User login
Health Care Utilization of Veterans With Serious Mental Illness (FULL)
About 10 million U.S. adults live with serious mental illness (SMI).1 Among military veterans, the number of mental health (MH) diagnoses is increasing with the return of troops from deployment in Iraq and Afghanistan.2-4 This increase has considerable implications for service use at the VA. An estimated 35% of army and marine veterans sought MH services within 1 year of returning from Iraq and Afghanistan.5 Furthermore, there is an association of MH disorders, physical illnesses, and mortality rates among veterans.2,6 Rising MH needs will increase the need for services; not unexpectedly, the VA is one of the largest providers of integrated health care in the U.S.7
Many patients with SMI have additional health issues, secondary to medication adverse effects, medical comorbidities, and other factors.8-12 Furthermore, their rates of preventable risk factors (eg, smoking, alcohol abuse, and poor exercise and diet13,14) are higher. Comorbid medical illnesses can sideline the treatment of mental illness and lead to negative health outcomes.15,16 These medical conditions coupled with SMI may increase overall rates of health care utilization in terms of outpatient visits, procedures, and inpatient hospitalizations. However, the literature on factors associated with health care utilization in veterans with SMI is scant and generally inconclusive.
Findings on utilization of non-MH medical services for veterans with comorbid MH diagnoses are mixed. Operation Enduring Freedom (OEF) and Operation Iraqi Freedom (OIF) veterans with MH diagnoses have been found to use significantly more non-MH medical services than do OEF and OIF veterans without these diagnoses.17 However, other studies have found that veterans with SMI seem to be less likely to use medical services.18,19 For example, in a study of the rate of medical visits for veterans with psychiatric diagnoses, veterans with SMI were found to have fewer outpatient medical visits than do other veterans without SMI.20 Given the high rates of medical comorbidities in veterans with SMI, this finding of reduced rates of medical care is both informative and concerning. However, more information on utilization rates for other types of health care system services is needed.
In addition to MH diagnoses, multiple factors influence the use of health care services. Lower income predicts overall use of mental and medical services for female but not male veterans.21 A large proportion of VA patients are male, and that disparity may affect female veterans’ perceptions and use of VA health care, underscoring the importance of examining the effects of sex in health care utilization.22 Unmarried status, unemployment, and combat experience are other factors associated with higher health care utilization.23,24 Sociodemographic factors, including income and possession of private health insurance, are associated with veterans’ use of VA health care services.25 It is important to understand the effects of these factors on service utilization by veterans with SMI in order to provide them with optimal, targeted health care.
The authors conducted this study to examine factors affecting MH service utilization and health outcomes in veterans with SMI at the VA Palo Alto Health Care System (VAPAHCS). A retrospective data analysis of medical records was performed. More specifically, this study aimed to identify veteran-specific variables (eg, demographics, psychiatric diagnosis, comorbid medical conditions, combat status) associated with health care utilization and outcomes in veterans with SMI. Dependent variables of interest included service utilization, such as rate and length of inpatient hospitalization and frequency of outpatient encounters. Examining predictors of inpatient medical and psychiatric hospitalization (demographic, clinical, or treatment-related factors) can provide insight into which veterans can benefit from targeted, intensive interventions. A better understanding of the factors affecting comprehensive health care service use for veterans with SMI can clarify targeted interventions and follow-up care for an expanding population.
Methods
Study approval was obtained from the institutional review board at Stanford University and the VAPAHCS research and development committee. Medical record data for veterans treated at the VAPAHCS were collected for a 10-year period (fiscal years 2003-2012). The Computerized Patient Record System (CPRS) data were accessed by VA decision support system staff and analyzed with SPSS Version 21.0 (Armonk, NY). Veterans were identified by ICD-9 codes 295.00 through 298.9, as documented in CPRS.
For this study, schizophrenic, psychotic, bipolar, depressive, and mood disorders were classified as SMI. VA clinic codes were used to categorize visits by service: medical (general medical clinic, surgery, pharmacy, laboratory tests), MH (outpatient visits, intakes and assessments), ancillary services (chaplain, social work, administration), residential MH treatment (substance use disorder, domiciliary care), home-based primary care, and home-based MH care. Psychiatric diagnoses were grouped into schizophrenic disorders, unspecified psychotic disorders, bipolar disorders, major depressive disorders (MDD), and mood disorders not otherwise specified (NOS). The demographic variables obtained through medical records included age, service connection, combat flag (deployment to combat zone), service period, and marital status at start of fiscal year 2003.
Over the 10-year period, 15,414 unique patients were classified as having SMI. Veterans who died during this period were removed from the dataset because of concerns about incomplete data (with specific dates of death unavailable, it was difficult to determine whether missed VA visits during a particular year indicated low utilization or death). In addition, inpatient medical and psychiatric visits were excluded from the analysis in an effort to identify how outpatient utilization uniquely affects the frequency of inpatient hospitalization. Last, because the authors wanted to exclude from the analysis all patients who may have had a single consultation at the VA during the 10-year period, they required at least 2 visits per patient. The final dataset consisted of 11,135 patients.
Age differences reported in the literature included a lesser likelihood of MH diagnosis for older returning OEF/OIF veterans,26 and older age as a factor related to veterans’ increased health care use.27 Therefore, age was included as a covariate in the analyses.
Results
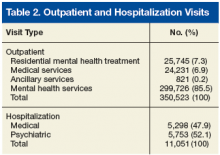
The 11,135 patients included in the study, made a total of 350,523 outpatient visits over the period (Table 1). The mean number of outpatient visits was 6.6 (SD, 11.7; median, 3.5; mode, 2.0; range, 1.0-219.9). Over the 10-year period, 14.0% of patients averaged 1 visit per year, 23.4% averaged 2 visits, 15.6% averaged 3 visits, 10.7% averaged 4 visits, 7.4% averaged 5 visits, and 28.9% averaged 6 or more visits. Table 2 lists the frequencies and percentages of outpatient visits and hospitalizations.
Group Difference T-Test Analysis
Of the 553 OEF/OIF veterans in the study, 225 (40.7%) had combat flags, and 328 (59.3%) did not. Independent- groups t test showed significant group differences in mean number of visits (t[2,964.48] = 9.94, P < .01). Veterans with combat flags averaged significantly fewer visits per year (mean, 4.79; SD, 5.89) than did veterans without flags (mean, 6.89; SD, 12.50), even with age as a covariate (F[1, 10,675] = 34.63, P < .01).
Independent-groups t test showed significant group differences in mean number of nonpsychiatric hospitalizations (t[2,328.68] = 5.00, P < .01). Veterans with combat flags averaged significantly fewer nonpsychiatric hospitalizations per year (mean, 0.34; SD, .99) than did veterans without flags (mean, 0.51; SD, 1.71), even with age as a covariate (F[1, 10,675] = 9.81, P < .01).
Independent-groups t test showed significant group differences in mean number of psychiatric hospitalizations (t[2,706.75] = 7.69, P < .01). Veterans with combat flags averaged significantly fewer psychiatric hospitalizations per year (mean, 0.31; SD, 0.91) than did veterans without flags (mean, 0.55; SD, 1.80), even with age as a covariate (F[1, 10,675] = 23.51, P < .01).
Group Differences by SMI Diagnosis
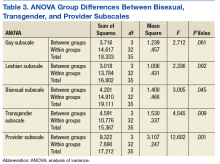
Mean number of visits. Analysis of covariance (ANCOVA) was used to determine differences among the SMI groups (schizophrenic, psychotic, bipolar, depressive, and mood disorders) in mean number of outpatient visits per year with age as a covariate. With age accounted for, there were significant differences among the SMI groups (F[4, 11,129] = 285.02, P < .01). Therefore, pairwise comparisons were used to examine differences among individual disorders. Veterans with schizophrenic disorders averaged more visits per year (mean, 16.2; SD, 27.12) than did veterans with bipolar disorders (mean, 6.4; SD, 7.8, P < .01) and veterans with MDD (mean, 4.8; SD, 5.5, P < .01). Veterans with schizophrenia also averaged more visits per year than did veterans with mood disorders NOS (mean, 5.2; SD, 5.9, P < .01) and veterans with unspecified psychotic disorders (mean, 5.9; SD, 9.6, P < .01).
Nonpsychiatric hospitalizations. An ANCOVA analysis was used to determine group differences in nonpsychiatric hospitalizations per year with SMI diagnosis as the independent variable and age as a covariate. With age accounted for, there were significant differences among the SMI groups (F[4, 11,129] = 143.02, P < .01). Furthermore, pairwise comparisons were used to examine differences among individual disorders. Veterans with schizophrenic disorders averaged more nonpsychiatric hospitalizations (mean, 1.4; SD, 3.4) than did veterans with bipolar disorders (mean, 0.7; SD, 1.8, P < .01) and veterans with MDD (mean, 0.3; SD, 0.9, P < .01). Veterans with schizophrenia averaged more nonpsychiatric hospitalizations per year than did veterans with mood disorders NOS (mean, 0.3; SD, 0.8, P < .01) and veterans with unspecified psychotic disorders (mean, 0.5; SD, 1.2, P < .01).
Psychiatric hospitalizations. An ANCOVA analysis was used to determine group differences in psychiatric hospitalizations per year with SMI diagnosis as the independent variable and age as a covariate. With age accounted for, there were significant differences among the SMI groups (F[4, 11,129] = 204.64, P < .01). Furthermore, pairwise comparisons were used to examine differences among individual disorders. Veterans with schizophrenic disorders averaged more psychiatric hospitalizations per year (mean, 1.6; SD, 3.4) than did veterans with bipolar disorders (mean, 0.7; SD, 1.9, P < .01) and veterans with MDD (mean, 0.3; SD, 1.0, P < .01). Veterans with schizophrenia averaged more psychiatric hospitalizations per year than did veterans with mood disorders NOS (mean, 0.2; SD, 0.8, P < .01) and veterans with unspecified psychotic disorders (mean, 0.9; SD, 1.8, P < .01).
Group Differences by Marital Status
Mean number of visits. An ANCOVA analysis was used to determine differences among the marital status groups (married, divorced, never married) in mean number of outpatient visits per year with age as a covariate. With age accounted for, there were significant differences among the marital status groups (F[3, 11,130] = 104.37, P < .01). Married veterans averaged fewer visits per year (mean, 4.6; SD, 5.2) than did divorced veterans (mean, 6.2; SD, 9.9, P < .01) and never-married veterans (mean, 9.8; SD, 18.8, P < .01).
Nonpsychiatric hospitalizations. An ANCOVA analysis was used to determine group differences in nonpsychiatric hospitalizations per year with marital status as the independent variable and age as a covariate. With age accounted for, there were significant differences among the marital status groups (F[3, 11,130] = 43.97, P < .01). Married veterans averaged fewer nonpsychiatric hospitalizations per year (mean, 0.27; SD, 1.52) than did divorced veterans (mean, 0.47; SD, 1.30, P < .01) and never-married veterans (mean, 0.75; SD, 2.08, P <.01) Last, married veterans averaged fewer nonpsychiatric hospitalizations than did veterans with other/unknown marital status (mean, 0.5; SD, 1.5, P < .01).
Psychiatric hospitalizations. An ANCOVA analysis was used to determine group differences in mean number of psychiatric hospitalizations per year with marital status as the independent variable and age as a covariate. With age accounted for, there were significant differences among the marital status groups (F[3, 11,130] = 68.94, P < .01). Married veterans averaged fewer psychiatric hospitalizations per year (mean, 0.23; SD, 0.86) than did divorced veterans (mean, 0.55; SD, 1.58, P < .01) and nevermarried veterans (mean, 0.86; SD, 2.53, P < .01).
Predictors of Hospitalization
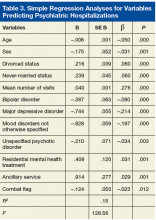
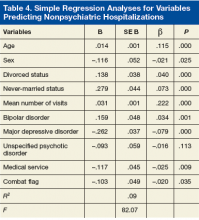
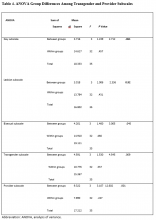
Regression analyses revealed that age, sex, divorced status, never-married status, mean number of visits, bipolar disorders, MDD, mood disorders NOS, unspecified psychotic disorders, residential MH treatment, ancillary services, and combat flag were all significant predictors of total number of both psychiatric hospitalizations (adjusted R2 = .15, F[128.56] = .00) and nonpsychiatric hospitalizations (adjusted R2 = .09, F[82.07] = .00) (Tables 3 & 4).
Discussion
This study examined medical and psychiatric service utilization in veterans with SMI diagnoses. For this population, 85.5% of the total number of VA visits were for MH services, and 52.1% of hospitalizations were for psychiatric care. Medical visits accounted for only 6.9% of outpatient services provided. Although the high utilization of psychiatric services by this SMI population may not be striking in itself, the considerably lower utilization of medical services is notable given the high rates of medical comorbidities associated with SMI. This result confirms findings from other studies.8-10,20 Possibly, VA health care guidelines may account for the lower medical service utilization. For example, the outpatient MH service has a metabolic syndrome clinic with psychiatrist prescribing guidelines for treating veterans who meet the criteria for metabolic syndrome.
The total number of outpatient visits varied widely. The largest percentage of veterans (29%) averaged 6 or more visits per year, followed by 2, 3, 1, and 4 visits. These findings suggest many patients are frequently utilizing services. The implications for inpatient hospitalization are notable, as the study’s data also suggest veterans with more total outpatient visits are at increased risk for hospitalization. The higher number of visits may be attributable to the SMI population’s increased health care needs and may suggest a need to target heavy users of outpatient services with more intensive programs to minimize costly hospitalizations. Mean number of outpatient visits was associated with a slightly higher risk for psychiatric hospitalization, possibly because of increased accessibility to care. Outpatient visits increased the risk for overall hospitalization, and psychiatric hospitalization specifically.
The type of SMI diagnosis predicted total number of outpatient visits, demonstrating that veterans with schizophrenic disorders averaged significantly more outpatient visits per year than did veterans with other SMI diagnoses. Interestingly, results showed that veterans with combat flags averaged significantly fewer outpatient visits than did veterans without flags. This finding is notable, as combat exposure is associated with higher rates of MH diagnoses and relatedly higher service utilization. However, combat veterans with SMI but not posttraumatic stress disorder (PTSD), averaged fewer outpatient visits than did veterans without combat exposure, which was not consistent with earlier findings.28 More research specifically on the effects of combat on veterans with PTSD may help in reconciling these findings. Married veterans averaged significantly fewer outpatient visits per year than did divorced or nevermarried veterans. It may be that social support among married couples is protective in veterans with SMI, or that veterans with SMI are unable to maintain marriages.
Hospitalization Predictors
Several significant predictors of psychiatric hospitalization were noted. Increasing age was negatively related to psychiatric hospitalization. It is possible that older patients with SMI receive other types of care, including board and care homes, or MH intensive case management, in which psychiatric outpatient care is readily provided—reducing the need for psychiatric hospitalization. In addition, married veterans were significantly less likely than were divorced and never-married veterans to have been hospitalized. It is possible that being married is protective for MH and physical health or that being unhealthy is a risk factor for divorce.
The type of SMI diagnosis also significantly predicted hospitalization. Having received specific types of services, including residential MH treatment and ancillary services, was a significant predictor of the total number of psychiatric hospitalizations. Last, having a combat flag negatively predicted psychiatric hospitalization—incongruent with earlier findings.24 These results suggest a profile of a veteran who likely could benefit either from a targeted intervention or from having ready access to a social worker.
Improved understanding of service utilization is vital to providing care to returning veterans. Mental disorders are very common among recent OEF, OIF, and Operation New Dawn veterans.29 As the rate of MH diagnoses climbs, the cost of providing health care grows exponentially. The cost of providing care to veterans with SMI can be addressed by identifying veteran-specific factors to provide intensive outreach and prevent costly hospitalization.
Limitations
The results of this study should be interpreted in light of several limitations. It is plausible that at least some veterans received health care services outside the VA, but non-VA data were not included in this study. Subsequent studies should include a group of veterans with SMI and a control group of veterans without SMI so that patterns of hospitalization and utilization rates can be compared. Also, the present study did not include data on missed appointments, an important variable in service utilization.
Missed appointments may suggest lack of follow-through with regular outpatient services, placing patients at risk for emergency services that require hospitalization. Veterans who had a single consultation were excluded in an effort to examine service utilization patterns of established patients. In future studies, including these patients could be informative in identifying specific patterns in this subpopulation. Fifth, PTSD was excluded from this study in order to identify utilization differences for veterans without PTSD.
Conclusion
Results of this study indicate that special attention should be given to veterans’ demographic and clinical factors, including age, sex, combat flags, marital status, and SMI diagnosis. Through identification of and outreach regarding these veteran-specific factors, it may be possible to use targeted interventions to reduce the need for inpatient hospitalization of veterans with chronic mental illness. Historically, the emphasis on access and outpatient care within the VA health care system drastically reduced the number of inpatient MH days.30 This outcome underscores the importance of outpatient services and suggests that targeted outpatient care can further reduce the need for inpatient MH care. Veterans with these outlined risk factors may benefit from implementation of early preventive measures.
Click here to read the digital edition.
1. Substance Abuse and Mental Health Services Administration. Results From the 2012 National Survey on Drug Use and Health: Mental Health Findings. NSDUH Series H-47, HHS publication SMA 13-4805. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2013.
2. Hoge CW, Terhakopian A, Castro CA, Messer SC, Engel CC. Association of posttraumatic stress disorder with somatic symptoms, health care visits, and absenteeism among Iraq war veterans. Am J Psychiatry. 2007;164(1):150-153.
3. Seal KH, Bertenthal D, Miner CR, Sen S, Marmar C. Bringing the war back home: mental health disorders among 103,788 US veterans returning from Iraq and Afghanistan seen at Department of Veterans Affairs facilities. Arch Intern Med. 2007;167(5):476-482.
4. Lee SE, Fonseca VP, Wolters CL, et al. Health care utilization behavior of veterans who deployed to Afghanistan and Iraq. Mil Med. 2015;180(4):374-379.
5. Hoge CW, Auchterlonie JL, Milliken CS. Mental health problems, use of mental health services, and attrition from military service after returning from deployment to Iraq or Afghanistan. JAMA. 2006;295(9):1023-1032.
6. Kramarow EA, Pastor PN. The health of male veterans and nonveterans aged 25-64: United States, 2007-2010. NCHS Data Brief. 2012;(101):1-8.
7. Elbogen EB, Wagner HR, Johnson SC, et al. Are Iraq and Afghanistan veterans using mental health services? New data from a national random-sample survey. Psychiatr Serv. 2013;64(2):134-141.
8. Bermudes RA, Keck PE Jr, Welge JA. The prevalence of the metabolic syndrome in psychiatric inpatients with primary psychotic and mood disorders. Psychosomatics. 2006;47(6):491-497.
9. Newcomer JW. Metabolic syndrome and mental illness. Am J Manag Care. 2007;13(7 suppl):S170-S177.
10. Toalson P, Ahmed S, Hardy T, Kabinoff G. The metabolic syndrome in patients with severe mental illnesses. Prim Care Companion J Clin Psychiatry. 2004;6(4):152-158.
11. Khatana SA, Kane J, Taveira TH, Bauer MS, Wu WC. Monitoring and prevalence rates of metabolic syndrome in military veterans with serious mental illness. PLoS One. 2011;6(4):e19298.
12. De Hert M, Correll CU, Bobes J, et al. Physical illness in patients with severe mental disorders. I. Prevalence, impact of medications and disparities in health care. World Psychiatry. 2011;10(1):52-77.
13. Lambert TJ, Velakoulis D, Pantelis C. Medical comorbidity in schizophrenia. Med J Aust. 2003;178(suppl):S67-S70.
14. Osborn DP, Nazareth I, King MB. Physical activity, dietary habits, and coronary heart disease risk factor knowledge amongst people with severe mental illness: a cross sectional comparative study in primary care. Soc Psychiatry Psychiatr Epidemiol. 2007;42(10):787-793.
15. Newcomer JW. Medical risk in patients with bipolar disorder and schizophrenia. J Clin Psychiatry. 2007;67(suppl 9):25-30, discussion 36-42.
16. Chwastiak LA, Rosenheck RA, Kazis LE. Association of psychiatric illness and obesity, physical inactivity, and smoking among a national sample of veterans. Psychosomatics. 2011;52(3):230-236.
17. Cohen BE, Gima K, Bertenthal D, Kim S, Marmar CR, Seal KH. Mental health diagnoses and utilization of VA non-mental health medical services among returning Iraq and Afghanistan veterans. J Gen Intern Med. 2010;25(1):18-24.
18. Chwastiak LA, Rosenheck RA, Kazis LE. Utilization of primary care of veterans with psychiatric illness in the national Department of Veterans Affairs Health Care System. J Gen Intern Med. 2008;23(11):1835-1840.
19. VA Office of Research and Development, Health Services Research and Development Service, Quality Enhancement Research Initiative. Mental health. QUERI fact sheet. http://www.hsrd.research.va.gov/publications/internal/mh_factsheet.pdf. Published December 2008. Accessed January 13, 2017.
20. Cradock-O’Leary J, Young AS, Yano EM, Wang M, Lee ML. Use of general medical services by VA patients with psychiatric disorders. Psychiatr Serv. 2002;53(7):874-878.
21. Di Leone BA, Vogt D, Gradus JL, Street AE, Giasson HL, Resick PA. Predictors of mental health care use among male and female veterans deployed in support of the wars in Afghanistan and Iraq. Psychol Serv. 2013;10(2):145-151.
22. Vogt DS, Barry AA, King LA. Toward gender-aware health care: evaluation of an intervention to enhance care for female patients in the VA setting. J Health Psychol. 2008;13(5):624-638.
23. Marshall RP, Jorm AF, Grayson DA, O’Toole BI. Posttraumatic stress disorder and other predictors of health care consumption by Vietnam veterans. Psychiatr Serv. 1998;49(12):1609-1611.
24. Rosenheck R, Massari L. Wartime military service and utilization of VA health care services. Mil Med. 1993;158(4):223-228.
25. Elhai JD, Grubaugh AL, Richardson JD, Egede LE, Creamer M. Outpatient medical and mental healthcare utilization models among military veterans: results from the 2001 National Survey of Veterans. J Psychiatr Res. 2008;42(10):858-867.
26. Seal KH, Bertenthal D, Miner CR, Sen S, Marmar C. Bringing the war back home: mental health disorders among 103,788 US veterans returning from Iraq and Afghanistan seen at Department of Veterans Affairs facilities. Arch Intern Med. 2007;167(5):476-482.
27. Fasoli DR, Glickman ME, Eisen SV. Predisposing characteristics, enabling resources and need as predictors of utilization and clinical outcomes for veterans receiving mental health services. Med Care. 2010;48(4):288-295.
28. Maguen S, Schumm JA, Norris RL, et al. Predictors of mental and physical health service utilization among Vietnam veterans. Psychol Serv. 2007;4(3):168-180.
29. Office of Public Health, Veterans Health Administration. Analysis of VA Health Care Utilization Among Operation Enduring Freedom (OEF), Operation Iraqi Freedom (OIF), and Operation New Dawn (OND) Veterans—Revised. http://www.publichealth.va.gov/docs/epidemiology/healthcare-utilization-report-fy2012-qtr3.pdf. Revised December 2012. Accessed February 6, 2017.
30. Wagner TH, Sinnott P, Siroka AM. Mental health and substance use disorder spending in the Department of Veterans Affairs, fiscal years 2000-2007. Psychiatr Serv. 2011;62(4):389-395.
About 10 million U.S. adults live with serious mental illness (SMI).1 Among military veterans, the number of mental health (MH) diagnoses is increasing with the return of troops from deployment in Iraq and Afghanistan.2-4 This increase has considerable implications for service use at the VA. An estimated 35% of army and marine veterans sought MH services within 1 year of returning from Iraq and Afghanistan.5 Furthermore, there is an association of MH disorders, physical illnesses, and mortality rates among veterans.2,6 Rising MH needs will increase the need for services; not unexpectedly, the VA is one of the largest providers of integrated health care in the U.S.7
Many patients with SMI have additional health issues, secondary to medication adverse effects, medical comorbidities, and other factors.8-12 Furthermore, their rates of preventable risk factors (eg, smoking, alcohol abuse, and poor exercise and diet13,14) are higher. Comorbid medical illnesses can sideline the treatment of mental illness and lead to negative health outcomes.15,16 These medical conditions coupled with SMI may increase overall rates of health care utilization in terms of outpatient visits, procedures, and inpatient hospitalizations. However, the literature on factors associated with health care utilization in veterans with SMI is scant and generally inconclusive.
Findings on utilization of non-MH medical services for veterans with comorbid MH diagnoses are mixed. Operation Enduring Freedom (OEF) and Operation Iraqi Freedom (OIF) veterans with MH diagnoses have been found to use significantly more non-MH medical services than do OEF and OIF veterans without these diagnoses.17 However, other studies have found that veterans with SMI seem to be less likely to use medical services.18,19 For example, in a study of the rate of medical visits for veterans with psychiatric diagnoses, veterans with SMI were found to have fewer outpatient medical visits than do other veterans without SMI.20 Given the high rates of medical comorbidities in veterans with SMI, this finding of reduced rates of medical care is both informative and concerning. However, more information on utilization rates for other types of health care system services is needed.
In addition to MH diagnoses, multiple factors influence the use of health care services. Lower income predicts overall use of mental and medical services for female but not male veterans.21 A large proportion of VA patients are male, and that disparity may affect female veterans’ perceptions and use of VA health care, underscoring the importance of examining the effects of sex in health care utilization.22 Unmarried status, unemployment, and combat experience are other factors associated with higher health care utilization.23,24 Sociodemographic factors, including income and possession of private health insurance, are associated with veterans’ use of VA health care services.25 It is important to understand the effects of these factors on service utilization by veterans with SMI in order to provide them with optimal, targeted health care.
The authors conducted this study to examine factors affecting MH service utilization and health outcomes in veterans with SMI at the VA Palo Alto Health Care System (VAPAHCS). A retrospective data analysis of medical records was performed. More specifically, this study aimed to identify veteran-specific variables (eg, demographics, psychiatric diagnosis, comorbid medical conditions, combat status) associated with health care utilization and outcomes in veterans with SMI. Dependent variables of interest included service utilization, such as rate and length of inpatient hospitalization and frequency of outpatient encounters. Examining predictors of inpatient medical and psychiatric hospitalization (demographic, clinical, or treatment-related factors) can provide insight into which veterans can benefit from targeted, intensive interventions. A better understanding of the factors affecting comprehensive health care service use for veterans with SMI can clarify targeted interventions and follow-up care for an expanding population.
Methods
Study approval was obtained from the institutional review board at Stanford University and the VAPAHCS research and development committee. Medical record data for veterans treated at the VAPAHCS were collected for a 10-year period (fiscal years 2003-2012). The Computerized Patient Record System (CPRS) data were accessed by VA decision support system staff and analyzed with SPSS Version 21.0 (Armonk, NY). Veterans were identified by ICD-9 codes 295.00 through 298.9, as documented in CPRS.
For this study, schizophrenic, psychotic, bipolar, depressive, and mood disorders were classified as SMI. VA clinic codes were used to categorize visits by service: medical (general medical clinic, surgery, pharmacy, laboratory tests), MH (outpatient visits, intakes and assessments), ancillary services (chaplain, social work, administration), residential MH treatment (substance use disorder, domiciliary care), home-based primary care, and home-based MH care. Psychiatric diagnoses were grouped into schizophrenic disorders, unspecified psychotic disorders, bipolar disorders, major depressive disorders (MDD), and mood disorders not otherwise specified (NOS). The demographic variables obtained through medical records included age, service connection, combat flag (deployment to combat zone), service period, and marital status at start of fiscal year 2003.
Over the 10-year period, 15,414 unique patients were classified as having SMI. Veterans who died during this period were removed from the dataset because of concerns about incomplete data (with specific dates of death unavailable, it was difficult to determine whether missed VA visits during a particular year indicated low utilization or death). In addition, inpatient medical and psychiatric visits were excluded from the analysis in an effort to identify how outpatient utilization uniquely affects the frequency of inpatient hospitalization. Last, because the authors wanted to exclude from the analysis all patients who may have had a single consultation at the VA during the 10-year period, they required at least 2 visits per patient. The final dataset consisted of 11,135 patients.
Age differences reported in the literature included a lesser likelihood of MH diagnosis for older returning OEF/OIF veterans,26 and older age as a factor related to veterans’ increased health care use.27 Therefore, age was included as a covariate in the analyses.
Results
The 11,135 patients included in the study, made a total of 350,523 outpatient visits over the period (Table 1). The mean number of outpatient visits was 6.6 (SD, 11.7; median, 3.5; mode, 2.0; range, 1.0-219.9). Over the 10-year period, 14.0% of patients averaged 1 visit per year, 23.4% averaged 2 visits, 15.6% averaged 3 visits, 10.7% averaged 4 visits, 7.4% averaged 5 visits, and 28.9% averaged 6 or more visits. Table 2 lists the frequencies and percentages of outpatient visits and hospitalizations.
Group Difference T-Test Analysis
Of the 553 OEF/OIF veterans in the study, 225 (40.7%) had combat flags, and 328 (59.3%) did not. Independent- groups t test showed significant group differences in mean number of visits (t[2,964.48] = 9.94, P < .01). Veterans with combat flags averaged significantly fewer visits per year (mean, 4.79; SD, 5.89) than did veterans without flags (mean, 6.89; SD, 12.50), even with age as a covariate (F[1, 10,675] = 34.63, P < .01).
Independent-groups t test showed significant group differences in mean number of nonpsychiatric hospitalizations (t[2,328.68] = 5.00, P < .01). Veterans with combat flags averaged significantly fewer nonpsychiatric hospitalizations per year (mean, 0.34; SD, .99) than did veterans without flags (mean, 0.51; SD, 1.71), even with age as a covariate (F[1, 10,675] = 9.81, P < .01).
Independent-groups t test showed significant group differences in mean number of psychiatric hospitalizations (t[2,706.75] = 7.69, P < .01). Veterans with combat flags averaged significantly fewer psychiatric hospitalizations per year (mean, 0.31; SD, 0.91) than did veterans without flags (mean, 0.55; SD, 1.80), even with age as a covariate (F[1, 10,675] = 23.51, P < .01).
Group Differences by SMI Diagnosis
Mean number of visits. Analysis of covariance (ANCOVA) was used to determine differences among the SMI groups (schizophrenic, psychotic, bipolar, depressive, and mood disorders) in mean number of outpatient visits per year with age as a covariate. With age accounted for, there were significant differences among the SMI groups (F[4, 11,129] = 285.02, P < .01). Therefore, pairwise comparisons were used to examine differences among individual disorders. Veterans with schizophrenic disorders averaged more visits per year (mean, 16.2; SD, 27.12) than did veterans with bipolar disorders (mean, 6.4; SD, 7.8, P < .01) and veterans with MDD (mean, 4.8; SD, 5.5, P < .01). Veterans with schizophrenia also averaged more visits per year than did veterans with mood disorders NOS (mean, 5.2; SD, 5.9, P < .01) and veterans with unspecified psychotic disorders (mean, 5.9; SD, 9.6, P < .01).
Nonpsychiatric hospitalizations. An ANCOVA analysis was used to determine group differences in nonpsychiatric hospitalizations per year with SMI diagnosis as the independent variable and age as a covariate. With age accounted for, there were significant differences among the SMI groups (F[4, 11,129] = 143.02, P < .01). Furthermore, pairwise comparisons were used to examine differences among individual disorders. Veterans with schizophrenic disorders averaged more nonpsychiatric hospitalizations (mean, 1.4; SD, 3.4) than did veterans with bipolar disorders (mean, 0.7; SD, 1.8, P < .01) and veterans with MDD (mean, 0.3; SD, 0.9, P < .01). Veterans with schizophrenia averaged more nonpsychiatric hospitalizations per year than did veterans with mood disorders NOS (mean, 0.3; SD, 0.8, P < .01) and veterans with unspecified psychotic disorders (mean, 0.5; SD, 1.2, P < .01).
Psychiatric hospitalizations. An ANCOVA analysis was used to determine group differences in psychiatric hospitalizations per year with SMI diagnosis as the independent variable and age as a covariate. With age accounted for, there were significant differences among the SMI groups (F[4, 11,129] = 204.64, P < .01). Furthermore, pairwise comparisons were used to examine differences among individual disorders. Veterans with schizophrenic disorders averaged more psychiatric hospitalizations per year (mean, 1.6; SD, 3.4) than did veterans with bipolar disorders (mean, 0.7; SD, 1.9, P < .01) and veterans with MDD (mean, 0.3; SD, 1.0, P < .01). Veterans with schizophrenia averaged more psychiatric hospitalizations per year than did veterans with mood disorders NOS (mean, 0.2; SD, 0.8, P < .01) and veterans with unspecified psychotic disorders (mean, 0.9; SD, 1.8, P < .01).
Group Differences by Marital Status
Mean number of visits. An ANCOVA analysis was used to determine differences among the marital status groups (married, divorced, never married) in mean number of outpatient visits per year with age as a covariate. With age accounted for, there were significant differences among the marital status groups (F[3, 11,130] = 104.37, P < .01). Married veterans averaged fewer visits per year (mean, 4.6; SD, 5.2) than did divorced veterans (mean, 6.2; SD, 9.9, P < .01) and never-married veterans (mean, 9.8; SD, 18.8, P < .01).
Nonpsychiatric hospitalizations. An ANCOVA analysis was used to determine group differences in nonpsychiatric hospitalizations per year with marital status as the independent variable and age as a covariate. With age accounted for, there were significant differences among the marital status groups (F[3, 11,130] = 43.97, P < .01). Married veterans averaged fewer nonpsychiatric hospitalizations per year (mean, 0.27; SD, 1.52) than did divorced veterans (mean, 0.47; SD, 1.30, P < .01) and never-married veterans (mean, 0.75; SD, 2.08, P <.01) Last, married veterans averaged fewer nonpsychiatric hospitalizations than did veterans with other/unknown marital status (mean, 0.5; SD, 1.5, P < .01).
Psychiatric hospitalizations. An ANCOVA analysis was used to determine group differences in mean number of psychiatric hospitalizations per year with marital status as the independent variable and age as a covariate. With age accounted for, there were significant differences among the marital status groups (F[3, 11,130] = 68.94, P < .01). Married veterans averaged fewer psychiatric hospitalizations per year (mean, 0.23; SD, 0.86) than did divorced veterans (mean, 0.55; SD, 1.58, P < .01) and nevermarried veterans (mean, 0.86; SD, 2.53, P < .01).
Predictors of Hospitalization
Regression analyses revealed that age, sex, divorced status, never-married status, mean number of visits, bipolar disorders, MDD, mood disorders NOS, unspecified psychotic disorders, residential MH treatment, ancillary services, and combat flag were all significant predictors of total number of both psychiatric hospitalizations (adjusted R2 = .15, F[128.56] = .00) and nonpsychiatric hospitalizations (adjusted R2 = .09, F[82.07] = .00) (Tables 3 & 4).
Discussion
This study examined medical and psychiatric service utilization in veterans with SMI diagnoses. For this population, 85.5% of the total number of VA visits were for MH services, and 52.1% of hospitalizations were for psychiatric care. Medical visits accounted for only 6.9% of outpatient services provided. Although the high utilization of psychiatric services by this SMI population may not be striking in itself, the considerably lower utilization of medical services is notable given the high rates of medical comorbidities associated with SMI. This result confirms findings from other studies.8-10,20 Possibly, VA health care guidelines may account for the lower medical service utilization. For example, the outpatient MH service has a metabolic syndrome clinic with psychiatrist prescribing guidelines for treating veterans who meet the criteria for metabolic syndrome.
The total number of outpatient visits varied widely. The largest percentage of veterans (29%) averaged 6 or more visits per year, followed by 2, 3, 1, and 4 visits. These findings suggest many patients are frequently utilizing services. The implications for inpatient hospitalization are notable, as the study’s data also suggest veterans with more total outpatient visits are at increased risk for hospitalization. The higher number of visits may be attributable to the SMI population’s increased health care needs and may suggest a need to target heavy users of outpatient services with more intensive programs to minimize costly hospitalizations. Mean number of outpatient visits was associated with a slightly higher risk for psychiatric hospitalization, possibly because of increased accessibility to care. Outpatient visits increased the risk for overall hospitalization, and psychiatric hospitalization specifically.
The type of SMI diagnosis predicted total number of outpatient visits, demonstrating that veterans with schizophrenic disorders averaged significantly more outpatient visits per year than did veterans with other SMI diagnoses. Interestingly, results showed that veterans with combat flags averaged significantly fewer outpatient visits than did veterans without flags. This finding is notable, as combat exposure is associated with higher rates of MH diagnoses and relatedly higher service utilization. However, combat veterans with SMI but not posttraumatic stress disorder (PTSD), averaged fewer outpatient visits than did veterans without combat exposure, which was not consistent with earlier findings.28 More research specifically on the effects of combat on veterans with PTSD may help in reconciling these findings. Married veterans averaged significantly fewer outpatient visits per year than did divorced or nevermarried veterans. It may be that social support among married couples is protective in veterans with SMI, or that veterans with SMI are unable to maintain marriages.
Hospitalization Predictors
Several significant predictors of psychiatric hospitalization were noted. Increasing age was negatively related to psychiatric hospitalization. It is possible that older patients with SMI receive other types of care, including board and care homes, or MH intensive case management, in which psychiatric outpatient care is readily provided—reducing the need for psychiatric hospitalization. In addition, married veterans were significantly less likely than were divorced and never-married veterans to have been hospitalized. It is possible that being married is protective for MH and physical health or that being unhealthy is a risk factor for divorce.
The type of SMI diagnosis also significantly predicted hospitalization. Having received specific types of services, including residential MH treatment and ancillary services, was a significant predictor of the total number of psychiatric hospitalizations. Last, having a combat flag negatively predicted psychiatric hospitalization—incongruent with earlier findings.24 These results suggest a profile of a veteran who likely could benefit either from a targeted intervention or from having ready access to a social worker.
Improved understanding of service utilization is vital to providing care to returning veterans. Mental disorders are very common among recent OEF, OIF, and Operation New Dawn veterans.29 As the rate of MH diagnoses climbs, the cost of providing health care grows exponentially. The cost of providing care to veterans with SMI can be addressed by identifying veteran-specific factors to provide intensive outreach and prevent costly hospitalization.
Limitations
The results of this study should be interpreted in light of several limitations. It is plausible that at least some veterans received health care services outside the VA, but non-VA data were not included in this study. Subsequent studies should include a group of veterans with SMI and a control group of veterans without SMI so that patterns of hospitalization and utilization rates can be compared. Also, the present study did not include data on missed appointments, an important variable in service utilization.
Missed appointments may suggest lack of follow-through with regular outpatient services, placing patients at risk for emergency services that require hospitalization. Veterans who had a single consultation were excluded in an effort to examine service utilization patterns of established patients. In future studies, including these patients could be informative in identifying specific patterns in this subpopulation. Fifth, PTSD was excluded from this study in order to identify utilization differences for veterans without PTSD.
Conclusion
Results of this study indicate that special attention should be given to veterans’ demographic and clinical factors, including age, sex, combat flags, marital status, and SMI diagnosis. Through identification of and outreach regarding these veteran-specific factors, it may be possible to use targeted interventions to reduce the need for inpatient hospitalization of veterans with chronic mental illness. Historically, the emphasis on access and outpatient care within the VA health care system drastically reduced the number of inpatient MH days.30 This outcome underscores the importance of outpatient services and suggests that targeted outpatient care can further reduce the need for inpatient MH care. Veterans with these outlined risk factors may benefit from implementation of early preventive measures.
Click here to read the digital edition.
About 10 million U.S. adults live with serious mental illness (SMI).1 Among military veterans, the number of mental health (MH) diagnoses is increasing with the return of troops from deployment in Iraq and Afghanistan.2-4 This increase has considerable implications for service use at the VA. An estimated 35% of army and marine veterans sought MH services within 1 year of returning from Iraq and Afghanistan.5 Furthermore, there is an association of MH disorders, physical illnesses, and mortality rates among veterans.2,6 Rising MH needs will increase the need for services; not unexpectedly, the VA is one of the largest providers of integrated health care in the U.S.7
Many patients with SMI have additional health issues, secondary to medication adverse effects, medical comorbidities, and other factors.8-12 Furthermore, their rates of preventable risk factors (eg, smoking, alcohol abuse, and poor exercise and diet13,14) are higher. Comorbid medical illnesses can sideline the treatment of mental illness and lead to negative health outcomes.15,16 These medical conditions coupled with SMI may increase overall rates of health care utilization in terms of outpatient visits, procedures, and inpatient hospitalizations. However, the literature on factors associated with health care utilization in veterans with SMI is scant and generally inconclusive.
Findings on utilization of non-MH medical services for veterans with comorbid MH diagnoses are mixed. Operation Enduring Freedom (OEF) and Operation Iraqi Freedom (OIF) veterans with MH diagnoses have been found to use significantly more non-MH medical services than do OEF and OIF veterans without these diagnoses.17 However, other studies have found that veterans with SMI seem to be less likely to use medical services.18,19 For example, in a study of the rate of medical visits for veterans with psychiatric diagnoses, veterans with SMI were found to have fewer outpatient medical visits than do other veterans without SMI.20 Given the high rates of medical comorbidities in veterans with SMI, this finding of reduced rates of medical care is both informative and concerning. However, more information on utilization rates for other types of health care system services is needed.
In addition to MH diagnoses, multiple factors influence the use of health care services. Lower income predicts overall use of mental and medical services for female but not male veterans.21 A large proportion of VA patients are male, and that disparity may affect female veterans’ perceptions and use of VA health care, underscoring the importance of examining the effects of sex in health care utilization.22 Unmarried status, unemployment, and combat experience are other factors associated with higher health care utilization.23,24 Sociodemographic factors, including income and possession of private health insurance, are associated with veterans’ use of VA health care services.25 It is important to understand the effects of these factors on service utilization by veterans with SMI in order to provide them with optimal, targeted health care.
The authors conducted this study to examine factors affecting MH service utilization and health outcomes in veterans with SMI at the VA Palo Alto Health Care System (VAPAHCS). A retrospective data analysis of medical records was performed. More specifically, this study aimed to identify veteran-specific variables (eg, demographics, psychiatric diagnosis, comorbid medical conditions, combat status) associated with health care utilization and outcomes in veterans with SMI. Dependent variables of interest included service utilization, such as rate and length of inpatient hospitalization and frequency of outpatient encounters. Examining predictors of inpatient medical and psychiatric hospitalization (demographic, clinical, or treatment-related factors) can provide insight into which veterans can benefit from targeted, intensive interventions. A better understanding of the factors affecting comprehensive health care service use for veterans with SMI can clarify targeted interventions and follow-up care for an expanding population.
Methods
Study approval was obtained from the institutional review board at Stanford University and the VAPAHCS research and development committee. Medical record data for veterans treated at the VAPAHCS were collected for a 10-year period (fiscal years 2003-2012). The Computerized Patient Record System (CPRS) data were accessed by VA decision support system staff and analyzed with SPSS Version 21.0 (Armonk, NY). Veterans were identified by ICD-9 codes 295.00 through 298.9, as documented in CPRS.
For this study, schizophrenic, psychotic, bipolar, depressive, and mood disorders were classified as SMI. VA clinic codes were used to categorize visits by service: medical (general medical clinic, surgery, pharmacy, laboratory tests), MH (outpatient visits, intakes and assessments), ancillary services (chaplain, social work, administration), residential MH treatment (substance use disorder, domiciliary care), home-based primary care, and home-based MH care. Psychiatric diagnoses were grouped into schizophrenic disorders, unspecified psychotic disorders, bipolar disorders, major depressive disorders (MDD), and mood disorders not otherwise specified (NOS). The demographic variables obtained through medical records included age, service connection, combat flag (deployment to combat zone), service period, and marital status at start of fiscal year 2003.
Over the 10-year period, 15,414 unique patients were classified as having SMI. Veterans who died during this period were removed from the dataset because of concerns about incomplete data (with specific dates of death unavailable, it was difficult to determine whether missed VA visits during a particular year indicated low utilization or death). In addition, inpatient medical and psychiatric visits were excluded from the analysis in an effort to identify how outpatient utilization uniquely affects the frequency of inpatient hospitalization. Last, because the authors wanted to exclude from the analysis all patients who may have had a single consultation at the VA during the 10-year period, they required at least 2 visits per patient. The final dataset consisted of 11,135 patients.
Age differences reported in the literature included a lesser likelihood of MH diagnosis for older returning OEF/OIF veterans,26 and older age as a factor related to veterans’ increased health care use.27 Therefore, age was included as a covariate in the analyses.
Results
The 11,135 patients included in the study, made a total of 350,523 outpatient visits over the period (Table 1). The mean number of outpatient visits was 6.6 (SD, 11.7; median, 3.5; mode, 2.0; range, 1.0-219.9). Over the 10-year period, 14.0% of patients averaged 1 visit per year, 23.4% averaged 2 visits, 15.6% averaged 3 visits, 10.7% averaged 4 visits, 7.4% averaged 5 visits, and 28.9% averaged 6 or more visits. Table 2 lists the frequencies and percentages of outpatient visits and hospitalizations.
Group Difference T-Test Analysis
Of the 553 OEF/OIF veterans in the study, 225 (40.7%) had combat flags, and 328 (59.3%) did not. Independent- groups t test showed significant group differences in mean number of visits (t[2,964.48] = 9.94, P < .01). Veterans with combat flags averaged significantly fewer visits per year (mean, 4.79; SD, 5.89) than did veterans without flags (mean, 6.89; SD, 12.50), even with age as a covariate (F[1, 10,675] = 34.63, P < .01).
Independent-groups t test showed significant group differences in mean number of nonpsychiatric hospitalizations (t[2,328.68] = 5.00, P < .01). Veterans with combat flags averaged significantly fewer nonpsychiatric hospitalizations per year (mean, 0.34; SD, .99) than did veterans without flags (mean, 0.51; SD, 1.71), even with age as a covariate (F[1, 10,675] = 9.81, P < .01).
Independent-groups t test showed significant group differences in mean number of psychiatric hospitalizations (t[2,706.75] = 7.69, P < .01). Veterans with combat flags averaged significantly fewer psychiatric hospitalizations per year (mean, 0.31; SD, 0.91) than did veterans without flags (mean, 0.55; SD, 1.80), even with age as a covariate (F[1, 10,675] = 23.51, P < .01).
Group Differences by SMI Diagnosis
Mean number of visits. Analysis of covariance (ANCOVA) was used to determine differences among the SMI groups (schizophrenic, psychotic, bipolar, depressive, and mood disorders) in mean number of outpatient visits per year with age as a covariate. With age accounted for, there were significant differences among the SMI groups (F[4, 11,129] = 285.02, P < .01). Therefore, pairwise comparisons were used to examine differences among individual disorders. Veterans with schizophrenic disorders averaged more visits per year (mean, 16.2; SD, 27.12) than did veterans with bipolar disorders (mean, 6.4; SD, 7.8, P < .01) and veterans with MDD (mean, 4.8; SD, 5.5, P < .01). Veterans with schizophrenia also averaged more visits per year than did veterans with mood disorders NOS (mean, 5.2; SD, 5.9, P < .01) and veterans with unspecified psychotic disorders (mean, 5.9; SD, 9.6, P < .01).
Nonpsychiatric hospitalizations. An ANCOVA analysis was used to determine group differences in nonpsychiatric hospitalizations per year with SMI diagnosis as the independent variable and age as a covariate. With age accounted for, there were significant differences among the SMI groups (F[4, 11,129] = 143.02, P < .01). Furthermore, pairwise comparisons were used to examine differences among individual disorders. Veterans with schizophrenic disorders averaged more nonpsychiatric hospitalizations (mean, 1.4; SD, 3.4) than did veterans with bipolar disorders (mean, 0.7; SD, 1.8, P < .01) and veterans with MDD (mean, 0.3; SD, 0.9, P < .01). Veterans with schizophrenia averaged more nonpsychiatric hospitalizations per year than did veterans with mood disorders NOS (mean, 0.3; SD, 0.8, P < .01) and veterans with unspecified psychotic disorders (mean, 0.5; SD, 1.2, P < .01).
Psychiatric hospitalizations. An ANCOVA analysis was used to determine group differences in psychiatric hospitalizations per year with SMI diagnosis as the independent variable and age as a covariate. With age accounted for, there were significant differences among the SMI groups (F[4, 11,129] = 204.64, P < .01). Furthermore, pairwise comparisons were used to examine differences among individual disorders. Veterans with schizophrenic disorders averaged more psychiatric hospitalizations per year (mean, 1.6; SD, 3.4) than did veterans with bipolar disorders (mean, 0.7; SD, 1.9, P < .01) and veterans with MDD (mean, 0.3; SD, 1.0, P < .01). Veterans with schizophrenia averaged more psychiatric hospitalizations per year than did veterans with mood disorders NOS (mean, 0.2; SD, 0.8, P < .01) and veterans with unspecified psychotic disorders (mean, 0.9; SD, 1.8, P < .01).
Group Differences by Marital Status
Mean number of visits. An ANCOVA analysis was used to determine differences among the marital status groups (married, divorced, never married) in mean number of outpatient visits per year with age as a covariate. With age accounted for, there were significant differences among the marital status groups (F[3, 11,130] = 104.37, P < .01). Married veterans averaged fewer visits per year (mean, 4.6; SD, 5.2) than did divorced veterans (mean, 6.2; SD, 9.9, P < .01) and never-married veterans (mean, 9.8; SD, 18.8, P < .01).
Nonpsychiatric hospitalizations. An ANCOVA analysis was used to determine group differences in nonpsychiatric hospitalizations per year with marital status as the independent variable and age as a covariate. With age accounted for, there were significant differences among the marital status groups (F[3, 11,130] = 43.97, P < .01). Married veterans averaged fewer nonpsychiatric hospitalizations per year (mean, 0.27; SD, 1.52) than did divorced veterans (mean, 0.47; SD, 1.30, P < .01) and never-married veterans (mean, 0.75; SD, 2.08, P <.01) Last, married veterans averaged fewer nonpsychiatric hospitalizations than did veterans with other/unknown marital status (mean, 0.5; SD, 1.5, P < .01).
Psychiatric hospitalizations. An ANCOVA analysis was used to determine group differences in mean number of psychiatric hospitalizations per year with marital status as the independent variable and age as a covariate. With age accounted for, there were significant differences among the marital status groups (F[3, 11,130] = 68.94, P < .01). Married veterans averaged fewer psychiatric hospitalizations per year (mean, 0.23; SD, 0.86) than did divorced veterans (mean, 0.55; SD, 1.58, P < .01) and nevermarried veterans (mean, 0.86; SD, 2.53, P < .01).
Predictors of Hospitalization
Regression analyses revealed that age, sex, divorced status, never-married status, mean number of visits, bipolar disorders, MDD, mood disorders NOS, unspecified psychotic disorders, residential MH treatment, ancillary services, and combat flag were all significant predictors of total number of both psychiatric hospitalizations (adjusted R2 = .15, F[128.56] = .00) and nonpsychiatric hospitalizations (adjusted R2 = .09, F[82.07] = .00) (Tables 3 & 4).
Discussion
This study examined medical and psychiatric service utilization in veterans with SMI diagnoses. For this population, 85.5% of the total number of VA visits were for MH services, and 52.1% of hospitalizations were for psychiatric care. Medical visits accounted for only 6.9% of outpatient services provided. Although the high utilization of psychiatric services by this SMI population may not be striking in itself, the considerably lower utilization of medical services is notable given the high rates of medical comorbidities associated with SMI. This result confirms findings from other studies.8-10,20 Possibly, VA health care guidelines may account for the lower medical service utilization. For example, the outpatient MH service has a metabolic syndrome clinic with psychiatrist prescribing guidelines for treating veterans who meet the criteria for metabolic syndrome.
The total number of outpatient visits varied widely. The largest percentage of veterans (29%) averaged 6 or more visits per year, followed by 2, 3, 1, and 4 visits. These findings suggest many patients are frequently utilizing services. The implications for inpatient hospitalization are notable, as the study’s data also suggest veterans with more total outpatient visits are at increased risk for hospitalization. The higher number of visits may be attributable to the SMI population’s increased health care needs and may suggest a need to target heavy users of outpatient services with more intensive programs to minimize costly hospitalizations. Mean number of outpatient visits was associated with a slightly higher risk for psychiatric hospitalization, possibly because of increased accessibility to care. Outpatient visits increased the risk for overall hospitalization, and psychiatric hospitalization specifically.
The type of SMI diagnosis predicted total number of outpatient visits, demonstrating that veterans with schizophrenic disorders averaged significantly more outpatient visits per year than did veterans with other SMI diagnoses. Interestingly, results showed that veterans with combat flags averaged significantly fewer outpatient visits than did veterans without flags. This finding is notable, as combat exposure is associated with higher rates of MH diagnoses and relatedly higher service utilization. However, combat veterans with SMI but not posttraumatic stress disorder (PTSD), averaged fewer outpatient visits than did veterans without combat exposure, which was not consistent with earlier findings.28 More research specifically on the effects of combat on veterans with PTSD may help in reconciling these findings. Married veterans averaged significantly fewer outpatient visits per year than did divorced or nevermarried veterans. It may be that social support among married couples is protective in veterans with SMI, or that veterans with SMI are unable to maintain marriages.
Hospitalization Predictors
Several significant predictors of psychiatric hospitalization were noted. Increasing age was negatively related to psychiatric hospitalization. It is possible that older patients with SMI receive other types of care, including board and care homes, or MH intensive case management, in which psychiatric outpatient care is readily provided—reducing the need for psychiatric hospitalization. In addition, married veterans were significantly less likely than were divorced and never-married veterans to have been hospitalized. It is possible that being married is protective for MH and physical health or that being unhealthy is a risk factor for divorce.
The type of SMI diagnosis also significantly predicted hospitalization. Having received specific types of services, including residential MH treatment and ancillary services, was a significant predictor of the total number of psychiatric hospitalizations. Last, having a combat flag negatively predicted psychiatric hospitalization—incongruent with earlier findings.24 These results suggest a profile of a veteran who likely could benefit either from a targeted intervention or from having ready access to a social worker.
Improved understanding of service utilization is vital to providing care to returning veterans. Mental disorders are very common among recent OEF, OIF, and Operation New Dawn veterans.29 As the rate of MH diagnoses climbs, the cost of providing health care grows exponentially. The cost of providing care to veterans with SMI can be addressed by identifying veteran-specific factors to provide intensive outreach and prevent costly hospitalization.
Limitations
The results of this study should be interpreted in light of several limitations. It is plausible that at least some veterans received health care services outside the VA, but non-VA data were not included in this study. Subsequent studies should include a group of veterans with SMI and a control group of veterans without SMI so that patterns of hospitalization and utilization rates can be compared. Also, the present study did not include data on missed appointments, an important variable in service utilization.
Missed appointments may suggest lack of follow-through with regular outpatient services, placing patients at risk for emergency services that require hospitalization. Veterans who had a single consultation were excluded in an effort to examine service utilization patterns of established patients. In future studies, including these patients could be informative in identifying specific patterns in this subpopulation. Fifth, PTSD was excluded from this study in order to identify utilization differences for veterans without PTSD.
Conclusion
Results of this study indicate that special attention should be given to veterans’ demographic and clinical factors, including age, sex, combat flags, marital status, and SMI diagnosis. Through identification of and outreach regarding these veteran-specific factors, it may be possible to use targeted interventions to reduce the need for inpatient hospitalization of veterans with chronic mental illness. Historically, the emphasis on access and outpatient care within the VA health care system drastically reduced the number of inpatient MH days.30 This outcome underscores the importance of outpatient services and suggests that targeted outpatient care can further reduce the need for inpatient MH care. Veterans with these outlined risk factors may benefit from implementation of early preventive measures.
Click here to read the digital edition.
1. Substance Abuse and Mental Health Services Administration. Results From the 2012 National Survey on Drug Use and Health: Mental Health Findings. NSDUH Series H-47, HHS publication SMA 13-4805. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2013.
2. Hoge CW, Terhakopian A, Castro CA, Messer SC, Engel CC. Association of posttraumatic stress disorder with somatic symptoms, health care visits, and absenteeism among Iraq war veterans. Am J Psychiatry. 2007;164(1):150-153.
3. Seal KH, Bertenthal D, Miner CR, Sen S, Marmar C. Bringing the war back home: mental health disorders among 103,788 US veterans returning from Iraq and Afghanistan seen at Department of Veterans Affairs facilities. Arch Intern Med. 2007;167(5):476-482.
4. Lee SE, Fonseca VP, Wolters CL, et al. Health care utilization behavior of veterans who deployed to Afghanistan and Iraq. Mil Med. 2015;180(4):374-379.
5. Hoge CW, Auchterlonie JL, Milliken CS. Mental health problems, use of mental health services, and attrition from military service after returning from deployment to Iraq or Afghanistan. JAMA. 2006;295(9):1023-1032.
6. Kramarow EA, Pastor PN. The health of male veterans and nonveterans aged 25-64: United States, 2007-2010. NCHS Data Brief. 2012;(101):1-8.
7. Elbogen EB, Wagner HR, Johnson SC, et al. Are Iraq and Afghanistan veterans using mental health services? New data from a national random-sample survey. Psychiatr Serv. 2013;64(2):134-141.
8. Bermudes RA, Keck PE Jr, Welge JA. The prevalence of the metabolic syndrome in psychiatric inpatients with primary psychotic and mood disorders. Psychosomatics. 2006;47(6):491-497.
9. Newcomer JW. Metabolic syndrome and mental illness. Am J Manag Care. 2007;13(7 suppl):S170-S177.
10. Toalson P, Ahmed S, Hardy T, Kabinoff G. The metabolic syndrome in patients with severe mental illnesses. Prim Care Companion J Clin Psychiatry. 2004;6(4):152-158.
11. Khatana SA, Kane J, Taveira TH, Bauer MS, Wu WC. Monitoring and prevalence rates of metabolic syndrome in military veterans with serious mental illness. PLoS One. 2011;6(4):e19298.
12. De Hert M, Correll CU, Bobes J, et al. Physical illness in patients with severe mental disorders. I. Prevalence, impact of medications and disparities in health care. World Psychiatry. 2011;10(1):52-77.
13. Lambert TJ, Velakoulis D, Pantelis C. Medical comorbidity in schizophrenia. Med J Aust. 2003;178(suppl):S67-S70.
14. Osborn DP, Nazareth I, King MB. Physical activity, dietary habits, and coronary heart disease risk factor knowledge amongst people with severe mental illness: a cross sectional comparative study in primary care. Soc Psychiatry Psychiatr Epidemiol. 2007;42(10):787-793.
15. Newcomer JW. Medical risk in patients with bipolar disorder and schizophrenia. J Clin Psychiatry. 2007;67(suppl 9):25-30, discussion 36-42.
16. Chwastiak LA, Rosenheck RA, Kazis LE. Association of psychiatric illness and obesity, physical inactivity, and smoking among a national sample of veterans. Psychosomatics. 2011;52(3):230-236.
17. Cohen BE, Gima K, Bertenthal D, Kim S, Marmar CR, Seal KH. Mental health diagnoses and utilization of VA non-mental health medical services among returning Iraq and Afghanistan veterans. J Gen Intern Med. 2010;25(1):18-24.
18. Chwastiak LA, Rosenheck RA, Kazis LE. Utilization of primary care of veterans with psychiatric illness in the national Department of Veterans Affairs Health Care System. J Gen Intern Med. 2008;23(11):1835-1840.
19. VA Office of Research and Development, Health Services Research and Development Service, Quality Enhancement Research Initiative. Mental health. QUERI fact sheet. http://www.hsrd.research.va.gov/publications/internal/mh_factsheet.pdf. Published December 2008. Accessed January 13, 2017.
20. Cradock-O’Leary J, Young AS, Yano EM, Wang M, Lee ML. Use of general medical services by VA patients with psychiatric disorders. Psychiatr Serv. 2002;53(7):874-878.
21. Di Leone BA, Vogt D, Gradus JL, Street AE, Giasson HL, Resick PA. Predictors of mental health care use among male and female veterans deployed in support of the wars in Afghanistan and Iraq. Psychol Serv. 2013;10(2):145-151.
22. Vogt DS, Barry AA, King LA. Toward gender-aware health care: evaluation of an intervention to enhance care for female patients in the VA setting. J Health Psychol. 2008;13(5):624-638.
23. Marshall RP, Jorm AF, Grayson DA, O’Toole BI. Posttraumatic stress disorder and other predictors of health care consumption by Vietnam veterans. Psychiatr Serv. 1998;49(12):1609-1611.
24. Rosenheck R, Massari L. Wartime military service and utilization of VA health care services. Mil Med. 1993;158(4):223-228.
25. Elhai JD, Grubaugh AL, Richardson JD, Egede LE, Creamer M. Outpatient medical and mental healthcare utilization models among military veterans: results from the 2001 National Survey of Veterans. J Psychiatr Res. 2008;42(10):858-867.
26. Seal KH, Bertenthal D, Miner CR, Sen S, Marmar C. Bringing the war back home: mental health disorders among 103,788 US veterans returning from Iraq and Afghanistan seen at Department of Veterans Affairs facilities. Arch Intern Med. 2007;167(5):476-482.
27. Fasoli DR, Glickman ME, Eisen SV. Predisposing characteristics, enabling resources and need as predictors of utilization and clinical outcomes for veterans receiving mental health services. Med Care. 2010;48(4):288-295.
28. Maguen S, Schumm JA, Norris RL, et al. Predictors of mental and physical health service utilization among Vietnam veterans. Psychol Serv. 2007;4(3):168-180.
29. Office of Public Health, Veterans Health Administration. Analysis of VA Health Care Utilization Among Operation Enduring Freedom (OEF), Operation Iraqi Freedom (OIF), and Operation New Dawn (OND) Veterans—Revised. http://www.publichealth.va.gov/docs/epidemiology/healthcare-utilization-report-fy2012-qtr3.pdf. Revised December 2012. Accessed February 6, 2017.
30. Wagner TH, Sinnott P, Siroka AM. Mental health and substance use disorder spending in the Department of Veterans Affairs, fiscal years 2000-2007. Psychiatr Serv. 2011;62(4):389-395.
1. Substance Abuse and Mental Health Services Administration. Results From the 2012 National Survey on Drug Use and Health: Mental Health Findings. NSDUH Series H-47, HHS publication SMA 13-4805. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2013.
2. Hoge CW, Terhakopian A, Castro CA, Messer SC, Engel CC. Association of posttraumatic stress disorder with somatic symptoms, health care visits, and absenteeism among Iraq war veterans. Am J Psychiatry. 2007;164(1):150-153.
3. Seal KH, Bertenthal D, Miner CR, Sen S, Marmar C. Bringing the war back home: mental health disorders among 103,788 US veterans returning from Iraq and Afghanistan seen at Department of Veterans Affairs facilities. Arch Intern Med. 2007;167(5):476-482.
4. Lee SE, Fonseca VP, Wolters CL, et al. Health care utilization behavior of veterans who deployed to Afghanistan and Iraq. Mil Med. 2015;180(4):374-379.
5. Hoge CW, Auchterlonie JL, Milliken CS. Mental health problems, use of mental health services, and attrition from military service after returning from deployment to Iraq or Afghanistan. JAMA. 2006;295(9):1023-1032.
6. Kramarow EA, Pastor PN. The health of male veterans and nonveterans aged 25-64: United States, 2007-2010. NCHS Data Brief. 2012;(101):1-8.
7. Elbogen EB, Wagner HR, Johnson SC, et al. Are Iraq and Afghanistan veterans using mental health services? New data from a national random-sample survey. Psychiatr Serv. 2013;64(2):134-141.
8. Bermudes RA, Keck PE Jr, Welge JA. The prevalence of the metabolic syndrome in psychiatric inpatients with primary psychotic and mood disorders. Psychosomatics. 2006;47(6):491-497.
9. Newcomer JW. Metabolic syndrome and mental illness. Am J Manag Care. 2007;13(7 suppl):S170-S177.
10. Toalson P, Ahmed S, Hardy T, Kabinoff G. The metabolic syndrome in patients with severe mental illnesses. Prim Care Companion J Clin Psychiatry. 2004;6(4):152-158.
11. Khatana SA, Kane J, Taveira TH, Bauer MS, Wu WC. Monitoring and prevalence rates of metabolic syndrome in military veterans with serious mental illness. PLoS One. 2011;6(4):e19298.
12. De Hert M, Correll CU, Bobes J, et al. Physical illness in patients with severe mental disorders. I. Prevalence, impact of medications and disparities in health care. World Psychiatry. 2011;10(1):52-77.
13. Lambert TJ, Velakoulis D, Pantelis C. Medical comorbidity in schizophrenia. Med J Aust. 2003;178(suppl):S67-S70.
14. Osborn DP, Nazareth I, King MB. Physical activity, dietary habits, and coronary heart disease risk factor knowledge amongst people with severe mental illness: a cross sectional comparative study in primary care. Soc Psychiatry Psychiatr Epidemiol. 2007;42(10):787-793.
15. Newcomer JW. Medical risk in patients with bipolar disorder and schizophrenia. J Clin Psychiatry. 2007;67(suppl 9):25-30, discussion 36-42.
16. Chwastiak LA, Rosenheck RA, Kazis LE. Association of psychiatric illness and obesity, physical inactivity, and smoking among a national sample of veterans. Psychosomatics. 2011;52(3):230-236.
17. Cohen BE, Gima K, Bertenthal D, Kim S, Marmar CR, Seal KH. Mental health diagnoses and utilization of VA non-mental health medical services among returning Iraq and Afghanistan veterans. J Gen Intern Med. 2010;25(1):18-24.
18. Chwastiak LA, Rosenheck RA, Kazis LE. Utilization of primary care of veterans with psychiatric illness in the national Department of Veterans Affairs Health Care System. J Gen Intern Med. 2008;23(11):1835-1840.
19. VA Office of Research and Development, Health Services Research and Development Service, Quality Enhancement Research Initiative. Mental health. QUERI fact sheet. http://www.hsrd.research.va.gov/publications/internal/mh_factsheet.pdf. Published December 2008. Accessed January 13, 2017.
20. Cradock-O’Leary J, Young AS, Yano EM, Wang M, Lee ML. Use of general medical services by VA patients with psychiatric disorders. Psychiatr Serv. 2002;53(7):874-878.
21. Di Leone BA, Vogt D, Gradus JL, Street AE, Giasson HL, Resick PA. Predictors of mental health care use among male and female veterans deployed in support of the wars in Afghanistan and Iraq. Psychol Serv. 2013;10(2):145-151.
22. Vogt DS, Barry AA, King LA. Toward gender-aware health care: evaluation of an intervention to enhance care for female patients in the VA setting. J Health Psychol. 2008;13(5):624-638.
23. Marshall RP, Jorm AF, Grayson DA, O’Toole BI. Posttraumatic stress disorder and other predictors of health care consumption by Vietnam veterans. Psychiatr Serv. 1998;49(12):1609-1611.
24. Rosenheck R, Massari L. Wartime military service and utilization of VA health care services. Mil Med. 1993;158(4):223-228.
25. Elhai JD, Grubaugh AL, Richardson JD, Egede LE, Creamer M. Outpatient medical and mental healthcare utilization models among military veterans: results from the 2001 National Survey of Veterans. J Psychiatr Res. 2008;42(10):858-867.
26. Seal KH, Bertenthal D, Miner CR, Sen S, Marmar C. Bringing the war back home: mental health disorders among 103,788 US veterans returning from Iraq and Afghanistan seen at Department of Veterans Affairs facilities. Arch Intern Med. 2007;167(5):476-482.
27. Fasoli DR, Glickman ME, Eisen SV. Predisposing characteristics, enabling resources and need as predictors of utilization and clinical outcomes for veterans receiving mental health services. Med Care. 2010;48(4):288-295.
28. Maguen S, Schumm JA, Norris RL, et al. Predictors of mental and physical health service utilization among Vietnam veterans. Psychol Serv. 2007;4(3):168-180.
29. Office of Public Health, Veterans Health Administration. Analysis of VA Health Care Utilization Among Operation Enduring Freedom (OEF), Operation Iraqi Freedom (OIF), and Operation New Dawn (OND) Veterans—Revised. http://www.publichealth.va.gov/docs/epidemiology/healthcare-utilization-report-fy2012-qtr3.pdf. Revised December 2012. Accessed February 6, 2017.
30. Wagner TH, Sinnott P, Siroka AM. Mental health and substance use disorder spending in the Department of Veterans Affairs, fiscal years 2000-2007. Psychiatr Serv. 2011;62(4):389-395.
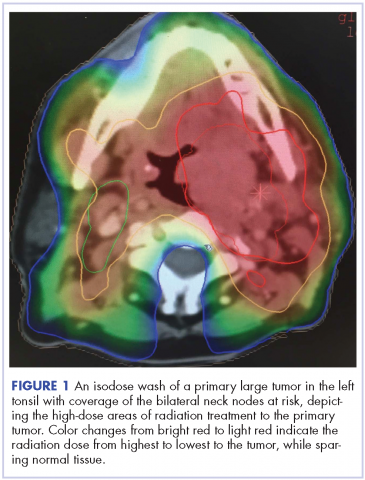
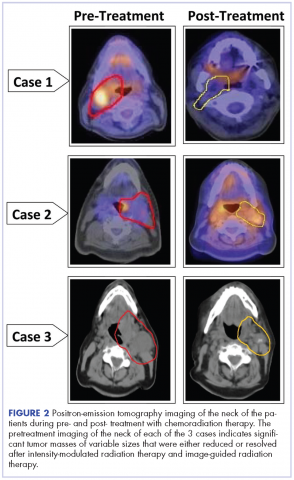
Management of tonsillar carcinoma with advanced radiation therapy and chemotherapy techniques
Tonsillar carcinoma is the most common of the oropharyngeal malignancies of the head and neck region after thyroid and laryngeal carcinoma. Squamous cell carcinoma is the most frequent histologic type of these tumors.1 Tonsillar tumors may originate in the oral cavity, oropharynx, hypopharynx, or larynx. In the United States, more than 5,000 new cases of oropharynx cancer are diagnosed annually.2 Men are affected three to four times more often than are women, and the rate of incidence increases after the 4th decade of life.3 Surveillance, Epidemiology, and End Results data from 1975-2004 show that tonsillar squamous cell carcinoma has had one of the largest increases in the male-to-female incidence rate ratios.4 The overall incidence of tonsillar carcinoma is increasing, especially in the younger population, and this may be attributed to increasing rates of human papilloma virus.5,6
Squamous cell carcinoma in the head and neck originate from subsites within the oral cavity, oropharynx, hypopharynx, larynx, and nasopharynx.7 Traditionally, alcohol consumption and tobacco use were considered the most significant risk factors for the development of tonsillar cancer.8 More recently, however, the high-risk oncogenic human papilloma virus has emerged as a clinical entity in the pathogenesis of squamous cell carcinoma in the head and neck. Other risk factors include poor oral hygiene, mechanical irritation, chewing of betel quid preparations, and a lack of vegetables and fruits in the diet.9-11 Squamous cell carcinoma of the oropharynx often presents late with lymph node involvement at the time of diagnosis. Nonspecific symptoms such as a sore throat and dysphagia can allow head and neck cancer to evade early detection. Many patients with tonsillar carcinoma present with advanced disease because early lesions are generally asymptomatic when small. This absence of symptoms is responsible for 67%-77% of patients presenting with tumors larger than 2.0 cm and often with regional nodal metastasis. At presentation, 45% of anterior tonsillar pillar lesions and 76% of tonsillar fossa lesions have clinically positive necks.12
Despite significant treatment advances, the management of advanced squamous cell carcinoma of the tonsil remains challenging. Historically, surgery was considered the standard of care for patients with tonsillar carcinoma with or without postoperative adjuvant radiotherapy. In locally advanced tonsillar carcinoma, extensive surgery with major tissue reconstruction was necessary, leading to speech dysfunction, cosmetic deformities, and difficulties in swallowing, all of which are detrimental to patient quality of life.13 Given the critical role of the oropharynx in speech and swallowing, nonsurgical therapy with organ-preserving chemoradiation has gained a greater role in the treatment of tonsil carcinoma.13 Over the past decade, innovations in radiation therapy techniques have led to the introduction of intensity-modulated radiation therapy (IMRT) and image-guided radiation therapy (IGRT) for the treatment of various cancers including tonsillar carcinoma.14,15 IMRT is an advanced mode of conformal high-precision radiotherapy that uses computer-controlled multiple small radiation beams of varying intensities to deliver precise radiation doses to the target tissues while sparing adjacent healthy tissues.14 By incorporating three-dimensional computed-tomography (CT) or positron-emission–tomography (PET) imaging technology, IMRT allows the radiation dose to conform more precisely to the three-dimensional shape of the tumor while modulating the intensity of the radiation beam and minimizing its dose to those adjacent sensitive and unaffected organs. IGRT uses a range of two-, three-, and four-dimensional imaging techniques that improve the precision and accuracy of the delivery of the radiation dose to the targeted tumor tissue while minimizing the dose to the surrounding normal tissue during the course of radiation therapy (Figure 1). In this report, we present challenging cases of advanced tonsillar carcinoma and describe our experience in managing the disease using a hyperfractionated IMRT-IGRT based three-dimensional conformal radiation therapy protocol with concurrent chemotherapy.
Case presentations and summaries
Case 1
A 52-year-old white, nonsmoking man who worked in a research chemical laboratory, presented with complaints of throat pain and difficulty in swallowing. The patient had a history of asthma and allergies and had been seen by an ear, nose, and throat (ENT) specialist prior to his visit to our oncology center. A biopsy was performed on a right tonsillar mass measuring 2.7 x 3.6 cm. A computed-tomography (CT) scan showed 2 enlarged inhomogeneous lymph nodes measuring 2.9 cm and 1.7 cm. The nodes were well defined with no soft tissue edema. Neoplasm was favored as a diagnosis and biopsy of the mass was carried out. A biopsy specimen measuring 1.0 x 0.4 x 0.3 cm revealed a moderately differentiated infiltrating squamous cell carcinoma, which extended to the edge of the biopsy specimen. The patient’s Karnofsky performance status was 90% (ie, able to carry on normal activity; minor signs or symptoms of disease).
A CT scan of the chest was clear with no evidence of malignant involvement. A subsequent CT scan of the neck revealed a primary neoplasm of the right faucial tonsil measuring 3.3 x 3.0 cm and associated with right level II, level III, and level IV pathological lymphadenopathy. Positron-emission tomography (PET) imaging of the neck revealed a right tonsillar lesion of 2.7 x 3.0 cm involving the right parapharyngeal space (Figure 2, Case 1). The standardized uptake value (SUV) of the PET scan of the primary lesion was measured at 7.3. A cluster of right level II cervical nodes measuring 3.2 x 2.5 cm had an SUV of 3.5. A 1.0-cm right level III jugular node was also seen with an SUV of 1.6, and a right level IV lymph node measuring 1.5 x 1.0 cm was seen with an SUV of 1.8. No other lesions were noted. The tumor stage was T2N2bM0, a stage IVa disease.
The patient had a percutaneous endoscopic gastrostomy (PEG) tube placement before starting radiation. He underwent a course of hyperfractionated intensity-modulated radiation therapy with image guidance (IMRT-IGRT) in 67 fractions of 120 cGy twice a day to a final tumor dose of 8,040 cGy.16 Concurrently, the patient received systemic chemotherapy with carboplatin at a dose of 240 mg weekly. To optimize the treatment, molecular profiling was performed to identify the sensitive genetic targets to systemic chemotherapy drugs.17, 18 Targets sensitive to paclitaxel and docetaxel were identified by molecular profiling of the tumor tissue, then chemotherapy with paclitaxel or docetaxel (25 mg/m2 weekly for 3 weeks and 1 week off) was also administered to the patient.
The follow-up after 41 months indicated that the patient had no evidence of recurrent disease (Figure 2, Case 1). Posttreatment magnetic-resonance imaging (MRI) of the neck also indicated no evidence of residual tonsillar cancer. The patient’s demographics, tumor characteristics, and the treatment details are summarized in the Table.
Case 2
A 49-year-old black male presented with throat pain and a mass seen initially by his family physician. The patient had a history of tobacco use (at least 1 cigar a day) periodically for about 10 years and had quit cigar smoking 15 years prior to developing his disease. An initial evaluation indicated that the patient had a hypopharyngeal mass in the left inferior pole of his tonsil with near occlusion of the hypopharyngeal airway. His larynx could not be visualized because of the obstructive mass. A neck lymph node measuring 3.0 cm in the left jugulodigastric region was also noted. The patient’s Karnofsky performance status was 90%. Subsequently, the patient underwent excision of the right tonsil and left tonsillar region.
The pathology of the right tonsil was found to be benign. Histology of the left tonsil revealed invasive squamous cell carcinoma. The resected tumor size measured 3.7 x 2.7 x 2.5 cm. The tumor was moderately differentiated involving the deep surgical margins. No lymphovascular invasion was seen. A PET scan revealed a mass arising from the left tonsillar pillar measuring 3.6 x 2.6 x 3.3 cm with deviation of the epiglottis posteriorly nearing the left vallecula. In addition, multiple large cervical nodal lesions in the left level II nodal chain were seen, with the largest measuring 3.1 x 3.0 x 4.5 cm with an SUV of 3.4. Displacement of the left submandibular gland with several further enlarged level II lymph nodes was observed. In the region of left vallecula, there was soft tissue thickening with increased activity measuring 2.7 x 1.5 cm, likely crossing the midline with an SUV of 5.5. The rest of the neck was negative for metastatic involvement (Figure 2, Case 2). The tumor stage was T3N2Mx, a stage IVa disease.
The patient had a Port-A-Cath placed, which caused a hemothorax after placement of the port and delayed initiating his treatment. A pretreatment MRI scan of the neck revealed multiple conglomerate hypodense peripherally enhancing nodular areas in the left neck posterior to the left submandibular gland deep to the parotid tail worrisome for necrotic lymphadenopathy. The patient underwent a course of hyperfractionated IMRT-IGRT in 67 fractions of 120 cGy twice daily for a total dose of 8,040 cGy to the primary tumor site.16 The patient had a port and PEG tube prior to initiating his radiation therapy. He received IMRT-IGRT with concurrent chemotherapy that was selected based on the recommendation of his genomic testing.17,18 The chemotherapy regimen used included carboplatin (300 mg weekly) and docetaxel (400 mg weekly). The patient had a treatment break because he was hospitalized for anemia and pancytopenia from his chemotherapy and he received supportive cancer care with epoetin alfa.A post therapy PET scan was negative for evidence of hypermetabolic malignancy; however, a 3.3 x 2.7 cm calcified lesion representing likely level III jugular lymph node exhibited no measurable activity at that time. The follow-up after 40 months indicated that the patient had no reported recurrence of the disease (Figure 2, Case 2). The patient’s demographics, tumor characteristics, and the treatment details are summarized in the Table.
Case 3
A 53-year-old white man, who had no smoking or tobacco history but who was exposed to chemicals including sulfuric acid, hydrogen chloride gas, and glycols at work, presented initially with a sore throat that became more painful over time. His ENT specialist referred him for a CT scan of the neck, which revealed a left-sided neck mass measuring 2.5 cm in diameter posterior to the submandibular gland and lateral to carotid sheath and anterior to the triangle (Figure 2, Case 3). The mass appeared to be encapsulated. There was a lobulated spherical mass in the left supraglottic area with formation of the airway of the pyriform sinus and additional anterior vascular involvement was noted. The mass measured 3.6 cm in transverse diameter.
A left tonsillar biopsy specimen measuring 1.4 x 0.6 x 0.2 cm was obtained, and its pathology revealed that the patient had a metastatic squamous cell carcinoma. The left neck lymph node mass aspiration also revealed the presence of squamous cell carcinoma. A PET-CT scan staging showed a dominant tonsillar fossa mass extending from the soft palate down to the pyriform sinus measuring 4.2 x 3.8 cm, with an SUV uptake of 7.3. There was a dominant left level II necrotic lymph node presence measuring 5.0 x 3.7 cm, with an SUV of 3.0. The patient’s Karnofsky performance status was 90%. The tumor stage was T4N2M0, a stage IVa disease. The patient received a course of conformal hyperfractionated IMRT-IGRT delivered to the primary tumor in 67 fractions at 120 cGy twice daily for a total dose of 8,040 cGy16 and concurrent carboplatin chemotherapy at a weekly dose of 200 mg.
After completion of his radiation therapy, chemotherapy was changed based on genomic testing from single agent to doublet with carboplatin (area under the curve (AUC) dose of 2 or 200 mg, weekly) plus docetaxel (25 mg/m2 weekly for 3 weeks and 1 week off ).17,18 A PET scan after chemoradiation therapy revealed a marked anatomical improvement in the primary neoplastic disease seen in the faucial tonsil. The tonsillar mass noted previously had almost completely resolved over the interval, with only a mild persistent asymmetrical thickening of around 1.5 cm, with a peak SUV of 2.0. A lymph node of 2.8 x 2.0 cm was present anterior to the left sternocleidomastoid muscle exhibiting SUV of only 1.8. No other abnormal lesions were noted (Figure 2, Case 3). The patient continues to do extremely well without local recurrence of the disease 46 months after radiation therapy (see Table for patient demographics, tumor characteristics, and therapy details.)
Discussion
The management of patients with primary squamous cell carcinoma of the oropharyngeal remains controversial. Traditionally, early-stage tonsillar squamous cell carcinoma was managed by a single modality treatment, either by surgery or radiation therapy, each showing similar efficacy and outcomes.19 For late-stage disease, a combined approach using surgery and radiation therapy was found to be superior to single modality treatment. However, surgery in conjugation with radiation therapy has been associated with significant toxicities compared with the radiation therapy alone.13Therefore, the use of radiation therapy without surgery is becoming more common with increasingly sophisticated radiation therapy techniques and organ preservation approach in patients with squamous cell carcinoma of the tonsil.
Findings from several studies have shown that in stage I or II oropharyngeal cancer, single modality treatment with radiation therapy achieves 80%-90% of local control of the disease, but poorer outcomes are reported for locally advanced stages III/IV with a local control rate of 63%-74%.20 These findings and others have led to a shift to evaluate the clinical benefits of radiation therapy given with concurrent chemotherapy for the primary treatment of advanced stage oropharyngeal squamous cell carcinoma.20,21 Findings from a number of studies have since reported comparable efficacy and toxicity outcomes using this regimen with concurrent chemotherapy in patients with locally advanced head and neck squamous cell cancer.22-24 Synchronous carboplatin chemotherapy was used effectively as an alternative to cisplatin with fewer potential adverse effects in the good prognosis group of patients with oropharyngeal squamous cell carcinoma.25,26 For our 3 patients, we used carboplatin-based chemotherapy with concurrent advanced hyperfractionated radiation therapy techniques to successfully manage tonsillar squamous cell carcinoma and reduce renal toxicity and neuropathy.
Advanced radiation therapy techniques such as IMRT-IGRT are used routinely at the University Cancer and Diagnostic Centers in Houston, Texas, to manage a range of malignant cancers.27 These innovative techniques have the potential to deliver highly conformal dose-intense radiation to targeted regions of disease, while sparing adjacent critical nonmalignant tissue. The improved shaping of high-dose distributions with IMRT-IGRT could mitigate treatment-related toxicities. For example, the use of advanced radiation therapy techniques has been associated with increased preservation of parotid salivary flow.28-30 The use of advanced radiation therapy techniques in head and neck squamous cell carcinoma is growing, and early evidence confirms its ability to secure excellent local and regional disease control.31,32 In this study, we have demonstrated that by using hyperfractionated conformal three-dimensional IMRT-IGRT we were able not only to manage advanced tonsillar squamous cell carcinoma and treat the malignant metastasis, but also spare adjacent critical organs that were not involved in the disease, thus reducing many of the detrimental side effects associated with hyperfractionated chemoradiation.
All 3 patients were followed for between 40 and 46 months. They continue to do extremely well without local recurrence of their disease, indicating a 100% disease control and overall survival rate. The disease control and survival outcomes for our patients with stage IVA disease compare favorably to other published reports in the literature.33,34 Findings from a study by Prestwich and colleagues33 of 41 patients with stage IV tonsillar carcinoma showed that the radiation therapy with concurrent chemotherapy achieved local and regional disease control in 91% of complete responders and an overall survival rate of 66% at 3 years. Similarly, Setton and colleagues34 reported on 442 patients – 50% with tonsillar cancer, 46% with base-of-tongue cancer – who underwent IMRT and concurrent chemotherapy and who achieved a 3-year overall survival of 84.9%. Our study findings demonstrate that hyperfractionated conformal three-dimensional IMRT-IGRT with concurrent chemotherapy can be delivered safely and effectively to patients with advanced tonsillar squamous cell carcinoma.
Acknowledgment
The authors thank Ms June Lyliston, LVN, for editing and proofreading the manuscript.
1. Stambuk HE, Karimi S, Lee N, Patel SG. Oral cavity and oropharynx tumors. Radiol Clin North Am. 2007;45(1):1-20.
2. Lin DT, Cohen SM, Coppit GL, Burkey BB. Squamous cell carcinoma of the oropharynx and hypopharynx. Otolaryngol Clin North Am. 2005;38(1):59-74, viii.
3. Golas SM. Trends in palatine tonsillar cancer incidence and mortality rates in the United States. Community Dent Oral Epidemiol. 2007;35(2):98-108.
4. Cook MB, Dawsey SM, Freedman ND, et al. Sex disparities in cancer incidence by period and age. Cancer Epidemiol Biomarkers Prev. 2009;18(4):1174-1182.
5. Enomoto LM, Bann DV, Hollenbeak CS, Goldenberg D. Trends in the Incidence of oropharyngeal cancers in the United States. Otolaryngol Head Neck Surg. 2016.
6. Shiboski CH, Schmidt BL, Jordan RC. Tongue and tonsil carcinoma: increasing trends in the U.S. population ages 20-44 years. Cancer. 2005;103(9):1843-1849.
7. Marur S, Forastiere AA. Head and neck cancer: changing epidemiology, diagnosis, and treatment. Mayo Clin Proc. 2008;83(4):489-501.
8. Hong AM, Martin A, Chatfield M, et al. Human papillomavirus, smoking status and outcomes in tonsillar squamous cell carcinoma. Int J Cancer. 2013;132(12):2748-2754.
9. Velly AM, Franco EL, Schlecht N, et al. Relationship between dental factors and risk of upper aerodigestive tract cancer. Oral Oncol. 1998;34(4):284-291.
10. Farrow DC, Vaughan TL, Berwick M, et al. Diet and nasopharyngeal cancer in a low-risk population. Int J Cancer. 1998;78(6):675-679.
11. Freedman ND, Park Y, Subar AF, et al. Fruit and vegetable intake and head and neck cancer risk in a large United States prospective cohort study. Int J Cancer. 2008;122(10):2330-2336.
12. Guay ME, Lavertu P. Tonsillar carcinoma. Eur Arch Otorhinolaryngol. 1995;252(5):259-264.
13. Parsons JT, Mendenhall WM, Stringer SP, et al. Squamous cell carcinoma of the oropharynx: surgery, radiation therapy, or both. Cancer. 2002;94(11):2967-2980.
14. Yao M, Dornfeld KJ, Buatti JM, et al. Intensity-modulated radiation treatment for head-and-neck squamous cell carcinoma--the University of Iowa experience. Int J Radiat Oncol Biol Phys. 2005;63(2):410-421.
15. Yang ES, Murphy BM, Chung CH, et al. Evolution of clinical trials in head and neck cancer. Crit Rev Oncol Hematol. 2009;71(1):29-42.
16. Beitler JJ, Zhang Q, Fu KK, et al. Final results of local-regional control and late toxicity of RTOG 9003: a randomized trial of altered fractionation radiation for locally advanced head and neck cancer. Int J Radiat Oncol Biol Phys. 2014;89(1):13-20.
17. Tomkiewicz C, Hans S, Mucchielli MH, et al. A head and neck cancer tumor response-specific gene signature for cisplatin, 5-fluorouracil induction chemotherapy fails with added taxanes. PLoS One. 2012;7(10):e47170.
18. Feldman R, Gatalica Z, Knezetic J, et al. Molecular profiling of head and neck squamous cell carcinoma. Head Neck. 2016;38 Suppl 1:E1625-1638.
19. Moose BD, Kelly MD, Levine PA, et al. Definitive radiotherapy for T1 and T2 squamous cell carcinoma of the tonsil. Head Neck. 1995;17(4):334-338.
20. Chen AY, Schrag N, Hao Y, Stewart A, Ward E. Changes in treatment of advanced oropharyngeal cancer, 1985-2001. Laryngoscope. 2007;117(1):16-21.
21. Machtay M, Rosenthal DI, Hershock D, et al. Organ preservation therapy using induction plus concurrent chemoradiation for advanced resectable oropharyngeal carcinoma: a University of Pennsylvania Phase II Trial. J Clin Oncol. 2002;20(19):3964-3971.
22. Jegannathen A, Swindell R, Yap B, et al. Can synchronous chemotherapy be added to accelerated hypofractionated radiotherapy in patients with base of tongue cancer? Clin Oncol (R Coll Radiol). 2010;22(3):185-191.
23. Budach V, Becker ET, Boehmer D, et al. Concurrent hyperfractionated accelerated radiotherapy with 5-FU and once weekly cisplatin in locally advanced head and neck cancer. The 10-year results of a prospective phase II trial. Strahlenther Onkol. 2014;190(3):250-255.
24. Tobias JS, Monson K, Gupta N, et al. Chemoradiotherapy for locally advanced head and neck cancer: 10-year follow-up of the UK Head and Neck (UKHAN1) trial. Lancet Oncol. 2010;11(1):66-74.
25. Wilkins AC, Rosenfelder N, Schick U, et al. Equivalence of cisplatin and carboplatin-based chemoradiation for locally advanced squamous cell carcinoma of the head and neck: a matched-pair analysis. Oral Oncol. 2013;49(6):615-619.
26. Benghiat H, Sanghera P, Cashmore1 J, et al. Four week hypofractionated accelerated intensity modulated radiotherapy and synchronous carboplatin or cetuximab in biologically staged oropharyngeal carcinoma. Cancer and Clinical Oncology. 2014;3:1-9.
27. D’Andrea MA, Reddy GK. Management of metastatic malignant thymoma with advanced radiation and chemotherapy techniques: report of a rare case. World J Surg Oncol. 2015;13:77.
28. Little M, Schipper M, Feng FY, et al. Reducing xerostomia after chemo-IMRT for head-and-neck cancer: beyond sparing the parotid glands. Int J Radiat Oncol Biol Phys. 2012;83(3):1007-1014.
29. Eisbruch A. Reducing xerostomia by IMRT: what may, and may not, be achieved. J Clin Oncol. 2007;25(31):4863-4864.
30. Pow EH, Kwong DL, McMillan AS, et al. Xerostomia and quality of life after intensity-modulated radiotherapy vs. conventional radiotherapy for early-stage nasopharyngeal carcinoma: initial report on a randomized controlled clinical trial. Int J Radiat Oncol Biol Phys. 2006;66(4):981-991.
31. Lee NY, de Arruda FF, Puri DR, et al. A comparison of intensity-modulated radiation therapy and concomitant boost radiotherapy in the setting of concurrent chemotherapy for locally advanced oropharyngeal carcinoma. Int J Radiat Oncol Biol Phys. 2006;66(4):966-974.
32. Daly ME, Lieskovsky Y, Pawlicki T, et al. Evaluation of patterns of failure and subjective salivary function in patients treated with intensity modulated radiotherapy for head and neck squamous cell carcinoma. Head Neck. 2007;29(3):211-220.
33. Prestwich RJ, Kancherla K, Oksuz DC, et al. A single centre experience with sequential and concomitant chemoradiotherapy in locally advanced stage IV tonsillar cancer. Radiat Oncol. 2010;5:121.
34. Setton J, Caria N, Romanyshyn J, et al. Intensity-modulated radiotherapy in the treatment of oropharyngeal cancer: an update of the Memorial Sloan-Kettering Cancer Center experience. Int J Radiat Oncol Biol Phys. 2012;82(1):291-298.
Tonsillar carcinoma is the most common of the oropharyngeal malignancies of the head and neck region after thyroid and laryngeal carcinoma. Squamous cell carcinoma is the most frequent histologic type of these tumors.1 Tonsillar tumors may originate in the oral cavity, oropharynx, hypopharynx, or larynx. In the United States, more than 5,000 new cases of oropharynx cancer are diagnosed annually.2 Men are affected three to four times more often than are women, and the rate of incidence increases after the 4th decade of life.3 Surveillance, Epidemiology, and End Results data from 1975-2004 show that tonsillar squamous cell carcinoma has had one of the largest increases in the male-to-female incidence rate ratios.4 The overall incidence of tonsillar carcinoma is increasing, especially in the younger population, and this may be attributed to increasing rates of human papilloma virus.5,6
Squamous cell carcinoma in the head and neck originate from subsites within the oral cavity, oropharynx, hypopharynx, larynx, and nasopharynx.7 Traditionally, alcohol consumption and tobacco use were considered the most significant risk factors for the development of tonsillar cancer.8 More recently, however, the high-risk oncogenic human papilloma virus has emerged as a clinical entity in the pathogenesis of squamous cell carcinoma in the head and neck. Other risk factors include poor oral hygiene, mechanical irritation, chewing of betel quid preparations, and a lack of vegetables and fruits in the diet.9-11 Squamous cell carcinoma of the oropharynx often presents late with lymph node involvement at the time of diagnosis. Nonspecific symptoms such as a sore throat and dysphagia can allow head and neck cancer to evade early detection. Many patients with tonsillar carcinoma present with advanced disease because early lesions are generally asymptomatic when small. This absence of symptoms is responsible for 67%-77% of patients presenting with tumors larger than 2.0 cm and often with regional nodal metastasis. At presentation, 45% of anterior tonsillar pillar lesions and 76% of tonsillar fossa lesions have clinically positive necks.12
Despite significant treatment advances, the management of advanced squamous cell carcinoma of the tonsil remains challenging. Historically, surgery was considered the standard of care for patients with tonsillar carcinoma with or without postoperative adjuvant radiotherapy. In locally advanced tonsillar carcinoma, extensive surgery with major tissue reconstruction was necessary, leading to speech dysfunction, cosmetic deformities, and difficulties in swallowing, all of which are detrimental to patient quality of life.13 Given the critical role of the oropharynx in speech and swallowing, nonsurgical therapy with organ-preserving chemoradiation has gained a greater role in the treatment of tonsil carcinoma.13 Over the past decade, innovations in radiation therapy techniques have led to the introduction of intensity-modulated radiation therapy (IMRT) and image-guided radiation therapy (IGRT) for the treatment of various cancers including tonsillar carcinoma.14,15 IMRT is an advanced mode of conformal high-precision radiotherapy that uses computer-controlled multiple small radiation beams of varying intensities to deliver precise radiation doses to the target tissues while sparing adjacent healthy tissues.14 By incorporating three-dimensional computed-tomography (CT) or positron-emission–tomography (PET) imaging technology, IMRT allows the radiation dose to conform more precisely to the three-dimensional shape of the tumor while modulating the intensity of the radiation beam and minimizing its dose to those adjacent sensitive and unaffected organs. IGRT uses a range of two-, three-, and four-dimensional imaging techniques that improve the precision and accuracy of the delivery of the radiation dose to the targeted tumor tissue while minimizing the dose to the surrounding normal tissue during the course of radiation therapy (Figure 1). In this report, we present challenging cases of advanced tonsillar carcinoma and describe our experience in managing the disease using a hyperfractionated IMRT-IGRT based three-dimensional conformal radiation therapy protocol with concurrent chemotherapy.
Case presentations and summaries
Case 1
A 52-year-old white, nonsmoking man who worked in a research chemical laboratory, presented with complaints of throat pain and difficulty in swallowing. The patient had a history of asthma and allergies and had been seen by an ear, nose, and throat (ENT) specialist prior to his visit to our oncology center. A biopsy was performed on a right tonsillar mass measuring 2.7 x 3.6 cm. A computed-tomography (CT) scan showed 2 enlarged inhomogeneous lymph nodes measuring 2.9 cm and 1.7 cm. The nodes were well defined with no soft tissue edema. Neoplasm was favored as a diagnosis and biopsy of the mass was carried out. A biopsy specimen measuring 1.0 x 0.4 x 0.3 cm revealed a moderately differentiated infiltrating squamous cell carcinoma, which extended to the edge of the biopsy specimen. The patient’s Karnofsky performance status was 90% (ie, able to carry on normal activity; minor signs or symptoms of disease).
A CT scan of the chest was clear with no evidence of malignant involvement. A subsequent CT scan of the neck revealed a primary neoplasm of the right faucial tonsil measuring 3.3 x 3.0 cm and associated with right level II, level III, and level IV pathological lymphadenopathy. Positron-emission tomography (PET) imaging of the neck revealed a right tonsillar lesion of 2.7 x 3.0 cm involving the right parapharyngeal space (Figure 2, Case 1). The standardized uptake value (SUV) of the PET scan of the primary lesion was measured at 7.3. A cluster of right level II cervical nodes measuring 3.2 x 2.5 cm had an SUV of 3.5. A 1.0-cm right level III jugular node was also seen with an SUV of 1.6, and a right level IV lymph node measuring 1.5 x 1.0 cm was seen with an SUV of 1.8. No other lesions were noted. The tumor stage was T2N2bM0, a stage IVa disease.
The patient had a percutaneous endoscopic gastrostomy (PEG) tube placement before starting radiation. He underwent a course of hyperfractionated intensity-modulated radiation therapy with image guidance (IMRT-IGRT) in 67 fractions of 120 cGy twice a day to a final tumor dose of 8,040 cGy.16 Concurrently, the patient received systemic chemotherapy with carboplatin at a dose of 240 mg weekly. To optimize the treatment, molecular profiling was performed to identify the sensitive genetic targets to systemic chemotherapy drugs.17, 18 Targets sensitive to paclitaxel and docetaxel were identified by molecular profiling of the tumor tissue, then chemotherapy with paclitaxel or docetaxel (25 mg/m2 weekly for 3 weeks and 1 week off) was also administered to the patient.
The follow-up after 41 months indicated that the patient had no evidence of recurrent disease (Figure 2, Case 1). Posttreatment magnetic-resonance imaging (MRI) of the neck also indicated no evidence of residual tonsillar cancer. The patient’s demographics, tumor characteristics, and the treatment details are summarized in the Table.
Case 2
A 49-year-old black male presented with throat pain and a mass seen initially by his family physician. The patient had a history of tobacco use (at least 1 cigar a day) periodically for about 10 years and had quit cigar smoking 15 years prior to developing his disease. An initial evaluation indicated that the patient had a hypopharyngeal mass in the left inferior pole of his tonsil with near occlusion of the hypopharyngeal airway. His larynx could not be visualized because of the obstructive mass. A neck lymph node measuring 3.0 cm in the left jugulodigastric region was also noted. The patient’s Karnofsky performance status was 90%. Subsequently, the patient underwent excision of the right tonsil and left tonsillar region.
The pathology of the right tonsil was found to be benign. Histology of the left tonsil revealed invasive squamous cell carcinoma. The resected tumor size measured 3.7 x 2.7 x 2.5 cm. The tumor was moderately differentiated involving the deep surgical margins. No lymphovascular invasion was seen. A PET scan revealed a mass arising from the left tonsillar pillar measuring 3.6 x 2.6 x 3.3 cm with deviation of the epiglottis posteriorly nearing the left vallecula. In addition, multiple large cervical nodal lesions in the left level II nodal chain were seen, with the largest measuring 3.1 x 3.0 x 4.5 cm with an SUV of 3.4. Displacement of the left submandibular gland with several further enlarged level II lymph nodes was observed. In the region of left vallecula, there was soft tissue thickening with increased activity measuring 2.7 x 1.5 cm, likely crossing the midline with an SUV of 5.5. The rest of the neck was negative for metastatic involvement (Figure 2, Case 2). The tumor stage was T3N2Mx, a stage IVa disease.
The patient had a Port-A-Cath placed, which caused a hemothorax after placement of the port and delayed initiating his treatment. A pretreatment MRI scan of the neck revealed multiple conglomerate hypodense peripherally enhancing nodular areas in the left neck posterior to the left submandibular gland deep to the parotid tail worrisome for necrotic lymphadenopathy. The patient underwent a course of hyperfractionated IMRT-IGRT in 67 fractions of 120 cGy twice daily for a total dose of 8,040 cGy to the primary tumor site.16 The patient had a port and PEG tube prior to initiating his radiation therapy. He received IMRT-IGRT with concurrent chemotherapy that was selected based on the recommendation of his genomic testing.17,18 The chemotherapy regimen used included carboplatin (300 mg weekly) and docetaxel (400 mg weekly). The patient had a treatment break because he was hospitalized for anemia and pancytopenia from his chemotherapy and he received supportive cancer care with epoetin alfa.A post therapy PET scan was negative for evidence of hypermetabolic malignancy; however, a 3.3 x 2.7 cm calcified lesion representing likely level III jugular lymph node exhibited no measurable activity at that time. The follow-up after 40 months indicated that the patient had no reported recurrence of the disease (Figure 2, Case 2). The patient’s demographics, tumor characteristics, and the treatment details are summarized in the Table.
Case 3
A 53-year-old white man, who had no smoking or tobacco history but who was exposed to chemicals including sulfuric acid, hydrogen chloride gas, and glycols at work, presented initially with a sore throat that became more painful over time. His ENT specialist referred him for a CT scan of the neck, which revealed a left-sided neck mass measuring 2.5 cm in diameter posterior to the submandibular gland and lateral to carotid sheath and anterior to the triangle (Figure 2, Case 3). The mass appeared to be encapsulated. There was a lobulated spherical mass in the left supraglottic area with formation of the airway of the pyriform sinus and additional anterior vascular involvement was noted. The mass measured 3.6 cm in transverse diameter.
A left tonsillar biopsy specimen measuring 1.4 x 0.6 x 0.2 cm was obtained, and its pathology revealed that the patient had a metastatic squamous cell carcinoma. The left neck lymph node mass aspiration also revealed the presence of squamous cell carcinoma. A PET-CT scan staging showed a dominant tonsillar fossa mass extending from the soft palate down to the pyriform sinus measuring 4.2 x 3.8 cm, with an SUV uptake of 7.3. There was a dominant left level II necrotic lymph node presence measuring 5.0 x 3.7 cm, with an SUV of 3.0. The patient’s Karnofsky performance status was 90%. The tumor stage was T4N2M0, a stage IVa disease. The patient received a course of conformal hyperfractionated IMRT-IGRT delivered to the primary tumor in 67 fractions at 120 cGy twice daily for a total dose of 8,040 cGy16 and concurrent carboplatin chemotherapy at a weekly dose of 200 mg.
After completion of his radiation therapy, chemotherapy was changed based on genomic testing from single agent to doublet with carboplatin (area under the curve (AUC) dose of 2 or 200 mg, weekly) plus docetaxel (25 mg/m2 weekly for 3 weeks and 1 week off ).17,18 A PET scan after chemoradiation therapy revealed a marked anatomical improvement in the primary neoplastic disease seen in the faucial tonsil. The tonsillar mass noted previously had almost completely resolved over the interval, with only a mild persistent asymmetrical thickening of around 1.5 cm, with a peak SUV of 2.0. A lymph node of 2.8 x 2.0 cm was present anterior to the left sternocleidomastoid muscle exhibiting SUV of only 1.8. No other abnormal lesions were noted (Figure 2, Case 3). The patient continues to do extremely well without local recurrence of the disease 46 months after radiation therapy (see Table for patient demographics, tumor characteristics, and therapy details.)
Discussion
The management of patients with primary squamous cell carcinoma of the oropharyngeal remains controversial. Traditionally, early-stage tonsillar squamous cell carcinoma was managed by a single modality treatment, either by surgery or radiation therapy, each showing similar efficacy and outcomes.19 For late-stage disease, a combined approach using surgery and radiation therapy was found to be superior to single modality treatment. However, surgery in conjugation with radiation therapy has been associated with significant toxicities compared with the radiation therapy alone.13Therefore, the use of radiation therapy without surgery is becoming more common with increasingly sophisticated radiation therapy techniques and organ preservation approach in patients with squamous cell carcinoma of the tonsil.
Findings from several studies have shown that in stage I or II oropharyngeal cancer, single modality treatment with radiation therapy achieves 80%-90% of local control of the disease, but poorer outcomes are reported for locally advanced stages III/IV with a local control rate of 63%-74%.20 These findings and others have led to a shift to evaluate the clinical benefits of radiation therapy given with concurrent chemotherapy for the primary treatment of advanced stage oropharyngeal squamous cell carcinoma.20,21 Findings from a number of studies have since reported comparable efficacy and toxicity outcomes using this regimen with concurrent chemotherapy in patients with locally advanced head and neck squamous cell cancer.22-24 Synchronous carboplatin chemotherapy was used effectively as an alternative to cisplatin with fewer potential adverse effects in the good prognosis group of patients with oropharyngeal squamous cell carcinoma.25,26 For our 3 patients, we used carboplatin-based chemotherapy with concurrent advanced hyperfractionated radiation therapy techniques to successfully manage tonsillar squamous cell carcinoma and reduce renal toxicity and neuropathy.
Advanced radiation therapy techniques such as IMRT-IGRT are used routinely at the University Cancer and Diagnostic Centers in Houston, Texas, to manage a range of malignant cancers.27 These innovative techniques have the potential to deliver highly conformal dose-intense radiation to targeted regions of disease, while sparing adjacent critical nonmalignant tissue. The improved shaping of high-dose distributions with IMRT-IGRT could mitigate treatment-related toxicities. For example, the use of advanced radiation therapy techniques has been associated with increased preservation of parotid salivary flow.28-30 The use of advanced radiation therapy techniques in head and neck squamous cell carcinoma is growing, and early evidence confirms its ability to secure excellent local and regional disease control.31,32 In this study, we have demonstrated that by using hyperfractionated conformal three-dimensional IMRT-IGRT we were able not only to manage advanced tonsillar squamous cell carcinoma and treat the malignant metastasis, but also spare adjacent critical organs that were not involved in the disease, thus reducing many of the detrimental side effects associated with hyperfractionated chemoradiation.
All 3 patients were followed for between 40 and 46 months. They continue to do extremely well without local recurrence of their disease, indicating a 100% disease control and overall survival rate. The disease control and survival outcomes for our patients with stage IVA disease compare favorably to other published reports in the literature.33,34 Findings from a study by Prestwich and colleagues33 of 41 patients with stage IV tonsillar carcinoma showed that the radiation therapy with concurrent chemotherapy achieved local and regional disease control in 91% of complete responders and an overall survival rate of 66% at 3 years. Similarly, Setton and colleagues34 reported on 442 patients – 50% with tonsillar cancer, 46% with base-of-tongue cancer – who underwent IMRT and concurrent chemotherapy and who achieved a 3-year overall survival of 84.9%. Our study findings demonstrate that hyperfractionated conformal three-dimensional IMRT-IGRT with concurrent chemotherapy can be delivered safely and effectively to patients with advanced tonsillar squamous cell carcinoma.
Acknowledgment
The authors thank Ms June Lyliston, LVN, for editing and proofreading the manuscript.
Tonsillar carcinoma is the most common of the oropharyngeal malignancies of the head and neck region after thyroid and laryngeal carcinoma. Squamous cell carcinoma is the most frequent histologic type of these tumors.1 Tonsillar tumors may originate in the oral cavity, oropharynx, hypopharynx, or larynx. In the United States, more than 5,000 new cases of oropharynx cancer are diagnosed annually.2 Men are affected three to four times more often than are women, and the rate of incidence increases after the 4th decade of life.3 Surveillance, Epidemiology, and End Results data from 1975-2004 show that tonsillar squamous cell carcinoma has had one of the largest increases in the male-to-female incidence rate ratios.4 The overall incidence of tonsillar carcinoma is increasing, especially in the younger population, and this may be attributed to increasing rates of human papilloma virus.5,6
Squamous cell carcinoma in the head and neck originate from subsites within the oral cavity, oropharynx, hypopharynx, larynx, and nasopharynx.7 Traditionally, alcohol consumption and tobacco use were considered the most significant risk factors for the development of tonsillar cancer.8 More recently, however, the high-risk oncogenic human papilloma virus has emerged as a clinical entity in the pathogenesis of squamous cell carcinoma in the head and neck. Other risk factors include poor oral hygiene, mechanical irritation, chewing of betel quid preparations, and a lack of vegetables and fruits in the diet.9-11 Squamous cell carcinoma of the oropharynx often presents late with lymph node involvement at the time of diagnosis. Nonspecific symptoms such as a sore throat and dysphagia can allow head and neck cancer to evade early detection. Many patients with tonsillar carcinoma present with advanced disease because early lesions are generally asymptomatic when small. This absence of symptoms is responsible for 67%-77% of patients presenting with tumors larger than 2.0 cm and often with regional nodal metastasis. At presentation, 45% of anterior tonsillar pillar lesions and 76% of tonsillar fossa lesions have clinically positive necks.12
Despite significant treatment advances, the management of advanced squamous cell carcinoma of the tonsil remains challenging. Historically, surgery was considered the standard of care for patients with tonsillar carcinoma with or without postoperative adjuvant radiotherapy. In locally advanced tonsillar carcinoma, extensive surgery with major tissue reconstruction was necessary, leading to speech dysfunction, cosmetic deformities, and difficulties in swallowing, all of which are detrimental to patient quality of life.13 Given the critical role of the oropharynx in speech and swallowing, nonsurgical therapy with organ-preserving chemoradiation has gained a greater role in the treatment of tonsil carcinoma.13 Over the past decade, innovations in radiation therapy techniques have led to the introduction of intensity-modulated radiation therapy (IMRT) and image-guided radiation therapy (IGRT) for the treatment of various cancers including tonsillar carcinoma.14,15 IMRT is an advanced mode of conformal high-precision radiotherapy that uses computer-controlled multiple small radiation beams of varying intensities to deliver precise radiation doses to the target tissues while sparing adjacent healthy tissues.14 By incorporating three-dimensional computed-tomography (CT) or positron-emission–tomography (PET) imaging technology, IMRT allows the radiation dose to conform more precisely to the three-dimensional shape of the tumor while modulating the intensity of the radiation beam and minimizing its dose to those adjacent sensitive and unaffected organs. IGRT uses a range of two-, three-, and four-dimensional imaging techniques that improve the precision and accuracy of the delivery of the radiation dose to the targeted tumor tissue while minimizing the dose to the surrounding normal tissue during the course of radiation therapy (Figure 1). In this report, we present challenging cases of advanced tonsillar carcinoma and describe our experience in managing the disease using a hyperfractionated IMRT-IGRT based three-dimensional conformal radiation therapy protocol with concurrent chemotherapy.
Case presentations and summaries
Case 1
A 52-year-old white, nonsmoking man who worked in a research chemical laboratory, presented with complaints of throat pain and difficulty in swallowing. The patient had a history of asthma and allergies and had been seen by an ear, nose, and throat (ENT) specialist prior to his visit to our oncology center. A biopsy was performed on a right tonsillar mass measuring 2.7 x 3.6 cm. A computed-tomography (CT) scan showed 2 enlarged inhomogeneous lymph nodes measuring 2.9 cm and 1.7 cm. The nodes were well defined with no soft tissue edema. Neoplasm was favored as a diagnosis and biopsy of the mass was carried out. A biopsy specimen measuring 1.0 x 0.4 x 0.3 cm revealed a moderately differentiated infiltrating squamous cell carcinoma, which extended to the edge of the biopsy specimen. The patient’s Karnofsky performance status was 90% (ie, able to carry on normal activity; minor signs or symptoms of disease).
A CT scan of the chest was clear with no evidence of malignant involvement. A subsequent CT scan of the neck revealed a primary neoplasm of the right faucial tonsil measuring 3.3 x 3.0 cm and associated with right level II, level III, and level IV pathological lymphadenopathy. Positron-emission tomography (PET) imaging of the neck revealed a right tonsillar lesion of 2.7 x 3.0 cm involving the right parapharyngeal space (Figure 2, Case 1). The standardized uptake value (SUV) of the PET scan of the primary lesion was measured at 7.3. A cluster of right level II cervical nodes measuring 3.2 x 2.5 cm had an SUV of 3.5. A 1.0-cm right level III jugular node was also seen with an SUV of 1.6, and a right level IV lymph node measuring 1.5 x 1.0 cm was seen with an SUV of 1.8. No other lesions were noted. The tumor stage was T2N2bM0, a stage IVa disease.
The patient had a percutaneous endoscopic gastrostomy (PEG) tube placement before starting radiation. He underwent a course of hyperfractionated intensity-modulated radiation therapy with image guidance (IMRT-IGRT) in 67 fractions of 120 cGy twice a day to a final tumor dose of 8,040 cGy.16 Concurrently, the patient received systemic chemotherapy with carboplatin at a dose of 240 mg weekly. To optimize the treatment, molecular profiling was performed to identify the sensitive genetic targets to systemic chemotherapy drugs.17, 18 Targets sensitive to paclitaxel and docetaxel were identified by molecular profiling of the tumor tissue, then chemotherapy with paclitaxel or docetaxel (25 mg/m2 weekly for 3 weeks and 1 week off) was also administered to the patient.
The follow-up after 41 months indicated that the patient had no evidence of recurrent disease (Figure 2, Case 1). Posttreatment magnetic-resonance imaging (MRI) of the neck also indicated no evidence of residual tonsillar cancer. The patient’s demographics, tumor characteristics, and the treatment details are summarized in the Table.
Case 2
A 49-year-old black male presented with throat pain and a mass seen initially by his family physician. The patient had a history of tobacco use (at least 1 cigar a day) periodically for about 10 years and had quit cigar smoking 15 years prior to developing his disease. An initial evaluation indicated that the patient had a hypopharyngeal mass in the left inferior pole of his tonsil with near occlusion of the hypopharyngeal airway. His larynx could not be visualized because of the obstructive mass. A neck lymph node measuring 3.0 cm in the left jugulodigastric region was also noted. The patient’s Karnofsky performance status was 90%. Subsequently, the patient underwent excision of the right tonsil and left tonsillar region.
The pathology of the right tonsil was found to be benign. Histology of the left tonsil revealed invasive squamous cell carcinoma. The resected tumor size measured 3.7 x 2.7 x 2.5 cm. The tumor was moderately differentiated involving the deep surgical margins. No lymphovascular invasion was seen. A PET scan revealed a mass arising from the left tonsillar pillar measuring 3.6 x 2.6 x 3.3 cm with deviation of the epiglottis posteriorly nearing the left vallecula. In addition, multiple large cervical nodal lesions in the left level II nodal chain were seen, with the largest measuring 3.1 x 3.0 x 4.5 cm with an SUV of 3.4. Displacement of the left submandibular gland with several further enlarged level II lymph nodes was observed. In the region of left vallecula, there was soft tissue thickening with increased activity measuring 2.7 x 1.5 cm, likely crossing the midline with an SUV of 5.5. The rest of the neck was negative for metastatic involvement (Figure 2, Case 2). The tumor stage was T3N2Mx, a stage IVa disease.
The patient had a Port-A-Cath placed, which caused a hemothorax after placement of the port and delayed initiating his treatment. A pretreatment MRI scan of the neck revealed multiple conglomerate hypodense peripherally enhancing nodular areas in the left neck posterior to the left submandibular gland deep to the parotid tail worrisome for necrotic lymphadenopathy. The patient underwent a course of hyperfractionated IMRT-IGRT in 67 fractions of 120 cGy twice daily for a total dose of 8,040 cGy to the primary tumor site.16 The patient had a port and PEG tube prior to initiating his radiation therapy. He received IMRT-IGRT with concurrent chemotherapy that was selected based on the recommendation of his genomic testing.17,18 The chemotherapy regimen used included carboplatin (300 mg weekly) and docetaxel (400 mg weekly). The patient had a treatment break because he was hospitalized for anemia and pancytopenia from his chemotherapy and he received supportive cancer care with epoetin alfa.A post therapy PET scan was negative for evidence of hypermetabolic malignancy; however, a 3.3 x 2.7 cm calcified lesion representing likely level III jugular lymph node exhibited no measurable activity at that time. The follow-up after 40 months indicated that the patient had no reported recurrence of the disease (Figure 2, Case 2). The patient’s demographics, tumor characteristics, and the treatment details are summarized in the Table.
Case 3
A 53-year-old white man, who had no smoking or tobacco history but who was exposed to chemicals including sulfuric acid, hydrogen chloride gas, and glycols at work, presented initially with a sore throat that became more painful over time. His ENT specialist referred him for a CT scan of the neck, which revealed a left-sided neck mass measuring 2.5 cm in diameter posterior to the submandibular gland and lateral to carotid sheath and anterior to the triangle (Figure 2, Case 3). The mass appeared to be encapsulated. There was a lobulated spherical mass in the left supraglottic area with formation of the airway of the pyriform sinus and additional anterior vascular involvement was noted. The mass measured 3.6 cm in transverse diameter.
A left tonsillar biopsy specimen measuring 1.4 x 0.6 x 0.2 cm was obtained, and its pathology revealed that the patient had a metastatic squamous cell carcinoma. The left neck lymph node mass aspiration also revealed the presence of squamous cell carcinoma. A PET-CT scan staging showed a dominant tonsillar fossa mass extending from the soft palate down to the pyriform sinus measuring 4.2 x 3.8 cm, with an SUV uptake of 7.3. There was a dominant left level II necrotic lymph node presence measuring 5.0 x 3.7 cm, with an SUV of 3.0. The patient’s Karnofsky performance status was 90%. The tumor stage was T4N2M0, a stage IVa disease. The patient received a course of conformal hyperfractionated IMRT-IGRT delivered to the primary tumor in 67 fractions at 120 cGy twice daily for a total dose of 8,040 cGy16 and concurrent carboplatin chemotherapy at a weekly dose of 200 mg.
After completion of his radiation therapy, chemotherapy was changed based on genomic testing from single agent to doublet with carboplatin (area under the curve (AUC) dose of 2 or 200 mg, weekly) plus docetaxel (25 mg/m2 weekly for 3 weeks and 1 week off ).17,18 A PET scan after chemoradiation therapy revealed a marked anatomical improvement in the primary neoplastic disease seen in the faucial tonsil. The tonsillar mass noted previously had almost completely resolved over the interval, with only a mild persistent asymmetrical thickening of around 1.5 cm, with a peak SUV of 2.0. A lymph node of 2.8 x 2.0 cm was present anterior to the left sternocleidomastoid muscle exhibiting SUV of only 1.8. No other abnormal lesions were noted (Figure 2, Case 3). The patient continues to do extremely well without local recurrence of the disease 46 months after radiation therapy (see Table for patient demographics, tumor characteristics, and therapy details.)
Discussion
The management of patients with primary squamous cell carcinoma of the oropharyngeal remains controversial. Traditionally, early-stage tonsillar squamous cell carcinoma was managed by a single modality treatment, either by surgery or radiation therapy, each showing similar efficacy and outcomes.19 For late-stage disease, a combined approach using surgery and radiation therapy was found to be superior to single modality treatment. However, surgery in conjugation with radiation therapy has been associated with significant toxicities compared with the radiation therapy alone.13Therefore, the use of radiation therapy without surgery is becoming more common with increasingly sophisticated radiation therapy techniques and organ preservation approach in patients with squamous cell carcinoma of the tonsil.
Findings from several studies have shown that in stage I or II oropharyngeal cancer, single modality treatment with radiation therapy achieves 80%-90% of local control of the disease, but poorer outcomes are reported for locally advanced stages III/IV with a local control rate of 63%-74%.20 These findings and others have led to a shift to evaluate the clinical benefits of radiation therapy given with concurrent chemotherapy for the primary treatment of advanced stage oropharyngeal squamous cell carcinoma.20,21 Findings from a number of studies have since reported comparable efficacy and toxicity outcomes using this regimen with concurrent chemotherapy in patients with locally advanced head and neck squamous cell cancer.22-24 Synchronous carboplatin chemotherapy was used effectively as an alternative to cisplatin with fewer potential adverse effects in the good prognosis group of patients with oropharyngeal squamous cell carcinoma.25,26 For our 3 patients, we used carboplatin-based chemotherapy with concurrent advanced hyperfractionated radiation therapy techniques to successfully manage tonsillar squamous cell carcinoma and reduce renal toxicity and neuropathy.
Advanced radiation therapy techniques such as IMRT-IGRT are used routinely at the University Cancer and Diagnostic Centers in Houston, Texas, to manage a range of malignant cancers.27 These innovative techniques have the potential to deliver highly conformal dose-intense radiation to targeted regions of disease, while sparing adjacent critical nonmalignant tissue. The improved shaping of high-dose distributions with IMRT-IGRT could mitigate treatment-related toxicities. For example, the use of advanced radiation therapy techniques has been associated with increased preservation of parotid salivary flow.28-30 The use of advanced radiation therapy techniques in head and neck squamous cell carcinoma is growing, and early evidence confirms its ability to secure excellent local and regional disease control.31,32 In this study, we have demonstrated that by using hyperfractionated conformal three-dimensional IMRT-IGRT we were able not only to manage advanced tonsillar squamous cell carcinoma and treat the malignant metastasis, but also spare adjacent critical organs that were not involved in the disease, thus reducing many of the detrimental side effects associated with hyperfractionated chemoradiation.
All 3 patients were followed for between 40 and 46 months. They continue to do extremely well without local recurrence of their disease, indicating a 100% disease control and overall survival rate. The disease control and survival outcomes for our patients with stage IVA disease compare favorably to other published reports in the literature.33,34 Findings from a study by Prestwich and colleagues33 of 41 patients with stage IV tonsillar carcinoma showed that the radiation therapy with concurrent chemotherapy achieved local and regional disease control in 91% of complete responders and an overall survival rate of 66% at 3 years. Similarly, Setton and colleagues34 reported on 442 patients – 50% with tonsillar cancer, 46% with base-of-tongue cancer – who underwent IMRT and concurrent chemotherapy and who achieved a 3-year overall survival of 84.9%. Our study findings demonstrate that hyperfractionated conformal three-dimensional IMRT-IGRT with concurrent chemotherapy can be delivered safely and effectively to patients with advanced tonsillar squamous cell carcinoma.
Acknowledgment
The authors thank Ms June Lyliston, LVN, for editing and proofreading the manuscript.
1. Stambuk HE, Karimi S, Lee N, Patel SG. Oral cavity and oropharynx tumors. Radiol Clin North Am. 2007;45(1):1-20.
2. Lin DT, Cohen SM, Coppit GL, Burkey BB. Squamous cell carcinoma of the oropharynx and hypopharynx. Otolaryngol Clin North Am. 2005;38(1):59-74, viii.
3. Golas SM. Trends in palatine tonsillar cancer incidence and mortality rates in the United States. Community Dent Oral Epidemiol. 2007;35(2):98-108.
4. Cook MB, Dawsey SM, Freedman ND, et al. Sex disparities in cancer incidence by period and age. Cancer Epidemiol Biomarkers Prev. 2009;18(4):1174-1182.
5. Enomoto LM, Bann DV, Hollenbeak CS, Goldenberg D. Trends in the Incidence of oropharyngeal cancers in the United States. Otolaryngol Head Neck Surg. 2016.
6. Shiboski CH, Schmidt BL, Jordan RC. Tongue and tonsil carcinoma: increasing trends in the U.S. population ages 20-44 years. Cancer. 2005;103(9):1843-1849.
7. Marur S, Forastiere AA. Head and neck cancer: changing epidemiology, diagnosis, and treatment. Mayo Clin Proc. 2008;83(4):489-501.
8. Hong AM, Martin A, Chatfield M, et al. Human papillomavirus, smoking status and outcomes in tonsillar squamous cell carcinoma. Int J Cancer. 2013;132(12):2748-2754.
9. Velly AM, Franco EL, Schlecht N, et al. Relationship between dental factors and risk of upper aerodigestive tract cancer. Oral Oncol. 1998;34(4):284-291.
10. Farrow DC, Vaughan TL, Berwick M, et al. Diet and nasopharyngeal cancer in a low-risk population. Int J Cancer. 1998;78(6):675-679.
11. Freedman ND, Park Y, Subar AF, et al. Fruit and vegetable intake and head and neck cancer risk in a large United States prospective cohort study. Int J Cancer. 2008;122(10):2330-2336.
12. Guay ME, Lavertu P. Tonsillar carcinoma. Eur Arch Otorhinolaryngol. 1995;252(5):259-264.
13. Parsons JT, Mendenhall WM, Stringer SP, et al. Squamous cell carcinoma of the oropharynx: surgery, radiation therapy, or both. Cancer. 2002;94(11):2967-2980.
14. Yao M, Dornfeld KJ, Buatti JM, et al. Intensity-modulated radiation treatment for head-and-neck squamous cell carcinoma--the University of Iowa experience. Int J Radiat Oncol Biol Phys. 2005;63(2):410-421.
15. Yang ES, Murphy BM, Chung CH, et al. Evolution of clinical trials in head and neck cancer. Crit Rev Oncol Hematol. 2009;71(1):29-42.
16. Beitler JJ, Zhang Q, Fu KK, et al. Final results of local-regional control and late toxicity of RTOG 9003: a randomized trial of altered fractionation radiation for locally advanced head and neck cancer. Int J Radiat Oncol Biol Phys. 2014;89(1):13-20.
17. Tomkiewicz C, Hans S, Mucchielli MH, et al. A head and neck cancer tumor response-specific gene signature for cisplatin, 5-fluorouracil induction chemotherapy fails with added taxanes. PLoS One. 2012;7(10):e47170.
18. Feldman R, Gatalica Z, Knezetic J, et al. Molecular profiling of head and neck squamous cell carcinoma. Head Neck. 2016;38 Suppl 1:E1625-1638.
19. Moose BD, Kelly MD, Levine PA, et al. Definitive radiotherapy for T1 and T2 squamous cell carcinoma of the tonsil. Head Neck. 1995;17(4):334-338.
20. Chen AY, Schrag N, Hao Y, Stewart A, Ward E. Changes in treatment of advanced oropharyngeal cancer, 1985-2001. Laryngoscope. 2007;117(1):16-21.
21. Machtay M, Rosenthal DI, Hershock D, et al. Organ preservation therapy using induction plus concurrent chemoradiation for advanced resectable oropharyngeal carcinoma: a University of Pennsylvania Phase II Trial. J Clin Oncol. 2002;20(19):3964-3971.
22. Jegannathen A, Swindell R, Yap B, et al. Can synchronous chemotherapy be added to accelerated hypofractionated radiotherapy in patients with base of tongue cancer? Clin Oncol (R Coll Radiol). 2010;22(3):185-191.
23. Budach V, Becker ET, Boehmer D, et al. Concurrent hyperfractionated accelerated radiotherapy with 5-FU and once weekly cisplatin in locally advanced head and neck cancer. The 10-year results of a prospective phase II trial. Strahlenther Onkol. 2014;190(3):250-255.
24. Tobias JS, Monson K, Gupta N, et al. Chemoradiotherapy for locally advanced head and neck cancer: 10-year follow-up of the UK Head and Neck (UKHAN1) trial. Lancet Oncol. 2010;11(1):66-74.
25. Wilkins AC, Rosenfelder N, Schick U, et al. Equivalence of cisplatin and carboplatin-based chemoradiation for locally advanced squamous cell carcinoma of the head and neck: a matched-pair analysis. Oral Oncol. 2013;49(6):615-619.
26. Benghiat H, Sanghera P, Cashmore1 J, et al. Four week hypofractionated accelerated intensity modulated radiotherapy and synchronous carboplatin or cetuximab in biologically staged oropharyngeal carcinoma. Cancer and Clinical Oncology. 2014;3:1-9.
27. D’Andrea MA, Reddy GK. Management of metastatic malignant thymoma with advanced radiation and chemotherapy techniques: report of a rare case. World J Surg Oncol. 2015;13:77.
28. Little M, Schipper M, Feng FY, et al. Reducing xerostomia after chemo-IMRT for head-and-neck cancer: beyond sparing the parotid glands. Int J Radiat Oncol Biol Phys. 2012;83(3):1007-1014.
29. Eisbruch A. Reducing xerostomia by IMRT: what may, and may not, be achieved. J Clin Oncol. 2007;25(31):4863-4864.
30. Pow EH, Kwong DL, McMillan AS, et al. Xerostomia and quality of life after intensity-modulated radiotherapy vs. conventional radiotherapy for early-stage nasopharyngeal carcinoma: initial report on a randomized controlled clinical trial. Int J Radiat Oncol Biol Phys. 2006;66(4):981-991.
31. Lee NY, de Arruda FF, Puri DR, et al. A comparison of intensity-modulated radiation therapy and concomitant boost radiotherapy in the setting of concurrent chemotherapy for locally advanced oropharyngeal carcinoma. Int J Radiat Oncol Biol Phys. 2006;66(4):966-974.
32. Daly ME, Lieskovsky Y, Pawlicki T, et al. Evaluation of patterns of failure and subjective salivary function in patients treated with intensity modulated radiotherapy for head and neck squamous cell carcinoma. Head Neck. 2007;29(3):211-220.
33. Prestwich RJ, Kancherla K, Oksuz DC, et al. A single centre experience with sequential and concomitant chemoradiotherapy in locally advanced stage IV tonsillar cancer. Radiat Oncol. 2010;5:121.
34. Setton J, Caria N, Romanyshyn J, et al. Intensity-modulated radiotherapy in the treatment of oropharyngeal cancer: an update of the Memorial Sloan-Kettering Cancer Center experience. Int J Radiat Oncol Biol Phys. 2012;82(1):291-298.
1. Stambuk HE, Karimi S, Lee N, Patel SG. Oral cavity and oropharynx tumors. Radiol Clin North Am. 2007;45(1):1-20.
2. Lin DT, Cohen SM, Coppit GL, Burkey BB. Squamous cell carcinoma of the oropharynx and hypopharynx. Otolaryngol Clin North Am. 2005;38(1):59-74, viii.
3. Golas SM. Trends in palatine tonsillar cancer incidence and mortality rates in the United States. Community Dent Oral Epidemiol. 2007;35(2):98-108.
4. Cook MB, Dawsey SM, Freedman ND, et al. Sex disparities in cancer incidence by period and age. Cancer Epidemiol Biomarkers Prev. 2009;18(4):1174-1182.
5. Enomoto LM, Bann DV, Hollenbeak CS, Goldenberg D. Trends in the Incidence of oropharyngeal cancers in the United States. Otolaryngol Head Neck Surg. 2016.
6. Shiboski CH, Schmidt BL, Jordan RC. Tongue and tonsil carcinoma: increasing trends in the U.S. population ages 20-44 years. Cancer. 2005;103(9):1843-1849.
7. Marur S, Forastiere AA. Head and neck cancer: changing epidemiology, diagnosis, and treatment. Mayo Clin Proc. 2008;83(4):489-501.
8. Hong AM, Martin A, Chatfield M, et al. Human papillomavirus, smoking status and outcomes in tonsillar squamous cell carcinoma. Int J Cancer. 2013;132(12):2748-2754.
9. Velly AM, Franco EL, Schlecht N, et al. Relationship between dental factors and risk of upper aerodigestive tract cancer. Oral Oncol. 1998;34(4):284-291.
10. Farrow DC, Vaughan TL, Berwick M, et al. Diet and nasopharyngeal cancer in a low-risk population. Int J Cancer. 1998;78(6):675-679.
11. Freedman ND, Park Y, Subar AF, et al. Fruit and vegetable intake and head and neck cancer risk in a large United States prospective cohort study. Int J Cancer. 2008;122(10):2330-2336.
12. Guay ME, Lavertu P. Tonsillar carcinoma. Eur Arch Otorhinolaryngol. 1995;252(5):259-264.
13. Parsons JT, Mendenhall WM, Stringer SP, et al. Squamous cell carcinoma of the oropharynx: surgery, radiation therapy, or both. Cancer. 2002;94(11):2967-2980.
14. Yao M, Dornfeld KJ, Buatti JM, et al. Intensity-modulated radiation treatment for head-and-neck squamous cell carcinoma--the University of Iowa experience. Int J Radiat Oncol Biol Phys. 2005;63(2):410-421.
15. Yang ES, Murphy BM, Chung CH, et al. Evolution of clinical trials in head and neck cancer. Crit Rev Oncol Hematol. 2009;71(1):29-42.
16. Beitler JJ, Zhang Q, Fu KK, et al. Final results of local-regional control and late toxicity of RTOG 9003: a randomized trial of altered fractionation radiation for locally advanced head and neck cancer. Int J Radiat Oncol Biol Phys. 2014;89(1):13-20.
17. Tomkiewicz C, Hans S, Mucchielli MH, et al. A head and neck cancer tumor response-specific gene signature for cisplatin, 5-fluorouracil induction chemotherapy fails with added taxanes. PLoS One. 2012;7(10):e47170.
18. Feldman R, Gatalica Z, Knezetic J, et al. Molecular profiling of head and neck squamous cell carcinoma. Head Neck. 2016;38 Suppl 1:E1625-1638.
19. Moose BD, Kelly MD, Levine PA, et al. Definitive radiotherapy for T1 and T2 squamous cell carcinoma of the tonsil. Head Neck. 1995;17(4):334-338.
20. Chen AY, Schrag N, Hao Y, Stewart A, Ward E. Changes in treatment of advanced oropharyngeal cancer, 1985-2001. Laryngoscope. 2007;117(1):16-21.
21. Machtay M, Rosenthal DI, Hershock D, et al. Organ preservation therapy using induction plus concurrent chemoradiation for advanced resectable oropharyngeal carcinoma: a University of Pennsylvania Phase II Trial. J Clin Oncol. 2002;20(19):3964-3971.
22. Jegannathen A, Swindell R, Yap B, et al. Can synchronous chemotherapy be added to accelerated hypofractionated radiotherapy in patients with base of tongue cancer? Clin Oncol (R Coll Radiol). 2010;22(3):185-191.
23. Budach V, Becker ET, Boehmer D, et al. Concurrent hyperfractionated accelerated radiotherapy with 5-FU and once weekly cisplatin in locally advanced head and neck cancer. The 10-year results of a prospective phase II trial. Strahlenther Onkol. 2014;190(3):250-255.
24. Tobias JS, Monson K, Gupta N, et al. Chemoradiotherapy for locally advanced head and neck cancer: 10-year follow-up of the UK Head and Neck (UKHAN1) trial. Lancet Oncol. 2010;11(1):66-74.
25. Wilkins AC, Rosenfelder N, Schick U, et al. Equivalence of cisplatin and carboplatin-based chemoradiation for locally advanced squamous cell carcinoma of the head and neck: a matched-pair analysis. Oral Oncol. 2013;49(6):615-619.
26. Benghiat H, Sanghera P, Cashmore1 J, et al. Four week hypofractionated accelerated intensity modulated radiotherapy and synchronous carboplatin or cetuximab in biologically staged oropharyngeal carcinoma. Cancer and Clinical Oncology. 2014;3:1-9.
27. D’Andrea MA, Reddy GK. Management of metastatic malignant thymoma with advanced radiation and chemotherapy techniques: report of a rare case. World J Surg Oncol. 2015;13:77.
28. Little M, Schipper M, Feng FY, et al. Reducing xerostomia after chemo-IMRT for head-and-neck cancer: beyond sparing the parotid glands. Int J Radiat Oncol Biol Phys. 2012;83(3):1007-1014.
29. Eisbruch A. Reducing xerostomia by IMRT: what may, and may not, be achieved. J Clin Oncol. 2007;25(31):4863-4864.
30. Pow EH, Kwong DL, McMillan AS, et al. Xerostomia and quality of life after intensity-modulated radiotherapy vs. conventional radiotherapy for early-stage nasopharyngeal carcinoma: initial report on a randomized controlled clinical trial. Int J Radiat Oncol Biol Phys. 2006;66(4):981-991.
31. Lee NY, de Arruda FF, Puri DR, et al. A comparison of intensity-modulated radiation therapy and concomitant boost radiotherapy in the setting of concurrent chemotherapy for locally advanced oropharyngeal carcinoma. Int J Radiat Oncol Biol Phys. 2006;66(4):966-974.
32. Daly ME, Lieskovsky Y, Pawlicki T, et al. Evaluation of patterns of failure and subjective salivary function in patients treated with intensity modulated radiotherapy for head and neck squamous cell carcinoma. Head Neck. 2007;29(3):211-220.
33. Prestwich RJ, Kancherla K, Oksuz DC, et al. A single centre experience with sequential and concomitant chemoradiotherapy in locally advanced stage IV tonsillar cancer. Radiat Oncol. 2010;5:121.
34. Setton J, Caria N, Romanyshyn J, et al. Intensity-modulated radiotherapy in the treatment of oropharyngeal cancer: an update of the Memorial Sloan-Kettering Cancer Center experience. Int J Radiat Oncol Biol Phys. 2012;82(1):291-298.
Providers’ Attitudes and Knowledge of Lesbian, Gay, Bisexual, and Transgender Health
Lesbian, gay, bisexual and transgender (LGBT) populations face significant social stigmatization, discrimination, and marginalization that contribute to negative patient outcomes. Consequently, the LGBT population experiences high rates of mental health issues, such as suicide and depression, as well as sexually transmitted diseases (STDs), drug abuse, poverty, and homelessness.1,2
Background
According to the CDC, gay men are at highest risk and have increased incidences of gonorrhea, chlamydia, herpes, human papilloma virus (HPV), and HIV.3 Lesbians and bisexual women are less likely to get preventive cancer screenings, such as Pap smears and mammograms, and have higher incidences of HIV, hepatitis C, self-reported gonorrhea, and are more likely to be overweight or obese.3-6 In addition, LGBT populations have high rates of use of tobacco, alcohol, and other drugs.
The National Transgender Discrimination Survey of 6,450 transgender and nonconforming participants also provides extensive data on the challenges faced by transgender individuals. Discrimination was frequently experienced in accessing health care. Due to their transgender status, 19% were denied care, and 28% postponed care due to perceived harassment and violence within a health care setting.1 The LGBT populations experience personal and structural barriers that interfere with their ability to access high-quality care. Sexual gender minority individuals also experience health care barriers due to isolation, insufficient social services, and a lack of culturally competent providers.4 At the same time, many health care providers (HCPs) experience various barriers to providing LGBT care and need to increase their cultural competence by improving awareness, receptivity, and knowledge.7,8 One personal barrier to quality care is stigmatization toward LGBT persons as expressed through HCP prejudices, beliefs, attitudes, and behaviors.2 Factors such as gender, race, and religious beliefs also influence attitudes to LGBT health care.
A study by Chapman and colleagues found significant differences in attitudes toward gay men by male and female medical and nursing students.9 Male students had a significantly more negative attitude toward gay men compared with the attitudes of female students. Cultural competence, defined in the study as gay affirmative action principles scores, were statistically significant and strongly correlated with negative attitudes. In this study there also was a statistically significant negative correlation between attitudes and knowledge scores indicating a considerable potential for personal values to influence the provision of health care.9
Various barriers inherent in the health care system restrict access to high-quality care. Institutional barriers that include a lack of legal recognition of same-sex partners, equality in visitation rights, and the ability of same-sex partners to access partner’s medical records hamper health care quality. The HCPs’ lack of knowledge of the health risks or health care needs of the LGBT population also present a structural barrier to quality of care and affects patient outcomes.2
Culturally competent interventions in health care delivery also have been studied to reduce LGBT health disparities. A systematic review of 56 studies by Butler and colleagues found that the term cultural competence was not well defined and often was denoted with the terms patient-centered or individualized care.10 A review on the impact of these interventions in LGBT populations also noted that the long-term effects of culturally competent interventions on health disparities in LGBT populations are still unknown.
The Joint Commission has identified the health and welfare of LGBT populations as a major priority. Beginning in 2012, The Joint Commission started assessing compliance with standards for cultural competence and patient-centered care for LGBT recipients as part of the accreditation criteria.11 The Joint Commission recommended that health care facilities begin to transform the health care environment to be a more welcoming, safe, and inclusive environment for LGBT patients and their families.11 Health care providers can play an important role in reducing the significant health disparities and unequal treatment.12
Problem Identification
Improving health outcomes and reducing health disparities are an important part of the HCP’s role. Yet, many HCPs lack the significant knowledge, skills, and cultural competencies needed to provide quality LGBT care.10 Evidence suggests that HCPs continue to receive little or no training to prepare them to manage this vulnerable population.10 Due to the growing evidence of health disparities and negative health outcomes affecting LGBT populations, the federal government has identified LGBT care and patient outcomes as a major health concern and priority under the Healthy 2020 goals.2,4
About 3.5% (9 million) of the U.S. adult population are identified as lesbian, gay, or bisexual and 0.3% or 700,000 as transgender.13,14 The VHA serves 9 million veterans at 1,245 facilities.15
Because the cooperation of HCPs can play a significant part in reducing health disparities and unequal treatment in the care LGBT patients receive, the VHA launched several initiatives to create a more welcoming, inclusive, and empowering environment for LGBT veterans and families. Among the initiatives, VHA established the Office of Health Equity to address health disparities and ensure that patient-centered care is provided in a positive environment.19,20 The VHA also issued a national directive mandating standardized services be provided for transgender veterans.20
Despite these initiatives, obstacles remain to the delivery of patient-centered LGBT care at the VA. A first step to identifying barriers to patient-centered, high-quality care to LGBT veterans is to evaluate personal and institutional barriers as expressed through HCPs’ preceptions and knowledge about the health of LGBT patients. The magnitude of barriers to providing patient-centered care must first be identified and understood before institutional recommendations can be made and implemented at the facility or national level.
This study examined attitudes and knowledge about LGBT patient health among 45 primary care providers (PCPs) in 4 VA community-based outpatient clinics (CBOCs). The first clinical question examined whether PCPs desired more education and training on LGBT health. The second clinical question asked whether there were gender differences in attitudes among providers about the need for LGBT health education.
The model presented in The Process of Cultural Competence in the Delivery of Healthcare Services by Campinha-Bacote provides an applicable conceptualization to guide HCPs’ actions toward delivering culturally responsive health care services to increasingly diverse health populations.21 The model defines cultural competence as an ongoing dynamic process of striving to effectively work within the cultural context of the client (person, family, or community). The model integrates 5 constructs that are fundamental to becoming culturally competent to provide appropriate culturally responsive care to diverse clients: cultural awareness, cultural knowledge, cultural skill, cultural encounters, and cultural desires.21 The level of competence of the HCP is believed to have a direct relationship with their ability to provide culturally competent health care services. Table 1 provides the definitions of the 5 constructs and highlights the role of education and training in influencing competence in providing LGBT health services.21
This project used a descriptive, cross-sectional one-group design to target physicians, nurse practitioners (NPs), and physician assistants (PAs) at VA Southern Nevada Healthcare System (VASNHS) CBOCs. Participation in the project was voluntary. The duration of project from data collection to completion of analysis and summation of the results was 4 months. The study was approved by the institutional review board (IRB) at the University of Alabama in Huntsville, and it was exempt from the VA IRB.
The survey consisted of 74 questions, including 8 demographic questions and 66 LGBT-related questions. The survey instrument, renamed the Perception and Knowledge of Sexual and Gender Minority Health (PKSGMH) survey was adapted with permission from an original study by Jabson and colleagues and used its format without revision or restructuring.22
Attitudinal questions asked personal opinions on LGBT orientation and gender identity (eAppendix:
Measures
The survey instrument integrated components of 4 different measures on attitudes and knowledge of LGBT health with questions about familiarity with organizational policies on discrimination, visitation, and staff training in LGBT care. The PKSGMH survey measured attitudes and knowledge levels on LGBT health by calculating the mean scores for each of 4 measures.
General Attitudes Toward LGBT Nonpatients.
Physician Attitudes Toward LGBT (ATLG) Patients. The attitudes toward LGBT scale assessed physicians’ feelings toward providing care to LGBT patients. This scale of 6 questions had modest reliability with a Cronbach α of .5. The measure used a 5-point Likert scale (5 = strongly agree). For this project, this scale was renamed the Provider subscale.
Knowledge of LGBT (KLGBT) Patients. The knowledge of LGBT patients’ scale included 13 true/false questions and had a Cronbach α of .74.
Gender and Sexual Minority Affirmative Practice (GSMAP). The GSMAP affirmative practice scale evaluated HCPs’ attitudes and beliefs about the treatment of LGBT patients. The 11-question measure with 2 subscales used a 5-point Likert scale with high reliability on the clinicians’ beliefs and behaviors subscales. Both subscales had a Cronbach α of .93 and .94, respectively.
Demographics and Data Analyses
Health care providers answered demographic questions about gender, sexual orientation, and marital status. They also were asked whether they had ever received any focused training in LGBT patient care. Descriptive and demographic data analyses were performed using SPSS version 24.0 (Armonk, New York). A significance level of P < .05 was used for all analyses. Analysis of variance (ANOVA) statistical analysis was conducted to evaluate the differences in mean scores between male and female PCP groups on the 4 attitudes toward LGBT subscales and the Provider subscale.
Results
Seventy-two PCPs participated in completing the PKSGMH survey. Fifty-seven surveys were returned; however, only 45 surveys were completely answered and included in the final analysis. Twelve surveys containing unanswered questions to the knowledge sections were excluded from the data analysis, and 14 distributed surveys were not returned. The overall response rate for completed surveys was 62.5% (Table 2).
Attitudes Toward Care
Attitudes about competence in providing LGBT care was answered in question 23 of the PKSGMH survey. Overall, a total of 51.1% (n = 23) of PCPs agreed that they were competentto provide LGBT care, and 15.5% (n = 7) disagreed. By gender, 50% (n = 9) of males said they were competent in providing LGBT care compared with 51.8% (n = 14) of females.
Analysis of variance was used to test for differences between groups on the 5 ATLG subscales (gay, lesbian, bisexual, transgender, provider) of the PKSGMH survey (Table 3). A grouping variable was created by separating participants by gender and by their responses to a question that asked about their desire for more education about the health care needs of LGBT patients. The grouping resulted in 4 groups: (1) males who responded yes to need for additional education; (2) males who responded no to need for additional education; (3) females who responded yes to need for additional education; and (4) females who responded no to need for additional education. Results of the ANOVA demonstrated significant differences between groups for the bisexual subscale (F = 3.005, df = 3, 32; P = .045), transgender subscale (F = 4.545, df = 3, 32; P = .009), and the provider subscale (F = 12.602, df = 3, 32; P < .001).
Attitudes toward adequacy of their medical training to address the health needs of the LGBT population were answered in question 26 of the PKSGMH survey. Overall a total of 29% (n = 13) of PCPs agreed that their training adequately prepared them to address the needs of the LGBT population while 51.1% (n = 23) disagreed (Figure).
Knowledge of LGBT Care
Discussion
Federal health care agencies consider the health and welfare of LGBT persons to be a health priority despite the lack of available science-based knowledge about this population.2 In 2011, the National Academies of Medicine (NAM) noted that there are still research gaps concerning the well-being of LGBT individuals. The report stated that a significant contributor of health care inequities in LGBT patients is the lack of provider training and medical education.2 A major recommendation of the NAM is that additional training and education is needed to reduce barriers and improve patient outcomes in the LGBT population.
Provider attitudes and education are among the gaps that contribute to inequities in the health care of LGBT populations as previously discussed. The findings from this survey suggest that PCPs in the VHA perceive that they have deficits in competencies and knowledge levels on LGBT care and that education influences attitudes toward LGBT care. The association between providers’ self-assessment of their competency and their knowledge and attitudes toward care for LGBT patients was not stated in the clinical question and was not investigated in this study.
An online search of 12,966 courses at the VA Talent Management System (TMS) was conducted to find web-based and/or instructor-led training courses focused on LGBT care. The search found 4 LGBT-focused courses that targeted physicians and nurses. Two 90-minute courses presented clinical and public health data on sexual health and addressed how providers can improve skills on taking sexual histories and incorporating these data into routine practice. Training and skills development in sexual history taking by clinicians is vital in reducing health disparities, such as STDs, and in helping LGBT patients feel more comfortable in accessing health care.4
A 1-hour TMS course focused on training HCPs to develop as researchers, teachers, and leaders in improving the LGBT veteran experience by providing competent care. Information on LGBT-related subjects, such as sexual and physical trauma and STDs, were included in the content of some online courses. However, no other comprehensive courses specifically focused on LGBT care. The only exceptions were the Specialty Care Access Network Extension of Community Healthcare outcome video conferencing-based sequential training on transgender care and the VA Lesbian, Gay and Bisexual Education sharepoint and the transgender education sharepoint. At the VA, online LGBT health training is still limited.
Recommendations
Providing additional LGBT-focused TMS courses could help increase provider knowledge and cultural competencies. An online introductory LGBT health course could be offered at VA facilities for all new employees and HCPs as part of employee orientation. More specific courses with continuing education credits geared toward the HCP and focused on LGBT health could be included in the TMS learning portfolio for each HCP as annual learning refresher courses. This course could include specific knowledge questions on LGBT care and a posttest with a required passing score of 80%.
Training HCPs as designated LGBT educators in VA facilities should be considered. Classroom training could be offered annually or during times of increased LGBT patient focus, such as during LGBT Pride month when learning activities can be planned and/or coordinated with the VA Office of Diversity and Inclusion. Nurses, social workers, pharmacists, and medical assistants who provide supportive care to LGBT patients also should be included in the target audience for LGBT health education and training.
Limitations
The use of a cross-sectional project design with such a small convenience sample prevents generalizability of the findings to all PCPs. The lack of a survey design that included randomization and blinding in survey distribution could certainly deter participants from offering candid responses, particularly to some attitudinal questions that were emotional in context. The true-false responses wherein respondents had a 50% chance at guessing the correct response was not the most reliable method of measuring knowledge levels and thereby limited the ability to draw any strong conclusions about providers’ knowledge levels. Additionally, the project design did not allow for measures of other confounding factors, such as age, race/ethnicity, religion, and other social factors that could have influenced how participants responded. Additional randomized controlled studies with larger samples are needed to test specific interventions that evaluate the influence of gender on provider attitudes and knowledge as well as the effect of more HCP education and training on LGBT patient outcomes. Moreover, a literature review found no guidelines on how to specifically address provider gender differences in LGBT education and training or strategies for education and training interventions to address these differences.
Conclusion
Findings suggest that PCPs need additional education and training involving LGBT health issues. Although both male and female providers want more education, female PCPs as a group expressed a greater desire for more training compared with the responses of male PCPs. However, given the study’s small sample size, a strong conclusion regarding gender differences cannot be made. Research has shown that education is a factor that positively influences attitudes and feelings about providing LGBT care. The availability of education and training that is focused on LGBT health topics is still limited within the VHA. Within its stated mission to provide patient-centered care to all veterans, the
Better training on LGBT health topics is vital to improving health care delivery to LGBT populations. To meet this goal, ongoing HCP training to improve attitudes and knowledge and develop the skills necessary to effectively address LGBT health issues also must be a priority at VHA facilities. The VHA also should consider institutional changes that incorporate increased LGBT-focused health education into the learning activities of PCPs. This is essential to evaluating the quality of care given the impact on patient outcomes and health disparities in LGBT populations.
1. Grant JM, Mottet LA, Tanis J, Harrison J, Herman JL, Keisling M. Injustice at every turn: a report of the National Transgender Discrimination Survey. http://www.thetaskforce.org/static_html/downloads/reports/reports/ntds_full.pdf. Published 2011. Accessed October 5, 2017.
2. Institute of Medicine Committee on Lesbian, Gay, Bisexual, and Transgender Health Issues and Research Gaps and Opportunities.
3. Centers for Disease Control Prevention. Reported STDs in the United States. 2014 national data for chlamydia, gonorrhea, and syphilis. www.cdc.gov/std/stats14/std-trends-508.pdf. Published November 2015. Accessed October 5, 2017.
4. U.S. Department of Health and Human Services, Office of Disease Prevention and Health Promotion. Lesbian, gay, bisexual, and transgender health. http://www.healthypeople.gov/2020/topics-objectives/topic/lesbian-gay-bisexual-and-transgender-health. Updated October 4, 2017. Accessed October 5, 2017.
5. Kerker BD, Mostashari F, Thorpe L. Health care access and utilization among women who have sex with women: sexual behavior and identity. J Urban Health. 2006;83(5):970-979.
6. Khan MA, Evans AT, Shah S. Caring for uninsured patients with diabetes: designing and evaluating a novel chronic care model for diabetes care. J Eval Clin Pract. 2010;16(4):700-706.
7. Herek GM. Sexual prejudice. In: Nelson TD, ed. Handbook of Prejudice, Stereotyping, and Discrimination. New York: Psychology Press; 2009:441-467.
8. McKay B. Lesbian, gay, bisexual, and transgender health issues, disparities, and information resources. Med Ref Serv Q. 2011;30(4):393-401.
9. Chapman R, Watkins R, Zappia T, Nicol P, Shields L. Nursing and medical students’ attitude, knowledge and beliefs regarding lesbian, gay, bisexual and transgender parents seeking health care for their children. J Clin Nurs. 2012;21(7‐8):938-945.
10. Butler M, McCreedy E, Schwer N, et al. Improving cultural competence to reduce health disparities. Review No. 170. https://ahrq-ehc-application.s3.amazonaws.com/media/pdf/cultural-competence_research.pdf. Published March 29, 2016. Accessed October 5, 2017.
11. The Joint Commission. Advancing effective communication, cultural competence, and patient-and family-centered care: a roadmap for hospitals. https://www.jointcommission.org/assets/1/6/ARoadmapforHospitalsfinalversion727.pdf. Published 2010. Accessed October 5, 2017.
12. Snelgrove JW, Jasudavisius AM, Rowe BW, Head EM, Bauer GR. “Completely out-at-sea” with “two-gender medicine”: a qualitative analysis of physician-side barriers to providing healthcare for transgender patients. BMC Health Serv Res. 2012;12:110.
13. Gates GJ. How many people are lesbian, gay, bisexual, and transgender? http://williamsinstitute.law.ucla.edu/wp-content/uploads/Gates-How-Many-People-LGBT-Apr-2011.pdf. Published April 2011. Accessed October 5, 2017.
14. Gates GJ, Herman J. Transgender military service in the United States. https://williamsinstitute.law.ucla.edu/wp-content/uploads/Transgender-Military-Service-May-2014.pdf. Published May 2014. Accessed October 5, 2017.
15. U.S. Department of Veteran Affairs. Veterans Health Administration. http://www.va.gov/health. Updated October 2, 2017. Accessed October 5, 2017.
16. Gates GJ. Gay men and lesbians in the U.S. military: estimates from the 2000 census. http://www.lgbtdata.com/uploads/1/0/8/8/10884149/ds008_uscensus_gates.pdf. Published September 28, 2014. Accessed October 5, 2017.
17. Mattocks KM, Kauth MR, Sandfort T, Matza AR, Sullivan JC, Shipherd JC. Understanding health-care needs of sexual and gender minority veterans: how targeted research and policy can improve health. LGBT Health. 2014;1(1):50-57.
18. Kauth MR, Shipherd JC, Lindsay J, Blosnich JR, Brown GR, Jones KT. Access to care for transgender veterans in the Veterans Health Administration: 2006–2013. Am J Public Health. 2014;104(suppl 4):S532-S534.
19. Uchendu US. Veterans Health Administration Office of Health Equity: what is it a about? http://www.hsrd.research.va.gov/for_researchers/cyber_seminars/archives/769-notes.pdf. Published November 4, 2013. Accessed October 5, 2017.
20. U.S. Department of Veterans Affairs, Veterans Health Administration. VHA Directive 2013-003: Providing Health Care for Transgender and Intersex Veterans. http://www.transequality.org/sites/default/files/docs/resources/VHAHealthcareDirective_2013.pdf. Published February 8, 2013. Accessed October 5, 2017.
21. Campinha-Bacote J. The process of cultural competence in the delivery of healthcare services: a model of care. J Transcult Nurs. 2002;13(3):181-184.
22. Jabson JM, Mitchell JW, Doty SB. Associations between non-discrimination and training policies and physicians’ attitudes and knowledge about sexual and gender minority patients: a comparison of physicians from two hospitals. BMC Public Health. 2016;16:256
Lesbian, gay, bisexual and transgender (LGBT) populations face significant social stigmatization, discrimination, and marginalization that contribute to negative patient outcomes. Consequently, the LGBT population experiences high rates of mental health issues, such as suicide and depression, as well as sexually transmitted diseases (STDs), drug abuse, poverty, and homelessness.1,2
Background
According to the CDC, gay men are at highest risk and have increased incidences of gonorrhea, chlamydia, herpes, human papilloma virus (HPV), and HIV.3 Lesbians and bisexual women are less likely to get preventive cancer screenings, such as Pap smears and mammograms, and have higher incidences of HIV, hepatitis C, self-reported gonorrhea, and are more likely to be overweight or obese.3-6 In addition, LGBT populations have high rates of use of tobacco, alcohol, and other drugs.
The National Transgender Discrimination Survey of 6,450 transgender and nonconforming participants also provides extensive data on the challenges faced by transgender individuals. Discrimination was frequently experienced in accessing health care. Due to their transgender status, 19% were denied care, and 28% postponed care due to perceived harassment and violence within a health care setting.1 The LGBT populations experience personal and structural barriers that interfere with their ability to access high-quality care. Sexual gender minority individuals also experience health care barriers due to isolation, insufficient social services, and a lack of culturally competent providers.4 At the same time, many health care providers (HCPs) experience various barriers to providing LGBT care and need to increase their cultural competence by improving awareness, receptivity, and knowledge.7,8 One personal barrier to quality care is stigmatization toward LGBT persons as expressed through HCP prejudices, beliefs, attitudes, and behaviors.2 Factors such as gender, race, and religious beliefs also influence attitudes to LGBT health care.
A study by Chapman and colleagues found significant differences in attitudes toward gay men by male and female medical and nursing students.9 Male students had a significantly more negative attitude toward gay men compared with the attitudes of female students. Cultural competence, defined in the study as gay affirmative action principles scores, were statistically significant and strongly correlated with negative attitudes. In this study there also was a statistically significant negative correlation between attitudes and knowledge scores indicating a considerable potential for personal values to influence the provision of health care.9
Various barriers inherent in the health care system restrict access to high-quality care. Institutional barriers that include a lack of legal recognition of same-sex partners, equality in visitation rights, and the ability of same-sex partners to access partner’s medical records hamper health care quality. The HCPs’ lack of knowledge of the health risks or health care needs of the LGBT population also present a structural barrier to quality of care and affects patient outcomes.2
Culturally competent interventions in health care delivery also have been studied to reduce LGBT health disparities. A systematic review of 56 studies by Butler and colleagues found that the term cultural competence was not well defined and often was denoted with the terms patient-centered or individualized care.10 A review on the impact of these interventions in LGBT populations also noted that the long-term effects of culturally competent interventions on health disparities in LGBT populations are still unknown.
The Joint Commission has identified the health and welfare of LGBT populations as a major priority. Beginning in 2012, The Joint Commission started assessing compliance with standards for cultural competence and patient-centered care for LGBT recipients as part of the accreditation criteria.11 The Joint Commission recommended that health care facilities begin to transform the health care environment to be a more welcoming, safe, and inclusive environment for LGBT patients and their families.11 Health care providers can play an important role in reducing the significant health disparities and unequal treatment.12
Problem Identification
Improving health outcomes and reducing health disparities are an important part of the HCP’s role. Yet, many HCPs lack the significant knowledge, skills, and cultural competencies needed to provide quality LGBT care.10 Evidence suggests that HCPs continue to receive little or no training to prepare them to manage this vulnerable population.10 Due to the growing evidence of health disparities and negative health outcomes affecting LGBT populations, the federal government has identified LGBT care and patient outcomes as a major health concern and priority under the Healthy 2020 goals.2,4
About 3.5% (9 million) of the U.S. adult population are identified as lesbian, gay, or bisexual and 0.3% or 700,000 as transgender.13,14 The VHA serves 9 million veterans at 1,245 facilities.15
Because the cooperation of HCPs can play a significant part in reducing health disparities and unequal treatment in the care LGBT patients receive, the VHA launched several initiatives to create a more welcoming, inclusive, and empowering environment for LGBT veterans and families. Among the initiatives, VHA established the Office of Health Equity to address health disparities and ensure that patient-centered care is provided in a positive environment.19,20 The VHA also issued a national directive mandating standardized services be provided for transgender veterans.20
Despite these initiatives, obstacles remain to the delivery of patient-centered LGBT care at the VA. A first step to identifying barriers to patient-centered, high-quality care to LGBT veterans is to evaluate personal and institutional barriers as expressed through HCPs’ preceptions and knowledge about the health of LGBT patients. The magnitude of barriers to providing patient-centered care must first be identified and understood before institutional recommendations can be made and implemented at the facility or national level.
This study examined attitudes and knowledge about LGBT patient health among 45 primary care providers (PCPs) in 4 VA community-based outpatient clinics (CBOCs). The first clinical question examined whether PCPs desired more education and training on LGBT health. The second clinical question asked whether there were gender differences in attitudes among providers about the need for LGBT health education.
The model presented in The Process of Cultural Competence in the Delivery of Healthcare Services by Campinha-Bacote provides an applicable conceptualization to guide HCPs’ actions toward delivering culturally responsive health care services to increasingly diverse health populations.21 The model defines cultural competence as an ongoing dynamic process of striving to effectively work within the cultural context of the client (person, family, or community). The model integrates 5 constructs that are fundamental to becoming culturally competent to provide appropriate culturally responsive care to diverse clients: cultural awareness, cultural knowledge, cultural skill, cultural encounters, and cultural desires.21 The level of competence of the HCP is believed to have a direct relationship with their ability to provide culturally competent health care services. Table 1 provides the definitions of the 5 constructs and highlights the role of education and training in influencing competence in providing LGBT health services.21
This project used a descriptive, cross-sectional one-group design to target physicians, nurse practitioners (NPs), and physician assistants (PAs) at VA Southern Nevada Healthcare System (VASNHS) CBOCs. Participation in the project was voluntary. The duration of project from data collection to completion of analysis and summation of the results was 4 months. The study was approved by the institutional review board (IRB) at the University of Alabama in Huntsville, and it was exempt from the VA IRB.
The survey consisted of 74 questions, including 8 demographic questions and 66 LGBT-related questions. The survey instrument, renamed the Perception and Knowledge of Sexual and Gender Minority Health (PKSGMH) survey was adapted with permission from an original study by Jabson and colleagues and used its format without revision or restructuring.22
Attitudinal questions asked personal opinions on LGBT orientation and gender identity (eAppendix:
Measures
The survey instrument integrated components of 4 different measures on attitudes and knowledge of LGBT health with questions about familiarity with organizational policies on discrimination, visitation, and staff training in LGBT care. The PKSGMH survey measured attitudes and knowledge levels on LGBT health by calculating the mean scores for each of 4 measures.
General Attitudes Toward LGBT Nonpatients.
Physician Attitudes Toward LGBT (ATLG) Patients. The attitudes toward LGBT scale assessed physicians’ feelings toward providing care to LGBT patients. This scale of 6 questions had modest reliability with a Cronbach α of .5. The measure used a 5-point Likert scale (5 = strongly agree). For this project, this scale was renamed the Provider subscale.
Knowledge of LGBT (KLGBT) Patients. The knowledge of LGBT patients’ scale included 13 true/false questions and had a Cronbach α of .74.
Gender and Sexual Minority Affirmative Practice (GSMAP). The GSMAP affirmative practice scale evaluated HCPs’ attitudes and beliefs about the treatment of LGBT patients. The 11-question measure with 2 subscales used a 5-point Likert scale with high reliability on the clinicians’ beliefs and behaviors subscales. Both subscales had a Cronbach α of .93 and .94, respectively.
Demographics and Data Analyses
Health care providers answered demographic questions about gender, sexual orientation, and marital status. They also were asked whether they had ever received any focused training in LGBT patient care. Descriptive and demographic data analyses were performed using SPSS version 24.0 (Armonk, New York). A significance level of P < .05 was used for all analyses. Analysis of variance (ANOVA) statistical analysis was conducted to evaluate the differences in mean scores between male and female PCP groups on the 4 attitudes toward LGBT subscales and the Provider subscale.
Results
Seventy-two PCPs participated in completing the PKSGMH survey. Fifty-seven surveys were returned; however, only 45 surveys were completely answered and included in the final analysis. Twelve surveys containing unanswered questions to the knowledge sections were excluded from the data analysis, and 14 distributed surveys were not returned. The overall response rate for completed surveys was 62.5% (Table 2).
Attitudes Toward Care
Attitudes about competence in providing LGBT care was answered in question 23 of the PKSGMH survey. Overall, a total of 51.1% (n = 23) of PCPs agreed that they were competentto provide LGBT care, and 15.5% (n = 7) disagreed. By gender, 50% (n = 9) of males said they were competent in providing LGBT care compared with 51.8% (n = 14) of females.
Analysis of variance was used to test for differences between groups on the 5 ATLG subscales (gay, lesbian, bisexual, transgender, provider) of the PKSGMH survey (Table 3). A grouping variable was created by separating participants by gender and by their responses to a question that asked about their desire for more education about the health care needs of LGBT patients. The grouping resulted in 4 groups: (1) males who responded yes to need for additional education; (2) males who responded no to need for additional education; (3) females who responded yes to need for additional education; and (4) females who responded no to need for additional education. Results of the ANOVA demonstrated significant differences between groups for the bisexual subscale (F = 3.005, df = 3, 32; P = .045), transgender subscale (F = 4.545, df = 3, 32; P = .009), and the provider subscale (F = 12.602, df = 3, 32; P < .001).
Attitudes toward adequacy of their medical training to address the health needs of the LGBT population were answered in question 26 of the PKSGMH survey. Overall a total of 29% (n = 13) of PCPs agreed that their training adequately prepared them to address the needs of the LGBT population while 51.1% (n = 23) disagreed (Figure).
Knowledge of LGBT Care
Discussion
Federal health care agencies consider the health and welfare of LGBT persons to be a health priority despite the lack of available science-based knowledge about this population.2 In 2011, the National Academies of Medicine (NAM) noted that there are still research gaps concerning the well-being of LGBT individuals. The report stated that a significant contributor of health care inequities in LGBT patients is the lack of provider training and medical education.2 A major recommendation of the NAM is that additional training and education is needed to reduce barriers and improve patient outcomes in the LGBT population.
Provider attitudes and education are among the gaps that contribute to inequities in the health care of LGBT populations as previously discussed. The findings from this survey suggest that PCPs in the VHA perceive that they have deficits in competencies and knowledge levels on LGBT care and that education influences attitudes toward LGBT care. The association between providers’ self-assessment of their competency and their knowledge and attitudes toward care for LGBT patients was not stated in the clinical question and was not investigated in this study.
An online search of 12,966 courses at the VA Talent Management System (TMS) was conducted to find web-based and/or instructor-led training courses focused on LGBT care. The search found 4 LGBT-focused courses that targeted physicians and nurses. Two 90-minute courses presented clinical and public health data on sexual health and addressed how providers can improve skills on taking sexual histories and incorporating these data into routine practice. Training and skills development in sexual history taking by clinicians is vital in reducing health disparities, such as STDs, and in helping LGBT patients feel more comfortable in accessing health care.4
A 1-hour TMS course focused on training HCPs to develop as researchers, teachers, and leaders in improving the LGBT veteran experience by providing competent care. Information on LGBT-related subjects, such as sexual and physical trauma and STDs, were included in the content of some online courses. However, no other comprehensive courses specifically focused on LGBT care. The only exceptions were the Specialty Care Access Network Extension of Community Healthcare outcome video conferencing-based sequential training on transgender care and the VA Lesbian, Gay and Bisexual Education sharepoint and the transgender education sharepoint. At the VA, online LGBT health training is still limited.
Recommendations
Providing additional LGBT-focused TMS courses could help increase provider knowledge and cultural competencies. An online introductory LGBT health course could be offered at VA facilities for all new employees and HCPs as part of employee orientation. More specific courses with continuing education credits geared toward the HCP and focused on LGBT health could be included in the TMS learning portfolio for each HCP as annual learning refresher courses. This course could include specific knowledge questions on LGBT care and a posttest with a required passing score of 80%.
Training HCPs as designated LGBT educators in VA facilities should be considered. Classroom training could be offered annually or during times of increased LGBT patient focus, such as during LGBT Pride month when learning activities can be planned and/or coordinated with the VA Office of Diversity and Inclusion. Nurses, social workers, pharmacists, and medical assistants who provide supportive care to LGBT patients also should be included in the target audience for LGBT health education and training.
Limitations
The use of a cross-sectional project design with such a small convenience sample prevents generalizability of the findings to all PCPs. The lack of a survey design that included randomization and blinding in survey distribution could certainly deter participants from offering candid responses, particularly to some attitudinal questions that were emotional in context. The true-false responses wherein respondents had a 50% chance at guessing the correct response was not the most reliable method of measuring knowledge levels and thereby limited the ability to draw any strong conclusions about providers’ knowledge levels. Additionally, the project design did not allow for measures of other confounding factors, such as age, race/ethnicity, religion, and other social factors that could have influenced how participants responded. Additional randomized controlled studies with larger samples are needed to test specific interventions that evaluate the influence of gender on provider attitudes and knowledge as well as the effect of more HCP education and training on LGBT patient outcomes. Moreover, a literature review found no guidelines on how to specifically address provider gender differences in LGBT education and training or strategies for education and training interventions to address these differences.
Conclusion
Findings suggest that PCPs need additional education and training involving LGBT health issues. Although both male and female providers want more education, female PCPs as a group expressed a greater desire for more training compared with the responses of male PCPs. However, given the study’s small sample size, a strong conclusion regarding gender differences cannot be made. Research has shown that education is a factor that positively influences attitudes and feelings about providing LGBT care. The availability of education and training that is focused on LGBT health topics is still limited within the VHA. Within its stated mission to provide patient-centered care to all veterans, the
Better training on LGBT health topics is vital to improving health care delivery to LGBT populations. To meet this goal, ongoing HCP training to improve attitudes and knowledge and develop the skills necessary to effectively address LGBT health issues also must be a priority at VHA facilities. The VHA also should consider institutional changes that incorporate increased LGBT-focused health education into the learning activities of PCPs. This is essential to evaluating the quality of care given the impact on patient outcomes and health disparities in LGBT populations.
Lesbian, gay, bisexual and transgender (LGBT) populations face significant social stigmatization, discrimination, and marginalization that contribute to negative patient outcomes. Consequently, the LGBT population experiences high rates of mental health issues, such as suicide and depression, as well as sexually transmitted diseases (STDs), drug abuse, poverty, and homelessness.1,2
Background
According to the CDC, gay men are at highest risk and have increased incidences of gonorrhea, chlamydia, herpes, human papilloma virus (HPV), and HIV.3 Lesbians and bisexual women are less likely to get preventive cancer screenings, such as Pap smears and mammograms, and have higher incidences of HIV, hepatitis C, self-reported gonorrhea, and are more likely to be overweight or obese.3-6 In addition, LGBT populations have high rates of use of tobacco, alcohol, and other drugs.
The National Transgender Discrimination Survey of 6,450 transgender and nonconforming participants also provides extensive data on the challenges faced by transgender individuals. Discrimination was frequently experienced in accessing health care. Due to their transgender status, 19% were denied care, and 28% postponed care due to perceived harassment and violence within a health care setting.1 The LGBT populations experience personal and structural barriers that interfere with their ability to access high-quality care. Sexual gender minority individuals also experience health care barriers due to isolation, insufficient social services, and a lack of culturally competent providers.4 At the same time, many health care providers (HCPs) experience various barriers to providing LGBT care and need to increase their cultural competence by improving awareness, receptivity, and knowledge.7,8 One personal barrier to quality care is stigmatization toward LGBT persons as expressed through HCP prejudices, beliefs, attitudes, and behaviors.2 Factors such as gender, race, and religious beliefs also influence attitudes to LGBT health care.
A study by Chapman and colleagues found significant differences in attitudes toward gay men by male and female medical and nursing students.9 Male students had a significantly more negative attitude toward gay men compared with the attitudes of female students. Cultural competence, defined in the study as gay affirmative action principles scores, were statistically significant and strongly correlated with negative attitudes. In this study there also was a statistically significant negative correlation between attitudes and knowledge scores indicating a considerable potential for personal values to influence the provision of health care.9
Various barriers inherent in the health care system restrict access to high-quality care. Institutional barriers that include a lack of legal recognition of same-sex partners, equality in visitation rights, and the ability of same-sex partners to access partner’s medical records hamper health care quality. The HCPs’ lack of knowledge of the health risks or health care needs of the LGBT population also present a structural barrier to quality of care and affects patient outcomes.2
Culturally competent interventions in health care delivery also have been studied to reduce LGBT health disparities. A systematic review of 56 studies by Butler and colleagues found that the term cultural competence was not well defined and often was denoted with the terms patient-centered or individualized care.10 A review on the impact of these interventions in LGBT populations also noted that the long-term effects of culturally competent interventions on health disparities in LGBT populations are still unknown.
The Joint Commission has identified the health and welfare of LGBT populations as a major priority. Beginning in 2012, The Joint Commission started assessing compliance with standards for cultural competence and patient-centered care for LGBT recipients as part of the accreditation criteria.11 The Joint Commission recommended that health care facilities begin to transform the health care environment to be a more welcoming, safe, and inclusive environment for LGBT patients and their families.11 Health care providers can play an important role in reducing the significant health disparities and unequal treatment.12
Problem Identification
Improving health outcomes and reducing health disparities are an important part of the HCP’s role. Yet, many HCPs lack the significant knowledge, skills, and cultural competencies needed to provide quality LGBT care.10 Evidence suggests that HCPs continue to receive little or no training to prepare them to manage this vulnerable population.10 Due to the growing evidence of health disparities and negative health outcomes affecting LGBT populations, the federal government has identified LGBT care and patient outcomes as a major health concern and priority under the Healthy 2020 goals.2,4
About 3.5% (9 million) of the U.S. adult population are identified as lesbian, gay, or bisexual and 0.3% or 700,000 as transgender.13,14 The VHA serves 9 million veterans at 1,245 facilities.15
Because the cooperation of HCPs can play a significant part in reducing health disparities and unequal treatment in the care LGBT patients receive, the VHA launched several initiatives to create a more welcoming, inclusive, and empowering environment for LGBT veterans and families. Among the initiatives, VHA established the Office of Health Equity to address health disparities and ensure that patient-centered care is provided in a positive environment.19,20 The VHA also issued a national directive mandating standardized services be provided for transgender veterans.20
Despite these initiatives, obstacles remain to the delivery of patient-centered LGBT care at the VA. A first step to identifying barriers to patient-centered, high-quality care to LGBT veterans is to evaluate personal and institutional barriers as expressed through HCPs’ preceptions and knowledge about the health of LGBT patients. The magnitude of barriers to providing patient-centered care must first be identified and understood before institutional recommendations can be made and implemented at the facility or national level.
This study examined attitudes and knowledge about LGBT patient health among 45 primary care providers (PCPs) in 4 VA community-based outpatient clinics (CBOCs). The first clinical question examined whether PCPs desired more education and training on LGBT health. The second clinical question asked whether there were gender differences in attitudes among providers about the need for LGBT health education.
The model presented in The Process of Cultural Competence in the Delivery of Healthcare Services by Campinha-Bacote provides an applicable conceptualization to guide HCPs’ actions toward delivering culturally responsive health care services to increasingly diverse health populations.21 The model defines cultural competence as an ongoing dynamic process of striving to effectively work within the cultural context of the client (person, family, or community). The model integrates 5 constructs that are fundamental to becoming culturally competent to provide appropriate culturally responsive care to diverse clients: cultural awareness, cultural knowledge, cultural skill, cultural encounters, and cultural desires.21 The level of competence of the HCP is believed to have a direct relationship with their ability to provide culturally competent health care services. Table 1 provides the definitions of the 5 constructs and highlights the role of education and training in influencing competence in providing LGBT health services.21
This project used a descriptive, cross-sectional one-group design to target physicians, nurse practitioners (NPs), and physician assistants (PAs) at VA Southern Nevada Healthcare System (VASNHS) CBOCs. Participation in the project was voluntary. The duration of project from data collection to completion of analysis and summation of the results was 4 months. The study was approved by the institutional review board (IRB) at the University of Alabama in Huntsville, and it was exempt from the VA IRB.
The survey consisted of 74 questions, including 8 demographic questions and 66 LGBT-related questions. The survey instrument, renamed the Perception and Knowledge of Sexual and Gender Minority Health (PKSGMH) survey was adapted with permission from an original study by Jabson and colleagues and used its format without revision or restructuring.22
Attitudinal questions asked personal opinions on LGBT orientation and gender identity (eAppendix:
Measures
The survey instrument integrated components of 4 different measures on attitudes and knowledge of LGBT health with questions about familiarity with organizational policies on discrimination, visitation, and staff training in LGBT care. The PKSGMH survey measured attitudes and knowledge levels on LGBT health by calculating the mean scores for each of 4 measures.
General Attitudes Toward LGBT Nonpatients.
Physician Attitudes Toward LGBT (ATLG) Patients. The attitudes toward LGBT scale assessed physicians’ feelings toward providing care to LGBT patients. This scale of 6 questions had modest reliability with a Cronbach α of .5. The measure used a 5-point Likert scale (5 = strongly agree). For this project, this scale was renamed the Provider subscale.
Knowledge of LGBT (KLGBT) Patients. The knowledge of LGBT patients’ scale included 13 true/false questions and had a Cronbach α of .74.
Gender and Sexual Minority Affirmative Practice (GSMAP). The GSMAP affirmative practice scale evaluated HCPs’ attitudes and beliefs about the treatment of LGBT patients. The 11-question measure with 2 subscales used a 5-point Likert scale with high reliability on the clinicians’ beliefs and behaviors subscales. Both subscales had a Cronbach α of .93 and .94, respectively.
Demographics and Data Analyses
Health care providers answered demographic questions about gender, sexual orientation, and marital status. They also were asked whether they had ever received any focused training in LGBT patient care. Descriptive and demographic data analyses were performed using SPSS version 24.0 (Armonk, New York). A significance level of P < .05 was used for all analyses. Analysis of variance (ANOVA) statistical analysis was conducted to evaluate the differences in mean scores between male and female PCP groups on the 4 attitudes toward LGBT subscales and the Provider subscale.
Results
Seventy-two PCPs participated in completing the PKSGMH survey. Fifty-seven surveys were returned; however, only 45 surveys were completely answered and included in the final analysis. Twelve surveys containing unanswered questions to the knowledge sections were excluded from the data analysis, and 14 distributed surveys were not returned. The overall response rate for completed surveys was 62.5% (Table 2).
Attitudes Toward Care
Attitudes about competence in providing LGBT care was answered in question 23 of the PKSGMH survey. Overall, a total of 51.1% (n = 23) of PCPs agreed that they were competentto provide LGBT care, and 15.5% (n = 7) disagreed. By gender, 50% (n = 9) of males said they were competent in providing LGBT care compared with 51.8% (n = 14) of females.
Analysis of variance was used to test for differences between groups on the 5 ATLG subscales (gay, lesbian, bisexual, transgender, provider) of the PKSGMH survey (Table 3). A grouping variable was created by separating participants by gender and by their responses to a question that asked about their desire for more education about the health care needs of LGBT patients. The grouping resulted in 4 groups: (1) males who responded yes to need for additional education; (2) males who responded no to need for additional education; (3) females who responded yes to need for additional education; and (4) females who responded no to need for additional education. Results of the ANOVA demonstrated significant differences between groups for the bisexual subscale (F = 3.005, df = 3, 32; P = .045), transgender subscale (F = 4.545, df = 3, 32; P = .009), and the provider subscale (F = 12.602, df = 3, 32; P < .001).
Attitudes toward adequacy of their medical training to address the health needs of the LGBT population were answered in question 26 of the PKSGMH survey. Overall a total of 29% (n = 13) of PCPs agreed that their training adequately prepared them to address the needs of the LGBT population while 51.1% (n = 23) disagreed (Figure).
Knowledge of LGBT Care
Discussion
Federal health care agencies consider the health and welfare of LGBT persons to be a health priority despite the lack of available science-based knowledge about this population.2 In 2011, the National Academies of Medicine (NAM) noted that there are still research gaps concerning the well-being of LGBT individuals. The report stated that a significant contributor of health care inequities in LGBT patients is the lack of provider training and medical education.2 A major recommendation of the NAM is that additional training and education is needed to reduce barriers and improve patient outcomes in the LGBT population.
Provider attitudes and education are among the gaps that contribute to inequities in the health care of LGBT populations as previously discussed. The findings from this survey suggest that PCPs in the VHA perceive that they have deficits in competencies and knowledge levels on LGBT care and that education influences attitudes toward LGBT care. The association between providers’ self-assessment of their competency and their knowledge and attitudes toward care for LGBT patients was not stated in the clinical question and was not investigated in this study.
An online search of 12,966 courses at the VA Talent Management System (TMS) was conducted to find web-based and/or instructor-led training courses focused on LGBT care. The search found 4 LGBT-focused courses that targeted physicians and nurses. Two 90-minute courses presented clinical and public health data on sexual health and addressed how providers can improve skills on taking sexual histories and incorporating these data into routine practice. Training and skills development in sexual history taking by clinicians is vital in reducing health disparities, such as STDs, and in helping LGBT patients feel more comfortable in accessing health care.4
A 1-hour TMS course focused on training HCPs to develop as researchers, teachers, and leaders in improving the LGBT veteran experience by providing competent care. Information on LGBT-related subjects, such as sexual and physical trauma and STDs, were included in the content of some online courses. However, no other comprehensive courses specifically focused on LGBT care. The only exceptions were the Specialty Care Access Network Extension of Community Healthcare outcome video conferencing-based sequential training on transgender care and the VA Lesbian, Gay and Bisexual Education sharepoint and the transgender education sharepoint. At the VA, online LGBT health training is still limited.
Recommendations
Providing additional LGBT-focused TMS courses could help increase provider knowledge and cultural competencies. An online introductory LGBT health course could be offered at VA facilities for all new employees and HCPs as part of employee orientation. More specific courses with continuing education credits geared toward the HCP and focused on LGBT health could be included in the TMS learning portfolio for each HCP as annual learning refresher courses. This course could include specific knowledge questions on LGBT care and a posttest with a required passing score of 80%.
Training HCPs as designated LGBT educators in VA facilities should be considered. Classroom training could be offered annually or during times of increased LGBT patient focus, such as during LGBT Pride month when learning activities can be planned and/or coordinated with the VA Office of Diversity and Inclusion. Nurses, social workers, pharmacists, and medical assistants who provide supportive care to LGBT patients also should be included in the target audience for LGBT health education and training.
Limitations
The use of a cross-sectional project design with such a small convenience sample prevents generalizability of the findings to all PCPs. The lack of a survey design that included randomization and blinding in survey distribution could certainly deter participants from offering candid responses, particularly to some attitudinal questions that were emotional in context. The true-false responses wherein respondents had a 50% chance at guessing the correct response was not the most reliable method of measuring knowledge levels and thereby limited the ability to draw any strong conclusions about providers’ knowledge levels. Additionally, the project design did not allow for measures of other confounding factors, such as age, race/ethnicity, religion, and other social factors that could have influenced how participants responded. Additional randomized controlled studies with larger samples are needed to test specific interventions that evaluate the influence of gender on provider attitudes and knowledge as well as the effect of more HCP education and training on LGBT patient outcomes. Moreover, a literature review found no guidelines on how to specifically address provider gender differences in LGBT education and training or strategies for education and training interventions to address these differences.
Conclusion
Findings suggest that PCPs need additional education and training involving LGBT health issues. Although both male and female providers want more education, female PCPs as a group expressed a greater desire for more training compared with the responses of male PCPs. However, given the study’s small sample size, a strong conclusion regarding gender differences cannot be made. Research has shown that education is a factor that positively influences attitudes and feelings about providing LGBT care. The availability of education and training that is focused on LGBT health topics is still limited within the VHA. Within its stated mission to provide patient-centered care to all veterans, the
Better training on LGBT health topics is vital to improving health care delivery to LGBT populations. To meet this goal, ongoing HCP training to improve attitudes and knowledge and develop the skills necessary to effectively address LGBT health issues also must be a priority at VHA facilities. The VHA also should consider institutional changes that incorporate increased LGBT-focused health education into the learning activities of PCPs. This is essential to evaluating the quality of care given the impact on patient outcomes and health disparities in LGBT populations.
1. Grant JM, Mottet LA, Tanis J, Harrison J, Herman JL, Keisling M. Injustice at every turn: a report of the National Transgender Discrimination Survey. http://www.thetaskforce.org/static_html/downloads/reports/reports/ntds_full.pdf. Published 2011. Accessed October 5, 2017.
2. Institute of Medicine Committee on Lesbian, Gay, Bisexual, and Transgender Health Issues and Research Gaps and Opportunities.
3. Centers for Disease Control Prevention. Reported STDs in the United States. 2014 national data for chlamydia, gonorrhea, and syphilis. www.cdc.gov/std/stats14/std-trends-508.pdf. Published November 2015. Accessed October 5, 2017.
4. U.S. Department of Health and Human Services, Office of Disease Prevention and Health Promotion. Lesbian, gay, bisexual, and transgender health. http://www.healthypeople.gov/2020/topics-objectives/topic/lesbian-gay-bisexual-and-transgender-health. Updated October 4, 2017. Accessed October 5, 2017.
5. Kerker BD, Mostashari F, Thorpe L. Health care access and utilization among women who have sex with women: sexual behavior and identity. J Urban Health. 2006;83(5):970-979.
6. Khan MA, Evans AT, Shah S. Caring for uninsured patients with diabetes: designing and evaluating a novel chronic care model for diabetes care. J Eval Clin Pract. 2010;16(4):700-706.
7. Herek GM. Sexual prejudice. In: Nelson TD, ed. Handbook of Prejudice, Stereotyping, and Discrimination. New York: Psychology Press; 2009:441-467.
8. McKay B. Lesbian, gay, bisexual, and transgender health issues, disparities, and information resources. Med Ref Serv Q. 2011;30(4):393-401.
9. Chapman R, Watkins R, Zappia T, Nicol P, Shields L. Nursing and medical students’ attitude, knowledge and beliefs regarding lesbian, gay, bisexual and transgender parents seeking health care for their children. J Clin Nurs. 2012;21(7‐8):938-945.
10. Butler M, McCreedy E, Schwer N, et al. Improving cultural competence to reduce health disparities. Review No. 170. https://ahrq-ehc-application.s3.amazonaws.com/media/pdf/cultural-competence_research.pdf. Published March 29, 2016. Accessed October 5, 2017.
11. The Joint Commission. Advancing effective communication, cultural competence, and patient-and family-centered care: a roadmap for hospitals. https://www.jointcommission.org/assets/1/6/ARoadmapforHospitalsfinalversion727.pdf. Published 2010. Accessed October 5, 2017.
12. Snelgrove JW, Jasudavisius AM, Rowe BW, Head EM, Bauer GR. “Completely out-at-sea” with “two-gender medicine”: a qualitative analysis of physician-side barriers to providing healthcare for transgender patients. BMC Health Serv Res. 2012;12:110.
13. Gates GJ. How many people are lesbian, gay, bisexual, and transgender? http://williamsinstitute.law.ucla.edu/wp-content/uploads/Gates-How-Many-People-LGBT-Apr-2011.pdf. Published April 2011. Accessed October 5, 2017.
14. Gates GJ, Herman J. Transgender military service in the United States. https://williamsinstitute.law.ucla.edu/wp-content/uploads/Transgender-Military-Service-May-2014.pdf. Published May 2014. Accessed October 5, 2017.
15. U.S. Department of Veteran Affairs. Veterans Health Administration. http://www.va.gov/health. Updated October 2, 2017. Accessed October 5, 2017.
16. Gates GJ. Gay men and lesbians in the U.S. military: estimates from the 2000 census. http://www.lgbtdata.com/uploads/1/0/8/8/10884149/ds008_uscensus_gates.pdf. Published September 28, 2014. Accessed October 5, 2017.
17. Mattocks KM, Kauth MR, Sandfort T, Matza AR, Sullivan JC, Shipherd JC. Understanding health-care needs of sexual and gender minority veterans: how targeted research and policy can improve health. LGBT Health. 2014;1(1):50-57.
18. Kauth MR, Shipherd JC, Lindsay J, Blosnich JR, Brown GR, Jones KT. Access to care for transgender veterans in the Veterans Health Administration: 2006–2013. Am J Public Health. 2014;104(suppl 4):S532-S534.
19. Uchendu US. Veterans Health Administration Office of Health Equity: what is it a about? http://www.hsrd.research.va.gov/for_researchers/cyber_seminars/archives/769-notes.pdf. Published November 4, 2013. Accessed October 5, 2017.
20. U.S. Department of Veterans Affairs, Veterans Health Administration. VHA Directive 2013-003: Providing Health Care for Transgender and Intersex Veterans. http://www.transequality.org/sites/default/files/docs/resources/VHAHealthcareDirective_2013.pdf. Published February 8, 2013. Accessed October 5, 2017.
21. Campinha-Bacote J. The process of cultural competence in the delivery of healthcare services: a model of care. J Transcult Nurs. 2002;13(3):181-184.
22. Jabson JM, Mitchell JW, Doty SB. Associations between non-discrimination and training policies and physicians’ attitudes and knowledge about sexual and gender minority patients: a comparison of physicians from two hospitals. BMC Public Health. 2016;16:256
1. Grant JM, Mottet LA, Tanis J, Harrison J, Herman JL, Keisling M. Injustice at every turn: a report of the National Transgender Discrimination Survey. http://www.thetaskforce.org/static_html/downloads/reports/reports/ntds_full.pdf. Published 2011. Accessed October 5, 2017.
2. Institute of Medicine Committee on Lesbian, Gay, Bisexual, and Transgender Health Issues and Research Gaps and Opportunities.
3. Centers for Disease Control Prevention. Reported STDs in the United States. 2014 national data for chlamydia, gonorrhea, and syphilis. www.cdc.gov/std/stats14/std-trends-508.pdf. Published November 2015. Accessed October 5, 2017.
4. U.S. Department of Health and Human Services, Office of Disease Prevention and Health Promotion. Lesbian, gay, bisexual, and transgender health. http://www.healthypeople.gov/2020/topics-objectives/topic/lesbian-gay-bisexual-and-transgender-health. Updated October 4, 2017. Accessed October 5, 2017.
5. Kerker BD, Mostashari F, Thorpe L. Health care access and utilization among women who have sex with women: sexual behavior and identity. J Urban Health. 2006;83(5):970-979.
6. Khan MA, Evans AT, Shah S. Caring for uninsured patients with diabetes: designing and evaluating a novel chronic care model for diabetes care. J Eval Clin Pract. 2010;16(4):700-706.
7. Herek GM. Sexual prejudice. In: Nelson TD, ed. Handbook of Prejudice, Stereotyping, and Discrimination. New York: Psychology Press; 2009:441-467.
8. McKay B. Lesbian, gay, bisexual, and transgender health issues, disparities, and information resources. Med Ref Serv Q. 2011;30(4):393-401.
9. Chapman R, Watkins R, Zappia T, Nicol P, Shields L. Nursing and medical students’ attitude, knowledge and beliefs regarding lesbian, gay, bisexual and transgender parents seeking health care for their children. J Clin Nurs. 2012;21(7‐8):938-945.
10. Butler M, McCreedy E, Schwer N, et al. Improving cultural competence to reduce health disparities. Review No. 170. https://ahrq-ehc-application.s3.amazonaws.com/media/pdf/cultural-competence_research.pdf. Published March 29, 2016. Accessed October 5, 2017.
11. The Joint Commission. Advancing effective communication, cultural competence, and patient-and family-centered care: a roadmap for hospitals. https://www.jointcommission.org/assets/1/6/ARoadmapforHospitalsfinalversion727.pdf. Published 2010. Accessed October 5, 2017.
12. Snelgrove JW, Jasudavisius AM, Rowe BW, Head EM, Bauer GR. “Completely out-at-sea” with “two-gender medicine”: a qualitative analysis of physician-side barriers to providing healthcare for transgender patients. BMC Health Serv Res. 2012;12:110.
13. Gates GJ. How many people are lesbian, gay, bisexual, and transgender? http://williamsinstitute.law.ucla.edu/wp-content/uploads/Gates-How-Many-People-LGBT-Apr-2011.pdf. Published April 2011. Accessed October 5, 2017.
14. Gates GJ, Herman J. Transgender military service in the United States. https://williamsinstitute.law.ucla.edu/wp-content/uploads/Transgender-Military-Service-May-2014.pdf. Published May 2014. Accessed October 5, 2017.
15. U.S. Department of Veteran Affairs. Veterans Health Administration. http://www.va.gov/health. Updated October 2, 2017. Accessed October 5, 2017.
16. Gates GJ. Gay men and lesbians in the U.S. military: estimates from the 2000 census. http://www.lgbtdata.com/uploads/1/0/8/8/10884149/ds008_uscensus_gates.pdf. Published September 28, 2014. Accessed October 5, 2017.
17. Mattocks KM, Kauth MR, Sandfort T, Matza AR, Sullivan JC, Shipherd JC. Understanding health-care needs of sexual and gender minority veterans: how targeted research and policy can improve health. LGBT Health. 2014;1(1):50-57.
18. Kauth MR, Shipherd JC, Lindsay J, Blosnich JR, Brown GR, Jones KT. Access to care for transgender veterans in the Veterans Health Administration: 2006–2013. Am J Public Health. 2014;104(suppl 4):S532-S534.
19. Uchendu US. Veterans Health Administration Office of Health Equity: what is it a about? http://www.hsrd.research.va.gov/for_researchers/cyber_seminars/archives/769-notes.pdf. Published November 4, 2013. Accessed October 5, 2017.
20. U.S. Department of Veterans Affairs, Veterans Health Administration. VHA Directive 2013-003: Providing Health Care for Transgender and Intersex Veterans. http://www.transequality.org/sites/default/files/docs/resources/VHAHealthcareDirective_2013.pdf. Published February 8, 2013. Accessed October 5, 2017.
21. Campinha-Bacote J. The process of cultural competence in the delivery of healthcare services: a model of care. J Transcult Nurs. 2002;13(3):181-184.
22. Jabson JM, Mitchell JW, Doty SB. Associations between non-discrimination and training policies and physicians’ attitudes and knowledge about sexual and gender minority patients: a comparison of physicians from two hospitals. BMC Public Health. 2016;16:256
Improving Care and Reducing Length of Stay in Patients Undergoing Total Knee Replacement
Many improvements in health care today involve care coordination across the entire health care system. Active management of an orthopedic surgery service from a system perspective allows for improvements that can favorably impact readmissions and length of stay (LOS) for patients.1 The following is an example of a systemwide process improvement in total knee replacement (TKR) surgery that dramatically decreased 30-day readmissions and shortened the LOS during a 12-month period.\
Background
The VA is the largest integrated health care system in the U.S. VA hospitals use the VA Surgical Quality Improvement Program (VASQIP) to monitor surgical services. Initially known as the National Surgery Quality Improvement Program (NSQIP), the program began in 1994 to help provide reliable, valid information on patient presurgical factors, processes of care during surgery, and 30-day morbidity and mortality rates in VA hospitals.2 Since its inception, NSQIP has spread to the private sector and is now widely used throughout the U.S.
Using on-site data acquisition by specially trained and dedicated registered nurses, information on each surgical case is input into a quality program. Quarterly reports are distributed to each hospital, and a comparison of mortality, LOS, 30-day readmissions to the hospital, and other data are analyzed and presented by quarter and rolling 12-month time frames. Use of VASQIP data allows improvement of the structures and processes of care throughout the VA, providing safer surgery for veterans.
At the Phoenix VA Health Care System (PVAHCS) in Arizona, the third quarter 2014 report showed the rolling 12-month average LOS for orthopedic TKR patients was 3.5 days and corresponding 30-day readmissions were 7.9%. Using a systems improvement approach, the authors set a goal of reducing these metrics by 10%.
The orthopedic service engaged members of the hospitalist, anesthesia, physical therapy (PT), nursing, social work, primary care, and pharmacy services, as well as hospital administration. Twelve months later, the LOS for TKR patients declined 20% to 2.8 days. Corresponding 30-day readmissions declined for the patients with knee replacement to 3.4%—a 57% reduction in 1 year. Mortality for these 177 cases was zero.
To accomplish these improvements, the authors divided the surgical procedure into preoperative, perioperative, and postoperative time frames and looked at process improvement during each of these periods. The following is a summary of the various processes that the authors feel contributed to the reduced LOS and 30-day readmission rate. Although some of these interventions were in place before the study period, all the processes were standardized for TKRs through surgeon consensus, and each of the surgeons adopted all the processes during the study period.
Preoperative Processes
In the VA primary care-based model orthopedic surgery is accessed through a consult process in the electronic health record. The orthopedic surgery service reviews each new consult and makes recommendations for optimization at the time the consult was received. This process was used to work closely with primary care providers to preoperatively prepare patients. The orthopedic surgery service advocates smoking cessation, substance abuse treatment, weight loss with an ideal body mass index of ≤ 35, and diabetes mellitus (DM) management with a ≤ 7 hemoglobin A1c value.3-7
This management did not result in fewer patients receiving TKR. In fact, the volume of TKR patients increased by 8% over the study period. Although part of this increase could have been due to increased scheduling efficiency, the orthopedic surgery service worked closely with primary care, nutrition, and medicine services to optimize these patients so they could be placed on the schedule for surgery.
Preoperative Education
Physical therapy and the orthopedic preprocedure clinic provided preoperative education to patients, covering preoperative chlorhexidine body washes, home safety, use of a walker, anticipated LOS, use of ambulatory sequential compressive devices, use of a knee cooling device, as well as PT protocols during hospitalization.8 This helped increase postoperative patient adherence and helped patients anticipate an appropriate LOS. Health care providers worked with patients to understand their home environment and plan for caregivers to assist them in the immediate postoperative period.
Intraoperative Processes
Reducing Blood Loss
The orthopedic surgery service reviewed literature related to the efficacy and safety of tranexamic acid. Based on the literature, the orthopedic surgery service arrived at a consensus agreement to implement a topical tranexamic acid dose of 3 g/100 cc saline for each TKR. Presentation of the pertinent literature to the pharmacy service allowed placement of this medication on the formulary for intraoperative use in the TKR cases.
Specific processes were implemented that involved the orthopedic service ordering tranexamic acid in advance for each patient, pharmacy mixing the solution and having it ready in a timely manner, and the operating room sending a messenger to the pharmacy to pick up a sterile container of the tranexamic acid/saline solution. Postoperative blood loss and transfusions decreased. Less anemia contributed to better performance and less fatigue in PT, which helped move patients down a pathway for quicker discharge.9,10
DVT Mechanical Prophylaxis
The orthopedic surgery service was concerned about adherence with stationary sequential compressive devices for mechanical thromboembolic prophylaxis. Patients had to remove them for PT, ambulation in the halls, and visiting the restroom, and then nurses had to replace them. A literature review examined a mobile compressive device that could be maintained during ambulation, and a demonstration for the orthopedic surgery service was arranged. The orthopedic service decided to change to the newer device, and the mobile compression device was presented to the PVAHCS Therapeutics Committee. Subsequently the new device was implemented after the appropriate in-service of the various clinic, PT, ward, surgery, preoperative, and postoperative personnel.11 The device was initiated in the holding area prior to surgery, continued throughout the hospitalization, and taken home by the patient for 2 weeks of use following surgery. Patients were instructed to return the device to clinic at their 2-week follow-up appointment.
Infection Control
A dilute betadine lavage was instituted for each surgical case, using the pulsatile lavage followed by a lactated Ringer solution rinse prior to TKR implantation. Additionally, the wound was lavaged prior to closure with this dilute betadine solution.12
Pain Control
Immediately before surgery, patients received oral morphine sulfate and celecoxib. A local 2% lidocaine with epinephrine injection was used at the surgical incision and joint after the skin prep and immediately prior to the skin incision. Patients received a mixture of ropivicaine .5%/20 mL, morphine sulfate 10 mg, and toradol 30 mg at the capsular region prior to implantation of the total knee prosthesis. At the end of the procedure, an additional 20 mL of 2% lidocaine was injected into the joint once the capsule was closed. This improved postoperative pain, decreased postoperative opioid dosing, and allowed for earlier ambulation with PT.13
PostOperative Processes
Deep Vein Thrombosis (DVT) Chemoprophylaxis
Once the chest physician guidelines-approved stand-alone mobile compressive devices was implemented, orthopedic surgery service revisited the chemoprophylaxis for routine low-risk patients. Use of subcutaneously daily injections of 2.5 mg fondiparinux was switched to 81 mg enteric-coated aspirin administered orally twice daily. The authors believe this further reduced the postoperative bleeding and transfusion risks. There was not an increase in DVT or pulmonary embolism complications.14,15
Physical Therapy
Partnering with PT, a 2-day LOS protocol was established. Patients were introduced to this protocol in a preoperative PT teaching class, and it was reinforced during the hospital stay. Patients who had earlier cases in the day were seen by PT the day of surgery when staffing and scheduling permitted. Early ambulation contributed significantly to earlier discharge for patients.16 Early ambulation also has been shown to decrease thromboembolic complications in orthopedic total joint patients.
Pain and Nausea Management
Parenteral narcotics were avoided, and oral narcotics were implemented with a graduated dosing based on a 10-point pain scale
Use of a postoperative cooling device that circulated cool water through a pad over the patient’s knee was instituted to assist with pain control. The patient received instruction on this device at the preoperative education sessions and was given the device to continue at home postdischarge.
Hospitalist Comanagement
Comanagement of orthopedic patients with hospitalists has become a standard practice nationally. The orthopedic surgery service works closely with the hospitalist team who see each total joint patient on postoperative admission to the ward. The orthopedic team handles all aspects of PT, wound management, pain control, and DVT prophylaxis. The hospitalist focuses on the remainder of comorbid conditions such as DM, chronic obstructive pulmonary disease, and underlying cardiac conditions.
The American Society of Anesthesiologists (ASA) average score was 2.8 for these procedures. Despite comprehensive preoperative screening, older patients with more comorbidities (higher ASA score) are more prone to emerging complications.17 Integration of the hospitalist team into the care of every orthopedic total joint patient facilitates prompt recognition and mitigation of these complications as they occur, directly reducing overall severity and LOS and allowing safe recovery from the surgical procedure.18,19
Conclusion
At the start of this system improvement, the previous 12-month data showed 164 knee replacements with a 4.9-day VA national LOS and 3.5- day PVAHCS LOS. At the end of the 12-month system improvement, the VA national LOS for TKR was 4.8 days, and at PVAHCS it was 2.8 days.
The 30-day readmission rate was 8.4% nationally and 7.9% at PVAHCS. After the system improvements, the national 30-day readmission rate was 7.1%, while the PVAHCS rate dropped to less than half the national rate: 3.4%.
It is important to note, that the improvements in the aforementioned multiple processes could not have been possible without a dedicated effort from the multiple stakeholders involved. Hospitalists, primary care, PT, pharmacy, operating room staff, anesthesia, preprocedure staff, floor nurses, the Commodities and Therapeutics Committee, and administration all partnered with the orthopedic surgery service to produce the improvements in LOS and corresponding reduction in 30-day readmissions.
These data suggest that there does not need to be an inherent tradeoff between LOS and 30-day readmissions. Rather, both measures can be managed independently to produce improvements across the service. A team approach to process improvement can allow for increased efficiency while providing safer care for patients.
1. Dundon JM, Bosco J, Slover J, Yu S, Sayeed Y, Iorio R. Improvement in total joint replacement quality metrics, year one versus year three of the bundled payments for care improvement initiative. J Bone Joint Surg Am. 2016;98(23):1949-1953.
2. Itani KM. Fifteen years of the National Surgical Quality Improvement Program in review. Am J Surg. 2009;198(suppl 5):S9-S18.
3. Tayton ER, Frampton C, Hooper GJ, Young SW. The impact of patient and surgical factors on the rate of infection after primary total knee arthroplasty: an analysis of 64,566 joints from the New Zealand Joint Registry. Bone Joint J. 2016;98-B(3):334-340.
4. Heller S, Rezapoor M, Parvizi J. Minimising the risk of infection: a peri-operative checklist. Bone Joint J. 2016;98-B(1)(suppl A):18-22.
5. Thornqvist C, Gislason GH, Køber L, Jensen PF, Torp-Pedersen C, Andersson C. Body mass index and risk of perioperative cardiovascular adverse events and mortality in 34,744 Danish patients undergoing hip or knee replacement. Acta Orthop. 2014;85(5):456-462.
6. Stryker LS, Abdel MP, Morrey ME, Morrow MM, Kor DJ, Morrey BF. Elevated postoperative blood glucose and preoperative hemoglobin A1C are associated with increased wound complications following total joint arthroplasty. J Bone Joint Surg Am. 2013;95(9):808-814.
7. Akhavan S, Nguyen LC, Chan V, Saleh J, Bozic KJ. Impact of smoking cessation counseling prior to total joint arthroplasty. Orthopedics. 2017;40(2):e323-e328.
8. Kim DH, Spencer M, Davidson SM, et al. Institutional prescreening for detection and eradication of methicillin-resistant Staphylococcus aureus in patients undergoing elective orthopaedic surgery. J Bone Joint Surg Am. 2010;92(9):1820-1826.
9. Goyal N, Chen DB, Harris IA, Rowden NJ, Kirsh G, MacDessi SJ. Intravenous vs intra-articular tranexamic acid in total knee arthroplasty: a randomized, double-blind trial. J Arthroplasty. 2017;32(1):28-32.
10. Phan DL, Ani F, Schwarzkopf R. Cost analysis of tranexamic acid in anemic total joint arthroplasty patients. J Arthroplasty. 2016;31(3):579-582.
11. Colwell CW Jr, Froimson MI, Mont MA, et al. Thrombosis prevention after total hip arthroplasty a prospective, randomized trial comparing a mobile compression device with low-molecular-weight heparin. J Bone Joint Surg Am. 2010;92(3):527-535.
12. Chundamala J, Wright JG. The efficacy and risks of using povidone-iodine irrigation to prevent surgical site infection: an evidence-based review. Can J Surg. 2007;50(6):473-481.
13. Fang R, Liu Z, Alijiang A, et al. Efficacy of intra-articular local anesthetics in total knee arthroplasty. Orthopedics. 2015;38(7):e573-e581.
14. Odeh K, Doran J, Yu S, Bolz N, Bosco J, Iorio R. Risk-stratified venous thromboembolism prophylaxis after total joint arthroplasty: aspirin and sequential pneumatic compression devices vs aggressive chemoprophylaxis. J Arthroplasty. 2016;31(suppl 9):78-82.
15. Parvizi J, Huang R, Restrepo C, et al. Low-dose aspirin is effective chemoprophylaxis against clinically important venous thromboembolism following total joint arthroplasty: a preliminary analysis. J Bone Joint Surg Am. 2017;99(2):91-98.
16. Robertson NB, Warganich T, Ghazarossian J, Khatod M. Implementation of an accelerated rehabilitation protocol for total joint arthroplasty in the managed care setting: the experience of one institution. Adv Orthop Surg. 2015;(2015):387197.
17. Hooper GJ, Rothwell AG, Hooper NM, Frampton C. The relationship between the American Society of Anesthesiologists physical rating and outcome following total hip and knee arthroplasty: an analysis of the New Zealand Joint Registry. J Bone Joint Surg Am. 2012;94(12):1065-1070.
18. Parry MC, Smith AJ, Blom AW. Early death following primary total knee arthroplasty. J Bone Joint Surg Am. 2011;93(10):948-953.
19. Parvizi J, Mui A, Purtill JJ, Sharkey PF, Hozack WJ, Rothman RH. Total joint arthroplasty: when do fatal or near-fatal complications occur? J Bone Joint Surg Am. 2007;89(1):27-32.
Many improvements in health care today involve care coordination across the entire health care system. Active management of an orthopedic surgery service from a system perspective allows for improvements that can favorably impact readmissions and length of stay (LOS) for patients.1 The following is an example of a systemwide process improvement in total knee replacement (TKR) surgery that dramatically decreased 30-day readmissions and shortened the LOS during a 12-month period.\
Background
The VA is the largest integrated health care system in the U.S. VA hospitals use the VA Surgical Quality Improvement Program (VASQIP) to monitor surgical services. Initially known as the National Surgery Quality Improvement Program (NSQIP), the program began in 1994 to help provide reliable, valid information on patient presurgical factors, processes of care during surgery, and 30-day morbidity and mortality rates in VA hospitals.2 Since its inception, NSQIP has spread to the private sector and is now widely used throughout the U.S.
Using on-site data acquisition by specially trained and dedicated registered nurses, information on each surgical case is input into a quality program. Quarterly reports are distributed to each hospital, and a comparison of mortality, LOS, 30-day readmissions to the hospital, and other data are analyzed and presented by quarter and rolling 12-month time frames. Use of VASQIP data allows improvement of the structures and processes of care throughout the VA, providing safer surgery for veterans.
At the Phoenix VA Health Care System (PVAHCS) in Arizona, the third quarter 2014 report showed the rolling 12-month average LOS for orthopedic TKR patients was 3.5 days and corresponding 30-day readmissions were 7.9%. Using a systems improvement approach, the authors set a goal of reducing these metrics by 10%.
The orthopedic service engaged members of the hospitalist, anesthesia, physical therapy (PT), nursing, social work, primary care, and pharmacy services, as well as hospital administration. Twelve months later, the LOS for TKR patients declined 20% to 2.8 days. Corresponding 30-day readmissions declined for the patients with knee replacement to 3.4%—a 57% reduction in 1 year. Mortality for these 177 cases was zero.
To accomplish these improvements, the authors divided the surgical procedure into preoperative, perioperative, and postoperative time frames and looked at process improvement during each of these periods. The following is a summary of the various processes that the authors feel contributed to the reduced LOS and 30-day readmission rate. Although some of these interventions were in place before the study period, all the processes were standardized for TKRs through surgeon consensus, and each of the surgeons adopted all the processes during the study period.
Preoperative Processes
In the VA primary care-based model orthopedic surgery is accessed through a consult process in the electronic health record. The orthopedic surgery service reviews each new consult and makes recommendations for optimization at the time the consult was received. This process was used to work closely with primary care providers to preoperatively prepare patients. The orthopedic surgery service advocates smoking cessation, substance abuse treatment, weight loss with an ideal body mass index of ≤ 35, and diabetes mellitus (DM) management with a ≤ 7 hemoglobin A1c value.3-7
This management did not result in fewer patients receiving TKR. In fact, the volume of TKR patients increased by 8% over the study period. Although part of this increase could have been due to increased scheduling efficiency, the orthopedic surgery service worked closely with primary care, nutrition, and medicine services to optimize these patients so they could be placed on the schedule for surgery.
Preoperative Education
Physical therapy and the orthopedic preprocedure clinic provided preoperative education to patients, covering preoperative chlorhexidine body washes, home safety, use of a walker, anticipated LOS, use of ambulatory sequential compressive devices, use of a knee cooling device, as well as PT protocols during hospitalization.8 This helped increase postoperative patient adherence and helped patients anticipate an appropriate LOS. Health care providers worked with patients to understand their home environment and plan for caregivers to assist them in the immediate postoperative period.
Intraoperative Processes
Reducing Blood Loss
The orthopedic surgery service reviewed literature related to the efficacy and safety of tranexamic acid. Based on the literature, the orthopedic surgery service arrived at a consensus agreement to implement a topical tranexamic acid dose of 3 g/100 cc saline for each TKR. Presentation of the pertinent literature to the pharmacy service allowed placement of this medication on the formulary for intraoperative use in the TKR cases.
Specific processes were implemented that involved the orthopedic service ordering tranexamic acid in advance for each patient, pharmacy mixing the solution and having it ready in a timely manner, and the operating room sending a messenger to the pharmacy to pick up a sterile container of the tranexamic acid/saline solution. Postoperative blood loss and transfusions decreased. Less anemia contributed to better performance and less fatigue in PT, which helped move patients down a pathway for quicker discharge.9,10
DVT Mechanical Prophylaxis
The orthopedic surgery service was concerned about adherence with stationary sequential compressive devices for mechanical thromboembolic prophylaxis. Patients had to remove them for PT, ambulation in the halls, and visiting the restroom, and then nurses had to replace them. A literature review examined a mobile compressive device that could be maintained during ambulation, and a demonstration for the orthopedic surgery service was arranged. The orthopedic service decided to change to the newer device, and the mobile compression device was presented to the PVAHCS Therapeutics Committee. Subsequently the new device was implemented after the appropriate in-service of the various clinic, PT, ward, surgery, preoperative, and postoperative personnel.11 The device was initiated in the holding area prior to surgery, continued throughout the hospitalization, and taken home by the patient for 2 weeks of use following surgery. Patients were instructed to return the device to clinic at their 2-week follow-up appointment.
Infection Control
A dilute betadine lavage was instituted for each surgical case, using the pulsatile lavage followed by a lactated Ringer solution rinse prior to TKR implantation. Additionally, the wound was lavaged prior to closure with this dilute betadine solution.12
Pain Control
Immediately before surgery, patients received oral morphine sulfate and celecoxib. A local 2% lidocaine with epinephrine injection was used at the surgical incision and joint after the skin prep and immediately prior to the skin incision. Patients received a mixture of ropivicaine .5%/20 mL, morphine sulfate 10 mg, and toradol 30 mg at the capsular region prior to implantation of the total knee prosthesis. At the end of the procedure, an additional 20 mL of 2% lidocaine was injected into the joint once the capsule was closed. This improved postoperative pain, decreased postoperative opioid dosing, and allowed for earlier ambulation with PT.13
PostOperative Processes
Deep Vein Thrombosis (DVT) Chemoprophylaxis
Once the chest physician guidelines-approved stand-alone mobile compressive devices was implemented, orthopedic surgery service revisited the chemoprophylaxis for routine low-risk patients. Use of subcutaneously daily injections of 2.5 mg fondiparinux was switched to 81 mg enteric-coated aspirin administered orally twice daily. The authors believe this further reduced the postoperative bleeding and transfusion risks. There was not an increase in DVT or pulmonary embolism complications.14,15
Physical Therapy
Partnering with PT, a 2-day LOS protocol was established. Patients were introduced to this protocol in a preoperative PT teaching class, and it was reinforced during the hospital stay. Patients who had earlier cases in the day were seen by PT the day of surgery when staffing and scheduling permitted. Early ambulation contributed significantly to earlier discharge for patients.16 Early ambulation also has been shown to decrease thromboembolic complications in orthopedic total joint patients.
Pain and Nausea Management
Parenteral narcotics were avoided, and oral narcotics were implemented with a graduated dosing based on a 10-point pain scale
Use of a postoperative cooling device that circulated cool water through a pad over the patient’s knee was instituted to assist with pain control. The patient received instruction on this device at the preoperative education sessions and was given the device to continue at home postdischarge.
Hospitalist Comanagement
Comanagement of orthopedic patients with hospitalists has become a standard practice nationally. The orthopedic surgery service works closely with the hospitalist team who see each total joint patient on postoperative admission to the ward. The orthopedic team handles all aspects of PT, wound management, pain control, and DVT prophylaxis. The hospitalist focuses on the remainder of comorbid conditions such as DM, chronic obstructive pulmonary disease, and underlying cardiac conditions.
The American Society of Anesthesiologists (ASA) average score was 2.8 for these procedures. Despite comprehensive preoperative screening, older patients with more comorbidities (higher ASA score) are more prone to emerging complications.17 Integration of the hospitalist team into the care of every orthopedic total joint patient facilitates prompt recognition and mitigation of these complications as they occur, directly reducing overall severity and LOS and allowing safe recovery from the surgical procedure.18,19
Conclusion
At the start of this system improvement, the previous 12-month data showed 164 knee replacements with a 4.9-day VA national LOS and 3.5- day PVAHCS LOS. At the end of the 12-month system improvement, the VA national LOS for TKR was 4.8 days, and at PVAHCS it was 2.8 days.
The 30-day readmission rate was 8.4% nationally and 7.9% at PVAHCS. After the system improvements, the national 30-day readmission rate was 7.1%, while the PVAHCS rate dropped to less than half the national rate: 3.4%.
It is important to note, that the improvements in the aforementioned multiple processes could not have been possible without a dedicated effort from the multiple stakeholders involved. Hospitalists, primary care, PT, pharmacy, operating room staff, anesthesia, preprocedure staff, floor nurses, the Commodities and Therapeutics Committee, and administration all partnered with the orthopedic surgery service to produce the improvements in LOS and corresponding reduction in 30-day readmissions.
These data suggest that there does not need to be an inherent tradeoff between LOS and 30-day readmissions. Rather, both measures can be managed independently to produce improvements across the service. A team approach to process improvement can allow for increased efficiency while providing safer care for patients.
Many improvements in health care today involve care coordination across the entire health care system. Active management of an orthopedic surgery service from a system perspective allows for improvements that can favorably impact readmissions and length of stay (LOS) for patients.1 The following is an example of a systemwide process improvement in total knee replacement (TKR) surgery that dramatically decreased 30-day readmissions and shortened the LOS during a 12-month period.\
Background
The VA is the largest integrated health care system in the U.S. VA hospitals use the VA Surgical Quality Improvement Program (VASQIP) to monitor surgical services. Initially known as the National Surgery Quality Improvement Program (NSQIP), the program began in 1994 to help provide reliable, valid information on patient presurgical factors, processes of care during surgery, and 30-day morbidity and mortality rates in VA hospitals.2 Since its inception, NSQIP has spread to the private sector and is now widely used throughout the U.S.
Using on-site data acquisition by specially trained and dedicated registered nurses, information on each surgical case is input into a quality program. Quarterly reports are distributed to each hospital, and a comparison of mortality, LOS, 30-day readmissions to the hospital, and other data are analyzed and presented by quarter and rolling 12-month time frames. Use of VASQIP data allows improvement of the structures and processes of care throughout the VA, providing safer surgery for veterans.
At the Phoenix VA Health Care System (PVAHCS) in Arizona, the third quarter 2014 report showed the rolling 12-month average LOS for orthopedic TKR patients was 3.5 days and corresponding 30-day readmissions were 7.9%. Using a systems improvement approach, the authors set a goal of reducing these metrics by 10%.
The orthopedic service engaged members of the hospitalist, anesthesia, physical therapy (PT), nursing, social work, primary care, and pharmacy services, as well as hospital administration. Twelve months later, the LOS for TKR patients declined 20% to 2.8 days. Corresponding 30-day readmissions declined for the patients with knee replacement to 3.4%—a 57% reduction in 1 year. Mortality for these 177 cases was zero.
To accomplish these improvements, the authors divided the surgical procedure into preoperative, perioperative, and postoperative time frames and looked at process improvement during each of these periods. The following is a summary of the various processes that the authors feel contributed to the reduced LOS and 30-day readmission rate. Although some of these interventions were in place before the study period, all the processes were standardized for TKRs through surgeon consensus, and each of the surgeons adopted all the processes during the study period.
Preoperative Processes
In the VA primary care-based model orthopedic surgery is accessed through a consult process in the electronic health record. The orthopedic surgery service reviews each new consult and makes recommendations for optimization at the time the consult was received. This process was used to work closely with primary care providers to preoperatively prepare patients. The orthopedic surgery service advocates smoking cessation, substance abuse treatment, weight loss with an ideal body mass index of ≤ 35, and diabetes mellitus (DM) management with a ≤ 7 hemoglobin A1c value.3-7
This management did not result in fewer patients receiving TKR. In fact, the volume of TKR patients increased by 8% over the study period. Although part of this increase could have been due to increased scheduling efficiency, the orthopedic surgery service worked closely with primary care, nutrition, and medicine services to optimize these patients so they could be placed on the schedule for surgery.
Preoperative Education
Physical therapy and the orthopedic preprocedure clinic provided preoperative education to patients, covering preoperative chlorhexidine body washes, home safety, use of a walker, anticipated LOS, use of ambulatory sequential compressive devices, use of a knee cooling device, as well as PT protocols during hospitalization.8 This helped increase postoperative patient adherence and helped patients anticipate an appropriate LOS. Health care providers worked with patients to understand their home environment and plan for caregivers to assist them in the immediate postoperative period.
Intraoperative Processes
Reducing Blood Loss
The orthopedic surgery service reviewed literature related to the efficacy and safety of tranexamic acid. Based on the literature, the orthopedic surgery service arrived at a consensus agreement to implement a topical tranexamic acid dose of 3 g/100 cc saline for each TKR. Presentation of the pertinent literature to the pharmacy service allowed placement of this medication on the formulary for intraoperative use in the TKR cases.
Specific processes were implemented that involved the orthopedic service ordering tranexamic acid in advance for each patient, pharmacy mixing the solution and having it ready in a timely manner, and the operating room sending a messenger to the pharmacy to pick up a sterile container of the tranexamic acid/saline solution. Postoperative blood loss and transfusions decreased. Less anemia contributed to better performance and less fatigue in PT, which helped move patients down a pathway for quicker discharge.9,10
DVT Mechanical Prophylaxis
The orthopedic surgery service was concerned about adherence with stationary sequential compressive devices for mechanical thromboembolic prophylaxis. Patients had to remove them for PT, ambulation in the halls, and visiting the restroom, and then nurses had to replace them. A literature review examined a mobile compressive device that could be maintained during ambulation, and a demonstration for the orthopedic surgery service was arranged. The orthopedic service decided to change to the newer device, and the mobile compression device was presented to the PVAHCS Therapeutics Committee. Subsequently the new device was implemented after the appropriate in-service of the various clinic, PT, ward, surgery, preoperative, and postoperative personnel.11 The device was initiated in the holding area prior to surgery, continued throughout the hospitalization, and taken home by the patient for 2 weeks of use following surgery. Patients were instructed to return the device to clinic at their 2-week follow-up appointment.
Infection Control
A dilute betadine lavage was instituted for each surgical case, using the pulsatile lavage followed by a lactated Ringer solution rinse prior to TKR implantation. Additionally, the wound was lavaged prior to closure with this dilute betadine solution.12
Pain Control
Immediately before surgery, patients received oral morphine sulfate and celecoxib. A local 2% lidocaine with epinephrine injection was used at the surgical incision and joint after the skin prep and immediately prior to the skin incision. Patients received a mixture of ropivicaine .5%/20 mL, morphine sulfate 10 mg, and toradol 30 mg at the capsular region prior to implantation of the total knee prosthesis. At the end of the procedure, an additional 20 mL of 2% lidocaine was injected into the joint once the capsule was closed. This improved postoperative pain, decreased postoperative opioid dosing, and allowed for earlier ambulation with PT.13
PostOperative Processes
Deep Vein Thrombosis (DVT) Chemoprophylaxis
Once the chest physician guidelines-approved stand-alone mobile compressive devices was implemented, orthopedic surgery service revisited the chemoprophylaxis for routine low-risk patients. Use of subcutaneously daily injections of 2.5 mg fondiparinux was switched to 81 mg enteric-coated aspirin administered orally twice daily. The authors believe this further reduced the postoperative bleeding and transfusion risks. There was not an increase in DVT or pulmonary embolism complications.14,15
Physical Therapy
Partnering with PT, a 2-day LOS protocol was established. Patients were introduced to this protocol in a preoperative PT teaching class, and it was reinforced during the hospital stay. Patients who had earlier cases in the day were seen by PT the day of surgery when staffing and scheduling permitted. Early ambulation contributed significantly to earlier discharge for patients.16 Early ambulation also has been shown to decrease thromboembolic complications in orthopedic total joint patients.
Pain and Nausea Management
Parenteral narcotics were avoided, and oral narcotics were implemented with a graduated dosing based on a 10-point pain scale
Use of a postoperative cooling device that circulated cool water through a pad over the patient’s knee was instituted to assist with pain control. The patient received instruction on this device at the preoperative education sessions and was given the device to continue at home postdischarge.
Hospitalist Comanagement
Comanagement of orthopedic patients with hospitalists has become a standard practice nationally. The orthopedic surgery service works closely with the hospitalist team who see each total joint patient on postoperative admission to the ward. The orthopedic team handles all aspects of PT, wound management, pain control, and DVT prophylaxis. The hospitalist focuses on the remainder of comorbid conditions such as DM, chronic obstructive pulmonary disease, and underlying cardiac conditions.
The American Society of Anesthesiologists (ASA) average score was 2.8 for these procedures. Despite comprehensive preoperative screening, older patients with more comorbidities (higher ASA score) are more prone to emerging complications.17 Integration of the hospitalist team into the care of every orthopedic total joint patient facilitates prompt recognition and mitigation of these complications as they occur, directly reducing overall severity and LOS and allowing safe recovery from the surgical procedure.18,19
Conclusion
At the start of this system improvement, the previous 12-month data showed 164 knee replacements with a 4.9-day VA national LOS and 3.5- day PVAHCS LOS. At the end of the 12-month system improvement, the VA national LOS for TKR was 4.8 days, and at PVAHCS it was 2.8 days.
The 30-day readmission rate was 8.4% nationally and 7.9% at PVAHCS. After the system improvements, the national 30-day readmission rate was 7.1%, while the PVAHCS rate dropped to less than half the national rate: 3.4%.
It is important to note, that the improvements in the aforementioned multiple processes could not have been possible without a dedicated effort from the multiple stakeholders involved. Hospitalists, primary care, PT, pharmacy, operating room staff, anesthesia, preprocedure staff, floor nurses, the Commodities and Therapeutics Committee, and administration all partnered with the orthopedic surgery service to produce the improvements in LOS and corresponding reduction in 30-day readmissions.
These data suggest that there does not need to be an inherent tradeoff between LOS and 30-day readmissions. Rather, both measures can be managed independently to produce improvements across the service. A team approach to process improvement can allow for increased efficiency while providing safer care for patients.
1. Dundon JM, Bosco J, Slover J, Yu S, Sayeed Y, Iorio R. Improvement in total joint replacement quality metrics, year one versus year three of the bundled payments for care improvement initiative. J Bone Joint Surg Am. 2016;98(23):1949-1953.
2. Itani KM. Fifteen years of the National Surgical Quality Improvement Program in review. Am J Surg. 2009;198(suppl 5):S9-S18.
3. Tayton ER, Frampton C, Hooper GJ, Young SW. The impact of patient and surgical factors on the rate of infection after primary total knee arthroplasty: an analysis of 64,566 joints from the New Zealand Joint Registry. Bone Joint J. 2016;98-B(3):334-340.
4. Heller S, Rezapoor M, Parvizi J. Minimising the risk of infection: a peri-operative checklist. Bone Joint J. 2016;98-B(1)(suppl A):18-22.
5. Thornqvist C, Gislason GH, Køber L, Jensen PF, Torp-Pedersen C, Andersson C. Body mass index and risk of perioperative cardiovascular adverse events and mortality in 34,744 Danish patients undergoing hip or knee replacement. Acta Orthop. 2014;85(5):456-462.
6. Stryker LS, Abdel MP, Morrey ME, Morrow MM, Kor DJ, Morrey BF. Elevated postoperative blood glucose and preoperative hemoglobin A1C are associated with increased wound complications following total joint arthroplasty. J Bone Joint Surg Am. 2013;95(9):808-814.
7. Akhavan S, Nguyen LC, Chan V, Saleh J, Bozic KJ. Impact of smoking cessation counseling prior to total joint arthroplasty. Orthopedics. 2017;40(2):e323-e328.
8. Kim DH, Spencer M, Davidson SM, et al. Institutional prescreening for detection and eradication of methicillin-resistant Staphylococcus aureus in patients undergoing elective orthopaedic surgery. J Bone Joint Surg Am. 2010;92(9):1820-1826.
9. Goyal N, Chen DB, Harris IA, Rowden NJ, Kirsh G, MacDessi SJ. Intravenous vs intra-articular tranexamic acid in total knee arthroplasty: a randomized, double-blind trial. J Arthroplasty. 2017;32(1):28-32.
10. Phan DL, Ani F, Schwarzkopf R. Cost analysis of tranexamic acid in anemic total joint arthroplasty patients. J Arthroplasty. 2016;31(3):579-582.
11. Colwell CW Jr, Froimson MI, Mont MA, et al. Thrombosis prevention after total hip arthroplasty a prospective, randomized trial comparing a mobile compression device with low-molecular-weight heparin. J Bone Joint Surg Am. 2010;92(3):527-535.
12. Chundamala J, Wright JG. The efficacy and risks of using povidone-iodine irrigation to prevent surgical site infection: an evidence-based review. Can J Surg. 2007;50(6):473-481.
13. Fang R, Liu Z, Alijiang A, et al. Efficacy of intra-articular local anesthetics in total knee arthroplasty. Orthopedics. 2015;38(7):e573-e581.
14. Odeh K, Doran J, Yu S, Bolz N, Bosco J, Iorio R. Risk-stratified venous thromboembolism prophylaxis after total joint arthroplasty: aspirin and sequential pneumatic compression devices vs aggressive chemoprophylaxis. J Arthroplasty. 2016;31(suppl 9):78-82.
15. Parvizi J, Huang R, Restrepo C, et al. Low-dose aspirin is effective chemoprophylaxis against clinically important venous thromboembolism following total joint arthroplasty: a preliminary analysis. J Bone Joint Surg Am. 2017;99(2):91-98.
16. Robertson NB, Warganich T, Ghazarossian J, Khatod M. Implementation of an accelerated rehabilitation protocol for total joint arthroplasty in the managed care setting: the experience of one institution. Adv Orthop Surg. 2015;(2015):387197.
17. Hooper GJ, Rothwell AG, Hooper NM, Frampton C. The relationship between the American Society of Anesthesiologists physical rating and outcome following total hip and knee arthroplasty: an analysis of the New Zealand Joint Registry. J Bone Joint Surg Am. 2012;94(12):1065-1070.
18. Parry MC, Smith AJ, Blom AW. Early death following primary total knee arthroplasty. J Bone Joint Surg Am. 2011;93(10):948-953.
19. Parvizi J, Mui A, Purtill JJ, Sharkey PF, Hozack WJ, Rothman RH. Total joint arthroplasty: when do fatal or near-fatal complications occur? J Bone Joint Surg Am. 2007;89(1):27-32.
1. Dundon JM, Bosco J, Slover J, Yu S, Sayeed Y, Iorio R. Improvement in total joint replacement quality metrics, year one versus year three of the bundled payments for care improvement initiative. J Bone Joint Surg Am. 2016;98(23):1949-1953.
2. Itani KM. Fifteen years of the National Surgical Quality Improvement Program in review. Am J Surg. 2009;198(suppl 5):S9-S18.
3. Tayton ER, Frampton C, Hooper GJ, Young SW. The impact of patient and surgical factors on the rate of infection after primary total knee arthroplasty: an analysis of 64,566 joints from the New Zealand Joint Registry. Bone Joint J. 2016;98-B(3):334-340.
4. Heller S, Rezapoor M, Parvizi J. Minimising the risk of infection: a peri-operative checklist. Bone Joint J. 2016;98-B(1)(suppl A):18-22.
5. Thornqvist C, Gislason GH, Køber L, Jensen PF, Torp-Pedersen C, Andersson C. Body mass index and risk of perioperative cardiovascular adverse events and mortality in 34,744 Danish patients undergoing hip or knee replacement. Acta Orthop. 2014;85(5):456-462.
6. Stryker LS, Abdel MP, Morrey ME, Morrow MM, Kor DJ, Morrey BF. Elevated postoperative blood glucose and preoperative hemoglobin A1C are associated with increased wound complications following total joint arthroplasty. J Bone Joint Surg Am. 2013;95(9):808-814.
7. Akhavan S, Nguyen LC, Chan V, Saleh J, Bozic KJ. Impact of smoking cessation counseling prior to total joint arthroplasty. Orthopedics. 2017;40(2):e323-e328.
8. Kim DH, Spencer M, Davidson SM, et al. Institutional prescreening for detection and eradication of methicillin-resistant Staphylococcus aureus in patients undergoing elective orthopaedic surgery. J Bone Joint Surg Am. 2010;92(9):1820-1826.
9. Goyal N, Chen DB, Harris IA, Rowden NJ, Kirsh G, MacDessi SJ. Intravenous vs intra-articular tranexamic acid in total knee arthroplasty: a randomized, double-blind trial. J Arthroplasty. 2017;32(1):28-32.
10. Phan DL, Ani F, Schwarzkopf R. Cost analysis of tranexamic acid in anemic total joint arthroplasty patients. J Arthroplasty. 2016;31(3):579-582.
11. Colwell CW Jr, Froimson MI, Mont MA, et al. Thrombosis prevention after total hip arthroplasty a prospective, randomized trial comparing a mobile compression device with low-molecular-weight heparin. J Bone Joint Surg Am. 2010;92(3):527-535.
12. Chundamala J, Wright JG. The efficacy and risks of using povidone-iodine irrigation to prevent surgical site infection: an evidence-based review. Can J Surg. 2007;50(6):473-481.
13. Fang R, Liu Z, Alijiang A, et al. Efficacy of intra-articular local anesthetics in total knee arthroplasty. Orthopedics. 2015;38(7):e573-e581.
14. Odeh K, Doran J, Yu S, Bolz N, Bosco J, Iorio R. Risk-stratified venous thromboembolism prophylaxis after total joint arthroplasty: aspirin and sequential pneumatic compression devices vs aggressive chemoprophylaxis. J Arthroplasty. 2016;31(suppl 9):78-82.
15. Parvizi J, Huang R, Restrepo C, et al. Low-dose aspirin is effective chemoprophylaxis against clinically important venous thromboembolism following total joint arthroplasty: a preliminary analysis. J Bone Joint Surg Am. 2017;99(2):91-98.
16. Robertson NB, Warganich T, Ghazarossian J, Khatod M. Implementation of an accelerated rehabilitation protocol for total joint arthroplasty in the managed care setting: the experience of one institution. Adv Orthop Surg. 2015;(2015):387197.
17. Hooper GJ, Rothwell AG, Hooper NM, Frampton C. The relationship between the American Society of Anesthesiologists physical rating and outcome following total hip and knee arthroplasty: an analysis of the New Zealand Joint Registry. J Bone Joint Surg Am. 2012;94(12):1065-1070.
18. Parry MC, Smith AJ, Blom AW. Early death following primary total knee arthroplasty. J Bone Joint Surg Am. 2011;93(10):948-953.
19. Parvizi J, Mui A, Purtill JJ, Sharkey PF, Hozack WJ, Rothman RH. Total joint arthroplasty: when do fatal or near-fatal complications occur? J Bone Joint Surg Am. 2007;89(1):27-32.
The Future of Choice & VA Health Care
In late August, the President signed legislation that provided $2.1 billion to extend a program that gives veterans enrolled in the VHA a “Choice” in where they receive care. In the next few months, Congress will consider various plans to redesign the Veterans Choice Program. As policy makers consider these options, they should assess not only the plan’s ability to remedy any problems in veterans’ access to care, but also its broader impact. Congress must ensure that the next Choice Program does not compromise VHA’s overall quality of health care services delivered to veterans—care that has been demonstrated, with geographic variations, to be equal to, and often superior to, non-VA care.
Launched in 2014 as part of the Veterans Access, Choice and Accountability Act, the temporary Choice Program was meant to remedy a crisis of limited capacity, access, and excessive delays reported at many VHA facilities. The program offered non-VA options to veterans who had to wait long or travel far for their care. To date, the program has provided health care services to more than 1.6 million veterans.
As Senate and House VA committees began to draft new authorizing language for the program, many have spoken out about these issues and highlighted the unique importance of the VHA’s comprehensive, integrated model of care—one that is focused on the specific problems of veterans. NOVA, alongside its partners—Association of VA Psychologist Leaders, Association of VA Social Workers, and the organization Fighting for Veterans Healthcare—has provided their thoughts on the best solution to continue providing veterans timely access to this type of high-quality health care.
Congress must ensure far more than simply preserving the VHA’s innovative, integrated-care model. It must guarantee that the VHA’s system for clinically training the majority of U.S. health care professionals is maintained. The program funding must include a robust research department whose mission not only benefits veterans, but also the health care provided to every American. It must ensure that the community has the capacity to absorb an influx of veterans in a timely manner.
Community providers must be required to meet VHA’s elevated standards, use evidence-based treatments driven by measurement-based care, have knowledge of military culture and competence in veteran-specific problems, perform needed screenings, and be subject to the same training and continuing education requirements as VHA providers.
Given that non-VA care is more expensive than VHA care, Congress must ensure that any Choice care that veterans are offered is done so judiciously. Otherwise, the cost of Choice could wind up eroding VHA’s level of services. Finally, Congress also must ensure that the VHA is improved, not dismantled. As surveys and studies have shown, this is what the majority of veterans prefer and what they have been promised by administration and congressional leaders.
As VA nurses providing and coordinating care for veterans, we have a stake in how Choice and all community care is provided. As an organization, NOVA understands that community providers are a crucial part of an integrated network set up to provide care where there are shortages, but VHA must remain the first point of access and coordinator of that care.
Any new legislation addressing community-integrated care must include measures that hold providers accountable for performance and timeliness of care and services. It also must take into account the VHA’s unparalleled integration of primary and mental health care and the many wraparound services that are offered veterans.
Finally, the congressional budgeting process must include adequate funding for both VHA services and its integrated-community care accounts. The practice of reallocating funds from VHA health care accounts to pay for non-VA care cannot continue.
Making significant, lasting improvements in how VHA provides health care within its facilities and with partners in the community is unquestionably the right thing to do. It honors the sacred obligation we owe to veterans. Congress must be willing to invest in the VHA and provide veterans with the type of high-quality, veteran-centered care that serves their complex needs.
In late August, the President signed legislation that provided $2.1 billion to extend a program that gives veterans enrolled in the VHA a “Choice” in where they receive care. In the next few months, Congress will consider various plans to redesign the Veterans Choice Program. As policy makers consider these options, they should assess not only the plan’s ability to remedy any problems in veterans’ access to care, but also its broader impact. Congress must ensure that the next Choice Program does not compromise VHA’s overall quality of health care services delivered to veterans—care that has been demonstrated, with geographic variations, to be equal to, and often superior to, non-VA care.
Launched in 2014 as part of the Veterans Access, Choice and Accountability Act, the temporary Choice Program was meant to remedy a crisis of limited capacity, access, and excessive delays reported at many VHA facilities. The program offered non-VA options to veterans who had to wait long or travel far for their care. To date, the program has provided health care services to more than 1.6 million veterans.
As Senate and House VA committees began to draft new authorizing language for the program, many have spoken out about these issues and highlighted the unique importance of the VHA’s comprehensive, integrated model of care—one that is focused on the specific problems of veterans. NOVA, alongside its partners—Association of VA Psychologist Leaders, Association of VA Social Workers, and the organization Fighting for Veterans Healthcare—has provided their thoughts on the best solution to continue providing veterans timely access to this type of high-quality health care.
Congress must ensure far more than simply preserving the VHA’s innovative, integrated-care model. It must guarantee that the VHA’s system for clinically training the majority of U.S. health care professionals is maintained. The program funding must include a robust research department whose mission not only benefits veterans, but also the health care provided to every American. It must ensure that the community has the capacity to absorb an influx of veterans in a timely manner.
Community providers must be required to meet VHA’s elevated standards, use evidence-based treatments driven by measurement-based care, have knowledge of military culture and competence in veteran-specific problems, perform needed screenings, and be subject to the same training and continuing education requirements as VHA providers.
Given that non-VA care is more expensive than VHA care, Congress must ensure that any Choice care that veterans are offered is done so judiciously. Otherwise, the cost of Choice could wind up eroding VHA’s level of services. Finally, Congress also must ensure that the VHA is improved, not dismantled. As surveys and studies have shown, this is what the majority of veterans prefer and what they have been promised by administration and congressional leaders.
As VA nurses providing and coordinating care for veterans, we have a stake in how Choice and all community care is provided. As an organization, NOVA understands that community providers are a crucial part of an integrated network set up to provide care where there are shortages, but VHA must remain the first point of access and coordinator of that care.
Any new legislation addressing community-integrated care must include measures that hold providers accountable for performance and timeliness of care and services. It also must take into account the VHA’s unparalleled integration of primary and mental health care and the many wraparound services that are offered veterans.
Finally, the congressional budgeting process must include adequate funding for both VHA services and its integrated-community care accounts. The practice of reallocating funds from VHA health care accounts to pay for non-VA care cannot continue.
Making significant, lasting improvements in how VHA provides health care within its facilities and with partners in the community is unquestionably the right thing to do. It honors the sacred obligation we owe to veterans. Congress must be willing to invest in the VHA and provide veterans with the type of high-quality, veteran-centered care that serves their complex needs.
In late August, the President signed legislation that provided $2.1 billion to extend a program that gives veterans enrolled in the VHA a “Choice” in where they receive care. In the next few months, Congress will consider various plans to redesign the Veterans Choice Program. As policy makers consider these options, they should assess not only the plan’s ability to remedy any problems in veterans’ access to care, but also its broader impact. Congress must ensure that the next Choice Program does not compromise VHA’s overall quality of health care services delivered to veterans—care that has been demonstrated, with geographic variations, to be equal to, and often superior to, non-VA care.
Launched in 2014 as part of the Veterans Access, Choice and Accountability Act, the temporary Choice Program was meant to remedy a crisis of limited capacity, access, and excessive delays reported at many VHA facilities. The program offered non-VA options to veterans who had to wait long or travel far for their care. To date, the program has provided health care services to more than 1.6 million veterans.
As Senate and House VA committees began to draft new authorizing language for the program, many have spoken out about these issues and highlighted the unique importance of the VHA’s comprehensive, integrated model of care—one that is focused on the specific problems of veterans. NOVA, alongside its partners—Association of VA Psychologist Leaders, Association of VA Social Workers, and the organization Fighting for Veterans Healthcare—has provided their thoughts on the best solution to continue providing veterans timely access to this type of high-quality health care.
Congress must ensure far more than simply preserving the VHA’s innovative, integrated-care model. It must guarantee that the VHA’s system for clinically training the majority of U.S. health care professionals is maintained. The program funding must include a robust research department whose mission not only benefits veterans, but also the health care provided to every American. It must ensure that the community has the capacity to absorb an influx of veterans in a timely manner.
Community providers must be required to meet VHA’s elevated standards, use evidence-based treatments driven by measurement-based care, have knowledge of military culture and competence in veteran-specific problems, perform needed screenings, and be subject to the same training and continuing education requirements as VHA providers.
Given that non-VA care is more expensive than VHA care, Congress must ensure that any Choice care that veterans are offered is done so judiciously. Otherwise, the cost of Choice could wind up eroding VHA’s level of services. Finally, Congress also must ensure that the VHA is improved, not dismantled. As surveys and studies have shown, this is what the majority of veterans prefer and what they have been promised by administration and congressional leaders.
As VA nurses providing and coordinating care for veterans, we have a stake in how Choice and all community care is provided. As an organization, NOVA understands that community providers are a crucial part of an integrated network set up to provide care where there are shortages, but VHA must remain the first point of access and coordinator of that care.
Any new legislation addressing community-integrated care must include measures that hold providers accountable for performance and timeliness of care and services. It also must take into account the VHA’s unparalleled integration of primary and mental health care and the many wraparound services that are offered veterans.
Finally, the congressional budgeting process must include adequate funding for both VHA services and its integrated-community care accounts. The practice of reallocating funds from VHA health care accounts to pay for non-VA care cannot continue.
Making significant, lasting improvements in how VHA provides health care within its facilities and with partners in the community is unquestionably the right thing to do. It honors the sacred obligation we owe to veterans. Congress must be willing to invest in the VHA and provide veterans with the type of high-quality, veteran-centered care that serves their complex needs.
Current Approaches to Measuring Functional Status Among Older Adults in VA Primary Care Clinics
The ability to perform activities of daily living (ADLs), commonly called functional status, is central to older adults’ quality of life (QOL) and independence.1,2 Understanding functional status is key to improving outcomes for older adults. In community-dwelling older adults with difficulty performing basic ADLs, practical interventions, including physical and occupational therapy, can improve functioning and prevent functional decline.3,4 Understanding function also is important for delivering patient-centered care, including individualizing cancer screening,5 evaluating how patients will tolerate interventions,6-9 and helping patients and families determine the need for long-term services and supports.
For these reasons, assessing functional status is a cornerstone of geriatrics practice. However, most older adults are cared for in primary care settings where routine measurement of functional status is uncommon.10,11 Although policy leaders have long noted this gap and the obstacle it poses to improving the quality and outcomes of care for older adults, many health care systems have been slow to incorporate measurement of functional status into routine patient care.12-14
Over the past several years, the VA has been a leader in the efforts to address this barrier by implementing routine, standardized measurement of functional status in primary care clinics. Initially, the VA encouraged, but did not require, measurement of functional status among older adults, but the implementation barriers and facilitators were not formally assessed.15 In a postimplementation evaluation, the authors found that a relatively small number of medical centers implemented functional measures. Moreover, the level of implementation seemed to vary across sites. Some sites were collecting complete measures on all eligible older patients, while other sites were collecting measures less consistently.15
As part of a national VA initiative to learn how best to implement standardized functional status measurement, the authors are conducting a qualitative study, including a formal assessment of barriers and facilitators to implementing functional assessments in VA primary care clinics. In the current project, which serves as formative work for this larger ongoing study, the authors identified and described current processes for measuring functional status in VA primary care patient aligned care team (PACT) and Geriatric (GeriPACT) clinics.
Methods
A rapid qualitative analysis approach was used, which included semistructured interviews with primary care stakeholders and rapid data analysis to summarize each clinic’s approach to measuring functional status and develop process maps for each clinic (eFigures 1, 2, 3, and 4 ). Interviews and analyses were conducted by a team consisting of a geriatrician clinician-researcher, a medical anthropologist, and a research coordinator. The institutional review boards of the San Francisco VAMC and the University of California, San Francisco approved the study.
Sampling Strategy
In order to identify VAMCs with varying approaches to assessing functional status in older patients who attended primary care appointments, the study used a criterion sampling approach.16,17 First, national “health factors” data were extracted from the VA Corporate Data Warehouse (CDW). Health factors are patient data collected through screening tools called clinical reminders, which prompt clinic staff and providers to enter data into checkbox-formatted templates. The study then identified medical centers that collected health factors data from patients aged ≥ 65 years (157 of 165 medical centers). A keyword search identified health factors related to the Katz ADL (bathing, dressing, transferring, toileting, and eating), and Lawton Instrumental ADL (IADL) Scale (using the telephone, shopping, preparing food, housekeeping, doing laundry, using transportation, managing medications, and managing finances).18,19 Health factors that were not collected during a primary care appointment were excluded.
Of the original 157 medical centers, 139 met these initial inclusion criteria. Among these 139 medical centers, 66 centers did not collect complete data on these 5 ADLs and 8 IADLs (eg, only ADLs or only IADLs, or only certain ADLs or IADLs).
Two medical centers were selected in each of the following 3 categories: (1) routinely used clinical reminders to collect standardized data on the Katz ADL and the Lawton IADL Scale; (2) routinely used clinical reminders to collect functional status data but collected partial information; and (3) did not use a clinical reminder to collect functional status data. To ensure that these 6 medical centers were geographically representative, the sample included at least 1 site from each of the 5 VA regions: 1 North Atlantic, 1 Southeast, 1 Midwest, 2 Continental (1 from the northern Continental region and 1 from the southern), and 1 Pacific. Three sites that included GeriPACTs also were sampled.
Primary care PACT and GeriPACT members from these 6 medical centers were recruited to participate. These PACT members included individuals who can assess function or use functional status information to inform patient care, including front-line nursing staff (licensed practical nurses [LPNs], and registered nurses [RNs]), primary care providers (medical doctors [MDs] and nurse practitioners [NPs]), and social workers (SWs).
Local bargaining units, nurse managers, and clinic directors provided lists of all clinic staff. All members of each group then received recruitment e-mails. Phone interviews were scheduled with interested participants. In several cases, a snowball sampling approach was used to increase enrollment numbers by asking interview participants to recommend colleagues who might be interested in participating.17
Data Collection
Telephone interviews were conducted between March 2016 and October 2016 using semistructured guides developed from the project aims and from related literature in implementation science.20,21 Interview domains included clinic structure, team member roles and responsibilities, current practices for collecting functional status data, and opinions on barriers and facilitators to assessing and recording functional status (Appendix:
Data Analysis
Rapid analysis, a team-based qualitative approach was used to engage efficiently and systematically with the data.22,23 This approach allowed results to be analyzed more quickly than in traditional qualitative analysis in order to inform intervention design and develop implementation strategies.23 Rapid analysis typically includes organization of interview data into summary templates, followed by a matrix analysis, which was used to create process maps.24
Summary Templates
Summary templates were developed from the interview guides by shortening each question into a representative code. The project team then read the transcripts and summarized key points in the appropriate section of the template. This process, known as data reduction, is used to organize and highlight material so conclusions can be drawn from the data easily.22 In order to maintain rigor and trustworthiness, one team member conducted the interview, and a different team member created the interview summary. All team members reviewed each summary and met regularly to discuss results.
The summary templates were converted into matrix analyses, a method of displaying data to identify relationships, including commonalities and differences.24 The matrixes were organized by stakeholder group and clinic in order to compare functional status assessment and documentation workflows across clinics.
Process Maps
Finally, the team used the matrix data to create process maps for each clinic of when, where, and by whom functional status information was assessed and documented. These maps were created using Microsoft Visio (Redmond, WA). The maps integrated perspectives from all participants to give an overview of the process for collecting functional status data in each clinic setting. To ensure accuracy, participants at each site received process maps to solicit feedback and validation.
Results
Forty-six participants at 6 medical centers (20 MDs and NPs, 19 RNs and LPNs, and 7 SWs) from 9 primary care clinics provided samples and interviews. The study team identified 3 general approaches to functional status assessment: (1) Routine collection of functional status data via a standardized clinical reminder; (2) Routine collection of functional status data via methods other than a clinical reminder (eg, a previsit telephone screen or electronic note template); and (3) Ad hoc approaches to measuring functional status (ie, no standard or routine approach to assessing or documenting functional status). The study team selected 4 clinics (2 PACTs and 2 GeriPACTs) clinics to serve as examples of the 3 identified approaches.
The processes for functional status assessment in each of 4 clinics are summarized in the following detailed descriptions (Table).
Clinic 1
Clinic 1 is a GeriPACT clinic that routinely assesses and documents functional status for all patients (efigure 1, available at feprac.com). The clinic’s current process includes 4 elements: (1) a patient questionnaire; (2) an annual clinical reminder administered by an RN; (3) a primary care provider (PCP) assessment; and (4) a postvisit SW assessment if referred by the PCP.
All newly referred patients are mailed a paper questionnaire that includes questions about their medical history and functional status. The patient is asked to bring the completed questionnaire to the first appointment. The clinic RN completes this form for returning patients at every visit during patient intake.
Second, the clinic uses an annual functional status clinical reminder for patients aged ≥ 75 years. The reminder includes questions about a patient’s ability to perform ADLs and IADLs with 3 to 4 response options for each question. If the clinical reminder is due at the time of a patient appointment, the RN fills out the reminder using information from the paper questionnaire. The RN also records this functional status in the nursing intake note. The RN may elect to designate the PCP as a cosigner for the nursing intake note especially if there are concerns about or changes in the patient’s functional status.
Third, the RN brings the paper form to the PCP, who often uses the questionnaire to guide the patient history. The PCP then uses the questionnaire and patient history to complete a functional status template within their visit note. The PCP also may use this information to inform patient care (eg, to make referrals to physical or occupational therapy).
Finally, the PCP might refer the patient to SW. The SW may be able to see the patient immediately after the PCP appointment, but if not, the SW follows up with a phone call to complete further functional status assessment and eligibility forms.
In addition to the above assessments by individual team members, the PACT has an interdisciplinary team huddle at the end of each clinic to discuss any issues or concerns about specific patients. The huddles often focus on issues related to functional status.
Clinic 2
Clinic 2 is a primary care PACT clinic that routinely assesses and documents functional status (eFigure 2, available at fedprac.com). The clinic process includes 3 steps: an annual clinical reminder for patients aged ≥ 75 years; a PCP assessment; and a postvisit SW assessment if referred by the PCP.
First, patients see an LPN for the intake process. During intake, the LPN records vitals and completes relevant clinical reminders. Similar to Clinic 1, Clinic 2 requires an annual functional status clinical reminder that includes ADLs and IADLs for patients aged ≥ 75 years. Patient information from the intake and clinical reminders are recorded by the LPN in a preventative medicine note in the electronic health record. This note is printed and handed to the PCP.
The PCP may review the preventative medicine note prior to completing the patient history and physical, including the functional status clinical reminder when applicable. If the PCP follows up on any functional issues identified by the LPN or completes further assessment of patient function, he or she may use this information to refer the patient to services or to place a SW consult; the PCP’s functional assessment is documented in a free-form visit note.
When the SW receives a consult, a chart review for social history, demographic information, and previous functional status assessments is conducted. The SW then calls the patient to administer functional and cognitive assessments over the phone and refers the patient to appropriate services based on eligibility.
Clinic 3
Clinic 3 is a GeriPACT clinic where functional status information is routinely collected for all new patients but may or may not be collected for returning patients (eFigure 3, available at fedprac.com). The process for new patients includes a previsit SW assessment; an informal LPN screening (ie, not based on a standardized clinical reminder); a PCP assessment; and a postvisit SW assessment if referred by the provider. The process for returning patients is similar but omits the previsit social work assessment. New patients complete a comprehensive questionnaire with a SW before their first clinic visit. The questionnaire is completed by phone and involves an extensive social and medical history, including an assessment of ADLs and IADLs. This assessment is recorded in a free-form social work note.
Next, both new and returning patients see an LPN who completes the intake process, including vitals and clinical reminders. Clinic 3 does not have a clinical reminder for functional status. However, the LPN could elect to ask about ADLs or IADLs if the patient brings up a functional issue related to the chief symptom or if the LPN observes something that indicates possible functional impairment, such as difficulty walking or a disheveled appearance. If discussed, this information is recorded in the LPN intake note, and the LPN also could verbally inform the PCP of the patient’s functional status. The RN is not formally involved in intake or functional status assessment in this clinic.
Finally, the patient sees the PCP, who may or may not have reviewed the LPN note. The PCP may assess functional status at his or her discretion, but there was no required assessment. The PCP could complete an optional functional status assessment template included in the PCP visit note. The PCP can refer the patient to services or to SW for further evaluation.
Clinic 4
Clinic 4 is a primary care PACT clinic that does not routinely measure functional status (eFigure 4, available at fedprac.com). The approach includes an informal LPN screening (ie, not based on a standardized clinical reminder); a PCP assessment; and a postvisit social worker assessment if referred by the provider. These steps are very similar to those of clinic 3, but they do not include a previsit SW assessment for new patients.
Although not represented within the 4 clinics described in this article, the content of functional status clinical reminders differed across the 9 clinics in the larger sample. Clinical reminders differed across several domains, including the type of question stems (scripted questions for each ADL vs categories for each activity); response options (eg, dichotomous vs ≥ 3 options), and the presence of free-text boxes to allow staff to enter any additional notes.
Discussion
Approaches to assessing and documenting functional status varied widely. Whereas some clinics primarily used informal approaches to assessing and documenting functional status (ie, neither routine nor standardized), others used a routine, standardized clinical reminder, and some combined several standardized approaches to measuring function. The study team identified variability across several domains of the functional status assessment process, including documentation, workflow, and clinical reminder content.
Approaches to functional assessment differed between GeriPACT and PACT clinics. Consistent with the central role that functional status assessment plays in geriatrics practice, GeriPACTs tended to employ a routine, multidisciplinary approach to measuring functional status. This approach included standardized functional assessments by multiple primary care team members, including LPNs, SWs, and PCPs. In contrast, when PACTs completed standardized functional status assessment, it was generally carried out by a single team member (typically an LPN). The PCPs in PACTs used a nonroutine approach to assess functional status in which they performed detailed functional assessments for certain high-risk patients and referred a subset for further SW evaluation.
These processes are consistent with research showing that standardized functional status data are seldom collected routinely in nongeriatric primary care settings.11 Reports by PCPs that they did not always assess functional status also are consistent with previous research demonstrating that clinicians are not always aware of their patients’ functional ability.10
In addition to highlighting differences between GeriPACT and PACTs, the identified processes illustrate the variability in documentation, clinic workflow, and clinical reminder content across all clinics. Approaches to documentation included checkbox-formatted clinical reminders with and without associated nursing notes, patient questionnaires, and templated PCP and SW notes. Clinics employed varying approaches to collect functional status information and to ensure that those data were shared with the team. Clinic staff assessed functional status at different times during the clinical encounter. Clinics used several approaches to share this information with team members, including warm handoffs from LPNs to PCPs, interdisciplinary team huddles, and electronic signoffs. Finally, clinical reminder content varied between clinics, with differences in the wording of ADL and IADL questions as well as in the number and type of response options.
This variability highlights the challenges inherent in developing a routine, standardized approach to measuring functional status that can be adapted across primary care settings. Such an approach must be both flexible enough to accommodate variation in workflow and structured enough to capture accurate data that can be used to guide clinical decisions. Capturing accurate, standardized data in CDW also will inform efforts to improve population health by allowing VHA leaders to understand the scope of disability among older veterans and plan for service needs and interventions.
Whereas the larger qualitative study will identify the specific barriers and facilitators to developing and implementing such an approach, current clinic processes present here offer hints as to which features may be important. For example, several clinics collected functional status information before the visit by telephone or questionnaire. Therefore, it will be important to choose a functional status assessment instrument that is validated for both telephone and in-person use. Similarly, some clinics had structured clinical reminders with categoric response options, whereas others included free-text boxes. Incorporating both categoric responses (to ensure accurate data) as well as free-text (to allow for additional notes about a patient’s specific circumstances that may influence service needs) may be one approach.
Limitations
This study’s approach to identifying clinic processes had several limitations. First, the authors did not send process maps to clinic directors for verification. However, speaking with PACT members who carry out clinic processes is likely the most accurate way to identify practice. Second, the results may not be generalizable to all VA primary care settings. Due to resource limitations and project scope, community-based outpatient clinics (CBOCs) were not included. Compared with clinics based in medical centers, CBOCs may have different staffing levels, practice models, and needs regarding implementation of functional status assessment.
Although 46 participants from 9 clinics were interviewed, there are likely additional approaches to measuring functional status that are not represented within this sample. In addition, 3 of the 4 clinics included are affiliated with academic institutions, and all 4 are located in large cities. Efforts to include rural VAMCs were not successful. Finally, clinic-level characteristics were not reported, which may impact clinic processes. Although study participants were asked about clinic characteristics, they were often unsure or only able to provide rough estimates. In the ongoing qualitative study, the authors will attempt to collect more reliable data about these clinic-level characteristics and to examine the potential role these characteristics may play as barriers or facilitators to implementing routine assessment of functional status in primary care settings.
Conclusion
VA primary care clinics had widely varying approaches for assessing and documenting functional status. This work along with a larger ongoing qualitative study that includes interviews with veterans will directly inform the design and implementation of a standardized, patient-centered approach to functional assessment that can be adapted across varied primary care settings. Implementing standardized functional status measurement will allow the VA to serve veterans better by using functional status information to refer patients to appropriate services and to deliver patient-centered care with the potential to improve patient function and quality of life.
1.Covinsky KE, Wu AW, Landefeld CS, et al. Health status versus quality of life in older patients: does the distinction matter? Am J Med. 1999;106(4):435-440.
2. Fried TR, McGraw S, Agostini JV, Tinetti ME. Views of older persons with multiple morbidities on competing outcomes and clinical decision-making. J Am Geriatr Soc. 2008;56(10):1839-1844.
3. Beswick AD, Rees K, Dieppe P, et al. Complex interventions to improve physical function and maintain independent living in elderly people: a systematic review and meta-analysis. Lancet. 2008;371(9614):725-735.
4. Szanton SL, Leff B, Wolff JL, Roberts L, Gitlin LN. Home-based care program reduces disability and promotes aging in place. Health Aff (Millwood). 2016;35(9):1558-1563.
5. Walter LC, Covinsky KE. Cancer screening in elderly patients: a framework for individualized decision making. JAMA. 2001;285(21):2750-2756.
6. Kurella Tamura M, Covinsky KE, Chertow GM, Yaffe K, Landefeld CS, McCulloch CE. Functional status of elderly adults before and after initiation of dialysis. N Engl J Med. 2009;361(16):1539-1547.
7. Hurria A, Togawa K, Mohile SG, et al. Predicting chemotherapy toxicity in older adults with cancer: a prospective multicenter study. J Clin Oncol. 2011;29(25):3457-3465.
8. Crawford RS, Cambria RP, Abularrage CJ, et al. Preoperative functional status predicts perioperative outcomes after infrainguinal bypass surgery. J Vasc Surg. 2010;51(2):351-358; discussion 358-359.
9. Arnold SV, Reynolds MR, Lei Y, et al; PARTNER Investigators. Predictors of poor outcomes after transcatheter aortic valve replacement: results from the PARTNER (Placement of Aortic Transcatheter Valve) trial. Circulation. 2014;129(25):2682-2690.
10. Calkins DR, Rubenstein LV, Cleary PD, et al. Failure of physicians to recognize functional disability in ambulatory patients. Ann Intern Med. 1991;114(6):451-454.
11. Bogardus ST Jr, Towle V, Williams CS, Desai MM, Inouye SK. What does the medical record reveal about functional status? A comparison of medical record and interview data. J Gen Intern Med. 2001;16(11):728-736.
12. Bierman AS. Functional status: the six vital sign. J Gen Intern Med. 2001;16(11):785-786.
13. Iezzoni LI, Greenberg MS. Capturing and classifying functional status information in administrative databases. Health Care Financ Rev. 2003;24(3):61-76.
14. Clauser SB, Bierman AS. Significance of functional status data for payment and quality. Health Care Financ Rev. 2003;24(3):1-12.
15. Brown RT, Komaiko KD, Shi Y, et al. Bringing functional status into a big data world: validation of national Veterans Affairs functional status data. PloS One. 2017;12(6):e0178726.
16. Palinkas LA, Horwitz SM, Green CA, Wisdom JP, Duan N, Hoagwood K. Purposeful sampling for qualitative data collection and analysis in mixed method implementation research. Adm Policy Ment Health. 2015;42(5):533-544.
17. Patton MQ. Qualitative Research Evaluation and Methods. 4th ed. Thousand Oaks, CA: Sage; 2015.
18. Katz S, Ford AB, Moskowitz RW, Jackson BA, Jaffe MW. Studies of illness in the aged. The index of ADL: a standardized measure of biological and psychosocial function. JAMA. 1963;185(12):914-919.
19. Lawton MP, Brody EM. Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontologist. 1969;9(3):179-186.
20. Damschroder LJ, Aron DC, Keith RE, Kirsh SR, Alexander JA, Lowery JC. Fostering implementation of health services research findings into practice: a consolidated framework for advancing implementation science. Implement Sci. 2009;4(1):50.
21. Saleem JJ, Patterson ES, Militello L, Render ML, Orshansky G, Asch SM. Exploring barriers and facilitators to the use of computerized clinical reminders. J Am Med Inform Assoc. 2005;12(4):438-447.
22. Miles MB, Huberman AM, Saldana J. Qualitative Data Analysis: A Methods Sourcebook. 3rd ed. Thousand Oaks, CA: Sage; 2014.
23. Hamilton AB. Qualitative methods in rapid turn-around health services research. https://www.hsrd .research.va.gov/for_researchers/cyber_seminars /archives/video_archive.cfm?SessionID=780. Published December 11, 2013. Accessed August 9, 2017.
24. Averill JB. Matrix analysis as a complementary analytic strategy in qualitative inquiry. Qual Health Res. 2002;12(6):855-866.
The ability to perform activities of daily living (ADLs), commonly called functional status, is central to older adults’ quality of life (QOL) and independence.1,2 Understanding functional status is key to improving outcomes for older adults. In community-dwelling older adults with difficulty performing basic ADLs, practical interventions, including physical and occupational therapy, can improve functioning and prevent functional decline.3,4 Understanding function also is important for delivering patient-centered care, including individualizing cancer screening,5 evaluating how patients will tolerate interventions,6-9 and helping patients and families determine the need for long-term services and supports.
For these reasons, assessing functional status is a cornerstone of geriatrics practice. However, most older adults are cared for in primary care settings where routine measurement of functional status is uncommon.10,11 Although policy leaders have long noted this gap and the obstacle it poses to improving the quality and outcomes of care for older adults, many health care systems have been slow to incorporate measurement of functional status into routine patient care.12-14
Over the past several years, the VA has been a leader in the efforts to address this barrier by implementing routine, standardized measurement of functional status in primary care clinics. Initially, the VA encouraged, but did not require, measurement of functional status among older adults, but the implementation barriers and facilitators were not formally assessed.15 In a postimplementation evaluation, the authors found that a relatively small number of medical centers implemented functional measures. Moreover, the level of implementation seemed to vary across sites. Some sites were collecting complete measures on all eligible older patients, while other sites were collecting measures less consistently.15
As part of a national VA initiative to learn how best to implement standardized functional status measurement, the authors are conducting a qualitative study, including a formal assessment of barriers and facilitators to implementing functional assessments in VA primary care clinics. In the current project, which serves as formative work for this larger ongoing study, the authors identified and described current processes for measuring functional status in VA primary care patient aligned care team (PACT) and Geriatric (GeriPACT) clinics.
Methods
A rapid qualitative analysis approach was used, which included semistructured interviews with primary care stakeholders and rapid data analysis to summarize each clinic’s approach to measuring functional status and develop process maps for each clinic (eFigures 1, 2, 3, and 4 ). Interviews and analyses were conducted by a team consisting of a geriatrician clinician-researcher, a medical anthropologist, and a research coordinator. The institutional review boards of the San Francisco VAMC and the University of California, San Francisco approved the study.
Sampling Strategy
In order to identify VAMCs with varying approaches to assessing functional status in older patients who attended primary care appointments, the study used a criterion sampling approach.16,17 First, national “health factors” data were extracted from the VA Corporate Data Warehouse (CDW). Health factors are patient data collected through screening tools called clinical reminders, which prompt clinic staff and providers to enter data into checkbox-formatted templates. The study then identified medical centers that collected health factors data from patients aged ≥ 65 years (157 of 165 medical centers). A keyword search identified health factors related to the Katz ADL (bathing, dressing, transferring, toileting, and eating), and Lawton Instrumental ADL (IADL) Scale (using the telephone, shopping, preparing food, housekeeping, doing laundry, using transportation, managing medications, and managing finances).18,19 Health factors that were not collected during a primary care appointment were excluded.
Of the original 157 medical centers, 139 met these initial inclusion criteria. Among these 139 medical centers, 66 centers did not collect complete data on these 5 ADLs and 8 IADLs (eg, only ADLs or only IADLs, or only certain ADLs or IADLs).
Two medical centers were selected in each of the following 3 categories: (1) routinely used clinical reminders to collect standardized data on the Katz ADL and the Lawton IADL Scale; (2) routinely used clinical reminders to collect functional status data but collected partial information; and (3) did not use a clinical reminder to collect functional status data. To ensure that these 6 medical centers were geographically representative, the sample included at least 1 site from each of the 5 VA regions: 1 North Atlantic, 1 Southeast, 1 Midwest, 2 Continental (1 from the northern Continental region and 1 from the southern), and 1 Pacific. Three sites that included GeriPACTs also were sampled.
Primary care PACT and GeriPACT members from these 6 medical centers were recruited to participate. These PACT members included individuals who can assess function or use functional status information to inform patient care, including front-line nursing staff (licensed practical nurses [LPNs], and registered nurses [RNs]), primary care providers (medical doctors [MDs] and nurse practitioners [NPs]), and social workers (SWs).
Local bargaining units, nurse managers, and clinic directors provided lists of all clinic staff. All members of each group then received recruitment e-mails. Phone interviews were scheduled with interested participants. In several cases, a snowball sampling approach was used to increase enrollment numbers by asking interview participants to recommend colleagues who might be interested in participating.17
Data Collection
Telephone interviews were conducted between March 2016 and October 2016 using semistructured guides developed from the project aims and from related literature in implementation science.20,21 Interview domains included clinic structure, team member roles and responsibilities, current practices for collecting functional status data, and opinions on barriers and facilitators to assessing and recording functional status (Appendix:
Data Analysis
Rapid analysis, a team-based qualitative approach was used to engage efficiently and systematically with the data.22,23 This approach allowed results to be analyzed more quickly than in traditional qualitative analysis in order to inform intervention design and develop implementation strategies.23 Rapid analysis typically includes organization of interview data into summary templates, followed by a matrix analysis, which was used to create process maps.24
Summary Templates
Summary templates were developed from the interview guides by shortening each question into a representative code. The project team then read the transcripts and summarized key points in the appropriate section of the template. This process, known as data reduction, is used to organize and highlight material so conclusions can be drawn from the data easily.22 In order to maintain rigor and trustworthiness, one team member conducted the interview, and a different team member created the interview summary. All team members reviewed each summary and met regularly to discuss results.
The summary templates were converted into matrix analyses, a method of displaying data to identify relationships, including commonalities and differences.24 The matrixes were organized by stakeholder group and clinic in order to compare functional status assessment and documentation workflows across clinics.
Process Maps
Finally, the team used the matrix data to create process maps for each clinic of when, where, and by whom functional status information was assessed and documented. These maps were created using Microsoft Visio (Redmond, WA). The maps integrated perspectives from all participants to give an overview of the process for collecting functional status data in each clinic setting. To ensure accuracy, participants at each site received process maps to solicit feedback and validation.
Results
Forty-six participants at 6 medical centers (20 MDs and NPs, 19 RNs and LPNs, and 7 SWs) from 9 primary care clinics provided samples and interviews. The study team identified 3 general approaches to functional status assessment: (1) Routine collection of functional status data via a standardized clinical reminder; (2) Routine collection of functional status data via methods other than a clinical reminder (eg, a previsit telephone screen or electronic note template); and (3) Ad hoc approaches to measuring functional status (ie, no standard or routine approach to assessing or documenting functional status). The study team selected 4 clinics (2 PACTs and 2 GeriPACTs) clinics to serve as examples of the 3 identified approaches.
The processes for functional status assessment in each of 4 clinics are summarized in the following detailed descriptions (Table).
Clinic 1
Clinic 1 is a GeriPACT clinic that routinely assesses and documents functional status for all patients (efigure 1, available at feprac.com). The clinic’s current process includes 4 elements: (1) a patient questionnaire; (2) an annual clinical reminder administered by an RN; (3) a primary care provider (PCP) assessment; and (4) a postvisit SW assessment if referred by the PCP.
All newly referred patients are mailed a paper questionnaire that includes questions about their medical history and functional status. The patient is asked to bring the completed questionnaire to the first appointment. The clinic RN completes this form for returning patients at every visit during patient intake.
Second, the clinic uses an annual functional status clinical reminder for patients aged ≥ 75 years. The reminder includes questions about a patient’s ability to perform ADLs and IADLs with 3 to 4 response options for each question. If the clinical reminder is due at the time of a patient appointment, the RN fills out the reminder using information from the paper questionnaire. The RN also records this functional status in the nursing intake note. The RN may elect to designate the PCP as a cosigner for the nursing intake note especially if there are concerns about or changes in the patient’s functional status.
Third, the RN brings the paper form to the PCP, who often uses the questionnaire to guide the patient history. The PCP then uses the questionnaire and patient history to complete a functional status template within their visit note. The PCP also may use this information to inform patient care (eg, to make referrals to physical or occupational therapy).
Finally, the PCP might refer the patient to SW. The SW may be able to see the patient immediately after the PCP appointment, but if not, the SW follows up with a phone call to complete further functional status assessment and eligibility forms.
In addition to the above assessments by individual team members, the PACT has an interdisciplinary team huddle at the end of each clinic to discuss any issues or concerns about specific patients. The huddles often focus on issues related to functional status.
Clinic 2
Clinic 2 is a primary care PACT clinic that routinely assesses and documents functional status (eFigure 2, available at fedprac.com). The clinic process includes 3 steps: an annual clinical reminder for patients aged ≥ 75 years; a PCP assessment; and a postvisit SW assessment if referred by the PCP.
First, patients see an LPN for the intake process. During intake, the LPN records vitals and completes relevant clinical reminders. Similar to Clinic 1, Clinic 2 requires an annual functional status clinical reminder that includes ADLs and IADLs for patients aged ≥ 75 years. Patient information from the intake and clinical reminders are recorded by the LPN in a preventative medicine note in the electronic health record. This note is printed and handed to the PCP.
The PCP may review the preventative medicine note prior to completing the patient history and physical, including the functional status clinical reminder when applicable. If the PCP follows up on any functional issues identified by the LPN or completes further assessment of patient function, he or she may use this information to refer the patient to services or to place a SW consult; the PCP’s functional assessment is documented in a free-form visit note.
When the SW receives a consult, a chart review for social history, demographic information, and previous functional status assessments is conducted. The SW then calls the patient to administer functional and cognitive assessments over the phone and refers the patient to appropriate services based on eligibility.
Clinic 3
Clinic 3 is a GeriPACT clinic where functional status information is routinely collected for all new patients but may or may not be collected for returning patients (eFigure 3, available at fedprac.com). The process for new patients includes a previsit SW assessment; an informal LPN screening (ie, not based on a standardized clinical reminder); a PCP assessment; and a postvisit SW assessment if referred by the provider. The process for returning patients is similar but omits the previsit social work assessment. New patients complete a comprehensive questionnaire with a SW before their first clinic visit. The questionnaire is completed by phone and involves an extensive social and medical history, including an assessment of ADLs and IADLs. This assessment is recorded in a free-form social work note.
Next, both new and returning patients see an LPN who completes the intake process, including vitals and clinical reminders. Clinic 3 does not have a clinical reminder for functional status. However, the LPN could elect to ask about ADLs or IADLs if the patient brings up a functional issue related to the chief symptom or if the LPN observes something that indicates possible functional impairment, such as difficulty walking or a disheveled appearance. If discussed, this information is recorded in the LPN intake note, and the LPN also could verbally inform the PCP of the patient’s functional status. The RN is not formally involved in intake or functional status assessment in this clinic.
Finally, the patient sees the PCP, who may or may not have reviewed the LPN note. The PCP may assess functional status at his or her discretion, but there was no required assessment. The PCP could complete an optional functional status assessment template included in the PCP visit note. The PCP can refer the patient to services or to SW for further evaluation.
Clinic 4
Clinic 4 is a primary care PACT clinic that does not routinely measure functional status (eFigure 4, available at fedprac.com). The approach includes an informal LPN screening (ie, not based on a standardized clinical reminder); a PCP assessment; and a postvisit social worker assessment if referred by the provider. These steps are very similar to those of clinic 3, but they do not include a previsit SW assessment for new patients.
Although not represented within the 4 clinics described in this article, the content of functional status clinical reminders differed across the 9 clinics in the larger sample. Clinical reminders differed across several domains, including the type of question stems (scripted questions for each ADL vs categories for each activity); response options (eg, dichotomous vs ≥ 3 options), and the presence of free-text boxes to allow staff to enter any additional notes.
Discussion
Approaches to assessing and documenting functional status varied widely. Whereas some clinics primarily used informal approaches to assessing and documenting functional status (ie, neither routine nor standardized), others used a routine, standardized clinical reminder, and some combined several standardized approaches to measuring function. The study team identified variability across several domains of the functional status assessment process, including documentation, workflow, and clinical reminder content.
Approaches to functional assessment differed between GeriPACT and PACT clinics. Consistent with the central role that functional status assessment plays in geriatrics practice, GeriPACTs tended to employ a routine, multidisciplinary approach to measuring functional status. This approach included standardized functional assessments by multiple primary care team members, including LPNs, SWs, and PCPs. In contrast, when PACTs completed standardized functional status assessment, it was generally carried out by a single team member (typically an LPN). The PCPs in PACTs used a nonroutine approach to assess functional status in which they performed detailed functional assessments for certain high-risk patients and referred a subset for further SW evaluation.
These processes are consistent with research showing that standardized functional status data are seldom collected routinely in nongeriatric primary care settings.11 Reports by PCPs that they did not always assess functional status also are consistent with previous research demonstrating that clinicians are not always aware of their patients’ functional ability.10
In addition to highlighting differences between GeriPACT and PACTs, the identified processes illustrate the variability in documentation, clinic workflow, and clinical reminder content across all clinics. Approaches to documentation included checkbox-formatted clinical reminders with and without associated nursing notes, patient questionnaires, and templated PCP and SW notes. Clinics employed varying approaches to collect functional status information and to ensure that those data were shared with the team. Clinic staff assessed functional status at different times during the clinical encounter. Clinics used several approaches to share this information with team members, including warm handoffs from LPNs to PCPs, interdisciplinary team huddles, and electronic signoffs. Finally, clinical reminder content varied between clinics, with differences in the wording of ADL and IADL questions as well as in the number and type of response options.
This variability highlights the challenges inherent in developing a routine, standardized approach to measuring functional status that can be adapted across primary care settings. Such an approach must be both flexible enough to accommodate variation in workflow and structured enough to capture accurate data that can be used to guide clinical decisions. Capturing accurate, standardized data in CDW also will inform efforts to improve population health by allowing VHA leaders to understand the scope of disability among older veterans and plan for service needs and interventions.
Whereas the larger qualitative study will identify the specific barriers and facilitators to developing and implementing such an approach, current clinic processes present here offer hints as to which features may be important. For example, several clinics collected functional status information before the visit by telephone or questionnaire. Therefore, it will be important to choose a functional status assessment instrument that is validated for both telephone and in-person use. Similarly, some clinics had structured clinical reminders with categoric response options, whereas others included free-text boxes. Incorporating both categoric responses (to ensure accurate data) as well as free-text (to allow for additional notes about a patient’s specific circumstances that may influence service needs) may be one approach.
Limitations
This study’s approach to identifying clinic processes had several limitations. First, the authors did not send process maps to clinic directors for verification. However, speaking with PACT members who carry out clinic processes is likely the most accurate way to identify practice. Second, the results may not be generalizable to all VA primary care settings. Due to resource limitations and project scope, community-based outpatient clinics (CBOCs) were not included. Compared with clinics based in medical centers, CBOCs may have different staffing levels, practice models, and needs regarding implementation of functional status assessment.
Although 46 participants from 9 clinics were interviewed, there are likely additional approaches to measuring functional status that are not represented within this sample. In addition, 3 of the 4 clinics included are affiliated with academic institutions, and all 4 are located in large cities. Efforts to include rural VAMCs were not successful. Finally, clinic-level characteristics were not reported, which may impact clinic processes. Although study participants were asked about clinic characteristics, they were often unsure or only able to provide rough estimates. In the ongoing qualitative study, the authors will attempt to collect more reliable data about these clinic-level characteristics and to examine the potential role these characteristics may play as barriers or facilitators to implementing routine assessment of functional status in primary care settings.
Conclusion
VA primary care clinics had widely varying approaches for assessing and documenting functional status. This work along with a larger ongoing qualitative study that includes interviews with veterans will directly inform the design and implementation of a standardized, patient-centered approach to functional assessment that can be adapted across varied primary care settings. Implementing standardized functional status measurement will allow the VA to serve veterans better by using functional status information to refer patients to appropriate services and to deliver patient-centered care with the potential to improve patient function and quality of life.
The ability to perform activities of daily living (ADLs), commonly called functional status, is central to older adults’ quality of life (QOL) and independence.1,2 Understanding functional status is key to improving outcomes for older adults. In community-dwelling older adults with difficulty performing basic ADLs, practical interventions, including physical and occupational therapy, can improve functioning and prevent functional decline.3,4 Understanding function also is important for delivering patient-centered care, including individualizing cancer screening,5 evaluating how patients will tolerate interventions,6-9 and helping patients and families determine the need for long-term services and supports.
For these reasons, assessing functional status is a cornerstone of geriatrics practice. However, most older adults are cared for in primary care settings where routine measurement of functional status is uncommon.10,11 Although policy leaders have long noted this gap and the obstacle it poses to improving the quality and outcomes of care for older adults, many health care systems have been slow to incorporate measurement of functional status into routine patient care.12-14
Over the past several years, the VA has been a leader in the efforts to address this barrier by implementing routine, standardized measurement of functional status in primary care clinics. Initially, the VA encouraged, but did not require, measurement of functional status among older adults, but the implementation barriers and facilitators were not formally assessed.15 In a postimplementation evaluation, the authors found that a relatively small number of medical centers implemented functional measures. Moreover, the level of implementation seemed to vary across sites. Some sites were collecting complete measures on all eligible older patients, while other sites were collecting measures less consistently.15
As part of a national VA initiative to learn how best to implement standardized functional status measurement, the authors are conducting a qualitative study, including a formal assessment of barriers and facilitators to implementing functional assessments in VA primary care clinics. In the current project, which serves as formative work for this larger ongoing study, the authors identified and described current processes for measuring functional status in VA primary care patient aligned care team (PACT) and Geriatric (GeriPACT) clinics.
Methods
A rapid qualitative analysis approach was used, which included semistructured interviews with primary care stakeholders and rapid data analysis to summarize each clinic’s approach to measuring functional status and develop process maps for each clinic (eFigures 1, 2, 3, and 4 ). Interviews and analyses were conducted by a team consisting of a geriatrician clinician-researcher, a medical anthropologist, and a research coordinator. The institutional review boards of the San Francisco VAMC and the University of California, San Francisco approved the study.
Sampling Strategy
In order to identify VAMCs with varying approaches to assessing functional status in older patients who attended primary care appointments, the study used a criterion sampling approach.16,17 First, national “health factors” data were extracted from the VA Corporate Data Warehouse (CDW). Health factors are patient data collected through screening tools called clinical reminders, which prompt clinic staff and providers to enter data into checkbox-formatted templates. The study then identified medical centers that collected health factors data from patients aged ≥ 65 years (157 of 165 medical centers). A keyword search identified health factors related to the Katz ADL (bathing, dressing, transferring, toileting, and eating), and Lawton Instrumental ADL (IADL) Scale (using the telephone, shopping, preparing food, housekeeping, doing laundry, using transportation, managing medications, and managing finances).18,19 Health factors that were not collected during a primary care appointment were excluded.
Of the original 157 medical centers, 139 met these initial inclusion criteria. Among these 139 medical centers, 66 centers did not collect complete data on these 5 ADLs and 8 IADLs (eg, only ADLs or only IADLs, or only certain ADLs or IADLs).
Two medical centers were selected in each of the following 3 categories: (1) routinely used clinical reminders to collect standardized data on the Katz ADL and the Lawton IADL Scale; (2) routinely used clinical reminders to collect functional status data but collected partial information; and (3) did not use a clinical reminder to collect functional status data. To ensure that these 6 medical centers were geographically representative, the sample included at least 1 site from each of the 5 VA regions: 1 North Atlantic, 1 Southeast, 1 Midwest, 2 Continental (1 from the northern Continental region and 1 from the southern), and 1 Pacific. Three sites that included GeriPACTs also were sampled.
Primary care PACT and GeriPACT members from these 6 medical centers were recruited to participate. These PACT members included individuals who can assess function or use functional status information to inform patient care, including front-line nursing staff (licensed practical nurses [LPNs], and registered nurses [RNs]), primary care providers (medical doctors [MDs] and nurse practitioners [NPs]), and social workers (SWs).
Local bargaining units, nurse managers, and clinic directors provided lists of all clinic staff. All members of each group then received recruitment e-mails. Phone interviews were scheduled with interested participants. In several cases, a snowball sampling approach was used to increase enrollment numbers by asking interview participants to recommend colleagues who might be interested in participating.17
Data Collection
Telephone interviews were conducted between March 2016 and October 2016 using semistructured guides developed from the project aims and from related literature in implementation science.20,21 Interview domains included clinic structure, team member roles and responsibilities, current practices for collecting functional status data, and opinions on barriers and facilitators to assessing and recording functional status (Appendix:
Data Analysis
Rapid analysis, a team-based qualitative approach was used to engage efficiently and systematically with the data.22,23 This approach allowed results to be analyzed more quickly than in traditional qualitative analysis in order to inform intervention design and develop implementation strategies.23 Rapid analysis typically includes organization of interview data into summary templates, followed by a matrix analysis, which was used to create process maps.24
Summary Templates
Summary templates were developed from the interview guides by shortening each question into a representative code. The project team then read the transcripts and summarized key points in the appropriate section of the template. This process, known as data reduction, is used to organize and highlight material so conclusions can be drawn from the data easily.22 In order to maintain rigor and trustworthiness, one team member conducted the interview, and a different team member created the interview summary. All team members reviewed each summary and met regularly to discuss results.
The summary templates were converted into matrix analyses, a method of displaying data to identify relationships, including commonalities and differences.24 The matrixes were organized by stakeholder group and clinic in order to compare functional status assessment and documentation workflows across clinics.
Process Maps
Finally, the team used the matrix data to create process maps for each clinic of when, where, and by whom functional status information was assessed and documented. These maps were created using Microsoft Visio (Redmond, WA). The maps integrated perspectives from all participants to give an overview of the process for collecting functional status data in each clinic setting. To ensure accuracy, participants at each site received process maps to solicit feedback and validation.
Results
Forty-six participants at 6 medical centers (20 MDs and NPs, 19 RNs and LPNs, and 7 SWs) from 9 primary care clinics provided samples and interviews. The study team identified 3 general approaches to functional status assessment: (1) Routine collection of functional status data via a standardized clinical reminder; (2) Routine collection of functional status data via methods other than a clinical reminder (eg, a previsit telephone screen or electronic note template); and (3) Ad hoc approaches to measuring functional status (ie, no standard or routine approach to assessing or documenting functional status). The study team selected 4 clinics (2 PACTs and 2 GeriPACTs) clinics to serve as examples of the 3 identified approaches.
The processes for functional status assessment in each of 4 clinics are summarized in the following detailed descriptions (Table).
Clinic 1
Clinic 1 is a GeriPACT clinic that routinely assesses and documents functional status for all patients (efigure 1, available at feprac.com). The clinic’s current process includes 4 elements: (1) a patient questionnaire; (2) an annual clinical reminder administered by an RN; (3) a primary care provider (PCP) assessment; and (4) a postvisit SW assessment if referred by the PCP.
All newly referred patients are mailed a paper questionnaire that includes questions about their medical history and functional status. The patient is asked to bring the completed questionnaire to the first appointment. The clinic RN completes this form for returning patients at every visit during patient intake.
Second, the clinic uses an annual functional status clinical reminder for patients aged ≥ 75 years. The reminder includes questions about a patient’s ability to perform ADLs and IADLs with 3 to 4 response options for each question. If the clinical reminder is due at the time of a patient appointment, the RN fills out the reminder using information from the paper questionnaire. The RN also records this functional status in the nursing intake note. The RN may elect to designate the PCP as a cosigner for the nursing intake note especially if there are concerns about or changes in the patient’s functional status.
Third, the RN brings the paper form to the PCP, who often uses the questionnaire to guide the patient history. The PCP then uses the questionnaire and patient history to complete a functional status template within their visit note. The PCP also may use this information to inform patient care (eg, to make referrals to physical or occupational therapy).
Finally, the PCP might refer the patient to SW. The SW may be able to see the patient immediately after the PCP appointment, but if not, the SW follows up with a phone call to complete further functional status assessment and eligibility forms.
In addition to the above assessments by individual team members, the PACT has an interdisciplinary team huddle at the end of each clinic to discuss any issues or concerns about specific patients. The huddles often focus on issues related to functional status.
Clinic 2
Clinic 2 is a primary care PACT clinic that routinely assesses and documents functional status (eFigure 2, available at fedprac.com). The clinic process includes 3 steps: an annual clinical reminder for patients aged ≥ 75 years; a PCP assessment; and a postvisit SW assessment if referred by the PCP.
First, patients see an LPN for the intake process. During intake, the LPN records vitals and completes relevant clinical reminders. Similar to Clinic 1, Clinic 2 requires an annual functional status clinical reminder that includes ADLs and IADLs for patients aged ≥ 75 years. Patient information from the intake and clinical reminders are recorded by the LPN in a preventative medicine note in the electronic health record. This note is printed and handed to the PCP.
The PCP may review the preventative medicine note prior to completing the patient history and physical, including the functional status clinical reminder when applicable. If the PCP follows up on any functional issues identified by the LPN or completes further assessment of patient function, he or she may use this information to refer the patient to services or to place a SW consult; the PCP’s functional assessment is documented in a free-form visit note.
When the SW receives a consult, a chart review for social history, demographic information, and previous functional status assessments is conducted. The SW then calls the patient to administer functional and cognitive assessments over the phone and refers the patient to appropriate services based on eligibility.
Clinic 3
Clinic 3 is a GeriPACT clinic where functional status information is routinely collected for all new patients but may or may not be collected for returning patients (eFigure 3, available at fedprac.com). The process for new patients includes a previsit SW assessment; an informal LPN screening (ie, not based on a standardized clinical reminder); a PCP assessment; and a postvisit SW assessment if referred by the provider. The process for returning patients is similar but omits the previsit social work assessment. New patients complete a comprehensive questionnaire with a SW before their first clinic visit. The questionnaire is completed by phone and involves an extensive social and medical history, including an assessment of ADLs and IADLs. This assessment is recorded in a free-form social work note.
Next, both new and returning patients see an LPN who completes the intake process, including vitals and clinical reminders. Clinic 3 does not have a clinical reminder for functional status. However, the LPN could elect to ask about ADLs or IADLs if the patient brings up a functional issue related to the chief symptom or if the LPN observes something that indicates possible functional impairment, such as difficulty walking or a disheveled appearance. If discussed, this information is recorded in the LPN intake note, and the LPN also could verbally inform the PCP of the patient’s functional status. The RN is not formally involved in intake or functional status assessment in this clinic.
Finally, the patient sees the PCP, who may or may not have reviewed the LPN note. The PCP may assess functional status at his or her discretion, but there was no required assessment. The PCP could complete an optional functional status assessment template included in the PCP visit note. The PCP can refer the patient to services or to SW for further evaluation.
Clinic 4
Clinic 4 is a primary care PACT clinic that does not routinely measure functional status (eFigure 4, available at fedprac.com). The approach includes an informal LPN screening (ie, not based on a standardized clinical reminder); a PCP assessment; and a postvisit social worker assessment if referred by the provider. These steps are very similar to those of clinic 3, but they do not include a previsit SW assessment for new patients.
Although not represented within the 4 clinics described in this article, the content of functional status clinical reminders differed across the 9 clinics in the larger sample. Clinical reminders differed across several domains, including the type of question stems (scripted questions for each ADL vs categories for each activity); response options (eg, dichotomous vs ≥ 3 options), and the presence of free-text boxes to allow staff to enter any additional notes.
Discussion
Approaches to assessing and documenting functional status varied widely. Whereas some clinics primarily used informal approaches to assessing and documenting functional status (ie, neither routine nor standardized), others used a routine, standardized clinical reminder, and some combined several standardized approaches to measuring function. The study team identified variability across several domains of the functional status assessment process, including documentation, workflow, and clinical reminder content.
Approaches to functional assessment differed between GeriPACT and PACT clinics. Consistent with the central role that functional status assessment plays in geriatrics practice, GeriPACTs tended to employ a routine, multidisciplinary approach to measuring functional status. This approach included standardized functional assessments by multiple primary care team members, including LPNs, SWs, and PCPs. In contrast, when PACTs completed standardized functional status assessment, it was generally carried out by a single team member (typically an LPN). The PCPs in PACTs used a nonroutine approach to assess functional status in which they performed detailed functional assessments for certain high-risk patients and referred a subset for further SW evaluation.
These processes are consistent with research showing that standardized functional status data are seldom collected routinely in nongeriatric primary care settings.11 Reports by PCPs that they did not always assess functional status also are consistent with previous research demonstrating that clinicians are not always aware of their patients’ functional ability.10
In addition to highlighting differences between GeriPACT and PACTs, the identified processes illustrate the variability in documentation, clinic workflow, and clinical reminder content across all clinics. Approaches to documentation included checkbox-formatted clinical reminders with and without associated nursing notes, patient questionnaires, and templated PCP and SW notes. Clinics employed varying approaches to collect functional status information and to ensure that those data were shared with the team. Clinic staff assessed functional status at different times during the clinical encounter. Clinics used several approaches to share this information with team members, including warm handoffs from LPNs to PCPs, interdisciplinary team huddles, and electronic signoffs. Finally, clinical reminder content varied between clinics, with differences in the wording of ADL and IADL questions as well as in the number and type of response options.
This variability highlights the challenges inherent in developing a routine, standardized approach to measuring functional status that can be adapted across primary care settings. Such an approach must be both flexible enough to accommodate variation in workflow and structured enough to capture accurate data that can be used to guide clinical decisions. Capturing accurate, standardized data in CDW also will inform efforts to improve population health by allowing VHA leaders to understand the scope of disability among older veterans and plan for service needs and interventions.
Whereas the larger qualitative study will identify the specific barriers and facilitators to developing and implementing such an approach, current clinic processes present here offer hints as to which features may be important. For example, several clinics collected functional status information before the visit by telephone or questionnaire. Therefore, it will be important to choose a functional status assessment instrument that is validated for both telephone and in-person use. Similarly, some clinics had structured clinical reminders with categoric response options, whereas others included free-text boxes. Incorporating both categoric responses (to ensure accurate data) as well as free-text (to allow for additional notes about a patient’s specific circumstances that may influence service needs) may be one approach.
Limitations
This study’s approach to identifying clinic processes had several limitations. First, the authors did not send process maps to clinic directors for verification. However, speaking with PACT members who carry out clinic processes is likely the most accurate way to identify practice. Second, the results may not be generalizable to all VA primary care settings. Due to resource limitations and project scope, community-based outpatient clinics (CBOCs) were not included. Compared with clinics based in medical centers, CBOCs may have different staffing levels, practice models, and needs regarding implementation of functional status assessment.
Although 46 participants from 9 clinics were interviewed, there are likely additional approaches to measuring functional status that are not represented within this sample. In addition, 3 of the 4 clinics included are affiliated with academic institutions, and all 4 are located in large cities. Efforts to include rural VAMCs were not successful. Finally, clinic-level characteristics were not reported, which may impact clinic processes. Although study participants were asked about clinic characteristics, they were often unsure or only able to provide rough estimates. In the ongoing qualitative study, the authors will attempt to collect more reliable data about these clinic-level characteristics and to examine the potential role these characteristics may play as barriers or facilitators to implementing routine assessment of functional status in primary care settings.
Conclusion
VA primary care clinics had widely varying approaches for assessing and documenting functional status. This work along with a larger ongoing qualitative study that includes interviews with veterans will directly inform the design and implementation of a standardized, patient-centered approach to functional assessment that can be adapted across varied primary care settings. Implementing standardized functional status measurement will allow the VA to serve veterans better by using functional status information to refer patients to appropriate services and to deliver patient-centered care with the potential to improve patient function and quality of life.
1.Covinsky KE, Wu AW, Landefeld CS, et al. Health status versus quality of life in older patients: does the distinction matter? Am J Med. 1999;106(4):435-440.
2. Fried TR, McGraw S, Agostini JV, Tinetti ME. Views of older persons with multiple morbidities on competing outcomes and clinical decision-making. J Am Geriatr Soc. 2008;56(10):1839-1844.
3. Beswick AD, Rees K, Dieppe P, et al. Complex interventions to improve physical function and maintain independent living in elderly people: a systematic review and meta-analysis. Lancet. 2008;371(9614):725-735.
4. Szanton SL, Leff B, Wolff JL, Roberts L, Gitlin LN. Home-based care program reduces disability and promotes aging in place. Health Aff (Millwood). 2016;35(9):1558-1563.
5. Walter LC, Covinsky KE. Cancer screening in elderly patients: a framework for individualized decision making. JAMA. 2001;285(21):2750-2756.
6. Kurella Tamura M, Covinsky KE, Chertow GM, Yaffe K, Landefeld CS, McCulloch CE. Functional status of elderly adults before and after initiation of dialysis. N Engl J Med. 2009;361(16):1539-1547.
7. Hurria A, Togawa K, Mohile SG, et al. Predicting chemotherapy toxicity in older adults with cancer: a prospective multicenter study. J Clin Oncol. 2011;29(25):3457-3465.
8. Crawford RS, Cambria RP, Abularrage CJ, et al. Preoperative functional status predicts perioperative outcomes after infrainguinal bypass surgery. J Vasc Surg. 2010;51(2):351-358; discussion 358-359.
9. Arnold SV, Reynolds MR, Lei Y, et al; PARTNER Investigators. Predictors of poor outcomes after transcatheter aortic valve replacement: results from the PARTNER (Placement of Aortic Transcatheter Valve) trial. Circulation. 2014;129(25):2682-2690.
10. Calkins DR, Rubenstein LV, Cleary PD, et al. Failure of physicians to recognize functional disability in ambulatory patients. Ann Intern Med. 1991;114(6):451-454.
11. Bogardus ST Jr, Towle V, Williams CS, Desai MM, Inouye SK. What does the medical record reveal about functional status? A comparison of medical record and interview data. J Gen Intern Med. 2001;16(11):728-736.
12. Bierman AS. Functional status: the six vital sign. J Gen Intern Med. 2001;16(11):785-786.
13. Iezzoni LI, Greenberg MS. Capturing and classifying functional status information in administrative databases. Health Care Financ Rev. 2003;24(3):61-76.
14. Clauser SB, Bierman AS. Significance of functional status data for payment and quality. Health Care Financ Rev. 2003;24(3):1-12.
15. Brown RT, Komaiko KD, Shi Y, et al. Bringing functional status into a big data world: validation of national Veterans Affairs functional status data. PloS One. 2017;12(6):e0178726.
16. Palinkas LA, Horwitz SM, Green CA, Wisdom JP, Duan N, Hoagwood K. Purposeful sampling for qualitative data collection and analysis in mixed method implementation research. Adm Policy Ment Health. 2015;42(5):533-544.
17. Patton MQ. Qualitative Research Evaluation and Methods. 4th ed. Thousand Oaks, CA: Sage; 2015.
18. Katz S, Ford AB, Moskowitz RW, Jackson BA, Jaffe MW. Studies of illness in the aged. The index of ADL: a standardized measure of biological and psychosocial function. JAMA. 1963;185(12):914-919.
19. Lawton MP, Brody EM. Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontologist. 1969;9(3):179-186.
20. Damschroder LJ, Aron DC, Keith RE, Kirsh SR, Alexander JA, Lowery JC. Fostering implementation of health services research findings into practice: a consolidated framework for advancing implementation science. Implement Sci. 2009;4(1):50.
21. Saleem JJ, Patterson ES, Militello L, Render ML, Orshansky G, Asch SM. Exploring barriers and facilitators to the use of computerized clinical reminders. J Am Med Inform Assoc. 2005;12(4):438-447.
22. Miles MB, Huberman AM, Saldana J. Qualitative Data Analysis: A Methods Sourcebook. 3rd ed. Thousand Oaks, CA: Sage; 2014.
23. Hamilton AB. Qualitative methods in rapid turn-around health services research. https://www.hsrd .research.va.gov/for_researchers/cyber_seminars /archives/video_archive.cfm?SessionID=780. Published December 11, 2013. Accessed August 9, 2017.
24. Averill JB. Matrix analysis as a complementary analytic strategy in qualitative inquiry. Qual Health Res. 2002;12(6):855-866.
1.Covinsky KE, Wu AW, Landefeld CS, et al. Health status versus quality of life in older patients: does the distinction matter? Am J Med. 1999;106(4):435-440.
2. Fried TR, McGraw S, Agostini JV, Tinetti ME. Views of older persons with multiple morbidities on competing outcomes and clinical decision-making. J Am Geriatr Soc. 2008;56(10):1839-1844.
3. Beswick AD, Rees K, Dieppe P, et al. Complex interventions to improve physical function and maintain independent living in elderly people: a systematic review and meta-analysis. Lancet. 2008;371(9614):725-735.
4. Szanton SL, Leff B, Wolff JL, Roberts L, Gitlin LN. Home-based care program reduces disability and promotes aging in place. Health Aff (Millwood). 2016;35(9):1558-1563.
5. Walter LC, Covinsky KE. Cancer screening in elderly patients: a framework for individualized decision making. JAMA. 2001;285(21):2750-2756.
6. Kurella Tamura M, Covinsky KE, Chertow GM, Yaffe K, Landefeld CS, McCulloch CE. Functional status of elderly adults before and after initiation of dialysis. N Engl J Med. 2009;361(16):1539-1547.
7. Hurria A, Togawa K, Mohile SG, et al. Predicting chemotherapy toxicity in older adults with cancer: a prospective multicenter study. J Clin Oncol. 2011;29(25):3457-3465.
8. Crawford RS, Cambria RP, Abularrage CJ, et al. Preoperative functional status predicts perioperative outcomes after infrainguinal bypass surgery. J Vasc Surg. 2010;51(2):351-358; discussion 358-359.
9. Arnold SV, Reynolds MR, Lei Y, et al; PARTNER Investigators. Predictors of poor outcomes after transcatheter aortic valve replacement: results from the PARTNER (Placement of Aortic Transcatheter Valve) trial. Circulation. 2014;129(25):2682-2690.
10. Calkins DR, Rubenstein LV, Cleary PD, et al. Failure of physicians to recognize functional disability in ambulatory patients. Ann Intern Med. 1991;114(6):451-454.
11. Bogardus ST Jr, Towle V, Williams CS, Desai MM, Inouye SK. What does the medical record reveal about functional status? A comparison of medical record and interview data. J Gen Intern Med. 2001;16(11):728-736.
12. Bierman AS. Functional status: the six vital sign. J Gen Intern Med. 2001;16(11):785-786.
13. Iezzoni LI, Greenberg MS. Capturing and classifying functional status information in administrative databases. Health Care Financ Rev. 2003;24(3):61-76.
14. Clauser SB, Bierman AS. Significance of functional status data for payment and quality. Health Care Financ Rev. 2003;24(3):1-12.
15. Brown RT, Komaiko KD, Shi Y, et al. Bringing functional status into a big data world: validation of national Veterans Affairs functional status data. PloS One. 2017;12(6):e0178726.
16. Palinkas LA, Horwitz SM, Green CA, Wisdom JP, Duan N, Hoagwood K. Purposeful sampling for qualitative data collection and analysis in mixed method implementation research. Adm Policy Ment Health. 2015;42(5):533-544.
17. Patton MQ. Qualitative Research Evaluation and Methods. 4th ed. Thousand Oaks, CA: Sage; 2015.
18. Katz S, Ford AB, Moskowitz RW, Jackson BA, Jaffe MW. Studies of illness in the aged. The index of ADL: a standardized measure of biological and psychosocial function. JAMA. 1963;185(12):914-919.
19. Lawton MP, Brody EM. Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontologist. 1969;9(3):179-186.
20. Damschroder LJ, Aron DC, Keith RE, Kirsh SR, Alexander JA, Lowery JC. Fostering implementation of health services research findings into practice: a consolidated framework for advancing implementation science. Implement Sci. 2009;4(1):50.
21. Saleem JJ, Patterson ES, Militello L, Render ML, Orshansky G, Asch SM. Exploring barriers and facilitators to the use of computerized clinical reminders. J Am Med Inform Assoc. 2005;12(4):438-447.
22. Miles MB, Huberman AM, Saldana J. Qualitative Data Analysis: A Methods Sourcebook. 3rd ed. Thousand Oaks, CA: Sage; 2014.
23. Hamilton AB. Qualitative methods in rapid turn-around health services research. https://www.hsrd .research.va.gov/for_researchers/cyber_seminars /archives/video_archive.cfm?SessionID=780. Published December 11, 2013. Accessed August 9, 2017.
24. Averill JB. Matrix analysis as a complementary analytic strategy in qualitative inquiry. Qual Health Res. 2002;12(6):855-866.
Is Ketamine the New Wonder Drug for Treating Suicide?
In 2014 the suicide rate in the U.S. was 13/100,000, the highest recorded in 28 years.1 Suicide is now considered the 10th leading cause of death for all ages, and the rate has increased every year from 2000 to 2014 among both women and men and in every age group except those aged ≥ 75 years.1-3 For those aged 15 to 44 years, suicide is among the top 3 causes of death worldwide.4-6
Background
In 2013, more than 490,000 hospital visits related to suicide attempts were reported in the U.S.4 Health care expenditures related to suicide are estimated at $56.9 billion in combined medical and work loss costs annually and an unmeasurable cost to the affected families.7 The mental health care community is desperate for ways to address this epidemic, and the National Academies of Medicine (NAM) has declared that research that directly addresses comparative effectiveness of treatment strategies following a suicide attempt should be a national priority.8
The most recent reports from 2014 indicate that the suicide rates are higher for male veterans than for male nonveterans (32.1 vs 20.9 per 100,000, respectively) and are much higher for female veterans than for female nonveterans (28.7 vs 5.2 per 100,000, respectively).3 Suicide rates also may be associated with veteran-specific comorbidities, such as higher rates of depression, anxiety, posttraumatic stress disorder (PTSD), and war-related trauma.3 According to the VHA, the suicide rate for veterans aged > 30 years also is rapidly increasing, and VHA has echoed the calls from NAM to make suicide prevention research a national priority.3
The VA has tried to stem the tide of suicides in veterans by implementing many advances in suicide prevention, including hiring suicide prevention coordinators at every VA hospital, enhanced monitoring, and the availability of 24-hour crisis hotline services. Yet the suicide rates for veterans continue to rise and remain higher than the rates in the general population.3
About 90% of deaths by suicide are by persons who have a treatable psychiatric disorder, most commonly a mood disorder, such as depression.4 However, most studies show that antidepressant therapy does not provide rapid or significant relief of suicidal ideation (SI).4 Therefore, the current standard of care for the treatment of acutely suicidal patients includes a combination of hospitalization, cognitive behavioral therapy or psychotherapy, case management, antidepressant medications, and electroconvulsive therapy (ECT).4 Even though these therapies have become more widely available over the past decade, rates of suicide continue to increase.1,4 These interventions have limited effectiveness in acute settings. Although both intensive outpatient follow-up and routine outpatient care have been studied in relation to the decrease of suicidal behavior, neither intervention has been shown to immediately reduce suicidal behavior significantly in patients.
Suicidality Interventions
Therapy and case management require patients to be well enough to make office visits and follow through with care for periods as long as 1 year, which is often not possible for individuals with severe depression.5 One-third of patients who attended 6 months of outpatient therapy consistently still met the criteria for major depressive disorder (MDD), a major risk for suicide attempt.9 Antidepressant medications take a minimum of 4 weeks to reach full efficacy, and many patients stop taking the medications before that point because of concern that the medication is not helping or because of adverse effects (AEs), such as sleep disturbance, sexual dysfunction, or weight gain.9
Electroconvulsive therapy has been shown to be an effective treatment for patients with depression and suicidal behavior, but adherence with 12 weeks of recommended therapy has been a barrier for this intervention. Additionally, ECT may not provide reduction in SI for 1 to 2 weeks.4,10 A review of research studies showed that nearly 50% of patients with high-expressed SI did not complete the prescribed amount of ECT due to the length of time to complete the recommended 12 sessions.10 Therefore, current treatment barriers for suicidal patients include: (1) long periods in treatment for therapy, medication, and ECT before any relief of symptoms is noted; (2) high recidivism rates for MDD symptoms and risk of suicide following treatment; and (3) high treatment dropout rates.
Pharmacologic treatments currently used in suicidal patients have not fared much better. Many have received FDA approval for treatment of associated mental health diagnoses such as bipolar disorder, schizophrenia, or MDD, but there are no approved treatments that specifically target suicidal behavior. Lithium is approved for reducing the long-term risk of SI primarily because it reduces the risk of mood disorders associated with SI, but lithium has not been shown to be effective in acute settings.11 Clozapine is approved for reducing the long-term risk of recurrent suicide in patients with schizophrenia or schizoaffective disorder.4 Clozapine has not been shown to be effective in patients with mood disorders, which make up the majority of patients who attempt suicide.4 Additionally, both medications are plagued by the same barriers listed earlier, such as long time to effect (it takes an average 4 weeks to reach efficacy), lack of efficacy in acute settings, and AEs (eg, sleep problems, weight gain, and sexual dysfunction).9 Thus finding better pharmacologic interventions for suicidal patients is a priority for current research.
Ketamine
Recently, researchers have identified ketamine as a potential therapeutic option for depression and SI. A single ketamine infusion treatment has a rapid response, minimal AEs, and potentially long-lasting efficacy with SI, which would make it ideal for the treatment of acutely suicidal patients.4 Ketamine is an N-methyl-D-aspartate receptor (NMDAR) inhibitor that also has been found to be a weak μ- and κ-opioid receptor agonist and an inhibitor of the reuptake of serotonin, dopamine, and norepinephrine. Inhibition of the NMDAR results in analgesia, and ketamine is approved for the induction of anesthesia, pain relief, and sedation.12
Although AEs such as hallucinations and sedation create the potential for dangerous recreational use, ketamine is safely used in health care settings for a variety of indications. Effects are noted within 5 minutes of administration if given by infusion, and the main effects can last between 20 and 40 minutes.
Ketamine has a complex pharmacology and plays a role in other cell signaling mechanisms, but the significance of these additional mechanisms in the therapeutic effects of ketamine have only recently been elucidated. Preclinical studies indicate a probable NMDAR inhibition-independent mechanism responsible for the antidepressant response to ketamine.13,14 The complex associations with rapamycin signaling, eukaryotic elongation factor 2 dephosphorylation, increased synthesis of brain-derived neurotrophic factor, and activation of glutamatergic AMPA (α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid) receptors have been linked to its rapid antidepressant effect and ketamine’s induction of synaptogenesis within the limbic system.13,14
Clinical Research
Ketamine was studied as an adjunctive treatment to psychotherapy for addictions as far back as the 1970s.15 The available reports indicate a universally positive result, with increased rates of remission and decreased rates of relapse attributed to ketamine’s ability to alter one’s thought processes by reinforcing limbic-cortex interactions that facilitate the growth of more positive cognitive schemas and improved emotional attitudes about the self in support of the recovery process.15
Neurobiologic studies have shown that treatment with ketamine has a direct and immediate effect on neuronal pathways of the limbic system. It is known to regulate the mind’s reaction to positive stimuli by reversing the depressed subject’s blunted reaction to positive faces.16 This rapid normalization of the positive faces test is unique to ketamine infusion and is not seen in tests with traditional antidepressants.
In 2000, the first placebo-controlled trial using ketamine for treatment resistant depression (TRD) demonstrated the rapid antidepressant effects of a single dose of ketamine, but this study only looked at these effects for 1 week.17 In multiple double blind, placebo-controlled trials since then, IV infusion of ketamine was shown to be an effective intervention for TRD.13,18,19 More recently, a published investigation involving the treatment of MDD showed that ketamine in conjunction with a selective serotonin reuptake inhibitor (SSRI) accelerated and enhanced the effectiveness of the SSRI in reducing depressive symptoms.20
Based on the rapid resolution of depressive symptoms using ketamine, researchers have looked at its effect on suicidality as a secondary measure. A case study of a patient with severe depressive episodes and multiple previous suicidal attempts reported that the patient responded to a single dose of ketamine, described the experience as “being reborn,” and maintained complete remission of SI for the 6-month study period.21 In a larger study, 133 TRD patients received a single IV dose of ketamine with significant reductions in SI independent of depressive and anxiety symptoms.22
Depression Treatment
These results have led to an excitement for ketamine therapy as a novel treatment of depression, and off-label use by treatment centers now exists in several countries to aid those with TRD.23 This off-label use continues to be controversial, as research has yet to determine the safest most effective route and duration of treatment and whether the ketamine treatment AEs will exceed any accrued therapeutic benefit.13
The American Psychological Association Council of Research Task Force on Novel Biomarkers and Treatment critically examined the clinical evidence of ketamine use and has raised important concerns about the use of ketamine in the outpatient setting, administered in the absence of consensus therapeutic monitoring guidelines, and ambitiously marketed as a panacea for TRD.13,24 A study showed permanent impairment of brain function for both groups compared with monkeys treated with saline infusions.25 In 2016, the FDA gave fast-track approval for an intranasal ketamine that would make the treatment more easily available in the outpatient setting, but this could lead to certain patients developing a dependency on ketamine or engaging in its diversion for recreational use. There are case reports and anecdotes in the literature of patients and research subjects developing drug-seeking behaviors and overuse of ketamine.24 Additionally, the comorbidities associated with TRD and SI have not been fully evaluated. For instance, there is evidence that depressed patients with obsessive compulsive disorder may have worse outcomes that include delayed onset SI.26
There also is concern for the use of ketamine for chronic opioid users. The combination of ketamine with opioids may increase the response to the opioid in an otherwise drug tolerant patient, leading to risk of death by overdose in patients who have not increased their usual dose.27 However, this effect was noted only when ketamine and opioids were administered together, and the effect does not seem to last postinfusion.27
The challenges in treatment of TRD include finding an effective formulation—IV infusion of ketamine requires cardiovascular monitoring and is administered by anesthesiologists. The short duration of action for depression requires repeated infusions, and the frequency and quantity of infusions have not been determined. Efforts to find other NMDAR inhibitors (eg, memantine, nitrous oxide, D-cycloserine, and others) that match ketamine’s antidepressant efficacy but with easier delivery methods and fewer risks have thus far been unsuccessful.13 It is now believed that ketamine’s unique ability to activate intracellular signaling pathways linked to synaptic plasticity gives it the antidepressant function. Recent studies have further narrowed ketamine’s antidepressant function to the R- enantiomer of the ketamine metabolite, hydroxynorketamine.14 The nasal spray for ketamine is the S- enantiomer, which has better bioavailability but may have less antidepressant efficacy compared with the racemic mixture used in ketamine infusions.
Suicide Ideation Treatment
The many challenges faced by researchers and clinicians trying to develop ketamine treatment for TRD may not apply to the treatment of SI. Whereas repeated doses of ketamine cannot reliably produce sustained remission of depression, the few studies that have looked at the long-term effects of ketamine treatment on SI indicate the potential for long-term efficacy after a single IV infusion.21,22 Although treatment with IV infusions have additional costs and logistics, if it is found beneficial, it could be given in the emergency department (ED) prior to hospitalization and potentially lead to better outcomes.
In 2011, a small preliminary observational study of patients with depression and SI presenting to the ED indicated that SI was rapidly reduced following an infusion of ketamine.28 This study showed that both depressive symptoms and suicidality rapidly and significantly diminished within 40 minutes with no evidence of the recurrence of symptoms 10 days postadministration.
A more recent study used ketamine in a military field hospital to treat SI and also concluded that it could be effective and safe when administered in an ED setting. This preliminary study suggests that ketamine could be a safe and potentially effective medication for rapid reduction of depression and suicidality in a busy ED setting.29 These limited studies involving the use of ketamine in patients with SI show promise with long-term effectiveness. However, more research is needed to clarify whether the efficacy with SI will be similar to the clinical experience seen in TRD; a duration of effect limited to 2 weeks with recurrence after treatment discontinued.24
Conclusion
There has been a compelling accumulation of scientific data since 2000 to support the use of ketamine for the treatment of depression and SI. Ketamine use in patients with these diagnoses showed a rapid decrease of symptoms and minimal AEs among a significant number of patients.22,30
Although the initial findings involving the use of ketamine in suicidal patients are promising, the clinical use of ketamine needs further research, using larger sample sizes and exploring both the short-term and long-term effects of this medication. Researchers need to further establish the safe and effective route, point of care, and patient type that would best respond to this novel treatment. The initial evidence would suggest that health care providers have every right to be hopeful that ketamine will become the first pharmacologic treatment of acute SI in a majority of patients presenting to EDs, mental health clinics, community hospitals, and VA medical centers.
1. Curtin SC, Warner MA, Hedegaard H. Increase in suicide in the United States 199-2014. NCHS data brief, no. 241. https://www.cdc.gov/nchs/data/data -briefs/db241.pdf. Published April 2016. Accessed August 3, 2017.
2. Nock MK, Borges G, Bromet EJ, Cha CB, Kessler RC, Lee S. Suicide and suicidal behavior. Epidemiol Rev. 2008;30(1):133-154.
3. U.S. Department of Veteran Affairs Office of Suicide Prevention. Suicide among veterans and other Americans 2001-2014. https://www.mentalhealth .va.gov/docs/2016suicidedatareport.pdf Published August 3, 2016. Accessed August 11, 2017.
4. Wilkinson ST, Sanacora G. Ketamine: a potential rapid-acting antisuicidal agent? Depress Anxiety. 2016;33(8):711-717.
5. Aleman A, Denys D. Mental health: a road map for suicide research and prevention. Nature. 2014;509(7501):421-423.
6. Griffiths JJ, Zarate CA, Jr, Rasimas JJ. Existing and novel biological therapeutics in suicide prevention. Am J Prev Med. 2014;47(3)(suppl 2):S195-S203.
7. Centers for Disease Control and Prevention. Leading causes of death reports, 1981-2015. https://www.cdc.gov/injury/wisqars/leading_causes_death.html. Updated February 19, 2017. Accessed August 14, 2017.
8. Institute of Medicine of the National Academies; Board on Health Care Services; Committee on Comparative Effectiveness Research Prioritization. Initial National Priorities for Comparative Effectiveness Research. Washington, DC: The National Academies Press; 2009.
9. Weinberger MI, Sirey JA, Bruce ML, Heo M, Papademetriou E, Meyers BS. Predictors of major depression six months after admission for outpatient treatment. Psychiatr Serv. 2008;59(10):1211-1215.
10. Kellner CH, Fink M, Knapp R, et al. Relief of expressed suicidal intent by ECT: a consortium for research in ECT study. Am J Psychiatry. 2005;162(5):977-982.
11. Lewitzka U, Jabs B, Fülle M, et al. Does lithium reduce acute suicidal ideation and behavior? A protocol for a randomized, placebo-controlled multicenter trial of lithium plus treatment as usual (TAU) in patients with suicidal major depressive episode. BMC Psychiatry. 2015;15:117.
12. Vadivelu N, Schermer E, Kodumudi V, Belani K, Urman RD, Kaye AD. Role of ketamine for analgesia in adults and children. J Anaesthesiol Clin Pharmacol. 2016;32(3):298-306.
13. Newport DJ, Carpenter LL, McDonald WM, et al; APA Council of Research Task Force on Novel Biomarkers and Treatments. Ketamine and other NMDA antagonists: early clinical trials and possible mechanisms in depression. Am J Psychiatry. 2015;172(10):950-966.
14. Zanos P, Moaddel R, Morris PJ, et al. NMDAR inhibition-independent antidepressant actions of ketamine metabolites. Nature. 2016;533(7604):481-486.
15. Krupitsky EM, Grinenko AY. Ketamine psychedelic therapy (KPT): a review of the results of ten years of research. J Psychoactive Drugs. 1997;29(2):165-183.
16. Murrough JW, Collins KA, Fields J, et al. Regulation of neural responses to emotion perception by ketamine in individuals with treatment-resistant major depressive disorder. Transl Psychiatry. 2015;5:e509.
17. Berman RM, Cappiello A, Anand A, et al. Antidepressant effects of ketamine in depressed patients. Biol Psychiatry. 2000;47(4):351-354.
18. Murrough JW, Iosifescu DV, Chang LC, et al. Antidepressant efficacy of ketamine in treatment-resistant major depression: a two-site randomized controlled trial. Am J Psychiatry. 2013;170(10):1134-1142.
19. Zarate CA Jr, Singh JB, Carlson PJ, et al. A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Arch Gen Psychiatry. 2006;63(8):856-864.
20. Hu YD, Xiang YT, Fang JX, et al. Single i.v. ketamine augmentation of newly initiated escitalopram for major depression: results from a randomized, placebo-controlled 4-week study. Psychol Med. 2016;46(3):623-635.
21. Aligeti S, Quinones M, Salazar R. Rapid resolution of suicidal behavior and depression with single low-dose ketamine intravenous push even after 6 months of follow-up. J Clin Psychopharmacol. 2014;34(4):533-535.
22. Ballard ED, Ionescu DF, Vande Voort JL, et al. Improvement in suicidal ideation after ketamine infusion: relationship to reductions in depression and anxiety. J Psychiatr Res. 2014;58:161-166.
23. Henderson TA. Practical application of the neuroregenerative properties of ketamine: real world treatment experience. Neural Regen Res. 2016;11(2):195-200.
24. Newport DJ, Schatzberg AF, Nemeroff CB. Whither ketamine as an antidepressant: panacea or toxin? Depress Anxiety. 2016;33(8):685-688.
25. Sun L, Li Q, Li Q, et al. Chronic ketamine exposure induces permanent impairment of brain functions in adolescent cynomolgus monkeys. Addict Biol. 2014;19(2):185-194.
26. Niciu MJ, Grunschel BD, Corlett PR, Pittenger C, Bloch MH. Two cases of delayed-onset suicidal ideation, dysphoria and anxiety after ketamine infusion in patients with obsessive-compulsive disorder and a history of major depressive disorder. J Psychopharmacol. 2013;27(7):651-654.
27. Huxtable CA, Roberts LJ, Somogyi AA, MacIntyre PE. Acute pain management in opioid-tolerant patients: a growing challenge. Anaesth Intensive Care. 2011;39(5):804-823.
28. Larkin GL, Beautrais AL. A preliminary naturalistic study of low-dose ketamine for depression and suicide ideation in the emergency department. Int J Neuropsychopharmacol. 2011;14(8):1127-1131.
29. Burger J, Capobianco M, Lovem R, et al. A double-blinded, randomized, placebo-controlled sub-dissociative dose ketamine pilot study in the treatment of acute depression and suicidality in a military emergency department setting. Mil Med. 2016;181(10):1195-1199.
30. Wan LB, Levitch CF, Perez AM, et al. Ketamine safety and tolerability in clinical trials for treatment-resistant depression. J Clin Psychiatry. 2015;76(3):247-252.
In 2014 the suicide rate in the U.S. was 13/100,000, the highest recorded in 28 years.1 Suicide is now considered the 10th leading cause of death for all ages, and the rate has increased every year from 2000 to 2014 among both women and men and in every age group except those aged ≥ 75 years.1-3 For those aged 15 to 44 years, suicide is among the top 3 causes of death worldwide.4-6
Background
In 2013, more than 490,000 hospital visits related to suicide attempts were reported in the U.S.4 Health care expenditures related to suicide are estimated at $56.9 billion in combined medical and work loss costs annually and an unmeasurable cost to the affected families.7 The mental health care community is desperate for ways to address this epidemic, and the National Academies of Medicine (NAM) has declared that research that directly addresses comparative effectiveness of treatment strategies following a suicide attempt should be a national priority.8
The most recent reports from 2014 indicate that the suicide rates are higher for male veterans than for male nonveterans (32.1 vs 20.9 per 100,000, respectively) and are much higher for female veterans than for female nonveterans (28.7 vs 5.2 per 100,000, respectively).3 Suicide rates also may be associated with veteran-specific comorbidities, such as higher rates of depression, anxiety, posttraumatic stress disorder (PTSD), and war-related trauma.3 According to the VHA, the suicide rate for veterans aged > 30 years also is rapidly increasing, and VHA has echoed the calls from NAM to make suicide prevention research a national priority.3
The VA has tried to stem the tide of suicides in veterans by implementing many advances in suicide prevention, including hiring suicide prevention coordinators at every VA hospital, enhanced monitoring, and the availability of 24-hour crisis hotline services. Yet the suicide rates for veterans continue to rise and remain higher than the rates in the general population.3
About 90% of deaths by suicide are by persons who have a treatable psychiatric disorder, most commonly a mood disorder, such as depression.4 However, most studies show that antidepressant therapy does not provide rapid or significant relief of suicidal ideation (SI).4 Therefore, the current standard of care for the treatment of acutely suicidal patients includes a combination of hospitalization, cognitive behavioral therapy or psychotherapy, case management, antidepressant medications, and electroconvulsive therapy (ECT).4 Even though these therapies have become more widely available over the past decade, rates of suicide continue to increase.1,4 These interventions have limited effectiveness in acute settings. Although both intensive outpatient follow-up and routine outpatient care have been studied in relation to the decrease of suicidal behavior, neither intervention has been shown to immediately reduce suicidal behavior significantly in patients.
Suicidality Interventions
Therapy and case management require patients to be well enough to make office visits and follow through with care for periods as long as 1 year, which is often not possible for individuals with severe depression.5 One-third of patients who attended 6 months of outpatient therapy consistently still met the criteria for major depressive disorder (MDD), a major risk for suicide attempt.9 Antidepressant medications take a minimum of 4 weeks to reach full efficacy, and many patients stop taking the medications before that point because of concern that the medication is not helping or because of adverse effects (AEs), such as sleep disturbance, sexual dysfunction, or weight gain.9
Electroconvulsive therapy has been shown to be an effective treatment for patients with depression and suicidal behavior, but adherence with 12 weeks of recommended therapy has been a barrier for this intervention. Additionally, ECT may not provide reduction in SI for 1 to 2 weeks.4,10 A review of research studies showed that nearly 50% of patients with high-expressed SI did not complete the prescribed amount of ECT due to the length of time to complete the recommended 12 sessions.10 Therefore, current treatment barriers for suicidal patients include: (1) long periods in treatment for therapy, medication, and ECT before any relief of symptoms is noted; (2) high recidivism rates for MDD symptoms and risk of suicide following treatment; and (3) high treatment dropout rates.
Pharmacologic treatments currently used in suicidal patients have not fared much better. Many have received FDA approval for treatment of associated mental health diagnoses such as bipolar disorder, schizophrenia, or MDD, but there are no approved treatments that specifically target suicidal behavior. Lithium is approved for reducing the long-term risk of SI primarily because it reduces the risk of mood disorders associated with SI, but lithium has not been shown to be effective in acute settings.11 Clozapine is approved for reducing the long-term risk of recurrent suicide in patients with schizophrenia or schizoaffective disorder.4 Clozapine has not been shown to be effective in patients with mood disorders, which make up the majority of patients who attempt suicide.4 Additionally, both medications are plagued by the same barriers listed earlier, such as long time to effect (it takes an average 4 weeks to reach efficacy), lack of efficacy in acute settings, and AEs (eg, sleep problems, weight gain, and sexual dysfunction).9 Thus finding better pharmacologic interventions for suicidal patients is a priority for current research.
Ketamine
Recently, researchers have identified ketamine as a potential therapeutic option for depression and SI. A single ketamine infusion treatment has a rapid response, minimal AEs, and potentially long-lasting efficacy with SI, which would make it ideal for the treatment of acutely suicidal patients.4 Ketamine is an N-methyl-D-aspartate receptor (NMDAR) inhibitor that also has been found to be a weak μ- and κ-opioid receptor agonist and an inhibitor of the reuptake of serotonin, dopamine, and norepinephrine. Inhibition of the NMDAR results in analgesia, and ketamine is approved for the induction of anesthesia, pain relief, and sedation.12
Although AEs such as hallucinations and sedation create the potential for dangerous recreational use, ketamine is safely used in health care settings for a variety of indications. Effects are noted within 5 minutes of administration if given by infusion, and the main effects can last between 20 and 40 minutes.
Ketamine has a complex pharmacology and plays a role in other cell signaling mechanisms, but the significance of these additional mechanisms in the therapeutic effects of ketamine have only recently been elucidated. Preclinical studies indicate a probable NMDAR inhibition-independent mechanism responsible for the antidepressant response to ketamine.13,14 The complex associations with rapamycin signaling, eukaryotic elongation factor 2 dephosphorylation, increased synthesis of brain-derived neurotrophic factor, and activation of glutamatergic AMPA (α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid) receptors have been linked to its rapid antidepressant effect and ketamine’s induction of synaptogenesis within the limbic system.13,14
Clinical Research
Ketamine was studied as an adjunctive treatment to psychotherapy for addictions as far back as the 1970s.15 The available reports indicate a universally positive result, with increased rates of remission and decreased rates of relapse attributed to ketamine’s ability to alter one’s thought processes by reinforcing limbic-cortex interactions that facilitate the growth of more positive cognitive schemas and improved emotional attitudes about the self in support of the recovery process.15
Neurobiologic studies have shown that treatment with ketamine has a direct and immediate effect on neuronal pathways of the limbic system. It is known to regulate the mind’s reaction to positive stimuli by reversing the depressed subject’s blunted reaction to positive faces.16 This rapid normalization of the positive faces test is unique to ketamine infusion and is not seen in tests with traditional antidepressants.
In 2000, the first placebo-controlled trial using ketamine for treatment resistant depression (TRD) demonstrated the rapid antidepressant effects of a single dose of ketamine, but this study only looked at these effects for 1 week.17 In multiple double blind, placebo-controlled trials since then, IV infusion of ketamine was shown to be an effective intervention for TRD.13,18,19 More recently, a published investigation involving the treatment of MDD showed that ketamine in conjunction with a selective serotonin reuptake inhibitor (SSRI) accelerated and enhanced the effectiveness of the SSRI in reducing depressive symptoms.20
Based on the rapid resolution of depressive symptoms using ketamine, researchers have looked at its effect on suicidality as a secondary measure. A case study of a patient with severe depressive episodes and multiple previous suicidal attempts reported that the patient responded to a single dose of ketamine, described the experience as “being reborn,” and maintained complete remission of SI for the 6-month study period.21 In a larger study, 133 TRD patients received a single IV dose of ketamine with significant reductions in SI independent of depressive and anxiety symptoms.22
Depression Treatment
These results have led to an excitement for ketamine therapy as a novel treatment of depression, and off-label use by treatment centers now exists in several countries to aid those with TRD.23 This off-label use continues to be controversial, as research has yet to determine the safest most effective route and duration of treatment and whether the ketamine treatment AEs will exceed any accrued therapeutic benefit.13
The American Psychological Association Council of Research Task Force on Novel Biomarkers and Treatment critically examined the clinical evidence of ketamine use and has raised important concerns about the use of ketamine in the outpatient setting, administered in the absence of consensus therapeutic monitoring guidelines, and ambitiously marketed as a panacea for TRD.13,24 A study showed permanent impairment of brain function for both groups compared with monkeys treated with saline infusions.25 In 2016, the FDA gave fast-track approval for an intranasal ketamine that would make the treatment more easily available in the outpatient setting, but this could lead to certain patients developing a dependency on ketamine or engaging in its diversion for recreational use. There are case reports and anecdotes in the literature of patients and research subjects developing drug-seeking behaviors and overuse of ketamine.24 Additionally, the comorbidities associated with TRD and SI have not been fully evaluated. For instance, there is evidence that depressed patients with obsessive compulsive disorder may have worse outcomes that include delayed onset SI.26
There also is concern for the use of ketamine for chronic opioid users. The combination of ketamine with opioids may increase the response to the opioid in an otherwise drug tolerant patient, leading to risk of death by overdose in patients who have not increased their usual dose.27 However, this effect was noted only when ketamine and opioids were administered together, and the effect does not seem to last postinfusion.27
The challenges in treatment of TRD include finding an effective formulation—IV infusion of ketamine requires cardiovascular monitoring and is administered by anesthesiologists. The short duration of action for depression requires repeated infusions, and the frequency and quantity of infusions have not been determined. Efforts to find other NMDAR inhibitors (eg, memantine, nitrous oxide, D-cycloserine, and others) that match ketamine’s antidepressant efficacy but with easier delivery methods and fewer risks have thus far been unsuccessful.13 It is now believed that ketamine’s unique ability to activate intracellular signaling pathways linked to synaptic plasticity gives it the antidepressant function. Recent studies have further narrowed ketamine’s antidepressant function to the R- enantiomer of the ketamine metabolite, hydroxynorketamine.14 The nasal spray for ketamine is the S- enantiomer, which has better bioavailability but may have less antidepressant efficacy compared with the racemic mixture used in ketamine infusions.
Suicide Ideation Treatment
The many challenges faced by researchers and clinicians trying to develop ketamine treatment for TRD may not apply to the treatment of SI. Whereas repeated doses of ketamine cannot reliably produce sustained remission of depression, the few studies that have looked at the long-term effects of ketamine treatment on SI indicate the potential for long-term efficacy after a single IV infusion.21,22 Although treatment with IV infusions have additional costs and logistics, if it is found beneficial, it could be given in the emergency department (ED) prior to hospitalization and potentially lead to better outcomes.
In 2011, a small preliminary observational study of patients with depression and SI presenting to the ED indicated that SI was rapidly reduced following an infusion of ketamine.28 This study showed that both depressive symptoms and suicidality rapidly and significantly diminished within 40 minutes with no evidence of the recurrence of symptoms 10 days postadministration.
A more recent study used ketamine in a military field hospital to treat SI and also concluded that it could be effective and safe when administered in an ED setting. This preliminary study suggests that ketamine could be a safe and potentially effective medication for rapid reduction of depression and suicidality in a busy ED setting.29 These limited studies involving the use of ketamine in patients with SI show promise with long-term effectiveness. However, more research is needed to clarify whether the efficacy with SI will be similar to the clinical experience seen in TRD; a duration of effect limited to 2 weeks with recurrence after treatment discontinued.24
Conclusion
There has been a compelling accumulation of scientific data since 2000 to support the use of ketamine for the treatment of depression and SI. Ketamine use in patients with these diagnoses showed a rapid decrease of symptoms and minimal AEs among a significant number of patients.22,30
Although the initial findings involving the use of ketamine in suicidal patients are promising, the clinical use of ketamine needs further research, using larger sample sizes and exploring both the short-term and long-term effects of this medication. Researchers need to further establish the safe and effective route, point of care, and patient type that would best respond to this novel treatment. The initial evidence would suggest that health care providers have every right to be hopeful that ketamine will become the first pharmacologic treatment of acute SI in a majority of patients presenting to EDs, mental health clinics, community hospitals, and VA medical centers.
In 2014 the suicide rate in the U.S. was 13/100,000, the highest recorded in 28 years.1 Suicide is now considered the 10th leading cause of death for all ages, and the rate has increased every year from 2000 to 2014 among both women and men and in every age group except those aged ≥ 75 years.1-3 For those aged 15 to 44 years, suicide is among the top 3 causes of death worldwide.4-6
Background
In 2013, more than 490,000 hospital visits related to suicide attempts were reported in the U.S.4 Health care expenditures related to suicide are estimated at $56.9 billion in combined medical and work loss costs annually and an unmeasurable cost to the affected families.7 The mental health care community is desperate for ways to address this epidemic, and the National Academies of Medicine (NAM) has declared that research that directly addresses comparative effectiveness of treatment strategies following a suicide attempt should be a national priority.8
The most recent reports from 2014 indicate that the suicide rates are higher for male veterans than for male nonveterans (32.1 vs 20.9 per 100,000, respectively) and are much higher for female veterans than for female nonveterans (28.7 vs 5.2 per 100,000, respectively).3 Suicide rates also may be associated with veteran-specific comorbidities, such as higher rates of depression, anxiety, posttraumatic stress disorder (PTSD), and war-related trauma.3 According to the VHA, the suicide rate for veterans aged > 30 years also is rapidly increasing, and VHA has echoed the calls from NAM to make suicide prevention research a national priority.3
The VA has tried to stem the tide of suicides in veterans by implementing many advances in suicide prevention, including hiring suicide prevention coordinators at every VA hospital, enhanced monitoring, and the availability of 24-hour crisis hotline services. Yet the suicide rates for veterans continue to rise and remain higher than the rates in the general population.3
About 90% of deaths by suicide are by persons who have a treatable psychiatric disorder, most commonly a mood disorder, such as depression.4 However, most studies show that antidepressant therapy does not provide rapid or significant relief of suicidal ideation (SI).4 Therefore, the current standard of care for the treatment of acutely suicidal patients includes a combination of hospitalization, cognitive behavioral therapy or psychotherapy, case management, antidepressant medications, and electroconvulsive therapy (ECT).4 Even though these therapies have become more widely available over the past decade, rates of suicide continue to increase.1,4 These interventions have limited effectiveness in acute settings. Although both intensive outpatient follow-up and routine outpatient care have been studied in relation to the decrease of suicidal behavior, neither intervention has been shown to immediately reduce suicidal behavior significantly in patients.
Suicidality Interventions
Therapy and case management require patients to be well enough to make office visits and follow through with care for periods as long as 1 year, which is often not possible for individuals with severe depression.5 One-third of patients who attended 6 months of outpatient therapy consistently still met the criteria for major depressive disorder (MDD), a major risk for suicide attempt.9 Antidepressant medications take a minimum of 4 weeks to reach full efficacy, and many patients stop taking the medications before that point because of concern that the medication is not helping or because of adverse effects (AEs), such as sleep disturbance, sexual dysfunction, or weight gain.9
Electroconvulsive therapy has been shown to be an effective treatment for patients with depression and suicidal behavior, but adherence with 12 weeks of recommended therapy has been a barrier for this intervention. Additionally, ECT may not provide reduction in SI for 1 to 2 weeks.4,10 A review of research studies showed that nearly 50% of patients with high-expressed SI did not complete the prescribed amount of ECT due to the length of time to complete the recommended 12 sessions.10 Therefore, current treatment barriers for suicidal patients include: (1) long periods in treatment for therapy, medication, and ECT before any relief of symptoms is noted; (2) high recidivism rates for MDD symptoms and risk of suicide following treatment; and (3) high treatment dropout rates.
Pharmacologic treatments currently used in suicidal patients have not fared much better. Many have received FDA approval for treatment of associated mental health diagnoses such as bipolar disorder, schizophrenia, or MDD, but there are no approved treatments that specifically target suicidal behavior. Lithium is approved for reducing the long-term risk of SI primarily because it reduces the risk of mood disorders associated with SI, but lithium has not been shown to be effective in acute settings.11 Clozapine is approved for reducing the long-term risk of recurrent suicide in patients with schizophrenia or schizoaffective disorder.4 Clozapine has not been shown to be effective in patients with mood disorders, which make up the majority of patients who attempt suicide.4 Additionally, both medications are plagued by the same barriers listed earlier, such as long time to effect (it takes an average 4 weeks to reach efficacy), lack of efficacy in acute settings, and AEs (eg, sleep problems, weight gain, and sexual dysfunction).9 Thus finding better pharmacologic interventions for suicidal patients is a priority for current research.
Ketamine
Recently, researchers have identified ketamine as a potential therapeutic option for depression and SI. A single ketamine infusion treatment has a rapid response, minimal AEs, and potentially long-lasting efficacy with SI, which would make it ideal for the treatment of acutely suicidal patients.4 Ketamine is an N-methyl-D-aspartate receptor (NMDAR) inhibitor that also has been found to be a weak μ- and κ-opioid receptor agonist and an inhibitor of the reuptake of serotonin, dopamine, and norepinephrine. Inhibition of the NMDAR results in analgesia, and ketamine is approved for the induction of anesthesia, pain relief, and sedation.12
Although AEs such as hallucinations and sedation create the potential for dangerous recreational use, ketamine is safely used in health care settings for a variety of indications. Effects are noted within 5 minutes of administration if given by infusion, and the main effects can last between 20 and 40 minutes.
Ketamine has a complex pharmacology and plays a role in other cell signaling mechanisms, but the significance of these additional mechanisms in the therapeutic effects of ketamine have only recently been elucidated. Preclinical studies indicate a probable NMDAR inhibition-independent mechanism responsible for the antidepressant response to ketamine.13,14 The complex associations with rapamycin signaling, eukaryotic elongation factor 2 dephosphorylation, increased synthesis of brain-derived neurotrophic factor, and activation of glutamatergic AMPA (α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid) receptors have been linked to its rapid antidepressant effect and ketamine’s induction of synaptogenesis within the limbic system.13,14
Clinical Research
Ketamine was studied as an adjunctive treatment to psychotherapy for addictions as far back as the 1970s.15 The available reports indicate a universally positive result, with increased rates of remission and decreased rates of relapse attributed to ketamine’s ability to alter one’s thought processes by reinforcing limbic-cortex interactions that facilitate the growth of more positive cognitive schemas and improved emotional attitudes about the self in support of the recovery process.15
Neurobiologic studies have shown that treatment with ketamine has a direct and immediate effect on neuronal pathways of the limbic system. It is known to regulate the mind’s reaction to positive stimuli by reversing the depressed subject’s blunted reaction to positive faces.16 This rapid normalization of the positive faces test is unique to ketamine infusion and is not seen in tests with traditional antidepressants.
In 2000, the first placebo-controlled trial using ketamine for treatment resistant depression (TRD) demonstrated the rapid antidepressant effects of a single dose of ketamine, but this study only looked at these effects for 1 week.17 In multiple double blind, placebo-controlled trials since then, IV infusion of ketamine was shown to be an effective intervention for TRD.13,18,19 More recently, a published investigation involving the treatment of MDD showed that ketamine in conjunction with a selective serotonin reuptake inhibitor (SSRI) accelerated and enhanced the effectiveness of the SSRI in reducing depressive symptoms.20
Based on the rapid resolution of depressive symptoms using ketamine, researchers have looked at its effect on suicidality as a secondary measure. A case study of a patient with severe depressive episodes and multiple previous suicidal attempts reported that the patient responded to a single dose of ketamine, described the experience as “being reborn,” and maintained complete remission of SI for the 6-month study period.21 In a larger study, 133 TRD patients received a single IV dose of ketamine with significant reductions in SI independent of depressive and anxiety symptoms.22
Depression Treatment
These results have led to an excitement for ketamine therapy as a novel treatment of depression, and off-label use by treatment centers now exists in several countries to aid those with TRD.23 This off-label use continues to be controversial, as research has yet to determine the safest most effective route and duration of treatment and whether the ketamine treatment AEs will exceed any accrued therapeutic benefit.13
The American Psychological Association Council of Research Task Force on Novel Biomarkers and Treatment critically examined the clinical evidence of ketamine use and has raised important concerns about the use of ketamine in the outpatient setting, administered in the absence of consensus therapeutic monitoring guidelines, and ambitiously marketed as a panacea for TRD.13,24 A study showed permanent impairment of brain function for both groups compared with monkeys treated with saline infusions.25 In 2016, the FDA gave fast-track approval for an intranasal ketamine that would make the treatment more easily available in the outpatient setting, but this could lead to certain patients developing a dependency on ketamine or engaging in its diversion for recreational use. There are case reports and anecdotes in the literature of patients and research subjects developing drug-seeking behaviors and overuse of ketamine.24 Additionally, the comorbidities associated with TRD and SI have not been fully evaluated. For instance, there is evidence that depressed patients with obsessive compulsive disorder may have worse outcomes that include delayed onset SI.26
There also is concern for the use of ketamine for chronic opioid users. The combination of ketamine with opioids may increase the response to the opioid in an otherwise drug tolerant patient, leading to risk of death by overdose in patients who have not increased their usual dose.27 However, this effect was noted only when ketamine and opioids were administered together, and the effect does not seem to last postinfusion.27
The challenges in treatment of TRD include finding an effective formulation—IV infusion of ketamine requires cardiovascular monitoring and is administered by anesthesiologists. The short duration of action for depression requires repeated infusions, and the frequency and quantity of infusions have not been determined. Efforts to find other NMDAR inhibitors (eg, memantine, nitrous oxide, D-cycloserine, and others) that match ketamine’s antidepressant efficacy but with easier delivery methods and fewer risks have thus far been unsuccessful.13 It is now believed that ketamine’s unique ability to activate intracellular signaling pathways linked to synaptic plasticity gives it the antidepressant function. Recent studies have further narrowed ketamine’s antidepressant function to the R- enantiomer of the ketamine metabolite, hydroxynorketamine.14 The nasal spray for ketamine is the S- enantiomer, which has better bioavailability but may have less antidepressant efficacy compared with the racemic mixture used in ketamine infusions.
Suicide Ideation Treatment
The many challenges faced by researchers and clinicians trying to develop ketamine treatment for TRD may not apply to the treatment of SI. Whereas repeated doses of ketamine cannot reliably produce sustained remission of depression, the few studies that have looked at the long-term effects of ketamine treatment on SI indicate the potential for long-term efficacy after a single IV infusion.21,22 Although treatment with IV infusions have additional costs and logistics, if it is found beneficial, it could be given in the emergency department (ED) prior to hospitalization and potentially lead to better outcomes.
In 2011, a small preliminary observational study of patients with depression and SI presenting to the ED indicated that SI was rapidly reduced following an infusion of ketamine.28 This study showed that both depressive symptoms and suicidality rapidly and significantly diminished within 40 minutes with no evidence of the recurrence of symptoms 10 days postadministration.
A more recent study used ketamine in a military field hospital to treat SI and also concluded that it could be effective and safe when administered in an ED setting. This preliminary study suggests that ketamine could be a safe and potentially effective medication for rapid reduction of depression and suicidality in a busy ED setting.29 These limited studies involving the use of ketamine in patients with SI show promise with long-term effectiveness. However, more research is needed to clarify whether the efficacy with SI will be similar to the clinical experience seen in TRD; a duration of effect limited to 2 weeks with recurrence after treatment discontinued.24
Conclusion
There has been a compelling accumulation of scientific data since 2000 to support the use of ketamine for the treatment of depression and SI. Ketamine use in patients with these diagnoses showed a rapid decrease of symptoms and minimal AEs among a significant number of patients.22,30
Although the initial findings involving the use of ketamine in suicidal patients are promising, the clinical use of ketamine needs further research, using larger sample sizes and exploring both the short-term and long-term effects of this medication. Researchers need to further establish the safe and effective route, point of care, and patient type that would best respond to this novel treatment. The initial evidence would suggest that health care providers have every right to be hopeful that ketamine will become the first pharmacologic treatment of acute SI in a majority of patients presenting to EDs, mental health clinics, community hospitals, and VA medical centers.
1. Curtin SC, Warner MA, Hedegaard H. Increase in suicide in the United States 199-2014. NCHS data brief, no. 241. https://www.cdc.gov/nchs/data/data -briefs/db241.pdf. Published April 2016. Accessed August 3, 2017.
2. Nock MK, Borges G, Bromet EJ, Cha CB, Kessler RC, Lee S. Suicide and suicidal behavior. Epidemiol Rev. 2008;30(1):133-154.
3. U.S. Department of Veteran Affairs Office of Suicide Prevention. Suicide among veterans and other Americans 2001-2014. https://www.mentalhealth .va.gov/docs/2016suicidedatareport.pdf Published August 3, 2016. Accessed August 11, 2017.
4. Wilkinson ST, Sanacora G. Ketamine: a potential rapid-acting antisuicidal agent? Depress Anxiety. 2016;33(8):711-717.
5. Aleman A, Denys D. Mental health: a road map for suicide research and prevention. Nature. 2014;509(7501):421-423.
6. Griffiths JJ, Zarate CA, Jr, Rasimas JJ. Existing and novel biological therapeutics in suicide prevention. Am J Prev Med. 2014;47(3)(suppl 2):S195-S203.
7. Centers for Disease Control and Prevention. Leading causes of death reports, 1981-2015. https://www.cdc.gov/injury/wisqars/leading_causes_death.html. Updated February 19, 2017. Accessed August 14, 2017.
8. Institute of Medicine of the National Academies; Board on Health Care Services; Committee on Comparative Effectiveness Research Prioritization. Initial National Priorities for Comparative Effectiveness Research. Washington, DC: The National Academies Press; 2009.
9. Weinberger MI, Sirey JA, Bruce ML, Heo M, Papademetriou E, Meyers BS. Predictors of major depression six months after admission for outpatient treatment. Psychiatr Serv. 2008;59(10):1211-1215.
10. Kellner CH, Fink M, Knapp R, et al. Relief of expressed suicidal intent by ECT: a consortium for research in ECT study. Am J Psychiatry. 2005;162(5):977-982.
11. Lewitzka U, Jabs B, Fülle M, et al. Does lithium reduce acute suicidal ideation and behavior? A protocol for a randomized, placebo-controlled multicenter trial of lithium plus treatment as usual (TAU) in patients with suicidal major depressive episode. BMC Psychiatry. 2015;15:117.
12. Vadivelu N, Schermer E, Kodumudi V, Belani K, Urman RD, Kaye AD. Role of ketamine for analgesia in adults and children. J Anaesthesiol Clin Pharmacol. 2016;32(3):298-306.
13. Newport DJ, Carpenter LL, McDonald WM, et al; APA Council of Research Task Force on Novel Biomarkers and Treatments. Ketamine and other NMDA antagonists: early clinical trials and possible mechanisms in depression. Am J Psychiatry. 2015;172(10):950-966.
14. Zanos P, Moaddel R, Morris PJ, et al. NMDAR inhibition-independent antidepressant actions of ketamine metabolites. Nature. 2016;533(7604):481-486.
15. Krupitsky EM, Grinenko AY. Ketamine psychedelic therapy (KPT): a review of the results of ten years of research. J Psychoactive Drugs. 1997;29(2):165-183.
16. Murrough JW, Collins KA, Fields J, et al. Regulation of neural responses to emotion perception by ketamine in individuals with treatment-resistant major depressive disorder. Transl Psychiatry. 2015;5:e509.
17. Berman RM, Cappiello A, Anand A, et al. Antidepressant effects of ketamine in depressed patients. Biol Psychiatry. 2000;47(4):351-354.
18. Murrough JW, Iosifescu DV, Chang LC, et al. Antidepressant efficacy of ketamine in treatment-resistant major depression: a two-site randomized controlled trial. Am J Psychiatry. 2013;170(10):1134-1142.
19. Zarate CA Jr, Singh JB, Carlson PJ, et al. A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Arch Gen Psychiatry. 2006;63(8):856-864.
20. Hu YD, Xiang YT, Fang JX, et al. Single i.v. ketamine augmentation of newly initiated escitalopram for major depression: results from a randomized, placebo-controlled 4-week study. Psychol Med. 2016;46(3):623-635.
21. Aligeti S, Quinones M, Salazar R. Rapid resolution of suicidal behavior and depression with single low-dose ketamine intravenous push even after 6 months of follow-up. J Clin Psychopharmacol. 2014;34(4):533-535.
22. Ballard ED, Ionescu DF, Vande Voort JL, et al. Improvement in suicidal ideation after ketamine infusion: relationship to reductions in depression and anxiety. J Psychiatr Res. 2014;58:161-166.
23. Henderson TA. Practical application of the neuroregenerative properties of ketamine: real world treatment experience. Neural Regen Res. 2016;11(2):195-200.
24. Newport DJ, Schatzberg AF, Nemeroff CB. Whither ketamine as an antidepressant: panacea or toxin? Depress Anxiety. 2016;33(8):685-688.
25. Sun L, Li Q, Li Q, et al. Chronic ketamine exposure induces permanent impairment of brain functions in adolescent cynomolgus monkeys. Addict Biol. 2014;19(2):185-194.
26. Niciu MJ, Grunschel BD, Corlett PR, Pittenger C, Bloch MH. Two cases of delayed-onset suicidal ideation, dysphoria and anxiety after ketamine infusion in patients with obsessive-compulsive disorder and a history of major depressive disorder. J Psychopharmacol. 2013;27(7):651-654.
27. Huxtable CA, Roberts LJ, Somogyi AA, MacIntyre PE. Acute pain management in opioid-tolerant patients: a growing challenge. Anaesth Intensive Care. 2011;39(5):804-823.
28. Larkin GL, Beautrais AL. A preliminary naturalistic study of low-dose ketamine for depression and suicide ideation in the emergency department. Int J Neuropsychopharmacol. 2011;14(8):1127-1131.
29. Burger J, Capobianco M, Lovem R, et al. A double-blinded, randomized, placebo-controlled sub-dissociative dose ketamine pilot study in the treatment of acute depression and suicidality in a military emergency department setting. Mil Med. 2016;181(10):1195-1199.
30. Wan LB, Levitch CF, Perez AM, et al. Ketamine safety and tolerability in clinical trials for treatment-resistant depression. J Clin Psychiatry. 2015;76(3):247-252.
1. Curtin SC, Warner MA, Hedegaard H. Increase in suicide in the United States 199-2014. NCHS data brief, no. 241. https://www.cdc.gov/nchs/data/data -briefs/db241.pdf. Published April 2016. Accessed August 3, 2017.
2. Nock MK, Borges G, Bromet EJ, Cha CB, Kessler RC, Lee S. Suicide and suicidal behavior. Epidemiol Rev. 2008;30(1):133-154.
3. U.S. Department of Veteran Affairs Office of Suicide Prevention. Suicide among veterans and other Americans 2001-2014. https://www.mentalhealth .va.gov/docs/2016suicidedatareport.pdf Published August 3, 2016. Accessed August 11, 2017.
4. Wilkinson ST, Sanacora G. Ketamine: a potential rapid-acting antisuicidal agent? Depress Anxiety. 2016;33(8):711-717.
5. Aleman A, Denys D. Mental health: a road map for suicide research and prevention. Nature. 2014;509(7501):421-423.
6. Griffiths JJ, Zarate CA, Jr, Rasimas JJ. Existing and novel biological therapeutics in suicide prevention. Am J Prev Med. 2014;47(3)(suppl 2):S195-S203.
7. Centers for Disease Control and Prevention. Leading causes of death reports, 1981-2015. https://www.cdc.gov/injury/wisqars/leading_causes_death.html. Updated February 19, 2017. Accessed August 14, 2017.
8. Institute of Medicine of the National Academies; Board on Health Care Services; Committee on Comparative Effectiveness Research Prioritization. Initial National Priorities for Comparative Effectiveness Research. Washington, DC: The National Academies Press; 2009.
9. Weinberger MI, Sirey JA, Bruce ML, Heo M, Papademetriou E, Meyers BS. Predictors of major depression six months after admission for outpatient treatment. Psychiatr Serv. 2008;59(10):1211-1215.
10. Kellner CH, Fink M, Knapp R, et al. Relief of expressed suicidal intent by ECT: a consortium for research in ECT study. Am J Psychiatry. 2005;162(5):977-982.
11. Lewitzka U, Jabs B, Fülle M, et al. Does lithium reduce acute suicidal ideation and behavior? A protocol for a randomized, placebo-controlled multicenter trial of lithium plus treatment as usual (TAU) in patients with suicidal major depressive episode. BMC Psychiatry. 2015;15:117.
12. Vadivelu N, Schermer E, Kodumudi V, Belani K, Urman RD, Kaye AD. Role of ketamine for analgesia in adults and children. J Anaesthesiol Clin Pharmacol. 2016;32(3):298-306.
13. Newport DJ, Carpenter LL, McDonald WM, et al; APA Council of Research Task Force on Novel Biomarkers and Treatments. Ketamine and other NMDA antagonists: early clinical trials and possible mechanisms in depression. Am J Psychiatry. 2015;172(10):950-966.
14. Zanos P, Moaddel R, Morris PJ, et al. NMDAR inhibition-independent antidepressant actions of ketamine metabolites. Nature. 2016;533(7604):481-486.
15. Krupitsky EM, Grinenko AY. Ketamine psychedelic therapy (KPT): a review of the results of ten years of research. J Psychoactive Drugs. 1997;29(2):165-183.
16. Murrough JW, Collins KA, Fields J, et al. Regulation of neural responses to emotion perception by ketamine in individuals with treatment-resistant major depressive disorder. Transl Psychiatry. 2015;5:e509.
17. Berman RM, Cappiello A, Anand A, et al. Antidepressant effects of ketamine in depressed patients. Biol Psychiatry. 2000;47(4):351-354.
18. Murrough JW, Iosifescu DV, Chang LC, et al. Antidepressant efficacy of ketamine in treatment-resistant major depression: a two-site randomized controlled trial. Am J Psychiatry. 2013;170(10):1134-1142.
19. Zarate CA Jr, Singh JB, Carlson PJ, et al. A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Arch Gen Psychiatry. 2006;63(8):856-864.
20. Hu YD, Xiang YT, Fang JX, et al. Single i.v. ketamine augmentation of newly initiated escitalopram for major depression: results from a randomized, placebo-controlled 4-week study. Psychol Med. 2016;46(3):623-635.
21. Aligeti S, Quinones M, Salazar R. Rapid resolution of suicidal behavior and depression with single low-dose ketamine intravenous push even after 6 months of follow-up. J Clin Psychopharmacol. 2014;34(4):533-535.
22. Ballard ED, Ionescu DF, Vande Voort JL, et al. Improvement in suicidal ideation after ketamine infusion: relationship to reductions in depression and anxiety. J Psychiatr Res. 2014;58:161-166.
23. Henderson TA. Practical application of the neuroregenerative properties of ketamine: real world treatment experience. Neural Regen Res. 2016;11(2):195-200.
24. Newport DJ, Schatzberg AF, Nemeroff CB. Whither ketamine as an antidepressant: panacea or toxin? Depress Anxiety. 2016;33(8):685-688.
25. Sun L, Li Q, Li Q, et al. Chronic ketamine exposure induces permanent impairment of brain functions in adolescent cynomolgus monkeys. Addict Biol. 2014;19(2):185-194.
26. Niciu MJ, Grunschel BD, Corlett PR, Pittenger C, Bloch MH. Two cases of delayed-onset suicidal ideation, dysphoria and anxiety after ketamine infusion in patients with obsessive-compulsive disorder and a history of major depressive disorder. J Psychopharmacol. 2013;27(7):651-654.
27. Huxtable CA, Roberts LJ, Somogyi AA, MacIntyre PE. Acute pain management in opioid-tolerant patients: a growing challenge. Anaesth Intensive Care. 2011;39(5):804-823.
28. Larkin GL, Beautrais AL. A preliminary naturalistic study of low-dose ketamine for depression and suicide ideation in the emergency department. Int J Neuropsychopharmacol. 2011;14(8):1127-1131.
29. Burger J, Capobianco M, Lovem R, et al. A double-blinded, randomized, placebo-controlled sub-dissociative dose ketamine pilot study in the treatment of acute depression and suicidality in a military emergency department setting. Mil Med. 2016;181(10):1195-1199.
30. Wan LB, Levitch CF, Perez AM, et al. Ketamine safety and tolerability in clinical trials for treatment-resistant depression. J Clin Psychiatry. 2015;76(3):247-252.
The Search for Meaning After Surviving Cancer
Until now, research on meaning in cancer patients has focused mostly on patients with advanced cancer, who may be facing existential issues like the desire for hastened death. But as more people survive cancer, a sense of meaning is also an important issue for them, say researchers from VU University in Amsterdam. Those patients may be facing “fundamental uncertainties,” such as possible recurrence, long-term adverse effects of treatment, and physical, personal, and social losses. Helping them come to terms with those stressors can have benefits: higher psychological well-being, more successful adjustment, better quality of life.
Related: Social Interaction May Enhance Patient Survival After Chemotherapy
Noting the results of meaning-centered group psychotherapy (MCGP) for patients with advanced cancer, the researchers decided to compare MCGP with supportive group therapy (SGP) and usual care. Their study included 170 survivors who were diagnosed in the past 5 years, were treated with curative intent, and had completed their main treatment (surgery, radiotherapy, chemotherapy). Patients also had to have an expressed need for psychological care and at least 1 psychosocial condition, such as depressed mood, anxiety, or coping issues.
The researchers adapted the original MCGP intervention with different terminologies and topics more relevant for survivors (MCGP-CS [cancer survivors]). For instance, the topic “a good and meaningful death” was replaced by “carrying on in life despite limitations.” Topics included “The story of our life as a source of meaning: things we have done and want to do in the future.” The researchers also added mindfulness exercises to help patients with introspection.
Related: Women Living Longer With Metastatic Breast Cancer
The intervention consisted of 8 once-weekly sessions using didactics, group discussions, experimental exercises, and homework assignments. The SGP sessions, also 8 once-weekly meetings, did not pay specific attention to meaning. The psychotherapists leading the sessions, while maintaining an “unconditionally positive regard and empathetic understanding,” were trained to avoid group discussions on meaning-related topics. The primary outcome, measured before and after the intervention, then at 3 and 6 months, was personal meaning; secondary outcomes included psychological well-being, adjustment to cancer, optimism, and quality of life.
The researchers found “evidence for the efficacy of MCGP-CS to improve personal meaning among cancer survivors,” in both the short and longer terms. MCGP-CS participants scored significantly higher on goal-orientedness, psychological well-being, and adjustment to cancer. At 6 months, the intervention group also had lower scores for psychological distress and depressive symptoms.
Source:
van der Spek N, Vos J, van Uden-Kraan CF, et al. 2017;47(11):1990-2001.
doi: 10.1017/S0033291717000447.
Until now, research on meaning in cancer patients has focused mostly on patients with advanced cancer, who may be facing existential issues like the desire for hastened death. But as more people survive cancer, a sense of meaning is also an important issue for them, say researchers from VU University in Amsterdam. Those patients may be facing “fundamental uncertainties,” such as possible recurrence, long-term adverse effects of treatment, and physical, personal, and social losses. Helping them come to terms with those stressors can have benefits: higher psychological well-being, more successful adjustment, better quality of life.
Related: Social Interaction May Enhance Patient Survival After Chemotherapy
Noting the results of meaning-centered group psychotherapy (MCGP) for patients with advanced cancer, the researchers decided to compare MCGP with supportive group therapy (SGP) and usual care. Their study included 170 survivors who were diagnosed in the past 5 years, were treated with curative intent, and had completed their main treatment (surgery, radiotherapy, chemotherapy). Patients also had to have an expressed need for psychological care and at least 1 psychosocial condition, such as depressed mood, anxiety, or coping issues.
The researchers adapted the original MCGP intervention with different terminologies and topics more relevant for survivors (MCGP-CS [cancer survivors]). For instance, the topic “a good and meaningful death” was replaced by “carrying on in life despite limitations.” Topics included “The story of our life as a source of meaning: things we have done and want to do in the future.” The researchers also added mindfulness exercises to help patients with introspection.
Related: Women Living Longer With Metastatic Breast Cancer
The intervention consisted of 8 once-weekly sessions using didactics, group discussions, experimental exercises, and homework assignments. The SGP sessions, also 8 once-weekly meetings, did not pay specific attention to meaning. The psychotherapists leading the sessions, while maintaining an “unconditionally positive regard and empathetic understanding,” were trained to avoid group discussions on meaning-related topics. The primary outcome, measured before and after the intervention, then at 3 and 6 months, was personal meaning; secondary outcomes included psychological well-being, adjustment to cancer, optimism, and quality of life.
The researchers found “evidence for the efficacy of MCGP-CS to improve personal meaning among cancer survivors,” in both the short and longer terms. MCGP-CS participants scored significantly higher on goal-orientedness, psychological well-being, and adjustment to cancer. At 6 months, the intervention group also had lower scores for psychological distress and depressive symptoms.
Source:
van der Spek N, Vos J, van Uden-Kraan CF, et al. 2017;47(11):1990-2001.
doi: 10.1017/S0033291717000447.
Until now, research on meaning in cancer patients has focused mostly on patients with advanced cancer, who may be facing existential issues like the desire for hastened death. But as more people survive cancer, a sense of meaning is also an important issue for them, say researchers from VU University in Amsterdam. Those patients may be facing “fundamental uncertainties,” such as possible recurrence, long-term adverse effects of treatment, and physical, personal, and social losses. Helping them come to terms with those stressors can have benefits: higher psychological well-being, more successful adjustment, better quality of life.
Related: Social Interaction May Enhance Patient Survival After Chemotherapy
Noting the results of meaning-centered group psychotherapy (MCGP) for patients with advanced cancer, the researchers decided to compare MCGP with supportive group therapy (SGP) and usual care. Their study included 170 survivors who were diagnosed in the past 5 years, were treated with curative intent, and had completed their main treatment (surgery, radiotherapy, chemotherapy). Patients also had to have an expressed need for psychological care and at least 1 psychosocial condition, such as depressed mood, anxiety, or coping issues.
The researchers adapted the original MCGP intervention with different terminologies and topics more relevant for survivors (MCGP-CS [cancer survivors]). For instance, the topic “a good and meaningful death” was replaced by “carrying on in life despite limitations.” Topics included “The story of our life as a source of meaning: things we have done and want to do in the future.” The researchers also added mindfulness exercises to help patients with introspection.
Related: Women Living Longer With Metastatic Breast Cancer
The intervention consisted of 8 once-weekly sessions using didactics, group discussions, experimental exercises, and homework assignments. The SGP sessions, also 8 once-weekly meetings, did not pay specific attention to meaning. The psychotherapists leading the sessions, while maintaining an “unconditionally positive regard and empathetic understanding,” were trained to avoid group discussions on meaning-related topics. The primary outcome, measured before and after the intervention, then at 3 and 6 months, was personal meaning; secondary outcomes included psychological well-being, adjustment to cancer, optimism, and quality of life.
The researchers found “evidence for the efficacy of MCGP-CS to improve personal meaning among cancer survivors,” in both the short and longer terms. MCGP-CS participants scored significantly higher on goal-orientedness, psychological well-being, and adjustment to cancer. At 6 months, the intervention group also had lower scores for psychological distress and depressive symptoms.
Source:
van der Spek N, Vos J, van Uden-Kraan CF, et al. 2017;47(11):1990-2001.
doi: 10.1017/S0033291717000447.
Social Interaction May Enhance Patient Survival After Chemotherapy
Being with other patients during chemotherapy—rather than isolated—may have long-term effects after chemotherapy, according to researchers from the National Human Genome Research Institute and the University of Oxford in the United Kingdom.
“People model behavior based on what’s around them,” said Jeff Lienert, lead author. “For example, you will often eat more when you’re dining with friends, even if you can’t see what they’re eating.” The researchers wanted to find out whether similar social interaction would influence chemotherapy patients.
Related: Quality of Supportive Care for Patients With Advanced Lung Cancer in the VHA
The researchers used data on 4,691 cancer patients. As a proxy for social connection, the researchers gathered information on when patients checked in and out of the chemotherapy ward and how long they spent there, in “a small intimate space” where people could interact for a long period.
“Co-presence matters,” the researchers say. They found that when patients were around other patients who died in less than 5 years, they had a 72% chance of dying within 5 years. The best outcome, the researchers said, was when patients interacted with someone who survived for 5 or more years: Their risk of dying within 5 years dropped to 68%. “Being connected to a single survivor is similarly protective,” the researchers concluded, “as being connected to a single nonsurvivor is deleterious to patient survival.”
Because the study focused on “mere co-presence”—that is, just being together—their findings likely underestimate the influence of social forces, the researchers say. However, they note that “just being around others receiving treatment with similar stressors does not seem to impart any health effects”—suggesting that social facilitation and social support are not the underlying influence mechanism.
Related: Advances in Targeted Therapy for Breast Cancer
The study is the first to investigate, on a large scale, how social context in a treatment setting can play a significant role in disease outcomes. The researchers didn’t study why the difference in survival occurred, but they suggest that stress response may play a role. If a patient is unable to “fight or flee,” as in the situation of chemotherapy, Lienert says, the hormones can build. “Positive social support during the exact moments of greatest stress is crucial.”
Sources:
Lienert J, Marcum CS, Finney J, Reed-Tsochas F, Koehly L. Network Sci. 2017:1-20. https://www.cambridge.org/core/journals/network-science/article/social-influence-on-5year-survival-in-a-longitudinal-chemotherapy-ward-copresence-network/4E08D5F5A0D332AA5BB119310833A244. Accessed August 8, 2017.
National Institutes of Health. Social interaction affects cancer patients’ response to treatment. https://www.nih.gov/news-events/news-releases/social-interaction-affects-cancer-patients-response-treatment. Published July 19, 2017. Accessed August 8, 2017.
Being with other patients during chemotherapy—rather than isolated—may have long-term effects after chemotherapy, according to researchers from the National Human Genome Research Institute and the University of Oxford in the United Kingdom.
“People model behavior based on what’s around them,” said Jeff Lienert, lead author. “For example, you will often eat more when you’re dining with friends, even if you can’t see what they’re eating.” The researchers wanted to find out whether similar social interaction would influence chemotherapy patients.
Related: Quality of Supportive Care for Patients With Advanced Lung Cancer in the VHA
The researchers used data on 4,691 cancer patients. As a proxy for social connection, the researchers gathered information on when patients checked in and out of the chemotherapy ward and how long they spent there, in “a small intimate space” where people could interact for a long period.
“Co-presence matters,” the researchers say. They found that when patients were around other patients who died in less than 5 years, they had a 72% chance of dying within 5 years. The best outcome, the researchers said, was when patients interacted with someone who survived for 5 or more years: Their risk of dying within 5 years dropped to 68%. “Being connected to a single survivor is similarly protective,” the researchers concluded, “as being connected to a single nonsurvivor is deleterious to patient survival.”
Because the study focused on “mere co-presence”—that is, just being together—their findings likely underestimate the influence of social forces, the researchers say. However, they note that “just being around others receiving treatment with similar stressors does not seem to impart any health effects”—suggesting that social facilitation and social support are not the underlying influence mechanism.
Related: Advances in Targeted Therapy for Breast Cancer
The study is the first to investigate, on a large scale, how social context in a treatment setting can play a significant role in disease outcomes. The researchers didn’t study why the difference in survival occurred, but they suggest that stress response may play a role. If a patient is unable to “fight or flee,” as in the situation of chemotherapy, Lienert says, the hormones can build. “Positive social support during the exact moments of greatest stress is crucial.”
Sources:
Lienert J, Marcum CS, Finney J, Reed-Tsochas F, Koehly L. Network Sci. 2017:1-20. https://www.cambridge.org/core/journals/network-science/article/social-influence-on-5year-survival-in-a-longitudinal-chemotherapy-ward-copresence-network/4E08D5F5A0D332AA5BB119310833A244. Accessed August 8, 2017.
National Institutes of Health. Social interaction affects cancer patients’ response to treatment. https://www.nih.gov/news-events/news-releases/social-interaction-affects-cancer-patients-response-treatment. Published July 19, 2017. Accessed August 8, 2017.
Being with other patients during chemotherapy—rather than isolated—may have long-term effects after chemotherapy, according to researchers from the National Human Genome Research Institute and the University of Oxford in the United Kingdom.
“People model behavior based on what’s around them,” said Jeff Lienert, lead author. “For example, you will often eat more when you’re dining with friends, even if you can’t see what they’re eating.” The researchers wanted to find out whether similar social interaction would influence chemotherapy patients.
Related: Quality of Supportive Care for Patients With Advanced Lung Cancer in the VHA
The researchers used data on 4,691 cancer patients. As a proxy for social connection, the researchers gathered information on when patients checked in and out of the chemotherapy ward and how long they spent there, in “a small intimate space” where people could interact for a long period.
“Co-presence matters,” the researchers say. They found that when patients were around other patients who died in less than 5 years, they had a 72% chance of dying within 5 years. The best outcome, the researchers said, was when patients interacted with someone who survived for 5 or more years: Their risk of dying within 5 years dropped to 68%. “Being connected to a single survivor is similarly protective,” the researchers concluded, “as being connected to a single nonsurvivor is deleterious to patient survival.”
Because the study focused on “mere co-presence”—that is, just being together—their findings likely underestimate the influence of social forces, the researchers say. However, they note that “just being around others receiving treatment with similar stressors does not seem to impart any health effects”—suggesting that social facilitation and social support are not the underlying influence mechanism.
Related: Advances in Targeted Therapy for Breast Cancer
The study is the first to investigate, on a large scale, how social context in a treatment setting can play a significant role in disease outcomes. The researchers didn’t study why the difference in survival occurred, but they suggest that stress response may play a role. If a patient is unable to “fight or flee,” as in the situation of chemotherapy, Lienert says, the hormones can build. “Positive social support during the exact moments of greatest stress is crucial.”
Sources:
Lienert J, Marcum CS, Finney J, Reed-Tsochas F, Koehly L. Network Sci. 2017:1-20. https://www.cambridge.org/core/journals/network-science/article/social-influence-on-5year-survival-in-a-longitudinal-chemotherapy-ward-copresence-network/4E08D5F5A0D332AA5BB119310833A244. Accessed August 8, 2017.
National Institutes of Health. Social interaction affects cancer patients’ response to treatment. https://www.nih.gov/news-events/news-releases/social-interaction-affects-cancer-patients-response-treatment. Published July 19, 2017. Accessed August 8, 2017.
Working With Black Women to Tailor Weight-Loss Programs
For many black women, big is beautiful, and size is not only a cultural norm, but also an asset, say researchers from University of North Texas in Fort Worth. But African American women are nearly twice as likely to develop diabetes and more than twice as likely to develop end-stage kidney disease or die of complications of diabetes than are white women. They also have a higher prevalence of being overweight or obese.
Related: Genomic Variation May Reveal ‘Biological Pathway’ to Obesity
However, focusing on weight loss to reduce rates of chronic disease, disability, and premature death may be the wrong tack, the researchers say; weight-loss programs often don’t work well for black women. Even those programs that may have been tailored to the black woman through culturally appropriate artwork and language, African American media outlets, and “meaningful themes” (such as family and spirituality) tend not to produce sustainable results. Most programs aim for participants to lose 5% to 10% of body mass (or roughly 9 to 18 lb for a 185-lb woman) to reduce cardiovascular risk. On average, the researchers say, black women lose 4 to 10 lb—and they typically regain as much as 33% of the weight lost within the year.
The answer, the researchers say, is to not use body mass index (BMI) as a guide. Body mass index is a poor proxy, they say, for general health and health behaviors because it fails to account for differences in body composition, fitness levels, and nutritional differences that predict health and longevity. “BMI itself,” the researchers note, “as a measure of health is of limited value.” According to an analysis of National Health and Nutrition Examination Surveys data, using BMI to predict cardiometabolic risk misclassifies nearly 75 million U.S. adults.
Related: Some Patients With Diabetes Aren’t Getting Needed Weight Advice
The researchers suggest a collaborative approach may help: community-based participatory research. A key feature is bringing in the women as partners in research so that their subjective experiences inform the programs. The researchers cite a study in which the collaborative team ultimately “shifted its focus” from using weight loss alone as a metric to what they labeled as “a common-sense approach” more focused on physical activity and nutritional goals perceived by participants as relevant and valuable.
For many black women, big is beautiful, and size is not only a cultural norm, but also an asset, say researchers from University of North Texas in Fort Worth. But African American women are nearly twice as likely to develop diabetes and more than twice as likely to develop end-stage kidney disease or die of complications of diabetes than are white women. They also have a higher prevalence of being overweight or obese.
Related: Genomic Variation May Reveal ‘Biological Pathway’ to Obesity
However, focusing on weight loss to reduce rates of chronic disease, disability, and premature death may be the wrong tack, the researchers say; weight-loss programs often don’t work well for black women. Even those programs that may have been tailored to the black woman through culturally appropriate artwork and language, African American media outlets, and “meaningful themes” (such as family and spirituality) tend not to produce sustainable results. Most programs aim for participants to lose 5% to 10% of body mass (or roughly 9 to 18 lb for a 185-lb woman) to reduce cardiovascular risk. On average, the researchers say, black women lose 4 to 10 lb—and they typically regain as much as 33% of the weight lost within the year.
The answer, the researchers say, is to not use body mass index (BMI) as a guide. Body mass index is a poor proxy, they say, for general health and health behaviors because it fails to account for differences in body composition, fitness levels, and nutritional differences that predict health and longevity. “BMI itself,” the researchers note, “as a measure of health is of limited value.” According to an analysis of National Health and Nutrition Examination Surveys data, using BMI to predict cardiometabolic risk misclassifies nearly 75 million U.S. adults.
Related: Some Patients With Diabetes Aren’t Getting Needed Weight Advice
The researchers suggest a collaborative approach may help: community-based participatory research. A key feature is bringing in the women as partners in research so that their subjective experiences inform the programs. The researchers cite a study in which the collaborative team ultimately “shifted its focus” from using weight loss alone as a metric to what they labeled as “a common-sense approach” more focused on physical activity and nutritional goals perceived by participants as relevant and valuable.
For many black women, big is beautiful, and size is not only a cultural norm, but also an asset, say researchers from University of North Texas in Fort Worth. But African American women are nearly twice as likely to develop diabetes and more than twice as likely to develop end-stage kidney disease or die of complications of diabetes than are white women. They also have a higher prevalence of being overweight or obese.
Related: Genomic Variation May Reveal ‘Biological Pathway’ to Obesity
However, focusing on weight loss to reduce rates of chronic disease, disability, and premature death may be the wrong tack, the researchers say; weight-loss programs often don’t work well for black women. Even those programs that may have been tailored to the black woman through culturally appropriate artwork and language, African American media outlets, and “meaningful themes” (such as family and spirituality) tend not to produce sustainable results. Most programs aim for participants to lose 5% to 10% of body mass (or roughly 9 to 18 lb for a 185-lb woman) to reduce cardiovascular risk. On average, the researchers say, black women lose 4 to 10 lb—and they typically regain as much as 33% of the weight lost within the year.
The answer, the researchers say, is to not use body mass index (BMI) as a guide. Body mass index is a poor proxy, they say, for general health and health behaviors because it fails to account for differences in body composition, fitness levels, and nutritional differences that predict health and longevity. “BMI itself,” the researchers note, “as a measure of health is of limited value.” According to an analysis of National Health and Nutrition Examination Surveys data, using BMI to predict cardiometabolic risk misclassifies nearly 75 million U.S. adults.
Related: Some Patients With Diabetes Aren’t Getting Needed Weight Advice
The researchers suggest a collaborative approach may help: community-based participatory research. A key feature is bringing in the women as partners in research so that their subjective experiences inform the programs. The researchers cite a study in which the collaborative team ultimately “shifted its focus” from using weight loss alone as a metric to what they labeled as “a common-sense approach” more focused on physical activity and nutritional goals perceived by participants as relevant and valuable.