User login
For MD-IQ use only
Botulinum Toxin as a Tool to Reduce Hyperhidrosis in Amputees
Botulinum Toxin as a Tool to Reduce Hyperhidrosis in Amputees
Practice Gap
Hyperhidrosis poses a considerable challenge for many amputees who use prosthetic devices, particularly at the interface between the residual limb and the prosthetic socket. The enclosed environment of the socket often leads to excessive sweating, which can compromise suction fit and increase the risk for skin chafing, irritation, and slippage. Persistent moisture also promotes bacterial and fungal growth, raising the likelihood of infections and foul odors within the socket. Research has shown that skin complications are highly prevalent among amputees, affecting up to 73.9% of this population in the United States.1 Commonly reported complications include wounds, abscesses, and blisters, many of which can be triggered or worsened by hyperhidrosis.2 Current treatment options for residual limb sweating include topical antiperspirants, botulinum toxin (BTX) injections, iontophoresis, and liner-liner socks.
While BTX commonly is used to treat hyperhidrosis in areas such as the palms and axillae, it typically is not considered as a first-line therapy for residual limb sweating; however, both BTX type A and type B have shown safety and effectiveness in managing hyperhidrosis in amputees, enhancing prosthetic use, and improving overall quality of life.3 Despite these benefits, BTX remains relatively underutilized for
Tools and Techniques
A 64-year-old man initially presented to our dermatology clinic after undergoing an above-the-knee amputation of the left leg 1 year prior. The amputation had been performed due to chronic prosthetic joint infections with Escherichia coli. He reported persistent sweating of the residual limb, which severely limited his use of a prosthesis and led to frequent falls.
During the initial visit, treatment options for primary hyperhidrosis including topical and injectable therapies were discussed. Due to a fear of needles, the patient chose topical treatment, with the option to pursue BTX injections later if better control was needed. An aluminum chloride hexahydrate prosthetic antiperspirant was prescribed for nightly application on the anterior and posterior
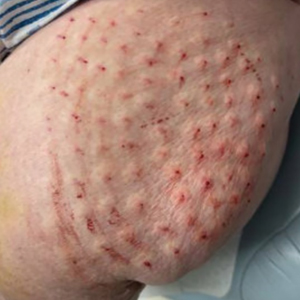
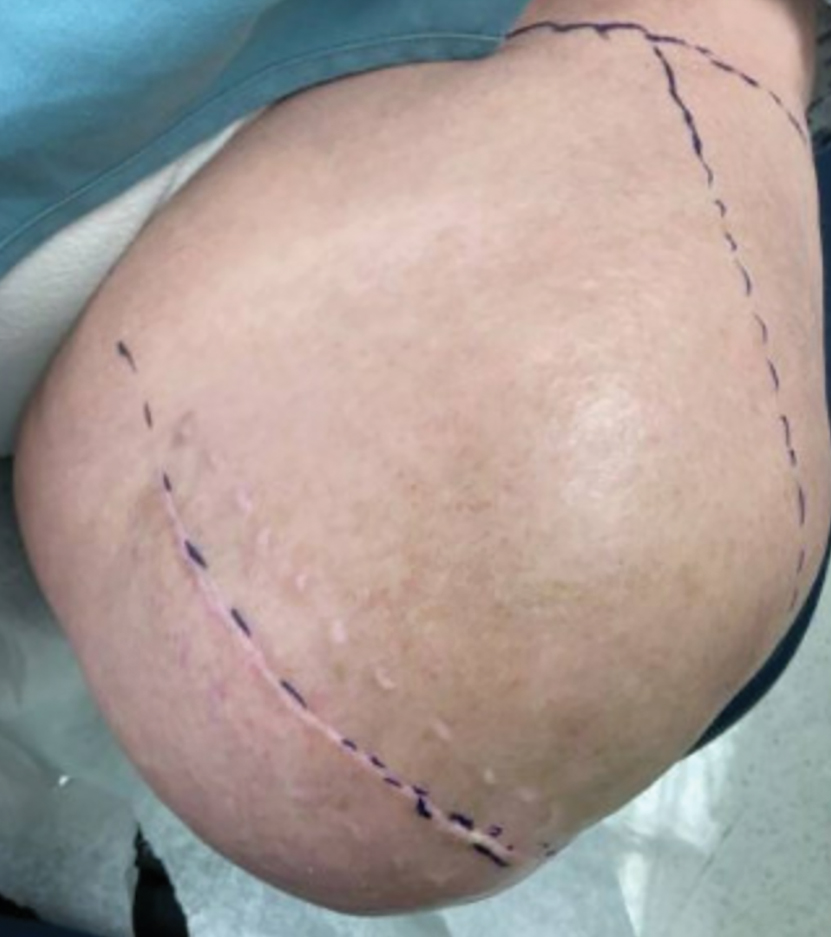
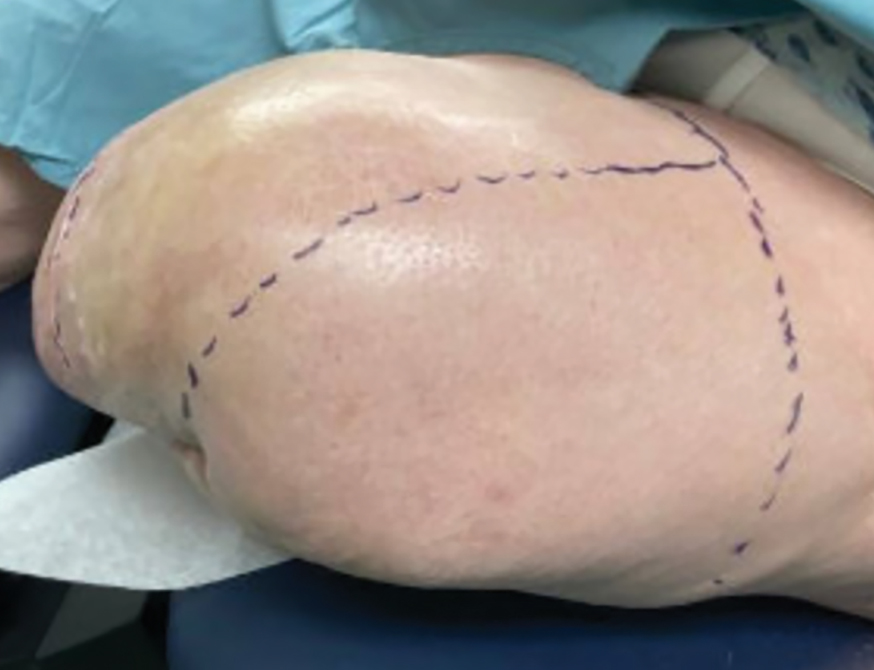
Botulinum toxin injections were administered in a grid-like pattern across the surface area where the residual limb made contact with the prosthetic. Using a surgical marker, the patient assisted the medical team in identifying the areas where sweating occurred most frequently. The area was divided into 4 equal sections, with each section treated per weekly interval sequentially over 4 weeks. The targeted areas included the left anterior (extending from the anterior tensor fasciae latae band to the lateral thigh) and left posterior residual limb (Figure 1 and eFigure 1, respectively).
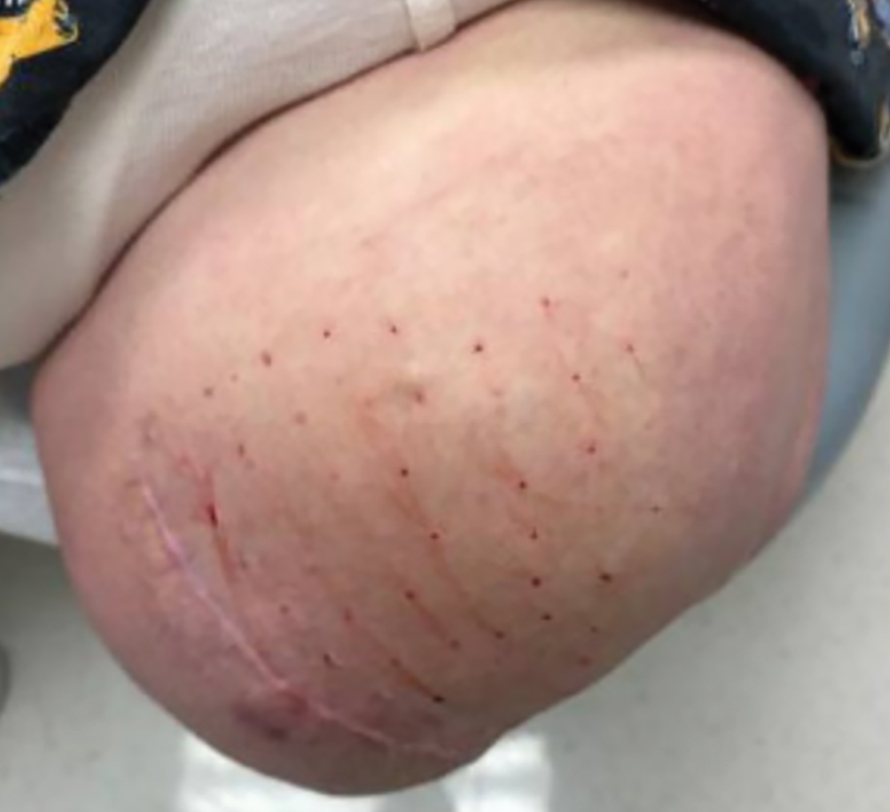
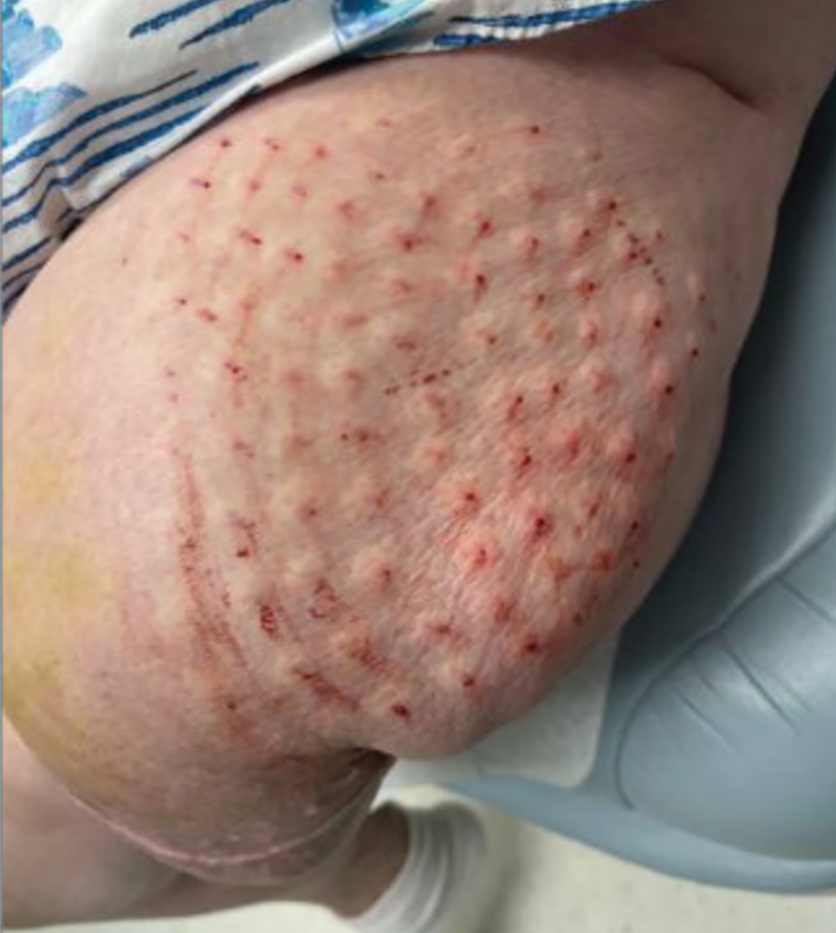
The treated section was cleaned with an alcohol wipe prior to each injection, and 50 units of BTX (diluted to 2.5 units per 0.1 mL in bacteriostatic saline) were injected intradermally into each section (Figure 2 and eFigure 2). The injections were administered in rows, with the needle inserted at evenly spaced intervals approximately 1 inch apart. A total of 100 units were administered per section at each weekly appointment. The patient tolerated the procedure well, and no complications were observed.
Practice Implications
This staged approach to administering BTX ensures even distribution of the injections, optimizes hyperhidrosis control, minimizes the risk for complications, and allows for precise targeting of the affected areas to maximize therapeutic benefit. Following the initial procedure, our patient was scheduled for follow-ups approximately every 3 to 4 months starting from the first set of injections for each area. Over 9 months, the patient successfully completed 3 treatment sessions using this method. The patient reported improved quality of life after starting the BTX injections.
After evaluating the initial treatment outcomes with 100 units per section, the dosage was increased to 200 units per section to reduce the number of visits from 4 every 3 months to cover the entire area to 2 visits every 3 months. This adjustment aimed to optimize results and better manage the patient’s ongoing symptoms. At about 1 to 2 weeks after beginning treatment, the patient noticed decreased sweating and discomfort during his daily activities and reduced friction with his prosthetic leg. No adverse effects were noted with the increased dosage during a clinical visit.
Our case highlights the importance of ensuring equitable access to hyperhidrosis treatment. Dermatologists should prioritize patient-centered care by factoring in financial constraints when recommending therapies. In this patient’s case, offering a range of options including over-the-counter antiperspirants and prescription treatments allowed for a management plan tailored to his individual needs and circumstances.
DaxibotulinumtoxinA, known for its longer duration of action compared to other BTX formulations, presents a promising alternative for treating hyperhidrosis.4 However, a gap in care emerged for our patient when prescription antiperspirant was not covered by his insurance, and daxibotulinumtoxinA, which could have offered a more durable solution, was not yet available at our clinic for hyperhidrosis management. Expanding insurance coverage for effective prescription treatments and improving access to newer treatment options are crucial for enhancing patient outcomes and ensuring more equitable care.
Focusing dermatologic care on amputees presents distinct challenges and opportunities for improving their care and decreasing discomfort. Amputees, particularly those with residual limb hyperhidrosis, often experience additional discomfort and difficulty while using prosthetics, as excessive sweating can interfere with fit and function.5,6 Dermatologists should proactively address these specific needs by tailoring treatment accordingly. Incorporating targeted therapies, such as BTX injections, in addition to education on lifestyle modifications and managing treatment expectations, ensures comprehensive care that enhances both quality of life and functional outcomes. Engaging patients in discussions about all available options, including emerging therapies, is essential for improving care for this underserved population.
- Koc E, Tunca M, Akar A, et al. Skin problems in amputees: a descriptive study. Int J Dermatol. 2008;47:463–466. doi:10.1111/j.1365-4632.2008.03604.x
- Bui KM, Raugi GJ, Nguyen VQ, et al. Skin problems in individuals with lower-limb loss: literature review and proposed classification system. J Rehabil Res Dev. 2009;46:1085-1090. doi:10.1682/jrrd.2009.04.0052
- Rocha Melo J, Rodrigues MA, Caetano M, et al. Botulinum toxin in the treatment of residual limb hyperhidrosis: a systematic review. Rehabilitacion (Madr). 2023;57:100754. doi:10.1016/j.rh.2022.07.003
- Hansen C, Godfrey B, Wixom J, et al. Incidence, severity, and impact of hyperhidrosis in people with lower-limb amputation. J Rehabil Res Dev. 2015;52:31-40. doi:10.1682/JRRD.2014.04.0108
- Lannan FM, Powell J, Kim GM, et al. Hyperhidrosis of the residual limb: a narrative review of the measurement and treatment of excess perspiration affecting individuals with amputation. Prosthet Orthot Int. 2021;45:477-486. doi:10.1097/PXR.0000000000000040
- Pace S, Kentosh J. Managing residual limb hyperhidrosis in wounded warriors. Cutis. 2016;97:401-403.
Practice Gap
Hyperhidrosis poses a considerable challenge for many amputees who use prosthetic devices, particularly at the interface between the residual limb and the prosthetic socket. The enclosed environment of the socket often leads to excessive sweating, which can compromise suction fit and increase the risk for skin chafing, irritation, and slippage. Persistent moisture also promotes bacterial and fungal growth, raising the likelihood of infections and foul odors within the socket. Research has shown that skin complications are highly prevalent among amputees, affecting up to 73.9% of this population in the United States.1 Commonly reported complications include wounds, abscesses, and blisters, many of which can be triggered or worsened by hyperhidrosis.2 Current treatment options for residual limb sweating include topical antiperspirants, botulinum toxin (BTX) injections, iontophoresis, and liner-liner socks.
While BTX commonly is used to treat hyperhidrosis in areas such as the palms and axillae, it typically is not considered as a first-line therapy for residual limb sweating; however, both BTX type A and type B have shown safety and effectiveness in managing hyperhidrosis in amputees, enhancing prosthetic use, and improving overall quality of life.3 Despite these benefits, BTX remains relatively underutilized for
Tools and Techniques
A 64-year-old man initially presented to our dermatology clinic after undergoing an above-the-knee amputation of the left leg 1 year prior. The amputation had been performed due to chronic prosthetic joint infections with Escherichia coli. He reported persistent sweating of the residual limb, which severely limited his use of a prosthesis and led to frequent falls.
During the initial visit, treatment options for primary hyperhidrosis including topical and injectable therapies were discussed. Due to a fear of needles, the patient chose topical treatment, with the option to pursue BTX injections later if better control was needed. An aluminum chloride hexahydrate prosthetic antiperspirant was prescribed for nightly application on the anterior and posterior
Botulinum toxin injections were administered in a grid-like pattern across the surface area where the residual limb made contact with the prosthetic. Using a surgical marker, the patient assisted the medical team in identifying the areas where sweating occurred most frequently. The area was divided into 4 equal sections, with each section treated per weekly interval sequentially over 4 weeks. The targeted areas included the left anterior (extending from the anterior tensor fasciae latae band to the lateral thigh) and left posterior residual limb (Figure 1 and eFigure 1, respectively).


The treated section was cleaned with an alcohol wipe prior to each injection, and 50 units of BTX (diluted to 2.5 units per 0.1 mL in bacteriostatic saline) were injected intradermally into each section (Figure 2 and eFigure 2). The injections were administered in rows, with the needle inserted at evenly spaced intervals approximately 1 inch apart. A total of 100 units were administered per section at each weekly appointment. The patient tolerated the procedure well, and no complications were observed.


Practice Implications
This staged approach to administering BTX ensures even distribution of the injections, optimizes hyperhidrosis control, minimizes the risk for complications, and allows for precise targeting of the affected areas to maximize therapeutic benefit. Following the initial procedure, our patient was scheduled for follow-ups approximately every 3 to 4 months starting from the first set of injections for each area. Over 9 months, the patient successfully completed 3 treatment sessions using this method. The patient reported improved quality of life after starting the BTX injections.
After evaluating the initial treatment outcomes with 100 units per section, the dosage was increased to 200 units per section to reduce the number of visits from 4 every 3 months to cover the entire area to 2 visits every 3 months. This adjustment aimed to optimize results and better manage the patient’s ongoing symptoms. At about 1 to 2 weeks after beginning treatment, the patient noticed decreased sweating and discomfort during his daily activities and reduced friction with his prosthetic leg. No adverse effects were noted with the increased dosage during a clinical visit.
Our case highlights the importance of ensuring equitable access to hyperhidrosis treatment. Dermatologists should prioritize patient-centered care by factoring in financial constraints when recommending therapies. In this patient’s case, offering a range of options including over-the-counter antiperspirants and prescription treatments allowed for a management plan tailored to his individual needs and circumstances.
DaxibotulinumtoxinA, known for its longer duration of action compared to other BTX formulations, presents a promising alternative for treating hyperhidrosis.4 However, a gap in care emerged for our patient when prescription antiperspirant was not covered by his insurance, and daxibotulinumtoxinA, which could have offered a more durable solution, was not yet available at our clinic for hyperhidrosis management. Expanding insurance coverage for effective prescription treatments and improving access to newer treatment options are crucial for enhancing patient outcomes and ensuring more equitable care.
Focusing dermatologic care on amputees presents distinct challenges and opportunities for improving their care and decreasing discomfort. Amputees, particularly those with residual limb hyperhidrosis, often experience additional discomfort and difficulty while using prosthetics, as excessive sweating can interfere with fit and function.5,6 Dermatologists should proactively address these specific needs by tailoring treatment accordingly. Incorporating targeted therapies, such as BTX injections, in addition to education on lifestyle modifications and managing treatment expectations, ensures comprehensive care that enhances both quality of life and functional outcomes. Engaging patients in discussions about all available options, including emerging therapies, is essential for improving care for this underserved population.
Practice Gap
Hyperhidrosis poses a considerable challenge for many amputees who use prosthetic devices, particularly at the interface between the residual limb and the prosthetic socket. The enclosed environment of the socket often leads to excessive sweating, which can compromise suction fit and increase the risk for skin chafing, irritation, and slippage. Persistent moisture also promotes bacterial and fungal growth, raising the likelihood of infections and foul odors within the socket. Research has shown that skin complications are highly prevalent among amputees, affecting up to 73.9% of this population in the United States.1 Commonly reported complications include wounds, abscesses, and blisters, many of which can be triggered or worsened by hyperhidrosis.2 Current treatment options for residual limb sweating include topical antiperspirants, botulinum toxin (BTX) injections, iontophoresis, and liner-liner socks.
While BTX commonly is used to treat hyperhidrosis in areas such as the palms and axillae, it typically is not considered as a first-line therapy for residual limb sweating; however, both BTX type A and type B have shown safety and effectiveness in managing hyperhidrosis in amputees, enhancing prosthetic use, and improving overall quality of life.3 Despite these benefits, BTX remains relatively underutilized for
Tools and Techniques
A 64-year-old man initially presented to our dermatology clinic after undergoing an above-the-knee amputation of the left leg 1 year prior. The amputation had been performed due to chronic prosthetic joint infections with Escherichia coli. He reported persistent sweating of the residual limb, which severely limited his use of a prosthesis and led to frequent falls.
During the initial visit, treatment options for primary hyperhidrosis including topical and injectable therapies were discussed. Due to a fear of needles, the patient chose topical treatment, with the option to pursue BTX injections later if better control was needed. An aluminum chloride hexahydrate prosthetic antiperspirant was prescribed for nightly application on the anterior and posterior
Botulinum toxin injections were administered in a grid-like pattern across the surface area where the residual limb made contact with the prosthetic. Using a surgical marker, the patient assisted the medical team in identifying the areas where sweating occurred most frequently. The area was divided into 4 equal sections, with each section treated per weekly interval sequentially over 4 weeks. The targeted areas included the left anterior (extending from the anterior tensor fasciae latae band to the lateral thigh) and left posterior residual limb (Figure 1 and eFigure 1, respectively).


The treated section was cleaned with an alcohol wipe prior to each injection, and 50 units of BTX (diluted to 2.5 units per 0.1 mL in bacteriostatic saline) were injected intradermally into each section (Figure 2 and eFigure 2). The injections were administered in rows, with the needle inserted at evenly spaced intervals approximately 1 inch apart. A total of 100 units were administered per section at each weekly appointment. The patient tolerated the procedure well, and no complications were observed.


Practice Implications
This staged approach to administering BTX ensures even distribution of the injections, optimizes hyperhidrosis control, minimizes the risk for complications, and allows for precise targeting of the affected areas to maximize therapeutic benefit. Following the initial procedure, our patient was scheduled for follow-ups approximately every 3 to 4 months starting from the first set of injections for each area. Over 9 months, the patient successfully completed 3 treatment sessions using this method. The patient reported improved quality of life after starting the BTX injections.
After evaluating the initial treatment outcomes with 100 units per section, the dosage was increased to 200 units per section to reduce the number of visits from 4 every 3 months to cover the entire area to 2 visits every 3 months. This adjustment aimed to optimize results and better manage the patient’s ongoing symptoms. At about 1 to 2 weeks after beginning treatment, the patient noticed decreased sweating and discomfort during his daily activities and reduced friction with his prosthetic leg. No adverse effects were noted with the increased dosage during a clinical visit.
Our case highlights the importance of ensuring equitable access to hyperhidrosis treatment. Dermatologists should prioritize patient-centered care by factoring in financial constraints when recommending therapies. In this patient’s case, offering a range of options including over-the-counter antiperspirants and prescription treatments allowed for a management plan tailored to his individual needs and circumstances.
DaxibotulinumtoxinA, known for its longer duration of action compared to other BTX formulations, presents a promising alternative for treating hyperhidrosis.4 However, a gap in care emerged for our patient when prescription antiperspirant was not covered by his insurance, and daxibotulinumtoxinA, which could have offered a more durable solution, was not yet available at our clinic for hyperhidrosis management. Expanding insurance coverage for effective prescription treatments and improving access to newer treatment options are crucial for enhancing patient outcomes and ensuring more equitable care.
Focusing dermatologic care on amputees presents distinct challenges and opportunities for improving their care and decreasing discomfort. Amputees, particularly those with residual limb hyperhidrosis, often experience additional discomfort and difficulty while using prosthetics, as excessive sweating can interfere with fit and function.5,6 Dermatologists should proactively address these specific needs by tailoring treatment accordingly. Incorporating targeted therapies, such as BTX injections, in addition to education on lifestyle modifications and managing treatment expectations, ensures comprehensive care that enhances both quality of life and functional outcomes. Engaging patients in discussions about all available options, including emerging therapies, is essential for improving care for this underserved population.
- Koc E, Tunca M, Akar A, et al. Skin problems in amputees: a descriptive study. Int J Dermatol. 2008;47:463–466. doi:10.1111/j.1365-4632.2008.03604.x
- Bui KM, Raugi GJ, Nguyen VQ, et al. Skin problems in individuals with lower-limb loss: literature review and proposed classification system. J Rehabil Res Dev. 2009;46:1085-1090. doi:10.1682/jrrd.2009.04.0052
- Rocha Melo J, Rodrigues MA, Caetano M, et al. Botulinum toxin in the treatment of residual limb hyperhidrosis: a systematic review. Rehabilitacion (Madr). 2023;57:100754. doi:10.1016/j.rh.2022.07.003
- Hansen C, Godfrey B, Wixom J, et al. Incidence, severity, and impact of hyperhidrosis in people with lower-limb amputation. J Rehabil Res Dev. 2015;52:31-40. doi:10.1682/JRRD.2014.04.0108
- Lannan FM, Powell J, Kim GM, et al. Hyperhidrosis of the residual limb: a narrative review of the measurement and treatment of excess perspiration affecting individuals with amputation. Prosthet Orthot Int. 2021;45:477-486. doi:10.1097/PXR.0000000000000040
- Pace S, Kentosh J. Managing residual limb hyperhidrosis in wounded warriors. Cutis. 2016;97:401-403.
- Koc E, Tunca M, Akar A, et al. Skin problems in amputees: a descriptive study. Int J Dermatol. 2008;47:463–466. doi:10.1111/j.1365-4632.2008.03604.x
- Bui KM, Raugi GJ, Nguyen VQ, et al. Skin problems in individuals with lower-limb loss: literature review and proposed classification system. J Rehabil Res Dev. 2009;46:1085-1090. doi:10.1682/jrrd.2009.04.0052
- Rocha Melo J, Rodrigues MA, Caetano M, et al. Botulinum toxin in the treatment of residual limb hyperhidrosis: a systematic review. Rehabilitacion (Madr). 2023;57:100754. doi:10.1016/j.rh.2022.07.003
- Hansen C, Godfrey B, Wixom J, et al. Incidence, severity, and impact of hyperhidrosis in people with lower-limb amputation. J Rehabil Res Dev. 2015;52:31-40. doi:10.1682/JRRD.2014.04.0108
- Lannan FM, Powell J, Kim GM, et al. Hyperhidrosis of the residual limb: a narrative review of the measurement and treatment of excess perspiration affecting individuals with amputation. Prosthet Orthot Int. 2021;45:477-486. doi:10.1097/PXR.0000000000000040
- Pace S, Kentosh J. Managing residual limb hyperhidrosis in wounded warriors. Cutis. 2016;97:401-403.
Botulinum Toxin as a Tool to Reduce Hyperhidrosis in Amputees
Botulinum Toxin as a Tool to Reduce Hyperhidrosis in Amputees
Dermatology Immediate Care: A Game Changer for the Health Care System?
Dermatology Immediate Care: A Game Changer for the Health Care System?
Emergency departments (EDs) and immediate care (IC) facilities often do not have prompt dermatologic care available for triage and treatment. Many EDs do not have staff dermatologists on call, instead relying on input from other specialists or quick outpatient dermatology appointments. It can be challenging to obtain a prompt appointment with a board-certified dermatologist, which is preferred for complex cases such as severe drug reactions or infection. In the United States, there are few well-established IC centers equipped to address dermatologic needs. The orthopedic specialty has modeled a concept that has led to the establishment of orthopedic urgent care/IC in many larger institutions,1 and many private practice clinics serve their communities as well. We present a rationale for why a similar IC concept for dermatology would be beneficial, particularly within a large institution or health system.
Dermatology Consultation Changes Disease Management
There is diagnostic and therapeutic utility in dermatology evaluation. In a prospective study of 591 patients who were either hospitalized or evaluated in an ED/urgent care setting, treatment was changed in more than 60% of cases when dermatology consultation was utilized.2 In another prospective review of 691 cases on an inpatient service, dermatology consultation resulted in treatment changes more than 80% of the time.3
Cellulitis has been a particularly well-studied diagnosis. Dermatologists often change the diagnosis of cellulitis in the hospital setting and reduce antibiotic exposure. In a prospective cohort study of 116 patients, 33.6% had their diagnosis of cellulitis changed to pseudocellulitis following evaluation by the dermatologist; of 34 patients who had started antibiotic therapy, 82.4% were recommended to discontinue the treatment, and all 39 patients with pseudocellulitis had a proven stable clinical course at 1-month follow-up.4 In another trial, 175 patients with presumed cellulitis were given standard management (provided by the medicine inpatient team) either alone or with the addition of dermatology consultation. Duration of antibiotic treatment (including intravenous therapy) was reduced when dermatology was consulted. Two weeks after discharge, patients who had dermatology consultations demonstrated greater clinical improvement.5
Improving ED and IC Access to Dermatology
Emergency department and IC teams across the United States work tirelessly to meet the demands of patients presenting with medically urgent conditions. In a study examining 861 ED cases, dermatology made up only 9.5% of specialist consultations, and in the opinion of the on-call dermatology resident, 51.0% (439/861) of cases warranted ED-level care.6
Data from the 2021 National Hospital Ambulatory Medical Care Survey showed that the mean wait time to see a physician, nurse, or physician assistant in an ED was 37.5 minutes, but wait times could range from less than 15 minutes to more than 6 hours.7 According to a study of 35,849 ED visits at nonfederal hospitals in the United States, only 47.7% of EDs admitted more than 90% of their patients within 6 hours.8 Moreover, perceived wait times in the ED have been shown to greatly impact patient satisfaction. Two predictors of perceived wait time include appropriate assessment of emergency level and the feeling of being forgotten.9 In a study of 2377 ED visits with primary dermatologic diagnoses, only 5.5% led to admission.10 This suggests many patients who come to the ED for dermatologic needs do not require inpatient hospital care. In these cases, patients with primary dermatologic concerns may experience longer ED wait times, as higher acuity or emergency cases take precedence. Studies also have shown that more vulnerable populations are utilizing ED visits most for primary dermatologic concerns.10,11 This includes individuals of lower income and/or those with Medicaid/Medicare or those without insurance.11 Predictors of high ED use for dermatologic concerns include prior frequent use of the ED (for nondermatologic concerns) instead of outpatient care, income below the poverty level, and lack of insurance; older individuals (>65 years) also were found to use the ED more frequently for dermatologic concerns when compared to younger individuals.10
Importantly, there is a great need for urgent dermatology consultation for pediatric patients. A single-institution study showed that over a 36-month period, there were 347 pediatric dermatology consultations from the pediatric ED mostly for children aged 0 days to 5 years; nearly half of these consultations required outpatient clinic follow-up.12 However, dermatology outpatient follow-up can be difficult to obtain, especially for vulnerable groups. In a study of 611 dermatology clinics, patients with Medicaid were shown to have longer wait times and less success in obtaining dermatology appointments compared to those with Medicare or private insurance.13 Only about 30% of private dermatology practices accept Medicaid patients, likely pushing these patients toward utilization of emergency services for dermatologic concerns.13,14
There is a clear role for a dermatology IC in our health care system, and the concept already has been identified and trialed in several institutions. At Oregon Health and Science University (Portland, Oregon), a retrospective chart review of patients with diagnoses of Morgellons disease and neurotic excoriations seen in dermatology urgent care between 2018 and 2020 showed an 88% decrease in annual rates of health care visits and a 77% decrease in ED visits after dermatology services were engaged compared to before the opening of the dermatology urgent care.15 Another study showed that uninsured or self-pay patients were more than 14 times more likely to access dermatology urgent care than to schedule a routine clinic appointment, suggesting that there is a barrier to making outpatient dermatologic appointments for uninsured patients. An urgent access model may facilitate the ability of underinsured patients to access care.16
Improving Dermatology Access for Other Specialties
Needs for dermatologic care are encountered in many other specialties. Having direct access to immediate dermatologic treatment is best for patients and may avoid inpatient care and trips to the ED for consultation access. Ideally, a dermatology IC would allow direct care to be provided alongside the oncology outpatient team. New immunologic therapies (cytotoxic T-lymphocyte–associated protein 4 and programmed cell death protein 1/programmed death-ligand 1 treatments) can cause dermatologic reactions in more than 40% of patients.17 Paraneoplastic syndromes can manifest with cutaneous symptoms, as can acute graft-vs-host-disease.18 In a study at Memorial Sloan Kettering (New York, New York) analyzing 426 same-day outpatient dermatology consultations, 17% of patients experienced interruptions in their cancer therapy, but 83% responded quickly to dermatologic treatment and resumed oncologic therapy—19% of them at a reduced dose.19 This is an important demonstration of prompt dermatologic consultation in an outpatient setting reducing interruptions to anticancer therapy. The heterogeneity of the cutaneous reactions seen from oncologic and immunomodulatory medications is profound, with more than 140 different types of skin-specific reactions.20
Solid-organ transplant recipients also could benefit from urgent access to dermatology services. These patients are at a much higher risk for skin cancers, and a study showed that those who receive referrals to dermatology are seen sooner after transplantation (5.6 years) than those who self-refer (7.2 years). Importantly, annual skin cancer screenings are recommended to begin 1 year after transplantation.21
Direct access to dermatology care could benefit patients with complicated rheumatologic conditions who present with skin findings; for example, patients with lupus erythematosus or dermatomyositis can have a spectrum of disease ranging from skin-predominant to systemic manifestations. Identification and treatment of such diseases require collaboration between dermatologists and rheumatologists.22 Likewise, a study of a joint rheumatology-dermatology clinic for psoriatic arthritis showed that a multidisciplinary approach to management leads to decreased time for patients to obtain proper rheumatologic and dermatologic examination and a faster time to diagnosis; however, such multidisciplinary clinic models and approaches to care often are found only at large university-based hospitals.23 In a patient population for whom time to diagnosis is crucial to avoid permanent changes such as joint destruction, a dermatology IC could fill this role in community hospitals and clinics. A dermatology IC also can serve patients with specific diagnoses who would benefit from more direct access to care; for example, in 2017 there were 131,430 ED visits for hidradenitis suppurativa (HS) in the United States. While HS is not uncommon, it usually is underdiagnosed because it can be challenging to differentiate from an uncomplicated abscess. Emergency department visits often are utilized for first-time presentations as well as flares of HS. In these situations, ED doctors can provide palliative treatment, but prompt referrals to dermatologists should be made for disease management to decrease recurrence.24
Final Thoughts
A huge caveat to the dermatology urgent care system is determining what is deemed “urgent.” We propose starting with a referral-based system only from other physicians (including IC and urgent care) rather than having patients walk in directly. Ideally, as support and staff increases, the availability can increase as well. In our institution, we suggested half-day clinics staffed by varying physicians, with compensation models similar to an ED or IC physician rather than by productivity. Each group considering this kind of addition to patient care will need to assess these points in building an IC for dermatology. The University of Pennsylvania’s (Philadelphia, Pennsylvania) system of rapid-access clinics to facilitate access to care for patients requiring urgent appointments may function as a model for future similar clinics.25 Creating a specialized IC/urgent care is not a novel concept. Orthopedic urgent care centers have increased greatly in the past decade, reducing ED burden for musculoskeletal complaints. In a study evaluating the utility of orthopedic urgent care settings, time to see an orthopedic specialist and cost were both greatly reduced with this system.1 The same has been shown in same-day access ophthalmology clinics, which are organized similarly to an urgent care.26
In 2021, there were 107.4 million treat-and-release visits to the ED in the United States for a total cost of $80.3 billion.27 This emphasizes the need to consider care models that not only provide excellent clinical care and treat the most acute diagnoses promptly and accurately but also reduce overall costs. While this may be convoluted for other specialties given the difficulty of having patients self-triage, dermatologic concerns are similar to orthopedic concerns for the patient to decipher the etiology of the concern. As in orthopedics, a dermatology IC could function similarly, increasing access, decreasing ED and IC wait times, saving overall health care spending, and allowing underserved and publicly insured individuals to have improved, prompt care.
- Anderson TJ, Althausen PL. The role of dedicated musculoskeletal urgent care centers in reducing cost and improving access to orthopaedic care. J Orthop Trauma. 2016;30:S3-S6.
- Falanga V, Schachner LA, Rae V, et al. Dermatologic consultations in the hospital setting. Arch Dermatol. 1994;130:1022-1025.
- Galimberti F, Guren L, Fernandez AP, et al. Dermatology consultations significantly contribute quality to care of hospitalized patients: a prospective study of dermatology inpatient consults at a tertiary care center. Int J Dermatol. 2016;55:E547-E551.
- Li DG, Xia FD, Khosravi H, et al. Outcomes of early dermatology consultation for inpatients diagnosed with cellulitis. JAMA Dermatol. 2018;154:537-543.
- Ko LN, Garza-Mayers AC, St John J, et al. Effect of dermatology consultation on outcomes for patients with presumed cellulitis: a randomized clinical trial. JAMA Dermatol. 2018;154:529-536.
- Grillo E, Vañó-Galván S, Jiménez-Gómez N, et al. Dermatologic emergencies: descriptive analysis of 861 patients in a tertiary care teaching hospital. Actas Dermosifiliogr. 2013;104:316-324.
- National Center for Health Statistics. National Hospital Ambulatory Medical Care Survey, 2021. Accessed September 23, 2025. https://www.cdc.gov/nchs/data/nhamcs/web_tables/2021-nhamcs-ed-web-tables-508.pdf
- Horwitz LI, Green J, Bradley EH. US emergency department performance on wait time and length of visit. Ann Emerg Med. 2010;55:133-141.
- Spechbach H, Rochat J, Gaspoz JM, et al. Patients’ time perception in the waiting room of an ambulatory emergency unit: a cross-sectional study. BMC Emerg Med. 2019;19:41.
- Yang JJ, Maloney NJ, Bach DQ, et al. Dermatology in the emergency department: prescriptions, rates of inpatient admission, and predictors of high utilization in the United States from 1996 to 2012. J Am Acad Dermatol. 2021;84:1480-1483.
- Chen CL, Fitzpatrick L, Kamel H. Who uses the emergency department for dermatologic care? a statewide analysis. J Am Acad Dermatol. 2014;71:308-313.
- Moon AT, Castelo-Soccio L, Yan AC. Emergency department utilization of pediatric dermatology (PD) consultations. J Am Acad Dermatol. 2016;74:1173-1177.
- Creadore A, Desai S, Li SJ, et al. Insurance acceptance, appointment wait time, and dermatologist access across practice types in the US. JAMA Dermatol. 2021;157:181-188.
- Mazmudar RS, Gupta N, Desai BJ, et al. Dermatologist appointment access and waiting times: a comparative study of insurance types. J Am Acad Dermatol. 2020;83:1468-1470.
- Johnson J, Cutler B, Latour E, et al. Dermatology urgent care model reduces costs and healthcare utilization for psychodermatology patients-a retrospective chart review. Dermatol Online J. 2022;28:5.
- Wintringham JA, Strock DM, Perkins-Holtsclaw K, et al. Dermatology in the urgent care setting: a retrospective review of patients seen in an urgent access dermatology clinic. J Am Acad Dermatol. 2023;89:1271-1273.
- Yoo MJ, Long B, Brady WJ, et al. Immune checkpoint inhibitors: an emergency medicine focused review. Am J Emerg Med. 2021;50:335-344.
- Merlo G, Cozzani E, Canale F, et al. Cutaneous manifestations of hematologic malignancies the experience of an Italian dermatology department. Hematol Oncol. 2019;37:285-290.
- Barrios D, Phillips G, Freites-Martinez A, et al. Outpatient dermatology consultations for oncology patients with acute dermatologic adverse events impact anticancer therapy interruption: a retrospective study.J Eur Acad Dermatol Venereol. 2020;34:1340-1347.
- Salah S, Kerob D, Pages Laurent C, et al. Evaluation of anticancer therapy-related dermatologic adverse events: insights from Food and Drug Administration’s Adverse Event Reporting System dataset. J Am Acad Dermatol. 2024;91:863-871. doi:10.1016/j.jaad.2024.07.1456
- Shope C, Andrews L, Girvin A, et al. Referrals to dermatology following solid organ transplant. J Am Acad Dermatol. 2023;88:1159-1160. doi:10.1016/j.jaad.2022.11.052
- Werth VP, Askanase AD, Lundberg IE. Importance of collaboration of dermatology and rheumatology to advance the field for lupus and dermatomyositis. Int J Womens Dermatol. 2021;7:583-587.
- Ziob J, Behning C, Brossart P, et al. Specialized dermatological-rheumatological patient management improves diagnostic outcome and patient journey in psoriasis and psoriatic arthritis: a four-year analysis. BMC Rheumatol. 2021;5:1-8. doi:10.1186/s41927-021-00217-z
- Okun MM, Flamm A, Werley EB, et al. Hidradenitis suppurativa: diagnosis and management in the emergency department. J Emerg Med. 2022;63:636-644.
- Jayakumar KL, Samimi SS, Vittorio CC, et al. Expediting patient appointments with dermatology rapid access clinics. Dermatol Online J. 2018;24:13030/qt2zv07510.
- Singman EL, Smith K, Mehta R, et al. Cost and visit duration of same-day access at an academic ophthalmology department vs emergency department. JAMA Ophthalmol. 2019;137:729-735. doi:10.1001/jamaophthalmol.2019.0864
- Roemer M. Costs of treat-and-release emergency department visits in the United States, 2021. Agency for Healthcare Research and Quality. Published September 2024. Accessed September 16, 2025. https://hcup-us.ahrq.gov/reports/statbriefs/sb311-ED-visit-costs-2021.pdf
Emergency departments (EDs) and immediate care (IC) facilities often do not have prompt dermatologic care available for triage and treatment. Many EDs do not have staff dermatologists on call, instead relying on input from other specialists or quick outpatient dermatology appointments. It can be challenging to obtain a prompt appointment with a board-certified dermatologist, which is preferred for complex cases such as severe drug reactions or infection. In the United States, there are few well-established IC centers equipped to address dermatologic needs. The orthopedic specialty has modeled a concept that has led to the establishment of orthopedic urgent care/IC in many larger institutions,1 and many private practice clinics serve their communities as well. We present a rationale for why a similar IC concept for dermatology would be beneficial, particularly within a large institution or health system.
Dermatology Consultation Changes Disease Management
There is diagnostic and therapeutic utility in dermatology evaluation. In a prospective study of 591 patients who were either hospitalized or evaluated in an ED/urgent care setting, treatment was changed in more than 60% of cases when dermatology consultation was utilized.2 In another prospective review of 691 cases on an inpatient service, dermatology consultation resulted in treatment changes more than 80% of the time.3
Cellulitis has been a particularly well-studied diagnosis. Dermatologists often change the diagnosis of cellulitis in the hospital setting and reduce antibiotic exposure. In a prospective cohort study of 116 patients, 33.6% had their diagnosis of cellulitis changed to pseudocellulitis following evaluation by the dermatologist; of 34 patients who had started antibiotic therapy, 82.4% were recommended to discontinue the treatment, and all 39 patients with pseudocellulitis had a proven stable clinical course at 1-month follow-up.4 In another trial, 175 patients with presumed cellulitis were given standard management (provided by the medicine inpatient team) either alone or with the addition of dermatology consultation. Duration of antibiotic treatment (including intravenous therapy) was reduced when dermatology was consulted. Two weeks after discharge, patients who had dermatology consultations demonstrated greater clinical improvement.5
Improving ED and IC Access to Dermatology
Emergency department and IC teams across the United States work tirelessly to meet the demands of patients presenting with medically urgent conditions. In a study examining 861 ED cases, dermatology made up only 9.5% of specialist consultations, and in the opinion of the on-call dermatology resident, 51.0% (439/861) of cases warranted ED-level care.6
Data from the 2021 National Hospital Ambulatory Medical Care Survey showed that the mean wait time to see a physician, nurse, or physician assistant in an ED was 37.5 minutes, but wait times could range from less than 15 minutes to more than 6 hours.7 According to a study of 35,849 ED visits at nonfederal hospitals in the United States, only 47.7% of EDs admitted more than 90% of their patients within 6 hours.8 Moreover, perceived wait times in the ED have been shown to greatly impact patient satisfaction. Two predictors of perceived wait time include appropriate assessment of emergency level and the feeling of being forgotten.9 In a study of 2377 ED visits with primary dermatologic diagnoses, only 5.5% led to admission.10 This suggests many patients who come to the ED for dermatologic needs do not require inpatient hospital care. In these cases, patients with primary dermatologic concerns may experience longer ED wait times, as higher acuity or emergency cases take precedence. Studies also have shown that more vulnerable populations are utilizing ED visits most for primary dermatologic concerns.10,11 This includes individuals of lower income and/or those with Medicaid/Medicare or those without insurance.11 Predictors of high ED use for dermatologic concerns include prior frequent use of the ED (for nondermatologic concerns) instead of outpatient care, income below the poverty level, and lack of insurance; older individuals (>65 years) also were found to use the ED more frequently for dermatologic concerns when compared to younger individuals.10
Importantly, there is a great need for urgent dermatology consultation for pediatric patients. A single-institution study showed that over a 36-month period, there were 347 pediatric dermatology consultations from the pediatric ED mostly for children aged 0 days to 5 years; nearly half of these consultations required outpatient clinic follow-up.12 However, dermatology outpatient follow-up can be difficult to obtain, especially for vulnerable groups. In a study of 611 dermatology clinics, patients with Medicaid were shown to have longer wait times and less success in obtaining dermatology appointments compared to those with Medicare or private insurance.13 Only about 30% of private dermatology practices accept Medicaid patients, likely pushing these patients toward utilization of emergency services for dermatologic concerns.13,14
There is a clear role for a dermatology IC in our health care system, and the concept already has been identified and trialed in several institutions. At Oregon Health and Science University (Portland, Oregon), a retrospective chart review of patients with diagnoses of Morgellons disease and neurotic excoriations seen in dermatology urgent care between 2018 and 2020 showed an 88% decrease in annual rates of health care visits and a 77% decrease in ED visits after dermatology services were engaged compared to before the opening of the dermatology urgent care.15 Another study showed that uninsured or self-pay patients were more than 14 times more likely to access dermatology urgent care than to schedule a routine clinic appointment, suggesting that there is a barrier to making outpatient dermatologic appointments for uninsured patients. An urgent access model may facilitate the ability of underinsured patients to access care.16
Improving Dermatology Access for Other Specialties
Needs for dermatologic care are encountered in many other specialties. Having direct access to immediate dermatologic treatment is best for patients and may avoid inpatient care and trips to the ED for consultation access. Ideally, a dermatology IC would allow direct care to be provided alongside the oncology outpatient team. New immunologic therapies (cytotoxic T-lymphocyte–associated protein 4 and programmed cell death protein 1/programmed death-ligand 1 treatments) can cause dermatologic reactions in more than 40% of patients.17 Paraneoplastic syndromes can manifest with cutaneous symptoms, as can acute graft-vs-host-disease.18 In a study at Memorial Sloan Kettering (New York, New York) analyzing 426 same-day outpatient dermatology consultations, 17% of patients experienced interruptions in their cancer therapy, but 83% responded quickly to dermatologic treatment and resumed oncologic therapy—19% of them at a reduced dose.19 This is an important demonstration of prompt dermatologic consultation in an outpatient setting reducing interruptions to anticancer therapy. The heterogeneity of the cutaneous reactions seen from oncologic and immunomodulatory medications is profound, with more than 140 different types of skin-specific reactions.20
Solid-organ transplant recipients also could benefit from urgent access to dermatology services. These patients are at a much higher risk for skin cancers, and a study showed that those who receive referrals to dermatology are seen sooner after transplantation (5.6 years) than those who self-refer (7.2 years). Importantly, annual skin cancer screenings are recommended to begin 1 year after transplantation.21
Direct access to dermatology care could benefit patients with complicated rheumatologic conditions who present with skin findings; for example, patients with lupus erythematosus or dermatomyositis can have a spectrum of disease ranging from skin-predominant to systemic manifestations. Identification and treatment of such diseases require collaboration between dermatologists and rheumatologists.22 Likewise, a study of a joint rheumatology-dermatology clinic for psoriatic arthritis showed that a multidisciplinary approach to management leads to decreased time for patients to obtain proper rheumatologic and dermatologic examination and a faster time to diagnosis; however, such multidisciplinary clinic models and approaches to care often are found only at large university-based hospitals.23 In a patient population for whom time to diagnosis is crucial to avoid permanent changes such as joint destruction, a dermatology IC could fill this role in community hospitals and clinics. A dermatology IC also can serve patients with specific diagnoses who would benefit from more direct access to care; for example, in 2017 there were 131,430 ED visits for hidradenitis suppurativa (HS) in the United States. While HS is not uncommon, it usually is underdiagnosed because it can be challenging to differentiate from an uncomplicated abscess. Emergency department visits often are utilized for first-time presentations as well as flares of HS. In these situations, ED doctors can provide palliative treatment, but prompt referrals to dermatologists should be made for disease management to decrease recurrence.24
Final Thoughts
A huge caveat to the dermatology urgent care system is determining what is deemed “urgent.” We propose starting with a referral-based system only from other physicians (including IC and urgent care) rather than having patients walk in directly. Ideally, as support and staff increases, the availability can increase as well. In our institution, we suggested half-day clinics staffed by varying physicians, with compensation models similar to an ED or IC physician rather than by productivity. Each group considering this kind of addition to patient care will need to assess these points in building an IC for dermatology. The University of Pennsylvania’s (Philadelphia, Pennsylvania) system of rapid-access clinics to facilitate access to care for patients requiring urgent appointments may function as a model for future similar clinics.25 Creating a specialized IC/urgent care is not a novel concept. Orthopedic urgent care centers have increased greatly in the past decade, reducing ED burden for musculoskeletal complaints. In a study evaluating the utility of orthopedic urgent care settings, time to see an orthopedic specialist and cost were both greatly reduced with this system.1 The same has been shown in same-day access ophthalmology clinics, which are organized similarly to an urgent care.26
In 2021, there were 107.4 million treat-and-release visits to the ED in the United States for a total cost of $80.3 billion.27 This emphasizes the need to consider care models that not only provide excellent clinical care and treat the most acute diagnoses promptly and accurately but also reduce overall costs. While this may be convoluted for other specialties given the difficulty of having patients self-triage, dermatologic concerns are similar to orthopedic concerns for the patient to decipher the etiology of the concern. As in orthopedics, a dermatology IC could function similarly, increasing access, decreasing ED and IC wait times, saving overall health care spending, and allowing underserved and publicly insured individuals to have improved, prompt care.
Emergency departments (EDs) and immediate care (IC) facilities often do not have prompt dermatologic care available for triage and treatment. Many EDs do not have staff dermatologists on call, instead relying on input from other specialists or quick outpatient dermatology appointments. It can be challenging to obtain a prompt appointment with a board-certified dermatologist, which is preferred for complex cases such as severe drug reactions or infection. In the United States, there are few well-established IC centers equipped to address dermatologic needs. The orthopedic specialty has modeled a concept that has led to the establishment of orthopedic urgent care/IC in many larger institutions,1 and many private practice clinics serve their communities as well. We present a rationale for why a similar IC concept for dermatology would be beneficial, particularly within a large institution or health system.
Dermatology Consultation Changes Disease Management
There is diagnostic and therapeutic utility in dermatology evaluation. In a prospective study of 591 patients who were either hospitalized or evaluated in an ED/urgent care setting, treatment was changed in more than 60% of cases when dermatology consultation was utilized.2 In another prospective review of 691 cases on an inpatient service, dermatology consultation resulted in treatment changes more than 80% of the time.3
Cellulitis has been a particularly well-studied diagnosis. Dermatologists often change the diagnosis of cellulitis in the hospital setting and reduce antibiotic exposure. In a prospective cohort study of 116 patients, 33.6% had their diagnosis of cellulitis changed to pseudocellulitis following evaluation by the dermatologist; of 34 patients who had started antibiotic therapy, 82.4% were recommended to discontinue the treatment, and all 39 patients with pseudocellulitis had a proven stable clinical course at 1-month follow-up.4 In another trial, 175 patients with presumed cellulitis were given standard management (provided by the medicine inpatient team) either alone or with the addition of dermatology consultation. Duration of antibiotic treatment (including intravenous therapy) was reduced when dermatology was consulted. Two weeks after discharge, patients who had dermatology consultations demonstrated greater clinical improvement.5
Improving ED and IC Access to Dermatology
Emergency department and IC teams across the United States work tirelessly to meet the demands of patients presenting with medically urgent conditions. In a study examining 861 ED cases, dermatology made up only 9.5% of specialist consultations, and in the opinion of the on-call dermatology resident, 51.0% (439/861) of cases warranted ED-level care.6
Data from the 2021 National Hospital Ambulatory Medical Care Survey showed that the mean wait time to see a physician, nurse, or physician assistant in an ED was 37.5 minutes, but wait times could range from less than 15 minutes to more than 6 hours.7 According to a study of 35,849 ED visits at nonfederal hospitals in the United States, only 47.7% of EDs admitted more than 90% of their patients within 6 hours.8 Moreover, perceived wait times in the ED have been shown to greatly impact patient satisfaction. Two predictors of perceived wait time include appropriate assessment of emergency level and the feeling of being forgotten.9 In a study of 2377 ED visits with primary dermatologic diagnoses, only 5.5% led to admission.10 This suggests many patients who come to the ED for dermatologic needs do not require inpatient hospital care. In these cases, patients with primary dermatologic concerns may experience longer ED wait times, as higher acuity or emergency cases take precedence. Studies also have shown that more vulnerable populations are utilizing ED visits most for primary dermatologic concerns.10,11 This includes individuals of lower income and/or those with Medicaid/Medicare or those without insurance.11 Predictors of high ED use for dermatologic concerns include prior frequent use of the ED (for nondermatologic concerns) instead of outpatient care, income below the poverty level, and lack of insurance; older individuals (>65 years) also were found to use the ED more frequently for dermatologic concerns when compared to younger individuals.10
Importantly, there is a great need for urgent dermatology consultation for pediatric patients. A single-institution study showed that over a 36-month period, there were 347 pediatric dermatology consultations from the pediatric ED mostly for children aged 0 days to 5 years; nearly half of these consultations required outpatient clinic follow-up.12 However, dermatology outpatient follow-up can be difficult to obtain, especially for vulnerable groups. In a study of 611 dermatology clinics, patients with Medicaid were shown to have longer wait times and less success in obtaining dermatology appointments compared to those with Medicare or private insurance.13 Only about 30% of private dermatology practices accept Medicaid patients, likely pushing these patients toward utilization of emergency services for dermatologic concerns.13,14
There is a clear role for a dermatology IC in our health care system, and the concept already has been identified and trialed in several institutions. At Oregon Health and Science University (Portland, Oregon), a retrospective chart review of patients with diagnoses of Morgellons disease and neurotic excoriations seen in dermatology urgent care between 2018 and 2020 showed an 88% decrease in annual rates of health care visits and a 77% decrease in ED visits after dermatology services were engaged compared to before the opening of the dermatology urgent care.15 Another study showed that uninsured or self-pay patients were more than 14 times more likely to access dermatology urgent care than to schedule a routine clinic appointment, suggesting that there is a barrier to making outpatient dermatologic appointments for uninsured patients. An urgent access model may facilitate the ability of underinsured patients to access care.16
Improving Dermatology Access for Other Specialties
Needs for dermatologic care are encountered in many other specialties. Having direct access to immediate dermatologic treatment is best for patients and may avoid inpatient care and trips to the ED for consultation access. Ideally, a dermatology IC would allow direct care to be provided alongside the oncology outpatient team. New immunologic therapies (cytotoxic T-lymphocyte–associated protein 4 and programmed cell death protein 1/programmed death-ligand 1 treatments) can cause dermatologic reactions in more than 40% of patients.17 Paraneoplastic syndromes can manifest with cutaneous symptoms, as can acute graft-vs-host-disease.18 In a study at Memorial Sloan Kettering (New York, New York) analyzing 426 same-day outpatient dermatology consultations, 17% of patients experienced interruptions in their cancer therapy, but 83% responded quickly to dermatologic treatment and resumed oncologic therapy—19% of them at a reduced dose.19 This is an important demonstration of prompt dermatologic consultation in an outpatient setting reducing interruptions to anticancer therapy. The heterogeneity of the cutaneous reactions seen from oncologic and immunomodulatory medications is profound, with more than 140 different types of skin-specific reactions.20
Solid-organ transplant recipients also could benefit from urgent access to dermatology services. These patients are at a much higher risk for skin cancers, and a study showed that those who receive referrals to dermatology are seen sooner after transplantation (5.6 years) than those who self-refer (7.2 years). Importantly, annual skin cancer screenings are recommended to begin 1 year after transplantation.21
Direct access to dermatology care could benefit patients with complicated rheumatologic conditions who present with skin findings; for example, patients with lupus erythematosus or dermatomyositis can have a spectrum of disease ranging from skin-predominant to systemic manifestations. Identification and treatment of such diseases require collaboration between dermatologists and rheumatologists.22 Likewise, a study of a joint rheumatology-dermatology clinic for psoriatic arthritis showed that a multidisciplinary approach to management leads to decreased time for patients to obtain proper rheumatologic and dermatologic examination and a faster time to diagnosis; however, such multidisciplinary clinic models and approaches to care often are found only at large university-based hospitals.23 In a patient population for whom time to diagnosis is crucial to avoid permanent changes such as joint destruction, a dermatology IC could fill this role in community hospitals and clinics. A dermatology IC also can serve patients with specific diagnoses who would benefit from more direct access to care; for example, in 2017 there were 131,430 ED visits for hidradenitis suppurativa (HS) in the United States. While HS is not uncommon, it usually is underdiagnosed because it can be challenging to differentiate from an uncomplicated abscess. Emergency department visits often are utilized for first-time presentations as well as flares of HS. In these situations, ED doctors can provide palliative treatment, but prompt referrals to dermatologists should be made for disease management to decrease recurrence.24
Final Thoughts
A huge caveat to the dermatology urgent care system is determining what is deemed “urgent.” We propose starting with a referral-based system only from other physicians (including IC and urgent care) rather than having patients walk in directly. Ideally, as support and staff increases, the availability can increase as well. In our institution, we suggested half-day clinics staffed by varying physicians, with compensation models similar to an ED or IC physician rather than by productivity. Each group considering this kind of addition to patient care will need to assess these points in building an IC for dermatology. The University of Pennsylvania’s (Philadelphia, Pennsylvania) system of rapid-access clinics to facilitate access to care for patients requiring urgent appointments may function as a model for future similar clinics.25 Creating a specialized IC/urgent care is not a novel concept. Orthopedic urgent care centers have increased greatly in the past decade, reducing ED burden for musculoskeletal complaints. In a study evaluating the utility of orthopedic urgent care settings, time to see an orthopedic specialist and cost were both greatly reduced with this system.1 The same has been shown in same-day access ophthalmology clinics, which are organized similarly to an urgent care.26
In 2021, there were 107.4 million treat-and-release visits to the ED in the United States for a total cost of $80.3 billion.27 This emphasizes the need to consider care models that not only provide excellent clinical care and treat the most acute diagnoses promptly and accurately but also reduce overall costs. While this may be convoluted for other specialties given the difficulty of having patients self-triage, dermatologic concerns are similar to orthopedic concerns for the patient to decipher the etiology of the concern. As in orthopedics, a dermatology IC could function similarly, increasing access, decreasing ED and IC wait times, saving overall health care spending, and allowing underserved and publicly insured individuals to have improved, prompt care.
- Anderson TJ, Althausen PL. The role of dedicated musculoskeletal urgent care centers in reducing cost and improving access to orthopaedic care. J Orthop Trauma. 2016;30:S3-S6.
- Falanga V, Schachner LA, Rae V, et al. Dermatologic consultations in the hospital setting. Arch Dermatol. 1994;130:1022-1025.
- Galimberti F, Guren L, Fernandez AP, et al. Dermatology consultations significantly contribute quality to care of hospitalized patients: a prospective study of dermatology inpatient consults at a tertiary care center. Int J Dermatol. 2016;55:E547-E551.
- Li DG, Xia FD, Khosravi H, et al. Outcomes of early dermatology consultation for inpatients diagnosed with cellulitis. JAMA Dermatol. 2018;154:537-543.
- Ko LN, Garza-Mayers AC, St John J, et al. Effect of dermatology consultation on outcomes for patients with presumed cellulitis: a randomized clinical trial. JAMA Dermatol. 2018;154:529-536.
- Grillo E, Vañó-Galván S, Jiménez-Gómez N, et al. Dermatologic emergencies: descriptive analysis of 861 patients in a tertiary care teaching hospital. Actas Dermosifiliogr. 2013;104:316-324.
- National Center for Health Statistics. National Hospital Ambulatory Medical Care Survey, 2021. Accessed September 23, 2025. https://www.cdc.gov/nchs/data/nhamcs/web_tables/2021-nhamcs-ed-web-tables-508.pdf
- Horwitz LI, Green J, Bradley EH. US emergency department performance on wait time and length of visit. Ann Emerg Med. 2010;55:133-141.
- Spechbach H, Rochat J, Gaspoz JM, et al. Patients’ time perception in the waiting room of an ambulatory emergency unit: a cross-sectional study. BMC Emerg Med. 2019;19:41.
- Yang JJ, Maloney NJ, Bach DQ, et al. Dermatology in the emergency department: prescriptions, rates of inpatient admission, and predictors of high utilization in the United States from 1996 to 2012. J Am Acad Dermatol. 2021;84:1480-1483.
- Chen CL, Fitzpatrick L, Kamel H. Who uses the emergency department for dermatologic care? a statewide analysis. J Am Acad Dermatol. 2014;71:308-313.
- Moon AT, Castelo-Soccio L, Yan AC. Emergency department utilization of pediatric dermatology (PD) consultations. J Am Acad Dermatol. 2016;74:1173-1177.
- Creadore A, Desai S, Li SJ, et al. Insurance acceptance, appointment wait time, and dermatologist access across practice types in the US. JAMA Dermatol. 2021;157:181-188.
- Mazmudar RS, Gupta N, Desai BJ, et al. Dermatologist appointment access and waiting times: a comparative study of insurance types. J Am Acad Dermatol. 2020;83:1468-1470.
- Johnson J, Cutler B, Latour E, et al. Dermatology urgent care model reduces costs and healthcare utilization for psychodermatology patients-a retrospective chart review. Dermatol Online J. 2022;28:5.
- Wintringham JA, Strock DM, Perkins-Holtsclaw K, et al. Dermatology in the urgent care setting: a retrospective review of patients seen in an urgent access dermatology clinic. J Am Acad Dermatol. 2023;89:1271-1273.
- Yoo MJ, Long B, Brady WJ, et al. Immune checkpoint inhibitors: an emergency medicine focused review. Am J Emerg Med. 2021;50:335-344.
- Merlo G, Cozzani E, Canale F, et al. Cutaneous manifestations of hematologic malignancies the experience of an Italian dermatology department. Hematol Oncol. 2019;37:285-290.
- Barrios D, Phillips G, Freites-Martinez A, et al. Outpatient dermatology consultations for oncology patients with acute dermatologic adverse events impact anticancer therapy interruption: a retrospective study.J Eur Acad Dermatol Venereol. 2020;34:1340-1347.
- Salah S, Kerob D, Pages Laurent C, et al. Evaluation of anticancer therapy-related dermatologic adverse events: insights from Food and Drug Administration’s Adverse Event Reporting System dataset. J Am Acad Dermatol. 2024;91:863-871. doi:10.1016/j.jaad.2024.07.1456
- Shope C, Andrews L, Girvin A, et al. Referrals to dermatology following solid organ transplant. J Am Acad Dermatol. 2023;88:1159-1160. doi:10.1016/j.jaad.2022.11.052
- Werth VP, Askanase AD, Lundberg IE. Importance of collaboration of dermatology and rheumatology to advance the field for lupus and dermatomyositis. Int J Womens Dermatol. 2021;7:583-587.
- Ziob J, Behning C, Brossart P, et al. Specialized dermatological-rheumatological patient management improves diagnostic outcome and patient journey in psoriasis and psoriatic arthritis: a four-year analysis. BMC Rheumatol. 2021;5:1-8. doi:10.1186/s41927-021-00217-z
- Okun MM, Flamm A, Werley EB, et al. Hidradenitis suppurativa: diagnosis and management in the emergency department. J Emerg Med. 2022;63:636-644.
- Jayakumar KL, Samimi SS, Vittorio CC, et al. Expediting patient appointments with dermatology rapid access clinics. Dermatol Online J. 2018;24:13030/qt2zv07510.
- Singman EL, Smith K, Mehta R, et al. Cost and visit duration of same-day access at an academic ophthalmology department vs emergency department. JAMA Ophthalmol. 2019;137:729-735. doi:10.1001/jamaophthalmol.2019.0864
- Roemer M. Costs of treat-and-release emergency department visits in the United States, 2021. Agency for Healthcare Research and Quality. Published September 2024. Accessed September 16, 2025. https://hcup-us.ahrq.gov/reports/statbriefs/sb311-ED-visit-costs-2021.pdf
- Anderson TJ, Althausen PL. The role of dedicated musculoskeletal urgent care centers in reducing cost and improving access to orthopaedic care. J Orthop Trauma. 2016;30:S3-S6.
- Falanga V, Schachner LA, Rae V, et al. Dermatologic consultations in the hospital setting. Arch Dermatol. 1994;130:1022-1025.
- Galimberti F, Guren L, Fernandez AP, et al. Dermatology consultations significantly contribute quality to care of hospitalized patients: a prospective study of dermatology inpatient consults at a tertiary care center. Int J Dermatol. 2016;55:E547-E551.
- Li DG, Xia FD, Khosravi H, et al. Outcomes of early dermatology consultation for inpatients diagnosed with cellulitis. JAMA Dermatol. 2018;154:537-543.
- Ko LN, Garza-Mayers AC, St John J, et al. Effect of dermatology consultation on outcomes for patients with presumed cellulitis: a randomized clinical trial. JAMA Dermatol. 2018;154:529-536.
- Grillo E, Vañó-Galván S, Jiménez-Gómez N, et al. Dermatologic emergencies: descriptive analysis of 861 patients in a tertiary care teaching hospital. Actas Dermosifiliogr. 2013;104:316-324.
- National Center for Health Statistics. National Hospital Ambulatory Medical Care Survey, 2021. Accessed September 23, 2025. https://www.cdc.gov/nchs/data/nhamcs/web_tables/2021-nhamcs-ed-web-tables-508.pdf
- Horwitz LI, Green J, Bradley EH. US emergency department performance on wait time and length of visit. Ann Emerg Med. 2010;55:133-141.
- Spechbach H, Rochat J, Gaspoz JM, et al. Patients’ time perception in the waiting room of an ambulatory emergency unit: a cross-sectional study. BMC Emerg Med. 2019;19:41.
- Yang JJ, Maloney NJ, Bach DQ, et al. Dermatology in the emergency department: prescriptions, rates of inpatient admission, and predictors of high utilization in the United States from 1996 to 2012. J Am Acad Dermatol. 2021;84:1480-1483.
- Chen CL, Fitzpatrick L, Kamel H. Who uses the emergency department for dermatologic care? a statewide analysis. J Am Acad Dermatol. 2014;71:308-313.
- Moon AT, Castelo-Soccio L, Yan AC. Emergency department utilization of pediatric dermatology (PD) consultations. J Am Acad Dermatol. 2016;74:1173-1177.
- Creadore A, Desai S, Li SJ, et al. Insurance acceptance, appointment wait time, and dermatologist access across practice types in the US. JAMA Dermatol. 2021;157:181-188.
- Mazmudar RS, Gupta N, Desai BJ, et al. Dermatologist appointment access and waiting times: a comparative study of insurance types. J Am Acad Dermatol. 2020;83:1468-1470.
- Johnson J, Cutler B, Latour E, et al. Dermatology urgent care model reduces costs and healthcare utilization for psychodermatology patients-a retrospective chart review. Dermatol Online J. 2022;28:5.
- Wintringham JA, Strock DM, Perkins-Holtsclaw K, et al. Dermatology in the urgent care setting: a retrospective review of patients seen in an urgent access dermatology clinic. J Am Acad Dermatol. 2023;89:1271-1273.
- Yoo MJ, Long B, Brady WJ, et al. Immune checkpoint inhibitors: an emergency medicine focused review. Am J Emerg Med. 2021;50:335-344.
- Merlo G, Cozzani E, Canale F, et al. Cutaneous manifestations of hematologic malignancies the experience of an Italian dermatology department. Hematol Oncol. 2019;37:285-290.
- Barrios D, Phillips G, Freites-Martinez A, et al. Outpatient dermatology consultations for oncology patients with acute dermatologic adverse events impact anticancer therapy interruption: a retrospective study.J Eur Acad Dermatol Venereol. 2020;34:1340-1347.
- Salah S, Kerob D, Pages Laurent C, et al. Evaluation of anticancer therapy-related dermatologic adverse events: insights from Food and Drug Administration’s Adverse Event Reporting System dataset. J Am Acad Dermatol. 2024;91:863-871. doi:10.1016/j.jaad.2024.07.1456
- Shope C, Andrews L, Girvin A, et al. Referrals to dermatology following solid organ transplant. J Am Acad Dermatol. 2023;88:1159-1160. doi:10.1016/j.jaad.2022.11.052
- Werth VP, Askanase AD, Lundberg IE. Importance of collaboration of dermatology and rheumatology to advance the field for lupus and dermatomyositis. Int J Womens Dermatol. 2021;7:583-587.
- Ziob J, Behning C, Brossart P, et al. Specialized dermatological-rheumatological patient management improves diagnostic outcome and patient journey in psoriasis and psoriatic arthritis: a four-year analysis. BMC Rheumatol. 2021;5:1-8. doi:10.1186/s41927-021-00217-z
- Okun MM, Flamm A, Werley EB, et al. Hidradenitis suppurativa: diagnosis and management in the emergency department. J Emerg Med. 2022;63:636-644.
- Jayakumar KL, Samimi SS, Vittorio CC, et al. Expediting patient appointments with dermatology rapid access clinics. Dermatol Online J. 2018;24:13030/qt2zv07510.
- Singman EL, Smith K, Mehta R, et al. Cost and visit duration of same-day access at an academic ophthalmology department vs emergency department. JAMA Ophthalmol. 2019;137:729-735. doi:10.1001/jamaophthalmol.2019.0864
- Roemer M. Costs of treat-and-release emergency department visits in the United States, 2021. Agency for Healthcare Research and Quality. Published September 2024. Accessed September 16, 2025. https://hcup-us.ahrq.gov/reports/statbriefs/sb311-ED-visit-costs-2021.pdf
Dermatology Immediate Care: A Game Changer for the Health Care System?
Dermatology Immediate Care: A Game Changer for the Health Care System?
Practice Points
- Emergency departments and most immediate care (IC) centers often lack prompt access to board-certified dermatologists.
- A dermatology urgent care/IC model may shorten wait times, improve access for vulnerable patients and pediatric populations, and reduce unnecessary hospital admissions and costs.
- Increased access to dermatology benefits other specialties by enabling multidisciplinary care leading to faster diagnosis and treatment.
- A staged referral-first dermatology IC pilot with defined staffing and triage rules is a practical path to demonstrate value and scale the service.
Tender Nodule on the Umbilicus
Tender Nodule on the Umbilicus
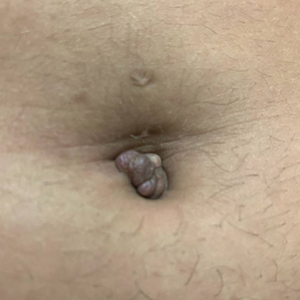
THE DIAGNOSIS: Villar Nodule
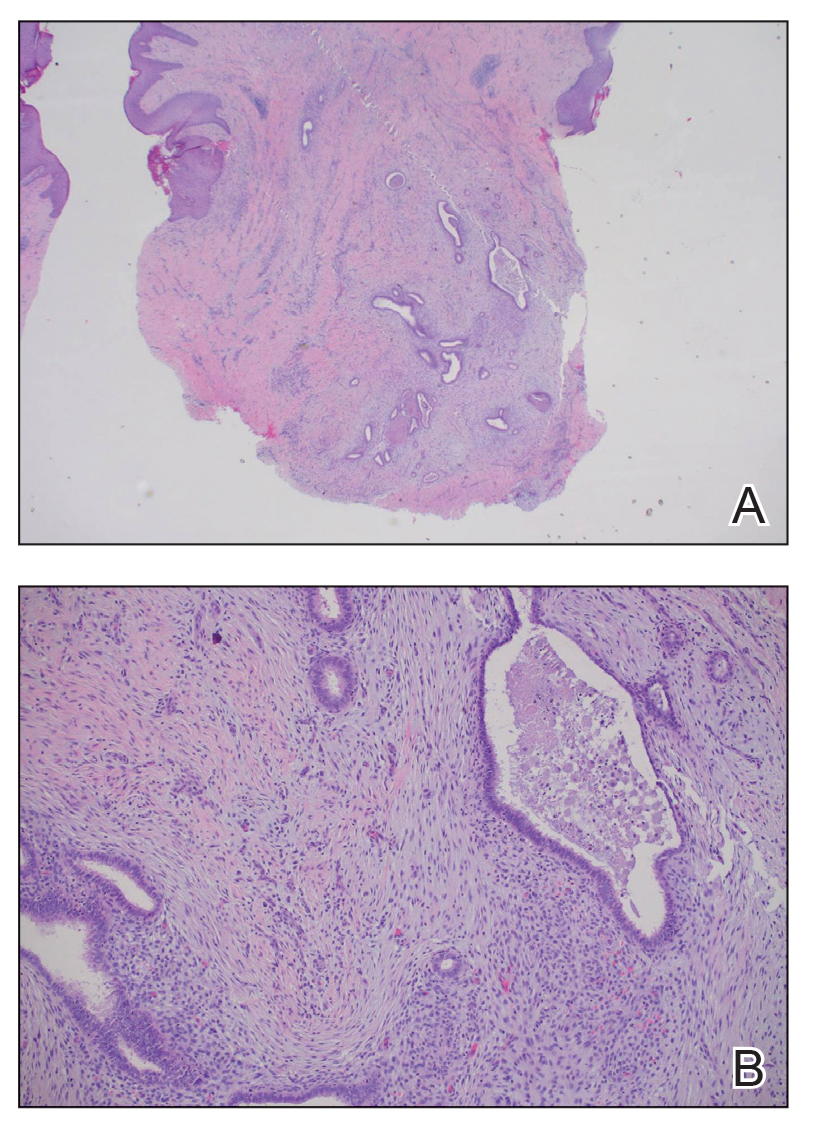
The biopsy revealed features consistent with cutaneous endometriosis in the setting of a painful, tender, multilobulated nodule with a cyclical bleeding pattern (Figure 1). The bleeding pattern of the nodule during menses and lack of surgical history supported the diagnosis of primary cutaneous endometriosis in our patient. She was diagnosed with endometriosis by gynecology, and her primary care physician started her on an oral contraceptive based on this diagnosis. She also was referred to gynecology and plastic surgery for a joint surgical consultation to remove the nodule. She initially decided to do a trial of the oral contraceptive but subsequently underwent umbilical endometrioma excision with neo-umbilicus creation with no evidence of recurrence.
Primary cutaneous endometriosis should be considered in young females who present with tender umbilical nodules. Endometriosis refers to the presence of an endometriumlike epithelium outside the endometrium and myometrium.1 The condition affects 10% to 15% of reproductive-aged (ie, 18-49 years) women in the United States and typically involves tissues within the pelvis, such as the ovaries, pouch of Douglas, or pelvic ligaments.2 Cutaneous endometriosis is the growth of endometrial tissue in the skin and is rare, accounting for less than 5.5% of cases of extrapelvic endometriosis worldwide, affecting primarily the umbilicus, abdominal wall, and vulva.3,4
The 2 main types of cutaneous endometriosis are primary (spontaneous) and secondary. Primary lesions develop in patients without prior surgical history, and secondary lesions occur within previous surgical incision sites, often scars from cesarean delivery.5 Less than 30% of cases of cutaneous endometriosis are primary disease.6 Primary cutaneous endometriosis of the umbilicus, known as Villar nodule, was first described in 1886.3,7 Up to 40% of patients with extrapelvic endometriosis worldwide presented with Villar nodules in a systematic literature review.6 The prevalence of these nodules is unknown, but the incidence is less than 1% of cases of extragenital endometriosis.4
There are 2 leading theories of primary cutaneous endometriosis pathogenesis. The first is the transportation theory, in which endometrial cells are transported outside the uterus via the lymphatic system.8 The second is the metaplasia theory, which proposed that endometrial cells develop in the coelomic mesothelium in the presence of high estrogen levels.8,9
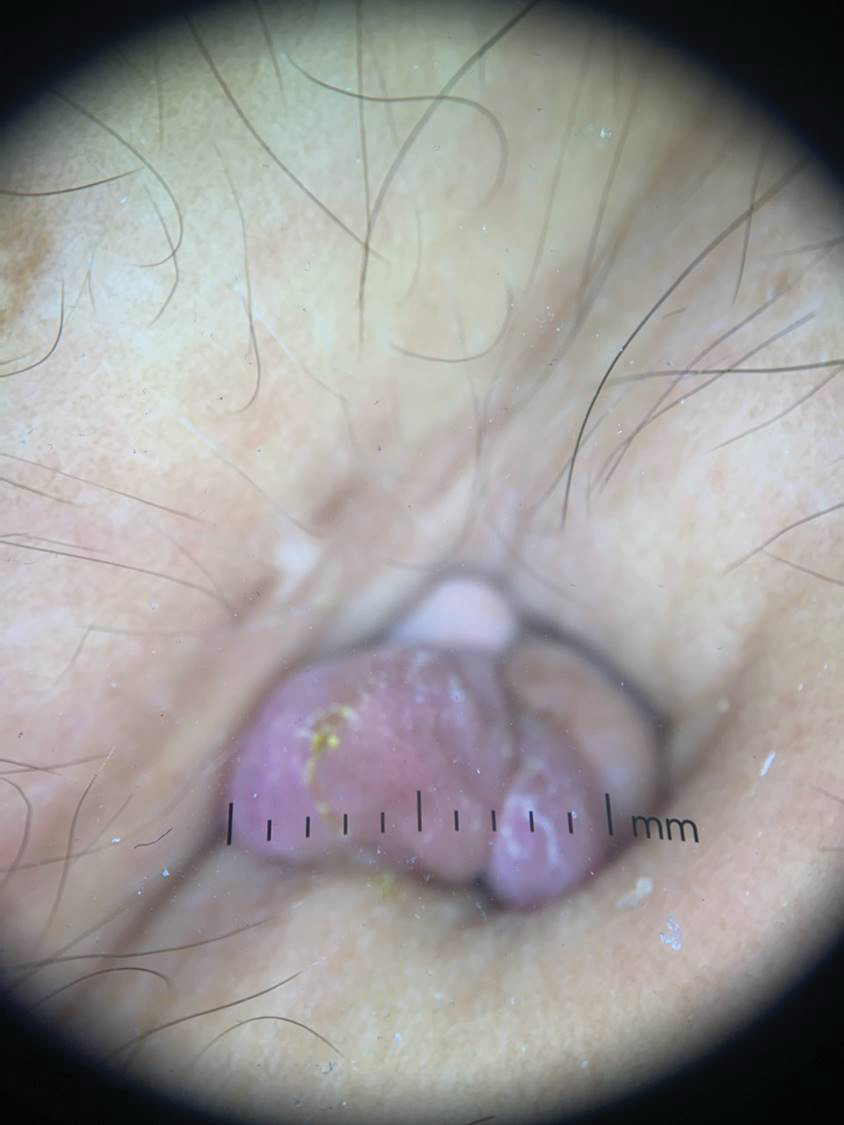
Secondary cutaneous endometriosis, also known as scar endometriosis, is suspected to be caused by an iatrogenic implantation of endometrial cells at the scar of a prior surgical site.9 Although our patient had an existing umbilicus scar from a piercing, it was improbable for that to have been the nidus, as the keloid scar was superficial and did not have contact with the abdominal cavity for iatrogenic implantation. Clinical diagnosis for secondary cutaneous endometriosis often is made based on a triad of features: a nonmalignant abdominal mass, recurring pain and bleeding of the lesion with menses, and prior history of abdominal surgery.9,10 On clinical examination, these features typically manifest as a palpable subcutaneous mass that is black, blue, brown, or red. Often, the lesions enlarge and bleed during the menstrual cycle, causing pain, tenderness, or pruritus.3 Dermoscopic features of secondary cutaneous endometriosis are erythematous umbilical nodules with a homogeneous vascular pattern that appears red with a brownish hue (Figure 2).9,11 Dermoscopic features may vary with the hormone cycle; for example, the follicular phase (correlating with day 7 of menses) demonstrates polypoid projections, erythematous violaceous color, dark-brown spots, and active bleeding of the lesion.12 Clinical and dermoscopic examination are useful tools in this diagnosis.
Imaging such as ultrasonography, computed tomography, or magnetic resonance imaging may be useful in identifying abdominal endometriomas.8,13,14 Pelvic involvement of endometriosis was found in approximately 15% of patients in a case series,4 with concurrent primary umbilical endometriosis. Imaging studies may assist evaluation for fistula formation, presence of malignancies, and the extent of endometriosis within the abdominal cavity.
Histopathology is key to confirming cutaneous endometriosis and shows multiple bland-appearing glands of varying sizes with loose, concentric, edematous, or fibromyxoid stroma (Figure 1).3 Red blood cells sometimes are found with hemosiderin within the stroma. Immunohistochemical staining with estrogen receptors may aid in identifying the endometriumlike epithelial cells.13
Standard treatment involves surgical excision with 1-cm margins and umbilical preservation, which results in a recurrence rate of less than 10%.4,10 Medical therapy, such as aromatase inhibitors, progestogens, antiprogestogens, combined oral contraceptives, or gonadotropin-releasing hormone agonists or antagonists may help manage pain or reduce the size of the nodule.4,15 Simple observation also is a potential course for patients who decline treatment options.
Differential diagnoses include lobular capillary hemangioma, also known as pyogenic granuloma; Sister Mary Joseph nodule; umbilical hernia; and dermatofibrosarcoma protuberans. Lobular capillary hemangiomas commonly are acquired benign vascular proliferations of the skin that are friable and tend to ulcerate.16 These lesions typically grow rapidly and often are located on the face, lips, mucosae, and fingers. Histopathologic examination may show an exophytic lesion with lobules of proliferating capillaries within an edematous matrix, superficial ulceration, and an epithelial collarette.17 Treatment includes surgical excision, cauterization, laser treatments, sclerotherapy, injectable medications, and topical medications, but recurrence is possible with any of these interventions.18
Cutaneous metastasis of an internal solid organ cancer, commonly known as a Sister Mary Joseph nodule, typically manifests as an erythematous, irregularly shaped nodule that may protrude from the umbilicus.14 Gastrointestinal symptoms such as change in bowel habits or obstructive symptoms in the setting of a progressive malignancy are common.14 Clinical features include a firm fixed lesion, oozing, and ulceration.19 On dermoscopy, polymorphous vascular patterns, milky red structureless areas, and white lines typically are present.11 Although dermoscopic features may differentiate this entity from cutaneous endometriosis, tissue sampling and histologic examination are crucial diagnostic tools to identify malignant vs benign lesions.
An umbilical hernia is a protrusion of omentum, bowel, or other intra-abdominal organs in an abdominal wall defect. Clinical presentation includes a soft protrusion that may be reduced on palpation if nonstrangulated.20 Treatment includes watchful waiting or surgical repair. The reducibility and presence of an abdominal wall defect may point to this diagnosis. Imaging also may aid in the diagnosis if the history and physical examination are unclear.
Dermatofibrosarcoma protuberans is a slow-developing, low- to intermediate-grade, soft-tissue sarcoma that occurs in less than 0.1% of all cancers in the United States.21 Lesions often manifest as small, firm, slow-growing, painless, flesh-colored dermal plaques; subcutaneous thickening; or atrophic nonprotuberant lesions typically involving the trunk.21 Histopathologically, they are composed of uniform spindle-cell proliferation growing in a storiform pattern and subcutaneous fat trapping that has strong and diffuse CD34 immunoreactivity.21,22 Pathologic examination typically distinguishes this diagnosis from cutaneous endometriosis. Treatment includes tumor resection that may or may not involve radiotherapy and targeted therapy, as recurrence and metastases are possible.
Primary cutaneous endometriosis is a rare but important diagnosis for dermatologists to consider when evaluating umbilical nodules. Clinical features may include bleeding masses during menses in females of reproductive age. Dermoscopic examination aids in workup, and histopathologic testing can confirm the diagnosis and rule out malignancies. Surgical excision is the treatment of choice with a low rate of recurrence.
- International Working Group of AAGL, ESGE, ESHRE and WES; Tomassetti C, Johnson NP, et al. An international terminology for endometriosis, 2021. Hum Reprod Open. 2021;2021:hoab029. doi:10.1093/hropen/hoab029
- Batista M, Alves F, Cardoso J, et al. Cutaneous endometriosis: a differential diagnosis of umbilical nodule. Acta Med Port. 2020; 33:282-284. doi:10.20344/amp.10966
- Brown ME, Osswald S, Biediger T. Cutaneous endometriosis of the umbilicus (Villar’s nodule). Int J Womens Dermatol. 2020;6:214-215. doi:10.1016/j.ijwd.2020.01.001
- Bindra V, Sampurna S, Kade S, et al. Primary umbilical endometriosis - case series and review of clinical presentation, diagnosis and management. Int J Surg Case Rep. 2022;94:107134. doi:10.1016/j.ijscr.2022.107134
- Loh SH, Lew BL, Sim WY. Primary cutaneous endometriosis of umbilicus. Ann Dermatol. 2017;29:621-625. doi:10.5021/ad.2017.29.5.621
- Victory R, Diamond MP, Johns DA. Villar’s nodule: a case report and systematic literature review of endometriosis externa of the umbilicus. J Minim Invasive Gynecol. 2007;14:23-32. doi:10.1016/j.jmig.2006.07.01
- Van den Nouland D, Kaur M. Primary umbilical endometriosis: a case report. Facts Views Vis Obgyn. 2017;9:115-119.
- Machairiotis N, Stylianaki A, Dryllis G, et al. Extrapelvic endometriosis: a rare entity or an under diagnosed condition? Diagn Pathol. 2013;8:194. doi:10.1186/1746-1596-8-194
- Huang QF, Jiang B, Yang X, et al. Primary versus secondary cutaneous endometriosis: literature review and case study. Heliyon. 2023;9:E20094. doi:10.1016/j.heliyon.2023.e20094
- Gonzalez RH, Singh MS, Hamza SA. Cutaneous endometriosis: a case report and review of the literature. Am J Case Rep. 2021;22:E932493. doi:10.12659/AJCR.932493
- Buljan M, Arzberger E, Šitum M, et al. The use of dermoscopy in differentiating Sister Mary Joseph nodule and cutaneous endometriosis. Australas J Dermatol. 2019;60:E233-E235. doi:10.1111/ajd.12980
- Costa IM, Gomes CM, Morais OO, et al. Cutaneous endometriosis: dermoscopic findings related to phases of the female hormonal cycle. Int J Dermatol. 2014;53:E130-E132. doi:10.1111 /j.1365-4632.2012.05854.x
- Mohaghegh F, Hatami P, Rajabi P, et al. Coexistence of cutaneous endometriosis and ovarian endometrioma: a case report. J Med Case Rep. 2022;16:256. doi:10.1186/s13256-022-03483-8
- Raffi L, Suresh R, McCalmont TH, et al. Cutaneous endometriosis. Int J Womens Dermatol. 2019;5:384-386. doi:10.1016 /j.ijwd.2019.06.025
- Saunders PTK, Horne AW. Endometriosis: etiology, pathobiology, and therapeutic prospects. Cell. 2021;184:2807-2824. doi:10.1016 /j.cell.2021.04.041
- Habif TP. Clinical Dermatology a Color Guide to Diagnosis and Therapy. St. Louis, Mo. Elsevier; 2016.
- Patrice SJ, Wiss K, Mulliken JB. Pyogenic granuloma (lobular capillary hemangioma): a clinicopathologic study of 178 cases. Pediatr Dermatol. 1991;8:267-276. doi:10.1111/j.15251470.1991.tb00931.x
- Kaleeny JD, Janis JE. Pyogenic granuloma diagnosis and management: a practical review. Plast Reconstr Surg Glob Open. 2024;12:E6160. doi:10.1097/GOX.0000000000006160
- Ha DL, Yang MY, Shin JO, et al. Benign umbilical tumors resembling Sister Mary Joseph nodule. Clin Med Insights Oncol. 2021;15:1179554921995022. doi:10.1177/1179554921995022
- Lawrence PF, Smeds M, Jessica Beth O’connell. Essentials of General Surgery and Surgical Specialties. Wolters Kluwer Health; 2019.
- Hao X, Billings SD, Wu F, et al. Dermatofibrosarcoma protuberans: update on the diagnosis and treatment. J Clin Med. 2020;9:1752. doi:10.3390/jcm9061752
- Allen A, Ahn C, Sangüeza OP. Dermatofibrosarcoma protuberans. Dermatol Clin. 2019;37:483-488. doi:10.1016/j.det.2019.05.006
THE DIAGNOSIS: Villar Nodule
The biopsy revealed features consistent with cutaneous endometriosis in the setting of a painful, tender, multilobulated nodule with a cyclical bleeding pattern (Figure 1). The bleeding pattern of the nodule during menses and lack of surgical history supported the diagnosis of primary cutaneous endometriosis in our patient. She was diagnosed with endometriosis by gynecology, and her primary care physician started her on an oral contraceptive based on this diagnosis. She also was referred to gynecology and plastic surgery for a joint surgical consultation to remove the nodule. She initially decided to do a trial of the oral contraceptive but subsequently underwent umbilical endometrioma excision with neo-umbilicus creation with no evidence of recurrence.

Primary cutaneous endometriosis should be considered in young females who present with tender umbilical nodules. Endometriosis refers to the presence of an endometriumlike epithelium outside the endometrium and myometrium.1 The condition affects 10% to 15% of reproductive-aged (ie, 18-49 years) women in the United States and typically involves tissues within the pelvis, such as the ovaries, pouch of Douglas, or pelvic ligaments.2 Cutaneous endometriosis is the growth of endometrial tissue in the skin and is rare, accounting for less than 5.5% of cases of extrapelvic endometriosis worldwide, affecting primarily the umbilicus, abdominal wall, and vulva.3,4
The 2 main types of cutaneous endometriosis are primary (spontaneous) and secondary. Primary lesions develop in patients without prior surgical history, and secondary lesions occur within previous surgical incision sites, often scars from cesarean delivery.5 Less than 30% of cases of cutaneous endometriosis are primary disease.6 Primary cutaneous endometriosis of the umbilicus, known as Villar nodule, was first described in 1886.3,7 Up to 40% of patients with extrapelvic endometriosis worldwide presented with Villar nodules in a systematic literature review.6 The prevalence of these nodules is unknown, but the incidence is less than 1% of cases of extragenital endometriosis.4
There are 2 leading theories of primary cutaneous endometriosis pathogenesis. The first is the transportation theory, in which endometrial cells are transported outside the uterus via the lymphatic system.8 The second is the metaplasia theory, which proposed that endometrial cells develop in the coelomic mesothelium in the presence of high estrogen levels.8,9
Secondary cutaneous endometriosis, also known as scar endometriosis, is suspected to be caused by an iatrogenic implantation of endometrial cells at the scar of a prior surgical site.9 Although our patient had an existing umbilicus scar from a piercing, it was improbable for that to have been the nidus, as the keloid scar was superficial and did not have contact with the abdominal cavity for iatrogenic implantation. Clinical diagnosis for secondary cutaneous endometriosis often is made based on a triad of features: a nonmalignant abdominal mass, recurring pain and bleeding of the lesion with menses, and prior history of abdominal surgery.9,10 On clinical examination, these features typically manifest as a palpable subcutaneous mass that is black, blue, brown, or red. Often, the lesions enlarge and bleed during the menstrual cycle, causing pain, tenderness, or pruritus.3 Dermoscopic features of secondary cutaneous endometriosis are erythematous umbilical nodules with a homogeneous vascular pattern that appears red with a brownish hue (Figure 2).9,11 Dermoscopic features may vary with the hormone cycle; for example, the follicular phase (correlating with day 7 of menses) demonstrates polypoid projections, erythematous violaceous color, dark-brown spots, and active bleeding of the lesion.12 Clinical and dermoscopic examination are useful tools in this diagnosis.

Imaging such as ultrasonography, computed tomography, or magnetic resonance imaging may be useful in identifying abdominal endometriomas.8,13,14 Pelvic involvement of endometriosis was found in approximately 15% of patients in a case series,4 with concurrent primary umbilical endometriosis. Imaging studies may assist evaluation for fistula formation, presence of malignancies, and the extent of endometriosis within the abdominal cavity.
Histopathology is key to confirming cutaneous endometriosis and shows multiple bland-appearing glands of varying sizes with loose, concentric, edematous, or fibromyxoid stroma (Figure 1).3 Red blood cells sometimes are found with hemosiderin within the stroma. Immunohistochemical staining with estrogen receptors may aid in identifying the endometriumlike epithelial cells.13
Standard treatment involves surgical excision with 1-cm margins and umbilical preservation, which results in a recurrence rate of less than 10%.4,10 Medical therapy, such as aromatase inhibitors, progestogens, antiprogestogens, combined oral contraceptives, or gonadotropin-releasing hormone agonists or antagonists may help manage pain or reduce the size of the nodule.4,15 Simple observation also is a potential course for patients who decline treatment options.
Differential diagnoses include lobular capillary hemangioma, also known as pyogenic granuloma; Sister Mary Joseph nodule; umbilical hernia; and dermatofibrosarcoma protuberans. Lobular capillary hemangiomas commonly are acquired benign vascular proliferations of the skin that are friable and tend to ulcerate.16 These lesions typically grow rapidly and often are located on the face, lips, mucosae, and fingers. Histopathologic examination may show an exophytic lesion with lobules of proliferating capillaries within an edematous matrix, superficial ulceration, and an epithelial collarette.17 Treatment includes surgical excision, cauterization, laser treatments, sclerotherapy, injectable medications, and topical medications, but recurrence is possible with any of these interventions.18
Cutaneous metastasis of an internal solid organ cancer, commonly known as a Sister Mary Joseph nodule, typically manifests as an erythematous, irregularly shaped nodule that may protrude from the umbilicus.14 Gastrointestinal symptoms such as change in bowel habits or obstructive symptoms in the setting of a progressive malignancy are common.14 Clinical features include a firm fixed lesion, oozing, and ulceration.19 On dermoscopy, polymorphous vascular patterns, milky red structureless areas, and white lines typically are present.11 Although dermoscopic features may differentiate this entity from cutaneous endometriosis, tissue sampling and histologic examination are crucial diagnostic tools to identify malignant vs benign lesions.
An umbilical hernia is a protrusion of omentum, bowel, or other intra-abdominal organs in an abdominal wall defect. Clinical presentation includes a soft protrusion that may be reduced on palpation if nonstrangulated.20 Treatment includes watchful waiting or surgical repair. The reducibility and presence of an abdominal wall defect may point to this diagnosis. Imaging also may aid in the diagnosis if the history and physical examination are unclear.
Dermatofibrosarcoma protuberans is a slow-developing, low- to intermediate-grade, soft-tissue sarcoma that occurs in less than 0.1% of all cancers in the United States.21 Lesions often manifest as small, firm, slow-growing, painless, flesh-colored dermal plaques; subcutaneous thickening; or atrophic nonprotuberant lesions typically involving the trunk.21 Histopathologically, they are composed of uniform spindle-cell proliferation growing in a storiform pattern and subcutaneous fat trapping that has strong and diffuse CD34 immunoreactivity.21,22 Pathologic examination typically distinguishes this diagnosis from cutaneous endometriosis. Treatment includes tumor resection that may or may not involve radiotherapy and targeted therapy, as recurrence and metastases are possible.
Primary cutaneous endometriosis is a rare but important diagnosis for dermatologists to consider when evaluating umbilical nodules. Clinical features may include bleeding masses during menses in females of reproductive age. Dermoscopic examination aids in workup, and histopathologic testing can confirm the diagnosis and rule out malignancies. Surgical excision is the treatment of choice with a low rate of recurrence.
THE DIAGNOSIS: Villar Nodule
The biopsy revealed features consistent with cutaneous endometriosis in the setting of a painful, tender, multilobulated nodule with a cyclical bleeding pattern (Figure 1). The bleeding pattern of the nodule during menses and lack of surgical history supported the diagnosis of primary cutaneous endometriosis in our patient. She was diagnosed with endometriosis by gynecology, and her primary care physician started her on an oral contraceptive based on this diagnosis. She also was referred to gynecology and plastic surgery for a joint surgical consultation to remove the nodule. She initially decided to do a trial of the oral contraceptive but subsequently underwent umbilical endometrioma excision with neo-umbilicus creation with no evidence of recurrence.

Primary cutaneous endometriosis should be considered in young females who present with tender umbilical nodules. Endometriosis refers to the presence of an endometriumlike epithelium outside the endometrium and myometrium.1 The condition affects 10% to 15% of reproductive-aged (ie, 18-49 years) women in the United States and typically involves tissues within the pelvis, such as the ovaries, pouch of Douglas, or pelvic ligaments.2 Cutaneous endometriosis is the growth of endometrial tissue in the skin and is rare, accounting for less than 5.5% of cases of extrapelvic endometriosis worldwide, affecting primarily the umbilicus, abdominal wall, and vulva.3,4
The 2 main types of cutaneous endometriosis are primary (spontaneous) and secondary. Primary lesions develop in patients without prior surgical history, and secondary lesions occur within previous surgical incision sites, often scars from cesarean delivery.5 Less than 30% of cases of cutaneous endometriosis are primary disease.6 Primary cutaneous endometriosis of the umbilicus, known as Villar nodule, was first described in 1886.3,7 Up to 40% of patients with extrapelvic endometriosis worldwide presented with Villar nodules in a systematic literature review.6 The prevalence of these nodules is unknown, but the incidence is less than 1% of cases of extragenital endometriosis.4
There are 2 leading theories of primary cutaneous endometriosis pathogenesis. The first is the transportation theory, in which endometrial cells are transported outside the uterus via the lymphatic system.8 The second is the metaplasia theory, which proposed that endometrial cells develop in the coelomic mesothelium in the presence of high estrogen levels.8,9
Secondary cutaneous endometriosis, also known as scar endometriosis, is suspected to be caused by an iatrogenic implantation of endometrial cells at the scar of a prior surgical site.9 Although our patient had an existing umbilicus scar from a piercing, it was improbable for that to have been the nidus, as the keloid scar was superficial and did not have contact with the abdominal cavity for iatrogenic implantation. Clinical diagnosis for secondary cutaneous endometriosis often is made based on a triad of features: a nonmalignant abdominal mass, recurring pain and bleeding of the lesion with menses, and prior history of abdominal surgery.9,10 On clinical examination, these features typically manifest as a palpable subcutaneous mass that is black, blue, brown, or red. Often, the lesions enlarge and bleed during the menstrual cycle, causing pain, tenderness, or pruritus.3 Dermoscopic features of secondary cutaneous endometriosis are erythematous umbilical nodules with a homogeneous vascular pattern that appears red with a brownish hue (Figure 2).9,11 Dermoscopic features may vary with the hormone cycle; for example, the follicular phase (correlating with day 7 of menses) demonstrates polypoid projections, erythematous violaceous color, dark-brown spots, and active bleeding of the lesion.12 Clinical and dermoscopic examination are useful tools in this diagnosis.

Imaging such as ultrasonography, computed tomography, or magnetic resonance imaging may be useful in identifying abdominal endometriomas.8,13,14 Pelvic involvement of endometriosis was found in approximately 15% of patients in a case series,4 with concurrent primary umbilical endometriosis. Imaging studies may assist evaluation for fistula formation, presence of malignancies, and the extent of endometriosis within the abdominal cavity.
Histopathology is key to confirming cutaneous endometriosis and shows multiple bland-appearing glands of varying sizes with loose, concentric, edematous, or fibromyxoid stroma (Figure 1).3 Red blood cells sometimes are found with hemosiderin within the stroma. Immunohistochemical staining with estrogen receptors may aid in identifying the endometriumlike epithelial cells.13
Standard treatment involves surgical excision with 1-cm margins and umbilical preservation, which results in a recurrence rate of less than 10%.4,10 Medical therapy, such as aromatase inhibitors, progestogens, antiprogestogens, combined oral contraceptives, or gonadotropin-releasing hormone agonists or antagonists may help manage pain or reduce the size of the nodule.4,15 Simple observation also is a potential course for patients who decline treatment options.
Differential diagnoses include lobular capillary hemangioma, also known as pyogenic granuloma; Sister Mary Joseph nodule; umbilical hernia; and dermatofibrosarcoma protuberans. Lobular capillary hemangiomas commonly are acquired benign vascular proliferations of the skin that are friable and tend to ulcerate.16 These lesions typically grow rapidly and often are located on the face, lips, mucosae, and fingers. Histopathologic examination may show an exophytic lesion with lobules of proliferating capillaries within an edematous matrix, superficial ulceration, and an epithelial collarette.17 Treatment includes surgical excision, cauterization, laser treatments, sclerotherapy, injectable medications, and topical medications, but recurrence is possible with any of these interventions.18
Cutaneous metastasis of an internal solid organ cancer, commonly known as a Sister Mary Joseph nodule, typically manifests as an erythematous, irregularly shaped nodule that may protrude from the umbilicus.14 Gastrointestinal symptoms such as change in bowel habits or obstructive symptoms in the setting of a progressive malignancy are common.14 Clinical features include a firm fixed lesion, oozing, and ulceration.19 On dermoscopy, polymorphous vascular patterns, milky red structureless areas, and white lines typically are present.11 Although dermoscopic features may differentiate this entity from cutaneous endometriosis, tissue sampling and histologic examination are crucial diagnostic tools to identify malignant vs benign lesions.
An umbilical hernia is a protrusion of omentum, bowel, or other intra-abdominal organs in an abdominal wall defect. Clinical presentation includes a soft protrusion that may be reduced on palpation if nonstrangulated.20 Treatment includes watchful waiting or surgical repair. The reducibility and presence of an abdominal wall defect may point to this diagnosis. Imaging also may aid in the diagnosis if the history and physical examination are unclear.
Dermatofibrosarcoma protuberans is a slow-developing, low- to intermediate-grade, soft-tissue sarcoma that occurs in less than 0.1% of all cancers in the United States.21 Lesions often manifest as small, firm, slow-growing, painless, flesh-colored dermal plaques; subcutaneous thickening; or atrophic nonprotuberant lesions typically involving the trunk.21 Histopathologically, they are composed of uniform spindle-cell proliferation growing in a storiform pattern and subcutaneous fat trapping that has strong and diffuse CD34 immunoreactivity.21,22 Pathologic examination typically distinguishes this diagnosis from cutaneous endometriosis. Treatment includes tumor resection that may or may not involve radiotherapy and targeted therapy, as recurrence and metastases are possible.
Primary cutaneous endometriosis is a rare but important diagnosis for dermatologists to consider when evaluating umbilical nodules. Clinical features may include bleeding masses during menses in females of reproductive age. Dermoscopic examination aids in workup, and histopathologic testing can confirm the diagnosis and rule out malignancies. Surgical excision is the treatment of choice with a low rate of recurrence.
- International Working Group of AAGL, ESGE, ESHRE and WES; Tomassetti C, Johnson NP, et al. An international terminology for endometriosis, 2021. Hum Reprod Open. 2021;2021:hoab029. doi:10.1093/hropen/hoab029
- Batista M, Alves F, Cardoso J, et al. Cutaneous endometriosis: a differential diagnosis of umbilical nodule. Acta Med Port. 2020; 33:282-284. doi:10.20344/amp.10966
- Brown ME, Osswald S, Biediger T. Cutaneous endometriosis of the umbilicus (Villar’s nodule). Int J Womens Dermatol. 2020;6:214-215. doi:10.1016/j.ijwd.2020.01.001
- Bindra V, Sampurna S, Kade S, et al. Primary umbilical endometriosis - case series and review of clinical presentation, diagnosis and management. Int J Surg Case Rep. 2022;94:107134. doi:10.1016/j.ijscr.2022.107134
- Loh SH, Lew BL, Sim WY. Primary cutaneous endometriosis of umbilicus. Ann Dermatol. 2017;29:621-625. doi:10.5021/ad.2017.29.5.621
- Victory R, Diamond MP, Johns DA. Villar’s nodule: a case report and systematic literature review of endometriosis externa of the umbilicus. J Minim Invasive Gynecol. 2007;14:23-32. doi:10.1016/j.jmig.2006.07.01
- Van den Nouland D, Kaur M. Primary umbilical endometriosis: a case report. Facts Views Vis Obgyn. 2017;9:115-119.
- Machairiotis N, Stylianaki A, Dryllis G, et al. Extrapelvic endometriosis: a rare entity or an under diagnosed condition? Diagn Pathol. 2013;8:194. doi:10.1186/1746-1596-8-194
- Huang QF, Jiang B, Yang X, et al. Primary versus secondary cutaneous endometriosis: literature review and case study. Heliyon. 2023;9:E20094. doi:10.1016/j.heliyon.2023.e20094
- Gonzalez RH, Singh MS, Hamza SA. Cutaneous endometriosis: a case report and review of the literature. Am J Case Rep. 2021;22:E932493. doi:10.12659/AJCR.932493
- Buljan M, Arzberger E, Šitum M, et al. The use of dermoscopy in differentiating Sister Mary Joseph nodule and cutaneous endometriosis. Australas J Dermatol. 2019;60:E233-E235. doi:10.1111/ajd.12980
- Costa IM, Gomes CM, Morais OO, et al. Cutaneous endometriosis: dermoscopic findings related to phases of the female hormonal cycle. Int J Dermatol. 2014;53:E130-E132. doi:10.1111 /j.1365-4632.2012.05854.x
- Mohaghegh F, Hatami P, Rajabi P, et al. Coexistence of cutaneous endometriosis and ovarian endometrioma: a case report. J Med Case Rep. 2022;16:256. doi:10.1186/s13256-022-03483-8
- Raffi L, Suresh R, McCalmont TH, et al. Cutaneous endometriosis. Int J Womens Dermatol. 2019;5:384-386. doi:10.1016 /j.ijwd.2019.06.025
- Saunders PTK, Horne AW. Endometriosis: etiology, pathobiology, and therapeutic prospects. Cell. 2021;184:2807-2824. doi:10.1016 /j.cell.2021.04.041
- Habif TP. Clinical Dermatology a Color Guide to Diagnosis and Therapy. St. Louis, Mo. Elsevier; 2016.
- Patrice SJ, Wiss K, Mulliken JB. Pyogenic granuloma (lobular capillary hemangioma): a clinicopathologic study of 178 cases. Pediatr Dermatol. 1991;8:267-276. doi:10.1111/j.15251470.1991.tb00931.x
- Kaleeny JD, Janis JE. Pyogenic granuloma diagnosis and management: a practical review. Plast Reconstr Surg Glob Open. 2024;12:E6160. doi:10.1097/GOX.0000000000006160
- Ha DL, Yang MY, Shin JO, et al. Benign umbilical tumors resembling Sister Mary Joseph nodule. Clin Med Insights Oncol. 2021;15:1179554921995022. doi:10.1177/1179554921995022
- Lawrence PF, Smeds M, Jessica Beth O’connell. Essentials of General Surgery and Surgical Specialties. Wolters Kluwer Health; 2019.
- Hao X, Billings SD, Wu F, et al. Dermatofibrosarcoma protuberans: update on the diagnosis and treatment. J Clin Med. 2020;9:1752. doi:10.3390/jcm9061752
- Allen A, Ahn C, Sangüeza OP. Dermatofibrosarcoma protuberans. Dermatol Clin. 2019;37:483-488. doi:10.1016/j.det.2019.05.006
- International Working Group of AAGL, ESGE, ESHRE and WES; Tomassetti C, Johnson NP, et al. An international terminology for endometriosis, 2021. Hum Reprod Open. 2021;2021:hoab029. doi:10.1093/hropen/hoab029
- Batista M, Alves F, Cardoso J, et al. Cutaneous endometriosis: a differential diagnosis of umbilical nodule. Acta Med Port. 2020; 33:282-284. doi:10.20344/amp.10966
- Brown ME, Osswald S, Biediger T. Cutaneous endometriosis of the umbilicus (Villar’s nodule). Int J Womens Dermatol. 2020;6:214-215. doi:10.1016/j.ijwd.2020.01.001
- Bindra V, Sampurna S, Kade S, et al. Primary umbilical endometriosis - case series and review of clinical presentation, diagnosis and management. Int J Surg Case Rep. 2022;94:107134. doi:10.1016/j.ijscr.2022.107134
- Loh SH, Lew BL, Sim WY. Primary cutaneous endometriosis of umbilicus. Ann Dermatol. 2017;29:621-625. doi:10.5021/ad.2017.29.5.621
- Victory R, Diamond MP, Johns DA. Villar’s nodule: a case report and systematic literature review of endometriosis externa of the umbilicus. J Minim Invasive Gynecol. 2007;14:23-32. doi:10.1016/j.jmig.2006.07.01
- Van den Nouland D, Kaur M. Primary umbilical endometriosis: a case report. Facts Views Vis Obgyn. 2017;9:115-119.
- Machairiotis N, Stylianaki A, Dryllis G, et al. Extrapelvic endometriosis: a rare entity or an under diagnosed condition? Diagn Pathol. 2013;8:194. doi:10.1186/1746-1596-8-194
- Huang QF, Jiang B, Yang X, et al. Primary versus secondary cutaneous endometriosis: literature review and case study. Heliyon. 2023;9:E20094. doi:10.1016/j.heliyon.2023.e20094
- Gonzalez RH, Singh MS, Hamza SA. Cutaneous endometriosis: a case report and review of the literature. Am J Case Rep. 2021;22:E932493. doi:10.12659/AJCR.932493
- Buljan M, Arzberger E, Šitum M, et al. The use of dermoscopy in differentiating Sister Mary Joseph nodule and cutaneous endometriosis. Australas J Dermatol. 2019;60:E233-E235. doi:10.1111/ajd.12980
- Costa IM, Gomes CM, Morais OO, et al. Cutaneous endometriosis: dermoscopic findings related to phases of the female hormonal cycle. Int J Dermatol. 2014;53:E130-E132. doi:10.1111 /j.1365-4632.2012.05854.x
- Mohaghegh F, Hatami P, Rajabi P, et al. Coexistence of cutaneous endometriosis and ovarian endometrioma: a case report. J Med Case Rep. 2022;16:256. doi:10.1186/s13256-022-03483-8
- Raffi L, Suresh R, McCalmont TH, et al. Cutaneous endometriosis. Int J Womens Dermatol. 2019;5:384-386. doi:10.1016 /j.ijwd.2019.06.025
- Saunders PTK, Horne AW. Endometriosis: etiology, pathobiology, and therapeutic prospects. Cell. 2021;184:2807-2824. doi:10.1016 /j.cell.2021.04.041
- Habif TP. Clinical Dermatology a Color Guide to Diagnosis and Therapy. St. Louis, Mo. Elsevier; 2016.
- Patrice SJ, Wiss K, Mulliken JB. Pyogenic granuloma (lobular capillary hemangioma): a clinicopathologic study of 178 cases. Pediatr Dermatol. 1991;8:267-276. doi:10.1111/j.15251470.1991.tb00931.x
- Kaleeny JD, Janis JE. Pyogenic granuloma diagnosis and management: a practical review. Plast Reconstr Surg Glob Open. 2024;12:E6160. doi:10.1097/GOX.0000000000006160
- Ha DL, Yang MY, Shin JO, et al. Benign umbilical tumors resembling Sister Mary Joseph nodule. Clin Med Insights Oncol. 2021;15:1179554921995022. doi:10.1177/1179554921995022
- Lawrence PF, Smeds M, Jessica Beth O’connell. Essentials of General Surgery and Surgical Specialties. Wolters Kluwer Health; 2019.
- Hao X, Billings SD, Wu F, et al. Dermatofibrosarcoma protuberans: update on the diagnosis and treatment. J Clin Med. 2020;9:1752. doi:10.3390/jcm9061752
- Allen A, Ahn C, Sangüeza OP. Dermatofibrosarcoma protuberans. Dermatol Clin. 2019;37:483-488. doi:10.1016/j.det.2019.05.006
Tender Nodule on the Umbilicus
Tender Nodule on the Umbilicus
A 25-year-old woman was referred to the dermatology clinic by her primary care provider for evaluation of a tender nodule on the inferior umbilicus of 2 years' duration at the site of a preexisting keloid scar. The patient reported that the lesion caused occasional pain and tenderness. A few weeks prior to the current presentation, a dark-red bloody discharge developed at the superior aspect of the lesion that subsequently crusted over. The patient denied any use of oral contraceptives or history of abdominal surgery.
The original keloid scar had been treated successfully by an outside physician with intralesional steroid injections, and the patient was interested in a similar procedure for the current nodule. She also had a history of a hyperpigmented hypertrophic scar on the superior periumbilical area from a previous piercing that had resolved several years prior to presentation.
Physical examination of the lesion revealed a 1.2-cm, soft, tender, violaceous nodule with scant yellow crust along the superior surface of the umbilicus. There was no palpable abdominal wall defect, and the nodule was not reducible into the abdominal cavity. An interval history revealed bleeding of the lesion during the patient's menstrual cycle with persistent pain and tenderness. A punch biopsy was performed.
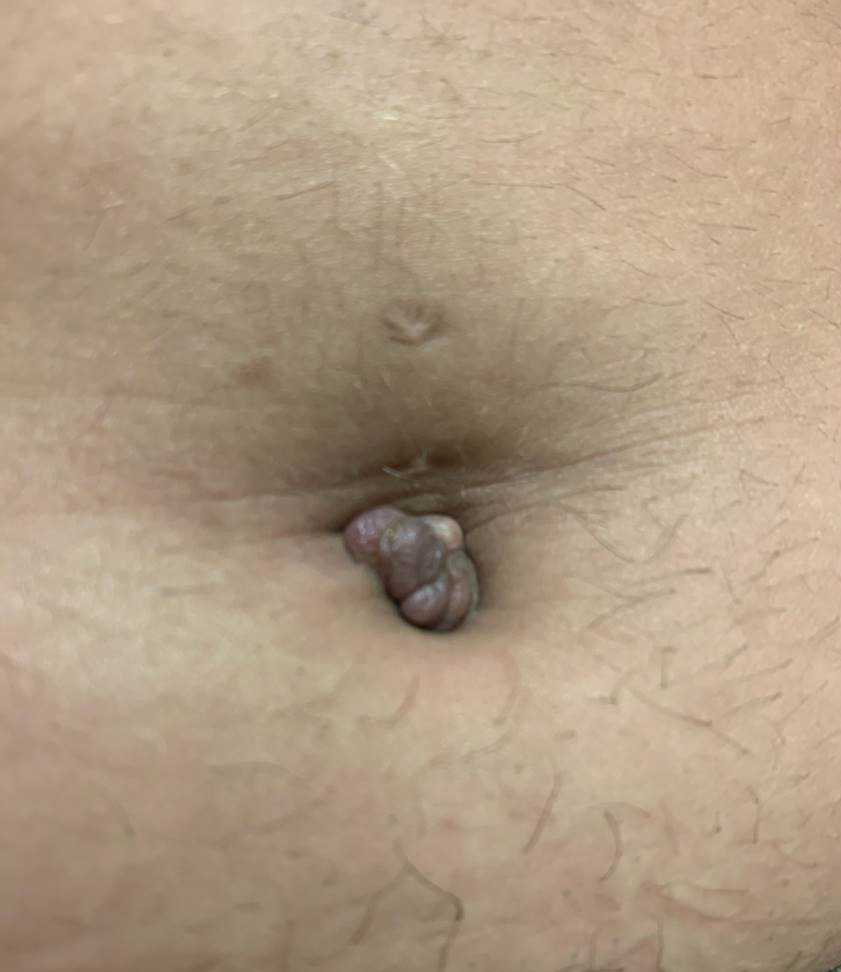
Diffuse Pruritic Keratotic Papules
Diffuse Pruritic Keratotic Papules
THE DIAGNOSIS: Reactive Perforating Collagenosis
Histopathology revealed invagination of the epidermis with hyperkeratosis; prominent epidermal hyperplasia; and a central basophilic plug of keratin, collagen, and inflammatory debris. Transepidermal elimination of bright eosinophilic altered collagen fibers was seen (Figure). The findings were consistent with a diagnosis of reactive perforating collagenosis (RPC).
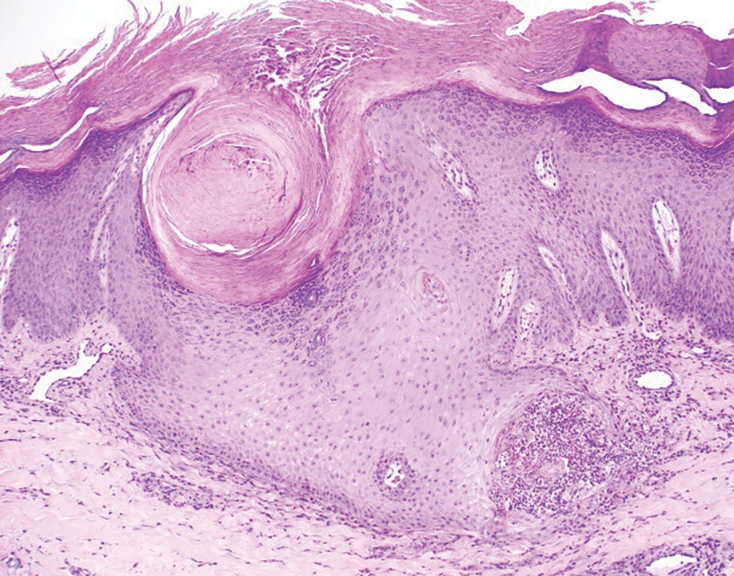
Reactive perforating collagenosis, a subtype of perforating dermatosis, is a rare skin condition in which altered collagen is eliminated through the epidermis.1 There are 2 forms of RPC: the inherited form, which is very rare and manifests in childhood, and the acquired form, which manifests in adulthood and is associated with systemic diseases, most notably diabetes and/or chronic renal failure, both of which our patient had been diagnosed with.1,2 The clinical presentation of RPC includes erythematous papules or nodules that evolve into umbilicated 4- to 10-mm craterlike ulcerations with a central keratotic plug. The lesions favor a linear distribution along the extensor surfaces of the arms and legs, trunk, and gluteal area. Involvement of the head, neck, and scalp has been reported less commonly, which makes our case particularly unique.3 Histopathologically, RPC is characterized by a cup-shaped depression of the epidermis with an overlying keratin plug containing inflammatory cells, keratinous debris, and collagen fibers. Vertically oriented collagen fibers are seen extruded through the epidermis.4,5
While the pathogenesis of RPC remains unknown, it is believed that superficial trauma due to chronic scratching results in transepithelial elimination of collagen. Due to the association of acquired RPC (ARPC) with diabetes, it also has been proposed that scratching can cause microtrauma and necrosis of the dermal structures, potentially due to diabetic microangiopathy.3 Additionally, RPC is associated with overexpression of transforming growth factor beta 3 in lesional skin, suggesting that transforming growth factor beta 3 is involved with tissue repair and extracellular remodeling in this condition.6
Treatment of ARPC should include the management of underlying disease. While no definitive treatment has been reported to date, topical corticosteroids, retinoids, keratolytics, emollients, antihistamines, narrow-band UVB phototherapy, and psoralen plus UVA phototherapy have been used with varying degrees of improvement. Typically, the lesions self-resolve within 6 to 8 weeks; however, they often recur and usually leave scarring with or without hyperpigmentation.2,7-10
Acquired RPC can be misdiagnosed initially, as it mimics several other conditions and commonly is associated with systemic diseases. While biopsy is necessary for diagnosis, if it cannot be performed or the results are indeterminate, dermoscopy can serve as a helpful diagnostic tool. The most common dermoscopic patterns seen in RPC include a yellow-brown structureless area in the center of the lesion with a peripheral surface crust and surrounding white rim—thought to represent epidermal invagination or keratinous debris. Additionally, inflammation with visible vessels both centrally and peripherally is represented by an outer pink circle on dermoscopy.5,11
The differential diagnoses for RPC include perforating folliculitis (PF), elastosis perforans serpiginosa (EPS), prurigo nodularis, and keratoacanthomas. The primary perforating dermatoses (PF, EPS, and RPC) are similarly characterized by elimination of altered dermal material through the epidermis. As these conditions manifest with similar features on clinical examination, differentiation is made by the type of epidermal damage and the features of elimination material, making histopathologic examination paramount for definitive diagnosis.
Perforating folliculitis manifests as erythematous, follicular papules with a small central keratotic core or a central hair. Histopathologically, PF reveals a widely dilated follicle containing keratin, necrotic debris, and degenerated inflammatory cells. Elastosis perforans serpiginosa manifests clinically as hyperkeratotic papules in serpiginous patterns rather than the linear pattern commonly seen with ARPC. Histopathologically, EPS reveals thickened elastic fibers, rather than collagen fibers as seen in ARPC, extruded through the epidermis. Prurigo nodularis manifests clinically as dome-shaped papules with possible excoriation and crusting. Histopathologic examination reveals epidermal hyperplasia and hyperkeratosis; however, the characteristic features of transepithelial elimination of collagen and invaginations of epidermis differentiate ARPC from prurigo nodularis.12,13 Keratoacanthomas manifest clinically as an eruption of small, round, pink papules that rapidly grow and evolve into 1- to 2-cm dome-shaped nodules with central keratinaceous plugs, mimicking a crateriform appearance. Histopathologic examination reveals a circumscribed proliferation of well-differentiated keratinocytes. Multilobular exophytic or endophytic cystlike invaginations of the epidermis also are noted. The expulsion of collagen from the epidermis is more consistent with ARPC.14
- Cohen RW, Auerbach R. Acquired reactive perforating collagenosis. J Am Acad Dermatol. 1989;20(2 pt 1):287-289. doi:10.1016/s0190 -9622(89)80059-3
- Bejjanki H, Siroy AE, Koratala A. Reactive perforating collagenosis in end-stage renal disease: not all that itches is uremic pruritis! Am J Med. 2019;132:E658-E660. doi:10.1016/j.amjmed.2019.03.015
- Gontijo JRV, Júnior FF, Pereira LB, et al. Trauma-induced acquired reactive perforating collagenosis. An Bras Dermatol. 2021;96:392-393. doi:10.1016/j.abd.2020.06.022
- Ambalathinkal JJ, Phiske MM, Someshwar SJ. Acquired reactive perforating collagenosis, a rare entity at uncommon site. Indian J Pathol Microbiol. 2022;65:895-897. doi:10.4103/ijpm.ijpm_333_21
- Ormerod E, Atwan A, Intzedy L, et al. Dermoscopy features of acquired reactive perforating collagenosis: a case series. Dermatol Pract Concept. 2018;8:303-305. doi:10.5826/dpc.0804a11
- Fei C, Wang Y, Gong Y, et al. Acquired reactive perforating collagenosis: a report of a typical case. Medicine (Baltimore). 2016;95:E4305. doi:10.1097/md.0000000000004305
- Bartling SJ, Naff JL, Canevari MM, et al. Pruritic rash in an elderly patient with uncontrolled diabetes mellitus. AACE Clin Case Rep. 2018;5:E146-E149. doi:10.4158/ACCR-2018-0388
- Kollipara H, Satya RS, Rao GR, et al. Acquired reactive perforating collagenosis: case series. Indian Dermatol Online J. 2023;14:72-76. doi:10.4103/idoj.idoj_373_22
- Wang C, Liu YH, Wang YX, et al. Acquired reactive perforating collagenosis. Chin Med J (Engl). 2020;133:2119-2120. doi:10.1097 /cm9.0000000000000906
- Harbaoui S, Litaiem N. Acquired perforating dermatosis. StatPearls [Internet]. Updated February 13, 2023. Accessed August 13, 2025. https://www.ncbi.nlm.nih.gov/books/NBK539715/
- Elmas ÖF, Kilitci A, Uyar B. Dermoscopic patterns of acquired reactive perforating collagenosis. Dermatol Pract Concept. 2021;11:E2020085. doi:10.5826/dpc.1101a85
- Patterson JW. The perforating disorders. J Am Acad Dermatol. 1984;10:561-581. doi:10.1016/s0190-9622(84)80259-5
- Huang AH, Williams KA, Kwatra SG. Prurigo nodularis: epidemiology and clinical features. J Am Acad Dermatol. 2020;83:1559-1565. doi:10.1016/j.jaad.2020.04.183
- Zito PM, Scharf R. Keratoacanthoma. StatPearls [Internet]. Updated August 8, 2023. Accessed August 13, 2025. https://www.ncbi.nlm.nih.gov/books/NBK499931/
THE DIAGNOSIS: Reactive Perforating Collagenosis
Histopathology revealed invagination of the epidermis with hyperkeratosis; prominent epidermal hyperplasia; and a central basophilic plug of keratin, collagen, and inflammatory debris. Transepidermal elimination of bright eosinophilic altered collagen fibers was seen (Figure). The findings were consistent with a diagnosis of reactive perforating collagenosis (RPC).

Reactive perforating collagenosis, a subtype of perforating dermatosis, is a rare skin condition in which altered collagen is eliminated through the epidermis.1 There are 2 forms of RPC: the inherited form, which is very rare and manifests in childhood, and the acquired form, which manifests in adulthood and is associated with systemic diseases, most notably diabetes and/or chronic renal failure, both of which our patient had been diagnosed with.1,2 The clinical presentation of RPC includes erythematous papules or nodules that evolve into umbilicated 4- to 10-mm craterlike ulcerations with a central keratotic plug. The lesions favor a linear distribution along the extensor surfaces of the arms and legs, trunk, and gluteal area. Involvement of the head, neck, and scalp has been reported less commonly, which makes our case particularly unique.3 Histopathologically, RPC is characterized by a cup-shaped depression of the epidermis with an overlying keratin plug containing inflammatory cells, keratinous debris, and collagen fibers. Vertically oriented collagen fibers are seen extruded through the epidermis.4,5
While the pathogenesis of RPC remains unknown, it is believed that superficial trauma due to chronic scratching results in transepithelial elimination of collagen. Due to the association of acquired RPC (ARPC) with diabetes, it also has been proposed that scratching can cause microtrauma and necrosis of the dermal structures, potentially due to diabetic microangiopathy.3 Additionally, RPC is associated with overexpression of transforming growth factor beta 3 in lesional skin, suggesting that transforming growth factor beta 3 is involved with tissue repair and extracellular remodeling in this condition.6
Treatment of ARPC should include the management of underlying disease. While no definitive treatment has been reported to date, topical corticosteroids, retinoids, keratolytics, emollients, antihistamines, narrow-band UVB phototherapy, and psoralen plus UVA phototherapy have been used with varying degrees of improvement. Typically, the lesions self-resolve within 6 to 8 weeks; however, they often recur and usually leave scarring with or without hyperpigmentation.2,7-10
Acquired RPC can be misdiagnosed initially, as it mimics several other conditions and commonly is associated with systemic diseases. While biopsy is necessary for diagnosis, if it cannot be performed or the results are indeterminate, dermoscopy can serve as a helpful diagnostic tool. The most common dermoscopic patterns seen in RPC include a yellow-brown structureless area in the center of the lesion with a peripheral surface crust and surrounding white rim—thought to represent epidermal invagination or keratinous debris. Additionally, inflammation with visible vessels both centrally and peripherally is represented by an outer pink circle on dermoscopy.5,11
The differential diagnoses for RPC include perforating folliculitis (PF), elastosis perforans serpiginosa (EPS), prurigo nodularis, and keratoacanthomas. The primary perforating dermatoses (PF, EPS, and RPC) are similarly characterized by elimination of altered dermal material through the epidermis. As these conditions manifest with similar features on clinical examination, differentiation is made by the type of epidermal damage and the features of elimination material, making histopathologic examination paramount for definitive diagnosis.
Perforating folliculitis manifests as erythematous, follicular papules with a small central keratotic core or a central hair. Histopathologically, PF reveals a widely dilated follicle containing keratin, necrotic debris, and degenerated inflammatory cells. Elastosis perforans serpiginosa manifests clinically as hyperkeratotic papules in serpiginous patterns rather than the linear pattern commonly seen with ARPC. Histopathologically, EPS reveals thickened elastic fibers, rather than collagen fibers as seen in ARPC, extruded through the epidermis. Prurigo nodularis manifests clinically as dome-shaped papules with possible excoriation and crusting. Histopathologic examination reveals epidermal hyperplasia and hyperkeratosis; however, the characteristic features of transepithelial elimination of collagen and invaginations of epidermis differentiate ARPC from prurigo nodularis.12,13 Keratoacanthomas manifest clinically as an eruption of small, round, pink papules that rapidly grow and evolve into 1- to 2-cm dome-shaped nodules with central keratinaceous plugs, mimicking a crateriform appearance. Histopathologic examination reveals a circumscribed proliferation of well-differentiated keratinocytes. Multilobular exophytic or endophytic cystlike invaginations of the epidermis also are noted. The expulsion of collagen from the epidermis is more consistent with ARPC.14
THE DIAGNOSIS: Reactive Perforating Collagenosis
Histopathology revealed invagination of the epidermis with hyperkeratosis; prominent epidermal hyperplasia; and a central basophilic plug of keratin, collagen, and inflammatory debris. Transepidermal elimination of bright eosinophilic altered collagen fibers was seen (Figure). The findings were consistent with a diagnosis of reactive perforating collagenosis (RPC).

Reactive perforating collagenosis, a subtype of perforating dermatosis, is a rare skin condition in which altered collagen is eliminated through the epidermis.1 There are 2 forms of RPC: the inherited form, which is very rare and manifests in childhood, and the acquired form, which manifests in adulthood and is associated with systemic diseases, most notably diabetes and/or chronic renal failure, both of which our patient had been diagnosed with.1,2 The clinical presentation of RPC includes erythematous papules or nodules that evolve into umbilicated 4- to 10-mm craterlike ulcerations with a central keratotic plug. The lesions favor a linear distribution along the extensor surfaces of the arms and legs, trunk, and gluteal area. Involvement of the head, neck, and scalp has been reported less commonly, which makes our case particularly unique.3 Histopathologically, RPC is characterized by a cup-shaped depression of the epidermis with an overlying keratin plug containing inflammatory cells, keratinous debris, and collagen fibers. Vertically oriented collagen fibers are seen extruded through the epidermis.4,5
While the pathogenesis of RPC remains unknown, it is believed that superficial trauma due to chronic scratching results in transepithelial elimination of collagen. Due to the association of acquired RPC (ARPC) with diabetes, it also has been proposed that scratching can cause microtrauma and necrosis of the dermal structures, potentially due to diabetic microangiopathy.3 Additionally, RPC is associated with overexpression of transforming growth factor beta 3 in lesional skin, suggesting that transforming growth factor beta 3 is involved with tissue repair and extracellular remodeling in this condition.6
Treatment of ARPC should include the management of underlying disease. While no definitive treatment has been reported to date, topical corticosteroids, retinoids, keratolytics, emollients, antihistamines, narrow-band UVB phototherapy, and psoralen plus UVA phototherapy have been used with varying degrees of improvement. Typically, the lesions self-resolve within 6 to 8 weeks; however, they often recur and usually leave scarring with or without hyperpigmentation.2,7-10
Acquired RPC can be misdiagnosed initially, as it mimics several other conditions and commonly is associated with systemic diseases. While biopsy is necessary for diagnosis, if it cannot be performed or the results are indeterminate, dermoscopy can serve as a helpful diagnostic tool. The most common dermoscopic patterns seen in RPC include a yellow-brown structureless area in the center of the lesion with a peripheral surface crust and surrounding white rim—thought to represent epidermal invagination or keratinous debris. Additionally, inflammation with visible vessels both centrally and peripherally is represented by an outer pink circle on dermoscopy.5,11
The differential diagnoses for RPC include perforating folliculitis (PF), elastosis perforans serpiginosa (EPS), prurigo nodularis, and keratoacanthomas. The primary perforating dermatoses (PF, EPS, and RPC) are similarly characterized by elimination of altered dermal material through the epidermis. As these conditions manifest with similar features on clinical examination, differentiation is made by the type of epidermal damage and the features of elimination material, making histopathologic examination paramount for definitive diagnosis.
Perforating folliculitis manifests as erythematous, follicular papules with a small central keratotic core or a central hair. Histopathologically, PF reveals a widely dilated follicle containing keratin, necrotic debris, and degenerated inflammatory cells. Elastosis perforans serpiginosa manifests clinically as hyperkeratotic papules in serpiginous patterns rather than the linear pattern commonly seen with ARPC. Histopathologically, EPS reveals thickened elastic fibers, rather than collagen fibers as seen in ARPC, extruded through the epidermis. Prurigo nodularis manifests clinically as dome-shaped papules with possible excoriation and crusting. Histopathologic examination reveals epidermal hyperplasia and hyperkeratosis; however, the characteristic features of transepithelial elimination of collagen and invaginations of epidermis differentiate ARPC from prurigo nodularis.12,13 Keratoacanthomas manifest clinically as an eruption of small, round, pink papules that rapidly grow and evolve into 1- to 2-cm dome-shaped nodules with central keratinaceous plugs, mimicking a crateriform appearance. Histopathologic examination reveals a circumscribed proliferation of well-differentiated keratinocytes. Multilobular exophytic or endophytic cystlike invaginations of the epidermis also are noted. The expulsion of collagen from the epidermis is more consistent with ARPC.14
- Cohen RW, Auerbach R. Acquired reactive perforating collagenosis. J Am Acad Dermatol. 1989;20(2 pt 1):287-289. doi:10.1016/s0190 -9622(89)80059-3
- Bejjanki H, Siroy AE, Koratala A. Reactive perforating collagenosis in end-stage renal disease: not all that itches is uremic pruritis! Am J Med. 2019;132:E658-E660. doi:10.1016/j.amjmed.2019.03.015
- Gontijo JRV, Júnior FF, Pereira LB, et al. Trauma-induced acquired reactive perforating collagenosis. An Bras Dermatol. 2021;96:392-393. doi:10.1016/j.abd.2020.06.022
- Ambalathinkal JJ, Phiske MM, Someshwar SJ. Acquired reactive perforating collagenosis, a rare entity at uncommon site. Indian J Pathol Microbiol. 2022;65:895-897. doi:10.4103/ijpm.ijpm_333_21
- Ormerod E, Atwan A, Intzedy L, et al. Dermoscopy features of acquired reactive perforating collagenosis: a case series. Dermatol Pract Concept. 2018;8:303-305. doi:10.5826/dpc.0804a11
- Fei C, Wang Y, Gong Y, et al. Acquired reactive perforating collagenosis: a report of a typical case. Medicine (Baltimore). 2016;95:E4305. doi:10.1097/md.0000000000004305
- Bartling SJ, Naff JL, Canevari MM, et al. Pruritic rash in an elderly patient with uncontrolled diabetes mellitus. AACE Clin Case Rep. 2018;5:E146-E149. doi:10.4158/ACCR-2018-0388
- Kollipara H, Satya RS, Rao GR, et al. Acquired reactive perforating collagenosis: case series. Indian Dermatol Online J. 2023;14:72-76. doi:10.4103/idoj.idoj_373_22
- Wang C, Liu YH, Wang YX, et al. Acquired reactive perforating collagenosis. Chin Med J (Engl). 2020;133:2119-2120. doi:10.1097 /cm9.0000000000000906
- Harbaoui S, Litaiem N. Acquired perforating dermatosis. StatPearls [Internet]. Updated February 13, 2023. Accessed August 13, 2025. https://www.ncbi.nlm.nih.gov/books/NBK539715/
- Elmas ÖF, Kilitci A, Uyar B. Dermoscopic patterns of acquired reactive perforating collagenosis. Dermatol Pract Concept. 2021;11:E2020085. doi:10.5826/dpc.1101a85
- Patterson JW. The perforating disorders. J Am Acad Dermatol. 1984;10:561-581. doi:10.1016/s0190-9622(84)80259-5
- Huang AH, Williams KA, Kwatra SG. Prurigo nodularis: epidemiology and clinical features. J Am Acad Dermatol. 2020;83:1559-1565. doi:10.1016/j.jaad.2020.04.183
- Zito PM, Scharf R. Keratoacanthoma. StatPearls [Internet]. Updated August 8, 2023. Accessed August 13, 2025. https://www.ncbi.nlm.nih.gov/books/NBK499931/
- Cohen RW, Auerbach R. Acquired reactive perforating collagenosis. J Am Acad Dermatol. 1989;20(2 pt 1):287-289. doi:10.1016/s0190 -9622(89)80059-3
- Bejjanki H, Siroy AE, Koratala A. Reactive perforating collagenosis in end-stage renal disease: not all that itches is uremic pruritis! Am J Med. 2019;132:E658-E660. doi:10.1016/j.amjmed.2019.03.015
- Gontijo JRV, Júnior FF, Pereira LB, et al. Trauma-induced acquired reactive perforating collagenosis. An Bras Dermatol. 2021;96:392-393. doi:10.1016/j.abd.2020.06.022
- Ambalathinkal JJ, Phiske MM, Someshwar SJ. Acquired reactive perforating collagenosis, a rare entity at uncommon site. Indian J Pathol Microbiol. 2022;65:895-897. doi:10.4103/ijpm.ijpm_333_21
- Ormerod E, Atwan A, Intzedy L, et al. Dermoscopy features of acquired reactive perforating collagenosis: a case series. Dermatol Pract Concept. 2018;8:303-305. doi:10.5826/dpc.0804a11
- Fei C, Wang Y, Gong Y, et al. Acquired reactive perforating collagenosis: a report of a typical case. Medicine (Baltimore). 2016;95:E4305. doi:10.1097/md.0000000000004305
- Bartling SJ, Naff JL, Canevari MM, et al. Pruritic rash in an elderly patient with uncontrolled diabetes mellitus. AACE Clin Case Rep. 2018;5:E146-E149. doi:10.4158/ACCR-2018-0388
- Kollipara H, Satya RS, Rao GR, et al. Acquired reactive perforating collagenosis: case series. Indian Dermatol Online J. 2023;14:72-76. doi:10.4103/idoj.idoj_373_22
- Wang C, Liu YH, Wang YX, et al. Acquired reactive perforating collagenosis. Chin Med J (Engl). 2020;133:2119-2120. doi:10.1097 /cm9.0000000000000906
- Harbaoui S, Litaiem N. Acquired perforating dermatosis. StatPearls [Internet]. Updated February 13, 2023. Accessed August 13, 2025. https://www.ncbi.nlm.nih.gov/books/NBK539715/
- Elmas ÖF, Kilitci A, Uyar B. Dermoscopic patterns of acquired reactive perforating collagenosis. Dermatol Pract Concept. 2021;11:E2020085. doi:10.5826/dpc.1101a85
- Patterson JW. The perforating disorders. J Am Acad Dermatol. 1984;10:561-581. doi:10.1016/s0190-9622(84)80259-5
- Huang AH, Williams KA, Kwatra SG. Prurigo nodularis: epidemiology and clinical features. J Am Acad Dermatol. 2020;83:1559-1565. doi:10.1016/j.jaad.2020.04.183
- Zito PM, Scharf R. Keratoacanthoma. StatPearls [Internet]. Updated August 8, 2023. Accessed August 13, 2025. https://www.ncbi.nlm.nih.gov/books/NBK499931/
Diffuse Pruritic Keratotic Papules
Diffuse Pruritic Keratotic Papules
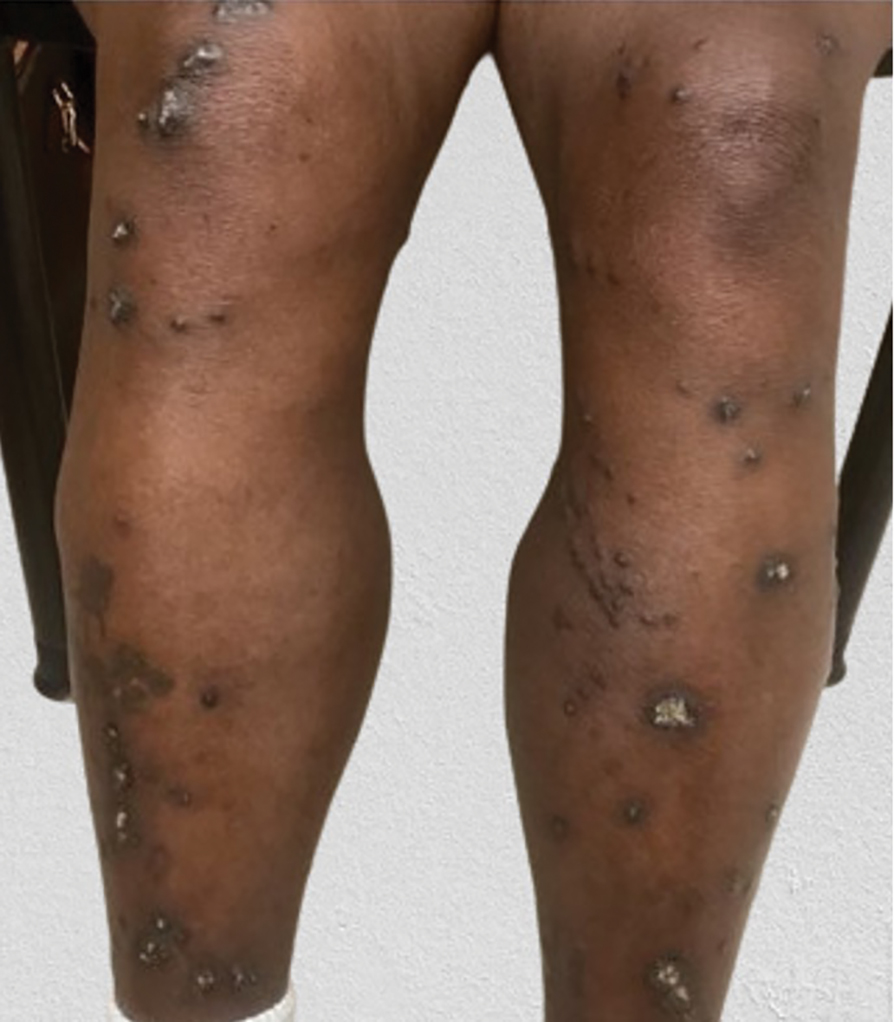
A 65-year-old woman presented to dermatology with an intensely pruritic rash on the arms, legs, neck, and face of several months’ duration. The patient reported scratching the lesions but denied any recent trauma to the affected areas. She previously had been evaluated by her primary care provider, who prescribed cephalexin with no improvement. Her medical history was remarkable for chronic renal failure on dialysis, diabetes, hypertension, and congestive heart failure. Physical examination of the skin revealed hard white cutaneous nodules distributed on the proximal posterior upper arms, bilateral proximal pretibial regions, right elbow, and left knee. Two shave biopsies from the right elbow and left knee were obtained for histopathology.
Bridging the Funding Gap
Federal grants have supported cutting-edge research in scientific and biomedical fields since the mid-20th century, fueling public health breakthroughs and health innovations. This crucial support has been greatly diminished in recent months with deep cuts to federal research dollars.
As these acute policy changes continue to disrupt academic institutions and their investigators, introducing financial strain and operational uncertainty, the importance of research support from professional societies and foundations has become increasingly evident. Their targeted funding plays a critical role in sustaining biomedical research, which directly impacts clinical innovation and patient care. As one example, the AGA Research Foundation provides over $2 million annually to spur discoveries in gastroenterology and hepatology. This vital research support, awarded to 74 unique recipients (including 7 early-career Research Scholar Award recipients) in 2025, represents one of the most important investments that AGA makes in the future of gastroenterology and the patients we treat.
While foundation awards such as these cannot completely close the federal funding gap, they serve as an important lifeline both in supporting the core work of early career and established investigators in an uncertain funding environment and in funding high-risk, high-reward research that more conservative funders are often hesitant to invest in. – now more than ever, the funding it provides has tremendous impact.
In this issue of GI & Hepatology News, we update you on the FDA’s recent approval of semaglutide as a treatment for MASH with fibrosis and highlight a recent target trial emulation study that casts doubt on our traditional understanding regarding the link between common medications such as PPIs and NSAIDs and microscopic colitis in older adults. We also summarize newly-released, global guidelines for pregnancy and IBD, which deserve a careful read. In this month’s Member Spotlight, we feature Pascale White, MD, MBA, MS (Mount Sinai), a recent recipient of the AGA-Pfizer Beacon of Hope Award for Gender and Health Equity, who shares her inspirational work to improve colorectal cancer screening among underserved, high-risk patients in East Harlem. We hope you enjoy this and all the exciting content in our October issue.
Megan A. Adams, MD, JD, MSc
Editor in Chief
Federal grants have supported cutting-edge research in scientific and biomedical fields since the mid-20th century, fueling public health breakthroughs and health innovations. This crucial support has been greatly diminished in recent months with deep cuts to federal research dollars.
As these acute policy changes continue to disrupt academic institutions and their investigators, introducing financial strain and operational uncertainty, the importance of research support from professional societies and foundations has become increasingly evident. Their targeted funding plays a critical role in sustaining biomedical research, which directly impacts clinical innovation and patient care. As one example, the AGA Research Foundation provides over $2 million annually to spur discoveries in gastroenterology and hepatology. This vital research support, awarded to 74 unique recipients (including 7 early-career Research Scholar Award recipients) in 2025, represents one of the most important investments that AGA makes in the future of gastroenterology and the patients we treat.
While foundation awards such as these cannot completely close the federal funding gap, they serve as an important lifeline both in supporting the core work of early career and established investigators in an uncertain funding environment and in funding high-risk, high-reward research that more conservative funders are often hesitant to invest in. – now more than ever, the funding it provides has tremendous impact.
In this issue of GI & Hepatology News, we update you on the FDA’s recent approval of semaglutide as a treatment for MASH with fibrosis and highlight a recent target trial emulation study that casts doubt on our traditional understanding regarding the link between common medications such as PPIs and NSAIDs and microscopic colitis in older adults. We also summarize newly-released, global guidelines for pregnancy and IBD, which deserve a careful read. In this month’s Member Spotlight, we feature Pascale White, MD, MBA, MS (Mount Sinai), a recent recipient of the AGA-Pfizer Beacon of Hope Award for Gender and Health Equity, who shares her inspirational work to improve colorectal cancer screening among underserved, high-risk patients in East Harlem. We hope you enjoy this and all the exciting content in our October issue.
Megan A. Adams, MD, JD, MSc
Editor in Chief
Federal grants have supported cutting-edge research in scientific and biomedical fields since the mid-20th century, fueling public health breakthroughs and health innovations. This crucial support has been greatly diminished in recent months with deep cuts to federal research dollars.
As these acute policy changes continue to disrupt academic institutions and their investigators, introducing financial strain and operational uncertainty, the importance of research support from professional societies and foundations has become increasingly evident. Their targeted funding plays a critical role in sustaining biomedical research, which directly impacts clinical innovation and patient care. As one example, the AGA Research Foundation provides over $2 million annually to spur discoveries in gastroenterology and hepatology. This vital research support, awarded to 74 unique recipients (including 7 early-career Research Scholar Award recipients) in 2025, represents one of the most important investments that AGA makes in the future of gastroenterology and the patients we treat.
While foundation awards such as these cannot completely close the federal funding gap, they serve as an important lifeline both in supporting the core work of early career and established investigators in an uncertain funding environment and in funding high-risk, high-reward research that more conservative funders are often hesitant to invest in. – now more than ever, the funding it provides has tremendous impact.
In this issue of GI & Hepatology News, we update you on the FDA’s recent approval of semaglutide as a treatment for MASH with fibrosis and highlight a recent target trial emulation study that casts doubt on our traditional understanding regarding the link between common medications such as PPIs and NSAIDs and microscopic colitis in older adults. We also summarize newly-released, global guidelines for pregnancy and IBD, which deserve a careful read. In this month’s Member Spotlight, we feature Pascale White, MD, MBA, MS (Mount Sinai), a recent recipient of the AGA-Pfizer Beacon of Hope Award for Gender and Health Equity, who shares her inspirational work to improve colorectal cancer screening among underserved, high-risk patients in East Harlem. We hope you enjoy this and all the exciting content in our October issue.
Megan A. Adams, MD, JD, MSc
Editor in Chief
Hyperpigmented Macules Caused by Burrowing Bugs (Cydnidae) May Mimic More Serious Conditions
Hyperpigmented Macules Caused by Burrowing Bugs (Cydnidae) May Mimic More Serious Conditions
Cydnidae is a family of small to medium-sized shield bugs with spiny legs that commonly are known as burrowing bugs (or burrower bugs). The family Cydnidae includes more than 100 genera and approximately 600 species worldwide.1 These insects are arthropods of the order Hemiptera (suborder: Heteroptera; superfamily: Pentatomoidae) and largely are concentrated in tropical and temperate regions. Approximately 145 species have been recorded in the Neotropical Region and have been included in the subfamilies Amnestinae, Cephalocteinae, and Sehirinae, in addition to Cydnidae.2 Burrowing bugs are ovoid in shape and 2 to 20 mm in length and morphologically are well adapted for burrowing. Their life span is 100 to 300 days. Being phytophagous, they burrow to feed on plants and roots. Adult burrowing bugs have wings and can fly. They have specialized glands located in either the abdomen (nymph) or thorax (adult) that secrete odorous chemicals for self-protection.3 The secretions contain hydrocarbonates that function as repellents and danger signals, can cause paralysis in prey, and act as a chemoattractant for mates.4-6 They also cause hyperpigmentation upon contact with the skin.
In this article, we present a series of cases from the same community to demonstrate the characteristic features of hyperpigmented macules caused by exposure to burrowing bugs. Dermatologists should be aware of this entity to prevent misdiagnosis and unnecessary investigations and treatment.
Case Series
A 36-year-old woman and 6 children (age range, 6-12 years) presented with a widespread, acute, brown-pigmented, macular eruption with lesions that increased in number over a 1-week period. All 7 patients resided in the same locality and were otherwise systemically healthy. Initially, the index case, a 7-year-old girl, was referred to our tertiary care center by a dermatologist with a provisional diagnosis of idiopathic macular eruptive pigmentation. The patient’s mother recalled noticing a tiny black insect on the child's scalp that left pigment on the skin when she crushed it between her fingers. The rest of the patients presented over the next few days: 3 of the children belonged to the same household as the index case, and there was history of all 6 children playing in the neighborhood park during late evening hours. The adult patient was the parent of one of the affected children. The lesions were associated with mild itching and tingling in 3 children but were asymptomatic in the other patients.
Clinical examination of the patients revealed multiple dark- to light-brown, discrete, irregularly shaped macules over the trunk, arms, and soles (eFigure 1). Dermoscopic examination of a pigmented macule showed an irregularly shaped, brownish, structureless area with accentuation of the pigment at skin creases and perieccrine pigmentation (eFigure 2). The pigmentation was unaffected by rubbing with alcohol or water. Clinicoepidemiologic parameters of the patients are summarized in the eTable.
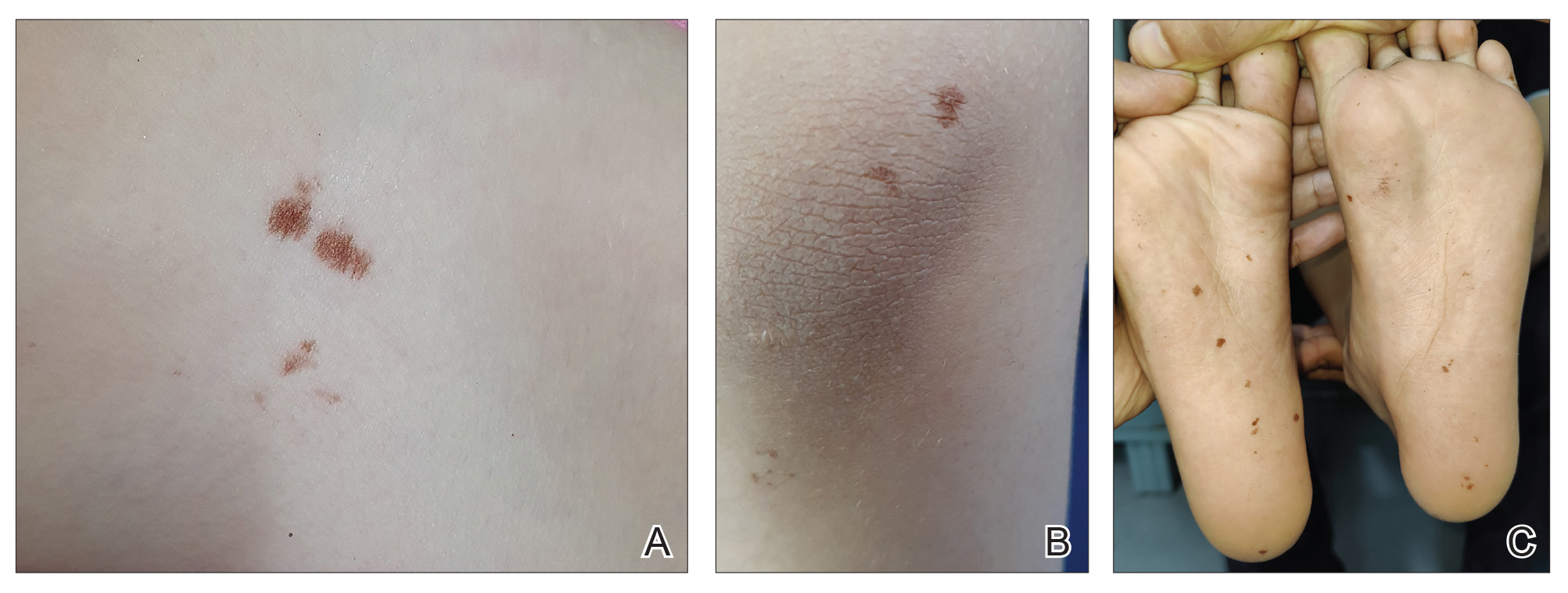
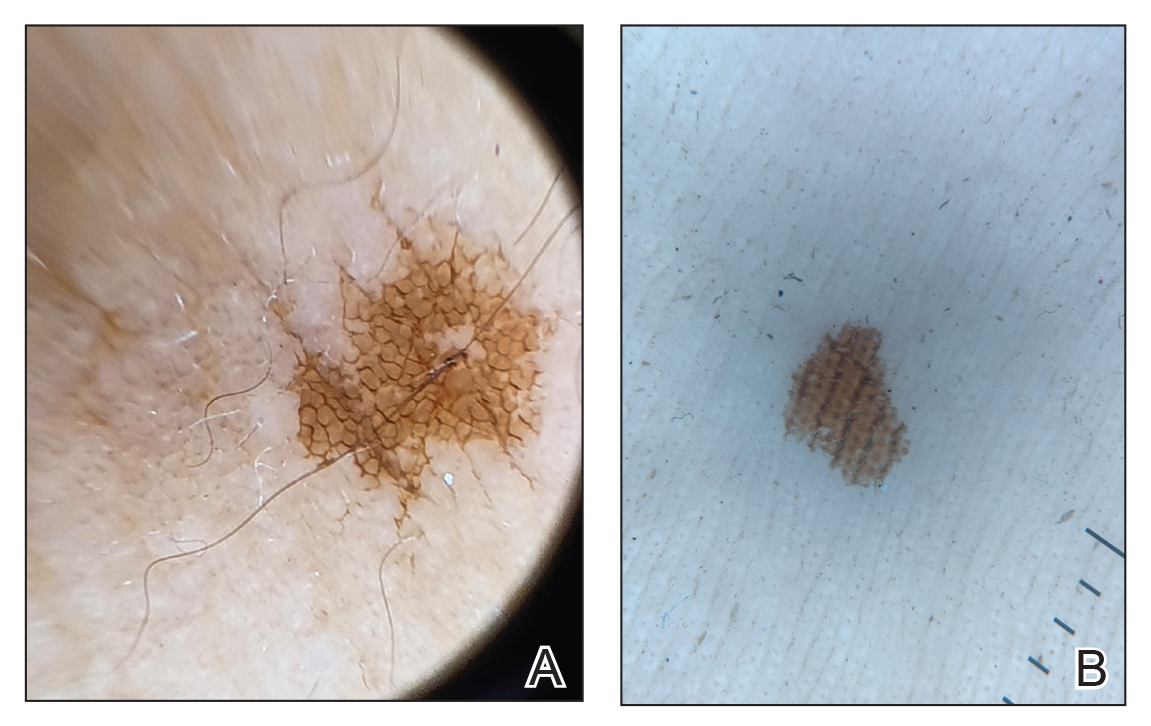
One of the children’s parents conducted a geological examination of the ground in the neighborhood park during evening hours and found tiny burrowing bugs (eFigure 3). When crushed between the fingers, these insects left a similar brownish hyperpigmentation on the skin. The parents were counseled on the nature of the eruption, and the patients were kept under observation for 2 weeks. On follow-up after 5 days, the lesions showed markedly decreased intensity of hyperpigmentation, and no new lesions were observed in any of the 7 patients.

Comment
Pentatomoidae insects generally are benign and harmless to humans. There have been isolated reports of erythematous plaques caused by Antiteuchus mixtus and Edessa maculate.7 Malhotra et al8 reported the first known series of cases with Cydnidae insect–induced hyperpigmented macules. The reported patients presented with asymptomatic, brown, hyperpigmented macules over exposed sites such as the feet, neck, and chest. All the cases occurred during the monsoon season in tropical and temperate regions of the world, and the patients were characteristically clustered in similar geographic areas. The causative insect was identified as Chilocoris assmuthi Breddin, 1904, belonging to the family Cydnidae. When it was crushed between the fingers, the skin became hyperpigmented, confirming the role of the secretions from the insect in the etiology.8
A second case was described by Sonthalia,9 who also described the dermoscopic features of hyperpigmented macules caused by burrowing bugs. The lesions showed a stuck-on, clustered appearance of ovoid and bizarre pigmented clods, globules, and granules.9 Although the lesions occur mainly over exposed sites, pigmented macules occurring over unusual sites such as the abdomen and back also have been reported in association with burrowing bugs.10 Characteristically, the lesions initially are faint and darken with time and usually fade within a week. They can be rubbed off with acetone but persist when washed with soap and water. The fleeting nature of the pigmentation also has led to the term transient pseudo-lentigines sign to describe hyperpigmentation caused by burrowing bugs.11
Soil and plants are burrowing bugs’ natural habitats, and the insects typically are seen in vegetation-rich, moist areas adjoining human dwellings (eg, parks, gardens), where clusters of cases can occur. These insects proliferate during the monsoon season in tropical and temperate areas, leading to more cases occurring during these months.
Compared to prior reports,8,9 a few of our patients had predominant trunk and neck involvement with an occasional tingling sensation or pruritus while the rest were asymptomatic. Dermoscopic features from our patients shared similar reported features of Cydnidae pigmentation.4,5 The accentuation of pigment over skin creases seen on dermoscopy was due to accumulation of Cydnidae secretion at these sites.
The differential diagnosis commonly includes idiopathic macular eruptive pigmentation, which is characterized by an asymptomatic progressive eruption of hyperpigmented macules over the trunk that persists from a few months up to 3 years. Other conditions in the differential include benign conditions such as acral benign melanocytic nevi, lentigines, pigmented purpuric dermatosis, and postinflammatory hyperpigmentation, as well as malignant conditions such as acral melanoma. Dermoscopy is a helpful, easy-to-use tool in differentiating these pigmentation disorders, obviating the need for an invasive investigation such as histopathologic analysis. Simultaneous involvement in a group of people living together or visiting the same place, abrupt onset, predominant involvement of the exposed sites, characteristic clinical and dermoscopic features, self-limiting course, and timing with the monsoon season should suggest a possibility of Cydnidae dermatitis/pigmentation, which can be confirmed by finding the causative bug in the affected locality.
Management
No specific treatment is required for the pigmentation caused by Cydnidae, as it is self-resolving. The macules can, however, be removed with acetone. Patients must be counseled regarding the benign and fleeting nature of this condition, as the abrupt onset may alarm them of a systemic disease. Affected patients should be advised against walking barefoot in areas where the insects can be found. Spraying insecticides in the affected locality also helps to reduce the presence of burrowing bugs.
- Hosokawa T, Kikuchi Y, Nikoh N, et al. Polyphyly of gut symbionts in stinkbugs of the family Cydnidae. Appl Environ Microbiol. 2012; 78:4758-4761.
- Schwertner CF, Nardi C. Burrower bugs (Cydnidae). In: Panizzi A, Grazia J, eds. True Bugs (Heteroptera) of the Neotropics. Entomology in Focus, vol 2. Springer; 2015.
- Lis JA. Burrower bugs of the Old World: a catalogue (Hemiptera: Heteroptera: Cydnidae). Genus (Wroclaw). 1999;10:165-249.
- Hayashi N, Yamamura Y, Ôhama S, et al. Defensive substances from stink bugs of Cydnidae. Experientia. 1976;32:418-419.
- Smith RM. The defensive secretion of the bugs Lampropharadifasciata, Adrisanumeensis, and Tectocorisdiophthalmus from Fiji. NZ J Zool. 1978;5:821-822.
- Krall BS, Zilkowski BW, Kight SL, et al. Chemistry and defensive efficacy of secretion of burrowing bugs. J Chem Ecol. 1997;23:1951-1962.
- Haddad V Jr, Cardoso J, Moraes R. Skin lesions caused by stink bugs (Insecta: Heteroptera: Pentatomidae): first report of dermatological injuries in humans. Wilderness Environ Med. 2002;13:48-50.
- Malhotra AK, Lis JA, Ramam M. Cydnidae (burrowing bug) pigmentation: a novel arthropod dermatosis. JAMA Dermatol. 2015;151:232-233.
- Sonthalia S. Dermoscopy of Cydnidae pigmentation: a novel disorder of pigmentation. Dermatol Pract Concept. 2019;9:228-229.
- Poojary S, Baddireddy K. Demystifying the stinking reddish brown stains through the dermoscope: Cydnidae pigmentation. Indian Dermatol Online J. 2019;10:757-758.
- Amrani A, Das A. Cydnidae pigmentation: unusual location on the abdomen and back. Br J Dermatol. 2021;184:E125.
Cydnidae is a family of small to medium-sized shield bugs with spiny legs that commonly are known as burrowing bugs (or burrower bugs). The family Cydnidae includes more than 100 genera and approximately 600 species worldwide.1 These insects are arthropods of the order Hemiptera (suborder: Heteroptera; superfamily: Pentatomoidae) and largely are concentrated in tropical and temperate regions. Approximately 145 species have been recorded in the Neotropical Region and have been included in the subfamilies Amnestinae, Cephalocteinae, and Sehirinae, in addition to Cydnidae.2 Burrowing bugs are ovoid in shape and 2 to 20 mm in length and morphologically are well adapted for burrowing. Their life span is 100 to 300 days. Being phytophagous, they burrow to feed on plants and roots. Adult burrowing bugs have wings and can fly. They have specialized glands located in either the abdomen (nymph) or thorax (adult) that secrete odorous chemicals for self-protection.3 The secretions contain hydrocarbonates that function as repellents and danger signals, can cause paralysis in prey, and act as a chemoattractant for mates.4-6 They also cause hyperpigmentation upon contact with the skin.
In this article, we present a series of cases from the same community to demonstrate the characteristic features of hyperpigmented macules caused by exposure to burrowing bugs. Dermatologists should be aware of this entity to prevent misdiagnosis and unnecessary investigations and treatment.
Case Series
A 36-year-old woman and 6 children (age range, 6-12 years) presented with a widespread, acute, brown-pigmented, macular eruption with lesions that increased in number over a 1-week period. All 7 patients resided in the same locality and were otherwise systemically healthy. Initially, the index case, a 7-year-old girl, was referred to our tertiary care center by a dermatologist with a provisional diagnosis of idiopathic macular eruptive pigmentation. The patient’s mother recalled noticing a tiny black insect on the child's scalp that left pigment on the skin when she crushed it between her fingers. The rest of the patients presented over the next few days: 3 of the children belonged to the same household as the index case, and there was history of all 6 children playing in the neighborhood park during late evening hours. The adult patient was the parent of one of the affected children. The lesions were associated with mild itching and tingling in 3 children but were asymptomatic in the other patients.
Clinical examination of the patients revealed multiple dark- to light-brown, discrete, irregularly shaped macules over the trunk, arms, and soles (eFigure 1). Dermoscopic examination of a pigmented macule showed an irregularly shaped, brownish, structureless area with accentuation of the pigment at skin creases and perieccrine pigmentation (eFigure 2). The pigmentation was unaffected by rubbing with alcohol or water. Clinicoepidemiologic parameters of the patients are summarized in the eTable.



One of the children’s parents conducted a geological examination of the ground in the neighborhood park during evening hours and found tiny burrowing bugs (eFigure 3). When crushed between the fingers, these insects left a similar brownish hyperpigmentation on the skin. The parents were counseled on the nature of the eruption, and the patients were kept under observation for 2 weeks. On follow-up after 5 days, the lesions showed markedly decreased intensity of hyperpigmentation, and no new lesions were observed in any of the 7 patients.

Comment
Pentatomoidae insects generally are benign and harmless to humans. There have been isolated reports of erythematous plaques caused by Antiteuchus mixtus and Edessa maculate.7 Malhotra et al8 reported the first known series of cases with Cydnidae insect–induced hyperpigmented macules. The reported patients presented with asymptomatic, brown, hyperpigmented macules over exposed sites such as the feet, neck, and chest. All the cases occurred during the monsoon season in tropical and temperate regions of the world, and the patients were characteristically clustered in similar geographic areas. The causative insect was identified as Chilocoris assmuthi Breddin, 1904, belonging to the family Cydnidae. When it was crushed between the fingers, the skin became hyperpigmented, confirming the role of the secretions from the insect in the etiology.8
A second case was described by Sonthalia,9 who also described the dermoscopic features of hyperpigmented macules caused by burrowing bugs. The lesions showed a stuck-on, clustered appearance of ovoid and bizarre pigmented clods, globules, and granules.9 Although the lesions occur mainly over exposed sites, pigmented macules occurring over unusual sites such as the abdomen and back also have been reported in association with burrowing bugs.10 Characteristically, the lesions initially are faint and darken with time and usually fade within a week. They can be rubbed off with acetone but persist when washed with soap and water. The fleeting nature of the pigmentation also has led to the term transient pseudo-lentigines sign to describe hyperpigmentation caused by burrowing bugs.11
Soil and plants are burrowing bugs’ natural habitats, and the insects typically are seen in vegetation-rich, moist areas adjoining human dwellings (eg, parks, gardens), where clusters of cases can occur. These insects proliferate during the monsoon season in tropical and temperate areas, leading to more cases occurring during these months.
Compared to prior reports,8,9 a few of our patients had predominant trunk and neck involvement with an occasional tingling sensation or pruritus while the rest were asymptomatic. Dermoscopic features from our patients shared similar reported features of Cydnidae pigmentation.4,5 The accentuation of pigment over skin creases seen on dermoscopy was due to accumulation of Cydnidae secretion at these sites.
The differential diagnosis commonly includes idiopathic macular eruptive pigmentation, which is characterized by an asymptomatic progressive eruption of hyperpigmented macules over the trunk that persists from a few months up to 3 years. Other conditions in the differential include benign conditions such as acral benign melanocytic nevi, lentigines, pigmented purpuric dermatosis, and postinflammatory hyperpigmentation, as well as malignant conditions such as acral melanoma. Dermoscopy is a helpful, easy-to-use tool in differentiating these pigmentation disorders, obviating the need for an invasive investigation such as histopathologic analysis. Simultaneous involvement in a group of people living together or visiting the same place, abrupt onset, predominant involvement of the exposed sites, characteristic clinical and dermoscopic features, self-limiting course, and timing with the monsoon season should suggest a possibility of Cydnidae dermatitis/pigmentation, which can be confirmed by finding the causative bug in the affected locality.
Management
No specific treatment is required for the pigmentation caused by Cydnidae, as it is self-resolving. The macules can, however, be removed with acetone. Patients must be counseled regarding the benign and fleeting nature of this condition, as the abrupt onset may alarm them of a systemic disease. Affected patients should be advised against walking barefoot in areas where the insects can be found. Spraying insecticides in the affected locality also helps to reduce the presence of burrowing bugs.
Cydnidae is a family of small to medium-sized shield bugs with spiny legs that commonly are known as burrowing bugs (or burrower bugs). The family Cydnidae includes more than 100 genera and approximately 600 species worldwide.1 These insects are arthropods of the order Hemiptera (suborder: Heteroptera; superfamily: Pentatomoidae) and largely are concentrated in tropical and temperate regions. Approximately 145 species have been recorded in the Neotropical Region and have been included in the subfamilies Amnestinae, Cephalocteinae, and Sehirinae, in addition to Cydnidae.2 Burrowing bugs are ovoid in shape and 2 to 20 mm in length and morphologically are well adapted for burrowing. Their life span is 100 to 300 days. Being phytophagous, they burrow to feed on plants and roots. Adult burrowing bugs have wings and can fly. They have specialized glands located in either the abdomen (nymph) or thorax (adult) that secrete odorous chemicals for self-protection.3 The secretions contain hydrocarbonates that function as repellents and danger signals, can cause paralysis in prey, and act as a chemoattractant for mates.4-6 They also cause hyperpigmentation upon contact with the skin.
In this article, we present a series of cases from the same community to demonstrate the characteristic features of hyperpigmented macules caused by exposure to burrowing bugs. Dermatologists should be aware of this entity to prevent misdiagnosis and unnecessary investigations and treatment.
Case Series
A 36-year-old woman and 6 children (age range, 6-12 years) presented with a widespread, acute, brown-pigmented, macular eruption with lesions that increased in number over a 1-week period. All 7 patients resided in the same locality and were otherwise systemically healthy. Initially, the index case, a 7-year-old girl, was referred to our tertiary care center by a dermatologist with a provisional diagnosis of idiopathic macular eruptive pigmentation. The patient’s mother recalled noticing a tiny black insect on the child's scalp that left pigment on the skin when she crushed it between her fingers. The rest of the patients presented over the next few days: 3 of the children belonged to the same household as the index case, and there was history of all 6 children playing in the neighborhood park during late evening hours. The adult patient was the parent of one of the affected children. The lesions were associated with mild itching and tingling in 3 children but were asymptomatic in the other patients.
Clinical examination of the patients revealed multiple dark- to light-brown, discrete, irregularly shaped macules over the trunk, arms, and soles (eFigure 1). Dermoscopic examination of a pigmented macule showed an irregularly shaped, brownish, structureless area with accentuation of the pigment at skin creases and perieccrine pigmentation (eFigure 2). The pigmentation was unaffected by rubbing with alcohol or water. Clinicoepidemiologic parameters of the patients are summarized in the eTable.



One of the children’s parents conducted a geological examination of the ground in the neighborhood park during evening hours and found tiny burrowing bugs (eFigure 3). When crushed between the fingers, these insects left a similar brownish hyperpigmentation on the skin. The parents were counseled on the nature of the eruption, and the patients were kept under observation for 2 weeks. On follow-up after 5 days, the lesions showed markedly decreased intensity of hyperpigmentation, and no new lesions were observed in any of the 7 patients.

Comment
Pentatomoidae insects generally are benign and harmless to humans. There have been isolated reports of erythematous plaques caused by Antiteuchus mixtus and Edessa maculate.7 Malhotra et al8 reported the first known series of cases with Cydnidae insect–induced hyperpigmented macules. The reported patients presented with asymptomatic, brown, hyperpigmented macules over exposed sites such as the feet, neck, and chest. All the cases occurred during the monsoon season in tropical and temperate regions of the world, and the patients were characteristically clustered in similar geographic areas. The causative insect was identified as Chilocoris assmuthi Breddin, 1904, belonging to the family Cydnidae. When it was crushed between the fingers, the skin became hyperpigmented, confirming the role of the secretions from the insect in the etiology.8
A second case was described by Sonthalia,9 who also described the dermoscopic features of hyperpigmented macules caused by burrowing bugs. The lesions showed a stuck-on, clustered appearance of ovoid and bizarre pigmented clods, globules, and granules.9 Although the lesions occur mainly over exposed sites, pigmented macules occurring over unusual sites such as the abdomen and back also have been reported in association with burrowing bugs.10 Characteristically, the lesions initially are faint and darken with time and usually fade within a week. They can be rubbed off with acetone but persist when washed with soap and water. The fleeting nature of the pigmentation also has led to the term transient pseudo-lentigines sign to describe hyperpigmentation caused by burrowing bugs.11
Soil and plants are burrowing bugs’ natural habitats, and the insects typically are seen in vegetation-rich, moist areas adjoining human dwellings (eg, parks, gardens), where clusters of cases can occur. These insects proliferate during the monsoon season in tropical and temperate areas, leading to more cases occurring during these months.
Compared to prior reports,8,9 a few of our patients had predominant trunk and neck involvement with an occasional tingling sensation or pruritus while the rest were asymptomatic. Dermoscopic features from our patients shared similar reported features of Cydnidae pigmentation.4,5 The accentuation of pigment over skin creases seen on dermoscopy was due to accumulation of Cydnidae secretion at these sites.
The differential diagnosis commonly includes idiopathic macular eruptive pigmentation, which is characterized by an asymptomatic progressive eruption of hyperpigmented macules over the trunk that persists from a few months up to 3 years. Other conditions in the differential include benign conditions such as acral benign melanocytic nevi, lentigines, pigmented purpuric dermatosis, and postinflammatory hyperpigmentation, as well as malignant conditions such as acral melanoma. Dermoscopy is a helpful, easy-to-use tool in differentiating these pigmentation disorders, obviating the need for an invasive investigation such as histopathologic analysis. Simultaneous involvement in a group of people living together or visiting the same place, abrupt onset, predominant involvement of the exposed sites, characteristic clinical and dermoscopic features, self-limiting course, and timing with the monsoon season should suggest a possibility of Cydnidae dermatitis/pigmentation, which can be confirmed by finding the causative bug in the affected locality.
Management
No specific treatment is required for the pigmentation caused by Cydnidae, as it is self-resolving. The macules can, however, be removed with acetone. Patients must be counseled regarding the benign and fleeting nature of this condition, as the abrupt onset may alarm them of a systemic disease. Affected patients should be advised against walking barefoot in areas where the insects can be found. Spraying insecticides in the affected locality also helps to reduce the presence of burrowing bugs.
- Hosokawa T, Kikuchi Y, Nikoh N, et al. Polyphyly of gut symbionts in stinkbugs of the family Cydnidae. Appl Environ Microbiol. 2012; 78:4758-4761.
- Schwertner CF, Nardi C. Burrower bugs (Cydnidae). In: Panizzi A, Grazia J, eds. True Bugs (Heteroptera) of the Neotropics. Entomology in Focus, vol 2. Springer; 2015.
- Lis JA. Burrower bugs of the Old World: a catalogue (Hemiptera: Heteroptera: Cydnidae). Genus (Wroclaw). 1999;10:165-249.
- Hayashi N, Yamamura Y, Ôhama S, et al. Defensive substances from stink bugs of Cydnidae. Experientia. 1976;32:418-419.
- Smith RM. The defensive secretion of the bugs Lampropharadifasciata, Adrisanumeensis, and Tectocorisdiophthalmus from Fiji. NZ J Zool. 1978;5:821-822.
- Krall BS, Zilkowski BW, Kight SL, et al. Chemistry and defensive efficacy of secretion of burrowing bugs. J Chem Ecol. 1997;23:1951-1962.
- Haddad V Jr, Cardoso J, Moraes R. Skin lesions caused by stink bugs (Insecta: Heteroptera: Pentatomidae): first report of dermatological injuries in humans. Wilderness Environ Med. 2002;13:48-50.
- Malhotra AK, Lis JA, Ramam M. Cydnidae (burrowing bug) pigmentation: a novel arthropod dermatosis. JAMA Dermatol. 2015;151:232-233.
- Sonthalia S. Dermoscopy of Cydnidae pigmentation: a novel disorder of pigmentation. Dermatol Pract Concept. 2019;9:228-229.
- Poojary S, Baddireddy K. Demystifying the stinking reddish brown stains through the dermoscope: Cydnidae pigmentation. Indian Dermatol Online J. 2019;10:757-758.
- Amrani A, Das A. Cydnidae pigmentation: unusual location on the abdomen and back. Br J Dermatol. 2021;184:E125.
- Hosokawa T, Kikuchi Y, Nikoh N, et al. Polyphyly of gut symbionts in stinkbugs of the family Cydnidae. Appl Environ Microbiol. 2012; 78:4758-4761.
- Schwertner CF, Nardi C. Burrower bugs (Cydnidae). In: Panizzi A, Grazia J, eds. True Bugs (Heteroptera) of the Neotropics. Entomology in Focus, vol 2. Springer; 2015.
- Lis JA. Burrower bugs of the Old World: a catalogue (Hemiptera: Heteroptera: Cydnidae). Genus (Wroclaw). 1999;10:165-249.
- Hayashi N, Yamamura Y, Ôhama S, et al. Defensive substances from stink bugs of Cydnidae. Experientia. 1976;32:418-419.
- Smith RM. The defensive secretion of the bugs Lampropharadifasciata, Adrisanumeensis, and Tectocorisdiophthalmus from Fiji. NZ J Zool. 1978;5:821-822.
- Krall BS, Zilkowski BW, Kight SL, et al. Chemistry and defensive efficacy of secretion of burrowing bugs. J Chem Ecol. 1997;23:1951-1962.
- Haddad V Jr, Cardoso J, Moraes R. Skin lesions caused by stink bugs (Insecta: Heteroptera: Pentatomidae): first report of dermatological injuries in humans. Wilderness Environ Med. 2002;13:48-50.
- Malhotra AK, Lis JA, Ramam M. Cydnidae (burrowing bug) pigmentation: a novel arthropod dermatosis. JAMA Dermatol. 2015;151:232-233.
- Sonthalia S. Dermoscopy of Cydnidae pigmentation: a novel disorder of pigmentation. Dermatol Pract Concept. 2019;9:228-229.
- Poojary S, Baddireddy K. Demystifying the stinking reddish brown stains through the dermoscope: Cydnidae pigmentation. Indian Dermatol Online J. 2019;10:757-758.
- Amrani A, Das A. Cydnidae pigmentation: unusual location on the abdomen and back. Br J Dermatol. 2021;184:E125.
Hyperpigmented Macules Caused by Burrowing Bugs (Cydnidae) May Mimic More Serious Conditions
Hyperpigmented Macules Caused by Burrowing Bugs (Cydnidae) May Mimic More Serious Conditions
Practice Points
- Burrowing bugs (Cydnidae) are phytophagous and burrow to feed on plants and roots. They are more numerous during the monsoon season in tropical and temperate regions.
- Secretions from burrowing bugs cause asymptomatic, hyperpigmented, irregularly shaped macules suggestive of an exogenous cause that commonly affect clusters of patients from the same geographic locality.
- The lesions are self-limiting and must be differentiated from close mimickers to ensure adequate and appropriate patient counseling.
Tapping Into Relief: A Distraction Technique to Reduce Pain During Dermatologic Procedures
Tapping Into Relief: A Distraction Technique to Reduce Pain During Dermatologic Procedures
Practice Gap
Pain during minimally invasive dermatologic procedures such as lidocaine injections, cryotherapy, nail unit injections, and cosmetic procedures including neurotoxin injections can cause patient discomfort leading to procedural anxiety, poor compliance with treatment regimens, and avoidance of necessary care. Current solutions to manage pain during dermatologic procedures present several limitations; for example, topical anesthetics seldom alleviate procedural pain,1 particularly in sensitive areas (eg, nail unit, face) or for patients with a needle phobia. Additionally, topical anesthetics often require up to 2 hours to take effect, making them impractical for quick outpatient procedures. Other pain reduction strategies including vibration devices or cold sprays2,3 can be effective but are an added expense to the physician or clinic, which may preclude their use in resource-limited settings. Psychological distraction techniques such as deep breathing require active patient participation and might reinforce pain expectations and increase patient anxiety.4 Given these challenges, there is a need for effective, affordable, nonpharmacologic pain reduction strategies that can be integrated seamlessly into clinical practice to enhance the patient experience.
The Technique
Tapping is a simple noninvasive distraction technique that may alleviate procedural pain by exploiting the gate control theory of pain.5 According to this theory, tactile stimuli activate mechanoreceptors that send inhibitory signals to the spinal cord, effectively closing the gate to pain transmission. Unlike the Helfer skin tap technique,6 which involves 15 preinjection taps and 3 postinjection taps directly on the injection site, our approach targets distant bony prominences. This modification allows for immediate needle insertion without interfering with the sterile field or increasing the risk for needlestick injuries from tapping near the injection site. Bony sites such as the shoulder or knee are ideal for this technique due to their high density and rigidity that efficiently transmit tactile stimuli––similar to how sound travels faster through solids than through liquids or gases.7
To implement this technique in practice, we first stabilize the injection site to reduce movement from tapping. This can be done by stabilizing the injection site (eg, resting the hand on an instrument stand during a nail unit injection). A second person—such as a medical assistant, medical student, resident, or even the patient’s family member—taps at a distant site at least an arm’s length away from the injection site (Figure). The tapping pressure should be firm enough for the patient to feel the vibration but not forceful enough that it becomes unpleasant or disrupts the injection area. Tapping starts just before needle insertion and continues through the injection. No warning is given to the patient, as the surprise element may help distract them from pain. Varying the rhythm, intensity, or location of the tapping can enhance its distracting effect.
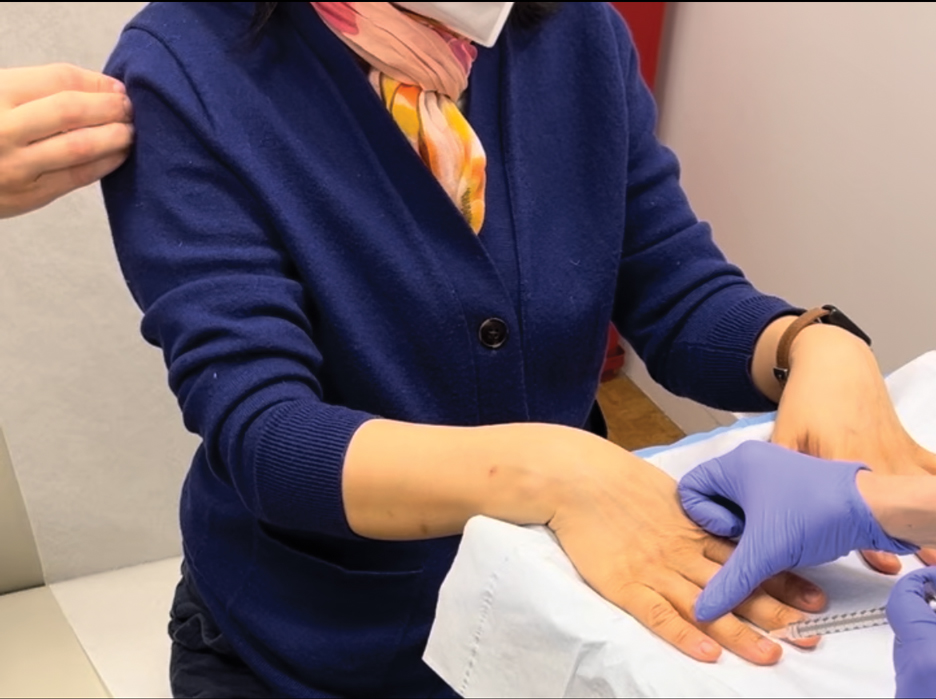
This tapping technique can be effectively combined with other pain reduction strategies in a multimodal approach; for example, when used concurrently with topical anesthetics, both the central (tapping) and peripheral (anesthetic) pain pathways are addressed, potentially yielding additive effects. For patients with a needle phobia, pairing tapping with cognitive distraction (eg, talkesthesia) may further reduce anxiety. In our nail specialty clinic at Weill Cornell Medicine (New York, New York), we often combine tapping with cold sprays and talkesthesia, which improves patient comfort without prolonging the visit. Importantly, the technique enables seamless integration with most pharmacologic and nonpharmacologic methods, eliminating the need for additional patient education or procedure time.
Practice Implications
The tapping technique described here is free, easy to implement, and requires no additional resources aside from another person to tap the patient during the procedure. It can be used for a wide range of dermatologic procedures, including biopsies, intralesional injections, and cosmetic treatments, including neurotoxin injections. The minimal learning curve and ease of integration into procedural workflows make this technique a valuable tool for dermatologists aiming to improve patient comfort without disrupting workflow. In our practice, we have observed that tapping reduces self-reported pain and helps ease anxiety, particularly in patients with a needle phobia. Its simplicity and accessibility make it a valuable addition to a wide range of dermatologic procedures. Prospective studies investigating patient-reported outcomes could help establish this technique’s role in clinical practice.
- Navarro-Rodriguez JM, Suarez-Serrano C, Martin-Valero R, et al. Effectiveness of topical anesthetics in pain management for dermal injuries: a systematic review. J Clin Med. 2021;10:2522. doi:10.3390/jcm10112522
- Lipner SR. Pain-minimizing strategies for nail surgery. Cutis. 2018;101:76-77.
- Ricardo JW, Lipner SR. Air cooling for improved analgesia during local anesthetic infiltration for nail surgery. J Am Acad Dermatol. 2021;84:e231-e232. doi:10.1016/j.jaad.2019.11.032
- Hill RC, Chernoff KA, Lipner SR. A breath of fresh air: use of deep breathing technique to minimize pain with nail injections. J Am Acad Dermatol. 2024;90:e163. doi:10.1016/j.jaad.2023.10.043
- Mendell LM. Constructing and deconstructing the gate theory of pain. Pain. 2014;155:210-216. doi:10.1016/j.pain.2013.12.010
- Jyoti G, Arora S, Sharma B. Helfer Skin Tap Tech Technique for the IM injection pain among adult patients. Nursing & Midwifery Research Journal. 2018;14:18-30. doi:10.1177/0974150X20180304
- Iowa State University. Nondestructive Evaluation Physics: Sound. Published 2021. Accessed July 31, 2025. https://www.nde-ed.org/Physics/Sound/speedinmaterials.xhtml
Practice Gap
Pain during minimally invasive dermatologic procedures such as lidocaine injections, cryotherapy, nail unit injections, and cosmetic procedures including neurotoxin injections can cause patient discomfort leading to procedural anxiety, poor compliance with treatment regimens, and avoidance of necessary care. Current solutions to manage pain during dermatologic procedures present several limitations; for example, topical anesthetics seldom alleviate procedural pain,1 particularly in sensitive areas (eg, nail unit, face) or for patients with a needle phobia. Additionally, topical anesthetics often require up to 2 hours to take effect, making them impractical for quick outpatient procedures. Other pain reduction strategies including vibration devices or cold sprays2,3 can be effective but are an added expense to the physician or clinic, which may preclude their use in resource-limited settings. Psychological distraction techniques such as deep breathing require active patient participation and might reinforce pain expectations and increase patient anxiety.4 Given these challenges, there is a need for effective, affordable, nonpharmacologic pain reduction strategies that can be integrated seamlessly into clinical practice to enhance the patient experience.
The Technique
Tapping is a simple noninvasive distraction technique that may alleviate procedural pain by exploiting the gate control theory of pain.5 According to this theory, tactile stimuli activate mechanoreceptors that send inhibitory signals to the spinal cord, effectively closing the gate to pain transmission. Unlike the Helfer skin tap technique,6 which involves 15 preinjection taps and 3 postinjection taps directly on the injection site, our approach targets distant bony prominences. This modification allows for immediate needle insertion without interfering with the sterile field or increasing the risk for needlestick injuries from tapping near the injection site. Bony sites such as the shoulder or knee are ideal for this technique due to their high density and rigidity that efficiently transmit tactile stimuli––similar to how sound travels faster through solids than through liquids or gases.7
To implement this technique in practice, we first stabilize the injection site to reduce movement from tapping. This can be done by stabilizing the injection site (eg, resting the hand on an instrument stand during a nail unit injection). A second person—such as a medical assistant, medical student, resident, or even the patient’s family member—taps at a distant site at least an arm’s length away from the injection site (Figure). The tapping pressure should be firm enough for the patient to feel the vibration but not forceful enough that it becomes unpleasant or disrupts the injection area. Tapping starts just before needle insertion and continues through the injection. No warning is given to the patient, as the surprise element may help distract them from pain. Varying the rhythm, intensity, or location of the tapping can enhance its distracting effect.

This tapping technique can be effectively combined with other pain reduction strategies in a multimodal approach; for example, when used concurrently with topical anesthetics, both the central (tapping) and peripheral (anesthetic) pain pathways are addressed, potentially yielding additive effects. For patients with a needle phobia, pairing tapping with cognitive distraction (eg, talkesthesia) may further reduce anxiety. In our nail specialty clinic at Weill Cornell Medicine (New York, New York), we often combine tapping with cold sprays and talkesthesia, which improves patient comfort without prolonging the visit. Importantly, the technique enables seamless integration with most pharmacologic and nonpharmacologic methods, eliminating the need for additional patient education or procedure time.
Practice Implications
The tapping technique described here is free, easy to implement, and requires no additional resources aside from another person to tap the patient during the procedure. It can be used for a wide range of dermatologic procedures, including biopsies, intralesional injections, and cosmetic treatments, including neurotoxin injections. The minimal learning curve and ease of integration into procedural workflows make this technique a valuable tool for dermatologists aiming to improve patient comfort without disrupting workflow. In our practice, we have observed that tapping reduces self-reported pain and helps ease anxiety, particularly in patients with a needle phobia. Its simplicity and accessibility make it a valuable addition to a wide range of dermatologic procedures. Prospective studies investigating patient-reported outcomes could help establish this technique’s role in clinical practice.
Practice Gap
Pain during minimally invasive dermatologic procedures such as lidocaine injections, cryotherapy, nail unit injections, and cosmetic procedures including neurotoxin injections can cause patient discomfort leading to procedural anxiety, poor compliance with treatment regimens, and avoidance of necessary care. Current solutions to manage pain during dermatologic procedures present several limitations; for example, topical anesthetics seldom alleviate procedural pain,1 particularly in sensitive areas (eg, nail unit, face) or for patients with a needle phobia. Additionally, topical anesthetics often require up to 2 hours to take effect, making them impractical for quick outpatient procedures. Other pain reduction strategies including vibration devices or cold sprays2,3 can be effective but are an added expense to the physician or clinic, which may preclude their use in resource-limited settings. Psychological distraction techniques such as deep breathing require active patient participation and might reinforce pain expectations and increase patient anxiety.4 Given these challenges, there is a need for effective, affordable, nonpharmacologic pain reduction strategies that can be integrated seamlessly into clinical practice to enhance the patient experience.
The Technique
Tapping is a simple noninvasive distraction technique that may alleviate procedural pain by exploiting the gate control theory of pain.5 According to this theory, tactile stimuli activate mechanoreceptors that send inhibitory signals to the spinal cord, effectively closing the gate to pain transmission. Unlike the Helfer skin tap technique,6 which involves 15 preinjection taps and 3 postinjection taps directly on the injection site, our approach targets distant bony prominences. This modification allows for immediate needle insertion without interfering with the sterile field or increasing the risk for needlestick injuries from tapping near the injection site. Bony sites such as the shoulder or knee are ideal for this technique due to their high density and rigidity that efficiently transmit tactile stimuli––similar to how sound travels faster through solids than through liquids or gases.7
To implement this technique in practice, we first stabilize the injection site to reduce movement from tapping. This can be done by stabilizing the injection site (eg, resting the hand on an instrument stand during a nail unit injection). A second person—such as a medical assistant, medical student, resident, or even the patient’s family member—taps at a distant site at least an arm’s length away from the injection site (Figure). The tapping pressure should be firm enough for the patient to feel the vibration but not forceful enough that it becomes unpleasant or disrupts the injection area. Tapping starts just before needle insertion and continues through the injection. No warning is given to the patient, as the surprise element may help distract them from pain. Varying the rhythm, intensity, or location of the tapping can enhance its distracting effect.

This tapping technique can be effectively combined with other pain reduction strategies in a multimodal approach; for example, when used concurrently with topical anesthetics, both the central (tapping) and peripheral (anesthetic) pain pathways are addressed, potentially yielding additive effects. For patients with a needle phobia, pairing tapping with cognitive distraction (eg, talkesthesia) may further reduce anxiety. In our nail specialty clinic at Weill Cornell Medicine (New York, New York), we often combine tapping with cold sprays and talkesthesia, which improves patient comfort without prolonging the visit. Importantly, the technique enables seamless integration with most pharmacologic and nonpharmacologic methods, eliminating the need for additional patient education or procedure time.
Practice Implications
The tapping technique described here is free, easy to implement, and requires no additional resources aside from another person to tap the patient during the procedure. It can be used for a wide range of dermatologic procedures, including biopsies, intralesional injections, and cosmetic treatments, including neurotoxin injections. The minimal learning curve and ease of integration into procedural workflows make this technique a valuable tool for dermatologists aiming to improve patient comfort without disrupting workflow. In our practice, we have observed that tapping reduces self-reported pain and helps ease anxiety, particularly in patients with a needle phobia. Its simplicity and accessibility make it a valuable addition to a wide range of dermatologic procedures. Prospective studies investigating patient-reported outcomes could help establish this technique’s role in clinical practice.
- Navarro-Rodriguez JM, Suarez-Serrano C, Martin-Valero R, et al. Effectiveness of topical anesthetics in pain management for dermal injuries: a systematic review. J Clin Med. 2021;10:2522. doi:10.3390/jcm10112522
- Lipner SR. Pain-minimizing strategies for nail surgery. Cutis. 2018;101:76-77.
- Ricardo JW, Lipner SR. Air cooling for improved analgesia during local anesthetic infiltration for nail surgery. J Am Acad Dermatol. 2021;84:e231-e232. doi:10.1016/j.jaad.2019.11.032
- Hill RC, Chernoff KA, Lipner SR. A breath of fresh air: use of deep breathing technique to minimize pain with nail injections. J Am Acad Dermatol. 2024;90:e163. doi:10.1016/j.jaad.2023.10.043
- Mendell LM. Constructing and deconstructing the gate theory of pain. Pain. 2014;155:210-216. doi:10.1016/j.pain.2013.12.010
- Jyoti G, Arora S, Sharma B. Helfer Skin Tap Tech Technique for the IM injection pain among adult patients. Nursing & Midwifery Research Journal. 2018;14:18-30. doi:10.1177/0974150X20180304
- Iowa State University. Nondestructive Evaluation Physics: Sound. Published 2021. Accessed July 31, 2025. https://www.nde-ed.org/Physics/Sound/speedinmaterials.xhtml
- Navarro-Rodriguez JM, Suarez-Serrano C, Martin-Valero R, et al. Effectiveness of topical anesthetics in pain management for dermal injuries: a systematic review. J Clin Med. 2021;10:2522. doi:10.3390/jcm10112522
- Lipner SR. Pain-minimizing strategies for nail surgery. Cutis. 2018;101:76-77.
- Ricardo JW, Lipner SR. Air cooling for improved analgesia during local anesthetic infiltration for nail surgery. J Am Acad Dermatol. 2021;84:e231-e232. doi:10.1016/j.jaad.2019.11.032
- Hill RC, Chernoff KA, Lipner SR. A breath of fresh air: use of deep breathing technique to minimize pain with nail injections. J Am Acad Dermatol. 2024;90:e163. doi:10.1016/j.jaad.2023.10.043
- Mendell LM. Constructing and deconstructing the gate theory of pain. Pain. 2014;155:210-216. doi:10.1016/j.pain.2013.12.010
- Jyoti G, Arora S, Sharma B. Helfer Skin Tap Tech Technique for the IM injection pain among adult patients. Nursing & Midwifery Research Journal. 2018;14:18-30. doi:10.1177/0974150X20180304
- Iowa State University. Nondestructive Evaluation Physics: Sound. Published 2021. Accessed July 31, 2025. https://www.nde-ed.org/Physics/Sound/speedinmaterials.xhtml
Tapping Into Relief: A Distraction Technique to Reduce Pain During Dermatologic Procedures
Tapping Into Relief: A Distraction Technique to Reduce Pain During Dermatologic Procedures
A Cross-Sectional Analysis of TikTok Skin Care Routines and the Associated Environmental Impact
A Cross-Sectional Analysis of TikTok Skin Care Routines and the Associated Environmental Impact
To the Editor:
The popularity of the social media platform TikTok, which is known for its short-form videos, has surged in recent years. Viral videos demonstrating skin care routines reach millions of viewers,1 showcasing specific products, detailing beauty regimens, and setting fads that many users eagerly follow. These trends often influence consumer behavior—in 2023, viral videos using the tag #TikTokMadeMeBuy lead to a 14% growth in the sale of skin care products.2 However, they also encourage purchasing decisions that may escalate environmental waste through plastic packaging and single-use products. In this study, we analyzed videos on TikTok to assess the environmental impact of trending skin care routines. By examining the types of products promoted, their packaging, and the frequency with which they appear in viral content, we aimed to investigate how these trends, which may be imitated by users, impact the environment.
A search of TikTok videos using #skincareroutine was conducted on June 21, 2024. Sponsored content, non–English language videos, videos without demonstrated skin care routines, and videos showing makeup routines were excluded from our analysis. Data collected from each video included username, date posted, number of likes, total number of skin care products used, number of single-use skin care products used, average amount of product used, number of skin care applicators used, and number of single-use applicators used. Single-use items, defined as those intended for one-time use and subsequent disposal, were identified visually by packaging, manufacturer intent, and common consumer usage patterns. The amount of product used per application was graded on a scale of 1 to 3 (1=pea-sized amount or less; 2=single full pump/spray; 3=multiple pumps/sprays). Videos were categorized as personal (ie, skin care routine walk-throughs by the creator) or autonomous sensory meridian response (ASMR)(focused on product sounds and aesthetics).3 A Mann-Whitney U test was utilized to statistically compare the 2 groups. Statistical analysis was performed using Microsoft Excel (α=0.05).
A total of 50 videos met the inclusion criteria and were included in the analysis. The average number of likes per video was 499,696.15, with skin care routines featuring an average of 6.4 unique products (Table). There was a weak positive correlation (r=0.1809) between the number of skin care products used and the number of likes. A total of 320 products were used across the videos, 23 of which were single-use (7.2%).On average, single-use skin care items were used 0.46 times per routine, comprising a mean 7.99% of total products per video. The average score for the amount of product used per application was 2.18. There was no difference in personal vs ASMR videos with regard to the total number of skin care products used or the average amount of product used per application (P>.05). Thirty-three (70.2%) of the 47 applicators used across all videos were single-use. An average of 0.94 applicators per routine were utilized, with a mean 68.83% being single-use applicators. Common single-use products were toner wipes and eye patches, and single-use applicators included cotton pads and plastic spatulas.
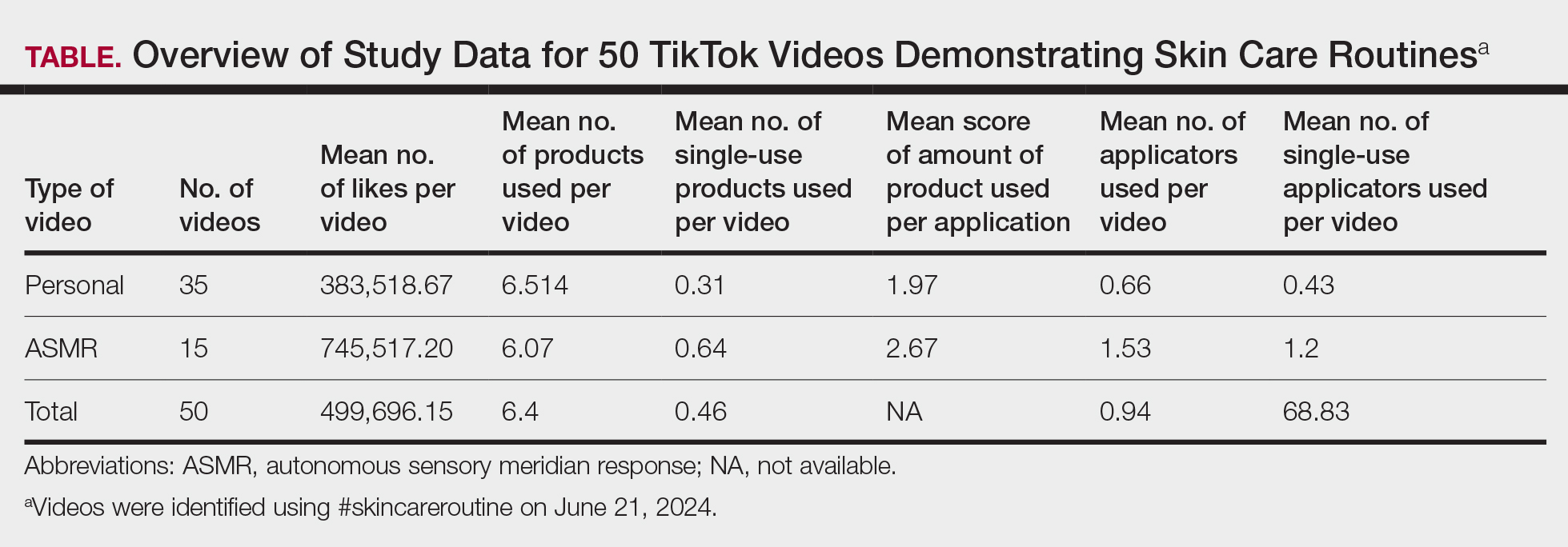
Our findings indicated a prevalence of multiple products and large amount of product used in trending skin care routines, suggesting a shift toward multistep skin care. This implies a high rate of product consumption that may accelerate the carbon footprint associated with skin care products,3 which could contribute to climate change and environmental degradation. Consumers also may feel compelled to purchase and discard numerous partially used products in order to keep up with the latest trends, exacerbating the environmental impact. Furthermore, the utilization of single-use products and applicators contributes to increased plastic waste, pollution, and resource depletion. Single-use items often are difficult to recycle due to their mixed materials and small size,4,5 and therefore they can accumulate in landfills and oceans. This impact can be mitigated by switching to reusable applicators, refillable packaging, and biodegradable materials.
The substantial average number of likes per video indicates high engagement with skin care content among TikTok users. The continued popularity of complex multistep skin care routines, despite a weak correlation between the number of skin care products used and the number of likes per video, likely stems from factors such as aesthetic appeal, ASMR effects, and creators’ established followings, which may drive user engagement to contribute to unsustainable consumption patterns. Factors such as presentation style, aesthetics, or creators’ pre-existing online following may have a major impact on how well a video performs on TikTok. The similarity between personal and ASMR videos, particularly in the number of products used and the amount applied, suggests that both formats employ common approaches to meet audience expectations and align with promotional trends, relying more on sensory and aesthetic strategies than substantive differences in skin care routines.
Our use of only one tag in our search as well as the subjective quantity scale limits the generalizability of these findings to broader TikTok skin care content.
Overall, our study underscores the role of brands and social media influencers in skin care education and promotion of sustainable practices. The extensive number of products used and generous application of each product in skin care routines demonstrated in TikTok videos may mislead viewers into believing that using more product improves outcomes, when often, less is more. We recommend that dermatologists counsel patients about informed skin care regimens that prioritize individual needs over social media fads.
- Pagani K, Lukac D, Martinez R, et al. Slugging: TikTokTM as a source of a viral “harmless” beauty trend. Clin Dermatol. 2022;40:810-812. doi:10.1016/j.clindermatol.2022.08.005
- Stern C. TikTok drives $31.7B in beauty sales: how viral trends are shaping the future of cosmetics. CosmeticsDesign. August 20, 2024. Accessed June 24, 2025. https://www.cosmeticsdesign.com/Article/2024/08/20/tiktok-drives-31.7b-in-beauty-sales-how-viral-trends-are-shaping-the-future-of-cosmetics/
- Fountain C. ASMR content saw huge growth on YouTube, but now creators are flocking to TikTok instead. Business Insider. July 4, 2022. Accessed June 24, 2025. https://www.businessinsider.com/asmr-tiktok-instead-of-youtube-growth-subscribers-2022-7
- Rathore S, Schuler B, Park J. Life cycle assessment of multiple dispensing systems used for cosmetic product packaging. Packaging Technol Sci. 2023;36:533-547. doi:10.1002/pts.2729
- Shaw S. How to actually recycle your empty beauty products. CNN Underscored. Updated April 17, 2024. Accessed June 24, 2025. https://www.cnn.com/cnn-underscored/beauty/how-to-recycle-beauty-products
To the Editor:
The popularity of the social media platform TikTok, which is known for its short-form videos, has surged in recent years. Viral videos demonstrating skin care routines reach millions of viewers,1 showcasing specific products, detailing beauty regimens, and setting fads that many users eagerly follow. These trends often influence consumer behavior—in 2023, viral videos using the tag #TikTokMadeMeBuy lead to a 14% growth in the sale of skin care products.2 However, they also encourage purchasing decisions that may escalate environmental waste through plastic packaging and single-use products. In this study, we analyzed videos on TikTok to assess the environmental impact of trending skin care routines. By examining the types of products promoted, their packaging, and the frequency with which they appear in viral content, we aimed to investigate how these trends, which may be imitated by users, impact the environment.
A search of TikTok videos using #skincareroutine was conducted on June 21, 2024. Sponsored content, non–English language videos, videos without demonstrated skin care routines, and videos showing makeup routines were excluded from our analysis. Data collected from each video included username, date posted, number of likes, total number of skin care products used, number of single-use skin care products used, average amount of product used, number of skin care applicators used, and number of single-use applicators used. Single-use items, defined as those intended for one-time use and subsequent disposal, were identified visually by packaging, manufacturer intent, and common consumer usage patterns. The amount of product used per application was graded on a scale of 1 to 3 (1=pea-sized amount or less; 2=single full pump/spray; 3=multiple pumps/sprays). Videos were categorized as personal (ie, skin care routine walk-throughs by the creator) or autonomous sensory meridian response (ASMR)(focused on product sounds and aesthetics).3 A Mann-Whitney U test was utilized to statistically compare the 2 groups. Statistical analysis was performed using Microsoft Excel (α=0.05).
A total of 50 videos met the inclusion criteria and were included in the analysis. The average number of likes per video was 499,696.15, with skin care routines featuring an average of 6.4 unique products (Table). There was a weak positive correlation (r=0.1809) between the number of skin care products used and the number of likes. A total of 320 products were used across the videos, 23 of which were single-use (7.2%).On average, single-use skin care items were used 0.46 times per routine, comprising a mean 7.99% of total products per video. The average score for the amount of product used per application was 2.18. There was no difference in personal vs ASMR videos with regard to the total number of skin care products used or the average amount of product used per application (P>.05). Thirty-three (70.2%) of the 47 applicators used across all videos were single-use. An average of 0.94 applicators per routine were utilized, with a mean 68.83% being single-use applicators. Common single-use products were toner wipes and eye patches, and single-use applicators included cotton pads and plastic spatulas.

Our findings indicated a prevalence of multiple products and large amount of product used in trending skin care routines, suggesting a shift toward multistep skin care. This implies a high rate of product consumption that may accelerate the carbon footprint associated with skin care products,3 which could contribute to climate change and environmental degradation. Consumers also may feel compelled to purchase and discard numerous partially used products in order to keep up with the latest trends, exacerbating the environmental impact. Furthermore, the utilization of single-use products and applicators contributes to increased plastic waste, pollution, and resource depletion. Single-use items often are difficult to recycle due to their mixed materials and small size,4,5 and therefore they can accumulate in landfills and oceans. This impact can be mitigated by switching to reusable applicators, refillable packaging, and biodegradable materials.
The substantial average number of likes per video indicates high engagement with skin care content among TikTok users. The continued popularity of complex multistep skin care routines, despite a weak correlation between the number of skin care products used and the number of likes per video, likely stems from factors such as aesthetic appeal, ASMR effects, and creators’ established followings, which may drive user engagement to contribute to unsustainable consumption patterns. Factors such as presentation style, aesthetics, or creators’ pre-existing online following may have a major impact on how well a video performs on TikTok. The similarity between personal and ASMR videos, particularly in the number of products used and the amount applied, suggests that both formats employ common approaches to meet audience expectations and align with promotional trends, relying more on sensory and aesthetic strategies than substantive differences in skin care routines.
Our use of only one tag in our search as well as the subjective quantity scale limits the generalizability of these findings to broader TikTok skin care content.
Overall, our study underscores the role of brands and social media influencers in skin care education and promotion of sustainable practices. The extensive number of products used and generous application of each product in skin care routines demonstrated in TikTok videos may mislead viewers into believing that using more product improves outcomes, when often, less is more. We recommend that dermatologists counsel patients about informed skin care regimens that prioritize individual needs over social media fads.
To the Editor:
The popularity of the social media platform TikTok, which is known for its short-form videos, has surged in recent years. Viral videos demonstrating skin care routines reach millions of viewers,1 showcasing specific products, detailing beauty regimens, and setting fads that many users eagerly follow. These trends often influence consumer behavior—in 2023, viral videos using the tag #TikTokMadeMeBuy lead to a 14% growth in the sale of skin care products.2 However, they also encourage purchasing decisions that may escalate environmental waste through plastic packaging and single-use products. In this study, we analyzed videos on TikTok to assess the environmental impact of trending skin care routines. By examining the types of products promoted, their packaging, and the frequency with which they appear in viral content, we aimed to investigate how these trends, which may be imitated by users, impact the environment.
A search of TikTok videos using #skincareroutine was conducted on June 21, 2024. Sponsored content, non–English language videos, videos without demonstrated skin care routines, and videos showing makeup routines were excluded from our analysis. Data collected from each video included username, date posted, number of likes, total number of skin care products used, number of single-use skin care products used, average amount of product used, number of skin care applicators used, and number of single-use applicators used. Single-use items, defined as those intended for one-time use and subsequent disposal, were identified visually by packaging, manufacturer intent, and common consumer usage patterns. The amount of product used per application was graded on a scale of 1 to 3 (1=pea-sized amount or less; 2=single full pump/spray; 3=multiple pumps/sprays). Videos were categorized as personal (ie, skin care routine walk-throughs by the creator) or autonomous sensory meridian response (ASMR)(focused on product sounds and aesthetics).3 A Mann-Whitney U test was utilized to statistically compare the 2 groups. Statistical analysis was performed using Microsoft Excel (α=0.05).
A total of 50 videos met the inclusion criteria and were included in the analysis. The average number of likes per video was 499,696.15, with skin care routines featuring an average of 6.4 unique products (Table). There was a weak positive correlation (r=0.1809) between the number of skin care products used and the number of likes. A total of 320 products were used across the videos, 23 of which were single-use (7.2%).On average, single-use skin care items were used 0.46 times per routine, comprising a mean 7.99% of total products per video. The average score for the amount of product used per application was 2.18. There was no difference in personal vs ASMR videos with regard to the total number of skin care products used or the average amount of product used per application (P>.05). Thirty-three (70.2%) of the 47 applicators used across all videos were single-use. An average of 0.94 applicators per routine were utilized, with a mean 68.83% being single-use applicators. Common single-use products were toner wipes and eye patches, and single-use applicators included cotton pads and plastic spatulas.

Our findings indicated a prevalence of multiple products and large amount of product used in trending skin care routines, suggesting a shift toward multistep skin care. This implies a high rate of product consumption that may accelerate the carbon footprint associated with skin care products,3 which could contribute to climate change and environmental degradation. Consumers also may feel compelled to purchase and discard numerous partially used products in order to keep up with the latest trends, exacerbating the environmental impact. Furthermore, the utilization of single-use products and applicators contributes to increased plastic waste, pollution, and resource depletion. Single-use items often are difficult to recycle due to their mixed materials and small size,4,5 and therefore they can accumulate in landfills and oceans. This impact can be mitigated by switching to reusable applicators, refillable packaging, and biodegradable materials.
The substantial average number of likes per video indicates high engagement with skin care content among TikTok users. The continued popularity of complex multistep skin care routines, despite a weak correlation between the number of skin care products used and the number of likes per video, likely stems from factors such as aesthetic appeal, ASMR effects, and creators’ established followings, which may drive user engagement to contribute to unsustainable consumption patterns. Factors such as presentation style, aesthetics, or creators’ pre-existing online following may have a major impact on how well a video performs on TikTok. The similarity between personal and ASMR videos, particularly in the number of products used and the amount applied, suggests that both formats employ common approaches to meet audience expectations and align with promotional trends, relying more on sensory and aesthetic strategies than substantive differences in skin care routines.
Our use of only one tag in our search as well as the subjective quantity scale limits the generalizability of these findings to broader TikTok skin care content.
Overall, our study underscores the role of brands and social media influencers in skin care education and promotion of sustainable practices. The extensive number of products used and generous application of each product in skin care routines demonstrated in TikTok videos may mislead viewers into believing that using more product improves outcomes, when often, less is more. We recommend that dermatologists counsel patients about informed skin care regimens that prioritize individual needs over social media fads.
- Pagani K, Lukac D, Martinez R, et al. Slugging: TikTokTM as a source of a viral “harmless” beauty trend. Clin Dermatol. 2022;40:810-812. doi:10.1016/j.clindermatol.2022.08.005
- Stern C. TikTok drives $31.7B in beauty sales: how viral trends are shaping the future of cosmetics. CosmeticsDesign. August 20, 2024. Accessed June 24, 2025. https://www.cosmeticsdesign.com/Article/2024/08/20/tiktok-drives-31.7b-in-beauty-sales-how-viral-trends-are-shaping-the-future-of-cosmetics/
- Fountain C. ASMR content saw huge growth on YouTube, but now creators are flocking to TikTok instead. Business Insider. July 4, 2022. Accessed June 24, 2025. https://www.businessinsider.com/asmr-tiktok-instead-of-youtube-growth-subscribers-2022-7
- Rathore S, Schuler B, Park J. Life cycle assessment of multiple dispensing systems used for cosmetic product packaging. Packaging Technol Sci. 2023;36:533-547. doi:10.1002/pts.2729
- Shaw S. How to actually recycle your empty beauty products. CNN Underscored. Updated April 17, 2024. Accessed June 24, 2025. https://www.cnn.com/cnn-underscored/beauty/how-to-recycle-beauty-products
- Pagani K, Lukac D, Martinez R, et al. Slugging: TikTokTM as a source of a viral “harmless” beauty trend. Clin Dermatol. 2022;40:810-812. doi:10.1016/j.clindermatol.2022.08.005
- Stern C. TikTok drives $31.7B in beauty sales: how viral trends are shaping the future of cosmetics. CosmeticsDesign. August 20, 2024. Accessed June 24, 2025. https://www.cosmeticsdesign.com/Article/2024/08/20/tiktok-drives-31.7b-in-beauty-sales-how-viral-trends-are-shaping-the-future-of-cosmetics/
- Fountain C. ASMR content saw huge growth on YouTube, but now creators are flocking to TikTok instead. Business Insider. July 4, 2022. Accessed June 24, 2025. https://www.businessinsider.com/asmr-tiktok-instead-of-youtube-growth-subscribers-2022-7
- Rathore S, Schuler B, Park J. Life cycle assessment of multiple dispensing systems used for cosmetic product packaging. Packaging Technol Sci. 2023;36:533-547. doi:10.1002/pts.2729
- Shaw S. How to actually recycle your empty beauty products. CNN Underscored. Updated April 17, 2024. Accessed June 24, 2025. https://www.cnn.com/cnn-underscored/beauty/how-to-recycle-beauty-products
A Cross-Sectional Analysis of TikTok Skin Care Routines and the Associated Environmental Impact
A Cross-Sectional Analysis of TikTok Skin Care Routines and the Associated Environmental Impact
PRACTICE POINTS
- Social media platforms are increasingly influential in shaping consumer skin care habits, particularly among younger demographics.
- Dermatologists should be aware of the aesthetic-driven nature of online skin care trends when advising patients on product use.
- Viral skin care routines often feature multiple products and applicators, potentially encouraging excessive product use and waste.
From Refractory to Responsive: The Expanding Therapeutic Landscape of Prurigo Nodularis
From Refractory to Responsive: The Expanding Therapeutic Landscape of Prurigo Nodularis
Prurigo nodularis (PN) is a chronic, severely pruritic neuroimmunologic skin disorder characterized by multiple firm hyperkeratotic nodules and intense pruritus, often leading to considerable impairment in quality of life and increased rates of depression and anxiety.1 It is considered difficult to treat due to its complex pathogenesis, the severity and chronicity of pruritus, and the limited efficacy of conventional therapies.2,3 The disease is driven by a self-perpetuating itch-scratch cycle, underpinned by dysregulation of both immune and neural pathways including type 2 (interleukin [IL] 4, IL-13, IL-31), Th17, and Th22 cytokines as well as neuropeptides and altered cutaneous nerve architecture.1,3 This results in persistent severe pruritus and nodular lesions that are highly refractory to standard treatments.1 Conventional therapies (eg, locally acting agents, phototherapy, and systemic immunomodulators and neuromodulators) have varied efficacy and notable adverse effect profiles.3 While the approval of targeted biologics has transformed the therapeutic landscape, several other treatment options also are being explored in clinical trials. Herein, we review all recently approved therapies as well as emerging treatments currently under investigation.
Dupilumab
Dupilumab, the first therapy for PN approved by the US Food and Drug Administration (FDA) in 2022—is a monoclonal antibody that inhibits signaling of IL-4 and IL-13, key drivers of type 2 inflammation implicated in PN pathogenesis.4,5 In 2 pivotal phase 3 randomized controlled trials (LIBERTY-PN PRIME and PRIME2),5 dupilumab demonstrated notable efficacy in adults with moderate to severe PN. A reduction of 4 points or more on the Worst Itch Numeric Rating Scale (WI-NRS) was achieved by 60.0% (45/75) of patients treated with dupilumab at week 24 compared with 18.4% (14/76) receiving placebo in the PRIME trial. In PRIME2, the same outcome was achieved by 37.2% (29/78) of patients receiving dupilumab at week 12 compared with 22.0% (18/82) of patients receiving placebo.5 Dupilumab also led to a greater proportion of patients achieving a substantial reduction in nodule count (≤5 nodules) and improved quality of life compared with placebo.5,6 The safety profile of dupilumab for treatment of PN was favorable and consistent with prior experience in atopic dermatitis; conjunctivitis was the most common adverse event.5,6
Nemolizumab
Nemolizumab, an IL-31 receptor A antagonist, is the most recent agent approved by the FDA for PN in 2024.7 In the OLYMPIA 1 and OLYMPIA 2 phase 3 trials,8 nemolizumab produced a clinically meaningful reduction in itch (defined as a ≥4-point improvement in the Peak Pruritus Numerical Rating Scale score) in 56.3% (103/183) of patients at week 16 compared with 20.9% (19/91) receiving placebo. Additionally, 37.7% (69/183) of patients receiving nemolizumab achieved clear or almost clear skin (Investigator’s Global Assessment score of 0 or 1 with a ≥2-point reduction) vs 11.0% with placebo (both P<.001). Benefits were observed as early as week 4, including rapid improvements in itch, sleep disturbance, and nodule count.8 Nemolizumab also improved quality of life and reduced symptoms of anxiety and depression. The safety profile was favorable, with headache and atopic dermatitis the most common adverse events; serious adverse events were infrequent and similar between groups.8
Abrocitinib
Abrocitinib, an oral selective Janus kinase 1 inhibitor, is an investigational therapy for PN and currently has not been approved by the FDA for this indication. In a phase 2 open-label trial, abrocitinib 200 mg daily for 12 weeks led to a 78.3% reduction in weekly Peak Pruritus Numerical Rating Scale scores in PN, with 80.0% (8/10) of patients achieving a clinically meaningful improvement of 4 points or higher. Nodule counts and quality of life also improved, with an onset of itch relief as early as week 2. The safety profile was favorable, with acneform eruptions the most common adverse event and no serious adverse events reported9; however, these results are based on small, nonrandomized studies and require confirmation in larger randomized controlled trials before abrocitinib can be considered a standard therapy for PN.
Cryosim-1
Transient receptor potential melastatin 8 (TRPM8) is a cold-sensing ion channel found in unmyelinated sensory neurons within the dorsal root and trigeminal ganglia.10 It is activated by cool temperatures (15-28 °C) and compounds such as menthol, leading to calcium influx and a cooling sensation. In a randomized, double-blind, vehicle-controlled trial, researchers investigated the efficacy of cryosim-1 (a synthetic TRPM8 agonist) in treating PN.10 Thirty patients were enrolled, with 18 (60.0%) receiving cryosim-1 and 12 (40.0%) receiving placebo over 8 weeks. By week 8, cryosim-1 significantly reduced itch severity (mean numerical rating scale score postapplication, 2.8 vs 4.3; P=.031) and improved sleep disturbances (2.2 vs 4.2; P=.031) compared to placebo. Patients reported higher satisfaction with itch relief, and no adverse effects were observed. The study concluded that cryosim-1 is a safe, effective topical therapy for PN, likely working by interrupting the itch-scratch cycle and potentially modulating inflammatory pathways involved in chronic itch.10
Nalbuphine
Nalbuphine is a κ opioid receptor agonist and μ opioid receptor antagonist that has been investigated for the treatment of PN.11 In a phase 2 randomized controlled trial, oral nalbuphine extended release (NAL-ER) 162 mg twice daily provided measurable antipruritic efficacy, with 44.4% (8/18) of patients achieving at least a 30% reduction in 7-day WI-NRS at week 10 compared with 36.4% (8/22) in the placebo group. Among those who completed the study, 66.7% (8/12) of patients receiving NAL-ER 162 mg achieved significant itch reduction vs 40% (8/20) receiving placebo (P=.03). At least a 50% reduction in WI-NRS was achieved by 33.3% (6/18) of patients receiving NAL-ER 162 mg twice daily. Extended open-label treatment was associated with further improvements in itch and lesion activity. Adverse events were mostly mild to moderate (eg, nausea, dizziness, headache, and fatigue) and occurred during dose titration. Physiologic opioid withdrawal symptoms were limited and resolved within a few days of discontinuing the medication.11
Final Thoughts
In conclusion, PN remains one of the most challenging chronic dermatologic conditions to manage and is driven by a complex interplay of neuroimmune mechanisms and resistance to many conventional therapies. The approval of dupilumab and nemolizumab has marked a pivotal shift in the therapeutic landscape, offering hope to patients who previously had limited options5,8; however, the burden of PN remains substantial, and many patients continue to experience relentless itch, poor sleep, and reduced quality of life.1 Emerging therapies such as TRPM8 agonists, Janus kinase inhibitors, and opioid modulators represent promising additions to the treatment options, targeting novel pathways beyond traditional immunosuppression.9-11
- Williams KA, Huang AH, Belzberg M, et al. Prurigo nodularis: pathogenesis and management. J Am Acad Dermatol. 2020;83:1567-1575. doi:10.1016/j.jaad.2020.04.182
- Gründel S, Pereira MP, Storck M, et al. Analysis of 325 patients with chronic nodular prurigo: clinics, burden of disease and course of treatment. Acta Derm Venereol. 2020;100:adv00269. doi:10.2340/00015555-3571
- Liao V, Cornman HL, Ma E, et al. Prurigo nodularis: new insights into pathogenesis and novel therapeutics. Br J Dermatol. 2024;190:798-810. doi:10.1093/bjd/ljae052
- Elmariah SB, Tao L, Valdes-Rodriguez R, et al. Individual article: management of prurigo nodularis. J Drugs Dermatol. 2023;22:SF365502s15-SF365502s22. doi:10.36849/JDD.SF365502
- Yosipovitch G, Mollanazar N, Ständer S, et al. Dupilumab in patients with prurigo nodularis: two randomized, double-blind, placebo-controlled phase 3 trials. Nat Med. 2023;29:1180-1190. doi:10.1038/s41591-023-02320-9
- Cao P, Xu W, Jiang S, et al. Dupilumab for the treatment of prurigo nodularis: a systematic review. Front Immunol. 2023;14:1092685. doi:10.3389/fimmu.2023.1092685
- Dagenet CB, Saadi C, Phillips MA, et al. Landscape of prurigo nodularis clinical trials. JAAD Rev. 2024;2:127-136. doi:10.1016/j.jdrv.2024.09.006
- Kwatra SG, Yosipovitch G, Legat FJ, et al. Phase 3 trial of nemolizumab in patients with prurigo nodularis. N Engl J Med. 2023;389:1579-1589. doi:10.1056/NEJMoa2301333
- Kwatra SG, Bordeaux ZA, Parthasarathy V, et al. Efficacy and safety of abrocitinib in prurigo nodularis and chronic pruritus of unknown origin: a nonrandomized controlled trial. JAMA Dermatol. 2024;160:717-724. doi:10.1001/jamadermatol.2024.1464
- Choi ME, Lee JH, Jung CJ, et al. A randomized, double-blinded, vehicle-controlled clinical trial of topical cryosim-1, a synthetic TRPM8 agonist, in prurigo nodularis. J Cosmet Dermatol. 2024;23:931-937. doi:10.1111/jocd.16079
- Weisshaar E, Szepietowski JC, Bernhard JD, et al. Efficacy and safety of oral nalbuphine extended release in prurigo nodularis: results of a phase 2 randomized controlled trial with an open-label extension phase. J Eur Acad Dermatol Venereol. 2022;36:453-461. doi:10.1111/jdv.17816
Prurigo nodularis (PN) is a chronic, severely pruritic neuroimmunologic skin disorder characterized by multiple firm hyperkeratotic nodules and intense pruritus, often leading to considerable impairment in quality of life and increased rates of depression and anxiety.1 It is considered difficult to treat due to its complex pathogenesis, the severity and chronicity of pruritus, and the limited efficacy of conventional therapies.2,3 The disease is driven by a self-perpetuating itch-scratch cycle, underpinned by dysregulation of both immune and neural pathways including type 2 (interleukin [IL] 4, IL-13, IL-31), Th17, and Th22 cytokines as well as neuropeptides and altered cutaneous nerve architecture.1,3 This results in persistent severe pruritus and nodular lesions that are highly refractory to standard treatments.1 Conventional therapies (eg, locally acting agents, phototherapy, and systemic immunomodulators and neuromodulators) have varied efficacy and notable adverse effect profiles.3 While the approval of targeted biologics has transformed the therapeutic landscape, several other treatment options also are being explored in clinical trials. Herein, we review all recently approved therapies as well as emerging treatments currently under investigation.
Dupilumab
Dupilumab, the first therapy for PN approved by the US Food and Drug Administration (FDA) in 2022—is a monoclonal antibody that inhibits signaling of IL-4 and IL-13, key drivers of type 2 inflammation implicated in PN pathogenesis.4,5 In 2 pivotal phase 3 randomized controlled trials (LIBERTY-PN PRIME and PRIME2),5 dupilumab demonstrated notable efficacy in adults with moderate to severe PN. A reduction of 4 points or more on the Worst Itch Numeric Rating Scale (WI-NRS) was achieved by 60.0% (45/75) of patients treated with dupilumab at week 24 compared with 18.4% (14/76) receiving placebo in the PRIME trial. In PRIME2, the same outcome was achieved by 37.2% (29/78) of patients receiving dupilumab at week 12 compared with 22.0% (18/82) of patients receiving placebo.5 Dupilumab also led to a greater proportion of patients achieving a substantial reduction in nodule count (≤5 nodules) and improved quality of life compared with placebo.5,6 The safety profile of dupilumab for treatment of PN was favorable and consistent with prior experience in atopic dermatitis; conjunctivitis was the most common adverse event.5,6
Nemolizumab
Nemolizumab, an IL-31 receptor A antagonist, is the most recent agent approved by the FDA for PN in 2024.7 In the OLYMPIA 1 and OLYMPIA 2 phase 3 trials,8 nemolizumab produced a clinically meaningful reduction in itch (defined as a ≥4-point improvement in the Peak Pruritus Numerical Rating Scale score) in 56.3% (103/183) of patients at week 16 compared with 20.9% (19/91) receiving placebo. Additionally, 37.7% (69/183) of patients receiving nemolizumab achieved clear or almost clear skin (Investigator’s Global Assessment score of 0 or 1 with a ≥2-point reduction) vs 11.0% with placebo (both P<.001). Benefits were observed as early as week 4, including rapid improvements in itch, sleep disturbance, and nodule count.8 Nemolizumab also improved quality of life and reduced symptoms of anxiety and depression. The safety profile was favorable, with headache and atopic dermatitis the most common adverse events; serious adverse events were infrequent and similar between groups.8
Abrocitinib
Abrocitinib, an oral selective Janus kinase 1 inhibitor, is an investigational therapy for PN and currently has not been approved by the FDA for this indication. In a phase 2 open-label trial, abrocitinib 200 mg daily for 12 weeks led to a 78.3% reduction in weekly Peak Pruritus Numerical Rating Scale scores in PN, with 80.0% (8/10) of patients achieving a clinically meaningful improvement of 4 points or higher. Nodule counts and quality of life also improved, with an onset of itch relief as early as week 2. The safety profile was favorable, with acneform eruptions the most common adverse event and no serious adverse events reported9; however, these results are based on small, nonrandomized studies and require confirmation in larger randomized controlled trials before abrocitinib can be considered a standard therapy for PN.
Cryosim-1
Transient receptor potential melastatin 8 (TRPM8) is a cold-sensing ion channel found in unmyelinated sensory neurons within the dorsal root and trigeminal ganglia.10 It is activated by cool temperatures (15-28 °C) and compounds such as menthol, leading to calcium influx and a cooling sensation. In a randomized, double-blind, vehicle-controlled trial, researchers investigated the efficacy of cryosim-1 (a synthetic TRPM8 agonist) in treating PN.10 Thirty patients were enrolled, with 18 (60.0%) receiving cryosim-1 and 12 (40.0%) receiving placebo over 8 weeks. By week 8, cryosim-1 significantly reduced itch severity (mean numerical rating scale score postapplication, 2.8 vs 4.3; P=.031) and improved sleep disturbances (2.2 vs 4.2; P=.031) compared to placebo. Patients reported higher satisfaction with itch relief, and no adverse effects were observed. The study concluded that cryosim-1 is a safe, effective topical therapy for PN, likely working by interrupting the itch-scratch cycle and potentially modulating inflammatory pathways involved in chronic itch.10
Nalbuphine
Nalbuphine is a κ opioid receptor agonist and μ opioid receptor antagonist that has been investigated for the treatment of PN.11 In a phase 2 randomized controlled trial, oral nalbuphine extended release (NAL-ER) 162 mg twice daily provided measurable antipruritic efficacy, with 44.4% (8/18) of patients achieving at least a 30% reduction in 7-day WI-NRS at week 10 compared with 36.4% (8/22) in the placebo group. Among those who completed the study, 66.7% (8/12) of patients receiving NAL-ER 162 mg achieved significant itch reduction vs 40% (8/20) receiving placebo (P=.03). At least a 50% reduction in WI-NRS was achieved by 33.3% (6/18) of patients receiving NAL-ER 162 mg twice daily. Extended open-label treatment was associated with further improvements in itch and lesion activity. Adverse events were mostly mild to moderate (eg, nausea, dizziness, headache, and fatigue) and occurred during dose titration. Physiologic opioid withdrawal symptoms were limited and resolved within a few days of discontinuing the medication.11
Final Thoughts
In conclusion, PN remains one of the most challenging chronic dermatologic conditions to manage and is driven by a complex interplay of neuroimmune mechanisms and resistance to many conventional therapies. The approval of dupilumab and nemolizumab has marked a pivotal shift in the therapeutic landscape, offering hope to patients who previously had limited options5,8; however, the burden of PN remains substantial, and many patients continue to experience relentless itch, poor sleep, and reduced quality of life.1 Emerging therapies such as TRPM8 agonists, Janus kinase inhibitors, and opioid modulators represent promising additions to the treatment options, targeting novel pathways beyond traditional immunosuppression.9-11
Prurigo nodularis (PN) is a chronic, severely pruritic neuroimmunologic skin disorder characterized by multiple firm hyperkeratotic nodules and intense pruritus, often leading to considerable impairment in quality of life and increased rates of depression and anxiety.1 It is considered difficult to treat due to its complex pathogenesis, the severity and chronicity of pruritus, and the limited efficacy of conventional therapies.2,3 The disease is driven by a self-perpetuating itch-scratch cycle, underpinned by dysregulation of both immune and neural pathways including type 2 (interleukin [IL] 4, IL-13, IL-31), Th17, and Th22 cytokines as well as neuropeptides and altered cutaneous nerve architecture.1,3 This results in persistent severe pruritus and nodular lesions that are highly refractory to standard treatments.1 Conventional therapies (eg, locally acting agents, phototherapy, and systemic immunomodulators and neuromodulators) have varied efficacy and notable adverse effect profiles.3 While the approval of targeted biologics has transformed the therapeutic landscape, several other treatment options also are being explored in clinical trials. Herein, we review all recently approved therapies as well as emerging treatments currently under investigation.
Dupilumab
Dupilumab, the first therapy for PN approved by the US Food and Drug Administration (FDA) in 2022—is a monoclonal antibody that inhibits signaling of IL-4 and IL-13, key drivers of type 2 inflammation implicated in PN pathogenesis.4,5 In 2 pivotal phase 3 randomized controlled trials (LIBERTY-PN PRIME and PRIME2),5 dupilumab demonstrated notable efficacy in adults with moderate to severe PN. A reduction of 4 points or more on the Worst Itch Numeric Rating Scale (WI-NRS) was achieved by 60.0% (45/75) of patients treated with dupilumab at week 24 compared with 18.4% (14/76) receiving placebo in the PRIME trial. In PRIME2, the same outcome was achieved by 37.2% (29/78) of patients receiving dupilumab at week 12 compared with 22.0% (18/82) of patients receiving placebo.5 Dupilumab also led to a greater proportion of patients achieving a substantial reduction in nodule count (≤5 nodules) and improved quality of life compared with placebo.5,6 The safety profile of dupilumab for treatment of PN was favorable and consistent with prior experience in atopic dermatitis; conjunctivitis was the most common adverse event.5,6
Nemolizumab
Nemolizumab, an IL-31 receptor A antagonist, is the most recent agent approved by the FDA for PN in 2024.7 In the OLYMPIA 1 and OLYMPIA 2 phase 3 trials,8 nemolizumab produced a clinically meaningful reduction in itch (defined as a ≥4-point improvement in the Peak Pruritus Numerical Rating Scale score) in 56.3% (103/183) of patients at week 16 compared with 20.9% (19/91) receiving placebo. Additionally, 37.7% (69/183) of patients receiving nemolizumab achieved clear or almost clear skin (Investigator’s Global Assessment score of 0 or 1 with a ≥2-point reduction) vs 11.0% with placebo (both P<.001). Benefits were observed as early as week 4, including rapid improvements in itch, sleep disturbance, and nodule count.8 Nemolizumab also improved quality of life and reduced symptoms of anxiety and depression. The safety profile was favorable, with headache and atopic dermatitis the most common adverse events; serious adverse events were infrequent and similar between groups.8
Abrocitinib
Abrocitinib, an oral selective Janus kinase 1 inhibitor, is an investigational therapy for PN and currently has not been approved by the FDA for this indication. In a phase 2 open-label trial, abrocitinib 200 mg daily for 12 weeks led to a 78.3% reduction in weekly Peak Pruritus Numerical Rating Scale scores in PN, with 80.0% (8/10) of patients achieving a clinically meaningful improvement of 4 points or higher. Nodule counts and quality of life also improved, with an onset of itch relief as early as week 2. The safety profile was favorable, with acneform eruptions the most common adverse event and no serious adverse events reported9; however, these results are based on small, nonrandomized studies and require confirmation in larger randomized controlled trials before abrocitinib can be considered a standard therapy for PN.
Cryosim-1
Transient receptor potential melastatin 8 (TRPM8) is a cold-sensing ion channel found in unmyelinated sensory neurons within the dorsal root and trigeminal ganglia.10 It is activated by cool temperatures (15-28 °C) and compounds such as menthol, leading to calcium influx and a cooling sensation. In a randomized, double-blind, vehicle-controlled trial, researchers investigated the efficacy of cryosim-1 (a synthetic TRPM8 agonist) in treating PN.10 Thirty patients were enrolled, with 18 (60.0%) receiving cryosim-1 and 12 (40.0%) receiving placebo over 8 weeks. By week 8, cryosim-1 significantly reduced itch severity (mean numerical rating scale score postapplication, 2.8 vs 4.3; P=.031) and improved sleep disturbances (2.2 vs 4.2; P=.031) compared to placebo. Patients reported higher satisfaction with itch relief, and no adverse effects were observed. The study concluded that cryosim-1 is a safe, effective topical therapy for PN, likely working by interrupting the itch-scratch cycle and potentially modulating inflammatory pathways involved in chronic itch.10
Nalbuphine
Nalbuphine is a κ opioid receptor agonist and μ opioid receptor antagonist that has been investigated for the treatment of PN.11 In a phase 2 randomized controlled trial, oral nalbuphine extended release (NAL-ER) 162 mg twice daily provided measurable antipruritic efficacy, with 44.4% (8/18) of patients achieving at least a 30% reduction in 7-day WI-NRS at week 10 compared with 36.4% (8/22) in the placebo group. Among those who completed the study, 66.7% (8/12) of patients receiving NAL-ER 162 mg achieved significant itch reduction vs 40% (8/20) receiving placebo (P=.03). At least a 50% reduction in WI-NRS was achieved by 33.3% (6/18) of patients receiving NAL-ER 162 mg twice daily. Extended open-label treatment was associated with further improvements in itch and lesion activity. Adverse events were mostly mild to moderate (eg, nausea, dizziness, headache, and fatigue) and occurred during dose titration. Physiologic opioid withdrawal symptoms were limited and resolved within a few days of discontinuing the medication.11
Final Thoughts
In conclusion, PN remains one of the most challenging chronic dermatologic conditions to manage and is driven by a complex interplay of neuroimmune mechanisms and resistance to many conventional therapies. The approval of dupilumab and nemolizumab has marked a pivotal shift in the therapeutic landscape, offering hope to patients who previously had limited options5,8; however, the burden of PN remains substantial, and many patients continue to experience relentless itch, poor sleep, and reduced quality of life.1 Emerging therapies such as TRPM8 agonists, Janus kinase inhibitors, and opioid modulators represent promising additions to the treatment options, targeting novel pathways beyond traditional immunosuppression.9-11
- Williams KA, Huang AH, Belzberg M, et al. Prurigo nodularis: pathogenesis and management. J Am Acad Dermatol. 2020;83:1567-1575. doi:10.1016/j.jaad.2020.04.182
- Gründel S, Pereira MP, Storck M, et al. Analysis of 325 patients with chronic nodular prurigo: clinics, burden of disease and course of treatment. Acta Derm Venereol. 2020;100:adv00269. doi:10.2340/00015555-3571
- Liao V, Cornman HL, Ma E, et al. Prurigo nodularis: new insights into pathogenesis and novel therapeutics. Br J Dermatol. 2024;190:798-810. doi:10.1093/bjd/ljae052
- Elmariah SB, Tao L, Valdes-Rodriguez R, et al. Individual article: management of prurigo nodularis. J Drugs Dermatol. 2023;22:SF365502s15-SF365502s22. doi:10.36849/JDD.SF365502
- Yosipovitch G, Mollanazar N, Ständer S, et al. Dupilumab in patients with prurigo nodularis: two randomized, double-blind, placebo-controlled phase 3 trials. Nat Med. 2023;29:1180-1190. doi:10.1038/s41591-023-02320-9
- Cao P, Xu W, Jiang S, et al. Dupilumab for the treatment of prurigo nodularis: a systematic review. Front Immunol. 2023;14:1092685. doi:10.3389/fimmu.2023.1092685
- Dagenet CB, Saadi C, Phillips MA, et al. Landscape of prurigo nodularis clinical trials. JAAD Rev. 2024;2:127-136. doi:10.1016/j.jdrv.2024.09.006
- Kwatra SG, Yosipovitch G, Legat FJ, et al. Phase 3 trial of nemolizumab in patients with prurigo nodularis. N Engl J Med. 2023;389:1579-1589. doi:10.1056/NEJMoa2301333
- Kwatra SG, Bordeaux ZA, Parthasarathy V, et al. Efficacy and safety of abrocitinib in prurigo nodularis and chronic pruritus of unknown origin: a nonrandomized controlled trial. JAMA Dermatol. 2024;160:717-724. doi:10.1001/jamadermatol.2024.1464
- Choi ME, Lee JH, Jung CJ, et al. A randomized, double-blinded, vehicle-controlled clinical trial of topical cryosim-1, a synthetic TRPM8 agonist, in prurigo nodularis. J Cosmet Dermatol. 2024;23:931-937. doi:10.1111/jocd.16079
- Weisshaar E, Szepietowski JC, Bernhard JD, et al. Efficacy and safety of oral nalbuphine extended release in prurigo nodularis: results of a phase 2 randomized controlled trial with an open-label extension phase. J Eur Acad Dermatol Venereol. 2022;36:453-461. doi:10.1111/jdv.17816
- Williams KA, Huang AH, Belzberg M, et al. Prurigo nodularis: pathogenesis and management. J Am Acad Dermatol. 2020;83:1567-1575. doi:10.1016/j.jaad.2020.04.182
- Gründel S, Pereira MP, Storck M, et al. Analysis of 325 patients with chronic nodular prurigo: clinics, burden of disease and course of treatment. Acta Derm Venereol. 2020;100:adv00269. doi:10.2340/00015555-3571
- Liao V, Cornman HL, Ma E, et al. Prurigo nodularis: new insights into pathogenesis and novel therapeutics. Br J Dermatol. 2024;190:798-810. doi:10.1093/bjd/ljae052
- Elmariah SB, Tao L, Valdes-Rodriguez R, et al. Individual article: management of prurigo nodularis. J Drugs Dermatol. 2023;22:SF365502s15-SF365502s22. doi:10.36849/JDD.SF365502
- Yosipovitch G, Mollanazar N, Ständer S, et al. Dupilumab in patients with prurigo nodularis: two randomized, double-blind, placebo-controlled phase 3 trials. Nat Med. 2023;29:1180-1190. doi:10.1038/s41591-023-02320-9
- Cao P, Xu W, Jiang S, et al. Dupilumab for the treatment of prurigo nodularis: a systematic review. Front Immunol. 2023;14:1092685. doi:10.3389/fimmu.2023.1092685
- Dagenet CB, Saadi C, Phillips MA, et al. Landscape of prurigo nodularis clinical trials. JAAD Rev. 2024;2:127-136. doi:10.1016/j.jdrv.2024.09.006
- Kwatra SG, Yosipovitch G, Legat FJ, et al. Phase 3 trial of nemolizumab in patients with prurigo nodularis. N Engl J Med. 2023;389:1579-1589. doi:10.1056/NEJMoa2301333
- Kwatra SG, Bordeaux ZA, Parthasarathy V, et al. Efficacy and safety of abrocitinib in prurigo nodularis and chronic pruritus of unknown origin: a nonrandomized controlled trial. JAMA Dermatol. 2024;160:717-724. doi:10.1001/jamadermatol.2024.1464
- Choi ME, Lee JH, Jung CJ, et al. A randomized, double-blinded, vehicle-controlled clinical trial of topical cryosim-1, a synthetic TRPM8 agonist, in prurigo nodularis. J Cosmet Dermatol. 2024;23:931-937. doi:10.1111/jocd.16079
- Weisshaar E, Szepietowski JC, Bernhard JD, et al. Efficacy and safety of oral nalbuphine extended release in prurigo nodularis: results of a phase 2 randomized controlled trial with an open-label extension phase. J Eur Acad Dermatol Venereol. 2022;36:453-461. doi:10.1111/jdv.17816
From Refractory to Responsive: The Expanding Therapeutic Landscape of Prurigo Nodularis
From Refractory to Responsive: The Expanding Therapeutic Landscape of Prurigo Nodularis