR. Physical and mental health effects of family caregiving. Am J Nurs. 2008;108(suppl 9):23-27.
3. Centers for Disease Control; Kimberly-Clark Corporation. Assuring healthy caregivers, a public health approach to translating research into practice: the RE-AIM framework. https://www.cdc.gov /aging/pdf/caregiving_monograph.pdf. Published 2008. Accessed January 9, 2017.
4. National Alliance for Caregiving. Caregivers of veterans—serving on the home front report of study findings. http://www.caregiving.org/data/2010 _Caregivers_of_Veterans_FULLREPORT_WEB _FINAL.pdf. Published 2010. Accessed January 9, 2017.
5. Whittier S, Coon D, Aaker J. Caregiving support interventions. http://cssr.berkeley.edu/pdfs/famcare_04.pdf. Updated 2001. Accessed January 9, 2017.
6. Alzheimer’s Association. Respite care. http://www .alz.org/care/alzheimers-dementia-caregiver-respite.asp. Updated 2016. Accessed January 9, 2017.
7. Chen YM, Hedrick SC, Young HM. A pilot evaluation of the family caregiver support program. Eval Program Plann. 2010;33(2):113-119.
1. VA Health Care. Actions needed to address higher-than-expected demand for the family caregiver program. http://www.gao.gov/assets/670/665928.pdf. Published September 18, 2014. Accessed January 9, 2017.
2. Schulz R, Sherwood PR. Physical and mental health effects of family caregiving. Am J Nurs. 2008;108(suppl 9):23-27.
3. Centers for Disease Control; Kimberly-Clark Corporation. Assuring healthy caregivers, a public health approach to translating research into practice: the RE-AIM framework. https://www.cdc.gov /aging/pdf/caregiving_monograph.pdf. Published 2008. Accessed January 9, 2017.
4. National Alliance for Caregiving. Caregivers of veterans—serving on the home front report of study findings. http://www.caregiving.org/data/2010 _Caregivers_of_Veterans_FULLREPORT_WEB _FINAL.pdf. Published 2010. Accessed January 9, 2017.
5. Whittier S, Coon D, Aaker J. Caregiving support interventions. http://cssr.berkeley.edu/pdfs/famcare_04.pdf. Updated 2001. Accessed January 9, 2017.
6. Alzheimer’s Association. Respite care. http://www .alz.org/care/alzheimers-dementia-caregiver-respite.asp. Updated 2016. Accessed January 9, 2017.
7. Chen YM, Hedrick SC, Young HM. A pilot evaluation of the family caregiver support program. Eval Program Plann. 2010;33(2):113-119.
Hospitalization Risk With Benzodiazepine and Opioid Use in Veterans With Posttraumatic Stress Disorder (FULL)
Posttraumatic stress disorder (PTSD) is a mental health condition that may develop in response to a traumatic event, such as that experienced by a soldier during active combat duty. In 2009, more than 495,000 veterans within the VA health care system were treated for PTSD—nearly triple the number a decade earlier.1 Core symptoms of PTSD include alterations in arousal and reactivity, avoidant behaviors, negative alterations in mood and cognition, and intrusive thoughts and nightmares. All of the symptoms can be debilitating. First-line pharmacotherapy options that target these core symptoms include selective serotonin reuptake inhibitors (SSRIs) and serotonin norepinephrine reuptake inhibitors (SNRIs).2
The anxiolytic and sedative effects of benzodiazepines may provide quick relief from many of the secondary symptoms of PTSD, including sleep disturbances, irritability, and panic attacks. However, benzodiazepines potentially interfere with the extinction of conditioned fear—a goal integral to certain types of psychotherapy, such as exposure therapy.3,4 In addition, the systematic review and meta-analysis by Guina and colleagues revealed that benzodiazepines are ineffective in the treatment of PTSD.5 The majority of the evaluated studies that used PTSD-specific measures (eg, Clinician-Administered PTSD Scale [CAPS]) found increased PTSD severity and worse prognosis with use of these medications.5 In 2010, the VA and the DoD released a joint guideline for PTSD management.2 According to the guideline, benzodiazepines cause harm when used in PTSD and are relatively contraindicated in combat veterans because of the higher incidence of comorbid substance use disorders (SUDs) in these veterans relative to the general population.2,6
Opioid use also has been linked to poor functional and clinical outcomes in veterans with PTSD. Among patients being treated for opioid use disorder, those with PTSD were less likely to endorse employment as a main source of income and had a higher incidence of recent attempted suicide.7 In a large retrospective cohort study, Operation Iraqi Freedom and Operation Enduring Freedom veterans with PTSD who were prescribed opioids were more likely to present to the emergency department (ED) and to be hospitalized for overdoses and injuries.8
Despite the risks of benzodiazepine and opioid use in this patient population, these medications are still often prescribed to veterans with PTSD for symptomatic relief. In fiscal year 2009, across the VHA system 37% of veterans diagnosed with PTSD were prescribed a benzodiazepine, 69% of the time by a mental health provider.9 Among Iraq and Afghanistan veterans, those with PTSD were significantly more likely to be prescribed an opioid for diagnosed pain—relative to those with a mental health disorder other than PTSD and those without a mental health disorder.8 Thus, there seems to be a disconnect between guideline recommendations and current practice.
The authors conducted a study to assess the potential risk of hospitalization for veterans with PTSD prescribed first-line pharmacotherapy and those also prescribed concurrent benzodiazepine and/or opioid therapy since the release of the PTSD guideline in 2010.2
Methods
In this retrospective cohort study, conducted at the Southern Arizona VA Health Care System (SAVAHCS), the authors analyzed electronic medical record data from November 1, 2009 to August 1, 2015. Study inclusion criteria were veteran, aged 18 to 89 years, diagnosis of PTSD (International Classification of Diseases, Ninth Revision, Clinical Modification code 309.81), and SSRI or SNRI newly prescribed between November 1, 2010 and August 1, 2013.
Any veteran prescribed at least one 30-day or longer supply of any benzodiazepine or opioid within 1 year before the SSRI/SNRI initial prescription date was excluded from the study. Also excluded was any patient treated for PTSD at a facility outside SAVAHCS or whose 2-year evaluation period extended past August 1, 2015.
Study Groups
An outpatient prescription was determined to be the initial SSRI/SNRI prescription for a patient who received less than a 30-day cumulative supply of any SSRI or SNRI within 1 year before that prescription date. Citalopram, desvenlafaxine, duloxetine, escitalopram, fluoxetine, fluvoxamine, levomilnacipran, milnacipran, paroxetine, sertraline, venlafaxine, vilazodone, and vortioxetine were the prespecified SSRI/SNRIs included in the study.
Patients who received at least 1 outpatient prescription for any benzodiazepine (minimum 30-day supply) within 1 year after the initial SSRI/SNRI prescription date were determined to be on concurrent SSRI/SNRI and benzodiazepine therapy. Alprazolam, chlordiazepoxide, clonazepam, clorazepate, diazepam, estazolam, flurazepam, lorazepam, oxazepam, temazepam, and triazolam were the prespecified benzodiazepines included in the study.
Patients who received at least 1 outpatient prescription for any opioid (minimum 30-day supply) within 1 year after the initial SSRI/SNRI prescription date were determined to be on concurrent SSRI/SNRI and opioid therapy. Codeine, fentanyl, hydrocodone, hydromorphone, levorphanol, meperidine, methadone, morphine, oxymorphone, pentazocine, propoxyphene, and tramadol were the prespecified opioids included in this study.
Patients who received at least 1 outpatient prescription for any benzodiazepine and any opioid (minimum 30-day supply) within 1 year after the initial SSRI/SNRI prescription date were determined to be on concurrent SSRI/SNRI, benzodiazepine, and opioid therapy.
The index date was defined as the first date of prescription overlap. If there was no benzodiazepine or opioid prescription within 1 year after the initial SSRI/SNRI prescription date, the patient was categorized as being on SSRI/SNRI monotherapy, and the index date was the date of the initial SSRI/SNRI prescription. For each patient, hospitalization data from the 2-year period after the index date were evaluated.
Outcomes and Data Collection
For evaluation of the primary outcome (2-year overall hospitalization risk), the number of unique mental health and medical/surgical hospitalizations was identified by the number of discharge summaries documented in the patient chart during the evaluation period. Time to first hospitalization was recorded for the survival data analysis. Secondary outcomes were mental health hospitalization risk, medical/surgical hospitalization risk, and all-cause mortality within 2 years.
Demographic data that were collected included age, sex, comorbid mental health disorders, comorbid SUDs, and concomitant use of psychotropic medications at index date (baseline). Select comorbid mental health disorders (anxiety, schizophrenia, depression, bipolar disorder) and substance use disorders (alcohol, opioid, illicit drug) also were identified. Data on insomnia and pain comorbidities (headaches or migraines; neuropathy; head, neck, back, arthritis, or joint pain) were collected, as these comorbidities could be indications for prescribing benzodiazepines and opioids. Concomitant baseline use of classes of psychotropic medications (antipsychotics, non-SSRI/SNRI antidepressants, mood stabilizers, anxiolytics, nonbenzodiazepine sedatives/hypnotics) also were documented. Last, hospitalizations within 6 months before the initial SSRI/SNRI prescription date were noted.
Statistical Analysis
Descriptive statistics were used to analyze all baseline demographic data. Continuous measures were evaluated with 1-way analyses of variance and post hoc Bonferroni-corrected pairwise comparisons, and categorical measures with contingency tables and χ2 tests or Fisher exact tests. When the overall χ2 test was significant across all 4 study groups, post hoc comparisons were performed between the SSRI/SNRI monotherapy group and each other group with Bonferroni adjusted for 3 comparisons.
Unadjusted and adjusted Weibull proportional hazard regression models were used to estimate hospitalization risk within 2 years after the index date for the different study groups with the SSRI/SNRI monotherapy group as the referent. Robust standard errors were used to estimate hazard ratios (HRs) and 95% confidence intervals (CIs). The Weibull model (and not the Cox model) was used because it does not assume hazard remains constant over time, which is appropriate in this instance, as the risk of an adverse event (AE) may be higher when first starting a medication or combination of medications relative to when doses are stabilized. Models were adjusted for age, sex, baseline mental health disorders, and baseline psychotropic medications. As earlier hospitalizations showed evidence of effect modification when this covariate was tested, hazard analyses were limited to patients not previously hospitalized.
The effect size of differences in hospitalization risk meeting statistical significance was assessed by estimating the number needed to harm (NNH) and 95% CIs (not shown) to observe 1 additional hospitalization in each medication group relative to the SSRI/SNRI monotherapy group over a 90-day period. A 95% CI for NNH that did not include 0 indicated the NNH was significant at the .05 level.10 All-cause mortality was evaluated with the Fisher exact test with post hoc Bonferroni-corrected comparisons as appropriate.
Results
Of 1,703 patients screened, 613 met all study inclusion criteria (Figure 1).
Baseline characteristics revealed no significant differences between groups in age or comorbid depression, schizophrenia, or SUDs (Table 1).
With the SSRI/SNRI monotherapy group as the referent, all concurrent therapy groups were at significantly increased risk for overall hospitalization within 2 years after the index date (Tables 2 & 3, Figure 2).
Risk for mental health hospitalization was significantly increased in all concurrent therapy groups relative to the referent group.
Although the risk for medical/surgical hospitalization was not significantly increased in the SSRI/SNRI and benzodiazepine therapy group (AHR, 1.9; 95% CI, 0.67-5.6), a significant difference was found in the SSRI/SNRI and opioid therapy group (AHR, 4.4; 95% CI, 1.6-12.0; NNH, 42).
Discussion
In 2013, Hawkins and colleagues evaluated hospitalization risk in veterans treated for PTSD within the Northwest VISN 20 between 2004 and 2010.11 Compared with patients treated with only an SSRI or SNRI, those treated with 1 of those medications and a benzodiazepine were at significantly higher risk for overall hospitalization (AHR, 1.79; 95% CI, 1.38-2.32; P < .001) and mental health hospitalization (AHR, 1.87; 95% CI, 1.37-2.53; P < .001). Furthermore, those prescribed a benzodiazepine and an opioid along with an SSRI or SNRI were at higher risk for overall hospitalization (AHR, 2.98; 95% CI, 2.22-4.00; P < .001), mental health hospitalization (AHR, 2.00; 95% CI, 1.35-2.98; P < .01), medical/surgical hospitalization (AHR, 4.86; 95% CI, 3.30-7.14; P < .001), and ED visits (AHR, 2.01; 95% CI, 1.53-2.65; P < .001).
Findings from the present study, which covered a period after the newest PTSD guideline was released,support findings reported by Hawkins and colleagues in their retrospective cohort study covering an earlier period.2,11 In the present study, compared with the monotherapy group, the SSRI/SNRI and benzodiazepine therapy group and the SSRI/SNRI, benzodiazepine, and opioid therapy group were at higher risk for both overall hospitalization and mental health hospitalization within 2 years. However, in a subset of PTSD patients prescribed opioids along with first-line pharmacotherapy, this study found that overall, mental health, and medical/surgical hospitalizations were significantly increased as well. Furthermore, this study found 2-year mortality was significantly higher for the SSRI/SNRI, benzodiazepine, and opioid therapy group than for the SSRI/SNRI monotherapy group.
Adjusted hazard ratios were higher in the present study than those in the study by Hawkins and colleagues,but CIs were wider as well.11 These differences may be attributable to the relatively smaller sample size of the present study and may explain why the HR was higher for the SSRI/SNRI and opioid therapy group than for the SSRI/SNRI, benzodiazepine, and opioid therapy group.
Nevertheless, these results support the growing body of evidence establishing the many risks for AEs when benzodiazepines and opioids are prescribed in the setting of PTSD. Unfortunately, it seems that, against clear guideline recommendations and literature findings, these medications still are being prescribed to this vulnerable, high-risk population.
In the last few months of 2013, the VA health care system launched 2 important medication safety initiatives. The Psychotropic Drug Safety Initiative (PDSI) was established as a quality improvement initiative for evidence-based provision of psychotropic medications. One PDSI metric in particular focused on reducing the proportion of veterans with PTSD being treated with benzodiazepines. The Opioid Safety Initiative (OSI) came as a response to a dramatic increase in the number of fatal overdoses related to prescription opioids—an increase linked to an unprecedented jump in opioid use for nonmalignant pain. As the present study’s inclusion cutoff date of August 1, 2013, preceded the debut of both PDSI and OSI, the benzodiazepine and opioid prescription rates reported here might be higher than those currently being found under the 2 initiatives.
Limitations
This study had several limitations that might affect the interpretation or generalizability of findings. Requiring at least a 30-day supply for prescription eligibility was an attempt to focus on chronic use of medications rather than on, for example, onetime supplies of opioids for dental procedures. However, prescription fill history was not assessed. Therefore, patients could have been included in certain study groups even if their SSRI, SNRI, benzodiazepine, or opioid prescription was not refilled. Furthermore, only VA medical records were used; non-VA prescriptions were not captured.
In addition, this study was limited to patients who at bare minimum were prescribed an SSRI or an SNRI. Some patients may have been prescribed a benzodiazepine and/or an opioid but were not on appropriate first-line pharmacotherapy for PTSD. These patients were excluded from the study, and their relative hospitalization risk went unexplored. Therefore, the magnitude of the issue at hand might have been underestimated.
Although psychotherapy is a first-line treatment option for PTSD, the study did not assess the potential impact of psychotherapy on outcomes or the groups’ relative proportions of patients undergoing psychotherapy. It is unknown whether the groups were equivalent at baseline in regards to psychotherapy participation rates.
This study did not characterize the specific reasons for hospitalization beyond whether it was for a mental health or a medical/surgical issue; thus, no distinction was made between hospitalizations for an elective procedure and hospitalizations for a drug overdose or an injury. Investigators could characterize admission diagnoses to better assess whether hospitalizations are truly associated with study medications or whether patients are being hospitalized for unrelated reasons. In addition, they could elucidate the true nature of hospitalization risk associated with SSRI/SNRI, benzodiazepine, and opioid use by comparing admission diagnoses made before and after initiation of these pharmacologic therapies.
This study also could not assess outcomes for patients who presented to the ED but were not admitted. If the hospital’s floor and ED beds were at full capacity, some patients might have been transferred to an outside facility. However, this scenario is not common at SAVAHCS, where the study was conducted.
Although some comorbid conditions were noted, the study did not evaluate whether its patients had a compelling indication for benzodiazepines in particular. Opioid use is very limited to the treatment of pain, and the majority of the patients on opioid therapy in this study had a diagnosed pain syndrome.
Because of the study’s sample size and power limitations, patients were eligible to be included in a concurrent therapy group if a benzodiazepine, an opioid, or both were added no later than 1 year after SSRI/SNRI initiation. This gap of up to 1 year might have introduced some variability in exposure to risk from earlier prescribed medications. However, sensitivity analyses were performed with multiple constructed Weibull models of time to hospitalization based on subsets with varying overlapping medication gaps. Analyses revealed relatively stable HRs, suggesting that potential bias did not occur.
Future Directions
Investigators could explore the higher all-cause mortality rates in the SSRI/SNRI, benzodiazepine, and opioid therapy group, as this study did not assess cause of death in these patients. Whether any patients died of reasons directly attributable to benzodiazepines or opioids is unknown.
That SSRIs and SNRIs are the only established first-line pharmacologic treatment options for PTSD symptoms partly accounts for the widespread use of benzodiazepines in this population. For that reason, beyond characterizing the many risks associated with using benzodiazepines to manage these symptoms, there is a huge need to research the viability of other pharmacologic agents in treating PTSD. This is especially important given the slower onset to efficacy of the SSRIs and SNRIs; per estimates, only up to 60% of patients respond to SSRIs, and 20% to 30% achieve full remission of PTSD.12 Furthermore, these rates likely are even lower for combat veterans than those for the general population. Several trials discussed in a 2009 guideline review of the treatment of patients with acute stress disorder and PTSD have called into question the efficacy of SSRIs for combat-related PTSD.13 In these randomized, controlled trials, change in PTSD symptom severity as measured with CAPS was not significantly reduced with SSRIs compared with placebo.
A systematic review revealed that, of the nonantidepressants used as adjuncts in treating patients who do not achieve remission with SSRIs, the atypical antipsychotic risperidone may have the strongest supporting evidence.12 However, the present study found high rates of antipsychotic use in the SSRI/SNRI, benzodiazepine, and opioid therapy group, which also had the highest all-cause mortality rate. The safety of risperidone as an alternative treatment needs further evaluation.
Some prospective studies have suggested that the α1 blockers doxazosin and prazosin, the latter of which is commonly used for PTSD nightmares, also may improve PTSD symptoms as assessed by CAPS.14,15 Although these results are promising, the trials to date have been conducted with relatively small sample sizes.
With more veterans being treated for PTSD within the VA health care system, the central treatment goal remains: Adequately address the symptoms of PTSD while minimizing the harm caused by medications. Prescribers should limit benzodiazepine and opioid use in this population and consider safer nonpharmacologic and pharmacologic treatment options when possible.
Conclusion
Combat veterans with PTSD who are prescribed benzodiazepines and/or opioids in addition to first-line pharmacotherapy are at significantly increased risk for overall and mental health hospitalization.
Click here to read the digital edition.
1. Bernardy NC, Lund BC, Alexander B, Jenkyn AB, Schnurr PP, Friedman MJ. Gender differences in prescribing among veterans diagnosed with posttraumatic stress disorder. J Gen Intern Med. 2013;28(suppl 2):S542-S548.
2. Management of Post-Traumatic Stress Working Group, Department of Veterans Affairs, Department of Defense. VA/DoD Clinical Practice Guideline for Management of Post-Traumatic Stress. http://www.healthquality.va.gov/PTSD-full-2010c .pdf. Published October 2010. Accessed July 12, 2015.
3. Marks IM, Swinson RP, Baso˘glu M, et al. Alprazolam and exposure alone and combined in panic disorder with agoraphobia. A controlled study in London and Toronto. Br J Psychiatry. 1993;162:776-787.
4. Wilhelm FH, Roth WT. Acute and delayed effects of alprazolam on flight phobics during exposure. Behav Res Ther. 1997;35(9):831-841.
5. Guina J, Rossetter SR, DeRhodes BJ, Nahhas RW, Welton RS. Benzodiazepines for PTSD: a systematic review and meta-analysis. J Psychiatr Pract. 2015;21(4):281-303.
6. Pietrzak RH, Goldstein RB, Southwick SM, Grant BF. Prevalence and Axis I comorbidity of full and partial posttraumatic stress disorder in the United States: results from wave 2 of the National Epidemiologic Survey on Alcohol and Related Conditions. J Anxiety Disord. 2011;25(3):456-465.
7. Mills KL, Teesson M, Ross J, Darke S, Shanahan M. The costs and outcomes of treatment for opioid dependence associated with posttraumatic stress disorder. Psychiatr Serv. 2005;56(8):940-945.
8. Seal KH, Shi Y, Cohen G, et al. Association of mental health disorders with prescription opioids and high-risk opioid use in US veterans of Iraq and Afghanistan. JAMA. 2012;307(9):940-947.
9. Abrams TE, Lund BC, Bernardy NC, Friedman MJ. Aligning clinical practice to PTSD treatment guidelines: medication prescribing by provider type. Psychiatr Serv. 2013;64(2):142-148.
10. Altman DG, Andersen PK. Calculating the number needed to treat for trials where the outcome is time to an event. BMJ. 1999;319(7223):1492-1495.
11. Hawkins EJ, Malte CA, Grossbard J, Saxon AJ, Imel ZE, Kivlahan DR. Comparative safety of benzodiazepines and opioids among Veterans Affairs patients with posttraumatic stress disorder. J Addict Med. 2013;7(5):354-362.
12. Berger W, Mendlowicz MV, Marques-Portella C, et al. Pharmacologic alternatives to antidepressants in posttraumatic stress disorder: a systematic review. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33(2):169-180.
13. Benedek DM, Friedman MJ, Zatzick D, Ursano RJ. Guideline watch (March 2009): practice guideline for the treatment of patients with acute stress disorder and posttraumatic stress disorder. Focus. 2009;7(2):204-213.
14. Raskind MA, Peterson K, Williams T, et al. A trial of prazosin for combat trauma PTSD with nightmares in active-duty soldiers returned from Iraq and Afghanistan. Am J Psychiatry. 2013;170(9):1003-1010.
15. Rodgman C, Verrico CD, Holst M, et al. Doxazosin XL reduces symptoms of posttraumatic stress disorder in veterans with PTSD: a pilot clinical trial. J Clin Psychiatry. 2016;77(5):e561-e565.
Posttraumatic stress disorder (PTSD) is a mental health condition that may develop in response to a traumatic event, such as that experienced by a soldier during active combat duty. In 2009, more than 495,000 veterans within the VA health care system were treated for PTSD—nearly triple the number a decade earlier.1 Core symptoms of PTSD include alterations in arousal and reactivity, avoidant behaviors, negative alterations in mood and cognition, and intrusive thoughts and nightmares. All of the symptoms can be debilitating. First-line pharmacotherapy options that target these core symptoms include selective serotonin reuptake inhibitors (SSRIs) and serotonin norepinephrine reuptake inhibitors (SNRIs).2
The anxiolytic and sedative effects of benzodiazepines may provide quick relief from many of the secondary symptoms of PTSD, including sleep disturbances, irritability, and panic attacks. However, benzodiazepines potentially interfere with the extinction of conditioned fear—a goal integral to certain types of psychotherapy, such as exposure therapy.3,4 In addition, the systematic review and meta-analysis by Guina and colleagues revealed that benzodiazepines are ineffective in the treatment of PTSD.5 The majority of the evaluated studies that used PTSD-specific measures (eg, Clinician-Administered PTSD Scale [CAPS]) found increased PTSD severity and worse prognosis with use of these medications.5 In 2010, the VA and the DoD released a joint guideline for PTSD management.2 According to the guideline, benzodiazepines cause harm when used in PTSD and are relatively contraindicated in combat veterans because of the higher incidence of comorbid substance use disorders (SUDs) in these veterans relative to the general population.2,6
Opioid use also has been linked to poor functional and clinical outcomes in veterans with PTSD. Among patients being treated for opioid use disorder, those with PTSD were less likely to endorse employment as a main source of income and had a higher incidence of recent attempted suicide.7 In a large retrospective cohort study, Operation Iraqi Freedom and Operation Enduring Freedom veterans with PTSD who were prescribed opioids were more likely to present to the emergency department (ED) and to be hospitalized for overdoses and injuries.8
Despite the risks of benzodiazepine and opioid use in this patient population, these medications are still often prescribed to veterans with PTSD for symptomatic relief. In fiscal year 2009, across the VHA system 37% of veterans diagnosed with PTSD were prescribed a benzodiazepine, 69% of the time by a mental health provider.9 Among Iraq and Afghanistan veterans, those with PTSD were significantly more likely to be prescribed an opioid for diagnosed pain—relative to those with a mental health disorder other than PTSD and those without a mental health disorder.8 Thus, there seems to be a disconnect between guideline recommendations and current practice.
The authors conducted a study to assess the potential risk of hospitalization for veterans with PTSD prescribed first-line pharmacotherapy and those also prescribed concurrent benzodiazepine and/or opioid therapy since the release of the PTSD guideline in 2010.2
Methods
In this retrospective cohort study, conducted at the Southern Arizona VA Health Care System (SAVAHCS), the authors analyzed electronic medical record data from November 1, 2009 to August 1, 2015. Study inclusion criteria were veteran, aged 18 to 89 years, diagnosis of PTSD (International Classification of Diseases, Ninth Revision, Clinical Modification code 309.81), and SSRI or SNRI newly prescribed between November 1, 2010 and August 1, 2013.
Any veteran prescribed at least one 30-day or longer supply of any benzodiazepine or opioid within 1 year before the SSRI/SNRI initial prescription date was excluded from the study. Also excluded was any patient treated for PTSD at a facility outside SAVAHCS or whose 2-year evaluation period extended past August 1, 2015.
Study Groups
An outpatient prescription was determined to be the initial SSRI/SNRI prescription for a patient who received less than a 30-day cumulative supply of any SSRI or SNRI within 1 year before that prescription date. Citalopram, desvenlafaxine, duloxetine, escitalopram, fluoxetine, fluvoxamine, levomilnacipran, milnacipran, paroxetine, sertraline, venlafaxine, vilazodone, and vortioxetine were the prespecified SSRI/SNRIs included in the study.
Patients who received at least 1 outpatient prescription for any benzodiazepine (minimum 30-day supply) within 1 year after the initial SSRI/SNRI prescription date were determined to be on concurrent SSRI/SNRI and benzodiazepine therapy. Alprazolam, chlordiazepoxide, clonazepam, clorazepate, diazepam, estazolam, flurazepam, lorazepam, oxazepam, temazepam, and triazolam were the prespecified benzodiazepines included in the study.
Patients who received at least 1 outpatient prescription for any opioid (minimum 30-day supply) within 1 year after the initial SSRI/SNRI prescription date were determined to be on concurrent SSRI/SNRI and opioid therapy. Codeine, fentanyl, hydrocodone, hydromorphone, levorphanol, meperidine, methadone, morphine, oxymorphone, pentazocine, propoxyphene, and tramadol were the prespecified opioids included in this study.
Patients who received at least 1 outpatient prescription for any benzodiazepine and any opioid (minimum 30-day supply) within 1 year after the initial SSRI/SNRI prescription date were determined to be on concurrent SSRI/SNRI, benzodiazepine, and opioid therapy.
The index date was defined as the first date of prescription overlap. If there was no benzodiazepine or opioid prescription within 1 year after the initial SSRI/SNRI prescription date, the patient was categorized as being on SSRI/SNRI monotherapy, and the index date was the date of the initial SSRI/SNRI prescription. For each patient, hospitalization data from the 2-year period after the index date were evaluated.
Outcomes and Data Collection
For evaluation of the primary outcome (2-year overall hospitalization risk), the number of unique mental health and medical/surgical hospitalizations was identified by the number of discharge summaries documented in the patient chart during the evaluation period. Time to first hospitalization was recorded for the survival data analysis. Secondary outcomes were mental health hospitalization risk, medical/surgical hospitalization risk, and all-cause mortality within 2 years.
Demographic data that were collected included age, sex, comorbid mental health disorders, comorbid SUDs, and concomitant use of psychotropic medications at index date (baseline). Select comorbid mental health disorders (anxiety, schizophrenia, depression, bipolar disorder) and substance use disorders (alcohol, opioid, illicit drug) also were identified. Data on insomnia and pain comorbidities (headaches or migraines; neuropathy; head, neck, back, arthritis, or joint pain) were collected, as these comorbidities could be indications for prescribing benzodiazepines and opioids. Concomitant baseline use of classes of psychotropic medications (antipsychotics, non-SSRI/SNRI antidepressants, mood stabilizers, anxiolytics, nonbenzodiazepine sedatives/hypnotics) also were documented. Last, hospitalizations within 6 months before the initial SSRI/SNRI prescription date were noted.
Statistical Analysis
Descriptive statistics were used to analyze all baseline demographic data. Continuous measures were evaluated with 1-way analyses of variance and post hoc Bonferroni-corrected pairwise comparisons, and categorical measures with contingency tables and χ2 tests or Fisher exact tests. When the overall χ2 test was significant across all 4 study groups, post hoc comparisons were performed between the SSRI/SNRI monotherapy group and each other group with Bonferroni adjusted for 3 comparisons.
Unadjusted and adjusted Weibull proportional hazard regression models were used to estimate hospitalization risk within 2 years after the index date for the different study groups with the SSRI/SNRI monotherapy group as the referent. Robust standard errors were used to estimate hazard ratios (HRs) and 95% confidence intervals (CIs). The Weibull model (and not the Cox model) was used because it does not assume hazard remains constant over time, which is appropriate in this instance, as the risk of an adverse event (AE) may be higher when first starting a medication or combination of medications relative to when doses are stabilized. Models were adjusted for age, sex, baseline mental health disorders, and baseline psychotropic medications. As earlier hospitalizations showed evidence of effect modification when this covariate was tested, hazard analyses were limited to patients not previously hospitalized.
The effect size of differences in hospitalization risk meeting statistical significance was assessed by estimating the number needed to harm (NNH) and 95% CIs (not shown) to observe 1 additional hospitalization in each medication group relative to the SSRI/SNRI monotherapy group over a 90-day period. A 95% CI for NNH that did not include 0 indicated the NNH was significant at the .05 level.10 All-cause mortality was evaluated with the Fisher exact test with post hoc Bonferroni-corrected comparisons as appropriate.
Results
Of 1,703 patients screened, 613 met all study inclusion criteria (Figure 1).
Baseline characteristics revealed no significant differences between groups in age or comorbid depression, schizophrenia, or SUDs (Table 1).
With the SSRI/SNRI monotherapy group as the referent, all concurrent therapy groups were at significantly increased risk for overall hospitalization within 2 years after the index date (Tables 2 & 3, Figure 2).
Risk for mental health hospitalization was significantly increased in all concurrent therapy groups relative to the referent group.
Although the risk for medical/surgical hospitalization was not significantly increased in the SSRI/SNRI and benzodiazepine therapy group (AHR, 1.9; 95% CI, 0.67-5.6), a significant difference was found in the SSRI/SNRI and opioid therapy group (AHR, 4.4; 95% CI, 1.6-12.0; NNH, 42).
Discussion
In 2013, Hawkins and colleagues evaluated hospitalization risk in veterans treated for PTSD within the Northwest VISN 20 between 2004 and 2010.11 Compared with patients treated with only an SSRI or SNRI, those treated with 1 of those medications and a benzodiazepine were at significantly higher risk for overall hospitalization (AHR, 1.79; 95% CI, 1.38-2.32; P < .001) and mental health hospitalization (AHR, 1.87; 95% CI, 1.37-2.53; P < .001). Furthermore, those prescribed a benzodiazepine and an opioid along with an SSRI or SNRI were at higher risk for overall hospitalization (AHR, 2.98; 95% CI, 2.22-4.00; P < .001), mental health hospitalization (AHR, 2.00; 95% CI, 1.35-2.98; P < .01), medical/surgical hospitalization (AHR, 4.86; 95% CI, 3.30-7.14; P < .001), and ED visits (AHR, 2.01; 95% CI, 1.53-2.65; P < .001).
Findings from the present study, which covered a period after the newest PTSD guideline was released,support findings reported by Hawkins and colleagues in their retrospective cohort study covering an earlier period.2,11 In the present study, compared with the monotherapy group, the SSRI/SNRI and benzodiazepine therapy group and the SSRI/SNRI, benzodiazepine, and opioid therapy group were at higher risk for both overall hospitalization and mental health hospitalization within 2 years. However, in a subset of PTSD patients prescribed opioids along with first-line pharmacotherapy, this study found that overall, mental health, and medical/surgical hospitalizations were significantly increased as well. Furthermore, this study found 2-year mortality was significantly higher for the SSRI/SNRI, benzodiazepine, and opioid therapy group than for the SSRI/SNRI monotherapy group.
Adjusted hazard ratios were higher in the present study than those in the study by Hawkins and colleagues,but CIs were wider as well.11 These differences may be attributable to the relatively smaller sample size of the present study and may explain why the HR was higher for the SSRI/SNRI and opioid therapy group than for the SSRI/SNRI, benzodiazepine, and opioid therapy group.
Nevertheless, these results support the growing body of evidence establishing the many risks for AEs when benzodiazepines and opioids are prescribed in the setting of PTSD. Unfortunately, it seems that, against clear guideline recommendations and literature findings, these medications still are being prescribed to this vulnerable, high-risk population.
In the last few months of 2013, the VA health care system launched 2 important medication safety initiatives. The Psychotropic Drug Safety Initiative (PDSI) was established as a quality improvement initiative for evidence-based provision of psychotropic medications. One PDSI metric in particular focused on reducing the proportion of veterans with PTSD being treated with benzodiazepines. The Opioid Safety Initiative (OSI) came as a response to a dramatic increase in the number of fatal overdoses related to prescription opioids—an increase linked to an unprecedented jump in opioid use for nonmalignant pain. As the present study’s inclusion cutoff date of August 1, 2013, preceded the debut of both PDSI and OSI, the benzodiazepine and opioid prescription rates reported here might be higher than those currently being found under the 2 initiatives.
Limitations
This study had several limitations that might affect the interpretation or generalizability of findings. Requiring at least a 30-day supply for prescription eligibility was an attempt to focus on chronic use of medications rather than on, for example, onetime supplies of opioids for dental procedures. However, prescription fill history was not assessed. Therefore, patients could have been included in certain study groups even if their SSRI, SNRI, benzodiazepine, or opioid prescription was not refilled. Furthermore, only VA medical records were used; non-VA prescriptions were not captured.
In addition, this study was limited to patients who at bare minimum were prescribed an SSRI or an SNRI. Some patients may have been prescribed a benzodiazepine and/or an opioid but were not on appropriate first-line pharmacotherapy for PTSD. These patients were excluded from the study, and their relative hospitalization risk went unexplored. Therefore, the magnitude of the issue at hand might have been underestimated.
Although psychotherapy is a first-line treatment option for PTSD, the study did not assess the potential impact of psychotherapy on outcomes or the groups’ relative proportions of patients undergoing psychotherapy. It is unknown whether the groups were equivalent at baseline in regards to psychotherapy participation rates.
This study did not characterize the specific reasons for hospitalization beyond whether it was for a mental health or a medical/surgical issue; thus, no distinction was made between hospitalizations for an elective procedure and hospitalizations for a drug overdose or an injury. Investigators could characterize admission diagnoses to better assess whether hospitalizations are truly associated with study medications or whether patients are being hospitalized for unrelated reasons. In addition, they could elucidate the true nature of hospitalization risk associated with SSRI/SNRI, benzodiazepine, and opioid use by comparing admission diagnoses made before and after initiation of these pharmacologic therapies.
This study also could not assess outcomes for patients who presented to the ED but were not admitted. If the hospital’s floor and ED beds were at full capacity, some patients might have been transferred to an outside facility. However, this scenario is not common at SAVAHCS, where the study was conducted.
Although some comorbid conditions were noted, the study did not evaluate whether its patients had a compelling indication for benzodiazepines in particular. Opioid use is very limited to the treatment of pain, and the majority of the patients on opioid therapy in this study had a diagnosed pain syndrome.
Because of the study’s sample size and power limitations, patients were eligible to be included in a concurrent therapy group if a benzodiazepine, an opioid, or both were added no later than 1 year after SSRI/SNRI initiation. This gap of up to 1 year might have introduced some variability in exposure to risk from earlier prescribed medications. However, sensitivity analyses were performed with multiple constructed Weibull models of time to hospitalization based on subsets with varying overlapping medication gaps. Analyses revealed relatively stable HRs, suggesting that potential bias did not occur.
Future Directions
Investigators could explore the higher all-cause mortality rates in the SSRI/SNRI, benzodiazepine, and opioid therapy group, as this study did not assess cause of death in these patients. Whether any patients died of reasons directly attributable to benzodiazepines or opioids is unknown.
That SSRIs and SNRIs are the only established first-line pharmacologic treatment options for PTSD symptoms partly accounts for the widespread use of benzodiazepines in this population. For that reason, beyond characterizing the many risks associated with using benzodiazepines to manage these symptoms, there is a huge need to research the viability of other pharmacologic agents in treating PTSD. This is especially important given the slower onset to efficacy of the SSRIs and SNRIs; per estimates, only up to 60% of patients respond to SSRIs, and 20% to 30% achieve full remission of PTSD.12 Furthermore, these rates likely are even lower for combat veterans than those for the general population. Several trials discussed in a 2009 guideline review of the treatment of patients with acute stress disorder and PTSD have called into question the efficacy of SSRIs for combat-related PTSD.13 In these randomized, controlled trials, change in PTSD symptom severity as measured with CAPS was not significantly reduced with SSRIs compared with placebo.
A systematic review revealed that, of the nonantidepressants used as adjuncts in treating patients who do not achieve remission with SSRIs, the atypical antipsychotic risperidone may have the strongest supporting evidence.12 However, the present study found high rates of antipsychotic use in the SSRI/SNRI, benzodiazepine, and opioid therapy group, which also had the highest all-cause mortality rate. The safety of risperidone as an alternative treatment needs further evaluation.
Some prospective studies have suggested that the α1 blockers doxazosin and prazosin, the latter of which is commonly used for PTSD nightmares, also may improve PTSD symptoms as assessed by CAPS.14,15 Although these results are promising, the trials to date have been conducted with relatively small sample sizes.
With more veterans being treated for PTSD within the VA health care system, the central treatment goal remains: Adequately address the symptoms of PTSD while minimizing the harm caused by medications. Prescribers should limit benzodiazepine and opioid use in this population and consider safer nonpharmacologic and pharmacologic treatment options when possible.
Conclusion
Combat veterans with PTSD who are prescribed benzodiazepines and/or opioids in addition to first-line pharmacotherapy are at significantly increased risk for overall and mental health hospitalization.
Click here to read the digital edition.
Posttraumatic stress disorder (PTSD) is a mental health condition that may develop in response to a traumatic event, such as that experienced by a soldier during active combat duty. In 2009, more than 495,000 veterans within the VA health care system were treated for PTSD—nearly triple the number a decade earlier.1 Core symptoms of PTSD include alterations in arousal and reactivity, avoidant behaviors, negative alterations in mood and cognition, and intrusive thoughts and nightmares. All of the symptoms can be debilitating. First-line pharmacotherapy options that target these core symptoms include selective serotonin reuptake inhibitors (SSRIs) and serotonin norepinephrine reuptake inhibitors (SNRIs).2
The anxiolytic and sedative effects of benzodiazepines may provide quick relief from many of the secondary symptoms of PTSD, including sleep disturbances, irritability, and panic attacks. However, benzodiazepines potentially interfere with the extinction of conditioned fear—a goal integral to certain types of psychotherapy, such as exposure therapy.3,4 In addition, the systematic review and meta-analysis by Guina and colleagues revealed that benzodiazepines are ineffective in the treatment of PTSD.5 The majority of the evaluated studies that used PTSD-specific measures (eg, Clinician-Administered PTSD Scale [CAPS]) found increased PTSD severity and worse prognosis with use of these medications.5 In 2010, the VA and the DoD released a joint guideline for PTSD management.2 According to the guideline, benzodiazepines cause harm when used in PTSD and are relatively contraindicated in combat veterans because of the higher incidence of comorbid substance use disorders (SUDs) in these veterans relative to the general population.2,6
Opioid use also has been linked to poor functional and clinical outcomes in veterans with PTSD. Among patients being treated for opioid use disorder, those with PTSD were less likely to endorse employment as a main source of income and had a higher incidence of recent attempted suicide.7 In a large retrospective cohort study, Operation Iraqi Freedom and Operation Enduring Freedom veterans with PTSD who were prescribed opioids were more likely to present to the emergency department (ED) and to be hospitalized for overdoses and injuries.8
Despite the risks of benzodiazepine and opioid use in this patient population, these medications are still often prescribed to veterans with PTSD for symptomatic relief. In fiscal year 2009, across the VHA system 37% of veterans diagnosed with PTSD were prescribed a benzodiazepine, 69% of the time by a mental health provider.9 Among Iraq and Afghanistan veterans, those with PTSD were significantly more likely to be prescribed an opioid for diagnosed pain—relative to those with a mental health disorder other than PTSD and those without a mental health disorder.8 Thus, there seems to be a disconnect between guideline recommendations and current practice.
The authors conducted a study to assess the potential risk of hospitalization for veterans with PTSD prescribed first-line pharmacotherapy and those also prescribed concurrent benzodiazepine and/or opioid therapy since the release of the PTSD guideline in 2010.2
Methods
In this retrospective cohort study, conducted at the Southern Arizona VA Health Care System (SAVAHCS), the authors analyzed electronic medical record data from November 1, 2009 to August 1, 2015. Study inclusion criteria were veteran, aged 18 to 89 years, diagnosis of PTSD (International Classification of Diseases, Ninth Revision, Clinical Modification code 309.81), and SSRI or SNRI newly prescribed between November 1, 2010 and August 1, 2013.
Any veteran prescribed at least one 30-day or longer supply of any benzodiazepine or opioid within 1 year before the SSRI/SNRI initial prescription date was excluded from the study. Also excluded was any patient treated for PTSD at a facility outside SAVAHCS or whose 2-year evaluation period extended past August 1, 2015.
Study Groups
An outpatient prescription was determined to be the initial SSRI/SNRI prescription for a patient who received less than a 30-day cumulative supply of any SSRI or SNRI within 1 year before that prescription date. Citalopram, desvenlafaxine, duloxetine, escitalopram, fluoxetine, fluvoxamine, levomilnacipran, milnacipran, paroxetine, sertraline, venlafaxine, vilazodone, and vortioxetine were the prespecified SSRI/SNRIs included in the study.
Patients who received at least 1 outpatient prescription for any benzodiazepine (minimum 30-day supply) within 1 year after the initial SSRI/SNRI prescription date were determined to be on concurrent SSRI/SNRI and benzodiazepine therapy. Alprazolam, chlordiazepoxide, clonazepam, clorazepate, diazepam, estazolam, flurazepam, lorazepam, oxazepam, temazepam, and triazolam were the prespecified benzodiazepines included in the study.
Patients who received at least 1 outpatient prescription for any opioid (minimum 30-day supply) within 1 year after the initial SSRI/SNRI prescription date were determined to be on concurrent SSRI/SNRI and opioid therapy. Codeine, fentanyl, hydrocodone, hydromorphone, levorphanol, meperidine, methadone, morphine, oxymorphone, pentazocine, propoxyphene, and tramadol were the prespecified opioids included in this study.
Patients who received at least 1 outpatient prescription for any benzodiazepine and any opioid (minimum 30-day supply) within 1 year after the initial SSRI/SNRI prescription date were determined to be on concurrent SSRI/SNRI, benzodiazepine, and opioid therapy.
The index date was defined as the first date of prescription overlap. If there was no benzodiazepine or opioid prescription within 1 year after the initial SSRI/SNRI prescription date, the patient was categorized as being on SSRI/SNRI monotherapy, and the index date was the date of the initial SSRI/SNRI prescription. For each patient, hospitalization data from the 2-year period after the index date were evaluated.
Outcomes and Data Collection
For evaluation of the primary outcome (2-year overall hospitalization risk), the number of unique mental health and medical/surgical hospitalizations was identified by the number of discharge summaries documented in the patient chart during the evaluation period. Time to first hospitalization was recorded for the survival data analysis. Secondary outcomes were mental health hospitalization risk, medical/surgical hospitalization risk, and all-cause mortality within 2 years.
Demographic data that were collected included age, sex, comorbid mental health disorders, comorbid SUDs, and concomitant use of psychotropic medications at index date (baseline). Select comorbid mental health disorders (anxiety, schizophrenia, depression, bipolar disorder) and substance use disorders (alcohol, opioid, illicit drug) also were identified. Data on insomnia and pain comorbidities (headaches or migraines; neuropathy; head, neck, back, arthritis, or joint pain) were collected, as these comorbidities could be indications for prescribing benzodiazepines and opioids. Concomitant baseline use of classes of psychotropic medications (antipsychotics, non-SSRI/SNRI antidepressants, mood stabilizers, anxiolytics, nonbenzodiazepine sedatives/hypnotics) also were documented. Last, hospitalizations within 6 months before the initial SSRI/SNRI prescription date were noted.
Statistical Analysis
Descriptive statistics were used to analyze all baseline demographic data. Continuous measures were evaluated with 1-way analyses of variance and post hoc Bonferroni-corrected pairwise comparisons, and categorical measures with contingency tables and χ2 tests or Fisher exact tests. When the overall χ2 test was significant across all 4 study groups, post hoc comparisons were performed between the SSRI/SNRI monotherapy group and each other group with Bonferroni adjusted for 3 comparisons.
Unadjusted and adjusted Weibull proportional hazard regression models were used to estimate hospitalization risk within 2 years after the index date for the different study groups with the SSRI/SNRI monotherapy group as the referent. Robust standard errors were used to estimate hazard ratios (HRs) and 95% confidence intervals (CIs). The Weibull model (and not the Cox model) was used because it does not assume hazard remains constant over time, which is appropriate in this instance, as the risk of an adverse event (AE) may be higher when first starting a medication or combination of medications relative to when doses are stabilized. Models were adjusted for age, sex, baseline mental health disorders, and baseline psychotropic medications. As earlier hospitalizations showed evidence of effect modification when this covariate was tested, hazard analyses were limited to patients not previously hospitalized.
The effect size of differences in hospitalization risk meeting statistical significance was assessed by estimating the number needed to harm (NNH) and 95% CIs (not shown) to observe 1 additional hospitalization in each medication group relative to the SSRI/SNRI monotherapy group over a 90-day period. A 95% CI for NNH that did not include 0 indicated the NNH was significant at the .05 level.10 All-cause mortality was evaluated with the Fisher exact test with post hoc Bonferroni-corrected comparisons as appropriate.
Results
Of 1,703 patients screened, 613 met all study inclusion criteria (Figure 1).
Baseline characteristics revealed no significant differences between groups in age or comorbid depression, schizophrenia, or SUDs (Table 1).
With the SSRI/SNRI monotherapy group as the referent, all concurrent therapy groups were at significantly increased risk for overall hospitalization within 2 years after the index date (Tables 2 & 3, Figure 2).
Risk for mental health hospitalization was significantly increased in all concurrent therapy groups relative to the referent group.
Although the risk for medical/surgical hospitalization was not significantly increased in the SSRI/SNRI and benzodiazepine therapy group (AHR, 1.9; 95% CI, 0.67-5.6), a significant difference was found in the SSRI/SNRI and opioid therapy group (AHR, 4.4; 95% CI, 1.6-12.0; NNH, 42).
Discussion
In 2013, Hawkins and colleagues evaluated hospitalization risk in veterans treated for PTSD within the Northwest VISN 20 between 2004 and 2010.11 Compared with patients treated with only an SSRI or SNRI, those treated with 1 of those medications and a benzodiazepine were at significantly higher risk for overall hospitalization (AHR, 1.79; 95% CI, 1.38-2.32; P < .001) and mental health hospitalization (AHR, 1.87; 95% CI, 1.37-2.53; P < .001). Furthermore, those prescribed a benzodiazepine and an opioid along with an SSRI or SNRI were at higher risk for overall hospitalization (AHR, 2.98; 95% CI, 2.22-4.00; P < .001), mental health hospitalization (AHR, 2.00; 95% CI, 1.35-2.98; P < .01), medical/surgical hospitalization (AHR, 4.86; 95% CI, 3.30-7.14; P < .001), and ED visits (AHR, 2.01; 95% CI, 1.53-2.65; P < .001).
Findings from the present study, which covered a period after the newest PTSD guideline was released,support findings reported by Hawkins and colleagues in their retrospective cohort study covering an earlier period.2,11 In the present study, compared with the monotherapy group, the SSRI/SNRI and benzodiazepine therapy group and the SSRI/SNRI, benzodiazepine, and opioid therapy group were at higher risk for both overall hospitalization and mental health hospitalization within 2 years. However, in a subset of PTSD patients prescribed opioids along with first-line pharmacotherapy, this study found that overall, mental health, and medical/surgical hospitalizations were significantly increased as well. Furthermore, this study found 2-year mortality was significantly higher for the SSRI/SNRI, benzodiazepine, and opioid therapy group than for the SSRI/SNRI monotherapy group.
Adjusted hazard ratios were higher in the present study than those in the study by Hawkins and colleagues,but CIs were wider as well.11 These differences may be attributable to the relatively smaller sample size of the present study and may explain why the HR was higher for the SSRI/SNRI and opioid therapy group than for the SSRI/SNRI, benzodiazepine, and opioid therapy group.
Nevertheless, these results support the growing body of evidence establishing the many risks for AEs when benzodiazepines and opioids are prescribed in the setting of PTSD. Unfortunately, it seems that, against clear guideline recommendations and literature findings, these medications still are being prescribed to this vulnerable, high-risk population.
In the last few months of 2013, the VA health care system launched 2 important medication safety initiatives. The Psychotropic Drug Safety Initiative (PDSI) was established as a quality improvement initiative for evidence-based provision of psychotropic medications. One PDSI metric in particular focused on reducing the proportion of veterans with PTSD being treated with benzodiazepines. The Opioid Safety Initiative (OSI) came as a response to a dramatic increase in the number of fatal overdoses related to prescription opioids—an increase linked to an unprecedented jump in opioid use for nonmalignant pain. As the present study’s inclusion cutoff date of August 1, 2013, preceded the debut of both PDSI and OSI, the benzodiazepine and opioid prescription rates reported here might be higher than those currently being found under the 2 initiatives.
Limitations
This study had several limitations that might affect the interpretation or generalizability of findings. Requiring at least a 30-day supply for prescription eligibility was an attempt to focus on chronic use of medications rather than on, for example, onetime supplies of opioids for dental procedures. However, prescription fill history was not assessed. Therefore, patients could have been included in certain study groups even if their SSRI, SNRI, benzodiazepine, or opioid prescription was not refilled. Furthermore, only VA medical records were used; non-VA prescriptions were not captured.
In addition, this study was limited to patients who at bare minimum were prescribed an SSRI or an SNRI. Some patients may have been prescribed a benzodiazepine and/or an opioid but were not on appropriate first-line pharmacotherapy for PTSD. These patients were excluded from the study, and their relative hospitalization risk went unexplored. Therefore, the magnitude of the issue at hand might have been underestimated.
Although psychotherapy is a first-line treatment option for PTSD, the study did not assess the potential impact of psychotherapy on outcomes or the groups’ relative proportions of patients undergoing psychotherapy. It is unknown whether the groups were equivalent at baseline in regards to psychotherapy participation rates.
This study did not characterize the specific reasons for hospitalization beyond whether it was for a mental health or a medical/surgical issue; thus, no distinction was made between hospitalizations for an elective procedure and hospitalizations for a drug overdose or an injury. Investigators could characterize admission diagnoses to better assess whether hospitalizations are truly associated with study medications or whether patients are being hospitalized for unrelated reasons. In addition, they could elucidate the true nature of hospitalization risk associated with SSRI/SNRI, benzodiazepine, and opioid use by comparing admission diagnoses made before and after initiation of these pharmacologic therapies.
This study also could not assess outcomes for patients who presented to the ED but were not admitted. If the hospital’s floor and ED beds were at full capacity, some patients might have been transferred to an outside facility. However, this scenario is not common at SAVAHCS, where the study was conducted.
Although some comorbid conditions were noted, the study did not evaluate whether its patients had a compelling indication for benzodiazepines in particular. Opioid use is very limited to the treatment of pain, and the majority of the patients on opioid therapy in this study had a diagnosed pain syndrome.
Because of the study’s sample size and power limitations, patients were eligible to be included in a concurrent therapy group if a benzodiazepine, an opioid, or both were added no later than 1 year after SSRI/SNRI initiation. This gap of up to 1 year might have introduced some variability in exposure to risk from earlier prescribed medications. However, sensitivity analyses were performed with multiple constructed Weibull models of time to hospitalization based on subsets with varying overlapping medication gaps. Analyses revealed relatively stable HRs, suggesting that potential bias did not occur.
Future Directions
Investigators could explore the higher all-cause mortality rates in the SSRI/SNRI, benzodiazepine, and opioid therapy group, as this study did not assess cause of death in these patients. Whether any patients died of reasons directly attributable to benzodiazepines or opioids is unknown.
That SSRIs and SNRIs are the only established first-line pharmacologic treatment options for PTSD symptoms partly accounts for the widespread use of benzodiazepines in this population. For that reason, beyond characterizing the many risks associated with using benzodiazepines to manage these symptoms, there is a huge need to research the viability of other pharmacologic agents in treating PTSD. This is especially important given the slower onset to efficacy of the SSRIs and SNRIs; per estimates, only up to 60% of patients respond to SSRIs, and 20% to 30% achieve full remission of PTSD.12 Furthermore, these rates likely are even lower for combat veterans than those for the general population. Several trials discussed in a 2009 guideline review of the treatment of patients with acute stress disorder and PTSD have called into question the efficacy of SSRIs for combat-related PTSD.13 In these randomized, controlled trials, change in PTSD symptom severity as measured with CAPS was not significantly reduced with SSRIs compared with placebo.
A systematic review revealed that, of the nonantidepressants used as adjuncts in treating patients who do not achieve remission with SSRIs, the atypical antipsychotic risperidone may have the strongest supporting evidence.12 However, the present study found high rates of antipsychotic use in the SSRI/SNRI, benzodiazepine, and opioid therapy group, which also had the highest all-cause mortality rate. The safety of risperidone as an alternative treatment needs further evaluation.
Some prospective studies have suggested that the α1 blockers doxazosin and prazosin, the latter of which is commonly used for PTSD nightmares, also may improve PTSD symptoms as assessed by CAPS.14,15 Although these results are promising, the trials to date have been conducted with relatively small sample sizes.
With more veterans being treated for PTSD within the VA health care system, the central treatment goal remains: Adequately address the symptoms of PTSD while minimizing the harm caused by medications. Prescribers should limit benzodiazepine and opioid use in this population and consider safer nonpharmacologic and pharmacologic treatment options when possible.
Conclusion
Combat veterans with PTSD who are prescribed benzodiazepines and/or opioids in addition to first-line pharmacotherapy are at significantly increased risk for overall and mental health hospitalization.
Click here to read the digital edition.
1. Bernardy NC, Lund BC, Alexander B, Jenkyn AB, Schnurr PP, Friedman MJ. Gender differences in prescribing among veterans diagnosed with posttraumatic stress disorder. J Gen Intern Med. 2013;28(suppl 2):S542-S548.
2. Management of Post-Traumatic Stress Working Group, Department of Veterans Affairs, Department of Defense. VA/DoD Clinical Practice Guideline for Management of Post-Traumatic Stress. http://www.healthquality.va.gov/PTSD-full-2010c .pdf. Published October 2010. Accessed July 12, 2015.
3. Marks IM, Swinson RP, Baso˘glu M, et al. Alprazolam and exposure alone and combined in panic disorder with agoraphobia. A controlled study in London and Toronto. Br J Psychiatry. 1993;162:776-787.
4. Wilhelm FH, Roth WT. Acute and delayed effects of alprazolam on flight phobics during exposure. Behav Res Ther. 1997;35(9):831-841.
5. Guina J, Rossetter SR, DeRhodes BJ, Nahhas RW, Welton RS. Benzodiazepines for PTSD: a systematic review and meta-analysis. J Psychiatr Pract. 2015;21(4):281-303.
6. Pietrzak RH, Goldstein RB, Southwick SM, Grant BF. Prevalence and Axis I comorbidity of full and partial posttraumatic stress disorder in the United States: results from wave 2 of the National Epidemiologic Survey on Alcohol and Related Conditions. J Anxiety Disord. 2011;25(3):456-465.
7. Mills KL, Teesson M, Ross J, Darke S, Shanahan M. The costs and outcomes of treatment for opioid dependence associated with posttraumatic stress disorder. Psychiatr Serv. 2005;56(8):940-945.
8. Seal KH, Shi Y, Cohen G, et al. Association of mental health disorders with prescription opioids and high-risk opioid use in US veterans of Iraq and Afghanistan. JAMA. 2012;307(9):940-947.
9. Abrams TE, Lund BC, Bernardy NC, Friedman MJ. Aligning clinical practice to PTSD treatment guidelines: medication prescribing by provider type. Psychiatr Serv. 2013;64(2):142-148.
10. Altman DG, Andersen PK. Calculating the number needed to treat for trials where the outcome is time to an event. BMJ. 1999;319(7223):1492-1495.
11. Hawkins EJ, Malte CA, Grossbard J, Saxon AJ, Imel ZE, Kivlahan DR. Comparative safety of benzodiazepines and opioids among Veterans Affairs patients with posttraumatic stress disorder. J Addict Med. 2013;7(5):354-362.
12. Berger W, Mendlowicz MV, Marques-Portella C, et al. Pharmacologic alternatives to antidepressants in posttraumatic stress disorder: a systematic review. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33(2):169-180.
13. Benedek DM, Friedman MJ, Zatzick D, Ursano RJ. Guideline watch (March 2009): practice guideline for the treatment of patients with acute stress disorder and posttraumatic stress disorder. Focus. 2009;7(2):204-213.
14. Raskind MA, Peterson K, Williams T, et al. A trial of prazosin for combat trauma PTSD with nightmares in active-duty soldiers returned from Iraq and Afghanistan. Am J Psychiatry. 2013;170(9):1003-1010.
15. Rodgman C, Verrico CD, Holst M, et al. Doxazosin XL reduces symptoms of posttraumatic stress disorder in veterans with PTSD: a pilot clinical trial. J Clin Psychiatry. 2016;77(5):e561-e565.
1. Bernardy NC, Lund BC, Alexander B, Jenkyn AB, Schnurr PP, Friedman MJ. Gender differences in prescribing among veterans diagnosed with posttraumatic stress disorder. J Gen Intern Med. 2013;28(suppl 2):S542-S548.
2. Management of Post-Traumatic Stress Working Group, Department of Veterans Affairs, Department of Defense. VA/DoD Clinical Practice Guideline for Management of Post-Traumatic Stress. http://www.healthquality.va.gov/PTSD-full-2010c .pdf. Published October 2010. Accessed July 12, 2015.
3. Marks IM, Swinson RP, Baso˘glu M, et al. Alprazolam and exposure alone and combined in panic disorder with agoraphobia. A controlled study in London and Toronto. Br J Psychiatry. 1993;162:776-787.
4. Wilhelm FH, Roth WT. Acute and delayed effects of alprazolam on flight phobics during exposure. Behav Res Ther. 1997;35(9):831-841.
5. Guina J, Rossetter SR, DeRhodes BJ, Nahhas RW, Welton RS. Benzodiazepines for PTSD: a systematic review and meta-analysis. J Psychiatr Pract. 2015;21(4):281-303.
6. Pietrzak RH, Goldstein RB, Southwick SM, Grant BF. Prevalence and Axis I comorbidity of full and partial posttraumatic stress disorder in the United States: results from wave 2 of the National Epidemiologic Survey on Alcohol and Related Conditions. J Anxiety Disord. 2011;25(3):456-465.
7. Mills KL, Teesson M, Ross J, Darke S, Shanahan M. The costs and outcomes of treatment for opioid dependence associated with posttraumatic stress disorder. Psychiatr Serv. 2005;56(8):940-945.
8. Seal KH, Shi Y, Cohen G, et al. Association of mental health disorders with prescription opioids and high-risk opioid use in US veterans of Iraq and Afghanistan. JAMA. 2012;307(9):940-947.
9. Abrams TE, Lund BC, Bernardy NC, Friedman MJ. Aligning clinical practice to PTSD treatment guidelines: medication prescribing by provider type. Psychiatr Serv. 2013;64(2):142-148.
10. Altman DG, Andersen PK. Calculating the number needed to treat for trials where the outcome is time to an event. BMJ. 1999;319(7223):1492-1495.
11. Hawkins EJ, Malte CA, Grossbard J, Saxon AJ, Imel ZE, Kivlahan DR. Comparative safety of benzodiazepines and opioids among Veterans Affairs patients with posttraumatic stress disorder. J Addict Med. 2013;7(5):354-362.
12. Berger W, Mendlowicz MV, Marques-Portella C, et al. Pharmacologic alternatives to antidepressants in posttraumatic stress disorder: a systematic review. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33(2):169-180.
13. Benedek DM, Friedman MJ, Zatzick D, Ursano RJ. Guideline watch (March 2009): practice guideline for the treatment of patients with acute stress disorder and posttraumatic stress disorder. Focus. 2009;7(2):204-213.
14. Raskind MA, Peterson K, Williams T, et al. A trial of prazosin for combat trauma PTSD with nightmares in active-duty soldiers returned from Iraq and Afghanistan. Am J Psychiatry. 2013;170(9):1003-1010.
15. Rodgman C, Verrico CD, Holst M, et al. Doxazosin XL reduces symptoms of posttraumatic stress disorder in veterans with PTSD: a pilot clinical trial. J Clin Psychiatry. 2016;77(5):e561-e565.
Sleep-Disordered Breathing in the Active-Duty Military Population and Its Civilian Care Cost
Sleep-disordered breathing (SDB) is a continuum of symptoms that range from primary snoring with upper airway resistance to frank obstruction seen in obstructive sleep apnea (OSA). This disease spectrum has been reported to affect 10% to 17% of men and 3% to 9% of women in the general population.1 The specific incidence of OSA has been estimated to be about 2% to 4% of the general adult population.2,3 Sleep-disordered breathing often leads to poor sleep quality, which has been associated with many medical comorbidities, including vascular disease, hypertension, major cardiac events, cardiomyopathies, impaired concentration, reduced psychomotor vigilance and cognition, and daytime somnolence.1,2,4-6 Furthermore, there is evidence that the prevalence of SDB continues to grow among the general population.1 However, the prevalence of SDB in various populations (eg, pediatric vs adult, varying body mass index, country of origin) varies widely due to the multifactorial nature of the risk factors and the difficulty in diagnosing SDB.
Some of the more intuitive medical sequelae of SDB are daytime somnolence and subsequent impaired concentration for those with disrupted sleep patterns. Medical literature has paid specific attention to cohorts of personnel who may be at heightened risk from impaired concentration or inability to focus. These populations include but are not limited to sleep-deprived resident physicians, firefighters, truck drivers, and heavy-machine operators.7,8
Military service members represent a distinct cohort that often is relied on to maintain vigilance even in austere environments. Concentration is paramount in order to perform combat operations or tasks that involve operating heavy machinery, such as nuclear submarines, aircraft, or tanks. Given the myriad of unique operational demands on service members, SDB can have detrimental consequences on an individual’s health and his or her military readiness and training. Ultimately, SDB may degrade a unit’s effectiveness and perhaps the country’s military capability.
Active-duty military service members seem to be more susceptible to clinically relevant sleep conditions. In the military, causes of disruptions in normal sleep patterns are multifactorial. Medical literature focuses on circadian disruptions due to shift work and frequent travel, frequent alternating use of caffeine and sedatives, exposure to combat/trauma, and chronic sleep deprivation.9-11 Studies have been published that focus on service members who have returned from combat deployment.10,12,13 However, these studies do not explore the overall burden of disease, and there are no specific data to suggest the prevalence, annual incidence, or associated costs.
To quantify this disease burden in the military, this study focused on the subset of sleep disorders that impact respiration during sleep and determined the prevalence and annual incidence for the entire active-duty population. Additionally, the authors fill a void in the literature by determining the financial burden of SDB on civilian care expenditures.
Methods
This study was a retrospective review of administrative military health care data spanning fiscal years (FYs) 2009 to 2013 (October 1, 2008 to September 30, 2013). The study protocol was approved by the Naval Medical Center Portsmouth Institutional Review Board, and approval was given to waive informed consent. The Health Analysis Department at the Navy and Marine Corps Public Health Center (NMCPHC) obtained and analyzed data from the Military Health System (MHS) Management Analysis and Reporting Tool (M2). The M2 system is an ad hoc query tool used for viewing population, clinical, and financial MHS data, including care received within military treatment facilities (MTFs) and care purchased through TRICARE at civilian facilities. Both inpatient and outpatient health care records were included.
The population included all active-duty service members and guard/reserve members on active duty within all military services, including air force, army, coast guard, and navy branches, between FY 2009 and FY 2013. The authors identified service members with SDB as those with at least 1 ICD-9 diagnosis code related to SDB: obstructive sleep apnea (327.23); sleep-related hypoventilation/hypoxemia (327.26); and other organic sleep disorder (327.80).
Due to the transient nature of the military population, a monthly average over the 5 years of the study determined the overall number of service members eligible for care (1,717,227 service members).
Data Analysis
Prevalence of diagnosed SDB per FY was calculated as the number of service members who received at least 1 SDB diagnostic code between October 1, 2008 and September 30, 2013, over the average total active-duty population. Incidence per year was calculated as the number of new cases per FY, using 2009 as the baseline. Data were stratified by demographic and enrollment information for diagnosed service members and analyzed using SAS 9.4 (Cary, NC) software.
Direct costs associated with SDB treatment fall into 2 categories for service members: (1) care delivered by civilian providers, calculated based on the amount TRICARE paid for the service, using insurance claim data; and (2) care received at MTFs by military providers. Costs for care at MTFs cannot be calculated, as the total cost amount for a single record is not directly attributed to SDB diagnosis.
Results
A total of 197,183 service members were diagnosed with SDB from FY 2009 to FY 2013. Both the annual incidence and prevalence of SDB for the active-duty military population showed upward trends for each of the years evaluated (Figure 1).
Notably, 72% of service members seen for SDB ranged in age from 25 to 44 years (Table).
Discussion
This study shows that the prevalence and incidence of SDB in the active-duty population are less than those reported for the civilian populace as a whole but are still greater than expected for an otherwise healthy and young population. Furthermore, the burden of disease and the cost to diagnose and treat have steadily increased for each of the past 5 fiscal years that were assessed.
The data show an upward trend in the incidence and prevalence of SDB in the military from FY 2009 to FY 2013 for reasons that are not clear but likely with many confounding contributions. As the spectrum of SDB has become better defined and the detrimental sequelae are better understood, it is likely that both service members and health care providers are more aware of the symptoms and more importantly, the potential for interventions that improve quality of life. It is also important to note that the U.S. military is a very transient organization with a nearly constant turnover between new enlistees/officers and those leaving the service or retiring after 20 years of service. Thus, despite an annual incidence of nearly 3% throughout the years evaluated, the annual increase in prevalence is not necessarily commensurate.
The FY 2013 prevalence (4.2%) and civilian care costs ($98,259,519) present traditional indications of the disease burden. Both metrics represent a sizable and increasing disease burden for the military. It is also important to note that these costs reflect only the short-term expenses for initial diagnosis and therapy. These costs in no way reflect the care for the long-term medical sequelae that have been recently linked to uncontrolled SDB/OSA, such as heart and vascular diseases, hypertension, and increased stroke risk. Additional costs will continue to grow.
Perhaps the most validated predictive factor for diagnosis of SDB or OSA is body habitus as measured by body mass index (BMI). In particular, nearly 60% to 90% of patients with OSA are obese.2 Weight gain seems to increase the OSA severity, whereas losing weight decreases it.14-16 Although the U.S. military employs height and weight standards that preclude those with persistently overweight or obese BMIs from continued service, these standards often are not rigid, and there are overweight or even obese active-duty members. Interestingly, despite a population that essentially controls for the most predictive risk factor, the prevalence of SDB is still approximately 1 in 20 (4.9%) in FY 2013.
Given the significant burden of disease represented by the incidence, prevalence, and cost data determined in this study and the growing recognition of long-term complications from poorly controlled SDB, it has become evident that more efficacious interventions are needed. Modern treatments for SDB can be classified as surgical or nonsurgical but with no single modality fitting the need for all patients secondary to poor adherence and/or limited efficacy.17-20 However, to mitigate the impact on military readiness and taxpayer-funded health care costs, it may be appropriate to begin exploring therapeutic options beyond the current standard of care. For example, an invasive and costly onetime surgical intervention using an implantable device to stimulate the hypoglossal nerve to open a person’s airway during inspiration is being investigated in a younger, nonobese cohort of patients.21 Further research is warranted into this specific model of therapeutic intervention and others for service members.
Limitations
Limitations in this study include possible reporting errors due to improper or insufficient medical coding as well as data entry errors at the clinic that may exist within medical billing databases. Therefore, the results of this analysis may be over- or underrepresented. The increase in incidence and prevalence may not necessarily reflect an increasing number of people who have the disease. The increase could be a result of better SDB detection practices or incentives to be diagnosed with SDB (VA disability claims upon retirement). The assumption is made that procedures corresponding with SDB diagnoses are directly related to SDB, and any costs incurred from those procedures are due to SDB.
It is important to note variability between services and institutions within the DoD in the diagnosis and treatment of SDB. Specifically, some institutions use ambulatory polysomnograms, or studies done at home, and autotitration of continuous positive airway pressure, whereas others require more costly hospital-based studies and laboratory titration. Another confounder in the cost data is the number of diagnoses and treatment deferred to the network as a result of the relatively small number of sleep-trained physicians within the military.
Conclusion
As the field of sleep medicine continues to develop its literature, it is becoming clearer that the detrimental sequelae of SDB are varied and pose significant short- and long-term risks. Active-duty service members represent a subset of the population with consequences that are potentially graver than those of civilians, especially when they are expected to operate complicated machinery or to make rapid and critical decisions in battle.
The prevalence and incidence of SDB increased each year during a 5-year review and currently affects 1 in 20 service members. Furthermore, the cost of civilian care for this disease process was nearly $100 million in FY 2012 to FY 2013, suggesting a growing financial burden for taxpayers. Further research is warranted to fully appreciate the impact of SDB on both service members and the U.S. military.
Acknowledgments
The authors thank the U.S. Navy and specifically the support within the Department of Otolaryngology at the Naval Medical Center Portsmouth for the time and effort allotted for completion of this study. This research was supported in part by an appointment to the Postgraduate Research Participation Program at the Navy and Marine Corps Public Health Center (NMCPHC) administered by the Oak Ridge Institute for Science and Education through an interagency agreement between the U.S. Department of Energy and NMCPHC.
1. Peppard PE, Young T, Barnet JH, Palta M, Hagen EW, Hla KM. Increased prevalence of sleep-disordered breathing in adults. Am J Epidemiol. 2013;177(9):1006-1014.
2. Young T, Peppard PE, Gottlieb DJ. Epidemiology of obstructive sleep apnea: a population health perspective. Am J Respir Crit Care Med. 2002;165(9):1217-1239.
3. Ram S, Seirawan H, Kumar SK, Clark GT. Prevalence and impact of sleep disorders and sleep habits in the United States. Sleep Breath. 2010;14(1):63-70.
4. Kim H, Dinges DF, Young T. Sleep-disordered breathing and psychomotor vigilance in a community-based sample. Sleep. 2007;30(10):1309-1316.
5. Yaffe K, Falvey CM, Hoang T. Connections between sleep and cognition in older adults. Lancet Neurol. 2014;13(10):1017-1028.
6. Gilat H, Vinker S, Buda I, Soudry E, Shani M, Bachar G. Obstructive sleep apnea and cardiovascular comorbidities: a large epidemiologic study. Medicine (Baltimore). 2014;93(9):e45.
7. Li X, Sundquist K, Sundquist J. Socioeconomic status and occupation as risk factors for obstructive sleep apnea in Sweden: a population-based study. Sleep Med. 2008;9(2):129-136.
8. Barger LK, Rajaratnam SM, Wang W, et al. Common sleep disorders increase risk of motor vehicle crashes and adverse health outcomes in firefighters. J Clin Sleep Med. 2015;11(3):233-240.
9. Mysliwiec V, Gill J, Lee H, et al. Sleep disorders in US military personnel: a high rate of comorbid insomnia and obstructive sleep apnea. Chest. 2013;144(2):549-557.
10. Mysliwiec V, McGraw L, Pierce R, Smith P, Trapp B, Roth BJ. Sleep disorders and associated medical comorbidities in active duty military personnel. Sleep. 2013;36(2):167-174.
11. Capaldi VF 2nd, Guerrero ML, Killgore WD. Sleep disruptions among returning combat veterans from Iraq and Afghanistan. Mil Med. 2011;176(8):879-888.
12. Collen J, Orr N, Lettieri CJ, Carter K, Holley AB. Sleep disturbances among soldiers with combat-related traumatic brain injury. Chest. 2012;142(3):622-630.
13. Peterson AL, Goodie JL, Satterfield WA, Brim WL. Sleep disturbance during military deployment. Mil Med. 2008;173(3):230-235.
14. Dixon JB, Schachter LM, O’Brien PE. Polysomnography before and after weight loss in obese patients with severe sleep apnea. Int J Obes (Lond). 2005;29(9):1048-1054.
15. Loube DI, Loube AA, Erman MK. Continuous positive airway pressure treatment results in weight less in obese and overweight patients with obstructive sleep apnea. J Am Diet Assoc. 1997;97(8):896-897.
16. Loube DI, Loube AA, Mitler MM. Weight loss for obstructive sleep apnea: the optimal therapy for obese patients. J Am Diet Assoc. 1994;94(11):1291-1295.
17. Malhotra A, Orr JE, Owens RL. On the cutting edge of obstructive sleep apnoea: where next? Lancet Respir Med. 2015;3(5):397-403.
18. Mysliwiec V, Capaldi VF, 2nd, Gill J, et al. Adherence to positive airway pressure therapy in U.S. military personnel with sleep apnea improves sleepiness, sleep quality, and depressive symptoms. Mil Med. 2015;180(4):475-482.
19. Salepci B, Caglayan B, Kiral N, et al. CPAP adherence of patients with obstructive sleep apnea. Respir Care. 2013;58(9):1467-1473.
20. Weaver TE, Grunstein RR. Adherence to continuous positive airway pressure therapy: the challenge to effective treatment. Proc Am Thorac Soc. 2008;5(2):173-178.
21. Pietzsch JB, Liu S, Garner AM, Kezirian EJ, Strollo PJ. Long-term cost-effectiveness of upper airway stimulation for the treatment of obstructive sleep apnea: a model-based projection based on the STAR trial. Sleep. 2015;38(5):735-744.
Sleep-disordered breathing (SDB) is a continuum of symptoms that range from primary snoring with upper airway resistance to frank obstruction seen in obstructive sleep apnea (OSA). This disease spectrum has been reported to affect 10% to 17% of men and 3% to 9% of women in the general population.1 The specific incidence of OSA has been estimated to be about 2% to 4% of the general adult population.2,3 Sleep-disordered breathing often leads to poor sleep quality, which has been associated with many medical comorbidities, including vascular disease, hypertension, major cardiac events, cardiomyopathies, impaired concentration, reduced psychomotor vigilance and cognition, and daytime somnolence.1,2,4-6 Furthermore, there is evidence that the prevalence of SDB continues to grow among the general population.1 However, the prevalence of SDB in various populations (eg, pediatric vs adult, varying body mass index, country of origin) varies widely due to the multifactorial nature of the risk factors and the difficulty in diagnosing SDB.
Some of the more intuitive medical sequelae of SDB are daytime somnolence and subsequent impaired concentration for those with disrupted sleep patterns. Medical literature has paid specific attention to cohorts of personnel who may be at heightened risk from impaired concentration or inability to focus. These populations include but are not limited to sleep-deprived resident physicians, firefighters, truck drivers, and heavy-machine operators.7,8
Military service members represent a distinct cohort that often is relied on to maintain vigilance even in austere environments. Concentration is paramount in order to perform combat operations or tasks that involve operating heavy machinery, such as nuclear submarines, aircraft, or tanks. Given the myriad of unique operational demands on service members, SDB can have detrimental consequences on an individual’s health and his or her military readiness and training. Ultimately, SDB may degrade a unit’s effectiveness and perhaps the country’s military capability.
Active-duty military service members seem to be more susceptible to clinically relevant sleep conditions. In the military, causes of disruptions in normal sleep patterns are multifactorial. Medical literature focuses on circadian disruptions due to shift work and frequent travel, frequent alternating use of caffeine and sedatives, exposure to combat/trauma, and chronic sleep deprivation.9-11 Studies have been published that focus on service members who have returned from combat deployment.10,12,13 However, these studies do not explore the overall burden of disease, and there are no specific data to suggest the prevalence, annual incidence, or associated costs.
To quantify this disease burden in the military, this study focused on the subset of sleep disorders that impact respiration during sleep and determined the prevalence and annual incidence for the entire active-duty population. Additionally, the authors fill a void in the literature by determining the financial burden of SDB on civilian care expenditures.
Methods
This study was a retrospective review of administrative military health care data spanning fiscal years (FYs) 2009 to 2013 (October 1, 2008 to September 30, 2013). The study protocol was approved by the Naval Medical Center Portsmouth Institutional Review Board, and approval was given to waive informed consent. The Health Analysis Department at the Navy and Marine Corps Public Health Center (NMCPHC) obtained and analyzed data from the Military Health System (MHS) Management Analysis and Reporting Tool (M2). The M2 system is an ad hoc query tool used for viewing population, clinical, and financial MHS data, including care received within military treatment facilities (MTFs) and care purchased through TRICARE at civilian facilities. Both inpatient and outpatient health care records were included.
The population included all active-duty service members and guard/reserve members on active duty within all military services, including air force, army, coast guard, and navy branches, between FY 2009 and FY 2013. The authors identified service members with SDB as those with at least 1 ICD-9 diagnosis code related to SDB: obstructive sleep apnea (327.23); sleep-related hypoventilation/hypoxemia (327.26); and other organic sleep disorder (327.80).
Due to the transient nature of the military population, a monthly average over the 5 years of the study determined the overall number of service members eligible for care (1,717,227 service members).
Data Analysis
Prevalence of diagnosed SDB per FY was calculated as the number of service members who received at least 1 SDB diagnostic code between October 1, 2008 and September 30, 2013, over the average total active-duty population. Incidence per year was calculated as the number of new cases per FY, using 2009 as the baseline. Data were stratified by demographic and enrollment information for diagnosed service members and analyzed using SAS 9.4 (Cary, NC) software.
Direct costs associated with SDB treatment fall into 2 categories for service members: (1) care delivered by civilian providers, calculated based on the amount TRICARE paid for the service, using insurance claim data; and (2) care received at MTFs by military providers. Costs for care at MTFs cannot be calculated, as the total cost amount for a single record is not directly attributed to SDB diagnosis.
Results
A total of 197,183 service members were diagnosed with SDB from FY 2009 to FY 2013. Both the annual incidence and prevalence of SDB for the active-duty military population showed upward trends for each of the years evaluated (Figure 1).
Notably, 72% of service members seen for SDB ranged in age from 25 to 44 years (Table).
Discussion
This study shows that the prevalence and incidence of SDB in the active-duty population are less than those reported for the civilian populace as a whole but are still greater than expected for an otherwise healthy and young population. Furthermore, the burden of disease and the cost to diagnose and treat have steadily increased for each of the past 5 fiscal years that were assessed.
The data show an upward trend in the incidence and prevalence of SDB in the military from FY 2009 to FY 2013 for reasons that are not clear but likely with many confounding contributions. As the spectrum of SDB has become better defined and the detrimental sequelae are better understood, it is likely that both service members and health care providers are more aware of the symptoms and more importantly, the potential for interventions that improve quality of life. It is also important to note that the U.S. military is a very transient organization with a nearly constant turnover between new enlistees/officers and those leaving the service or retiring after 20 years of service. Thus, despite an annual incidence of nearly 3% throughout the years evaluated, the annual increase in prevalence is not necessarily commensurate.
The FY 2013 prevalence (4.2%) and civilian care costs ($98,259,519) present traditional indications of the disease burden. Both metrics represent a sizable and increasing disease burden for the military. It is also important to note that these costs reflect only the short-term expenses for initial diagnosis and therapy. These costs in no way reflect the care for the long-term medical sequelae that have been recently linked to uncontrolled SDB/OSA, such as heart and vascular diseases, hypertension, and increased stroke risk. Additional costs will continue to grow.
Perhaps the most validated predictive factor for diagnosis of SDB or OSA is body habitus as measured by body mass index (BMI). In particular, nearly 60% to 90% of patients with OSA are obese.2 Weight gain seems to increase the OSA severity, whereas losing weight decreases it.14-16 Although the U.S. military employs height and weight standards that preclude those with persistently overweight or obese BMIs from continued service, these standards often are not rigid, and there are overweight or even obese active-duty members. Interestingly, despite a population that essentially controls for the most predictive risk factor, the prevalence of SDB is still approximately 1 in 20 (4.9%) in FY 2013.
Given the significant burden of disease represented by the incidence, prevalence, and cost data determined in this study and the growing recognition of long-term complications from poorly controlled SDB, it has become evident that more efficacious interventions are needed. Modern treatments for SDB can be classified as surgical or nonsurgical but with no single modality fitting the need for all patients secondary to poor adherence and/or limited efficacy.17-20 However, to mitigate the impact on military readiness and taxpayer-funded health care costs, it may be appropriate to begin exploring therapeutic options beyond the current standard of care. For example, an invasive and costly onetime surgical intervention using an implantable device to stimulate the hypoglossal nerve to open a person’s airway during inspiration is being investigated in a younger, nonobese cohort of patients.21 Further research is warranted into this specific model of therapeutic intervention and others for service members.
Limitations
Limitations in this study include possible reporting errors due to improper or insufficient medical coding as well as data entry errors at the clinic that may exist within medical billing databases. Therefore, the results of this analysis may be over- or underrepresented. The increase in incidence and prevalence may not necessarily reflect an increasing number of people who have the disease. The increase could be a result of better SDB detection practices or incentives to be diagnosed with SDB (VA disability claims upon retirement). The assumption is made that procedures corresponding with SDB diagnoses are directly related to SDB, and any costs incurred from those procedures are due to SDB.
It is important to note variability between services and institutions within the DoD in the diagnosis and treatment of SDB. Specifically, some institutions use ambulatory polysomnograms, or studies done at home, and autotitration of continuous positive airway pressure, whereas others require more costly hospital-based studies and laboratory titration. Another confounder in the cost data is the number of diagnoses and treatment deferred to the network as a result of the relatively small number of sleep-trained physicians within the military.
Conclusion
As the field of sleep medicine continues to develop its literature, it is becoming clearer that the detrimental sequelae of SDB are varied and pose significant short- and long-term risks. Active-duty service members represent a subset of the population with consequences that are potentially graver than those of civilians, especially when they are expected to operate complicated machinery or to make rapid and critical decisions in battle.
The prevalence and incidence of SDB increased each year during a 5-year review and currently affects 1 in 20 service members. Furthermore, the cost of civilian care for this disease process was nearly $100 million in FY 2012 to FY 2013, suggesting a growing financial burden for taxpayers. Further research is warranted to fully appreciate the impact of SDB on both service members and the U.S. military.
Acknowledgments
The authors thank the U.S. Navy and specifically the support within the Department of Otolaryngology at the Naval Medical Center Portsmouth for the time and effort allotted for completion of this study. This research was supported in part by an appointment to the Postgraduate Research Participation Program at the Navy and Marine Corps Public Health Center (NMCPHC) administered by the Oak Ridge Institute for Science and Education through an interagency agreement between the U.S. Department of Energy and NMCPHC.
Sleep-disordered breathing (SDB) is a continuum of symptoms that range from primary snoring with upper airway resistance to frank obstruction seen in obstructive sleep apnea (OSA). This disease spectrum has been reported to affect 10% to 17% of men and 3% to 9% of women in the general population.1 The specific incidence of OSA has been estimated to be about 2% to 4% of the general adult population.2,3 Sleep-disordered breathing often leads to poor sleep quality, which has been associated with many medical comorbidities, including vascular disease, hypertension, major cardiac events, cardiomyopathies, impaired concentration, reduced psychomotor vigilance and cognition, and daytime somnolence.1,2,4-6 Furthermore, there is evidence that the prevalence of SDB continues to grow among the general population.1 However, the prevalence of SDB in various populations (eg, pediatric vs adult, varying body mass index, country of origin) varies widely due to the multifactorial nature of the risk factors and the difficulty in diagnosing SDB.
Some of the more intuitive medical sequelae of SDB are daytime somnolence and subsequent impaired concentration for those with disrupted sleep patterns. Medical literature has paid specific attention to cohorts of personnel who may be at heightened risk from impaired concentration or inability to focus. These populations include but are not limited to sleep-deprived resident physicians, firefighters, truck drivers, and heavy-machine operators.7,8
Military service members represent a distinct cohort that often is relied on to maintain vigilance even in austere environments. Concentration is paramount in order to perform combat operations or tasks that involve operating heavy machinery, such as nuclear submarines, aircraft, or tanks. Given the myriad of unique operational demands on service members, SDB can have detrimental consequences on an individual’s health and his or her military readiness and training. Ultimately, SDB may degrade a unit’s effectiveness and perhaps the country’s military capability.
Active-duty military service members seem to be more susceptible to clinically relevant sleep conditions. In the military, causes of disruptions in normal sleep patterns are multifactorial. Medical literature focuses on circadian disruptions due to shift work and frequent travel, frequent alternating use of caffeine and sedatives, exposure to combat/trauma, and chronic sleep deprivation.9-11 Studies have been published that focus on service members who have returned from combat deployment.10,12,13 However, these studies do not explore the overall burden of disease, and there are no specific data to suggest the prevalence, annual incidence, or associated costs.
To quantify this disease burden in the military, this study focused on the subset of sleep disorders that impact respiration during sleep and determined the prevalence and annual incidence for the entire active-duty population. Additionally, the authors fill a void in the literature by determining the financial burden of SDB on civilian care expenditures.
Methods
This study was a retrospective review of administrative military health care data spanning fiscal years (FYs) 2009 to 2013 (October 1, 2008 to September 30, 2013). The study protocol was approved by the Naval Medical Center Portsmouth Institutional Review Board, and approval was given to waive informed consent. The Health Analysis Department at the Navy and Marine Corps Public Health Center (NMCPHC) obtained and analyzed data from the Military Health System (MHS) Management Analysis and Reporting Tool (M2). The M2 system is an ad hoc query tool used for viewing population, clinical, and financial MHS data, including care received within military treatment facilities (MTFs) and care purchased through TRICARE at civilian facilities. Both inpatient and outpatient health care records were included.
The population included all active-duty service members and guard/reserve members on active duty within all military services, including air force, army, coast guard, and navy branches, between FY 2009 and FY 2013. The authors identified service members with SDB as those with at least 1 ICD-9 diagnosis code related to SDB: obstructive sleep apnea (327.23); sleep-related hypoventilation/hypoxemia (327.26); and other organic sleep disorder (327.80).
Due to the transient nature of the military population, a monthly average over the 5 years of the study determined the overall number of service members eligible for care (1,717,227 service members).
Data Analysis
Prevalence of diagnosed SDB per FY was calculated as the number of service members who received at least 1 SDB diagnostic code between October 1, 2008 and September 30, 2013, over the average total active-duty population. Incidence per year was calculated as the number of new cases per FY, using 2009 as the baseline. Data were stratified by demographic and enrollment information for diagnosed service members and analyzed using SAS 9.4 (Cary, NC) software.
Direct costs associated with SDB treatment fall into 2 categories for service members: (1) care delivered by civilian providers, calculated based on the amount TRICARE paid for the service, using insurance claim data; and (2) care received at MTFs by military providers. Costs for care at MTFs cannot be calculated, as the total cost amount for a single record is not directly attributed to SDB diagnosis.
Results
A total of 197,183 service members were diagnosed with SDB from FY 2009 to FY 2013. Both the annual incidence and prevalence of SDB for the active-duty military population showed upward trends for each of the years evaluated (Figure 1).
Notably, 72% of service members seen for SDB ranged in age from 25 to 44 years (Table).
Discussion
This study shows that the prevalence and incidence of SDB in the active-duty population are less than those reported for the civilian populace as a whole but are still greater than expected for an otherwise healthy and young population. Furthermore, the burden of disease and the cost to diagnose and treat have steadily increased for each of the past 5 fiscal years that were assessed.
The data show an upward trend in the incidence and prevalence of SDB in the military from FY 2009 to FY 2013 for reasons that are not clear but likely with many confounding contributions. As the spectrum of SDB has become better defined and the detrimental sequelae are better understood, it is likely that both service members and health care providers are more aware of the symptoms and more importantly, the potential for interventions that improve quality of life. It is also important to note that the U.S. military is a very transient organization with a nearly constant turnover between new enlistees/officers and those leaving the service or retiring after 20 years of service. Thus, despite an annual incidence of nearly 3% throughout the years evaluated, the annual increase in prevalence is not necessarily commensurate.
The FY 2013 prevalence (4.2%) and civilian care costs ($98,259,519) present traditional indications of the disease burden. Both metrics represent a sizable and increasing disease burden for the military. It is also important to note that these costs reflect only the short-term expenses for initial diagnosis and therapy. These costs in no way reflect the care for the long-term medical sequelae that have been recently linked to uncontrolled SDB/OSA, such as heart and vascular diseases, hypertension, and increased stroke risk. Additional costs will continue to grow.
Perhaps the most validated predictive factor for diagnosis of SDB or OSA is body habitus as measured by body mass index (BMI). In particular, nearly 60% to 90% of patients with OSA are obese.2 Weight gain seems to increase the OSA severity, whereas losing weight decreases it.14-16 Although the U.S. military employs height and weight standards that preclude those with persistently overweight or obese BMIs from continued service, these standards often are not rigid, and there are overweight or even obese active-duty members. Interestingly, despite a population that essentially controls for the most predictive risk factor, the prevalence of SDB is still approximately 1 in 20 (4.9%) in FY 2013.
Given the significant burden of disease represented by the incidence, prevalence, and cost data determined in this study and the growing recognition of long-term complications from poorly controlled SDB, it has become evident that more efficacious interventions are needed. Modern treatments for SDB can be classified as surgical or nonsurgical but with no single modality fitting the need for all patients secondary to poor adherence and/or limited efficacy.17-20 However, to mitigate the impact on military readiness and taxpayer-funded health care costs, it may be appropriate to begin exploring therapeutic options beyond the current standard of care. For example, an invasive and costly onetime surgical intervention using an implantable device to stimulate the hypoglossal nerve to open a person’s airway during inspiration is being investigated in a younger, nonobese cohort of patients.21 Further research is warranted into this specific model of therapeutic intervention and others for service members.
Limitations
Limitations in this study include possible reporting errors due to improper or insufficient medical coding as well as data entry errors at the clinic that may exist within medical billing databases. Therefore, the results of this analysis may be over- or underrepresented. The increase in incidence and prevalence may not necessarily reflect an increasing number of people who have the disease. The increase could be a result of better SDB detection practices or incentives to be diagnosed with SDB (VA disability claims upon retirement). The assumption is made that procedures corresponding with SDB diagnoses are directly related to SDB, and any costs incurred from those procedures are due to SDB.
It is important to note variability between services and institutions within the DoD in the diagnosis and treatment of SDB. Specifically, some institutions use ambulatory polysomnograms, or studies done at home, and autotitration of continuous positive airway pressure, whereas others require more costly hospital-based studies and laboratory titration. Another confounder in the cost data is the number of diagnoses and treatment deferred to the network as a result of the relatively small number of sleep-trained physicians within the military.
Conclusion
As the field of sleep medicine continues to develop its literature, it is becoming clearer that the detrimental sequelae of SDB are varied and pose significant short- and long-term risks. Active-duty service members represent a subset of the population with consequences that are potentially graver than those of civilians, especially when they are expected to operate complicated machinery or to make rapid and critical decisions in battle.
The prevalence and incidence of SDB increased each year during a 5-year review and currently affects 1 in 20 service members. Furthermore, the cost of civilian care for this disease process was nearly $100 million in FY 2012 to FY 2013, suggesting a growing financial burden for taxpayers. Further research is warranted to fully appreciate the impact of SDB on both service members and the U.S. military.
Acknowledgments
The authors thank the U.S. Navy and specifically the support within the Department of Otolaryngology at the Naval Medical Center Portsmouth for the time and effort allotted for completion of this study. This research was supported in part by an appointment to the Postgraduate Research Participation Program at the Navy and Marine Corps Public Health Center (NMCPHC) administered by the Oak Ridge Institute for Science and Education through an interagency agreement between the U.S. Department of Energy and NMCPHC.
1. Peppard PE, Young T, Barnet JH, Palta M, Hagen EW, Hla KM. Increased prevalence of sleep-disordered breathing in adults. Am J Epidemiol. 2013;177(9):1006-1014.
2. Young T, Peppard PE, Gottlieb DJ. Epidemiology of obstructive sleep apnea: a population health perspective. Am J Respir Crit Care Med. 2002;165(9):1217-1239.
3. Ram S, Seirawan H, Kumar SK, Clark GT. Prevalence and impact of sleep disorders and sleep habits in the United States. Sleep Breath. 2010;14(1):63-70.
4. Kim H, Dinges DF, Young T. Sleep-disordered breathing and psychomotor vigilance in a community-based sample. Sleep. 2007;30(10):1309-1316.
5. Yaffe K, Falvey CM, Hoang T. Connections between sleep and cognition in older adults. Lancet Neurol. 2014;13(10):1017-1028.
6. Gilat H, Vinker S, Buda I, Soudry E, Shani M, Bachar G. Obstructive sleep apnea and cardiovascular comorbidities: a large epidemiologic study. Medicine (Baltimore). 2014;93(9):e45.
7. Li X, Sundquist K, Sundquist J. Socioeconomic status and occupation as risk factors for obstructive sleep apnea in Sweden: a population-based study. Sleep Med. 2008;9(2):129-136.
8. Barger LK, Rajaratnam SM, Wang W, et al. Common sleep disorders increase risk of motor vehicle crashes and adverse health outcomes in firefighters. J Clin Sleep Med. 2015;11(3):233-240.
9. Mysliwiec V, Gill J, Lee H, et al. Sleep disorders in US military personnel: a high rate of comorbid insomnia and obstructive sleep apnea. Chest. 2013;144(2):549-557.
10. Mysliwiec V, McGraw L, Pierce R, Smith P, Trapp B, Roth BJ. Sleep disorders and associated medical comorbidities in active duty military personnel. Sleep. 2013;36(2):167-174.
11. Capaldi VF 2nd, Guerrero ML, Killgore WD. Sleep disruptions among returning combat veterans from Iraq and Afghanistan. Mil Med. 2011;176(8):879-888.
12. Collen J, Orr N, Lettieri CJ, Carter K, Holley AB. Sleep disturbances among soldiers with combat-related traumatic brain injury. Chest. 2012;142(3):622-630.
13. Peterson AL, Goodie JL, Satterfield WA, Brim WL. Sleep disturbance during military deployment. Mil Med. 2008;173(3):230-235.
14. Dixon JB, Schachter LM, O’Brien PE. Polysomnography before and after weight loss in obese patients with severe sleep apnea. Int J Obes (Lond). 2005;29(9):1048-1054.
15. Loube DI, Loube AA, Erman MK. Continuous positive airway pressure treatment results in weight less in obese and overweight patients with obstructive sleep apnea. J Am Diet Assoc. 1997;97(8):896-897.
16. Loube DI, Loube AA, Mitler MM. Weight loss for obstructive sleep apnea: the optimal therapy for obese patients. J Am Diet Assoc. 1994;94(11):1291-1295.
17. Malhotra A, Orr JE, Owens RL. On the cutting edge of obstructive sleep apnoea: where next? Lancet Respir Med. 2015;3(5):397-403.
18. Mysliwiec V, Capaldi VF, 2nd, Gill J, et al. Adherence to positive airway pressure therapy in U.S. military personnel with sleep apnea improves sleepiness, sleep quality, and depressive symptoms. Mil Med. 2015;180(4):475-482.
19. Salepci B, Caglayan B, Kiral N, et al. CPAP adherence of patients with obstructive sleep apnea. Respir Care. 2013;58(9):1467-1473.
20. Weaver TE, Grunstein RR. Adherence to continuous positive airway pressure therapy: the challenge to effective treatment. Proc Am Thorac Soc. 2008;5(2):173-178.
21. Pietzsch JB, Liu S, Garner AM, Kezirian EJ, Strollo PJ. Long-term cost-effectiveness of upper airway stimulation for the treatment of obstructive sleep apnea: a model-based projection based on the STAR trial. Sleep. 2015;38(5):735-744.
1. Peppard PE, Young T, Barnet JH, Palta M, Hagen EW, Hla KM. Increased prevalence of sleep-disordered breathing in adults. Am J Epidemiol. 2013;177(9):1006-1014.
2. Young T, Peppard PE, Gottlieb DJ. Epidemiology of obstructive sleep apnea: a population health perspective. Am J Respir Crit Care Med. 2002;165(9):1217-1239.
3. Ram S, Seirawan H, Kumar SK, Clark GT. Prevalence and impact of sleep disorders and sleep habits in the United States. Sleep Breath. 2010;14(1):63-70.
4. Kim H, Dinges DF, Young T. Sleep-disordered breathing and psychomotor vigilance in a community-based sample. Sleep. 2007;30(10):1309-1316.
5. Yaffe K, Falvey CM, Hoang T. Connections between sleep and cognition in older adults. Lancet Neurol. 2014;13(10):1017-1028.
6. Gilat H, Vinker S, Buda I, Soudry E, Shani M, Bachar G. Obstructive sleep apnea and cardiovascular comorbidities: a large epidemiologic study. Medicine (Baltimore). 2014;93(9):e45.
7. Li X, Sundquist K, Sundquist J. Socioeconomic status and occupation as risk factors for obstructive sleep apnea in Sweden: a population-based study. Sleep Med. 2008;9(2):129-136.
8. Barger LK, Rajaratnam SM, Wang W, et al. Common sleep disorders increase risk of motor vehicle crashes and adverse health outcomes in firefighters. J Clin Sleep Med. 2015;11(3):233-240.
9. Mysliwiec V, Gill J, Lee H, et al. Sleep disorders in US military personnel: a high rate of comorbid insomnia and obstructive sleep apnea. Chest. 2013;144(2):549-557.
10. Mysliwiec V, McGraw L, Pierce R, Smith P, Trapp B, Roth BJ. Sleep disorders and associated medical comorbidities in active duty military personnel. Sleep. 2013;36(2):167-174.
11. Capaldi VF 2nd, Guerrero ML, Killgore WD. Sleep disruptions among returning combat veterans from Iraq and Afghanistan. Mil Med. 2011;176(8):879-888.
12. Collen J, Orr N, Lettieri CJ, Carter K, Holley AB. Sleep disturbances among soldiers with combat-related traumatic brain injury. Chest. 2012;142(3):622-630.
13. Peterson AL, Goodie JL, Satterfield WA, Brim WL. Sleep disturbance during military deployment. Mil Med. 2008;173(3):230-235.
14. Dixon JB, Schachter LM, O’Brien PE. Polysomnography before and after weight loss in obese patients with severe sleep apnea. Int J Obes (Lond). 2005;29(9):1048-1054.
15. Loube DI, Loube AA, Erman MK. Continuous positive airway pressure treatment results in weight less in obese and overweight patients with obstructive sleep apnea. J Am Diet Assoc. 1997;97(8):896-897.
16. Loube DI, Loube AA, Mitler MM. Weight loss for obstructive sleep apnea: the optimal therapy for obese patients. J Am Diet Assoc. 1994;94(11):1291-1295.
17. Malhotra A, Orr JE, Owens RL. On the cutting edge of obstructive sleep apnoea: where next? Lancet Respir Med. 2015;3(5):397-403.
18. Mysliwiec V, Capaldi VF, 2nd, Gill J, et al. Adherence to positive airway pressure therapy in U.S. military personnel with sleep apnea improves sleepiness, sleep quality, and depressive symptoms. Mil Med. 2015;180(4):475-482.
19. Salepci B, Caglayan B, Kiral N, et al. CPAP adherence of patients with obstructive sleep apnea. Respir Care. 2013;58(9):1467-1473.
20. Weaver TE, Grunstein RR. Adherence to continuous positive airway pressure therapy: the challenge to effective treatment. Proc Am Thorac Soc. 2008;5(2):173-178.
21. Pietzsch JB, Liu S, Garner AM, Kezirian EJ, Strollo PJ. Long-term cost-effectiveness of upper airway stimulation for the treatment of obstructive sleep apnea: a model-based projection based on the STAR trial. Sleep. 2015;38(5):735-744.
Minimizing Postdisaster Fatalities
Environmental disasters can overpower local medical resources. Fortunately, such crises are rare in the U.S. This situation, however, has not always been the case. For example, in 1812, an earthquake along the New Madrid fault of the Mississippi Valley caused the Mississippi River to flow backward for 3 days.1 Today, in urbanized America, an earthquake of that magnitude would be devastating and severely overwhelm medical systems. All nations, including highly modernized nations, would need help in such disasters.2 A response system that is nimble, well-trained, scalable, and rapidly deployable can mitigate disaster sequelae. This article focuses on key aspects of effective rapid response, including speed, appropriate triage, quick-response strike teams, and disaster dynamics.
Why Speed Matters Most
Response time arguably is the most important factor in increasing survival in a disaster. In a 1996 study of earthquake disasters worldwide, Schultz and colleagues found a lower survival rate for victims who received medical care outside a 24-hour window.1 Studies of earthquakes in China have suggested that unless aid is rendered within 2 to 6 hours, fewer than half the victims will survive.3 Regarding a 1980 earthquake in Italy, de Bruycker and colleagues emphasized the importance of engaging in rescue activities within the first 48 hours.4 Safar reviewed mass disasters and reported that 25% to 50% of the injured and dead could have been saved if first aid had been provided immediately.5 In 1992 and 1994, Pretto and colleagues wrote that in earthquakes in Armenia and Costa Rica, many deaths could have been prevented had the victims received medical attention within the first 6 hours.6,7 The question is: How can responses to such crises be improved? Confederate Army Lt. Gen. Nathan Bedford Forrest’s dictum “[Get] there first with the most men” holds true in disaster medicine as well: get there fast with the right people, training, equipment, and supplies.8
Deaths in disasters can be described in a 3-phase distribution: immediate, early, and delayed. Stringent building codes and public warnings and evacuations reduce immediate deaths, but victims also die of catastrophic injury soon after an event. Early deaths are preventable with use of rapid interventions, such as tourniquets and airway adjuncts, but these must be administered within minutes or hours. Delayed deaths occur days or weeks after injury secondary to infection or organ system failure—which emphasizes the value of early wound care.
Emergency Supplies
What items are most needed? As each disaster is different, it would be presumptuous to provide a one-size-fits-all list, but some common supplies have been suggested. In 2010, Ginzberg and colleagues reported that during the first 24 hours of the Haiti earthquake of 1996, the overwhelming need was for IV hydration, narcotic analgesics, and casting supplies for the splinting of fractures.9 During the next 24 hours, IV stabilization was key, along with monitoring by Foley and suprapubic catheters. In the third 24-hour period, providers began to see sepsis-related deaths. In response to this challenge, teams began aggressive treatment with open surgical debridement of wounds, amputation of severely injured limbs, and administration of broad-spectrum IV antibiotics. Regional anesthesia with conscious sedation was mandatory because supplemental oxygen and ventilators were unavailable. By day 4, wound debridement, amputations, and fasciotomies were being provided by newly arrived anesthesiologists and orthopedic surgeons. Ginzberg and colleagues emphasized that rapid response was key in maximizing survival and by day 4, there was a greater need for surgical teams and broad-spectrum antibiotics (eg, piperacillin, tazobactam) to combat sepsis.
Pereira and colleagues reported that in a catastrophe caused by a tropical storm and landslides in Brazil, the most common injuries involved the extremities; the majority of wounds required only cleaning, debridement, and suture; and the most commonly performed operations were for orthopedic injuries.10 Incidentally, population baseline morbidity and mortality continue during disasters, and rescue personnel invariably sustain injuries, which contribute to the total medical burden. These additional injuries must be anticipated, and plans to manage them must be included in any disaster contingency planning.
Triage
Speed and correct triage are essential building blocks of disaster response. When resources are limited, triage is crucial in providing the right treatment to the right patient. There are numerous triage methods, some more rapid and straightforward; others more effective and cumbersome.11 In 2012, Sasser and colleagues wrote that the purpose of triage is to ensure injured patients are transported to a trauma center or the hospital best equipped to manage their specific injuries in an appropriate and timely manner.12 Their report focused on prehospital emergent care, not mass-casualty or disaster situations.
Triage is sometimes performed inconsistently. In a 2013 study, Kleber and colleagues found that 24% of providers overtriage and 16% undertriage.13 In the U.S., simple triage and rapid treatment (START) is commonly used to sort traumatized patients. All these methods take a “worst gets first treatment” approach. Depending on the magnitude of an event, however, providers may take a reverse-triage approach, in which they better use resources for the least injured patients and provide palliative care to the gravely ill.
During pandemic disasters, trauma triage protocols are ineffective. Instead, these events demand assessments that are sensitive to infectious diseases. Timely, didactic, hands-on training must be conducted before the fact so responders can adapt to react appropriately to a given disaster.14
Accurate, timely triage in mass-casualty incidents was conceptually demonstrated by Mekel and colleagues who reviewed the medical management of bombing victims in metropolitan Haifa, Israel during the period 2000 to 2006.15 Providers initiated a predetermined triage system in which patients are assigned to the appropriate echelon of care. Of 342 injured patients, 9.5% had severe injuries, 2.4% had moderate-severe injuries, and 88.9% had mild injuries. Correct and timely triage directed trauma victims to the appropriate medical care. Such action prevents the highest level facility from becoming overcrowded with less severely injured patients and ensures that the more critically injured receive a level of care comparable to that given under nondisaster circumstances.
The handheld ultrasound device, which can be used to correctly diagnose fractures, is an efficient triage resource for prehospital teams. In a 2008 study, McManus and colleagues suggested that ultrasound (vs traditional radiography) could be used to identify fractures in an emergency room.16 A handheld ultrasound device could be used outside the hospital, in the field, potentially reducing the number of referrals to overwhelmed orthopedic hospitals.
In 2007, Dean and colleagues reported on using ultrasound to rapidly triage disease during an earthquake in Guatemala.17 In that disaster, 23% of injuries presented within the first 24 hours, and a handheld ultrasound device was used to assess orthopedic injuries—ruling in 12% and ruling out 42%. The handheld ultrasound device is an example of a tool that small medical teams can use to speed triage, enhance patient care, and relieve overcrowded medical centers of the unrelenting pressure.
On-Site vs Hospital
Complicating disaster response is self-triage. Victims with injuries of all severity levels go to the nearest hospital and overwhelm it. In 1991, Waeckerle reported that within the first 30 minutes of a disaster, a wave of victims arrives, usually with minor injuries, and impedes care for the more severely wounded.18 Correct triage instead would have directed these patients to a hospital other than the overwhelmed level I trauma center.15 This is not to say that patients with mild or moderate injuries are unimportant—just that their care may take scarce space and resources from the more severely injured.
Mallonee and colleagues reported that of the 759 people injured in the 1996 Oklahoma City bombing, 167 (22%) were fatalities, 83 (11%) were hospitalized, and 509 (67%) were treated on an outpatient basis.19 Most of the injuries could have been managed by quick-response medical teams operating in the affected area, outside the hospitals. This action would have reduced operational pressure on hospitals and improved severely injured patients’ access to care.
Specialized Teams
In 2008, Barillo and colleagues suggested that having standardized medic bags would allow a small detachment of medical professionals to provide care nimbly—and doing so would represent a leap forward in access to care.20
Because of their unique ability to understand the culture and coordinate military assets with local authorities, DoD international health specialists are crucial interfaces for any population, foreign or domestic. Seyedin and colleagues and Merin and colleagues suggested that in both the Bam earthquake in 2003 and the Nepal earthquake in 2015, understanding the culture played a vital role in health care delivery and in adhering to cultural norms in deciding when to perform surgery, making end-of-life decisions, communicating with family, establishing trust with local and regional leaders, and other matters.21,22
Strike teams are small groups of variably trained health care providers who are dispatched to underserved, outlying, or overwhelmed areas to deliver precached basic medical care and triage significant injuries to medical centers. The handheld ultrasound device is an example of a strike team tool. During a local emergency, it is understood or assumed that response is inundated and that people are going untreated.
Crucially, strike teams must be trained, prepared, and readily dispatched ahead of larger response elements. Though quickly deployable, disaster medical assistance teams (DMATs) and National Guard Chemical, Biological, Radiological, Nuclear and High-Yield Explosive Enhanced Response Force Package units, take time to mobilize. Therefore, strike teams should consist of community citizens or local National Guard assets, the latter being particularly suited to rapid response given their training, effective command and control, and intrinsic logistics.
The efficacy of strike teams was demonstrated during the 2011 earthquake in Japan.23 Disaster medical assistance teams were invaluable in triaging and treating patients during the first 3 days. A team left 34 minutes after the event to render aid to people caught in a roof collapse. During triage, 17% of the injuries were classified urgent, 22% intermediate, and 61% minor. On day 7, a DMAT was dispatched to assist with emergency medicine and primary care; 3% of the injuries were severe and required urgent care, 50% required intermediate care, and 47% required minor care.
The value of strike teams is 3-fold: It provides rapid, professional care at a crucial place and critical time; it correctly triages patients and thus allow hospitals to maintain resources for the more severely injured; and augments overwhelmed providers at crucial sites. The roles of strike teams were echoed in 2006 by Campos-Outcalt, who reported that DMATs deployed to austere locations had the flexibility to augment existing medical staff and to rapidly deploy, self-sustain, and treat patients until a situation was resolved.24 This nimble strike team mentality could become a rapid and flexible model to save more lives, relieve significant suffering, and offload pressure from local hospitals by treating the less critically injured.
After a disaster, space is at a premium, and nonmedical residents who make up 40% to 70% of the shelter population require resources as well.25 Family members and the lightly injured may be conscripted to augment the overwhelmed medical staff. In 1988, Halbert and colleagues described how Afghan volunteers with minimal medical experience were given training and supplies and served as advanced emergency medics, delivering medical care and performing well under austere conditions.26,27 Strike teams thus may provide on-scene training in addition to medical care.
In 2012, Kirsch and colleaguesfound that Haiti earthquake victims who received treatment and remained in camps showed no improvement in income, employment, or food access 1 year after the disaster compared with victims who remained outside the camps and in their own neighborhoods.28 This finding underscores the need for accurate and timely triage by strike teams outside hospitals and quick treatment and return of patients to their homes.
Conceptually, strike teams need not be confined to medical response. Team members also might be specialists in epidemiology, disease surveillance, public health, culinary water protection, municipal security, and civil engineering. Noji reported that malnutrition, diarrheal diseases, measles, acute respiratory infections, and malaria consistently accounted for 60% to 95% of reported deaths among refugees and displaced populations.29 In 2005, Spiegel found that the potential for epidemics of communicable diseases was increased by overcrowding and poor sanitation in both natural disasters and complex emergencies.30 In 2007, Watson and colleagues suggested that communicable diseases may account for two-thirds of the deaths in conflict areas, and malnutrition significantly increases the risk of these diseases.31 Effective disaster care may be better accomplished through decentralized strike team interventions, which avoid the pitfalls of displacement and overcrowding.
Conclusion
Crises of all magnitudes can be greatly eased by well-trained, quick-response, all-hazards medical detachments—small teams that can be rapidly mobilized and deployed to establish casualty collection points, provide accurate triage, and render emergency care. These highly mobile teams can bridge the gap between the occurrence of a disaster and the arrival of substantial assistance from state, federal, and nongovernmental organizations—a most vulnerable time. These competent, flexible teams then can be absorbed by the larger force when it arrives for sustained disaster operations. Predisaster planning must take into account the possibility of long-term care for casualties and the displaced. Careful attention should be given to the potential for epidemics—immunizations should be administered quickly to achieve herd immunity—and a program that will provide food, water, shelter, sanitation, and security should be established.
Acknowledgments
The authors thank Sarah M. Paulsen and members of the Utah Air National Guard and Morrocan military for their friendship and help in preparing the manuscript.
1. Schultz CH, Koenig KL, Noji EK. A medical disaster response to reduce immediate mortality after an earthquake. N Engl J Med. 1996;334(7):438-444.
2. Merin O, Blumberg N, Raveh D, Bar A, Nishizawa M, Cohen-Marom O. Global responsibility in mass casualty events: the Israeli experience in Japan. Am J Disaster Med. 2012;7(1):61-64.
3. Sheng ZY. Medical support in the Tangshan earthquake: a review of the management of mass casualties and certain major injuries. J Trauma. 1987;27(10):1130-1135.
4. de Bruycker M, Greco D, Annino I, et al. The 1980 earthquake in southern Italy: rescue of trapped victims and mortality. Bull World Health Organ. 1983;61(6):1021-1025.
5. Safar P. Resuscitation potentials in mass disasters. Prehosp Disaster Med. 1986;2:34-47.
6. Pretto EA, Ricci E, Klain M, et al. Disaster reanimatology potentials: a structured interview study in Armenia III. Results, conclusions and recommendations. Prehosp Disaster Med. 1992;7:327-338.
7. Pretto EA, Angus DC, Abrams JI, et al. An analysis of prehospital mortality in an earthquake. Disaster Reanimatology Study Group. Prehosp Disaster Med. 1994;9(2):107-124.
8. Keyes R. The Quote Verifier: Who Said What, Where, and When. New York, NY: St. Martin’s Griffin; 2006.
9. Ginzberg E, O’Neill WW, Goldschmidt-Clermont PJ, de Marchena E, Pust D, Green BA. Rapid medical relief—Project Medishare and the Haitian earthquake. N Engl J Med. 2010;362(10):e31.
10. Pereira BM, Morales W, Cardoso RG, Fiorelli R, Fraga GP, Briggs SM. Lessons learned from a landslide catastrophe in Rio de Janeiro, Brazil. Am J Disaster Med. 2013;8(4):253-258.
11. Cross KP, Cicero MX. Head-to-head comparison of disaster triage methods in pediatric, adult, and geriatric patients. Ann Emerg Med. 2013;61(6):668-676.e7.
12. Sasser SM, Hunt RC, Faul M, et al; Centers for Disease Control and Prevention (CDC). Guidelines for field triage of injured patients: recommendations of the National Expert Panel on Field Triage, 2011. MMWR Recomm Rep. 2012;61(RR-1):1-20.
13. Kleber C, Cwojdzinski D, Strehl M, Poloczek S, Haas NP. Results of in-hospital triage in 17 mass casualty trainings: underestimation of life-threatening injuries and need for re-triage. Am J Disaster Med. 2013;8(1):5-11.
14. Talmor D, Jones AE, Rubinson L, Howell MD, Shapiro NI. Simple triage scoring system predicting death and the need for critical care resources for use during epidemics. Crit Care Med. 2007;35(5):1251-1256.
15. Mekel M, Bumenfeld A, Feigenberg Z, et al. Terrorist suicide bombings: lessons learned in metropolitan Haifa from September 2000 to January 2006. Am J Disaster Med. 2009;4(4):233-248.
16. McManus JG, Morton MJ, Crystal CS, et al. Use of ultrasound to assess acute fracture reduction in emergency care settings. Am J Disaster Med. 2008;3(4):241-247.
17. Dean AJ, Ku BS, Zeserson EM. The utility of handheld ultrasound in an austere medical setting in Guatemala after a natural disaster. Am J Disaster Med. 2007;2(5):249-256.
18. Waeckerle JF. Disaster planning and response. N Engl J Med. 1991;324(12):815-821.
19. Mallonee S, Shariat S, Stennies G, Waxweiler R, Hogan D, Jordan F. Physical injuries and fatalities resulting from the Oklahoma City bombing. JAMA. 1996;276(5):382-387.
20. Barillo DJ, Renz E, Broger K, Moak B, Wright G, Holcomb JB. An emergency medical bag set for long-range aeromedical transportation. Am J Disaster Med. 2008;3(2):79-86.
21. Seyedin SH, Aflatoonian MR, Ryan J. Adverse impact of international NGOs during and after the Bam earthquake: health system’s consumers’ points of view. Am J Disaster Med. 2009;4(3):173-179.
22. Merin O, Yitzhak A, Bader T. Medicine in a disaster area: lessons from the 2015 earthquake in Nepal. JAMA. 2015;175(9):1437-1438.
23. Ushizawa H, Foxwell AR, Bice S, et al. Needs for disaster medicine: lessons from the field of the great east Japan earthquake. Western Pac Surveill Response J. 2013;4(1):51-55.
24. Campos-Outcalt D. Disaster medical response: maximizing your effectiveness. J Fam Pract. 2006;55(2):113-115.
25. Patton-Levine JK, Vest JR, Valadez AM. Caregivers and families in medical special needs shelters: an experience during Hurricane Rita. Am J Disaster Med. 2007;2(2):81-86.
26. Halbert RJ, Simon RR, Nasraty Q. Surgical theatre in rural Afghanistan. Ann Emerg Med. 1988;17(8):775-778.
27. Halbert RJ, Simon RR, Nasraty Q. Surgical training model for advanced emergency medics in Afghanistan. Ann Emerg Med. 1988;17(8):779-784.
28. Kirsch TD, Leidman E, Weiss W, Doocy S. The impact of the earthquake and humanitarian assistance on household economies and livelihoods of earthquake-affected populations in Haiti. Am J Disaster Med. 2012;7(2):85-94.
29. Noji EK. Public health in the aftermath of disasters. BMJ. 2005;330(7504):1379-1381.
30. Spiegel PB. Differences in world responses to natural disasters and complex emergencies. JAMA. 2005;293(15):1915-1918.
31. Watson JT, Gayer M, Connolly MA. Epidemics after natural disasters. Emerg Infect Dis. 2007;13(1):1-5.
Environmental disasters can overpower local medical resources. Fortunately, such crises are rare in the U.S. This situation, however, has not always been the case. For example, in 1812, an earthquake along the New Madrid fault of the Mississippi Valley caused the Mississippi River to flow backward for 3 days.1 Today, in urbanized America, an earthquake of that magnitude would be devastating and severely overwhelm medical systems. All nations, including highly modernized nations, would need help in such disasters.2 A response system that is nimble, well-trained, scalable, and rapidly deployable can mitigate disaster sequelae. This article focuses on key aspects of effective rapid response, including speed, appropriate triage, quick-response strike teams, and disaster dynamics.
Why Speed Matters Most
Response time arguably is the most important factor in increasing survival in a disaster. In a 1996 study of earthquake disasters worldwide, Schultz and colleagues found a lower survival rate for victims who received medical care outside a 24-hour window.1 Studies of earthquakes in China have suggested that unless aid is rendered within 2 to 6 hours, fewer than half the victims will survive.3 Regarding a 1980 earthquake in Italy, de Bruycker and colleagues emphasized the importance of engaging in rescue activities within the first 48 hours.4 Safar reviewed mass disasters and reported that 25% to 50% of the injured and dead could have been saved if first aid had been provided immediately.5 In 1992 and 1994, Pretto and colleagues wrote that in earthquakes in Armenia and Costa Rica, many deaths could have been prevented had the victims received medical attention within the first 6 hours.6,7 The question is: How can responses to such crises be improved? Confederate Army Lt. Gen. Nathan Bedford Forrest’s dictum “[Get] there first with the most men” holds true in disaster medicine as well: get there fast with the right people, training, equipment, and supplies.8
Deaths in disasters can be described in a 3-phase distribution: immediate, early, and delayed. Stringent building codes and public warnings and evacuations reduce immediate deaths, but victims also die of catastrophic injury soon after an event. Early deaths are preventable with use of rapid interventions, such as tourniquets and airway adjuncts, but these must be administered within minutes or hours. Delayed deaths occur days or weeks after injury secondary to infection or organ system failure—which emphasizes the value of early wound care.
Emergency Supplies
What items are most needed? As each disaster is different, it would be presumptuous to provide a one-size-fits-all list, but some common supplies have been suggested. In 2010, Ginzberg and colleagues reported that during the first 24 hours of the Haiti earthquake of 1996, the overwhelming need was for IV hydration, narcotic analgesics, and casting supplies for the splinting of fractures.9 During the next 24 hours, IV stabilization was key, along with monitoring by Foley and suprapubic catheters. In the third 24-hour period, providers began to see sepsis-related deaths. In response to this challenge, teams began aggressive treatment with open surgical debridement of wounds, amputation of severely injured limbs, and administration of broad-spectrum IV antibiotics. Regional anesthesia with conscious sedation was mandatory because supplemental oxygen and ventilators were unavailable. By day 4, wound debridement, amputations, and fasciotomies were being provided by newly arrived anesthesiologists and orthopedic surgeons. Ginzberg and colleagues emphasized that rapid response was key in maximizing survival and by day 4, there was a greater need for surgical teams and broad-spectrum antibiotics (eg, piperacillin, tazobactam) to combat sepsis.
Pereira and colleagues reported that in a catastrophe caused by a tropical storm and landslides in Brazil, the most common injuries involved the extremities; the majority of wounds required only cleaning, debridement, and suture; and the most commonly performed operations were for orthopedic injuries.10 Incidentally, population baseline morbidity and mortality continue during disasters, and rescue personnel invariably sustain injuries, which contribute to the total medical burden. These additional injuries must be anticipated, and plans to manage them must be included in any disaster contingency planning.
Triage
Speed and correct triage are essential building blocks of disaster response. When resources are limited, triage is crucial in providing the right treatment to the right patient. There are numerous triage methods, some more rapid and straightforward; others more effective and cumbersome.11 In 2012, Sasser and colleagues wrote that the purpose of triage is to ensure injured patients are transported to a trauma center or the hospital best equipped to manage their specific injuries in an appropriate and timely manner.12 Their report focused on prehospital emergent care, not mass-casualty or disaster situations.
Triage is sometimes performed inconsistently. In a 2013 study, Kleber and colleagues found that 24% of providers overtriage and 16% undertriage.13 In the U.S., simple triage and rapid treatment (START) is commonly used to sort traumatized patients. All these methods take a “worst gets first treatment” approach. Depending on the magnitude of an event, however, providers may take a reverse-triage approach, in which they better use resources for the least injured patients and provide palliative care to the gravely ill.
During pandemic disasters, trauma triage protocols are ineffective. Instead, these events demand assessments that are sensitive to infectious diseases. Timely, didactic, hands-on training must be conducted before the fact so responders can adapt to react appropriately to a given disaster.14
Accurate, timely triage in mass-casualty incidents was conceptually demonstrated by Mekel and colleagues who reviewed the medical management of bombing victims in metropolitan Haifa, Israel during the period 2000 to 2006.15 Providers initiated a predetermined triage system in which patients are assigned to the appropriate echelon of care. Of 342 injured patients, 9.5% had severe injuries, 2.4% had moderate-severe injuries, and 88.9% had mild injuries. Correct and timely triage directed trauma victims to the appropriate medical care. Such action prevents the highest level facility from becoming overcrowded with less severely injured patients and ensures that the more critically injured receive a level of care comparable to that given under nondisaster circumstances.
The handheld ultrasound device, which can be used to correctly diagnose fractures, is an efficient triage resource for prehospital teams. In a 2008 study, McManus and colleagues suggested that ultrasound (vs traditional radiography) could be used to identify fractures in an emergency room.16 A handheld ultrasound device could be used outside the hospital, in the field, potentially reducing the number of referrals to overwhelmed orthopedic hospitals.
In 2007, Dean and colleagues reported on using ultrasound to rapidly triage disease during an earthquake in Guatemala.17 In that disaster, 23% of injuries presented within the first 24 hours, and a handheld ultrasound device was used to assess orthopedic injuries—ruling in 12% and ruling out 42%. The handheld ultrasound device is an example of a tool that small medical teams can use to speed triage, enhance patient care, and relieve overcrowded medical centers of the unrelenting pressure.
On-Site vs Hospital
Complicating disaster response is self-triage. Victims with injuries of all severity levels go to the nearest hospital and overwhelm it. In 1991, Waeckerle reported that within the first 30 minutes of a disaster, a wave of victims arrives, usually with minor injuries, and impedes care for the more severely wounded.18 Correct triage instead would have directed these patients to a hospital other than the overwhelmed level I trauma center.15 This is not to say that patients with mild or moderate injuries are unimportant—just that their care may take scarce space and resources from the more severely injured.
Mallonee and colleagues reported that of the 759 people injured in the 1996 Oklahoma City bombing, 167 (22%) were fatalities, 83 (11%) were hospitalized, and 509 (67%) were treated on an outpatient basis.19 Most of the injuries could have been managed by quick-response medical teams operating in the affected area, outside the hospitals. This action would have reduced operational pressure on hospitals and improved severely injured patients’ access to care.
Specialized Teams
In 2008, Barillo and colleagues suggested that having standardized medic bags would allow a small detachment of medical professionals to provide care nimbly—and doing so would represent a leap forward in access to care.20
Because of their unique ability to understand the culture and coordinate military assets with local authorities, DoD international health specialists are crucial interfaces for any population, foreign or domestic. Seyedin and colleagues and Merin and colleagues suggested that in both the Bam earthquake in 2003 and the Nepal earthquake in 2015, understanding the culture played a vital role in health care delivery and in adhering to cultural norms in deciding when to perform surgery, making end-of-life decisions, communicating with family, establishing trust with local and regional leaders, and other matters.21,22
Strike teams are small groups of variably trained health care providers who are dispatched to underserved, outlying, or overwhelmed areas to deliver precached basic medical care and triage significant injuries to medical centers. The handheld ultrasound device is an example of a strike team tool. During a local emergency, it is understood or assumed that response is inundated and that people are going untreated.
Crucially, strike teams must be trained, prepared, and readily dispatched ahead of larger response elements. Though quickly deployable, disaster medical assistance teams (DMATs) and National Guard Chemical, Biological, Radiological, Nuclear and High-Yield Explosive Enhanced Response Force Package units, take time to mobilize. Therefore, strike teams should consist of community citizens or local National Guard assets, the latter being particularly suited to rapid response given their training, effective command and control, and intrinsic logistics.
The efficacy of strike teams was demonstrated during the 2011 earthquake in Japan.23 Disaster medical assistance teams were invaluable in triaging and treating patients during the first 3 days. A team left 34 minutes after the event to render aid to people caught in a roof collapse. During triage, 17% of the injuries were classified urgent, 22% intermediate, and 61% minor. On day 7, a DMAT was dispatched to assist with emergency medicine and primary care; 3% of the injuries were severe and required urgent care, 50% required intermediate care, and 47% required minor care.
The value of strike teams is 3-fold: It provides rapid, professional care at a crucial place and critical time; it correctly triages patients and thus allow hospitals to maintain resources for the more severely injured; and augments overwhelmed providers at crucial sites. The roles of strike teams were echoed in 2006 by Campos-Outcalt, who reported that DMATs deployed to austere locations had the flexibility to augment existing medical staff and to rapidly deploy, self-sustain, and treat patients until a situation was resolved.24 This nimble strike team mentality could become a rapid and flexible model to save more lives, relieve significant suffering, and offload pressure from local hospitals by treating the less critically injured.
After a disaster, space is at a premium, and nonmedical residents who make up 40% to 70% of the shelter population require resources as well.25 Family members and the lightly injured may be conscripted to augment the overwhelmed medical staff. In 1988, Halbert and colleagues described how Afghan volunteers with minimal medical experience were given training and supplies and served as advanced emergency medics, delivering medical care and performing well under austere conditions.26,27 Strike teams thus may provide on-scene training in addition to medical care.
In 2012, Kirsch and colleaguesfound that Haiti earthquake victims who received treatment and remained in camps showed no improvement in income, employment, or food access 1 year after the disaster compared with victims who remained outside the camps and in their own neighborhoods.28 This finding underscores the need for accurate and timely triage by strike teams outside hospitals and quick treatment and return of patients to their homes.
Conceptually, strike teams need not be confined to medical response. Team members also might be specialists in epidemiology, disease surveillance, public health, culinary water protection, municipal security, and civil engineering. Noji reported that malnutrition, diarrheal diseases, measles, acute respiratory infections, and malaria consistently accounted for 60% to 95% of reported deaths among refugees and displaced populations.29 In 2005, Spiegel found that the potential for epidemics of communicable diseases was increased by overcrowding and poor sanitation in both natural disasters and complex emergencies.30 In 2007, Watson and colleagues suggested that communicable diseases may account for two-thirds of the deaths in conflict areas, and malnutrition significantly increases the risk of these diseases.31 Effective disaster care may be better accomplished through decentralized strike team interventions, which avoid the pitfalls of displacement and overcrowding.
Conclusion
Crises of all magnitudes can be greatly eased by well-trained, quick-response, all-hazards medical detachments—small teams that can be rapidly mobilized and deployed to establish casualty collection points, provide accurate triage, and render emergency care. These highly mobile teams can bridge the gap between the occurrence of a disaster and the arrival of substantial assistance from state, federal, and nongovernmental organizations—a most vulnerable time. These competent, flexible teams then can be absorbed by the larger force when it arrives for sustained disaster operations. Predisaster planning must take into account the possibility of long-term care for casualties and the displaced. Careful attention should be given to the potential for epidemics—immunizations should be administered quickly to achieve herd immunity—and a program that will provide food, water, shelter, sanitation, and security should be established.
Acknowledgments
The authors thank Sarah M. Paulsen and members of the Utah Air National Guard and Morrocan military for their friendship and help in preparing the manuscript.
Environmental disasters can overpower local medical resources. Fortunately, such crises are rare in the U.S. This situation, however, has not always been the case. For example, in 1812, an earthquake along the New Madrid fault of the Mississippi Valley caused the Mississippi River to flow backward for 3 days.1 Today, in urbanized America, an earthquake of that magnitude would be devastating and severely overwhelm medical systems. All nations, including highly modernized nations, would need help in such disasters.2 A response system that is nimble, well-trained, scalable, and rapidly deployable can mitigate disaster sequelae. This article focuses on key aspects of effective rapid response, including speed, appropriate triage, quick-response strike teams, and disaster dynamics.
Why Speed Matters Most
Response time arguably is the most important factor in increasing survival in a disaster. In a 1996 study of earthquake disasters worldwide, Schultz and colleagues found a lower survival rate for victims who received medical care outside a 24-hour window.1 Studies of earthquakes in China have suggested that unless aid is rendered within 2 to 6 hours, fewer than half the victims will survive.3 Regarding a 1980 earthquake in Italy, de Bruycker and colleagues emphasized the importance of engaging in rescue activities within the first 48 hours.4 Safar reviewed mass disasters and reported that 25% to 50% of the injured and dead could have been saved if first aid had been provided immediately.5 In 1992 and 1994, Pretto and colleagues wrote that in earthquakes in Armenia and Costa Rica, many deaths could have been prevented had the victims received medical attention within the first 6 hours.6,7 The question is: How can responses to such crises be improved? Confederate Army Lt. Gen. Nathan Bedford Forrest’s dictum “[Get] there first with the most men” holds true in disaster medicine as well: get there fast with the right people, training, equipment, and supplies.8
Deaths in disasters can be described in a 3-phase distribution: immediate, early, and delayed. Stringent building codes and public warnings and evacuations reduce immediate deaths, but victims also die of catastrophic injury soon after an event. Early deaths are preventable with use of rapid interventions, such as tourniquets and airway adjuncts, but these must be administered within minutes or hours. Delayed deaths occur days or weeks after injury secondary to infection or organ system failure—which emphasizes the value of early wound care.
Emergency Supplies
What items are most needed? As each disaster is different, it would be presumptuous to provide a one-size-fits-all list, but some common supplies have been suggested. In 2010, Ginzberg and colleagues reported that during the first 24 hours of the Haiti earthquake of 1996, the overwhelming need was for IV hydration, narcotic analgesics, and casting supplies for the splinting of fractures.9 During the next 24 hours, IV stabilization was key, along with monitoring by Foley and suprapubic catheters. In the third 24-hour period, providers began to see sepsis-related deaths. In response to this challenge, teams began aggressive treatment with open surgical debridement of wounds, amputation of severely injured limbs, and administration of broad-spectrum IV antibiotics. Regional anesthesia with conscious sedation was mandatory because supplemental oxygen and ventilators were unavailable. By day 4, wound debridement, amputations, and fasciotomies were being provided by newly arrived anesthesiologists and orthopedic surgeons. Ginzberg and colleagues emphasized that rapid response was key in maximizing survival and by day 4, there was a greater need for surgical teams and broad-spectrum antibiotics (eg, piperacillin, tazobactam) to combat sepsis.
Pereira and colleagues reported that in a catastrophe caused by a tropical storm and landslides in Brazil, the most common injuries involved the extremities; the majority of wounds required only cleaning, debridement, and suture; and the most commonly performed operations were for orthopedic injuries.10 Incidentally, population baseline morbidity and mortality continue during disasters, and rescue personnel invariably sustain injuries, which contribute to the total medical burden. These additional injuries must be anticipated, and plans to manage them must be included in any disaster contingency planning.
Triage
Speed and correct triage are essential building blocks of disaster response. When resources are limited, triage is crucial in providing the right treatment to the right patient. There are numerous triage methods, some more rapid and straightforward; others more effective and cumbersome.11 In 2012, Sasser and colleagues wrote that the purpose of triage is to ensure injured patients are transported to a trauma center or the hospital best equipped to manage their specific injuries in an appropriate and timely manner.12 Their report focused on prehospital emergent care, not mass-casualty or disaster situations.
Triage is sometimes performed inconsistently. In a 2013 study, Kleber and colleagues found that 24% of providers overtriage and 16% undertriage.13 In the U.S., simple triage and rapid treatment (START) is commonly used to sort traumatized patients. All these methods take a “worst gets first treatment” approach. Depending on the magnitude of an event, however, providers may take a reverse-triage approach, in which they better use resources for the least injured patients and provide palliative care to the gravely ill.
During pandemic disasters, trauma triage protocols are ineffective. Instead, these events demand assessments that are sensitive to infectious diseases. Timely, didactic, hands-on training must be conducted before the fact so responders can adapt to react appropriately to a given disaster.14
Accurate, timely triage in mass-casualty incidents was conceptually demonstrated by Mekel and colleagues who reviewed the medical management of bombing victims in metropolitan Haifa, Israel during the period 2000 to 2006.15 Providers initiated a predetermined triage system in which patients are assigned to the appropriate echelon of care. Of 342 injured patients, 9.5% had severe injuries, 2.4% had moderate-severe injuries, and 88.9% had mild injuries. Correct and timely triage directed trauma victims to the appropriate medical care. Such action prevents the highest level facility from becoming overcrowded with less severely injured patients and ensures that the more critically injured receive a level of care comparable to that given under nondisaster circumstances.
The handheld ultrasound device, which can be used to correctly diagnose fractures, is an efficient triage resource for prehospital teams. In a 2008 study, McManus and colleagues suggested that ultrasound (vs traditional radiography) could be used to identify fractures in an emergency room.16 A handheld ultrasound device could be used outside the hospital, in the field, potentially reducing the number of referrals to overwhelmed orthopedic hospitals.
In 2007, Dean and colleagues reported on using ultrasound to rapidly triage disease during an earthquake in Guatemala.17 In that disaster, 23% of injuries presented within the first 24 hours, and a handheld ultrasound device was used to assess orthopedic injuries—ruling in 12% and ruling out 42%. The handheld ultrasound device is an example of a tool that small medical teams can use to speed triage, enhance patient care, and relieve overcrowded medical centers of the unrelenting pressure.
On-Site vs Hospital
Complicating disaster response is self-triage. Victims with injuries of all severity levels go to the nearest hospital and overwhelm it. In 1991, Waeckerle reported that within the first 30 minutes of a disaster, a wave of victims arrives, usually with minor injuries, and impedes care for the more severely wounded.18 Correct triage instead would have directed these patients to a hospital other than the overwhelmed level I trauma center.15 This is not to say that patients with mild or moderate injuries are unimportant—just that their care may take scarce space and resources from the more severely injured.
Mallonee and colleagues reported that of the 759 people injured in the 1996 Oklahoma City bombing, 167 (22%) were fatalities, 83 (11%) were hospitalized, and 509 (67%) were treated on an outpatient basis.19 Most of the injuries could have been managed by quick-response medical teams operating in the affected area, outside the hospitals. This action would have reduced operational pressure on hospitals and improved severely injured patients’ access to care.
Specialized Teams
In 2008, Barillo and colleagues suggested that having standardized medic bags would allow a small detachment of medical professionals to provide care nimbly—and doing so would represent a leap forward in access to care.20
Because of their unique ability to understand the culture and coordinate military assets with local authorities, DoD international health specialists are crucial interfaces for any population, foreign or domestic. Seyedin and colleagues and Merin and colleagues suggested that in both the Bam earthquake in 2003 and the Nepal earthquake in 2015, understanding the culture played a vital role in health care delivery and in adhering to cultural norms in deciding when to perform surgery, making end-of-life decisions, communicating with family, establishing trust with local and regional leaders, and other matters.21,22
Strike teams are small groups of variably trained health care providers who are dispatched to underserved, outlying, or overwhelmed areas to deliver precached basic medical care and triage significant injuries to medical centers. The handheld ultrasound device is an example of a strike team tool. During a local emergency, it is understood or assumed that response is inundated and that people are going untreated.
Crucially, strike teams must be trained, prepared, and readily dispatched ahead of larger response elements. Though quickly deployable, disaster medical assistance teams (DMATs) and National Guard Chemical, Biological, Radiological, Nuclear and High-Yield Explosive Enhanced Response Force Package units, take time to mobilize. Therefore, strike teams should consist of community citizens or local National Guard assets, the latter being particularly suited to rapid response given their training, effective command and control, and intrinsic logistics.
The efficacy of strike teams was demonstrated during the 2011 earthquake in Japan.23 Disaster medical assistance teams were invaluable in triaging and treating patients during the first 3 days. A team left 34 minutes after the event to render aid to people caught in a roof collapse. During triage, 17% of the injuries were classified urgent, 22% intermediate, and 61% minor. On day 7, a DMAT was dispatched to assist with emergency medicine and primary care; 3% of the injuries were severe and required urgent care, 50% required intermediate care, and 47% required minor care.
The value of strike teams is 3-fold: It provides rapid, professional care at a crucial place and critical time; it correctly triages patients and thus allow hospitals to maintain resources for the more severely injured; and augments overwhelmed providers at crucial sites. The roles of strike teams were echoed in 2006 by Campos-Outcalt, who reported that DMATs deployed to austere locations had the flexibility to augment existing medical staff and to rapidly deploy, self-sustain, and treat patients until a situation was resolved.24 This nimble strike team mentality could become a rapid and flexible model to save more lives, relieve significant suffering, and offload pressure from local hospitals by treating the less critically injured.
After a disaster, space is at a premium, and nonmedical residents who make up 40% to 70% of the shelter population require resources as well.25 Family members and the lightly injured may be conscripted to augment the overwhelmed medical staff. In 1988, Halbert and colleagues described how Afghan volunteers with minimal medical experience were given training and supplies and served as advanced emergency medics, delivering medical care and performing well under austere conditions.26,27 Strike teams thus may provide on-scene training in addition to medical care.
In 2012, Kirsch and colleaguesfound that Haiti earthquake victims who received treatment and remained in camps showed no improvement in income, employment, or food access 1 year after the disaster compared with victims who remained outside the camps and in their own neighborhoods.28 This finding underscores the need for accurate and timely triage by strike teams outside hospitals and quick treatment and return of patients to their homes.
Conceptually, strike teams need not be confined to medical response. Team members also might be specialists in epidemiology, disease surveillance, public health, culinary water protection, municipal security, and civil engineering. Noji reported that malnutrition, diarrheal diseases, measles, acute respiratory infections, and malaria consistently accounted for 60% to 95% of reported deaths among refugees and displaced populations.29 In 2005, Spiegel found that the potential for epidemics of communicable diseases was increased by overcrowding and poor sanitation in both natural disasters and complex emergencies.30 In 2007, Watson and colleagues suggested that communicable diseases may account for two-thirds of the deaths in conflict areas, and malnutrition significantly increases the risk of these diseases.31 Effective disaster care may be better accomplished through decentralized strike team interventions, which avoid the pitfalls of displacement and overcrowding.
Conclusion
Crises of all magnitudes can be greatly eased by well-trained, quick-response, all-hazards medical detachments—small teams that can be rapidly mobilized and deployed to establish casualty collection points, provide accurate triage, and render emergency care. These highly mobile teams can bridge the gap between the occurrence of a disaster and the arrival of substantial assistance from state, federal, and nongovernmental organizations—a most vulnerable time. These competent, flexible teams then can be absorbed by the larger force when it arrives for sustained disaster operations. Predisaster planning must take into account the possibility of long-term care for casualties and the displaced. Careful attention should be given to the potential for epidemics—immunizations should be administered quickly to achieve herd immunity—and a program that will provide food, water, shelter, sanitation, and security should be established.
Acknowledgments
The authors thank Sarah M. Paulsen and members of the Utah Air National Guard and Morrocan military for their friendship and help in preparing the manuscript.
1. Schultz CH, Koenig KL, Noji EK. A medical disaster response to reduce immediate mortality after an earthquake. N Engl J Med. 1996;334(7):438-444.
2. Merin O, Blumberg N, Raveh D, Bar A, Nishizawa M, Cohen-Marom O. Global responsibility in mass casualty events: the Israeli experience in Japan. Am J Disaster Med. 2012;7(1):61-64.
3. Sheng ZY. Medical support in the Tangshan earthquake: a review of the management of mass casualties and certain major injuries. J Trauma. 1987;27(10):1130-1135.
4. de Bruycker M, Greco D, Annino I, et al. The 1980 earthquake in southern Italy: rescue of trapped victims and mortality. Bull World Health Organ. 1983;61(6):1021-1025.
5. Safar P. Resuscitation potentials in mass disasters. Prehosp Disaster Med. 1986;2:34-47.
6. Pretto EA, Ricci E, Klain M, et al. Disaster reanimatology potentials: a structured interview study in Armenia III. Results, conclusions and recommendations. Prehosp Disaster Med. 1992;7:327-338.
7. Pretto EA, Angus DC, Abrams JI, et al. An analysis of prehospital mortality in an earthquake. Disaster Reanimatology Study Group. Prehosp Disaster Med. 1994;9(2):107-124.
8. Keyes R. The Quote Verifier: Who Said What, Where, and When. New York, NY: St. Martin’s Griffin; 2006.
9. Ginzberg E, O’Neill WW, Goldschmidt-Clermont PJ, de Marchena E, Pust D, Green BA. Rapid medical relief—Project Medishare and the Haitian earthquake. N Engl J Med. 2010;362(10):e31.
10. Pereira BM, Morales W, Cardoso RG, Fiorelli R, Fraga GP, Briggs SM. Lessons learned from a landslide catastrophe in Rio de Janeiro, Brazil. Am J Disaster Med. 2013;8(4):253-258.
11. Cross KP, Cicero MX. Head-to-head comparison of disaster triage methods in pediatric, adult, and geriatric patients. Ann Emerg Med. 2013;61(6):668-676.e7.
12. Sasser SM, Hunt RC, Faul M, et al; Centers for Disease Control and Prevention (CDC). Guidelines for field triage of injured patients: recommendations of the National Expert Panel on Field Triage, 2011. MMWR Recomm Rep. 2012;61(RR-1):1-20.
13. Kleber C, Cwojdzinski D, Strehl M, Poloczek S, Haas NP. Results of in-hospital triage in 17 mass casualty trainings: underestimation of life-threatening injuries and need for re-triage. Am J Disaster Med. 2013;8(1):5-11.
14. Talmor D, Jones AE, Rubinson L, Howell MD, Shapiro NI. Simple triage scoring system predicting death and the need for critical care resources for use during epidemics. Crit Care Med. 2007;35(5):1251-1256.
15. Mekel M, Bumenfeld A, Feigenberg Z, et al. Terrorist suicide bombings: lessons learned in metropolitan Haifa from September 2000 to January 2006. Am J Disaster Med. 2009;4(4):233-248.
16. McManus JG, Morton MJ, Crystal CS, et al. Use of ultrasound to assess acute fracture reduction in emergency care settings. Am J Disaster Med. 2008;3(4):241-247.
17. Dean AJ, Ku BS, Zeserson EM. The utility of handheld ultrasound in an austere medical setting in Guatemala after a natural disaster. Am J Disaster Med. 2007;2(5):249-256.
18. Waeckerle JF. Disaster planning and response. N Engl J Med. 1991;324(12):815-821.
19. Mallonee S, Shariat S, Stennies G, Waxweiler R, Hogan D, Jordan F. Physical injuries and fatalities resulting from the Oklahoma City bombing. JAMA. 1996;276(5):382-387.
20. Barillo DJ, Renz E, Broger K, Moak B, Wright G, Holcomb JB. An emergency medical bag set for long-range aeromedical transportation. Am J Disaster Med. 2008;3(2):79-86.
21. Seyedin SH, Aflatoonian MR, Ryan J. Adverse impact of international NGOs during and after the Bam earthquake: health system’s consumers’ points of view. Am J Disaster Med. 2009;4(3):173-179.
22. Merin O, Yitzhak A, Bader T. Medicine in a disaster area: lessons from the 2015 earthquake in Nepal. JAMA. 2015;175(9):1437-1438.
23. Ushizawa H, Foxwell AR, Bice S, et al. Needs for disaster medicine: lessons from the field of the great east Japan earthquake. Western Pac Surveill Response J. 2013;4(1):51-55.
24. Campos-Outcalt D. Disaster medical response: maximizing your effectiveness. J Fam Pract. 2006;55(2):113-115.
25. Patton-Levine JK, Vest JR, Valadez AM. Caregivers and families in medical special needs shelters: an experience during Hurricane Rita. Am J Disaster Med. 2007;2(2):81-86.
26. Halbert RJ, Simon RR, Nasraty Q. Surgical theatre in rural Afghanistan. Ann Emerg Med. 1988;17(8):775-778.
27. Halbert RJ, Simon RR, Nasraty Q. Surgical training model for advanced emergency medics in Afghanistan. Ann Emerg Med. 1988;17(8):779-784.
28. Kirsch TD, Leidman E, Weiss W, Doocy S. The impact of the earthquake and humanitarian assistance on household economies and livelihoods of earthquake-affected populations in Haiti. Am J Disaster Med. 2012;7(2):85-94.
29. Noji EK. Public health in the aftermath of disasters. BMJ. 2005;330(7504):1379-1381.
30. Spiegel PB. Differences in world responses to natural disasters and complex emergencies. JAMA. 2005;293(15):1915-1918.
31. Watson JT, Gayer M, Connolly MA. Epidemics after natural disasters. Emerg Infect Dis. 2007;13(1):1-5.
1. Schultz CH, Koenig KL, Noji EK. A medical disaster response to reduce immediate mortality after an earthquake. N Engl J Med. 1996;334(7):438-444.
2. Merin O, Blumberg N, Raveh D, Bar A, Nishizawa M, Cohen-Marom O. Global responsibility in mass casualty events: the Israeli experience in Japan. Am J Disaster Med. 2012;7(1):61-64.
3. Sheng ZY. Medical support in the Tangshan earthquake: a review of the management of mass casualties and certain major injuries. J Trauma. 1987;27(10):1130-1135.
4. de Bruycker M, Greco D, Annino I, et al. The 1980 earthquake in southern Italy: rescue of trapped victims and mortality. Bull World Health Organ. 1983;61(6):1021-1025.
5. Safar P. Resuscitation potentials in mass disasters. Prehosp Disaster Med. 1986;2:34-47.
6. Pretto EA, Ricci E, Klain M, et al. Disaster reanimatology potentials: a structured interview study in Armenia III. Results, conclusions and recommendations. Prehosp Disaster Med. 1992;7:327-338.
7. Pretto EA, Angus DC, Abrams JI, et al. An analysis of prehospital mortality in an earthquake. Disaster Reanimatology Study Group. Prehosp Disaster Med. 1994;9(2):107-124.
8. Keyes R. The Quote Verifier: Who Said What, Where, and When. New York, NY: St. Martin’s Griffin; 2006.
9. Ginzberg E, O’Neill WW, Goldschmidt-Clermont PJ, de Marchena E, Pust D, Green BA. Rapid medical relief—Project Medishare and the Haitian earthquake. N Engl J Med. 2010;362(10):e31.
10. Pereira BM, Morales W, Cardoso RG, Fiorelli R, Fraga GP, Briggs SM. Lessons learned from a landslide catastrophe in Rio de Janeiro, Brazil. Am J Disaster Med. 2013;8(4):253-258.
11. Cross KP, Cicero MX. Head-to-head comparison of disaster triage methods in pediatric, adult, and geriatric patients. Ann Emerg Med. 2013;61(6):668-676.e7.
12. Sasser SM, Hunt RC, Faul M, et al; Centers for Disease Control and Prevention (CDC). Guidelines for field triage of injured patients: recommendations of the National Expert Panel on Field Triage, 2011. MMWR Recomm Rep. 2012;61(RR-1):1-20.
13. Kleber C, Cwojdzinski D, Strehl M, Poloczek S, Haas NP. Results of in-hospital triage in 17 mass casualty trainings: underestimation of life-threatening injuries and need for re-triage. Am J Disaster Med. 2013;8(1):5-11.
14. Talmor D, Jones AE, Rubinson L, Howell MD, Shapiro NI. Simple triage scoring system predicting death and the need for critical care resources for use during epidemics. Crit Care Med. 2007;35(5):1251-1256.
15. Mekel M, Bumenfeld A, Feigenberg Z, et al. Terrorist suicide bombings: lessons learned in metropolitan Haifa from September 2000 to January 2006. Am J Disaster Med. 2009;4(4):233-248.
16. McManus JG, Morton MJ, Crystal CS, et al. Use of ultrasound to assess acute fracture reduction in emergency care settings. Am J Disaster Med. 2008;3(4):241-247.
17. Dean AJ, Ku BS, Zeserson EM. The utility of handheld ultrasound in an austere medical setting in Guatemala after a natural disaster. Am J Disaster Med. 2007;2(5):249-256.
18. Waeckerle JF. Disaster planning and response. N Engl J Med. 1991;324(12):815-821.
19. Mallonee S, Shariat S, Stennies G, Waxweiler R, Hogan D, Jordan F. Physical injuries and fatalities resulting from the Oklahoma City bombing. JAMA. 1996;276(5):382-387.
20. Barillo DJ, Renz E, Broger K, Moak B, Wright G, Holcomb JB. An emergency medical bag set for long-range aeromedical transportation. Am J Disaster Med. 2008;3(2):79-86.
21. Seyedin SH, Aflatoonian MR, Ryan J. Adverse impact of international NGOs during and after the Bam earthquake: health system’s consumers’ points of view. Am J Disaster Med. 2009;4(3):173-179.
22. Merin O, Yitzhak A, Bader T. Medicine in a disaster area: lessons from the 2015 earthquake in Nepal. JAMA. 2015;175(9):1437-1438.
23. Ushizawa H, Foxwell AR, Bice S, et al. Needs for disaster medicine: lessons from the field of the great east Japan earthquake. Western Pac Surveill Response J. 2013;4(1):51-55.
24. Campos-Outcalt D. Disaster medical response: maximizing your effectiveness. J Fam Pract. 2006;55(2):113-115.
25. Patton-Levine JK, Vest JR, Valadez AM. Caregivers and families in medical special needs shelters: an experience during Hurricane Rita. Am J Disaster Med. 2007;2(2):81-86.
26. Halbert RJ, Simon RR, Nasraty Q. Surgical theatre in rural Afghanistan. Ann Emerg Med. 1988;17(8):775-778.
27. Halbert RJ, Simon RR, Nasraty Q. Surgical training model for advanced emergency medics in Afghanistan. Ann Emerg Med. 1988;17(8):779-784.
28. Kirsch TD, Leidman E, Weiss W, Doocy S. The impact of the earthquake and humanitarian assistance on household economies and livelihoods of earthquake-affected populations in Haiti. Am J Disaster Med. 2012;7(2):85-94.
29. Noji EK. Public health in the aftermath of disasters. BMJ. 2005;330(7504):1379-1381.
30. Spiegel PB. Differences in world responses to natural disasters and complex emergencies. JAMA. 2005;293(15):1915-1918.
31. Watson JT, Gayer M, Connolly MA. Epidemics after natural disasters. Emerg Infect Dis. 2007;13(1):1-5.