User login
Valaciclovir Shows Promise in Preventing Herpes Zoster During Anifrolumab Treatment for Lupus
TOPLINE:
The use of valaciclovir as prophylaxis prevents herpes zoster (HZ) in patients with systemic lupus erythematosus (SLE) receiving anifrolumab treatment, with no cases of zoster reported during the follow-up period in patients receiving valaciclovir.
METHODOLOGY:
- Anifrolumab, a human monoclonal antibody binding to type I interferon receptor subunit 1, increases the risk for HZ in patients with SLE; however, specific recommendations to prevent HZ are currently nonexistent for patients with SLE receiving anifrolumab.
- Researchers conducted a multicenter observational study in France from November 2021 to July 2024 to evaluate the prophylactic benefits of valaciclovir in 132 patients with SLE (mean age, 42 years; 92% women) treated with anifrolumab for ≥ 3 months.
- Among these patients, 87 received either 500 mg/d valaciclovir (n = 69) or 1000 mg/d valaciclovir (n = 18) as prophylaxis, whereas 45 did not receive valaciclovir.
- The patients were followed up for a median duration of 234 days under anifrolumab treatment, with monitoring for the development of herpes zoster.
TAKEAWAY:
- The risk for HZ was significantly lower in patients who received valaciclovir than in those who did not (hazard ratio, 0.08; P = .01).
- None of the patients treated with valaciclovir developed HZ during the survey period.
- The frequency of HZ in patients who did not receive valaciclovir increased progressively from 2.2% at 3 months to 6.2% at 6 months, reaching 23% at 12 months.
- None of the reported cases of HZ required hospitalization or led to anifrolumab discontinuation, although one patient developed neuralgia.
IN PRACTICE:
“Prophylactic treatment with valaciclovir is effective for preventing HZ [herpes zoster] infection in SLE patients treated with anifrolumab,” the authors wrote. “This finding is particularly relevant for SLE patients who cannot receive the recombinant HZ vaccine or for whom it is unavailable,” they added.
SOURCE:
The study was led by Ludovic Trefond, MD, PhD, Centre Hospitalier Universitaire de Clermont-Ferrand in France. It was published online on January 4, 2025, in RMD Open.
LIMITATIONS:
The observational design of the study and the low number of herpes zoster events during the follow-up period may have affected the robustness of the findings.
DISCLOSURES:
The authors did not receive any specific grants. Some authors reported having financial relationships with various pharmaceutical companies.
This article was created using several editorial tools, including artificial intelligence, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
The use of valaciclovir as prophylaxis prevents herpes zoster (HZ) in patients with systemic lupus erythematosus (SLE) receiving anifrolumab treatment, with no cases of zoster reported during the follow-up period in patients receiving valaciclovir.
METHODOLOGY:
- Anifrolumab, a human monoclonal antibody binding to type I interferon receptor subunit 1, increases the risk for HZ in patients with SLE; however, specific recommendations to prevent HZ are currently nonexistent for patients with SLE receiving anifrolumab.
- Researchers conducted a multicenter observational study in France from November 2021 to July 2024 to evaluate the prophylactic benefits of valaciclovir in 132 patients with SLE (mean age, 42 years; 92% women) treated with anifrolumab for ≥ 3 months.
- Among these patients, 87 received either 500 mg/d valaciclovir (n = 69) or 1000 mg/d valaciclovir (n = 18) as prophylaxis, whereas 45 did not receive valaciclovir.
- The patients were followed up for a median duration of 234 days under anifrolumab treatment, with monitoring for the development of herpes zoster.
TAKEAWAY:
- The risk for HZ was significantly lower in patients who received valaciclovir than in those who did not (hazard ratio, 0.08; P = .01).
- None of the patients treated with valaciclovir developed HZ during the survey period.
- The frequency of HZ in patients who did not receive valaciclovir increased progressively from 2.2% at 3 months to 6.2% at 6 months, reaching 23% at 12 months.
- None of the reported cases of HZ required hospitalization or led to anifrolumab discontinuation, although one patient developed neuralgia.
IN PRACTICE:
“Prophylactic treatment with valaciclovir is effective for preventing HZ [herpes zoster] infection in SLE patients treated with anifrolumab,” the authors wrote. “This finding is particularly relevant for SLE patients who cannot receive the recombinant HZ vaccine or for whom it is unavailable,” they added.
SOURCE:
The study was led by Ludovic Trefond, MD, PhD, Centre Hospitalier Universitaire de Clermont-Ferrand in France. It was published online on January 4, 2025, in RMD Open.
LIMITATIONS:
The observational design of the study and the low number of herpes zoster events during the follow-up period may have affected the robustness of the findings.
DISCLOSURES:
The authors did not receive any specific grants. Some authors reported having financial relationships with various pharmaceutical companies.
This article was created using several editorial tools, including artificial intelligence, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
The use of valaciclovir as prophylaxis prevents herpes zoster (HZ) in patients with systemic lupus erythematosus (SLE) receiving anifrolumab treatment, with no cases of zoster reported during the follow-up period in patients receiving valaciclovir.
METHODOLOGY:
- Anifrolumab, a human monoclonal antibody binding to type I interferon receptor subunit 1, increases the risk for HZ in patients with SLE; however, specific recommendations to prevent HZ are currently nonexistent for patients with SLE receiving anifrolumab.
- Researchers conducted a multicenter observational study in France from November 2021 to July 2024 to evaluate the prophylactic benefits of valaciclovir in 132 patients with SLE (mean age, 42 years; 92% women) treated with anifrolumab for ≥ 3 months.
- Among these patients, 87 received either 500 mg/d valaciclovir (n = 69) or 1000 mg/d valaciclovir (n = 18) as prophylaxis, whereas 45 did not receive valaciclovir.
- The patients were followed up for a median duration of 234 days under anifrolumab treatment, with monitoring for the development of herpes zoster.
TAKEAWAY:
- The risk for HZ was significantly lower in patients who received valaciclovir than in those who did not (hazard ratio, 0.08; P = .01).
- None of the patients treated with valaciclovir developed HZ during the survey period.
- The frequency of HZ in patients who did not receive valaciclovir increased progressively from 2.2% at 3 months to 6.2% at 6 months, reaching 23% at 12 months.
- None of the reported cases of HZ required hospitalization or led to anifrolumab discontinuation, although one patient developed neuralgia.
IN PRACTICE:
“Prophylactic treatment with valaciclovir is effective for preventing HZ [herpes zoster] infection in SLE patients treated with anifrolumab,” the authors wrote. “This finding is particularly relevant for SLE patients who cannot receive the recombinant HZ vaccine or for whom it is unavailable,” they added.
SOURCE:
The study was led by Ludovic Trefond, MD, PhD, Centre Hospitalier Universitaire de Clermont-Ferrand in France. It was published online on January 4, 2025, in RMD Open.
LIMITATIONS:
The observational design of the study and the low number of herpes zoster events during the follow-up period may have affected the robustness of the findings.
DISCLOSURES:
The authors did not receive any specific grants. Some authors reported having financial relationships with various pharmaceutical companies.
This article was created using several editorial tools, including artificial intelligence, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
Patients With Refractory Systemic Sclerosis Have Early Success With CAR T-Cell Therapy
TOPLINE:
CD19-targeting chimeric antigen receptor (CAR) T-cell therapy shows potential to intercept fibrotic organ manifestations and improve disease measures in patients with diffuse cutaneous systemic sclerosis (SSc) who had disease progression despite multiple previous treatments.
METHODOLOGY:
- Researchers conducted a case series to examine the effect of CD19-targeting CAR T-cell therapy on fibrotic and vascular organ manifestations in six patients with diffuse cutaneous SSc (median age, 42 years; four men and two women) who had an insufficient response to at least two previous treatments.
- Participants received CD19-targeting CAR T-cell treatment at a dose of 1 × 106 CAR T cells per kilogram of body weight after lymphodepletion with fludarabine and cyclophosphamide.
- The primary outcome was event-free time or treatment intensification after study entry, with events defined as the progression of interstitial lung disease, onset of congestive heart or renal failure or arterial hypertension, or initiation of new therapy.
- The secondary outcomes included changes in the modified Rodnan skin score (mRSS), imaging and laboratory assessments, patient-reported outcomes, and the modified American College of Rheumatology Composite Response Index in Systemic Sclerosis (ACR-CRISS), assessed at baseline and 3, 6, 9, and 12 months after treatment.
TAKEAWAY:
- No progression of organ manifestations or new lung, cardiac, or renal events occurred within the median follow-up period of 487 days.
- The probability of improvement in the ACR-CRISS score increased to a median value of 100% within 6 and 12 months of CAR T-cell treatment compared with baseline.
- Skin involvement improved in all the patients after CAR T-cell treatment, with a median mRSS decrease of 8 points within 100 days; the improvements were maintained throughout the 1-year follow-up period.
- This treatment also led to a depletion of antinuclear antibodies and SSc-specific autoantibodies.
IN PRACTICE:
“This case series highlights the potential of CAR T-cell therapy to address a crucial unmet need in refractory systemic sclerosis treatment. The study’s most significant contribution is the demonstration that CD19-targeting CAR T-cell therapy can halt or reverse aspects of fibrosis in systemic sclerosis,” Jérôme Avouac, Service de Rhumatologie, Hôpital Cochin, AP-HP Centre-Université Paris Cité, Paris, France, wrote in an accompanying editorial.
SOURCE:
The study was led by Janina Auth, MD, Deutsches Zentrum Immuntherapie, Friedrich-Alexander-Universität Erlangen-Nürnberg and Universitätsklinikum Erlangen in Germany, and was published online on November 11, 2024, in The Lancet Rheumatology.
LIMITATIONS:
The study lacked a control group, which limited the ability to draw definitive conclusions about the efficacy of CD19-targeting CAR T-cell therapy compared with standard treatments. The unpredictable nature of SSc, in which periods of stability can occur spontaneously, makes it difficult to attribute the improvements merely to the intervention. Moreover, the effect of CAR T-cell therapy on other disease manifestations, such as pulmonary hypertension, myocardial involvement, and scleroderma renal crisis, remains unclear.
DISCLOSURES:
The study was funded by Deutsche Forschungsgemeinschaft, Deutsche Krebshilfe, ELAN Foundation Erlangen, Interdisziplinäres Zentrum für Klinische Forschung Erlangen, Bundesministerium für Bildung und Forschung, and the European Union. Some authors reported receiving research grants, consulting fees, speaker fees, honoraria, or travel grants from Boehringer Ingelheim, Novartis, Almirall, and other pharmaceutical companies.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
CD19-targeting chimeric antigen receptor (CAR) T-cell therapy shows potential to intercept fibrotic organ manifestations and improve disease measures in patients with diffuse cutaneous systemic sclerosis (SSc) who had disease progression despite multiple previous treatments.
METHODOLOGY:
- Researchers conducted a case series to examine the effect of CD19-targeting CAR T-cell therapy on fibrotic and vascular organ manifestations in six patients with diffuse cutaneous SSc (median age, 42 years; four men and two women) who had an insufficient response to at least two previous treatments.
- Participants received CD19-targeting CAR T-cell treatment at a dose of 1 × 106 CAR T cells per kilogram of body weight after lymphodepletion with fludarabine and cyclophosphamide.
- The primary outcome was event-free time or treatment intensification after study entry, with events defined as the progression of interstitial lung disease, onset of congestive heart or renal failure or arterial hypertension, or initiation of new therapy.
- The secondary outcomes included changes in the modified Rodnan skin score (mRSS), imaging and laboratory assessments, patient-reported outcomes, and the modified American College of Rheumatology Composite Response Index in Systemic Sclerosis (ACR-CRISS), assessed at baseline and 3, 6, 9, and 12 months after treatment.
TAKEAWAY:
- No progression of organ manifestations or new lung, cardiac, or renal events occurred within the median follow-up period of 487 days.
- The probability of improvement in the ACR-CRISS score increased to a median value of 100% within 6 and 12 months of CAR T-cell treatment compared with baseline.
- Skin involvement improved in all the patients after CAR T-cell treatment, with a median mRSS decrease of 8 points within 100 days; the improvements were maintained throughout the 1-year follow-up period.
- This treatment also led to a depletion of antinuclear antibodies and SSc-specific autoantibodies.
IN PRACTICE:
“This case series highlights the potential of CAR T-cell therapy to address a crucial unmet need in refractory systemic sclerosis treatment. The study’s most significant contribution is the demonstration that CD19-targeting CAR T-cell therapy can halt or reverse aspects of fibrosis in systemic sclerosis,” Jérôme Avouac, Service de Rhumatologie, Hôpital Cochin, AP-HP Centre-Université Paris Cité, Paris, France, wrote in an accompanying editorial.
SOURCE:
The study was led by Janina Auth, MD, Deutsches Zentrum Immuntherapie, Friedrich-Alexander-Universität Erlangen-Nürnberg and Universitätsklinikum Erlangen in Germany, and was published online on November 11, 2024, in The Lancet Rheumatology.
LIMITATIONS:
The study lacked a control group, which limited the ability to draw definitive conclusions about the efficacy of CD19-targeting CAR T-cell therapy compared with standard treatments. The unpredictable nature of SSc, in which periods of stability can occur spontaneously, makes it difficult to attribute the improvements merely to the intervention. Moreover, the effect of CAR T-cell therapy on other disease manifestations, such as pulmonary hypertension, myocardial involvement, and scleroderma renal crisis, remains unclear.
DISCLOSURES:
The study was funded by Deutsche Forschungsgemeinschaft, Deutsche Krebshilfe, ELAN Foundation Erlangen, Interdisziplinäres Zentrum für Klinische Forschung Erlangen, Bundesministerium für Bildung und Forschung, and the European Union. Some authors reported receiving research grants, consulting fees, speaker fees, honoraria, or travel grants from Boehringer Ingelheim, Novartis, Almirall, and other pharmaceutical companies.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
CD19-targeting chimeric antigen receptor (CAR) T-cell therapy shows potential to intercept fibrotic organ manifestations and improve disease measures in patients with diffuse cutaneous systemic sclerosis (SSc) who had disease progression despite multiple previous treatments.
METHODOLOGY:
- Researchers conducted a case series to examine the effect of CD19-targeting CAR T-cell therapy on fibrotic and vascular organ manifestations in six patients with diffuse cutaneous SSc (median age, 42 years; four men and two women) who had an insufficient response to at least two previous treatments.
- Participants received CD19-targeting CAR T-cell treatment at a dose of 1 × 106 CAR T cells per kilogram of body weight after lymphodepletion with fludarabine and cyclophosphamide.
- The primary outcome was event-free time or treatment intensification after study entry, with events defined as the progression of interstitial lung disease, onset of congestive heart or renal failure or arterial hypertension, or initiation of new therapy.
- The secondary outcomes included changes in the modified Rodnan skin score (mRSS), imaging and laboratory assessments, patient-reported outcomes, and the modified American College of Rheumatology Composite Response Index in Systemic Sclerosis (ACR-CRISS), assessed at baseline and 3, 6, 9, and 12 months after treatment.
TAKEAWAY:
- No progression of organ manifestations or new lung, cardiac, or renal events occurred within the median follow-up period of 487 days.
- The probability of improvement in the ACR-CRISS score increased to a median value of 100% within 6 and 12 months of CAR T-cell treatment compared with baseline.
- Skin involvement improved in all the patients after CAR T-cell treatment, with a median mRSS decrease of 8 points within 100 days; the improvements were maintained throughout the 1-year follow-up period.
- This treatment also led to a depletion of antinuclear antibodies and SSc-specific autoantibodies.
IN PRACTICE:
“This case series highlights the potential of CAR T-cell therapy to address a crucial unmet need in refractory systemic sclerosis treatment. The study’s most significant contribution is the demonstration that CD19-targeting CAR T-cell therapy can halt or reverse aspects of fibrosis in systemic sclerosis,” Jérôme Avouac, Service de Rhumatologie, Hôpital Cochin, AP-HP Centre-Université Paris Cité, Paris, France, wrote in an accompanying editorial.
SOURCE:
The study was led by Janina Auth, MD, Deutsches Zentrum Immuntherapie, Friedrich-Alexander-Universität Erlangen-Nürnberg and Universitätsklinikum Erlangen in Germany, and was published online on November 11, 2024, in The Lancet Rheumatology.
LIMITATIONS:
The study lacked a control group, which limited the ability to draw definitive conclusions about the efficacy of CD19-targeting CAR T-cell therapy compared with standard treatments. The unpredictable nature of SSc, in which periods of stability can occur spontaneously, makes it difficult to attribute the improvements merely to the intervention. Moreover, the effect of CAR T-cell therapy on other disease manifestations, such as pulmonary hypertension, myocardial involvement, and scleroderma renal crisis, remains unclear.
DISCLOSURES:
The study was funded by Deutsche Forschungsgemeinschaft, Deutsche Krebshilfe, ELAN Foundation Erlangen, Interdisziplinäres Zentrum für Klinische Forschung Erlangen, Bundesministerium für Bildung und Forschung, and the European Union. Some authors reported receiving research grants, consulting fees, speaker fees, honoraria, or travel grants from Boehringer Ingelheim, Novartis, Almirall, and other pharmaceutical companies.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
Painful Oral, Groin, and Scalp Lesions in a Young Man
Painful Oral, Groin, and Scalp Lesions in a Young Man
THE DIAGNOSIS: Pemphigus Vegetans
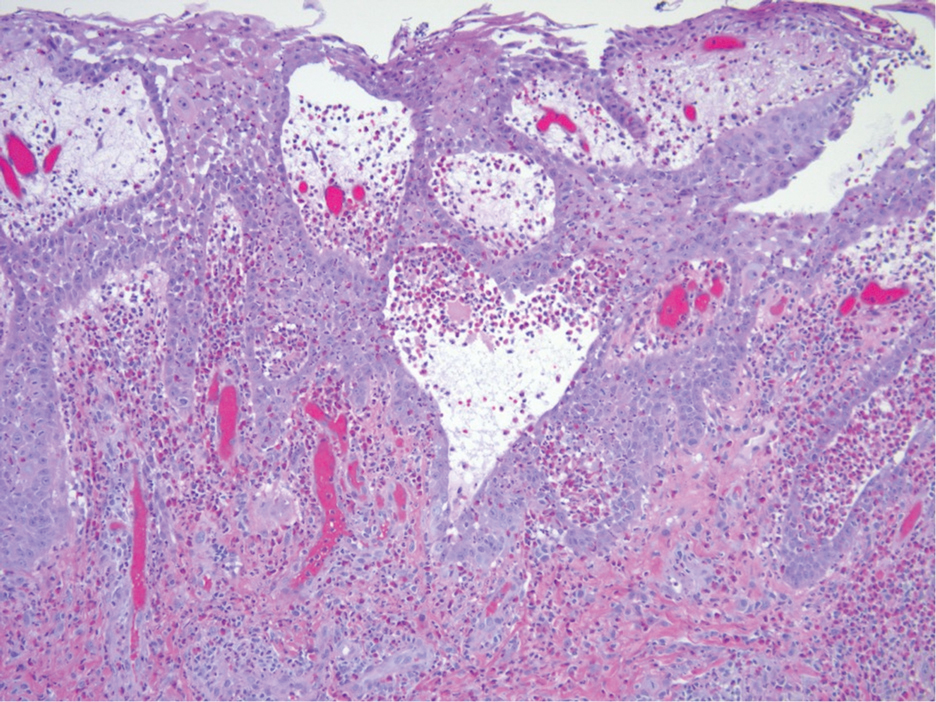
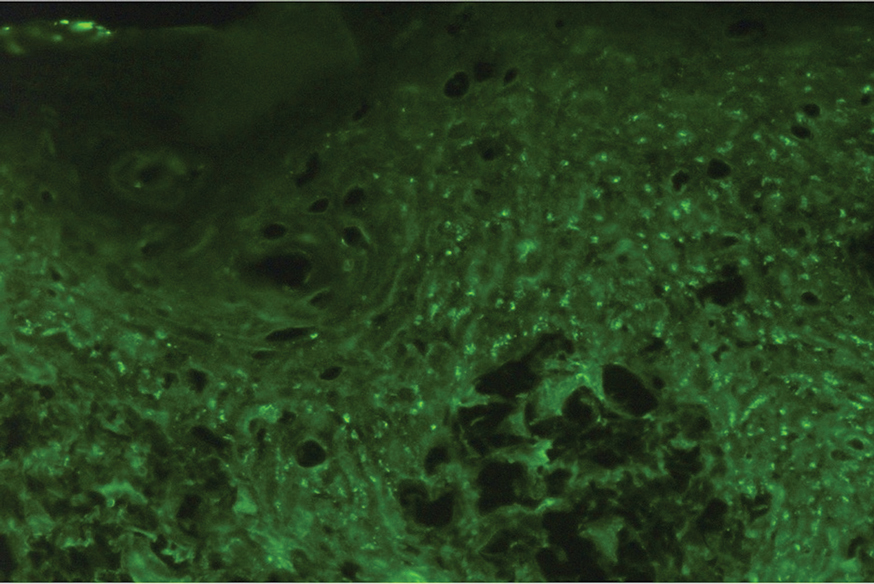
Histopathologic examination of the biopsies from the scalp and left anterior thigh revealed suprabasal clefting with acantholytic cells extending into the follicular infundibulum with eosinophilic pustules within the epidermis. The dermis contained perivascular lymphohistiocytic and eosinophilic inflammatory infiltrates without viral cytopathic effects (Figure 1). Direct immunofluorescence revealed strong IgG and moderate IgA pericellular deposition around keratinocyte cytoplasms (Figure 2). Serologic evaluation demonstrated anti–desmoglein 3 antibodies. Based on the clinical presentation and histopathologic correlation, a diagnosis of pemphigus vegetans was made.
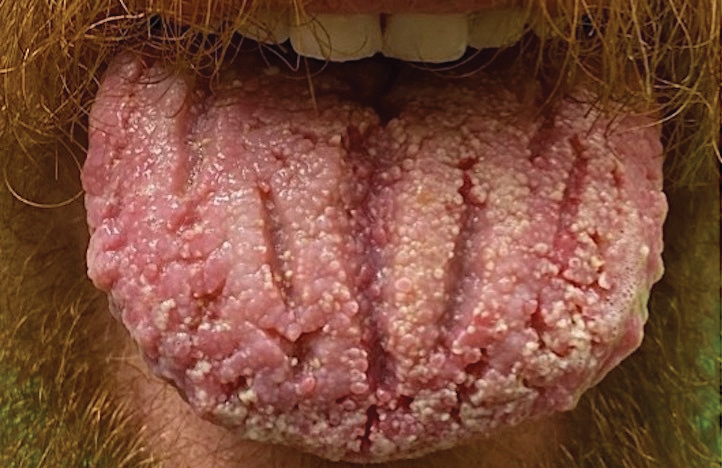
Pemphigus vegetans is a vesiculobullous autoimmune disease that is similar to pemphigus vulgaris but is characterized by the formation of vegetative plaques along the intertriginous areas and on the oral mucosa.1 It is the rarest variant of all pemphigus subtypes and was first described by Neumann in 1876.2 There are 2 subtypes of this variant: Hallopeau and Neumann, each with unique characteristics and physical manifestations. The Hallopeau type initially manifests with pustular lesions that rupture and evolve into erosions that commonly become infected. Gradually they merge and multiply to become more painful and vegetative.3 It has a more indolent course and typically responds well to treatment, and prolonged remission can be reached.4 The Neumann type is more severe and manifests with large vesiculobullous and erosive lesions that rupture and ulcerate, forming verrucous crusted vegetative plaques over the erosions.5 The erosions along the edge of the lesions induce new vegetation, becoming dry, hyperkeratotic, and fissured.3 The Neumann type often requires higher-dose steroids and typically is resistant to treatment.4 Patients can present with oral stomatitis and occasionally can develop a fissured or cerebriform appearance of the tongue, as seen in our patient (Figure 3).1,2 Nail changes include onychorrhexis, onychomadesis, subungual pustules, and ultimately nail atrophy.5
Pemphigus diseases are characterized by IgG autoantibodies against desmoglein 3 and/or desmoglein 1. These are components of desmosomes that are responsible for keratinocyte adhesion, disruption of which results in the blister formation seen in pemphigus subtypes. The unique physical manifestation of pemphigus vegetans is thought to be due not only to autoantibodies against desmogleins 1 and 3 but also to autoantibodies against desmocollin 1 and 2.1
Histopathologic examination reveals hyperkeratosis and pseudoepitheliomatous hyperplasia with acantholysis that creates a suprabasal cleft. Basal cells remain intact to the basement membrane by hemidesmosomes, resulting in a tombstone appearance. The Hallopeau type typically manifests with a large eosinophilic inflammatory response, leading to eosinophilic spongiosis and intraepidermal microabscesses. The Neumann type manifests with more of a neutrophilic and lymphocytic infiltrate, accompanied by the eosinophilic response.1 For evaluation, obtain histopathology as well as direct immunofluorescence or enzyme-linked immunosorbent assay to look for intracellular deposition of desmoglein autoantibodies.
First-line treatment for pemphigus vulgaris and its variants is rituximab, an anti-CD20 monoclonal antibody. It has also been shown to have therapeutic benefit with combination of corticosteroids and rituximab. Corticosteroids should be given at a dose of 1 mg/kg daily for 2 to 4 weeks. Other immunosuppressive agents (steroid sparing) include azathioprine, dapsone, mycophenolate mofetil, methotrexate, cyclophosphamide, cyclosporine, and intravenous immunoglobulin. Pulse therapy with intermittent intravenous corticosteroids and immunosuppressants is another second-line therapeutic option. Topical therapeutic options include steroids, tacrolimus, and nicotinamide with oral tetracycline at onset and relapse. The goal of therapy is to maintain remission for 1 year then slowly taper treatment over another year.1
Our patient initially was treated with prednisone, and subsequent courses of azathioprine and mycophenolate mofetil failed. He then was treated with 2 infusions of rituximab that were given 2 weeks apart. He was able to taper off the prednisone 1 month after the last infusion with complete remission of disease. He has been disease free for more than 9 months postinfusion.
Differential diagnoses for pemphigus vegetans can include bullous pemphigoid, bullous systemic lupus erythematosus, dermatitis herpetiformis, and pemphigus vulgaris. Lesion characteristics are key to differentiating pemphigus vegetans from other autoimmune blistering disorders. Bullous pemphigoid will manifest with tense blisters where pemphigus vulgaris will be flaccid; this is due to the difference in autoantibody targets between the conditions. Diagnosis depends on clinical presentation and histopathologic findings.
- Messersmith L, Krauland K. Pemphigus vegetans. StatPearls [Internet]. Updated June 26, 2023. Accessed December 16, 2024. https://www.ncbi.nlm.nih.gov/books/NBK545229/
- Rebello MS, Ramesh BM, Sukumar D, et al. Cerebriform cutaneous lesions in pemphigus vegetans. Indian J Dermatol. 2016;61:206-208.
- Ruocco V, Ruocco E, Caccavale S, et al. Pemphigus vegetans of the folds (intertriginous areas). Clin Dermatol. 2015;33:471-476.
- Ajbani AA, Mehta KS, Marfatia YS. Verrucous lesions over external genitalia as a presenting feature of pemphigus vegetans. Indian J Sex Transm Dis AIDS. 2019;40:176-179.
- Vinay K, De D, Handa S, et al. Pemphigus vegetans presenting as a verrucous plaque on the finger. Clin Exp Dermatol. 2016;41:316-317.
THE DIAGNOSIS: Pemphigus Vegetans
Histopathologic examination of the biopsies from the scalp and left anterior thigh revealed suprabasal clefting with acantholytic cells extending into the follicular infundibulum with eosinophilic pustules within the epidermis. The dermis contained perivascular lymphohistiocytic and eosinophilic inflammatory infiltrates without viral cytopathic effects (Figure 1). Direct immunofluorescence revealed strong IgG and moderate IgA pericellular deposition around keratinocyte cytoplasms (Figure 2). Serologic evaluation demonstrated anti–desmoglein 3 antibodies. Based on the clinical presentation and histopathologic correlation, a diagnosis of pemphigus vegetans was made.


Pemphigus vegetans is a vesiculobullous autoimmune disease that is similar to pemphigus vulgaris but is characterized by the formation of vegetative plaques along the intertriginous areas and on the oral mucosa.1 It is the rarest variant of all pemphigus subtypes and was first described by Neumann in 1876.2 There are 2 subtypes of this variant: Hallopeau and Neumann, each with unique characteristics and physical manifestations. The Hallopeau type initially manifests with pustular lesions that rupture and evolve into erosions that commonly become infected. Gradually they merge and multiply to become more painful and vegetative.3 It has a more indolent course and typically responds well to treatment, and prolonged remission can be reached.4 The Neumann type is more severe and manifests with large vesiculobullous and erosive lesions that rupture and ulcerate, forming verrucous crusted vegetative plaques over the erosions.5 The erosions along the edge of the lesions induce new vegetation, becoming dry, hyperkeratotic, and fissured.3 The Neumann type often requires higher-dose steroids and typically is resistant to treatment.4 Patients can present with oral stomatitis and occasionally can develop a fissured or cerebriform appearance of the tongue, as seen in our patient (Figure 3).1,2 Nail changes include onychorrhexis, onychomadesis, subungual pustules, and ultimately nail atrophy.5

Pemphigus diseases are characterized by IgG autoantibodies against desmoglein 3 and/or desmoglein 1. These are components of desmosomes that are responsible for keratinocyte adhesion, disruption of which results in the blister formation seen in pemphigus subtypes. The unique physical manifestation of pemphigus vegetans is thought to be due not only to autoantibodies against desmogleins 1 and 3 but also to autoantibodies against desmocollin 1 and 2.1
Histopathologic examination reveals hyperkeratosis and pseudoepitheliomatous hyperplasia with acantholysis that creates a suprabasal cleft. Basal cells remain intact to the basement membrane by hemidesmosomes, resulting in a tombstone appearance. The Hallopeau type typically manifests with a large eosinophilic inflammatory response, leading to eosinophilic spongiosis and intraepidermal microabscesses. The Neumann type manifests with more of a neutrophilic and lymphocytic infiltrate, accompanied by the eosinophilic response.1 For evaluation, obtain histopathology as well as direct immunofluorescence or enzyme-linked immunosorbent assay to look for intracellular deposition of desmoglein autoantibodies.
First-line treatment for pemphigus vulgaris and its variants is rituximab, an anti-CD20 monoclonal antibody. It has also been shown to have therapeutic benefit with combination of corticosteroids and rituximab. Corticosteroids should be given at a dose of 1 mg/kg daily for 2 to 4 weeks. Other immunosuppressive agents (steroid sparing) include azathioprine, dapsone, mycophenolate mofetil, methotrexate, cyclophosphamide, cyclosporine, and intravenous immunoglobulin. Pulse therapy with intermittent intravenous corticosteroids and immunosuppressants is another second-line therapeutic option. Topical therapeutic options include steroids, tacrolimus, and nicotinamide with oral tetracycline at onset and relapse. The goal of therapy is to maintain remission for 1 year then slowly taper treatment over another year.1
Our patient initially was treated with prednisone, and subsequent courses of azathioprine and mycophenolate mofetil failed. He then was treated with 2 infusions of rituximab that were given 2 weeks apart. He was able to taper off the prednisone 1 month after the last infusion with complete remission of disease. He has been disease free for more than 9 months postinfusion.
Differential diagnoses for pemphigus vegetans can include bullous pemphigoid, bullous systemic lupus erythematosus, dermatitis herpetiformis, and pemphigus vulgaris. Lesion characteristics are key to differentiating pemphigus vegetans from other autoimmune blistering disorders. Bullous pemphigoid will manifest with tense blisters where pemphigus vulgaris will be flaccid; this is due to the difference in autoantibody targets between the conditions. Diagnosis depends on clinical presentation and histopathologic findings.
THE DIAGNOSIS: Pemphigus Vegetans
Histopathologic examination of the biopsies from the scalp and left anterior thigh revealed suprabasal clefting with acantholytic cells extending into the follicular infundibulum with eosinophilic pustules within the epidermis. The dermis contained perivascular lymphohistiocytic and eosinophilic inflammatory infiltrates without viral cytopathic effects (Figure 1). Direct immunofluorescence revealed strong IgG and moderate IgA pericellular deposition around keratinocyte cytoplasms (Figure 2). Serologic evaluation demonstrated anti–desmoglein 3 antibodies. Based on the clinical presentation and histopathologic correlation, a diagnosis of pemphigus vegetans was made.


Pemphigus vegetans is a vesiculobullous autoimmune disease that is similar to pemphigus vulgaris but is characterized by the formation of vegetative plaques along the intertriginous areas and on the oral mucosa.1 It is the rarest variant of all pemphigus subtypes and was first described by Neumann in 1876.2 There are 2 subtypes of this variant: Hallopeau and Neumann, each with unique characteristics and physical manifestations. The Hallopeau type initially manifests with pustular lesions that rupture and evolve into erosions that commonly become infected. Gradually they merge and multiply to become more painful and vegetative.3 It has a more indolent course and typically responds well to treatment, and prolonged remission can be reached.4 The Neumann type is more severe and manifests with large vesiculobullous and erosive lesions that rupture and ulcerate, forming verrucous crusted vegetative plaques over the erosions.5 The erosions along the edge of the lesions induce new vegetation, becoming dry, hyperkeratotic, and fissured.3 The Neumann type often requires higher-dose steroids and typically is resistant to treatment.4 Patients can present with oral stomatitis and occasionally can develop a fissured or cerebriform appearance of the tongue, as seen in our patient (Figure 3).1,2 Nail changes include onychorrhexis, onychomadesis, subungual pustules, and ultimately nail atrophy.5

Pemphigus diseases are characterized by IgG autoantibodies against desmoglein 3 and/or desmoglein 1. These are components of desmosomes that are responsible for keratinocyte adhesion, disruption of which results in the blister formation seen in pemphigus subtypes. The unique physical manifestation of pemphigus vegetans is thought to be due not only to autoantibodies against desmogleins 1 and 3 but also to autoantibodies against desmocollin 1 and 2.1
Histopathologic examination reveals hyperkeratosis and pseudoepitheliomatous hyperplasia with acantholysis that creates a suprabasal cleft. Basal cells remain intact to the basement membrane by hemidesmosomes, resulting in a tombstone appearance. The Hallopeau type typically manifests with a large eosinophilic inflammatory response, leading to eosinophilic spongiosis and intraepidermal microabscesses. The Neumann type manifests with more of a neutrophilic and lymphocytic infiltrate, accompanied by the eosinophilic response.1 For evaluation, obtain histopathology as well as direct immunofluorescence or enzyme-linked immunosorbent assay to look for intracellular deposition of desmoglein autoantibodies.
First-line treatment for pemphigus vulgaris and its variants is rituximab, an anti-CD20 monoclonal antibody. It has also been shown to have therapeutic benefit with combination of corticosteroids and rituximab. Corticosteroids should be given at a dose of 1 mg/kg daily for 2 to 4 weeks. Other immunosuppressive agents (steroid sparing) include azathioprine, dapsone, mycophenolate mofetil, methotrexate, cyclophosphamide, cyclosporine, and intravenous immunoglobulin. Pulse therapy with intermittent intravenous corticosteroids and immunosuppressants is another second-line therapeutic option. Topical therapeutic options include steroids, tacrolimus, and nicotinamide with oral tetracycline at onset and relapse. The goal of therapy is to maintain remission for 1 year then slowly taper treatment over another year.1
Our patient initially was treated with prednisone, and subsequent courses of azathioprine and mycophenolate mofetil failed. He then was treated with 2 infusions of rituximab that were given 2 weeks apart. He was able to taper off the prednisone 1 month after the last infusion with complete remission of disease. He has been disease free for more than 9 months postinfusion.
Differential diagnoses for pemphigus vegetans can include bullous pemphigoid, bullous systemic lupus erythematosus, dermatitis herpetiformis, and pemphigus vulgaris. Lesion characteristics are key to differentiating pemphigus vegetans from other autoimmune blistering disorders. Bullous pemphigoid will manifest with tense blisters where pemphigus vulgaris will be flaccid; this is due to the difference in autoantibody targets between the conditions. Diagnosis depends on clinical presentation and histopathologic findings.
- Messersmith L, Krauland K. Pemphigus vegetans. StatPearls [Internet]. Updated June 26, 2023. Accessed December 16, 2024. https://www.ncbi.nlm.nih.gov/books/NBK545229/
- Rebello MS, Ramesh BM, Sukumar D, et al. Cerebriform cutaneous lesions in pemphigus vegetans. Indian J Dermatol. 2016;61:206-208.
- Ruocco V, Ruocco E, Caccavale S, et al. Pemphigus vegetans of the folds (intertriginous areas). Clin Dermatol. 2015;33:471-476.
- Ajbani AA, Mehta KS, Marfatia YS. Verrucous lesions over external genitalia as a presenting feature of pemphigus vegetans. Indian J Sex Transm Dis AIDS. 2019;40:176-179.
- Vinay K, De D, Handa S, et al. Pemphigus vegetans presenting as a verrucous plaque on the finger. Clin Exp Dermatol. 2016;41:316-317.
- Messersmith L, Krauland K. Pemphigus vegetans. StatPearls [Internet]. Updated June 26, 2023. Accessed December 16, 2024. https://www.ncbi.nlm.nih.gov/books/NBK545229/
- Rebello MS, Ramesh BM, Sukumar D, et al. Cerebriform cutaneous lesions in pemphigus vegetans. Indian J Dermatol. 2016;61:206-208.
- Ruocco V, Ruocco E, Caccavale S, et al. Pemphigus vegetans of the folds (intertriginous areas). Clin Dermatol. 2015;33:471-476.
- Ajbani AA, Mehta KS, Marfatia YS. Verrucous lesions over external genitalia as a presenting feature of pemphigus vegetans. Indian J Sex Transm Dis AIDS. 2019;40:176-179.
- Vinay K, De D, Handa S, et al. Pemphigus vegetans presenting as a verrucous plaque on the finger. Clin Exp Dermatol. 2016;41:316-317.
Painful Oral, Groin, and Scalp Lesions in a Young Man
Painful Oral, Groin, and Scalp Lesions in a Young Man
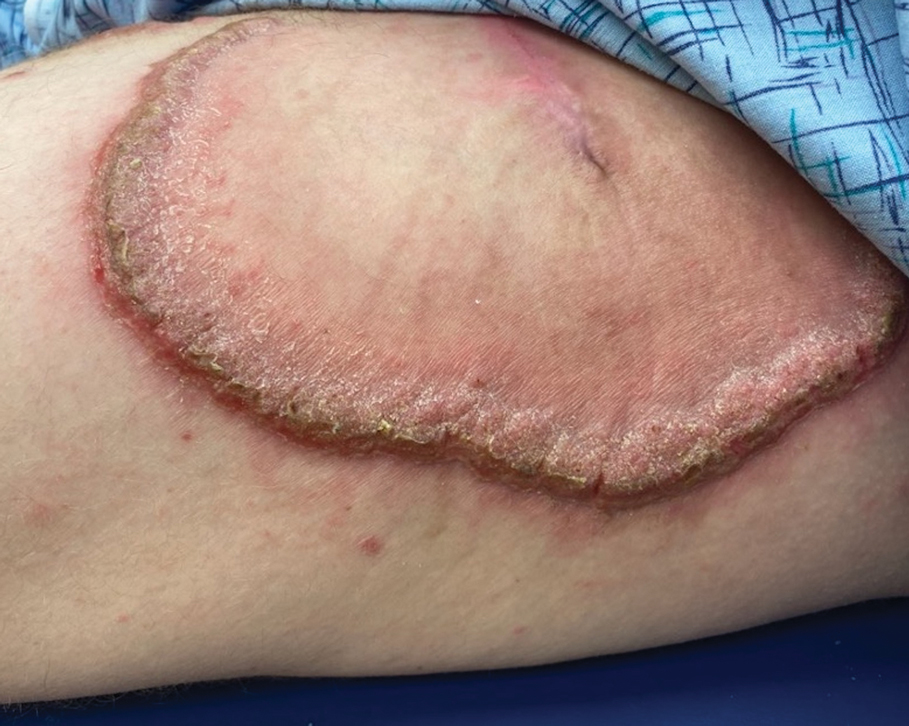
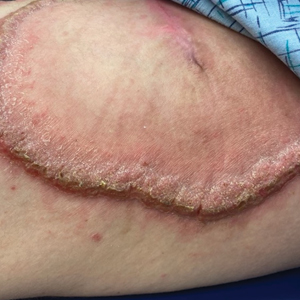
A 27-year-old man presented to the dermatology department with painful oral and groin lesions of 2 years’ duration as well as lip ulceration that had been present for 1 month. The patient also reported moderately tender scalp and face lesions that had been present for several weeks. The lip ulceration was previously treated by his primary care provider with valacyclovir (1 g daily for 2 weeks) without improvement. Six months prior to the current presentation, we treated the groin lesions as condyloma involving the perineum and genital region at our clinic with no response to cryotherapy, topical imiquimod, or extensive surgical excision with skin grafting. Pathology at the time showed condyloma but was negative for human papillomavirus. Physical examination at the current presentation revealed superficial erosions along the vermilion border. The oral mucosa exhibited cobblestoning, and fissures were present on the tongue. Eroded pink plaques studded with vesicles were present on the vertex scalp and left chin. The bilateral inguinal regions extending to anterior-lateral upper thighs and posterior buttocks revealed erythematous, arcuate, and annular erosive plaques with verrucous hyperkeratotic borders and fissuring on the leading edge. Pink erosive and verrucous erythematous plaques were noted on the penile shaft, scrotum, and perineum. Punch biopsies of the scalp and left anterior thigh as well as direct immunofluorescence were performed.
Cutaneous Lupus Associated with Greater Risk for Atherosclerotic Cardiovascular Disease
TOPLINE:
than with psoriasis.
METHODOLOGY:
- A retrospective matched longitudinal study compared the incidence and prevalence of ASCVD of 8138 individuals with CLE; 24,675 with SLE; 192,577 with psoriasis; and 81,380 control individuals.
- The disease-free control population was matched in a 10:1 ratio to the CLE population on the basis of age, sex, insurance type, and enrollment duration.
- Prevalent ASCVD was defined as coronary artery disease, prior myocardial infarction, or cerebrovascular accident, with ASCVD incidence assessed by number of hospitalizations over 3 years.
TAKEAWAY:
- Persons with CLE had higher ASCVD risk than control individuals (odds ratio [OR], 1.72; P < .001), similar to those with SLE (OR, 2.41; P < .001) but unlike those with psoriasis (OR, 1.03; P = .48).
- ASCVD incidence at 3 years was 24.8 per 1000 person-years for SLE, 15.2 per 1000 person-years for CLE, 14.0 per 1000 person-years for psoriasis, and 10.3 per 1000 person-years for controls.
- Multivariable Cox proportional regression modeling showed ASCVD risk was highest in those with SLE (hazard ratio [HR], 2.23; P < .001) vs CLE (HR, 1.32; P < .001) and psoriasis (HR, 1.06; P = .09).
- ASCVD prevalence was higher in individuals with CLE receiving systemic therapy (2.7%) than in those receiving no therapy (1.6%), suggesting a potential link between disease severity and CVD risk.
IN PRACTICE:
“Persons with CLE are at higher risk for ASCVD, and guidelines for the evaluation and management of ASCVD may improve their quality of care,” the authors wrote.
SOURCE:
The study was led by Henry W. Chen, MD, Department of Dermatology, University of Texas Southwestern Medical Center, Dallas. It was published online on December 4, 2024, in JAMA Dermatology.
LIMITATIONS:
The study was limited by its relatively young population (median age, 49 years) and the exclusion of adults aged > 65 years on Medicare insurance plans. The database lacked race and ethnicity data, and the analysis was restricted to a shorter 3-year period. The study could not fully evaluate detailed risk factors such as blood pressure levels, cholesterol measurements, or glycemic control, nor could it accurately assess smoking status.
DISCLOSURES:
The research was supported by the Department of Dermatology at the University of Texas Southwestern Medical Center and a grant from the National Institutes of Health. Several authors reported receiving grants or personal fees from various pharmaceutical companies. One author reported being a deputy editor for diversity, equity, and inclusion at JAMA Cardiology. Additional disclosures are noted in the original article.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
than with psoriasis.
METHODOLOGY:
- A retrospective matched longitudinal study compared the incidence and prevalence of ASCVD of 8138 individuals with CLE; 24,675 with SLE; 192,577 with psoriasis; and 81,380 control individuals.
- The disease-free control population was matched in a 10:1 ratio to the CLE population on the basis of age, sex, insurance type, and enrollment duration.
- Prevalent ASCVD was defined as coronary artery disease, prior myocardial infarction, or cerebrovascular accident, with ASCVD incidence assessed by number of hospitalizations over 3 years.
TAKEAWAY:
- Persons with CLE had higher ASCVD risk than control individuals (odds ratio [OR], 1.72; P < .001), similar to those with SLE (OR, 2.41; P < .001) but unlike those with psoriasis (OR, 1.03; P = .48).
- ASCVD incidence at 3 years was 24.8 per 1000 person-years for SLE, 15.2 per 1000 person-years for CLE, 14.0 per 1000 person-years for psoriasis, and 10.3 per 1000 person-years for controls.
- Multivariable Cox proportional regression modeling showed ASCVD risk was highest in those with SLE (hazard ratio [HR], 2.23; P < .001) vs CLE (HR, 1.32; P < .001) and psoriasis (HR, 1.06; P = .09).
- ASCVD prevalence was higher in individuals with CLE receiving systemic therapy (2.7%) than in those receiving no therapy (1.6%), suggesting a potential link between disease severity and CVD risk.
IN PRACTICE:
“Persons with CLE are at higher risk for ASCVD, and guidelines for the evaluation and management of ASCVD may improve their quality of care,” the authors wrote.
SOURCE:
The study was led by Henry W. Chen, MD, Department of Dermatology, University of Texas Southwestern Medical Center, Dallas. It was published online on December 4, 2024, in JAMA Dermatology.
LIMITATIONS:
The study was limited by its relatively young population (median age, 49 years) and the exclusion of adults aged > 65 years on Medicare insurance plans. The database lacked race and ethnicity data, and the analysis was restricted to a shorter 3-year period. The study could not fully evaluate detailed risk factors such as blood pressure levels, cholesterol measurements, or glycemic control, nor could it accurately assess smoking status.
DISCLOSURES:
The research was supported by the Department of Dermatology at the University of Texas Southwestern Medical Center and a grant from the National Institutes of Health. Several authors reported receiving grants or personal fees from various pharmaceutical companies. One author reported being a deputy editor for diversity, equity, and inclusion at JAMA Cardiology. Additional disclosures are noted in the original article.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
than with psoriasis.
METHODOLOGY:
- A retrospective matched longitudinal study compared the incidence and prevalence of ASCVD of 8138 individuals with CLE; 24,675 with SLE; 192,577 with psoriasis; and 81,380 control individuals.
- The disease-free control population was matched in a 10:1 ratio to the CLE population on the basis of age, sex, insurance type, and enrollment duration.
- Prevalent ASCVD was defined as coronary artery disease, prior myocardial infarction, or cerebrovascular accident, with ASCVD incidence assessed by number of hospitalizations over 3 years.
TAKEAWAY:
- Persons with CLE had higher ASCVD risk than control individuals (odds ratio [OR], 1.72; P < .001), similar to those with SLE (OR, 2.41; P < .001) but unlike those with psoriasis (OR, 1.03; P = .48).
- ASCVD incidence at 3 years was 24.8 per 1000 person-years for SLE, 15.2 per 1000 person-years for CLE, 14.0 per 1000 person-years for psoriasis, and 10.3 per 1000 person-years for controls.
- Multivariable Cox proportional regression modeling showed ASCVD risk was highest in those with SLE (hazard ratio [HR], 2.23; P < .001) vs CLE (HR, 1.32; P < .001) and psoriasis (HR, 1.06; P = .09).
- ASCVD prevalence was higher in individuals with CLE receiving systemic therapy (2.7%) than in those receiving no therapy (1.6%), suggesting a potential link between disease severity and CVD risk.
IN PRACTICE:
“Persons with CLE are at higher risk for ASCVD, and guidelines for the evaluation and management of ASCVD may improve their quality of care,” the authors wrote.
SOURCE:
The study was led by Henry W. Chen, MD, Department of Dermatology, University of Texas Southwestern Medical Center, Dallas. It was published online on December 4, 2024, in JAMA Dermatology.
LIMITATIONS:
The study was limited by its relatively young population (median age, 49 years) and the exclusion of adults aged > 65 years on Medicare insurance plans. The database lacked race and ethnicity data, and the analysis was restricted to a shorter 3-year period. The study could not fully evaluate detailed risk factors such as blood pressure levels, cholesterol measurements, or glycemic control, nor could it accurately assess smoking status.
DISCLOSURES:
The research was supported by the Department of Dermatology at the University of Texas Southwestern Medical Center and a grant from the National Institutes of Health. Several authors reported receiving grants or personal fees from various pharmaceutical companies. One author reported being a deputy editor for diversity, equity, and inclusion at JAMA Cardiology. Additional disclosures are noted in the original article.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
Study Addresses Lichen Planus Prevalence, Treatment
TOPLINE:
METHODOLOGY:
- To evaluate the prevalence of LP, researchers analyzed 566,851 eligible patients from the Explorys database, comprising electronic medical records from over 40 healthcare networks and 53 million patients across the United States.
- They also assessed treatment plans separately among 1998 newly diagnosed patients with LP between October 2015 and January 2020, who required at least one dermatology encounter within the first year following diagnosis.
- The primary outcome was overall prevalence of LP in the United States, including prevalence across specific age, sex, and racial subgroups. Additionally, dermatologist-prescribed treatments for non-oral LP were also reported.
TAKEAWAY:
- Overall, there were 1098 cases of LP (median age, 66 years; 74% women); the crude prevalence of LP was 0.19% and the age- and sex-standardized overall prevalence was 0.15%. Prevalence in women was 1.77 times higher than in men.
- Asian patients showed the highest standardized prevalence (0.2%), followed by Black patients (0.16). Prevalence increased with age, ranging from 0.04% among those aged 18-29 years to 0.26% among those aged 60-69 years and 0.33% among those aged 70-79 years.
IN PRACTICE:
“LP is a fairly common disease, which disproportionately affects women and individuals older than 60 years of age,” the authors wrote. “Future research to help identify patients who may need systemic treatment and determine appropriate treatments for patients with LP to limit sequelae is important as no medication is currently FDA approved for LP.”
SOURCE:
The study was led by Natalia Pelet Del Toro, MD, Department of Dermatology, Northwell Health, New Hyde Park, New York, and was published online in The Journal of the American Academy of Dermatology.
LIMITATIONS:
The absence of a precise diagnosis code for non-oral LP introduces potential misclassification risks. Additionally, the study design did not allow for the establishment of disease severity levels, limiting the ability to correlate treatment choices with disease severity.
DISCLOSURES:
The study did not receive any funding. Two authors reported to have received advisory fees, grants, and/or honoraria from several pharmaceutical companies. Pelet Del Toro and another author did not declare any conflict of interests.
This article was created using several editorial tools, including artificial intelligence, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- To evaluate the prevalence of LP, researchers analyzed 566,851 eligible patients from the Explorys database, comprising electronic medical records from over 40 healthcare networks and 53 million patients across the United States.
- They also assessed treatment plans separately among 1998 newly diagnosed patients with LP between October 2015 and January 2020, who required at least one dermatology encounter within the first year following diagnosis.
- The primary outcome was overall prevalence of LP in the United States, including prevalence across specific age, sex, and racial subgroups. Additionally, dermatologist-prescribed treatments for non-oral LP were also reported.
TAKEAWAY:
- Overall, there were 1098 cases of LP (median age, 66 years; 74% women); the crude prevalence of LP was 0.19% and the age- and sex-standardized overall prevalence was 0.15%. Prevalence in women was 1.77 times higher than in men.
- Asian patients showed the highest standardized prevalence (0.2%), followed by Black patients (0.16). Prevalence increased with age, ranging from 0.04% among those aged 18-29 years to 0.26% among those aged 60-69 years and 0.33% among those aged 70-79 years.
IN PRACTICE:
“LP is a fairly common disease, which disproportionately affects women and individuals older than 60 years of age,” the authors wrote. “Future research to help identify patients who may need systemic treatment and determine appropriate treatments for patients with LP to limit sequelae is important as no medication is currently FDA approved for LP.”
SOURCE:
The study was led by Natalia Pelet Del Toro, MD, Department of Dermatology, Northwell Health, New Hyde Park, New York, and was published online in The Journal of the American Academy of Dermatology.
LIMITATIONS:
The absence of a precise diagnosis code for non-oral LP introduces potential misclassification risks. Additionally, the study design did not allow for the establishment of disease severity levels, limiting the ability to correlate treatment choices with disease severity.
DISCLOSURES:
The study did not receive any funding. Two authors reported to have received advisory fees, grants, and/or honoraria from several pharmaceutical companies. Pelet Del Toro and another author did not declare any conflict of interests.
This article was created using several editorial tools, including artificial intelligence, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- To evaluate the prevalence of LP, researchers analyzed 566,851 eligible patients from the Explorys database, comprising electronic medical records from over 40 healthcare networks and 53 million patients across the United States.
- They also assessed treatment plans separately among 1998 newly diagnosed patients with LP between October 2015 and January 2020, who required at least one dermatology encounter within the first year following diagnosis.
- The primary outcome was overall prevalence of LP in the United States, including prevalence across specific age, sex, and racial subgroups. Additionally, dermatologist-prescribed treatments for non-oral LP were also reported.
TAKEAWAY:
- Overall, there were 1098 cases of LP (median age, 66 years; 74% women); the crude prevalence of LP was 0.19% and the age- and sex-standardized overall prevalence was 0.15%. Prevalence in women was 1.77 times higher than in men.
- Asian patients showed the highest standardized prevalence (0.2%), followed by Black patients (0.16). Prevalence increased with age, ranging from 0.04% among those aged 18-29 years to 0.26% among those aged 60-69 years and 0.33% among those aged 70-79 years.
IN PRACTICE:
“LP is a fairly common disease, which disproportionately affects women and individuals older than 60 years of age,” the authors wrote. “Future research to help identify patients who may need systemic treatment and determine appropriate treatments for patients with LP to limit sequelae is important as no medication is currently FDA approved for LP.”
SOURCE:
The study was led by Natalia Pelet Del Toro, MD, Department of Dermatology, Northwell Health, New Hyde Park, New York, and was published online in The Journal of the American Academy of Dermatology.
LIMITATIONS:
The absence of a precise diagnosis code for non-oral LP introduces potential misclassification risks. Additionally, the study design did not allow for the establishment of disease severity levels, limiting the ability to correlate treatment choices with disease severity.
DISCLOSURES:
The study did not receive any funding. Two authors reported to have received advisory fees, grants, and/or honoraria from several pharmaceutical companies. Pelet Del Toro and another author did not declare any conflict of interests.
This article was created using several editorial tools, including artificial intelligence, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
Phase 3 Lupus Trial Shows Promising Results for Dapirolizumab Pegol
WASHINGTON — The investigational anti-CD40 ligand agent dapirolizumab pegol (DZP) outperformed placebo in improving disease activity and reducing high-dose corticosteroid use in patients with systemic lupus erythematosus (SLE) in the phase 3 PHOENYCS GO trial.
“We really think that dapirolizumab pegol may represent a novel treatment for lupus, particularly given its broad immune modulatory effects,” said Megan Clowse, MD, MPH, associate professor of medicine and chief of the Division of Rheumatology and Immunology at Duke University School of Medicine in Durham, North Carolina. She presented the study in a late-breaking poster session at the American College of Rheumatology (ACR) 2024 Annual Meeting.
There is a “huge unmet need” for drugs for lupus, Clowse told this news organization. Patients with SLE continue to have high disease burden, including ongoing symptoms often driven by inflammation. Corticosteroids are often the best medications to control disease activity, she said, but they can result in long-term toxicity.
What Makes DZP Unique?
Through CD40 ligand signaling, DZP has been shown to reduce B- and T-cell activation and to downregulate interferon pathways. Previous antibodies targeting the CD40 ligand have been associated with an increased risk for thromboembolic events. However, DZP lacks the Fc portion of the antibody, which can bind to platelets and cause clotting. Data from phase 1, 2, and 3 trials thus far do not show an elevated risk for these events, Clowse explained. In fact, safety signals were strong enough that patients with antiphospholipid antibodies — a key driver for blood clots in patients with SLE — were included in the trial.
In PHOENYCS GO, investigators enrolled 321 patients with moderate to severe SLE with persistently active or frequently flaring/relapsing-remitting disease activity despite stable standard of care (SOC) medications such as antimalarials, corticosteroids, and immunosuppressants.
Patients were randomized 2:1 to receive intravenous DZP (24 mg/kg) plus SOC or intravenous placebo plus SOC every 4 weeks, with patients and investigators blinded to treatment assignments.
Patients taking a corticosteroid dose > 7.5 mg/day began a mandatory steroid taper by week 8 of the trial, with the goal of reducing that to < 7.5 mg/day. The tapering regimen was at the discretion of providers and was adapted to each patient’s individual disease activity.
The primary endpoint was British Isles Lupus Assessment Group–based Composite Lupus Assessment (BICLA) response at week 48.
Patients in the DZP and placebo groups were on average 43.5 and 41.5 years old, respectively. More than 90% of patients were women, all on concomitant SLE medications. About half of the participants took a daily corticosteroid dose > 7.5 mg.
At 48 weeks, half of the DZP group (49.5%) achieved BICLA response compared with 34.6% in the placebo group (P = .0110). A higher proportion of patients taking DZP achieved SLE Responder Index-4 response than those taking placebo (60.1% vs 41.1%, respectively; P = .0014), and the rate of severe British Isles Lupus Assessment Group flares in the DZP group was half that of the placebo group (11.6% vs 23.4%; P = .0257). In the subgroup of patients who underwent corticosteroid tapering, 72.4% receiving DZP and 52.9% taking placebo reduced their dose to < 7.5 mg/day by 48 weeks (P = .0404).
DZP was generally well tolerated. Over 48 weeks, 82.6% of the DZP group and 75% of the placebo group reported treatment-emergent adverse events, but serious occurrences were more common in the placebo group (14.8%) than in the DZP group (9.9%). Herpes viral infections were higher in the placebo group, although there were three ophthalmic herpes cases in the DZP group. There was one case of acute myocardial infarction and one death linked to gangrene-related sepsis in patients receiving DZP.
A ‘Mild to Moderate’ Response
Although these are definitely positive results, they show a “mild to moderate response” to DZP, commented Gregory Gardner, MD, an emeritus professor in the Division of Rheumatology at the University of Washington, Seattle, and chair of the American College of Rheumatology’s annual meeting planning committee. He moderated the session where the research was presented. Although DZP showed efficacy among some patients, he noted, “there were still 51% patients that it didn’t work for.”
The drug uses an alternative pathway to current lupus drugs, Gardner added, and more research is needed to understand how best to use this medication in practice.
Clowse noted that DZP could be particularly beneficial for patients with SLE who want to get pregnant. Many drugs used to treat the disease are teratogenic; however, “because of the lack of Fc portion on this drug, it very likely does not cross the placenta in any kind of significant amount,” she said. Although there are not yet any reproductive safety data on DZP, she added, “that is a great potential niche.”
Biogen and UCB, which are jointly developing DZP, aim to start a second phase 3 trial of DZP in patients with SLE, called PHOENYCS FLY, in 2024.
The trial was sponsored by UCB. Clowse is a consultant and has received grant/research support from GSK and UCB. Gardner had no relevant disclosures.
A version of this article appeared on Medscape.com.
WASHINGTON — The investigational anti-CD40 ligand agent dapirolizumab pegol (DZP) outperformed placebo in improving disease activity and reducing high-dose corticosteroid use in patients with systemic lupus erythematosus (SLE) in the phase 3 PHOENYCS GO trial.
“We really think that dapirolizumab pegol may represent a novel treatment for lupus, particularly given its broad immune modulatory effects,” said Megan Clowse, MD, MPH, associate professor of medicine and chief of the Division of Rheumatology and Immunology at Duke University School of Medicine in Durham, North Carolina. She presented the study in a late-breaking poster session at the American College of Rheumatology (ACR) 2024 Annual Meeting.
There is a “huge unmet need” for drugs for lupus, Clowse told this news organization. Patients with SLE continue to have high disease burden, including ongoing symptoms often driven by inflammation. Corticosteroids are often the best medications to control disease activity, she said, but they can result in long-term toxicity.
What Makes DZP Unique?
Through CD40 ligand signaling, DZP has been shown to reduce B- and T-cell activation and to downregulate interferon pathways. Previous antibodies targeting the CD40 ligand have been associated with an increased risk for thromboembolic events. However, DZP lacks the Fc portion of the antibody, which can bind to platelets and cause clotting. Data from phase 1, 2, and 3 trials thus far do not show an elevated risk for these events, Clowse explained. In fact, safety signals were strong enough that patients with antiphospholipid antibodies — a key driver for blood clots in patients with SLE — were included in the trial.
In PHOENYCS GO, investigators enrolled 321 patients with moderate to severe SLE with persistently active or frequently flaring/relapsing-remitting disease activity despite stable standard of care (SOC) medications such as antimalarials, corticosteroids, and immunosuppressants.
Patients were randomized 2:1 to receive intravenous DZP (24 mg/kg) plus SOC or intravenous placebo plus SOC every 4 weeks, with patients and investigators blinded to treatment assignments.
Patients taking a corticosteroid dose > 7.5 mg/day began a mandatory steroid taper by week 8 of the trial, with the goal of reducing that to < 7.5 mg/day. The tapering regimen was at the discretion of providers and was adapted to each patient’s individual disease activity.
The primary endpoint was British Isles Lupus Assessment Group–based Composite Lupus Assessment (BICLA) response at week 48.
Patients in the DZP and placebo groups were on average 43.5 and 41.5 years old, respectively. More than 90% of patients were women, all on concomitant SLE medications. About half of the participants took a daily corticosteroid dose > 7.5 mg.
At 48 weeks, half of the DZP group (49.5%) achieved BICLA response compared with 34.6% in the placebo group (P = .0110). A higher proportion of patients taking DZP achieved SLE Responder Index-4 response than those taking placebo (60.1% vs 41.1%, respectively; P = .0014), and the rate of severe British Isles Lupus Assessment Group flares in the DZP group was half that of the placebo group (11.6% vs 23.4%; P = .0257). In the subgroup of patients who underwent corticosteroid tapering, 72.4% receiving DZP and 52.9% taking placebo reduced their dose to < 7.5 mg/day by 48 weeks (P = .0404).
DZP was generally well tolerated. Over 48 weeks, 82.6% of the DZP group and 75% of the placebo group reported treatment-emergent adverse events, but serious occurrences were more common in the placebo group (14.8%) than in the DZP group (9.9%). Herpes viral infections were higher in the placebo group, although there were three ophthalmic herpes cases in the DZP group. There was one case of acute myocardial infarction and one death linked to gangrene-related sepsis in patients receiving DZP.
A ‘Mild to Moderate’ Response
Although these are definitely positive results, they show a “mild to moderate response” to DZP, commented Gregory Gardner, MD, an emeritus professor in the Division of Rheumatology at the University of Washington, Seattle, and chair of the American College of Rheumatology’s annual meeting planning committee. He moderated the session where the research was presented. Although DZP showed efficacy among some patients, he noted, “there were still 51% patients that it didn’t work for.”
The drug uses an alternative pathway to current lupus drugs, Gardner added, and more research is needed to understand how best to use this medication in practice.
Clowse noted that DZP could be particularly beneficial for patients with SLE who want to get pregnant. Many drugs used to treat the disease are teratogenic; however, “because of the lack of Fc portion on this drug, it very likely does not cross the placenta in any kind of significant amount,” she said. Although there are not yet any reproductive safety data on DZP, she added, “that is a great potential niche.”
Biogen and UCB, which are jointly developing DZP, aim to start a second phase 3 trial of DZP in patients with SLE, called PHOENYCS FLY, in 2024.
The trial was sponsored by UCB. Clowse is a consultant and has received grant/research support from GSK and UCB. Gardner had no relevant disclosures.
A version of this article appeared on Medscape.com.
WASHINGTON — The investigational anti-CD40 ligand agent dapirolizumab pegol (DZP) outperformed placebo in improving disease activity and reducing high-dose corticosteroid use in patients with systemic lupus erythematosus (SLE) in the phase 3 PHOENYCS GO trial.
“We really think that dapirolizumab pegol may represent a novel treatment for lupus, particularly given its broad immune modulatory effects,” said Megan Clowse, MD, MPH, associate professor of medicine and chief of the Division of Rheumatology and Immunology at Duke University School of Medicine in Durham, North Carolina. She presented the study in a late-breaking poster session at the American College of Rheumatology (ACR) 2024 Annual Meeting.
There is a “huge unmet need” for drugs for lupus, Clowse told this news organization. Patients with SLE continue to have high disease burden, including ongoing symptoms often driven by inflammation. Corticosteroids are often the best medications to control disease activity, she said, but they can result in long-term toxicity.
What Makes DZP Unique?
Through CD40 ligand signaling, DZP has been shown to reduce B- and T-cell activation and to downregulate interferon pathways. Previous antibodies targeting the CD40 ligand have been associated with an increased risk for thromboembolic events. However, DZP lacks the Fc portion of the antibody, which can bind to platelets and cause clotting. Data from phase 1, 2, and 3 trials thus far do not show an elevated risk for these events, Clowse explained. In fact, safety signals were strong enough that patients with antiphospholipid antibodies — a key driver for blood clots in patients with SLE — were included in the trial.
In PHOENYCS GO, investigators enrolled 321 patients with moderate to severe SLE with persistently active or frequently flaring/relapsing-remitting disease activity despite stable standard of care (SOC) medications such as antimalarials, corticosteroids, and immunosuppressants.
Patients were randomized 2:1 to receive intravenous DZP (24 mg/kg) plus SOC or intravenous placebo plus SOC every 4 weeks, with patients and investigators blinded to treatment assignments.
Patients taking a corticosteroid dose > 7.5 mg/day began a mandatory steroid taper by week 8 of the trial, with the goal of reducing that to < 7.5 mg/day. The tapering regimen was at the discretion of providers and was adapted to each patient’s individual disease activity.
The primary endpoint was British Isles Lupus Assessment Group–based Composite Lupus Assessment (BICLA) response at week 48.
Patients in the DZP and placebo groups were on average 43.5 and 41.5 years old, respectively. More than 90% of patients were women, all on concomitant SLE medications. About half of the participants took a daily corticosteroid dose > 7.5 mg.
At 48 weeks, half of the DZP group (49.5%) achieved BICLA response compared with 34.6% in the placebo group (P = .0110). A higher proportion of patients taking DZP achieved SLE Responder Index-4 response than those taking placebo (60.1% vs 41.1%, respectively; P = .0014), and the rate of severe British Isles Lupus Assessment Group flares in the DZP group was half that of the placebo group (11.6% vs 23.4%; P = .0257). In the subgroup of patients who underwent corticosteroid tapering, 72.4% receiving DZP and 52.9% taking placebo reduced their dose to < 7.5 mg/day by 48 weeks (P = .0404).
DZP was generally well tolerated. Over 48 weeks, 82.6% of the DZP group and 75% of the placebo group reported treatment-emergent adverse events, but serious occurrences were more common in the placebo group (14.8%) than in the DZP group (9.9%). Herpes viral infections were higher in the placebo group, although there were three ophthalmic herpes cases in the DZP group. There was one case of acute myocardial infarction and one death linked to gangrene-related sepsis in patients receiving DZP.
A ‘Mild to Moderate’ Response
Although these are definitely positive results, they show a “mild to moderate response” to DZP, commented Gregory Gardner, MD, an emeritus professor in the Division of Rheumatology at the University of Washington, Seattle, and chair of the American College of Rheumatology’s annual meeting planning committee. He moderated the session where the research was presented. Although DZP showed efficacy among some patients, he noted, “there were still 51% patients that it didn’t work for.”
The drug uses an alternative pathway to current lupus drugs, Gardner added, and more research is needed to understand how best to use this medication in practice.
Clowse noted that DZP could be particularly beneficial for patients with SLE who want to get pregnant. Many drugs used to treat the disease are teratogenic; however, “because of the lack of Fc portion on this drug, it very likely does not cross the placenta in any kind of significant amount,” she said. Although there are not yet any reproductive safety data on DZP, she added, “that is a great potential niche.”
Biogen and UCB, which are jointly developing DZP, aim to start a second phase 3 trial of DZP in patients with SLE, called PHOENYCS FLY, in 2024.
The trial was sponsored by UCB. Clowse is a consultant and has received grant/research support from GSK and UCB. Gardner had no relevant disclosures.
A version of this article appeared on Medscape.com.
FROM ACR 2024
Cancer Mortality Not Higher for Patients With Autoimmune Disease on Checkpoint Inhibitors
WASHINGTON — Immune checkpoint inhibitor (ICI) therapy does not increase mortality in people with preexisting autoimmune diseases, new research has found.
Results from a large database analysis of patients with and without autoimmune diseases suggest it is safe to treat them with ICI if they develop a cancer for which it is indicated, Greg Challener, MD, a postdoctoral fellow at the Rheumatology and Allergy Clinical Epidemiology Research Center, Massachusetts General Hospital, Boston, said at the American College of Rheumatology 2024 Annual Meeting.
“One message is that, when rheumatologists are asked by oncologists about patients with rheumatoid arthritis or vasculitis or other autoimmune diseases and whether it’s safe to treat them with immune checkpoint inhibitors, this result provides some evidence that it probably is safe…. Checkpoint inhibitors are really incredible drugs, and they’ve improved mortality for a lot of cancers, particularly melanoma, and so I think there should be a pretty high threshold for us to say a patient shouldn’t receive them because of an autoimmune condition,” he told this news organization.
Another implication, Challener said, is that people with autoimmune diseases shouldn’t routinely be excluded from clinical trials of ICIs. Currently they are excluded because of concerns about exacerbation of underlying autoimmunity, possible interference between the ICI and the immunosuppressive drugs used to treat the autoimmune condition, and a theoretical risk for serious adverse events.
“Clinical trials are continuing to exclude these patients, and they paint with a very broad brush anyone with underlying autoimmunity ... I’m hoping that that changes. I don’t think there’s a great evidence base to support that practice, and it’s unfortunate that patients with underlying autoimmune diseases are excluded from important studies,” Challener said.
Asked to comment, session moderator Matlock Jeffries, MD, director of the Arthritis Research Unit at the Oklahoma Medical Research Foundation, Oklahoma City, told this news organization that he agrees the data are generally reassuring. “If one of our patients gets cancer and their oncologist wants to use a checkpoint inhibitor, we’d obviously still monitor them for complications, but we wouldn’t automatically assume the combination of a checkpoint inhibitor and autoimmune disease would increase their mortality.”
No Difference in Mortality for Those With and Without Autoimmune Disease
Challener and colleagues used administrative health data from the TriNetX Diamond network of 92 US healthcare sites with 212 million patients. All patients included in the study were receiving anti-programmed death protein 1/programmed death ligand 1 to treat malignancies involving the skin, lung/bronchus, digestive organs, or urinary tract. The study population also had at least one rheumatologic, gastrointestinal, neurologic, dermatologic, or endocrine autoimmune disease.
Propensity score matching between those with and without autoimmune disease was performed for about 100 covariates. Prior to the matching, the autoimmune disease group had significantly higher rates of cardiovascular and other comorbidities. The matching yielded 23,714 individuals with autoimmune disease and the same number without who had similar demographics and comorbidity rates, as well as malignancy type, alcohol/tobacco use, and medication use.
At a median follow-up of 250 days, the risk for mortality prior to propensity matching was 40.0% in the autoimmune disease group and 38.1% for those without, a significant difference with hazard ratio 1.07 (95% CI, 1.05-1.10). But after the matching, the difference was no longer significant: 39.8% vs 40.2%, respectively (0.97, 0.94-1.00).
The Kaplan-Meier curves for survival probability for those with or without autoimmune disease were nearly superimposed, showing no difference up to 1600 days. An analysis of just the patients with rheumatic diseases yielded similar results, Challener said.
Some Caveats About the Data
Jeffries, who is also an associate professor of medicine at the University of Oklahoma Health Sciences Center, Oklahoma City, and the Oklahoma VA, said he would like to see additional data on outcomes, both for the autoimmune conditions and the cancers. Challener said there are plans to look at other hard endpoints such as myocardial infarction and end-stage renal disease, but that the database is limited.
Both Challener and Jeffries also cautioned that the reassurance may not apply to patients with active disease.
“One thing this research doesn’t address is whether active autoimmune disease might have a different outcome compared to more kind of quiet disease…. If you have a patient who has extremely active rheumatoid arthritis or extremely active giant cell arthritis, for instance, I think that could be more challenging. I would be frightened to put a patient with really active GCA on pembrolizumab or say that it’s safe without their disease being controlled. But for someone who has well-controlled disease or minimally active disease, this is very reassuring,” Challener told this news organization.
“I think this may also be important in that it’s a good argument to tell the drug companies to include autoimmune patients in these trials so we can get better data,” Jeffries said.
Challener and Jeffries had no relevant disclosures.
A version of this article appeared on Medscape.com.
WASHINGTON — Immune checkpoint inhibitor (ICI) therapy does not increase mortality in people with preexisting autoimmune diseases, new research has found.
Results from a large database analysis of patients with and without autoimmune diseases suggest it is safe to treat them with ICI if they develop a cancer for which it is indicated, Greg Challener, MD, a postdoctoral fellow at the Rheumatology and Allergy Clinical Epidemiology Research Center, Massachusetts General Hospital, Boston, said at the American College of Rheumatology 2024 Annual Meeting.
“One message is that, when rheumatologists are asked by oncologists about patients with rheumatoid arthritis or vasculitis or other autoimmune diseases and whether it’s safe to treat them with immune checkpoint inhibitors, this result provides some evidence that it probably is safe…. Checkpoint inhibitors are really incredible drugs, and they’ve improved mortality for a lot of cancers, particularly melanoma, and so I think there should be a pretty high threshold for us to say a patient shouldn’t receive them because of an autoimmune condition,” he told this news organization.
Another implication, Challener said, is that people with autoimmune diseases shouldn’t routinely be excluded from clinical trials of ICIs. Currently they are excluded because of concerns about exacerbation of underlying autoimmunity, possible interference between the ICI and the immunosuppressive drugs used to treat the autoimmune condition, and a theoretical risk for serious adverse events.
“Clinical trials are continuing to exclude these patients, and they paint with a very broad brush anyone with underlying autoimmunity ... I’m hoping that that changes. I don’t think there’s a great evidence base to support that practice, and it’s unfortunate that patients with underlying autoimmune diseases are excluded from important studies,” Challener said.
Asked to comment, session moderator Matlock Jeffries, MD, director of the Arthritis Research Unit at the Oklahoma Medical Research Foundation, Oklahoma City, told this news organization that he agrees the data are generally reassuring. “If one of our patients gets cancer and their oncologist wants to use a checkpoint inhibitor, we’d obviously still monitor them for complications, but we wouldn’t automatically assume the combination of a checkpoint inhibitor and autoimmune disease would increase their mortality.”
No Difference in Mortality for Those With and Without Autoimmune Disease
Challener and colleagues used administrative health data from the TriNetX Diamond network of 92 US healthcare sites with 212 million patients. All patients included in the study were receiving anti-programmed death protein 1/programmed death ligand 1 to treat malignancies involving the skin, lung/bronchus, digestive organs, or urinary tract. The study population also had at least one rheumatologic, gastrointestinal, neurologic, dermatologic, or endocrine autoimmune disease.
Propensity score matching between those with and without autoimmune disease was performed for about 100 covariates. Prior to the matching, the autoimmune disease group had significantly higher rates of cardiovascular and other comorbidities. The matching yielded 23,714 individuals with autoimmune disease and the same number without who had similar demographics and comorbidity rates, as well as malignancy type, alcohol/tobacco use, and medication use.
At a median follow-up of 250 days, the risk for mortality prior to propensity matching was 40.0% in the autoimmune disease group and 38.1% for those without, a significant difference with hazard ratio 1.07 (95% CI, 1.05-1.10). But after the matching, the difference was no longer significant: 39.8% vs 40.2%, respectively (0.97, 0.94-1.00).
The Kaplan-Meier curves for survival probability for those with or without autoimmune disease were nearly superimposed, showing no difference up to 1600 days. An analysis of just the patients with rheumatic diseases yielded similar results, Challener said.
Some Caveats About the Data
Jeffries, who is also an associate professor of medicine at the University of Oklahoma Health Sciences Center, Oklahoma City, and the Oklahoma VA, said he would like to see additional data on outcomes, both for the autoimmune conditions and the cancers. Challener said there are plans to look at other hard endpoints such as myocardial infarction and end-stage renal disease, but that the database is limited.
Both Challener and Jeffries also cautioned that the reassurance may not apply to patients with active disease.
“One thing this research doesn’t address is whether active autoimmune disease might have a different outcome compared to more kind of quiet disease…. If you have a patient who has extremely active rheumatoid arthritis or extremely active giant cell arthritis, for instance, I think that could be more challenging. I would be frightened to put a patient with really active GCA on pembrolizumab or say that it’s safe without their disease being controlled. But for someone who has well-controlled disease or minimally active disease, this is very reassuring,” Challener told this news organization.
“I think this may also be important in that it’s a good argument to tell the drug companies to include autoimmune patients in these trials so we can get better data,” Jeffries said.
Challener and Jeffries had no relevant disclosures.
A version of this article appeared on Medscape.com.
WASHINGTON — Immune checkpoint inhibitor (ICI) therapy does not increase mortality in people with preexisting autoimmune diseases, new research has found.
Results from a large database analysis of patients with and without autoimmune diseases suggest it is safe to treat them with ICI if they develop a cancer for which it is indicated, Greg Challener, MD, a postdoctoral fellow at the Rheumatology and Allergy Clinical Epidemiology Research Center, Massachusetts General Hospital, Boston, said at the American College of Rheumatology 2024 Annual Meeting.
“One message is that, when rheumatologists are asked by oncologists about patients with rheumatoid arthritis or vasculitis or other autoimmune diseases and whether it’s safe to treat them with immune checkpoint inhibitors, this result provides some evidence that it probably is safe…. Checkpoint inhibitors are really incredible drugs, and they’ve improved mortality for a lot of cancers, particularly melanoma, and so I think there should be a pretty high threshold for us to say a patient shouldn’t receive them because of an autoimmune condition,” he told this news organization.
Another implication, Challener said, is that people with autoimmune diseases shouldn’t routinely be excluded from clinical trials of ICIs. Currently they are excluded because of concerns about exacerbation of underlying autoimmunity, possible interference between the ICI and the immunosuppressive drugs used to treat the autoimmune condition, and a theoretical risk for serious adverse events.
“Clinical trials are continuing to exclude these patients, and they paint with a very broad brush anyone with underlying autoimmunity ... I’m hoping that that changes. I don’t think there’s a great evidence base to support that practice, and it’s unfortunate that patients with underlying autoimmune diseases are excluded from important studies,” Challener said.
Asked to comment, session moderator Matlock Jeffries, MD, director of the Arthritis Research Unit at the Oklahoma Medical Research Foundation, Oklahoma City, told this news organization that he agrees the data are generally reassuring. “If one of our patients gets cancer and their oncologist wants to use a checkpoint inhibitor, we’d obviously still monitor them for complications, but we wouldn’t automatically assume the combination of a checkpoint inhibitor and autoimmune disease would increase their mortality.”
No Difference in Mortality for Those With and Without Autoimmune Disease
Challener and colleagues used administrative health data from the TriNetX Diamond network of 92 US healthcare sites with 212 million patients. All patients included in the study were receiving anti-programmed death protein 1/programmed death ligand 1 to treat malignancies involving the skin, lung/bronchus, digestive organs, or urinary tract. The study population also had at least one rheumatologic, gastrointestinal, neurologic, dermatologic, or endocrine autoimmune disease.
Propensity score matching between those with and without autoimmune disease was performed for about 100 covariates. Prior to the matching, the autoimmune disease group had significantly higher rates of cardiovascular and other comorbidities. The matching yielded 23,714 individuals with autoimmune disease and the same number without who had similar demographics and comorbidity rates, as well as malignancy type, alcohol/tobacco use, and medication use.
At a median follow-up of 250 days, the risk for mortality prior to propensity matching was 40.0% in the autoimmune disease group and 38.1% for those without, a significant difference with hazard ratio 1.07 (95% CI, 1.05-1.10). But after the matching, the difference was no longer significant: 39.8% vs 40.2%, respectively (0.97, 0.94-1.00).
The Kaplan-Meier curves for survival probability for those with or without autoimmune disease were nearly superimposed, showing no difference up to 1600 days. An analysis of just the patients with rheumatic diseases yielded similar results, Challener said.
Some Caveats About the Data
Jeffries, who is also an associate professor of medicine at the University of Oklahoma Health Sciences Center, Oklahoma City, and the Oklahoma VA, said he would like to see additional data on outcomes, both for the autoimmune conditions and the cancers. Challener said there are plans to look at other hard endpoints such as myocardial infarction and end-stage renal disease, but that the database is limited.
Both Challener and Jeffries also cautioned that the reassurance may not apply to patients with active disease.
“One thing this research doesn’t address is whether active autoimmune disease might have a different outcome compared to more kind of quiet disease…. If you have a patient who has extremely active rheumatoid arthritis or extremely active giant cell arthritis, for instance, I think that could be more challenging. I would be frightened to put a patient with really active GCA on pembrolizumab or say that it’s safe without their disease being controlled. But for someone who has well-controlled disease or minimally active disease, this is very reassuring,” Challener told this news organization.
“I think this may also be important in that it’s a good argument to tell the drug companies to include autoimmune patients in these trials so we can get better data,” Jeffries said.
Challener and Jeffries had no relevant disclosures.
A version of this article appeared on Medscape.com.
FROM ACR 2024
Post COVID-19, Long-term Risk for Autoimmune, Autoinflammatory Skin Disorders Increased, Study Finds
In addition, the authors reported that COVID-19 vaccination appears to reduce these risks.
The study was published in JAMA Dermatology.
‘Compelling Evidence’
“This well-executed study by Heo et al provides compelling evidence to support an association between COVID-19 infection and the development of subsequent autoimmune and autoinflammatory skin diseases,” wrote authors led by Lisa M. Arkin, MD, of the Department of Dermatology, University of Wisconsin School of Medicine and Public Health in Madison, in an accompanying editorial.
Using databases from Korea’s National Health Insurance Service and the Korea Disease Control and Prevention Agency, investigators led by Yeon-Woo Heo, MD, a dermatology resident at Yonsei University Wonju College of Medicine, Wonju, Republic of Korea, compared 3.1 million people who had COVID-19 with 3.8 million controls, all with at least 180 days’ follow-up through December 31, 2022.
At a mean follow-up of 287 days in both cohorts, authors found significantly elevated risks for AA and vitiligo (adjusted hazard ratio [aHR], 1.11 for both), AT (aHR, 1.24), Behçet disease (aHR, 1.45), and BP (aHR, 1.62) in the post–COVID-19 cohort. The infection also raised the risk for other conditions such as systemic lupus erythematosus (aHR, 1.14) and Crohn’s disease (aHR, 1.35).
In subgroup analyses, demographic factors were associated with diverse effects: COVID-19 infection was associated with significantly higher odds of developing AA (for both men and women), vitiligo (men), Behçet disease (men and women), Crohn’s disease (men), ulcerative colitis (men), rheumatoid arthritis (men and women), systemic lupus erythematosus (men), ankylosing spondylitis (men), AT (women), and BP (women) than controls.
Those aged under 40 years were more likely to develop AA, primary cicatricial alopecia, Behçet disease, and ulcerative colitis, while those aged 40 years or older were more likely to develop AA, AT, vitiligo, Behçet disease, Crohn’s disease, rheumatoid arthritis, systemic lupus erythematosus, Sjögren’s syndrome, ankylosing spondylitis, and BP.
Additionally, severe COVID-19 requiring intensive care unit admission was associated with a significantly increased risk for autoimmune diseases, including AA, psoriasis, BP, and sarcoidosis. By timeframe, risks for AA, AT, and psoriasis were significantly higher during the initial Delta-dominant period.
Vaccination Effect
Moreover, vaccinated individuals were less likely to develop AA, AT, psoriasis, Behçet disease, and various nondermatologic conditions than were those who were unvaccinated. This finding, wrote Heo and colleagues, “may provide evidence to support the hypothesis that COVID-19 vaccines can help prevent autoimmune diseases.”
“That’s the part we all need to take into our offices tomorrow,” said Brett King, MD, PhD, a Fairfield, Connecticut–based dermatologist in private practice. He was not involved with the study but was asked to comment.
Overall, King said, the study carries two main messages. “The first is that COVID-19 infection increases the likelihood of developing an autoimmune or autoinflammatory disease in a large population.” The second and very important message is that being vaccinated against COVID-19 provides protection against developing an autoimmune or autoinflammatory disease.
“My concern is that the popular media highlights the first part,” said King, “and everybody who develops alopecia areata, vitiligo, or sarcoidosis blames COVID-19. That’s not what this work says.”
The foregoing distinction is especially important during the fall and winter, he added, when people getting influenza vaccines are routinely offered COVID-19 vaccines. “Many patients have said, ‘I got the COVID vaccine and developed alopecia areata 6 months later.’ Nearly everybody who has developed a new or worsening health condition in the last almost 5 years has had the perfect fall guy — the COVID vaccine or infection.”
With virtually all patients asking if they should get an updated COVID-19 vaccine or booster, he added, many report having heard that such vaccines cause AA, vitiligo, or other diseases. “To anchor these conversations in real data and not just anecdotes from a blog or Facebook is very useful,” said King, “and now we have very good data saying that the COVID vaccine is protective against these disorders.”
George Han, MD, PhD, associate professor of dermatology at the Donald and Barbara Zucker School of Medicine at Hofstra/Northwell in Hempstead, New York, applauds investigators’ use of a large, robust database but suggests interpreting results cautiously. He was not involved with the study but was asked to comment.
“You could do a large, well-done study,” Han said, “but it could still not necessarily be generalizable. These autoimmune conditions they’re looking at have clear ethnic and racial biases.” Heo and colleagues acknowledged shortcomings including their study population’s monomorphic nature.
Additional issues that limit the study’s impact, said Han, include the difficulty of conceptualizing a 10%-20% increase in conditions that at baseline are rare. And many of the findings reflected natural patterns, he said. For instance, BP more commonly affects older people, COVID-19 notwithstanding.
Han said that for him, the study’s main value going forward is helping to explain a rash of worsening inflammatory skin disease that many dermatologists saw early in the pandemic. “We would regularly see patients who were well controlled with, for example, psoriasis or eczema. But after COVID-19 infection or a vaccine (usually mRNA-type), in some cases they would come in flaring badly.” This happened at least a dozen times during the first year of post-shutdown appointments, he said.
“We’ve seen patients who have flared multiple times — they get the booster, then flare again,” Han added. Similar patterns occurred with pyoderma gangrenosum and other inflammatory skin diseases, he said.
Given the modest effect sizes of the associations reported in the Korean study, Arkin and colleagues wrote in their JAMA Dermatology editorial that surveillance for autoimmune disease is probably not warranted without new examination findings or symptoms. “For certain,” King said, “we should not go hunting for things that aren’t obviously there.”
Rather, Arkin and colleagues wrote, the higher autoimmunity rates seen among the unvaccinated, as well as during the Delta phase (when patients were sicker and hospitalizations were more likely) and in patients requiring intensive care, suggest that “interventions that reduce disease severity could also potentially reduce long-term risk of subsequent autoimmune sequelae.”
Future research addressing whether people with preexisting autoimmune conditions are at greater risk for flares or developing new autoimmune diseases following COVID-19 infection “would help to frame an evidence-based approach for patients with autoimmune disorders who develop COVID-19 infection, including the role for antiviral treatments,” they added.
The study was supported by grants from the Research Program of the Korea Medical Institute, the Korea Health Industry Development Institute, and the National Research Foundation of Korea. Han and King reported no relevant financial relationships. Arkin disclosed receiving research grants to her institution from Amgen and Eli Lilly, personal fees from Sanofi/Regeneron for consulting, and personal consulting fees from Merck outside the submitted work. Another author reported personal consulting fees from Dexcel Pharma and Honeydew outside the submitted work. No other disclosures were reported.
A version of this article appeared on Medscape.com.
In addition, the authors reported that COVID-19 vaccination appears to reduce these risks.
The study was published in JAMA Dermatology.
‘Compelling Evidence’
“This well-executed study by Heo et al provides compelling evidence to support an association between COVID-19 infection and the development of subsequent autoimmune and autoinflammatory skin diseases,” wrote authors led by Lisa M. Arkin, MD, of the Department of Dermatology, University of Wisconsin School of Medicine and Public Health in Madison, in an accompanying editorial.
Using databases from Korea’s National Health Insurance Service and the Korea Disease Control and Prevention Agency, investigators led by Yeon-Woo Heo, MD, a dermatology resident at Yonsei University Wonju College of Medicine, Wonju, Republic of Korea, compared 3.1 million people who had COVID-19 with 3.8 million controls, all with at least 180 days’ follow-up through December 31, 2022.
At a mean follow-up of 287 days in both cohorts, authors found significantly elevated risks for AA and vitiligo (adjusted hazard ratio [aHR], 1.11 for both), AT (aHR, 1.24), Behçet disease (aHR, 1.45), and BP (aHR, 1.62) in the post–COVID-19 cohort. The infection also raised the risk for other conditions such as systemic lupus erythematosus (aHR, 1.14) and Crohn’s disease (aHR, 1.35).
In subgroup analyses, demographic factors were associated with diverse effects: COVID-19 infection was associated with significantly higher odds of developing AA (for both men and women), vitiligo (men), Behçet disease (men and women), Crohn’s disease (men), ulcerative colitis (men), rheumatoid arthritis (men and women), systemic lupus erythematosus (men), ankylosing spondylitis (men), AT (women), and BP (women) than controls.
Those aged under 40 years were more likely to develop AA, primary cicatricial alopecia, Behçet disease, and ulcerative colitis, while those aged 40 years or older were more likely to develop AA, AT, vitiligo, Behçet disease, Crohn’s disease, rheumatoid arthritis, systemic lupus erythematosus, Sjögren’s syndrome, ankylosing spondylitis, and BP.
Additionally, severe COVID-19 requiring intensive care unit admission was associated with a significantly increased risk for autoimmune diseases, including AA, psoriasis, BP, and sarcoidosis. By timeframe, risks for AA, AT, and psoriasis were significantly higher during the initial Delta-dominant period.
Vaccination Effect
Moreover, vaccinated individuals were less likely to develop AA, AT, psoriasis, Behçet disease, and various nondermatologic conditions than were those who were unvaccinated. This finding, wrote Heo and colleagues, “may provide evidence to support the hypothesis that COVID-19 vaccines can help prevent autoimmune diseases.”
“That’s the part we all need to take into our offices tomorrow,” said Brett King, MD, PhD, a Fairfield, Connecticut–based dermatologist in private practice. He was not involved with the study but was asked to comment.
Overall, King said, the study carries two main messages. “The first is that COVID-19 infection increases the likelihood of developing an autoimmune or autoinflammatory disease in a large population.” The second and very important message is that being vaccinated against COVID-19 provides protection against developing an autoimmune or autoinflammatory disease.
“My concern is that the popular media highlights the first part,” said King, “and everybody who develops alopecia areata, vitiligo, or sarcoidosis blames COVID-19. That’s not what this work says.”
The foregoing distinction is especially important during the fall and winter, he added, when people getting influenza vaccines are routinely offered COVID-19 vaccines. “Many patients have said, ‘I got the COVID vaccine and developed alopecia areata 6 months later.’ Nearly everybody who has developed a new or worsening health condition in the last almost 5 years has had the perfect fall guy — the COVID vaccine or infection.”
With virtually all patients asking if they should get an updated COVID-19 vaccine or booster, he added, many report having heard that such vaccines cause AA, vitiligo, or other diseases. “To anchor these conversations in real data and not just anecdotes from a blog or Facebook is very useful,” said King, “and now we have very good data saying that the COVID vaccine is protective against these disorders.”
George Han, MD, PhD, associate professor of dermatology at the Donald and Barbara Zucker School of Medicine at Hofstra/Northwell in Hempstead, New York, applauds investigators’ use of a large, robust database but suggests interpreting results cautiously. He was not involved with the study but was asked to comment.
“You could do a large, well-done study,” Han said, “but it could still not necessarily be generalizable. These autoimmune conditions they’re looking at have clear ethnic and racial biases.” Heo and colleagues acknowledged shortcomings including their study population’s monomorphic nature.
Additional issues that limit the study’s impact, said Han, include the difficulty of conceptualizing a 10%-20% increase in conditions that at baseline are rare. And many of the findings reflected natural patterns, he said. For instance, BP more commonly affects older people, COVID-19 notwithstanding.
Han said that for him, the study’s main value going forward is helping to explain a rash of worsening inflammatory skin disease that many dermatologists saw early in the pandemic. “We would regularly see patients who were well controlled with, for example, psoriasis or eczema. But after COVID-19 infection or a vaccine (usually mRNA-type), in some cases they would come in flaring badly.” This happened at least a dozen times during the first year of post-shutdown appointments, he said.
“We’ve seen patients who have flared multiple times — they get the booster, then flare again,” Han added. Similar patterns occurred with pyoderma gangrenosum and other inflammatory skin diseases, he said.
Given the modest effect sizes of the associations reported in the Korean study, Arkin and colleagues wrote in their JAMA Dermatology editorial that surveillance for autoimmune disease is probably not warranted without new examination findings or symptoms. “For certain,” King said, “we should not go hunting for things that aren’t obviously there.”
Rather, Arkin and colleagues wrote, the higher autoimmunity rates seen among the unvaccinated, as well as during the Delta phase (when patients were sicker and hospitalizations were more likely) and in patients requiring intensive care, suggest that “interventions that reduce disease severity could also potentially reduce long-term risk of subsequent autoimmune sequelae.”
Future research addressing whether people with preexisting autoimmune conditions are at greater risk for flares or developing new autoimmune diseases following COVID-19 infection “would help to frame an evidence-based approach for patients with autoimmune disorders who develop COVID-19 infection, including the role for antiviral treatments,” they added.
The study was supported by grants from the Research Program of the Korea Medical Institute, the Korea Health Industry Development Institute, and the National Research Foundation of Korea. Han and King reported no relevant financial relationships. Arkin disclosed receiving research grants to her institution from Amgen and Eli Lilly, personal fees from Sanofi/Regeneron for consulting, and personal consulting fees from Merck outside the submitted work. Another author reported personal consulting fees from Dexcel Pharma and Honeydew outside the submitted work. No other disclosures were reported.
A version of this article appeared on Medscape.com.
In addition, the authors reported that COVID-19 vaccination appears to reduce these risks.
The study was published in JAMA Dermatology.
‘Compelling Evidence’
“This well-executed study by Heo et al provides compelling evidence to support an association between COVID-19 infection and the development of subsequent autoimmune and autoinflammatory skin diseases,” wrote authors led by Lisa M. Arkin, MD, of the Department of Dermatology, University of Wisconsin School of Medicine and Public Health in Madison, in an accompanying editorial.
Using databases from Korea’s National Health Insurance Service and the Korea Disease Control and Prevention Agency, investigators led by Yeon-Woo Heo, MD, a dermatology resident at Yonsei University Wonju College of Medicine, Wonju, Republic of Korea, compared 3.1 million people who had COVID-19 with 3.8 million controls, all with at least 180 days’ follow-up through December 31, 2022.
At a mean follow-up of 287 days in both cohorts, authors found significantly elevated risks for AA and vitiligo (adjusted hazard ratio [aHR], 1.11 for both), AT (aHR, 1.24), Behçet disease (aHR, 1.45), and BP (aHR, 1.62) in the post–COVID-19 cohort. The infection also raised the risk for other conditions such as systemic lupus erythematosus (aHR, 1.14) and Crohn’s disease (aHR, 1.35).
In subgroup analyses, demographic factors were associated with diverse effects: COVID-19 infection was associated with significantly higher odds of developing AA (for both men and women), vitiligo (men), Behçet disease (men and women), Crohn’s disease (men), ulcerative colitis (men), rheumatoid arthritis (men and women), systemic lupus erythematosus (men), ankylosing spondylitis (men), AT (women), and BP (women) than controls.
Those aged under 40 years were more likely to develop AA, primary cicatricial alopecia, Behçet disease, and ulcerative colitis, while those aged 40 years or older were more likely to develop AA, AT, vitiligo, Behçet disease, Crohn’s disease, rheumatoid arthritis, systemic lupus erythematosus, Sjögren’s syndrome, ankylosing spondylitis, and BP.
Additionally, severe COVID-19 requiring intensive care unit admission was associated with a significantly increased risk for autoimmune diseases, including AA, psoriasis, BP, and sarcoidosis. By timeframe, risks for AA, AT, and psoriasis were significantly higher during the initial Delta-dominant period.
Vaccination Effect
Moreover, vaccinated individuals were less likely to develop AA, AT, psoriasis, Behçet disease, and various nondermatologic conditions than were those who were unvaccinated. This finding, wrote Heo and colleagues, “may provide evidence to support the hypothesis that COVID-19 vaccines can help prevent autoimmune diseases.”
“That’s the part we all need to take into our offices tomorrow,” said Brett King, MD, PhD, a Fairfield, Connecticut–based dermatologist in private practice. He was not involved with the study but was asked to comment.
Overall, King said, the study carries two main messages. “The first is that COVID-19 infection increases the likelihood of developing an autoimmune or autoinflammatory disease in a large population.” The second and very important message is that being vaccinated against COVID-19 provides protection against developing an autoimmune or autoinflammatory disease.
“My concern is that the popular media highlights the first part,” said King, “and everybody who develops alopecia areata, vitiligo, or sarcoidosis blames COVID-19. That’s not what this work says.”
The foregoing distinction is especially important during the fall and winter, he added, when people getting influenza vaccines are routinely offered COVID-19 vaccines. “Many patients have said, ‘I got the COVID vaccine and developed alopecia areata 6 months later.’ Nearly everybody who has developed a new or worsening health condition in the last almost 5 years has had the perfect fall guy — the COVID vaccine or infection.”
With virtually all patients asking if they should get an updated COVID-19 vaccine or booster, he added, many report having heard that such vaccines cause AA, vitiligo, or other diseases. “To anchor these conversations in real data and not just anecdotes from a blog or Facebook is very useful,” said King, “and now we have very good data saying that the COVID vaccine is protective against these disorders.”
George Han, MD, PhD, associate professor of dermatology at the Donald and Barbara Zucker School of Medicine at Hofstra/Northwell in Hempstead, New York, applauds investigators’ use of a large, robust database but suggests interpreting results cautiously. He was not involved with the study but was asked to comment.
“You could do a large, well-done study,” Han said, “but it could still not necessarily be generalizable. These autoimmune conditions they’re looking at have clear ethnic and racial biases.” Heo and colleagues acknowledged shortcomings including their study population’s monomorphic nature.
Additional issues that limit the study’s impact, said Han, include the difficulty of conceptualizing a 10%-20% increase in conditions that at baseline are rare. And many of the findings reflected natural patterns, he said. For instance, BP more commonly affects older people, COVID-19 notwithstanding.
Han said that for him, the study’s main value going forward is helping to explain a rash of worsening inflammatory skin disease that many dermatologists saw early in the pandemic. “We would regularly see patients who were well controlled with, for example, psoriasis or eczema. But after COVID-19 infection or a vaccine (usually mRNA-type), in some cases they would come in flaring badly.” This happened at least a dozen times during the first year of post-shutdown appointments, he said.
“We’ve seen patients who have flared multiple times — they get the booster, then flare again,” Han added. Similar patterns occurred with pyoderma gangrenosum and other inflammatory skin diseases, he said.
Given the modest effect sizes of the associations reported in the Korean study, Arkin and colleagues wrote in their JAMA Dermatology editorial that surveillance for autoimmune disease is probably not warranted without new examination findings or symptoms. “For certain,” King said, “we should not go hunting for things that aren’t obviously there.”
Rather, Arkin and colleagues wrote, the higher autoimmunity rates seen among the unvaccinated, as well as during the Delta phase (when patients were sicker and hospitalizations were more likely) and in patients requiring intensive care, suggest that “interventions that reduce disease severity could also potentially reduce long-term risk of subsequent autoimmune sequelae.”
Future research addressing whether people with preexisting autoimmune conditions are at greater risk for flares or developing new autoimmune diseases following COVID-19 infection “would help to frame an evidence-based approach for patients with autoimmune disorders who develop COVID-19 infection, including the role for antiviral treatments,” they added.
The study was supported by grants from the Research Program of the Korea Medical Institute, the Korea Health Industry Development Institute, and the National Research Foundation of Korea. Han and King reported no relevant financial relationships. Arkin disclosed receiving research grants to her institution from Amgen and Eli Lilly, personal fees from Sanofi/Regeneron for consulting, and personal consulting fees from Merck outside the submitted work. Another author reported personal consulting fees from Dexcel Pharma and Honeydew outside the submitted work. No other disclosures were reported.
A version of this article appeared on Medscape.com.
FROM JAMA DERMATOLOGY
Home Spirometry Has Potential for Detecting Pulmonary Decline in Systemic Sclerosis
TOPLINE:
Home spirometry shows potential for early detection of pulmonary function decline in patients with systemic sclerosis–associated interstitial lung disease (SSc-ILD). It shows good cross-sectional correlation with hospital tests, along with 60% sensitivity and 87% specificity for detecting progressive ILD.
METHODOLOGY:
- Researchers conducted a prospective, observational study to examine the validity of home spirometry for detecting a decline in pulmonary function in patients with SSc-ILD.
- They included 43 patients aged 18 years or older with SSc-ILD from two tertiary referral centers in the Netherlands who received treatment with immunosuppressives for a maximum duration of 8 weeks prior to baseline.
- All participants were required to take weekly home spirometry measurements using a handheld spirometer for 1 year, with 35 completing 6 months of follow-up and 31 completing 12 months.
- Pulmonary function tests were conducted in the hospital at baseline and semiannual visits.
- The primary outcome was the κ (kappa statistic) agreement between home and hospital measurements after 1 year to detect a decline in forced vital capacity (FVC) of 5% or more; the sensitivity and specificity of home spirometry were also evaluated to detect an absolute decline in FVC%, using hospital tests as the gold standard.
TAKEAWAY:
- Home spirometry showed a fair agreement with the pulmonary function tests conducted at the hospital (κ, 0.40; 95% CI, 0.01-0.79).
- Home spirometry showed a sensitivity of 60% and specificity of 87% in detecting a decline in FVC% predicted of 5% or more.
- The intraclass correlation coefficient between home and hospital FVC measurements was moderate to high, with values of 0.85 at baseline, 0.84 at 6 months, and 0.72 at 12 months (P < .0001 for all).
- However, the longitudinal agreement between home and hospital measurements was lower with a correlation coefficient of 0.55.
IN PRACTICE:
“These findings suggest that home spirometry is both feasible and moderately accurate in patients with systemic sclerosis–associated ILD. However, where home spirometry fell short was the low sensitivity in detecting a decline in FVC% predicted,” experts wrote in an accompanying editorial.
“The results of this study support further evaluation of the implementation of home spirometry in addition to regular healthcare management but do not endorse relying solely on home monitoring to detect a decline in pulmonary function,” study authors wrote.
SOURCE:
The study was led by Arthiha Velauthapillai, MD, Department of Rheumatology, Radboud University Medical Center, Nijmegen, the Netherlands, and was published online November 8, 2024, in The Lancet Rheumatology.
LIMITATIONS:
The study might have been underpowered because of inaccuracies in initial assumptions, with a lower-than-anticipated prevalence of progressive ILD and a higher dropout rate. The study included only Dutch patients, which may have limited the generalizability of its findings to other settings with lower internet access or literacy rates.
DISCLOSURES:
This study was partly supported by grants from Galapagos and Boehringer Ingelheim. Some authors received grants or consulting or speaker fees from Boehringer Ingelheim, AstraZeneca, and other pharmaceutical companies.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
TOPLINE:
Home spirometry shows potential for early detection of pulmonary function decline in patients with systemic sclerosis–associated interstitial lung disease (SSc-ILD). It shows good cross-sectional correlation with hospital tests, along with 60% sensitivity and 87% specificity for detecting progressive ILD.
METHODOLOGY:
- Researchers conducted a prospective, observational study to examine the validity of home spirometry for detecting a decline in pulmonary function in patients with SSc-ILD.
- They included 43 patients aged 18 years or older with SSc-ILD from two tertiary referral centers in the Netherlands who received treatment with immunosuppressives for a maximum duration of 8 weeks prior to baseline.
- All participants were required to take weekly home spirometry measurements using a handheld spirometer for 1 year, with 35 completing 6 months of follow-up and 31 completing 12 months.
- Pulmonary function tests were conducted in the hospital at baseline and semiannual visits.
- The primary outcome was the κ (kappa statistic) agreement between home and hospital measurements after 1 year to detect a decline in forced vital capacity (FVC) of 5% or more; the sensitivity and specificity of home spirometry were also evaluated to detect an absolute decline in FVC%, using hospital tests as the gold standard.
TAKEAWAY:
- Home spirometry showed a fair agreement with the pulmonary function tests conducted at the hospital (κ, 0.40; 95% CI, 0.01-0.79).
- Home spirometry showed a sensitivity of 60% and specificity of 87% in detecting a decline in FVC% predicted of 5% or more.
- The intraclass correlation coefficient between home and hospital FVC measurements was moderate to high, with values of 0.85 at baseline, 0.84 at 6 months, and 0.72 at 12 months (P < .0001 for all).
- However, the longitudinal agreement between home and hospital measurements was lower with a correlation coefficient of 0.55.
IN PRACTICE:
“These findings suggest that home spirometry is both feasible and moderately accurate in patients with systemic sclerosis–associated ILD. However, where home spirometry fell short was the low sensitivity in detecting a decline in FVC% predicted,” experts wrote in an accompanying editorial.
“The results of this study support further evaluation of the implementation of home spirometry in addition to regular healthcare management but do not endorse relying solely on home monitoring to detect a decline in pulmonary function,” study authors wrote.
SOURCE:
The study was led by Arthiha Velauthapillai, MD, Department of Rheumatology, Radboud University Medical Center, Nijmegen, the Netherlands, and was published online November 8, 2024, in The Lancet Rheumatology.
LIMITATIONS:
The study might have been underpowered because of inaccuracies in initial assumptions, with a lower-than-anticipated prevalence of progressive ILD and a higher dropout rate. The study included only Dutch patients, which may have limited the generalizability of its findings to other settings with lower internet access or literacy rates.
DISCLOSURES:
This study was partly supported by grants from Galapagos and Boehringer Ingelheim. Some authors received grants or consulting or speaker fees from Boehringer Ingelheim, AstraZeneca, and other pharmaceutical companies.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
TOPLINE:
Home spirometry shows potential for early detection of pulmonary function decline in patients with systemic sclerosis–associated interstitial lung disease (SSc-ILD). It shows good cross-sectional correlation with hospital tests, along with 60% sensitivity and 87% specificity for detecting progressive ILD.
METHODOLOGY:
- Researchers conducted a prospective, observational study to examine the validity of home spirometry for detecting a decline in pulmonary function in patients with SSc-ILD.
- They included 43 patients aged 18 years or older with SSc-ILD from two tertiary referral centers in the Netherlands who received treatment with immunosuppressives for a maximum duration of 8 weeks prior to baseline.
- All participants were required to take weekly home spirometry measurements using a handheld spirometer for 1 year, with 35 completing 6 months of follow-up and 31 completing 12 months.
- Pulmonary function tests were conducted in the hospital at baseline and semiannual visits.
- The primary outcome was the κ (kappa statistic) agreement between home and hospital measurements after 1 year to detect a decline in forced vital capacity (FVC) of 5% or more; the sensitivity and specificity of home spirometry were also evaluated to detect an absolute decline in FVC%, using hospital tests as the gold standard.
TAKEAWAY:
- Home spirometry showed a fair agreement with the pulmonary function tests conducted at the hospital (κ, 0.40; 95% CI, 0.01-0.79).
- Home spirometry showed a sensitivity of 60% and specificity of 87% in detecting a decline in FVC% predicted of 5% or more.
- The intraclass correlation coefficient between home and hospital FVC measurements was moderate to high, with values of 0.85 at baseline, 0.84 at 6 months, and 0.72 at 12 months (P < .0001 for all).
- However, the longitudinal agreement between home and hospital measurements was lower with a correlation coefficient of 0.55.
IN PRACTICE:
“These findings suggest that home spirometry is both feasible and moderately accurate in patients with systemic sclerosis–associated ILD. However, where home spirometry fell short was the low sensitivity in detecting a decline in FVC% predicted,” experts wrote in an accompanying editorial.
“The results of this study support further evaluation of the implementation of home spirometry in addition to regular healthcare management but do not endorse relying solely on home monitoring to detect a decline in pulmonary function,” study authors wrote.
SOURCE:
The study was led by Arthiha Velauthapillai, MD, Department of Rheumatology, Radboud University Medical Center, Nijmegen, the Netherlands, and was published online November 8, 2024, in The Lancet Rheumatology.
LIMITATIONS:
The study might have been underpowered because of inaccuracies in initial assumptions, with a lower-than-anticipated prevalence of progressive ILD and a higher dropout rate. The study included only Dutch patients, which may have limited the generalizability of its findings to other settings with lower internet access or literacy rates.
DISCLOSURES:
This study was partly supported by grants from Galapagos and Boehringer Ingelheim. Some authors received grants or consulting or speaker fees from Boehringer Ingelheim, AstraZeneca, and other pharmaceutical companies.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
Lichen Planus Responds to Treatment with Topical Ruxolitinib in Phase 2 Study
both when given twice daily and as needed, according to data from a phase 2 trial.
The research, presented at the European Academy of Dermatology and Venereology (EADV) 2024 Congress, involved 64 patients older than 18 years. Ruxolitinib cream (Opzelura) is a topical formulation of a Janus kinase (JAK)1/JAK2 inhibitor, approved by the Food and Drug Administration (FDA) for treating mild to moderate atopic dermatitis and for nonsegmental vitiligo in adults and children aged 12 years or older.
Ruxolitinib cream twice daily resulted in “significant improvements in cutaneous lichen planus disease severity vs vehicle” after 16 weeks of treatment, said the study presenter, Aaron R. Mangold, MD, a dermatologist at Mayo Clinic, Scottsdale, Arizona.
Further improvements were seen during another 16 weeks of additional open-label, as-needed application, he added, and the topical treatment was “generally well tolerated.”
Consequently, “ruxolitinib cream represents a promising potential treatment for cutaneous lichen planus,” Mangold concluded.
Asked to comment on the results, Adam Friedman, MD, Professor and Chair of Dermatology, George Washington University, Washington, DC, who was not involved with the study, said that in keeping with the characterization of lichen planus using the four Ps — purple, polygonal, pruritic, papules — it is “Pretty common, Predictably disabling and disfiguring, and Passed over again and again in the drug development world.”
He said in an interview that this chronic inflammatory skin condition, which affects roughly 2% of the population, also “lacks consensus on work-up and management, likely in part owing to the absence of sizable clinical trial data.”
A recent survey conducted at a meeting indicated that dermatologists “heavily lean on topical therapies for the management of all severity levels,” noted Friedman, one of the survey authors. “Therefore, the phase 2 data presented at EADV is a welcome addition to the mix.”
Phase 2 Study Results
At the meeting, Mangold said that a previous proof-of-concept single-arm study in 12 patients suggested that topical ruxolitinib was highly effective in treating cutaneous lichen planus.
The current phase 2 trial enrolled 64 patients with predominantly cutaneous disease who had an Investigator’s Global Assessment (IGA) score of 3 or 4 and an Itch Numeric Rating Scale (NRS) score of ≥ 4. Their median age was 57 years, and 71.9% were women. Nearly 63% were White, 28.1% were Black, and 6.3% were Asian. The median duration of disease was 4.9 years, and 90.6% had received prior treatment for their lichen planus.
They were randomized to receive 1.5% ruxolitinib cream or a vehicle cream twice daily for 16 weeks, and following a primary endpoint assessment, they were transferred to an open-label extension period, during which they used ruxolitinib cream as needed for another 16 weeks. There was an additional 30-day safety follow-up period.
At week 16, significantly more patients treated with the ruxolitinib cream (50.0%) vs vehicle cream (21.9%) achieved IGA treatment success (the primary endpoint), defined as an IGA score of 0 or 1 with ≥ 2-grade improvement from baseline (odds ratio, 4.04; P = .0129).
In the open-label extension, when all patients used the active cream as needed, the proportion achieving IGA treatment success increased to 60% among the patients originally treated with ruxolitinib cream and 60.9% among those who switched from the vehicle cream.
A similar pattern was seen with Itch NRS scores. At 16 weeks, 57.7% of those treated with the ruxolitinib cream and 19.2% of those given the vehicle cream achieved an Itch NRS score of ≥ 4 (P < .01), rising to 84.2% and 73.3%, respectively, during the open-label extension.
The time to achievement of an Itch NRS of ≥ 4 was also significantly shorter with the ruxolitinib cream than with the vehicle cream (median days, 17 vs 97; hazard ratio, 2.85; P = .0008).
In both treatment groups, Skin Pain NRS scores decreased by a mean of 3.0 with ruxolitinib cream and 1.3 with the vehicle cream at week 16. By the end of the open-label extension, scores dropped by 4.3 among those who continued on active treatment and by 3.5 among those who switched from vehicle to topical ruxolitinib.
There were few treatment-emergent adverse events, with just three ruxolitinib patients affected during the randomized phase of the trial. There was one grade ≥ 3 event considered unrelated to the study drug, and no serious treatment-emergent adverse events were reported.
The most common adverse events during the randomized period were nasopharyngitis, hypertension, and contusion, all experienced by fewer than 10% of patients, whereas sinusitis, increased blood cholesterol levels, and increased blood creatine phosphokinase were most common in the open-label extension, experienced by no more than 5% of patients.
In the interview, Friedman commented that “these data provide hope that one day soon, there will be an FDA-approved, effective, and well-tolerated approach for this condition, validating the patient and supporting the dermatologist with an evidence-based option.”
The study was funded by Incyte. Mangold declared relationships with Argenx, Boehringer Ingelheim, Bristol-Myers Squibb, Clarivate, Incyte Corporation, Janssen, Nuvig Therapeutics, Pfizer, Regeneron Pharmaceuticals, Soligenix, Tourmaline Bio, AbbVie, Corbus, Eli Lilly, Kyowa, Merck, miRagen Therapeutics, Palvella Therapeutics, Priovant Therapeutics, and Adelphi Values. Friedman declared a relationship with Incyte, but it is not related to this topic.
A version of this article first appeared on Medscape.com.
both when given twice daily and as needed, according to data from a phase 2 trial.
The research, presented at the European Academy of Dermatology and Venereology (EADV) 2024 Congress, involved 64 patients older than 18 years. Ruxolitinib cream (Opzelura) is a topical formulation of a Janus kinase (JAK)1/JAK2 inhibitor, approved by the Food and Drug Administration (FDA) for treating mild to moderate atopic dermatitis and for nonsegmental vitiligo in adults and children aged 12 years or older.
Ruxolitinib cream twice daily resulted in “significant improvements in cutaneous lichen planus disease severity vs vehicle” after 16 weeks of treatment, said the study presenter, Aaron R. Mangold, MD, a dermatologist at Mayo Clinic, Scottsdale, Arizona.
Further improvements were seen during another 16 weeks of additional open-label, as-needed application, he added, and the topical treatment was “generally well tolerated.”
Consequently, “ruxolitinib cream represents a promising potential treatment for cutaneous lichen planus,” Mangold concluded.
Asked to comment on the results, Adam Friedman, MD, Professor and Chair of Dermatology, George Washington University, Washington, DC, who was not involved with the study, said that in keeping with the characterization of lichen planus using the four Ps — purple, polygonal, pruritic, papules — it is “Pretty common, Predictably disabling and disfiguring, and Passed over again and again in the drug development world.”
He said in an interview that this chronic inflammatory skin condition, which affects roughly 2% of the population, also “lacks consensus on work-up and management, likely in part owing to the absence of sizable clinical trial data.”
A recent survey conducted at a meeting indicated that dermatologists “heavily lean on topical therapies for the management of all severity levels,” noted Friedman, one of the survey authors. “Therefore, the phase 2 data presented at EADV is a welcome addition to the mix.”
Phase 2 Study Results
At the meeting, Mangold said that a previous proof-of-concept single-arm study in 12 patients suggested that topical ruxolitinib was highly effective in treating cutaneous lichen planus.
The current phase 2 trial enrolled 64 patients with predominantly cutaneous disease who had an Investigator’s Global Assessment (IGA) score of 3 or 4 and an Itch Numeric Rating Scale (NRS) score of ≥ 4. Their median age was 57 years, and 71.9% were women. Nearly 63% were White, 28.1% were Black, and 6.3% were Asian. The median duration of disease was 4.9 years, and 90.6% had received prior treatment for their lichen planus.
They were randomized to receive 1.5% ruxolitinib cream or a vehicle cream twice daily for 16 weeks, and following a primary endpoint assessment, they were transferred to an open-label extension period, during which they used ruxolitinib cream as needed for another 16 weeks. There was an additional 30-day safety follow-up period.
At week 16, significantly more patients treated with the ruxolitinib cream (50.0%) vs vehicle cream (21.9%) achieved IGA treatment success (the primary endpoint), defined as an IGA score of 0 or 1 with ≥ 2-grade improvement from baseline (odds ratio, 4.04; P = .0129).
In the open-label extension, when all patients used the active cream as needed, the proportion achieving IGA treatment success increased to 60% among the patients originally treated with ruxolitinib cream and 60.9% among those who switched from the vehicle cream.
A similar pattern was seen with Itch NRS scores. At 16 weeks, 57.7% of those treated with the ruxolitinib cream and 19.2% of those given the vehicle cream achieved an Itch NRS score of ≥ 4 (P < .01), rising to 84.2% and 73.3%, respectively, during the open-label extension.
The time to achievement of an Itch NRS of ≥ 4 was also significantly shorter with the ruxolitinib cream than with the vehicle cream (median days, 17 vs 97; hazard ratio, 2.85; P = .0008).
In both treatment groups, Skin Pain NRS scores decreased by a mean of 3.0 with ruxolitinib cream and 1.3 with the vehicle cream at week 16. By the end of the open-label extension, scores dropped by 4.3 among those who continued on active treatment and by 3.5 among those who switched from vehicle to topical ruxolitinib.
There were few treatment-emergent adverse events, with just three ruxolitinib patients affected during the randomized phase of the trial. There was one grade ≥ 3 event considered unrelated to the study drug, and no serious treatment-emergent adverse events were reported.
The most common adverse events during the randomized period were nasopharyngitis, hypertension, and contusion, all experienced by fewer than 10% of patients, whereas sinusitis, increased blood cholesterol levels, and increased blood creatine phosphokinase were most common in the open-label extension, experienced by no more than 5% of patients.
In the interview, Friedman commented that “these data provide hope that one day soon, there will be an FDA-approved, effective, and well-tolerated approach for this condition, validating the patient and supporting the dermatologist with an evidence-based option.”
The study was funded by Incyte. Mangold declared relationships with Argenx, Boehringer Ingelheim, Bristol-Myers Squibb, Clarivate, Incyte Corporation, Janssen, Nuvig Therapeutics, Pfizer, Regeneron Pharmaceuticals, Soligenix, Tourmaline Bio, AbbVie, Corbus, Eli Lilly, Kyowa, Merck, miRagen Therapeutics, Palvella Therapeutics, Priovant Therapeutics, and Adelphi Values. Friedman declared a relationship with Incyte, but it is not related to this topic.
A version of this article first appeared on Medscape.com.
both when given twice daily and as needed, according to data from a phase 2 trial.
The research, presented at the European Academy of Dermatology and Venereology (EADV) 2024 Congress, involved 64 patients older than 18 years. Ruxolitinib cream (Opzelura) is a topical formulation of a Janus kinase (JAK)1/JAK2 inhibitor, approved by the Food and Drug Administration (FDA) for treating mild to moderate atopic dermatitis and for nonsegmental vitiligo in adults and children aged 12 years or older.
Ruxolitinib cream twice daily resulted in “significant improvements in cutaneous lichen planus disease severity vs vehicle” after 16 weeks of treatment, said the study presenter, Aaron R. Mangold, MD, a dermatologist at Mayo Clinic, Scottsdale, Arizona.
Further improvements were seen during another 16 weeks of additional open-label, as-needed application, he added, and the topical treatment was “generally well tolerated.”
Consequently, “ruxolitinib cream represents a promising potential treatment for cutaneous lichen planus,” Mangold concluded.
Asked to comment on the results, Adam Friedman, MD, Professor and Chair of Dermatology, George Washington University, Washington, DC, who was not involved with the study, said that in keeping with the characterization of lichen planus using the four Ps — purple, polygonal, pruritic, papules — it is “Pretty common, Predictably disabling and disfiguring, and Passed over again and again in the drug development world.”
He said in an interview that this chronic inflammatory skin condition, which affects roughly 2% of the population, also “lacks consensus on work-up and management, likely in part owing to the absence of sizable clinical trial data.”
A recent survey conducted at a meeting indicated that dermatologists “heavily lean on topical therapies for the management of all severity levels,” noted Friedman, one of the survey authors. “Therefore, the phase 2 data presented at EADV is a welcome addition to the mix.”
Phase 2 Study Results
At the meeting, Mangold said that a previous proof-of-concept single-arm study in 12 patients suggested that topical ruxolitinib was highly effective in treating cutaneous lichen planus.
The current phase 2 trial enrolled 64 patients with predominantly cutaneous disease who had an Investigator’s Global Assessment (IGA) score of 3 or 4 and an Itch Numeric Rating Scale (NRS) score of ≥ 4. Their median age was 57 years, and 71.9% were women. Nearly 63% were White, 28.1% were Black, and 6.3% were Asian. The median duration of disease was 4.9 years, and 90.6% had received prior treatment for their lichen planus.
They were randomized to receive 1.5% ruxolitinib cream or a vehicle cream twice daily for 16 weeks, and following a primary endpoint assessment, they were transferred to an open-label extension period, during which they used ruxolitinib cream as needed for another 16 weeks. There was an additional 30-day safety follow-up period.
At week 16, significantly more patients treated with the ruxolitinib cream (50.0%) vs vehicle cream (21.9%) achieved IGA treatment success (the primary endpoint), defined as an IGA score of 0 or 1 with ≥ 2-grade improvement from baseline (odds ratio, 4.04; P = .0129).
In the open-label extension, when all patients used the active cream as needed, the proportion achieving IGA treatment success increased to 60% among the patients originally treated with ruxolitinib cream and 60.9% among those who switched from the vehicle cream.
A similar pattern was seen with Itch NRS scores. At 16 weeks, 57.7% of those treated with the ruxolitinib cream and 19.2% of those given the vehicle cream achieved an Itch NRS score of ≥ 4 (P < .01), rising to 84.2% and 73.3%, respectively, during the open-label extension.
The time to achievement of an Itch NRS of ≥ 4 was also significantly shorter with the ruxolitinib cream than with the vehicle cream (median days, 17 vs 97; hazard ratio, 2.85; P = .0008).
In both treatment groups, Skin Pain NRS scores decreased by a mean of 3.0 with ruxolitinib cream and 1.3 with the vehicle cream at week 16. By the end of the open-label extension, scores dropped by 4.3 among those who continued on active treatment and by 3.5 among those who switched from vehicle to topical ruxolitinib.
There were few treatment-emergent adverse events, with just three ruxolitinib patients affected during the randomized phase of the trial. There was one grade ≥ 3 event considered unrelated to the study drug, and no serious treatment-emergent adverse events were reported.
The most common adverse events during the randomized period were nasopharyngitis, hypertension, and contusion, all experienced by fewer than 10% of patients, whereas sinusitis, increased blood cholesterol levels, and increased blood creatine phosphokinase were most common in the open-label extension, experienced by no more than 5% of patients.
In the interview, Friedman commented that “these data provide hope that one day soon, there will be an FDA-approved, effective, and well-tolerated approach for this condition, validating the patient and supporting the dermatologist with an evidence-based option.”
The study was funded by Incyte. Mangold declared relationships with Argenx, Boehringer Ingelheim, Bristol-Myers Squibb, Clarivate, Incyte Corporation, Janssen, Nuvig Therapeutics, Pfizer, Regeneron Pharmaceuticals, Soligenix, Tourmaline Bio, AbbVie, Corbus, Eli Lilly, Kyowa, Merck, miRagen Therapeutics, Palvella Therapeutics, Priovant Therapeutics, and Adelphi Values. Friedman declared a relationship with Incyte, but it is not related to this topic.
A version of this article first appeared on Medscape.com.
FROM EADV 2024

