User login
The Challenges of Precision Medicine and New Advances in Molecular Diagnostic Testing in Hematolymphoid Malignancies: Impact on the VHA (FULL)
In January 2015, President Obama introduced the Precision Medicine Initiative, a program set up to identify new biomedical discoveries for the development of a personalized knowledge base of disease entities and individualized treatments. Advances in precision medicine typically involve the use of targeted therapies tailored to individual genetic characteristics identified with molecular testing. The goals are to improve survival and reduce adverse effects. With an initial budget of $215 million, this initiative presented a unique opportunity to combine efforts in genomic discovery, bioinformatic analysis, and health information technology to move toward data-driven, evidence-based precision medicine.1
The VHA is the largest comprehensive health care system in the U. S. and has more than 1,700 care sites serving nearly 9 million veterans each year. The budget for this single-payer system is proposed by the President and approved by Congress. As the VHA must treat a diverse and aging veteran population in an environment of rising costs and budget constraints, limited resources must be monitored and appropriated for the most cost-effective health care delivery. Precision medicine offers a model in which physicians can select the most appropriate diagnostic tests in defined clinical settings to direct clinical care. It supports the testing needed to subdivide each disease category into distinct subcategories. Nevertheless, the need for fiscal responsibility in a capitated health care system recommends testing in cases in which it can change therapy or prognosis rather than for purely academic reasons.
Pathology and Laboratory Medicine Service
Given limited resources and an increasing number of requests for advanced molecular testing, the VA Pathology and Laboratory Medicine Service (P&LMS) formed the Molecular Genetics Pathology Workgroup (MGPW) in September 2013. The charter listed the tasks of the MGPW to “provide recommendations on how to effectively use molecular genetics tests, promote increased quality and availability of testing within the VHA, encourage internal referral testing, provide an organizational structure for Molecular Genetics Testing Consortia, and create a P&LMS policy for molecular genetic testing in general, specifically addressing the issues surrounding laboratory developed testing.” The MGPW has 4 subcommittees: molecular oncology, pharmacogenetics, hematopathology molecular genetics (HMG), and genetic medicine. Since its inception, the HMG subcommittee has had several objectives:
- Standardize the molecular testing nomenclature for and develop practice guidelines for acute myeloid leukemia (AML), myeloproliferative neoplasms (MPN), myelodysplastic syndrome (MDS), chronic lymphocytic leukemia (CLL)/small lymphocytic lymphoma, lymphoma, and plasma cell neoplasms;
- Develop standardized reporting guidelines for current VA molecular laboratories;
- Identify new tests as they are being reported in the literature and collaborate with hematology and oncology services to evaluate the clinical utility of these tests for VA patients;
- Network current VA molecular laboratories, perform fact-finding for these laboratories, and compile test menus; and
- Assess for the formation of VA-wide interfacility consultation services for hematopathology so that all VA facilities, regardless of their complexity, will be able to access the expertise of hematopathology-trained pathologists (Appendix).
The HMG subcommittee met monthly and discussed various diagnostic entities in hematopathology. For hematolymphoid malignancies, it was generally agreed that the traditional laboratory tools of morphology, flow cytometry, and immunohistochemistry (IHC) are standard in initial assessment and often in diagnosis. As the clinical molecular and cytogenetic assays of karyotype, fluorescence in situ hybridization (FISH), advanced DNA sequencing, microarray, and highly sensitive polymerase chain reaction (PCR) analysis affect diagnosis, subclassification, minimal residual disease (MRD) monitoring, prognosis, and therapy selection, their use is marked by a high degree of variability. As a result, standardization is needed. As each laboratory develops and reports ancillary testing, the variable reporting formats may generate postanalytic errors.
A detailed description of all molecular methodologies is beyond the scope of this article. For practicing pathologists, challenges remain in overall cost and reimbursement, extensive and time-consuming data analysis, and in some cases, interpretation differences.
Myeloid Neoplasms
Myeloid malignancies were divided into AML, MPN, and MDS. Next-generation sequencing (NGS) information for these malignancies was used to identify various contributory functional categories, including cell signaling (FLT3, KIT, JAK2, MPL, KRAS/NRAS, PTPN11, NF1, CSF3R); transcription (CEBPA, RUNX1, GATA1/GATA2, PHF6, ETV6); splicing (SF3B1, SRSF2, ZRSR2, U2AF1); epigenetics (DNMT3A, TET2, IDH1/IDH2, ASXL1, EZH2, SUZ12, KDM6A); cohesin complex (STAG2, SMC1A, SMC3, RAD21); and cell cycle (TP53, NPM1).2
Acute Myeloid Leukemia
The HMG subcommittee reviewed the literature on prognostically significant genes in myeloid leukemias. Karyotype abnormalities, such as t(8;21) and inv(16), collectively known as the core-binding factor (CBF) leukemias, t(15;17), t(11q23) (KMT2A/MLL), and so forth, are recurrent lesions in AML. Included in the minimum set of genes recommended by the National Comprehensive Cancer Network (NCCN) for AML prognosis evaluation are nucleolar protein nucleophosmin (NPM1), CCAAT/enhancer-binding protein
Some of the chromosomal translocations, such as inv(16)/t(16;16) in AML and t(15;17) in acute promyelocytic leukemia, can be monitored with FISH or reverse transcription–PCR (RT-PCR) analysis. As NPM1 mutations tend to be seen in recurrence, they can be used as molecular markers for MRD. Other mutations that provide important prognostic information in AML include:
- Activating insertions/duplications in the FLT3 receptor tyrosine kinase, which can be detected with PCR sizing assays;
- Mutations in the KIT receptor tyrosine kinase, which can be detected with DNA sequencing or more limited hotspot PCR;
- Mutations in the DNA methyltransferase, DNMT3A, a poor prognostic indicator seen in 22% of cases of AML, also detected with gene sequencing or more limited hotspot PCR; and
- Another set of genes, TET2, IDH1, IDH2, KRAS, NRAS, EZH2, and ASXL1, is mutated in MPN as well as AML and MDS, making a common molecular panel with next-generation sequencing useful in diagnosing and risk-stratifying all myeloid neoplasms.
The HMG subcommittee agreed that, for de novo AML, chromosomal karyotype is the standard of care, necessary in detecting known cytogenetic abnormalities as well as a wide range of lesions that might indicate a diagnosis of AML with myelodysplasia-related changes at time of diagnosis. In addition, molecular analysis of FLT3 is useful in determining prognosis, and CEBPA (biallelic) and NPM1 mutations are good prognostic factors in normal-karyotype AML. KMT2A (MLL) rearrangements should be tested with FISH if the lineage is ambiguous. The PML-RARA fusion gene also should be tested with FISH if morphologic and flow cytometry results suggest acute promyelocytic leukemia (Table). At this time, testing for TP53, DNMT3A, RAS, and other such mutations is not recommended because it is not cost-effective for the VA.
Myeloproliferative Neoplasms
Myeloproliferative neoplasms are clonal hematopoietic stem cell disorders characterized by proliferation of at least 1 myeloid lineage: granulocytic, erythroid, or megakaryocytic. Myeloproliferative neoplasms show a range of recurrent chromosomal translocations, such as BCR-ABL1 fusion in chronic myelogenous leukemia (CML) that can be detected with RT-PCR analysis as well as FISH. In CML, BCR-ABL1 fusion transcript levels detected by a quantitative PCR (qPCR) method are now used to monitor the course of CML therapy with tyrosine kinase inhibitors (TKIs) and to trigger a treatment change in drug-resistant cases. Given the importance of qPCR in clinical management, significant progress has been made in standardizing both the PCR protocol and the reference materials used to calibrate the BCR-ABL1 PCR assay. BCR-ABL1–negative MPN, including polycythemia vera (PV), essential thrombocythemia (ET), and primary myelofibrosis (PMF), are most commonly associated with mutations in the tyrosine kinase JAK2. Mutations in CALR and MPL are seen in a subset of patients with ET and PMF as well, whereas PV is essentially exclusively a disease of JAK2 mutations.
Chronic myelogenous leukemia is the prototypical MPN. To establish the initial diagnosis, FISH and/or qPCR for BCR-ABL1 fusion should be used. If CML is confirmed, the sample can be reflexed to qPCR BCR-ABL1 on the initial peripheral blood and/or bone marrow sample(s) to establish the patient’s baseline. In addition, a bone marrow sample (aspirate) should be used for a complete karyotype and for morphologic confirmation of disease phase.
For follow-up assessment of CML patients’ response to TKI treatment, qPCR for BCR-ABL1 should be tested with a peripheral blood sample or a bone marrow sample every 3 months.4 A peripheral blood sample is more commonly used because it is conveniently obtained. Early molecular response as indicated by a BCR-ABL1 transcript ratio of < 10% on the International Scale at 3 months, has a strong prognostic value.5 Major molecular response as indicated by a BCR-ABL1 transcript ratio of < 0.1% on the International Scale at 12 to 18 months is also highly prognostic.5
After the peripheral blood sample becomes negative for BCR-ABL1 by qPCR, testing bone marrow samples may be considered. If important treatment response benchmarks are not achieved, or response is lost with rising BCR-ABL1 levels (TKI resistance), ABL1 kinase domain mutation analysis as well as repeat FISH (to assess for copy number multiplication) should be performed to guide further management. Patients with the ABL1 T315I mutation are resistant to all first-line TKIs but may respond to later third-generation TKIs.6
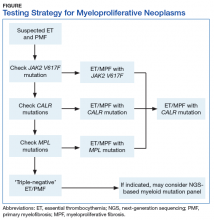
BCR-ABL1–negative MPNs include PV, ET, and PMF. Bone marrow morphology remains the cornerstone of ET and PMF diagnosis. The discovery of JAK2, CALR, and MPL mutations has contributed to how these disorders are diagnosed.7-12 Besides providing the clonality proof that is crucial for diagnosis, the molecular markers influence the prognosis. The JAK2 (p.V617F) or less common JAK2 exon 12 mutations, which are detected in more than 95% of PV cases, are used as molecular markers to confirm diagnosis.7 Further, the JAK2 (p.V617F), CALR (exon 9), and MPL (exon 10) mutations are detected in ET (~60%, 25%, and 3%-5%, respectively) and PMF (~55%, 30%, and 5%, respectively).12 If ET or PMF is suspected clinically, first JAK2 (p.V617F) mutation analysis should be performed, then CALR mutation analysis, and finally MPL mutation analysis. Although novel gain-of-function JAK2 and MPL mutations were recently discovered in triple-negative ET (negative for canonical mutations in JAK2, CALR, and MPL) and PMF by whole exome sequencing,13 clinical testing is not readily available. Besides its utility in the initial diagnosis of ET and PMF, the JAK2 or CALR mutation assay also may be considered for bone marrow transplantation follow-up (Table).14
Despite the continuing debate on the classification of eosinophilic myeloid disorders, the discovery of the FIP1L1-PDGFRA fusion represents a major milestone in the understanding of these disorders.15,16 Unlike PDGFRB (5q33) and FGFR1 (8p11) rearrangements, which can be detected with routine chromosomal analysis (cytogenetics), the cryptic FIP1L1-PDGFRA fusion must be detected with FISH (for CHIC2 deletion) or RT-PCR analysis. It should be pointed out that, as most eosinophilia is reactive or secondary, molecular testing for FIP1L1-PDGFRA fusion is indicated only when primary hypereosinophilia or hypereosinophilic syndrome (HES) is suspected. This is particularly the case in the following hypereosinophilia accompanying conditions: CML-like morphology, but BCR-ABL1–negative; chronic myelomonocytic leukemia (CMML)–like morphology with a normal karyotype; and new onset of cardiac damage or dysfunction.17
Primary eosinophilic myeloid disorders with PDGFRA or PDGFRB rearrangements can be treated with TKIs (eg, imatinib). Next-generation sequencing may be considered in cases of presumed HES when there is no identifiable karyotypic or FISH abnormality. Recent studies have found that cases of HES with somatic mutations indicating clonality had adverse clinical outcomes similar to those of cases of chronic eosinophilic leukemia.18
The discovery of CSF3R mutations offers a new molecular marker for the diagnosis of chronic neutrophilic leukemia (CNL), an MPN.19 The CSF3R (p.T618I) mutation or another activating CSF3R mutation is now used as a diagnostic criterion for CNL. Identification of specific CSF3R mutations may have therapeutic implications as well. The test should be ordered only for patients with clinical and morphologic findings suggestive of CNL; reactive neutrophilic leukocytosis (eg, infection, inflammation) should be ruled out before the test is ordered.
Myelodysplastic Syndrome
Myelodysplastic syndrome is a group of clonal bone marrow disorders characterized by ineffective hematopoiesis, manifested by morphologic dysplasia in ≥ 1 hematopoietic lineages and peripheral cytopenias (hemoglobin level, < 10 g/dL; platelet count, < 100×103/µL; absolute neutrophil count, < 1.8×103/µL). Diagnosis and classification of MDS depend mainly on the degree of morphologic dysplasia and blast percentages, as determined by examining well-prepared cellular bone marrow aspirate smears and/or biopsy touch preparations and peripheral blood smears.
Conventional karyotyping is an essential part of the diagnostic workup for all presumptive cases of MDS and is of both diagnostic and prognostic importance.20 About 60% of MDS cases have recurrent cytogenetic abnormalities, which can be detected with conventional karyotyping. If a high-quality cytogenetic analysis cannot be performed (eg, the bone marrow sample is inadequate), or if quick turnaround is required, an alternative FISH panel may be used to detect some of the common MDS-associated chromosomal abnormalities (eg, 5q deletion, 7q deletion/monosomy 7, +8, 20q deletion).21 Sequencing with FISH also can be useful for assessing MRD by detecting a previously identified chromosomal abnormality.
Targeted sequencing of a limited number of genes can detect mutations in the vast majority of patients with MDS. The most commonly mutated genes in MDS are SF3B1, TET2, SRSF2, ASXL1, DNMT3A, RUNX1, U2AF1, TP53, and EZH2. Mutations in SRSF2 cause RNA splicing abnormalities. In addition, mutations in TP53, EZH2, RUNX1, and ASXL1 are associated with poor prognosis,22,23 whereas mutations in SF3B1 confer better event-free survival.24 Despite these developments, the HMG subcommittee agreed that NGS-based mutation panels are not cost-effective for the VA population at this time and should not be included in a MDS workup. Only in rare situations and when clinically indicated (to change disease classification or patient management) should evaluation for specific gene mutations be considered—for instance, the SF3B1 mutation for patients with probable MDS with ring sideroblasts, if ring sideroblasts are < 15%.25
Myelodysplastic/Myeloproliferative Neoplasms
Myelodysplastic/myeloproliferative neoplasms are a group of myeloid neoplasms with clinical, laboratory, and morphologic features that overlap both MDS and MPN. In MDS/MPN, the karyotype is often normal or shows abnormalities in common with MDS.
In cases of unexplained monocytosis for which there is clinical concern for CMML, morphologic evaluation and conventional chromosomal karyotyping should be performed after other secondary causes and known myeloproliferative and myelodysplastic entities have been excluded. If concomitant hypereosinophilia is present and the karyotype is normal, FISH or PCR-based assay should be performed to rule out FIP1L1-PDGFRA rearrangements. BCR-ABL1, PDGFRB, FGFR1, and t(8;9)/PCM1-JAK2 rearrangements typically are detected with high-quality cytogenetic analysis and thus do not require targeted molecular assays. Although certain gene mutations (eg, SRSF2, TET2, ASXL1, CBL) are commonly detected in CMML, the HMG subcommittee does not recommend sequencing-based mutation panels, as there is insufficient information for testing for prognostic or treatment stratification.
If MDS/MPN with ring sideroblasts and thrombocytosis is suspected on the basis of the clinical and morphologic criteria, molecular tests for the JAK2 (p.V617F) and SF3B1 mutations may be considered in an effort to help confirm the diagnosis.
Atypical CML is a rare MDS/MPN subtype that is now better characterized molecularly with SETBP1 and/or ETNK1 mutations, which are detectable in up to a third of cases. If clinical suspicion is high, sequencing may be diagnostically helpful.
Lymphoid Neoplasms
Chronic Lymphocytic Leukemia
In CLL, recurrent chromosomal abnormalities (eg, deletions of 13q, trisomy 12, deletions of 11q, deletions of 17p) have clear prognostic value and can be detected with FISH. Other prognostic information, such as somatic mutation of immunoglobulin heavy chain variable (IgHV) genes, TP53 mutations, SF3B1, and NOTCH1 mutation, are mostly derived from PCR-based assays. The discovery of recurrently mutated genes in CLL has increased with the use of highly sensitive sequencing methods constructing a more detailed landscape of CLL at genetic, epigenetic, and cellular levels. A recent literature review summarizes the vast heterogeneity of CLL with recurrent pathogenetic findings in MYD88, SF3B1, TP53, ATM, and NOTCH1 signaling pathways.26 The treatment of CLL is rapidly evolving, and many clinical trials are proposing a change from the “watch and wait” paradigm to treatment upon initial presentation based on molecular findings. Additional testing based on new treatment options from current clinical trials will be recommended.
Flow cytometry and morphology are standard for CLL diagnosis. The HMG subcommittee recommends FISH for del(13q14), del(11q), trisomy 12, and del(17p) at time of diagnosis or immediately before therapy initiation. Zeta-chain (
Other B-Cell Lymphoproliferative Disorders
Unlike the common molecular changes in CLL, in other mature B-cell lymphomas, chromosomal translocations that juxtapose a variety of different oncogenes next to an Ig gene enhancer usually are—and those that switch regions less commonly are—important initiating events that can be detected with PCR, DNA sequencing, or FISH. In follicular lymphoma (FL), Burkitt lymphoma, marginal zone lymphoma (MZL), and mantle cell lymphoma (MCL), these oncogenes driven by an Ig gene enhancer typically include BCL2, MYC, MALT1, and CCND1 (cyclin D1), respectively. Molecular variants of these lymphomas that lack these classical translocations often activate homologous genes (eg, cyclin D3/CCND3 is activated in variants of MCL).
Morphology, flow cytometry, and IHC are routinely used for diagnosis. In inconclusive cases, Ig gene rearrangement by PCR may be used. The Table summarizes common molecular changes in B-cell lymphomas.
Mantle cell lymphoma. MCL is a non-Hodgkin lymphoma subtype characterized by t(11;14) (q13;q32) translocations that in the majority of cases lead to overexpression of cyclin D1 (BCL1). Recent molecular profiling has identified an MCL variant that is cyclin D1–negative but SOX11-positive and may have a more aggressive clinical course.30SOX11 regulates PAX5 expression and blocks terminal B-cell differentiation in aggressive MCL.
Lymphoplasmacytic lymphoma. Lymphoplasmacytic lymphoma (LPL), MZL, and CLL/small lymphocytic lymphoma are well-defined clinicopathologic entities. However, distinguishing LPL from MZL and atypical cases of CLL can sometimes be difficult because of overlapping clinical and morphologic features. Recent studies have identified a recurrent L265P mutation in the MYD88 gene in 90% to 95% of LPL cases with IgM paraprotein and in 40% to 50% of the rare non-IgM LPL cases. In contrast, the mutation is much less frequently present in MZL and other low-grade B-cell neoplasms (2%-7%).31 Therefore, testing for this abnormality can be a diagnostic aid in these difficult-to-classify cases. In addition, from a therapeutic perspective, presence or absence of MYD88 mutation may prove more significant than presence of a specific paraprotein or histopathologic features. Ibrutinib has shown efficacy in LPL and demonstrates improved response rates in patients with MYD88 mutation compared with that of their mutation-negative counterparts.32 Several MYD88 inhibitors are in clinical trials. This again indicates the need to more accurately identify and subclassify these non-IgM LPL cases to ensure appropriate molecular evaluation.
Hairy cell leukemia. Flow cytometry and morphology are usually sufficient for a hairy cell leukemia (HCL) diagnosis. However, rare cases are difficult to distinguish variant HCL from other mimics. The BRAF V600E mutation recently was described as a disease-defining molecular marker for HCL—present in nearly all HCL cases but virtually absent in HCL mimics. Therefore, detection of the BRAF mutation by IHC stain with specific antibody or PCR analysis is highly sensitive and specific for the diagnosis of HCL.33
Diffuse large B-cell lymphoma. Recent molecular analysis has created various risk stratification schemata for diffuse large B-cell lymphoma (DLBCL). The HGM subcommittee agrees that well-preserved morphology, IHC, flow cytometry, and FISH-specific markers (BCL2, BCL6, cMYC) are sufficient for diagnostic, prognostic, and therapeutic purposes. Although a wide range of genes have been implicated in the pathogenesis of DLBCL, sequencing and gene expression profiling are not cost-effective at this time and do not add benefit to patient treatment.
The MYD88 L265P mutation has been identified in DLBCL, particularly the activated B-cell-like type and primary central nervous system lymphoma (PCNSL), and may have implications for ibrutinib therapy. PCNSL commonly manifests aggressive clinical behavior and has a poor prognosis. It has been proposed that the MYD88 mutation can be used as a genetic hallmark for PCNSL to distinguish CNS involvement by systemic DLBCL from PCNSL.34
Plasma cell neoplasms. Flow cytometry is acceptable for the diagnosis of plasma cell neoplasms and for residual disease follow-up. Chromosomal karyotype or FISH for IGH/CCND1, IGH/MMSET, and IGH/CMAF dual fusion probes is recommended in conjunction with morphology, IHC, and flow cytometry. In plasma cell myeloma, several genetic mutations can be detected with NGS, including mutations in NRAS, KRAS, TP53, BCL7A, DIS3, and FAM46C.35 Less commonly, BRAF mutations, previously described in melanoma and several other solid tumors, can be detected with DNA sequencing in 4% of multiple myeloma cases, which may prove promising for targeted therapy with BRAF inhibitors. However, current therapeutic decisions are based on genetic and clinical factors, and sequence-based assays are not recommended at this time.
Follicular lymphoma. Cytology, histology, and IHC typically are sufficient for diagnosing FL. In difficult-to-diagnose cases and in cases with scant material, additional tests may help with diagnosis. Eighty to ninety percent of FL cases have t(14;18)(q32;q21), which places the BCL2 gene transcription under the control of the IGH promoter. In addition, about 10% of FL cases have 3q27 aberrancies at the BCL6 gene.36-38 More recently, cases of FL with bulky inguinal disease negative for IGH-BCL2 and BCL6 translocations were found to have 1p36 deletions. These 1p36-deleted FLs typically have a diffuse pattern and a good prognosis.39 For t(14;18), 3q27, or 1p36, FISH is a sensitive means for detecting these translocations, as is PCR for IGH-BCL2.40 There are reports that t(14;18) can be detected in a substantial fraction of otherwise healthy donors at levels and rates that depend on the type of detection test used.41-43 In addition, between one-fourth and one-third of de novo DLBCLs show t(14;18), and about one-third show BCL6 abnormalities at 3q27. Therefore, these genetic changes are not specific for FL and should not be used to subtype a lymphoma as follicular in origin.
Use of IGH-BCL2 as a marker for MRD is still controversial. Some studies have found that a postinduction and posttransplantation IGH-BCL2-positive finding by PCR predicted relapse.44,45 However, others studies have not found significance to postinduction IGH-BCL2 positivity.46 The NCCN guidelines recommend testing for IGH-BCL2 or BCL6 translocations or 1p36 deletion only if this testing is needed for diagnosis. The guidelines do not recommend using these genetic assays in follow-up biopsies, as the importance of treating early relapse has not been definitively demonstrated.
Therefore, if a lymphoma has morphologic, histologic, and IHC findings consistent with FL, then cytogenetic, FISH, or PCR testing is not needed for diagnosis but may be used as confirmation. Follow-up molecular and cytogenetic testing should be avoided if the original cytogenetic abnormality is unknown. That is, IGH-BCL2 FISH should be performed in follow-up samples only if the original lymphoma is known to contain the translocation. As follow-up genetic testing is of disputed clinical significance even in cases in which the original molecular change is known, the NCCN recommendations for therapy are no different. The HMG subcommittee does not recommend molecular or cytogenetic testing in FL beyond what is required for initial diagnosis.
T-Cell Lymphomas
Mature T-Cell Lymphoma and Leukemia
For mature T-cell lymphoma (TCL) and leukemia, the clinical and morphologic criteria have a very important role in the initial workup. However, IHC immunophenotyping is crucial for definitive diagnosis and subclassification. Flow cytometry is routinely used in diagnosing diseases such as T-cell prolymphocytic leukemia (TPLL), T-cell large granular lymphocytic (LGL) leukemia, and Sézary syndrome. T-cell clonality studies, preferably with BIOMED-II–validated primers against targets such as T-cell receptor
Significant advances in TCL classification have led to revisions and the inclusion of new provisional entities in the 2016 World Health Organization classification of lymphoid neoplasms.47 Many of these changes originated in studies of gene expression profiling and the genetic landscape of T-cell neoplasms. Even though subsets of peripheral TCL not otherwise specified (PTCL-NOS) have been recognized on the basis of phenotypic and molecular abnormalities with possible clinical implications, in most cases molecular testing is not part of routine practice. Typically, only a few cytogenetic abnormalities and genetic mutations are used in the evaluation of TCL and T-cell leukemia.
A group of T-cell lymphoproliferative disorders with expression of T follicular helper cell markers can be identified with IHC. These disorders include angioimmunoblastic TCL; follicular TCL, a new entity that is a PTCL-NOS subset; and primary cutaneous CD4-positive small/medium T-cell lymphoproliferative disorder. The neoplastic cells should express at least 2 or 3 T follicular helper cell–related antigens, including CD279/PD1, CD10, BCL6, CXCL13, ICOS, SAP, and CCR5; the most commonly used are PD1, BCL6, and CD10. Recurrent fusion of ITK-SYK translocation t(5;9) or CTLA4-CD28 is also common in follicular TCL. Although recurrent mutation is found in these entities, conventional karyotyping or IHC should be sufficient for diagnosis.
Cutaneous γ -Δ T-Cell Lymphoma
Among cutaneous TCLs, primary cutaneous
Peripheral T-Cell Lymphoma
Gene expression profiling analysis of PTCLs has identified at least 3 subtypes characterized by overexpression of GATA3, TBX21, and cytotoxic genes and expression of the corresponding proteins with IHC.47 These subtypes are associated with different clinical behavior and therapy responses. The GATA3 subtype has an inferior prognosis and shows a high level of T helper type 2 cytokines, which can be identified with IHC. As IHC-stained GATA3 has been available as a marker of urothelial carcinoma at most IHC laboratories, GATA3 IHC staining also may be considered in the evaluation of PTCLs.
Many monoclonal antibody therapies are being used as primary or secondary regimens in the treatment of TCL. Clinical trials are working to establish their efficacy. If treatment with a monoclonal antibody is being considered, it is appropriate to conduct IHC to demonstrate the presence of the target antigen and at follow-up, to demonstrate the efficacy of treatment. These therapies include alemtuzumab, which targets CD52, and brentuximab, which targets CD30.
T-Cell Large Granular Lymphocytic Leukemia
T-cell LGL leukemia is a complex diagnosis that requires persistent clonal expansion of LGLs and clinically peripheral blood cytopenia. In many cases, the diagnosis is difficult to establish, as benign large granular lymphocytosis with clonal T cells may occur in conjunction with viral infections or autoimmune disorders. Somatic mutations in the STAT3 (signal transducer and activator of transcription 3) gene are found in 40% of patients with T-cell LGL leukemia.49 More recently, somatic mutations in the STAT5B gene were identified in 2% of T-cell LGL leukemia subsets. The clinical course of T-cell LGL leukemia in patients with the STAT5B mutation is aggressive and fatal, clearly different from the relatively favorable course of typical T-cell LGL leukemia.50 The HMG subcommittee recommends considering a STAT3 and STAT5B mutation study for selected cases in which it is difficult to distinguish true T-cell LGL leukemia from its reactive expansions.
T-Cell Prolymphocytic Leukemia
T-cell prolymphocytic leukemia (T-PLL) is a rare, aggressive disease and is most commonly associated with a prolymphocytic morphology and expression of CD4. However, since a specific immunophenotypic profile of T-PLL has not been identified, flow cytometry is not adequate in isolation for definitive classification as T-PLL.51 A diagnosis of T-PLL often requires cytogenetics or a FISH study to confirm a suspected case. Most TPLL cases harbor characteristic chromosomal abnormalities involving 14q11.2 (TCR
Anaplastic Large Cell Lymphoma
The World Health Organization recognizes 3 distinct types of anaplastic large cell lymphoma (ALCL): systemic anaplastic lymphoma kinase (ALK)–positive ALCL, systemic ALK-negative ALCL, and primary cutaneous ALCL. Systemic ALK-positive ALCLs consistently have ALK gene rearrangements and favorable outcomes. The most common translocation is the t(2;5) rearrangement of NPM1 and ALK, though other ALK partners are also possible. In contrast, systemic ALK-negative ALCLs lack ALK gene rearrangements and as a whole have outcomes inferior to those of systemic ALK-positive ALCLs. However, studies have found systemic ALK-negative ALCL to be a genetically and clinically heterogeneous entity.54 About 30% of cases have rearrangements of the DUSP22-IRF4 locus on 6p25.3 (DUSP22 rearrangement), and these cases have favorable outcomes similar to those of systemic ALK-positive ALCL.55 Only 8% of patients have TP63 rearrangements and very poor outcomes. The remaining cases lack ALK, DUSP22, and TP63 rearrangements and have intermediate outcomes. The HMG subcommittee recommends considering DUSP22 rearrangement by FISH in the evaluation of systemic ALK-negative ALCL.
Conclusion
The pathologic diagnosis, classification, and risk stratification of lymphoma and leukemia require an approach that integrates morphology, flow cytometry, cytogenetics, and molecular pathology. Rapidly evolving molecular techniques currently allow for detailed description of the molecular defects in lymphoma and leukemia, including driver mutations, amplification/deletion events, and clonal evolution. Unfortunately, the technical ability to catalogue the molecular defects in lymphoma and leukemia, often at great expense, is outpacing the ability to use this detailed information in treating patients with hematologic malignancies. The challenge, then, is to identify best practices for the diagnosis and classification of lymphoma and leukemia in VHA hospitals that incorporate the most useful molecular tests without wasting financial resources.
In this report, the HMG subcommittee of the MGPW has presented its recommendations for molecular testing in AML, MPN, MDS, and lymphomas in the context of standard morphologic and immunophenotypic approaches to hematopathology diagnosis and classification. Adoption of these recommendations by VHA hospitals and clinics should help ensure that all VA patients with hematologic malignancies benefit from the latest advances in precision medicine.
Within the vast and comprehensive national VHA health care system are multiple centers of expertise in hematopathology. In addition, multiple VA clinical molecular diagnostic laboratories are performing state-of-the-art testing. The HMG subcommittee proposes that, to make best use of these expert resources, the VHA should establish an interfacility hematopathology consultation service. This service would allow any VA pathologist to consult a board-certified hematopathologist regarding use of ancillary molecular genetic testing in the diagnosis of hematologic malignancy.
In addition, the HMG subcommittee recommends consolidating VA molecular diagnostic reference laboratories and having them perform molecular testing for other VA hospitals rather than using commercial reference laboratories, where testing standards are not uniform and results may be difficult to interpret. Several well-established VA clinical laboratories with technical expertise and informatics support are already performing selected molecular diagnostic testing. These laboratories’ resources should be expanded, where practical, to cost-effectively provide VA expertise to all veterans and to improve access to appropriate molecular diagnostic testing.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of
Click here to read the digital edition.
1. Collins FS, Varmus H. A new initiative on precision medicine. N Engl J Med. 2015;372(9):793-795.
2. Matynia AP, Szankasi P, Shen W, Kelley TW. Molecular genetic biomarkers in myeloid malignancies. Arch Pathol Lab Med. 2015;139(5):594-601.
3. Wang ML, Bailey NG. Acute myeloid leukemia genetics: risk stratification and implications for therapy. Arch Pathol Lab Med. 2015;139(10):1215-1223.
4. Marum JE, Branford S. Current developments in molecular monitoring in chronic myeloid leukemia. Ther Adv Hematol. 2016;7(5):237-251.
5. Hughes TP, Saglio G, Kantarjian HM, et al. Early molecular response predicts outcomes in patients with chronic myeloid leukemia in chronic phase treated with frontline nilotinib or imatinib. Blood. 2014;123(9):1353-1360.
6. Pallera A, Altman JK, Berman E, et al. Guidelines insights: chronic myeloid leukemia, version 1.2017. J Natl Compr Canc Netw. 2016;14:1505-1512.
7. James C, Ugo V, Le Couédic JP, et al. A unique clonal JAK2 mutation leading to constitutive signalling causes polycythaemia vera. Nature. 2005;434(7037):1144-1148.
8. Kralovics R, Passamonti F, Buser AS, et al. A gain-of-function mutation of JAK2 in myeloproliferative disorders. N Engl J Med. 2005;352(17):1779-1790.
9. Beer PA, Campbell PJ, Scott LM, et al. MPL mutations in myeloproliferative disorders: analysis of the PT-1 cohort. Blood. 2008;112(1):141-149.
10. Vannucchi AM, Antonioli E, Guglielmelli P, et al. Characteristics and clinical correlates of MPL 515W>L/K mutation in essential thrombocythemia. Blood. 2008;112(3):844-847.
11. Nangalia J, Massie CE, Baxter EJ, et al. Somatic CALR mutations in myeloproliferative neoplasms with nonmutated JAK2. N Engl J Med. 2013;369(25):2391-2405.
12. Klampfl T, Gisslinger H, Harutyunyan AS, et al. Somatic mutations of calreticulin in myeloproliferative neoplasms. N Engl J Med. 2013;369(25):2379-2390.
13. Harrison CN, Vannucchi AM. Closing the gap: genetic landscape of MPN. Blood. 2016;127(3):276-278.
14. Tefferi A, Barbui T. Essential thrombocythemia and polycythemia vera: focus on clinical practice. Mayo Clin Proc. 2015;90(9):1283-1293.
15. Cools J, DeAngelo DJ, Gotlib J, et al. A tyrosine kinase created by fusion of the PDGFRA and FIP1L1 genes as a therapeutic target of imatinib in idiopathic hypereosinophilic syndrome. N Engl J Med. 2003;348(13):1201-1214.
16. Valent P, Klion AD, Horny HP, et al. Contemporary consensus proposal on criteria and classification of eosinophilic disorders and related syndromes. J Allergy Clin Immunol. 2012;130(3):607-612.e609.
17. Bain B, Billiland D, Horny H, Verdiman J. Myeloid and lymphoid neoplasms with eosinophilia and abnormalities of PDGFRA, PDGFRB, or FGFR1. In: Swerdlow S, Campo E, Harris N, et al, eds. WHO Classification of Tumours of the Haematopoietic and Lymphoid Tissues. Vol 2. Lyon, France: IRAC Press; 2008:68-73.
18. Wang SA, Tam W, Tsai AG, et al. Targeted next-generation sequencing identifies a subset of idiopathic hypereosinophilic syndrome with features similar to chronic eosinophilic leukemia, not otherwise specified. Mod Pathol. 2016;29(8):854-864.
19. Maxson JE, Gotlib J, Pollyea DA, et al. Oncogenic CSF3R mutations in chronic neutrophilic leukemia and atypical CML. N Engl J Med. 2013;368(19):1781-1790.
20. Schanz J, Tüchler H, Solé F, et al. New comprehensive cytogenetic scoring system for primary myelodysplastic syndromes (MDS) and oligoblastic acute myeloid leukemia after MDS derived from an international database merge. J Clin Oncol. 2012;30(8):820-829.
21. Marshall D, Roboz GJ. Standardizing the initial evaluation for myelodysplastic syndromes. Curr Hematol Malig Rep. 2013;8(4):361-369.
22. Damm F, Chesnais V, Nagata Y, et al. BCOR and BCORL1 mutations in myelodysplastic syndromes and related disorders. Blood. 2013;122(18):3169-3177.
23. Malcovati L, Papaemmanuil E, Ambaglio I, et al. Driver somatic mutations identify distinct disease entities within myeloid neoplasms with myelodysplasia. Blood. 2014;124(9):1513-1521.
24. Papaemmanuil E, Cazzola M, Boultwood J, et al. Somatic SF3B1 mutation in myelodysplasia with ring sideroblasts. N Engl J Med. 2011;365(15):1384-1395.
25. Patnaik MM, Hanson CA, Sulai NH, et al. Prognostic irrelevance of ring sideroblast percentage in World Health Organization-defined myelodysplastic syndromes without excess blasts. Blood. 2012;119(24):5674-5677.
26. Guièze R, Wu CJ. Genomic and epigenomic heterogeneity in chronic lymphocytic leukemia. Blood. 2015;126(4):445-453.
27. Jeromin S, Weissmann S, Haferlach C, et al. SF3B1 mutations correlated to cytogenetics and mutations in NOTCH1, FBXW7, MYD88, XPO1 and TP53 in 1160 untreated CLL patients. Leukemia. 2014;28(1):108-117.
28. Del Giudice I, Rossi D, Chiaretti S, et al. NOTCH1 mutations in +12 chronic lymphocytic leukemia (CLL) confer an unfavorable prognosis, induce a distinctive transcriptional profiling and refine the intermediate prognosis of +12 CLL. Haematologica. 2012;97(3):437-441.
29. Weissmann S, Roller A, Jeromin S, et al. Prognostic impact and landscape of NOTCH1 mutations in chronic lymphocytic leukemia (CLL): a study on 852 patients. Leukemia. 2013;27(12):2393-2396.
30. Vegliante MC, Palomero J, Pérez-Galán P, et al. SOX11 regulates PAX5 expression and blocks terminal B-cell differentiation in aggressive mantle cell lymphoma. Blood. 2013;121(12):2175-2185.
31. King RL, Gonsalves WI, Ansell SM, et al. Lymphoplasmacytic lymphoma with a non-IgM paraprotein shows clinical and pathologic heterogeneity and may harbor MYD88 L265P mutations. Am J Clin Pathol. 2016;145(6):843-851.
32. Insuasti-Beltran G, Gale JM, Wilson CS, Foucar K, Czuchlewski DR. Significance of MYD88 L265P mutation status in the subclassification of low-grade B-cell lymphoma/leukemia. Arch Pathol Lab Med. 2015;139(8):1035-1041.
33. Wang XJ, Kim A, Li S. Immunohistochemical analysis using a BRAF V600E mutation specific antibody is highly sensitive and specific for the diagnosis of hairy cell leukemia. Int J Clin Exp Pathol. 2014;7(7):4323-4328.
34. Nakamura T, Tateishi K, Niwa T, et al. Recurrent mutations of CD79B and MYD88 are the hallmark of primary central nervous system lymphomas. Neuropathol Appl Neurobiol. 2016;42(3):279-290.
35. Chapman MA, Lawrence MS, Keats JJ, et al. Initial genome sequencing and analysis of multiple myeloma. Nature. 2011;471(7339):467-472.
36. Bosga-Bouwer AG, van Imhoff GW, Boonstra R, et al. Follicular lymphoma grade 3B includes 3 cytogenetically defined subgroups with primary t(14;18), 3q27, or other translocations: t(14;18) and 3q27 are mutually exclusive. Blood. 2003;101(3):1149-1154.
37. Gu K, Fu K, Jain S, et al. t(14;18)-negative follicular lymphomas are associated with a high frequency of BCL6 rearrangement at the alternative breakpoint region. Mod Pathol. 2009;22(9):1251-1257.
38. Katzenberger T, Ott G, Klein T, Kalla J, Müller-Hermelink HK, Ott MM. Cytogenetic alterations affecting BCL6 are predominantly found in follicular lymphomas grade 3B with a diffuse large B-cell component. Am J Pathol. 2004;165(2):481-490.
39. Katzenberger T, Kalla J, Leich E, et al. A distinctive subtype of t(14;18)-negative nodal follicular non-Hodgkin lymphoma characterized by a predominantly diffuse growth pattern and deletions in the chromosomal region 1p36. Blood. 2009;113(5):1053-1061.
40. Belaud-Rotureau MA, Parrens M, Carrere N, et al. Interphase fluorescence in situ hybridization is more sensitive than BIOMED-2 polymerase chain reaction protocol in detecting IGH-BCL2 rearrangement in both fixed and frozen lymph node with follicular lymphoma. Hum Pathol. 2007;38(2):365-372.
41. Limpens J, Stad R, Vos C, et al. Lymphoma-associated translocation t(14;18) in blood B cells of normal individuals. Blood. 1995;85(9):2528-2536.
42. Schmitt C, Balogh B, Grundt A, et al. The bcl-2/IgH rearrangement in a population of 204 healthy individuals: occurrence, age and gender distribution, breakpoints, and detection method validity. Leuk Res. 2006;30(6):745-750.
43. Summers KE, Goff LK, Wilson AG, Gupta RK, Lister TA, Fitzgibbon J. Frequency of the Bcl-2/IgH rearrangement in normal individuals: implications for the monitoring of disease in patients with follicular lymphoma. J Clin Oncol. 2001;19(2):420-424.
44. Galimberti S, Luminari S, Ciabatti E, et al. Minimal residual disease after conventional treatment significantly impacts on progression-free survival of patients with follicular lymphoma: the FIL FOLL05 trial. Clin Cancer Res. 2014;20(24):6398-6405.
45. Ladetto M, Lobetti-Bodoni C, Mantoan B, et al; Fondazione Italiana Linfomi. Persistence of minimal residual disease in bone marrow predicts outcome in follicular lymphomas treated with a rituximab-intensive program. Blood. 2013;122(23):3759-3766.
46. van Oers MHJ, Tönnissen E, Van Glabbeke M, et al. BCL-2/IgH polymerase chain reaction status at the end of induction treatment is not predictive for progression-free survival in relapsed/resistant follicular lymphoma: results of a prospective randomized EORTC 20981 phase III intergroup study. J Clin Oncol. 2010;28(13):2246-2252.
47. Swerdlow SH, Campo E, Pileri SA, et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood. 2016;127(20):2375-2390.
48. Dewar R, Andea AA, Guitart J, Arber DA, Weiss LM. Best practices in diagnostic immunohistochemistry: workup of cutaneous lymphoid lesions in the diagnosis of primary cutaneous lymphoma. Arch Pathol Lab Med. 2015;139(3):338-350.
49. Koskela HLM, Eldfors S, Ellonen P, et al. Somatic STAT3 mutations in large granular lymphocytic leukemia. N Engl J Med. 2012;366(20):1905-1913.
50. Rajala HL, Eldfors S, Kuusanmäki H, et al. Discovery of somatic STAT5b mutations in large granular lymphocytic leukemia. Blood. 2013;121(22):4541-4550.
51. Chen X, Cherian S. Immunophenotypic characterization of T-cell prolymphocytic leukemia. Am J Clin Pathol. 2013;140(5):727-735.
52. Delgado P, Starshak P, Rao N, Tirado C. A comprehensive update on molecular and cytogenetic abnormalities in T-cell prolymphocytic leukemia (T-PLL). J Assoc Genet Technol. 2012;38(4):193-198.
53. Stengel A, Kern W, Zenger M, et al. Genetic characterization of T-PLL reveals two major biologic subgroups and JAK3 mutations as prognostic marker. Genes Chromosomes Cancer. 2016;55(1):82-94.
54. Thompson MA, Stumph J, Henrickson SE, et al. Differential gene expression in anaplastic lymphoma kinase-positive and anaplastic lymphoma kinase-negative anaplastic large cell lymphomas. Hum Pathol. 2005;36(5):494-504.
55. King R, Dao L, McPhail E, et al. Morphologic features of ALK-negative anaplastic large cell lymphomas with DUSP22 rearrangements. Am J Surg Pathol. 2016;40(1):36-43.
In January 2015, President Obama introduced the Precision Medicine Initiative, a program set up to identify new biomedical discoveries for the development of a personalized knowledge base of disease entities and individualized treatments. Advances in precision medicine typically involve the use of targeted therapies tailored to individual genetic characteristics identified with molecular testing. The goals are to improve survival and reduce adverse effects. With an initial budget of $215 million, this initiative presented a unique opportunity to combine efforts in genomic discovery, bioinformatic analysis, and health information technology to move toward data-driven, evidence-based precision medicine.1
The VHA is the largest comprehensive health care system in the U. S. and has more than 1,700 care sites serving nearly 9 million veterans each year. The budget for this single-payer system is proposed by the President and approved by Congress. As the VHA must treat a diverse and aging veteran population in an environment of rising costs and budget constraints, limited resources must be monitored and appropriated for the most cost-effective health care delivery. Precision medicine offers a model in which physicians can select the most appropriate diagnostic tests in defined clinical settings to direct clinical care. It supports the testing needed to subdivide each disease category into distinct subcategories. Nevertheless, the need for fiscal responsibility in a capitated health care system recommends testing in cases in which it can change therapy or prognosis rather than for purely academic reasons.
Pathology and Laboratory Medicine Service
Given limited resources and an increasing number of requests for advanced molecular testing, the VA Pathology and Laboratory Medicine Service (P&LMS) formed the Molecular Genetics Pathology Workgroup (MGPW) in September 2013. The charter listed the tasks of the MGPW to “provide recommendations on how to effectively use molecular genetics tests, promote increased quality and availability of testing within the VHA, encourage internal referral testing, provide an organizational structure for Molecular Genetics Testing Consortia, and create a P&LMS policy for molecular genetic testing in general, specifically addressing the issues surrounding laboratory developed testing.” The MGPW has 4 subcommittees: molecular oncology, pharmacogenetics, hematopathology molecular genetics (HMG), and genetic medicine. Since its inception, the HMG subcommittee has had several objectives:
- Standardize the molecular testing nomenclature for and develop practice guidelines for acute myeloid leukemia (AML), myeloproliferative neoplasms (MPN), myelodysplastic syndrome (MDS), chronic lymphocytic leukemia (CLL)/small lymphocytic lymphoma, lymphoma, and plasma cell neoplasms;
- Develop standardized reporting guidelines for current VA molecular laboratories;
- Identify new tests as they are being reported in the literature and collaborate with hematology and oncology services to evaluate the clinical utility of these tests for VA patients;
- Network current VA molecular laboratories, perform fact-finding for these laboratories, and compile test menus; and
- Assess for the formation of VA-wide interfacility consultation services for hematopathology so that all VA facilities, regardless of their complexity, will be able to access the expertise of hematopathology-trained pathologists (Appendix).
The HMG subcommittee met monthly and discussed various diagnostic entities in hematopathology. For hematolymphoid malignancies, it was generally agreed that the traditional laboratory tools of morphology, flow cytometry, and immunohistochemistry (IHC) are standard in initial assessment and often in diagnosis. As the clinical molecular and cytogenetic assays of karyotype, fluorescence in situ hybridization (FISH), advanced DNA sequencing, microarray, and highly sensitive polymerase chain reaction (PCR) analysis affect diagnosis, subclassification, minimal residual disease (MRD) monitoring, prognosis, and therapy selection, their use is marked by a high degree of variability. As a result, standardization is needed. As each laboratory develops and reports ancillary testing, the variable reporting formats may generate postanalytic errors.
A detailed description of all molecular methodologies is beyond the scope of this article. For practicing pathologists, challenges remain in overall cost and reimbursement, extensive and time-consuming data analysis, and in some cases, interpretation differences.
Myeloid Neoplasms
Myeloid malignancies were divided into AML, MPN, and MDS. Next-generation sequencing (NGS) information for these malignancies was used to identify various contributory functional categories, including cell signaling (FLT3, KIT, JAK2, MPL, KRAS/NRAS, PTPN11, NF1, CSF3R); transcription (CEBPA, RUNX1, GATA1/GATA2, PHF6, ETV6); splicing (SF3B1, SRSF2, ZRSR2, U2AF1); epigenetics (DNMT3A, TET2, IDH1/IDH2, ASXL1, EZH2, SUZ12, KDM6A); cohesin complex (STAG2, SMC1A, SMC3, RAD21); and cell cycle (TP53, NPM1).2
Acute Myeloid Leukemia
The HMG subcommittee reviewed the literature on prognostically significant genes in myeloid leukemias. Karyotype abnormalities, such as t(8;21) and inv(16), collectively known as the core-binding factor (CBF) leukemias, t(15;17), t(11q23) (KMT2A/MLL), and so forth, are recurrent lesions in AML. Included in the minimum set of genes recommended by the National Comprehensive Cancer Network (NCCN) for AML prognosis evaluation are nucleolar protein nucleophosmin (NPM1), CCAAT/enhancer-binding protein
Some of the chromosomal translocations, such as inv(16)/t(16;16) in AML and t(15;17) in acute promyelocytic leukemia, can be monitored with FISH or reverse transcription–PCR (RT-PCR) analysis. As NPM1 mutations tend to be seen in recurrence, they can be used as molecular markers for MRD. Other mutations that provide important prognostic information in AML include:
- Activating insertions/duplications in the FLT3 receptor tyrosine kinase, which can be detected with PCR sizing assays;
- Mutations in the KIT receptor tyrosine kinase, which can be detected with DNA sequencing or more limited hotspot PCR;
- Mutations in the DNA methyltransferase, DNMT3A, a poor prognostic indicator seen in 22% of cases of AML, also detected with gene sequencing or more limited hotspot PCR; and
- Another set of genes, TET2, IDH1, IDH2, KRAS, NRAS, EZH2, and ASXL1, is mutated in MPN as well as AML and MDS, making a common molecular panel with next-generation sequencing useful in diagnosing and risk-stratifying all myeloid neoplasms.
The HMG subcommittee agreed that, for de novo AML, chromosomal karyotype is the standard of care, necessary in detecting known cytogenetic abnormalities as well as a wide range of lesions that might indicate a diagnosis of AML with myelodysplasia-related changes at time of diagnosis. In addition, molecular analysis of FLT3 is useful in determining prognosis, and CEBPA (biallelic) and NPM1 mutations are good prognostic factors in normal-karyotype AML. KMT2A (MLL) rearrangements should be tested with FISH if the lineage is ambiguous. The PML-RARA fusion gene also should be tested with FISH if morphologic and flow cytometry results suggest acute promyelocytic leukemia (Table). At this time, testing for TP53, DNMT3A, RAS, and other such mutations is not recommended because it is not cost-effective for the VA.
Myeloproliferative Neoplasms
Myeloproliferative neoplasms are clonal hematopoietic stem cell disorders characterized by proliferation of at least 1 myeloid lineage: granulocytic, erythroid, or megakaryocytic. Myeloproliferative neoplasms show a range of recurrent chromosomal translocations, such as BCR-ABL1 fusion in chronic myelogenous leukemia (CML) that can be detected with RT-PCR analysis as well as FISH. In CML, BCR-ABL1 fusion transcript levels detected by a quantitative PCR (qPCR) method are now used to monitor the course of CML therapy with tyrosine kinase inhibitors (TKIs) and to trigger a treatment change in drug-resistant cases. Given the importance of qPCR in clinical management, significant progress has been made in standardizing both the PCR protocol and the reference materials used to calibrate the BCR-ABL1 PCR assay. BCR-ABL1–negative MPN, including polycythemia vera (PV), essential thrombocythemia (ET), and primary myelofibrosis (PMF), are most commonly associated with mutations in the tyrosine kinase JAK2. Mutations in CALR and MPL are seen in a subset of patients with ET and PMF as well, whereas PV is essentially exclusively a disease of JAK2 mutations.
Chronic myelogenous leukemia is the prototypical MPN. To establish the initial diagnosis, FISH and/or qPCR for BCR-ABL1 fusion should be used. If CML is confirmed, the sample can be reflexed to qPCR BCR-ABL1 on the initial peripheral blood and/or bone marrow sample(s) to establish the patient’s baseline. In addition, a bone marrow sample (aspirate) should be used for a complete karyotype and for morphologic confirmation of disease phase.
For follow-up assessment of CML patients’ response to TKI treatment, qPCR for BCR-ABL1 should be tested with a peripheral blood sample or a bone marrow sample every 3 months.4 A peripheral blood sample is more commonly used because it is conveniently obtained. Early molecular response as indicated by a BCR-ABL1 transcript ratio of < 10% on the International Scale at 3 months, has a strong prognostic value.5 Major molecular response as indicated by a BCR-ABL1 transcript ratio of < 0.1% on the International Scale at 12 to 18 months is also highly prognostic.5
After the peripheral blood sample becomes negative for BCR-ABL1 by qPCR, testing bone marrow samples may be considered. If important treatment response benchmarks are not achieved, or response is lost with rising BCR-ABL1 levels (TKI resistance), ABL1 kinase domain mutation analysis as well as repeat FISH (to assess for copy number multiplication) should be performed to guide further management. Patients with the ABL1 T315I mutation are resistant to all first-line TKIs but may respond to later third-generation TKIs.6
BCR-ABL1–negative MPNs include PV, ET, and PMF. Bone marrow morphology remains the cornerstone of ET and PMF diagnosis. The discovery of JAK2, CALR, and MPL mutations has contributed to how these disorders are diagnosed.7-12 Besides providing the clonality proof that is crucial for diagnosis, the molecular markers influence the prognosis. The JAK2 (p.V617F) or less common JAK2 exon 12 mutations, which are detected in more than 95% of PV cases, are used as molecular markers to confirm diagnosis.7 Further, the JAK2 (p.V617F), CALR (exon 9), and MPL (exon 10) mutations are detected in ET (~60%, 25%, and 3%-5%, respectively) and PMF (~55%, 30%, and 5%, respectively).12 If ET or PMF is suspected clinically, first JAK2 (p.V617F) mutation analysis should be performed, then CALR mutation analysis, and finally MPL mutation analysis. Although novel gain-of-function JAK2 and MPL mutations were recently discovered in triple-negative ET (negative for canonical mutations in JAK2, CALR, and MPL) and PMF by whole exome sequencing,13 clinical testing is not readily available. Besides its utility in the initial diagnosis of ET and PMF, the JAK2 or CALR mutation assay also may be considered for bone marrow transplantation follow-up (Table).14
Despite the continuing debate on the classification of eosinophilic myeloid disorders, the discovery of the FIP1L1-PDGFRA fusion represents a major milestone in the understanding of these disorders.15,16 Unlike PDGFRB (5q33) and FGFR1 (8p11) rearrangements, which can be detected with routine chromosomal analysis (cytogenetics), the cryptic FIP1L1-PDGFRA fusion must be detected with FISH (for CHIC2 deletion) or RT-PCR analysis. It should be pointed out that, as most eosinophilia is reactive or secondary, molecular testing for FIP1L1-PDGFRA fusion is indicated only when primary hypereosinophilia or hypereosinophilic syndrome (HES) is suspected. This is particularly the case in the following hypereosinophilia accompanying conditions: CML-like morphology, but BCR-ABL1–negative; chronic myelomonocytic leukemia (CMML)–like morphology with a normal karyotype; and new onset of cardiac damage or dysfunction.17
Primary eosinophilic myeloid disorders with PDGFRA or PDGFRB rearrangements can be treated with TKIs (eg, imatinib). Next-generation sequencing may be considered in cases of presumed HES when there is no identifiable karyotypic or FISH abnormality. Recent studies have found that cases of HES with somatic mutations indicating clonality had adverse clinical outcomes similar to those of cases of chronic eosinophilic leukemia.18
The discovery of CSF3R mutations offers a new molecular marker for the diagnosis of chronic neutrophilic leukemia (CNL), an MPN.19 The CSF3R (p.T618I) mutation or another activating CSF3R mutation is now used as a diagnostic criterion for CNL. Identification of specific CSF3R mutations may have therapeutic implications as well. The test should be ordered only for patients with clinical and morphologic findings suggestive of CNL; reactive neutrophilic leukocytosis (eg, infection, inflammation) should be ruled out before the test is ordered.
Myelodysplastic Syndrome
Myelodysplastic syndrome is a group of clonal bone marrow disorders characterized by ineffective hematopoiesis, manifested by morphologic dysplasia in ≥ 1 hematopoietic lineages and peripheral cytopenias (hemoglobin level, < 10 g/dL; platelet count, < 100×103/µL; absolute neutrophil count, < 1.8×103/µL). Diagnosis and classification of MDS depend mainly on the degree of morphologic dysplasia and blast percentages, as determined by examining well-prepared cellular bone marrow aspirate smears and/or biopsy touch preparations and peripheral blood smears.
Conventional karyotyping is an essential part of the diagnostic workup for all presumptive cases of MDS and is of both diagnostic and prognostic importance.20 About 60% of MDS cases have recurrent cytogenetic abnormalities, which can be detected with conventional karyotyping. If a high-quality cytogenetic analysis cannot be performed (eg, the bone marrow sample is inadequate), or if quick turnaround is required, an alternative FISH panel may be used to detect some of the common MDS-associated chromosomal abnormalities (eg, 5q deletion, 7q deletion/monosomy 7, +8, 20q deletion).21 Sequencing with FISH also can be useful for assessing MRD by detecting a previously identified chromosomal abnormality.
Targeted sequencing of a limited number of genes can detect mutations in the vast majority of patients with MDS. The most commonly mutated genes in MDS are SF3B1, TET2, SRSF2, ASXL1, DNMT3A, RUNX1, U2AF1, TP53, and EZH2. Mutations in SRSF2 cause RNA splicing abnormalities. In addition, mutations in TP53, EZH2, RUNX1, and ASXL1 are associated with poor prognosis,22,23 whereas mutations in SF3B1 confer better event-free survival.24 Despite these developments, the HMG subcommittee agreed that NGS-based mutation panels are not cost-effective for the VA population at this time and should not be included in a MDS workup. Only in rare situations and when clinically indicated (to change disease classification or patient management) should evaluation for specific gene mutations be considered—for instance, the SF3B1 mutation for patients with probable MDS with ring sideroblasts, if ring sideroblasts are < 15%.25
Myelodysplastic/Myeloproliferative Neoplasms
Myelodysplastic/myeloproliferative neoplasms are a group of myeloid neoplasms with clinical, laboratory, and morphologic features that overlap both MDS and MPN. In MDS/MPN, the karyotype is often normal or shows abnormalities in common with MDS.
In cases of unexplained monocytosis for which there is clinical concern for CMML, morphologic evaluation and conventional chromosomal karyotyping should be performed after other secondary causes and known myeloproliferative and myelodysplastic entities have been excluded. If concomitant hypereosinophilia is present and the karyotype is normal, FISH or PCR-based assay should be performed to rule out FIP1L1-PDGFRA rearrangements. BCR-ABL1, PDGFRB, FGFR1, and t(8;9)/PCM1-JAK2 rearrangements typically are detected with high-quality cytogenetic analysis and thus do not require targeted molecular assays. Although certain gene mutations (eg, SRSF2, TET2, ASXL1, CBL) are commonly detected in CMML, the HMG subcommittee does not recommend sequencing-based mutation panels, as there is insufficient information for testing for prognostic or treatment stratification.
If MDS/MPN with ring sideroblasts and thrombocytosis is suspected on the basis of the clinical and morphologic criteria, molecular tests for the JAK2 (p.V617F) and SF3B1 mutations may be considered in an effort to help confirm the diagnosis.
Atypical CML is a rare MDS/MPN subtype that is now better characterized molecularly with SETBP1 and/or ETNK1 mutations, which are detectable in up to a third of cases. If clinical suspicion is high, sequencing may be diagnostically helpful.
Lymphoid Neoplasms
Chronic Lymphocytic Leukemia
In CLL, recurrent chromosomal abnormalities (eg, deletions of 13q, trisomy 12, deletions of 11q, deletions of 17p) have clear prognostic value and can be detected with FISH. Other prognostic information, such as somatic mutation of immunoglobulin heavy chain variable (IgHV) genes, TP53 mutations, SF3B1, and NOTCH1 mutation, are mostly derived from PCR-based assays. The discovery of recurrently mutated genes in CLL has increased with the use of highly sensitive sequencing methods constructing a more detailed landscape of CLL at genetic, epigenetic, and cellular levels. A recent literature review summarizes the vast heterogeneity of CLL with recurrent pathogenetic findings in MYD88, SF3B1, TP53, ATM, and NOTCH1 signaling pathways.26 The treatment of CLL is rapidly evolving, and many clinical trials are proposing a change from the “watch and wait” paradigm to treatment upon initial presentation based on molecular findings. Additional testing based on new treatment options from current clinical trials will be recommended.
Flow cytometry and morphology are standard for CLL diagnosis. The HMG subcommittee recommends FISH for del(13q14), del(11q), trisomy 12, and del(17p) at time of diagnosis or immediately before therapy initiation. Zeta-chain (
Other B-Cell Lymphoproliferative Disorders
Unlike the common molecular changes in CLL, in other mature B-cell lymphomas, chromosomal translocations that juxtapose a variety of different oncogenes next to an Ig gene enhancer usually are—and those that switch regions less commonly are—important initiating events that can be detected with PCR, DNA sequencing, or FISH. In follicular lymphoma (FL), Burkitt lymphoma, marginal zone lymphoma (MZL), and mantle cell lymphoma (MCL), these oncogenes driven by an Ig gene enhancer typically include BCL2, MYC, MALT1, and CCND1 (cyclin D1), respectively. Molecular variants of these lymphomas that lack these classical translocations often activate homologous genes (eg, cyclin D3/CCND3 is activated in variants of MCL).
Morphology, flow cytometry, and IHC are routinely used for diagnosis. In inconclusive cases, Ig gene rearrangement by PCR may be used. The Table summarizes common molecular changes in B-cell lymphomas.
Mantle cell lymphoma. MCL is a non-Hodgkin lymphoma subtype characterized by t(11;14) (q13;q32) translocations that in the majority of cases lead to overexpression of cyclin D1 (BCL1). Recent molecular profiling has identified an MCL variant that is cyclin D1–negative but SOX11-positive and may have a more aggressive clinical course.30SOX11 regulates PAX5 expression and blocks terminal B-cell differentiation in aggressive MCL.
Lymphoplasmacytic lymphoma. Lymphoplasmacytic lymphoma (LPL), MZL, and CLL/small lymphocytic lymphoma are well-defined clinicopathologic entities. However, distinguishing LPL from MZL and atypical cases of CLL can sometimes be difficult because of overlapping clinical and morphologic features. Recent studies have identified a recurrent L265P mutation in the MYD88 gene in 90% to 95% of LPL cases with IgM paraprotein and in 40% to 50% of the rare non-IgM LPL cases. In contrast, the mutation is much less frequently present in MZL and other low-grade B-cell neoplasms (2%-7%).31 Therefore, testing for this abnormality can be a diagnostic aid in these difficult-to-classify cases. In addition, from a therapeutic perspective, presence or absence of MYD88 mutation may prove more significant than presence of a specific paraprotein or histopathologic features. Ibrutinib has shown efficacy in LPL and demonstrates improved response rates in patients with MYD88 mutation compared with that of their mutation-negative counterparts.32 Several MYD88 inhibitors are in clinical trials. This again indicates the need to more accurately identify and subclassify these non-IgM LPL cases to ensure appropriate molecular evaluation.
Hairy cell leukemia. Flow cytometry and morphology are usually sufficient for a hairy cell leukemia (HCL) diagnosis. However, rare cases are difficult to distinguish variant HCL from other mimics. The BRAF V600E mutation recently was described as a disease-defining molecular marker for HCL—present in nearly all HCL cases but virtually absent in HCL mimics. Therefore, detection of the BRAF mutation by IHC stain with specific antibody or PCR analysis is highly sensitive and specific for the diagnosis of HCL.33
Diffuse large B-cell lymphoma. Recent molecular analysis has created various risk stratification schemata for diffuse large B-cell lymphoma (DLBCL). The HGM subcommittee agrees that well-preserved morphology, IHC, flow cytometry, and FISH-specific markers (BCL2, BCL6, cMYC) are sufficient for diagnostic, prognostic, and therapeutic purposes. Although a wide range of genes have been implicated in the pathogenesis of DLBCL, sequencing and gene expression profiling are not cost-effective at this time and do not add benefit to patient treatment.
The MYD88 L265P mutation has been identified in DLBCL, particularly the activated B-cell-like type and primary central nervous system lymphoma (PCNSL), and may have implications for ibrutinib therapy. PCNSL commonly manifests aggressive clinical behavior and has a poor prognosis. It has been proposed that the MYD88 mutation can be used as a genetic hallmark for PCNSL to distinguish CNS involvement by systemic DLBCL from PCNSL.34
Plasma cell neoplasms. Flow cytometry is acceptable for the diagnosis of plasma cell neoplasms and for residual disease follow-up. Chromosomal karyotype or FISH for IGH/CCND1, IGH/MMSET, and IGH/CMAF dual fusion probes is recommended in conjunction with morphology, IHC, and flow cytometry. In plasma cell myeloma, several genetic mutations can be detected with NGS, including mutations in NRAS, KRAS, TP53, BCL7A, DIS3, and FAM46C.35 Less commonly, BRAF mutations, previously described in melanoma and several other solid tumors, can be detected with DNA sequencing in 4% of multiple myeloma cases, which may prove promising for targeted therapy with BRAF inhibitors. However, current therapeutic decisions are based on genetic and clinical factors, and sequence-based assays are not recommended at this time.
Follicular lymphoma. Cytology, histology, and IHC typically are sufficient for diagnosing FL. In difficult-to-diagnose cases and in cases with scant material, additional tests may help with diagnosis. Eighty to ninety percent of FL cases have t(14;18)(q32;q21), which places the BCL2 gene transcription under the control of the IGH promoter. In addition, about 10% of FL cases have 3q27 aberrancies at the BCL6 gene.36-38 More recently, cases of FL with bulky inguinal disease negative for IGH-BCL2 and BCL6 translocations were found to have 1p36 deletions. These 1p36-deleted FLs typically have a diffuse pattern and a good prognosis.39 For t(14;18), 3q27, or 1p36, FISH is a sensitive means for detecting these translocations, as is PCR for IGH-BCL2.40 There are reports that t(14;18) can be detected in a substantial fraction of otherwise healthy donors at levels and rates that depend on the type of detection test used.41-43 In addition, between one-fourth and one-third of de novo DLBCLs show t(14;18), and about one-third show BCL6 abnormalities at 3q27. Therefore, these genetic changes are not specific for FL and should not be used to subtype a lymphoma as follicular in origin.
Use of IGH-BCL2 as a marker for MRD is still controversial. Some studies have found that a postinduction and posttransplantation IGH-BCL2-positive finding by PCR predicted relapse.44,45 However, others studies have not found significance to postinduction IGH-BCL2 positivity.46 The NCCN guidelines recommend testing for IGH-BCL2 or BCL6 translocations or 1p36 deletion only if this testing is needed for diagnosis. The guidelines do not recommend using these genetic assays in follow-up biopsies, as the importance of treating early relapse has not been definitively demonstrated.
Therefore, if a lymphoma has morphologic, histologic, and IHC findings consistent with FL, then cytogenetic, FISH, or PCR testing is not needed for diagnosis but may be used as confirmation. Follow-up molecular and cytogenetic testing should be avoided if the original cytogenetic abnormality is unknown. That is, IGH-BCL2 FISH should be performed in follow-up samples only if the original lymphoma is known to contain the translocation. As follow-up genetic testing is of disputed clinical significance even in cases in which the original molecular change is known, the NCCN recommendations for therapy are no different. The HMG subcommittee does not recommend molecular or cytogenetic testing in FL beyond what is required for initial diagnosis.
T-Cell Lymphomas
Mature T-Cell Lymphoma and Leukemia
For mature T-cell lymphoma (TCL) and leukemia, the clinical and morphologic criteria have a very important role in the initial workup. However, IHC immunophenotyping is crucial for definitive diagnosis and subclassification. Flow cytometry is routinely used in diagnosing diseases such as T-cell prolymphocytic leukemia (TPLL), T-cell large granular lymphocytic (LGL) leukemia, and Sézary syndrome. T-cell clonality studies, preferably with BIOMED-II–validated primers against targets such as T-cell receptor
Significant advances in TCL classification have led to revisions and the inclusion of new provisional entities in the 2016 World Health Organization classification of lymphoid neoplasms.47 Many of these changes originated in studies of gene expression profiling and the genetic landscape of T-cell neoplasms. Even though subsets of peripheral TCL not otherwise specified (PTCL-NOS) have been recognized on the basis of phenotypic and molecular abnormalities with possible clinical implications, in most cases molecular testing is not part of routine practice. Typically, only a few cytogenetic abnormalities and genetic mutations are used in the evaluation of TCL and T-cell leukemia.
A group of T-cell lymphoproliferative disorders with expression of T follicular helper cell markers can be identified with IHC. These disorders include angioimmunoblastic TCL; follicular TCL, a new entity that is a PTCL-NOS subset; and primary cutaneous CD4-positive small/medium T-cell lymphoproliferative disorder. The neoplastic cells should express at least 2 or 3 T follicular helper cell–related antigens, including CD279/PD1, CD10, BCL6, CXCL13, ICOS, SAP, and CCR5; the most commonly used are PD1, BCL6, and CD10. Recurrent fusion of ITK-SYK translocation t(5;9) or CTLA4-CD28 is also common in follicular TCL. Although recurrent mutation is found in these entities, conventional karyotyping or IHC should be sufficient for diagnosis.
Cutaneous γ -Δ T-Cell Lymphoma
Among cutaneous TCLs, primary cutaneous
Peripheral T-Cell Lymphoma
Gene expression profiling analysis of PTCLs has identified at least 3 subtypes characterized by overexpression of GATA3, TBX21, and cytotoxic genes and expression of the corresponding proteins with IHC.47 These subtypes are associated with different clinical behavior and therapy responses. The GATA3 subtype has an inferior prognosis and shows a high level of T helper type 2 cytokines, which can be identified with IHC. As IHC-stained GATA3 has been available as a marker of urothelial carcinoma at most IHC laboratories, GATA3 IHC staining also may be considered in the evaluation of PTCLs.
Many monoclonal antibody therapies are being used as primary or secondary regimens in the treatment of TCL. Clinical trials are working to establish their efficacy. If treatment with a monoclonal antibody is being considered, it is appropriate to conduct IHC to demonstrate the presence of the target antigen and at follow-up, to demonstrate the efficacy of treatment. These therapies include alemtuzumab, which targets CD52, and brentuximab, which targets CD30.
T-Cell Large Granular Lymphocytic Leukemia
T-cell LGL leukemia is a complex diagnosis that requires persistent clonal expansion of LGLs and clinically peripheral blood cytopenia. In many cases, the diagnosis is difficult to establish, as benign large granular lymphocytosis with clonal T cells may occur in conjunction with viral infections or autoimmune disorders. Somatic mutations in the STAT3 (signal transducer and activator of transcription 3) gene are found in 40% of patients with T-cell LGL leukemia.49 More recently, somatic mutations in the STAT5B gene were identified in 2% of T-cell LGL leukemia subsets. The clinical course of T-cell LGL leukemia in patients with the STAT5B mutation is aggressive and fatal, clearly different from the relatively favorable course of typical T-cell LGL leukemia.50 The HMG subcommittee recommends considering a STAT3 and STAT5B mutation study for selected cases in which it is difficult to distinguish true T-cell LGL leukemia from its reactive expansions.
T-Cell Prolymphocytic Leukemia
T-cell prolymphocytic leukemia (T-PLL) is a rare, aggressive disease and is most commonly associated with a prolymphocytic morphology and expression of CD4. However, since a specific immunophenotypic profile of T-PLL has not been identified, flow cytometry is not adequate in isolation for definitive classification as T-PLL.51 A diagnosis of T-PLL often requires cytogenetics or a FISH study to confirm a suspected case. Most TPLL cases harbor characteristic chromosomal abnormalities involving 14q11.2 (TCR
Anaplastic Large Cell Lymphoma
The World Health Organization recognizes 3 distinct types of anaplastic large cell lymphoma (ALCL): systemic anaplastic lymphoma kinase (ALK)–positive ALCL, systemic ALK-negative ALCL, and primary cutaneous ALCL. Systemic ALK-positive ALCLs consistently have ALK gene rearrangements and favorable outcomes. The most common translocation is the t(2;5) rearrangement of NPM1 and ALK, though other ALK partners are also possible. In contrast, systemic ALK-negative ALCLs lack ALK gene rearrangements and as a whole have outcomes inferior to those of systemic ALK-positive ALCLs. However, studies have found systemic ALK-negative ALCL to be a genetically and clinically heterogeneous entity.54 About 30% of cases have rearrangements of the DUSP22-IRF4 locus on 6p25.3 (DUSP22 rearrangement), and these cases have favorable outcomes similar to those of systemic ALK-positive ALCL.55 Only 8% of patients have TP63 rearrangements and very poor outcomes. The remaining cases lack ALK, DUSP22, and TP63 rearrangements and have intermediate outcomes. The HMG subcommittee recommends considering DUSP22 rearrangement by FISH in the evaluation of systemic ALK-negative ALCL.
Conclusion
The pathologic diagnosis, classification, and risk stratification of lymphoma and leukemia require an approach that integrates morphology, flow cytometry, cytogenetics, and molecular pathology. Rapidly evolving molecular techniques currently allow for detailed description of the molecular defects in lymphoma and leukemia, including driver mutations, amplification/deletion events, and clonal evolution. Unfortunately, the technical ability to catalogue the molecular defects in lymphoma and leukemia, often at great expense, is outpacing the ability to use this detailed information in treating patients with hematologic malignancies. The challenge, then, is to identify best practices for the diagnosis and classification of lymphoma and leukemia in VHA hospitals that incorporate the most useful molecular tests without wasting financial resources.
In this report, the HMG subcommittee of the MGPW has presented its recommendations for molecular testing in AML, MPN, MDS, and lymphomas in the context of standard morphologic and immunophenotypic approaches to hematopathology diagnosis and classification. Adoption of these recommendations by VHA hospitals and clinics should help ensure that all VA patients with hematologic malignancies benefit from the latest advances in precision medicine.
Within the vast and comprehensive national VHA health care system are multiple centers of expertise in hematopathology. In addition, multiple VA clinical molecular diagnostic laboratories are performing state-of-the-art testing. The HMG subcommittee proposes that, to make best use of these expert resources, the VHA should establish an interfacility hematopathology consultation service. This service would allow any VA pathologist to consult a board-certified hematopathologist regarding use of ancillary molecular genetic testing in the diagnosis of hematologic malignancy.
In addition, the HMG subcommittee recommends consolidating VA molecular diagnostic reference laboratories and having them perform molecular testing for other VA hospitals rather than using commercial reference laboratories, where testing standards are not uniform and results may be difficult to interpret. Several well-established VA clinical laboratories with technical expertise and informatics support are already performing selected molecular diagnostic testing. These laboratories’ resources should be expanded, where practical, to cost-effectively provide VA expertise to all veterans and to improve access to appropriate molecular diagnostic testing.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of
Click here to read the digital edition.
In January 2015, President Obama introduced the Precision Medicine Initiative, a program set up to identify new biomedical discoveries for the development of a personalized knowledge base of disease entities and individualized treatments. Advances in precision medicine typically involve the use of targeted therapies tailored to individual genetic characteristics identified with molecular testing. The goals are to improve survival and reduce adverse effects. With an initial budget of $215 million, this initiative presented a unique opportunity to combine efforts in genomic discovery, bioinformatic analysis, and health information technology to move toward data-driven, evidence-based precision medicine.1
The VHA is the largest comprehensive health care system in the U. S. and has more than 1,700 care sites serving nearly 9 million veterans each year. The budget for this single-payer system is proposed by the President and approved by Congress. As the VHA must treat a diverse and aging veteran population in an environment of rising costs and budget constraints, limited resources must be monitored and appropriated for the most cost-effective health care delivery. Precision medicine offers a model in which physicians can select the most appropriate diagnostic tests in defined clinical settings to direct clinical care. It supports the testing needed to subdivide each disease category into distinct subcategories. Nevertheless, the need for fiscal responsibility in a capitated health care system recommends testing in cases in which it can change therapy or prognosis rather than for purely academic reasons.
Pathology and Laboratory Medicine Service
Given limited resources and an increasing number of requests for advanced molecular testing, the VA Pathology and Laboratory Medicine Service (P&LMS) formed the Molecular Genetics Pathology Workgroup (MGPW) in September 2013. The charter listed the tasks of the MGPW to “provide recommendations on how to effectively use molecular genetics tests, promote increased quality and availability of testing within the VHA, encourage internal referral testing, provide an organizational structure for Molecular Genetics Testing Consortia, and create a P&LMS policy for molecular genetic testing in general, specifically addressing the issues surrounding laboratory developed testing.” The MGPW has 4 subcommittees: molecular oncology, pharmacogenetics, hematopathology molecular genetics (HMG), and genetic medicine. Since its inception, the HMG subcommittee has had several objectives:
- Standardize the molecular testing nomenclature for and develop practice guidelines for acute myeloid leukemia (AML), myeloproliferative neoplasms (MPN), myelodysplastic syndrome (MDS), chronic lymphocytic leukemia (CLL)/small lymphocytic lymphoma, lymphoma, and plasma cell neoplasms;
- Develop standardized reporting guidelines for current VA molecular laboratories;
- Identify new tests as they are being reported in the literature and collaborate with hematology and oncology services to evaluate the clinical utility of these tests for VA patients;
- Network current VA molecular laboratories, perform fact-finding for these laboratories, and compile test menus; and
- Assess for the formation of VA-wide interfacility consultation services for hematopathology so that all VA facilities, regardless of their complexity, will be able to access the expertise of hematopathology-trained pathologists (Appendix).
The HMG subcommittee met monthly and discussed various diagnostic entities in hematopathology. For hematolymphoid malignancies, it was generally agreed that the traditional laboratory tools of morphology, flow cytometry, and immunohistochemistry (IHC) are standard in initial assessment and often in diagnosis. As the clinical molecular and cytogenetic assays of karyotype, fluorescence in situ hybridization (FISH), advanced DNA sequencing, microarray, and highly sensitive polymerase chain reaction (PCR) analysis affect diagnosis, subclassification, minimal residual disease (MRD) monitoring, prognosis, and therapy selection, their use is marked by a high degree of variability. As a result, standardization is needed. As each laboratory develops and reports ancillary testing, the variable reporting formats may generate postanalytic errors.
A detailed description of all molecular methodologies is beyond the scope of this article. For practicing pathologists, challenges remain in overall cost and reimbursement, extensive and time-consuming data analysis, and in some cases, interpretation differences.
Myeloid Neoplasms
Myeloid malignancies were divided into AML, MPN, and MDS. Next-generation sequencing (NGS) information for these malignancies was used to identify various contributory functional categories, including cell signaling (FLT3, KIT, JAK2, MPL, KRAS/NRAS, PTPN11, NF1, CSF3R); transcription (CEBPA, RUNX1, GATA1/GATA2, PHF6, ETV6); splicing (SF3B1, SRSF2, ZRSR2, U2AF1); epigenetics (DNMT3A, TET2, IDH1/IDH2, ASXL1, EZH2, SUZ12, KDM6A); cohesin complex (STAG2, SMC1A, SMC3, RAD21); and cell cycle (TP53, NPM1).2
Acute Myeloid Leukemia
The HMG subcommittee reviewed the literature on prognostically significant genes in myeloid leukemias. Karyotype abnormalities, such as t(8;21) and inv(16), collectively known as the core-binding factor (CBF) leukemias, t(15;17), t(11q23) (KMT2A/MLL), and so forth, are recurrent lesions in AML. Included in the minimum set of genes recommended by the National Comprehensive Cancer Network (NCCN) for AML prognosis evaluation are nucleolar protein nucleophosmin (NPM1), CCAAT/enhancer-binding protein
Some of the chromosomal translocations, such as inv(16)/t(16;16) in AML and t(15;17) in acute promyelocytic leukemia, can be monitored with FISH or reverse transcription–PCR (RT-PCR) analysis. As NPM1 mutations tend to be seen in recurrence, they can be used as molecular markers for MRD. Other mutations that provide important prognostic information in AML include:
- Activating insertions/duplications in the FLT3 receptor tyrosine kinase, which can be detected with PCR sizing assays;
- Mutations in the KIT receptor tyrosine kinase, which can be detected with DNA sequencing or more limited hotspot PCR;
- Mutations in the DNA methyltransferase, DNMT3A, a poor prognostic indicator seen in 22% of cases of AML, also detected with gene sequencing or more limited hotspot PCR; and
- Another set of genes, TET2, IDH1, IDH2, KRAS, NRAS, EZH2, and ASXL1, is mutated in MPN as well as AML and MDS, making a common molecular panel with next-generation sequencing useful in diagnosing and risk-stratifying all myeloid neoplasms.
The HMG subcommittee agreed that, for de novo AML, chromosomal karyotype is the standard of care, necessary in detecting known cytogenetic abnormalities as well as a wide range of lesions that might indicate a diagnosis of AML with myelodysplasia-related changes at time of diagnosis. In addition, molecular analysis of FLT3 is useful in determining prognosis, and CEBPA (biallelic) and NPM1 mutations are good prognostic factors in normal-karyotype AML. KMT2A (MLL) rearrangements should be tested with FISH if the lineage is ambiguous. The PML-RARA fusion gene also should be tested with FISH if morphologic and flow cytometry results suggest acute promyelocytic leukemia (Table). At this time, testing for TP53, DNMT3A, RAS, and other such mutations is not recommended because it is not cost-effective for the VA.
Myeloproliferative Neoplasms
Myeloproliferative neoplasms are clonal hematopoietic stem cell disorders characterized by proliferation of at least 1 myeloid lineage: granulocytic, erythroid, or megakaryocytic. Myeloproliferative neoplasms show a range of recurrent chromosomal translocations, such as BCR-ABL1 fusion in chronic myelogenous leukemia (CML) that can be detected with RT-PCR analysis as well as FISH. In CML, BCR-ABL1 fusion transcript levels detected by a quantitative PCR (qPCR) method are now used to monitor the course of CML therapy with tyrosine kinase inhibitors (TKIs) and to trigger a treatment change in drug-resistant cases. Given the importance of qPCR in clinical management, significant progress has been made in standardizing both the PCR protocol and the reference materials used to calibrate the BCR-ABL1 PCR assay. BCR-ABL1–negative MPN, including polycythemia vera (PV), essential thrombocythemia (ET), and primary myelofibrosis (PMF), are most commonly associated with mutations in the tyrosine kinase JAK2. Mutations in CALR and MPL are seen in a subset of patients with ET and PMF as well, whereas PV is essentially exclusively a disease of JAK2 mutations.
Chronic myelogenous leukemia is the prototypical MPN. To establish the initial diagnosis, FISH and/or qPCR for BCR-ABL1 fusion should be used. If CML is confirmed, the sample can be reflexed to qPCR BCR-ABL1 on the initial peripheral blood and/or bone marrow sample(s) to establish the patient’s baseline. In addition, a bone marrow sample (aspirate) should be used for a complete karyotype and for morphologic confirmation of disease phase.
For follow-up assessment of CML patients’ response to TKI treatment, qPCR for BCR-ABL1 should be tested with a peripheral blood sample or a bone marrow sample every 3 months.4 A peripheral blood sample is more commonly used because it is conveniently obtained. Early molecular response as indicated by a BCR-ABL1 transcript ratio of < 10% on the International Scale at 3 months, has a strong prognostic value.5 Major molecular response as indicated by a BCR-ABL1 transcript ratio of < 0.1% on the International Scale at 12 to 18 months is also highly prognostic.5
After the peripheral blood sample becomes negative for BCR-ABL1 by qPCR, testing bone marrow samples may be considered. If important treatment response benchmarks are not achieved, or response is lost with rising BCR-ABL1 levels (TKI resistance), ABL1 kinase domain mutation analysis as well as repeat FISH (to assess for copy number multiplication) should be performed to guide further management. Patients with the ABL1 T315I mutation are resistant to all first-line TKIs but may respond to later third-generation TKIs.6
BCR-ABL1–negative MPNs include PV, ET, and PMF. Bone marrow morphology remains the cornerstone of ET and PMF diagnosis. The discovery of JAK2, CALR, and MPL mutations has contributed to how these disorders are diagnosed.7-12 Besides providing the clonality proof that is crucial for diagnosis, the molecular markers influence the prognosis. The JAK2 (p.V617F) or less common JAK2 exon 12 mutations, which are detected in more than 95% of PV cases, are used as molecular markers to confirm diagnosis.7 Further, the JAK2 (p.V617F), CALR (exon 9), and MPL (exon 10) mutations are detected in ET (~60%, 25%, and 3%-5%, respectively) and PMF (~55%, 30%, and 5%, respectively).12 If ET or PMF is suspected clinically, first JAK2 (p.V617F) mutation analysis should be performed, then CALR mutation analysis, and finally MPL mutation analysis. Although novel gain-of-function JAK2 and MPL mutations were recently discovered in triple-negative ET (negative for canonical mutations in JAK2, CALR, and MPL) and PMF by whole exome sequencing,13 clinical testing is not readily available. Besides its utility in the initial diagnosis of ET and PMF, the JAK2 or CALR mutation assay also may be considered for bone marrow transplantation follow-up (Table).14
Despite the continuing debate on the classification of eosinophilic myeloid disorders, the discovery of the FIP1L1-PDGFRA fusion represents a major milestone in the understanding of these disorders.15,16 Unlike PDGFRB (5q33) and FGFR1 (8p11) rearrangements, which can be detected with routine chromosomal analysis (cytogenetics), the cryptic FIP1L1-PDGFRA fusion must be detected with FISH (for CHIC2 deletion) or RT-PCR analysis. It should be pointed out that, as most eosinophilia is reactive or secondary, molecular testing for FIP1L1-PDGFRA fusion is indicated only when primary hypereosinophilia or hypereosinophilic syndrome (HES) is suspected. This is particularly the case in the following hypereosinophilia accompanying conditions: CML-like morphology, but BCR-ABL1–negative; chronic myelomonocytic leukemia (CMML)–like morphology with a normal karyotype; and new onset of cardiac damage or dysfunction.17
Primary eosinophilic myeloid disorders with PDGFRA or PDGFRB rearrangements can be treated with TKIs (eg, imatinib). Next-generation sequencing may be considered in cases of presumed HES when there is no identifiable karyotypic or FISH abnormality. Recent studies have found that cases of HES with somatic mutations indicating clonality had adverse clinical outcomes similar to those of cases of chronic eosinophilic leukemia.18
The discovery of CSF3R mutations offers a new molecular marker for the diagnosis of chronic neutrophilic leukemia (CNL), an MPN.19 The CSF3R (p.T618I) mutation or another activating CSF3R mutation is now used as a diagnostic criterion for CNL. Identification of specific CSF3R mutations may have therapeutic implications as well. The test should be ordered only for patients with clinical and morphologic findings suggestive of CNL; reactive neutrophilic leukocytosis (eg, infection, inflammation) should be ruled out before the test is ordered.
Myelodysplastic Syndrome
Myelodysplastic syndrome is a group of clonal bone marrow disorders characterized by ineffective hematopoiesis, manifested by morphologic dysplasia in ≥ 1 hematopoietic lineages and peripheral cytopenias (hemoglobin level, < 10 g/dL; platelet count, < 100×103/µL; absolute neutrophil count, < 1.8×103/µL). Diagnosis and classification of MDS depend mainly on the degree of morphologic dysplasia and blast percentages, as determined by examining well-prepared cellular bone marrow aspirate smears and/or biopsy touch preparations and peripheral blood smears.
Conventional karyotyping is an essential part of the diagnostic workup for all presumptive cases of MDS and is of both diagnostic and prognostic importance.20 About 60% of MDS cases have recurrent cytogenetic abnormalities, which can be detected with conventional karyotyping. If a high-quality cytogenetic analysis cannot be performed (eg, the bone marrow sample is inadequate), or if quick turnaround is required, an alternative FISH panel may be used to detect some of the common MDS-associated chromosomal abnormalities (eg, 5q deletion, 7q deletion/monosomy 7, +8, 20q deletion).21 Sequencing with FISH also can be useful for assessing MRD by detecting a previously identified chromosomal abnormality.
Targeted sequencing of a limited number of genes can detect mutations in the vast majority of patients with MDS. The most commonly mutated genes in MDS are SF3B1, TET2, SRSF2, ASXL1, DNMT3A, RUNX1, U2AF1, TP53, and EZH2. Mutations in SRSF2 cause RNA splicing abnormalities. In addition, mutations in TP53, EZH2, RUNX1, and ASXL1 are associated with poor prognosis,22,23 whereas mutations in SF3B1 confer better event-free survival.24 Despite these developments, the HMG subcommittee agreed that NGS-based mutation panels are not cost-effective for the VA population at this time and should not be included in a MDS workup. Only in rare situations and when clinically indicated (to change disease classification or patient management) should evaluation for specific gene mutations be considered—for instance, the SF3B1 mutation for patients with probable MDS with ring sideroblasts, if ring sideroblasts are < 15%.25
Myelodysplastic/Myeloproliferative Neoplasms
Myelodysplastic/myeloproliferative neoplasms are a group of myeloid neoplasms with clinical, laboratory, and morphologic features that overlap both MDS and MPN. In MDS/MPN, the karyotype is often normal or shows abnormalities in common with MDS.
In cases of unexplained monocytosis for which there is clinical concern for CMML, morphologic evaluation and conventional chromosomal karyotyping should be performed after other secondary causes and known myeloproliferative and myelodysplastic entities have been excluded. If concomitant hypereosinophilia is present and the karyotype is normal, FISH or PCR-based assay should be performed to rule out FIP1L1-PDGFRA rearrangements. BCR-ABL1, PDGFRB, FGFR1, and t(8;9)/PCM1-JAK2 rearrangements typically are detected with high-quality cytogenetic analysis and thus do not require targeted molecular assays. Although certain gene mutations (eg, SRSF2, TET2, ASXL1, CBL) are commonly detected in CMML, the HMG subcommittee does not recommend sequencing-based mutation panels, as there is insufficient information for testing for prognostic or treatment stratification.
If MDS/MPN with ring sideroblasts and thrombocytosis is suspected on the basis of the clinical and morphologic criteria, molecular tests for the JAK2 (p.V617F) and SF3B1 mutations may be considered in an effort to help confirm the diagnosis.
Atypical CML is a rare MDS/MPN subtype that is now better characterized molecularly with SETBP1 and/or ETNK1 mutations, which are detectable in up to a third of cases. If clinical suspicion is high, sequencing may be diagnostically helpful.
Lymphoid Neoplasms
Chronic Lymphocytic Leukemia
In CLL, recurrent chromosomal abnormalities (eg, deletions of 13q, trisomy 12, deletions of 11q, deletions of 17p) have clear prognostic value and can be detected with FISH. Other prognostic information, such as somatic mutation of immunoglobulin heavy chain variable (IgHV) genes, TP53 mutations, SF3B1, and NOTCH1 mutation, are mostly derived from PCR-based assays. The discovery of recurrently mutated genes in CLL has increased with the use of highly sensitive sequencing methods constructing a more detailed landscape of CLL at genetic, epigenetic, and cellular levels. A recent literature review summarizes the vast heterogeneity of CLL with recurrent pathogenetic findings in MYD88, SF3B1, TP53, ATM, and NOTCH1 signaling pathways.26 The treatment of CLL is rapidly evolving, and many clinical trials are proposing a change from the “watch and wait” paradigm to treatment upon initial presentation based on molecular findings. Additional testing based on new treatment options from current clinical trials will be recommended.
Flow cytometry and morphology are standard for CLL diagnosis. The HMG subcommittee recommends FISH for del(13q14), del(11q), trisomy 12, and del(17p) at time of diagnosis or immediately before therapy initiation. Zeta-chain (
Other B-Cell Lymphoproliferative Disorders
Unlike the common molecular changes in CLL, in other mature B-cell lymphomas, chromosomal translocations that juxtapose a variety of different oncogenes next to an Ig gene enhancer usually are—and those that switch regions less commonly are—important initiating events that can be detected with PCR, DNA sequencing, or FISH. In follicular lymphoma (FL), Burkitt lymphoma, marginal zone lymphoma (MZL), and mantle cell lymphoma (MCL), these oncogenes driven by an Ig gene enhancer typically include BCL2, MYC, MALT1, and CCND1 (cyclin D1), respectively. Molecular variants of these lymphomas that lack these classical translocations often activate homologous genes (eg, cyclin D3/CCND3 is activated in variants of MCL).
Morphology, flow cytometry, and IHC are routinely used for diagnosis. In inconclusive cases, Ig gene rearrangement by PCR may be used. The Table summarizes common molecular changes in B-cell lymphomas.
Mantle cell lymphoma. MCL is a non-Hodgkin lymphoma subtype characterized by t(11;14) (q13;q32) translocations that in the majority of cases lead to overexpression of cyclin D1 (BCL1). Recent molecular profiling has identified an MCL variant that is cyclin D1–negative but SOX11-positive and may have a more aggressive clinical course.30SOX11 regulates PAX5 expression and blocks terminal B-cell differentiation in aggressive MCL.
Lymphoplasmacytic lymphoma. Lymphoplasmacytic lymphoma (LPL), MZL, and CLL/small lymphocytic lymphoma are well-defined clinicopathologic entities. However, distinguishing LPL from MZL and atypical cases of CLL can sometimes be difficult because of overlapping clinical and morphologic features. Recent studies have identified a recurrent L265P mutation in the MYD88 gene in 90% to 95% of LPL cases with IgM paraprotein and in 40% to 50% of the rare non-IgM LPL cases. In contrast, the mutation is much less frequently present in MZL and other low-grade B-cell neoplasms (2%-7%).31 Therefore, testing for this abnormality can be a diagnostic aid in these difficult-to-classify cases. In addition, from a therapeutic perspective, presence or absence of MYD88 mutation may prove more significant than presence of a specific paraprotein or histopathologic features. Ibrutinib has shown efficacy in LPL and demonstrates improved response rates in patients with MYD88 mutation compared with that of their mutation-negative counterparts.32 Several MYD88 inhibitors are in clinical trials. This again indicates the need to more accurately identify and subclassify these non-IgM LPL cases to ensure appropriate molecular evaluation.
Hairy cell leukemia. Flow cytometry and morphology are usually sufficient for a hairy cell leukemia (HCL) diagnosis. However, rare cases are difficult to distinguish variant HCL from other mimics. The BRAF V600E mutation recently was described as a disease-defining molecular marker for HCL—present in nearly all HCL cases but virtually absent in HCL mimics. Therefore, detection of the BRAF mutation by IHC stain with specific antibody or PCR analysis is highly sensitive and specific for the diagnosis of HCL.33
Diffuse large B-cell lymphoma. Recent molecular analysis has created various risk stratification schemata for diffuse large B-cell lymphoma (DLBCL). The HGM subcommittee agrees that well-preserved morphology, IHC, flow cytometry, and FISH-specific markers (BCL2, BCL6, cMYC) are sufficient for diagnostic, prognostic, and therapeutic purposes. Although a wide range of genes have been implicated in the pathogenesis of DLBCL, sequencing and gene expression profiling are not cost-effective at this time and do not add benefit to patient treatment.
The MYD88 L265P mutation has been identified in DLBCL, particularly the activated B-cell-like type and primary central nervous system lymphoma (PCNSL), and may have implications for ibrutinib therapy. PCNSL commonly manifests aggressive clinical behavior and has a poor prognosis. It has been proposed that the MYD88 mutation can be used as a genetic hallmark for PCNSL to distinguish CNS involvement by systemic DLBCL from PCNSL.34
Plasma cell neoplasms. Flow cytometry is acceptable for the diagnosis of plasma cell neoplasms and for residual disease follow-up. Chromosomal karyotype or FISH for IGH/CCND1, IGH/MMSET, and IGH/CMAF dual fusion probes is recommended in conjunction with morphology, IHC, and flow cytometry. In plasma cell myeloma, several genetic mutations can be detected with NGS, including mutations in NRAS, KRAS, TP53, BCL7A, DIS3, and FAM46C.35 Less commonly, BRAF mutations, previously described in melanoma and several other solid tumors, can be detected with DNA sequencing in 4% of multiple myeloma cases, which may prove promising for targeted therapy with BRAF inhibitors. However, current therapeutic decisions are based on genetic and clinical factors, and sequence-based assays are not recommended at this time.
Follicular lymphoma. Cytology, histology, and IHC typically are sufficient for diagnosing FL. In difficult-to-diagnose cases and in cases with scant material, additional tests may help with diagnosis. Eighty to ninety percent of FL cases have t(14;18)(q32;q21), which places the BCL2 gene transcription under the control of the IGH promoter. In addition, about 10% of FL cases have 3q27 aberrancies at the BCL6 gene.36-38 More recently, cases of FL with bulky inguinal disease negative for IGH-BCL2 and BCL6 translocations were found to have 1p36 deletions. These 1p36-deleted FLs typically have a diffuse pattern and a good prognosis.39 For t(14;18), 3q27, or 1p36, FISH is a sensitive means for detecting these translocations, as is PCR for IGH-BCL2.40 There are reports that t(14;18) can be detected in a substantial fraction of otherwise healthy donors at levels and rates that depend on the type of detection test used.41-43 In addition, between one-fourth and one-third of de novo DLBCLs show t(14;18), and about one-third show BCL6 abnormalities at 3q27. Therefore, these genetic changes are not specific for FL and should not be used to subtype a lymphoma as follicular in origin.
Use of IGH-BCL2 as a marker for MRD is still controversial. Some studies have found that a postinduction and posttransplantation IGH-BCL2-positive finding by PCR predicted relapse.44,45 However, others studies have not found significance to postinduction IGH-BCL2 positivity.46 The NCCN guidelines recommend testing for IGH-BCL2 or BCL6 translocations or 1p36 deletion only if this testing is needed for diagnosis. The guidelines do not recommend using these genetic assays in follow-up biopsies, as the importance of treating early relapse has not been definitively demonstrated.
Therefore, if a lymphoma has morphologic, histologic, and IHC findings consistent with FL, then cytogenetic, FISH, or PCR testing is not needed for diagnosis but may be used as confirmation. Follow-up molecular and cytogenetic testing should be avoided if the original cytogenetic abnormality is unknown. That is, IGH-BCL2 FISH should be performed in follow-up samples only if the original lymphoma is known to contain the translocation. As follow-up genetic testing is of disputed clinical significance even in cases in which the original molecular change is known, the NCCN recommendations for therapy are no different. The HMG subcommittee does not recommend molecular or cytogenetic testing in FL beyond what is required for initial diagnosis.
T-Cell Lymphomas
Mature T-Cell Lymphoma and Leukemia
For mature T-cell lymphoma (TCL) and leukemia, the clinical and morphologic criteria have a very important role in the initial workup. However, IHC immunophenotyping is crucial for definitive diagnosis and subclassification. Flow cytometry is routinely used in diagnosing diseases such as T-cell prolymphocytic leukemia (TPLL), T-cell large granular lymphocytic (LGL) leukemia, and Sézary syndrome. T-cell clonality studies, preferably with BIOMED-II–validated primers against targets such as T-cell receptor
Significant advances in TCL classification have led to revisions and the inclusion of new provisional entities in the 2016 World Health Organization classification of lymphoid neoplasms.47 Many of these changes originated in studies of gene expression profiling and the genetic landscape of T-cell neoplasms. Even though subsets of peripheral TCL not otherwise specified (PTCL-NOS) have been recognized on the basis of phenotypic and molecular abnormalities with possible clinical implications, in most cases molecular testing is not part of routine practice. Typically, only a few cytogenetic abnormalities and genetic mutations are used in the evaluation of TCL and T-cell leukemia.
A group of T-cell lymphoproliferative disorders with expression of T follicular helper cell markers can be identified with IHC. These disorders include angioimmunoblastic TCL; follicular TCL, a new entity that is a PTCL-NOS subset; and primary cutaneous CD4-positive small/medium T-cell lymphoproliferative disorder. The neoplastic cells should express at least 2 or 3 T follicular helper cell–related antigens, including CD279/PD1, CD10, BCL6, CXCL13, ICOS, SAP, and CCR5; the most commonly used are PD1, BCL6, and CD10. Recurrent fusion of ITK-SYK translocation t(5;9) or CTLA4-CD28 is also common in follicular TCL. Although recurrent mutation is found in these entities, conventional karyotyping or IHC should be sufficient for diagnosis.
Cutaneous γ -Δ T-Cell Lymphoma
Among cutaneous TCLs, primary cutaneous
Peripheral T-Cell Lymphoma
Gene expression profiling analysis of PTCLs has identified at least 3 subtypes characterized by overexpression of GATA3, TBX21, and cytotoxic genes and expression of the corresponding proteins with IHC.47 These subtypes are associated with different clinical behavior and therapy responses. The GATA3 subtype has an inferior prognosis and shows a high level of T helper type 2 cytokines, which can be identified with IHC. As IHC-stained GATA3 has been available as a marker of urothelial carcinoma at most IHC laboratories, GATA3 IHC staining also may be considered in the evaluation of PTCLs.
Many monoclonal antibody therapies are being used as primary or secondary regimens in the treatment of TCL. Clinical trials are working to establish their efficacy. If treatment with a monoclonal antibody is being considered, it is appropriate to conduct IHC to demonstrate the presence of the target antigen and at follow-up, to demonstrate the efficacy of treatment. These therapies include alemtuzumab, which targets CD52, and brentuximab, which targets CD30.
T-Cell Large Granular Lymphocytic Leukemia
T-cell LGL leukemia is a complex diagnosis that requires persistent clonal expansion of LGLs and clinically peripheral blood cytopenia. In many cases, the diagnosis is difficult to establish, as benign large granular lymphocytosis with clonal T cells may occur in conjunction with viral infections or autoimmune disorders. Somatic mutations in the STAT3 (signal transducer and activator of transcription 3) gene are found in 40% of patients with T-cell LGL leukemia.49 More recently, somatic mutations in the STAT5B gene were identified in 2% of T-cell LGL leukemia subsets. The clinical course of T-cell LGL leukemia in patients with the STAT5B mutation is aggressive and fatal, clearly different from the relatively favorable course of typical T-cell LGL leukemia.50 The HMG subcommittee recommends considering a STAT3 and STAT5B mutation study for selected cases in which it is difficult to distinguish true T-cell LGL leukemia from its reactive expansions.
T-Cell Prolymphocytic Leukemia
T-cell prolymphocytic leukemia (T-PLL) is a rare, aggressive disease and is most commonly associated with a prolymphocytic morphology and expression of CD4. However, since a specific immunophenotypic profile of T-PLL has not been identified, flow cytometry is not adequate in isolation for definitive classification as T-PLL.51 A diagnosis of T-PLL often requires cytogenetics or a FISH study to confirm a suspected case. Most TPLL cases harbor characteristic chromosomal abnormalities involving 14q11.2 (TCR
Anaplastic Large Cell Lymphoma
The World Health Organization recognizes 3 distinct types of anaplastic large cell lymphoma (ALCL): systemic anaplastic lymphoma kinase (ALK)–positive ALCL, systemic ALK-negative ALCL, and primary cutaneous ALCL. Systemic ALK-positive ALCLs consistently have ALK gene rearrangements and favorable outcomes. The most common translocation is the t(2;5) rearrangement of NPM1 and ALK, though other ALK partners are also possible. In contrast, systemic ALK-negative ALCLs lack ALK gene rearrangements and as a whole have outcomes inferior to those of systemic ALK-positive ALCLs. However, studies have found systemic ALK-negative ALCL to be a genetically and clinically heterogeneous entity.54 About 30% of cases have rearrangements of the DUSP22-IRF4 locus on 6p25.3 (DUSP22 rearrangement), and these cases have favorable outcomes similar to those of systemic ALK-positive ALCL.55 Only 8% of patients have TP63 rearrangements and very poor outcomes. The remaining cases lack ALK, DUSP22, and TP63 rearrangements and have intermediate outcomes. The HMG subcommittee recommends considering DUSP22 rearrangement by FISH in the evaluation of systemic ALK-negative ALCL.
Conclusion
The pathologic diagnosis, classification, and risk stratification of lymphoma and leukemia require an approach that integrates morphology, flow cytometry, cytogenetics, and molecular pathology. Rapidly evolving molecular techniques currently allow for detailed description of the molecular defects in lymphoma and leukemia, including driver mutations, amplification/deletion events, and clonal evolution. Unfortunately, the technical ability to catalogue the molecular defects in lymphoma and leukemia, often at great expense, is outpacing the ability to use this detailed information in treating patients with hematologic malignancies. The challenge, then, is to identify best practices for the diagnosis and classification of lymphoma and leukemia in VHA hospitals that incorporate the most useful molecular tests without wasting financial resources.
In this report, the HMG subcommittee of the MGPW has presented its recommendations for molecular testing in AML, MPN, MDS, and lymphomas in the context of standard morphologic and immunophenotypic approaches to hematopathology diagnosis and classification. Adoption of these recommendations by VHA hospitals and clinics should help ensure that all VA patients with hematologic malignancies benefit from the latest advances in precision medicine.
Within the vast and comprehensive national VHA health care system are multiple centers of expertise in hematopathology. In addition, multiple VA clinical molecular diagnostic laboratories are performing state-of-the-art testing. The HMG subcommittee proposes that, to make best use of these expert resources, the VHA should establish an interfacility hematopathology consultation service. This service would allow any VA pathologist to consult a board-certified hematopathologist regarding use of ancillary molecular genetic testing in the diagnosis of hematologic malignancy.
In addition, the HMG subcommittee recommends consolidating VA molecular diagnostic reference laboratories and having them perform molecular testing for other VA hospitals rather than using commercial reference laboratories, where testing standards are not uniform and results may be difficult to interpret. Several well-established VA clinical laboratories with technical expertise and informatics support are already performing selected molecular diagnostic testing. These laboratories’ resources should be expanded, where practical, to cost-effectively provide VA expertise to all veterans and to improve access to appropriate molecular diagnostic testing.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of
Click here to read the digital edition.
1. Collins FS, Varmus H. A new initiative on precision medicine. N Engl J Med. 2015;372(9):793-795.
2. Matynia AP, Szankasi P, Shen W, Kelley TW. Molecular genetic biomarkers in myeloid malignancies. Arch Pathol Lab Med. 2015;139(5):594-601.
3. Wang ML, Bailey NG. Acute myeloid leukemia genetics: risk stratification and implications for therapy. Arch Pathol Lab Med. 2015;139(10):1215-1223.
4. Marum JE, Branford S. Current developments in molecular monitoring in chronic myeloid leukemia. Ther Adv Hematol. 2016;7(5):237-251.
5. Hughes TP, Saglio G, Kantarjian HM, et al. Early molecular response predicts outcomes in patients with chronic myeloid leukemia in chronic phase treated with frontline nilotinib or imatinib. Blood. 2014;123(9):1353-1360.
6. Pallera A, Altman JK, Berman E, et al. Guidelines insights: chronic myeloid leukemia, version 1.2017. J Natl Compr Canc Netw. 2016;14:1505-1512.
7. James C, Ugo V, Le Couédic JP, et al. A unique clonal JAK2 mutation leading to constitutive signalling causes polycythaemia vera. Nature. 2005;434(7037):1144-1148.
8. Kralovics R, Passamonti F, Buser AS, et al. A gain-of-function mutation of JAK2 in myeloproliferative disorders. N Engl J Med. 2005;352(17):1779-1790.
9. Beer PA, Campbell PJ, Scott LM, et al. MPL mutations in myeloproliferative disorders: analysis of the PT-1 cohort. Blood. 2008;112(1):141-149.
10. Vannucchi AM, Antonioli E, Guglielmelli P, et al. Characteristics and clinical correlates of MPL 515W>L/K mutation in essential thrombocythemia. Blood. 2008;112(3):844-847.
11. Nangalia J, Massie CE, Baxter EJ, et al. Somatic CALR mutations in myeloproliferative neoplasms with nonmutated JAK2. N Engl J Med. 2013;369(25):2391-2405.
12. Klampfl T, Gisslinger H, Harutyunyan AS, et al. Somatic mutations of calreticulin in myeloproliferative neoplasms. N Engl J Med. 2013;369(25):2379-2390.
13. Harrison CN, Vannucchi AM. Closing the gap: genetic landscape of MPN. Blood. 2016;127(3):276-278.
14. Tefferi A, Barbui T. Essential thrombocythemia and polycythemia vera: focus on clinical practice. Mayo Clin Proc. 2015;90(9):1283-1293.
15. Cools J, DeAngelo DJ, Gotlib J, et al. A tyrosine kinase created by fusion of the PDGFRA and FIP1L1 genes as a therapeutic target of imatinib in idiopathic hypereosinophilic syndrome. N Engl J Med. 2003;348(13):1201-1214.
16. Valent P, Klion AD, Horny HP, et al. Contemporary consensus proposal on criteria and classification of eosinophilic disorders and related syndromes. J Allergy Clin Immunol. 2012;130(3):607-612.e609.
17. Bain B, Billiland D, Horny H, Verdiman J. Myeloid and lymphoid neoplasms with eosinophilia and abnormalities of PDGFRA, PDGFRB, or FGFR1. In: Swerdlow S, Campo E, Harris N, et al, eds. WHO Classification of Tumours of the Haematopoietic and Lymphoid Tissues. Vol 2. Lyon, France: IRAC Press; 2008:68-73.
18. Wang SA, Tam W, Tsai AG, et al. Targeted next-generation sequencing identifies a subset of idiopathic hypereosinophilic syndrome with features similar to chronic eosinophilic leukemia, not otherwise specified. Mod Pathol. 2016;29(8):854-864.
19. Maxson JE, Gotlib J, Pollyea DA, et al. Oncogenic CSF3R mutations in chronic neutrophilic leukemia and atypical CML. N Engl J Med. 2013;368(19):1781-1790.
20. Schanz J, Tüchler H, Solé F, et al. New comprehensive cytogenetic scoring system for primary myelodysplastic syndromes (MDS) and oligoblastic acute myeloid leukemia after MDS derived from an international database merge. J Clin Oncol. 2012;30(8):820-829.
21. Marshall D, Roboz GJ. Standardizing the initial evaluation for myelodysplastic syndromes. Curr Hematol Malig Rep. 2013;8(4):361-369.
22. Damm F, Chesnais V, Nagata Y, et al. BCOR and BCORL1 mutations in myelodysplastic syndromes and related disorders. Blood. 2013;122(18):3169-3177.
23. Malcovati L, Papaemmanuil E, Ambaglio I, et al. Driver somatic mutations identify distinct disease entities within myeloid neoplasms with myelodysplasia. Blood. 2014;124(9):1513-1521.
24. Papaemmanuil E, Cazzola M, Boultwood J, et al. Somatic SF3B1 mutation in myelodysplasia with ring sideroblasts. N Engl J Med. 2011;365(15):1384-1395.
25. Patnaik MM, Hanson CA, Sulai NH, et al. Prognostic irrelevance of ring sideroblast percentage in World Health Organization-defined myelodysplastic syndromes without excess blasts. Blood. 2012;119(24):5674-5677.
26. Guièze R, Wu CJ. Genomic and epigenomic heterogeneity in chronic lymphocytic leukemia. Blood. 2015;126(4):445-453.
27. Jeromin S, Weissmann S, Haferlach C, et al. SF3B1 mutations correlated to cytogenetics and mutations in NOTCH1, FBXW7, MYD88, XPO1 and TP53 in 1160 untreated CLL patients. Leukemia. 2014;28(1):108-117.
28. Del Giudice I, Rossi D, Chiaretti S, et al. NOTCH1 mutations in +12 chronic lymphocytic leukemia (CLL) confer an unfavorable prognosis, induce a distinctive transcriptional profiling and refine the intermediate prognosis of +12 CLL. Haematologica. 2012;97(3):437-441.
29. Weissmann S, Roller A, Jeromin S, et al. Prognostic impact and landscape of NOTCH1 mutations in chronic lymphocytic leukemia (CLL): a study on 852 patients. Leukemia. 2013;27(12):2393-2396.
30. Vegliante MC, Palomero J, Pérez-Galán P, et al. SOX11 regulates PAX5 expression and blocks terminal B-cell differentiation in aggressive mantle cell lymphoma. Blood. 2013;121(12):2175-2185.
31. King RL, Gonsalves WI, Ansell SM, et al. Lymphoplasmacytic lymphoma with a non-IgM paraprotein shows clinical and pathologic heterogeneity and may harbor MYD88 L265P mutations. Am J Clin Pathol. 2016;145(6):843-851.
32. Insuasti-Beltran G, Gale JM, Wilson CS, Foucar K, Czuchlewski DR. Significance of MYD88 L265P mutation status in the subclassification of low-grade B-cell lymphoma/leukemia. Arch Pathol Lab Med. 2015;139(8):1035-1041.
33. Wang XJ, Kim A, Li S. Immunohistochemical analysis using a BRAF V600E mutation specific antibody is highly sensitive and specific for the diagnosis of hairy cell leukemia. Int J Clin Exp Pathol. 2014;7(7):4323-4328.
34. Nakamura T, Tateishi K, Niwa T, et al. Recurrent mutations of CD79B and MYD88 are the hallmark of primary central nervous system lymphomas. Neuropathol Appl Neurobiol. 2016;42(3):279-290.
35. Chapman MA, Lawrence MS, Keats JJ, et al. Initial genome sequencing and analysis of multiple myeloma. Nature. 2011;471(7339):467-472.
36. Bosga-Bouwer AG, van Imhoff GW, Boonstra R, et al. Follicular lymphoma grade 3B includes 3 cytogenetically defined subgroups with primary t(14;18), 3q27, or other translocations: t(14;18) and 3q27 are mutually exclusive. Blood. 2003;101(3):1149-1154.
37. Gu K, Fu K, Jain S, et al. t(14;18)-negative follicular lymphomas are associated with a high frequency of BCL6 rearrangement at the alternative breakpoint region. Mod Pathol. 2009;22(9):1251-1257.
38. Katzenberger T, Ott G, Klein T, Kalla J, Müller-Hermelink HK, Ott MM. Cytogenetic alterations affecting BCL6 are predominantly found in follicular lymphomas grade 3B with a diffuse large B-cell component. Am J Pathol. 2004;165(2):481-490.
39. Katzenberger T, Kalla J, Leich E, et al. A distinctive subtype of t(14;18)-negative nodal follicular non-Hodgkin lymphoma characterized by a predominantly diffuse growth pattern and deletions in the chromosomal region 1p36. Blood. 2009;113(5):1053-1061.
40. Belaud-Rotureau MA, Parrens M, Carrere N, et al. Interphase fluorescence in situ hybridization is more sensitive than BIOMED-2 polymerase chain reaction protocol in detecting IGH-BCL2 rearrangement in both fixed and frozen lymph node with follicular lymphoma. Hum Pathol. 2007;38(2):365-372.
41. Limpens J, Stad R, Vos C, et al. Lymphoma-associated translocation t(14;18) in blood B cells of normal individuals. Blood. 1995;85(9):2528-2536.
42. Schmitt C, Balogh B, Grundt A, et al. The bcl-2/IgH rearrangement in a population of 204 healthy individuals: occurrence, age and gender distribution, breakpoints, and detection method validity. Leuk Res. 2006;30(6):745-750.
43. Summers KE, Goff LK, Wilson AG, Gupta RK, Lister TA, Fitzgibbon J. Frequency of the Bcl-2/IgH rearrangement in normal individuals: implications for the monitoring of disease in patients with follicular lymphoma. J Clin Oncol. 2001;19(2):420-424.
44. Galimberti S, Luminari S, Ciabatti E, et al. Minimal residual disease after conventional treatment significantly impacts on progression-free survival of patients with follicular lymphoma: the FIL FOLL05 trial. Clin Cancer Res. 2014;20(24):6398-6405.
45. Ladetto M, Lobetti-Bodoni C, Mantoan B, et al; Fondazione Italiana Linfomi. Persistence of minimal residual disease in bone marrow predicts outcome in follicular lymphomas treated with a rituximab-intensive program. Blood. 2013;122(23):3759-3766.
46. van Oers MHJ, Tönnissen E, Van Glabbeke M, et al. BCL-2/IgH polymerase chain reaction status at the end of induction treatment is not predictive for progression-free survival in relapsed/resistant follicular lymphoma: results of a prospective randomized EORTC 20981 phase III intergroup study. J Clin Oncol. 2010;28(13):2246-2252.
47. Swerdlow SH, Campo E, Pileri SA, et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood. 2016;127(20):2375-2390.
48. Dewar R, Andea AA, Guitart J, Arber DA, Weiss LM. Best practices in diagnostic immunohistochemistry: workup of cutaneous lymphoid lesions in the diagnosis of primary cutaneous lymphoma. Arch Pathol Lab Med. 2015;139(3):338-350.
49. Koskela HLM, Eldfors S, Ellonen P, et al. Somatic STAT3 mutations in large granular lymphocytic leukemia. N Engl J Med. 2012;366(20):1905-1913.
50. Rajala HL, Eldfors S, Kuusanmäki H, et al. Discovery of somatic STAT5b mutations in large granular lymphocytic leukemia. Blood. 2013;121(22):4541-4550.
51. Chen X, Cherian S. Immunophenotypic characterization of T-cell prolymphocytic leukemia. Am J Clin Pathol. 2013;140(5):727-735.
52. Delgado P, Starshak P, Rao N, Tirado C. A comprehensive update on molecular and cytogenetic abnormalities in T-cell prolymphocytic leukemia (T-PLL). J Assoc Genet Technol. 2012;38(4):193-198.
53. Stengel A, Kern W, Zenger M, et al. Genetic characterization of T-PLL reveals two major biologic subgroups and JAK3 mutations as prognostic marker. Genes Chromosomes Cancer. 2016;55(1):82-94.
54. Thompson MA, Stumph J, Henrickson SE, et al. Differential gene expression in anaplastic lymphoma kinase-positive and anaplastic lymphoma kinase-negative anaplastic large cell lymphomas. Hum Pathol. 2005;36(5):494-504.
55. King R, Dao L, McPhail E, et al. Morphologic features of ALK-negative anaplastic large cell lymphomas with DUSP22 rearrangements. Am J Surg Pathol. 2016;40(1):36-43.
1. Collins FS, Varmus H. A new initiative on precision medicine. N Engl J Med. 2015;372(9):793-795.
2. Matynia AP, Szankasi P, Shen W, Kelley TW. Molecular genetic biomarkers in myeloid malignancies. Arch Pathol Lab Med. 2015;139(5):594-601.
3. Wang ML, Bailey NG. Acute myeloid leukemia genetics: risk stratification and implications for therapy. Arch Pathol Lab Med. 2015;139(10):1215-1223.
4. Marum JE, Branford S. Current developments in molecular monitoring in chronic myeloid leukemia. Ther Adv Hematol. 2016;7(5):237-251.
5. Hughes TP, Saglio G, Kantarjian HM, et al. Early molecular response predicts outcomes in patients with chronic myeloid leukemia in chronic phase treated with frontline nilotinib or imatinib. Blood. 2014;123(9):1353-1360.
6. Pallera A, Altman JK, Berman E, et al. Guidelines insights: chronic myeloid leukemia, version 1.2017. J Natl Compr Canc Netw. 2016;14:1505-1512.
7. James C, Ugo V, Le Couédic JP, et al. A unique clonal JAK2 mutation leading to constitutive signalling causes polycythaemia vera. Nature. 2005;434(7037):1144-1148.
8. Kralovics R, Passamonti F, Buser AS, et al. A gain-of-function mutation of JAK2 in myeloproliferative disorders. N Engl J Med. 2005;352(17):1779-1790.
9. Beer PA, Campbell PJ, Scott LM, et al. MPL mutations in myeloproliferative disorders: analysis of the PT-1 cohort. Blood. 2008;112(1):141-149.
10. Vannucchi AM, Antonioli E, Guglielmelli P, et al. Characteristics and clinical correlates of MPL 515W>L/K mutation in essential thrombocythemia. Blood. 2008;112(3):844-847.
11. Nangalia J, Massie CE, Baxter EJ, et al. Somatic CALR mutations in myeloproliferative neoplasms with nonmutated JAK2. N Engl J Med. 2013;369(25):2391-2405.
12. Klampfl T, Gisslinger H, Harutyunyan AS, et al. Somatic mutations of calreticulin in myeloproliferative neoplasms. N Engl J Med. 2013;369(25):2379-2390.
13. Harrison CN, Vannucchi AM. Closing the gap: genetic landscape of MPN. Blood. 2016;127(3):276-278.
14. Tefferi A, Barbui T. Essential thrombocythemia and polycythemia vera: focus on clinical practice. Mayo Clin Proc. 2015;90(9):1283-1293.
15. Cools J, DeAngelo DJ, Gotlib J, et al. A tyrosine kinase created by fusion of the PDGFRA and FIP1L1 genes as a therapeutic target of imatinib in idiopathic hypereosinophilic syndrome. N Engl J Med. 2003;348(13):1201-1214.
16. Valent P, Klion AD, Horny HP, et al. Contemporary consensus proposal on criteria and classification of eosinophilic disorders and related syndromes. J Allergy Clin Immunol. 2012;130(3):607-612.e609.
17. Bain B, Billiland D, Horny H, Verdiman J. Myeloid and lymphoid neoplasms with eosinophilia and abnormalities of PDGFRA, PDGFRB, or FGFR1. In: Swerdlow S, Campo E, Harris N, et al, eds. WHO Classification of Tumours of the Haematopoietic and Lymphoid Tissues. Vol 2. Lyon, France: IRAC Press; 2008:68-73.
18. Wang SA, Tam W, Tsai AG, et al. Targeted next-generation sequencing identifies a subset of idiopathic hypereosinophilic syndrome with features similar to chronic eosinophilic leukemia, not otherwise specified. Mod Pathol. 2016;29(8):854-864.
19. Maxson JE, Gotlib J, Pollyea DA, et al. Oncogenic CSF3R mutations in chronic neutrophilic leukemia and atypical CML. N Engl J Med. 2013;368(19):1781-1790.
20. Schanz J, Tüchler H, Solé F, et al. New comprehensive cytogenetic scoring system for primary myelodysplastic syndromes (MDS) and oligoblastic acute myeloid leukemia after MDS derived from an international database merge. J Clin Oncol. 2012;30(8):820-829.
21. Marshall D, Roboz GJ. Standardizing the initial evaluation for myelodysplastic syndromes. Curr Hematol Malig Rep. 2013;8(4):361-369.
22. Damm F, Chesnais V, Nagata Y, et al. BCOR and BCORL1 mutations in myelodysplastic syndromes and related disorders. Blood. 2013;122(18):3169-3177.
23. Malcovati L, Papaemmanuil E, Ambaglio I, et al. Driver somatic mutations identify distinct disease entities within myeloid neoplasms with myelodysplasia. Blood. 2014;124(9):1513-1521.
24. Papaemmanuil E, Cazzola M, Boultwood J, et al. Somatic SF3B1 mutation in myelodysplasia with ring sideroblasts. N Engl J Med. 2011;365(15):1384-1395.
25. Patnaik MM, Hanson CA, Sulai NH, et al. Prognostic irrelevance of ring sideroblast percentage in World Health Organization-defined myelodysplastic syndromes without excess blasts. Blood. 2012;119(24):5674-5677.
26. Guièze R, Wu CJ. Genomic and epigenomic heterogeneity in chronic lymphocytic leukemia. Blood. 2015;126(4):445-453.
27. Jeromin S, Weissmann S, Haferlach C, et al. SF3B1 mutations correlated to cytogenetics and mutations in NOTCH1, FBXW7, MYD88, XPO1 and TP53 in 1160 untreated CLL patients. Leukemia. 2014;28(1):108-117.
28. Del Giudice I, Rossi D, Chiaretti S, et al. NOTCH1 mutations in +12 chronic lymphocytic leukemia (CLL) confer an unfavorable prognosis, induce a distinctive transcriptional profiling and refine the intermediate prognosis of +12 CLL. Haematologica. 2012;97(3):437-441.
29. Weissmann S, Roller A, Jeromin S, et al. Prognostic impact and landscape of NOTCH1 mutations in chronic lymphocytic leukemia (CLL): a study on 852 patients. Leukemia. 2013;27(12):2393-2396.
30. Vegliante MC, Palomero J, Pérez-Galán P, et al. SOX11 regulates PAX5 expression and blocks terminal B-cell differentiation in aggressive mantle cell lymphoma. Blood. 2013;121(12):2175-2185.
31. King RL, Gonsalves WI, Ansell SM, et al. Lymphoplasmacytic lymphoma with a non-IgM paraprotein shows clinical and pathologic heterogeneity and may harbor MYD88 L265P mutations. Am J Clin Pathol. 2016;145(6):843-851.
32. Insuasti-Beltran G, Gale JM, Wilson CS, Foucar K, Czuchlewski DR. Significance of MYD88 L265P mutation status in the subclassification of low-grade B-cell lymphoma/leukemia. Arch Pathol Lab Med. 2015;139(8):1035-1041.
33. Wang XJ, Kim A, Li S. Immunohistochemical analysis using a BRAF V600E mutation specific antibody is highly sensitive and specific for the diagnosis of hairy cell leukemia. Int J Clin Exp Pathol. 2014;7(7):4323-4328.
34. Nakamura T, Tateishi K, Niwa T, et al. Recurrent mutations of CD79B and MYD88 are the hallmark of primary central nervous system lymphomas. Neuropathol Appl Neurobiol. 2016;42(3):279-290.
35. Chapman MA, Lawrence MS, Keats JJ, et al. Initial genome sequencing and analysis of multiple myeloma. Nature. 2011;471(7339):467-472.
36. Bosga-Bouwer AG, van Imhoff GW, Boonstra R, et al. Follicular lymphoma grade 3B includes 3 cytogenetically defined subgroups with primary t(14;18), 3q27, or other translocations: t(14;18) and 3q27 are mutually exclusive. Blood. 2003;101(3):1149-1154.
37. Gu K, Fu K, Jain S, et al. t(14;18)-negative follicular lymphomas are associated with a high frequency of BCL6 rearrangement at the alternative breakpoint region. Mod Pathol. 2009;22(9):1251-1257.
38. Katzenberger T, Ott G, Klein T, Kalla J, Müller-Hermelink HK, Ott MM. Cytogenetic alterations affecting BCL6 are predominantly found in follicular lymphomas grade 3B with a diffuse large B-cell component. Am J Pathol. 2004;165(2):481-490.
39. Katzenberger T, Kalla J, Leich E, et al. A distinctive subtype of t(14;18)-negative nodal follicular non-Hodgkin lymphoma characterized by a predominantly diffuse growth pattern and deletions in the chromosomal region 1p36. Blood. 2009;113(5):1053-1061.
40. Belaud-Rotureau MA, Parrens M, Carrere N, et al. Interphase fluorescence in situ hybridization is more sensitive than BIOMED-2 polymerase chain reaction protocol in detecting IGH-BCL2 rearrangement in both fixed and frozen lymph node with follicular lymphoma. Hum Pathol. 2007;38(2):365-372.
41. Limpens J, Stad R, Vos C, et al. Lymphoma-associated translocation t(14;18) in blood B cells of normal individuals. Blood. 1995;85(9):2528-2536.
42. Schmitt C, Balogh B, Grundt A, et al. The bcl-2/IgH rearrangement in a population of 204 healthy individuals: occurrence, age and gender distribution, breakpoints, and detection method validity. Leuk Res. 2006;30(6):745-750.
43. Summers KE, Goff LK, Wilson AG, Gupta RK, Lister TA, Fitzgibbon J. Frequency of the Bcl-2/IgH rearrangement in normal individuals: implications for the monitoring of disease in patients with follicular lymphoma. J Clin Oncol. 2001;19(2):420-424.
44. Galimberti S, Luminari S, Ciabatti E, et al. Minimal residual disease after conventional treatment significantly impacts on progression-free survival of patients with follicular lymphoma: the FIL FOLL05 trial. Clin Cancer Res. 2014;20(24):6398-6405.
45. Ladetto M, Lobetti-Bodoni C, Mantoan B, et al; Fondazione Italiana Linfomi. Persistence of minimal residual disease in bone marrow predicts outcome in follicular lymphomas treated with a rituximab-intensive program. Blood. 2013;122(23):3759-3766.
46. van Oers MHJ, Tönnissen E, Van Glabbeke M, et al. BCL-2/IgH polymerase chain reaction status at the end of induction treatment is not predictive for progression-free survival in relapsed/resistant follicular lymphoma: results of a prospective randomized EORTC 20981 phase III intergroup study. J Clin Oncol. 2010;28(13):2246-2252.
47. Swerdlow SH, Campo E, Pileri SA, et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood. 2016;127(20):2375-2390.
48. Dewar R, Andea AA, Guitart J, Arber DA, Weiss LM. Best practices in diagnostic immunohistochemistry: workup of cutaneous lymphoid lesions in the diagnosis of primary cutaneous lymphoma. Arch Pathol Lab Med. 2015;139(3):338-350.
49. Koskela HLM, Eldfors S, Ellonen P, et al. Somatic STAT3 mutations in large granular lymphocytic leukemia. N Engl J Med. 2012;366(20):1905-1913.
50. Rajala HL, Eldfors S, Kuusanmäki H, et al. Discovery of somatic STAT5b mutations in large granular lymphocytic leukemia. Blood. 2013;121(22):4541-4550.
51. Chen X, Cherian S. Immunophenotypic characterization of T-cell prolymphocytic leukemia. Am J Clin Pathol. 2013;140(5):727-735.
52. Delgado P, Starshak P, Rao N, Tirado C. A comprehensive update on molecular and cytogenetic abnormalities in T-cell prolymphocytic leukemia (T-PLL). J Assoc Genet Technol. 2012;38(4):193-198.
53. Stengel A, Kern W, Zenger M, et al. Genetic characterization of T-PLL reveals two major biologic subgroups and JAK3 mutations as prognostic marker. Genes Chromosomes Cancer. 2016;55(1):82-94.
54. Thompson MA, Stumph J, Henrickson SE, et al. Differential gene expression in anaplastic lymphoma kinase-positive and anaplastic lymphoma kinase-negative anaplastic large cell lymphomas. Hum Pathol. 2005;36(5):494-504.
55. King R, Dao L, McPhail E, et al. Morphologic features of ALK-negative anaplastic large cell lymphomas with DUSP22 rearrangements. Am J Surg Pathol. 2016;40(1):36-43.
The Potential Dangers of Treating Chronic Lyme Disease
“Chronic Lyme disease” is sometimes a catchall diagnosis for patients with a wide spectrum of musculoskeletal and neuropsychiatric symptoms, fatigue, and generalized pain. That, in turn, has led to a variety of treatments: courses of antibiotics lasting for months to years, IV infusions of hydrogen peroxide, immunoglobulin therapy, even stem cell transplants. Those treatments, though, may not lead to substantial long-term improvement—in fact, they can be harmful.
Clinicians, health departments, and patients have contacted the CDC reporting life-threatening complications resulting from treatment for chronic Lyme disease, including metastatic bacterial infections, septic shock, Clostridium difficile (C diff) colitis, and abscess. An article in Morbidity and Mortality Weekly Report (MMWR) described 5 cases that “highlight the severity and scope” of adverse effects caused by the use of unproven treatments for chronic Lyme disease.
One patient with fatigue and joint pain, was diagnosed with chronic Lyme disease, babesiosis, and Bartonella infection. When the symptoms worsened despite multiple courses of oral antibiotics, the patient was switched to IV ceftriaxone and cefotaxime. However, the pain did not lessen; the patient became hypotensive and tachycardic and was placed in intensive care. Her condition continued to worsen, and she died. The patient’s death was attributed to septic shock related to central venous catheter–associated bacteremia.
In another case, a woman was first diagnosed with amyotrophic lateral sclerosis, then as a second opinion, with chronic Lyme disease. After 7 months of intensive antimicrobial treatment, the pain improved but she got weaker. She also developed intractable C diff infection that required prolonged treatment. However, the patient died of complications of amyotrophic lateral sclerosis—an example, the researchers say, of a missed opportunity for appropriate treatment due to misdiagnosis.
Antibiotics and immunoglobulin therapies are effective and necessary treatments for many conditions, MMWR emphasized—“however, unnecessary antibiotic and immunoglobulin use provides no benefit to patients while putting them at risk for adverse events.”
“Chronic Lyme disease” is sometimes a catchall diagnosis for patients with a wide spectrum of musculoskeletal and neuropsychiatric symptoms, fatigue, and generalized pain. That, in turn, has led to a variety of treatments: courses of antibiotics lasting for months to years, IV infusions of hydrogen peroxide, immunoglobulin therapy, even stem cell transplants. Those treatments, though, may not lead to substantial long-term improvement—in fact, they can be harmful.
Clinicians, health departments, and patients have contacted the CDC reporting life-threatening complications resulting from treatment for chronic Lyme disease, including metastatic bacterial infections, septic shock, Clostridium difficile (C diff) colitis, and abscess. An article in Morbidity and Mortality Weekly Report (MMWR) described 5 cases that “highlight the severity and scope” of adverse effects caused by the use of unproven treatments for chronic Lyme disease.
One patient with fatigue and joint pain, was diagnosed with chronic Lyme disease, babesiosis, and Bartonella infection. When the symptoms worsened despite multiple courses of oral antibiotics, the patient was switched to IV ceftriaxone and cefotaxime. However, the pain did not lessen; the patient became hypotensive and tachycardic and was placed in intensive care. Her condition continued to worsen, and she died. The patient’s death was attributed to septic shock related to central venous catheter–associated bacteremia.
In another case, a woman was first diagnosed with amyotrophic lateral sclerosis, then as a second opinion, with chronic Lyme disease. After 7 months of intensive antimicrobial treatment, the pain improved but she got weaker. She also developed intractable C diff infection that required prolonged treatment. However, the patient died of complications of amyotrophic lateral sclerosis—an example, the researchers say, of a missed opportunity for appropriate treatment due to misdiagnosis.
Antibiotics and immunoglobulin therapies are effective and necessary treatments for many conditions, MMWR emphasized—“however, unnecessary antibiotic and immunoglobulin use provides no benefit to patients while putting them at risk for adverse events.”
“Chronic Lyme disease” is sometimes a catchall diagnosis for patients with a wide spectrum of musculoskeletal and neuropsychiatric symptoms, fatigue, and generalized pain. That, in turn, has led to a variety of treatments: courses of antibiotics lasting for months to years, IV infusions of hydrogen peroxide, immunoglobulin therapy, even stem cell transplants. Those treatments, though, may not lead to substantial long-term improvement—in fact, they can be harmful.
Clinicians, health departments, and patients have contacted the CDC reporting life-threatening complications resulting from treatment for chronic Lyme disease, including metastatic bacterial infections, septic shock, Clostridium difficile (C diff) colitis, and abscess. An article in Morbidity and Mortality Weekly Report (MMWR) described 5 cases that “highlight the severity and scope” of adverse effects caused by the use of unproven treatments for chronic Lyme disease.
One patient with fatigue and joint pain, was diagnosed with chronic Lyme disease, babesiosis, and Bartonella infection. When the symptoms worsened despite multiple courses of oral antibiotics, the patient was switched to IV ceftriaxone and cefotaxime. However, the pain did not lessen; the patient became hypotensive and tachycardic and was placed in intensive care. Her condition continued to worsen, and she died. The patient’s death was attributed to septic shock related to central venous catheter–associated bacteremia.
In another case, a woman was first diagnosed with amyotrophic lateral sclerosis, then as a second opinion, with chronic Lyme disease. After 7 months of intensive antimicrobial treatment, the pain improved but she got weaker. She also developed intractable C diff infection that required prolonged treatment. However, the patient died of complications of amyotrophic lateral sclerosis—an example, the researchers say, of a missed opportunity for appropriate treatment due to misdiagnosis.
Antibiotics and immunoglobulin therapies are effective and necessary treatments for many conditions, MMWR emphasized—“however, unnecessary antibiotic and immunoglobulin use provides no benefit to patients while putting them at risk for adverse events.”
Implementation of a Patient Medication Disposal Program at a VA Medical Center
Opioid overdoses have quadrupled since 1999, with 78 Americans dying every day of opioid overdoses. More than half of all opioid overdose deaths involve prescription opioids.1 Attacking this problem from both ends—prescribing and disposal—can have a greater impact than focusing on a single strategy.
Background
In 2016, the CDC issued opioid prescription guidelines that included encouraging health care providers to discuss “options for safe disposal of unused opioids.”2 Pharmacies are prohibited from directly taking possession of controlled substances from a user. Historically, the Richard L. Roudebush VAMC (RLRVAMC) in Indianapolis, Indiana, recommended that patients follow FDA guidance for household medication disposal, which includes a list of medications that should be flushed down the toilet.3 As more data became available about the negative downstream environmental effects of pharmaceuticals on the water supply, this method of destruction made many patients feel uncomfortable.4,5
The Secure and Responsible Drug Disposal Act of 2010 presented additional options for hospitals and pharmacies to assist the public with medication disposal.6,7 These options offer convenience and anonymity for the end user, reduce potential for diversion, and enhance patient safety by ridding homes of unwanted and expired medications.
Prior to the 2010 act, the only legal methods of controlled substance disposal were via trash disposal, flushing, or delivery to law enforcement, typically at a community-based drug take-back event. These methods were not always convenient, environmentally friendly, or safe for other family members and pets in the home. The Secure and Responsible Drug Disposal Act of 2010 added 2 additional collection options for pharmacies: collection receptacles and mail-back programs.
The RLRVAMC treats > 62,000 veterans annually. The RLRVAMC Pharmacy Service had been providing pharmaceutical mail-back envelopes to patients since May 2015 with moderate success (271 lb of medications returned and a 22.8% envelope return rate through September 2016). Although the mail-back envelopes offer at-home convenience, there was no on-site disposal option. It is not uncommon for patients to bring medications to their appointment or the emergency department (ED). When medication reconciliation is performed, some medications are discontinued, and the prescriber wants them to be safely out of the patient’s possession to avoid confusion and/or accidental overdose. The purpose of this project was to offer more disposal options to patients through the addition of an on-site medication collection receptacle that would be in compliance with U.S. Drug Enforcement Agency (DEA) regulations.
Methods
A policy was developed between the RLRVAMC pharmacy and police departments for the management of a medication collection receptacle. Police would oversee the disposal program so that the pharmacy would not have to change its DEA registration to collector status. The 2 access keys to the receptacle were maintained by police and secured within a key accountability system in the police station.
Full liners are removed from the receptacle by 2 police officers and sealed securely according to vendor guidelines. In accordance with DEA regulations, a form is completed that documents the dates the inner liner was acquired, installed, removed, and transferred for destruction as well as the unique identification number and size of the liner, the address of the location where it was installed, and the names and signatures of the 2 employees that witnessed the removal.
Arrangements are made to have a mail courier present during the removal of the liner. Once removed, the liner is immediately sealed and released to the mail courier who transports the liner to the DEA authorized reverse distributor. The reverse distributor is a licensed entity that has the authority to take control of the medications, including controlled substances, for disposal. The liner tracking numbers are kept in a police log book so that delivery can be confirmed and destruction certificates obtained from the vendor’s website at a later date. The records are kept for 3 years.
Funding was obtained for a 38-gallon collection receptacle and 12 liners from Pharmacy Benefits Management Services (PBM). Approval was obtained from RLRVAMC leadership to locate the receptacle in a high-traffic area, anchored to the floor, under video surveillance, and away from the ED entrance (a DEA requirement). Public Affairs promoted the receptacle to veterans. E-mails were sent to staff to provide education on regulatory requirements. Weight and frequency of medications returned were obtained from data collected by the reverse distributor. Descriptive statistics are reported.
Results
The Federal Supply Schedule cost to procure a DEA-compliant receptacle was $1,450. The additional 12 inner liners cost $2,024.97.8 Staff from Engineering Service were able to install the receptacle at no additional cost to the facility. The collection receptacle was opened to the public in May 2016. From May through October 2016, the facility collected and returned 10 liners to the reverse distributor containing 452 lb of medications. An additional 30 lb of drugs were returned through the mailback envelope program for a total weight of 482 lb over the 6-month period (Figure). The average time between inner liner changes was 2.6 weeks.
Discussion
The most challenging aspect of implementation was identification of a location for the receptacle. The location chosen, an alcove in the hallway between the coffee shop and outpatient pharmacy, was most appropriate. It is a high-traffic area, under video surveillance, and provides easy access for patients. Another challenge was determining the frequency of liner changes. There was no historic data to assist with predicting how quickly the liner would fill up. Initially, Police Service checked the receptacle every week, and it was emptied about every 2 weeks. Aspatients cleaned out their medicine cabinets, the liner needed to be replaced closer to every 4 weeks. An ongoing challenge has been determining how full the liner is without requiring the police to open the receptacle. Consideration is being given to installing a scale in the receptacle under the liner and having the display affixed to the outside of the container.
The receptacle seemed to be the preferred method of disposal, considering that it generated nearly 15 times more waste than did the mail-back envelopes during the same time period. Anecdotal patient feedback has been extremely positive on social media and by word-of-mouth.
Limitations
One limitation of this disposal program is that the specific amount of controlled substance waste vs noncontrolled substance waste cannot be determined since the liner contents are not inventoried. The University of Findlay in Ohio partnered with local law enforcement to host 7 community medication take-back events over a 3-year period, inventoried the drugs, and found that about one-third of the dosing units (eg, tablet or capsule) returned in the analgesic category were controlled substances, suggesting that take-back events may play a role in reducing unauthorized access to prescription painkillers.9 By witnessing the changing of inner liners, it can be anecdotally confirmed that a significant amount of controlled substances were collected and returned at RLRVAMC. These results have been shared with respective VISN leadership, and additional facilities are installing receptacles.
Conclusion
Changes to DEA regulations offer medical centers more options for developing a comprehensive drug disposal program. Implementation of a pharmaceutical take-back program can assist patients with disposal of unwanted and expired medications, promote safety and environmental stewardship, and reduce the risk of diversion.
1. Centers for Disease Control and Prevention. Opioid overdose. http://www.cdc.gov/drugoverdose/index.html. Updated April 16, 2017. Accessed June 5, 2017.
2. Dowell D, Haegerich TM, Chou R. CDC guideline for prescribing opioids for chronic pain—United States, 2016. MMWR Recomm Rep. 2016;65(1):1-49.
3. U.S. Food and Drug Administration. Disposal of unused medicines: what you should know. http://www.fda.gov/Drugs/ResourcesForYou/Consumers/BuyingUsingMedicineSafely/EnsuringSafeUseofMedicine/SafeDisposalofMedicines/ucm186187.htm#Flush_List. Updated April 21, 2017. Accessed June 5, 2017.
4. Li WC. Occurrence, sources, and fate of pharmaceuticals in aquatic environment and soil. Environ Pollut. 2014;187:193-201.
5. Boxall AB. The environmental side effects of medication. EMBO Rep. 2004;5(12):1110-1116.
6. Peterson DM. New DEA rules expand options for controlled substance disposal. J Pain Palliat Care Pharmacother. 2015;29(1):22-26.
7. U.S. Department of Justice, Drug Enforcement Administration. Drug disposal information. https:// www.deadiversion.usdoj.gov/drug_disposal/index.html. Accessed June 5, 2016.
8. GSA Advantage! Online shopping. https://www.gsaadvantage.gov. Accessed June 5, 2017.
9. Perry LA, Shinn BW, Stanovich J. Quantification of an ongoing community-based medication take-back program. J Am Pharm Assoc (2003). 2014;54(3):275-279.
Opioid overdoses have quadrupled since 1999, with 78 Americans dying every day of opioid overdoses. More than half of all opioid overdose deaths involve prescription opioids.1 Attacking this problem from both ends—prescribing and disposal—can have a greater impact than focusing on a single strategy.
Background
In 2016, the CDC issued opioid prescription guidelines that included encouraging health care providers to discuss “options for safe disposal of unused opioids.”2 Pharmacies are prohibited from directly taking possession of controlled substances from a user. Historically, the Richard L. Roudebush VAMC (RLRVAMC) in Indianapolis, Indiana, recommended that patients follow FDA guidance for household medication disposal, which includes a list of medications that should be flushed down the toilet.3 As more data became available about the negative downstream environmental effects of pharmaceuticals on the water supply, this method of destruction made many patients feel uncomfortable.4,5
The Secure and Responsible Drug Disposal Act of 2010 presented additional options for hospitals and pharmacies to assist the public with medication disposal.6,7 These options offer convenience and anonymity for the end user, reduce potential for diversion, and enhance patient safety by ridding homes of unwanted and expired medications.
Prior to the 2010 act, the only legal methods of controlled substance disposal were via trash disposal, flushing, or delivery to law enforcement, typically at a community-based drug take-back event. These methods were not always convenient, environmentally friendly, or safe for other family members and pets in the home. The Secure and Responsible Drug Disposal Act of 2010 added 2 additional collection options for pharmacies: collection receptacles and mail-back programs.
The RLRVAMC treats > 62,000 veterans annually. The RLRVAMC Pharmacy Service had been providing pharmaceutical mail-back envelopes to patients since May 2015 with moderate success (271 lb of medications returned and a 22.8% envelope return rate through September 2016). Although the mail-back envelopes offer at-home convenience, there was no on-site disposal option. It is not uncommon for patients to bring medications to their appointment or the emergency department (ED). When medication reconciliation is performed, some medications are discontinued, and the prescriber wants them to be safely out of the patient’s possession to avoid confusion and/or accidental overdose. The purpose of this project was to offer more disposal options to patients through the addition of an on-site medication collection receptacle that would be in compliance with U.S. Drug Enforcement Agency (DEA) regulations.
Methods
A policy was developed between the RLRVAMC pharmacy and police departments for the management of a medication collection receptacle. Police would oversee the disposal program so that the pharmacy would not have to change its DEA registration to collector status. The 2 access keys to the receptacle were maintained by police and secured within a key accountability system in the police station.
Full liners are removed from the receptacle by 2 police officers and sealed securely according to vendor guidelines. In accordance with DEA regulations, a form is completed that documents the dates the inner liner was acquired, installed, removed, and transferred for destruction as well as the unique identification number and size of the liner, the address of the location where it was installed, and the names and signatures of the 2 employees that witnessed the removal.
Arrangements are made to have a mail courier present during the removal of the liner. Once removed, the liner is immediately sealed and released to the mail courier who transports the liner to the DEA authorized reverse distributor. The reverse distributor is a licensed entity that has the authority to take control of the medications, including controlled substances, for disposal. The liner tracking numbers are kept in a police log book so that delivery can be confirmed and destruction certificates obtained from the vendor’s website at a later date. The records are kept for 3 years.
Funding was obtained for a 38-gallon collection receptacle and 12 liners from Pharmacy Benefits Management Services (PBM). Approval was obtained from RLRVAMC leadership to locate the receptacle in a high-traffic area, anchored to the floor, under video surveillance, and away from the ED entrance (a DEA requirement). Public Affairs promoted the receptacle to veterans. E-mails were sent to staff to provide education on regulatory requirements. Weight and frequency of medications returned were obtained from data collected by the reverse distributor. Descriptive statistics are reported.
Results
The Federal Supply Schedule cost to procure a DEA-compliant receptacle was $1,450. The additional 12 inner liners cost $2,024.97.8 Staff from Engineering Service were able to install the receptacle at no additional cost to the facility. The collection receptacle was opened to the public in May 2016. From May through October 2016, the facility collected and returned 10 liners to the reverse distributor containing 452 lb of medications. An additional 30 lb of drugs were returned through the mailback envelope program for a total weight of 482 lb over the 6-month period (Figure). The average time between inner liner changes was 2.6 weeks.
Discussion
The most challenging aspect of implementation was identification of a location for the receptacle. The location chosen, an alcove in the hallway between the coffee shop and outpatient pharmacy, was most appropriate. It is a high-traffic area, under video surveillance, and provides easy access for patients. Another challenge was determining the frequency of liner changes. There was no historic data to assist with predicting how quickly the liner would fill up. Initially, Police Service checked the receptacle every week, and it was emptied about every 2 weeks. Aspatients cleaned out their medicine cabinets, the liner needed to be replaced closer to every 4 weeks. An ongoing challenge has been determining how full the liner is without requiring the police to open the receptacle. Consideration is being given to installing a scale in the receptacle under the liner and having the display affixed to the outside of the container.
The receptacle seemed to be the preferred method of disposal, considering that it generated nearly 15 times more waste than did the mail-back envelopes during the same time period. Anecdotal patient feedback has been extremely positive on social media and by word-of-mouth.
Limitations
One limitation of this disposal program is that the specific amount of controlled substance waste vs noncontrolled substance waste cannot be determined since the liner contents are not inventoried. The University of Findlay in Ohio partnered with local law enforcement to host 7 community medication take-back events over a 3-year period, inventoried the drugs, and found that about one-third of the dosing units (eg, tablet or capsule) returned in the analgesic category were controlled substances, suggesting that take-back events may play a role in reducing unauthorized access to prescription painkillers.9 By witnessing the changing of inner liners, it can be anecdotally confirmed that a significant amount of controlled substances were collected and returned at RLRVAMC. These results have been shared with respective VISN leadership, and additional facilities are installing receptacles.
Conclusion
Changes to DEA regulations offer medical centers more options for developing a comprehensive drug disposal program. Implementation of a pharmaceutical take-back program can assist patients with disposal of unwanted and expired medications, promote safety and environmental stewardship, and reduce the risk of diversion.
Opioid overdoses have quadrupled since 1999, with 78 Americans dying every day of opioid overdoses. More than half of all opioid overdose deaths involve prescription opioids.1 Attacking this problem from both ends—prescribing and disposal—can have a greater impact than focusing on a single strategy.
Background
In 2016, the CDC issued opioid prescription guidelines that included encouraging health care providers to discuss “options for safe disposal of unused opioids.”2 Pharmacies are prohibited from directly taking possession of controlled substances from a user. Historically, the Richard L. Roudebush VAMC (RLRVAMC) in Indianapolis, Indiana, recommended that patients follow FDA guidance for household medication disposal, which includes a list of medications that should be flushed down the toilet.3 As more data became available about the negative downstream environmental effects of pharmaceuticals on the water supply, this method of destruction made many patients feel uncomfortable.4,5
The Secure and Responsible Drug Disposal Act of 2010 presented additional options for hospitals and pharmacies to assist the public with medication disposal.6,7 These options offer convenience and anonymity for the end user, reduce potential for diversion, and enhance patient safety by ridding homes of unwanted and expired medications.
Prior to the 2010 act, the only legal methods of controlled substance disposal were via trash disposal, flushing, or delivery to law enforcement, typically at a community-based drug take-back event. These methods were not always convenient, environmentally friendly, or safe for other family members and pets in the home. The Secure and Responsible Drug Disposal Act of 2010 added 2 additional collection options for pharmacies: collection receptacles and mail-back programs.
The RLRVAMC treats > 62,000 veterans annually. The RLRVAMC Pharmacy Service had been providing pharmaceutical mail-back envelopes to patients since May 2015 with moderate success (271 lb of medications returned and a 22.8% envelope return rate through September 2016). Although the mail-back envelopes offer at-home convenience, there was no on-site disposal option. It is not uncommon for patients to bring medications to their appointment or the emergency department (ED). When medication reconciliation is performed, some medications are discontinued, and the prescriber wants them to be safely out of the patient’s possession to avoid confusion and/or accidental overdose. The purpose of this project was to offer more disposal options to patients through the addition of an on-site medication collection receptacle that would be in compliance with U.S. Drug Enforcement Agency (DEA) regulations.
Methods
A policy was developed between the RLRVAMC pharmacy and police departments for the management of a medication collection receptacle. Police would oversee the disposal program so that the pharmacy would not have to change its DEA registration to collector status. The 2 access keys to the receptacle were maintained by police and secured within a key accountability system in the police station.
Full liners are removed from the receptacle by 2 police officers and sealed securely according to vendor guidelines. In accordance with DEA regulations, a form is completed that documents the dates the inner liner was acquired, installed, removed, and transferred for destruction as well as the unique identification number and size of the liner, the address of the location where it was installed, and the names and signatures of the 2 employees that witnessed the removal.
Arrangements are made to have a mail courier present during the removal of the liner. Once removed, the liner is immediately sealed and released to the mail courier who transports the liner to the DEA authorized reverse distributor. The reverse distributor is a licensed entity that has the authority to take control of the medications, including controlled substances, for disposal. The liner tracking numbers are kept in a police log book so that delivery can be confirmed and destruction certificates obtained from the vendor’s website at a later date. The records are kept for 3 years.
Funding was obtained for a 38-gallon collection receptacle and 12 liners from Pharmacy Benefits Management Services (PBM). Approval was obtained from RLRVAMC leadership to locate the receptacle in a high-traffic area, anchored to the floor, under video surveillance, and away from the ED entrance (a DEA requirement). Public Affairs promoted the receptacle to veterans. E-mails were sent to staff to provide education on regulatory requirements. Weight and frequency of medications returned were obtained from data collected by the reverse distributor. Descriptive statistics are reported.
Results
The Federal Supply Schedule cost to procure a DEA-compliant receptacle was $1,450. The additional 12 inner liners cost $2,024.97.8 Staff from Engineering Service were able to install the receptacle at no additional cost to the facility. The collection receptacle was opened to the public in May 2016. From May through October 2016, the facility collected and returned 10 liners to the reverse distributor containing 452 lb of medications. An additional 30 lb of drugs were returned through the mailback envelope program for a total weight of 482 lb over the 6-month period (Figure). The average time between inner liner changes was 2.6 weeks.
Discussion
The most challenging aspect of implementation was identification of a location for the receptacle. The location chosen, an alcove in the hallway between the coffee shop and outpatient pharmacy, was most appropriate. It is a high-traffic area, under video surveillance, and provides easy access for patients. Another challenge was determining the frequency of liner changes. There was no historic data to assist with predicting how quickly the liner would fill up. Initially, Police Service checked the receptacle every week, and it was emptied about every 2 weeks. Aspatients cleaned out their medicine cabinets, the liner needed to be replaced closer to every 4 weeks. An ongoing challenge has been determining how full the liner is without requiring the police to open the receptacle. Consideration is being given to installing a scale in the receptacle under the liner and having the display affixed to the outside of the container.
The receptacle seemed to be the preferred method of disposal, considering that it generated nearly 15 times more waste than did the mail-back envelopes during the same time period. Anecdotal patient feedback has been extremely positive on social media and by word-of-mouth.
Limitations
One limitation of this disposal program is that the specific amount of controlled substance waste vs noncontrolled substance waste cannot be determined since the liner contents are not inventoried. The University of Findlay in Ohio partnered with local law enforcement to host 7 community medication take-back events over a 3-year period, inventoried the drugs, and found that about one-third of the dosing units (eg, tablet or capsule) returned in the analgesic category were controlled substances, suggesting that take-back events may play a role in reducing unauthorized access to prescription painkillers.9 By witnessing the changing of inner liners, it can be anecdotally confirmed that a significant amount of controlled substances were collected and returned at RLRVAMC. These results have been shared with respective VISN leadership, and additional facilities are installing receptacles.
Conclusion
Changes to DEA regulations offer medical centers more options for developing a comprehensive drug disposal program. Implementation of a pharmaceutical take-back program can assist patients with disposal of unwanted and expired medications, promote safety and environmental stewardship, and reduce the risk of diversion.
1. Centers for Disease Control and Prevention. Opioid overdose. http://www.cdc.gov/drugoverdose/index.html. Updated April 16, 2017. Accessed June 5, 2017.
2. Dowell D, Haegerich TM, Chou R. CDC guideline for prescribing opioids for chronic pain—United States, 2016. MMWR Recomm Rep. 2016;65(1):1-49.
3. U.S. Food and Drug Administration. Disposal of unused medicines: what you should know. http://www.fda.gov/Drugs/ResourcesForYou/Consumers/BuyingUsingMedicineSafely/EnsuringSafeUseofMedicine/SafeDisposalofMedicines/ucm186187.htm#Flush_List. Updated April 21, 2017. Accessed June 5, 2017.
4. Li WC. Occurrence, sources, and fate of pharmaceuticals in aquatic environment and soil. Environ Pollut. 2014;187:193-201.
5. Boxall AB. The environmental side effects of medication. EMBO Rep. 2004;5(12):1110-1116.
6. Peterson DM. New DEA rules expand options for controlled substance disposal. J Pain Palliat Care Pharmacother. 2015;29(1):22-26.
7. U.S. Department of Justice, Drug Enforcement Administration. Drug disposal information. https:// www.deadiversion.usdoj.gov/drug_disposal/index.html. Accessed June 5, 2016.
8. GSA Advantage! Online shopping. https://www.gsaadvantage.gov. Accessed June 5, 2017.
9. Perry LA, Shinn BW, Stanovich J. Quantification of an ongoing community-based medication take-back program. J Am Pharm Assoc (2003). 2014;54(3):275-279.
1. Centers for Disease Control and Prevention. Opioid overdose. http://www.cdc.gov/drugoverdose/index.html. Updated April 16, 2017. Accessed June 5, 2017.
2. Dowell D, Haegerich TM, Chou R. CDC guideline for prescribing opioids for chronic pain—United States, 2016. MMWR Recomm Rep. 2016;65(1):1-49.
3. U.S. Food and Drug Administration. Disposal of unused medicines: what you should know. http://www.fda.gov/Drugs/ResourcesForYou/Consumers/BuyingUsingMedicineSafely/EnsuringSafeUseofMedicine/SafeDisposalofMedicines/ucm186187.htm#Flush_List. Updated April 21, 2017. Accessed June 5, 2017.
4. Li WC. Occurrence, sources, and fate of pharmaceuticals in aquatic environment and soil. Environ Pollut. 2014;187:193-201.
5. Boxall AB. The environmental side effects of medication. EMBO Rep. 2004;5(12):1110-1116.
6. Peterson DM. New DEA rules expand options for controlled substance disposal. J Pain Palliat Care Pharmacother. 2015;29(1):22-26.
7. U.S. Department of Justice, Drug Enforcement Administration. Drug disposal information. https:// www.deadiversion.usdoj.gov/drug_disposal/index.html. Accessed June 5, 2016.
8. GSA Advantage! Online shopping. https://www.gsaadvantage.gov. Accessed June 5, 2017.
9. Perry LA, Shinn BW, Stanovich J. Quantification of an ongoing community-based medication take-back program. J Am Pharm Assoc (2003). 2014;54(3):275-279.
Outcomes Associated With a Multidisciplinary Pain Oversight Committee to Facilitate Appropriate Management of Chronic Opioid Therapy
The use of opioids to treat chronic noncancer pain (CNCP) has become increasingly common over the previous 2 decades. The Office of National Drug Control Policy (ONDCP) reported that from 1997 to 2007, there was a 4-fold increase in the mg per person per year sale of prescription opioids, from 74 mg to 369 mg.1 The number of opioid prescriptions dispensed by pharmacies also has increased by 48% from 2000 to 2009.2 Within the VA population, about half of the 1.44 million patients with a diagnosis of pain (excluding cancer pain) received opioids during 2011, and 57% of these patients received chronic opioid therapy (COT), which is at least 90 days of opioid use in a year.3
Despite this increased use of opioids, data regarding the efficacy of long-term opioid use for noncancer pain remain limited.1,4-8 Instead, there is a growing body of evidence describing potential adverse effects (AEs) of long-term opioid use at even relatively modest doses, including sexual dysfunction, hyperalgesia, and altered brain structure.9-11 Additionally, increases in the misuse and abuse of opioids as well as mortality associated with opioid toxicity have been observed.12-14 Opioid pain relievers were involved in nearly 17,000 deaths in the U.S. in 2010, which represents a 3-fold increase since 1999. This number also represents 75% of all deaths that were attributed to prescription drug poisoning in 2010.13 Unfortunately, this alarming trend parallels the aforementioned increases in the utilization of prescription opioids for CNCP.
Given this accumulating data regarding the profound risks and limited benefit of COT, many organizations have advocated a reassessment of the upward trajectory of opioid utilization. In 2009, the American Pain Society (APS) in partnership with the American Academy of Pain Medicine (AAPM) released clinical guidelines for the use of COT in CNCP.6 In this guideline, the authors advocate a balanced approach to opioid use: Clinicians consider both the legitimate medical need for opioids in some patients with CNCP as well as the serious public health problem of abuse, addiction, and diversion.6 In 2011, the FDA, Drug Enforcement Agency (DEA), and ONDCP enacted the Prescription Drug Abuse Prevention Plan, which focused on 4 major areas: education, prescription monitoring, proper medication disposal, and law enforcement.4
In March 2016, the CDC released a new guideline for prescribing opioids for chronic pain that included 12 recommendations based on 3 key principles. First, nonopioids are preferred for chronic pain in all settings except for active cancer, palliative, and end-of-life care. Next, when opioids are used for chronic pain, they always should be prescribed at the lowest possible effective dose to reduce the risk of opioid use disorder and overdose. Finally, clinicians should exercise caution when prescribing opioids and monitor all patients closely for opioid-related risk.15
Recently, an August 2016 FDA review found that the combined use of opioids and benzodiazepines (BZDs) resulted in serious AEs, including respiratory depression and death. Based on these findings, the FDA requires that updated boxed warnings be added to the labeling of prescription opioid and BZDs.16
The VHA also has been at the forefront of this national movement to promote the appropriate use of opioids. In 2009, the VHA released a pain management directive that highlighted the risks of COT and required adoption of a stepped-care approach to opioid prescribing that focused on quality of life as the primary determinant of treatment quality.17 In 2010, the VHA released its guideline on opioid therapy for chronic pain, which also included tools for providers, such as a sample opioid therapy agreement, equivalent potency tables, and a urine drug screening guide.18 In 2014, the VHA released the Opioid Safety Initiative (OSI), which advocates for a team-based approach to reduce the use of opioids for veterans through a focus on alternate methods to alleviate pain.
At the Ralph H. Johnson VAMC (RHJVAMC) in Charleston, South Carolina, a multidisciplinary pain oversight committee (POC) was tasked with assisting in achieving the goals set forth in the VHA OSI. To reach these goals, the POC sought to develop and implement a population-based initiative targeting modifiable factors that are known to increase the risk of opioid-related toxicity and overdose. These factors included patient utilization of multiple prescribers or multiple pharmacies, high-dose COT (defined in the APS/AAPM guidelines as a morphine equivalent daily dose [MEDD] > 200 mg6), and use of concomitant central nervous system-active medications, chiefly BZDs.19-23 The POC consisted of the RHJVAMC chiefs of mental health, primary care, and pharmacy; a physician specializing in pain and addiction medicine; a pharmacist specializing in pain and palliative care; quality management personnel; a patient advocate; and multiple physicians from the mental health and primary care departments.
Previous studies have described the successful implementation of opioid management initiatives in a variety of health care settings.2,21,24-27 However, most of this work focused only on strategies to decrease prescribing of high-dose and long-acting opioid formulations. The study presented here sought to add to the existing body of knowledge through evaluation of an initiative aimed at increasing appropriate monitoring as a tool to decrease opioid-related patient risk. The primary aim of this study was to describe the types of interventions implemented by the POC during the study period. The secondary aim was to evaluate the effect of these interventions on the appropriate monitoring of COT as well as the appropriate management of high-risk COT > 200 mg MEDD.
Methods
This study involved a qualitative description of individual POC interventions as well as a retrospective data analysis that examined the clinical impact of these interventions during the study period from April 1, 2012 to September 30, 2015. This study was reviewed and approved by the Medical University of South Carolina Institutional Review Board and the RHJVAMC Research and Development Committee.
Setting
The RHJVAMC is a tertiary care teaching hospital with primary and specialized outpatient services that are provided at the main medical center in Charleston, South Carolina, and at 6 community-based outpatient clinics (CBOCs) located throughout southeastern South Carolina and parts of Georgia. Primary care is delivered by patient aligned care teams (PACTs) based on the patient-centered medical home model.28 The PACT consists of a primary care provider (PCP) who is aided by dedicated nursing, pharmacy, and mental health care providers. In most cases, COT is prescribed and managed in the PACT setting. At the time of this initiative, a broad range of specialty services were available, including a multidisciplinary pain management team, orthopedics, and physical medicine and rehabilitation. In 2012, about 55,000 patients were enrolled and received care at RHJVAMC. The POC interventions were carried out at all clinic sites.
Patients
The study population included all patients prescribed COT at RHJVAMC during the study period. A patient was considered to be prescribed COT if at least 1 opioid-containing medication was dispensed to the patient in a selected fiscal quarter during the study period and the total cumulative supply of opioid-containing medications was ≥ 90 days for both the selected quarter and the prior quarter.
Furthermore, a high-risk COT subpopulation included any patient who satisfied either of the following criteria: (1) Receipt of outpatient prescription(s) for opioid-containing medication(s) (including tramadol) and a benzodiazepine derivative in the same fiscal quarter; patients were included in this subpopulation regardless of whether they met COT criteria; (2) Receipt of outpatient prescription(s) for opioid-containing medication(s) with at least 1 instance in which the MEDD was ≥ 200 mg in the designated quarter (Table 1). The MEDD was calculated for each fill in the fiscal quarter using the following equation:
If medication fills were within 3 days of each other, the prescriptions were considered to be taken together and the MEDD was summed.
Intervention Descriptions
The primary aim of this study was to qualitatively describe each intervention implemented by the POC. The POC monthly meeting minutes were recorded and reviewed for the study period, and descriptive information regarding each intervention was extracted. Extracted information included implementation date(s), the responsible POC member, and a general description of each intervention. Interventions were then categorized as informatics tool, targeted patient intervention, provider education, or patient education.
Impact of Interventions on Monitoring
In order to characterize the impact of POC interventions on appropriate monitoring of COT, the electronic medical record (EMR) of each patient satisfying COT criteria was queried for the presence of an annual urine drug screen (UDS) result and a note in the chart signaling that a prescription drug monitoring program (PDMP) review had been performed. The authors defined appropriate UDS monitoring and PDMP review as the presence of a UDS result and a PDMP review note in the EMR in the year prior to the query date.
Prior to the start of the POC interventions, 4.1% of RJVAMC patients had an annual PDMP review and 47.8% had an annual UDS. Although more frequent UDS results and PDMP reviews are appropriate in most cases, yearly monitoring was considered by the POC to be a reasonable initial goal. The percentage of veterans receiving COT who had received appropriate monitoring for each measure was collected for each fiscal quarter during the study period. In addition, the difference between the initial and final fiscal quarter during the study period was calculated for each measure.
Impact of Interventions on High-Risk Opioid Prescribing
To assess the impact of POC interventions on appropriate management of high-dose COT, clinical variables were collected for patients who were prescribed high-dose COT and received targeted intervention in the form of a pain clinic e-consult. These variables were MEDD, presence of annual UDS, presence of annual PDMP review, and active BZD prescription. Each variable was assessed on the date of intervention (e-consult submission) and at 6 months postintervention. Changes in each clinical variable between baseline and at 6 months postintervention were then evaluated.
Data Sources
All patient data were obtained from the VHA Corporate Data Warehouse (CDW). The CDW contains extracts from VHA clinical and administrative systems that contain complete clinical data from October 1999 to the present. Population-level data were obtained from the Opioid Safety Initiative Master Dashboard National Report where available. Data not contained in this national dashboard were obtained through local data extractions from the CDW.
Informatics Tool
In September 2014, an e-consult tool was created to enable PCPs to efficiently consult the RHJVAMC pain clinic for advice on opioid-related issues in patients who require specialized attention. On activation of this EMR-based tool, the following patient data autopopulated in the consult: recent and active opioid prescription(s), UDS data from the previous 365 days, and PDMP review data from the previous 365 days. The consulting provider was then required to enter data on concomitant mental health disorders that were deemed pertinent to opioid safety as well as obstructive sleep apnea (OSA) status (OSA diagnosis and continuous positive airway pressure machine receipt and adherence).
The consulting provider was required to indicate whether the patient had an active BZD prescription. If yes, a text field allowed the provider to enter the specific agent(s) prescribed and dose(s). Data were required in all fields for the e-consult to be considered ready for pain clinic review. Common pain clinic recommendations included orders for additional laboratory tests to assess adherence and potential toxicity, drug tapers, and consideration for complementary and alternative medicine (CAM). If a drug taper was recommended (either opioid or BZD), specific taper schedules would be provided by a pharmacist specializing in pain management.
Targeted Patient Intervention
In April 2014, the POC and Mental Health service began a targeted review of all outpatients receiving combination opioid and BZD therapy. First, the POC distributed to each mental health provider a list of patients who were receiving combination opioid and BZD therapy. An opioid/BZD combination risk assessment tool (Table 2) was developed by the POC and made available to assist with these patient reviews. This tool prompted a provider to assess a patient’s stability on the current regimen as well as the presence of any absolute or relative contraindications to concomitant BZD and opioid use. Providers documented whether a discussion regarding the risks and benefits of opioids and BZDs had occurred with the patient. The tool encouraged providers to document a continued indication for combined BZD and opioid therapy use and whether the lowest effective BZD dose was being prescribed. A standardized BZD taper protocol also was developed by the POC to assist providers if a BZD taper was indicated. A total of 222 patients were reviewed over 7 months from April 2014 to October 2015.
Following completion of this targeted review in October 2015, the POC required that starting any patients on opioid and BZD combination therapy would require a specialist consult. For existing COT patients, a mental health consult would be required to initiate BZD therapy. For stable patients on BZD therapy, a pain clinic consult was required before initiating an opioid prescription. The Pharmacy service acted as a gatekeeper for these agents and refused to dispense either new agent until the proper consults had been submitted unless clinical necessity of an agent was apparent (ie, opioid prescription following invasive surgery).
The final targeted patient intervention occurred following deployment of the opioid safety review e-consult tool in September 2015. To review the highest risk COT patients, each PCP was given a list of their patients who were taking ≥ 200 mg MEDD. With support from the primary care service chief, PCPs were required to submit an e-consult for every patient who did not meet the e-consult exclusion criteria. In the fourth quarter of fiscal year (FY) 2014, and first quarter of FY 2015, 116 RHJVAMC patients received ≥ 200 mg MEDD with 49 meeting the exclusion criteria. Of the 67 patients eligible for pain clinic review, e-consults were placed for 58 patients over a 7-month period. The remaining 9 patients did not receive an e-consult because taper was initiated by the patient’s PCP without pain clinic assistance (6), aberrant patient behavior was identified during data collection (2), and patient was transitioned to palliative care (1).
Provider Education
A primary goal of the POC was to educate PCPs on opioid safety, to ensure that each provider was able to use evidence-based medicine and identify potential high-risk situations during patient encounters. Provider education was delivered by physician and pharmacist pain specialists and took place from September 2013 to January 2015 at existing primary care meetings. Topics included UDS interpretation, opioid/BZD combination risks, the goals and requirements of the VA OSI, and legal requirements of the South Carolina Reporting and Identification Prescription Tracking System (SCRIPTS) PDMP.
Patient Education
Patient education was delivered through informational brochures either mailed or given out during clinic visits. The first brochure was mailed to patients and described the VA OSI goals and its potential impact on patients. A second handout described the risks associated with opioid/BZD combination therapy and encouraged patients to discuss these risks and alternate options with their providers. It was made available to primary care and mental health teams for distribution to patients.
Results
Interventions spanned 19 months, with an average of 1 intervention per month. The highest number of POC interventions in a single month was observed in October 2014, with 3 individual interventions from 3 separate categories.
Impact of POC Interventions on COT Monitoring
During the study period, patients meeting COT criteria who received an annual UDS increased from 47.8% to 75.5%, a 56.7% increase from baseline (Figure 1). During the same period, patients with an annual PDMP review note in their medical record also increased from 4.1% to 19.6%, a 324% increase from baseline (Figure 2). Although the study period began in FY 2012 third quarter, FY 2014 first quarter was the baseline for PDMP review note data collection because VA providers were not legally allowed to access the SCRIPTS database prior to FY 2014.
Impact of Interventions on High-Risk Opioid Prescribing
Patients who received an opioid prescription and a BZD derivative in the same fiscal quarter decreased 41.7% during the study period (Figure 3). A significant improvement was observed in each clinical variable at 6 months postintervention among high-dose COT patients who received an opioid safety review e-consult (Table 3). The median opioid dose per patient decreased 20% from baseline, from 300 mg MEDD to 240 mg MEDD. The number of patients with an annual UDS increased 31.7% from 41 to 54 patients. The number of patients with an annual PDMP review also increased 345%, from 11 patients to 49 patients. Finally, the number of patients with an active BZD order decreased > 75% from 17 patients at baseline to 4 patients at 6-month follow-up.
In the FY 2014 third quarter, prior to activation of the opioid safety review e-consult tool, 100 patients received high-dose COT. Follow-up at the conclusion of fourth quarter FY 2015 revealed 64 such patients, which represented a 36% decrease from baseline.
Discussion
During the study period, the POC used a variety of interventions from 4 distinct categories. Overall, these interventions successfully increased measures of appropriate COT monitoring (ie, UDS and PDMP utilization) and management of high-risk COT. Substantial improvements also were seen in the subgroup of patients receiving high-dose COT following creation and use of the opioid safety review e-consult tool.
Other VHA opioid management improvement initiatives were successful at reducing high-dose opioid prescribing through interventions similar to those described in this study. However, these initiatives did not address opioid monitoring practices or opioid/BZD combination therapy.25,26 To the authors’ knowledge, no previous opioid management improvement initiatives have reported improvements in provider use of a state PDMP database.
There are a number of factors that also may have helped lead to the successful outcomes observed during the study period. First, the creation of an informatics tool allowed for sustained interventions over time. While targeted interventions and patient/provider education were certainly beneficial, the impact of these efforts wanes as time moves forward. Inevitably, a patient’s and a provider’s focus move to the next important issue, and new patients meet the criteria of the original targeted intervention.
Group Health Cooperative implemented an opioid risk reduction initiative that successfully increased UDS use over a 2-year postimplementation period.21,26 While this initiative used a number of similar interventions to those implemented in this study (patient and provider education, targeted patient intervention), an informatics tool was not used. The annual UDS rate at the conclusion of the Group Health initiative was 50%, which contrasts with a final rate of 75.5% in this study. Although it is difficult to draw comparisons between the studies given differences in populations studied, periods of evaluation, and varying baseline annual UDS rates, the current study results demonstrate the potential effectiveness of informatics tools to help drive enduring changes in practice.
An additional factor that had a positive impact on outcomes the continued support and advocacy from RHJVAMC clinical and administrative leadership. A targeted review of all patients receiving concomitant BZDs and opioids would not be possible without mental health department leaders who believed in the value of the time consuming undertaking. Furthermore, an e-consult tool is effective only if actually submitted for patients and if a specialist’s recommendations are then followed by a PCP.
Finally, the interdisciplinary nature of the POC contributed to the success of each intervention described in this study. Patients receiving COT often have many complex physical, psychological, and social issues that must be considered in order to make a positive impact on patient care. To appropriately and effectively address these issues requires close collaboration between specialists from multiple disciplines.
Limitations
This study has several important limitations. First, its retrospective nature presents obvious documentation challenges. A second limitation is the brief period of evaluation following a number of POC interventions. For instance, 3 interventions took place in January 2015, leaving only 3 FY quarters of effectiveness data. Furthermore, increased awareness of the risks associated with opioid therapy in the VHA and the health care industry across the study period may have independently impacted the improvements observed in this study.
The lack of an assessment of both patient-centered and clinical outcomes is an additional limitation of this study. Rates of annual UDS and PDMP database reviews and the number of patients receiving high-risk COT are only surrogate metrics that may indicate appropriate prescribing and monitoring of these. Obtaining a UDS or PDMP review is meant to provide a practitioner with additional information to interpret when caring for a patient. These data are only meant to complement—not replace—skilled patient assessment by a provider. Although the authors observed no major patient or clinical adverse events during the study period, the possibility exists that a patient may have been negatively impacted by a population-level initiative to improve surrogate measures of appropriate drug use.
Future studies should assess changes in measures, such as pain scores, legitimate adverse events, and overdose occurrences in order to evaluate whether such opioid improvement initiatives truly benefit the patients who are ultimately affected by each intervention.
Conclusion
This study demonstrates the successful implementation of a VHA-based opioid management initiative to increase appropriate COT monitoring and appropriate management of high-risk patients. It is the authors’ hope that the findings may add to the growing body of literature describing successful opioid improvement initiatives and serve as a tool for other health systems that are confronted with these same issues.
1. Manchikanti L, Fellows B, Ailinani H, Pampati V. Therapeutic use, abuse, and nonmedical use of opioids: a ten-year perspective. Pain Physician. 2010;13(5):401-435.
2. Garcia MM, Angelini MC, Thomas T, Lenz K, Jeffrey P. Implementation of an opioid management initiative by a state Medicaid program. J Manag Care Spec Pharm. 2014;20(5):447-454.
3. Edlund MJ, Austen MA, Sullivan MD, et al. Patterns of opioid use for chronic noncancer pain in the Veterans Health Administration from 2009 to 2011. Pain. 2014;155(11):2337-2343.
4. Office of National Drug Control Policy. Prescription drug abuse. https://obamawhitehouse.archives.gov /ondcp/prescription-drug-abuse1. Accessed April 18, 2017.
5. Chou R, Ballantyne JC, Fanciullo GJ, Fine PG, Miaskowski C. Research gaps on use of opioids for chronic noncancer pain: findings from a review of the evidence for an American Pain Society and American Academy of Pain Medicine clinical practice guideline. J Pain. 2009;10(2):147-159.
6. Chou R, Fanciullo GJ, Fine PG, et al; American Pain Society-American Academy of Pain Medicine Opioids Guidelines Panel. Clinical guidelines for the use of chronic opioid therapy in chronic noncancer pain. J Pain. 2009;10(2):113-130.
7. The American Pain Society, The American Academy of Pain Medicine. Guideline for the use of chronic opioid therapy in chronic non-cancer pain: evidence review. http://americanpainsociety.org/uploads/education/guidelines/chronic-opioid-therapy -cncp.pdf. Accessed April 18, 2017.
8. Von Korff M, Deyo RA. Potent opioids for chronic musculoskeletal pain: flying blind? Pain. 2004;109(3):207-209.
9. Angst MS, Clark JD. Opioid-induced hyperalgesia: a qualitative systematic review. Anesthesiology. 2006;104(3):570-587.
10. Abs R, Verhelst J, Maeyaert J, et al. Endocrine consequences of long-term intrathecal administration of opioids. J Clin Endocrinol Metab. 2000;85(6):2215-2222.
11. Younger JW, Chu LF, D’Arcy NT, Trott KE, Jastrzab LE, Mackey SC. Prescription opioid analgesics rapidly change the human brain. Pain. 2011;152(8):1803-1810.
12. U.S Department of Health and Human Services, Substance Abuse and Mental Health Service Administration Office of Applied Studies. Results from the 2004 national survey on drug use and health: national findings. http://medicalmarijuana.procon .org/sourcefiles/2k4results.pdf. Updated September 8, 2005. Accessed April 18, 2017.
13. Dunn KM, Saunders KW, Rutter CM, et al. Opioid prescriptions for chronic pain and overdose: a cohort study. Ann Intern Med. 2010;152(2):85-92.
14. Centers for Disease Control and Prevention (CDC). Emergency department visits involving nonmedical use of selected prescription drugs—United States, 2004-2008. MMWR Morb Mortal Wkly Rep. 2010;59(23):705-709.
15. Dowell D, Haegerich TM, Chou R. CDC guideline for prescribing opioids for chronic pain—United States, 2016. JAMA. 2016;315(15):1624-1645.
16. U.S. Federal Drug Administration. FDA Drug Safety Communication: FDA warns about serious risks and death when combining opioid pain or cough medicines with benzodiazepines; requires its strongest warning. http://www.fda.gov/Drugs/DrugSafety /ucm518473.htm. Published August 31, 2016. Accessed April 18, 2017.
17. U.S. Department of Veterans Affairs. Pain management, VHA directive 2009-053. https://www.va.gov/painmanagement/docs/vha09paindirective.pdf. Published October 28, 2009. Accessed April 18, 2017.
18. U.S. Department of Veteran Affairs, U.S. Department of Defense. VA/DoD clinical practice guideline for management of opioid therapy for chronic pain. https://www.va.gov/painmanagement/docs/cpg_opioidtherapy_summary.pdf. Published May 2010. Accessed May 8, 2017.
19. Jones CM, Mack KA, Paulozzi LJ. Pharmaceutical overdose deaths, United States, 2010. JAMA. 2013;309(7):657-659.
20. Gwira Baumblatt JA, Wiedeman C, Dunn JR, Schaffner W, Paulozzi LJ, Jones TF. High-risk use by patients prescribed opioids for pain and its role in overdose deaths. JAMA Intern Med. 2014;174(5):796-801.
21. Zedler B, Xie L, Wang L, et al. Risk factors for serious prescription opioid-related toxicity or overdose among Veterans Health Administration patients. Pain Med. 2014;15(11):1911-1929.
22. Centers for Disease Control and Prevention. CDC grand rounds: prescription drug overdoses—a U.S. epidemic. MMWR Morb Mortal Wkly Rep. 2012;61(1):10-13.
23. Jones CM, McAninch JK. Emergency department visits and overdose deaths from combined use of opioids and benzodiazepines. Am J Prev Med. 2015;49(4):493-501.
24. Morden NE, Zerzan JT, Rue TC, et al. Medicaid prior authorization and controlled-release oxycodone. Med Care. 2008;46(6):573-580.
25. Westanmo A, Marshall P, Jones E, Burns K, Krebs EE. Opioid dose reduction in a VA health care system—implementation of a primary care population-level initiative. Pain Med. 2015;16(5):1019-1026.
26. Kryskalla J, Kern S, Gray D, Hauser P. Using dashboard technology to monitor overdose risk. Fed Pract. 2014;31(9):8-14.
27. Centers for Disease Control and Prevention. CDC grand rounds: prescription drug overdoses—a U.S. epidemic. MMWR Morb Mortal Wkly Rep. 2012;61(1):10-13.
28. U.S. Department of Veterans Affairs. Patient aligned care team (PACT). https://www.patientcare.va.gov /primarycare/PACT.asp. Updated September 22, 2016. Accessed April 18, 2017.
29. Liu Y, Logan JE, Paulozzi LJ, Zhang K, Jones CM. Potential misuse and inappropriate prescription practices involving opioid analgesics. Am J Manag Care. 2013;19(8):648-665.
30. Reisfield GM, Webster LR. Benzodiazepines in long-term opioid therapy. Pain Med. 2013;14(10):1441-1446.
The use of opioids to treat chronic noncancer pain (CNCP) has become increasingly common over the previous 2 decades. The Office of National Drug Control Policy (ONDCP) reported that from 1997 to 2007, there was a 4-fold increase in the mg per person per year sale of prescription opioids, from 74 mg to 369 mg.1 The number of opioid prescriptions dispensed by pharmacies also has increased by 48% from 2000 to 2009.2 Within the VA population, about half of the 1.44 million patients with a diagnosis of pain (excluding cancer pain) received opioids during 2011, and 57% of these patients received chronic opioid therapy (COT), which is at least 90 days of opioid use in a year.3
Despite this increased use of opioids, data regarding the efficacy of long-term opioid use for noncancer pain remain limited.1,4-8 Instead, there is a growing body of evidence describing potential adverse effects (AEs) of long-term opioid use at even relatively modest doses, including sexual dysfunction, hyperalgesia, and altered brain structure.9-11 Additionally, increases in the misuse and abuse of opioids as well as mortality associated with opioid toxicity have been observed.12-14 Opioid pain relievers were involved in nearly 17,000 deaths in the U.S. in 2010, which represents a 3-fold increase since 1999. This number also represents 75% of all deaths that were attributed to prescription drug poisoning in 2010.13 Unfortunately, this alarming trend parallels the aforementioned increases in the utilization of prescription opioids for CNCP.
Given this accumulating data regarding the profound risks and limited benefit of COT, many organizations have advocated a reassessment of the upward trajectory of opioid utilization. In 2009, the American Pain Society (APS) in partnership with the American Academy of Pain Medicine (AAPM) released clinical guidelines for the use of COT in CNCP.6 In this guideline, the authors advocate a balanced approach to opioid use: Clinicians consider both the legitimate medical need for opioids in some patients with CNCP as well as the serious public health problem of abuse, addiction, and diversion.6 In 2011, the FDA, Drug Enforcement Agency (DEA), and ONDCP enacted the Prescription Drug Abuse Prevention Plan, which focused on 4 major areas: education, prescription monitoring, proper medication disposal, and law enforcement.4
In March 2016, the CDC released a new guideline for prescribing opioids for chronic pain that included 12 recommendations based on 3 key principles. First, nonopioids are preferred for chronic pain in all settings except for active cancer, palliative, and end-of-life care. Next, when opioids are used for chronic pain, they always should be prescribed at the lowest possible effective dose to reduce the risk of opioid use disorder and overdose. Finally, clinicians should exercise caution when prescribing opioids and monitor all patients closely for opioid-related risk.15
Recently, an August 2016 FDA review found that the combined use of opioids and benzodiazepines (BZDs) resulted in serious AEs, including respiratory depression and death. Based on these findings, the FDA requires that updated boxed warnings be added to the labeling of prescription opioid and BZDs.16
The VHA also has been at the forefront of this national movement to promote the appropriate use of opioids. In 2009, the VHA released a pain management directive that highlighted the risks of COT and required adoption of a stepped-care approach to opioid prescribing that focused on quality of life as the primary determinant of treatment quality.17 In 2010, the VHA released its guideline on opioid therapy for chronic pain, which also included tools for providers, such as a sample opioid therapy agreement, equivalent potency tables, and a urine drug screening guide.18 In 2014, the VHA released the Opioid Safety Initiative (OSI), which advocates for a team-based approach to reduce the use of opioids for veterans through a focus on alternate methods to alleviate pain.
At the Ralph H. Johnson VAMC (RHJVAMC) in Charleston, South Carolina, a multidisciplinary pain oversight committee (POC) was tasked with assisting in achieving the goals set forth in the VHA OSI. To reach these goals, the POC sought to develop and implement a population-based initiative targeting modifiable factors that are known to increase the risk of opioid-related toxicity and overdose. These factors included patient utilization of multiple prescribers or multiple pharmacies, high-dose COT (defined in the APS/AAPM guidelines as a morphine equivalent daily dose [MEDD] > 200 mg6), and use of concomitant central nervous system-active medications, chiefly BZDs.19-23 The POC consisted of the RHJVAMC chiefs of mental health, primary care, and pharmacy; a physician specializing in pain and addiction medicine; a pharmacist specializing in pain and palliative care; quality management personnel; a patient advocate; and multiple physicians from the mental health and primary care departments.
Previous studies have described the successful implementation of opioid management initiatives in a variety of health care settings.2,21,24-27 However, most of this work focused only on strategies to decrease prescribing of high-dose and long-acting opioid formulations. The study presented here sought to add to the existing body of knowledge through evaluation of an initiative aimed at increasing appropriate monitoring as a tool to decrease opioid-related patient risk. The primary aim of this study was to describe the types of interventions implemented by the POC during the study period. The secondary aim was to evaluate the effect of these interventions on the appropriate monitoring of COT as well as the appropriate management of high-risk COT > 200 mg MEDD.
Methods
This study involved a qualitative description of individual POC interventions as well as a retrospective data analysis that examined the clinical impact of these interventions during the study period from April 1, 2012 to September 30, 2015. This study was reviewed and approved by the Medical University of South Carolina Institutional Review Board and the RHJVAMC Research and Development Committee.
Setting
The RHJVAMC is a tertiary care teaching hospital with primary and specialized outpatient services that are provided at the main medical center in Charleston, South Carolina, and at 6 community-based outpatient clinics (CBOCs) located throughout southeastern South Carolina and parts of Georgia. Primary care is delivered by patient aligned care teams (PACTs) based on the patient-centered medical home model.28 The PACT consists of a primary care provider (PCP) who is aided by dedicated nursing, pharmacy, and mental health care providers. In most cases, COT is prescribed and managed in the PACT setting. At the time of this initiative, a broad range of specialty services were available, including a multidisciplinary pain management team, orthopedics, and physical medicine and rehabilitation. In 2012, about 55,000 patients were enrolled and received care at RHJVAMC. The POC interventions were carried out at all clinic sites.
Patients
The study population included all patients prescribed COT at RHJVAMC during the study period. A patient was considered to be prescribed COT if at least 1 opioid-containing medication was dispensed to the patient in a selected fiscal quarter during the study period and the total cumulative supply of opioid-containing medications was ≥ 90 days for both the selected quarter and the prior quarter.
Furthermore, a high-risk COT subpopulation included any patient who satisfied either of the following criteria: (1) Receipt of outpatient prescription(s) for opioid-containing medication(s) (including tramadol) and a benzodiazepine derivative in the same fiscal quarter; patients were included in this subpopulation regardless of whether they met COT criteria; (2) Receipt of outpatient prescription(s) for opioid-containing medication(s) with at least 1 instance in which the MEDD was ≥ 200 mg in the designated quarter (Table 1). The MEDD was calculated for each fill in the fiscal quarter using the following equation:
If medication fills were within 3 days of each other, the prescriptions were considered to be taken together and the MEDD was summed.
Intervention Descriptions
The primary aim of this study was to qualitatively describe each intervention implemented by the POC. The POC monthly meeting minutes were recorded and reviewed for the study period, and descriptive information regarding each intervention was extracted. Extracted information included implementation date(s), the responsible POC member, and a general description of each intervention. Interventions were then categorized as informatics tool, targeted patient intervention, provider education, or patient education.
Impact of Interventions on Monitoring
In order to characterize the impact of POC interventions on appropriate monitoring of COT, the electronic medical record (EMR) of each patient satisfying COT criteria was queried for the presence of an annual urine drug screen (UDS) result and a note in the chart signaling that a prescription drug monitoring program (PDMP) review had been performed. The authors defined appropriate UDS monitoring and PDMP review as the presence of a UDS result and a PDMP review note in the EMR in the year prior to the query date.
Prior to the start of the POC interventions, 4.1% of RJVAMC patients had an annual PDMP review and 47.8% had an annual UDS. Although more frequent UDS results and PDMP reviews are appropriate in most cases, yearly monitoring was considered by the POC to be a reasonable initial goal. The percentage of veterans receiving COT who had received appropriate monitoring for each measure was collected for each fiscal quarter during the study period. In addition, the difference between the initial and final fiscal quarter during the study period was calculated for each measure.
Impact of Interventions on High-Risk Opioid Prescribing
To assess the impact of POC interventions on appropriate management of high-dose COT, clinical variables were collected for patients who were prescribed high-dose COT and received targeted intervention in the form of a pain clinic e-consult. These variables were MEDD, presence of annual UDS, presence of annual PDMP review, and active BZD prescription. Each variable was assessed on the date of intervention (e-consult submission) and at 6 months postintervention. Changes in each clinical variable between baseline and at 6 months postintervention were then evaluated.
Data Sources
All patient data were obtained from the VHA Corporate Data Warehouse (CDW). The CDW contains extracts from VHA clinical and administrative systems that contain complete clinical data from October 1999 to the present. Population-level data were obtained from the Opioid Safety Initiative Master Dashboard National Report where available. Data not contained in this national dashboard were obtained through local data extractions from the CDW.
Informatics Tool
In September 2014, an e-consult tool was created to enable PCPs to efficiently consult the RHJVAMC pain clinic for advice on opioid-related issues in patients who require specialized attention. On activation of this EMR-based tool, the following patient data autopopulated in the consult: recent and active opioid prescription(s), UDS data from the previous 365 days, and PDMP review data from the previous 365 days. The consulting provider was then required to enter data on concomitant mental health disorders that were deemed pertinent to opioid safety as well as obstructive sleep apnea (OSA) status (OSA diagnosis and continuous positive airway pressure machine receipt and adherence).
The consulting provider was required to indicate whether the patient had an active BZD prescription. If yes, a text field allowed the provider to enter the specific agent(s) prescribed and dose(s). Data were required in all fields for the e-consult to be considered ready for pain clinic review. Common pain clinic recommendations included orders for additional laboratory tests to assess adherence and potential toxicity, drug tapers, and consideration for complementary and alternative medicine (CAM). If a drug taper was recommended (either opioid or BZD), specific taper schedules would be provided by a pharmacist specializing in pain management.
Targeted Patient Intervention
In April 2014, the POC and Mental Health service began a targeted review of all outpatients receiving combination opioid and BZD therapy. First, the POC distributed to each mental health provider a list of patients who were receiving combination opioid and BZD therapy. An opioid/BZD combination risk assessment tool (Table 2) was developed by the POC and made available to assist with these patient reviews. This tool prompted a provider to assess a patient’s stability on the current regimen as well as the presence of any absolute or relative contraindications to concomitant BZD and opioid use. Providers documented whether a discussion regarding the risks and benefits of opioids and BZDs had occurred with the patient. The tool encouraged providers to document a continued indication for combined BZD and opioid therapy use and whether the lowest effective BZD dose was being prescribed. A standardized BZD taper protocol also was developed by the POC to assist providers if a BZD taper was indicated. A total of 222 patients were reviewed over 7 months from April 2014 to October 2015.
Following completion of this targeted review in October 2015, the POC required that starting any patients on opioid and BZD combination therapy would require a specialist consult. For existing COT patients, a mental health consult would be required to initiate BZD therapy. For stable patients on BZD therapy, a pain clinic consult was required before initiating an opioid prescription. The Pharmacy service acted as a gatekeeper for these agents and refused to dispense either new agent until the proper consults had been submitted unless clinical necessity of an agent was apparent (ie, opioid prescription following invasive surgery).
The final targeted patient intervention occurred following deployment of the opioid safety review e-consult tool in September 2015. To review the highest risk COT patients, each PCP was given a list of their patients who were taking ≥ 200 mg MEDD. With support from the primary care service chief, PCPs were required to submit an e-consult for every patient who did not meet the e-consult exclusion criteria. In the fourth quarter of fiscal year (FY) 2014, and first quarter of FY 2015, 116 RHJVAMC patients received ≥ 200 mg MEDD with 49 meeting the exclusion criteria. Of the 67 patients eligible for pain clinic review, e-consults were placed for 58 patients over a 7-month period. The remaining 9 patients did not receive an e-consult because taper was initiated by the patient’s PCP without pain clinic assistance (6), aberrant patient behavior was identified during data collection (2), and patient was transitioned to palliative care (1).
Provider Education
A primary goal of the POC was to educate PCPs on opioid safety, to ensure that each provider was able to use evidence-based medicine and identify potential high-risk situations during patient encounters. Provider education was delivered by physician and pharmacist pain specialists and took place from September 2013 to January 2015 at existing primary care meetings. Topics included UDS interpretation, opioid/BZD combination risks, the goals and requirements of the VA OSI, and legal requirements of the South Carolina Reporting and Identification Prescription Tracking System (SCRIPTS) PDMP.
Patient Education
Patient education was delivered through informational brochures either mailed or given out during clinic visits. The first brochure was mailed to patients and described the VA OSI goals and its potential impact on patients. A second handout described the risks associated with opioid/BZD combination therapy and encouraged patients to discuss these risks and alternate options with their providers. It was made available to primary care and mental health teams for distribution to patients.
Results
Interventions spanned 19 months, with an average of 1 intervention per month. The highest number of POC interventions in a single month was observed in October 2014, with 3 individual interventions from 3 separate categories.
Impact of POC Interventions on COT Monitoring
During the study period, patients meeting COT criteria who received an annual UDS increased from 47.8% to 75.5%, a 56.7% increase from baseline (Figure 1). During the same period, patients with an annual PDMP review note in their medical record also increased from 4.1% to 19.6%, a 324% increase from baseline (Figure 2). Although the study period began in FY 2012 third quarter, FY 2014 first quarter was the baseline for PDMP review note data collection because VA providers were not legally allowed to access the SCRIPTS database prior to FY 2014.
Impact of Interventions on High-Risk Opioid Prescribing
Patients who received an opioid prescription and a BZD derivative in the same fiscal quarter decreased 41.7% during the study period (Figure 3). A significant improvement was observed in each clinical variable at 6 months postintervention among high-dose COT patients who received an opioid safety review e-consult (Table 3). The median opioid dose per patient decreased 20% from baseline, from 300 mg MEDD to 240 mg MEDD. The number of patients with an annual UDS increased 31.7% from 41 to 54 patients. The number of patients with an annual PDMP review also increased 345%, from 11 patients to 49 patients. Finally, the number of patients with an active BZD order decreased > 75% from 17 patients at baseline to 4 patients at 6-month follow-up.
In the FY 2014 third quarter, prior to activation of the opioid safety review e-consult tool, 100 patients received high-dose COT. Follow-up at the conclusion of fourth quarter FY 2015 revealed 64 such patients, which represented a 36% decrease from baseline.
Discussion
During the study period, the POC used a variety of interventions from 4 distinct categories. Overall, these interventions successfully increased measures of appropriate COT monitoring (ie, UDS and PDMP utilization) and management of high-risk COT. Substantial improvements also were seen in the subgroup of patients receiving high-dose COT following creation and use of the opioid safety review e-consult tool.
Other VHA opioid management improvement initiatives were successful at reducing high-dose opioid prescribing through interventions similar to those described in this study. However, these initiatives did not address opioid monitoring practices or opioid/BZD combination therapy.25,26 To the authors’ knowledge, no previous opioid management improvement initiatives have reported improvements in provider use of a state PDMP database.
There are a number of factors that also may have helped lead to the successful outcomes observed during the study period. First, the creation of an informatics tool allowed for sustained interventions over time. While targeted interventions and patient/provider education were certainly beneficial, the impact of these efforts wanes as time moves forward. Inevitably, a patient’s and a provider’s focus move to the next important issue, and new patients meet the criteria of the original targeted intervention.
Group Health Cooperative implemented an opioid risk reduction initiative that successfully increased UDS use over a 2-year postimplementation period.21,26 While this initiative used a number of similar interventions to those implemented in this study (patient and provider education, targeted patient intervention), an informatics tool was not used. The annual UDS rate at the conclusion of the Group Health initiative was 50%, which contrasts with a final rate of 75.5% in this study. Although it is difficult to draw comparisons between the studies given differences in populations studied, periods of evaluation, and varying baseline annual UDS rates, the current study results demonstrate the potential effectiveness of informatics tools to help drive enduring changes in practice.
An additional factor that had a positive impact on outcomes the continued support and advocacy from RHJVAMC clinical and administrative leadership. A targeted review of all patients receiving concomitant BZDs and opioids would not be possible without mental health department leaders who believed in the value of the time consuming undertaking. Furthermore, an e-consult tool is effective only if actually submitted for patients and if a specialist’s recommendations are then followed by a PCP.
Finally, the interdisciplinary nature of the POC contributed to the success of each intervention described in this study. Patients receiving COT often have many complex physical, psychological, and social issues that must be considered in order to make a positive impact on patient care. To appropriately and effectively address these issues requires close collaboration between specialists from multiple disciplines.
Limitations
This study has several important limitations. First, its retrospective nature presents obvious documentation challenges. A second limitation is the brief period of evaluation following a number of POC interventions. For instance, 3 interventions took place in January 2015, leaving only 3 FY quarters of effectiveness data. Furthermore, increased awareness of the risks associated with opioid therapy in the VHA and the health care industry across the study period may have independently impacted the improvements observed in this study.
The lack of an assessment of both patient-centered and clinical outcomes is an additional limitation of this study. Rates of annual UDS and PDMP database reviews and the number of patients receiving high-risk COT are only surrogate metrics that may indicate appropriate prescribing and monitoring of these. Obtaining a UDS or PDMP review is meant to provide a practitioner with additional information to interpret when caring for a patient. These data are only meant to complement—not replace—skilled patient assessment by a provider. Although the authors observed no major patient or clinical adverse events during the study period, the possibility exists that a patient may have been negatively impacted by a population-level initiative to improve surrogate measures of appropriate drug use.
Future studies should assess changes in measures, such as pain scores, legitimate adverse events, and overdose occurrences in order to evaluate whether such opioid improvement initiatives truly benefit the patients who are ultimately affected by each intervention.
Conclusion
This study demonstrates the successful implementation of a VHA-based opioid management initiative to increase appropriate COT monitoring and appropriate management of high-risk patients. It is the authors’ hope that the findings may add to the growing body of literature describing successful opioid improvement initiatives and serve as a tool for other health systems that are confronted with these same issues.
The use of opioids to treat chronic noncancer pain (CNCP) has become increasingly common over the previous 2 decades. The Office of National Drug Control Policy (ONDCP) reported that from 1997 to 2007, there was a 4-fold increase in the mg per person per year sale of prescription opioids, from 74 mg to 369 mg.1 The number of opioid prescriptions dispensed by pharmacies also has increased by 48% from 2000 to 2009.2 Within the VA population, about half of the 1.44 million patients with a diagnosis of pain (excluding cancer pain) received opioids during 2011, and 57% of these patients received chronic opioid therapy (COT), which is at least 90 days of opioid use in a year.3
Despite this increased use of opioids, data regarding the efficacy of long-term opioid use for noncancer pain remain limited.1,4-8 Instead, there is a growing body of evidence describing potential adverse effects (AEs) of long-term opioid use at even relatively modest doses, including sexual dysfunction, hyperalgesia, and altered brain structure.9-11 Additionally, increases in the misuse and abuse of opioids as well as mortality associated with opioid toxicity have been observed.12-14 Opioid pain relievers were involved in nearly 17,000 deaths in the U.S. in 2010, which represents a 3-fold increase since 1999. This number also represents 75% of all deaths that were attributed to prescription drug poisoning in 2010.13 Unfortunately, this alarming trend parallels the aforementioned increases in the utilization of prescription opioids for CNCP.
Given this accumulating data regarding the profound risks and limited benefit of COT, many organizations have advocated a reassessment of the upward trajectory of opioid utilization. In 2009, the American Pain Society (APS) in partnership with the American Academy of Pain Medicine (AAPM) released clinical guidelines for the use of COT in CNCP.6 In this guideline, the authors advocate a balanced approach to opioid use: Clinicians consider both the legitimate medical need for opioids in some patients with CNCP as well as the serious public health problem of abuse, addiction, and diversion.6 In 2011, the FDA, Drug Enforcement Agency (DEA), and ONDCP enacted the Prescription Drug Abuse Prevention Plan, which focused on 4 major areas: education, prescription monitoring, proper medication disposal, and law enforcement.4
In March 2016, the CDC released a new guideline for prescribing opioids for chronic pain that included 12 recommendations based on 3 key principles. First, nonopioids are preferred for chronic pain in all settings except for active cancer, palliative, and end-of-life care. Next, when opioids are used for chronic pain, they always should be prescribed at the lowest possible effective dose to reduce the risk of opioid use disorder and overdose. Finally, clinicians should exercise caution when prescribing opioids and monitor all patients closely for opioid-related risk.15
Recently, an August 2016 FDA review found that the combined use of opioids and benzodiazepines (BZDs) resulted in serious AEs, including respiratory depression and death. Based on these findings, the FDA requires that updated boxed warnings be added to the labeling of prescription opioid and BZDs.16
The VHA also has been at the forefront of this national movement to promote the appropriate use of opioids. In 2009, the VHA released a pain management directive that highlighted the risks of COT and required adoption of a stepped-care approach to opioid prescribing that focused on quality of life as the primary determinant of treatment quality.17 In 2010, the VHA released its guideline on opioid therapy for chronic pain, which also included tools for providers, such as a sample opioid therapy agreement, equivalent potency tables, and a urine drug screening guide.18 In 2014, the VHA released the Opioid Safety Initiative (OSI), which advocates for a team-based approach to reduce the use of opioids for veterans through a focus on alternate methods to alleviate pain.
At the Ralph H. Johnson VAMC (RHJVAMC) in Charleston, South Carolina, a multidisciplinary pain oversight committee (POC) was tasked with assisting in achieving the goals set forth in the VHA OSI. To reach these goals, the POC sought to develop and implement a population-based initiative targeting modifiable factors that are known to increase the risk of opioid-related toxicity and overdose. These factors included patient utilization of multiple prescribers or multiple pharmacies, high-dose COT (defined in the APS/AAPM guidelines as a morphine equivalent daily dose [MEDD] > 200 mg6), and use of concomitant central nervous system-active medications, chiefly BZDs.19-23 The POC consisted of the RHJVAMC chiefs of mental health, primary care, and pharmacy; a physician specializing in pain and addiction medicine; a pharmacist specializing in pain and palliative care; quality management personnel; a patient advocate; and multiple physicians from the mental health and primary care departments.
Previous studies have described the successful implementation of opioid management initiatives in a variety of health care settings.2,21,24-27 However, most of this work focused only on strategies to decrease prescribing of high-dose and long-acting opioid formulations. The study presented here sought to add to the existing body of knowledge through evaluation of an initiative aimed at increasing appropriate monitoring as a tool to decrease opioid-related patient risk. The primary aim of this study was to describe the types of interventions implemented by the POC during the study period. The secondary aim was to evaluate the effect of these interventions on the appropriate monitoring of COT as well as the appropriate management of high-risk COT > 200 mg MEDD.
Methods
This study involved a qualitative description of individual POC interventions as well as a retrospective data analysis that examined the clinical impact of these interventions during the study period from April 1, 2012 to September 30, 2015. This study was reviewed and approved by the Medical University of South Carolina Institutional Review Board and the RHJVAMC Research and Development Committee.
Setting
The RHJVAMC is a tertiary care teaching hospital with primary and specialized outpatient services that are provided at the main medical center in Charleston, South Carolina, and at 6 community-based outpatient clinics (CBOCs) located throughout southeastern South Carolina and parts of Georgia. Primary care is delivered by patient aligned care teams (PACTs) based on the patient-centered medical home model.28 The PACT consists of a primary care provider (PCP) who is aided by dedicated nursing, pharmacy, and mental health care providers. In most cases, COT is prescribed and managed in the PACT setting. At the time of this initiative, a broad range of specialty services were available, including a multidisciplinary pain management team, orthopedics, and physical medicine and rehabilitation. In 2012, about 55,000 patients were enrolled and received care at RHJVAMC. The POC interventions were carried out at all clinic sites.
Patients
The study population included all patients prescribed COT at RHJVAMC during the study period. A patient was considered to be prescribed COT if at least 1 opioid-containing medication was dispensed to the patient in a selected fiscal quarter during the study period and the total cumulative supply of opioid-containing medications was ≥ 90 days for both the selected quarter and the prior quarter.
Furthermore, a high-risk COT subpopulation included any patient who satisfied either of the following criteria: (1) Receipt of outpatient prescription(s) for opioid-containing medication(s) (including tramadol) and a benzodiazepine derivative in the same fiscal quarter; patients were included in this subpopulation regardless of whether they met COT criteria; (2) Receipt of outpatient prescription(s) for opioid-containing medication(s) with at least 1 instance in which the MEDD was ≥ 200 mg in the designated quarter (Table 1). The MEDD was calculated for each fill in the fiscal quarter using the following equation:
If medication fills were within 3 days of each other, the prescriptions were considered to be taken together and the MEDD was summed.
Intervention Descriptions
The primary aim of this study was to qualitatively describe each intervention implemented by the POC. The POC monthly meeting minutes were recorded and reviewed for the study period, and descriptive information regarding each intervention was extracted. Extracted information included implementation date(s), the responsible POC member, and a general description of each intervention. Interventions were then categorized as informatics tool, targeted patient intervention, provider education, or patient education.
Impact of Interventions on Monitoring
In order to characterize the impact of POC interventions on appropriate monitoring of COT, the electronic medical record (EMR) of each patient satisfying COT criteria was queried for the presence of an annual urine drug screen (UDS) result and a note in the chart signaling that a prescription drug monitoring program (PDMP) review had been performed. The authors defined appropriate UDS monitoring and PDMP review as the presence of a UDS result and a PDMP review note in the EMR in the year prior to the query date.
Prior to the start of the POC interventions, 4.1% of RJVAMC patients had an annual PDMP review and 47.8% had an annual UDS. Although more frequent UDS results and PDMP reviews are appropriate in most cases, yearly monitoring was considered by the POC to be a reasonable initial goal. The percentage of veterans receiving COT who had received appropriate monitoring for each measure was collected for each fiscal quarter during the study period. In addition, the difference between the initial and final fiscal quarter during the study period was calculated for each measure.
Impact of Interventions on High-Risk Opioid Prescribing
To assess the impact of POC interventions on appropriate management of high-dose COT, clinical variables were collected for patients who were prescribed high-dose COT and received targeted intervention in the form of a pain clinic e-consult. These variables were MEDD, presence of annual UDS, presence of annual PDMP review, and active BZD prescription. Each variable was assessed on the date of intervention (e-consult submission) and at 6 months postintervention. Changes in each clinical variable between baseline and at 6 months postintervention were then evaluated.
Data Sources
All patient data were obtained from the VHA Corporate Data Warehouse (CDW). The CDW contains extracts from VHA clinical and administrative systems that contain complete clinical data from October 1999 to the present. Population-level data were obtained from the Opioid Safety Initiative Master Dashboard National Report where available. Data not contained in this national dashboard were obtained through local data extractions from the CDW.
Informatics Tool
In September 2014, an e-consult tool was created to enable PCPs to efficiently consult the RHJVAMC pain clinic for advice on opioid-related issues in patients who require specialized attention. On activation of this EMR-based tool, the following patient data autopopulated in the consult: recent and active opioid prescription(s), UDS data from the previous 365 days, and PDMP review data from the previous 365 days. The consulting provider was then required to enter data on concomitant mental health disorders that were deemed pertinent to opioid safety as well as obstructive sleep apnea (OSA) status (OSA diagnosis and continuous positive airway pressure machine receipt and adherence).
The consulting provider was required to indicate whether the patient had an active BZD prescription. If yes, a text field allowed the provider to enter the specific agent(s) prescribed and dose(s). Data were required in all fields for the e-consult to be considered ready for pain clinic review. Common pain clinic recommendations included orders for additional laboratory tests to assess adherence and potential toxicity, drug tapers, and consideration for complementary and alternative medicine (CAM). If a drug taper was recommended (either opioid or BZD), specific taper schedules would be provided by a pharmacist specializing in pain management.
Targeted Patient Intervention
In April 2014, the POC and Mental Health service began a targeted review of all outpatients receiving combination opioid and BZD therapy. First, the POC distributed to each mental health provider a list of patients who were receiving combination opioid and BZD therapy. An opioid/BZD combination risk assessment tool (Table 2) was developed by the POC and made available to assist with these patient reviews. This tool prompted a provider to assess a patient’s stability on the current regimen as well as the presence of any absolute or relative contraindications to concomitant BZD and opioid use. Providers documented whether a discussion regarding the risks and benefits of opioids and BZDs had occurred with the patient. The tool encouraged providers to document a continued indication for combined BZD and opioid therapy use and whether the lowest effective BZD dose was being prescribed. A standardized BZD taper protocol also was developed by the POC to assist providers if a BZD taper was indicated. A total of 222 patients were reviewed over 7 months from April 2014 to October 2015.
Following completion of this targeted review in October 2015, the POC required that starting any patients on opioid and BZD combination therapy would require a specialist consult. For existing COT patients, a mental health consult would be required to initiate BZD therapy. For stable patients on BZD therapy, a pain clinic consult was required before initiating an opioid prescription. The Pharmacy service acted as a gatekeeper for these agents and refused to dispense either new agent until the proper consults had been submitted unless clinical necessity of an agent was apparent (ie, opioid prescription following invasive surgery).
The final targeted patient intervention occurred following deployment of the opioid safety review e-consult tool in September 2015. To review the highest risk COT patients, each PCP was given a list of their patients who were taking ≥ 200 mg MEDD. With support from the primary care service chief, PCPs were required to submit an e-consult for every patient who did not meet the e-consult exclusion criteria. In the fourth quarter of fiscal year (FY) 2014, and first quarter of FY 2015, 116 RHJVAMC patients received ≥ 200 mg MEDD with 49 meeting the exclusion criteria. Of the 67 patients eligible for pain clinic review, e-consults were placed for 58 patients over a 7-month period. The remaining 9 patients did not receive an e-consult because taper was initiated by the patient’s PCP without pain clinic assistance (6), aberrant patient behavior was identified during data collection (2), and patient was transitioned to palliative care (1).
Provider Education
A primary goal of the POC was to educate PCPs on opioid safety, to ensure that each provider was able to use evidence-based medicine and identify potential high-risk situations during patient encounters. Provider education was delivered by physician and pharmacist pain specialists and took place from September 2013 to January 2015 at existing primary care meetings. Topics included UDS interpretation, opioid/BZD combination risks, the goals and requirements of the VA OSI, and legal requirements of the South Carolina Reporting and Identification Prescription Tracking System (SCRIPTS) PDMP.
Patient Education
Patient education was delivered through informational brochures either mailed or given out during clinic visits. The first brochure was mailed to patients and described the VA OSI goals and its potential impact on patients. A second handout described the risks associated with opioid/BZD combination therapy and encouraged patients to discuss these risks and alternate options with their providers. It was made available to primary care and mental health teams for distribution to patients.
Results
Interventions spanned 19 months, with an average of 1 intervention per month. The highest number of POC interventions in a single month was observed in October 2014, with 3 individual interventions from 3 separate categories.
Impact of POC Interventions on COT Monitoring
During the study period, patients meeting COT criteria who received an annual UDS increased from 47.8% to 75.5%, a 56.7% increase from baseline (Figure 1). During the same period, patients with an annual PDMP review note in their medical record also increased from 4.1% to 19.6%, a 324% increase from baseline (Figure 2). Although the study period began in FY 2012 third quarter, FY 2014 first quarter was the baseline for PDMP review note data collection because VA providers were not legally allowed to access the SCRIPTS database prior to FY 2014.
Impact of Interventions on High-Risk Opioid Prescribing
Patients who received an opioid prescription and a BZD derivative in the same fiscal quarter decreased 41.7% during the study period (Figure 3). A significant improvement was observed in each clinical variable at 6 months postintervention among high-dose COT patients who received an opioid safety review e-consult (Table 3). The median opioid dose per patient decreased 20% from baseline, from 300 mg MEDD to 240 mg MEDD. The number of patients with an annual UDS increased 31.7% from 41 to 54 patients. The number of patients with an annual PDMP review also increased 345%, from 11 patients to 49 patients. Finally, the number of patients with an active BZD order decreased > 75% from 17 patients at baseline to 4 patients at 6-month follow-up.
In the FY 2014 third quarter, prior to activation of the opioid safety review e-consult tool, 100 patients received high-dose COT. Follow-up at the conclusion of fourth quarter FY 2015 revealed 64 such patients, which represented a 36% decrease from baseline.
Discussion
During the study period, the POC used a variety of interventions from 4 distinct categories. Overall, these interventions successfully increased measures of appropriate COT monitoring (ie, UDS and PDMP utilization) and management of high-risk COT. Substantial improvements also were seen in the subgroup of patients receiving high-dose COT following creation and use of the opioid safety review e-consult tool.
Other VHA opioid management improvement initiatives were successful at reducing high-dose opioid prescribing through interventions similar to those described in this study. However, these initiatives did not address opioid monitoring practices or opioid/BZD combination therapy.25,26 To the authors’ knowledge, no previous opioid management improvement initiatives have reported improvements in provider use of a state PDMP database.
There are a number of factors that also may have helped lead to the successful outcomes observed during the study period. First, the creation of an informatics tool allowed for sustained interventions over time. While targeted interventions and patient/provider education were certainly beneficial, the impact of these efforts wanes as time moves forward. Inevitably, a patient’s and a provider’s focus move to the next important issue, and new patients meet the criteria of the original targeted intervention.
Group Health Cooperative implemented an opioid risk reduction initiative that successfully increased UDS use over a 2-year postimplementation period.21,26 While this initiative used a number of similar interventions to those implemented in this study (patient and provider education, targeted patient intervention), an informatics tool was not used. The annual UDS rate at the conclusion of the Group Health initiative was 50%, which contrasts with a final rate of 75.5% in this study. Although it is difficult to draw comparisons between the studies given differences in populations studied, periods of evaluation, and varying baseline annual UDS rates, the current study results demonstrate the potential effectiveness of informatics tools to help drive enduring changes in practice.
An additional factor that had a positive impact on outcomes the continued support and advocacy from RHJVAMC clinical and administrative leadership. A targeted review of all patients receiving concomitant BZDs and opioids would not be possible without mental health department leaders who believed in the value of the time consuming undertaking. Furthermore, an e-consult tool is effective only if actually submitted for patients and if a specialist’s recommendations are then followed by a PCP.
Finally, the interdisciplinary nature of the POC contributed to the success of each intervention described in this study. Patients receiving COT often have many complex physical, psychological, and social issues that must be considered in order to make a positive impact on patient care. To appropriately and effectively address these issues requires close collaboration between specialists from multiple disciplines.
Limitations
This study has several important limitations. First, its retrospective nature presents obvious documentation challenges. A second limitation is the brief period of evaluation following a number of POC interventions. For instance, 3 interventions took place in January 2015, leaving only 3 FY quarters of effectiveness data. Furthermore, increased awareness of the risks associated with opioid therapy in the VHA and the health care industry across the study period may have independently impacted the improvements observed in this study.
The lack of an assessment of both patient-centered and clinical outcomes is an additional limitation of this study. Rates of annual UDS and PDMP database reviews and the number of patients receiving high-risk COT are only surrogate metrics that may indicate appropriate prescribing and monitoring of these. Obtaining a UDS or PDMP review is meant to provide a practitioner with additional information to interpret when caring for a patient. These data are only meant to complement—not replace—skilled patient assessment by a provider. Although the authors observed no major patient or clinical adverse events during the study period, the possibility exists that a patient may have been negatively impacted by a population-level initiative to improve surrogate measures of appropriate drug use.
Future studies should assess changes in measures, such as pain scores, legitimate adverse events, and overdose occurrences in order to evaluate whether such opioid improvement initiatives truly benefit the patients who are ultimately affected by each intervention.
Conclusion
This study demonstrates the successful implementation of a VHA-based opioid management initiative to increase appropriate COT monitoring and appropriate management of high-risk patients. It is the authors’ hope that the findings may add to the growing body of literature describing successful opioid improvement initiatives and serve as a tool for other health systems that are confronted with these same issues.
1. Manchikanti L, Fellows B, Ailinani H, Pampati V. Therapeutic use, abuse, and nonmedical use of opioids: a ten-year perspective. Pain Physician. 2010;13(5):401-435.
2. Garcia MM, Angelini MC, Thomas T, Lenz K, Jeffrey P. Implementation of an opioid management initiative by a state Medicaid program. J Manag Care Spec Pharm. 2014;20(5):447-454.
3. Edlund MJ, Austen MA, Sullivan MD, et al. Patterns of opioid use for chronic noncancer pain in the Veterans Health Administration from 2009 to 2011. Pain. 2014;155(11):2337-2343.
4. Office of National Drug Control Policy. Prescription drug abuse. https://obamawhitehouse.archives.gov /ondcp/prescription-drug-abuse1. Accessed April 18, 2017.
5. Chou R, Ballantyne JC, Fanciullo GJ, Fine PG, Miaskowski C. Research gaps on use of opioids for chronic noncancer pain: findings from a review of the evidence for an American Pain Society and American Academy of Pain Medicine clinical practice guideline. J Pain. 2009;10(2):147-159.
6. Chou R, Fanciullo GJ, Fine PG, et al; American Pain Society-American Academy of Pain Medicine Opioids Guidelines Panel. Clinical guidelines for the use of chronic opioid therapy in chronic noncancer pain. J Pain. 2009;10(2):113-130.
7. The American Pain Society, The American Academy of Pain Medicine. Guideline for the use of chronic opioid therapy in chronic non-cancer pain: evidence review. http://americanpainsociety.org/uploads/education/guidelines/chronic-opioid-therapy -cncp.pdf. Accessed April 18, 2017.
8. Von Korff M, Deyo RA. Potent opioids for chronic musculoskeletal pain: flying blind? Pain. 2004;109(3):207-209.
9. Angst MS, Clark JD. Opioid-induced hyperalgesia: a qualitative systematic review. Anesthesiology. 2006;104(3):570-587.
10. Abs R, Verhelst J, Maeyaert J, et al. Endocrine consequences of long-term intrathecal administration of opioids. J Clin Endocrinol Metab. 2000;85(6):2215-2222.
11. Younger JW, Chu LF, D’Arcy NT, Trott KE, Jastrzab LE, Mackey SC. Prescription opioid analgesics rapidly change the human brain. Pain. 2011;152(8):1803-1810.
12. U.S Department of Health and Human Services, Substance Abuse and Mental Health Service Administration Office of Applied Studies. Results from the 2004 national survey on drug use and health: national findings. http://medicalmarijuana.procon .org/sourcefiles/2k4results.pdf. Updated September 8, 2005. Accessed April 18, 2017.
13. Dunn KM, Saunders KW, Rutter CM, et al. Opioid prescriptions for chronic pain and overdose: a cohort study. Ann Intern Med. 2010;152(2):85-92.
14. Centers for Disease Control and Prevention (CDC). Emergency department visits involving nonmedical use of selected prescription drugs—United States, 2004-2008. MMWR Morb Mortal Wkly Rep. 2010;59(23):705-709.
15. Dowell D, Haegerich TM, Chou R. CDC guideline for prescribing opioids for chronic pain—United States, 2016. JAMA. 2016;315(15):1624-1645.
16. U.S. Federal Drug Administration. FDA Drug Safety Communication: FDA warns about serious risks and death when combining opioid pain or cough medicines with benzodiazepines; requires its strongest warning. http://www.fda.gov/Drugs/DrugSafety /ucm518473.htm. Published August 31, 2016. Accessed April 18, 2017.
17. U.S. Department of Veterans Affairs. Pain management, VHA directive 2009-053. https://www.va.gov/painmanagement/docs/vha09paindirective.pdf. Published October 28, 2009. Accessed April 18, 2017.
18. U.S. Department of Veteran Affairs, U.S. Department of Defense. VA/DoD clinical practice guideline for management of opioid therapy for chronic pain. https://www.va.gov/painmanagement/docs/cpg_opioidtherapy_summary.pdf. Published May 2010. Accessed May 8, 2017.
19. Jones CM, Mack KA, Paulozzi LJ. Pharmaceutical overdose deaths, United States, 2010. JAMA. 2013;309(7):657-659.
20. Gwira Baumblatt JA, Wiedeman C, Dunn JR, Schaffner W, Paulozzi LJ, Jones TF. High-risk use by patients prescribed opioids for pain and its role in overdose deaths. JAMA Intern Med. 2014;174(5):796-801.
21. Zedler B, Xie L, Wang L, et al. Risk factors for serious prescription opioid-related toxicity or overdose among Veterans Health Administration patients. Pain Med. 2014;15(11):1911-1929.
22. Centers for Disease Control and Prevention. CDC grand rounds: prescription drug overdoses—a U.S. epidemic. MMWR Morb Mortal Wkly Rep. 2012;61(1):10-13.
23. Jones CM, McAninch JK. Emergency department visits and overdose deaths from combined use of opioids and benzodiazepines. Am J Prev Med. 2015;49(4):493-501.
24. Morden NE, Zerzan JT, Rue TC, et al. Medicaid prior authorization and controlled-release oxycodone. Med Care. 2008;46(6):573-580.
25. Westanmo A, Marshall P, Jones E, Burns K, Krebs EE. Opioid dose reduction in a VA health care system—implementation of a primary care population-level initiative. Pain Med. 2015;16(5):1019-1026.
26. Kryskalla J, Kern S, Gray D, Hauser P. Using dashboard technology to monitor overdose risk. Fed Pract. 2014;31(9):8-14.
27. Centers for Disease Control and Prevention. CDC grand rounds: prescription drug overdoses—a U.S. epidemic. MMWR Morb Mortal Wkly Rep. 2012;61(1):10-13.
28. U.S. Department of Veterans Affairs. Patient aligned care team (PACT). https://www.patientcare.va.gov /primarycare/PACT.asp. Updated September 22, 2016. Accessed April 18, 2017.
29. Liu Y, Logan JE, Paulozzi LJ, Zhang K, Jones CM. Potential misuse and inappropriate prescription practices involving opioid analgesics. Am J Manag Care. 2013;19(8):648-665.
30. Reisfield GM, Webster LR. Benzodiazepines in long-term opioid therapy. Pain Med. 2013;14(10):1441-1446.
1. Manchikanti L, Fellows B, Ailinani H, Pampati V. Therapeutic use, abuse, and nonmedical use of opioids: a ten-year perspective. Pain Physician. 2010;13(5):401-435.
2. Garcia MM, Angelini MC, Thomas T, Lenz K, Jeffrey P. Implementation of an opioid management initiative by a state Medicaid program. J Manag Care Spec Pharm. 2014;20(5):447-454.
3. Edlund MJ, Austen MA, Sullivan MD, et al. Patterns of opioid use for chronic noncancer pain in the Veterans Health Administration from 2009 to 2011. Pain. 2014;155(11):2337-2343.
4. Office of National Drug Control Policy. Prescription drug abuse. https://obamawhitehouse.archives.gov /ondcp/prescription-drug-abuse1. Accessed April 18, 2017.
5. Chou R, Ballantyne JC, Fanciullo GJ, Fine PG, Miaskowski C. Research gaps on use of opioids for chronic noncancer pain: findings from a review of the evidence for an American Pain Society and American Academy of Pain Medicine clinical practice guideline. J Pain. 2009;10(2):147-159.
6. Chou R, Fanciullo GJ, Fine PG, et al; American Pain Society-American Academy of Pain Medicine Opioids Guidelines Panel. Clinical guidelines for the use of chronic opioid therapy in chronic noncancer pain. J Pain. 2009;10(2):113-130.
7. The American Pain Society, The American Academy of Pain Medicine. Guideline for the use of chronic opioid therapy in chronic non-cancer pain: evidence review. http://americanpainsociety.org/uploads/education/guidelines/chronic-opioid-therapy -cncp.pdf. Accessed April 18, 2017.
8. Von Korff M, Deyo RA. Potent opioids for chronic musculoskeletal pain: flying blind? Pain. 2004;109(3):207-209.
9. Angst MS, Clark JD. Opioid-induced hyperalgesia: a qualitative systematic review. Anesthesiology. 2006;104(3):570-587.
10. Abs R, Verhelst J, Maeyaert J, et al. Endocrine consequences of long-term intrathecal administration of opioids. J Clin Endocrinol Metab. 2000;85(6):2215-2222.
11. Younger JW, Chu LF, D’Arcy NT, Trott KE, Jastrzab LE, Mackey SC. Prescription opioid analgesics rapidly change the human brain. Pain. 2011;152(8):1803-1810.
12. U.S Department of Health and Human Services, Substance Abuse and Mental Health Service Administration Office of Applied Studies. Results from the 2004 national survey on drug use and health: national findings. http://medicalmarijuana.procon .org/sourcefiles/2k4results.pdf. Updated September 8, 2005. Accessed April 18, 2017.
13. Dunn KM, Saunders KW, Rutter CM, et al. Opioid prescriptions for chronic pain and overdose: a cohort study. Ann Intern Med. 2010;152(2):85-92.
14. Centers for Disease Control and Prevention (CDC). Emergency department visits involving nonmedical use of selected prescription drugs—United States, 2004-2008. MMWR Morb Mortal Wkly Rep. 2010;59(23):705-709.
15. Dowell D, Haegerich TM, Chou R. CDC guideline for prescribing opioids for chronic pain—United States, 2016. JAMA. 2016;315(15):1624-1645.
16. U.S. Federal Drug Administration. FDA Drug Safety Communication: FDA warns about serious risks and death when combining opioid pain or cough medicines with benzodiazepines; requires its strongest warning. http://www.fda.gov/Drugs/DrugSafety /ucm518473.htm. Published August 31, 2016. Accessed April 18, 2017.
17. U.S. Department of Veterans Affairs. Pain management, VHA directive 2009-053. https://www.va.gov/painmanagement/docs/vha09paindirective.pdf. Published October 28, 2009. Accessed April 18, 2017.
18. U.S. Department of Veteran Affairs, U.S. Department of Defense. VA/DoD clinical practice guideline for management of opioid therapy for chronic pain. https://www.va.gov/painmanagement/docs/cpg_opioidtherapy_summary.pdf. Published May 2010. Accessed May 8, 2017.
19. Jones CM, Mack KA, Paulozzi LJ. Pharmaceutical overdose deaths, United States, 2010. JAMA. 2013;309(7):657-659.
20. Gwira Baumblatt JA, Wiedeman C, Dunn JR, Schaffner W, Paulozzi LJ, Jones TF. High-risk use by patients prescribed opioids for pain and its role in overdose deaths. JAMA Intern Med. 2014;174(5):796-801.
21. Zedler B, Xie L, Wang L, et al. Risk factors for serious prescription opioid-related toxicity or overdose among Veterans Health Administration patients. Pain Med. 2014;15(11):1911-1929.
22. Centers for Disease Control and Prevention. CDC grand rounds: prescription drug overdoses—a U.S. epidemic. MMWR Morb Mortal Wkly Rep. 2012;61(1):10-13.
23. Jones CM, McAninch JK. Emergency department visits and overdose deaths from combined use of opioids and benzodiazepines. Am J Prev Med. 2015;49(4):493-501.
24. Morden NE, Zerzan JT, Rue TC, et al. Medicaid prior authorization and controlled-release oxycodone. Med Care. 2008;46(6):573-580.
25. Westanmo A, Marshall P, Jones E, Burns K, Krebs EE. Opioid dose reduction in a VA health care system—implementation of a primary care population-level initiative. Pain Med. 2015;16(5):1019-1026.
26. Kryskalla J, Kern S, Gray D, Hauser P. Using dashboard technology to monitor overdose risk. Fed Pract. 2014;31(9):8-14.
27. Centers for Disease Control and Prevention. CDC grand rounds: prescription drug overdoses—a U.S. epidemic. MMWR Morb Mortal Wkly Rep. 2012;61(1):10-13.
28. U.S. Department of Veterans Affairs. Patient aligned care team (PACT). https://www.patientcare.va.gov /primarycare/PACT.asp. Updated September 22, 2016. Accessed April 18, 2017.
29. Liu Y, Logan JE, Paulozzi LJ, Zhang K, Jones CM. Potential misuse and inappropriate prescription practices involving opioid analgesics. Am J Manag Care. 2013;19(8):648-665.
30. Reisfield GM, Webster LR. Benzodiazepines in long-term opioid therapy. Pain Med. 2013;14(10):1441-1446.
Tracking the Relationship Between Unintentional Weight Loss and Cancer
Unintentional weight loss can present diagnostic challenges, according to researchers from University of Barcelona. So many factors are involved, and so many causes are possible, extensive and invasive investigation often is needed. And because cancer may be the underlying cause of weight loss in patients with few or no symptoms, a swift workup is crucial. Most studies of unintentional weight loss (UWL) have been limited by small sample sizes, short or variable follow-up, or focus on older patients, the researchers say. So they conducted the largest and longest prospective study thus far, following 2,677 patients for > 5 years.
The patients were referred to an outpatient diagnosis unit for evaluation of UWL as a dominant or isolated feature of disease and underwent a standard baseline evaluation with laboratory tests and chest X-ray. Those without identifiable causes 6 months after presentation were followed for 60 more months. Older patients also were given an oral cavity examination, a videofluoroscopy or swallowing study, and a depression and cognitive assessment.
At 6 months, 582 patients had unexplained UWL. Of those, 27 had malignancies: 11 patients had pancreatic cancer; nine had lymphoma. Nearly half of the cancers were digestive; of those, pancreatic cancer accounted for 19%, followed by lung (17%); lymphoma (11%); and kidney, ureteral, and bladder cancers (10%). Of 450 nonmalignant organic disorders, 45% were digestive.
Cancer patients had the highest mortality rate (69% vs 5%-6% in other groups). Decreasing weight was substantially more common in survivors with malignancies (66%) than in the rest of the patients studied, as well as in survivors with nonmalignant organic disorders (10%) than in unexplained UWL and psychosocial disorders. Patients with cancers were older, male, and active smokers, with a greater weight loss than patients in other groups. They also were more likely to have accompanying symptoms and abnormalities on physical examination, lab tests, and chest X-ray.
Cancer evading early diagnosis is a major concern, the researchers say. One of the main findings of the study was the long follow-up of patients with unexplained UWL: a mean of 47.5 months. Based on their findings, occult malignancies detected during a follow-up of up to 66 months after presentation “do not seem to be as rare as thought.” Moreover, autopsies of 13 patients showed malignancies in eight.
However, the fact that only 1 in 20 patients received a cancer diagnosis within that period—most notably within the first 28 months after referral for workup—is “fairly reassuring.” However, they recommend continuing to regularly evaluate patients with UWL for longer periods even if they had a normal evaluation and workup.
Unintentional weight loss can present diagnostic challenges, according to researchers from University of Barcelona. So many factors are involved, and so many causes are possible, extensive and invasive investigation often is needed. And because cancer may be the underlying cause of weight loss in patients with few or no symptoms, a swift workup is crucial. Most studies of unintentional weight loss (UWL) have been limited by small sample sizes, short or variable follow-up, or focus on older patients, the researchers say. So they conducted the largest and longest prospective study thus far, following 2,677 patients for > 5 years.
The patients were referred to an outpatient diagnosis unit for evaluation of UWL as a dominant or isolated feature of disease and underwent a standard baseline evaluation with laboratory tests and chest X-ray. Those without identifiable causes 6 months after presentation were followed for 60 more months. Older patients also were given an oral cavity examination, a videofluoroscopy or swallowing study, and a depression and cognitive assessment.
At 6 months, 582 patients had unexplained UWL. Of those, 27 had malignancies: 11 patients had pancreatic cancer; nine had lymphoma. Nearly half of the cancers were digestive; of those, pancreatic cancer accounted for 19%, followed by lung (17%); lymphoma (11%); and kidney, ureteral, and bladder cancers (10%). Of 450 nonmalignant organic disorders, 45% were digestive.
Cancer patients had the highest mortality rate (69% vs 5%-6% in other groups). Decreasing weight was substantially more common in survivors with malignancies (66%) than in the rest of the patients studied, as well as in survivors with nonmalignant organic disorders (10%) than in unexplained UWL and psychosocial disorders. Patients with cancers were older, male, and active smokers, with a greater weight loss than patients in other groups. They also were more likely to have accompanying symptoms and abnormalities on physical examination, lab tests, and chest X-ray.
Cancer evading early diagnosis is a major concern, the researchers say. One of the main findings of the study was the long follow-up of patients with unexplained UWL: a mean of 47.5 months. Based on their findings, occult malignancies detected during a follow-up of up to 66 months after presentation “do not seem to be as rare as thought.” Moreover, autopsies of 13 patients showed malignancies in eight.
However, the fact that only 1 in 20 patients received a cancer diagnosis within that period—most notably within the first 28 months after referral for workup—is “fairly reassuring.” However, they recommend continuing to regularly evaluate patients with UWL for longer periods even if they had a normal evaluation and workup.
Unintentional weight loss can present diagnostic challenges, according to researchers from University of Barcelona. So many factors are involved, and so many causes are possible, extensive and invasive investigation often is needed. And because cancer may be the underlying cause of weight loss in patients with few or no symptoms, a swift workup is crucial. Most studies of unintentional weight loss (UWL) have been limited by small sample sizes, short or variable follow-up, or focus on older patients, the researchers say. So they conducted the largest and longest prospective study thus far, following 2,677 patients for > 5 years.
The patients were referred to an outpatient diagnosis unit for evaluation of UWL as a dominant or isolated feature of disease and underwent a standard baseline evaluation with laboratory tests and chest X-ray. Those without identifiable causes 6 months after presentation were followed for 60 more months. Older patients also were given an oral cavity examination, a videofluoroscopy or swallowing study, and a depression and cognitive assessment.
At 6 months, 582 patients had unexplained UWL. Of those, 27 had malignancies: 11 patients had pancreatic cancer; nine had lymphoma. Nearly half of the cancers were digestive; of those, pancreatic cancer accounted for 19%, followed by lung (17%); lymphoma (11%); and kidney, ureteral, and bladder cancers (10%). Of 450 nonmalignant organic disorders, 45% were digestive.
Cancer patients had the highest mortality rate (69% vs 5%-6% in other groups). Decreasing weight was substantially more common in survivors with malignancies (66%) than in the rest of the patients studied, as well as in survivors with nonmalignant organic disorders (10%) than in unexplained UWL and psychosocial disorders. Patients with cancers were older, male, and active smokers, with a greater weight loss than patients in other groups. They also were more likely to have accompanying symptoms and abnormalities on physical examination, lab tests, and chest X-ray.
Cancer evading early diagnosis is a major concern, the researchers say. One of the main findings of the study was the long follow-up of patients with unexplained UWL: a mean of 47.5 months. Based on their findings, occult malignancies detected during a follow-up of up to 66 months after presentation “do not seem to be as rare as thought.” Moreover, autopsies of 13 patients showed malignancies in eight.
However, the fact that only 1 in 20 patients received a cancer diagnosis within that period—most notably within the first 28 months after referral for workup—is “fairly reassuring.” However, they recommend continuing to regularly evaluate patients with UWL for longer periods even if they had a normal evaluation and workup.
The Personal Health Inventory: Current Use, Perceived Barriers, and Benefits
To better meet the needs and values of patients, the VA has been promulgating a paradigm shift away from the disease-focused model toward a whole health, patient-centered focus.1 To achieve this goal, the VA Office of Patient Centered Care and Cultural Transformation has advocated the use of the personal health inventory (PHI). This inventory asks patients to mindfully assess why their health is important to them and to determine where they feel they are and where they want to be with respect to 8 areas of self-care (working the body, physical and emotional surroundings, personal development, food and drink, sleep, human relationships, spirituality/purpose, and awareness of relationship between mind and body).
Personal health inventory written responses are then discussed with a member of the health care team to develop a proactive, patient-driven health plan unique to that veteran’s circumstances and aspirations.2 The PHI is applicable not only to veterans, but also in primary care and other practices outside the VA to improve shared decision making and produce more effective clinician-patient partnerships.
After national PHI promotion by the VA, the authors observed that there was not widespread adoption of this practice at their institution, despite its introduction and discussion at several primary care staff meetings. The authors surveyed primary care providers (PCPs) at VA Connecticut Healthcare System (VACHS) to understand perceived barriers and benefits to the use of PHIs in clinical practice.
Methods
The authors surveyed PCPs at VACHS sites about their current use of the PHI as well as their perceptions of barriers and benefits for future implementation of the PHI in clinical settings. Current use of the PHI was captured in a free response question. The authors assessed comfort with the PHI using a 5-point Likert scale, asking participants how comfortable they would feel explaining the PHI to a patient and or a coworker (1 = very uncomfortable, 5 = very comfortable). Barriers and benefits of future PHI implementation were chosen from preselected lists (Figure 1). Participants also were asked how important they feel it is for VA PCPs to use the PHI (1 = very unimportant, 5 = very important).
Finally, participants were asked whether they plan to use the PHI with their patients and how often (1 = less than once a month, 5 = daily). Participants were initially asked at staff meetings to complete the survey in a paper format. Nonrespondents then were asked to complete the survey electronically. This research protocol was reviewed and approved by the institutional review board of the participating institutions.
Study Population
The survey was delivered to all PCPs in the VACHS, which consisted of 2 main facilities (West Haven and Newington campuses) and 7 community-based outpatient clinics. The VACHS provides care to Connecticut’s eligible veteran population of > 55,000 patients who are enrolled in care. Survey participants included physicians, physician assistants, and nurse practitioners. Trainees were excluded.
Statistical Analyses
Summary statistics were calculated to assess current use of the PHI, barriers to and benefits of future implementation, and other scaled responses. Chi-square tests were used to compare the responses of participants who were completing the survey online with those completing it on paper for major study outcomes. Mann-Whitney tests were conducted to assess whether responses to certain questions (eg, future plans to use the PHI) were associated with responses to other related questions (eg, importance of VACHS providers pursuing the PHI). Significance was determined as P ≤ .05.
Results
Thirty-eight (53%) of 72 PCPs completed the survey. Thirteen providers completed the survey in the online format and 25 on paper. There was no significant difference between participants who completed the survey online vs paper for each of the major outcomes assessed. Most participants were aged between 40 and 60 years (64%), female (70%), and white (76%), similar to the entire PCP population at VACHS. The majority of participants worked in a hospital-based outpatient primary care setting (58%) (Table).
Current Use of PHI
Of respondents, 84% stated that they had heard of the PHI. Of those, 68% felt very or somewhat comfortable explaining the PHI to a patient, with slightly fewer, 64%, very or somewhat comfortable explaining the PHI to a coworker. Forty-eight percent stated that they had implemented the PHI in their clinical practices. Examples of current use included “can refer to RN to complete a true PHI,” “giving blank PHI to patients to fill out and bring back/mail,” and “occasional patient who I am trying to achieve some sort of lifestyle modification or change in behavior.”
PHI Barriers and Benefits
Almost all participants (95%) stated that lack of time was a barrier to using the PHI in their clinical settings (Figure 2). The next most common barriers were cumbersome paper forms (37%) and lack of support from upper management (24%). Very few participants listed discomfort as a reason for not discussing the PHI with patients (5%).
Respondents were divided evenly when identifying the benefits of the PHI. The top 3 selections were greater focus on what patients want (55%), greater patient engagement (55%), and improved patient/provider communication (53%) (Figure 3).
PHI Importance and Future Use
The majority of participants (71%) stated that it was very or somewhat important for VA PCPs to pursue the PHI. However, only 45% planned to use the PHI with their patients. Respondents who said they had implemented the PHI in the past were not more likely than others to state that pursuing the PHI was very important (P = .81). However, respondents who stated that it was very important to pursue the PHI were significantly more likely to plan to implement the PHI (P = .04). Of those planning on its use, the frequency of expected use varied from 31% planning to use the PHI daily with patients to 25% expecting to use it less than once a month.
Discussion
The traditional model of care has been fraught with problems. For example, patients are frequently nonadherent to medical therapies and lifestyle recommendations.3-6 Clearly, changes need to be made. To improve health care outcomes by delivering more patient-centered care, the VA initiated the PHI.7
Although nearly three-fourths of the respondents believed that the PHI was an important tool that the VA should pursue, more than half of all respondents did not intend to use it. Of those planning on using it, a large proportion planned on using it infrequently.
The authors found that despite PCP knowledge of PHI and its acceptance as a tool to focus more on what patients want to accomplish, to enhance patient engagement, and to improve communication between patients and providers, time constraints were a universal barrier to implementation, followed by cumbersome paper forms, and not enough perceived support from local upper management.
Measures to decrease PCP time investment and involvement with paper forms, such as having the patient complete the PHI outside of an office visit with a PCP, either at home, with the assistance of a team member with less training than a PCP, or electronically could help address an identified barrier. Further, if the PHI is to be more broadly adopted, support of local upper management should be enlisted to vociferously advocate its use, thus it will be deemed more essential to enhance care and introduce an organizational system for its effective implementation.
Interestingly, only about one-third of respondents believed that the use of the PHI would lead to better health outcomes for patients. Future studies should address whether the use of the PHI improves surrogate goals, such as cholesterol levels, blood pressures, hemoglobin A1c, or medication adherence as well as harder outcomes, such as risk of cardiovascular outcomes, diabetic complications, and mortality.
Limitations
The questionnaire was used at only 1 health care system within the VA. Whether it could be generalizable to PCPs with other baseline demographic information, non-VA facilities, or even other VA facilities, is not known. Since this survey was administered to PCPs, the authors also do not know the impact of implementing the PHI in specialty settings.
Conclusion
Although the concept of the PHI is favored by the majority of PCPs within VACHS, significant barriers, the most common being time constraints, need to be overcome before it is widely adopted. Implementation of novel collaborative systems of PHI administration may be needed.
1. U.S. Department of Veterans Affairs.VA patient centered care. http://www.va.gov/patientcenteredcare/about.asp. Updated March 3, 2016. Accessed March 30, 2017.
2. U.S. Department of Veterans Affairs. MyStory: personal health inventory. http://www.va.gov/patientcenteredcare/docs/va-opcc-personal-health-inventory-final-508.pdf. Published October 7, 2013. Accessed March 30, 2017.
3. Martin LR, Williams SL, Haskard KB, Dimatteo MR. The challenge of patient adherence. Ther Clin Risk Manag. 2005;1(3):189-199.
4. Nieuwlaat R, Wilczynski N, Navarro T, et al. Interventions for enhancing medication adherence. Cochrane Database Syst Rev. 2014;(11):CD000011.
5. Iuga AO, McGuire MJ. Adherence and health care costs. Risk Manag Healthc Policy. 2014;7:35-44.
6. Viswanathan M, Golin CE, Jones CD, et al. Interventions to improve adherence to self-administered medications for chronic diseases in the United States: a systematic review. Ann Intern Med. 2012;157(11):785-795. 7. Simmons LA, Drake CD, Gaudet TW, Snyderman R. Personalized health planning in primary care settings. Fed Pract. 2016;33(1):27-34.
To better meet the needs and values of patients, the VA has been promulgating a paradigm shift away from the disease-focused model toward a whole health, patient-centered focus.1 To achieve this goal, the VA Office of Patient Centered Care and Cultural Transformation has advocated the use of the personal health inventory (PHI). This inventory asks patients to mindfully assess why their health is important to them and to determine where they feel they are and where they want to be with respect to 8 areas of self-care (working the body, physical and emotional surroundings, personal development, food and drink, sleep, human relationships, spirituality/purpose, and awareness of relationship between mind and body).
Personal health inventory written responses are then discussed with a member of the health care team to develop a proactive, patient-driven health plan unique to that veteran’s circumstances and aspirations.2 The PHI is applicable not only to veterans, but also in primary care and other practices outside the VA to improve shared decision making and produce more effective clinician-patient partnerships.
After national PHI promotion by the VA, the authors observed that there was not widespread adoption of this practice at their institution, despite its introduction and discussion at several primary care staff meetings. The authors surveyed primary care providers (PCPs) at VA Connecticut Healthcare System (VACHS) to understand perceived barriers and benefits to the use of PHIs in clinical practice.
Methods
The authors surveyed PCPs at VACHS sites about their current use of the PHI as well as their perceptions of barriers and benefits for future implementation of the PHI in clinical settings. Current use of the PHI was captured in a free response question. The authors assessed comfort with the PHI using a 5-point Likert scale, asking participants how comfortable they would feel explaining the PHI to a patient and or a coworker (1 = very uncomfortable, 5 = very comfortable). Barriers and benefits of future PHI implementation were chosen from preselected lists (Figure 1). Participants also were asked how important they feel it is for VA PCPs to use the PHI (1 = very unimportant, 5 = very important).
Finally, participants were asked whether they plan to use the PHI with their patients and how often (1 = less than once a month, 5 = daily). Participants were initially asked at staff meetings to complete the survey in a paper format. Nonrespondents then were asked to complete the survey electronically. This research protocol was reviewed and approved by the institutional review board of the participating institutions.
Study Population
The survey was delivered to all PCPs in the VACHS, which consisted of 2 main facilities (West Haven and Newington campuses) and 7 community-based outpatient clinics. The VACHS provides care to Connecticut’s eligible veteran population of > 55,000 patients who are enrolled in care. Survey participants included physicians, physician assistants, and nurse practitioners. Trainees were excluded.
Statistical Analyses
Summary statistics were calculated to assess current use of the PHI, barriers to and benefits of future implementation, and other scaled responses. Chi-square tests were used to compare the responses of participants who were completing the survey online with those completing it on paper for major study outcomes. Mann-Whitney tests were conducted to assess whether responses to certain questions (eg, future plans to use the PHI) were associated with responses to other related questions (eg, importance of VACHS providers pursuing the PHI). Significance was determined as P ≤ .05.
Results
Thirty-eight (53%) of 72 PCPs completed the survey. Thirteen providers completed the survey in the online format and 25 on paper. There was no significant difference between participants who completed the survey online vs paper for each of the major outcomes assessed. Most participants were aged between 40 and 60 years (64%), female (70%), and white (76%), similar to the entire PCP population at VACHS. The majority of participants worked in a hospital-based outpatient primary care setting (58%) (Table).
Current Use of PHI
Of respondents, 84% stated that they had heard of the PHI. Of those, 68% felt very or somewhat comfortable explaining the PHI to a patient, with slightly fewer, 64%, very or somewhat comfortable explaining the PHI to a coworker. Forty-eight percent stated that they had implemented the PHI in their clinical practices. Examples of current use included “can refer to RN to complete a true PHI,” “giving blank PHI to patients to fill out and bring back/mail,” and “occasional patient who I am trying to achieve some sort of lifestyle modification or change in behavior.”
PHI Barriers and Benefits
Almost all participants (95%) stated that lack of time was a barrier to using the PHI in their clinical settings (Figure 2). The next most common barriers were cumbersome paper forms (37%) and lack of support from upper management (24%). Very few participants listed discomfort as a reason for not discussing the PHI with patients (5%).
Respondents were divided evenly when identifying the benefits of the PHI. The top 3 selections were greater focus on what patients want (55%), greater patient engagement (55%), and improved patient/provider communication (53%) (Figure 3).
PHI Importance and Future Use
The majority of participants (71%) stated that it was very or somewhat important for VA PCPs to pursue the PHI. However, only 45% planned to use the PHI with their patients. Respondents who said they had implemented the PHI in the past were not more likely than others to state that pursuing the PHI was very important (P = .81). However, respondents who stated that it was very important to pursue the PHI were significantly more likely to plan to implement the PHI (P = .04). Of those planning on its use, the frequency of expected use varied from 31% planning to use the PHI daily with patients to 25% expecting to use it less than once a month.
Discussion
The traditional model of care has been fraught with problems. For example, patients are frequently nonadherent to medical therapies and lifestyle recommendations.3-6 Clearly, changes need to be made. To improve health care outcomes by delivering more patient-centered care, the VA initiated the PHI.7
Although nearly three-fourths of the respondents believed that the PHI was an important tool that the VA should pursue, more than half of all respondents did not intend to use it. Of those planning on using it, a large proportion planned on using it infrequently.
The authors found that despite PCP knowledge of PHI and its acceptance as a tool to focus more on what patients want to accomplish, to enhance patient engagement, and to improve communication between patients and providers, time constraints were a universal barrier to implementation, followed by cumbersome paper forms, and not enough perceived support from local upper management.
Measures to decrease PCP time investment and involvement with paper forms, such as having the patient complete the PHI outside of an office visit with a PCP, either at home, with the assistance of a team member with less training than a PCP, or electronically could help address an identified barrier. Further, if the PHI is to be more broadly adopted, support of local upper management should be enlisted to vociferously advocate its use, thus it will be deemed more essential to enhance care and introduce an organizational system for its effective implementation.
Interestingly, only about one-third of respondents believed that the use of the PHI would lead to better health outcomes for patients. Future studies should address whether the use of the PHI improves surrogate goals, such as cholesterol levels, blood pressures, hemoglobin A1c, or medication adherence as well as harder outcomes, such as risk of cardiovascular outcomes, diabetic complications, and mortality.
Limitations
The questionnaire was used at only 1 health care system within the VA. Whether it could be generalizable to PCPs with other baseline demographic information, non-VA facilities, or even other VA facilities, is not known. Since this survey was administered to PCPs, the authors also do not know the impact of implementing the PHI in specialty settings.
Conclusion
Although the concept of the PHI is favored by the majority of PCPs within VACHS, significant barriers, the most common being time constraints, need to be overcome before it is widely adopted. Implementation of novel collaborative systems of PHI administration may be needed.
To better meet the needs and values of patients, the VA has been promulgating a paradigm shift away from the disease-focused model toward a whole health, patient-centered focus.1 To achieve this goal, the VA Office of Patient Centered Care and Cultural Transformation has advocated the use of the personal health inventory (PHI). This inventory asks patients to mindfully assess why their health is important to them and to determine where they feel they are and where they want to be with respect to 8 areas of self-care (working the body, physical and emotional surroundings, personal development, food and drink, sleep, human relationships, spirituality/purpose, and awareness of relationship between mind and body).
Personal health inventory written responses are then discussed with a member of the health care team to develop a proactive, patient-driven health plan unique to that veteran’s circumstances and aspirations.2 The PHI is applicable not only to veterans, but also in primary care and other practices outside the VA to improve shared decision making and produce more effective clinician-patient partnerships.
After national PHI promotion by the VA, the authors observed that there was not widespread adoption of this practice at their institution, despite its introduction and discussion at several primary care staff meetings. The authors surveyed primary care providers (PCPs) at VA Connecticut Healthcare System (VACHS) to understand perceived barriers and benefits to the use of PHIs in clinical practice.
Methods
The authors surveyed PCPs at VACHS sites about their current use of the PHI as well as their perceptions of barriers and benefits for future implementation of the PHI in clinical settings. Current use of the PHI was captured in a free response question. The authors assessed comfort with the PHI using a 5-point Likert scale, asking participants how comfortable they would feel explaining the PHI to a patient and or a coworker (1 = very uncomfortable, 5 = very comfortable). Barriers and benefits of future PHI implementation were chosen from preselected lists (Figure 1). Participants also were asked how important they feel it is for VA PCPs to use the PHI (1 = very unimportant, 5 = very important).
Finally, participants were asked whether they plan to use the PHI with their patients and how often (1 = less than once a month, 5 = daily). Participants were initially asked at staff meetings to complete the survey in a paper format. Nonrespondents then were asked to complete the survey electronically. This research protocol was reviewed and approved by the institutional review board of the participating institutions.
Study Population
The survey was delivered to all PCPs in the VACHS, which consisted of 2 main facilities (West Haven and Newington campuses) and 7 community-based outpatient clinics. The VACHS provides care to Connecticut’s eligible veteran population of > 55,000 patients who are enrolled in care. Survey participants included physicians, physician assistants, and nurse practitioners. Trainees were excluded.
Statistical Analyses
Summary statistics were calculated to assess current use of the PHI, barriers to and benefits of future implementation, and other scaled responses. Chi-square tests were used to compare the responses of participants who were completing the survey online with those completing it on paper for major study outcomes. Mann-Whitney tests were conducted to assess whether responses to certain questions (eg, future plans to use the PHI) were associated with responses to other related questions (eg, importance of VACHS providers pursuing the PHI). Significance was determined as P ≤ .05.
Results
Thirty-eight (53%) of 72 PCPs completed the survey. Thirteen providers completed the survey in the online format and 25 on paper. There was no significant difference between participants who completed the survey online vs paper for each of the major outcomes assessed. Most participants were aged between 40 and 60 years (64%), female (70%), and white (76%), similar to the entire PCP population at VACHS. The majority of participants worked in a hospital-based outpatient primary care setting (58%) (Table).
Current Use of PHI
Of respondents, 84% stated that they had heard of the PHI. Of those, 68% felt very or somewhat comfortable explaining the PHI to a patient, with slightly fewer, 64%, very or somewhat comfortable explaining the PHI to a coworker. Forty-eight percent stated that they had implemented the PHI in their clinical practices. Examples of current use included “can refer to RN to complete a true PHI,” “giving blank PHI to patients to fill out and bring back/mail,” and “occasional patient who I am trying to achieve some sort of lifestyle modification or change in behavior.”
PHI Barriers and Benefits
Almost all participants (95%) stated that lack of time was a barrier to using the PHI in their clinical settings (Figure 2). The next most common barriers were cumbersome paper forms (37%) and lack of support from upper management (24%). Very few participants listed discomfort as a reason for not discussing the PHI with patients (5%).
Respondents were divided evenly when identifying the benefits of the PHI. The top 3 selections were greater focus on what patients want (55%), greater patient engagement (55%), and improved patient/provider communication (53%) (Figure 3).
PHI Importance and Future Use
The majority of participants (71%) stated that it was very or somewhat important for VA PCPs to pursue the PHI. However, only 45% planned to use the PHI with their patients. Respondents who said they had implemented the PHI in the past were not more likely than others to state that pursuing the PHI was very important (P = .81). However, respondents who stated that it was very important to pursue the PHI were significantly more likely to plan to implement the PHI (P = .04). Of those planning on its use, the frequency of expected use varied from 31% planning to use the PHI daily with patients to 25% expecting to use it less than once a month.
Discussion
The traditional model of care has been fraught with problems. For example, patients are frequently nonadherent to medical therapies and lifestyle recommendations.3-6 Clearly, changes need to be made. To improve health care outcomes by delivering more patient-centered care, the VA initiated the PHI.7
Although nearly three-fourths of the respondents believed that the PHI was an important tool that the VA should pursue, more than half of all respondents did not intend to use it. Of those planning on using it, a large proportion planned on using it infrequently.
The authors found that despite PCP knowledge of PHI and its acceptance as a tool to focus more on what patients want to accomplish, to enhance patient engagement, and to improve communication between patients and providers, time constraints were a universal barrier to implementation, followed by cumbersome paper forms, and not enough perceived support from local upper management.
Measures to decrease PCP time investment and involvement with paper forms, such as having the patient complete the PHI outside of an office visit with a PCP, either at home, with the assistance of a team member with less training than a PCP, or electronically could help address an identified barrier. Further, if the PHI is to be more broadly adopted, support of local upper management should be enlisted to vociferously advocate its use, thus it will be deemed more essential to enhance care and introduce an organizational system for its effective implementation.
Interestingly, only about one-third of respondents believed that the use of the PHI would lead to better health outcomes for patients. Future studies should address whether the use of the PHI improves surrogate goals, such as cholesterol levels, blood pressures, hemoglobin A1c, or medication adherence as well as harder outcomes, such as risk of cardiovascular outcomes, diabetic complications, and mortality.
Limitations
The questionnaire was used at only 1 health care system within the VA. Whether it could be generalizable to PCPs with other baseline demographic information, non-VA facilities, or even other VA facilities, is not known. Since this survey was administered to PCPs, the authors also do not know the impact of implementing the PHI in specialty settings.
Conclusion
Although the concept of the PHI is favored by the majority of PCPs within VACHS, significant barriers, the most common being time constraints, need to be overcome before it is widely adopted. Implementation of novel collaborative systems of PHI administration may be needed.
1. U.S. Department of Veterans Affairs.VA patient centered care. http://www.va.gov/patientcenteredcare/about.asp. Updated March 3, 2016. Accessed March 30, 2017.
2. U.S. Department of Veterans Affairs. MyStory: personal health inventory. http://www.va.gov/patientcenteredcare/docs/va-opcc-personal-health-inventory-final-508.pdf. Published October 7, 2013. Accessed March 30, 2017.
3. Martin LR, Williams SL, Haskard KB, Dimatteo MR. The challenge of patient adherence. Ther Clin Risk Manag. 2005;1(3):189-199.
4. Nieuwlaat R, Wilczynski N, Navarro T, et al. Interventions for enhancing medication adherence. Cochrane Database Syst Rev. 2014;(11):CD000011.
5. Iuga AO, McGuire MJ. Adherence and health care costs. Risk Manag Healthc Policy. 2014;7:35-44.
6. Viswanathan M, Golin CE, Jones CD, et al. Interventions to improve adherence to self-administered medications for chronic diseases in the United States: a systematic review. Ann Intern Med. 2012;157(11):785-795. 7. Simmons LA, Drake CD, Gaudet TW, Snyderman R. Personalized health planning in primary care settings. Fed Pract. 2016;33(1):27-34.
1. U.S. Department of Veterans Affairs.VA patient centered care. http://www.va.gov/patientcenteredcare/about.asp. Updated March 3, 2016. Accessed March 30, 2017.
2. U.S. Department of Veterans Affairs. MyStory: personal health inventory. http://www.va.gov/patientcenteredcare/docs/va-opcc-personal-health-inventory-final-508.pdf. Published October 7, 2013. Accessed March 30, 2017.
3. Martin LR, Williams SL, Haskard KB, Dimatteo MR. The challenge of patient adherence. Ther Clin Risk Manag. 2005;1(3):189-199.
4. Nieuwlaat R, Wilczynski N, Navarro T, et al. Interventions for enhancing medication adherence. Cochrane Database Syst Rev. 2014;(11):CD000011.
5. Iuga AO, McGuire MJ. Adherence and health care costs. Risk Manag Healthc Policy. 2014;7:35-44.
6. Viswanathan M, Golin CE, Jones CD, et al. Interventions to improve adherence to self-administered medications for chronic diseases in the United States: a systematic review. Ann Intern Med. 2012;157(11):785-795. 7. Simmons LA, Drake CD, Gaudet TW, Snyderman R. Personalized health planning in primary care settings. Fed Pract. 2016;33(1):27-34.
Dabigatran vs Warfarin Before Cardioversion of Atrial Arrhythmias
Atrial fibrillation (AF) is the most common cardiac arrhythmia, followed by atrial flutter. Both arrhythmias may increase the risk of stroke. Atrial fibrillation affects about 1% to 2% of the population.1 Patients with atrial flutter often have episodes of AF.
Direct current cardioversion (DCCV) treats atrial arrhythmias by attempting to return the patient to a normal sinus rhythm. When sinus rhythm is restored, cardiac structural changes that might have occurred as a result of AF or atrial flutter may be reversed.2 However, patients undergoing cardioversion are at an increased risk of stroke if a thrombus is present in the left atria. This thrombus may become dislodged during the procedure. Although sinus rhythm may be restored during cardioversion, restoration of the atrial mechanical function may take several weeks, and new thrombi may form during that time. Stroke risk is significantly decreased with anticoagulation.3,4
Current guidelines on antithrombotic therapy for AF and atrial flutter recommend that patients who are appropriate candidates for electrical cardioversion need to be properly anticoagulated for 3 to 4 weeks before and after the procedure if the duration of AF or flutter is > 48 hours or is unknown.5 The practice of anticoagulating candidates needing cardioversion for 3 to 4 weeks before the procedure and 4 weeks after the procedure is based on the theory that it takes about 14 days for a new thrombus to firmly adhere to the atrial wall.6 Therefore 3 to 4 weeks of anticoagulation before cardioversion will prevent new thrombi from forming and theoretically allows enough time for older thrombi to adhere to the atrial wall. Anticoagulation for 4 weeks after cardioversion will prevent new thrombi from forming in the atria during the several weeks that atrial remodeling takes place.3,7 These practices are based on physiologic concepts and observational studies and have not been evaluated in randomized, controlled clinical trials.7
To receive an electrical cardioversion, patients at the VA Portland Health Care System (VAPORHCS) should maintain a therapeutic international normalized ratio (INR), defined as 2.0 to 3.0, for 4 consecutive weeks. The Anticoagulation Clinic monitors patients receiving warfarin for planned DCCV at least weekly. The estimated average time for cardioversion candidates at the VAPORHCS to achieve stability on warfarin is 2 months. Prolonging the time to DCCV may expose symptomatic patients to additional discomfort, lead to further cardiac remodeling, and result in poorer outcomes.
In response to the delays attributed to time needed to achieve INR stability, the VISN 20 Pharmacy and Therapeutics (P&T) committee approved the use of dabigatran prior to cardioversion of AF in October 2011. This quality improvement (QI) project evaluated the time elapsed between initiation of anticoagulation with dabigatran vs warfarin and DCCV and the associated costs of anticoagulation before DCCV.
Methods
A single site, retrospective chart review of patients scheduled for cardioversion from November 2011 to December 2013 was conducted. This QI project was considered exempt from institutional review board approval. VAPORHCS patients aged > 18 years who initiated dabigatran or
warfarin for planned cardioversion of AF or atrial flutter were included in the study. Exclusion criteria included use of dabigatran or warfarin within 3 months before the decision to cardiovert and emergency cardioversion performed within 48 hours of symptom onset. Patients were assigned to either the dabigatran or warfarin group, based on the prescribed anticoagulant. The primary objectives were to evaluate the time elapsed from initiation of anticoagulation to planned cardioversion of AF or atrial flutter and to evaluate treatment costs associated with dabigatran vs warfarin before planned cardioversion of AF or atrial flutter. The secondary objective was to identify reasons for rescheduled or cancelled cardioversions.
Data Collection
Potential patients were identified using the computerized patient record system and VistA. Demographics, including age, gender, indication for cardioversion, calculated CHADS2 score for thromboembolic risk, and calculated HAS-BLED score for bleeding risk were collected to evaluate the potential differences between the 2 groups. Anticoagulation time before cardioversion was evaluated by collecting the first fill date of dabigatran or warfarin and the date that cardioversion was performed. An internal cost analysis was completed. The cost analysis for dabigatran included medication and laboratory costs. The cost analysis for warfarin included costs associated with the medication, laboratory, and pharmacists’ monitoring time.
Statistical Analysis
Statistical analysis was performed using Sigma Plot, Version 12.5 for Windows (System Software, Inc., Chicago, Illinois). Demographic parameters and the primary objectives of time and cost were analyzed using the Mann-Whitney U test. The secondary objective of reasons for rescheduled or cancelled cardioversions was reported using descriptive statistics. A P value of ≥ .05 was considered statistically significant.
Results
Forty dabigatran patients and 68 warfarin patients met inclusion criteria (Table 1). All patients were male with a median age of 65 years in both groups, which is representative of the VA patient population of mostly older adult males. The CHADS2 and HAS-BLED scores were similar between the groups.
Primary Objectives
There was a difference in anticoagulation time before cardioversion between the 2 groups (Table 2). The median number of days that elapsed between initiation of dabigatran and cardioversion was 43 (range 28-120 days) vs 76 days (range 27-278 days) in the warfarin group (P < .001). Patients whose cardioversions were cancelled were not included in the time analysis. The difference in total cost per patient was not statistically significant. The median cost for dabigatran was $277.65 (range: $114.00-$633.65) per patient and $262.58 (range $121.0-$599.31) per patient in the warfarin group (P = .139). All patients, including those whose cardioversions were cancelled, were included in the cost analysis. Costs for cancellations were evaluated from the date of initiation to the date of the cardioversion cancellation decision.
Secondary Objective
In the dabigatran group, 3 patients rescheduled cardioversions and 5 patients cancelled cardioversions. Fourteen warfarin patients rescheduled cardioversions, and 10 patients cancelled cardioversions (Tables 3 and 4). Two dabigatran patients were rescheduled due to missed doses of dabigatran or propafenone and 7 warfarin patients were rescheduled due to out of range INRs (< 2.0) at their preprocedure appointment. Three dabigatran patients presented without symptoms at their preprocedure appointments and their cardioversions were cancelled. Similarly, 5 warfarin patients spontaneously returned to sinus rhythm, and their cardioversions were cancelled.
Discussion
Currently, there are 4 target-specific oral anticoagulants (TSOACs) approved by the FDA for nonvalvular AF: dabigatran, rivaroxaban, apixaban, and edoxaban. Dabigatran is a direct thrombin inhibitor, and rivaroxaban apixaban and edoxaban are factor Xa inhibitors.8-11 The American College of Chest Physicians (CHEST) 2012 guidelines on antithrombotic therapy for AF recommend anticoagulation with warfarin, low molecular weight heparin (LMWH) or dabigatran before cardioversion (grade 1B for all 3 options).5
Anticoagulation with warfarin (Class Ia, Level B), dabigatran, rivaroxaban, or apixaban (Class IIa, Level C) before and after cardioversion was also recommended by the recently published American College of Cardiology/ American Heart Association/ Heart Rhythm Society (ACC/AHA/ HRS) 2014 guidelines for the management of patients with atrial fibrillation.1 Edoxaban was not included since the guidelines were published prior to FDA approval. The main evidence supporting the inclusion of the 3 TSOACs in the 2014 ACC/AHA/HRS guidelines are based on post hoc analyses of the major landmark trials (RE-LY, ROCKET-AF, and ARISTOTLE) evaluating the use of dabigatran, rivaroxaban, and apixaban, respectively, before and after DCCV.12-15 Major adverse events (AEs) were similar between warfarin and the TSOAC comparator in all 3 post hoc analyses.
Low molecular weight heparin was not included as an option for anticoagulation before cardioversion in the ACC/AHA/HRS 2014 guidelines. This is likely due to lack of evidence, as most of the evidence supporting anticoagulation included warfarin and not heparin. The 2 guidelines did not differ in their recommendations on the duration of pre- and postprocedure anticoagulation of 3 and 4 weeks, respectively.1,5 Nor did they differ on the use of transesophageal echocardiogram (TEE) to rule out left atrial thrombus if a patient has not been anticoagulated for 3 weeks before cardioversion.
A recent nonrandomized cohort study by Choo and colleagues evaluated the timing, rescheduling, and cancellation of scheduled DCCV in 193 patients receiving warfarin or dabigatran.16 The study found that patients receiving dabigatran waited 22 fewer days until scheduled DCCV and had lower rates of rescheduled cardioversions than did patients receiving warfarin. The results of this study were similar to the findings at VAPORHCS. The most common reasons for rescheduled or cancelled DCCVs at VAPORHCS were out of range INRs and spontaneous return to sinus rhythm, respectively, which were the same reasons that Choo and colleagues found for rescheduling or cancellations in their study.
Dabigatran patients received drug therapy at VAPORHCS for fewer days before cardioversion than did the patients taking warfarin. The median total cost per patient was about $15 higher in the dabigatran group. Based on these findings and the recommendations of the 2 guidelines, both drugs remain reasonable and appropriate options for patients before cardioversion.
Reasons to Select Dabigatran
If warfarin or a TSOAC is clinically indicated for anticoagulation, then patient preference and nonclinical barriers to safe monitoring may also factor in the decision. Some patients from the surrounding states are referred to VAPORHCS for cardioversions and continue to receive primary care from their facility. Patients receiving primary care and anticoagulation management outside VAPORHCS were not included in this QI project. It may add an additional layer of difficulty to initiate warfarin on a remote patient if the patient does not have access to anticoagulation monitoring locally. Additionally, it may be difficult for the remote anticoagulation providers to communicate information efficiently with the cardiology team at VAPORHCS. It also may be challenging for VAPORHCS to safely manage warfarin in a remote patient without full access to laboratory results and the patient’s primary care provider. For these reasons, dabigatran may be a more favorable option in remote patients referred to VAPORHCS for their cardioversion.
Additionally, dabigatran may be a more appropriate anticoagulant in highly symptomatic AF patients in whom the potential for longer wait times may expose the patient to more symptoms and decreased quality of life. The longer the duration of AF or atrial flutter, the less likely that sinus rhythm will be restored in patients undergoing DCCV.2 A study of 157 patients with AF showed that the adjusted risk for return to AF after DCCV increased if the AF was present for > 2 months before the DCCV.17 If returning patients to sinus rhythm is the highest priority for reversal of cardiac restructuring and symptoms, then a shorter time to DCCV may be preferred, and a TSOAC may be the preferred agent in this case.
Reasons to Select Warfarin
Warfarin may be a more appropriate option in patients with a high bleeding risk due to the current lack of a reversal agent for dabigatran. Dabigatran is not recommended in patients with creatinine clearances < 30 mL/min; thus, warfarin may be a better choice in patients with impaired renal function. It may be reasonable to consider switching a current warfarin patient with a history of variable INRs to a TSOAC in preparation for cardioversion to potentially shorten the time to cardioversion if the patient is highly symptomatic. Low molecular weight heparin may be considered as a last resort for patients who may not be able to tolerate warfarin or TSOACs. However, if LMWH were to be used, it may be more reasonable to consider a TEE-guided DCCV rather than 3 full weeks of anticoagulation with LMWH.
Limitations
There were several limitations to this single site, retrospective, QI project with a small sample size. All patients were older, adult males. Results may not be relevant to other institutions and patient populations, including females and younger patients.
Standardized anticoagulation clinic encounter times (15 minutes for phone call and 5 minutes for letter) were used to calculate pharmacist’s monitoring time costs for warfarin patients. This standardized time did not account for the amount of time spent in monitoring and creating dosing plans that may vary drastically between patients. The time and cost analyses did not account for pharmacy technician reminder phone calls for missed or late INR draws or home health nurse INR draws and visits. Theoretically, patients with home health services have fewer missed or late INRs, and phone encounter times may be shorter between the pharmacist and the nurse vs the pharmacist and the patient.
Finally, it was difficult to capture administrative reasons for delayed DCCV in both groups. In the warfarin group, communication between the anticoagulation clinic and the cardiology team may have been delayed due to staff vacations, sick time, or differences in staff work schedules. In both groups, assessing how procedure scheduling affected wait times was difficult. Procedure room availability, clinic schedules, staff schedules, and preprocedure appointment availability likely impacted patient wait times for DCCV but were difficult to assess and quantify. Finally, power was not calculated for this project.
Conclusions
Based on the recommendations of the CHEST 2012 guidelines, the ACC/AHA/HRS 2014 guidelines, and recent literature, TSOACs are reasonable anticoagulants to consider before and after planned cardioversion of atrial arrhythmias. The findings of this QI project support the
use of either dabigatran or warfarin before a planned cardioversion at VAPORHCS. Several factors should be considered when choosing an oral anticoagulant before a planned DCCV, including indication, duration of anticoagulation, previous anticoagulant use, medication adherence, renal function, risk of thromboembolism vs bleeding risk, and potential need for a reversal agent.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
1. January CT, Wann LS, Alpert JS, et al; ACC/AHA Task Force Members. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines and the Heart Rhythm Society. Circulation. 2014;130(2):2071-2104.
2. Van Gelder IC, Crijns HJ, van Gilst WH, Hamer HP, Lie KI. Decrease of right and left atrial sizes after direct-current electrical cardioversion in chronic atrial fibrillation. Am J Cardiol. 1991;67(1):93-95.
3. Manning WJ, Leeman DE, Gotch PJ, Come PC. Pulsed Doppler evaluation of atrial mechanical function after electrical cardioversion of atrial fibrillation. J Am Coll Cardiol. 1989;13(3):617-623.
4. Design of a clinical trial for the assessment of cardioversion using transesophageal echocardiography (The ACUTE Multicenter Study). Steering and Publications Committees of the ACUTE Study. Am J Cardiol. 1998;81(7):877-883.
5. You JJ, Singer DE, Howard PA, et al; American College of Chest Physicians. Antithrombotic therapy for atrial fibrillation: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(suppl 2):e531S-e575S.
6. Mancini GB, Goldberger AL. Cardioversion of atrial fibrillation: consideration of embolization, anticoagulation, prophylactic pacemaker, and long-term success. Am Heart J. 1982;104(3):617-621.
7. Berger M, Schweitzer P. Timing of thromboembolic events after electrical cardioversion of atrial fibrillation or flutter: a retrospective analysis. Am J Cardiol. 1998;82(12):1545-1547, A8.
8. Pradaxa [package insert]. Ridgefield, CT: Boehringer Ingelheim Pharmaceuticals Inc; 2015.
9. Xarelto [package insert]. Titusville, NJ: Janssen Pharmaceuticals Inc; 2015.
10. Eliquis [package insert]. Princeton, NJ: Bristol Myers Squibb Company; 2015.
11. Savaysa [package insert]. Parsippany, NJ: Daiichi Sankyo, Inc; 2015.
12. Nagarakanti R, Ezekowitz MD, Oldgren J, et al. Dabigatran versus warfarin in patients with atrial fibrillation: an analysis of patients undergoing cardioversion. Circulation. 2011;123(2):131-136.
13. Connolly SJ, Ezekowitz MD, Yusuf S, et al; RE-LY Steering Committee and Investigators. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361(12):1139-1151.
14. Piccini JP, Stevens SR, Lokhnygina Y, et al; ROCKET AF Steering Committee & Investigators. Outcomes after cardioversion and atrial fibrillation ablation in patients treated with rivaroxaban and warfarin in the ROCKET AF trial. J Am Coll Cardiol. 2013;61(19):1998-2006.
15. Flaker G, Lopes RD, Al-Khatib SM, et al; ARISTOTLE Committees and Investigators. Efficacy and safety of apixaban in patients after cardioversion for atrial fibrillation: insights from the ARISTOTLE trial (Apixaban for Reduction in Stroke and Other Thromboembolic Events in Atrial Fibrillation). J Am Coll Cardiol. 2014;63(11):1082-1087.
16. Choo WK, Fraser S, Padfield G, et al. Dabigatran improves the efficiency of an elective direct current cardioversion service. Br J Cardiol. 2014;21(1):29-32.
17. Alt E, Ammer R, Lehmann G, et al. Patient characteristics and underlying heart disease as predictors of recurrent atrial fibrillation after internal and external cardioversion in patients treated with oral sotalol. Am Heart J. 1997;134(3):419-425.
Atrial fibrillation (AF) is the most common cardiac arrhythmia, followed by atrial flutter. Both arrhythmias may increase the risk of stroke. Atrial fibrillation affects about 1% to 2% of the population.1 Patients with atrial flutter often have episodes of AF.
Direct current cardioversion (DCCV) treats atrial arrhythmias by attempting to return the patient to a normal sinus rhythm. When sinus rhythm is restored, cardiac structural changes that might have occurred as a result of AF or atrial flutter may be reversed.2 However, patients undergoing cardioversion are at an increased risk of stroke if a thrombus is present in the left atria. This thrombus may become dislodged during the procedure. Although sinus rhythm may be restored during cardioversion, restoration of the atrial mechanical function may take several weeks, and new thrombi may form during that time. Stroke risk is significantly decreased with anticoagulation.3,4
Current guidelines on antithrombotic therapy for AF and atrial flutter recommend that patients who are appropriate candidates for electrical cardioversion need to be properly anticoagulated for 3 to 4 weeks before and after the procedure if the duration of AF or flutter is > 48 hours or is unknown.5 The practice of anticoagulating candidates needing cardioversion for 3 to 4 weeks before the procedure and 4 weeks after the procedure is based on the theory that it takes about 14 days for a new thrombus to firmly adhere to the atrial wall.6 Therefore 3 to 4 weeks of anticoagulation before cardioversion will prevent new thrombi from forming and theoretically allows enough time for older thrombi to adhere to the atrial wall. Anticoagulation for 4 weeks after cardioversion will prevent new thrombi from forming in the atria during the several weeks that atrial remodeling takes place.3,7 These practices are based on physiologic concepts and observational studies and have not been evaluated in randomized, controlled clinical trials.7
To receive an electrical cardioversion, patients at the VA Portland Health Care System (VAPORHCS) should maintain a therapeutic international normalized ratio (INR), defined as 2.0 to 3.0, for 4 consecutive weeks. The Anticoagulation Clinic monitors patients receiving warfarin for planned DCCV at least weekly. The estimated average time for cardioversion candidates at the VAPORHCS to achieve stability on warfarin is 2 months. Prolonging the time to DCCV may expose symptomatic patients to additional discomfort, lead to further cardiac remodeling, and result in poorer outcomes.
In response to the delays attributed to time needed to achieve INR stability, the VISN 20 Pharmacy and Therapeutics (P&T) committee approved the use of dabigatran prior to cardioversion of AF in October 2011. This quality improvement (QI) project evaluated the time elapsed between initiation of anticoagulation with dabigatran vs warfarin and DCCV and the associated costs of anticoagulation before DCCV.
Methods
A single site, retrospective chart review of patients scheduled for cardioversion from November 2011 to December 2013 was conducted. This QI project was considered exempt from institutional review board approval. VAPORHCS patients aged > 18 years who initiated dabigatran or
warfarin for planned cardioversion of AF or atrial flutter were included in the study. Exclusion criteria included use of dabigatran or warfarin within 3 months before the decision to cardiovert and emergency cardioversion performed within 48 hours of symptom onset. Patients were assigned to either the dabigatran or warfarin group, based on the prescribed anticoagulant. The primary objectives were to evaluate the time elapsed from initiation of anticoagulation to planned cardioversion of AF or atrial flutter and to evaluate treatment costs associated with dabigatran vs warfarin before planned cardioversion of AF or atrial flutter. The secondary objective was to identify reasons for rescheduled or cancelled cardioversions.
Data Collection
Potential patients were identified using the computerized patient record system and VistA. Demographics, including age, gender, indication for cardioversion, calculated CHADS2 score for thromboembolic risk, and calculated HAS-BLED score for bleeding risk were collected to evaluate the potential differences between the 2 groups. Anticoagulation time before cardioversion was evaluated by collecting the first fill date of dabigatran or warfarin and the date that cardioversion was performed. An internal cost analysis was completed. The cost analysis for dabigatran included medication and laboratory costs. The cost analysis for warfarin included costs associated with the medication, laboratory, and pharmacists’ monitoring time.
Statistical Analysis
Statistical analysis was performed using Sigma Plot, Version 12.5 for Windows (System Software, Inc., Chicago, Illinois). Demographic parameters and the primary objectives of time and cost were analyzed using the Mann-Whitney U test. The secondary objective of reasons for rescheduled or cancelled cardioversions was reported using descriptive statistics. A P value of ≥ .05 was considered statistically significant.
Results
Forty dabigatran patients and 68 warfarin patients met inclusion criteria (Table 1). All patients were male with a median age of 65 years in both groups, which is representative of the VA patient population of mostly older adult males. The CHADS2 and HAS-BLED scores were similar between the groups.
Primary Objectives
There was a difference in anticoagulation time before cardioversion between the 2 groups (Table 2). The median number of days that elapsed between initiation of dabigatran and cardioversion was 43 (range 28-120 days) vs 76 days (range 27-278 days) in the warfarin group (P < .001). Patients whose cardioversions were cancelled were not included in the time analysis. The difference in total cost per patient was not statistically significant. The median cost for dabigatran was $277.65 (range: $114.00-$633.65) per patient and $262.58 (range $121.0-$599.31) per patient in the warfarin group (P = .139). All patients, including those whose cardioversions were cancelled, were included in the cost analysis. Costs for cancellations were evaluated from the date of initiation to the date of the cardioversion cancellation decision.
Secondary Objective
In the dabigatran group, 3 patients rescheduled cardioversions and 5 patients cancelled cardioversions. Fourteen warfarin patients rescheduled cardioversions, and 10 patients cancelled cardioversions (Tables 3 and 4). Two dabigatran patients were rescheduled due to missed doses of dabigatran or propafenone and 7 warfarin patients were rescheduled due to out of range INRs (< 2.0) at their preprocedure appointment. Three dabigatran patients presented without symptoms at their preprocedure appointments and their cardioversions were cancelled. Similarly, 5 warfarin patients spontaneously returned to sinus rhythm, and their cardioversions were cancelled.
Discussion
Currently, there are 4 target-specific oral anticoagulants (TSOACs) approved by the FDA for nonvalvular AF: dabigatran, rivaroxaban, apixaban, and edoxaban. Dabigatran is a direct thrombin inhibitor, and rivaroxaban apixaban and edoxaban are factor Xa inhibitors.8-11 The American College of Chest Physicians (CHEST) 2012 guidelines on antithrombotic therapy for AF recommend anticoagulation with warfarin, low molecular weight heparin (LMWH) or dabigatran before cardioversion (grade 1B for all 3 options).5
Anticoagulation with warfarin (Class Ia, Level B), dabigatran, rivaroxaban, or apixaban (Class IIa, Level C) before and after cardioversion was also recommended by the recently published American College of Cardiology/ American Heart Association/ Heart Rhythm Society (ACC/AHA/ HRS) 2014 guidelines for the management of patients with atrial fibrillation.1 Edoxaban was not included since the guidelines were published prior to FDA approval. The main evidence supporting the inclusion of the 3 TSOACs in the 2014 ACC/AHA/HRS guidelines are based on post hoc analyses of the major landmark trials (RE-LY, ROCKET-AF, and ARISTOTLE) evaluating the use of dabigatran, rivaroxaban, and apixaban, respectively, before and after DCCV.12-15 Major adverse events (AEs) were similar between warfarin and the TSOAC comparator in all 3 post hoc analyses.
Low molecular weight heparin was not included as an option for anticoagulation before cardioversion in the ACC/AHA/HRS 2014 guidelines. This is likely due to lack of evidence, as most of the evidence supporting anticoagulation included warfarin and not heparin. The 2 guidelines did not differ in their recommendations on the duration of pre- and postprocedure anticoagulation of 3 and 4 weeks, respectively.1,5 Nor did they differ on the use of transesophageal echocardiogram (TEE) to rule out left atrial thrombus if a patient has not been anticoagulated for 3 weeks before cardioversion.
A recent nonrandomized cohort study by Choo and colleagues evaluated the timing, rescheduling, and cancellation of scheduled DCCV in 193 patients receiving warfarin or dabigatran.16 The study found that patients receiving dabigatran waited 22 fewer days until scheduled DCCV and had lower rates of rescheduled cardioversions than did patients receiving warfarin. The results of this study were similar to the findings at VAPORHCS. The most common reasons for rescheduled or cancelled DCCVs at VAPORHCS were out of range INRs and spontaneous return to sinus rhythm, respectively, which were the same reasons that Choo and colleagues found for rescheduling or cancellations in their study.
Dabigatran patients received drug therapy at VAPORHCS for fewer days before cardioversion than did the patients taking warfarin. The median total cost per patient was about $15 higher in the dabigatran group. Based on these findings and the recommendations of the 2 guidelines, both drugs remain reasonable and appropriate options for patients before cardioversion.
Reasons to Select Dabigatran
If warfarin or a TSOAC is clinically indicated for anticoagulation, then patient preference and nonclinical barriers to safe monitoring may also factor in the decision. Some patients from the surrounding states are referred to VAPORHCS for cardioversions and continue to receive primary care from their facility. Patients receiving primary care and anticoagulation management outside VAPORHCS were not included in this QI project. It may add an additional layer of difficulty to initiate warfarin on a remote patient if the patient does not have access to anticoagulation monitoring locally. Additionally, it may be difficult for the remote anticoagulation providers to communicate information efficiently with the cardiology team at VAPORHCS. It also may be challenging for VAPORHCS to safely manage warfarin in a remote patient without full access to laboratory results and the patient’s primary care provider. For these reasons, dabigatran may be a more favorable option in remote patients referred to VAPORHCS for their cardioversion.
Additionally, dabigatran may be a more appropriate anticoagulant in highly symptomatic AF patients in whom the potential for longer wait times may expose the patient to more symptoms and decreased quality of life. The longer the duration of AF or atrial flutter, the less likely that sinus rhythm will be restored in patients undergoing DCCV.2 A study of 157 patients with AF showed that the adjusted risk for return to AF after DCCV increased if the AF was present for > 2 months before the DCCV.17 If returning patients to sinus rhythm is the highest priority for reversal of cardiac restructuring and symptoms, then a shorter time to DCCV may be preferred, and a TSOAC may be the preferred agent in this case.
Reasons to Select Warfarin
Warfarin may be a more appropriate option in patients with a high bleeding risk due to the current lack of a reversal agent for dabigatran. Dabigatran is not recommended in patients with creatinine clearances < 30 mL/min; thus, warfarin may be a better choice in patients with impaired renal function. It may be reasonable to consider switching a current warfarin patient with a history of variable INRs to a TSOAC in preparation for cardioversion to potentially shorten the time to cardioversion if the patient is highly symptomatic. Low molecular weight heparin may be considered as a last resort for patients who may not be able to tolerate warfarin or TSOACs. However, if LMWH were to be used, it may be more reasonable to consider a TEE-guided DCCV rather than 3 full weeks of anticoagulation with LMWH.
Limitations
There were several limitations to this single site, retrospective, QI project with a small sample size. All patients were older, adult males. Results may not be relevant to other institutions and patient populations, including females and younger patients.
Standardized anticoagulation clinic encounter times (15 minutes for phone call and 5 minutes for letter) were used to calculate pharmacist’s monitoring time costs for warfarin patients. This standardized time did not account for the amount of time spent in monitoring and creating dosing plans that may vary drastically between patients. The time and cost analyses did not account for pharmacy technician reminder phone calls for missed or late INR draws or home health nurse INR draws and visits. Theoretically, patients with home health services have fewer missed or late INRs, and phone encounter times may be shorter between the pharmacist and the nurse vs the pharmacist and the patient.
Finally, it was difficult to capture administrative reasons for delayed DCCV in both groups. In the warfarin group, communication between the anticoagulation clinic and the cardiology team may have been delayed due to staff vacations, sick time, or differences in staff work schedules. In both groups, assessing how procedure scheduling affected wait times was difficult. Procedure room availability, clinic schedules, staff schedules, and preprocedure appointment availability likely impacted patient wait times for DCCV but were difficult to assess and quantify. Finally, power was not calculated for this project.
Conclusions
Based on the recommendations of the CHEST 2012 guidelines, the ACC/AHA/HRS 2014 guidelines, and recent literature, TSOACs are reasonable anticoagulants to consider before and after planned cardioversion of atrial arrhythmias. The findings of this QI project support the
use of either dabigatran or warfarin before a planned cardioversion at VAPORHCS. Several factors should be considered when choosing an oral anticoagulant before a planned DCCV, including indication, duration of anticoagulation, previous anticoagulant use, medication adherence, renal function, risk of thromboembolism vs bleeding risk, and potential need for a reversal agent.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
Atrial fibrillation (AF) is the most common cardiac arrhythmia, followed by atrial flutter. Both arrhythmias may increase the risk of stroke. Atrial fibrillation affects about 1% to 2% of the population.1 Patients with atrial flutter often have episodes of AF.
Direct current cardioversion (DCCV) treats atrial arrhythmias by attempting to return the patient to a normal sinus rhythm. When sinus rhythm is restored, cardiac structural changes that might have occurred as a result of AF or atrial flutter may be reversed.2 However, patients undergoing cardioversion are at an increased risk of stroke if a thrombus is present in the left atria. This thrombus may become dislodged during the procedure. Although sinus rhythm may be restored during cardioversion, restoration of the atrial mechanical function may take several weeks, and new thrombi may form during that time. Stroke risk is significantly decreased with anticoagulation.3,4
Current guidelines on antithrombotic therapy for AF and atrial flutter recommend that patients who are appropriate candidates for electrical cardioversion need to be properly anticoagulated for 3 to 4 weeks before and after the procedure if the duration of AF or flutter is > 48 hours or is unknown.5 The practice of anticoagulating candidates needing cardioversion for 3 to 4 weeks before the procedure and 4 weeks after the procedure is based on the theory that it takes about 14 days for a new thrombus to firmly adhere to the atrial wall.6 Therefore 3 to 4 weeks of anticoagulation before cardioversion will prevent new thrombi from forming and theoretically allows enough time for older thrombi to adhere to the atrial wall. Anticoagulation for 4 weeks after cardioversion will prevent new thrombi from forming in the atria during the several weeks that atrial remodeling takes place.3,7 These practices are based on physiologic concepts and observational studies and have not been evaluated in randomized, controlled clinical trials.7
To receive an electrical cardioversion, patients at the VA Portland Health Care System (VAPORHCS) should maintain a therapeutic international normalized ratio (INR), defined as 2.0 to 3.0, for 4 consecutive weeks. The Anticoagulation Clinic monitors patients receiving warfarin for planned DCCV at least weekly. The estimated average time for cardioversion candidates at the VAPORHCS to achieve stability on warfarin is 2 months. Prolonging the time to DCCV may expose symptomatic patients to additional discomfort, lead to further cardiac remodeling, and result in poorer outcomes.
In response to the delays attributed to time needed to achieve INR stability, the VISN 20 Pharmacy and Therapeutics (P&T) committee approved the use of dabigatran prior to cardioversion of AF in October 2011. This quality improvement (QI) project evaluated the time elapsed between initiation of anticoagulation with dabigatran vs warfarin and DCCV and the associated costs of anticoagulation before DCCV.
Methods
A single site, retrospective chart review of patients scheduled for cardioversion from November 2011 to December 2013 was conducted. This QI project was considered exempt from institutional review board approval. VAPORHCS patients aged > 18 years who initiated dabigatran or
warfarin for planned cardioversion of AF or atrial flutter were included in the study. Exclusion criteria included use of dabigatran or warfarin within 3 months before the decision to cardiovert and emergency cardioversion performed within 48 hours of symptom onset. Patients were assigned to either the dabigatran or warfarin group, based on the prescribed anticoagulant. The primary objectives were to evaluate the time elapsed from initiation of anticoagulation to planned cardioversion of AF or atrial flutter and to evaluate treatment costs associated with dabigatran vs warfarin before planned cardioversion of AF or atrial flutter. The secondary objective was to identify reasons for rescheduled or cancelled cardioversions.
Data Collection
Potential patients were identified using the computerized patient record system and VistA. Demographics, including age, gender, indication for cardioversion, calculated CHADS2 score for thromboembolic risk, and calculated HAS-BLED score for bleeding risk were collected to evaluate the potential differences between the 2 groups. Anticoagulation time before cardioversion was evaluated by collecting the first fill date of dabigatran or warfarin and the date that cardioversion was performed. An internal cost analysis was completed. The cost analysis for dabigatran included medication and laboratory costs. The cost analysis for warfarin included costs associated with the medication, laboratory, and pharmacists’ monitoring time.
Statistical Analysis
Statistical analysis was performed using Sigma Plot, Version 12.5 for Windows (System Software, Inc., Chicago, Illinois). Demographic parameters and the primary objectives of time and cost were analyzed using the Mann-Whitney U test. The secondary objective of reasons for rescheduled or cancelled cardioversions was reported using descriptive statistics. A P value of ≥ .05 was considered statistically significant.
Results
Forty dabigatran patients and 68 warfarin patients met inclusion criteria (Table 1). All patients were male with a median age of 65 years in both groups, which is representative of the VA patient population of mostly older adult males. The CHADS2 and HAS-BLED scores were similar between the groups.
Primary Objectives
There was a difference in anticoagulation time before cardioversion between the 2 groups (Table 2). The median number of days that elapsed between initiation of dabigatran and cardioversion was 43 (range 28-120 days) vs 76 days (range 27-278 days) in the warfarin group (P < .001). Patients whose cardioversions were cancelled were not included in the time analysis. The difference in total cost per patient was not statistically significant. The median cost for dabigatran was $277.65 (range: $114.00-$633.65) per patient and $262.58 (range $121.0-$599.31) per patient in the warfarin group (P = .139). All patients, including those whose cardioversions were cancelled, were included in the cost analysis. Costs for cancellations were evaluated from the date of initiation to the date of the cardioversion cancellation decision.
Secondary Objective
In the dabigatran group, 3 patients rescheduled cardioversions and 5 patients cancelled cardioversions. Fourteen warfarin patients rescheduled cardioversions, and 10 patients cancelled cardioversions (Tables 3 and 4). Two dabigatran patients were rescheduled due to missed doses of dabigatran or propafenone and 7 warfarin patients were rescheduled due to out of range INRs (< 2.0) at their preprocedure appointment. Three dabigatran patients presented without symptoms at their preprocedure appointments and their cardioversions were cancelled. Similarly, 5 warfarin patients spontaneously returned to sinus rhythm, and their cardioversions were cancelled.
Discussion
Currently, there are 4 target-specific oral anticoagulants (TSOACs) approved by the FDA for nonvalvular AF: dabigatran, rivaroxaban, apixaban, and edoxaban. Dabigatran is a direct thrombin inhibitor, and rivaroxaban apixaban and edoxaban are factor Xa inhibitors.8-11 The American College of Chest Physicians (CHEST) 2012 guidelines on antithrombotic therapy for AF recommend anticoagulation with warfarin, low molecular weight heparin (LMWH) or dabigatran before cardioversion (grade 1B for all 3 options).5
Anticoagulation with warfarin (Class Ia, Level B), dabigatran, rivaroxaban, or apixaban (Class IIa, Level C) before and after cardioversion was also recommended by the recently published American College of Cardiology/ American Heart Association/ Heart Rhythm Society (ACC/AHA/ HRS) 2014 guidelines for the management of patients with atrial fibrillation.1 Edoxaban was not included since the guidelines were published prior to FDA approval. The main evidence supporting the inclusion of the 3 TSOACs in the 2014 ACC/AHA/HRS guidelines are based on post hoc analyses of the major landmark trials (RE-LY, ROCKET-AF, and ARISTOTLE) evaluating the use of dabigatran, rivaroxaban, and apixaban, respectively, before and after DCCV.12-15 Major adverse events (AEs) were similar between warfarin and the TSOAC comparator in all 3 post hoc analyses.
Low molecular weight heparin was not included as an option for anticoagulation before cardioversion in the ACC/AHA/HRS 2014 guidelines. This is likely due to lack of evidence, as most of the evidence supporting anticoagulation included warfarin and not heparin. The 2 guidelines did not differ in their recommendations on the duration of pre- and postprocedure anticoagulation of 3 and 4 weeks, respectively.1,5 Nor did they differ on the use of transesophageal echocardiogram (TEE) to rule out left atrial thrombus if a patient has not been anticoagulated for 3 weeks before cardioversion.
A recent nonrandomized cohort study by Choo and colleagues evaluated the timing, rescheduling, and cancellation of scheduled DCCV in 193 patients receiving warfarin or dabigatran.16 The study found that patients receiving dabigatran waited 22 fewer days until scheduled DCCV and had lower rates of rescheduled cardioversions than did patients receiving warfarin. The results of this study were similar to the findings at VAPORHCS. The most common reasons for rescheduled or cancelled DCCVs at VAPORHCS were out of range INRs and spontaneous return to sinus rhythm, respectively, which were the same reasons that Choo and colleagues found for rescheduling or cancellations in their study.
Dabigatran patients received drug therapy at VAPORHCS for fewer days before cardioversion than did the patients taking warfarin. The median total cost per patient was about $15 higher in the dabigatran group. Based on these findings and the recommendations of the 2 guidelines, both drugs remain reasonable and appropriate options for patients before cardioversion.
Reasons to Select Dabigatran
If warfarin or a TSOAC is clinically indicated for anticoagulation, then patient preference and nonclinical barriers to safe monitoring may also factor in the decision. Some patients from the surrounding states are referred to VAPORHCS for cardioversions and continue to receive primary care from their facility. Patients receiving primary care and anticoagulation management outside VAPORHCS were not included in this QI project. It may add an additional layer of difficulty to initiate warfarin on a remote patient if the patient does not have access to anticoagulation monitoring locally. Additionally, it may be difficult for the remote anticoagulation providers to communicate information efficiently with the cardiology team at VAPORHCS. It also may be challenging for VAPORHCS to safely manage warfarin in a remote patient without full access to laboratory results and the patient’s primary care provider. For these reasons, dabigatran may be a more favorable option in remote patients referred to VAPORHCS for their cardioversion.
Additionally, dabigatran may be a more appropriate anticoagulant in highly symptomatic AF patients in whom the potential for longer wait times may expose the patient to more symptoms and decreased quality of life. The longer the duration of AF or atrial flutter, the less likely that sinus rhythm will be restored in patients undergoing DCCV.2 A study of 157 patients with AF showed that the adjusted risk for return to AF after DCCV increased if the AF was present for > 2 months before the DCCV.17 If returning patients to sinus rhythm is the highest priority for reversal of cardiac restructuring and symptoms, then a shorter time to DCCV may be preferred, and a TSOAC may be the preferred agent in this case.
Reasons to Select Warfarin
Warfarin may be a more appropriate option in patients with a high bleeding risk due to the current lack of a reversal agent for dabigatran. Dabigatran is not recommended in patients with creatinine clearances < 30 mL/min; thus, warfarin may be a better choice in patients with impaired renal function. It may be reasonable to consider switching a current warfarin patient with a history of variable INRs to a TSOAC in preparation for cardioversion to potentially shorten the time to cardioversion if the patient is highly symptomatic. Low molecular weight heparin may be considered as a last resort for patients who may not be able to tolerate warfarin or TSOACs. However, if LMWH were to be used, it may be more reasonable to consider a TEE-guided DCCV rather than 3 full weeks of anticoagulation with LMWH.
Limitations
There were several limitations to this single site, retrospective, QI project with a small sample size. All patients were older, adult males. Results may not be relevant to other institutions and patient populations, including females and younger patients.
Standardized anticoagulation clinic encounter times (15 minutes for phone call and 5 minutes for letter) were used to calculate pharmacist’s monitoring time costs for warfarin patients. This standardized time did not account for the amount of time spent in monitoring and creating dosing plans that may vary drastically between patients. The time and cost analyses did not account for pharmacy technician reminder phone calls for missed or late INR draws or home health nurse INR draws and visits. Theoretically, patients with home health services have fewer missed or late INRs, and phone encounter times may be shorter between the pharmacist and the nurse vs the pharmacist and the patient.
Finally, it was difficult to capture administrative reasons for delayed DCCV in both groups. In the warfarin group, communication between the anticoagulation clinic and the cardiology team may have been delayed due to staff vacations, sick time, or differences in staff work schedules. In both groups, assessing how procedure scheduling affected wait times was difficult. Procedure room availability, clinic schedules, staff schedules, and preprocedure appointment availability likely impacted patient wait times for DCCV but were difficult to assess and quantify. Finally, power was not calculated for this project.
Conclusions
Based on the recommendations of the CHEST 2012 guidelines, the ACC/AHA/HRS 2014 guidelines, and recent literature, TSOACs are reasonable anticoagulants to consider before and after planned cardioversion of atrial arrhythmias. The findings of this QI project support the
use of either dabigatran or warfarin before a planned cardioversion at VAPORHCS. Several factors should be considered when choosing an oral anticoagulant before a planned DCCV, including indication, duration of anticoagulation, previous anticoagulant use, medication adherence, renal function, risk of thromboembolism vs bleeding risk, and potential need for a reversal agent.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
1. January CT, Wann LS, Alpert JS, et al; ACC/AHA Task Force Members. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines and the Heart Rhythm Society. Circulation. 2014;130(2):2071-2104.
2. Van Gelder IC, Crijns HJ, van Gilst WH, Hamer HP, Lie KI. Decrease of right and left atrial sizes after direct-current electrical cardioversion in chronic atrial fibrillation. Am J Cardiol. 1991;67(1):93-95.
3. Manning WJ, Leeman DE, Gotch PJ, Come PC. Pulsed Doppler evaluation of atrial mechanical function after electrical cardioversion of atrial fibrillation. J Am Coll Cardiol. 1989;13(3):617-623.
4. Design of a clinical trial for the assessment of cardioversion using transesophageal echocardiography (The ACUTE Multicenter Study). Steering and Publications Committees of the ACUTE Study. Am J Cardiol. 1998;81(7):877-883.
5. You JJ, Singer DE, Howard PA, et al; American College of Chest Physicians. Antithrombotic therapy for atrial fibrillation: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(suppl 2):e531S-e575S.
6. Mancini GB, Goldberger AL. Cardioversion of atrial fibrillation: consideration of embolization, anticoagulation, prophylactic pacemaker, and long-term success. Am Heart J. 1982;104(3):617-621.
7. Berger M, Schweitzer P. Timing of thromboembolic events after electrical cardioversion of atrial fibrillation or flutter: a retrospective analysis. Am J Cardiol. 1998;82(12):1545-1547, A8.
8. Pradaxa [package insert]. Ridgefield, CT: Boehringer Ingelheim Pharmaceuticals Inc; 2015.
9. Xarelto [package insert]. Titusville, NJ: Janssen Pharmaceuticals Inc; 2015.
10. Eliquis [package insert]. Princeton, NJ: Bristol Myers Squibb Company; 2015.
11. Savaysa [package insert]. Parsippany, NJ: Daiichi Sankyo, Inc; 2015.
12. Nagarakanti R, Ezekowitz MD, Oldgren J, et al. Dabigatran versus warfarin in patients with atrial fibrillation: an analysis of patients undergoing cardioversion. Circulation. 2011;123(2):131-136.
13. Connolly SJ, Ezekowitz MD, Yusuf S, et al; RE-LY Steering Committee and Investigators. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361(12):1139-1151.
14. Piccini JP, Stevens SR, Lokhnygina Y, et al; ROCKET AF Steering Committee & Investigators. Outcomes after cardioversion and atrial fibrillation ablation in patients treated with rivaroxaban and warfarin in the ROCKET AF trial. J Am Coll Cardiol. 2013;61(19):1998-2006.
15. Flaker G, Lopes RD, Al-Khatib SM, et al; ARISTOTLE Committees and Investigators. Efficacy and safety of apixaban in patients after cardioversion for atrial fibrillation: insights from the ARISTOTLE trial (Apixaban for Reduction in Stroke and Other Thromboembolic Events in Atrial Fibrillation). J Am Coll Cardiol. 2014;63(11):1082-1087.
16. Choo WK, Fraser S, Padfield G, et al. Dabigatran improves the efficiency of an elective direct current cardioversion service. Br J Cardiol. 2014;21(1):29-32.
17. Alt E, Ammer R, Lehmann G, et al. Patient characteristics and underlying heart disease as predictors of recurrent atrial fibrillation after internal and external cardioversion in patients treated with oral sotalol. Am Heart J. 1997;134(3):419-425.
1. January CT, Wann LS, Alpert JS, et al; ACC/AHA Task Force Members. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines and the Heart Rhythm Society. Circulation. 2014;130(2):2071-2104.
2. Van Gelder IC, Crijns HJ, van Gilst WH, Hamer HP, Lie KI. Decrease of right and left atrial sizes after direct-current electrical cardioversion in chronic atrial fibrillation. Am J Cardiol. 1991;67(1):93-95.
3. Manning WJ, Leeman DE, Gotch PJ, Come PC. Pulsed Doppler evaluation of atrial mechanical function after electrical cardioversion of atrial fibrillation. J Am Coll Cardiol. 1989;13(3):617-623.
4. Design of a clinical trial for the assessment of cardioversion using transesophageal echocardiography (The ACUTE Multicenter Study). Steering and Publications Committees of the ACUTE Study. Am J Cardiol. 1998;81(7):877-883.
5. You JJ, Singer DE, Howard PA, et al; American College of Chest Physicians. Antithrombotic therapy for atrial fibrillation: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(suppl 2):e531S-e575S.
6. Mancini GB, Goldberger AL. Cardioversion of atrial fibrillation: consideration of embolization, anticoagulation, prophylactic pacemaker, and long-term success. Am Heart J. 1982;104(3):617-621.
7. Berger M, Schweitzer P. Timing of thromboembolic events after electrical cardioversion of atrial fibrillation or flutter: a retrospective analysis. Am J Cardiol. 1998;82(12):1545-1547, A8.
8. Pradaxa [package insert]. Ridgefield, CT: Boehringer Ingelheim Pharmaceuticals Inc; 2015.
9. Xarelto [package insert]. Titusville, NJ: Janssen Pharmaceuticals Inc; 2015.
10. Eliquis [package insert]. Princeton, NJ: Bristol Myers Squibb Company; 2015.
11. Savaysa [package insert]. Parsippany, NJ: Daiichi Sankyo, Inc; 2015.
12. Nagarakanti R, Ezekowitz MD, Oldgren J, et al. Dabigatran versus warfarin in patients with atrial fibrillation: an analysis of patients undergoing cardioversion. Circulation. 2011;123(2):131-136.
13. Connolly SJ, Ezekowitz MD, Yusuf S, et al; RE-LY Steering Committee and Investigators. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361(12):1139-1151.
14. Piccini JP, Stevens SR, Lokhnygina Y, et al; ROCKET AF Steering Committee & Investigators. Outcomes after cardioversion and atrial fibrillation ablation in patients treated with rivaroxaban and warfarin in the ROCKET AF trial. J Am Coll Cardiol. 2013;61(19):1998-2006.
15. Flaker G, Lopes RD, Al-Khatib SM, et al; ARISTOTLE Committees and Investigators. Efficacy and safety of apixaban in patients after cardioversion for atrial fibrillation: insights from the ARISTOTLE trial (Apixaban for Reduction in Stroke and Other Thromboembolic Events in Atrial Fibrillation). J Am Coll Cardiol. 2014;63(11):1082-1087.
16. Choo WK, Fraser S, Padfield G, et al. Dabigatran improves the efficiency of an elective direct current cardioversion service. Br J Cardiol. 2014;21(1):29-32.
17. Alt E, Ammer R, Lehmann G, et al. Patient characteristics and underlying heart disease as predictors of recurrent atrial fibrillation after internal and external cardioversion in patients treated with oral sotalol. Am Heart J. 1997;134(3):419-425.
A Heart Failure Management Program Using Shared Medical Appointments
Rising health care costs have led to threats of nonreimbursement for rehospitalization within 30 days postdischarge.1,2 Heart failure (HF) in particular is characterized by the highest 30-day rehospitalization rate (23.5% in 2013), which accounts for more than two-thirds of HF expenditures.3,4
Much of HF-related health care costs can be addressed with effective self-management by patients with HF. Therefore, developing and implementing effective disease management programs for this high-risk patient population is essential. Heart failure management programs may include optimizing HF medications, improving patient understanding of the importance of appropriate diet and physical activity, and cultivating psychological health and well-being. In a 2013 systematic review and meta-analysis, Wakefield and colleagues found that disease management programs improved nearly all HF outcomes, including lower mortality rates, lower hospital readmission rates, fewer clinic visits, higher satisfaction with care, and higher quality of life, compared with a no-treatment control or standard care.5 Moreover, these programs demonstrated cost-effectiveness by reducing HF-related hospitalizations and health care expenditures.5
One method to deliver specialized disease management programs to a greater number of patients may be to use shared medical appointments (SMAs). In a randomized controlled trial, Smith and colleagues demonstrated improved HF outcomes through 7 months among veterans who attended SMAs for HF management.6 However, the trial enrolled only 25% of patients screened, and 63% of the patients who did not enroll were classified as not interested. These findings suggest that patients with HF, and veterans in particular, may face additional barriers to enrolling in HF management programs, and these results may not be fully representative of veterans with HF.
In this study, the authors used a naturalistic study design via retrospective review of the electronic health record (EHR) to evaluate whether patients with acute HF who chose to attend SMAs promoting self-management skills for HF would have better hospitalization outcomes compared with those who received individual disease management instructions in a HF specialty clinic (ie, usual care). The authors hypothesized that veterans who participated in the HF SMA clinic would have fewer 12-month HF-related and all-cause hospitalizations, fewer days in the hospital, and more days to first hospitalization compared with patients in usual care.
Methods
The clinic for veterans with acute HF was initiated in October, 2010 at the Jesse Brown VAMC (JBVAMC) in Chicago, Illinois, to reduce readmissions by targeting patients who had been previously hospitalized for HF. In September 2011, the multidisciplinary SMA clinic was developed within the HF clinic to provide enhanced care focused on self-management strategies for patients with HF. The HF SMA program comprised 4 weekly face-to-face sessions co-led by a nurse practitioner (NP), a dietitian, and a clinical psychologist, similar to what has been shown to be successful and cost-effective in nonveteran populations.6-8 Patients attended at least 4 sessions before graduating to the advanced HF SMA program where they could attend monthly booster sessions. The program promoted self-management by providing education about and support for the HF process, HF medications, diet adherence, physical activity, psychological well-being, and stress management via interactive presentations. During the visit, patients’ medication and food logs were reviewed. Patients were encouraged to discuss successes and obstacles in achieving their goals. All study procedures were approved by the institutional review board at JBVAMC.
Study Design
Data were collected by retrospective review of the JBVAMC EHR. The EHR was reviewed for all veterans scheduled for ≥ 1 SMA clinic visit within the HF specialty clinic using predetermined, convenient selection between January 1, 2012, and December 31, 2013. Outcome data were collected through 12-month follow-up (through December 31, 2014).
Patients in both treatment arms received HF care through the HF clinic, including one-on-one education regarding HF self-management provided by a NP. Patients were assigned to the HF SMA group if they also attended the HF SMA clinic within 3 months of their initial HF clinic consult. The number of SMAs attended was included as a covariate in the models. Patients who were scheduled for, but did not attend, the HF SMA clinic were assigned to the HF clinic group. Patients who attended the initial HF consult before September 1, 2011, were excluded, thereby ensuring that all patients included in the present analyses had the opportunity to attend the HF SMA appointment within the predetermined period of chart review.
Data for all VA hospitalizations that occurred between January 1, 2012 and December 31, 2014, were extracted from the EHR. Extracted data included admission date, discharge date, and discharge diagnoses. From these data, the authors assessed 4 hospitalization outcomes for each HF hospitalization and all-cause hospitalization within 12 months of the initial HF clinic consult date: hospitalization (yes/no), number of hospitalizations, number of days in the hospital, and days to first hospitalization.
Data Analysis
Demographic, HF characteristics, and HF outcome variables for the HF SMA and HF clinic groups were compared using t tests and chi-square analyses. Logistic regressions were used to predict 12-month hospitalization, linear regressions were used to predict number of hospitalizations and number of days hospitalized, and Cox proportional hazards regressions were used to predict time from initial HF consult to first hospitalization for each HF-related hospitalization variable and all-cause hospitalization variable. A separate logistic regression was conducted to predict 12-month all-cause mortality. The primary predictor variable of interest for all models was group membership (HF SMA vs HF clinic). Covariates in all models included race (black vs nonblack), ethnicity (Hispanic/Latino vs non-Hispanic/Latino), age, and number of HF SMAs attended.
Results
Of 709 HF SMA clinic appointments made for 141 patients between January 1, 2012, and December 31, 2013, 54 patients were assigned to the HF SMA group and 37 patients were assigned to the HF clinic group (Figure). The majority of the sample was black (87%), non-Hispanic/Latino (96%), and the average age was 68 years. Patients were more likely to have nonischemic (rather than ischemic) cardiomyopathy (66%) and more likely to have HF with reduced (rather than preserved) ejection fraction (76%; ie, systolic HF). Furthermore, 40% of the sample was diagnosed with atrial fibrillation (AF) or atrial flutter (A-flutter), and 24% had an implantable cardioverter-defibrillator or pacemaker. There were no significant differences in demographics or HF characteristics between the HF SMA group and the HF clinic group (Table).
HF Hospitalization Outcomes
During the 12-month follow-up, 32 patients were hospitalized for HF, 18 (33.3%) in the SMA group and 14 (37.8%) in the HF clinic group, P = .658. Patients were hospitalized up to 4 times for between 1 and 38 days, and from 1 to 352 days postconsult. No differences between the HF SMA group and HF clinic group were observed on any of the HF hospitalization outcomes (Table). Group membership did not predict HF hospitalization (odds ratio [OR]: 0.39, 95% confidence interval [CI]: 0.11-1.42), number of HF hospitalizations (β: 0.15, SE: 0.29), number of days hospitalized for HF (β: 0.1.66, SE: 2.01), or time to first HF hospitalization (hazard ratio [HR]: 1.35, 95% CI: 0.66-2.77), all Ps > .10. In the Cox proportional hazards regression predicting time to HF hospitalization, the coefficients did not converge when the model included demographic covariates; therefore, the model was run only with HF group as a predictor variable. For all other models, no covariates significantly predicted HF hospitalization outcomes.
All-Cause Hospitalization Outcomes
During the 12-month follow-up, 57 patients were hospitalized for any cause (including HF hospitalizations), 32 (59.3%) in the SMA group and 25 (67.6%) in the HF clinic group, P = .421. Patients were hospitalized up to 6 times for between 1 and 106 days and from 1 to 352 days postconsult. No differences were observed between the groups on any of the all-cause hospitalization outcomes (Table). Group membership did not predict all-cause hospitalization (OR: 0.34, 95% CI: 0.10-1.19), number of all-cause hospitalizations (β: 0.49, SE: 0.41), number of days hospitalized for any cause (β: 5.15, SE: 5.15), or time to first all-cause hospitalization (HR: 0.98, 95% CI: 0.56-1.72), all P > .05. None of the covariates predicted any of the all-cause hospitalization outcomes.
All-Cause Mortality Outcomes
During the 12-month follow-up, 14 patients (15%) died of any cause, 8 (15%) in the SMA group and 6 (16%) in the HF clinic group, P = .856. Group membership did not predict all-cause mortality (OR: 2.32, 95% CI: 0.44-12.18), and likewise none of the covariates were associated with 12-month all-cause mortality.
Discussion
This study was a naturalistic, retrospective examination of a HF management program promoting self-management delivered via multidisciplinary SMAs among veterans who enrolled in an acute HF specialty clinic. The authors’ hypothesis was not supported: patients who attended the HF SMA clinic did not have lower 12-month hospitalization or mortality rates, shorter hospital stays, or longer time to hospitalization compared with patients in the HF clinic only.
In contrast to the patient-centered approach of this study, a randomized trial delivering a similar disease management program found that patients with acute HF in the SMA group had better short-term (< 7 months) hospitalization outcomes, specifically greater time to first HF-related hospitalization (HR 0.45, 95% CI: 0.21-0.98), but this effect did not last through 12 months when compared with patients in standard care.6 These disparate findings may be explained by the gap in bench-to-bedside research, where despite scientific evidence indicating better outcomes among patients randomized to an intervention, when patients are given a choice, they may not choose to engage in the best option for their HF treatment.
In the present study, veterans who chose not to attend the HF SMA clinic may have done so for numerous reasons that may have influenced the outcomes. For example, those veterans who did not attend the HF SMA clinic may have had higher health literacy and less need for an educational program. Health literacy has been inversely associated with HF outcomes, such that patients with HF with lower health literacy have greater risk of HF rehospitalization or mortality.9,10 In addition, many of the veterans who were followed in the HF clinic were taught the same disease management strategies by the NP during one-on-one visits, and they may have gained the same self-management skills in a different setting.
Another possibility is that the veterans enrolled in the HF clinic were less likely to be followed exclusively at the VA and therefore may have had external hospitalizations not recorded in their VA health records. In 2000, more than half the veterans who received health care services at the VA reported that they did not receive their care exclusively at the VA.11 This may be especially true since the Veteran’s Choice Program permits veterans who reside > 40 miles from a VA hospital to receive care closer to home.
Disease Management Programs
Disease management programs for HF in general promote better outcomes and lower health care expenditures.5,12 Self-management instruction delivered via SMAs may have greater potential for reducing HF-associated health care costs if it were to be integrated earlier in the continuum of care. The sample in this study was composed of veterans who were referred to a specialty clinic only after being hospitalized for HF. These patients likely were experiencing more advanced disease and/or low adherence, as indicated by the relatively high prevalence of AF diagnoses and pacemakers; these null findings are consistent with those from a randomized controlled trial of a disease management program among veterans with heavy HF symptom burden and impaired functional status.13 However, integrating self-management programs earlier in HF clinical care (eg, primary care or cardiology clinics) may be more effective in promoting proactive disease management and delaying or preventing initial HF hospitalizations.
For example, a disease management plan implemented by general practitioners for veterans with HF in Australia was associated with a 23% reduction in potentially preventable hospitalization rates.14 Veterans with HF enrolled in a NP-led disease management intervention, relative to those followed only in primary care, had significantly fewer hospitalizations and nearly half the risk mortality (15% vs 28% after 2 years; HR 0.55).15 Furthermore, some have suggested that SMAs may be more effective for patients for whom risk of disease is high but current disease burden (ie, symptoms) is low, such as diabetes mellitus management programs.16 Early intervention also may allow providers to reach more patients more quickly and before they experience advanced symptoms, thereby reducing specialty clinic wait times and overall health expenditures. Developing more effective disease management programs for patients with acute HF and veterans in particular remains a critical matter for future study.
Additional and novel components of HF management programs show promise for future interventions. First, various facets of social support, including emotional support, instrumental/tangible support, informational support, and appraisal support, are associated with improved self-care.17 For example, the levels of family functioning and family support predict HF outcomes, perhaps because between-appointment monitoring allows patients to report problems that might otherwise go unidentified and receive more external feedback about their disease and symptoms.18,19 Patients report that family members or especially supportive members of their health care team are invaluable contributors to their successful management of HF.20 A recently published feasibility trial of a couple-based disease management program observed positive trends in HF management for veterans, as well as improvements in caregiver’s depressive symptoms and burden, indicating that even support from informal caregivers may improve HF outcomes.21
Advances in technology-delivered disease management programs show promise in improving adherence to chronic disease management programs.22,23 Specifically for HF, veterans who enrolled in a daily telehealth intervention employing daily vital signs and symptom reporting, automated reminders and tips for self-management, and proactive monitoring and intervention telephone calls from a nurse successfully lowered their blood pressure, lost weight, reduced their HF medication dosages, and spent 80% fewer days in the hospital.24 Among patients with coronary artery disease, a text messaging service was shown to improve a number of cardiovascular risk factors.25 Moreover, mobile applications can be used to support informal caregivers of patients with HF.26 To the authors knowledge, no research studies have been conducted using text messaging or mobile applications among veterans with HF.
Limitations
Some limitations of the present study warrant discussion. First, as discussed earlier, patients were not randomized to the treatment arms. Second, veterans are referred to the HF clinic only after being hospitalized for HF. As a result, all the referred veterans likely were experiencing more advanced disease and/or low adherence, and these results may not be representative of patients with less advanced disease. Finally, the sample used in the present analysis was a small, homogeneous group of 91 male veterans who were 85% black and 95% non-Hispanic. These demographics are largely representative of the JBVAMC. Therefore, the present results may not be generalizable to more racially or ethnically diverse populations, women, or nonveterans.
Conclusion
Minimizing rehospitalization rates for patients with HF continues to be a priority. Health care costs of HF are more than double those of patients in the general population, primarily due to hospitalizations—in 2013, HF hospitalization costs in the U.S. exceeded $10 billion.27,28 Given the current emphasis on economical, patient-centered care, SMAs may be a cost-effective alternative to individualized disease management plans while continuing to allow providers to tailor treatment to individual patient needs.
Although this study did not find better outcomes among veterans whose specialty HF care was augmented by clinic-based SMAs, the authors believe that this type of program has great potential. Heart failure SMAs may improve HF outcomes, enhance efficiency of health care delivery, and reduce overall HF-associated health care costs if it is integrated earlier along the continuum of care or if other novel components, such as caregiver support or technology-based delivery, is included. Further studies are needed to systematically evaluate HF management programs delivered via SMAs to improve outcomes and reduce the economic burden that HF places on the health care system.
1. Boccuti C, Casillas G. Aiming for fewer hospital U-turns: the Medicare hospital readmissions reduction program. http://kff.org/medicare/issue-brief/aiming-for-fewer-hospital-u-turns-the-medicare-hospital-readmission-reduction-program/. Published September 30, 2016. Accessed March 6, 2017.
2. Barrett ML, Wier LM, Jiang HJ, Steiner CA. All-cause readmissions by payer and age, 2009-2013. Statistical Brief #199. https://www.hcup-us.ahrq .gov/reports/statbriefs/sb199-Readmissions-Payer -Age.jsp. Published December 2015. Accessed March 6, 2017.
3. Fingar K, Washington R. Trends in hospital readmissions for four high-volume conditions, 2009-2013. Statistical Brief #196. https://www.hcup-us .ahrq.gov/reports/statbriefs/sb196-Readmissions -Trends-High-Volume-Conditions.jsp. Published November 2015. Accessed March 6, 2017.
4. McMurray JJ, Adamopoulos S, Anker SD, et al; ESC Committee for Practice Guidelines. ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: the Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2012;33(14):1787-1847.
5. Wakefield BJ, Boren SA, Groves PS, Conn VS. Heart failure care management programs: a review of study interventions and meta-analysis of outcomes. J Cardiovasc Nurs. 2013;28(1):8-19.
6. Smith CE, Piamjariyakul U, Wick JA, et al. Multidisciplinary group clinic appointments: the Self-Management and Care of Heart Failure (SMAC-HF) trial. Circ Heart Fail. 2014;7(6):888-894.
7. Sochalski J, Jaarsma T, Krumholz HM, et al. What works in chronic care management: the case of heart failure. Health Aff (Millwood). 2009;28(1): 179-189.
8. Ågren S, Evangelista LS, Davidson T, Strömberg A. Cost-effectiveness of a nurse-led education and psychosocial programme for patients with chronic heart failure and their partners. J Clin Nurs. 2013;22(15-16):2347-2353.
9. Moser DK, Robinson S, Biddle MJ, et al. Health literacy predicts morbidity and mortality in rural patients with heart failure. J Card Fail. 2015;21(8):612-618.
10. McNaughton CD, Cawthon C, Kripalani S, Liu D, Storrow AB, Roumie CL. Health literacy and mortality: a cohort study of patients hospitalized for acute heart failure. J Am Heart Assoc. 2015;4(6). pii:e001799.
11. Nelson KM, Starkebaum GA, Reiber GE. Veterans using and uninsured veterans not using Veterans Affairs (VA) health care. Public Health Rep. 2007;122(1):93-100.
12. Whellan DJ, Hasselblad V, Peterson E, O’Connor CM, Schulman KA. Metaanalysis and review of heart failure disease management randomized controlled clinical trials. Am Heart J. 2005;149(4):722-729.
13. Bekelman DB, Plomondon ME, Carey EP, et al. Primary results of the Patient-Centered Disease Management (PCDM) for Heart Failure Study: a randomized clinical trial. JAMA Intern Med. 2015;175(5):725-732.
14. Vitry AI, Nguyen TA, Ramsay EN, et al. General practitioner management plans delaying time to next potentially preventable hospitalisation for patients with heart failure. Intern Med J. 2014;44(11):1117-1123.
15. Lowery J, Hopp F, Subramanian U, et al. Evaluation of a nurse practitioner disease management model for chronic heart failure: a multi-site implementation study. Congest Heart Fail. 2012;18(1):64-71.
16. Edelman D, McDuffie JR, Oddone E, Gierisch JM, Nagi A, Williams JW Jr. Shared medical appointments for chronic medical conditions: a systematic review. VAESP Project #09-010. http://www.hsrd.research.va.gov/publications/esp/shared -med- appt-REPORT.pdf. Published July 2012. Accessed March 6, 2017.
17. Graven LJ, Grant JS. Social support and self-care behaviors in individuals with heart failure: an integrative review. Int J Nurs Stud. 2014;51(2):320-333.
18. Dunbar SB, Clark PC, Quinn C, Gary RA, Kaslow NJ. Family influences on heart failure self-care and outcomes. J Cardiovasc Nurs. 2008;23(3):258-265.
19. Piette JD, Gregor MA, Share D, et al. Improving heart failure self-management support by actively engaging out-of-home caregivers: results of a feasibility study. Congest Heart Fail. 2008;14(1):12-18.
20. Skaperdas E, Tuepker A, Nicolaidis C, Robb JK, Kansagara D, Hickam DH. Congestive heart failure self-management among US veterans: the role of personal and professional advocates. Patient Educ Couns. 2014;95(3):371-377.
21. Trivedi R, Slightam C, Fan VS, et al. A couples’ based self-management program for heart failure: results of a feasibility study. Front Public Health. 2016;4:171.
22. Hamine S, Gerth-Guyette E, Faulx D, Green BB, Ginsburg SA. Impact of mHealth chronic disease management on treatment adherence and patient outcomes: a systematic review. J Med Internet Res. 2015;17(2):e52.
23. Free C, Phillips G, Galli L, et al. The effectiveness of mobile-health technology-based health behaviour change or disease management interventions for health care consumers: a systematic review. PLoS Med. 2013;10(1):e1001362.
24. Schofield RS, Kline SE, Schmalfuss CM, et al. Early outcomes of a care coordination-enhanced telehome care program for elderly veterans with chronic heart failure. Telemed J E Health. 2005;11(1):20-27.
25. Chow CK, Redfern J, Hillis GS, et al. Effect of lifestyle-focused text messaging on risk factor modification in patients with coronary heart disease: a randomized clinical trial. JAMA. 2015;314(12):1255-1263.
26. Piette JD, Striplin D, Marinec N, et al. A mobile health intervention supporting heart failure patients and their informal caregivers: a randomized comparative efficacy trial. J Med Internet Res. 2015;17(6):e142.
27. Mejhert M, Lindgren P, Schill O, Edner M, Persson H, Kahan T. Long term health care consumption and cost expenditure in systolic heart failure. Eur J Intern Med. 2013;24(3):260-265.
28. Torio CM, Moore BJ. National inpatient hospital costs: the most expensive conditions by payer, 2013. Statistical Brief #204. https://www.hcup-us.ahrq.gov/reports/statbriefs/sb204-Most-Expensive-Hospital-Conditions.jsp. Published May 2016. Accessed March 6, 2017.
Rising health care costs have led to threats of nonreimbursement for rehospitalization within 30 days postdischarge.1,2 Heart failure (HF) in particular is characterized by the highest 30-day rehospitalization rate (23.5% in 2013), which accounts for more than two-thirds of HF expenditures.3,4
Much of HF-related health care costs can be addressed with effective self-management by patients with HF. Therefore, developing and implementing effective disease management programs for this high-risk patient population is essential. Heart failure management programs may include optimizing HF medications, improving patient understanding of the importance of appropriate diet and physical activity, and cultivating psychological health and well-being. In a 2013 systematic review and meta-analysis, Wakefield and colleagues found that disease management programs improved nearly all HF outcomes, including lower mortality rates, lower hospital readmission rates, fewer clinic visits, higher satisfaction with care, and higher quality of life, compared with a no-treatment control or standard care.5 Moreover, these programs demonstrated cost-effectiveness by reducing HF-related hospitalizations and health care expenditures.5
One method to deliver specialized disease management programs to a greater number of patients may be to use shared medical appointments (SMAs). In a randomized controlled trial, Smith and colleagues demonstrated improved HF outcomes through 7 months among veterans who attended SMAs for HF management.6 However, the trial enrolled only 25% of patients screened, and 63% of the patients who did not enroll were classified as not interested. These findings suggest that patients with HF, and veterans in particular, may face additional barriers to enrolling in HF management programs, and these results may not be fully representative of veterans with HF.
In this study, the authors used a naturalistic study design via retrospective review of the electronic health record (EHR) to evaluate whether patients with acute HF who chose to attend SMAs promoting self-management skills for HF would have better hospitalization outcomes compared with those who received individual disease management instructions in a HF specialty clinic (ie, usual care). The authors hypothesized that veterans who participated in the HF SMA clinic would have fewer 12-month HF-related and all-cause hospitalizations, fewer days in the hospital, and more days to first hospitalization compared with patients in usual care.
Methods
The clinic for veterans with acute HF was initiated in October, 2010 at the Jesse Brown VAMC (JBVAMC) in Chicago, Illinois, to reduce readmissions by targeting patients who had been previously hospitalized for HF. In September 2011, the multidisciplinary SMA clinic was developed within the HF clinic to provide enhanced care focused on self-management strategies for patients with HF. The HF SMA program comprised 4 weekly face-to-face sessions co-led by a nurse practitioner (NP), a dietitian, and a clinical psychologist, similar to what has been shown to be successful and cost-effective in nonveteran populations.6-8 Patients attended at least 4 sessions before graduating to the advanced HF SMA program where they could attend monthly booster sessions. The program promoted self-management by providing education about and support for the HF process, HF medications, diet adherence, physical activity, psychological well-being, and stress management via interactive presentations. During the visit, patients’ medication and food logs were reviewed. Patients were encouraged to discuss successes and obstacles in achieving their goals. All study procedures were approved by the institutional review board at JBVAMC.
Study Design
Data were collected by retrospective review of the JBVAMC EHR. The EHR was reviewed for all veterans scheduled for ≥ 1 SMA clinic visit within the HF specialty clinic using predetermined, convenient selection between January 1, 2012, and December 31, 2013. Outcome data were collected through 12-month follow-up (through December 31, 2014).
Patients in both treatment arms received HF care through the HF clinic, including one-on-one education regarding HF self-management provided by a NP. Patients were assigned to the HF SMA group if they also attended the HF SMA clinic within 3 months of their initial HF clinic consult. The number of SMAs attended was included as a covariate in the models. Patients who were scheduled for, but did not attend, the HF SMA clinic were assigned to the HF clinic group. Patients who attended the initial HF consult before September 1, 2011, were excluded, thereby ensuring that all patients included in the present analyses had the opportunity to attend the HF SMA appointment within the predetermined period of chart review.
Data for all VA hospitalizations that occurred between January 1, 2012 and December 31, 2014, were extracted from the EHR. Extracted data included admission date, discharge date, and discharge diagnoses. From these data, the authors assessed 4 hospitalization outcomes for each HF hospitalization and all-cause hospitalization within 12 months of the initial HF clinic consult date: hospitalization (yes/no), number of hospitalizations, number of days in the hospital, and days to first hospitalization.
Data Analysis
Demographic, HF characteristics, and HF outcome variables for the HF SMA and HF clinic groups were compared using t tests and chi-square analyses. Logistic regressions were used to predict 12-month hospitalization, linear regressions were used to predict number of hospitalizations and number of days hospitalized, and Cox proportional hazards regressions were used to predict time from initial HF consult to first hospitalization for each HF-related hospitalization variable and all-cause hospitalization variable. A separate logistic regression was conducted to predict 12-month all-cause mortality. The primary predictor variable of interest for all models was group membership (HF SMA vs HF clinic). Covariates in all models included race (black vs nonblack), ethnicity (Hispanic/Latino vs non-Hispanic/Latino), age, and number of HF SMAs attended.
Results
Of 709 HF SMA clinic appointments made for 141 patients between January 1, 2012, and December 31, 2013, 54 patients were assigned to the HF SMA group and 37 patients were assigned to the HF clinic group (Figure). The majority of the sample was black (87%), non-Hispanic/Latino (96%), and the average age was 68 years. Patients were more likely to have nonischemic (rather than ischemic) cardiomyopathy (66%) and more likely to have HF with reduced (rather than preserved) ejection fraction (76%; ie, systolic HF). Furthermore, 40% of the sample was diagnosed with atrial fibrillation (AF) or atrial flutter (A-flutter), and 24% had an implantable cardioverter-defibrillator or pacemaker. There were no significant differences in demographics or HF characteristics between the HF SMA group and the HF clinic group (Table).
HF Hospitalization Outcomes
During the 12-month follow-up, 32 patients were hospitalized for HF, 18 (33.3%) in the SMA group and 14 (37.8%) in the HF clinic group, P = .658. Patients were hospitalized up to 4 times for between 1 and 38 days, and from 1 to 352 days postconsult. No differences between the HF SMA group and HF clinic group were observed on any of the HF hospitalization outcomes (Table). Group membership did not predict HF hospitalization (odds ratio [OR]: 0.39, 95% confidence interval [CI]: 0.11-1.42), number of HF hospitalizations (β: 0.15, SE: 0.29), number of days hospitalized for HF (β: 0.1.66, SE: 2.01), or time to first HF hospitalization (hazard ratio [HR]: 1.35, 95% CI: 0.66-2.77), all Ps > .10. In the Cox proportional hazards regression predicting time to HF hospitalization, the coefficients did not converge when the model included demographic covariates; therefore, the model was run only with HF group as a predictor variable. For all other models, no covariates significantly predicted HF hospitalization outcomes.
All-Cause Hospitalization Outcomes
During the 12-month follow-up, 57 patients were hospitalized for any cause (including HF hospitalizations), 32 (59.3%) in the SMA group and 25 (67.6%) in the HF clinic group, P = .421. Patients were hospitalized up to 6 times for between 1 and 106 days and from 1 to 352 days postconsult. No differences were observed between the groups on any of the all-cause hospitalization outcomes (Table). Group membership did not predict all-cause hospitalization (OR: 0.34, 95% CI: 0.10-1.19), number of all-cause hospitalizations (β: 0.49, SE: 0.41), number of days hospitalized for any cause (β: 5.15, SE: 5.15), or time to first all-cause hospitalization (HR: 0.98, 95% CI: 0.56-1.72), all P > .05. None of the covariates predicted any of the all-cause hospitalization outcomes.
All-Cause Mortality Outcomes
During the 12-month follow-up, 14 patients (15%) died of any cause, 8 (15%) in the SMA group and 6 (16%) in the HF clinic group, P = .856. Group membership did not predict all-cause mortality (OR: 2.32, 95% CI: 0.44-12.18), and likewise none of the covariates were associated with 12-month all-cause mortality.
Discussion
This study was a naturalistic, retrospective examination of a HF management program promoting self-management delivered via multidisciplinary SMAs among veterans who enrolled in an acute HF specialty clinic. The authors’ hypothesis was not supported: patients who attended the HF SMA clinic did not have lower 12-month hospitalization or mortality rates, shorter hospital stays, or longer time to hospitalization compared with patients in the HF clinic only.
In contrast to the patient-centered approach of this study, a randomized trial delivering a similar disease management program found that patients with acute HF in the SMA group had better short-term (< 7 months) hospitalization outcomes, specifically greater time to first HF-related hospitalization (HR 0.45, 95% CI: 0.21-0.98), but this effect did not last through 12 months when compared with patients in standard care.6 These disparate findings may be explained by the gap in bench-to-bedside research, where despite scientific evidence indicating better outcomes among patients randomized to an intervention, when patients are given a choice, they may not choose to engage in the best option for their HF treatment.
In the present study, veterans who chose not to attend the HF SMA clinic may have done so for numerous reasons that may have influenced the outcomes. For example, those veterans who did not attend the HF SMA clinic may have had higher health literacy and less need for an educational program. Health literacy has been inversely associated with HF outcomes, such that patients with HF with lower health literacy have greater risk of HF rehospitalization or mortality.9,10 In addition, many of the veterans who were followed in the HF clinic were taught the same disease management strategies by the NP during one-on-one visits, and they may have gained the same self-management skills in a different setting.
Another possibility is that the veterans enrolled in the HF clinic were less likely to be followed exclusively at the VA and therefore may have had external hospitalizations not recorded in their VA health records. In 2000, more than half the veterans who received health care services at the VA reported that they did not receive their care exclusively at the VA.11 This may be especially true since the Veteran’s Choice Program permits veterans who reside > 40 miles from a VA hospital to receive care closer to home.
Disease Management Programs
Disease management programs for HF in general promote better outcomes and lower health care expenditures.5,12 Self-management instruction delivered via SMAs may have greater potential for reducing HF-associated health care costs if it were to be integrated earlier in the continuum of care. The sample in this study was composed of veterans who were referred to a specialty clinic only after being hospitalized for HF. These patients likely were experiencing more advanced disease and/or low adherence, as indicated by the relatively high prevalence of AF diagnoses and pacemakers; these null findings are consistent with those from a randomized controlled trial of a disease management program among veterans with heavy HF symptom burden and impaired functional status.13 However, integrating self-management programs earlier in HF clinical care (eg, primary care or cardiology clinics) may be more effective in promoting proactive disease management and delaying or preventing initial HF hospitalizations.
For example, a disease management plan implemented by general practitioners for veterans with HF in Australia was associated with a 23% reduction in potentially preventable hospitalization rates.14 Veterans with HF enrolled in a NP-led disease management intervention, relative to those followed only in primary care, had significantly fewer hospitalizations and nearly half the risk mortality (15% vs 28% after 2 years; HR 0.55).15 Furthermore, some have suggested that SMAs may be more effective for patients for whom risk of disease is high but current disease burden (ie, symptoms) is low, such as diabetes mellitus management programs.16 Early intervention also may allow providers to reach more patients more quickly and before they experience advanced symptoms, thereby reducing specialty clinic wait times and overall health expenditures. Developing more effective disease management programs for patients with acute HF and veterans in particular remains a critical matter for future study.
Additional and novel components of HF management programs show promise for future interventions. First, various facets of social support, including emotional support, instrumental/tangible support, informational support, and appraisal support, are associated with improved self-care.17 For example, the levels of family functioning and family support predict HF outcomes, perhaps because between-appointment monitoring allows patients to report problems that might otherwise go unidentified and receive more external feedback about their disease and symptoms.18,19 Patients report that family members or especially supportive members of their health care team are invaluable contributors to their successful management of HF.20 A recently published feasibility trial of a couple-based disease management program observed positive trends in HF management for veterans, as well as improvements in caregiver’s depressive symptoms and burden, indicating that even support from informal caregivers may improve HF outcomes.21
Advances in technology-delivered disease management programs show promise in improving adherence to chronic disease management programs.22,23 Specifically for HF, veterans who enrolled in a daily telehealth intervention employing daily vital signs and symptom reporting, automated reminders and tips for self-management, and proactive monitoring and intervention telephone calls from a nurse successfully lowered their blood pressure, lost weight, reduced their HF medication dosages, and spent 80% fewer days in the hospital.24 Among patients with coronary artery disease, a text messaging service was shown to improve a number of cardiovascular risk factors.25 Moreover, mobile applications can be used to support informal caregivers of patients with HF.26 To the authors knowledge, no research studies have been conducted using text messaging or mobile applications among veterans with HF.
Limitations
Some limitations of the present study warrant discussion. First, as discussed earlier, patients were not randomized to the treatment arms. Second, veterans are referred to the HF clinic only after being hospitalized for HF. As a result, all the referred veterans likely were experiencing more advanced disease and/or low adherence, and these results may not be representative of patients with less advanced disease. Finally, the sample used in the present analysis was a small, homogeneous group of 91 male veterans who were 85% black and 95% non-Hispanic. These demographics are largely representative of the JBVAMC. Therefore, the present results may not be generalizable to more racially or ethnically diverse populations, women, or nonveterans.
Conclusion
Minimizing rehospitalization rates for patients with HF continues to be a priority. Health care costs of HF are more than double those of patients in the general population, primarily due to hospitalizations—in 2013, HF hospitalization costs in the U.S. exceeded $10 billion.27,28 Given the current emphasis on economical, patient-centered care, SMAs may be a cost-effective alternative to individualized disease management plans while continuing to allow providers to tailor treatment to individual patient needs.
Although this study did not find better outcomes among veterans whose specialty HF care was augmented by clinic-based SMAs, the authors believe that this type of program has great potential. Heart failure SMAs may improve HF outcomes, enhance efficiency of health care delivery, and reduce overall HF-associated health care costs if it is integrated earlier along the continuum of care or if other novel components, such as caregiver support or technology-based delivery, is included. Further studies are needed to systematically evaluate HF management programs delivered via SMAs to improve outcomes and reduce the economic burden that HF places on the health care system.
Rising health care costs have led to threats of nonreimbursement for rehospitalization within 30 days postdischarge.1,2 Heart failure (HF) in particular is characterized by the highest 30-day rehospitalization rate (23.5% in 2013), which accounts for more than two-thirds of HF expenditures.3,4
Much of HF-related health care costs can be addressed with effective self-management by patients with HF. Therefore, developing and implementing effective disease management programs for this high-risk patient population is essential. Heart failure management programs may include optimizing HF medications, improving patient understanding of the importance of appropriate diet and physical activity, and cultivating psychological health and well-being. In a 2013 systematic review and meta-analysis, Wakefield and colleagues found that disease management programs improved nearly all HF outcomes, including lower mortality rates, lower hospital readmission rates, fewer clinic visits, higher satisfaction with care, and higher quality of life, compared with a no-treatment control or standard care.5 Moreover, these programs demonstrated cost-effectiveness by reducing HF-related hospitalizations and health care expenditures.5
One method to deliver specialized disease management programs to a greater number of patients may be to use shared medical appointments (SMAs). In a randomized controlled trial, Smith and colleagues demonstrated improved HF outcomes through 7 months among veterans who attended SMAs for HF management.6 However, the trial enrolled only 25% of patients screened, and 63% of the patients who did not enroll were classified as not interested. These findings suggest that patients with HF, and veterans in particular, may face additional barriers to enrolling in HF management programs, and these results may not be fully representative of veterans with HF.
In this study, the authors used a naturalistic study design via retrospective review of the electronic health record (EHR) to evaluate whether patients with acute HF who chose to attend SMAs promoting self-management skills for HF would have better hospitalization outcomes compared with those who received individual disease management instructions in a HF specialty clinic (ie, usual care). The authors hypothesized that veterans who participated in the HF SMA clinic would have fewer 12-month HF-related and all-cause hospitalizations, fewer days in the hospital, and more days to first hospitalization compared with patients in usual care.
Methods
The clinic for veterans with acute HF was initiated in October, 2010 at the Jesse Brown VAMC (JBVAMC) in Chicago, Illinois, to reduce readmissions by targeting patients who had been previously hospitalized for HF. In September 2011, the multidisciplinary SMA clinic was developed within the HF clinic to provide enhanced care focused on self-management strategies for patients with HF. The HF SMA program comprised 4 weekly face-to-face sessions co-led by a nurse practitioner (NP), a dietitian, and a clinical psychologist, similar to what has been shown to be successful and cost-effective in nonveteran populations.6-8 Patients attended at least 4 sessions before graduating to the advanced HF SMA program where they could attend monthly booster sessions. The program promoted self-management by providing education about and support for the HF process, HF medications, diet adherence, physical activity, psychological well-being, and stress management via interactive presentations. During the visit, patients’ medication and food logs were reviewed. Patients were encouraged to discuss successes and obstacles in achieving their goals. All study procedures were approved by the institutional review board at JBVAMC.
Study Design
Data were collected by retrospective review of the JBVAMC EHR. The EHR was reviewed for all veterans scheduled for ≥ 1 SMA clinic visit within the HF specialty clinic using predetermined, convenient selection between January 1, 2012, and December 31, 2013. Outcome data were collected through 12-month follow-up (through December 31, 2014).
Patients in both treatment arms received HF care through the HF clinic, including one-on-one education regarding HF self-management provided by a NP. Patients were assigned to the HF SMA group if they also attended the HF SMA clinic within 3 months of their initial HF clinic consult. The number of SMAs attended was included as a covariate in the models. Patients who were scheduled for, but did not attend, the HF SMA clinic were assigned to the HF clinic group. Patients who attended the initial HF consult before September 1, 2011, were excluded, thereby ensuring that all patients included in the present analyses had the opportunity to attend the HF SMA appointment within the predetermined period of chart review.
Data for all VA hospitalizations that occurred between January 1, 2012 and December 31, 2014, were extracted from the EHR. Extracted data included admission date, discharge date, and discharge diagnoses. From these data, the authors assessed 4 hospitalization outcomes for each HF hospitalization and all-cause hospitalization within 12 months of the initial HF clinic consult date: hospitalization (yes/no), number of hospitalizations, number of days in the hospital, and days to first hospitalization.
Data Analysis
Demographic, HF characteristics, and HF outcome variables for the HF SMA and HF clinic groups were compared using t tests and chi-square analyses. Logistic regressions were used to predict 12-month hospitalization, linear regressions were used to predict number of hospitalizations and number of days hospitalized, and Cox proportional hazards regressions were used to predict time from initial HF consult to first hospitalization for each HF-related hospitalization variable and all-cause hospitalization variable. A separate logistic regression was conducted to predict 12-month all-cause mortality. The primary predictor variable of interest for all models was group membership (HF SMA vs HF clinic). Covariates in all models included race (black vs nonblack), ethnicity (Hispanic/Latino vs non-Hispanic/Latino), age, and number of HF SMAs attended.
Results
Of 709 HF SMA clinic appointments made for 141 patients between January 1, 2012, and December 31, 2013, 54 patients were assigned to the HF SMA group and 37 patients were assigned to the HF clinic group (Figure). The majority of the sample was black (87%), non-Hispanic/Latino (96%), and the average age was 68 years. Patients were more likely to have nonischemic (rather than ischemic) cardiomyopathy (66%) and more likely to have HF with reduced (rather than preserved) ejection fraction (76%; ie, systolic HF). Furthermore, 40% of the sample was diagnosed with atrial fibrillation (AF) or atrial flutter (A-flutter), and 24% had an implantable cardioverter-defibrillator or pacemaker. There were no significant differences in demographics or HF characteristics between the HF SMA group and the HF clinic group (Table).
HF Hospitalization Outcomes
During the 12-month follow-up, 32 patients were hospitalized for HF, 18 (33.3%) in the SMA group and 14 (37.8%) in the HF clinic group, P = .658. Patients were hospitalized up to 4 times for between 1 and 38 days, and from 1 to 352 days postconsult. No differences between the HF SMA group and HF clinic group were observed on any of the HF hospitalization outcomes (Table). Group membership did not predict HF hospitalization (odds ratio [OR]: 0.39, 95% confidence interval [CI]: 0.11-1.42), number of HF hospitalizations (β: 0.15, SE: 0.29), number of days hospitalized for HF (β: 0.1.66, SE: 2.01), or time to first HF hospitalization (hazard ratio [HR]: 1.35, 95% CI: 0.66-2.77), all Ps > .10. In the Cox proportional hazards regression predicting time to HF hospitalization, the coefficients did not converge when the model included demographic covariates; therefore, the model was run only with HF group as a predictor variable. For all other models, no covariates significantly predicted HF hospitalization outcomes.
All-Cause Hospitalization Outcomes
During the 12-month follow-up, 57 patients were hospitalized for any cause (including HF hospitalizations), 32 (59.3%) in the SMA group and 25 (67.6%) in the HF clinic group, P = .421. Patients were hospitalized up to 6 times for between 1 and 106 days and from 1 to 352 days postconsult. No differences were observed between the groups on any of the all-cause hospitalization outcomes (Table). Group membership did not predict all-cause hospitalization (OR: 0.34, 95% CI: 0.10-1.19), number of all-cause hospitalizations (β: 0.49, SE: 0.41), number of days hospitalized for any cause (β: 5.15, SE: 5.15), or time to first all-cause hospitalization (HR: 0.98, 95% CI: 0.56-1.72), all P > .05. None of the covariates predicted any of the all-cause hospitalization outcomes.
All-Cause Mortality Outcomes
During the 12-month follow-up, 14 patients (15%) died of any cause, 8 (15%) in the SMA group and 6 (16%) in the HF clinic group, P = .856. Group membership did not predict all-cause mortality (OR: 2.32, 95% CI: 0.44-12.18), and likewise none of the covariates were associated with 12-month all-cause mortality.
Discussion
This study was a naturalistic, retrospective examination of a HF management program promoting self-management delivered via multidisciplinary SMAs among veterans who enrolled in an acute HF specialty clinic. The authors’ hypothesis was not supported: patients who attended the HF SMA clinic did not have lower 12-month hospitalization or mortality rates, shorter hospital stays, or longer time to hospitalization compared with patients in the HF clinic only.
In contrast to the patient-centered approach of this study, a randomized trial delivering a similar disease management program found that patients with acute HF in the SMA group had better short-term (< 7 months) hospitalization outcomes, specifically greater time to first HF-related hospitalization (HR 0.45, 95% CI: 0.21-0.98), but this effect did not last through 12 months when compared with patients in standard care.6 These disparate findings may be explained by the gap in bench-to-bedside research, where despite scientific evidence indicating better outcomes among patients randomized to an intervention, when patients are given a choice, they may not choose to engage in the best option for their HF treatment.
In the present study, veterans who chose not to attend the HF SMA clinic may have done so for numerous reasons that may have influenced the outcomes. For example, those veterans who did not attend the HF SMA clinic may have had higher health literacy and less need for an educational program. Health literacy has been inversely associated with HF outcomes, such that patients with HF with lower health literacy have greater risk of HF rehospitalization or mortality.9,10 In addition, many of the veterans who were followed in the HF clinic were taught the same disease management strategies by the NP during one-on-one visits, and they may have gained the same self-management skills in a different setting.
Another possibility is that the veterans enrolled in the HF clinic were less likely to be followed exclusively at the VA and therefore may have had external hospitalizations not recorded in their VA health records. In 2000, more than half the veterans who received health care services at the VA reported that they did not receive their care exclusively at the VA.11 This may be especially true since the Veteran’s Choice Program permits veterans who reside > 40 miles from a VA hospital to receive care closer to home.
Disease Management Programs
Disease management programs for HF in general promote better outcomes and lower health care expenditures.5,12 Self-management instruction delivered via SMAs may have greater potential for reducing HF-associated health care costs if it were to be integrated earlier in the continuum of care. The sample in this study was composed of veterans who were referred to a specialty clinic only after being hospitalized for HF. These patients likely were experiencing more advanced disease and/or low adherence, as indicated by the relatively high prevalence of AF diagnoses and pacemakers; these null findings are consistent with those from a randomized controlled trial of a disease management program among veterans with heavy HF symptom burden and impaired functional status.13 However, integrating self-management programs earlier in HF clinical care (eg, primary care or cardiology clinics) may be more effective in promoting proactive disease management and delaying or preventing initial HF hospitalizations.
For example, a disease management plan implemented by general practitioners for veterans with HF in Australia was associated with a 23% reduction in potentially preventable hospitalization rates.14 Veterans with HF enrolled in a NP-led disease management intervention, relative to those followed only in primary care, had significantly fewer hospitalizations and nearly half the risk mortality (15% vs 28% after 2 years; HR 0.55).15 Furthermore, some have suggested that SMAs may be more effective for patients for whom risk of disease is high but current disease burden (ie, symptoms) is low, such as diabetes mellitus management programs.16 Early intervention also may allow providers to reach more patients more quickly and before they experience advanced symptoms, thereby reducing specialty clinic wait times and overall health expenditures. Developing more effective disease management programs for patients with acute HF and veterans in particular remains a critical matter for future study.
Additional and novel components of HF management programs show promise for future interventions. First, various facets of social support, including emotional support, instrumental/tangible support, informational support, and appraisal support, are associated with improved self-care.17 For example, the levels of family functioning and family support predict HF outcomes, perhaps because between-appointment monitoring allows patients to report problems that might otherwise go unidentified and receive more external feedback about their disease and symptoms.18,19 Patients report that family members or especially supportive members of their health care team are invaluable contributors to their successful management of HF.20 A recently published feasibility trial of a couple-based disease management program observed positive trends in HF management for veterans, as well as improvements in caregiver’s depressive symptoms and burden, indicating that even support from informal caregivers may improve HF outcomes.21
Advances in technology-delivered disease management programs show promise in improving adherence to chronic disease management programs.22,23 Specifically for HF, veterans who enrolled in a daily telehealth intervention employing daily vital signs and symptom reporting, automated reminders and tips for self-management, and proactive monitoring and intervention telephone calls from a nurse successfully lowered their blood pressure, lost weight, reduced their HF medication dosages, and spent 80% fewer days in the hospital.24 Among patients with coronary artery disease, a text messaging service was shown to improve a number of cardiovascular risk factors.25 Moreover, mobile applications can be used to support informal caregivers of patients with HF.26 To the authors knowledge, no research studies have been conducted using text messaging or mobile applications among veterans with HF.
Limitations
Some limitations of the present study warrant discussion. First, as discussed earlier, patients were not randomized to the treatment arms. Second, veterans are referred to the HF clinic only after being hospitalized for HF. As a result, all the referred veterans likely were experiencing more advanced disease and/or low adherence, and these results may not be representative of patients with less advanced disease. Finally, the sample used in the present analysis was a small, homogeneous group of 91 male veterans who were 85% black and 95% non-Hispanic. These demographics are largely representative of the JBVAMC. Therefore, the present results may not be generalizable to more racially or ethnically diverse populations, women, or nonveterans.
Conclusion
Minimizing rehospitalization rates for patients with HF continues to be a priority. Health care costs of HF are more than double those of patients in the general population, primarily due to hospitalizations—in 2013, HF hospitalization costs in the U.S. exceeded $10 billion.27,28 Given the current emphasis on economical, patient-centered care, SMAs may be a cost-effective alternative to individualized disease management plans while continuing to allow providers to tailor treatment to individual patient needs.
Although this study did not find better outcomes among veterans whose specialty HF care was augmented by clinic-based SMAs, the authors believe that this type of program has great potential. Heart failure SMAs may improve HF outcomes, enhance efficiency of health care delivery, and reduce overall HF-associated health care costs if it is integrated earlier along the continuum of care or if other novel components, such as caregiver support or technology-based delivery, is included. Further studies are needed to systematically evaluate HF management programs delivered via SMAs to improve outcomes and reduce the economic burden that HF places on the health care system.
1. Boccuti C, Casillas G. Aiming for fewer hospital U-turns: the Medicare hospital readmissions reduction program. http://kff.org/medicare/issue-brief/aiming-for-fewer-hospital-u-turns-the-medicare-hospital-readmission-reduction-program/. Published September 30, 2016. Accessed March 6, 2017.
2. Barrett ML, Wier LM, Jiang HJ, Steiner CA. All-cause readmissions by payer and age, 2009-2013. Statistical Brief #199. https://www.hcup-us.ahrq .gov/reports/statbriefs/sb199-Readmissions-Payer -Age.jsp. Published December 2015. Accessed March 6, 2017.
3. Fingar K, Washington R. Trends in hospital readmissions for four high-volume conditions, 2009-2013. Statistical Brief #196. https://www.hcup-us .ahrq.gov/reports/statbriefs/sb196-Readmissions -Trends-High-Volume-Conditions.jsp. Published November 2015. Accessed March 6, 2017.
4. McMurray JJ, Adamopoulos S, Anker SD, et al; ESC Committee for Practice Guidelines. ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: the Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2012;33(14):1787-1847.
5. Wakefield BJ, Boren SA, Groves PS, Conn VS. Heart failure care management programs: a review of study interventions and meta-analysis of outcomes. J Cardiovasc Nurs. 2013;28(1):8-19.
6. Smith CE, Piamjariyakul U, Wick JA, et al. Multidisciplinary group clinic appointments: the Self-Management and Care of Heart Failure (SMAC-HF) trial. Circ Heart Fail. 2014;7(6):888-894.
7. Sochalski J, Jaarsma T, Krumholz HM, et al. What works in chronic care management: the case of heart failure. Health Aff (Millwood). 2009;28(1): 179-189.
8. Ågren S, Evangelista LS, Davidson T, Strömberg A. Cost-effectiveness of a nurse-led education and psychosocial programme for patients with chronic heart failure and their partners. J Clin Nurs. 2013;22(15-16):2347-2353.
9. Moser DK, Robinson S, Biddle MJ, et al. Health literacy predicts morbidity and mortality in rural patients with heart failure. J Card Fail. 2015;21(8):612-618.
10. McNaughton CD, Cawthon C, Kripalani S, Liu D, Storrow AB, Roumie CL. Health literacy and mortality: a cohort study of patients hospitalized for acute heart failure. J Am Heart Assoc. 2015;4(6). pii:e001799.
11. Nelson KM, Starkebaum GA, Reiber GE. Veterans using and uninsured veterans not using Veterans Affairs (VA) health care. Public Health Rep. 2007;122(1):93-100.
12. Whellan DJ, Hasselblad V, Peterson E, O’Connor CM, Schulman KA. Metaanalysis and review of heart failure disease management randomized controlled clinical trials. Am Heart J. 2005;149(4):722-729.
13. Bekelman DB, Plomondon ME, Carey EP, et al. Primary results of the Patient-Centered Disease Management (PCDM) for Heart Failure Study: a randomized clinical trial. JAMA Intern Med. 2015;175(5):725-732.
14. Vitry AI, Nguyen TA, Ramsay EN, et al. General practitioner management plans delaying time to next potentially preventable hospitalisation for patients with heart failure. Intern Med J. 2014;44(11):1117-1123.
15. Lowery J, Hopp F, Subramanian U, et al. Evaluation of a nurse practitioner disease management model for chronic heart failure: a multi-site implementation study. Congest Heart Fail. 2012;18(1):64-71.
16. Edelman D, McDuffie JR, Oddone E, Gierisch JM, Nagi A, Williams JW Jr. Shared medical appointments for chronic medical conditions: a systematic review. VAESP Project #09-010. http://www.hsrd.research.va.gov/publications/esp/shared -med- appt-REPORT.pdf. Published July 2012. Accessed March 6, 2017.
17. Graven LJ, Grant JS. Social support and self-care behaviors in individuals with heart failure: an integrative review. Int J Nurs Stud. 2014;51(2):320-333.
18. Dunbar SB, Clark PC, Quinn C, Gary RA, Kaslow NJ. Family influences on heart failure self-care and outcomes. J Cardiovasc Nurs. 2008;23(3):258-265.
19. Piette JD, Gregor MA, Share D, et al. Improving heart failure self-management support by actively engaging out-of-home caregivers: results of a feasibility study. Congest Heart Fail. 2008;14(1):12-18.
20. Skaperdas E, Tuepker A, Nicolaidis C, Robb JK, Kansagara D, Hickam DH. Congestive heart failure self-management among US veterans: the role of personal and professional advocates. Patient Educ Couns. 2014;95(3):371-377.
21. Trivedi R, Slightam C, Fan VS, et al. A couples’ based self-management program for heart failure: results of a feasibility study. Front Public Health. 2016;4:171.
22. Hamine S, Gerth-Guyette E, Faulx D, Green BB, Ginsburg SA. Impact of mHealth chronic disease management on treatment adherence and patient outcomes: a systematic review. J Med Internet Res. 2015;17(2):e52.
23. Free C, Phillips G, Galli L, et al. The effectiveness of mobile-health technology-based health behaviour change or disease management interventions for health care consumers: a systematic review. PLoS Med. 2013;10(1):e1001362.
24. Schofield RS, Kline SE, Schmalfuss CM, et al. Early outcomes of a care coordination-enhanced telehome care program for elderly veterans with chronic heart failure. Telemed J E Health. 2005;11(1):20-27.
25. Chow CK, Redfern J, Hillis GS, et al. Effect of lifestyle-focused text messaging on risk factor modification in patients with coronary heart disease: a randomized clinical trial. JAMA. 2015;314(12):1255-1263.
26. Piette JD, Striplin D, Marinec N, et al. A mobile health intervention supporting heart failure patients and their informal caregivers: a randomized comparative efficacy trial. J Med Internet Res. 2015;17(6):e142.
27. Mejhert M, Lindgren P, Schill O, Edner M, Persson H, Kahan T. Long term health care consumption and cost expenditure in systolic heart failure. Eur J Intern Med. 2013;24(3):260-265.
28. Torio CM, Moore BJ. National inpatient hospital costs: the most expensive conditions by payer, 2013. Statistical Brief #204. https://www.hcup-us.ahrq.gov/reports/statbriefs/sb204-Most-Expensive-Hospital-Conditions.jsp. Published May 2016. Accessed March 6, 2017.
1. Boccuti C, Casillas G. Aiming for fewer hospital U-turns: the Medicare hospital readmissions reduction program. http://kff.org/medicare/issue-brief/aiming-for-fewer-hospital-u-turns-the-medicare-hospital-readmission-reduction-program/. Published September 30, 2016. Accessed March 6, 2017.
2. Barrett ML, Wier LM, Jiang HJ, Steiner CA. All-cause readmissions by payer and age, 2009-2013. Statistical Brief #199. https://www.hcup-us.ahrq .gov/reports/statbriefs/sb199-Readmissions-Payer -Age.jsp. Published December 2015. Accessed March 6, 2017.
3. Fingar K, Washington R. Trends in hospital readmissions for four high-volume conditions, 2009-2013. Statistical Brief #196. https://www.hcup-us .ahrq.gov/reports/statbriefs/sb196-Readmissions -Trends-High-Volume-Conditions.jsp. Published November 2015. Accessed March 6, 2017.
4. McMurray JJ, Adamopoulos S, Anker SD, et al; ESC Committee for Practice Guidelines. ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: the Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2012;33(14):1787-1847.
5. Wakefield BJ, Boren SA, Groves PS, Conn VS. Heart failure care management programs: a review of study interventions and meta-analysis of outcomes. J Cardiovasc Nurs. 2013;28(1):8-19.
6. Smith CE, Piamjariyakul U, Wick JA, et al. Multidisciplinary group clinic appointments: the Self-Management and Care of Heart Failure (SMAC-HF) trial. Circ Heart Fail. 2014;7(6):888-894.
7. Sochalski J, Jaarsma T, Krumholz HM, et al. What works in chronic care management: the case of heart failure. Health Aff (Millwood). 2009;28(1): 179-189.
8. Ågren S, Evangelista LS, Davidson T, Strömberg A. Cost-effectiveness of a nurse-led education and psychosocial programme for patients with chronic heart failure and their partners. J Clin Nurs. 2013;22(15-16):2347-2353.
9. Moser DK, Robinson S, Biddle MJ, et al. Health literacy predicts morbidity and mortality in rural patients with heart failure. J Card Fail. 2015;21(8):612-618.
10. McNaughton CD, Cawthon C, Kripalani S, Liu D, Storrow AB, Roumie CL. Health literacy and mortality: a cohort study of patients hospitalized for acute heart failure. J Am Heart Assoc. 2015;4(6). pii:e001799.
11. Nelson KM, Starkebaum GA, Reiber GE. Veterans using and uninsured veterans not using Veterans Affairs (VA) health care. Public Health Rep. 2007;122(1):93-100.
12. Whellan DJ, Hasselblad V, Peterson E, O’Connor CM, Schulman KA. Metaanalysis and review of heart failure disease management randomized controlled clinical trials. Am Heart J. 2005;149(4):722-729.
13. Bekelman DB, Plomondon ME, Carey EP, et al. Primary results of the Patient-Centered Disease Management (PCDM) for Heart Failure Study: a randomized clinical trial. JAMA Intern Med. 2015;175(5):725-732.
14. Vitry AI, Nguyen TA, Ramsay EN, et al. General practitioner management plans delaying time to next potentially preventable hospitalisation for patients with heart failure. Intern Med J. 2014;44(11):1117-1123.
15. Lowery J, Hopp F, Subramanian U, et al. Evaluation of a nurse practitioner disease management model for chronic heart failure: a multi-site implementation study. Congest Heart Fail. 2012;18(1):64-71.
16. Edelman D, McDuffie JR, Oddone E, Gierisch JM, Nagi A, Williams JW Jr. Shared medical appointments for chronic medical conditions: a systematic review. VAESP Project #09-010. http://www.hsrd.research.va.gov/publications/esp/shared -med- appt-REPORT.pdf. Published July 2012. Accessed March 6, 2017.
17. Graven LJ, Grant JS. Social support and self-care behaviors in individuals with heart failure: an integrative review. Int J Nurs Stud. 2014;51(2):320-333.
18. Dunbar SB, Clark PC, Quinn C, Gary RA, Kaslow NJ. Family influences on heart failure self-care and outcomes. J Cardiovasc Nurs. 2008;23(3):258-265.
19. Piette JD, Gregor MA, Share D, et al. Improving heart failure self-management support by actively engaging out-of-home caregivers: results of a feasibility study. Congest Heart Fail. 2008;14(1):12-18.
20. Skaperdas E, Tuepker A, Nicolaidis C, Robb JK, Kansagara D, Hickam DH. Congestive heart failure self-management among US veterans: the role of personal and professional advocates. Patient Educ Couns. 2014;95(3):371-377.
21. Trivedi R, Slightam C, Fan VS, et al. A couples’ based self-management program for heart failure: results of a feasibility study. Front Public Health. 2016;4:171.
22. Hamine S, Gerth-Guyette E, Faulx D, Green BB, Ginsburg SA. Impact of mHealth chronic disease management on treatment adherence and patient outcomes: a systematic review. J Med Internet Res. 2015;17(2):e52.
23. Free C, Phillips G, Galli L, et al. The effectiveness of mobile-health technology-based health behaviour change or disease management interventions for health care consumers: a systematic review. PLoS Med. 2013;10(1):e1001362.
24. Schofield RS, Kline SE, Schmalfuss CM, et al. Early outcomes of a care coordination-enhanced telehome care program for elderly veterans with chronic heart failure. Telemed J E Health. 2005;11(1):20-27.
25. Chow CK, Redfern J, Hillis GS, et al. Effect of lifestyle-focused text messaging on risk factor modification in patients with coronary heart disease: a randomized clinical trial. JAMA. 2015;314(12):1255-1263.
26. Piette JD, Striplin D, Marinec N, et al. A mobile health intervention supporting heart failure patients and their informal caregivers: a randomized comparative efficacy trial. J Med Internet Res. 2015;17(6):e142.
27. Mejhert M, Lindgren P, Schill O, Edner M, Persson H, Kahan T. Long term health care consumption and cost expenditure in systolic heart failure. Eur J Intern Med. 2013;24(3):260-265.
28. Torio CM, Moore BJ. National inpatient hospital costs: the most expensive conditions by payer, 2013. Statistical Brief #204. https://www.hcup-us.ahrq.gov/reports/statbriefs/sb204-Most-Expensive-Hospital-Conditions.jsp. Published May 2016. Accessed March 6, 2017.
Military Sexual Trauma and Sexual Health: Practice and Future Research for Mental Health Professionals
About 24% of women and 1% of men will experience military sexual trauma (MST) during their service.1 Despite the higher percentage of women reporting MST, the estimated number of men (55,491) and women (72,497) who endorse MST is relatively similar. Military sexual trauma is associated with negative psychosocial (eg, decreased quality of life) and psychiatric (eg, posttraumatic stress disorder [PTSD], depression) sequelae. Surís and colleagues provided a full review of sequelae, with PTSD being the most discussed consequence of MST.2 However, sexually transmitted infections (STIs) during or after MST are a consequence of growing concern.
Sexually Transmitted Infections
The link between sexual trauma and increased incidence of STIs is well established. Survivors of rape are at a higher risk of exposure to STIs due to unprotected sexual contact that may occur during the assault(s).3 Numerous studies have demonstrated that sexual trauma is directly related to greater engagement in risky sexual behaviors (eg, more sexual partners, unprotected sex, and “sex trading”).2,4
This relationship is particularly concerning given that individuals in the military tend to report sexual trauma with greater propensity than that reported in civilian populations.4 Additionally, military personnel and veteran populations tend to engage in high-risk behaviors (eg, alcohol and drug use) more often than their civilian counterparts, increasing their potential susceptibility to predatory sexual trauma(s) and victimization.2,5 Taken in aggregate, military personnel and veterans may be at increased risk for STIs compared with the civilian population due to the increased incidence of risky sexual behavior and sexual traumatization during military service.
Sexually transmitted infections potentially lead to immediate-term (eg, physical discomfort, sexual dysfunction) and long-term (eg, cancer, infertility) adverse health consequences.6 Early detection is crucial in the treatment of STIs because it can aid in preventing STI transmission and allow for early intervention. See Table for a list of common STIs and their prevalence, testing method, method of sexual transmission, and treatment. Early detection is also important because many STIs may be asymptomatic (eg, HIV, human papillomavirus [HPV]), which decreases the likelihood of seeking testing or treatment as well as increases the likelihood of transmission.7
Current Research
To date, only 1 study has explicitly examined the relationship between MST and STIs. In 2011, researchers analyzed a large national database of 420,725 male and female Operation Enduring Freedom/Operation Iraqi Freedom (OEF/OIF) veterans.6 In the study, both male and female OEF/OIF veterans who endorsed MST were significantly more likely than those who did not endorse MST to have a STI diagnosis. The researchers noted that this finding underscored the necessity for sexual health assessment in survivors of MST, to facilitate early detection and treatment.
STI Risk Assessment
Because military personnel and veterans often initially disclose their MST to a mental health provider (MHP), these providers operate in a unique circumstance where they may be the individual’s first point of contact for determining STI risk. In these circumstances, the MHP should consider the utility of briefly assessing the patient’s sexual health and making subsequent medical referrals as necessary.
To accurately assess a patient’s STI risk, the MHP should gather information regarding both current/acute risk (eg, “Have you had unprotected sexual contact, for instance, genital contact without a condom or oral sex without a dental dam, in the past month?”) as well as longer standing (eg, “Have you had unprotected sexual contact... in the past year?”) STI risk. Providers also should consider oral, anal, and genital modes of sexual contact as well as common STI symptoms (eg, warts, sores, genital discharge, and/or pain or burning sensation when peeing or during sex). Additionally, psychoeducation should be provided, especially information regarding the asymptomatic nature of certain STIs, such as HIV and HPV, and the risk of transmission in all forms of sexual contact, including nongenital anal contact. Appropriate referrals to sexual health education, including safe sex practices, also should be considered in order to minimize risk of future STI transmission.
Mental health providers also should determine the date of patient’s most recent STI test. Not all STIs can be detected via a blood test or routine yearly Papanicolaou (Pap test) physicals. Additionally, MHPs should be aware that risky practices, including substance misuse, are more common in survivors of MST and that there is an association between substance misuse and STIs.2,6 If testing has not occurred recently, MHPs should strongly encourage the individual to access STI testing and provide resources as necessary, such as access to low-cost STI testing. Further, if the MHP has reason to suspect the presence of an STI after the brief assessment (eg, individual endorses unprotected sexual contact or risky behaviors, including substance misuse, sex trading, and STI symptomatology; a positive STI test but the patient has not accessed treatment), an appropriate sexual health referral should be made.
During the assessment and psychoeducation processes, terminology and language plays an integral role. If a MHP assumes an individual has sexual contact only with opposite sex partners (eg, asking a male “How many women have you had sexual contact with in the past 30 days?” vs “How many partners have you had sexual contact with in the past 30 days?”), the MHP will not accurately assess the individual’s level of current risk. Additionally, it is important to remember that sexual behavior does not always align with sexual identity: A man who identifies as heterosexual may still have sexual contact with men. Due to the sensitive nature of sexual health, MHPs should be careful to use nonjudgmental language, such as using the term sex work rather than the more pejorative term prostitution, to avoid offending patients and to increase their likelihood to disclose sexual health information.
Nonjudgmental language is especially relevant when working with gender-minority veterans (eg, transgender, gender nonconforming, gender transitioning), because this clinical population has a higher risk of victimization and lower rates of help-seeking health behavior.7 In particular, a sizable portion of individuals who identify as transgender do not seek services out of fear that they will be discriminated against, humiliated, or misunderstood.7 To assuage these concerns, MHPs should ensure they refer to the veteran with the veteran’s preferred pronouns. For example, a MHP could ask “I would like to be respectful, how would you like to be addressed?” or “What name and pronoun would you like me/us to use?” Providers also should consider nonbinary pronouns when appropriate (eg, singular: ze/hir/hirs; plural: they/them/theirs). Providers also should recognize that making a mistake is not uncommon, and they should apologize to maintain rapport and maximize the patient’s comfort during this distressing process. Further, MHPs should consider additional training, education, and/or consultation if they feel uncomfortable or ill prepared when working with gender-minority veterans.
Future Research
Research has attempted to understand the consequences of MST on sexual health; however, despite these efforts, more research is necessary. The majority of published studies have focused on females even though a similar number of males have reported MST.2 This dearth of published studies likely is due to hesitation by male active-duty personnel and veterans to disclose or seek treatment for MST and because the percentage of females reporting MST is much higher. Males are less likely to report or seek treatment for MST because of stigma-based concerns (eg, shame, self-blame, privacy concerns).8,9 Therefore, it is difficult to acquire a sizable research sample to study. As previously noted, a single study has specifically examined MST and STI risk. Although this study included a sizable population of male OEF/OIF veterans, results have yet to be replicated in other clinical populations of interest, such as male military personnel and male veterans of other service eras.
Research is even more limited regarding lesbian, gay, bisexual, transgender, and other gender-minority military personnel and veterans. Although researchers propose that these populations may experience a similar, or even heightened, likelihood of MST during their service, no empirical research yet exists to fully examine this hypothesis.10,11 It is important to note that the paucity of research attention may be related to the Don’t Ask, Don’t Tell (DADT) policy, which obstructed the open discussion and empirical examination of sexual and gender minorities within military populations.11 The DADT policy led to limited awareness and greater stigmatization among sexual- and gender-minority personnel, resulting in poorer sexual health outcomes in these populations.10,11 With the end of DADT in 2011, it is now imperative for future research to examine the prevalence and associated consequences of MST in sexual- and gender-minority military personnel and veterans.
Conclusion
The DoD and VA should be commended for their continued focus on understanding the health consequences of MST. These efforts have yielded substantial information regarding the negative effects of MST on sexual health; in particular, increased risk for STIs. These findings suggest that MHPs may, at times, be the first point of contact for MST-related sexual health concerns. These providers should be aware of their ability to assess for STI risk and make appropriate referrals to facilitate early detection and access to treatment. Despite the presence of MST-related sexual health research, continued research remains necessary. In particular, a broader focus that includes other genders (eg, male, transgender) and sexual minorities would further inform research and clinical practice.
1. Military Sexual Trauma Support Team. Military sexual trauma (MST) screening report fiscal year 2012. Washington DC: U.S. Department of Veterans Affairs, Office of Patient Care Services, Mental Health Services; 2013.
2. Surís A, Holliday R, Weitlauf JC, North CS; the Veteran Safety Initiative Writing Collaborative. Military sexual trauma in the context of veterans’ life experiences. Fed Pract. 2013;30(suppl 3):16S-20S.
3. Jenny C, Hooton TM, Bowers A, et al. Sexually transmitted diseases in victims of rape. N Eng J Med. 1990;322(11):713-716.
4. Senn TE, Carey MP, Vanable PA, Coury-Doniger P, Urban MA. Childhood sexual abuse and sexual risk behavior among men and women attending a sexually transmitted disease clinic. J Consult Clin Psychol. 2006;74(4):720-731.
5. Schultz JR, Bell KM, Naugle AE, Polusny MA. Child sexual abuse and adult sexual assault among military veteran and civilian women. Mil Med. 2006;171(8):723-728.
6. Turchik JA, Pavao J, Nazarian D, Iqbal S, McLean C, Kimerling R. Sexually transmitted infections and sexual dysfunctions among newly returned veterans with and without military sexual trauma. Int J Sex Health. 2012;24(1):45-59.
7. National LGBT Health Education Center. Affirmative care for transgender and gender non-conforming people: best practices for front-line health care staff. https://www.lgbthealth education.org/wp-content/uploads/2016/12/Affirmative-Care-for-Transgender -and-Gender-Non-conforming-People-Best-Practices -for-Frontline-Health-Care-Staff.pdf. Published 2016. Accessed February 16, 2017.
8. Morris EE, Smith JC, Farooqui SY, Surís AM. Unseen battles: the recognition, assessment, and treatment issues of men with military sexual trauma (MST). Trauma Violence Abuse. 2014;15(2):94-101.
9. Turchik JA, Edwards KM. Myths about male rape: a literature review. Psychol Men Masc. 2012;13(2):211-226.
10. Mattocks KM, Kauth MR, Sandfort T, Matza AR, Sullivan JC, Shipherd J. Understanding health-care needs of sexual and gender minority veterans: how targeted research and policy can improve health. LGBT Health. 2014;1(1):50-57.
11. Burks DJ. Lesbian, gay, and bisexual victimization in the military: an unintended consequence of “Don’t Ask, Don’t Tell”? Am Psychol. 2011;66(7):604-613.
About 24% of women and 1% of men will experience military sexual trauma (MST) during their service.1 Despite the higher percentage of women reporting MST, the estimated number of men (55,491) and women (72,497) who endorse MST is relatively similar. Military sexual trauma is associated with negative psychosocial (eg, decreased quality of life) and psychiatric (eg, posttraumatic stress disorder [PTSD], depression) sequelae. Surís and colleagues provided a full review of sequelae, with PTSD being the most discussed consequence of MST.2 However, sexually transmitted infections (STIs) during or after MST are a consequence of growing concern.
Sexually Transmitted Infections
The link between sexual trauma and increased incidence of STIs is well established. Survivors of rape are at a higher risk of exposure to STIs due to unprotected sexual contact that may occur during the assault(s).3 Numerous studies have demonstrated that sexual trauma is directly related to greater engagement in risky sexual behaviors (eg, more sexual partners, unprotected sex, and “sex trading”).2,4
This relationship is particularly concerning given that individuals in the military tend to report sexual trauma with greater propensity than that reported in civilian populations.4 Additionally, military personnel and veteran populations tend to engage in high-risk behaviors (eg, alcohol and drug use) more often than their civilian counterparts, increasing their potential susceptibility to predatory sexual trauma(s) and victimization.2,5 Taken in aggregate, military personnel and veterans may be at increased risk for STIs compared with the civilian population due to the increased incidence of risky sexual behavior and sexual traumatization during military service.
Sexually transmitted infections potentially lead to immediate-term (eg, physical discomfort, sexual dysfunction) and long-term (eg, cancer, infertility) adverse health consequences.6 Early detection is crucial in the treatment of STIs because it can aid in preventing STI transmission and allow for early intervention. See Table for a list of common STIs and their prevalence, testing method, method of sexual transmission, and treatment. Early detection is also important because many STIs may be asymptomatic (eg, HIV, human papillomavirus [HPV]), which decreases the likelihood of seeking testing or treatment as well as increases the likelihood of transmission.7
Current Research
To date, only 1 study has explicitly examined the relationship between MST and STIs. In 2011, researchers analyzed a large national database of 420,725 male and female Operation Enduring Freedom/Operation Iraqi Freedom (OEF/OIF) veterans.6 In the study, both male and female OEF/OIF veterans who endorsed MST were significantly more likely than those who did not endorse MST to have a STI diagnosis. The researchers noted that this finding underscored the necessity for sexual health assessment in survivors of MST, to facilitate early detection and treatment.
STI Risk Assessment
Because military personnel and veterans often initially disclose their MST to a mental health provider (MHP), these providers operate in a unique circumstance where they may be the individual’s first point of contact for determining STI risk. In these circumstances, the MHP should consider the utility of briefly assessing the patient’s sexual health and making subsequent medical referrals as necessary.
To accurately assess a patient’s STI risk, the MHP should gather information regarding both current/acute risk (eg, “Have you had unprotected sexual contact, for instance, genital contact without a condom or oral sex without a dental dam, in the past month?”) as well as longer standing (eg, “Have you had unprotected sexual contact... in the past year?”) STI risk. Providers also should consider oral, anal, and genital modes of sexual contact as well as common STI symptoms (eg, warts, sores, genital discharge, and/or pain or burning sensation when peeing or during sex). Additionally, psychoeducation should be provided, especially information regarding the asymptomatic nature of certain STIs, such as HIV and HPV, and the risk of transmission in all forms of sexual contact, including nongenital anal contact. Appropriate referrals to sexual health education, including safe sex practices, also should be considered in order to minimize risk of future STI transmission.
Mental health providers also should determine the date of patient’s most recent STI test. Not all STIs can be detected via a blood test or routine yearly Papanicolaou (Pap test) physicals. Additionally, MHPs should be aware that risky practices, including substance misuse, are more common in survivors of MST and that there is an association between substance misuse and STIs.2,6 If testing has not occurred recently, MHPs should strongly encourage the individual to access STI testing and provide resources as necessary, such as access to low-cost STI testing. Further, if the MHP has reason to suspect the presence of an STI after the brief assessment (eg, individual endorses unprotected sexual contact or risky behaviors, including substance misuse, sex trading, and STI symptomatology; a positive STI test but the patient has not accessed treatment), an appropriate sexual health referral should be made.
During the assessment and psychoeducation processes, terminology and language plays an integral role. If a MHP assumes an individual has sexual contact only with opposite sex partners (eg, asking a male “How many women have you had sexual contact with in the past 30 days?” vs “How many partners have you had sexual contact with in the past 30 days?”), the MHP will not accurately assess the individual’s level of current risk. Additionally, it is important to remember that sexual behavior does not always align with sexual identity: A man who identifies as heterosexual may still have sexual contact with men. Due to the sensitive nature of sexual health, MHPs should be careful to use nonjudgmental language, such as using the term sex work rather than the more pejorative term prostitution, to avoid offending patients and to increase their likelihood to disclose sexual health information.
Nonjudgmental language is especially relevant when working with gender-minority veterans (eg, transgender, gender nonconforming, gender transitioning), because this clinical population has a higher risk of victimization and lower rates of help-seeking health behavior.7 In particular, a sizable portion of individuals who identify as transgender do not seek services out of fear that they will be discriminated against, humiliated, or misunderstood.7 To assuage these concerns, MHPs should ensure they refer to the veteran with the veteran’s preferred pronouns. For example, a MHP could ask “I would like to be respectful, how would you like to be addressed?” or “What name and pronoun would you like me/us to use?” Providers also should consider nonbinary pronouns when appropriate (eg, singular: ze/hir/hirs; plural: they/them/theirs). Providers also should recognize that making a mistake is not uncommon, and they should apologize to maintain rapport and maximize the patient’s comfort during this distressing process. Further, MHPs should consider additional training, education, and/or consultation if they feel uncomfortable or ill prepared when working with gender-minority veterans.
Future Research
Research has attempted to understand the consequences of MST on sexual health; however, despite these efforts, more research is necessary. The majority of published studies have focused on females even though a similar number of males have reported MST.2 This dearth of published studies likely is due to hesitation by male active-duty personnel and veterans to disclose or seek treatment for MST and because the percentage of females reporting MST is much higher. Males are less likely to report or seek treatment for MST because of stigma-based concerns (eg, shame, self-blame, privacy concerns).8,9 Therefore, it is difficult to acquire a sizable research sample to study. As previously noted, a single study has specifically examined MST and STI risk. Although this study included a sizable population of male OEF/OIF veterans, results have yet to be replicated in other clinical populations of interest, such as male military personnel and male veterans of other service eras.
Research is even more limited regarding lesbian, gay, bisexual, transgender, and other gender-minority military personnel and veterans. Although researchers propose that these populations may experience a similar, or even heightened, likelihood of MST during their service, no empirical research yet exists to fully examine this hypothesis.10,11 It is important to note that the paucity of research attention may be related to the Don’t Ask, Don’t Tell (DADT) policy, which obstructed the open discussion and empirical examination of sexual and gender minorities within military populations.11 The DADT policy led to limited awareness and greater stigmatization among sexual- and gender-minority personnel, resulting in poorer sexual health outcomes in these populations.10,11 With the end of DADT in 2011, it is now imperative for future research to examine the prevalence and associated consequences of MST in sexual- and gender-minority military personnel and veterans.
Conclusion
The DoD and VA should be commended for their continued focus on understanding the health consequences of MST. These efforts have yielded substantial information regarding the negative effects of MST on sexual health; in particular, increased risk for STIs. These findings suggest that MHPs may, at times, be the first point of contact for MST-related sexual health concerns. These providers should be aware of their ability to assess for STI risk and make appropriate referrals to facilitate early detection and access to treatment. Despite the presence of MST-related sexual health research, continued research remains necessary. In particular, a broader focus that includes other genders (eg, male, transgender) and sexual minorities would further inform research and clinical practice.
About 24% of women and 1% of men will experience military sexual trauma (MST) during their service.1 Despite the higher percentage of women reporting MST, the estimated number of men (55,491) and women (72,497) who endorse MST is relatively similar. Military sexual trauma is associated with negative psychosocial (eg, decreased quality of life) and psychiatric (eg, posttraumatic stress disorder [PTSD], depression) sequelae. Surís and colleagues provided a full review of sequelae, with PTSD being the most discussed consequence of MST.2 However, sexually transmitted infections (STIs) during or after MST are a consequence of growing concern.
Sexually Transmitted Infections
The link between sexual trauma and increased incidence of STIs is well established. Survivors of rape are at a higher risk of exposure to STIs due to unprotected sexual contact that may occur during the assault(s).3 Numerous studies have demonstrated that sexual trauma is directly related to greater engagement in risky sexual behaviors (eg, more sexual partners, unprotected sex, and “sex trading”).2,4
This relationship is particularly concerning given that individuals in the military tend to report sexual trauma with greater propensity than that reported in civilian populations.4 Additionally, military personnel and veteran populations tend to engage in high-risk behaviors (eg, alcohol and drug use) more often than their civilian counterparts, increasing their potential susceptibility to predatory sexual trauma(s) and victimization.2,5 Taken in aggregate, military personnel and veterans may be at increased risk for STIs compared with the civilian population due to the increased incidence of risky sexual behavior and sexual traumatization during military service.
Sexually transmitted infections potentially lead to immediate-term (eg, physical discomfort, sexual dysfunction) and long-term (eg, cancer, infertility) adverse health consequences.6 Early detection is crucial in the treatment of STIs because it can aid in preventing STI transmission and allow for early intervention. See Table for a list of common STIs and their prevalence, testing method, method of sexual transmission, and treatment. Early detection is also important because many STIs may be asymptomatic (eg, HIV, human papillomavirus [HPV]), which decreases the likelihood of seeking testing or treatment as well as increases the likelihood of transmission.7
Current Research
To date, only 1 study has explicitly examined the relationship between MST and STIs. In 2011, researchers analyzed a large national database of 420,725 male and female Operation Enduring Freedom/Operation Iraqi Freedom (OEF/OIF) veterans.6 In the study, both male and female OEF/OIF veterans who endorsed MST were significantly more likely than those who did not endorse MST to have a STI diagnosis. The researchers noted that this finding underscored the necessity for sexual health assessment in survivors of MST, to facilitate early detection and treatment.
STI Risk Assessment
Because military personnel and veterans often initially disclose their MST to a mental health provider (MHP), these providers operate in a unique circumstance where they may be the individual’s first point of contact for determining STI risk. In these circumstances, the MHP should consider the utility of briefly assessing the patient’s sexual health and making subsequent medical referrals as necessary.
To accurately assess a patient’s STI risk, the MHP should gather information regarding both current/acute risk (eg, “Have you had unprotected sexual contact, for instance, genital contact without a condom or oral sex without a dental dam, in the past month?”) as well as longer standing (eg, “Have you had unprotected sexual contact... in the past year?”) STI risk. Providers also should consider oral, anal, and genital modes of sexual contact as well as common STI symptoms (eg, warts, sores, genital discharge, and/or pain or burning sensation when peeing or during sex). Additionally, psychoeducation should be provided, especially information regarding the asymptomatic nature of certain STIs, such as HIV and HPV, and the risk of transmission in all forms of sexual contact, including nongenital anal contact. Appropriate referrals to sexual health education, including safe sex practices, also should be considered in order to minimize risk of future STI transmission.
Mental health providers also should determine the date of patient’s most recent STI test. Not all STIs can be detected via a blood test or routine yearly Papanicolaou (Pap test) physicals. Additionally, MHPs should be aware that risky practices, including substance misuse, are more common in survivors of MST and that there is an association between substance misuse and STIs.2,6 If testing has not occurred recently, MHPs should strongly encourage the individual to access STI testing and provide resources as necessary, such as access to low-cost STI testing. Further, if the MHP has reason to suspect the presence of an STI after the brief assessment (eg, individual endorses unprotected sexual contact or risky behaviors, including substance misuse, sex trading, and STI symptomatology; a positive STI test but the patient has not accessed treatment), an appropriate sexual health referral should be made.
During the assessment and psychoeducation processes, terminology and language plays an integral role. If a MHP assumes an individual has sexual contact only with opposite sex partners (eg, asking a male “How many women have you had sexual contact with in the past 30 days?” vs “How many partners have you had sexual contact with in the past 30 days?”), the MHP will not accurately assess the individual’s level of current risk. Additionally, it is important to remember that sexual behavior does not always align with sexual identity: A man who identifies as heterosexual may still have sexual contact with men. Due to the sensitive nature of sexual health, MHPs should be careful to use nonjudgmental language, such as using the term sex work rather than the more pejorative term prostitution, to avoid offending patients and to increase their likelihood to disclose sexual health information.
Nonjudgmental language is especially relevant when working with gender-minority veterans (eg, transgender, gender nonconforming, gender transitioning), because this clinical population has a higher risk of victimization and lower rates of help-seeking health behavior.7 In particular, a sizable portion of individuals who identify as transgender do not seek services out of fear that they will be discriminated against, humiliated, or misunderstood.7 To assuage these concerns, MHPs should ensure they refer to the veteran with the veteran’s preferred pronouns. For example, a MHP could ask “I would like to be respectful, how would you like to be addressed?” or “What name and pronoun would you like me/us to use?” Providers also should consider nonbinary pronouns when appropriate (eg, singular: ze/hir/hirs; plural: they/them/theirs). Providers also should recognize that making a mistake is not uncommon, and they should apologize to maintain rapport and maximize the patient’s comfort during this distressing process. Further, MHPs should consider additional training, education, and/or consultation if they feel uncomfortable or ill prepared when working with gender-minority veterans.
Future Research
Research has attempted to understand the consequences of MST on sexual health; however, despite these efforts, more research is necessary. The majority of published studies have focused on females even though a similar number of males have reported MST.2 This dearth of published studies likely is due to hesitation by male active-duty personnel and veterans to disclose or seek treatment for MST and because the percentage of females reporting MST is much higher. Males are less likely to report or seek treatment for MST because of stigma-based concerns (eg, shame, self-blame, privacy concerns).8,9 Therefore, it is difficult to acquire a sizable research sample to study. As previously noted, a single study has specifically examined MST and STI risk. Although this study included a sizable population of male OEF/OIF veterans, results have yet to be replicated in other clinical populations of interest, such as male military personnel and male veterans of other service eras.
Research is even more limited regarding lesbian, gay, bisexual, transgender, and other gender-minority military personnel and veterans. Although researchers propose that these populations may experience a similar, or even heightened, likelihood of MST during their service, no empirical research yet exists to fully examine this hypothesis.10,11 It is important to note that the paucity of research attention may be related to the Don’t Ask, Don’t Tell (DADT) policy, which obstructed the open discussion and empirical examination of sexual and gender minorities within military populations.11 The DADT policy led to limited awareness and greater stigmatization among sexual- and gender-minority personnel, resulting in poorer sexual health outcomes in these populations.10,11 With the end of DADT in 2011, it is now imperative for future research to examine the prevalence and associated consequences of MST in sexual- and gender-minority military personnel and veterans.
Conclusion
The DoD and VA should be commended for their continued focus on understanding the health consequences of MST. These efforts have yielded substantial information regarding the negative effects of MST on sexual health; in particular, increased risk for STIs. These findings suggest that MHPs may, at times, be the first point of contact for MST-related sexual health concerns. These providers should be aware of their ability to assess for STI risk and make appropriate referrals to facilitate early detection and access to treatment. Despite the presence of MST-related sexual health research, continued research remains necessary. In particular, a broader focus that includes other genders (eg, male, transgender) and sexual minorities would further inform research and clinical practice.
1. Military Sexual Trauma Support Team. Military sexual trauma (MST) screening report fiscal year 2012. Washington DC: U.S. Department of Veterans Affairs, Office of Patient Care Services, Mental Health Services; 2013.
2. Surís A, Holliday R, Weitlauf JC, North CS; the Veteran Safety Initiative Writing Collaborative. Military sexual trauma in the context of veterans’ life experiences. Fed Pract. 2013;30(suppl 3):16S-20S.
3. Jenny C, Hooton TM, Bowers A, et al. Sexually transmitted diseases in victims of rape. N Eng J Med. 1990;322(11):713-716.
4. Senn TE, Carey MP, Vanable PA, Coury-Doniger P, Urban MA. Childhood sexual abuse and sexual risk behavior among men and women attending a sexually transmitted disease clinic. J Consult Clin Psychol. 2006;74(4):720-731.
5. Schultz JR, Bell KM, Naugle AE, Polusny MA. Child sexual abuse and adult sexual assault among military veteran and civilian women. Mil Med. 2006;171(8):723-728.
6. Turchik JA, Pavao J, Nazarian D, Iqbal S, McLean C, Kimerling R. Sexually transmitted infections and sexual dysfunctions among newly returned veterans with and without military sexual trauma. Int J Sex Health. 2012;24(1):45-59.
7. National LGBT Health Education Center. Affirmative care for transgender and gender non-conforming people: best practices for front-line health care staff. https://www.lgbthealth education.org/wp-content/uploads/2016/12/Affirmative-Care-for-Transgender -and-Gender-Non-conforming-People-Best-Practices -for-Frontline-Health-Care-Staff.pdf. Published 2016. Accessed February 16, 2017.
8. Morris EE, Smith JC, Farooqui SY, Surís AM. Unseen battles: the recognition, assessment, and treatment issues of men with military sexual trauma (MST). Trauma Violence Abuse. 2014;15(2):94-101.
9. Turchik JA, Edwards KM. Myths about male rape: a literature review. Psychol Men Masc. 2012;13(2):211-226.
10. Mattocks KM, Kauth MR, Sandfort T, Matza AR, Sullivan JC, Shipherd J. Understanding health-care needs of sexual and gender minority veterans: how targeted research and policy can improve health. LGBT Health. 2014;1(1):50-57.
11. Burks DJ. Lesbian, gay, and bisexual victimization in the military: an unintended consequence of “Don’t Ask, Don’t Tell”? Am Psychol. 2011;66(7):604-613.
1. Military Sexual Trauma Support Team. Military sexual trauma (MST) screening report fiscal year 2012. Washington DC: U.S. Department of Veterans Affairs, Office of Patient Care Services, Mental Health Services; 2013.
2. Surís A, Holliday R, Weitlauf JC, North CS; the Veteran Safety Initiative Writing Collaborative. Military sexual trauma in the context of veterans’ life experiences. Fed Pract. 2013;30(suppl 3):16S-20S.
3. Jenny C, Hooton TM, Bowers A, et al. Sexually transmitted diseases in victims of rape. N Eng J Med. 1990;322(11):713-716.
4. Senn TE, Carey MP, Vanable PA, Coury-Doniger P, Urban MA. Childhood sexual abuse and sexual risk behavior among men and women attending a sexually transmitted disease clinic. J Consult Clin Psychol. 2006;74(4):720-731.
5. Schultz JR, Bell KM, Naugle AE, Polusny MA. Child sexual abuse and adult sexual assault among military veteran and civilian women. Mil Med. 2006;171(8):723-728.
6. Turchik JA, Pavao J, Nazarian D, Iqbal S, McLean C, Kimerling R. Sexually transmitted infections and sexual dysfunctions among newly returned veterans with and without military sexual trauma. Int J Sex Health. 2012;24(1):45-59.
7. National LGBT Health Education Center. Affirmative care for transgender and gender non-conforming people: best practices for front-line health care staff. https://www.lgbthealth education.org/wp-content/uploads/2016/12/Affirmative-Care-for-Transgender -and-Gender-Non-conforming-People-Best-Practices -for-Frontline-Health-Care-Staff.pdf. Published 2016. Accessed February 16, 2017.
8. Morris EE, Smith JC, Farooqui SY, Surís AM. Unseen battles: the recognition, assessment, and treatment issues of men with military sexual trauma (MST). Trauma Violence Abuse. 2014;15(2):94-101.
9. Turchik JA, Edwards KM. Myths about male rape: a literature review. Psychol Men Masc. 2012;13(2):211-226.
10. Mattocks KM, Kauth MR, Sandfort T, Matza AR, Sullivan JC, Shipherd J. Understanding health-care needs of sexual and gender minority veterans: how targeted research and policy can improve health. LGBT Health. 2014;1(1):50-57.
11. Burks DJ. Lesbian, gay, and bisexual victimization in the military: an unintended consequence of “Don’t Ask, Don’t Tell”? Am Psychol. 2011;66(7):604-613.
Advances in Targeted Therapy for Breast Cancer
It is estimated that there were more than 3.1 million women living in the U.S. with a history of invasive breast cancer as of January 1, 2014, and an additional 231,840 women will be newly diagnosed with invasive breast cancer in 2015.1,2 The median age at the time of breast cancer diagnosis is 61 years. About 20% of breast cancers occur among women aged < 50 years, and 43% occur in women aged > 65 years.
The treatment and prognosis for breast cancer depend on the stage at diagnosis, the biologic characteristics of the tumor, and the age and health of the patient. The overall 5-year relative survival rate for female patients with breast cancer has improved from 75% to 90% from 1975 to 1977 and from 2003 to 2009, respectively, largely due to improvements in treatment (ie, chemotherapy, hormone therapy, and targeted drugs) and because of earlier diagnosis resulting from the widespread use of mammography and other screening tools.2
Estrogen Receptor-Positive Therapies
Women with breast cancer who test positive for hormone receptors are candidates for treatment with hormone therapy to reduce the likelihood of recurrence or as a core component of treatment for advanced disease. Currently available endocrine strategies for the treatment of estrogen receptor- (ER) positive breast cancer include targeting the ER with the antiestrogen drug tamoxifen. Another option is suppressing the amount of available ligand (estrogen) for the receptor either with gonadal suppression in premenopausal oophorectomy, or luteinizing hormonereleasing hormone agonists, or with the aromatase inhibitors (AIs) anastrozole, exemestane, and letrozole in postmenopausal women and by downregulating the receptor with fulvestrant. Given their proven efficacy and generally favorable adverse effect (AE) profile, these endocrine therapies are widely used in the treatment of both early-stage and recurrent and/or metastatic breast cancer.
Recent studies have offered new treatments for patients with hormone receptor-positive, human epidermal growth factor receptor 2 (HER2)-negative breast cancer. Innovative hormonal and targeted therapies for advanced disease as well as new data on adjuvant hormonal therapy for young high-risk patients are changing the available therapeutic options.
Advanced Metastatic Treatments
Treatment for metastatic hormone receptor-positive breast cancer has shifted from traditional cytotoxic chemotherapies to targeted therapeutic options. Most treatment guidelines, including the National Comprehensive Cancer Network guidelines, recommend targeted therapy with AIs or selective ER modulators rather than chemotherapy, except in the case of visceral crisis.3
Until recently, there had been relatively little guidance to inform which hormonal therapy was most appropriate. Aromatase inhibitors were generally reserved for postmenopausal women, whereas tamoxifen was preferred in premenopausal women.
Fulvestrant
The FDA initially approved fulvestrant, a hormone receptor downregulator, in 2002 at a 250-mg dose, following progression on an anti-estrogen therapy, such as tamoxifen in postmenopausal women with stage IV breast cancer. The FDA approval was based on similar response rates for the already approved agent anastrozole.4 However, pharmacokinetic findings from the phase 3 EFECT trial in 2008 prompted researchers to explore a 500-mg dose of fulvestrant.5
The recently published FIRST study is a phase 2, randomized, open-label study comparing fulvestrant 500 mg with anastrozole 1 mg as first-line hormonal therapy for postmenopausal women with hormone receptorpositive advanced breast cancer. Fulvestrant was given 500 mg once monthly with an extra dose given on day 14 of month 1. The trial enrolled 233 patients. The median time to progression was 23.4 months for fulvestrant and 13.1 months for anastrozole. These results translate into a 34% reduction in the risk of progression.6
These outcomes suggest that fulvestrant is as viable and perhaps even preferred first-line therapy for postmenopausal women with hormone receptor-positive, HER2-negative advanced breast cancer. The impressive results from this trial are likely, because the study used the 500-mg dose of fulvestrant, which is twice the dose used in the original trials. However, the 500-mg dose has previously been studied, and long-term outcome data suggest both safety and efficiency. The large randomized, double-blinded phase 3 CONFIRM trial, published in 2013, compared the 250-mg dose with the 500-mg dose and found that the higher dose was associated with a 19% reduction in the risk of death and a 4.1 month increase in median overall survival (OS) without any new safety concerns.5
Palbociclib
The FDA recently granted accelerated approval to palbociclib in combination with letrozole for the first-line therapy of advanced hormone receptor-positive, HER2-negative breast cancer in postmenopausal women. Palbociclib is an oral small-molecular inhibitor of cyclindependent kinases 4 and 6. Preclinical data suggested synergy with anti-estrogen therapies and inhibition of breast cancer cell growth.7
A phase 2, open-label randomized trial (PALOMA-1/TRIO-18) enrolled 165 patients. Progression-free survival (PFS) was 20.2 months for the palbociclib plus letrozole arm and 10.2 months for the letrozole alone arm. Significant toxicities were noted in the palbociclib arm, including 54% of people experiencing grade 3 to 4 neutropenia (vs 1% in the letrozole arm), leukopenia in 19% (vs 0%) and fatigue in 4% (vs 1%). A phase 3 trial is currently enrolling patients.7 While we await the results of the phase 3 trial and long-term follow-up data, palbociclib plus letrozole is a new, viable option for metastatic hormone receptor-positive advanced breast cancer.
Although many practitioners will continue to reasonably use any AI or selective ER modulator when treating metastatic breast cancer, both fulvestrant and palbociclib in combination with letrozole are new evidence-based, first-line options worth considering.
Early-Stage Treatment Options
There are many acceptable therapeutic options for treating early stage breast cancer. Tamoxifen has traditionally been used in the adjuvant setting for premenopausal women, whereas AIs are often used in postmenopausal women. There has also been a long-standing debate about the role of ovarian suppression in premenopausal women.
The recently published phase 3 TEXT and SOFT trials attempted to provide answers to these long-standing therapeutic dilemmas. The SOFT trial randomly assigned 3,066 premenopausal women to 5 years of tamoxifen, 5 years of tamoxifen plus ovarian suppression, or exemestane plus ovarian suppression. The TEXT trial randomly assigned 2,672 women to receive either exemestane plus ovarian suppression or tamoxifen plus ovarian suppression. The studies showed that subjecting all women receiving tamoxifen to ovarian suppression did not provide any significant benefit.8,9
However, the subgroup of women with high-risk disease who required adjuvant chemotherapy and remained premenopausal experienced improved outcomes from ovarian suppression. This high-risk subgroup when given tamoxifen plus ovarian suppression had a 4.5% absolute reduction in breast cancer recurrence at 5 years compared with the group that received tamoxifen alone. When this high-risk subgroup was given exemestane plus ovarian suppression, the women had a 7.7% absolute reduction in breast cancer recurrence at 5 years compared with the group that received tamoxifen alone.8
Ovarian suppression resulted in significant additional AEs, including depression and menopausal symptoms. The authors of the study also pointed out the additional risk of hypertension, musculoskeletal AEs, and decreased bone density. Furthermore, the OS data from these studies are premature, because the patients had fewer AEs than initially anticipated; this resulted in an only 5% mortality at publication.
The study design also raised several interesting questions. The primary endpoint was disease-free survival. The authors defined this as the time from randomization to the first appearance of invasive recurrence of breast cancer (local, regional, or distant), invasive contralateral breast cancer, second (non-breast) invasive cancer, or death without breast cancer recurrence or second invasive cancer. When studying adjuvant therapy for diseases, such as breast cancer, which carry long-term survival, studies often use PFS with various modified definitions as a surrogate marker for OS. Clinicians are then left to decide whether this surrogate marker is an accurate predictor of OS or other important clinical outcomes.
In the combined analysis of the TEXT and SOFT trials, only 60% of the first recurrences, second invasive cancers, or deaths involved recurrence of breast cancer
at a distant site.9 Because locally recurrent breast cancer is highly treatable and often curable, clinicians must ask whether the increased toxicities of ovarian suppression are worth the large number of women who experienced local recurrence given the still relatively small absolute reduction in recurrence risk.
Last, the study authors retrospectively reviewed data from the International Breast Cancer Study Group and U.S. Intergroup trials and concluded that women aged < 35 years were most likely to be at high-risk for AEs.10,11 A subgroup analysis of women aged < 35 years in the SOFT trial noted that breast cancer recurred within 5 years in one-third of women receiving tamoxifen alone, whereas only in one-sixth of women receiving exemestane plus ovarian suppression.8 This is the basis for the conclusion that premenopausal women, particularly those aged < 35 years, with high-risk disease who receive chemotherapy and remain premenopausal after chemotherapy, benefit from ovarian suppression in combination with tamoxifen, and even more impressively from ovarian suppression combined with exemestane.
The problem is that the study did not risk-stratify patients based on those aged < 35 years, and the conclusion is based on a subgroup analysis using a primary endpoint that may not accurately predict OS. Nonetheless, although not definitive, the data from the TEXT and SOFT trials raise interesting therapeutic questions that require further study and certainly provide tempting therapeutic options in patients who are clinically at high risk for recurrence.
HER2-Positive Breast Cancer
Up to 20% of invasive breast cancers are a result of HER2 gene amplification or overexpression of the HER2 protein, a tyrosine kinase transmembrane receptor, resulting in a more aggressive phenotype and a poor prognosis. Anti-HER2 drugs have changed the landscape of the disease previously known as aggressive breast cancer with a poor survival rate.
Treatment with the anti-HER2 humanized monoclonal antibody trastuzumab in addition to chemotherapy, compared with chemotherapy alone, significantly improves PFS and OS among patients with HER2-positive metastatic as well as early breast cancer. However, in most patients with HER2-positive metastatic breast cancer, the disease progresses, highlighting the need for new, targeted therapies for advanced disease.
New Standard of Care
The original studies of trastuzumab showed improved OS in late-stage (metastatic) breast cancer from 20.3 to 25.1 months, and in early-stage breast cancer, it reduced the risk of cancer returning after surgery by an absolute risk of 9.5% and the risk of death by an absolute risk of 3%.
New therapies directed at HER2 are being developed, among them pertuzumab, a humanized monoclonal antibody that binds HER2 at a different epitope of the HER2 extracellular domain (subdomain 2) than that at which trastuzumab binds. Pertuzumab prevents HER2 from dimerizing with other ligand-activated HER receptors, most notably HER3. Like trastuzumab, pertuzumab stimulates antibody-dependent, cell-mediated cytotoxicity. Because pertuzumab and trastuzumab bind to different HER2 epitopes and have complementary mechanisms of action, these 2 agents, when given together, provide a more comprehensive blockade of HER2 signaling and result in greater antitumor activity than does either agent alone in HER2-positive tumor models.12 In phase 2 studies, a pertuzumab–trastuzumab regimen has shown activity in patients with HER2-positive metastatic breast cancer and in patients with early breast cancer.13
In the phase 3 CLEOPATRA study, the combination of pertuzumab plus trastuzumab plus docetaxel, used as first-line treatment for HER2-positive metastatic breast cancer compared with placebo plus trastuzumab plus docetaxel, significantly prolonged PFS (18.5 months vs 12.4 months), with no increase in cardiac toxic effects.12 In a recent updated follow-up of the CLEOPATRA study, the addition of pertuzumab to trastuzumab and docetaxel showed a significantly better median OS (56.5 months vs 40.8 months; hazard ratio, 0.68; P < .001).14 From these results, this combination regimen is now considered a first-line therapy for patients with HER2-positive metastatic breast cancer.
However, the cost of cancer treatment has become a mounting concern during the past decade, as new therapies come down the pipeline with ever-increasing price tags. Trastuzumab costs about $4,500 a month, and the newer pertuzumab runs about 30% higher, at $6,000 a month. For a full course of treatment, the cost of the pertuzumab and trastuzumab combination could go as high as $195,000, depending on the duration of therapy and the choice of taxanes.
Conclusions
The landscape of therapeutic options in high-risk, young patients with early-stage breast cancer as well as patients with advanced or metastatic disease is changing rapidly.
Clinicians now have 2 new first-line options for the treatment of advanced hormone receptor-positive, HER2-negative breast cancer. A phase 3 trial demonstrated that fulvestrant monotherapy offers improved PFS and some improvement in OS compared with anastrazole in postmenopausal women. A phase 2 trial showed that palbociclib plus letrozole offers improved PFS in postmenopausal women. Based on the SOFT and TEXT trials, clinicians treating high-risk premenopausal women now have some data to inform the debate about whether ovarian suppression should be added to hormone therapy.
Based on the CLEOPATRA trial, clinicians can now consider combination pertuzumab and trastuzumab and docetaxel as first-line therapy for patients with HER2-positive metastatic breast cancer.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
Click here to read the digital edition.
1. American Cancer Society. Cancer facts & figures, 2015. Atlanta, GA: American Cancer Society; 2015.
2. American Cancer Society. Cancer treatment & survivorship facts & figures, 2014-2015. Atlanta, GA: American Cancer Society; 2014.
3. National Comprehensive Cancer Network. NCCN clinical Practice guidelines in oncology: breast Cancer. Version 1. 2015. Fort Washington, PA: National Comprehensive Cancer Network; 2015:BINV-19.
4. Howell A, Robertson JF, Quaresma Albano J. Fulvestrant, formerly ICI 182,780, is as effective as anastrozole in postmenopausal women with advanced breast cancer progressing after prior endocrine treatment. J Clin Oncol. 2002;20(16):3396-3403.
5. Di Leo A, Jerusalem G, Petruzelka L, et al. Final overall survival: fulvestrant 500 mg vs 250 mg in the randomized CONFIRM trial. J Natl Cancer Inst. 2014;106(1):djt337.
6. Robertson JF, Lindemann JB, Llombart-Cussac A, et al. Fulvestrant 500 mg versus anastrozole 1 mg for the first-line treatment of advanced breast cancer: follow-up analysis from the randomized ‘FIRST’ study. Breast Cancer Res Treat. 2012;136(2):503-511.
7. Finn RS, Crown JP, Lang I, et al. The cyclin-dependent kinase 4/6 inhibitor palbociclib in combination with letrozole versus letrozole alone as first-line treatment of oestrogen receptor-positive, HER2-negative, advanced breast cancer (PALOMA-1/TRIO-18): a randomised phase 2 study. Lancet Oncol. 2015;16(1):25-35.
8. Francis PA, Regan MM, Fleming GF, et al; SOFT Investigators; International Breast Cancer Study Group. Adjuvant ovarian suppression in premenopausal breast cancer. N Engl J Med. 2015;372(5):436-446.
9. Pagani O. Regan MM, Walley BA, et al. TEXT and SOFT Investigators; International Breast Cancer Study Group. Adjuvant exemestane with ovarian suppression in premenopausal breast cancer. N Engl J Med. 2014;371(2):107-118.
10. Aebi S, Gelber S, Castiglione-Gertsch M, et al. Is chemotherapy alone adequate for young women with oestrogen-receptor-positive breast cancer? Lancet. 2000;355:1869-1874.
11. Goldhirsch A, Gelber RD, Yothers G, et al. Adjuvant therapy for very young women with breast cancer: need for tailored treatments. J Natl Cancer Inst Monogr. 2001;(30):44-51
12. Hudis CA. Trastuzumab—mechanism of action and use in clinical practice. N Engl J Med. 2007;357(1):39-51.
13. Baselga J, Cortés J, Kim SB, et al; CLEOPATRA Study Group. Pertuzumab plus trastuzumab plus docetaxel for metastatic breast cancer. N Engl J Med. 2012;366(2):109-119.
14. Swain SM, Baselga J, Kim SB, et al; CLEOPATRA Study Group. Pertuzumab, trastuzumab, and docetaxel in HER2-positive metastatic breast cancer. N Engl J Med. 2015;372(8):724-734.
It is estimated that there were more than 3.1 million women living in the U.S. with a history of invasive breast cancer as of January 1, 2014, and an additional 231,840 women will be newly diagnosed with invasive breast cancer in 2015.1,2 The median age at the time of breast cancer diagnosis is 61 years. About 20% of breast cancers occur among women aged < 50 years, and 43% occur in women aged > 65 years.
The treatment and prognosis for breast cancer depend on the stage at diagnosis, the biologic characteristics of the tumor, and the age and health of the patient. The overall 5-year relative survival rate for female patients with breast cancer has improved from 75% to 90% from 1975 to 1977 and from 2003 to 2009, respectively, largely due to improvements in treatment (ie, chemotherapy, hormone therapy, and targeted drugs) and because of earlier diagnosis resulting from the widespread use of mammography and other screening tools.2
Estrogen Receptor-Positive Therapies
Women with breast cancer who test positive for hormone receptors are candidates for treatment with hormone therapy to reduce the likelihood of recurrence or as a core component of treatment for advanced disease. Currently available endocrine strategies for the treatment of estrogen receptor- (ER) positive breast cancer include targeting the ER with the antiestrogen drug tamoxifen. Another option is suppressing the amount of available ligand (estrogen) for the receptor either with gonadal suppression in premenopausal oophorectomy, or luteinizing hormonereleasing hormone agonists, or with the aromatase inhibitors (AIs) anastrozole, exemestane, and letrozole in postmenopausal women and by downregulating the receptor with fulvestrant. Given their proven efficacy and generally favorable adverse effect (AE) profile, these endocrine therapies are widely used in the treatment of both early-stage and recurrent and/or metastatic breast cancer.
Recent studies have offered new treatments for patients with hormone receptor-positive, human epidermal growth factor receptor 2 (HER2)-negative breast cancer. Innovative hormonal and targeted therapies for advanced disease as well as new data on adjuvant hormonal therapy for young high-risk patients are changing the available therapeutic options.
Advanced Metastatic Treatments
Treatment for metastatic hormone receptor-positive breast cancer has shifted from traditional cytotoxic chemotherapies to targeted therapeutic options. Most treatment guidelines, including the National Comprehensive Cancer Network guidelines, recommend targeted therapy with AIs or selective ER modulators rather than chemotherapy, except in the case of visceral crisis.3
Until recently, there had been relatively little guidance to inform which hormonal therapy was most appropriate. Aromatase inhibitors were generally reserved for postmenopausal women, whereas tamoxifen was preferred in premenopausal women.
Fulvestrant
The FDA initially approved fulvestrant, a hormone receptor downregulator, in 2002 at a 250-mg dose, following progression on an anti-estrogen therapy, such as tamoxifen in postmenopausal women with stage IV breast cancer. The FDA approval was based on similar response rates for the already approved agent anastrozole.4 However, pharmacokinetic findings from the phase 3 EFECT trial in 2008 prompted researchers to explore a 500-mg dose of fulvestrant.5
The recently published FIRST study is a phase 2, randomized, open-label study comparing fulvestrant 500 mg with anastrozole 1 mg as first-line hormonal therapy for postmenopausal women with hormone receptorpositive advanced breast cancer. Fulvestrant was given 500 mg once monthly with an extra dose given on day 14 of month 1. The trial enrolled 233 patients. The median time to progression was 23.4 months for fulvestrant and 13.1 months for anastrozole. These results translate into a 34% reduction in the risk of progression.6
These outcomes suggest that fulvestrant is as viable and perhaps even preferred first-line therapy for postmenopausal women with hormone receptor-positive, HER2-negative advanced breast cancer. The impressive results from this trial are likely, because the study used the 500-mg dose of fulvestrant, which is twice the dose used in the original trials. However, the 500-mg dose has previously been studied, and long-term outcome data suggest both safety and efficiency. The large randomized, double-blinded phase 3 CONFIRM trial, published in 2013, compared the 250-mg dose with the 500-mg dose and found that the higher dose was associated with a 19% reduction in the risk of death and a 4.1 month increase in median overall survival (OS) without any new safety concerns.5
Palbociclib
The FDA recently granted accelerated approval to palbociclib in combination with letrozole for the first-line therapy of advanced hormone receptor-positive, HER2-negative breast cancer in postmenopausal women. Palbociclib is an oral small-molecular inhibitor of cyclindependent kinases 4 and 6. Preclinical data suggested synergy with anti-estrogen therapies and inhibition of breast cancer cell growth.7
A phase 2, open-label randomized trial (PALOMA-1/TRIO-18) enrolled 165 patients. Progression-free survival (PFS) was 20.2 months for the palbociclib plus letrozole arm and 10.2 months for the letrozole alone arm. Significant toxicities were noted in the palbociclib arm, including 54% of people experiencing grade 3 to 4 neutropenia (vs 1% in the letrozole arm), leukopenia in 19% (vs 0%) and fatigue in 4% (vs 1%). A phase 3 trial is currently enrolling patients.7 While we await the results of the phase 3 trial and long-term follow-up data, palbociclib plus letrozole is a new, viable option for metastatic hormone receptor-positive advanced breast cancer.
Although many practitioners will continue to reasonably use any AI or selective ER modulator when treating metastatic breast cancer, both fulvestrant and palbociclib in combination with letrozole are new evidence-based, first-line options worth considering.
Early-Stage Treatment Options
There are many acceptable therapeutic options for treating early stage breast cancer. Tamoxifen has traditionally been used in the adjuvant setting for premenopausal women, whereas AIs are often used in postmenopausal women. There has also been a long-standing debate about the role of ovarian suppression in premenopausal women.
The recently published phase 3 TEXT and SOFT trials attempted to provide answers to these long-standing therapeutic dilemmas. The SOFT trial randomly assigned 3,066 premenopausal women to 5 years of tamoxifen, 5 years of tamoxifen plus ovarian suppression, or exemestane plus ovarian suppression. The TEXT trial randomly assigned 2,672 women to receive either exemestane plus ovarian suppression or tamoxifen plus ovarian suppression. The studies showed that subjecting all women receiving tamoxifen to ovarian suppression did not provide any significant benefit.8,9
However, the subgroup of women with high-risk disease who required adjuvant chemotherapy and remained premenopausal experienced improved outcomes from ovarian suppression. This high-risk subgroup when given tamoxifen plus ovarian suppression had a 4.5% absolute reduction in breast cancer recurrence at 5 years compared with the group that received tamoxifen alone. When this high-risk subgroup was given exemestane plus ovarian suppression, the women had a 7.7% absolute reduction in breast cancer recurrence at 5 years compared with the group that received tamoxifen alone.8
Ovarian suppression resulted in significant additional AEs, including depression and menopausal symptoms. The authors of the study also pointed out the additional risk of hypertension, musculoskeletal AEs, and decreased bone density. Furthermore, the OS data from these studies are premature, because the patients had fewer AEs than initially anticipated; this resulted in an only 5% mortality at publication.
The study design also raised several interesting questions. The primary endpoint was disease-free survival. The authors defined this as the time from randomization to the first appearance of invasive recurrence of breast cancer (local, regional, or distant), invasive contralateral breast cancer, second (non-breast) invasive cancer, or death without breast cancer recurrence or second invasive cancer. When studying adjuvant therapy for diseases, such as breast cancer, which carry long-term survival, studies often use PFS with various modified definitions as a surrogate marker for OS. Clinicians are then left to decide whether this surrogate marker is an accurate predictor of OS or other important clinical outcomes.
In the combined analysis of the TEXT and SOFT trials, only 60% of the first recurrences, second invasive cancers, or deaths involved recurrence of breast cancer
at a distant site.9 Because locally recurrent breast cancer is highly treatable and often curable, clinicians must ask whether the increased toxicities of ovarian suppression are worth the large number of women who experienced local recurrence given the still relatively small absolute reduction in recurrence risk.
Last, the study authors retrospectively reviewed data from the International Breast Cancer Study Group and U.S. Intergroup trials and concluded that women aged < 35 years were most likely to be at high-risk for AEs.10,11 A subgroup analysis of women aged < 35 years in the SOFT trial noted that breast cancer recurred within 5 years in one-third of women receiving tamoxifen alone, whereas only in one-sixth of women receiving exemestane plus ovarian suppression.8 This is the basis for the conclusion that premenopausal women, particularly those aged < 35 years, with high-risk disease who receive chemotherapy and remain premenopausal after chemotherapy, benefit from ovarian suppression in combination with tamoxifen, and even more impressively from ovarian suppression combined with exemestane.
The problem is that the study did not risk-stratify patients based on those aged < 35 years, and the conclusion is based on a subgroup analysis using a primary endpoint that may not accurately predict OS. Nonetheless, although not definitive, the data from the TEXT and SOFT trials raise interesting therapeutic questions that require further study and certainly provide tempting therapeutic options in patients who are clinically at high risk for recurrence.
HER2-Positive Breast Cancer
Up to 20% of invasive breast cancers are a result of HER2 gene amplification or overexpression of the HER2 protein, a tyrosine kinase transmembrane receptor, resulting in a more aggressive phenotype and a poor prognosis. Anti-HER2 drugs have changed the landscape of the disease previously known as aggressive breast cancer with a poor survival rate.
Treatment with the anti-HER2 humanized monoclonal antibody trastuzumab in addition to chemotherapy, compared with chemotherapy alone, significantly improves PFS and OS among patients with HER2-positive metastatic as well as early breast cancer. However, in most patients with HER2-positive metastatic breast cancer, the disease progresses, highlighting the need for new, targeted therapies for advanced disease.
New Standard of Care
The original studies of trastuzumab showed improved OS in late-stage (metastatic) breast cancer from 20.3 to 25.1 months, and in early-stage breast cancer, it reduced the risk of cancer returning after surgery by an absolute risk of 9.5% and the risk of death by an absolute risk of 3%.
New therapies directed at HER2 are being developed, among them pertuzumab, a humanized monoclonal antibody that binds HER2 at a different epitope of the HER2 extracellular domain (subdomain 2) than that at which trastuzumab binds. Pertuzumab prevents HER2 from dimerizing with other ligand-activated HER receptors, most notably HER3. Like trastuzumab, pertuzumab stimulates antibody-dependent, cell-mediated cytotoxicity. Because pertuzumab and trastuzumab bind to different HER2 epitopes and have complementary mechanisms of action, these 2 agents, when given together, provide a more comprehensive blockade of HER2 signaling and result in greater antitumor activity than does either agent alone in HER2-positive tumor models.12 In phase 2 studies, a pertuzumab–trastuzumab regimen has shown activity in patients with HER2-positive metastatic breast cancer and in patients with early breast cancer.13
In the phase 3 CLEOPATRA study, the combination of pertuzumab plus trastuzumab plus docetaxel, used as first-line treatment for HER2-positive metastatic breast cancer compared with placebo plus trastuzumab plus docetaxel, significantly prolonged PFS (18.5 months vs 12.4 months), with no increase in cardiac toxic effects.12 In a recent updated follow-up of the CLEOPATRA study, the addition of pertuzumab to trastuzumab and docetaxel showed a significantly better median OS (56.5 months vs 40.8 months; hazard ratio, 0.68; P < .001).14 From these results, this combination regimen is now considered a first-line therapy for patients with HER2-positive metastatic breast cancer.
However, the cost of cancer treatment has become a mounting concern during the past decade, as new therapies come down the pipeline with ever-increasing price tags. Trastuzumab costs about $4,500 a month, and the newer pertuzumab runs about 30% higher, at $6,000 a month. For a full course of treatment, the cost of the pertuzumab and trastuzumab combination could go as high as $195,000, depending on the duration of therapy and the choice of taxanes.
Conclusions
The landscape of therapeutic options in high-risk, young patients with early-stage breast cancer as well as patients with advanced or metastatic disease is changing rapidly.
Clinicians now have 2 new first-line options for the treatment of advanced hormone receptor-positive, HER2-negative breast cancer. A phase 3 trial demonstrated that fulvestrant monotherapy offers improved PFS and some improvement in OS compared with anastrazole in postmenopausal women. A phase 2 trial showed that palbociclib plus letrozole offers improved PFS in postmenopausal women. Based on the SOFT and TEXT trials, clinicians treating high-risk premenopausal women now have some data to inform the debate about whether ovarian suppression should be added to hormone therapy.
Based on the CLEOPATRA trial, clinicians can now consider combination pertuzumab and trastuzumab and docetaxel as first-line therapy for patients with HER2-positive metastatic breast cancer.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
Click here to read the digital edition.
It is estimated that there were more than 3.1 million women living in the U.S. with a history of invasive breast cancer as of January 1, 2014, and an additional 231,840 women will be newly diagnosed with invasive breast cancer in 2015.1,2 The median age at the time of breast cancer diagnosis is 61 years. About 20% of breast cancers occur among women aged < 50 years, and 43% occur in women aged > 65 years.
The treatment and prognosis for breast cancer depend on the stage at diagnosis, the biologic characteristics of the tumor, and the age and health of the patient. The overall 5-year relative survival rate for female patients with breast cancer has improved from 75% to 90% from 1975 to 1977 and from 2003 to 2009, respectively, largely due to improvements in treatment (ie, chemotherapy, hormone therapy, and targeted drugs) and because of earlier diagnosis resulting from the widespread use of mammography and other screening tools.2
Estrogen Receptor-Positive Therapies
Women with breast cancer who test positive for hormone receptors are candidates for treatment with hormone therapy to reduce the likelihood of recurrence or as a core component of treatment for advanced disease. Currently available endocrine strategies for the treatment of estrogen receptor- (ER) positive breast cancer include targeting the ER with the antiestrogen drug tamoxifen. Another option is suppressing the amount of available ligand (estrogen) for the receptor either with gonadal suppression in premenopausal oophorectomy, or luteinizing hormonereleasing hormone agonists, or with the aromatase inhibitors (AIs) anastrozole, exemestane, and letrozole in postmenopausal women and by downregulating the receptor with fulvestrant. Given their proven efficacy and generally favorable adverse effect (AE) profile, these endocrine therapies are widely used in the treatment of both early-stage and recurrent and/or metastatic breast cancer.
Recent studies have offered new treatments for patients with hormone receptor-positive, human epidermal growth factor receptor 2 (HER2)-negative breast cancer. Innovative hormonal and targeted therapies for advanced disease as well as new data on adjuvant hormonal therapy for young high-risk patients are changing the available therapeutic options.
Advanced Metastatic Treatments
Treatment for metastatic hormone receptor-positive breast cancer has shifted from traditional cytotoxic chemotherapies to targeted therapeutic options. Most treatment guidelines, including the National Comprehensive Cancer Network guidelines, recommend targeted therapy with AIs or selective ER modulators rather than chemotherapy, except in the case of visceral crisis.3
Until recently, there had been relatively little guidance to inform which hormonal therapy was most appropriate. Aromatase inhibitors were generally reserved for postmenopausal women, whereas tamoxifen was preferred in premenopausal women.
Fulvestrant
The FDA initially approved fulvestrant, a hormone receptor downregulator, in 2002 at a 250-mg dose, following progression on an anti-estrogen therapy, such as tamoxifen in postmenopausal women with stage IV breast cancer. The FDA approval was based on similar response rates for the already approved agent anastrozole.4 However, pharmacokinetic findings from the phase 3 EFECT trial in 2008 prompted researchers to explore a 500-mg dose of fulvestrant.5
The recently published FIRST study is a phase 2, randomized, open-label study comparing fulvestrant 500 mg with anastrozole 1 mg as first-line hormonal therapy for postmenopausal women with hormone receptorpositive advanced breast cancer. Fulvestrant was given 500 mg once monthly with an extra dose given on day 14 of month 1. The trial enrolled 233 patients. The median time to progression was 23.4 months for fulvestrant and 13.1 months for anastrozole. These results translate into a 34% reduction in the risk of progression.6
These outcomes suggest that fulvestrant is as viable and perhaps even preferred first-line therapy for postmenopausal women with hormone receptor-positive, HER2-negative advanced breast cancer. The impressive results from this trial are likely, because the study used the 500-mg dose of fulvestrant, which is twice the dose used in the original trials. However, the 500-mg dose has previously been studied, and long-term outcome data suggest both safety and efficiency. The large randomized, double-blinded phase 3 CONFIRM trial, published in 2013, compared the 250-mg dose with the 500-mg dose and found that the higher dose was associated with a 19% reduction in the risk of death and a 4.1 month increase in median overall survival (OS) without any new safety concerns.5
Palbociclib
The FDA recently granted accelerated approval to palbociclib in combination with letrozole for the first-line therapy of advanced hormone receptor-positive, HER2-negative breast cancer in postmenopausal women. Palbociclib is an oral small-molecular inhibitor of cyclindependent kinases 4 and 6. Preclinical data suggested synergy with anti-estrogen therapies and inhibition of breast cancer cell growth.7
A phase 2, open-label randomized trial (PALOMA-1/TRIO-18) enrolled 165 patients. Progression-free survival (PFS) was 20.2 months for the palbociclib plus letrozole arm and 10.2 months for the letrozole alone arm. Significant toxicities were noted in the palbociclib arm, including 54% of people experiencing grade 3 to 4 neutropenia (vs 1% in the letrozole arm), leukopenia in 19% (vs 0%) and fatigue in 4% (vs 1%). A phase 3 trial is currently enrolling patients.7 While we await the results of the phase 3 trial and long-term follow-up data, palbociclib plus letrozole is a new, viable option for metastatic hormone receptor-positive advanced breast cancer.
Although many practitioners will continue to reasonably use any AI or selective ER modulator when treating metastatic breast cancer, both fulvestrant and palbociclib in combination with letrozole are new evidence-based, first-line options worth considering.
Early-Stage Treatment Options
There are many acceptable therapeutic options for treating early stage breast cancer. Tamoxifen has traditionally been used in the adjuvant setting for premenopausal women, whereas AIs are often used in postmenopausal women. There has also been a long-standing debate about the role of ovarian suppression in premenopausal women.
The recently published phase 3 TEXT and SOFT trials attempted to provide answers to these long-standing therapeutic dilemmas. The SOFT trial randomly assigned 3,066 premenopausal women to 5 years of tamoxifen, 5 years of tamoxifen plus ovarian suppression, or exemestane plus ovarian suppression. The TEXT trial randomly assigned 2,672 women to receive either exemestane plus ovarian suppression or tamoxifen plus ovarian suppression. The studies showed that subjecting all women receiving tamoxifen to ovarian suppression did not provide any significant benefit.8,9
However, the subgroup of women with high-risk disease who required adjuvant chemotherapy and remained premenopausal experienced improved outcomes from ovarian suppression. This high-risk subgroup when given tamoxifen plus ovarian suppression had a 4.5% absolute reduction in breast cancer recurrence at 5 years compared with the group that received tamoxifen alone. When this high-risk subgroup was given exemestane plus ovarian suppression, the women had a 7.7% absolute reduction in breast cancer recurrence at 5 years compared with the group that received tamoxifen alone.8
Ovarian suppression resulted in significant additional AEs, including depression and menopausal symptoms. The authors of the study also pointed out the additional risk of hypertension, musculoskeletal AEs, and decreased bone density. Furthermore, the OS data from these studies are premature, because the patients had fewer AEs than initially anticipated; this resulted in an only 5% mortality at publication.
The study design also raised several interesting questions. The primary endpoint was disease-free survival. The authors defined this as the time from randomization to the first appearance of invasive recurrence of breast cancer (local, regional, or distant), invasive contralateral breast cancer, second (non-breast) invasive cancer, or death without breast cancer recurrence or second invasive cancer. When studying adjuvant therapy for diseases, such as breast cancer, which carry long-term survival, studies often use PFS with various modified definitions as a surrogate marker for OS. Clinicians are then left to decide whether this surrogate marker is an accurate predictor of OS or other important clinical outcomes.
In the combined analysis of the TEXT and SOFT trials, only 60% of the first recurrences, second invasive cancers, or deaths involved recurrence of breast cancer
at a distant site.9 Because locally recurrent breast cancer is highly treatable and often curable, clinicians must ask whether the increased toxicities of ovarian suppression are worth the large number of women who experienced local recurrence given the still relatively small absolute reduction in recurrence risk.
Last, the study authors retrospectively reviewed data from the International Breast Cancer Study Group and U.S. Intergroup trials and concluded that women aged < 35 years were most likely to be at high-risk for AEs.10,11 A subgroup analysis of women aged < 35 years in the SOFT trial noted that breast cancer recurred within 5 years in one-third of women receiving tamoxifen alone, whereas only in one-sixth of women receiving exemestane plus ovarian suppression.8 This is the basis for the conclusion that premenopausal women, particularly those aged < 35 years, with high-risk disease who receive chemotherapy and remain premenopausal after chemotherapy, benefit from ovarian suppression in combination with tamoxifen, and even more impressively from ovarian suppression combined with exemestane.
The problem is that the study did not risk-stratify patients based on those aged < 35 years, and the conclusion is based on a subgroup analysis using a primary endpoint that may not accurately predict OS. Nonetheless, although not definitive, the data from the TEXT and SOFT trials raise interesting therapeutic questions that require further study and certainly provide tempting therapeutic options in patients who are clinically at high risk for recurrence.
HER2-Positive Breast Cancer
Up to 20% of invasive breast cancers are a result of HER2 gene amplification or overexpression of the HER2 protein, a tyrosine kinase transmembrane receptor, resulting in a more aggressive phenotype and a poor prognosis. Anti-HER2 drugs have changed the landscape of the disease previously known as aggressive breast cancer with a poor survival rate.
Treatment with the anti-HER2 humanized monoclonal antibody trastuzumab in addition to chemotherapy, compared with chemotherapy alone, significantly improves PFS and OS among patients with HER2-positive metastatic as well as early breast cancer. However, in most patients with HER2-positive metastatic breast cancer, the disease progresses, highlighting the need for new, targeted therapies for advanced disease.
New Standard of Care
The original studies of trastuzumab showed improved OS in late-stage (metastatic) breast cancer from 20.3 to 25.1 months, and in early-stage breast cancer, it reduced the risk of cancer returning after surgery by an absolute risk of 9.5% and the risk of death by an absolute risk of 3%.
New therapies directed at HER2 are being developed, among them pertuzumab, a humanized monoclonal antibody that binds HER2 at a different epitope of the HER2 extracellular domain (subdomain 2) than that at which trastuzumab binds. Pertuzumab prevents HER2 from dimerizing with other ligand-activated HER receptors, most notably HER3. Like trastuzumab, pertuzumab stimulates antibody-dependent, cell-mediated cytotoxicity. Because pertuzumab and trastuzumab bind to different HER2 epitopes and have complementary mechanisms of action, these 2 agents, when given together, provide a more comprehensive blockade of HER2 signaling and result in greater antitumor activity than does either agent alone in HER2-positive tumor models.12 In phase 2 studies, a pertuzumab–trastuzumab regimen has shown activity in patients with HER2-positive metastatic breast cancer and in patients with early breast cancer.13
In the phase 3 CLEOPATRA study, the combination of pertuzumab plus trastuzumab plus docetaxel, used as first-line treatment for HER2-positive metastatic breast cancer compared with placebo plus trastuzumab plus docetaxel, significantly prolonged PFS (18.5 months vs 12.4 months), with no increase in cardiac toxic effects.12 In a recent updated follow-up of the CLEOPATRA study, the addition of pertuzumab to trastuzumab and docetaxel showed a significantly better median OS (56.5 months vs 40.8 months; hazard ratio, 0.68; P < .001).14 From these results, this combination regimen is now considered a first-line therapy for patients with HER2-positive metastatic breast cancer.
However, the cost of cancer treatment has become a mounting concern during the past decade, as new therapies come down the pipeline with ever-increasing price tags. Trastuzumab costs about $4,500 a month, and the newer pertuzumab runs about 30% higher, at $6,000 a month. For a full course of treatment, the cost of the pertuzumab and trastuzumab combination could go as high as $195,000, depending on the duration of therapy and the choice of taxanes.
Conclusions
The landscape of therapeutic options in high-risk, young patients with early-stage breast cancer as well as patients with advanced or metastatic disease is changing rapidly.
Clinicians now have 2 new first-line options for the treatment of advanced hormone receptor-positive, HER2-negative breast cancer. A phase 3 trial demonstrated that fulvestrant monotherapy offers improved PFS and some improvement in OS compared with anastrazole in postmenopausal women. A phase 2 trial showed that palbociclib plus letrozole offers improved PFS in postmenopausal women. Based on the SOFT and TEXT trials, clinicians treating high-risk premenopausal women now have some data to inform the debate about whether ovarian suppression should be added to hormone therapy.
Based on the CLEOPATRA trial, clinicians can now consider combination pertuzumab and trastuzumab and docetaxel as first-line therapy for patients with HER2-positive metastatic breast cancer.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
Click here to read the digital edition.
1. American Cancer Society. Cancer facts & figures, 2015. Atlanta, GA: American Cancer Society; 2015.
2. American Cancer Society. Cancer treatment & survivorship facts & figures, 2014-2015. Atlanta, GA: American Cancer Society; 2014.
3. National Comprehensive Cancer Network. NCCN clinical Practice guidelines in oncology: breast Cancer. Version 1. 2015. Fort Washington, PA: National Comprehensive Cancer Network; 2015:BINV-19.
4. Howell A, Robertson JF, Quaresma Albano J. Fulvestrant, formerly ICI 182,780, is as effective as anastrozole in postmenopausal women with advanced breast cancer progressing after prior endocrine treatment. J Clin Oncol. 2002;20(16):3396-3403.
5. Di Leo A, Jerusalem G, Petruzelka L, et al. Final overall survival: fulvestrant 500 mg vs 250 mg in the randomized CONFIRM trial. J Natl Cancer Inst. 2014;106(1):djt337.
6. Robertson JF, Lindemann JB, Llombart-Cussac A, et al. Fulvestrant 500 mg versus anastrozole 1 mg for the first-line treatment of advanced breast cancer: follow-up analysis from the randomized ‘FIRST’ study. Breast Cancer Res Treat. 2012;136(2):503-511.
7. Finn RS, Crown JP, Lang I, et al. The cyclin-dependent kinase 4/6 inhibitor palbociclib in combination with letrozole versus letrozole alone as first-line treatment of oestrogen receptor-positive, HER2-negative, advanced breast cancer (PALOMA-1/TRIO-18): a randomised phase 2 study. Lancet Oncol. 2015;16(1):25-35.
8. Francis PA, Regan MM, Fleming GF, et al; SOFT Investigators; International Breast Cancer Study Group. Adjuvant ovarian suppression in premenopausal breast cancer. N Engl J Med. 2015;372(5):436-446.
9. Pagani O. Regan MM, Walley BA, et al. TEXT and SOFT Investigators; International Breast Cancer Study Group. Adjuvant exemestane with ovarian suppression in premenopausal breast cancer. N Engl J Med. 2014;371(2):107-118.
10. Aebi S, Gelber S, Castiglione-Gertsch M, et al. Is chemotherapy alone adequate for young women with oestrogen-receptor-positive breast cancer? Lancet. 2000;355:1869-1874.
11. Goldhirsch A, Gelber RD, Yothers G, et al. Adjuvant therapy for very young women with breast cancer: need for tailored treatments. J Natl Cancer Inst Monogr. 2001;(30):44-51
12. Hudis CA. Trastuzumab—mechanism of action and use in clinical practice. N Engl J Med. 2007;357(1):39-51.
13. Baselga J, Cortés J, Kim SB, et al; CLEOPATRA Study Group. Pertuzumab plus trastuzumab plus docetaxel for metastatic breast cancer. N Engl J Med. 2012;366(2):109-119.
14. Swain SM, Baselga J, Kim SB, et al; CLEOPATRA Study Group. Pertuzumab, trastuzumab, and docetaxel in HER2-positive metastatic breast cancer. N Engl J Med. 2015;372(8):724-734.
1. American Cancer Society. Cancer facts & figures, 2015. Atlanta, GA: American Cancer Society; 2015.
2. American Cancer Society. Cancer treatment & survivorship facts & figures, 2014-2015. Atlanta, GA: American Cancer Society; 2014.
3. National Comprehensive Cancer Network. NCCN clinical Practice guidelines in oncology: breast Cancer. Version 1. 2015. Fort Washington, PA: National Comprehensive Cancer Network; 2015:BINV-19.
4. Howell A, Robertson JF, Quaresma Albano J. Fulvestrant, formerly ICI 182,780, is as effective as anastrozole in postmenopausal women with advanced breast cancer progressing after prior endocrine treatment. J Clin Oncol. 2002;20(16):3396-3403.
5. Di Leo A, Jerusalem G, Petruzelka L, et al. Final overall survival: fulvestrant 500 mg vs 250 mg in the randomized CONFIRM trial. J Natl Cancer Inst. 2014;106(1):djt337.
6. Robertson JF, Lindemann JB, Llombart-Cussac A, et al. Fulvestrant 500 mg versus anastrozole 1 mg for the first-line treatment of advanced breast cancer: follow-up analysis from the randomized ‘FIRST’ study. Breast Cancer Res Treat. 2012;136(2):503-511.
7. Finn RS, Crown JP, Lang I, et al. The cyclin-dependent kinase 4/6 inhibitor palbociclib in combination with letrozole versus letrozole alone as first-line treatment of oestrogen receptor-positive, HER2-negative, advanced breast cancer (PALOMA-1/TRIO-18): a randomised phase 2 study. Lancet Oncol. 2015;16(1):25-35.
8. Francis PA, Regan MM, Fleming GF, et al; SOFT Investigators; International Breast Cancer Study Group. Adjuvant ovarian suppression in premenopausal breast cancer. N Engl J Med. 2015;372(5):436-446.
9. Pagani O. Regan MM, Walley BA, et al. TEXT and SOFT Investigators; International Breast Cancer Study Group. Adjuvant exemestane with ovarian suppression in premenopausal breast cancer. N Engl J Med. 2014;371(2):107-118.
10. Aebi S, Gelber S, Castiglione-Gertsch M, et al. Is chemotherapy alone adequate for young women with oestrogen-receptor-positive breast cancer? Lancet. 2000;355:1869-1874.
11. Goldhirsch A, Gelber RD, Yothers G, et al. Adjuvant therapy for very young women with breast cancer: need for tailored treatments. J Natl Cancer Inst Monogr. 2001;(30):44-51
12. Hudis CA. Trastuzumab—mechanism of action and use in clinical practice. N Engl J Med. 2007;357(1):39-51.
13. Baselga J, Cortés J, Kim SB, et al; CLEOPATRA Study Group. Pertuzumab plus trastuzumab plus docetaxel for metastatic breast cancer. N Engl J Med. 2012;366(2):109-119.
14. Swain SM, Baselga J, Kim SB, et al; CLEOPATRA Study Group. Pertuzumab, trastuzumab, and docetaxel in HER2-positive metastatic breast cancer. N Engl J Med. 2015;372(8):724-734.