User login
Diagnostic Value of Deep Punch Biopsies in Intravascular Large B-cell Lymphoma
Diagnostic Value of Deep Punch Biopsies in Intravascular Large B-cell Lymphoma
Intravascular large B-cell lymphoma (IVBCL) is an exceedingly rare aggressive form of non-Hodgkin lymphoma with tumor cells growing selectively in vascular lumina.1 The annual incidence of IVBCL is fewer than 0.5 cases per 1,000,000 individuals worldwide.2 Only about 500 known cases of IVBCL have been recorded in the literature,3 and it accounts for less than 1% of all lymphomas. It generally affects middle-aged to elderly individuals, with an average age at diagnosis of 70 years.2 It has a predilection for men and commonly develops in individuals who are immunosuppressed.3,4
Multiple variants of IVBCL have been described in the literature, with central nervous system and cutaneous involvement being the most classic findings.5 Bone marrow involvement with hepatosplenomegaly also has been noted in the literature.6,7 Diagnosis of IVBCL and its variants requires a high index of suspicion, as the clinical manifestations and tissues involved typically are nonspecific and highly variable. Even in the classic variant of IVBCL, skin involvement is only reported in approximately half of cases.3 When present, cutaneous manifestations can range from nodules and violaceous plaques to induration and telangiectasias.3 Lymphadenopathy and lymphoma (leukemic) cells are not seen on a peripheral blood smear.2,8,9
The lack of lymphadenopathy or identifiable leukemic cells in the peripheral blood presents a diagnostic dilemma, as sufficient information for accurate diagnosis must be obtained while minimizing invasive procedures and resource expenditure. Because IVBCL cells can reside in the vascular lumina of various organs, numerous biopsy sites have been proposed for diagnosis of lymphoma, including the bone marrow, skin, prostate, adrenal gland, brain, liver, and kidneys.10 While some studies have reported that the optimal diagnostic site is the bone marrow, skin biopsies are more routinely carried out, as they represent a convenient and cost-effective alternative to other more invasive techniques.6,7,10 Studies have shown biopsy sensitivity values ranging from 77.8% to 83.3% for detection of IVBCL in normal-appearing skin, which is comparable to the sensitivities of a bone marrow biopsy.7,8 Although skin biopsy of random sites has shown diagnostic efficacy, some studies have proposed that biopsies taken from hemangiomas and other hypervascular lesions can further improve diagnostic yield, as lymphoma cells often are present in capillaries of subcutaneous adipose tissue.6,10,11 However, no obvious clinicopathologic differences were observed between IVBCL with and without involvement of a cutaneous hemangioma.11
The purpose of this study was to determine the diagnostic efficacy of skin biopsies for detecting IVBCL at various body sites and to establish whether biopsies from hemangiomas yield higher diagnostic value.
Methods
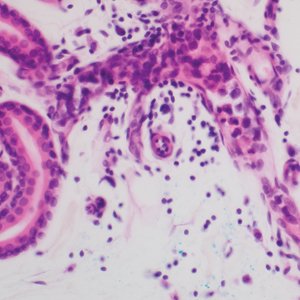
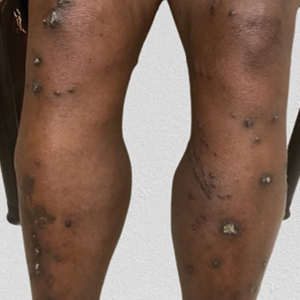
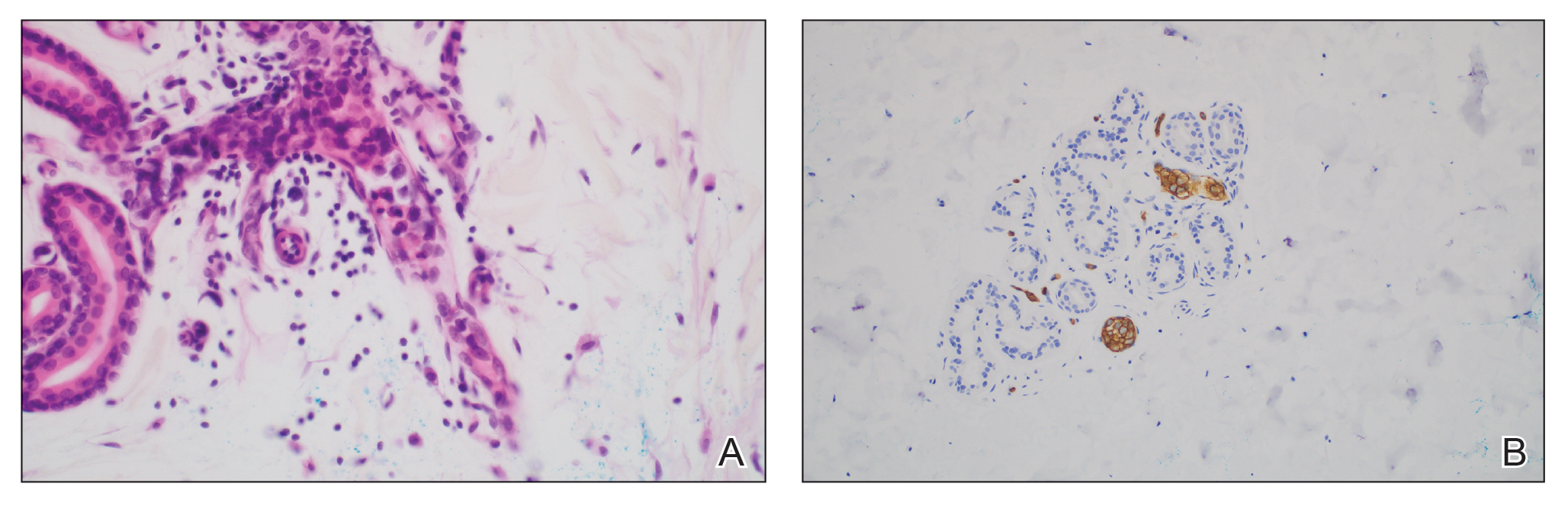
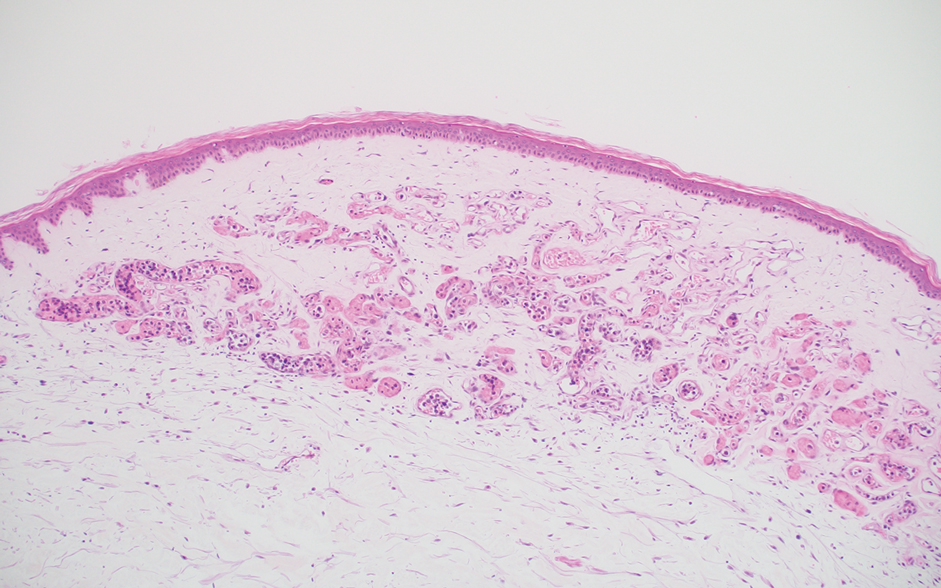
A 66-year-old man recently died at our institution secondary to IVBCL. His disease course was characterized by multiple hospital admissions in a 6-month period for fever of unknown origin and tachycardia unresponsive to broad-spectrum antibiotics and systemic steroids. The patient declined over the course of 3 to 4 weeks with findings suggestive of lymphoma and tumor lysis syndrome, and he eventually developed shock, hypoxic respiratory failure, and acute renal failure. As imaging studies and examinations had not shown lymphadenopathy, bone marrow biopsy was performed, and dermatology was consulted to perform skin biopsies to evaluate for IVBCL. Both bone marrow biopsies and random skin biopsies from the abdomen showed large and atypical CD20+ B cells within select vascular lumina (Figure). No extravascular lymphoma cells were seen. Based on the bone marrow and skin biopsies, a diagnosis of IVBCL was made. Unfortunately, no progress was made clinically, and the patient was transitioned to comfort measures. Upon the patient’s death, his family expressed interest in participating in IVBCL research and agreed to a limited autopsy consisting of numerous skin biopsies to evaluate different body sites and biopsy types (normal skin vs hemangiomas) to ascertain whether diagnostic yield could be increased by performing selective biopsies of hemangiomas if IVBCL was suspected.
Twenty-four postmortem 4-mm punch biopsies containing subcutaneous adipose tissue were taken within 24 hours of the death of the patient before embalming. The biopsies were taken from all regions of the body except the head and neck for cosmetic preservation of the decedent. Eighteen of the biopsies were taken from random sites of normal-appearing skin; the remaining 6 were taken from clinically identifiable cherry hemangiomas (5 on the trunk and 1 on the thigh). There was a variable degree of livor mortis in the dependent areas of the body, which was included in the random biopsies from the back to ensure any pooling of dependent blood would not alter the findings.
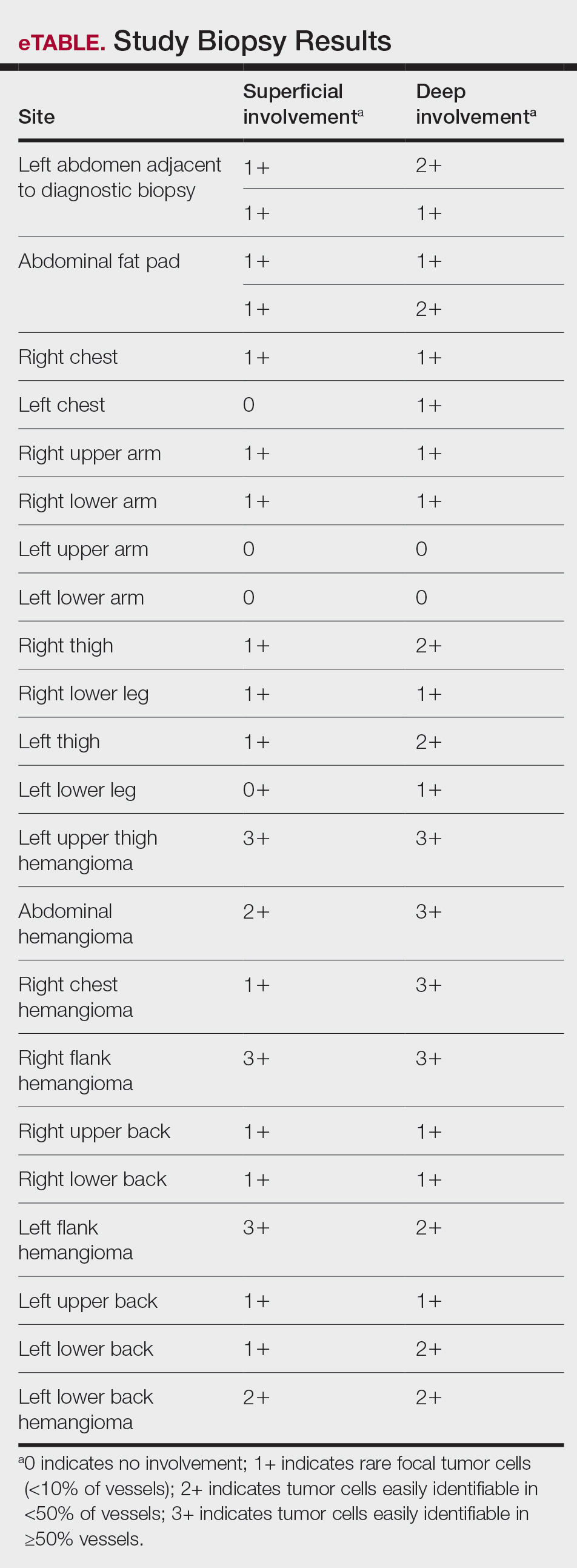
A histopathologic examination by a board-certified dermatopathologist (M.P.) on a single hematoxylin-eosin–stained level was performed to evaluate each biopsy for superficial involvement and deep involvement by IVBCL. Superficial involvement was defined as dermal involvement at or above the level of the eccrine sweat glands; deep involvement was defined as dermal involvement beneath the eccrine sweat glands and all subcutaneous fat present. Skin and bone marrow biopsies used to make the original diagnosis prior to the patient’s death were reviewed, including CD20 immunohistochemistry for morphologic comparison to the study slides. Involvement was graded as 0 to 3+ (eTable).
Results
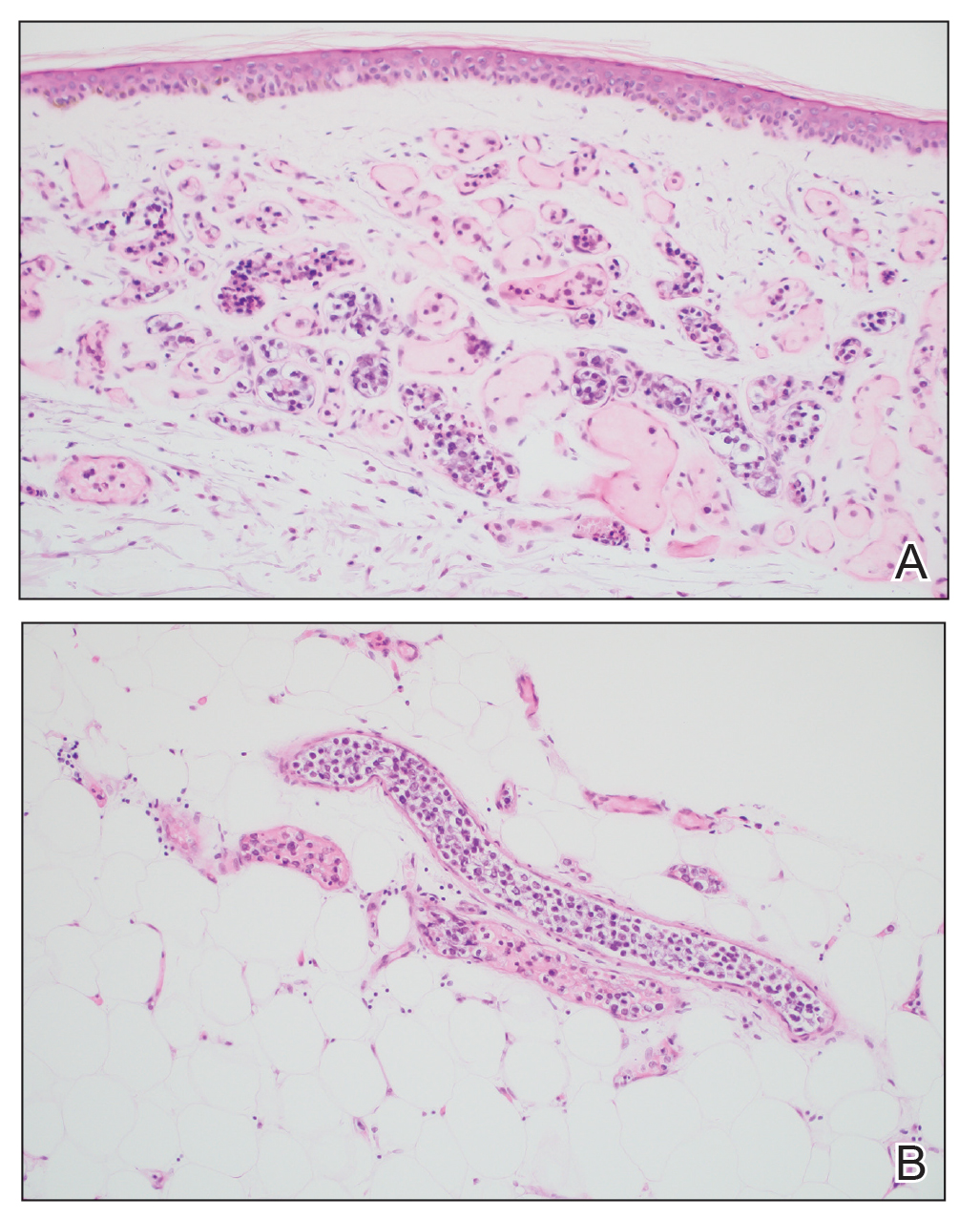
Results from all 24 biopsies are shown in the eTable. Twenty-two (91.7%) biopsies showed at least focal involvement by IVBCL. Nine (37.5%) biopsies showed more deep vs superficial involvement of the same site. On average, the 6 biopsies from clinically detected hemangiomas showed more involvement by IVBCL than the random biopsies (eFigures 1 and 2A). The superficial involvement of skin with a hemangioma showed an average score of 2.33 v 0.78 when compared with the superficial aspect of the random biopsies; the deep involvement of skin with a hemangioma showed an average score of 2.67 vs 1.16 when compared with the deep aspect of the random biopsies (eFigure 2B).
Comment
Intravascular large B-cell lymphoma is an aggressive malignancy that traditionally is difficult to diagnose. Many efforts have been made to improve detection and early diagnosis. As cutaneous involvement is common and sometimes the only sign of disease, dermatologists may be called upon to evaluate and biopsy patients with this suspected diagnosis. The purpose of our study was to improve diagnostic efficacy by methodically performing numerous biopsies and assessing the level of involvement of the superficial and deep skin as well as involvement of hemangiomas. The goal of this meticulous approach was to identify the highest-yield areas for biopsy with minimal impact on the patient. Our results showed that random skin biopsies are an effective way to identify IVBCL. Twenty-two (91.7%) biopsies contained at least focal lymphoma cells. Although the 2 biopsies that showed no tumor cells at all happened to both be from the left arm, this is believed to be coincidental. No discernable pattern was identified regarding involvement and anatomic region. Even though 20 (83.3%) biopsies showed superficial involvement, deep biopsy is essential, as 9 (37.5%) biopsies showed increased deep involvement compared to superficial involvement. Therefore, a deep punch biopsy is essential for maximum sensitivity.
Hemangiomas provide a potential target that could increase the sensitivity of a biopsy in the absence of clinical findings, when the disease in question is exclusively intravascular. The data gathered in this study support this idea, as biopsies from hemangiomas showed increased involvement compared to random biopsies, both superficially and deep (2.33 vs 0.78 and 2.67 vs 1.16, respectively). Interestingly, the hemangioma biopsy sites showed increased deep and superficial involvement, despite these typical cherry hemangiomas only involving the superficial dermis. One possible explanation for this is that the hemangiomas have larger-caliber feeder vessels with increased blood flow beneath them. It would then follow that this increased vasculature would increase the chances of identifying intravascular lymphoma cells. This finding further accentuates the need for a deep punch biopsy containing subcutaneous fat.
Completing the study in the setting of an autopsy provided the advantage of being able to take numerous biopsies without increased harm to the patient. This extensive set of biopsies would not be reasonable to complete on a living patient. This study also has limitations. Although this patient did fall within the typical demographics for patients with IVBCL, the data were still limited to 1 patient. This autopsy format (on a patient whose cause of death was indeed IVBCL) also implies terminal disease, which may mean the patient had a larger disease burden than a living patient who would typically be biopsied. Although this increased disease burden may have increased the sensitivity of finding IVBCL in the biopsies of this study, this further emphasizes the importance of trying to determine any factors that could increase sensitivity in a living patient with a lower disease burden.
Conclusion
Skin biopsies can provide a sensitive, low-cost, and low-morbidity method to assess a patient for IVBCL. Though random skin biopsies can yield valuable information, deep, 4-mm punch biopsies of clinically identifiable hemangiomas may provide the highest sensitivity for IVBCL.
- Ponzoni M, Campo E, Nakamura S. Intravascular large B-cell lymphoma: a chameleon with multiple faces and many masks. Blood. 2018;132:1561-1567. doi:10.1182/blood-2017-04-737445
- Roy AM, Pandey Y, Middleton D, et al. Intravascular large B-cell lymphoma: a diagnostic dilemma. Cureus. 2021;13:e16459. doi:10.7759/cureus.16459
- Bayçelebi D, Yildiz L, S?entürk N. A case report and literature review of cutaneous intravascular large B-cell lymphoma presenting clinically as panniculitis: a difficult diagnosis, but a good prognosis. An Bras Dermatol. 2021;96:72-75. doi:10.1016/j.abd.2020.08.004
- Orwat DE, Batalis NI. Intravascular large B-cell lymphoma. Arch Pathol Lab Med. 2012;136:333-338. doi:10.5858/arpa.2010-0747-RS
- Breakell T, Waibel H, Schliep S, et al. Intravascular large B-cell lymphoma: a review with a focus on the prognostic value of skininvolvement. Curr Oncol. 2022;29:2909-2919. doi:10.3390/curroncol29050237
- Oppegard L, O’Donnell M, Piro K, et al. Going skin deep: excavating a diagnosis of intravascular large B cell lymphoma. J Gen Intern Med. 2020;35:3368-3371. doi:10.1007/s11606-020-06141-1
- Barker JL, Swarup O, Puliyayil A. Intravascular large B-cell lymphoma: representative cases and approach to diagnosis. BMJ Case Rep. 2021;14:e244069. doi:10.1136/bcr-2021-244069
- Matsue K, Asada N, Odawara J, et al. Random skin biopsy and bone marrow biopsy for diagnosis of intravascular large B cell lymphoma. Ann Hematol. 2011;90:417-421. doi:10.1007/s00277-010-1101-3
- Shimada K, Kinoshita T, Naoe T, et al. Presentation and management of intravascular large B-cell lymphoma. Lancet Oncol. 2009;10:895-902. doi:10.1016/S1470-2045(09)70140-8
- Adachi Y, Kosami K, Mizuta N, et al. Benefits of skin biopsy of senile hemangioma in intravascular large B-cell lymphoma: a case report and review of the literature. Oncol Lett. 2014;7:2003-2006. doi:10.3892/ol.2014.2017
- Ishida M, Hodohara K, Yoshida T, et al. Intravascular large B-cell lymphoma colonizing in senile hemangioma: a case report and proposal of possible diagnostic strategy for intravascular lymphoma. Pathol Int. 2011;61:555-557. doi:10.1111/j.1440-1827.2011.02697.x
Intravascular large B-cell lymphoma (IVBCL) is an exceedingly rare aggressive form of non-Hodgkin lymphoma with tumor cells growing selectively in vascular lumina.1 The annual incidence of IVBCL is fewer than 0.5 cases per 1,000,000 individuals worldwide.2 Only about 500 known cases of IVBCL have been recorded in the literature,3 and it accounts for less than 1% of all lymphomas. It generally affects middle-aged to elderly individuals, with an average age at diagnosis of 70 years.2 It has a predilection for men and commonly develops in individuals who are immunosuppressed.3,4
Multiple variants of IVBCL have been described in the literature, with central nervous system and cutaneous involvement being the most classic findings.5 Bone marrow involvement with hepatosplenomegaly also has been noted in the literature.6,7 Diagnosis of IVBCL and its variants requires a high index of suspicion, as the clinical manifestations and tissues involved typically are nonspecific and highly variable. Even in the classic variant of IVBCL, skin involvement is only reported in approximately half of cases.3 When present, cutaneous manifestations can range from nodules and violaceous plaques to induration and telangiectasias.3 Lymphadenopathy and lymphoma (leukemic) cells are not seen on a peripheral blood smear.2,8,9
The lack of lymphadenopathy or identifiable leukemic cells in the peripheral blood presents a diagnostic dilemma, as sufficient information for accurate diagnosis must be obtained while minimizing invasive procedures and resource expenditure. Because IVBCL cells can reside in the vascular lumina of various organs, numerous biopsy sites have been proposed for diagnosis of lymphoma, including the bone marrow, skin, prostate, adrenal gland, brain, liver, and kidneys.10 While some studies have reported that the optimal diagnostic site is the bone marrow, skin biopsies are more routinely carried out, as they represent a convenient and cost-effective alternative to other more invasive techniques.6,7,10 Studies have shown biopsy sensitivity values ranging from 77.8% to 83.3% for detection of IVBCL in normal-appearing skin, which is comparable to the sensitivities of a bone marrow biopsy.7,8 Although skin biopsy of random sites has shown diagnostic efficacy, some studies have proposed that biopsies taken from hemangiomas and other hypervascular lesions can further improve diagnostic yield, as lymphoma cells often are present in capillaries of subcutaneous adipose tissue.6,10,11 However, no obvious clinicopathologic differences were observed between IVBCL with and without involvement of a cutaneous hemangioma.11
The purpose of this study was to determine the diagnostic efficacy of skin biopsies for detecting IVBCL at various body sites and to establish whether biopsies from hemangiomas yield higher diagnostic value.
Methods
A 66-year-old man recently died at our institution secondary to IVBCL. His disease course was characterized by multiple hospital admissions in a 6-month period for fever of unknown origin and tachycardia unresponsive to broad-spectrum antibiotics and systemic steroids. The patient declined over the course of 3 to 4 weeks with findings suggestive of lymphoma and tumor lysis syndrome, and he eventually developed shock, hypoxic respiratory failure, and acute renal failure. As imaging studies and examinations had not shown lymphadenopathy, bone marrow biopsy was performed, and dermatology was consulted to perform skin biopsies to evaluate for IVBCL. Both bone marrow biopsies and random skin biopsies from the abdomen showed large and atypical CD20+ B cells within select vascular lumina (Figure). No extravascular lymphoma cells were seen. Based on the bone marrow and skin biopsies, a diagnosis of IVBCL was made. Unfortunately, no progress was made clinically, and the patient was transitioned to comfort measures. Upon the patient’s death, his family expressed interest in participating in IVBCL research and agreed to a limited autopsy consisting of numerous skin biopsies to evaluate different body sites and biopsy types (normal skin vs hemangiomas) to ascertain whether diagnostic yield could be increased by performing selective biopsies of hemangiomas if IVBCL was suspected.

Twenty-four postmortem 4-mm punch biopsies containing subcutaneous adipose tissue were taken within 24 hours of the death of the patient before embalming. The biopsies were taken from all regions of the body except the head and neck for cosmetic preservation of the decedent. Eighteen of the biopsies were taken from random sites of normal-appearing skin; the remaining 6 were taken from clinically identifiable cherry hemangiomas (5 on the trunk and 1 on the thigh). There was a variable degree of livor mortis in the dependent areas of the body, which was included in the random biopsies from the back to ensure any pooling of dependent blood would not alter the findings.
A histopathologic examination by a board-certified dermatopathologist (M.P.) on a single hematoxylin-eosin–stained level was performed to evaluate each biopsy for superficial involvement and deep involvement by IVBCL. Superficial involvement was defined as dermal involvement at or above the level of the eccrine sweat glands; deep involvement was defined as dermal involvement beneath the eccrine sweat glands and all subcutaneous fat present. Skin and bone marrow biopsies used to make the original diagnosis prior to the patient’s death were reviewed, including CD20 immunohistochemistry for morphologic comparison to the study slides. Involvement was graded as 0 to 3+ (eTable).

Results
Results from all 24 biopsies are shown in the eTable. Twenty-two (91.7%) biopsies showed at least focal involvement by IVBCL. Nine (37.5%) biopsies showed more deep vs superficial involvement of the same site. On average, the 6 biopsies from clinically detected hemangiomas showed more involvement by IVBCL than the random biopsies (eFigures 1 and 2A). The superficial involvement of skin with a hemangioma showed an average score of 2.33 v 0.78 when compared with the superficial aspect of the random biopsies; the deep involvement of skin with a hemangioma showed an average score of 2.67 vs 1.16 when compared with the deep aspect of the random biopsies (eFigure 2B).


Comment
Intravascular large B-cell lymphoma is an aggressive malignancy that traditionally is difficult to diagnose. Many efforts have been made to improve detection and early diagnosis. As cutaneous involvement is common and sometimes the only sign of disease, dermatologists may be called upon to evaluate and biopsy patients with this suspected diagnosis. The purpose of our study was to improve diagnostic efficacy by methodically performing numerous biopsies and assessing the level of involvement of the superficial and deep skin as well as involvement of hemangiomas. The goal of this meticulous approach was to identify the highest-yield areas for biopsy with minimal impact on the patient. Our results showed that random skin biopsies are an effective way to identify IVBCL. Twenty-two (91.7%) biopsies contained at least focal lymphoma cells. Although the 2 biopsies that showed no tumor cells at all happened to both be from the left arm, this is believed to be coincidental. No discernable pattern was identified regarding involvement and anatomic region. Even though 20 (83.3%) biopsies showed superficial involvement, deep biopsy is essential, as 9 (37.5%) biopsies showed increased deep involvement compared to superficial involvement. Therefore, a deep punch biopsy is essential for maximum sensitivity.
Hemangiomas provide a potential target that could increase the sensitivity of a biopsy in the absence of clinical findings, when the disease in question is exclusively intravascular. The data gathered in this study support this idea, as biopsies from hemangiomas showed increased involvement compared to random biopsies, both superficially and deep (2.33 vs 0.78 and 2.67 vs 1.16, respectively). Interestingly, the hemangioma biopsy sites showed increased deep and superficial involvement, despite these typical cherry hemangiomas only involving the superficial dermis. One possible explanation for this is that the hemangiomas have larger-caliber feeder vessels with increased blood flow beneath them. It would then follow that this increased vasculature would increase the chances of identifying intravascular lymphoma cells. This finding further accentuates the need for a deep punch biopsy containing subcutaneous fat.
Completing the study in the setting of an autopsy provided the advantage of being able to take numerous biopsies without increased harm to the patient. This extensive set of biopsies would not be reasonable to complete on a living patient. This study also has limitations. Although this patient did fall within the typical demographics for patients with IVBCL, the data were still limited to 1 patient. This autopsy format (on a patient whose cause of death was indeed IVBCL) also implies terminal disease, which may mean the patient had a larger disease burden than a living patient who would typically be biopsied. Although this increased disease burden may have increased the sensitivity of finding IVBCL in the biopsies of this study, this further emphasizes the importance of trying to determine any factors that could increase sensitivity in a living patient with a lower disease burden.
Conclusion
Skin biopsies can provide a sensitive, low-cost, and low-morbidity method to assess a patient for IVBCL. Though random skin biopsies can yield valuable information, deep, 4-mm punch biopsies of clinically identifiable hemangiomas may provide the highest sensitivity for IVBCL.
Intravascular large B-cell lymphoma (IVBCL) is an exceedingly rare aggressive form of non-Hodgkin lymphoma with tumor cells growing selectively in vascular lumina.1 The annual incidence of IVBCL is fewer than 0.5 cases per 1,000,000 individuals worldwide.2 Only about 500 known cases of IVBCL have been recorded in the literature,3 and it accounts for less than 1% of all lymphomas. It generally affects middle-aged to elderly individuals, with an average age at diagnosis of 70 years.2 It has a predilection for men and commonly develops in individuals who are immunosuppressed.3,4
Multiple variants of IVBCL have been described in the literature, with central nervous system and cutaneous involvement being the most classic findings.5 Bone marrow involvement with hepatosplenomegaly also has been noted in the literature.6,7 Diagnosis of IVBCL and its variants requires a high index of suspicion, as the clinical manifestations and tissues involved typically are nonspecific and highly variable. Even in the classic variant of IVBCL, skin involvement is only reported in approximately half of cases.3 When present, cutaneous manifestations can range from nodules and violaceous plaques to induration and telangiectasias.3 Lymphadenopathy and lymphoma (leukemic) cells are not seen on a peripheral blood smear.2,8,9
The lack of lymphadenopathy or identifiable leukemic cells in the peripheral blood presents a diagnostic dilemma, as sufficient information for accurate diagnosis must be obtained while minimizing invasive procedures and resource expenditure. Because IVBCL cells can reside in the vascular lumina of various organs, numerous biopsy sites have been proposed for diagnosis of lymphoma, including the bone marrow, skin, prostate, adrenal gland, brain, liver, and kidneys.10 While some studies have reported that the optimal diagnostic site is the bone marrow, skin biopsies are more routinely carried out, as they represent a convenient and cost-effective alternative to other more invasive techniques.6,7,10 Studies have shown biopsy sensitivity values ranging from 77.8% to 83.3% for detection of IVBCL in normal-appearing skin, which is comparable to the sensitivities of a bone marrow biopsy.7,8 Although skin biopsy of random sites has shown diagnostic efficacy, some studies have proposed that biopsies taken from hemangiomas and other hypervascular lesions can further improve diagnostic yield, as lymphoma cells often are present in capillaries of subcutaneous adipose tissue.6,10,11 However, no obvious clinicopathologic differences were observed between IVBCL with and without involvement of a cutaneous hemangioma.11
The purpose of this study was to determine the diagnostic efficacy of skin biopsies for detecting IVBCL at various body sites and to establish whether biopsies from hemangiomas yield higher diagnostic value.
Methods
A 66-year-old man recently died at our institution secondary to IVBCL. His disease course was characterized by multiple hospital admissions in a 6-month period for fever of unknown origin and tachycardia unresponsive to broad-spectrum antibiotics and systemic steroids. The patient declined over the course of 3 to 4 weeks with findings suggestive of lymphoma and tumor lysis syndrome, and he eventually developed shock, hypoxic respiratory failure, and acute renal failure. As imaging studies and examinations had not shown lymphadenopathy, bone marrow biopsy was performed, and dermatology was consulted to perform skin biopsies to evaluate for IVBCL. Both bone marrow biopsies and random skin biopsies from the abdomen showed large and atypical CD20+ B cells within select vascular lumina (Figure). No extravascular lymphoma cells were seen. Based on the bone marrow and skin biopsies, a diagnosis of IVBCL was made. Unfortunately, no progress was made clinically, and the patient was transitioned to comfort measures. Upon the patient’s death, his family expressed interest in participating in IVBCL research and agreed to a limited autopsy consisting of numerous skin biopsies to evaluate different body sites and biopsy types (normal skin vs hemangiomas) to ascertain whether diagnostic yield could be increased by performing selective biopsies of hemangiomas if IVBCL was suspected.

Twenty-four postmortem 4-mm punch biopsies containing subcutaneous adipose tissue were taken within 24 hours of the death of the patient before embalming. The biopsies were taken from all regions of the body except the head and neck for cosmetic preservation of the decedent. Eighteen of the biopsies were taken from random sites of normal-appearing skin; the remaining 6 were taken from clinically identifiable cherry hemangiomas (5 on the trunk and 1 on the thigh). There was a variable degree of livor mortis in the dependent areas of the body, which was included in the random biopsies from the back to ensure any pooling of dependent blood would not alter the findings.
A histopathologic examination by a board-certified dermatopathologist (M.P.) on a single hematoxylin-eosin–stained level was performed to evaluate each biopsy for superficial involvement and deep involvement by IVBCL. Superficial involvement was defined as dermal involvement at or above the level of the eccrine sweat glands; deep involvement was defined as dermal involvement beneath the eccrine sweat glands and all subcutaneous fat present. Skin and bone marrow biopsies used to make the original diagnosis prior to the patient’s death were reviewed, including CD20 immunohistochemistry for morphologic comparison to the study slides. Involvement was graded as 0 to 3+ (eTable).

Results
Results from all 24 biopsies are shown in the eTable. Twenty-two (91.7%) biopsies showed at least focal involvement by IVBCL. Nine (37.5%) biopsies showed more deep vs superficial involvement of the same site. On average, the 6 biopsies from clinically detected hemangiomas showed more involvement by IVBCL than the random biopsies (eFigures 1 and 2A). The superficial involvement of skin with a hemangioma showed an average score of 2.33 v 0.78 when compared with the superficial aspect of the random biopsies; the deep involvement of skin with a hemangioma showed an average score of 2.67 vs 1.16 when compared with the deep aspect of the random biopsies (eFigure 2B).


Comment
Intravascular large B-cell lymphoma is an aggressive malignancy that traditionally is difficult to diagnose. Many efforts have been made to improve detection and early diagnosis. As cutaneous involvement is common and sometimes the only sign of disease, dermatologists may be called upon to evaluate and biopsy patients with this suspected diagnosis. The purpose of our study was to improve diagnostic efficacy by methodically performing numerous biopsies and assessing the level of involvement of the superficial and deep skin as well as involvement of hemangiomas. The goal of this meticulous approach was to identify the highest-yield areas for biopsy with minimal impact on the patient. Our results showed that random skin biopsies are an effective way to identify IVBCL. Twenty-two (91.7%) biopsies contained at least focal lymphoma cells. Although the 2 biopsies that showed no tumor cells at all happened to both be from the left arm, this is believed to be coincidental. No discernable pattern was identified regarding involvement and anatomic region. Even though 20 (83.3%) biopsies showed superficial involvement, deep biopsy is essential, as 9 (37.5%) biopsies showed increased deep involvement compared to superficial involvement. Therefore, a deep punch biopsy is essential for maximum sensitivity.
Hemangiomas provide a potential target that could increase the sensitivity of a biopsy in the absence of clinical findings, when the disease in question is exclusively intravascular. The data gathered in this study support this idea, as biopsies from hemangiomas showed increased involvement compared to random biopsies, both superficially and deep (2.33 vs 0.78 and 2.67 vs 1.16, respectively). Interestingly, the hemangioma biopsy sites showed increased deep and superficial involvement, despite these typical cherry hemangiomas only involving the superficial dermis. One possible explanation for this is that the hemangiomas have larger-caliber feeder vessels with increased blood flow beneath them. It would then follow that this increased vasculature would increase the chances of identifying intravascular lymphoma cells. This finding further accentuates the need for a deep punch biopsy containing subcutaneous fat.
Completing the study in the setting of an autopsy provided the advantage of being able to take numerous biopsies without increased harm to the patient. This extensive set of biopsies would not be reasonable to complete on a living patient. This study also has limitations. Although this patient did fall within the typical demographics for patients with IVBCL, the data were still limited to 1 patient. This autopsy format (on a patient whose cause of death was indeed IVBCL) also implies terminal disease, which may mean the patient had a larger disease burden than a living patient who would typically be biopsied. Although this increased disease burden may have increased the sensitivity of finding IVBCL in the biopsies of this study, this further emphasizes the importance of trying to determine any factors that could increase sensitivity in a living patient with a lower disease burden.
Conclusion
Skin biopsies can provide a sensitive, low-cost, and low-morbidity method to assess a patient for IVBCL. Though random skin biopsies can yield valuable information, deep, 4-mm punch biopsies of clinically identifiable hemangiomas may provide the highest sensitivity for IVBCL.
- Ponzoni M, Campo E, Nakamura S. Intravascular large B-cell lymphoma: a chameleon with multiple faces and many masks. Blood. 2018;132:1561-1567. doi:10.1182/blood-2017-04-737445
- Roy AM, Pandey Y, Middleton D, et al. Intravascular large B-cell lymphoma: a diagnostic dilemma. Cureus. 2021;13:e16459. doi:10.7759/cureus.16459
- Bayçelebi D, Yildiz L, S?entürk N. A case report and literature review of cutaneous intravascular large B-cell lymphoma presenting clinically as panniculitis: a difficult diagnosis, but a good prognosis. An Bras Dermatol. 2021;96:72-75. doi:10.1016/j.abd.2020.08.004
- Orwat DE, Batalis NI. Intravascular large B-cell lymphoma. Arch Pathol Lab Med. 2012;136:333-338. doi:10.5858/arpa.2010-0747-RS
- Breakell T, Waibel H, Schliep S, et al. Intravascular large B-cell lymphoma: a review with a focus on the prognostic value of skininvolvement. Curr Oncol. 2022;29:2909-2919. doi:10.3390/curroncol29050237
- Oppegard L, O’Donnell M, Piro K, et al. Going skin deep: excavating a diagnosis of intravascular large B cell lymphoma. J Gen Intern Med. 2020;35:3368-3371. doi:10.1007/s11606-020-06141-1
- Barker JL, Swarup O, Puliyayil A. Intravascular large B-cell lymphoma: representative cases and approach to diagnosis. BMJ Case Rep. 2021;14:e244069. doi:10.1136/bcr-2021-244069
- Matsue K, Asada N, Odawara J, et al. Random skin biopsy and bone marrow biopsy for diagnosis of intravascular large B cell lymphoma. Ann Hematol. 2011;90:417-421. doi:10.1007/s00277-010-1101-3
- Shimada K, Kinoshita T, Naoe T, et al. Presentation and management of intravascular large B-cell lymphoma. Lancet Oncol. 2009;10:895-902. doi:10.1016/S1470-2045(09)70140-8
- Adachi Y, Kosami K, Mizuta N, et al. Benefits of skin biopsy of senile hemangioma in intravascular large B-cell lymphoma: a case report and review of the literature. Oncol Lett. 2014;7:2003-2006. doi:10.3892/ol.2014.2017
- Ishida M, Hodohara K, Yoshida T, et al. Intravascular large B-cell lymphoma colonizing in senile hemangioma: a case report and proposal of possible diagnostic strategy for intravascular lymphoma. Pathol Int. 2011;61:555-557. doi:10.1111/j.1440-1827.2011.02697.x
- Ponzoni M, Campo E, Nakamura S. Intravascular large B-cell lymphoma: a chameleon with multiple faces and many masks. Blood. 2018;132:1561-1567. doi:10.1182/blood-2017-04-737445
- Roy AM, Pandey Y, Middleton D, et al. Intravascular large B-cell lymphoma: a diagnostic dilemma. Cureus. 2021;13:e16459. doi:10.7759/cureus.16459
- Bayçelebi D, Yildiz L, S?entürk N. A case report and literature review of cutaneous intravascular large B-cell lymphoma presenting clinically as panniculitis: a difficult diagnosis, but a good prognosis. An Bras Dermatol. 2021;96:72-75. doi:10.1016/j.abd.2020.08.004
- Orwat DE, Batalis NI. Intravascular large B-cell lymphoma. Arch Pathol Lab Med. 2012;136:333-338. doi:10.5858/arpa.2010-0747-RS
- Breakell T, Waibel H, Schliep S, et al. Intravascular large B-cell lymphoma: a review with a focus on the prognostic value of skininvolvement. Curr Oncol. 2022;29:2909-2919. doi:10.3390/curroncol29050237
- Oppegard L, O’Donnell M, Piro K, et al. Going skin deep: excavating a diagnosis of intravascular large B cell lymphoma. J Gen Intern Med. 2020;35:3368-3371. doi:10.1007/s11606-020-06141-1
- Barker JL, Swarup O, Puliyayil A. Intravascular large B-cell lymphoma: representative cases and approach to diagnosis. BMJ Case Rep. 2021;14:e244069. doi:10.1136/bcr-2021-244069
- Matsue K, Asada N, Odawara J, et al. Random skin biopsy and bone marrow biopsy for diagnosis of intravascular large B cell lymphoma. Ann Hematol. 2011;90:417-421. doi:10.1007/s00277-010-1101-3
- Shimada K, Kinoshita T, Naoe T, et al. Presentation and management of intravascular large B-cell lymphoma. Lancet Oncol. 2009;10:895-902. doi:10.1016/S1470-2045(09)70140-8
- Adachi Y, Kosami K, Mizuta N, et al. Benefits of skin biopsy of senile hemangioma in intravascular large B-cell lymphoma: a case report and review of the literature. Oncol Lett. 2014;7:2003-2006. doi:10.3892/ol.2014.2017
- Ishida M, Hodohara K, Yoshida T, et al. Intravascular large B-cell lymphoma colonizing in senile hemangioma: a case report and proposal of possible diagnostic strategy for intravascular lymphoma. Pathol Int. 2011;61:555-557. doi:10.1111/j.1440-1827.2011.02697.x
Diagnostic Value of Deep Punch Biopsies in Intravascular Large B-cell Lymphoma
Diagnostic Value of Deep Punch Biopsies in Intravascular Large B-cell Lymphoma
Practice Points
- Skin biopsy is an effective method for identifying intravascular large B-cell lymphoma (IVBCL).
- Deep punch biopsies of sites involving hemangiomas may further heighten sensitivity for detection of IVBCL, as these lesions may harbor increased numbers of intravascular lymphoma cells.
- Deep and strategically placed skin biopsies offer potential improvements in timely diagnosis and outcomes of patients with IVBCL.
Botulinum Toxin as a Tool to Reduce Hyperhidrosis in Amputees
Botulinum Toxin as a Tool to Reduce Hyperhidrosis in Amputees
Practice Gap
Hyperhidrosis poses a considerable challenge for many amputees who use prosthetic devices, particularly at the interface between the residual limb and the prosthetic socket. The enclosed environment of the socket often leads to excessive sweating, which can compromise suction fit and increase the risk for skin chafing, irritation, and slippage. Persistent moisture also promotes bacterial and fungal growth, raising the likelihood of infections and foul odors within the socket. Research has shown that skin complications are highly prevalent among amputees, affecting up to 73.9% of this population in the United States.1 Commonly reported complications include wounds, abscesses, and blisters, many of which can be triggered or worsened by hyperhidrosis.2 Current treatment options for residual limb sweating include topical antiperspirants, botulinum toxin (BTX) injections, iontophoresis, and liner-liner socks.
While BTX commonly is used to treat hyperhidrosis in areas such as the palms and axillae, it typically is not considered as a first-line therapy for residual limb sweating; however, both BTX type A and type B have shown safety and effectiveness in managing hyperhidrosis in amputees, enhancing prosthetic use, and improving overall quality of life.3 Despite these benefits, BTX remains relatively underutilized for
Tools and Techniques
A 64-year-old man initially presented to our dermatology clinic after undergoing an above-the-knee amputation of the left leg 1 year prior. The amputation had been performed due to chronic prosthetic joint infections with Escherichia coli. He reported persistent sweating of the residual limb, which severely limited his use of a prosthesis and led to frequent falls.
During the initial visit, treatment options for primary hyperhidrosis including topical and injectable therapies were discussed. Due to a fear of needles, the patient chose topical treatment, with the option to pursue BTX injections later if better control was needed. An aluminum chloride hexahydrate prosthetic antiperspirant was prescribed for nightly application on the anterior and posterior
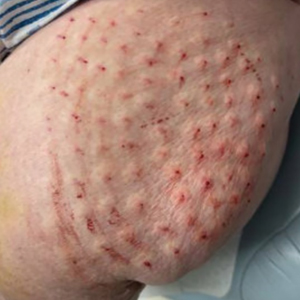
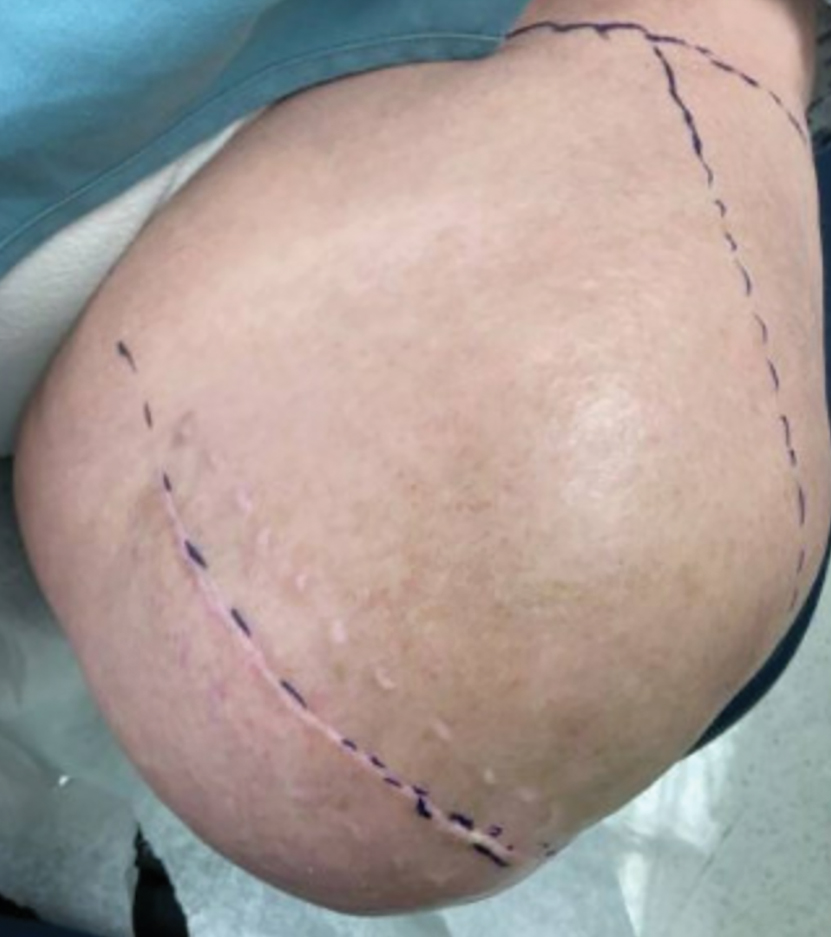
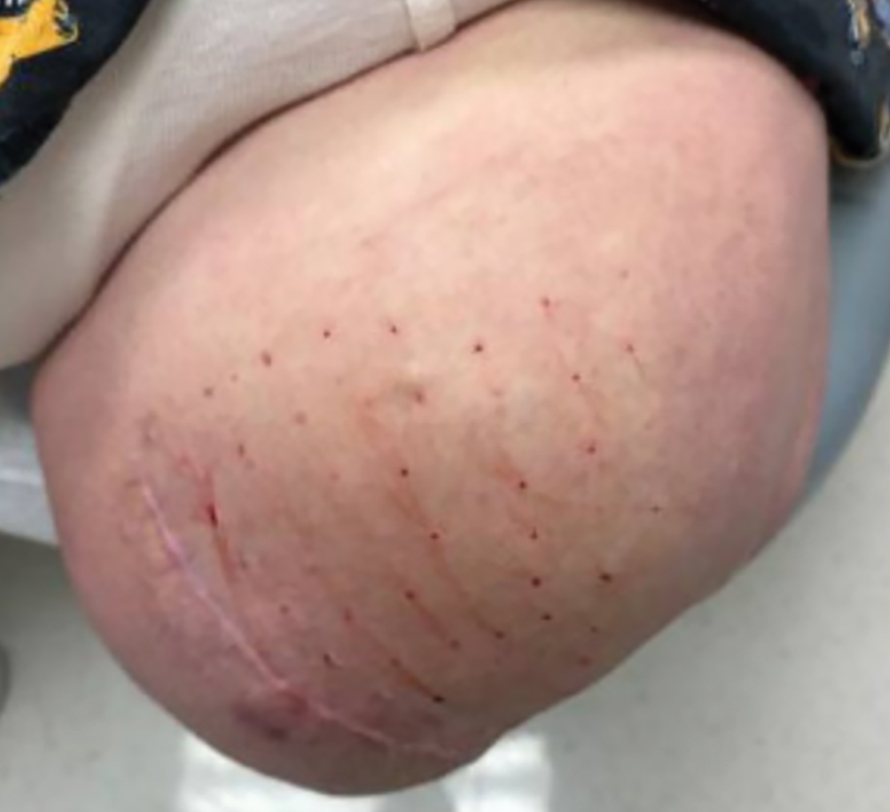
Botulinum toxin injections were administered in a grid-like pattern across the surface area where the residual limb made contact with the prosthetic. Using a surgical marker, the patient assisted the medical team in identifying the areas where sweating occurred most frequently. The area was divided into 4 equal sections, with each section treated per weekly interval sequentially over 4 weeks. The targeted areas included the left anterior (extending from the anterior tensor fasciae latae band to the lateral thigh) and left posterior residual limb (Figure 1 and eFigure 1, respectively).
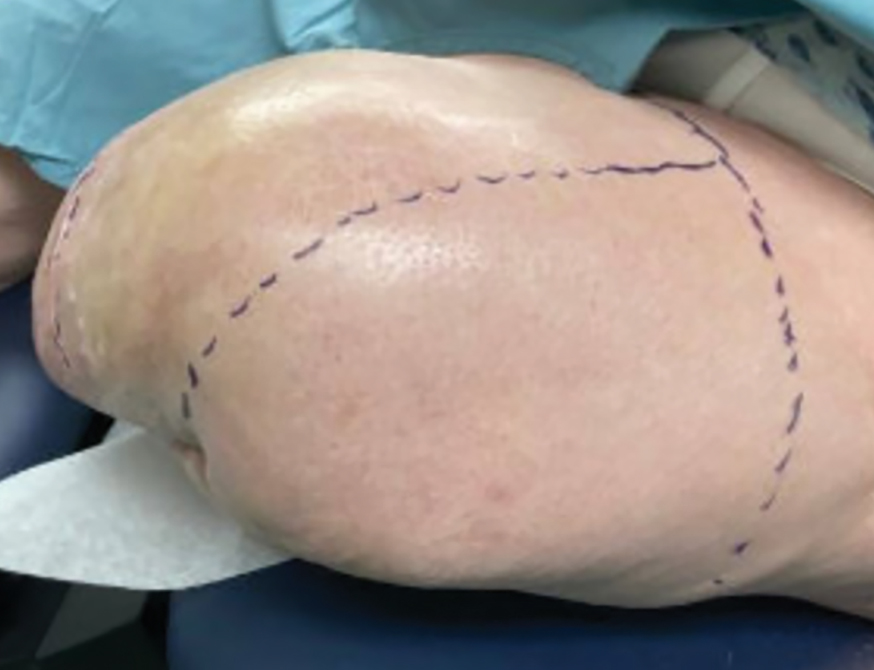
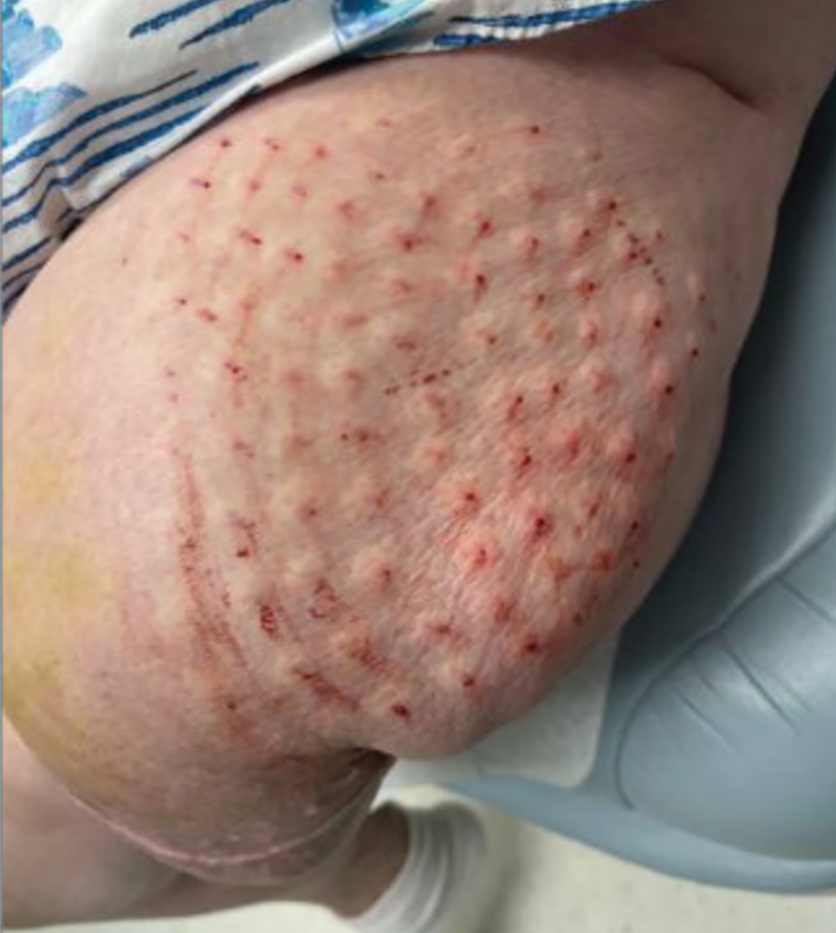
The treated section was cleaned with an alcohol wipe prior to each injection, and 50 units of BTX (diluted to 2.5 units per 0.1 mL in bacteriostatic saline) were injected intradermally into each section (Figure 2 and eFigure 2). The injections were administered in rows, with the needle inserted at evenly spaced intervals approximately 1 inch apart. A total of 100 units were administered per section at each weekly appointment. The patient tolerated the procedure well, and no complications were observed.
Practice Implications
This staged approach to administering BTX ensures even distribution of the injections, optimizes hyperhidrosis control, minimizes the risk for complications, and allows for precise targeting of the affected areas to maximize therapeutic benefit. Following the initial procedure, our patient was scheduled for follow-ups approximately every 3 to 4 months starting from the first set of injections for each area. Over 9 months, the patient successfully completed 3 treatment sessions using this method. The patient reported improved quality of life after starting the BTX injections.
After evaluating the initial treatment outcomes with 100 units per section, the dosage was increased to 200 units per section to reduce the number of visits from 4 every 3 months to cover the entire area to 2 visits every 3 months. This adjustment aimed to optimize results and better manage the patient’s ongoing symptoms. At about 1 to 2 weeks after beginning treatment, the patient noticed decreased sweating and discomfort during his daily activities and reduced friction with his prosthetic leg. No adverse effects were noted with the increased dosage during a clinical visit.
Our case highlights the importance of ensuring equitable access to hyperhidrosis treatment. Dermatologists should prioritize patient-centered care by factoring in financial constraints when recommending therapies. In this patient’s case, offering a range of options including over-the-counter antiperspirants and prescription treatments allowed for a management plan tailored to his individual needs and circumstances.
DaxibotulinumtoxinA, known for its longer duration of action compared to other BTX formulations, presents a promising alternative for treating hyperhidrosis.4 However, a gap in care emerged for our patient when prescription antiperspirant was not covered by his insurance, and daxibotulinumtoxinA, which could have offered a more durable solution, was not yet available at our clinic for hyperhidrosis management. Expanding insurance coverage for effective prescription treatments and improving access to newer treatment options are crucial for enhancing patient outcomes and ensuring more equitable care.
Focusing dermatologic care on amputees presents distinct challenges and opportunities for improving their care and decreasing discomfort. Amputees, particularly those with residual limb hyperhidrosis, often experience additional discomfort and difficulty while using prosthetics, as excessive sweating can interfere with fit and function.5,6 Dermatologists should proactively address these specific needs by tailoring treatment accordingly. Incorporating targeted therapies, such as BTX injections, in addition to education on lifestyle modifications and managing treatment expectations, ensures comprehensive care that enhances both quality of life and functional outcomes. Engaging patients in discussions about all available options, including emerging therapies, is essential for improving care for this underserved population.
- Koc E, Tunca M, Akar A, et al. Skin problems in amputees: a descriptive study. Int J Dermatol. 2008;47:463–466. doi:10.1111/j.1365-4632.2008.03604.x
- Bui KM, Raugi GJ, Nguyen VQ, et al. Skin problems in individuals with lower-limb loss: literature review and proposed classification system. J Rehabil Res Dev. 2009;46:1085-1090. doi:10.1682/jrrd.2009.04.0052
- Rocha Melo J, Rodrigues MA, Caetano M, et al. Botulinum toxin in the treatment of residual limb hyperhidrosis: a systematic review. Rehabilitacion (Madr). 2023;57:100754. doi:10.1016/j.rh.2022.07.003
- Hansen C, Godfrey B, Wixom J, et al. Incidence, severity, and impact of hyperhidrosis in people with lower-limb amputation. J Rehabil Res Dev. 2015;52:31-40. doi:10.1682/JRRD.2014.04.0108
- Lannan FM, Powell J, Kim GM, et al. Hyperhidrosis of the residual limb: a narrative review of the measurement and treatment of excess perspiration affecting individuals with amputation. Prosthet Orthot Int. 2021;45:477-486. doi:10.1097/PXR.0000000000000040
- Pace S, Kentosh J. Managing residual limb hyperhidrosis in wounded warriors. Cutis. 2016;97:401-403.
Practice Gap
Hyperhidrosis poses a considerable challenge for many amputees who use prosthetic devices, particularly at the interface between the residual limb and the prosthetic socket. The enclosed environment of the socket often leads to excessive sweating, which can compromise suction fit and increase the risk for skin chafing, irritation, and slippage. Persistent moisture also promotes bacterial and fungal growth, raising the likelihood of infections and foul odors within the socket. Research has shown that skin complications are highly prevalent among amputees, affecting up to 73.9% of this population in the United States.1 Commonly reported complications include wounds, abscesses, and blisters, many of which can be triggered or worsened by hyperhidrosis.2 Current treatment options for residual limb sweating include topical antiperspirants, botulinum toxin (BTX) injections, iontophoresis, and liner-liner socks.
While BTX commonly is used to treat hyperhidrosis in areas such as the palms and axillae, it typically is not considered as a first-line therapy for residual limb sweating; however, both BTX type A and type B have shown safety and effectiveness in managing hyperhidrosis in amputees, enhancing prosthetic use, and improving overall quality of life.3 Despite these benefits, BTX remains relatively underutilized for
Tools and Techniques
A 64-year-old man initially presented to our dermatology clinic after undergoing an above-the-knee amputation of the left leg 1 year prior. The amputation had been performed due to chronic prosthetic joint infections with Escherichia coli. He reported persistent sweating of the residual limb, which severely limited his use of a prosthesis and led to frequent falls.
During the initial visit, treatment options for primary hyperhidrosis including topical and injectable therapies were discussed. Due to a fear of needles, the patient chose topical treatment, with the option to pursue BTX injections later if better control was needed. An aluminum chloride hexahydrate prosthetic antiperspirant was prescribed for nightly application on the anterior and posterior
Botulinum toxin injections were administered in a grid-like pattern across the surface area where the residual limb made contact with the prosthetic. Using a surgical marker, the patient assisted the medical team in identifying the areas where sweating occurred most frequently. The area was divided into 4 equal sections, with each section treated per weekly interval sequentially over 4 weeks. The targeted areas included the left anterior (extending from the anterior tensor fasciae latae band to the lateral thigh) and left posterior residual limb (Figure 1 and eFigure 1, respectively).


The treated section was cleaned with an alcohol wipe prior to each injection, and 50 units of BTX (diluted to 2.5 units per 0.1 mL in bacteriostatic saline) were injected intradermally into each section (Figure 2 and eFigure 2). The injections were administered in rows, with the needle inserted at evenly spaced intervals approximately 1 inch apart. A total of 100 units were administered per section at each weekly appointment. The patient tolerated the procedure well, and no complications were observed.


Practice Implications
This staged approach to administering BTX ensures even distribution of the injections, optimizes hyperhidrosis control, minimizes the risk for complications, and allows for precise targeting of the affected areas to maximize therapeutic benefit. Following the initial procedure, our patient was scheduled for follow-ups approximately every 3 to 4 months starting from the first set of injections for each area. Over 9 months, the patient successfully completed 3 treatment sessions using this method. The patient reported improved quality of life after starting the BTX injections.
After evaluating the initial treatment outcomes with 100 units per section, the dosage was increased to 200 units per section to reduce the number of visits from 4 every 3 months to cover the entire area to 2 visits every 3 months. This adjustment aimed to optimize results and better manage the patient’s ongoing symptoms. At about 1 to 2 weeks after beginning treatment, the patient noticed decreased sweating and discomfort during his daily activities and reduced friction with his prosthetic leg. No adverse effects were noted with the increased dosage during a clinical visit.
Our case highlights the importance of ensuring equitable access to hyperhidrosis treatment. Dermatologists should prioritize patient-centered care by factoring in financial constraints when recommending therapies. In this patient’s case, offering a range of options including over-the-counter antiperspirants and prescription treatments allowed for a management plan tailored to his individual needs and circumstances.
DaxibotulinumtoxinA, known for its longer duration of action compared to other BTX formulations, presents a promising alternative for treating hyperhidrosis.4 However, a gap in care emerged for our patient when prescription antiperspirant was not covered by his insurance, and daxibotulinumtoxinA, which could have offered a more durable solution, was not yet available at our clinic for hyperhidrosis management. Expanding insurance coverage for effective prescription treatments and improving access to newer treatment options are crucial for enhancing patient outcomes and ensuring more equitable care.
Focusing dermatologic care on amputees presents distinct challenges and opportunities for improving their care and decreasing discomfort. Amputees, particularly those with residual limb hyperhidrosis, often experience additional discomfort and difficulty while using prosthetics, as excessive sweating can interfere with fit and function.5,6 Dermatologists should proactively address these specific needs by tailoring treatment accordingly. Incorporating targeted therapies, such as BTX injections, in addition to education on lifestyle modifications and managing treatment expectations, ensures comprehensive care that enhances both quality of life and functional outcomes. Engaging patients in discussions about all available options, including emerging therapies, is essential for improving care for this underserved population.
Practice Gap
Hyperhidrosis poses a considerable challenge for many amputees who use prosthetic devices, particularly at the interface between the residual limb and the prosthetic socket. The enclosed environment of the socket often leads to excessive sweating, which can compromise suction fit and increase the risk for skin chafing, irritation, and slippage. Persistent moisture also promotes bacterial and fungal growth, raising the likelihood of infections and foul odors within the socket. Research has shown that skin complications are highly prevalent among amputees, affecting up to 73.9% of this population in the United States.1 Commonly reported complications include wounds, abscesses, and blisters, many of which can be triggered or worsened by hyperhidrosis.2 Current treatment options for residual limb sweating include topical antiperspirants, botulinum toxin (BTX) injections, iontophoresis, and liner-liner socks.
While BTX commonly is used to treat hyperhidrosis in areas such as the palms and axillae, it typically is not considered as a first-line therapy for residual limb sweating; however, both BTX type A and type B have shown safety and effectiveness in managing hyperhidrosis in amputees, enhancing prosthetic use, and improving overall quality of life.3 Despite these benefits, BTX remains relatively underutilized for
Tools and Techniques
A 64-year-old man initially presented to our dermatology clinic after undergoing an above-the-knee amputation of the left leg 1 year prior. The amputation had been performed due to chronic prosthetic joint infections with Escherichia coli. He reported persistent sweating of the residual limb, which severely limited his use of a prosthesis and led to frequent falls.
During the initial visit, treatment options for primary hyperhidrosis including topical and injectable therapies were discussed. Due to a fear of needles, the patient chose topical treatment, with the option to pursue BTX injections later if better control was needed. An aluminum chloride hexahydrate prosthetic antiperspirant was prescribed for nightly application on the anterior and posterior
Botulinum toxin injections were administered in a grid-like pattern across the surface area where the residual limb made contact with the prosthetic. Using a surgical marker, the patient assisted the medical team in identifying the areas where sweating occurred most frequently. The area was divided into 4 equal sections, with each section treated per weekly interval sequentially over 4 weeks. The targeted areas included the left anterior (extending from the anterior tensor fasciae latae band to the lateral thigh) and left posterior residual limb (Figure 1 and eFigure 1, respectively).


The treated section was cleaned with an alcohol wipe prior to each injection, and 50 units of BTX (diluted to 2.5 units per 0.1 mL in bacteriostatic saline) were injected intradermally into each section (Figure 2 and eFigure 2). The injections were administered in rows, with the needle inserted at evenly spaced intervals approximately 1 inch apart. A total of 100 units were administered per section at each weekly appointment. The patient tolerated the procedure well, and no complications were observed.


Practice Implications
This staged approach to administering BTX ensures even distribution of the injections, optimizes hyperhidrosis control, minimizes the risk for complications, and allows for precise targeting of the affected areas to maximize therapeutic benefit. Following the initial procedure, our patient was scheduled for follow-ups approximately every 3 to 4 months starting from the first set of injections for each area. Over 9 months, the patient successfully completed 3 treatment sessions using this method. The patient reported improved quality of life after starting the BTX injections.
After evaluating the initial treatment outcomes with 100 units per section, the dosage was increased to 200 units per section to reduce the number of visits from 4 every 3 months to cover the entire area to 2 visits every 3 months. This adjustment aimed to optimize results and better manage the patient’s ongoing symptoms. At about 1 to 2 weeks after beginning treatment, the patient noticed decreased sweating and discomfort during his daily activities and reduced friction with his prosthetic leg. No adverse effects were noted with the increased dosage during a clinical visit.
Our case highlights the importance of ensuring equitable access to hyperhidrosis treatment. Dermatologists should prioritize patient-centered care by factoring in financial constraints when recommending therapies. In this patient’s case, offering a range of options including over-the-counter antiperspirants and prescription treatments allowed for a management plan tailored to his individual needs and circumstances.
DaxibotulinumtoxinA, known for its longer duration of action compared to other BTX formulations, presents a promising alternative for treating hyperhidrosis.4 However, a gap in care emerged for our patient when prescription antiperspirant was not covered by his insurance, and daxibotulinumtoxinA, which could have offered a more durable solution, was not yet available at our clinic for hyperhidrosis management. Expanding insurance coverage for effective prescription treatments and improving access to newer treatment options are crucial for enhancing patient outcomes and ensuring more equitable care.
Focusing dermatologic care on amputees presents distinct challenges and opportunities for improving their care and decreasing discomfort. Amputees, particularly those with residual limb hyperhidrosis, often experience additional discomfort and difficulty while using prosthetics, as excessive sweating can interfere with fit and function.5,6 Dermatologists should proactively address these specific needs by tailoring treatment accordingly. Incorporating targeted therapies, such as BTX injections, in addition to education on lifestyle modifications and managing treatment expectations, ensures comprehensive care that enhances both quality of life and functional outcomes. Engaging patients in discussions about all available options, including emerging therapies, is essential for improving care for this underserved population.
- Koc E, Tunca M, Akar A, et al. Skin problems in amputees: a descriptive study. Int J Dermatol. 2008;47:463–466. doi:10.1111/j.1365-4632.2008.03604.x
- Bui KM, Raugi GJ, Nguyen VQ, et al. Skin problems in individuals with lower-limb loss: literature review and proposed classification system. J Rehabil Res Dev. 2009;46:1085-1090. doi:10.1682/jrrd.2009.04.0052
- Rocha Melo J, Rodrigues MA, Caetano M, et al. Botulinum toxin in the treatment of residual limb hyperhidrosis: a systematic review. Rehabilitacion (Madr). 2023;57:100754. doi:10.1016/j.rh.2022.07.003
- Hansen C, Godfrey B, Wixom J, et al. Incidence, severity, and impact of hyperhidrosis in people with lower-limb amputation. J Rehabil Res Dev. 2015;52:31-40. doi:10.1682/JRRD.2014.04.0108
- Lannan FM, Powell J, Kim GM, et al. Hyperhidrosis of the residual limb: a narrative review of the measurement and treatment of excess perspiration affecting individuals with amputation. Prosthet Orthot Int. 2021;45:477-486. doi:10.1097/PXR.0000000000000040
- Pace S, Kentosh J. Managing residual limb hyperhidrosis in wounded warriors. Cutis. 2016;97:401-403.
- Koc E, Tunca M, Akar A, et al. Skin problems in amputees: a descriptive study. Int J Dermatol. 2008;47:463–466. doi:10.1111/j.1365-4632.2008.03604.x
- Bui KM, Raugi GJ, Nguyen VQ, et al. Skin problems in individuals with lower-limb loss: literature review and proposed classification system. J Rehabil Res Dev. 2009;46:1085-1090. doi:10.1682/jrrd.2009.04.0052
- Rocha Melo J, Rodrigues MA, Caetano M, et al. Botulinum toxin in the treatment of residual limb hyperhidrosis: a systematic review. Rehabilitacion (Madr). 2023;57:100754. doi:10.1016/j.rh.2022.07.003
- Hansen C, Godfrey B, Wixom J, et al. Incidence, severity, and impact of hyperhidrosis in people with lower-limb amputation. J Rehabil Res Dev. 2015;52:31-40. doi:10.1682/JRRD.2014.04.0108
- Lannan FM, Powell J, Kim GM, et al. Hyperhidrosis of the residual limb: a narrative review of the measurement and treatment of excess perspiration affecting individuals with amputation. Prosthet Orthot Int. 2021;45:477-486. doi:10.1097/PXR.0000000000000040
- Pace S, Kentosh J. Managing residual limb hyperhidrosis in wounded warriors. Cutis. 2016;97:401-403.
Botulinum Toxin as a Tool to Reduce Hyperhidrosis in Amputees
Botulinum Toxin as a Tool to Reduce Hyperhidrosis in Amputees
Dermatology Immediate Care: A Game Changer for the Health Care System?
Dermatology Immediate Care: A Game Changer for the Health Care System?
Emergency departments (EDs) and immediate care (IC) facilities often do not have prompt dermatologic care available for triage and treatment. Many EDs do not have staff dermatologists on call, instead relying on input from other specialists or quick outpatient dermatology appointments. It can be challenging to obtain a prompt appointment with a board-certified dermatologist, which is preferred for complex cases such as severe drug reactions or infection. In the United States, there are few well-established IC centers equipped to address dermatologic needs. The orthopedic specialty has modeled a concept that has led to the establishment of orthopedic urgent care/IC in many larger institutions,1 and many private practice clinics serve their communities as well. We present a rationale for why a similar IC concept for dermatology would be beneficial, particularly within a large institution or health system.
Dermatology Consultation Changes Disease Management
There is diagnostic and therapeutic utility in dermatology evaluation. In a prospective study of 591 patients who were either hospitalized or evaluated in an ED/urgent care setting, treatment was changed in more than 60% of cases when dermatology consultation was utilized.2 In another prospective review of 691 cases on an inpatient service, dermatology consultation resulted in treatment changes more than 80% of the time.3
Cellulitis has been a particularly well-studied diagnosis. Dermatologists often change the diagnosis of cellulitis in the hospital setting and reduce antibiotic exposure. In a prospective cohort study of 116 patients, 33.6% had their diagnosis of cellulitis changed to pseudocellulitis following evaluation by the dermatologist; of 34 patients who had started antibiotic therapy, 82.4% were recommended to discontinue the treatment, and all 39 patients with pseudocellulitis had a proven stable clinical course at 1-month follow-up.4 In another trial, 175 patients with presumed cellulitis were given standard management (provided by the medicine inpatient team) either alone or with the addition of dermatology consultation. Duration of antibiotic treatment (including intravenous therapy) was reduced when dermatology was consulted. Two weeks after discharge, patients who had dermatology consultations demonstrated greater clinical improvement.5
Improving ED and IC Access to Dermatology
Emergency department and IC teams across the United States work tirelessly to meet the demands of patients presenting with medically urgent conditions. In a study examining 861 ED cases, dermatology made up only 9.5% of specialist consultations, and in the opinion of the on-call dermatology resident, 51.0% (439/861) of cases warranted ED-level care.6
Data from the 2021 National Hospital Ambulatory Medical Care Survey showed that the mean wait time to see a physician, nurse, or physician assistant in an ED was 37.5 minutes, but wait times could range from less than 15 minutes to more than 6 hours.7 According to a study of 35,849 ED visits at nonfederal hospitals in the United States, only 47.7% of EDs admitted more than 90% of their patients within 6 hours.8 Moreover, perceived wait times in the ED have been shown to greatly impact patient satisfaction. Two predictors of perceived wait time include appropriate assessment of emergency level and the feeling of being forgotten.9 In a study of 2377 ED visits with primary dermatologic diagnoses, only 5.5% led to admission.10 This suggests many patients who come to the ED for dermatologic needs do not require inpatient hospital care. In these cases, patients with primary dermatologic concerns may experience longer ED wait times, as higher acuity or emergency cases take precedence. Studies also have shown that more vulnerable populations are utilizing ED visits most for primary dermatologic concerns.10,11 This includes individuals of lower income and/or those with Medicaid/Medicare or those without insurance.11 Predictors of high ED use for dermatologic concerns include prior frequent use of the ED (for nondermatologic concerns) instead of outpatient care, income below the poverty level, and lack of insurance; older individuals (>65 years) also were found to use the ED more frequently for dermatologic concerns when compared to younger individuals.10
Importantly, there is a great need for urgent dermatology consultation for pediatric patients. A single-institution study showed that over a 36-month period, there were 347 pediatric dermatology consultations from the pediatric ED mostly for children aged 0 days to 5 years; nearly half of these consultations required outpatient clinic follow-up.12 However, dermatology outpatient follow-up can be difficult to obtain, especially for vulnerable groups. In a study of 611 dermatology clinics, patients with Medicaid were shown to have longer wait times and less success in obtaining dermatology appointments compared to those with Medicare or private insurance.13 Only about 30% of private dermatology practices accept Medicaid patients, likely pushing these patients toward utilization of emergency services for dermatologic concerns.13,14
There is a clear role for a dermatology IC in our health care system, and the concept already has been identified and trialed in several institutions. At Oregon Health and Science University (Portland, Oregon), a retrospective chart review of patients with diagnoses of Morgellons disease and neurotic excoriations seen in dermatology urgent care between 2018 and 2020 showed an 88% decrease in annual rates of health care visits and a 77% decrease in ED visits after dermatology services were engaged compared to before the opening of the dermatology urgent care.15 Another study showed that uninsured or self-pay patients were more than 14 times more likely to access dermatology urgent care than to schedule a routine clinic appointment, suggesting that there is a barrier to making outpatient dermatologic appointments for uninsured patients. An urgent access model may facilitate the ability of underinsured patients to access care.16
Improving Dermatology Access for Other Specialties
Needs for dermatologic care are encountered in many other specialties. Having direct access to immediate dermatologic treatment is best for patients and may avoid inpatient care and trips to the ED for consultation access. Ideally, a dermatology IC would allow direct care to be provided alongside the oncology outpatient team. New immunologic therapies (cytotoxic T-lymphocyte–associated protein 4 and programmed cell death protein 1/programmed death-ligand 1 treatments) can cause dermatologic reactions in more than 40% of patients.17 Paraneoplastic syndromes can manifest with cutaneous symptoms, as can acute graft-vs-host-disease.18 In a study at Memorial Sloan Kettering (New York, New York) analyzing 426 same-day outpatient dermatology consultations, 17% of patients experienced interruptions in their cancer therapy, but 83% responded quickly to dermatologic treatment and resumed oncologic therapy—19% of them at a reduced dose.19 This is an important demonstration of prompt dermatologic consultation in an outpatient setting reducing interruptions to anticancer therapy. The heterogeneity of the cutaneous reactions seen from oncologic and immunomodulatory medications is profound, with more than 140 different types of skin-specific reactions.20
Solid-organ transplant recipients also could benefit from urgent access to dermatology services. These patients are at a much higher risk for skin cancers, and a study showed that those who receive referrals to dermatology are seen sooner after transplantation (5.6 years) than those who self-refer (7.2 years). Importantly, annual skin cancer screenings are recommended to begin 1 year after transplantation.21
Direct access to dermatology care could benefit patients with complicated rheumatologic conditions who present with skin findings; for example, patients with lupus erythematosus or dermatomyositis can have a spectrum of disease ranging from skin-predominant to systemic manifestations. Identification and treatment of such diseases require collaboration between dermatologists and rheumatologists.22 Likewise, a study of a joint rheumatology-dermatology clinic for psoriatic arthritis showed that a multidisciplinary approach to management leads to decreased time for patients to obtain proper rheumatologic and dermatologic examination and a faster time to diagnosis; however, such multidisciplinary clinic models and approaches to care often are found only at large university-based hospitals.23 In a patient population for whom time to diagnosis is crucial to avoid permanent changes such as joint destruction, a dermatology IC could fill this role in community hospitals and clinics. A dermatology IC also can serve patients with specific diagnoses who would benefit from more direct access to care; for example, in 2017 there were 131,430 ED visits for hidradenitis suppurativa (HS) in the United States. While HS is not uncommon, it usually is underdiagnosed because it can be challenging to differentiate from an uncomplicated abscess. Emergency department visits often are utilized for first-time presentations as well as flares of HS. In these situations, ED doctors can provide palliative treatment, but prompt referrals to dermatologists should be made for disease management to decrease recurrence.24
Final Thoughts
A huge caveat to the dermatology urgent care system is determining what is deemed “urgent.” We propose starting with a referral-based system only from other physicians (including IC and urgent care) rather than having patients walk in directly. Ideally, as support and staff increases, the availability can increase as well. In our institution, we suggested half-day clinics staffed by varying physicians, with compensation models similar to an ED or IC physician rather than by productivity. Each group considering this kind of addition to patient care will need to assess these points in building an IC for dermatology. The University of Pennsylvania’s (Philadelphia, Pennsylvania) system of rapid-access clinics to facilitate access to care for patients requiring urgent appointments may function as a model for future similar clinics.25 Creating a specialized IC/urgent care is not a novel concept. Orthopedic urgent care centers have increased greatly in the past decade, reducing ED burden for musculoskeletal complaints. In a study evaluating the utility of orthopedic urgent care settings, time to see an orthopedic specialist and cost were both greatly reduced with this system.1 The same has been shown in same-day access ophthalmology clinics, which are organized similarly to an urgent care.26
In 2021, there were 107.4 million treat-and-release visits to the ED in the United States for a total cost of $80.3 billion.27 This emphasizes the need to consider care models that not only provide excellent clinical care and treat the most acute diagnoses promptly and accurately but also reduce overall costs. While this may be convoluted for other specialties given the difficulty of having patients self-triage, dermatologic concerns are similar to orthopedic concerns for the patient to decipher the etiology of the concern. As in orthopedics, a dermatology IC could function similarly, increasing access, decreasing ED and IC wait times, saving overall health care spending, and allowing underserved and publicly insured individuals to have improved, prompt care.
- Anderson TJ, Althausen PL. The role of dedicated musculoskeletal urgent care centers in reducing cost and improving access to orthopaedic care. J Orthop Trauma. 2016;30:S3-S6.
- Falanga V, Schachner LA, Rae V, et al. Dermatologic consultations in the hospital setting. Arch Dermatol. 1994;130:1022-1025.
- Galimberti F, Guren L, Fernandez AP, et al. Dermatology consultations significantly contribute quality to care of hospitalized patients: a prospective study of dermatology inpatient consults at a tertiary care center. Int J Dermatol. 2016;55:E547-E551.
- Li DG, Xia FD, Khosravi H, et al. Outcomes of early dermatology consultation for inpatients diagnosed with cellulitis. JAMA Dermatol. 2018;154:537-543.
- Ko LN, Garza-Mayers AC, St John J, et al. Effect of dermatology consultation on outcomes for patients with presumed cellulitis: a randomized clinical trial. JAMA Dermatol. 2018;154:529-536.
- Grillo E, Vañó-Galván S, Jiménez-Gómez N, et al. Dermatologic emergencies: descriptive analysis of 861 patients in a tertiary care teaching hospital. Actas Dermosifiliogr. 2013;104:316-324.
- National Center for Health Statistics. National Hospital Ambulatory Medical Care Survey, 2021. Accessed September 23, 2025. https://www.cdc.gov/nchs/data/nhamcs/web_tables/2021-nhamcs-ed-web-tables-508.pdf
- Horwitz LI, Green J, Bradley EH. US emergency department performance on wait time and length of visit. Ann Emerg Med. 2010;55:133-141.
- Spechbach H, Rochat J, Gaspoz JM, et al. Patients’ time perception in the waiting room of an ambulatory emergency unit: a cross-sectional study. BMC Emerg Med. 2019;19:41.
- Yang JJ, Maloney NJ, Bach DQ, et al. Dermatology in the emergency department: prescriptions, rates of inpatient admission, and predictors of high utilization in the United States from 1996 to 2012. J Am Acad Dermatol. 2021;84:1480-1483.
- Chen CL, Fitzpatrick L, Kamel H. Who uses the emergency department for dermatologic care? a statewide analysis. J Am Acad Dermatol. 2014;71:308-313.
- Moon AT, Castelo-Soccio L, Yan AC. Emergency department utilization of pediatric dermatology (PD) consultations. J Am Acad Dermatol. 2016;74:1173-1177.
- Creadore A, Desai S, Li SJ, et al. Insurance acceptance, appointment wait time, and dermatologist access across practice types in the US. JAMA Dermatol. 2021;157:181-188.
- Mazmudar RS, Gupta N, Desai BJ, et al. Dermatologist appointment access and waiting times: a comparative study of insurance types. J Am Acad Dermatol. 2020;83:1468-1470.
- Johnson J, Cutler B, Latour E, et al. Dermatology urgent care model reduces costs and healthcare utilization for psychodermatology patients-a retrospective chart review. Dermatol Online J. 2022;28:5.
- Wintringham JA, Strock DM, Perkins-Holtsclaw K, et al. Dermatology in the urgent care setting: a retrospective review of patients seen in an urgent access dermatology clinic. J Am Acad Dermatol. 2023;89:1271-1273.
- Yoo MJ, Long B, Brady WJ, et al. Immune checkpoint inhibitors: an emergency medicine focused review. Am J Emerg Med. 2021;50:335-344.
- Merlo G, Cozzani E, Canale F, et al. Cutaneous manifestations of hematologic malignancies the experience of an Italian dermatology department. Hematol Oncol. 2019;37:285-290.
- Barrios D, Phillips G, Freites-Martinez A, et al. Outpatient dermatology consultations for oncology patients with acute dermatologic adverse events impact anticancer therapy interruption: a retrospective study.J Eur Acad Dermatol Venereol. 2020;34:1340-1347.
- Salah S, Kerob D, Pages Laurent C, et al. Evaluation of anticancer therapy-related dermatologic adverse events: insights from Food and Drug Administration’s Adverse Event Reporting System dataset. J Am Acad Dermatol. 2024;91:863-871. doi:10.1016/j.jaad.2024.07.1456
- Shope C, Andrews L, Girvin A, et al. Referrals to dermatology following solid organ transplant. J Am Acad Dermatol. 2023;88:1159-1160. doi:10.1016/j.jaad.2022.11.052
- Werth VP, Askanase AD, Lundberg IE. Importance of collaboration of dermatology and rheumatology to advance the field for lupus and dermatomyositis. Int J Womens Dermatol. 2021;7:583-587.
- Ziob J, Behning C, Brossart P, et al. Specialized dermatological-rheumatological patient management improves diagnostic outcome and patient journey in psoriasis and psoriatic arthritis: a four-year analysis. BMC Rheumatol. 2021;5:1-8. doi:10.1186/s41927-021-00217-z
- Okun MM, Flamm A, Werley EB, et al. Hidradenitis suppurativa: diagnosis and management in the emergency department. J Emerg Med. 2022;63:636-644.
- Jayakumar KL, Samimi SS, Vittorio CC, et al. Expediting patient appointments with dermatology rapid access clinics. Dermatol Online J. 2018;24:13030/qt2zv07510.
- Singman EL, Smith K, Mehta R, et al. Cost and visit duration of same-day access at an academic ophthalmology department vs emergency department. JAMA Ophthalmol. 2019;137:729-735. doi:10.1001/jamaophthalmol.2019.0864
- Roemer M. Costs of treat-and-release emergency department visits in the United States, 2021. Agency for Healthcare Research and Quality. Published September 2024. Accessed September 16, 2025. https://hcup-us.ahrq.gov/reports/statbriefs/sb311-ED-visit-costs-2021.pdf
Emergency departments (EDs) and immediate care (IC) facilities often do not have prompt dermatologic care available for triage and treatment. Many EDs do not have staff dermatologists on call, instead relying on input from other specialists or quick outpatient dermatology appointments. It can be challenging to obtain a prompt appointment with a board-certified dermatologist, which is preferred for complex cases such as severe drug reactions or infection. In the United States, there are few well-established IC centers equipped to address dermatologic needs. The orthopedic specialty has modeled a concept that has led to the establishment of orthopedic urgent care/IC in many larger institutions,1 and many private practice clinics serve their communities as well. We present a rationale for why a similar IC concept for dermatology would be beneficial, particularly within a large institution or health system.
Dermatology Consultation Changes Disease Management
There is diagnostic and therapeutic utility in dermatology evaluation. In a prospective study of 591 patients who were either hospitalized or evaluated in an ED/urgent care setting, treatment was changed in more than 60% of cases when dermatology consultation was utilized.2 In another prospective review of 691 cases on an inpatient service, dermatology consultation resulted in treatment changes more than 80% of the time.3
Cellulitis has been a particularly well-studied diagnosis. Dermatologists often change the diagnosis of cellulitis in the hospital setting and reduce antibiotic exposure. In a prospective cohort study of 116 patients, 33.6% had their diagnosis of cellulitis changed to pseudocellulitis following evaluation by the dermatologist; of 34 patients who had started antibiotic therapy, 82.4% were recommended to discontinue the treatment, and all 39 patients with pseudocellulitis had a proven stable clinical course at 1-month follow-up.4 In another trial, 175 patients with presumed cellulitis were given standard management (provided by the medicine inpatient team) either alone or with the addition of dermatology consultation. Duration of antibiotic treatment (including intravenous therapy) was reduced when dermatology was consulted. Two weeks after discharge, patients who had dermatology consultations demonstrated greater clinical improvement.5
Improving ED and IC Access to Dermatology
Emergency department and IC teams across the United States work tirelessly to meet the demands of patients presenting with medically urgent conditions. In a study examining 861 ED cases, dermatology made up only 9.5% of specialist consultations, and in the opinion of the on-call dermatology resident, 51.0% (439/861) of cases warranted ED-level care.6
Data from the 2021 National Hospital Ambulatory Medical Care Survey showed that the mean wait time to see a physician, nurse, or physician assistant in an ED was 37.5 minutes, but wait times could range from less than 15 minutes to more than 6 hours.7 According to a study of 35,849 ED visits at nonfederal hospitals in the United States, only 47.7% of EDs admitted more than 90% of their patients within 6 hours.8 Moreover, perceived wait times in the ED have been shown to greatly impact patient satisfaction. Two predictors of perceived wait time include appropriate assessment of emergency level and the feeling of being forgotten.9 In a study of 2377 ED visits with primary dermatologic diagnoses, only 5.5% led to admission.10 This suggests many patients who come to the ED for dermatologic needs do not require inpatient hospital care. In these cases, patients with primary dermatologic concerns may experience longer ED wait times, as higher acuity or emergency cases take precedence. Studies also have shown that more vulnerable populations are utilizing ED visits most for primary dermatologic concerns.10,11 This includes individuals of lower income and/or those with Medicaid/Medicare or those without insurance.11 Predictors of high ED use for dermatologic concerns include prior frequent use of the ED (for nondermatologic concerns) instead of outpatient care, income below the poverty level, and lack of insurance; older individuals (>65 years) also were found to use the ED more frequently for dermatologic concerns when compared to younger individuals.10
Importantly, there is a great need for urgent dermatology consultation for pediatric patients. A single-institution study showed that over a 36-month period, there were 347 pediatric dermatology consultations from the pediatric ED mostly for children aged 0 days to 5 years; nearly half of these consultations required outpatient clinic follow-up.12 However, dermatology outpatient follow-up can be difficult to obtain, especially for vulnerable groups. In a study of 611 dermatology clinics, patients with Medicaid were shown to have longer wait times and less success in obtaining dermatology appointments compared to those with Medicare or private insurance.13 Only about 30% of private dermatology practices accept Medicaid patients, likely pushing these patients toward utilization of emergency services for dermatologic concerns.13,14
There is a clear role for a dermatology IC in our health care system, and the concept already has been identified and trialed in several institutions. At Oregon Health and Science University (Portland, Oregon), a retrospective chart review of patients with diagnoses of Morgellons disease and neurotic excoriations seen in dermatology urgent care between 2018 and 2020 showed an 88% decrease in annual rates of health care visits and a 77% decrease in ED visits after dermatology services were engaged compared to before the opening of the dermatology urgent care.15 Another study showed that uninsured or self-pay patients were more than 14 times more likely to access dermatology urgent care than to schedule a routine clinic appointment, suggesting that there is a barrier to making outpatient dermatologic appointments for uninsured patients. An urgent access model may facilitate the ability of underinsured patients to access care.16
Improving Dermatology Access for Other Specialties
Needs for dermatologic care are encountered in many other specialties. Having direct access to immediate dermatologic treatment is best for patients and may avoid inpatient care and trips to the ED for consultation access. Ideally, a dermatology IC would allow direct care to be provided alongside the oncology outpatient team. New immunologic therapies (cytotoxic T-lymphocyte–associated protein 4 and programmed cell death protein 1/programmed death-ligand 1 treatments) can cause dermatologic reactions in more than 40% of patients.17 Paraneoplastic syndromes can manifest with cutaneous symptoms, as can acute graft-vs-host-disease.18 In a study at Memorial Sloan Kettering (New York, New York) analyzing 426 same-day outpatient dermatology consultations, 17% of patients experienced interruptions in their cancer therapy, but 83% responded quickly to dermatologic treatment and resumed oncologic therapy—19% of them at a reduced dose.19 This is an important demonstration of prompt dermatologic consultation in an outpatient setting reducing interruptions to anticancer therapy. The heterogeneity of the cutaneous reactions seen from oncologic and immunomodulatory medications is profound, with more than 140 different types of skin-specific reactions.20
Solid-organ transplant recipients also could benefit from urgent access to dermatology services. These patients are at a much higher risk for skin cancers, and a study showed that those who receive referrals to dermatology are seen sooner after transplantation (5.6 years) than those who self-refer (7.2 years). Importantly, annual skin cancer screenings are recommended to begin 1 year after transplantation.21
Direct access to dermatology care could benefit patients with complicated rheumatologic conditions who present with skin findings; for example, patients with lupus erythematosus or dermatomyositis can have a spectrum of disease ranging from skin-predominant to systemic manifestations. Identification and treatment of such diseases require collaboration between dermatologists and rheumatologists.22 Likewise, a study of a joint rheumatology-dermatology clinic for psoriatic arthritis showed that a multidisciplinary approach to management leads to decreased time for patients to obtain proper rheumatologic and dermatologic examination and a faster time to diagnosis; however, such multidisciplinary clinic models and approaches to care often are found only at large university-based hospitals.23 In a patient population for whom time to diagnosis is crucial to avoid permanent changes such as joint destruction, a dermatology IC could fill this role in community hospitals and clinics. A dermatology IC also can serve patients with specific diagnoses who would benefit from more direct access to care; for example, in 2017 there were 131,430 ED visits for hidradenitis suppurativa (HS) in the United States. While HS is not uncommon, it usually is underdiagnosed because it can be challenging to differentiate from an uncomplicated abscess. Emergency department visits often are utilized for first-time presentations as well as flares of HS. In these situations, ED doctors can provide palliative treatment, but prompt referrals to dermatologists should be made for disease management to decrease recurrence.24
Final Thoughts
A huge caveat to the dermatology urgent care system is determining what is deemed “urgent.” We propose starting with a referral-based system only from other physicians (including IC and urgent care) rather than having patients walk in directly. Ideally, as support and staff increases, the availability can increase as well. In our institution, we suggested half-day clinics staffed by varying physicians, with compensation models similar to an ED or IC physician rather than by productivity. Each group considering this kind of addition to patient care will need to assess these points in building an IC for dermatology. The University of Pennsylvania’s (Philadelphia, Pennsylvania) system of rapid-access clinics to facilitate access to care for patients requiring urgent appointments may function as a model for future similar clinics.25 Creating a specialized IC/urgent care is not a novel concept. Orthopedic urgent care centers have increased greatly in the past decade, reducing ED burden for musculoskeletal complaints. In a study evaluating the utility of orthopedic urgent care settings, time to see an orthopedic specialist and cost were both greatly reduced with this system.1 The same has been shown in same-day access ophthalmology clinics, which are organized similarly to an urgent care.26
In 2021, there were 107.4 million treat-and-release visits to the ED in the United States for a total cost of $80.3 billion.27 This emphasizes the need to consider care models that not only provide excellent clinical care and treat the most acute diagnoses promptly and accurately but also reduce overall costs. While this may be convoluted for other specialties given the difficulty of having patients self-triage, dermatologic concerns are similar to orthopedic concerns for the patient to decipher the etiology of the concern. As in orthopedics, a dermatology IC could function similarly, increasing access, decreasing ED and IC wait times, saving overall health care spending, and allowing underserved and publicly insured individuals to have improved, prompt care.
Emergency departments (EDs) and immediate care (IC) facilities often do not have prompt dermatologic care available for triage and treatment. Many EDs do not have staff dermatologists on call, instead relying on input from other specialists or quick outpatient dermatology appointments. It can be challenging to obtain a prompt appointment with a board-certified dermatologist, which is preferred for complex cases such as severe drug reactions or infection. In the United States, there are few well-established IC centers equipped to address dermatologic needs. The orthopedic specialty has modeled a concept that has led to the establishment of orthopedic urgent care/IC in many larger institutions,1 and many private practice clinics serve their communities as well. We present a rationale for why a similar IC concept for dermatology would be beneficial, particularly within a large institution or health system.
Dermatology Consultation Changes Disease Management
There is diagnostic and therapeutic utility in dermatology evaluation. In a prospective study of 591 patients who were either hospitalized or evaluated in an ED/urgent care setting, treatment was changed in more than 60% of cases when dermatology consultation was utilized.2 In another prospective review of 691 cases on an inpatient service, dermatology consultation resulted in treatment changes more than 80% of the time.3
Cellulitis has been a particularly well-studied diagnosis. Dermatologists often change the diagnosis of cellulitis in the hospital setting and reduce antibiotic exposure. In a prospective cohort study of 116 patients, 33.6% had their diagnosis of cellulitis changed to pseudocellulitis following evaluation by the dermatologist; of 34 patients who had started antibiotic therapy, 82.4% were recommended to discontinue the treatment, and all 39 patients with pseudocellulitis had a proven stable clinical course at 1-month follow-up.4 In another trial, 175 patients with presumed cellulitis were given standard management (provided by the medicine inpatient team) either alone or with the addition of dermatology consultation. Duration of antibiotic treatment (including intravenous therapy) was reduced when dermatology was consulted. Two weeks after discharge, patients who had dermatology consultations demonstrated greater clinical improvement.5
Improving ED and IC Access to Dermatology
Emergency department and IC teams across the United States work tirelessly to meet the demands of patients presenting with medically urgent conditions. In a study examining 861 ED cases, dermatology made up only 9.5% of specialist consultations, and in the opinion of the on-call dermatology resident, 51.0% (439/861) of cases warranted ED-level care.6
Data from the 2021 National Hospital Ambulatory Medical Care Survey showed that the mean wait time to see a physician, nurse, or physician assistant in an ED was 37.5 minutes, but wait times could range from less than 15 minutes to more than 6 hours.7 According to a study of 35,849 ED visits at nonfederal hospitals in the United States, only 47.7% of EDs admitted more than 90% of their patients within 6 hours.8 Moreover, perceived wait times in the ED have been shown to greatly impact patient satisfaction. Two predictors of perceived wait time include appropriate assessment of emergency level and the feeling of being forgotten.9 In a study of 2377 ED visits with primary dermatologic diagnoses, only 5.5% led to admission.10 This suggests many patients who come to the ED for dermatologic needs do not require inpatient hospital care. In these cases, patients with primary dermatologic concerns may experience longer ED wait times, as higher acuity or emergency cases take precedence. Studies also have shown that more vulnerable populations are utilizing ED visits most for primary dermatologic concerns.10,11 This includes individuals of lower income and/or those with Medicaid/Medicare or those without insurance.11 Predictors of high ED use for dermatologic concerns include prior frequent use of the ED (for nondermatologic concerns) instead of outpatient care, income below the poverty level, and lack of insurance; older individuals (>65 years) also were found to use the ED more frequently for dermatologic concerns when compared to younger individuals.10
Importantly, there is a great need for urgent dermatology consultation for pediatric patients. A single-institution study showed that over a 36-month period, there were 347 pediatric dermatology consultations from the pediatric ED mostly for children aged 0 days to 5 years; nearly half of these consultations required outpatient clinic follow-up.12 However, dermatology outpatient follow-up can be difficult to obtain, especially for vulnerable groups. In a study of 611 dermatology clinics, patients with Medicaid were shown to have longer wait times and less success in obtaining dermatology appointments compared to those with Medicare or private insurance.13 Only about 30% of private dermatology practices accept Medicaid patients, likely pushing these patients toward utilization of emergency services for dermatologic concerns.13,14
There is a clear role for a dermatology IC in our health care system, and the concept already has been identified and trialed in several institutions. At Oregon Health and Science University (Portland, Oregon), a retrospective chart review of patients with diagnoses of Morgellons disease and neurotic excoriations seen in dermatology urgent care between 2018 and 2020 showed an 88% decrease in annual rates of health care visits and a 77% decrease in ED visits after dermatology services were engaged compared to before the opening of the dermatology urgent care.15 Another study showed that uninsured or self-pay patients were more than 14 times more likely to access dermatology urgent care than to schedule a routine clinic appointment, suggesting that there is a barrier to making outpatient dermatologic appointments for uninsured patients. An urgent access model may facilitate the ability of underinsured patients to access care.16
Improving Dermatology Access for Other Specialties
Needs for dermatologic care are encountered in many other specialties. Having direct access to immediate dermatologic treatment is best for patients and may avoid inpatient care and trips to the ED for consultation access. Ideally, a dermatology IC would allow direct care to be provided alongside the oncology outpatient team. New immunologic therapies (cytotoxic T-lymphocyte–associated protein 4 and programmed cell death protein 1/programmed death-ligand 1 treatments) can cause dermatologic reactions in more than 40% of patients.17 Paraneoplastic syndromes can manifest with cutaneous symptoms, as can acute graft-vs-host-disease.18 In a study at Memorial Sloan Kettering (New York, New York) analyzing 426 same-day outpatient dermatology consultations, 17% of patients experienced interruptions in their cancer therapy, but 83% responded quickly to dermatologic treatment and resumed oncologic therapy—19% of them at a reduced dose.19 This is an important demonstration of prompt dermatologic consultation in an outpatient setting reducing interruptions to anticancer therapy. The heterogeneity of the cutaneous reactions seen from oncologic and immunomodulatory medications is profound, with more than 140 different types of skin-specific reactions.20
Solid-organ transplant recipients also could benefit from urgent access to dermatology services. These patients are at a much higher risk for skin cancers, and a study showed that those who receive referrals to dermatology are seen sooner after transplantation (5.6 years) than those who self-refer (7.2 years). Importantly, annual skin cancer screenings are recommended to begin 1 year after transplantation.21
Direct access to dermatology care could benefit patients with complicated rheumatologic conditions who present with skin findings; for example, patients with lupus erythematosus or dermatomyositis can have a spectrum of disease ranging from skin-predominant to systemic manifestations. Identification and treatment of such diseases require collaboration between dermatologists and rheumatologists.22 Likewise, a study of a joint rheumatology-dermatology clinic for psoriatic arthritis showed that a multidisciplinary approach to management leads to decreased time for patients to obtain proper rheumatologic and dermatologic examination and a faster time to diagnosis; however, such multidisciplinary clinic models and approaches to care often are found only at large university-based hospitals.23 In a patient population for whom time to diagnosis is crucial to avoid permanent changes such as joint destruction, a dermatology IC could fill this role in community hospitals and clinics. A dermatology IC also can serve patients with specific diagnoses who would benefit from more direct access to care; for example, in 2017 there were 131,430 ED visits for hidradenitis suppurativa (HS) in the United States. While HS is not uncommon, it usually is underdiagnosed because it can be challenging to differentiate from an uncomplicated abscess. Emergency department visits often are utilized for first-time presentations as well as flares of HS. In these situations, ED doctors can provide palliative treatment, but prompt referrals to dermatologists should be made for disease management to decrease recurrence.24
Final Thoughts
A huge caveat to the dermatology urgent care system is determining what is deemed “urgent.” We propose starting with a referral-based system only from other physicians (including IC and urgent care) rather than having patients walk in directly. Ideally, as support and staff increases, the availability can increase as well. In our institution, we suggested half-day clinics staffed by varying physicians, with compensation models similar to an ED or IC physician rather than by productivity. Each group considering this kind of addition to patient care will need to assess these points in building an IC for dermatology. The University of Pennsylvania’s (Philadelphia, Pennsylvania) system of rapid-access clinics to facilitate access to care for patients requiring urgent appointments may function as a model for future similar clinics.25 Creating a specialized IC/urgent care is not a novel concept. Orthopedic urgent care centers have increased greatly in the past decade, reducing ED burden for musculoskeletal complaints. In a study evaluating the utility of orthopedic urgent care settings, time to see an orthopedic specialist and cost were both greatly reduced with this system.1 The same has been shown in same-day access ophthalmology clinics, which are organized similarly to an urgent care.26
In 2021, there were 107.4 million treat-and-release visits to the ED in the United States for a total cost of $80.3 billion.27 This emphasizes the need to consider care models that not only provide excellent clinical care and treat the most acute diagnoses promptly and accurately but also reduce overall costs. While this may be convoluted for other specialties given the difficulty of having patients self-triage, dermatologic concerns are similar to orthopedic concerns for the patient to decipher the etiology of the concern. As in orthopedics, a dermatology IC could function similarly, increasing access, decreasing ED and IC wait times, saving overall health care spending, and allowing underserved and publicly insured individuals to have improved, prompt care.
- Anderson TJ, Althausen PL. The role of dedicated musculoskeletal urgent care centers in reducing cost and improving access to orthopaedic care. J Orthop Trauma. 2016;30:S3-S6.
- Falanga V, Schachner LA, Rae V, et al. Dermatologic consultations in the hospital setting. Arch Dermatol. 1994;130:1022-1025.
- Galimberti F, Guren L, Fernandez AP, et al. Dermatology consultations significantly contribute quality to care of hospitalized patients: a prospective study of dermatology inpatient consults at a tertiary care center. Int J Dermatol. 2016;55:E547-E551.
- Li DG, Xia FD, Khosravi H, et al. Outcomes of early dermatology consultation for inpatients diagnosed with cellulitis. JAMA Dermatol. 2018;154:537-543.
- Ko LN, Garza-Mayers AC, St John J, et al. Effect of dermatology consultation on outcomes for patients with presumed cellulitis: a randomized clinical trial. JAMA Dermatol. 2018;154:529-536.
- Grillo E, Vañó-Galván S, Jiménez-Gómez N, et al. Dermatologic emergencies: descriptive analysis of 861 patients in a tertiary care teaching hospital. Actas Dermosifiliogr. 2013;104:316-324.
- National Center for Health Statistics. National Hospital Ambulatory Medical Care Survey, 2021. Accessed September 23, 2025. https://www.cdc.gov/nchs/data/nhamcs/web_tables/2021-nhamcs-ed-web-tables-508.pdf
- Horwitz LI, Green J, Bradley EH. US emergency department performance on wait time and length of visit. Ann Emerg Med. 2010;55:133-141.
- Spechbach H, Rochat J, Gaspoz JM, et al. Patients’ time perception in the waiting room of an ambulatory emergency unit: a cross-sectional study. BMC Emerg Med. 2019;19:41.
- Yang JJ, Maloney NJ, Bach DQ, et al. Dermatology in the emergency department: prescriptions, rates of inpatient admission, and predictors of high utilization in the United States from 1996 to 2012. J Am Acad Dermatol. 2021;84:1480-1483.
- Chen CL, Fitzpatrick L, Kamel H. Who uses the emergency department for dermatologic care? a statewide analysis. J Am Acad Dermatol. 2014;71:308-313.
- Moon AT, Castelo-Soccio L, Yan AC. Emergency department utilization of pediatric dermatology (PD) consultations. J Am Acad Dermatol. 2016;74:1173-1177.
- Creadore A, Desai S, Li SJ, et al. Insurance acceptance, appointment wait time, and dermatologist access across practice types in the US. JAMA Dermatol. 2021;157:181-188.
- Mazmudar RS, Gupta N, Desai BJ, et al. Dermatologist appointment access and waiting times: a comparative study of insurance types. J Am Acad Dermatol. 2020;83:1468-1470.
- Johnson J, Cutler B, Latour E, et al. Dermatology urgent care model reduces costs and healthcare utilization for psychodermatology patients-a retrospective chart review. Dermatol Online J. 2022;28:5.
- Wintringham JA, Strock DM, Perkins-Holtsclaw K, et al. Dermatology in the urgent care setting: a retrospective review of patients seen in an urgent access dermatology clinic. J Am Acad Dermatol. 2023;89:1271-1273.
- Yoo MJ, Long B, Brady WJ, et al. Immune checkpoint inhibitors: an emergency medicine focused review. Am J Emerg Med. 2021;50:335-344.
- Merlo G, Cozzani E, Canale F, et al. Cutaneous manifestations of hematologic malignancies the experience of an Italian dermatology department. Hematol Oncol. 2019;37:285-290.
- Barrios D, Phillips G, Freites-Martinez A, et al. Outpatient dermatology consultations for oncology patients with acute dermatologic adverse events impact anticancer therapy interruption: a retrospective study.J Eur Acad Dermatol Venereol. 2020;34:1340-1347.
- Salah S, Kerob D, Pages Laurent C, et al. Evaluation of anticancer therapy-related dermatologic adverse events: insights from Food and Drug Administration’s Adverse Event Reporting System dataset. J Am Acad Dermatol. 2024;91:863-871. doi:10.1016/j.jaad.2024.07.1456
- Shope C, Andrews L, Girvin A, et al. Referrals to dermatology following solid organ transplant. J Am Acad Dermatol. 2023;88:1159-1160. doi:10.1016/j.jaad.2022.11.052
- Werth VP, Askanase AD, Lundberg IE. Importance of collaboration of dermatology and rheumatology to advance the field for lupus and dermatomyositis. Int J Womens Dermatol. 2021;7:583-587.
- Ziob J, Behning C, Brossart P, et al. Specialized dermatological-rheumatological patient management improves diagnostic outcome and patient journey in psoriasis and psoriatic arthritis: a four-year analysis. BMC Rheumatol. 2021;5:1-8. doi:10.1186/s41927-021-00217-z
- Okun MM, Flamm A, Werley EB, et al. Hidradenitis suppurativa: diagnosis and management in the emergency department. J Emerg Med. 2022;63:636-644.
- Jayakumar KL, Samimi SS, Vittorio CC, et al. Expediting patient appointments with dermatology rapid access clinics. Dermatol Online J. 2018;24:13030/qt2zv07510.
- Singman EL, Smith K, Mehta R, et al. Cost and visit duration of same-day access at an academic ophthalmology department vs emergency department. JAMA Ophthalmol. 2019;137:729-735. doi:10.1001/jamaophthalmol.2019.0864
- Roemer M. Costs of treat-and-release emergency department visits in the United States, 2021. Agency for Healthcare Research and Quality. Published September 2024. Accessed September 16, 2025. https://hcup-us.ahrq.gov/reports/statbriefs/sb311-ED-visit-costs-2021.pdf
- Anderson TJ, Althausen PL. The role of dedicated musculoskeletal urgent care centers in reducing cost and improving access to orthopaedic care. J Orthop Trauma. 2016;30:S3-S6.
- Falanga V, Schachner LA, Rae V, et al. Dermatologic consultations in the hospital setting. Arch Dermatol. 1994;130:1022-1025.
- Galimberti F, Guren L, Fernandez AP, et al. Dermatology consultations significantly contribute quality to care of hospitalized patients: a prospective study of dermatology inpatient consults at a tertiary care center. Int J Dermatol. 2016;55:E547-E551.
- Li DG, Xia FD, Khosravi H, et al. Outcomes of early dermatology consultation for inpatients diagnosed with cellulitis. JAMA Dermatol. 2018;154:537-543.
- Ko LN, Garza-Mayers AC, St John J, et al. Effect of dermatology consultation on outcomes for patients with presumed cellulitis: a randomized clinical trial. JAMA Dermatol. 2018;154:529-536.
- Grillo E, Vañó-Galván S, Jiménez-Gómez N, et al. Dermatologic emergencies: descriptive analysis of 861 patients in a tertiary care teaching hospital. Actas Dermosifiliogr. 2013;104:316-324.
- National Center for Health Statistics. National Hospital Ambulatory Medical Care Survey, 2021. Accessed September 23, 2025. https://www.cdc.gov/nchs/data/nhamcs/web_tables/2021-nhamcs-ed-web-tables-508.pdf
- Horwitz LI, Green J, Bradley EH. US emergency department performance on wait time and length of visit. Ann Emerg Med. 2010;55:133-141.
- Spechbach H, Rochat J, Gaspoz JM, et al. Patients’ time perception in the waiting room of an ambulatory emergency unit: a cross-sectional study. BMC Emerg Med. 2019;19:41.
- Yang JJ, Maloney NJ, Bach DQ, et al. Dermatology in the emergency department: prescriptions, rates of inpatient admission, and predictors of high utilization in the United States from 1996 to 2012. J Am Acad Dermatol. 2021;84:1480-1483.
- Chen CL, Fitzpatrick L, Kamel H. Who uses the emergency department for dermatologic care? a statewide analysis. J Am Acad Dermatol. 2014;71:308-313.
- Moon AT, Castelo-Soccio L, Yan AC. Emergency department utilization of pediatric dermatology (PD) consultations. J Am Acad Dermatol. 2016;74:1173-1177.
- Creadore A, Desai S, Li SJ, et al. Insurance acceptance, appointment wait time, and dermatologist access across practice types in the US. JAMA Dermatol. 2021;157:181-188.
- Mazmudar RS, Gupta N, Desai BJ, et al. Dermatologist appointment access and waiting times: a comparative study of insurance types. J Am Acad Dermatol. 2020;83:1468-1470.
- Johnson J, Cutler B, Latour E, et al. Dermatology urgent care model reduces costs and healthcare utilization for psychodermatology patients-a retrospective chart review. Dermatol Online J. 2022;28:5.
- Wintringham JA, Strock DM, Perkins-Holtsclaw K, et al. Dermatology in the urgent care setting: a retrospective review of patients seen in an urgent access dermatology clinic. J Am Acad Dermatol. 2023;89:1271-1273.
- Yoo MJ, Long B, Brady WJ, et al. Immune checkpoint inhibitors: an emergency medicine focused review. Am J Emerg Med. 2021;50:335-344.
- Merlo G, Cozzani E, Canale F, et al. Cutaneous manifestations of hematologic malignancies the experience of an Italian dermatology department. Hematol Oncol. 2019;37:285-290.
- Barrios D, Phillips G, Freites-Martinez A, et al. Outpatient dermatology consultations for oncology patients with acute dermatologic adverse events impact anticancer therapy interruption: a retrospective study.J Eur Acad Dermatol Venereol. 2020;34:1340-1347.
- Salah S, Kerob D, Pages Laurent C, et al. Evaluation of anticancer therapy-related dermatologic adverse events: insights from Food and Drug Administration’s Adverse Event Reporting System dataset. J Am Acad Dermatol. 2024;91:863-871. doi:10.1016/j.jaad.2024.07.1456
- Shope C, Andrews L, Girvin A, et al. Referrals to dermatology following solid organ transplant. J Am Acad Dermatol. 2023;88:1159-1160. doi:10.1016/j.jaad.2022.11.052
- Werth VP, Askanase AD, Lundberg IE. Importance of collaboration of dermatology and rheumatology to advance the field for lupus and dermatomyositis. Int J Womens Dermatol. 2021;7:583-587.
- Ziob J, Behning C, Brossart P, et al. Specialized dermatological-rheumatological patient management improves diagnostic outcome and patient journey in psoriasis and psoriatic arthritis: a four-year analysis. BMC Rheumatol. 2021;5:1-8. doi:10.1186/s41927-021-00217-z
- Okun MM, Flamm A, Werley EB, et al. Hidradenitis suppurativa: diagnosis and management in the emergency department. J Emerg Med. 2022;63:636-644.
- Jayakumar KL, Samimi SS, Vittorio CC, et al. Expediting patient appointments with dermatology rapid access clinics. Dermatol Online J. 2018;24:13030/qt2zv07510.
- Singman EL, Smith K, Mehta R, et al. Cost and visit duration of same-day access at an academic ophthalmology department vs emergency department. JAMA Ophthalmol. 2019;137:729-735. doi:10.1001/jamaophthalmol.2019.0864
- Roemer M. Costs of treat-and-release emergency department visits in the United States, 2021. Agency for Healthcare Research and Quality. Published September 2024. Accessed September 16, 2025. https://hcup-us.ahrq.gov/reports/statbriefs/sb311-ED-visit-costs-2021.pdf
Dermatology Immediate Care: A Game Changer for the Health Care System?
Dermatology Immediate Care: A Game Changer for the Health Care System?
Practice Points
- Emergency departments and most immediate care (IC) centers often lack prompt access to board-certified dermatologists.
- A dermatology urgent care/IC model may shorten wait times, improve access for vulnerable patients and pediatric populations, and reduce unnecessary hospital admissions and costs.
- Increased access to dermatology benefits other specialties by enabling multidisciplinary care leading to faster diagnosis and treatment.
- A staged referral-first dermatology IC pilot with defined staffing and triage rules is a practical path to demonstrate value and scale the service.
Pseudofolliculitis Barbae in the Military: Policy, Stigma, and Practical Solutions
Pseudofolliculitis Barbae in the Military: Policy, Stigma, and Practical Solutions
The impact of pseudofolliculitis barbae (PFB) on military service members and other uniformed professionals has been a topic of recent interest due to the announcement of the US Army’s new shaving rule in July 2025.1 The policy prohibits permanent shaving waivers, requires medical re-evaluation of shaving profiles within 90 days, and allows for administrative separation if a service member accumulates shaving exceptions totaling more than 12 months over a 24-month period.2 A common skin condition triggered or worsened by shaving, PFB causes painful bumps, pustules, and hyperpigmentation most often in the beard and cheek areas and negatively impacts quality of life. It disproportionately affects 45% to 83% of men in the United States, particularly those of African, Hispanic, or Middle Eastern descent.3,4 Genetic factors, particularly tightly coiled or coarse curly hair, can predispose individuals to PFB. The most successful treatment for PFB is to stop shaving, but this conflicts with military shaving standards and interferes with the use of protective equipment (eg, masks). Herein, we highlight the adverse impact of PFB on military career progression and provide context for clinicians who treat patients with PFB, especially as policies recently have shifted to allow nonmilitary clinicians to evaluate PFB in service members.5
Shaving Waivers and Advancement
Pseudofolliculitis barbae disproportionately prolongs the time to advancement of many service members, and those with PFB also are overburdened by policy changes related to shaving.6 In the US military, nearly 18% of the active-duty force is Black,7 a population that is more susceptible to PFB. Military personnel may request PFB-related accommodations, including medical shaving waivers that vary by branch. Through a formal documentation process, waivers allow service members to maintain facial hair up to one-quarter inch in length.5 Previously, waivers could be temporary (eg, up to 90 days) or permanent as subjectively determined based on clinician-documented disease severity. Almost 65% of US Air Force medical shaving waivers are held by Black men, and PFB is one of the most common reasons.6 Notably, the US Navy discontinued permanent shaving waivers in October 2019.8 A US Marine Corps policy issued in March 2025 now allows administrative separation of service members with PFB if symptoms do not improve after a 1-year medical shaving waiver due to “incompatibility with service.”9 This change reversed a 2022 policy that protected Marines from separation based on PFB.10 A Marine Corps spokesperson stated that this change aims to clarify how medical conditions can impact uniform compliance and standardize medical condition management while prioritizing compliance and duty readiness.1
Even in the absence of policy changes, obtaining a medical shaving waiver for PFB can be challenging. Service members may have little to no access to military dermatologists who specialize in management of PFB and experience long wait times for civilian network deferment. Service members seen in civilian clinics may have restricted treatment options due to limited insurance coverage for laser hair reduction, even in the most difficult-to-manage areas (eg, neck, jawline). Expanding access to military dermatologists, civilian dermatologists who are experienced with PFB and understand the impact and necessity of military waivers, and teledermatology services could help improve and streamline care. Other challenges include the subjective nature of documenting PFB disease severity, the need for validated assessment tools, a lack of standardized policies across military branches, and stigma. A standardized approach to documentation may reduce variability in how shaving waivers are evaluated across service branches, but at a minimum, clinicians should document the diagnosis, clinical findings, severity of PFB, and the treatment used. Having a waiver would help these service members focus on mastering critical skillsets and performing duties without the time pressures, angst, and expense dedicated to caring for and managing PFB.
Clinical and Policy Barriers
Unfortunately, service members with PFB or shaving waivers often face stigma that can hinder career advancement.6 In a recent analysis of 9339 US Air Force personnel, those with shaving waivers experienced longer times to promotion compared to those without waivers: in the waiver group, 94.47% were enlisted and 5.53% were officers; in the nonwaiver group, 72.11% were enlisted and 27.89% were officers (P=.0003).6 While delays in promotion were consistent across racial groups, most of the waiver holders identified as Black (64.8%), despite this demographic group representing only a small portion of the overall cohort (12.9%).6 Promotion delays may be linked to perceptions of unprofessionalism and exclusion from high-profile assignments, which notably require “the highest standards of military appearance and professional conduct.”11 The burden of career-limiting shaving policies falls disproportionately on military personnel with PFB who self-identify as Black. Perceptions about unprofessional appearance or job readiness often unintentionally introduce bias, unjustly restricting career advancement.6
Safety Equipment and Shaving Standards
Conditions that potentially affect the use of masks and chemical defense equipment extend beyond the military. Firefighters and law enforcement officers generally are required to maintain a clean-shaven face for proper fit of respirator masks; the standard is that no respirator fit test shall be conducted if hair—including stubble, beards, mustaches, or sideburns—grows between the skin and the facepiece sealing surface, and any apparel interfering with a proper seal must be altered or removed.12 This creates challenges for uniformed professionals with PFB who must manage their condition while adhering to safety requirements. Some endure long-term pain and scarring in order to comply, while others seek waivers to treat and prevent symptoms while also facing the stigma of doing so.13 One of the most effective treatments for PFB is to discontinue shaving,14 which may not be feasible for those in uniformed professions with strict grooming standards. Research on mask seal effectiveness in individuals with neatly trimmed beards or PFB remains limited.5 Studies evaluating mask fit across facial hair types and lengths are needed, along with the development of protective equipment that accommodates career-limiting conditions such as PFB, cystic acne, and acne keloidalis nuchae. This also may encourage development of equipment that does not induce such conditions (eg, mechanical acne from friction). These efforts would promote safety, scientific innovation for dermatologic follicular-based disorders, and overall quality of life for service members as well as increase their ability to serve without stigma. These developments also would positively impact other fields that require intermittent or full-time use of masks, including health care and some food service industries.
Final Thoughts
The disproportionate impact of PFB in the military highlights the need for improved access to treatment, culturally informed care, and policies that avoid penalizing service members with tightly coiled hair and a desire to serve. We discussed PFB management strategies, clinical features, and implications across various skin tones in a previous publication.14 It is important to consider insights from individuals with PFB who are serving in the military as well as the medical personnel who care for them. Ensuring or creating effective treatment options drives innovation, and evidence-based accommodation plans can help individuals in uniformed professions avoid choosing between PFB management and their career. Promoting awareness about the impact of PFB beyond the razor is key to reducing disparities and supporting excellence among those who serve and desire to continue to do so.
- Lawrence DF. Marines with skin condition affecting mostly black men could now be booted under new policy. Military.com. March 14, 2025. Accessed May 4, 2025. https://www.military.com/daily-news/2025/03/14/marines-can-now-be-kicked-out-skin-condition-affects-mostly-black-men.html
- Secretary of the Army. Army directive 2025-13 (facial hair grooming standards). Published July 7, 2025. Accessed September 19, 2025. https://lyster.tricare.mil/Portals/61/ARN44307-ARMY_DIR_2025-13-000.pdf
- Alexis A, Heath CR, Halder RM. Folliculitis keloidalis nuchae and pseudofolliculitis barbae: are prevention and effective treatment within reach? Dermatol Clin. 2014;32:183-191. doi:10.1016/j.det.2013.12.001
- Gray J, McMichael AJ. Pseudofolliculitis barbae: understanding the condition and the role of facial grooming. Int J Cosmet Sci. 2016;38:24-27. doi:10.1111/ics.12331
- Jung I, Lannan FM, Weiss A, et al. Treatment and current policies on pseudofolliculitis barbae in the US military. Cutis. 2023;112:299-302. doi:10.12788/cutis.0907
- Ritchie S, Park J, Banta J, et al. Shaving waivers in the United States Air Force and their impact on promotions of Black/African-American members. Mil Med. 2023;188:E242-E247. doi:10.1093/milmed/usab272
- Defense Manpower Data Center. Active-duty military personnel master file and reserve components common personnel data system. Military OneSource. September 2023. Accessed May 3, 2025. https://download.militaryonesource.mil/12038/MOS/Reports/2023-demographics-report.pdf
- Tshudy MT, Cho S. Pseudofolliculitis barbae in the US. Military, a review. Mil Med. 2021;186:E52-E57. doi:10.1093/milmed/usaa243
- US Marine Corps. Uniform and grooming standards for medical conditions (MARADMINS number: 124/25). Published March 13, 2025. Accessed September 19, 2025. https://www.marines.mil/News/Messages/Messages-Display/Article/4119098/uniform-and-grooming-standards-for-medical-conditions/
- US Marine Corps. Advance notification of change to MCO 6310.1C (Pseudofolliculitis Barbae), MCO 1900.16 CH2 (Marine Corps Retirement and Separation Manual), and MCO 1040.31 (Enlisted Retention and Career Development Program). Published January 21, 2022. Accessed September 19, 2025. https://www.marines.mil/News/Messages/Messages-Display/Article/2907104/advance-notification-of-change-to-mco-63101c-pseudofolliculitis-barbae-mco-1900/
- US Department of Defense. Special duty catalog (SPECAT). Published August 15, 2013. Accessed September 19, 2025. https://share.google/iuMrVMIASWx4EFLVN
- Occupational Safety and Health Administration. Appendix A to §1910.134—fit testing procedures (mandatory). Accessed September 19, 2025. https://www.osha.gov/laws-regs/regulations/standardnumber/1910/1910.134AppA
- Jiang YR. Reasonable accommodation and disparate impact: clean shave policy discrimination in today’s workplace. J Law Med Ethics. 2023;51:185-195. doi:10.1017/jme.2023.55
- Welch D, Usatine R, Heath C. Implications of PFB beyond the razor. Cutis. 2025;115:135-136. doi:10.12788/cutis.1194
The impact of pseudofolliculitis barbae (PFB) on military service members and other uniformed professionals has been a topic of recent interest due to the announcement of the US Army’s new shaving rule in July 2025.1 The policy prohibits permanent shaving waivers, requires medical re-evaluation of shaving profiles within 90 days, and allows for administrative separation if a service member accumulates shaving exceptions totaling more than 12 months over a 24-month period.2 A common skin condition triggered or worsened by shaving, PFB causes painful bumps, pustules, and hyperpigmentation most often in the beard and cheek areas and negatively impacts quality of life. It disproportionately affects 45% to 83% of men in the United States, particularly those of African, Hispanic, or Middle Eastern descent.3,4 Genetic factors, particularly tightly coiled or coarse curly hair, can predispose individuals to PFB. The most successful treatment for PFB is to stop shaving, but this conflicts with military shaving standards and interferes with the use of protective equipment (eg, masks). Herein, we highlight the adverse impact of PFB on military career progression and provide context for clinicians who treat patients with PFB, especially as policies recently have shifted to allow nonmilitary clinicians to evaluate PFB in service members.5
Shaving Waivers and Advancement
Pseudofolliculitis barbae disproportionately prolongs the time to advancement of many service members, and those with PFB also are overburdened by policy changes related to shaving.6 In the US military, nearly 18% of the active-duty force is Black,7 a population that is more susceptible to PFB. Military personnel may request PFB-related accommodations, including medical shaving waivers that vary by branch. Through a formal documentation process, waivers allow service members to maintain facial hair up to one-quarter inch in length.5 Previously, waivers could be temporary (eg, up to 90 days) or permanent as subjectively determined based on clinician-documented disease severity. Almost 65% of US Air Force medical shaving waivers are held by Black men, and PFB is one of the most common reasons.6 Notably, the US Navy discontinued permanent shaving waivers in October 2019.8 A US Marine Corps policy issued in March 2025 now allows administrative separation of service members with PFB if symptoms do not improve after a 1-year medical shaving waiver due to “incompatibility with service.”9 This change reversed a 2022 policy that protected Marines from separation based on PFB.10 A Marine Corps spokesperson stated that this change aims to clarify how medical conditions can impact uniform compliance and standardize medical condition management while prioritizing compliance and duty readiness.1
Even in the absence of policy changes, obtaining a medical shaving waiver for PFB can be challenging. Service members may have little to no access to military dermatologists who specialize in management of PFB and experience long wait times for civilian network deferment. Service members seen in civilian clinics may have restricted treatment options due to limited insurance coverage for laser hair reduction, even in the most difficult-to-manage areas (eg, neck, jawline). Expanding access to military dermatologists, civilian dermatologists who are experienced with PFB and understand the impact and necessity of military waivers, and teledermatology services could help improve and streamline care. Other challenges include the subjective nature of documenting PFB disease severity, the need for validated assessment tools, a lack of standardized policies across military branches, and stigma. A standardized approach to documentation may reduce variability in how shaving waivers are evaluated across service branches, but at a minimum, clinicians should document the diagnosis, clinical findings, severity of PFB, and the treatment used. Having a waiver would help these service members focus on mastering critical skillsets and performing duties without the time pressures, angst, and expense dedicated to caring for and managing PFB.
Clinical and Policy Barriers
Unfortunately, service members with PFB or shaving waivers often face stigma that can hinder career advancement.6 In a recent analysis of 9339 US Air Force personnel, those with shaving waivers experienced longer times to promotion compared to those without waivers: in the waiver group, 94.47% were enlisted and 5.53% were officers; in the nonwaiver group, 72.11% were enlisted and 27.89% were officers (P=.0003).6 While delays in promotion were consistent across racial groups, most of the waiver holders identified as Black (64.8%), despite this demographic group representing only a small portion of the overall cohort (12.9%).6 Promotion delays may be linked to perceptions of unprofessionalism and exclusion from high-profile assignments, which notably require “the highest standards of military appearance and professional conduct.”11 The burden of career-limiting shaving policies falls disproportionately on military personnel with PFB who self-identify as Black. Perceptions about unprofessional appearance or job readiness often unintentionally introduce bias, unjustly restricting career advancement.6
Safety Equipment and Shaving Standards
Conditions that potentially affect the use of masks and chemical defense equipment extend beyond the military. Firefighters and law enforcement officers generally are required to maintain a clean-shaven face for proper fit of respirator masks; the standard is that no respirator fit test shall be conducted if hair—including stubble, beards, mustaches, or sideburns—grows between the skin and the facepiece sealing surface, and any apparel interfering with a proper seal must be altered or removed.12 This creates challenges for uniformed professionals with PFB who must manage their condition while adhering to safety requirements. Some endure long-term pain and scarring in order to comply, while others seek waivers to treat and prevent symptoms while also facing the stigma of doing so.13 One of the most effective treatments for PFB is to discontinue shaving,14 which may not be feasible for those in uniformed professions with strict grooming standards. Research on mask seal effectiveness in individuals with neatly trimmed beards or PFB remains limited.5 Studies evaluating mask fit across facial hair types and lengths are needed, along with the development of protective equipment that accommodates career-limiting conditions such as PFB, cystic acne, and acne keloidalis nuchae. This also may encourage development of equipment that does not induce such conditions (eg, mechanical acne from friction). These efforts would promote safety, scientific innovation for dermatologic follicular-based disorders, and overall quality of life for service members as well as increase their ability to serve without stigma. These developments also would positively impact other fields that require intermittent or full-time use of masks, including health care and some food service industries.
Final Thoughts
The disproportionate impact of PFB in the military highlights the need for improved access to treatment, culturally informed care, and policies that avoid penalizing service members with tightly coiled hair and a desire to serve. We discussed PFB management strategies, clinical features, and implications across various skin tones in a previous publication.14 It is important to consider insights from individuals with PFB who are serving in the military as well as the medical personnel who care for them. Ensuring or creating effective treatment options drives innovation, and evidence-based accommodation plans can help individuals in uniformed professions avoid choosing between PFB management and their career. Promoting awareness about the impact of PFB beyond the razor is key to reducing disparities and supporting excellence among those who serve and desire to continue to do so.
The impact of pseudofolliculitis barbae (PFB) on military service members and other uniformed professionals has been a topic of recent interest due to the announcement of the US Army’s new shaving rule in July 2025.1 The policy prohibits permanent shaving waivers, requires medical re-evaluation of shaving profiles within 90 days, and allows for administrative separation if a service member accumulates shaving exceptions totaling more than 12 months over a 24-month period.2 A common skin condition triggered or worsened by shaving, PFB causes painful bumps, pustules, and hyperpigmentation most often in the beard and cheek areas and negatively impacts quality of life. It disproportionately affects 45% to 83% of men in the United States, particularly those of African, Hispanic, or Middle Eastern descent.3,4 Genetic factors, particularly tightly coiled or coarse curly hair, can predispose individuals to PFB. The most successful treatment for PFB is to stop shaving, but this conflicts with military shaving standards and interferes with the use of protective equipment (eg, masks). Herein, we highlight the adverse impact of PFB on military career progression and provide context for clinicians who treat patients with PFB, especially as policies recently have shifted to allow nonmilitary clinicians to evaluate PFB in service members.5
Shaving Waivers and Advancement
Pseudofolliculitis barbae disproportionately prolongs the time to advancement of many service members, and those with PFB also are overburdened by policy changes related to shaving.6 In the US military, nearly 18% of the active-duty force is Black,7 a population that is more susceptible to PFB. Military personnel may request PFB-related accommodations, including medical shaving waivers that vary by branch. Through a formal documentation process, waivers allow service members to maintain facial hair up to one-quarter inch in length.5 Previously, waivers could be temporary (eg, up to 90 days) or permanent as subjectively determined based on clinician-documented disease severity. Almost 65% of US Air Force medical shaving waivers are held by Black men, and PFB is one of the most common reasons.6 Notably, the US Navy discontinued permanent shaving waivers in October 2019.8 A US Marine Corps policy issued in March 2025 now allows administrative separation of service members with PFB if symptoms do not improve after a 1-year medical shaving waiver due to “incompatibility with service.”9 This change reversed a 2022 policy that protected Marines from separation based on PFB.10 A Marine Corps spokesperson stated that this change aims to clarify how medical conditions can impact uniform compliance and standardize medical condition management while prioritizing compliance and duty readiness.1
Even in the absence of policy changes, obtaining a medical shaving waiver for PFB can be challenging. Service members may have little to no access to military dermatologists who specialize in management of PFB and experience long wait times for civilian network deferment. Service members seen in civilian clinics may have restricted treatment options due to limited insurance coverage for laser hair reduction, even in the most difficult-to-manage areas (eg, neck, jawline). Expanding access to military dermatologists, civilian dermatologists who are experienced with PFB and understand the impact and necessity of military waivers, and teledermatology services could help improve and streamline care. Other challenges include the subjective nature of documenting PFB disease severity, the need for validated assessment tools, a lack of standardized policies across military branches, and stigma. A standardized approach to documentation may reduce variability in how shaving waivers are evaluated across service branches, but at a minimum, clinicians should document the diagnosis, clinical findings, severity of PFB, and the treatment used. Having a waiver would help these service members focus on mastering critical skillsets and performing duties without the time pressures, angst, and expense dedicated to caring for and managing PFB.
Clinical and Policy Barriers
Unfortunately, service members with PFB or shaving waivers often face stigma that can hinder career advancement.6 In a recent analysis of 9339 US Air Force personnel, those with shaving waivers experienced longer times to promotion compared to those without waivers: in the waiver group, 94.47% were enlisted and 5.53% were officers; in the nonwaiver group, 72.11% were enlisted and 27.89% were officers (P=.0003).6 While delays in promotion were consistent across racial groups, most of the waiver holders identified as Black (64.8%), despite this demographic group representing only a small portion of the overall cohort (12.9%).6 Promotion delays may be linked to perceptions of unprofessionalism and exclusion from high-profile assignments, which notably require “the highest standards of military appearance and professional conduct.”11 The burden of career-limiting shaving policies falls disproportionately on military personnel with PFB who self-identify as Black. Perceptions about unprofessional appearance or job readiness often unintentionally introduce bias, unjustly restricting career advancement.6
Safety Equipment and Shaving Standards
Conditions that potentially affect the use of masks and chemical defense equipment extend beyond the military. Firefighters and law enforcement officers generally are required to maintain a clean-shaven face for proper fit of respirator masks; the standard is that no respirator fit test shall be conducted if hair—including stubble, beards, mustaches, or sideburns—grows between the skin and the facepiece sealing surface, and any apparel interfering with a proper seal must be altered or removed.12 This creates challenges for uniformed professionals with PFB who must manage their condition while adhering to safety requirements. Some endure long-term pain and scarring in order to comply, while others seek waivers to treat and prevent symptoms while also facing the stigma of doing so.13 One of the most effective treatments for PFB is to discontinue shaving,14 which may not be feasible for those in uniformed professions with strict grooming standards. Research on mask seal effectiveness in individuals with neatly trimmed beards or PFB remains limited.5 Studies evaluating mask fit across facial hair types and lengths are needed, along with the development of protective equipment that accommodates career-limiting conditions such as PFB, cystic acne, and acne keloidalis nuchae. This also may encourage development of equipment that does not induce such conditions (eg, mechanical acne from friction). These efforts would promote safety, scientific innovation for dermatologic follicular-based disorders, and overall quality of life for service members as well as increase their ability to serve without stigma. These developments also would positively impact other fields that require intermittent or full-time use of masks, including health care and some food service industries.
Final Thoughts
The disproportionate impact of PFB in the military highlights the need for improved access to treatment, culturally informed care, and policies that avoid penalizing service members with tightly coiled hair and a desire to serve. We discussed PFB management strategies, clinical features, and implications across various skin tones in a previous publication.14 It is important to consider insights from individuals with PFB who are serving in the military as well as the medical personnel who care for them. Ensuring or creating effective treatment options drives innovation, and evidence-based accommodation plans can help individuals in uniformed professions avoid choosing between PFB management and their career. Promoting awareness about the impact of PFB beyond the razor is key to reducing disparities and supporting excellence among those who serve and desire to continue to do so.
- Lawrence DF. Marines with skin condition affecting mostly black men could now be booted under new policy. Military.com. March 14, 2025. Accessed May 4, 2025. https://www.military.com/daily-news/2025/03/14/marines-can-now-be-kicked-out-skin-condition-affects-mostly-black-men.html
- Secretary of the Army. Army directive 2025-13 (facial hair grooming standards). Published July 7, 2025. Accessed September 19, 2025. https://lyster.tricare.mil/Portals/61/ARN44307-ARMY_DIR_2025-13-000.pdf
- Alexis A, Heath CR, Halder RM. Folliculitis keloidalis nuchae and pseudofolliculitis barbae: are prevention and effective treatment within reach? Dermatol Clin. 2014;32:183-191. doi:10.1016/j.det.2013.12.001
- Gray J, McMichael AJ. Pseudofolliculitis barbae: understanding the condition and the role of facial grooming. Int J Cosmet Sci. 2016;38:24-27. doi:10.1111/ics.12331
- Jung I, Lannan FM, Weiss A, et al. Treatment and current policies on pseudofolliculitis barbae in the US military. Cutis. 2023;112:299-302. doi:10.12788/cutis.0907
- Ritchie S, Park J, Banta J, et al. Shaving waivers in the United States Air Force and their impact on promotions of Black/African-American members. Mil Med. 2023;188:E242-E247. doi:10.1093/milmed/usab272
- Defense Manpower Data Center. Active-duty military personnel master file and reserve components common personnel data system. Military OneSource. September 2023. Accessed May 3, 2025. https://download.militaryonesource.mil/12038/MOS/Reports/2023-demographics-report.pdf
- Tshudy MT, Cho S. Pseudofolliculitis barbae in the US. Military, a review. Mil Med. 2021;186:E52-E57. doi:10.1093/milmed/usaa243
- US Marine Corps. Uniform and grooming standards for medical conditions (MARADMINS number: 124/25). Published March 13, 2025. Accessed September 19, 2025. https://www.marines.mil/News/Messages/Messages-Display/Article/4119098/uniform-and-grooming-standards-for-medical-conditions/
- US Marine Corps. Advance notification of change to MCO 6310.1C (Pseudofolliculitis Barbae), MCO 1900.16 CH2 (Marine Corps Retirement and Separation Manual), and MCO 1040.31 (Enlisted Retention and Career Development Program). Published January 21, 2022. Accessed September 19, 2025. https://www.marines.mil/News/Messages/Messages-Display/Article/2907104/advance-notification-of-change-to-mco-63101c-pseudofolliculitis-barbae-mco-1900/
- US Department of Defense. Special duty catalog (SPECAT). Published August 15, 2013. Accessed September 19, 2025. https://share.google/iuMrVMIASWx4EFLVN
- Occupational Safety and Health Administration. Appendix A to §1910.134—fit testing procedures (mandatory). Accessed September 19, 2025. https://www.osha.gov/laws-regs/regulations/standardnumber/1910/1910.134AppA
- Jiang YR. Reasonable accommodation and disparate impact: clean shave policy discrimination in today’s workplace. J Law Med Ethics. 2023;51:185-195. doi:10.1017/jme.2023.55
- Welch D, Usatine R, Heath C. Implications of PFB beyond the razor. Cutis. 2025;115:135-136. doi:10.12788/cutis.1194
- Lawrence DF. Marines with skin condition affecting mostly black men could now be booted under new policy. Military.com. March 14, 2025. Accessed May 4, 2025. https://www.military.com/daily-news/2025/03/14/marines-can-now-be-kicked-out-skin-condition-affects-mostly-black-men.html
- Secretary of the Army. Army directive 2025-13 (facial hair grooming standards). Published July 7, 2025. Accessed September 19, 2025. https://lyster.tricare.mil/Portals/61/ARN44307-ARMY_DIR_2025-13-000.pdf
- Alexis A, Heath CR, Halder RM. Folliculitis keloidalis nuchae and pseudofolliculitis barbae: are prevention and effective treatment within reach? Dermatol Clin. 2014;32:183-191. doi:10.1016/j.det.2013.12.001
- Gray J, McMichael AJ. Pseudofolliculitis barbae: understanding the condition and the role of facial grooming. Int J Cosmet Sci. 2016;38:24-27. doi:10.1111/ics.12331
- Jung I, Lannan FM, Weiss A, et al. Treatment and current policies on pseudofolliculitis barbae in the US military. Cutis. 2023;112:299-302. doi:10.12788/cutis.0907
- Ritchie S, Park J, Banta J, et al. Shaving waivers in the United States Air Force and their impact on promotions of Black/African-American members. Mil Med. 2023;188:E242-E247. doi:10.1093/milmed/usab272
- Defense Manpower Data Center. Active-duty military personnel master file and reserve components common personnel data system. Military OneSource. September 2023. Accessed May 3, 2025. https://download.militaryonesource.mil/12038/MOS/Reports/2023-demographics-report.pdf
- Tshudy MT, Cho S. Pseudofolliculitis barbae in the US. Military, a review. Mil Med. 2021;186:E52-E57. doi:10.1093/milmed/usaa243
- US Marine Corps. Uniform and grooming standards for medical conditions (MARADMINS number: 124/25). Published March 13, 2025. Accessed September 19, 2025. https://www.marines.mil/News/Messages/Messages-Display/Article/4119098/uniform-and-grooming-standards-for-medical-conditions/
- US Marine Corps. Advance notification of change to MCO 6310.1C (Pseudofolliculitis Barbae), MCO 1900.16 CH2 (Marine Corps Retirement and Separation Manual), and MCO 1040.31 (Enlisted Retention and Career Development Program). Published January 21, 2022. Accessed September 19, 2025. https://www.marines.mil/News/Messages/Messages-Display/Article/2907104/advance-notification-of-change-to-mco-63101c-pseudofolliculitis-barbae-mco-1900/
- US Department of Defense. Special duty catalog (SPECAT). Published August 15, 2013. Accessed September 19, 2025. https://share.google/iuMrVMIASWx4EFLVN
- Occupational Safety and Health Administration. Appendix A to §1910.134—fit testing procedures (mandatory). Accessed September 19, 2025. https://www.osha.gov/laws-regs/regulations/standardnumber/1910/1910.134AppA
- Jiang YR. Reasonable accommodation and disparate impact: clean shave policy discrimination in today’s workplace. J Law Med Ethics. 2023;51:185-195. doi:10.1017/jme.2023.55
- Welch D, Usatine R, Heath C. Implications of PFB beyond the razor. Cutis. 2025;115:135-136. doi:10.12788/cutis.1194
Pseudofolliculitis Barbae in the Military: Policy, Stigma, and Practical Solutions
Pseudofolliculitis Barbae in the Military: Policy, Stigma, and Practical Solutions
Tender Nodule on the Umbilicus
Tender Nodule on the Umbilicus
THE DIAGNOSIS: Villar Nodule
The biopsy revealed features consistent with cutaneous endometriosis in the setting of a painful, tender, multilobulated nodule with a cyclical bleeding pattern (Figure 1). The bleeding pattern of the nodule during menses and lack of surgical history supported the diagnosis of primary cutaneous endometriosis in our patient. She was diagnosed with endometriosis by gynecology, and her primary care physician started her on an oral contraceptive based on this diagnosis. She also was referred to gynecology and plastic surgery for a joint surgical consultation to remove the nodule. She initially decided to do a trial of the oral contraceptive but subsequently underwent umbilical endometrioma excision with neo-umbilicus creation with no evidence of recurrence.
Primary cutaneous endometriosis should be considered in young females who present with tender umbilical nodules. Endometriosis refers to the presence of an endometriumlike epithelium outside the endometrium and myometrium.1 The condition affects 10% to 15% of reproductive-aged (ie, 18-49 years) women in the United States and typically involves tissues within the pelvis, such as the ovaries, pouch of Douglas, or pelvic ligaments.2 Cutaneous endometriosis is the growth of endometrial tissue in the skin and is rare, accounting for less than 5.5% of cases of extrapelvic endometriosis worldwide, affecting primarily the umbilicus, abdominal wall, and vulva.3,4
The 2 main types of cutaneous endometriosis are primary (spontaneous) and secondary. Primary lesions develop in patients without prior surgical history, and secondary lesions occur within previous surgical incision sites, often scars from cesarean delivery.5 Less than 30% of cases of cutaneous endometriosis are primary disease.6 Primary cutaneous endometriosis of the umbilicus, known as Villar nodule, was first described in 1886.3,7 Up to 40% of patients with extrapelvic endometriosis worldwide presented with Villar nodules in a systematic literature review.6 The prevalence of these nodules is unknown, but the incidence is less than 1% of cases of extragenital endometriosis.4
There are 2 leading theories of primary cutaneous endometriosis pathogenesis. The first is the transportation theory, in which endometrial cells are transported outside the uterus via the lymphatic system.8 The second is the metaplasia theory, which proposed that endometrial cells develop in the coelomic mesothelium in the presence of high estrogen levels.8,9
Secondary cutaneous endometriosis, also known as scar endometriosis, is suspected to be caused by an iatrogenic implantation of endometrial cells at the scar of a prior surgical site.9 Although our patient had an existing umbilicus scar from a piercing, it was improbable for that to have been the nidus, as the keloid scar was superficial and did not have contact with the abdominal cavity for iatrogenic implantation. Clinical diagnosis for secondary cutaneous endometriosis often is made based on a triad of features: a nonmalignant abdominal mass, recurring pain and bleeding of the lesion with menses, and prior history of abdominal surgery.9,10 On clinical examination, these features typically manifest as a palpable subcutaneous mass that is black, blue, brown, or red. Often, the lesions enlarge and bleed during the menstrual cycle, causing pain, tenderness, or pruritus.3 Dermoscopic features of secondary cutaneous endometriosis are erythematous umbilical nodules with a homogeneous vascular pattern that appears red with a brownish hue (Figure 2).9,11 Dermoscopic features may vary with the hormone cycle; for example, the follicular phase (correlating with day 7 of menses) demonstrates polypoid projections, erythematous violaceous color, dark-brown spots, and active bleeding of the lesion.12 Clinical and dermoscopic examination are useful tools in this diagnosis.
Imaging such as ultrasonography, computed tomography, or magnetic resonance imaging may be useful in identifying abdominal endometriomas.8,13,14 Pelvic involvement of endometriosis was found in approximately 15% of patients in a case series,4 with concurrent primary umbilical endometriosis. Imaging studies may assist evaluation for fistula formation, presence of malignancies, and the extent of endometriosis within the abdominal cavity.
Histopathology is key to confirming cutaneous endometriosis and shows multiple bland-appearing glands of varying sizes with loose, concentric, edematous, or fibromyxoid stroma (Figure 1).3 Red blood cells sometimes are found with hemosiderin within the stroma. Immunohistochemical staining with estrogen receptors may aid in identifying the endometriumlike epithelial cells.13
Standard treatment involves surgical excision with 1-cm margins and umbilical preservation, which results in a recurrence rate of less than 10%.4,10 Medical therapy, such as aromatase inhibitors, progestogens, antiprogestogens, combined oral contraceptives, or gonadotropin-releasing hormone agonists or antagonists may help manage pain or reduce the size of the nodule.4,15 Simple observation also is a potential course for patients who decline treatment options.
Differential diagnoses include lobular capillary hemangioma, also known as pyogenic granuloma; Sister Mary Joseph nodule; umbilical hernia; and dermatofibrosarcoma protuberans. Lobular capillary hemangiomas commonly are acquired benign vascular proliferations of the skin that are friable and tend to ulcerate.16 These lesions typically grow rapidly and often are located on the face, lips, mucosae, and fingers. Histopathologic examination may show an exophytic lesion with lobules of proliferating capillaries within an edematous matrix, superficial ulceration, and an epithelial collarette.17 Treatment includes surgical excision, cauterization, laser treatments, sclerotherapy, injectable medications, and topical medications, but recurrence is possible with any of these interventions.18
Cutaneous metastasis of an internal solid organ cancer, commonly known as a Sister Mary Joseph nodule, typically manifests as an erythematous, irregularly shaped nodule that may protrude from the umbilicus.14 Gastrointestinal symptoms such as change in bowel habits or obstructive symptoms in the setting of a progressive malignancy are common.14 Clinical features include a firm fixed lesion, oozing, and ulceration.19 On dermoscopy, polymorphous vascular patterns, milky red structureless areas, and white lines typically are present.11 Although dermoscopic features may differentiate this entity from cutaneous endometriosis, tissue sampling and histologic examination are crucial diagnostic tools to identify malignant vs benign lesions.
An umbilical hernia is a protrusion of omentum, bowel, or other intra-abdominal organs in an abdominal wall defect. Clinical presentation includes a soft protrusion that may be reduced on palpation if nonstrangulated.20 Treatment includes watchful waiting or surgical repair. The reducibility and presence of an abdominal wall defect may point to this diagnosis. Imaging also may aid in the diagnosis if the history and physical examination are unclear.
Dermatofibrosarcoma protuberans is a slow-developing, low- to intermediate-grade, soft-tissue sarcoma that occurs in less than 0.1% of all cancers in the United States.21 Lesions often manifest as small, firm, slow-growing, painless, flesh-colored dermal plaques; subcutaneous thickening; or atrophic nonprotuberant lesions typically involving the trunk.21 Histopathologically, they are composed of uniform spindle-cell proliferation growing in a storiform pattern and subcutaneous fat trapping that has strong and diffuse CD34 immunoreactivity.21,22 Pathologic examination typically distinguishes this diagnosis from cutaneous endometriosis. Treatment includes tumor resection that may or may not involve radiotherapy and targeted therapy, as recurrence and metastases are possible.
Primary cutaneous endometriosis is a rare but important diagnosis for dermatologists to consider when evaluating umbilical nodules. Clinical features may include bleeding masses during menses in females of reproductive age. Dermoscopic examination aids in workup, and histopathologic testing can confirm the diagnosis and rule out malignancies. Surgical excision is the treatment of choice with a low rate of recurrence.
- International Working Group of AAGL, ESGE, ESHRE and WES; Tomassetti C, Johnson NP, et al. An international terminology for endometriosis, 2021. Hum Reprod Open. 2021;2021:hoab029. doi:10.1093/hropen/hoab029
- Batista M, Alves F, Cardoso J, et al. Cutaneous endometriosis: a differential diagnosis of umbilical nodule. Acta Med Port. 2020; 33:282-284. doi:10.20344/amp.10966
- Brown ME, Osswald S, Biediger T. Cutaneous endometriosis of the umbilicus (Villar’s nodule). Int J Womens Dermatol. 2020;6:214-215. doi:10.1016/j.ijwd.2020.01.001
- Bindra V, Sampurna S, Kade S, et al. Primary umbilical endometriosis - case series and review of clinical presentation, diagnosis and management. Int J Surg Case Rep. 2022;94:107134. doi:10.1016/j.ijscr.2022.107134
- Loh SH, Lew BL, Sim WY. Primary cutaneous endometriosis of umbilicus. Ann Dermatol. 2017;29:621-625. doi:10.5021/ad.2017.29.5.621
- Victory R, Diamond MP, Johns DA. Villar’s nodule: a case report and systematic literature review of endometriosis externa of the umbilicus. J Minim Invasive Gynecol. 2007;14:23-32. doi:10.1016/j.jmig.2006.07.01
- Van den Nouland D, Kaur M. Primary umbilical endometriosis: a case report. Facts Views Vis Obgyn. 2017;9:115-119.
- Machairiotis N, Stylianaki A, Dryllis G, et al. Extrapelvic endometriosis: a rare entity or an under diagnosed condition? Diagn Pathol. 2013;8:194. doi:10.1186/1746-1596-8-194
- Huang QF, Jiang B, Yang X, et al. Primary versus secondary cutaneous endometriosis: literature review and case study. Heliyon. 2023;9:E20094. doi:10.1016/j.heliyon.2023.e20094
- Gonzalez RH, Singh MS, Hamza SA. Cutaneous endometriosis: a case report and review of the literature. Am J Case Rep. 2021;22:E932493. doi:10.12659/AJCR.932493
- Buljan M, Arzberger E, Šitum M, et al. The use of dermoscopy in differentiating Sister Mary Joseph nodule and cutaneous endometriosis. Australas J Dermatol. 2019;60:E233-E235. doi:10.1111/ajd.12980
- Costa IM, Gomes CM, Morais OO, et al. Cutaneous endometriosis: dermoscopic findings related to phases of the female hormonal cycle. Int J Dermatol. 2014;53:E130-E132. doi:10.1111 /j.1365-4632.2012.05854.x
- Mohaghegh F, Hatami P, Rajabi P, et al. Coexistence of cutaneous endometriosis and ovarian endometrioma: a case report. J Med Case Rep. 2022;16:256. doi:10.1186/s13256-022-03483-8
- Raffi L, Suresh R, McCalmont TH, et al. Cutaneous endometriosis. Int J Womens Dermatol. 2019;5:384-386. doi:10.1016 /j.ijwd.2019.06.025
- Saunders PTK, Horne AW. Endometriosis: etiology, pathobiology, and therapeutic prospects. Cell. 2021;184:2807-2824. doi:10.1016 /j.cell.2021.04.041
- Habif TP. Clinical Dermatology a Color Guide to Diagnosis and Therapy. St. Louis, Mo. Elsevier; 2016.
- Patrice SJ, Wiss K, Mulliken JB. Pyogenic granuloma (lobular capillary hemangioma): a clinicopathologic study of 178 cases. Pediatr Dermatol. 1991;8:267-276. doi:10.1111/j.15251470.1991.tb00931.x
- Kaleeny JD, Janis JE. Pyogenic granuloma diagnosis and management: a practical review. Plast Reconstr Surg Glob Open. 2024;12:E6160. doi:10.1097/GOX.0000000000006160
- Ha DL, Yang MY, Shin JO, et al. Benign umbilical tumors resembling Sister Mary Joseph nodule. Clin Med Insights Oncol. 2021;15:1179554921995022. doi:10.1177/1179554921995022
- Lawrence PF, Smeds M, Jessica Beth O’connell. Essentials of General Surgery and Surgical Specialties. Wolters Kluwer Health; 2019.
- Hao X, Billings SD, Wu F, et al. Dermatofibrosarcoma protuberans: update on the diagnosis and treatment. J Clin Med. 2020;9:1752. doi:10.3390/jcm9061752
- Allen A, Ahn C, Sangüeza OP. Dermatofibrosarcoma protuberans. Dermatol Clin. 2019;37:483-488. doi:10.1016/j.det.2019.05.006
THE DIAGNOSIS: Villar Nodule
The biopsy revealed features consistent with cutaneous endometriosis in the setting of a painful, tender, multilobulated nodule with a cyclical bleeding pattern (Figure 1). The bleeding pattern of the nodule during menses and lack of surgical history supported the diagnosis of primary cutaneous endometriosis in our patient. She was diagnosed with endometriosis by gynecology, and her primary care physician started her on an oral contraceptive based on this diagnosis. She also was referred to gynecology and plastic surgery for a joint surgical consultation to remove the nodule. She initially decided to do a trial of the oral contraceptive but subsequently underwent umbilical endometrioma excision with neo-umbilicus creation with no evidence of recurrence.

Primary cutaneous endometriosis should be considered in young females who present with tender umbilical nodules. Endometriosis refers to the presence of an endometriumlike epithelium outside the endometrium and myometrium.1 The condition affects 10% to 15% of reproductive-aged (ie, 18-49 years) women in the United States and typically involves tissues within the pelvis, such as the ovaries, pouch of Douglas, or pelvic ligaments.2 Cutaneous endometriosis is the growth of endometrial tissue in the skin and is rare, accounting for less than 5.5% of cases of extrapelvic endometriosis worldwide, affecting primarily the umbilicus, abdominal wall, and vulva.3,4
The 2 main types of cutaneous endometriosis are primary (spontaneous) and secondary. Primary lesions develop in patients without prior surgical history, and secondary lesions occur within previous surgical incision sites, often scars from cesarean delivery.5 Less than 30% of cases of cutaneous endometriosis are primary disease.6 Primary cutaneous endometriosis of the umbilicus, known as Villar nodule, was first described in 1886.3,7 Up to 40% of patients with extrapelvic endometriosis worldwide presented with Villar nodules in a systematic literature review.6 The prevalence of these nodules is unknown, but the incidence is less than 1% of cases of extragenital endometriosis.4
There are 2 leading theories of primary cutaneous endometriosis pathogenesis. The first is the transportation theory, in which endometrial cells are transported outside the uterus via the lymphatic system.8 The second is the metaplasia theory, which proposed that endometrial cells develop in the coelomic mesothelium in the presence of high estrogen levels.8,9
Secondary cutaneous endometriosis, also known as scar endometriosis, is suspected to be caused by an iatrogenic implantation of endometrial cells at the scar of a prior surgical site.9 Although our patient had an existing umbilicus scar from a piercing, it was improbable for that to have been the nidus, as the keloid scar was superficial and did not have contact with the abdominal cavity for iatrogenic implantation. Clinical diagnosis for secondary cutaneous endometriosis often is made based on a triad of features: a nonmalignant abdominal mass, recurring pain and bleeding of the lesion with menses, and prior history of abdominal surgery.9,10 On clinical examination, these features typically manifest as a palpable subcutaneous mass that is black, blue, brown, or red. Often, the lesions enlarge and bleed during the menstrual cycle, causing pain, tenderness, or pruritus.3 Dermoscopic features of secondary cutaneous endometriosis are erythematous umbilical nodules with a homogeneous vascular pattern that appears red with a brownish hue (Figure 2).9,11 Dermoscopic features may vary with the hormone cycle; for example, the follicular phase (correlating with day 7 of menses) demonstrates polypoid projections, erythematous violaceous color, dark-brown spots, and active bleeding of the lesion.12 Clinical and dermoscopic examination are useful tools in this diagnosis.

Imaging such as ultrasonography, computed tomography, or magnetic resonance imaging may be useful in identifying abdominal endometriomas.8,13,14 Pelvic involvement of endometriosis was found in approximately 15% of patients in a case series,4 with concurrent primary umbilical endometriosis. Imaging studies may assist evaluation for fistula formation, presence of malignancies, and the extent of endometriosis within the abdominal cavity.
Histopathology is key to confirming cutaneous endometriosis and shows multiple bland-appearing glands of varying sizes with loose, concentric, edematous, or fibromyxoid stroma (Figure 1).3 Red blood cells sometimes are found with hemosiderin within the stroma. Immunohistochemical staining with estrogen receptors may aid in identifying the endometriumlike epithelial cells.13
Standard treatment involves surgical excision with 1-cm margins and umbilical preservation, which results in a recurrence rate of less than 10%.4,10 Medical therapy, such as aromatase inhibitors, progestogens, antiprogestogens, combined oral contraceptives, or gonadotropin-releasing hormone agonists or antagonists may help manage pain or reduce the size of the nodule.4,15 Simple observation also is a potential course for patients who decline treatment options.
Differential diagnoses include lobular capillary hemangioma, also known as pyogenic granuloma; Sister Mary Joseph nodule; umbilical hernia; and dermatofibrosarcoma protuberans. Lobular capillary hemangiomas commonly are acquired benign vascular proliferations of the skin that are friable and tend to ulcerate.16 These lesions typically grow rapidly and often are located on the face, lips, mucosae, and fingers. Histopathologic examination may show an exophytic lesion with lobules of proliferating capillaries within an edematous matrix, superficial ulceration, and an epithelial collarette.17 Treatment includes surgical excision, cauterization, laser treatments, sclerotherapy, injectable medications, and topical medications, but recurrence is possible with any of these interventions.18
Cutaneous metastasis of an internal solid organ cancer, commonly known as a Sister Mary Joseph nodule, typically manifests as an erythematous, irregularly shaped nodule that may protrude from the umbilicus.14 Gastrointestinal symptoms such as change in bowel habits or obstructive symptoms in the setting of a progressive malignancy are common.14 Clinical features include a firm fixed lesion, oozing, and ulceration.19 On dermoscopy, polymorphous vascular patterns, milky red structureless areas, and white lines typically are present.11 Although dermoscopic features may differentiate this entity from cutaneous endometriosis, tissue sampling and histologic examination are crucial diagnostic tools to identify malignant vs benign lesions.
An umbilical hernia is a protrusion of omentum, bowel, or other intra-abdominal organs in an abdominal wall defect. Clinical presentation includes a soft protrusion that may be reduced on palpation if nonstrangulated.20 Treatment includes watchful waiting or surgical repair. The reducibility and presence of an abdominal wall defect may point to this diagnosis. Imaging also may aid in the diagnosis if the history and physical examination are unclear.
Dermatofibrosarcoma protuberans is a slow-developing, low- to intermediate-grade, soft-tissue sarcoma that occurs in less than 0.1% of all cancers in the United States.21 Lesions often manifest as small, firm, slow-growing, painless, flesh-colored dermal plaques; subcutaneous thickening; or atrophic nonprotuberant lesions typically involving the trunk.21 Histopathologically, they are composed of uniform spindle-cell proliferation growing in a storiform pattern and subcutaneous fat trapping that has strong and diffuse CD34 immunoreactivity.21,22 Pathologic examination typically distinguishes this diagnosis from cutaneous endometriosis. Treatment includes tumor resection that may or may not involve radiotherapy and targeted therapy, as recurrence and metastases are possible.
Primary cutaneous endometriosis is a rare but important diagnosis for dermatologists to consider when evaluating umbilical nodules. Clinical features may include bleeding masses during menses in females of reproductive age. Dermoscopic examination aids in workup, and histopathologic testing can confirm the diagnosis and rule out malignancies. Surgical excision is the treatment of choice with a low rate of recurrence.
THE DIAGNOSIS: Villar Nodule
The biopsy revealed features consistent with cutaneous endometriosis in the setting of a painful, tender, multilobulated nodule with a cyclical bleeding pattern (Figure 1). The bleeding pattern of the nodule during menses and lack of surgical history supported the diagnosis of primary cutaneous endometriosis in our patient. She was diagnosed with endometriosis by gynecology, and her primary care physician started her on an oral contraceptive based on this diagnosis. She also was referred to gynecology and plastic surgery for a joint surgical consultation to remove the nodule. She initially decided to do a trial of the oral contraceptive but subsequently underwent umbilical endometrioma excision with neo-umbilicus creation with no evidence of recurrence.

Primary cutaneous endometriosis should be considered in young females who present with tender umbilical nodules. Endometriosis refers to the presence of an endometriumlike epithelium outside the endometrium and myometrium.1 The condition affects 10% to 15% of reproductive-aged (ie, 18-49 years) women in the United States and typically involves tissues within the pelvis, such as the ovaries, pouch of Douglas, or pelvic ligaments.2 Cutaneous endometriosis is the growth of endometrial tissue in the skin and is rare, accounting for less than 5.5% of cases of extrapelvic endometriosis worldwide, affecting primarily the umbilicus, abdominal wall, and vulva.3,4
The 2 main types of cutaneous endometriosis are primary (spontaneous) and secondary. Primary lesions develop in patients without prior surgical history, and secondary lesions occur within previous surgical incision sites, often scars from cesarean delivery.5 Less than 30% of cases of cutaneous endometriosis are primary disease.6 Primary cutaneous endometriosis of the umbilicus, known as Villar nodule, was first described in 1886.3,7 Up to 40% of patients with extrapelvic endometriosis worldwide presented with Villar nodules in a systematic literature review.6 The prevalence of these nodules is unknown, but the incidence is less than 1% of cases of extragenital endometriosis.4
There are 2 leading theories of primary cutaneous endometriosis pathogenesis. The first is the transportation theory, in which endometrial cells are transported outside the uterus via the lymphatic system.8 The second is the metaplasia theory, which proposed that endometrial cells develop in the coelomic mesothelium in the presence of high estrogen levels.8,9
Secondary cutaneous endometriosis, also known as scar endometriosis, is suspected to be caused by an iatrogenic implantation of endometrial cells at the scar of a prior surgical site.9 Although our patient had an existing umbilicus scar from a piercing, it was improbable for that to have been the nidus, as the keloid scar was superficial and did not have contact with the abdominal cavity for iatrogenic implantation. Clinical diagnosis for secondary cutaneous endometriosis often is made based on a triad of features: a nonmalignant abdominal mass, recurring pain and bleeding of the lesion with menses, and prior history of abdominal surgery.9,10 On clinical examination, these features typically manifest as a palpable subcutaneous mass that is black, blue, brown, or red. Often, the lesions enlarge and bleed during the menstrual cycle, causing pain, tenderness, or pruritus.3 Dermoscopic features of secondary cutaneous endometriosis are erythematous umbilical nodules with a homogeneous vascular pattern that appears red with a brownish hue (Figure 2).9,11 Dermoscopic features may vary with the hormone cycle; for example, the follicular phase (correlating with day 7 of menses) demonstrates polypoid projections, erythematous violaceous color, dark-brown spots, and active bleeding of the lesion.12 Clinical and dermoscopic examination are useful tools in this diagnosis.

Imaging such as ultrasonography, computed tomography, or magnetic resonance imaging may be useful in identifying abdominal endometriomas.8,13,14 Pelvic involvement of endometriosis was found in approximately 15% of patients in a case series,4 with concurrent primary umbilical endometriosis. Imaging studies may assist evaluation for fistula formation, presence of malignancies, and the extent of endometriosis within the abdominal cavity.
Histopathology is key to confirming cutaneous endometriosis and shows multiple bland-appearing glands of varying sizes with loose, concentric, edematous, or fibromyxoid stroma (Figure 1).3 Red blood cells sometimes are found with hemosiderin within the stroma. Immunohistochemical staining with estrogen receptors may aid in identifying the endometriumlike epithelial cells.13
Standard treatment involves surgical excision with 1-cm margins and umbilical preservation, which results in a recurrence rate of less than 10%.4,10 Medical therapy, such as aromatase inhibitors, progestogens, antiprogestogens, combined oral contraceptives, or gonadotropin-releasing hormone agonists or antagonists may help manage pain or reduce the size of the nodule.4,15 Simple observation also is a potential course for patients who decline treatment options.
Differential diagnoses include lobular capillary hemangioma, also known as pyogenic granuloma; Sister Mary Joseph nodule; umbilical hernia; and dermatofibrosarcoma protuberans. Lobular capillary hemangiomas commonly are acquired benign vascular proliferations of the skin that are friable and tend to ulcerate.16 These lesions typically grow rapidly and often are located on the face, lips, mucosae, and fingers. Histopathologic examination may show an exophytic lesion with lobules of proliferating capillaries within an edematous matrix, superficial ulceration, and an epithelial collarette.17 Treatment includes surgical excision, cauterization, laser treatments, sclerotherapy, injectable medications, and topical medications, but recurrence is possible with any of these interventions.18
Cutaneous metastasis of an internal solid organ cancer, commonly known as a Sister Mary Joseph nodule, typically manifests as an erythematous, irregularly shaped nodule that may protrude from the umbilicus.14 Gastrointestinal symptoms such as change in bowel habits or obstructive symptoms in the setting of a progressive malignancy are common.14 Clinical features include a firm fixed lesion, oozing, and ulceration.19 On dermoscopy, polymorphous vascular patterns, milky red structureless areas, and white lines typically are present.11 Although dermoscopic features may differentiate this entity from cutaneous endometriosis, tissue sampling and histologic examination are crucial diagnostic tools to identify malignant vs benign lesions.
An umbilical hernia is a protrusion of omentum, bowel, or other intra-abdominal organs in an abdominal wall defect. Clinical presentation includes a soft protrusion that may be reduced on palpation if nonstrangulated.20 Treatment includes watchful waiting or surgical repair. The reducibility and presence of an abdominal wall defect may point to this diagnosis. Imaging also may aid in the diagnosis if the history and physical examination are unclear.
Dermatofibrosarcoma protuberans is a slow-developing, low- to intermediate-grade, soft-tissue sarcoma that occurs in less than 0.1% of all cancers in the United States.21 Lesions often manifest as small, firm, slow-growing, painless, flesh-colored dermal plaques; subcutaneous thickening; or atrophic nonprotuberant lesions typically involving the trunk.21 Histopathologically, they are composed of uniform spindle-cell proliferation growing in a storiform pattern and subcutaneous fat trapping that has strong and diffuse CD34 immunoreactivity.21,22 Pathologic examination typically distinguishes this diagnosis from cutaneous endometriosis. Treatment includes tumor resection that may or may not involve radiotherapy and targeted therapy, as recurrence and metastases are possible.
Primary cutaneous endometriosis is a rare but important diagnosis for dermatologists to consider when evaluating umbilical nodules. Clinical features may include bleeding masses during menses in females of reproductive age. Dermoscopic examination aids in workup, and histopathologic testing can confirm the diagnosis and rule out malignancies. Surgical excision is the treatment of choice with a low rate of recurrence.
- International Working Group of AAGL, ESGE, ESHRE and WES; Tomassetti C, Johnson NP, et al. An international terminology for endometriosis, 2021. Hum Reprod Open. 2021;2021:hoab029. doi:10.1093/hropen/hoab029
- Batista M, Alves F, Cardoso J, et al. Cutaneous endometriosis: a differential diagnosis of umbilical nodule. Acta Med Port. 2020; 33:282-284. doi:10.20344/amp.10966
- Brown ME, Osswald S, Biediger T. Cutaneous endometriosis of the umbilicus (Villar’s nodule). Int J Womens Dermatol. 2020;6:214-215. doi:10.1016/j.ijwd.2020.01.001
- Bindra V, Sampurna S, Kade S, et al. Primary umbilical endometriosis - case series and review of clinical presentation, diagnosis and management. Int J Surg Case Rep. 2022;94:107134. doi:10.1016/j.ijscr.2022.107134
- Loh SH, Lew BL, Sim WY. Primary cutaneous endometriosis of umbilicus. Ann Dermatol. 2017;29:621-625. doi:10.5021/ad.2017.29.5.621
- Victory R, Diamond MP, Johns DA. Villar’s nodule: a case report and systematic literature review of endometriosis externa of the umbilicus. J Minim Invasive Gynecol. 2007;14:23-32. doi:10.1016/j.jmig.2006.07.01
- Van den Nouland D, Kaur M. Primary umbilical endometriosis: a case report. Facts Views Vis Obgyn. 2017;9:115-119.
- Machairiotis N, Stylianaki A, Dryllis G, et al. Extrapelvic endometriosis: a rare entity or an under diagnosed condition? Diagn Pathol. 2013;8:194. doi:10.1186/1746-1596-8-194
- Huang QF, Jiang B, Yang X, et al. Primary versus secondary cutaneous endometriosis: literature review and case study. Heliyon. 2023;9:E20094. doi:10.1016/j.heliyon.2023.e20094
- Gonzalez RH, Singh MS, Hamza SA. Cutaneous endometriosis: a case report and review of the literature. Am J Case Rep. 2021;22:E932493. doi:10.12659/AJCR.932493
- Buljan M, Arzberger E, Šitum M, et al. The use of dermoscopy in differentiating Sister Mary Joseph nodule and cutaneous endometriosis. Australas J Dermatol. 2019;60:E233-E235. doi:10.1111/ajd.12980
- Costa IM, Gomes CM, Morais OO, et al. Cutaneous endometriosis: dermoscopic findings related to phases of the female hormonal cycle. Int J Dermatol. 2014;53:E130-E132. doi:10.1111 /j.1365-4632.2012.05854.x
- Mohaghegh F, Hatami P, Rajabi P, et al. Coexistence of cutaneous endometriosis and ovarian endometrioma: a case report. J Med Case Rep. 2022;16:256. doi:10.1186/s13256-022-03483-8
- Raffi L, Suresh R, McCalmont TH, et al. Cutaneous endometriosis. Int J Womens Dermatol. 2019;5:384-386. doi:10.1016 /j.ijwd.2019.06.025
- Saunders PTK, Horne AW. Endometriosis: etiology, pathobiology, and therapeutic prospects. Cell. 2021;184:2807-2824. doi:10.1016 /j.cell.2021.04.041
- Habif TP. Clinical Dermatology a Color Guide to Diagnosis and Therapy. St. Louis, Mo. Elsevier; 2016.
- Patrice SJ, Wiss K, Mulliken JB. Pyogenic granuloma (lobular capillary hemangioma): a clinicopathologic study of 178 cases. Pediatr Dermatol. 1991;8:267-276. doi:10.1111/j.15251470.1991.tb00931.x
- Kaleeny JD, Janis JE. Pyogenic granuloma diagnosis and management: a practical review. Plast Reconstr Surg Glob Open. 2024;12:E6160. doi:10.1097/GOX.0000000000006160
- Ha DL, Yang MY, Shin JO, et al. Benign umbilical tumors resembling Sister Mary Joseph nodule. Clin Med Insights Oncol. 2021;15:1179554921995022. doi:10.1177/1179554921995022
- Lawrence PF, Smeds M, Jessica Beth O’connell. Essentials of General Surgery and Surgical Specialties. Wolters Kluwer Health; 2019.
- Hao X, Billings SD, Wu F, et al. Dermatofibrosarcoma protuberans: update on the diagnosis and treatment. J Clin Med. 2020;9:1752. doi:10.3390/jcm9061752
- Allen A, Ahn C, Sangüeza OP. Dermatofibrosarcoma protuberans. Dermatol Clin. 2019;37:483-488. doi:10.1016/j.det.2019.05.006
- International Working Group of AAGL, ESGE, ESHRE and WES; Tomassetti C, Johnson NP, et al. An international terminology for endometriosis, 2021. Hum Reprod Open. 2021;2021:hoab029. doi:10.1093/hropen/hoab029
- Batista M, Alves F, Cardoso J, et al. Cutaneous endometriosis: a differential diagnosis of umbilical nodule. Acta Med Port. 2020; 33:282-284. doi:10.20344/amp.10966
- Brown ME, Osswald S, Biediger T. Cutaneous endometriosis of the umbilicus (Villar’s nodule). Int J Womens Dermatol. 2020;6:214-215. doi:10.1016/j.ijwd.2020.01.001
- Bindra V, Sampurna S, Kade S, et al. Primary umbilical endometriosis - case series and review of clinical presentation, diagnosis and management. Int J Surg Case Rep. 2022;94:107134. doi:10.1016/j.ijscr.2022.107134
- Loh SH, Lew BL, Sim WY. Primary cutaneous endometriosis of umbilicus. Ann Dermatol. 2017;29:621-625. doi:10.5021/ad.2017.29.5.621
- Victory R, Diamond MP, Johns DA. Villar’s nodule: a case report and systematic literature review of endometriosis externa of the umbilicus. J Minim Invasive Gynecol. 2007;14:23-32. doi:10.1016/j.jmig.2006.07.01
- Van den Nouland D, Kaur M. Primary umbilical endometriosis: a case report. Facts Views Vis Obgyn. 2017;9:115-119.
- Machairiotis N, Stylianaki A, Dryllis G, et al. Extrapelvic endometriosis: a rare entity or an under diagnosed condition? Diagn Pathol. 2013;8:194. doi:10.1186/1746-1596-8-194
- Huang QF, Jiang B, Yang X, et al. Primary versus secondary cutaneous endometriosis: literature review and case study. Heliyon. 2023;9:E20094. doi:10.1016/j.heliyon.2023.e20094
- Gonzalez RH, Singh MS, Hamza SA. Cutaneous endometriosis: a case report and review of the literature. Am J Case Rep. 2021;22:E932493. doi:10.12659/AJCR.932493
- Buljan M, Arzberger E, Šitum M, et al. The use of dermoscopy in differentiating Sister Mary Joseph nodule and cutaneous endometriosis. Australas J Dermatol. 2019;60:E233-E235. doi:10.1111/ajd.12980
- Costa IM, Gomes CM, Morais OO, et al. Cutaneous endometriosis: dermoscopic findings related to phases of the female hormonal cycle. Int J Dermatol. 2014;53:E130-E132. doi:10.1111 /j.1365-4632.2012.05854.x
- Mohaghegh F, Hatami P, Rajabi P, et al. Coexistence of cutaneous endometriosis and ovarian endometrioma: a case report. J Med Case Rep. 2022;16:256. doi:10.1186/s13256-022-03483-8
- Raffi L, Suresh R, McCalmont TH, et al. Cutaneous endometriosis. Int J Womens Dermatol. 2019;5:384-386. doi:10.1016 /j.ijwd.2019.06.025
- Saunders PTK, Horne AW. Endometriosis: etiology, pathobiology, and therapeutic prospects. Cell. 2021;184:2807-2824. doi:10.1016 /j.cell.2021.04.041
- Habif TP. Clinical Dermatology a Color Guide to Diagnosis and Therapy. St. Louis, Mo. Elsevier; 2016.
- Patrice SJ, Wiss K, Mulliken JB. Pyogenic granuloma (lobular capillary hemangioma): a clinicopathologic study of 178 cases. Pediatr Dermatol. 1991;8:267-276. doi:10.1111/j.15251470.1991.tb00931.x
- Kaleeny JD, Janis JE. Pyogenic granuloma diagnosis and management: a practical review. Plast Reconstr Surg Glob Open. 2024;12:E6160. doi:10.1097/GOX.0000000000006160
- Ha DL, Yang MY, Shin JO, et al. Benign umbilical tumors resembling Sister Mary Joseph nodule. Clin Med Insights Oncol. 2021;15:1179554921995022. doi:10.1177/1179554921995022
- Lawrence PF, Smeds M, Jessica Beth O’connell. Essentials of General Surgery and Surgical Specialties. Wolters Kluwer Health; 2019.
- Hao X, Billings SD, Wu F, et al. Dermatofibrosarcoma protuberans: update on the diagnosis and treatment. J Clin Med. 2020;9:1752. doi:10.3390/jcm9061752
- Allen A, Ahn C, Sangüeza OP. Dermatofibrosarcoma protuberans. Dermatol Clin. 2019;37:483-488. doi:10.1016/j.det.2019.05.006
Tender Nodule on the Umbilicus
Tender Nodule on the Umbilicus
A 25-year-old woman was referred to the dermatology clinic by her primary care provider for evaluation of a tender nodule on the inferior umbilicus of 2 years' duration at the site of a preexisting keloid scar. The patient reported that the lesion caused occasional pain and tenderness. A few weeks prior to the current presentation, a dark-red bloody discharge developed at the superior aspect of the lesion that subsequently crusted over. The patient denied any use of oral contraceptives or history of abdominal surgery.
The original keloid scar had been treated successfully by an outside physician with intralesional steroid injections, and the patient was interested in a similar procedure for the current nodule. She also had a history of a hyperpigmented hypertrophic scar on the superior periumbilical area from a previous piercing that had resolved several years prior to presentation.
Physical examination of the lesion revealed a 1.2-cm, soft, tender, violaceous nodule with scant yellow crust along the superior surface of the umbilicus. There was no palpable abdominal wall defect, and the nodule was not reducible into the abdominal cavity. An interval history revealed bleeding of the lesion during the patient's menstrual cycle with persistent pain and tenderness. A punch biopsy was performed.
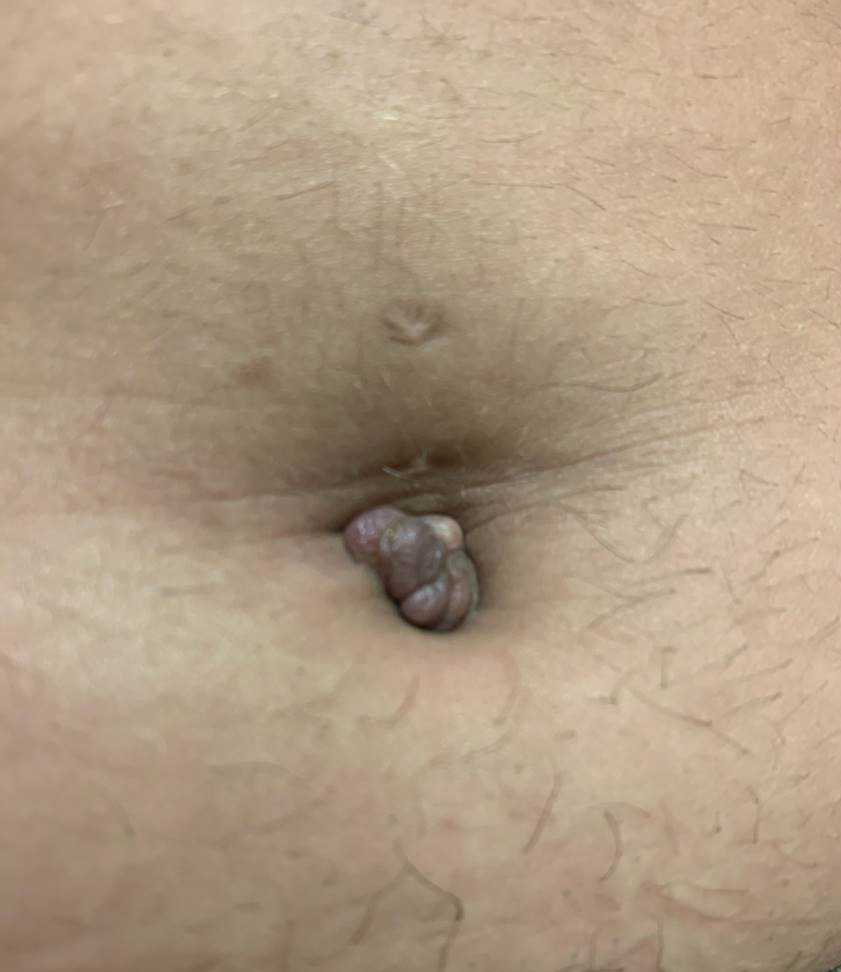
Diffuse Pruritic Keratotic Papules
Diffuse Pruritic Keratotic Papules
THE DIAGNOSIS: Reactive Perforating Collagenosis
Histopathology revealed invagination of the epidermis with hyperkeratosis; prominent epidermal hyperplasia; and a central basophilic plug of keratin, collagen, and inflammatory debris. Transepidermal elimination of bright eosinophilic altered collagen fibers was seen (Figure). The findings were consistent with a diagnosis of reactive perforating collagenosis (RPC).
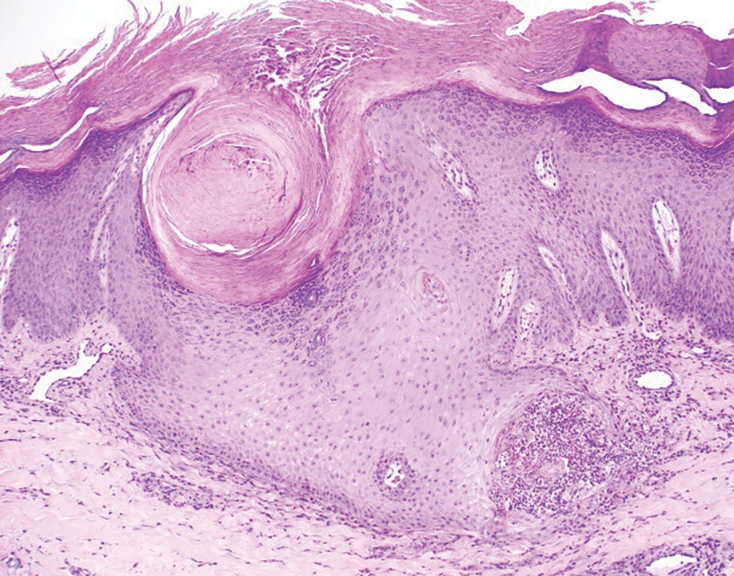
Reactive perforating collagenosis, a subtype of perforating dermatosis, is a rare skin condition in which altered collagen is eliminated through the epidermis.1 There are 2 forms of RPC: the inherited form, which is very rare and manifests in childhood, and the acquired form, which manifests in adulthood and is associated with systemic diseases, most notably diabetes and/or chronic renal failure, both of which our patient had been diagnosed with.1,2 The clinical presentation of RPC includes erythematous papules or nodules that evolve into umbilicated 4- to 10-mm craterlike ulcerations with a central keratotic plug. The lesions favor a linear distribution along the extensor surfaces of the arms and legs, trunk, and gluteal area. Involvement of the head, neck, and scalp has been reported less commonly, which makes our case particularly unique.3 Histopathologically, RPC is characterized by a cup-shaped depression of the epidermis with an overlying keratin plug containing inflammatory cells, keratinous debris, and collagen fibers. Vertically oriented collagen fibers are seen extruded through the epidermis.4,5
While the pathogenesis of RPC remains unknown, it is believed that superficial trauma due to chronic scratching results in transepithelial elimination of collagen. Due to the association of acquired RPC (ARPC) with diabetes, it also has been proposed that scratching can cause microtrauma and necrosis of the dermal structures, potentially due to diabetic microangiopathy.3 Additionally, RPC is associated with overexpression of transforming growth factor beta 3 in lesional skin, suggesting that transforming growth factor beta 3 is involved with tissue repair and extracellular remodeling in this condition.6
Treatment of ARPC should include the management of underlying disease. While no definitive treatment has been reported to date, topical corticosteroids, retinoids, keratolytics, emollients, antihistamines, narrow-band UVB phototherapy, and psoralen plus UVA phototherapy have been used with varying degrees of improvement. Typically, the lesions self-resolve within 6 to 8 weeks; however, they often recur and usually leave scarring with or without hyperpigmentation.2,7-10
Acquired RPC can be misdiagnosed initially, as it mimics several other conditions and commonly is associated with systemic diseases. While biopsy is necessary for diagnosis, if it cannot be performed or the results are indeterminate, dermoscopy can serve as a helpful diagnostic tool. The most common dermoscopic patterns seen in RPC include a yellow-brown structureless area in the center of the lesion with a peripheral surface crust and surrounding white rim—thought to represent epidermal invagination or keratinous debris. Additionally, inflammation with visible vessels both centrally and peripherally is represented by an outer pink circle on dermoscopy.5,11
The differential diagnoses for RPC include perforating folliculitis (PF), elastosis perforans serpiginosa (EPS), prurigo nodularis, and keratoacanthomas. The primary perforating dermatoses (PF, EPS, and RPC) are similarly characterized by elimination of altered dermal material through the epidermis. As these conditions manifest with similar features on clinical examination, differentiation is made by the type of epidermal damage and the features of elimination material, making histopathologic examination paramount for definitive diagnosis.
Perforating folliculitis manifests as erythematous, follicular papules with a small central keratotic core or a central hair. Histopathologically, PF reveals a widely dilated follicle containing keratin, necrotic debris, and degenerated inflammatory cells. Elastosis perforans serpiginosa manifests clinically as hyperkeratotic papules in serpiginous patterns rather than the linear pattern commonly seen with ARPC. Histopathologically, EPS reveals thickened elastic fibers, rather than collagen fibers as seen in ARPC, extruded through the epidermis. Prurigo nodularis manifests clinically as dome-shaped papules with possible excoriation and crusting. Histopathologic examination reveals epidermal hyperplasia and hyperkeratosis; however, the characteristic features of transepithelial elimination of collagen and invaginations of epidermis differentiate ARPC from prurigo nodularis.12,13 Keratoacanthomas manifest clinically as an eruption of small, round, pink papules that rapidly grow and evolve into 1- to 2-cm dome-shaped nodules with central keratinaceous plugs, mimicking a crateriform appearance. Histopathologic examination reveals a circumscribed proliferation of well-differentiated keratinocytes. Multilobular exophytic or endophytic cystlike invaginations of the epidermis also are noted. The expulsion of collagen from the epidermis is more consistent with ARPC.14
- Cohen RW, Auerbach R. Acquired reactive perforating collagenosis. J Am Acad Dermatol. 1989;20(2 pt 1):287-289. doi:10.1016/s0190 -9622(89)80059-3
- Bejjanki H, Siroy AE, Koratala A. Reactive perforating collagenosis in end-stage renal disease: not all that itches is uremic pruritis! Am J Med. 2019;132:E658-E660. doi:10.1016/j.amjmed.2019.03.015
- Gontijo JRV, Júnior FF, Pereira LB, et al. Trauma-induced acquired reactive perforating collagenosis. An Bras Dermatol. 2021;96:392-393. doi:10.1016/j.abd.2020.06.022
- Ambalathinkal JJ, Phiske MM, Someshwar SJ. Acquired reactive perforating collagenosis, a rare entity at uncommon site. Indian J Pathol Microbiol. 2022;65:895-897. doi:10.4103/ijpm.ijpm_333_21
- Ormerod E, Atwan A, Intzedy L, et al. Dermoscopy features of acquired reactive perforating collagenosis: a case series. Dermatol Pract Concept. 2018;8:303-305. doi:10.5826/dpc.0804a11
- Fei C, Wang Y, Gong Y, et al. Acquired reactive perforating collagenosis: a report of a typical case. Medicine (Baltimore). 2016;95:E4305. doi:10.1097/md.0000000000004305
- Bartling SJ, Naff JL, Canevari MM, et al. Pruritic rash in an elderly patient with uncontrolled diabetes mellitus. AACE Clin Case Rep. 2018;5:E146-E149. doi:10.4158/ACCR-2018-0388
- Kollipara H, Satya RS, Rao GR, et al. Acquired reactive perforating collagenosis: case series. Indian Dermatol Online J. 2023;14:72-76. doi:10.4103/idoj.idoj_373_22
- Wang C, Liu YH, Wang YX, et al. Acquired reactive perforating collagenosis. Chin Med J (Engl). 2020;133:2119-2120. doi:10.1097 /cm9.0000000000000906
- Harbaoui S, Litaiem N. Acquired perforating dermatosis. StatPearls [Internet]. Updated February 13, 2023. Accessed August 13, 2025. https://www.ncbi.nlm.nih.gov/books/NBK539715/
- Elmas ÖF, Kilitci A, Uyar B. Dermoscopic patterns of acquired reactive perforating collagenosis. Dermatol Pract Concept. 2021;11:E2020085. doi:10.5826/dpc.1101a85
- Patterson JW. The perforating disorders. J Am Acad Dermatol. 1984;10:561-581. doi:10.1016/s0190-9622(84)80259-5
- Huang AH, Williams KA, Kwatra SG. Prurigo nodularis: epidemiology and clinical features. J Am Acad Dermatol. 2020;83:1559-1565. doi:10.1016/j.jaad.2020.04.183
- Zito PM, Scharf R. Keratoacanthoma. StatPearls [Internet]. Updated August 8, 2023. Accessed August 13, 2025. https://www.ncbi.nlm.nih.gov/books/NBK499931/
THE DIAGNOSIS: Reactive Perforating Collagenosis
Histopathology revealed invagination of the epidermis with hyperkeratosis; prominent epidermal hyperplasia; and a central basophilic plug of keratin, collagen, and inflammatory debris. Transepidermal elimination of bright eosinophilic altered collagen fibers was seen (Figure). The findings were consistent with a diagnosis of reactive perforating collagenosis (RPC).

Reactive perforating collagenosis, a subtype of perforating dermatosis, is a rare skin condition in which altered collagen is eliminated through the epidermis.1 There are 2 forms of RPC: the inherited form, which is very rare and manifests in childhood, and the acquired form, which manifests in adulthood and is associated with systemic diseases, most notably diabetes and/or chronic renal failure, both of which our patient had been diagnosed with.1,2 The clinical presentation of RPC includes erythematous papules or nodules that evolve into umbilicated 4- to 10-mm craterlike ulcerations with a central keratotic plug. The lesions favor a linear distribution along the extensor surfaces of the arms and legs, trunk, and gluteal area. Involvement of the head, neck, and scalp has been reported less commonly, which makes our case particularly unique.3 Histopathologically, RPC is characterized by a cup-shaped depression of the epidermis with an overlying keratin plug containing inflammatory cells, keratinous debris, and collagen fibers. Vertically oriented collagen fibers are seen extruded through the epidermis.4,5
While the pathogenesis of RPC remains unknown, it is believed that superficial trauma due to chronic scratching results in transepithelial elimination of collagen. Due to the association of acquired RPC (ARPC) with diabetes, it also has been proposed that scratching can cause microtrauma and necrosis of the dermal structures, potentially due to diabetic microangiopathy.3 Additionally, RPC is associated with overexpression of transforming growth factor beta 3 in lesional skin, suggesting that transforming growth factor beta 3 is involved with tissue repair and extracellular remodeling in this condition.6
Treatment of ARPC should include the management of underlying disease. While no definitive treatment has been reported to date, topical corticosteroids, retinoids, keratolytics, emollients, antihistamines, narrow-band UVB phototherapy, and psoralen plus UVA phototherapy have been used with varying degrees of improvement. Typically, the lesions self-resolve within 6 to 8 weeks; however, they often recur and usually leave scarring with or without hyperpigmentation.2,7-10
Acquired RPC can be misdiagnosed initially, as it mimics several other conditions and commonly is associated with systemic diseases. While biopsy is necessary for diagnosis, if it cannot be performed or the results are indeterminate, dermoscopy can serve as a helpful diagnostic tool. The most common dermoscopic patterns seen in RPC include a yellow-brown structureless area in the center of the lesion with a peripheral surface crust and surrounding white rim—thought to represent epidermal invagination or keratinous debris. Additionally, inflammation with visible vessels both centrally and peripherally is represented by an outer pink circle on dermoscopy.5,11
The differential diagnoses for RPC include perforating folliculitis (PF), elastosis perforans serpiginosa (EPS), prurigo nodularis, and keratoacanthomas. The primary perforating dermatoses (PF, EPS, and RPC) are similarly characterized by elimination of altered dermal material through the epidermis. As these conditions manifest with similar features on clinical examination, differentiation is made by the type of epidermal damage and the features of elimination material, making histopathologic examination paramount for definitive diagnosis.
Perforating folliculitis manifests as erythematous, follicular papules with a small central keratotic core or a central hair. Histopathologically, PF reveals a widely dilated follicle containing keratin, necrotic debris, and degenerated inflammatory cells. Elastosis perforans serpiginosa manifests clinically as hyperkeratotic papules in serpiginous patterns rather than the linear pattern commonly seen with ARPC. Histopathologically, EPS reveals thickened elastic fibers, rather than collagen fibers as seen in ARPC, extruded through the epidermis. Prurigo nodularis manifests clinically as dome-shaped papules with possible excoriation and crusting. Histopathologic examination reveals epidermal hyperplasia and hyperkeratosis; however, the characteristic features of transepithelial elimination of collagen and invaginations of epidermis differentiate ARPC from prurigo nodularis.12,13 Keratoacanthomas manifest clinically as an eruption of small, round, pink papules that rapidly grow and evolve into 1- to 2-cm dome-shaped nodules with central keratinaceous plugs, mimicking a crateriform appearance. Histopathologic examination reveals a circumscribed proliferation of well-differentiated keratinocytes. Multilobular exophytic or endophytic cystlike invaginations of the epidermis also are noted. The expulsion of collagen from the epidermis is more consistent with ARPC.14
THE DIAGNOSIS: Reactive Perforating Collagenosis
Histopathology revealed invagination of the epidermis with hyperkeratosis; prominent epidermal hyperplasia; and a central basophilic plug of keratin, collagen, and inflammatory debris. Transepidermal elimination of bright eosinophilic altered collagen fibers was seen (Figure). The findings were consistent with a diagnosis of reactive perforating collagenosis (RPC).

Reactive perforating collagenosis, a subtype of perforating dermatosis, is a rare skin condition in which altered collagen is eliminated through the epidermis.1 There are 2 forms of RPC: the inherited form, which is very rare and manifests in childhood, and the acquired form, which manifests in adulthood and is associated with systemic diseases, most notably diabetes and/or chronic renal failure, both of which our patient had been diagnosed with.1,2 The clinical presentation of RPC includes erythematous papules or nodules that evolve into umbilicated 4- to 10-mm craterlike ulcerations with a central keratotic plug. The lesions favor a linear distribution along the extensor surfaces of the arms and legs, trunk, and gluteal area. Involvement of the head, neck, and scalp has been reported less commonly, which makes our case particularly unique.3 Histopathologically, RPC is characterized by a cup-shaped depression of the epidermis with an overlying keratin plug containing inflammatory cells, keratinous debris, and collagen fibers. Vertically oriented collagen fibers are seen extruded through the epidermis.4,5
While the pathogenesis of RPC remains unknown, it is believed that superficial trauma due to chronic scratching results in transepithelial elimination of collagen. Due to the association of acquired RPC (ARPC) with diabetes, it also has been proposed that scratching can cause microtrauma and necrosis of the dermal structures, potentially due to diabetic microangiopathy.3 Additionally, RPC is associated with overexpression of transforming growth factor beta 3 in lesional skin, suggesting that transforming growth factor beta 3 is involved with tissue repair and extracellular remodeling in this condition.6
Treatment of ARPC should include the management of underlying disease. While no definitive treatment has been reported to date, topical corticosteroids, retinoids, keratolytics, emollients, antihistamines, narrow-band UVB phototherapy, and psoralen plus UVA phototherapy have been used with varying degrees of improvement. Typically, the lesions self-resolve within 6 to 8 weeks; however, they often recur and usually leave scarring with or without hyperpigmentation.2,7-10
Acquired RPC can be misdiagnosed initially, as it mimics several other conditions and commonly is associated with systemic diseases. While biopsy is necessary for diagnosis, if it cannot be performed or the results are indeterminate, dermoscopy can serve as a helpful diagnostic tool. The most common dermoscopic patterns seen in RPC include a yellow-brown structureless area in the center of the lesion with a peripheral surface crust and surrounding white rim—thought to represent epidermal invagination or keratinous debris. Additionally, inflammation with visible vessels both centrally and peripherally is represented by an outer pink circle on dermoscopy.5,11
The differential diagnoses for RPC include perforating folliculitis (PF), elastosis perforans serpiginosa (EPS), prurigo nodularis, and keratoacanthomas. The primary perforating dermatoses (PF, EPS, and RPC) are similarly characterized by elimination of altered dermal material through the epidermis. As these conditions manifest with similar features on clinical examination, differentiation is made by the type of epidermal damage and the features of elimination material, making histopathologic examination paramount for definitive diagnosis.
Perforating folliculitis manifests as erythematous, follicular papules with a small central keratotic core or a central hair. Histopathologically, PF reveals a widely dilated follicle containing keratin, necrotic debris, and degenerated inflammatory cells. Elastosis perforans serpiginosa manifests clinically as hyperkeratotic papules in serpiginous patterns rather than the linear pattern commonly seen with ARPC. Histopathologically, EPS reveals thickened elastic fibers, rather than collagen fibers as seen in ARPC, extruded through the epidermis. Prurigo nodularis manifests clinically as dome-shaped papules with possible excoriation and crusting. Histopathologic examination reveals epidermal hyperplasia and hyperkeratosis; however, the characteristic features of transepithelial elimination of collagen and invaginations of epidermis differentiate ARPC from prurigo nodularis.12,13 Keratoacanthomas manifest clinically as an eruption of small, round, pink papules that rapidly grow and evolve into 1- to 2-cm dome-shaped nodules with central keratinaceous plugs, mimicking a crateriform appearance. Histopathologic examination reveals a circumscribed proliferation of well-differentiated keratinocytes. Multilobular exophytic or endophytic cystlike invaginations of the epidermis also are noted. The expulsion of collagen from the epidermis is more consistent with ARPC.14
- Cohen RW, Auerbach R. Acquired reactive perforating collagenosis. J Am Acad Dermatol. 1989;20(2 pt 1):287-289. doi:10.1016/s0190 -9622(89)80059-3
- Bejjanki H, Siroy AE, Koratala A. Reactive perforating collagenosis in end-stage renal disease: not all that itches is uremic pruritis! Am J Med. 2019;132:E658-E660. doi:10.1016/j.amjmed.2019.03.015
- Gontijo JRV, Júnior FF, Pereira LB, et al. Trauma-induced acquired reactive perforating collagenosis. An Bras Dermatol. 2021;96:392-393. doi:10.1016/j.abd.2020.06.022
- Ambalathinkal JJ, Phiske MM, Someshwar SJ. Acquired reactive perforating collagenosis, a rare entity at uncommon site. Indian J Pathol Microbiol. 2022;65:895-897. doi:10.4103/ijpm.ijpm_333_21
- Ormerod E, Atwan A, Intzedy L, et al. Dermoscopy features of acquired reactive perforating collagenosis: a case series. Dermatol Pract Concept. 2018;8:303-305. doi:10.5826/dpc.0804a11
- Fei C, Wang Y, Gong Y, et al. Acquired reactive perforating collagenosis: a report of a typical case. Medicine (Baltimore). 2016;95:E4305. doi:10.1097/md.0000000000004305
- Bartling SJ, Naff JL, Canevari MM, et al. Pruritic rash in an elderly patient with uncontrolled diabetes mellitus. AACE Clin Case Rep. 2018;5:E146-E149. doi:10.4158/ACCR-2018-0388
- Kollipara H, Satya RS, Rao GR, et al. Acquired reactive perforating collagenosis: case series. Indian Dermatol Online J. 2023;14:72-76. doi:10.4103/idoj.idoj_373_22
- Wang C, Liu YH, Wang YX, et al. Acquired reactive perforating collagenosis. Chin Med J (Engl). 2020;133:2119-2120. doi:10.1097 /cm9.0000000000000906
- Harbaoui S, Litaiem N. Acquired perforating dermatosis. StatPearls [Internet]. Updated February 13, 2023. Accessed August 13, 2025. https://www.ncbi.nlm.nih.gov/books/NBK539715/
- Elmas ÖF, Kilitci A, Uyar B. Dermoscopic patterns of acquired reactive perforating collagenosis. Dermatol Pract Concept. 2021;11:E2020085. doi:10.5826/dpc.1101a85
- Patterson JW. The perforating disorders. J Am Acad Dermatol. 1984;10:561-581. doi:10.1016/s0190-9622(84)80259-5
- Huang AH, Williams KA, Kwatra SG. Prurigo nodularis: epidemiology and clinical features. J Am Acad Dermatol. 2020;83:1559-1565. doi:10.1016/j.jaad.2020.04.183
- Zito PM, Scharf R. Keratoacanthoma. StatPearls [Internet]. Updated August 8, 2023. Accessed August 13, 2025. https://www.ncbi.nlm.nih.gov/books/NBK499931/
- Cohen RW, Auerbach R. Acquired reactive perforating collagenosis. J Am Acad Dermatol. 1989;20(2 pt 1):287-289. doi:10.1016/s0190 -9622(89)80059-3
- Bejjanki H, Siroy AE, Koratala A. Reactive perforating collagenosis in end-stage renal disease: not all that itches is uremic pruritis! Am J Med. 2019;132:E658-E660. doi:10.1016/j.amjmed.2019.03.015
- Gontijo JRV, Júnior FF, Pereira LB, et al. Trauma-induced acquired reactive perforating collagenosis. An Bras Dermatol. 2021;96:392-393. doi:10.1016/j.abd.2020.06.022
- Ambalathinkal JJ, Phiske MM, Someshwar SJ. Acquired reactive perforating collagenosis, a rare entity at uncommon site. Indian J Pathol Microbiol. 2022;65:895-897. doi:10.4103/ijpm.ijpm_333_21
- Ormerod E, Atwan A, Intzedy L, et al. Dermoscopy features of acquired reactive perforating collagenosis: a case series. Dermatol Pract Concept. 2018;8:303-305. doi:10.5826/dpc.0804a11
- Fei C, Wang Y, Gong Y, et al. Acquired reactive perforating collagenosis: a report of a typical case. Medicine (Baltimore). 2016;95:E4305. doi:10.1097/md.0000000000004305
- Bartling SJ, Naff JL, Canevari MM, et al. Pruritic rash in an elderly patient with uncontrolled diabetes mellitus. AACE Clin Case Rep. 2018;5:E146-E149. doi:10.4158/ACCR-2018-0388
- Kollipara H, Satya RS, Rao GR, et al. Acquired reactive perforating collagenosis: case series. Indian Dermatol Online J. 2023;14:72-76. doi:10.4103/idoj.idoj_373_22
- Wang C, Liu YH, Wang YX, et al. Acquired reactive perforating collagenosis. Chin Med J (Engl). 2020;133:2119-2120. doi:10.1097 /cm9.0000000000000906
- Harbaoui S, Litaiem N. Acquired perforating dermatosis. StatPearls [Internet]. Updated February 13, 2023. Accessed August 13, 2025. https://www.ncbi.nlm.nih.gov/books/NBK539715/
- Elmas ÖF, Kilitci A, Uyar B. Dermoscopic patterns of acquired reactive perforating collagenosis. Dermatol Pract Concept. 2021;11:E2020085. doi:10.5826/dpc.1101a85
- Patterson JW. The perforating disorders. J Am Acad Dermatol. 1984;10:561-581. doi:10.1016/s0190-9622(84)80259-5
- Huang AH, Williams KA, Kwatra SG. Prurigo nodularis: epidemiology and clinical features. J Am Acad Dermatol. 2020;83:1559-1565. doi:10.1016/j.jaad.2020.04.183
- Zito PM, Scharf R. Keratoacanthoma. StatPearls [Internet]. Updated August 8, 2023. Accessed August 13, 2025. https://www.ncbi.nlm.nih.gov/books/NBK499931/
Diffuse Pruritic Keratotic Papules
Diffuse Pruritic Keratotic Papules
A 65-year-old woman presented to dermatology with an intensely pruritic rash on the arms, legs, neck, and face of several months’ duration. The patient reported scratching the lesions but denied any recent trauma to the affected areas. She previously had been evaluated by her primary care provider, who prescribed cephalexin with no improvement. Her medical history was remarkable for chronic renal failure on dialysis, diabetes, hypertension, and congestive heart failure. Physical examination of the skin revealed hard white cutaneous nodules distributed on the proximal posterior upper arms, bilateral proximal pretibial regions, right elbow, and left knee. Two shave biopsies from the right elbow and left knee were obtained for histopathology.
Advantages and Disadvantages of Private vs Academic Dermatology Practices
Advantages and Disadvantages of Private vs Academic Dermatology Practices
Dermatology is a rapidly growing, highly competitive specialty with patients that can be served via private practice, academic medicine, hybrid settings, and rural health clinics. Medical residents’ choice of a career path has been rapidly evolving alongside shifts in health care policy, increasing demand for dermatologic services, stagnant fees falling behind inflation for more than a decade, and payment methods that no longer reflect the traditional fee-for-service model. This places a lot of pressure on young dermatologists to evaluate which practice structure best fits their career goals. A nuanced understanding of the strengths and limitations of each practice model is essential for dermatologists to make informed career decisions that are aligned with their values.
While there are many health care practice models, the first decision dermatology residents must make is whether they would prefer working in the private sector or an academic practice. Of course, it is not uncommon for academic dermatologists to embark on a midcareer segue into private practice and, less commonly, for private dermatologists to culminate their careers with a move to academics. The private sector includes private practice, private equity (PE)–owned group practices that often are single-specialty focused, and hospital-owned group practices that usually are multispecialty. Traditionally, private practices are health care businesses owned by one physician (solo practice) or a group of physicians (group practice) operated independently from hospitals, health systems, or private investors. Financially, these practices rely heavily on volume-based services, especially clinic visits and cosmetic procedures, which provide higher reimbursement rates and usually cash payments at the time of service.1 Roughly 35% of dermatologists in the United States work in private practice, and a dwindling 15% work in solo practice.2,3
Medical practices that are not self-owned by physicians vary widely, and they include hospital- or medical center–owned, private equity, and university-based academic practices. Private equity practices typically are characterized as profit driven. Hospital-owned practices shoulder business decisions and administrative duties for the physician at the cost of provider autonomy. Academic medicine is the most different from the other practice types. In contrast to private practice dermatologists, university-based dermatologists practice at academic medical centers (AMCs) with the core goals of patient care, education, and research. Compensation generally is based on the relative value unit (RVU), which is supplemented by government support and research grants.
As evidenced in this brief discussion, health care practice models are complex, and choosing the right model to align with professional goals can pose a major challenge for many physicians. The advantages and disadvantages of various practice models will be reviewed, highlighting trends and emerging models.
Solo or Small-Group Single-Specialty Private Practice
Private practice offers dermatologists the advantage of higher income potential but with greater economic risk; it often requires physicians to be more involved in the business aspects of dermatologic practice. In the early 1990s, a survey of private practice dermatologists revealed that income was the first or second most important factor that contributed to their career choice of private vs academic practice.4 Earning potential in private practice largely is driven by the autonomy afforded in this setting. Physicians have the liberty of choosing their practice location, structure, schedule, and staff in addition to tailoring services toward profitability; this typically leads to a higher volume of cosmetic and procedural visits, which may be attractive to providers wishing to focus on aesthetics. Private practice dermatologists also are not subject to institutional requirements that may include the preparation of grant submissions, research productivity targets, and devotion of time to teaching. Many private dermatologists find satisfaction in tailoring their work environments to align with personal values and goals and in cultivating long-term relationships with patients in a more personal and less bureaucratic context.
There also are drawbacks to private practice. The profitability often can be attributed to the higher patient load and more hours devoted to practice.5 A 2006 study found that academics saw 32% to 41% fewer patients per week than private practice dermatologists.6 Along with the opportunity for financial gain is the risk of financial ruin. Cost is the largest hurdle for establishing a practice, and most practices do not turn a profit for the first few years.1,5 The financial burden of running a practice includes pressure from the federal government to adopt expensive electronic health record systems to achieve maximum Medicare payment through the Merit-Based Incentive Payment System, liability insurance, health insurance, and staff salaries.7 These challenges require strong business acumen, including managing overhead costs, navigating insurance negotiations, marketing a practice, and maintaining compliance with evolving health care regulations. The purchase of a $100,000 laser could be a boon or bust, requiring the development of a business plan that ensures a positive return on investment. Additionally, private practice profitability has the potential to dwindle as governmental reimbursements fail to match inflation rates. Securing business advisors or even obtaining a Master of Business Administration degree can be helpful.
Insurance and government agencies also are infringing upon some of the autonomy of private practice dermatologists, as evidenced by a 2017 survey of dermatologists that found that more than half of respondents altered treatment plans based on insurance coverage more than 20% of the time.2 Private equity firms also could infringe on private practice autonomy, as providers are beholden to the firm’s restrictions—from which company’s product will be stocked to which partner will be on call. Lastly, private practice is less conducive to consistent referral patterns and strong relationships with specialists when compared to academic practice. Additionally, reliance on high patient throughput or cosmetic services for financial sustainability can shift focus away from complex medical dermatology, which often is referred to AMCs.
Academic Medicine
Academic dermatology offers a stimulating and collaborative environment with opportunities to advance the field through research and education. Often, the opportunity to teach medical students, residents, and peers is the deciding factor for academic dermatologists, as supported by a 2016 survey that found teaching opportunities are a major influence on career decision.8 The mixture of patient care, education, and research roles can be satisfying when compared to the grind of seeing large numbers of patients every day. Because they typically are salaried with an RVU-based income, academic dermatologists often are less concerned with the costs associated with medical treatment, and they typically treat more medically complex patients and underserved populations.9 The salary structure of academic roles also provides the benefit of a stable and predictable income. Physicians in this setting often are considered experts in their field, positioning them to have a strong built-in referral system along with frequent participation in multidisciplinary care alongside colleagues in rheumatology, oncology, and infectious diseases. The benefits of downstream income from dermatopathology, Mohs surgery, and other ancillary testing can provide great financial advantages for an academic or large group practice.10 Academic medical centers also afford the benefit of resources, such as research offices, clinical trial units, and institutional support for scholarly publication.
Despite its benefits, academic dermatology is not without unique demands. The resources afforded by research work come with grant application deadlines and the pressure to maintain research productivity as measured by grant dollars. Academic providers also must navigate institutional political dynamics and deal with limits on autonomy. Additionally, the administrative burden associated with committee work, mentorship obligations, and publishing requirements further limit clinical time and contribute to burnout. According to Loo et al,5 92% of 89 dermatology department chairmen responding to a poll believed that the lower compensation was the primary factor preventing more residents from pursuing academia.
The adoption of RVU-based and incentive compensation models at many AMCs, along with dwindling government funds available for research, also have created pressure to increase patient volume, sometimes at the expense of teaching and research. Of those academic dermatologists spending more than half their time seeing patients, a majority reported that they lack the time to also conduct research, teach, and mentor students and resident physicians.6 A survey of academic dermatologists suggested that, for those already serving in academic positions, salary was less of a concern than the lack of protected academic time.4 While competing demands can erode the appeal of academic dermatology, academia continues to offer a meaningful and fulfilling career path for those motivated by scholarship, mentorship, teaching opportunities, and systemic impact.
Hybrid and Emerging Models
To reconcile the trade-offs inherent in private and academic models, hybrid roles are becoming increasingly common. In these arrangements, dermatologists split their time between private practice and academic appointments settings, allowing for participation in resident education and research while also benefiting from the operational and financial structure of a private office. In some cases, private groups formally affiliate with academic institutions, creating academic-private practices that host trainees and produce scholarly work while operating financially outside of traditional hospital systems. Individual dermatologists also may choose to accept part-time academic roles that allow residents and medical students to rotate in their offices. Hybrid roles may be of most interest to individuals who feel that they are missing out on the mentorship and teaching opportunities afforded at AMCs.
Government-funded systems such as Veterans Affairs (VA) hospitals offer another alternative. Dermatologists at VA hospitals often hold faculty appointments, treat a wide range of conditions in a population with great need, and engage in teaching without the intensity of productivity requirements seen at AMCs. These roles can be attractive to physicians who value public service, work-life balance, and minimal malpractice risk, as well as dermatologists who wish to introduce variety in their practice through an additional clinical setting. Notably, these roles are limited, as roughly 80% of VA hospitals employ part-time dermatologists and 72% reported being understaffed.11 Despite the challenges of limited resources and increased bureaucracy, the VA is the largest health care delivery system in the United States, offering the benefits of protection from most malpractice risk and participation in medical education at 80% of VA hospitals.12 A VA-based practice may be most attractive to physicians with prior military service or those looking for a stable practice that serves the underserved and the mission of medical education.
Similarly, rural health clinics are private practices with special subsidies from the federal government that bring Medicaid payments up to the level of Medicare.13 Rural dermatology also mirrors that of a VA-based practice by offering the opportunity to treat an array of conditions in a population of great need, as rural patients often are in care deserts and would otherwise need to travel for miles to receive dermatologic care. There is a shortage of dermatologists working in rural areas, and rural dermatologists are more likely than those in suburban or urban areas to practice alone.2 Although potentially more physically isolating, rural dermatology offers providers the opportunity to establish a lucrative practice with minimal competition and development of meaningful patient relationships.
The most rapidly increasing practice model emerging in dermatology over the past decade is the private equity (PE) group. Rajabi-Estarabadi et al14 estimated that at least 184 dermatology practices have been acquired by PE groups between 2010 and 2019. An estimated 15% of all PE acquisitions in health care have been within the field of dermatology.9 Private equity firms typically acquire 1 or more practices, then consolidate the operations with the short-term goals of reducing costs and maximizing profits and longer-term goals of selling the practice for further profit in 3 to 7 years.9 They often rely heavily on a dermatologist supervising a number of nurse practitioners.15 While PE acquisition may provide additional financial stability and income, providers have less autonomy and potentially risk a shift in their focus from patient care to profit.
The blurred lines between practice settings reflect a broader shift in the profession. Dermatologists have increasingly crafted flexible, individualized careers that align with their goals and values while drawing from both academic and private models. Hybrid roles may prove critical in preserving the educational and research missions of dermatology while adapting to economic and institutional realities.
Gender Trends, Career Satisfaction, and Other Factors Influencing Career Choice
The gender demographics of dermatology have changed greatly in recent decades. In the years 2010 to 2021, the percentage of women in the field rose from 41% to 52.2%, mirroring the rise in female medical students.16 Despite this, gender disparities persist through differences in pay, promotion rates, leadership opportunities, and research productivity.17 Women who are academic dermatologists are less likely to have protected research time and often shoulder a disproportionate share of mentorship and administrative responsibilities, which frequently are undervalued in promotion and compensation structures. Similarly, women physicians are less likely to own their own private practice.18 Notably, women physicians work part-time more often than their male counterparts, which likely impacts their income.19 Interestingly, no differences were noted in job satisfaction between men and women in academic or private practice settings, suggesting that dermatology is a fulfilling field for female physicians.16 Similar data were observed in the field of dermatopathology; in fact, there is no difference in job satisfaction when comparing providers in academics vs private practice.20
Geographic factors also influence career decisions. Some dermatologists may choose private practice to remain close to family or serve a rural area, while some choose academic centers typically located in major metropolitan areas. Others are drawn to AMCs due to their reputation, resources, or opportunities for specialization. The number of practicing dermatologists in an area also may be considered, as areas with fewer providers likely have more individuals seeking a provider and thus more earning potential.
In summary, career satisfaction is influenced by many factors, including practice setting, colleagues, institutional leadership, work environment, and professional goals. For individuals who are seeking intellectual stimulation and teaching opportunities, academic dermatology may be a great career option. Academic or large group practices may come with a large group of clinical dermatologists to provide a steady stream of specimens. Private practice appeals to those seeking autonomy, reduced bureaucracy, and higher earning potential. Tierney et al21 found that the greatest predictor of a future career in academics among Mohs surgeons was the number of publications a fellow had before and during fellowship training. These data suggest that personal interests greatly influence career decisions.
The Role of Mentorship in Career Decision-Making
Just as personal preferences guide career decisions, so too do interpersonal interactions. Mentorship plays a large role in career success, and the involvement of faculty mentors in society meetings and editorial boards has been shown to positively correlate with the number of residents pursuing academia.14 Similarly, negative interactions have strong impacts, as the top cited reason for Mohs surgeons leaving academia was lack of support from their academic chair.21 While many academic dermatologists report fulfillment from the collegial environment, retention remains an issue. Tierney et al21 found that, among 455 academic Mohs surgeons, only 28% of those who began in academia remained in those roles over the long term, and this trend of low retention holds true across the field of academic dermatology. Lack of autonomy, insufficient institutional support, and more lucrative private practice opportunities were all cited as reasons for leaving. For dermatologists seeking separation from academics but continued research opportunities, data suggest that private practice allows for continued research and publications, indicating that scholarly engagement is not exclusive to academic settings. These trends point to the increasing viability of hybrid or academic-private models that combine academic productivity with greater flexibility and financial stability.
Final Thoughts
Academic and private practice dermatology each offer compelling advantages and distinct challenges (Table). The growing popularity of hybrid models reflects a desire among dermatologists to balance the intellectual fulfillment associated with academic medicine with professional sustainability and autonomy of private practice. Whether through part-time academic appointments, rural health clinics, VA employment, or affiliations between private groups and academic institutions, these emerging roles offer a flexible and adaptive approach to career development.
Ultimately, the ideal practice model is one that aligns with a physician’s personal values, long-term goals, and lifestyle preferences. No single path fits all, but thoughtful career planning supported by mentorship and institutional transparency can help dermatologists thrive in a rapidly evolving health care landscape.
- Kaplan J. Part I: private practice versus academic medicine. BoardVitals Blog. June 5, 2018. Accessed August 5, 2025. https://www.boardvitals.com/blog/private-practice-academic-medicine/
- Ehrlich A, Kostecki J, Olkaba H. Trends in dermatology practices and the implications for the workforce. J Am Acad Dermatol. 2017;77:746-752. doi:10.1016/j.jaad.2017.06.030
- Parthasarathy V, Pollock JR, McNeely GL, et al. A cross-sectional analysis of trends in dermatology practice size in the United States from 2012 to 2020. Arch Dermatol Res. 2022;315:223-229. doi:10.1007/s00403-022-02344-0
- Bergstresser PR. Perceptions of the academic environment: a national survey. J Am Acad Dermatol. 1991;25:1092-1096. doi:10.1016/0190-9622(91)70311-o
- Loo DS, Liu CL, Geller AC, et al. Academic dermatology manpower: issues of recruitment and retention. Arch Dermatol. 2007;143:341-347. doi:10.1001/archderm.143.3.341
- Resneck JS, Tierney EP, Kimball AB. Challenges facing academic dermatology: survey data on the faculty workforce. J Am Acad Dermatol. 2006;54:211-216. doi:10.1016/j.jaad.2005.10.013
- Salmen N, Brodell R, Brodell Dolohanty L. The electronic health record: should small practices adopt this technology? J of Skin. 2024;8:1269-1273. doi:10.25251/skin.8.1.8
- Morales-Pico BM, Cotton CC, Morrell DS. Factors correlated with residents’ decisions to enter academic dermatology. Dermatol Online J. 2016;22:13030/qt7295783b.
- DeWane ME, Mostow E, Grant-Kels JM. The corporatization of care in academic dermatology. Clin Dermatol. 2020;38:289-295. doi:10.1016/j.clindermatol.2020.02.003
- Pearlman RL, Nahar VK, Sisson WT, et al. Understanding downstream service profitability generated by dermatology faculty in an academic medical center: a key driver to promotion of access-to-care. Arch Dermatol Res. 2023;315:1425-1427. doi:10.1007/s00403-022-02406-3
- Huang WW, Tsoukas MM, Bhutani T, et al. Benchmarking U.S. Department of Veterans Affairs dermatologic services: a nationwide survey of VA dermatologists. J Am Acad Dermatol. 2011;65:50-54. doi:10.1016/j.jaad.2010.04.035
- 20 reasons doctors like working for the Veterans Health Administration. US Department of Veterans Affairs. August 2016. Accessed August 5, 2025. https://www.va.gov/HEALTH/docs/20ReasonsVHA_508_IB10935.pdf
- Rural health clinics (RHCs). Rural Health Information Hub. Updated April 7, 2025. Accessed August 5, 2025. https://www .ruralhealthinfo.org/topics/rural-health-clinics
- Rajabi-Estarabadi A, Jones VA, Zheng C, et al. Dermatologist transitions: academics into private practices and vice versa. Clin Dermatol. 2020;38:541-546. doi:10.1016/j.clindermatol.2020.05.012
- Bruch JD, Foot C, Singh Y, et al. Workforce composition in private equity–acquired versus non–private equity–acquired physician practices. Health Affairs. 2023;42:121-129. doi:10.1377/hlthaff.2022.00308
- Zlakishvili B, Horev A. Gender disparities in high-quality dermatology research over the past 15 years. Int J Womens Dermatol. 2024;10:e160. doi:10.1097/JW9.0000000000000160
- Jambusaria-Pahlajani A, Crow LD, Levender MM, et al. Practice patterns and job satisfaction of Mohs surgeons: a gender-based survey. J Drugs Dermatol. 2017;16:1103-1108. https://pubmed.ncbi.nlm.nih.gov/29140863/
- Kane CK. Policy Research Perspectives. Recent changes in physician practice arrangements: shifts away from private practice and towards larger practice size continue through 2022. American Medical Association website. 2023. Accessed August 5, 2025. https://www.ama-assn.org/system/files/2022-prp-practice-arrangement.pdf
- Frank E, Zhao Z, Sen S, et al. Gender disparities in work and parental status among early career physicians. JAMA Netw Open. 2019;2:e198340. doi:10.1001/jamanetworkopen.2019.8340
- Boyd AS, Fang F. A survey-based evaluation of dermatopathology in the United States. Am J Dermatopathol. 2011;33:173-176. doi:10.1097/dad.0b013e3181f0ed84
- Tierney EP, Hanke CW, Kimball AB. Career trajectory and job satisfaction trends in Mohs micrographic surgeons. Dermatol Surg. 2011;37:1229-1238. doi:10.1111/j.1524-4725.2011.02076.x
Dermatology is a rapidly growing, highly competitive specialty with patients that can be served via private practice, academic medicine, hybrid settings, and rural health clinics. Medical residents’ choice of a career path has been rapidly evolving alongside shifts in health care policy, increasing demand for dermatologic services, stagnant fees falling behind inflation for more than a decade, and payment methods that no longer reflect the traditional fee-for-service model. This places a lot of pressure on young dermatologists to evaluate which practice structure best fits their career goals. A nuanced understanding of the strengths and limitations of each practice model is essential for dermatologists to make informed career decisions that are aligned with their values.
While there are many health care practice models, the first decision dermatology residents must make is whether they would prefer working in the private sector or an academic practice. Of course, it is not uncommon for academic dermatologists to embark on a midcareer segue into private practice and, less commonly, for private dermatologists to culminate their careers with a move to academics. The private sector includes private practice, private equity (PE)–owned group practices that often are single-specialty focused, and hospital-owned group practices that usually are multispecialty. Traditionally, private practices are health care businesses owned by one physician (solo practice) or a group of physicians (group practice) operated independently from hospitals, health systems, or private investors. Financially, these practices rely heavily on volume-based services, especially clinic visits and cosmetic procedures, which provide higher reimbursement rates and usually cash payments at the time of service.1 Roughly 35% of dermatologists in the United States work in private practice, and a dwindling 15% work in solo practice.2,3
Medical practices that are not self-owned by physicians vary widely, and they include hospital- or medical center–owned, private equity, and university-based academic practices. Private equity practices typically are characterized as profit driven. Hospital-owned practices shoulder business decisions and administrative duties for the physician at the cost of provider autonomy. Academic medicine is the most different from the other practice types. In contrast to private practice dermatologists, university-based dermatologists practice at academic medical centers (AMCs) with the core goals of patient care, education, and research. Compensation generally is based on the relative value unit (RVU), which is supplemented by government support and research grants.
As evidenced in this brief discussion, health care practice models are complex, and choosing the right model to align with professional goals can pose a major challenge for many physicians. The advantages and disadvantages of various practice models will be reviewed, highlighting trends and emerging models.
Solo or Small-Group Single-Specialty Private Practice
Private practice offers dermatologists the advantage of higher income potential but with greater economic risk; it often requires physicians to be more involved in the business aspects of dermatologic practice. In the early 1990s, a survey of private practice dermatologists revealed that income was the first or second most important factor that contributed to their career choice of private vs academic practice.4 Earning potential in private practice largely is driven by the autonomy afforded in this setting. Physicians have the liberty of choosing their practice location, structure, schedule, and staff in addition to tailoring services toward profitability; this typically leads to a higher volume of cosmetic and procedural visits, which may be attractive to providers wishing to focus on aesthetics. Private practice dermatologists also are not subject to institutional requirements that may include the preparation of grant submissions, research productivity targets, and devotion of time to teaching. Many private dermatologists find satisfaction in tailoring their work environments to align with personal values and goals and in cultivating long-term relationships with patients in a more personal and less bureaucratic context.
There also are drawbacks to private practice. The profitability often can be attributed to the higher patient load and more hours devoted to practice.5 A 2006 study found that academics saw 32% to 41% fewer patients per week than private practice dermatologists.6 Along with the opportunity for financial gain is the risk of financial ruin. Cost is the largest hurdle for establishing a practice, and most practices do not turn a profit for the first few years.1,5 The financial burden of running a practice includes pressure from the federal government to adopt expensive electronic health record systems to achieve maximum Medicare payment through the Merit-Based Incentive Payment System, liability insurance, health insurance, and staff salaries.7 These challenges require strong business acumen, including managing overhead costs, navigating insurance negotiations, marketing a practice, and maintaining compliance with evolving health care regulations. The purchase of a $100,000 laser could be a boon or bust, requiring the development of a business plan that ensures a positive return on investment. Additionally, private practice profitability has the potential to dwindle as governmental reimbursements fail to match inflation rates. Securing business advisors or even obtaining a Master of Business Administration degree can be helpful.
Insurance and government agencies also are infringing upon some of the autonomy of private practice dermatologists, as evidenced by a 2017 survey of dermatologists that found that more than half of respondents altered treatment plans based on insurance coverage more than 20% of the time.2 Private equity firms also could infringe on private practice autonomy, as providers are beholden to the firm’s restrictions—from which company’s product will be stocked to which partner will be on call. Lastly, private practice is less conducive to consistent referral patterns and strong relationships with specialists when compared to academic practice. Additionally, reliance on high patient throughput or cosmetic services for financial sustainability can shift focus away from complex medical dermatology, which often is referred to AMCs.
Academic Medicine
Academic dermatology offers a stimulating and collaborative environment with opportunities to advance the field through research and education. Often, the opportunity to teach medical students, residents, and peers is the deciding factor for academic dermatologists, as supported by a 2016 survey that found teaching opportunities are a major influence on career decision.8 The mixture of patient care, education, and research roles can be satisfying when compared to the grind of seeing large numbers of patients every day. Because they typically are salaried with an RVU-based income, academic dermatologists often are less concerned with the costs associated with medical treatment, and they typically treat more medically complex patients and underserved populations.9 The salary structure of academic roles also provides the benefit of a stable and predictable income. Physicians in this setting often are considered experts in their field, positioning them to have a strong built-in referral system along with frequent participation in multidisciplinary care alongside colleagues in rheumatology, oncology, and infectious diseases. The benefits of downstream income from dermatopathology, Mohs surgery, and other ancillary testing can provide great financial advantages for an academic or large group practice.10 Academic medical centers also afford the benefit of resources, such as research offices, clinical trial units, and institutional support for scholarly publication.
Despite its benefits, academic dermatology is not without unique demands. The resources afforded by research work come with grant application deadlines and the pressure to maintain research productivity as measured by grant dollars. Academic providers also must navigate institutional political dynamics and deal with limits on autonomy. Additionally, the administrative burden associated with committee work, mentorship obligations, and publishing requirements further limit clinical time and contribute to burnout. According to Loo et al,5 92% of 89 dermatology department chairmen responding to a poll believed that the lower compensation was the primary factor preventing more residents from pursuing academia.
The adoption of RVU-based and incentive compensation models at many AMCs, along with dwindling government funds available for research, also have created pressure to increase patient volume, sometimes at the expense of teaching and research. Of those academic dermatologists spending more than half their time seeing patients, a majority reported that they lack the time to also conduct research, teach, and mentor students and resident physicians.6 A survey of academic dermatologists suggested that, for those already serving in academic positions, salary was less of a concern than the lack of protected academic time.4 While competing demands can erode the appeal of academic dermatology, academia continues to offer a meaningful and fulfilling career path for those motivated by scholarship, mentorship, teaching opportunities, and systemic impact.
Hybrid and Emerging Models
To reconcile the trade-offs inherent in private and academic models, hybrid roles are becoming increasingly common. In these arrangements, dermatologists split their time between private practice and academic appointments settings, allowing for participation in resident education and research while also benefiting from the operational and financial structure of a private office. In some cases, private groups formally affiliate with academic institutions, creating academic-private practices that host trainees and produce scholarly work while operating financially outside of traditional hospital systems. Individual dermatologists also may choose to accept part-time academic roles that allow residents and medical students to rotate in their offices. Hybrid roles may be of most interest to individuals who feel that they are missing out on the mentorship and teaching opportunities afforded at AMCs.
Government-funded systems such as Veterans Affairs (VA) hospitals offer another alternative. Dermatologists at VA hospitals often hold faculty appointments, treat a wide range of conditions in a population with great need, and engage in teaching without the intensity of productivity requirements seen at AMCs. These roles can be attractive to physicians who value public service, work-life balance, and minimal malpractice risk, as well as dermatologists who wish to introduce variety in their practice through an additional clinical setting. Notably, these roles are limited, as roughly 80% of VA hospitals employ part-time dermatologists and 72% reported being understaffed.11 Despite the challenges of limited resources and increased bureaucracy, the VA is the largest health care delivery system in the United States, offering the benefits of protection from most malpractice risk and participation in medical education at 80% of VA hospitals.12 A VA-based practice may be most attractive to physicians with prior military service or those looking for a stable practice that serves the underserved and the mission of medical education.
Similarly, rural health clinics are private practices with special subsidies from the federal government that bring Medicaid payments up to the level of Medicare.13 Rural dermatology also mirrors that of a VA-based practice by offering the opportunity to treat an array of conditions in a population of great need, as rural patients often are in care deserts and would otherwise need to travel for miles to receive dermatologic care. There is a shortage of dermatologists working in rural areas, and rural dermatologists are more likely than those in suburban or urban areas to practice alone.2 Although potentially more physically isolating, rural dermatology offers providers the opportunity to establish a lucrative practice with minimal competition and development of meaningful patient relationships.
The most rapidly increasing practice model emerging in dermatology over the past decade is the private equity (PE) group. Rajabi-Estarabadi et al14 estimated that at least 184 dermatology practices have been acquired by PE groups between 2010 and 2019. An estimated 15% of all PE acquisitions in health care have been within the field of dermatology.9 Private equity firms typically acquire 1 or more practices, then consolidate the operations with the short-term goals of reducing costs and maximizing profits and longer-term goals of selling the practice for further profit in 3 to 7 years.9 They often rely heavily on a dermatologist supervising a number of nurse practitioners.15 While PE acquisition may provide additional financial stability and income, providers have less autonomy and potentially risk a shift in their focus from patient care to profit.
The blurred lines between practice settings reflect a broader shift in the profession. Dermatologists have increasingly crafted flexible, individualized careers that align with their goals and values while drawing from both academic and private models. Hybrid roles may prove critical in preserving the educational and research missions of dermatology while adapting to economic and institutional realities.
Gender Trends, Career Satisfaction, and Other Factors Influencing Career Choice
The gender demographics of dermatology have changed greatly in recent decades. In the years 2010 to 2021, the percentage of women in the field rose from 41% to 52.2%, mirroring the rise in female medical students.16 Despite this, gender disparities persist through differences in pay, promotion rates, leadership opportunities, and research productivity.17 Women who are academic dermatologists are less likely to have protected research time and often shoulder a disproportionate share of mentorship and administrative responsibilities, which frequently are undervalued in promotion and compensation structures. Similarly, women physicians are less likely to own their own private practice.18 Notably, women physicians work part-time more often than their male counterparts, which likely impacts their income.19 Interestingly, no differences were noted in job satisfaction between men and women in academic or private practice settings, suggesting that dermatology is a fulfilling field for female physicians.16 Similar data were observed in the field of dermatopathology; in fact, there is no difference in job satisfaction when comparing providers in academics vs private practice.20
Geographic factors also influence career decisions. Some dermatologists may choose private practice to remain close to family or serve a rural area, while some choose academic centers typically located in major metropolitan areas. Others are drawn to AMCs due to their reputation, resources, or opportunities for specialization. The number of practicing dermatologists in an area also may be considered, as areas with fewer providers likely have more individuals seeking a provider and thus more earning potential.
In summary, career satisfaction is influenced by many factors, including practice setting, colleagues, institutional leadership, work environment, and professional goals. For individuals who are seeking intellectual stimulation and teaching opportunities, academic dermatology may be a great career option. Academic or large group practices may come with a large group of clinical dermatologists to provide a steady stream of specimens. Private practice appeals to those seeking autonomy, reduced bureaucracy, and higher earning potential. Tierney et al21 found that the greatest predictor of a future career in academics among Mohs surgeons was the number of publications a fellow had before and during fellowship training. These data suggest that personal interests greatly influence career decisions.
The Role of Mentorship in Career Decision-Making
Just as personal preferences guide career decisions, so too do interpersonal interactions. Mentorship plays a large role in career success, and the involvement of faculty mentors in society meetings and editorial boards has been shown to positively correlate with the number of residents pursuing academia.14 Similarly, negative interactions have strong impacts, as the top cited reason for Mohs surgeons leaving academia was lack of support from their academic chair.21 While many academic dermatologists report fulfillment from the collegial environment, retention remains an issue. Tierney et al21 found that, among 455 academic Mohs surgeons, only 28% of those who began in academia remained in those roles over the long term, and this trend of low retention holds true across the field of academic dermatology. Lack of autonomy, insufficient institutional support, and more lucrative private practice opportunities were all cited as reasons for leaving. For dermatologists seeking separation from academics but continued research opportunities, data suggest that private practice allows for continued research and publications, indicating that scholarly engagement is not exclusive to academic settings. These trends point to the increasing viability of hybrid or academic-private models that combine academic productivity with greater flexibility and financial stability.
Final Thoughts
Academic and private practice dermatology each offer compelling advantages and distinct challenges (Table). The growing popularity of hybrid models reflects a desire among dermatologists to balance the intellectual fulfillment associated with academic medicine with professional sustainability and autonomy of private practice. Whether through part-time academic appointments, rural health clinics, VA employment, or affiliations between private groups and academic institutions, these emerging roles offer a flexible and adaptive approach to career development.

Ultimately, the ideal practice model is one that aligns with a physician’s personal values, long-term goals, and lifestyle preferences. No single path fits all, but thoughtful career planning supported by mentorship and institutional transparency can help dermatologists thrive in a rapidly evolving health care landscape.
Dermatology is a rapidly growing, highly competitive specialty with patients that can be served via private practice, academic medicine, hybrid settings, and rural health clinics. Medical residents’ choice of a career path has been rapidly evolving alongside shifts in health care policy, increasing demand for dermatologic services, stagnant fees falling behind inflation for more than a decade, and payment methods that no longer reflect the traditional fee-for-service model. This places a lot of pressure on young dermatologists to evaluate which practice structure best fits their career goals. A nuanced understanding of the strengths and limitations of each practice model is essential for dermatologists to make informed career decisions that are aligned with their values.
While there are many health care practice models, the first decision dermatology residents must make is whether they would prefer working in the private sector or an academic practice. Of course, it is not uncommon for academic dermatologists to embark on a midcareer segue into private practice and, less commonly, for private dermatologists to culminate their careers with a move to academics. The private sector includes private practice, private equity (PE)–owned group practices that often are single-specialty focused, and hospital-owned group practices that usually are multispecialty. Traditionally, private practices are health care businesses owned by one physician (solo practice) or a group of physicians (group practice) operated independently from hospitals, health systems, or private investors. Financially, these practices rely heavily on volume-based services, especially clinic visits and cosmetic procedures, which provide higher reimbursement rates and usually cash payments at the time of service.1 Roughly 35% of dermatologists in the United States work in private practice, and a dwindling 15% work in solo practice.2,3
Medical practices that are not self-owned by physicians vary widely, and they include hospital- or medical center–owned, private equity, and university-based academic practices. Private equity practices typically are characterized as profit driven. Hospital-owned practices shoulder business decisions and administrative duties for the physician at the cost of provider autonomy. Academic medicine is the most different from the other practice types. In contrast to private practice dermatologists, university-based dermatologists practice at academic medical centers (AMCs) with the core goals of patient care, education, and research. Compensation generally is based on the relative value unit (RVU), which is supplemented by government support and research grants.
As evidenced in this brief discussion, health care practice models are complex, and choosing the right model to align with professional goals can pose a major challenge for many physicians. The advantages and disadvantages of various practice models will be reviewed, highlighting trends and emerging models.
Solo or Small-Group Single-Specialty Private Practice
Private practice offers dermatologists the advantage of higher income potential but with greater economic risk; it often requires physicians to be more involved in the business aspects of dermatologic practice. In the early 1990s, a survey of private practice dermatologists revealed that income was the first or second most important factor that contributed to their career choice of private vs academic practice.4 Earning potential in private practice largely is driven by the autonomy afforded in this setting. Physicians have the liberty of choosing their practice location, structure, schedule, and staff in addition to tailoring services toward profitability; this typically leads to a higher volume of cosmetic and procedural visits, which may be attractive to providers wishing to focus on aesthetics. Private practice dermatologists also are not subject to institutional requirements that may include the preparation of grant submissions, research productivity targets, and devotion of time to teaching. Many private dermatologists find satisfaction in tailoring their work environments to align with personal values and goals and in cultivating long-term relationships with patients in a more personal and less bureaucratic context.
There also are drawbacks to private practice. The profitability often can be attributed to the higher patient load and more hours devoted to practice.5 A 2006 study found that academics saw 32% to 41% fewer patients per week than private practice dermatologists.6 Along with the opportunity for financial gain is the risk of financial ruin. Cost is the largest hurdle for establishing a practice, and most practices do not turn a profit for the first few years.1,5 The financial burden of running a practice includes pressure from the federal government to adopt expensive electronic health record systems to achieve maximum Medicare payment through the Merit-Based Incentive Payment System, liability insurance, health insurance, and staff salaries.7 These challenges require strong business acumen, including managing overhead costs, navigating insurance negotiations, marketing a practice, and maintaining compliance with evolving health care regulations. The purchase of a $100,000 laser could be a boon or bust, requiring the development of a business plan that ensures a positive return on investment. Additionally, private practice profitability has the potential to dwindle as governmental reimbursements fail to match inflation rates. Securing business advisors or even obtaining a Master of Business Administration degree can be helpful.
Insurance and government agencies also are infringing upon some of the autonomy of private practice dermatologists, as evidenced by a 2017 survey of dermatologists that found that more than half of respondents altered treatment plans based on insurance coverage more than 20% of the time.2 Private equity firms also could infringe on private practice autonomy, as providers are beholden to the firm’s restrictions—from which company’s product will be stocked to which partner will be on call. Lastly, private practice is less conducive to consistent referral patterns and strong relationships with specialists when compared to academic practice. Additionally, reliance on high patient throughput or cosmetic services for financial sustainability can shift focus away from complex medical dermatology, which often is referred to AMCs.
Academic Medicine
Academic dermatology offers a stimulating and collaborative environment with opportunities to advance the field through research and education. Often, the opportunity to teach medical students, residents, and peers is the deciding factor for academic dermatologists, as supported by a 2016 survey that found teaching opportunities are a major influence on career decision.8 The mixture of patient care, education, and research roles can be satisfying when compared to the grind of seeing large numbers of patients every day. Because they typically are salaried with an RVU-based income, academic dermatologists often are less concerned with the costs associated with medical treatment, and they typically treat more medically complex patients and underserved populations.9 The salary structure of academic roles also provides the benefit of a stable and predictable income. Physicians in this setting often are considered experts in their field, positioning them to have a strong built-in referral system along with frequent participation in multidisciplinary care alongside colleagues in rheumatology, oncology, and infectious diseases. The benefits of downstream income from dermatopathology, Mohs surgery, and other ancillary testing can provide great financial advantages for an academic or large group practice.10 Academic medical centers also afford the benefit of resources, such as research offices, clinical trial units, and institutional support for scholarly publication.
Despite its benefits, academic dermatology is not without unique demands. The resources afforded by research work come with grant application deadlines and the pressure to maintain research productivity as measured by grant dollars. Academic providers also must navigate institutional political dynamics and deal with limits on autonomy. Additionally, the administrative burden associated with committee work, mentorship obligations, and publishing requirements further limit clinical time and contribute to burnout. According to Loo et al,5 92% of 89 dermatology department chairmen responding to a poll believed that the lower compensation was the primary factor preventing more residents from pursuing academia.
The adoption of RVU-based and incentive compensation models at many AMCs, along with dwindling government funds available for research, also have created pressure to increase patient volume, sometimes at the expense of teaching and research. Of those academic dermatologists spending more than half their time seeing patients, a majority reported that they lack the time to also conduct research, teach, and mentor students and resident physicians.6 A survey of academic dermatologists suggested that, for those already serving in academic positions, salary was less of a concern than the lack of protected academic time.4 While competing demands can erode the appeal of academic dermatology, academia continues to offer a meaningful and fulfilling career path for those motivated by scholarship, mentorship, teaching opportunities, and systemic impact.
Hybrid and Emerging Models
To reconcile the trade-offs inherent in private and academic models, hybrid roles are becoming increasingly common. In these arrangements, dermatologists split their time between private practice and academic appointments settings, allowing for participation in resident education and research while also benefiting from the operational and financial structure of a private office. In some cases, private groups formally affiliate with academic institutions, creating academic-private practices that host trainees and produce scholarly work while operating financially outside of traditional hospital systems. Individual dermatologists also may choose to accept part-time academic roles that allow residents and medical students to rotate in their offices. Hybrid roles may be of most interest to individuals who feel that they are missing out on the mentorship and teaching opportunities afforded at AMCs.
Government-funded systems such as Veterans Affairs (VA) hospitals offer another alternative. Dermatologists at VA hospitals often hold faculty appointments, treat a wide range of conditions in a population with great need, and engage in teaching without the intensity of productivity requirements seen at AMCs. These roles can be attractive to physicians who value public service, work-life balance, and minimal malpractice risk, as well as dermatologists who wish to introduce variety in their practice through an additional clinical setting. Notably, these roles are limited, as roughly 80% of VA hospitals employ part-time dermatologists and 72% reported being understaffed.11 Despite the challenges of limited resources and increased bureaucracy, the VA is the largest health care delivery system in the United States, offering the benefits of protection from most malpractice risk and participation in medical education at 80% of VA hospitals.12 A VA-based practice may be most attractive to physicians with prior military service or those looking for a stable practice that serves the underserved and the mission of medical education.
Similarly, rural health clinics are private practices with special subsidies from the federal government that bring Medicaid payments up to the level of Medicare.13 Rural dermatology also mirrors that of a VA-based practice by offering the opportunity to treat an array of conditions in a population of great need, as rural patients often are in care deserts and would otherwise need to travel for miles to receive dermatologic care. There is a shortage of dermatologists working in rural areas, and rural dermatologists are more likely than those in suburban or urban areas to practice alone.2 Although potentially more physically isolating, rural dermatology offers providers the opportunity to establish a lucrative practice with minimal competition and development of meaningful patient relationships.
The most rapidly increasing practice model emerging in dermatology over the past decade is the private equity (PE) group. Rajabi-Estarabadi et al14 estimated that at least 184 dermatology practices have been acquired by PE groups between 2010 and 2019. An estimated 15% of all PE acquisitions in health care have been within the field of dermatology.9 Private equity firms typically acquire 1 or more practices, then consolidate the operations with the short-term goals of reducing costs and maximizing profits and longer-term goals of selling the practice for further profit in 3 to 7 years.9 They often rely heavily on a dermatologist supervising a number of nurse practitioners.15 While PE acquisition may provide additional financial stability and income, providers have less autonomy and potentially risk a shift in their focus from patient care to profit.
The blurred lines between practice settings reflect a broader shift in the profession. Dermatologists have increasingly crafted flexible, individualized careers that align with their goals and values while drawing from both academic and private models. Hybrid roles may prove critical in preserving the educational and research missions of dermatology while adapting to economic and institutional realities.
Gender Trends, Career Satisfaction, and Other Factors Influencing Career Choice
The gender demographics of dermatology have changed greatly in recent decades. In the years 2010 to 2021, the percentage of women in the field rose from 41% to 52.2%, mirroring the rise in female medical students.16 Despite this, gender disparities persist through differences in pay, promotion rates, leadership opportunities, and research productivity.17 Women who are academic dermatologists are less likely to have protected research time and often shoulder a disproportionate share of mentorship and administrative responsibilities, which frequently are undervalued in promotion and compensation structures. Similarly, women physicians are less likely to own their own private practice.18 Notably, women physicians work part-time more often than their male counterparts, which likely impacts their income.19 Interestingly, no differences were noted in job satisfaction between men and women in academic or private practice settings, suggesting that dermatology is a fulfilling field for female physicians.16 Similar data were observed in the field of dermatopathology; in fact, there is no difference in job satisfaction when comparing providers in academics vs private practice.20
Geographic factors also influence career decisions. Some dermatologists may choose private practice to remain close to family or serve a rural area, while some choose academic centers typically located in major metropolitan areas. Others are drawn to AMCs due to their reputation, resources, or opportunities for specialization. The number of practicing dermatologists in an area also may be considered, as areas with fewer providers likely have more individuals seeking a provider and thus more earning potential.
In summary, career satisfaction is influenced by many factors, including practice setting, colleagues, institutional leadership, work environment, and professional goals. For individuals who are seeking intellectual stimulation and teaching opportunities, academic dermatology may be a great career option. Academic or large group practices may come with a large group of clinical dermatologists to provide a steady stream of specimens. Private practice appeals to those seeking autonomy, reduced bureaucracy, and higher earning potential. Tierney et al21 found that the greatest predictor of a future career in academics among Mohs surgeons was the number of publications a fellow had before and during fellowship training. These data suggest that personal interests greatly influence career decisions.
The Role of Mentorship in Career Decision-Making
Just as personal preferences guide career decisions, so too do interpersonal interactions. Mentorship plays a large role in career success, and the involvement of faculty mentors in society meetings and editorial boards has been shown to positively correlate with the number of residents pursuing academia.14 Similarly, negative interactions have strong impacts, as the top cited reason for Mohs surgeons leaving academia was lack of support from their academic chair.21 While many academic dermatologists report fulfillment from the collegial environment, retention remains an issue. Tierney et al21 found that, among 455 academic Mohs surgeons, only 28% of those who began in academia remained in those roles over the long term, and this trend of low retention holds true across the field of academic dermatology. Lack of autonomy, insufficient institutional support, and more lucrative private practice opportunities were all cited as reasons for leaving. For dermatologists seeking separation from academics but continued research opportunities, data suggest that private practice allows for continued research and publications, indicating that scholarly engagement is not exclusive to academic settings. These trends point to the increasing viability of hybrid or academic-private models that combine academic productivity with greater flexibility and financial stability.
Final Thoughts
Academic and private practice dermatology each offer compelling advantages and distinct challenges (Table). The growing popularity of hybrid models reflects a desire among dermatologists to balance the intellectual fulfillment associated with academic medicine with professional sustainability and autonomy of private practice. Whether through part-time academic appointments, rural health clinics, VA employment, or affiliations between private groups and academic institutions, these emerging roles offer a flexible and adaptive approach to career development.

Ultimately, the ideal practice model is one that aligns with a physician’s personal values, long-term goals, and lifestyle preferences. No single path fits all, but thoughtful career planning supported by mentorship and institutional transparency can help dermatologists thrive in a rapidly evolving health care landscape.
- Kaplan J. Part I: private practice versus academic medicine. BoardVitals Blog. June 5, 2018. Accessed August 5, 2025. https://www.boardvitals.com/blog/private-practice-academic-medicine/
- Ehrlich A, Kostecki J, Olkaba H. Trends in dermatology practices and the implications for the workforce. J Am Acad Dermatol. 2017;77:746-752. doi:10.1016/j.jaad.2017.06.030
- Parthasarathy V, Pollock JR, McNeely GL, et al. A cross-sectional analysis of trends in dermatology practice size in the United States from 2012 to 2020. Arch Dermatol Res. 2022;315:223-229. doi:10.1007/s00403-022-02344-0
- Bergstresser PR. Perceptions of the academic environment: a national survey. J Am Acad Dermatol. 1991;25:1092-1096. doi:10.1016/0190-9622(91)70311-o
- Loo DS, Liu CL, Geller AC, et al. Academic dermatology manpower: issues of recruitment and retention. Arch Dermatol. 2007;143:341-347. doi:10.1001/archderm.143.3.341
- Resneck JS, Tierney EP, Kimball AB. Challenges facing academic dermatology: survey data on the faculty workforce. J Am Acad Dermatol. 2006;54:211-216. doi:10.1016/j.jaad.2005.10.013
- Salmen N, Brodell R, Brodell Dolohanty L. The electronic health record: should small practices adopt this technology? J of Skin. 2024;8:1269-1273. doi:10.25251/skin.8.1.8
- Morales-Pico BM, Cotton CC, Morrell DS. Factors correlated with residents’ decisions to enter academic dermatology. Dermatol Online J. 2016;22:13030/qt7295783b.
- DeWane ME, Mostow E, Grant-Kels JM. The corporatization of care in academic dermatology. Clin Dermatol. 2020;38:289-295. doi:10.1016/j.clindermatol.2020.02.003
- Pearlman RL, Nahar VK, Sisson WT, et al. Understanding downstream service profitability generated by dermatology faculty in an academic medical center: a key driver to promotion of access-to-care. Arch Dermatol Res. 2023;315:1425-1427. doi:10.1007/s00403-022-02406-3
- Huang WW, Tsoukas MM, Bhutani T, et al. Benchmarking U.S. Department of Veterans Affairs dermatologic services: a nationwide survey of VA dermatologists. J Am Acad Dermatol. 2011;65:50-54. doi:10.1016/j.jaad.2010.04.035
- 20 reasons doctors like working for the Veterans Health Administration. US Department of Veterans Affairs. August 2016. Accessed August 5, 2025. https://www.va.gov/HEALTH/docs/20ReasonsVHA_508_IB10935.pdf
- Rural health clinics (RHCs). Rural Health Information Hub. Updated April 7, 2025. Accessed August 5, 2025. https://www .ruralhealthinfo.org/topics/rural-health-clinics
- Rajabi-Estarabadi A, Jones VA, Zheng C, et al. Dermatologist transitions: academics into private practices and vice versa. Clin Dermatol. 2020;38:541-546. doi:10.1016/j.clindermatol.2020.05.012
- Bruch JD, Foot C, Singh Y, et al. Workforce composition in private equity–acquired versus non–private equity–acquired physician practices. Health Affairs. 2023;42:121-129. doi:10.1377/hlthaff.2022.00308
- Zlakishvili B, Horev A. Gender disparities in high-quality dermatology research over the past 15 years. Int J Womens Dermatol. 2024;10:e160. doi:10.1097/JW9.0000000000000160
- Jambusaria-Pahlajani A, Crow LD, Levender MM, et al. Practice patterns and job satisfaction of Mohs surgeons: a gender-based survey. J Drugs Dermatol. 2017;16:1103-1108. https://pubmed.ncbi.nlm.nih.gov/29140863/
- Kane CK. Policy Research Perspectives. Recent changes in physician practice arrangements: shifts away from private practice and towards larger practice size continue through 2022. American Medical Association website. 2023. Accessed August 5, 2025. https://www.ama-assn.org/system/files/2022-prp-practice-arrangement.pdf
- Frank E, Zhao Z, Sen S, et al. Gender disparities in work and parental status among early career physicians. JAMA Netw Open. 2019;2:e198340. doi:10.1001/jamanetworkopen.2019.8340
- Boyd AS, Fang F. A survey-based evaluation of dermatopathology in the United States. Am J Dermatopathol. 2011;33:173-176. doi:10.1097/dad.0b013e3181f0ed84
- Tierney EP, Hanke CW, Kimball AB. Career trajectory and job satisfaction trends in Mohs micrographic surgeons. Dermatol Surg. 2011;37:1229-1238. doi:10.1111/j.1524-4725.2011.02076.x
- Kaplan J. Part I: private practice versus academic medicine. BoardVitals Blog. June 5, 2018. Accessed August 5, 2025. https://www.boardvitals.com/blog/private-practice-academic-medicine/
- Ehrlich A, Kostecki J, Olkaba H. Trends in dermatology practices and the implications for the workforce. J Am Acad Dermatol. 2017;77:746-752. doi:10.1016/j.jaad.2017.06.030
- Parthasarathy V, Pollock JR, McNeely GL, et al. A cross-sectional analysis of trends in dermatology practice size in the United States from 2012 to 2020. Arch Dermatol Res. 2022;315:223-229. doi:10.1007/s00403-022-02344-0
- Bergstresser PR. Perceptions of the academic environment: a national survey. J Am Acad Dermatol. 1991;25:1092-1096. doi:10.1016/0190-9622(91)70311-o
- Loo DS, Liu CL, Geller AC, et al. Academic dermatology manpower: issues of recruitment and retention. Arch Dermatol. 2007;143:341-347. doi:10.1001/archderm.143.3.341
- Resneck JS, Tierney EP, Kimball AB. Challenges facing academic dermatology: survey data on the faculty workforce. J Am Acad Dermatol. 2006;54:211-216. doi:10.1016/j.jaad.2005.10.013
- Salmen N, Brodell R, Brodell Dolohanty L. The electronic health record: should small practices adopt this technology? J of Skin. 2024;8:1269-1273. doi:10.25251/skin.8.1.8
- Morales-Pico BM, Cotton CC, Morrell DS. Factors correlated with residents’ decisions to enter academic dermatology. Dermatol Online J. 2016;22:13030/qt7295783b.
- DeWane ME, Mostow E, Grant-Kels JM. The corporatization of care in academic dermatology. Clin Dermatol. 2020;38:289-295. doi:10.1016/j.clindermatol.2020.02.003
- Pearlman RL, Nahar VK, Sisson WT, et al. Understanding downstream service profitability generated by dermatology faculty in an academic medical center: a key driver to promotion of access-to-care. Arch Dermatol Res. 2023;315:1425-1427. doi:10.1007/s00403-022-02406-3
- Huang WW, Tsoukas MM, Bhutani T, et al. Benchmarking U.S. Department of Veterans Affairs dermatologic services: a nationwide survey of VA dermatologists. J Am Acad Dermatol. 2011;65:50-54. doi:10.1016/j.jaad.2010.04.035
- 20 reasons doctors like working for the Veterans Health Administration. US Department of Veterans Affairs. August 2016. Accessed August 5, 2025. https://www.va.gov/HEALTH/docs/20ReasonsVHA_508_IB10935.pdf
- Rural health clinics (RHCs). Rural Health Information Hub. Updated April 7, 2025. Accessed August 5, 2025. https://www .ruralhealthinfo.org/topics/rural-health-clinics
- Rajabi-Estarabadi A, Jones VA, Zheng C, et al. Dermatologist transitions: academics into private practices and vice versa. Clin Dermatol. 2020;38:541-546. doi:10.1016/j.clindermatol.2020.05.012
- Bruch JD, Foot C, Singh Y, et al. Workforce composition in private equity–acquired versus non–private equity–acquired physician practices. Health Affairs. 2023;42:121-129. doi:10.1377/hlthaff.2022.00308
- Zlakishvili B, Horev A. Gender disparities in high-quality dermatology research over the past 15 years. Int J Womens Dermatol. 2024;10:e160. doi:10.1097/JW9.0000000000000160
- Jambusaria-Pahlajani A, Crow LD, Levender MM, et al. Practice patterns and job satisfaction of Mohs surgeons: a gender-based survey. J Drugs Dermatol. 2017;16:1103-1108. https://pubmed.ncbi.nlm.nih.gov/29140863/
- Kane CK. Policy Research Perspectives. Recent changes in physician practice arrangements: shifts away from private practice and towards larger practice size continue through 2022. American Medical Association website. 2023. Accessed August 5, 2025. https://www.ama-assn.org/system/files/2022-prp-practice-arrangement.pdf
- Frank E, Zhao Z, Sen S, et al. Gender disparities in work and parental status among early career physicians. JAMA Netw Open. 2019;2:e198340. doi:10.1001/jamanetworkopen.2019.8340
- Boyd AS, Fang F. A survey-based evaluation of dermatopathology in the United States. Am J Dermatopathol. 2011;33:173-176. doi:10.1097/dad.0b013e3181f0ed84
- Tierney EP, Hanke CW, Kimball AB. Career trajectory and job satisfaction trends in Mohs micrographic surgeons. Dermatol Surg. 2011;37:1229-1238. doi:10.1111/j.1524-4725.2011.02076.x
Advantages and Disadvantages of Private vs Academic Dermatology Practices
Advantages and Disadvantages of Private vs Academic Dermatology Practices
PRACTICE POINTS
- In the field of dermatology, solo and small-group single-specialty private practices are shrinking while academic medicine is growing.
- Hybrid models reflect a desire among some dermatologists to balance the intellectual fulfillment and sustainability associated with academic medicine and the professional autonomy of private practice.
Quality of Life for Males With Abdominal Aortic Aneurysm
Quality of Life for Males With Abdominal Aortic Aneurysm
Abdominal aortic aneurysm (AAA) is a public health threat, with a global prevalence of 4.8% and a prevalence in males that increases with age, from 1.3% between ages 45 and 54 years to 12.5% between ages 75 and 84 years.1 AAA is often asymptomatic until it ruptures and can become life-threatening, with mortality rates near 90% in the event of rupture with survival rates of about 50% to 70% for individuals with rupture who require urgent surgical intervention.2,3 Males experience AAA at 4 times the rate of females.4
Previous research has found that the awareness of having an AAA causes anxiety that some have described as “living with a ticking time bomb.”5 Others reported worries and concerns about life’s fragility and mortality due to an AAA diagnosis.6 However, the psychological impact on the individuals’ quality of life (QoL) remains unclear, especially for individuals with a small AAA (< 5.5 cm).7 Factors such as age, male sex, smoking, family history, hypertension, carotid artery disease, and hypercholesterolemia have been strongly associated with increased growth rate and the risk of small AAA ruptures.8,9
Most patients with a small AAA enter surveillance awaiting future repair and not only have the anxiety of living with an AAA despite the low risk of rupture, but also a worse QoL than those who have undergone repair.10,11 However, data are sparse regarding the effects on QoL of knowing they have an AAA, whether repaired or not. This study sought to examine the impact an AAA diagnosis had on male QoL at the initial investigation and after 12 months.
Methods
This prospective study was examined and approved by the Veterans Affairs Northern California Health Care System (NCHCS) Institutional Review Board. It was conducted at the Sacramento US Department of Veterans Affairs (VA) Medical Center from January 1, 2019, to February 28, 2022. Patients were identified through the vascular clinic. One hundred sixteen patients with AAA were eligible and agreed to participate. Of these, 91 (78%) completed the survey at baseline and 12 months later. Participation was voluntary; written informed consent was obtained from every patient before completing the survey. This study included only male patients due to their higher prevalence than female patients.4 Patients were also eligible if they were aged > 18 years and had a previously known AAA that was being followed with a recorded clinical imaging study in the NCHCS vascular clinic. Patients were excluded if they were unable to return for their 12-month follow-up investigation, were incapable of giving informed consent, were unable to complete the 12-item short form health survey version 2 (SF-12v2), had a documented history of psychiatric illness, or refused to participate. The SF-12v2, an abbreviated version of the 36-item short form health survey (SF-36), is a generic health-related quality-of-life survey that measures 8 domains of general health status: general health (GH), physical functioning (PF), role limitations due to physical problems (RP), bodily pain (BP), vitality (VT), social functioning (SF), role emotional (RE), and mental health (MH). A higher number on the QoL scale indicates better QoL. The GH, PF, RP, and BP scales yield a physical component score (PCS), and the VT, SF, RE, and MH scales generate a mental component score (MCS). Although SF-12v2 has not been validated for patients with AAA, it has been widely used and validated to measure health-related QoL in cohorts of healthy and chronically ill individuals.12,13
Analysis
Descriptive statistics, including means, SDs, frequency, percentages, 95% CIs, and correlations were calculated. The t test was used to analyze differences in mean scores. For continuous variables, such as SF-12v2 domains, PCS, and MCS, mean, SD, 95% CI, and range were determined. Comparisons were performed using X2 or t test. P < .05 was considered statistically significant. Clinical risk factors, including age, race, body mass index (BMI), diabetes, hypertension, hyperlipidemia, coronary artery disease, cerebrovascular accident, myocardial infarction, and smoking status, were also recorded.
Results
Between January 1, 2019, and February 28, 2022, 91 patients were diagnosed with an AAA and completed the survey at the initial and 12-month investigations. Patients had a mean (SD) age of 76.0 (5.6) years (range, 64-93) and BMI of 29.7 (6.4). Comorbid diabetes was present in 31% of patients, hypertension in 75%, hyperlipidemia 66%, and coronary artery disease in 12% (Table 1). Most patients smoked tobacco: 71% indicated previous use and 22% were current users.
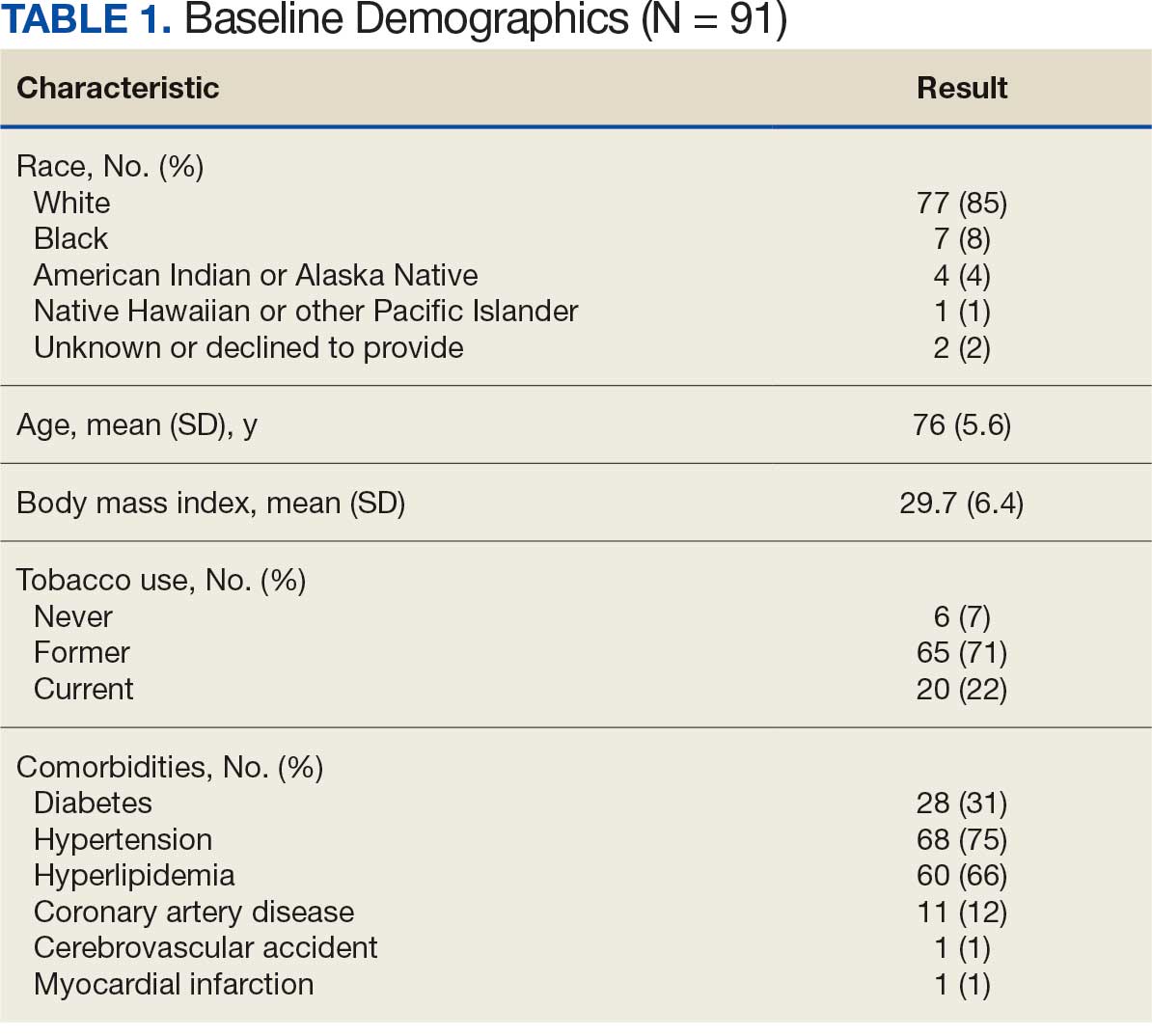
When comparing baseline vs 12-month follow-up, patients indicated a higher QoL in GH (3.2 vs 3.5, respectively; P < .05) and BP (3.1 vs 3.6, respectively; P < .05). No statistically significant difference was seen PF, RP, VT, SF, RE, MH, as well as PCS and MCS between baseline and follow-up with respect to QoL (P < .05). However, the 5 domains of SF-12v2: PF, RP, SF, RE, MH, and PCS had lower QoL scores at the 12-month follow-up when compared with baseline, but with no statistically significant difference between both investigations (Table 2).
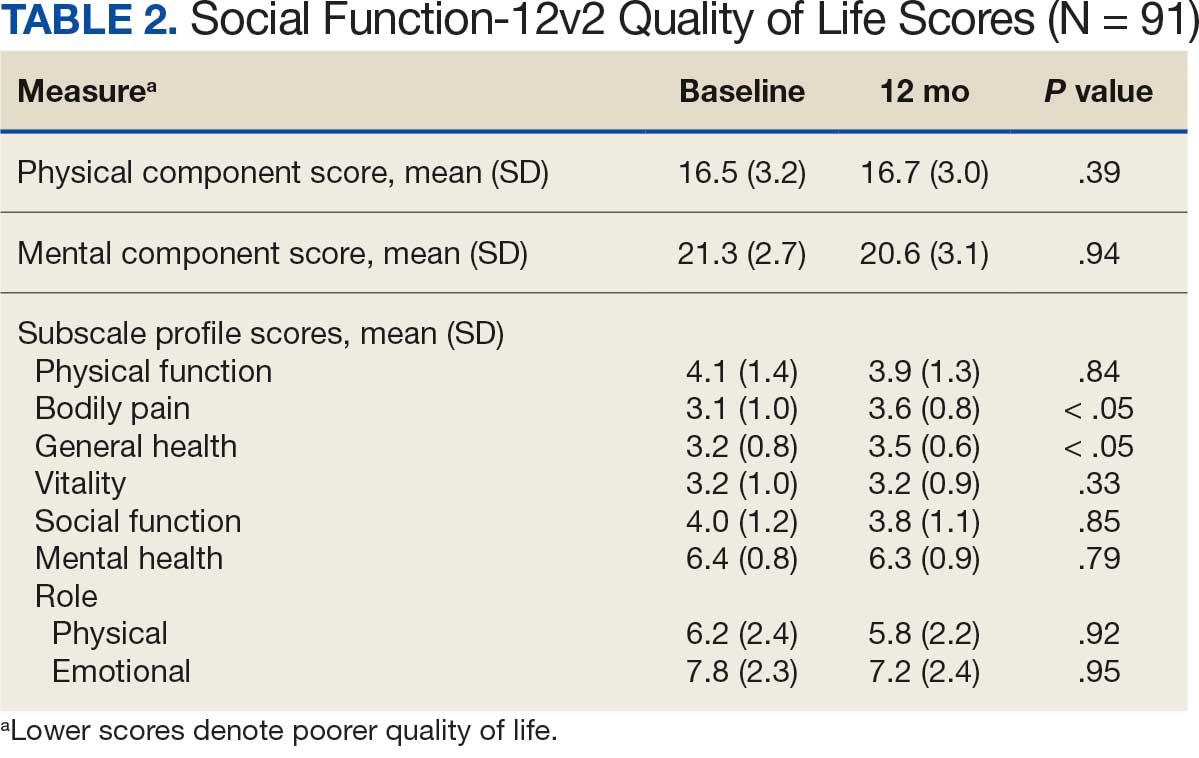
Discussion
Previous studies have characterized the results of QoL measures as subjective because they are based on patient perceptions of their physical and psychological condition.14,15 However, SF-36 and SF-12v2 responses provide a multifaceted account that encompasses the physical, psychological, and social aspects of QoL. Despite being the most widely used generic instrument in many fields of medicine, SF-36 is time consuming for clinicians who may prefer simpler and more time-efficient instruments.16-18 The SF-12v2 not only imposes less burden on respondents but also generates accurate summary scores for patients physical and mental health.19
The replicability of SF-12v2 PCS and MCS scores has been demonstrated. In the United Kingdom, Jenkinson and Layte constructed SF-12v2 summary measures from a large scale dataset by sending the SF-36 and other questions on health and lifestyles to 9332 individuals and compared the results of the SF-36 and SF-12v2 across diverse patient groups (eg, Parkinson disease, congestive heart failure, sleep apnea, benign prostatic hypertrophy). Results from SF-36 PCS, SF-36 MCS, and PCS-12v2 (ρ, 0.94; P < .001) and SF-12v2 MCS (ρ, 0.96; P < .001) were found to be highly correlated, and also produced similar results, both in the community sample and across a variety of disease-specific groups.20
The aim of this longitudinal observational study was to measure the QoL of males with an AAA ≥ 3.0 cm at baseline and 12 months later. The mean age of participants was 76 years, which aligns with previous research that found the prevalence of AAAs increased with age.1 Study participants had a mean BMI of 29.7, which also supports previous research that indicated that obesity is independently associated with an AAA.21 Patients with an AAA and a history of smoking (former or current), hypertension, or hyperlipidemia had lower mean scores for 3 of 8 SF-12v2 domains at the 12-month follow-up.
These findings support previous research that indicated smoking is not only a very strong risk factor for the presence of an AAA but also associated with increased rates of expansion and the risk of rupture in patients with an AAA.22 Bath et al found that patients with an AAA compared to patients without an AAA were older (age 72.6 vs 69.8 years; P < .001), had a higher BMI (28.1 vs 27.0; P < .001), were more likely to be a current smoker (15.1% vs 5.2%; P < .001), and were more likely to have diabetes (18.8% vs 10.0%; P < .001), ischemic heart disease (12.2% vs 4.4%; P < .001), high cholesterol (53.2% vs 30.8%; P <. 001), previous stroke (6.1% vs 2.9%; P < .001), and a previous myocardial infarction (21.1% vs 5.8%; P < .001).23 Lesjak et al found that men with AAA reported significantly lower scores in the domains of social functioning, pain, and general health 6 months after ultrasound compared with men without AAA.24
Previous research indicates that patients with an AAA have a higher risk of cardiovascular diseases and comorbidities that may impact their perceived QoL. In a study assessing cardiovascular risk in 2323 patients with a small AAA, Bath et al found a high prevalence of coronary artery disease (44.9%), myocardial infarction (26.8%), heart failure (4.4%) and cerebrovascular accident (14.0%) which may have contributed to the decreased level of self-perceived QoL in these patients.25
This aligned with a study by Golledge et al, who found that participants diagnosed with an AAA and peripheral artery disease not only had significantly poorer QoL scores in 5 SF-36 domains (PF, RP, GH, VT, and PCS)when compared with participants diagnosed with an AAA alone. They also had significantly poorer QoL scores in 7 domains of the SF-36 (PF, RP, GH, VT, SF, RE, and PCS) when compared with controls without an AAA.26
Our analysis found that males with an AAA had a rise in SF-12v2 QoL scores from baseline to 12-month follow-up in the GH and BP domains. There was no statistically significant difference in QoL in the other 6 domains (PF, RP, VT, SF, RE, and MH) between the initial and 12-month investigations. Bath et al also found that men with an AAA had a transient reduction in mental QoL during the first year after the initial screening but returned to baseline.23
Strengths and Limitations
This study is notable for its sample of patients who previously had a diagnosed AAA that were followed with a recorded clinical imaging study and the use of a validated QoL measure (SF-12v2) that provided virtually identical summary scores (PCS and MCS) as the SF-36.27 However, this study was limited by the brevity of the SF-12v2 instrument which made it difficult to extract sufficient reliable information for the 8 domains.28 Subjective perception of patients is another limitation inherent to any QoL study. QoL scores were not available before the initial investigation. Measuring QoL at baseline and 12 months later does not capture the potential fluctuations and changes in QoL that the patient may experience some months later. Another limitation arises from the fact that the AAA patient population in the study included patients under surveillance and patients who had undergone repair.
Fourteen patients (15%) had received AAA repair: 10 had endovascular reconstruction and 4 had open surgical repair. Including patients with a previous AAA repair may have influenced reported QoL levels. Suckow et al performed a 2-phase study on 1008 patients, 351 (35%) were under surveillance and 657 (65%) had undergone repair. In that study, patients under AAA surveillance had worse emotional impact scores compared with patients with repair (22 vs 13; P < .001).11 Additionally, the size of the abdominal aorta at the time of survey was not addressed in the study, which could constitute explanatory variables.
Conclusions
This study found higher QoL at 12-month follow-up compared to baseline in both the GH and BP domains of the SF-12v2 health survey for male veterans with an AAA. Periodic QoL assessments for patients with an AAA may be helpful in tracking QoL course, minimizing their physical and psychological concerns, and improving overall care and support. However, further research is necessary to assess the QoL of patients with an AAA who are under surveillance compared with those who had an aneurysm repair to accurately measure the impact of an AAA on QoL.
- Altobelli E, Rapacchietta L, Profeta VF, et al. Risk factors for abdominal aortic aneurysm in population- based studies: a systematic review and meta-analysis. Int J Environ Res Public Health. 2018;15:2805. doi:10.3390/ijerph15122805
- Chaikof EL, Dalman RL, Eskandari MK, et al. The society for vascular surgery practice guidelines on the care of patients with an abdominal aortic aneurysm. J Vasc Surg. 2018;67:2-77.e2. doi:10.1016/j.jvs.2017.10.044
- Kent KC. Abdominal aortic aneurysms. N Engl J Med. 2014;371:2101-2108. doi:10.1056/NEJMcp1401430
- Harthun NL. Current issues in the treatment of women with abdominal aortic aneurysm. Gend Med. 2008;5:36-43.
- Aoki H. Taking control of the time bomb in abdominal aortic aneurysm. Circ J. 2016;80:314-315. doi:10.1253/circj.CJ-15-1350
- Damhus CS, Siersma V, Hansson A, Bang CW, Brodersen J. Psychosocial consequences of screeningdetected abdominal aortic aneurisms: a cross-sectional study. Scand J Prim Health Care. 2021;39:459-465. doi:10.1080/02813432.2021.2004713
- Ericsson A, Kumlien C, Ching S, Carlson E, Molassiotis A. Impact on quality of life of men with screening-detected abdominal aortic aneurysms attending regular follow ups: a narrative literature review. Eur J Vasc Endovasc Surg. 2019;57:589-596. doi:10.1016/j.ejvs.2018.10.012
- Galyfos G, Voulalas G, Stamatatos I, et al. Small abdominal aortic aneurysms: should we wait? Vasc Dis Manag. 2015;12:E152-E159.
- Kristensen KL, Dahl M, Rasmussen LM, et al. Glycated hemoglobin is associated with the growth rate of abdominal aortic aneurysms. Arterioscler Thromb Vasc Biol. 2017;37:730-736. doi:10.1161/ATVBAHA.116.308874
- Xiao-Yan L, Yu-Kui M, Li-Hui L. Risk factors for preoperative anxiety and depression in patients scheduled for abdominal aortic aneurysm repair. Chine Med J. 2018;131:1951-1957. doi:10.4103/0366-6999.238154
- Suckow BD, Schanzer AS, Hoel AW, et al. A novel quality of life instrument for patients with an abdominal aortic aneurysm. Eur J Vasc Endovasc Surg. 2019;57:809-815. doi:10.1016/j.ejvs.2019.01.018
- Flatz A, Casillas A, Stringhini S, et al. Association between education and quality of diabetes care in Switzerland. Int J Gen Med. 2015;8:87-92. doi:10.2147/IJGM.S77139
- Christensen AV, Bjorner JB, Ekholm O, et al. Increased risk of mortality and readmission associated with lower SF-12 scores in cardiac patients: Results from the national DenHeart study. Eur J Cardiovasc Nurs. 2020;19:330-338. doi:10.1177/1474515119885480
- Hamming JF, De Vries J. Measuring quality of life. Br J Surg. 2007;94:923-924. doi:10.1002/bjs.5948
- Urbach DR. Measuring quality of life after surgery. Surg Innov. 2005;12:161-165. doi:10.1177/ 155335060501200216
- Gandek B, Sinclair SJ, Kosinski M, et al. Psychometric evaluation of the SF-36® health survey in medicare managed care. Health Care Financ Rev. 2004;25:5.
- Ware JE, Sherbourne CD. The MOS 36-item short form health survey (SF-36). Med Care. 1992;30:473-483. doi:10.1097/00005650-199206000-00002
- Takayoshi K, Mototsugu T, Tomohiro T, et al. Health-related quality of life prospectively evaluated by the 8-item short form after endovascular repair versus open surgery for abdominal aortic aneurysms. Heart Vessels. 2017;32:960- 968. doi:10.1007/s00380-017-0956-9
- Pickard AS, Johnson JA, Penn A, et al. Replicability of SF-36 summary scores by the SF-12 in stroke patients. Stroke. 1999;30:1213-1217. doi:10.1161/01.str.30.6.1213
- Jenkinson C, Layte R. The development and testing of the UK SF-12. J Health Serv Res Policy. 1997;2:14-18. doi:10.1177/135581969700200105
- Golledge J, Clancy P, Jamrozik K, et al. Obesity, adipokines, and abdominal aortic aneurysm: Health in Men study. Circulation. 2007;116:2275-2279. doi:10.1161/CIRCULATIONAHA.107.717926
- Norman PE, Curci JA. Understanding the effects of tobacco smoke on the pathogenesis of aortic aneurysm. Arterioscler Thromb Vasc Biol. 2013;33:1473-1477. doi:10.1161/ATVBAHA.112.300158
- Bath MF, Sidloff D, Saratzis A, et al. Impact of abdominal aortic aneurysm screening on quality of life. BJS. 2018;105:203-208. doi:10.1002/bjs.10721
- Lesjak M, Boreland F, Lyle D, Sidford J, Flecknoe-Brown S, Fletcher J. Screening for abdominal aortic aneurysm: does it affect men’s quality of life? Aust J Prim Health. 2012;18:284-288. doi:10.1071/PY11131
- Bath MF, Gokani VJ, Sidloff DA, et al. Systematic review of cardiovascular disease and cardiovascular death in patients with a small abdominal aortic aneurysm. Br J Surg. 2015;102:866-872. doi:10.1002/bjs.9837
- Golledge J, Pinchbeck J, Rowbotham SE, et al. Health-related quality of life amongst people diagnosed with abdominal aortic aneurysm and peripheral artery disease and the effect of fenofibrate. Sci Rep. 2020;10:14583. doi:10.1038/s41598-020-71454-4
- Jenkinson C, Layte R, Jenkinson D. A shorter form health survey: can the SF-12 replicate results from the SF-36 in longitudinal studies? J Public Health Med. 1997;19:179- 186. doi:10.1093/oxfordjournals.pubmed.a024606
- White MK, Maher SM, Rizio AA, et al. A meta-analytic review of measurement equivalence study findings of the SF-36® and SF-12® Health Surveys across electronic modes compared to paper administration. Qual Life Res. 2018;27:1757-1767. doi:10.1007/s11136-018-1851-2
Abdominal aortic aneurysm (AAA) is a public health threat, with a global prevalence of 4.8% and a prevalence in males that increases with age, from 1.3% between ages 45 and 54 years to 12.5% between ages 75 and 84 years.1 AAA is often asymptomatic until it ruptures and can become life-threatening, with mortality rates near 90% in the event of rupture with survival rates of about 50% to 70% for individuals with rupture who require urgent surgical intervention.2,3 Males experience AAA at 4 times the rate of females.4
Previous research has found that the awareness of having an AAA causes anxiety that some have described as “living with a ticking time bomb.”5 Others reported worries and concerns about life’s fragility and mortality due to an AAA diagnosis.6 However, the psychological impact on the individuals’ quality of life (QoL) remains unclear, especially for individuals with a small AAA (< 5.5 cm).7 Factors such as age, male sex, smoking, family history, hypertension, carotid artery disease, and hypercholesterolemia have been strongly associated with increased growth rate and the risk of small AAA ruptures.8,9
Most patients with a small AAA enter surveillance awaiting future repair and not only have the anxiety of living with an AAA despite the low risk of rupture, but also a worse QoL than those who have undergone repair.10,11 However, data are sparse regarding the effects on QoL of knowing they have an AAA, whether repaired or not. This study sought to examine the impact an AAA diagnosis had on male QoL at the initial investigation and after 12 months.
Methods
This prospective study was examined and approved by the Veterans Affairs Northern California Health Care System (NCHCS) Institutional Review Board. It was conducted at the Sacramento US Department of Veterans Affairs (VA) Medical Center from January 1, 2019, to February 28, 2022. Patients were identified through the vascular clinic. One hundred sixteen patients with AAA were eligible and agreed to participate. Of these, 91 (78%) completed the survey at baseline and 12 months later. Participation was voluntary; written informed consent was obtained from every patient before completing the survey. This study included only male patients due to their higher prevalence than female patients.4 Patients were also eligible if they were aged > 18 years and had a previously known AAA that was being followed with a recorded clinical imaging study in the NCHCS vascular clinic. Patients were excluded if they were unable to return for their 12-month follow-up investigation, were incapable of giving informed consent, were unable to complete the 12-item short form health survey version 2 (SF-12v2), had a documented history of psychiatric illness, or refused to participate. The SF-12v2, an abbreviated version of the 36-item short form health survey (SF-36), is a generic health-related quality-of-life survey that measures 8 domains of general health status: general health (GH), physical functioning (PF), role limitations due to physical problems (RP), bodily pain (BP), vitality (VT), social functioning (SF), role emotional (RE), and mental health (MH). A higher number on the QoL scale indicates better QoL. The GH, PF, RP, and BP scales yield a physical component score (PCS), and the VT, SF, RE, and MH scales generate a mental component score (MCS). Although SF-12v2 has not been validated for patients with AAA, it has been widely used and validated to measure health-related QoL in cohorts of healthy and chronically ill individuals.12,13
Analysis
Descriptive statistics, including means, SDs, frequency, percentages, 95% CIs, and correlations were calculated. The t test was used to analyze differences in mean scores. For continuous variables, such as SF-12v2 domains, PCS, and MCS, mean, SD, 95% CI, and range were determined. Comparisons were performed using X2 or t test. P < .05 was considered statistically significant. Clinical risk factors, including age, race, body mass index (BMI), diabetes, hypertension, hyperlipidemia, coronary artery disease, cerebrovascular accident, myocardial infarction, and smoking status, were also recorded.
Results
Between January 1, 2019, and February 28, 2022, 91 patients were diagnosed with an AAA and completed the survey at the initial and 12-month investigations. Patients had a mean (SD) age of 76.0 (5.6) years (range, 64-93) and BMI of 29.7 (6.4). Comorbid diabetes was present in 31% of patients, hypertension in 75%, hyperlipidemia 66%, and coronary artery disease in 12% (Table 1). Most patients smoked tobacco: 71% indicated previous use and 22% were current users.

When comparing baseline vs 12-month follow-up, patients indicated a higher QoL in GH (3.2 vs 3.5, respectively; P < .05) and BP (3.1 vs 3.6, respectively; P < .05). No statistically significant difference was seen PF, RP, VT, SF, RE, MH, as well as PCS and MCS between baseline and follow-up with respect to QoL (P < .05). However, the 5 domains of SF-12v2: PF, RP, SF, RE, MH, and PCS had lower QoL scores at the 12-month follow-up when compared with baseline, but with no statistically significant difference between both investigations (Table 2).

Discussion
Previous studies have characterized the results of QoL measures as subjective because they are based on patient perceptions of their physical and psychological condition.14,15 However, SF-36 and SF-12v2 responses provide a multifaceted account that encompasses the physical, psychological, and social aspects of QoL. Despite being the most widely used generic instrument in many fields of medicine, SF-36 is time consuming for clinicians who may prefer simpler and more time-efficient instruments.16-18 The SF-12v2 not only imposes less burden on respondents but also generates accurate summary scores for patients physical and mental health.19
The replicability of SF-12v2 PCS and MCS scores has been demonstrated. In the United Kingdom, Jenkinson and Layte constructed SF-12v2 summary measures from a large scale dataset by sending the SF-36 and other questions on health and lifestyles to 9332 individuals and compared the results of the SF-36 and SF-12v2 across diverse patient groups (eg, Parkinson disease, congestive heart failure, sleep apnea, benign prostatic hypertrophy). Results from SF-36 PCS, SF-36 MCS, and PCS-12v2 (ρ, 0.94; P < .001) and SF-12v2 MCS (ρ, 0.96; P < .001) were found to be highly correlated, and also produced similar results, both in the community sample and across a variety of disease-specific groups.20
The aim of this longitudinal observational study was to measure the QoL of males with an AAA ≥ 3.0 cm at baseline and 12 months later. The mean age of participants was 76 years, which aligns with previous research that found the prevalence of AAAs increased with age.1 Study participants had a mean BMI of 29.7, which also supports previous research that indicated that obesity is independently associated with an AAA.21 Patients with an AAA and a history of smoking (former or current), hypertension, or hyperlipidemia had lower mean scores for 3 of 8 SF-12v2 domains at the 12-month follow-up.
These findings support previous research that indicated smoking is not only a very strong risk factor for the presence of an AAA but also associated with increased rates of expansion and the risk of rupture in patients with an AAA.22 Bath et al found that patients with an AAA compared to patients without an AAA were older (age 72.6 vs 69.8 years; P < .001), had a higher BMI (28.1 vs 27.0; P < .001), were more likely to be a current smoker (15.1% vs 5.2%; P < .001), and were more likely to have diabetes (18.8% vs 10.0%; P < .001), ischemic heart disease (12.2% vs 4.4%; P < .001), high cholesterol (53.2% vs 30.8%; P <. 001), previous stroke (6.1% vs 2.9%; P < .001), and a previous myocardial infarction (21.1% vs 5.8%; P < .001).23 Lesjak et al found that men with AAA reported significantly lower scores in the domains of social functioning, pain, and general health 6 months after ultrasound compared with men without AAA.24
Previous research indicates that patients with an AAA have a higher risk of cardiovascular diseases and comorbidities that may impact their perceived QoL. In a study assessing cardiovascular risk in 2323 patients with a small AAA, Bath et al found a high prevalence of coronary artery disease (44.9%), myocardial infarction (26.8%), heart failure (4.4%) and cerebrovascular accident (14.0%) which may have contributed to the decreased level of self-perceived QoL in these patients.25
This aligned with a study by Golledge et al, who found that participants diagnosed with an AAA and peripheral artery disease not only had significantly poorer QoL scores in 5 SF-36 domains (PF, RP, GH, VT, and PCS)when compared with participants diagnosed with an AAA alone. They also had significantly poorer QoL scores in 7 domains of the SF-36 (PF, RP, GH, VT, SF, RE, and PCS) when compared with controls without an AAA.26
Our analysis found that males with an AAA had a rise in SF-12v2 QoL scores from baseline to 12-month follow-up in the GH and BP domains. There was no statistically significant difference in QoL in the other 6 domains (PF, RP, VT, SF, RE, and MH) between the initial and 12-month investigations. Bath et al also found that men with an AAA had a transient reduction in mental QoL during the first year after the initial screening but returned to baseline.23
Strengths and Limitations
This study is notable for its sample of patients who previously had a diagnosed AAA that were followed with a recorded clinical imaging study and the use of a validated QoL measure (SF-12v2) that provided virtually identical summary scores (PCS and MCS) as the SF-36.27 However, this study was limited by the brevity of the SF-12v2 instrument which made it difficult to extract sufficient reliable information for the 8 domains.28 Subjective perception of patients is another limitation inherent to any QoL study. QoL scores were not available before the initial investigation. Measuring QoL at baseline and 12 months later does not capture the potential fluctuations and changes in QoL that the patient may experience some months later. Another limitation arises from the fact that the AAA patient population in the study included patients under surveillance and patients who had undergone repair.
Fourteen patients (15%) had received AAA repair: 10 had endovascular reconstruction and 4 had open surgical repair. Including patients with a previous AAA repair may have influenced reported QoL levels. Suckow et al performed a 2-phase study on 1008 patients, 351 (35%) were under surveillance and 657 (65%) had undergone repair. In that study, patients under AAA surveillance had worse emotional impact scores compared with patients with repair (22 vs 13; P < .001).11 Additionally, the size of the abdominal aorta at the time of survey was not addressed in the study, which could constitute explanatory variables.
Conclusions
This study found higher QoL at 12-month follow-up compared to baseline in both the GH and BP domains of the SF-12v2 health survey for male veterans with an AAA. Periodic QoL assessments for patients with an AAA may be helpful in tracking QoL course, minimizing their physical and psychological concerns, and improving overall care and support. However, further research is necessary to assess the QoL of patients with an AAA who are under surveillance compared with those who had an aneurysm repair to accurately measure the impact of an AAA on QoL.
Abdominal aortic aneurysm (AAA) is a public health threat, with a global prevalence of 4.8% and a prevalence in males that increases with age, from 1.3% between ages 45 and 54 years to 12.5% between ages 75 and 84 years.1 AAA is often asymptomatic until it ruptures and can become life-threatening, with mortality rates near 90% in the event of rupture with survival rates of about 50% to 70% for individuals with rupture who require urgent surgical intervention.2,3 Males experience AAA at 4 times the rate of females.4
Previous research has found that the awareness of having an AAA causes anxiety that some have described as “living with a ticking time bomb.”5 Others reported worries and concerns about life’s fragility and mortality due to an AAA diagnosis.6 However, the psychological impact on the individuals’ quality of life (QoL) remains unclear, especially for individuals with a small AAA (< 5.5 cm).7 Factors such as age, male sex, smoking, family history, hypertension, carotid artery disease, and hypercholesterolemia have been strongly associated with increased growth rate and the risk of small AAA ruptures.8,9
Most patients with a small AAA enter surveillance awaiting future repair and not only have the anxiety of living with an AAA despite the low risk of rupture, but also a worse QoL than those who have undergone repair.10,11 However, data are sparse regarding the effects on QoL of knowing they have an AAA, whether repaired or not. This study sought to examine the impact an AAA diagnosis had on male QoL at the initial investigation and after 12 months.
Methods
This prospective study was examined and approved by the Veterans Affairs Northern California Health Care System (NCHCS) Institutional Review Board. It was conducted at the Sacramento US Department of Veterans Affairs (VA) Medical Center from January 1, 2019, to February 28, 2022. Patients were identified through the vascular clinic. One hundred sixteen patients with AAA were eligible and agreed to participate. Of these, 91 (78%) completed the survey at baseline and 12 months later. Participation was voluntary; written informed consent was obtained from every patient before completing the survey. This study included only male patients due to their higher prevalence than female patients.4 Patients were also eligible if they were aged > 18 years and had a previously known AAA that was being followed with a recorded clinical imaging study in the NCHCS vascular clinic. Patients were excluded if they were unable to return for their 12-month follow-up investigation, were incapable of giving informed consent, were unable to complete the 12-item short form health survey version 2 (SF-12v2), had a documented history of psychiatric illness, or refused to participate. The SF-12v2, an abbreviated version of the 36-item short form health survey (SF-36), is a generic health-related quality-of-life survey that measures 8 domains of general health status: general health (GH), physical functioning (PF), role limitations due to physical problems (RP), bodily pain (BP), vitality (VT), social functioning (SF), role emotional (RE), and mental health (MH). A higher number on the QoL scale indicates better QoL. The GH, PF, RP, and BP scales yield a physical component score (PCS), and the VT, SF, RE, and MH scales generate a mental component score (MCS). Although SF-12v2 has not been validated for patients with AAA, it has been widely used and validated to measure health-related QoL in cohorts of healthy and chronically ill individuals.12,13
Analysis
Descriptive statistics, including means, SDs, frequency, percentages, 95% CIs, and correlations were calculated. The t test was used to analyze differences in mean scores. For continuous variables, such as SF-12v2 domains, PCS, and MCS, mean, SD, 95% CI, and range were determined. Comparisons were performed using X2 or t test. P < .05 was considered statistically significant. Clinical risk factors, including age, race, body mass index (BMI), diabetes, hypertension, hyperlipidemia, coronary artery disease, cerebrovascular accident, myocardial infarction, and smoking status, were also recorded.
Results
Between January 1, 2019, and February 28, 2022, 91 patients were diagnosed with an AAA and completed the survey at the initial and 12-month investigations. Patients had a mean (SD) age of 76.0 (5.6) years (range, 64-93) and BMI of 29.7 (6.4). Comorbid diabetes was present in 31% of patients, hypertension in 75%, hyperlipidemia 66%, and coronary artery disease in 12% (Table 1). Most patients smoked tobacco: 71% indicated previous use and 22% were current users.

When comparing baseline vs 12-month follow-up, patients indicated a higher QoL in GH (3.2 vs 3.5, respectively; P < .05) and BP (3.1 vs 3.6, respectively; P < .05). No statistically significant difference was seen PF, RP, VT, SF, RE, MH, as well as PCS and MCS between baseline and follow-up with respect to QoL (P < .05). However, the 5 domains of SF-12v2: PF, RP, SF, RE, MH, and PCS had lower QoL scores at the 12-month follow-up when compared with baseline, but with no statistically significant difference between both investigations (Table 2).

Discussion
Previous studies have characterized the results of QoL measures as subjective because they are based on patient perceptions of their physical and psychological condition.14,15 However, SF-36 and SF-12v2 responses provide a multifaceted account that encompasses the physical, psychological, and social aspects of QoL. Despite being the most widely used generic instrument in many fields of medicine, SF-36 is time consuming for clinicians who may prefer simpler and more time-efficient instruments.16-18 The SF-12v2 not only imposes less burden on respondents but also generates accurate summary scores for patients physical and mental health.19
The replicability of SF-12v2 PCS and MCS scores has been demonstrated. In the United Kingdom, Jenkinson and Layte constructed SF-12v2 summary measures from a large scale dataset by sending the SF-36 and other questions on health and lifestyles to 9332 individuals and compared the results of the SF-36 and SF-12v2 across diverse patient groups (eg, Parkinson disease, congestive heart failure, sleep apnea, benign prostatic hypertrophy). Results from SF-36 PCS, SF-36 MCS, and PCS-12v2 (ρ, 0.94; P < .001) and SF-12v2 MCS (ρ, 0.96; P < .001) were found to be highly correlated, and also produced similar results, both in the community sample and across a variety of disease-specific groups.20
The aim of this longitudinal observational study was to measure the QoL of males with an AAA ≥ 3.0 cm at baseline and 12 months later. The mean age of participants was 76 years, which aligns with previous research that found the prevalence of AAAs increased with age.1 Study participants had a mean BMI of 29.7, which also supports previous research that indicated that obesity is independently associated with an AAA.21 Patients with an AAA and a history of smoking (former or current), hypertension, or hyperlipidemia had lower mean scores for 3 of 8 SF-12v2 domains at the 12-month follow-up.
These findings support previous research that indicated smoking is not only a very strong risk factor for the presence of an AAA but also associated with increased rates of expansion and the risk of rupture in patients with an AAA.22 Bath et al found that patients with an AAA compared to patients without an AAA were older (age 72.6 vs 69.8 years; P < .001), had a higher BMI (28.1 vs 27.0; P < .001), were more likely to be a current smoker (15.1% vs 5.2%; P < .001), and were more likely to have diabetes (18.8% vs 10.0%; P < .001), ischemic heart disease (12.2% vs 4.4%; P < .001), high cholesterol (53.2% vs 30.8%; P <. 001), previous stroke (6.1% vs 2.9%; P < .001), and a previous myocardial infarction (21.1% vs 5.8%; P < .001).23 Lesjak et al found that men with AAA reported significantly lower scores in the domains of social functioning, pain, and general health 6 months after ultrasound compared with men without AAA.24
Previous research indicates that patients with an AAA have a higher risk of cardiovascular diseases and comorbidities that may impact their perceived QoL. In a study assessing cardiovascular risk in 2323 patients with a small AAA, Bath et al found a high prevalence of coronary artery disease (44.9%), myocardial infarction (26.8%), heart failure (4.4%) and cerebrovascular accident (14.0%) which may have contributed to the decreased level of self-perceived QoL in these patients.25
This aligned with a study by Golledge et al, who found that participants diagnosed with an AAA and peripheral artery disease not only had significantly poorer QoL scores in 5 SF-36 domains (PF, RP, GH, VT, and PCS)when compared with participants diagnosed with an AAA alone. They also had significantly poorer QoL scores in 7 domains of the SF-36 (PF, RP, GH, VT, SF, RE, and PCS) when compared with controls without an AAA.26
Our analysis found that males with an AAA had a rise in SF-12v2 QoL scores from baseline to 12-month follow-up in the GH and BP domains. There was no statistically significant difference in QoL in the other 6 domains (PF, RP, VT, SF, RE, and MH) between the initial and 12-month investigations. Bath et al also found that men with an AAA had a transient reduction in mental QoL during the first year after the initial screening but returned to baseline.23
Strengths and Limitations
This study is notable for its sample of patients who previously had a diagnosed AAA that were followed with a recorded clinical imaging study and the use of a validated QoL measure (SF-12v2) that provided virtually identical summary scores (PCS and MCS) as the SF-36.27 However, this study was limited by the brevity of the SF-12v2 instrument which made it difficult to extract sufficient reliable information for the 8 domains.28 Subjective perception of patients is another limitation inherent to any QoL study. QoL scores were not available before the initial investigation. Measuring QoL at baseline and 12 months later does not capture the potential fluctuations and changes in QoL that the patient may experience some months later. Another limitation arises from the fact that the AAA patient population in the study included patients under surveillance and patients who had undergone repair.
Fourteen patients (15%) had received AAA repair: 10 had endovascular reconstruction and 4 had open surgical repair. Including patients with a previous AAA repair may have influenced reported QoL levels. Suckow et al performed a 2-phase study on 1008 patients, 351 (35%) were under surveillance and 657 (65%) had undergone repair. In that study, patients under AAA surveillance had worse emotional impact scores compared with patients with repair (22 vs 13; P < .001).11 Additionally, the size of the abdominal aorta at the time of survey was not addressed in the study, which could constitute explanatory variables.
Conclusions
This study found higher QoL at 12-month follow-up compared to baseline in both the GH and BP domains of the SF-12v2 health survey for male veterans with an AAA. Periodic QoL assessments for patients with an AAA may be helpful in tracking QoL course, minimizing their physical and psychological concerns, and improving overall care and support. However, further research is necessary to assess the QoL of patients with an AAA who are under surveillance compared with those who had an aneurysm repair to accurately measure the impact of an AAA on QoL.
- Altobelli E, Rapacchietta L, Profeta VF, et al. Risk factors for abdominal aortic aneurysm in population- based studies: a systematic review and meta-analysis. Int J Environ Res Public Health. 2018;15:2805. doi:10.3390/ijerph15122805
- Chaikof EL, Dalman RL, Eskandari MK, et al. The society for vascular surgery practice guidelines on the care of patients with an abdominal aortic aneurysm. J Vasc Surg. 2018;67:2-77.e2. doi:10.1016/j.jvs.2017.10.044
- Kent KC. Abdominal aortic aneurysms. N Engl J Med. 2014;371:2101-2108. doi:10.1056/NEJMcp1401430
- Harthun NL. Current issues in the treatment of women with abdominal aortic aneurysm. Gend Med. 2008;5:36-43.
- Aoki H. Taking control of the time bomb in abdominal aortic aneurysm. Circ J. 2016;80:314-315. doi:10.1253/circj.CJ-15-1350
- Damhus CS, Siersma V, Hansson A, Bang CW, Brodersen J. Psychosocial consequences of screeningdetected abdominal aortic aneurisms: a cross-sectional study. Scand J Prim Health Care. 2021;39:459-465. doi:10.1080/02813432.2021.2004713
- Ericsson A, Kumlien C, Ching S, Carlson E, Molassiotis A. Impact on quality of life of men with screening-detected abdominal aortic aneurysms attending regular follow ups: a narrative literature review. Eur J Vasc Endovasc Surg. 2019;57:589-596. doi:10.1016/j.ejvs.2018.10.012
- Galyfos G, Voulalas G, Stamatatos I, et al. Small abdominal aortic aneurysms: should we wait? Vasc Dis Manag. 2015;12:E152-E159.
- Kristensen KL, Dahl M, Rasmussen LM, et al. Glycated hemoglobin is associated with the growth rate of abdominal aortic aneurysms. Arterioscler Thromb Vasc Biol. 2017;37:730-736. doi:10.1161/ATVBAHA.116.308874
- Xiao-Yan L, Yu-Kui M, Li-Hui L. Risk factors for preoperative anxiety and depression in patients scheduled for abdominal aortic aneurysm repair. Chine Med J. 2018;131:1951-1957. doi:10.4103/0366-6999.238154
- Suckow BD, Schanzer AS, Hoel AW, et al. A novel quality of life instrument for patients with an abdominal aortic aneurysm. Eur J Vasc Endovasc Surg. 2019;57:809-815. doi:10.1016/j.ejvs.2019.01.018
- Flatz A, Casillas A, Stringhini S, et al. Association between education and quality of diabetes care in Switzerland. Int J Gen Med. 2015;8:87-92. doi:10.2147/IJGM.S77139
- Christensen AV, Bjorner JB, Ekholm O, et al. Increased risk of mortality and readmission associated with lower SF-12 scores in cardiac patients: Results from the national DenHeart study. Eur J Cardiovasc Nurs. 2020;19:330-338. doi:10.1177/1474515119885480
- Hamming JF, De Vries J. Measuring quality of life. Br J Surg. 2007;94:923-924. doi:10.1002/bjs.5948
- Urbach DR. Measuring quality of life after surgery. Surg Innov. 2005;12:161-165. doi:10.1177/ 155335060501200216
- Gandek B, Sinclair SJ, Kosinski M, et al. Psychometric evaluation of the SF-36® health survey in medicare managed care. Health Care Financ Rev. 2004;25:5.
- Ware JE, Sherbourne CD. The MOS 36-item short form health survey (SF-36). Med Care. 1992;30:473-483. doi:10.1097/00005650-199206000-00002
- Takayoshi K, Mototsugu T, Tomohiro T, et al. Health-related quality of life prospectively evaluated by the 8-item short form after endovascular repair versus open surgery for abdominal aortic aneurysms. Heart Vessels. 2017;32:960- 968. doi:10.1007/s00380-017-0956-9
- Pickard AS, Johnson JA, Penn A, et al. Replicability of SF-36 summary scores by the SF-12 in stroke patients. Stroke. 1999;30:1213-1217. doi:10.1161/01.str.30.6.1213
- Jenkinson C, Layte R. The development and testing of the UK SF-12. J Health Serv Res Policy. 1997;2:14-18. doi:10.1177/135581969700200105
- Golledge J, Clancy P, Jamrozik K, et al. Obesity, adipokines, and abdominal aortic aneurysm: Health in Men study. Circulation. 2007;116:2275-2279. doi:10.1161/CIRCULATIONAHA.107.717926
- Norman PE, Curci JA. Understanding the effects of tobacco smoke on the pathogenesis of aortic aneurysm. Arterioscler Thromb Vasc Biol. 2013;33:1473-1477. doi:10.1161/ATVBAHA.112.300158
- Bath MF, Sidloff D, Saratzis A, et al. Impact of abdominal aortic aneurysm screening on quality of life. BJS. 2018;105:203-208. doi:10.1002/bjs.10721
- Lesjak M, Boreland F, Lyle D, Sidford J, Flecknoe-Brown S, Fletcher J. Screening for abdominal aortic aneurysm: does it affect men’s quality of life? Aust J Prim Health. 2012;18:284-288. doi:10.1071/PY11131
- Bath MF, Gokani VJ, Sidloff DA, et al. Systematic review of cardiovascular disease and cardiovascular death in patients with a small abdominal aortic aneurysm. Br J Surg. 2015;102:866-872. doi:10.1002/bjs.9837
- Golledge J, Pinchbeck J, Rowbotham SE, et al. Health-related quality of life amongst people diagnosed with abdominal aortic aneurysm and peripheral artery disease and the effect of fenofibrate. Sci Rep. 2020;10:14583. doi:10.1038/s41598-020-71454-4
- Jenkinson C, Layte R, Jenkinson D. A shorter form health survey: can the SF-12 replicate results from the SF-36 in longitudinal studies? J Public Health Med. 1997;19:179- 186. doi:10.1093/oxfordjournals.pubmed.a024606
- White MK, Maher SM, Rizio AA, et al. A meta-analytic review of measurement equivalence study findings of the SF-36® and SF-12® Health Surveys across electronic modes compared to paper administration. Qual Life Res. 2018;27:1757-1767. doi:10.1007/s11136-018-1851-2
- Altobelli E, Rapacchietta L, Profeta VF, et al. Risk factors for abdominal aortic aneurysm in population- based studies: a systematic review and meta-analysis. Int J Environ Res Public Health. 2018;15:2805. doi:10.3390/ijerph15122805
- Chaikof EL, Dalman RL, Eskandari MK, et al. The society for vascular surgery practice guidelines on the care of patients with an abdominal aortic aneurysm. J Vasc Surg. 2018;67:2-77.e2. doi:10.1016/j.jvs.2017.10.044
- Kent KC. Abdominal aortic aneurysms. N Engl J Med. 2014;371:2101-2108. doi:10.1056/NEJMcp1401430
- Harthun NL. Current issues in the treatment of women with abdominal aortic aneurysm. Gend Med. 2008;5:36-43.
- Aoki H. Taking control of the time bomb in abdominal aortic aneurysm. Circ J. 2016;80:314-315. doi:10.1253/circj.CJ-15-1350
- Damhus CS, Siersma V, Hansson A, Bang CW, Brodersen J. Psychosocial consequences of screeningdetected abdominal aortic aneurisms: a cross-sectional study. Scand J Prim Health Care. 2021;39:459-465. doi:10.1080/02813432.2021.2004713
- Ericsson A, Kumlien C, Ching S, Carlson E, Molassiotis A. Impact on quality of life of men with screening-detected abdominal aortic aneurysms attending regular follow ups: a narrative literature review. Eur J Vasc Endovasc Surg. 2019;57:589-596. doi:10.1016/j.ejvs.2018.10.012
- Galyfos G, Voulalas G, Stamatatos I, et al. Small abdominal aortic aneurysms: should we wait? Vasc Dis Manag. 2015;12:E152-E159.
- Kristensen KL, Dahl M, Rasmussen LM, et al. Glycated hemoglobin is associated with the growth rate of abdominal aortic aneurysms. Arterioscler Thromb Vasc Biol. 2017;37:730-736. doi:10.1161/ATVBAHA.116.308874
- Xiao-Yan L, Yu-Kui M, Li-Hui L. Risk factors for preoperative anxiety and depression in patients scheduled for abdominal aortic aneurysm repair. Chine Med J. 2018;131:1951-1957. doi:10.4103/0366-6999.238154
- Suckow BD, Schanzer AS, Hoel AW, et al. A novel quality of life instrument for patients with an abdominal aortic aneurysm. Eur J Vasc Endovasc Surg. 2019;57:809-815. doi:10.1016/j.ejvs.2019.01.018
- Flatz A, Casillas A, Stringhini S, et al. Association between education and quality of diabetes care in Switzerland. Int J Gen Med. 2015;8:87-92. doi:10.2147/IJGM.S77139
- Christensen AV, Bjorner JB, Ekholm O, et al. Increased risk of mortality and readmission associated with lower SF-12 scores in cardiac patients: Results from the national DenHeart study. Eur J Cardiovasc Nurs. 2020;19:330-338. doi:10.1177/1474515119885480
- Hamming JF, De Vries J. Measuring quality of life. Br J Surg. 2007;94:923-924. doi:10.1002/bjs.5948
- Urbach DR. Measuring quality of life after surgery. Surg Innov. 2005;12:161-165. doi:10.1177/ 155335060501200216
- Gandek B, Sinclair SJ, Kosinski M, et al. Psychometric evaluation of the SF-36® health survey in medicare managed care. Health Care Financ Rev. 2004;25:5.
- Ware JE, Sherbourne CD. The MOS 36-item short form health survey (SF-36). Med Care. 1992;30:473-483. doi:10.1097/00005650-199206000-00002
- Takayoshi K, Mototsugu T, Tomohiro T, et al. Health-related quality of life prospectively evaluated by the 8-item short form after endovascular repair versus open surgery for abdominal aortic aneurysms. Heart Vessels. 2017;32:960- 968. doi:10.1007/s00380-017-0956-9
- Pickard AS, Johnson JA, Penn A, et al. Replicability of SF-36 summary scores by the SF-12 in stroke patients. Stroke. 1999;30:1213-1217. doi:10.1161/01.str.30.6.1213
- Jenkinson C, Layte R. The development and testing of the UK SF-12. J Health Serv Res Policy. 1997;2:14-18. doi:10.1177/135581969700200105
- Golledge J, Clancy P, Jamrozik K, et al. Obesity, adipokines, and abdominal aortic aneurysm: Health in Men study. Circulation. 2007;116:2275-2279. doi:10.1161/CIRCULATIONAHA.107.717926
- Norman PE, Curci JA. Understanding the effects of tobacco smoke on the pathogenesis of aortic aneurysm. Arterioscler Thromb Vasc Biol. 2013;33:1473-1477. doi:10.1161/ATVBAHA.112.300158
- Bath MF, Sidloff D, Saratzis A, et al. Impact of abdominal aortic aneurysm screening on quality of life. BJS. 2018;105:203-208. doi:10.1002/bjs.10721
- Lesjak M, Boreland F, Lyle D, Sidford J, Flecknoe-Brown S, Fletcher J. Screening for abdominal aortic aneurysm: does it affect men’s quality of life? Aust J Prim Health. 2012;18:284-288. doi:10.1071/PY11131
- Bath MF, Gokani VJ, Sidloff DA, et al. Systematic review of cardiovascular disease and cardiovascular death in patients with a small abdominal aortic aneurysm. Br J Surg. 2015;102:866-872. doi:10.1002/bjs.9837
- Golledge J, Pinchbeck J, Rowbotham SE, et al. Health-related quality of life amongst people diagnosed with abdominal aortic aneurysm and peripheral artery disease and the effect of fenofibrate. Sci Rep. 2020;10:14583. doi:10.1038/s41598-020-71454-4
- Jenkinson C, Layte R, Jenkinson D. A shorter form health survey: can the SF-12 replicate results from the SF-36 in longitudinal studies? J Public Health Med. 1997;19:179- 186. doi:10.1093/oxfordjournals.pubmed.a024606
- White MK, Maher SM, Rizio AA, et al. A meta-analytic review of measurement equivalence study findings of the SF-36® and SF-12® Health Surveys across electronic modes compared to paper administration. Qual Life Res. 2018;27:1757-1767. doi:10.1007/s11136-018-1851-2
Quality of Life for Males With Abdominal Aortic Aneurysm
Quality of Life for Males With Abdominal Aortic Aneurysm
VIP Boot Camp: Expanding the Impact of VA Primary Care Mental Health With a Transdiagnostic Modular Group Program
VIP Boot Camp: Expanding the Impact of VA Primary Care Mental Health With a Transdiagnostic Modular Group Program
Since 2007, Primary Care Mental Health Integration (PCMHI) at the Veterans Health Administration (VHA) has improved access to mental health care services for veterans by directly embedding mental health care professionals (HCPs) within primary care teams.1 Veterans referred to PCMHI often have co-occurring physical and mental health disorders.2 Untreated chronic physical and mental comorbidities can diminish the effectiveness of medical and mental health interventions. Growing evidence suggests that treatment of mental health conditions can improve physical health outcomes and management of physical conditions can improve mental health outcomes.2,3
Chronic pain and sleep disorders are common reasons patients present to primary care, and often coexist together with mental health comorbidities.4 Sleep disorders affect 50% to 88% of patients with chronic pain, and 40% of patients with sleep disorders report chronic pain.4 Research has found that chronic pain and sleep disorders increase the risk of suicide attempts and deaths by suicide. Addressing suicide prevention simultaneously with treating chronic pain and insomnia is encouraged.5
Background
PCMHI treats physical and mental health comorbidities with a collaborative framework and a biopsychosocial integrative model.6 PCMHI staff provide mental health services as members of primary care teams. An interdisciplinary PCMHI team can include, but is not limited to, psychologists, mental health social workers, psychiatrists, nurse practitioners, clinical pharmacists, and mental health nurses. Quality of care within this model is elevated, as mental and physical health are recognized as interconnected. Collaboration between primary care and mental health benefits veterans and the VHA by increasing access to mental health care, decreasing stigma associated with mental health treatment, improving health outcomes, and enhancing the likelihood of recovery, resulting in high patient satisfaction.6-8
In the existing PCMHI model, HCPs are encouraged to use short-term, evidence-based psychotherapies (EBPs).9 Veterans referred to PCMHI from primary care are typically able to attend 1 to 6 brief sessions of mental health treatment, often 20 to 30 minutes long. Most EBPs in PCMHI are disorder- specific, providing interventions focused on a single presenting problem (eg, insomnia, chronic pain, or posttraumatic stress disorder [PTSD]). For veterans with a single issue, this model can be very effective. 1,10 However, the high rate of co-occurrence of mental and physical health issues can make it difficult to fully treat interrelated problems if the focus is on 1 specific diagnosis. Veterans with a need for additional (more comprehensive or intensive) mental health treatment are frequently referred to a higher, more resource-intensive level of mental health care, either in the VHA or the community. Examples of higher levels of mental health care include the longer term behavioral health interdisciplinary program (BHIP), sometimes called a mental health clinic (MHC), or a specialty mental health program such as a PTSD clinic.
As PCMHI continues to grow, new challenges have emerged related to staffing shortages and gaps in the clinical delivery of mental health treatment within the VHA. At the same time, demand for VHA mental health treatment has increased. However, a mental health professional shortage severely limits the ability of the VHA to meet this demand. In many systems, this shortage may result in more referrals being made to a higher level of mental health care because of fewer resources to provide comprehensive treatment in a less intensive PCMHI setting.8,10,11 This referral pattern can overburden higher level care, often with long wait times for treatment and lengthy lag times between appointments. Furthermore, these gaps in the clinical delivery of care cannot be effectively addressed by hiring additional mental health professionals. This strain on resources can impede access to care and negatively affect outcomes.10
Recent congressional reports highlight these issues, noting that demand for mental health services continues to outpace the capacity of both PCMHI and higher levels of mental health care, leading to delays in treatment that may negatively affect outcomes.8,10,11 These delays can be particularly detrimental for individuals with conditions requiring timely intervention.8,11 Some veterans are willing to engage with PCMHI in a primary care setting but may be reluctant to engage in general mental health treatment. These veterans might not receive the mental health care they need without PCMHI.
Group Psychotherapy
A group psychotherapy format can address gaps in care delivery and provide advantages for patients, mental health professionals, and the VHA. Group psychotherapy aligns with the US Department of Veterans Affairs (VA) 2018 Blueprint for Excellence and 2018 to 2024 strategic plan, underscoring the need for more timely and efficient mental health services.12,13
Benefits of group psychotherapy include reductions in symptoms, decreased feelings of isolation, increased social support, decreased emotional suppression, and enhanced satisfaction with overall quality of life.14-17 Studies of veterans with PTSD have found less attrition among those who chose group therapy compared with individual therapy.14,18 Group psychotherapy improves access to care by enabling delivery to more patients.14 When compared with individual therapy, the group format allows for a large number of patients to be treated simultaneously, maximizing resources and reducing costs.3,19-21
VISN 9 CRH Innovation
The VA provides care to veterans through regionally distinct administrative systems known as Veterans Integrated Service Networks (VISNs). Clinical resource hubs (CRH) are VISN-based programs created to cover VA staffing shortages by virtually deploying HCPs into local VA systems until vacancies are filled. The national CRH vision of effectively using resources and innovative technologies to meet veterans’ health care needs, along with the above-referenced clinical gaps in the delivery of care, inspired the development of VIP Boot Camp within the VISN 9 CRH.22
Program Description
VIP Boot Camp is an evidence-informed group psychotherapy program designed to provide timely, brief, and comprehensive mental health treatment for veterans. VIP Boot Camp was developed to address the needs of veterans accessing PCMHI services who experience ≥ 1 of the often overlapping problems of anxiety/emotion regulation/stress, sleep difficulties, and chronic pain (Figure). VIP Boot Camp uses an integrative approach to highlight interconnections and similarities among these difficulties and their treatment. A primary vision of the program is to provide this comprehensive treatment within PCMHI (upstream) so additional referrals to higher levels of mental health care (downstream) may not be needed.
This design is intentional because it increases the number of individuals who can be treated upstream with comprehensive, preventive, and proactive care within PCMHI which, over time, frees up resources in the BHIP for individuals requiring higher levels of care. This approach also aligns with the importance of early treatment for chronic pain and sleep disturbances, which are linked to increased risk of suicide attempts and deaths by suicide for veterans.5 National interest for VIP Boot Camp grew during fiscal year 2024 after it received the Gold Medal Recognition for Most Adoptable and Greatest Potential for Impact during VHA National Access Sprint Wave 3—Mental Health Call of Champions.
History
VIP Boot Camp began in August 2021 at VISN 9 as a 6-week virtual group for veterans with chronic pain. It was established to assist a large VA medical center experiencing PCMHI staffing shortages and lacking available PCMHI groups. Many veterans in the chronic pain group discussed co-occurring issues such as sleep disturbances, anxiety, and stress. The CRH team considered launching 2 separate groups to address these additional PCMHI-level issues; however, in developing the group material which drew from multiple clinical approaches, the team recognized significant overlapping and interconnected themes.
The team discussed EBPs within the VHA and how certain interventions within these treatments could be helpful across many other co-occurring disorders. Integrated tactics (clinical interventions) were drawn from cognitive-behavioral therapy (for depression, insomnia, or chronic pain), acceptance and commitment therapy, prolonged exposure, cognitive processing therapy, dialectical behavior therapy, unified protocol, pain reprocessing therapy, emotional awareness and expression therapy, interpersonal neurobiology, and mindfulness. We collaborated with veterans during VIP Boot Camp groups to determine how to present and discuss complex interventions in ways that were clinically accurate, understandable, relatable, and relevant to their experiences.
To address accessibility issues, the chronic pain group was reduced to 4 weeks. A second 4-week module for anxiety, emotion regulation, and stress was developed, mirroring the tactics, language, and integrative approach of the revised chronic pain module. A similar integrative approach led to the development of the third and final 4-week module for sleep disturbances.
Current Program
The VIP Boot Camp consists of three 4-week integrated modules, each highlighting a critical area: sleep disturbances (Improving Sleep), chronic pain difficulties (Outsmarting Chronic Pain), and emotion regulation difficulties (Rewiring Your Brain). VIP Boot Camp is designed for veterans who are at the PCMHI level of care. Referrals are accepted for patients receiving treatment from primary care or PCMHI.
Guidelines for participation in VIP Boot Camp may differ across sites or VISNs. For example, a veteran who has been referred to the BHIP for medication management only or to a specialty MHC such as a pain clinic or PTSD clinic might also be appropriate and eligible for VIP Boot Camp.
Given the interconnectedness of foundational themes, elements, and practices across the VIP Boot Camp modules, the modules are offered in a rolling format with a veteran-centric “choose your own adventure” approach. Tactics are presented in the modules in a way that allows patients to begin with any 1 of the 3 modules and receive treatment that will help in the other areas. Participants choose their core module and initial treatment focus based on their values, needs, and goals. Individuals who complete a core module can end their VIP Boot Camp experience or continue to the next 4-week module for up to 3 modules.
The group is open to new individuals at the start of any 4-week module and closed for the remainder of its 4-week duration. This innovative rolling modular approach combines elements of open- and closed-group format, allowing for the flexibility and accessibility of an open group with the stability and peer support of a closed group.
Given the complicated and overlapping nature of chronic pain, emotion regulation/ stress, and sleep disturbances, VIP Boot Camp acknowledges that everything is interconnected and difficulties in 1 area may impact other areas. The 3 interconnected modules with repeating themes provide coherence and consistency. Veterans learn how interconnections across difficulties can be leveraged so that tactics learned and practiced in 1 area can assist in other areas, changing the cycle of suffering into a cycle of growth.
VIP Boot Camp sessions are 90 minutes long, once weekly for 4 weeks, with 2 mental health professionals trained to lead a dynamic group psychotherapy experience that aims to be fun for participants. VIP Boot Camp synthesizes evidence-based and evidence-informed interventions, as well as techniques from VHA complementary and integrative health programs, psychoeducation, and interpersonal interventions that model connection, playfulness, and healthy boundaries. These varied strategies combine to equip veterans with practical tactics for self-management outside of sessions, a process described as “finding puzzle pieces.” VIP Boot Camp is built on the idea that people are more likely to adopt and practice any tactic after being taught why that tactic is important, and how it fits into their larger interconnected puzzle. After each session, participants are provided with additional asynchronous educational material to help reinforce their learnings and practices.
Although individuals may hesitate to participate in a group setting, they often find the experience of community enhances and accelerates their treatment and gains. This involvement is highlighted in a core aspect of a VIP Boot Camp session called wins, during which participants learn how others on their Boot Camp team are implementing new skills and moving toward their personal values and objectives in a stepwise manner. Through these shared experiences, veterans discover how tactics working for others may serve as a model for their own personal objectives and plans for practice. The sense of relief described by many upon realizing they are not alone in their experiences, along with the satisfaction felt in discovering their ability to support others in Boot Camp, is described by many participants as deeply meaningful and in line with their personal values.
While developed as a fully virtual group program, VIP Boot Camp can also be conducted in person. The virtual program has been successful and continues to spread across VISN 9. There are 8 virtual VIP Boot Camps running in VISN 9, with plans for continued expansion. In the VISN 9 CRH, Boot Camps typically have 10 to 12 participants. Additionally, as VIP Boot Camp grows within a location there are frequently sufficient referrals to support a second rolling group, which enables staggering of the module offerings to allow for even more timely treatment.
Training Program
VISN 9 CRH also developed a VIP Boot Camp 3-day intensive training program for PCMHI HCPs that consists of learning and practicing VIP Boot Camp material for chronic pain, emotion regulation/ stress, sleep disturbances, mindfulness, and guided imagery, along with gaining experience as a VIP Boot Camp coleader. Feedback received from PCMHI HCPs who completed training has been positive. There is also a private Microsoft Teams channel for HCPs, which allows for resource sharing and community building among coleaders. More than 75 PCMHI HCPs have completed VIP Boot Camp training and > 25 VIP Boot Camps have been established at 4 additional VISNs.
The VISN 9 CRH VIP Boot Camp program initiated an implementation and effectiveness project with the Michael E. DeBakey VA Medical Center and the South Central Mental Illness Research, Education and Clinical Center. The focus of this collaboration is support for implementation and treatment effectiveness research with reports, articles, and a white paper on findings and best practices, alongside continued dissemination of the VIP Boot Camp program and training.
Conclusions
VIP Boot Camp is a PCMHI group program offering readily available, comprehensive, and integrative group psychotherapy services to veterans experiencing . 1 of the following: chronic pain, emotion regulation/ stress, and sleep disturbances. It was launched at the VISN 9 CRH with a goal of addressing clinical gaps in the delivery of mental health care, by increasing the number of patients treated within PCMHI. The VIP Boot Camp model provides veterans the opportunity to transform cycles of suffering into cycles of growth through a single approach that can address multiple presenting and interconnected issues.
A 3-day VIP Boot Camp training program provides a quick and effective path for a PCMHI program to train HCPs to launch a VIP Boot Camp. The VISN 9 CRH will continue to champion VIP Boot Camp as a model for the successful provision of comprehensive and integrative mental health treatment within PCMHI at the VA. Through readily available access to comprehensive mental health treatment in an environment that promotes participant empowerment and social engagement, VIP Boot Camp represents an integrative and innovative model of mental health treatment that offers benefits to veteran participants, HCPs, and the VHA.
- Leung LB, Yoon J, Escarce JJ, et al. Primary care-mental health integration in the VA: shifting mental health services for common mental illnesses to primary care. Psychiatr Serv. 2018;69:403-409. doi:10.1176/appi.ps.201700190
- Zhang A, Park S, Sullivan JE, et al. The effectiveness of problem-solving therapy for primary care patients’ depressive and/or anxiety disorders: a systematic review and meta-analysis. J Am Board Fam Med. 2018;31:139-150. doi:10.3122/jabfm.2018.01.170270
- Hundt NE, Barrera TL, Robinson A, et al. A systematic review of cognitive behavioral therapy for depression in veterans. Mil Med. 2014;179:942-949. doi:10.7205/milmed-d-14-00128
- Jank R, Gallee A, Boeckle M, et al. Chronic pain and sleep disorders in primary care. Pain Res Treat. 2017;2017:1-9. doi:10.1155/2017/9081802
- Ashrafioun L, Bishop TM, Pigeon WR. The relationship between pain severity, insomnia, and suicide attempts among a national veteran sample initiating pain care. Psychosom Med. 2021;83:733- 738. doi:10.1097/psy.0000000000000975
- Ramanuj P, Ferenchik E, Docherty M, et al. Evolving models of integrated behavioral health and primary care. Curr Psychiatry Rep. 2019;21:1. doi:10.1007/s11920-019-0985-4
- Post EP, Metzger M, Dumas P, et al. Integrating mental health into primary care within the Veterans Health Administration. Fam Syst Health. 2010;28:83-90. doi:10.1037/a0020130
- Smith TL, Kim B, Benzer JK, et al. FLOW: early results from a clinical demonstration project to improve the transition of patients with mental health disorders back to primary care. Psychol Serv. 2021;18:23-32. doi:10.1037/ser0000336
- Kearney LK, Post EP, Pomerantz AS, et al. Applying the interprofessional patient aligned care team in the department of veterans affairs transforming primary care. Am Psychol. 2014;69(4):399-408. doi:10.1037/a0035909
- US Government Accountability Office. Veterans health care: staffing challenges persist for fully integrating mental health and primary care services. December 15, 2022. Accessed July 9, 2025. https://www.gao.gov/products/gao-23-105372
- National Academies of Science and Engineering. Evaluation of the Department of Veterans Affairs Mental Health Services. National Academies Press; 2018. Accessed July 9, 2025. https://nap.nationalacademies.org/catalog/24915/evaluation-of-the-department-of-veterans-affairs-mental-health-services
- US Department of Veterans Affairs. Blueprint for excellence: achieving veterans’ excellence. October 6, 2014. Accessed July 9, 2025. https://www.volunteer.va.gov/docs/blueprintforexcellence_factsheet.PDF
- US Department of Veterans Affairs. Department of Veterans Affairs FY 2018-2024 strategic plan. Accessed July 9, 2025. https://www.calvet.ca.gov/Regulations/USDVA%20Strategic%20Plan%202018-2024.pdf
- Sripada RK, Bohnert KM, Ganoczy D, et al. Initial group versus individual therapy for posttraumatic stress disorder and subsequent follow-up treatment adequacy. Psychol Serv. 2016;13:349-355. doi:10.1037/ser0000077
- Burnett-Zeigler IE, Pfeiffer P, Zivin K, et al. Psychotherapy utilization for acute depression within the Veterans Affairs health care system. Psychol Serv. 2012;9:325-335. doi:10.1037/a0027957
- Kim JS, Prins A, Hirschhorn EW, et al. Preliminary investigation into the effectiveness of group webSTAIR for trauma-exposed veterans in primary care. Mil Med. 2024;189:e1403-e1408. doi:10.1093/milmed/usae052
- Jakupcak M, Blais RK, Grossbard J, et al. “Toughness” in association with mental health symptoms among Iraq and Afghanistan war veterans seeking Veterans Affairs health care. Psychol Men Masc. 2014;15:100-104. doi:10.1037/a0031508
- Stoycos SA, Berzenski SR, Beck JG, et al. Predictors of treatment completion in group psychotherapy for male veterans with posttraumatic stress disorder. J Trauma Stress. 2023;36:346-358. doi:10.1002/jts.22915
- Possemato K. The current state of intervention research for posttraumatic stress disorder within the primary care setting. J Clin Psychol Med Settings. 2011;18:268-280. doi:10.1007/s10880-011-9237-4
- Hunt MG, Rosenheck RA. Psychotherapy in mental health clinics of the Department of Veterans Affairs. J Clin Psychol. 2011;67:561-573. doi:10.1002/jclp.20788
- Khatri N, Marziali E, Tchernikov I, et al. Comparing telehealth-based and clinic-based group cognitive behavioral therapy for adults with depression and anxiety: a pilot study. Clin Interv Aging. 2014;9:765. doi:10.2147/cia.s57832
- Dangel J. Clinical resource hub increases veterans' access to care. VA News. January 12, 2025. Accessed September 3, 2025. https://news.va.gov/137439/clinical-resource-hub-increases-access-to-care/
Since 2007, Primary Care Mental Health Integration (PCMHI) at the Veterans Health Administration (VHA) has improved access to mental health care services for veterans by directly embedding mental health care professionals (HCPs) within primary care teams.1 Veterans referred to PCMHI often have co-occurring physical and mental health disorders.2 Untreated chronic physical and mental comorbidities can diminish the effectiveness of medical and mental health interventions. Growing evidence suggests that treatment of mental health conditions can improve physical health outcomes and management of physical conditions can improve mental health outcomes.2,3
Chronic pain and sleep disorders are common reasons patients present to primary care, and often coexist together with mental health comorbidities.4 Sleep disorders affect 50% to 88% of patients with chronic pain, and 40% of patients with sleep disorders report chronic pain.4 Research has found that chronic pain and sleep disorders increase the risk of suicide attempts and deaths by suicide. Addressing suicide prevention simultaneously with treating chronic pain and insomnia is encouraged.5
Background
PCMHI treats physical and mental health comorbidities with a collaborative framework and a biopsychosocial integrative model.6 PCMHI staff provide mental health services as members of primary care teams. An interdisciplinary PCMHI team can include, but is not limited to, psychologists, mental health social workers, psychiatrists, nurse practitioners, clinical pharmacists, and mental health nurses. Quality of care within this model is elevated, as mental and physical health are recognized as interconnected. Collaboration between primary care and mental health benefits veterans and the VHA by increasing access to mental health care, decreasing stigma associated with mental health treatment, improving health outcomes, and enhancing the likelihood of recovery, resulting in high patient satisfaction.6-8
In the existing PCMHI model, HCPs are encouraged to use short-term, evidence-based psychotherapies (EBPs).9 Veterans referred to PCMHI from primary care are typically able to attend 1 to 6 brief sessions of mental health treatment, often 20 to 30 minutes long. Most EBPs in PCMHI are disorder- specific, providing interventions focused on a single presenting problem (eg, insomnia, chronic pain, or posttraumatic stress disorder [PTSD]). For veterans with a single issue, this model can be very effective. 1,10 However, the high rate of co-occurrence of mental and physical health issues can make it difficult to fully treat interrelated problems if the focus is on 1 specific diagnosis. Veterans with a need for additional (more comprehensive or intensive) mental health treatment are frequently referred to a higher, more resource-intensive level of mental health care, either in the VHA or the community. Examples of higher levels of mental health care include the longer term behavioral health interdisciplinary program (BHIP), sometimes called a mental health clinic (MHC), or a specialty mental health program such as a PTSD clinic.
As PCMHI continues to grow, new challenges have emerged related to staffing shortages and gaps in the clinical delivery of mental health treatment within the VHA. At the same time, demand for VHA mental health treatment has increased. However, a mental health professional shortage severely limits the ability of the VHA to meet this demand. In many systems, this shortage may result in more referrals being made to a higher level of mental health care because of fewer resources to provide comprehensive treatment in a less intensive PCMHI setting.8,10,11 This referral pattern can overburden higher level care, often with long wait times for treatment and lengthy lag times between appointments. Furthermore, these gaps in the clinical delivery of care cannot be effectively addressed by hiring additional mental health professionals. This strain on resources can impede access to care and negatively affect outcomes.10
Recent congressional reports highlight these issues, noting that demand for mental health services continues to outpace the capacity of both PCMHI and higher levels of mental health care, leading to delays in treatment that may negatively affect outcomes.8,10,11 These delays can be particularly detrimental for individuals with conditions requiring timely intervention.8,11 Some veterans are willing to engage with PCMHI in a primary care setting but may be reluctant to engage in general mental health treatment. These veterans might not receive the mental health care they need without PCMHI.
Group Psychotherapy
A group psychotherapy format can address gaps in care delivery and provide advantages for patients, mental health professionals, and the VHA. Group psychotherapy aligns with the US Department of Veterans Affairs (VA) 2018 Blueprint for Excellence and 2018 to 2024 strategic plan, underscoring the need for more timely and efficient mental health services.12,13
Benefits of group psychotherapy include reductions in symptoms, decreased feelings of isolation, increased social support, decreased emotional suppression, and enhanced satisfaction with overall quality of life.14-17 Studies of veterans with PTSD have found less attrition among those who chose group therapy compared with individual therapy.14,18 Group psychotherapy improves access to care by enabling delivery to more patients.14 When compared with individual therapy, the group format allows for a large number of patients to be treated simultaneously, maximizing resources and reducing costs.3,19-21
VISN 9 CRH Innovation
The VA provides care to veterans through regionally distinct administrative systems known as Veterans Integrated Service Networks (VISNs). Clinical resource hubs (CRH) are VISN-based programs created to cover VA staffing shortages by virtually deploying HCPs into local VA systems until vacancies are filled. The national CRH vision of effectively using resources and innovative technologies to meet veterans’ health care needs, along with the above-referenced clinical gaps in the delivery of care, inspired the development of VIP Boot Camp within the VISN 9 CRH.22
Program Description
VIP Boot Camp is an evidence-informed group psychotherapy program designed to provide timely, brief, and comprehensive mental health treatment for veterans. VIP Boot Camp was developed to address the needs of veterans accessing PCMHI services who experience ≥ 1 of the often overlapping problems of anxiety/emotion regulation/stress, sleep difficulties, and chronic pain (Figure). VIP Boot Camp uses an integrative approach to highlight interconnections and similarities among these difficulties and their treatment. A primary vision of the program is to provide this comprehensive treatment within PCMHI (upstream) so additional referrals to higher levels of mental health care (downstream) may not be needed.

This design is intentional because it increases the number of individuals who can be treated upstream with comprehensive, preventive, and proactive care within PCMHI which, over time, frees up resources in the BHIP for individuals requiring higher levels of care. This approach also aligns with the importance of early treatment for chronic pain and sleep disturbances, which are linked to increased risk of suicide attempts and deaths by suicide for veterans.5 National interest for VIP Boot Camp grew during fiscal year 2024 after it received the Gold Medal Recognition for Most Adoptable and Greatest Potential for Impact during VHA National Access Sprint Wave 3—Mental Health Call of Champions.
History
VIP Boot Camp began in August 2021 at VISN 9 as a 6-week virtual group for veterans with chronic pain. It was established to assist a large VA medical center experiencing PCMHI staffing shortages and lacking available PCMHI groups. Many veterans in the chronic pain group discussed co-occurring issues such as sleep disturbances, anxiety, and stress. The CRH team considered launching 2 separate groups to address these additional PCMHI-level issues; however, in developing the group material which drew from multiple clinical approaches, the team recognized significant overlapping and interconnected themes.
The team discussed EBPs within the VHA and how certain interventions within these treatments could be helpful across many other co-occurring disorders. Integrated tactics (clinical interventions) were drawn from cognitive-behavioral therapy (for depression, insomnia, or chronic pain), acceptance and commitment therapy, prolonged exposure, cognitive processing therapy, dialectical behavior therapy, unified protocol, pain reprocessing therapy, emotional awareness and expression therapy, interpersonal neurobiology, and mindfulness. We collaborated with veterans during VIP Boot Camp groups to determine how to present and discuss complex interventions in ways that were clinically accurate, understandable, relatable, and relevant to their experiences.
To address accessibility issues, the chronic pain group was reduced to 4 weeks. A second 4-week module for anxiety, emotion regulation, and stress was developed, mirroring the tactics, language, and integrative approach of the revised chronic pain module. A similar integrative approach led to the development of the third and final 4-week module for sleep disturbances.
Current Program
The VIP Boot Camp consists of three 4-week integrated modules, each highlighting a critical area: sleep disturbances (Improving Sleep), chronic pain difficulties (Outsmarting Chronic Pain), and emotion regulation difficulties (Rewiring Your Brain). VIP Boot Camp is designed for veterans who are at the PCMHI level of care. Referrals are accepted for patients receiving treatment from primary care or PCMHI.
Guidelines for participation in VIP Boot Camp may differ across sites or VISNs. For example, a veteran who has been referred to the BHIP for medication management only or to a specialty MHC such as a pain clinic or PTSD clinic might also be appropriate and eligible for VIP Boot Camp.
Given the interconnectedness of foundational themes, elements, and practices across the VIP Boot Camp modules, the modules are offered in a rolling format with a veteran-centric “choose your own adventure” approach. Tactics are presented in the modules in a way that allows patients to begin with any 1 of the 3 modules and receive treatment that will help in the other areas. Participants choose their core module and initial treatment focus based on their values, needs, and goals. Individuals who complete a core module can end their VIP Boot Camp experience or continue to the next 4-week module for up to 3 modules.
The group is open to new individuals at the start of any 4-week module and closed for the remainder of its 4-week duration. This innovative rolling modular approach combines elements of open- and closed-group format, allowing for the flexibility and accessibility of an open group with the stability and peer support of a closed group.
Given the complicated and overlapping nature of chronic pain, emotion regulation/ stress, and sleep disturbances, VIP Boot Camp acknowledges that everything is interconnected and difficulties in 1 area may impact other areas. The 3 interconnected modules with repeating themes provide coherence and consistency. Veterans learn how interconnections across difficulties can be leveraged so that tactics learned and practiced in 1 area can assist in other areas, changing the cycle of suffering into a cycle of growth.
VIP Boot Camp sessions are 90 minutes long, once weekly for 4 weeks, with 2 mental health professionals trained to lead a dynamic group psychotherapy experience that aims to be fun for participants. VIP Boot Camp synthesizes evidence-based and evidence-informed interventions, as well as techniques from VHA complementary and integrative health programs, psychoeducation, and interpersonal interventions that model connection, playfulness, and healthy boundaries. These varied strategies combine to equip veterans with practical tactics for self-management outside of sessions, a process described as “finding puzzle pieces.” VIP Boot Camp is built on the idea that people are more likely to adopt and practice any tactic after being taught why that tactic is important, and how it fits into their larger interconnected puzzle. After each session, participants are provided with additional asynchronous educational material to help reinforce their learnings and practices.
Although individuals may hesitate to participate in a group setting, they often find the experience of community enhances and accelerates their treatment and gains. This involvement is highlighted in a core aspect of a VIP Boot Camp session called wins, during which participants learn how others on their Boot Camp team are implementing new skills and moving toward their personal values and objectives in a stepwise manner. Through these shared experiences, veterans discover how tactics working for others may serve as a model for their own personal objectives and plans for practice. The sense of relief described by many upon realizing they are not alone in their experiences, along with the satisfaction felt in discovering their ability to support others in Boot Camp, is described by many participants as deeply meaningful and in line with their personal values.
While developed as a fully virtual group program, VIP Boot Camp can also be conducted in person. The virtual program has been successful and continues to spread across VISN 9. There are 8 virtual VIP Boot Camps running in VISN 9, with plans for continued expansion. In the VISN 9 CRH, Boot Camps typically have 10 to 12 participants. Additionally, as VIP Boot Camp grows within a location there are frequently sufficient referrals to support a second rolling group, which enables staggering of the module offerings to allow for even more timely treatment.
Training Program
VISN 9 CRH also developed a VIP Boot Camp 3-day intensive training program for PCMHI HCPs that consists of learning and practicing VIP Boot Camp material for chronic pain, emotion regulation/ stress, sleep disturbances, mindfulness, and guided imagery, along with gaining experience as a VIP Boot Camp coleader. Feedback received from PCMHI HCPs who completed training has been positive. There is also a private Microsoft Teams channel for HCPs, which allows for resource sharing and community building among coleaders. More than 75 PCMHI HCPs have completed VIP Boot Camp training and > 25 VIP Boot Camps have been established at 4 additional VISNs.
The VISN 9 CRH VIP Boot Camp program initiated an implementation and effectiveness project with the Michael E. DeBakey VA Medical Center and the South Central Mental Illness Research, Education and Clinical Center. The focus of this collaboration is support for implementation and treatment effectiveness research with reports, articles, and a white paper on findings and best practices, alongside continued dissemination of the VIP Boot Camp program and training.
Conclusions
VIP Boot Camp is a PCMHI group program offering readily available, comprehensive, and integrative group psychotherapy services to veterans experiencing . 1 of the following: chronic pain, emotion regulation/ stress, and sleep disturbances. It was launched at the VISN 9 CRH with a goal of addressing clinical gaps in the delivery of mental health care, by increasing the number of patients treated within PCMHI. The VIP Boot Camp model provides veterans the opportunity to transform cycles of suffering into cycles of growth through a single approach that can address multiple presenting and interconnected issues.
A 3-day VIP Boot Camp training program provides a quick and effective path for a PCMHI program to train HCPs to launch a VIP Boot Camp. The VISN 9 CRH will continue to champion VIP Boot Camp as a model for the successful provision of comprehensive and integrative mental health treatment within PCMHI at the VA. Through readily available access to comprehensive mental health treatment in an environment that promotes participant empowerment and social engagement, VIP Boot Camp represents an integrative and innovative model of mental health treatment that offers benefits to veteran participants, HCPs, and the VHA.
Since 2007, Primary Care Mental Health Integration (PCMHI) at the Veterans Health Administration (VHA) has improved access to mental health care services for veterans by directly embedding mental health care professionals (HCPs) within primary care teams.1 Veterans referred to PCMHI often have co-occurring physical and mental health disorders.2 Untreated chronic physical and mental comorbidities can diminish the effectiveness of medical and mental health interventions. Growing evidence suggests that treatment of mental health conditions can improve physical health outcomes and management of physical conditions can improve mental health outcomes.2,3
Chronic pain and sleep disorders are common reasons patients present to primary care, and often coexist together with mental health comorbidities.4 Sleep disorders affect 50% to 88% of patients with chronic pain, and 40% of patients with sleep disorders report chronic pain.4 Research has found that chronic pain and sleep disorders increase the risk of suicide attempts and deaths by suicide. Addressing suicide prevention simultaneously with treating chronic pain and insomnia is encouraged.5
Background
PCMHI treats physical and mental health comorbidities with a collaborative framework and a biopsychosocial integrative model.6 PCMHI staff provide mental health services as members of primary care teams. An interdisciplinary PCMHI team can include, but is not limited to, psychologists, mental health social workers, psychiatrists, nurse practitioners, clinical pharmacists, and mental health nurses. Quality of care within this model is elevated, as mental and physical health are recognized as interconnected. Collaboration between primary care and mental health benefits veterans and the VHA by increasing access to mental health care, decreasing stigma associated with mental health treatment, improving health outcomes, and enhancing the likelihood of recovery, resulting in high patient satisfaction.6-8
In the existing PCMHI model, HCPs are encouraged to use short-term, evidence-based psychotherapies (EBPs).9 Veterans referred to PCMHI from primary care are typically able to attend 1 to 6 brief sessions of mental health treatment, often 20 to 30 minutes long. Most EBPs in PCMHI are disorder- specific, providing interventions focused on a single presenting problem (eg, insomnia, chronic pain, or posttraumatic stress disorder [PTSD]). For veterans with a single issue, this model can be very effective. 1,10 However, the high rate of co-occurrence of mental and physical health issues can make it difficult to fully treat interrelated problems if the focus is on 1 specific diagnosis. Veterans with a need for additional (more comprehensive or intensive) mental health treatment are frequently referred to a higher, more resource-intensive level of mental health care, either in the VHA or the community. Examples of higher levels of mental health care include the longer term behavioral health interdisciplinary program (BHIP), sometimes called a mental health clinic (MHC), or a specialty mental health program such as a PTSD clinic.
As PCMHI continues to grow, new challenges have emerged related to staffing shortages and gaps in the clinical delivery of mental health treatment within the VHA. At the same time, demand for VHA mental health treatment has increased. However, a mental health professional shortage severely limits the ability of the VHA to meet this demand. In many systems, this shortage may result in more referrals being made to a higher level of mental health care because of fewer resources to provide comprehensive treatment in a less intensive PCMHI setting.8,10,11 This referral pattern can overburden higher level care, often with long wait times for treatment and lengthy lag times between appointments. Furthermore, these gaps in the clinical delivery of care cannot be effectively addressed by hiring additional mental health professionals. This strain on resources can impede access to care and negatively affect outcomes.10
Recent congressional reports highlight these issues, noting that demand for mental health services continues to outpace the capacity of both PCMHI and higher levels of mental health care, leading to delays in treatment that may negatively affect outcomes.8,10,11 These delays can be particularly detrimental for individuals with conditions requiring timely intervention.8,11 Some veterans are willing to engage with PCMHI in a primary care setting but may be reluctant to engage in general mental health treatment. These veterans might not receive the mental health care they need without PCMHI.
Group Psychotherapy
A group psychotherapy format can address gaps in care delivery and provide advantages for patients, mental health professionals, and the VHA. Group psychotherapy aligns with the US Department of Veterans Affairs (VA) 2018 Blueprint for Excellence and 2018 to 2024 strategic plan, underscoring the need for more timely and efficient mental health services.12,13
Benefits of group psychotherapy include reductions in symptoms, decreased feelings of isolation, increased social support, decreased emotional suppression, and enhanced satisfaction with overall quality of life.14-17 Studies of veterans with PTSD have found less attrition among those who chose group therapy compared with individual therapy.14,18 Group psychotherapy improves access to care by enabling delivery to more patients.14 When compared with individual therapy, the group format allows for a large number of patients to be treated simultaneously, maximizing resources and reducing costs.3,19-21
VISN 9 CRH Innovation
The VA provides care to veterans through regionally distinct administrative systems known as Veterans Integrated Service Networks (VISNs). Clinical resource hubs (CRH) are VISN-based programs created to cover VA staffing shortages by virtually deploying HCPs into local VA systems until vacancies are filled. The national CRH vision of effectively using resources and innovative technologies to meet veterans’ health care needs, along with the above-referenced clinical gaps in the delivery of care, inspired the development of VIP Boot Camp within the VISN 9 CRH.22
Program Description
VIP Boot Camp is an evidence-informed group psychotherapy program designed to provide timely, brief, and comprehensive mental health treatment for veterans. VIP Boot Camp was developed to address the needs of veterans accessing PCMHI services who experience ≥ 1 of the often overlapping problems of anxiety/emotion regulation/stress, sleep difficulties, and chronic pain (Figure). VIP Boot Camp uses an integrative approach to highlight interconnections and similarities among these difficulties and their treatment. A primary vision of the program is to provide this comprehensive treatment within PCMHI (upstream) so additional referrals to higher levels of mental health care (downstream) may not be needed.

This design is intentional because it increases the number of individuals who can be treated upstream with comprehensive, preventive, and proactive care within PCMHI which, over time, frees up resources in the BHIP for individuals requiring higher levels of care. This approach also aligns with the importance of early treatment for chronic pain and sleep disturbances, which are linked to increased risk of suicide attempts and deaths by suicide for veterans.5 National interest for VIP Boot Camp grew during fiscal year 2024 after it received the Gold Medal Recognition for Most Adoptable and Greatest Potential for Impact during VHA National Access Sprint Wave 3—Mental Health Call of Champions.
History
VIP Boot Camp began in August 2021 at VISN 9 as a 6-week virtual group for veterans with chronic pain. It was established to assist a large VA medical center experiencing PCMHI staffing shortages and lacking available PCMHI groups. Many veterans in the chronic pain group discussed co-occurring issues such as sleep disturbances, anxiety, and stress. The CRH team considered launching 2 separate groups to address these additional PCMHI-level issues; however, in developing the group material which drew from multiple clinical approaches, the team recognized significant overlapping and interconnected themes.
The team discussed EBPs within the VHA and how certain interventions within these treatments could be helpful across many other co-occurring disorders. Integrated tactics (clinical interventions) were drawn from cognitive-behavioral therapy (for depression, insomnia, or chronic pain), acceptance and commitment therapy, prolonged exposure, cognitive processing therapy, dialectical behavior therapy, unified protocol, pain reprocessing therapy, emotional awareness and expression therapy, interpersonal neurobiology, and mindfulness. We collaborated with veterans during VIP Boot Camp groups to determine how to present and discuss complex interventions in ways that were clinically accurate, understandable, relatable, and relevant to their experiences.
To address accessibility issues, the chronic pain group was reduced to 4 weeks. A second 4-week module for anxiety, emotion regulation, and stress was developed, mirroring the tactics, language, and integrative approach of the revised chronic pain module. A similar integrative approach led to the development of the third and final 4-week module for sleep disturbances.
Current Program
The VIP Boot Camp consists of three 4-week integrated modules, each highlighting a critical area: sleep disturbances (Improving Sleep), chronic pain difficulties (Outsmarting Chronic Pain), and emotion regulation difficulties (Rewiring Your Brain). VIP Boot Camp is designed for veterans who are at the PCMHI level of care. Referrals are accepted for patients receiving treatment from primary care or PCMHI.
Guidelines for participation in VIP Boot Camp may differ across sites or VISNs. For example, a veteran who has been referred to the BHIP for medication management only or to a specialty MHC such as a pain clinic or PTSD clinic might also be appropriate and eligible for VIP Boot Camp.
Given the interconnectedness of foundational themes, elements, and practices across the VIP Boot Camp modules, the modules are offered in a rolling format with a veteran-centric “choose your own adventure” approach. Tactics are presented in the modules in a way that allows patients to begin with any 1 of the 3 modules and receive treatment that will help in the other areas. Participants choose their core module and initial treatment focus based on their values, needs, and goals. Individuals who complete a core module can end their VIP Boot Camp experience or continue to the next 4-week module for up to 3 modules.
The group is open to new individuals at the start of any 4-week module and closed for the remainder of its 4-week duration. This innovative rolling modular approach combines elements of open- and closed-group format, allowing for the flexibility and accessibility of an open group with the stability and peer support of a closed group.
Given the complicated and overlapping nature of chronic pain, emotion regulation/ stress, and sleep disturbances, VIP Boot Camp acknowledges that everything is interconnected and difficulties in 1 area may impact other areas. The 3 interconnected modules with repeating themes provide coherence and consistency. Veterans learn how interconnections across difficulties can be leveraged so that tactics learned and practiced in 1 area can assist in other areas, changing the cycle of suffering into a cycle of growth.
VIP Boot Camp sessions are 90 minutes long, once weekly for 4 weeks, with 2 mental health professionals trained to lead a dynamic group psychotherapy experience that aims to be fun for participants. VIP Boot Camp synthesizes evidence-based and evidence-informed interventions, as well as techniques from VHA complementary and integrative health programs, psychoeducation, and interpersonal interventions that model connection, playfulness, and healthy boundaries. These varied strategies combine to equip veterans with practical tactics for self-management outside of sessions, a process described as “finding puzzle pieces.” VIP Boot Camp is built on the idea that people are more likely to adopt and practice any tactic after being taught why that tactic is important, and how it fits into their larger interconnected puzzle. After each session, participants are provided with additional asynchronous educational material to help reinforce their learnings and practices.
Although individuals may hesitate to participate in a group setting, they often find the experience of community enhances and accelerates their treatment and gains. This involvement is highlighted in a core aspect of a VIP Boot Camp session called wins, during which participants learn how others on their Boot Camp team are implementing new skills and moving toward their personal values and objectives in a stepwise manner. Through these shared experiences, veterans discover how tactics working for others may serve as a model for their own personal objectives and plans for practice. The sense of relief described by many upon realizing they are not alone in their experiences, along with the satisfaction felt in discovering their ability to support others in Boot Camp, is described by many participants as deeply meaningful and in line with their personal values.
While developed as a fully virtual group program, VIP Boot Camp can also be conducted in person. The virtual program has been successful and continues to spread across VISN 9. There are 8 virtual VIP Boot Camps running in VISN 9, with plans for continued expansion. In the VISN 9 CRH, Boot Camps typically have 10 to 12 participants. Additionally, as VIP Boot Camp grows within a location there are frequently sufficient referrals to support a second rolling group, which enables staggering of the module offerings to allow for even more timely treatment.
Training Program
VISN 9 CRH also developed a VIP Boot Camp 3-day intensive training program for PCMHI HCPs that consists of learning and practicing VIP Boot Camp material for chronic pain, emotion regulation/ stress, sleep disturbances, mindfulness, and guided imagery, along with gaining experience as a VIP Boot Camp coleader. Feedback received from PCMHI HCPs who completed training has been positive. There is also a private Microsoft Teams channel for HCPs, which allows for resource sharing and community building among coleaders. More than 75 PCMHI HCPs have completed VIP Boot Camp training and > 25 VIP Boot Camps have been established at 4 additional VISNs.
The VISN 9 CRH VIP Boot Camp program initiated an implementation and effectiveness project with the Michael E. DeBakey VA Medical Center and the South Central Mental Illness Research, Education and Clinical Center. The focus of this collaboration is support for implementation and treatment effectiveness research with reports, articles, and a white paper on findings and best practices, alongside continued dissemination of the VIP Boot Camp program and training.
Conclusions
VIP Boot Camp is a PCMHI group program offering readily available, comprehensive, and integrative group psychotherapy services to veterans experiencing . 1 of the following: chronic pain, emotion regulation/ stress, and sleep disturbances. It was launched at the VISN 9 CRH with a goal of addressing clinical gaps in the delivery of mental health care, by increasing the number of patients treated within PCMHI. The VIP Boot Camp model provides veterans the opportunity to transform cycles of suffering into cycles of growth through a single approach that can address multiple presenting and interconnected issues.
A 3-day VIP Boot Camp training program provides a quick and effective path for a PCMHI program to train HCPs to launch a VIP Boot Camp. The VISN 9 CRH will continue to champion VIP Boot Camp as a model for the successful provision of comprehensive and integrative mental health treatment within PCMHI at the VA. Through readily available access to comprehensive mental health treatment in an environment that promotes participant empowerment and social engagement, VIP Boot Camp represents an integrative and innovative model of mental health treatment that offers benefits to veteran participants, HCPs, and the VHA.
- Leung LB, Yoon J, Escarce JJ, et al. Primary care-mental health integration in the VA: shifting mental health services for common mental illnesses to primary care. Psychiatr Serv. 2018;69:403-409. doi:10.1176/appi.ps.201700190
- Zhang A, Park S, Sullivan JE, et al. The effectiveness of problem-solving therapy for primary care patients’ depressive and/or anxiety disorders: a systematic review and meta-analysis. J Am Board Fam Med. 2018;31:139-150. doi:10.3122/jabfm.2018.01.170270
- Hundt NE, Barrera TL, Robinson A, et al. A systematic review of cognitive behavioral therapy for depression in veterans. Mil Med. 2014;179:942-949. doi:10.7205/milmed-d-14-00128
- Jank R, Gallee A, Boeckle M, et al. Chronic pain and sleep disorders in primary care. Pain Res Treat. 2017;2017:1-9. doi:10.1155/2017/9081802
- Ashrafioun L, Bishop TM, Pigeon WR. The relationship between pain severity, insomnia, and suicide attempts among a national veteran sample initiating pain care. Psychosom Med. 2021;83:733- 738. doi:10.1097/psy.0000000000000975
- Ramanuj P, Ferenchik E, Docherty M, et al. Evolving models of integrated behavioral health and primary care. Curr Psychiatry Rep. 2019;21:1. doi:10.1007/s11920-019-0985-4
- Post EP, Metzger M, Dumas P, et al. Integrating mental health into primary care within the Veterans Health Administration. Fam Syst Health. 2010;28:83-90. doi:10.1037/a0020130
- Smith TL, Kim B, Benzer JK, et al. FLOW: early results from a clinical demonstration project to improve the transition of patients with mental health disorders back to primary care. Psychol Serv. 2021;18:23-32. doi:10.1037/ser0000336
- Kearney LK, Post EP, Pomerantz AS, et al. Applying the interprofessional patient aligned care team in the department of veterans affairs transforming primary care. Am Psychol. 2014;69(4):399-408. doi:10.1037/a0035909
- US Government Accountability Office. Veterans health care: staffing challenges persist for fully integrating mental health and primary care services. December 15, 2022. Accessed July 9, 2025. https://www.gao.gov/products/gao-23-105372
- National Academies of Science and Engineering. Evaluation of the Department of Veterans Affairs Mental Health Services. National Academies Press; 2018. Accessed July 9, 2025. https://nap.nationalacademies.org/catalog/24915/evaluation-of-the-department-of-veterans-affairs-mental-health-services
- US Department of Veterans Affairs. Blueprint for excellence: achieving veterans’ excellence. October 6, 2014. Accessed July 9, 2025. https://www.volunteer.va.gov/docs/blueprintforexcellence_factsheet.PDF
- US Department of Veterans Affairs. Department of Veterans Affairs FY 2018-2024 strategic plan. Accessed July 9, 2025. https://www.calvet.ca.gov/Regulations/USDVA%20Strategic%20Plan%202018-2024.pdf
- Sripada RK, Bohnert KM, Ganoczy D, et al. Initial group versus individual therapy for posttraumatic stress disorder and subsequent follow-up treatment adequacy. Psychol Serv. 2016;13:349-355. doi:10.1037/ser0000077
- Burnett-Zeigler IE, Pfeiffer P, Zivin K, et al. Psychotherapy utilization for acute depression within the Veterans Affairs health care system. Psychol Serv. 2012;9:325-335. doi:10.1037/a0027957
- Kim JS, Prins A, Hirschhorn EW, et al. Preliminary investigation into the effectiveness of group webSTAIR for trauma-exposed veterans in primary care. Mil Med. 2024;189:e1403-e1408. doi:10.1093/milmed/usae052
- Jakupcak M, Blais RK, Grossbard J, et al. “Toughness” in association with mental health symptoms among Iraq and Afghanistan war veterans seeking Veterans Affairs health care. Psychol Men Masc. 2014;15:100-104. doi:10.1037/a0031508
- Stoycos SA, Berzenski SR, Beck JG, et al. Predictors of treatment completion in group psychotherapy for male veterans with posttraumatic stress disorder. J Trauma Stress. 2023;36:346-358. doi:10.1002/jts.22915
- Possemato K. The current state of intervention research for posttraumatic stress disorder within the primary care setting. J Clin Psychol Med Settings. 2011;18:268-280. doi:10.1007/s10880-011-9237-4
- Hunt MG, Rosenheck RA. Psychotherapy in mental health clinics of the Department of Veterans Affairs. J Clin Psychol. 2011;67:561-573. doi:10.1002/jclp.20788
- Khatri N, Marziali E, Tchernikov I, et al. Comparing telehealth-based and clinic-based group cognitive behavioral therapy for adults with depression and anxiety: a pilot study. Clin Interv Aging. 2014;9:765. doi:10.2147/cia.s57832
- Dangel J. Clinical resource hub increases veterans' access to care. VA News. January 12, 2025. Accessed September 3, 2025. https://news.va.gov/137439/clinical-resource-hub-increases-access-to-care/
- Leung LB, Yoon J, Escarce JJ, et al. Primary care-mental health integration in the VA: shifting mental health services for common mental illnesses to primary care. Psychiatr Serv. 2018;69:403-409. doi:10.1176/appi.ps.201700190
- Zhang A, Park S, Sullivan JE, et al. The effectiveness of problem-solving therapy for primary care patients’ depressive and/or anxiety disorders: a systematic review and meta-analysis. J Am Board Fam Med. 2018;31:139-150. doi:10.3122/jabfm.2018.01.170270
- Hundt NE, Barrera TL, Robinson A, et al. A systematic review of cognitive behavioral therapy for depression in veterans. Mil Med. 2014;179:942-949. doi:10.7205/milmed-d-14-00128
- Jank R, Gallee A, Boeckle M, et al. Chronic pain and sleep disorders in primary care. Pain Res Treat. 2017;2017:1-9. doi:10.1155/2017/9081802
- Ashrafioun L, Bishop TM, Pigeon WR. The relationship between pain severity, insomnia, and suicide attempts among a national veteran sample initiating pain care. Psychosom Med. 2021;83:733- 738. doi:10.1097/psy.0000000000000975
- Ramanuj P, Ferenchik E, Docherty M, et al. Evolving models of integrated behavioral health and primary care. Curr Psychiatry Rep. 2019;21:1. doi:10.1007/s11920-019-0985-4
- Post EP, Metzger M, Dumas P, et al. Integrating mental health into primary care within the Veterans Health Administration. Fam Syst Health. 2010;28:83-90. doi:10.1037/a0020130
- Smith TL, Kim B, Benzer JK, et al. FLOW: early results from a clinical demonstration project to improve the transition of patients with mental health disorders back to primary care. Psychol Serv. 2021;18:23-32. doi:10.1037/ser0000336
- Kearney LK, Post EP, Pomerantz AS, et al. Applying the interprofessional patient aligned care team in the department of veterans affairs transforming primary care. Am Psychol. 2014;69(4):399-408. doi:10.1037/a0035909
- US Government Accountability Office. Veterans health care: staffing challenges persist for fully integrating mental health and primary care services. December 15, 2022. Accessed July 9, 2025. https://www.gao.gov/products/gao-23-105372
- National Academies of Science and Engineering. Evaluation of the Department of Veterans Affairs Mental Health Services. National Academies Press; 2018. Accessed July 9, 2025. https://nap.nationalacademies.org/catalog/24915/evaluation-of-the-department-of-veterans-affairs-mental-health-services
- US Department of Veterans Affairs. Blueprint for excellence: achieving veterans’ excellence. October 6, 2014. Accessed July 9, 2025. https://www.volunteer.va.gov/docs/blueprintforexcellence_factsheet.PDF
- US Department of Veterans Affairs. Department of Veterans Affairs FY 2018-2024 strategic plan. Accessed July 9, 2025. https://www.calvet.ca.gov/Regulations/USDVA%20Strategic%20Plan%202018-2024.pdf
- Sripada RK, Bohnert KM, Ganoczy D, et al. Initial group versus individual therapy for posttraumatic stress disorder and subsequent follow-up treatment adequacy. Psychol Serv. 2016;13:349-355. doi:10.1037/ser0000077
- Burnett-Zeigler IE, Pfeiffer P, Zivin K, et al. Psychotherapy utilization for acute depression within the Veterans Affairs health care system. Psychol Serv. 2012;9:325-335. doi:10.1037/a0027957
- Kim JS, Prins A, Hirschhorn EW, et al. Preliminary investigation into the effectiveness of group webSTAIR for trauma-exposed veterans in primary care. Mil Med. 2024;189:e1403-e1408. doi:10.1093/milmed/usae052
- Jakupcak M, Blais RK, Grossbard J, et al. “Toughness” in association with mental health symptoms among Iraq and Afghanistan war veterans seeking Veterans Affairs health care. Psychol Men Masc. 2014;15:100-104. doi:10.1037/a0031508
- Stoycos SA, Berzenski SR, Beck JG, et al. Predictors of treatment completion in group psychotherapy for male veterans with posttraumatic stress disorder. J Trauma Stress. 2023;36:346-358. doi:10.1002/jts.22915
- Possemato K. The current state of intervention research for posttraumatic stress disorder within the primary care setting. J Clin Psychol Med Settings. 2011;18:268-280. doi:10.1007/s10880-011-9237-4
- Hunt MG, Rosenheck RA. Psychotherapy in mental health clinics of the Department of Veterans Affairs. J Clin Psychol. 2011;67:561-573. doi:10.1002/jclp.20788
- Khatri N, Marziali E, Tchernikov I, et al. Comparing telehealth-based and clinic-based group cognitive behavioral therapy for adults with depression and anxiety: a pilot study. Clin Interv Aging. 2014;9:765. doi:10.2147/cia.s57832
- Dangel J. Clinical resource hub increases veterans' access to care. VA News. January 12, 2025. Accessed September 3, 2025. https://news.va.gov/137439/clinical-resource-hub-increases-access-to-care/
VIP Boot Camp: Expanding the Impact of VA Primary Care Mental Health With a Transdiagnostic Modular Group Program
VIP Boot Camp: Expanding the Impact of VA Primary Care Mental Health With a Transdiagnostic Modular Group Program