User login
For MD-IQ use only
Many Patients With Cancer Visit EDs Before Diagnosis
Researchers examined Institute for Clinical Evaluative Sciences (ICES) data that had been gathered from January 1, 2014, to December 31, 2021. The study focused on patients aged 18 years or older with confirmed primary cancer diagnoses.
Factors associated with an increased likelihood of an ED visit ahead of diagnosis included having certain cancers, living in rural areas, and having less access to primary care, according to study author Keerat Grewal, MD, an emergency physician and clinician scientist at the Schwartz/Reisman Emergency Medicine Institute at Sinai Health in Toronto, Ontario, Canada, and coauthors.
“The ED is a distressing environment for patients to receive a possible cancer diagnosis,” the authors wrote. “Moreover, it is frequently ill equipped to provide ongoing continuity of care, which can lead patients down a poorly defined diagnostic pathway before receiving a confirmed diagnosis based on tissue and a subsequent treatment plan.”
The findings were published online on November 4 in CMAJ).
Neurologic Cancers Prominent
In an interview, Grewal said in an interview that the study reflects her desire as an emergency room physician to understand why so many patients with cancer get the initial reports about their disease from clinicians whom they often have just met for the first time.
Among patients with an ED visit before cancer diagnosis, 51.4% were admitted to hospital from the most recent visit.
Compared with patients with a family physician on whom they could rely for routine care, those who had no outpatient visits (odds ratio [OR], 2.09) or fewer than three outpatient visits (OR, 1.41) in the 6-30 months before cancer diagnosis were more likely to have an ED visit before their cancer diagnosis.
Other factors associated with increased odds of ED use before cancer diagnosis included rurality (OR, 1.15), residence in northern Ontario (northeast region: OR, 1.14 and northwest region: OR, 1.27 vs Toronto region), and living in the most marginalized areas (material resource deprivation: OR, 1.37 and housing stability: OR, 1.09 vs least marginalized area).
The researchers also found that patients with certain cancers were more likely to have sought care in the ED. They compared these cancers with breast cancer, which is often detected through screening.
“Patients with neurologic cancers had extremely high odds of ED use before cancer diagnosis,” the authors wrote. “This is likely because of the emergent nature of presentation, with acute neurologic symptoms such as weakness, confusion, or seizures, which require urgent assessment.” On the other hand, pancreatic, liver, or thoracic cancer can trigger nonspecific symptoms that may be ignored until they reach a crisis level that prompts an ED visit.
The limitations of the study included its inability to identify cancer-related ED visits and its narrow focus on patients in Ontario, according to the researchers. But the use of the ICES databases also allowed researchers access to a broader pool of data than are available in many other cases.
The findings in the new paper echo those of previous research, the authors noted. Research in the United Kingdom found that 24%-31% of cancer diagnoses involved the ED. In addition, a study of people enrolled in the US Medicare program, which serves patients aged 65 years or older, found that 23% were seen in the ED in the 30 days before diagnosis.
‘Unpacking the Data’
The current findings also are consistent with those of an International Cancer Benchmarking Partnership study that was published in 2022 in The Lancet Oncology, said Erika Nicholson, MHS, vice president of cancer systems and innovation at the Canadian Partnership Against Cancer. The latter study analyzed cancer registration and linked hospital admissions data from 14 jurisdictions in Australia, Canada, Denmark, New Zealand, Norway, and the United Kingdom.
“We see similar trends in terms of people visiting EDs and being diagnosed through EDs internationally,” Nicholson said. “We’re working with partners to put in place different strategies to address the challenges” that this phenomenon presents in terms of improving screening and follow-up care.
“Cancer is not one disease, but many diseases,” she said. “They present differently. We’re focused on really unpacking the data and understanding them.”
All this research highlights the need for more services and personnel to address cancer, including people who are trained to help patients cope after getting concerning news through emergency care, she said.
“That means having a system that fully supports you and helps you navigate through that diagnostic process,” Nicholson said. Addressing the added challenges for patients who don’t have secure housing is a special need, she added.
This study was supported by the Canadian Institutes of Health Research (CIHR). Grewal reported receiving grants from CIHR and the Canadian Association of Emergency Physicians. Nicholson reported no relevant financial relationships.
A version of this article appeared on Medscape.com.
Researchers examined Institute for Clinical Evaluative Sciences (ICES) data that had been gathered from January 1, 2014, to December 31, 2021. The study focused on patients aged 18 years or older with confirmed primary cancer diagnoses.
Factors associated with an increased likelihood of an ED visit ahead of diagnosis included having certain cancers, living in rural areas, and having less access to primary care, according to study author Keerat Grewal, MD, an emergency physician and clinician scientist at the Schwartz/Reisman Emergency Medicine Institute at Sinai Health in Toronto, Ontario, Canada, and coauthors.
“The ED is a distressing environment for patients to receive a possible cancer diagnosis,” the authors wrote. “Moreover, it is frequently ill equipped to provide ongoing continuity of care, which can lead patients down a poorly defined diagnostic pathway before receiving a confirmed diagnosis based on tissue and a subsequent treatment plan.”
The findings were published online on November 4 in CMAJ).
Neurologic Cancers Prominent
In an interview, Grewal said in an interview that the study reflects her desire as an emergency room physician to understand why so many patients with cancer get the initial reports about their disease from clinicians whom they often have just met for the first time.
Among patients with an ED visit before cancer diagnosis, 51.4% were admitted to hospital from the most recent visit.
Compared with patients with a family physician on whom they could rely for routine care, those who had no outpatient visits (odds ratio [OR], 2.09) or fewer than three outpatient visits (OR, 1.41) in the 6-30 months before cancer diagnosis were more likely to have an ED visit before their cancer diagnosis.
Other factors associated with increased odds of ED use before cancer diagnosis included rurality (OR, 1.15), residence in northern Ontario (northeast region: OR, 1.14 and northwest region: OR, 1.27 vs Toronto region), and living in the most marginalized areas (material resource deprivation: OR, 1.37 and housing stability: OR, 1.09 vs least marginalized area).
The researchers also found that patients with certain cancers were more likely to have sought care in the ED. They compared these cancers with breast cancer, which is often detected through screening.
“Patients with neurologic cancers had extremely high odds of ED use before cancer diagnosis,” the authors wrote. “This is likely because of the emergent nature of presentation, with acute neurologic symptoms such as weakness, confusion, or seizures, which require urgent assessment.” On the other hand, pancreatic, liver, or thoracic cancer can trigger nonspecific symptoms that may be ignored until they reach a crisis level that prompts an ED visit.
The limitations of the study included its inability to identify cancer-related ED visits and its narrow focus on patients in Ontario, according to the researchers. But the use of the ICES databases also allowed researchers access to a broader pool of data than are available in many other cases.
The findings in the new paper echo those of previous research, the authors noted. Research in the United Kingdom found that 24%-31% of cancer diagnoses involved the ED. In addition, a study of people enrolled in the US Medicare program, which serves patients aged 65 years or older, found that 23% were seen in the ED in the 30 days before diagnosis.
‘Unpacking the Data’
The current findings also are consistent with those of an International Cancer Benchmarking Partnership study that was published in 2022 in The Lancet Oncology, said Erika Nicholson, MHS, vice president of cancer systems and innovation at the Canadian Partnership Against Cancer. The latter study analyzed cancer registration and linked hospital admissions data from 14 jurisdictions in Australia, Canada, Denmark, New Zealand, Norway, and the United Kingdom.
“We see similar trends in terms of people visiting EDs and being diagnosed through EDs internationally,” Nicholson said. “We’re working with partners to put in place different strategies to address the challenges” that this phenomenon presents in terms of improving screening and follow-up care.
“Cancer is not one disease, but many diseases,” she said. “They present differently. We’re focused on really unpacking the data and understanding them.”
All this research highlights the need for more services and personnel to address cancer, including people who are trained to help patients cope after getting concerning news through emergency care, she said.
“That means having a system that fully supports you and helps you navigate through that diagnostic process,” Nicholson said. Addressing the added challenges for patients who don’t have secure housing is a special need, she added.
This study was supported by the Canadian Institutes of Health Research (CIHR). Grewal reported receiving grants from CIHR and the Canadian Association of Emergency Physicians. Nicholson reported no relevant financial relationships.
A version of this article appeared on Medscape.com.
Researchers examined Institute for Clinical Evaluative Sciences (ICES) data that had been gathered from January 1, 2014, to December 31, 2021. The study focused on patients aged 18 years or older with confirmed primary cancer diagnoses.
Factors associated with an increased likelihood of an ED visit ahead of diagnosis included having certain cancers, living in rural areas, and having less access to primary care, according to study author Keerat Grewal, MD, an emergency physician and clinician scientist at the Schwartz/Reisman Emergency Medicine Institute at Sinai Health in Toronto, Ontario, Canada, and coauthors.
“The ED is a distressing environment for patients to receive a possible cancer diagnosis,” the authors wrote. “Moreover, it is frequently ill equipped to provide ongoing continuity of care, which can lead patients down a poorly defined diagnostic pathway before receiving a confirmed diagnosis based on tissue and a subsequent treatment plan.”
The findings were published online on November 4 in CMAJ).
Neurologic Cancers Prominent
In an interview, Grewal said in an interview that the study reflects her desire as an emergency room physician to understand why so many patients with cancer get the initial reports about their disease from clinicians whom they often have just met for the first time.
Among patients with an ED visit before cancer diagnosis, 51.4% were admitted to hospital from the most recent visit.
Compared with patients with a family physician on whom they could rely for routine care, those who had no outpatient visits (odds ratio [OR], 2.09) or fewer than three outpatient visits (OR, 1.41) in the 6-30 months before cancer diagnosis were more likely to have an ED visit before their cancer diagnosis.
Other factors associated with increased odds of ED use before cancer diagnosis included rurality (OR, 1.15), residence in northern Ontario (northeast region: OR, 1.14 and northwest region: OR, 1.27 vs Toronto region), and living in the most marginalized areas (material resource deprivation: OR, 1.37 and housing stability: OR, 1.09 vs least marginalized area).
The researchers also found that patients with certain cancers were more likely to have sought care in the ED. They compared these cancers with breast cancer, which is often detected through screening.
“Patients with neurologic cancers had extremely high odds of ED use before cancer diagnosis,” the authors wrote. “This is likely because of the emergent nature of presentation, with acute neurologic symptoms such as weakness, confusion, or seizures, which require urgent assessment.” On the other hand, pancreatic, liver, or thoracic cancer can trigger nonspecific symptoms that may be ignored until they reach a crisis level that prompts an ED visit.
The limitations of the study included its inability to identify cancer-related ED visits and its narrow focus on patients in Ontario, according to the researchers. But the use of the ICES databases also allowed researchers access to a broader pool of data than are available in many other cases.
The findings in the new paper echo those of previous research, the authors noted. Research in the United Kingdom found that 24%-31% of cancer diagnoses involved the ED. In addition, a study of people enrolled in the US Medicare program, which serves patients aged 65 years or older, found that 23% were seen in the ED in the 30 days before diagnosis.
‘Unpacking the Data’
The current findings also are consistent with those of an International Cancer Benchmarking Partnership study that was published in 2022 in The Lancet Oncology, said Erika Nicholson, MHS, vice president of cancer systems and innovation at the Canadian Partnership Against Cancer. The latter study analyzed cancer registration and linked hospital admissions data from 14 jurisdictions in Australia, Canada, Denmark, New Zealand, Norway, and the United Kingdom.
“We see similar trends in terms of people visiting EDs and being diagnosed through EDs internationally,” Nicholson said. “We’re working with partners to put in place different strategies to address the challenges” that this phenomenon presents in terms of improving screening and follow-up care.
“Cancer is not one disease, but many diseases,” she said. “They present differently. We’re focused on really unpacking the data and understanding them.”
All this research highlights the need for more services and personnel to address cancer, including people who are trained to help patients cope after getting concerning news through emergency care, she said.
“That means having a system that fully supports you and helps you navigate through that diagnostic process,” Nicholson said. Addressing the added challenges for patients who don’t have secure housing is a special need, she added.
This study was supported by the Canadian Institutes of Health Research (CIHR). Grewal reported receiving grants from CIHR and the Canadian Association of Emergency Physicians. Nicholson reported no relevant financial relationships.
A version of this article appeared on Medscape.com.
FROM CMAJ
Plasma Omega-6 and Omega-3 Fatty Acids Inversely Associated With Cancer
TOPLINE:
Higher plasma levels of omega-6 and omega-3 fatty acids are associated with a lower incidence of cancer. However, omega-3 fatty acids are linked to an increased risk for prostate cancer, specifically.
METHODOLOGY:
- Researchers looked for associations of plasma omega-3 and omega-6 polyunsaturated fatty acids (PUFAs) with the incidence of cancer overall and 19 site-specific cancers in the large population-based prospective UK Biobank cohort.
- They included 253,138 participants aged 37-73 years who were followed for an average of 12.9 years, with 29,838 diagnosed with cancer.
- Plasma levels of omega-3 and omega-6 fatty acids were measured using nuclear magnetic resonance and expressed as percentages of total fatty acids.
- Participants with cancer diagnoses at baseline, those who withdrew from the study, and those with missing data on plasma PUFAs were excluded.
- The study adjusted for multiple covariates, including age, sex, ethnicity, socioeconomic status, lifestyle behaviors, and family history of diseases.
TAKEAWAY:
- Higher plasma levels of omega-6 and omega-3 fatty acids were associated with a 2% and 1% reduction in overall cancer risk per SD increase, respectively (P = .001 and P = .03).
- Omega-6 fatty acids were inversely associated with 14 site-specific cancers, whereas omega-3 fatty acids were inversely associated with five site-specific cancers.
- Prostate cancer was positively associated with omega-3 fatty acids, with a 3% increased risk per SD increase (P = .049).
- A higher omega-6/omega-3 ratio was associated with an increased risk for overall cancer, and three site-specific cancers showed positive associations with the ratio. “Each standard deviation increase, corresponding to a 13.13 increase in the omega ratio, was associated with a 2% increase in the risk of rectum cancer,” for example, the authors wrote.
IN PRACTICE:
“Overall, our findings provide support for possible small net protective roles of omega-3 and omega-6 PUFAs in the development of new cancer incidence. Our study also suggests that the usage of circulating blood biomarkers captures different aspects of dietary intake, reduces measurement errors, and thus enhances statistical power. The differential effects of omega-6% and omega-3% in age and sex subgroups warrant future investigation,” wrote the authors of the study.
SOURCE:
The study was led by Yuchen Zhang of the University of Georgia in Athens, Georgia. It was published online in the International Journal of Cancer.
LIMITATIONS:
The study’s potential for selective bias persists due to the participant sample skewing heavily toward European ancestry and White ethnicity. The number of events was small for some specific cancer sites, which may have limited the statistical power. The study focused on total omega-3 and omega-6 PUFAs, with only two individual fatty acids measured. Future studies are needed to examine the roles of other individual PUFAs and specific genetic variants.
DISCLOSURES:
This study was supported by grants from the National Institute of General Medical Sciences of the National Institutes of Health. No relevant conflicts of interest were disclosed by the authors.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
TOPLINE:
Higher plasma levels of omega-6 and omega-3 fatty acids are associated with a lower incidence of cancer. However, omega-3 fatty acids are linked to an increased risk for prostate cancer, specifically.
METHODOLOGY:
- Researchers looked for associations of plasma omega-3 and omega-6 polyunsaturated fatty acids (PUFAs) with the incidence of cancer overall and 19 site-specific cancers in the large population-based prospective UK Biobank cohort.
- They included 253,138 participants aged 37-73 years who were followed for an average of 12.9 years, with 29,838 diagnosed with cancer.
- Plasma levels of omega-3 and omega-6 fatty acids were measured using nuclear magnetic resonance and expressed as percentages of total fatty acids.
- Participants with cancer diagnoses at baseline, those who withdrew from the study, and those with missing data on plasma PUFAs were excluded.
- The study adjusted for multiple covariates, including age, sex, ethnicity, socioeconomic status, lifestyle behaviors, and family history of diseases.
TAKEAWAY:
- Higher plasma levels of omega-6 and omega-3 fatty acids were associated with a 2% and 1% reduction in overall cancer risk per SD increase, respectively (P = .001 and P = .03).
- Omega-6 fatty acids were inversely associated with 14 site-specific cancers, whereas omega-3 fatty acids were inversely associated with five site-specific cancers.
- Prostate cancer was positively associated with omega-3 fatty acids, with a 3% increased risk per SD increase (P = .049).
- A higher omega-6/omega-3 ratio was associated with an increased risk for overall cancer, and three site-specific cancers showed positive associations with the ratio. “Each standard deviation increase, corresponding to a 13.13 increase in the omega ratio, was associated with a 2% increase in the risk of rectum cancer,” for example, the authors wrote.
IN PRACTICE:
“Overall, our findings provide support for possible small net protective roles of omega-3 and omega-6 PUFAs in the development of new cancer incidence. Our study also suggests that the usage of circulating blood biomarkers captures different aspects of dietary intake, reduces measurement errors, and thus enhances statistical power. The differential effects of omega-6% and omega-3% in age and sex subgroups warrant future investigation,” wrote the authors of the study.
SOURCE:
The study was led by Yuchen Zhang of the University of Georgia in Athens, Georgia. It was published online in the International Journal of Cancer.
LIMITATIONS:
The study’s potential for selective bias persists due to the participant sample skewing heavily toward European ancestry and White ethnicity. The number of events was small for some specific cancer sites, which may have limited the statistical power. The study focused on total omega-3 and omega-6 PUFAs, with only two individual fatty acids measured. Future studies are needed to examine the roles of other individual PUFAs and specific genetic variants.
DISCLOSURES:
This study was supported by grants from the National Institute of General Medical Sciences of the National Institutes of Health. No relevant conflicts of interest were disclosed by the authors.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
TOPLINE:
Higher plasma levels of omega-6 and omega-3 fatty acids are associated with a lower incidence of cancer. However, omega-3 fatty acids are linked to an increased risk for prostate cancer, specifically.
METHODOLOGY:
- Researchers looked for associations of plasma omega-3 and omega-6 polyunsaturated fatty acids (PUFAs) with the incidence of cancer overall and 19 site-specific cancers in the large population-based prospective UK Biobank cohort.
- They included 253,138 participants aged 37-73 years who were followed for an average of 12.9 years, with 29,838 diagnosed with cancer.
- Plasma levels of omega-3 and omega-6 fatty acids were measured using nuclear magnetic resonance and expressed as percentages of total fatty acids.
- Participants with cancer diagnoses at baseline, those who withdrew from the study, and those with missing data on plasma PUFAs were excluded.
- The study adjusted for multiple covariates, including age, sex, ethnicity, socioeconomic status, lifestyle behaviors, and family history of diseases.
TAKEAWAY:
- Higher plasma levels of omega-6 and omega-3 fatty acids were associated with a 2% and 1% reduction in overall cancer risk per SD increase, respectively (P = .001 and P = .03).
- Omega-6 fatty acids were inversely associated with 14 site-specific cancers, whereas omega-3 fatty acids were inversely associated with five site-specific cancers.
- Prostate cancer was positively associated with omega-3 fatty acids, with a 3% increased risk per SD increase (P = .049).
- A higher omega-6/omega-3 ratio was associated with an increased risk for overall cancer, and three site-specific cancers showed positive associations with the ratio. “Each standard deviation increase, corresponding to a 13.13 increase in the omega ratio, was associated with a 2% increase in the risk of rectum cancer,” for example, the authors wrote.
IN PRACTICE:
“Overall, our findings provide support for possible small net protective roles of omega-3 and omega-6 PUFAs in the development of new cancer incidence. Our study also suggests that the usage of circulating blood biomarkers captures different aspects of dietary intake, reduces measurement errors, and thus enhances statistical power. The differential effects of omega-6% and omega-3% in age and sex subgroups warrant future investigation,” wrote the authors of the study.
SOURCE:
The study was led by Yuchen Zhang of the University of Georgia in Athens, Georgia. It was published online in the International Journal of Cancer.
LIMITATIONS:
The study’s potential for selective bias persists due to the participant sample skewing heavily toward European ancestry and White ethnicity. The number of events was small for some specific cancer sites, which may have limited the statistical power. The study focused on total omega-3 and omega-6 PUFAs, with only two individual fatty acids measured. Future studies are needed to examine the roles of other individual PUFAs and specific genetic variants.
DISCLOSURES:
This study was supported by grants from the National Institute of General Medical Sciences of the National Institutes of Health. No relevant conflicts of interest were disclosed by the authors.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
Pinto Bean Pressure Wraps: A Novel Approach to Treating Digital Warts
Practice Gap
Verruca vulgaris is a common dermatologic challenge due to its high prevalence and tendency to recur following routinely employed destructive modalities (eg, cryotherapy, electrosurgery), which can incur a considerable amount of pain and some risk for scarring.1,2 Other treatment methods for warts such as topical salicylic acid preparations, topical immunotherapy, or intralesional allergen injections often require multiple treatment sessions.3,4 Furthermore, the financial burden of traditional wart treatment can be substantial.4 Better techniques are needed to improve the clinician’s approach to treating warts. We describe a home-based technique to treat common digital warts using pinto bean pressure wraps to induce ischemic changes in wart tissue with similar response rates to commonly used modalities.
Technique
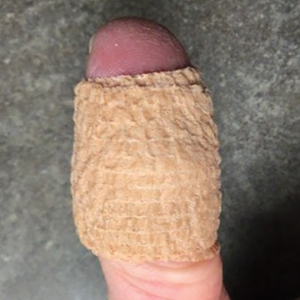
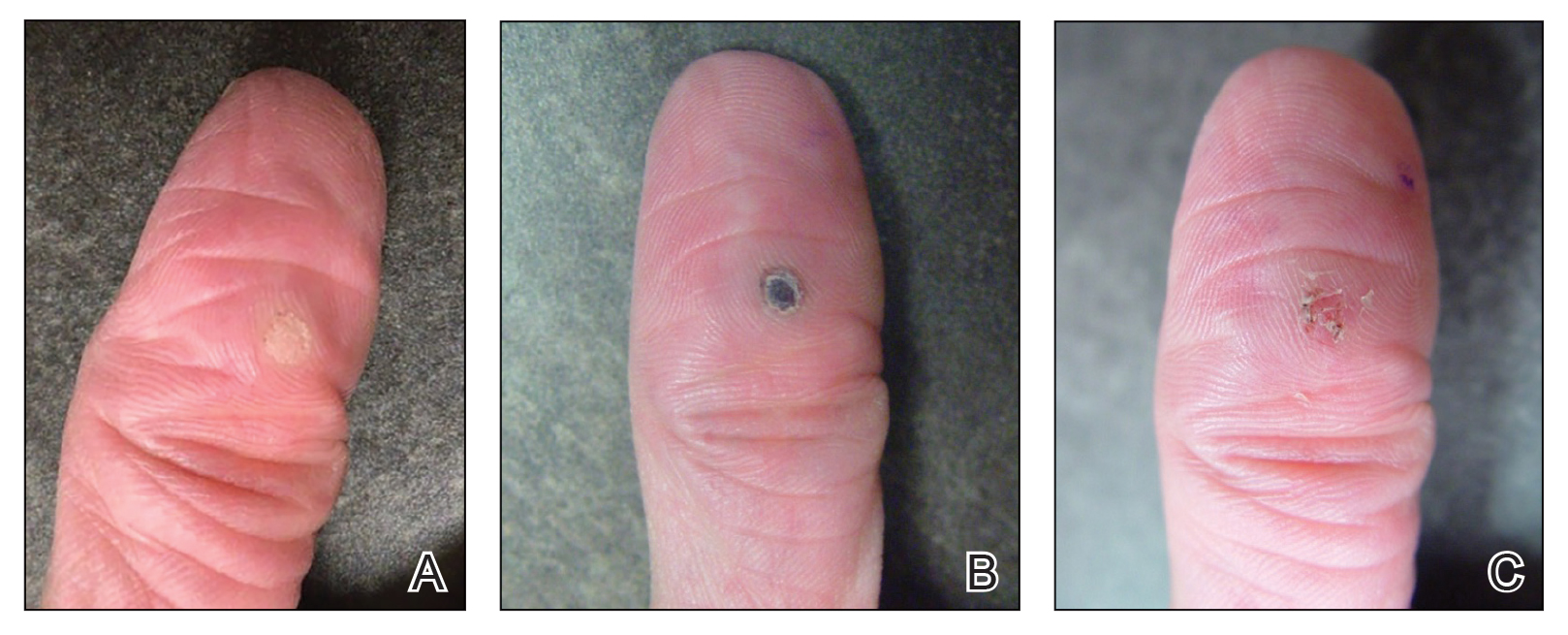
Our technique utilizes a small, hard, convex object that is applied directly over the digital wart. A simple self-adhesive wrap is used to cover the object and maintain constant pressure on the wart overnight. We typically use a dried pinto bean (a variety of the common bean Phaseolus vulgaris) acquired from a local grocery store due to its ideal size, hard surface, and convex shape (Figure 1). The bean is taped in place directly overlying the wart and covered with a self-adhesive wrap overnight. The wrap is removed in the morning, and often no further treatment is needed. The ischemic wart tissue is allowed to slough spontaneously over 1 to 2 weeks. No wound care or dressing is necessary (Figure 2). Larger warts may require application of the pressure wraps for 2 to 3 additional nights. While most warts resolve with this technique, we have observed a recurrence rate similar to that for cryotherapy. Patients are advised that any recurrent warts can be re-treated monthly, if needed, until resolution.
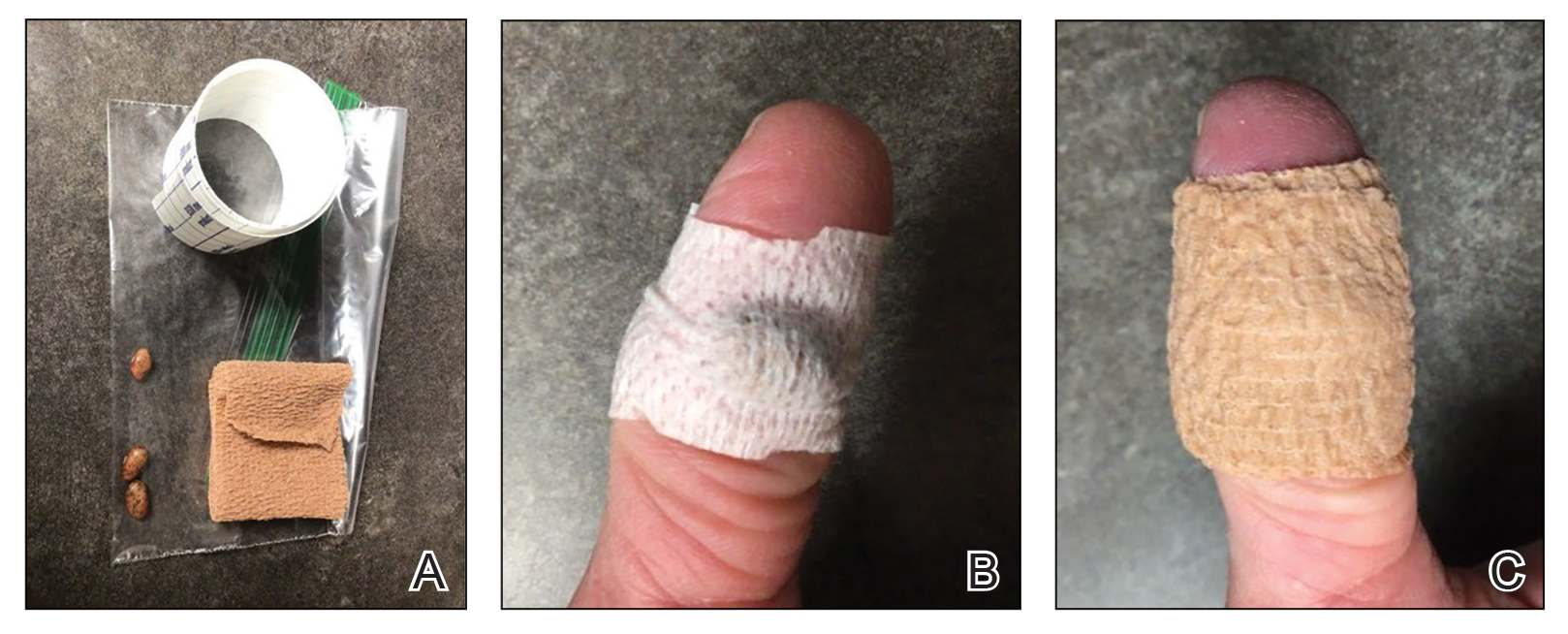
What to Use and How to Prepare—Any small, hard, convex object can be used for the pressure wrap; we also have used appropriately sized and shaped plastic shirt buttons with similar results. Home kits can be assembled in advance and provided to patients at their initial visit along with appropriate instructions (Figure 1A).
Effects on the Skin and Distal Digit—Application of pressure wraps does not harm normal skin; however, care should be taken when the self-adherent wrap is applied so as not to induce ischemia of the distal digit. The wrap should be applied using gentle pressure with patients experiencing minimal discomfort from the overnight application.
Indications—This pressure wrap technique can be employed on most digital warts, including periungual warts, which can be difficult to treat by other means. However, in our experience this technique is not effective for nondigital warts, likely due to the inability to maintain adequate pressure with the overlying dressing. Patients at risk for compromised digital perfusion, such as those with Raynaud phenomenon or systemic sclerosis, should not be treated with pressure wraps due to possible digital ischemia.
Precautions—Patients should be advised that the pinto bean should only be used if dry and should not be ingested. The bean can be a choking hazard for small children, therefore appropriate precautions should be used. Allergic contact dermatitis to the materials used in this technique is possible, but we have never observed this. The pinto bean can be reused for future application as long as it remains dry and provides a hard convex surface.
Practice Implications
The probable mechanism of the ischemic changes to the wart tissue likely is the occlusion of tortuous blood vessels in the dermal papillae, which are intrinsic to wart tissue and absent in normal skin.1 This pressure-induced ischemic injury allows for selective destruction of the wart tissue with sparing of the normal skin. Our technique is fairly novel, although at least one report in the literature has described the use of a mechanical device to induce ischemic changes in skin tags.5
The use of pinto bean pressure wraps to induce ischemic change in digital warts provides a low-risk and nearly pain-free alternative to more expensive and invasive treatment methods. Moreover, this technique allows for a low-cost home-based therapy that can be repeated easily for other digital sites or if recurrence is noted.
- Cardoso J, Calonje E. Cutaneous manifestations of human papillomaviruses: a review. Acta Dermatovenerol Alp Pannonica Adriat. 2011;20:145-154.
- Lipke M. An armamentarium of wart treatments. Clin Med Res. 2006;4:273-293. doi:10.3121/cmr.4.4.273
- Muse M, Stiff K, Glines K, et al. A review of intralesional wart therapy. Dermatol Online J. 2020;26:2. doi:10.5070/D3263048027
- Berna R, Margolis D, Barbieri J. Annual health care utilization and costs for treatment of cutaneous and anogenital warts among a commercially insured population in the US, 2017-2019. JAMA Dermatol. 2022;158:695-697. doi:10.1001/jamadermatol.2022.0964
- Fredriksson C, Ilias M, Anderson C. New mechanical device for effective removal of skin tags in routine health care. Dermatol Online J. 2009;15:9. doi:10.5070/D37tj2800k
Practice Gap
Verruca vulgaris is a common dermatologic challenge due to its high prevalence and tendency to recur following routinely employed destructive modalities (eg, cryotherapy, electrosurgery), which can incur a considerable amount of pain and some risk for scarring.1,2 Other treatment methods for warts such as topical salicylic acid preparations, topical immunotherapy, or intralesional allergen injections often require multiple treatment sessions.3,4 Furthermore, the financial burden of traditional wart treatment can be substantial.4 Better techniques are needed to improve the clinician’s approach to treating warts. We describe a home-based technique to treat common digital warts using pinto bean pressure wraps to induce ischemic changes in wart tissue with similar response rates to commonly used modalities.
Technique
Our technique utilizes a small, hard, convex object that is applied directly over the digital wart. A simple self-adhesive wrap is used to cover the object and maintain constant pressure on the wart overnight. We typically use a dried pinto bean (a variety of the common bean Phaseolus vulgaris) acquired from a local grocery store due to its ideal size, hard surface, and convex shape (Figure 1). The bean is taped in place directly overlying the wart and covered with a self-adhesive wrap overnight. The wrap is removed in the morning, and often no further treatment is needed. The ischemic wart tissue is allowed to slough spontaneously over 1 to 2 weeks. No wound care or dressing is necessary (Figure 2). Larger warts may require application of the pressure wraps for 2 to 3 additional nights. While most warts resolve with this technique, we have observed a recurrence rate similar to that for cryotherapy. Patients are advised that any recurrent warts can be re-treated monthly, if needed, until resolution.


What to Use and How to Prepare—Any small, hard, convex object can be used for the pressure wrap; we also have used appropriately sized and shaped plastic shirt buttons with similar results. Home kits can be assembled in advance and provided to patients at their initial visit along with appropriate instructions (Figure 1A).
Effects on the Skin and Distal Digit—Application of pressure wraps does not harm normal skin; however, care should be taken when the self-adherent wrap is applied so as not to induce ischemia of the distal digit. The wrap should be applied using gentle pressure with patients experiencing minimal discomfort from the overnight application.
Indications—This pressure wrap technique can be employed on most digital warts, including periungual warts, which can be difficult to treat by other means. However, in our experience this technique is not effective for nondigital warts, likely due to the inability to maintain adequate pressure with the overlying dressing. Patients at risk for compromised digital perfusion, such as those with Raynaud phenomenon or systemic sclerosis, should not be treated with pressure wraps due to possible digital ischemia.
Precautions—Patients should be advised that the pinto bean should only be used if dry and should not be ingested. The bean can be a choking hazard for small children, therefore appropriate precautions should be used. Allergic contact dermatitis to the materials used in this technique is possible, but we have never observed this. The pinto bean can be reused for future application as long as it remains dry and provides a hard convex surface.
Practice Implications
The probable mechanism of the ischemic changes to the wart tissue likely is the occlusion of tortuous blood vessels in the dermal papillae, which are intrinsic to wart tissue and absent in normal skin.1 This pressure-induced ischemic injury allows for selective destruction of the wart tissue with sparing of the normal skin. Our technique is fairly novel, although at least one report in the literature has described the use of a mechanical device to induce ischemic changes in skin tags.5
The use of pinto bean pressure wraps to induce ischemic change in digital warts provides a low-risk and nearly pain-free alternative to more expensive and invasive treatment methods. Moreover, this technique allows for a low-cost home-based therapy that can be repeated easily for other digital sites or if recurrence is noted.
Practice Gap
Verruca vulgaris is a common dermatologic challenge due to its high prevalence and tendency to recur following routinely employed destructive modalities (eg, cryotherapy, electrosurgery), which can incur a considerable amount of pain and some risk for scarring.1,2 Other treatment methods for warts such as topical salicylic acid preparations, topical immunotherapy, or intralesional allergen injections often require multiple treatment sessions.3,4 Furthermore, the financial burden of traditional wart treatment can be substantial.4 Better techniques are needed to improve the clinician’s approach to treating warts. We describe a home-based technique to treat common digital warts using pinto bean pressure wraps to induce ischemic changes in wart tissue with similar response rates to commonly used modalities.
Technique
Our technique utilizes a small, hard, convex object that is applied directly over the digital wart. A simple self-adhesive wrap is used to cover the object and maintain constant pressure on the wart overnight. We typically use a dried pinto bean (a variety of the common bean Phaseolus vulgaris) acquired from a local grocery store due to its ideal size, hard surface, and convex shape (Figure 1). The bean is taped in place directly overlying the wart and covered with a self-adhesive wrap overnight. The wrap is removed in the morning, and often no further treatment is needed. The ischemic wart tissue is allowed to slough spontaneously over 1 to 2 weeks. No wound care or dressing is necessary (Figure 2). Larger warts may require application of the pressure wraps for 2 to 3 additional nights. While most warts resolve with this technique, we have observed a recurrence rate similar to that for cryotherapy. Patients are advised that any recurrent warts can be re-treated monthly, if needed, until resolution.


What to Use and How to Prepare—Any small, hard, convex object can be used for the pressure wrap; we also have used appropriately sized and shaped plastic shirt buttons with similar results. Home kits can be assembled in advance and provided to patients at their initial visit along with appropriate instructions (Figure 1A).
Effects on the Skin and Distal Digit—Application of pressure wraps does not harm normal skin; however, care should be taken when the self-adherent wrap is applied so as not to induce ischemia of the distal digit. The wrap should be applied using gentle pressure with patients experiencing minimal discomfort from the overnight application.
Indications—This pressure wrap technique can be employed on most digital warts, including periungual warts, which can be difficult to treat by other means. However, in our experience this technique is not effective for nondigital warts, likely due to the inability to maintain adequate pressure with the overlying dressing. Patients at risk for compromised digital perfusion, such as those with Raynaud phenomenon or systemic sclerosis, should not be treated with pressure wraps due to possible digital ischemia.
Precautions—Patients should be advised that the pinto bean should only be used if dry and should not be ingested. The bean can be a choking hazard for small children, therefore appropriate precautions should be used. Allergic contact dermatitis to the materials used in this technique is possible, but we have never observed this. The pinto bean can be reused for future application as long as it remains dry and provides a hard convex surface.
Practice Implications
The probable mechanism of the ischemic changes to the wart tissue likely is the occlusion of tortuous blood vessels in the dermal papillae, which are intrinsic to wart tissue and absent in normal skin.1 This pressure-induced ischemic injury allows for selective destruction of the wart tissue with sparing of the normal skin. Our technique is fairly novel, although at least one report in the literature has described the use of a mechanical device to induce ischemic changes in skin tags.5
The use of pinto bean pressure wraps to induce ischemic change in digital warts provides a low-risk and nearly pain-free alternative to more expensive and invasive treatment methods. Moreover, this technique allows for a low-cost home-based therapy that can be repeated easily for other digital sites or if recurrence is noted.
- Cardoso J, Calonje E. Cutaneous manifestations of human papillomaviruses: a review. Acta Dermatovenerol Alp Pannonica Adriat. 2011;20:145-154.
- Lipke M. An armamentarium of wart treatments. Clin Med Res. 2006;4:273-293. doi:10.3121/cmr.4.4.273
- Muse M, Stiff K, Glines K, et al. A review of intralesional wart therapy. Dermatol Online J. 2020;26:2. doi:10.5070/D3263048027
- Berna R, Margolis D, Barbieri J. Annual health care utilization and costs for treatment of cutaneous and anogenital warts among a commercially insured population in the US, 2017-2019. JAMA Dermatol. 2022;158:695-697. doi:10.1001/jamadermatol.2022.0964
- Fredriksson C, Ilias M, Anderson C. New mechanical device for effective removal of skin tags in routine health care. Dermatol Online J. 2009;15:9. doi:10.5070/D37tj2800k
- Cardoso J, Calonje E. Cutaneous manifestations of human papillomaviruses: a review. Acta Dermatovenerol Alp Pannonica Adriat. 2011;20:145-154.
- Lipke M. An armamentarium of wart treatments. Clin Med Res. 2006;4:273-293. doi:10.3121/cmr.4.4.273
- Muse M, Stiff K, Glines K, et al. A review of intralesional wart therapy. Dermatol Online J. 2020;26:2. doi:10.5070/D3263048027
- Berna R, Margolis D, Barbieri J. Annual health care utilization and costs for treatment of cutaneous and anogenital warts among a commercially insured population in the US, 2017-2019. JAMA Dermatol. 2022;158:695-697. doi:10.1001/jamadermatol.2022.0964
- Fredriksson C, Ilias M, Anderson C. New mechanical device for effective removal of skin tags in routine health care. Dermatol Online J. 2009;15:9. doi:10.5070/D37tj2800k
Hospital Dermatology: Review of Research in 2023-2024
Inpatient consultative dermatology has advanced as a subspecialty and increasingly gained recognition in recent years. Since its founding in 2009, the Society of Dermatology Hospitalists has fostered research and education in hospital dermatology. Last year, we reviewed the 2022-2023 literature with a focus on developments in severe cutaneous adverse reactions, supportive oncodermatology, cost of inpatient services, and teledermatology.1 In this review, we highlight 3 areas of interest from the 2023-2024 literature: severe cutaneous adverse drug reactions, skin and soft tissue infections, and autoimmune blistering diseases (AIBDs).
Severe Cutaneous Adverse Drug Reactions
Adverse drug reactions are among the most common diagnoses encountered by inpatient dermatology consultants.2,3 Severe cutaneous adverse drug reactions are associated with substantial morbidity and mortality. Efforts to characterize these conditions and standardize their diagnosis and management continue to be a major focus of ongoing research.
A single-center retrospective analysis of 102 cases of drug reaction with eosinophilia and systemic symptoms (DRESS) syndrome evaluated differences in clinical manifestations depending on the culprit drug, offering insights into the heterogeneity of DRESS syndrome and the potential for diagnostic uncertainty.4 The shortest median latency was observed in a case caused by penicillin and cephalosporins (12 and 18 days, respectively), while DRESS syndrome secondary to allopurinol had the longest median latency (36 days). Nonsteroidal anti-inflammatory drug–induced DRESS syndrome was associated with the shortest hospital stay (6.5 days), while cephalosporin and vancomycin cases had the highest mortality rates.4
In the first international Delphi consensus study on the diagnostic workup, severity assessment, and management of DRESS syndrome, 54 dermatology and/or allergy experts reached consensus on 93 statements.5 Specific recommendations included basic evaluation with complete blood count with differential, kidney and liver function parameters, and electrocardiogram for all patients with suspected DRESS syndrome, with additional complementary workup considered in patients with evidence of specific organ damage and/or severe disease. In the proposed DRESS syndrome severity grading scheme, laboratory values that reached consensus for inclusion were hemoglobin, neutrophil, and platelet counts and creatinine, transaminases, and alkaline phosphatase levels. Although treatment of DRESS syndrome should be based on assessed disease severity, treatment with corticosteroids should be initiated in all patients with confirmed DRESS syndrome. Cyclosporine, antibodies interfering with the IL-5 axis, and intravenous immunoglobulins can be considered in patients with corticosteroid-refractory DRESS syndrome, and antiviral treatment can be considered in patients with a high serum cytomegalovirus viral load. Regularly following up with laboratory evaluation of involved organs; screening for autoantibodies, thyroid dysfunction, and steroid adverse effects; and offering of psychological support also were consensus recommendations.5
Identifying causative agents in drug hypersensitivity reactions remains challenging. A retrospective cohort study of 48 patients with Stevens-Johnson syndrome (SJS)/toxic epidermal necrolysis (TEN) highlighted the need for a systematic unbiased approach to identifying culprit drugs. Using the RegiSCAR database and algorithm for drug causality for epidermal necrolysis to analyze the cohort, more than half of causative agents were determined to be different from those initially identified by the treating physicians. Nine additional suspected culprit drugs were identified, while 43 drugs initially identified as allergens were exonerated.6
Etiology-associated definitions for blistering reactions in children have been proposed to replace the existing terms Stevens-Johnson syndrome, toxic epidermal necrolysis, and others.7 Investigators in a recent study reclassified cases of SJS and TEN as reactive infectious mucocutaneous eruption (RIME) or drug-induced epidermal necrolysis (DEN), respectively. In RIME cases, Mycoplasma pneumoniae was the most commonly identified trigger, and in DEN cases, anticonvulsants were the most common class of culprit medications. Cases of RIME were less severe and were most often treated with antibiotics, whereas patients with DEN were more likely to receive supportive care, corticosteroids, intravenous immunoglobulins, and other immunosuppressive therapies.7
In addition to causing acute devastating mucocutaneous complications, SJS and TEN have long-lasting effects that require ongoing care. In a cohort of 6552 incident SJS/TEN cases over an 11-year period, survivors of SJS/TEN endured a mean loss of 9.4 years in life expectancy and excess health care expenditures of $3752 per year compared with age- and sex-matched controls. Patients with more severe disease, comorbid malignancy, diabetes, end-stage renal disease, or SJS/TEN sequelae experienced greater loss in life expectancy and lifetime health care expenditures.8 Separately, a qualitative study investigating the psychological impact of SJS/TEN in pediatric patients described sequelae including night terrors, posttraumatic stress disorder, depression, and anxiety for many years after the acute phase. Many patients reported a desire for increased support for their physical and emotional needs following hospital discharge.9
Skin and Soft Tissue Infections: Diagnosis, Management, and Prevention
Dermatology consultation has been shown to be a cost-effective intervention to improve outcomes in hospitalized patients with skin and soft tissue infections.10,11 In particular, cellulitis frequently is misdiagnosed, leading to unnecessary antibiotic use, hospitalizations, and major health care expenditures.12 Recognizing this challenge, researchers have worked to develop objective tools to improve diagnostic accuracy. In a large prospective prognostic validation study, Pulia et al13 found that thermal imaging alone or in combination with the ALT-70 prediction model (asymmetry, leukocytosis, tachycardia, and age ≥70 years) could be used successfully to reduce overdiagnosis of cellulitis. Both thermal imaging and the ALT-70 prediction model demonstrated robust sensitivity (93.5% and 98.8%, respectively) but low specificity (38.4% and 22.0%, respectively, and 53.9% when combined).13
In a systematic review, Kovacs et al14 analyzed case reports of pseudocellulitis caused by chemotherapeutic medications. Of the 81 cases selected, 58 (71.6%) were associated with gemcitabine, with the remaining 23 (28.4%) attributed to pemetrexed. Within this group, two-thirds of the patients received antibiotic treatment prior to receiving the correct diagnosis, and 36% experienced interruptions to their oncologic therapies. In contrast to infectious cellulitis, which tends to be unilateral and associated with elevated erythrocyte sedimentation rate or C-reactive protein, most chemotherapy-induced pseudocellulitis cases occurred bilaterally on the lower extremities, while erythrocyte sedimentation rate and C-reactive protein seldom were elevated.14
Necrotizing soft tissue infections (NSTIs) are severe life-threatening conditions characterized by widespread tissue destruction, signs of systemic toxicity, hemodynamic collapse, organ failure, and high mortality. Surgical inspection along with intraoperative tissue culture is the gold standard for diagnosis. Early detection, prompt surgical intervention, and appropriate antibiotic treatment are essential to reduce mortality and improve outcomes.15 A retrospective study of patients with surgically confirmed NSTIs assessed the incidence and risk factors for recurrence within 1 year following an initial NSTI of the lower extremity. Among 93 included patients, 32 (34.4%) had recurrence within 1 year, and more than half of recurrences occurred in the first 3 months (median, 66 days). The comparison of patients with and without recurrence showed similar proportions of antibiotic prophylaxis use after the first NSTI. There was significantly less compression therapy use (33.3% vs 62.3%; P=.13) and more negative pressure wound therapy use (83.3% vs 63.3%; P=.03) in the recurrence group, though the authors acknowledged that factors such as severity of pain and size of soft tissue defect may have affected the decisions for compression and negative pressure wound therapy.16
Residents of nursing homes are a particularly vulnerable population at high risk for health care–associated infections due to older age and a higher likelihood of having wounds, indwelling medical devices, and/or coexisting conditions.17 One cluster-randomized trial compared universal decolonization with routine-care bathing practices in nursing homes (N=28,956 residents). Decolonization entailed the use of chlorhexidine for all routine bathing and showering and administration of nasal povidone-iodine twice daily for the first 5 days after admission and then twice daily for 5 days every other week. Transfer to a hospital due to infection decreased from 62.9% to 52.2% with decolonization, for a difference in risk ratio of 16.6% (P<.001) compared with routine care. Additionally, the difference in risk ratio of the secondary end point (transfer to a hospital for any reason) was 14.6%. The number needed to treat was 9.7 to prevent 1 infection-related hospitalization and 8.9 to prevent 1 hospitalization for any reason.17
Autoimmune Blistering Diseases
Although rare, AIBDs are potentially life-threatening cutaneous diseases that often require inpatient management. While corticosteroids remain the mainstay of initial AIBD management, rituximab is now well recognized as the steroid-sparing treatment of choice for patients with moderate to severe pemphigus. In a long-term follow-up study of Ritux 318—the trial that led to the US Food and Drug Administration approval of rituximab in the treatment of moderate to severe pemphigus vulgaris—researchers assessed the long-term efficacy and safety of rituximab as a first-line treatment in patients with pemphigus.19 The 5- and 7-year disease-free survival rates without corticosteroid therapy for patients treated with rituximab were 76.7% and 72.1%, respectively, compared with 35.3% and 35.3% in those treated with prednisone alone (P<.001). Fewer serious adverse events were reported in those treated with rituximab plus prednisone compared with those treated with prednisone alone. None of the patients who maintained complete remission off corticosteroid therapy received any additional maintenance infusions of rituximab after the end of the Ritux 3 regimen (1 g of rituximab at day 0 and day 14, then 500 mg at months 12 and 18).19
By contrast, treatment of severe bullous pemphigoid (BP) often is less clear-cut, as no single therapeutic option has been shown to be superior to other immunomodulatory and immunosuppressive regimens, and the medical comorbidities of elderly patients with BP can be limiting. Fortunately, newer therapies with favorable safety profiles have emerged in recent years. In a multicenter retrospective study, 100 patients with BP received omalizumab after previously failing to respond to at least one alternative therapy. Disease control was obtained after a median of 10 days, and complete remission was achieved in 77% of patients in a median time of 3 months.20 In a multicenter retrospective cohort study of 146 patients with BP treated with dupilumab following the atopic dermatitis dosing schedule (one 600-mg dose followed by 300 mg every 2 weeks), disease control was achieved in a median of 14 days, while complete remission was achieved in 35.6% of patients, with 8.9% relapsing during the observation period.21 A retrospective case series of 30 patients with BP treated with dupilumab with maintenance dosing frequency tailored to individual patient response showed complete remission or marked response in 76.7% (23/30) of patients.22 A phase 2/3 randomized controlled trial of dupilumab in BP is currently ongoing (ClinicalTrials.gov identifier NCT04206553).
Pemphigoid gestationis is a rare autoimmune subepidermal bullous dermatosis of pregnancy that may be difficult to distinguish clinically from polymorphic eruption of pregnancy but confers notably different maternal and fetal risks. Researchers developed and validated a scoring system using clinical factors—history of pemphigoid gestationis, primigravidae, timing of rash onset, and specific clinical examination findings—that was able to differentiate between the 2 diseases with 79% sensitivity, 95% specificity, and an area under the curve of 0.93 without the need for advanced immunologic testing.23
Final Thoughts
Highlights of the literature from 2023-2024 demonstrate advancements in hospital-based dermatology as well as ongoing challenges. This year’s review emphasizes key developments in severe cutaneous adverse drug reactions, skin and soft tissue infections, and AIBDs. Continued expansion of knowledge in these areas and others informs patient care and demonstrates the value of dermatologic expertise in the inpatient setting.
- Berk-Krauss J, Micheletti RG. Hospital dermatology: review of research in 2022-2023. Cutis. 2023;112:236-239.
- Falanga V, Schachner LA, Rae V, et al. Dermatologic consultations in the hospital setting. Arch Dermatol. 1994;130:1022-1025.
- Kroshinsky D, Cotliar J, Hughey LC, et al. Association of dermatology consultation with accuracy of cutaneous disorder diagnoses in hospitalized patients: a multicenter analysis. JAMA Dermatol. 2016;152:477-480.
- Blumenthal KG, Alvarez-Arango S, Kroshinsky D, et al. Drug reaction eosinophilia and systemic symptoms: clinical phenotypic patterns according to causative drug. J Am Acad Dermatol. 2024;90:1240-1242.
- Brüggen MC, Walsh S, Ameri MM, et al. Management of adult patients with drug reaction with eosinophilia and systemic symptoms: a Delphi-based international consensus. JAMA Dermatol. 2024;160:37-44.
- Li DJ, Velasquez GA, Romar GA, et al. Assessment of need for improved identification of a culprit drug in Stevens-Johnson syndrome/toxic epidermal necrolysis. JAMA Dermatol. 2023;159:830-836.
- Martinez-Cabriales S, Coulombe J, Aaron M, et al. Preliminary summary and reclassification of cases from the Pediatric Research of Management in Stevens-Johnson syndrome and Epidermonecrolysis (PROMISE) study: a North American, multisite retrospective cohort. J Am Acad Dermatol. 2024;90:635-637.
- Chiu YM, Chiu HY. Lifetime risk, life expectancy, loss-of-life expectancy and lifetime healthcare expenditure for Stevens-Johnson syndrome/toxic epidermal necrolysis in Taiwan: follow-up of a nationwide cohort from 2008 to 2019. Br J Dermatol. 2023;189:553-560.
- Phillips C, Russell E, McNiven A, et al. A qualitative study of psychological morbidity in paediatric survivors of Stevens-Johnson syndrome/toxic epidermal necrolysis. Br J Dermatol. 2024;191:293-295.
- Li DG, Xia FD, Khosravi H, et al. Outcomes of early dermatology consultation for inpatients diagnosed with cellulitis. JAMA Dermatol. 2018;154:537-543.
- Milani-Nejad N, Zhang M, Kaffenberger BH. Association of dermatology consultations with patient care outcomes in hospitalized patients with inflammatory skin diseases. JAMA Dermatol. 2017;153:523-528.
- Weng QY, Raff AB, Cohen JM, et al. Costs and consequences associated with misdiagnosed lower extremity cellulitis. JAMA Dermatol. 2017;153:141-146.
- Pulia MS, Schwei RJ, Alexandridis R, et al. Validation of thermal imaging and the ALT-70 prediction model to differentiate cellulitis from pseudocellulitis. JAMA Dermatol. 2024;160:511-517.
- Kovacs LD, O’Donoghue M, Cogen AL. Chemotherapy-induced pseudocellulitis without prior radiation exposure: a systematic review. JAMA Dermatol. 2023;159:870-874.
- Yildiz H, Yombi JC. Necrotizing soft-tissue infections. comment. N Engl J Med. 2018;378:970.
- Traineau H, Charpentier C, Lepeule R, et al. First-year recurrence rate of skin and soft tissue infections following an initial necrotizing soft tissue infection of the lower extremities: a retrospective cohort study of 93 patients. J Am Acad Dermatol. 2023;88:1360-1363.
- Miller LG, McKinnell JA, Singh RD, et al. Decolonization in nursing homes to prevent infection and hospitalization. N Engl J Med. 2023;389:1766-1777.
- Joly P, Maho-Vaillant M, Prost-Squarcioni C, et al; French Study Group on Autoimmune Bullous Skin Diseases. First-line rituximab combined with short-term prednisone versus prednisone alone for the treatment of pemphigus (Ritux 3): a prospective, multicentre, parallel-group, open-label randomised trial. Lancet. 2017;389:2031-2040.
- Tedbirt B, Maho-Vaillant M, Houivet E, et al; French Reference Center for Autoimmune Blistering Diseases MALIBUL. Sustained remission without corticosteroids among patients with pemphigus who had rituximab as first-line therapy: follow-up of the Ritux 3 Trial. JAMA Dermatol. 2024;160:290-296.
- Chebani R, Lombart F, Chaby G, et al; French Study Group on Autoimmune Bullous Diseases. Omalizumab in the treatment of bullous pemphigoid resistant to first-line therapy: a French national multicentre retrospective study of 100 patients. Br J Dermatol. 2024;190:258-265.
- Zhao L, Wang Q, Liang G, et al. Evaluation of dupilumab in patients with bullous pemphigoid. JAMA Dermatol. 2023;159:953-960.
- Miller AC, Temiz LA, Adjei S, et al. Treatment of bullous pemphigoid with dupilumab: a case series of 30 patients. J Drugs Dermatol. 2024;23:E144-E148.
- Xie F, Davis DMR, Baban F, et al. Development and multicenter international validation of a diagnostic tool to differentiate between pemphigoid gestationis and polymorphic eruption of pregnancy. J Am Acad Dermatol. 2023;89:106-113.
Inpatient consultative dermatology has advanced as a subspecialty and increasingly gained recognition in recent years. Since its founding in 2009, the Society of Dermatology Hospitalists has fostered research and education in hospital dermatology. Last year, we reviewed the 2022-2023 literature with a focus on developments in severe cutaneous adverse reactions, supportive oncodermatology, cost of inpatient services, and teledermatology.1 In this review, we highlight 3 areas of interest from the 2023-2024 literature: severe cutaneous adverse drug reactions, skin and soft tissue infections, and autoimmune blistering diseases (AIBDs).
Severe Cutaneous Adverse Drug Reactions
Adverse drug reactions are among the most common diagnoses encountered by inpatient dermatology consultants.2,3 Severe cutaneous adverse drug reactions are associated with substantial morbidity and mortality. Efforts to characterize these conditions and standardize their diagnosis and management continue to be a major focus of ongoing research.
A single-center retrospective analysis of 102 cases of drug reaction with eosinophilia and systemic symptoms (DRESS) syndrome evaluated differences in clinical manifestations depending on the culprit drug, offering insights into the heterogeneity of DRESS syndrome and the potential for diagnostic uncertainty.4 The shortest median latency was observed in a case caused by penicillin and cephalosporins (12 and 18 days, respectively), while DRESS syndrome secondary to allopurinol had the longest median latency (36 days). Nonsteroidal anti-inflammatory drug–induced DRESS syndrome was associated with the shortest hospital stay (6.5 days), while cephalosporin and vancomycin cases had the highest mortality rates.4
In the first international Delphi consensus study on the diagnostic workup, severity assessment, and management of DRESS syndrome, 54 dermatology and/or allergy experts reached consensus on 93 statements.5 Specific recommendations included basic evaluation with complete blood count with differential, kidney and liver function parameters, and electrocardiogram for all patients with suspected DRESS syndrome, with additional complementary workup considered in patients with evidence of specific organ damage and/or severe disease. In the proposed DRESS syndrome severity grading scheme, laboratory values that reached consensus for inclusion were hemoglobin, neutrophil, and platelet counts and creatinine, transaminases, and alkaline phosphatase levels. Although treatment of DRESS syndrome should be based on assessed disease severity, treatment with corticosteroids should be initiated in all patients with confirmed DRESS syndrome. Cyclosporine, antibodies interfering with the IL-5 axis, and intravenous immunoglobulins can be considered in patients with corticosteroid-refractory DRESS syndrome, and antiviral treatment can be considered in patients with a high serum cytomegalovirus viral load. Regularly following up with laboratory evaluation of involved organs; screening for autoantibodies, thyroid dysfunction, and steroid adverse effects; and offering of psychological support also were consensus recommendations.5
Identifying causative agents in drug hypersensitivity reactions remains challenging. A retrospective cohort study of 48 patients with Stevens-Johnson syndrome (SJS)/toxic epidermal necrolysis (TEN) highlighted the need for a systematic unbiased approach to identifying culprit drugs. Using the RegiSCAR database and algorithm for drug causality for epidermal necrolysis to analyze the cohort, more than half of causative agents were determined to be different from those initially identified by the treating physicians. Nine additional suspected culprit drugs were identified, while 43 drugs initially identified as allergens were exonerated.6
Etiology-associated definitions for blistering reactions in children have been proposed to replace the existing terms Stevens-Johnson syndrome, toxic epidermal necrolysis, and others.7 Investigators in a recent study reclassified cases of SJS and TEN as reactive infectious mucocutaneous eruption (RIME) or drug-induced epidermal necrolysis (DEN), respectively. In RIME cases, Mycoplasma pneumoniae was the most commonly identified trigger, and in DEN cases, anticonvulsants were the most common class of culprit medications. Cases of RIME were less severe and were most often treated with antibiotics, whereas patients with DEN were more likely to receive supportive care, corticosteroids, intravenous immunoglobulins, and other immunosuppressive therapies.7
In addition to causing acute devastating mucocutaneous complications, SJS and TEN have long-lasting effects that require ongoing care. In a cohort of 6552 incident SJS/TEN cases over an 11-year period, survivors of SJS/TEN endured a mean loss of 9.4 years in life expectancy and excess health care expenditures of $3752 per year compared with age- and sex-matched controls. Patients with more severe disease, comorbid malignancy, diabetes, end-stage renal disease, or SJS/TEN sequelae experienced greater loss in life expectancy and lifetime health care expenditures.8 Separately, a qualitative study investigating the psychological impact of SJS/TEN in pediatric patients described sequelae including night terrors, posttraumatic stress disorder, depression, and anxiety for many years after the acute phase. Many patients reported a desire for increased support for their physical and emotional needs following hospital discharge.9
Skin and Soft Tissue Infections: Diagnosis, Management, and Prevention
Dermatology consultation has been shown to be a cost-effective intervention to improve outcomes in hospitalized patients with skin and soft tissue infections.10,11 In particular, cellulitis frequently is misdiagnosed, leading to unnecessary antibiotic use, hospitalizations, and major health care expenditures.12 Recognizing this challenge, researchers have worked to develop objective tools to improve diagnostic accuracy. In a large prospective prognostic validation study, Pulia et al13 found that thermal imaging alone or in combination with the ALT-70 prediction model (asymmetry, leukocytosis, tachycardia, and age ≥70 years) could be used successfully to reduce overdiagnosis of cellulitis. Both thermal imaging and the ALT-70 prediction model demonstrated robust sensitivity (93.5% and 98.8%, respectively) but low specificity (38.4% and 22.0%, respectively, and 53.9% when combined).13
In a systematic review, Kovacs et al14 analyzed case reports of pseudocellulitis caused by chemotherapeutic medications. Of the 81 cases selected, 58 (71.6%) were associated with gemcitabine, with the remaining 23 (28.4%) attributed to pemetrexed. Within this group, two-thirds of the patients received antibiotic treatment prior to receiving the correct diagnosis, and 36% experienced interruptions to their oncologic therapies. In contrast to infectious cellulitis, which tends to be unilateral and associated with elevated erythrocyte sedimentation rate or C-reactive protein, most chemotherapy-induced pseudocellulitis cases occurred bilaterally on the lower extremities, while erythrocyte sedimentation rate and C-reactive protein seldom were elevated.14
Necrotizing soft tissue infections (NSTIs) are severe life-threatening conditions characterized by widespread tissue destruction, signs of systemic toxicity, hemodynamic collapse, organ failure, and high mortality. Surgical inspection along with intraoperative tissue culture is the gold standard for diagnosis. Early detection, prompt surgical intervention, and appropriate antibiotic treatment are essential to reduce mortality and improve outcomes.15 A retrospective study of patients with surgically confirmed NSTIs assessed the incidence and risk factors for recurrence within 1 year following an initial NSTI of the lower extremity. Among 93 included patients, 32 (34.4%) had recurrence within 1 year, and more than half of recurrences occurred in the first 3 months (median, 66 days). The comparison of patients with and without recurrence showed similar proportions of antibiotic prophylaxis use after the first NSTI. There was significantly less compression therapy use (33.3% vs 62.3%; P=.13) and more negative pressure wound therapy use (83.3% vs 63.3%; P=.03) in the recurrence group, though the authors acknowledged that factors such as severity of pain and size of soft tissue defect may have affected the decisions for compression and negative pressure wound therapy.16
Residents of nursing homes are a particularly vulnerable population at high risk for health care–associated infections due to older age and a higher likelihood of having wounds, indwelling medical devices, and/or coexisting conditions.17 One cluster-randomized trial compared universal decolonization with routine-care bathing practices in nursing homes (N=28,956 residents). Decolonization entailed the use of chlorhexidine for all routine bathing and showering and administration of nasal povidone-iodine twice daily for the first 5 days after admission and then twice daily for 5 days every other week. Transfer to a hospital due to infection decreased from 62.9% to 52.2% with decolonization, for a difference in risk ratio of 16.6% (P<.001) compared with routine care. Additionally, the difference in risk ratio of the secondary end point (transfer to a hospital for any reason) was 14.6%. The number needed to treat was 9.7 to prevent 1 infection-related hospitalization and 8.9 to prevent 1 hospitalization for any reason.17
Autoimmune Blistering Diseases
Although rare, AIBDs are potentially life-threatening cutaneous diseases that often require inpatient management. While corticosteroids remain the mainstay of initial AIBD management, rituximab is now well recognized as the steroid-sparing treatment of choice for patients with moderate to severe pemphigus. In a long-term follow-up study of Ritux 318—the trial that led to the US Food and Drug Administration approval of rituximab in the treatment of moderate to severe pemphigus vulgaris—researchers assessed the long-term efficacy and safety of rituximab as a first-line treatment in patients with pemphigus.19 The 5- and 7-year disease-free survival rates without corticosteroid therapy for patients treated with rituximab were 76.7% and 72.1%, respectively, compared with 35.3% and 35.3% in those treated with prednisone alone (P<.001). Fewer serious adverse events were reported in those treated with rituximab plus prednisone compared with those treated with prednisone alone. None of the patients who maintained complete remission off corticosteroid therapy received any additional maintenance infusions of rituximab after the end of the Ritux 3 regimen (1 g of rituximab at day 0 and day 14, then 500 mg at months 12 and 18).19
By contrast, treatment of severe bullous pemphigoid (BP) often is less clear-cut, as no single therapeutic option has been shown to be superior to other immunomodulatory and immunosuppressive regimens, and the medical comorbidities of elderly patients with BP can be limiting. Fortunately, newer therapies with favorable safety profiles have emerged in recent years. In a multicenter retrospective study, 100 patients with BP received omalizumab after previously failing to respond to at least one alternative therapy. Disease control was obtained after a median of 10 days, and complete remission was achieved in 77% of patients in a median time of 3 months.20 In a multicenter retrospective cohort study of 146 patients with BP treated with dupilumab following the atopic dermatitis dosing schedule (one 600-mg dose followed by 300 mg every 2 weeks), disease control was achieved in a median of 14 days, while complete remission was achieved in 35.6% of patients, with 8.9% relapsing during the observation period.21 A retrospective case series of 30 patients with BP treated with dupilumab with maintenance dosing frequency tailored to individual patient response showed complete remission or marked response in 76.7% (23/30) of patients.22 A phase 2/3 randomized controlled trial of dupilumab in BP is currently ongoing (ClinicalTrials.gov identifier NCT04206553).
Pemphigoid gestationis is a rare autoimmune subepidermal bullous dermatosis of pregnancy that may be difficult to distinguish clinically from polymorphic eruption of pregnancy but confers notably different maternal and fetal risks. Researchers developed and validated a scoring system using clinical factors—history of pemphigoid gestationis, primigravidae, timing of rash onset, and specific clinical examination findings—that was able to differentiate between the 2 diseases with 79% sensitivity, 95% specificity, and an area under the curve of 0.93 without the need for advanced immunologic testing.23
Final Thoughts
Highlights of the literature from 2023-2024 demonstrate advancements in hospital-based dermatology as well as ongoing challenges. This year’s review emphasizes key developments in severe cutaneous adverse drug reactions, skin and soft tissue infections, and AIBDs. Continued expansion of knowledge in these areas and others informs patient care and demonstrates the value of dermatologic expertise in the inpatient setting.
Inpatient consultative dermatology has advanced as a subspecialty and increasingly gained recognition in recent years. Since its founding in 2009, the Society of Dermatology Hospitalists has fostered research and education in hospital dermatology. Last year, we reviewed the 2022-2023 literature with a focus on developments in severe cutaneous adverse reactions, supportive oncodermatology, cost of inpatient services, and teledermatology.1 In this review, we highlight 3 areas of interest from the 2023-2024 literature: severe cutaneous adverse drug reactions, skin and soft tissue infections, and autoimmune blistering diseases (AIBDs).
Severe Cutaneous Adverse Drug Reactions
Adverse drug reactions are among the most common diagnoses encountered by inpatient dermatology consultants.2,3 Severe cutaneous adverse drug reactions are associated with substantial morbidity and mortality. Efforts to characterize these conditions and standardize their diagnosis and management continue to be a major focus of ongoing research.
A single-center retrospective analysis of 102 cases of drug reaction with eosinophilia and systemic symptoms (DRESS) syndrome evaluated differences in clinical manifestations depending on the culprit drug, offering insights into the heterogeneity of DRESS syndrome and the potential for diagnostic uncertainty.4 The shortest median latency was observed in a case caused by penicillin and cephalosporins (12 and 18 days, respectively), while DRESS syndrome secondary to allopurinol had the longest median latency (36 days). Nonsteroidal anti-inflammatory drug–induced DRESS syndrome was associated with the shortest hospital stay (6.5 days), while cephalosporin and vancomycin cases had the highest mortality rates.4
In the first international Delphi consensus study on the diagnostic workup, severity assessment, and management of DRESS syndrome, 54 dermatology and/or allergy experts reached consensus on 93 statements.5 Specific recommendations included basic evaluation with complete blood count with differential, kidney and liver function parameters, and electrocardiogram for all patients with suspected DRESS syndrome, with additional complementary workup considered in patients with evidence of specific organ damage and/or severe disease. In the proposed DRESS syndrome severity grading scheme, laboratory values that reached consensus for inclusion were hemoglobin, neutrophil, and platelet counts and creatinine, transaminases, and alkaline phosphatase levels. Although treatment of DRESS syndrome should be based on assessed disease severity, treatment with corticosteroids should be initiated in all patients with confirmed DRESS syndrome. Cyclosporine, antibodies interfering with the IL-5 axis, and intravenous immunoglobulins can be considered in patients with corticosteroid-refractory DRESS syndrome, and antiviral treatment can be considered in patients with a high serum cytomegalovirus viral load. Regularly following up with laboratory evaluation of involved organs; screening for autoantibodies, thyroid dysfunction, and steroid adverse effects; and offering of psychological support also were consensus recommendations.5
Identifying causative agents in drug hypersensitivity reactions remains challenging. A retrospective cohort study of 48 patients with Stevens-Johnson syndrome (SJS)/toxic epidermal necrolysis (TEN) highlighted the need for a systematic unbiased approach to identifying culprit drugs. Using the RegiSCAR database and algorithm for drug causality for epidermal necrolysis to analyze the cohort, more than half of causative agents were determined to be different from those initially identified by the treating physicians. Nine additional suspected culprit drugs were identified, while 43 drugs initially identified as allergens were exonerated.6
Etiology-associated definitions for blistering reactions in children have been proposed to replace the existing terms Stevens-Johnson syndrome, toxic epidermal necrolysis, and others.7 Investigators in a recent study reclassified cases of SJS and TEN as reactive infectious mucocutaneous eruption (RIME) or drug-induced epidermal necrolysis (DEN), respectively. In RIME cases, Mycoplasma pneumoniae was the most commonly identified trigger, and in DEN cases, anticonvulsants were the most common class of culprit medications. Cases of RIME were less severe and were most often treated with antibiotics, whereas patients with DEN were more likely to receive supportive care, corticosteroids, intravenous immunoglobulins, and other immunosuppressive therapies.7
In addition to causing acute devastating mucocutaneous complications, SJS and TEN have long-lasting effects that require ongoing care. In a cohort of 6552 incident SJS/TEN cases over an 11-year period, survivors of SJS/TEN endured a mean loss of 9.4 years in life expectancy and excess health care expenditures of $3752 per year compared with age- and sex-matched controls. Patients with more severe disease, comorbid malignancy, diabetes, end-stage renal disease, or SJS/TEN sequelae experienced greater loss in life expectancy and lifetime health care expenditures.8 Separately, a qualitative study investigating the psychological impact of SJS/TEN in pediatric patients described sequelae including night terrors, posttraumatic stress disorder, depression, and anxiety for many years after the acute phase. Many patients reported a desire for increased support for their physical and emotional needs following hospital discharge.9
Skin and Soft Tissue Infections: Diagnosis, Management, and Prevention
Dermatology consultation has been shown to be a cost-effective intervention to improve outcomes in hospitalized patients with skin and soft tissue infections.10,11 In particular, cellulitis frequently is misdiagnosed, leading to unnecessary antibiotic use, hospitalizations, and major health care expenditures.12 Recognizing this challenge, researchers have worked to develop objective tools to improve diagnostic accuracy. In a large prospective prognostic validation study, Pulia et al13 found that thermal imaging alone or in combination with the ALT-70 prediction model (asymmetry, leukocytosis, tachycardia, and age ≥70 years) could be used successfully to reduce overdiagnosis of cellulitis. Both thermal imaging and the ALT-70 prediction model demonstrated robust sensitivity (93.5% and 98.8%, respectively) but low specificity (38.4% and 22.0%, respectively, and 53.9% when combined).13
In a systematic review, Kovacs et al14 analyzed case reports of pseudocellulitis caused by chemotherapeutic medications. Of the 81 cases selected, 58 (71.6%) were associated with gemcitabine, with the remaining 23 (28.4%) attributed to pemetrexed. Within this group, two-thirds of the patients received antibiotic treatment prior to receiving the correct diagnosis, and 36% experienced interruptions to their oncologic therapies. In contrast to infectious cellulitis, which tends to be unilateral and associated with elevated erythrocyte sedimentation rate or C-reactive protein, most chemotherapy-induced pseudocellulitis cases occurred bilaterally on the lower extremities, while erythrocyte sedimentation rate and C-reactive protein seldom were elevated.14
Necrotizing soft tissue infections (NSTIs) are severe life-threatening conditions characterized by widespread tissue destruction, signs of systemic toxicity, hemodynamic collapse, organ failure, and high mortality. Surgical inspection along with intraoperative tissue culture is the gold standard for diagnosis. Early detection, prompt surgical intervention, and appropriate antibiotic treatment are essential to reduce mortality and improve outcomes.15 A retrospective study of patients with surgically confirmed NSTIs assessed the incidence and risk factors for recurrence within 1 year following an initial NSTI of the lower extremity. Among 93 included patients, 32 (34.4%) had recurrence within 1 year, and more than half of recurrences occurred in the first 3 months (median, 66 days). The comparison of patients with and without recurrence showed similar proportions of antibiotic prophylaxis use after the first NSTI. There was significantly less compression therapy use (33.3% vs 62.3%; P=.13) and more negative pressure wound therapy use (83.3% vs 63.3%; P=.03) in the recurrence group, though the authors acknowledged that factors such as severity of pain and size of soft tissue defect may have affected the decisions for compression and negative pressure wound therapy.16
Residents of nursing homes are a particularly vulnerable population at high risk for health care–associated infections due to older age and a higher likelihood of having wounds, indwelling medical devices, and/or coexisting conditions.17 One cluster-randomized trial compared universal decolonization with routine-care bathing practices in nursing homes (N=28,956 residents). Decolonization entailed the use of chlorhexidine for all routine bathing and showering and administration of nasal povidone-iodine twice daily for the first 5 days after admission and then twice daily for 5 days every other week. Transfer to a hospital due to infection decreased from 62.9% to 52.2% with decolonization, for a difference in risk ratio of 16.6% (P<.001) compared with routine care. Additionally, the difference in risk ratio of the secondary end point (transfer to a hospital for any reason) was 14.6%. The number needed to treat was 9.7 to prevent 1 infection-related hospitalization and 8.9 to prevent 1 hospitalization for any reason.17
Autoimmune Blistering Diseases
Although rare, AIBDs are potentially life-threatening cutaneous diseases that often require inpatient management. While corticosteroids remain the mainstay of initial AIBD management, rituximab is now well recognized as the steroid-sparing treatment of choice for patients with moderate to severe pemphigus. In a long-term follow-up study of Ritux 318—the trial that led to the US Food and Drug Administration approval of rituximab in the treatment of moderate to severe pemphigus vulgaris—researchers assessed the long-term efficacy and safety of rituximab as a first-line treatment in patients with pemphigus.19 The 5- and 7-year disease-free survival rates without corticosteroid therapy for patients treated with rituximab were 76.7% and 72.1%, respectively, compared with 35.3% and 35.3% in those treated with prednisone alone (P<.001). Fewer serious adverse events were reported in those treated with rituximab plus prednisone compared with those treated with prednisone alone. None of the patients who maintained complete remission off corticosteroid therapy received any additional maintenance infusions of rituximab after the end of the Ritux 3 regimen (1 g of rituximab at day 0 and day 14, then 500 mg at months 12 and 18).19
By contrast, treatment of severe bullous pemphigoid (BP) often is less clear-cut, as no single therapeutic option has been shown to be superior to other immunomodulatory and immunosuppressive regimens, and the medical comorbidities of elderly patients with BP can be limiting. Fortunately, newer therapies with favorable safety profiles have emerged in recent years. In a multicenter retrospective study, 100 patients with BP received omalizumab after previously failing to respond to at least one alternative therapy. Disease control was obtained after a median of 10 days, and complete remission was achieved in 77% of patients in a median time of 3 months.20 In a multicenter retrospective cohort study of 146 patients with BP treated with dupilumab following the atopic dermatitis dosing schedule (one 600-mg dose followed by 300 mg every 2 weeks), disease control was achieved in a median of 14 days, while complete remission was achieved in 35.6% of patients, with 8.9% relapsing during the observation period.21 A retrospective case series of 30 patients with BP treated with dupilumab with maintenance dosing frequency tailored to individual patient response showed complete remission or marked response in 76.7% (23/30) of patients.22 A phase 2/3 randomized controlled trial of dupilumab in BP is currently ongoing (ClinicalTrials.gov identifier NCT04206553).
Pemphigoid gestationis is a rare autoimmune subepidermal bullous dermatosis of pregnancy that may be difficult to distinguish clinically from polymorphic eruption of pregnancy but confers notably different maternal and fetal risks. Researchers developed and validated a scoring system using clinical factors—history of pemphigoid gestationis, primigravidae, timing of rash onset, and specific clinical examination findings—that was able to differentiate between the 2 diseases with 79% sensitivity, 95% specificity, and an area under the curve of 0.93 without the need for advanced immunologic testing.23
Final Thoughts
Highlights of the literature from 2023-2024 demonstrate advancements in hospital-based dermatology as well as ongoing challenges. This year’s review emphasizes key developments in severe cutaneous adverse drug reactions, skin and soft tissue infections, and AIBDs. Continued expansion of knowledge in these areas and others informs patient care and demonstrates the value of dermatologic expertise in the inpatient setting.
- Berk-Krauss J, Micheletti RG. Hospital dermatology: review of research in 2022-2023. Cutis. 2023;112:236-239.
- Falanga V, Schachner LA, Rae V, et al. Dermatologic consultations in the hospital setting. Arch Dermatol. 1994;130:1022-1025.
- Kroshinsky D, Cotliar J, Hughey LC, et al. Association of dermatology consultation with accuracy of cutaneous disorder diagnoses in hospitalized patients: a multicenter analysis. JAMA Dermatol. 2016;152:477-480.
- Blumenthal KG, Alvarez-Arango S, Kroshinsky D, et al. Drug reaction eosinophilia and systemic symptoms: clinical phenotypic patterns according to causative drug. J Am Acad Dermatol. 2024;90:1240-1242.
- Brüggen MC, Walsh S, Ameri MM, et al. Management of adult patients with drug reaction with eosinophilia and systemic symptoms: a Delphi-based international consensus. JAMA Dermatol. 2024;160:37-44.
- Li DJ, Velasquez GA, Romar GA, et al. Assessment of need for improved identification of a culprit drug in Stevens-Johnson syndrome/toxic epidermal necrolysis. JAMA Dermatol. 2023;159:830-836.
- Martinez-Cabriales S, Coulombe J, Aaron M, et al. Preliminary summary and reclassification of cases from the Pediatric Research of Management in Stevens-Johnson syndrome and Epidermonecrolysis (PROMISE) study: a North American, multisite retrospective cohort. J Am Acad Dermatol. 2024;90:635-637.
- Chiu YM, Chiu HY. Lifetime risk, life expectancy, loss-of-life expectancy and lifetime healthcare expenditure for Stevens-Johnson syndrome/toxic epidermal necrolysis in Taiwan: follow-up of a nationwide cohort from 2008 to 2019. Br J Dermatol. 2023;189:553-560.
- Phillips C, Russell E, McNiven A, et al. A qualitative study of psychological morbidity in paediatric survivors of Stevens-Johnson syndrome/toxic epidermal necrolysis. Br J Dermatol. 2024;191:293-295.
- Li DG, Xia FD, Khosravi H, et al. Outcomes of early dermatology consultation for inpatients diagnosed with cellulitis. JAMA Dermatol. 2018;154:537-543.
- Milani-Nejad N, Zhang M, Kaffenberger BH. Association of dermatology consultations with patient care outcomes in hospitalized patients with inflammatory skin diseases. JAMA Dermatol. 2017;153:523-528.
- Weng QY, Raff AB, Cohen JM, et al. Costs and consequences associated with misdiagnosed lower extremity cellulitis. JAMA Dermatol. 2017;153:141-146.
- Pulia MS, Schwei RJ, Alexandridis R, et al. Validation of thermal imaging and the ALT-70 prediction model to differentiate cellulitis from pseudocellulitis. JAMA Dermatol. 2024;160:511-517.
- Kovacs LD, O’Donoghue M, Cogen AL. Chemotherapy-induced pseudocellulitis without prior radiation exposure: a systematic review. JAMA Dermatol. 2023;159:870-874.
- Yildiz H, Yombi JC. Necrotizing soft-tissue infections. comment. N Engl J Med. 2018;378:970.
- Traineau H, Charpentier C, Lepeule R, et al. First-year recurrence rate of skin and soft tissue infections following an initial necrotizing soft tissue infection of the lower extremities: a retrospective cohort study of 93 patients. J Am Acad Dermatol. 2023;88:1360-1363.
- Miller LG, McKinnell JA, Singh RD, et al. Decolonization in nursing homes to prevent infection and hospitalization. N Engl J Med. 2023;389:1766-1777.
- Joly P, Maho-Vaillant M, Prost-Squarcioni C, et al; French Study Group on Autoimmune Bullous Skin Diseases. First-line rituximab combined with short-term prednisone versus prednisone alone for the treatment of pemphigus (Ritux 3): a prospective, multicentre, parallel-group, open-label randomised trial. Lancet. 2017;389:2031-2040.
- Tedbirt B, Maho-Vaillant M, Houivet E, et al; French Reference Center for Autoimmune Blistering Diseases MALIBUL. Sustained remission without corticosteroids among patients with pemphigus who had rituximab as first-line therapy: follow-up of the Ritux 3 Trial. JAMA Dermatol. 2024;160:290-296.
- Chebani R, Lombart F, Chaby G, et al; French Study Group on Autoimmune Bullous Diseases. Omalizumab in the treatment of bullous pemphigoid resistant to first-line therapy: a French national multicentre retrospective study of 100 patients. Br J Dermatol. 2024;190:258-265.
- Zhao L, Wang Q, Liang G, et al. Evaluation of dupilumab in patients with bullous pemphigoid. JAMA Dermatol. 2023;159:953-960.
- Miller AC, Temiz LA, Adjei S, et al. Treatment of bullous pemphigoid with dupilumab: a case series of 30 patients. J Drugs Dermatol. 2024;23:E144-E148.
- Xie F, Davis DMR, Baban F, et al. Development and multicenter international validation of a diagnostic tool to differentiate between pemphigoid gestationis and polymorphic eruption of pregnancy. J Am Acad Dermatol. 2023;89:106-113.
- Berk-Krauss J, Micheletti RG. Hospital dermatology: review of research in 2022-2023. Cutis. 2023;112:236-239.
- Falanga V, Schachner LA, Rae V, et al. Dermatologic consultations in the hospital setting. Arch Dermatol. 1994;130:1022-1025.
- Kroshinsky D, Cotliar J, Hughey LC, et al. Association of dermatology consultation with accuracy of cutaneous disorder diagnoses in hospitalized patients: a multicenter analysis. JAMA Dermatol. 2016;152:477-480.
- Blumenthal KG, Alvarez-Arango S, Kroshinsky D, et al. Drug reaction eosinophilia and systemic symptoms: clinical phenotypic patterns according to causative drug. J Am Acad Dermatol. 2024;90:1240-1242.
- Brüggen MC, Walsh S, Ameri MM, et al. Management of adult patients with drug reaction with eosinophilia and systemic symptoms: a Delphi-based international consensus. JAMA Dermatol. 2024;160:37-44.
- Li DJ, Velasquez GA, Romar GA, et al. Assessment of need for improved identification of a culprit drug in Stevens-Johnson syndrome/toxic epidermal necrolysis. JAMA Dermatol. 2023;159:830-836.
- Martinez-Cabriales S, Coulombe J, Aaron M, et al. Preliminary summary and reclassification of cases from the Pediatric Research of Management in Stevens-Johnson syndrome and Epidermonecrolysis (PROMISE) study: a North American, multisite retrospective cohort. J Am Acad Dermatol. 2024;90:635-637.
- Chiu YM, Chiu HY. Lifetime risk, life expectancy, loss-of-life expectancy and lifetime healthcare expenditure for Stevens-Johnson syndrome/toxic epidermal necrolysis in Taiwan: follow-up of a nationwide cohort from 2008 to 2019. Br J Dermatol. 2023;189:553-560.
- Phillips C, Russell E, McNiven A, et al. A qualitative study of psychological morbidity in paediatric survivors of Stevens-Johnson syndrome/toxic epidermal necrolysis. Br J Dermatol. 2024;191:293-295.
- Li DG, Xia FD, Khosravi H, et al. Outcomes of early dermatology consultation for inpatients diagnosed with cellulitis. JAMA Dermatol. 2018;154:537-543.
- Milani-Nejad N, Zhang M, Kaffenberger BH. Association of dermatology consultations with patient care outcomes in hospitalized patients with inflammatory skin diseases. JAMA Dermatol. 2017;153:523-528.
- Weng QY, Raff AB, Cohen JM, et al. Costs and consequences associated with misdiagnosed lower extremity cellulitis. JAMA Dermatol. 2017;153:141-146.
- Pulia MS, Schwei RJ, Alexandridis R, et al. Validation of thermal imaging and the ALT-70 prediction model to differentiate cellulitis from pseudocellulitis. JAMA Dermatol. 2024;160:511-517.
- Kovacs LD, O’Donoghue M, Cogen AL. Chemotherapy-induced pseudocellulitis without prior radiation exposure: a systematic review. JAMA Dermatol. 2023;159:870-874.
- Yildiz H, Yombi JC. Necrotizing soft-tissue infections. comment. N Engl J Med. 2018;378:970.
- Traineau H, Charpentier C, Lepeule R, et al. First-year recurrence rate of skin and soft tissue infections following an initial necrotizing soft tissue infection of the lower extremities: a retrospective cohort study of 93 patients. J Am Acad Dermatol. 2023;88:1360-1363.
- Miller LG, McKinnell JA, Singh RD, et al. Decolonization in nursing homes to prevent infection and hospitalization. N Engl J Med. 2023;389:1766-1777.
- Joly P, Maho-Vaillant M, Prost-Squarcioni C, et al; French Study Group on Autoimmune Bullous Skin Diseases. First-line rituximab combined with short-term prednisone versus prednisone alone for the treatment of pemphigus (Ritux 3): a prospective, multicentre, parallel-group, open-label randomised trial. Lancet. 2017;389:2031-2040.
- Tedbirt B, Maho-Vaillant M, Houivet E, et al; French Reference Center for Autoimmune Blistering Diseases MALIBUL. Sustained remission without corticosteroids among patients with pemphigus who had rituximab as first-line therapy: follow-up of the Ritux 3 Trial. JAMA Dermatol. 2024;160:290-296.
- Chebani R, Lombart F, Chaby G, et al; French Study Group on Autoimmune Bullous Diseases. Omalizumab in the treatment of bullous pemphigoid resistant to first-line therapy: a French national multicentre retrospective study of 100 patients. Br J Dermatol. 2024;190:258-265.
- Zhao L, Wang Q, Liang G, et al. Evaluation of dupilumab in patients with bullous pemphigoid. JAMA Dermatol. 2023;159:953-960.
- Miller AC, Temiz LA, Adjei S, et al. Treatment of bullous pemphigoid with dupilumab: a case series of 30 patients. J Drugs Dermatol. 2024;23:E144-E148.
- Xie F, Davis DMR, Baban F, et al. Development and multicenter international validation of a diagnostic tool to differentiate between pemphigoid gestationis and polymorphic eruption of pregnancy. J Am Acad Dermatol. 2023;89:106-113.
Practice Points
- An international Delphi study reached consensus on 93 statements regarding workup, severity assessment, and management of DRESS syndrome.
- In nursing homes, universal decolonization with chlorhexidine and nasal iodophor greatly reduced the risk for hospital transfers due to infection compared to routine care.
- Rituximab as the first-line therapy for pemphigus vulgaris is associated with long-term sustained complete remission without corticosteroid therapy.
- Dupilumab and omalizumab are emerging safe and effective treatment options for bullous pemphigoid.
On Second Thought: Aspirin for Primary Prevention — What We Really Know
This transcript has been edited for clarity.
Our recommendations vis-à-vis aspirin have evolved at a dizzying pace. The young’uns watching us right now don’t know what things were like in the 1980s. The Reagan era was a wild, heady time where nuclear war was imminent and we didn’t prescribe aspirin to patients.
That only started in 1988, which was a banner year in human history. Not because a number of doves were incinerated by the lighting of the Olympic torch at the Seoul Olympics — look it up if you don’t know what I’m talking about — but because 1988 saw the publication of the ISIS-2 trial, which first showed a mortality benefit to prescribing aspirin post–myocardial infarction (MI).
Giving patients aspirin during or after a heart attack is not controversial. It’s one of the few things in this business that isn’t, but that’s secondary prevention — treating somebody after they develop a disease. Primary prevention, treating them before they have their incident event, is a very different ballgame. Here, things are messy.
For one thing, the doses used have been very inconsistent. We should point out that the reason for 81 mg of aspirin is very arbitrary and is rooted in the old apothecary system of weights and measurements. A standard dose of aspirin was 5 grains, where 20 grains made 1 scruple, 3 scruples made 1 dram, 8 drams made 1 oz, and 12 oz made 1 lb - because screw you, metric system. Therefore, 5 grains was 325 mg of aspirin, and 1 quarter of the standard dose became 81 mg if you rounded out the decimal.
People have tried all kinds of dosing structures with aspirin prophylaxis. The Physicians’ Health Study used a full-dose aspirin, 325 mg every 2 days, while the Hypertension Optimal Treatment (HOT) trial tested 75 mg daily and the Women’s Health Study tested 100 mg, but every other day.
Ironically, almost no one has studied 81 mg every day, which is weird if you think about it. The bigger problem here is not the variability of doses used, but the discrepancy when you look at older vs newer studies.
Older studies, like the Physicians’ Health Study, did show a benefit, at least in the subgroup of patients over age 50 years, which is probably where the “everybody over 50 should be taking an aspirin” idea comes from, at least as near as I can tell.
More recent studies, like the Women’s Health Study, ASPREE, or ASPIRE, didn’t show a benefit. I know what you’re thinking: Newer stuff is always better. That’s why you should never trust anybody over age 40 years. The context of primary prevention studies has changed. In the ‘80s and ‘90s, people smoked more and we didn’t have the same medications that we have today. We talked about all this in the beta-blocker video to explain why beta-blockers don’t seem to have a benefit post MI.
We have a similar issue here. The magnitude of the benefit with aspirin primary prevention has decreased because we’re all just healthier overall. So, yay! Progress! Here’s where the numbers matter. No one is saying that aspirin doesn’t help. It does.
If we look at the 2019 meta-analysis published in JAMA, there is a cardiovascular benefit. The numbers bear that out. I know you’re all here for the math, so here we go. Aspirin reduced the composite cardiovascular endpoint from 65.2 to 60.2 events per 10,000 patient-years; or to put it more meaningfully in absolute risk reduction terms, because that’s my jam, an absolute risk reduction of 0.41%, which means a number needed to treat of 241, which is okay-ish. It’s not super-great, but it may be justifiable for something that costs next to nothing.
The tradeoff is bleeding. Major bleeding increased from 16.4 to 23.1 bleeds per 10,000 patient-years, or an absolute risk increase of 0.47%, which is a number needed to harm of 210. That’s the problem. Aspirin does prevent heart disease. The benefit is small, for sure, but the real problem is that it’s outweighed by the risk of bleeding, so you’re not really coming out ahead.
The real tragedy here is that the public is locked into this idea of everyone over age 50 years should be taking an aspirin. Even today, even though guidelines have recommended against aspirin for primary prevention for some time, data from the National Health Interview Survey sample found that nearly one in three older adults take aspirin for primary prevention when they shouldn’t be. That’s a large number of people. That’s millions of Americans — and Canadians, but nobody cares about us. It’s fine.
That’s the point. We’re not debunking aspirin. It does work. The benefits are just really small in a primary prevention population and offset by the admittedly also really small risks of bleeding. It’s a tradeoff that doesn’t really work in your favor.
But that’s aspirin for cardiovascular disease. When it comes to cancer or DVT prophylaxis, that’s another really interesting story. We might have to save that for another time. Do I know how to tease a sequel or what?
Labos, a cardiologist at Kirkland Medical Center, Montreal, Quebec, Canada, has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
Our recommendations vis-à-vis aspirin have evolved at a dizzying pace. The young’uns watching us right now don’t know what things were like in the 1980s. The Reagan era was a wild, heady time where nuclear war was imminent and we didn’t prescribe aspirin to patients.
That only started in 1988, which was a banner year in human history. Not because a number of doves were incinerated by the lighting of the Olympic torch at the Seoul Olympics — look it up if you don’t know what I’m talking about — but because 1988 saw the publication of the ISIS-2 trial, which first showed a mortality benefit to prescribing aspirin post–myocardial infarction (MI).
Giving patients aspirin during or after a heart attack is not controversial. It’s one of the few things in this business that isn’t, but that’s secondary prevention — treating somebody after they develop a disease. Primary prevention, treating them before they have their incident event, is a very different ballgame. Here, things are messy.
For one thing, the doses used have been very inconsistent. We should point out that the reason for 81 mg of aspirin is very arbitrary and is rooted in the old apothecary system of weights and measurements. A standard dose of aspirin was 5 grains, where 20 grains made 1 scruple, 3 scruples made 1 dram, 8 drams made 1 oz, and 12 oz made 1 lb - because screw you, metric system. Therefore, 5 grains was 325 mg of aspirin, and 1 quarter of the standard dose became 81 mg if you rounded out the decimal.
People have tried all kinds of dosing structures with aspirin prophylaxis. The Physicians’ Health Study used a full-dose aspirin, 325 mg every 2 days, while the Hypertension Optimal Treatment (HOT) trial tested 75 mg daily and the Women’s Health Study tested 100 mg, but every other day.
Ironically, almost no one has studied 81 mg every day, which is weird if you think about it. The bigger problem here is not the variability of doses used, but the discrepancy when you look at older vs newer studies.
Older studies, like the Physicians’ Health Study, did show a benefit, at least in the subgroup of patients over age 50 years, which is probably where the “everybody over 50 should be taking an aspirin” idea comes from, at least as near as I can tell.
More recent studies, like the Women’s Health Study, ASPREE, or ASPIRE, didn’t show a benefit. I know what you’re thinking: Newer stuff is always better. That’s why you should never trust anybody over age 40 years. The context of primary prevention studies has changed. In the ‘80s and ‘90s, people smoked more and we didn’t have the same medications that we have today. We talked about all this in the beta-blocker video to explain why beta-blockers don’t seem to have a benefit post MI.
We have a similar issue here. The magnitude of the benefit with aspirin primary prevention has decreased because we’re all just healthier overall. So, yay! Progress! Here’s where the numbers matter. No one is saying that aspirin doesn’t help. It does.
If we look at the 2019 meta-analysis published in JAMA, there is a cardiovascular benefit. The numbers bear that out. I know you’re all here for the math, so here we go. Aspirin reduced the composite cardiovascular endpoint from 65.2 to 60.2 events per 10,000 patient-years; or to put it more meaningfully in absolute risk reduction terms, because that’s my jam, an absolute risk reduction of 0.41%, which means a number needed to treat of 241, which is okay-ish. It’s not super-great, but it may be justifiable for something that costs next to nothing.
The tradeoff is bleeding. Major bleeding increased from 16.4 to 23.1 bleeds per 10,000 patient-years, or an absolute risk increase of 0.47%, which is a number needed to harm of 210. That’s the problem. Aspirin does prevent heart disease. The benefit is small, for sure, but the real problem is that it’s outweighed by the risk of bleeding, so you’re not really coming out ahead.
The real tragedy here is that the public is locked into this idea of everyone over age 50 years should be taking an aspirin. Even today, even though guidelines have recommended against aspirin for primary prevention for some time, data from the National Health Interview Survey sample found that nearly one in three older adults take aspirin for primary prevention when they shouldn’t be. That’s a large number of people. That’s millions of Americans — and Canadians, but nobody cares about us. It’s fine.
That’s the point. We’re not debunking aspirin. It does work. The benefits are just really small in a primary prevention population and offset by the admittedly also really small risks of bleeding. It’s a tradeoff that doesn’t really work in your favor.
But that’s aspirin for cardiovascular disease. When it comes to cancer or DVT prophylaxis, that’s another really interesting story. We might have to save that for another time. Do I know how to tease a sequel or what?
Labos, a cardiologist at Kirkland Medical Center, Montreal, Quebec, Canada, has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
Our recommendations vis-à-vis aspirin have evolved at a dizzying pace. The young’uns watching us right now don’t know what things were like in the 1980s. The Reagan era was a wild, heady time where nuclear war was imminent and we didn’t prescribe aspirin to patients.
That only started in 1988, which was a banner year in human history. Not because a number of doves were incinerated by the lighting of the Olympic torch at the Seoul Olympics — look it up if you don’t know what I’m talking about — but because 1988 saw the publication of the ISIS-2 trial, which first showed a mortality benefit to prescribing aspirin post–myocardial infarction (MI).
Giving patients aspirin during or after a heart attack is not controversial. It’s one of the few things in this business that isn’t, but that’s secondary prevention — treating somebody after they develop a disease. Primary prevention, treating them before they have their incident event, is a very different ballgame. Here, things are messy.
For one thing, the doses used have been very inconsistent. We should point out that the reason for 81 mg of aspirin is very arbitrary and is rooted in the old apothecary system of weights and measurements. A standard dose of aspirin was 5 grains, where 20 grains made 1 scruple, 3 scruples made 1 dram, 8 drams made 1 oz, and 12 oz made 1 lb - because screw you, metric system. Therefore, 5 grains was 325 mg of aspirin, and 1 quarter of the standard dose became 81 mg if you rounded out the decimal.
People have tried all kinds of dosing structures with aspirin prophylaxis. The Physicians’ Health Study used a full-dose aspirin, 325 mg every 2 days, while the Hypertension Optimal Treatment (HOT) trial tested 75 mg daily and the Women’s Health Study tested 100 mg, but every other day.
Ironically, almost no one has studied 81 mg every day, which is weird if you think about it. The bigger problem here is not the variability of doses used, but the discrepancy when you look at older vs newer studies.
Older studies, like the Physicians’ Health Study, did show a benefit, at least in the subgroup of patients over age 50 years, which is probably where the “everybody over 50 should be taking an aspirin” idea comes from, at least as near as I can tell.
More recent studies, like the Women’s Health Study, ASPREE, or ASPIRE, didn’t show a benefit. I know what you’re thinking: Newer stuff is always better. That’s why you should never trust anybody over age 40 years. The context of primary prevention studies has changed. In the ‘80s and ‘90s, people smoked more and we didn’t have the same medications that we have today. We talked about all this in the beta-blocker video to explain why beta-blockers don’t seem to have a benefit post MI.
We have a similar issue here. The magnitude of the benefit with aspirin primary prevention has decreased because we’re all just healthier overall. So, yay! Progress! Here’s where the numbers matter. No one is saying that aspirin doesn’t help. It does.
If we look at the 2019 meta-analysis published in JAMA, there is a cardiovascular benefit. The numbers bear that out. I know you’re all here for the math, so here we go. Aspirin reduced the composite cardiovascular endpoint from 65.2 to 60.2 events per 10,000 patient-years; or to put it more meaningfully in absolute risk reduction terms, because that’s my jam, an absolute risk reduction of 0.41%, which means a number needed to treat of 241, which is okay-ish. It’s not super-great, but it may be justifiable for something that costs next to nothing.
The tradeoff is bleeding. Major bleeding increased from 16.4 to 23.1 bleeds per 10,000 patient-years, or an absolute risk increase of 0.47%, which is a number needed to harm of 210. That’s the problem. Aspirin does prevent heart disease. The benefit is small, for sure, but the real problem is that it’s outweighed by the risk of bleeding, so you’re not really coming out ahead.
The real tragedy here is that the public is locked into this idea of everyone over age 50 years should be taking an aspirin. Even today, even though guidelines have recommended against aspirin for primary prevention for some time, data from the National Health Interview Survey sample found that nearly one in three older adults take aspirin for primary prevention when they shouldn’t be. That’s a large number of people. That’s millions of Americans — and Canadians, but nobody cares about us. It’s fine.
That’s the point. We’re not debunking aspirin. It does work. The benefits are just really small in a primary prevention population and offset by the admittedly also really small risks of bleeding. It’s a tradeoff that doesn’t really work in your favor.
But that’s aspirin for cardiovascular disease. When it comes to cancer or DVT prophylaxis, that’s another really interesting story. We might have to save that for another time. Do I know how to tease a sequel or what?
Labos, a cardiologist at Kirkland Medical Center, Montreal, Quebec, Canada, has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
Outpatient CAR T: Safe, Effective, Accessible
In one recent study, an industry-funded phase 2 trial, researchers found similar outcomes from outpatient and inpatient CAR T-cell therapy for relapsed/refractory large B-cell lymphoma with lisocabtagene maraleucel (Breyanzi).
Another recent study reported that outpatient treatment of B cell non-Hodgkin lymphoma with tisagenlecleucel (Kymriah) had similar efficacy to inpatient treatment. Meanwhile, a 2023 review of CAR T-cell therapy in various settings found similar outcomes in outpatient and inpatient treatment.
“The future of CAR T-cell therapy lies in balancing safety with accessibility,” said Rayne Rouce, MD, a pediatric oncologist at Texas Children’s Cancer Center in Houston, Texas, in an interview. “Expanding CAR T-cell therapy beyond large medical centers is a critical next step.”
Great Outcomes, Low Access
Since 2017, the FDA has approved six CAR T-cell therapies, which target cancer by harnessing the power of a patient’s own T cells. As an Oregon Health & Sciences University/Knight Cancer Center website explains, T cells are removed from the patient’s body, “genetically modified to make the chimeric antigen receptor, or CAR, [which] protein binds to specific proteins on the surface of cancer cells.”
Modified cells are grown and then infused back into the body, where they “multiply and may be able to destroy all the cancer cells.”
As Rouce puts it, “CAR T-cells have revolutionized the treatment of relapsed or refractory blood cancers.” One or more of the therapies have been approved to treat types of lymphoblastic leukemia, B-cell lymphoma, follicular lymphoma, mantle cell lymphoma, and multiple myeloma.
A 2023 review of clinical trial data reported complete response rates of 40%-54% in aggressive B-cell lymphoma, 67% in mantle cell lymphoma, and 69%-74% in indolent B cell lymphoma.
“Commercialization of CAR T-cell therapy brought hope that access would expand beyond the major academic medical centers with the highly specialized infrastructure and advanced laboratories required to manufacture and ultimately treat patients,” Rouce said. “However, it quickly became clear that patients who are underinsured or uninsured — or who live outside the network of the well-resourced institutions that house these therapies — are still unable to access these potentially life-saving therapies.”
A 2024 report estimated the cost of CAR T-cell therapy as $700,000-$1 million and said only a small percentage of those who could benefit from the treatment actually get it. For example, an estimated 10,000 patients with diffuse large B-cell lymphoma alone could benefit from CAR T therapy annually, but a survey of 200 US healthcare centers in 2021 found that 1900 procedures were performed overall for all indications.
Distance to Treatment Is a Major Obstacle
Even if patients have insurance plans willing to cover CAR T-cell therapy, they may not be able get care. While more than 150 US centers are certified to administer the therapy, “distance to major medical centers with CAR T capabilities is a major obstacle,” Yuliya Linhares, MD, chief of lymphoma at Miami Cancer Institute in Miami, Florida, said in an interview.
“I have had patients who chose to not proceed with CAR T therapy due to inability to travel the distance to the medical center for pre-CAR T appointments and assessments and a lack of caretakers who are available to stay nearby,” Linhares said.
Indeed, the challenges facing patients in rural and underserved urban areas can be overwhelming, Hoda Badr, PhD, professor of medicine at Baylor College of Medicine in Houston, Texas, said in an interview.
“They must take time off work, arrange accommodations near treatment sites, and manage travel costs, all of which strain limited financial resources. The inability to afford these additional expenses can lead to delays in receiving care or patients forgoing the treatment altogether,” Badr said. She added that “the psychological and social burden of being away from family and community support systems during treatment can intensify the stress of an already difficult situation.”
A statistic tells the story of the urban/community divide. CAR T-cell therapy administration at academic centers after leukapheresis — the separation and collection of white blood cells — is reported to be at around 90%, while it’s only 47% in community-based practices that have to refer patients elsewhere, Linhares noted.
Researchers Explore CAR T-Cell Therapy in the Community
Linhares is lead author of the phase 2 trial that explored administration of lisocabtagene maraleucel in 82 patients with relapsed/refractory large B-cell lymphoma. The findings were published Sept. 30 in Blood Advances.
The OUTREACH trial, funded by Juno/Bristol-Myers Squibb, treated patients in the third line and beyond at community medical centers (outpatient-monitored, 70%; inpatient-monitored, 30%). The trial didn’t require facilities to be certified by the Foundation for the Accreditation of Cellular Therapy (FACT); all had to be non-tertiary cancer centers that weren’t associated with a university. In order to administer therapy on the outpatient basis, the centers had to have phase 1 or hematopoietic stem cell transplant capabilities.
As Linhares explained, 72% of participating centers hadn’t provided CAR T-cell therapy before, and 44% did not have FACT accreditation. “About 32% of patients received CAR T at CAR T naive sites, while 70% of patients received CAR T as outpatients. Investigators had to decide whether patients qualified for the outpatient observation or had to be admitted for the inpatient observation,” she noted.
Community Outcomes Were Comparable to Major Trial
As for the results, grade 3 or higher adverse events occurred at a similar frequency among outpatients and inpatients at 74% and 76%, Linhares said. There were no grade 5 adverse events, and 25% of patients treated as outpatients were never hospitalized.
Response rates were similar to those in the major TRANSCEND trial with the objective response rates rate of 80% and complete response rates of 54%.
“Overall,” Linhares said, “our study demonstrated that with the availability of standard operating procedures, specially trained staff and a multidisciplinary team trained in CAR T toxicity management, inpatient and outpatient CAR T administration is feasible at specialized community medical centers.”
In 2023, another study examined patients with B-cell non-Hodgkin lymphoma who were treated on an outpatient basis with tisagenlecleucel. Researchers reported that outpatient therapy was “feasible and associated with similar efficacy outcomes as inpatient treatment.”
And a 2023 systematic literature review identified 11 studies that reported outpatient vs inpatient outcomes in CAR T-cell therapy and found “comparable response rates (80-82% in outpatient and 72-80% in inpatient).” Costs were cheaper in the outpatient setting.
Research findings like these are good news, Baylor College of Medicine’s Badr said. “Outpatient administration could help to scale the availability of this therapy to a broader range of healthcare settings, including those serving underserved populations. Findings indicate promising safety profiles, which is encouraging for expanding access.”
Not Every Patient Can Tolerate Outpatient Care
Linhares noted that the patients who received outpatient care in the lisocabtagene maraleucel study were in better shape than those in the inpatient group. Those selected for inpatient care had “higher disease risk characteristics, including high grade B cell lymphoma histology, higher disease burden, and having received bridging therapy. This points to the fact that the investigators properly selected patients who were at a higher risk of complications for inpatient observation. Additionally, some patients stayed as inpatient due to social factors, which increases length of stay independently of disease characteristics.”
Specifically, reasons for inpatient monitoring were disease characteristics (48%) including tumor burden and risk of adverse events; psychosocial factors (32%) including lack of caregiver support or transportation; COVID-19 precautions (8%); pre-infusion adverse events (8%) of fever and vasovagal reaction; and principal investigator decision (4%) due to limited hospital experience with CAR T-cell therapy.
Texas Children’s Cancer Center’s Rouce said “certain patients, particularly those with higher risk for complications or those who require intensive monitoring, may not be suited for outpatient CAR T-cell therapy. This may be due to other comorbidities or baseline factors known to predispose to CAR T-related toxicities. However, evidence-based risk mitigation algorithms may still allow closely monitored outpatient treatment, with recognition that hospital admission for incipient side effects may be necessary.”
What’s Next for Access to Therapy?
Rouce noted that her institution, like many others, is offering CAR T-cell therapy on an outpatient basis. “Additionally, continued scientific innovation, such as immediately available, off-the-shelf cell therapies and inducible safety switches, will ultimately improve access,” she said.
Linhares noted a recent advance and highlighted research that’s now in progress. “CAR Ts now have an indication as a second-line therapy in relapsed/refractory large B-cell lymphoma, and there are ongoing clinical trials that will potentially move CAR Ts into the first line,” she said. “Some trials are exploring allogeneic, readily available off-the-shelf CAR T for the treatment of minimal residual disease positive large B-cell lymphoma after completion of first-line therapy.”
These potential advances “are increasing the need for CAR T-capable medical centers,” Linhares noted. “More and more medical centers with expert hematology teams are becoming CAR T-certified, with more patients having access to CAR T.”
Still, she said, “I don’t think access is nearly as good as it should be. Many patients in rural areas are still unable to get this life-saving treatment. “However, “it is very possible that other novel targeted therapies, such as bispecific antibodies, will be used in place of CAR T in areas with poor CAR T access. Bispecific antibody efficacy in various B cell lymphoma histologies are being currently explored.”
Rouce discloses relationships with Novartis and Pfizer. Linhares reports ties with Kyowa Kirin, AbbVie, ADC, BeiGene, Genentech, Gilead, GlaxoSmithKline, Seagen, and TG. Badr has no disclosures.
A version of this article appeared on Medscape.com.
In one recent study, an industry-funded phase 2 trial, researchers found similar outcomes from outpatient and inpatient CAR T-cell therapy for relapsed/refractory large B-cell lymphoma with lisocabtagene maraleucel (Breyanzi).
Another recent study reported that outpatient treatment of B cell non-Hodgkin lymphoma with tisagenlecleucel (Kymriah) had similar efficacy to inpatient treatment. Meanwhile, a 2023 review of CAR T-cell therapy in various settings found similar outcomes in outpatient and inpatient treatment.
“The future of CAR T-cell therapy lies in balancing safety with accessibility,” said Rayne Rouce, MD, a pediatric oncologist at Texas Children’s Cancer Center in Houston, Texas, in an interview. “Expanding CAR T-cell therapy beyond large medical centers is a critical next step.”
Great Outcomes, Low Access
Since 2017, the FDA has approved six CAR T-cell therapies, which target cancer by harnessing the power of a patient’s own T cells. As an Oregon Health & Sciences University/Knight Cancer Center website explains, T cells are removed from the patient’s body, “genetically modified to make the chimeric antigen receptor, or CAR, [which] protein binds to specific proteins on the surface of cancer cells.”
Modified cells are grown and then infused back into the body, where they “multiply and may be able to destroy all the cancer cells.”
As Rouce puts it, “CAR T-cells have revolutionized the treatment of relapsed or refractory blood cancers.” One or more of the therapies have been approved to treat types of lymphoblastic leukemia, B-cell lymphoma, follicular lymphoma, mantle cell lymphoma, and multiple myeloma.
A 2023 review of clinical trial data reported complete response rates of 40%-54% in aggressive B-cell lymphoma, 67% in mantle cell lymphoma, and 69%-74% in indolent B cell lymphoma.
“Commercialization of CAR T-cell therapy brought hope that access would expand beyond the major academic medical centers with the highly specialized infrastructure and advanced laboratories required to manufacture and ultimately treat patients,” Rouce said. “However, it quickly became clear that patients who are underinsured or uninsured — or who live outside the network of the well-resourced institutions that house these therapies — are still unable to access these potentially life-saving therapies.”
A 2024 report estimated the cost of CAR T-cell therapy as $700,000-$1 million and said only a small percentage of those who could benefit from the treatment actually get it. For example, an estimated 10,000 patients with diffuse large B-cell lymphoma alone could benefit from CAR T therapy annually, but a survey of 200 US healthcare centers in 2021 found that 1900 procedures were performed overall for all indications.
Distance to Treatment Is a Major Obstacle
Even if patients have insurance plans willing to cover CAR T-cell therapy, they may not be able get care. While more than 150 US centers are certified to administer the therapy, “distance to major medical centers with CAR T capabilities is a major obstacle,” Yuliya Linhares, MD, chief of lymphoma at Miami Cancer Institute in Miami, Florida, said in an interview.
“I have had patients who chose to not proceed with CAR T therapy due to inability to travel the distance to the medical center for pre-CAR T appointments and assessments and a lack of caretakers who are available to stay nearby,” Linhares said.
Indeed, the challenges facing patients in rural and underserved urban areas can be overwhelming, Hoda Badr, PhD, professor of medicine at Baylor College of Medicine in Houston, Texas, said in an interview.
“They must take time off work, arrange accommodations near treatment sites, and manage travel costs, all of which strain limited financial resources. The inability to afford these additional expenses can lead to delays in receiving care or patients forgoing the treatment altogether,” Badr said. She added that “the psychological and social burden of being away from family and community support systems during treatment can intensify the stress of an already difficult situation.”
A statistic tells the story of the urban/community divide. CAR T-cell therapy administration at academic centers after leukapheresis — the separation and collection of white blood cells — is reported to be at around 90%, while it’s only 47% in community-based practices that have to refer patients elsewhere, Linhares noted.
Researchers Explore CAR T-Cell Therapy in the Community
Linhares is lead author of the phase 2 trial that explored administration of lisocabtagene maraleucel in 82 patients with relapsed/refractory large B-cell lymphoma. The findings were published Sept. 30 in Blood Advances.
The OUTREACH trial, funded by Juno/Bristol-Myers Squibb, treated patients in the third line and beyond at community medical centers (outpatient-monitored, 70%; inpatient-monitored, 30%). The trial didn’t require facilities to be certified by the Foundation for the Accreditation of Cellular Therapy (FACT); all had to be non-tertiary cancer centers that weren’t associated with a university. In order to administer therapy on the outpatient basis, the centers had to have phase 1 or hematopoietic stem cell transplant capabilities.
As Linhares explained, 72% of participating centers hadn’t provided CAR T-cell therapy before, and 44% did not have FACT accreditation. “About 32% of patients received CAR T at CAR T naive sites, while 70% of patients received CAR T as outpatients. Investigators had to decide whether patients qualified for the outpatient observation or had to be admitted for the inpatient observation,” she noted.
Community Outcomes Were Comparable to Major Trial
As for the results, grade 3 or higher adverse events occurred at a similar frequency among outpatients and inpatients at 74% and 76%, Linhares said. There were no grade 5 adverse events, and 25% of patients treated as outpatients were never hospitalized.
Response rates were similar to those in the major TRANSCEND trial with the objective response rates rate of 80% and complete response rates of 54%.
“Overall,” Linhares said, “our study demonstrated that with the availability of standard operating procedures, specially trained staff and a multidisciplinary team trained in CAR T toxicity management, inpatient and outpatient CAR T administration is feasible at specialized community medical centers.”
In 2023, another study examined patients with B-cell non-Hodgkin lymphoma who were treated on an outpatient basis with tisagenlecleucel. Researchers reported that outpatient therapy was “feasible and associated with similar efficacy outcomes as inpatient treatment.”
And a 2023 systematic literature review identified 11 studies that reported outpatient vs inpatient outcomes in CAR T-cell therapy and found “comparable response rates (80-82% in outpatient and 72-80% in inpatient).” Costs were cheaper in the outpatient setting.
Research findings like these are good news, Baylor College of Medicine’s Badr said. “Outpatient administration could help to scale the availability of this therapy to a broader range of healthcare settings, including those serving underserved populations. Findings indicate promising safety profiles, which is encouraging for expanding access.”
Not Every Patient Can Tolerate Outpatient Care
Linhares noted that the patients who received outpatient care in the lisocabtagene maraleucel study were in better shape than those in the inpatient group. Those selected for inpatient care had “higher disease risk characteristics, including high grade B cell lymphoma histology, higher disease burden, and having received bridging therapy. This points to the fact that the investigators properly selected patients who were at a higher risk of complications for inpatient observation. Additionally, some patients stayed as inpatient due to social factors, which increases length of stay independently of disease characteristics.”
Specifically, reasons for inpatient monitoring were disease characteristics (48%) including tumor burden and risk of adverse events; psychosocial factors (32%) including lack of caregiver support or transportation; COVID-19 precautions (8%); pre-infusion adverse events (8%) of fever and vasovagal reaction; and principal investigator decision (4%) due to limited hospital experience with CAR T-cell therapy.
Texas Children’s Cancer Center’s Rouce said “certain patients, particularly those with higher risk for complications or those who require intensive monitoring, may not be suited for outpatient CAR T-cell therapy. This may be due to other comorbidities or baseline factors known to predispose to CAR T-related toxicities. However, evidence-based risk mitigation algorithms may still allow closely monitored outpatient treatment, with recognition that hospital admission for incipient side effects may be necessary.”
What’s Next for Access to Therapy?
Rouce noted that her institution, like many others, is offering CAR T-cell therapy on an outpatient basis. “Additionally, continued scientific innovation, such as immediately available, off-the-shelf cell therapies and inducible safety switches, will ultimately improve access,” she said.
Linhares noted a recent advance and highlighted research that’s now in progress. “CAR Ts now have an indication as a second-line therapy in relapsed/refractory large B-cell lymphoma, and there are ongoing clinical trials that will potentially move CAR Ts into the first line,” she said. “Some trials are exploring allogeneic, readily available off-the-shelf CAR T for the treatment of minimal residual disease positive large B-cell lymphoma after completion of first-line therapy.”
These potential advances “are increasing the need for CAR T-capable medical centers,” Linhares noted. “More and more medical centers with expert hematology teams are becoming CAR T-certified, with more patients having access to CAR T.”
Still, she said, “I don’t think access is nearly as good as it should be. Many patients in rural areas are still unable to get this life-saving treatment. “However, “it is very possible that other novel targeted therapies, such as bispecific antibodies, will be used in place of CAR T in areas with poor CAR T access. Bispecific antibody efficacy in various B cell lymphoma histologies are being currently explored.”
Rouce discloses relationships with Novartis and Pfizer. Linhares reports ties with Kyowa Kirin, AbbVie, ADC, BeiGene, Genentech, Gilead, GlaxoSmithKline, Seagen, and TG. Badr has no disclosures.
A version of this article appeared on Medscape.com.
In one recent study, an industry-funded phase 2 trial, researchers found similar outcomes from outpatient and inpatient CAR T-cell therapy for relapsed/refractory large B-cell lymphoma with lisocabtagene maraleucel (Breyanzi).
Another recent study reported that outpatient treatment of B cell non-Hodgkin lymphoma with tisagenlecleucel (Kymriah) had similar efficacy to inpatient treatment. Meanwhile, a 2023 review of CAR T-cell therapy in various settings found similar outcomes in outpatient and inpatient treatment.
“The future of CAR T-cell therapy lies in balancing safety with accessibility,” said Rayne Rouce, MD, a pediatric oncologist at Texas Children’s Cancer Center in Houston, Texas, in an interview. “Expanding CAR T-cell therapy beyond large medical centers is a critical next step.”
Great Outcomes, Low Access
Since 2017, the FDA has approved six CAR T-cell therapies, which target cancer by harnessing the power of a patient’s own T cells. As an Oregon Health & Sciences University/Knight Cancer Center website explains, T cells are removed from the patient’s body, “genetically modified to make the chimeric antigen receptor, or CAR, [which] protein binds to specific proteins on the surface of cancer cells.”
Modified cells are grown and then infused back into the body, where they “multiply and may be able to destroy all the cancer cells.”
As Rouce puts it, “CAR T-cells have revolutionized the treatment of relapsed or refractory blood cancers.” One or more of the therapies have been approved to treat types of lymphoblastic leukemia, B-cell lymphoma, follicular lymphoma, mantle cell lymphoma, and multiple myeloma.
A 2023 review of clinical trial data reported complete response rates of 40%-54% in aggressive B-cell lymphoma, 67% in mantle cell lymphoma, and 69%-74% in indolent B cell lymphoma.
“Commercialization of CAR T-cell therapy brought hope that access would expand beyond the major academic medical centers with the highly specialized infrastructure and advanced laboratories required to manufacture and ultimately treat patients,” Rouce said. “However, it quickly became clear that patients who are underinsured or uninsured — or who live outside the network of the well-resourced institutions that house these therapies — are still unable to access these potentially life-saving therapies.”
A 2024 report estimated the cost of CAR T-cell therapy as $700,000-$1 million and said only a small percentage of those who could benefit from the treatment actually get it. For example, an estimated 10,000 patients with diffuse large B-cell lymphoma alone could benefit from CAR T therapy annually, but a survey of 200 US healthcare centers in 2021 found that 1900 procedures were performed overall for all indications.
Distance to Treatment Is a Major Obstacle
Even if patients have insurance plans willing to cover CAR T-cell therapy, they may not be able get care. While more than 150 US centers are certified to administer the therapy, “distance to major medical centers with CAR T capabilities is a major obstacle,” Yuliya Linhares, MD, chief of lymphoma at Miami Cancer Institute in Miami, Florida, said in an interview.
“I have had patients who chose to not proceed with CAR T therapy due to inability to travel the distance to the medical center for pre-CAR T appointments and assessments and a lack of caretakers who are available to stay nearby,” Linhares said.
Indeed, the challenges facing patients in rural and underserved urban areas can be overwhelming, Hoda Badr, PhD, professor of medicine at Baylor College of Medicine in Houston, Texas, said in an interview.
“They must take time off work, arrange accommodations near treatment sites, and manage travel costs, all of which strain limited financial resources. The inability to afford these additional expenses can lead to delays in receiving care or patients forgoing the treatment altogether,” Badr said. She added that “the psychological and social burden of being away from family and community support systems during treatment can intensify the stress of an already difficult situation.”
A statistic tells the story of the urban/community divide. CAR T-cell therapy administration at academic centers after leukapheresis — the separation and collection of white blood cells — is reported to be at around 90%, while it’s only 47% in community-based practices that have to refer patients elsewhere, Linhares noted.
Researchers Explore CAR T-Cell Therapy in the Community
Linhares is lead author of the phase 2 trial that explored administration of lisocabtagene maraleucel in 82 patients with relapsed/refractory large B-cell lymphoma. The findings were published Sept. 30 in Blood Advances.
The OUTREACH trial, funded by Juno/Bristol-Myers Squibb, treated patients in the third line and beyond at community medical centers (outpatient-monitored, 70%; inpatient-monitored, 30%). The trial didn’t require facilities to be certified by the Foundation for the Accreditation of Cellular Therapy (FACT); all had to be non-tertiary cancer centers that weren’t associated with a university. In order to administer therapy on the outpatient basis, the centers had to have phase 1 or hematopoietic stem cell transplant capabilities.
As Linhares explained, 72% of participating centers hadn’t provided CAR T-cell therapy before, and 44% did not have FACT accreditation. “About 32% of patients received CAR T at CAR T naive sites, while 70% of patients received CAR T as outpatients. Investigators had to decide whether patients qualified for the outpatient observation or had to be admitted for the inpatient observation,” she noted.
Community Outcomes Were Comparable to Major Trial
As for the results, grade 3 or higher adverse events occurred at a similar frequency among outpatients and inpatients at 74% and 76%, Linhares said. There were no grade 5 adverse events, and 25% of patients treated as outpatients were never hospitalized.
Response rates were similar to those in the major TRANSCEND trial with the objective response rates rate of 80% and complete response rates of 54%.
“Overall,” Linhares said, “our study demonstrated that with the availability of standard operating procedures, specially trained staff and a multidisciplinary team trained in CAR T toxicity management, inpatient and outpatient CAR T administration is feasible at specialized community medical centers.”
In 2023, another study examined patients with B-cell non-Hodgkin lymphoma who were treated on an outpatient basis with tisagenlecleucel. Researchers reported that outpatient therapy was “feasible and associated with similar efficacy outcomes as inpatient treatment.”
And a 2023 systematic literature review identified 11 studies that reported outpatient vs inpatient outcomes in CAR T-cell therapy and found “comparable response rates (80-82% in outpatient and 72-80% in inpatient).” Costs were cheaper in the outpatient setting.
Research findings like these are good news, Baylor College of Medicine’s Badr said. “Outpatient administration could help to scale the availability of this therapy to a broader range of healthcare settings, including those serving underserved populations. Findings indicate promising safety profiles, which is encouraging for expanding access.”
Not Every Patient Can Tolerate Outpatient Care
Linhares noted that the patients who received outpatient care in the lisocabtagene maraleucel study were in better shape than those in the inpatient group. Those selected for inpatient care had “higher disease risk characteristics, including high grade B cell lymphoma histology, higher disease burden, and having received bridging therapy. This points to the fact that the investigators properly selected patients who were at a higher risk of complications for inpatient observation. Additionally, some patients stayed as inpatient due to social factors, which increases length of stay independently of disease characteristics.”
Specifically, reasons for inpatient monitoring were disease characteristics (48%) including tumor burden and risk of adverse events; psychosocial factors (32%) including lack of caregiver support or transportation; COVID-19 precautions (8%); pre-infusion adverse events (8%) of fever and vasovagal reaction; and principal investigator decision (4%) due to limited hospital experience with CAR T-cell therapy.
Texas Children’s Cancer Center’s Rouce said “certain patients, particularly those with higher risk for complications or those who require intensive monitoring, may not be suited for outpatient CAR T-cell therapy. This may be due to other comorbidities or baseline factors known to predispose to CAR T-related toxicities. However, evidence-based risk mitigation algorithms may still allow closely monitored outpatient treatment, with recognition that hospital admission for incipient side effects may be necessary.”
What’s Next for Access to Therapy?
Rouce noted that her institution, like many others, is offering CAR T-cell therapy on an outpatient basis. “Additionally, continued scientific innovation, such as immediately available, off-the-shelf cell therapies and inducible safety switches, will ultimately improve access,” she said.
Linhares noted a recent advance and highlighted research that’s now in progress. “CAR Ts now have an indication as a second-line therapy in relapsed/refractory large B-cell lymphoma, and there are ongoing clinical trials that will potentially move CAR Ts into the first line,” she said. “Some trials are exploring allogeneic, readily available off-the-shelf CAR T for the treatment of minimal residual disease positive large B-cell lymphoma after completion of first-line therapy.”
These potential advances “are increasing the need for CAR T-capable medical centers,” Linhares noted. “More and more medical centers with expert hematology teams are becoming CAR T-certified, with more patients having access to CAR T.”
Still, she said, “I don’t think access is nearly as good as it should be. Many patients in rural areas are still unable to get this life-saving treatment. “However, “it is very possible that other novel targeted therapies, such as bispecific antibodies, will be used in place of CAR T in areas with poor CAR T access. Bispecific antibody efficacy in various B cell lymphoma histologies are being currently explored.”
Rouce discloses relationships with Novartis and Pfizer. Linhares reports ties with Kyowa Kirin, AbbVie, ADC, BeiGene, Genentech, Gilead, GlaxoSmithKline, Seagen, and TG. Badr has no disclosures.
A version of this article appeared on Medscape.com.
Cosmetic Dermatology Product Recalls Still Common, Analysis Finds
TOPLINE:
Between 2011 and 2023, the US Food and Drug Administration (FDA) reported recalls of 334 cosmetic dermatology products in the United States, affecting over 77 million units, predominantly due to bacterial contamination.
METHODOLOGY:
- Researchers conducted a cross-sectional analysis of the FDA Enforcement Report database for cosmetic dermatology products from 2011 to 2023.
- Cosmetic products are any article “intended for body cleaning or beauty enhancement,” as defined by the FDA.
- Recalls were categorized by product type, reason for the recall, microbial contaminant, inorganic contaminant, distribution, and risk classification.
TAKEAWAY:
- During the study period, 334 voluntary and manufacturer-initiated recalls of cosmetic products were reported, affecting 77,135,700 units.
- A total of 297 recalls (88.9%) were categorized as Class II, indicating that they caused “medically reversible health consequences.” The median recall duration was 307 days.
- Hygiene and cleaning products accounted for most of the recalls (51.5%). Makeup gels, soaps, shampoos, tattoo ink, wipes, and lotions were the most recalled product categories. Nearly 51% of the products were distributed internationally.
- Microbial and inorganic contamination accounted for 76.8% and 10.2% of the recalls (the two most common reasons for the recall), respectively, with bacteria (80%) the most common contaminating pathogen (primarily Pseudomonas and Burkholderia species).
IN PRACTICE:
With 77 million units recalled by the FDA over 12 years, cosmetic recalls have remained common, the authors concluded, adding that “dermatologists should be key voices in pharmacovigilance given scientific expertise and frontline experience managing products and associated concerns.” Dermatologists, they added, “should also be aware of FDA enforcement reports for recall updates given that average recall termination took approximately 1 year.”
SOURCE:
The study was led by Kaushik P. Venkatesh, MBA, MPH, Harvard Medical School, Boston, and was published online on October 29 in the Journal of the American Academy of Dermatology.
LIMITATIONS:
The study’s limitations include the potential underreporting of Class III recalls (products that are unlikely to cause any adverse health reaction but violate FDA labeling or manufacturing laws) and lack of complete information on contaminants.
DISCLOSURES:
No information on funding was provided in the study. No conflicts of interest were reported.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
Between 2011 and 2023, the US Food and Drug Administration (FDA) reported recalls of 334 cosmetic dermatology products in the United States, affecting over 77 million units, predominantly due to bacterial contamination.
METHODOLOGY:
- Researchers conducted a cross-sectional analysis of the FDA Enforcement Report database for cosmetic dermatology products from 2011 to 2023.
- Cosmetic products are any article “intended for body cleaning or beauty enhancement,” as defined by the FDA.
- Recalls were categorized by product type, reason for the recall, microbial contaminant, inorganic contaminant, distribution, and risk classification.
TAKEAWAY:
- During the study period, 334 voluntary and manufacturer-initiated recalls of cosmetic products were reported, affecting 77,135,700 units.
- A total of 297 recalls (88.9%) were categorized as Class II, indicating that they caused “medically reversible health consequences.” The median recall duration was 307 days.
- Hygiene and cleaning products accounted for most of the recalls (51.5%). Makeup gels, soaps, shampoos, tattoo ink, wipes, and lotions were the most recalled product categories. Nearly 51% of the products were distributed internationally.
- Microbial and inorganic contamination accounted for 76.8% and 10.2% of the recalls (the two most common reasons for the recall), respectively, with bacteria (80%) the most common contaminating pathogen (primarily Pseudomonas and Burkholderia species).
IN PRACTICE:
With 77 million units recalled by the FDA over 12 years, cosmetic recalls have remained common, the authors concluded, adding that “dermatologists should be key voices in pharmacovigilance given scientific expertise and frontline experience managing products and associated concerns.” Dermatologists, they added, “should also be aware of FDA enforcement reports for recall updates given that average recall termination took approximately 1 year.”
SOURCE:
The study was led by Kaushik P. Venkatesh, MBA, MPH, Harvard Medical School, Boston, and was published online on October 29 in the Journal of the American Academy of Dermatology.
LIMITATIONS:
The study’s limitations include the potential underreporting of Class III recalls (products that are unlikely to cause any adverse health reaction but violate FDA labeling or manufacturing laws) and lack of complete information on contaminants.
DISCLOSURES:
No information on funding was provided in the study. No conflicts of interest were reported.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
Between 2011 and 2023, the US Food and Drug Administration (FDA) reported recalls of 334 cosmetic dermatology products in the United States, affecting over 77 million units, predominantly due to bacterial contamination.
METHODOLOGY:
- Researchers conducted a cross-sectional analysis of the FDA Enforcement Report database for cosmetic dermatology products from 2011 to 2023.
- Cosmetic products are any article “intended for body cleaning or beauty enhancement,” as defined by the FDA.
- Recalls were categorized by product type, reason for the recall, microbial contaminant, inorganic contaminant, distribution, and risk classification.
TAKEAWAY:
- During the study period, 334 voluntary and manufacturer-initiated recalls of cosmetic products were reported, affecting 77,135,700 units.
- A total of 297 recalls (88.9%) were categorized as Class II, indicating that they caused “medically reversible health consequences.” The median recall duration was 307 days.
- Hygiene and cleaning products accounted for most of the recalls (51.5%). Makeup gels, soaps, shampoos, tattoo ink, wipes, and lotions were the most recalled product categories. Nearly 51% of the products were distributed internationally.
- Microbial and inorganic contamination accounted for 76.8% and 10.2% of the recalls (the two most common reasons for the recall), respectively, with bacteria (80%) the most common contaminating pathogen (primarily Pseudomonas and Burkholderia species).
IN PRACTICE:
With 77 million units recalled by the FDA over 12 years, cosmetic recalls have remained common, the authors concluded, adding that “dermatologists should be key voices in pharmacovigilance given scientific expertise and frontline experience managing products and associated concerns.” Dermatologists, they added, “should also be aware of FDA enforcement reports for recall updates given that average recall termination took approximately 1 year.”
SOURCE:
The study was led by Kaushik P. Venkatesh, MBA, MPH, Harvard Medical School, Boston, and was published online on October 29 in the Journal of the American Academy of Dermatology.
LIMITATIONS:
The study’s limitations include the potential underreporting of Class III recalls (products that are unlikely to cause any adverse health reaction but violate FDA labeling or manufacturing laws) and lack of complete information on contaminants.
DISCLOSURES:
No information on funding was provided in the study. No conflicts of interest were reported.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
Treating Digestive Disease Across the Lifespan
Pediatric gastroenterologists are a vital, yet often overlooked segment of the GI workforce and an important part of AGA’s diverse membership. Per the American Board of Pediatrics, 2,232 pediatricians have been board certified in pediatric gastroenterology since formal certification was first offered in 1990, and AGA Institute Council’s Pediatric Gastroenterology and Developmental Biology Section has nearly 1,900 members.
According to a recently published study in the journal Pediatrics, the pediatric GI workforce is expected to double by 2040, growing at a rate faster than that of most other pediatric subspecialties. This is largely due to the increased scope and complexity of the field driven by scientific advances and the increasing prevalence of digestive and liver diseases in children, including inflammatory bowel and other diseases.
In this month’s Member Spotlight, we highlight Dr. Yoyo Zhang, a pediatric gastroenterologist at Stanford Children’s Health specializing in intestinal and liver transplantation. Her passion for her profession and for improving the lives of her patients shines brightly, and her interview provides fascinating insights into the complexities and rewards of the rapidly expanding field of pediatric gastroenterology.
Also in our November issue, we update you on the FDA’s recent approval of the “next-gen” Cologuard test and query a panel of primary care and GI experts on their thoughts regarding the role that newly FDA-approved (but not yet guideline-recommended) Guardant blood-based CRC screening test should play in CRC screening moving forward.
In our Perspectives feature, we offer expert insights on how to appropriately screen patients for certain rare malignancies. Is it worthwhile screening for pancreatic cancer, and if so, how should it be done? Likewise, diagnosing cholangiocarcinoma is challenging; how best should one evaluate for this in higher-risk populations?
We hope you enjoy all the content in our November issue – as always, thanks for reading!
Megan A. Adams, MD, JD, MSc
Editor in Chief
Pediatric gastroenterologists are a vital, yet often overlooked segment of the GI workforce and an important part of AGA’s diverse membership. Per the American Board of Pediatrics, 2,232 pediatricians have been board certified in pediatric gastroenterology since formal certification was first offered in 1990, and AGA Institute Council’s Pediatric Gastroenterology and Developmental Biology Section has nearly 1,900 members.
According to a recently published study in the journal Pediatrics, the pediatric GI workforce is expected to double by 2040, growing at a rate faster than that of most other pediatric subspecialties. This is largely due to the increased scope and complexity of the field driven by scientific advances and the increasing prevalence of digestive and liver diseases in children, including inflammatory bowel and other diseases.
In this month’s Member Spotlight, we highlight Dr. Yoyo Zhang, a pediatric gastroenterologist at Stanford Children’s Health specializing in intestinal and liver transplantation. Her passion for her profession and for improving the lives of her patients shines brightly, and her interview provides fascinating insights into the complexities and rewards of the rapidly expanding field of pediatric gastroenterology.
Also in our November issue, we update you on the FDA’s recent approval of the “next-gen” Cologuard test and query a panel of primary care and GI experts on their thoughts regarding the role that newly FDA-approved (but not yet guideline-recommended) Guardant blood-based CRC screening test should play in CRC screening moving forward.
In our Perspectives feature, we offer expert insights on how to appropriately screen patients for certain rare malignancies. Is it worthwhile screening for pancreatic cancer, and if so, how should it be done? Likewise, diagnosing cholangiocarcinoma is challenging; how best should one evaluate for this in higher-risk populations?
We hope you enjoy all the content in our November issue – as always, thanks for reading!
Megan A. Adams, MD, JD, MSc
Editor in Chief
Pediatric gastroenterologists are a vital, yet often overlooked segment of the GI workforce and an important part of AGA’s diverse membership. Per the American Board of Pediatrics, 2,232 pediatricians have been board certified in pediatric gastroenterology since formal certification was first offered in 1990, and AGA Institute Council’s Pediatric Gastroenterology and Developmental Biology Section has nearly 1,900 members.
According to a recently published study in the journal Pediatrics, the pediatric GI workforce is expected to double by 2040, growing at a rate faster than that of most other pediatric subspecialties. This is largely due to the increased scope and complexity of the field driven by scientific advances and the increasing prevalence of digestive and liver diseases in children, including inflammatory bowel and other diseases.
In this month’s Member Spotlight, we highlight Dr. Yoyo Zhang, a pediatric gastroenterologist at Stanford Children’s Health specializing in intestinal and liver transplantation. Her passion for her profession and for improving the lives of her patients shines brightly, and her interview provides fascinating insights into the complexities and rewards of the rapidly expanding field of pediatric gastroenterology.
Also in our November issue, we update you on the FDA’s recent approval of the “next-gen” Cologuard test and query a panel of primary care and GI experts on their thoughts regarding the role that newly FDA-approved (but not yet guideline-recommended) Guardant blood-based CRC screening test should play in CRC screening moving forward.
In our Perspectives feature, we offer expert insights on how to appropriately screen patients for certain rare malignancies. Is it worthwhile screening for pancreatic cancer, and if so, how should it be done? Likewise, diagnosing cholangiocarcinoma is challenging; how best should one evaluate for this in higher-risk populations?
We hope you enjoy all the content in our November issue – as always, thanks for reading!
Megan A. Adams, MD, JD, MSc
Editor in Chief
Michigan Oncologist Charged in Scheme to Illegally Sell Cancer Drugs
In late October, a federal grand jury charged a Detroit-area medical oncologist Naveed Aslam, MD, in an indictment for his part in a scheme to illegally sell cancer drugs.
According to the indictment, Aslam acquired and sold more than $17 million in cancer drugs and personally netted more than $2.5 million during the scheme.
The charges against Aslam, filed on October 23 in the US District Court for the Eastern District of Michigan, include 10 counts of illegally selling or trading prescription drugs and one count of conspiring to do so.
“Dr. Aslam’s alleged participation in this scheme not only allowed him to profit unlawfully from the sale of cancer drugs but it also posed a serious threat by potentially placing these medications into the wrong hands,” Cheyvoryea Gibson, special agent in charge of the FBI in Michigan, said in a press release announcing the indictment.
The investigation is being conducted jointly by the FBI, the US Food and Drug Administration (FDA), the US Department of Health and Human Services Office of Inspector General, and Homeland Security Investigations.
The indictment alleges that Aslam was recruited by an unnamed operator of a Michigan corporation that engaged in business as a retail pharmacy and in the wholesale distribution of expensive prescription drugs, largely oncology drugs.
According to the indictment, Aslam and the operator came to an agreement where Aslam would purchase these expensive drugs from an authorized distributor under the false pretense that he was going to prescribe them to patients.
Instead, Aslam allegedly “sold and transferred the prescription drugs” to or through the Michigan business, with involvement from the unnamed operator and a second unnamed individual.
The unnamed individuals “identified customers interested in buying prescription cancer drugs” and “communicated with Dr. Aslam about what cancer drugs were requested,” according to the press release. “Dr. Aslam used his access to certain cancer drugs through his medical practice, Somerset Hematology and Oncology, P.C., to order and purchase the cancer drugs from his supplier.”
The indictment lays out that Aslam allegedly profited from this scheme in several ways, which included charging the Michigan business more than he paid the distributor for the drugs, sharing the profits when the business resold the drugs at a markup, and receiving rebates and discounts from the distributor “based on the amount of qualifying drugs he purchased and resold.”
According to the indictment, the scheme ran from early 2019 to mid-2023 and included four antibody drug conjugates — trastuzumab deruxtecan (Enhertu), enfortumab vedotin (Padcev), tisotumab vedotin (Tivdak), and sacituzumab govitecan (Trodelvy) — and the monoclonal antibody mogamulizumab (Poteligeo) for cutaneous T-cell lymphoma.
By working with Aslam, the operatives “obtained prescription drugs from an authorized distributor that they would not otherwise have been permitted to purchase, and which they were able to sell at a profit,” according to the indictment.
Both the prosecuting assistant US attorney, Andrew Lievense, and Aslam’s defense lawyer, Daniel Dena, declined to comment for this news organization.
The prosecutor is seeking to recoup the more than $2.5 million Aslam allegedly pocketed, according to the indictment. The press release also noted that an “indictment is only a charge and is not evidence of guilt.”
A version of this article first appeared on Medscape.com.
In late October, a federal grand jury charged a Detroit-area medical oncologist Naveed Aslam, MD, in an indictment for his part in a scheme to illegally sell cancer drugs.
According to the indictment, Aslam acquired and sold more than $17 million in cancer drugs and personally netted more than $2.5 million during the scheme.
The charges against Aslam, filed on October 23 in the US District Court for the Eastern District of Michigan, include 10 counts of illegally selling or trading prescription drugs and one count of conspiring to do so.
“Dr. Aslam’s alleged participation in this scheme not only allowed him to profit unlawfully from the sale of cancer drugs but it also posed a serious threat by potentially placing these medications into the wrong hands,” Cheyvoryea Gibson, special agent in charge of the FBI in Michigan, said in a press release announcing the indictment.
The investigation is being conducted jointly by the FBI, the US Food and Drug Administration (FDA), the US Department of Health and Human Services Office of Inspector General, and Homeland Security Investigations.
The indictment alleges that Aslam was recruited by an unnamed operator of a Michigan corporation that engaged in business as a retail pharmacy and in the wholesale distribution of expensive prescription drugs, largely oncology drugs.
According to the indictment, Aslam and the operator came to an agreement where Aslam would purchase these expensive drugs from an authorized distributor under the false pretense that he was going to prescribe them to patients.
Instead, Aslam allegedly “sold and transferred the prescription drugs” to or through the Michigan business, with involvement from the unnamed operator and a second unnamed individual.
The unnamed individuals “identified customers interested in buying prescription cancer drugs” and “communicated with Dr. Aslam about what cancer drugs were requested,” according to the press release. “Dr. Aslam used his access to certain cancer drugs through his medical practice, Somerset Hematology and Oncology, P.C., to order and purchase the cancer drugs from his supplier.”
The indictment lays out that Aslam allegedly profited from this scheme in several ways, which included charging the Michigan business more than he paid the distributor for the drugs, sharing the profits when the business resold the drugs at a markup, and receiving rebates and discounts from the distributor “based on the amount of qualifying drugs he purchased and resold.”
According to the indictment, the scheme ran from early 2019 to mid-2023 and included four antibody drug conjugates — trastuzumab deruxtecan (Enhertu), enfortumab vedotin (Padcev), tisotumab vedotin (Tivdak), and sacituzumab govitecan (Trodelvy) — and the monoclonal antibody mogamulizumab (Poteligeo) for cutaneous T-cell lymphoma.
By working with Aslam, the operatives “obtained prescription drugs from an authorized distributor that they would not otherwise have been permitted to purchase, and which they were able to sell at a profit,” according to the indictment.
Both the prosecuting assistant US attorney, Andrew Lievense, and Aslam’s defense lawyer, Daniel Dena, declined to comment for this news organization.
The prosecutor is seeking to recoup the more than $2.5 million Aslam allegedly pocketed, according to the indictment. The press release also noted that an “indictment is only a charge and is not evidence of guilt.”
A version of this article first appeared on Medscape.com.
In late October, a federal grand jury charged a Detroit-area medical oncologist Naveed Aslam, MD, in an indictment for his part in a scheme to illegally sell cancer drugs.
According to the indictment, Aslam acquired and sold more than $17 million in cancer drugs and personally netted more than $2.5 million during the scheme.
The charges against Aslam, filed on October 23 in the US District Court for the Eastern District of Michigan, include 10 counts of illegally selling or trading prescription drugs and one count of conspiring to do so.
“Dr. Aslam’s alleged participation in this scheme not only allowed him to profit unlawfully from the sale of cancer drugs but it also posed a serious threat by potentially placing these medications into the wrong hands,” Cheyvoryea Gibson, special agent in charge of the FBI in Michigan, said in a press release announcing the indictment.
The investigation is being conducted jointly by the FBI, the US Food and Drug Administration (FDA), the US Department of Health and Human Services Office of Inspector General, and Homeland Security Investigations.
The indictment alleges that Aslam was recruited by an unnamed operator of a Michigan corporation that engaged in business as a retail pharmacy and in the wholesale distribution of expensive prescription drugs, largely oncology drugs.
According to the indictment, Aslam and the operator came to an agreement where Aslam would purchase these expensive drugs from an authorized distributor under the false pretense that he was going to prescribe them to patients.
Instead, Aslam allegedly “sold and transferred the prescription drugs” to or through the Michigan business, with involvement from the unnamed operator and a second unnamed individual.
The unnamed individuals “identified customers interested in buying prescription cancer drugs” and “communicated with Dr. Aslam about what cancer drugs were requested,” according to the press release. “Dr. Aslam used his access to certain cancer drugs through his medical practice, Somerset Hematology and Oncology, P.C., to order and purchase the cancer drugs from his supplier.”
The indictment lays out that Aslam allegedly profited from this scheme in several ways, which included charging the Michigan business more than he paid the distributor for the drugs, sharing the profits when the business resold the drugs at a markup, and receiving rebates and discounts from the distributor “based on the amount of qualifying drugs he purchased and resold.”
According to the indictment, the scheme ran from early 2019 to mid-2023 and included four antibody drug conjugates — trastuzumab deruxtecan (Enhertu), enfortumab vedotin (Padcev), tisotumab vedotin (Tivdak), and sacituzumab govitecan (Trodelvy) — and the monoclonal antibody mogamulizumab (Poteligeo) for cutaneous T-cell lymphoma.
By working with Aslam, the operatives “obtained prescription drugs from an authorized distributor that they would not otherwise have been permitted to purchase, and which they were able to sell at a profit,” according to the indictment.
Both the prosecuting assistant US attorney, Andrew Lievense, and Aslam’s defense lawyer, Daniel Dena, declined to comment for this news organization.
The prosecutor is seeking to recoup the more than $2.5 million Aslam allegedly pocketed, according to the indictment. The press release also noted that an “indictment is only a charge and is not evidence of guilt.”
A version of this article first appeared on Medscape.com.
Should the Body Roundness Index Replace BMI?
In daily practice, physicians need a quick and simple way to assess whether a patient’s weight presents a health risk. For decades, the body mass index (BMI) has been used for this purpose, with calculations based on height and weight. Despite its convenience, BMI has faced increasing criticism.
According to experts, BRI may more accurately identify people with high levels of visceral fat than BMI. It’s well documented that abdominal fat is strongly linked to higher risks for obesity-related diseases.
Studies Support BRI
Several studies have suggested that BRI could be a valuable tool for assessing health risks. In June of this year, researchers from China reported a significant U-shaped association between BRI and overall mortality in a paper published in JAMA Network Open. People with very low or very high BRI had an increased risk for death, noted Xiaoqian Zhang, MD, from Beijing University of Chinese Medicine, Beijing, China, and his colleagues.
A study published in September in the Journal of the American Heart Association showed that elevated BRI over several years was associated with an increased risk for cardiovascular diseases. “The BRI can be included as a predictive factor for cardiovascular disease incidence,” stated the authors, led by Man Yang, MD, from Nanjing Medical University in Nanjing, China.
Why Replace BMI?
Why is a replacement for BMI necessary? When asked by this news organization, Manfred Müller, MD, senior professor at the Institute of Human Nutrition and Food Science at the University of Kiel, in Germany, explained: “BMI was designed to provide a simple value that was as independent of body size as possible, that could detect obesity and estimate related disease risks. But scientifically, BMI has always been a very crude measure to characterize disease risks.”
Müller was part of a research group led by US mathematician Diana Thomas, PhD, who, at the time, worked at Montclair State University, Montclair, New Jersey, and now holds a position at the US Military Academy at West Point, in New York. The group developed and published the BRI in 2013.
BMI Classifies Bodybuilders as Obese
The researchers justified their search for a “better” anthropometric measure with two aspects of BMI that still constitute the main points of criticism of the widely used index today:
BMI incorrectly classifies individuals with significant muscle mass, like bodybuilders, as obese, as it doesn’t distinguish between fat and muscle mass.
BMI provides no information about fat distribution in the body — whether it’s concentrated in the hips or the abdomen, for example.
In practice, this means that a person with a normal BMI could already have prediabetes, high blood pressure, and high cholesterol, which might go undetected if no further investigations are conducted based solely on their BMI.
The BRI aims to solve this problem. As the name suggests, this index seeks to capture a person’s “roundness.” The formula for calculating BRI includes waist circumference and height but excludes body weight:
BRI = 364.2 − 365.5 × √(1 − [Waist circumference in cm/2π]²/[0.5 × Height in cm]²)
In their 2013 article, Thomas, Müller, and colleagues wrote that it still needed to be proven whether their newly developed index correlated with mortality and the risk for cardiovascular and metabolic diseases — and whether it was sufficiently better than BMI to justify the more complex calculation.
Could BRI Replace BMI?
Opinions differ on whether the BRI should replace the BMI. Zhang’s team concluded that the BRI needs to be validated in additional independent cohorts. If it does, it could become a practical screening tool in patient care.
Yang’s research group is optimistic about the BRI’s future: “The longitudinal trajectory of the BRI could be used as a novel indicator of cardiovascular disease risk, which provides a new possibility for cardiovascular disease prevention,” they wrote.
However, even BRI Co-creator Thomas has concerns. “Our entire medical system has been built around the BMI,” she told JAMA, referring to factors such as children’s growth charts and dosage recommendations for medications. That cannot be changed overnight.
Any anthropometric measure intended to replace BMI would need to be rigorously validated across all age groups, genders, and ethnicities. The impact of interventions such as bariatric surgery, diet, and exercise on the new measure would also need to be demonstrated.
Anthropometric Measures Only for Clinical Use
Even if BRI proves to be a “better” metric than BMI for patient care, Müller believes it would be no more suitable for research than BMI. “Regardless of the anthropometric measure, these are practical tools for everyday use,” he stressed.
“A high BRI, like a high BMI, is a risk factor — similar to high blood pressure, high cholesterol levels, or smoking — but it is not a disease,” he added. “In practice, as a physician, I know that a patient with a high BMI or BRI has an increased risk. I need to pay attention to that patient.”
Problems arise when indices like BMI or BRI are used in research. “These ‘invented’ anthropometric measures have no biological basis, which can harm obesity research,” Müller emphasized.
He cited the example of genetic research into obesity, which seeks to identify associations between specific genetic patterns and BMI values. “Why should weight in kilograms divided by height in meters squared be genetically determined?” he asked. “These measures are human-made constructs that have nothing to do with biology.”
Müller believes that the use of BMI has created a “gray area in obesity research” that may account for many of the “unexplained” phenomena in this field.
The BMI Might Be Responsible for the ‘Healthy Obese’
One such phenomenon is the much-discussed “healthy obese,” referring to individuals with a BMI over 30 who do not have high blood sugar, high blood pressure, metabolic disorders, or elevated uric acid levels. “It’s speculated that it must be due to genetic factors, but in reality, the classification is simply wrong,” Müller said.
According to Müller, research should rely on other methods to determine obesity or relevant fat. For example, to assess diabetes risk, liver fat needs to be measured through enzyme tests, ultrasonography, CT, or MRI.
Visceral fat is also important in assessing cardiometabolic risk. “In the doctor’s office, it’s acceptable to estimate this by looking at waist circumference or even BRI. But for research, that’s inadequate,” noted Müller. Direct measurement of trunk fat with dual-energy x-ray absorptiometry or visceral fat with CT or MRI is needed.
“You always have to distinguish between research and patient care. In daily practice, measures like BRI or BMI are sufficient for assessing cardiometabolic risk. But in research, they are not,” Müller explained. To accurately study the disease risks associated with obesity, one must be aware that “with BMI, you cannot create scientifically valid patient or population groups because this value is far too imprecise.”
This story was translated from Medscape’s German edition using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
In daily practice, physicians need a quick and simple way to assess whether a patient’s weight presents a health risk. For decades, the body mass index (BMI) has been used for this purpose, with calculations based on height and weight. Despite its convenience, BMI has faced increasing criticism.
According to experts, BRI may more accurately identify people with high levels of visceral fat than BMI. It’s well documented that abdominal fat is strongly linked to higher risks for obesity-related diseases.
Studies Support BRI
Several studies have suggested that BRI could be a valuable tool for assessing health risks. In June of this year, researchers from China reported a significant U-shaped association between BRI and overall mortality in a paper published in JAMA Network Open. People with very low or very high BRI had an increased risk for death, noted Xiaoqian Zhang, MD, from Beijing University of Chinese Medicine, Beijing, China, and his colleagues.
A study published in September in the Journal of the American Heart Association showed that elevated BRI over several years was associated with an increased risk for cardiovascular diseases. “The BRI can be included as a predictive factor for cardiovascular disease incidence,” stated the authors, led by Man Yang, MD, from Nanjing Medical University in Nanjing, China.
Why Replace BMI?
Why is a replacement for BMI necessary? When asked by this news organization, Manfred Müller, MD, senior professor at the Institute of Human Nutrition and Food Science at the University of Kiel, in Germany, explained: “BMI was designed to provide a simple value that was as independent of body size as possible, that could detect obesity and estimate related disease risks. But scientifically, BMI has always been a very crude measure to characterize disease risks.”
Müller was part of a research group led by US mathematician Diana Thomas, PhD, who, at the time, worked at Montclair State University, Montclair, New Jersey, and now holds a position at the US Military Academy at West Point, in New York. The group developed and published the BRI in 2013.
BMI Classifies Bodybuilders as Obese
The researchers justified their search for a “better” anthropometric measure with two aspects of BMI that still constitute the main points of criticism of the widely used index today:
BMI incorrectly classifies individuals with significant muscle mass, like bodybuilders, as obese, as it doesn’t distinguish between fat and muscle mass.
BMI provides no information about fat distribution in the body — whether it’s concentrated in the hips or the abdomen, for example.
In practice, this means that a person with a normal BMI could already have prediabetes, high blood pressure, and high cholesterol, which might go undetected if no further investigations are conducted based solely on their BMI.
The BRI aims to solve this problem. As the name suggests, this index seeks to capture a person’s “roundness.” The formula for calculating BRI includes waist circumference and height but excludes body weight:
BRI = 364.2 − 365.5 × √(1 − [Waist circumference in cm/2π]²/[0.5 × Height in cm]²)
In their 2013 article, Thomas, Müller, and colleagues wrote that it still needed to be proven whether their newly developed index correlated with mortality and the risk for cardiovascular and metabolic diseases — and whether it was sufficiently better than BMI to justify the more complex calculation.
Could BRI Replace BMI?
Opinions differ on whether the BRI should replace the BMI. Zhang’s team concluded that the BRI needs to be validated in additional independent cohorts. If it does, it could become a practical screening tool in patient care.
Yang’s research group is optimistic about the BRI’s future: “The longitudinal trajectory of the BRI could be used as a novel indicator of cardiovascular disease risk, which provides a new possibility for cardiovascular disease prevention,” they wrote.
However, even BRI Co-creator Thomas has concerns. “Our entire medical system has been built around the BMI,” she told JAMA, referring to factors such as children’s growth charts and dosage recommendations for medications. That cannot be changed overnight.
Any anthropometric measure intended to replace BMI would need to be rigorously validated across all age groups, genders, and ethnicities. The impact of interventions such as bariatric surgery, diet, and exercise on the new measure would also need to be demonstrated.
Anthropometric Measures Only for Clinical Use
Even if BRI proves to be a “better” metric than BMI for patient care, Müller believes it would be no more suitable for research than BMI. “Regardless of the anthropometric measure, these are practical tools for everyday use,” he stressed.
“A high BRI, like a high BMI, is a risk factor — similar to high blood pressure, high cholesterol levels, or smoking — but it is not a disease,” he added. “In practice, as a physician, I know that a patient with a high BMI or BRI has an increased risk. I need to pay attention to that patient.”
Problems arise when indices like BMI or BRI are used in research. “These ‘invented’ anthropometric measures have no biological basis, which can harm obesity research,” Müller emphasized.
He cited the example of genetic research into obesity, which seeks to identify associations between specific genetic patterns and BMI values. “Why should weight in kilograms divided by height in meters squared be genetically determined?” he asked. “These measures are human-made constructs that have nothing to do with biology.”
Müller believes that the use of BMI has created a “gray area in obesity research” that may account for many of the “unexplained” phenomena in this field.
The BMI Might Be Responsible for the ‘Healthy Obese’
One such phenomenon is the much-discussed “healthy obese,” referring to individuals with a BMI over 30 who do not have high blood sugar, high blood pressure, metabolic disorders, or elevated uric acid levels. “It’s speculated that it must be due to genetic factors, but in reality, the classification is simply wrong,” Müller said.
According to Müller, research should rely on other methods to determine obesity or relevant fat. For example, to assess diabetes risk, liver fat needs to be measured through enzyme tests, ultrasonography, CT, or MRI.
Visceral fat is also important in assessing cardiometabolic risk. “In the doctor’s office, it’s acceptable to estimate this by looking at waist circumference or even BRI. But for research, that’s inadequate,” noted Müller. Direct measurement of trunk fat with dual-energy x-ray absorptiometry or visceral fat with CT or MRI is needed.
“You always have to distinguish between research and patient care. In daily practice, measures like BRI or BMI are sufficient for assessing cardiometabolic risk. But in research, they are not,” Müller explained. To accurately study the disease risks associated with obesity, one must be aware that “with BMI, you cannot create scientifically valid patient or population groups because this value is far too imprecise.”
This story was translated from Medscape’s German edition using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
In daily practice, physicians need a quick and simple way to assess whether a patient’s weight presents a health risk. For decades, the body mass index (BMI) has been used for this purpose, with calculations based on height and weight. Despite its convenience, BMI has faced increasing criticism.
According to experts, BRI may more accurately identify people with high levels of visceral fat than BMI. It’s well documented that abdominal fat is strongly linked to higher risks for obesity-related diseases.
Studies Support BRI
Several studies have suggested that BRI could be a valuable tool for assessing health risks. In June of this year, researchers from China reported a significant U-shaped association between BRI and overall mortality in a paper published in JAMA Network Open. People with very low or very high BRI had an increased risk for death, noted Xiaoqian Zhang, MD, from Beijing University of Chinese Medicine, Beijing, China, and his colleagues.
A study published in September in the Journal of the American Heart Association showed that elevated BRI over several years was associated with an increased risk for cardiovascular diseases. “The BRI can be included as a predictive factor for cardiovascular disease incidence,” stated the authors, led by Man Yang, MD, from Nanjing Medical University in Nanjing, China.
Why Replace BMI?
Why is a replacement for BMI necessary? When asked by this news organization, Manfred Müller, MD, senior professor at the Institute of Human Nutrition and Food Science at the University of Kiel, in Germany, explained: “BMI was designed to provide a simple value that was as independent of body size as possible, that could detect obesity and estimate related disease risks. But scientifically, BMI has always been a very crude measure to characterize disease risks.”
Müller was part of a research group led by US mathematician Diana Thomas, PhD, who, at the time, worked at Montclair State University, Montclair, New Jersey, and now holds a position at the US Military Academy at West Point, in New York. The group developed and published the BRI in 2013.
BMI Classifies Bodybuilders as Obese
The researchers justified their search for a “better” anthropometric measure with two aspects of BMI that still constitute the main points of criticism of the widely used index today:
BMI incorrectly classifies individuals with significant muscle mass, like bodybuilders, as obese, as it doesn’t distinguish between fat and muscle mass.
BMI provides no information about fat distribution in the body — whether it’s concentrated in the hips or the abdomen, for example.
In practice, this means that a person with a normal BMI could already have prediabetes, high blood pressure, and high cholesterol, which might go undetected if no further investigations are conducted based solely on their BMI.
The BRI aims to solve this problem. As the name suggests, this index seeks to capture a person’s “roundness.” The formula for calculating BRI includes waist circumference and height but excludes body weight:
BRI = 364.2 − 365.5 × √(1 − [Waist circumference in cm/2π]²/[0.5 × Height in cm]²)
In their 2013 article, Thomas, Müller, and colleagues wrote that it still needed to be proven whether their newly developed index correlated with mortality and the risk for cardiovascular and metabolic diseases — and whether it was sufficiently better than BMI to justify the more complex calculation.
Could BRI Replace BMI?
Opinions differ on whether the BRI should replace the BMI. Zhang’s team concluded that the BRI needs to be validated in additional independent cohorts. If it does, it could become a practical screening tool in patient care.
Yang’s research group is optimistic about the BRI’s future: “The longitudinal trajectory of the BRI could be used as a novel indicator of cardiovascular disease risk, which provides a new possibility for cardiovascular disease prevention,” they wrote.
However, even BRI Co-creator Thomas has concerns. “Our entire medical system has been built around the BMI,” she told JAMA, referring to factors such as children’s growth charts and dosage recommendations for medications. That cannot be changed overnight.
Any anthropometric measure intended to replace BMI would need to be rigorously validated across all age groups, genders, and ethnicities. The impact of interventions such as bariatric surgery, diet, and exercise on the new measure would also need to be demonstrated.
Anthropometric Measures Only for Clinical Use
Even if BRI proves to be a “better” metric than BMI for patient care, Müller believes it would be no more suitable for research than BMI. “Regardless of the anthropometric measure, these are practical tools for everyday use,” he stressed.
“A high BRI, like a high BMI, is a risk factor — similar to high blood pressure, high cholesterol levels, or smoking — but it is not a disease,” he added. “In practice, as a physician, I know that a patient with a high BMI or BRI has an increased risk. I need to pay attention to that patient.”
Problems arise when indices like BMI or BRI are used in research. “These ‘invented’ anthropometric measures have no biological basis, which can harm obesity research,” Müller emphasized.
He cited the example of genetic research into obesity, which seeks to identify associations between specific genetic patterns and BMI values. “Why should weight in kilograms divided by height in meters squared be genetically determined?” he asked. “These measures are human-made constructs that have nothing to do with biology.”
Müller believes that the use of BMI has created a “gray area in obesity research” that may account for many of the “unexplained” phenomena in this field.
The BMI Might Be Responsible for the ‘Healthy Obese’
One such phenomenon is the much-discussed “healthy obese,” referring to individuals with a BMI over 30 who do not have high blood sugar, high blood pressure, metabolic disorders, or elevated uric acid levels. “It’s speculated that it must be due to genetic factors, but in reality, the classification is simply wrong,” Müller said.
According to Müller, research should rely on other methods to determine obesity or relevant fat. For example, to assess diabetes risk, liver fat needs to be measured through enzyme tests, ultrasonography, CT, or MRI.
Visceral fat is also important in assessing cardiometabolic risk. “In the doctor’s office, it’s acceptable to estimate this by looking at waist circumference or even BRI. But for research, that’s inadequate,” noted Müller. Direct measurement of trunk fat with dual-energy x-ray absorptiometry or visceral fat with CT or MRI is needed.
“You always have to distinguish between research and patient care. In daily practice, measures like BRI or BMI are sufficient for assessing cardiometabolic risk. But in research, they are not,” Müller explained. To accurately study the disease risks associated with obesity, one must be aware that “with BMI, you cannot create scientifically valid patient or population groups because this value is far too imprecise.”
This story was translated from Medscape’s German edition using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.