User login
Fluoroscopy-Induced Chronic Radiation Dermatitis: A Comprehensive Review and Reappraisal
Fluoroscopy-Induced Chronic Radiation Dermatitis: A Comprehensive Review and Reappraisal
Fluoroscopy is an imaging technique that allows for real-time visualization of internal structures in the body using continuous radiography beams. More than 1 million fluoroscopy-guided procedures are performed annually in the United States.1 Utilization of these procedures continues to increase, and so does the probability of related complications, as prolonged exposure to ionizing radiation can cause skin injuries.2 Fortunately, the incidence of radiation-induced skin injuries compared with the total number of fluoroscopic procedures performed remains small,2 although one study suggested the incidence may be as high as 8.9% in at-risk populations.3
Radiation dermatitis is well recognized in dermatology as a complication of oncologic management; however, radiation dermatitis as a complication of fluoroscopic procedures is underrecognized.4 Fluoroscopy-induced radiation dermatitis can be categorized as acute, subacute, or chronic.5 Common fluoroscopic procedures that have been associated with fluoroscopy-induced radiation dermatitis include interventional cardiac procedures, neurovascular procedures, transjugular intrahepatic portosystemic shunt procedures, and endovascular abdominal aortic aneurysm repairs.6,7
Patients with fluoroscopy-induced radiation dermatitis, particularly fluoroscopy-induced chronic radiation dermatitis (FICRD), can present to dermatology up to several years after the initial fluoroscopy procedure with no awareness of the association between the procedure and their skin findings. This presents a diagnostic challenge, and FICRD often is overlooked.5,8-10
We conducted a literature search of PubMed articles indexed for MEDLINE using the search terms fluoroscopy and dermatitis. In this reappraisal, we will provide a comprehensive overview of fluoroscopy-induced radiation dermatitis with an emphasis on FICRD, covering its clinical manifestations, pathophysiology, risk factors, differential diagnosis, histology, and management. The aim of this review is to highlight the salient features and mimickers of FICRD and inform readers how to approach suspected cases, leading to accurate diagnosis and effective management.
Pathophysiology
Fluoroscopy-induced radiation dermatitis is the result of dose-dependent radiation-induced tissue damage. As the peak skin dosage (PSD) of radiation increases over the course of a procedure or multiple procedures, the severity of skin injury predictably increases. During fluoroscopic procedures, the standard irradiation dosage ranges from 0.02 Gy/min to 0.05 Gy/min.11 Transient skin changes may start to be seen around 2 Gy of cumulative exposure. Fluoroscopic procedures typically range in duration from 60 to 120 minutes; however, complex cases may exceed that. Additionally, multiple procedures performed within shorter intervals can result in greater PSD accumulation. Shorter intervals between procedures do not allow enough time for damage repair from the previous procedure and can result in further severe damage when the skin is re-exposed to radiation.2 The American College of Radiology recommends medical follow-up after 10 Gy of cumulative exposure, while cumulative exposure above 15 Gy within a 6- to 12-month period is defined as a sentinel event, according to The Joint Commission.12-14
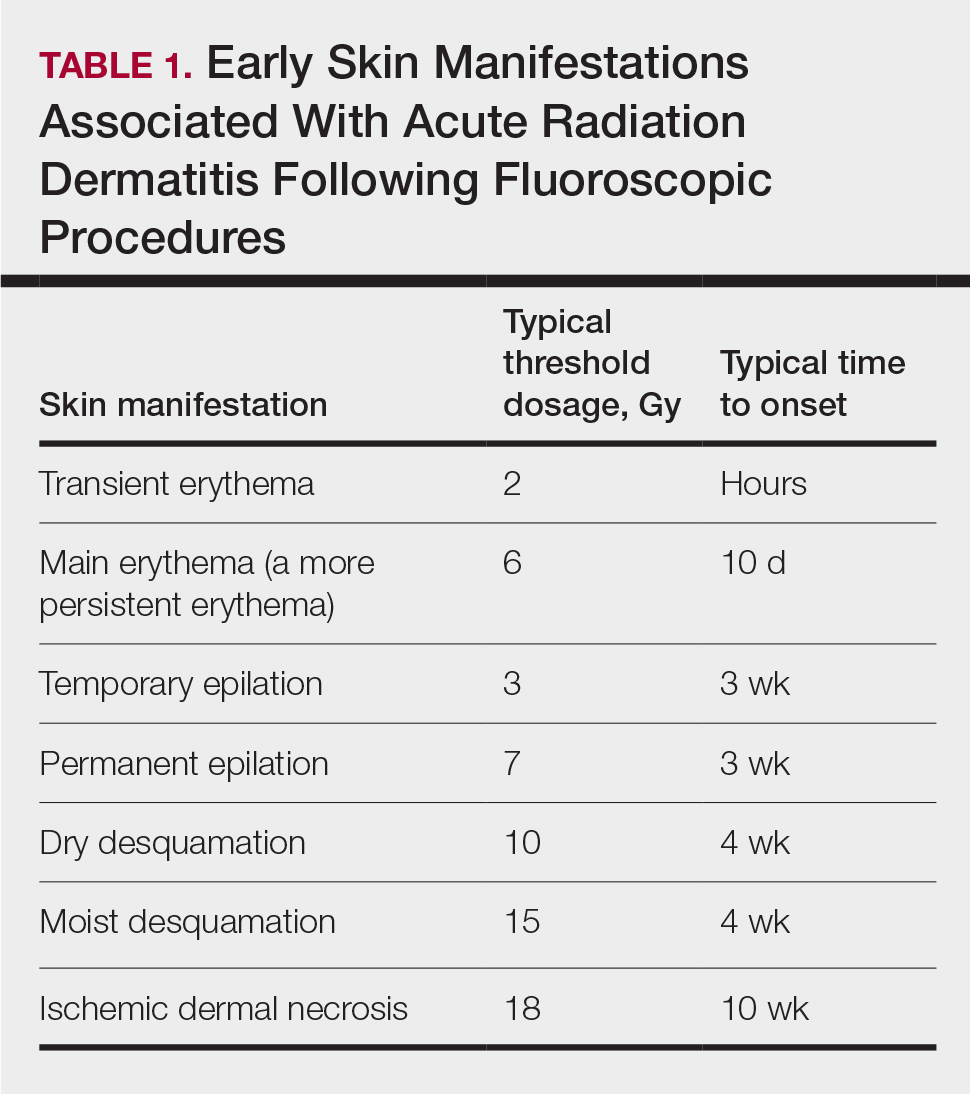
Depending on the patient’s total radiation dosage during one or more procedures, the result of the tissue damage manifests differently at varying times: early skin changes are categorized as fluoroscopy-induced acute radiation dermatitis, and late skin changes are categorized as FICRD (Table 1).
Clinical Manifestations
Acute radiation dermatitis from fluoroscopic procedures manifests within hours to days up to 90 days following radiation exposure and can be characterized by erythema with blistering, desquamation, epilation, pigmentation changes, and even necrosis if the accumulated dosage exceeds 15 Gy.15 Chronic radiation dermatitis (which as related to fluoroscopic procedures is termed FICRD) has a longer onset of weeks to years and is clinically characterized by telangiectasias, permanent erythema, dermal atrophy, or ulcerations. Clinically, subacute radiation dermatitis shares features of both acute and chronic radiation dermatitis; therefore, it is differentiated based on its histologic features.5,16
Although fluoroscopy-induced acute radiation dermatitis (Table 1) may precede FICRD, acute manifestations of fluoroscopy-related dermatitis can be subtle and often manifest in areas not easily visualized. Because referrals to dermatologists for full-skin examinations after fluoroscopy procedures are not standard, patients may not be aware of the association between these procedures and the development of skin lesions. Nonetheless, some patients may report a history of skin changes such as redness days or weeks after a fluoroscopic procedure with accompanying pain and pruritus limited to the fluoroscopy-exposed region, which tend to self-resolve.17 The risk for FICRD is thought to increase if a history of fluoroscopy-induced acute radiation dermatitis is present.18
The location of the skin findings correlates to the area exposed to prolonged radiation during the procedure(s). The most common areas include the scapular and subscapular regions, the right lateral trunk inferior to the axilla, the mid back, and the right anterolateral chest.16,19,20 These regions are associated with more complex (eg, cardiac) procedures that have been reported to lead to prolonged radiation exposure. The skin findings in FICRD are described as geometric, corresponding to the squarish or rectangular radiography beam that is directed at the patient. Additionally, radiography beams spread outward as they travel in space; therefore, skin injuries are common at the region more distal to the path of origination of the beam.21-23 Subsequently, a geometric, dyspigmented, indurated or atrophic plaque with telangiectasias and erosions or ulcerations with progressive worsening is a common manifestation of FICRD.5,16,23 Patients also commonly present with pruritus or severe pain associated with the lesion.24,25
Dermatologic Manifestations of FICRD
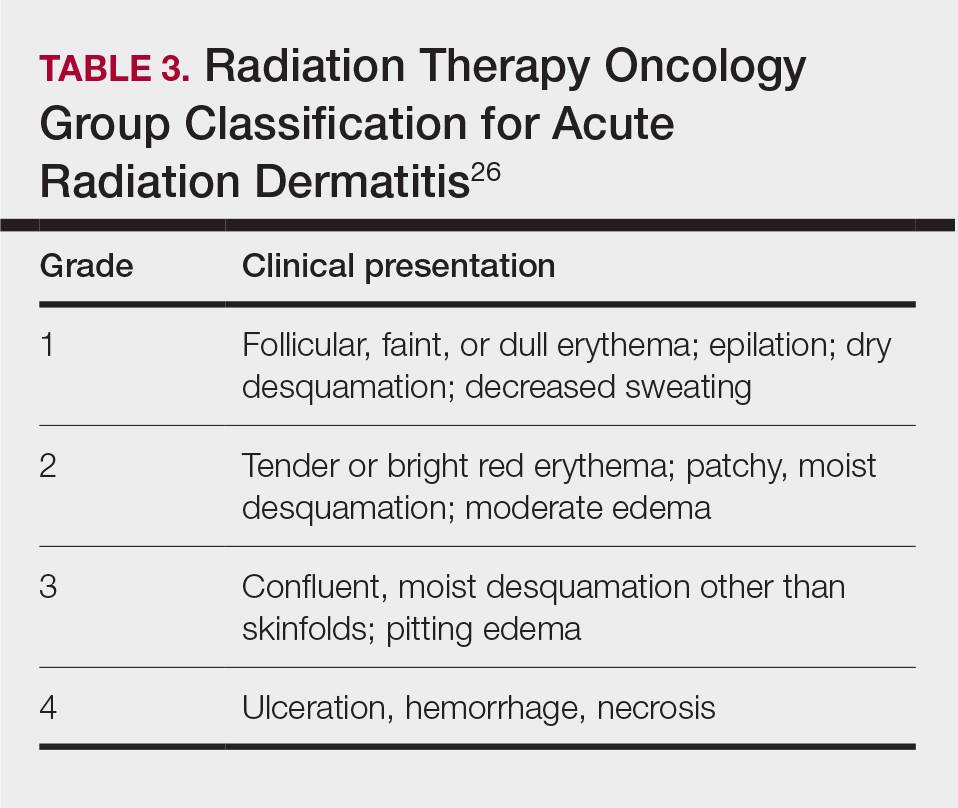
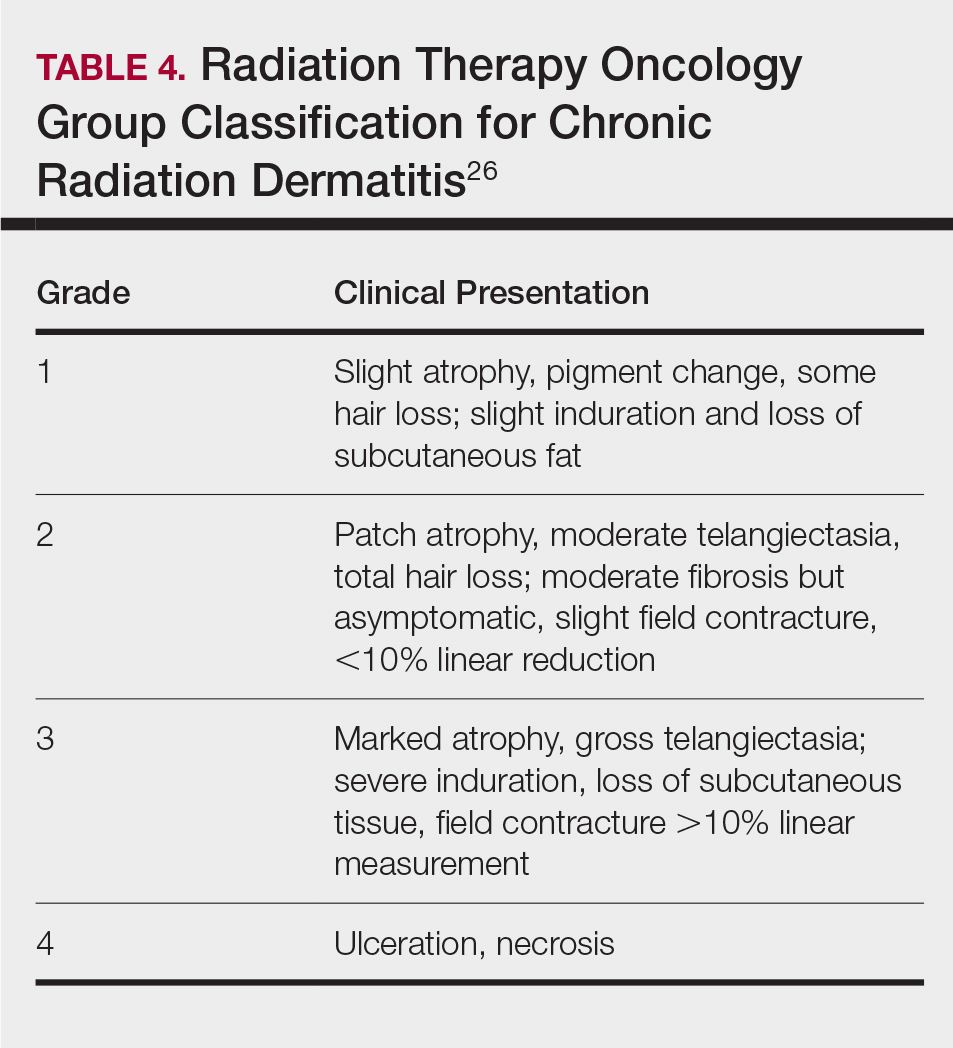
Skin responses seen weeks to years after a fluoroscopic procedure and typically after cumulative radiation exposure of 10 Gy or greater are categorized as FICRD (Table 2). These changes also can be clinically graded based on the Radiation Therapy Oncology Group classification of radiation dermatitis (Tables 3 and 4).26 Chronic changes in the skin largely result from remodeling of the vasculature and the subcutaneous tissue over time. Unlike acute changes, chronic changes typically persist and continue to worsen.27

Telangiectasias—Anywhere from months to 1 year after exposure to 10 Gy of radiation, proliferation of atypical superficial vessels in the dermis can be seen, typically manifesting as telangiectasias on physical examination. Telangiectasias can increase with time and can even exhibit a dose-dependent relationship to the radiation exposure.28
Atrophy—Atrophic-appearing skin after radiation exposure is the result of direct injury to both the epidermis and fibroblasts in the dermis. The destruction of keratinocytes leads to a thin epidermis, and destruction of dermal fibroblasts causes insufficient collagen production.29 Clinically, this process manifests as an atrophic plaque that can be seen 12 weeks to 1 year after the procedure.
Fibrosis—Approximately 1 year after the exposure, the initial damage can lead to disruption of molecular pathways, causing fibrosis. Transforming growth factor (TGF) β1 is the main factor involved.29 Damage to the endothelial cells results in increased TGF-β1 levels, which causes increased stimulation of remaining atypical fibroblasts and thus increased irregular collagen deposition.30 Further adding to this knowledge, Wei et al31 recently proposed that damage to the epidermal keratinocytes leads to disruption of yes-associated protein 1, which is a protective factor released from keratinocytes that regulates the dermal fibroblasts. However, extensive damage to the keratinocytes can lead to lower yes-associated protein 1 levels and its downstream activity, leading to increased levels of TGF-β1 and fibroblast activity.31 Clinically, this fibrotic stage is seen as indurated plaques in patients.
Necrosis—There are 2 forms of necrosis that can be seen. Ischemic dermal necrosis typically occurs in the acute phase after 10 weeks and approximately 18 Gy of cumulative exposure. It results from substantial skin damage, including microvascular damage and reduction in dermal capillaries, leading to ischemia of the tissue.2 Late dermal necrosis is the process seen in the chronic stage of FICRD and radiation dermatitis not related to fluoroscopy. It results from the inability of the fibrotic dermis to vascularly support the epidermis above it.2 It can be seen anywhere from 1 to 4 years after the procedure. This stage clinically manifests as worsening ulcerations with major pain and increased risk for secondary infections.16
Dyspigmentation—Dyspigmentation at the site of the radiation exposure can be seen acutely and chronically. Dosage above 15 to 18 Gy can lead to destruction of melanocytes, which can cause hypopigmentation in exposed areas. However, melanocytes are relatively resistant to radiation; therefore, dosages below the threshold of destruction of 15 to 18 Gy can cause melanocytic hyperactivity leading to hyperpigmentation.32 Hence, pigmentary changes can vary greatly. Classically, a central area of hypopigmentation with surrounding hyperpigmentation is seen.
Histology
Histologic appearance of radiation dermatitis varies depending on its stage. Acute radiation dermatitis primarily demonstrates superficial dermal edema, damage to the basal cell layer, small vessel dilation with thrombi, and hemorrhage along with a sparse inflammatory cell infiltrate.33 Histology typically is the only way to characterize subacute radiation dermatitis.5 Lichenoid tissue reaction is its characteristic feature. Mononuclear cells are found adjected to necrotic keratinocytes along with prominent vacuolization of the basal cell layer.33
The key histologic features of chronic radiation dermatitis include epidermal atrophy, hyperkeratosis, telangiectasias, loss of adnexal structures, and dermal fibrosis along with sparse atypical stellate fibroblasts.34 However, clinical context of fluoroscopic exposure is required for the dermatopathologist to differentiate chronic radiation dermatitis from its histologic differential of morphea and lichen sclerosus. In a cross-sectional study, only 1 of 6 cases (16.7%) was correctly diagnosed as chronic radiation dermatitis in the absence of correlating clinical history.35
Risk Factors for FICRD
Since the diagnosis of FICRD can be a clinical challenge, understanding the risk factors can be helpful. The general likelihood of developing FICRD is related to the duration, frequency, interval, intensity, and area of radiation exposure. Procedures exceeding the normal duration of 60 to 120 minutes have been well documented as a substantial risk factor for radiation dermatitis and FICRD.36-38 The risk tends to be higher in longer procedures because they result in more radiation exposure and higher accumulated PSD. Obesity (ie, body mass index >26) is the major risk factor that has been associated with longer procedure times, as higher radiation dosages are necessary to penetrate the body of a larger patient and a larger skin surface area is exposed.37-39
Other risk factors associated with FICRD relate to how prone a patient is to radiation-induced DNA damage. Older patients are at higher risk due to lower intrinsic ability of the tissue to repair itself.11 Patients with a history of connective tissue diseases—particularly lupus, scleroderma, and mixed connective tissue disease—are at an increased risk.40 Furthermore, patients with genetic disorders that impair DNA repair are more susceptible to radiation-induced DNA damage; therefore, patients with ataxia-telangiectasia, xeroderma pigmentosum, Fanconi anemia, and hereditary nevoid basal cell carcinoma are at higher risk for FICRD.39 Similarly, medications that can affect DNA repair also have been shown to be risk factors. These medications include chemotherapeutic agents such as actinomycin D, cyclophosphamide, doxorubicin, methotrexate, and 5-fluorouracil.2,39 Diabetes, hyperthyroidism, and tobacco use also have been shown to increase a patient’s risk for FICRD.39 It also is reasonable to believe that patients with defects in fibroblasts or with elastin or collagen disorders (eg, Ehlers-Danlos syndrome) would be at higher risk, but there are no known studies highlighting the association in the literature.
Differential Diagnosis of FICRD
Acute allergic or irritant contact dermatitis manifests with a localized area of erythematous skin accompanied by pruritus.41 Patients with FICRD can present with a localized area of erythema and hyperpigmentation with minimal atrophy. The lesion may accompany substantial pruritus, which can favor the more common diagnosis of contact dermatitis.35,42,43
Fixed-drug eruption manifests as a well-defined, hyperpigmented plaque in a fixed location that occurs upon ingestion of a drug.44 Fluoroscopy-induced chronic radiation dermatitis lesions are well demarcated and geometrically shaped and therefore can mimic lesions seen in fixed-drug eruptions.45 Additionally, the patient population undergoing fluoroscopic procedures tends to have major comorbidities requiring multiple medications.4
Decubitus ulcers are a result of vascular compromise to an area of skin due to constant pressure and are most commonly seen in the sacral region of patients with obesity.46 Ulcerated FICRD lesions can manifest on the lower midback. These lesions can be seen after endovascular repair of abdominal aortic aneurysm or prostatic artery embolization.20,21 The location of these lesions can mimic decubitus ulcers if fluoroscopic history is unknown. As mentioned, obesity also increases the risk for FICRD.
Morphea can manifest as a localized area of induration and hyperpigmentation of the skin.47 When FICRD has progressed to dermal fibrosis, patients can present with indurated plaques without ulcerations, which can be hard to differentiate from morphea.16,48 However, the presence of ulcerations or hyperkeratosis can differentiate morphea from FICRD.16
Ultimately, it is the location of FICRD lesions that remains the biggest diagnostic clue. Any suspicious lesion present on the scapular or subscapular areas, anterolateral chest, and/or mid back should prompt an investigation into recent or remote history of fluoroscopic procedures.
Management of FICRD
Diagnosis of FICRD should be made clinically based on the history and physical examination whenever possible, since a biopsy is not recommended.35 Wound healing in FICRD is delayed, and biopsies can lead to ulcerations or secondary infections.17 Therefore, it is important to remain suspicious for FICRD. Management of FICRD should correspond to the clinical findings outlined by a recent Delphi consensus survey.49 Regardless, the core of FICRD management framework should always include good hygiene, maintenance of skin hydration to improve epithelialization, and sufficient photoprotection.49,50
Among the first signs of FICRD are telangiectasias. Although asymptomatic, their appearance can be distressing for patients. Pulsed dye laser therapy is a first-line option that has been studied and has shown clinical efficacy for treatment of telangiectasias and vascular changes in patients with FICRD.49,51
If patients develop fibrotic changes, treatment options are limited. Fibrosis is hard to reverse, and the management approach is limited to symptomatic relief. Mechanical and deep-friction massages have been shown to be effective at reducing skin induration in patients.52 Fractional ablative lasers also may be utilized for skin contractures, especially if range of motion is affected.53,54 Although it comes with its own challenges, autologous fat grafting has shown promise in reducing postradiation fibrosis and inducing angiogenesis in tissue.55 Oral pentoxifylline also has shown mild efficacy, as it may be able to suppress TGF-β1 levels.53 However, prevention of fibrotic changes may be the most important. Wei et al31 suggested that low-dose oral prednisolone at 5 mg twice daily for 3 weeks might be an option to prevent the progression of skin changes and even reverse fibrosis to an extent; however, further evidence regarding its efficacy still is necessary. Additionally, no evidence was identified to support the use of topical corticosteroids for fibrotic changes seen in FICRD.56
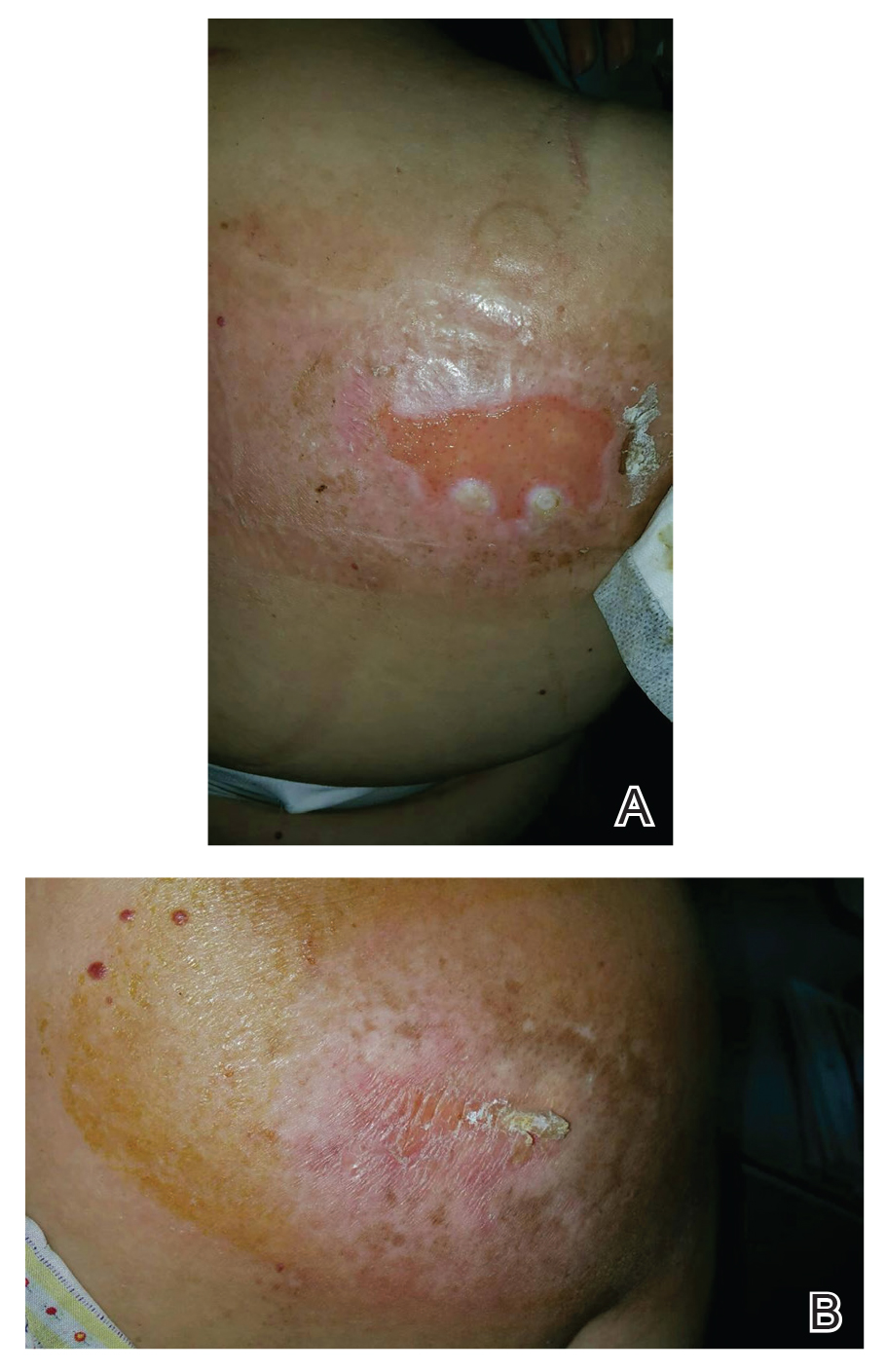
Patients with FICRD or even acute radiation dermatitis after fluoroscopy tend to develop superficial ulcerations from minor traumas. Good wound hygiene, antiseptic care, and absorbent dressings, such as hydrogel and hydrocolloid, may be sufficient for treating these wounds, as seen in the Figure.42,48 However, once patients develop refractory ulcerations or necrosis, treatment options are then limited to surgical removal with a flap or graft.5,33,42,45
Risk for basal cell carcinomas and squamous cell carcinomas is higher in patients with radiation exposure; however, the exact risk from fluoroscopic procedures is unknown. One study demonstrated an increased risk of 6.9% in development of skin cancer after a median radiation exposure of 15.5 Gy and a mean latency period of 38.3 years,57 and in another retrospective study, the risk was higher in Fitzpatrick skin types I and II.58 Unlike the development of radiodermatitis itself, which shows a dose-dependent response, development of skin cancers follows a stochastic pattern (not dose dependent).59 Therefore, it is important to identify these high-risk patients and establish follow-up.
Conclusion
Fluoroscopy-induced chronic radiation dermatitis can be a diagnostic challenge, as skin changes may not be readily associated with the procedure by patients. Therefore, any lesion with a geometric shape and accompanying chronic radiation dermatitis features located on the scapular or subscapular areas, anterolateral chest, and midback should prompt an investigation into history of fluoroscopic procedures. Treatment of chronic skin changes in FICRD depends on the clinical manifestations. Good hygiene, skin hydration, and sufficient photoprotection are crucial. Finally, long-term monitoring with skin examinations is important to assess for the development of skin cancers in the treated area.
- Benjamin EJ, Muntner P, Alonso A, et al. Heart Disease and Stroke Statistics-2019 Update: a report from the American Heart Association. Circulation. 2019;139:E56-E528. doi:10.1161/CIR.0000000000000659. Published correction appears in Circulation. 2020;141:E33.
- Koenig TR, Wolff D, Mettler FA, et al. Skin injuries from fluoroscopically guided procedures: part 1, characteristics of radiation injury. AJR Am J Roentgenol. 2001;177:3-11. doi:10.2214/ajr.177.1.1770003
- Guesnier-Dopagne M, Boyer L, Pereira B, et al. Incidence of chronic radiodermatitis after fluoroscopically guided interventions: a retrospective study. J Vasc Interv Radiol. 2019;30:692-698.e13. doi:10.1016/j.jvir.2019.01.010
- Cunha N, Cardoso P, Cabete J. Subacute radiation dermatitis following an interventional cardiology procedure. Cutan Ocul Toxicol. 2017;36:297-299. doi:10.1080/15569527.2016.1254649
- Frazier TH, Richardson JB, Fabré VC, et al. Fluoroscopy-induced chronic radiation skin injury: a disease perhaps often overlooked. Arch Dermatol. 2007;143:637-640. doi:10.1001/archderm.143.5.637
- Koenig TR, Mettler FA, Wagner LK. Skin injuries from fluoroscopically guided procedures: part 2, review of 73 cases and recommendations for minimizing dose delivered to patient. AJR Am J Roentgenol. 2001;177:13-20. doi:10.2214/ajr.177.1.1770013
- Shope TB. Radiation-induced skin injuries from fluoroscopy. Radiographics. 1996;16:1195-1199. doi:10.1148/radiographics.16.5.8888398
- Tchanque-Fossuo CN, Isseroff RR, Silverstein MA. Fluoroscopy induced chronic radiation dermatitis should be included in the differential diagnosis of notalgia paresthetica. Dermatol Online J. 2016;22:13030/qt0kh726m9.
- Berlin L. Radiation-induced skin injuries and fluoroscopy. AJR Am J Roentgenol. 2001;177:21-25. doi:10.2214/ajr.177.1.1770021
- Tchanque-Fossuo CN, Kamangar F, Ho B, et al. Fluoroscopy-induced radionecrosis. Dermatol Online J. 2016;22:13030/qt68w910t2.
- Wagner LK, Eifel PJ, Geise RA. Potential biological effects following high X-ray dose interventional procedures. J Vasc Interv Radiol. 1994;5:71-84. doi:10.1016/s1051-0443(94)71456-1
- Balter S, Hopewell JW, Miller DL, et al. Fluoroscopically guided interventional procedures: a review of radiation effects on patients’ skin and hair. Radiology. 2010;254:326-341. doi:10.1148/radiol.2542082312
- Vance AZ, Weinberg BD, Arbique GM, et al. Fluoroscopic sentinel events in neuroendovascular procedures: how to screen, prevent, and address occurrence. AJNR Am J Neuroradiol. 2013;34:1513-1515. doi:10.3174/ajnr.A3185
- Aerts A, Decraene T, van den Oord JJ, et al. Chronic radiodermatitis following percutaneous coronary interventions: a report of two cases. J Eur Acad Dermatol Venereol. 2003;17:340-343. doi:10.1046/j.1468-3083.2003.00687.x
- Rosenthal A, Israilevich R, Moy R. Management of acute radiation dermatitis: a review of the literature and proposal for treatment algorithm. J Am Acad Dermatol. 2019;81:558-567. doi:10.1016/j.jaad.2019.02.047
- Boncher J, Bergfeld WF. Fluoroscopy-induced chronic radiation dermatitis: a report of two additional cases and a brief review of the literature. J Cutan Pathol. 2012;39:63-67. doi:10.1111/j.1600-0560.2011.01754.x
- Spiker A, Zinn Z, Carter WH, et al. Fluoroscopy-induced chronic radiation dermatitis. Am J Cardiol. 2012;110:1861-1863. doi:10.1016/j.amjcard.2012.08.023
- Batrani M, Kubba A, Sundharam J. Fluoroscopy-induced chronic radiation dermatitis masquerading as morphea: a diagnostic pitfall. Indian J Pathol Microbiol. 2018;61:393-396. doi:10.4103/IJPM.IJPM_566_17
- Jeskowiak A, Hubmer M, Prenner G, et al. Radiation induced cutaneous ulcer on the back in a patient with congenital anomaly of the upper cava system. Interact Cardiovasc Thorac Surg. 2011;12:290-292.
- Laborda A, De Assis AM, Ioakeim I, et al. Radiodermitis after prostatic artery embolization: case report and review of the literature. Cardiovasc Intervent Radiol. 2015;38:755-759. doi:10.1007/s00270-015-1083-6
- Lyons AB, Harvey VM, Gusev J. Fluoroscopy-induced chronic radiation dermatitis (FICRD) after endovascular abdominal aortic aneurysm endoleak repair. JAAD Case Rep. 2015;1:403-405. doi:10.1016/j.jdcr.2015.09.022
- Mossman KL. Analysis of risk in computerized tomography and other diagnostic radiology procedures. Comput Radiol. 1982;6:251-256. doi:10.1016/0730-4862(82)90109-3
- Henry MF, Maender JL, Shen Y, et al. Fluoroscopy-induced chronic radiation dermatitis: a report of three cases. Dermatol Online J. 2009;15:3.
- Balter S, Miller DL. Patient skin reactions from interventional fluoroscopy procedures. AJR Am J Roentgenol. 2014;202:W335-W342. doi:10.2214/AJR.13.12029
- Nishimoto S, Fukuda K, Kawai K, et al. Supplementation of bone marrow aspirate-derived platelet-rich plasma for treating radiation-induced ulcer after cardiac fluoroscopic procedures: a preliminary report. Indian J Plast Surg. 2012;45:109-114. doi:10.4103/0970-0358.96599
- Cox JD, Stetz J, Pajak TF. Toxicity criteria of the Radiation Therapy Oncology Group (RTOG) and the European Organization for Research and Treatment of Cancer (EORTC). Int J Radiat Oncol Biol Phys. 1995;31:1341-1346. doi:10.1016/0360-3016(95)00060-C
- Wong RK, Bensadoun RJ, Boers-Doets CB, et al. Clinical practice guidelines for the prevention and treatment of acute and late radiation reactions from the MASCC Skin Toxicity Study Group. Support Care Cancer. 2013;21:2933-2948. doi:10.1007/s00520-013-1896-2
- Turesson I, Notter G. The predictive value of skin telangiectasia for late radiation effects in different normal tissues. Int J Radiat Oncol Biol Phys. 1986;12:603-609. doi:10.1016/0360-3016(86)90069-6
- Hegedus F, Mathew LM, Schwartz RA. Radiation dermatitis: an overview. Int J Dermatol. 2017;56:909-914. doi:10.1111/ijd.13371
- Denham JW, Hauer-Jensen M. The radiotherapeutic injury—a complex ‘wound.’ Radiother Oncol. 2002;63:129-145. doi:10.1016/s0167-8140(02)00060-9
- Wei KC, Lai SF, Huang WL, et al. An innovative targeted therapy for fluoroscopy-induced chronic radiation dermatitis. J Mol Med (Berl). 2022;100:135-146. doi:10.1007/s00109-021-02146-3
- Sitton E. Early and late radiation-induced skin alterations. part I: mechanisms of skin changes. Oncol Nurs Forum. 1992;19:801-807.
- Pruitt LG, Rogers W, Byarlay JA, et al. Subacute radiation dermatitis after fluoroscopy. J Cutan Pathol. 2016;43:1091-1095. doi:10.1111/cup.12815
- Anderson EB, Draft KS, Lee RA, et al. Update in dermatopathology. Am J Clin Pathol. 2006;125(Suppl):S50-S70. doi:10.1309/GMUFNP6LFMPNR86R
- Wei KC, Yang KC, Mar GY, et al. STROBE—radiation ulcer: an overlooked complication of fluoroscopic intervention: a cross-sectional study. Medicine (Baltimore). 2015;94:e2178. doi:10.1097/MD.0000000000002178
- Otterburn D, Losken A. Iatrogenic fluoroscopy injury to the skin. Ann Plast Surg. 2010;65:462-465. doi:10.1097/SAP.0b013e3181d6e2d3
- Cha MJ, Jo SJ, Cho Y, et al. Patient characteristics and the incidence of radiation-induced dermatitis following radiofrequency catheter ablation. Korean Circ J. 2016;46:646-653. doi:10.4070/kcj.2016.46.5.646
- Dehen L, Vilmer C, Humilière C, et al. Chronic radiodermatitis following cardiac catheterisation: a report of two cases and a brief review of the literature. Heart. 1999;81:308-312. doi:10.1136/hrt.81.3.308
- Brown KR, Rzucidlo E. Acute and chronic radiation injury. J Vasc Surg. 2011;53(Suppl 1):15S-21S. doi:10.1016/j.jvs.2010.06.175. Published correction appears in J Vasc Surg. 2012;55:627.
- Hymes SR, Strom EA, Fife C. Radiation dermatitis: clinical presentation, pathophysiology, and treatment 2006. J Am Acad Dermatol. 2006;54:28-46. doi:10.1016/j.jaad.2005.08.054
- Scheinman PL, Vocanson M, Thyssen JP, et al. Contact dermatitis. Nat Rev Dis Primers. 2021;7:38. doi:10.1038/s41572-021-00271-4
- Cheng TT, Yang HJ. Chronic radiation dermatitis induced by cardiac catheterization: a case report and literature review. Acta Dermatovenerol Alp Pannonica Adriat. 2022;31:147-149.
- Minni JP, Nowak M, Usmani A, et al. A unique case of subacute radiodermatitis. Cutis. 2013;91:230-232.
- Flowers H, Brodell R, Brents M, et al. Fixed drug eruptions: presentation, diagnosis, and management. South Med J. 2014;107:724-727. doi:10.14423/SMJ.0000000000000195
- Hashimoto I, Sedo H, Inatsugi K, et al. Severe radiation-induced injury after cardiac catheter ablation: a case requiring free anterolateral thigh flap and vastus lateralis muscle flap reconstruction on the upper arm. J Plast Reconstr Aesthet Surg. 2008;61:704-708. doi:10.1016/j.bjps.2007.01.003
- Mervis JS, Phillips TJ. Pressure ulcers: pathophysiology, epidemiology, risk factors, and presentation. J Am Acad Dermatol. 2019;81:881-890. doi:10.1016/j.jaad.2018.12.069
- Careta MF, Romiti R. Localized scleroderma: clinical spectrum and therapeutic update. An Bras Dermatol. 2015;90:62-73. doi:10.1590/abd1806-4841.20152890
- Herz-Ruelas ME, Gómez-Flores M, Moxica-Del Angel J, et al. Ulcerated radiodermatitis induced after fluoroscopically guided stent implantation angioplasty. Case Rep Dermatol Med. 2014;2014:768624. doi:10.1155/2014/768624
- Wilson BN, Shah R, Menzer C, et al. Consensus on the clinical management of chronic radiation dermatitis and radiation fibrosis: a Delphi survey. Br J Dermatol. 2022;187:1054-1056. doi:10.1111/bjd.21852
- Khanna NR, Kumar DP, Laskar SG, et al. Radiation dermatitis: an overview. Indian J Burns. 2013;21:24-31. doi:10.4103/0971-653x.121877
- Spalek M. Chronic radiation-induced dermatitis: challenges and solutions. Clin Cosmet Investig Dermatol. 2016;9:473-482. doi:10.2147/CCID.S94320
- Bourgeois JF, Gourgou S, Kramar A, et al. A randomized, prospective study using the LPG technique in treating radiation-induced skin fibrosis: clinical and profilometric analysis. Skin Res Technol. 2008;14:71-76. doi:10.1111/j.1600-0846.2007.00263.x
- Borrelli MR, Shen AH, Lee GK, et al. Radiation-induced skinfibrosis: pathogenesis, current treatment options, and emerging therapeutics. Ann Plast Surg. 2019;83(4S Suppl 1):S59-S64. doi:10.1097/SAP.0000000000002098
- Wilson B, Shah R, Menzer C, et al. Laser therapy as a treatment for chronic radiation fibrosis. Lasers Surg Med. 2023;55:82-88. doi:10.1002/lsm.23617
- Rigotti G, Marchi A, Galiè M, et al. Clinical treatment of radiotherapy tissue damage by lipoaspirate transplant: a healing process mediated by adipose-derived adult stem cells. Plast Reconstr Surg. 2007;119:1409-1422. doi:10.1097/01.prs.0000256047.47909.71
- Leventhal J, Young MR. Radiation dermatitis: recognition, prevention, and management. Oncology (Williston Park). 2017;31:885-899.
- van Vloten WA, Hermans J, van Daal WA. Radiation-induced skin cancer and radiodermatitis of the head and neck. Cancer. 1987;59:411-414. doi:10.1002/1097-0142(19870201)59:3<411::aid-cncr2820590310>3.0.co;2-z
- Davis MM, Hanke CW, Zollinger TW, et al. Skin cancer in patients with chronic radiation dermatitis. J Am Acad Dermatol. 1989;20:608-616. doi:10.1016/s0190-9622(89)70072-4
- Miller DL, Balter S, Schueler BA, et al. Clinical radiation management for fluoroscopically guided interventional procedures. Radiology. 2010;257:321-332. doi:10.1148/radiol.10091269
Fluoroscopy is an imaging technique that allows for real-time visualization of internal structures in the body using continuous radiography beams. More than 1 million fluoroscopy-guided procedures are performed annually in the United States.1 Utilization of these procedures continues to increase, and so does the probability of related complications, as prolonged exposure to ionizing radiation can cause skin injuries.2 Fortunately, the incidence of radiation-induced skin injuries compared with the total number of fluoroscopic procedures performed remains small,2 although one study suggested the incidence may be as high as 8.9% in at-risk populations.3
Radiation dermatitis is well recognized in dermatology as a complication of oncologic management; however, radiation dermatitis as a complication of fluoroscopic procedures is underrecognized.4 Fluoroscopy-induced radiation dermatitis can be categorized as acute, subacute, or chronic.5 Common fluoroscopic procedures that have been associated with fluoroscopy-induced radiation dermatitis include interventional cardiac procedures, neurovascular procedures, transjugular intrahepatic portosystemic shunt procedures, and endovascular abdominal aortic aneurysm repairs.6,7
Patients with fluoroscopy-induced radiation dermatitis, particularly fluoroscopy-induced chronic radiation dermatitis (FICRD), can present to dermatology up to several years after the initial fluoroscopy procedure with no awareness of the association between the procedure and their skin findings. This presents a diagnostic challenge, and FICRD often is overlooked.5,8-10
We conducted a literature search of PubMed articles indexed for MEDLINE using the search terms fluoroscopy and dermatitis. In this reappraisal, we will provide a comprehensive overview of fluoroscopy-induced radiation dermatitis with an emphasis on FICRD, covering its clinical manifestations, pathophysiology, risk factors, differential diagnosis, histology, and management. The aim of this review is to highlight the salient features and mimickers of FICRD and inform readers how to approach suspected cases, leading to accurate diagnosis and effective management.
Pathophysiology
Fluoroscopy-induced radiation dermatitis is the result of dose-dependent radiation-induced tissue damage. As the peak skin dosage (PSD) of radiation increases over the course of a procedure or multiple procedures, the severity of skin injury predictably increases. During fluoroscopic procedures, the standard irradiation dosage ranges from 0.02 Gy/min to 0.05 Gy/min.11 Transient skin changes may start to be seen around 2 Gy of cumulative exposure. Fluoroscopic procedures typically range in duration from 60 to 120 minutes; however, complex cases may exceed that. Additionally, multiple procedures performed within shorter intervals can result in greater PSD accumulation. Shorter intervals between procedures do not allow enough time for damage repair from the previous procedure and can result in further severe damage when the skin is re-exposed to radiation.2 The American College of Radiology recommends medical follow-up after 10 Gy of cumulative exposure, while cumulative exposure above 15 Gy within a 6- to 12-month period is defined as a sentinel event, according to The Joint Commission.12-14
Depending on the patient’s total radiation dosage during one or more procedures, the result of the tissue damage manifests differently at varying times: early skin changes are categorized as fluoroscopy-induced acute radiation dermatitis, and late skin changes are categorized as FICRD (Table 1).

Clinical Manifestations
Acute radiation dermatitis from fluoroscopic procedures manifests within hours to days up to 90 days following radiation exposure and can be characterized by erythema with blistering, desquamation, epilation, pigmentation changes, and even necrosis if the accumulated dosage exceeds 15 Gy.15 Chronic radiation dermatitis (which as related to fluoroscopic procedures is termed FICRD) has a longer onset of weeks to years and is clinically characterized by telangiectasias, permanent erythema, dermal atrophy, or ulcerations. Clinically, subacute radiation dermatitis shares features of both acute and chronic radiation dermatitis; therefore, it is differentiated based on its histologic features.5,16
Although fluoroscopy-induced acute radiation dermatitis (Table 1) may precede FICRD, acute manifestations of fluoroscopy-related dermatitis can be subtle and often manifest in areas not easily visualized. Because referrals to dermatologists for full-skin examinations after fluoroscopy procedures are not standard, patients may not be aware of the association between these procedures and the development of skin lesions. Nonetheless, some patients may report a history of skin changes such as redness days or weeks after a fluoroscopic procedure with accompanying pain and pruritus limited to the fluoroscopy-exposed region, which tend to self-resolve.17 The risk for FICRD is thought to increase if a history of fluoroscopy-induced acute radiation dermatitis is present.18
The location of the skin findings correlates to the area exposed to prolonged radiation during the procedure(s). The most common areas include the scapular and subscapular regions, the right lateral trunk inferior to the axilla, the mid back, and the right anterolateral chest.16,19,20 These regions are associated with more complex (eg, cardiac) procedures that have been reported to lead to prolonged radiation exposure. The skin findings in FICRD are described as geometric, corresponding to the squarish or rectangular radiography beam that is directed at the patient. Additionally, radiography beams spread outward as they travel in space; therefore, skin injuries are common at the region more distal to the path of origination of the beam.21-23 Subsequently, a geometric, dyspigmented, indurated or atrophic plaque with telangiectasias and erosions or ulcerations with progressive worsening is a common manifestation of FICRD.5,16,23 Patients also commonly present with pruritus or severe pain associated with the lesion.24,25
Dermatologic Manifestations of FICRD
Skin responses seen weeks to years after a fluoroscopic procedure and typically after cumulative radiation exposure of 10 Gy or greater are categorized as FICRD (Table 2). These changes also can be clinically graded based on the Radiation Therapy Oncology Group classification of radiation dermatitis (Tables 3 and 4).26 Chronic changes in the skin largely result from remodeling of the vasculature and the subcutaneous tissue over time. Unlike acute changes, chronic changes typically persist and continue to worsen.27



Telangiectasias—Anywhere from months to 1 year after exposure to 10 Gy of radiation, proliferation of atypical superficial vessels in the dermis can be seen, typically manifesting as telangiectasias on physical examination. Telangiectasias can increase with time and can even exhibit a dose-dependent relationship to the radiation exposure.28
Atrophy—Atrophic-appearing skin after radiation exposure is the result of direct injury to both the epidermis and fibroblasts in the dermis. The destruction of keratinocytes leads to a thin epidermis, and destruction of dermal fibroblasts causes insufficient collagen production.29 Clinically, this process manifests as an atrophic plaque that can be seen 12 weeks to 1 year after the procedure.
Fibrosis—Approximately 1 year after the exposure, the initial damage can lead to disruption of molecular pathways, causing fibrosis. Transforming growth factor (TGF) β1 is the main factor involved.29 Damage to the endothelial cells results in increased TGF-β1 levels, which causes increased stimulation of remaining atypical fibroblasts and thus increased irregular collagen deposition.30 Further adding to this knowledge, Wei et al31 recently proposed that damage to the epidermal keratinocytes leads to disruption of yes-associated protein 1, which is a protective factor released from keratinocytes that regulates the dermal fibroblasts. However, extensive damage to the keratinocytes can lead to lower yes-associated protein 1 levels and its downstream activity, leading to increased levels of TGF-β1 and fibroblast activity.31 Clinically, this fibrotic stage is seen as indurated plaques in patients.
Necrosis—There are 2 forms of necrosis that can be seen. Ischemic dermal necrosis typically occurs in the acute phase after 10 weeks and approximately 18 Gy of cumulative exposure. It results from substantial skin damage, including microvascular damage and reduction in dermal capillaries, leading to ischemia of the tissue.2 Late dermal necrosis is the process seen in the chronic stage of FICRD and radiation dermatitis not related to fluoroscopy. It results from the inability of the fibrotic dermis to vascularly support the epidermis above it.2 It can be seen anywhere from 1 to 4 years after the procedure. This stage clinically manifests as worsening ulcerations with major pain and increased risk for secondary infections.16
Dyspigmentation—Dyspigmentation at the site of the radiation exposure can be seen acutely and chronically. Dosage above 15 to 18 Gy can lead to destruction of melanocytes, which can cause hypopigmentation in exposed areas. However, melanocytes are relatively resistant to radiation; therefore, dosages below the threshold of destruction of 15 to 18 Gy can cause melanocytic hyperactivity leading to hyperpigmentation.32 Hence, pigmentary changes can vary greatly. Classically, a central area of hypopigmentation with surrounding hyperpigmentation is seen.
Histology
Histologic appearance of radiation dermatitis varies depending on its stage. Acute radiation dermatitis primarily demonstrates superficial dermal edema, damage to the basal cell layer, small vessel dilation with thrombi, and hemorrhage along with a sparse inflammatory cell infiltrate.33 Histology typically is the only way to characterize subacute radiation dermatitis.5 Lichenoid tissue reaction is its characteristic feature. Mononuclear cells are found adjected to necrotic keratinocytes along with prominent vacuolization of the basal cell layer.33
The key histologic features of chronic radiation dermatitis include epidermal atrophy, hyperkeratosis, telangiectasias, loss of adnexal structures, and dermal fibrosis along with sparse atypical stellate fibroblasts.34 However, clinical context of fluoroscopic exposure is required for the dermatopathologist to differentiate chronic radiation dermatitis from its histologic differential of morphea and lichen sclerosus. In a cross-sectional study, only 1 of 6 cases (16.7%) was correctly diagnosed as chronic radiation dermatitis in the absence of correlating clinical history.35
Risk Factors for FICRD
Since the diagnosis of FICRD can be a clinical challenge, understanding the risk factors can be helpful. The general likelihood of developing FICRD is related to the duration, frequency, interval, intensity, and area of radiation exposure. Procedures exceeding the normal duration of 60 to 120 minutes have been well documented as a substantial risk factor for radiation dermatitis and FICRD.36-38 The risk tends to be higher in longer procedures because they result in more radiation exposure and higher accumulated PSD. Obesity (ie, body mass index >26) is the major risk factor that has been associated with longer procedure times, as higher radiation dosages are necessary to penetrate the body of a larger patient and a larger skin surface area is exposed.37-39
Other risk factors associated with FICRD relate to how prone a patient is to radiation-induced DNA damage. Older patients are at higher risk due to lower intrinsic ability of the tissue to repair itself.11 Patients with a history of connective tissue diseases—particularly lupus, scleroderma, and mixed connective tissue disease—are at an increased risk.40 Furthermore, patients with genetic disorders that impair DNA repair are more susceptible to radiation-induced DNA damage; therefore, patients with ataxia-telangiectasia, xeroderma pigmentosum, Fanconi anemia, and hereditary nevoid basal cell carcinoma are at higher risk for FICRD.39 Similarly, medications that can affect DNA repair also have been shown to be risk factors. These medications include chemotherapeutic agents such as actinomycin D, cyclophosphamide, doxorubicin, methotrexate, and 5-fluorouracil.2,39 Diabetes, hyperthyroidism, and tobacco use also have been shown to increase a patient’s risk for FICRD.39 It also is reasonable to believe that patients with defects in fibroblasts or with elastin or collagen disorders (eg, Ehlers-Danlos syndrome) would be at higher risk, but there are no known studies highlighting the association in the literature.
Differential Diagnosis of FICRD
Acute allergic or irritant contact dermatitis manifests with a localized area of erythematous skin accompanied by pruritus.41 Patients with FICRD can present with a localized area of erythema and hyperpigmentation with minimal atrophy. The lesion may accompany substantial pruritus, which can favor the more common diagnosis of contact dermatitis.35,42,43
Fixed-drug eruption manifests as a well-defined, hyperpigmented plaque in a fixed location that occurs upon ingestion of a drug.44 Fluoroscopy-induced chronic radiation dermatitis lesions are well demarcated and geometrically shaped and therefore can mimic lesions seen in fixed-drug eruptions.45 Additionally, the patient population undergoing fluoroscopic procedures tends to have major comorbidities requiring multiple medications.4
Decubitus ulcers are a result of vascular compromise to an area of skin due to constant pressure and are most commonly seen in the sacral region of patients with obesity.46 Ulcerated FICRD lesions can manifest on the lower midback. These lesions can be seen after endovascular repair of abdominal aortic aneurysm or prostatic artery embolization.20,21 The location of these lesions can mimic decubitus ulcers if fluoroscopic history is unknown. As mentioned, obesity also increases the risk for FICRD.
Morphea can manifest as a localized area of induration and hyperpigmentation of the skin.47 When FICRD has progressed to dermal fibrosis, patients can present with indurated plaques without ulcerations, which can be hard to differentiate from morphea.16,48 However, the presence of ulcerations or hyperkeratosis can differentiate morphea from FICRD.16
Ultimately, it is the location of FICRD lesions that remains the biggest diagnostic clue. Any suspicious lesion present on the scapular or subscapular areas, anterolateral chest, and/or mid back should prompt an investigation into recent or remote history of fluoroscopic procedures.
Management of FICRD
Diagnosis of FICRD should be made clinically based on the history and physical examination whenever possible, since a biopsy is not recommended.35 Wound healing in FICRD is delayed, and biopsies can lead to ulcerations or secondary infections.17 Therefore, it is important to remain suspicious for FICRD. Management of FICRD should correspond to the clinical findings outlined by a recent Delphi consensus survey.49 Regardless, the core of FICRD management framework should always include good hygiene, maintenance of skin hydration to improve epithelialization, and sufficient photoprotection.49,50
Among the first signs of FICRD are telangiectasias. Although asymptomatic, their appearance can be distressing for patients. Pulsed dye laser therapy is a first-line option that has been studied and has shown clinical efficacy for treatment of telangiectasias and vascular changes in patients with FICRD.49,51
If patients develop fibrotic changes, treatment options are limited. Fibrosis is hard to reverse, and the management approach is limited to symptomatic relief. Mechanical and deep-friction massages have been shown to be effective at reducing skin induration in patients.52 Fractional ablative lasers also may be utilized for skin contractures, especially if range of motion is affected.53,54 Although it comes with its own challenges, autologous fat grafting has shown promise in reducing postradiation fibrosis and inducing angiogenesis in tissue.55 Oral pentoxifylline also has shown mild efficacy, as it may be able to suppress TGF-β1 levels.53 However, prevention of fibrotic changes may be the most important. Wei et al31 suggested that low-dose oral prednisolone at 5 mg twice daily for 3 weeks might be an option to prevent the progression of skin changes and even reverse fibrosis to an extent; however, further evidence regarding its efficacy still is necessary. Additionally, no evidence was identified to support the use of topical corticosteroids for fibrotic changes seen in FICRD.56
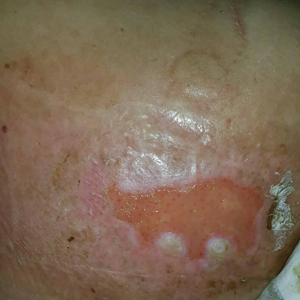
Patients with FICRD or even acute radiation dermatitis after fluoroscopy tend to develop superficial ulcerations from minor traumas. Good wound hygiene, antiseptic care, and absorbent dressings, such as hydrogel and hydrocolloid, may be sufficient for treating these wounds, as seen in the Figure.42,48 However, once patients develop refractory ulcerations or necrosis, treatment options are then limited to surgical removal with a flap or graft.5,33,42,45

Risk for basal cell carcinomas and squamous cell carcinomas is higher in patients with radiation exposure; however, the exact risk from fluoroscopic procedures is unknown. One study demonstrated an increased risk of 6.9% in development of skin cancer after a median radiation exposure of 15.5 Gy and a mean latency period of 38.3 years,57 and in another retrospective study, the risk was higher in Fitzpatrick skin types I and II.58 Unlike the development of radiodermatitis itself, which shows a dose-dependent response, development of skin cancers follows a stochastic pattern (not dose dependent).59 Therefore, it is important to identify these high-risk patients and establish follow-up.
Conclusion
Fluoroscopy-induced chronic radiation dermatitis can be a diagnostic challenge, as skin changes may not be readily associated with the procedure by patients. Therefore, any lesion with a geometric shape and accompanying chronic radiation dermatitis features located on the scapular or subscapular areas, anterolateral chest, and midback should prompt an investigation into history of fluoroscopic procedures. Treatment of chronic skin changes in FICRD depends on the clinical manifestations. Good hygiene, skin hydration, and sufficient photoprotection are crucial. Finally, long-term monitoring with skin examinations is important to assess for the development of skin cancers in the treated area.
Fluoroscopy is an imaging technique that allows for real-time visualization of internal structures in the body using continuous radiography beams. More than 1 million fluoroscopy-guided procedures are performed annually in the United States.1 Utilization of these procedures continues to increase, and so does the probability of related complications, as prolonged exposure to ionizing radiation can cause skin injuries.2 Fortunately, the incidence of radiation-induced skin injuries compared with the total number of fluoroscopic procedures performed remains small,2 although one study suggested the incidence may be as high as 8.9% in at-risk populations.3
Radiation dermatitis is well recognized in dermatology as a complication of oncologic management; however, radiation dermatitis as a complication of fluoroscopic procedures is underrecognized.4 Fluoroscopy-induced radiation dermatitis can be categorized as acute, subacute, or chronic.5 Common fluoroscopic procedures that have been associated with fluoroscopy-induced radiation dermatitis include interventional cardiac procedures, neurovascular procedures, transjugular intrahepatic portosystemic shunt procedures, and endovascular abdominal aortic aneurysm repairs.6,7
Patients with fluoroscopy-induced radiation dermatitis, particularly fluoroscopy-induced chronic radiation dermatitis (FICRD), can present to dermatology up to several years after the initial fluoroscopy procedure with no awareness of the association between the procedure and their skin findings. This presents a diagnostic challenge, and FICRD often is overlooked.5,8-10
We conducted a literature search of PubMed articles indexed for MEDLINE using the search terms fluoroscopy and dermatitis. In this reappraisal, we will provide a comprehensive overview of fluoroscopy-induced radiation dermatitis with an emphasis on FICRD, covering its clinical manifestations, pathophysiology, risk factors, differential diagnosis, histology, and management. The aim of this review is to highlight the salient features and mimickers of FICRD and inform readers how to approach suspected cases, leading to accurate diagnosis and effective management.
Pathophysiology
Fluoroscopy-induced radiation dermatitis is the result of dose-dependent radiation-induced tissue damage. As the peak skin dosage (PSD) of radiation increases over the course of a procedure or multiple procedures, the severity of skin injury predictably increases. During fluoroscopic procedures, the standard irradiation dosage ranges from 0.02 Gy/min to 0.05 Gy/min.11 Transient skin changes may start to be seen around 2 Gy of cumulative exposure. Fluoroscopic procedures typically range in duration from 60 to 120 minutes; however, complex cases may exceed that. Additionally, multiple procedures performed within shorter intervals can result in greater PSD accumulation. Shorter intervals between procedures do not allow enough time for damage repair from the previous procedure and can result in further severe damage when the skin is re-exposed to radiation.2 The American College of Radiology recommends medical follow-up after 10 Gy of cumulative exposure, while cumulative exposure above 15 Gy within a 6- to 12-month period is defined as a sentinel event, according to The Joint Commission.12-14
Depending on the patient’s total radiation dosage during one or more procedures, the result of the tissue damage manifests differently at varying times: early skin changes are categorized as fluoroscopy-induced acute radiation dermatitis, and late skin changes are categorized as FICRD (Table 1).

Clinical Manifestations
Acute radiation dermatitis from fluoroscopic procedures manifests within hours to days up to 90 days following radiation exposure and can be characterized by erythema with blistering, desquamation, epilation, pigmentation changes, and even necrosis if the accumulated dosage exceeds 15 Gy.15 Chronic radiation dermatitis (which as related to fluoroscopic procedures is termed FICRD) has a longer onset of weeks to years and is clinically characterized by telangiectasias, permanent erythema, dermal atrophy, or ulcerations. Clinically, subacute radiation dermatitis shares features of both acute and chronic radiation dermatitis; therefore, it is differentiated based on its histologic features.5,16
Although fluoroscopy-induced acute radiation dermatitis (Table 1) may precede FICRD, acute manifestations of fluoroscopy-related dermatitis can be subtle and often manifest in areas not easily visualized. Because referrals to dermatologists for full-skin examinations after fluoroscopy procedures are not standard, patients may not be aware of the association between these procedures and the development of skin lesions. Nonetheless, some patients may report a history of skin changes such as redness days or weeks after a fluoroscopic procedure with accompanying pain and pruritus limited to the fluoroscopy-exposed region, which tend to self-resolve.17 The risk for FICRD is thought to increase if a history of fluoroscopy-induced acute radiation dermatitis is present.18
The location of the skin findings correlates to the area exposed to prolonged radiation during the procedure(s). The most common areas include the scapular and subscapular regions, the right lateral trunk inferior to the axilla, the mid back, and the right anterolateral chest.16,19,20 These regions are associated with more complex (eg, cardiac) procedures that have been reported to lead to prolonged radiation exposure. The skin findings in FICRD are described as geometric, corresponding to the squarish or rectangular radiography beam that is directed at the patient. Additionally, radiography beams spread outward as they travel in space; therefore, skin injuries are common at the region more distal to the path of origination of the beam.21-23 Subsequently, a geometric, dyspigmented, indurated or atrophic plaque with telangiectasias and erosions or ulcerations with progressive worsening is a common manifestation of FICRD.5,16,23 Patients also commonly present with pruritus or severe pain associated with the lesion.24,25
Dermatologic Manifestations of FICRD
Skin responses seen weeks to years after a fluoroscopic procedure and typically after cumulative radiation exposure of 10 Gy or greater are categorized as FICRD (Table 2). These changes also can be clinically graded based on the Radiation Therapy Oncology Group classification of radiation dermatitis (Tables 3 and 4).26 Chronic changes in the skin largely result from remodeling of the vasculature and the subcutaneous tissue over time. Unlike acute changes, chronic changes typically persist and continue to worsen.27



Telangiectasias—Anywhere from months to 1 year after exposure to 10 Gy of radiation, proliferation of atypical superficial vessels in the dermis can be seen, typically manifesting as telangiectasias on physical examination. Telangiectasias can increase with time and can even exhibit a dose-dependent relationship to the radiation exposure.28
Atrophy—Atrophic-appearing skin after radiation exposure is the result of direct injury to both the epidermis and fibroblasts in the dermis. The destruction of keratinocytes leads to a thin epidermis, and destruction of dermal fibroblasts causes insufficient collagen production.29 Clinically, this process manifests as an atrophic plaque that can be seen 12 weeks to 1 year after the procedure.
Fibrosis—Approximately 1 year after the exposure, the initial damage can lead to disruption of molecular pathways, causing fibrosis. Transforming growth factor (TGF) β1 is the main factor involved.29 Damage to the endothelial cells results in increased TGF-β1 levels, which causes increased stimulation of remaining atypical fibroblasts and thus increased irregular collagen deposition.30 Further adding to this knowledge, Wei et al31 recently proposed that damage to the epidermal keratinocytes leads to disruption of yes-associated protein 1, which is a protective factor released from keratinocytes that regulates the dermal fibroblasts. However, extensive damage to the keratinocytes can lead to lower yes-associated protein 1 levels and its downstream activity, leading to increased levels of TGF-β1 and fibroblast activity.31 Clinically, this fibrotic stage is seen as indurated plaques in patients.
Necrosis—There are 2 forms of necrosis that can be seen. Ischemic dermal necrosis typically occurs in the acute phase after 10 weeks and approximately 18 Gy of cumulative exposure. It results from substantial skin damage, including microvascular damage and reduction in dermal capillaries, leading to ischemia of the tissue.2 Late dermal necrosis is the process seen in the chronic stage of FICRD and radiation dermatitis not related to fluoroscopy. It results from the inability of the fibrotic dermis to vascularly support the epidermis above it.2 It can be seen anywhere from 1 to 4 years after the procedure. This stage clinically manifests as worsening ulcerations with major pain and increased risk for secondary infections.16
Dyspigmentation—Dyspigmentation at the site of the radiation exposure can be seen acutely and chronically. Dosage above 15 to 18 Gy can lead to destruction of melanocytes, which can cause hypopigmentation in exposed areas. However, melanocytes are relatively resistant to radiation; therefore, dosages below the threshold of destruction of 15 to 18 Gy can cause melanocytic hyperactivity leading to hyperpigmentation.32 Hence, pigmentary changes can vary greatly. Classically, a central area of hypopigmentation with surrounding hyperpigmentation is seen.
Histology
Histologic appearance of radiation dermatitis varies depending on its stage. Acute radiation dermatitis primarily demonstrates superficial dermal edema, damage to the basal cell layer, small vessel dilation with thrombi, and hemorrhage along with a sparse inflammatory cell infiltrate.33 Histology typically is the only way to characterize subacute radiation dermatitis.5 Lichenoid tissue reaction is its characteristic feature. Mononuclear cells are found adjected to necrotic keratinocytes along with prominent vacuolization of the basal cell layer.33
The key histologic features of chronic radiation dermatitis include epidermal atrophy, hyperkeratosis, telangiectasias, loss of adnexal structures, and dermal fibrosis along with sparse atypical stellate fibroblasts.34 However, clinical context of fluoroscopic exposure is required for the dermatopathologist to differentiate chronic radiation dermatitis from its histologic differential of morphea and lichen sclerosus. In a cross-sectional study, only 1 of 6 cases (16.7%) was correctly diagnosed as chronic radiation dermatitis in the absence of correlating clinical history.35
Risk Factors for FICRD
Since the diagnosis of FICRD can be a clinical challenge, understanding the risk factors can be helpful. The general likelihood of developing FICRD is related to the duration, frequency, interval, intensity, and area of radiation exposure. Procedures exceeding the normal duration of 60 to 120 minutes have been well documented as a substantial risk factor for radiation dermatitis and FICRD.36-38 The risk tends to be higher in longer procedures because they result in more radiation exposure and higher accumulated PSD. Obesity (ie, body mass index >26) is the major risk factor that has been associated with longer procedure times, as higher radiation dosages are necessary to penetrate the body of a larger patient and a larger skin surface area is exposed.37-39
Other risk factors associated with FICRD relate to how prone a patient is to radiation-induced DNA damage. Older patients are at higher risk due to lower intrinsic ability of the tissue to repair itself.11 Patients with a history of connective tissue diseases—particularly lupus, scleroderma, and mixed connective tissue disease—are at an increased risk.40 Furthermore, patients with genetic disorders that impair DNA repair are more susceptible to radiation-induced DNA damage; therefore, patients with ataxia-telangiectasia, xeroderma pigmentosum, Fanconi anemia, and hereditary nevoid basal cell carcinoma are at higher risk for FICRD.39 Similarly, medications that can affect DNA repair also have been shown to be risk factors. These medications include chemotherapeutic agents such as actinomycin D, cyclophosphamide, doxorubicin, methotrexate, and 5-fluorouracil.2,39 Diabetes, hyperthyroidism, and tobacco use also have been shown to increase a patient’s risk for FICRD.39 It also is reasonable to believe that patients with defects in fibroblasts or with elastin or collagen disorders (eg, Ehlers-Danlos syndrome) would be at higher risk, but there are no known studies highlighting the association in the literature.
Differential Diagnosis of FICRD
Acute allergic or irritant contact dermatitis manifests with a localized area of erythematous skin accompanied by pruritus.41 Patients with FICRD can present with a localized area of erythema and hyperpigmentation with minimal atrophy. The lesion may accompany substantial pruritus, which can favor the more common diagnosis of contact dermatitis.35,42,43
Fixed-drug eruption manifests as a well-defined, hyperpigmented plaque in a fixed location that occurs upon ingestion of a drug.44 Fluoroscopy-induced chronic radiation dermatitis lesions are well demarcated and geometrically shaped and therefore can mimic lesions seen in fixed-drug eruptions.45 Additionally, the patient population undergoing fluoroscopic procedures tends to have major comorbidities requiring multiple medications.4
Decubitus ulcers are a result of vascular compromise to an area of skin due to constant pressure and are most commonly seen in the sacral region of patients with obesity.46 Ulcerated FICRD lesions can manifest on the lower midback. These lesions can be seen after endovascular repair of abdominal aortic aneurysm or prostatic artery embolization.20,21 The location of these lesions can mimic decubitus ulcers if fluoroscopic history is unknown. As mentioned, obesity also increases the risk for FICRD.
Morphea can manifest as a localized area of induration and hyperpigmentation of the skin.47 When FICRD has progressed to dermal fibrosis, patients can present with indurated plaques without ulcerations, which can be hard to differentiate from morphea.16,48 However, the presence of ulcerations or hyperkeratosis can differentiate morphea from FICRD.16
Ultimately, it is the location of FICRD lesions that remains the biggest diagnostic clue. Any suspicious lesion present on the scapular or subscapular areas, anterolateral chest, and/or mid back should prompt an investigation into recent or remote history of fluoroscopic procedures.
Management of FICRD
Diagnosis of FICRD should be made clinically based on the history and physical examination whenever possible, since a biopsy is not recommended.35 Wound healing in FICRD is delayed, and biopsies can lead to ulcerations or secondary infections.17 Therefore, it is important to remain suspicious for FICRD. Management of FICRD should correspond to the clinical findings outlined by a recent Delphi consensus survey.49 Regardless, the core of FICRD management framework should always include good hygiene, maintenance of skin hydration to improve epithelialization, and sufficient photoprotection.49,50
Among the first signs of FICRD are telangiectasias. Although asymptomatic, their appearance can be distressing for patients. Pulsed dye laser therapy is a first-line option that has been studied and has shown clinical efficacy for treatment of telangiectasias and vascular changes in patients with FICRD.49,51
If patients develop fibrotic changes, treatment options are limited. Fibrosis is hard to reverse, and the management approach is limited to symptomatic relief. Mechanical and deep-friction massages have been shown to be effective at reducing skin induration in patients.52 Fractional ablative lasers also may be utilized for skin contractures, especially if range of motion is affected.53,54 Although it comes with its own challenges, autologous fat grafting has shown promise in reducing postradiation fibrosis and inducing angiogenesis in tissue.55 Oral pentoxifylline also has shown mild efficacy, as it may be able to suppress TGF-β1 levels.53 However, prevention of fibrotic changes may be the most important. Wei et al31 suggested that low-dose oral prednisolone at 5 mg twice daily for 3 weeks might be an option to prevent the progression of skin changes and even reverse fibrosis to an extent; however, further evidence regarding its efficacy still is necessary. Additionally, no evidence was identified to support the use of topical corticosteroids for fibrotic changes seen in FICRD.56
Patients with FICRD or even acute radiation dermatitis after fluoroscopy tend to develop superficial ulcerations from minor traumas. Good wound hygiene, antiseptic care, and absorbent dressings, such as hydrogel and hydrocolloid, may be sufficient for treating these wounds, as seen in the Figure.42,48 However, once patients develop refractory ulcerations or necrosis, treatment options are then limited to surgical removal with a flap or graft.5,33,42,45

Risk for basal cell carcinomas and squamous cell carcinomas is higher in patients with radiation exposure; however, the exact risk from fluoroscopic procedures is unknown. One study demonstrated an increased risk of 6.9% in development of skin cancer after a median radiation exposure of 15.5 Gy and a mean latency period of 38.3 years,57 and in another retrospective study, the risk was higher in Fitzpatrick skin types I and II.58 Unlike the development of radiodermatitis itself, which shows a dose-dependent response, development of skin cancers follows a stochastic pattern (not dose dependent).59 Therefore, it is important to identify these high-risk patients and establish follow-up.
Conclusion
Fluoroscopy-induced chronic radiation dermatitis can be a diagnostic challenge, as skin changes may not be readily associated with the procedure by patients. Therefore, any lesion with a geometric shape and accompanying chronic radiation dermatitis features located on the scapular or subscapular areas, anterolateral chest, and midback should prompt an investigation into history of fluoroscopic procedures. Treatment of chronic skin changes in FICRD depends on the clinical manifestations. Good hygiene, skin hydration, and sufficient photoprotection are crucial. Finally, long-term monitoring with skin examinations is important to assess for the development of skin cancers in the treated area.
- Benjamin EJ, Muntner P, Alonso A, et al. Heart Disease and Stroke Statistics-2019 Update: a report from the American Heart Association. Circulation. 2019;139:E56-E528. doi:10.1161/CIR.0000000000000659. Published correction appears in Circulation. 2020;141:E33.
- Koenig TR, Wolff D, Mettler FA, et al. Skin injuries from fluoroscopically guided procedures: part 1, characteristics of radiation injury. AJR Am J Roentgenol. 2001;177:3-11. doi:10.2214/ajr.177.1.1770003
- Guesnier-Dopagne M, Boyer L, Pereira B, et al. Incidence of chronic radiodermatitis after fluoroscopically guided interventions: a retrospective study. J Vasc Interv Radiol. 2019;30:692-698.e13. doi:10.1016/j.jvir.2019.01.010
- Cunha N, Cardoso P, Cabete J. Subacute radiation dermatitis following an interventional cardiology procedure. Cutan Ocul Toxicol. 2017;36:297-299. doi:10.1080/15569527.2016.1254649
- Frazier TH, Richardson JB, Fabré VC, et al. Fluoroscopy-induced chronic radiation skin injury: a disease perhaps often overlooked. Arch Dermatol. 2007;143:637-640. doi:10.1001/archderm.143.5.637
- Koenig TR, Mettler FA, Wagner LK. Skin injuries from fluoroscopically guided procedures: part 2, review of 73 cases and recommendations for minimizing dose delivered to patient. AJR Am J Roentgenol. 2001;177:13-20. doi:10.2214/ajr.177.1.1770013
- Shope TB. Radiation-induced skin injuries from fluoroscopy. Radiographics. 1996;16:1195-1199. doi:10.1148/radiographics.16.5.8888398
- Tchanque-Fossuo CN, Isseroff RR, Silverstein MA. Fluoroscopy induced chronic radiation dermatitis should be included in the differential diagnosis of notalgia paresthetica. Dermatol Online J. 2016;22:13030/qt0kh726m9.
- Berlin L. Radiation-induced skin injuries and fluoroscopy. AJR Am J Roentgenol. 2001;177:21-25. doi:10.2214/ajr.177.1.1770021
- Tchanque-Fossuo CN, Kamangar F, Ho B, et al. Fluoroscopy-induced radionecrosis. Dermatol Online J. 2016;22:13030/qt68w910t2.
- Wagner LK, Eifel PJ, Geise RA. Potential biological effects following high X-ray dose interventional procedures. J Vasc Interv Radiol. 1994;5:71-84. doi:10.1016/s1051-0443(94)71456-1
- Balter S, Hopewell JW, Miller DL, et al. Fluoroscopically guided interventional procedures: a review of radiation effects on patients’ skin and hair. Radiology. 2010;254:326-341. doi:10.1148/radiol.2542082312
- Vance AZ, Weinberg BD, Arbique GM, et al. Fluoroscopic sentinel events in neuroendovascular procedures: how to screen, prevent, and address occurrence. AJNR Am J Neuroradiol. 2013;34:1513-1515. doi:10.3174/ajnr.A3185
- Aerts A, Decraene T, van den Oord JJ, et al. Chronic radiodermatitis following percutaneous coronary interventions: a report of two cases. J Eur Acad Dermatol Venereol. 2003;17:340-343. doi:10.1046/j.1468-3083.2003.00687.x
- Rosenthal A, Israilevich R, Moy R. Management of acute radiation dermatitis: a review of the literature and proposal for treatment algorithm. J Am Acad Dermatol. 2019;81:558-567. doi:10.1016/j.jaad.2019.02.047
- Boncher J, Bergfeld WF. Fluoroscopy-induced chronic radiation dermatitis: a report of two additional cases and a brief review of the literature. J Cutan Pathol. 2012;39:63-67. doi:10.1111/j.1600-0560.2011.01754.x
- Spiker A, Zinn Z, Carter WH, et al. Fluoroscopy-induced chronic radiation dermatitis. Am J Cardiol. 2012;110:1861-1863. doi:10.1016/j.amjcard.2012.08.023
- Batrani M, Kubba A, Sundharam J. Fluoroscopy-induced chronic radiation dermatitis masquerading as morphea: a diagnostic pitfall. Indian J Pathol Microbiol. 2018;61:393-396. doi:10.4103/IJPM.IJPM_566_17
- Jeskowiak A, Hubmer M, Prenner G, et al. Radiation induced cutaneous ulcer on the back in a patient with congenital anomaly of the upper cava system. Interact Cardiovasc Thorac Surg. 2011;12:290-292.
- Laborda A, De Assis AM, Ioakeim I, et al. Radiodermitis after prostatic artery embolization: case report and review of the literature. Cardiovasc Intervent Radiol. 2015;38:755-759. doi:10.1007/s00270-015-1083-6
- Lyons AB, Harvey VM, Gusev J. Fluoroscopy-induced chronic radiation dermatitis (FICRD) after endovascular abdominal aortic aneurysm endoleak repair. JAAD Case Rep. 2015;1:403-405. doi:10.1016/j.jdcr.2015.09.022
- Mossman KL. Analysis of risk in computerized tomography and other diagnostic radiology procedures. Comput Radiol. 1982;6:251-256. doi:10.1016/0730-4862(82)90109-3
- Henry MF, Maender JL, Shen Y, et al. Fluoroscopy-induced chronic radiation dermatitis: a report of three cases. Dermatol Online J. 2009;15:3.
- Balter S, Miller DL. Patient skin reactions from interventional fluoroscopy procedures. AJR Am J Roentgenol. 2014;202:W335-W342. doi:10.2214/AJR.13.12029
- Nishimoto S, Fukuda K, Kawai K, et al. Supplementation of bone marrow aspirate-derived platelet-rich plasma for treating radiation-induced ulcer after cardiac fluoroscopic procedures: a preliminary report. Indian J Plast Surg. 2012;45:109-114. doi:10.4103/0970-0358.96599
- Cox JD, Stetz J, Pajak TF. Toxicity criteria of the Radiation Therapy Oncology Group (RTOG) and the European Organization for Research and Treatment of Cancer (EORTC). Int J Radiat Oncol Biol Phys. 1995;31:1341-1346. doi:10.1016/0360-3016(95)00060-C
- Wong RK, Bensadoun RJ, Boers-Doets CB, et al. Clinical practice guidelines for the prevention and treatment of acute and late radiation reactions from the MASCC Skin Toxicity Study Group. Support Care Cancer. 2013;21:2933-2948. doi:10.1007/s00520-013-1896-2
- Turesson I, Notter G. The predictive value of skin telangiectasia for late radiation effects in different normal tissues. Int J Radiat Oncol Biol Phys. 1986;12:603-609. doi:10.1016/0360-3016(86)90069-6
- Hegedus F, Mathew LM, Schwartz RA. Radiation dermatitis: an overview. Int J Dermatol. 2017;56:909-914. doi:10.1111/ijd.13371
- Denham JW, Hauer-Jensen M. The radiotherapeutic injury—a complex ‘wound.’ Radiother Oncol. 2002;63:129-145. doi:10.1016/s0167-8140(02)00060-9
- Wei KC, Lai SF, Huang WL, et al. An innovative targeted therapy for fluoroscopy-induced chronic radiation dermatitis. J Mol Med (Berl). 2022;100:135-146. doi:10.1007/s00109-021-02146-3
- Sitton E. Early and late radiation-induced skin alterations. part I: mechanisms of skin changes. Oncol Nurs Forum. 1992;19:801-807.
- Pruitt LG, Rogers W, Byarlay JA, et al. Subacute radiation dermatitis after fluoroscopy. J Cutan Pathol. 2016;43:1091-1095. doi:10.1111/cup.12815
- Anderson EB, Draft KS, Lee RA, et al. Update in dermatopathology. Am J Clin Pathol. 2006;125(Suppl):S50-S70. doi:10.1309/GMUFNP6LFMPNR86R
- Wei KC, Yang KC, Mar GY, et al. STROBE—radiation ulcer: an overlooked complication of fluoroscopic intervention: a cross-sectional study. Medicine (Baltimore). 2015;94:e2178. doi:10.1097/MD.0000000000002178
- Otterburn D, Losken A. Iatrogenic fluoroscopy injury to the skin. Ann Plast Surg. 2010;65:462-465. doi:10.1097/SAP.0b013e3181d6e2d3
- Cha MJ, Jo SJ, Cho Y, et al. Patient characteristics and the incidence of radiation-induced dermatitis following radiofrequency catheter ablation. Korean Circ J. 2016;46:646-653. doi:10.4070/kcj.2016.46.5.646
- Dehen L, Vilmer C, Humilière C, et al. Chronic radiodermatitis following cardiac catheterisation: a report of two cases and a brief review of the literature. Heart. 1999;81:308-312. doi:10.1136/hrt.81.3.308
- Brown KR, Rzucidlo E. Acute and chronic radiation injury. J Vasc Surg. 2011;53(Suppl 1):15S-21S. doi:10.1016/j.jvs.2010.06.175. Published correction appears in J Vasc Surg. 2012;55:627.
- Hymes SR, Strom EA, Fife C. Radiation dermatitis: clinical presentation, pathophysiology, and treatment 2006. J Am Acad Dermatol. 2006;54:28-46. doi:10.1016/j.jaad.2005.08.054
- Scheinman PL, Vocanson M, Thyssen JP, et al. Contact dermatitis. Nat Rev Dis Primers. 2021;7:38. doi:10.1038/s41572-021-00271-4
- Cheng TT, Yang HJ. Chronic radiation dermatitis induced by cardiac catheterization: a case report and literature review. Acta Dermatovenerol Alp Pannonica Adriat. 2022;31:147-149.
- Minni JP, Nowak M, Usmani A, et al. A unique case of subacute radiodermatitis. Cutis. 2013;91:230-232.
- Flowers H, Brodell R, Brents M, et al. Fixed drug eruptions: presentation, diagnosis, and management. South Med J. 2014;107:724-727. doi:10.14423/SMJ.0000000000000195
- Hashimoto I, Sedo H, Inatsugi K, et al. Severe radiation-induced injury after cardiac catheter ablation: a case requiring free anterolateral thigh flap and vastus lateralis muscle flap reconstruction on the upper arm. J Plast Reconstr Aesthet Surg. 2008;61:704-708. doi:10.1016/j.bjps.2007.01.003
- Mervis JS, Phillips TJ. Pressure ulcers: pathophysiology, epidemiology, risk factors, and presentation. J Am Acad Dermatol. 2019;81:881-890. doi:10.1016/j.jaad.2018.12.069
- Careta MF, Romiti R. Localized scleroderma: clinical spectrum and therapeutic update. An Bras Dermatol. 2015;90:62-73. doi:10.1590/abd1806-4841.20152890
- Herz-Ruelas ME, Gómez-Flores M, Moxica-Del Angel J, et al. Ulcerated radiodermatitis induced after fluoroscopically guided stent implantation angioplasty. Case Rep Dermatol Med. 2014;2014:768624. doi:10.1155/2014/768624
- Wilson BN, Shah R, Menzer C, et al. Consensus on the clinical management of chronic radiation dermatitis and radiation fibrosis: a Delphi survey. Br J Dermatol. 2022;187:1054-1056. doi:10.1111/bjd.21852
- Khanna NR, Kumar DP, Laskar SG, et al. Radiation dermatitis: an overview. Indian J Burns. 2013;21:24-31. doi:10.4103/0971-653x.121877
- Spalek M. Chronic radiation-induced dermatitis: challenges and solutions. Clin Cosmet Investig Dermatol. 2016;9:473-482. doi:10.2147/CCID.S94320
- Bourgeois JF, Gourgou S, Kramar A, et al. A randomized, prospective study using the LPG technique in treating radiation-induced skin fibrosis: clinical and profilometric analysis. Skin Res Technol. 2008;14:71-76. doi:10.1111/j.1600-0846.2007.00263.x
- Borrelli MR, Shen AH, Lee GK, et al. Radiation-induced skinfibrosis: pathogenesis, current treatment options, and emerging therapeutics. Ann Plast Surg. 2019;83(4S Suppl 1):S59-S64. doi:10.1097/SAP.0000000000002098
- Wilson B, Shah R, Menzer C, et al. Laser therapy as a treatment for chronic radiation fibrosis. Lasers Surg Med. 2023;55:82-88. doi:10.1002/lsm.23617
- Rigotti G, Marchi A, Galiè M, et al. Clinical treatment of radiotherapy tissue damage by lipoaspirate transplant: a healing process mediated by adipose-derived adult stem cells. Plast Reconstr Surg. 2007;119:1409-1422. doi:10.1097/01.prs.0000256047.47909.71
- Leventhal J, Young MR. Radiation dermatitis: recognition, prevention, and management. Oncology (Williston Park). 2017;31:885-899.
- van Vloten WA, Hermans J, van Daal WA. Radiation-induced skin cancer and radiodermatitis of the head and neck. Cancer. 1987;59:411-414. doi:10.1002/1097-0142(19870201)59:3<411::aid-cncr2820590310>3.0.co;2-z
- Davis MM, Hanke CW, Zollinger TW, et al. Skin cancer in patients with chronic radiation dermatitis. J Am Acad Dermatol. 1989;20:608-616. doi:10.1016/s0190-9622(89)70072-4
- Miller DL, Balter S, Schueler BA, et al. Clinical radiation management for fluoroscopically guided interventional procedures. Radiology. 2010;257:321-332. doi:10.1148/radiol.10091269
- Benjamin EJ, Muntner P, Alonso A, et al. Heart Disease and Stroke Statistics-2019 Update: a report from the American Heart Association. Circulation. 2019;139:E56-E528. doi:10.1161/CIR.0000000000000659. Published correction appears in Circulation. 2020;141:E33.
- Koenig TR, Wolff D, Mettler FA, et al. Skin injuries from fluoroscopically guided procedures: part 1, characteristics of radiation injury. AJR Am J Roentgenol. 2001;177:3-11. doi:10.2214/ajr.177.1.1770003
- Guesnier-Dopagne M, Boyer L, Pereira B, et al. Incidence of chronic radiodermatitis after fluoroscopically guided interventions: a retrospective study. J Vasc Interv Radiol. 2019;30:692-698.e13. doi:10.1016/j.jvir.2019.01.010
- Cunha N, Cardoso P, Cabete J. Subacute radiation dermatitis following an interventional cardiology procedure. Cutan Ocul Toxicol. 2017;36:297-299. doi:10.1080/15569527.2016.1254649
- Frazier TH, Richardson JB, Fabré VC, et al. Fluoroscopy-induced chronic radiation skin injury: a disease perhaps often overlooked. Arch Dermatol. 2007;143:637-640. doi:10.1001/archderm.143.5.637
- Koenig TR, Mettler FA, Wagner LK. Skin injuries from fluoroscopically guided procedures: part 2, review of 73 cases and recommendations for minimizing dose delivered to patient. AJR Am J Roentgenol. 2001;177:13-20. doi:10.2214/ajr.177.1.1770013
- Shope TB. Radiation-induced skin injuries from fluoroscopy. Radiographics. 1996;16:1195-1199. doi:10.1148/radiographics.16.5.8888398
- Tchanque-Fossuo CN, Isseroff RR, Silverstein MA. Fluoroscopy induced chronic radiation dermatitis should be included in the differential diagnosis of notalgia paresthetica. Dermatol Online J. 2016;22:13030/qt0kh726m9.
- Berlin L. Radiation-induced skin injuries and fluoroscopy. AJR Am J Roentgenol. 2001;177:21-25. doi:10.2214/ajr.177.1.1770021
- Tchanque-Fossuo CN, Kamangar F, Ho B, et al. Fluoroscopy-induced radionecrosis. Dermatol Online J. 2016;22:13030/qt68w910t2.
- Wagner LK, Eifel PJ, Geise RA. Potential biological effects following high X-ray dose interventional procedures. J Vasc Interv Radiol. 1994;5:71-84. doi:10.1016/s1051-0443(94)71456-1
- Balter S, Hopewell JW, Miller DL, et al. Fluoroscopically guided interventional procedures: a review of radiation effects on patients’ skin and hair. Radiology. 2010;254:326-341. doi:10.1148/radiol.2542082312
- Vance AZ, Weinberg BD, Arbique GM, et al. Fluoroscopic sentinel events in neuroendovascular procedures: how to screen, prevent, and address occurrence. AJNR Am J Neuroradiol. 2013;34:1513-1515. doi:10.3174/ajnr.A3185
- Aerts A, Decraene T, van den Oord JJ, et al. Chronic radiodermatitis following percutaneous coronary interventions: a report of two cases. J Eur Acad Dermatol Venereol. 2003;17:340-343. doi:10.1046/j.1468-3083.2003.00687.x
- Rosenthal A, Israilevich R, Moy R. Management of acute radiation dermatitis: a review of the literature and proposal for treatment algorithm. J Am Acad Dermatol. 2019;81:558-567. doi:10.1016/j.jaad.2019.02.047
- Boncher J, Bergfeld WF. Fluoroscopy-induced chronic radiation dermatitis: a report of two additional cases and a brief review of the literature. J Cutan Pathol. 2012;39:63-67. doi:10.1111/j.1600-0560.2011.01754.x
- Spiker A, Zinn Z, Carter WH, et al. Fluoroscopy-induced chronic radiation dermatitis. Am J Cardiol. 2012;110:1861-1863. doi:10.1016/j.amjcard.2012.08.023
- Batrani M, Kubba A, Sundharam J. Fluoroscopy-induced chronic radiation dermatitis masquerading as morphea: a diagnostic pitfall. Indian J Pathol Microbiol. 2018;61:393-396. doi:10.4103/IJPM.IJPM_566_17
- Jeskowiak A, Hubmer M, Prenner G, et al. Radiation induced cutaneous ulcer on the back in a patient with congenital anomaly of the upper cava system. Interact Cardiovasc Thorac Surg. 2011;12:290-292.
- Laborda A, De Assis AM, Ioakeim I, et al. Radiodermitis after prostatic artery embolization: case report and review of the literature. Cardiovasc Intervent Radiol. 2015;38:755-759. doi:10.1007/s00270-015-1083-6
- Lyons AB, Harvey VM, Gusev J. Fluoroscopy-induced chronic radiation dermatitis (FICRD) after endovascular abdominal aortic aneurysm endoleak repair. JAAD Case Rep. 2015;1:403-405. doi:10.1016/j.jdcr.2015.09.022
- Mossman KL. Analysis of risk in computerized tomography and other diagnostic radiology procedures. Comput Radiol. 1982;6:251-256. doi:10.1016/0730-4862(82)90109-3
- Henry MF, Maender JL, Shen Y, et al. Fluoroscopy-induced chronic radiation dermatitis: a report of three cases. Dermatol Online J. 2009;15:3.
- Balter S, Miller DL. Patient skin reactions from interventional fluoroscopy procedures. AJR Am J Roentgenol. 2014;202:W335-W342. doi:10.2214/AJR.13.12029
- Nishimoto S, Fukuda K, Kawai K, et al. Supplementation of bone marrow aspirate-derived platelet-rich plasma for treating radiation-induced ulcer after cardiac fluoroscopic procedures: a preliminary report. Indian J Plast Surg. 2012;45:109-114. doi:10.4103/0970-0358.96599
- Cox JD, Stetz J, Pajak TF. Toxicity criteria of the Radiation Therapy Oncology Group (RTOG) and the European Organization for Research and Treatment of Cancer (EORTC). Int J Radiat Oncol Biol Phys. 1995;31:1341-1346. doi:10.1016/0360-3016(95)00060-C
- Wong RK, Bensadoun RJ, Boers-Doets CB, et al. Clinical practice guidelines for the prevention and treatment of acute and late radiation reactions from the MASCC Skin Toxicity Study Group. Support Care Cancer. 2013;21:2933-2948. doi:10.1007/s00520-013-1896-2
- Turesson I, Notter G. The predictive value of skin telangiectasia for late radiation effects in different normal tissues. Int J Radiat Oncol Biol Phys. 1986;12:603-609. doi:10.1016/0360-3016(86)90069-6
- Hegedus F, Mathew LM, Schwartz RA. Radiation dermatitis: an overview. Int J Dermatol. 2017;56:909-914. doi:10.1111/ijd.13371
- Denham JW, Hauer-Jensen M. The radiotherapeutic injury—a complex ‘wound.’ Radiother Oncol. 2002;63:129-145. doi:10.1016/s0167-8140(02)00060-9
- Wei KC, Lai SF, Huang WL, et al. An innovative targeted therapy for fluoroscopy-induced chronic radiation dermatitis. J Mol Med (Berl). 2022;100:135-146. doi:10.1007/s00109-021-02146-3
- Sitton E. Early and late radiation-induced skin alterations. part I: mechanisms of skin changes. Oncol Nurs Forum. 1992;19:801-807.
- Pruitt LG, Rogers W, Byarlay JA, et al. Subacute radiation dermatitis after fluoroscopy. J Cutan Pathol. 2016;43:1091-1095. doi:10.1111/cup.12815
- Anderson EB, Draft KS, Lee RA, et al. Update in dermatopathology. Am J Clin Pathol. 2006;125(Suppl):S50-S70. doi:10.1309/GMUFNP6LFMPNR86R
- Wei KC, Yang KC, Mar GY, et al. STROBE—radiation ulcer: an overlooked complication of fluoroscopic intervention: a cross-sectional study. Medicine (Baltimore). 2015;94:e2178. doi:10.1097/MD.0000000000002178
- Otterburn D, Losken A. Iatrogenic fluoroscopy injury to the skin. Ann Plast Surg. 2010;65:462-465. doi:10.1097/SAP.0b013e3181d6e2d3
- Cha MJ, Jo SJ, Cho Y, et al. Patient characteristics and the incidence of radiation-induced dermatitis following radiofrequency catheter ablation. Korean Circ J. 2016;46:646-653. doi:10.4070/kcj.2016.46.5.646
- Dehen L, Vilmer C, Humilière C, et al. Chronic radiodermatitis following cardiac catheterisation: a report of two cases and a brief review of the literature. Heart. 1999;81:308-312. doi:10.1136/hrt.81.3.308
- Brown KR, Rzucidlo E. Acute and chronic radiation injury. J Vasc Surg. 2011;53(Suppl 1):15S-21S. doi:10.1016/j.jvs.2010.06.175. Published correction appears in J Vasc Surg. 2012;55:627.
- Hymes SR, Strom EA, Fife C. Radiation dermatitis: clinical presentation, pathophysiology, and treatment 2006. J Am Acad Dermatol. 2006;54:28-46. doi:10.1016/j.jaad.2005.08.054
- Scheinman PL, Vocanson M, Thyssen JP, et al. Contact dermatitis. Nat Rev Dis Primers. 2021;7:38. doi:10.1038/s41572-021-00271-4
- Cheng TT, Yang HJ. Chronic radiation dermatitis induced by cardiac catheterization: a case report and literature review. Acta Dermatovenerol Alp Pannonica Adriat. 2022;31:147-149.
- Minni JP, Nowak M, Usmani A, et al. A unique case of subacute radiodermatitis. Cutis. 2013;91:230-232.
- Flowers H, Brodell R, Brents M, et al. Fixed drug eruptions: presentation, diagnosis, and management. South Med J. 2014;107:724-727. doi:10.14423/SMJ.0000000000000195
- Hashimoto I, Sedo H, Inatsugi K, et al. Severe radiation-induced injury after cardiac catheter ablation: a case requiring free anterolateral thigh flap and vastus lateralis muscle flap reconstruction on the upper arm. J Plast Reconstr Aesthet Surg. 2008;61:704-708. doi:10.1016/j.bjps.2007.01.003
- Mervis JS, Phillips TJ. Pressure ulcers: pathophysiology, epidemiology, risk factors, and presentation. J Am Acad Dermatol. 2019;81:881-890. doi:10.1016/j.jaad.2018.12.069
- Careta MF, Romiti R. Localized scleroderma: clinical spectrum and therapeutic update. An Bras Dermatol. 2015;90:62-73. doi:10.1590/abd1806-4841.20152890
- Herz-Ruelas ME, Gómez-Flores M, Moxica-Del Angel J, et al. Ulcerated radiodermatitis induced after fluoroscopically guided stent implantation angioplasty. Case Rep Dermatol Med. 2014;2014:768624. doi:10.1155/2014/768624
- Wilson BN, Shah R, Menzer C, et al. Consensus on the clinical management of chronic radiation dermatitis and radiation fibrosis: a Delphi survey. Br J Dermatol. 2022;187:1054-1056. doi:10.1111/bjd.21852
- Khanna NR, Kumar DP, Laskar SG, et al. Radiation dermatitis: an overview. Indian J Burns. 2013;21:24-31. doi:10.4103/0971-653x.121877
- Spalek M. Chronic radiation-induced dermatitis: challenges and solutions. Clin Cosmet Investig Dermatol. 2016;9:473-482. doi:10.2147/CCID.S94320
- Bourgeois JF, Gourgou S, Kramar A, et al. A randomized, prospective study using the LPG technique in treating radiation-induced skin fibrosis: clinical and profilometric analysis. Skin Res Technol. 2008;14:71-76. doi:10.1111/j.1600-0846.2007.00263.x
- Borrelli MR, Shen AH, Lee GK, et al. Radiation-induced skinfibrosis: pathogenesis, current treatment options, and emerging therapeutics. Ann Plast Surg. 2019;83(4S Suppl 1):S59-S64. doi:10.1097/SAP.0000000000002098
- Wilson B, Shah R, Menzer C, et al. Laser therapy as a treatment for chronic radiation fibrosis. Lasers Surg Med. 2023;55:82-88. doi:10.1002/lsm.23617
- Rigotti G, Marchi A, Galiè M, et al. Clinical treatment of radiotherapy tissue damage by lipoaspirate transplant: a healing process mediated by adipose-derived adult stem cells. Plast Reconstr Surg. 2007;119:1409-1422. doi:10.1097/01.prs.0000256047.47909.71
- Leventhal J, Young MR. Radiation dermatitis: recognition, prevention, and management. Oncology (Williston Park). 2017;31:885-899.
- van Vloten WA, Hermans J, van Daal WA. Radiation-induced skin cancer and radiodermatitis of the head and neck. Cancer. 1987;59:411-414. doi:10.1002/1097-0142(19870201)59:3<411::aid-cncr2820590310>3.0.co;2-z
- Davis MM, Hanke CW, Zollinger TW, et al. Skin cancer in patients with chronic radiation dermatitis. J Am Acad Dermatol. 1989;20:608-616. doi:10.1016/s0190-9622(89)70072-4
- Miller DL, Balter S, Schueler BA, et al. Clinical radiation management for fluoroscopically guided interventional procedures. Radiology. 2010;257:321-332. doi:10.1148/radiol.10091269
Fluoroscopy-Induced Chronic Radiation Dermatitis: A Comprehensive Review and Reappraisal
Fluoroscopy-Induced Chronic Radiation Dermatitis: A Comprehensive Review and Reappraisal
PRACTICE POINTS
- Fluoroscopy-induced chronic radiation dermatitis poses diagnostic challenges, as patients often are unable to associate a history of fluoroscopic procedures with the development of skin lesions.
- Scapular and subscapular lesions as well as those on the anterolateral chest and mid back should prompt clinicians to inquire about the patient’s history of fluoroscopic procedures.
- Because lesions can remain refractory to treatment, longterm monitoring is necessary if they are not excised.
COPD CARE Academy: Design of Purposeful Training Guided by Implementation Strategies
COPD CARE Academy: Design of Purposeful Training Guided by Implementation Strategies
Quality improvement (QI) initiatives within the US Department of Veterans Affairs (VA) play an important role in enhancing health care for veterans.1,2 While effective QI programs are often developed, veterans benefit only if they receive care at sites where the program is offered.3 It is estimated only 1% to 5% of patients receive benefit from evidence-based programs, limiting the opportunity for widespread impact.4,5
The Chronic Obstructive Pulmonary Disease (COPD) Coordinated Access to Reduce Exacerbations (CARE) Academy is a national training program designed to promote the adoption of a COPD primary care service.6 The Academy was created and iteratively refined by VA staff to include both clinical training emphasizing COPD management and program implementation strategies. Training programs such as COPD CARE are commonly described as a method to support adoption of health care services, but there is no consensus on a universal approach to training design.
This article describes COPD CARE training and implementation strategies (Table). The Academy began as a training program at 1 VA medical center (VAMC) and has expanded to 49 diverse VAMCs. The Academy illustrates how implementation strategies can be leveraged to develop pragmatic and impactful training. Highlights from the Academy's 9-year history are outlined in this article.

COPD CARE
One in 4 veterans have a COPD diagnosis, and the 5-year mortality rate following a COPD flare is ≥ 50%.7,8 In 2015, a pharmacy resident designed and piloted COPD CARE, a program that used evidence-based practice to optimize management of the disease.9,10
The COPD CARE program is delivered by interprofessional team members. It includes a postacute care call completed 48 hours postdischarge, a wellness visit (face-to-face or virtual) 1 month postdischarge, and a follow-up visit scheduled 2 months postdischarge. Clinical pharmacist practitioners (CPPs) prescribe and collaborate with the COPD CARE health care team. Evidence-based practices embedded within COPD CARE include treatment optimization, symptom evaluation, severity staging, vaccination promotion, referrals, tobacco treatment, and comorbidity management.11-16 The initial COPD CARE pilot demonstrated promising results; patients received timely care and high rates of COPD best practices.11
Academy Design and Implementation
Initial COPD CARE training was tailored to the culture, context, and workflow of the William S. Middleton Memorial Veteran’s Hospital in Madison, Wisconsin. Further service expansion required integration of implementation strategies that enable learners to apply and adapt content to fit different processes, staffing, and patient needs.
Formal Implementation Blueprint
A key aspect of the Academy is the integration of a formal implementation blueprint that includes training goals, scope, and key milestones to guide implementation. The Academy blueprint includes 4 phased training workbooks: (1) preimplementation support from local stakeholders; (2) integration of COPD CARE operational infrastructure into workflows; (3) preparing clinical champions; and (4) leading clinical training (Figure 1). Five weekly 1-hour synchronous virtual discussions are used for learning the workbook content that include learning objectives and opportunities to strategize how to overcome implementation barriers.
Promoting and Facilitating Implementation
As clinicians apply content from the Academy to install informatics tools, coordinate clinical training, and build relationships across service lines, implementation barriers may occur. A learning collaborative allows peer-mentorship and shared problem solving. The Academy learning collaborative includes attendees across multiple VAMCs, allowing for diverse perspectives and cross-site learning. Within the field of dissemination and implementation science, this process of shared problem-solving to support individuals is referred to as implementation facilitation.17 Academy facilitators with prior experience provide a unique perspective and external facilitation from outside local VAMCs. Academy learners form local teams to engage in shared decision-making when applying Academy content. Following Academy completion, learning collaboratives continue to meet monthly to share clinical insights and operational updates.
Local Champions Promote Adaptability
One or more local champions were identified at each VAMC who were focused on the implementation of clinical training content and operational implementation of Academy content.18 Champions have helped develop adaptations of Academy content, such as integrating telehealth nursing within the COPD CARE referral process, which have become new best practices. Champions attend Academy sessions, which provide an opportunity to share adaptations to meet local needs.19
Using a Train-The-Trainer Model
Clinical training was designed to be dynamic and included video modeling, such as recorded examples of CPPs conducting COPD CARE visits and video clips highlighting clinical content. Each learner received a clinical workbook summarizing the content. The champion shares discussion questions to relate training content to the local clinical practice setting. The combination of live training, with videos of clinic visits and case-based discussion was intended to address differing learning styles. Clinical training was delivered using a train-the-trainer model led by the local champion, which allows clinicians with expertise to tailor their training. The use of a train-the-trainer model was intended to promote local buy-in and was often completed by frontline clinicians.
Informatics note templates provide clinicians with information needed to deliver training content during clinic visits. Direct hyperlinks to symptomatic scoring tools, resources to promote evidence-based medication optimization, and patient education resources were embedded within the electronic health record note templates. Direct links to consults for COPD referrals services discussed during clinical training were also included to promote ease of care coordination and awareness of referral opportunities. The integration of clinical training with informatics note template support was intentional to directly relate clinical training to clinical care delivery.
Audit and Feedback
To inform COPD CARE practice, the Academy included informatics infrastructure that allowed for timely local quality monitoring. Electronic health record note templates with embedded data fields track COPD CARE service implementation, including timely completion of patient visits, completion of patient medication reviews, appropriate testing, symptom assessment, and interventions made. Champions can organize template installation and integrate templates into COPD CARE clinical training. Data are included on a COPD CARE implementation dashboard.
An audit and feedback process is allows for the review of performance metrics and development of action plans.20,21 Data reports from note templates are described during the Academy, along with resources to help teams enhance delivery of their program based on performance metrics.
Building a Coalition
Within VA primary care, clinical care delivery is optimized through a team-based coalition of clinicians using the patient aligned care team (PACT) framework. The VA patient-centered team-based care delivery model, patient facilitates coordination of patient referrals, including patient review, scheduling, and completion of patient visits.22
Partnerships with VA Pharmacy Benefits Manager, VA Diffusion of Excellence, VA Quality Enhancement Research Initiative, VA Office of Pulmonary Medicine, and the VA Office of Rural Health have facilitated COPD CARE successes. Collaborations with VA Centers of Innovation helped benchmark the Academy’s impact. An academic partnership with the University of Wisconsin-Madison was established in 2017 and has provided evaluation expertise and leadership as the Academy has been iteratively developed, and revised.
Preliminary Metrics
COPD CARE has delivered > 2000 visits. CPPs have delivered COPD care, with a mean 9.4 of 10 best practices per patient visit. Improvements in veteran COPD symptoms have also been observed following COPD CARE patient visits.
DISCUSSION
The COPD CARE Academy was developed to promote rapid scale-up of a complex, team-based COPD service delivered during veteran care transitions. The implementation blueprint for the Academy is multifaceted and integrates both clinical-focused and implementation-focused infrastructure to apply training content.23 A randomized control trial evaluating the efficacy of training modalities found a need to expand implementation blueprints beyond clinical training alone, as training by itself may not be sufficient to change behavior.24 VA staff designed the Academy using clinical- and implementation-focused content within its implementation blueprint. Key components included leveraging clinical champions, using a train-the-trainer approach, and incorporating facilitation strategies to overcome adoption barriers.
Lewis et al emphasize matching implementation strategies to barriers within VA staff who identify care coordination as a key challenge.23 The informatics infrastructure developed for Academy learners, including standardized note templates, video modeling examples of clinic visits, and data capture for audit and feedback, was designed to complement clinical training and standardize service workflows (Figure 2). There are opportunities to explore how to optimize technology in the Academy.
While Academy clinical training specifically focuses on COPD management, many implementation strategies can be considered to promote care delivery services for other chronic conditions. The Academy blueprint and implementation infrastructure, are strategies that may be considered within and outside the federal health care system. The opportunity for adaptations to Academy training enables clinical champions to promote tailored content to the needs of each unique VAMC. The translation of Academy implementation strategies for new chronic conditions will similarly require adaptations at each VAMC to promote adoption of content.
CONCLUSIONS
COPD CARE Academy is an example of the collaborative spirit within VA, and the opportunity for further advancement of health care programs. The VA is a national leader in Learning Health Systems implementation, in which “science, informatics, incentives and culture are aligned for continuous improvement and innovation.”25,26 There are many opportunities for VA staff to learn from one another to form partnerships between leaders, clinicians, and scientists to optimize health care delivery and further the VA’s work as a learning health system.
- Robinson CH, Thompto AJ, Lima EN, Damschroder LJ. Continuous quality improvement at the frontline: one interdisciplinary clinical team's four-year journey after completing a virtual learning program. Learn Health Syst. 2022;6(4):e10345. doi:10.1002/lrh2.10345
- US Department of Veterans Affairs. Continuous quality improvement (CQI) for clinical teams: a systematic review of reviews. Accessed July 24, 2025. https://www.hsrd.research.va.gov/for_researchers/cyber_seminars/archives/video_archive.cfm?SessionID=4151
- Dondanville KA, Fina BA, Straud CL, et al. Launching a competency-based training program in evidence-based treatments for PTSD: supporting veteran-serving mental health providers in Texas. Community Ment Health J. 2021;57(5):910-919. doi:10.1007/S10597-020-00676-7
- Abildso CG, Zizzi SJ, Reger-Nash B. Evaluating an insurance- sponsored weight management program with the RE-AIM model, West Virginia, 2004-2008. Prev Chronic Dis. 2010;7(3):A46.
- Glasgow RE, Vinson C, Chambers D, Khoury MJ, Kaplan RM, Hunter C. National institutes of health approaches to dissemination and implementation science: current and future directions. Am J Public Health. 2012;102(7):1274- 1281. doi:10.2105/AJPH.2012.300755
- Portillo EC, Maurer MA, Kettner JT, et al. Applying RE-AIM to examine the impact of an implementation facilitation package to scale up a program for veterans with chronic obstructive pulmonary disease. Implement Sci Commun. 2023;4(1):143. doi:10.1186/S43058-023-00520-5
- McGhan R, Radcliff T, Fish R, Sutherland ER, Welsh C, Make B. Predictors of rehospitalization and death after a severe exacerbation of COPD. Chest. 2007;132(6):1748- 1755. doi:10.1378/chest.06-3018
- Anderson E, Wiener RS, Resnick K, Elwy AR, Rinne ST. Care coordination for veterans with COPD: a positive deviance study. Am J Manag Care. 2020;26(2):63-68. doi:10.37765/AJMC.2020.42394
- 2024 GOLD Report. Global Initiative for Chronic Obstructive Lung Disease - GOLD. Accessed July 24, 2025. https://goldcopd.org/2024-gold-report/
- Nici L, Mammen MJ, Charbek E, et al. Pharmacologic management of chronic obstructive pulmonary disease. An official American Thoracic Society clinical practice guideline. Am J Respir Crit Care Med. 2020;201(9):e56-e69. doi:10.1164/rccm.202003-0625ST
- Portillo EC, Wilcox A, Seckel E, et al. Reducing COPD readmission rates: using a COPD care service during care transitions. Fed Pract. 2018;35(11):30-36.
- Portillo EC, Gruber S, Lehmann M, et al. Application of the replicating effective programs framework to design a COPD training program. J Am Pharm Assoc. 2021;61(2):e129-e135. doi:10.1016/J.JAPH.2020.10.023
- Portillo EC, Lehmann MR, Hagen TL, et al. Integration of the patient-centered medical home to deliver a care bundle for chronic obstructive pulmonary disease management. J Am Pharm Assoc. 2023;63(1):212-219. doi:10.1016/j.japh.2022.10.003
- Portillo E, Lehmann M, Hagen T, et al. Evaluation of an implementation package to deliver the COPD CARE service. BMJ Open Qual. 2023;12(1). doi:10.1136/BMJOQ-2022-002074
- Portillo E, Lehmann M, Maurer M, et al. Barriers to implementing a pharmacist-led COPD care bundle in rural settings: A qualitative evaluation. 2025 (under review).
- Population Health Management. American Hospital Association. Accessed July 24, 2025. https://www.aha.org/center/population-health-management
- Ritchie MJ, Dollar KM, Miller CK, et al. Using implementation facilitation to improve healthcare: implementation facilitation training manual. Accessed July 11, 2024. https:// www.queri.research.va.gov/tools/Facilitation-Manual.pdf
- Morena AL, Gaias LM, Larkin C. Understanding the role of clinical champions and their impact on clinician behavior change: the need for causal pathway mechanisms. Front Health Serv. 2022;2:896885. doi:10.3389/FRHS.2022.896885
- Ayele RA, Rabin BA, McCreight M, Battaglia C. Editorial: understanding, assessing, and guiding adaptations in public health and health systems interventions: current and future directions. Front Public Health. 2023;11:1228437. doi:10.3389/fpubh.2023.1228437
- Jamtvedt G, Flottorp S, Ivers N. Audit and feedback as a quality strategy. In: Improving Healthcare Services. World Health Organization; 2019. Accessed July 24, 2025. https://www.ncbi.nlm.nih.gov/books/NBK549284/
- Snider MDH, Boyd MR, Walker MR, Powell BJ, Lewis CC. Using audit and feedback to guide tailored implementations of measurement-based care in community mental health: a multiple case study. Implement Sci Commun. 2023;4(1):94. doi:10.1186/s43058-023-00474-8
- Patient Aligned Care Team (PACT) – Patient Care Services. US Department of Veterans Affairs. Accessed July 24, 2025. https://www.patientcare.va.gov/primarycare/PACT.asp
- Lewis CC, Scott K, Marriott BR. A methodology for generating a tailored implementation blueprint: an exemplar from a youth residential setting. Implementat Sci. 2018;13(1):68. doi:10.1186/s13012-018-0761-6
- Beidas RS, Edmunds JM, Marcus SC, Kendall PC. Training and consultation to promote implementation of an empirically supported treatment: a randomized trial. Psychiatr Serv. 2012;63(7):660-665. doi:10.1176/appi.ps.201100401
- Kilbourne AM, Schmidt J, Edmunds M, Vega R, Bowersox N, Atkins D. How the VA is training the next-generation workforce for learning health systems. Learn Health Syst. 2022;6(4):e10333. doi:10.1002/LRH2.10333
- Easterling D, Perry AC, Woodside R, Patel T, Gesell SB. Clarifying the concept of a learning health system for healthcare delivery organizations: implications from a qualitative analysis of the scientific literature. Learn Health Syst. 2021;6(2):e10287. doi:10.1002/LRH2.10287
Quality improvement (QI) initiatives within the US Department of Veterans Affairs (VA) play an important role in enhancing health care for veterans.1,2 While effective QI programs are often developed, veterans benefit only if they receive care at sites where the program is offered.3 It is estimated only 1% to 5% of patients receive benefit from evidence-based programs, limiting the opportunity for widespread impact.4,5
The Chronic Obstructive Pulmonary Disease (COPD) Coordinated Access to Reduce Exacerbations (CARE) Academy is a national training program designed to promote the adoption of a COPD primary care service.6 The Academy was created and iteratively refined by VA staff to include both clinical training emphasizing COPD management and program implementation strategies. Training programs such as COPD CARE are commonly described as a method to support adoption of health care services, but there is no consensus on a universal approach to training design.
This article describes COPD CARE training and implementation strategies (Table). The Academy began as a training program at 1 VA medical center (VAMC) and has expanded to 49 diverse VAMCs. The Academy illustrates how implementation strategies can be leveraged to develop pragmatic and impactful training. Highlights from the Academy's 9-year history are outlined in this article.

COPD CARE
One in 4 veterans have a COPD diagnosis, and the 5-year mortality rate following a COPD flare is ≥ 50%.7,8 In 2015, a pharmacy resident designed and piloted COPD CARE, a program that used evidence-based practice to optimize management of the disease.9,10
The COPD CARE program is delivered by interprofessional team members. It includes a postacute care call completed 48 hours postdischarge, a wellness visit (face-to-face or virtual) 1 month postdischarge, and a follow-up visit scheduled 2 months postdischarge. Clinical pharmacist practitioners (CPPs) prescribe and collaborate with the COPD CARE health care team. Evidence-based practices embedded within COPD CARE include treatment optimization, symptom evaluation, severity staging, vaccination promotion, referrals, tobacco treatment, and comorbidity management.11-16 The initial COPD CARE pilot demonstrated promising results; patients received timely care and high rates of COPD best practices.11
Academy Design and Implementation
Initial COPD CARE training was tailored to the culture, context, and workflow of the William S. Middleton Memorial Veteran’s Hospital in Madison, Wisconsin. Further service expansion required integration of implementation strategies that enable learners to apply and adapt content to fit different processes, staffing, and patient needs.
Formal Implementation Blueprint
A key aspect of the Academy is the integration of a formal implementation blueprint that includes training goals, scope, and key milestones to guide implementation. The Academy blueprint includes 4 phased training workbooks: (1) preimplementation support from local stakeholders; (2) integration of COPD CARE operational infrastructure into workflows; (3) preparing clinical champions; and (4) leading clinical training (Figure 1). Five weekly 1-hour synchronous virtual discussions are used for learning the workbook content that include learning objectives and opportunities to strategize how to overcome implementation barriers.

Promoting and Facilitating Implementation
As clinicians apply content from the Academy to install informatics tools, coordinate clinical training, and build relationships across service lines, implementation barriers may occur. A learning collaborative allows peer-mentorship and shared problem solving. The Academy learning collaborative includes attendees across multiple VAMCs, allowing for diverse perspectives and cross-site learning. Within the field of dissemination and implementation science, this process of shared problem-solving to support individuals is referred to as implementation facilitation.17 Academy facilitators with prior experience provide a unique perspective and external facilitation from outside local VAMCs. Academy learners form local teams to engage in shared decision-making when applying Academy content. Following Academy completion, learning collaboratives continue to meet monthly to share clinical insights and operational updates.
Local Champions Promote Adaptability
One or more local champions were identified at each VAMC who were focused on the implementation of clinical training content and operational implementation of Academy content.18 Champions have helped develop adaptations of Academy content, such as integrating telehealth nursing within the COPD CARE referral process, which have become new best practices. Champions attend Academy sessions, which provide an opportunity to share adaptations to meet local needs.19
Using a Train-The-Trainer Model
Clinical training was designed to be dynamic and included video modeling, such as recorded examples of CPPs conducting COPD CARE visits and video clips highlighting clinical content. Each learner received a clinical workbook summarizing the content. The champion shares discussion questions to relate training content to the local clinical practice setting. The combination of live training, with videos of clinic visits and case-based discussion was intended to address differing learning styles. Clinical training was delivered using a train-the-trainer model led by the local champion, which allows clinicians with expertise to tailor their training. The use of a train-the-trainer model was intended to promote local buy-in and was often completed by frontline clinicians.
Informatics note templates provide clinicians with information needed to deliver training content during clinic visits. Direct hyperlinks to symptomatic scoring tools, resources to promote evidence-based medication optimization, and patient education resources were embedded within the electronic health record note templates. Direct links to consults for COPD referrals services discussed during clinical training were also included to promote ease of care coordination and awareness of referral opportunities. The integration of clinical training with informatics note template support was intentional to directly relate clinical training to clinical care delivery.
Audit and Feedback
To inform COPD CARE practice, the Academy included informatics infrastructure that allowed for timely local quality monitoring. Electronic health record note templates with embedded data fields track COPD CARE service implementation, including timely completion of patient visits, completion of patient medication reviews, appropriate testing, symptom assessment, and interventions made. Champions can organize template installation and integrate templates into COPD CARE clinical training. Data are included on a COPD CARE implementation dashboard.
An audit and feedback process is allows for the review of performance metrics and development of action plans.20,21 Data reports from note templates are described during the Academy, along with resources to help teams enhance delivery of their program based on performance metrics.
Building a Coalition
Within VA primary care, clinical care delivery is optimized through a team-based coalition of clinicians using the patient aligned care team (PACT) framework. The VA patient-centered team-based care delivery model, patient facilitates coordination of patient referrals, including patient review, scheduling, and completion of patient visits.22
Partnerships with VA Pharmacy Benefits Manager, VA Diffusion of Excellence, VA Quality Enhancement Research Initiative, VA Office of Pulmonary Medicine, and the VA Office of Rural Health have facilitated COPD CARE successes. Collaborations with VA Centers of Innovation helped benchmark the Academy’s impact. An academic partnership with the University of Wisconsin-Madison was established in 2017 and has provided evaluation expertise and leadership as the Academy has been iteratively developed, and revised.
Preliminary Metrics
COPD CARE has delivered > 2000 visits. CPPs have delivered COPD care, with a mean 9.4 of 10 best practices per patient visit. Improvements in veteran COPD symptoms have also been observed following COPD CARE patient visits.
DISCUSSION
The COPD CARE Academy was developed to promote rapid scale-up of a complex, team-based COPD service delivered during veteran care transitions. The implementation blueprint for the Academy is multifaceted and integrates both clinical-focused and implementation-focused infrastructure to apply training content.23 A randomized control trial evaluating the efficacy of training modalities found a need to expand implementation blueprints beyond clinical training alone, as training by itself may not be sufficient to change behavior.24 VA staff designed the Academy using clinical- and implementation-focused content within its implementation blueprint. Key components included leveraging clinical champions, using a train-the-trainer approach, and incorporating facilitation strategies to overcome adoption barriers.
Lewis et al emphasize matching implementation strategies to barriers within VA staff who identify care coordination as a key challenge.23 The informatics infrastructure developed for Academy learners, including standardized note templates, video modeling examples of clinic visits, and data capture for audit and feedback, was designed to complement clinical training and standardize service workflows (Figure 2). There are opportunities to explore how to optimize technology in the Academy.

While Academy clinical training specifically focuses on COPD management, many implementation strategies can be considered to promote care delivery services for other chronic conditions. The Academy blueprint and implementation infrastructure, are strategies that may be considered within and outside the federal health care system. The opportunity for adaptations to Academy training enables clinical champions to promote tailored content to the needs of each unique VAMC. The translation of Academy implementation strategies for new chronic conditions will similarly require adaptations at each VAMC to promote adoption of content.
CONCLUSIONS
COPD CARE Academy is an example of the collaborative spirit within VA, and the opportunity for further advancement of health care programs. The VA is a national leader in Learning Health Systems implementation, in which “science, informatics, incentives and culture are aligned for continuous improvement and innovation.”25,26 There are many opportunities for VA staff to learn from one another to form partnerships between leaders, clinicians, and scientists to optimize health care delivery and further the VA’s work as a learning health system.
Quality improvement (QI) initiatives within the US Department of Veterans Affairs (VA) play an important role in enhancing health care for veterans.1,2 While effective QI programs are often developed, veterans benefit only if they receive care at sites where the program is offered.3 It is estimated only 1% to 5% of patients receive benefit from evidence-based programs, limiting the opportunity for widespread impact.4,5
The Chronic Obstructive Pulmonary Disease (COPD) Coordinated Access to Reduce Exacerbations (CARE) Academy is a national training program designed to promote the adoption of a COPD primary care service.6 The Academy was created and iteratively refined by VA staff to include both clinical training emphasizing COPD management and program implementation strategies. Training programs such as COPD CARE are commonly described as a method to support adoption of health care services, but there is no consensus on a universal approach to training design.
This article describes COPD CARE training and implementation strategies (Table). The Academy began as a training program at 1 VA medical center (VAMC) and has expanded to 49 diverse VAMCs. The Academy illustrates how implementation strategies can be leveraged to develop pragmatic and impactful training. Highlights from the Academy's 9-year history are outlined in this article.

COPD CARE
One in 4 veterans have a COPD diagnosis, and the 5-year mortality rate following a COPD flare is ≥ 50%.7,8 In 2015, a pharmacy resident designed and piloted COPD CARE, a program that used evidence-based practice to optimize management of the disease.9,10
The COPD CARE program is delivered by interprofessional team members. It includes a postacute care call completed 48 hours postdischarge, a wellness visit (face-to-face or virtual) 1 month postdischarge, and a follow-up visit scheduled 2 months postdischarge. Clinical pharmacist practitioners (CPPs) prescribe and collaborate with the COPD CARE health care team. Evidence-based practices embedded within COPD CARE include treatment optimization, symptom evaluation, severity staging, vaccination promotion, referrals, tobacco treatment, and comorbidity management.11-16 The initial COPD CARE pilot demonstrated promising results; patients received timely care and high rates of COPD best practices.11
Academy Design and Implementation
Initial COPD CARE training was tailored to the culture, context, and workflow of the William S. Middleton Memorial Veteran’s Hospital in Madison, Wisconsin. Further service expansion required integration of implementation strategies that enable learners to apply and adapt content to fit different processes, staffing, and patient needs.
Formal Implementation Blueprint
A key aspect of the Academy is the integration of a formal implementation blueprint that includes training goals, scope, and key milestones to guide implementation. The Academy blueprint includes 4 phased training workbooks: (1) preimplementation support from local stakeholders; (2) integration of COPD CARE operational infrastructure into workflows; (3) preparing clinical champions; and (4) leading clinical training (Figure 1). Five weekly 1-hour synchronous virtual discussions are used for learning the workbook content that include learning objectives and opportunities to strategize how to overcome implementation barriers.

Promoting and Facilitating Implementation
As clinicians apply content from the Academy to install informatics tools, coordinate clinical training, and build relationships across service lines, implementation barriers may occur. A learning collaborative allows peer-mentorship and shared problem solving. The Academy learning collaborative includes attendees across multiple VAMCs, allowing for diverse perspectives and cross-site learning. Within the field of dissemination and implementation science, this process of shared problem-solving to support individuals is referred to as implementation facilitation.17 Academy facilitators with prior experience provide a unique perspective and external facilitation from outside local VAMCs. Academy learners form local teams to engage in shared decision-making when applying Academy content. Following Academy completion, learning collaboratives continue to meet monthly to share clinical insights and operational updates.
Local Champions Promote Adaptability
One or more local champions were identified at each VAMC who were focused on the implementation of clinical training content and operational implementation of Academy content.18 Champions have helped develop adaptations of Academy content, such as integrating telehealth nursing within the COPD CARE referral process, which have become new best practices. Champions attend Academy sessions, which provide an opportunity to share adaptations to meet local needs.19
Using a Train-The-Trainer Model
Clinical training was designed to be dynamic and included video modeling, such as recorded examples of CPPs conducting COPD CARE visits and video clips highlighting clinical content. Each learner received a clinical workbook summarizing the content. The champion shares discussion questions to relate training content to the local clinical practice setting. The combination of live training, with videos of clinic visits and case-based discussion was intended to address differing learning styles. Clinical training was delivered using a train-the-trainer model led by the local champion, which allows clinicians with expertise to tailor their training. The use of a train-the-trainer model was intended to promote local buy-in and was often completed by frontline clinicians.
Informatics note templates provide clinicians with information needed to deliver training content during clinic visits. Direct hyperlinks to symptomatic scoring tools, resources to promote evidence-based medication optimization, and patient education resources were embedded within the electronic health record note templates. Direct links to consults for COPD referrals services discussed during clinical training were also included to promote ease of care coordination and awareness of referral opportunities. The integration of clinical training with informatics note template support was intentional to directly relate clinical training to clinical care delivery.
Audit and Feedback
To inform COPD CARE practice, the Academy included informatics infrastructure that allowed for timely local quality monitoring. Electronic health record note templates with embedded data fields track COPD CARE service implementation, including timely completion of patient visits, completion of patient medication reviews, appropriate testing, symptom assessment, and interventions made. Champions can organize template installation and integrate templates into COPD CARE clinical training. Data are included on a COPD CARE implementation dashboard.
An audit and feedback process is allows for the review of performance metrics and development of action plans.20,21 Data reports from note templates are described during the Academy, along with resources to help teams enhance delivery of their program based on performance metrics.
Building a Coalition
Within VA primary care, clinical care delivery is optimized through a team-based coalition of clinicians using the patient aligned care team (PACT) framework. The VA patient-centered team-based care delivery model, patient facilitates coordination of patient referrals, including patient review, scheduling, and completion of patient visits.22
Partnerships with VA Pharmacy Benefits Manager, VA Diffusion of Excellence, VA Quality Enhancement Research Initiative, VA Office of Pulmonary Medicine, and the VA Office of Rural Health have facilitated COPD CARE successes. Collaborations with VA Centers of Innovation helped benchmark the Academy’s impact. An academic partnership with the University of Wisconsin-Madison was established in 2017 and has provided evaluation expertise and leadership as the Academy has been iteratively developed, and revised.
Preliminary Metrics
COPD CARE has delivered > 2000 visits. CPPs have delivered COPD care, with a mean 9.4 of 10 best practices per patient visit. Improvements in veteran COPD symptoms have also been observed following COPD CARE patient visits.
DISCUSSION
The COPD CARE Academy was developed to promote rapid scale-up of a complex, team-based COPD service delivered during veteran care transitions. The implementation blueprint for the Academy is multifaceted and integrates both clinical-focused and implementation-focused infrastructure to apply training content.23 A randomized control trial evaluating the efficacy of training modalities found a need to expand implementation blueprints beyond clinical training alone, as training by itself may not be sufficient to change behavior.24 VA staff designed the Academy using clinical- and implementation-focused content within its implementation blueprint. Key components included leveraging clinical champions, using a train-the-trainer approach, and incorporating facilitation strategies to overcome adoption barriers.
Lewis et al emphasize matching implementation strategies to barriers within VA staff who identify care coordination as a key challenge.23 The informatics infrastructure developed for Academy learners, including standardized note templates, video modeling examples of clinic visits, and data capture for audit and feedback, was designed to complement clinical training and standardize service workflows (Figure 2). There are opportunities to explore how to optimize technology in the Academy.

While Academy clinical training specifically focuses on COPD management, many implementation strategies can be considered to promote care delivery services for other chronic conditions. The Academy blueprint and implementation infrastructure, are strategies that may be considered within and outside the federal health care system. The opportunity for adaptations to Academy training enables clinical champions to promote tailored content to the needs of each unique VAMC. The translation of Academy implementation strategies for new chronic conditions will similarly require adaptations at each VAMC to promote adoption of content.
CONCLUSIONS
COPD CARE Academy is an example of the collaborative spirit within VA, and the opportunity for further advancement of health care programs. The VA is a national leader in Learning Health Systems implementation, in which “science, informatics, incentives and culture are aligned for continuous improvement and innovation.”25,26 There are many opportunities for VA staff to learn from one another to form partnerships between leaders, clinicians, and scientists to optimize health care delivery and further the VA’s work as a learning health system.
- Robinson CH, Thompto AJ, Lima EN, Damschroder LJ. Continuous quality improvement at the frontline: one interdisciplinary clinical team's four-year journey after completing a virtual learning program. Learn Health Syst. 2022;6(4):e10345. doi:10.1002/lrh2.10345
- US Department of Veterans Affairs. Continuous quality improvement (CQI) for clinical teams: a systematic review of reviews. Accessed July 24, 2025. https://www.hsrd.research.va.gov/for_researchers/cyber_seminars/archives/video_archive.cfm?SessionID=4151
- Dondanville KA, Fina BA, Straud CL, et al. Launching a competency-based training program in evidence-based treatments for PTSD: supporting veteran-serving mental health providers in Texas. Community Ment Health J. 2021;57(5):910-919. doi:10.1007/S10597-020-00676-7
- Abildso CG, Zizzi SJ, Reger-Nash B. Evaluating an insurance- sponsored weight management program with the RE-AIM model, West Virginia, 2004-2008. Prev Chronic Dis. 2010;7(3):A46.
- Glasgow RE, Vinson C, Chambers D, Khoury MJ, Kaplan RM, Hunter C. National institutes of health approaches to dissemination and implementation science: current and future directions. Am J Public Health. 2012;102(7):1274- 1281. doi:10.2105/AJPH.2012.300755
- Portillo EC, Maurer MA, Kettner JT, et al. Applying RE-AIM to examine the impact of an implementation facilitation package to scale up a program for veterans with chronic obstructive pulmonary disease. Implement Sci Commun. 2023;4(1):143. doi:10.1186/S43058-023-00520-5
- McGhan R, Radcliff T, Fish R, Sutherland ER, Welsh C, Make B. Predictors of rehospitalization and death after a severe exacerbation of COPD. Chest. 2007;132(6):1748- 1755. doi:10.1378/chest.06-3018
- Anderson E, Wiener RS, Resnick K, Elwy AR, Rinne ST. Care coordination for veterans with COPD: a positive deviance study. Am J Manag Care. 2020;26(2):63-68. doi:10.37765/AJMC.2020.42394
- 2024 GOLD Report. Global Initiative for Chronic Obstructive Lung Disease - GOLD. Accessed July 24, 2025. https://goldcopd.org/2024-gold-report/
- Nici L, Mammen MJ, Charbek E, et al. Pharmacologic management of chronic obstructive pulmonary disease. An official American Thoracic Society clinical practice guideline. Am J Respir Crit Care Med. 2020;201(9):e56-e69. doi:10.1164/rccm.202003-0625ST
- Portillo EC, Wilcox A, Seckel E, et al. Reducing COPD readmission rates: using a COPD care service during care transitions. Fed Pract. 2018;35(11):30-36.
- Portillo EC, Gruber S, Lehmann M, et al. Application of the replicating effective programs framework to design a COPD training program. J Am Pharm Assoc. 2021;61(2):e129-e135. doi:10.1016/J.JAPH.2020.10.023
- Portillo EC, Lehmann MR, Hagen TL, et al. Integration of the patient-centered medical home to deliver a care bundle for chronic obstructive pulmonary disease management. J Am Pharm Assoc. 2023;63(1):212-219. doi:10.1016/j.japh.2022.10.003
- Portillo E, Lehmann M, Hagen T, et al. Evaluation of an implementation package to deliver the COPD CARE service. BMJ Open Qual. 2023;12(1). doi:10.1136/BMJOQ-2022-002074
- Portillo E, Lehmann M, Maurer M, et al. Barriers to implementing a pharmacist-led COPD care bundle in rural settings: A qualitative evaluation. 2025 (under review).
- Population Health Management. American Hospital Association. Accessed July 24, 2025. https://www.aha.org/center/population-health-management
- Ritchie MJ, Dollar KM, Miller CK, et al. Using implementation facilitation to improve healthcare: implementation facilitation training manual. Accessed July 11, 2024. https:// www.queri.research.va.gov/tools/Facilitation-Manual.pdf
- Morena AL, Gaias LM, Larkin C. Understanding the role of clinical champions and their impact on clinician behavior change: the need for causal pathway mechanisms. Front Health Serv. 2022;2:896885. doi:10.3389/FRHS.2022.896885
- Ayele RA, Rabin BA, McCreight M, Battaglia C. Editorial: understanding, assessing, and guiding adaptations in public health and health systems interventions: current and future directions. Front Public Health. 2023;11:1228437. doi:10.3389/fpubh.2023.1228437
- Jamtvedt G, Flottorp S, Ivers N. Audit and feedback as a quality strategy. In: Improving Healthcare Services. World Health Organization; 2019. Accessed July 24, 2025. https://www.ncbi.nlm.nih.gov/books/NBK549284/
- Snider MDH, Boyd MR, Walker MR, Powell BJ, Lewis CC. Using audit and feedback to guide tailored implementations of measurement-based care in community mental health: a multiple case study. Implement Sci Commun. 2023;4(1):94. doi:10.1186/s43058-023-00474-8
- Patient Aligned Care Team (PACT) – Patient Care Services. US Department of Veterans Affairs. Accessed July 24, 2025. https://www.patientcare.va.gov/primarycare/PACT.asp
- Lewis CC, Scott K, Marriott BR. A methodology for generating a tailored implementation blueprint: an exemplar from a youth residential setting. Implementat Sci. 2018;13(1):68. doi:10.1186/s13012-018-0761-6
- Beidas RS, Edmunds JM, Marcus SC, Kendall PC. Training and consultation to promote implementation of an empirically supported treatment: a randomized trial. Psychiatr Serv. 2012;63(7):660-665. doi:10.1176/appi.ps.201100401
- Kilbourne AM, Schmidt J, Edmunds M, Vega R, Bowersox N, Atkins D. How the VA is training the next-generation workforce for learning health systems. Learn Health Syst. 2022;6(4):e10333. doi:10.1002/LRH2.10333
- Easterling D, Perry AC, Woodside R, Patel T, Gesell SB. Clarifying the concept of a learning health system for healthcare delivery organizations: implications from a qualitative analysis of the scientific literature. Learn Health Syst. 2021;6(2):e10287. doi:10.1002/LRH2.10287
- Robinson CH, Thompto AJ, Lima EN, Damschroder LJ. Continuous quality improvement at the frontline: one interdisciplinary clinical team's four-year journey after completing a virtual learning program. Learn Health Syst. 2022;6(4):e10345. doi:10.1002/lrh2.10345
- US Department of Veterans Affairs. Continuous quality improvement (CQI) for clinical teams: a systematic review of reviews. Accessed July 24, 2025. https://www.hsrd.research.va.gov/for_researchers/cyber_seminars/archives/video_archive.cfm?SessionID=4151
- Dondanville KA, Fina BA, Straud CL, et al. Launching a competency-based training program in evidence-based treatments for PTSD: supporting veteran-serving mental health providers in Texas. Community Ment Health J. 2021;57(5):910-919. doi:10.1007/S10597-020-00676-7
- Abildso CG, Zizzi SJ, Reger-Nash B. Evaluating an insurance- sponsored weight management program with the RE-AIM model, West Virginia, 2004-2008. Prev Chronic Dis. 2010;7(3):A46.
- Glasgow RE, Vinson C, Chambers D, Khoury MJ, Kaplan RM, Hunter C. National institutes of health approaches to dissemination and implementation science: current and future directions. Am J Public Health. 2012;102(7):1274- 1281. doi:10.2105/AJPH.2012.300755
- Portillo EC, Maurer MA, Kettner JT, et al. Applying RE-AIM to examine the impact of an implementation facilitation package to scale up a program for veterans with chronic obstructive pulmonary disease. Implement Sci Commun. 2023;4(1):143. doi:10.1186/S43058-023-00520-5
- McGhan R, Radcliff T, Fish R, Sutherland ER, Welsh C, Make B. Predictors of rehospitalization and death after a severe exacerbation of COPD. Chest. 2007;132(6):1748- 1755. doi:10.1378/chest.06-3018
- Anderson E, Wiener RS, Resnick K, Elwy AR, Rinne ST. Care coordination for veterans with COPD: a positive deviance study. Am J Manag Care. 2020;26(2):63-68. doi:10.37765/AJMC.2020.42394
- 2024 GOLD Report. Global Initiative for Chronic Obstructive Lung Disease - GOLD. Accessed July 24, 2025. https://goldcopd.org/2024-gold-report/
- Nici L, Mammen MJ, Charbek E, et al. Pharmacologic management of chronic obstructive pulmonary disease. An official American Thoracic Society clinical practice guideline. Am J Respir Crit Care Med. 2020;201(9):e56-e69. doi:10.1164/rccm.202003-0625ST
- Portillo EC, Wilcox A, Seckel E, et al. Reducing COPD readmission rates: using a COPD care service during care transitions. Fed Pract. 2018;35(11):30-36.
- Portillo EC, Gruber S, Lehmann M, et al. Application of the replicating effective programs framework to design a COPD training program. J Am Pharm Assoc. 2021;61(2):e129-e135. doi:10.1016/J.JAPH.2020.10.023
- Portillo EC, Lehmann MR, Hagen TL, et al. Integration of the patient-centered medical home to deliver a care bundle for chronic obstructive pulmonary disease management. J Am Pharm Assoc. 2023;63(1):212-219. doi:10.1016/j.japh.2022.10.003
- Portillo E, Lehmann M, Hagen T, et al. Evaluation of an implementation package to deliver the COPD CARE service. BMJ Open Qual. 2023;12(1). doi:10.1136/BMJOQ-2022-002074
- Portillo E, Lehmann M, Maurer M, et al. Barriers to implementing a pharmacist-led COPD care bundle in rural settings: A qualitative evaluation. 2025 (under review).
- Population Health Management. American Hospital Association. Accessed July 24, 2025. https://www.aha.org/center/population-health-management
- Ritchie MJ, Dollar KM, Miller CK, et al. Using implementation facilitation to improve healthcare: implementation facilitation training manual. Accessed July 11, 2024. https:// www.queri.research.va.gov/tools/Facilitation-Manual.pdf
- Morena AL, Gaias LM, Larkin C. Understanding the role of clinical champions and their impact on clinician behavior change: the need for causal pathway mechanisms. Front Health Serv. 2022;2:896885. doi:10.3389/FRHS.2022.896885
- Ayele RA, Rabin BA, McCreight M, Battaglia C. Editorial: understanding, assessing, and guiding adaptations in public health and health systems interventions: current and future directions. Front Public Health. 2023;11:1228437. doi:10.3389/fpubh.2023.1228437
- Jamtvedt G, Flottorp S, Ivers N. Audit and feedback as a quality strategy. In: Improving Healthcare Services. World Health Organization; 2019. Accessed July 24, 2025. https://www.ncbi.nlm.nih.gov/books/NBK549284/
- Snider MDH, Boyd MR, Walker MR, Powell BJ, Lewis CC. Using audit and feedback to guide tailored implementations of measurement-based care in community mental health: a multiple case study. Implement Sci Commun. 2023;4(1):94. doi:10.1186/s43058-023-00474-8
- Patient Aligned Care Team (PACT) – Patient Care Services. US Department of Veterans Affairs. Accessed July 24, 2025. https://www.patientcare.va.gov/primarycare/PACT.asp
- Lewis CC, Scott K, Marriott BR. A methodology for generating a tailored implementation blueprint: an exemplar from a youth residential setting. Implementat Sci. 2018;13(1):68. doi:10.1186/s13012-018-0761-6
- Beidas RS, Edmunds JM, Marcus SC, Kendall PC. Training and consultation to promote implementation of an empirically supported treatment: a randomized trial. Psychiatr Serv. 2012;63(7):660-665. doi:10.1176/appi.ps.201100401
- Kilbourne AM, Schmidt J, Edmunds M, Vega R, Bowersox N, Atkins D. How the VA is training the next-generation workforce for learning health systems. Learn Health Syst. 2022;6(4):e10333. doi:10.1002/LRH2.10333
- Easterling D, Perry AC, Woodside R, Patel T, Gesell SB. Clarifying the concept of a learning health system for healthcare delivery organizations: implications from a qualitative analysis of the scientific literature. Learn Health Syst. 2021;6(2):e10287. doi:10.1002/LRH2.10287
COPD CARE Academy: Design of Purposeful Training Guided by Implementation Strategies
COPD CARE Academy: Design of Purposeful Training Guided by Implementation Strategies
Consider Cultural Practices and Barriers to Care When Treating Alopecia Areata
Consider Cultural Practices and Barriers to Care When Treating Alopecia Areata
The Comparison
A. Alopecia areata in a young girl with a lighter skin tone. The fine white vellus hairs are signs of regrowth.
B. Alopecia areata in a 49-year-old man with tightly coiled hair and darker skin tone. Coiled white hairs are noted in the alopecia patches.
young girl with a lighter skin
tone. The fine white vellus
hairs are signs of regrowth. Photographs courtesy of
Richard P. Usatine, MD.

49-year-old man with tightly
coiled hair and darker skin
tone. Coiled white hairs
are noted in the alopecia
patches. Photographs courtesy of
Richard P. Usatine, MD.
Alopecia areata (AA) is a common autoimmune condition characterized by hair loss resulting from a T cell–mediated attack on the hair follicles. It manifests as nonscarring patches of hair loss on the scalp, eyebrows, eyelashes, and beard area as well as more extensive complete loss of scalp and body hair. While AA may affect individuals of any age, most patients develop their first patch(es) of hair loss during childhood.1 The treatment landscape for AA has evolved considerably in recent years, but barriers to access to newer treatments persist.
Epidemiology
AA is most prevalent among pediatric and adult individuals of African, Asian, or Hispanic/Latino descent.2-4 In some studies, Black individuals had higher odds and Asian individuals had lower odds of developing AA, while other studies have reported the highest standardized prevalence among Asian individuals.5 In the United States, AA affects about 1.47% of adults and as many as 0.11% of children.6-8 In Black patients, AA often manifests early with a female predominance.5
AA frequently is associated with autoimmune comorbidities, the most common being thyroid disease.3,5 In Black patients, AA is associated with more atopic comorbidities, including asthma, atopic dermatitis, and allergic rhinitis.5
Key Clinical Features
AA clinically manifests similarly across different skin tones; however, in patients with more tightly coiled or curly hair, the extent of scalp hair loss may be underestimated without a full examination. Culturally sensitive approaches to hair and scalp evaluation are essential, especially for Black women, whose hair care practices and scalp conditions may be overlooked or misunderstood during visits to evaluate hair loss. A thoughtful history and gentle examination of the hair and scalp that considers hair texture, cultural practices such as head coverings (eg, headwraps, turbans, hijabs), use of hair adornments (eg, clips, beads, bows), traditional braiding, and use of natural oils or herbal treatments, as well as styling methods including tight hairstyles, use of heat styling tools (eg, flat irons, curling irons), chemical application (eg, straighteners, hair color), and washing or styling frequency can improve diagnostic accuracy and help build trust in the patient-provider relationship.
Classic signs of AA visualized with dermoscopy include yellow and/or black dots on the scalp and exclamation point hairs. The appearance of fine white vellus hairs within the alopecic patches also may indicate early regrowth. On scalp trichoscopy, black dots are more prominent, and yellow dots are less prominent, in individuals with darker skin tones vs lighter skin tones.9
Worth Noting
In addition to a full examination of the scalp, documenting the extent of hair loss using validated severity scales, including the severity of alopecia tool (SALT), AA severity index (AASI), clinician-reported outcome assessment, and patient-reported outcome measures, can standardize disease severity assessment, facilitate timely insurance or medication approvals, and support objective tracking of treatment response, which may ultimately enhance access to care.10
Prompt treatment of AA is essential. Not surprisingly, patients given a diagnosis of AA may experience considerable emotional and psychological distress—regardless of the extent of the loss.11 Treatment options include mid- to high-potency topical or intralesional corticosteroids and newer and more targeted systemic options, including 3 Janus kinase (JAK) inhibitors—baricitinib, ritlecitinib, and deuruxolitinib—for more extensive disease.12 Treatment with intralesional corticosteroids may cause transient hypopigmentation, which may be more noticeable in patients with darker skin tones. Delays in treatment with JAK inhibitors can lead to a less-than-optimal response. Of the 3 JAK inhibitors that are approved by the US Food and Drug Administration for AA, only ritlecitinib is approved for children 12 years and older, leaving a therapeutic gap for younger patients that often leads to uncomfortable scalp injections, delayed or no treatment, off-label use of JAK inhibitors as well as the pairing of off-label dupilumab with oral minoxidil.12
Based on adult data, patients with severe disease and a shorter duration of hair loss (ie, < 4 years) tend to respond better to JAK inhibitors than those experiencing hair loss for longer periods. Also, those with more severe AA tend to have poorer outcomes than those with less severe disease.13 If treatment proves less than optimal, wigs and hair pieces may need to be considered. It is worth noting that some insurance companies will cover the cost of wigs for patients when prescribed as cranial prostheses.
Health Disparity Highlight
Health disparities in AA can be influenced by socioeconomic status and access to care. Patients from lower-income backgrounds often face barriers to accessing dermatologic care and treatments such as JAK inhibitors, which may remain inaccessible due to high costs and insurance limitations.14 These barriers can intersect with other factors such as age, sex, and race, potentially exacerbating disparities. Women with skin of color in underserved communities may experience delayed diagnosis, limited treatment options, and greater psychosocial distress from hair loss.14 Addressing these inequities requires advocacy, education for both patients and clinicians, and improved access to treatment to ensure comprehensive care for all patients.
- Kara T, Topkarcı Z. Interactions between posttraumatic stress disorder and alopecia areata in child with trauma exposure: two case reports. Int J Trichology. 2018;10:131-134. doi:10.4103/ijt.ijt_2_18
- Sy N, Mastacouris N, Strunk A, et al. Overall and racial and ethnic subgroup prevalences of alopecia areata, alopecia totalis, and alopecia universalis. JAMA Dermatol. 2023;159:419-423.
- Lee H, Jung SJ, Patel AB, et al. Racial characteristics of alopecia areata in the United States. J Am Acad Dermatol. 2020;83:1064-1070.
- Feaster B, McMichael AJ. Epidemiology of alopecia areata in Black patients: a retrospective chart review. J Am Acad Dermatol. 2022;87:1121-1123.
- Lee HH, Gwillim E, Patel KR, et al. Epidemiology of alopecia areata, ophiasis, totalis, and universalis: a systematic review and meta-analysis. J Am Acad Dermatol. 2020;82:675-682.
- Mostaghimi A, Gao W, Ray M, et al. Trends in prevalence and incidence of alopecia areata, alopecia totalis, and alopecia universalis among adults and children in a US employer-sponsored insured population. JAMA Dermatol. 2023;159:411-418.
- Adhanom R, Ansbro B, Castelo-Soccio L. Epidemiology of pediatric alopecia areata. Pediatr Dermatol. 2025;42 suppl 1(suppl 1):12-23.
- Karampinis E, Toli O, Georgopoulou KE, et al. Exploring pediatric dermatology in skin of color: focus on dermoscopy. Life (Basel). 2024;14:1604.
- King BA, Senna MM, Ohyama M, et al. Defining severity in alopecia areata: current perspectives and a multidimensional framework. Dermatol Ther (Heidelb). 2022;12:825-834.
- Toussi A, Barton VR, Le ST, et al. Psychosocial and psychiatric comorbidities and health-related quality of life in alopecia areata: a systematic review. J Am Acad Dermatol. 2021;85:162-175.
- Kalil L, Welch D, Heath CR, et al. Systemic therapies for pediatric alopecia areata. Pediatr Dermatol. 2025;42 suppl 1:36-42.
- King BA, Craiglow BG. Janus kinase inhibitors for alopecia areata. J Am Acad Dermatol. 2023;89:S29-S32.
- Klein EJ, Taiwò D, Kakpovbia E, et al. Disparities in Janus kinase inhibitor access for alopecia areata: a retrospective analysis. Int J Womens Dermatol. 2024;10:E155.
- McKenzie PL, Maltenfort M, Bruckner AL, et al. Evaluation of the prevalence and incidence of pediatric alopecia areata using electronic health record data. JAMA Dermatol. 2022;158:547-551. doi:10.1001/jamadermatol.2022.0351
The Comparison
A. Alopecia areata in a young girl with a lighter skin tone. The fine white vellus hairs are signs of regrowth.
B. Alopecia areata in a 49-year-old man with tightly coiled hair and darker skin tone. Coiled white hairs are noted in the alopecia patches.

young girl with a lighter skin
tone. The fine white vellus
hairs are signs of regrowth. Photographs courtesy of
Richard P. Usatine, MD.

49-year-old man with tightly
coiled hair and darker skin
tone. Coiled white hairs
are noted in the alopecia
patches. Photographs courtesy of
Richard P. Usatine, MD.
Alopecia areata (AA) is a common autoimmune condition characterized by hair loss resulting from a T cell–mediated attack on the hair follicles. It manifests as nonscarring patches of hair loss on the scalp, eyebrows, eyelashes, and beard area as well as more extensive complete loss of scalp and body hair. While AA may affect individuals of any age, most patients develop their first patch(es) of hair loss during childhood.1 The treatment landscape for AA has evolved considerably in recent years, but barriers to access to newer treatments persist.
Epidemiology
AA is most prevalent among pediatric and adult individuals of African, Asian, or Hispanic/Latino descent.2-4 In some studies, Black individuals had higher odds and Asian individuals had lower odds of developing AA, while other studies have reported the highest standardized prevalence among Asian individuals.5 In the United States, AA affects about 1.47% of adults and as many as 0.11% of children.6-8 In Black patients, AA often manifests early with a female predominance.5
AA frequently is associated with autoimmune comorbidities, the most common being thyroid disease.3,5 In Black patients, AA is associated with more atopic comorbidities, including asthma, atopic dermatitis, and allergic rhinitis.5
Key Clinical Features
AA clinically manifests similarly across different skin tones; however, in patients with more tightly coiled or curly hair, the extent of scalp hair loss may be underestimated without a full examination. Culturally sensitive approaches to hair and scalp evaluation are essential, especially for Black women, whose hair care practices and scalp conditions may be overlooked or misunderstood during visits to evaluate hair loss. A thoughtful history and gentle examination of the hair and scalp that considers hair texture, cultural practices such as head coverings (eg, headwraps, turbans, hijabs), use of hair adornments (eg, clips, beads, bows), traditional braiding, and use of natural oils or herbal treatments, as well as styling methods including tight hairstyles, use of heat styling tools (eg, flat irons, curling irons), chemical application (eg, straighteners, hair color), and washing or styling frequency can improve diagnostic accuracy and help build trust in the patient-provider relationship.
Classic signs of AA visualized with dermoscopy include yellow and/or black dots on the scalp and exclamation point hairs. The appearance of fine white vellus hairs within the alopecic patches also may indicate early regrowth. On scalp trichoscopy, black dots are more prominent, and yellow dots are less prominent, in individuals with darker skin tones vs lighter skin tones.9
Worth Noting
In addition to a full examination of the scalp, documenting the extent of hair loss using validated severity scales, including the severity of alopecia tool (SALT), AA severity index (AASI), clinician-reported outcome assessment, and patient-reported outcome measures, can standardize disease severity assessment, facilitate timely insurance or medication approvals, and support objective tracking of treatment response, which may ultimately enhance access to care.10
Prompt treatment of AA is essential. Not surprisingly, patients given a diagnosis of AA may experience considerable emotional and psychological distress—regardless of the extent of the loss.11 Treatment options include mid- to high-potency topical or intralesional corticosteroids and newer and more targeted systemic options, including 3 Janus kinase (JAK) inhibitors—baricitinib, ritlecitinib, and deuruxolitinib—for more extensive disease.12 Treatment with intralesional corticosteroids may cause transient hypopigmentation, which may be more noticeable in patients with darker skin tones. Delays in treatment with JAK inhibitors can lead to a less-than-optimal response. Of the 3 JAK inhibitors that are approved by the US Food and Drug Administration for AA, only ritlecitinib is approved for children 12 years and older, leaving a therapeutic gap for younger patients that often leads to uncomfortable scalp injections, delayed or no treatment, off-label use of JAK inhibitors as well as the pairing of off-label dupilumab with oral minoxidil.12
Based on adult data, patients with severe disease and a shorter duration of hair loss (ie, < 4 years) tend to respond better to JAK inhibitors than those experiencing hair loss for longer periods. Also, those with more severe AA tend to have poorer outcomes than those with less severe disease.13 If treatment proves less than optimal, wigs and hair pieces may need to be considered. It is worth noting that some insurance companies will cover the cost of wigs for patients when prescribed as cranial prostheses.
Health Disparity Highlight
Health disparities in AA can be influenced by socioeconomic status and access to care. Patients from lower-income backgrounds often face barriers to accessing dermatologic care and treatments such as JAK inhibitors, which may remain inaccessible due to high costs and insurance limitations.14 These barriers can intersect with other factors such as age, sex, and race, potentially exacerbating disparities. Women with skin of color in underserved communities may experience delayed diagnosis, limited treatment options, and greater psychosocial distress from hair loss.14 Addressing these inequities requires advocacy, education for both patients and clinicians, and improved access to treatment to ensure comprehensive care for all patients.
The Comparison
A. Alopecia areata in a young girl with a lighter skin tone. The fine white vellus hairs are signs of regrowth.
B. Alopecia areata in a 49-year-old man with tightly coiled hair and darker skin tone. Coiled white hairs are noted in the alopecia patches.

young girl with a lighter skin
tone. The fine white vellus
hairs are signs of regrowth. Photographs courtesy of
Richard P. Usatine, MD.

49-year-old man with tightly
coiled hair and darker skin
tone. Coiled white hairs
are noted in the alopecia
patches. Photographs courtesy of
Richard P. Usatine, MD.
Alopecia areata (AA) is a common autoimmune condition characterized by hair loss resulting from a T cell–mediated attack on the hair follicles. It manifests as nonscarring patches of hair loss on the scalp, eyebrows, eyelashes, and beard area as well as more extensive complete loss of scalp and body hair. While AA may affect individuals of any age, most patients develop their first patch(es) of hair loss during childhood.1 The treatment landscape for AA has evolved considerably in recent years, but barriers to access to newer treatments persist.
Epidemiology
AA is most prevalent among pediatric and adult individuals of African, Asian, or Hispanic/Latino descent.2-4 In some studies, Black individuals had higher odds and Asian individuals had lower odds of developing AA, while other studies have reported the highest standardized prevalence among Asian individuals.5 In the United States, AA affects about 1.47% of adults and as many as 0.11% of children.6-8 In Black patients, AA often manifests early with a female predominance.5
AA frequently is associated with autoimmune comorbidities, the most common being thyroid disease.3,5 In Black patients, AA is associated with more atopic comorbidities, including asthma, atopic dermatitis, and allergic rhinitis.5
Key Clinical Features
AA clinically manifests similarly across different skin tones; however, in patients with more tightly coiled or curly hair, the extent of scalp hair loss may be underestimated without a full examination. Culturally sensitive approaches to hair and scalp evaluation are essential, especially for Black women, whose hair care practices and scalp conditions may be overlooked or misunderstood during visits to evaluate hair loss. A thoughtful history and gentle examination of the hair and scalp that considers hair texture, cultural practices such as head coverings (eg, headwraps, turbans, hijabs), use of hair adornments (eg, clips, beads, bows), traditional braiding, and use of natural oils or herbal treatments, as well as styling methods including tight hairstyles, use of heat styling tools (eg, flat irons, curling irons), chemical application (eg, straighteners, hair color), and washing or styling frequency can improve diagnostic accuracy and help build trust in the patient-provider relationship.
Classic signs of AA visualized with dermoscopy include yellow and/or black dots on the scalp and exclamation point hairs. The appearance of fine white vellus hairs within the alopecic patches also may indicate early regrowth. On scalp trichoscopy, black dots are more prominent, and yellow dots are less prominent, in individuals with darker skin tones vs lighter skin tones.9
Worth Noting
In addition to a full examination of the scalp, documenting the extent of hair loss using validated severity scales, including the severity of alopecia tool (SALT), AA severity index (AASI), clinician-reported outcome assessment, and patient-reported outcome measures, can standardize disease severity assessment, facilitate timely insurance or medication approvals, and support objective tracking of treatment response, which may ultimately enhance access to care.10
Prompt treatment of AA is essential. Not surprisingly, patients given a diagnosis of AA may experience considerable emotional and psychological distress—regardless of the extent of the loss.11 Treatment options include mid- to high-potency topical or intralesional corticosteroids and newer and more targeted systemic options, including 3 Janus kinase (JAK) inhibitors—baricitinib, ritlecitinib, and deuruxolitinib—for more extensive disease.12 Treatment with intralesional corticosteroids may cause transient hypopigmentation, which may be more noticeable in patients with darker skin tones. Delays in treatment with JAK inhibitors can lead to a less-than-optimal response. Of the 3 JAK inhibitors that are approved by the US Food and Drug Administration for AA, only ritlecitinib is approved for children 12 years and older, leaving a therapeutic gap for younger patients that often leads to uncomfortable scalp injections, delayed or no treatment, off-label use of JAK inhibitors as well as the pairing of off-label dupilumab with oral minoxidil.12
Based on adult data, patients with severe disease and a shorter duration of hair loss (ie, < 4 years) tend to respond better to JAK inhibitors than those experiencing hair loss for longer periods. Also, those with more severe AA tend to have poorer outcomes than those with less severe disease.13 If treatment proves less than optimal, wigs and hair pieces may need to be considered. It is worth noting that some insurance companies will cover the cost of wigs for patients when prescribed as cranial prostheses.
Health Disparity Highlight
Health disparities in AA can be influenced by socioeconomic status and access to care. Patients from lower-income backgrounds often face barriers to accessing dermatologic care and treatments such as JAK inhibitors, which may remain inaccessible due to high costs and insurance limitations.14 These barriers can intersect with other factors such as age, sex, and race, potentially exacerbating disparities. Women with skin of color in underserved communities may experience delayed diagnosis, limited treatment options, and greater psychosocial distress from hair loss.14 Addressing these inequities requires advocacy, education for both patients and clinicians, and improved access to treatment to ensure comprehensive care for all patients.
- Kara T, Topkarcı Z. Interactions between posttraumatic stress disorder and alopecia areata in child with trauma exposure: two case reports. Int J Trichology. 2018;10:131-134. doi:10.4103/ijt.ijt_2_18
- Sy N, Mastacouris N, Strunk A, et al. Overall and racial and ethnic subgroup prevalences of alopecia areata, alopecia totalis, and alopecia universalis. JAMA Dermatol. 2023;159:419-423.
- Lee H, Jung SJ, Patel AB, et al. Racial characteristics of alopecia areata in the United States. J Am Acad Dermatol. 2020;83:1064-1070.
- Feaster B, McMichael AJ. Epidemiology of alopecia areata in Black patients: a retrospective chart review. J Am Acad Dermatol. 2022;87:1121-1123.
- Lee HH, Gwillim E, Patel KR, et al. Epidemiology of alopecia areata, ophiasis, totalis, and universalis: a systematic review and meta-analysis. J Am Acad Dermatol. 2020;82:675-682.
- Mostaghimi A, Gao W, Ray M, et al. Trends in prevalence and incidence of alopecia areata, alopecia totalis, and alopecia universalis among adults and children in a US employer-sponsored insured population. JAMA Dermatol. 2023;159:411-418.
- Adhanom R, Ansbro B, Castelo-Soccio L. Epidemiology of pediatric alopecia areata. Pediatr Dermatol. 2025;42 suppl 1(suppl 1):12-23.
- Karampinis E, Toli O, Georgopoulou KE, et al. Exploring pediatric dermatology in skin of color: focus on dermoscopy. Life (Basel). 2024;14:1604.
- King BA, Senna MM, Ohyama M, et al. Defining severity in alopecia areata: current perspectives and a multidimensional framework. Dermatol Ther (Heidelb). 2022;12:825-834.
- Toussi A, Barton VR, Le ST, et al. Psychosocial and psychiatric comorbidities and health-related quality of life in alopecia areata: a systematic review. J Am Acad Dermatol. 2021;85:162-175.
- Kalil L, Welch D, Heath CR, et al. Systemic therapies for pediatric alopecia areata. Pediatr Dermatol. 2025;42 suppl 1:36-42.
- King BA, Craiglow BG. Janus kinase inhibitors for alopecia areata. J Am Acad Dermatol. 2023;89:S29-S32.
- Klein EJ, Taiwò D, Kakpovbia E, et al. Disparities in Janus kinase inhibitor access for alopecia areata: a retrospective analysis. Int J Womens Dermatol. 2024;10:E155.
- McKenzie PL, Maltenfort M, Bruckner AL, et al. Evaluation of the prevalence and incidence of pediatric alopecia areata using electronic health record data. JAMA Dermatol. 2022;158:547-551. doi:10.1001/jamadermatol.2022.0351
- Kara T, Topkarcı Z. Interactions between posttraumatic stress disorder and alopecia areata in child with trauma exposure: two case reports. Int J Trichology. 2018;10:131-134. doi:10.4103/ijt.ijt_2_18
- Sy N, Mastacouris N, Strunk A, et al. Overall and racial and ethnic subgroup prevalences of alopecia areata, alopecia totalis, and alopecia universalis. JAMA Dermatol. 2023;159:419-423.
- Lee H, Jung SJ, Patel AB, et al. Racial characteristics of alopecia areata in the United States. J Am Acad Dermatol. 2020;83:1064-1070.
- Feaster B, McMichael AJ. Epidemiology of alopecia areata in Black patients: a retrospective chart review. J Am Acad Dermatol. 2022;87:1121-1123.
- Lee HH, Gwillim E, Patel KR, et al. Epidemiology of alopecia areata, ophiasis, totalis, and universalis: a systematic review and meta-analysis. J Am Acad Dermatol. 2020;82:675-682.
- Mostaghimi A, Gao W, Ray M, et al. Trends in prevalence and incidence of alopecia areata, alopecia totalis, and alopecia universalis among adults and children in a US employer-sponsored insured population. JAMA Dermatol. 2023;159:411-418.
- Adhanom R, Ansbro B, Castelo-Soccio L. Epidemiology of pediatric alopecia areata. Pediatr Dermatol. 2025;42 suppl 1(suppl 1):12-23.
- Karampinis E, Toli O, Georgopoulou KE, et al. Exploring pediatric dermatology in skin of color: focus on dermoscopy. Life (Basel). 2024;14:1604.
- King BA, Senna MM, Ohyama M, et al. Defining severity in alopecia areata: current perspectives and a multidimensional framework. Dermatol Ther (Heidelb). 2022;12:825-834.
- Toussi A, Barton VR, Le ST, et al. Psychosocial and psychiatric comorbidities and health-related quality of life in alopecia areata: a systematic review. J Am Acad Dermatol. 2021;85:162-175.
- Kalil L, Welch D, Heath CR, et al. Systemic therapies for pediatric alopecia areata. Pediatr Dermatol. 2025;42 suppl 1:36-42.
- King BA, Craiglow BG. Janus kinase inhibitors for alopecia areata. J Am Acad Dermatol. 2023;89:S29-S32.
- Klein EJ, Taiwò D, Kakpovbia E, et al. Disparities in Janus kinase inhibitor access for alopecia areata: a retrospective analysis. Int J Womens Dermatol. 2024;10:E155.
- McKenzie PL, Maltenfort M, Bruckner AL, et al. Evaluation of the prevalence and incidence of pediatric alopecia areata using electronic health record data. JAMA Dermatol. 2022;158:547-551. doi:10.1001/jamadermatol.2022.0351
Consider Cultural Practices and Barriers to Care When Treating Alopecia Areata
Consider Cultural Practices and Barriers to Care When Treating Alopecia Areata
A Voice for Those Caring for Veterans With Cancer
A Voice for Those Caring for Veterans With Cancer
At some point, most Americans will experience the anxiety associated with an organizational restructure or a corporate budget cut that leads to job loss. Self-assurances may follow by telling ourselves we will be fine, and we could even start a new position that (if we're lucky) will be better than our previous one. It can be devastating, but is not a life-or-death scenario.
Unless you care for veterans with cancer.
The recent workforce reductions across the US Department of Veterans of Affairs (VA) health care system, whether through voluntary retirements or forced layoffs, is a life-threatening crisis. Every position lost has the potential to directly impact whether a veteran receives the necessary care in their battle with cancer.
Veterans deserve every opportunity, treatment plan, and resource available to ensure their comfort and survival. They are entitled to the specialized, comprehensive, and thorough care they receive through the VA—care that cannot be duplicated in community health care. Because many of the health challenges they face are a direct result of serving our country, we owe it to them to provide the best care available from the most highly-trained and competent clinicians. This level of excellence cannot be achieved in a gutted or chaotic system.
Reducing or eliminating VA health care positions is a decision that demands careful examination. Like any organization, the VA experiences some measure of waste or inefficiency that should be eliminated. But that cannot be done swiftly or in large-scale action.
Consider these examples: the reduction of force resulting in the removal of those deemed to hold unnecessary administrative positions—such as continuing education or physician oversight—has a direct impact on a clinician's ability to provide the most current and precise care. Reduced research funding limits the VA's contribution to health care innovation. The loss of contract positions that appear superfluous on paper represent the staff who schedule appointments, chemotherapy or radiation therapy, and wrap-around services for veterans. Even reducing auxiliary services like laundry may seem like a cost-saving measure—until the hospital can't admit new patients due to lack of sanitized linens.
VA employees know that veterans need specialized care for their complex and unique challenges. That individualized care has led to the VA nearly eliminating disparity gaps experienced in traditional health care. The removal of support positions and opportunities in professional development demands coordination with less-prepared community-based health care; overpopulated work environments will have a lasting impact. Limiting the workforce will make it impossible to provide coordinated and exceptional care.
The Association of VA Hematology/Oncology (AVAHO) is a leader in professional development opportunities for those who care for veterans with cancer. As a nonprofit organization, AVAHO is also a voice for those working with veterans with cancer to ensure they receive the care they deserve. AVAHO is calling on its colleagues, veterans, and those committed to supporting veterans to voice their opposition to reducing critical staff, research, and resources within the VA.
We ask veterans to share stories describing the difference VA care makes. We ask clinicians—including those within the federal system—to explain how a system that is well-staffed, supported, and with ample resources can impact patient care. Americans must stand for the care our veterans have earned.
Most importantly, we call on policymakers to carefully consider the impact each position has on the outcome of excellent, well-coordinated, and state-of-the-art care. The lives of our veterans depend on it.
AVAHO is a 501(c)3 nonprofit organization dedicated to supporting and educating health care providers who serve veterans with cancer and hematological disorders. You can find out more and support their advocacy initiatives at www.avaho.org.
At some point, most Americans will experience the anxiety associated with an organizational restructure or a corporate budget cut that leads to job loss. Self-assurances may follow by telling ourselves we will be fine, and we could even start a new position that (if we're lucky) will be better than our previous one. It can be devastating, but is not a life-or-death scenario.
Unless you care for veterans with cancer.
The recent workforce reductions across the US Department of Veterans of Affairs (VA) health care system, whether through voluntary retirements or forced layoffs, is a life-threatening crisis. Every position lost has the potential to directly impact whether a veteran receives the necessary care in their battle with cancer.
Veterans deserve every opportunity, treatment plan, and resource available to ensure their comfort and survival. They are entitled to the specialized, comprehensive, and thorough care they receive through the VA—care that cannot be duplicated in community health care. Because many of the health challenges they face are a direct result of serving our country, we owe it to them to provide the best care available from the most highly-trained and competent clinicians. This level of excellence cannot be achieved in a gutted or chaotic system.
Reducing or eliminating VA health care positions is a decision that demands careful examination. Like any organization, the VA experiences some measure of waste or inefficiency that should be eliminated. But that cannot be done swiftly or in large-scale action.
Consider these examples: the reduction of force resulting in the removal of those deemed to hold unnecessary administrative positions—such as continuing education or physician oversight—has a direct impact on a clinician's ability to provide the most current and precise care. Reduced research funding limits the VA's contribution to health care innovation. The loss of contract positions that appear superfluous on paper represent the staff who schedule appointments, chemotherapy or radiation therapy, and wrap-around services for veterans. Even reducing auxiliary services like laundry may seem like a cost-saving measure—until the hospital can't admit new patients due to lack of sanitized linens.
VA employees know that veterans need specialized care for their complex and unique challenges. That individualized care has led to the VA nearly eliminating disparity gaps experienced in traditional health care. The removal of support positions and opportunities in professional development demands coordination with less-prepared community-based health care; overpopulated work environments will have a lasting impact. Limiting the workforce will make it impossible to provide coordinated and exceptional care.
The Association of VA Hematology/Oncology (AVAHO) is a leader in professional development opportunities for those who care for veterans with cancer. As a nonprofit organization, AVAHO is also a voice for those working with veterans with cancer to ensure they receive the care they deserve. AVAHO is calling on its colleagues, veterans, and those committed to supporting veterans to voice their opposition to reducing critical staff, research, and resources within the VA.
We ask veterans to share stories describing the difference VA care makes. We ask clinicians—including those within the federal system—to explain how a system that is well-staffed, supported, and with ample resources can impact patient care. Americans must stand for the care our veterans have earned.
Most importantly, we call on policymakers to carefully consider the impact each position has on the outcome of excellent, well-coordinated, and state-of-the-art care. The lives of our veterans depend on it.
AVAHO is a 501(c)3 nonprofit organization dedicated to supporting and educating health care providers who serve veterans with cancer and hematological disorders. You can find out more and support their advocacy initiatives at www.avaho.org.
At some point, most Americans will experience the anxiety associated with an organizational restructure or a corporate budget cut that leads to job loss. Self-assurances may follow by telling ourselves we will be fine, and we could even start a new position that (if we're lucky) will be better than our previous one. It can be devastating, but is not a life-or-death scenario.
Unless you care for veterans with cancer.
The recent workforce reductions across the US Department of Veterans of Affairs (VA) health care system, whether through voluntary retirements or forced layoffs, is a life-threatening crisis. Every position lost has the potential to directly impact whether a veteran receives the necessary care in their battle with cancer.
Veterans deserve every opportunity, treatment plan, and resource available to ensure their comfort and survival. They are entitled to the specialized, comprehensive, and thorough care they receive through the VA—care that cannot be duplicated in community health care. Because many of the health challenges they face are a direct result of serving our country, we owe it to them to provide the best care available from the most highly-trained and competent clinicians. This level of excellence cannot be achieved in a gutted or chaotic system.
Reducing or eliminating VA health care positions is a decision that demands careful examination. Like any organization, the VA experiences some measure of waste or inefficiency that should be eliminated. But that cannot be done swiftly or in large-scale action.
Consider these examples: the reduction of force resulting in the removal of those deemed to hold unnecessary administrative positions—such as continuing education or physician oversight—has a direct impact on a clinician's ability to provide the most current and precise care. Reduced research funding limits the VA's contribution to health care innovation. The loss of contract positions that appear superfluous on paper represent the staff who schedule appointments, chemotherapy or radiation therapy, and wrap-around services for veterans. Even reducing auxiliary services like laundry may seem like a cost-saving measure—until the hospital can't admit new patients due to lack of sanitized linens.
VA employees know that veterans need specialized care for their complex and unique challenges. That individualized care has led to the VA nearly eliminating disparity gaps experienced in traditional health care. The removal of support positions and opportunities in professional development demands coordination with less-prepared community-based health care; overpopulated work environments will have a lasting impact. Limiting the workforce will make it impossible to provide coordinated and exceptional care.
The Association of VA Hematology/Oncology (AVAHO) is a leader in professional development opportunities for those who care for veterans with cancer. As a nonprofit organization, AVAHO is also a voice for those working with veterans with cancer to ensure they receive the care they deserve. AVAHO is calling on its colleagues, veterans, and those committed to supporting veterans to voice their opposition to reducing critical staff, research, and resources within the VA.
We ask veterans to share stories describing the difference VA care makes. We ask clinicians—including those within the federal system—to explain how a system that is well-staffed, supported, and with ample resources can impact patient care. Americans must stand for the care our veterans have earned.
Most importantly, we call on policymakers to carefully consider the impact each position has on the outcome of excellent, well-coordinated, and state-of-the-art care. The lives of our veterans depend on it.
AVAHO is a 501(c)3 nonprofit organization dedicated to supporting and educating health care providers who serve veterans with cancer and hematological disorders. You can find out more and support their advocacy initiatives at www.avaho.org.
A Voice for Those Caring for Veterans With Cancer
A Voice for Those Caring for Veterans With Cancer
Painless Nodule on the Lower Eyelid
Painless Nodule on the Lower Eyelid
THE DIAGNOSIS: Idiopathic Facial Aseptic Granuloma
Histopathology showed a ruptured follicle, perifollicular granulomatous inflammation, and admixed multinucleated giant cells in the superficial dermis. The deeper tissue exhibited edema, histiocytic/granulomatous inflammation forming ill-defined loose granulomas, and a single neutrophilic microabscess (Figure). Stains for periodic acid-Schiff with diastase and acid-fast bacillus were negative for microorganisms. The clinical examination and pathology findings supported a diagnosis of idiopathic facial aseptic granuloma (IFAG).
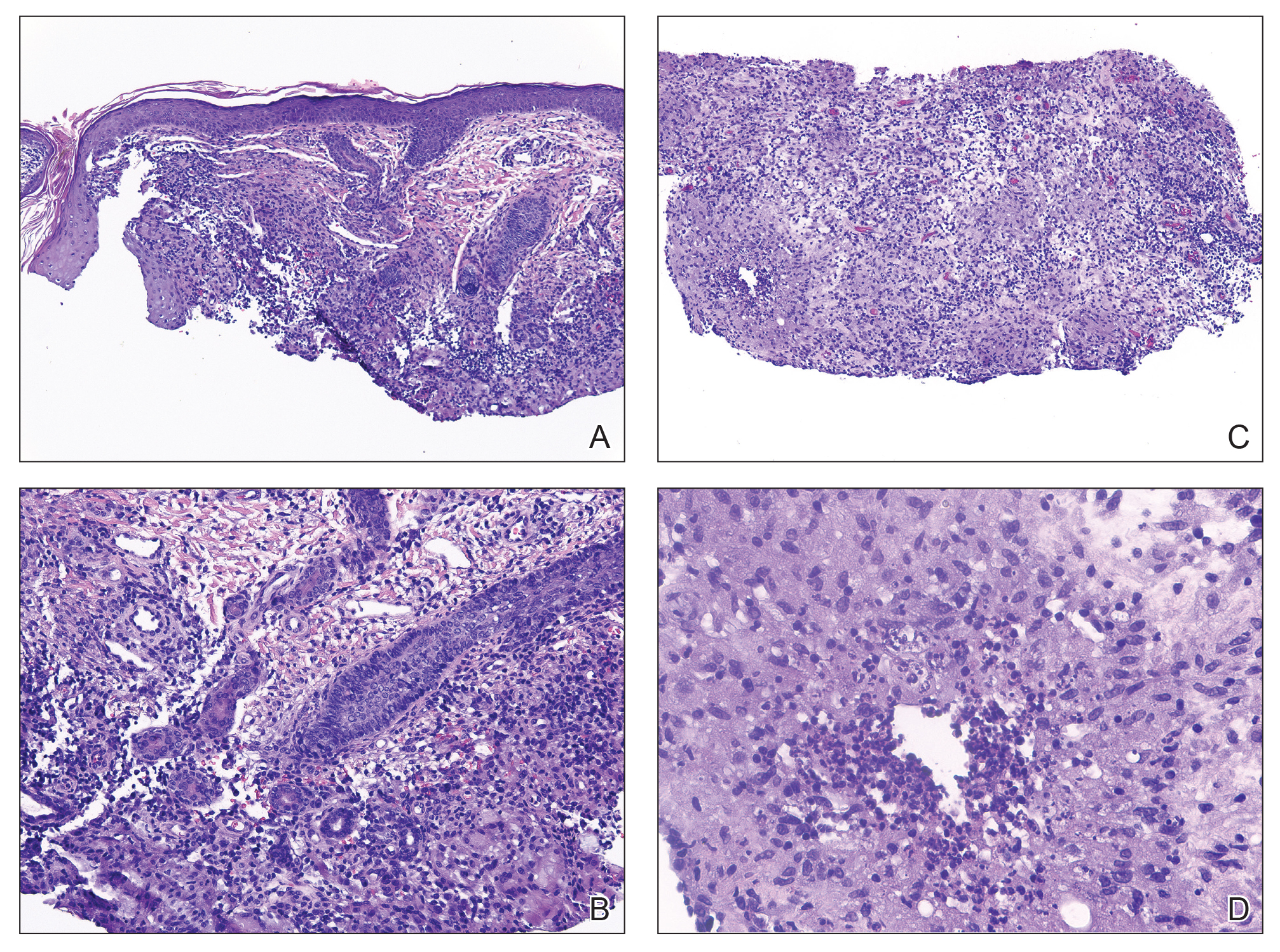
First reported in 1999, IFAG was described using the French term pyodermite froide du visage, which translates to “cold pyoderma of the face”; however, it was renamed to represent its granulomatous characteristics and noninfectious etiology.1 The pathogenesis of IFAG is unknown, but the leading hypothesis is that it may be a type of childhood granulomatous rosacea, given its association with relapsing chalazions, papulopustular eruptions on the face, and facial flushing.2 Other hypotheses are that IFAG is idiopathic or a granulomatous response to an insect bite, minor trauma, or embryologic remnant.3
A rare condition arising in early childhood, IFAG manifests as a single or multiple, painless, erythematous or violaceous nodule(s) on the face, most often on the cheeks or eyelids.4 A thorough history and clinical examination often suffice for diagnosis. Dermoscopy may reveal white perifollicular halos and follicular plugs on an erythematous base with linear vessels.4 If diagnostic tests are performed, there are notable characteristic findings: ultrasonography often shows a well-circumscribed, hypoechoic, ovoid dermal lesion without calcifications. Bacterial, fungal, and mycobacterial cultures commonly are negative.4 On biopsy, histopathology may reveal granulomatous inflammation in the superficial and deep dermis, multinucleated giant cells, and surrounding lymphocytic, neutrophilic, and eosinophilic infiltration with no calcium deposits.3,5,6 Histopathology findings for IFAG and rosacea lesions are similar; both may demonstrate folliculitis, perifollicular granulomas, and admixed lymphohistiocytic inflammation.7
Differentiating IFAG from other dermatologic lesions can be challenging, as the differential includes benign neoplasms (eg, dermoid cyst, chalazion, pilomatricoma, xanthoma, xanthogranuloma2) and infectious etiologies such as bacterial pyoderma and mycobacterial, fungal, and parasitic infections (eg, cutaneous leishmaniasis). Pilomatricomas, although often seen on the face or extremities in young girls, more often are well circumscribed and located in the dermis. Ultrasonography of a pilomatricoma classically shows variable foci of calcification. Xanthoma and xanthogranuloma also were considered in our case since the lesion was subtly yellowish on examination. Similar to IFAG, these conditions may manifest as single or multiple lesions. Abnormalities in the patient’s blood lipid panel or family history may be needed to diagnose xanthoma. Biopsy of a juvenile xanthogranuloma would exhibit a dense dermal nodular proliferation of histiocytic cells with a smaller number of admixed lymphocytes, neutrophils, and eosinophils, in contrast to the multiple smaller loose epithelioid granulomas seen in IFAG. Additional diagnoses in the differential for IFAG include pyogenic granuloma, Spitz nevus, nodulocystic infantile acne, granulomatous rosacea, and hemangioma.1,3,9 In particular, granulomatous rosacea is challenging to differentiate from IFAG given the overlapping clinical findings. Multiple lesions, the presence of papules and pustules, and associated rosacea symptoms such as flushing suggest a diagnosis of granulomatous rosacea over IFAG.2
The prognosis for IFAG is excellent; most lesions self-resolve without treatment or procedural intervention within 1 year without scarring or relapse.3 Topical and oral antibiotic treatments such as metronidazole 0.75% gel or cream, oral erythromycin, oral clarithromycin, and doxycycline (in patients older than 8 years) have been used to treat IFAG with variable clinic responses.2,3,6,8 Persistent IFAG has been treated with surgical excision.3 Our patient was treated with a combination of gentamicin ointment 0.3% and tacrolimus ointment 0.3% and experienced approximately 50% improvement in the first month of treatment.
- Roul S, Léauté-Labrèze C, Boralevi F, et al. Idiopathic aseptic facial granuloma (pyodermite froide du visage): a pediatric entity? Arch Dermatol. 2001;137:1253-1255.
- Prey S, Ezzedine K, Mazereeuw-Hautier J, et al. IFAG and childhood rosacea: a possible link? Pediatr Dermatol. 2013;30:429-432. doi:10.1111/pde.12137
- Boralevi F, Léauté-Labrèze C, Lepreux S, et al. Idiopathic facial aseptic granuloma: a multicentre prospective study of 30 cases. Br J Dermatol. 2007;156:705-708. doi:10.1111/j.1365-2133.2006.07741.x
- Lobato-Berezo A, Montoro-Romero S, Pujol RM, et al. Dermoscopic features of idiopathic facial aseptic granuloma. Pediatr Dermatol. 2018;35:E308-E309. doi:10.1111/pde.13582
- González Rodríguez AJ, Jordá Cuevas E. Idiopathic facial aseptic granuloma. Clin Exp Dermatol. 2015;40:298-300. doi:10.1111/ced.12535
- Orion C, Sfecci A, Tisseau L, et al. Idiopathic facial aseptic granuloma in a 13-year-old boy dramatically improved with oral doxycycline and topical metronidazole: evidence for a link with childhood rosacea. Case Rep Dermatol. 2016;8:197-201. doi:10.1159/000447624
- Neri I, Raone B, Dondi A, et al. Should idiopathic facial aseptic granuloma be considered granulomatous rosacea? report of three pediatric cases. Pediatr Dermatol. 2013;30:109-111. doi:10.1111 /j.1525-1470.2011.01689.x
- Miconi F, Principi N, Cassiani L, et al. A cheek nodule in a child: be aware of idiopathic facial aseptic granuloma and its differential diagnosis. Int J Environ Res Public Health. 2019;16:2471. doi:10.3390/ijerph16142471
- Baroni A, Russo T, Faccenda F, et al. Idiopathic facial aseptic granuloma in a child: a possible expression of childhood rosacea. Pediatr Dermatol. 2013;30:394-395. doi:10.1111/j.1525-1470.2012.01805.x
THE DIAGNOSIS: Idiopathic Facial Aseptic Granuloma
Histopathology showed a ruptured follicle, perifollicular granulomatous inflammation, and admixed multinucleated giant cells in the superficial dermis. The deeper tissue exhibited edema, histiocytic/granulomatous inflammation forming ill-defined loose granulomas, and a single neutrophilic microabscess (Figure). Stains for periodic acid-Schiff with diastase and acid-fast bacillus were negative for microorganisms. The clinical examination and pathology findings supported a diagnosis of idiopathic facial aseptic granuloma (IFAG).

First reported in 1999, IFAG was described using the French term pyodermite froide du visage, which translates to “cold pyoderma of the face”; however, it was renamed to represent its granulomatous characteristics and noninfectious etiology.1 The pathogenesis of IFAG is unknown, but the leading hypothesis is that it may be a type of childhood granulomatous rosacea, given its association with relapsing chalazions, papulopustular eruptions on the face, and facial flushing.2 Other hypotheses are that IFAG is idiopathic or a granulomatous response to an insect bite, minor trauma, or embryologic remnant.3
A rare condition arising in early childhood, IFAG manifests as a single or multiple, painless, erythematous or violaceous nodule(s) on the face, most often on the cheeks or eyelids.4 A thorough history and clinical examination often suffice for diagnosis. Dermoscopy may reveal white perifollicular halos and follicular plugs on an erythematous base with linear vessels.4 If diagnostic tests are performed, there are notable characteristic findings: ultrasonography often shows a well-circumscribed, hypoechoic, ovoid dermal lesion without calcifications. Bacterial, fungal, and mycobacterial cultures commonly are negative.4 On biopsy, histopathology may reveal granulomatous inflammation in the superficial and deep dermis, multinucleated giant cells, and surrounding lymphocytic, neutrophilic, and eosinophilic infiltration with no calcium deposits.3,5,6 Histopathology findings for IFAG and rosacea lesions are similar; both may demonstrate folliculitis, perifollicular granulomas, and admixed lymphohistiocytic inflammation.7
Differentiating IFAG from other dermatologic lesions can be challenging, as the differential includes benign neoplasms (eg, dermoid cyst, chalazion, pilomatricoma, xanthoma, xanthogranuloma2) and infectious etiologies such as bacterial pyoderma and mycobacterial, fungal, and parasitic infections (eg, cutaneous leishmaniasis). Pilomatricomas, although often seen on the face or extremities in young girls, more often are well circumscribed and located in the dermis. Ultrasonography of a pilomatricoma classically shows variable foci of calcification. Xanthoma and xanthogranuloma also were considered in our case since the lesion was subtly yellowish on examination. Similar to IFAG, these conditions may manifest as single or multiple lesions. Abnormalities in the patient’s blood lipid panel or family history may be needed to diagnose xanthoma. Biopsy of a juvenile xanthogranuloma would exhibit a dense dermal nodular proliferation of histiocytic cells with a smaller number of admixed lymphocytes, neutrophils, and eosinophils, in contrast to the multiple smaller loose epithelioid granulomas seen in IFAG. Additional diagnoses in the differential for IFAG include pyogenic granuloma, Spitz nevus, nodulocystic infantile acne, granulomatous rosacea, and hemangioma.1,3,9 In particular, granulomatous rosacea is challenging to differentiate from IFAG given the overlapping clinical findings. Multiple lesions, the presence of papules and pustules, and associated rosacea symptoms such as flushing suggest a diagnosis of granulomatous rosacea over IFAG.2
The prognosis for IFAG is excellent; most lesions self-resolve without treatment or procedural intervention within 1 year without scarring or relapse.3 Topical and oral antibiotic treatments such as metronidazole 0.75% gel or cream, oral erythromycin, oral clarithromycin, and doxycycline (in patients older than 8 years) have been used to treat IFAG with variable clinic responses.2,3,6,8 Persistent IFAG has been treated with surgical excision.3 Our patient was treated with a combination of gentamicin ointment 0.3% and tacrolimus ointment 0.3% and experienced approximately 50% improvement in the first month of treatment.
THE DIAGNOSIS: Idiopathic Facial Aseptic Granuloma
Histopathology showed a ruptured follicle, perifollicular granulomatous inflammation, and admixed multinucleated giant cells in the superficial dermis. The deeper tissue exhibited edema, histiocytic/granulomatous inflammation forming ill-defined loose granulomas, and a single neutrophilic microabscess (Figure). Stains for periodic acid-Schiff with diastase and acid-fast bacillus were negative for microorganisms. The clinical examination and pathology findings supported a diagnosis of idiopathic facial aseptic granuloma (IFAG).

First reported in 1999, IFAG was described using the French term pyodermite froide du visage, which translates to “cold pyoderma of the face”; however, it was renamed to represent its granulomatous characteristics and noninfectious etiology.1 The pathogenesis of IFAG is unknown, but the leading hypothesis is that it may be a type of childhood granulomatous rosacea, given its association with relapsing chalazions, papulopustular eruptions on the face, and facial flushing.2 Other hypotheses are that IFAG is idiopathic or a granulomatous response to an insect bite, minor trauma, or embryologic remnant.3
A rare condition arising in early childhood, IFAG manifests as a single or multiple, painless, erythematous or violaceous nodule(s) on the face, most often on the cheeks or eyelids.4 A thorough history and clinical examination often suffice for diagnosis. Dermoscopy may reveal white perifollicular halos and follicular plugs on an erythematous base with linear vessels.4 If diagnostic tests are performed, there are notable characteristic findings: ultrasonography often shows a well-circumscribed, hypoechoic, ovoid dermal lesion without calcifications. Bacterial, fungal, and mycobacterial cultures commonly are negative.4 On biopsy, histopathology may reveal granulomatous inflammation in the superficial and deep dermis, multinucleated giant cells, and surrounding lymphocytic, neutrophilic, and eosinophilic infiltration with no calcium deposits.3,5,6 Histopathology findings for IFAG and rosacea lesions are similar; both may demonstrate folliculitis, perifollicular granulomas, and admixed lymphohistiocytic inflammation.7
Differentiating IFAG from other dermatologic lesions can be challenging, as the differential includes benign neoplasms (eg, dermoid cyst, chalazion, pilomatricoma, xanthoma, xanthogranuloma2) and infectious etiologies such as bacterial pyoderma and mycobacterial, fungal, and parasitic infections (eg, cutaneous leishmaniasis). Pilomatricomas, although often seen on the face or extremities in young girls, more often are well circumscribed and located in the dermis. Ultrasonography of a pilomatricoma classically shows variable foci of calcification. Xanthoma and xanthogranuloma also were considered in our case since the lesion was subtly yellowish on examination. Similar to IFAG, these conditions may manifest as single or multiple lesions. Abnormalities in the patient’s blood lipid panel or family history may be needed to diagnose xanthoma. Biopsy of a juvenile xanthogranuloma would exhibit a dense dermal nodular proliferation of histiocytic cells with a smaller number of admixed lymphocytes, neutrophils, and eosinophils, in contrast to the multiple smaller loose epithelioid granulomas seen in IFAG. Additional diagnoses in the differential for IFAG include pyogenic granuloma, Spitz nevus, nodulocystic infantile acne, granulomatous rosacea, and hemangioma.1,3,9 In particular, granulomatous rosacea is challenging to differentiate from IFAG given the overlapping clinical findings. Multiple lesions, the presence of papules and pustules, and associated rosacea symptoms such as flushing suggest a diagnosis of granulomatous rosacea over IFAG.2
The prognosis for IFAG is excellent; most lesions self-resolve without treatment or procedural intervention within 1 year without scarring or relapse.3 Topical and oral antibiotic treatments such as metronidazole 0.75% gel or cream, oral erythromycin, oral clarithromycin, and doxycycline (in patients older than 8 years) have been used to treat IFAG with variable clinic responses.2,3,6,8 Persistent IFAG has been treated with surgical excision.3 Our patient was treated with a combination of gentamicin ointment 0.3% and tacrolimus ointment 0.3% and experienced approximately 50% improvement in the first month of treatment.
- Roul S, Léauté-Labrèze C, Boralevi F, et al. Idiopathic aseptic facial granuloma (pyodermite froide du visage): a pediatric entity? Arch Dermatol. 2001;137:1253-1255.
- Prey S, Ezzedine K, Mazereeuw-Hautier J, et al. IFAG and childhood rosacea: a possible link? Pediatr Dermatol. 2013;30:429-432. doi:10.1111/pde.12137
- Boralevi F, Léauté-Labrèze C, Lepreux S, et al. Idiopathic facial aseptic granuloma: a multicentre prospective study of 30 cases. Br J Dermatol. 2007;156:705-708. doi:10.1111/j.1365-2133.2006.07741.x
- Lobato-Berezo A, Montoro-Romero S, Pujol RM, et al. Dermoscopic features of idiopathic facial aseptic granuloma. Pediatr Dermatol. 2018;35:E308-E309. doi:10.1111/pde.13582
- González Rodríguez AJ, Jordá Cuevas E. Idiopathic facial aseptic granuloma. Clin Exp Dermatol. 2015;40:298-300. doi:10.1111/ced.12535
- Orion C, Sfecci A, Tisseau L, et al. Idiopathic facial aseptic granuloma in a 13-year-old boy dramatically improved with oral doxycycline and topical metronidazole: evidence for a link with childhood rosacea. Case Rep Dermatol. 2016;8:197-201. doi:10.1159/000447624
- Neri I, Raone B, Dondi A, et al. Should idiopathic facial aseptic granuloma be considered granulomatous rosacea? report of three pediatric cases. Pediatr Dermatol. 2013;30:109-111. doi:10.1111 /j.1525-1470.2011.01689.x
- Miconi F, Principi N, Cassiani L, et al. A cheek nodule in a child: be aware of idiopathic facial aseptic granuloma and its differential diagnosis. Int J Environ Res Public Health. 2019;16:2471. doi:10.3390/ijerph16142471
- Baroni A, Russo T, Faccenda F, et al. Idiopathic facial aseptic granuloma in a child: a possible expression of childhood rosacea. Pediatr Dermatol. 2013;30:394-395. doi:10.1111/j.1525-1470.2012.01805.x
- Roul S, Léauté-Labrèze C, Boralevi F, et al. Idiopathic aseptic facial granuloma (pyodermite froide du visage): a pediatric entity? Arch Dermatol. 2001;137:1253-1255.
- Prey S, Ezzedine K, Mazereeuw-Hautier J, et al. IFAG and childhood rosacea: a possible link? Pediatr Dermatol. 2013;30:429-432. doi:10.1111/pde.12137
- Boralevi F, Léauté-Labrèze C, Lepreux S, et al. Idiopathic facial aseptic granuloma: a multicentre prospective study of 30 cases. Br J Dermatol. 2007;156:705-708. doi:10.1111/j.1365-2133.2006.07741.x
- Lobato-Berezo A, Montoro-Romero S, Pujol RM, et al. Dermoscopic features of idiopathic facial aseptic granuloma. Pediatr Dermatol. 2018;35:E308-E309. doi:10.1111/pde.13582
- González Rodríguez AJ, Jordá Cuevas E. Idiopathic facial aseptic granuloma. Clin Exp Dermatol. 2015;40:298-300. doi:10.1111/ced.12535
- Orion C, Sfecci A, Tisseau L, et al. Idiopathic facial aseptic granuloma in a 13-year-old boy dramatically improved with oral doxycycline and topical metronidazole: evidence for a link with childhood rosacea. Case Rep Dermatol. 2016;8:197-201. doi:10.1159/000447624
- Neri I, Raone B, Dondi A, et al. Should idiopathic facial aseptic granuloma be considered granulomatous rosacea? report of three pediatric cases. Pediatr Dermatol. 2013;30:109-111. doi:10.1111 /j.1525-1470.2011.01689.x
- Miconi F, Principi N, Cassiani L, et al. A cheek nodule in a child: be aware of idiopathic facial aseptic granuloma and its differential diagnosis. Int J Environ Res Public Health. 2019;16:2471. doi:10.3390/ijerph16142471
- Baroni A, Russo T, Faccenda F, et al. Idiopathic facial aseptic granuloma in a child: a possible expression of childhood rosacea. Pediatr Dermatol. 2013;30:394-395. doi:10.1111/j.1525-1470.2012.01805.x
Painless Nodule on the Lower Eyelid
Painless Nodule on the Lower Eyelid
A 4-year-old girl presented to the dermatology clinic with a painless, red to golden-yellowish nodule on the right lower eyelid of 4 months’ duration. The patient had no history of skin disease and was otherwise healthy. Physical examination revealed a single 1-cm, soft, erythematous and yellowish plaque on the right lower eyelid that was subtly fluctuant on palpation. She had no associated systemic symptoms or lymphadenopathy. A punch biopsy of the lesion was performed.
Evaluation of Subcutaneous Contraception for Patient Self-Administration at North Florida/South Georgia Veterans Health System
Evaluation of Subcutaneous Contraception for Patient Self-Administration at North Florida/South Georgia Veterans Health System
Medroxyprogesterone acetate is an injectable medication indicated for contraception and management of endometriosis-associated pain in females of reproductive age.1 Medroxyprogesterone inhibits gonadotropin secretion, which prevents follicular maturation and ovulation. This leads to endometrial thinning and a contraceptive effect. Adverse drug reactions (ADRs), such as weight gain, menstrual bleeding irregularities, and bone loss appear to be dose- and time-related. Two formulations of medroxyprogesterone acetate are available: 150 mg depot medroxyprogesterone acetate intramuscular (DMPA-IM) and 104 mg DMPA subcutaneous (DMPA-SC).2 Originally, medroxyprogesterone acetate injections required administration by a health care worker. While the current labeling for DMPA-SC still indicates a requirement for administration by a health care worker, data show that the medication can be safe and effective when self-administered.3
Self-Administered Contraception
The 2019 World Health Organization (WHO) guideline on self-care interventions recommends making self-administered injectable contraception available to individuals of reproductive age.3 The WHO recommendation is based on evidence from the Depo Self-Administration Study, which included 401 patients randomized 1:1 to receive self-administered or clinic-administered DMPA-SC. This study concluded that self-administration improved continuation of contraception.4
The North Florida/South Georgia Veterans Health System (NFSGVHS) is the largest US Department of Veterans Affairs (VA) health care system, serving > 22,000 female veterans. All primary care practitioners (PCP) have been trained in women’s health (WH).
The WH patient-aligned care team (PACT) clinical pharmacy practitioner (CPP) proposed using DMPA-SC for outpatient self-administration to increase access, improve patient satisfaction, and reduce burden on patients and nurses for administration appointments. The Pharmacy and Therapeutics Committee (P&T), WH Medical Director, and Chief of Gynecology approved the proposal. DMPA-SC was added to the ordering menu with order sets. The order set included instructions that outlined the 12-week dosing interval, instructions to contact the prescriber if the injection was > 2 weeks overdue (aligning with dosing recommendations for administration every 12 to 14 weeks), and an optional order for a home pregnancy test if necessary. These instructions were designed to ensure proper self-administration of the medication and timely follow-up care.
The gynecology and PACT health care practitioners (HCPs), including physicians, pharmacists, nurses, and medical assistants, received DMPA-SC education, which consisted of a review of medication, ADRs, contraindications, and administration. An NFSGVHS procedure was developed to ensure patients received self-administration education. DMPA-SC prescriptions were mailed to patients with scheduled nursing appointments. The patient would then bring DMPA-SC to the nursing appointment where they received administration instruction and completed the first injection under nurse supervision to ensure appropriate technique. Patients were offered supplementary educational documents and a calendar to keep track of injection days. The patients were responsible for ordering refills and administering subsequent injections at home. Once all stakeholders received education and order sets were in place, prescribers and nurses could begin offering the option for initiation of self-administered DMPA-SC to patients. All conversions or new prescriptions were initiated by prescribers as a part of usual care.
Medication Use Evaluation
A medication use evaluation was conducted about 1 year after the rollout to assess use, adherence, and impact of DMPA-SC for patient-self administration as a new contraceptive option for NFSGVHS patients.
A retrospective chart review was conducted for patients dispensed DMPA-SC from June 1, 2022, to July 1, 2023. Baseline body mass index (BMI), recorded prior to initiation of DMPA-SC, was compared with the most recent BMI on record at the completion of the study to evaluate weight change. Nursing visit attendance for the first injection was also assessed. Adherence was evaluated by reviewing the date of the initial DMPA-SC prescription, the date of the patient's first nursing visit, and subsequent refill patterns. A 2-week margin of error was established to account for the flexibility within the recommended dosing interval and delays in postal service delivery.
Forty patients were initiated on DMPA-SC for patient self-administration. The mean age of patients was 37.2 years. All 40 patients were female. Twenty-two patients (55%) identified as Black, 17 (43%) as White, and 1 (3%) as Asian. The majority (90%) of patients were non-Hispanic. The mean baseline BMI was 30 and BMI after DMPA-SC initiation was 30.4.
Twenty-eight (70%) patients had a nursing appointment, adhering to the NFSGVHS protocol. Five patients (13%) discontinued use and switched to DMPA-IM administered by an HCP and 4 (10%) discontinued use following an ADR (hives, mood changes, bruising, and menometrorrhagia). Of the 31 patients who continued therapy, 25 (81%) were refilling appropriately (Table).

Six patients with unidentified reasons for nonadherence were contacted to determine if there were unmet contraceptive needs. This subgroup included patients with an active prescription for DMPA-SC that did not meet refill expectations. Nonadherence was mostly due to forgetfulness, however 1 patient was unable to refill her DMPA-SC in a timely manner due to an outside hospital admission and another was unreachable. These conversations were documented in the electronic health record (EHR) and all patients requesting follow-up, reinitiation of therapy, or alternative regimens, the appropriate parties were notified to coordinate care.
Discussion
The uptake in DMPA-SC prescribing suggests prescribers and patients have embraced self-administration as an option for contraception. Most patients were appropriately scheduled for nursing appointments to reinforce education and ensure appropriate self-injection technique, as outlined in the NFSGVHS procedure.
The need to improve adherence to NFSGVHS procedure was identified because not all patients had scheduled nursing appointments. This is concerning because some patients may have started self-injecting DMPA-SC without proper education, which could lead to improper injection technique and diminished effectiveness. Nursing appointments ensure appropriate self-injection techniques and reinforce the importance of refilling every 12 weeks for proper effectiveness. Nonadherence to contraceptive therapy may result in unintended pregnancy, although no pregnancies were reported by patients in this study. Pharmacist involvement in DMPA-SC initiation and follow-up monitoring may help ensure adherence to local procedure for initiation and improve patient adherence.
There is limited evidence comparing weight gain related to DMPA-SC vs DMPA-IM. However, in a small, 2-year, randomized study, weight changes were considered comparable for both cohorts with a mean increase of 3.5 kg in the DMPA-IM group vs 3.4 kg in the DMPA-SC group.5 While our analysis did not formally evaluate weight changes, BMI data were collected to evaluate for evidence of weight change. The duration of therapy varied per patient and may not have been long enough to see comparable weight changes.
Strengths of this project include the use of the PACT multidisciplinary approach in primary care including physicians, pharmacists, and nurses. The NFSGVHS EHR is comprehensive, and data including appointments and pharmacy refill information was readily available for collection and evaluation. Limitations included inconsistent documentation in the patient’s EHR which made collection of some data difficult.
Cost Estimates
NFSGVHS had 231 patients prescribed DMPA-IM at the time of DMPA-SC rollout and 40 patients initiated DMPA-SC therapy in the first year. There are possible cost savings associated with the use of DMPA-SC compared to DMPA-IM. Although DMPA-IM costs about $120 annually and DMPA-SC costs about $252 annually, this does not account for indirect costs such as supplies, overhead cost, nursing visits, and patient travel.6 Additionally, allowing patients to self-administer the DMPA-SC injection at home provides nurses time to care for other patients.
Moving forward, the PACT and gynecology teams will receive instruction on the importance of adhering to NFSGVHS procedures to ensure new patients prescribed DMPA-SC receive education and present for nursing appointments to ensure appropriate self-injection.
DMPA has historically been administered in the clinic setting by an HCP; therefore, the prescriber was available to assess adherence to therapy based on patient’s attendance to scheduled clinic appointments. Some prescribers may feel apprehensive about shifting the onus of medication adherence to the patient when prescribing DMPA-SC. However, this model is comparable to any other prescription form of birth control, such as combined hormonal contraceptive pills, where the prescriber expects the patient to take the medication as prescribed and refill their prescriptions in a timely manner to avoid gaps in therapy. The findings of this project suggest the majority of patients who were prescribed self-administered DMPA-SC for contraception were adherent to therapy. The utility of self-administration of DMPA-SC for other labeled or off-label indications was not evaluated; however, it is possible that patients who are motivated to self-administer the medication (regardless of indication) would also demonstrate similar adherence rates.
Conclusions
The majority of patients who started DMPA-SC tolerated the medication well and continued to refill therapy within the recommended time period. Patient self-administration of DMPA-SC can enhance access by removing barriers to administration, increase patient autonomy and contraceptive continuation rates. Overall, the increase in DMPA-SC prescriptions suggests that patients and HCPs support the option for DMPA-SC self-administration at NFSGVHS.
- Depo-SubQ Provera. Package insert. Pharmacia & Upjohn Co; 2019.
- Kaunitz AM. Depot medroxyprogesterone acetate. UpToDate. Updated June 12, 2025. Accessed July 11, 2025. https://www.uptodate.com/contents/depot-medroxyprogesterone-acetate-dmpa-formulations-patient-selection-and-drug-administration
- World Health Organization. WHO guideline on self-care interventions for health and well-being, 2022 revision. World Health Organization. 2022. Accessed July 17, 2025. https://iris.who.int/bitstream/handle/10665/357828/9789240052192-eng.pdf
- Kohn JE, Simons HR, Della Badia L, et al. Increased 1-year continuation of DMPA among women randomized to self-administration: results from a randomized controlled trial at Planned Parenthood. Contraception. 2018;97(3):198-204. doi:10.1016/j.contraception.2017.11.009
- Kaunitz AM, Darney PD, Ross D, Wolter KD, Speroff L. Subcutaneous DMPA vs. intramuscular DMPA: a 2-year randomized study of contraceptive efficacy and bone mineral density. Contraception. 2009;80(1):7-17. doi:10.1016/j.contraception.2009.02.005
- UpToDate, Lexidrug. Medroxyprogesterone acetate. Accessed July 16, 2025. https://online.lexi.com
Medroxyprogesterone acetate is an injectable medication indicated for contraception and management of endometriosis-associated pain in females of reproductive age.1 Medroxyprogesterone inhibits gonadotropin secretion, which prevents follicular maturation and ovulation. This leads to endometrial thinning and a contraceptive effect. Adverse drug reactions (ADRs), such as weight gain, menstrual bleeding irregularities, and bone loss appear to be dose- and time-related. Two formulations of medroxyprogesterone acetate are available: 150 mg depot medroxyprogesterone acetate intramuscular (DMPA-IM) and 104 mg DMPA subcutaneous (DMPA-SC).2 Originally, medroxyprogesterone acetate injections required administration by a health care worker. While the current labeling for DMPA-SC still indicates a requirement for administration by a health care worker, data show that the medication can be safe and effective when self-administered.3
Self-Administered Contraception
The 2019 World Health Organization (WHO) guideline on self-care interventions recommends making self-administered injectable contraception available to individuals of reproductive age.3 The WHO recommendation is based on evidence from the Depo Self-Administration Study, which included 401 patients randomized 1:1 to receive self-administered or clinic-administered DMPA-SC. This study concluded that self-administration improved continuation of contraception.4
The North Florida/South Georgia Veterans Health System (NFSGVHS) is the largest US Department of Veterans Affairs (VA) health care system, serving > 22,000 female veterans. All primary care practitioners (PCP) have been trained in women’s health (WH).
The WH patient-aligned care team (PACT) clinical pharmacy practitioner (CPP) proposed using DMPA-SC for outpatient self-administration to increase access, improve patient satisfaction, and reduce burden on patients and nurses for administration appointments. The Pharmacy and Therapeutics Committee (P&T), WH Medical Director, and Chief of Gynecology approved the proposal. DMPA-SC was added to the ordering menu with order sets. The order set included instructions that outlined the 12-week dosing interval, instructions to contact the prescriber if the injection was > 2 weeks overdue (aligning with dosing recommendations for administration every 12 to 14 weeks), and an optional order for a home pregnancy test if necessary. These instructions were designed to ensure proper self-administration of the medication and timely follow-up care.
The gynecology and PACT health care practitioners (HCPs), including physicians, pharmacists, nurses, and medical assistants, received DMPA-SC education, which consisted of a review of medication, ADRs, contraindications, and administration. An NFSGVHS procedure was developed to ensure patients received self-administration education. DMPA-SC prescriptions were mailed to patients with scheduled nursing appointments. The patient would then bring DMPA-SC to the nursing appointment where they received administration instruction and completed the first injection under nurse supervision to ensure appropriate technique. Patients were offered supplementary educational documents and a calendar to keep track of injection days. The patients were responsible for ordering refills and administering subsequent injections at home. Once all stakeholders received education and order sets were in place, prescribers and nurses could begin offering the option for initiation of self-administered DMPA-SC to patients. All conversions or new prescriptions were initiated by prescribers as a part of usual care.
Medication Use Evaluation
A medication use evaluation was conducted about 1 year after the rollout to assess use, adherence, and impact of DMPA-SC for patient-self administration as a new contraceptive option for NFSGVHS patients.
A retrospective chart review was conducted for patients dispensed DMPA-SC from June 1, 2022, to July 1, 2023. Baseline body mass index (BMI), recorded prior to initiation of DMPA-SC, was compared with the most recent BMI on record at the completion of the study to evaluate weight change. Nursing visit attendance for the first injection was also assessed. Adherence was evaluated by reviewing the date of the initial DMPA-SC prescription, the date of the patient's first nursing visit, and subsequent refill patterns. A 2-week margin of error was established to account for the flexibility within the recommended dosing interval and delays in postal service delivery.
Forty patients were initiated on DMPA-SC for patient self-administration. The mean age of patients was 37.2 years. All 40 patients were female. Twenty-two patients (55%) identified as Black, 17 (43%) as White, and 1 (3%) as Asian. The majority (90%) of patients were non-Hispanic. The mean baseline BMI was 30 and BMI after DMPA-SC initiation was 30.4.
Twenty-eight (70%) patients had a nursing appointment, adhering to the NFSGVHS protocol. Five patients (13%) discontinued use and switched to DMPA-IM administered by an HCP and 4 (10%) discontinued use following an ADR (hives, mood changes, bruising, and menometrorrhagia). Of the 31 patients who continued therapy, 25 (81%) were refilling appropriately (Table).

Six patients with unidentified reasons for nonadherence were contacted to determine if there were unmet contraceptive needs. This subgroup included patients with an active prescription for DMPA-SC that did not meet refill expectations. Nonadherence was mostly due to forgetfulness, however 1 patient was unable to refill her DMPA-SC in a timely manner due to an outside hospital admission and another was unreachable. These conversations were documented in the electronic health record (EHR) and all patients requesting follow-up, reinitiation of therapy, or alternative regimens, the appropriate parties were notified to coordinate care.
Discussion
The uptake in DMPA-SC prescribing suggests prescribers and patients have embraced self-administration as an option for contraception. Most patients were appropriately scheduled for nursing appointments to reinforce education and ensure appropriate self-injection technique, as outlined in the NFSGVHS procedure.
The need to improve adherence to NFSGVHS procedure was identified because not all patients had scheduled nursing appointments. This is concerning because some patients may have started self-injecting DMPA-SC without proper education, which could lead to improper injection technique and diminished effectiveness. Nursing appointments ensure appropriate self-injection techniques and reinforce the importance of refilling every 12 weeks for proper effectiveness. Nonadherence to contraceptive therapy may result in unintended pregnancy, although no pregnancies were reported by patients in this study. Pharmacist involvement in DMPA-SC initiation and follow-up monitoring may help ensure adherence to local procedure for initiation and improve patient adherence.
There is limited evidence comparing weight gain related to DMPA-SC vs DMPA-IM. However, in a small, 2-year, randomized study, weight changes were considered comparable for both cohorts with a mean increase of 3.5 kg in the DMPA-IM group vs 3.4 kg in the DMPA-SC group.5 While our analysis did not formally evaluate weight changes, BMI data were collected to evaluate for evidence of weight change. The duration of therapy varied per patient and may not have been long enough to see comparable weight changes.
Strengths of this project include the use of the PACT multidisciplinary approach in primary care including physicians, pharmacists, and nurses. The NFSGVHS EHR is comprehensive, and data including appointments and pharmacy refill information was readily available for collection and evaluation. Limitations included inconsistent documentation in the patient’s EHR which made collection of some data difficult.
Cost Estimates
NFSGVHS had 231 patients prescribed DMPA-IM at the time of DMPA-SC rollout and 40 patients initiated DMPA-SC therapy in the first year. There are possible cost savings associated with the use of DMPA-SC compared to DMPA-IM. Although DMPA-IM costs about $120 annually and DMPA-SC costs about $252 annually, this does not account for indirect costs such as supplies, overhead cost, nursing visits, and patient travel.6 Additionally, allowing patients to self-administer the DMPA-SC injection at home provides nurses time to care for other patients.
Moving forward, the PACT and gynecology teams will receive instruction on the importance of adhering to NFSGVHS procedures to ensure new patients prescribed DMPA-SC receive education and present for nursing appointments to ensure appropriate self-injection.
DMPA has historically been administered in the clinic setting by an HCP; therefore, the prescriber was available to assess adherence to therapy based on patient’s attendance to scheduled clinic appointments. Some prescribers may feel apprehensive about shifting the onus of medication adherence to the patient when prescribing DMPA-SC. However, this model is comparable to any other prescription form of birth control, such as combined hormonal contraceptive pills, where the prescriber expects the patient to take the medication as prescribed and refill their prescriptions in a timely manner to avoid gaps in therapy. The findings of this project suggest the majority of patients who were prescribed self-administered DMPA-SC for contraception were adherent to therapy. The utility of self-administration of DMPA-SC for other labeled or off-label indications was not evaluated; however, it is possible that patients who are motivated to self-administer the medication (regardless of indication) would also demonstrate similar adherence rates.
Conclusions
The majority of patients who started DMPA-SC tolerated the medication well and continued to refill therapy within the recommended time period. Patient self-administration of DMPA-SC can enhance access by removing barriers to administration, increase patient autonomy and contraceptive continuation rates. Overall, the increase in DMPA-SC prescriptions suggests that patients and HCPs support the option for DMPA-SC self-administration at NFSGVHS.
Medroxyprogesterone acetate is an injectable medication indicated for contraception and management of endometriosis-associated pain in females of reproductive age.1 Medroxyprogesterone inhibits gonadotropin secretion, which prevents follicular maturation and ovulation. This leads to endometrial thinning and a contraceptive effect. Adverse drug reactions (ADRs), such as weight gain, menstrual bleeding irregularities, and bone loss appear to be dose- and time-related. Two formulations of medroxyprogesterone acetate are available: 150 mg depot medroxyprogesterone acetate intramuscular (DMPA-IM) and 104 mg DMPA subcutaneous (DMPA-SC).2 Originally, medroxyprogesterone acetate injections required administration by a health care worker. While the current labeling for DMPA-SC still indicates a requirement for administration by a health care worker, data show that the medication can be safe and effective when self-administered.3
Self-Administered Contraception
The 2019 World Health Organization (WHO) guideline on self-care interventions recommends making self-administered injectable contraception available to individuals of reproductive age.3 The WHO recommendation is based on evidence from the Depo Self-Administration Study, which included 401 patients randomized 1:1 to receive self-administered or clinic-administered DMPA-SC. This study concluded that self-administration improved continuation of contraception.4
The North Florida/South Georgia Veterans Health System (NFSGVHS) is the largest US Department of Veterans Affairs (VA) health care system, serving > 22,000 female veterans. All primary care practitioners (PCP) have been trained in women’s health (WH).
The WH patient-aligned care team (PACT) clinical pharmacy practitioner (CPP) proposed using DMPA-SC for outpatient self-administration to increase access, improve patient satisfaction, and reduce burden on patients and nurses for administration appointments. The Pharmacy and Therapeutics Committee (P&T), WH Medical Director, and Chief of Gynecology approved the proposal. DMPA-SC was added to the ordering menu with order sets. The order set included instructions that outlined the 12-week dosing interval, instructions to contact the prescriber if the injection was > 2 weeks overdue (aligning with dosing recommendations for administration every 12 to 14 weeks), and an optional order for a home pregnancy test if necessary. These instructions were designed to ensure proper self-administration of the medication and timely follow-up care.
The gynecology and PACT health care practitioners (HCPs), including physicians, pharmacists, nurses, and medical assistants, received DMPA-SC education, which consisted of a review of medication, ADRs, contraindications, and administration. An NFSGVHS procedure was developed to ensure patients received self-administration education. DMPA-SC prescriptions were mailed to patients with scheduled nursing appointments. The patient would then bring DMPA-SC to the nursing appointment where they received administration instruction and completed the first injection under nurse supervision to ensure appropriate technique. Patients were offered supplementary educational documents and a calendar to keep track of injection days. The patients were responsible for ordering refills and administering subsequent injections at home. Once all stakeholders received education and order sets were in place, prescribers and nurses could begin offering the option for initiation of self-administered DMPA-SC to patients. All conversions or new prescriptions were initiated by prescribers as a part of usual care.
Medication Use Evaluation
A medication use evaluation was conducted about 1 year after the rollout to assess use, adherence, and impact of DMPA-SC for patient-self administration as a new contraceptive option for NFSGVHS patients.
A retrospective chart review was conducted for patients dispensed DMPA-SC from June 1, 2022, to July 1, 2023. Baseline body mass index (BMI), recorded prior to initiation of DMPA-SC, was compared with the most recent BMI on record at the completion of the study to evaluate weight change. Nursing visit attendance for the first injection was also assessed. Adherence was evaluated by reviewing the date of the initial DMPA-SC prescription, the date of the patient's first nursing visit, and subsequent refill patterns. A 2-week margin of error was established to account for the flexibility within the recommended dosing interval and delays in postal service delivery.
Forty patients were initiated on DMPA-SC for patient self-administration. The mean age of patients was 37.2 years. All 40 patients were female. Twenty-two patients (55%) identified as Black, 17 (43%) as White, and 1 (3%) as Asian. The majority (90%) of patients were non-Hispanic. The mean baseline BMI was 30 and BMI after DMPA-SC initiation was 30.4.
Twenty-eight (70%) patients had a nursing appointment, adhering to the NFSGVHS protocol. Five patients (13%) discontinued use and switched to DMPA-IM administered by an HCP and 4 (10%) discontinued use following an ADR (hives, mood changes, bruising, and menometrorrhagia). Of the 31 patients who continued therapy, 25 (81%) were refilling appropriately (Table).

Six patients with unidentified reasons for nonadherence were contacted to determine if there were unmet contraceptive needs. This subgroup included patients with an active prescription for DMPA-SC that did not meet refill expectations. Nonadherence was mostly due to forgetfulness, however 1 patient was unable to refill her DMPA-SC in a timely manner due to an outside hospital admission and another was unreachable. These conversations were documented in the electronic health record (EHR) and all patients requesting follow-up, reinitiation of therapy, or alternative regimens, the appropriate parties were notified to coordinate care.
Discussion
The uptake in DMPA-SC prescribing suggests prescribers and patients have embraced self-administration as an option for contraception. Most patients were appropriately scheduled for nursing appointments to reinforce education and ensure appropriate self-injection technique, as outlined in the NFSGVHS procedure.
The need to improve adherence to NFSGVHS procedure was identified because not all patients had scheduled nursing appointments. This is concerning because some patients may have started self-injecting DMPA-SC without proper education, which could lead to improper injection technique and diminished effectiveness. Nursing appointments ensure appropriate self-injection techniques and reinforce the importance of refilling every 12 weeks for proper effectiveness. Nonadherence to contraceptive therapy may result in unintended pregnancy, although no pregnancies were reported by patients in this study. Pharmacist involvement in DMPA-SC initiation and follow-up monitoring may help ensure adherence to local procedure for initiation and improve patient adherence.
There is limited evidence comparing weight gain related to DMPA-SC vs DMPA-IM. However, in a small, 2-year, randomized study, weight changes were considered comparable for both cohorts with a mean increase of 3.5 kg in the DMPA-IM group vs 3.4 kg in the DMPA-SC group.5 While our analysis did not formally evaluate weight changes, BMI data were collected to evaluate for evidence of weight change. The duration of therapy varied per patient and may not have been long enough to see comparable weight changes.
Strengths of this project include the use of the PACT multidisciplinary approach in primary care including physicians, pharmacists, and nurses. The NFSGVHS EHR is comprehensive, and data including appointments and pharmacy refill information was readily available for collection and evaluation. Limitations included inconsistent documentation in the patient’s EHR which made collection of some data difficult.
Cost Estimates
NFSGVHS had 231 patients prescribed DMPA-IM at the time of DMPA-SC rollout and 40 patients initiated DMPA-SC therapy in the first year. There are possible cost savings associated with the use of DMPA-SC compared to DMPA-IM. Although DMPA-IM costs about $120 annually and DMPA-SC costs about $252 annually, this does not account for indirect costs such as supplies, overhead cost, nursing visits, and patient travel.6 Additionally, allowing patients to self-administer the DMPA-SC injection at home provides nurses time to care for other patients.
Moving forward, the PACT and gynecology teams will receive instruction on the importance of adhering to NFSGVHS procedures to ensure new patients prescribed DMPA-SC receive education and present for nursing appointments to ensure appropriate self-injection.
DMPA has historically been administered in the clinic setting by an HCP; therefore, the prescriber was available to assess adherence to therapy based on patient’s attendance to scheduled clinic appointments. Some prescribers may feel apprehensive about shifting the onus of medication adherence to the patient when prescribing DMPA-SC. However, this model is comparable to any other prescription form of birth control, such as combined hormonal contraceptive pills, where the prescriber expects the patient to take the medication as prescribed and refill their prescriptions in a timely manner to avoid gaps in therapy. The findings of this project suggest the majority of patients who were prescribed self-administered DMPA-SC for contraception were adherent to therapy. The utility of self-administration of DMPA-SC for other labeled or off-label indications was not evaluated; however, it is possible that patients who are motivated to self-administer the medication (regardless of indication) would also demonstrate similar adherence rates.
Conclusions
The majority of patients who started DMPA-SC tolerated the medication well and continued to refill therapy within the recommended time period. Patient self-administration of DMPA-SC can enhance access by removing barriers to administration, increase patient autonomy and contraceptive continuation rates. Overall, the increase in DMPA-SC prescriptions suggests that patients and HCPs support the option for DMPA-SC self-administration at NFSGVHS.
- Depo-SubQ Provera. Package insert. Pharmacia & Upjohn Co; 2019.
- Kaunitz AM. Depot medroxyprogesterone acetate. UpToDate. Updated June 12, 2025. Accessed July 11, 2025. https://www.uptodate.com/contents/depot-medroxyprogesterone-acetate-dmpa-formulations-patient-selection-and-drug-administration
- World Health Organization. WHO guideline on self-care interventions for health and well-being, 2022 revision. World Health Organization. 2022. Accessed July 17, 2025. https://iris.who.int/bitstream/handle/10665/357828/9789240052192-eng.pdf
- Kohn JE, Simons HR, Della Badia L, et al. Increased 1-year continuation of DMPA among women randomized to self-administration: results from a randomized controlled trial at Planned Parenthood. Contraception. 2018;97(3):198-204. doi:10.1016/j.contraception.2017.11.009
- Kaunitz AM, Darney PD, Ross D, Wolter KD, Speroff L. Subcutaneous DMPA vs. intramuscular DMPA: a 2-year randomized study of contraceptive efficacy and bone mineral density. Contraception. 2009;80(1):7-17. doi:10.1016/j.contraception.2009.02.005
- UpToDate, Lexidrug. Medroxyprogesterone acetate. Accessed July 16, 2025. https://online.lexi.com
- Depo-SubQ Provera. Package insert. Pharmacia & Upjohn Co; 2019.
- Kaunitz AM. Depot medroxyprogesterone acetate. UpToDate. Updated June 12, 2025. Accessed July 11, 2025. https://www.uptodate.com/contents/depot-medroxyprogesterone-acetate-dmpa-formulations-patient-selection-and-drug-administration
- World Health Organization. WHO guideline on self-care interventions for health and well-being, 2022 revision. World Health Organization. 2022. Accessed July 17, 2025. https://iris.who.int/bitstream/handle/10665/357828/9789240052192-eng.pdf
- Kohn JE, Simons HR, Della Badia L, et al. Increased 1-year continuation of DMPA among women randomized to self-administration: results from a randomized controlled trial at Planned Parenthood. Contraception. 2018;97(3):198-204. doi:10.1016/j.contraception.2017.11.009
- Kaunitz AM, Darney PD, Ross D, Wolter KD, Speroff L. Subcutaneous DMPA vs. intramuscular DMPA: a 2-year randomized study of contraceptive efficacy and bone mineral density. Contraception. 2009;80(1):7-17. doi:10.1016/j.contraception.2009.02.005
- UpToDate, Lexidrug. Medroxyprogesterone acetate. Accessed July 16, 2025. https://online.lexi.com
Evaluation of Subcutaneous Contraception for Patient Self-Administration at North Florida/South Georgia Veterans Health System
Evaluation of Subcutaneous Contraception for Patient Self-Administration at North Florida/South Georgia Veterans Health System
Proactive Penicillin Allergy Delabeling: Lessons Learned From a Quality Improvement Project
Proactive Penicillin Allergy Delabeling: Lessons Learned From a Quality Improvement Project
Penicillin allergy is common in the United States. About 9.0% to 13.8% of patients have a diagnosed penicillin allergy documented in their electronic health record. The annual incidence rates is 1.1% in males and 1.4% in females.1,2
Penicillin hypersensitivity likely wanes over time. A 1981 study found that 93% of patients who experienced an allergic reaction to penicillin had a positive skin test 7 to 12 months postreaction, but only 22% still had a positive test after 10 years.3 Confirmed type 1 hypersensitivity penicillin allergies, as demonstrated by positive skin prick testing, also are decreasing over time.4 Furthermore, many patients’ reactions may have been misdiagnosed as a penicillin allergy. Upon actual confirmatory testing of penicillin allergy, only 8.5% to 13.8% of patients believed to have a penicillin allergy were positive on skin prick testing of penicillin products.5,6 A 2024 US study found that 11% of individuals with a history of a penicillin reaction tested positive on skin testing.7
The positive predictive value of penicillin allergy skin testing is poorly defined due to the ethical dilemma of orally challenging a patient who demonstrates skin test reactivity. Due to its high negative predictive value (NPV), skin prick combined with intradermal testing has been the gold-standard test in cases of clinical concern.6 Patients with positive skin testing are assumed to be truly positive, and therefore penicillin allergic, even though false-positive results to penicillin skin testing are known to occur.8
Misdiagnosis of penicillin allergy carries substantial clinical and economic consequences. A 2011 study suggested a statistically significant 1.8% increased absolute risk of mortality and 5.5% increased absolute risk of intensive care unit admission for those labeled with penicillin allergy and admitted for an infection.9 Another study found a 14% increase in mortality associated with the diagnosis of penicillin allergy.10 In a 2014 case-control study, penicillin allergy also was associated with a 23.4% greater risk of Clostridioides difficile, 14.1% more methicillin-resistant Staphylococcus aureus, and 30.1% more vancomycin-resistant enterococci infections.11Direct cost savings during an inpatient admission for infection were as much as $609 per patient with additional indirect cost savings of up to $4254 per admission.12 When viewed from the perspective of a health care system, these costs quickly accumulate, negatively impacting the fiscal stability of our patients and placing additional financial strain on an over-burdened system.
If 10% of US patients have penicillin allergy labels, then about 33 million patients might be eligible for delabeling. There are only 6309 board-certified allergists actively practicing in the US, which could amount to about 5231 potential penicillin challenges per allergist, not even including the 3.3 million new patients per year (assuming a 1% incidence).13 Clarifying each patient’s tolerance of penicillin products will clearly require nonallergist cooperation.
The 2022 drug allergy practice parameter update recommends several consensus-based statements (CBSs) to directly address penicillin allergy.14 This guideline recommends proactive efforts to delabel patients with a reported penicillin allergy (CBS 4); advise against testing in cases where the history is inconsistent with a true allergic reaction, though a challenge may be offered (CBS 5); skin testing for those with a history of anaphylaxis or a recent reaction (CBS 6); advise against multiple-day penicillin challenges (CBS 7); advise against skin testing for pediatric patients with benign cutaneous reactions (CBS 8); and recommends direct oral challenge for adults with distant or benign cutaneous reactions (CBS 9). These recommendations create a potentially high demand for delabeling with allergy specialists. One potential solution is to perform direct oral challenges in primary care, emergency departments, and urgent care clinics.
Evidence supporting the safety of direct oral penicillin challenges in low-risk patients was initially noted in the allergy community, but now evidence for their use in primary care clinics is growing—including in children.15 In a military-specific population, an amoxicillin challenge of Marine recruits with suspected penicillin allergy revealed that only 1.5% of those challenged acutely reacted and should be considered allergic to penicillin.16 Historically, in order to refute the diagnosis of penicillin allergy, an allergist would order penicillin skin prick testing. If the test was negative, an allergist would proceed to intradermal testing and if negative again (NPV of 97.9%), proceed to a graded oral challenge.6 However, this process is not fully reproducible in most clinics because the minor determinants mixture used in skin testing is not commercially available.17 Additionally, the full skin testing procedure requires specialized training, is more time-consuming, causes more discomfort, lacks US Food and Drug Administration approval for children, and has a higher cost ($220 per test for each patient as of 2016).18 As such, the movement toward direct oral challenges is progressing. Nonetheless, the best method for primary care or emergency department clinicians to determine who the appropriate patients are for this procedure has not been fully established. Risk tools have been created in the past to help delineate low-risk patients who would be appropriate for direct oral amoxicillin challenges, but these were not widely replicated or validated.19 The PEN-FAST standardized risk score was first published in 2020 and has since been validated in different groups with additional safety data. This scoring system ranges from 0 to 5 points, assigning 2 points for a penicillin reaction within the past five (F) years, 2 points for angioedema/anaphylaxis (A) or a severe (S) cutaneous reaction, and 1 point if treatment (T) was required for the reaction. A score < 3 is considered low-risk and safe for direct oral challenge, although most of the safety data are in patients with a score of 0 or 1.20 The PEN-FAST guided direct oral challenge with an NPV of 96.3% has now been prospectively shown to be noninferior to standard skin prick test/intradermal test/graded challenge for low-risk patients with a PEN-FAST score < 3.21 The PEN-FAST validating study was conducted predominantly with an Australian population of adult White women, but now it also has been validated in children aged > 12 years, as well as in European and North American cohorts.22-24
Air Force Delabeling Program
This article describes a method for proactively, safely, and efficiently delabeling penicillin allergic patients at an Air Force clinic. This quality improvement (QI) report provides a successful model for penicillin allergy delabeling, illustrates lessons learned, and suggests next steps toward improving patient options for an invaluable antibiotic class.
The first step was to proactively delabel penicillin allergy from a population of active duty service members and their dependents. Electronic health record (EHR) allergy search functions are a helpful tool in finding patients with allergy labels. The Kadena Medical Clinic, in Okinawa, Japan, uses the Military Health System GENESIS EHR, which includes a discern reporting portal with a patient allergy search that creates a patient-specific medication allergy report. To compile the most complete database of patients with a penicillin allergy, all 15 potential allergy search options for “penicillin” were selected, as were 4 relevant options for amoxicillin (including options with clavulanate). Including so many options for specific penicillin medication allergies helps add specificity to the diagnosis in the EHR but can make aggregation of data more difficult. The
The complete compiled list was manually reviewed for high-risk patients with severe cutaneous adverse reactions (SCARs) of any age. Patients with pregnancy, unsuitable medical histories (ie, severe asthma), or taking β-blockers were excluded. Patients remaining on the list were contacted by telephone and offered appointments during a single week that was dedicated to penicillin allergy delabeling. Allergists in the Air Force are assigned to a region where they offer allergy services at clinics without a regular allergist. The allergist for the region traveled to the QI site for a 1-week campaign at an estimated cost of $4600. When the patients were contacted, they were briefly informed of the goal of the penicillin delabeling campaign, and if interested, they were scheduled for 1 of 50 available appointments that week. Patients were contacted with enough lead time to stop oral antihistamines (OAH) for ≥ 7 days before the appointment.
Patients were given an intake questionnaire and interviewed about their penicillin allergy history. This questionnaire inquired about the nature of the allergy, mental and physical health impacts of the allergy label, PEN-FAST scoring questions, and posttest attitude toward delabeling, if applicable. Patients with a PEN-FAST score < 3 were offered direct, graded oral challenge or the standard skin prick, followed by intradermal, followed by graded oral challenge protocol. Patients with PEN-FAST scores of ≥ 3 were offered skin testing prior to oral challenge protocol. Patients could decline further testing. If patients wished to proceed, they were asked to complete a written informed consent document.
Oral challenges followed a 10%/90% protocol, beginning with 50 mg of liquid amoxicillin followed by 450 mg after 15 minutes, as long as the patient remained asymptomatic. Challenge forms are available in the eAppendix . After receiving the 450-mg amoxicillin dose, the patient remained in the clinic for 60 minutes before a final clinical evaluation. If the patient remained asymptomatic after this period, the penicillin or amoxicillin allergy was marked as resolved in the EHR. The patients were given contact information for the clinic for follow-up if a delayed reaction was noted and they wished the medication allergy to be re-entered. An EHR encounter note was created for each patient detailing the allergy testing and delabeling.
This campaign was conducted at a basic life support-only facility by a single clinician without medical technician support. An allergic reaction medication kit was available and contained OAHs, intramuscular antihistamines, intramuscular epinephrine, intramuscular corticosteroids, and short-acting β-agonists for nebulization. The facility also had an urgent care room (staffed by primary care practitioners [PCPs]) that could help establish intravenous access and administer fluids if necessary and had previously established plans for emergency patient transport to a higher level of care, if necessary.
Program Outcomes
A list of 65 patients that included both active-duty service members and dependents with penicillin or amoxicillin allergy was created. This list was reviewed by an allergist to identify high-risk individuals, which required about 90 minutes. Two patients (3%) were excluded; 1 had a history of SCAR to penicillin and 1 had a complex medical history requiring continued OAH use. Sixty-three patients were contacted via telephone, and 29 patients (46%) scheduled an appointment. One patient (2%) was identified as penicillin-tolerant during the booking process, and the penicillin allergy was removed without testing (Figure 1).
Of the 29 scheduled patients, 5 patients (17%) failed to present for care. Of the potential appointments set aside for the program, only 42% were used. One patient (4%) who was seen in clinic was delabeled based on history alone as they had previously successfully tolerated a course of amoxicillin. Four patients (17%) declined further testing with a PEN-FAST score > 2 due to a clear history of acute immunoglobulin (Ig) E-mediated reaction to a penicillin product within the past year. One patient (4%) was unable to be tested due to ongoing OAH use and 1 patient (4%) declined further penicillin testing after the discussion about risks, benefits, and alternatives to the procedures offered.
Of the 24 patients who arrived for a clinic appointment, 17 (71%) underwent penicillin allergy delabeling testing: 14 (82%) underwent direct challenge, and 3 (18%) underwent the skin testing before oral amoxicillin challenge procedure. Of the 17 who were tested, 16 (94%) tolerated a total dose of 500 mg of oral amoxicillin within the 1-hour observation period. One tested patient (6%) in the direct oral challenge group experienced an adverse reaction that was described as dull headache and hand tremor after the 50-mg dose; although it self-resolved within 15 minutes, this prompted the patient to discontinue the challenge. This adverse reaction was determined to be very unlikely IgE-mediated. None of the 3 patients who underwent the skin testing before oral challenge protocol experienced an adverse drug reaction (ADR). None of the 17 patients who received any oral amoxicillin required follow-up or reported a delayed cutaneous ADR to the challenge. No OAHs or epinephrine were used for any of the challenges.
Data collected from patient questionnaires displayed perceived health impacts of a penicillin allergy on the patient population. Patients reported a variety of ADRs to previous administration of penicillin products: 17 (71%) reported urticaria, 2 (8%) reported anaphylaxis, and 3 (13%) were unable to recall the reaction (Figure 2). Nine patients (38%) felt their initial reaction was distressing. Fifteen patients (88%) felt relief following negative testing (Table).
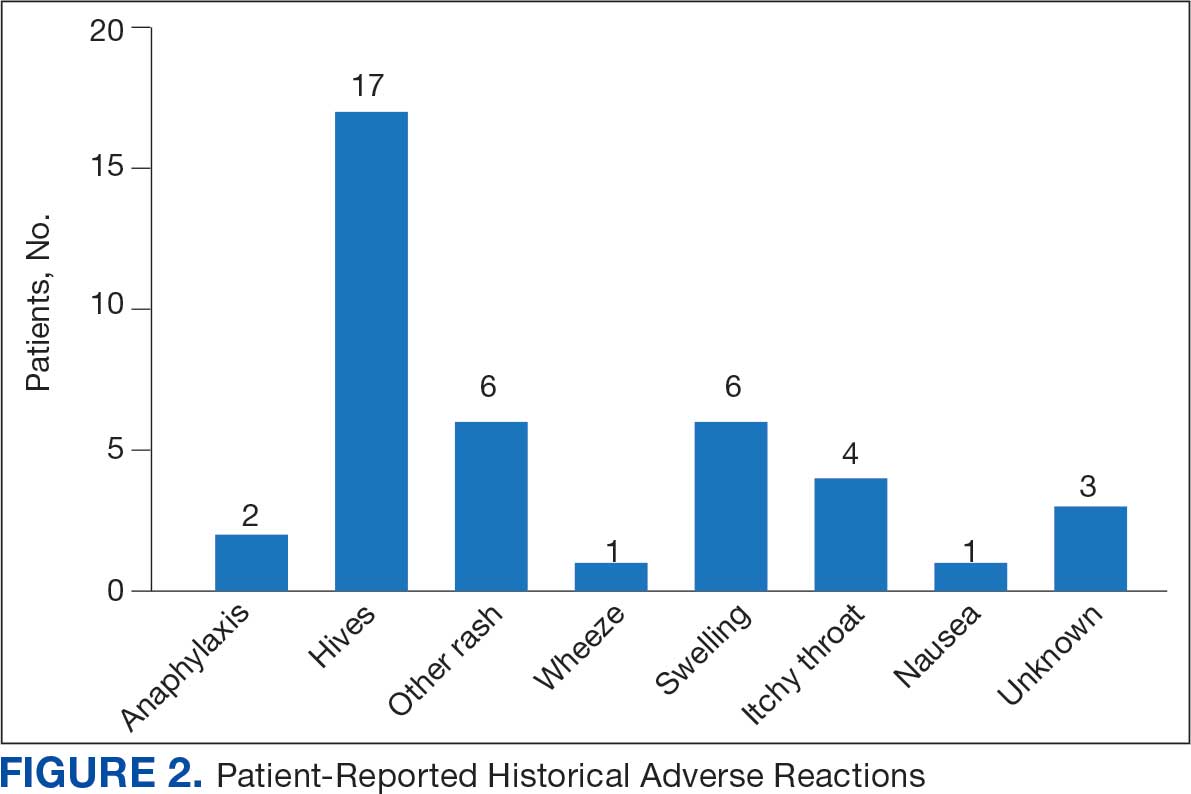
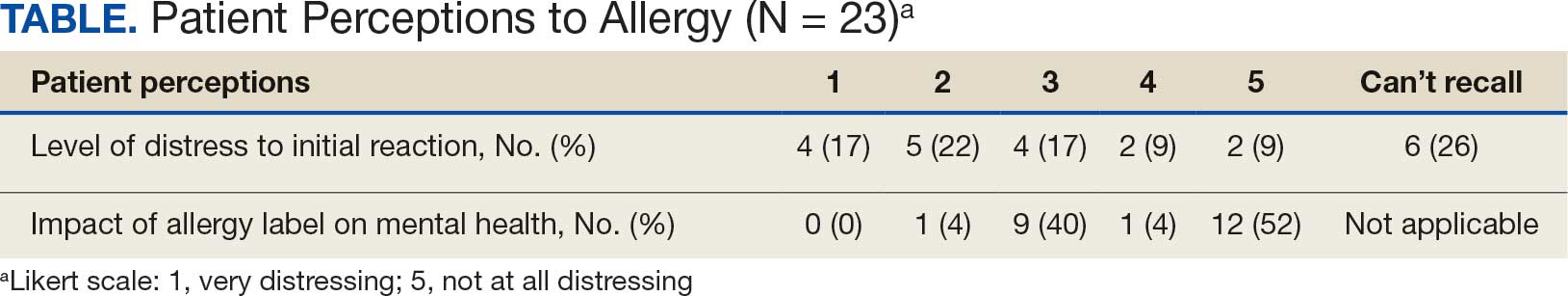
Discussion
To our knowledge, this was the first documented proactive penicillin delabeling QI project in a military clinic treating both active-duty service members and their dependents, modeled on the 2022 drug allergy guidelines.14 Several interesting lessons were learned that may improve future similar QI projects. Only 46% of patients identified as having penicillin allergy presented for evaluation, leaving 42% of available appointments unused. Without prior data on anticipated participation rates, these data provide a crude benchmark for utilization rates, which can inform future resource planning. While attempts were made to contact each patient, additional efforts to publicize the penicillin allergy delabeling campaign would have been useful to improve efficiency.
In addition, when patients with a PEN-FAST score of < 3 were educated about the risks and benefits of each procedure and offered the direct oral graded challenge and skin testing prior to oral challenge, 82% preferred the direct challenge. None of the patients who experienced a penicillin ADR in the past year wished to undergo skin testing or oral challenge, though each was educated on penicillin allergy and the possibility of testing in the future, making each encounter beneficial. Of the 17 patients tested, 16 (94%) tolerated oral amoxicillin and 1 (6%) experienced a mild, self-resolving ADR that was very unlikely of an IgE-mediated origin. Additionally, while plans and preparations for ADRs to the challenges were available, none were required. Patient questionnaires demonstrated the heterogeneity of previous ADRs and their attitude toward their allergy diagnosis. The positive impact of delabeling on patient well-being noted by 88% of patients reinforced the benefit of the effort.
This project was limited by a relatively small sample size, which may not have been large enough to detect ADRs, especially IgE-mediated allergic reactions. Herein lies the importance of having clinicians equipped to treat allergic ADRs to conduct penicillin challenges in the primary care setting. It is prudent to ensure not only proper training of physicians performing these challenges, but also appropriate equipment, medication, and response personnel. Medications that are useful include epinephrine, OAHs, albuterol, steroids, and intravenous fluids.
Having a response area and plan are essential to ensure appropriate care in the rare instance of allergic ADRs progressing to anaphylaxis. In rare cases, emergency medical services may be required and having a plan with appropriate response and transport time is essential to patient safety. This may not be practical in more rural or smaller practices. In those scenarios, it may be helpful to partner with a larger practice to send patients for delabeling or to use clinical space in closer proximity to emergency services. Perhaps an ideal setting might be urgent or emergent care centers due to high acuity resources and frequent prescription of amoxicillin antibiotics; however, this may be complicated by concurrent infections raising the incidence of delayed benign eruptions with amoxicillin ingestion and complicating the patient’s allergy records. Further training of urgent and emergent care practitioners would be helpful for proper patient education regarding antibiotic-associated reactions.
Full testing integration into other primary care clinics may be limited due to the specialized training required for complete skin testing. Nevertheless, as shown in this project, most patients may be delabeled based on a PEN-FAST evaluation followed by oral challenge alone. Incorporation in other QI projects could involve continuing medical education to train staff physicians on PEN-FAST, teaching primary care residents during training, and site visits by allergists to train local physicians on testing. This project involved training 2 PCPs to conduct skin and oral challenge testing using PEN-FAST to guide clinical decision-making with an allergist available for consultation if needed. Future projects might model a similar approach or perhaps focus on training more physicians on oral challenges alone to reach a high percentage of the target population.
Conclusions
This project demonstrates a safe, efficient, and cost-effective model for penicillin allergy delabeling in clinics without regular access to allergy services. The use of PEN-FAST allows a quick and simple method to screen patients with penicillin allergy to meet the goals of the 2022 CBSs, but data are still accumulating to validate this method of screening across populations. This project demonstrates additional support for the use of PEN-FAST, while illustrating appropriate education regarding oral testing technique and its limitations.
Using an EHR report limited the patients in the testing pool and subsequent sample size. This suggests that a primary care identification-driven enrollment in testing may offer even more benefit both in allergy detection and education of testing benefits. Oral challenges are more cost effective, shorter in duration, and have fewer training requirements when compared with antecedent skin testing, making them an ideal option for PCPs in a clinic setting. Trained PCPs may opt to offer periodic appointments for delabeling, or offer days dedicated to delabeling as many patients as possible. Penicillin delabeling is an urgent and expansive charge; this study offers a replicable model for executing this important task.
- Macy E, Poon KYT. Self-reported antibiotic allergy incidence and prevalence: age and sex effects. Am J Med. 2009;122(8):778.e1-778.e7787. doi:10.1016/j.amjmed.2009.01.034
- Zhou L, Dhopeshwarkar N, Blumenthal KG, et al. Drug allergies documented in electronic health records of a large healthcare system. Allergy. 2016;71(9):1305-1313. doi:10.1111/all.12881
- Sullivan TJ, Wedner HJ, Shatz GS, Yecies LD, Parker CW. Skin testing to detect penicillin allergy. J Allergy Clin Immunol. 1981;68(3):171-180. doi:10.1016/0091-6749(81)90180-9
- Macy E, Schatz M, Lin C, Poon KY. The falling rate of positive penicillin skin tests from 1995 to 2007. Perm J. 2009;13(2):12-18. doi:10.7812/TPP/08-073
- Fox SJ, Park MA. Penicillin skin testing is a safe and effective tool for evaluating penicillin allergy in the pediatric population. J Allergy Clin Immunol Pract. 2014;2(4):439-444. doi:10.1016/j.jaip.2014.04.013
- Solensky R, Jacobs J, Lester M, et al. Penicillin Allergy Evaluation: A Prospective, Multicenter, Open-Label Evaluation of a Comprehensive Penicillin Skin Test Kit. J Allergy Clin Immunol Pract. 2019;7(6):1876-1885.e3. doi:10.1016/j.jaip.2019.02.040 7.
- Gonzalez-Estrada A, Park MA, Accarino JJO, et al. Predicting penicillin allergy: A United States multicenter retrospective study. J Allergy Clin Immunol Pract. 2024;12(5):1181-1191.e10. doi:10.1016/j.jaip.2024.01.010
- Stüwe HT, Geissler W, Paap A, Cromwell O. The presence of latex can induce false-positive skin tests in subjects tested with penicillin determinants. Allergy. 1997;52(12):1243. doi:10.1111/j.1398-9995.1997.tb00975.x
- Charneski L, Deshpande G, Smith SW. Impact of an antimicrobial allergy label in the medical record on clinical outcomes in hospitalized patients. Pharmacotherapy. 2011;31(8):742-747. doi:10.1592/phco.31.8.742
- Blumenthal KG, Lu N, Zhang Y, Walensky RP, Choi HK. Recorded penicillin allergy and risk of mortality: a population-based matched cohort study. J Gen Intern Med. 2019;34(9):1685-1687. doi:10.1007/s11606-019-04991-y
- Macy E, Contreras R. Health care use and serious infection prevalence associated with penicillin “allergy” in hospitalized patients: A cohort study. J Allergy Clin Immunol. 2014;133(3):790-796. doi:10.1016/j.jaci.2013.09.021
- Mattingly TJ II, Fulton A, Lumish RA, et al. The cost of self-reported penicillin allergy: a systematic review. J Allergy Clin Immunol Pract. 2018;6(5):1649-1654.e4. doi:10.1016/j.jaip.2017.12.033
- Diplomate Statistics. American Board of Allergy and Immunology website. Published February, 18 2021. Accessed July 28, 2025. https://www.abai.org/statistics_diplomates.asp
- Khan DA, Banerji A, Blumenthal KG, et al. Drug allergy: a 2022 practice parameter update. J Allergy Clin Immunol. 2022;150(6):1333-1393. doi:10.1016/j.jaci.2022.08.028
- Mill C, Primeau MN, Medoff E, et al. Assessing the diagnostic properties of a graded oral provocation challenge for the diagnosis of immediate and nonimmediate reactions to amoxicillin in children. JAMA Pediatr. 2016;170:e160033. doi:10.1001/jamapediatrics.2016.0033
- Tucker MH, Lomas CM, Ramchandar N, Waldram JD. Amoxicillin challenge without penicillin skin testing in evaluation of penicillin allergy in a cohort of Marine recruits. J Allergy Clin Immunol Pract. 2017;5(3):813-815. doi:10.1016/j.jaip.2017.01.023
- Shenoy ES, Macy E, Rowe T, Blumenthal KG. Evaluation and management of penicillin allergy: a review. JAMA. 2019;321(2):188–99. doi:10.1001/jama.2018.19283
- Blumenthal KG, Li Y, Banerji A, et al. The cost of penicillin allergy evaluation. J Allergy Clin Immunol Pract. 2018;6(3):1019-1027.e2. doi:10.1016/j.jaip.2017.08.006
- Banks TA, Tucker M, Macy E. Evaluating penicillin allergies without skin testing. Curr Allergy Asthma Rep. 2019;19(5):27. doi:10.1007/s11882-019-0854-6
- Trubiano JA, Vogrin S, Chua KYL, et al. Development and validation of a penicillin allergy clinical decision rule. JAMA Intern Med. 2020;180(5):745-752. doi:10.1001/jamainternmed.2020.0403
- Copaescu AM, Vogrin S, James F, et al. Efficacy of a clinical decision rule to enable direct oral challenge in patients with low-risk penicillin allergy: the PALACE randomized clinical trial. JAMA Intern Med. 2023;183(9):944-952. doi:10.1001/jamainternmed.2023.2986
- Copaescu AM, Vogrin S, Shand G, et al. Validation of the PEN-FAST score in a pediatric population. JAMA Netw Open. 2022;5(9):e2233703. doi:10.1001/jamanetworkopen.2022.33703
- Piotin A, Godet J, Trubiano JA, et al. Predictive factors of amoxicillin immediate hypersensitivity and validation of PEN-FAST clinical decision rule. Ann Allergy Asthma Immunol. 2022;128(1):27-32. doi:10.1016/j.anai.2021.07.005
- Su C, Belmont A, Liao J, et al. Evaluating the PEN-FAST clinical decision-making tool to enhance penicillin allergy delabeling. JAMA Intern Med. 2023;183(8):883-885. doi:10.1001/jamainternmed.2023.1572
Penicillin allergy is common in the United States. About 9.0% to 13.8% of patients have a diagnosed penicillin allergy documented in their electronic health record. The annual incidence rates is 1.1% in males and 1.4% in females.1,2
Penicillin hypersensitivity likely wanes over time. A 1981 study found that 93% of patients who experienced an allergic reaction to penicillin had a positive skin test 7 to 12 months postreaction, but only 22% still had a positive test after 10 years.3 Confirmed type 1 hypersensitivity penicillin allergies, as demonstrated by positive skin prick testing, also are decreasing over time.4 Furthermore, many patients’ reactions may have been misdiagnosed as a penicillin allergy. Upon actual confirmatory testing of penicillin allergy, only 8.5% to 13.8% of patients believed to have a penicillin allergy were positive on skin prick testing of penicillin products.5,6 A 2024 US study found that 11% of individuals with a history of a penicillin reaction tested positive on skin testing.7
The positive predictive value of penicillin allergy skin testing is poorly defined due to the ethical dilemma of orally challenging a patient who demonstrates skin test reactivity. Due to its high negative predictive value (NPV), skin prick combined with intradermal testing has been the gold-standard test in cases of clinical concern.6 Patients with positive skin testing are assumed to be truly positive, and therefore penicillin allergic, even though false-positive results to penicillin skin testing are known to occur.8
Misdiagnosis of penicillin allergy carries substantial clinical and economic consequences. A 2011 study suggested a statistically significant 1.8% increased absolute risk of mortality and 5.5% increased absolute risk of intensive care unit admission for those labeled with penicillin allergy and admitted for an infection.9 Another study found a 14% increase in mortality associated with the diagnosis of penicillin allergy.10 In a 2014 case-control study, penicillin allergy also was associated with a 23.4% greater risk of Clostridioides difficile, 14.1% more methicillin-resistant Staphylococcus aureus, and 30.1% more vancomycin-resistant enterococci infections.11Direct cost savings during an inpatient admission for infection were as much as $609 per patient with additional indirect cost savings of up to $4254 per admission.12 When viewed from the perspective of a health care system, these costs quickly accumulate, negatively impacting the fiscal stability of our patients and placing additional financial strain on an over-burdened system.
If 10% of US patients have penicillin allergy labels, then about 33 million patients might be eligible for delabeling. There are only 6309 board-certified allergists actively practicing in the US, which could amount to about 5231 potential penicillin challenges per allergist, not even including the 3.3 million new patients per year (assuming a 1% incidence).13 Clarifying each patient’s tolerance of penicillin products will clearly require nonallergist cooperation.
The 2022 drug allergy practice parameter update recommends several consensus-based statements (CBSs) to directly address penicillin allergy.14 This guideline recommends proactive efforts to delabel patients with a reported penicillin allergy (CBS 4); advise against testing in cases where the history is inconsistent with a true allergic reaction, though a challenge may be offered (CBS 5); skin testing for those with a history of anaphylaxis or a recent reaction (CBS 6); advise against multiple-day penicillin challenges (CBS 7); advise against skin testing for pediatric patients with benign cutaneous reactions (CBS 8); and recommends direct oral challenge for adults with distant or benign cutaneous reactions (CBS 9). These recommendations create a potentially high demand for delabeling with allergy specialists. One potential solution is to perform direct oral challenges in primary care, emergency departments, and urgent care clinics.
Evidence supporting the safety of direct oral penicillin challenges in low-risk patients was initially noted in the allergy community, but now evidence for their use in primary care clinics is growing—including in children.15 In a military-specific population, an amoxicillin challenge of Marine recruits with suspected penicillin allergy revealed that only 1.5% of those challenged acutely reacted and should be considered allergic to penicillin.16 Historically, in order to refute the diagnosis of penicillin allergy, an allergist would order penicillin skin prick testing. If the test was negative, an allergist would proceed to intradermal testing and if negative again (NPV of 97.9%), proceed to a graded oral challenge.6 However, this process is not fully reproducible in most clinics because the minor determinants mixture used in skin testing is not commercially available.17 Additionally, the full skin testing procedure requires specialized training, is more time-consuming, causes more discomfort, lacks US Food and Drug Administration approval for children, and has a higher cost ($220 per test for each patient as of 2016).18 As such, the movement toward direct oral challenges is progressing. Nonetheless, the best method for primary care or emergency department clinicians to determine who the appropriate patients are for this procedure has not been fully established. Risk tools have been created in the past to help delineate low-risk patients who would be appropriate for direct oral amoxicillin challenges, but these were not widely replicated or validated.19 The PEN-FAST standardized risk score was first published in 2020 and has since been validated in different groups with additional safety data. This scoring system ranges from 0 to 5 points, assigning 2 points for a penicillin reaction within the past five (F) years, 2 points for angioedema/anaphylaxis (A) or a severe (S) cutaneous reaction, and 1 point if treatment (T) was required for the reaction. A score < 3 is considered low-risk and safe for direct oral challenge, although most of the safety data are in patients with a score of 0 or 1.20 The PEN-FAST guided direct oral challenge with an NPV of 96.3% has now been prospectively shown to be noninferior to standard skin prick test/intradermal test/graded challenge for low-risk patients with a PEN-FAST score < 3.21 The PEN-FAST validating study was conducted predominantly with an Australian population of adult White women, but now it also has been validated in children aged > 12 years, as well as in European and North American cohorts.22-24
Air Force Delabeling Program
This article describes a method for proactively, safely, and efficiently delabeling penicillin allergic patients at an Air Force clinic. This quality improvement (QI) report provides a successful model for penicillin allergy delabeling, illustrates lessons learned, and suggests next steps toward improving patient options for an invaluable antibiotic class.
The first step was to proactively delabel penicillin allergy from a population of active duty service members and their dependents. Electronic health record (EHR) allergy search functions are a helpful tool in finding patients with allergy labels. The Kadena Medical Clinic, in Okinawa, Japan, uses the Military Health System GENESIS EHR, which includes a discern reporting portal with a patient allergy search that creates a patient-specific medication allergy report. To compile the most complete database of patients with a penicillin allergy, all 15 potential allergy search options for “penicillin” were selected, as were 4 relevant options for amoxicillin (including options with clavulanate). Including so many options for specific penicillin medication allergies helps add specificity to the diagnosis in the EHR but can make aggregation of data more difficult. The
The complete compiled list was manually reviewed for high-risk patients with severe cutaneous adverse reactions (SCARs) of any age. Patients with pregnancy, unsuitable medical histories (ie, severe asthma), or taking β-blockers were excluded. Patients remaining on the list were contacted by telephone and offered appointments during a single week that was dedicated to penicillin allergy delabeling. Allergists in the Air Force are assigned to a region where they offer allergy services at clinics without a regular allergist. The allergist for the region traveled to the QI site for a 1-week campaign at an estimated cost of $4600. When the patients were contacted, they were briefly informed of the goal of the penicillin delabeling campaign, and if interested, they were scheduled for 1 of 50 available appointments that week. Patients were contacted with enough lead time to stop oral antihistamines (OAH) for ≥ 7 days before the appointment.
Patients were given an intake questionnaire and interviewed about their penicillin allergy history. This questionnaire inquired about the nature of the allergy, mental and physical health impacts of the allergy label, PEN-FAST scoring questions, and posttest attitude toward delabeling, if applicable. Patients with a PEN-FAST score < 3 were offered direct, graded oral challenge or the standard skin prick, followed by intradermal, followed by graded oral challenge protocol. Patients with PEN-FAST scores of ≥ 3 were offered skin testing prior to oral challenge protocol. Patients could decline further testing. If patients wished to proceed, they were asked to complete a written informed consent document.
Oral challenges followed a 10%/90% protocol, beginning with 50 mg of liquid amoxicillin followed by 450 mg after 15 minutes, as long as the patient remained asymptomatic. Challenge forms are available in the eAppendix . After receiving the 450-mg amoxicillin dose, the patient remained in the clinic for 60 minutes before a final clinical evaluation. If the patient remained asymptomatic after this period, the penicillin or amoxicillin allergy was marked as resolved in the EHR. The patients were given contact information for the clinic for follow-up if a delayed reaction was noted and they wished the medication allergy to be re-entered. An EHR encounter note was created for each patient detailing the allergy testing and delabeling.

This campaign was conducted at a basic life support-only facility by a single clinician without medical technician support. An allergic reaction medication kit was available and contained OAHs, intramuscular antihistamines, intramuscular epinephrine, intramuscular corticosteroids, and short-acting β-agonists for nebulization. The facility also had an urgent care room (staffed by primary care practitioners [PCPs]) that could help establish intravenous access and administer fluids if necessary and had previously established plans for emergency patient transport to a higher level of care, if necessary.
Program Outcomes
A list of 65 patients that included both active-duty service members and dependents with penicillin or amoxicillin allergy was created. This list was reviewed by an allergist to identify high-risk individuals, which required about 90 minutes. Two patients (3%) were excluded; 1 had a history of SCAR to penicillin and 1 had a complex medical history requiring continued OAH use. Sixty-three patients were contacted via telephone, and 29 patients (46%) scheduled an appointment. One patient (2%) was identified as penicillin-tolerant during the booking process, and the penicillin allergy was removed without testing (Figure 1).

Of the 29 scheduled patients, 5 patients (17%) failed to present for care. Of the potential appointments set aside for the program, only 42% were used. One patient (4%) who was seen in clinic was delabeled based on history alone as they had previously successfully tolerated a course of amoxicillin. Four patients (17%) declined further testing with a PEN-FAST score > 2 due to a clear history of acute immunoglobulin (Ig) E-mediated reaction to a penicillin product within the past year. One patient (4%) was unable to be tested due to ongoing OAH use and 1 patient (4%) declined further penicillin testing after the discussion about risks, benefits, and alternatives to the procedures offered.
Of the 24 patients who arrived for a clinic appointment, 17 (71%) underwent penicillin allergy delabeling testing: 14 (82%) underwent direct challenge, and 3 (18%) underwent the skin testing before oral amoxicillin challenge procedure. Of the 17 who were tested, 16 (94%) tolerated a total dose of 500 mg of oral amoxicillin within the 1-hour observation period. One tested patient (6%) in the direct oral challenge group experienced an adverse reaction that was described as dull headache and hand tremor after the 50-mg dose; although it self-resolved within 15 minutes, this prompted the patient to discontinue the challenge. This adverse reaction was determined to be very unlikely IgE-mediated. None of the 3 patients who underwent the skin testing before oral challenge protocol experienced an adverse drug reaction (ADR). None of the 17 patients who received any oral amoxicillin required follow-up or reported a delayed cutaneous ADR to the challenge. No OAHs or epinephrine were used for any of the challenges.
Data collected from patient questionnaires displayed perceived health impacts of a penicillin allergy on the patient population. Patients reported a variety of ADRs to previous administration of penicillin products: 17 (71%) reported urticaria, 2 (8%) reported anaphylaxis, and 3 (13%) were unable to recall the reaction (Figure 2). Nine patients (38%) felt their initial reaction was distressing. Fifteen patients (88%) felt relief following negative testing (Table).


Discussion
To our knowledge, this was the first documented proactive penicillin delabeling QI project in a military clinic treating both active-duty service members and their dependents, modeled on the 2022 drug allergy guidelines.14 Several interesting lessons were learned that may improve future similar QI projects. Only 46% of patients identified as having penicillin allergy presented for evaluation, leaving 42% of available appointments unused. Without prior data on anticipated participation rates, these data provide a crude benchmark for utilization rates, which can inform future resource planning. While attempts were made to contact each patient, additional efforts to publicize the penicillin allergy delabeling campaign would have been useful to improve efficiency.
In addition, when patients with a PEN-FAST score of < 3 were educated about the risks and benefits of each procedure and offered the direct oral graded challenge and skin testing prior to oral challenge, 82% preferred the direct challenge. None of the patients who experienced a penicillin ADR in the past year wished to undergo skin testing or oral challenge, though each was educated on penicillin allergy and the possibility of testing in the future, making each encounter beneficial. Of the 17 patients tested, 16 (94%) tolerated oral amoxicillin and 1 (6%) experienced a mild, self-resolving ADR that was very unlikely of an IgE-mediated origin. Additionally, while plans and preparations for ADRs to the challenges were available, none were required. Patient questionnaires demonstrated the heterogeneity of previous ADRs and their attitude toward their allergy diagnosis. The positive impact of delabeling on patient well-being noted by 88% of patients reinforced the benefit of the effort.
This project was limited by a relatively small sample size, which may not have been large enough to detect ADRs, especially IgE-mediated allergic reactions. Herein lies the importance of having clinicians equipped to treat allergic ADRs to conduct penicillin challenges in the primary care setting. It is prudent to ensure not only proper training of physicians performing these challenges, but also appropriate equipment, medication, and response personnel. Medications that are useful include epinephrine, OAHs, albuterol, steroids, and intravenous fluids.
Having a response area and plan are essential to ensure appropriate care in the rare instance of allergic ADRs progressing to anaphylaxis. In rare cases, emergency medical services may be required and having a plan with appropriate response and transport time is essential to patient safety. This may not be practical in more rural or smaller practices. In those scenarios, it may be helpful to partner with a larger practice to send patients for delabeling or to use clinical space in closer proximity to emergency services. Perhaps an ideal setting might be urgent or emergent care centers due to high acuity resources and frequent prescription of amoxicillin antibiotics; however, this may be complicated by concurrent infections raising the incidence of delayed benign eruptions with amoxicillin ingestion and complicating the patient’s allergy records. Further training of urgent and emergent care practitioners would be helpful for proper patient education regarding antibiotic-associated reactions.
Full testing integration into other primary care clinics may be limited due to the specialized training required for complete skin testing. Nevertheless, as shown in this project, most patients may be delabeled based on a PEN-FAST evaluation followed by oral challenge alone. Incorporation in other QI projects could involve continuing medical education to train staff physicians on PEN-FAST, teaching primary care residents during training, and site visits by allergists to train local physicians on testing. This project involved training 2 PCPs to conduct skin and oral challenge testing using PEN-FAST to guide clinical decision-making with an allergist available for consultation if needed. Future projects might model a similar approach or perhaps focus on training more physicians on oral challenges alone to reach a high percentage of the target population.
Conclusions
This project demonstrates a safe, efficient, and cost-effective model for penicillin allergy delabeling in clinics without regular access to allergy services. The use of PEN-FAST allows a quick and simple method to screen patients with penicillin allergy to meet the goals of the 2022 CBSs, but data are still accumulating to validate this method of screening across populations. This project demonstrates additional support for the use of PEN-FAST, while illustrating appropriate education regarding oral testing technique and its limitations.
Using an EHR report limited the patients in the testing pool and subsequent sample size. This suggests that a primary care identification-driven enrollment in testing may offer even more benefit both in allergy detection and education of testing benefits. Oral challenges are more cost effective, shorter in duration, and have fewer training requirements when compared with antecedent skin testing, making them an ideal option for PCPs in a clinic setting. Trained PCPs may opt to offer periodic appointments for delabeling, or offer days dedicated to delabeling as many patients as possible. Penicillin delabeling is an urgent and expansive charge; this study offers a replicable model for executing this important task.
Penicillin allergy is common in the United States. About 9.0% to 13.8% of patients have a diagnosed penicillin allergy documented in their electronic health record. The annual incidence rates is 1.1% in males and 1.4% in females.1,2
Penicillin hypersensitivity likely wanes over time. A 1981 study found that 93% of patients who experienced an allergic reaction to penicillin had a positive skin test 7 to 12 months postreaction, but only 22% still had a positive test after 10 years.3 Confirmed type 1 hypersensitivity penicillin allergies, as demonstrated by positive skin prick testing, also are decreasing over time.4 Furthermore, many patients’ reactions may have been misdiagnosed as a penicillin allergy. Upon actual confirmatory testing of penicillin allergy, only 8.5% to 13.8% of patients believed to have a penicillin allergy were positive on skin prick testing of penicillin products.5,6 A 2024 US study found that 11% of individuals with a history of a penicillin reaction tested positive on skin testing.7
The positive predictive value of penicillin allergy skin testing is poorly defined due to the ethical dilemma of orally challenging a patient who demonstrates skin test reactivity. Due to its high negative predictive value (NPV), skin prick combined with intradermal testing has been the gold-standard test in cases of clinical concern.6 Patients with positive skin testing are assumed to be truly positive, and therefore penicillin allergic, even though false-positive results to penicillin skin testing are known to occur.8
Misdiagnosis of penicillin allergy carries substantial clinical and economic consequences. A 2011 study suggested a statistically significant 1.8% increased absolute risk of mortality and 5.5% increased absolute risk of intensive care unit admission for those labeled with penicillin allergy and admitted for an infection.9 Another study found a 14% increase in mortality associated with the diagnosis of penicillin allergy.10 In a 2014 case-control study, penicillin allergy also was associated with a 23.4% greater risk of Clostridioides difficile, 14.1% more methicillin-resistant Staphylococcus aureus, and 30.1% more vancomycin-resistant enterococci infections.11Direct cost savings during an inpatient admission for infection were as much as $609 per patient with additional indirect cost savings of up to $4254 per admission.12 When viewed from the perspective of a health care system, these costs quickly accumulate, negatively impacting the fiscal stability of our patients and placing additional financial strain on an over-burdened system.
If 10% of US patients have penicillin allergy labels, then about 33 million patients might be eligible for delabeling. There are only 6309 board-certified allergists actively practicing in the US, which could amount to about 5231 potential penicillin challenges per allergist, not even including the 3.3 million new patients per year (assuming a 1% incidence).13 Clarifying each patient’s tolerance of penicillin products will clearly require nonallergist cooperation.
The 2022 drug allergy practice parameter update recommends several consensus-based statements (CBSs) to directly address penicillin allergy.14 This guideline recommends proactive efforts to delabel patients with a reported penicillin allergy (CBS 4); advise against testing in cases where the history is inconsistent with a true allergic reaction, though a challenge may be offered (CBS 5); skin testing for those with a history of anaphylaxis or a recent reaction (CBS 6); advise against multiple-day penicillin challenges (CBS 7); advise against skin testing for pediatric patients with benign cutaneous reactions (CBS 8); and recommends direct oral challenge for adults with distant or benign cutaneous reactions (CBS 9). These recommendations create a potentially high demand for delabeling with allergy specialists. One potential solution is to perform direct oral challenges in primary care, emergency departments, and urgent care clinics.
Evidence supporting the safety of direct oral penicillin challenges in low-risk patients was initially noted in the allergy community, but now evidence for their use in primary care clinics is growing—including in children.15 In a military-specific population, an amoxicillin challenge of Marine recruits with suspected penicillin allergy revealed that only 1.5% of those challenged acutely reacted and should be considered allergic to penicillin.16 Historically, in order to refute the diagnosis of penicillin allergy, an allergist would order penicillin skin prick testing. If the test was negative, an allergist would proceed to intradermal testing and if negative again (NPV of 97.9%), proceed to a graded oral challenge.6 However, this process is not fully reproducible in most clinics because the minor determinants mixture used in skin testing is not commercially available.17 Additionally, the full skin testing procedure requires specialized training, is more time-consuming, causes more discomfort, lacks US Food and Drug Administration approval for children, and has a higher cost ($220 per test for each patient as of 2016).18 As such, the movement toward direct oral challenges is progressing. Nonetheless, the best method for primary care or emergency department clinicians to determine who the appropriate patients are for this procedure has not been fully established. Risk tools have been created in the past to help delineate low-risk patients who would be appropriate for direct oral amoxicillin challenges, but these were not widely replicated or validated.19 The PEN-FAST standardized risk score was first published in 2020 and has since been validated in different groups with additional safety data. This scoring system ranges from 0 to 5 points, assigning 2 points for a penicillin reaction within the past five (F) years, 2 points for angioedema/anaphylaxis (A) or a severe (S) cutaneous reaction, and 1 point if treatment (T) was required for the reaction. A score < 3 is considered low-risk and safe for direct oral challenge, although most of the safety data are in patients with a score of 0 or 1.20 The PEN-FAST guided direct oral challenge with an NPV of 96.3% has now been prospectively shown to be noninferior to standard skin prick test/intradermal test/graded challenge for low-risk patients with a PEN-FAST score < 3.21 The PEN-FAST validating study was conducted predominantly with an Australian population of adult White women, but now it also has been validated in children aged > 12 years, as well as in European and North American cohorts.22-24
Air Force Delabeling Program
This article describes a method for proactively, safely, and efficiently delabeling penicillin allergic patients at an Air Force clinic. This quality improvement (QI) report provides a successful model for penicillin allergy delabeling, illustrates lessons learned, and suggests next steps toward improving patient options for an invaluable antibiotic class.
The first step was to proactively delabel penicillin allergy from a population of active duty service members and their dependents. Electronic health record (EHR) allergy search functions are a helpful tool in finding patients with allergy labels. The Kadena Medical Clinic, in Okinawa, Japan, uses the Military Health System GENESIS EHR, which includes a discern reporting portal with a patient allergy search that creates a patient-specific medication allergy report. To compile the most complete database of patients with a penicillin allergy, all 15 potential allergy search options for “penicillin” were selected, as were 4 relevant options for amoxicillin (including options with clavulanate). Including so many options for specific penicillin medication allergies helps add specificity to the diagnosis in the EHR but can make aggregation of data more difficult. The
The complete compiled list was manually reviewed for high-risk patients with severe cutaneous adverse reactions (SCARs) of any age. Patients with pregnancy, unsuitable medical histories (ie, severe asthma), or taking β-blockers were excluded. Patients remaining on the list were contacted by telephone and offered appointments during a single week that was dedicated to penicillin allergy delabeling. Allergists in the Air Force are assigned to a region where they offer allergy services at clinics without a regular allergist. The allergist for the region traveled to the QI site for a 1-week campaign at an estimated cost of $4600. When the patients were contacted, they were briefly informed of the goal of the penicillin delabeling campaign, and if interested, they were scheduled for 1 of 50 available appointments that week. Patients were contacted with enough lead time to stop oral antihistamines (OAH) for ≥ 7 days before the appointment.
Patients were given an intake questionnaire and interviewed about their penicillin allergy history. This questionnaire inquired about the nature of the allergy, mental and physical health impacts of the allergy label, PEN-FAST scoring questions, and posttest attitude toward delabeling, if applicable. Patients with a PEN-FAST score < 3 were offered direct, graded oral challenge or the standard skin prick, followed by intradermal, followed by graded oral challenge protocol. Patients with PEN-FAST scores of ≥ 3 were offered skin testing prior to oral challenge protocol. Patients could decline further testing. If patients wished to proceed, they were asked to complete a written informed consent document.
Oral challenges followed a 10%/90% protocol, beginning with 50 mg of liquid amoxicillin followed by 450 mg after 15 minutes, as long as the patient remained asymptomatic. Challenge forms are available in the eAppendix . After receiving the 450-mg amoxicillin dose, the patient remained in the clinic for 60 minutes before a final clinical evaluation. If the patient remained asymptomatic after this period, the penicillin or amoxicillin allergy was marked as resolved in the EHR. The patients were given contact information for the clinic for follow-up if a delayed reaction was noted and they wished the medication allergy to be re-entered. An EHR encounter note was created for each patient detailing the allergy testing and delabeling.

This campaign was conducted at a basic life support-only facility by a single clinician without medical technician support. An allergic reaction medication kit was available and contained OAHs, intramuscular antihistamines, intramuscular epinephrine, intramuscular corticosteroids, and short-acting β-agonists for nebulization. The facility also had an urgent care room (staffed by primary care practitioners [PCPs]) that could help establish intravenous access and administer fluids if necessary and had previously established plans for emergency patient transport to a higher level of care, if necessary.
Program Outcomes
A list of 65 patients that included both active-duty service members and dependents with penicillin or amoxicillin allergy was created. This list was reviewed by an allergist to identify high-risk individuals, which required about 90 minutes. Two patients (3%) were excluded; 1 had a history of SCAR to penicillin and 1 had a complex medical history requiring continued OAH use. Sixty-three patients were contacted via telephone, and 29 patients (46%) scheduled an appointment. One patient (2%) was identified as penicillin-tolerant during the booking process, and the penicillin allergy was removed without testing (Figure 1).

Of the 29 scheduled patients, 5 patients (17%) failed to present for care. Of the potential appointments set aside for the program, only 42% were used. One patient (4%) who was seen in clinic was delabeled based on history alone as they had previously successfully tolerated a course of amoxicillin. Four patients (17%) declined further testing with a PEN-FAST score > 2 due to a clear history of acute immunoglobulin (Ig) E-mediated reaction to a penicillin product within the past year. One patient (4%) was unable to be tested due to ongoing OAH use and 1 patient (4%) declined further penicillin testing after the discussion about risks, benefits, and alternatives to the procedures offered.
Of the 24 patients who arrived for a clinic appointment, 17 (71%) underwent penicillin allergy delabeling testing: 14 (82%) underwent direct challenge, and 3 (18%) underwent the skin testing before oral amoxicillin challenge procedure. Of the 17 who were tested, 16 (94%) tolerated a total dose of 500 mg of oral amoxicillin within the 1-hour observation period. One tested patient (6%) in the direct oral challenge group experienced an adverse reaction that was described as dull headache and hand tremor after the 50-mg dose; although it self-resolved within 15 minutes, this prompted the patient to discontinue the challenge. This adverse reaction was determined to be very unlikely IgE-mediated. None of the 3 patients who underwent the skin testing before oral challenge protocol experienced an adverse drug reaction (ADR). None of the 17 patients who received any oral amoxicillin required follow-up or reported a delayed cutaneous ADR to the challenge. No OAHs or epinephrine were used for any of the challenges.
Data collected from patient questionnaires displayed perceived health impacts of a penicillin allergy on the patient population. Patients reported a variety of ADRs to previous administration of penicillin products: 17 (71%) reported urticaria, 2 (8%) reported anaphylaxis, and 3 (13%) were unable to recall the reaction (Figure 2). Nine patients (38%) felt their initial reaction was distressing. Fifteen patients (88%) felt relief following negative testing (Table).


Discussion
To our knowledge, this was the first documented proactive penicillin delabeling QI project in a military clinic treating both active-duty service members and their dependents, modeled on the 2022 drug allergy guidelines.14 Several interesting lessons were learned that may improve future similar QI projects. Only 46% of patients identified as having penicillin allergy presented for evaluation, leaving 42% of available appointments unused. Without prior data on anticipated participation rates, these data provide a crude benchmark for utilization rates, which can inform future resource planning. While attempts were made to contact each patient, additional efforts to publicize the penicillin allergy delabeling campaign would have been useful to improve efficiency.
In addition, when patients with a PEN-FAST score of < 3 were educated about the risks and benefits of each procedure and offered the direct oral graded challenge and skin testing prior to oral challenge, 82% preferred the direct challenge. None of the patients who experienced a penicillin ADR in the past year wished to undergo skin testing or oral challenge, though each was educated on penicillin allergy and the possibility of testing in the future, making each encounter beneficial. Of the 17 patients tested, 16 (94%) tolerated oral amoxicillin and 1 (6%) experienced a mild, self-resolving ADR that was very unlikely of an IgE-mediated origin. Additionally, while plans and preparations for ADRs to the challenges were available, none were required. Patient questionnaires demonstrated the heterogeneity of previous ADRs and their attitude toward their allergy diagnosis. The positive impact of delabeling on patient well-being noted by 88% of patients reinforced the benefit of the effort.
This project was limited by a relatively small sample size, which may not have been large enough to detect ADRs, especially IgE-mediated allergic reactions. Herein lies the importance of having clinicians equipped to treat allergic ADRs to conduct penicillin challenges in the primary care setting. It is prudent to ensure not only proper training of physicians performing these challenges, but also appropriate equipment, medication, and response personnel. Medications that are useful include epinephrine, OAHs, albuterol, steroids, and intravenous fluids.
Having a response area and plan are essential to ensure appropriate care in the rare instance of allergic ADRs progressing to anaphylaxis. In rare cases, emergency medical services may be required and having a plan with appropriate response and transport time is essential to patient safety. This may not be practical in more rural or smaller practices. In those scenarios, it may be helpful to partner with a larger practice to send patients for delabeling or to use clinical space in closer proximity to emergency services. Perhaps an ideal setting might be urgent or emergent care centers due to high acuity resources and frequent prescription of amoxicillin antibiotics; however, this may be complicated by concurrent infections raising the incidence of delayed benign eruptions with amoxicillin ingestion and complicating the patient’s allergy records. Further training of urgent and emergent care practitioners would be helpful for proper patient education regarding antibiotic-associated reactions.
Full testing integration into other primary care clinics may be limited due to the specialized training required for complete skin testing. Nevertheless, as shown in this project, most patients may be delabeled based on a PEN-FAST evaluation followed by oral challenge alone. Incorporation in other QI projects could involve continuing medical education to train staff physicians on PEN-FAST, teaching primary care residents during training, and site visits by allergists to train local physicians on testing. This project involved training 2 PCPs to conduct skin and oral challenge testing using PEN-FAST to guide clinical decision-making with an allergist available for consultation if needed. Future projects might model a similar approach or perhaps focus on training more physicians on oral challenges alone to reach a high percentage of the target population.
Conclusions
This project demonstrates a safe, efficient, and cost-effective model for penicillin allergy delabeling in clinics without regular access to allergy services. The use of PEN-FAST allows a quick and simple method to screen patients with penicillin allergy to meet the goals of the 2022 CBSs, but data are still accumulating to validate this method of screening across populations. This project demonstrates additional support for the use of PEN-FAST, while illustrating appropriate education regarding oral testing technique and its limitations.
Using an EHR report limited the patients in the testing pool and subsequent sample size. This suggests that a primary care identification-driven enrollment in testing may offer even more benefit both in allergy detection and education of testing benefits. Oral challenges are more cost effective, shorter in duration, and have fewer training requirements when compared with antecedent skin testing, making them an ideal option for PCPs in a clinic setting. Trained PCPs may opt to offer periodic appointments for delabeling, or offer days dedicated to delabeling as many patients as possible. Penicillin delabeling is an urgent and expansive charge; this study offers a replicable model for executing this important task.
- Macy E, Poon KYT. Self-reported antibiotic allergy incidence and prevalence: age and sex effects. Am J Med. 2009;122(8):778.e1-778.e7787. doi:10.1016/j.amjmed.2009.01.034
- Zhou L, Dhopeshwarkar N, Blumenthal KG, et al. Drug allergies documented in electronic health records of a large healthcare system. Allergy. 2016;71(9):1305-1313. doi:10.1111/all.12881
- Sullivan TJ, Wedner HJ, Shatz GS, Yecies LD, Parker CW. Skin testing to detect penicillin allergy. J Allergy Clin Immunol. 1981;68(3):171-180. doi:10.1016/0091-6749(81)90180-9
- Macy E, Schatz M, Lin C, Poon KY. The falling rate of positive penicillin skin tests from 1995 to 2007. Perm J. 2009;13(2):12-18. doi:10.7812/TPP/08-073
- Fox SJ, Park MA. Penicillin skin testing is a safe and effective tool for evaluating penicillin allergy in the pediatric population. J Allergy Clin Immunol Pract. 2014;2(4):439-444. doi:10.1016/j.jaip.2014.04.013
- Solensky R, Jacobs J, Lester M, et al. Penicillin Allergy Evaluation: A Prospective, Multicenter, Open-Label Evaluation of a Comprehensive Penicillin Skin Test Kit. J Allergy Clin Immunol Pract. 2019;7(6):1876-1885.e3. doi:10.1016/j.jaip.2019.02.040 7.
- Gonzalez-Estrada A, Park MA, Accarino JJO, et al. Predicting penicillin allergy: A United States multicenter retrospective study. J Allergy Clin Immunol Pract. 2024;12(5):1181-1191.e10. doi:10.1016/j.jaip.2024.01.010
- Stüwe HT, Geissler W, Paap A, Cromwell O. The presence of latex can induce false-positive skin tests in subjects tested with penicillin determinants. Allergy. 1997;52(12):1243. doi:10.1111/j.1398-9995.1997.tb00975.x
- Charneski L, Deshpande G, Smith SW. Impact of an antimicrobial allergy label in the medical record on clinical outcomes in hospitalized patients. Pharmacotherapy. 2011;31(8):742-747. doi:10.1592/phco.31.8.742
- Blumenthal KG, Lu N, Zhang Y, Walensky RP, Choi HK. Recorded penicillin allergy and risk of mortality: a population-based matched cohort study. J Gen Intern Med. 2019;34(9):1685-1687. doi:10.1007/s11606-019-04991-y
- Macy E, Contreras R. Health care use and serious infection prevalence associated with penicillin “allergy” in hospitalized patients: A cohort study. J Allergy Clin Immunol. 2014;133(3):790-796. doi:10.1016/j.jaci.2013.09.021
- Mattingly TJ II, Fulton A, Lumish RA, et al. The cost of self-reported penicillin allergy: a systematic review. J Allergy Clin Immunol Pract. 2018;6(5):1649-1654.e4. doi:10.1016/j.jaip.2017.12.033
- Diplomate Statistics. American Board of Allergy and Immunology website. Published February, 18 2021. Accessed July 28, 2025. https://www.abai.org/statistics_diplomates.asp
- Khan DA, Banerji A, Blumenthal KG, et al. Drug allergy: a 2022 practice parameter update. J Allergy Clin Immunol. 2022;150(6):1333-1393. doi:10.1016/j.jaci.2022.08.028
- Mill C, Primeau MN, Medoff E, et al. Assessing the diagnostic properties of a graded oral provocation challenge for the diagnosis of immediate and nonimmediate reactions to amoxicillin in children. JAMA Pediatr. 2016;170:e160033. doi:10.1001/jamapediatrics.2016.0033
- Tucker MH, Lomas CM, Ramchandar N, Waldram JD. Amoxicillin challenge without penicillin skin testing in evaluation of penicillin allergy in a cohort of Marine recruits. J Allergy Clin Immunol Pract. 2017;5(3):813-815. doi:10.1016/j.jaip.2017.01.023
- Shenoy ES, Macy E, Rowe T, Blumenthal KG. Evaluation and management of penicillin allergy: a review. JAMA. 2019;321(2):188–99. doi:10.1001/jama.2018.19283
- Blumenthal KG, Li Y, Banerji A, et al. The cost of penicillin allergy evaluation. J Allergy Clin Immunol Pract. 2018;6(3):1019-1027.e2. doi:10.1016/j.jaip.2017.08.006
- Banks TA, Tucker M, Macy E. Evaluating penicillin allergies without skin testing. Curr Allergy Asthma Rep. 2019;19(5):27. doi:10.1007/s11882-019-0854-6
- Trubiano JA, Vogrin S, Chua KYL, et al. Development and validation of a penicillin allergy clinical decision rule. JAMA Intern Med. 2020;180(5):745-752. doi:10.1001/jamainternmed.2020.0403
- Copaescu AM, Vogrin S, James F, et al. Efficacy of a clinical decision rule to enable direct oral challenge in patients with low-risk penicillin allergy: the PALACE randomized clinical trial. JAMA Intern Med. 2023;183(9):944-952. doi:10.1001/jamainternmed.2023.2986
- Copaescu AM, Vogrin S, Shand G, et al. Validation of the PEN-FAST score in a pediatric population. JAMA Netw Open. 2022;5(9):e2233703. doi:10.1001/jamanetworkopen.2022.33703
- Piotin A, Godet J, Trubiano JA, et al. Predictive factors of amoxicillin immediate hypersensitivity and validation of PEN-FAST clinical decision rule. Ann Allergy Asthma Immunol. 2022;128(1):27-32. doi:10.1016/j.anai.2021.07.005
- Su C, Belmont A, Liao J, et al. Evaluating the PEN-FAST clinical decision-making tool to enhance penicillin allergy delabeling. JAMA Intern Med. 2023;183(8):883-885. doi:10.1001/jamainternmed.2023.1572
- Macy E, Poon KYT. Self-reported antibiotic allergy incidence and prevalence: age and sex effects. Am J Med. 2009;122(8):778.e1-778.e7787. doi:10.1016/j.amjmed.2009.01.034
- Zhou L, Dhopeshwarkar N, Blumenthal KG, et al. Drug allergies documented in electronic health records of a large healthcare system. Allergy. 2016;71(9):1305-1313. doi:10.1111/all.12881
- Sullivan TJ, Wedner HJ, Shatz GS, Yecies LD, Parker CW. Skin testing to detect penicillin allergy. J Allergy Clin Immunol. 1981;68(3):171-180. doi:10.1016/0091-6749(81)90180-9
- Macy E, Schatz M, Lin C, Poon KY. The falling rate of positive penicillin skin tests from 1995 to 2007. Perm J. 2009;13(2):12-18. doi:10.7812/TPP/08-073
- Fox SJ, Park MA. Penicillin skin testing is a safe and effective tool for evaluating penicillin allergy in the pediatric population. J Allergy Clin Immunol Pract. 2014;2(4):439-444. doi:10.1016/j.jaip.2014.04.013
- Solensky R, Jacobs J, Lester M, et al. Penicillin Allergy Evaluation: A Prospective, Multicenter, Open-Label Evaluation of a Comprehensive Penicillin Skin Test Kit. J Allergy Clin Immunol Pract. 2019;7(6):1876-1885.e3. doi:10.1016/j.jaip.2019.02.040 7.
- Gonzalez-Estrada A, Park MA, Accarino JJO, et al. Predicting penicillin allergy: A United States multicenter retrospective study. J Allergy Clin Immunol Pract. 2024;12(5):1181-1191.e10. doi:10.1016/j.jaip.2024.01.010
- Stüwe HT, Geissler W, Paap A, Cromwell O. The presence of latex can induce false-positive skin tests in subjects tested with penicillin determinants. Allergy. 1997;52(12):1243. doi:10.1111/j.1398-9995.1997.tb00975.x
- Charneski L, Deshpande G, Smith SW. Impact of an antimicrobial allergy label in the medical record on clinical outcomes in hospitalized patients. Pharmacotherapy. 2011;31(8):742-747. doi:10.1592/phco.31.8.742
- Blumenthal KG, Lu N, Zhang Y, Walensky RP, Choi HK. Recorded penicillin allergy and risk of mortality: a population-based matched cohort study. J Gen Intern Med. 2019;34(9):1685-1687. doi:10.1007/s11606-019-04991-y
- Macy E, Contreras R. Health care use and serious infection prevalence associated with penicillin “allergy” in hospitalized patients: A cohort study. J Allergy Clin Immunol. 2014;133(3):790-796. doi:10.1016/j.jaci.2013.09.021
- Mattingly TJ II, Fulton A, Lumish RA, et al. The cost of self-reported penicillin allergy: a systematic review. J Allergy Clin Immunol Pract. 2018;6(5):1649-1654.e4. doi:10.1016/j.jaip.2017.12.033
- Diplomate Statistics. American Board of Allergy and Immunology website. Published February, 18 2021. Accessed July 28, 2025. https://www.abai.org/statistics_diplomates.asp
- Khan DA, Banerji A, Blumenthal KG, et al. Drug allergy: a 2022 practice parameter update. J Allergy Clin Immunol. 2022;150(6):1333-1393. doi:10.1016/j.jaci.2022.08.028
- Mill C, Primeau MN, Medoff E, et al. Assessing the diagnostic properties of a graded oral provocation challenge for the diagnosis of immediate and nonimmediate reactions to amoxicillin in children. JAMA Pediatr. 2016;170:e160033. doi:10.1001/jamapediatrics.2016.0033
- Tucker MH, Lomas CM, Ramchandar N, Waldram JD. Amoxicillin challenge without penicillin skin testing in evaluation of penicillin allergy in a cohort of Marine recruits. J Allergy Clin Immunol Pract. 2017;5(3):813-815. doi:10.1016/j.jaip.2017.01.023
- Shenoy ES, Macy E, Rowe T, Blumenthal KG. Evaluation and management of penicillin allergy: a review. JAMA. 2019;321(2):188–99. doi:10.1001/jama.2018.19283
- Blumenthal KG, Li Y, Banerji A, et al. The cost of penicillin allergy evaluation. J Allergy Clin Immunol Pract. 2018;6(3):1019-1027.e2. doi:10.1016/j.jaip.2017.08.006
- Banks TA, Tucker M, Macy E. Evaluating penicillin allergies without skin testing. Curr Allergy Asthma Rep. 2019;19(5):27. doi:10.1007/s11882-019-0854-6
- Trubiano JA, Vogrin S, Chua KYL, et al. Development and validation of a penicillin allergy clinical decision rule. JAMA Intern Med. 2020;180(5):745-752. doi:10.1001/jamainternmed.2020.0403
- Copaescu AM, Vogrin S, James F, et al. Efficacy of a clinical decision rule to enable direct oral challenge in patients with low-risk penicillin allergy: the PALACE randomized clinical trial. JAMA Intern Med. 2023;183(9):944-952. doi:10.1001/jamainternmed.2023.2986
- Copaescu AM, Vogrin S, Shand G, et al. Validation of the PEN-FAST score in a pediatric population. JAMA Netw Open. 2022;5(9):e2233703. doi:10.1001/jamanetworkopen.2022.33703
- Piotin A, Godet J, Trubiano JA, et al. Predictive factors of amoxicillin immediate hypersensitivity and validation of PEN-FAST clinical decision rule. Ann Allergy Asthma Immunol. 2022;128(1):27-32. doi:10.1016/j.anai.2021.07.005
- Su C, Belmont A, Liao J, et al. Evaluating the PEN-FAST clinical decision-making tool to enhance penicillin allergy delabeling. JAMA Intern Med. 2023;183(8):883-885. doi:10.1001/jamainternmed.2023.1572
Proactive Penicillin Allergy Delabeling: Lessons Learned From a Quality Improvement Project
Proactive Penicillin Allergy Delabeling: Lessons Learned From a Quality Improvement Project
Profound Hypoxemia in a Patient With Hypertriglyceridemia-Induced Pancreatitis
Profound Hypoxemia in a Patient With Hypertriglyceridemia-Induced Pancreatitis
Acute pancreatitis can be associated with multiorgan system failure, including respiratory failure, which has a high mortality rate. Acute respiratory distress syndrome (ARDS) is a known complication of severe, acute pancreatitis, and is fatal in up to 40% of cases. Mortality rates exceed 80% in patients with PaO2/FiO2 < 100 mm Hg.2 Although ARDS is typically associated with bilateral pulmonary infiltrates, severe hypoxemia in pancreatitis may not be visible in radiography in up to 50% of cases.1
Hypertriglyceridemia is the third-most common cause of acute pancreatitis, with an incidence of 2% to 10% among patients diagnosed with acute pancreatitis.3.4 Elevated serum triglycerides have been proposed to trigger acute pancreatitis by increasing plasma viscosity, which leads to ischemia and inflammation of the pancreas.4 In severe cases of hypertriglyceridemia-induced acute pancreatitis, plasmapheresis is used to rapidly reduce serum chylomicron and triglyceride levels.3
This case report discusses a patient with acute pancreatitis whose hypoxemia coincided with the severity of hypertriglyceridemia, but without radiographic evidence of pulmonary infiltrates or other known pulmonary causes.
Case Presentation
A 60-year-old male presented to the emergency department with several hours of diffuse abdominal pain, nausea, and vomiting. The patient reported that his symptoms began after eating fried chicken. He reported no dyspnea, fever, chills, or other symptoms. His medical history included type 2 diabetes (hemoglobin A1c, 11.1%), Hashimoto hypothyroidism, severe obstructive sleep apnea not on continuous positive airway pressure (apnea-hypoxia index, 59/h), and obesity (body mass index, 52). Initial vital signs were afebrile, heart rate of 90 beats/min, and oxygen saturation (SpO2) of 85% on 6L oxygen via nasal cannula. He was admitted to the intensive care unit and quickly maximized on high flow nasal cannula, ultimately requiring endotracheal intubation and mechanical ventilation.
Initial laboratory studies were remarkable for serum sodium of 120 mmol/L (reference range, 136-146 mmol/L), creatinine of 1.65 mg/dL (reference range, 0.52-1.28 mg/dL), anion gap of 18 mEq/L (reference range, 3-11 mEq/L), lipase level of 1115 U/L (reference range, 11-82 U/L), glucose level of 334 mg/dL (reference range, 70-110 mg/dL), white blood count of 13.1 K/uL (reference range, 4.5-11.0 K/uL), lactate level of 3.8 mmol/L (reference range, 0.5-2.2 mmol/L), triglyceride level of 1605 mg/dL (reference range, 40-160 mg/dL), cholesterol level of 565 mg/dL (reference range, < 200 mg/dL), aminotransferase of 21 U/L (reference range, 13-36 U/L), alanine aminotransferase of < 3 U/L (reference range, 7-45 U/L), and total bilirubin level of 1.6 mg/dL (reference range, 0.2-1 mg/dL).
The patient had an initial arterial blood gas pH of 7.26, partial pressure of CO2 and O2 of 64.1 mm Hg and 74.1 mm Hg, respectively, on volume control with a tidal volume of 500 mL, positive end-expiratory pressure of 10 cm H2O, respiratory rate of 26 breaths/min, and FiO2 was 100%, which yielded a PaO2/FiO2 of 74 mm Hg. The patient was maintained in steep reverse-Trendelenburg position with moderate improvement in his SpO2.
Chest X-ray and computed tomography angiogram did not reveal pleural effusions, pulmonary infiltrates, or pulmonary embolism (Figure 1). Computed tomography of the abdomen and pelvis demonstrated severe acute interstitial edematous pancreatitis with no evidence of pancreatic necrosis or evidence of gallstones (Figure 2). A transthoracic echocardiogram with bubble was negative for intracardiac right to left shunting.
The leading diagnosis was ARDS secondary to acute pancreatitis with hypoxemia exacerbated by morbid obesity and untreated obstructive sleep apnea leading to hypoventilation.
Treatment
The patient was intubated and restricted to nothing by mouth and provided fluid resuscitation with crystalloids. On hospital day 1, he remained intubated and on mechanical ventilation, started on plasmapheresis and continued insulin infusion for severe hypertriglyceridemia. The patient’s PaO2/FiO2 ratio remained persistently < 100 mm Hg despite maximal ventilatory support. After 3 sessions of plasmapheresis, the serum triglyceride levels and oxygen requirements improved (Figure 3).
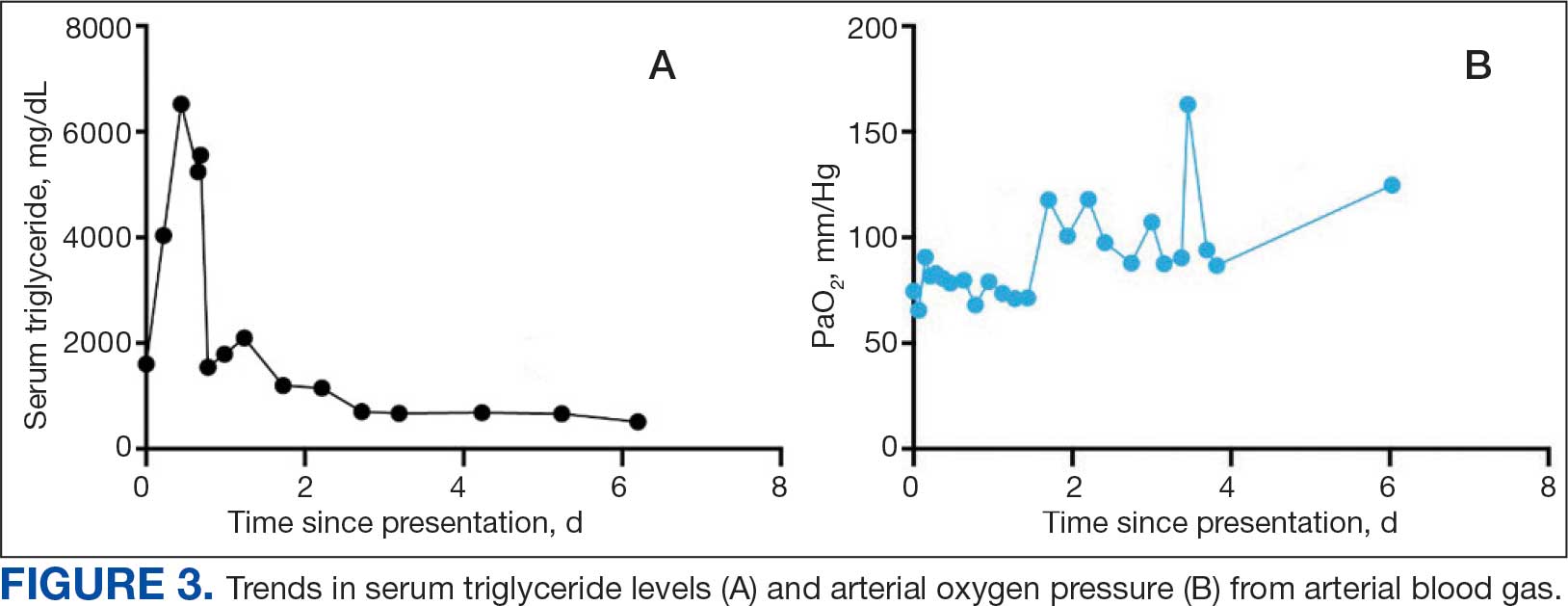
Due to prolonged intubation, the patient ultimately required a tracheostomy. By hospital day 48, the patient was successfully weaned off mechanical ventilation. His tracheostomy was decannulated uneventfully on hospital day 55 and the stoma was closed. The patient was discharged to a skilled nursing home for rehabilitation and received intensive physical therapy for deconditioning from prolonged hospitalization.
Discussion
Respiratory insufficiency is a common and potentially lethal complication observed in one-third of patients with acute pancreatitis.1 Radiographic evidence of pleural effusions, atelectasis and pulmonary infiltrates are often present. Acute lung injury (ALI) and ARDS are the most severe pulmonary complications of acute pancreatitis.5 It has been proposed that ALI and ARDS are driven by a hyperinflammatory state, which has multiple downstream effects. Pulmonary parenchymal and vascular damage has been associated with activated proinflammatory cytokines, trypsin, phospholipase A, and free fatty acids (Figure 4).1
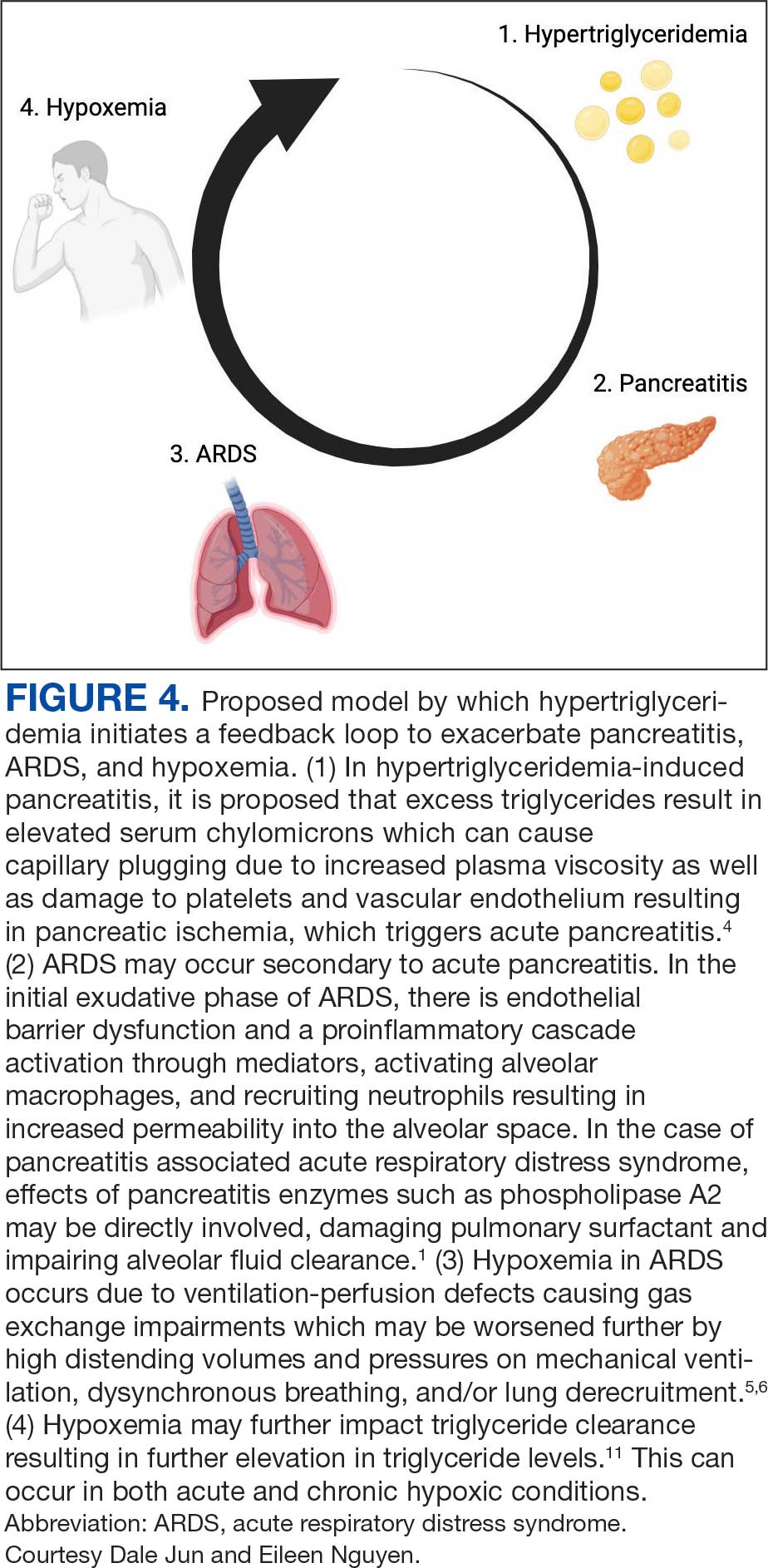
Hypoxemia secondary to acute pancreatitis may occur without initial radiographic findings and has been observed in up to half of patients.1 Hypoxemia in ARDS occurs due to ventilation-perfusion defects causing gas exchange impairments which may be worsened further by high distending volumes and pressures on mechanical ventilation, dyssynchronous breathing, and/or lung derecruitment.6 Patients who require intubation for pancreatitis-associated ALI or ARDS eventually exhibit imaging findings consistent with their disease.1 The patient in this case exhibited severe hypoxemia for several days despite persistently negative radiographic studies. His history of obstructive sleep apnea and a body mass index of 52 may have contributed to respiratory failure; however, assessment of other contributors to the acute and profound hypoxemia yielded largely unremarkable results. The patient did not have a history or evidence of heart failure and his hypoxemia did not improve with diuresis. He tested positive for COVID-19 on admission and was briefly treated with remdesivir and dexamethasone, but it was determined that the test was likely a false positive due to negative subsequent tests and elevated cycle thresholds (> 40). A concomitant COVID-19 infection likely did not contribute to his symptoms.
Ventilation-perfusion mismatch is a well-recognized complication of pancreatitis, which results in right-to-left shunting.5 While we considered whether an intracardiac shunt may have contributed to the patient’s hypoxemia, a transthoracic echocardiogram with bubble contrast was negative.
The patient had a peak serum triglyceride of > 6000 mg/dl, which meets the criteria for very severe hypertriglyceridemia.7 As observed in prior reports, the extent of the hypertriglyceridemia in this patient resulted in pronounced lipemic blood, which was appreciable by the eye and necessitated several rounds of centrifugation to analyze the laboratory studies.8 In this case, plasmapheresis was used to rapidly treat the hypertriglyceridemia, thereby reducing inflammation and further damage to the pancreas.9
It is possible the patient’s hypertriglyceridemia may have been associated with his hypoxemia. His hypoxemia was most pronounced approximately 24 hours postadmission, which coincided with the peak of the hypertriglyceridemia. It remains unclear whether the severity of triglyceride elevation could accurately predict the severity of respiratory insufficiency. Hypoxemia is thought to modulate triglyceride metabolism through stimulation of intracellular lipolysis, upregulation of very low-density lipoproteins production in the liver, and inhibition of triglyceride-rich lipoprotein metabolism.10 Evidence from rodent studies supports the idea that acute hypoxemia increases triglycerides, and the degree of hypoxemia correlates with the elevated triglyceride levels.11 However, this has not been consistently observed in humans and may vary by prandial state.12,13 Thus, dysfunction of lipid metabolism may be a relevant clinical indicator of hypoxemia; further work is needed to elucidate this association.
Patient Perspective
The patient continues to undergo extensive rehabilitation following his prolonged illness and hospitalization. He expressed gratitude for the care received. However, he has limited and distorted recollection of the events during his hospitalization and stated that it felt “like an extraterrestrial state.”
Conclusions
This report describes a case of marked hypoxemia in the setting of acute pancreatitis. Pulmonary insufficiency in acute pancreatitis is commonly associated with imaging findings such as atelectasis, pleural effusions, and pulmonary infiltrates; however, up to half of cases initially lack any radiographic findings. Plasmapheresis is an effective treatment for hypertriglyceridemia-induced pancreatitis to both directly reduce circulating triglycerides and inflammation. Plasmapheresis also represents a promising therapy for the prevention of further episodes of pancreatitis in patients with recurrent pancreatitis. We propose a feedback mechanism through which pancreatitis induces severe hypoxemia, which may modulate lipid metabolism and severe hypertriglyceridemia correlates with respiratory failure.
- Zhou M-T, Chen C-S, Chen B-C, Zhang Q-Y, Andersson R. Acute lung injury and ARDS in acute pancreatitis: mechanisms and potential intervention. World J Gastroenterol. 2010;16(17):2094-2099. doi:10.3748/wjg.v16.i17.2094
- Peek GJ, White S, Scott AD, et al. Severe acute respiratory distress syndrome secondary to acute pancreatitis successfully treated with extracorporeal membrane oxygenation in three patients. Ann Surg. 1998;227(4):572-574. doi:10.1097/00000658-199804000-00020
- Searles GE, Ooi TC. Underrecognition of chylomicronemia as a cause of acute pancreatitis. Can Med Assoc J. 1992;147(12):1806-1808.
- de Pretis N, Amodio A, Frulloni L. Hypertriglyceridemic pancreatitis: Epidemiology, pathophysiology and clinical management. United European Gastroenterol J. 2018;6(5):649-655. doi:10.1177/2050640618755002
- Ranson JH, Turner JW, Roses DF, et al. Respiratory compli cations in acute pancreatitis. Ann Surg. 1974;179(5):557-566. doi:10.1097/00000658-197405000-00006 6. Swenson KE, Swenson ER. Pathophysiology of acute respiratory distress syndrome and COVID-19 lung injury. Crit Care Clin. 2021;37(4):749-776. doi:10.1016/j.ccc.2021.05.003
- Swenson KE, Swenson ER. Pathophysiology of acute respiratory distress syndrome and COVID- 19 lung injury. Crit Care Clin. 2021;37(4):749-776. doi:10.1016/j.ccc.2021.05.003
- Berglund L, Brunzell JD, Goldberg AC, et al. Evaluation and treatment of hypertriglyceridemia: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2012;97(9):2969-2989. doi:10.1210/jc.2011-3213
- Ahern BJ, Yi HJ, Somma CL. Hypertriglyceridemia-induced pancreatitis and a lipemic blood sample: a case report and brief clinical review. J Emerg Nurs. 2022;48(4):455-459. doi:10.1016/j.jen.2022.02.001
- Garg R, Rustagi T. Management of hypertriglyceridemia induced acute pancreatitis. Biomed Res Int. 2018;2018:4721357. doi:10.1155/2018/4721357
- Morin R, Goulet N, Mauger J-F, Imbeault P. Physiological responses to hypoxia on triglyceride levels. Front Physiol. 2021;12:730935. doi:10.3389/fphys.2021.730935
- Jun JC, Shin M-K, Yao Q, et al. Acute hypoxia induces hypertriglyceridemia by decreasing plasma triglyceride clearance in mice. Am J Physiol Endocrinol Metab. 2012;303(3):E377-88. doi:10.1152/ajpendo.00641.2011
- Mahat B, Chassé É, Lindon C, Mauger J-F, Imbeault P. No effect of acute normobaric hypoxia on plasma triglyceride levels in fasting healthy men. Appl Physiol Nutr Metab. 2018;43(7):727-732. doi:10.1139/apnm-2017-0505
- Mauger J-F, Chassé É, Mahat B, Lindon C, Bordenave N, Imbeault P. The effect of acute continuous hypoxia on triglyceride levels in constantly fed healthy men. Front Physiol. 2019;10:752. doi:10.3389/fphys.2019.00752
Acute pancreatitis can be associated with multiorgan system failure, including respiratory failure, which has a high mortality rate. Acute respiratory distress syndrome (ARDS) is a known complication of severe, acute pancreatitis, and is fatal in up to 40% of cases. Mortality rates exceed 80% in patients with PaO2/FiO2 < 100 mm Hg.2 Although ARDS is typically associated with bilateral pulmonary infiltrates, severe hypoxemia in pancreatitis may not be visible in radiography in up to 50% of cases.1
Hypertriglyceridemia is the third-most common cause of acute pancreatitis, with an incidence of 2% to 10% among patients diagnosed with acute pancreatitis.3.4 Elevated serum triglycerides have been proposed to trigger acute pancreatitis by increasing plasma viscosity, which leads to ischemia and inflammation of the pancreas.4 In severe cases of hypertriglyceridemia-induced acute pancreatitis, plasmapheresis is used to rapidly reduce serum chylomicron and triglyceride levels.3
This case report discusses a patient with acute pancreatitis whose hypoxemia coincided with the severity of hypertriglyceridemia, but without radiographic evidence of pulmonary infiltrates or other known pulmonary causes.
Case Presentation
A 60-year-old male presented to the emergency department with several hours of diffuse abdominal pain, nausea, and vomiting. The patient reported that his symptoms began after eating fried chicken. He reported no dyspnea, fever, chills, or other symptoms. His medical history included type 2 diabetes (hemoglobin A1c, 11.1%), Hashimoto hypothyroidism, severe obstructive sleep apnea not on continuous positive airway pressure (apnea-hypoxia index, 59/h), and obesity (body mass index, 52). Initial vital signs were afebrile, heart rate of 90 beats/min, and oxygen saturation (SpO2) of 85% on 6L oxygen via nasal cannula. He was admitted to the intensive care unit and quickly maximized on high flow nasal cannula, ultimately requiring endotracheal intubation and mechanical ventilation.
Initial laboratory studies were remarkable for serum sodium of 120 mmol/L (reference range, 136-146 mmol/L), creatinine of 1.65 mg/dL (reference range, 0.52-1.28 mg/dL), anion gap of 18 mEq/L (reference range, 3-11 mEq/L), lipase level of 1115 U/L (reference range, 11-82 U/L), glucose level of 334 mg/dL (reference range, 70-110 mg/dL), white blood count of 13.1 K/uL (reference range, 4.5-11.0 K/uL), lactate level of 3.8 mmol/L (reference range, 0.5-2.2 mmol/L), triglyceride level of 1605 mg/dL (reference range, 40-160 mg/dL), cholesterol level of 565 mg/dL (reference range, < 200 mg/dL), aminotransferase of 21 U/L (reference range, 13-36 U/L), alanine aminotransferase of < 3 U/L (reference range, 7-45 U/L), and total bilirubin level of 1.6 mg/dL (reference range, 0.2-1 mg/dL).
The patient had an initial arterial blood gas pH of 7.26, partial pressure of CO2 and O2 of 64.1 mm Hg and 74.1 mm Hg, respectively, on volume control with a tidal volume of 500 mL, positive end-expiratory pressure of 10 cm H2O, respiratory rate of 26 breaths/min, and FiO2 was 100%, which yielded a PaO2/FiO2 of 74 mm Hg. The patient was maintained in steep reverse-Trendelenburg position with moderate improvement in his SpO2.
Chest X-ray and computed tomography angiogram did not reveal pleural effusions, pulmonary infiltrates, or pulmonary embolism (Figure 1). Computed tomography of the abdomen and pelvis demonstrated severe acute interstitial edematous pancreatitis with no evidence of pancreatic necrosis or evidence of gallstones (Figure 2). A transthoracic echocardiogram with bubble was negative for intracardiac right to left shunting.


The leading diagnosis was ARDS secondary to acute pancreatitis with hypoxemia exacerbated by morbid obesity and untreated obstructive sleep apnea leading to hypoventilation.
Treatment
The patient was intubated and restricted to nothing by mouth and provided fluid resuscitation with crystalloids. On hospital day 1, he remained intubated and on mechanical ventilation, started on plasmapheresis and continued insulin infusion for severe hypertriglyceridemia. The patient’s PaO2/FiO2 ratio remained persistently < 100 mm Hg despite maximal ventilatory support. After 3 sessions of plasmapheresis, the serum triglyceride levels and oxygen requirements improved (Figure 3).

Due to prolonged intubation, the patient ultimately required a tracheostomy. By hospital day 48, the patient was successfully weaned off mechanical ventilation. His tracheostomy was decannulated uneventfully on hospital day 55 and the stoma was closed. The patient was discharged to a skilled nursing home for rehabilitation and received intensive physical therapy for deconditioning from prolonged hospitalization.
Discussion
Respiratory insufficiency is a common and potentially lethal complication observed in one-third of patients with acute pancreatitis.1 Radiographic evidence of pleural effusions, atelectasis and pulmonary infiltrates are often present. Acute lung injury (ALI) and ARDS are the most severe pulmonary complications of acute pancreatitis.5 It has been proposed that ALI and ARDS are driven by a hyperinflammatory state, which has multiple downstream effects. Pulmonary parenchymal and vascular damage has been associated with activated proinflammatory cytokines, trypsin, phospholipase A, and free fatty acids (Figure 4).1

Hypoxemia secondary to acute pancreatitis may occur without initial radiographic findings and has been observed in up to half of patients.1 Hypoxemia in ARDS occurs due to ventilation-perfusion defects causing gas exchange impairments which may be worsened further by high distending volumes and pressures on mechanical ventilation, dyssynchronous breathing, and/or lung derecruitment.6 Patients who require intubation for pancreatitis-associated ALI or ARDS eventually exhibit imaging findings consistent with their disease.1 The patient in this case exhibited severe hypoxemia for several days despite persistently negative radiographic studies. His history of obstructive sleep apnea and a body mass index of 52 may have contributed to respiratory failure; however, assessment of other contributors to the acute and profound hypoxemia yielded largely unremarkable results. The patient did not have a history or evidence of heart failure and his hypoxemia did not improve with diuresis. He tested positive for COVID-19 on admission and was briefly treated with remdesivir and dexamethasone, but it was determined that the test was likely a false positive due to negative subsequent tests and elevated cycle thresholds (> 40). A concomitant COVID-19 infection likely did not contribute to his symptoms.
Ventilation-perfusion mismatch is a well-recognized complication of pancreatitis, which results in right-to-left shunting.5 While we considered whether an intracardiac shunt may have contributed to the patient’s hypoxemia, a transthoracic echocardiogram with bubble contrast was negative.
The patient had a peak serum triglyceride of > 6000 mg/dl, which meets the criteria for very severe hypertriglyceridemia.7 As observed in prior reports, the extent of the hypertriglyceridemia in this patient resulted in pronounced lipemic blood, which was appreciable by the eye and necessitated several rounds of centrifugation to analyze the laboratory studies.8 In this case, plasmapheresis was used to rapidly treat the hypertriglyceridemia, thereby reducing inflammation and further damage to the pancreas.9
It is possible the patient’s hypertriglyceridemia may have been associated with his hypoxemia. His hypoxemia was most pronounced approximately 24 hours postadmission, which coincided with the peak of the hypertriglyceridemia. It remains unclear whether the severity of triglyceride elevation could accurately predict the severity of respiratory insufficiency. Hypoxemia is thought to modulate triglyceride metabolism through stimulation of intracellular lipolysis, upregulation of very low-density lipoproteins production in the liver, and inhibition of triglyceride-rich lipoprotein metabolism.10 Evidence from rodent studies supports the idea that acute hypoxemia increases triglycerides, and the degree of hypoxemia correlates with the elevated triglyceride levels.11 However, this has not been consistently observed in humans and may vary by prandial state.12,13 Thus, dysfunction of lipid metabolism may be a relevant clinical indicator of hypoxemia; further work is needed to elucidate this association.
Patient Perspective
The patient continues to undergo extensive rehabilitation following his prolonged illness and hospitalization. He expressed gratitude for the care received. However, he has limited and distorted recollection of the events during his hospitalization and stated that it felt “like an extraterrestrial state.”
Conclusions
This report describes a case of marked hypoxemia in the setting of acute pancreatitis. Pulmonary insufficiency in acute pancreatitis is commonly associated with imaging findings such as atelectasis, pleural effusions, and pulmonary infiltrates; however, up to half of cases initially lack any radiographic findings. Plasmapheresis is an effective treatment for hypertriglyceridemia-induced pancreatitis to both directly reduce circulating triglycerides and inflammation. Plasmapheresis also represents a promising therapy for the prevention of further episodes of pancreatitis in patients with recurrent pancreatitis. We propose a feedback mechanism through which pancreatitis induces severe hypoxemia, which may modulate lipid metabolism and severe hypertriglyceridemia correlates with respiratory failure.
Acute pancreatitis can be associated with multiorgan system failure, including respiratory failure, which has a high mortality rate. Acute respiratory distress syndrome (ARDS) is a known complication of severe, acute pancreatitis, and is fatal in up to 40% of cases. Mortality rates exceed 80% in patients with PaO2/FiO2 < 100 mm Hg.2 Although ARDS is typically associated with bilateral pulmonary infiltrates, severe hypoxemia in pancreatitis may not be visible in radiography in up to 50% of cases.1
Hypertriglyceridemia is the third-most common cause of acute pancreatitis, with an incidence of 2% to 10% among patients diagnosed with acute pancreatitis.3.4 Elevated serum triglycerides have been proposed to trigger acute pancreatitis by increasing plasma viscosity, which leads to ischemia and inflammation of the pancreas.4 In severe cases of hypertriglyceridemia-induced acute pancreatitis, plasmapheresis is used to rapidly reduce serum chylomicron and triglyceride levels.3
This case report discusses a patient with acute pancreatitis whose hypoxemia coincided with the severity of hypertriglyceridemia, but without radiographic evidence of pulmonary infiltrates or other known pulmonary causes.
Case Presentation
A 60-year-old male presented to the emergency department with several hours of diffuse abdominal pain, nausea, and vomiting. The patient reported that his symptoms began after eating fried chicken. He reported no dyspnea, fever, chills, or other symptoms. His medical history included type 2 diabetes (hemoglobin A1c, 11.1%), Hashimoto hypothyroidism, severe obstructive sleep apnea not on continuous positive airway pressure (apnea-hypoxia index, 59/h), and obesity (body mass index, 52). Initial vital signs were afebrile, heart rate of 90 beats/min, and oxygen saturation (SpO2) of 85% on 6L oxygen via nasal cannula. He was admitted to the intensive care unit and quickly maximized on high flow nasal cannula, ultimately requiring endotracheal intubation and mechanical ventilation.
Initial laboratory studies were remarkable for serum sodium of 120 mmol/L (reference range, 136-146 mmol/L), creatinine of 1.65 mg/dL (reference range, 0.52-1.28 mg/dL), anion gap of 18 mEq/L (reference range, 3-11 mEq/L), lipase level of 1115 U/L (reference range, 11-82 U/L), glucose level of 334 mg/dL (reference range, 70-110 mg/dL), white blood count of 13.1 K/uL (reference range, 4.5-11.0 K/uL), lactate level of 3.8 mmol/L (reference range, 0.5-2.2 mmol/L), triglyceride level of 1605 mg/dL (reference range, 40-160 mg/dL), cholesterol level of 565 mg/dL (reference range, < 200 mg/dL), aminotransferase of 21 U/L (reference range, 13-36 U/L), alanine aminotransferase of < 3 U/L (reference range, 7-45 U/L), and total bilirubin level of 1.6 mg/dL (reference range, 0.2-1 mg/dL).
The patient had an initial arterial blood gas pH of 7.26, partial pressure of CO2 and O2 of 64.1 mm Hg and 74.1 mm Hg, respectively, on volume control with a tidal volume of 500 mL, positive end-expiratory pressure of 10 cm H2O, respiratory rate of 26 breaths/min, and FiO2 was 100%, which yielded a PaO2/FiO2 of 74 mm Hg. The patient was maintained in steep reverse-Trendelenburg position with moderate improvement in his SpO2.
Chest X-ray and computed tomography angiogram did not reveal pleural effusions, pulmonary infiltrates, or pulmonary embolism (Figure 1). Computed tomography of the abdomen and pelvis demonstrated severe acute interstitial edematous pancreatitis with no evidence of pancreatic necrosis or evidence of gallstones (Figure 2). A transthoracic echocardiogram with bubble was negative for intracardiac right to left shunting.


The leading diagnosis was ARDS secondary to acute pancreatitis with hypoxemia exacerbated by morbid obesity and untreated obstructive sleep apnea leading to hypoventilation.
Treatment
The patient was intubated and restricted to nothing by mouth and provided fluid resuscitation with crystalloids. On hospital day 1, he remained intubated and on mechanical ventilation, started on plasmapheresis and continued insulin infusion for severe hypertriglyceridemia. The patient’s PaO2/FiO2 ratio remained persistently < 100 mm Hg despite maximal ventilatory support. After 3 sessions of plasmapheresis, the serum triglyceride levels and oxygen requirements improved (Figure 3).

Due to prolonged intubation, the patient ultimately required a tracheostomy. By hospital day 48, the patient was successfully weaned off mechanical ventilation. His tracheostomy was decannulated uneventfully on hospital day 55 and the stoma was closed. The patient was discharged to a skilled nursing home for rehabilitation and received intensive physical therapy for deconditioning from prolonged hospitalization.
Discussion
Respiratory insufficiency is a common and potentially lethal complication observed in one-third of patients with acute pancreatitis.1 Radiographic evidence of pleural effusions, atelectasis and pulmonary infiltrates are often present. Acute lung injury (ALI) and ARDS are the most severe pulmonary complications of acute pancreatitis.5 It has been proposed that ALI and ARDS are driven by a hyperinflammatory state, which has multiple downstream effects. Pulmonary parenchymal and vascular damage has been associated with activated proinflammatory cytokines, trypsin, phospholipase A, and free fatty acids (Figure 4).1

Hypoxemia secondary to acute pancreatitis may occur without initial radiographic findings and has been observed in up to half of patients.1 Hypoxemia in ARDS occurs due to ventilation-perfusion defects causing gas exchange impairments which may be worsened further by high distending volumes and pressures on mechanical ventilation, dyssynchronous breathing, and/or lung derecruitment.6 Patients who require intubation for pancreatitis-associated ALI or ARDS eventually exhibit imaging findings consistent with their disease.1 The patient in this case exhibited severe hypoxemia for several days despite persistently negative radiographic studies. His history of obstructive sleep apnea and a body mass index of 52 may have contributed to respiratory failure; however, assessment of other contributors to the acute and profound hypoxemia yielded largely unremarkable results. The patient did not have a history or evidence of heart failure and his hypoxemia did not improve with diuresis. He tested positive for COVID-19 on admission and was briefly treated with remdesivir and dexamethasone, but it was determined that the test was likely a false positive due to negative subsequent tests and elevated cycle thresholds (> 40). A concomitant COVID-19 infection likely did not contribute to his symptoms.
Ventilation-perfusion mismatch is a well-recognized complication of pancreatitis, which results in right-to-left shunting.5 While we considered whether an intracardiac shunt may have contributed to the patient’s hypoxemia, a transthoracic echocardiogram with bubble contrast was negative.
The patient had a peak serum triglyceride of > 6000 mg/dl, which meets the criteria for very severe hypertriglyceridemia.7 As observed in prior reports, the extent of the hypertriglyceridemia in this patient resulted in pronounced lipemic blood, which was appreciable by the eye and necessitated several rounds of centrifugation to analyze the laboratory studies.8 In this case, plasmapheresis was used to rapidly treat the hypertriglyceridemia, thereby reducing inflammation and further damage to the pancreas.9
It is possible the patient’s hypertriglyceridemia may have been associated with his hypoxemia. His hypoxemia was most pronounced approximately 24 hours postadmission, which coincided with the peak of the hypertriglyceridemia. It remains unclear whether the severity of triglyceride elevation could accurately predict the severity of respiratory insufficiency. Hypoxemia is thought to modulate triglyceride metabolism through stimulation of intracellular lipolysis, upregulation of very low-density lipoproteins production in the liver, and inhibition of triglyceride-rich lipoprotein metabolism.10 Evidence from rodent studies supports the idea that acute hypoxemia increases triglycerides, and the degree of hypoxemia correlates with the elevated triglyceride levels.11 However, this has not been consistently observed in humans and may vary by prandial state.12,13 Thus, dysfunction of lipid metabolism may be a relevant clinical indicator of hypoxemia; further work is needed to elucidate this association.
Patient Perspective
The patient continues to undergo extensive rehabilitation following his prolonged illness and hospitalization. He expressed gratitude for the care received. However, he has limited and distorted recollection of the events during his hospitalization and stated that it felt “like an extraterrestrial state.”
Conclusions
This report describes a case of marked hypoxemia in the setting of acute pancreatitis. Pulmonary insufficiency in acute pancreatitis is commonly associated with imaging findings such as atelectasis, pleural effusions, and pulmonary infiltrates; however, up to half of cases initially lack any radiographic findings. Plasmapheresis is an effective treatment for hypertriglyceridemia-induced pancreatitis to both directly reduce circulating triglycerides and inflammation. Plasmapheresis also represents a promising therapy for the prevention of further episodes of pancreatitis in patients with recurrent pancreatitis. We propose a feedback mechanism through which pancreatitis induces severe hypoxemia, which may modulate lipid metabolism and severe hypertriglyceridemia correlates with respiratory failure.
- Zhou M-T, Chen C-S, Chen B-C, Zhang Q-Y, Andersson R. Acute lung injury and ARDS in acute pancreatitis: mechanisms and potential intervention. World J Gastroenterol. 2010;16(17):2094-2099. doi:10.3748/wjg.v16.i17.2094
- Peek GJ, White S, Scott AD, et al. Severe acute respiratory distress syndrome secondary to acute pancreatitis successfully treated with extracorporeal membrane oxygenation in three patients. Ann Surg. 1998;227(4):572-574. doi:10.1097/00000658-199804000-00020
- Searles GE, Ooi TC. Underrecognition of chylomicronemia as a cause of acute pancreatitis. Can Med Assoc J. 1992;147(12):1806-1808.
- de Pretis N, Amodio A, Frulloni L. Hypertriglyceridemic pancreatitis: Epidemiology, pathophysiology and clinical management. United European Gastroenterol J. 2018;6(5):649-655. doi:10.1177/2050640618755002
- Ranson JH, Turner JW, Roses DF, et al. Respiratory compli cations in acute pancreatitis. Ann Surg. 1974;179(5):557-566. doi:10.1097/00000658-197405000-00006 6. Swenson KE, Swenson ER. Pathophysiology of acute respiratory distress syndrome and COVID-19 lung injury. Crit Care Clin. 2021;37(4):749-776. doi:10.1016/j.ccc.2021.05.003
- Swenson KE, Swenson ER. Pathophysiology of acute respiratory distress syndrome and COVID- 19 lung injury. Crit Care Clin. 2021;37(4):749-776. doi:10.1016/j.ccc.2021.05.003
- Berglund L, Brunzell JD, Goldberg AC, et al. Evaluation and treatment of hypertriglyceridemia: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2012;97(9):2969-2989. doi:10.1210/jc.2011-3213
- Ahern BJ, Yi HJ, Somma CL. Hypertriglyceridemia-induced pancreatitis and a lipemic blood sample: a case report and brief clinical review. J Emerg Nurs. 2022;48(4):455-459. doi:10.1016/j.jen.2022.02.001
- Garg R, Rustagi T. Management of hypertriglyceridemia induced acute pancreatitis. Biomed Res Int. 2018;2018:4721357. doi:10.1155/2018/4721357
- Morin R, Goulet N, Mauger J-F, Imbeault P. Physiological responses to hypoxia on triglyceride levels. Front Physiol. 2021;12:730935. doi:10.3389/fphys.2021.730935
- Jun JC, Shin M-K, Yao Q, et al. Acute hypoxia induces hypertriglyceridemia by decreasing plasma triglyceride clearance in mice. Am J Physiol Endocrinol Metab. 2012;303(3):E377-88. doi:10.1152/ajpendo.00641.2011
- Mahat B, Chassé É, Lindon C, Mauger J-F, Imbeault P. No effect of acute normobaric hypoxia on plasma triglyceride levels in fasting healthy men. Appl Physiol Nutr Metab. 2018;43(7):727-732. doi:10.1139/apnm-2017-0505
- Mauger J-F, Chassé É, Mahat B, Lindon C, Bordenave N, Imbeault P. The effect of acute continuous hypoxia on triglyceride levels in constantly fed healthy men. Front Physiol. 2019;10:752. doi:10.3389/fphys.2019.00752
- Zhou M-T, Chen C-S, Chen B-C, Zhang Q-Y, Andersson R. Acute lung injury and ARDS in acute pancreatitis: mechanisms and potential intervention. World J Gastroenterol. 2010;16(17):2094-2099. doi:10.3748/wjg.v16.i17.2094
- Peek GJ, White S, Scott AD, et al. Severe acute respiratory distress syndrome secondary to acute pancreatitis successfully treated with extracorporeal membrane oxygenation in three patients. Ann Surg. 1998;227(4):572-574. doi:10.1097/00000658-199804000-00020
- Searles GE, Ooi TC. Underrecognition of chylomicronemia as a cause of acute pancreatitis. Can Med Assoc J. 1992;147(12):1806-1808.
- de Pretis N, Amodio A, Frulloni L. Hypertriglyceridemic pancreatitis: Epidemiology, pathophysiology and clinical management. United European Gastroenterol J. 2018;6(5):649-655. doi:10.1177/2050640618755002
- Ranson JH, Turner JW, Roses DF, et al. Respiratory compli cations in acute pancreatitis. Ann Surg. 1974;179(5):557-566. doi:10.1097/00000658-197405000-00006 6. Swenson KE, Swenson ER. Pathophysiology of acute respiratory distress syndrome and COVID-19 lung injury. Crit Care Clin. 2021;37(4):749-776. doi:10.1016/j.ccc.2021.05.003
- Swenson KE, Swenson ER. Pathophysiology of acute respiratory distress syndrome and COVID- 19 lung injury. Crit Care Clin. 2021;37(4):749-776. doi:10.1016/j.ccc.2021.05.003
- Berglund L, Brunzell JD, Goldberg AC, et al. Evaluation and treatment of hypertriglyceridemia: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2012;97(9):2969-2989. doi:10.1210/jc.2011-3213
- Ahern BJ, Yi HJ, Somma CL. Hypertriglyceridemia-induced pancreatitis and a lipemic blood sample: a case report and brief clinical review. J Emerg Nurs. 2022;48(4):455-459. doi:10.1016/j.jen.2022.02.001
- Garg R, Rustagi T. Management of hypertriglyceridemia induced acute pancreatitis. Biomed Res Int. 2018;2018:4721357. doi:10.1155/2018/4721357
- Morin R, Goulet N, Mauger J-F, Imbeault P. Physiological responses to hypoxia on triglyceride levels. Front Physiol. 2021;12:730935. doi:10.3389/fphys.2021.730935
- Jun JC, Shin M-K, Yao Q, et al. Acute hypoxia induces hypertriglyceridemia by decreasing plasma triglyceride clearance in mice. Am J Physiol Endocrinol Metab. 2012;303(3):E377-88. doi:10.1152/ajpendo.00641.2011
- Mahat B, Chassé É, Lindon C, Mauger J-F, Imbeault P. No effect of acute normobaric hypoxia on plasma triglyceride levels in fasting healthy men. Appl Physiol Nutr Metab. 2018;43(7):727-732. doi:10.1139/apnm-2017-0505
- Mauger J-F, Chassé É, Mahat B, Lindon C, Bordenave N, Imbeault P. The effect of acute continuous hypoxia on triglyceride levels in constantly fed healthy men. Front Physiol. 2019;10:752. doi:10.3389/fphys.2019.00752
Profound Hypoxemia in a Patient With Hypertriglyceridemia-Induced Pancreatitis
Profound Hypoxemia in a Patient With Hypertriglyceridemia-Induced Pancreatitis
Insights Into Veterans’ Motivations and Hesitancies for COVID-19 Vaccine Uptake: A Mixed-Methods Analysis
Insights Into Veterans’ Motivations and Hesitancies for COVID-19 Vaccine Uptake: A Mixed-Methods Analysis
The SARS-CoV-2 virus has resulted in > 778 million reported COVID-19 cases and > 7 million deaths worldwide. 1 About 70% of the eligible US population has completed a primary COVID-19 vaccination series, yet only 17% have received an updated bivalent booster dose.2 These immunization rates fall below the World Health Organization (WHO) target of 70%.3
Early in the pandemic, US Department of Veterans Affairs (VA) vaccination rates ranged from 46% to 71%.4,5 Ensuring a high level of COVID-19 vaccination in the largest integrated US health care system aligns with the VA priority to provide high-quality, evidence-based care to a patient population that is older and has more comorbidities than the overall US population.6-9
Vaccine hesitancy, defined as a “delay in acceptance or refusal of vaccination despite availability of vaccination service,” is a major contributor to suboptimal vaccination rates.10-13 Previous studies used cluster analyses to identify the unique combinations of behavioral and social factors responsible for COVID-19 vaccine hesitancy.10,11 Lack of perceived vaccine effectiveness and low perceived risk of the health consequences from COVID-19 infection were frequently identified in clusters where patients had the lowest intent for vaccination.10,11 Similarly, low trust in health care practitioners (HCPs), government, and pharmaceutical companies diminished intent for vaccination in these clusters.10 These quantitative studies were limited by their exclusive focus on unvaccinated individuals, reliance on self-reported intent, and lack of assessment of a health care system with a COVID-19 vaccine delivery program designed to overcome barriers to health care access, such as the VA.
Prior qualitative studies of vaccine uptake in distinct veteran subgroups (ie, unhoused and in VA facilities with low vaccination rates) demonstrated that overriding medical priorities among the unhoused and vaccine safety concerns were associated with decreased vaccine uptake, and positive perceptions of HCPs and the health care system were associated with increased vaccine uptake.11,12 However, these studies were conducted during periods of greater COVID-19 vaccine availability and acceptance, and prior to booster recommendations.4,12,13
This mixed-methods quality improvement (QI) project assessed the barriers and facilitators of COVID-19 vaccination among veterans receiving primary care at a single VA health care facility. We assessed whether unique patient clusters could be identified based on COVID-19–related and vaccine-related thoughts and feelings and whether cluster membership was associated with COVID-19 vaccination. This analysis also explored how individuals’ beliefs and trust shaped motivations and hesitancies for vaccine uptake in quantitatively derived clusters with varying vaccination rates.
Methods
This QI project was conducted at the VA Pittsburgh Healthcare System (VAPHS), a tertiary care facility serving > 75,000 veterans in Pennsylvania, West Virginia, and Ohio. The VAPHS Institutional Review Board determined this QI study was exempt from review.14-17 Participation was voluntary and had no bearing on VA health care or benefits. Financial support for the project, including key personnel and participant compensation, was provided by VAPHS. We followed the STROBE reporting guideline for cross-sectional studies and the COREQ checklist for qualitative research.18,19
Quantitative Survey
The 32,271 veterans assigned to a VAPHS primary care HCP, effective April 1, 2020, were eligible. To ensure representation of subgroups underrecognized in research and/or QI projects, the sample included all 1980 female patients at VAPHS and a random sample of 500 White and 500 Hispanic and/or non-White men within 4 age categories (< 50, 50-64, 65-84, and > 84 years). For the < 50 years or > 84 years categories, all Hispanic and/or non-White men were included due to small sample sizes.20-22 The nonrandom sampling frame comprised 1708 Hispanic and/or non-White men and 2000 White men. After assigning the 5688 potentially eligible individuals a unique identifier, 31 opted out, resulting in a final sample of 5657 individuals.
The 5657 individuals received a letter requesting their completion of a future questionnaire about COVID-19 infection and vaccines. An electronic Qualtrics questionnaire link was emailed to 3221 individuals; nonresponders received 2 follow-up email reminders. For the 2436 veterans without an email address on file, trained interviewers conducted phone surveys and entered responses. Those patients who completed the questionnaire could enter a drawing to win 1 of 100 cash prizes valued at $100. We collected questionnaire data from July to September 2021.
Questionnaire Items
We constructed a 60-item questionnaire based on prior research on COVID-19 vaccine hesitancy and the WHO Guidebook for Immunization Programs and Implementing Partners.4,23-25 The WHO Guidebook comprises survey items organized within 4 domains reflecting the behavioral and social determinants of vaccination: thoughts and feelings; social processes; motivation and hesitancy; and practical factors.23
Sociodemographic, clinical, and personal characteristics. The survey assessed respondent ethnicity and race and used these data to create a composite race and ethnicity variable. Highest educational level was also attained using 8 response options. The survey also assessed prior COVID-19 infection; prior receipt of vaccines for influenza, pneumonia, tetanus, or shingles; and presence of comorbidities that increase the risk of severe COVID-19 infection. We used administrative data from the VA Corporate Data Warehouse to determine respondent age, sex, geographic residence (urban, rural), and to fill in missing self-reported data on sex (n = 4) and ethnicity and race (n = 12). The survey assessed political views using a 5-point Likert scale (1, very liberal; 5, very conservative) and was collapsed into 3 categories (ie, very conservative or conservative, moderate, very liberal or liberal), with prefer not to answer reported separately
COVID-19 infection and vaccine. We asked veterans if they had ever been infected with COVID-19, whether they had been offered and/or received a COVID-19 vaccine, and type (Pfizer, Moderna, or Johnson & Johnson), and number of doses received. Positive vaccination status was defined as the receipt of ≥ 1 dose of a COVID-19 vaccine approved by the US Food and Drug Administration.
COVID-19 opinions. Respondents were asked about perceived risk of COVID-19 infection and related health outcomes, as well as beliefs about COVID-19 vaccines, using a 4-point Likert scale for all items: (1, not at all concerned; 4, very concerned). Respondents were asked about concerns related to COVID-19 infection and severe illness. They also were asked about vaccine-related short-term adverse effects (AEs) and long-term complications. Respondents were asked how effective they believed COVID-19 vaccines were at preventing infection, serious illness, or death. Unvaccinated and vaccinated veterans were asked similar items, with a qualifier of “before getting vaccinated…” for those who were vaccinated.
Social processes. Respondents were asked to rate their level of trust in various sources of COVID-19 vaccine information using a 4-point Likert scale (1, trust not at all; 4, trust very much). Respondents were asked whether community or religious leaders or close family or friends wanted them to get vaccinated (yes, no, or unsure).
Practical factors. Respondents were asked to rate the logistical difficulty of getting vaccinated or trying to get vaccinated using a 4-point Likert scale (1, not at all; 4, extremely).
Participants
Respondents were asked to participate in a follow-up qualitative interview. Among 293 participants who agreed, we sampled all 86 unvaccinated individuals regardless of cluster assignment, a random sample of 88 individuals in the cluster with the lowest vaccination rate, and all 33 vaccinated individuals in the cluster with the second-lowest vaccination rate. Forty-nine veterans completed qualitative interviews.
Two research staff trained in qualitative research completed telephone interviews, averaging 16.5 minutes (March to May 2022), using semistructured scripts to elicit vaccine-related motivations, hesitancies, or concerns. Interviews were recorded, transcribed, and deidentified. Participants provided written consent for recording and received $50 cash-equivalent compensation for interview completion.
Qualitative Interview Script
The interview script consisted of open-ended questions related to vaccine uptake across WHO domains.23 Both unvaccinated and vaccinated respondents were asked similar questions and customized questions about boosters for the vaccinated subgroup. To assess motivations and hesitancies, respondents were asked how they made their decisions about vaccination and what they considered when deciding. Vaccinated participants were asked about motivations and overcoming concerns. Unvaccinated respondents were asked about reasons for concern. To assess social processes, the interviewers asked participants whose opinion or counsel they trusted when deciding whether to get vaccinated. Questions also focused on positive experiences and vaccination barriers. Vaccinated participants were asked what could have improved their vaccination experiences. Finally, the interviewers asked participants who received a complete primary vaccine series about their motivations and plans related to booster vaccines, and whether information about emerging COVID-19 variants influenced their decisions.
Data Analyses
This analysis used X2 and Fisher exact tests to assess the associations among respondent characteristics, questionnaire responses, vaccination status, and cluster membership. Items phrased similarly were handled in a similar fashion for vaccinated and unvaccinated respondents.
Cluster analysis assessed the possible groupings in responses to the quantitative questionnaire items focused on thoughts and feelings about COVID-19 infection risk and severity, vaccine effectiveness, and vaccine safety. This analysis treated the items’ ordinal response categories as continuous. We performed factor analysis using principal component analysis to explore dimension reduction and account for covariance between items. Two principal components were calculated and applied k-means clustering, determining the number of clusters through agreement from the elbow, gap statistic, and silhouette methods.26 Each cluster was named based on its unique pattern of responses to the items used to define them (eAppendix 1).
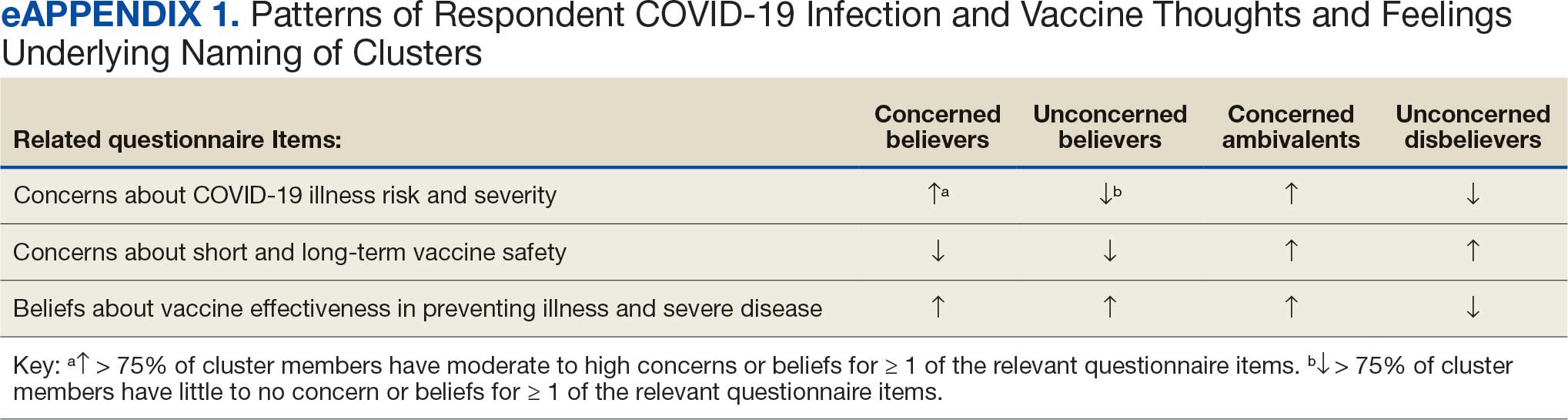
Multivariable logistic regression analyses assessed the independent association between cluster membership as the independent measure and vaccination status as the dependent measure, adjusting for respondent sociodemographic and personal characteristics and 2 measures of trust (ie, local VA HCP and the CDC). We selected these trust measures because they represent objective sources of medical information and were independently associated with COVID-19 vaccination status in a logistic regression model comprising all 6 trust items assessed.
This study defined statistical significance as a 2-tailed P value < .05. SAS 9.4 was used for all statistical analyses and Python 3.7.4 and the Scikit-learn package for cluster analyses.27 For qualitative analyses, this study used an inductive thematic approach guided by conventional qualitative content analysis, NVivo 12 Plus for Windows to code and analyze interview transcripts.28,29 We created an initial codebook based on 10 transcripts that were selected for high complexity and represented cluster membership and vaccination status.30,31 After 2 qualitative staff developed the initial codebook, 11 of 49 (22%) transcripts were independently coded by a primary and secondary coder to ensure consistent code application. Both coders reviewed the cocoded transcripts and resolved all discrepancies through negotiated consensus.32 After the cocoding process was complete, the primary coder coded the remaining transcripts. The primary and secondary coder met as needed to review and discuss any questions that arose during the primary coder’s work.
Results
Of 5657 eligible participants, 1208 (21.4%) completed a questionnaire. Overall, 674 (55.8%) were aged < 65 years, 530 (43.9%) were women, 828 (68.5%) were non-Hispanic White, 303 (25.1%) were Black, and 47 (3.9%) were Hispanic, and 1034 (85.6%) were vaccinated (Table 1). Compared to the total sampled population, respondents were more often older, female, and White (eAppendix 2).
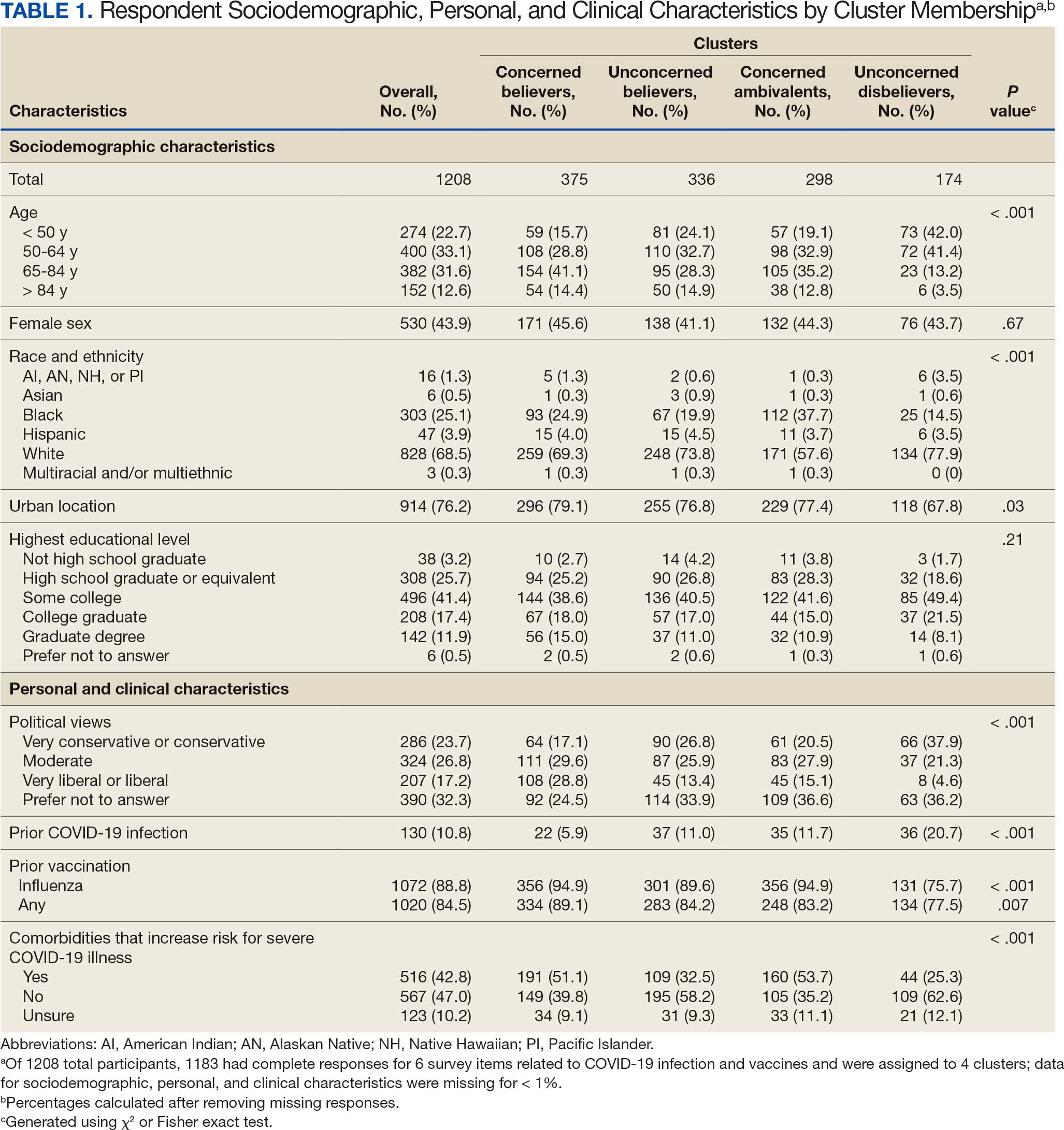
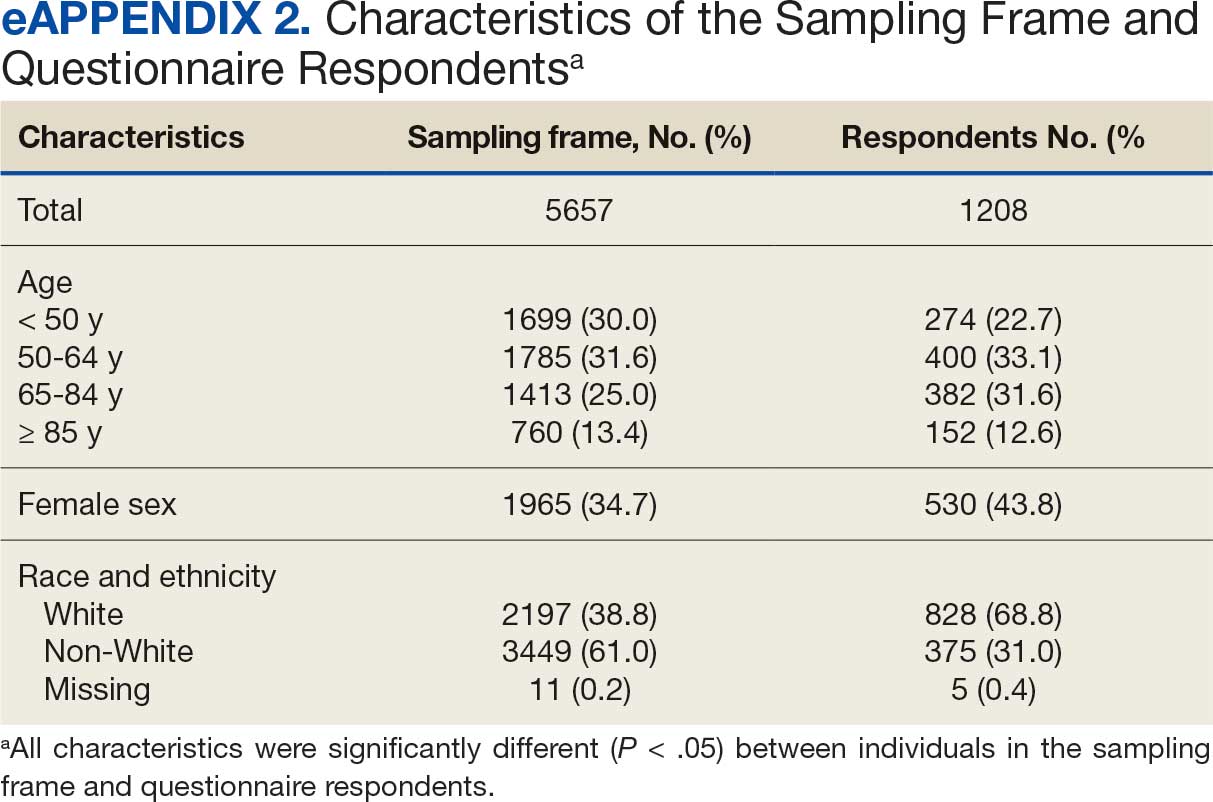
Cluster Membership
Four clusters were identified from 1183 (97.9%) participants who provided complete responses to 6 items assessing thoughts and feelings about COVID-19 infection and vaccines (Table 2). Of the 1183 respondents, 375 (31.7%) were Concerned Believers (cluster 1), 336 (28.4%) were Unconcerned Believers (cluster 2), 298 (25.2%) were Concerned Ambivalents (cluster 3), and 174 (14.7%) were Unconcerned Disbelievers (cluster 4). The Concerned Believers were moderately/ very concerned about COVID-19 infection (96.0%) and becoming very ill from infection (94.6%), believed the vaccine was moderately/very effective in preventing COVID-19 infection (100%) and severe illness or death from infection (98.7%), and had slight concern about short-term AEs (92.6%) or long-term complications (92.0%) from the vaccine. The Unconcerned Believers had no/slight concern about COVID-19 infection (76.5%) or becoming very ill (79.2%), believed the vaccine was effective in preventing infection (82.4%) and severe illness and death (83.6%), and had no/slight concern about short-term AEs (94.0%) or long-term complications (87.2%) from the vaccine. The Concerned Ambivalents were moderately/ very concerned about COVID-19 infection (94.3%) and becoming very ill (93.6%), believed the vaccine was moderately/very effective in preventing infection (86.6%) and severe illness or death (86.9%), and were moderately/very concerned about short-term AEs (81.9%) or long-term complications (89.3%) from the vaccine. The Unconcerned Disbelievers had no/slight concern about COVID-19 infection (90.8%) and becoming very ill (88.6%), believed the vaccine was not at all/slightly effective in preventing infection (90.3%) and severe illness or death (87.4%), and were moderately/very concerned about short-term AEs (52.8%) or long-term complications (75.9%) from the vaccine.
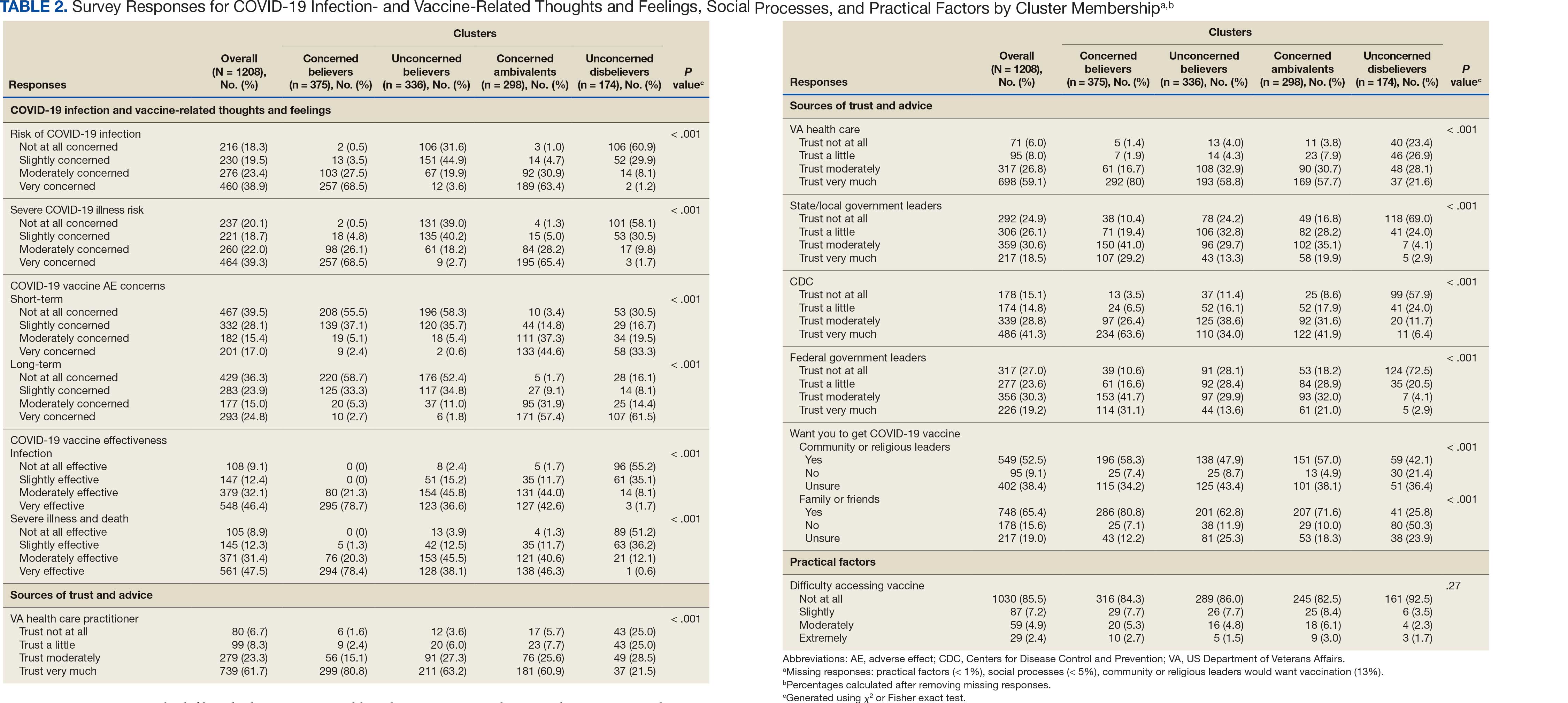
Cluster Membership
Respondent age, race and ethnicity, and political viewpoints differed significantly by cluster (P < .001). Compared with the other clusters, the Concerned Believer cluster was older (55.5% age ≥ 65 years vs 16.7%-48.0%) and more frequently reported liberal political views (28.8% vs 4.6%-15.1%). In contrast, the Unconcerned Disbeliever cluster was younger (83.4% age ≤ 64 years vs 44.5%-56.8%) and more frequently reported conservative political views (37.9% vs 17.1%-26.8%) than the other clusters. Whereas the Concerned Ambivalent cluster had the highest proportion of Black (37.7%) and the lowest proportion of White respondents (57.6%), the Unconcerned Disbelievers cluster had the lowest proportion of Black respondents (14.5%) and the highest proportion of White respondents (77.9%). The Unconcerned Disbelievers cluster were significantly less likely to trust COVID-19 vaccine information from any source and to believe those close to them wanted them to get vaccinated.
Association of Cluster Membership and COVID-19 Vaccination
COVID-19 vaccination rates varied more than 3-fold (P < .001) by cluster, with 29.9% of Unconcerned Disbelievers, 93.3% of Concerned Ambivalents, 93.5% of Unconcerned Believers, and 98.9% of Concerned Believers reporting being vaccinated. (Figure). Cluster membership was independently associated with vaccination, with adjusted odds ratios (AORs) of 12.0 (95% CI, 6.1-23.8) for the Concerned Ambivalent, 13.0 (95% CI, 6.9-24.5) for Unconcerned Believer, and 48.6 (95% CI, 15.5-152.1) for Concerned Believer clusters (Table 3). Respondent trust in COVID-19 vaccine information from their VA HCP (AOR 2.1; 95% CI, 1.6-2.8) and the CDC (AOR 1.6; 95% CI, 1.2-2.1) were independently associated with vaccination status, while the remaining respondent sociodemographic or personal characteristics were not.
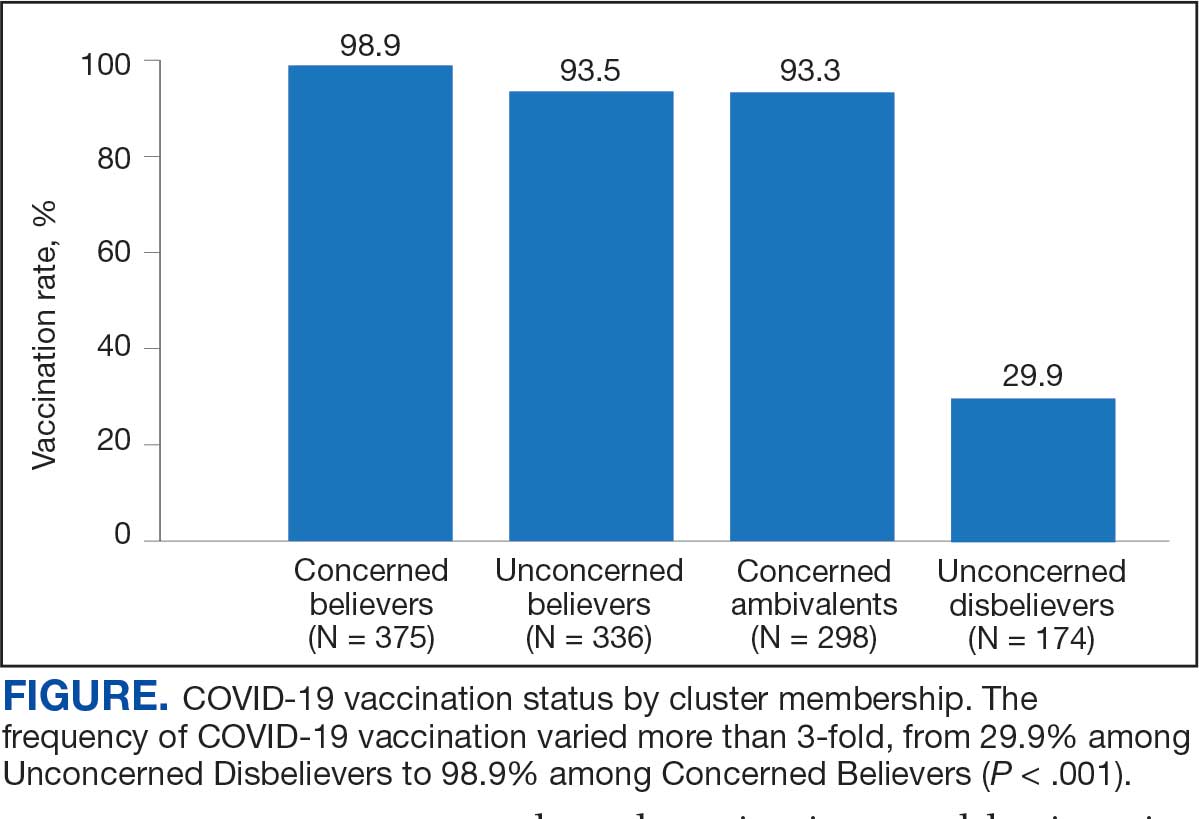
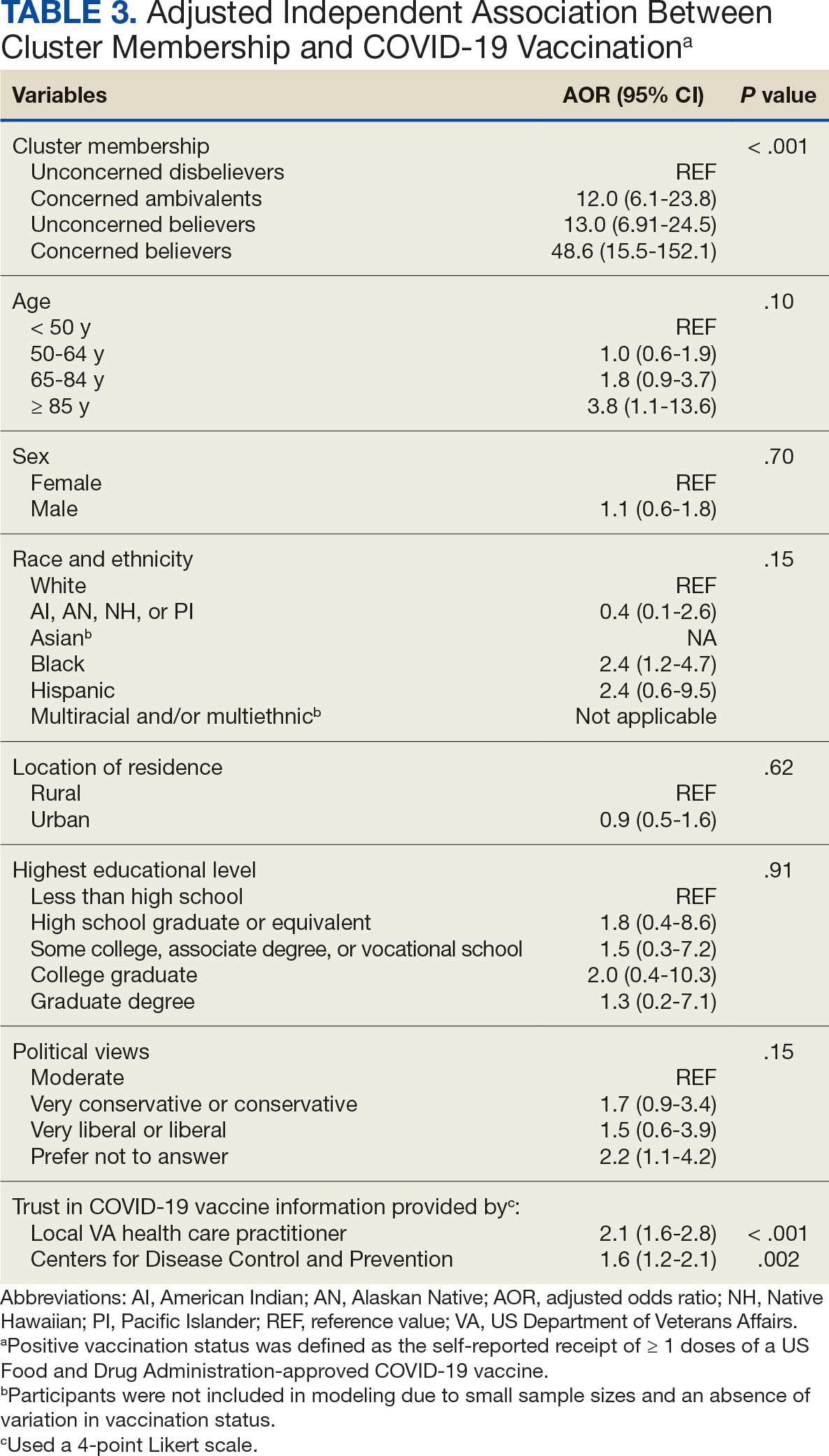
Qualitative Interview Participants
A 49-participant convenience sample completed interviews, including 30 Concerned Ambivalent, 17 Unconcerned Disbeliever, and 2 Unconcerned Believer respondents cluster. The data were not calculated for Unconcerned Believers due to the small sample size. Interview participants were more likely to be younger, female, non-Hispanic, White, less educated, and more politically conservative than the questionnaire respondents as a whole (Appendix). The vaccination rate for the interview participants was 73.5%, ranging from 29.9% in the Unconcerned Disbeliever to 93.3% in the Concerned Ambivalent cluster. Qualitative themes and participant quotes for Concerned Ambivalent and Unconcerned Disbeliever respondents are in eAppendix 3.
Motivations. Wanting personal protection from becoming infected or severely ill from COVID-19 (63.8%), caregiver wanting to protect others (17.0%), and employment vaccine requirements (14.9%) were frequent motivations for vaccination. Whereas personal protection (90.0%) and protection of others (23.3%) were identified more frequently in the Concerned Ambivalents cluster, employment vaccine requirements (35.3%) were more frequently identified in the Unconcerned Disbelievers cluster.
Hesitancies or concerns. Lack of sufficient information related to rapid vaccine development (55.3%), vaccine AEs (38.3%), and low confidence in vaccine efficacy (23.4%) were frequent concerns or hesitancies about vaccination. Unconcerned Disbelievers expressed higher levels of concern about the vaccine’s rapid development (82.4%), low perceived vaccine efficacy (47.1%), and a lack of trust in governmental vaccine promotion (23.5%) than did the Concerned Ambivalents.
Overcoming concerns. Not wanting to get sick or die from infection coupled with an understanding that vaccine benefits exceed risks (23.4%) and receiving information from a trusted source (10.6%) were common ways of overcoming concerns for vaccination. Although the Unconcerned Disbelievers infrequently identified reasons for overcoming concerns, they identified employment requirements (17.6%) as a reason for vaccination despite concerns. They also identified seeing others with positive vaccine experiences and pressure from family or friends as ways of overcoming concerns (11.8% each).
Social influences. Family members or partners (38.3%), personal opinions (38.3%), and HCPs (23.4%) were frequent social influences for vaccination. Concerned Ambivalents mentioned family members and partners (46.7%), HCPs (26.7%), and friends (20.0%) as common influences, while Unconcerned Disbelievers more frequently relied on their opinion (41.2%) and quoted specific scientifically reputable data sources (17.6%) to guide vaccine decision-making, although it is unclear whether these sources were accessed directly or if this information was obtained indirectly through scientifically unvetted data platforms.
Practical factors. Most participants had positive vaccination experiences (68.1%), determined mainly by the Concerned Ambivalents (90.0%), who were more highly vaccinated. Barriers to vaccination were reported by 9 (19.1%) participants, driven by those in the Concerned Ambivalent cluster (26.7%). Eight (17.0%) participants suggested improvements for vaccination processes, with similar overall reporting frequencies across clusters.
COVID-19 boosters and variants. Wanting continued protection from COVID-19 (36.2%), recommendations from a doctor or trusted source (17.0%), and news about emerging variants (10.6%) were frequent motivations for receiving a vaccine booster (eAppendix 4). These motivations were largely driven by the Concerned Ambivalents, of whom 25 of 30 were booster eligible and 24 received a booster dose. Belief that boosters were unnecessary (8.5%), concerns about efficacy (6.4%), and concerns about AEs (6.4%) were frequently identified hesitancies. These concerns were expressed largely by the Unconcerned Disbelievers, of whom 7 of 17 were booster dose eligible, but only 1 received a dose.
Evolving knowledge about variants was not a major concern overall and did not change existing opinions about the vaccine (36.2%). Concerned Ambivalents believed vaccination provided extra protection against variants (36.7%) and the emergence of variants served as a reminder of the ongoing pandemic (30.0%). In contrast, Unconcerned Disbelievers believed that the threat of variants was overblown (35.3%) and mutations are to be expected (17.6%).
Discussion
This study used a complementary mixed-methods approach to understand the motivations, hesitancies, and social and practical drivers of COVID-19 vaccine uptake among VA beneficiaries. Our quantitative analyses identified 4 distinct clusters based on respondents’ opinions on COVID-19 infection severity and vaccine effectiveness and safety. Veterans in 3 clusters were 12 to 49 times more likely to be vaccinated than those in the remaining cluster, even when controlling for baseline respondent characteristics and level of trust in credible sources of COVID-19 information. The observed vaccination rate of nearly 86% was higher than the contemporaneous national average of 62% for vaccine-eligible individuals, likely reflecting the comprehensive VA vaccine promotion strategies tailored to a patient demographic with a high COVID-19 risk profile.2,10
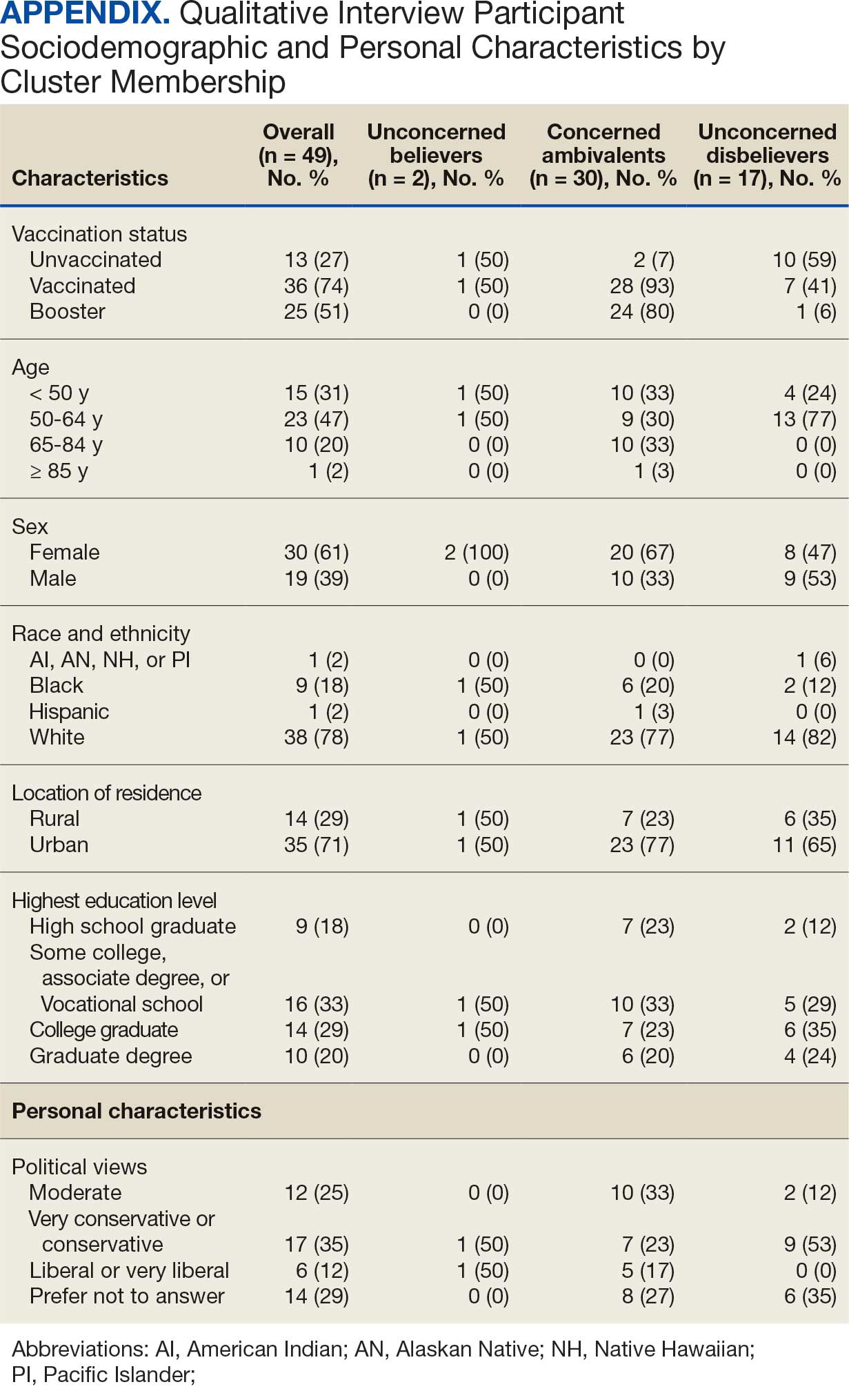
This cluster analyses demonstrated the importance of thoughts and feelings about COVID-19 infection and vaccination as influential social and behavioral drivers of vaccine uptake. These opinions help explain the strong association between cluster membership and vaccination status in this multivariable modeling. The cluster composition was consistent with findings from studies of nonveteran populations that identified perceived vulnerability to COVID-19 infection, beliefs in vaccine effectiveness, and adherence with protective behaviors during the pandemic as contributors to vaccine uptake.13,33 Qualitative themes showed that personal protection, protecting others, and vaccine mandates were frequent motivators for vaccination. Whereas protection of self and others from COVID-19 infection were more often expressed by the highly vaccinated Concerned Ambivalents, employment and travel vaccine mandates were more often identified by Unconcerned Disbelievers, who had a lower vaccination rate. Among Unconcerned Disbelievers, an employer vaccine requirement was the most frequent qualitative theme for overcoming vaccination concerns.
In addition to cluster membership, our modeling showed that trust in local VA HCPs and the CDC were independently associated with COVID-19 vaccination, which has been found in prior research.20 This qualitative analyses regarding vaccine hesitancy identified trust-related concerns that were more frequently expressed by Unconcerned Disbelievers than Concerned Ambivalents. Concerns included the rapid development of the vaccines potentially limiting the generation of scientifically sound effectiveness and safety data, and potential biases involving the entities promoting vaccine uptake.
Whereas the Concerned Believers, Unconcerned Believers, and Concerned Ambivalents all had high COVID-19 vaccination rates (≥ 93%), the decision-making pathways to vaccine uptake likely differ by their concerns about COVID-19 infection and perceptions of vaccine safety and effectiveness. For example, this mixed-methods analysis consistently showed that people in the Concerned Ambivalent cluster were positively motivated by concerns about COVID-19 infection and severity and beliefs about vaccine effectiveness that were tempered by concerns about vaccine AEs. For this cluster, their frequent thematic expression that the benefits of the vaccine exceed the risks, and the positive social influences of family, friends, and HCPs may explain their high vaccination rate.
Such insights into how the patterns of COVID-19–related thoughts and feelings vary across clusters can be used to design interventions to encourage initial and booster doses of COVID-19 vaccines. For example, messaging that highlights the infectivity and severity of COVID-19 and the potential for persistent negative health outcomes associated with long COVID could reinforce the beliefs of Concerned Believers and Concerned Ambivalents, and such messaging could also be used as a targeted intervention for Unconcerned Believers who expressed fewer concerns about the health consequences of COVID-19.23 Likewise, messaging about the safety profile of COVID-19 vaccines may reduce vaccine hesitancy for Concerned Ambivalents. Importantly, purposeful attention to health equity, community engagement, and involvement of racially diverse HCPs in patient discussions represent successful strategies to increase COVID-19 vaccine uptake among Black individuals, who were disproportionately represented in the Concerned Ambivalent cluster and may possess higher levels of mistrust due to racism experienced within the health care system.24
Our findings suggest that the greatest challenge for overcoming vaccine hesitancy is for individuals in the suboptimally vaccinated (30%) Unconcerned Disbeliever cluster. These individuals had low levels of concern about COVID-19 infection and severity, high levels of concern about vaccine safety, low perceived vaccine effectiveness, and low levels of trust in all information sources about COVID-19. While the Unconcerned Disbelievers cited scientifically reputable data sources, we were unable to verify whether participants accessed these reputable sources of information directly or obtained such information indirectly through potentially biased online sources. Nearly half of this cluster trusted their VA HCP and believed their community or religious leaders would want them to get vaccinated. This qualitative analyses found that Unconcerned Disbelievers relied on personal beliefs for vaccine decision-making more than Concerned Ambivalents. While Unconcerned Disbelievers were less likely to be socially influenced by family, friends, or religious leaders, they still acknowledged some impact from these sources. These findings suggest that addressing vaccine hesitancy among Unconcerned Disbelievers may require a multifaceted approach that respects their reliance on personal research while also leveraging the potential social influences. This approach supports the promising, previously reported practices of harnessing the social influences of HCPs and other community and religious leaders to promote vaccine uptake among Unconcerned Disbelievers.34,35 One evidence-based approach to effectively change patient health care behaviors is through motivational interviewing strategies that use open-ended questions, nonjudgmental interactions, and collaborative decision-making when discussing the risks and benefits of vaccination.21,22
Limitations
This study was conducted at a single VA health care facility and our sampling technique was nonrandom, suggesting that these results may not be generalizable to all veterans or non-VA patient populations. The 21% questionnaire response rate could have introduced selection bias into the respondent sample. All questionnaire data were self-reported, including vaccination status. Finally, the qualitative interviews consisted of a small number of unvaccinated individuals in 2 clusters (ie, Concerned Ambivalents and Unconcerned Disbelievers) and may not have reached thematic saturation in these subgroups.
Conclusions
Quantitative analyses identified 4 clusters based on individual thoughts and feelings about COVID-19 infection and vaccines. Cluster membership and levels of trust in COVID-19 information sources were independently associated with vaccination. Understanding the quantitative patterns of thoughts and beliefs across clusters, enriched by common qualitative themes for vaccine hesitancy, help inform tailored interventions to augment COVID-19 vaccine uptake and highlight the importance of targeted, trust-based communication and culturally sensitive interventions to enhance vaccine uptake across diverse populations.
- World Health Organization. WHO COVID-19 dashboard. Accessed July 18, 2025. https://covid19.who.int/
- Centers for Disease Control and Prevention. COVIDVax- View: Weekly COVID-19 Vaccination Coverage and Intent among Adults. Accessed June 10, 2025. https://www.cdc.gov/covidvaxview/weekly-dashboard/adult-vaccination-coverage.html
- World Health Organization. Strategy to achieve global Covid-19 vaccination by mid-2022. 2021. Accessed April 30, 2025. https://cdn.who.int/media/docs/default-source/immunization/covid-19/strategy-to-achieve-global-covid-19-vaccination-by-mid-2022.pdf
- Jasuja GK, Meterko M, Bradshaw LD, et al. Attitudes and intentions of US veterans regarding COVID-19 vaccination. JAMA Netw Open. 2021;4(11):e2132548. doi:10.1001/jamanetworkopen.2021.32548
- Der-Martirosian C, Steers WN, Northcraft H, Chu K, Dobalian A. Vaccinating veterans for COVID-19 at the U.S. Department of Veterans Affairs. Am J Prev Med. 2022;62(6):e317-e324. doi:10.1016/j.amepre.2021.12.016
- Bloeser K, Lipkowitz-Eaton J. Disproportionate multimorbidity among veterans in middle age. J Public Health (Oxf). 2022;44(1):28-35. doi:10.1093/pubmed/fdab149
- US Department of Veterans Affairs. National Center for Veterans Analysis and Statistics: veteran population. Updated March 26, 2025. Accessed April 30, 2025. https://www.va.gov/vetdata/Veteran_Population.asp
- Olenick M, Flowers M, Diaz VJ. US veterans and their unique issues: enhancing health care professional awareness. Adv Med Educ Pract. 2015;6:635-639. doi:10.2147/AMEP.S89479
- Orkaby AR, Nussbaum L, Ho YL, et al. The burden of frailty among U.S. veterans and its association with mortality, 2002-2012. J Gerontol A Biol Sci Med Sci. 2019;74(8):1257-1264. doi:10.1093/gerona/gly232
- Bass SB, Kelly PJ, Hoadley A, Arroyo Lloret A, Organtini T. Mapping perceptual differences to understand COVID-19 beliefs in those with vaccine hesitancy. J Health Commun. 2022;27(1):49-61. doi:10.1080/10810730.2022.2042627
- Meng L, Masters NB, Lu PJ, et al. Cluster analysis of adults unvaccinated for COVID-19 based on behavioral and social factors, National Immunization Survey-Adult COVID Module, United States. Prev Med. 2023;167:107415. doi:10.1016/j.ypmed.2022.107415
- Gin JL, Balut MD, Dobalian A. COVID-19 vaccination uptake and receptivity among veterans enrolled in homelessness- tailored primary health care clinics: provider trust vs. misinformation. BMC Prim Care. 2024;25(1):24. doi:10.1186/s12875-023-02251-x
- Wilson GM, Ray CE, Kale IO, et al. Age and beliefs about vaccines associated with COVID-19 vaccination among US veterans. Antimicrob Steward Healthc Epidemiol. 2023;3(1):e184. doi:10.1017/ash.2023.446
- VA Pittsburgh Healthcare System (VAPHS). Human Research Protection Program (HRPP) policy for quality assurance/ quality improvement projects. Policy H-013. December 31, 2021. Accessed April 30, 2025. https://www.va.gov/files/2020-11/H-013_QAQI%20Project_revised_updated%20format_clean_508.pdf
- Burkitt KH, Rodriguez KL, Mor MK, et al. Evaluation of a collaborative VA network initiative to reduce racial disparities in blood pressure control among veterans with severe hypertension. Healthc (Amst). 2021;8(suppl 1):100485. doi:10.1016/j.hjdsi.2020.100485
- Sinkowitz-Cochran RL, Burkitt KH, Cuerdon T, et al. The associations between organizational culture and knowledge, attitudes, and practices in a multicenter Veterans Affairs quality improvement initiative to prevent methicillin-resistant Staphylococcus aureus. Am J Infect Control. 2012;40(2):138-143. doi:10.1016/j.ajic.2011.04.332
- Burkitt KH, Sinkowitz-Cochran RL, Obrosky DS, et al. Survey of employee knowledge and attitudes before and after a multicenter Veterans’ Administration quality improvement initiative to reduce nosocomial methicillin-resistant Staphylococcus aureus infections. Am J Infect Control. 2010;38(4):274-282. doi:10.1016/j.ajic.2009.08.019
- STROBE - strengthening the reporting of observational studies in epidemiology. What is STROBE? Accessed April 30, 2025. https://www.strobe-statement.org/
- Tong A, Sainsbury P, Craig J. Consolidated criteria for reporting qualitative research (COREQ): a 32-item checklist for interviews and focus groups. Int J Qual Health Care. 2007;19(6):349-357. doi:10.1093/intqhc/mzm042
- Ward RE, Nguyen XT, Li Y, et al; on behalf of the VA Million Veteran Program. Racial and ethnic disparities in U.S. veteran health characteristics. Int J Environ Res Public Health. 2021;18(5):2411. doi:10.3390/ijerph18052411
- Harrington KM, Nguyen XT, Song RJ, et al; VA Million Veteran Program. Gender differences in demographic and health characteristics of the Million Veteran Program cohort. Womens Health Issues. 2019;29(suppl 1):S56-S66. doi:10.1016/j.whi.2019.04.012
- Washington DL, ed. National Veteran Health Equity Report 2021. Focus on Veterans Health Administration Patient Experience and Health Care Quality. VHA Office of Health Equity; September 2022. Accessed April 30, 2025. https://www.va.gov/healthequity/nvher.asp
- World Health Organization. Data for action: achieving high uptake of COVID-19 vaccines. April 1, 2021. Accessed April 30, 2025. https://www.who.int/publications/i/item/WHO-2019-nCoV-vaccination-demand-planning-2021.1
- Hoffman BL, Boness CL, Chu KH, et al. COVID- 19 vaccine hesitancy, acceptance, and promotion among healthcare workers: a mixed-methods analysis. J Community Health. 2022;47(5):750-758. doi:10.1007/s10900-022-01095-3
- Vasudevan L, Bruening R, Hung A, et al. COVID- 19 vaccination intention and activation among health care system employees: a mixed methods study. Vaccine. 2022;40(35):5141-5152. doi:10.1016/j.vaccine.2022.07.010
- Tibshirani R, Walther G, Hastie T. Estimating the number of clusters in a data set via the gap statistic. J R Stat Soc Series B Stat Methodol. 2001;63(2):411-423. doi:10.1111/1467-9868.00293
- Pedregosa FP, Varoquaux G, Gramfort A, et al. Scikitlearn: machine learning in Python. J Mach Learn Res. 2011;12:2825-2830.
- Proudfoot K. Inductive/deductive hybrid thematic analysis in mixed methods research. J Mix Methods Res. 2022;17(3): 308-326. doi:10.1177/15586898221126816
- Chapman AL, Hadfield M, Chapman CJ. Qualitative research in healthcare: an introduction to grounded theory using thematic analysis. J R Coll Physicians Edinb. 2015;45(3):201-205. doi:10.4997/jrcpe.2015.305
- Grandheim UH, Lundman B. Qualitative content analysis in nursing research: concepts, procedures and measures to achieve trustworthiness. Nurse Educ Today. 2004;24(2):105-112. doi:10.1016/j.nedt.2003.1001
- Sandelowski M. Whatever happened to qualitative description? Res Nurs Health. 2000;23(4):334-340. doi:10.1002/1098-240x(200008)23:4<334::aid-nur9 >3.0.co;2-g
- Garrison DR, Cleveland-Innes M, Koole M, Kappelman J. Revisiting methodological issues in transcript analysis: negotiated coding and reliability. Internet High Educ. 2006;9(1):1-8. doi:10.1016/j.iheduc.2005.11.001
- Wagner AL, Porth JM, Wu Z, Boulton ML, Finlay JM, Kobayashi LC. Vaccine hesitancy during the COVID-19 pandemic: a latent class analysis of middle-aged and older US adults. J Community Health. 2022;47(3):408- 415. doi:10.1007/s10900-022-01064-w
- Syed U, Kapera O, Chandrasekhar A, et al. The role of faith-based organizations in improving vaccination confidence & addressing vaccination disparities to help improve vaccine uptake: a systematic review. Vaccines (Basel). 2023;11(2):449. doi:10.3390/vaccines11020449
- Evans D, Norrbom C, Schmidt S, Powell R, McReynolds J, Sidibe T. Engaging community-based organizations to address barriers in public health programs: lessons learned from COVID-19 vaccine acceptance programs in diverse rural communities. Health Secur. 2023;21(S1):S17-S24. doi:10.1089/hs.2023.0017
The SARS-CoV-2 virus has resulted in > 778 million reported COVID-19 cases and > 7 million deaths worldwide. 1 About 70% of the eligible US population has completed a primary COVID-19 vaccination series, yet only 17% have received an updated bivalent booster dose.2 These immunization rates fall below the World Health Organization (WHO) target of 70%.3
Early in the pandemic, US Department of Veterans Affairs (VA) vaccination rates ranged from 46% to 71%.4,5 Ensuring a high level of COVID-19 vaccination in the largest integrated US health care system aligns with the VA priority to provide high-quality, evidence-based care to a patient population that is older and has more comorbidities than the overall US population.6-9
Vaccine hesitancy, defined as a “delay in acceptance or refusal of vaccination despite availability of vaccination service,” is a major contributor to suboptimal vaccination rates.10-13 Previous studies used cluster analyses to identify the unique combinations of behavioral and social factors responsible for COVID-19 vaccine hesitancy.10,11 Lack of perceived vaccine effectiveness and low perceived risk of the health consequences from COVID-19 infection were frequently identified in clusters where patients had the lowest intent for vaccination.10,11 Similarly, low trust in health care practitioners (HCPs), government, and pharmaceutical companies diminished intent for vaccination in these clusters.10 These quantitative studies were limited by their exclusive focus on unvaccinated individuals, reliance on self-reported intent, and lack of assessment of a health care system with a COVID-19 vaccine delivery program designed to overcome barriers to health care access, such as the VA.
Prior qualitative studies of vaccine uptake in distinct veteran subgroups (ie, unhoused and in VA facilities with low vaccination rates) demonstrated that overriding medical priorities among the unhoused and vaccine safety concerns were associated with decreased vaccine uptake, and positive perceptions of HCPs and the health care system were associated with increased vaccine uptake.11,12 However, these studies were conducted during periods of greater COVID-19 vaccine availability and acceptance, and prior to booster recommendations.4,12,13
This mixed-methods quality improvement (QI) project assessed the barriers and facilitators of COVID-19 vaccination among veterans receiving primary care at a single VA health care facility. We assessed whether unique patient clusters could be identified based on COVID-19–related and vaccine-related thoughts and feelings and whether cluster membership was associated with COVID-19 vaccination. This analysis also explored how individuals’ beliefs and trust shaped motivations and hesitancies for vaccine uptake in quantitatively derived clusters with varying vaccination rates.
Methods
This QI project was conducted at the VA Pittsburgh Healthcare System (VAPHS), a tertiary care facility serving > 75,000 veterans in Pennsylvania, West Virginia, and Ohio. The VAPHS Institutional Review Board determined this QI study was exempt from review.14-17 Participation was voluntary and had no bearing on VA health care or benefits. Financial support for the project, including key personnel and participant compensation, was provided by VAPHS. We followed the STROBE reporting guideline for cross-sectional studies and the COREQ checklist for qualitative research.18,19
Quantitative Survey
The 32,271 veterans assigned to a VAPHS primary care HCP, effective April 1, 2020, were eligible. To ensure representation of subgroups underrecognized in research and/or QI projects, the sample included all 1980 female patients at VAPHS and a random sample of 500 White and 500 Hispanic and/or non-White men within 4 age categories (< 50, 50-64, 65-84, and > 84 years). For the < 50 years or > 84 years categories, all Hispanic and/or non-White men were included due to small sample sizes.20-22 The nonrandom sampling frame comprised 1708 Hispanic and/or non-White men and 2000 White men. After assigning the 5688 potentially eligible individuals a unique identifier, 31 opted out, resulting in a final sample of 5657 individuals.
The 5657 individuals received a letter requesting their completion of a future questionnaire about COVID-19 infection and vaccines. An electronic Qualtrics questionnaire link was emailed to 3221 individuals; nonresponders received 2 follow-up email reminders. For the 2436 veterans without an email address on file, trained interviewers conducted phone surveys and entered responses. Those patients who completed the questionnaire could enter a drawing to win 1 of 100 cash prizes valued at $100. We collected questionnaire data from July to September 2021.
Questionnaire Items
We constructed a 60-item questionnaire based on prior research on COVID-19 vaccine hesitancy and the WHO Guidebook for Immunization Programs and Implementing Partners.4,23-25 The WHO Guidebook comprises survey items organized within 4 domains reflecting the behavioral and social determinants of vaccination: thoughts and feelings; social processes; motivation and hesitancy; and practical factors.23
Sociodemographic, clinical, and personal characteristics. The survey assessed respondent ethnicity and race and used these data to create a composite race and ethnicity variable. Highest educational level was also attained using 8 response options. The survey also assessed prior COVID-19 infection; prior receipt of vaccines for influenza, pneumonia, tetanus, or shingles; and presence of comorbidities that increase the risk of severe COVID-19 infection. We used administrative data from the VA Corporate Data Warehouse to determine respondent age, sex, geographic residence (urban, rural), and to fill in missing self-reported data on sex (n = 4) and ethnicity and race (n = 12). The survey assessed political views using a 5-point Likert scale (1, very liberal; 5, very conservative) and was collapsed into 3 categories (ie, very conservative or conservative, moderate, very liberal or liberal), with prefer not to answer reported separately
COVID-19 infection and vaccine. We asked veterans if they had ever been infected with COVID-19, whether they had been offered and/or received a COVID-19 vaccine, and type (Pfizer, Moderna, or Johnson & Johnson), and number of doses received. Positive vaccination status was defined as the receipt of ≥ 1 dose of a COVID-19 vaccine approved by the US Food and Drug Administration.
COVID-19 opinions. Respondents were asked about perceived risk of COVID-19 infection and related health outcomes, as well as beliefs about COVID-19 vaccines, using a 4-point Likert scale for all items: (1, not at all concerned; 4, very concerned). Respondents were asked about concerns related to COVID-19 infection and severe illness. They also were asked about vaccine-related short-term adverse effects (AEs) and long-term complications. Respondents were asked how effective they believed COVID-19 vaccines were at preventing infection, serious illness, or death. Unvaccinated and vaccinated veterans were asked similar items, with a qualifier of “before getting vaccinated…” for those who were vaccinated.
Social processes. Respondents were asked to rate their level of trust in various sources of COVID-19 vaccine information using a 4-point Likert scale (1, trust not at all; 4, trust very much). Respondents were asked whether community or religious leaders or close family or friends wanted them to get vaccinated (yes, no, or unsure).
Practical factors. Respondents were asked to rate the logistical difficulty of getting vaccinated or trying to get vaccinated using a 4-point Likert scale (1, not at all; 4, extremely).
Participants
Respondents were asked to participate in a follow-up qualitative interview. Among 293 participants who agreed, we sampled all 86 unvaccinated individuals regardless of cluster assignment, a random sample of 88 individuals in the cluster with the lowest vaccination rate, and all 33 vaccinated individuals in the cluster with the second-lowest vaccination rate. Forty-nine veterans completed qualitative interviews.
Two research staff trained in qualitative research completed telephone interviews, averaging 16.5 minutes (March to May 2022), using semistructured scripts to elicit vaccine-related motivations, hesitancies, or concerns. Interviews were recorded, transcribed, and deidentified. Participants provided written consent for recording and received $50 cash-equivalent compensation for interview completion.
Qualitative Interview Script
The interview script consisted of open-ended questions related to vaccine uptake across WHO domains.23 Both unvaccinated and vaccinated respondents were asked similar questions and customized questions about boosters for the vaccinated subgroup. To assess motivations and hesitancies, respondents were asked how they made their decisions about vaccination and what they considered when deciding. Vaccinated participants were asked about motivations and overcoming concerns. Unvaccinated respondents were asked about reasons for concern. To assess social processes, the interviewers asked participants whose opinion or counsel they trusted when deciding whether to get vaccinated. Questions also focused on positive experiences and vaccination barriers. Vaccinated participants were asked what could have improved their vaccination experiences. Finally, the interviewers asked participants who received a complete primary vaccine series about their motivations and plans related to booster vaccines, and whether information about emerging COVID-19 variants influenced their decisions.
Data Analyses
This analysis used X2 and Fisher exact tests to assess the associations among respondent characteristics, questionnaire responses, vaccination status, and cluster membership. Items phrased similarly were handled in a similar fashion for vaccinated and unvaccinated respondents.
Cluster analysis assessed the possible groupings in responses to the quantitative questionnaire items focused on thoughts and feelings about COVID-19 infection risk and severity, vaccine effectiveness, and vaccine safety. This analysis treated the items’ ordinal response categories as continuous. We performed factor analysis using principal component analysis to explore dimension reduction and account for covariance between items. Two principal components were calculated and applied k-means clustering, determining the number of clusters through agreement from the elbow, gap statistic, and silhouette methods.26 Each cluster was named based on its unique pattern of responses to the items used to define them (eAppendix 1).

Multivariable logistic regression analyses assessed the independent association between cluster membership as the independent measure and vaccination status as the dependent measure, adjusting for respondent sociodemographic and personal characteristics and 2 measures of trust (ie, local VA HCP and the CDC). We selected these trust measures because they represent objective sources of medical information and were independently associated with COVID-19 vaccination status in a logistic regression model comprising all 6 trust items assessed.
This study defined statistical significance as a 2-tailed P value < .05. SAS 9.4 was used for all statistical analyses and Python 3.7.4 and the Scikit-learn package for cluster analyses.27 For qualitative analyses, this study used an inductive thematic approach guided by conventional qualitative content analysis, NVivo 12 Plus for Windows to code and analyze interview transcripts.28,29 We created an initial codebook based on 10 transcripts that were selected for high complexity and represented cluster membership and vaccination status.30,31 After 2 qualitative staff developed the initial codebook, 11 of 49 (22%) transcripts were independently coded by a primary and secondary coder to ensure consistent code application. Both coders reviewed the cocoded transcripts and resolved all discrepancies through negotiated consensus.32 After the cocoding process was complete, the primary coder coded the remaining transcripts. The primary and secondary coder met as needed to review and discuss any questions that arose during the primary coder’s work.
Results
Of 5657 eligible participants, 1208 (21.4%) completed a questionnaire. Overall, 674 (55.8%) were aged < 65 years, 530 (43.9%) were women, 828 (68.5%) were non-Hispanic White, 303 (25.1%) were Black, and 47 (3.9%) were Hispanic, and 1034 (85.6%) were vaccinated (Table 1). Compared to the total sampled population, respondents were more often older, female, and White (eAppendix 2).


Cluster Membership
Four clusters were identified from 1183 (97.9%) participants who provided complete responses to 6 items assessing thoughts and feelings about COVID-19 infection and vaccines (Table 2). Of the 1183 respondents, 375 (31.7%) were Concerned Believers (cluster 1), 336 (28.4%) were Unconcerned Believers (cluster 2), 298 (25.2%) were Concerned Ambivalents (cluster 3), and 174 (14.7%) were Unconcerned Disbelievers (cluster 4). The Concerned Believers were moderately/ very concerned about COVID-19 infection (96.0%) and becoming very ill from infection (94.6%), believed the vaccine was moderately/very effective in preventing COVID-19 infection (100%) and severe illness or death from infection (98.7%), and had slight concern about short-term AEs (92.6%) or long-term complications (92.0%) from the vaccine. The Unconcerned Believers had no/slight concern about COVID-19 infection (76.5%) or becoming very ill (79.2%), believed the vaccine was effective in preventing infection (82.4%) and severe illness and death (83.6%), and had no/slight concern about short-term AEs (94.0%) or long-term complications (87.2%) from the vaccine. The Concerned Ambivalents were moderately/ very concerned about COVID-19 infection (94.3%) and becoming very ill (93.6%), believed the vaccine was moderately/very effective in preventing infection (86.6%) and severe illness or death (86.9%), and were moderately/very concerned about short-term AEs (81.9%) or long-term complications (89.3%) from the vaccine. The Unconcerned Disbelievers had no/slight concern about COVID-19 infection (90.8%) and becoming very ill (88.6%), believed the vaccine was not at all/slightly effective in preventing infection (90.3%) and severe illness or death (87.4%), and were moderately/very concerned about short-term AEs (52.8%) or long-term complications (75.9%) from the vaccine.

Cluster Membership
Respondent age, race and ethnicity, and political viewpoints differed significantly by cluster (P < .001). Compared with the other clusters, the Concerned Believer cluster was older (55.5% age ≥ 65 years vs 16.7%-48.0%) and more frequently reported liberal political views (28.8% vs 4.6%-15.1%). In contrast, the Unconcerned Disbeliever cluster was younger (83.4% age ≤ 64 years vs 44.5%-56.8%) and more frequently reported conservative political views (37.9% vs 17.1%-26.8%) than the other clusters. Whereas the Concerned Ambivalent cluster had the highest proportion of Black (37.7%) and the lowest proportion of White respondents (57.6%), the Unconcerned Disbelievers cluster had the lowest proportion of Black respondents (14.5%) and the highest proportion of White respondents (77.9%). The Unconcerned Disbelievers cluster were significantly less likely to trust COVID-19 vaccine information from any source and to believe those close to them wanted them to get vaccinated.
Association of Cluster Membership and COVID-19 Vaccination
COVID-19 vaccination rates varied more than 3-fold (P < .001) by cluster, with 29.9% of Unconcerned Disbelievers, 93.3% of Concerned Ambivalents, 93.5% of Unconcerned Believers, and 98.9% of Concerned Believers reporting being vaccinated. (Figure). Cluster membership was independently associated with vaccination, with adjusted odds ratios (AORs) of 12.0 (95% CI, 6.1-23.8) for the Concerned Ambivalent, 13.0 (95% CI, 6.9-24.5) for Unconcerned Believer, and 48.6 (95% CI, 15.5-152.1) for Concerned Believer clusters (Table 3). Respondent trust in COVID-19 vaccine information from their VA HCP (AOR 2.1; 95% CI, 1.6-2.8) and the CDC (AOR 1.6; 95% CI, 1.2-2.1) were independently associated with vaccination status, while the remaining respondent sociodemographic or personal characteristics were not.


Qualitative Interview Participants
A 49-participant convenience sample completed interviews, including 30 Concerned Ambivalent, 17 Unconcerned Disbeliever, and 2 Unconcerned Believer respondents cluster. The data were not calculated for Unconcerned Believers due to the small sample size. Interview participants were more likely to be younger, female, non-Hispanic, White, less educated, and more politically conservative than the questionnaire respondents as a whole (Appendix). The vaccination rate for the interview participants was 73.5%, ranging from 29.9% in the Unconcerned Disbeliever to 93.3% in the Concerned Ambivalent cluster. Qualitative themes and participant quotes for Concerned Ambivalent and Unconcerned Disbeliever respondents are in eAppendix 3.
Motivations. Wanting personal protection from becoming infected or severely ill from COVID-19 (63.8%), caregiver wanting to protect others (17.0%), and employment vaccine requirements (14.9%) were frequent motivations for vaccination. Whereas personal protection (90.0%) and protection of others (23.3%) were identified more frequently in the Concerned Ambivalents cluster, employment vaccine requirements (35.3%) were more frequently identified in the Unconcerned Disbelievers cluster.
Hesitancies or concerns. Lack of sufficient information related to rapid vaccine development (55.3%), vaccine AEs (38.3%), and low confidence in vaccine efficacy (23.4%) were frequent concerns or hesitancies about vaccination. Unconcerned Disbelievers expressed higher levels of concern about the vaccine’s rapid development (82.4%), low perceived vaccine efficacy (47.1%), and a lack of trust in governmental vaccine promotion (23.5%) than did the Concerned Ambivalents.
Overcoming concerns. Not wanting to get sick or die from infection coupled with an understanding that vaccine benefits exceed risks (23.4%) and receiving information from a trusted source (10.6%) were common ways of overcoming concerns for vaccination. Although the Unconcerned Disbelievers infrequently identified reasons for overcoming concerns, they identified employment requirements (17.6%) as a reason for vaccination despite concerns. They also identified seeing others with positive vaccine experiences and pressure from family or friends as ways of overcoming concerns (11.8% each).
Social influences. Family members or partners (38.3%), personal opinions (38.3%), and HCPs (23.4%) were frequent social influences for vaccination. Concerned Ambivalents mentioned family members and partners (46.7%), HCPs (26.7%), and friends (20.0%) as common influences, while Unconcerned Disbelievers more frequently relied on their opinion (41.2%) and quoted specific scientifically reputable data sources (17.6%) to guide vaccine decision-making, although it is unclear whether these sources were accessed directly or if this information was obtained indirectly through scientifically unvetted data platforms.
Practical factors. Most participants had positive vaccination experiences (68.1%), determined mainly by the Concerned Ambivalents (90.0%), who were more highly vaccinated. Barriers to vaccination were reported by 9 (19.1%) participants, driven by those in the Concerned Ambivalent cluster (26.7%). Eight (17.0%) participants suggested improvements for vaccination processes, with similar overall reporting frequencies across clusters.
COVID-19 boosters and variants. Wanting continued protection from COVID-19 (36.2%), recommendations from a doctor or trusted source (17.0%), and news about emerging variants (10.6%) were frequent motivations for receiving a vaccine booster (eAppendix 4). These motivations were largely driven by the Concerned Ambivalents, of whom 25 of 30 were booster eligible and 24 received a booster dose. Belief that boosters were unnecessary (8.5%), concerns about efficacy (6.4%), and concerns about AEs (6.4%) were frequently identified hesitancies. These concerns were expressed largely by the Unconcerned Disbelievers, of whom 7 of 17 were booster dose eligible, but only 1 received a dose.
Evolving knowledge about variants was not a major concern overall and did not change existing opinions about the vaccine (36.2%). Concerned Ambivalents believed vaccination provided extra protection against variants (36.7%) and the emergence of variants served as a reminder of the ongoing pandemic (30.0%). In contrast, Unconcerned Disbelievers believed that the threat of variants was overblown (35.3%) and mutations are to be expected (17.6%).
Discussion
This study used a complementary mixed-methods approach to understand the motivations, hesitancies, and social and practical drivers of COVID-19 vaccine uptake among VA beneficiaries. Our quantitative analyses identified 4 distinct clusters based on respondents’ opinions on COVID-19 infection severity and vaccine effectiveness and safety. Veterans in 3 clusters were 12 to 49 times more likely to be vaccinated than those in the remaining cluster, even when controlling for baseline respondent characteristics and level of trust in credible sources of COVID-19 information. The observed vaccination rate of nearly 86% was higher than the contemporaneous national average of 62% for vaccine-eligible individuals, likely reflecting the comprehensive VA vaccine promotion strategies tailored to a patient demographic with a high COVID-19 risk profile.2,10

This cluster analyses demonstrated the importance of thoughts and feelings about COVID-19 infection and vaccination as influential social and behavioral drivers of vaccine uptake. These opinions help explain the strong association between cluster membership and vaccination status in this multivariable modeling. The cluster composition was consistent with findings from studies of nonveteran populations that identified perceived vulnerability to COVID-19 infection, beliefs in vaccine effectiveness, and adherence with protective behaviors during the pandemic as contributors to vaccine uptake.13,33 Qualitative themes showed that personal protection, protecting others, and vaccine mandates were frequent motivators for vaccination. Whereas protection of self and others from COVID-19 infection were more often expressed by the highly vaccinated Concerned Ambivalents, employment and travel vaccine mandates were more often identified by Unconcerned Disbelievers, who had a lower vaccination rate. Among Unconcerned Disbelievers, an employer vaccine requirement was the most frequent qualitative theme for overcoming vaccination concerns.
In addition to cluster membership, our modeling showed that trust in local VA HCPs and the CDC were independently associated with COVID-19 vaccination, which has been found in prior research.20 This qualitative analyses regarding vaccine hesitancy identified trust-related concerns that were more frequently expressed by Unconcerned Disbelievers than Concerned Ambivalents. Concerns included the rapid development of the vaccines potentially limiting the generation of scientifically sound effectiveness and safety data, and potential biases involving the entities promoting vaccine uptake.
Whereas the Concerned Believers, Unconcerned Believers, and Concerned Ambivalents all had high COVID-19 vaccination rates (≥ 93%), the decision-making pathways to vaccine uptake likely differ by their concerns about COVID-19 infection and perceptions of vaccine safety and effectiveness. For example, this mixed-methods analysis consistently showed that people in the Concerned Ambivalent cluster were positively motivated by concerns about COVID-19 infection and severity and beliefs about vaccine effectiveness that were tempered by concerns about vaccine AEs. For this cluster, their frequent thematic expression that the benefits of the vaccine exceed the risks, and the positive social influences of family, friends, and HCPs may explain their high vaccination rate.
Such insights into how the patterns of COVID-19–related thoughts and feelings vary across clusters can be used to design interventions to encourage initial and booster doses of COVID-19 vaccines. For example, messaging that highlights the infectivity and severity of COVID-19 and the potential for persistent negative health outcomes associated with long COVID could reinforce the beliefs of Concerned Believers and Concerned Ambivalents, and such messaging could also be used as a targeted intervention for Unconcerned Believers who expressed fewer concerns about the health consequences of COVID-19.23 Likewise, messaging about the safety profile of COVID-19 vaccines may reduce vaccine hesitancy for Concerned Ambivalents. Importantly, purposeful attention to health equity, community engagement, and involvement of racially diverse HCPs in patient discussions represent successful strategies to increase COVID-19 vaccine uptake among Black individuals, who were disproportionately represented in the Concerned Ambivalent cluster and may possess higher levels of mistrust due to racism experienced within the health care system.24
Our findings suggest that the greatest challenge for overcoming vaccine hesitancy is for individuals in the suboptimally vaccinated (30%) Unconcerned Disbeliever cluster. These individuals had low levels of concern about COVID-19 infection and severity, high levels of concern about vaccine safety, low perceived vaccine effectiveness, and low levels of trust in all information sources about COVID-19. While the Unconcerned Disbelievers cited scientifically reputable data sources, we were unable to verify whether participants accessed these reputable sources of information directly or obtained such information indirectly through potentially biased online sources. Nearly half of this cluster trusted their VA HCP and believed their community or religious leaders would want them to get vaccinated. This qualitative analyses found that Unconcerned Disbelievers relied on personal beliefs for vaccine decision-making more than Concerned Ambivalents. While Unconcerned Disbelievers were less likely to be socially influenced by family, friends, or religious leaders, they still acknowledged some impact from these sources. These findings suggest that addressing vaccine hesitancy among Unconcerned Disbelievers may require a multifaceted approach that respects their reliance on personal research while also leveraging the potential social influences. This approach supports the promising, previously reported practices of harnessing the social influences of HCPs and other community and religious leaders to promote vaccine uptake among Unconcerned Disbelievers.34,35 One evidence-based approach to effectively change patient health care behaviors is through motivational interviewing strategies that use open-ended questions, nonjudgmental interactions, and collaborative decision-making when discussing the risks and benefits of vaccination.21,22
Limitations
This study was conducted at a single VA health care facility and our sampling technique was nonrandom, suggesting that these results may not be generalizable to all veterans or non-VA patient populations. The 21% questionnaire response rate could have introduced selection bias into the respondent sample. All questionnaire data were self-reported, including vaccination status. Finally, the qualitative interviews consisted of a small number of unvaccinated individuals in 2 clusters (ie, Concerned Ambivalents and Unconcerned Disbelievers) and may not have reached thematic saturation in these subgroups.
Conclusions
Quantitative analyses identified 4 clusters based on individual thoughts and feelings about COVID-19 infection and vaccines. Cluster membership and levels of trust in COVID-19 information sources were independently associated with vaccination. Understanding the quantitative patterns of thoughts and beliefs across clusters, enriched by common qualitative themes for vaccine hesitancy, help inform tailored interventions to augment COVID-19 vaccine uptake and highlight the importance of targeted, trust-based communication and culturally sensitive interventions to enhance vaccine uptake across diverse populations.
The SARS-CoV-2 virus has resulted in > 778 million reported COVID-19 cases and > 7 million deaths worldwide. 1 About 70% of the eligible US population has completed a primary COVID-19 vaccination series, yet only 17% have received an updated bivalent booster dose.2 These immunization rates fall below the World Health Organization (WHO) target of 70%.3
Early in the pandemic, US Department of Veterans Affairs (VA) vaccination rates ranged from 46% to 71%.4,5 Ensuring a high level of COVID-19 vaccination in the largest integrated US health care system aligns with the VA priority to provide high-quality, evidence-based care to a patient population that is older and has more comorbidities than the overall US population.6-9
Vaccine hesitancy, defined as a “delay in acceptance or refusal of vaccination despite availability of vaccination service,” is a major contributor to suboptimal vaccination rates.10-13 Previous studies used cluster analyses to identify the unique combinations of behavioral and social factors responsible for COVID-19 vaccine hesitancy.10,11 Lack of perceived vaccine effectiveness and low perceived risk of the health consequences from COVID-19 infection were frequently identified in clusters where patients had the lowest intent for vaccination.10,11 Similarly, low trust in health care practitioners (HCPs), government, and pharmaceutical companies diminished intent for vaccination in these clusters.10 These quantitative studies were limited by their exclusive focus on unvaccinated individuals, reliance on self-reported intent, and lack of assessment of a health care system with a COVID-19 vaccine delivery program designed to overcome barriers to health care access, such as the VA.
Prior qualitative studies of vaccine uptake in distinct veteran subgroups (ie, unhoused and in VA facilities with low vaccination rates) demonstrated that overriding medical priorities among the unhoused and vaccine safety concerns were associated with decreased vaccine uptake, and positive perceptions of HCPs and the health care system were associated with increased vaccine uptake.11,12 However, these studies were conducted during periods of greater COVID-19 vaccine availability and acceptance, and prior to booster recommendations.4,12,13
This mixed-methods quality improvement (QI) project assessed the barriers and facilitators of COVID-19 vaccination among veterans receiving primary care at a single VA health care facility. We assessed whether unique patient clusters could be identified based on COVID-19–related and vaccine-related thoughts and feelings and whether cluster membership was associated with COVID-19 vaccination. This analysis also explored how individuals’ beliefs and trust shaped motivations and hesitancies for vaccine uptake in quantitatively derived clusters with varying vaccination rates.
Methods
This QI project was conducted at the VA Pittsburgh Healthcare System (VAPHS), a tertiary care facility serving > 75,000 veterans in Pennsylvania, West Virginia, and Ohio. The VAPHS Institutional Review Board determined this QI study was exempt from review.14-17 Participation was voluntary and had no bearing on VA health care or benefits. Financial support for the project, including key personnel and participant compensation, was provided by VAPHS. We followed the STROBE reporting guideline for cross-sectional studies and the COREQ checklist for qualitative research.18,19
Quantitative Survey
The 32,271 veterans assigned to a VAPHS primary care HCP, effective April 1, 2020, were eligible. To ensure representation of subgroups underrecognized in research and/or QI projects, the sample included all 1980 female patients at VAPHS and a random sample of 500 White and 500 Hispanic and/or non-White men within 4 age categories (< 50, 50-64, 65-84, and > 84 years). For the < 50 years or > 84 years categories, all Hispanic and/or non-White men were included due to small sample sizes.20-22 The nonrandom sampling frame comprised 1708 Hispanic and/or non-White men and 2000 White men. After assigning the 5688 potentially eligible individuals a unique identifier, 31 opted out, resulting in a final sample of 5657 individuals.
The 5657 individuals received a letter requesting their completion of a future questionnaire about COVID-19 infection and vaccines. An electronic Qualtrics questionnaire link was emailed to 3221 individuals; nonresponders received 2 follow-up email reminders. For the 2436 veterans without an email address on file, trained interviewers conducted phone surveys and entered responses. Those patients who completed the questionnaire could enter a drawing to win 1 of 100 cash prizes valued at $100. We collected questionnaire data from July to September 2021.
Questionnaire Items
We constructed a 60-item questionnaire based on prior research on COVID-19 vaccine hesitancy and the WHO Guidebook for Immunization Programs and Implementing Partners.4,23-25 The WHO Guidebook comprises survey items organized within 4 domains reflecting the behavioral and social determinants of vaccination: thoughts and feelings; social processes; motivation and hesitancy; and practical factors.23
Sociodemographic, clinical, and personal characteristics. The survey assessed respondent ethnicity and race and used these data to create a composite race and ethnicity variable. Highest educational level was also attained using 8 response options. The survey also assessed prior COVID-19 infection; prior receipt of vaccines for influenza, pneumonia, tetanus, or shingles; and presence of comorbidities that increase the risk of severe COVID-19 infection. We used administrative data from the VA Corporate Data Warehouse to determine respondent age, sex, geographic residence (urban, rural), and to fill in missing self-reported data on sex (n = 4) and ethnicity and race (n = 12). The survey assessed political views using a 5-point Likert scale (1, very liberal; 5, very conservative) and was collapsed into 3 categories (ie, very conservative or conservative, moderate, very liberal or liberal), with prefer not to answer reported separately
COVID-19 infection and vaccine. We asked veterans if they had ever been infected with COVID-19, whether they had been offered and/or received a COVID-19 vaccine, and type (Pfizer, Moderna, or Johnson & Johnson), and number of doses received. Positive vaccination status was defined as the receipt of ≥ 1 dose of a COVID-19 vaccine approved by the US Food and Drug Administration.
COVID-19 opinions. Respondents were asked about perceived risk of COVID-19 infection and related health outcomes, as well as beliefs about COVID-19 vaccines, using a 4-point Likert scale for all items: (1, not at all concerned; 4, very concerned). Respondents were asked about concerns related to COVID-19 infection and severe illness. They also were asked about vaccine-related short-term adverse effects (AEs) and long-term complications. Respondents were asked how effective they believed COVID-19 vaccines were at preventing infection, serious illness, or death. Unvaccinated and vaccinated veterans were asked similar items, with a qualifier of “before getting vaccinated…” for those who were vaccinated.
Social processes. Respondents were asked to rate their level of trust in various sources of COVID-19 vaccine information using a 4-point Likert scale (1, trust not at all; 4, trust very much). Respondents were asked whether community or religious leaders or close family or friends wanted them to get vaccinated (yes, no, or unsure).
Practical factors. Respondents were asked to rate the logistical difficulty of getting vaccinated or trying to get vaccinated using a 4-point Likert scale (1, not at all; 4, extremely).
Participants
Respondents were asked to participate in a follow-up qualitative interview. Among 293 participants who agreed, we sampled all 86 unvaccinated individuals regardless of cluster assignment, a random sample of 88 individuals in the cluster with the lowest vaccination rate, and all 33 vaccinated individuals in the cluster with the second-lowest vaccination rate. Forty-nine veterans completed qualitative interviews.
Two research staff trained in qualitative research completed telephone interviews, averaging 16.5 minutes (March to May 2022), using semistructured scripts to elicit vaccine-related motivations, hesitancies, or concerns. Interviews were recorded, transcribed, and deidentified. Participants provided written consent for recording and received $50 cash-equivalent compensation for interview completion.
Qualitative Interview Script
The interview script consisted of open-ended questions related to vaccine uptake across WHO domains.23 Both unvaccinated and vaccinated respondents were asked similar questions and customized questions about boosters for the vaccinated subgroup. To assess motivations and hesitancies, respondents were asked how they made their decisions about vaccination and what they considered when deciding. Vaccinated participants were asked about motivations and overcoming concerns. Unvaccinated respondents were asked about reasons for concern. To assess social processes, the interviewers asked participants whose opinion or counsel they trusted when deciding whether to get vaccinated. Questions also focused on positive experiences and vaccination barriers. Vaccinated participants were asked what could have improved their vaccination experiences. Finally, the interviewers asked participants who received a complete primary vaccine series about their motivations and plans related to booster vaccines, and whether information about emerging COVID-19 variants influenced their decisions.
Data Analyses
This analysis used X2 and Fisher exact tests to assess the associations among respondent characteristics, questionnaire responses, vaccination status, and cluster membership. Items phrased similarly were handled in a similar fashion for vaccinated and unvaccinated respondents.
Cluster analysis assessed the possible groupings in responses to the quantitative questionnaire items focused on thoughts and feelings about COVID-19 infection risk and severity, vaccine effectiveness, and vaccine safety. This analysis treated the items’ ordinal response categories as continuous. We performed factor analysis using principal component analysis to explore dimension reduction and account for covariance between items. Two principal components were calculated and applied k-means clustering, determining the number of clusters through agreement from the elbow, gap statistic, and silhouette methods.26 Each cluster was named based on its unique pattern of responses to the items used to define them (eAppendix 1).

Multivariable logistic regression analyses assessed the independent association between cluster membership as the independent measure and vaccination status as the dependent measure, adjusting for respondent sociodemographic and personal characteristics and 2 measures of trust (ie, local VA HCP and the CDC). We selected these trust measures because they represent objective sources of medical information and were independently associated with COVID-19 vaccination status in a logistic regression model comprising all 6 trust items assessed.
This study defined statistical significance as a 2-tailed P value < .05. SAS 9.4 was used for all statistical analyses and Python 3.7.4 and the Scikit-learn package for cluster analyses.27 For qualitative analyses, this study used an inductive thematic approach guided by conventional qualitative content analysis, NVivo 12 Plus for Windows to code and analyze interview transcripts.28,29 We created an initial codebook based on 10 transcripts that were selected for high complexity and represented cluster membership and vaccination status.30,31 After 2 qualitative staff developed the initial codebook, 11 of 49 (22%) transcripts were independently coded by a primary and secondary coder to ensure consistent code application. Both coders reviewed the cocoded transcripts and resolved all discrepancies through negotiated consensus.32 After the cocoding process was complete, the primary coder coded the remaining transcripts. The primary and secondary coder met as needed to review and discuss any questions that arose during the primary coder’s work.
Results
Of 5657 eligible participants, 1208 (21.4%) completed a questionnaire. Overall, 674 (55.8%) were aged < 65 years, 530 (43.9%) were women, 828 (68.5%) were non-Hispanic White, 303 (25.1%) were Black, and 47 (3.9%) were Hispanic, and 1034 (85.6%) were vaccinated (Table 1). Compared to the total sampled population, respondents were more often older, female, and White (eAppendix 2).


Cluster Membership
Four clusters were identified from 1183 (97.9%) participants who provided complete responses to 6 items assessing thoughts and feelings about COVID-19 infection and vaccines (Table 2). Of the 1183 respondents, 375 (31.7%) were Concerned Believers (cluster 1), 336 (28.4%) were Unconcerned Believers (cluster 2), 298 (25.2%) were Concerned Ambivalents (cluster 3), and 174 (14.7%) were Unconcerned Disbelievers (cluster 4). The Concerned Believers were moderately/ very concerned about COVID-19 infection (96.0%) and becoming very ill from infection (94.6%), believed the vaccine was moderately/very effective in preventing COVID-19 infection (100%) and severe illness or death from infection (98.7%), and had slight concern about short-term AEs (92.6%) or long-term complications (92.0%) from the vaccine. The Unconcerned Believers had no/slight concern about COVID-19 infection (76.5%) or becoming very ill (79.2%), believed the vaccine was effective in preventing infection (82.4%) and severe illness and death (83.6%), and had no/slight concern about short-term AEs (94.0%) or long-term complications (87.2%) from the vaccine. The Concerned Ambivalents were moderately/ very concerned about COVID-19 infection (94.3%) and becoming very ill (93.6%), believed the vaccine was moderately/very effective in preventing infection (86.6%) and severe illness or death (86.9%), and were moderately/very concerned about short-term AEs (81.9%) or long-term complications (89.3%) from the vaccine. The Unconcerned Disbelievers had no/slight concern about COVID-19 infection (90.8%) and becoming very ill (88.6%), believed the vaccine was not at all/slightly effective in preventing infection (90.3%) and severe illness or death (87.4%), and were moderately/very concerned about short-term AEs (52.8%) or long-term complications (75.9%) from the vaccine.

Cluster Membership
Respondent age, race and ethnicity, and political viewpoints differed significantly by cluster (P < .001). Compared with the other clusters, the Concerned Believer cluster was older (55.5% age ≥ 65 years vs 16.7%-48.0%) and more frequently reported liberal political views (28.8% vs 4.6%-15.1%). In contrast, the Unconcerned Disbeliever cluster was younger (83.4% age ≤ 64 years vs 44.5%-56.8%) and more frequently reported conservative political views (37.9% vs 17.1%-26.8%) than the other clusters. Whereas the Concerned Ambivalent cluster had the highest proportion of Black (37.7%) and the lowest proportion of White respondents (57.6%), the Unconcerned Disbelievers cluster had the lowest proportion of Black respondents (14.5%) and the highest proportion of White respondents (77.9%). The Unconcerned Disbelievers cluster were significantly less likely to trust COVID-19 vaccine information from any source and to believe those close to them wanted them to get vaccinated.
Association of Cluster Membership and COVID-19 Vaccination
COVID-19 vaccination rates varied more than 3-fold (P < .001) by cluster, with 29.9% of Unconcerned Disbelievers, 93.3% of Concerned Ambivalents, 93.5% of Unconcerned Believers, and 98.9% of Concerned Believers reporting being vaccinated. (Figure). Cluster membership was independently associated with vaccination, with adjusted odds ratios (AORs) of 12.0 (95% CI, 6.1-23.8) for the Concerned Ambivalent, 13.0 (95% CI, 6.9-24.5) for Unconcerned Believer, and 48.6 (95% CI, 15.5-152.1) for Concerned Believer clusters (Table 3). Respondent trust in COVID-19 vaccine information from their VA HCP (AOR 2.1; 95% CI, 1.6-2.8) and the CDC (AOR 1.6; 95% CI, 1.2-2.1) were independently associated with vaccination status, while the remaining respondent sociodemographic or personal characteristics were not.


Qualitative Interview Participants
A 49-participant convenience sample completed interviews, including 30 Concerned Ambivalent, 17 Unconcerned Disbeliever, and 2 Unconcerned Believer respondents cluster. The data were not calculated for Unconcerned Believers due to the small sample size. Interview participants were more likely to be younger, female, non-Hispanic, White, less educated, and more politically conservative than the questionnaire respondents as a whole (Appendix). The vaccination rate for the interview participants was 73.5%, ranging from 29.9% in the Unconcerned Disbeliever to 93.3% in the Concerned Ambivalent cluster. Qualitative themes and participant quotes for Concerned Ambivalent and Unconcerned Disbeliever respondents are in eAppendix 3.
Motivations. Wanting personal protection from becoming infected or severely ill from COVID-19 (63.8%), caregiver wanting to protect others (17.0%), and employment vaccine requirements (14.9%) were frequent motivations for vaccination. Whereas personal protection (90.0%) and protection of others (23.3%) were identified more frequently in the Concerned Ambivalents cluster, employment vaccine requirements (35.3%) were more frequently identified in the Unconcerned Disbelievers cluster.
Hesitancies or concerns. Lack of sufficient information related to rapid vaccine development (55.3%), vaccine AEs (38.3%), and low confidence in vaccine efficacy (23.4%) were frequent concerns or hesitancies about vaccination. Unconcerned Disbelievers expressed higher levels of concern about the vaccine’s rapid development (82.4%), low perceived vaccine efficacy (47.1%), and a lack of trust in governmental vaccine promotion (23.5%) than did the Concerned Ambivalents.
Overcoming concerns. Not wanting to get sick or die from infection coupled with an understanding that vaccine benefits exceed risks (23.4%) and receiving information from a trusted source (10.6%) were common ways of overcoming concerns for vaccination. Although the Unconcerned Disbelievers infrequently identified reasons for overcoming concerns, they identified employment requirements (17.6%) as a reason for vaccination despite concerns. They also identified seeing others with positive vaccine experiences and pressure from family or friends as ways of overcoming concerns (11.8% each).
Social influences. Family members or partners (38.3%), personal opinions (38.3%), and HCPs (23.4%) were frequent social influences for vaccination. Concerned Ambivalents mentioned family members and partners (46.7%), HCPs (26.7%), and friends (20.0%) as common influences, while Unconcerned Disbelievers more frequently relied on their opinion (41.2%) and quoted specific scientifically reputable data sources (17.6%) to guide vaccine decision-making, although it is unclear whether these sources were accessed directly or if this information was obtained indirectly through scientifically unvetted data platforms.
Practical factors. Most participants had positive vaccination experiences (68.1%), determined mainly by the Concerned Ambivalents (90.0%), who were more highly vaccinated. Barriers to vaccination were reported by 9 (19.1%) participants, driven by those in the Concerned Ambivalent cluster (26.7%). Eight (17.0%) participants suggested improvements for vaccination processes, with similar overall reporting frequencies across clusters.
COVID-19 boosters and variants. Wanting continued protection from COVID-19 (36.2%), recommendations from a doctor or trusted source (17.0%), and news about emerging variants (10.6%) were frequent motivations for receiving a vaccine booster (eAppendix 4). These motivations were largely driven by the Concerned Ambivalents, of whom 25 of 30 were booster eligible and 24 received a booster dose. Belief that boosters were unnecessary (8.5%), concerns about efficacy (6.4%), and concerns about AEs (6.4%) were frequently identified hesitancies. These concerns were expressed largely by the Unconcerned Disbelievers, of whom 7 of 17 were booster dose eligible, but only 1 received a dose.
Evolving knowledge about variants was not a major concern overall and did not change existing opinions about the vaccine (36.2%). Concerned Ambivalents believed vaccination provided extra protection against variants (36.7%) and the emergence of variants served as a reminder of the ongoing pandemic (30.0%). In contrast, Unconcerned Disbelievers believed that the threat of variants was overblown (35.3%) and mutations are to be expected (17.6%).
Discussion
This study used a complementary mixed-methods approach to understand the motivations, hesitancies, and social and practical drivers of COVID-19 vaccine uptake among VA beneficiaries. Our quantitative analyses identified 4 distinct clusters based on respondents’ opinions on COVID-19 infection severity and vaccine effectiveness and safety. Veterans in 3 clusters were 12 to 49 times more likely to be vaccinated than those in the remaining cluster, even when controlling for baseline respondent characteristics and level of trust in credible sources of COVID-19 information. The observed vaccination rate of nearly 86% was higher than the contemporaneous national average of 62% for vaccine-eligible individuals, likely reflecting the comprehensive VA vaccine promotion strategies tailored to a patient demographic with a high COVID-19 risk profile.2,10

This cluster analyses demonstrated the importance of thoughts and feelings about COVID-19 infection and vaccination as influential social and behavioral drivers of vaccine uptake. These opinions help explain the strong association between cluster membership and vaccination status in this multivariable modeling. The cluster composition was consistent with findings from studies of nonveteran populations that identified perceived vulnerability to COVID-19 infection, beliefs in vaccine effectiveness, and adherence with protective behaviors during the pandemic as contributors to vaccine uptake.13,33 Qualitative themes showed that personal protection, protecting others, and vaccine mandates were frequent motivators for vaccination. Whereas protection of self and others from COVID-19 infection were more often expressed by the highly vaccinated Concerned Ambivalents, employment and travel vaccine mandates were more often identified by Unconcerned Disbelievers, who had a lower vaccination rate. Among Unconcerned Disbelievers, an employer vaccine requirement was the most frequent qualitative theme for overcoming vaccination concerns.
In addition to cluster membership, our modeling showed that trust in local VA HCPs and the CDC were independently associated with COVID-19 vaccination, which has been found in prior research.20 This qualitative analyses regarding vaccine hesitancy identified trust-related concerns that were more frequently expressed by Unconcerned Disbelievers than Concerned Ambivalents. Concerns included the rapid development of the vaccines potentially limiting the generation of scientifically sound effectiveness and safety data, and potential biases involving the entities promoting vaccine uptake.
Whereas the Concerned Believers, Unconcerned Believers, and Concerned Ambivalents all had high COVID-19 vaccination rates (≥ 93%), the decision-making pathways to vaccine uptake likely differ by their concerns about COVID-19 infection and perceptions of vaccine safety and effectiveness. For example, this mixed-methods analysis consistently showed that people in the Concerned Ambivalent cluster were positively motivated by concerns about COVID-19 infection and severity and beliefs about vaccine effectiveness that were tempered by concerns about vaccine AEs. For this cluster, their frequent thematic expression that the benefits of the vaccine exceed the risks, and the positive social influences of family, friends, and HCPs may explain their high vaccination rate.
Such insights into how the patterns of COVID-19–related thoughts and feelings vary across clusters can be used to design interventions to encourage initial and booster doses of COVID-19 vaccines. For example, messaging that highlights the infectivity and severity of COVID-19 and the potential for persistent negative health outcomes associated with long COVID could reinforce the beliefs of Concerned Believers and Concerned Ambivalents, and such messaging could also be used as a targeted intervention for Unconcerned Believers who expressed fewer concerns about the health consequences of COVID-19.23 Likewise, messaging about the safety profile of COVID-19 vaccines may reduce vaccine hesitancy for Concerned Ambivalents. Importantly, purposeful attention to health equity, community engagement, and involvement of racially diverse HCPs in patient discussions represent successful strategies to increase COVID-19 vaccine uptake among Black individuals, who were disproportionately represented in the Concerned Ambivalent cluster and may possess higher levels of mistrust due to racism experienced within the health care system.24
Our findings suggest that the greatest challenge for overcoming vaccine hesitancy is for individuals in the suboptimally vaccinated (30%) Unconcerned Disbeliever cluster. These individuals had low levels of concern about COVID-19 infection and severity, high levels of concern about vaccine safety, low perceived vaccine effectiveness, and low levels of trust in all information sources about COVID-19. While the Unconcerned Disbelievers cited scientifically reputable data sources, we were unable to verify whether participants accessed these reputable sources of information directly or obtained such information indirectly through potentially biased online sources. Nearly half of this cluster trusted their VA HCP and believed their community or religious leaders would want them to get vaccinated. This qualitative analyses found that Unconcerned Disbelievers relied on personal beliefs for vaccine decision-making more than Concerned Ambivalents. While Unconcerned Disbelievers were less likely to be socially influenced by family, friends, or religious leaders, they still acknowledged some impact from these sources. These findings suggest that addressing vaccine hesitancy among Unconcerned Disbelievers may require a multifaceted approach that respects their reliance on personal research while also leveraging the potential social influences. This approach supports the promising, previously reported practices of harnessing the social influences of HCPs and other community and religious leaders to promote vaccine uptake among Unconcerned Disbelievers.34,35 One evidence-based approach to effectively change patient health care behaviors is through motivational interviewing strategies that use open-ended questions, nonjudgmental interactions, and collaborative decision-making when discussing the risks and benefits of vaccination.21,22
Limitations
This study was conducted at a single VA health care facility and our sampling technique was nonrandom, suggesting that these results may not be generalizable to all veterans or non-VA patient populations. The 21% questionnaire response rate could have introduced selection bias into the respondent sample. All questionnaire data were self-reported, including vaccination status. Finally, the qualitative interviews consisted of a small number of unvaccinated individuals in 2 clusters (ie, Concerned Ambivalents and Unconcerned Disbelievers) and may not have reached thematic saturation in these subgroups.
Conclusions
Quantitative analyses identified 4 clusters based on individual thoughts and feelings about COVID-19 infection and vaccines. Cluster membership and levels of trust in COVID-19 information sources were independently associated with vaccination. Understanding the quantitative patterns of thoughts and beliefs across clusters, enriched by common qualitative themes for vaccine hesitancy, help inform tailored interventions to augment COVID-19 vaccine uptake and highlight the importance of targeted, trust-based communication and culturally sensitive interventions to enhance vaccine uptake across diverse populations.
- World Health Organization. WHO COVID-19 dashboard. Accessed July 18, 2025. https://covid19.who.int/
- Centers for Disease Control and Prevention. COVIDVax- View: Weekly COVID-19 Vaccination Coverage and Intent among Adults. Accessed June 10, 2025. https://www.cdc.gov/covidvaxview/weekly-dashboard/adult-vaccination-coverage.html
- World Health Organization. Strategy to achieve global Covid-19 vaccination by mid-2022. 2021. Accessed April 30, 2025. https://cdn.who.int/media/docs/default-source/immunization/covid-19/strategy-to-achieve-global-covid-19-vaccination-by-mid-2022.pdf
- Jasuja GK, Meterko M, Bradshaw LD, et al. Attitudes and intentions of US veterans regarding COVID-19 vaccination. JAMA Netw Open. 2021;4(11):e2132548. doi:10.1001/jamanetworkopen.2021.32548
- Der-Martirosian C, Steers WN, Northcraft H, Chu K, Dobalian A. Vaccinating veterans for COVID-19 at the U.S. Department of Veterans Affairs. Am J Prev Med. 2022;62(6):e317-e324. doi:10.1016/j.amepre.2021.12.016
- Bloeser K, Lipkowitz-Eaton J. Disproportionate multimorbidity among veterans in middle age. J Public Health (Oxf). 2022;44(1):28-35. doi:10.1093/pubmed/fdab149
- US Department of Veterans Affairs. National Center for Veterans Analysis and Statistics: veteran population. Updated March 26, 2025. Accessed April 30, 2025. https://www.va.gov/vetdata/Veteran_Population.asp
- Olenick M, Flowers M, Diaz VJ. US veterans and their unique issues: enhancing health care professional awareness. Adv Med Educ Pract. 2015;6:635-639. doi:10.2147/AMEP.S89479
- Orkaby AR, Nussbaum L, Ho YL, et al. The burden of frailty among U.S. veterans and its association with mortality, 2002-2012. J Gerontol A Biol Sci Med Sci. 2019;74(8):1257-1264. doi:10.1093/gerona/gly232
- Bass SB, Kelly PJ, Hoadley A, Arroyo Lloret A, Organtini T. Mapping perceptual differences to understand COVID-19 beliefs in those with vaccine hesitancy. J Health Commun. 2022;27(1):49-61. doi:10.1080/10810730.2022.2042627
- Meng L, Masters NB, Lu PJ, et al. Cluster analysis of adults unvaccinated for COVID-19 based on behavioral and social factors, National Immunization Survey-Adult COVID Module, United States. Prev Med. 2023;167:107415. doi:10.1016/j.ypmed.2022.107415
- Gin JL, Balut MD, Dobalian A. COVID-19 vaccination uptake and receptivity among veterans enrolled in homelessness- tailored primary health care clinics: provider trust vs. misinformation. BMC Prim Care. 2024;25(1):24. doi:10.1186/s12875-023-02251-x
- Wilson GM, Ray CE, Kale IO, et al. Age and beliefs about vaccines associated with COVID-19 vaccination among US veterans. Antimicrob Steward Healthc Epidemiol. 2023;3(1):e184. doi:10.1017/ash.2023.446
- VA Pittsburgh Healthcare System (VAPHS). Human Research Protection Program (HRPP) policy for quality assurance/ quality improvement projects. Policy H-013. December 31, 2021. Accessed April 30, 2025. https://www.va.gov/files/2020-11/H-013_QAQI%20Project_revised_updated%20format_clean_508.pdf
- Burkitt KH, Rodriguez KL, Mor MK, et al. Evaluation of a collaborative VA network initiative to reduce racial disparities in blood pressure control among veterans with severe hypertension. Healthc (Amst). 2021;8(suppl 1):100485. doi:10.1016/j.hjdsi.2020.100485
- Sinkowitz-Cochran RL, Burkitt KH, Cuerdon T, et al. The associations between organizational culture and knowledge, attitudes, and practices in a multicenter Veterans Affairs quality improvement initiative to prevent methicillin-resistant Staphylococcus aureus. Am J Infect Control. 2012;40(2):138-143. doi:10.1016/j.ajic.2011.04.332
- Burkitt KH, Sinkowitz-Cochran RL, Obrosky DS, et al. Survey of employee knowledge and attitudes before and after a multicenter Veterans’ Administration quality improvement initiative to reduce nosocomial methicillin-resistant Staphylococcus aureus infections. Am J Infect Control. 2010;38(4):274-282. doi:10.1016/j.ajic.2009.08.019
- STROBE - strengthening the reporting of observational studies in epidemiology. What is STROBE? Accessed April 30, 2025. https://www.strobe-statement.org/
- Tong A, Sainsbury P, Craig J. Consolidated criteria for reporting qualitative research (COREQ): a 32-item checklist for interviews and focus groups. Int J Qual Health Care. 2007;19(6):349-357. doi:10.1093/intqhc/mzm042
- Ward RE, Nguyen XT, Li Y, et al; on behalf of the VA Million Veteran Program. Racial and ethnic disparities in U.S. veteran health characteristics. Int J Environ Res Public Health. 2021;18(5):2411. doi:10.3390/ijerph18052411
- Harrington KM, Nguyen XT, Song RJ, et al; VA Million Veteran Program. Gender differences in demographic and health characteristics of the Million Veteran Program cohort. Womens Health Issues. 2019;29(suppl 1):S56-S66. doi:10.1016/j.whi.2019.04.012
- Washington DL, ed. National Veteran Health Equity Report 2021. Focus on Veterans Health Administration Patient Experience and Health Care Quality. VHA Office of Health Equity; September 2022. Accessed April 30, 2025. https://www.va.gov/healthequity/nvher.asp
- World Health Organization. Data for action: achieving high uptake of COVID-19 vaccines. April 1, 2021. Accessed April 30, 2025. https://www.who.int/publications/i/item/WHO-2019-nCoV-vaccination-demand-planning-2021.1
- Hoffman BL, Boness CL, Chu KH, et al. COVID- 19 vaccine hesitancy, acceptance, and promotion among healthcare workers: a mixed-methods analysis. J Community Health. 2022;47(5):750-758. doi:10.1007/s10900-022-01095-3
- Vasudevan L, Bruening R, Hung A, et al. COVID- 19 vaccination intention and activation among health care system employees: a mixed methods study. Vaccine. 2022;40(35):5141-5152. doi:10.1016/j.vaccine.2022.07.010
- Tibshirani R, Walther G, Hastie T. Estimating the number of clusters in a data set via the gap statistic. J R Stat Soc Series B Stat Methodol. 2001;63(2):411-423. doi:10.1111/1467-9868.00293
- Pedregosa FP, Varoquaux G, Gramfort A, et al. Scikitlearn: machine learning in Python. J Mach Learn Res. 2011;12:2825-2830.
- Proudfoot K. Inductive/deductive hybrid thematic analysis in mixed methods research. J Mix Methods Res. 2022;17(3): 308-326. doi:10.1177/15586898221126816
- Chapman AL, Hadfield M, Chapman CJ. Qualitative research in healthcare: an introduction to grounded theory using thematic analysis. J R Coll Physicians Edinb. 2015;45(3):201-205. doi:10.4997/jrcpe.2015.305
- Grandheim UH, Lundman B. Qualitative content analysis in nursing research: concepts, procedures and measures to achieve trustworthiness. Nurse Educ Today. 2004;24(2):105-112. doi:10.1016/j.nedt.2003.1001
- Sandelowski M. Whatever happened to qualitative description? Res Nurs Health. 2000;23(4):334-340. doi:10.1002/1098-240x(200008)23:4<334::aid-nur9 >3.0.co;2-g
- Garrison DR, Cleveland-Innes M, Koole M, Kappelman J. Revisiting methodological issues in transcript analysis: negotiated coding and reliability. Internet High Educ. 2006;9(1):1-8. doi:10.1016/j.iheduc.2005.11.001
- Wagner AL, Porth JM, Wu Z, Boulton ML, Finlay JM, Kobayashi LC. Vaccine hesitancy during the COVID-19 pandemic: a latent class analysis of middle-aged and older US adults. J Community Health. 2022;47(3):408- 415. doi:10.1007/s10900-022-01064-w
- Syed U, Kapera O, Chandrasekhar A, et al. The role of faith-based organizations in improving vaccination confidence & addressing vaccination disparities to help improve vaccine uptake: a systematic review. Vaccines (Basel). 2023;11(2):449. doi:10.3390/vaccines11020449
- Evans D, Norrbom C, Schmidt S, Powell R, McReynolds J, Sidibe T. Engaging community-based organizations to address barriers in public health programs: lessons learned from COVID-19 vaccine acceptance programs in diverse rural communities. Health Secur. 2023;21(S1):S17-S24. doi:10.1089/hs.2023.0017
- World Health Organization. WHO COVID-19 dashboard. Accessed July 18, 2025. https://covid19.who.int/
- Centers for Disease Control and Prevention. COVIDVax- View: Weekly COVID-19 Vaccination Coverage and Intent among Adults. Accessed June 10, 2025. https://www.cdc.gov/covidvaxview/weekly-dashboard/adult-vaccination-coverage.html
- World Health Organization. Strategy to achieve global Covid-19 vaccination by mid-2022. 2021. Accessed April 30, 2025. https://cdn.who.int/media/docs/default-source/immunization/covid-19/strategy-to-achieve-global-covid-19-vaccination-by-mid-2022.pdf
- Jasuja GK, Meterko M, Bradshaw LD, et al. Attitudes and intentions of US veterans regarding COVID-19 vaccination. JAMA Netw Open. 2021;4(11):e2132548. doi:10.1001/jamanetworkopen.2021.32548
- Der-Martirosian C, Steers WN, Northcraft H, Chu K, Dobalian A. Vaccinating veterans for COVID-19 at the U.S. Department of Veterans Affairs. Am J Prev Med. 2022;62(6):e317-e324. doi:10.1016/j.amepre.2021.12.016
- Bloeser K, Lipkowitz-Eaton J. Disproportionate multimorbidity among veterans in middle age. J Public Health (Oxf). 2022;44(1):28-35. doi:10.1093/pubmed/fdab149
- US Department of Veterans Affairs. National Center for Veterans Analysis and Statistics: veteran population. Updated March 26, 2025. Accessed April 30, 2025. https://www.va.gov/vetdata/Veteran_Population.asp
- Olenick M, Flowers M, Diaz VJ. US veterans and their unique issues: enhancing health care professional awareness. Adv Med Educ Pract. 2015;6:635-639. doi:10.2147/AMEP.S89479
- Orkaby AR, Nussbaum L, Ho YL, et al. The burden of frailty among U.S. veterans and its association with mortality, 2002-2012. J Gerontol A Biol Sci Med Sci. 2019;74(8):1257-1264. doi:10.1093/gerona/gly232
- Bass SB, Kelly PJ, Hoadley A, Arroyo Lloret A, Organtini T. Mapping perceptual differences to understand COVID-19 beliefs in those with vaccine hesitancy. J Health Commun. 2022;27(1):49-61. doi:10.1080/10810730.2022.2042627
- Meng L, Masters NB, Lu PJ, et al. Cluster analysis of adults unvaccinated for COVID-19 based on behavioral and social factors, National Immunization Survey-Adult COVID Module, United States. Prev Med. 2023;167:107415. doi:10.1016/j.ypmed.2022.107415
- Gin JL, Balut MD, Dobalian A. COVID-19 vaccination uptake and receptivity among veterans enrolled in homelessness- tailored primary health care clinics: provider trust vs. misinformation. BMC Prim Care. 2024;25(1):24. doi:10.1186/s12875-023-02251-x
- Wilson GM, Ray CE, Kale IO, et al. Age and beliefs about vaccines associated with COVID-19 vaccination among US veterans. Antimicrob Steward Healthc Epidemiol. 2023;3(1):e184. doi:10.1017/ash.2023.446
- VA Pittsburgh Healthcare System (VAPHS). Human Research Protection Program (HRPP) policy for quality assurance/ quality improvement projects. Policy H-013. December 31, 2021. Accessed April 30, 2025. https://www.va.gov/files/2020-11/H-013_QAQI%20Project_revised_updated%20format_clean_508.pdf
- Burkitt KH, Rodriguez KL, Mor MK, et al. Evaluation of a collaborative VA network initiative to reduce racial disparities in blood pressure control among veterans with severe hypertension. Healthc (Amst). 2021;8(suppl 1):100485. doi:10.1016/j.hjdsi.2020.100485
- Sinkowitz-Cochran RL, Burkitt KH, Cuerdon T, et al. The associations between organizational culture and knowledge, attitudes, and practices in a multicenter Veterans Affairs quality improvement initiative to prevent methicillin-resistant Staphylococcus aureus. Am J Infect Control. 2012;40(2):138-143. doi:10.1016/j.ajic.2011.04.332
- Burkitt KH, Sinkowitz-Cochran RL, Obrosky DS, et al. Survey of employee knowledge and attitudes before and after a multicenter Veterans’ Administration quality improvement initiative to reduce nosocomial methicillin-resistant Staphylococcus aureus infections. Am J Infect Control. 2010;38(4):274-282. doi:10.1016/j.ajic.2009.08.019
- STROBE - strengthening the reporting of observational studies in epidemiology. What is STROBE? Accessed April 30, 2025. https://www.strobe-statement.org/
- Tong A, Sainsbury P, Craig J. Consolidated criteria for reporting qualitative research (COREQ): a 32-item checklist for interviews and focus groups. Int J Qual Health Care. 2007;19(6):349-357. doi:10.1093/intqhc/mzm042
- Ward RE, Nguyen XT, Li Y, et al; on behalf of the VA Million Veteran Program. Racial and ethnic disparities in U.S. veteran health characteristics. Int J Environ Res Public Health. 2021;18(5):2411. doi:10.3390/ijerph18052411
- Harrington KM, Nguyen XT, Song RJ, et al; VA Million Veteran Program. Gender differences in demographic and health characteristics of the Million Veteran Program cohort. Womens Health Issues. 2019;29(suppl 1):S56-S66. doi:10.1016/j.whi.2019.04.012
- Washington DL, ed. National Veteran Health Equity Report 2021. Focus on Veterans Health Administration Patient Experience and Health Care Quality. VHA Office of Health Equity; September 2022. Accessed April 30, 2025. https://www.va.gov/healthequity/nvher.asp
- World Health Organization. Data for action: achieving high uptake of COVID-19 vaccines. April 1, 2021. Accessed April 30, 2025. https://www.who.int/publications/i/item/WHO-2019-nCoV-vaccination-demand-planning-2021.1
- Hoffman BL, Boness CL, Chu KH, et al. COVID- 19 vaccine hesitancy, acceptance, and promotion among healthcare workers: a mixed-methods analysis. J Community Health. 2022;47(5):750-758. doi:10.1007/s10900-022-01095-3
- Vasudevan L, Bruening R, Hung A, et al. COVID- 19 vaccination intention and activation among health care system employees: a mixed methods study. Vaccine. 2022;40(35):5141-5152. doi:10.1016/j.vaccine.2022.07.010
- Tibshirani R, Walther G, Hastie T. Estimating the number of clusters in a data set via the gap statistic. J R Stat Soc Series B Stat Methodol. 2001;63(2):411-423. doi:10.1111/1467-9868.00293
- Pedregosa FP, Varoquaux G, Gramfort A, et al. Scikitlearn: machine learning in Python. J Mach Learn Res. 2011;12:2825-2830.
- Proudfoot K. Inductive/deductive hybrid thematic analysis in mixed methods research. J Mix Methods Res. 2022;17(3): 308-326. doi:10.1177/15586898221126816
- Chapman AL, Hadfield M, Chapman CJ. Qualitative research in healthcare: an introduction to grounded theory using thematic analysis. J R Coll Physicians Edinb. 2015;45(3):201-205. doi:10.4997/jrcpe.2015.305
- Grandheim UH, Lundman B. Qualitative content analysis in nursing research: concepts, procedures and measures to achieve trustworthiness. Nurse Educ Today. 2004;24(2):105-112. doi:10.1016/j.nedt.2003.1001
- Sandelowski M. Whatever happened to qualitative description? Res Nurs Health. 2000;23(4):334-340. doi:10.1002/1098-240x(200008)23:4<334::aid-nur9 >3.0.co;2-g
- Garrison DR, Cleveland-Innes M, Koole M, Kappelman J. Revisiting methodological issues in transcript analysis: negotiated coding and reliability. Internet High Educ. 2006;9(1):1-8. doi:10.1016/j.iheduc.2005.11.001
- Wagner AL, Porth JM, Wu Z, Boulton ML, Finlay JM, Kobayashi LC. Vaccine hesitancy during the COVID-19 pandemic: a latent class analysis of middle-aged and older US adults. J Community Health. 2022;47(3):408- 415. doi:10.1007/s10900-022-01064-w
- Syed U, Kapera O, Chandrasekhar A, et al. The role of faith-based organizations in improving vaccination confidence & addressing vaccination disparities to help improve vaccine uptake: a systematic review. Vaccines (Basel). 2023;11(2):449. doi:10.3390/vaccines11020449
- Evans D, Norrbom C, Schmidt S, Powell R, McReynolds J, Sidibe T. Engaging community-based organizations to address barriers in public health programs: lessons learned from COVID-19 vaccine acceptance programs in diverse rural communities. Health Secur. 2023;21(S1):S17-S24. doi:10.1089/hs.2023.0017
Insights Into Veterans’ Motivations and Hesitancies for COVID-19 Vaccine Uptake: A Mixed-Methods Analysis
Insights Into Veterans’ Motivations and Hesitancies for COVID-19 Vaccine Uptake: A Mixed-Methods Analysis
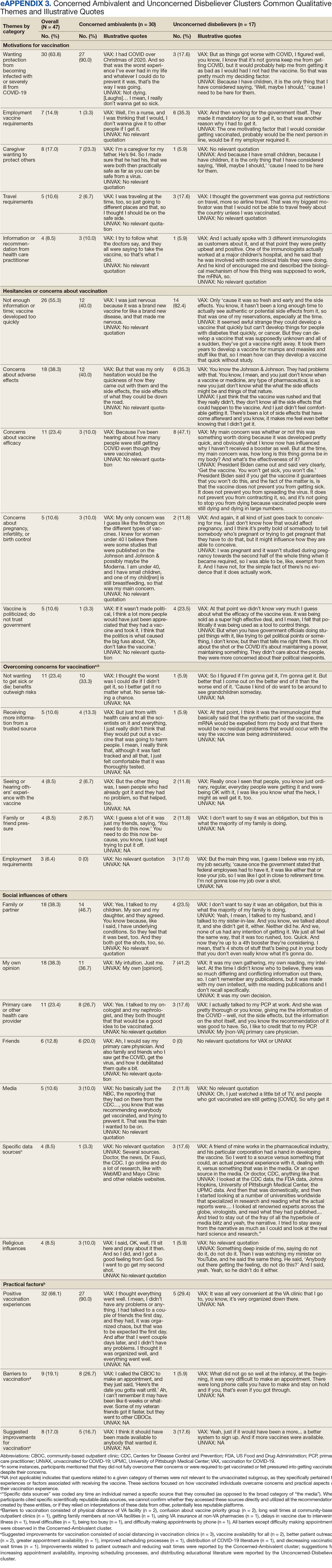
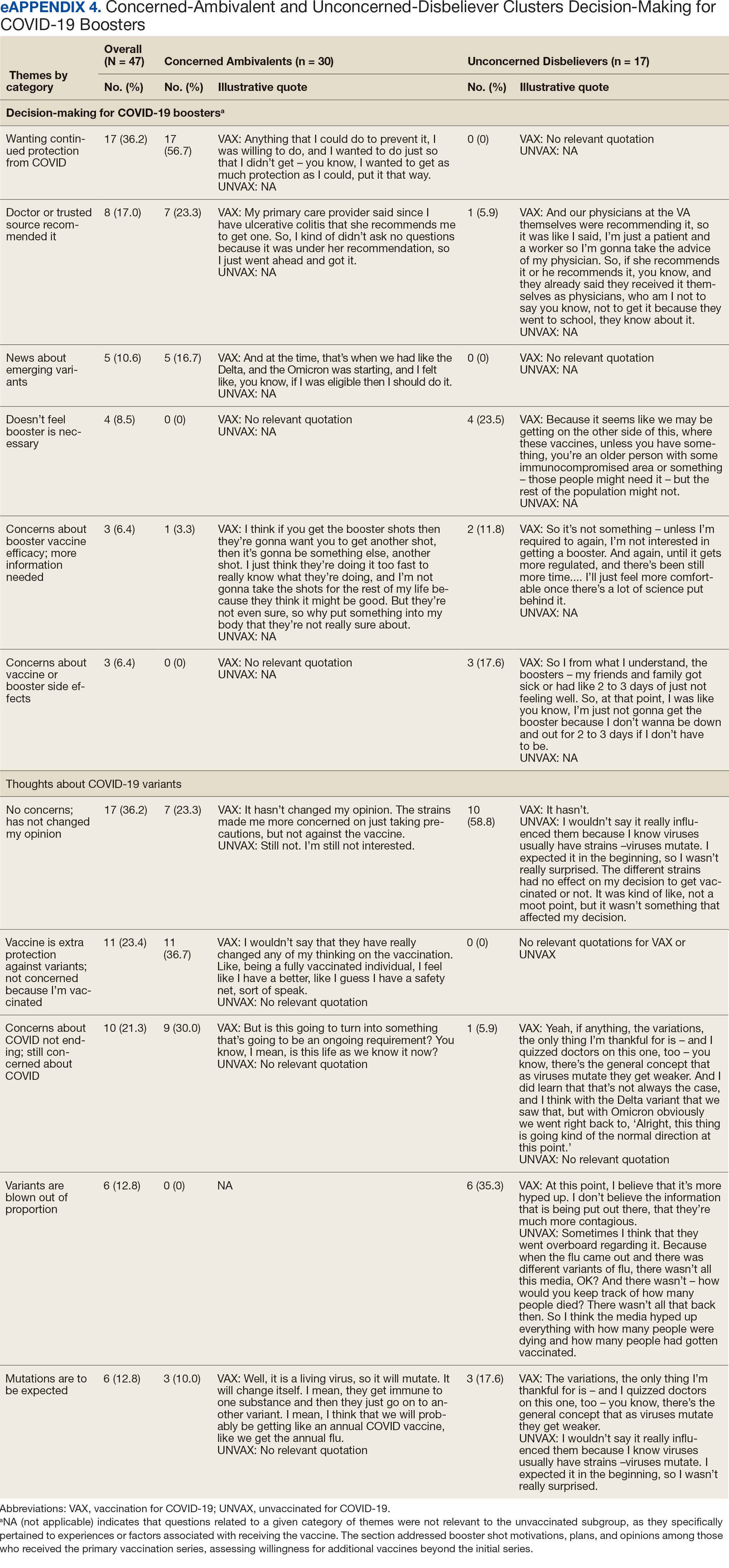
Military Imposters: What Drives Them and How They Damage Us All
Military Imposters: What Drives Them and How They Damage Us All
The better part of valor is discretion.
Henry IV, Part 1 by William Shakespeare1
This is the second part of an exploration of the phenomenon of stolen valor, where individuals claim military exploits or acts of heroism that are either fabricated or exaggerated, and/or awards and medals they did not earn.2 In June, I focused on the unsettling story of Sarah Cavanaugh, a young US Department of Veterans Affairs (VA) social worker who posed as a decorated, heroic, and seriously wounded Marine veteran for years. Cavanaugh’s manipulative masquerade allowed her to receive coveted spots in veteran recovery programs, thousands of dollars in fraudulent donations, the leadership of a local Veterans of Foreign Wars post, and eventually a federal conviction and prison sentence.3 The first column focused on the legal history of stolen valor; this editorial analyzes the clinical import and ethical impact of the behavior of military imposters. Military imposters are the culprits who steal valor.
It would be easy and perhaps reassuring to assume that stolen valor has emerged as another deplorable example of a national culture in which the betrayal of trust in human beings and loss of faith in institutions and aspirations has reached a nadir. Ironically, stolen valor is inextricably linked to the founding of the United States. When General George Washington inaugurated the American military tradition of awarding decorations to honor the bravery and sacrifices of the patriot Army, he anticipated military imposters. He tried to deter stolen valor through the threat of chastisement: “Should any who are not entitled to these honors have the insolence to assume the badges of them, they shall be severely punished,” Washington warned.4
It is plausible to think such despicable conduct occurs only as the ugly side of the beauty of our unparalleled national freedom, but this is a mistake. Cases of stolen valor have been reported in many countries around the world, with some of the most infamous found in the United Kingdom.5
While many brazen military imposters like Cavanaugh never serve, there is a small subset who honorably wore a uniform yet embellish their service record with secret missions and meritorious gallantry that purportedly earned them high rank and even higher awards. A most puzzling and disturbing example of this group is an allegation that surfaced when celebrated Navy SEAL Chris Kyle declared in American Sniper that he had won 3 additional combat awards for combat valor in addition to the Silver Star and 3 Bronze Stars actually listed in his service record.6
The fact that for centuries stolen valor has plagued multiple nations suggests, at least to this psychiatrically trained mind, that something deeper and darker in human nature than profit alone drives military imposters. Philosopher Verna Gehring has distilled these less tangible motivations into the concept of virtue imposters. According to Gehring, military phonies are a notorious exemplar: “The military phony adopts a past not her own, acts of courage she did not perform—she impersonates the heroic character and virtues she does not possess.”7 There could be no more apposite depiction of Cavanaugh, other military imposters, or a legion of other offenders of honor. 8
As with Cavanaugh, financial gain is a byproduct of the machinations of military imposters and is usually secondary to the pursuit of nonmaterial rewards such as power, influence, admiration, emulation, empathy, and charity. Gehring contends, and I agree, that virtue imposters are more pernicious and culpable than the plethora of more prosaic scammers and swindlers who use deceit primarily as a means of economic exploitation: “The virtue impostor by contrast plays on people’s better natures—their generosity, humility, and their need for heroes.”7
Military imposters cause real and lasting harm. Every veteran who exaggerates claims or scams the VA unjustly steals human and monetary resources from other deserving veterans whose integrity would not permit them to break the rules.9 Yet, even more harmful is the potential damage to therapeutic relationships: federal practitioners may become skeptical of a veteran’s history even when there is little to no grounds for suspicion. Veterans, in turn, may experience a breach of trust and betrayal not only from health care professionals and VA leaders but from their brothers and sisters in arms. On an ever-wider scale, every military impostor who is exposed may diminish the respect and honor all veterans have earned.
It is clear, then, why a small group of former service members has adopted the cause of uncovering military imposters and adroitly using the media to identify signs of stolen valor.10 Yet deception mars even these mostly well-intentioned campaigns, as some more zealous stolen valor hunters may make allegations that turn out to be false.11 Nevertheless, 500 years ago and in a very different context Shakespeare was, right on the mark: the better part of valor is discretion in describing one’s achievements, in relying on the veracity of our veteran’s narratives, and when there are sound reasons to do so verifying the truth of what our patients, friends, and even family tell us about their time in the military.1
- Shakespeare W. Introduction in: Henry IV, Part 1. Folger Sharespeare Library. Accessed July 24, 2025. https://www.folger.edu/explore/shakespeares-works/henry-iv-part-1/
- Geppert CM. What about stolen valor actually is illegal? Fed Pract. 2025;42(6):218-219. doi:10.12788/fp.0599
- Lehrfeld J. Woman who faked being cancer-stricken Marine gets 6 years in prison. Military Times. March 15, 2023. Accessed July 24, 2025. https://www.militarytimes.com/news/your-military/2023/03/15/woman-who-faked-being-sick-marine-purple-heart-gets-6-years-in-prison/
- Washington G. General Orders, 7 August 1782 in: Papers of George Washington. Founders Online. August 7, 1782. Accessed July 24, 2025. https://founders.archives.gov/documents/Washington/99-01-02-09056 5. Simpson LK. The men who impersonate military personnel for stolen glory. The Conversation. Updated November 17, 2016. Accessed July 24, 2025. https://theconversation.com/the-men-who-impersonate-military-personnel-for-stolen-glory-62233
- Larter DB. New questions cast doubt on ‘American Sniper‘ Chris Kyle‘s combat record. Navy Times. May 25, 2016. Accessed July 24, 2025. https://www.navytimes.com/news/your-navy/2016/05/25/new-questions-cast-doubt-on-american-sniper-chris-kyle-s-combat-record
- Gehring VV. Phonies, fakes, and frauds—and the social harms they cause. Philos Public Policy Q. 2003;23:14-20.
- Liem, E. The 6 most shocking military imposters ever. Military.com. July 7, 2015. Accessed July 29, 2025. https://www.military.com/undertheradar/2015/07/the-6-most-shocking-military-impostors-ever 9. Sisk R. Some vets with PTSD are scamming the VA: testimony. Military.com. June 8, 2017. Accessed July 24, 2025. https://www.military.com/daily-news/2017/06/08/some-vets-with-ptsd-are-scamming-va-testimony.html
- Bushatz A. How to spot a veteran. Military.com. October 3, 2022. Updated September 16, 2024. Accessed July 24, 2025. https://www.military.com/veterans-day/how-spot-veteran.html
- Monroe R. How to spot a military imposter. The New Yorker. October 19, 2020. Accessed July 24, 2025. https://www.newyorker.com/magazine/2020/10/26/how-to-spot-a-military-impostor
The better part of valor is discretion.
Henry IV, Part 1 by William Shakespeare1
This is the second part of an exploration of the phenomenon of stolen valor, where individuals claim military exploits or acts of heroism that are either fabricated or exaggerated, and/or awards and medals they did not earn.2 In June, I focused on the unsettling story of Sarah Cavanaugh, a young US Department of Veterans Affairs (VA) social worker who posed as a decorated, heroic, and seriously wounded Marine veteran for years. Cavanaugh’s manipulative masquerade allowed her to receive coveted spots in veteran recovery programs, thousands of dollars in fraudulent donations, the leadership of a local Veterans of Foreign Wars post, and eventually a federal conviction and prison sentence.3 The first column focused on the legal history of stolen valor; this editorial analyzes the clinical import and ethical impact of the behavior of military imposters. Military imposters are the culprits who steal valor.
It would be easy and perhaps reassuring to assume that stolen valor has emerged as another deplorable example of a national culture in which the betrayal of trust in human beings and loss of faith in institutions and aspirations has reached a nadir. Ironically, stolen valor is inextricably linked to the founding of the United States. When General George Washington inaugurated the American military tradition of awarding decorations to honor the bravery and sacrifices of the patriot Army, he anticipated military imposters. He tried to deter stolen valor through the threat of chastisement: “Should any who are not entitled to these honors have the insolence to assume the badges of them, they shall be severely punished,” Washington warned.4
It is plausible to think such despicable conduct occurs only as the ugly side of the beauty of our unparalleled national freedom, but this is a mistake. Cases of stolen valor have been reported in many countries around the world, with some of the most infamous found in the United Kingdom.5
While many brazen military imposters like Cavanaugh never serve, there is a small subset who honorably wore a uniform yet embellish their service record with secret missions and meritorious gallantry that purportedly earned them high rank and even higher awards. A most puzzling and disturbing example of this group is an allegation that surfaced when celebrated Navy SEAL Chris Kyle declared in American Sniper that he had won 3 additional combat awards for combat valor in addition to the Silver Star and 3 Bronze Stars actually listed in his service record.6
The fact that for centuries stolen valor has plagued multiple nations suggests, at least to this psychiatrically trained mind, that something deeper and darker in human nature than profit alone drives military imposters. Philosopher Verna Gehring has distilled these less tangible motivations into the concept of virtue imposters. According to Gehring, military phonies are a notorious exemplar: “The military phony adopts a past not her own, acts of courage she did not perform—she impersonates the heroic character and virtues she does not possess.”7 There could be no more apposite depiction of Cavanaugh, other military imposters, or a legion of other offenders of honor. 8
As with Cavanaugh, financial gain is a byproduct of the machinations of military imposters and is usually secondary to the pursuit of nonmaterial rewards such as power, influence, admiration, emulation, empathy, and charity. Gehring contends, and I agree, that virtue imposters are more pernicious and culpable than the plethora of more prosaic scammers and swindlers who use deceit primarily as a means of economic exploitation: “The virtue impostor by contrast plays on people’s better natures—their generosity, humility, and their need for heroes.”7
Military imposters cause real and lasting harm. Every veteran who exaggerates claims or scams the VA unjustly steals human and monetary resources from other deserving veterans whose integrity would not permit them to break the rules.9 Yet, even more harmful is the potential damage to therapeutic relationships: federal practitioners may become skeptical of a veteran’s history even when there is little to no grounds for suspicion. Veterans, in turn, may experience a breach of trust and betrayal not only from health care professionals and VA leaders but from their brothers and sisters in arms. On an ever-wider scale, every military impostor who is exposed may diminish the respect and honor all veterans have earned.
It is clear, then, why a small group of former service members has adopted the cause of uncovering military imposters and adroitly using the media to identify signs of stolen valor.10 Yet deception mars even these mostly well-intentioned campaigns, as some more zealous stolen valor hunters may make allegations that turn out to be false.11 Nevertheless, 500 years ago and in a very different context Shakespeare was, right on the mark: the better part of valor is discretion in describing one’s achievements, in relying on the veracity of our veteran’s narratives, and when there are sound reasons to do so verifying the truth of what our patients, friends, and even family tell us about their time in the military.1
The better part of valor is discretion.
Henry IV, Part 1 by William Shakespeare1
This is the second part of an exploration of the phenomenon of stolen valor, where individuals claim military exploits or acts of heroism that are either fabricated or exaggerated, and/or awards and medals they did not earn.2 In June, I focused on the unsettling story of Sarah Cavanaugh, a young US Department of Veterans Affairs (VA) social worker who posed as a decorated, heroic, and seriously wounded Marine veteran for years. Cavanaugh’s manipulative masquerade allowed her to receive coveted spots in veteran recovery programs, thousands of dollars in fraudulent donations, the leadership of a local Veterans of Foreign Wars post, and eventually a federal conviction and prison sentence.3 The first column focused on the legal history of stolen valor; this editorial analyzes the clinical import and ethical impact of the behavior of military imposters. Military imposters are the culprits who steal valor.
It would be easy and perhaps reassuring to assume that stolen valor has emerged as another deplorable example of a national culture in which the betrayal of trust in human beings and loss of faith in institutions and aspirations has reached a nadir. Ironically, stolen valor is inextricably linked to the founding of the United States. When General George Washington inaugurated the American military tradition of awarding decorations to honor the bravery and sacrifices of the patriot Army, he anticipated military imposters. He tried to deter stolen valor through the threat of chastisement: “Should any who are not entitled to these honors have the insolence to assume the badges of them, they shall be severely punished,” Washington warned.4
It is plausible to think such despicable conduct occurs only as the ugly side of the beauty of our unparalleled national freedom, but this is a mistake. Cases of stolen valor have been reported in many countries around the world, with some of the most infamous found in the United Kingdom.5
While many brazen military imposters like Cavanaugh never serve, there is a small subset who honorably wore a uniform yet embellish their service record with secret missions and meritorious gallantry that purportedly earned them high rank and even higher awards. A most puzzling and disturbing example of this group is an allegation that surfaced when celebrated Navy SEAL Chris Kyle declared in American Sniper that he had won 3 additional combat awards for combat valor in addition to the Silver Star and 3 Bronze Stars actually listed in his service record.6
The fact that for centuries stolen valor has plagued multiple nations suggests, at least to this psychiatrically trained mind, that something deeper and darker in human nature than profit alone drives military imposters. Philosopher Verna Gehring has distilled these less tangible motivations into the concept of virtue imposters. According to Gehring, military phonies are a notorious exemplar: “The military phony adopts a past not her own, acts of courage she did not perform—she impersonates the heroic character and virtues she does not possess.”7 There could be no more apposite depiction of Cavanaugh, other military imposters, or a legion of other offenders of honor. 8
As with Cavanaugh, financial gain is a byproduct of the machinations of military imposters and is usually secondary to the pursuit of nonmaterial rewards such as power, influence, admiration, emulation, empathy, and charity. Gehring contends, and I agree, that virtue imposters are more pernicious and culpable than the plethora of more prosaic scammers and swindlers who use deceit primarily as a means of economic exploitation: “The virtue impostor by contrast plays on people’s better natures—their generosity, humility, and their need for heroes.”7
Military imposters cause real and lasting harm. Every veteran who exaggerates claims or scams the VA unjustly steals human and monetary resources from other deserving veterans whose integrity would not permit them to break the rules.9 Yet, even more harmful is the potential damage to therapeutic relationships: federal practitioners may become skeptical of a veteran’s history even when there is little to no grounds for suspicion. Veterans, in turn, may experience a breach of trust and betrayal not only from health care professionals and VA leaders but from their brothers and sisters in arms. On an ever-wider scale, every military impostor who is exposed may diminish the respect and honor all veterans have earned.
It is clear, then, why a small group of former service members has adopted the cause of uncovering military imposters and adroitly using the media to identify signs of stolen valor.10 Yet deception mars even these mostly well-intentioned campaigns, as some more zealous stolen valor hunters may make allegations that turn out to be false.11 Nevertheless, 500 years ago and in a very different context Shakespeare was, right on the mark: the better part of valor is discretion in describing one’s achievements, in relying on the veracity of our veteran’s narratives, and when there are sound reasons to do so verifying the truth of what our patients, friends, and even family tell us about their time in the military.1
- Shakespeare W. Introduction in: Henry IV, Part 1. Folger Sharespeare Library. Accessed July 24, 2025. https://www.folger.edu/explore/shakespeares-works/henry-iv-part-1/
- Geppert CM. What about stolen valor actually is illegal? Fed Pract. 2025;42(6):218-219. doi:10.12788/fp.0599
- Lehrfeld J. Woman who faked being cancer-stricken Marine gets 6 years in prison. Military Times. March 15, 2023. Accessed July 24, 2025. https://www.militarytimes.com/news/your-military/2023/03/15/woman-who-faked-being-sick-marine-purple-heart-gets-6-years-in-prison/
- Washington G. General Orders, 7 August 1782 in: Papers of George Washington. Founders Online. August 7, 1782. Accessed July 24, 2025. https://founders.archives.gov/documents/Washington/99-01-02-09056 5. Simpson LK. The men who impersonate military personnel for stolen glory. The Conversation. Updated November 17, 2016. Accessed July 24, 2025. https://theconversation.com/the-men-who-impersonate-military-personnel-for-stolen-glory-62233
- Larter DB. New questions cast doubt on ‘American Sniper‘ Chris Kyle‘s combat record. Navy Times. May 25, 2016. Accessed July 24, 2025. https://www.navytimes.com/news/your-navy/2016/05/25/new-questions-cast-doubt-on-american-sniper-chris-kyle-s-combat-record
- Gehring VV. Phonies, fakes, and frauds—and the social harms they cause. Philos Public Policy Q. 2003;23:14-20.
- Liem, E. The 6 most shocking military imposters ever. Military.com. July 7, 2015. Accessed July 29, 2025. https://www.military.com/undertheradar/2015/07/the-6-most-shocking-military-impostors-ever 9. Sisk R. Some vets with PTSD are scamming the VA: testimony. Military.com. June 8, 2017. Accessed July 24, 2025. https://www.military.com/daily-news/2017/06/08/some-vets-with-ptsd-are-scamming-va-testimony.html
- Bushatz A. How to spot a veteran. Military.com. October 3, 2022. Updated September 16, 2024. Accessed July 24, 2025. https://www.military.com/veterans-day/how-spot-veteran.html
- Monroe R. How to spot a military imposter. The New Yorker. October 19, 2020. Accessed July 24, 2025. https://www.newyorker.com/magazine/2020/10/26/how-to-spot-a-military-impostor
- Shakespeare W. Introduction in: Henry IV, Part 1. Folger Sharespeare Library. Accessed July 24, 2025. https://www.folger.edu/explore/shakespeares-works/henry-iv-part-1/
- Geppert CM. What about stolen valor actually is illegal? Fed Pract. 2025;42(6):218-219. doi:10.12788/fp.0599
- Lehrfeld J. Woman who faked being cancer-stricken Marine gets 6 years in prison. Military Times. March 15, 2023. Accessed July 24, 2025. https://www.militarytimes.com/news/your-military/2023/03/15/woman-who-faked-being-sick-marine-purple-heart-gets-6-years-in-prison/
- Washington G. General Orders, 7 August 1782 in: Papers of George Washington. Founders Online. August 7, 1782. Accessed July 24, 2025. https://founders.archives.gov/documents/Washington/99-01-02-09056 5. Simpson LK. The men who impersonate military personnel for stolen glory. The Conversation. Updated November 17, 2016. Accessed July 24, 2025. https://theconversation.com/the-men-who-impersonate-military-personnel-for-stolen-glory-62233
- Larter DB. New questions cast doubt on ‘American Sniper‘ Chris Kyle‘s combat record. Navy Times. May 25, 2016. Accessed July 24, 2025. https://www.navytimes.com/news/your-navy/2016/05/25/new-questions-cast-doubt-on-american-sniper-chris-kyle-s-combat-record
- Gehring VV. Phonies, fakes, and frauds—and the social harms they cause. Philos Public Policy Q. 2003;23:14-20.
- Liem, E. The 6 most shocking military imposters ever. Military.com. July 7, 2015. Accessed July 29, 2025. https://www.military.com/undertheradar/2015/07/the-6-most-shocking-military-impostors-ever 9. Sisk R. Some vets with PTSD are scamming the VA: testimony. Military.com. June 8, 2017. Accessed July 24, 2025. https://www.military.com/daily-news/2017/06/08/some-vets-with-ptsd-are-scamming-va-testimony.html
- Bushatz A. How to spot a veteran. Military.com. October 3, 2022. Updated September 16, 2024. Accessed July 24, 2025. https://www.military.com/veterans-day/how-spot-veteran.html
- Monroe R. How to spot a military imposter. The New Yorker. October 19, 2020. Accessed July 24, 2025. https://www.newyorker.com/magazine/2020/10/26/how-to-spot-a-military-impostor
Military Imposters: What Drives Them and How They Damage Us All
Military Imposters: What Drives Them and How They Damage Us All