User login
Simulation Training, Coaching, and Cue Cards Improve Delirium Care
Many clinicians continue to think that delirium or acute confusion is inevitable, untreatable, and harmless. However, nothing could be further from the truth.1 Delirium is a common and costly clinical syndrome that plagues a large percentage of older adults in a variety of settings. Numerous clinical studies conducted over the past 20 years have validated a high incidence of delirium in acute care hospitals.2-5 Reported rates of delirium among veteran and nonveteran populations vary widely, from 20% to 80%, but even these rates may reflect underrecognition and underreporting.6
Veterans with delirium pose a unique challenge to clinicians and health care systems because they often concurrently experience dementia, depression, posttraumatic stress disorder, and delirium. This complex syndrome caused by a myriad of environmental, physiologic, and psychological factors has been associated with profoundly poor clinical outcomes, including increased institutionalization, hospital length of stay, medication use, restraint use, falls, and mortality.3,7,8
Financial costs associated with delirium have been estimated at between $38 billion and $152 billion per year.9 In addition, this syndrome is costly in human resource expenditures, including increased burden on family members and the need for additional care providers, such as “sitters.” Families and clinicians report increased burden and stress in their interactions with these patients.10-12 Mounting evidence exists that some people with delirium never return to baseline cognitive function after even a single episode of delirium.1,8 Unfortunately, many clinicians do not recognize the seriousness of acute confusion.
Clinical practices related to routine screening for delirium vary widely. Although the Confusion Assessment Method (CAM) screening tool has high sensitivity and specificity, only 17% of hospitals consistently use this tool in clinical practice.2,6,13,14 According to a survey by Ely and colleagues, physicians reported being aware of delirium but inconsistently applying treatment protocols in clinical practice.2 Nurses noted similar difficulties in consistently screening patients and using delirium management protocols.15 Given the high incidence of delirium and its associated morbidity, including long-term cognitive impairment and human and financial costs, there is an urgent need to implement programs that enhance delirium prevention, timely recognition, and effective management to improve patient outcomes and address caregiver burden.
Over the past decade, educational strategies for improving delirium prevention, recognition, and management have included didactic education, consultation, and use of protocols.2,3,5,16 Bedside mentoring, implementation of protocols, and other interventions have been proposed as well.16,17 Several program models, including consultation by psychiatrists or psychiatric advanced practice nurses, have been implemented to increase detection and treatment of delirium.2,3,5,15 These pilot programs have been successful to varying degrees but in general have not shown independent effects beyond intervention or significantly increased recognition and management of adverse consequences for most patients. The cause of these outcomes seem to be multifactorial, but the complexity of the syndrome is part of the problem.18-20
Other possible barriers to change regarding delirium-related issues in clinical practice are lack of knowledge and skills and individual attitudes.20-22 Continuing evidence exists that clinicians feel ill-prepared to help delirious patients and frustrated enough to resort to using restraints and medication as first-line treatment.17 Yanamadala and colleagues reviewed 26 studies that identified strategies for delirium education.23 Most of the studies reported on didactic teaching methods that included information on resources. Only 1 study with nursing students reported using actors for simulation training. The programs most successful in improving knowledge, skills, attitudes, practice changes, and patient outcomes seemed to be those that used multiple educational methods, including information dissemination, use of guidelines and protocols, and peer and expert feedback.
This finding is consistent with the report that didactic learning alone, though improving competency, is less likely to change behavior or improve outcomes.24 A constellation of didactic education, mentoring, use of protocols to target high-risk patients, and a therapeutic environment has helped to reduce delirium incidence.4,25,26 Rudolph and colleagues found an association between multimodal education (risk assessment, sensory improvement, sleep promotion) and shorter hospital stays and less use of restraints.27 In clinical practice,however, implementation of evidence-based nonpharmacologic interventions, such as enhanced communication, mobility, nutrition, and meaningful activities, continues to lag despite education.28,29
Multimodal Education
To address these gaps in knowledge and skills, a multidisciplinary delirium resource team at the Louis Stokes Cleveland VAMC in Ohio developed a multimodal educational program incorporating simulation. The team of physicians, nurses, care coordinators, and social workers met regularly and developed interventions, educational materials, cue cards (eFigure 1), sense-enhancing aids (hearing amplifiers, puzzles, books, music CDs, prism glasses), clinical protocols, and delirium resources, such as CARES (Confusion Assessment Resource Enhancement Supplies) activity carts.
The CARES carts are small, rolling wooden carts stocked with various resources that focus on comfort and entertainment. The carts hold guided imagery CDs and Playaways (small audio players that come with ear buds for individual use and preloaded with a specific guided imagery session). The carts also hold books, books on tape, magazines, portable CD players, music CDs, games, exercise bands, healthful snacks, DVDs, and a portable DVD player.
Bedside mentoring continued throughout this quality improvement (QI) project, and a CARES teaching tool kit was developed. This kit, which continues to be used, includes videos and webinars for professionals and family caregivers; delirium pocket cue cards for physicians, nurses, aides, and sitters; a list of patient diversion supplies; and a family brochure. Delirium resource team members continue to provide the health care team with education and support. Given the emphasis on clinicians and patient outcomes in intensive care units (ICUs), the teaching tool kit is a valuable guide for assessing and treating patients with delirium during rounds and consultations.
Simulation
Using a simulation center and standardized patients (SPs), teams of interdisciplinary care providers practiced communication techniques and recommended treatment strategies with the help of a delirium coach. Sessions were videotaped. This intervention, which used simulation training, was supported by VA grant T-21, to reduce institutionalization and promote patient-centered care.
In a clinical context, simulation involves activities that mimick the reality of the clinical environment, including physical symptoms, communication patterns, and critical decision making. Trained SPs have the unique advantage of providing interactive practice and immediate feedback in a safe, controlled setting.30
Standardized patient programs provide learners with real-life interactions for the development and practice of interpersonal communication and clinical skills. In a laboratory setting, SP programs use role-play scenarios that allow learners to practice complex assessment and communication skills. Standardized patients are effective in teaching clinical, interviewing, and communication skills to learners from a variety of disciplines, including medicine, nursing, dental, and law.31 Standardized patients also provide a safe, supportive environment conducive to learning and standardized assessment.
Standardized patients can serve as practice models and participate in sophisticated assessment and feedback of learners’ abilities and services. Interacting with SPs gives learners a chance to practice clinical and interpersonal skills with an emphasis on communication before meeting actual patients. After interacting with an SP, a learner receives feedback from a preceptor and/or the learner’s peers. The SP also may be asked to provide brief feedback—a component of the SP training process. Allowing time for feedback is an integral part of student learning.
For this QI project, the delirium team used the Mt. Sinai Skills and Simulation Center at Case Western Reserve University School of Medicine. The facility focuses on creative, innovative continuing learning for health care providers at all levels and is certified by the American College of Surgeons as a level 1 Comprehensive Accredited Education Institute.
After a literature review and several brainstorming sessions, the delirium team tailored case studies to veterans to simulate the intervention and train SPs for the delirium program. During training, SPs reviewed scenarios, engaged in practice sessions, and answered questions. Several SPs were familiar with the behavior of delirious patients from personal experience.
The goals of the program were to increase knowledge of delirium signs and symptoms as a medical condition that requires immediate attention; increase competency in administering CAM and in documenting its results; increase interdisciplinary communication; and increase knowledge using nonpharmacologic interventions for sensory enhancement and agitation. Enhanced interdisciplinary communication was accomplished during the simulation by assigning individuals from different disciplines to work in teams. To maximize the use of resources and limit participants’ time away from the clinical area, the administration planned and supported a daylong program that included didactic education, videos, group work sessions, and the simulation sessions with resource team members as coaches.
Methods
All participants attended an hour-long introductory didactic lecture together. Then, they were randomly assigned to 1 of 4 remaining 45-minute training sessions. Each participant attended a session that combined a video and a case study; a session of role-playing with group discussion; and 2 simulation scenario sessions. Concurrent training sessions were needed to facilitate having all 100 attendees participate within 6 hours. Attendees were multidisciplinary providers from various non-ICU medical/surgical units and outpatient geriatric clinics. They rotated among sessions to accommodate all participants.
For the simulation scenarios, 8 simulation rooms were used over 2 periods—for 16 simulation sessions total. Participants were randomly assigned to multidisciplinary groups that worked in teams to assess and recommend care and treatments for SPs who were stimulating delirium. During the simulation, delirium coaches used cue cards and verbal hints to direct teams (Simulation Exercise). After the session, participants received verbal and
Outcomes
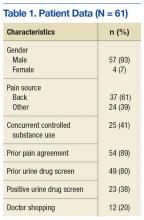
The impact of this multimodal intervention was measured in a variety of ways—with preintervention and postintervention knowledge tests, postsimulation surveys, program surveys, and patient chart reviews. Simulation sessions had 100 attendees, including mentors (interdisciplinary resource team), champions, and nursing staff from various hospital units. Champions represented multiple disciplines and had varying levels of experience. Most of the participants were nurses (62%), followed by social workers (12%), nursing assistants (12%), psychologists (6%), and others (11%). Participants’ years of experience were < 1 year (6%), 1 to 5 years (21%), 6 to 10 years (21%), and > 10 years (52%).
Mean knowledge survey score was 84% before training and 92% after training. Recognition of delirium as a medical emergency requiring immediate follow-up was increased (P = .02), as was knowledge about delirium management, as in increasing daytime activities (P < .001) and using distraction techniques (P = .03) (Table).
More than 94% of participants said the simulation training fulfilled their education needs. More than 80% reported using the information from the delirium workshop in their practice. In reviewing the techniques presented during the workshop, participants reported that they would approach situations differently from before the workshop by using more nonpharmacologic interventions (40%), enhanced communication (24%), and more in-depth assessment for medical causes of delirium (19%). Thirteen percent said they would not change their approach.
Thirty-five percent of respondents had positive feelings after the simulation exercise, 40% had cared for patients in similar situations, and 35% knew delirium care should start with assessment for medical causes.
The team reviewed patient charts for documentation of confusion assessment (signs and symptoms of confusion), including the standardized CAM method and nonpharmacologic interventions. Random monthly audits, 1 month before training and 5 months after, indicated an increase in confusion assessment and documentation. For veterans with delirium, nonpharmacologic interventions increased from 9% at baseline to 53% at the 5-month audit. Hospital length of stay, however, trended toward a slight increase in number of days. These findings are consistent with those reported by Rudolph and colleagues, who also piloted multimodal education and sensory enhancement.27
Discussion
Delirium assessment and management are complex skills that require well-coordinated interdisciplinary care and significant administrative support. Clinicians are becoming increasingly aware of the mounting evidence that patients with delirium feel immediate and often long-term negative effects. Strategies that support clinicians and enhance clinical care must include multimodal education and support.
In this QI project, participants supported use of simulation education, bedside coaching, and pocket cue cards to enhance delirium caregiving knowledge and skills. The majority of participants indicated that, though the simulation sessions were challenging, they also were realistic and helpful. Standardized patients provided feedback and often advised teams of needed improvement, such as spending more time in helping patients feel safe and comfortable. Coaches noted that many team members collaborated with one another but often neglected to use the pocket cue cards, family brochures, and other resources in the room. The reason is not clear. Perhaps the novelty of the resources and potential participant anxiety during the simulation were contributing factors. In future sessions, coaches must address making use of available resources.
Chart reviews indicated that nonpharmacologic management of delirium increased to 53% from < 10%. The increased use of resources in caring for patients with delirium was confirmed by the need to restock the CARES activity carts with patient diversion supplies. Given the success of this first program, another ICU education and simulation program was initiated. Findings from this QI project support using multimodal education that incorporates simulation training, bedside coaching, and pocket cue cards to enhance clinical practices for care of patients with delirium.33
Methods facilitated team collaboration, patient family communication, and synthesis of much information over a relatively short period. Didactic education alone may be insufficient to adequately enhance clinical care for delirium. The impact of a multimodal strategy—including a delirium resource consultation team that provided bedside mentoring, encouraged use of pocket cue cards, and supported evidence-based nonpharmacologic interventions—cannot be underestimated. In addition, simulation education also provided a unique opportunity for the health care teams to “practice” assessment, communication, and collaboration skills in a supportive setting with real-time feedback. These resources are being disseminated throughout the Louis Stokes Cleveland VAMC. Plans to disseminate this information to a broader national audience are under way.
Although not all facilities can access the simulation laboratory, many may be able to implement use of videos, case studies, and role-playing to enhance didactic education, to improve outcomes for patients with delirium. Enhanced clinical management of complex syndromes such as delirium may be influenced most by a combination of education, practice, and mentoring methods. Use of simulation as an adjunct teaching method is a promising strategy that may enhance care of patients with delirium. This QI project demonstrated positive educational and clinical trends in a VA setting. More studies, including randomized clinical trials, are needed in a variety of settings to further test these strategies.
Acknowledgments
The authors give special thanks to Brigid Wilson, PhD, Heather O’Leary, RN, and Patrick Kilroy, SN, for their assistance in this project. The project was developed by the Cleveland VAMC interdisciplinary delirium resource team with support of a VA T-21 grant and the VISN 10 Geriatric Research Education and Clinical Centers.
1. ICU Delirium and Cognitive Impairment Study Group, Center for Health Services Research, Vanderbilt University Medical Center. Delirium prevention and safety: starting with the ABCDEF’s. http://www.icudelirium.org/medicalprofessionals .html. Accessed November 9, 2016.
2. Ely EW, Siegel MD, Inouye SK. Delirium in the intensive care unit: an under-recognized syndrome of organ dysfunction. Semin Respir Crit Care Med. 2001;22(2):115-126.
3. Inouye SK, Bogardus ST Jr, Vitagliano G, et al. Burden of illness score for elderly persons: risk adjustment incorporating the cumulative impact of diseases, physiologic abnormalities, and functional impairments. Med Care. 2003;41(1):70-83.
4. Marcantonio ER, Flacker JM, Wright RJ, Resnick NM. Reducing delirium after hip fracture: a randomized trial. J Am Geriatr Soc. 2001;49(5):516-522.
5. Foreman MD, Wakefield B, Culp K, Milisen K. Delirium in elderly patients: an overview of the state of the science. J Gerontol Nurs. 2001;27(4):12-20.
6. Inouye SK, van Dyck CH, Alessi CA, Balkin S, Siegal AP, Horwitz RI. Clarifying confusion: the confusion assessment method. A new method for detection of delirium. Ann Intern Med. 1990;113(12):941-948.
7. Maldonado JR. Pathoetiological model of delirium: a comprehensive understanding of the neurobiology of delirium and an evidence-based approach to prevention and treatment. Crit Care Clin. 2008;24(4):789-856.
8. Witlox J, Eurelings LS, de Jonghe JF, Kalisvaart KJ, Eikelenboom P, van Gool WA. Delirium in elderly patients and the risk of postdischarge mortality, institutionalization, and dementia: a meta-analysis. JAMA. 2010;304(4):443-451.
9. Leslie DL, Marcantonio ER, Zhang Y, Leo-Summers L, Inouye SK. One-year health care costs associated with delirium in the elderly population. Arch Intern Med. 2008;168(1):27-32.
10. Bruera E, Bush SH, Willey J, et al. Impact of delirium and recall on the level of distress in patients with advanced cancer and their family caregivers. Cancer. 2009;115(9):2004-2012.
11. Cohen MZ, Pace EA, Kaur G, Bruera E. Delirium in advanced cancer leading to distress in patients and family caregivers. J Palliat Care. 2009;25(3):164-171.
12. Patel RP, Gambrell M, Speroff T, et al. Delirium and sedation in the intensive care unit: survey of behaviors and attitudes of 1384 healthcare professionals. Crit Care Med. 2009;37(3):825-832.
13. Wei LA, Fearing MA, Sternberg EJ, Inouye SK. The confusion assessment method: a systematic review of current usage. J Am Geriatr Soc. 2008;56(5):823-830.
14. Neuman MD, Speck RM, Karlawish JH, Schwartz JS, Shea JA. Hospital protocols for the inpatient care of older adults: results from a statewide survey. J Am Geriatr Soc. 2010;58(10):1959-1964.
15. Idemoto BK. The assessment of delirium and depression in the intensive care unit [doctoral dissertation]. Cleveland, OH: Case Western Reserve University; 2005. No. 137.
16. Balas MC, Vasilevskis EE, Burke WJ, et al. Critical care nurses’ role in implementing the “ABCDE bundle” into practice. Crit Care Nurse. 2012;32(2):35-38, 40-47.
17. Barr J, Fraser GL, Puntillo K, et al; American College of Critical Care Medicine. Clinical practice guidelines for the management of pain, agitation, and delirium in adult patients in the intensive care unit: executive summary. Am J Health Syst Pharm. 2013;70(1):53-58.
18. Pun BT, Ely EW. The importance of diagnosing and managing ICU delirium. Chest. 2007;132(2):624-636.
19. Pisani MA, Araujo KL, Van Ness PH, Zhang Y, Ely EW, Inouye SK. A research algorithm to improve detection of delirium in the intensive care unit. Crit Care. 2006;10(4):R121.
20. Voyer P, Cole MG, McCusker J, St-Jacques S, Laplante J. Accuracy of nurse documentation of delirium symptoms in medical charts. Int J Nurs Pract. 2008;14(2):165-167.
21. Pandharipande P, Cotton BA, Shintani A, et al. Prevalence and risk factors for development of delirium in surgical and trauma intensive care unit patients. J Trauma. 2008;65(1):34-41.
22. Steis MR, Fick DM. Are nurses recognizing delirium? A systematic review. J Gerontol Nurs. 2008;34(9):40-48.
23. Yanamadala M, Wieland D, Heflin MT. Educational interventions to improve recognition of delirium: a systematic review. J Am Geriatr Soc. 2013;61(11):1983-1993.
24. Ramaswamy R, Dix EF, Drew JE, Diamond JJ, Inouye SK, Roehl BJ. Beyond grand rounds: a comprehensive and sequential intervention to improve identification of delirium. Gerontologist. 2011;51(1):122-131.
25. Lundström M, Olofsson B, Stenvall M, et al. Postoperative delirium in old patients with femoral neck fracture: a randomized intervention study. Aging Clin Exp Res. 2007;19(3):178-186.
26. Inouye SK. Predisposing and precipitating factors for delirium in hospitalized older patients. Dement Geriatr Cogn Disord. 1999;10(5):393-400.
27. Rudolph JL, Archambault E, Kelly B; VA Boston Delirium Task Force. A delirium risk modification program is associated with hospital outcomes. J Am Med Dir Assoc. 2014;15(12):957.e7-e11.
28. Hartford Institute for Geriatric Nursing. http://hartfordign.org. Accessed November 9, 2016.
29. Inouye SK. A practical program for preventing delirium in hospitalized elderly patients. Cleve Clin J Med. 2004;71(11):890-896.
30. Raurell-Torredà M, Olivet-Pujol J, Romero-Collado À, Malagon-Aguilera MC, Patiño-Masó J, Baltasar-Bagué A. Case-based learning and simulation: useful tools to enhance nurses’ education? Nonrandomized controlled trial. J Nurs Scholarsh. 2015;47(1):34-42.
31. Mast TA, Coulson LR, Meyer TC, et al. Self directed learning: wisdom from independent study programs. Res Med Educ. 1985;24:305-311.
32. Massachusetts Department of Higher Education Nursing Initiative—Simulation Scenario Library. http://www.mass.edu/nahi/Sim/Welcome.asp. Accessed November 1, 2016.
33. Vancouver Island Health Authority. Delirium in the older person: a medical emergency. Pre-Post Test. http://www.viha.ca/NR/rdonlyres/03D50FE6-AF90 -4F98-8B3B-7AE87A4B481D/0/preposttest.pdf. Reviewed August 2014. Accessed November 9, 2016.
Many clinicians continue to think that delirium or acute confusion is inevitable, untreatable, and harmless. However, nothing could be further from the truth.1 Delirium is a common and costly clinical syndrome that plagues a large percentage of older adults in a variety of settings. Numerous clinical studies conducted over the past 20 years have validated a high incidence of delirium in acute care hospitals.2-5 Reported rates of delirium among veteran and nonveteran populations vary widely, from 20% to 80%, but even these rates may reflect underrecognition and underreporting.6
Veterans with delirium pose a unique challenge to clinicians and health care systems because they often concurrently experience dementia, depression, posttraumatic stress disorder, and delirium. This complex syndrome caused by a myriad of environmental, physiologic, and psychological factors has been associated with profoundly poor clinical outcomes, including increased institutionalization, hospital length of stay, medication use, restraint use, falls, and mortality.3,7,8
Financial costs associated with delirium have been estimated at between $38 billion and $152 billion per year.9 In addition, this syndrome is costly in human resource expenditures, including increased burden on family members and the need for additional care providers, such as “sitters.” Families and clinicians report increased burden and stress in their interactions with these patients.10-12 Mounting evidence exists that some people with delirium never return to baseline cognitive function after even a single episode of delirium.1,8 Unfortunately, many clinicians do not recognize the seriousness of acute confusion.
Clinical practices related to routine screening for delirium vary widely. Although the Confusion Assessment Method (CAM) screening tool has high sensitivity and specificity, only 17% of hospitals consistently use this tool in clinical practice.2,6,13,14 According to a survey by Ely and colleagues, physicians reported being aware of delirium but inconsistently applying treatment protocols in clinical practice.2 Nurses noted similar difficulties in consistently screening patients and using delirium management protocols.15 Given the high incidence of delirium and its associated morbidity, including long-term cognitive impairment and human and financial costs, there is an urgent need to implement programs that enhance delirium prevention, timely recognition, and effective management to improve patient outcomes and address caregiver burden.
Over the past decade, educational strategies for improving delirium prevention, recognition, and management have included didactic education, consultation, and use of protocols.2,3,5,16 Bedside mentoring, implementation of protocols, and other interventions have been proposed as well.16,17 Several program models, including consultation by psychiatrists or psychiatric advanced practice nurses, have been implemented to increase detection and treatment of delirium.2,3,5,15 These pilot programs have been successful to varying degrees but in general have not shown independent effects beyond intervention or significantly increased recognition and management of adverse consequences for most patients. The cause of these outcomes seem to be multifactorial, but the complexity of the syndrome is part of the problem.18-20
Other possible barriers to change regarding delirium-related issues in clinical practice are lack of knowledge and skills and individual attitudes.20-22 Continuing evidence exists that clinicians feel ill-prepared to help delirious patients and frustrated enough to resort to using restraints and medication as first-line treatment.17 Yanamadala and colleagues reviewed 26 studies that identified strategies for delirium education.23 Most of the studies reported on didactic teaching methods that included information on resources. Only 1 study with nursing students reported using actors for simulation training. The programs most successful in improving knowledge, skills, attitudes, practice changes, and patient outcomes seemed to be those that used multiple educational methods, including information dissemination, use of guidelines and protocols, and peer and expert feedback.
This finding is consistent with the report that didactic learning alone, though improving competency, is less likely to change behavior or improve outcomes.24 A constellation of didactic education, mentoring, use of protocols to target high-risk patients, and a therapeutic environment has helped to reduce delirium incidence.4,25,26 Rudolph and colleagues found an association between multimodal education (risk assessment, sensory improvement, sleep promotion) and shorter hospital stays and less use of restraints.27 In clinical practice,however, implementation of evidence-based nonpharmacologic interventions, such as enhanced communication, mobility, nutrition, and meaningful activities, continues to lag despite education.28,29
Multimodal Education
To address these gaps in knowledge and skills, a multidisciplinary delirium resource team at the Louis Stokes Cleveland VAMC in Ohio developed a multimodal educational program incorporating simulation. The team of physicians, nurses, care coordinators, and social workers met regularly and developed interventions, educational materials, cue cards (eFigure 1), sense-enhancing aids (hearing amplifiers, puzzles, books, music CDs, prism glasses), clinical protocols, and delirium resources, such as CARES (Confusion Assessment Resource Enhancement Supplies) activity carts.
The CARES carts are small, rolling wooden carts stocked with various resources that focus on comfort and entertainment. The carts hold guided imagery CDs and Playaways (small audio players that come with ear buds for individual use and preloaded with a specific guided imagery session). The carts also hold books, books on tape, magazines, portable CD players, music CDs, games, exercise bands, healthful snacks, DVDs, and a portable DVD player.
Bedside mentoring continued throughout this quality improvement (QI) project, and a CARES teaching tool kit was developed. This kit, which continues to be used, includes videos and webinars for professionals and family caregivers; delirium pocket cue cards for physicians, nurses, aides, and sitters; a list of patient diversion supplies; and a family brochure. Delirium resource team members continue to provide the health care team with education and support. Given the emphasis on clinicians and patient outcomes in intensive care units (ICUs), the teaching tool kit is a valuable guide for assessing and treating patients with delirium during rounds and consultations.
Simulation
Using a simulation center and standardized patients (SPs), teams of interdisciplinary care providers practiced communication techniques and recommended treatment strategies with the help of a delirium coach. Sessions were videotaped. This intervention, which used simulation training, was supported by VA grant T-21, to reduce institutionalization and promote patient-centered care.
In a clinical context, simulation involves activities that mimick the reality of the clinical environment, including physical symptoms, communication patterns, and critical decision making. Trained SPs have the unique advantage of providing interactive practice and immediate feedback in a safe, controlled setting.30
Standardized patient programs provide learners with real-life interactions for the development and practice of interpersonal communication and clinical skills. In a laboratory setting, SP programs use role-play scenarios that allow learners to practice complex assessment and communication skills. Standardized patients are effective in teaching clinical, interviewing, and communication skills to learners from a variety of disciplines, including medicine, nursing, dental, and law.31 Standardized patients also provide a safe, supportive environment conducive to learning and standardized assessment.
Standardized patients can serve as practice models and participate in sophisticated assessment and feedback of learners’ abilities and services. Interacting with SPs gives learners a chance to practice clinical and interpersonal skills with an emphasis on communication before meeting actual patients. After interacting with an SP, a learner receives feedback from a preceptor and/or the learner’s peers. The SP also may be asked to provide brief feedback—a component of the SP training process. Allowing time for feedback is an integral part of student learning.
For this QI project, the delirium team used the Mt. Sinai Skills and Simulation Center at Case Western Reserve University School of Medicine. The facility focuses on creative, innovative continuing learning for health care providers at all levels and is certified by the American College of Surgeons as a level 1 Comprehensive Accredited Education Institute.
After a literature review and several brainstorming sessions, the delirium team tailored case studies to veterans to simulate the intervention and train SPs for the delirium program. During training, SPs reviewed scenarios, engaged in practice sessions, and answered questions. Several SPs were familiar with the behavior of delirious patients from personal experience.
The goals of the program were to increase knowledge of delirium signs and symptoms as a medical condition that requires immediate attention; increase competency in administering CAM and in documenting its results; increase interdisciplinary communication; and increase knowledge using nonpharmacologic interventions for sensory enhancement and agitation. Enhanced interdisciplinary communication was accomplished during the simulation by assigning individuals from different disciplines to work in teams. To maximize the use of resources and limit participants’ time away from the clinical area, the administration planned and supported a daylong program that included didactic education, videos, group work sessions, and the simulation sessions with resource team members as coaches.
Methods
All participants attended an hour-long introductory didactic lecture together. Then, they were randomly assigned to 1 of 4 remaining 45-minute training sessions. Each participant attended a session that combined a video and a case study; a session of role-playing with group discussion; and 2 simulation scenario sessions. Concurrent training sessions were needed to facilitate having all 100 attendees participate within 6 hours. Attendees were multidisciplinary providers from various non-ICU medical/surgical units and outpatient geriatric clinics. They rotated among sessions to accommodate all participants.
For the simulation scenarios, 8 simulation rooms were used over 2 periods—for 16 simulation sessions total. Participants were randomly assigned to multidisciplinary groups that worked in teams to assess and recommend care and treatments for SPs who were stimulating delirium. During the simulation, delirium coaches used cue cards and verbal hints to direct teams (Simulation Exercise). After the session, participants received verbal and
Outcomes
The impact of this multimodal intervention was measured in a variety of ways—with preintervention and postintervention knowledge tests, postsimulation surveys, program surveys, and patient chart reviews. Simulation sessions had 100 attendees, including mentors (interdisciplinary resource team), champions, and nursing staff from various hospital units. Champions represented multiple disciplines and had varying levels of experience. Most of the participants were nurses (62%), followed by social workers (12%), nursing assistants (12%), psychologists (6%), and others (11%). Participants’ years of experience were < 1 year (6%), 1 to 5 years (21%), 6 to 10 years (21%), and > 10 years (52%).
Mean knowledge survey score was 84% before training and 92% after training. Recognition of delirium as a medical emergency requiring immediate follow-up was increased (P = .02), as was knowledge about delirium management, as in increasing daytime activities (P < .001) and using distraction techniques (P = .03) (Table).
More than 94% of participants said the simulation training fulfilled their education needs. More than 80% reported using the information from the delirium workshop in their practice. In reviewing the techniques presented during the workshop, participants reported that they would approach situations differently from before the workshop by using more nonpharmacologic interventions (40%), enhanced communication (24%), and more in-depth assessment for medical causes of delirium (19%). Thirteen percent said they would not change their approach.
Thirty-five percent of respondents had positive feelings after the simulation exercise, 40% had cared for patients in similar situations, and 35% knew delirium care should start with assessment for medical causes.
The team reviewed patient charts for documentation of confusion assessment (signs and symptoms of confusion), including the standardized CAM method and nonpharmacologic interventions. Random monthly audits, 1 month before training and 5 months after, indicated an increase in confusion assessment and documentation. For veterans with delirium, nonpharmacologic interventions increased from 9% at baseline to 53% at the 5-month audit. Hospital length of stay, however, trended toward a slight increase in number of days. These findings are consistent with those reported by Rudolph and colleagues, who also piloted multimodal education and sensory enhancement.27
Discussion
Delirium assessment and management are complex skills that require well-coordinated interdisciplinary care and significant administrative support. Clinicians are becoming increasingly aware of the mounting evidence that patients with delirium feel immediate and often long-term negative effects. Strategies that support clinicians and enhance clinical care must include multimodal education and support.
In this QI project, participants supported use of simulation education, bedside coaching, and pocket cue cards to enhance delirium caregiving knowledge and skills. The majority of participants indicated that, though the simulation sessions were challenging, they also were realistic and helpful. Standardized patients provided feedback and often advised teams of needed improvement, such as spending more time in helping patients feel safe and comfortable. Coaches noted that many team members collaborated with one another but often neglected to use the pocket cue cards, family brochures, and other resources in the room. The reason is not clear. Perhaps the novelty of the resources and potential participant anxiety during the simulation were contributing factors. In future sessions, coaches must address making use of available resources.
Chart reviews indicated that nonpharmacologic management of delirium increased to 53% from < 10%. The increased use of resources in caring for patients with delirium was confirmed by the need to restock the CARES activity carts with patient diversion supplies. Given the success of this first program, another ICU education and simulation program was initiated. Findings from this QI project support using multimodal education that incorporates simulation training, bedside coaching, and pocket cue cards to enhance clinical practices for care of patients with delirium.33
Methods facilitated team collaboration, patient family communication, and synthesis of much information over a relatively short period. Didactic education alone may be insufficient to adequately enhance clinical care for delirium. The impact of a multimodal strategy—including a delirium resource consultation team that provided bedside mentoring, encouraged use of pocket cue cards, and supported evidence-based nonpharmacologic interventions—cannot be underestimated. In addition, simulation education also provided a unique opportunity for the health care teams to “practice” assessment, communication, and collaboration skills in a supportive setting with real-time feedback. These resources are being disseminated throughout the Louis Stokes Cleveland VAMC. Plans to disseminate this information to a broader national audience are under way.
Although not all facilities can access the simulation laboratory, many may be able to implement use of videos, case studies, and role-playing to enhance didactic education, to improve outcomes for patients with delirium. Enhanced clinical management of complex syndromes such as delirium may be influenced most by a combination of education, practice, and mentoring methods. Use of simulation as an adjunct teaching method is a promising strategy that may enhance care of patients with delirium. This QI project demonstrated positive educational and clinical trends in a VA setting. More studies, including randomized clinical trials, are needed in a variety of settings to further test these strategies.
Acknowledgments
The authors give special thanks to Brigid Wilson, PhD, Heather O’Leary, RN, and Patrick Kilroy, SN, for their assistance in this project. The project was developed by the Cleveland VAMC interdisciplinary delirium resource team with support of a VA T-21 grant and the VISN 10 Geriatric Research Education and Clinical Centers.
Many clinicians continue to think that delirium or acute confusion is inevitable, untreatable, and harmless. However, nothing could be further from the truth.1 Delirium is a common and costly clinical syndrome that plagues a large percentage of older adults in a variety of settings. Numerous clinical studies conducted over the past 20 years have validated a high incidence of delirium in acute care hospitals.2-5 Reported rates of delirium among veteran and nonveteran populations vary widely, from 20% to 80%, but even these rates may reflect underrecognition and underreporting.6
Veterans with delirium pose a unique challenge to clinicians and health care systems because they often concurrently experience dementia, depression, posttraumatic stress disorder, and delirium. This complex syndrome caused by a myriad of environmental, physiologic, and psychological factors has been associated with profoundly poor clinical outcomes, including increased institutionalization, hospital length of stay, medication use, restraint use, falls, and mortality.3,7,8
Financial costs associated with delirium have been estimated at between $38 billion and $152 billion per year.9 In addition, this syndrome is costly in human resource expenditures, including increased burden on family members and the need for additional care providers, such as “sitters.” Families and clinicians report increased burden and stress in their interactions with these patients.10-12 Mounting evidence exists that some people with delirium never return to baseline cognitive function after even a single episode of delirium.1,8 Unfortunately, many clinicians do not recognize the seriousness of acute confusion.
Clinical practices related to routine screening for delirium vary widely. Although the Confusion Assessment Method (CAM) screening tool has high sensitivity and specificity, only 17% of hospitals consistently use this tool in clinical practice.2,6,13,14 According to a survey by Ely and colleagues, physicians reported being aware of delirium but inconsistently applying treatment protocols in clinical practice.2 Nurses noted similar difficulties in consistently screening patients and using delirium management protocols.15 Given the high incidence of delirium and its associated morbidity, including long-term cognitive impairment and human and financial costs, there is an urgent need to implement programs that enhance delirium prevention, timely recognition, and effective management to improve patient outcomes and address caregiver burden.
Over the past decade, educational strategies for improving delirium prevention, recognition, and management have included didactic education, consultation, and use of protocols.2,3,5,16 Bedside mentoring, implementation of protocols, and other interventions have been proposed as well.16,17 Several program models, including consultation by psychiatrists or psychiatric advanced practice nurses, have been implemented to increase detection and treatment of delirium.2,3,5,15 These pilot programs have been successful to varying degrees but in general have not shown independent effects beyond intervention or significantly increased recognition and management of adverse consequences for most patients. The cause of these outcomes seem to be multifactorial, but the complexity of the syndrome is part of the problem.18-20
Other possible barriers to change regarding delirium-related issues in clinical practice are lack of knowledge and skills and individual attitudes.20-22 Continuing evidence exists that clinicians feel ill-prepared to help delirious patients and frustrated enough to resort to using restraints and medication as first-line treatment.17 Yanamadala and colleagues reviewed 26 studies that identified strategies for delirium education.23 Most of the studies reported on didactic teaching methods that included information on resources. Only 1 study with nursing students reported using actors for simulation training. The programs most successful in improving knowledge, skills, attitudes, practice changes, and patient outcomes seemed to be those that used multiple educational methods, including information dissemination, use of guidelines and protocols, and peer and expert feedback.
This finding is consistent with the report that didactic learning alone, though improving competency, is less likely to change behavior or improve outcomes.24 A constellation of didactic education, mentoring, use of protocols to target high-risk patients, and a therapeutic environment has helped to reduce delirium incidence.4,25,26 Rudolph and colleagues found an association between multimodal education (risk assessment, sensory improvement, sleep promotion) and shorter hospital stays and less use of restraints.27 In clinical practice,however, implementation of evidence-based nonpharmacologic interventions, such as enhanced communication, mobility, nutrition, and meaningful activities, continues to lag despite education.28,29
Multimodal Education
To address these gaps in knowledge and skills, a multidisciplinary delirium resource team at the Louis Stokes Cleveland VAMC in Ohio developed a multimodal educational program incorporating simulation. The team of physicians, nurses, care coordinators, and social workers met regularly and developed interventions, educational materials, cue cards (eFigure 1), sense-enhancing aids (hearing amplifiers, puzzles, books, music CDs, prism glasses), clinical protocols, and delirium resources, such as CARES (Confusion Assessment Resource Enhancement Supplies) activity carts.
The CARES carts are small, rolling wooden carts stocked with various resources that focus on comfort and entertainment. The carts hold guided imagery CDs and Playaways (small audio players that come with ear buds for individual use and preloaded with a specific guided imagery session). The carts also hold books, books on tape, magazines, portable CD players, music CDs, games, exercise bands, healthful snacks, DVDs, and a portable DVD player.
Bedside mentoring continued throughout this quality improvement (QI) project, and a CARES teaching tool kit was developed. This kit, which continues to be used, includes videos and webinars for professionals and family caregivers; delirium pocket cue cards for physicians, nurses, aides, and sitters; a list of patient diversion supplies; and a family brochure. Delirium resource team members continue to provide the health care team with education and support. Given the emphasis on clinicians and patient outcomes in intensive care units (ICUs), the teaching tool kit is a valuable guide for assessing and treating patients with delirium during rounds and consultations.
Simulation
Using a simulation center and standardized patients (SPs), teams of interdisciplinary care providers practiced communication techniques and recommended treatment strategies with the help of a delirium coach. Sessions were videotaped. This intervention, which used simulation training, was supported by VA grant T-21, to reduce institutionalization and promote patient-centered care.
In a clinical context, simulation involves activities that mimick the reality of the clinical environment, including physical symptoms, communication patterns, and critical decision making. Trained SPs have the unique advantage of providing interactive practice and immediate feedback in a safe, controlled setting.30
Standardized patient programs provide learners with real-life interactions for the development and practice of interpersonal communication and clinical skills. In a laboratory setting, SP programs use role-play scenarios that allow learners to practice complex assessment and communication skills. Standardized patients are effective in teaching clinical, interviewing, and communication skills to learners from a variety of disciplines, including medicine, nursing, dental, and law.31 Standardized patients also provide a safe, supportive environment conducive to learning and standardized assessment.
Standardized patients can serve as practice models and participate in sophisticated assessment and feedback of learners’ abilities and services. Interacting with SPs gives learners a chance to practice clinical and interpersonal skills with an emphasis on communication before meeting actual patients. After interacting with an SP, a learner receives feedback from a preceptor and/or the learner’s peers. The SP also may be asked to provide brief feedback—a component of the SP training process. Allowing time for feedback is an integral part of student learning.
For this QI project, the delirium team used the Mt. Sinai Skills and Simulation Center at Case Western Reserve University School of Medicine. The facility focuses on creative, innovative continuing learning for health care providers at all levels and is certified by the American College of Surgeons as a level 1 Comprehensive Accredited Education Institute.
After a literature review and several brainstorming sessions, the delirium team tailored case studies to veterans to simulate the intervention and train SPs for the delirium program. During training, SPs reviewed scenarios, engaged in practice sessions, and answered questions. Several SPs were familiar with the behavior of delirious patients from personal experience.
The goals of the program were to increase knowledge of delirium signs and symptoms as a medical condition that requires immediate attention; increase competency in administering CAM and in documenting its results; increase interdisciplinary communication; and increase knowledge using nonpharmacologic interventions for sensory enhancement and agitation. Enhanced interdisciplinary communication was accomplished during the simulation by assigning individuals from different disciplines to work in teams. To maximize the use of resources and limit participants’ time away from the clinical area, the administration planned and supported a daylong program that included didactic education, videos, group work sessions, and the simulation sessions with resource team members as coaches.
Methods
All participants attended an hour-long introductory didactic lecture together. Then, they were randomly assigned to 1 of 4 remaining 45-minute training sessions. Each participant attended a session that combined a video and a case study; a session of role-playing with group discussion; and 2 simulation scenario sessions. Concurrent training sessions were needed to facilitate having all 100 attendees participate within 6 hours. Attendees were multidisciplinary providers from various non-ICU medical/surgical units and outpatient geriatric clinics. They rotated among sessions to accommodate all participants.
For the simulation scenarios, 8 simulation rooms were used over 2 periods—for 16 simulation sessions total. Participants were randomly assigned to multidisciplinary groups that worked in teams to assess and recommend care and treatments for SPs who were stimulating delirium. During the simulation, delirium coaches used cue cards and verbal hints to direct teams (Simulation Exercise). After the session, participants received verbal and
Outcomes
The impact of this multimodal intervention was measured in a variety of ways—with preintervention and postintervention knowledge tests, postsimulation surveys, program surveys, and patient chart reviews. Simulation sessions had 100 attendees, including mentors (interdisciplinary resource team), champions, and nursing staff from various hospital units. Champions represented multiple disciplines and had varying levels of experience. Most of the participants were nurses (62%), followed by social workers (12%), nursing assistants (12%), psychologists (6%), and others (11%). Participants’ years of experience were < 1 year (6%), 1 to 5 years (21%), 6 to 10 years (21%), and > 10 years (52%).
Mean knowledge survey score was 84% before training and 92% after training. Recognition of delirium as a medical emergency requiring immediate follow-up was increased (P = .02), as was knowledge about delirium management, as in increasing daytime activities (P < .001) and using distraction techniques (P = .03) (Table).
More than 94% of participants said the simulation training fulfilled their education needs. More than 80% reported using the information from the delirium workshop in their practice. In reviewing the techniques presented during the workshop, participants reported that they would approach situations differently from before the workshop by using more nonpharmacologic interventions (40%), enhanced communication (24%), and more in-depth assessment for medical causes of delirium (19%). Thirteen percent said they would not change their approach.
Thirty-five percent of respondents had positive feelings after the simulation exercise, 40% had cared for patients in similar situations, and 35% knew delirium care should start with assessment for medical causes.
The team reviewed patient charts for documentation of confusion assessment (signs and symptoms of confusion), including the standardized CAM method and nonpharmacologic interventions. Random monthly audits, 1 month before training and 5 months after, indicated an increase in confusion assessment and documentation. For veterans with delirium, nonpharmacologic interventions increased from 9% at baseline to 53% at the 5-month audit. Hospital length of stay, however, trended toward a slight increase in number of days. These findings are consistent with those reported by Rudolph and colleagues, who also piloted multimodal education and sensory enhancement.27
Discussion
Delirium assessment and management are complex skills that require well-coordinated interdisciplinary care and significant administrative support. Clinicians are becoming increasingly aware of the mounting evidence that patients with delirium feel immediate and often long-term negative effects. Strategies that support clinicians and enhance clinical care must include multimodal education and support.
In this QI project, participants supported use of simulation education, bedside coaching, and pocket cue cards to enhance delirium caregiving knowledge and skills. The majority of participants indicated that, though the simulation sessions were challenging, they also were realistic and helpful. Standardized patients provided feedback and often advised teams of needed improvement, such as spending more time in helping patients feel safe and comfortable. Coaches noted that many team members collaborated with one another but often neglected to use the pocket cue cards, family brochures, and other resources in the room. The reason is not clear. Perhaps the novelty of the resources and potential participant anxiety during the simulation were contributing factors. In future sessions, coaches must address making use of available resources.
Chart reviews indicated that nonpharmacologic management of delirium increased to 53% from < 10%. The increased use of resources in caring for patients with delirium was confirmed by the need to restock the CARES activity carts with patient diversion supplies. Given the success of this first program, another ICU education and simulation program was initiated. Findings from this QI project support using multimodal education that incorporates simulation training, bedside coaching, and pocket cue cards to enhance clinical practices for care of patients with delirium.33
Methods facilitated team collaboration, patient family communication, and synthesis of much information over a relatively short period. Didactic education alone may be insufficient to adequately enhance clinical care for delirium. The impact of a multimodal strategy—including a delirium resource consultation team that provided bedside mentoring, encouraged use of pocket cue cards, and supported evidence-based nonpharmacologic interventions—cannot be underestimated. In addition, simulation education also provided a unique opportunity for the health care teams to “practice” assessment, communication, and collaboration skills in a supportive setting with real-time feedback. These resources are being disseminated throughout the Louis Stokes Cleveland VAMC. Plans to disseminate this information to a broader national audience are under way.
Although not all facilities can access the simulation laboratory, many may be able to implement use of videos, case studies, and role-playing to enhance didactic education, to improve outcomes for patients with delirium. Enhanced clinical management of complex syndromes such as delirium may be influenced most by a combination of education, practice, and mentoring methods. Use of simulation as an adjunct teaching method is a promising strategy that may enhance care of patients with delirium. This QI project demonstrated positive educational and clinical trends in a VA setting. More studies, including randomized clinical trials, are needed in a variety of settings to further test these strategies.
Acknowledgments
The authors give special thanks to Brigid Wilson, PhD, Heather O’Leary, RN, and Patrick Kilroy, SN, for their assistance in this project. The project was developed by the Cleveland VAMC interdisciplinary delirium resource team with support of a VA T-21 grant and the VISN 10 Geriatric Research Education and Clinical Centers.
1. ICU Delirium and Cognitive Impairment Study Group, Center for Health Services Research, Vanderbilt University Medical Center. Delirium prevention and safety: starting with the ABCDEF’s. http://www.icudelirium.org/medicalprofessionals .html. Accessed November 9, 2016.
2. Ely EW, Siegel MD, Inouye SK. Delirium in the intensive care unit: an under-recognized syndrome of organ dysfunction. Semin Respir Crit Care Med. 2001;22(2):115-126.
3. Inouye SK, Bogardus ST Jr, Vitagliano G, et al. Burden of illness score for elderly persons: risk adjustment incorporating the cumulative impact of diseases, physiologic abnormalities, and functional impairments. Med Care. 2003;41(1):70-83.
4. Marcantonio ER, Flacker JM, Wright RJ, Resnick NM. Reducing delirium after hip fracture: a randomized trial. J Am Geriatr Soc. 2001;49(5):516-522.
5. Foreman MD, Wakefield B, Culp K, Milisen K. Delirium in elderly patients: an overview of the state of the science. J Gerontol Nurs. 2001;27(4):12-20.
6. Inouye SK, van Dyck CH, Alessi CA, Balkin S, Siegal AP, Horwitz RI. Clarifying confusion: the confusion assessment method. A new method for detection of delirium. Ann Intern Med. 1990;113(12):941-948.
7. Maldonado JR. Pathoetiological model of delirium: a comprehensive understanding of the neurobiology of delirium and an evidence-based approach to prevention and treatment. Crit Care Clin. 2008;24(4):789-856.
8. Witlox J, Eurelings LS, de Jonghe JF, Kalisvaart KJ, Eikelenboom P, van Gool WA. Delirium in elderly patients and the risk of postdischarge mortality, institutionalization, and dementia: a meta-analysis. JAMA. 2010;304(4):443-451.
9. Leslie DL, Marcantonio ER, Zhang Y, Leo-Summers L, Inouye SK. One-year health care costs associated with delirium in the elderly population. Arch Intern Med. 2008;168(1):27-32.
10. Bruera E, Bush SH, Willey J, et al. Impact of delirium and recall on the level of distress in patients with advanced cancer and their family caregivers. Cancer. 2009;115(9):2004-2012.
11. Cohen MZ, Pace EA, Kaur G, Bruera E. Delirium in advanced cancer leading to distress in patients and family caregivers. J Palliat Care. 2009;25(3):164-171.
12. Patel RP, Gambrell M, Speroff T, et al. Delirium and sedation in the intensive care unit: survey of behaviors and attitudes of 1384 healthcare professionals. Crit Care Med. 2009;37(3):825-832.
13. Wei LA, Fearing MA, Sternberg EJ, Inouye SK. The confusion assessment method: a systematic review of current usage. J Am Geriatr Soc. 2008;56(5):823-830.
14. Neuman MD, Speck RM, Karlawish JH, Schwartz JS, Shea JA. Hospital protocols for the inpatient care of older adults: results from a statewide survey. J Am Geriatr Soc. 2010;58(10):1959-1964.
15. Idemoto BK. The assessment of delirium and depression in the intensive care unit [doctoral dissertation]. Cleveland, OH: Case Western Reserve University; 2005. No. 137.
16. Balas MC, Vasilevskis EE, Burke WJ, et al. Critical care nurses’ role in implementing the “ABCDE bundle” into practice. Crit Care Nurse. 2012;32(2):35-38, 40-47.
17. Barr J, Fraser GL, Puntillo K, et al; American College of Critical Care Medicine. Clinical practice guidelines for the management of pain, agitation, and delirium in adult patients in the intensive care unit: executive summary. Am J Health Syst Pharm. 2013;70(1):53-58.
18. Pun BT, Ely EW. The importance of diagnosing and managing ICU delirium. Chest. 2007;132(2):624-636.
19. Pisani MA, Araujo KL, Van Ness PH, Zhang Y, Ely EW, Inouye SK. A research algorithm to improve detection of delirium in the intensive care unit. Crit Care. 2006;10(4):R121.
20. Voyer P, Cole MG, McCusker J, St-Jacques S, Laplante J. Accuracy of nurse documentation of delirium symptoms in medical charts. Int J Nurs Pract. 2008;14(2):165-167.
21. Pandharipande P, Cotton BA, Shintani A, et al. Prevalence and risk factors for development of delirium in surgical and trauma intensive care unit patients. J Trauma. 2008;65(1):34-41.
22. Steis MR, Fick DM. Are nurses recognizing delirium? A systematic review. J Gerontol Nurs. 2008;34(9):40-48.
23. Yanamadala M, Wieland D, Heflin MT. Educational interventions to improve recognition of delirium: a systematic review. J Am Geriatr Soc. 2013;61(11):1983-1993.
24. Ramaswamy R, Dix EF, Drew JE, Diamond JJ, Inouye SK, Roehl BJ. Beyond grand rounds: a comprehensive and sequential intervention to improve identification of delirium. Gerontologist. 2011;51(1):122-131.
25. Lundström M, Olofsson B, Stenvall M, et al. Postoperative delirium in old patients with femoral neck fracture: a randomized intervention study. Aging Clin Exp Res. 2007;19(3):178-186.
26. Inouye SK. Predisposing and precipitating factors for delirium in hospitalized older patients. Dement Geriatr Cogn Disord. 1999;10(5):393-400.
27. Rudolph JL, Archambault E, Kelly B; VA Boston Delirium Task Force. A delirium risk modification program is associated with hospital outcomes. J Am Med Dir Assoc. 2014;15(12):957.e7-e11.
28. Hartford Institute for Geriatric Nursing. http://hartfordign.org. Accessed November 9, 2016.
29. Inouye SK. A practical program for preventing delirium in hospitalized elderly patients. Cleve Clin J Med. 2004;71(11):890-896.
30. Raurell-Torredà M, Olivet-Pujol J, Romero-Collado À, Malagon-Aguilera MC, Patiño-Masó J, Baltasar-Bagué A. Case-based learning and simulation: useful tools to enhance nurses’ education? Nonrandomized controlled trial. J Nurs Scholarsh. 2015;47(1):34-42.
31. Mast TA, Coulson LR, Meyer TC, et al. Self directed learning: wisdom from independent study programs. Res Med Educ. 1985;24:305-311.
32. Massachusetts Department of Higher Education Nursing Initiative—Simulation Scenario Library. http://www.mass.edu/nahi/Sim/Welcome.asp. Accessed November 1, 2016.
33. Vancouver Island Health Authority. Delirium in the older person: a medical emergency. Pre-Post Test. http://www.viha.ca/NR/rdonlyres/03D50FE6-AF90 -4F98-8B3B-7AE87A4B481D/0/preposttest.pdf. Reviewed August 2014. Accessed November 9, 2016.
1. ICU Delirium and Cognitive Impairment Study Group, Center for Health Services Research, Vanderbilt University Medical Center. Delirium prevention and safety: starting with the ABCDEF’s. http://www.icudelirium.org/medicalprofessionals .html. Accessed November 9, 2016.
2. Ely EW, Siegel MD, Inouye SK. Delirium in the intensive care unit: an under-recognized syndrome of organ dysfunction. Semin Respir Crit Care Med. 2001;22(2):115-126.
3. Inouye SK, Bogardus ST Jr, Vitagliano G, et al. Burden of illness score for elderly persons: risk adjustment incorporating the cumulative impact of diseases, physiologic abnormalities, and functional impairments. Med Care. 2003;41(1):70-83.
4. Marcantonio ER, Flacker JM, Wright RJ, Resnick NM. Reducing delirium after hip fracture: a randomized trial. J Am Geriatr Soc. 2001;49(5):516-522.
5. Foreman MD, Wakefield B, Culp K, Milisen K. Delirium in elderly patients: an overview of the state of the science. J Gerontol Nurs. 2001;27(4):12-20.
6. Inouye SK, van Dyck CH, Alessi CA, Balkin S, Siegal AP, Horwitz RI. Clarifying confusion: the confusion assessment method. A new method for detection of delirium. Ann Intern Med. 1990;113(12):941-948.
7. Maldonado JR. Pathoetiological model of delirium: a comprehensive understanding of the neurobiology of delirium and an evidence-based approach to prevention and treatment. Crit Care Clin. 2008;24(4):789-856.
8. Witlox J, Eurelings LS, de Jonghe JF, Kalisvaart KJ, Eikelenboom P, van Gool WA. Delirium in elderly patients and the risk of postdischarge mortality, institutionalization, and dementia: a meta-analysis. JAMA. 2010;304(4):443-451.
9. Leslie DL, Marcantonio ER, Zhang Y, Leo-Summers L, Inouye SK. One-year health care costs associated with delirium in the elderly population. Arch Intern Med. 2008;168(1):27-32.
10. Bruera E, Bush SH, Willey J, et al. Impact of delirium and recall on the level of distress in patients with advanced cancer and their family caregivers. Cancer. 2009;115(9):2004-2012.
11. Cohen MZ, Pace EA, Kaur G, Bruera E. Delirium in advanced cancer leading to distress in patients and family caregivers. J Palliat Care. 2009;25(3):164-171.
12. Patel RP, Gambrell M, Speroff T, et al. Delirium and sedation in the intensive care unit: survey of behaviors and attitudes of 1384 healthcare professionals. Crit Care Med. 2009;37(3):825-832.
13. Wei LA, Fearing MA, Sternberg EJ, Inouye SK. The confusion assessment method: a systematic review of current usage. J Am Geriatr Soc. 2008;56(5):823-830.
14. Neuman MD, Speck RM, Karlawish JH, Schwartz JS, Shea JA. Hospital protocols for the inpatient care of older adults: results from a statewide survey. J Am Geriatr Soc. 2010;58(10):1959-1964.
15. Idemoto BK. The assessment of delirium and depression in the intensive care unit [doctoral dissertation]. Cleveland, OH: Case Western Reserve University; 2005. No. 137.
16. Balas MC, Vasilevskis EE, Burke WJ, et al. Critical care nurses’ role in implementing the “ABCDE bundle” into practice. Crit Care Nurse. 2012;32(2):35-38, 40-47.
17. Barr J, Fraser GL, Puntillo K, et al; American College of Critical Care Medicine. Clinical practice guidelines for the management of pain, agitation, and delirium in adult patients in the intensive care unit: executive summary. Am J Health Syst Pharm. 2013;70(1):53-58.
18. Pun BT, Ely EW. The importance of diagnosing and managing ICU delirium. Chest. 2007;132(2):624-636.
19. Pisani MA, Araujo KL, Van Ness PH, Zhang Y, Ely EW, Inouye SK. A research algorithm to improve detection of delirium in the intensive care unit. Crit Care. 2006;10(4):R121.
20. Voyer P, Cole MG, McCusker J, St-Jacques S, Laplante J. Accuracy of nurse documentation of delirium symptoms in medical charts. Int J Nurs Pract. 2008;14(2):165-167.
21. Pandharipande P, Cotton BA, Shintani A, et al. Prevalence and risk factors for development of delirium in surgical and trauma intensive care unit patients. J Trauma. 2008;65(1):34-41.
22. Steis MR, Fick DM. Are nurses recognizing delirium? A systematic review. J Gerontol Nurs. 2008;34(9):40-48.
23. Yanamadala M, Wieland D, Heflin MT. Educational interventions to improve recognition of delirium: a systematic review. J Am Geriatr Soc. 2013;61(11):1983-1993.
24. Ramaswamy R, Dix EF, Drew JE, Diamond JJ, Inouye SK, Roehl BJ. Beyond grand rounds: a comprehensive and sequential intervention to improve identification of delirium. Gerontologist. 2011;51(1):122-131.
25. Lundström M, Olofsson B, Stenvall M, et al. Postoperative delirium in old patients with femoral neck fracture: a randomized intervention study. Aging Clin Exp Res. 2007;19(3):178-186.
26. Inouye SK. Predisposing and precipitating factors for delirium in hospitalized older patients. Dement Geriatr Cogn Disord. 1999;10(5):393-400.
27. Rudolph JL, Archambault E, Kelly B; VA Boston Delirium Task Force. A delirium risk modification program is associated with hospital outcomes. J Am Med Dir Assoc. 2014;15(12):957.e7-e11.
28. Hartford Institute for Geriatric Nursing. http://hartfordign.org. Accessed November 9, 2016.
29. Inouye SK. A practical program for preventing delirium in hospitalized elderly patients. Cleve Clin J Med. 2004;71(11):890-896.
30. Raurell-Torredà M, Olivet-Pujol J, Romero-Collado À, Malagon-Aguilera MC, Patiño-Masó J, Baltasar-Bagué A. Case-based learning and simulation: useful tools to enhance nurses’ education? Nonrandomized controlled trial. J Nurs Scholarsh. 2015;47(1):34-42.
31. Mast TA, Coulson LR, Meyer TC, et al. Self directed learning: wisdom from independent study programs. Res Med Educ. 1985;24:305-311.
32. Massachusetts Department of Higher Education Nursing Initiative—Simulation Scenario Library. http://www.mass.edu/nahi/Sim/Welcome.asp. Accessed November 1, 2016.
33. Vancouver Island Health Authority. Delirium in the older person: a medical emergency. Pre-Post Test. http://www.viha.ca/NR/rdonlyres/03D50FE6-AF90 -4F98-8B3B-7AE87A4B481D/0/preposttest.pdf. Reviewed August 2014. Accessed November 9, 2016.
Hospitalists See Benefit from Working with ‘Surgicalists’
Time was critical. He needed surgery right away to remove his gallbladder. But for that, he needed a surgeon.
“There was a surgeon on call, but the surgeon was not picking up the phone,” Dr. Singh says. “I’m scratching my head. Why is the surgeon not calling back? Where is the surgeon? Did the pager get lost? What if the patient has a bad outcome?”
Eventually, Dr. Singh had to give up on the on-call surgeon, and the patient was flown to a hospital 45 miles away in downtown Sacramento. His surgery had been delayed for almost 12 hours.
The man lived largely due to good luck, Dr. Singh says. The unresponsive surgeon had disciplinary proceedings started against his license but retired rather than face the consequences.
Today, hospitalists at Sutter Amador no longer have to anxiously wait for those responses to emergency pages. It’s one of many hospitals that have turned to a “surgicalist” model, with a surgeon always on hand at the hospital. Surgicalists perform both emergency procedures and procedures that are tied to a hospital admission, without which a patient can’t be discharged. Although it is growing in popularity, the model is still only seen in a small fraction of hospitals.
The model is widely supported by hospitalists because it brings several advantages, mainly a greater availability of the surgeon for consult.
“We don’t have to hunt them down, trying to call their office, trying to see if they’re available to call back,” says Dr. Singh, who is now also the chair of medical staff performance at Sutter Amador and adds that the change has helped with his job satisfaction.
A Clear Delineation
Arrangements between hospitalists and surgicalists vary depending on the hospital, but there typically are clearly delineated criteria on who cares for whom, with the more urgent surgical cases tending to fall under the surgicalists’ care and those with less urgent problems, even though surgery might be involved, tending to go to hospitalists.
When a surgery-related question or the need for actual surgery arises, the model calls for a quick response time from the surgicalist. Hospitalists and surgicalists collaborate on ways to reduce length of stay and prevent readmissions since they share the same institutional goals. Hospitalists are also more in tune with the needs of the surgeons, for instance, not feeding a patient who is going to need quick surgery and not administering blood thinners when a surgery is imminent unless there’s an overriding reason not to do so.
One advantage of this collaboration is that a hospitalist working alongside a surgicalist can get extra surgery-related guidance even when surgery probably isn’t needed, says John Nelson, MD, MHM, a hospitalist at Overlake Medical Center in Bellevue, Wash., a hospitalist management consultant, and a past president of SHM.
“Maybe the opinion of a general surgeon could be useful, but maybe I can get along without it because the general surgeons are busy. It’s going to be hard for them to find time to see this patient, and they’re not going to be very interested in it,” he says. “But if instead I have a surgical hospitalist who’s there all day, it’s much less of a bother for them to come by and take a look at my patient.”
Remaining Challenges
The model is not without its hurdles. When surgicalists are on a 24-hour shift, the patients will see a new one each day, sometimes prompting them to ask, “Who’s my doctor?” Also, complex cases can pose a challenge as they move from one surgicalist to another day to day.
John Maa, MD, who wrote a seminal paper on surgicalists in 2007 based on an early surgicalist model he started at San Mateo Medical Center in California,1 says he is now concerned that the principles he helped make popular—the absorption of surgeons into a system as they work hand in hand with other hospital staff all the time—might be eroding. Some small staffing companies are calling themselves surgicalists, promising fast response times, but are actually locum tenens surgeons under a surgicalist guise, he says.
Properly rolled out, surgicalist programs mean a much better working relationship between hospitalists and surgeons, says Lynette Scherer, MD, FACS, chief medical officer at Surgical Affiliates Management Group in Sacramento. The company, founded in 1996, employs about 200 surgeons, twice as many as three years ago, Dr. Scherer says, but the company declined to share what that amounts to in full-time equivalent positions.
“The hospitalists know all of our algorithms, and they know when to call us,” Dr. Scherer says. “We share the patients on the inpatient side as we need to. We keep the ones that are appropriate for us, and they keep the ones that are appropriate for them.”
The details depend on the hospital, she says.
“Whenever we go to a new site, we sit down with the hospitalist team and say, ‘What do you need here?’ And our admitting grids are different based on what the different needs of the hospitals are.”
To stay on top of complex cases with very sick patients, the medical director rounds with the team nearly every day to help guide that care, Dr. Scherer says.
At Sutter Amador, the arrival of the surgicalist model has helped shorten the length of stay by almost one day for surgery admissions, Dr. Singh says.
Reported outcomes, however, seem to be mixed.
In 2008, Sutter Medical Center in Sacramento switched from a nine-surgeon call panel to four surgeons who covered the acute-care surgery service in 24-hour shifts. Researchers looked at outcomes from 2007, before the new model was adopted, and from the four subsequent years. The results were published in 2014 in the Journal of the American College of Surgeons.2
The total number of operations rose significantly, with 497 performed in 2007 and 640 in 2011. The percentage of cases with complications also fell significantly, from 21% in 2007 to 12% in 2011, with a low of 11% in 2010.
But the mortality rate rose significantly, from 1.4% in 2007 to 2.2% in 2011, with a high of 4.1% in 2008. The study authors note that the mortality rate ultimately fell back to levels not statistically significantly higher than the rate before the service. They suggested the spike could have been due to a greater willingness by the service to treat severely ill patients and due to the “immaturity” of the service in its earlier years. The percentage of cases with a readmission fell from 6.4% in 2007 to 4.7% in 2011, with a low of 3% in 2009, but that change wasn’t quite statistically significant.
“The data’s really bearing out that emergency patients are different in terms of the care they demand,” Dr. Scherer says. “So the patient with alcoholic cirrhosis who presents with a hole in his colon is very different than somebody who presents for an elective colon resection. And you can really reduce complications when you have a team of educated people taking care of these patients.”
Dr. Nelson says adopting the model “just means you’re a smoother operator and you can provide better service to people.” He adds that for any hospital that is getting poor surgical coverage and is paying for it, “it might make sense to consider it.”
Thomas R. Collins is a freelance medical writer based in Florida.
References
- Maa J, Carter JT, Gosnell JE, Wachter R, Harris HW. The surgical hospitalist: a new model for emergency surgical care. J Am Coll Surg. 2007;205(5):704-711.
- O’Mara MS, Scherer L, Wisner D, Owens LJ. Sustainability and success of the acute care surgery model in the nontrauma setting. J Am Coll Surg. 2014;219(1):90-98.
Time was critical. He needed surgery right away to remove his gallbladder. But for that, he needed a surgeon.
“There was a surgeon on call, but the surgeon was not picking up the phone,” Dr. Singh says. “I’m scratching my head. Why is the surgeon not calling back? Where is the surgeon? Did the pager get lost? What if the patient has a bad outcome?”
Eventually, Dr. Singh had to give up on the on-call surgeon, and the patient was flown to a hospital 45 miles away in downtown Sacramento. His surgery had been delayed for almost 12 hours.
The man lived largely due to good luck, Dr. Singh says. The unresponsive surgeon had disciplinary proceedings started against his license but retired rather than face the consequences.
Today, hospitalists at Sutter Amador no longer have to anxiously wait for those responses to emergency pages. It’s one of many hospitals that have turned to a “surgicalist” model, with a surgeon always on hand at the hospital. Surgicalists perform both emergency procedures and procedures that are tied to a hospital admission, without which a patient can’t be discharged. Although it is growing in popularity, the model is still only seen in a small fraction of hospitals.
The model is widely supported by hospitalists because it brings several advantages, mainly a greater availability of the surgeon for consult.
“We don’t have to hunt them down, trying to call their office, trying to see if they’re available to call back,” says Dr. Singh, who is now also the chair of medical staff performance at Sutter Amador and adds that the change has helped with his job satisfaction.
A Clear Delineation
Arrangements between hospitalists and surgicalists vary depending on the hospital, but there typically are clearly delineated criteria on who cares for whom, with the more urgent surgical cases tending to fall under the surgicalists’ care and those with less urgent problems, even though surgery might be involved, tending to go to hospitalists.
When a surgery-related question or the need for actual surgery arises, the model calls for a quick response time from the surgicalist. Hospitalists and surgicalists collaborate on ways to reduce length of stay and prevent readmissions since they share the same institutional goals. Hospitalists are also more in tune with the needs of the surgeons, for instance, not feeding a patient who is going to need quick surgery and not administering blood thinners when a surgery is imminent unless there’s an overriding reason not to do so.
One advantage of this collaboration is that a hospitalist working alongside a surgicalist can get extra surgery-related guidance even when surgery probably isn’t needed, says John Nelson, MD, MHM, a hospitalist at Overlake Medical Center in Bellevue, Wash., a hospitalist management consultant, and a past president of SHM.
“Maybe the opinion of a general surgeon could be useful, but maybe I can get along without it because the general surgeons are busy. It’s going to be hard for them to find time to see this patient, and they’re not going to be very interested in it,” he says. “But if instead I have a surgical hospitalist who’s there all day, it’s much less of a bother for them to come by and take a look at my patient.”
Remaining Challenges
The model is not without its hurdles. When surgicalists are on a 24-hour shift, the patients will see a new one each day, sometimes prompting them to ask, “Who’s my doctor?” Also, complex cases can pose a challenge as they move from one surgicalist to another day to day.
John Maa, MD, who wrote a seminal paper on surgicalists in 2007 based on an early surgicalist model he started at San Mateo Medical Center in California,1 says he is now concerned that the principles he helped make popular—the absorption of surgeons into a system as they work hand in hand with other hospital staff all the time—might be eroding. Some small staffing companies are calling themselves surgicalists, promising fast response times, but are actually locum tenens surgeons under a surgicalist guise, he says.
Properly rolled out, surgicalist programs mean a much better working relationship between hospitalists and surgeons, says Lynette Scherer, MD, FACS, chief medical officer at Surgical Affiliates Management Group in Sacramento. The company, founded in 1996, employs about 200 surgeons, twice as many as three years ago, Dr. Scherer says, but the company declined to share what that amounts to in full-time equivalent positions.
“The hospitalists know all of our algorithms, and they know when to call us,” Dr. Scherer says. “We share the patients on the inpatient side as we need to. We keep the ones that are appropriate for us, and they keep the ones that are appropriate for them.”
The details depend on the hospital, she says.
“Whenever we go to a new site, we sit down with the hospitalist team and say, ‘What do you need here?’ And our admitting grids are different based on what the different needs of the hospitals are.”
To stay on top of complex cases with very sick patients, the medical director rounds with the team nearly every day to help guide that care, Dr. Scherer says.
At Sutter Amador, the arrival of the surgicalist model has helped shorten the length of stay by almost one day for surgery admissions, Dr. Singh says.
Reported outcomes, however, seem to be mixed.
In 2008, Sutter Medical Center in Sacramento switched from a nine-surgeon call panel to four surgeons who covered the acute-care surgery service in 24-hour shifts. Researchers looked at outcomes from 2007, before the new model was adopted, and from the four subsequent years. The results were published in 2014 in the Journal of the American College of Surgeons.2
The total number of operations rose significantly, with 497 performed in 2007 and 640 in 2011. The percentage of cases with complications also fell significantly, from 21% in 2007 to 12% in 2011, with a low of 11% in 2010.
But the mortality rate rose significantly, from 1.4% in 2007 to 2.2% in 2011, with a high of 4.1% in 2008. The study authors note that the mortality rate ultimately fell back to levels not statistically significantly higher than the rate before the service. They suggested the spike could have been due to a greater willingness by the service to treat severely ill patients and due to the “immaturity” of the service in its earlier years. The percentage of cases with a readmission fell from 6.4% in 2007 to 4.7% in 2011, with a low of 3% in 2009, but that change wasn’t quite statistically significant.
“The data’s really bearing out that emergency patients are different in terms of the care they demand,” Dr. Scherer says. “So the patient with alcoholic cirrhosis who presents with a hole in his colon is very different than somebody who presents for an elective colon resection. And you can really reduce complications when you have a team of educated people taking care of these patients.”
Dr. Nelson says adopting the model “just means you’re a smoother operator and you can provide better service to people.” He adds that for any hospital that is getting poor surgical coverage and is paying for it, “it might make sense to consider it.”
Thomas R. Collins is a freelance medical writer based in Florida.
References
- Maa J, Carter JT, Gosnell JE, Wachter R, Harris HW. The surgical hospitalist: a new model for emergency surgical care. J Am Coll Surg. 2007;205(5):704-711.
- O’Mara MS, Scherer L, Wisner D, Owens LJ. Sustainability and success of the acute care surgery model in the nontrauma setting. J Am Coll Surg. 2014;219(1):90-98.
Time was critical. He needed surgery right away to remove his gallbladder. But for that, he needed a surgeon.
“There was a surgeon on call, but the surgeon was not picking up the phone,” Dr. Singh says. “I’m scratching my head. Why is the surgeon not calling back? Where is the surgeon? Did the pager get lost? What if the patient has a bad outcome?”
Eventually, Dr. Singh had to give up on the on-call surgeon, and the patient was flown to a hospital 45 miles away in downtown Sacramento. His surgery had been delayed for almost 12 hours.
The man lived largely due to good luck, Dr. Singh says. The unresponsive surgeon had disciplinary proceedings started against his license but retired rather than face the consequences.
Today, hospitalists at Sutter Amador no longer have to anxiously wait for those responses to emergency pages. It’s one of many hospitals that have turned to a “surgicalist” model, with a surgeon always on hand at the hospital. Surgicalists perform both emergency procedures and procedures that are tied to a hospital admission, without which a patient can’t be discharged. Although it is growing in popularity, the model is still only seen in a small fraction of hospitals.
The model is widely supported by hospitalists because it brings several advantages, mainly a greater availability of the surgeon for consult.
“We don’t have to hunt them down, trying to call their office, trying to see if they’re available to call back,” says Dr. Singh, who is now also the chair of medical staff performance at Sutter Amador and adds that the change has helped with his job satisfaction.
A Clear Delineation
Arrangements between hospitalists and surgicalists vary depending on the hospital, but there typically are clearly delineated criteria on who cares for whom, with the more urgent surgical cases tending to fall under the surgicalists’ care and those with less urgent problems, even though surgery might be involved, tending to go to hospitalists.
When a surgery-related question or the need for actual surgery arises, the model calls for a quick response time from the surgicalist. Hospitalists and surgicalists collaborate on ways to reduce length of stay and prevent readmissions since they share the same institutional goals. Hospitalists are also more in tune with the needs of the surgeons, for instance, not feeding a patient who is going to need quick surgery and not administering blood thinners when a surgery is imminent unless there’s an overriding reason not to do so.
One advantage of this collaboration is that a hospitalist working alongside a surgicalist can get extra surgery-related guidance even when surgery probably isn’t needed, says John Nelson, MD, MHM, a hospitalist at Overlake Medical Center in Bellevue, Wash., a hospitalist management consultant, and a past president of SHM.
“Maybe the opinion of a general surgeon could be useful, but maybe I can get along without it because the general surgeons are busy. It’s going to be hard for them to find time to see this patient, and they’re not going to be very interested in it,” he says. “But if instead I have a surgical hospitalist who’s there all day, it’s much less of a bother for them to come by and take a look at my patient.”
Remaining Challenges
The model is not without its hurdles. When surgicalists are on a 24-hour shift, the patients will see a new one each day, sometimes prompting them to ask, “Who’s my doctor?” Also, complex cases can pose a challenge as they move from one surgicalist to another day to day.
John Maa, MD, who wrote a seminal paper on surgicalists in 2007 based on an early surgicalist model he started at San Mateo Medical Center in California,1 says he is now concerned that the principles he helped make popular—the absorption of surgeons into a system as they work hand in hand with other hospital staff all the time—might be eroding. Some small staffing companies are calling themselves surgicalists, promising fast response times, but are actually locum tenens surgeons under a surgicalist guise, he says.
Properly rolled out, surgicalist programs mean a much better working relationship between hospitalists and surgeons, says Lynette Scherer, MD, FACS, chief medical officer at Surgical Affiliates Management Group in Sacramento. The company, founded in 1996, employs about 200 surgeons, twice as many as three years ago, Dr. Scherer says, but the company declined to share what that amounts to in full-time equivalent positions.
“The hospitalists know all of our algorithms, and they know when to call us,” Dr. Scherer says. “We share the patients on the inpatient side as we need to. We keep the ones that are appropriate for us, and they keep the ones that are appropriate for them.”
The details depend on the hospital, she says.
“Whenever we go to a new site, we sit down with the hospitalist team and say, ‘What do you need here?’ And our admitting grids are different based on what the different needs of the hospitals are.”
To stay on top of complex cases with very sick patients, the medical director rounds with the team nearly every day to help guide that care, Dr. Scherer says.
At Sutter Amador, the arrival of the surgicalist model has helped shorten the length of stay by almost one day for surgery admissions, Dr. Singh says.
Reported outcomes, however, seem to be mixed.
In 2008, Sutter Medical Center in Sacramento switched from a nine-surgeon call panel to four surgeons who covered the acute-care surgery service in 24-hour shifts. Researchers looked at outcomes from 2007, before the new model was adopted, and from the four subsequent years. The results were published in 2014 in the Journal of the American College of Surgeons.2
The total number of operations rose significantly, with 497 performed in 2007 and 640 in 2011. The percentage of cases with complications also fell significantly, from 21% in 2007 to 12% in 2011, with a low of 11% in 2010.
But the mortality rate rose significantly, from 1.4% in 2007 to 2.2% in 2011, with a high of 4.1% in 2008. The study authors note that the mortality rate ultimately fell back to levels not statistically significantly higher than the rate before the service. They suggested the spike could have been due to a greater willingness by the service to treat severely ill patients and due to the “immaturity” of the service in its earlier years. The percentage of cases with a readmission fell from 6.4% in 2007 to 4.7% in 2011, with a low of 3% in 2009, but that change wasn’t quite statistically significant.
“The data’s really bearing out that emergency patients are different in terms of the care they demand,” Dr. Scherer says. “So the patient with alcoholic cirrhosis who presents with a hole in his colon is very different than somebody who presents for an elective colon resection. And you can really reduce complications when you have a team of educated people taking care of these patients.”
Dr. Nelson says adopting the model “just means you’re a smoother operator and you can provide better service to people.” He adds that for any hospital that is getting poor surgical coverage and is paying for it, “it might make sense to consider it.”
Thomas R. Collins is a freelance medical writer based in Florida.
References
- Maa J, Carter JT, Gosnell JE, Wachter R, Harris HW. The surgical hospitalist: a new model for emergency surgical care. J Am Coll Surg. 2007;205(5):704-711.
- O’Mara MS, Scherer L, Wisner D, Owens LJ. Sustainability and success of the acute care surgery model in the nontrauma setting. J Am Coll Surg. 2014;219(1):90-98.
Updated ACCP Guideline for Antithrombotic Therapy for VTE Disease
Venous thromboembolism (VTE), which includes deep venous thrombosis (DVT) and pulmonary embolism (PE), continues to be a major cause of morbidity and mortality among hospitalized patients. Although it is well-known that anticoagulation therapy is effective in the prevention and treatment of VTE events, these agents are some of the highest-risk medications a hospitalist will prescribe given the danger of major bleeding. With the recent approval of several newer anticoagulants, it is important for the practicing hospitalist to be comfortable initiating, maintaining, and stopping these agents in a wide variety of patient populations.
Guideline Updates
In February 2016, an update to the ninth edition of the antithrombotic guideline from the American College of Chest Physician (ACCP) was published and included updated recommendations on 12 topics in addition to three new topics. This 10th-edition guideline update is referred to as AT10.1
One of the most notable changes in the updated guideline is the recommended choice of anticoagulant in patients with acute DVT or PE without cancer. Now, the direct oral anticoagulants (DOACs) dabigatran, rivaroxaban, apixaban, or edoxaban are recommended over warfarin. Although this is a weak recommendation based on moderate-quality evidence (grade 2B), this is the first time that warfarin is not considered first-line therapy. It should be emphasized that none of the four FDA-approved DOACs are preferred over another, and they should be avoided in patients who are pregnant or have severe renal disease. In patients with DVT or PE and cancer, low-molecular-weight heparin (LMWH) is still the preferred medication. If LMWH is not prescribed, AT10 does not have a preference for either a DOAC or warfarin for patients with cancer.
When it comes to duration of anticoagulation following a VTE event, the updated guideline continues to recommend three months for a provoked VTE event, with consideration for lifelong anticoagulation for an unprovoked event for patients at low or moderate bleeding risk. However, it now suggests that the recurrence risk factors of male sex and a positive D-dimer measured one month after stopping anticoagulant therapy should be taken into consideration when deciding whether extended anticoagulation is indicated.
AT10 also includes new recommendations concerning the role of aspirin for extended VTE treatment. Interestingly, the 2008 ACCP guideline gave a strong recommendation against the use of aspirin for VTE management in any patient population. In the 2012 guideline, the role of aspirin was not addressed for VTE treatment. Now, AT10 states that low-dose aspirin can be used in patients who stop anticoagulant therapy for treatment of an unprovoked proximal DVT or PE as an extended therapy (grade 2B). The significant change in this recommendation stems from two recent randomized trials that compared aspirin with placebo for the prevention of VTE recurrence in patients who have completed a course of anticoagulation for a first unprovoked proximal DVT or PE.2,3 Although the guideline doesn’t consider aspirin to be a reasonable alternative to anticoagulation for patients who require extended therapy and are agreeable to continue, for patients who have decided to stop anticoagulation, aspirin appears to reduce recurrent VTE by approximately one-third, with no significant increased risk of bleeding.
Another significant change in AT10 is the recommendation against the routine use of compression stockings to prevent postthrombotic syndrome (PTS). This change was influenced by a recent multicenter randomized trial showing that elastic compression stockings did not prevent PTS after an acute proximal DVT.4 The guideline authors remark that this recommendation focuses on the prevention of the chronic complications of PTS rather than treatment of the symptoms. Thus, for patients with acute or chronic leg pain or swelling from DVT, compression stockings may be justified.
A topic that was not addressed in the previous guideline was whether patients with a subsegmental PE should be treated. The guideline now suggests that patients with only subsegmental PE and no ultrasound-proven proximal DVT of the legs should undergo “clinical surveillance” rather than anticoagulation (grade 2C). Exceptions include patients at high risk for recurrent VTE (e.g., hospitalization, reduced mobility, active cancer, or irreversible VTE risk factors) and those with a low cardiopulmonary reserve or marked symptoms thought to be from PE. AT10 also states that patient preferences regarding anticoagulation treatment as well as the patient’s risk of bleeding should be taken into consideration. If the decision is made to not prescribe anticoagulation for subsegmental PE, patients should be advised to seek reevaluation if their symptoms persist or worsen.
The 2012 guideline included a new recommendation that patients with low-risk PE (typically defined by a low Pulmonary Embolism Severity Index [PESI] score) could be discharged “early” from the hospital. This recommendation has now been modified to state that patients with low-risk PE may be treated entirely at home. It is worth noting that outpatient management of low-risk PE has become much less complicated if using a DOAC, particularly rivaroxaban and apixaban as neither require initial treatment with parenteral anticoagulation.
AT10 has not changed the recommendation for which patients should receive thrombolytic therapy for treatment of PE. It recommends systemic thrombolytic therapy for patients with acute PE associated with hypotension (defined as systolic blood pressure less than 90 mmHg for 15 minutes) who are not at high risk for bleeding (grade 2B). Likewise, for patients with acute PE not associated with hypotension, the guideline recommends against systemic thrombolytics (grade 1B). If thrombolytics are implemented, AT10 favors systemic administration over catheter-directed thrombolysis (CDT) due to the higher-quality evidence available. However, the authors state that CDT may be preferred for patients at higher risk of bleeding and when local expertise is available. Lastly, catheter-assisted thrombus removal should be considered in patients with acute PE and hypotension who have a high bleeding risk, who have failed systemic thrombolytics, or who are in shock and likely to die before systemic thrombolytics become therapeutic.
Although no prospective trials have evaluated the management of patients with recurrent VTE events while on anticoagulation therapy, AT10 offers some guidance. After ensuring the patient truly had a recurrent VTE event while on therapeutic warfarin or compliant with a DOAC, the authors suggest switching to LMWH for at least one month (grade 2C). Furthermore, for patients who have a recurrent VTE event while compliant on long-term LMWH, the guideline suggests increasing the dose of LMWH by about one-quarter to one-third (grade 2C).
Guideline Analysis
It is important to note that of the 54 recommendations included in the complete guideline update, only 20 were strong recommendations (grade 1), and none were based on high-quality evidence (level A). It is obvious that more research is needed in this field. Regardless, the ACCP antithrombotic guideline remains the authoritative source in VTE management and has a strong influence on practice behavior. With the recent addition of several newer anticoagulants, AT10 is particularly useful in helping providers understand when and when not to use them. The authors indicate that future iterations will be continually updated, describing them as “living guidelines.” The format of AT10 was designed to facilitate this method with the goal of having discrete topics discussed as new evidence becomes available.
Hospital Medicine Takeaways
Despite the lack of randomized and prospective clinical trials, the updated recommendations from AT10 provide important information on challenging VTE issues that the hospitalist can apply to most patients most of the time. Important updates include:
- Prescribe DOACs as first-line agents for the treatment of acute VTE in patients without cancer.
- Use aspirin for the prevention of recurrent VTE in patients who stop anticoagulation for treatment of an unprovoked DVT or PE.
- Avoid compression stockings for the sole purpose of preventing postthrombotic syndrome.
- Do not admit patients with low-risk PE (as determined by the PESI score) to the hospital but rather treat them entirely at home.
Lastly, it is important to remember that VTE treatment decisions need to be individualized based on the clinical, imaging, and biochemical features of your patient.
Paul J. Grant, MD, SFHM, is assistant professor of medicine and director of perioperative and consultative medicine within the Department of Internal Medicine at the University of Michigan Health System in Ann Arbor.
References
- Kearon C, Akl EA, Ornelas J, et al. Antithrombotic therapy for VTE disease: CHEST guideline and expert panel report. Chest. 2016;149(2):315-352.
- Brighton TA, Eikelboom JW, Mann K, et al. Low-dose aspirin for preventing recurrent venous thromboembolism. N Engl J Med. 2012;367(21):1979-1987.
- Becattini C, Agnelli G, Schenone A, et al. Aspirin for preventing the recurrence of venous thromboembolism. N Engl J Med. 2012;366(21):1959-1967.
- Kahn SR, Shapiro S, Wells PS, et al. Compression stockings to prevent post-thrombotic syndrome: a randomised placebo controlled trial. Lancet. 2014;383(9920):880-888.
Venous thromboembolism (VTE), which includes deep venous thrombosis (DVT) and pulmonary embolism (PE), continues to be a major cause of morbidity and mortality among hospitalized patients. Although it is well-known that anticoagulation therapy is effective in the prevention and treatment of VTE events, these agents are some of the highest-risk medications a hospitalist will prescribe given the danger of major bleeding. With the recent approval of several newer anticoagulants, it is important for the practicing hospitalist to be comfortable initiating, maintaining, and stopping these agents in a wide variety of patient populations.
Guideline Updates
In February 2016, an update to the ninth edition of the antithrombotic guideline from the American College of Chest Physician (ACCP) was published and included updated recommendations on 12 topics in addition to three new topics. This 10th-edition guideline update is referred to as AT10.1
One of the most notable changes in the updated guideline is the recommended choice of anticoagulant in patients with acute DVT or PE without cancer. Now, the direct oral anticoagulants (DOACs) dabigatran, rivaroxaban, apixaban, or edoxaban are recommended over warfarin. Although this is a weak recommendation based on moderate-quality evidence (grade 2B), this is the first time that warfarin is not considered first-line therapy. It should be emphasized that none of the four FDA-approved DOACs are preferred over another, and they should be avoided in patients who are pregnant or have severe renal disease. In patients with DVT or PE and cancer, low-molecular-weight heparin (LMWH) is still the preferred medication. If LMWH is not prescribed, AT10 does not have a preference for either a DOAC or warfarin for patients with cancer.
When it comes to duration of anticoagulation following a VTE event, the updated guideline continues to recommend three months for a provoked VTE event, with consideration for lifelong anticoagulation for an unprovoked event for patients at low or moderate bleeding risk. However, it now suggests that the recurrence risk factors of male sex and a positive D-dimer measured one month after stopping anticoagulant therapy should be taken into consideration when deciding whether extended anticoagulation is indicated.
AT10 also includes new recommendations concerning the role of aspirin for extended VTE treatment. Interestingly, the 2008 ACCP guideline gave a strong recommendation against the use of aspirin for VTE management in any patient population. In the 2012 guideline, the role of aspirin was not addressed for VTE treatment. Now, AT10 states that low-dose aspirin can be used in patients who stop anticoagulant therapy for treatment of an unprovoked proximal DVT or PE as an extended therapy (grade 2B). The significant change in this recommendation stems from two recent randomized trials that compared aspirin with placebo for the prevention of VTE recurrence in patients who have completed a course of anticoagulation for a first unprovoked proximal DVT or PE.2,3 Although the guideline doesn’t consider aspirin to be a reasonable alternative to anticoagulation for patients who require extended therapy and are agreeable to continue, for patients who have decided to stop anticoagulation, aspirin appears to reduce recurrent VTE by approximately one-third, with no significant increased risk of bleeding.
Another significant change in AT10 is the recommendation against the routine use of compression stockings to prevent postthrombotic syndrome (PTS). This change was influenced by a recent multicenter randomized trial showing that elastic compression stockings did not prevent PTS after an acute proximal DVT.4 The guideline authors remark that this recommendation focuses on the prevention of the chronic complications of PTS rather than treatment of the symptoms. Thus, for patients with acute or chronic leg pain or swelling from DVT, compression stockings may be justified.
A topic that was not addressed in the previous guideline was whether patients with a subsegmental PE should be treated. The guideline now suggests that patients with only subsegmental PE and no ultrasound-proven proximal DVT of the legs should undergo “clinical surveillance” rather than anticoagulation (grade 2C). Exceptions include patients at high risk for recurrent VTE (e.g., hospitalization, reduced mobility, active cancer, or irreversible VTE risk factors) and those with a low cardiopulmonary reserve or marked symptoms thought to be from PE. AT10 also states that patient preferences regarding anticoagulation treatment as well as the patient’s risk of bleeding should be taken into consideration. If the decision is made to not prescribe anticoagulation for subsegmental PE, patients should be advised to seek reevaluation if their symptoms persist or worsen.
The 2012 guideline included a new recommendation that patients with low-risk PE (typically defined by a low Pulmonary Embolism Severity Index [PESI] score) could be discharged “early” from the hospital. This recommendation has now been modified to state that patients with low-risk PE may be treated entirely at home. It is worth noting that outpatient management of low-risk PE has become much less complicated if using a DOAC, particularly rivaroxaban and apixaban as neither require initial treatment with parenteral anticoagulation.
AT10 has not changed the recommendation for which patients should receive thrombolytic therapy for treatment of PE. It recommends systemic thrombolytic therapy for patients with acute PE associated with hypotension (defined as systolic blood pressure less than 90 mmHg for 15 minutes) who are not at high risk for bleeding (grade 2B). Likewise, for patients with acute PE not associated with hypotension, the guideline recommends against systemic thrombolytics (grade 1B). If thrombolytics are implemented, AT10 favors systemic administration over catheter-directed thrombolysis (CDT) due to the higher-quality evidence available. However, the authors state that CDT may be preferred for patients at higher risk of bleeding and when local expertise is available. Lastly, catheter-assisted thrombus removal should be considered in patients with acute PE and hypotension who have a high bleeding risk, who have failed systemic thrombolytics, or who are in shock and likely to die before systemic thrombolytics become therapeutic.
Although no prospective trials have evaluated the management of patients with recurrent VTE events while on anticoagulation therapy, AT10 offers some guidance. After ensuring the patient truly had a recurrent VTE event while on therapeutic warfarin or compliant with a DOAC, the authors suggest switching to LMWH for at least one month (grade 2C). Furthermore, for patients who have a recurrent VTE event while compliant on long-term LMWH, the guideline suggests increasing the dose of LMWH by about one-quarter to one-third (grade 2C).
Guideline Analysis
It is important to note that of the 54 recommendations included in the complete guideline update, only 20 were strong recommendations (grade 1), and none were based on high-quality evidence (level A). It is obvious that more research is needed in this field. Regardless, the ACCP antithrombotic guideline remains the authoritative source in VTE management and has a strong influence on practice behavior. With the recent addition of several newer anticoagulants, AT10 is particularly useful in helping providers understand when and when not to use them. The authors indicate that future iterations will be continually updated, describing them as “living guidelines.” The format of AT10 was designed to facilitate this method with the goal of having discrete topics discussed as new evidence becomes available.
Hospital Medicine Takeaways
Despite the lack of randomized and prospective clinical trials, the updated recommendations from AT10 provide important information on challenging VTE issues that the hospitalist can apply to most patients most of the time. Important updates include:
- Prescribe DOACs as first-line agents for the treatment of acute VTE in patients without cancer.
- Use aspirin for the prevention of recurrent VTE in patients who stop anticoagulation for treatment of an unprovoked DVT or PE.
- Avoid compression stockings for the sole purpose of preventing postthrombotic syndrome.
- Do not admit patients with low-risk PE (as determined by the PESI score) to the hospital but rather treat them entirely at home.
Lastly, it is important to remember that VTE treatment decisions need to be individualized based on the clinical, imaging, and biochemical features of your patient.
Paul J. Grant, MD, SFHM, is assistant professor of medicine and director of perioperative and consultative medicine within the Department of Internal Medicine at the University of Michigan Health System in Ann Arbor.
References
- Kearon C, Akl EA, Ornelas J, et al. Antithrombotic therapy for VTE disease: CHEST guideline and expert panel report. Chest. 2016;149(2):315-352.
- Brighton TA, Eikelboom JW, Mann K, et al. Low-dose aspirin for preventing recurrent venous thromboembolism. N Engl J Med. 2012;367(21):1979-1987.
- Becattini C, Agnelli G, Schenone A, et al. Aspirin for preventing the recurrence of venous thromboembolism. N Engl J Med. 2012;366(21):1959-1967.
- Kahn SR, Shapiro S, Wells PS, et al. Compression stockings to prevent post-thrombotic syndrome: a randomised placebo controlled trial. Lancet. 2014;383(9920):880-888.
Venous thromboembolism (VTE), which includes deep venous thrombosis (DVT) and pulmonary embolism (PE), continues to be a major cause of morbidity and mortality among hospitalized patients. Although it is well-known that anticoagulation therapy is effective in the prevention and treatment of VTE events, these agents are some of the highest-risk medications a hospitalist will prescribe given the danger of major bleeding. With the recent approval of several newer anticoagulants, it is important for the practicing hospitalist to be comfortable initiating, maintaining, and stopping these agents in a wide variety of patient populations.
Guideline Updates
In February 2016, an update to the ninth edition of the antithrombotic guideline from the American College of Chest Physician (ACCP) was published and included updated recommendations on 12 topics in addition to three new topics. This 10th-edition guideline update is referred to as AT10.1
One of the most notable changes in the updated guideline is the recommended choice of anticoagulant in patients with acute DVT or PE without cancer. Now, the direct oral anticoagulants (DOACs) dabigatran, rivaroxaban, apixaban, or edoxaban are recommended over warfarin. Although this is a weak recommendation based on moderate-quality evidence (grade 2B), this is the first time that warfarin is not considered first-line therapy. It should be emphasized that none of the four FDA-approved DOACs are preferred over another, and they should be avoided in patients who are pregnant or have severe renal disease. In patients with DVT or PE and cancer, low-molecular-weight heparin (LMWH) is still the preferred medication. If LMWH is not prescribed, AT10 does not have a preference for either a DOAC or warfarin for patients with cancer.
When it comes to duration of anticoagulation following a VTE event, the updated guideline continues to recommend three months for a provoked VTE event, with consideration for lifelong anticoagulation for an unprovoked event for patients at low or moderate bleeding risk. However, it now suggests that the recurrence risk factors of male sex and a positive D-dimer measured one month after stopping anticoagulant therapy should be taken into consideration when deciding whether extended anticoagulation is indicated.
AT10 also includes new recommendations concerning the role of aspirin for extended VTE treatment. Interestingly, the 2008 ACCP guideline gave a strong recommendation against the use of aspirin for VTE management in any patient population. In the 2012 guideline, the role of aspirin was not addressed for VTE treatment. Now, AT10 states that low-dose aspirin can be used in patients who stop anticoagulant therapy for treatment of an unprovoked proximal DVT or PE as an extended therapy (grade 2B). The significant change in this recommendation stems from two recent randomized trials that compared aspirin with placebo for the prevention of VTE recurrence in patients who have completed a course of anticoagulation for a first unprovoked proximal DVT or PE.2,3 Although the guideline doesn’t consider aspirin to be a reasonable alternative to anticoagulation for patients who require extended therapy and are agreeable to continue, for patients who have decided to stop anticoagulation, aspirin appears to reduce recurrent VTE by approximately one-third, with no significant increased risk of bleeding.
Another significant change in AT10 is the recommendation against the routine use of compression stockings to prevent postthrombotic syndrome (PTS). This change was influenced by a recent multicenter randomized trial showing that elastic compression stockings did not prevent PTS after an acute proximal DVT.4 The guideline authors remark that this recommendation focuses on the prevention of the chronic complications of PTS rather than treatment of the symptoms. Thus, for patients with acute or chronic leg pain or swelling from DVT, compression stockings may be justified.
A topic that was not addressed in the previous guideline was whether patients with a subsegmental PE should be treated. The guideline now suggests that patients with only subsegmental PE and no ultrasound-proven proximal DVT of the legs should undergo “clinical surveillance” rather than anticoagulation (grade 2C). Exceptions include patients at high risk for recurrent VTE (e.g., hospitalization, reduced mobility, active cancer, or irreversible VTE risk factors) and those with a low cardiopulmonary reserve or marked symptoms thought to be from PE. AT10 also states that patient preferences regarding anticoagulation treatment as well as the patient’s risk of bleeding should be taken into consideration. If the decision is made to not prescribe anticoagulation for subsegmental PE, patients should be advised to seek reevaluation if their symptoms persist or worsen.
The 2012 guideline included a new recommendation that patients with low-risk PE (typically defined by a low Pulmonary Embolism Severity Index [PESI] score) could be discharged “early” from the hospital. This recommendation has now been modified to state that patients with low-risk PE may be treated entirely at home. It is worth noting that outpatient management of low-risk PE has become much less complicated if using a DOAC, particularly rivaroxaban and apixaban as neither require initial treatment with parenteral anticoagulation.
AT10 has not changed the recommendation for which patients should receive thrombolytic therapy for treatment of PE. It recommends systemic thrombolytic therapy for patients with acute PE associated with hypotension (defined as systolic blood pressure less than 90 mmHg for 15 minutes) who are not at high risk for bleeding (grade 2B). Likewise, for patients with acute PE not associated with hypotension, the guideline recommends against systemic thrombolytics (grade 1B). If thrombolytics are implemented, AT10 favors systemic administration over catheter-directed thrombolysis (CDT) due to the higher-quality evidence available. However, the authors state that CDT may be preferred for patients at higher risk of bleeding and when local expertise is available. Lastly, catheter-assisted thrombus removal should be considered in patients with acute PE and hypotension who have a high bleeding risk, who have failed systemic thrombolytics, or who are in shock and likely to die before systemic thrombolytics become therapeutic.
Although no prospective trials have evaluated the management of patients with recurrent VTE events while on anticoagulation therapy, AT10 offers some guidance. After ensuring the patient truly had a recurrent VTE event while on therapeutic warfarin or compliant with a DOAC, the authors suggest switching to LMWH for at least one month (grade 2C). Furthermore, for patients who have a recurrent VTE event while compliant on long-term LMWH, the guideline suggests increasing the dose of LMWH by about one-quarter to one-third (grade 2C).
Guideline Analysis
It is important to note that of the 54 recommendations included in the complete guideline update, only 20 were strong recommendations (grade 1), and none were based on high-quality evidence (level A). It is obvious that more research is needed in this field. Regardless, the ACCP antithrombotic guideline remains the authoritative source in VTE management and has a strong influence on practice behavior. With the recent addition of several newer anticoagulants, AT10 is particularly useful in helping providers understand when and when not to use them. The authors indicate that future iterations will be continually updated, describing them as “living guidelines.” The format of AT10 was designed to facilitate this method with the goal of having discrete topics discussed as new evidence becomes available.
Hospital Medicine Takeaways
Despite the lack of randomized and prospective clinical trials, the updated recommendations from AT10 provide important information on challenging VTE issues that the hospitalist can apply to most patients most of the time. Important updates include:
- Prescribe DOACs as first-line agents for the treatment of acute VTE in patients without cancer.
- Use aspirin for the prevention of recurrent VTE in patients who stop anticoagulation for treatment of an unprovoked DVT or PE.
- Avoid compression stockings for the sole purpose of preventing postthrombotic syndrome.
- Do not admit patients with low-risk PE (as determined by the PESI score) to the hospital but rather treat them entirely at home.
Lastly, it is important to remember that VTE treatment decisions need to be individualized based on the clinical, imaging, and biochemical features of your patient.
Paul J. Grant, MD, SFHM, is assistant professor of medicine and director of perioperative and consultative medicine within the Department of Internal Medicine at the University of Michigan Health System in Ann Arbor.
References
- Kearon C, Akl EA, Ornelas J, et al. Antithrombotic therapy for VTE disease: CHEST guideline and expert panel report. Chest. 2016;149(2):315-352.
- Brighton TA, Eikelboom JW, Mann K, et al. Low-dose aspirin for preventing recurrent venous thromboembolism. N Engl J Med. 2012;367(21):1979-1987.
- Becattini C, Agnelli G, Schenone A, et al. Aspirin for preventing the recurrence of venous thromboembolism. N Engl J Med. 2012;366(21):1959-1967.
- Kahn SR, Shapiro S, Wells PS, et al. Compression stockings to prevent post-thrombotic syndrome: a randomised placebo controlled trial. Lancet. 2014;383(9920):880-888.
Implementation and Evaluation of an APRN-Led Opioid Monitoring Clinic
Chronic pain, defined as pain lasting longer than 3 to 6 months in duration, affects about 100 million Americans.1 The use of opioids in the management of chronic nonmalignant pain is common in primary care. The U.S., with only 5% of the global population, nevertheless is the world’s leading opioid consumer.2 For example, it is estimated that the U.S. consumes 56% of the global supply of morphine, 99% of hydrocodone, and 83% of oxycodone; this consumption is a growing problem in the use of chronic opioid therapy in managing chronic nonmalignant pain.2,3 The high rates of use of opioids continues, despite a lack of solid evidence on the long-term effectiveness of opioids for managing chronic nonmalignant pain and on the associated risks of opioid addiction, abuse, and misuse.3,4 Among veterans, the prevalence of opioid abuse and misuse has been reported to be about 30%, a nearly 7-fold occurrence compared with that in the general population.5,6
Due to the pervasiveness of opioid abuse and misuse among veterans, a project was initiated to develop, implement, and evaluate an Opioid Monitoring Clinic (OMC) as a clinical referral system within the primary care service of the VA Southern Nevada Healthcare System (VASNHS) in North Las Vegas. A health care provider (HCP) needs assessment was conducted at the facility, resulting in recommendations to improve adherence to evidence-based clinical practice guidelines in opioid management and regular monitoring of veterans on chronic opioid therapy for the identification of opioid abuse and misuse. Based on the results, an advanced practice registered nurse (APRN) in consultation with the chief of primary care at VASNHS and teamlet support (a registered nurse, licensed practical nurse, and medical support assistant) started the OMC. The OMC was developed consistent with the 2010 VA/DoD clinical practice guidelines for managing opioid therapy for chronic pain.7
After 6 months of OMC operation, the project also was evaluated for efficacy. First, a retrospective chart review of participants was conducted to identify the use of opioid pain agreements, prescription drug monitoring programs (PDMP) for controlled substance use review, and urine drug screens (UDSs). The chart review also included the average daily morphine equivalent dose (MED) for patients and OMC retention rates. Second, an online survey of primary care providers (PCPs) assessed their adherence to evidence-based guidelines in opioid management and satisfaction of the OMC services.
Background
In 1997, the average sales and distribution of opioids in the U.S. was 96 mg MED per individual, which increased to 710 mg per individual in 2010.8,9 The MED is a standardized daily dose measure for all opioids.10 At VASNHS North Las Vegas, there were 5,881 patients on opioid therapy in 2013 with about 13% of patients on opioid therapy using about 100 mg MED/d. The potential for abuse and misuse was great. Almost 30% of patients on chronic opioid therapy for chronic nonmalignant pain abuse their opioid prescriptions.5,7 Subsequently, opioid analgesics were responsible for nearly 60% of overdose deaths in 2010.11
In 2010, there were about 12 million people in the U.S. who abused or misused prescription opioids, using them for nonmedical reasons; annually, the prevalence rate of Americans who abuse and misuse opioids is about 2 million people.12 The 5-year prevalence rate of opioid abuse among veterans is at least 3%.6 This results in health care expenditures with an average excess medical cost of $20,546 per year for patients who misuse opioids compared with those who do not.13 The economic burden among veterans is even higher. Baser and colleagues reported that the annual economic cost for veterans who abused their opioid prescriptions was nearly $29,000.6
Unfortunately, injudicious opioid prescribing by HCPs is often cited as a contributor to the growing problem of opioid abuse and misuse in the U.S.14 Health care provider education on the proper and judicious use of opioids and adherence to clinical practice guidelines in the management of chronic pain is a crucial factor in reducing the complications of chron
Method
This project evaluated the APRN-led OMC with both quantitative and qualitative data from patients and PCPs. The OMC was implemented at VASNHS North Las Vegas, which serves almost 60,000 veterans. Patients referred to the OMC who were eligible for admission were veterans aged ≥ 18 years, who had chronic nonmalignant pain for at least 3 months, were receiving chronic opioid therapy, and were considered high risk for abuse or misuse of opioids. Patients were considered high risk if they had documented aberrant behaviors, such as multiple early refill requests, history of lost medications, drug screens not showing prescribed opioid(s), positive drug screens for controlled substance not prescribed, nonadherence with plan of care, or a history of substance abuse, including alcohol, cocaine, heroin, and marijuana. Veterans found to be suicidal or homicidal were excluded from the OMC and instead were referred to appropriate specialty care for further evaluation. A total of 61 veteran participants were successfully recruited.
Primary care physicians, physician assistants, and APRNs working at VASNHS on a full-time and part-time basis were eligible for participation as PCPs in this project. Thirty of 42 eligible PCPs participated and responded to the secure online provider survey (71% response rate).
Risks and benefits were discussed, and a written informed consent was obtained for each participant. There were no apparent risks or adverse effects encountered during project implementation and evaluation. Prior to data collection, permission for the study was obtained from the VA facility. An institutional review board application through the University of Nevada, Las Vegas’ Office of Research Integrity-Human Subjects was also submitted and approved through an expedited review.
Results
Table 1 details patient data, including demographics on the veteran participants. The av
Review of PDMPs revealed that 20% of the participants received controlled substances from other HCPs. Consequently, all OMC patients completed and had reinforced OPAs that served 2 purposes. First, the content in the agreement provided patient education regarding the potential hazards of opioid use. Second, the content provided specific expectations from the patient if opioid therapy is continued, including opioid risk reduction strategies (ie, random UDSs and pill counts, PDMP for controlled substance utilization review), and the consequences of lack of adherence to opioid management.
The majority of patients remained as patients and continued to be monitored after 6 months at the OMC (Table 2). However, only 64% were retained in the OMC, in large part because 36% had their opioids discontinued due to discovery of active illicit substance use, opioi
The mean MED/d among OMC participants was 54 mg/d before admission, which decreased gradually to 22 mg/d after 6 months of OMC care; this represented a 59% MED reduction (Table 3). Using the exact single-tailed Wilcoxon signed rank
Primary Care Providers
Based on the survey of PCPs, 40% of respondents referred at least 1 patient to the OMC. The majority (90%) acknowledged following the VA/DoD guidelines for opioid management, with 80% using an OPA more than usual since the implementation of the OMC, and 54% routinely accessing a PDMP for controlled substance utilization review. Moreover, 93% of PCPs routinely ordered a UDS when indicated.
For 3 months prior to opening the OMC in 2013, 1,606 UDSs were ordered at VASNHS. The UDS number steadily increased, and 6 months after opening the OMC, UDSs increased to 2,293 from months 4 to 6, representing a 30% increase. This was an expected outcome from the OMC—more PCPs now were following the VA/DoD clinical practice guidelines regarding the monitoring of patients on chronic opioid therapy.
A survey assessed the level of satisfaction among PCPs who had referred their patients to the OMC. Although only 11 PCPs responded to this question, a large majority found the OMC to have a positive effect on primary care services; many noted receiving fewer complaints regarding pain medications and fewer walk-ins. The majority recognized the advantage of the OMC in facilitating more PCP time for managing other medical problems beyond opioid use, which tends to be challenging and time consuming. Overall, 100% were satisfied with the OMC service.
Finally, 82% of PCPs reported that the OMC referral process did not need improvement. Two PCPs (18%) left positive feedback, reporting that the OMC was “simple, easy and accessible” and that the referral process “so far, it is great.” Another PCP noted, “I want patients who are suicidal but still need pain control with narcotics to be addressed.”
Discussion
The OMC has shown great promise in identifying abuse and misuse of opioids through evidence-based guidelines and risk-mitigation strategies. In the past, VA clinics specifically focused on opioid renewal have been implemented. In 2002, the Philadelphia VAMC opened an opioid renewal clinic (ORC) to assist PCPs in the management of patients with chronic pain on chronic opioid therapy.15 The Philadelphia VAMC ORC was operated collaboratively by PCPs and Pharmacy Service. They reported that 51% of their patients initially had documented aberrant behaviors, and 45% of these patients resolved their aberrant behaviors through intensive opioid monitoring using random UDSs.14 Thirteen percent of their patients were found to have an opioid addiction disorder and eventually were referred to addiction treatment; and 4% were weaned off opioids due to consistently negative UDSs.14
In the same manner, the OMC has effectively identified patients who abused and misused their opioids and consequently referred these patients for pain interventional management or to the VASNHS alcohol and drug treatment program as appropriate, which falls under the VASNHS Mental Health Care line, a service that is vital for veterans who are suicidal and homicidal. The importance of mental health care cannot be understated, as many patients with chronic pain also experience mental health challenges.
The Malcom Randall VAMC in Gainesville, Florida, structured a nurse-led, multidisciplinary ORC in 2003.16 A retrospective review of their program showed that 33% of patients had a positive UDS for marijuana, cocaine, or alcohol. The ORC had increased patient involvement in substance abuse treatment, resulting in some patients taking lower opioid dosages than before.
The New York Harbor VAMC reduced opioid cost by effectively switching veterans on expensive long-acting opioids, such as oxycodone and fentanyl, to less expensive alternatives, such a long-acting morphine.17 A secondary purpose of the New York initiative was to reduce the potential for inappropriate use of expensive long-acting opioids. Accordingly, the initiative reduced the number of expensive long-acting and potentially inappropriate opioids from 165 to 69 prescriptions in less than 6 months (November 2007 through March 2008). Similarly, after 6 months of operation, the OMC significantly reduced opioid prescription from 54 mg to 22 MED/d. This reduction represents a significant pharmacy cost savings. The combination of the discontinuation of opioids for patients found to be abusing and misusing opioids coupled with the decrease in pill burden resulting from changes from short-acting to long-acting opioids also resulted in significant savings for VA facilities.
The impact of the OMC on PCP adherence to opioid management guidelines as well as PCP satisfaction with the OMC services was significant. Similarly, Wiedemer and colleagues found significant PCP satisfaction with the ORC.15 The ability to spend more time with patients on other medical problems while allowing the OMC to focus on opioid and pain management was found to be beneficial. Buy-in among PCPs coupled with their concerns for chronic opioid therapy for high-risk patients facilitated the success of the OMC. The commitment of the VASNHS leadership to the OMC and their support for the APRN in leading this initiative were important facilitators in the success of the OMC.
Limitations
Long-term evaluation of the OMC with a larger sample is needed to fully evaluate its impact on decreasing opioid misuse and abuse. This project was limited by a small sample size, although the results are promising. Pharmacy costs, emergency department visits, as well as patient satisfaction, physical and emotional function, and pain levels are outcomes that need to be considered over the long term. Incorporating mental health counseling, cognitive behavior therapy, self-management programs, and group educational sessions have the potential to be important OMC services. The continued success and cost-effectiveness of the OMC can be a potentially significant model for this type of service that can be applied to clinics outside the VA system.
Implications
Possible implications to practice settings that are considering an OMC or ORC include the chance that patients will want to be discharged from such a clinic and return to the PCP for opioid management. Collaborative relationships and communication between PCPs and OMC providers are important to facilitate adherence and consistency with pain care. Collaboration and effective communication can be facilitated by electronic recording and reporting. For example, the VA Computerized Patient Record System can alert PCPs to patient discharges from the OMC along with OMC provider recommendations for patient care. Another challenge for the OMC would be a lack of referrals for patients who are at high risk for opioid abuse or misuse. These challenges can be mitigated by providing in-services, educational flyers, and advertisement promotions regarding OMC services. With the high prevalence of opioid abuse and misuse as well as the subsequent exorbitant health-related costs and deaths associated with opioids, OMCs and ORCs are viable options for improving opioid management in the treatment of patients with nonmalignant chronic pain.
Conclusion
The OMC effectively reduced the MED of patients referred to the clinic by 59%. The significant reduction in the opioid dose of patients referred to the OMC resulted from the implementation of evidence-based strategies that were used to identify abuse of prescription opioids, the use of illicit substances that can cause opioid-related complications, and the discovery of doctor shopping, coupled with gradual dose reductions for patients when appropriate. Provider satisfaction and increased use of evidence-based guidelines in opioid management and risk mitigation strategies, such as OPAs, PDMP databases, and UDS were evident. These results suggest that an OMC can be an effective program to help identify abuse and misuse of prescription opioids among high-risk patients and can improve patient safety and provider satisfaction.
Acknowledgments
This material is the result of work supported with resources and the use of facilities at the VA Southern Nevada Healthcare System.
1. Institute of Medicine (IOM). Relieving pain in America: a blueprint for transforming prevention, care, education, and research. http://iom.nationalacademies.org/~/media/Files/Report%20Files/2011/Relieving-Pain-in-America-A-Blueprint-for-Transforming-Prevention-Care-Education-Research/Pain%20Research%202011%20Report%20Brief.pdf. Published June 2011. Accessed August 25, 2016.
2. International Narcotics Control Board. Report of the International Narcotics Control Board on the availability of internationally controlled drugs. https://www.incb.org/documents/Publications/AnnualReports/AR2010/Supplement-AR10_availability_English.pdf. Published January 2011. Accessed August 25, 2016.
3. Manchikanti L, Helm S II, Fellows B, et al. Opioid epidemic in the United States. Pain Physician. 2012;15(3)(suppl):ES9-ES38.
4. Krebs EE, Ramsey DC, Miloshoff JM, Bair MJ. Primary care monitoring of long-term opioid therapy among veterans with chronic pain. Pain Med. 2011;12(5):740-746.
5. Von Korff M, Kolodny A, Deyo RA, Chou R. Long-term opioid therapy reconsidered. Ann Intern Med. 2011;155(5):325-328.
6. Baser O, Xie L, Mardekian J, Schaaf D, Wang L, Joshi AV. Prevalence of diagnosed opioid abuse and its economic burden in the Veterans’ Health Administration. Pain Pract. 2014;14(5):437-445.
7. Department of Veterans Affairs, Department of Defense. VA/DoD Clinical Practice Guideline for Management of Opioid Therapy for Chronic Pain. Guideline summary. http://www.va.gov/painmanagement/docs/cpg_opioidtherapy_summary.pdf. Published May 2010. Accessed August 25, 2016.
8. Centers for Disease Control and Prevention (CDC). CDC grand rounds: prescription drug overdoses—a U.S. epidemic. MMWR Morb Mortal Wkly Rep. 2012;61(1):10-13.
9. Hansen H, Noe CE, Racz GB. The evolving role of opioid treatment in chronic pain management. In: Hansen H, Racz GB, Noe CE, eds. Pain and Treatment. http://www.intechopen.com/books/pain-and-treatment/the-evolving-role-of-opioid-treatment-in-chronic-pain-management. Published July 10, 2014. Accessed October 5, 2016.
10. McAuley D. Opioids—equianalgesic dosages. http://www.globalrph.com/narcotic.htm. Updated August 5, 2016. Accessed October 5, 2016.
11. U.S. Department of Health and Human Services. Addressing prescription drug abuse in the United States: current activities and future opportunities. https://www.cdc.gov/drugoverdose/pdf/hhs_prescription_drug_abuse_report_09.2013.pdf. Accessed August 25, 2016.
12. Center for Behavioral Health Statistics and Quality, Substance Abuse and Mental Health Services Administration, U.S. Department of Health and Human Services, RTI International. Results from the 2010 National survey on drug use and health: summary of national findings. http://archive.samhsa.gov/data/NSDUH/2k10NSDUH/2k10Results.htm. Published September 2011. Accessed October 5, 2016.
13. Birnbaum HG, White AG, Schiller M, Waldman T, Cleveland JM, Roland CL. Societal costs of prescription opioid abuse, dependence, and misuse in the United States. Pain Med. 2011;12(4):657-667.
14. Federation of State Medical Boards. Model policy on the use of opioid analgesics in the treatment of chronic pain. http://www.fsmb.org/Media/Default/PDF/FSMB/Advocacy/pain_policy_july2013.pdf.Published July 2013. Accessed October 5, 2016.
15. Wiedemer NL, Harden PS, Arndt IO, Gallagher RM. The opioid renewal clinic: a primary care, managed approach to opioid therapy in chronic pain patients at risk for substance abuse. Pain Med. 2007;8(7):573-584.
16. Sampson JM, Havens S, Marsh B, Murrhee R. Managing chronic, nonmalignant pain in patients with a substance use disorder. Fed Pract. 2005;22(11):10, 16, 18, 25-26, 29.
17. Kharlamb V. (2008). VISN Opioid cost avoidance plan. New York Harbor VA, New York. Unpublished raw data.
Chronic pain, defined as pain lasting longer than 3 to 6 months in duration, affects about 100 million Americans.1 The use of opioids in the management of chronic nonmalignant pain is common in primary care. The U.S., with only 5% of the global population, nevertheless is the world’s leading opioid consumer.2 For example, it is estimated that the U.S. consumes 56% of the global supply of morphine, 99% of hydrocodone, and 83% of oxycodone; this consumption is a growing problem in the use of chronic opioid therapy in managing chronic nonmalignant pain.2,3 The high rates of use of opioids continues, despite a lack of solid evidence on the long-term effectiveness of opioids for managing chronic nonmalignant pain and on the associated risks of opioid addiction, abuse, and misuse.3,4 Among veterans, the prevalence of opioid abuse and misuse has been reported to be about 30%, a nearly 7-fold occurrence compared with that in the general population.5,6
Due to the pervasiveness of opioid abuse and misuse among veterans, a project was initiated to develop, implement, and evaluate an Opioid Monitoring Clinic (OMC) as a clinical referral system within the primary care service of the VA Southern Nevada Healthcare System (VASNHS) in North Las Vegas. A health care provider (HCP) needs assessment was conducted at the facility, resulting in recommendations to improve adherence to evidence-based clinical practice guidelines in opioid management and regular monitoring of veterans on chronic opioid therapy for the identification of opioid abuse and misuse. Based on the results, an advanced practice registered nurse (APRN) in consultation with the chief of primary care at VASNHS and teamlet support (a registered nurse, licensed practical nurse, and medical support assistant) started the OMC. The OMC was developed consistent with the 2010 VA/DoD clinical practice guidelines for managing opioid therapy for chronic pain.7
After 6 months of OMC operation, the project also was evaluated for efficacy. First, a retrospective chart review of participants was conducted to identify the use of opioid pain agreements, prescription drug monitoring programs (PDMP) for controlled substance use review, and urine drug screens (UDSs). The chart review also included the average daily morphine equivalent dose (MED) for patients and OMC retention rates. Second, an online survey of primary care providers (PCPs) assessed their adherence to evidence-based guidelines in opioid management and satisfaction of the OMC services.
Background
In 1997, the average sales and distribution of opioids in the U.S. was 96 mg MED per individual, which increased to 710 mg per individual in 2010.8,9 The MED is a standardized daily dose measure for all opioids.10 At VASNHS North Las Vegas, there were 5,881 patients on opioid therapy in 2013 with about 13% of patients on opioid therapy using about 100 mg MED/d. The potential for abuse and misuse was great. Almost 30% of patients on chronic opioid therapy for chronic nonmalignant pain abuse their opioid prescriptions.5,7 Subsequently, opioid analgesics were responsible for nearly 60% of overdose deaths in 2010.11
In 2010, there were about 12 million people in the U.S. who abused or misused prescription opioids, using them for nonmedical reasons; annually, the prevalence rate of Americans who abuse and misuse opioids is about 2 million people.12 The 5-year prevalence rate of opioid abuse among veterans is at least 3%.6 This results in health care expenditures with an average excess medical cost of $20,546 per year for patients who misuse opioids compared with those who do not.13 The economic burden among veterans is even higher. Baser and colleagues reported that the annual economic cost for veterans who abused their opioid prescriptions was nearly $29,000.6
Unfortunately, injudicious opioid prescribing by HCPs is often cited as a contributor to the growing problem of opioid abuse and misuse in the U.S.14 Health care provider education on the proper and judicious use of opioids and adherence to clinical practice guidelines in the management of chronic pain is a crucial factor in reducing the complications of chron
Method
This project evaluated the APRN-led OMC with both quantitative and qualitative data from patients and PCPs. The OMC was implemented at VASNHS North Las Vegas, which serves almost 60,000 veterans. Patients referred to the OMC who were eligible for admission were veterans aged ≥ 18 years, who had chronic nonmalignant pain for at least 3 months, were receiving chronic opioid therapy, and were considered high risk for abuse or misuse of opioids. Patients were considered high risk if they had documented aberrant behaviors, such as multiple early refill requests, history of lost medications, drug screens not showing prescribed opioid(s), positive drug screens for controlled substance not prescribed, nonadherence with plan of care, or a history of substance abuse, including alcohol, cocaine, heroin, and marijuana. Veterans found to be suicidal or homicidal were excluded from the OMC and instead were referred to appropriate specialty care for further evaluation. A total of 61 veteran participants were successfully recruited.
Primary care physicians, physician assistants, and APRNs working at VASNHS on a full-time and part-time basis were eligible for participation as PCPs in this project. Thirty of 42 eligible PCPs participated and responded to the secure online provider survey (71% response rate).
Risks and benefits were discussed, and a written informed consent was obtained for each participant. There were no apparent risks or adverse effects encountered during project implementation and evaluation. Prior to data collection, permission for the study was obtained from the VA facility. An institutional review board application through the University of Nevada, Las Vegas’ Office of Research Integrity-Human Subjects was also submitted and approved through an expedited review.
Results
Table 1 details patient data, including demographics on the veteran participants. The av
Review of PDMPs revealed that 20% of the participants received controlled substances from other HCPs. Consequently, all OMC patients completed and had reinforced OPAs that served 2 purposes. First, the content in the agreement provided patient education regarding the potential hazards of opioid use. Second, the content provided specific expectations from the patient if opioid therapy is continued, including opioid risk reduction strategies (ie, random UDSs and pill counts, PDMP for controlled substance utilization review), and the consequences of lack of adherence to opioid management.
The majority of patients remained as patients and continued to be monitored after 6 months at the OMC (Table 2). However, only 64% were retained in the OMC, in large part because 36% had their opioids discontinued due to discovery of active illicit substance use, opioi
The mean MED/d among OMC participants was 54 mg/d before admission, which decreased gradually to 22 mg/d after 6 months of OMC care; this represented a 59% MED reduction (Table 3). Using the exact single-tailed Wilcoxon signed rank
Primary Care Providers
Based on the survey of PCPs, 40% of respondents referred at least 1 patient to the OMC. The majority (90%) acknowledged following the VA/DoD guidelines for opioid management, with 80% using an OPA more than usual since the implementation of the OMC, and 54% routinely accessing a PDMP for controlled substance utilization review. Moreover, 93% of PCPs routinely ordered a UDS when indicated.
For 3 months prior to opening the OMC in 2013, 1,606 UDSs were ordered at VASNHS. The UDS number steadily increased, and 6 months after opening the OMC, UDSs increased to 2,293 from months 4 to 6, representing a 30% increase. This was an expected outcome from the OMC—more PCPs now were following the VA/DoD clinical practice guidelines regarding the monitoring of patients on chronic opioid therapy.
A survey assessed the level of satisfaction among PCPs who had referred their patients to the OMC. Although only 11 PCPs responded to this question, a large majority found the OMC to have a positive effect on primary care services; many noted receiving fewer complaints regarding pain medications and fewer walk-ins. The majority recognized the advantage of the OMC in facilitating more PCP time for managing other medical problems beyond opioid use, which tends to be challenging and time consuming. Overall, 100% were satisfied with the OMC service.
Finally, 82% of PCPs reported that the OMC referral process did not need improvement. Two PCPs (18%) left positive feedback, reporting that the OMC was “simple, easy and accessible” and that the referral process “so far, it is great.” Another PCP noted, “I want patients who are suicidal but still need pain control with narcotics to be addressed.”
Discussion
The OMC has shown great promise in identifying abuse and misuse of opioids through evidence-based guidelines and risk-mitigation strategies. In the past, VA clinics specifically focused on opioid renewal have been implemented. In 2002, the Philadelphia VAMC opened an opioid renewal clinic (ORC) to assist PCPs in the management of patients with chronic pain on chronic opioid therapy.15 The Philadelphia VAMC ORC was operated collaboratively by PCPs and Pharmacy Service. They reported that 51% of their patients initially had documented aberrant behaviors, and 45% of these patients resolved their aberrant behaviors through intensive opioid monitoring using random UDSs.14 Thirteen percent of their patients were found to have an opioid addiction disorder and eventually were referred to addiction treatment; and 4% were weaned off opioids due to consistently negative UDSs.14
In the same manner, the OMC has effectively identified patients who abused and misused their opioids and consequently referred these patients for pain interventional management or to the VASNHS alcohol and drug treatment program as appropriate, which falls under the VASNHS Mental Health Care line, a service that is vital for veterans who are suicidal and homicidal. The importance of mental health care cannot be understated, as many patients with chronic pain also experience mental health challenges.
The Malcom Randall VAMC in Gainesville, Florida, structured a nurse-led, multidisciplinary ORC in 2003.16 A retrospective review of their program showed that 33% of patients had a positive UDS for marijuana, cocaine, or alcohol. The ORC had increased patient involvement in substance abuse treatment, resulting in some patients taking lower opioid dosages than before.
The New York Harbor VAMC reduced opioid cost by effectively switching veterans on expensive long-acting opioids, such as oxycodone and fentanyl, to less expensive alternatives, such a long-acting morphine.17 A secondary purpose of the New York initiative was to reduce the potential for inappropriate use of expensive long-acting opioids. Accordingly, the initiative reduced the number of expensive long-acting and potentially inappropriate opioids from 165 to 69 prescriptions in less than 6 months (November 2007 through March 2008). Similarly, after 6 months of operation, the OMC significantly reduced opioid prescription from 54 mg to 22 MED/d. This reduction represents a significant pharmacy cost savings. The combination of the discontinuation of opioids for patients found to be abusing and misusing opioids coupled with the decrease in pill burden resulting from changes from short-acting to long-acting opioids also resulted in significant savings for VA facilities.
The impact of the OMC on PCP adherence to opioid management guidelines as well as PCP satisfaction with the OMC services was significant. Similarly, Wiedemer and colleagues found significant PCP satisfaction with the ORC.15 The ability to spend more time with patients on other medical problems while allowing the OMC to focus on opioid and pain management was found to be beneficial. Buy-in among PCPs coupled with their concerns for chronic opioid therapy for high-risk patients facilitated the success of the OMC. The commitment of the VASNHS leadership to the OMC and their support for the APRN in leading this initiative were important facilitators in the success of the OMC.
Limitations
Long-term evaluation of the OMC with a larger sample is needed to fully evaluate its impact on decreasing opioid misuse and abuse. This project was limited by a small sample size, although the results are promising. Pharmacy costs, emergency department visits, as well as patient satisfaction, physical and emotional function, and pain levels are outcomes that need to be considered over the long term. Incorporating mental health counseling, cognitive behavior therapy, self-management programs, and group educational sessions have the potential to be important OMC services. The continued success and cost-effectiveness of the OMC can be a potentially significant model for this type of service that can be applied to clinics outside the VA system.
Implications
Possible implications to practice settings that are considering an OMC or ORC include the chance that patients will want to be discharged from such a clinic and return to the PCP for opioid management. Collaborative relationships and communication between PCPs and OMC providers are important to facilitate adherence and consistency with pain care. Collaboration and effective communication can be facilitated by electronic recording and reporting. For example, the VA Computerized Patient Record System can alert PCPs to patient discharges from the OMC along with OMC provider recommendations for patient care. Another challenge for the OMC would be a lack of referrals for patients who are at high risk for opioid abuse or misuse. These challenges can be mitigated by providing in-services, educational flyers, and advertisement promotions regarding OMC services. With the high prevalence of opioid abuse and misuse as well as the subsequent exorbitant health-related costs and deaths associated with opioids, OMCs and ORCs are viable options for improving opioid management in the treatment of patients with nonmalignant chronic pain.
Conclusion
The OMC effectively reduced the MED of patients referred to the clinic by 59%. The significant reduction in the opioid dose of patients referred to the OMC resulted from the implementation of evidence-based strategies that were used to identify abuse of prescription opioids, the use of illicit substances that can cause opioid-related complications, and the discovery of doctor shopping, coupled with gradual dose reductions for patients when appropriate. Provider satisfaction and increased use of evidence-based guidelines in opioid management and risk mitigation strategies, such as OPAs, PDMP databases, and UDS were evident. These results suggest that an OMC can be an effective program to help identify abuse and misuse of prescription opioids among high-risk patients and can improve patient safety and provider satisfaction.
Acknowledgments
This material is the result of work supported with resources and the use of facilities at the VA Southern Nevada Healthcare System.
Chronic pain, defined as pain lasting longer than 3 to 6 months in duration, affects about 100 million Americans.1 The use of opioids in the management of chronic nonmalignant pain is common in primary care. The U.S., with only 5% of the global population, nevertheless is the world’s leading opioid consumer.2 For example, it is estimated that the U.S. consumes 56% of the global supply of morphine, 99% of hydrocodone, and 83% of oxycodone; this consumption is a growing problem in the use of chronic opioid therapy in managing chronic nonmalignant pain.2,3 The high rates of use of opioids continues, despite a lack of solid evidence on the long-term effectiveness of opioids for managing chronic nonmalignant pain and on the associated risks of opioid addiction, abuse, and misuse.3,4 Among veterans, the prevalence of opioid abuse and misuse has been reported to be about 30%, a nearly 7-fold occurrence compared with that in the general population.5,6
Due to the pervasiveness of opioid abuse and misuse among veterans, a project was initiated to develop, implement, and evaluate an Opioid Monitoring Clinic (OMC) as a clinical referral system within the primary care service of the VA Southern Nevada Healthcare System (VASNHS) in North Las Vegas. A health care provider (HCP) needs assessment was conducted at the facility, resulting in recommendations to improve adherence to evidence-based clinical practice guidelines in opioid management and regular monitoring of veterans on chronic opioid therapy for the identification of opioid abuse and misuse. Based on the results, an advanced practice registered nurse (APRN) in consultation with the chief of primary care at VASNHS and teamlet support (a registered nurse, licensed practical nurse, and medical support assistant) started the OMC. The OMC was developed consistent with the 2010 VA/DoD clinical practice guidelines for managing opioid therapy for chronic pain.7
After 6 months of OMC operation, the project also was evaluated for efficacy. First, a retrospective chart review of participants was conducted to identify the use of opioid pain agreements, prescription drug monitoring programs (PDMP) for controlled substance use review, and urine drug screens (UDSs). The chart review also included the average daily morphine equivalent dose (MED) for patients and OMC retention rates. Second, an online survey of primary care providers (PCPs) assessed their adherence to evidence-based guidelines in opioid management and satisfaction of the OMC services.
Background
In 1997, the average sales and distribution of opioids in the U.S. was 96 mg MED per individual, which increased to 710 mg per individual in 2010.8,9 The MED is a standardized daily dose measure for all opioids.10 At VASNHS North Las Vegas, there were 5,881 patients on opioid therapy in 2013 with about 13% of patients on opioid therapy using about 100 mg MED/d. The potential for abuse and misuse was great. Almost 30% of patients on chronic opioid therapy for chronic nonmalignant pain abuse their opioid prescriptions.5,7 Subsequently, opioid analgesics were responsible for nearly 60% of overdose deaths in 2010.11
In 2010, there were about 12 million people in the U.S. who abused or misused prescription opioids, using them for nonmedical reasons; annually, the prevalence rate of Americans who abuse and misuse opioids is about 2 million people.12 The 5-year prevalence rate of opioid abuse among veterans is at least 3%.6 This results in health care expenditures with an average excess medical cost of $20,546 per year for patients who misuse opioids compared with those who do not.13 The economic burden among veterans is even higher. Baser and colleagues reported that the annual economic cost for veterans who abused their opioid prescriptions was nearly $29,000.6
Unfortunately, injudicious opioid prescribing by HCPs is often cited as a contributor to the growing problem of opioid abuse and misuse in the U.S.14 Health care provider education on the proper and judicious use of opioids and adherence to clinical practice guidelines in the management of chronic pain is a crucial factor in reducing the complications of chron
Method
This project evaluated the APRN-led OMC with both quantitative and qualitative data from patients and PCPs. The OMC was implemented at VASNHS North Las Vegas, which serves almost 60,000 veterans. Patients referred to the OMC who were eligible for admission were veterans aged ≥ 18 years, who had chronic nonmalignant pain for at least 3 months, were receiving chronic opioid therapy, and were considered high risk for abuse or misuse of opioids. Patients were considered high risk if they had documented aberrant behaviors, such as multiple early refill requests, history of lost medications, drug screens not showing prescribed opioid(s), positive drug screens for controlled substance not prescribed, nonadherence with plan of care, or a history of substance abuse, including alcohol, cocaine, heroin, and marijuana. Veterans found to be suicidal or homicidal were excluded from the OMC and instead were referred to appropriate specialty care for further evaluation. A total of 61 veteran participants were successfully recruited.
Primary care physicians, physician assistants, and APRNs working at VASNHS on a full-time and part-time basis were eligible for participation as PCPs in this project. Thirty of 42 eligible PCPs participated and responded to the secure online provider survey (71% response rate).
Risks and benefits were discussed, and a written informed consent was obtained for each participant. There were no apparent risks or adverse effects encountered during project implementation and evaluation. Prior to data collection, permission for the study was obtained from the VA facility. An institutional review board application through the University of Nevada, Las Vegas’ Office of Research Integrity-Human Subjects was also submitted and approved through an expedited review.
Results
Table 1 details patient data, including demographics on the veteran participants. The av
Review of PDMPs revealed that 20% of the participants received controlled substances from other HCPs. Consequently, all OMC patients completed and had reinforced OPAs that served 2 purposes. First, the content in the agreement provided patient education regarding the potential hazards of opioid use. Second, the content provided specific expectations from the patient if opioid therapy is continued, including opioid risk reduction strategies (ie, random UDSs and pill counts, PDMP for controlled substance utilization review), and the consequences of lack of adherence to opioid management.
The majority of patients remained as patients and continued to be monitored after 6 months at the OMC (Table 2). However, only 64% were retained in the OMC, in large part because 36% had their opioids discontinued due to discovery of active illicit substance use, opioi
The mean MED/d among OMC participants was 54 mg/d before admission, which decreased gradually to 22 mg/d after 6 months of OMC care; this represented a 59% MED reduction (Table 3). Using the exact single-tailed Wilcoxon signed rank
Primary Care Providers
Based on the survey of PCPs, 40% of respondents referred at least 1 patient to the OMC. The majority (90%) acknowledged following the VA/DoD guidelines for opioid management, with 80% using an OPA more than usual since the implementation of the OMC, and 54% routinely accessing a PDMP for controlled substance utilization review. Moreover, 93% of PCPs routinely ordered a UDS when indicated.
For 3 months prior to opening the OMC in 2013, 1,606 UDSs were ordered at VASNHS. The UDS number steadily increased, and 6 months after opening the OMC, UDSs increased to 2,293 from months 4 to 6, representing a 30% increase. This was an expected outcome from the OMC—more PCPs now were following the VA/DoD clinical practice guidelines regarding the monitoring of patients on chronic opioid therapy.
A survey assessed the level of satisfaction among PCPs who had referred their patients to the OMC. Although only 11 PCPs responded to this question, a large majority found the OMC to have a positive effect on primary care services; many noted receiving fewer complaints regarding pain medications and fewer walk-ins. The majority recognized the advantage of the OMC in facilitating more PCP time for managing other medical problems beyond opioid use, which tends to be challenging and time consuming. Overall, 100% were satisfied with the OMC service.
Finally, 82% of PCPs reported that the OMC referral process did not need improvement. Two PCPs (18%) left positive feedback, reporting that the OMC was “simple, easy and accessible” and that the referral process “so far, it is great.” Another PCP noted, “I want patients who are suicidal but still need pain control with narcotics to be addressed.”
Discussion
The OMC has shown great promise in identifying abuse and misuse of opioids through evidence-based guidelines and risk-mitigation strategies. In the past, VA clinics specifically focused on opioid renewal have been implemented. In 2002, the Philadelphia VAMC opened an opioid renewal clinic (ORC) to assist PCPs in the management of patients with chronic pain on chronic opioid therapy.15 The Philadelphia VAMC ORC was operated collaboratively by PCPs and Pharmacy Service. They reported that 51% of their patients initially had documented aberrant behaviors, and 45% of these patients resolved their aberrant behaviors through intensive opioid monitoring using random UDSs.14 Thirteen percent of their patients were found to have an opioid addiction disorder and eventually were referred to addiction treatment; and 4% were weaned off opioids due to consistently negative UDSs.14
In the same manner, the OMC has effectively identified patients who abused and misused their opioids and consequently referred these patients for pain interventional management or to the VASNHS alcohol and drug treatment program as appropriate, which falls under the VASNHS Mental Health Care line, a service that is vital for veterans who are suicidal and homicidal. The importance of mental health care cannot be understated, as many patients with chronic pain also experience mental health challenges.
The Malcom Randall VAMC in Gainesville, Florida, structured a nurse-led, multidisciplinary ORC in 2003.16 A retrospective review of their program showed that 33% of patients had a positive UDS for marijuana, cocaine, or alcohol. The ORC had increased patient involvement in substance abuse treatment, resulting in some patients taking lower opioid dosages than before.
The New York Harbor VAMC reduced opioid cost by effectively switching veterans on expensive long-acting opioids, such as oxycodone and fentanyl, to less expensive alternatives, such a long-acting morphine.17 A secondary purpose of the New York initiative was to reduce the potential for inappropriate use of expensive long-acting opioids. Accordingly, the initiative reduced the number of expensive long-acting and potentially inappropriate opioids from 165 to 69 prescriptions in less than 6 months (November 2007 through March 2008). Similarly, after 6 months of operation, the OMC significantly reduced opioid prescription from 54 mg to 22 MED/d. This reduction represents a significant pharmacy cost savings. The combination of the discontinuation of opioids for patients found to be abusing and misusing opioids coupled with the decrease in pill burden resulting from changes from short-acting to long-acting opioids also resulted in significant savings for VA facilities.
The impact of the OMC on PCP adherence to opioid management guidelines as well as PCP satisfaction with the OMC services was significant. Similarly, Wiedemer and colleagues found significant PCP satisfaction with the ORC.15 The ability to spend more time with patients on other medical problems while allowing the OMC to focus on opioid and pain management was found to be beneficial. Buy-in among PCPs coupled with their concerns for chronic opioid therapy for high-risk patients facilitated the success of the OMC. The commitment of the VASNHS leadership to the OMC and their support for the APRN in leading this initiative were important facilitators in the success of the OMC.
Limitations
Long-term evaluation of the OMC with a larger sample is needed to fully evaluate its impact on decreasing opioid misuse and abuse. This project was limited by a small sample size, although the results are promising. Pharmacy costs, emergency department visits, as well as patient satisfaction, physical and emotional function, and pain levels are outcomes that need to be considered over the long term. Incorporating mental health counseling, cognitive behavior therapy, self-management programs, and group educational sessions have the potential to be important OMC services. The continued success and cost-effectiveness of the OMC can be a potentially significant model for this type of service that can be applied to clinics outside the VA system.
Implications
Possible implications to practice settings that are considering an OMC or ORC include the chance that patients will want to be discharged from such a clinic and return to the PCP for opioid management. Collaborative relationships and communication between PCPs and OMC providers are important to facilitate adherence and consistency with pain care. Collaboration and effective communication can be facilitated by electronic recording and reporting. For example, the VA Computerized Patient Record System can alert PCPs to patient discharges from the OMC along with OMC provider recommendations for patient care. Another challenge for the OMC would be a lack of referrals for patients who are at high risk for opioid abuse or misuse. These challenges can be mitigated by providing in-services, educational flyers, and advertisement promotions regarding OMC services. With the high prevalence of opioid abuse and misuse as well as the subsequent exorbitant health-related costs and deaths associated with opioids, OMCs and ORCs are viable options for improving opioid management in the treatment of patients with nonmalignant chronic pain.
Conclusion
The OMC effectively reduced the MED of patients referred to the clinic by 59%. The significant reduction in the opioid dose of patients referred to the OMC resulted from the implementation of evidence-based strategies that were used to identify abuse of prescription opioids, the use of illicit substances that can cause opioid-related complications, and the discovery of doctor shopping, coupled with gradual dose reductions for patients when appropriate. Provider satisfaction and increased use of evidence-based guidelines in opioid management and risk mitigation strategies, such as OPAs, PDMP databases, and UDS were evident. These results suggest that an OMC can be an effective program to help identify abuse and misuse of prescription opioids among high-risk patients and can improve patient safety and provider satisfaction.
Acknowledgments
This material is the result of work supported with resources and the use of facilities at the VA Southern Nevada Healthcare System.
1. Institute of Medicine (IOM). Relieving pain in America: a blueprint for transforming prevention, care, education, and research. http://iom.nationalacademies.org/~/media/Files/Report%20Files/2011/Relieving-Pain-in-America-A-Blueprint-for-Transforming-Prevention-Care-Education-Research/Pain%20Research%202011%20Report%20Brief.pdf. Published June 2011. Accessed August 25, 2016.
2. International Narcotics Control Board. Report of the International Narcotics Control Board on the availability of internationally controlled drugs. https://www.incb.org/documents/Publications/AnnualReports/AR2010/Supplement-AR10_availability_English.pdf. Published January 2011. Accessed August 25, 2016.
3. Manchikanti L, Helm S II, Fellows B, et al. Opioid epidemic in the United States. Pain Physician. 2012;15(3)(suppl):ES9-ES38.
4. Krebs EE, Ramsey DC, Miloshoff JM, Bair MJ. Primary care monitoring of long-term opioid therapy among veterans with chronic pain. Pain Med. 2011;12(5):740-746.
5. Von Korff M, Kolodny A, Deyo RA, Chou R. Long-term opioid therapy reconsidered. Ann Intern Med. 2011;155(5):325-328.
6. Baser O, Xie L, Mardekian J, Schaaf D, Wang L, Joshi AV. Prevalence of diagnosed opioid abuse and its economic burden in the Veterans’ Health Administration. Pain Pract. 2014;14(5):437-445.
7. Department of Veterans Affairs, Department of Defense. VA/DoD Clinical Practice Guideline for Management of Opioid Therapy for Chronic Pain. Guideline summary. http://www.va.gov/painmanagement/docs/cpg_opioidtherapy_summary.pdf. Published May 2010. Accessed August 25, 2016.
8. Centers for Disease Control and Prevention (CDC). CDC grand rounds: prescription drug overdoses—a U.S. epidemic. MMWR Morb Mortal Wkly Rep. 2012;61(1):10-13.
9. Hansen H, Noe CE, Racz GB. The evolving role of opioid treatment in chronic pain management. In: Hansen H, Racz GB, Noe CE, eds. Pain and Treatment. http://www.intechopen.com/books/pain-and-treatment/the-evolving-role-of-opioid-treatment-in-chronic-pain-management. Published July 10, 2014. Accessed October 5, 2016.
10. McAuley D. Opioids—equianalgesic dosages. http://www.globalrph.com/narcotic.htm. Updated August 5, 2016. Accessed October 5, 2016.
11. U.S. Department of Health and Human Services. Addressing prescription drug abuse in the United States: current activities and future opportunities. https://www.cdc.gov/drugoverdose/pdf/hhs_prescription_drug_abuse_report_09.2013.pdf. Accessed August 25, 2016.
12. Center for Behavioral Health Statistics and Quality, Substance Abuse and Mental Health Services Administration, U.S. Department of Health and Human Services, RTI International. Results from the 2010 National survey on drug use and health: summary of national findings. http://archive.samhsa.gov/data/NSDUH/2k10NSDUH/2k10Results.htm. Published September 2011. Accessed October 5, 2016.
13. Birnbaum HG, White AG, Schiller M, Waldman T, Cleveland JM, Roland CL. Societal costs of prescription opioid abuse, dependence, and misuse in the United States. Pain Med. 2011;12(4):657-667.
14. Federation of State Medical Boards. Model policy on the use of opioid analgesics in the treatment of chronic pain. http://www.fsmb.org/Media/Default/PDF/FSMB/Advocacy/pain_policy_july2013.pdf.Published July 2013. Accessed October 5, 2016.
15. Wiedemer NL, Harden PS, Arndt IO, Gallagher RM. The opioid renewal clinic: a primary care, managed approach to opioid therapy in chronic pain patients at risk for substance abuse. Pain Med. 2007;8(7):573-584.
16. Sampson JM, Havens S, Marsh B, Murrhee R. Managing chronic, nonmalignant pain in patients with a substance use disorder. Fed Pract. 2005;22(11):10, 16, 18, 25-26, 29.
17. Kharlamb V. (2008). VISN Opioid cost avoidance plan. New York Harbor VA, New York. Unpublished raw data.
1. Institute of Medicine (IOM). Relieving pain in America: a blueprint for transforming prevention, care, education, and research. http://iom.nationalacademies.org/~/media/Files/Report%20Files/2011/Relieving-Pain-in-America-A-Blueprint-for-Transforming-Prevention-Care-Education-Research/Pain%20Research%202011%20Report%20Brief.pdf. Published June 2011. Accessed August 25, 2016.
2. International Narcotics Control Board. Report of the International Narcotics Control Board on the availability of internationally controlled drugs. https://www.incb.org/documents/Publications/AnnualReports/AR2010/Supplement-AR10_availability_English.pdf. Published January 2011. Accessed August 25, 2016.
3. Manchikanti L, Helm S II, Fellows B, et al. Opioid epidemic in the United States. Pain Physician. 2012;15(3)(suppl):ES9-ES38.
4. Krebs EE, Ramsey DC, Miloshoff JM, Bair MJ. Primary care monitoring of long-term opioid therapy among veterans with chronic pain. Pain Med. 2011;12(5):740-746.
5. Von Korff M, Kolodny A, Deyo RA, Chou R. Long-term opioid therapy reconsidered. Ann Intern Med. 2011;155(5):325-328.
6. Baser O, Xie L, Mardekian J, Schaaf D, Wang L, Joshi AV. Prevalence of diagnosed opioid abuse and its economic burden in the Veterans’ Health Administration. Pain Pract. 2014;14(5):437-445.
7. Department of Veterans Affairs, Department of Defense. VA/DoD Clinical Practice Guideline for Management of Opioid Therapy for Chronic Pain. Guideline summary. http://www.va.gov/painmanagement/docs/cpg_opioidtherapy_summary.pdf. Published May 2010. Accessed August 25, 2016.
8. Centers for Disease Control and Prevention (CDC). CDC grand rounds: prescription drug overdoses—a U.S. epidemic. MMWR Morb Mortal Wkly Rep. 2012;61(1):10-13.
9. Hansen H, Noe CE, Racz GB. The evolving role of opioid treatment in chronic pain management. In: Hansen H, Racz GB, Noe CE, eds. Pain and Treatment. http://www.intechopen.com/books/pain-and-treatment/the-evolving-role-of-opioid-treatment-in-chronic-pain-management. Published July 10, 2014. Accessed October 5, 2016.
10. McAuley D. Opioids—equianalgesic dosages. http://www.globalrph.com/narcotic.htm. Updated August 5, 2016. Accessed October 5, 2016.
11. U.S. Department of Health and Human Services. Addressing prescription drug abuse in the United States: current activities and future opportunities. https://www.cdc.gov/drugoverdose/pdf/hhs_prescription_drug_abuse_report_09.2013.pdf. Accessed August 25, 2016.
12. Center for Behavioral Health Statistics and Quality, Substance Abuse and Mental Health Services Administration, U.S. Department of Health and Human Services, RTI International. Results from the 2010 National survey on drug use and health: summary of national findings. http://archive.samhsa.gov/data/NSDUH/2k10NSDUH/2k10Results.htm. Published September 2011. Accessed October 5, 2016.
13. Birnbaum HG, White AG, Schiller M, Waldman T, Cleveland JM, Roland CL. Societal costs of prescription opioid abuse, dependence, and misuse in the United States. Pain Med. 2011;12(4):657-667.
14. Federation of State Medical Boards. Model policy on the use of opioid analgesics in the treatment of chronic pain. http://www.fsmb.org/Media/Default/PDF/FSMB/Advocacy/pain_policy_july2013.pdf.Published July 2013. Accessed October 5, 2016.
15. Wiedemer NL, Harden PS, Arndt IO, Gallagher RM. The opioid renewal clinic: a primary care, managed approach to opioid therapy in chronic pain patients at risk for substance abuse. Pain Med. 2007;8(7):573-584.
16. Sampson JM, Havens S, Marsh B, Murrhee R. Managing chronic, nonmalignant pain in patients with a substance use disorder. Fed Pract. 2005;22(11):10, 16, 18, 25-26, 29.
17. Kharlamb V. (2008). VISN Opioid cost avoidance plan. New York Harbor VA, New York. Unpublished raw data.
The Overdose Education and Naloxone Distribution Program at a VA Hospital
Fatal opioid overdoses have quadrupled in the U.S. from 1999 to 2013.1 In 2013, 43,982 deaths in the U.S. were attributable to drug overdoses, and 37% of them involved opioids.1 Data from 2005 suggested that veterans are at an increased risk with a 2-fold rise in overdose observed.2 In 2013, there were 17,124 encounters at VA facilities for the treatment of opioid overdose.3 In response to this opioid epidemic, individual VA facilities began implementing Overdose Education and Naloxone Distribution (OEND) programs in 2013.4 In May 2014, the Under Secretary for Health mandated the implementation of OEND programs across all VA facilities, and the VA became the only health care system in the U.S. with a national OEND program.4
Naloxone
Naloxone is a pure opioid antagonist that competes with and displaces opioids at opioid receptors. In an overdose, opioids occupying opioid receptor sites can lead to respiratory depression, which can lead to death. Patients can experience rapid reversal of respiratory depression and opioid overdose with the first dose of naloxone 0.4 mg. Naloxone has a short half-life relative to opioids, whereas opioids can bind to opioid receptors once again after naloxone is eliminated, risking the reemergence of overdose symptoms. For this reason, a second dose of naloxone is often given. When administered appropriately with close monitoring, naloxone can provide a safe and effective way to reverse a potentially life-threatening opioid overdose.
Availability
In April 2014, Evzio, an intramuscular/subcutaneous formulation of naloxone, was FDA approved as an emergency treatment for known or suspected opioid overdose. Shortly after, in November 2015, Narcan, a nasal spray formulation of naloxone, also was approved. Each formulation has been instrumental in fulfilling an unmet medical need in the U.S. Both Evzio and Narcan have been used in opioid-overdose prevention programs to train health care professionals and lay persons to respond in the event of suspected overdose and administer naloxone.
Maxwell and colleagues implemented a naloxone distribution program in Chicago, Illinois, in January 2001 after a 4-fold increase in heroin-related overdose deaths was observed.5 Within a year, Chicago experienced a 20% decrease in heroin-related overdose deaths, and this trend continued with additional 10% reductions in heroin-related overdoses in 2002 and 2003. Similarly, Piper and colleagues implemented a naloxone distribution program and found that 82% of injection drug users felt “comfortable” to “very comfortable” administering naloxone in the event of an overdose, and 86% of subjects reported they would want naloxone in the event of an overdose.6
Naloxone is available for over-the-counter purchase in Rhode Island, which is a bordering state to VA Connecticut Healthcare System (VACT). In 2015 new legislation permitted the training and certification of Connecticut community pharmacists to prescribe naloxone. Furthermore, in May 2016, Connecticut passed legislation to permit qualified individuals to carry naloxone and administer it to another person that he or she, believes is experiencing an opioid-related drug overdose. Given the increase in naloxone availability both nationally and locally, VACT sought to implement a naloxone distribution program to offer the same access to veterans. The primary objective of this article is to describe the development, implementation, and preliminary results of this OEND program.
Development
In accordance with VA guidance, the VACT OEND program development and implementation process included 4 steps that required program coordinators to identify target populations, garner support, train staff members, and implement the OEND program.
A multidisciplinary team of representatives from pharmacy, mental health (MH), and substance abuse (SA) departments was established to draft hospital policy. The hospital policy detailed job functions related to the OEND program for prescribing, educating, verifying, and dispensing naloxone. This policy also served as a document to identify the target population.
With resources from the National VA Pharmacy Benefits Manager, the target population for VACT listed veterans with an opioid use disorder, prescription opioid use disorder, and injection opioid use disorder. Also listed were veterans at risk of an opioid overdose, such as those encountered in medication-assisted treatment programs for opioid use disorder, recent inpatient withdrawal management for opioid use disorder, HIV education/prevention programs, syringe access programs, outpatient and residential opioid use disorder treatment programs, community meetings/support group programs for opioid use disorder, emergency departments for opioid overdose or intoxication, domiciliary care or community-based treatment for homeless veterans, and primary health care for follow-up of recent opioid overdose or intoxication. The aim of the broad description of veterans was to ensure that naloxone access remained inclusive.
The program overview and hospital policy were presented to the VACT medication management committee (MMC), which approved a small pilot project for veterans engaged in SA treatment from January 2015 to April 2015. Barriers to implementation were encountered at this time, which included a belief that only MH or SA providers should prescribe naloxone rescue kits. Reasons for this were largely related to the belief that MH and SA providers are more familiar with substance use disorders. These barriers were addressed by increasing education to providers by detailing previous success stories regarding naloxone programs to highlight that the benefits outweighed any associated risks.
Implementation
Naloxone kits were assembled in preparation for program implementation. Each kit contained 2 vials of naloxone (each vial contains 1 dose), 2 needleless luer-lock syringes, and 1 atomizer with visual instructions for product assembly. Next, a template was created for entry into the veteran’s electronic health record (EHR) each time a naloxone kit was dispensed. The template tracked diagnosis, history of naloxone kit use, and aspects of the OEND program that were discussed with the veteran. Embedded within the template is a hyperlink to the Substance Abuse and Mental Health Services Administration Opioid Overdose Prevention Toolkit booklet, which serves as an education guide and take-home material for the veteran.
Once the naloxone kits were assembled and the template created, SA providers were able to engage in OEND program training. Training required providers to attend a 60-minute, 75-slide PowerPoint seminar, highlighting risk factors and prevention strategies for opioid overdose, proper identification and management of an opioid overdose, appropriate administration of naloxone, and monitoring parameters after administration. Providers were not screened for baseline knowledge, nor was a posttraining proficiency evaluation performed. However, providers were allowed to ask questions and request further clarification about any unclear aspects of training.
Each month, data were collected on the number of providers trained and naloxone kits dispensed. Implementation began in January 2015, and veterans were either educated during individual or group sessions led by a trained OEND provider. An institutional review board-approved quality improvement (QI) process ran concurrently that sought to improve the OEND program. Phase 1 of this QI project anonymously surveyed OEND providers to evaluate knowledge, comfort, attitude, and fear of consequences regarding naloxone distribution in an attempt to understand barriers to program implementation or success.7 In phase 2, veterans who received a naloxone kit will be interviewed regarding knowledge retention of OEND program education and subsequent opioid abuse and naloxone use after receiving a naloxone kit.
Results
From January 2015 through preliminary data analysis in April 2015, the facility trained 19 providers and dispensed naloxone kits to 49 veterans. In the first 4 months of program implementation, there were no reports of attempted opioid overdose reversals with naloxone in the 49 veterans who had received a naloxone kit. Of note, past studies have demonstrated that 1 death is prevented for every 227 naloxone kits distributed.8 Therefore, it is possible that the analysis was too early to detect an impact.
As of October 2016, VACT has dispensed 538 naloxone kits to 441 veterans, as some patients have received naloxone kit refills for various reasons (ie, lost, used, confiscated, stolen, etc). The OEND program implementation team has been made aware that at least 5 of the 441 veterans have used naloxone kits, though it is unclear whether naloxone kits were used on the veteran themselves or someone else. This is likely an underestimate of naloxone kit use, as veterans could be sent to alternative hospitals in the event of an opioid overdose, or veterans may not disclose actual use of naloxone kit to VACT providers. Phase 2 of the QI project is designed to extract an estimation of actual naloxone kit use in the VACT veteran population.
Discussion
Naloxone, a pure opioid antagonist, is readily available in many communities. In order to extend the same continuity of care to veterans, VACT implemented the OEND program to increase access to naloxone kits. This program serves as an opportunity to educate veterans regarding safe practices with opioids and provides a life-saving measure in the event of an overdose.
The 4-step approach to implementation was a feasible method of quickly implementing an OEND program. Despite ongoing prescriber interest in participating in the OEND program, barriers to implementation were encountered. The largest barrier was provider concerns related to an increase in opioid use given the availability of a reversal agent. However, the medical literature suggests that opioid use tends to decrease when naloxone programs are implemented. Additionally, OEND implementation barriers were internally analyzed in an effort to foster a successful OEND program with continuous evaluation and improvement.7
Clark and colleagues examined 19 published studies regarding community-based naloxone programs in order to assess whether naloxone distribution reduced fatal and nonfatal overdose rates.9 Naloxone was used successfully across 1,949 patients, and 8 studies reported survival rates of 83% to 96% after naloxone, while 11 studies reported 100% survival rate. The authors of a study of the Chicago Recovery Alliance program found an overall decrease in heroin overdose, and the initial downward trend was seen during the year of naloxone implementation.9
In addition, the cost of program implementation was minimal at the local level. Given that implementation of OENDs is a national VA mandate, all naloxone kits are provided to individual VA facilities at no cost from the Consolidated Mail Outpatient Pharmacy, which is the only foreseeable tangible cost. Therefore, intangible costs may include time spent implementing the OEND program, time spent maintaining naloxone inventory, time spent discussing and educating patients on naloxone, and time spent dispensing naloxone kits to patients. However, the intangible costs of the program are predicted to be reduced or offset by the cost-savings measure of reduced emergency department visits and hospitalizations for opioid overdose.
By implementing the OEND program at VACT, veterans are able to engage in primary prophylaxis via education regarding safe practices of opioids. These veterans are also able to obtain tertiary prophylaxis by receiving a naloxone kit and associated training. It is predicted that deaths due to opioid overdose will decrease in this veteran population with the expansion of the OEND program.
Scientific Significance
The OEND programs increase naloxone access and can potentially decrease the incidence of opioid misuse and fatal opioid overdose.
Conclusion
An OEND program can be easily implemented to dispense naloxone kits and deliver education regarding safe opioid use.
1. Chen LH, Hedegaard H, Warner M. Rates of deaths from drug poisoning and drug poisoning involving opioid analgesics—United States, 1999-2013. MMWR. 2015;64(1):32.
2. Bohnert AS, Ilgen MA, Galea S, McCarthy JF, Blow FC. Accidental poisoning mortality among patients in the Department of Veterans Affairs Health System. Med Care. 2011;49(4):393-396.
3. Bohnert AS, Ilgen MA, Trafton JA, et al. Trends and regional variation in opioid overdose mortality among Veterans Health Administration patients, fiscal year 2001 to 2009. Clin J Pain. 2014;30(7):605-612.
4. Oliva EM, Nevedal A, Lewis ET, et al. Patient perspectives on an opioid overdose education and naloxone distribution program in the U.S. Department of Veterans Affairs. Subst Abus. 2016;37(1):118-126.
5. Maxwell S, Bigg D, Stanczykiewicz K, Carlberg-Racich S. Prescribing naloxone to actively injecting heroin users: a program to reduce heroin overdose deaths. J Addict Dis. 2006;25(3):89-96.
6. Piper TM, Stancliff S, Rudenstine S, et al. Evaluation of a naloxone distribution and administration program in New York City. Subst Use Misuse. 2008;43(7):858-870.
7. Peckham AM, Niculete ME, Steinberg, HR, et al. A survey of a Veterans Administration Healthcare System to determine medication provider acceptance of the Opioid Overdose Education and Naloxone Distribution Program (OEND). Subst Abus. In press.
8. Coffin PO, Sullivan SD. Cost-effectiveness of distributing naloxone to heroin users for lay overdose reversal. Ann Intern Med. 2013;158(1):1-9.
9. Clark AK, Wilder CM, Winstanley EL. A systematic review of community opioid overdose prevention and naloxone distribution programs. J Addict Med. 2014;8(3):153-163.
Fatal opioid overdoses have quadrupled in the U.S. from 1999 to 2013.1 In 2013, 43,982 deaths in the U.S. were attributable to drug overdoses, and 37% of them involved opioids.1 Data from 2005 suggested that veterans are at an increased risk with a 2-fold rise in overdose observed.2 In 2013, there were 17,124 encounters at VA facilities for the treatment of opioid overdose.3 In response to this opioid epidemic, individual VA facilities began implementing Overdose Education and Naloxone Distribution (OEND) programs in 2013.4 In May 2014, the Under Secretary for Health mandated the implementation of OEND programs across all VA facilities, and the VA became the only health care system in the U.S. with a national OEND program.4
Naloxone
Naloxone is a pure opioid antagonist that competes with and displaces opioids at opioid receptors. In an overdose, opioids occupying opioid receptor sites can lead to respiratory depression, which can lead to death. Patients can experience rapid reversal of respiratory depression and opioid overdose with the first dose of naloxone 0.4 mg. Naloxone has a short half-life relative to opioids, whereas opioids can bind to opioid receptors once again after naloxone is eliminated, risking the reemergence of overdose symptoms. For this reason, a second dose of naloxone is often given. When administered appropriately with close monitoring, naloxone can provide a safe and effective way to reverse a potentially life-threatening opioid overdose.
Availability
In April 2014, Evzio, an intramuscular/subcutaneous formulation of naloxone, was FDA approved as an emergency treatment for known or suspected opioid overdose. Shortly after, in November 2015, Narcan, a nasal spray formulation of naloxone, also was approved. Each formulation has been instrumental in fulfilling an unmet medical need in the U.S. Both Evzio and Narcan have been used in opioid-overdose prevention programs to train health care professionals and lay persons to respond in the event of suspected overdose and administer naloxone.
Maxwell and colleagues implemented a naloxone distribution program in Chicago, Illinois, in January 2001 after a 4-fold increase in heroin-related overdose deaths was observed.5 Within a year, Chicago experienced a 20% decrease in heroin-related overdose deaths, and this trend continued with additional 10% reductions in heroin-related overdoses in 2002 and 2003. Similarly, Piper and colleagues implemented a naloxone distribution program and found that 82% of injection drug users felt “comfortable” to “very comfortable” administering naloxone in the event of an overdose, and 86% of subjects reported they would want naloxone in the event of an overdose.6
Naloxone is available for over-the-counter purchase in Rhode Island, which is a bordering state to VA Connecticut Healthcare System (VACT). In 2015 new legislation permitted the training and certification of Connecticut community pharmacists to prescribe naloxone. Furthermore, in May 2016, Connecticut passed legislation to permit qualified individuals to carry naloxone and administer it to another person that he or she, believes is experiencing an opioid-related drug overdose. Given the increase in naloxone availability both nationally and locally, VACT sought to implement a naloxone distribution program to offer the same access to veterans. The primary objective of this article is to describe the development, implementation, and preliminary results of this OEND program.
Development
In accordance with VA guidance, the VACT OEND program development and implementation process included 4 steps that required program coordinators to identify target populations, garner support, train staff members, and implement the OEND program.
A multidisciplinary team of representatives from pharmacy, mental health (MH), and substance abuse (SA) departments was established to draft hospital policy. The hospital policy detailed job functions related to the OEND program for prescribing, educating, verifying, and dispensing naloxone. This policy also served as a document to identify the target population.
With resources from the National VA Pharmacy Benefits Manager, the target population for VACT listed veterans with an opioid use disorder, prescription opioid use disorder, and injection opioid use disorder. Also listed were veterans at risk of an opioid overdose, such as those encountered in medication-assisted treatment programs for opioid use disorder, recent inpatient withdrawal management for opioid use disorder, HIV education/prevention programs, syringe access programs, outpatient and residential opioid use disorder treatment programs, community meetings/support group programs for opioid use disorder, emergency departments for opioid overdose or intoxication, domiciliary care or community-based treatment for homeless veterans, and primary health care for follow-up of recent opioid overdose or intoxication. The aim of the broad description of veterans was to ensure that naloxone access remained inclusive.
The program overview and hospital policy were presented to the VACT medication management committee (MMC), which approved a small pilot project for veterans engaged in SA treatment from January 2015 to April 2015. Barriers to implementation were encountered at this time, which included a belief that only MH or SA providers should prescribe naloxone rescue kits. Reasons for this were largely related to the belief that MH and SA providers are more familiar with substance use disorders. These barriers were addressed by increasing education to providers by detailing previous success stories regarding naloxone programs to highlight that the benefits outweighed any associated risks.
Implementation
Naloxone kits were assembled in preparation for program implementation. Each kit contained 2 vials of naloxone (each vial contains 1 dose), 2 needleless luer-lock syringes, and 1 atomizer with visual instructions for product assembly. Next, a template was created for entry into the veteran’s electronic health record (EHR) each time a naloxone kit was dispensed. The template tracked diagnosis, history of naloxone kit use, and aspects of the OEND program that were discussed with the veteran. Embedded within the template is a hyperlink to the Substance Abuse and Mental Health Services Administration Opioid Overdose Prevention Toolkit booklet, which serves as an education guide and take-home material for the veteran.
Once the naloxone kits were assembled and the template created, SA providers were able to engage in OEND program training. Training required providers to attend a 60-minute, 75-slide PowerPoint seminar, highlighting risk factors and prevention strategies for opioid overdose, proper identification and management of an opioid overdose, appropriate administration of naloxone, and monitoring parameters after administration. Providers were not screened for baseline knowledge, nor was a posttraining proficiency evaluation performed. However, providers were allowed to ask questions and request further clarification about any unclear aspects of training.
Each month, data were collected on the number of providers trained and naloxone kits dispensed. Implementation began in January 2015, and veterans were either educated during individual or group sessions led by a trained OEND provider. An institutional review board-approved quality improvement (QI) process ran concurrently that sought to improve the OEND program. Phase 1 of this QI project anonymously surveyed OEND providers to evaluate knowledge, comfort, attitude, and fear of consequences regarding naloxone distribution in an attempt to understand barriers to program implementation or success.7 In phase 2, veterans who received a naloxone kit will be interviewed regarding knowledge retention of OEND program education and subsequent opioid abuse and naloxone use after receiving a naloxone kit.
Results
From January 2015 through preliminary data analysis in April 2015, the facility trained 19 providers and dispensed naloxone kits to 49 veterans. In the first 4 months of program implementation, there were no reports of attempted opioid overdose reversals with naloxone in the 49 veterans who had received a naloxone kit. Of note, past studies have demonstrated that 1 death is prevented for every 227 naloxone kits distributed.8 Therefore, it is possible that the analysis was too early to detect an impact.
As of October 2016, VACT has dispensed 538 naloxone kits to 441 veterans, as some patients have received naloxone kit refills for various reasons (ie, lost, used, confiscated, stolen, etc). The OEND program implementation team has been made aware that at least 5 of the 441 veterans have used naloxone kits, though it is unclear whether naloxone kits were used on the veteran themselves or someone else. This is likely an underestimate of naloxone kit use, as veterans could be sent to alternative hospitals in the event of an opioid overdose, or veterans may not disclose actual use of naloxone kit to VACT providers. Phase 2 of the QI project is designed to extract an estimation of actual naloxone kit use in the VACT veteran population.
Discussion
Naloxone, a pure opioid antagonist, is readily available in many communities. In order to extend the same continuity of care to veterans, VACT implemented the OEND program to increase access to naloxone kits. This program serves as an opportunity to educate veterans regarding safe practices with opioids and provides a life-saving measure in the event of an overdose.
The 4-step approach to implementation was a feasible method of quickly implementing an OEND program. Despite ongoing prescriber interest in participating in the OEND program, barriers to implementation were encountered. The largest barrier was provider concerns related to an increase in opioid use given the availability of a reversal agent. However, the medical literature suggests that opioid use tends to decrease when naloxone programs are implemented. Additionally, OEND implementation barriers were internally analyzed in an effort to foster a successful OEND program with continuous evaluation and improvement.7
Clark and colleagues examined 19 published studies regarding community-based naloxone programs in order to assess whether naloxone distribution reduced fatal and nonfatal overdose rates.9 Naloxone was used successfully across 1,949 patients, and 8 studies reported survival rates of 83% to 96% after naloxone, while 11 studies reported 100% survival rate. The authors of a study of the Chicago Recovery Alliance program found an overall decrease in heroin overdose, and the initial downward trend was seen during the year of naloxone implementation.9
In addition, the cost of program implementation was minimal at the local level. Given that implementation of OENDs is a national VA mandate, all naloxone kits are provided to individual VA facilities at no cost from the Consolidated Mail Outpatient Pharmacy, which is the only foreseeable tangible cost. Therefore, intangible costs may include time spent implementing the OEND program, time spent maintaining naloxone inventory, time spent discussing and educating patients on naloxone, and time spent dispensing naloxone kits to patients. However, the intangible costs of the program are predicted to be reduced or offset by the cost-savings measure of reduced emergency department visits and hospitalizations for opioid overdose.
By implementing the OEND program at VACT, veterans are able to engage in primary prophylaxis via education regarding safe practices of opioids. These veterans are also able to obtain tertiary prophylaxis by receiving a naloxone kit and associated training. It is predicted that deaths due to opioid overdose will decrease in this veteran population with the expansion of the OEND program.
Scientific Significance
The OEND programs increase naloxone access and can potentially decrease the incidence of opioid misuse and fatal opioid overdose.
Conclusion
An OEND program can be easily implemented to dispense naloxone kits and deliver education regarding safe opioid use.
Fatal opioid overdoses have quadrupled in the U.S. from 1999 to 2013.1 In 2013, 43,982 deaths in the U.S. were attributable to drug overdoses, and 37% of them involved opioids.1 Data from 2005 suggested that veterans are at an increased risk with a 2-fold rise in overdose observed.2 In 2013, there were 17,124 encounters at VA facilities for the treatment of opioid overdose.3 In response to this opioid epidemic, individual VA facilities began implementing Overdose Education and Naloxone Distribution (OEND) programs in 2013.4 In May 2014, the Under Secretary for Health mandated the implementation of OEND programs across all VA facilities, and the VA became the only health care system in the U.S. with a national OEND program.4
Naloxone
Naloxone is a pure opioid antagonist that competes with and displaces opioids at opioid receptors. In an overdose, opioids occupying opioid receptor sites can lead to respiratory depression, which can lead to death. Patients can experience rapid reversal of respiratory depression and opioid overdose with the first dose of naloxone 0.4 mg. Naloxone has a short half-life relative to opioids, whereas opioids can bind to opioid receptors once again after naloxone is eliminated, risking the reemergence of overdose symptoms. For this reason, a second dose of naloxone is often given. When administered appropriately with close monitoring, naloxone can provide a safe and effective way to reverse a potentially life-threatening opioid overdose.
Availability
In April 2014, Evzio, an intramuscular/subcutaneous formulation of naloxone, was FDA approved as an emergency treatment for known or suspected opioid overdose. Shortly after, in November 2015, Narcan, a nasal spray formulation of naloxone, also was approved. Each formulation has been instrumental in fulfilling an unmet medical need in the U.S. Both Evzio and Narcan have been used in opioid-overdose prevention programs to train health care professionals and lay persons to respond in the event of suspected overdose and administer naloxone.
Maxwell and colleagues implemented a naloxone distribution program in Chicago, Illinois, in January 2001 after a 4-fold increase in heroin-related overdose deaths was observed.5 Within a year, Chicago experienced a 20% decrease in heroin-related overdose deaths, and this trend continued with additional 10% reductions in heroin-related overdoses in 2002 and 2003. Similarly, Piper and colleagues implemented a naloxone distribution program and found that 82% of injection drug users felt “comfortable” to “very comfortable” administering naloxone in the event of an overdose, and 86% of subjects reported they would want naloxone in the event of an overdose.6
Naloxone is available for over-the-counter purchase in Rhode Island, which is a bordering state to VA Connecticut Healthcare System (VACT). In 2015 new legislation permitted the training and certification of Connecticut community pharmacists to prescribe naloxone. Furthermore, in May 2016, Connecticut passed legislation to permit qualified individuals to carry naloxone and administer it to another person that he or she, believes is experiencing an opioid-related drug overdose. Given the increase in naloxone availability both nationally and locally, VACT sought to implement a naloxone distribution program to offer the same access to veterans. The primary objective of this article is to describe the development, implementation, and preliminary results of this OEND program.
Development
In accordance with VA guidance, the VACT OEND program development and implementation process included 4 steps that required program coordinators to identify target populations, garner support, train staff members, and implement the OEND program.
A multidisciplinary team of representatives from pharmacy, mental health (MH), and substance abuse (SA) departments was established to draft hospital policy. The hospital policy detailed job functions related to the OEND program for prescribing, educating, verifying, and dispensing naloxone. This policy also served as a document to identify the target population.
With resources from the National VA Pharmacy Benefits Manager, the target population for VACT listed veterans with an opioid use disorder, prescription opioid use disorder, and injection opioid use disorder. Also listed were veterans at risk of an opioid overdose, such as those encountered in medication-assisted treatment programs for opioid use disorder, recent inpatient withdrawal management for opioid use disorder, HIV education/prevention programs, syringe access programs, outpatient and residential opioid use disorder treatment programs, community meetings/support group programs for opioid use disorder, emergency departments for opioid overdose or intoxication, domiciliary care or community-based treatment for homeless veterans, and primary health care for follow-up of recent opioid overdose or intoxication. The aim of the broad description of veterans was to ensure that naloxone access remained inclusive.
The program overview and hospital policy were presented to the VACT medication management committee (MMC), which approved a small pilot project for veterans engaged in SA treatment from January 2015 to April 2015. Barriers to implementation were encountered at this time, which included a belief that only MH or SA providers should prescribe naloxone rescue kits. Reasons for this were largely related to the belief that MH and SA providers are more familiar with substance use disorders. These barriers were addressed by increasing education to providers by detailing previous success stories regarding naloxone programs to highlight that the benefits outweighed any associated risks.
Implementation
Naloxone kits were assembled in preparation for program implementation. Each kit contained 2 vials of naloxone (each vial contains 1 dose), 2 needleless luer-lock syringes, and 1 atomizer with visual instructions for product assembly. Next, a template was created for entry into the veteran’s electronic health record (EHR) each time a naloxone kit was dispensed. The template tracked diagnosis, history of naloxone kit use, and aspects of the OEND program that were discussed with the veteran. Embedded within the template is a hyperlink to the Substance Abuse and Mental Health Services Administration Opioid Overdose Prevention Toolkit booklet, which serves as an education guide and take-home material for the veteran.
Once the naloxone kits were assembled and the template created, SA providers were able to engage in OEND program training. Training required providers to attend a 60-minute, 75-slide PowerPoint seminar, highlighting risk factors and prevention strategies for opioid overdose, proper identification and management of an opioid overdose, appropriate administration of naloxone, and monitoring parameters after administration. Providers were not screened for baseline knowledge, nor was a posttraining proficiency evaluation performed. However, providers were allowed to ask questions and request further clarification about any unclear aspects of training.
Each month, data were collected on the number of providers trained and naloxone kits dispensed. Implementation began in January 2015, and veterans were either educated during individual or group sessions led by a trained OEND provider. An institutional review board-approved quality improvement (QI) process ran concurrently that sought to improve the OEND program. Phase 1 of this QI project anonymously surveyed OEND providers to evaluate knowledge, comfort, attitude, and fear of consequences regarding naloxone distribution in an attempt to understand barriers to program implementation or success.7 In phase 2, veterans who received a naloxone kit will be interviewed regarding knowledge retention of OEND program education and subsequent opioid abuse and naloxone use after receiving a naloxone kit.
Results
From January 2015 through preliminary data analysis in April 2015, the facility trained 19 providers and dispensed naloxone kits to 49 veterans. In the first 4 months of program implementation, there were no reports of attempted opioid overdose reversals with naloxone in the 49 veterans who had received a naloxone kit. Of note, past studies have demonstrated that 1 death is prevented for every 227 naloxone kits distributed.8 Therefore, it is possible that the analysis was too early to detect an impact.
As of October 2016, VACT has dispensed 538 naloxone kits to 441 veterans, as some patients have received naloxone kit refills for various reasons (ie, lost, used, confiscated, stolen, etc). The OEND program implementation team has been made aware that at least 5 of the 441 veterans have used naloxone kits, though it is unclear whether naloxone kits were used on the veteran themselves or someone else. This is likely an underestimate of naloxone kit use, as veterans could be sent to alternative hospitals in the event of an opioid overdose, or veterans may not disclose actual use of naloxone kit to VACT providers. Phase 2 of the QI project is designed to extract an estimation of actual naloxone kit use in the VACT veteran population.
Discussion
Naloxone, a pure opioid antagonist, is readily available in many communities. In order to extend the same continuity of care to veterans, VACT implemented the OEND program to increase access to naloxone kits. This program serves as an opportunity to educate veterans regarding safe practices with opioids and provides a life-saving measure in the event of an overdose.
The 4-step approach to implementation was a feasible method of quickly implementing an OEND program. Despite ongoing prescriber interest in participating in the OEND program, barriers to implementation were encountered. The largest barrier was provider concerns related to an increase in opioid use given the availability of a reversal agent. However, the medical literature suggests that opioid use tends to decrease when naloxone programs are implemented. Additionally, OEND implementation barriers were internally analyzed in an effort to foster a successful OEND program with continuous evaluation and improvement.7
Clark and colleagues examined 19 published studies regarding community-based naloxone programs in order to assess whether naloxone distribution reduced fatal and nonfatal overdose rates.9 Naloxone was used successfully across 1,949 patients, and 8 studies reported survival rates of 83% to 96% after naloxone, while 11 studies reported 100% survival rate. The authors of a study of the Chicago Recovery Alliance program found an overall decrease in heroin overdose, and the initial downward trend was seen during the year of naloxone implementation.9
In addition, the cost of program implementation was minimal at the local level. Given that implementation of OENDs is a national VA mandate, all naloxone kits are provided to individual VA facilities at no cost from the Consolidated Mail Outpatient Pharmacy, which is the only foreseeable tangible cost. Therefore, intangible costs may include time spent implementing the OEND program, time spent maintaining naloxone inventory, time spent discussing and educating patients on naloxone, and time spent dispensing naloxone kits to patients. However, the intangible costs of the program are predicted to be reduced or offset by the cost-savings measure of reduced emergency department visits and hospitalizations for opioid overdose.
By implementing the OEND program at VACT, veterans are able to engage in primary prophylaxis via education regarding safe practices of opioids. These veterans are also able to obtain tertiary prophylaxis by receiving a naloxone kit and associated training. It is predicted that deaths due to opioid overdose will decrease in this veteran population with the expansion of the OEND program.
Scientific Significance
The OEND programs increase naloxone access and can potentially decrease the incidence of opioid misuse and fatal opioid overdose.
Conclusion
An OEND program can be easily implemented to dispense naloxone kits and deliver education regarding safe opioid use.
1. Chen LH, Hedegaard H, Warner M. Rates of deaths from drug poisoning and drug poisoning involving opioid analgesics—United States, 1999-2013. MMWR. 2015;64(1):32.
2. Bohnert AS, Ilgen MA, Galea S, McCarthy JF, Blow FC. Accidental poisoning mortality among patients in the Department of Veterans Affairs Health System. Med Care. 2011;49(4):393-396.
3. Bohnert AS, Ilgen MA, Trafton JA, et al. Trends and regional variation in opioid overdose mortality among Veterans Health Administration patients, fiscal year 2001 to 2009. Clin J Pain. 2014;30(7):605-612.
4. Oliva EM, Nevedal A, Lewis ET, et al. Patient perspectives on an opioid overdose education and naloxone distribution program in the U.S. Department of Veterans Affairs. Subst Abus. 2016;37(1):118-126.
5. Maxwell S, Bigg D, Stanczykiewicz K, Carlberg-Racich S. Prescribing naloxone to actively injecting heroin users: a program to reduce heroin overdose deaths. J Addict Dis. 2006;25(3):89-96.
6. Piper TM, Stancliff S, Rudenstine S, et al. Evaluation of a naloxone distribution and administration program in New York City. Subst Use Misuse. 2008;43(7):858-870.
7. Peckham AM, Niculete ME, Steinberg, HR, et al. A survey of a Veterans Administration Healthcare System to determine medication provider acceptance of the Opioid Overdose Education and Naloxone Distribution Program (OEND). Subst Abus. In press.
8. Coffin PO, Sullivan SD. Cost-effectiveness of distributing naloxone to heroin users for lay overdose reversal. Ann Intern Med. 2013;158(1):1-9.
9. Clark AK, Wilder CM, Winstanley EL. A systematic review of community opioid overdose prevention and naloxone distribution programs. J Addict Med. 2014;8(3):153-163.
1. Chen LH, Hedegaard H, Warner M. Rates of deaths from drug poisoning and drug poisoning involving opioid analgesics—United States, 1999-2013. MMWR. 2015;64(1):32.
2. Bohnert AS, Ilgen MA, Galea S, McCarthy JF, Blow FC. Accidental poisoning mortality among patients in the Department of Veterans Affairs Health System. Med Care. 2011;49(4):393-396.
3. Bohnert AS, Ilgen MA, Trafton JA, et al. Trends and regional variation in opioid overdose mortality among Veterans Health Administration patients, fiscal year 2001 to 2009. Clin J Pain. 2014;30(7):605-612.
4. Oliva EM, Nevedal A, Lewis ET, et al. Patient perspectives on an opioid overdose education and naloxone distribution program in the U.S. Department of Veterans Affairs. Subst Abus. 2016;37(1):118-126.
5. Maxwell S, Bigg D, Stanczykiewicz K, Carlberg-Racich S. Prescribing naloxone to actively injecting heroin users: a program to reduce heroin overdose deaths. J Addict Dis. 2006;25(3):89-96.
6. Piper TM, Stancliff S, Rudenstine S, et al. Evaluation of a naloxone distribution and administration program in New York City. Subst Use Misuse. 2008;43(7):858-870.
7. Peckham AM, Niculete ME, Steinberg, HR, et al. A survey of a Veterans Administration Healthcare System to determine medication provider acceptance of the Opioid Overdose Education and Naloxone Distribution Program (OEND). Subst Abus. In press.
8. Coffin PO, Sullivan SD. Cost-effectiveness of distributing naloxone to heroin users for lay overdose reversal. Ann Intern Med. 2013;158(1):1-9.
9. Clark AK, Wilder CM, Winstanley EL. A systematic review of community opioid overdose prevention and naloxone distribution programs. J Addict Med. 2014;8(3):153-163.
Tips for Hospitalists on Solving Difficult Situations
At Bay Area Medical Center in Marinette, Wis., the time had come to start talking about an elderly woman’s end-of-life care.
Her hospitalist thought that those discussions should take place with the patient present, but the woman’s family felt otherwise and made this known to the hospitalist, who stood his ground.
Eventually, the family told a nurse that they wanted to fire the physician. But the only other hospitalist on shift didn’t want to take the patient.
As case managers and hospital administrators tried to wrap their heads around the situation, it became clear: They didn’t really know what to do.
Could the patient fire a physician? Was the second physician obligated to take what he knew from the outset would be a difficult case? What if nobody wanted to take care of this patient?
“There was no black-and-white to this,” says Robin Dequaine, director of medical staff services at the hospital, who was involved in the case.
Some “difficult patient” scenarios are fairly straightforward. A patient is violent? Enact your security measures. An addict wants narcotics? Don’t give them.
But there are other situations that enter murkier territory: What if a patient makes inappropriate or abusive remarks? How much should a hospitalist put up with? What if a patient’s request for treatment might not be the hospitalist’s first choice but could be seen as reasonable? Is the patient’s request accommodated? And what about those firings?
Hospitalists, administrators, and patient advocates say these tense situations with patients involving firings, or would-be firings, while not a daily occurrence, are actually fairly common.1 Getting to the root of the problem is essential. And as with so much in healthcare, good communication is the absolute crux of it all, they say.
“These are almost all communication issues,” says John Bulger, DO, MBA, FACP, SFHM, chief medical officer at Geisinger Health Plan in Danville, Pa., who has had a long career as a hospitalist and administrator handling and trying to resolve these situations. “They’re all [about] the way the hospitalist and the team is relating to the patient.”
Jackie O’Doherty, a private patient advocate who practices in New Jersey and New York across a gamut of hospital types, has a similar view.
“For me, the biggest problem, period, against hospitalists, doctors, everybody in the hospital, is communication—the lack of it,” she says. “Their communication skills are really poor.”
Patients accustomed to choice in the outpatient setting might not handle it well when they don’t have an established relationship with their hospitalist, says John Vazquez, MD, associate director for the Emory University School of Medicine’s Division of Hospital Medicine in Atlanta.
But the system, he says, “does not allow for, unfortunately, that much patient choice.”
End-of-life Discussion at a Small Hospital
Dequaine says the staff at Bay Area Medical Center was caught flat-footed with the case of the family not wanting end-of-life care discussed with their elderly mother.
“The doctor felt very confident that he was in a position that he could have that discussion in front of the patient,” she says.
At the 99-bed center, there were just two hospitalists, who were also employees of the hospital, on shift. And the communication channels involving the medical director of hospital medicine, a case manager, and the chief nursing executive were not well-controlled, Dequaine says.
“It didn’t go up the ladder correctly,” she says. “Too many people got involved, not knowing that somebody else was already involved.”
The second hospitalist at first said he would take the case, but later Dequaine learned that he changed his mind.
“He knew his care would be no different, and we were very, very busy, so they both had a high census already,” Dequaine says.
A third physician reluctantly took over until the issue subsided. And the family still brings the patient to the hospital for care.
Ultimately, the center adopted a new policy that doesn’t guarantee a patient a new doctor, only that the hospital will have frank discussions to try to resolve the issue and then try to arrange for a transfer if the situation can’t be resolved.
“The goal is not to get rid of the patient or to force them to keep the provider,” Dequaine says. “The goal is to resolve it in a mutually satisfactory way.”
A Patient Demands a Contraindicated Medication
A middle-aged woman with Crohn’s disease was hospitalized at Emory with an infection. The woman, worried about her disease flaring, wanted to keep getting her immunosuppressant, but the hospitalist suspended it because she needed to fight off the infection. The patient became upset. At a point when the hospitalist wasn’t in the room, the woman insisted to a nurse that she get her medication. The nurse called a doctor who was on call, but that doctor wouldn’t give the immunosuppressant either.
The patient began to think she wasn’t being listened to. Dr. Vazquez went in to see the patient and apologized for the misunderstanding.
“I went back into the room and explained here’s why I’m doing it: ‘I totally understand where you’re coming from; you don’t want your disease to be out of control. I appreciate that. What I’m worried about is killing you if we give you an immunosuppressant at the wrong time,’” Dr. Vazquez says.
Dr. Vazquez has underscored at his center how important it is for the physicians to be consulted and go back into the room when patients want to fire them, even though the expedient step might be to just bring in a new doctor. At previous centers, he says, it wouldn’t be unusual for the director to get a call from a nurse, who would say, “Yeah, they want to fire this physician, so let me know who’s going to see the patient.”
But simply switching doctors, he cautions, is like saying, “I agree with you we have incompetent doctors here, so we’re going to remove that doctor and I’m going to put a doctor on who actually knows what they’re doing.”
When doctors try to resolve the issues, good things tend to happen, Dr. Vazquez says.
“There’s generally a large amount of appreciation that someone comes back into the room and says, ‘We want to do this right.’”
Of course, there are times when, if tension remains after such discussions, patient care might be better served by a swap. At large centers, that might be possible, Dr. Bulger of Geisinger says.
“If the patient doesn’t tell the doctor something because he or she doesn’t like the doctor, then the doctor’s decisions are made on partial information—that’s the issue,” he says.
O’Doherty, the patient advocate, says that if patients frustrated with poor communication actually fired physicians as often as they would like, there would be more firings.
“Patients don’t like firing the doctors because they don’t want to be the patient who everybody doesn’t like,” she says. “They’re afraid that if they argue or disagree or ask too many questions, that they’re not going to get the care they need. And the family is afraid of that as well, especially in the older population. They think doctors are like God, they hold your life in their hands. So they don’t want to really question doctors.”
She says patients don’t necessarily need a particular finesse or expert bedside manner. In many cases, she says, it’s “just giving the information.”
A Patient Demands Pain Medication
Martin Austin, MD, SFHM, recently cared for a patient with chronic headaches. The patient asked for higher doses of pain medication, insinuating that she might turn to heroin if denied.
“I was trying to make the argument that I kind of disagreed with that but, ‘I respect your opinion,’” says Dr. Austin, medical director at the Gwinnett Medical Center Inpatient Medical Group in Georgia. “We came to a negotiation about how long we would use narcotics acutely until her other acute issues were over, but then we would try to get her away from narcotics.”
A good approach, he says, is to “outline to the patient why you’re doing what you’re doing. We try not to pick battles and give the patient some degree of control if it’s not contraindicated.”
But sometimes there can be no negotiating these kinds of requests, he says.
“Sometimes we’ll just say, ‘Look, it’s not a good thing for you to continue on this medication. You’re showing side effects, you’re sedated. … We think that the risk outweighs the benefit in this case,” he says.
A Patient Feels Left in the Dark
One patient at Emory wanted to fire his hospitalist because he wouldn’t tell him what was on his CT scan.
Dr. Vazquez held a discussion between the patient and the doctor. If not for the seriousness of the patient’s condition (he had tremors and neurological concerns), it would have been almost comical.
The patient had asked, “What’s on my scan?” The patient interpreted the doctor’s response, “It’s negative,” to mean that he wasn’t being told something about the scan.
Dr. Vazquez realized that the patient had felt dismissed.
“He was a sick gentleman,” Dr. Vazquez says. “And what he wanted to hear was, ‘Look, the great news is your CT scan looks good. There’s not an anatomical abnormality. It’s not a tumor. It’s not a big bleed. … That’s great news, but I, as a physician, I am concerned about you. You’re sick. We’ve got to really figure out what’s going on with you.’… He wanted a pat on the back, and that’s all it took.”
After that, the patient no longer wanted to fire the hospitalist.
Verbal Abuse
One case at Gwinnett involves a hospitalist who was quite shy and easily intimidated and was not comfortable with a patient.
“They were struggling with a patient who was very difficult and very angry and a little abusive,” Dr. Austin says. “This doctor was really suffering psychically from this whole thing, and we switched.” Another doctor, who would not be thrown by the situation, took over the case. And Dr. Austin says he had great respect for the first doctor’s request to hand over the case.
“They needed a different personality,” he says. “It worked out beautifully. The patient and the doctor got along much better. The doctor was firm with the patient but respectful, and the other doctor felt relieved. And the [original] doctor is great with patients who need a lot of emotional support, probably better than the other doctor. So that worked out really well.”
It might be a challenge during a busy day, but it’s helpful to step back and see the situation as a whole, Dr. Bulger says. Sometimes, hospitalists can get flustered when patients are not acting rationally. But there’s usually a good reason they’re acting that way, he says.
“The patient is sick. And if it’s the patient’s family, they’re stressed by the fact that the patient’s sick. So you really need to take a step back and understand that.” TH
Thomas R. Collins is a freelance writer based in West Palm Beach, Fla.
Reference
- Centor R. Can I fire my hospitalist? SGIM Forum. 32(5):112-13.
At Bay Area Medical Center in Marinette, Wis., the time had come to start talking about an elderly woman’s end-of-life care.
Her hospitalist thought that those discussions should take place with the patient present, but the woman’s family felt otherwise and made this known to the hospitalist, who stood his ground.
Eventually, the family told a nurse that they wanted to fire the physician. But the only other hospitalist on shift didn’t want to take the patient.
As case managers and hospital administrators tried to wrap their heads around the situation, it became clear: They didn’t really know what to do.
Could the patient fire a physician? Was the second physician obligated to take what he knew from the outset would be a difficult case? What if nobody wanted to take care of this patient?
“There was no black-and-white to this,” says Robin Dequaine, director of medical staff services at the hospital, who was involved in the case.
Some “difficult patient” scenarios are fairly straightforward. A patient is violent? Enact your security measures. An addict wants narcotics? Don’t give them.
But there are other situations that enter murkier territory: What if a patient makes inappropriate or abusive remarks? How much should a hospitalist put up with? What if a patient’s request for treatment might not be the hospitalist’s first choice but could be seen as reasonable? Is the patient’s request accommodated? And what about those firings?
Hospitalists, administrators, and patient advocates say these tense situations with patients involving firings, or would-be firings, while not a daily occurrence, are actually fairly common.1 Getting to the root of the problem is essential. And as with so much in healthcare, good communication is the absolute crux of it all, they say.
“These are almost all communication issues,” says John Bulger, DO, MBA, FACP, SFHM, chief medical officer at Geisinger Health Plan in Danville, Pa., who has had a long career as a hospitalist and administrator handling and trying to resolve these situations. “They’re all [about] the way the hospitalist and the team is relating to the patient.”
Jackie O’Doherty, a private patient advocate who practices in New Jersey and New York across a gamut of hospital types, has a similar view.
“For me, the biggest problem, period, against hospitalists, doctors, everybody in the hospital, is communication—the lack of it,” she says. “Their communication skills are really poor.”
Patients accustomed to choice in the outpatient setting might not handle it well when they don’t have an established relationship with their hospitalist, says John Vazquez, MD, associate director for the Emory University School of Medicine’s Division of Hospital Medicine in Atlanta.
But the system, he says, “does not allow for, unfortunately, that much patient choice.”
End-of-life Discussion at a Small Hospital
Dequaine says the staff at Bay Area Medical Center was caught flat-footed with the case of the family not wanting end-of-life care discussed with their elderly mother.
“The doctor felt very confident that he was in a position that he could have that discussion in front of the patient,” she says.
At the 99-bed center, there were just two hospitalists, who were also employees of the hospital, on shift. And the communication channels involving the medical director of hospital medicine, a case manager, and the chief nursing executive were not well-controlled, Dequaine says.
“It didn’t go up the ladder correctly,” she says. “Too many people got involved, not knowing that somebody else was already involved.”
The second hospitalist at first said he would take the case, but later Dequaine learned that he changed his mind.
“He knew his care would be no different, and we were very, very busy, so they both had a high census already,” Dequaine says.
A third physician reluctantly took over until the issue subsided. And the family still brings the patient to the hospital for care.
Ultimately, the center adopted a new policy that doesn’t guarantee a patient a new doctor, only that the hospital will have frank discussions to try to resolve the issue and then try to arrange for a transfer if the situation can’t be resolved.
“The goal is not to get rid of the patient or to force them to keep the provider,” Dequaine says. “The goal is to resolve it in a mutually satisfactory way.”
A Patient Demands a Contraindicated Medication
A middle-aged woman with Crohn’s disease was hospitalized at Emory with an infection. The woman, worried about her disease flaring, wanted to keep getting her immunosuppressant, but the hospitalist suspended it because she needed to fight off the infection. The patient became upset. At a point when the hospitalist wasn’t in the room, the woman insisted to a nurse that she get her medication. The nurse called a doctor who was on call, but that doctor wouldn’t give the immunosuppressant either.
The patient began to think she wasn’t being listened to. Dr. Vazquez went in to see the patient and apologized for the misunderstanding.
“I went back into the room and explained here’s why I’m doing it: ‘I totally understand where you’re coming from; you don’t want your disease to be out of control. I appreciate that. What I’m worried about is killing you if we give you an immunosuppressant at the wrong time,’” Dr. Vazquez says.
Dr. Vazquez has underscored at his center how important it is for the physicians to be consulted and go back into the room when patients want to fire them, even though the expedient step might be to just bring in a new doctor. At previous centers, he says, it wouldn’t be unusual for the director to get a call from a nurse, who would say, “Yeah, they want to fire this physician, so let me know who’s going to see the patient.”
But simply switching doctors, he cautions, is like saying, “I agree with you we have incompetent doctors here, so we’re going to remove that doctor and I’m going to put a doctor on who actually knows what they’re doing.”
When doctors try to resolve the issues, good things tend to happen, Dr. Vazquez says.
“There’s generally a large amount of appreciation that someone comes back into the room and says, ‘We want to do this right.’”
Of course, there are times when, if tension remains after such discussions, patient care might be better served by a swap. At large centers, that might be possible, Dr. Bulger of Geisinger says.
“If the patient doesn’t tell the doctor something because he or she doesn’t like the doctor, then the doctor’s decisions are made on partial information—that’s the issue,” he says.
O’Doherty, the patient advocate, says that if patients frustrated with poor communication actually fired physicians as often as they would like, there would be more firings.
“Patients don’t like firing the doctors because they don’t want to be the patient who everybody doesn’t like,” she says. “They’re afraid that if they argue or disagree or ask too many questions, that they’re not going to get the care they need. And the family is afraid of that as well, especially in the older population. They think doctors are like God, they hold your life in their hands. So they don’t want to really question doctors.”
She says patients don’t necessarily need a particular finesse or expert bedside manner. In many cases, she says, it’s “just giving the information.”
A Patient Demands Pain Medication
Martin Austin, MD, SFHM, recently cared for a patient with chronic headaches. The patient asked for higher doses of pain medication, insinuating that she might turn to heroin if denied.
“I was trying to make the argument that I kind of disagreed with that but, ‘I respect your opinion,’” says Dr. Austin, medical director at the Gwinnett Medical Center Inpatient Medical Group in Georgia. “We came to a negotiation about how long we would use narcotics acutely until her other acute issues were over, but then we would try to get her away from narcotics.”
A good approach, he says, is to “outline to the patient why you’re doing what you’re doing. We try not to pick battles and give the patient some degree of control if it’s not contraindicated.”
But sometimes there can be no negotiating these kinds of requests, he says.
“Sometimes we’ll just say, ‘Look, it’s not a good thing for you to continue on this medication. You’re showing side effects, you’re sedated. … We think that the risk outweighs the benefit in this case,” he says.
A Patient Feels Left in the Dark
One patient at Emory wanted to fire his hospitalist because he wouldn’t tell him what was on his CT scan.
Dr. Vazquez held a discussion between the patient and the doctor. If not for the seriousness of the patient’s condition (he had tremors and neurological concerns), it would have been almost comical.
The patient had asked, “What’s on my scan?” The patient interpreted the doctor’s response, “It’s negative,” to mean that he wasn’t being told something about the scan.
Dr. Vazquez realized that the patient had felt dismissed.
“He was a sick gentleman,” Dr. Vazquez says. “And what he wanted to hear was, ‘Look, the great news is your CT scan looks good. There’s not an anatomical abnormality. It’s not a tumor. It’s not a big bleed. … That’s great news, but I, as a physician, I am concerned about you. You’re sick. We’ve got to really figure out what’s going on with you.’… He wanted a pat on the back, and that’s all it took.”
After that, the patient no longer wanted to fire the hospitalist.
Verbal Abuse
One case at Gwinnett involves a hospitalist who was quite shy and easily intimidated and was not comfortable with a patient.
“They were struggling with a patient who was very difficult and very angry and a little abusive,” Dr. Austin says. “This doctor was really suffering psychically from this whole thing, and we switched.” Another doctor, who would not be thrown by the situation, took over the case. And Dr. Austin says he had great respect for the first doctor’s request to hand over the case.
“They needed a different personality,” he says. “It worked out beautifully. The patient and the doctor got along much better. The doctor was firm with the patient but respectful, and the other doctor felt relieved. And the [original] doctor is great with patients who need a lot of emotional support, probably better than the other doctor. So that worked out really well.”
It might be a challenge during a busy day, but it’s helpful to step back and see the situation as a whole, Dr. Bulger says. Sometimes, hospitalists can get flustered when patients are not acting rationally. But there’s usually a good reason they’re acting that way, he says.
“The patient is sick. And if it’s the patient’s family, they’re stressed by the fact that the patient’s sick. So you really need to take a step back and understand that.” TH
Thomas R. Collins is a freelance writer based in West Palm Beach, Fla.
Reference
- Centor R. Can I fire my hospitalist? SGIM Forum. 32(5):112-13.
At Bay Area Medical Center in Marinette, Wis., the time had come to start talking about an elderly woman’s end-of-life care.
Her hospitalist thought that those discussions should take place with the patient present, but the woman’s family felt otherwise and made this known to the hospitalist, who stood his ground.
Eventually, the family told a nurse that they wanted to fire the physician. But the only other hospitalist on shift didn’t want to take the patient.
As case managers and hospital administrators tried to wrap their heads around the situation, it became clear: They didn’t really know what to do.
Could the patient fire a physician? Was the second physician obligated to take what he knew from the outset would be a difficult case? What if nobody wanted to take care of this patient?
“There was no black-and-white to this,” says Robin Dequaine, director of medical staff services at the hospital, who was involved in the case.
Some “difficult patient” scenarios are fairly straightforward. A patient is violent? Enact your security measures. An addict wants narcotics? Don’t give them.
But there are other situations that enter murkier territory: What if a patient makes inappropriate or abusive remarks? How much should a hospitalist put up with? What if a patient’s request for treatment might not be the hospitalist’s first choice but could be seen as reasonable? Is the patient’s request accommodated? And what about those firings?
Hospitalists, administrators, and patient advocates say these tense situations with patients involving firings, or would-be firings, while not a daily occurrence, are actually fairly common.1 Getting to the root of the problem is essential. And as with so much in healthcare, good communication is the absolute crux of it all, they say.
“These are almost all communication issues,” says John Bulger, DO, MBA, FACP, SFHM, chief medical officer at Geisinger Health Plan in Danville, Pa., who has had a long career as a hospitalist and administrator handling and trying to resolve these situations. “They’re all [about] the way the hospitalist and the team is relating to the patient.”
Jackie O’Doherty, a private patient advocate who practices in New Jersey and New York across a gamut of hospital types, has a similar view.
“For me, the biggest problem, period, against hospitalists, doctors, everybody in the hospital, is communication—the lack of it,” she says. “Their communication skills are really poor.”
Patients accustomed to choice in the outpatient setting might not handle it well when they don’t have an established relationship with their hospitalist, says John Vazquez, MD, associate director for the Emory University School of Medicine’s Division of Hospital Medicine in Atlanta.
But the system, he says, “does not allow for, unfortunately, that much patient choice.”
End-of-life Discussion at a Small Hospital
Dequaine says the staff at Bay Area Medical Center was caught flat-footed with the case of the family not wanting end-of-life care discussed with their elderly mother.
“The doctor felt very confident that he was in a position that he could have that discussion in front of the patient,” she says.
At the 99-bed center, there were just two hospitalists, who were also employees of the hospital, on shift. And the communication channels involving the medical director of hospital medicine, a case manager, and the chief nursing executive were not well-controlled, Dequaine says.
“It didn’t go up the ladder correctly,” she says. “Too many people got involved, not knowing that somebody else was already involved.”
The second hospitalist at first said he would take the case, but later Dequaine learned that he changed his mind.
“He knew his care would be no different, and we were very, very busy, so they both had a high census already,” Dequaine says.
A third physician reluctantly took over until the issue subsided. And the family still brings the patient to the hospital for care.
Ultimately, the center adopted a new policy that doesn’t guarantee a patient a new doctor, only that the hospital will have frank discussions to try to resolve the issue and then try to arrange for a transfer if the situation can’t be resolved.
“The goal is not to get rid of the patient or to force them to keep the provider,” Dequaine says. “The goal is to resolve it in a mutually satisfactory way.”
A Patient Demands a Contraindicated Medication
A middle-aged woman with Crohn’s disease was hospitalized at Emory with an infection. The woman, worried about her disease flaring, wanted to keep getting her immunosuppressant, but the hospitalist suspended it because she needed to fight off the infection. The patient became upset. At a point when the hospitalist wasn’t in the room, the woman insisted to a nurse that she get her medication. The nurse called a doctor who was on call, but that doctor wouldn’t give the immunosuppressant either.
The patient began to think she wasn’t being listened to. Dr. Vazquez went in to see the patient and apologized for the misunderstanding.
“I went back into the room and explained here’s why I’m doing it: ‘I totally understand where you’re coming from; you don’t want your disease to be out of control. I appreciate that. What I’m worried about is killing you if we give you an immunosuppressant at the wrong time,’” Dr. Vazquez says.
Dr. Vazquez has underscored at his center how important it is for the physicians to be consulted and go back into the room when patients want to fire them, even though the expedient step might be to just bring in a new doctor. At previous centers, he says, it wouldn’t be unusual for the director to get a call from a nurse, who would say, “Yeah, they want to fire this physician, so let me know who’s going to see the patient.”
But simply switching doctors, he cautions, is like saying, “I agree with you we have incompetent doctors here, so we’re going to remove that doctor and I’m going to put a doctor on who actually knows what they’re doing.”
When doctors try to resolve the issues, good things tend to happen, Dr. Vazquez says.
“There’s generally a large amount of appreciation that someone comes back into the room and says, ‘We want to do this right.’”
Of course, there are times when, if tension remains after such discussions, patient care might be better served by a swap. At large centers, that might be possible, Dr. Bulger of Geisinger says.
“If the patient doesn’t tell the doctor something because he or she doesn’t like the doctor, then the doctor’s decisions are made on partial information—that’s the issue,” he says.
O’Doherty, the patient advocate, says that if patients frustrated with poor communication actually fired physicians as often as they would like, there would be more firings.
“Patients don’t like firing the doctors because they don’t want to be the patient who everybody doesn’t like,” she says. “They’re afraid that if they argue or disagree or ask too many questions, that they’re not going to get the care they need. And the family is afraid of that as well, especially in the older population. They think doctors are like God, they hold your life in their hands. So they don’t want to really question doctors.”
She says patients don’t necessarily need a particular finesse or expert bedside manner. In many cases, she says, it’s “just giving the information.”
A Patient Demands Pain Medication
Martin Austin, MD, SFHM, recently cared for a patient with chronic headaches. The patient asked for higher doses of pain medication, insinuating that she might turn to heroin if denied.
“I was trying to make the argument that I kind of disagreed with that but, ‘I respect your opinion,’” says Dr. Austin, medical director at the Gwinnett Medical Center Inpatient Medical Group in Georgia. “We came to a negotiation about how long we would use narcotics acutely until her other acute issues were over, but then we would try to get her away from narcotics.”
A good approach, he says, is to “outline to the patient why you’re doing what you’re doing. We try not to pick battles and give the patient some degree of control if it’s not contraindicated.”
But sometimes there can be no negotiating these kinds of requests, he says.
“Sometimes we’ll just say, ‘Look, it’s not a good thing for you to continue on this medication. You’re showing side effects, you’re sedated. … We think that the risk outweighs the benefit in this case,” he says.
A Patient Feels Left in the Dark
One patient at Emory wanted to fire his hospitalist because he wouldn’t tell him what was on his CT scan.
Dr. Vazquez held a discussion between the patient and the doctor. If not for the seriousness of the patient’s condition (he had tremors and neurological concerns), it would have been almost comical.
The patient had asked, “What’s on my scan?” The patient interpreted the doctor’s response, “It’s negative,” to mean that he wasn’t being told something about the scan.
Dr. Vazquez realized that the patient had felt dismissed.
“He was a sick gentleman,” Dr. Vazquez says. “And what he wanted to hear was, ‘Look, the great news is your CT scan looks good. There’s not an anatomical abnormality. It’s not a tumor. It’s not a big bleed. … That’s great news, but I, as a physician, I am concerned about you. You’re sick. We’ve got to really figure out what’s going on with you.’… He wanted a pat on the back, and that’s all it took.”
After that, the patient no longer wanted to fire the hospitalist.
Verbal Abuse
One case at Gwinnett involves a hospitalist who was quite shy and easily intimidated and was not comfortable with a patient.
“They were struggling with a patient who was very difficult and very angry and a little abusive,” Dr. Austin says. “This doctor was really suffering psychically from this whole thing, and we switched.” Another doctor, who would not be thrown by the situation, took over the case. And Dr. Austin says he had great respect for the first doctor’s request to hand over the case.
“They needed a different personality,” he says. “It worked out beautifully. The patient and the doctor got along much better. The doctor was firm with the patient but respectful, and the other doctor felt relieved. And the [original] doctor is great with patients who need a lot of emotional support, probably better than the other doctor. So that worked out really well.”
It might be a challenge during a busy day, but it’s helpful to step back and see the situation as a whole, Dr. Bulger says. Sometimes, hospitalists can get flustered when patients are not acting rationally. But there’s usually a good reason they’re acting that way, he says.
“The patient is sick. And if it’s the patient’s family, they’re stressed by the fact that the patient’s sick. So you really need to take a step back and understand that.” TH
Thomas R. Collins is a freelance writer based in West Palm Beach, Fla.
Reference
- Centor R. Can I fire my hospitalist? SGIM Forum. 32(5):112-13.
Anecdotal Failures in the Diagnosis of Serotonin Syndrome
Clinical Question: What is the validity of commonly held beliefs regarding serotonin syndrome (SS)?
Background: SS is a potentially life-threatening condition caused by serotonin excess in the central nervous system. The authors tested the validity of four widely accepted tenets about SS: that the Hunter criteria are superior, that the onset of SS is rapid compared to neuroleptic malignant syndrome (NMS), that hyperthermia is common with SS, and that SS can be distinguished from NMS based on medication history.
Study Design: Systematic review and meta-analysis.
Setting: PubMed and Web of Science.
Synopsis: Researchers identified 299 case reports from 2004 to 2014 in which SS was the most likely diagnosis based on one of three available diagnostic systems. Rhabdomyolysis with creatine kinase >1,500 and ICU treatment were used as proxies for SS severity. The Hunter criteria (the current gold standard) identified fewer overdoses, episodes of rhabdomyolysis, and ICU cases than the Sternbach or Radomski criteria. Combinations of antidepressants with methylene blue, opiates, or linezolid were the most common reasons for ICU admission. Symptom onset was within six hours in only 27.5% of cases. Hyperthermia was present in only 9.2% of patients with SS.
Hospitalists cannot rely on any one set of criteria to diagnose SS. The typical combinations of opiates or linezolid with antidepressants should raise the level of suspicion for SS. Rigidity and rhabdomyolysis occur commonly in both NMS and SS. Hyperthermia and timing of onset are not good indicators to the diagnosis of SS.
Bottom line: A high index of suspicion rather than reliance on classification systems or anecdotal key symptoms is necessary when considering SS.
Citation: Werneke U, Jamshidi F, Taylor DM, Ott M. Conundrums in neurology: diagnosing serotonin syndrome – a meta-analysis of cases. BMC Neurol. 2016;16:97.
Clinical Question: What is the validity of commonly held beliefs regarding serotonin syndrome (SS)?
Background: SS is a potentially life-threatening condition caused by serotonin excess in the central nervous system. The authors tested the validity of four widely accepted tenets about SS: that the Hunter criteria are superior, that the onset of SS is rapid compared to neuroleptic malignant syndrome (NMS), that hyperthermia is common with SS, and that SS can be distinguished from NMS based on medication history.
Study Design: Systematic review and meta-analysis.
Setting: PubMed and Web of Science.
Synopsis: Researchers identified 299 case reports from 2004 to 2014 in which SS was the most likely diagnosis based on one of three available diagnostic systems. Rhabdomyolysis with creatine kinase >1,500 and ICU treatment were used as proxies for SS severity. The Hunter criteria (the current gold standard) identified fewer overdoses, episodes of rhabdomyolysis, and ICU cases than the Sternbach or Radomski criteria. Combinations of antidepressants with methylene blue, opiates, or linezolid were the most common reasons for ICU admission. Symptom onset was within six hours in only 27.5% of cases. Hyperthermia was present in only 9.2% of patients with SS.
Hospitalists cannot rely on any one set of criteria to diagnose SS. The typical combinations of opiates or linezolid with antidepressants should raise the level of suspicion for SS. Rigidity and rhabdomyolysis occur commonly in both NMS and SS. Hyperthermia and timing of onset are not good indicators to the diagnosis of SS.
Bottom line: A high index of suspicion rather than reliance on classification systems or anecdotal key symptoms is necessary when considering SS.
Citation: Werneke U, Jamshidi F, Taylor DM, Ott M. Conundrums in neurology: diagnosing serotonin syndrome – a meta-analysis of cases. BMC Neurol. 2016;16:97.
Clinical Question: What is the validity of commonly held beliefs regarding serotonin syndrome (SS)?
Background: SS is a potentially life-threatening condition caused by serotonin excess in the central nervous system. The authors tested the validity of four widely accepted tenets about SS: that the Hunter criteria are superior, that the onset of SS is rapid compared to neuroleptic malignant syndrome (NMS), that hyperthermia is common with SS, and that SS can be distinguished from NMS based on medication history.
Study Design: Systematic review and meta-analysis.
Setting: PubMed and Web of Science.
Synopsis: Researchers identified 299 case reports from 2004 to 2014 in which SS was the most likely diagnosis based on one of three available diagnostic systems. Rhabdomyolysis with creatine kinase >1,500 and ICU treatment were used as proxies for SS severity. The Hunter criteria (the current gold standard) identified fewer overdoses, episodes of rhabdomyolysis, and ICU cases than the Sternbach or Radomski criteria. Combinations of antidepressants with methylene blue, opiates, or linezolid were the most common reasons for ICU admission. Symptom onset was within six hours in only 27.5% of cases. Hyperthermia was present in only 9.2% of patients with SS.
Hospitalists cannot rely on any one set of criteria to diagnose SS. The typical combinations of opiates or linezolid with antidepressants should raise the level of suspicion for SS. Rigidity and rhabdomyolysis occur commonly in both NMS and SS. Hyperthermia and timing of onset are not good indicators to the diagnosis of SS.
Bottom line: A high index of suspicion rather than reliance on classification systems or anecdotal key symptoms is necessary when considering SS.
Citation: Werneke U, Jamshidi F, Taylor DM, Ott M. Conundrums in neurology: diagnosing serotonin syndrome – a meta-analysis of cases. BMC Neurol. 2016;16:97.
Restrictive Blood Transfusion Strategies May Increase the Risk of Mortality, Morbidity for Elderly Patients Undergoing Orthopedic Surgery
Clinical Question: Are there particular groups of patients in which lower transfusion thresholds (transfusion only at lower hemoglobin levels) may be harmful?
Background: Previously published meta-analyses have examined transfusion thresholds for critically ill, surgical, and medical patients. By combining these patients, previous meta-analyses are limited in the identification of intervention effects. A more refined understanding of how transfusion thresholds impact outcomes for a variety of patients in different clinical settings is needed.
Study Design: Context-specific systematic review and meta-analysis of randomized clinical trials.
Setting: Adult patients in perioperative, emergency, or intensive-care settings.
Synopsis: Patient information was extracted from 31 randomized clinical trials. The authors found that among 3,465 elderly patients undergoing orthopedic surgery, those given restrictive transfusion strategies had significantly more events reflecting inadequate oxygen supply (relative risk, 1.41; 95% CI, 1.03–1.92). No statistically significant effect from restrictive transfusions was seen in 3,322 patients with cardiovascular disease undergoing cardiac or vascular procedures; 3,590 mixed medical-surgical patients in emergency and intensive-care settings; and 823 patients in a combined group of postpartum women, hematologic malignancy patients, and younger patients with neurologic injury.
The authors argue that even statistically nonsignificant differences in morbidity and mortality should encourage more liberal transfusion; apart from orthopedic surgery patients, this argument is not well-supported by the available data.
Bottom Line: It remains unclear whether restrictive transfusion strategies have a negative impact on certain types of patients, although the authors argue that there may be a trend in that direction. Further study is needed for specific patient populations.
Citation: Hovaguimian F, Myles PS. Restrictive versus liberal transfusion strategy in the perioperative and acute care settings: a context-specific systematic review and meta-analysis of randomized clinical trials. Anesthesiology. 2016;125(1):46-61.
Clinical Question: Are there particular groups of patients in which lower transfusion thresholds (transfusion only at lower hemoglobin levels) may be harmful?
Background: Previously published meta-analyses have examined transfusion thresholds for critically ill, surgical, and medical patients. By combining these patients, previous meta-analyses are limited in the identification of intervention effects. A more refined understanding of how transfusion thresholds impact outcomes for a variety of patients in different clinical settings is needed.
Study Design: Context-specific systematic review and meta-analysis of randomized clinical trials.
Setting: Adult patients in perioperative, emergency, or intensive-care settings.
Synopsis: Patient information was extracted from 31 randomized clinical trials. The authors found that among 3,465 elderly patients undergoing orthopedic surgery, those given restrictive transfusion strategies had significantly more events reflecting inadequate oxygen supply (relative risk, 1.41; 95% CI, 1.03–1.92). No statistically significant effect from restrictive transfusions was seen in 3,322 patients with cardiovascular disease undergoing cardiac or vascular procedures; 3,590 mixed medical-surgical patients in emergency and intensive-care settings; and 823 patients in a combined group of postpartum women, hematologic malignancy patients, and younger patients with neurologic injury.
The authors argue that even statistically nonsignificant differences in morbidity and mortality should encourage more liberal transfusion; apart from orthopedic surgery patients, this argument is not well-supported by the available data.
Bottom Line: It remains unclear whether restrictive transfusion strategies have a negative impact on certain types of patients, although the authors argue that there may be a trend in that direction. Further study is needed for specific patient populations.
Citation: Hovaguimian F, Myles PS. Restrictive versus liberal transfusion strategy in the perioperative and acute care settings: a context-specific systematic review and meta-analysis of randomized clinical trials. Anesthesiology. 2016;125(1):46-61.
Clinical Question: Are there particular groups of patients in which lower transfusion thresholds (transfusion only at lower hemoglobin levels) may be harmful?
Background: Previously published meta-analyses have examined transfusion thresholds for critically ill, surgical, and medical patients. By combining these patients, previous meta-analyses are limited in the identification of intervention effects. A more refined understanding of how transfusion thresholds impact outcomes for a variety of patients in different clinical settings is needed.
Study Design: Context-specific systematic review and meta-analysis of randomized clinical trials.
Setting: Adult patients in perioperative, emergency, or intensive-care settings.
Synopsis: Patient information was extracted from 31 randomized clinical trials. The authors found that among 3,465 elderly patients undergoing orthopedic surgery, those given restrictive transfusion strategies had significantly more events reflecting inadequate oxygen supply (relative risk, 1.41; 95% CI, 1.03–1.92). No statistically significant effect from restrictive transfusions was seen in 3,322 patients with cardiovascular disease undergoing cardiac or vascular procedures; 3,590 mixed medical-surgical patients in emergency and intensive-care settings; and 823 patients in a combined group of postpartum women, hematologic malignancy patients, and younger patients with neurologic injury.
The authors argue that even statistically nonsignificant differences in morbidity and mortality should encourage more liberal transfusion; apart from orthopedic surgery patients, this argument is not well-supported by the available data.
Bottom Line: It remains unclear whether restrictive transfusion strategies have a negative impact on certain types of patients, although the authors argue that there may be a trend in that direction. Further study is needed for specific patient populations.
Citation: Hovaguimian F, Myles PS. Restrictive versus liberal transfusion strategy in the perioperative and acute care settings: a context-specific systematic review and meta-analysis of randomized clinical trials. Anesthesiology. 2016;125(1):46-61.
Taking Steps to Reduce Arthritis Pain
Severe joint pain (SJP) is common in arthritis but it disproportionately affects certain groups, such as women, blacks, Hispanics, and people with comorbidities. In the US, it’s a leading cause of disability that affects nearly one quarter of adults in 2010-2012, according to data from the CDC, which also says an estimated 78 million people will have arthritis by 2040.
Related: Complementary and Alternative Medicine for Chronic Musculoskeletal Pain
Severe joint pain can limit someone’s ability to perform basic functions, researchers note, and seriously compromise their quality of life. To find out how prevalent severe joint pain is among adults with arthritis, the CDC analyzed data from the National Health Interview Survey. In 2014, approximately one fourth of adults with arthritis had SJP.
Two major objectives of the 2016 National Pain Strategy are to reduce barriers to pain care and increase patient knowledge of treatment options and risks, the researchers say. They cite the CDC Guideline for Prescribing Opioids for Chronic Pain–United States, 2016, for guidance on managing arthritis pain. (The CDC also currently funds arthritis programs in 12 states.)
Related: Lessons Learned From the RACAT Trial: A Comparison of Rheumatoid Arthritis Therapies
But the researchers also point out that many non-drug options exist for managing arthritis pain. For example, low-impact physical activity, such as walking, biking, and swimming is a “nonpharmacological and underused way of reducing joint pain,” they comment. They also note that community-based programs such as EnhanceFitness and Walk with Ease, as well as self-management education interventions such as the Chronic Disease Self-Management Program, have been shown to improve health-related quality of life and confidence in managing health conditions.
Severe joint pain (SJP) is common in arthritis but it disproportionately affects certain groups, such as women, blacks, Hispanics, and people with comorbidities. In the US, it’s a leading cause of disability that affects nearly one quarter of adults in 2010-2012, according to data from the CDC, which also says an estimated 78 million people will have arthritis by 2040.
Related: Complementary and Alternative Medicine for Chronic Musculoskeletal Pain
Severe joint pain can limit someone’s ability to perform basic functions, researchers note, and seriously compromise their quality of life. To find out how prevalent severe joint pain is among adults with arthritis, the CDC analyzed data from the National Health Interview Survey. In 2014, approximately one fourth of adults with arthritis had SJP.
Two major objectives of the 2016 National Pain Strategy are to reduce barriers to pain care and increase patient knowledge of treatment options and risks, the researchers say. They cite the CDC Guideline for Prescribing Opioids for Chronic Pain–United States, 2016, for guidance on managing arthritis pain. (The CDC also currently funds arthritis programs in 12 states.)
Related: Lessons Learned From the RACAT Trial: A Comparison of Rheumatoid Arthritis Therapies
But the researchers also point out that many non-drug options exist for managing arthritis pain. For example, low-impact physical activity, such as walking, biking, and swimming is a “nonpharmacological and underused way of reducing joint pain,” they comment. They also note that community-based programs such as EnhanceFitness and Walk with Ease, as well as self-management education interventions such as the Chronic Disease Self-Management Program, have been shown to improve health-related quality of life and confidence in managing health conditions.
Severe joint pain (SJP) is common in arthritis but it disproportionately affects certain groups, such as women, blacks, Hispanics, and people with comorbidities. In the US, it’s a leading cause of disability that affects nearly one quarter of adults in 2010-2012, according to data from the CDC, which also says an estimated 78 million people will have arthritis by 2040.
Related: Complementary and Alternative Medicine for Chronic Musculoskeletal Pain
Severe joint pain can limit someone’s ability to perform basic functions, researchers note, and seriously compromise their quality of life. To find out how prevalent severe joint pain is among adults with arthritis, the CDC analyzed data from the National Health Interview Survey. In 2014, approximately one fourth of adults with arthritis had SJP.
Two major objectives of the 2016 National Pain Strategy are to reduce barriers to pain care and increase patient knowledge of treatment options and risks, the researchers say. They cite the CDC Guideline for Prescribing Opioids for Chronic Pain–United States, 2016, for guidance on managing arthritis pain. (The CDC also currently funds arthritis programs in 12 states.)
Related: Lessons Learned From the RACAT Trial: A Comparison of Rheumatoid Arthritis Therapies
But the researchers also point out that many non-drug options exist for managing arthritis pain. For example, low-impact physical activity, such as walking, biking, and swimming is a “nonpharmacological and underused way of reducing joint pain,” they comment. They also note that community-based programs such as EnhanceFitness and Walk with Ease, as well as self-management education interventions such as the Chronic Disease Self-Management Program, have been shown to improve health-related quality of life and confidence in managing health conditions.
Heart failure readmission metric not linked to care quality
Metrics used by the Centers for Medicare & Medicaid Services to determine penalties for heart failure hospital readmissions are not associated with quality of care or overall clinical outcomes, according to data presented at the annual scientific sessions of the American Heart Association.
Ambarish Pandey, MD, of the University of Texas Southwestern Medical Center in Dallas, and his colleagues analyzed data from centers participating in the American Heart Association’s Get With The Guidelines-Heart Failure (GWTG-HF) registry linked to Medicare claims from July 2008 to June 2011. Centers were stratified as having low risk-adjusted readmission rates and high risk-adjusted readmission rates based on publicly available data from 2013.
The study included 171 centers with 43,143 patients. Centers were almost evenly split between low- and high-risk–adjusted 30-day readmission rates, with just a few more (51%) falling in the low-risk–adjusted category.
Performance was nearly equal (95.7% for centers with a low risk-adjusted readmission rate vs. 96.5% for those with high risk-adjusted rate) for median adherence to all performance measures, as was the case for median percentage of defect-free care (90.0% vs. 91.1%, respectively) and composite 1-year outcome of death or all-cause readmission rates (median 62.9% vs. 65.3%, respectively). The higher readmission group had higher 1-year all-cause readmission rates (median, 59.1% vs. 54.7%), Dr. Pandey and his colleagues reported in the study that was published simultaneously in JACC: Heart Failure (2016 Nov 15. doi. org/10.1016/j.jchf.2016). One-year mortality rates were lower in the higher readmission group with a trend toward statistical significance (median, 28.2% vs. 31.7%; P = 0.07).
Taken together, the findings suggest the 30-day readmission metrics currently used by CMS to determine readmission penalties are not associated with quality of care or overall clinical outcomes, Dr. Pandey and his colleagues wrote. Results showing higher 30-day readmissions do not necessarily reflect poor quality of care and may be related to other factors.
“These findings question the usefulness of the [hospital readmission reduction program] metric in identifying and penalizing hospitals with low quality of care,” Dr. Pandey wrote, adding that the findings were consistent with previous studies that have demonstrated a lack of association between in-hospital quality of care and 30-day readmission rates.
CMS implemented the federal Hospital Readmissions Reduction Program (HRRP) in 2012 to provide financial incentives for hospitals to reduce readmissions. Under the program, CMS uses claims data to determine whether readmission rates for heart failure, acute myocardial infarction, and pneumonia at eligible hospitals are higher than would be predicted by CMS models. Centers with higher than expected readmission rates face up to a 3% reimbursement penalty.
agallegos@frontlinemedcom.com
On Twitter @legal_med
These authors add to a chorus of voices expressing concern regarding the appropriateness and validity of the 30-day readmission metric. Arguably, this metric has driven our entire provider workforce to construct machinery designed to reduce short-term posthospitalization utilization, while doing little to improve quality for the 5.7 million (and counting) Americans with heart failure.
Marvin A. Konstam, MD, of Tufts University, Boston, made these comments in an accompanying editorial (JACC: Heart Fail. 2016 Nov 15. doi: 10.1016/j.jchf.2016.10.004). He reported no relevant disclosures.
These authors add to a chorus of voices expressing concern regarding the appropriateness and validity of the 30-day readmission metric. Arguably, this metric has driven our entire provider workforce to construct machinery designed to reduce short-term posthospitalization utilization, while doing little to improve quality for the 5.7 million (and counting) Americans with heart failure.
Marvin A. Konstam, MD, of Tufts University, Boston, made these comments in an accompanying editorial (JACC: Heart Fail. 2016 Nov 15. doi: 10.1016/j.jchf.2016.10.004). He reported no relevant disclosures.
These authors add to a chorus of voices expressing concern regarding the appropriateness and validity of the 30-day readmission metric. Arguably, this metric has driven our entire provider workforce to construct machinery designed to reduce short-term posthospitalization utilization, while doing little to improve quality for the 5.7 million (and counting) Americans with heart failure.
Marvin A. Konstam, MD, of Tufts University, Boston, made these comments in an accompanying editorial (JACC: Heart Fail. 2016 Nov 15. doi: 10.1016/j.jchf.2016.10.004). He reported no relevant disclosures.
Metrics used by the Centers for Medicare & Medicaid Services to determine penalties for heart failure hospital readmissions are not associated with quality of care or overall clinical outcomes, according to data presented at the annual scientific sessions of the American Heart Association.
Ambarish Pandey, MD, of the University of Texas Southwestern Medical Center in Dallas, and his colleagues analyzed data from centers participating in the American Heart Association’s Get With The Guidelines-Heart Failure (GWTG-HF) registry linked to Medicare claims from July 2008 to June 2011. Centers were stratified as having low risk-adjusted readmission rates and high risk-adjusted readmission rates based on publicly available data from 2013.
The study included 171 centers with 43,143 patients. Centers were almost evenly split between low- and high-risk–adjusted 30-day readmission rates, with just a few more (51%) falling in the low-risk–adjusted category.
Performance was nearly equal (95.7% for centers with a low risk-adjusted readmission rate vs. 96.5% for those with high risk-adjusted rate) for median adherence to all performance measures, as was the case for median percentage of defect-free care (90.0% vs. 91.1%, respectively) and composite 1-year outcome of death or all-cause readmission rates (median 62.9% vs. 65.3%, respectively). The higher readmission group had higher 1-year all-cause readmission rates (median, 59.1% vs. 54.7%), Dr. Pandey and his colleagues reported in the study that was published simultaneously in JACC: Heart Failure (2016 Nov 15. doi. org/10.1016/j.jchf.2016). One-year mortality rates were lower in the higher readmission group with a trend toward statistical significance (median, 28.2% vs. 31.7%; P = 0.07).
Taken together, the findings suggest the 30-day readmission metrics currently used by CMS to determine readmission penalties are not associated with quality of care or overall clinical outcomes, Dr. Pandey and his colleagues wrote. Results showing higher 30-day readmissions do not necessarily reflect poor quality of care and may be related to other factors.
“These findings question the usefulness of the [hospital readmission reduction program] metric in identifying and penalizing hospitals with low quality of care,” Dr. Pandey wrote, adding that the findings were consistent with previous studies that have demonstrated a lack of association between in-hospital quality of care and 30-day readmission rates.
CMS implemented the federal Hospital Readmissions Reduction Program (HRRP) in 2012 to provide financial incentives for hospitals to reduce readmissions. Under the program, CMS uses claims data to determine whether readmission rates for heart failure, acute myocardial infarction, and pneumonia at eligible hospitals are higher than would be predicted by CMS models. Centers with higher than expected readmission rates face up to a 3% reimbursement penalty.
agallegos@frontlinemedcom.com
On Twitter @legal_med
Metrics used by the Centers for Medicare & Medicaid Services to determine penalties for heart failure hospital readmissions are not associated with quality of care or overall clinical outcomes, according to data presented at the annual scientific sessions of the American Heart Association.
Ambarish Pandey, MD, of the University of Texas Southwestern Medical Center in Dallas, and his colleagues analyzed data from centers participating in the American Heart Association’s Get With The Guidelines-Heart Failure (GWTG-HF) registry linked to Medicare claims from July 2008 to June 2011. Centers were stratified as having low risk-adjusted readmission rates and high risk-adjusted readmission rates based on publicly available data from 2013.
The study included 171 centers with 43,143 patients. Centers were almost evenly split between low- and high-risk–adjusted 30-day readmission rates, with just a few more (51%) falling in the low-risk–adjusted category.
Performance was nearly equal (95.7% for centers with a low risk-adjusted readmission rate vs. 96.5% for those with high risk-adjusted rate) for median adherence to all performance measures, as was the case for median percentage of defect-free care (90.0% vs. 91.1%, respectively) and composite 1-year outcome of death or all-cause readmission rates (median 62.9% vs. 65.3%, respectively). The higher readmission group had higher 1-year all-cause readmission rates (median, 59.1% vs. 54.7%), Dr. Pandey and his colleagues reported in the study that was published simultaneously in JACC: Heart Failure (2016 Nov 15. doi. org/10.1016/j.jchf.2016). One-year mortality rates were lower in the higher readmission group with a trend toward statistical significance (median, 28.2% vs. 31.7%; P = 0.07).
Taken together, the findings suggest the 30-day readmission metrics currently used by CMS to determine readmission penalties are not associated with quality of care or overall clinical outcomes, Dr. Pandey and his colleagues wrote. Results showing higher 30-day readmissions do not necessarily reflect poor quality of care and may be related to other factors.
“These findings question the usefulness of the [hospital readmission reduction program] metric in identifying and penalizing hospitals with low quality of care,” Dr. Pandey wrote, adding that the findings were consistent with previous studies that have demonstrated a lack of association between in-hospital quality of care and 30-day readmission rates.
CMS implemented the federal Hospital Readmissions Reduction Program (HRRP) in 2012 to provide financial incentives for hospitals to reduce readmissions. Under the program, CMS uses claims data to determine whether readmission rates for heart failure, acute myocardial infarction, and pneumonia at eligible hospitals are higher than would be predicted by CMS models. Centers with higher than expected readmission rates face up to a 3% reimbursement penalty.
agallegos@frontlinemedcom.com
On Twitter @legal_med
FROM THE AHA SCIENTIFIC SESSIONS
Key clinical point:
Major finding: Performance was nearly equal (95.7% for centers with a low risk-adjusted readmission rate vs. 96.5% for those with high risk-adjusted rate) for median adherence to all performance measures.
Data source: Analysis of publicly available data reported to the CMS Hospital Readmission Reduction program.
Disclosures: No relevant conflicts of interest.