User login
What’s Eating You? Ixodes Tick and Related Diseases, Part 1: Life Cycle, Local Reactions, and Lyme Disease
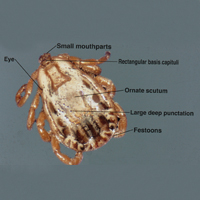
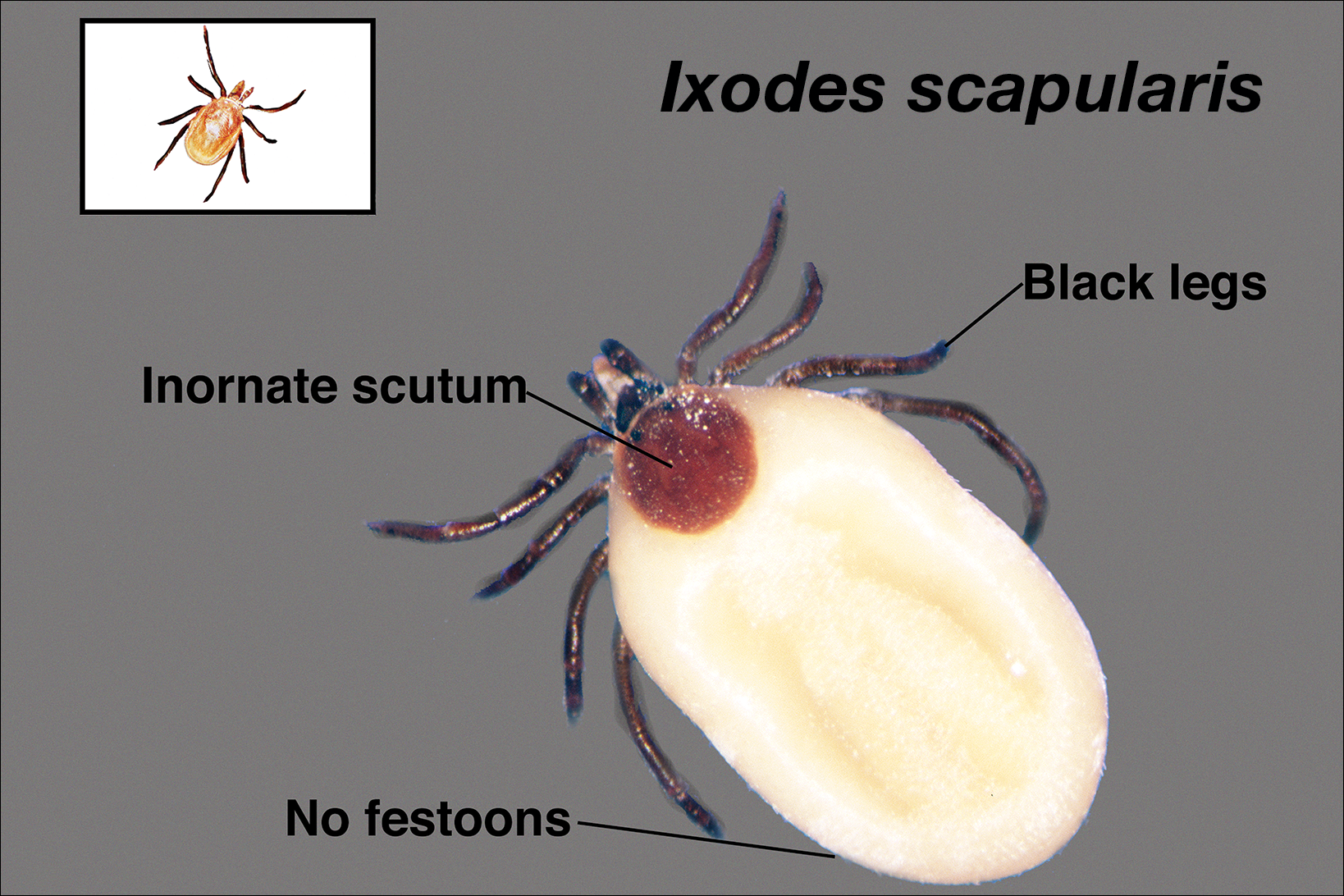
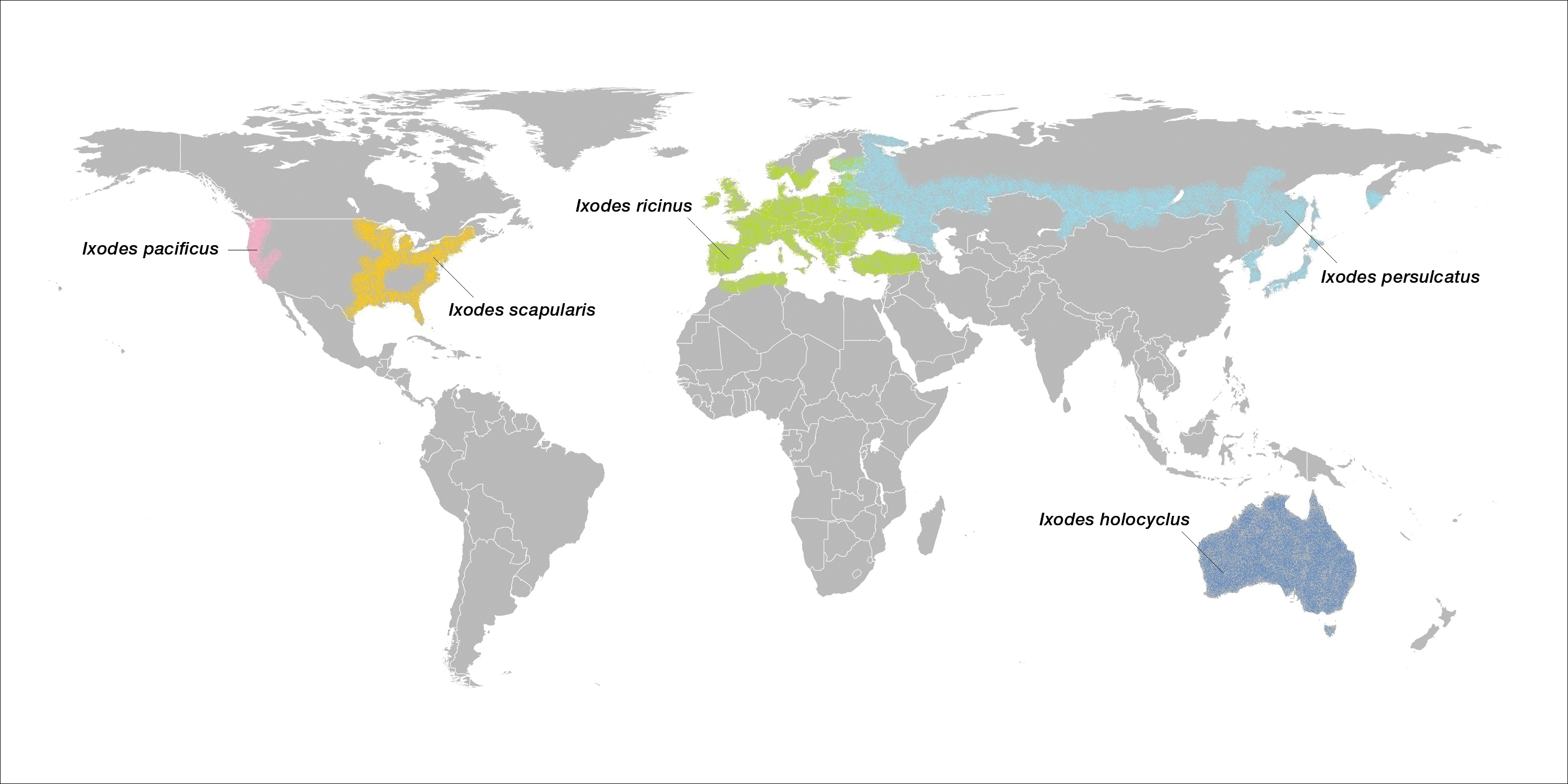
Ticks are ectoparasitic hemophages that feed on mammals, reptiles, and birds. The Ixodidae family comprises the hard ticks. A hard dorsal plate, scutum, and capitulum that extends outward from the body are features that distinguish the hard tick. 1Ixodes is the largest genus of hard ticks, with more than 250 species localized in temperate climates.2 It has an inornate scutum and lacks festoons (Figure 1).1 The Ixodes ricinus species complex accounts for most species relevant to the spread of human disease (Figure 2), with Ixodes scapularis in the northeastern, north midwestern, and southern United States; Ixodes pacificus in western United States; I ricinus in Europe and North Africa; and Ixodes persulcatus in Russia and Asia. Ixodes holocyclus is endemic to Australia.3,4
Life Cycle
Ixodes species progress through 4 life stages—egg, larvae, nymph, and adult—during their 3-host life cycle. Lifespan is 2 to 6 years, varying with environmental factors. A blood meal is required between each stage. Female ticks have a small scutum, allowing the abdomen to engorge during meals (Figure 3).

Larvae hatch in the early summer and remain dormant until the spring, emerging as a nymph. Following a blood meal, the nymph molts and reemerges as an adult in autumn. During autumn and winter, the female lays as many as 2000 eggs that emerge in early summer.5 Nymphs are small and easily undetected for the duration required for pathogen transmission, making nymphs the stage most likely to transmit disease.6
The majority of tick-borne diseases present from May to July, corresponding to nymph activity. Fewer cases present in the autumn and early spring because the adult female feeds during cooler months.7
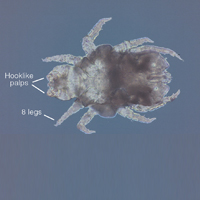
Larvae have 6 legs and are about the size of a sesame seed when engorged. Nymphs are slightly larger with 8 legs. Adults are largest and have 8 legs. Following a blood meal, the tick becomes engorged, increasing in size and lightening in color (Figure 3).1
Ticks are found in low-lying shrubs and tall grass as well as on the forest floor. They search for a host by detecting CO2, warmth, the smell of sweat, and the color white, prompting attachment.8 Habitats hospitable to Ixodes have expanded in the wake of climate, environmental, and socioeconomic changes, potentially contributing to the increasing incidence and expansion of zoonoses associated with this vector.9,10
Local Reactions
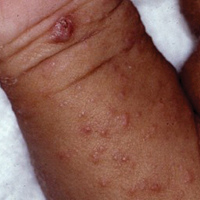
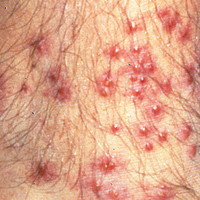
A tick bite may induce local hypersensitivity, leading to a red papule or plaque at the bite site, followed by swelling, warmth, and erythema. A cellular immune reaction induces induration and pruritus. Hard ticks are less likely than soft ticks to cause a serious local reaction.11,12
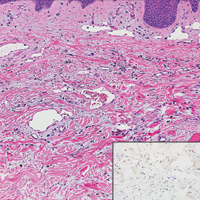
A variety of clinical and histologic features are observed following an arthropod bite. Histologically, acute tick bites show a neutrophilic infiltrate with fibrin deposition. Chronic reactions demonstrate a wedge-shaped, mixed infiltrate with prominent endothelial swelling. Eosinophilic cellulitis, or Wells syndrome, reveals tissue eosinophilia and flame figures.13 Tick mouthparts may be identified in the tissue. B-cell hyperplasia is seen in Borrelia lymphocytoma and is more common in Europe, presenting as erythematous to plum–colored nodules on the ear and areola.14
Lyme Disease
Disease manifestations vary by location. Lyme disease is associated with Borrelia burgdorferi and the recently identified Borrelia mayonii in the United States15; in Europe and Asia, acrodermatitis chronica atrophicans is associated with Borrelia afzelii and neuroborreliosis, with Borrelia garinii. Lyme disease is the most common tick-borne illness in the United States.16 The I ricinus species complex is the most common vector harboring Borrelia species.17 At least 36 hours of tick adherence is required for disease transmission.18 The incubation period is 3 to 20 days (median, 12 days).19
Clinical Findings
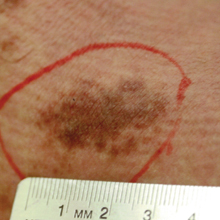
Erythema migrans is the most characteristic sign, seen in 80% of cases of Lyme disease. The typical rash is a centrifugally spreading, erythematous, annular patch with central clearing at the site of the tick bite.20 Atypical rashes include vesicular, indurated, ulcerated, and follicular variants.21 Histopathology commonly shows a superficial and deep perivascular lymphocytic infiltrate with plasma cells, histiocytes, and eosinophils.22 Typically, the rash resolves in 3 to 5 weeks.18
Early disseminated Lyme disease can present with any of the following findings: multiple erythema migrans; neurologic involvement, including cranial nerve palsy and meningitis; and Lyme carditis, which may result in atrioventricular block.23,24 Late findings include arthritis, encephalopathy, and polyneuropathy. A late cutaneous manifestation, acrodermatitis chronica atrophicans, is rare in the United States but occurs in as many as 10% of Lyme disease cases in Europe. An initial inflammatory response manifests as blue-red erythema and edema of the extensor surfaces of the extremities, commonly on the dorsal hands, feet, elbows, and knees. Firm fibrotic nodules may develop later over the olecranon and patella.23,24
The term chronic Lyme disease has been used to describe the persistence of symptoms after treatment; however, large clinical trials have not detected a difference in symptom frequency between patients with a history of Lyme disease and matched controls.25,26 Many patients with chronic Lyme disease may instead have posttreatment Lyme disease syndrome, described as nonspecific symptoms including fatigue, arthralgia, and decreased mental acuity following treatment of confirmed Lyme disease. Symptoms generally improve within 1 year.27
Laboratory Testing
The gold standard for laboratory diagnosis of Lyme disease is 2-tiered serologic testing. First, an enzyme immunoassay or immunofluorescence assay is used to screen for antibodies. A Western blot follows if the result of the screen is positive or equivocal. Western blot testing for IgM and IgG is used when illness duration is less than 4 weeks; after 4 weeks, a Western blot for IgG alone is sufficient.27,28 The 2-tiered test has 99% specificity. Sensitivity increases with duration of disease (29%–40% with erythema migrans; 42%–87% in early disseminated disease; 97%–100% in late disease).29,30 A false-positive result can occur in the presence of infectious mononucleosis, an autoimmune disorder, and syphilis. If serologic testing is negative and suspicion remains high, testing should be repeated in 2 to 4 weeks.31 When a patient in a Lyme-endemic area presents with typical erythema migrans, serologic testing is unnecessary prior to treatment.32
Management
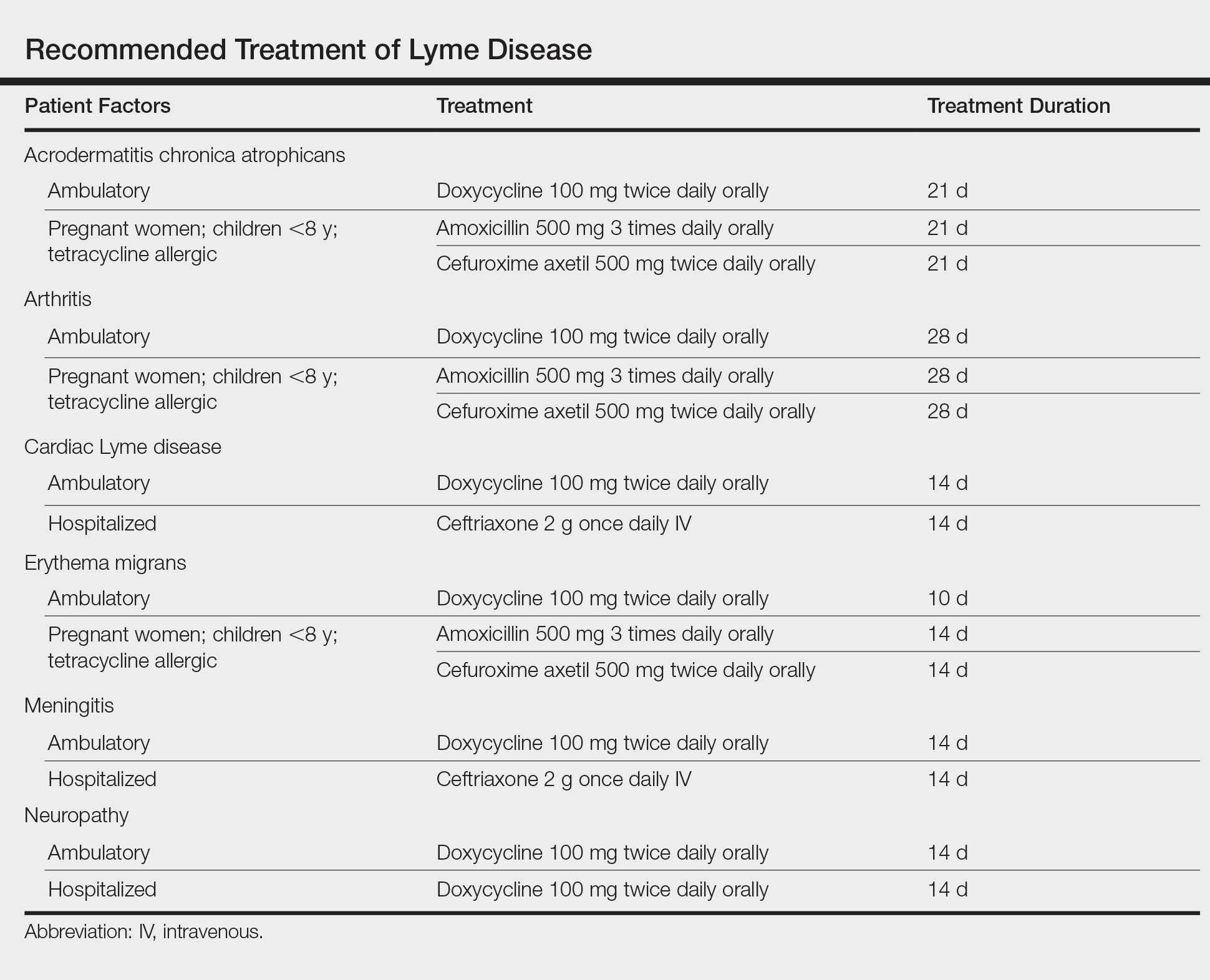
Treatment of Lyme disease centers on antibiotic therapy (Table). First-line treatment of early disseminated disease is doxycycline for 14 days (range, 10–21 days).27 In pregnant women, children younger than 8 years, and tetracycline-allergic patients, amoxicillin or cefuroxime axetil for 14 days (range, 14–21 days) may be used.33 For erythema migrans without complications, doxycycline for 10 days is effective. Complications that require hospitalization are treated with intravenous ceftriaxone.27 Re-treatment in patients with posttreatment Lyme disease syndrome is not recommended.34 Prophylaxis with a single dose of doxycycline 200 mg may be indicated when all of the following conditions are met: (1) the patient is in an area where more than 20% of Ixodes ticks are infected with B burgdorferi, (2) the attached tick is I scapularis, (3) the tick has been attached for more than 36 hours, and (4) treatment is begun within 72 hours of tick removal.27
- Anderson JF, Magnarelli LA. Biology of ticks. Infect Dis Clin North Am. 2008;22:195-215.
- Jongejan F, Uilenberg G. The global importance of ticks. Parasitology. 2004;129(suppl):S3-S14.
- Xu G, Fang QQ, Keirans JE, et al. Molecular phylogenetic analyses indicate that the Ixodes ricinus complex is a paraphyletic group. J Parasitol. 2003;89:452-457.
- Swanson SJ, Neitzel D, Reed DK, et al. Coinfections acquired from Ixodes ticks. Clin Microbiol Rev. 2006;19:708-727.
- Mathison BA, Pritt BS. Laboratory identification of arthropod ectoparasites. Clin Microbol Rev. 2014;27:48-67.
- Falco RC, Fish D, Piesman J. Duration of tick bites in a Lyme disease-endemic area. Am J Epidemiol. 1996;143:187-192.
- Centers for Disease Control and Prevention. Lyme disease graphs. http://www.cdc.gov/lyme/stats/graphs.html. Updated November 21, 2016. Accessed November 21, 2017.
- Randolph SE. The impact of tick ecology on pathogen transmission dynamics. In: Bowman AS, Nuttall PA, eds. Ticks: Biology, Disease and Control. Cambridge, UK: Cambridge University Press; 2008:40-72.
- Ostfeld RS, Brunner JL. Climate change and Ixodes tick-borne diseases of humans. Philos Trans R Soc Lond B Biol Sci. 2015;370. pii:20140051. doi:10.1098/rstb.2014.0051.
- Medlock JM, Hansford KM, Bormane A, et al. Driving forces for changes in geographical distribution of Ixodes ricinus ticks in Europe. Parasit Vectors. 2013;6:1.
- McGinley-Smith DE, Tsao SS. Dermatoses from ticks. J Am Acad Dermatol. 2003;49:393-396.
- Middleton DB. Tick-borne infections. What starts as a tiny bite may have a serious outcome. Postgrad Med. 1994;95:131-139.
- Melski JW. Wells’ syndrome, insect bites, and eosinophils. Dermatol Clin. 2015;8:287-293.
- Castelli E, Caputo V, Morello V, et al. Local reactions to tick bites. Am J Dermatopathol. 2008;30:241-248.
- Pritt BS, Mead PS, Johnson DK, et al. Identification of a novel pathogenic Borrelia species causing Lyme borreliosis with unusually high spirochaetaemia: a descriptive study. Lancet Infect Dis. 2016;16:556-564.
- Orloski KA, Hayes EB, Campbell GL, et al. Surveillance for Lyme disease—United States, 1992-1998. MMWR CDC Surveill Summ. 2000;49:1-11.
- Gray JS. The ecology of ticks transmitting Lyme borreliosis. Exp Appl Acarol. 1998;22:249-258.
- Piesman J, Mather TN, Sinsky RJ, et al. Duration of tick attachment and Borrelia burgdorferi transmission. J Clin Microbiol. 1987;25:557-558.
- Richardson M, Elliman D, Maguire H, et al. Evidence base of incubation periods, periods of infectiousness and exclusion policies for the control of communicable diseases in schools and preschools. Pediatr Infect Dis J. 2001;20:380-391.
- Myers SA, Sexton DJ. Dermatologic manifestations of arthropod-borne diseases. Infect Dis Clin North Am. 1994;8:689-712.
- Ducroux E, Debarbieux S, Boibieux A, et al. Follicular borreliosis: an atypical presentation of erythema chronicum migrans. Dermatology. 2009;219:84-85.
- Miraflor AP, Seidel GD, Perry AE, et al. The many masks of cutaneous Lyme disease. J Cutan Pathol. 2016:43:32-40.
- Lenormand C, Jaulhac B, Debarbieux S, et al. Expanding the clinicopathological spectrum of late cutaneous Lyme borreliosis (acrodermatitis chronica atrophicans): a prospective study of 20 culture and/or polymerase chain reaction (PCR) documented cases. J Am Acad Dermatol. 2016;74:685-692.
- Zajkowska J, Czupryna P, Pancewicz SA, et al. Acrodermatitis chronica atrophicans. Lancet Infect Dis. 2011;11:800.
- Seltzer EG, Gerber MA, Cartter ML, et al. Long-term outcomes of persons with Lyme disease. JAMA. 2000;283:609-616.
- Shadick NA, Phillips CB, Sangha O, et al. Musculoskeletal and neurologic outcomes in patients with previously treated Lyme disease. Ann Intern Med. 1999;131:919-926.
- Wormser GP, Dattwyler RJ, Shapiro ED, et al. The clinical assessment, treatment, and prevention of Lyme disease, human granulocytic anaplasmosis, and babesiosis: clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis. 2006;43:1089-1134.
- Schriefer ME. Lyme disease diagnosis: serology. Clin Lab Med. 2015;35:797-814.
- Wormser GP, Nowakowski J, Nadelman RB, et al. Impact of clinical variables on Borrelia burgdorferi-specific antibody seropositivity in acute-phase sera from patients in North America with culture-confirmed early Lyme disease. Clin Vaccine Immunol. 2008;15:1519-1522.
- Leeflang MM, Ang CW, Berkhout J, et al. The diagnostic accuracy of serological tests for Lyme borreliosis in Europe: a systematic review and meta-analysis. BMC Infect Dis. 2016;16:140.
- Sanchez E, Vannier E, Wormser GP, et al. Diagnosis, treatment, and prevention of Lyme disease, human granulocytic anaplasmosis, and babesiosis: a review. JAMA. 2016;315:1767-1777.
- Lantos PM, Brinkerhoff RJ, Wormser GP, et al. Empiric antibiotic treatment of erythema migrans-like skin lesions as a function of geography: a clinical and cost effectiveness modeling study. Vector Borne Zoonotic Dis. 2013;13:877-883.
- Smith GN, Gemmill I, Moore KM. Management of tick bites and Lyme disease during pregnancy. J Obstet Gynaecol Can. 2012;34:1087-1091.
- Berende A, ter Hofstede HJ, Vos FJ, et al. Randomized trial of longer-term therapy for symptoms attributed to Lyme disease. N Engl J Med. 2016;374:1209-1220.
Ticks are ectoparasitic hemophages that feed on mammals, reptiles, and birds. The Ixodidae family comprises the hard ticks. A hard dorsal plate, scutum, and capitulum that extends outward from the body are features that distinguish the hard tick. 1Ixodes is the largest genus of hard ticks, with more than 250 species localized in temperate climates.2 It has an inornate scutum and lacks festoons (Figure 1).1 The Ixodes ricinus species complex accounts for most species relevant to the spread of human disease (Figure 2), with Ixodes scapularis in the northeastern, north midwestern, and southern United States; Ixodes pacificus in western United States; I ricinus in Europe and North Africa; and Ixodes persulcatus in Russia and Asia. Ixodes holocyclus is endemic to Australia.3,4


Life Cycle
Ixodes species progress through 4 life stages—egg, larvae, nymph, and adult—during their 3-host life cycle. Lifespan is 2 to 6 years, varying with environmental factors. A blood meal is required between each stage. Female ticks have a small scutum, allowing the abdomen to engorge during meals (Figure 3).

Larvae hatch in the early summer and remain dormant until the spring, emerging as a nymph. Following a blood meal, the nymph molts and reemerges as an adult in autumn. During autumn and winter, the female lays as many as 2000 eggs that emerge in early summer.5 Nymphs are small and easily undetected for the duration required for pathogen transmission, making nymphs the stage most likely to transmit disease.6
The majority of tick-borne diseases present from May to July, corresponding to nymph activity. Fewer cases present in the autumn and early spring because the adult female feeds during cooler months.7
Larvae have 6 legs and are about the size of a sesame seed when engorged. Nymphs are slightly larger with 8 legs. Adults are largest and have 8 legs. Following a blood meal, the tick becomes engorged, increasing in size and lightening in color (Figure 3).1
Ticks are found in low-lying shrubs and tall grass as well as on the forest floor. They search for a host by detecting CO2, warmth, the smell of sweat, and the color white, prompting attachment.8 Habitats hospitable to Ixodes have expanded in the wake of climate, environmental, and socioeconomic changes, potentially contributing to the increasing incidence and expansion of zoonoses associated with this vector.9,10
Local Reactions
A tick bite may induce local hypersensitivity, leading to a red papule or plaque at the bite site, followed by swelling, warmth, and erythema. A cellular immune reaction induces induration and pruritus. Hard ticks are less likely than soft ticks to cause a serious local reaction.11,12
A variety of clinical and histologic features are observed following an arthropod bite. Histologically, acute tick bites show a neutrophilic infiltrate with fibrin deposition. Chronic reactions demonstrate a wedge-shaped, mixed infiltrate with prominent endothelial swelling. Eosinophilic cellulitis, or Wells syndrome, reveals tissue eosinophilia and flame figures.13 Tick mouthparts may be identified in the tissue. B-cell hyperplasia is seen in Borrelia lymphocytoma and is more common in Europe, presenting as erythematous to plum–colored nodules on the ear and areola.14
Lyme Disease
Disease manifestations vary by location. Lyme disease is associated with Borrelia burgdorferi and the recently identified Borrelia mayonii in the United States15; in Europe and Asia, acrodermatitis chronica atrophicans is associated with Borrelia afzelii and neuroborreliosis, with Borrelia garinii. Lyme disease is the most common tick-borne illness in the United States.16 The I ricinus species complex is the most common vector harboring Borrelia species.17 At least 36 hours of tick adherence is required for disease transmission.18 The incubation period is 3 to 20 days (median, 12 days).19
Clinical Findings
Erythema migrans is the most characteristic sign, seen in 80% of cases of Lyme disease. The typical rash is a centrifugally spreading, erythematous, annular patch with central clearing at the site of the tick bite.20 Atypical rashes include vesicular, indurated, ulcerated, and follicular variants.21 Histopathology commonly shows a superficial and deep perivascular lymphocytic infiltrate with plasma cells, histiocytes, and eosinophils.22 Typically, the rash resolves in 3 to 5 weeks.18
Early disseminated Lyme disease can present with any of the following findings: multiple erythema migrans; neurologic involvement, including cranial nerve palsy and meningitis; and Lyme carditis, which may result in atrioventricular block.23,24 Late findings include arthritis, encephalopathy, and polyneuropathy. A late cutaneous manifestation, acrodermatitis chronica atrophicans, is rare in the United States but occurs in as many as 10% of Lyme disease cases in Europe. An initial inflammatory response manifests as blue-red erythema and edema of the extensor surfaces of the extremities, commonly on the dorsal hands, feet, elbows, and knees. Firm fibrotic nodules may develop later over the olecranon and patella.23,24
The term chronic Lyme disease has been used to describe the persistence of symptoms after treatment; however, large clinical trials have not detected a difference in symptom frequency between patients with a history of Lyme disease and matched controls.25,26 Many patients with chronic Lyme disease may instead have posttreatment Lyme disease syndrome, described as nonspecific symptoms including fatigue, arthralgia, and decreased mental acuity following treatment of confirmed Lyme disease. Symptoms generally improve within 1 year.27
Laboratory Testing
The gold standard for laboratory diagnosis of Lyme disease is 2-tiered serologic testing. First, an enzyme immunoassay or immunofluorescence assay is used to screen for antibodies. A Western blot follows if the result of the screen is positive or equivocal. Western blot testing for IgM and IgG is used when illness duration is less than 4 weeks; after 4 weeks, a Western blot for IgG alone is sufficient.27,28 The 2-tiered test has 99% specificity. Sensitivity increases with duration of disease (29%–40% with erythema migrans; 42%–87% in early disseminated disease; 97%–100% in late disease).29,30 A false-positive result can occur in the presence of infectious mononucleosis, an autoimmune disorder, and syphilis. If serologic testing is negative and suspicion remains high, testing should be repeated in 2 to 4 weeks.31 When a patient in a Lyme-endemic area presents with typical erythema migrans, serologic testing is unnecessary prior to treatment.32
Management
Treatment of Lyme disease centers on antibiotic therapy (Table). First-line treatment of early disseminated disease is doxycycline for 14 days (range, 10–21 days).27 In pregnant women, children younger than 8 years, and tetracycline-allergic patients, amoxicillin or cefuroxime axetil for 14 days (range, 14–21 days) may be used.33 For erythema migrans without complications, doxycycline for 10 days is effective. Complications that require hospitalization are treated with intravenous ceftriaxone.27 Re-treatment in patients with posttreatment Lyme disease syndrome is not recommended.34 Prophylaxis with a single dose of doxycycline 200 mg may be indicated when all of the following conditions are met: (1) the patient is in an area where more than 20% of Ixodes ticks are infected with B burgdorferi, (2) the attached tick is I scapularis, (3) the tick has been attached for more than 36 hours, and (4) treatment is begun within 72 hours of tick removal.27

Ticks are ectoparasitic hemophages that feed on mammals, reptiles, and birds. The Ixodidae family comprises the hard ticks. A hard dorsal plate, scutum, and capitulum that extends outward from the body are features that distinguish the hard tick. 1Ixodes is the largest genus of hard ticks, with more than 250 species localized in temperate climates.2 It has an inornate scutum and lacks festoons (Figure 1).1 The Ixodes ricinus species complex accounts for most species relevant to the spread of human disease (Figure 2), with Ixodes scapularis in the northeastern, north midwestern, and southern United States; Ixodes pacificus in western United States; I ricinus in Europe and North Africa; and Ixodes persulcatus in Russia and Asia. Ixodes holocyclus is endemic to Australia.3,4


Life Cycle
Ixodes species progress through 4 life stages—egg, larvae, nymph, and adult—during their 3-host life cycle. Lifespan is 2 to 6 years, varying with environmental factors. A blood meal is required between each stage. Female ticks have a small scutum, allowing the abdomen to engorge during meals (Figure 3).

Larvae hatch in the early summer and remain dormant until the spring, emerging as a nymph. Following a blood meal, the nymph molts and reemerges as an adult in autumn. During autumn and winter, the female lays as many as 2000 eggs that emerge in early summer.5 Nymphs are small and easily undetected for the duration required for pathogen transmission, making nymphs the stage most likely to transmit disease.6
The majority of tick-borne diseases present from May to July, corresponding to nymph activity. Fewer cases present in the autumn and early spring because the adult female feeds during cooler months.7
Larvae have 6 legs and are about the size of a sesame seed when engorged. Nymphs are slightly larger with 8 legs. Adults are largest and have 8 legs. Following a blood meal, the tick becomes engorged, increasing in size and lightening in color (Figure 3).1
Ticks are found in low-lying shrubs and tall grass as well as on the forest floor. They search for a host by detecting CO2, warmth, the smell of sweat, and the color white, prompting attachment.8 Habitats hospitable to Ixodes have expanded in the wake of climate, environmental, and socioeconomic changes, potentially contributing to the increasing incidence and expansion of zoonoses associated with this vector.9,10
Local Reactions
A tick bite may induce local hypersensitivity, leading to a red papule or plaque at the bite site, followed by swelling, warmth, and erythema. A cellular immune reaction induces induration and pruritus. Hard ticks are less likely than soft ticks to cause a serious local reaction.11,12
A variety of clinical and histologic features are observed following an arthropod bite. Histologically, acute tick bites show a neutrophilic infiltrate with fibrin deposition. Chronic reactions demonstrate a wedge-shaped, mixed infiltrate with prominent endothelial swelling. Eosinophilic cellulitis, or Wells syndrome, reveals tissue eosinophilia and flame figures.13 Tick mouthparts may be identified in the tissue. B-cell hyperplasia is seen in Borrelia lymphocytoma and is more common in Europe, presenting as erythematous to plum–colored nodules on the ear and areola.14
Lyme Disease
Disease manifestations vary by location. Lyme disease is associated with Borrelia burgdorferi and the recently identified Borrelia mayonii in the United States15; in Europe and Asia, acrodermatitis chronica atrophicans is associated with Borrelia afzelii and neuroborreliosis, with Borrelia garinii. Lyme disease is the most common tick-borne illness in the United States.16 The I ricinus species complex is the most common vector harboring Borrelia species.17 At least 36 hours of tick adherence is required for disease transmission.18 The incubation period is 3 to 20 days (median, 12 days).19
Clinical Findings
Erythema migrans is the most characteristic sign, seen in 80% of cases of Lyme disease. The typical rash is a centrifugally spreading, erythematous, annular patch with central clearing at the site of the tick bite.20 Atypical rashes include vesicular, indurated, ulcerated, and follicular variants.21 Histopathology commonly shows a superficial and deep perivascular lymphocytic infiltrate with plasma cells, histiocytes, and eosinophils.22 Typically, the rash resolves in 3 to 5 weeks.18
Early disseminated Lyme disease can present with any of the following findings: multiple erythema migrans; neurologic involvement, including cranial nerve palsy and meningitis; and Lyme carditis, which may result in atrioventricular block.23,24 Late findings include arthritis, encephalopathy, and polyneuropathy. A late cutaneous manifestation, acrodermatitis chronica atrophicans, is rare in the United States but occurs in as many as 10% of Lyme disease cases in Europe. An initial inflammatory response manifests as blue-red erythema and edema of the extensor surfaces of the extremities, commonly on the dorsal hands, feet, elbows, and knees. Firm fibrotic nodules may develop later over the olecranon and patella.23,24
The term chronic Lyme disease has been used to describe the persistence of symptoms after treatment; however, large clinical trials have not detected a difference in symptom frequency between patients with a history of Lyme disease and matched controls.25,26 Many patients with chronic Lyme disease may instead have posttreatment Lyme disease syndrome, described as nonspecific symptoms including fatigue, arthralgia, and decreased mental acuity following treatment of confirmed Lyme disease. Symptoms generally improve within 1 year.27
Laboratory Testing
The gold standard for laboratory diagnosis of Lyme disease is 2-tiered serologic testing. First, an enzyme immunoassay or immunofluorescence assay is used to screen for antibodies. A Western blot follows if the result of the screen is positive or equivocal. Western blot testing for IgM and IgG is used when illness duration is less than 4 weeks; after 4 weeks, a Western blot for IgG alone is sufficient.27,28 The 2-tiered test has 99% specificity. Sensitivity increases with duration of disease (29%–40% with erythema migrans; 42%–87% in early disseminated disease; 97%–100% in late disease).29,30 A false-positive result can occur in the presence of infectious mononucleosis, an autoimmune disorder, and syphilis. If serologic testing is negative and suspicion remains high, testing should be repeated in 2 to 4 weeks.31 When a patient in a Lyme-endemic area presents with typical erythema migrans, serologic testing is unnecessary prior to treatment.32
Management
Treatment of Lyme disease centers on antibiotic therapy (Table). First-line treatment of early disseminated disease is doxycycline for 14 days (range, 10–21 days).27 In pregnant women, children younger than 8 years, and tetracycline-allergic patients, amoxicillin or cefuroxime axetil for 14 days (range, 14–21 days) may be used.33 For erythema migrans without complications, doxycycline for 10 days is effective. Complications that require hospitalization are treated with intravenous ceftriaxone.27 Re-treatment in patients with posttreatment Lyme disease syndrome is not recommended.34 Prophylaxis with a single dose of doxycycline 200 mg may be indicated when all of the following conditions are met: (1) the patient is in an area where more than 20% of Ixodes ticks are infected with B burgdorferi, (2) the attached tick is I scapularis, (3) the tick has been attached for more than 36 hours, and (4) treatment is begun within 72 hours of tick removal.27

- Anderson JF, Magnarelli LA. Biology of ticks. Infect Dis Clin North Am. 2008;22:195-215.
- Jongejan F, Uilenberg G. The global importance of ticks. Parasitology. 2004;129(suppl):S3-S14.
- Xu G, Fang QQ, Keirans JE, et al. Molecular phylogenetic analyses indicate that the Ixodes ricinus complex is a paraphyletic group. J Parasitol. 2003;89:452-457.
- Swanson SJ, Neitzel D, Reed DK, et al. Coinfections acquired from Ixodes ticks. Clin Microbiol Rev. 2006;19:708-727.
- Mathison BA, Pritt BS. Laboratory identification of arthropod ectoparasites. Clin Microbol Rev. 2014;27:48-67.
- Falco RC, Fish D, Piesman J. Duration of tick bites in a Lyme disease-endemic area. Am J Epidemiol. 1996;143:187-192.
- Centers for Disease Control and Prevention. Lyme disease graphs. http://www.cdc.gov/lyme/stats/graphs.html. Updated November 21, 2016. Accessed November 21, 2017.
- Randolph SE. The impact of tick ecology on pathogen transmission dynamics. In: Bowman AS, Nuttall PA, eds. Ticks: Biology, Disease and Control. Cambridge, UK: Cambridge University Press; 2008:40-72.
- Ostfeld RS, Brunner JL. Climate change and Ixodes tick-borne diseases of humans. Philos Trans R Soc Lond B Biol Sci. 2015;370. pii:20140051. doi:10.1098/rstb.2014.0051.
- Medlock JM, Hansford KM, Bormane A, et al. Driving forces for changes in geographical distribution of Ixodes ricinus ticks in Europe. Parasit Vectors. 2013;6:1.
- McGinley-Smith DE, Tsao SS. Dermatoses from ticks. J Am Acad Dermatol. 2003;49:393-396.
- Middleton DB. Tick-borne infections. What starts as a tiny bite may have a serious outcome. Postgrad Med. 1994;95:131-139.
- Melski JW. Wells’ syndrome, insect bites, and eosinophils. Dermatol Clin. 2015;8:287-293.
- Castelli E, Caputo V, Morello V, et al. Local reactions to tick bites. Am J Dermatopathol. 2008;30:241-248.
- Pritt BS, Mead PS, Johnson DK, et al. Identification of a novel pathogenic Borrelia species causing Lyme borreliosis with unusually high spirochaetaemia: a descriptive study. Lancet Infect Dis. 2016;16:556-564.
- Orloski KA, Hayes EB, Campbell GL, et al. Surveillance for Lyme disease—United States, 1992-1998. MMWR CDC Surveill Summ. 2000;49:1-11.
- Gray JS. The ecology of ticks transmitting Lyme borreliosis. Exp Appl Acarol. 1998;22:249-258.
- Piesman J, Mather TN, Sinsky RJ, et al. Duration of tick attachment and Borrelia burgdorferi transmission. J Clin Microbiol. 1987;25:557-558.
- Richardson M, Elliman D, Maguire H, et al. Evidence base of incubation periods, periods of infectiousness and exclusion policies for the control of communicable diseases in schools and preschools. Pediatr Infect Dis J. 2001;20:380-391.
- Myers SA, Sexton DJ. Dermatologic manifestations of arthropod-borne diseases. Infect Dis Clin North Am. 1994;8:689-712.
- Ducroux E, Debarbieux S, Boibieux A, et al. Follicular borreliosis: an atypical presentation of erythema chronicum migrans. Dermatology. 2009;219:84-85.
- Miraflor AP, Seidel GD, Perry AE, et al. The many masks of cutaneous Lyme disease. J Cutan Pathol. 2016:43:32-40.
- Lenormand C, Jaulhac B, Debarbieux S, et al. Expanding the clinicopathological spectrum of late cutaneous Lyme borreliosis (acrodermatitis chronica atrophicans): a prospective study of 20 culture and/or polymerase chain reaction (PCR) documented cases. J Am Acad Dermatol. 2016;74:685-692.
- Zajkowska J, Czupryna P, Pancewicz SA, et al. Acrodermatitis chronica atrophicans. Lancet Infect Dis. 2011;11:800.
- Seltzer EG, Gerber MA, Cartter ML, et al. Long-term outcomes of persons with Lyme disease. JAMA. 2000;283:609-616.
- Shadick NA, Phillips CB, Sangha O, et al. Musculoskeletal and neurologic outcomes in patients with previously treated Lyme disease. Ann Intern Med. 1999;131:919-926.
- Wormser GP, Dattwyler RJ, Shapiro ED, et al. The clinical assessment, treatment, and prevention of Lyme disease, human granulocytic anaplasmosis, and babesiosis: clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis. 2006;43:1089-1134.
- Schriefer ME. Lyme disease diagnosis: serology. Clin Lab Med. 2015;35:797-814.
- Wormser GP, Nowakowski J, Nadelman RB, et al. Impact of clinical variables on Borrelia burgdorferi-specific antibody seropositivity in acute-phase sera from patients in North America with culture-confirmed early Lyme disease. Clin Vaccine Immunol. 2008;15:1519-1522.
- Leeflang MM, Ang CW, Berkhout J, et al. The diagnostic accuracy of serological tests for Lyme borreliosis in Europe: a systematic review and meta-analysis. BMC Infect Dis. 2016;16:140.
- Sanchez E, Vannier E, Wormser GP, et al. Diagnosis, treatment, and prevention of Lyme disease, human granulocytic anaplasmosis, and babesiosis: a review. JAMA. 2016;315:1767-1777.
- Lantos PM, Brinkerhoff RJ, Wormser GP, et al. Empiric antibiotic treatment of erythema migrans-like skin lesions as a function of geography: a clinical and cost effectiveness modeling study. Vector Borne Zoonotic Dis. 2013;13:877-883.
- Smith GN, Gemmill I, Moore KM. Management of tick bites and Lyme disease during pregnancy. J Obstet Gynaecol Can. 2012;34:1087-1091.
- Berende A, ter Hofstede HJ, Vos FJ, et al. Randomized trial of longer-term therapy for symptoms attributed to Lyme disease. N Engl J Med. 2016;374:1209-1220.
- Anderson JF, Magnarelli LA. Biology of ticks. Infect Dis Clin North Am. 2008;22:195-215.
- Jongejan F, Uilenberg G. The global importance of ticks. Parasitology. 2004;129(suppl):S3-S14.
- Xu G, Fang QQ, Keirans JE, et al. Molecular phylogenetic analyses indicate that the Ixodes ricinus complex is a paraphyletic group. J Parasitol. 2003;89:452-457.
- Swanson SJ, Neitzel D, Reed DK, et al. Coinfections acquired from Ixodes ticks. Clin Microbiol Rev. 2006;19:708-727.
- Mathison BA, Pritt BS. Laboratory identification of arthropod ectoparasites. Clin Microbol Rev. 2014;27:48-67.
- Falco RC, Fish D, Piesman J. Duration of tick bites in a Lyme disease-endemic area. Am J Epidemiol. 1996;143:187-192.
- Centers for Disease Control and Prevention. Lyme disease graphs. http://www.cdc.gov/lyme/stats/graphs.html. Updated November 21, 2016. Accessed November 21, 2017.
- Randolph SE. The impact of tick ecology on pathogen transmission dynamics. In: Bowman AS, Nuttall PA, eds. Ticks: Biology, Disease and Control. Cambridge, UK: Cambridge University Press; 2008:40-72.
- Ostfeld RS, Brunner JL. Climate change and Ixodes tick-borne diseases of humans. Philos Trans R Soc Lond B Biol Sci. 2015;370. pii:20140051. doi:10.1098/rstb.2014.0051.
- Medlock JM, Hansford KM, Bormane A, et al. Driving forces for changes in geographical distribution of Ixodes ricinus ticks in Europe. Parasit Vectors. 2013;6:1.
- McGinley-Smith DE, Tsao SS. Dermatoses from ticks. J Am Acad Dermatol. 2003;49:393-396.
- Middleton DB. Tick-borne infections. What starts as a tiny bite may have a serious outcome. Postgrad Med. 1994;95:131-139.
- Melski JW. Wells’ syndrome, insect bites, and eosinophils. Dermatol Clin. 2015;8:287-293.
- Castelli E, Caputo V, Morello V, et al. Local reactions to tick bites. Am J Dermatopathol. 2008;30:241-248.
- Pritt BS, Mead PS, Johnson DK, et al. Identification of a novel pathogenic Borrelia species causing Lyme borreliosis with unusually high spirochaetaemia: a descriptive study. Lancet Infect Dis. 2016;16:556-564.
- Orloski KA, Hayes EB, Campbell GL, et al. Surveillance for Lyme disease—United States, 1992-1998. MMWR CDC Surveill Summ. 2000;49:1-11.
- Gray JS. The ecology of ticks transmitting Lyme borreliosis. Exp Appl Acarol. 1998;22:249-258.
- Piesman J, Mather TN, Sinsky RJ, et al. Duration of tick attachment and Borrelia burgdorferi transmission. J Clin Microbiol. 1987;25:557-558.
- Richardson M, Elliman D, Maguire H, et al. Evidence base of incubation periods, periods of infectiousness and exclusion policies for the control of communicable diseases in schools and preschools. Pediatr Infect Dis J. 2001;20:380-391.
- Myers SA, Sexton DJ. Dermatologic manifestations of arthropod-borne diseases. Infect Dis Clin North Am. 1994;8:689-712.
- Ducroux E, Debarbieux S, Boibieux A, et al. Follicular borreliosis: an atypical presentation of erythema chronicum migrans. Dermatology. 2009;219:84-85.
- Miraflor AP, Seidel GD, Perry AE, et al. The many masks of cutaneous Lyme disease. J Cutan Pathol. 2016:43:32-40.
- Lenormand C, Jaulhac B, Debarbieux S, et al. Expanding the clinicopathological spectrum of late cutaneous Lyme borreliosis (acrodermatitis chronica atrophicans): a prospective study of 20 culture and/or polymerase chain reaction (PCR) documented cases. J Am Acad Dermatol. 2016;74:685-692.
- Zajkowska J, Czupryna P, Pancewicz SA, et al. Acrodermatitis chronica atrophicans. Lancet Infect Dis. 2011;11:800.
- Seltzer EG, Gerber MA, Cartter ML, et al. Long-term outcomes of persons with Lyme disease. JAMA. 2000;283:609-616.
- Shadick NA, Phillips CB, Sangha O, et al. Musculoskeletal and neurologic outcomes in patients with previously treated Lyme disease. Ann Intern Med. 1999;131:919-926.
- Wormser GP, Dattwyler RJ, Shapiro ED, et al. The clinical assessment, treatment, and prevention of Lyme disease, human granulocytic anaplasmosis, and babesiosis: clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis. 2006;43:1089-1134.
- Schriefer ME. Lyme disease diagnosis: serology. Clin Lab Med. 2015;35:797-814.
- Wormser GP, Nowakowski J, Nadelman RB, et al. Impact of clinical variables on Borrelia burgdorferi-specific antibody seropositivity in acute-phase sera from patients in North America with culture-confirmed early Lyme disease. Clin Vaccine Immunol. 2008;15:1519-1522.
- Leeflang MM, Ang CW, Berkhout J, et al. The diagnostic accuracy of serological tests for Lyme borreliosis in Europe: a systematic review and meta-analysis. BMC Infect Dis. 2016;16:140.
- Sanchez E, Vannier E, Wormser GP, et al. Diagnosis, treatment, and prevention of Lyme disease, human granulocytic anaplasmosis, and babesiosis: a review. JAMA. 2016;315:1767-1777.
- Lantos PM, Brinkerhoff RJ, Wormser GP, et al. Empiric antibiotic treatment of erythema migrans-like skin lesions as a function of geography: a clinical and cost effectiveness modeling study. Vector Borne Zoonotic Dis. 2013;13:877-883.
- Smith GN, Gemmill I, Moore KM. Management of tick bites and Lyme disease during pregnancy. J Obstet Gynaecol Can. 2012;34:1087-1091.
- Berende A, ter Hofstede HJ, Vos FJ, et al. Randomized trial of longer-term therapy for symptoms attributed to Lyme disease. N Engl J Med. 2016;374:1209-1220.
Practice Points
- Lyme disease is transmitted by Ixodes ticks in the northeastern, midwestern, and far western United States.
- Most tick-borne illnesses, including Lyme disease, respond to treatment with doxycycline.
- Babesiosis, a malarialike illness, can be transmitted concurrently with Lyme disease.
What’s Eating You? Sand Flies
Identification
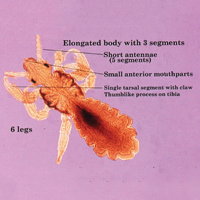
Phlebotomine sand flies are the only member of the Psychodidae family that are capable of taking blood.1 The mouthparts of the sand fly are toothed distally, and the maxilla and mandible are utilized in a sawtooth fashion to take a bloodmeal.2 The flies are very small (ie, only 1.5–3.5 mm in length), which makes their identification difficult.1 Sand flies can be distinguished by the appearance of their wings, which often are covered in hair and extend across the back in a V shape.3 The adult sand fly is hairy with a 6- to 8-segmented abdomen, and the color can range from gray to yellow to brown.2 Phlebotomine sand flies can be further identified by their long antennae, dark eyes, and small heads (Figure).2

As is the case with all Diptera, the sand fly goes through 4 complete life stages from egg to larva to pupa to adult.3 Female sand flies will lay their eggs following a blood meal and have been found to take multiple blood meals in a single cycle.2 On average, the eggs will hatch in 6 to 17 days but are temperature dependent.3 The subsequent larvae and pupa stages last 20 to 30 days and 6 to 13 days, respectively.1 The larvae are white in color with short antennae and dark heads.4 Sand flies prefer to lay their eggs in areas where adequate resting places are available and where their larvae will thrive.4,5 The larvae require warm moist environments to succeed and thus are commonly found in animal burrows.3 Once fully developed, the adult sand fly can live up to 6 weeks.2
Sand Fly Vector
Although it is more common in rural forested areas, the sand fly also can be found in urban areas, including heavily populated cities in Brazil.6 Sand flies are most active during hot humid seasons but depending on the local climate may remain active year-round.1,7 For example, in tropical regions of Asia, the number of sand flies increases substantially during the monsoon season compared to the dry season.2 Phlebotomine sand flies are most active at dusk and during the night5 but may become agitated during the daytime if their environment is disturbed.1
Host selection usually is broad and includes a wide variety of vertebrates.2 In the United States, host species are thought to include small rodents, foxes, armadillos, and opossums.8 One study found that visceral leishmaniasis in foxhounds is able to develop fully in sand flies, thus posing an emerging risk to the American population.9
Distribution
The Phlebotominae family contains approximately 700 different species of sand flies but only 21 are known vectors of disease.10 The great majority belong to 1 of 3 genuses: Phlebotomus, Sergentomyia, and Lutzomyia.11 The vectors are commonly divided into Old World species, dominated by the Phlebotomus genus, and New World species, which exclusively refers to the Lutzomyia genus.3 The Old World and New World distinction helps to classify the various vectors and subsequently the diseases they transmit. Old World refers to those vectors found in Southwest and Central Asia, the Indian subcontinent, the Middle East, and East Africa, as well as Southern Europe.6 New World refers to vectors found predominantly in Brazil and other parts of Latin America but also Mexico and the United States.6 Sand flies are found to be endemic in 90 countries and on each continent, except Australia.5 Although the vector can be found in a variety of environments, sand flies prefer moist environments that typify tropical and subtropical climates, thus it is not surprising that the highest diversity of Phlebotominae in the world can be found in the Amazon basin.12
Disease Transmission
Leishmania refers to a genus of intracellular protozoa found in both the Old World and the New World that causes a variety of clinical syndromes.5 Approximately 20 Leishmania species are known to cause human disease that includes localized cutaneous, diffuse cutaneous, mucosal cutaneous, and visceral infections.13 Cases of all forms of leishmaniasis worldwide have increased rapidly over the last few decades from multiple factors including war in endemic regions, increased numbers of immunodeficient individuals, and increased travel to endemic areas.14 In the United States, leishmaniasis is caused by both imported and autochthonous forms of transmission and often mirrors recent travel and immigration patterns.14,15
Sand flies also serve as vectors for sandfly fever, also known as Pappataci fever. Although sandfly fever commonly causes a mild febrile illness, it has been shown to be a considerable cause of aseptic meningitis.16 A number of novel Phleboviruses have been isolated as causes of sandfly fever, including Massilia virus, Granada virus, and Punique virus.16-18 A form of sandfly fever caused by the Toscana virus has a predilection for the nervous system and can cause encephalitis.19 Sandfly fever can be found in both the Old World and New World and thus poses a global risk.2 Additionally, Phlebotominae also have been found to transmit the Changuinola virus, a type of bunyavirus that is known to cause febrile illness in Panama.20 Vesicular stomatitis, also carried by sand flies, is a known cause of febrile disease in North and South America, including the United States.2 In 2013, the Niakha virus, a novel type of Rhabdoviridae, was isolated from Phlebotominae in Senegal.21 The sand fly is noted to transmit another type of Rhabdoviridae in India and Africa, known as the Chandipura virus.22 Although originally thought to cause mild febrile disease, it was the primary cause of multiple outbreaks of fatal encephalitis in India in 200323,24 and again in 2012.22
Sand flies also are known to serve as vectors for the bacterium Bartonella bacilliformis, which is responsible for bartonellosis.25 The disease is divided into 2 forms, which can occur separately or in succession, and is endemic to the Andes region of Peru, Ecuador, and Colombia. The first form is Oroya fever, an acute febrile hemolytic anemia that is fatal in 40% to 88% of cases without intervention.25 This bacterium also causes verruga peruana, an endemic form of bacillary angiomatosis that can persist for years.2 Two reports suggested that bartonellosis also can be caused by Bartonella rochalimae and Candidatus Bartonella ancashi.26,27
Vector Control
Prevention is key to reducing the risk of the various diseases caused by the Phlebotominae vector. Vector control often falls into a few categories, including residual sprays, barriers, and topical repellants.3 It appears that residual sprays applied to houses and animal shelters are the most utilized and effective form of control, with the pyrethroid insecticides having the highest sand fly–specific toxicity.3,28 Insecticides also have been applied to animal burrows where sand flies are known to reproduce; one study in Kenya showed a 90% reduction in the sand fly population following treatment of termite and animal burrows with a pyrethroid spray.29 Studies by Perich et al30,31 in 1995 and 2003 showed that using barrier sprays can be an effective protective measure. The investigators applied a 100-m barrier using a pyrethroid spray on vegetation and reported a notable decrease in sand flies for over an 80-day period.30,31
For personal protection, barrier methods are important adjunct methods of preventing individual exposures. Due to the small size of sand flies, ordinary bed nets are not effective and those treated with insecticides should be used,15 which may ultimately prove to be the most sustainable way to prevent sand fly–borne disease.32 Protective attire also should be worn, as sand flies are not able to penetrate clothing.2 N,N-diethyl-meta-toluamide (DEET)–based repellants should be applied to exposed skin.15 Finally, it is important to avoid exposure from dusk to dawn when sand flies are most active.15
Rise in Autochthonous Cutaneous Leishmaniasis in the United States
With the increased amount of worldwide tourism, especially to endemic areas, providers will continue to see rising numbers of leishmaniasis in the United States. It is difficult to determine the incidence of the disease in the United States, but one study has shown that leishmaniasis accounts for 143 of every 1000 dermatologic diseases acquired by South American tourists.33,34 In addition, the number of autochthonous cases reported in the United States continues to grow. Although only 29 cases were reported between 1903 and 1996, 13 cases were reported between 2000 and 2008.35 Another report in 2013 described an additional 3 cases in the states of Texas and Oklahoma.35 The cases have continued to move in a northeasterly pattern, suggesting a possible shift in the location of sand fly populations. Each of these cases in which a specific species of Leishmania was identified showed transmission of Leishmania mexicana.35 Most cases of cutaneous disease have occurred in Texas and Oklahoma. The first known case outside of this region was reported in 2014 in North Dakota.8 Leishmania donovani, brought into the United States with European foxhounds, also is spreading.8 One species of sand fly, Leishmania shannoni, has now been discovered in 16 states,36-42 where it serves as a potential vector for L mexicana.43,44
- European Centre for Disease Prevention and Control. Phlebotomine sand flies—factsheet for experts. https://ecdc.europa.eu/en/disease-vectors/facts/phlebotomine-sand-flies. Accessed January 24, 2018.
- Durden L, Mullen G. Moth flies and sand flies (Psychodidae). Medical And Veterinary Entomology. San Diego, CA: Academic Press; 2002.
- Claborn DM. The biology and control of leishmaniasis vectors. J Glob Infect Dis. 2010;2:127-134.
- Young DG, Duncan MA. Guide to the identification and geographic distribution of Lutzomyia sand flies in Mexico, the West Indies, Central and South America (Diptera: Psychodidae). Mem Am Entomol Inst. 1994;54:1-881.
- Wolff K, Johnson R, Saavedra AP. Systemic parasitic infections. In: Wolff K, Johnson R, Saavedra AP, eds. Fitzpatrick’s Color Atlas and Synopsis of Clinical Dermatology. 7th ed. New York, NY: McGraw-Hill; 2013.
- Herwaldt BL, Magill AJ. Leishmaniasis, visceral. In: Centers for Disease Control and Prevention. CDC Yellow Book. https://wwwnc.cdc.gov/travel/yellowbook/2018/infectious-diseases-related-to-travel/leishmaniasis-visceral. Updated May 31, 2017. Accessed January 24, 2018.
- Lawyer PG, Perkins PV. Leishmaniasis and trypanosomiasis. In: Eldridge BF, Edman JD, eds. Medical Entomology. Dordrecht, Netherlands: Kluwer Academic; 2000.
- Douvoyiannis M, Khromachou T, Byers N, et al. Cutaneous leishmaniasis in North Dakota. Clin Infect Dis. 2014;59:73-75.
- Schaut RG, Robles-Murguia M, Juelsgaard R, et al. Vectorborne transmission of Leishmania infantum from hounds, United States. Emerg Infect Dis. 2015;21:2209-2212 .
- Hennings C, Bloch K, Miller J, et al. What is your diagnosis? New World cutaneous leishmaniasis. Cutis. 2015;95:208, 229-230.
- Lewis DJ. Phlebotomid sandflies. Bull World Health Organ. 1971;44:535-551.
- Alves VR, Freitas RA, Santos FL, et al. Sand flies (Diptera, Psychodidae, Phlebotominae) from Central Amazonia and four new records for the Amazonas state, Brazil. Rev Bras Entomol. 2012;56:220-227.
- Hashiguchi Y, Gomez EL, Kato H, et al. Diffuse and disseminated cutaneous leishmaniasis: clinical cases experienced in Ecuador and a brief review. Trop Med Health. 2016;44:2.
- Shaw J. The leishmaniases—survival and expansion in a changing world. a mini-review. Mem Inst Oswaldo Cruz. 2007;102:541-547.
- Centers for Disease Control and Prevention. CDC Health Information for International Travel 2016. New York, NY: Oxford University Press; 2016.
- Zhioua E, Moureau G, Chelbi I, et al. Punique virus, a novel phlebovirus, related to sandfly fever Naples virus, isolated from sandflies collected in Tunisia. J Gen Virol. 2010;91:1275-1283.
- Charrel RN, Moureau G, Temmam S, et al. Massilia virus, a novel phlebovirus (Bunyaviridae) isolated from sandflies in the Mediterranean. Vector Borne Zoonotic Dis. 2009;9:519-530.
- Collao X, Palacios G, de Ory F, et al. SecoGranada virus: a natural phlebovirus reassortant of the sandfly fever Naples serocomplex with low seroprevalence in humans. Am J Trop Med Hyg. 2010;83:760-765.
- Alkan C, Bichaud L, de Lamballerie X, et al. Sandfly-borne phleboviruses of Eurasia and Africa: epidemiology, genetic diversity, geographic range, control measures. Antiviral Res. 2013;100:54-74.
- Travassos da Rosa AP, Tesh RB, Pinheiro FP, et al. Characterization of the Changuinola serogroup viruses (Reoviridae: Orbivirus). Intervirology. 1984;21:38-49.
- Vasilakis N, Widen S, Mayer SV, et al. Niakha virus: a novel member of the family Rhabdoviridae isolated from phlebotomine sandflies in Senegal. Virology. 2013;444:80-89.
- Sudeep AB, Bondre VP, Gurav YK, et al. Isolation of Chandipura virus (Vesiculovirus: Rhabdoviridae) from Sergentomyia species of sandflies from Nagpur, Maharashtra, India. Indian J Med Res. 2014;139:769-772.
- Rao BL, Basu A, Wairagkar NS, et al. A large outbreak of acute encephalitis with high fatality rate in children in Andhra Pradesh, India, in 2003, associated with Chandipura virus. Lancet. 2004;364:869-874.
- Chadha MS, Arankalle VA, Jadi RS, et al. An outbreak of Chandipura virus encephalitis in the eastern districts of Gujarat state, India. Am J Trop Med Hyg. 2005;73:566-570.
- Minnick MF, Anderson BE, Lima A, et al. Oroya fever and verruga peruana: bartonelloses unique to South America. PLoS Negl Trop Dis. 2014;8:E2919.
- Eremeeva ME, Gerns HL, Lydy SL, et al. Bacteremia, fever, and splenomegaly caused by a newly recognized bartonella species. N Engl J Med. 2007;356:2381-2387.
- Blazes DL, Mullins K, Smoak BL, et al. Novel bartonella agent as cause of verruga peruana. Emerg Infect Dis. 2013;19:1111-1114.
- Tetreault GE, Zayed AB, Hanafi HA, et al. Suseptibility of sand flies to selected insecticides in North Africa and the Middle East. J Am Mosq Control Assoc. 2001;17:23-27.
- Robert LL, Perich MJ. Phlebotomine sand fly (Diptera:Psychodidae) control using a residual pyrethroid insecticide. J Am Mosq Control Assoc. 1995;11:195-199.
- Perich MJ, Hoch AL, Rizzo N, et al. Insecticide barrier spraying for the control of sandfly vectors of cutaneous leishmaniasis in rural Guatemala. Am J Trop Med Hyg. 1995;52:485-488.
- Perich MJ, Kardec A, Braga IA, et al. Field evaluation of a lethal ovitrap against dengue vectors in Brazil. Med Vet Entomol. 2003;17:205-210.
- Alexander B, Maroli M. Control of phlebotomine sandflies. Medical and Veterinary Entomology. 2003;17:1-18.
- Freedman DO, Weld LH, Kozarsky PE, et al. Spectrum of disease and relation to place of exposure among ill returned travelers. New Engl J Med. 2006;354:119-130.
- Ergen EN, King AH, Tull M. Cutaneous leishmaniasis: an emerging infectious disease in travelers. Cutis. 2015;96:E22-E26.
- Clarke CF, Bradley KK, Wright JH, et al. Emergence of autochthonous cutaneous leishmaniasis in northeastern Texas and southeastern Oklahoma. Am J Trop Med Hyg. 2013;88:157-161.
- Young DG, Perkins PV. Phlebotomine sand flies of North America (Diptera:Psychodidae). Mosq News. 1984;44:263-304.
- Comer JA, Tesh RB, Modi GB, et al. Vesicular stomatitis virus, New Jersey serotype: replication in and transmission by Lutzomyia shannoni (Diptera: Psychodidae). Am J Trop Med Hyg. 1990;42:483-490.
- Haddow A, Curler G, Moulton J. New records of Lutzomyia shannoni and Lutzomyia vexator (Diptera: Psychodidae) in eastern Tennessee. J Vector Ecol. 2008;33:393-396.
- Claborn DM, Rowton ED, Lawyer PG, et al. Species diversity and relative abundance of phlebotomine sand flies (Diptera: Psychodidae) on three Army installations in the southern United States and susceptibility of a domestic sand fly to infection with Old World Leishmania major. Mil Med. 2009;174:1203-1208.
- Minter L, Kovacic B, Claborn DM, et al. New state records for Lutzomyia shannoni (Dyar) and Lutzomyia vexator (Coquillett). J Med Entomol. 2009;46:965-968.
- Price DC, Gunther DE, Gaugler R. First collection records of phlebotomine sand flies (Diptera: Psychodidae) from New Jersey. J Med Entomol. 2011;48:476-478.
- Weng J, Young SL, Gordon DM, et al. First report of phlebotomine sand flies (Diptera: Psychodidae) in Kansas and Missouri, and a PCR method to distinguish Lutzomyia shannoni from Lutzomyia vexator. J Med Entomol. 2012;49:1460-1465.
- Pech-May A, Escobedo-Ortegón FJ, Berzunza-Cruz M, et al. Incrimination of four sandfly species previously unrecognized as vectors of leishmania parasites in Mexico. Med Vet Entomol. 2010;24:150-161.
- González C, Rebollar-Téllez EA, Ibáñez-Bernal S, et al. Current knowledge of leishmania vectors in Mexico: how geographic distributions of species relate to transmission areas. Am J Trop Med Hyg. 2011;85:839-846.
Identification
Phlebotomine sand flies are the only member of the Psychodidae family that are capable of taking blood.1 The mouthparts of the sand fly are toothed distally, and the maxilla and mandible are utilized in a sawtooth fashion to take a bloodmeal.2 The flies are very small (ie, only 1.5–3.5 mm in length), which makes their identification difficult.1 Sand flies can be distinguished by the appearance of their wings, which often are covered in hair and extend across the back in a V shape.3 The adult sand fly is hairy with a 6- to 8-segmented abdomen, and the color can range from gray to yellow to brown.2 Phlebotomine sand flies can be further identified by their long antennae, dark eyes, and small heads (Figure).2

As is the case with all Diptera, the sand fly goes through 4 complete life stages from egg to larva to pupa to adult.3 Female sand flies will lay their eggs following a blood meal and have been found to take multiple blood meals in a single cycle.2 On average, the eggs will hatch in 6 to 17 days but are temperature dependent.3 The subsequent larvae and pupa stages last 20 to 30 days and 6 to 13 days, respectively.1 The larvae are white in color with short antennae and dark heads.4 Sand flies prefer to lay their eggs in areas where adequate resting places are available and where their larvae will thrive.4,5 The larvae require warm moist environments to succeed and thus are commonly found in animal burrows.3 Once fully developed, the adult sand fly can live up to 6 weeks.2
Sand Fly Vector
Although it is more common in rural forested areas, the sand fly also can be found in urban areas, including heavily populated cities in Brazil.6 Sand flies are most active during hot humid seasons but depending on the local climate may remain active year-round.1,7 For example, in tropical regions of Asia, the number of sand flies increases substantially during the monsoon season compared to the dry season.2 Phlebotomine sand flies are most active at dusk and during the night5 but may become agitated during the daytime if their environment is disturbed.1
Host selection usually is broad and includes a wide variety of vertebrates.2 In the United States, host species are thought to include small rodents, foxes, armadillos, and opossums.8 One study found that visceral leishmaniasis in foxhounds is able to develop fully in sand flies, thus posing an emerging risk to the American population.9
Distribution
The Phlebotominae family contains approximately 700 different species of sand flies but only 21 are known vectors of disease.10 The great majority belong to 1 of 3 genuses: Phlebotomus, Sergentomyia, and Lutzomyia.11 The vectors are commonly divided into Old World species, dominated by the Phlebotomus genus, and New World species, which exclusively refers to the Lutzomyia genus.3 The Old World and New World distinction helps to classify the various vectors and subsequently the diseases they transmit. Old World refers to those vectors found in Southwest and Central Asia, the Indian subcontinent, the Middle East, and East Africa, as well as Southern Europe.6 New World refers to vectors found predominantly in Brazil and other parts of Latin America but also Mexico and the United States.6 Sand flies are found to be endemic in 90 countries and on each continent, except Australia.5 Although the vector can be found in a variety of environments, sand flies prefer moist environments that typify tropical and subtropical climates, thus it is not surprising that the highest diversity of Phlebotominae in the world can be found in the Amazon basin.12
Disease Transmission
Leishmania refers to a genus of intracellular protozoa found in both the Old World and the New World that causes a variety of clinical syndromes.5 Approximately 20 Leishmania species are known to cause human disease that includes localized cutaneous, diffuse cutaneous, mucosal cutaneous, and visceral infections.13 Cases of all forms of leishmaniasis worldwide have increased rapidly over the last few decades from multiple factors including war in endemic regions, increased numbers of immunodeficient individuals, and increased travel to endemic areas.14 In the United States, leishmaniasis is caused by both imported and autochthonous forms of transmission and often mirrors recent travel and immigration patterns.14,15
Sand flies also serve as vectors for sandfly fever, also known as Pappataci fever. Although sandfly fever commonly causes a mild febrile illness, it has been shown to be a considerable cause of aseptic meningitis.16 A number of novel Phleboviruses have been isolated as causes of sandfly fever, including Massilia virus, Granada virus, and Punique virus.16-18 A form of sandfly fever caused by the Toscana virus has a predilection for the nervous system and can cause encephalitis.19 Sandfly fever can be found in both the Old World and New World and thus poses a global risk.2 Additionally, Phlebotominae also have been found to transmit the Changuinola virus, a type of bunyavirus that is known to cause febrile illness in Panama.20 Vesicular stomatitis, also carried by sand flies, is a known cause of febrile disease in North and South America, including the United States.2 In 2013, the Niakha virus, a novel type of Rhabdoviridae, was isolated from Phlebotominae in Senegal.21 The sand fly is noted to transmit another type of Rhabdoviridae in India and Africa, known as the Chandipura virus.22 Although originally thought to cause mild febrile disease, it was the primary cause of multiple outbreaks of fatal encephalitis in India in 200323,24 and again in 2012.22
Sand flies also are known to serve as vectors for the bacterium Bartonella bacilliformis, which is responsible for bartonellosis.25 The disease is divided into 2 forms, which can occur separately or in succession, and is endemic to the Andes region of Peru, Ecuador, and Colombia. The first form is Oroya fever, an acute febrile hemolytic anemia that is fatal in 40% to 88% of cases without intervention.25 This bacterium also causes verruga peruana, an endemic form of bacillary angiomatosis that can persist for years.2 Two reports suggested that bartonellosis also can be caused by Bartonella rochalimae and Candidatus Bartonella ancashi.26,27
Vector Control
Prevention is key to reducing the risk of the various diseases caused by the Phlebotominae vector. Vector control often falls into a few categories, including residual sprays, barriers, and topical repellants.3 It appears that residual sprays applied to houses and animal shelters are the most utilized and effective form of control, with the pyrethroid insecticides having the highest sand fly–specific toxicity.3,28 Insecticides also have been applied to animal burrows where sand flies are known to reproduce; one study in Kenya showed a 90% reduction in the sand fly population following treatment of termite and animal burrows with a pyrethroid spray.29 Studies by Perich et al30,31 in 1995 and 2003 showed that using barrier sprays can be an effective protective measure. The investigators applied a 100-m barrier using a pyrethroid spray on vegetation and reported a notable decrease in sand flies for over an 80-day period.30,31
For personal protection, barrier methods are important adjunct methods of preventing individual exposures. Due to the small size of sand flies, ordinary bed nets are not effective and those treated with insecticides should be used,15 which may ultimately prove to be the most sustainable way to prevent sand fly–borne disease.32 Protective attire also should be worn, as sand flies are not able to penetrate clothing.2 N,N-diethyl-meta-toluamide (DEET)–based repellants should be applied to exposed skin.15 Finally, it is important to avoid exposure from dusk to dawn when sand flies are most active.15
Rise in Autochthonous Cutaneous Leishmaniasis in the United States
With the increased amount of worldwide tourism, especially to endemic areas, providers will continue to see rising numbers of leishmaniasis in the United States. It is difficult to determine the incidence of the disease in the United States, but one study has shown that leishmaniasis accounts for 143 of every 1000 dermatologic diseases acquired by South American tourists.33,34 In addition, the number of autochthonous cases reported in the United States continues to grow. Although only 29 cases were reported between 1903 and 1996, 13 cases were reported between 2000 and 2008.35 Another report in 2013 described an additional 3 cases in the states of Texas and Oklahoma.35 The cases have continued to move in a northeasterly pattern, suggesting a possible shift in the location of sand fly populations. Each of these cases in which a specific species of Leishmania was identified showed transmission of Leishmania mexicana.35 Most cases of cutaneous disease have occurred in Texas and Oklahoma. The first known case outside of this region was reported in 2014 in North Dakota.8 Leishmania donovani, brought into the United States with European foxhounds, also is spreading.8 One species of sand fly, Leishmania shannoni, has now been discovered in 16 states,36-42 where it serves as a potential vector for L mexicana.43,44
Identification
Phlebotomine sand flies are the only member of the Psychodidae family that are capable of taking blood.1 The mouthparts of the sand fly are toothed distally, and the maxilla and mandible are utilized in a sawtooth fashion to take a bloodmeal.2 The flies are very small (ie, only 1.5–3.5 mm in length), which makes their identification difficult.1 Sand flies can be distinguished by the appearance of their wings, which often are covered in hair and extend across the back in a V shape.3 The adult sand fly is hairy with a 6- to 8-segmented abdomen, and the color can range from gray to yellow to brown.2 Phlebotomine sand flies can be further identified by their long antennae, dark eyes, and small heads (Figure).2

As is the case with all Diptera, the sand fly goes through 4 complete life stages from egg to larva to pupa to adult.3 Female sand flies will lay their eggs following a blood meal and have been found to take multiple blood meals in a single cycle.2 On average, the eggs will hatch in 6 to 17 days but are temperature dependent.3 The subsequent larvae and pupa stages last 20 to 30 days and 6 to 13 days, respectively.1 The larvae are white in color with short antennae and dark heads.4 Sand flies prefer to lay their eggs in areas where adequate resting places are available and where their larvae will thrive.4,5 The larvae require warm moist environments to succeed and thus are commonly found in animal burrows.3 Once fully developed, the adult sand fly can live up to 6 weeks.2
Sand Fly Vector
Although it is more common in rural forested areas, the sand fly also can be found in urban areas, including heavily populated cities in Brazil.6 Sand flies are most active during hot humid seasons but depending on the local climate may remain active year-round.1,7 For example, in tropical regions of Asia, the number of sand flies increases substantially during the monsoon season compared to the dry season.2 Phlebotomine sand flies are most active at dusk and during the night5 but may become agitated during the daytime if their environment is disturbed.1
Host selection usually is broad and includes a wide variety of vertebrates.2 In the United States, host species are thought to include small rodents, foxes, armadillos, and opossums.8 One study found that visceral leishmaniasis in foxhounds is able to develop fully in sand flies, thus posing an emerging risk to the American population.9
Distribution
The Phlebotominae family contains approximately 700 different species of sand flies but only 21 are known vectors of disease.10 The great majority belong to 1 of 3 genuses: Phlebotomus, Sergentomyia, and Lutzomyia.11 The vectors are commonly divided into Old World species, dominated by the Phlebotomus genus, and New World species, which exclusively refers to the Lutzomyia genus.3 The Old World and New World distinction helps to classify the various vectors and subsequently the diseases they transmit. Old World refers to those vectors found in Southwest and Central Asia, the Indian subcontinent, the Middle East, and East Africa, as well as Southern Europe.6 New World refers to vectors found predominantly in Brazil and other parts of Latin America but also Mexico and the United States.6 Sand flies are found to be endemic in 90 countries and on each continent, except Australia.5 Although the vector can be found in a variety of environments, sand flies prefer moist environments that typify tropical and subtropical climates, thus it is not surprising that the highest diversity of Phlebotominae in the world can be found in the Amazon basin.12
Disease Transmission
Leishmania refers to a genus of intracellular protozoa found in both the Old World and the New World that causes a variety of clinical syndromes.5 Approximately 20 Leishmania species are known to cause human disease that includes localized cutaneous, diffuse cutaneous, mucosal cutaneous, and visceral infections.13 Cases of all forms of leishmaniasis worldwide have increased rapidly over the last few decades from multiple factors including war in endemic regions, increased numbers of immunodeficient individuals, and increased travel to endemic areas.14 In the United States, leishmaniasis is caused by both imported and autochthonous forms of transmission and often mirrors recent travel and immigration patterns.14,15
Sand flies also serve as vectors for sandfly fever, also known as Pappataci fever. Although sandfly fever commonly causes a mild febrile illness, it has been shown to be a considerable cause of aseptic meningitis.16 A number of novel Phleboviruses have been isolated as causes of sandfly fever, including Massilia virus, Granada virus, and Punique virus.16-18 A form of sandfly fever caused by the Toscana virus has a predilection for the nervous system and can cause encephalitis.19 Sandfly fever can be found in both the Old World and New World and thus poses a global risk.2 Additionally, Phlebotominae also have been found to transmit the Changuinola virus, a type of bunyavirus that is known to cause febrile illness in Panama.20 Vesicular stomatitis, also carried by sand flies, is a known cause of febrile disease in North and South America, including the United States.2 In 2013, the Niakha virus, a novel type of Rhabdoviridae, was isolated from Phlebotominae in Senegal.21 The sand fly is noted to transmit another type of Rhabdoviridae in India and Africa, known as the Chandipura virus.22 Although originally thought to cause mild febrile disease, it was the primary cause of multiple outbreaks of fatal encephalitis in India in 200323,24 and again in 2012.22
Sand flies also are known to serve as vectors for the bacterium Bartonella bacilliformis, which is responsible for bartonellosis.25 The disease is divided into 2 forms, which can occur separately or in succession, and is endemic to the Andes region of Peru, Ecuador, and Colombia. The first form is Oroya fever, an acute febrile hemolytic anemia that is fatal in 40% to 88% of cases without intervention.25 This bacterium also causes verruga peruana, an endemic form of bacillary angiomatosis that can persist for years.2 Two reports suggested that bartonellosis also can be caused by Bartonella rochalimae and Candidatus Bartonella ancashi.26,27
Vector Control
Prevention is key to reducing the risk of the various diseases caused by the Phlebotominae vector. Vector control often falls into a few categories, including residual sprays, barriers, and topical repellants.3 It appears that residual sprays applied to houses and animal shelters are the most utilized and effective form of control, with the pyrethroid insecticides having the highest sand fly–specific toxicity.3,28 Insecticides also have been applied to animal burrows where sand flies are known to reproduce; one study in Kenya showed a 90% reduction in the sand fly population following treatment of termite and animal burrows with a pyrethroid spray.29 Studies by Perich et al30,31 in 1995 and 2003 showed that using barrier sprays can be an effective protective measure. The investigators applied a 100-m barrier using a pyrethroid spray on vegetation and reported a notable decrease in sand flies for over an 80-day period.30,31
For personal protection, barrier methods are important adjunct methods of preventing individual exposures. Due to the small size of sand flies, ordinary bed nets are not effective and those treated with insecticides should be used,15 which may ultimately prove to be the most sustainable way to prevent sand fly–borne disease.32 Protective attire also should be worn, as sand flies are not able to penetrate clothing.2 N,N-diethyl-meta-toluamide (DEET)–based repellants should be applied to exposed skin.15 Finally, it is important to avoid exposure from dusk to dawn when sand flies are most active.15
Rise in Autochthonous Cutaneous Leishmaniasis in the United States
With the increased amount of worldwide tourism, especially to endemic areas, providers will continue to see rising numbers of leishmaniasis in the United States. It is difficult to determine the incidence of the disease in the United States, but one study has shown that leishmaniasis accounts for 143 of every 1000 dermatologic diseases acquired by South American tourists.33,34 In addition, the number of autochthonous cases reported in the United States continues to grow. Although only 29 cases were reported between 1903 and 1996, 13 cases were reported between 2000 and 2008.35 Another report in 2013 described an additional 3 cases in the states of Texas and Oklahoma.35 The cases have continued to move in a northeasterly pattern, suggesting a possible shift in the location of sand fly populations. Each of these cases in which a specific species of Leishmania was identified showed transmission of Leishmania mexicana.35 Most cases of cutaneous disease have occurred in Texas and Oklahoma. The first known case outside of this region was reported in 2014 in North Dakota.8 Leishmania donovani, brought into the United States with European foxhounds, also is spreading.8 One species of sand fly, Leishmania shannoni, has now been discovered in 16 states,36-42 where it serves as a potential vector for L mexicana.43,44
- European Centre for Disease Prevention and Control. Phlebotomine sand flies—factsheet for experts. https://ecdc.europa.eu/en/disease-vectors/facts/phlebotomine-sand-flies. Accessed January 24, 2018.
- Durden L, Mullen G. Moth flies and sand flies (Psychodidae). Medical And Veterinary Entomology. San Diego, CA: Academic Press; 2002.
- Claborn DM. The biology and control of leishmaniasis vectors. J Glob Infect Dis. 2010;2:127-134.
- Young DG, Duncan MA. Guide to the identification and geographic distribution of Lutzomyia sand flies in Mexico, the West Indies, Central and South America (Diptera: Psychodidae). Mem Am Entomol Inst. 1994;54:1-881.
- Wolff K, Johnson R, Saavedra AP. Systemic parasitic infections. In: Wolff K, Johnson R, Saavedra AP, eds. Fitzpatrick’s Color Atlas and Synopsis of Clinical Dermatology. 7th ed. New York, NY: McGraw-Hill; 2013.
- Herwaldt BL, Magill AJ. Leishmaniasis, visceral. In: Centers for Disease Control and Prevention. CDC Yellow Book. https://wwwnc.cdc.gov/travel/yellowbook/2018/infectious-diseases-related-to-travel/leishmaniasis-visceral. Updated May 31, 2017. Accessed January 24, 2018.
- Lawyer PG, Perkins PV. Leishmaniasis and trypanosomiasis. In: Eldridge BF, Edman JD, eds. Medical Entomology. Dordrecht, Netherlands: Kluwer Academic; 2000.
- Douvoyiannis M, Khromachou T, Byers N, et al. Cutaneous leishmaniasis in North Dakota. Clin Infect Dis. 2014;59:73-75.
- Schaut RG, Robles-Murguia M, Juelsgaard R, et al. Vectorborne transmission of Leishmania infantum from hounds, United States. Emerg Infect Dis. 2015;21:2209-2212 .
- Hennings C, Bloch K, Miller J, et al. What is your diagnosis? New World cutaneous leishmaniasis. Cutis. 2015;95:208, 229-230.
- Lewis DJ. Phlebotomid sandflies. Bull World Health Organ. 1971;44:535-551.
- Alves VR, Freitas RA, Santos FL, et al. Sand flies (Diptera, Psychodidae, Phlebotominae) from Central Amazonia and four new records for the Amazonas state, Brazil. Rev Bras Entomol. 2012;56:220-227.
- Hashiguchi Y, Gomez EL, Kato H, et al. Diffuse and disseminated cutaneous leishmaniasis: clinical cases experienced in Ecuador and a brief review. Trop Med Health. 2016;44:2.
- Shaw J. The leishmaniases—survival and expansion in a changing world. a mini-review. Mem Inst Oswaldo Cruz. 2007;102:541-547.
- Centers for Disease Control and Prevention. CDC Health Information for International Travel 2016. New York, NY: Oxford University Press; 2016.
- Zhioua E, Moureau G, Chelbi I, et al. Punique virus, a novel phlebovirus, related to sandfly fever Naples virus, isolated from sandflies collected in Tunisia. J Gen Virol. 2010;91:1275-1283.
- Charrel RN, Moureau G, Temmam S, et al. Massilia virus, a novel phlebovirus (Bunyaviridae) isolated from sandflies in the Mediterranean. Vector Borne Zoonotic Dis. 2009;9:519-530.
- Collao X, Palacios G, de Ory F, et al. SecoGranada virus: a natural phlebovirus reassortant of the sandfly fever Naples serocomplex with low seroprevalence in humans. Am J Trop Med Hyg. 2010;83:760-765.
- Alkan C, Bichaud L, de Lamballerie X, et al. Sandfly-borne phleboviruses of Eurasia and Africa: epidemiology, genetic diversity, geographic range, control measures. Antiviral Res. 2013;100:54-74.
- Travassos da Rosa AP, Tesh RB, Pinheiro FP, et al. Characterization of the Changuinola serogroup viruses (Reoviridae: Orbivirus). Intervirology. 1984;21:38-49.
- Vasilakis N, Widen S, Mayer SV, et al. Niakha virus: a novel member of the family Rhabdoviridae isolated from phlebotomine sandflies in Senegal. Virology. 2013;444:80-89.
- Sudeep AB, Bondre VP, Gurav YK, et al. Isolation of Chandipura virus (Vesiculovirus: Rhabdoviridae) from Sergentomyia species of sandflies from Nagpur, Maharashtra, India. Indian J Med Res. 2014;139:769-772.
- Rao BL, Basu A, Wairagkar NS, et al. A large outbreak of acute encephalitis with high fatality rate in children in Andhra Pradesh, India, in 2003, associated with Chandipura virus. Lancet. 2004;364:869-874.
- Chadha MS, Arankalle VA, Jadi RS, et al. An outbreak of Chandipura virus encephalitis in the eastern districts of Gujarat state, India. Am J Trop Med Hyg. 2005;73:566-570.
- Minnick MF, Anderson BE, Lima A, et al. Oroya fever and verruga peruana: bartonelloses unique to South America. PLoS Negl Trop Dis. 2014;8:E2919.
- Eremeeva ME, Gerns HL, Lydy SL, et al. Bacteremia, fever, and splenomegaly caused by a newly recognized bartonella species. N Engl J Med. 2007;356:2381-2387.
- Blazes DL, Mullins K, Smoak BL, et al. Novel bartonella agent as cause of verruga peruana. Emerg Infect Dis. 2013;19:1111-1114.
- Tetreault GE, Zayed AB, Hanafi HA, et al. Suseptibility of sand flies to selected insecticides in North Africa and the Middle East. J Am Mosq Control Assoc. 2001;17:23-27.
- Robert LL, Perich MJ. Phlebotomine sand fly (Diptera:Psychodidae) control using a residual pyrethroid insecticide. J Am Mosq Control Assoc. 1995;11:195-199.
- Perich MJ, Hoch AL, Rizzo N, et al. Insecticide barrier spraying for the control of sandfly vectors of cutaneous leishmaniasis in rural Guatemala. Am J Trop Med Hyg. 1995;52:485-488.
- Perich MJ, Kardec A, Braga IA, et al. Field evaluation of a lethal ovitrap against dengue vectors in Brazil. Med Vet Entomol. 2003;17:205-210.
- Alexander B, Maroli M. Control of phlebotomine sandflies. Medical and Veterinary Entomology. 2003;17:1-18.
- Freedman DO, Weld LH, Kozarsky PE, et al. Spectrum of disease and relation to place of exposure among ill returned travelers. New Engl J Med. 2006;354:119-130.
- Ergen EN, King AH, Tull M. Cutaneous leishmaniasis: an emerging infectious disease in travelers. Cutis. 2015;96:E22-E26.
- Clarke CF, Bradley KK, Wright JH, et al. Emergence of autochthonous cutaneous leishmaniasis in northeastern Texas and southeastern Oklahoma. Am J Trop Med Hyg. 2013;88:157-161.
- Young DG, Perkins PV. Phlebotomine sand flies of North America (Diptera:Psychodidae). Mosq News. 1984;44:263-304.
- Comer JA, Tesh RB, Modi GB, et al. Vesicular stomatitis virus, New Jersey serotype: replication in and transmission by Lutzomyia shannoni (Diptera: Psychodidae). Am J Trop Med Hyg. 1990;42:483-490.
- Haddow A, Curler G, Moulton J. New records of Lutzomyia shannoni and Lutzomyia vexator (Diptera: Psychodidae) in eastern Tennessee. J Vector Ecol. 2008;33:393-396.
- Claborn DM, Rowton ED, Lawyer PG, et al. Species diversity and relative abundance of phlebotomine sand flies (Diptera: Psychodidae) on three Army installations in the southern United States and susceptibility of a domestic sand fly to infection with Old World Leishmania major. Mil Med. 2009;174:1203-1208.
- Minter L, Kovacic B, Claborn DM, et al. New state records for Lutzomyia shannoni (Dyar) and Lutzomyia vexator (Coquillett). J Med Entomol. 2009;46:965-968.
- Price DC, Gunther DE, Gaugler R. First collection records of phlebotomine sand flies (Diptera: Psychodidae) from New Jersey. J Med Entomol. 2011;48:476-478.
- Weng J, Young SL, Gordon DM, et al. First report of phlebotomine sand flies (Diptera: Psychodidae) in Kansas and Missouri, and a PCR method to distinguish Lutzomyia shannoni from Lutzomyia vexator. J Med Entomol. 2012;49:1460-1465.
- Pech-May A, Escobedo-Ortegón FJ, Berzunza-Cruz M, et al. Incrimination of four sandfly species previously unrecognized as vectors of leishmania parasites in Mexico. Med Vet Entomol. 2010;24:150-161.
- González C, Rebollar-Téllez EA, Ibáñez-Bernal S, et al. Current knowledge of leishmania vectors in Mexico: how geographic distributions of species relate to transmission areas. Am J Trop Med Hyg. 2011;85:839-846.
- European Centre for Disease Prevention and Control. Phlebotomine sand flies—factsheet for experts. https://ecdc.europa.eu/en/disease-vectors/facts/phlebotomine-sand-flies. Accessed January 24, 2018.
- Durden L, Mullen G. Moth flies and sand flies (Psychodidae). Medical And Veterinary Entomology. San Diego, CA: Academic Press; 2002.
- Claborn DM. The biology and control of leishmaniasis vectors. J Glob Infect Dis. 2010;2:127-134.
- Young DG, Duncan MA. Guide to the identification and geographic distribution of Lutzomyia sand flies in Mexico, the West Indies, Central and South America (Diptera: Psychodidae). Mem Am Entomol Inst. 1994;54:1-881.
- Wolff K, Johnson R, Saavedra AP. Systemic parasitic infections. In: Wolff K, Johnson R, Saavedra AP, eds. Fitzpatrick’s Color Atlas and Synopsis of Clinical Dermatology. 7th ed. New York, NY: McGraw-Hill; 2013.
- Herwaldt BL, Magill AJ. Leishmaniasis, visceral. In: Centers for Disease Control and Prevention. CDC Yellow Book. https://wwwnc.cdc.gov/travel/yellowbook/2018/infectious-diseases-related-to-travel/leishmaniasis-visceral. Updated May 31, 2017. Accessed January 24, 2018.
- Lawyer PG, Perkins PV. Leishmaniasis and trypanosomiasis. In: Eldridge BF, Edman JD, eds. Medical Entomology. Dordrecht, Netherlands: Kluwer Academic; 2000.
- Douvoyiannis M, Khromachou T, Byers N, et al. Cutaneous leishmaniasis in North Dakota. Clin Infect Dis. 2014;59:73-75.
- Schaut RG, Robles-Murguia M, Juelsgaard R, et al. Vectorborne transmission of Leishmania infantum from hounds, United States. Emerg Infect Dis. 2015;21:2209-2212 .
- Hennings C, Bloch K, Miller J, et al. What is your diagnosis? New World cutaneous leishmaniasis. Cutis. 2015;95:208, 229-230.
- Lewis DJ. Phlebotomid sandflies. Bull World Health Organ. 1971;44:535-551.
- Alves VR, Freitas RA, Santos FL, et al. Sand flies (Diptera, Psychodidae, Phlebotominae) from Central Amazonia and four new records for the Amazonas state, Brazil. Rev Bras Entomol. 2012;56:220-227.
- Hashiguchi Y, Gomez EL, Kato H, et al. Diffuse and disseminated cutaneous leishmaniasis: clinical cases experienced in Ecuador and a brief review. Trop Med Health. 2016;44:2.
- Shaw J. The leishmaniases—survival and expansion in a changing world. a mini-review. Mem Inst Oswaldo Cruz. 2007;102:541-547.
- Centers for Disease Control and Prevention. CDC Health Information for International Travel 2016. New York, NY: Oxford University Press; 2016.
- Zhioua E, Moureau G, Chelbi I, et al. Punique virus, a novel phlebovirus, related to sandfly fever Naples virus, isolated from sandflies collected in Tunisia. J Gen Virol. 2010;91:1275-1283.
- Charrel RN, Moureau G, Temmam S, et al. Massilia virus, a novel phlebovirus (Bunyaviridae) isolated from sandflies in the Mediterranean. Vector Borne Zoonotic Dis. 2009;9:519-530.
- Collao X, Palacios G, de Ory F, et al. SecoGranada virus: a natural phlebovirus reassortant of the sandfly fever Naples serocomplex with low seroprevalence in humans. Am J Trop Med Hyg. 2010;83:760-765.
- Alkan C, Bichaud L, de Lamballerie X, et al. Sandfly-borne phleboviruses of Eurasia and Africa: epidemiology, genetic diversity, geographic range, control measures. Antiviral Res. 2013;100:54-74.
- Travassos da Rosa AP, Tesh RB, Pinheiro FP, et al. Characterization of the Changuinola serogroup viruses (Reoviridae: Orbivirus). Intervirology. 1984;21:38-49.
- Vasilakis N, Widen S, Mayer SV, et al. Niakha virus: a novel member of the family Rhabdoviridae isolated from phlebotomine sandflies in Senegal. Virology. 2013;444:80-89.
- Sudeep AB, Bondre VP, Gurav YK, et al. Isolation of Chandipura virus (Vesiculovirus: Rhabdoviridae) from Sergentomyia species of sandflies from Nagpur, Maharashtra, India. Indian J Med Res. 2014;139:769-772.
- Rao BL, Basu A, Wairagkar NS, et al. A large outbreak of acute encephalitis with high fatality rate in children in Andhra Pradesh, India, in 2003, associated with Chandipura virus. Lancet. 2004;364:869-874.
- Chadha MS, Arankalle VA, Jadi RS, et al. An outbreak of Chandipura virus encephalitis in the eastern districts of Gujarat state, India. Am J Trop Med Hyg. 2005;73:566-570.
- Minnick MF, Anderson BE, Lima A, et al. Oroya fever and verruga peruana: bartonelloses unique to South America. PLoS Negl Trop Dis. 2014;8:E2919.
- Eremeeva ME, Gerns HL, Lydy SL, et al. Bacteremia, fever, and splenomegaly caused by a newly recognized bartonella species. N Engl J Med. 2007;356:2381-2387.
- Blazes DL, Mullins K, Smoak BL, et al. Novel bartonella agent as cause of verruga peruana. Emerg Infect Dis. 2013;19:1111-1114.
- Tetreault GE, Zayed AB, Hanafi HA, et al. Suseptibility of sand flies to selected insecticides in North Africa and the Middle East. J Am Mosq Control Assoc. 2001;17:23-27.
- Robert LL, Perich MJ. Phlebotomine sand fly (Diptera:Psychodidae) control using a residual pyrethroid insecticide. J Am Mosq Control Assoc. 1995;11:195-199.
- Perich MJ, Hoch AL, Rizzo N, et al. Insecticide barrier spraying for the control of sandfly vectors of cutaneous leishmaniasis in rural Guatemala. Am J Trop Med Hyg. 1995;52:485-488.
- Perich MJ, Kardec A, Braga IA, et al. Field evaluation of a lethal ovitrap against dengue vectors in Brazil. Med Vet Entomol. 2003;17:205-210.
- Alexander B, Maroli M. Control of phlebotomine sandflies. Medical and Veterinary Entomology. 2003;17:1-18.
- Freedman DO, Weld LH, Kozarsky PE, et al. Spectrum of disease and relation to place of exposure among ill returned travelers. New Engl J Med. 2006;354:119-130.
- Ergen EN, King AH, Tull M. Cutaneous leishmaniasis: an emerging infectious disease in travelers. Cutis. 2015;96:E22-E26.
- Clarke CF, Bradley KK, Wright JH, et al. Emergence of autochthonous cutaneous leishmaniasis in northeastern Texas and southeastern Oklahoma. Am J Trop Med Hyg. 2013;88:157-161.
- Young DG, Perkins PV. Phlebotomine sand flies of North America (Diptera:Psychodidae). Mosq News. 1984;44:263-304.
- Comer JA, Tesh RB, Modi GB, et al. Vesicular stomatitis virus, New Jersey serotype: replication in and transmission by Lutzomyia shannoni (Diptera: Psychodidae). Am J Trop Med Hyg. 1990;42:483-490.
- Haddow A, Curler G, Moulton J. New records of Lutzomyia shannoni and Lutzomyia vexator (Diptera: Psychodidae) in eastern Tennessee. J Vector Ecol. 2008;33:393-396.
- Claborn DM, Rowton ED, Lawyer PG, et al. Species diversity and relative abundance of phlebotomine sand flies (Diptera: Psychodidae) on three Army installations in the southern United States and susceptibility of a domestic sand fly to infection with Old World Leishmania major. Mil Med. 2009;174:1203-1208.
- Minter L, Kovacic B, Claborn DM, et al. New state records for Lutzomyia shannoni (Dyar) and Lutzomyia vexator (Coquillett). J Med Entomol. 2009;46:965-968.
- Price DC, Gunther DE, Gaugler R. First collection records of phlebotomine sand flies (Diptera: Psychodidae) from New Jersey. J Med Entomol. 2011;48:476-478.
- Weng J, Young SL, Gordon DM, et al. First report of phlebotomine sand flies (Diptera: Psychodidae) in Kansas and Missouri, and a PCR method to distinguish Lutzomyia shannoni from Lutzomyia vexator. J Med Entomol. 2012;49:1460-1465.
- Pech-May A, Escobedo-Ortegón FJ, Berzunza-Cruz M, et al. Incrimination of four sandfly species previously unrecognized as vectors of leishmania parasites in Mexico. Med Vet Entomol. 2010;24:150-161.
- González C, Rebollar-Téllez EA, Ibáñez-Bernal S, et al. Current knowledge of leishmania vectors in Mexico: how geographic distributions of species relate to transmission areas. Am J Trop Med Hyg. 2011;85:839-846.
Practice Points
- Sand flies cause a wide array of cutaneous and systemic diseases worldwide.
- Identification and treatment of leishmaniasis and other diseases transmitted by sand flies requires a high degree of clinical suspicion.
- With the increase in global travel and the rise of autochthonous disease in the United States, American physicians must increase their awareness of diseases for which sand flies serve as vectors.
Purpuric Macule of the Right Axilla
The Diagnosis: Atypical Vascular Lesion
Atypical vascular lesion (AVL)(quiz image), named by Fineberg and Rosen,1 is a vascular lesion that arises on mammary skin with a history of radiation exposure. Clinically, AVL can present as a papule or erythematous patch that manifests 3 to 7 years after radiation therapy.2,3 There are 2 histologic subtypes of AVL: lymphatic and vascular.2,4 Lymphatic-type AVL is comprised of a symmetric distribution of thin, dilated, and anastomosing vessels usually found in the superficial and mid dermis. The vessels are lined by flat or hobnail protuberant endothelial cells that lack nuclear irregularity or pleomorphism; however, hyperchromatism of endothelial cell nuclei is a common finding. Vascular-type AVL is morphologically similar to a capillary hemangioma, and histologic features include irregular growth of capillary-sized vessels that extend to the dermis and subcutis.2,4 Atypical vascular lesions are benign lesions but may be a precursor to angiosarcoma. Along with vascular markers, D2-40 typically is positive. Surgical excision with clear margins is recommended when the lesion is small.4,5 Observation is more appropriate for extensive lesions.
Angiosarcoma can arise spontaneously or in association with radiation or chronic lymphedema. Given the shared risk factors and presentation with AVL, it is essential to differentiate angiosarcoma from AVL. Primary cutaneous angiosarcoma usually presents on the head of elderly patients as an ecchymotic patch or plaque with ulceration.4 Secondary angiosarcoma may arise following radiation or chronic lymphedema (Stewart-Treves syndrome); however, some authors now prefer to consider lymphangiosarcoma arising in chronic lymphedematous limbs a distinct entity.6 Surgical excision with wide margins is the mainstay of therapy, but angiosarcoma has high recurrence rates, and the 5-year survival rate has been reported to be as low as 35%.7 Histologic overlap with AVL includes dissecting anastomosing vessels lined by hyperchromatic nuclei; however, angiosarcoma is distinguished by endothelial cell layering, nuclear pleomorphism, and prominent nucleoli (Figure 1).4,8 Increased positivity for Ki-67 immunostain, which indicates cell proliferation, may be used to distinguish angiosarcoma from an AVL (Figure 1 [inset]).9 Further, in contrast to AVL, radiation-induced angiosarcoma is characterized by amplification of C-MYC, a regulator gene, and FLT4 (FMS-related tyrosine kinase 4), a gene encoding vascular endothelial growth factor receptor 3. Gene amplification may be detected through immunohistochemistry or fluorescence in situ hybridization.10 Ki-67 labeling showed less than 10% staining in endothelial cells in our case (quiz image [inset]), and fluorescence in situ hybridization was negative for C-MYC amplification, supporting the diagnosis of AVL.
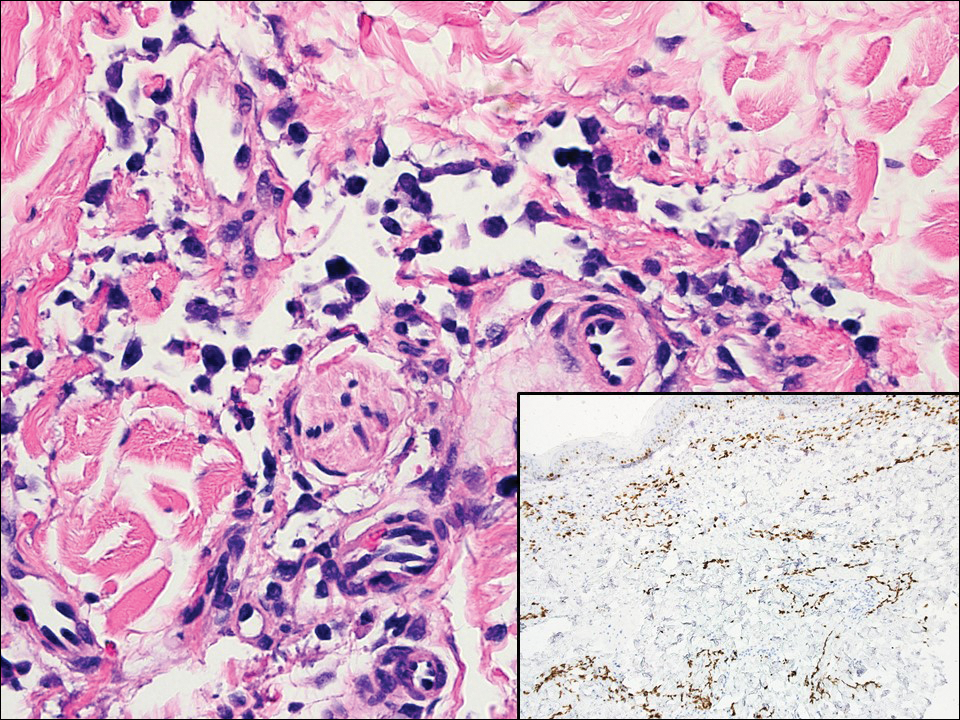
Lymphangioma circumscriptum, the most common superficial lymphangioma, is a hamartomatous malformation that usually occurs at the axillary folds, neck, and trunk. It clinically presents as small agminated vesicles with a characteristic frog spawn appearance.11 Dermoscopic features include yellow lacunae that may alternate with a dark red color secondary to extravasation of erythrocytes.12 These clinical features often lead to a differential diagnosis of verrucae, angiokeratoma, and angiosarcoma. Lymphangioma circumscriptum histologically is characterized by an overgrowth of dilated lymphatic vessels that fill the papillary dermis. The vessels are composed of flat endothelial cells typically filled with acellular proteinaceous debris and occasional erythrocytes (Figure 2). As the lesion traverses deeper into the dermis, the caliber of the lymphatic channel becomes narrower. The presence of deep lymphatic cisterns with surrounding smooth muscle is helpful to differentiate lymphangioma circumscriptum from other lymphatic malformations such as acquired lymphangiectasia. Treatment options include surgical excision, sclerosing agents, and destructive modalities such as cryotherapy.
Hobnail hemangioma, originally termed targetoid hemosiderotic hemangioma by Santa Cruz and Aronberg,13 presents as a violaceous papule or nodule surrounded by a characteristic brown halo on the leg. Trauma has been proposed as the inciting factor for the clinical appearance of hobnail hemangioma.14 Microscopically, the lesion shows vessels in a wedge shape. The superficial component has telangiectatic vessels with focal areas of papillary projections lined by endothelial cells. Although the endothelial nuclei typically project into the lumen, the nuclei are small, bland, and without mitotic activity.15 Deeper components show slit-shaped vasculature with dermal collagen dissection. Hemosiderin, extravasated red blood cells, and inflammation are found adjacent to the vessels (Figure 3). Given the benign nature, hobnail hemangiomas may be monitored.
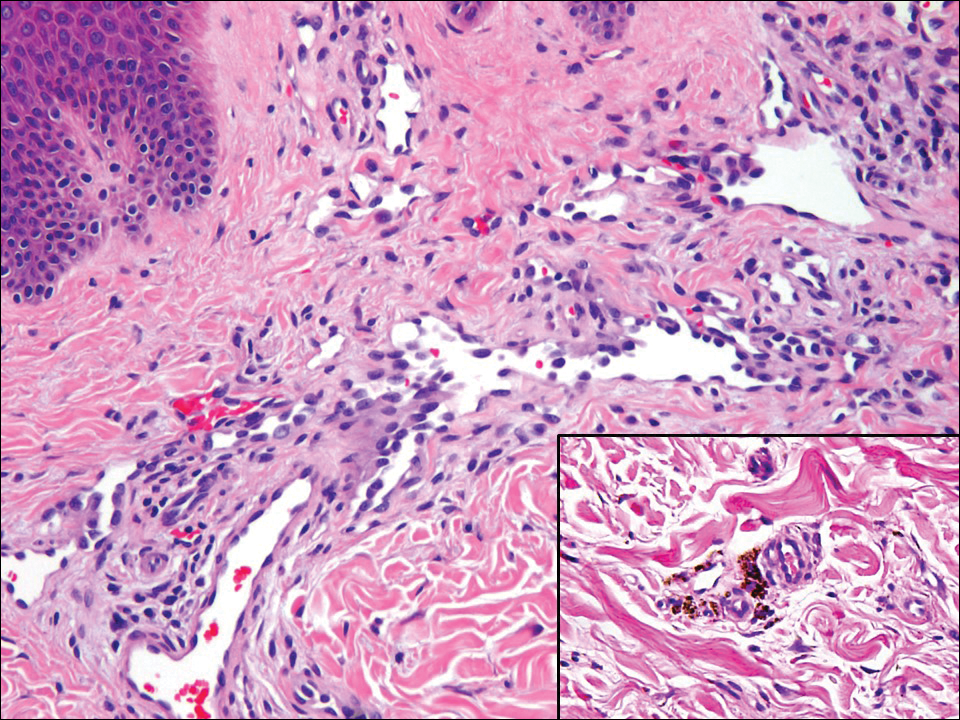
Kaposi sarcoma (KS) is a low-grade vascular neoplasm associated with human herpesvirus 8 that arises in multiple clinical settings, especially in immunosuppression secondary to human immunodeficiency virus. There are 3 distinct clinical stages: patch, plaque, and tumor. The patch stage appears as red macules that blend into larger plaques; the tumor stage is defined as larger nodules developing from plaques. Histologic features differ by stage. Similar to angiosarcoma, KS is comprised of anastomosing vessels that dissect collagen bundles; endothelial cell atypia is minimal. A useful feature of KS is its propensity to involve adnexa and display the promontory sign, which involves the tumor growing into normal vasculature (Figure 4).16 Positive immunohistochemistry for human herpesvirus 8 aids in confirmation of the diagnosis. Treatment options for KS are numerous but include destructive modalities, chemotherapeutic agents such as doxorubicin, or highly active antiretroviral therapy for AIDS-related KS.17
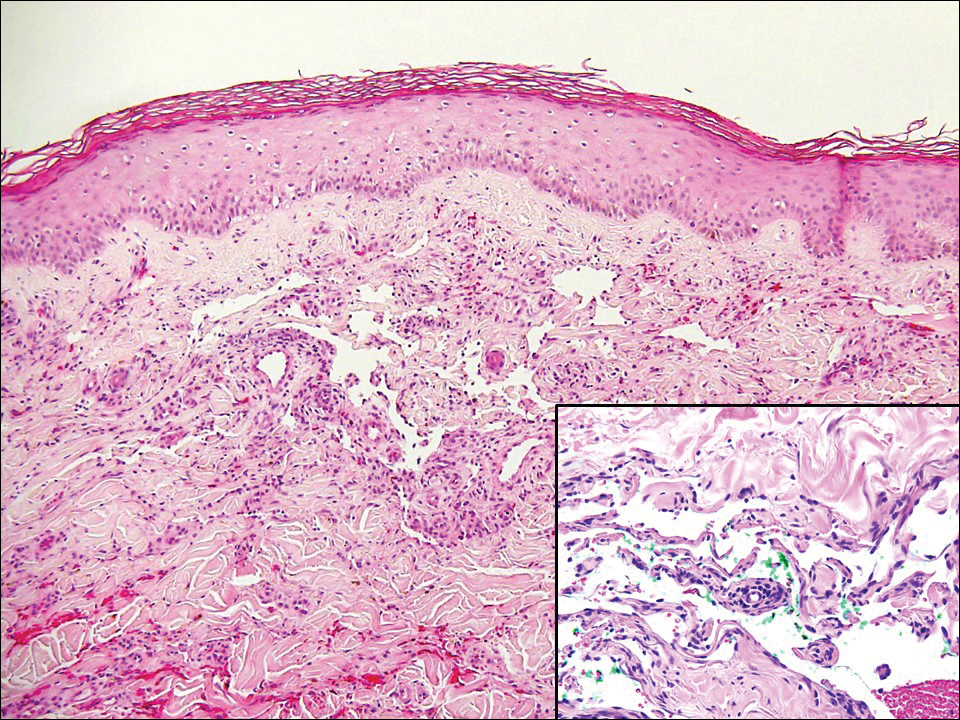
- Fineberg S, Rosen PP. Cutaneous angiosarcoma and atypical vascular lesions of the skin and breast after radiation therapy for breast carcinoma. Am J Clin Pathol. 1994;102:757-763.
- Patton KT, Deyrup AT, Weiss SW. Atypical vascular lesions after surgery and radiation of the breast: a clinicopathologic study of 32 cases analyzing histologic heterogeneity and association with angiosarcoma. Am J Surg Pathol. 2008;32:943-950.
- Billings SD, McKenney JK, Folpe AL, et al. Cutaneous angiosarcoma following breast-conserving surgery and radiation: an analysis of 27 cases. Am J Surg Pathol. 2004;28:781-788.
- Lucas DR. Angiosarcoma, radiation-associated angiosarcoma, and atypical vascular lesion. Arch Pathol Lab Med. 2009;133:1804-1809.
- Udager AM, Ishikawa MK, Lucas DR, et al. MYC immunohistochemistry in angiosarcoma and atypical vascular lesions: practical considerations based on a single institutional experience. Pathology. 2016;48:697-704.
- Patterson JW, Hosler GA. Weedon's Skin Pathology. 4th ed. Philadelphia, PA: Elsevier; 2016:1069-1115.
- Shin JY, Roh SG, Lee NH, et al. Predisposing factors for poor prognosis of angiosarcoma of the scalp and face: systematic review and meta-analysis. Head Neck. 2017;39:380-386.
- Fraga-Guedes C, Gobbi H, Mastropasqua MG, et al. Clinicopathological and immunohistochemical study of 30 cases of post-radiation atypical vascular lesion of the breast. Breast Cancer Res Treat. 2014;146:347-354.
- Shin SJ, Lesser M, Rosen PP. Hemangiomas and angiosarcomas of the breast: diagnostic utility of cell cycle markers with emphasis on Ki-67. Arch Pathol Lab Med. 2007;131:538-544.
- Cornejo KM, Deng A, Wu H, et al. The utility of MYC and FLT4 in the diagnosis and treatment of postradiation atypical vascular lesion and angiosarcoma of the breast. Hum Pathol. 2015;46:868-875.
- Patel GA, Schwartz RA. Cutaneous lymphangioma circumscriptum: frog spawn on the skin. Int J Dermatol. 2009;48:1290-1295.
- Massa AF, Menezes N, Baptista A, et al. Cutaneous lymphangioma circumscriptum--dermoscopic features. An Bras Dermatol. 2015;90:262-264.
- Santa Cruz DJ, Aronberg J. Targetoid hemosiderotic hemangioma. J Am Acad Dermatol. 1988;19:550-558.
- Christenson LJ, Stone MS. Trauma-induced simulator of targetoid hemosiderotic hemangioma. Am J Dermatopathol. 2001;23:221-223.
- Trindade F, Kutzner H, Tellechea O, et al. Hobnail hemangioma reclassified as superficial lymphatic malformation: a study of 52 cases. J Am Acad Dermatol. 2012;66:112-115.
- Radu O, Pantanowitz L. Kaposi sarcoma. Arch Pathol Lab Med. 2013;137:289-294.
- Di Lorenzo G, Di Trolio R, Montesarchio V, et al. Pegylated liposomal doxorubicin as second-line therapy in the treatment of patients with advanced classic Kaposi sarcoma: a retrospective study. Cancer. 2008;112:1147-1152.
The Diagnosis: Atypical Vascular Lesion
Atypical vascular lesion (AVL)(quiz image), named by Fineberg and Rosen,1 is a vascular lesion that arises on mammary skin with a history of radiation exposure. Clinically, AVL can present as a papule or erythematous patch that manifests 3 to 7 years after radiation therapy.2,3 There are 2 histologic subtypes of AVL: lymphatic and vascular.2,4 Lymphatic-type AVL is comprised of a symmetric distribution of thin, dilated, and anastomosing vessels usually found in the superficial and mid dermis. The vessels are lined by flat or hobnail protuberant endothelial cells that lack nuclear irregularity or pleomorphism; however, hyperchromatism of endothelial cell nuclei is a common finding. Vascular-type AVL is morphologically similar to a capillary hemangioma, and histologic features include irregular growth of capillary-sized vessels that extend to the dermis and subcutis.2,4 Atypical vascular lesions are benign lesions but may be a precursor to angiosarcoma. Along with vascular markers, D2-40 typically is positive. Surgical excision with clear margins is recommended when the lesion is small.4,5 Observation is more appropriate for extensive lesions.
Angiosarcoma can arise spontaneously or in association with radiation or chronic lymphedema. Given the shared risk factors and presentation with AVL, it is essential to differentiate angiosarcoma from AVL. Primary cutaneous angiosarcoma usually presents on the head of elderly patients as an ecchymotic patch or plaque with ulceration.4 Secondary angiosarcoma may arise following radiation or chronic lymphedema (Stewart-Treves syndrome); however, some authors now prefer to consider lymphangiosarcoma arising in chronic lymphedematous limbs a distinct entity.6 Surgical excision with wide margins is the mainstay of therapy, but angiosarcoma has high recurrence rates, and the 5-year survival rate has been reported to be as low as 35%.7 Histologic overlap with AVL includes dissecting anastomosing vessels lined by hyperchromatic nuclei; however, angiosarcoma is distinguished by endothelial cell layering, nuclear pleomorphism, and prominent nucleoli (Figure 1).4,8 Increased positivity for Ki-67 immunostain, which indicates cell proliferation, may be used to distinguish angiosarcoma from an AVL (Figure 1 [inset]).9 Further, in contrast to AVL, radiation-induced angiosarcoma is characterized by amplification of C-MYC, a regulator gene, and FLT4 (FMS-related tyrosine kinase 4), a gene encoding vascular endothelial growth factor receptor 3. Gene amplification may be detected through immunohistochemistry or fluorescence in situ hybridization.10 Ki-67 labeling showed less than 10% staining in endothelial cells in our case (quiz image [inset]), and fluorescence in situ hybridization was negative for C-MYC amplification, supporting the diagnosis of AVL.

Lymphangioma circumscriptum, the most common superficial lymphangioma, is a hamartomatous malformation that usually occurs at the axillary folds, neck, and trunk. It clinically presents as small agminated vesicles with a characteristic frog spawn appearance.11 Dermoscopic features include yellow lacunae that may alternate with a dark red color secondary to extravasation of erythrocytes.12 These clinical features often lead to a differential diagnosis of verrucae, angiokeratoma, and angiosarcoma. Lymphangioma circumscriptum histologically is characterized by an overgrowth of dilated lymphatic vessels that fill the papillary dermis. The vessels are composed of flat endothelial cells typically filled with acellular proteinaceous debris and occasional erythrocytes (Figure 2). As the lesion traverses deeper into the dermis, the caliber of the lymphatic channel becomes narrower. The presence of deep lymphatic cisterns with surrounding smooth muscle is helpful to differentiate lymphangioma circumscriptum from other lymphatic malformations such as acquired lymphangiectasia. Treatment options include surgical excision, sclerosing agents, and destructive modalities such as cryotherapy.

Hobnail hemangioma, originally termed targetoid hemosiderotic hemangioma by Santa Cruz and Aronberg,13 presents as a violaceous papule or nodule surrounded by a characteristic brown halo on the leg. Trauma has been proposed as the inciting factor for the clinical appearance of hobnail hemangioma.14 Microscopically, the lesion shows vessels in a wedge shape. The superficial component has telangiectatic vessels with focal areas of papillary projections lined by endothelial cells. Although the endothelial nuclei typically project into the lumen, the nuclei are small, bland, and without mitotic activity.15 Deeper components show slit-shaped vasculature with dermal collagen dissection. Hemosiderin, extravasated red blood cells, and inflammation are found adjacent to the vessels (Figure 3). Given the benign nature, hobnail hemangiomas may be monitored.

Kaposi sarcoma (KS) is a low-grade vascular neoplasm associated with human herpesvirus 8 that arises in multiple clinical settings, especially in immunosuppression secondary to human immunodeficiency virus. There are 3 distinct clinical stages: patch, plaque, and tumor. The patch stage appears as red macules that blend into larger plaques; the tumor stage is defined as larger nodules developing from plaques. Histologic features differ by stage. Similar to angiosarcoma, KS is comprised of anastomosing vessels that dissect collagen bundles; endothelial cell atypia is minimal. A useful feature of KS is its propensity to involve adnexa and display the promontory sign, which involves the tumor growing into normal vasculature (Figure 4).16 Positive immunohistochemistry for human herpesvirus 8 aids in confirmation of the diagnosis. Treatment options for KS are numerous but include destructive modalities, chemotherapeutic agents such as doxorubicin, or highly active antiretroviral therapy for AIDS-related KS.17

The Diagnosis: Atypical Vascular Lesion
Atypical vascular lesion (AVL)(quiz image), named by Fineberg and Rosen,1 is a vascular lesion that arises on mammary skin with a history of radiation exposure. Clinically, AVL can present as a papule or erythematous patch that manifests 3 to 7 years after radiation therapy.2,3 There are 2 histologic subtypes of AVL: lymphatic and vascular.2,4 Lymphatic-type AVL is comprised of a symmetric distribution of thin, dilated, and anastomosing vessels usually found in the superficial and mid dermis. The vessels are lined by flat or hobnail protuberant endothelial cells that lack nuclear irregularity or pleomorphism; however, hyperchromatism of endothelial cell nuclei is a common finding. Vascular-type AVL is morphologically similar to a capillary hemangioma, and histologic features include irregular growth of capillary-sized vessels that extend to the dermis and subcutis.2,4 Atypical vascular lesions are benign lesions but may be a precursor to angiosarcoma. Along with vascular markers, D2-40 typically is positive. Surgical excision with clear margins is recommended when the lesion is small.4,5 Observation is more appropriate for extensive lesions.
Angiosarcoma can arise spontaneously or in association with radiation or chronic lymphedema. Given the shared risk factors and presentation with AVL, it is essential to differentiate angiosarcoma from AVL. Primary cutaneous angiosarcoma usually presents on the head of elderly patients as an ecchymotic patch or plaque with ulceration.4 Secondary angiosarcoma may arise following radiation or chronic lymphedema (Stewart-Treves syndrome); however, some authors now prefer to consider lymphangiosarcoma arising in chronic lymphedematous limbs a distinct entity.6 Surgical excision with wide margins is the mainstay of therapy, but angiosarcoma has high recurrence rates, and the 5-year survival rate has been reported to be as low as 35%.7 Histologic overlap with AVL includes dissecting anastomosing vessels lined by hyperchromatic nuclei; however, angiosarcoma is distinguished by endothelial cell layering, nuclear pleomorphism, and prominent nucleoli (Figure 1).4,8 Increased positivity for Ki-67 immunostain, which indicates cell proliferation, may be used to distinguish angiosarcoma from an AVL (Figure 1 [inset]).9 Further, in contrast to AVL, radiation-induced angiosarcoma is characterized by amplification of C-MYC, a regulator gene, and FLT4 (FMS-related tyrosine kinase 4), a gene encoding vascular endothelial growth factor receptor 3. Gene amplification may be detected through immunohistochemistry or fluorescence in situ hybridization.10 Ki-67 labeling showed less than 10% staining in endothelial cells in our case (quiz image [inset]), and fluorescence in situ hybridization was negative for C-MYC amplification, supporting the diagnosis of AVL.

Lymphangioma circumscriptum, the most common superficial lymphangioma, is a hamartomatous malformation that usually occurs at the axillary folds, neck, and trunk. It clinically presents as small agminated vesicles with a characteristic frog spawn appearance.11 Dermoscopic features include yellow lacunae that may alternate with a dark red color secondary to extravasation of erythrocytes.12 These clinical features often lead to a differential diagnosis of verrucae, angiokeratoma, and angiosarcoma. Lymphangioma circumscriptum histologically is characterized by an overgrowth of dilated lymphatic vessels that fill the papillary dermis. The vessels are composed of flat endothelial cells typically filled with acellular proteinaceous debris and occasional erythrocytes (Figure 2). As the lesion traverses deeper into the dermis, the caliber of the lymphatic channel becomes narrower. The presence of deep lymphatic cisterns with surrounding smooth muscle is helpful to differentiate lymphangioma circumscriptum from other lymphatic malformations such as acquired lymphangiectasia. Treatment options include surgical excision, sclerosing agents, and destructive modalities such as cryotherapy.

Hobnail hemangioma, originally termed targetoid hemosiderotic hemangioma by Santa Cruz and Aronberg,13 presents as a violaceous papule or nodule surrounded by a characteristic brown halo on the leg. Trauma has been proposed as the inciting factor for the clinical appearance of hobnail hemangioma.14 Microscopically, the lesion shows vessels in a wedge shape. The superficial component has telangiectatic vessels with focal areas of papillary projections lined by endothelial cells. Although the endothelial nuclei typically project into the lumen, the nuclei are small, bland, and without mitotic activity.15 Deeper components show slit-shaped vasculature with dermal collagen dissection. Hemosiderin, extravasated red blood cells, and inflammation are found adjacent to the vessels (Figure 3). Given the benign nature, hobnail hemangiomas may be monitored.

Kaposi sarcoma (KS) is a low-grade vascular neoplasm associated with human herpesvirus 8 that arises in multiple clinical settings, especially in immunosuppression secondary to human immunodeficiency virus. There are 3 distinct clinical stages: patch, plaque, and tumor. The patch stage appears as red macules that blend into larger plaques; the tumor stage is defined as larger nodules developing from plaques. Histologic features differ by stage. Similar to angiosarcoma, KS is comprised of anastomosing vessels that dissect collagen bundles; endothelial cell atypia is minimal. A useful feature of KS is its propensity to involve adnexa and display the promontory sign, which involves the tumor growing into normal vasculature (Figure 4).16 Positive immunohistochemistry for human herpesvirus 8 aids in confirmation of the diagnosis. Treatment options for KS are numerous but include destructive modalities, chemotherapeutic agents such as doxorubicin, or highly active antiretroviral therapy for AIDS-related KS.17

- Fineberg S, Rosen PP. Cutaneous angiosarcoma and atypical vascular lesions of the skin and breast after radiation therapy for breast carcinoma. Am J Clin Pathol. 1994;102:757-763.
- Patton KT, Deyrup AT, Weiss SW. Atypical vascular lesions after surgery and radiation of the breast: a clinicopathologic study of 32 cases analyzing histologic heterogeneity and association with angiosarcoma. Am J Surg Pathol. 2008;32:943-950.
- Billings SD, McKenney JK, Folpe AL, et al. Cutaneous angiosarcoma following breast-conserving surgery and radiation: an analysis of 27 cases. Am J Surg Pathol. 2004;28:781-788.
- Lucas DR. Angiosarcoma, radiation-associated angiosarcoma, and atypical vascular lesion. Arch Pathol Lab Med. 2009;133:1804-1809.
- Udager AM, Ishikawa MK, Lucas DR, et al. MYC immunohistochemistry in angiosarcoma and atypical vascular lesions: practical considerations based on a single institutional experience. Pathology. 2016;48:697-704.
- Patterson JW, Hosler GA. Weedon's Skin Pathology. 4th ed. Philadelphia, PA: Elsevier; 2016:1069-1115.
- Shin JY, Roh SG, Lee NH, et al. Predisposing factors for poor prognosis of angiosarcoma of the scalp and face: systematic review and meta-analysis. Head Neck. 2017;39:380-386.
- Fraga-Guedes C, Gobbi H, Mastropasqua MG, et al. Clinicopathological and immunohistochemical study of 30 cases of post-radiation atypical vascular lesion of the breast. Breast Cancer Res Treat. 2014;146:347-354.
- Shin SJ, Lesser M, Rosen PP. Hemangiomas and angiosarcomas of the breast: diagnostic utility of cell cycle markers with emphasis on Ki-67. Arch Pathol Lab Med. 2007;131:538-544.
- Cornejo KM, Deng A, Wu H, et al. The utility of MYC and FLT4 in the diagnosis and treatment of postradiation atypical vascular lesion and angiosarcoma of the breast. Hum Pathol. 2015;46:868-875.
- Patel GA, Schwartz RA. Cutaneous lymphangioma circumscriptum: frog spawn on the skin. Int J Dermatol. 2009;48:1290-1295.
- Massa AF, Menezes N, Baptista A, et al. Cutaneous lymphangioma circumscriptum--dermoscopic features. An Bras Dermatol. 2015;90:262-264.
- Santa Cruz DJ, Aronberg J. Targetoid hemosiderotic hemangioma. J Am Acad Dermatol. 1988;19:550-558.
- Christenson LJ, Stone MS. Trauma-induced simulator of targetoid hemosiderotic hemangioma. Am J Dermatopathol. 2001;23:221-223.
- Trindade F, Kutzner H, Tellechea O, et al. Hobnail hemangioma reclassified as superficial lymphatic malformation: a study of 52 cases. J Am Acad Dermatol. 2012;66:112-115.
- Radu O, Pantanowitz L. Kaposi sarcoma. Arch Pathol Lab Med. 2013;137:289-294.
- Di Lorenzo G, Di Trolio R, Montesarchio V, et al. Pegylated liposomal doxorubicin as second-line therapy in the treatment of patients with advanced classic Kaposi sarcoma: a retrospective study. Cancer. 2008;112:1147-1152.
- Fineberg S, Rosen PP. Cutaneous angiosarcoma and atypical vascular lesions of the skin and breast after radiation therapy for breast carcinoma. Am J Clin Pathol. 1994;102:757-763.
- Patton KT, Deyrup AT, Weiss SW. Atypical vascular lesions after surgery and radiation of the breast: a clinicopathologic study of 32 cases analyzing histologic heterogeneity and association with angiosarcoma. Am J Surg Pathol. 2008;32:943-950.
- Billings SD, McKenney JK, Folpe AL, et al. Cutaneous angiosarcoma following breast-conserving surgery and radiation: an analysis of 27 cases. Am J Surg Pathol. 2004;28:781-788.
- Lucas DR. Angiosarcoma, radiation-associated angiosarcoma, and atypical vascular lesion. Arch Pathol Lab Med. 2009;133:1804-1809.
- Udager AM, Ishikawa MK, Lucas DR, et al. MYC immunohistochemistry in angiosarcoma and atypical vascular lesions: practical considerations based on a single institutional experience. Pathology. 2016;48:697-704.
- Patterson JW, Hosler GA. Weedon's Skin Pathology. 4th ed. Philadelphia, PA: Elsevier; 2016:1069-1115.
- Shin JY, Roh SG, Lee NH, et al. Predisposing factors for poor prognosis of angiosarcoma of the scalp and face: systematic review and meta-analysis. Head Neck. 2017;39:380-386.
- Fraga-Guedes C, Gobbi H, Mastropasqua MG, et al. Clinicopathological and immunohistochemical study of 30 cases of post-radiation atypical vascular lesion of the breast. Breast Cancer Res Treat. 2014;146:347-354.
- Shin SJ, Lesser M, Rosen PP. Hemangiomas and angiosarcomas of the breast: diagnostic utility of cell cycle markers with emphasis on Ki-67. Arch Pathol Lab Med. 2007;131:538-544.
- Cornejo KM, Deng A, Wu H, et al. The utility of MYC and FLT4 in the diagnosis and treatment of postradiation atypical vascular lesion and angiosarcoma of the breast. Hum Pathol. 2015;46:868-875.
- Patel GA, Schwartz RA. Cutaneous lymphangioma circumscriptum: frog spawn on the skin. Int J Dermatol. 2009;48:1290-1295.
- Massa AF, Menezes N, Baptista A, et al. Cutaneous lymphangioma circumscriptum--dermoscopic features. An Bras Dermatol. 2015;90:262-264.
- Santa Cruz DJ, Aronberg J. Targetoid hemosiderotic hemangioma. J Am Acad Dermatol. 1988;19:550-558.
- Christenson LJ, Stone MS. Trauma-induced simulator of targetoid hemosiderotic hemangioma. Am J Dermatopathol. 2001;23:221-223.
- Trindade F, Kutzner H, Tellechea O, et al. Hobnail hemangioma reclassified as superficial lymphatic malformation: a study of 52 cases. J Am Acad Dermatol. 2012;66:112-115.
- Radu O, Pantanowitz L. Kaposi sarcoma. Arch Pathol Lab Med. 2013;137:289-294.
- Di Lorenzo G, Di Trolio R, Montesarchio V, et al. Pegylated liposomal doxorubicin as second-line therapy in the treatment of patients with advanced classic Kaposi sarcoma: a retrospective study. Cancer. 2008;112:1147-1152.
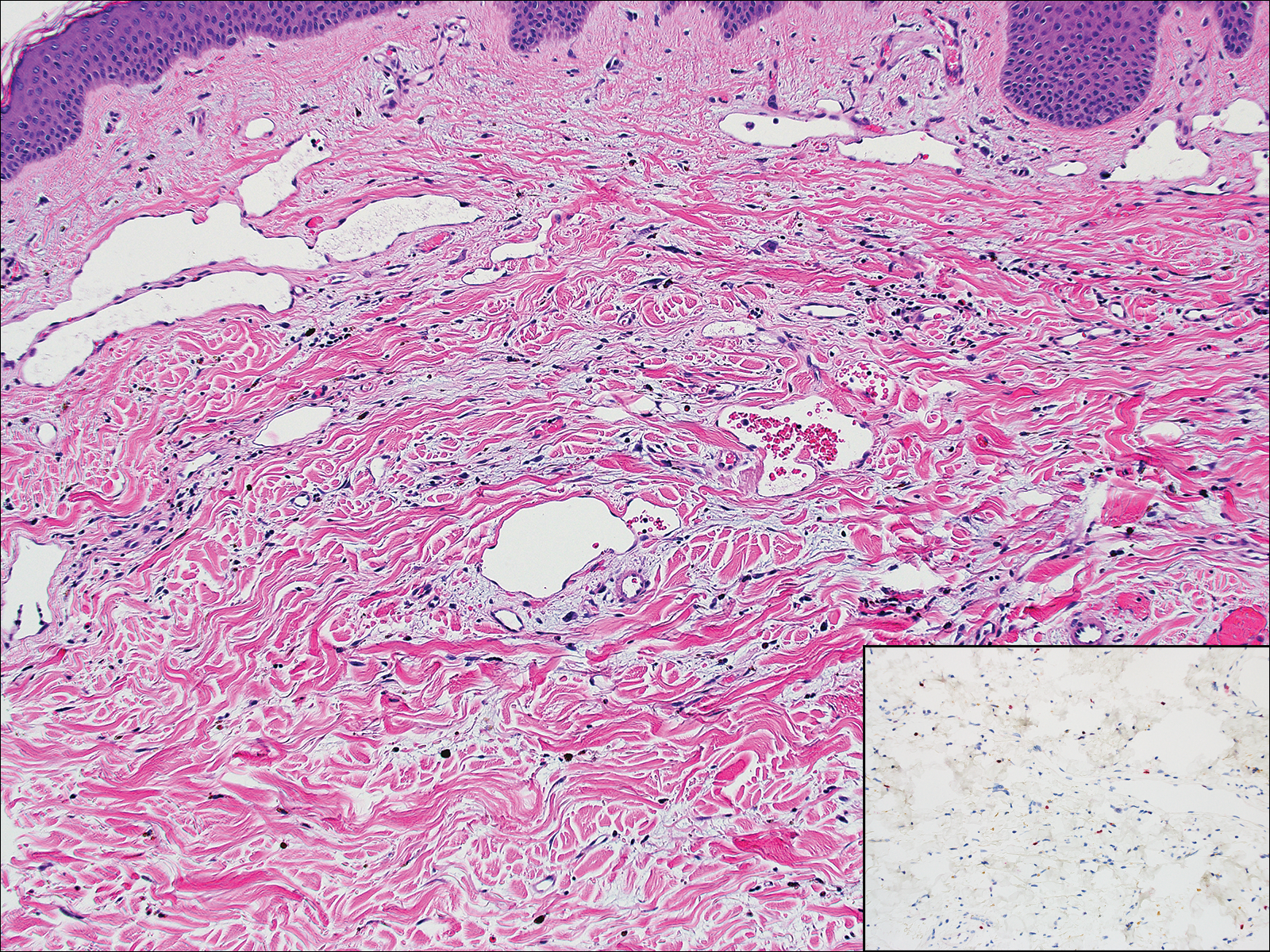
A 67-year-old woman presented with a lesion on the medial aspect of the right axilla of 2 weeks' duration. The patient had a history of cancer of the right breast treated with a mastectomy and adjuvant radiation. She denied pain, bleeding, pruritus, or rapid growth, as well as any changes in medication or recent trauma. Physical examination revealed a 5-mm purpuric macule of the right axilla. A punch biopsy was performed. Amplification for the C-MYC gene was negative by fluorescence in situ hybridization.
What’s Eating You? Clinical Manifestations of Dermacentor Tick Bites
Background and Distribution
The Dermacentor ticks belong to the family Ixodidae (hard ticks). The 2 best-known ticks of the genus are Dermacentor andersoni (Rocky Mountain wood tick)(Figure, A) and Dermacentor variabilis (American dog tick)(Figure, B). The Dermacentor ticks are large ticks with small anterior mouthparts that attach to a rectangular basis capituli (Figure, A). Both ticks exhibit widely spaced eyes and posterior festoons as well as bifid coxa 1 (the attachment site for the first pair of legs) and enlarged coxa 4. As adults, these ticks display an ornate hard dorsal plate, or scutum, with numerous pits. Female ticks have a much smaller scutum, allowing for abdominal engorgement during feeding.1 Although D andersoni tends to have a brown to yellow hue, the specimens of D variabilis display a somewhat silver color pattern.
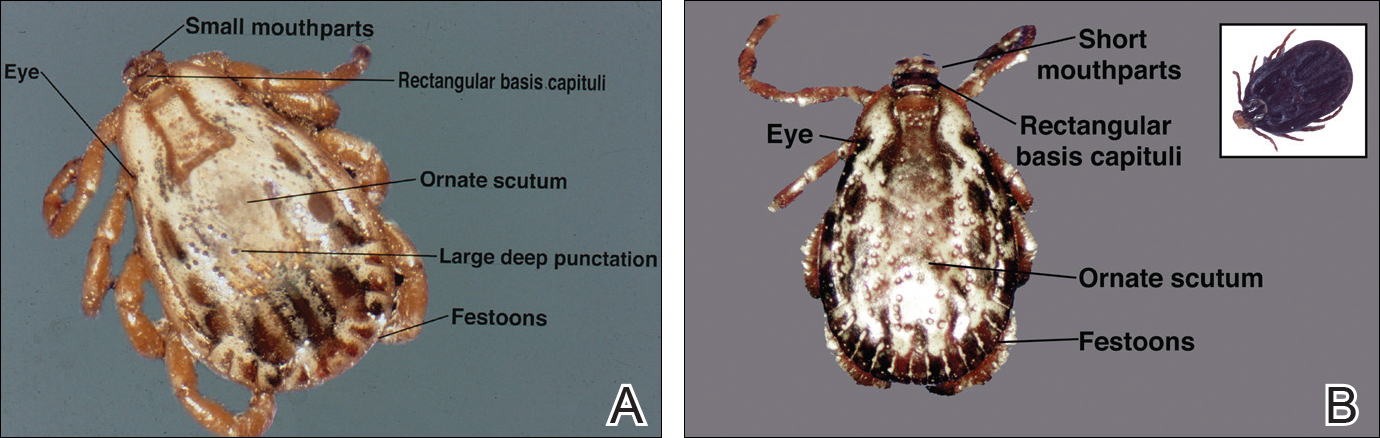
Dermacentor ticks can be found throughout most of North America, with the northern distribution limits of both species previously occurring in the province of Saskatchewan, Canada. Although the range of D andersoni has remained relatively stable within this distribution, the distribution of D variabilis recently has expanded westward and northward of these limits.2 The ranges of the 2 species overlap in certain areas, though D andersoni primarily is found in the Rocky Mountain and northwestern states as well as southwestern Canada, whereas D variabilis can be found throughout most parts of the United States, except in the Rocky Mountain states.3 Within these regions the ticks can be found in heavily wooded areas, but they most commonly inhabit fields with tall grass, crops, bushes, and shrubbery, often clustering where these types of vegetation form clearly defined edges.4 The diseases transmitted by the Dermacentor ticks include Rocky Mountain spotted fever (RMSF), Colorado tick fever, tularemia, tick paralysis, and even human monocytic erlichiosis, though Amblyomma americanum is the major vector for human monocytic erlichiosis.
Rocky Mountain Spotted Fever
Both species of ticks are known to serve as vectors for RMSF, but D variabilis is the major vector in the United States, especially in the eastern and southeastern parts of the United States. Overall, the majority of cases occur in North Carolina, South Carolina, Tennessee, and Oklahoma,5 with North Carolina having the highest incidence. In endemic areas, RMSF should be suspected in any patient with fever and headache, and empiric treatment with antibiotics should be started while awaiting the results of diagnostic tests. Serologic testing with indirect fluorescent antibodies is widely available and is considered the best method for detection; although the sensitivity is poor during the first 10 to 12 days of infection, it increases to 94% during days 14 to 21.6 Therapeutic decisions should be influenced by clinical suspicion and epidemiologic data. Treatment should be started promptly and should never be delayed until confirmatory tests are available. Doxycycline is considered the gold standard therapy in both adults and children, with a typical treatment duration of 10 days. The only other recommended agent for pregnant women in the first or second trimesters or patients with severe hypersensitivity reactions to tetracyclines is chloramphenicol.7
Colorado Tick Fever
Colorado tick fever, also known as mountain fever, is an arboviral infection transmitted by D andersoni. Its distribution coincides with the tick’s natural geographic range in the western United States and Rocky Mountains. Colorado tick fever causes an acute febrile illness consisting of chills, headaches, myalgia, retro-orbital pain, and malaise, which tend to occur within 3 to 5 days of the tick bite. Some cases may be accompanied by a nonspecific rash that may be morbilliform or petechial in appearance. Notably, approximately half of all patients will experience transient resolution of symptoms for 24 to 48 hours followed by a recurrence of fever, a phenomenon that has been referred to as saddleback fever. Routine laboratory findings may include leukopenia, thrombocytopenia, and a peripheral smear with atypical lymphocytes. Reverse transcription polymerase chain reaction is both sensitive and specific for detecting viral loads in the blood during the first week of infection, though testing does not alter management, which is largely supportive.8
Tularemia
Tularemia is a relatively rare disease but has been documented in every US state except Hawaii.9 The disease is caused by Francisella tularensis, a small, aerobic, gram-negative coccobacillus transmitted via inhalation, bitingflies, or tick bites; the most common ticks to transmit the disease include D andersoni, D variabilis, and A americanum.10 Clinical manifestations depend on the form of exposure, with tick bites most often resulting in an ulcerated skin lesion at the site of the vector bite accompanied by regional lymphadenopathy and systemic symptoms such as fever, chills, myalgia, and headache.11 Mucosal manifestations such as pharyngitis, conjunctivitis, and other ocular lesions also are commonly seen. Diagnosis most frequently is made using serology because F tularensis is both challenging and dangerous to culture; in fact, because of the high risk of contagion, F tularensis should only be cultured in biosafety level 3 laboratories. Polymerase chain reaction assays can be used on tissue samples with decent sensitivity (78%) and specificity (96%); however, these assays cannot distinguish between Francisella subspecies and are not readily available to most clinicians.12 First-line therapy for the treatment of tularemia is streptomycin given as twice-daily intramuscular injections over the course of 7 to 10 days. Alternative agents include gentamicin, ciprofloxacin, imipenem, doxycycline, and chloramphenicol.10 Because tularemia is relatively rare, a high index of suspicion is necessary to reduce the morbidity and mortality associated with the disease.
Tick Paralysis
More than 40 different species of ticks have been implicated worldwide as causes of tick paralysis, though D andersoni has been the most common in North America. Female patients account for most cases, possibly because long hair conceals ticks on the scalp or neck, the preferred attachment locations for Dermacentor ticks.13 The classic presentation of tick paralysis is an acute, flaccid, ascending paralysis that occurs from a neurotoxin in the tick saliva that impairs afferent nerve signal propagation.14,15 The paralysis progresses over hours to days and typically occurs 5 to 6 days after attachment of the tick. Notably, there is no associated fever with tick paralysis, and without intervention, patients may die of respiratory failure. Overall, the condition carries a fatality rate of nearly 10%16 but reverses rapidly if the tick is identified and removed.
Protection against tick bites and tick-borne illnesses includes avoidance of infested areas, treatment of populated habitats with insecticide sprays, use of topical repellants prior to outdoor activities, and diligent full-body tick checks upon return from tick-heavy areas. Permethrin can be used to treat clothing and remains protective through multiple washings. Ticks typically survive washing of untreated clothing but are killed by prolonged drying in a dryer. Pets may be treated with oral, intramuscular, or topical agents prescribed by a veterinarian to prevent tick attachments.
Conclusion
Accurate identification of Dermacentor ticks allows for appropriate surveillance for associated diseases and can improve patient outcomes. Patients who engage in outdoor activities in endemic areas should take steps to avoid exposure, use appropriate acaricides and repellents, and perform tick checks after returning indoors.
- Bowman DD. Georgis’ Parasitology for Veterinarians. 8th ed. New York, NY: Saunders; 2002.
- Dergousoff SJ, Galloway TD, Lindsay LR, et al. Range expansion of Dermacentor variabilis and Dermacentor andersoni near their northern distributional limits. J Med Entomol. 2013;50:510-520.
- Centers for Disease Control and Prevention. Geographic distribution of ticks that bite humans. Center for Disease Control and Prevention website. http://www.cdc.gov/ticks/geographic_distribution.html. Updated August 11, 2017. Accessed December 15
, 2017. - Trout Fryxell RT, Moore JE, Collins MD, et al. Habitat and vegetation variables are not enough when predicting tick populations in the southeastern United States. PLoS One. 2015;10:e0144092.
- Chapman AS, Bakken JS, Folk SM, et al. Diagnosis and management of tickborne rickettsial diseases: Rocky Mountain spotted fever, erlichiosis, and anaplasmosis—United States: a practical guide for physicians and other health-care and public health professionals. MMWR Recomm Rep. 2006;55:1-27.
- Nathavitharana RR, Mitty JA. Diseases from North America: focus on tick-borne infections. Clin Med. 2015;15:74-77.
- Chen LF, Sexton DJ. What’s new in Rocky Mountain spotted fever? Infect Dis Clin North Am. 2008;22:415-432.
- Lambert AJ, Kosoy O, Velez JO, et al. Detection of Colorado tick fever viral RNA in acute human serum samples by a quantitative real-time RT-PCR assay. J Virol Methods. 2007;140:43-48.
- Centers for Disease Control and Prevention (CDC). Tularemia—United States, 1990-2000. MMWR Morb Mortal Wkly Rep. 2002;51:182-184.
- Nigrovic LE, Wingerter SL. Tularemia. Infect Dis Clin North Am. 2008;22:489-504.
- Evans ME, Gregory DW, Schaffner W, et al. Tularemia: a 30-year experience with 88 cases. Medicine (Baltimore). 1985;64:251-269.
- Eliasson H, Sjöstedt A, Bäck E. Clinical use of diagnostic PCR for Francisella tularensis in patients with suspected ulceroglandular tularaemia. Scand J Infect Dis. 2005;37:833-837.
- Edlow JA, McGillicuddy DC. Tick paralysis. Infect Dis Clin North Am. 2008;22:397-413.
- Felz MW, Smith CD, Swift TR. A six-year-old girl with tick paralysis. N Engl J Med. 2000;342:90-94.
- Rose I. A review of tick paralysis. Can Med Assoc J. 1954;70:175-176.
- Dworkin MS, Shoemaker PC, Anderson DE. Tick paralysis: 33 human cases in Washington State, 1946-1996. Clin Infect Dis. 1999;29:1435-1439.
Background and Distribution
The Dermacentor ticks belong to the family Ixodidae (hard ticks). The 2 best-known ticks of the genus are Dermacentor andersoni (Rocky Mountain wood tick)(Figure, A) and Dermacentor variabilis (American dog tick)(Figure, B). The Dermacentor ticks are large ticks with small anterior mouthparts that attach to a rectangular basis capituli (Figure, A). Both ticks exhibit widely spaced eyes and posterior festoons as well as bifid coxa 1 (the attachment site for the first pair of legs) and enlarged coxa 4. As adults, these ticks display an ornate hard dorsal plate, or scutum, with numerous pits. Female ticks have a much smaller scutum, allowing for abdominal engorgement during feeding.1 Although D andersoni tends to have a brown to yellow hue, the specimens of D variabilis display a somewhat silver color pattern.

Dermacentor ticks can be found throughout most of North America, with the northern distribution limits of both species previously occurring in the province of Saskatchewan, Canada. Although the range of D andersoni has remained relatively stable within this distribution, the distribution of D variabilis recently has expanded westward and northward of these limits.2 The ranges of the 2 species overlap in certain areas, though D andersoni primarily is found in the Rocky Mountain and northwestern states as well as southwestern Canada, whereas D variabilis can be found throughout most parts of the United States, except in the Rocky Mountain states.3 Within these regions the ticks can be found in heavily wooded areas, but they most commonly inhabit fields with tall grass, crops, bushes, and shrubbery, often clustering where these types of vegetation form clearly defined edges.4 The diseases transmitted by the Dermacentor ticks include Rocky Mountain spotted fever (RMSF), Colorado tick fever, tularemia, tick paralysis, and even human monocytic erlichiosis, though Amblyomma americanum is the major vector for human monocytic erlichiosis.
Rocky Mountain Spotted Fever
Both species of ticks are known to serve as vectors for RMSF, but D variabilis is the major vector in the United States, especially in the eastern and southeastern parts of the United States. Overall, the majority of cases occur in North Carolina, South Carolina, Tennessee, and Oklahoma,5 with North Carolina having the highest incidence. In endemic areas, RMSF should be suspected in any patient with fever and headache, and empiric treatment with antibiotics should be started while awaiting the results of diagnostic tests. Serologic testing with indirect fluorescent antibodies is widely available and is considered the best method for detection; although the sensitivity is poor during the first 10 to 12 days of infection, it increases to 94% during days 14 to 21.6 Therapeutic decisions should be influenced by clinical suspicion and epidemiologic data. Treatment should be started promptly and should never be delayed until confirmatory tests are available. Doxycycline is considered the gold standard therapy in both adults and children, with a typical treatment duration of 10 days. The only other recommended agent for pregnant women in the first or second trimesters or patients with severe hypersensitivity reactions to tetracyclines is chloramphenicol.7
Colorado Tick Fever
Colorado tick fever, also known as mountain fever, is an arboviral infection transmitted by D andersoni. Its distribution coincides with the tick’s natural geographic range in the western United States and Rocky Mountains. Colorado tick fever causes an acute febrile illness consisting of chills, headaches, myalgia, retro-orbital pain, and malaise, which tend to occur within 3 to 5 days of the tick bite. Some cases may be accompanied by a nonspecific rash that may be morbilliform or petechial in appearance. Notably, approximately half of all patients will experience transient resolution of symptoms for 24 to 48 hours followed by a recurrence of fever, a phenomenon that has been referred to as saddleback fever. Routine laboratory findings may include leukopenia, thrombocytopenia, and a peripheral smear with atypical lymphocytes. Reverse transcription polymerase chain reaction is both sensitive and specific for detecting viral loads in the blood during the first week of infection, though testing does not alter management, which is largely supportive.8
Tularemia
Tularemia is a relatively rare disease but has been documented in every US state except Hawaii.9 The disease is caused by Francisella tularensis, a small, aerobic, gram-negative coccobacillus transmitted via inhalation, bitingflies, or tick bites; the most common ticks to transmit the disease include D andersoni, D variabilis, and A americanum.10 Clinical manifestations depend on the form of exposure, with tick bites most often resulting in an ulcerated skin lesion at the site of the vector bite accompanied by regional lymphadenopathy and systemic symptoms such as fever, chills, myalgia, and headache.11 Mucosal manifestations such as pharyngitis, conjunctivitis, and other ocular lesions also are commonly seen. Diagnosis most frequently is made using serology because F tularensis is both challenging and dangerous to culture; in fact, because of the high risk of contagion, F tularensis should only be cultured in biosafety level 3 laboratories. Polymerase chain reaction assays can be used on tissue samples with decent sensitivity (78%) and specificity (96%); however, these assays cannot distinguish between Francisella subspecies and are not readily available to most clinicians.12 First-line therapy for the treatment of tularemia is streptomycin given as twice-daily intramuscular injections over the course of 7 to 10 days. Alternative agents include gentamicin, ciprofloxacin, imipenem, doxycycline, and chloramphenicol.10 Because tularemia is relatively rare, a high index of suspicion is necessary to reduce the morbidity and mortality associated with the disease.
Tick Paralysis
More than 40 different species of ticks have been implicated worldwide as causes of tick paralysis, though D andersoni has been the most common in North America. Female patients account for most cases, possibly because long hair conceals ticks on the scalp or neck, the preferred attachment locations for Dermacentor ticks.13 The classic presentation of tick paralysis is an acute, flaccid, ascending paralysis that occurs from a neurotoxin in the tick saliva that impairs afferent nerve signal propagation.14,15 The paralysis progresses over hours to days and typically occurs 5 to 6 days after attachment of the tick. Notably, there is no associated fever with tick paralysis, and without intervention, patients may die of respiratory failure. Overall, the condition carries a fatality rate of nearly 10%16 but reverses rapidly if the tick is identified and removed.
Protection against tick bites and tick-borne illnesses includes avoidance of infested areas, treatment of populated habitats with insecticide sprays, use of topical repellants prior to outdoor activities, and diligent full-body tick checks upon return from tick-heavy areas. Permethrin can be used to treat clothing and remains protective through multiple washings. Ticks typically survive washing of untreated clothing but are killed by prolonged drying in a dryer. Pets may be treated with oral, intramuscular, or topical agents prescribed by a veterinarian to prevent tick attachments.
Conclusion
Accurate identification of Dermacentor ticks allows for appropriate surveillance for associated diseases and can improve patient outcomes. Patients who engage in outdoor activities in endemic areas should take steps to avoid exposure, use appropriate acaricides and repellents, and perform tick checks after returning indoors.
Background and Distribution
The Dermacentor ticks belong to the family Ixodidae (hard ticks). The 2 best-known ticks of the genus are Dermacentor andersoni (Rocky Mountain wood tick)(Figure, A) and Dermacentor variabilis (American dog tick)(Figure, B). The Dermacentor ticks are large ticks with small anterior mouthparts that attach to a rectangular basis capituli (Figure, A). Both ticks exhibit widely spaced eyes and posterior festoons as well as bifid coxa 1 (the attachment site for the first pair of legs) and enlarged coxa 4. As adults, these ticks display an ornate hard dorsal plate, or scutum, with numerous pits. Female ticks have a much smaller scutum, allowing for abdominal engorgement during feeding.1 Although D andersoni tends to have a brown to yellow hue, the specimens of D variabilis display a somewhat silver color pattern.

Dermacentor ticks can be found throughout most of North America, with the northern distribution limits of both species previously occurring in the province of Saskatchewan, Canada. Although the range of D andersoni has remained relatively stable within this distribution, the distribution of D variabilis recently has expanded westward and northward of these limits.2 The ranges of the 2 species overlap in certain areas, though D andersoni primarily is found in the Rocky Mountain and northwestern states as well as southwestern Canada, whereas D variabilis can be found throughout most parts of the United States, except in the Rocky Mountain states.3 Within these regions the ticks can be found in heavily wooded areas, but they most commonly inhabit fields with tall grass, crops, bushes, and shrubbery, often clustering where these types of vegetation form clearly defined edges.4 The diseases transmitted by the Dermacentor ticks include Rocky Mountain spotted fever (RMSF), Colorado tick fever, tularemia, tick paralysis, and even human monocytic erlichiosis, though Amblyomma americanum is the major vector for human monocytic erlichiosis.
Rocky Mountain Spotted Fever
Both species of ticks are known to serve as vectors for RMSF, but D variabilis is the major vector in the United States, especially in the eastern and southeastern parts of the United States. Overall, the majority of cases occur in North Carolina, South Carolina, Tennessee, and Oklahoma,5 with North Carolina having the highest incidence. In endemic areas, RMSF should be suspected in any patient with fever and headache, and empiric treatment with antibiotics should be started while awaiting the results of diagnostic tests. Serologic testing with indirect fluorescent antibodies is widely available and is considered the best method for detection; although the sensitivity is poor during the first 10 to 12 days of infection, it increases to 94% during days 14 to 21.6 Therapeutic decisions should be influenced by clinical suspicion and epidemiologic data. Treatment should be started promptly and should never be delayed until confirmatory tests are available. Doxycycline is considered the gold standard therapy in both adults and children, with a typical treatment duration of 10 days. The only other recommended agent for pregnant women in the first or second trimesters or patients with severe hypersensitivity reactions to tetracyclines is chloramphenicol.7
Colorado Tick Fever
Colorado tick fever, also known as mountain fever, is an arboviral infection transmitted by D andersoni. Its distribution coincides with the tick’s natural geographic range in the western United States and Rocky Mountains. Colorado tick fever causes an acute febrile illness consisting of chills, headaches, myalgia, retro-orbital pain, and malaise, which tend to occur within 3 to 5 days of the tick bite. Some cases may be accompanied by a nonspecific rash that may be morbilliform or petechial in appearance. Notably, approximately half of all patients will experience transient resolution of symptoms for 24 to 48 hours followed by a recurrence of fever, a phenomenon that has been referred to as saddleback fever. Routine laboratory findings may include leukopenia, thrombocytopenia, and a peripheral smear with atypical lymphocytes. Reverse transcription polymerase chain reaction is both sensitive and specific for detecting viral loads in the blood during the first week of infection, though testing does not alter management, which is largely supportive.8
Tularemia
Tularemia is a relatively rare disease but has been documented in every US state except Hawaii.9 The disease is caused by Francisella tularensis, a small, aerobic, gram-negative coccobacillus transmitted via inhalation, bitingflies, or tick bites; the most common ticks to transmit the disease include D andersoni, D variabilis, and A americanum.10 Clinical manifestations depend on the form of exposure, with tick bites most often resulting in an ulcerated skin lesion at the site of the vector bite accompanied by regional lymphadenopathy and systemic symptoms such as fever, chills, myalgia, and headache.11 Mucosal manifestations such as pharyngitis, conjunctivitis, and other ocular lesions also are commonly seen. Diagnosis most frequently is made using serology because F tularensis is both challenging and dangerous to culture; in fact, because of the high risk of contagion, F tularensis should only be cultured in biosafety level 3 laboratories. Polymerase chain reaction assays can be used on tissue samples with decent sensitivity (78%) and specificity (96%); however, these assays cannot distinguish between Francisella subspecies and are not readily available to most clinicians.12 First-line therapy for the treatment of tularemia is streptomycin given as twice-daily intramuscular injections over the course of 7 to 10 days. Alternative agents include gentamicin, ciprofloxacin, imipenem, doxycycline, and chloramphenicol.10 Because tularemia is relatively rare, a high index of suspicion is necessary to reduce the morbidity and mortality associated with the disease.
Tick Paralysis
More than 40 different species of ticks have been implicated worldwide as causes of tick paralysis, though D andersoni has been the most common in North America. Female patients account for most cases, possibly because long hair conceals ticks on the scalp or neck, the preferred attachment locations for Dermacentor ticks.13 The classic presentation of tick paralysis is an acute, flaccid, ascending paralysis that occurs from a neurotoxin in the tick saliva that impairs afferent nerve signal propagation.14,15 The paralysis progresses over hours to days and typically occurs 5 to 6 days after attachment of the tick. Notably, there is no associated fever with tick paralysis, and without intervention, patients may die of respiratory failure. Overall, the condition carries a fatality rate of nearly 10%16 but reverses rapidly if the tick is identified and removed.
Protection against tick bites and tick-borne illnesses includes avoidance of infested areas, treatment of populated habitats with insecticide sprays, use of topical repellants prior to outdoor activities, and diligent full-body tick checks upon return from tick-heavy areas. Permethrin can be used to treat clothing and remains protective through multiple washings. Ticks typically survive washing of untreated clothing but are killed by prolonged drying in a dryer. Pets may be treated with oral, intramuscular, or topical agents prescribed by a veterinarian to prevent tick attachments.
Conclusion
Accurate identification of Dermacentor ticks allows for appropriate surveillance for associated diseases and can improve patient outcomes. Patients who engage in outdoor activities in endemic areas should take steps to avoid exposure, use appropriate acaricides and repellents, and perform tick checks after returning indoors.
- Bowman DD. Georgis’ Parasitology for Veterinarians. 8th ed. New York, NY: Saunders; 2002.
- Dergousoff SJ, Galloway TD, Lindsay LR, et al. Range expansion of Dermacentor variabilis and Dermacentor andersoni near their northern distributional limits. J Med Entomol. 2013;50:510-520.
- Centers for Disease Control and Prevention. Geographic distribution of ticks that bite humans. Center for Disease Control and Prevention website. http://www.cdc.gov/ticks/geographic_distribution.html. Updated August 11, 2017. Accessed December 15
, 2017. - Trout Fryxell RT, Moore JE, Collins MD, et al. Habitat and vegetation variables are not enough when predicting tick populations in the southeastern United States. PLoS One. 2015;10:e0144092.
- Chapman AS, Bakken JS, Folk SM, et al. Diagnosis and management of tickborne rickettsial diseases: Rocky Mountain spotted fever, erlichiosis, and anaplasmosis—United States: a practical guide for physicians and other health-care and public health professionals. MMWR Recomm Rep. 2006;55:1-27.
- Nathavitharana RR, Mitty JA. Diseases from North America: focus on tick-borne infections. Clin Med. 2015;15:74-77.
- Chen LF, Sexton DJ. What’s new in Rocky Mountain spotted fever? Infect Dis Clin North Am. 2008;22:415-432.
- Lambert AJ, Kosoy O, Velez JO, et al. Detection of Colorado tick fever viral RNA in acute human serum samples by a quantitative real-time RT-PCR assay. J Virol Methods. 2007;140:43-48.
- Centers for Disease Control and Prevention (CDC). Tularemia—United States, 1990-2000. MMWR Morb Mortal Wkly Rep. 2002;51:182-184.
- Nigrovic LE, Wingerter SL. Tularemia. Infect Dis Clin North Am. 2008;22:489-504.
- Evans ME, Gregory DW, Schaffner W, et al. Tularemia: a 30-year experience with 88 cases. Medicine (Baltimore). 1985;64:251-269.
- Eliasson H, Sjöstedt A, Bäck E. Clinical use of diagnostic PCR for Francisella tularensis in patients with suspected ulceroglandular tularaemia. Scand J Infect Dis. 2005;37:833-837.
- Edlow JA, McGillicuddy DC. Tick paralysis. Infect Dis Clin North Am. 2008;22:397-413.
- Felz MW, Smith CD, Swift TR. A six-year-old girl with tick paralysis. N Engl J Med. 2000;342:90-94.
- Rose I. A review of tick paralysis. Can Med Assoc J. 1954;70:175-176.
- Dworkin MS, Shoemaker PC, Anderson DE. Tick paralysis: 33 human cases in Washington State, 1946-1996. Clin Infect Dis. 1999;29:1435-1439.
- Bowman DD. Georgis’ Parasitology for Veterinarians. 8th ed. New York, NY: Saunders; 2002.
- Dergousoff SJ, Galloway TD, Lindsay LR, et al. Range expansion of Dermacentor variabilis and Dermacentor andersoni near their northern distributional limits. J Med Entomol. 2013;50:510-520.
- Centers for Disease Control and Prevention. Geographic distribution of ticks that bite humans. Center for Disease Control and Prevention website. http://www.cdc.gov/ticks/geographic_distribution.html. Updated August 11, 2017. Accessed December 15
, 2017. - Trout Fryxell RT, Moore JE, Collins MD, et al. Habitat and vegetation variables are not enough when predicting tick populations in the southeastern United States. PLoS One. 2015;10:e0144092.
- Chapman AS, Bakken JS, Folk SM, et al. Diagnosis and management of tickborne rickettsial diseases: Rocky Mountain spotted fever, erlichiosis, and anaplasmosis—United States: a practical guide for physicians and other health-care and public health professionals. MMWR Recomm Rep. 2006;55:1-27.
- Nathavitharana RR, Mitty JA. Diseases from North America: focus on tick-borne infections. Clin Med. 2015;15:74-77.
- Chen LF, Sexton DJ. What’s new in Rocky Mountain spotted fever? Infect Dis Clin North Am. 2008;22:415-432.
- Lambert AJ, Kosoy O, Velez JO, et al. Detection of Colorado tick fever viral RNA in acute human serum samples by a quantitative real-time RT-PCR assay. J Virol Methods. 2007;140:43-48.
- Centers for Disease Control and Prevention (CDC). Tularemia—United States, 1990-2000. MMWR Morb Mortal Wkly Rep. 2002;51:182-184.
- Nigrovic LE, Wingerter SL. Tularemia. Infect Dis Clin North Am. 2008;22:489-504.
- Evans ME, Gregory DW, Schaffner W, et al. Tularemia: a 30-year experience with 88 cases. Medicine (Baltimore). 1985;64:251-269.
- Eliasson H, Sjöstedt A, Bäck E. Clinical use of diagnostic PCR for Francisella tularensis in patients with suspected ulceroglandular tularaemia. Scand J Infect Dis. 2005;37:833-837.
- Edlow JA, McGillicuddy DC. Tick paralysis. Infect Dis Clin North Am. 2008;22:397-413.
- Felz MW, Smith CD, Swift TR. A six-year-old girl with tick paralysis. N Engl J Med. 2000;342:90-94.
- Rose I. A review of tick paralysis. Can Med Assoc J. 1954;70:175-176.
- Dworkin MS, Shoemaker PC, Anderson DE. Tick paralysis: 33 human cases in Washington State, 1946-1996. Clin Infect Dis. 1999;29:1435-1439.
What’s Eating You? Head Lice (Pediculus humanus capitis)
The head louse (Pediculus humanus capitis) is a blood-sucking arthropod of the suborder Anoplura. Lice are obligate human parasites that have infested humans since antiquity. Pediculosis capitis is an infestation of the scalp by head lice. It is estimated that 6 to 12 million individuals in the United States are affected with head lice per year.1 Resistance to topical chemical pediculicides is widespread, and new agents have been developed to address this gap in care.
Characteristics of Head Lice
The head louse is a tan-gray–colored, wingless insect measuring approximately 2- to 3-mm long with 3 body segments. It has 6 legs with claws used to grasp individual hairs, and it moves by crawling; it does not fly or jump.2,3 The head louse has an elongated abdomen and a small head with short antennae and anterior piercing mouthparts (Figure 1).4 Nits are transparent, flask-shaped, 0.5- to 0.8-mm egg cases found firmly cemented to the hair shafts approximately 1 to 4 mm above the level of the scalp (Figure 2).5 The head louse resides on scalp hair and feeds off the scalp itself. Both lice and nits can be present throughout the scalp but are most commonly found in the postauricular and occipital scalp.3,4
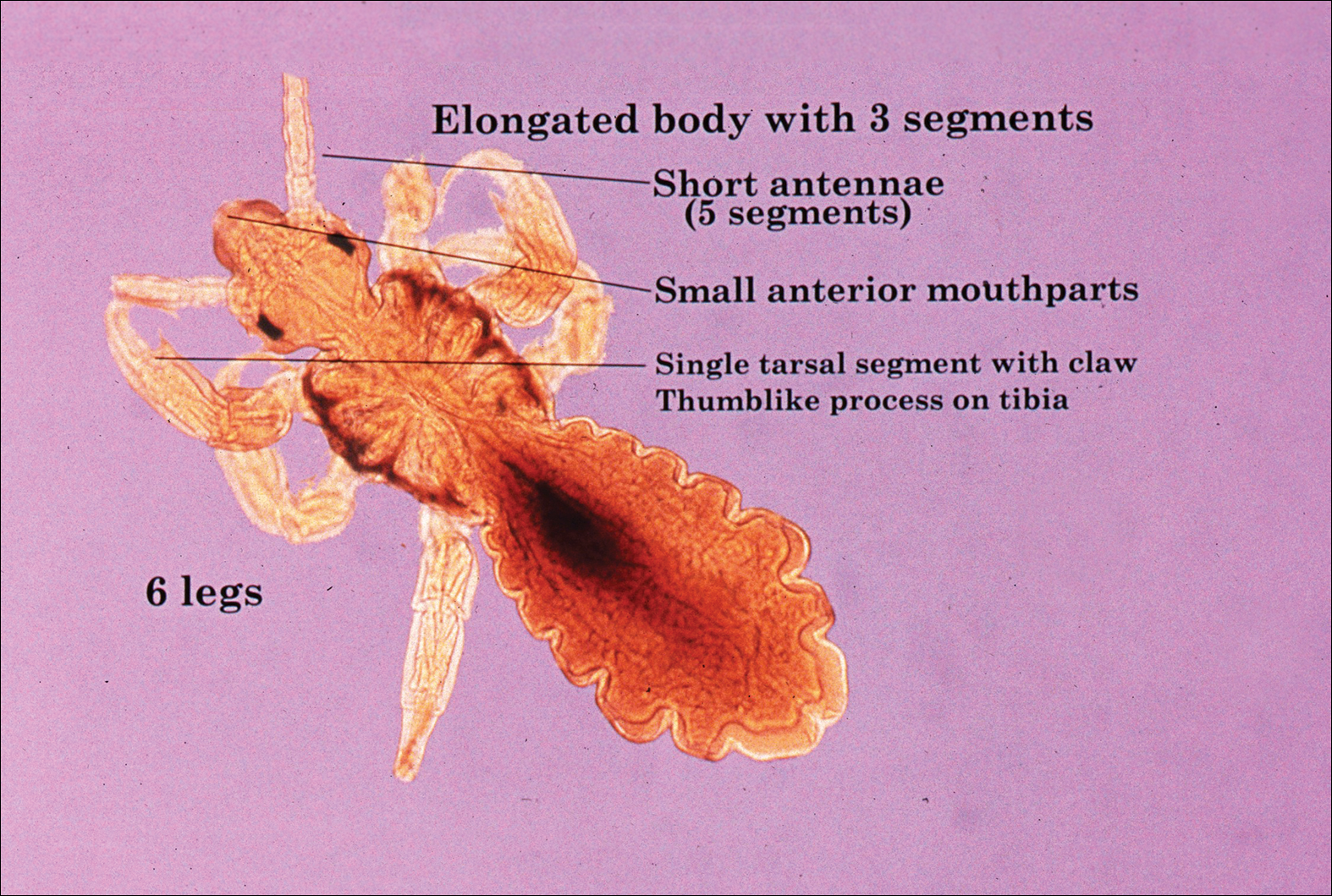
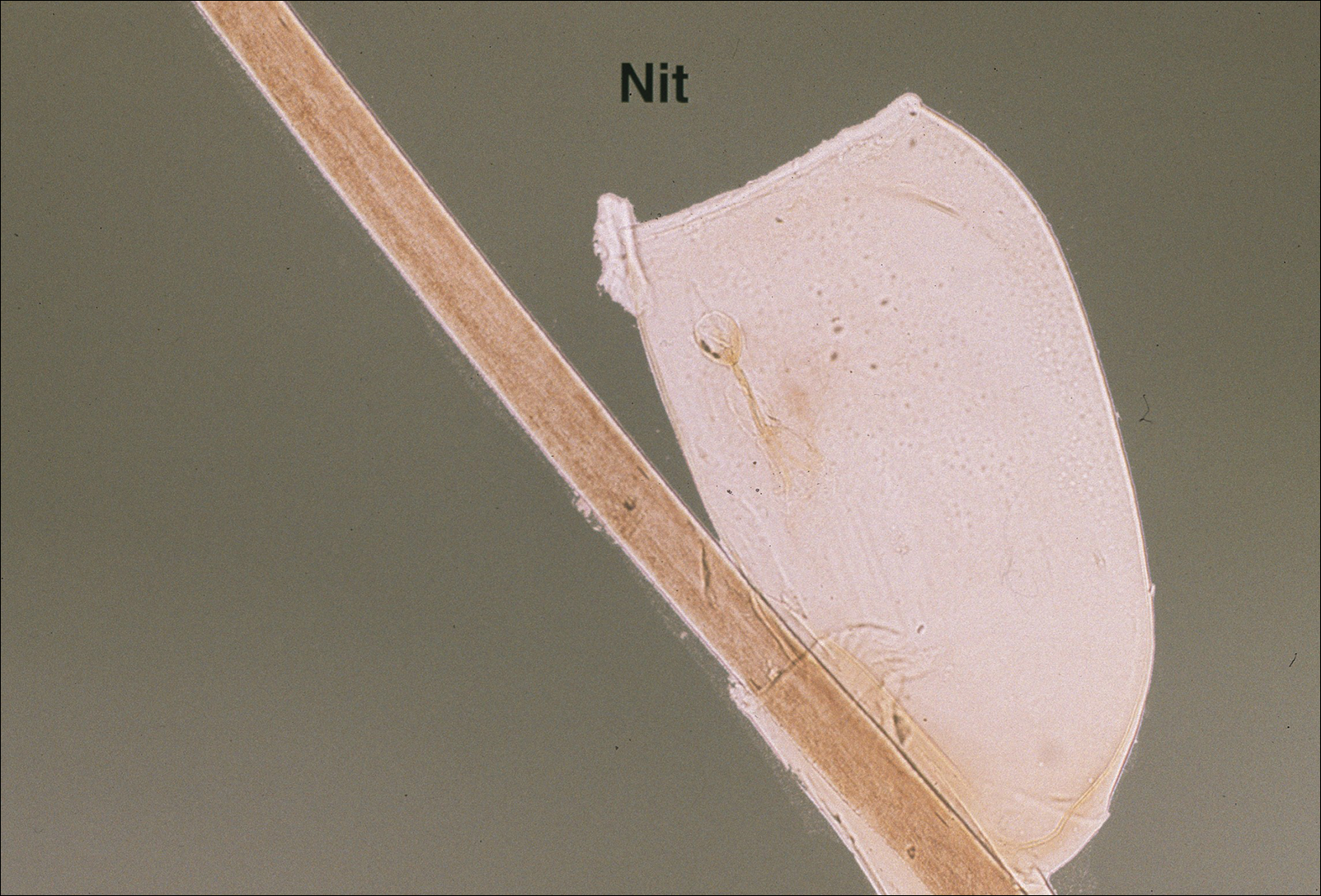
Female lice live approximately 30 days and lay 5 to 10 eggs per day. Eggs incubate individually in nits laid close to the scalp for 8 to 10 days before hatching.1,6 The newly hatched nymphs (also called instars) have multiple exoskeletons that are shed as they grow.7 Nymphs mature into adults in approximately 2 weeks, and the life cycle begins again.8 Head lice are obligate human parasites, feeding approximately every 4 to 6 hours on the blood of the host; however, they can survive up to 4 days without a blood meal on fomites if the climate and conditions are favorable.5,9
Epidemiology and Transmission
Head lice infestations commonly occur in children aged 3 to 11 years and are more prevalent in girls and women.1,10 Infestation rates are not reliably recorded, and few population-based studies have been performed; however, it is estimated that 6 to 12 million individuals are infested annually in the United States.1 Prevalence in some European populations has been estimated to range from 1% to 20%.11 A 2008 literature review found that worldwide prevalence varied across populations from 0.7% to 59%.10
Transmission occurs most frequently from direct head-to-head contact. One study found that transmission is most likely to occur when hairs are arranged in a parallel alignment and move slowly in relation to one another.12 Although controversial and probably less notable, transmission also may occur indirectly via fomites or the sharing of hairbrushes, hats, or other headgear.13,14 Classrooms are a common place for transmission.1 A 2009 study in Germany found an increase in health department consultations for head lice when schools reopened after vacations. The investigators also found that pediculicide sales peaked from mid-September through October, subsequent to schools reopening after the summer holiday.15 There is some evidence that overcrowded housing also can lead to increased incidence and transmission.16,17 There is no consistent correlation of infestation with socioeconomic status.1,17,18
Clinical Manifestations and Diagnosis
Clinically, patients with head lice present with scalp pruritus and sometimes posterior cervical or occipital lymphadenopathy. Pediculosis also can be asymptomatic. With the first exposure, symptoms may not develop for up to 4 to 6 weeks as the immune system develops sensitivity to the louse saliva.6 Bite reactions consisting of papules or wheals are related to immune sensitization.5 Louse feces and excoriations from scratching to relieve itch also may be present on examination. Secondary infection of excoriations also is possible.1
Diagnosis of an active infestation is made by identifying living lice. Because lice move quickly and can be difficult to detect, tightly attached nits on the hair shaft within 4 mm of the scalp are at least indicative of a historic infestation and can be suggestive of active infestation.1,19 Dermoscopy is a helpful tool in differentiating eggs containing nymphs from the empty cases of hatched lice and also from amorphous pseudonits (hair casts)(Figure 3).19,20 Wet combing improves the accuracy of diagnosing an active infection.21

Treatment
Effective treatment of head lice requires eradication of all living lice as well as louse eggs. Topically applied pyrethroids, including pyrethrin shampoos and mousses and permethrin lotion 1%, are considered the first-line therapy.8 Pyrethroids are over-the-counter treatments that act by interfering with sodium transport in the louse, causing depolarization of the neuromembranes and respiratory paralysis.22 Pyrethrins are natural compounds derived from the chrysanthemum plant; permethrin is a synthetic compound. Pyrethrins often are combined with piperonyl butoxide, an insecticide synergist that improves efficacy by inhibiting pyrethrin catabolism.23 Resistance to pyrethroids has become an increasingly important problem in the United States and worldwide.
Malathion lotion 0.5% is another therapeutic option for head lice. Malathion is a prescription organophosphate cholinesterase inhibitor that also causes respiratory paralysis of the louse and is one of the few treatments that is ovicidal.22 It was withdrawn from the market in 1995 due to its flammability and a theoretical risk of respiratory depression if ingested; however, it was reintroduced in 1999 and remains an effective treatment option with little resistance in the United States.24
Lindane 1% (shampoo and lotion), an organochloride compound that acts by causing neuronal hyperstimulation and eventual paralysis of lice, is no longer recommended due to its serious side effects, including central nervous system toxicity and increased risk of seizure.8,24
New US Food and Drug Administration–Approved Therapies
Newer topical treatments include benzyl alcohol lotion 5%, spinosad topical suspension 0.9%, ivermectin lotion 0.5%, and dimethicone-based products. Benzyl alcohol was approved by the US Food and Drug Administration (FDA) in 2009 and is available in the United States by prescription.25 Benzyl alcohol kills lice by asphyxiation. Phase 2 and 3 clinical trials showed significant treatment success 1 day posttreatment (fewer live lice than the vehicle alone; P=.004) and 2 weeks posttreatment (absence of live lice compared to the vehicle alone; P=.001).26
Spinosad was approved by the FDA in 2011 and is available in the United States by prescription.25 It contains the compounds spinosyn A and spinosyn D, which are naturally derived through fermentation by the soil bacterium Saccharopolyspora spinosa. It also contains benzyl alcohol. Spinosad paralyzes lice by disrupting neuronal activity and is at least partially ovicidal.27 Phase 3 clinical trials published in 2009 showed that spinosad was significantly more effective than permethrin in eradicating head lice (P<.001).28
Topical ivermectin was approved by the FDA in 2012 for prescription use.25 It acts on chloride ion channels, causing hyperpolarization of the muscle cells of lice and resulting in paralysis and death. Oral ivermectin (200 μg/kg) given once and repeated in 10 days is not FDA approved for the treatment of head lice but has shown some effectiveness and is sometimes used.8 A comparison study of topical versus oral ivermectin published in 2014 found that eradication was achieved in 88% (n=27) of topical ivermectin users after 1 treatment and 100% (n=31) after 2 treatments. Oral ivermectin produced cure rates of 45% (n=14) after 1 treatment and 97% (n=30) after 2 treatments. Both topical and oral ivermectin treatments are well tolerated.29
Physically Acting Preparations
Products with a physical mode of action are a new attractive option for treatment of pediculosis because the development of resistance is less likely. Studies of silicone-based fluids that physically occlude the respiratory system of the louse, such as dimethicone liquid gel 4%, have shown superiority over treatment with pyrethroids.30,31 Although the safety of dimethicone has been demonstrated, silicone-based treatments have not yet been widely adopted in the United States and are not currently used as a first-line treatment.32 However, use of such physically acting pediculicides may in time surpass traditional neurotoxic treatments due to their low susceptibility to resistance and good safety profile.33,34
Alternative Therapies
Nonchemical treatments for head lice that have shown variable success include wet combing, hot air treatments, and varying occlusive treatments. Physical removal via wet combing requires persistent repeated treatments over several weeks; for example, wet combing may be performed every 3 days for at least 2 weeks or until no head lice are detected on 4 consecutive occasions.35 Cure rates range from 38% to 75% with wet combing as a sole treatment of head lice.36 Because this treatment has minimal risks and no adverse side effects, it can be considered as an alternative treatment for some patients.
Hot air treatments also have been studied. A 2006 study showed that a hot air treatment device had the potential to eradicate head lice, most likely by desiccation. Specifically, 30 minutes of exposure to hot air (at 58.9°F, slightly cooler than a standard hair dryer) using the custom-built device resulted in 98% mortality of eggs and 80% mortality of hatched lice.37 Large randomized controlled trials of hot air treatments have not been performed.
Other alternative treatments include plant-derived oils. A laboratory study of essential oils found that spearmint, cassia, and clove showed pediculicidal activity similar to malathion with improved ovicidal activity.38 However, there is a potential for development of contact dermatitis from essential oils.
Complete Eradication of Head Lice
Removal of nits is an important component of effective lice eradication. Biochemical analysis has revealed that the nit sheath of the head louse is similar in composition to amyloid, rendering it difficult to design products that will unravel the nit sheath while leaving human hair undamaged.39 Because pediculicides are not necessarily ovicidal and complete physical nit removal is difficult to achieve, re-treatment in 7 to 10 days often is advisable to ensure that lice in all stages of the life cycle have been killed.4 Treatment of any secondary bacterial infection also is important. Although transmission of lice via fomites is less likely than from head-to-head contact, the cleaning of hats, hairbrushes, and linens is prudent. Diagnosing and treating infested close contacts also is essential to achieving eradication.4 Coordinated surveillance, education, and treatment efforts in high-risk communities can help detect asymptomatic cases and control local epidemics in a cost-effective manner.40 However, “no nit” policies at schools likely cause a net harm, as nit removal is difficult and children with nonviable nits are then excluded from the classroom.5
Treatment Resistance
Resistance to topical neurotoxic treatments is becoming increasingly common.41-43 Therefore, it is important to identify local patterns of resistance, if possible, when selecting a therapy for head lice. Improper usage, changes in pediculicide formulations and packaging, decreased product efficacy, and natural selection have all contributed to this rise in resistance.7 Additionally, due to protection from multiple exoskeletons and the natural molting process as they mature into adults, nymphs may only receive a sublethal dose when exposed to pediculicides, contributing further to resistance.7 Resistance to synthetic pyrethroids is most predominant, likely due to selection pressure because permethrin historically has been the most widely used insecticide for pediculosis. A 2014 study found that the frequency of sodium-channel insensitivity to pyrethroids, also known as knockdown resistance (or kdr), in US head louse populations collected over a 10-year period was 84.4% and approached 100% in some communities in recent years.44 This evidence strongly supports the use of alternative therapeutic categories to effectively eradicate head lice infestations.
Conclusion
Head lice infestation is common in children, and although it is not harmful to the host, it can be an irritating and symptomatic problem and can lead to notable distress, missed days of school, and secondary infections. Identifying active adult lice is the gold standard for diagnosis. Current recommended treatments include pyrethroids as the first-line therapy; however, resistance to these neurotoxic agents is becoming increasingly common. Alternative therapies such as newer neurotoxic agents or pediculicides with physical mechanisms of action (eg, dimethicone-based products) should be considered, particularly in regions where resistance is known to be high. Education about head lice, proper use of treatment, and coordinated diagnosis are necessary for effective management of this problem.
- Chosidow O. Scabies and pediculosis. Lancet. 2000;355:819-826.
- Centers for Disease Control and Prevention. Head lice. http://www.cdc.gov/parasites/lice/head/index.html. Updated September 24, 2013. Accessed November 9, 2017.
- Hurwitz S. Lice (pediculosis). In: Hurwitz S. Hurwitz Clinical Pediatric Dermatology: A Textbook of Skin Disorders of Childhood and Adolescence. 2nd ed. Philadelphia, PA: WB Saunders Company; 1993:416-419.
- Elston DM. What’s eating you? Pediculus humanus (head louse and body louse). Cutis. 1999;63:259-264.
- Ko CJ, Elston DM. Pediculosis. J Am Acad Dermatol. 2004;50:1-12.
- Frankowski BL, Weiner LB. Head lice. Pediatrics. 2002;110:638-643.
- Meinking TL. Clinical update on resistance and treatment of pediculosis capitis. Am J Manag Care. 2004;10(9 suppl):S264-S268.
- Devore CD, Schutze GE. Head lice. Pediatrics. 2015;135:E1355-E1365.
- Burkhart CN. Fomite transmission with head lice: a continuing controversy. Lancet. 2003;361:99-100.
- Falagas ME, Matthaiou DK, Rafailidis PI, et al. Worldwide prevalence of head lice. Emerg Infect Dis. 2008;14:1493-1494.
- Feldmeier H. Pediculosis capitis: new insights into epidemiology, diagnosis and treatment. Eur J Clin Microbiol Infect Dis. 2012;31:2105-2110.
- Canyon DV, Speare R, Muller R. Spatial and kinetic factors for the transfer of head lice (Pediculus capitis) between hairs. J Invest Dermatol. 2002;119:629-631.
- Burkhart CN, Burkhart CG. Fomite transmission in head lice. J Am Acad Dermatol. 2007;56:1044-1047.
- Canyon DV, Speare R. Indirect transmission of head lice via inanimate objects. Open Dermatol J. 2010;4:72-76.
- Bauer E, Jahnke C, Feldmeier H. Seasonal fluctuations of head lice infestation in Germany. Parasitol Res. 2009;104:677-681.
- Balcioglu IC, Kurt O, Limoncu ME, et al. Rural life, lower socioeconomic status and parasitic infections. Parasitol Int. 2007;56:129-133.
- Lesshafft H, Baier A, Guerra H, et al. Prevalence and risk factors associated with pediculosis capitis in an impoverished urban community in Lima, Peru. J Glob Infect Dis. 2013;5:138-143.
- Tagka A, Lambrou GI, Braoudaki M, et al. Socioeconomical factors associated with pediculosis (Phthiraptera: Pediculidae) in Athens, Greece. J Med Entomol. 2016;53:919-922.
- Di Stefani A, Hofmann-Wellenhof R, Zalaudek I. Dermoscopy for diagnosis and treatment monitoring of pediculosis capitis. J Am Acad Dermatol. 2006;54:909-911.
- Bakos RM, Bakos L. Dermoscopy for diagnosis of pediculosis capitis. J Am Acad Dermatol. 2007;57:727-728.
- Jahnke C, Bauer E, Hengge UR, et al. Accuracy of diagnosis of pediculosis capitis: visual inspection vs wet combing. Arch Dermatol. 2009;145:309-313.
- Elston DM. Drugs used in the treatment of pediculosis. J Drugs Dermatol. 2005;4:207-211.
- National Pesticide Information Center. Piperonyl butoxide (general fact sheet). http://npic.orst.edu/factsheets/pbogen.pdf/. Accessed November 13, 2017.
- Diamantis SA, Morrell DS, Burkhart CN. Treatment of head lice. Dermatol Ther. 2009;22:273-278.
- United States Food and Drug Administration. Treating and preventing head lice. http://www.fda.gov/forconsumers/consumerupdates/ucm171730.htm. Published July 13, 2010. Updated November 8, 2017. Accessed November 13, 2017.
- Meinking TL, Villar ME, Vicaria M, et al. The clinical trials supporting benzyl alcohol lotion 5% (UlesfiaTM): a safe and effective topical treatment for head lice (Pediculosis Humanus Capitis). Pediatr Dermatol. 2010;27:19-24.
- McCormack PL. Spinosad in pediculosis capitis. Am J Clin Dermatol. 2011;12:349-353.
- Stough D, Shellabarger S, Quiring J, et al. Efficacy and safety of spinosad and permethrin creme rinses for pediculosis capitis (head lice). Pediatrics. 2009;124:E389-E395.
- Ahmad HM, Abdel-Azim ES, Abdel-Aziz RT. Assessment of topical versus oral ivermectin as a treatment for head lice. Dermatol Ther. 2014;27:307-310.
- Heukelbach J, Pilger D, Oliveira FA, et al. A highly efficacious pediculicide based on dimethicone: randomized observer blinded comparative trial. BMC Infect Dis. 2008;8:115.
- Burgess IF, Brunton ER, Burgess NA. Single application of 4% dimethicone liquid gel versus two applications of 1% permethrin creme rinse for treatment of head louse infestation: a randomised controlled trial. BMC Dermatol. 2013;13:5.
- Ihde ES, Boscamp JR, Loh JM, et al. Safety and efficacy of a 100% dimethicone pediculocide in school-age children. BMC Pediatr. 2015;15:70.
- Heukelbach J, Oliveira FA, Richter J, et al. Dimethicone-based pediculicides: a physical approach to eradicate head lice. Open Dermatol J. 2010;4:77-81.
- Feldmeier H. Treatment of pediculosis capitis: a critical appraisal of the current literature. Am J Clin Dermatol. 2014;15:401-412.
- Glasziou P, Bennett J, Greenberg P, et al; Handbook Of Non Drug Intervention (HANDI) Project Team. Wet combing for the eradication of head lice. Aust Fam Physician. 2013;42:129-130.
- Tebruegge M, Runnacles J. Is wet combing effective in children with pediculosis capitis infestation? Arch Dis Child. 2007;92:818-820.
- Goates BM, Atkin JS, Wilding KG, et al. An effective nonchemical treatment for head lice: a lot of hot air. Pediatrics. 2006;118:1962-1970.
- Yones DA, Bakir HY, Bayoumi SA. Chemical composition and efficacy of some selected plant oils against Pediculus humanus capitis in vitro. Parasitol Res. 2016;115:3209-3218.
- Burkhart CN, Burkhart CG. Head lice: scientific assessment of the nit sheath with clinical ramifications and therapeutic options. J Am Acad Dermatol. 2005;53:129-133.
- Ibarra J, Fry F, Wickenden C, et al. The impact of well-developed preventative strategies on the eradication of head lice. Perspect Public Health. 2009;129:165-173.
- Mumcuoglu KY, Hemingway J, Miller J, et al. Permethrin resistance in the head louse pediculus humanus capitis from Israel. Med Vet Entomol. 1995;9:427-432.
- Meinking TL, Serrano L, Hard B, et al. Comparative in vitro pediculicidal efficacy of treatments in a resistant head lice population in the United States. Arch Dermatol. 2002;138:220-224.
- Hemingway J, Miller J, Mumcuoglu KY. Pyrethroid resistance mechanisms in the head louse Pediculus capitis from Israel: implications for control. Med Vet Entomol. 1999;13:89-96.
- Yoon KS, Previte DJ, Hodgdon HE, et al. Knockdown resistance allele frequencies in North American head louse (Anoplura: Pediculidae) populations. J Med Entomol. 2014;51:450-457.
The head louse (Pediculus humanus capitis) is a blood-sucking arthropod of the suborder Anoplura. Lice are obligate human parasites that have infested humans since antiquity. Pediculosis capitis is an infestation of the scalp by head lice. It is estimated that 6 to 12 million individuals in the United States are affected with head lice per year.1 Resistance to topical chemical pediculicides is widespread, and new agents have been developed to address this gap in care.
Characteristics of Head Lice
The head louse is a tan-gray–colored, wingless insect measuring approximately 2- to 3-mm long with 3 body segments. It has 6 legs with claws used to grasp individual hairs, and it moves by crawling; it does not fly or jump.2,3 The head louse has an elongated abdomen and a small head with short antennae and anterior piercing mouthparts (Figure 1).4 Nits are transparent, flask-shaped, 0.5- to 0.8-mm egg cases found firmly cemented to the hair shafts approximately 1 to 4 mm above the level of the scalp (Figure 2).5 The head louse resides on scalp hair and feeds off the scalp itself. Both lice and nits can be present throughout the scalp but are most commonly found in the postauricular and occipital scalp.3,4


Female lice live approximately 30 days and lay 5 to 10 eggs per day. Eggs incubate individually in nits laid close to the scalp for 8 to 10 days before hatching.1,6 The newly hatched nymphs (also called instars) have multiple exoskeletons that are shed as they grow.7 Nymphs mature into adults in approximately 2 weeks, and the life cycle begins again.8 Head lice are obligate human parasites, feeding approximately every 4 to 6 hours on the blood of the host; however, they can survive up to 4 days without a blood meal on fomites if the climate and conditions are favorable.5,9
Epidemiology and Transmission
Head lice infestations commonly occur in children aged 3 to 11 years and are more prevalent in girls and women.1,10 Infestation rates are not reliably recorded, and few population-based studies have been performed; however, it is estimated that 6 to 12 million individuals are infested annually in the United States.1 Prevalence in some European populations has been estimated to range from 1% to 20%.11 A 2008 literature review found that worldwide prevalence varied across populations from 0.7% to 59%.10
Transmission occurs most frequently from direct head-to-head contact. One study found that transmission is most likely to occur when hairs are arranged in a parallel alignment and move slowly in relation to one another.12 Although controversial and probably less notable, transmission also may occur indirectly via fomites or the sharing of hairbrushes, hats, or other headgear.13,14 Classrooms are a common place for transmission.1 A 2009 study in Germany found an increase in health department consultations for head lice when schools reopened after vacations. The investigators also found that pediculicide sales peaked from mid-September through October, subsequent to schools reopening after the summer holiday.15 There is some evidence that overcrowded housing also can lead to increased incidence and transmission.16,17 There is no consistent correlation of infestation with socioeconomic status.1,17,18
Clinical Manifestations and Diagnosis
Clinically, patients with head lice present with scalp pruritus and sometimes posterior cervical or occipital lymphadenopathy. Pediculosis also can be asymptomatic. With the first exposure, symptoms may not develop for up to 4 to 6 weeks as the immune system develops sensitivity to the louse saliva.6 Bite reactions consisting of papules or wheals are related to immune sensitization.5 Louse feces and excoriations from scratching to relieve itch also may be present on examination. Secondary infection of excoriations also is possible.1
Diagnosis of an active infestation is made by identifying living lice. Because lice move quickly and can be difficult to detect, tightly attached nits on the hair shaft within 4 mm of the scalp are at least indicative of a historic infestation and can be suggestive of active infestation.1,19 Dermoscopy is a helpful tool in differentiating eggs containing nymphs from the empty cases of hatched lice and also from amorphous pseudonits (hair casts)(Figure 3).19,20 Wet combing improves the accuracy of diagnosing an active infection.21

Treatment
Effective treatment of head lice requires eradication of all living lice as well as louse eggs. Topically applied pyrethroids, including pyrethrin shampoos and mousses and permethrin lotion 1%, are considered the first-line therapy.8 Pyrethroids are over-the-counter treatments that act by interfering with sodium transport in the louse, causing depolarization of the neuromembranes and respiratory paralysis.22 Pyrethrins are natural compounds derived from the chrysanthemum plant; permethrin is a synthetic compound. Pyrethrins often are combined with piperonyl butoxide, an insecticide synergist that improves efficacy by inhibiting pyrethrin catabolism.23 Resistance to pyrethroids has become an increasingly important problem in the United States and worldwide.
Malathion lotion 0.5% is another therapeutic option for head lice. Malathion is a prescription organophosphate cholinesterase inhibitor that also causes respiratory paralysis of the louse and is one of the few treatments that is ovicidal.22 It was withdrawn from the market in 1995 due to its flammability and a theoretical risk of respiratory depression if ingested; however, it was reintroduced in 1999 and remains an effective treatment option with little resistance in the United States.24
Lindane 1% (shampoo and lotion), an organochloride compound that acts by causing neuronal hyperstimulation and eventual paralysis of lice, is no longer recommended due to its serious side effects, including central nervous system toxicity and increased risk of seizure.8,24
New US Food and Drug Administration–Approved Therapies
Newer topical treatments include benzyl alcohol lotion 5%, spinosad topical suspension 0.9%, ivermectin lotion 0.5%, and dimethicone-based products. Benzyl alcohol was approved by the US Food and Drug Administration (FDA) in 2009 and is available in the United States by prescription.25 Benzyl alcohol kills lice by asphyxiation. Phase 2 and 3 clinical trials showed significant treatment success 1 day posttreatment (fewer live lice than the vehicle alone; P=.004) and 2 weeks posttreatment (absence of live lice compared to the vehicle alone; P=.001).26
Spinosad was approved by the FDA in 2011 and is available in the United States by prescription.25 It contains the compounds spinosyn A and spinosyn D, which are naturally derived through fermentation by the soil bacterium Saccharopolyspora spinosa. It also contains benzyl alcohol. Spinosad paralyzes lice by disrupting neuronal activity and is at least partially ovicidal.27 Phase 3 clinical trials published in 2009 showed that spinosad was significantly more effective than permethrin in eradicating head lice (P<.001).28
Topical ivermectin was approved by the FDA in 2012 for prescription use.25 It acts on chloride ion channels, causing hyperpolarization of the muscle cells of lice and resulting in paralysis and death. Oral ivermectin (200 μg/kg) given once and repeated in 10 days is not FDA approved for the treatment of head lice but has shown some effectiveness and is sometimes used.8 A comparison study of topical versus oral ivermectin published in 2014 found that eradication was achieved in 88% (n=27) of topical ivermectin users after 1 treatment and 100% (n=31) after 2 treatments. Oral ivermectin produced cure rates of 45% (n=14) after 1 treatment and 97% (n=30) after 2 treatments. Both topical and oral ivermectin treatments are well tolerated.29
Physically Acting Preparations
Products with a physical mode of action are a new attractive option for treatment of pediculosis because the development of resistance is less likely. Studies of silicone-based fluids that physically occlude the respiratory system of the louse, such as dimethicone liquid gel 4%, have shown superiority over treatment with pyrethroids.30,31 Although the safety of dimethicone has been demonstrated, silicone-based treatments have not yet been widely adopted in the United States and are not currently used as a first-line treatment.32 However, use of such physically acting pediculicides may in time surpass traditional neurotoxic treatments due to their low susceptibility to resistance and good safety profile.33,34
Alternative Therapies
Nonchemical treatments for head lice that have shown variable success include wet combing, hot air treatments, and varying occlusive treatments. Physical removal via wet combing requires persistent repeated treatments over several weeks; for example, wet combing may be performed every 3 days for at least 2 weeks or until no head lice are detected on 4 consecutive occasions.35 Cure rates range from 38% to 75% with wet combing as a sole treatment of head lice.36 Because this treatment has minimal risks and no adverse side effects, it can be considered as an alternative treatment for some patients.
Hot air treatments also have been studied. A 2006 study showed that a hot air treatment device had the potential to eradicate head lice, most likely by desiccation. Specifically, 30 minutes of exposure to hot air (at 58.9°F, slightly cooler than a standard hair dryer) using the custom-built device resulted in 98% mortality of eggs and 80% mortality of hatched lice.37 Large randomized controlled trials of hot air treatments have not been performed.
Other alternative treatments include plant-derived oils. A laboratory study of essential oils found that spearmint, cassia, and clove showed pediculicidal activity similar to malathion with improved ovicidal activity.38 However, there is a potential for development of contact dermatitis from essential oils.
Complete Eradication of Head Lice
Removal of nits is an important component of effective lice eradication. Biochemical analysis has revealed that the nit sheath of the head louse is similar in composition to amyloid, rendering it difficult to design products that will unravel the nit sheath while leaving human hair undamaged.39 Because pediculicides are not necessarily ovicidal and complete physical nit removal is difficult to achieve, re-treatment in 7 to 10 days often is advisable to ensure that lice in all stages of the life cycle have been killed.4 Treatment of any secondary bacterial infection also is important. Although transmission of lice via fomites is less likely than from head-to-head contact, the cleaning of hats, hairbrushes, and linens is prudent. Diagnosing and treating infested close contacts also is essential to achieving eradication.4 Coordinated surveillance, education, and treatment efforts in high-risk communities can help detect asymptomatic cases and control local epidemics in a cost-effective manner.40 However, “no nit” policies at schools likely cause a net harm, as nit removal is difficult and children with nonviable nits are then excluded from the classroom.5
Treatment Resistance
Resistance to topical neurotoxic treatments is becoming increasingly common.41-43 Therefore, it is important to identify local patterns of resistance, if possible, when selecting a therapy for head lice. Improper usage, changes in pediculicide formulations and packaging, decreased product efficacy, and natural selection have all contributed to this rise in resistance.7 Additionally, due to protection from multiple exoskeletons and the natural molting process as they mature into adults, nymphs may only receive a sublethal dose when exposed to pediculicides, contributing further to resistance.7 Resistance to synthetic pyrethroids is most predominant, likely due to selection pressure because permethrin historically has been the most widely used insecticide for pediculosis. A 2014 study found that the frequency of sodium-channel insensitivity to pyrethroids, also known as knockdown resistance (or kdr), in US head louse populations collected over a 10-year period was 84.4% and approached 100% in some communities in recent years.44 This evidence strongly supports the use of alternative therapeutic categories to effectively eradicate head lice infestations.
Conclusion
Head lice infestation is common in children, and although it is not harmful to the host, it can be an irritating and symptomatic problem and can lead to notable distress, missed days of school, and secondary infections. Identifying active adult lice is the gold standard for diagnosis. Current recommended treatments include pyrethroids as the first-line therapy; however, resistance to these neurotoxic agents is becoming increasingly common. Alternative therapies such as newer neurotoxic agents or pediculicides with physical mechanisms of action (eg, dimethicone-based products) should be considered, particularly in regions where resistance is known to be high. Education about head lice, proper use of treatment, and coordinated diagnosis are necessary for effective management of this problem.
The head louse (Pediculus humanus capitis) is a blood-sucking arthropod of the suborder Anoplura. Lice are obligate human parasites that have infested humans since antiquity. Pediculosis capitis is an infestation of the scalp by head lice. It is estimated that 6 to 12 million individuals in the United States are affected with head lice per year.1 Resistance to topical chemical pediculicides is widespread, and new agents have been developed to address this gap in care.
Characteristics of Head Lice
The head louse is a tan-gray–colored, wingless insect measuring approximately 2- to 3-mm long with 3 body segments. It has 6 legs with claws used to grasp individual hairs, and it moves by crawling; it does not fly or jump.2,3 The head louse has an elongated abdomen and a small head with short antennae and anterior piercing mouthparts (Figure 1).4 Nits are transparent, flask-shaped, 0.5- to 0.8-mm egg cases found firmly cemented to the hair shafts approximately 1 to 4 mm above the level of the scalp (Figure 2).5 The head louse resides on scalp hair and feeds off the scalp itself. Both lice and nits can be present throughout the scalp but are most commonly found in the postauricular and occipital scalp.3,4


Female lice live approximately 30 days and lay 5 to 10 eggs per day. Eggs incubate individually in nits laid close to the scalp for 8 to 10 days before hatching.1,6 The newly hatched nymphs (also called instars) have multiple exoskeletons that are shed as they grow.7 Nymphs mature into adults in approximately 2 weeks, and the life cycle begins again.8 Head lice are obligate human parasites, feeding approximately every 4 to 6 hours on the blood of the host; however, they can survive up to 4 days without a blood meal on fomites if the climate and conditions are favorable.5,9
Epidemiology and Transmission
Head lice infestations commonly occur in children aged 3 to 11 years and are more prevalent in girls and women.1,10 Infestation rates are not reliably recorded, and few population-based studies have been performed; however, it is estimated that 6 to 12 million individuals are infested annually in the United States.1 Prevalence in some European populations has been estimated to range from 1% to 20%.11 A 2008 literature review found that worldwide prevalence varied across populations from 0.7% to 59%.10
Transmission occurs most frequently from direct head-to-head contact. One study found that transmission is most likely to occur when hairs are arranged in a parallel alignment and move slowly in relation to one another.12 Although controversial and probably less notable, transmission also may occur indirectly via fomites or the sharing of hairbrushes, hats, or other headgear.13,14 Classrooms are a common place for transmission.1 A 2009 study in Germany found an increase in health department consultations for head lice when schools reopened after vacations. The investigators also found that pediculicide sales peaked from mid-September through October, subsequent to schools reopening after the summer holiday.15 There is some evidence that overcrowded housing also can lead to increased incidence and transmission.16,17 There is no consistent correlation of infestation with socioeconomic status.1,17,18
Clinical Manifestations and Diagnosis
Clinically, patients with head lice present with scalp pruritus and sometimes posterior cervical or occipital lymphadenopathy. Pediculosis also can be asymptomatic. With the first exposure, symptoms may not develop for up to 4 to 6 weeks as the immune system develops sensitivity to the louse saliva.6 Bite reactions consisting of papules or wheals are related to immune sensitization.5 Louse feces and excoriations from scratching to relieve itch also may be present on examination. Secondary infection of excoriations also is possible.1
Diagnosis of an active infestation is made by identifying living lice. Because lice move quickly and can be difficult to detect, tightly attached nits on the hair shaft within 4 mm of the scalp are at least indicative of a historic infestation and can be suggestive of active infestation.1,19 Dermoscopy is a helpful tool in differentiating eggs containing nymphs from the empty cases of hatched lice and also from amorphous pseudonits (hair casts)(Figure 3).19,20 Wet combing improves the accuracy of diagnosing an active infection.21

Treatment
Effective treatment of head lice requires eradication of all living lice as well as louse eggs. Topically applied pyrethroids, including pyrethrin shampoos and mousses and permethrin lotion 1%, are considered the first-line therapy.8 Pyrethroids are over-the-counter treatments that act by interfering with sodium transport in the louse, causing depolarization of the neuromembranes and respiratory paralysis.22 Pyrethrins are natural compounds derived from the chrysanthemum plant; permethrin is a synthetic compound. Pyrethrins often are combined with piperonyl butoxide, an insecticide synergist that improves efficacy by inhibiting pyrethrin catabolism.23 Resistance to pyrethroids has become an increasingly important problem in the United States and worldwide.
Malathion lotion 0.5% is another therapeutic option for head lice. Malathion is a prescription organophosphate cholinesterase inhibitor that also causes respiratory paralysis of the louse and is one of the few treatments that is ovicidal.22 It was withdrawn from the market in 1995 due to its flammability and a theoretical risk of respiratory depression if ingested; however, it was reintroduced in 1999 and remains an effective treatment option with little resistance in the United States.24
Lindane 1% (shampoo and lotion), an organochloride compound that acts by causing neuronal hyperstimulation and eventual paralysis of lice, is no longer recommended due to its serious side effects, including central nervous system toxicity and increased risk of seizure.8,24
New US Food and Drug Administration–Approved Therapies
Newer topical treatments include benzyl alcohol lotion 5%, spinosad topical suspension 0.9%, ivermectin lotion 0.5%, and dimethicone-based products. Benzyl alcohol was approved by the US Food and Drug Administration (FDA) in 2009 and is available in the United States by prescription.25 Benzyl alcohol kills lice by asphyxiation. Phase 2 and 3 clinical trials showed significant treatment success 1 day posttreatment (fewer live lice than the vehicle alone; P=.004) and 2 weeks posttreatment (absence of live lice compared to the vehicle alone; P=.001).26
Spinosad was approved by the FDA in 2011 and is available in the United States by prescription.25 It contains the compounds spinosyn A and spinosyn D, which are naturally derived through fermentation by the soil bacterium Saccharopolyspora spinosa. It also contains benzyl alcohol. Spinosad paralyzes lice by disrupting neuronal activity and is at least partially ovicidal.27 Phase 3 clinical trials published in 2009 showed that spinosad was significantly more effective than permethrin in eradicating head lice (P<.001).28
Topical ivermectin was approved by the FDA in 2012 for prescription use.25 It acts on chloride ion channels, causing hyperpolarization of the muscle cells of lice and resulting in paralysis and death. Oral ivermectin (200 μg/kg) given once and repeated in 10 days is not FDA approved for the treatment of head lice but has shown some effectiveness and is sometimes used.8 A comparison study of topical versus oral ivermectin published in 2014 found that eradication was achieved in 88% (n=27) of topical ivermectin users after 1 treatment and 100% (n=31) after 2 treatments. Oral ivermectin produced cure rates of 45% (n=14) after 1 treatment and 97% (n=30) after 2 treatments. Both topical and oral ivermectin treatments are well tolerated.29
Physically Acting Preparations
Products with a physical mode of action are a new attractive option for treatment of pediculosis because the development of resistance is less likely. Studies of silicone-based fluids that physically occlude the respiratory system of the louse, such as dimethicone liquid gel 4%, have shown superiority over treatment with pyrethroids.30,31 Although the safety of dimethicone has been demonstrated, silicone-based treatments have not yet been widely adopted in the United States and are not currently used as a first-line treatment.32 However, use of such physically acting pediculicides may in time surpass traditional neurotoxic treatments due to their low susceptibility to resistance and good safety profile.33,34
Alternative Therapies
Nonchemical treatments for head lice that have shown variable success include wet combing, hot air treatments, and varying occlusive treatments. Physical removal via wet combing requires persistent repeated treatments over several weeks; for example, wet combing may be performed every 3 days for at least 2 weeks or until no head lice are detected on 4 consecutive occasions.35 Cure rates range from 38% to 75% with wet combing as a sole treatment of head lice.36 Because this treatment has minimal risks and no adverse side effects, it can be considered as an alternative treatment for some patients.
Hot air treatments also have been studied. A 2006 study showed that a hot air treatment device had the potential to eradicate head lice, most likely by desiccation. Specifically, 30 minutes of exposure to hot air (at 58.9°F, slightly cooler than a standard hair dryer) using the custom-built device resulted in 98% mortality of eggs and 80% mortality of hatched lice.37 Large randomized controlled trials of hot air treatments have not been performed.
Other alternative treatments include plant-derived oils. A laboratory study of essential oils found that spearmint, cassia, and clove showed pediculicidal activity similar to malathion with improved ovicidal activity.38 However, there is a potential for development of contact dermatitis from essential oils.
Complete Eradication of Head Lice
Removal of nits is an important component of effective lice eradication. Biochemical analysis has revealed that the nit sheath of the head louse is similar in composition to amyloid, rendering it difficult to design products that will unravel the nit sheath while leaving human hair undamaged.39 Because pediculicides are not necessarily ovicidal and complete physical nit removal is difficult to achieve, re-treatment in 7 to 10 days often is advisable to ensure that lice in all stages of the life cycle have been killed.4 Treatment of any secondary bacterial infection also is important. Although transmission of lice via fomites is less likely than from head-to-head contact, the cleaning of hats, hairbrushes, and linens is prudent. Diagnosing and treating infested close contacts also is essential to achieving eradication.4 Coordinated surveillance, education, and treatment efforts in high-risk communities can help detect asymptomatic cases and control local epidemics in a cost-effective manner.40 However, “no nit” policies at schools likely cause a net harm, as nit removal is difficult and children with nonviable nits are then excluded from the classroom.5
Treatment Resistance
Resistance to topical neurotoxic treatments is becoming increasingly common.41-43 Therefore, it is important to identify local patterns of resistance, if possible, when selecting a therapy for head lice. Improper usage, changes in pediculicide formulations and packaging, decreased product efficacy, and natural selection have all contributed to this rise in resistance.7 Additionally, due to protection from multiple exoskeletons and the natural molting process as they mature into adults, nymphs may only receive a sublethal dose when exposed to pediculicides, contributing further to resistance.7 Resistance to synthetic pyrethroids is most predominant, likely due to selection pressure because permethrin historically has been the most widely used insecticide for pediculosis. A 2014 study found that the frequency of sodium-channel insensitivity to pyrethroids, also known as knockdown resistance (or kdr), in US head louse populations collected over a 10-year period was 84.4% and approached 100% in some communities in recent years.44 This evidence strongly supports the use of alternative therapeutic categories to effectively eradicate head lice infestations.
Conclusion
Head lice infestation is common in children, and although it is not harmful to the host, it can be an irritating and symptomatic problem and can lead to notable distress, missed days of school, and secondary infections. Identifying active adult lice is the gold standard for diagnosis. Current recommended treatments include pyrethroids as the first-line therapy; however, resistance to these neurotoxic agents is becoming increasingly common. Alternative therapies such as newer neurotoxic agents or pediculicides with physical mechanisms of action (eg, dimethicone-based products) should be considered, particularly in regions where resistance is known to be high. Education about head lice, proper use of treatment, and coordinated diagnosis are necessary for effective management of this problem.
- Chosidow O. Scabies and pediculosis. Lancet. 2000;355:819-826.
- Centers for Disease Control and Prevention. Head lice. http://www.cdc.gov/parasites/lice/head/index.html. Updated September 24, 2013. Accessed November 9, 2017.
- Hurwitz S. Lice (pediculosis). In: Hurwitz S. Hurwitz Clinical Pediatric Dermatology: A Textbook of Skin Disorders of Childhood and Adolescence. 2nd ed. Philadelphia, PA: WB Saunders Company; 1993:416-419.
- Elston DM. What’s eating you? Pediculus humanus (head louse and body louse). Cutis. 1999;63:259-264.
- Ko CJ, Elston DM. Pediculosis. J Am Acad Dermatol. 2004;50:1-12.
- Frankowski BL, Weiner LB. Head lice. Pediatrics. 2002;110:638-643.
- Meinking TL. Clinical update on resistance and treatment of pediculosis capitis. Am J Manag Care. 2004;10(9 suppl):S264-S268.
- Devore CD, Schutze GE. Head lice. Pediatrics. 2015;135:E1355-E1365.
- Burkhart CN. Fomite transmission with head lice: a continuing controversy. Lancet. 2003;361:99-100.
- Falagas ME, Matthaiou DK, Rafailidis PI, et al. Worldwide prevalence of head lice. Emerg Infect Dis. 2008;14:1493-1494.
- Feldmeier H. Pediculosis capitis: new insights into epidemiology, diagnosis and treatment. Eur J Clin Microbiol Infect Dis. 2012;31:2105-2110.
- Canyon DV, Speare R, Muller R. Spatial and kinetic factors for the transfer of head lice (Pediculus capitis) between hairs. J Invest Dermatol. 2002;119:629-631.
- Burkhart CN, Burkhart CG. Fomite transmission in head lice. J Am Acad Dermatol. 2007;56:1044-1047.
- Canyon DV, Speare R. Indirect transmission of head lice via inanimate objects. Open Dermatol J. 2010;4:72-76.
- Bauer E, Jahnke C, Feldmeier H. Seasonal fluctuations of head lice infestation in Germany. Parasitol Res. 2009;104:677-681.
- Balcioglu IC, Kurt O, Limoncu ME, et al. Rural life, lower socioeconomic status and parasitic infections. Parasitol Int. 2007;56:129-133.
- Lesshafft H, Baier A, Guerra H, et al. Prevalence and risk factors associated with pediculosis capitis in an impoverished urban community in Lima, Peru. J Glob Infect Dis. 2013;5:138-143.
- Tagka A, Lambrou GI, Braoudaki M, et al. Socioeconomical factors associated with pediculosis (Phthiraptera: Pediculidae) in Athens, Greece. J Med Entomol. 2016;53:919-922.
- Di Stefani A, Hofmann-Wellenhof R, Zalaudek I. Dermoscopy for diagnosis and treatment monitoring of pediculosis capitis. J Am Acad Dermatol. 2006;54:909-911.
- Bakos RM, Bakos L. Dermoscopy for diagnosis of pediculosis capitis. J Am Acad Dermatol. 2007;57:727-728.
- Jahnke C, Bauer E, Hengge UR, et al. Accuracy of diagnosis of pediculosis capitis: visual inspection vs wet combing. Arch Dermatol. 2009;145:309-313.
- Elston DM. Drugs used in the treatment of pediculosis. J Drugs Dermatol. 2005;4:207-211.
- National Pesticide Information Center. Piperonyl butoxide (general fact sheet). http://npic.orst.edu/factsheets/pbogen.pdf/. Accessed November 13, 2017.
- Diamantis SA, Morrell DS, Burkhart CN. Treatment of head lice. Dermatol Ther. 2009;22:273-278.
- United States Food and Drug Administration. Treating and preventing head lice. http://www.fda.gov/forconsumers/consumerupdates/ucm171730.htm. Published July 13, 2010. Updated November 8, 2017. Accessed November 13, 2017.
- Meinking TL, Villar ME, Vicaria M, et al. The clinical trials supporting benzyl alcohol lotion 5% (UlesfiaTM): a safe and effective topical treatment for head lice (Pediculosis Humanus Capitis). Pediatr Dermatol. 2010;27:19-24.
- McCormack PL. Spinosad in pediculosis capitis. Am J Clin Dermatol. 2011;12:349-353.
- Stough D, Shellabarger S, Quiring J, et al. Efficacy and safety of spinosad and permethrin creme rinses for pediculosis capitis (head lice). Pediatrics. 2009;124:E389-E395.
- Ahmad HM, Abdel-Azim ES, Abdel-Aziz RT. Assessment of topical versus oral ivermectin as a treatment for head lice. Dermatol Ther. 2014;27:307-310.
- Heukelbach J, Pilger D, Oliveira FA, et al. A highly efficacious pediculicide based on dimethicone: randomized observer blinded comparative trial. BMC Infect Dis. 2008;8:115.
- Burgess IF, Brunton ER, Burgess NA. Single application of 4% dimethicone liquid gel versus two applications of 1% permethrin creme rinse for treatment of head louse infestation: a randomised controlled trial. BMC Dermatol. 2013;13:5.
- Ihde ES, Boscamp JR, Loh JM, et al. Safety and efficacy of a 100% dimethicone pediculocide in school-age children. BMC Pediatr. 2015;15:70.
- Heukelbach J, Oliveira FA, Richter J, et al. Dimethicone-based pediculicides: a physical approach to eradicate head lice. Open Dermatol J. 2010;4:77-81.
- Feldmeier H. Treatment of pediculosis capitis: a critical appraisal of the current literature. Am J Clin Dermatol. 2014;15:401-412.
- Glasziou P, Bennett J, Greenberg P, et al; Handbook Of Non Drug Intervention (HANDI) Project Team. Wet combing for the eradication of head lice. Aust Fam Physician. 2013;42:129-130.
- Tebruegge M, Runnacles J. Is wet combing effective in children with pediculosis capitis infestation? Arch Dis Child. 2007;92:818-820.
- Goates BM, Atkin JS, Wilding KG, et al. An effective nonchemical treatment for head lice: a lot of hot air. Pediatrics. 2006;118:1962-1970.
- Yones DA, Bakir HY, Bayoumi SA. Chemical composition and efficacy of some selected plant oils against Pediculus humanus capitis in vitro. Parasitol Res. 2016;115:3209-3218.
- Burkhart CN, Burkhart CG. Head lice: scientific assessment of the nit sheath with clinical ramifications and therapeutic options. J Am Acad Dermatol. 2005;53:129-133.
- Ibarra J, Fry F, Wickenden C, et al. The impact of well-developed preventative strategies on the eradication of head lice. Perspect Public Health. 2009;129:165-173.
- Mumcuoglu KY, Hemingway J, Miller J, et al. Permethrin resistance in the head louse pediculus humanus capitis from Israel. Med Vet Entomol. 1995;9:427-432.
- Meinking TL, Serrano L, Hard B, et al. Comparative in vitro pediculicidal efficacy of treatments in a resistant head lice population in the United States. Arch Dermatol. 2002;138:220-224.
- Hemingway J, Miller J, Mumcuoglu KY. Pyrethroid resistance mechanisms in the head louse Pediculus capitis from Israel: implications for control. Med Vet Entomol. 1999;13:89-96.
- Yoon KS, Previte DJ, Hodgdon HE, et al. Knockdown resistance allele frequencies in North American head louse (Anoplura: Pediculidae) populations. J Med Entomol. 2014;51:450-457.
- Chosidow O. Scabies and pediculosis. Lancet. 2000;355:819-826.
- Centers for Disease Control and Prevention. Head lice. http://www.cdc.gov/parasites/lice/head/index.html. Updated September 24, 2013. Accessed November 9, 2017.
- Hurwitz S. Lice (pediculosis). In: Hurwitz S. Hurwitz Clinical Pediatric Dermatology: A Textbook of Skin Disorders of Childhood and Adolescence. 2nd ed. Philadelphia, PA: WB Saunders Company; 1993:416-419.
- Elston DM. What’s eating you? Pediculus humanus (head louse and body louse). Cutis. 1999;63:259-264.
- Ko CJ, Elston DM. Pediculosis. J Am Acad Dermatol. 2004;50:1-12.
- Frankowski BL, Weiner LB. Head lice. Pediatrics. 2002;110:638-643.
- Meinking TL. Clinical update on resistance and treatment of pediculosis capitis. Am J Manag Care. 2004;10(9 suppl):S264-S268.
- Devore CD, Schutze GE. Head lice. Pediatrics. 2015;135:E1355-E1365.
- Burkhart CN. Fomite transmission with head lice: a continuing controversy. Lancet. 2003;361:99-100.
- Falagas ME, Matthaiou DK, Rafailidis PI, et al. Worldwide prevalence of head lice. Emerg Infect Dis. 2008;14:1493-1494.
- Feldmeier H. Pediculosis capitis: new insights into epidemiology, diagnosis and treatment. Eur J Clin Microbiol Infect Dis. 2012;31:2105-2110.
- Canyon DV, Speare R, Muller R. Spatial and kinetic factors for the transfer of head lice (Pediculus capitis) between hairs. J Invest Dermatol. 2002;119:629-631.
- Burkhart CN, Burkhart CG. Fomite transmission in head lice. J Am Acad Dermatol. 2007;56:1044-1047.
- Canyon DV, Speare R. Indirect transmission of head lice via inanimate objects. Open Dermatol J. 2010;4:72-76.
- Bauer E, Jahnke C, Feldmeier H. Seasonal fluctuations of head lice infestation in Germany. Parasitol Res. 2009;104:677-681.
- Balcioglu IC, Kurt O, Limoncu ME, et al. Rural life, lower socioeconomic status and parasitic infections. Parasitol Int. 2007;56:129-133.
- Lesshafft H, Baier A, Guerra H, et al. Prevalence and risk factors associated with pediculosis capitis in an impoverished urban community in Lima, Peru. J Glob Infect Dis. 2013;5:138-143.
- Tagka A, Lambrou GI, Braoudaki M, et al. Socioeconomical factors associated with pediculosis (Phthiraptera: Pediculidae) in Athens, Greece. J Med Entomol. 2016;53:919-922.
- Di Stefani A, Hofmann-Wellenhof R, Zalaudek I. Dermoscopy for diagnosis and treatment monitoring of pediculosis capitis. J Am Acad Dermatol. 2006;54:909-911.
- Bakos RM, Bakos L. Dermoscopy for diagnosis of pediculosis capitis. J Am Acad Dermatol. 2007;57:727-728.
- Jahnke C, Bauer E, Hengge UR, et al. Accuracy of diagnosis of pediculosis capitis: visual inspection vs wet combing. Arch Dermatol. 2009;145:309-313.
- Elston DM. Drugs used in the treatment of pediculosis. J Drugs Dermatol. 2005;4:207-211.
- National Pesticide Information Center. Piperonyl butoxide (general fact sheet). http://npic.orst.edu/factsheets/pbogen.pdf/. Accessed November 13, 2017.
- Diamantis SA, Morrell DS, Burkhart CN. Treatment of head lice. Dermatol Ther. 2009;22:273-278.
- United States Food and Drug Administration. Treating and preventing head lice. http://www.fda.gov/forconsumers/consumerupdates/ucm171730.htm. Published July 13, 2010. Updated November 8, 2017. Accessed November 13, 2017.
- Meinking TL, Villar ME, Vicaria M, et al. The clinical trials supporting benzyl alcohol lotion 5% (UlesfiaTM): a safe and effective topical treatment for head lice (Pediculosis Humanus Capitis). Pediatr Dermatol. 2010;27:19-24.
- McCormack PL. Spinosad in pediculosis capitis. Am J Clin Dermatol. 2011;12:349-353.
- Stough D, Shellabarger S, Quiring J, et al. Efficacy and safety of spinosad and permethrin creme rinses for pediculosis capitis (head lice). Pediatrics. 2009;124:E389-E395.
- Ahmad HM, Abdel-Azim ES, Abdel-Aziz RT. Assessment of topical versus oral ivermectin as a treatment for head lice. Dermatol Ther. 2014;27:307-310.
- Heukelbach J, Pilger D, Oliveira FA, et al. A highly efficacious pediculicide based on dimethicone: randomized observer blinded comparative trial. BMC Infect Dis. 2008;8:115.
- Burgess IF, Brunton ER, Burgess NA. Single application of 4% dimethicone liquid gel versus two applications of 1% permethrin creme rinse for treatment of head louse infestation: a randomised controlled trial. BMC Dermatol. 2013;13:5.
- Ihde ES, Boscamp JR, Loh JM, et al. Safety and efficacy of a 100% dimethicone pediculocide in school-age children. BMC Pediatr. 2015;15:70.
- Heukelbach J, Oliveira FA, Richter J, et al. Dimethicone-based pediculicides: a physical approach to eradicate head lice. Open Dermatol J. 2010;4:77-81.
- Feldmeier H. Treatment of pediculosis capitis: a critical appraisal of the current literature. Am J Clin Dermatol. 2014;15:401-412.
- Glasziou P, Bennett J, Greenberg P, et al; Handbook Of Non Drug Intervention (HANDI) Project Team. Wet combing for the eradication of head lice. Aust Fam Physician. 2013;42:129-130.
- Tebruegge M, Runnacles J. Is wet combing effective in children with pediculosis capitis infestation? Arch Dis Child. 2007;92:818-820.
- Goates BM, Atkin JS, Wilding KG, et al. An effective nonchemical treatment for head lice: a lot of hot air. Pediatrics. 2006;118:1962-1970.
- Yones DA, Bakir HY, Bayoumi SA. Chemical composition and efficacy of some selected plant oils against Pediculus humanus capitis in vitro. Parasitol Res. 2016;115:3209-3218.
- Burkhart CN, Burkhart CG. Head lice: scientific assessment of the nit sheath with clinical ramifications and therapeutic options. J Am Acad Dermatol. 2005;53:129-133.
- Ibarra J, Fry F, Wickenden C, et al. The impact of well-developed preventative strategies on the eradication of head lice. Perspect Public Health. 2009;129:165-173.
- Mumcuoglu KY, Hemingway J, Miller J, et al. Permethrin resistance in the head louse pediculus humanus capitis from Israel. Med Vet Entomol. 1995;9:427-432.
- Meinking TL, Serrano L, Hard B, et al. Comparative in vitro pediculicidal efficacy of treatments in a resistant head lice population in the United States. Arch Dermatol. 2002;138:220-224.
- Hemingway J, Miller J, Mumcuoglu KY. Pyrethroid resistance mechanisms in the head louse Pediculus capitis from Israel: implications for control. Med Vet Entomol. 1999;13:89-96.
- Yoon KS, Previte DJ, Hodgdon HE, et al. Knockdown resistance allele frequencies in North American head louse (Anoplura: Pediculidae) populations. J Med Entomol. 2014;51:450-457.
Practice Points
- Transmission of head lice occurs most frequently from direct head-to-head contact; however, head lice can survive up to 4 days on fomites.
- Patients present with scalp pruritus and bite reactions (papules or wheals), but pediculosis can be asymptomatic, particularly with the first exposure before the immune system has developed sensitivity to the louse saliva.
- Topical pyrethroids are available over-the-counter and are considered first-line therapy; however, resistance to pyrethroids has become an important problem in the United States and worldwide.
- Newer topical treatments such as benzyl alcohol lotion 5%, spinosad topical suspension 0.9%, and ivermectin lotion 0.5% can be prescribed as alternative therapies, particularly if resistance to pyrethroids is a concern.
What’s Eating You? Scabies in the Developing World
Scabies is caused by the mite Sarcoptes scabiei var hominis.1 It is in the arthropod class Arachnida, subclass Acari, and family Sarcoptidae.2 Historically, scabies was first described in the Old Testament and by Aristotle,2 but the causative organism was not identified until 1687 using a light microscope.3 Scabies affects all age groups, races, and social classes and is globally widespread. It is most prevalent in developing tropical countries.1 It is estimated that 300 million individuals worldwide are infested with scabies mites annually, with the highest burden in young children.4-7 In industrialized societies, infections often are seen in young adults and in institutional settings such as nursing homes.8 Scabies disproportionately impacts impoverished communities with crowded living conditions, poor hygiene and nutrition, and substandard housing.5,9 Controlling the spread of the disease in these communities presents challenges but is important because of the connection between scabies and chronic kidney disease.10 As such, scabies represents a major health problem in the developing world and has been the focus of major health initiatives.1,11
Identifying Characteristics
Adult females are 0.4-mm long and 0.3-mm wide, with males being smaller. Adult nymphs have 8 legs and larvae have 6 legs. Scabies mites are distinguishable from other arachnids by the position of a distinct gnathosoma and the lack of a division between the abdomen and cephalothorax.12 They are ovoid with a small anterior cephalic and caudal thoracoabdominal portion with hairlike projections coming off from the rudimentary legs. They can crawl as fast as 2.5 cm per minute on warm skin.2 The life cycle of the mite begins after mating: the male mite dies, and the female lays up to 3 eggs per day, which hatch in 3 to 4 days,2 in skin burrows within the stratum granulosum.12 Maturation from larva to adult takes 10 to 14 days.12 A female mite can live for 4 to 6 weeks and can produce up to 40 ova (Figure 1).
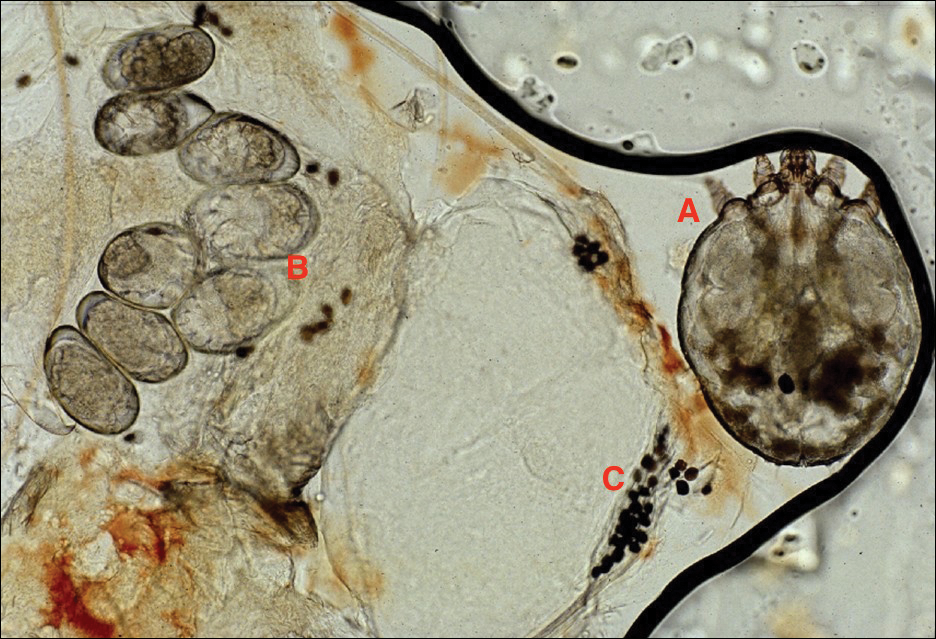
Disease Transmission
Without a host, mites are able to survive and remain capable of infestation for 24 to 36 hours at 21°C and 40% to 80% relative humidity. Lower temperatures and higher humidity prolong survival, but infectivity decreases the longer they are without a host.13
An adult human with ordinary scabies will have an average of 12 adult female mites on the body surface at a given time.14 However, hundreds of mites can be found in neglected children in underprivileged communities and millions in patients with crusted scabies.13 Transmission of typical scabies requires close direct skin-to-skin contact for 15 to 20 minutes.2,8 Transmission from clothing or fomites are an unlikely source of infestation with the exception of patients who are heavily infested such as in crusted scabies.12 In adults, sexual contact is an important method of transmission,12 and patients with scabies should be screened for other sexually transmitted diseases.8
Clinical Manifestations
Signs of scabies on the skin include burrows, erythematous papules, and generalized pruritus (Figure 2).12 The scalp, face, and neck frequently are involved in infants and children,2 and the hands, wrists, elbows, genitalia, axillae, umbilicus, belt line, nipples, and buttocks commonly are involved in adults.12 Itching is characteristically worse at night.8 In tropical climates, patients with scabies are predisposed to secondary bacterial skin infections, particularly Streptococcus pyogenes (group A streptococci). The association between scabies and pyoderma caused by group A streptococci has been well established.15,16 Mika et al10 suggested that local complement inhibition plays an important role in the development of pyoderma in scabies-infested skin.
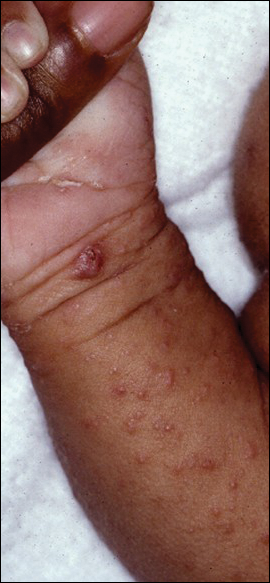
Prevention and Control in the Developing World
Low-cost diagnostic equipment can play a key role in the definitive diagnosis and management of scabies outbreaks in the developing world. Micali et al28 found that a $30 videomicroscope was as effective in scabies diagnosis as a $20,000 videodermatoscope. Because of the low cost of benzyl benzoate, it is commonly used as a first-line drug in many parts of the world,13 whereas permethrin cream 5% is the standard treatment in the developed world.29 Recognition of the role of scabies in patients with pyoderma is key, and one study indicated clinically apparent scabies went unnoticed by physicians in 52% of patients presenting with skin lesions.30 Drug shortages also can contribute to a high prevalence of scabies infestation in the community.31 Mass treatment with ivermectin has proven to be an effective means of reducing the prevalence of many parasitic diseases,1,32,33 and it shows great promise for crusted scabies, institutional outbreaks, and mass administration in highly endemic communites.8 However, there is evidence of ivermectin tolerance among mites, which could undermine the success of mass drug administration.34 Another important consideration is population mobility and the risk for rapid reintroduction of scabies infection across regions.35
Complicating disease control are the socioeconomic factors associated with scabies in the developing world. Families with scabies infestation typically do not own their homes, are less likely to have constant electricity, have a lower monthly income, and live in substandard housing.20 Families can spend a substantial part of their household income on treatment, impacting what they can spend on food.8,11 In addition to medication, control of scabies requires community education and involvement, along with access to primary care and attention to living conditions and environmental factors.34,36
- Romani L, Whitfeld MJ, Koroivueta J, et al. Mass drug administration for scabies control in a population with endemic disease. N Engl J Med. 2015;373:2305-2313.
- Hicks MI, Elston DM. Scabies. Dermatol Ther. 2009;22:279-292.
- Ramos-e-Silva M. Giovan Cosimo Bonomo (1663-1696): discoverer of the etiology of scabies. Int J Dermatol. 1998;37:625-630.
- Chung SD, Wang KH, Huang CC, et al. Scabies increased the risk of chronic kidney disease: a 5-year follow-up study. J Eur Acad Dermatol Venereol. 2014;28:286-292.
- Wong SS, Poon RW, Chau S, et al. Development of conventional and real-time quantitative PCR assays for diagnosis and monitoring of scabies. J Clin Microbiol. 2015;53:2095-2102.
- Kearns TM, Speare R, Cheng AC, et al. Impact of an ivermectin mass drug administration on scabies prevalence in a remote Australian aboriginal community. PLoS Negl Trop Dis. 2015;9:e0004151.
- Gilmore SJ. Control strategies for endemic childhood scabies. PLoS One. 2011;6:e15990.
- Hay RJ, Steer AC, Engelman D, Walton S. Scabies in the developing world—its prevalence, complications, and management. Clin Microbiol Infect. 2012;18:313-323.
- Hoy WE, White AV, Dowling A, et al. Post-streptococcal glomerulonephritis is a strong risk factor for chronic kidney disease in later life. Kidney Int. 2012;81:1026-1032.
- Mika A, Reynolds SL, Pickering D, et al. Complement inhibitors from scabies mites promote streptococcal growth—a novel mechanism in infected epidermis? PLoS Negl Trop Dis. 2012;6:e1563.
- McLean FE. The elimination of scabies: a task for our generation. Int J Dermatol. 2013;52:1215-1223.
- Hengge UR, Currie BJ, Jäger G, et al. Scabies: a ubiquitous neglected skin disease. Lancet Infect Dis. 2006;6:769-779.
- Heukelbach J, Feldmeier H. Scabies. Lancet. 2006;367:1767-1774.
- Johnston G, Sladden M. Scabies: diagnosis and treatment. BMJ. 2005;331:619-622.
- Yeoh DK, Bowen AC, Carapetis JR. Impetigo and scabies—disease burden and modern treatment strategies [published online May 11, 2016]. J Infect. 2016;(72 suppl):S61-S67.
- Bowen AC, Mahé A, Hay RJ, et al. The global epidemiology of impetigo: a systematic review of the population prevalence of impetigo and pyoderma. PLoS One. 2015;10:e0136789.
- Bowen AC, Tong SY, Chatfield MD, et al. The microbiology of impetigo in indigenous children: associations between Streptococcus pyogenes, Staphylococcus aureus, scabies, and nasal carriage. BMC Infect Dis. 2014;14:727.
- Sesso R, Pinto SW. Five-year follow-up of patients with epidemic glomerulonephritis due to Streptococcus zooepidemicus. Nephrol Dial Transplant. 2005;20:1808-1812.
- Singh GR. Glomerulonephritis and managing the risks of chronic renal disease. Pediatr Clin North Am. 2009;56:1363-1382.
- La Vincente S, Kearns T, Connors C, et al. Community management of endemic scabies in remote aboriginal communities of northern Australia: low treatment uptake and high ongoing acquisition. PLoS Negl Trop Dis. 2009;3:e444.
- Clucas DB, Carville KS, Connors C, et al. Disease burden and health-care clinic attendances for young children in remote aboriginal communities of northern Australia. Bull World Health Organ. 2008;86:275-281.
- Stanton B, Khanam S, Nazrul H, et al. Scabies in urban Bangladesh. J Trop Med Hyg. 1987;90:219-226.
- Heukelbach J, de Oliveira FA, Feldmeier H. Ecoparasitoses and public health in Brazil: challenges for control [in Portuguese]. Cad Saude Publica. 2003;19:1535-1540.
- Edison L, Beaudoin A, Goh L, et al. Scabies and bacterial superinfection among American Samoan children, 2011-2012. PLoS One. 2015;10:e0139336.
- Steer AC, Jenney AW, Kado J, et al. High burden of impetigo and scabies in a tropical country. PLoS Negl Trop Dis. 2009;3:e467.
- Romani L, Steer AC, Whitfeld MJ, et al. Prevalence of scabies and impetigo worldwide: a systematic review. Lancet Infect Dis. 2015;15:960-967.
- Romani L, Koroivueta J, Steer AC, et al. Scabies and impetigo prevalence and risk factors in Fiji: a national survey. PLoS Negl Trop Dis. 2015;9:e0003452.
- Micali G, Lacarrubba F, Verzì AE, et al. Low-cost equipment for diagnosis and management of endemic scabies outbreaks in underserved populations. Clin Infect Dis. 2015;60:327-329.
- Pasay C, Walton S, Fischer K, et al. PCR-based assay to survey for knockdown resistance to pyrethroid acaricides in human scabies mites (Sarcoptes scabiei var hominis). Am J Trop Med Hyg. 2006;74:649-657.
- Heukelbach J, van Haeff E, Rump B, et al. Parasitic skin diseases: health care-seeking in a slum in north-east Brazil. Trop Med Int Health. 2003;8:368-373.
- Potter EV, Mayon-White R, Poon-King T, et al. Acute glomerulonephritis as a complication of scabies. In: Orkin M, Maibach HI, eds. Cutaneous Infestations and Insect Bites. New York, NY: Marcel Dekker; 1985.
- Mahé A. Mass drug administration for scabies control. N Engl J Med. 2016;374:1689.
- Steer AC, Romani L, Kaldor JM. Mass drug administration for scabies control. N Engl J Med. 2016;374:1690.
- Mounsey KE, Holt DC, McCarthy JS, et al. Longitudinal evidence of increasing in vitro tolerance of scabies mites to ivermectin in scabies-endemic communities. Arch Dermatol. 2009;145:840-841.
- Currie BJ. Scabies and global control of neglected tropical diseases. N Engl J Med. 2015;373:2371-2372.
- O’Donnell V, Morris S, Ward J. Mass drug administration for scabies control. N Engl J Med. 2016;374:1689-1690.
Scabies is caused by the mite Sarcoptes scabiei var hominis.1 It is in the arthropod class Arachnida, subclass Acari, and family Sarcoptidae.2 Historically, scabies was first described in the Old Testament and by Aristotle,2 but the causative organism was not identified until 1687 using a light microscope.3 Scabies affects all age groups, races, and social classes and is globally widespread. It is most prevalent in developing tropical countries.1 It is estimated that 300 million individuals worldwide are infested with scabies mites annually, with the highest burden in young children.4-7 In industrialized societies, infections often are seen in young adults and in institutional settings such as nursing homes.8 Scabies disproportionately impacts impoverished communities with crowded living conditions, poor hygiene and nutrition, and substandard housing.5,9 Controlling the spread of the disease in these communities presents challenges but is important because of the connection between scabies and chronic kidney disease.10 As such, scabies represents a major health problem in the developing world and has been the focus of major health initiatives.1,11
Identifying Characteristics
Adult females are 0.4-mm long and 0.3-mm wide, with males being smaller. Adult nymphs have 8 legs and larvae have 6 legs. Scabies mites are distinguishable from other arachnids by the position of a distinct gnathosoma and the lack of a division between the abdomen and cephalothorax.12 They are ovoid with a small anterior cephalic and caudal thoracoabdominal portion with hairlike projections coming off from the rudimentary legs. They can crawl as fast as 2.5 cm per minute on warm skin.2 The life cycle of the mite begins after mating: the male mite dies, and the female lays up to 3 eggs per day, which hatch in 3 to 4 days,2 in skin burrows within the stratum granulosum.12 Maturation from larva to adult takes 10 to 14 days.12 A female mite can live for 4 to 6 weeks and can produce up to 40 ova (Figure 1).

Disease Transmission
Without a host, mites are able to survive and remain capable of infestation for 24 to 36 hours at 21°C and 40% to 80% relative humidity. Lower temperatures and higher humidity prolong survival, but infectivity decreases the longer they are without a host.13
An adult human with ordinary scabies will have an average of 12 adult female mites on the body surface at a given time.14 However, hundreds of mites can be found in neglected children in underprivileged communities and millions in patients with crusted scabies.13 Transmission of typical scabies requires close direct skin-to-skin contact for 15 to 20 minutes.2,8 Transmission from clothing or fomites are an unlikely source of infestation with the exception of patients who are heavily infested such as in crusted scabies.12 In adults, sexual contact is an important method of transmission,12 and patients with scabies should be screened for other sexually transmitted diseases.8
Clinical Manifestations
Signs of scabies on the skin include burrows, erythematous papules, and generalized pruritus (Figure 2).12 The scalp, face, and neck frequently are involved in infants and children,2 and the hands, wrists, elbows, genitalia, axillae, umbilicus, belt line, nipples, and buttocks commonly are involved in adults.12 Itching is characteristically worse at night.8 In tropical climates, patients with scabies are predisposed to secondary bacterial skin infections, particularly Streptococcus pyogenes (group A streptococci). The association between scabies and pyoderma caused by group A streptococci has been well established.15,16 Mika et al10 suggested that local complement inhibition plays an important role in the development of pyoderma in scabies-infested skin.

Prevention and Control in the Developing World
Low-cost diagnostic equipment can play a key role in the definitive diagnosis and management of scabies outbreaks in the developing world. Micali et al28 found that a $30 videomicroscope was as effective in scabies diagnosis as a $20,000 videodermatoscope. Because of the low cost of benzyl benzoate, it is commonly used as a first-line drug in many parts of the world,13 whereas permethrin cream 5% is the standard treatment in the developed world.29 Recognition of the role of scabies in patients with pyoderma is key, and one study indicated clinically apparent scabies went unnoticed by physicians in 52% of patients presenting with skin lesions.30 Drug shortages also can contribute to a high prevalence of scabies infestation in the community.31 Mass treatment with ivermectin has proven to be an effective means of reducing the prevalence of many parasitic diseases,1,32,33 and it shows great promise for crusted scabies, institutional outbreaks, and mass administration in highly endemic communites.8 However, there is evidence of ivermectin tolerance among mites, which could undermine the success of mass drug administration.34 Another important consideration is population mobility and the risk for rapid reintroduction of scabies infection across regions.35
Complicating disease control are the socioeconomic factors associated with scabies in the developing world. Families with scabies infestation typically do not own their homes, are less likely to have constant electricity, have a lower monthly income, and live in substandard housing.20 Families can spend a substantial part of their household income on treatment, impacting what they can spend on food.8,11 In addition to medication, control of scabies requires community education and involvement, along with access to primary care and attention to living conditions and environmental factors.34,36
Scabies is caused by the mite Sarcoptes scabiei var hominis.1 It is in the arthropod class Arachnida, subclass Acari, and family Sarcoptidae.2 Historically, scabies was first described in the Old Testament and by Aristotle,2 but the causative organism was not identified until 1687 using a light microscope.3 Scabies affects all age groups, races, and social classes and is globally widespread. It is most prevalent in developing tropical countries.1 It is estimated that 300 million individuals worldwide are infested with scabies mites annually, with the highest burden in young children.4-7 In industrialized societies, infections often are seen in young adults and in institutional settings such as nursing homes.8 Scabies disproportionately impacts impoverished communities with crowded living conditions, poor hygiene and nutrition, and substandard housing.5,9 Controlling the spread of the disease in these communities presents challenges but is important because of the connection between scabies and chronic kidney disease.10 As such, scabies represents a major health problem in the developing world and has been the focus of major health initiatives.1,11
Identifying Characteristics
Adult females are 0.4-mm long and 0.3-mm wide, with males being smaller. Adult nymphs have 8 legs and larvae have 6 legs. Scabies mites are distinguishable from other arachnids by the position of a distinct gnathosoma and the lack of a division between the abdomen and cephalothorax.12 They are ovoid with a small anterior cephalic and caudal thoracoabdominal portion with hairlike projections coming off from the rudimentary legs. They can crawl as fast as 2.5 cm per minute on warm skin.2 The life cycle of the mite begins after mating: the male mite dies, and the female lays up to 3 eggs per day, which hatch in 3 to 4 days,2 in skin burrows within the stratum granulosum.12 Maturation from larva to adult takes 10 to 14 days.12 A female mite can live for 4 to 6 weeks and can produce up to 40 ova (Figure 1).

Disease Transmission
Without a host, mites are able to survive and remain capable of infestation for 24 to 36 hours at 21°C and 40% to 80% relative humidity. Lower temperatures and higher humidity prolong survival, but infectivity decreases the longer they are without a host.13
An adult human with ordinary scabies will have an average of 12 adult female mites on the body surface at a given time.14 However, hundreds of mites can be found in neglected children in underprivileged communities and millions in patients with crusted scabies.13 Transmission of typical scabies requires close direct skin-to-skin contact for 15 to 20 minutes.2,8 Transmission from clothing or fomites are an unlikely source of infestation with the exception of patients who are heavily infested such as in crusted scabies.12 In adults, sexual contact is an important method of transmission,12 and patients with scabies should be screened for other sexually transmitted diseases.8
Clinical Manifestations
Signs of scabies on the skin include burrows, erythematous papules, and generalized pruritus (Figure 2).12 The scalp, face, and neck frequently are involved in infants and children,2 and the hands, wrists, elbows, genitalia, axillae, umbilicus, belt line, nipples, and buttocks commonly are involved in adults.12 Itching is characteristically worse at night.8 In tropical climates, patients with scabies are predisposed to secondary bacterial skin infections, particularly Streptococcus pyogenes (group A streptococci). The association between scabies and pyoderma caused by group A streptococci has been well established.15,16 Mika et al10 suggested that local complement inhibition plays an important role in the development of pyoderma in scabies-infested skin.

Prevention and Control in the Developing World
Low-cost diagnostic equipment can play a key role in the definitive diagnosis and management of scabies outbreaks in the developing world. Micali et al28 found that a $30 videomicroscope was as effective in scabies diagnosis as a $20,000 videodermatoscope. Because of the low cost of benzyl benzoate, it is commonly used as a first-line drug in many parts of the world,13 whereas permethrin cream 5% is the standard treatment in the developed world.29 Recognition of the role of scabies in patients with pyoderma is key, and one study indicated clinically apparent scabies went unnoticed by physicians in 52% of patients presenting with skin lesions.30 Drug shortages also can contribute to a high prevalence of scabies infestation in the community.31 Mass treatment with ivermectin has proven to be an effective means of reducing the prevalence of many parasitic diseases,1,32,33 and it shows great promise for crusted scabies, institutional outbreaks, and mass administration in highly endemic communites.8 However, there is evidence of ivermectin tolerance among mites, which could undermine the success of mass drug administration.34 Another important consideration is population mobility and the risk for rapid reintroduction of scabies infection across regions.35
Complicating disease control are the socioeconomic factors associated with scabies in the developing world. Families with scabies infestation typically do not own their homes, are less likely to have constant electricity, have a lower monthly income, and live in substandard housing.20 Families can spend a substantial part of their household income on treatment, impacting what they can spend on food.8,11 In addition to medication, control of scabies requires community education and involvement, along with access to primary care and attention to living conditions and environmental factors.34,36
- Romani L, Whitfeld MJ, Koroivueta J, et al. Mass drug administration for scabies control in a population with endemic disease. N Engl J Med. 2015;373:2305-2313.
- Hicks MI, Elston DM. Scabies. Dermatol Ther. 2009;22:279-292.
- Ramos-e-Silva M. Giovan Cosimo Bonomo (1663-1696): discoverer of the etiology of scabies. Int J Dermatol. 1998;37:625-630.
- Chung SD, Wang KH, Huang CC, et al. Scabies increased the risk of chronic kidney disease: a 5-year follow-up study. J Eur Acad Dermatol Venereol. 2014;28:286-292.
- Wong SS, Poon RW, Chau S, et al. Development of conventional and real-time quantitative PCR assays for diagnosis and monitoring of scabies. J Clin Microbiol. 2015;53:2095-2102.
- Kearns TM, Speare R, Cheng AC, et al. Impact of an ivermectin mass drug administration on scabies prevalence in a remote Australian aboriginal community. PLoS Negl Trop Dis. 2015;9:e0004151.
- Gilmore SJ. Control strategies for endemic childhood scabies. PLoS One. 2011;6:e15990.
- Hay RJ, Steer AC, Engelman D, Walton S. Scabies in the developing world—its prevalence, complications, and management. Clin Microbiol Infect. 2012;18:313-323.
- Hoy WE, White AV, Dowling A, et al. Post-streptococcal glomerulonephritis is a strong risk factor for chronic kidney disease in later life. Kidney Int. 2012;81:1026-1032.
- Mika A, Reynolds SL, Pickering D, et al. Complement inhibitors from scabies mites promote streptococcal growth—a novel mechanism in infected epidermis? PLoS Negl Trop Dis. 2012;6:e1563.
- McLean FE. The elimination of scabies: a task for our generation. Int J Dermatol. 2013;52:1215-1223.
- Hengge UR, Currie BJ, Jäger G, et al. Scabies: a ubiquitous neglected skin disease. Lancet Infect Dis. 2006;6:769-779.
- Heukelbach J, Feldmeier H. Scabies. Lancet. 2006;367:1767-1774.
- Johnston G, Sladden M. Scabies: diagnosis and treatment. BMJ. 2005;331:619-622.
- Yeoh DK, Bowen AC, Carapetis JR. Impetigo and scabies—disease burden and modern treatment strategies [published online May 11, 2016]. J Infect. 2016;(72 suppl):S61-S67.
- Bowen AC, Mahé A, Hay RJ, et al. The global epidemiology of impetigo: a systematic review of the population prevalence of impetigo and pyoderma. PLoS One. 2015;10:e0136789.
- Bowen AC, Tong SY, Chatfield MD, et al. The microbiology of impetigo in indigenous children: associations between Streptococcus pyogenes, Staphylococcus aureus, scabies, and nasal carriage. BMC Infect Dis. 2014;14:727.
- Sesso R, Pinto SW. Five-year follow-up of patients with epidemic glomerulonephritis due to Streptococcus zooepidemicus. Nephrol Dial Transplant. 2005;20:1808-1812.
- Singh GR. Glomerulonephritis and managing the risks of chronic renal disease. Pediatr Clin North Am. 2009;56:1363-1382.
- La Vincente S, Kearns T, Connors C, et al. Community management of endemic scabies in remote aboriginal communities of northern Australia: low treatment uptake and high ongoing acquisition. PLoS Negl Trop Dis. 2009;3:e444.
- Clucas DB, Carville KS, Connors C, et al. Disease burden and health-care clinic attendances for young children in remote aboriginal communities of northern Australia. Bull World Health Organ. 2008;86:275-281.
- Stanton B, Khanam S, Nazrul H, et al. Scabies in urban Bangladesh. J Trop Med Hyg. 1987;90:219-226.
- Heukelbach J, de Oliveira FA, Feldmeier H. Ecoparasitoses and public health in Brazil: challenges for control [in Portuguese]. Cad Saude Publica. 2003;19:1535-1540.
- Edison L, Beaudoin A, Goh L, et al. Scabies and bacterial superinfection among American Samoan children, 2011-2012. PLoS One. 2015;10:e0139336.
- Steer AC, Jenney AW, Kado J, et al. High burden of impetigo and scabies in a tropical country. PLoS Negl Trop Dis. 2009;3:e467.
- Romani L, Steer AC, Whitfeld MJ, et al. Prevalence of scabies and impetigo worldwide: a systematic review. Lancet Infect Dis. 2015;15:960-967.
- Romani L, Koroivueta J, Steer AC, et al. Scabies and impetigo prevalence and risk factors in Fiji: a national survey. PLoS Negl Trop Dis. 2015;9:e0003452.
- Micali G, Lacarrubba F, Verzì AE, et al. Low-cost equipment for diagnosis and management of endemic scabies outbreaks in underserved populations. Clin Infect Dis. 2015;60:327-329.
- Pasay C, Walton S, Fischer K, et al. PCR-based assay to survey for knockdown resistance to pyrethroid acaricides in human scabies mites (Sarcoptes scabiei var hominis). Am J Trop Med Hyg. 2006;74:649-657.
- Heukelbach J, van Haeff E, Rump B, et al. Parasitic skin diseases: health care-seeking in a slum in north-east Brazil. Trop Med Int Health. 2003;8:368-373.
- Potter EV, Mayon-White R, Poon-King T, et al. Acute glomerulonephritis as a complication of scabies. In: Orkin M, Maibach HI, eds. Cutaneous Infestations and Insect Bites. New York, NY: Marcel Dekker; 1985.
- Mahé A. Mass drug administration for scabies control. N Engl J Med. 2016;374:1689.
- Steer AC, Romani L, Kaldor JM. Mass drug administration for scabies control. N Engl J Med. 2016;374:1690.
- Mounsey KE, Holt DC, McCarthy JS, et al. Longitudinal evidence of increasing in vitro tolerance of scabies mites to ivermectin in scabies-endemic communities. Arch Dermatol. 2009;145:840-841.
- Currie BJ. Scabies and global control of neglected tropical diseases. N Engl J Med. 2015;373:2371-2372.
- O’Donnell V, Morris S, Ward J. Mass drug administration for scabies control. N Engl J Med. 2016;374:1689-1690.
- Romani L, Whitfeld MJ, Koroivueta J, et al. Mass drug administration for scabies control in a population with endemic disease. N Engl J Med. 2015;373:2305-2313.
- Hicks MI, Elston DM. Scabies. Dermatol Ther. 2009;22:279-292.
- Ramos-e-Silva M. Giovan Cosimo Bonomo (1663-1696): discoverer of the etiology of scabies. Int J Dermatol. 1998;37:625-630.
- Chung SD, Wang KH, Huang CC, et al. Scabies increased the risk of chronic kidney disease: a 5-year follow-up study. J Eur Acad Dermatol Venereol. 2014;28:286-292.
- Wong SS, Poon RW, Chau S, et al. Development of conventional and real-time quantitative PCR assays for diagnosis and monitoring of scabies. J Clin Microbiol. 2015;53:2095-2102.
- Kearns TM, Speare R, Cheng AC, et al. Impact of an ivermectin mass drug administration on scabies prevalence in a remote Australian aboriginal community. PLoS Negl Trop Dis. 2015;9:e0004151.
- Gilmore SJ. Control strategies for endemic childhood scabies. PLoS One. 2011;6:e15990.
- Hay RJ, Steer AC, Engelman D, Walton S. Scabies in the developing world—its prevalence, complications, and management. Clin Microbiol Infect. 2012;18:313-323.
- Hoy WE, White AV, Dowling A, et al. Post-streptococcal glomerulonephritis is a strong risk factor for chronic kidney disease in later life. Kidney Int. 2012;81:1026-1032.
- Mika A, Reynolds SL, Pickering D, et al. Complement inhibitors from scabies mites promote streptococcal growth—a novel mechanism in infected epidermis? PLoS Negl Trop Dis. 2012;6:e1563.
- McLean FE. The elimination of scabies: a task for our generation. Int J Dermatol. 2013;52:1215-1223.
- Hengge UR, Currie BJ, Jäger G, et al. Scabies: a ubiquitous neglected skin disease. Lancet Infect Dis. 2006;6:769-779.
- Heukelbach J, Feldmeier H. Scabies. Lancet. 2006;367:1767-1774.
- Johnston G, Sladden M. Scabies: diagnosis and treatment. BMJ. 2005;331:619-622.
- Yeoh DK, Bowen AC, Carapetis JR. Impetigo and scabies—disease burden and modern treatment strategies [published online May 11, 2016]. J Infect. 2016;(72 suppl):S61-S67.
- Bowen AC, Mahé A, Hay RJ, et al. The global epidemiology of impetigo: a systematic review of the population prevalence of impetigo and pyoderma. PLoS One. 2015;10:e0136789.
- Bowen AC, Tong SY, Chatfield MD, et al. The microbiology of impetigo in indigenous children: associations between Streptococcus pyogenes, Staphylococcus aureus, scabies, and nasal carriage. BMC Infect Dis. 2014;14:727.
- Sesso R, Pinto SW. Five-year follow-up of patients with epidemic glomerulonephritis due to Streptococcus zooepidemicus. Nephrol Dial Transplant. 2005;20:1808-1812.
- Singh GR. Glomerulonephritis and managing the risks of chronic renal disease. Pediatr Clin North Am. 2009;56:1363-1382.
- La Vincente S, Kearns T, Connors C, et al. Community management of endemic scabies in remote aboriginal communities of northern Australia: low treatment uptake and high ongoing acquisition. PLoS Negl Trop Dis. 2009;3:e444.
- Clucas DB, Carville KS, Connors C, et al. Disease burden and health-care clinic attendances for young children in remote aboriginal communities of northern Australia. Bull World Health Organ. 2008;86:275-281.
- Stanton B, Khanam S, Nazrul H, et al. Scabies in urban Bangladesh. J Trop Med Hyg. 1987;90:219-226.
- Heukelbach J, de Oliveira FA, Feldmeier H. Ecoparasitoses and public health in Brazil: challenges for control [in Portuguese]. Cad Saude Publica. 2003;19:1535-1540.
- Edison L, Beaudoin A, Goh L, et al. Scabies and bacterial superinfection among American Samoan children, 2011-2012. PLoS One. 2015;10:e0139336.
- Steer AC, Jenney AW, Kado J, et al. High burden of impetigo and scabies in a tropical country. PLoS Negl Trop Dis. 2009;3:e467.
- Romani L, Steer AC, Whitfeld MJ, et al. Prevalence of scabies and impetigo worldwide: a systematic review. Lancet Infect Dis. 2015;15:960-967.
- Romani L, Koroivueta J, Steer AC, et al. Scabies and impetigo prevalence and risk factors in Fiji: a national survey. PLoS Negl Trop Dis. 2015;9:e0003452.
- Micali G, Lacarrubba F, Verzì AE, et al. Low-cost equipment for diagnosis and management of endemic scabies outbreaks in underserved populations. Clin Infect Dis. 2015;60:327-329.
- Pasay C, Walton S, Fischer K, et al. PCR-based assay to survey for knockdown resistance to pyrethroid acaricides in human scabies mites (Sarcoptes scabiei var hominis). Am J Trop Med Hyg. 2006;74:649-657.
- Heukelbach J, van Haeff E, Rump B, et al. Parasitic skin diseases: health care-seeking in a slum in north-east Brazil. Trop Med Int Health. 2003;8:368-373.
- Potter EV, Mayon-White R, Poon-King T, et al. Acute glomerulonephritis as a complication of scabies. In: Orkin M, Maibach HI, eds. Cutaneous Infestations and Insect Bites. New York, NY: Marcel Dekker; 1985.
- Mahé A. Mass drug administration for scabies control. N Engl J Med. 2016;374:1689.
- Steer AC, Romani L, Kaldor JM. Mass drug administration for scabies control. N Engl J Med. 2016;374:1690.
- Mounsey KE, Holt DC, McCarthy JS, et al. Longitudinal evidence of increasing in vitro tolerance of scabies mites to ivermectin in scabies-endemic communities. Arch Dermatol. 2009;145:840-841.
- Currie BJ. Scabies and global control of neglected tropical diseases. N Engl J Med. 2015;373:2371-2372.
- O’Donnell V, Morris S, Ward J. Mass drug administration for scabies control. N Engl J Med. 2016;374:1689-1690.
Practice Points
- Scabies infestation is one of the world’s leading causes of chronic kidney disease.
- Ivermectin can be used to treat mass infestations, and older topical therapies also are commonly used.
Hyperpigmented Patch on the Leg
The Diagnosis: Lichen Aureus
The clinicopathological findings were diagnostic of lichen aureus (LA). Microscopic examination revealed a relatively sparse, superficial, perivascular and interstitial lymphohistiocytic infiltrate with scattered siderophages in the upper dermis. Extravasation of red blood cells also was noted (Figure 1). An immunohistochemical stain for Melan-A highlighted a normal number and distribution of single melanocytes at the dermoepidermal junction with no evidence of pagetoid scatter. A Perls Prussian blue stain for iron demonstrated abundant hemosiderin in the dermis (Figure 2).
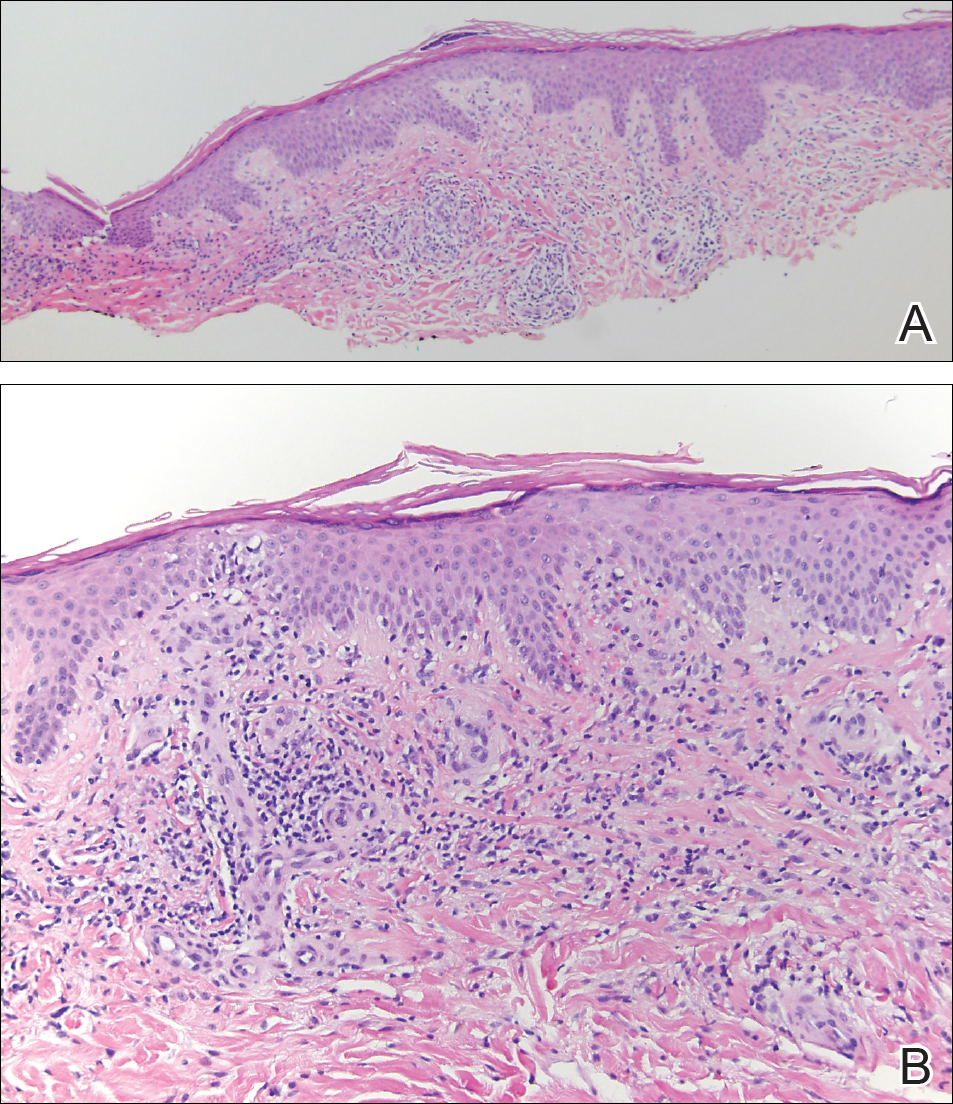
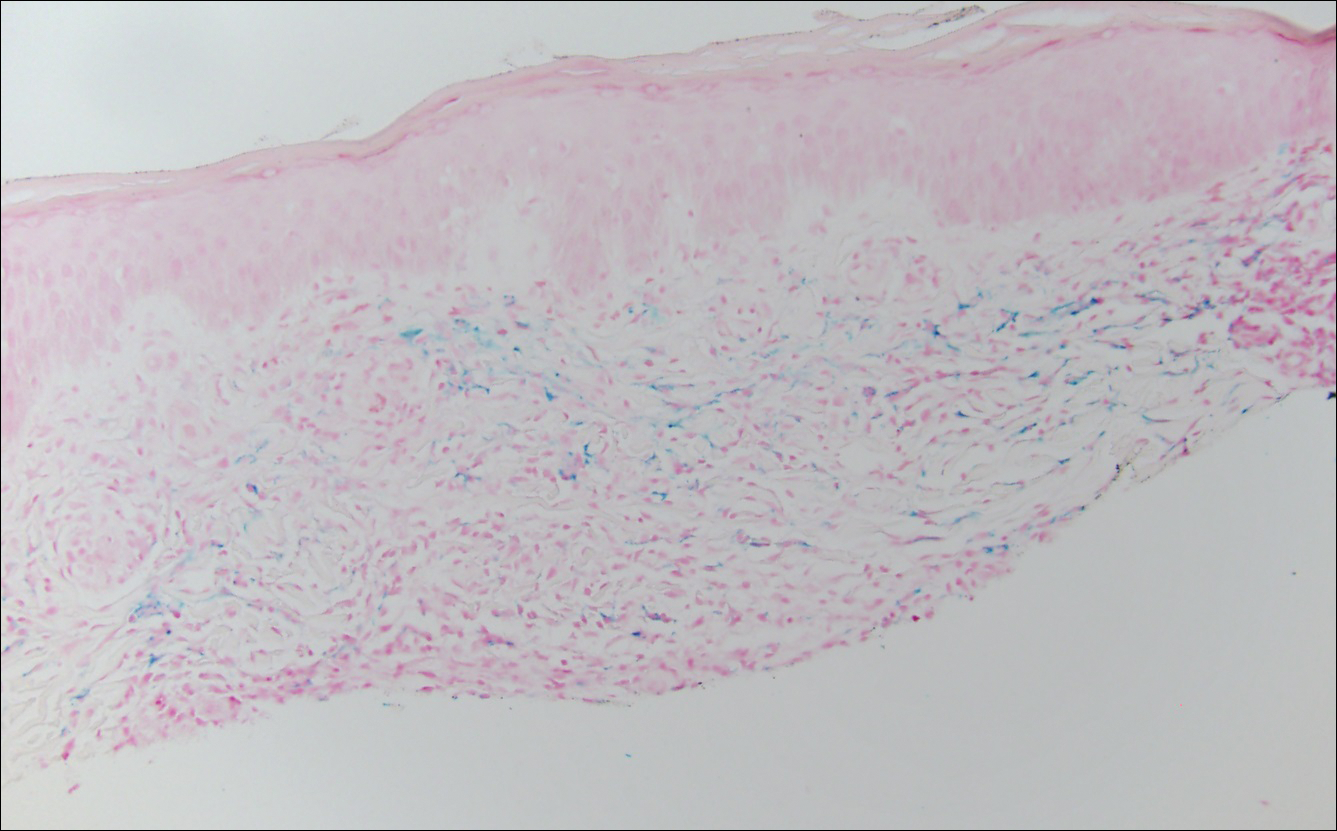
Pigmented purpuric dermatosis (PPD) describes a group of cutaneous lesions that are characterized by petechiae and pigmentary changes. These lesions most commonly present on the lower limbs; however, other sites have been reported.1 This group includes several major clinical forms such as Schamberg disease, LA, purpura annularis telangiectodes of Majocchi, eczematidlike purpura of Doucas and Kapetanakis, and lichenoid PPD of Gougerot and Blum. Lesions typically demonstrate a striking golden brown color clinically and by definition occur in the absence of platelet defects or vasculitis.1
Factors implicated in the pathogenesis of pigmented purpura include gravitational dependency, venous stasis, infection, and drugs.2 It is suggested that cellular immunity may play a role in the development of the disease based on the presence of CD4+ T lymphocytes in the infiltrate and the expression of HLA-DR by these lymphocytes and the keratinocytes.3 Lichen aureus differs in that it relates to increased intravascular pressure from an incompetent valve in an underlying perforating vein.4
Lichen aureus, also referred to as lichen purpuricus, is one major variant of PPD. The name reflects both the characteristic golden brown color and the histopathologic pattern of inflammation.1 Lichen aureus usually presents as a unilateral, asymptomatic, confined single lesion located mainly on the leg,1 though it can develop at other sites or as a localized group of lesions. Extensive lesions have been reported5 and cases with a segmental distribution have been described.6 In contrast, Schamberg disease demonstrates pinhead-sized reddish lesions giving the characteristic cayenne pepper pigmentation. These lesions coalesce to form thumbprint patches that progress proximally.1 Majocchi purpura is annular and telangiectatic, while lichenoid purpura of Gougerot and Blum presents with flat-topped, polygonal, violaceous papules that turn brown over time.
Some authors have championed a role for dermoscopy in diagnosis of LA.7 By dermoscopy, LA demonstrates a diffuse copper background reflecting the lymphohistiocytic dermal infiltrate, red dots and globules representing the extravasated red blood cells and the dilated swollen vessels, and grey dots that reflect the hemosiderin present in the dermis.8
Histologically, LA demonstrates a superficial perivascular infiltrate composed mainly of CD4+ lymphocytes surrounding the superficial capillaries. Over time, red cell extravasation leads to the formation of hemosiderin-laden macrophages, which can be highlighted with Perls Prussian blue stain. A bandlike infiltrate with thin strands of collagen separating it from the epidermis also may be noted.9
An important consideration in the differential diagnosis of PPD is mycosis fungoides (MF). Mycosis fungoides is a cutaneous T-cell lymphoma that clinically presents as a single or multiple hypopigmented or hyperpigmented patches or as erythematous scaly lesions in the patch or plaque stage. These lesions eventually may evolve into tumor stage.10 Mycosis fungoides may mimic PPD clinically and/or histopathologically, and rarely PPD also may precede MF.11 Involvement of the trunk, especially the lower abdomen and buttock region, favors a diagnosis of MF. Typically, histopathologic examination of MF demonstrates an epidermotropic lymphocytic infiltrate composed of atypical cerebriform lymphocytes overlying papillary dermal fibrosis. Although classic MF would be difficult to confuse with PPD, the atrophic lichenoid pattern of MF may show remarkable overlap with PPD.12 Such cases require clinicopathologic correlation, immunophenotyping of the epidermotropic lymphocytes, and occasionally T-cell clonality studies.
Lichen aureus is a chronic persistent disease unless the underlying incompetent perforator vessel is ligated. Various treatments have been used for other forms of pigmented purpura including topical corticosteroids, topical tacrolimus, systemic vasodilators such as prostacyclin and pentoxifylline, and phototherapy.1 Clinical follow-up is recommended for lesions that show some clinical or histopathological overlap with MF. Additional biopsies also may prove useful in establishing a definitive diagnosis in ambiguous cases.
- Sardana K, Sarkar R, Sehgal VN. Pigmented purpuric dermatoses: an overview. Int J Dermatol. 2004;43:482-488.
- Newton RC, Raimer SS. Pigmented purpuric eruptions. Dermatol Clin. 1985;3:165-169.
- Aiba S, Tagami H. Immunohistologic studies in Schamberg's disease. evidence for cellular immune reaction in lesional skin. Arch Dermatol. 1988;124:1058-1062.
- English J. Lichen aureus. J Am Acad Dermatol. 1985;12(2, pt 1):377-379.
- Duhra P, Tan CY. Lichen aureus. Br J Dermatol. 1986;114:395.
- Moche J, Glassman S, Modi D, et al. Segmental lichen aureus: a report of two cases treated with methylprednisolone aceponate. Australas J Dermatol. 2011;52:E15-E18.
- Zaballos P, Puig S, Malvehy J. Dermoscopy of pigmented purpuric dermatoses (lichen aureus): a useful tool for clinical diagnosis. Arch Dermatol. 2004;140:1290-1291.
- Portela PS, Melo DF, Ormiga P, et al. Dermoscopy of lichen aureus. An Bras Dermatol. 2013;88:253-255.
- Smoller BR, Kamel OW. Pigmented purpuric eruptions: immunopathologic studies supportive of a common immunophenotype. J Cutan Pathol. 1991;18:423-427.
- Jaffe ES, Harris NL, Diebold J, et al. World Health Organization classification of neoplastic diseases of the hematopoietic and lymphoid tissues. a progress report. Am J Clin Pathol. 1999;111(1 suppl 1):S8-S12.
- Hanna S, Walsh N, D'Intino Y, et al. Mycosis fungoides presenting as pigmented purpuric dermatitis. Pediatr Dermatol. 2006;23:350-354.
- Toro JR, Sander CA, LeBoit PE. Persistent pigmented purpuric dermatitis and mycosis fungoides: simulant, precursor, or both? a study by light microscopy and molecular methods. Am J Dermatopathol. 1997;19:108-118.
The Diagnosis: Lichen Aureus
The clinicopathological findings were diagnostic of lichen aureus (LA). Microscopic examination revealed a relatively sparse, superficial, perivascular and interstitial lymphohistiocytic infiltrate with scattered siderophages in the upper dermis. Extravasation of red blood cells also was noted (Figure 1). An immunohistochemical stain for Melan-A highlighted a normal number and distribution of single melanocytes at the dermoepidermal junction with no evidence of pagetoid scatter. A Perls Prussian blue stain for iron demonstrated abundant hemosiderin in the dermis (Figure 2).


Pigmented purpuric dermatosis (PPD) describes a group of cutaneous lesions that are characterized by petechiae and pigmentary changes. These lesions most commonly present on the lower limbs; however, other sites have been reported.1 This group includes several major clinical forms such as Schamberg disease, LA, purpura annularis telangiectodes of Majocchi, eczematidlike purpura of Doucas and Kapetanakis, and lichenoid PPD of Gougerot and Blum. Lesions typically demonstrate a striking golden brown color clinically and by definition occur in the absence of platelet defects or vasculitis.1
Factors implicated in the pathogenesis of pigmented purpura include gravitational dependency, venous stasis, infection, and drugs.2 It is suggested that cellular immunity may play a role in the development of the disease based on the presence of CD4+ T lymphocytes in the infiltrate and the expression of HLA-DR by these lymphocytes and the keratinocytes.3 Lichen aureus differs in that it relates to increased intravascular pressure from an incompetent valve in an underlying perforating vein.4
Lichen aureus, also referred to as lichen purpuricus, is one major variant of PPD. The name reflects both the characteristic golden brown color and the histopathologic pattern of inflammation.1 Lichen aureus usually presents as a unilateral, asymptomatic, confined single lesion located mainly on the leg,1 though it can develop at other sites or as a localized group of lesions. Extensive lesions have been reported5 and cases with a segmental distribution have been described.6 In contrast, Schamberg disease demonstrates pinhead-sized reddish lesions giving the characteristic cayenne pepper pigmentation. These lesions coalesce to form thumbprint patches that progress proximally.1 Majocchi purpura is annular and telangiectatic, while lichenoid purpura of Gougerot and Blum presents with flat-topped, polygonal, violaceous papules that turn brown over time.
Some authors have championed a role for dermoscopy in diagnosis of LA.7 By dermoscopy, LA demonstrates a diffuse copper background reflecting the lymphohistiocytic dermal infiltrate, red dots and globules representing the extravasated red blood cells and the dilated swollen vessels, and grey dots that reflect the hemosiderin present in the dermis.8
Histologically, LA demonstrates a superficial perivascular infiltrate composed mainly of CD4+ lymphocytes surrounding the superficial capillaries. Over time, red cell extravasation leads to the formation of hemosiderin-laden macrophages, which can be highlighted with Perls Prussian blue stain. A bandlike infiltrate with thin strands of collagen separating it from the epidermis also may be noted.9
An important consideration in the differential diagnosis of PPD is mycosis fungoides (MF). Mycosis fungoides is a cutaneous T-cell lymphoma that clinically presents as a single or multiple hypopigmented or hyperpigmented patches or as erythematous scaly lesions in the patch or plaque stage. These lesions eventually may evolve into tumor stage.10 Mycosis fungoides may mimic PPD clinically and/or histopathologically, and rarely PPD also may precede MF.11 Involvement of the trunk, especially the lower abdomen and buttock region, favors a diagnosis of MF. Typically, histopathologic examination of MF demonstrates an epidermotropic lymphocytic infiltrate composed of atypical cerebriform lymphocytes overlying papillary dermal fibrosis. Although classic MF would be difficult to confuse with PPD, the atrophic lichenoid pattern of MF may show remarkable overlap with PPD.12 Such cases require clinicopathologic correlation, immunophenotyping of the epidermotropic lymphocytes, and occasionally T-cell clonality studies.
Lichen aureus is a chronic persistent disease unless the underlying incompetent perforator vessel is ligated. Various treatments have been used for other forms of pigmented purpura including topical corticosteroids, topical tacrolimus, systemic vasodilators such as prostacyclin and pentoxifylline, and phototherapy.1 Clinical follow-up is recommended for lesions that show some clinical or histopathological overlap with MF. Additional biopsies also may prove useful in establishing a definitive diagnosis in ambiguous cases.
The Diagnosis: Lichen Aureus
The clinicopathological findings were diagnostic of lichen aureus (LA). Microscopic examination revealed a relatively sparse, superficial, perivascular and interstitial lymphohistiocytic infiltrate with scattered siderophages in the upper dermis. Extravasation of red blood cells also was noted (Figure 1). An immunohistochemical stain for Melan-A highlighted a normal number and distribution of single melanocytes at the dermoepidermal junction with no evidence of pagetoid scatter. A Perls Prussian blue stain for iron demonstrated abundant hemosiderin in the dermis (Figure 2).


Pigmented purpuric dermatosis (PPD) describes a group of cutaneous lesions that are characterized by petechiae and pigmentary changes. These lesions most commonly present on the lower limbs; however, other sites have been reported.1 This group includes several major clinical forms such as Schamberg disease, LA, purpura annularis telangiectodes of Majocchi, eczematidlike purpura of Doucas and Kapetanakis, and lichenoid PPD of Gougerot and Blum. Lesions typically demonstrate a striking golden brown color clinically and by definition occur in the absence of platelet defects or vasculitis.1
Factors implicated in the pathogenesis of pigmented purpura include gravitational dependency, venous stasis, infection, and drugs.2 It is suggested that cellular immunity may play a role in the development of the disease based on the presence of CD4+ T lymphocytes in the infiltrate and the expression of HLA-DR by these lymphocytes and the keratinocytes.3 Lichen aureus differs in that it relates to increased intravascular pressure from an incompetent valve in an underlying perforating vein.4
Lichen aureus, also referred to as lichen purpuricus, is one major variant of PPD. The name reflects both the characteristic golden brown color and the histopathologic pattern of inflammation.1 Lichen aureus usually presents as a unilateral, asymptomatic, confined single lesion located mainly on the leg,1 though it can develop at other sites or as a localized group of lesions. Extensive lesions have been reported5 and cases with a segmental distribution have been described.6 In contrast, Schamberg disease demonstrates pinhead-sized reddish lesions giving the characteristic cayenne pepper pigmentation. These lesions coalesce to form thumbprint patches that progress proximally.1 Majocchi purpura is annular and telangiectatic, while lichenoid purpura of Gougerot and Blum presents with flat-topped, polygonal, violaceous papules that turn brown over time.
Some authors have championed a role for dermoscopy in diagnosis of LA.7 By dermoscopy, LA demonstrates a diffuse copper background reflecting the lymphohistiocytic dermal infiltrate, red dots and globules representing the extravasated red blood cells and the dilated swollen vessels, and grey dots that reflect the hemosiderin present in the dermis.8
Histologically, LA demonstrates a superficial perivascular infiltrate composed mainly of CD4+ lymphocytes surrounding the superficial capillaries. Over time, red cell extravasation leads to the formation of hemosiderin-laden macrophages, which can be highlighted with Perls Prussian blue stain. A bandlike infiltrate with thin strands of collagen separating it from the epidermis also may be noted.9
An important consideration in the differential diagnosis of PPD is mycosis fungoides (MF). Mycosis fungoides is a cutaneous T-cell lymphoma that clinically presents as a single or multiple hypopigmented or hyperpigmented patches or as erythematous scaly lesions in the patch or plaque stage. These lesions eventually may evolve into tumor stage.10 Mycosis fungoides may mimic PPD clinically and/or histopathologically, and rarely PPD also may precede MF.11 Involvement of the trunk, especially the lower abdomen and buttock region, favors a diagnosis of MF. Typically, histopathologic examination of MF demonstrates an epidermotropic lymphocytic infiltrate composed of atypical cerebriform lymphocytes overlying papillary dermal fibrosis. Although classic MF would be difficult to confuse with PPD, the atrophic lichenoid pattern of MF may show remarkable overlap with PPD.12 Such cases require clinicopathologic correlation, immunophenotyping of the epidermotropic lymphocytes, and occasionally T-cell clonality studies.
Lichen aureus is a chronic persistent disease unless the underlying incompetent perforator vessel is ligated. Various treatments have been used for other forms of pigmented purpura including topical corticosteroids, topical tacrolimus, systemic vasodilators such as prostacyclin and pentoxifylline, and phototherapy.1 Clinical follow-up is recommended for lesions that show some clinical or histopathological overlap with MF. Additional biopsies also may prove useful in establishing a definitive diagnosis in ambiguous cases.
- Sardana K, Sarkar R, Sehgal VN. Pigmented purpuric dermatoses: an overview. Int J Dermatol. 2004;43:482-488.
- Newton RC, Raimer SS. Pigmented purpuric eruptions. Dermatol Clin. 1985;3:165-169.
- Aiba S, Tagami H. Immunohistologic studies in Schamberg's disease. evidence for cellular immune reaction in lesional skin. Arch Dermatol. 1988;124:1058-1062.
- English J. Lichen aureus. J Am Acad Dermatol. 1985;12(2, pt 1):377-379.
- Duhra P, Tan CY. Lichen aureus. Br J Dermatol. 1986;114:395.
- Moche J, Glassman S, Modi D, et al. Segmental lichen aureus: a report of two cases treated with methylprednisolone aceponate. Australas J Dermatol. 2011;52:E15-E18.
- Zaballos P, Puig S, Malvehy J. Dermoscopy of pigmented purpuric dermatoses (lichen aureus): a useful tool for clinical diagnosis. Arch Dermatol. 2004;140:1290-1291.
- Portela PS, Melo DF, Ormiga P, et al. Dermoscopy of lichen aureus. An Bras Dermatol. 2013;88:253-255.
- Smoller BR, Kamel OW. Pigmented purpuric eruptions: immunopathologic studies supportive of a common immunophenotype. J Cutan Pathol. 1991;18:423-427.
- Jaffe ES, Harris NL, Diebold J, et al. World Health Organization classification of neoplastic diseases of the hematopoietic and lymphoid tissues. a progress report. Am J Clin Pathol. 1999;111(1 suppl 1):S8-S12.
- Hanna S, Walsh N, D'Intino Y, et al. Mycosis fungoides presenting as pigmented purpuric dermatitis. Pediatr Dermatol. 2006;23:350-354.
- Toro JR, Sander CA, LeBoit PE. Persistent pigmented purpuric dermatitis and mycosis fungoides: simulant, precursor, or both? a study by light microscopy and molecular methods. Am J Dermatopathol. 1997;19:108-118.
- Sardana K, Sarkar R, Sehgal VN. Pigmented purpuric dermatoses: an overview. Int J Dermatol. 2004;43:482-488.
- Newton RC, Raimer SS. Pigmented purpuric eruptions. Dermatol Clin. 1985;3:165-169.
- Aiba S, Tagami H. Immunohistologic studies in Schamberg's disease. evidence for cellular immune reaction in lesional skin. Arch Dermatol. 1988;124:1058-1062.
- English J. Lichen aureus. J Am Acad Dermatol. 1985;12(2, pt 1):377-379.
- Duhra P, Tan CY. Lichen aureus. Br J Dermatol. 1986;114:395.
- Moche J, Glassman S, Modi D, et al. Segmental lichen aureus: a report of two cases treated with methylprednisolone aceponate. Australas J Dermatol. 2011;52:E15-E18.
- Zaballos P, Puig S, Malvehy J. Dermoscopy of pigmented purpuric dermatoses (lichen aureus): a useful tool for clinical diagnosis. Arch Dermatol. 2004;140:1290-1291.
- Portela PS, Melo DF, Ormiga P, et al. Dermoscopy of lichen aureus. An Bras Dermatol. 2013;88:253-255.
- Smoller BR, Kamel OW. Pigmented purpuric eruptions: immunopathologic studies supportive of a common immunophenotype. J Cutan Pathol. 1991;18:423-427.
- Jaffe ES, Harris NL, Diebold J, et al. World Health Organization classification of neoplastic diseases of the hematopoietic and lymphoid tissues. a progress report. Am J Clin Pathol. 1999;111(1 suppl 1):S8-S12.
- Hanna S, Walsh N, D'Intino Y, et al. Mycosis fungoides presenting as pigmented purpuric dermatitis. Pediatr Dermatol. 2006;23:350-354.
- Toro JR, Sander CA, LeBoit PE. Persistent pigmented purpuric dermatitis and mycosis fungoides: simulant, precursor, or both? a study by light microscopy and molecular methods. Am J Dermatopathol. 1997;19:108-118.
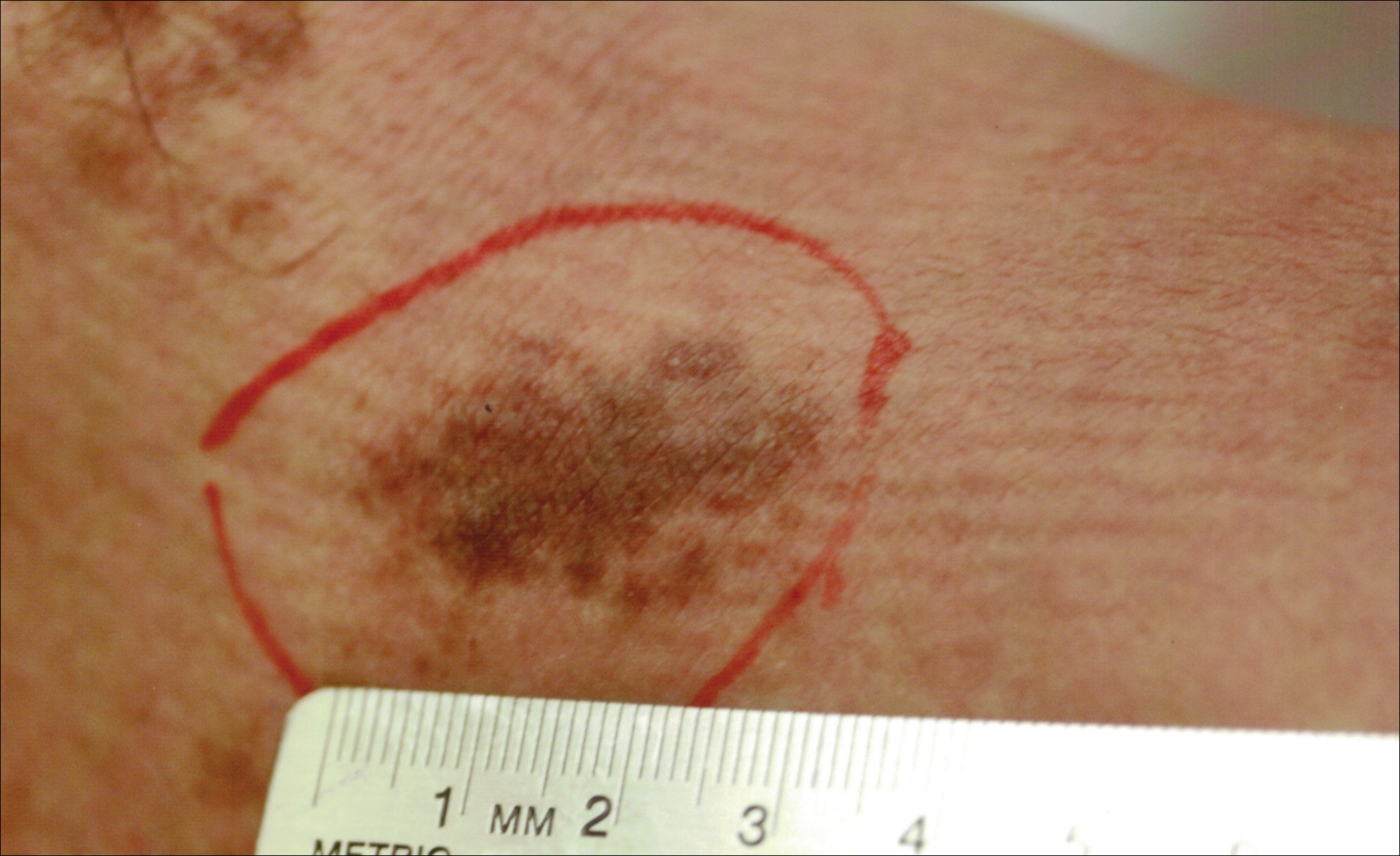
A 32-year-old man presented with an asymptomatic pigmented lesion on the left foot that developed over the course of 4 months. Physical examination revealed a 4-cm asymmetrical, deeply pigmented macule on the left foot. A shave biopsy of the lesion was performed.
What's Eating You? Sticktight Flea Revisited
Identifying Characteristics
The sticktight flea (Echidnophaga gallinacea) earns its name by embedding its head in the host's skin using broad and serrated laciniae and can feed at one site for up to 19 days.1 It differs in morphology from dog (Ctenocephalides canis) and cat (Ctenocephalides felis) fleas, lacking genal (mustache area) and promotal (back of the head) ctenidia (combs), and is half the size of the cat flea. It has 2 pairs of setae (hairs) behind the antennae with an anteriorly flattened head (Figure).
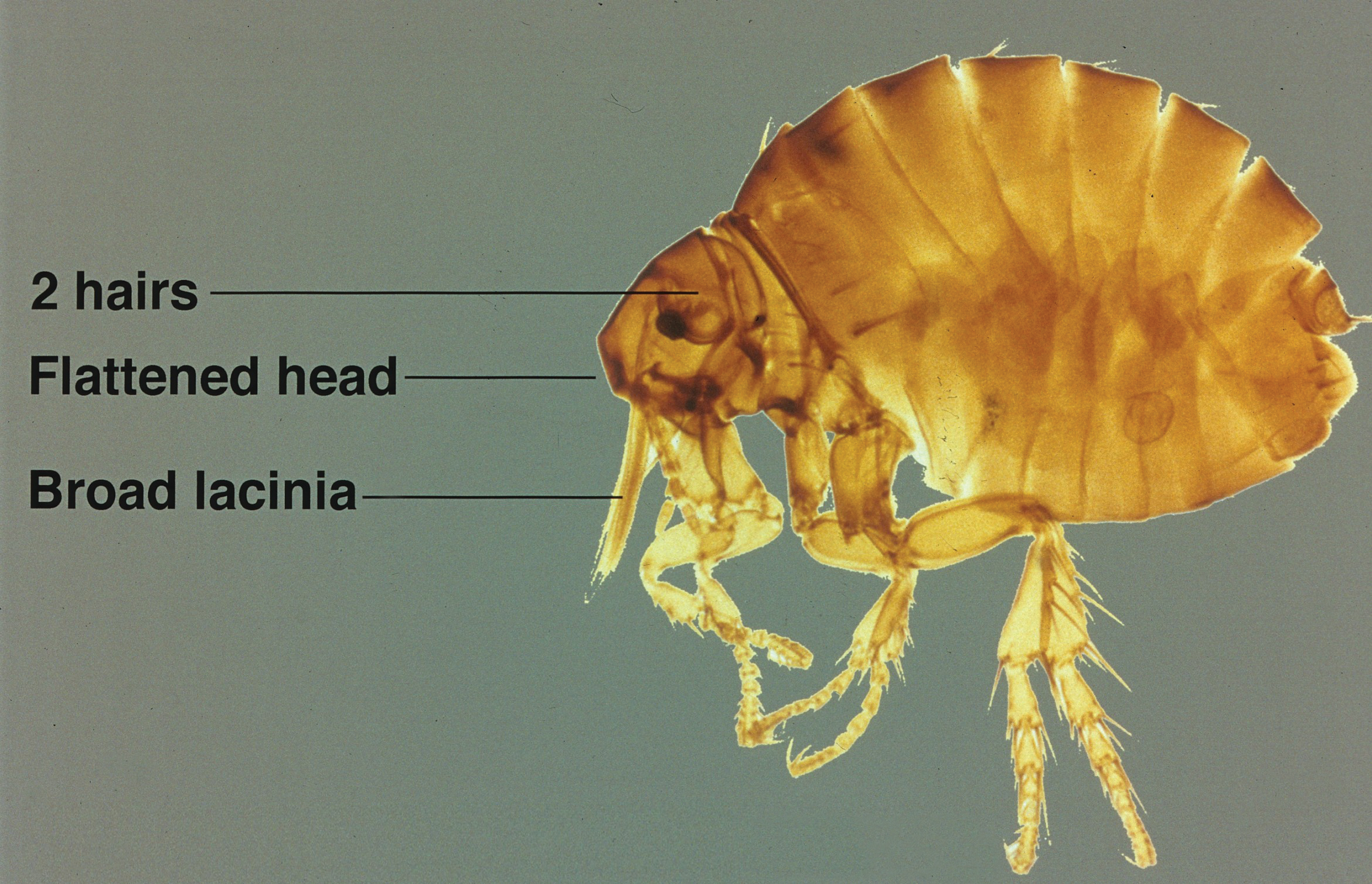
Disease Transmission
Although its primary host is poultry and it also is known as the stickfast or chicken flea, the sticktight flea has been found in many species of birds and mammals, including humans. It is becoming more common in dogs in many parts of the world, including the United States,2-5 and has been found to be the most common flea on dogs in areas of South Africa.6 Other noted hosts of E gallinacea are rodents, cottontail rabbits, cats, ground squirrels, and pigs.7-14 Human infestation occurs from exposure to affected animals.15 As blood feeders, fleas have long been known to serve as vectors for many diseases, including bubonic plague, typhus, and tularemia, as well as an intermediate host of the dog tapeworm (Dipylidium caninum).5Rickettsia felis, belonging to the spotted fever group, is an emerging infectious disease in humans commonly found in the cat flea (C felis) but also has been detected in E gallinacea.7Echidnophaga gallinacea is found worldwide in the tropics, subtropics, and temperate zones, and it is the only representative of the genus found in the United States.1 Given the wide range of wild and domestic animal hosts and wide geographic distribution for E gallinacea, it represents an increasing risk for humans.
Echidnophaga gallinacea favors feeding from fleshy areas without thick fur or plumage. In birds, the area around the eyes, comb, and wattles is included; in dogs, it can be the eyes, in between the toes, and in the genital area.1 Flea bites cause irritation and itching for hosts including humans, typically resulting in clusters of firm, pruritic, erythematous papules with a central punctum.15 Severe bites also may lead to bullous lesions. In birds, symptoms can be extreme, with infestation around the eyes leading to swelling and blindness, a decline in egg production, weight loss, and death in young birds.1 Similar to other fleas, E gallinacea is wingless and depends on jumping onto a host for transmission, which can be from the ground, carpeting and flooring, furniture, or another host. Fleas are champion jumpers (relative to body size) and can jump 100 times their length.16
Management
Treating sticktight fleas can be tricky, as they embed tightly into the host's skin. Animals should be treated by a qualified veterinarian. Removal of attached fleas in humans requires grasping the flea firmly with tweezers and pulling from the skin. If the infestation is considerable, malathion 5% liquid or gel can be applied. Patients can treat itching with topical steroids and antipruritic creams, and oral antihistamines can be used to relieve symptoms and reduce the likelihood of damaged skin as well as the potential for secondary infection. The flea-infested environment should be treated with insecticides. For treatment of hard surfaces, dichlorvos and propetamphos are effective. Organophosphates work well on fabric and carpeting. Domestic pets and livestock may be treated by a veterinarian with agents such as fipronil, selamectin, imidacloprid, metaflumizone, nitenpyram, lufenuron, methoprene, and pyriproxyfen.
- Gyimesi ZS, Hayden ER, Greiner EC. Sticktight flea (Echidnophaga gallinacea) infestation in a Victoria crowned pigeon (Goura victoria). J Zoo Wildl Med. 2007;38:594-596.
- Kalkofen UP, Greenberg J. Echidnophaga gallinacea infestation in dogs. J Am Vet Med Assoc. 1974;165:447-448.
- Harman DW, Halliwell RE, Greiner EC. Flea species from dogs and cats in north-central Florida. Vet Parasitol. 1987;23:135-140.
- Boughton RK, Atwell JW, Schoech SJ. An introduced generalist parasite, the sticktight flea (Echidnophaga gallinacea), and its pathology in the threatened Florida scrub-jay (Aphelocoma coerulescens). J Parasitol. 2006;92:941-948.
- Durden LA, Judy TN, Martin JE, et al. Fleas parasitizing domestic dogs in Georgia, USA: species composition and seasonal abundance. Vet Parasitol. 2005;130:157-162.
- Rautenbach GH, Boomker J, de Villiers IL. A descriptive study of the canine population in a rural town in southern Africa. J S Afr Vet Assoc. 1991;62:158-162.
- Leulmi H, Socolovschi C, Laudisoit A, et al. Detection of Rickettsia felis, Rickettsia typhi, Bartonella species and Yersinia pestis in fleas (Siphonaptera) from Africa. PLoS Negl Trop Dis. 2014;8:e3152.
- Guernier V, Lagadec E, LeMinter G, et al. Fleas of small mammals on Reunion Island: diversity, distribution and epidemiological consequences. PLoS Negl Trop Dis. 2014;8:e3129.
- Cantó GJ, Guerrero RI, Olvera-Ramírez AM, et al. Prevalence of fleas and gastrointestinal parasites in free-roaming cats in central Mexico [published online April 3, 2013]. PLoS One. 2013;8:e60744.
- Akucewich LH, Philman K, Clark A, et al. Prevalence of ectoparasites in a population of feral cats from north central Florida during the summer. Vet Parasitol. 2002;109:129-139.
- Linardi PM, Gomes AF, Botelho JR, et al. Some ectoparasites of commensal rodents from Huambo, Angola. J Med Entomol. 1994;31:754-756.
- Pfaffenberger GS, Valencia VB. Ectoparasites of sympatric cottontails (Sylvilagus audubonii Nelson) and jack rabbits (Lepus californicus Mearns) from the high plains of eastern New Mexico. J Parasitol. 1988;74:842-846.
- Hubbart JA, Jachowski DS, Eads DA. Seasonal and among-site variation in the occurrence and abundance of fleas on California ground squirrels (Otospermophilus beecheyi). J Vector Ecol. 2011;36:117-123.
- Braae UC, Ngowi HA, Johansen MV. Smallholder pig production: prevalence and risk factors of ectoparasites. Vet Parasitol. 2013;196:241-244.
- Carlson JC, Fox MS. A sticktight flea removed from the cheek of a two-year-old boy from Los Angeles. Dermatol Online J. 2009;15:4.
- Rothschild M, Schlein Y, Parker K, et al. The flying leap of the flea. Scientific American. 1973;229:92.
Identifying Characteristics
The sticktight flea (Echidnophaga gallinacea) earns its name by embedding its head in the host's skin using broad and serrated laciniae and can feed at one site for up to 19 days.1 It differs in morphology from dog (Ctenocephalides canis) and cat (Ctenocephalides felis) fleas, lacking genal (mustache area) and promotal (back of the head) ctenidia (combs), and is half the size of the cat flea. It has 2 pairs of setae (hairs) behind the antennae with an anteriorly flattened head (Figure).

Disease Transmission
Although its primary host is poultry and it also is known as the stickfast or chicken flea, the sticktight flea has been found in many species of birds and mammals, including humans. It is becoming more common in dogs in many parts of the world, including the United States,2-5 and has been found to be the most common flea on dogs in areas of South Africa.6 Other noted hosts of E gallinacea are rodents, cottontail rabbits, cats, ground squirrels, and pigs.7-14 Human infestation occurs from exposure to affected animals.15 As blood feeders, fleas have long been known to serve as vectors for many diseases, including bubonic plague, typhus, and tularemia, as well as an intermediate host of the dog tapeworm (Dipylidium caninum).5Rickettsia felis, belonging to the spotted fever group, is an emerging infectious disease in humans commonly found in the cat flea (C felis) but also has been detected in E gallinacea.7Echidnophaga gallinacea is found worldwide in the tropics, subtropics, and temperate zones, and it is the only representative of the genus found in the United States.1 Given the wide range of wild and domestic animal hosts and wide geographic distribution for E gallinacea, it represents an increasing risk for humans.
Echidnophaga gallinacea favors feeding from fleshy areas without thick fur or plumage. In birds, the area around the eyes, comb, and wattles is included; in dogs, it can be the eyes, in between the toes, and in the genital area.1 Flea bites cause irritation and itching for hosts including humans, typically resulting in clusters of firm, pruritic, erythematous papules with a central punctum.15 Severe bites also may lead to bullous lesions. In birds, symptoms can be extreme, with infestation around the eyes leading to swelling and blindness, a decline in egg production, weight loss, and death in young birds.1 Similar to other fleas, E gallinacea is wingless and depends on jumping onto a host for transmission, which can be from the ground, carpeting and flooring, furniture, or another host. Fleas are champion jumpers (relative to body size) and can jump 100 times their length.16
Management
Treating sticktight fleas can be tricky, as they embed tightly into the host's skin. Animals should be treated by a qualified veterinarian. Removal of attached fleas in humans requires grasping the flea firmly with tweezers and pulling from the skin. If the infestation is considerable, malathion 5% liquid or gel can be applied. Patients can treat itching with topical steroids and antipruritic creams, and oral antihistamines can be used to relieve symptoms and reduce the likelihood of damaged skin as well as the potential for secondary infection. The flea-infested environment should be treated with insecticides. For treatment of hard surfaces, dichlorvos and propetamphos are effective. Organophosphates work well on fabric and carpeting. Domestic pets and livestock may be treated by a veterinarian with agents such as fipronil, selamectin, imidacloprid, metaflumizone, nitenpyram, lufenuron, methoprene, and pyriproxyfen.
Identifying Characteristics
The sticktight flea (Echidnophaga gallinacea) earns its name by embedding its head in the host's skin using broad and serrated laciniae and can feed at one site for up to 19 days.1 It differs in morphology from dog (Ctenocephalides canis) and cat (Ctenocephalides felis) fleas, lacking genal (mustache area) and promotal (back of the head) ctenidia (combs), and is half the size of the cat flea. It has 2 pairs of setae (hairs) behind the antennae with an anteriorly flattened head (Figure).

Disease Transmission
Although its primary host is poultry and it also is known as the stickfast or chicken flea, the sticktight flea has been found in many species of birds and mammals, including humans. It is becoming more common in dogs in many parts of the world, including the United States,2-5 and has been found to be the most common flea on dogs in areas of South Africa.6 Other noted hosts of E gallinacea are rodents, cottontail rabbits, cats, ground squirrels, and pigs.7-14 Human infestation occurs from exposure to affected animals.15 As blood feeders, fleas have long been known to serve as vectors for many diseases, including bubonic plague, typhus, and tularemia, as well as an intermediate host of the dog tapeworm (Dipylidium caninum).5Rickettsia felis, belonging to the spotted fever group, is an emerging infectious disease in humans commonly found in the cat flea (C felis) but also has been detected in E gallinacea.7Echidnophaga gallinacea is found worldwide in the tropics, subtropics, and temperate zones, and it is the only representative of the genus found in the United States.1 Given the wide range of wild and domestic animal hosts and wide geographic distribution for E gallinacea, it represents an increasing risk for humans.
Echidnophaga gallinacea favors feeding from fleshy areas without thick fur or plumage. In birds, the area around the eyes, comb, and wattles is included; in dogs, it can be the eyes, in between the toes, and in the genital area.1 Flea bites cause irritation and itching for hosts including humans, typically resulting in clusters of firm, pruritic, erythematous papules with a central punctum.15 Severe bites also may lead to bullous lesions. In birds, symptoms can be extreme, with infestation around the eyes leading to swelling and blindness, a decline in egg production, weight loss, and death in young birds.1 Similar to other fleas, E gallinacea is wingless and depends on jumping onto a host for transmission, which can be from the ground, carpeting and flooring, furniture, or another host. Fleas are champion jumpers (relative to body size) and can jump 100 times their length.16
Management
Treating sticktight fleas can be tricky, as they embed tightly into the host's skin. Animals should be treated by a qualified veterinarian. Removal of attached fleas in humans requires grasping the flea firmly with tweezers and pulling from the skin. If the infestation is considerable, malathion 5% liquid or gel can be applied. Patients can treat itching with topical steroids and antipruritic creams, and oral antihistamines can be used to relieve symptoms and reduce the likelihood of damaged skin as well as the potential for secondary infection. The flea-infested environment should be treated with insecticides. For treatment of hard surfaces, dichlorvos and propetamphos are effective. Organophosphates work well on fabric and carpeting. Domestic pets and livestock may be treated by a veterinarian with agents such as fipronil, selamectin, imidacloprid, metaflumizone, nitenpyram, lufenuron, methoprene, and pyriproxyfen.
- Gyimesi ZS, Hayden ER, Greiner EC. Sticktight flea (Echidnophaga gallinacea) infestation in a Victoria crowned pigeon (Goura victoria). J Zoo Wildl Med. 2007;38:594-596.
- Kalkofen UP, Greenberg J. Echidnophaga gallinacea infestation in dogs. J Am Vet Med Assoc. 1974;165:447-448.
- Harman DW, Halliwell RE, Greiner EC. Flea species from dogs and cats in north-central Florida. Vet Parasitol. 1987;23:135-140.
- Boughton RK, Atwell JW, Schoech SJ. An introduced generalist parasite, the sticktight flea (Echidnophaga gallinacea), and its pathology in the threatened Florida scrub-jay (Aphelocoma coerulescens). J Parasitol. 2006;92:941-948.
- Durden LA, Judy TN, Martin JE, et al. Fleas parasitizing domestic dogs in Georgia, USA: species composition and seasonal abundance. Vet Parasitol. 2005;130:157-162.
- Rautenbach GH, Boomker J, de Villiers IL. A descriptive study of the canine population in a rural town in southern Africa. J S Afr Vet Assoc. 1991;62:158-162.
- Leulmi H, Socolovschi C, Laudisoit A, et al. Detection of Rickettsia felis, Rickettsia typhi, Bartonella species and Yersinia pestis in fleas (Siphonaptera) from Africa. PLoS Negl Trop Dis. 2014;8:e3152.
- Guernier V, Lagadec E, LeMinter G, et al. Fleas of small mammals on Reunion Island: diversity, distribution and epidemiological consequences. PLoS Negl Trop Dis. 2014;8:e3129.
- Cantó GJ, Guerrero RI, Olvera-Ramírez AM, et al. Prevalence of fleas and gastrointestinal parasites in free-roaming cats in central Mexico [published online April 3, 2013]. PLoS One. 2013;8:e60744.
- Akucewich LH, Philman K, Clark A, et al. Prevalence of ectoparasites in a population of feral cats from north central Florida during the summer. Vet Parasitol. 2002;109:129-139.
- Linardi PM, Gomes AF, Botelho JR, et al. Some ectoparasites of commensal rodents from Huambo, Angola. J Med Entomol. 1994;31:754-756.
- Pfaffenberger GS, Valencia VB. Ectoparasites of sympatric cottontails (Sylvilagus audubonii Nelson) and jack rabbits (Lepus californicus Mearns) from the high plains of eastern New Mexico. J Parasitol. 1988;74:842-846.
- Hubbart JA, Jachowski DS, Eads DA. Seasonal and among-site variation in the occurrence and abundance of fleas on California ground squirrels (Otospermophilus beecheyi). J Vector Ecol. 2011;36:117-123.
- Braae UC, Ngowi HA, Johansen MV. Smallholder pig production: prevalence and risk factors of ectoparasites. Vet Parasitol. 2013;196:241-244.
- Carlson JC, Fox MS. A sticktight flea removed from the cheek of a two-year-old boy from Los Angeles. Dermatol Online J. 2009;15:4.
- Rothschild M, Schlein Y, Parker K, et al. The flying leap of the flea. Scientific American. 1973;229:92.
- Gyimesi ZS, Hayden ER, Greiner EC. Sticktight flea (Echidnophaga gallinacea) infestation in a Victoria crowned pigeon (Goura victoria). J Zoo Wildl Med. 2007;38:594-596.
- Kalkofen UP, Greenberg J. Echidnophaga gallinacea infestation in dogs. J Am Vet Med Assoc. 1974;165:447-448.
- Harman DW, Halliwell RE, Greiner EC. Flea species from dogs and cats in north-central Florida. Vet Parasitol. 1987;23:135-140.
- Boughton RK, Atwell JW, Schoech SJ. An introduced generalist parasite, the sticktight flea (Echidnophaga gallinacea), and its pathology in the threatened Florida scrub-jay (Aphelocoma coerulescens). J Parasitol. 2006;92:941-948.
- Durden LA, Judy TN, Martin JE, et al. Fleas parasitizing domestic dogs in Georgia, USA: species composition and seasonal abundance. Vet Parasitol. 2005;130:157-162.
- Rautenbach GH, Boomker J, de Villiers IL. A descriptive study of the canine population in a rural town in southern Africa. J S Afr Vet Assoc. 1991;62:158-162.
- Leulmi H, Socolovschi C, Laudisoit A, et al. Detection of Rickettsia felis, Rickettsia typhi, Bartonella species and Yersinia pestis in fleas (Siphonaptera) from Africa. PLoS Negl Trop Dis. 2014;8:e3152.
- Guernier V, Lagadec E, LeMinter G, et al. Fleas of small mammals on Reunion Island: diversity, distribution and epidemiological consequences. PLoS Negl Trop Dis. 2014;8:e3129.
- Cantó GJ, Guerrero RI, Olvera-Ramírez AM, et al. Prevalence of fleas and gastrointestinal parasites in free-roaming cats in central Mexico [published online April 3, 2013]. PLoS One. 2013;8:e60744.
- Akucewich LH, Philman K, Clark A, et al. Prevalence of ectoparasites in a population of feral cats from north central Florida during the summer. Vet Parasitol. 2002;109:129-139.
- Linardi PM, Gomes AF, Botelho JR, et al. Some ectoparasites of commensal rodents from Huambo, Angola. J Med Entomol. 1994;31:754-756.
- Pfaffenberger GS, Valencia VB. Ectoparasites of sympatric cottontails (Sylvilagus audubonii Nelson) and jack rabbits (Lepus californicus Mearns) from the high plains of eastern New Mexico. J Parasitol. 1988;74:842-846.
- Hubbart JA, Jachowski DS, Eads DA. Seasonal and among-site variation in the occurrence and abundance of fleas on California ground squirrels (Otospermophilus beecheyi). J Vector Ecol. 2011;36:117-123.
- Braae UC, Ngowi HA, Johansen MV. Smallholder pig production: prevalence and risk factors of ectoparasites. Vet Parasitol. 2013;196:241-244.
- Carlson JC, Fox MS. A sticktight flea removed from the cheek of a two-year-old boy from Los Angeles. Dermatol Online J. 2009;15:4.
- Rothschild M, Schlein Y, Parker K, et al. The flying leap of the flea. Scientific American. 1973;229:92.
Practice Points
- Although the primary host of the sticktight flea is poultry, it has been found in many species of birds and mammals, including humans.
- Flea bites cause irritation and itching for hosts, typically resulting in clusters of firm, pruritic, erythematous papules with a central punctum.
- Removal of attached fleas in humans requires grasping the flea firmly with tweezers and pulling from the skin.
What’s Eating You? Chiggers
Identifying Characteristics and Disease Transmission
Chiggers belong to the Trombiculidae family of mites and also are referred to as harvest mites, harvest bugs, harvest lice, mower’s mites, and redbugs.1 The term chigger specifically describes the larval stage of this mite’s life cycle, as it is the only stage responsible for chigger bites. The nymph and adult phases feed on vegetable matter. Trombiculid mites are most often found in forests, grassy areas, gardens, and moist areas of soil near bodies of water. Trombicula alfreddugesi is the most common species in the United States, and these mites mainly live in the southeastern and south central regions of the country. Conversely, Trombicula autumnalis is most predominant in Western Europe and East Asia.1
The life cycle of the mite includes the egg, larval, nymphal, and adult stages.2 Due to their need for air humidity greater than 80%, mites lay their eggs on low leaves, blades of grass, or on the ground. They spend most of their lives on vegetation no more than 30 cm above ground level.3 Eggs remain dormant for approximately 6 days until the hatching of the prelarvae, which have 6 legs and are nonfeeding. It takes another 6 days for the prelarvae to mature into larvae. Measuring 0.15 to 0.3 mm in length, mite larvae are a mere fraction of the size of adult mites, which generally are 1 to 2 mm in length, and are bright red or brown-red in color (Figure 1).
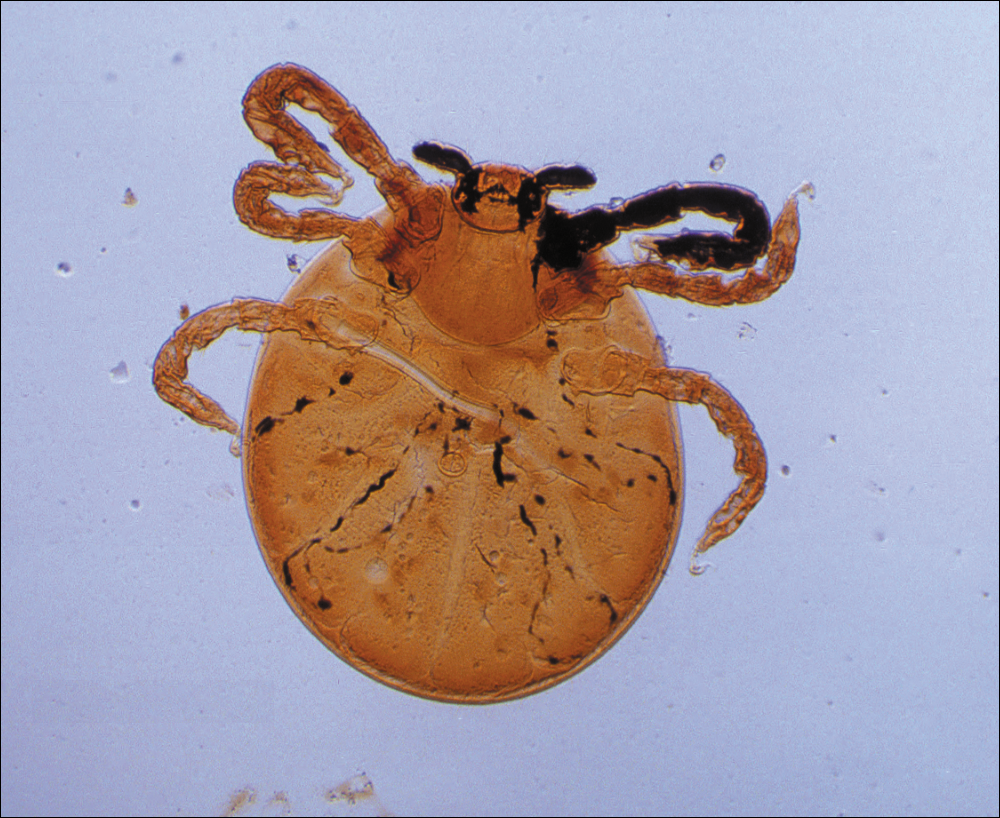
The biting larvae have many acceptable hosts including turtles, toads, birds, small mammals, and humans, which act as accidental hosts. Larvae remain on vegetation waiting for a suitable host to pass by so they may attach to its skin and remain there for several days. In the exploration for an ideal area to begin feeding (eg, thin epidermis,4 localized increased air humidity5), larvae can travel extensively on the skin; however, they often are stopped by tight-fitting sections of clothing (eg, waistbands), so bites are mostly found in clusters. To feed, mite larvae latch onto the skin using chelicerae, jawlike appendages found in the front of the mouth in arachnids.6 They then inject digestive enzymes that liquefy epidermal cells on direct contact, which results in the formation of a stylostome from which the mites may suck up lymph fluid and broken down tissue.7 Although the actual initial bite is painless, this feeding process leads to the localized inflammation and irritation noticed by infested patients.8
The classic clinical presentation includes severe pruritus and cutaneous swelling as well as erythema caused by the combination of several factors, such as enzyme-induced cellular mechanical damage, human immune response, and sometimes a superimposed bacterial infection. Papules and papulovesicles appear in groups, most commonly affecting the legs and waistline (Figure 2).9 Itching generally occurs within hours of larval latching and subsides within 72 hours. Cutaneous lesions typically take 1 to 2 weeks to heal. In some rare cases, patients may react with urticarial, bullous, or morbilliform eruptions, and the inflammation and pruritus can last for weeks.6 Summer penile syndrome has been noted in boys who display a local hypersensitivity to chigger bites.10 This syndrome represents a triad of penile swelling, dysuria, and pruritus, which lasts for a few days to a few weeks.
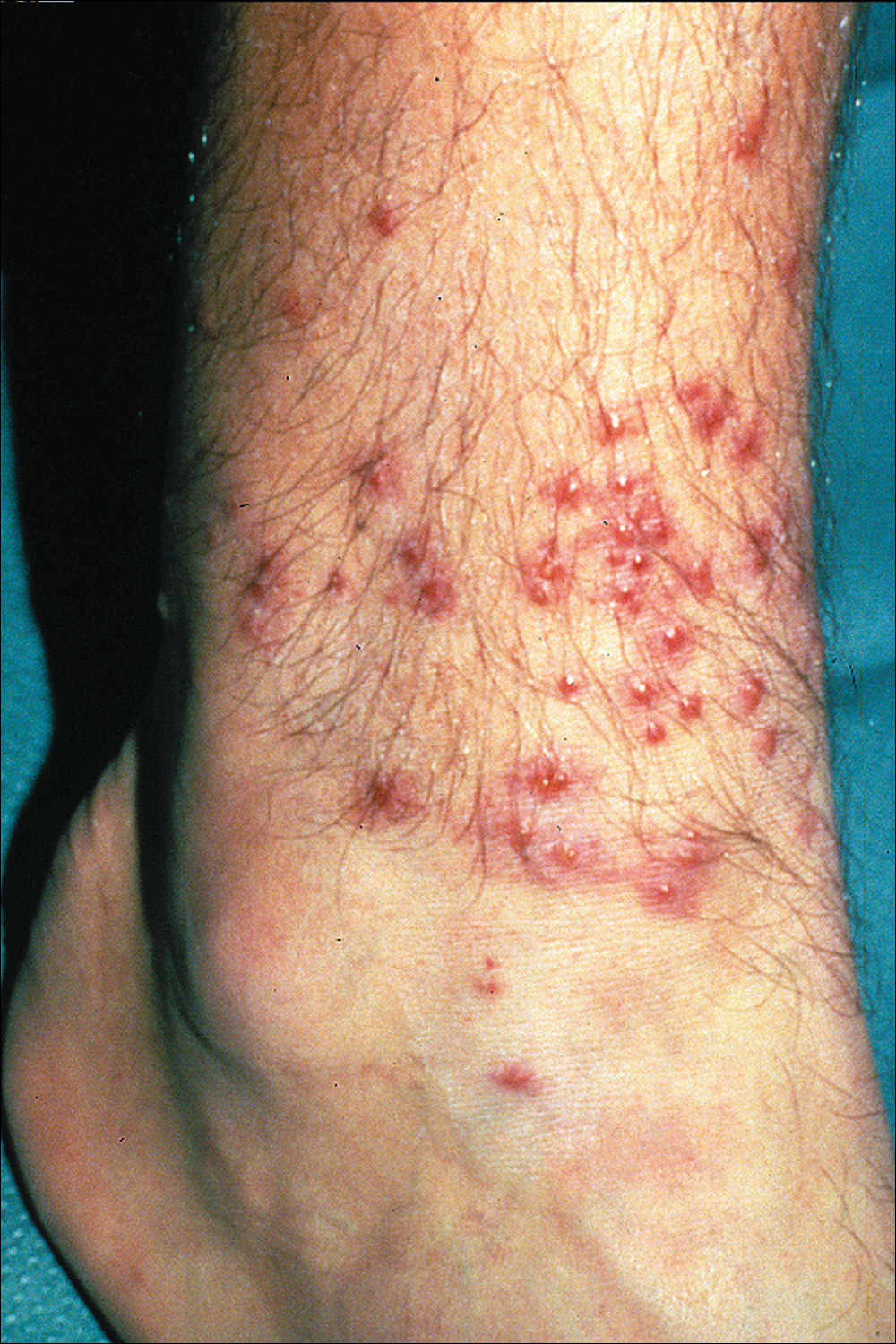
Disease Management
Because the lesions are self-healing, treatment is focused on symptomatic relief of itching by means of topical antipruritics (eg, camphor and menthol, pramoxine lotion) or oral antihistamines (eg, diphenhydramine, hydroxyzine). Potent topical corticosteroids may be used to alleviate inflammation and pruritus, especially when occluded under plastic wrap to increase absorption. In severe cases, an intralesional triamcinolone acetonide (2.5–5 mg/mL) injection may be required.9 The best practice, however, is to take preventative measures to avoid becoming a host for the mites. Patients should take special care when traveling in infested areas by completely covering their skin, tucking pant cuffs into their socks, and applying products containing DEET (N,N-diethyl-meta-toluamide or N,N-diethyl-3-methylbenzamide) to the skin and clothing. The odds of prevention are increased even further when clothing also is treated with permethrin.11
In parts of Asia and Australia, these mites may transmit Orientia tsutsugamushi, the organism responsible for scrub typhus, through their saliva during a bite.12 Scrub typhus is associated with an eschar, as well as fever, intense headache, and diffuse myalgia. It responds well to treatment with doxycycline 100 mg twice daily.13 Studies investigating genetic material found in trombiculid mites across the globe have detected Ehrlichia-specific DNA in Spain,14Borrelia-specific DNA in the Czech Republic,15,16 and Hantavirus-specific RNA in Texas.17 There is evidence that the mites play a role in maintenance of zoonotic reservoirs, while humans are infected via ingestion or inhalation of infectious rodent extreta.18
- McClain D, Dana AN, Goldenberg G. Mite infestations. Dermatol Ther. 2009;22:327-346.
- Lane RP, Crosskey RW. Medical Insects and Arachnids. London, England: Chapman & Hall; 1993.
- Gasser R, Wyniger R. Distribution and control of Trombiculidae with special reference to Trombicula autumnalis [article in German]. Acta Trop. 1955;12:308-326.
- Jones BM. The penetration of the host tissue by the harvest mite, Trombicula autumnalis Shaw. Parasitology. 1950;40:247-260.
- Farkas J. Concerning the predilected localisation of the manifestations of trombidiosis. predilected localisation and its relation to the ways of invasion [article in German]. Dermatol Monatsschr. 1979;165:858-861.
- Jones JG. Chiggers. Am Fam Physician. 1987;36:149-152.
- Shatrov AB. Stylostome formation in trombiculid mites (Acariformes: Trombiculidae). Exp Appl Acarol. 2009;49:261-280.
- Potts J. Eradication of ectoparasites in children. how to treat infestations of lice, scabies, and chiggers. Postgrad Med. 2001;110:57-59, 63-64.
- Elston DM. Arthropods and infestations. Infectious Diseases of the Skin. Boca Raton, FL; CRC Press; 2009:112-116.
- Smith GA, Sharma V, Knapp JF, et al. The summer penile syndrome: seasonal acute hypersensitivity reaction caused by chigger bites on the penis. Pediatr Emerg Care. 1998;14:116-118.
- Young GD, Evans S. Safety of DEET and permethrin in the prevention of arthropod attack. Military Med. 1998;163:324-330.
- Watt G, Parola P. Scrub typhus and tropical rickettsioses. Curr Opin Infect Dis. 2003;16:429-436.
- Panpanich R, Garner P. Antibiotics for treating scrub typhus. Cochrane Database Syst Rev. 2000;2:CD002150.
- Fernández-Soto P, Pérez-Sánchez R, Encinas-Grandes A. Molecular detection of Ehrlichia phagocytophila genogroup organisms in larvae of Neotrombicula autumnalis (Acari: Trombiculidae) captured in Spain. J Parasitol. 2001;87:1482-1483.
- Literak I, Stekolnikov AA, Sychra O, et al. Larvae of chigger mites Neotrombicula spp. (Acari: Trombiculidae) exhibited Borrelia but no Anaplasma infections: a field study including birds from the Czech Carpathians as hosts of chiggers. Exp Appl Acarol. 2008;44:307-314.
- Kampen H, Schöler A, Metzen M, et al. Neotrombicula autumnalis (Acari, Trombiculidae) as a vector for Borrelia burgdorferi sensu lato? Exp Appl Acarol. 2004;33:93-102.
- Houck MA, Qin H, Roberts HR. Hantavirus transmission: potential role of ectoparasites. Vector Borne Zoonotic Dis. 2001;1:75-79.
- Yu XJ, Tesh RB. The role of mites in the transmission and maintenance of Hantaan virus (Hantavirus: Bunyaviridae). J Infect Dis. 2014;210:1693-1699.
Identifying Characteristics and Disease Transmission
Chiggers belong to the Trombiculidae family of mites and also are referred to as harvest mites, harvest bugs, harvest lice, mower’s mites, and redbugs.1 The term chigger specifically describes the larval stage of this mite’s life cycle, as it is the only stage responsible for chigger bites. The nymph and adult phases feed on vegetable matter. Trombiculid mites are most often found in forests, grassy areas, gardens, and moist areas of soil near bodies of water. Trombicula alfreddugesi is the most common species in the United States, and these mites mainly live in the southeastern and south central regions of the country. Conversely, Trombicula autumnalis is most predominant in Western Europe and East Asia.1
The life cycle of the mite includes the egg, larval, nymphal, and adult stages.2 Due to their need for air humidity greater than 80%, mites lay their eggs on low leaves, blades of grass, or on the ground. They spend most of their lives on vegetation no more than 30 cm above ground level.3 Eggs remain dormant for approximately 6 days until the hatching of the prelarvae, which have 6 legs and are nonfeeding. It takes another 6 days for the prelarvae to mature into larvae. Measuring 0.15 to 0.3 mm in length, mite larvae are a mere fraction of the size of adult mites, which generally are 1 to 2 mm in length, and are bright red or brown-red in color (Figure 1).

The biting larvae have many acceptable hosts including turtles, toads, birds, small mammals, and humans, which act as accidental hosts. Larvae remain on vegetation waiting for a suitable host to pass by so they may attach to its skin and remain there for several days. In the exploration for an ideal area to begin feeding (eg, thin epidermis,4 localized increased air humidity5), larvae can travel extensively on the skin; however, they often are stopped by tight-fitting sections of clothing (eg, waistbands), so bites are mostly found in clusters. To feed, mite larvae latch onto the skin using chelicerae, jawlike appendages found in the front of the mouth in arachnids.6 They then inject digestive enzymes that liquefy epidermal cells on direct contact, which results in the formation of a stylostome from which the mites may suck up lymph fluid and broken down tissue.7 Although the actual initial bite is painless, this feeding process leads to the localized inflammation and irritation noticed by infested patients.8
The classic clinical presentation includes severe pruritus and cutaneous swelling as well as erythema caused by the combination of several factors, such as enzyme-induced cellular mechanical damage, human immune response, and sometimes a superimposed bacterial infection. Papules and papulovesicles appear in groups, most commonly affecting the legs and waistline (Figure 2).9 Itching generally occurs within hours of larval latching and subsides within 72 hours. Cutaneous lesions typically take 1 to 2 weeks to heal. In some rare cases, patients may react with urticarial, bullous, or morbilliform eruptions, and the inflammation and pruritus can last for weeks.6 Summer penile syndrome has been noted in boys who display a local hypersensitivity to chigger bites.10 This syndrome represents a triad of penile swelling, dysuria, and pruritus, which lasts for a few days to a few weeks.

Disease Management
Because the lesions are self-healing, treatment is focused on symptomatic relief of itching by means of topical antipruritics (eg, camphor and menthol, pramoxine lotion) or oral antihistamines (eg, diphenhydramine, hydroxyzine). Potent topical corticosteroids may be used to alleviate inflammation and pruritus, especially when occluded under plastic wrap to increase absorption. In severe cases, an intralesional triamcinolone acetonide (2.5–5 mg/mL) injection may be required.9 The best practice, however, is to take preventative measures to avoid becoming a host for the mites. Patients should take special care when traveling in infested areas by completely covering their skin, tucking pant cuffs into their socks, and applying products containing DEET (N,N-diethyl-meta-toluamide or N,N-diethyl-3-methylbenzamide) to the skin and clothing. The odds of prevention are increased even further when clothing also is treated with permethrin.11
In parts of Asia and Australia, these mites may transmit Orientia tsutsugamushi, the organism responsible for scrub typhus, through their saliva during a bite.12 Scrub typhus is associated with an eschar, as well as fever, intense headache, and diffuse myalgia. It responds well to treatment with doxycycline 100 mg twice daily.13 Studies investigating genetic material found in trombiculid mites across the globe have detected Ehrlichia-specific DNA in Spain,14Borrelia-specific DNA in the Czech Republic,15,16 and Hantavirus-specific RNA in Texas.17 There is evidence that the mites play a role in maintenance of zoonotic reservoirs, while humans are infected via ingestion or inhalation of infectious rodent extreta.18
Identifying Characteristics and Disease Transmission
Chiggers belong to the Trombiculidae family of mites and also are referred to as harvest mites, harvest bugs, harvest lice, mower’s mites, and redbugs.1 The term chigger specifically describes the larval stage of this mite’s life cycle, as it is the only stage responsible for chigger bites. The nymph and adult phases feed on vegetable matter. Trombiculid mites are most often found in forests, grassy areas, gardens, and moist areas of soil near bodies of water. Trombicula alfreddugesi is the most common species in the United States, and these mites mainly live in the southeastern and south central regions of the country. Conversely, Trombicula autumnalis is most predominant in Western Europe and East Asia.1
The life cycle of the mite includes the egg, larval, nymphal, and adult stages.2 Due to their need for air humidity greater than 80%, mites lay their eggs on low leaves, blades of grass, or on the ground. They spend most of their lives on vegetation no more than 30 cm above ground level.3 Eggs remain dormant for approximately 6 days until the hatching of the prelarvae, which have 6 legs and are nonfeeding. It takes another 6 days for the prelarvae to mature into larvae. Measuring 0.15 to 0.3 mm in length, mite larvae are a mere fraction of the size of adult mites, which generally are 1 to 2 mm in length, and are bright red or brown-red in color (Figure 1).

The biting larvae have many acceptable hosts including turtles, toads, birds, small mammals, and humans, which act as accidental hosts. Larvae remain on vegetation waiting for a suitable host to pass by so they may attach to its skin and remain there for several days. In the exploration for an ideal area to begin feeding (eg, thin epidermis,4 localized increased air humidity5), larvae can travel extensively on the skin; however, they often are stopped by tight-fitting sections of clothing (eg, waistbands), so bites are mostly found in clusters. To feed, mite larvae latch onto the skin using chelicerae, jawlike appendages found in the front of the mouth in arachnids.6 They then inject digestive enzymes that liquefy epidermal cells on direct contact, which results in the formation of a stylostome from which the mites may suck up lymph fluid and broken down tissue.7 Although the actual initial bite is painless, this feeding process leads to the localized inflammation and irritation noticed by infested patients.8
The classic clinical presentation includes severe pruritus and cutaneous swelling as well as erythema caused by the combination of several factors, such as enzyme-induced cellular mechanical damage, human immune response, and sometimes a superimposed bacterial infection. Papules and papulovesicles appear in groups, most commonly affecting the legs and waistline (Figure 2).9 Itching generally occurs within hours of larval latching and subsides within 72 hours. Cutaneous lesions typically take 1 to 2 weeks to heal. In some rare cases, patients may react with urticarial, bullous, or morbilliform eruptions, and the inflammation and pruritus can last for weeks.6 Summer penile syndrome has been noted in boys who display a local hypersensitivity to chigger bites.10 This syndrome represents a triad of penile swelling, dysuria, and pruritus, which lasts for a few days to a few weeks.

Disease Management
Because the lesions are self-healing, treatment is focused on symptomatic relief of itching by means of topical antipruritics (eg, camphor and menthol, pramoxine lotion) or oral antihistamines (eg, diphenhydramine, hydroxyzine). Potent topical corticosteroids may be used to alleviate inflammation and pruritus, especially when occluded under plastic wrap to increase absorption. In severe cases, an intralesional triamcinolone acetonide (2.5–5 mg/mL) injection may be required.9 The best practice, however, is to take preventative measures to avoid becoming a host for the mites. Patients should take special care when traveling in infested areas by completely covering their skin, tucking pant cuffs into their socks, and applying products containing DEET (N,N-diethyl-meta-toluamide or N,N-diethyl-3-methylbenzamide) to the skin and clothing. The odds of prevention are increased even further when clothing also is treated with permethrin.11
In parts of Asia and Australia, these mites may transmit Orientia tsutsugamushi, the organism responsible for scrub typhus, through their saliva during a bite.12 Scrub typhus is associated with an eschar, as well as fever, intense headache, and diffuse myalgia. It responds well to treatment with doxycycline 100 mg twice daily.13 Studies investigating genetic material found in trombiculid mites across the globe have detected Ehrlichia-specific DNA in Spain,14Borrelia-specific DNA in the Czech Republic,15,16 and Hantavirus-specific RNA in Texas.17 There is evidence that the mites play a role in maintenance of zoonotic reservoirs, while humans are infected via ingestion or inhalation of infectious rodent extreta.18
- McClain D, Dana AN, Goldenberg G. Mite infestations. Dermatol Ther. 2009;22:327-346.
- Lane RP, Crosskey RW. Medical Insects and Arachnids. London, England: Chapman & Hall; 1993.
- Gasser R, Wyniger R. Distribution and control of Trombiculidae with special reference to Trombicula autumnalis [article in German]. Acta Trop. 1955;12:308-326.
- Jones BM. The penetration of the host tissue by the harvest mite, Trombicula autumnalis Shaw. Parasitology. 1950;40:247-260.
- Farkas J. Concerning the predilected localisation of the manifestations of trombidiosis. predilected localisation and its relation to the ways of invasion [article in German]. Dermatol Monatsschr. 1979;165:858-861.
- Jones JG. Chiggers. Am Fam Physician. 1987;36:149-152.
- Shatrov AB. Stylostome formation in trombiculid mites (Acariformes: Trombiculidae). Exp Appl Acarol. 2009;49:261-280.
- Potts J. Eradication of ectoparasites in children. how to treat infestations of lice, scabies, and chiggers. Postgrad Med. 2001;110:57-59, 63-64.
- Elston DM. Arthropods and infestations. Infectious Diseases of the Skin. Boca Raton, FL; CRC Press; 2009:112-116.
- Smith GA, Sharma V, Knapp JF, et al. The summer penile syndrome: seasonal acute hypersensitivity reaction caused by chigger bites on the penis. Pediatr Emerg Care. 1998;14:116-118.
- Young GD, Evans S. Safety of DEET and permethrin in the prevention of arthropod attack. Military Med. 1998;163:324-330.
- Watt G, Parola P. Scrub typhus and tropical rickettsioses. Curr Opin Infect Dis. 2003;16:429-436.
- Panpanich R, Garner P. Antibiotics for treating scrub typhus. Cochrane Database Syst Rev. 2000;2:CD002150.
- Fernández-Soto P, Pérez-Sánchez R, Encinas-Grandes A. Molecular detection of Ehrlichia phagocytophila genogroup organisms in larvae of Neotrombicula autumnalis (Acari: Trombiculidae) captured in Spain. J Parasitol. 2001;87:1482-1483.
- Literak I, Stekolnikov AA, Sychra O, et al. Larvae of chigger mites Neotrombicula spp. (Acari: Trombiculidae) exhibited Borrelia but no Anaplasma infections: a field study including birds from the Czech Carpathians as hosts of chiggers. Exp Appl Acarol. 2008;44:307-314.
- Kampen H, Schöler A, Metzen M, et al. Neotrombicula autumnalis (Acari, Trombiculidae) as a vector for Borrelia burgdorferi sensu lato? Exp Appl Acarol. 2004;33:93-102.
- Houck MA, Qin H, Roberts HR. Hantavirus transmission: potential role of ectoparasites. Vector Borne Zoonotic Dis. 2001;1:75-79.
- Yu XJ, Tesh RB. The role of mites in the transmission and maintenance of Hantaan virus (Hantavirus: Bunyaviridae). J Infect Dis. 2014;210:1693-1699.
- McClain D, Dana AN, Goldenberg G. Mite infestations. Dermatol Ther. 2009;22:327-346.
- Lane RP, Crosskey RW. Medical Insects and Arachnids. London, England: Chapman & Hall; 1993.
- Gasser R, Wyniger R. Distribution and control of Trombiculidae with special reference to Trombicula autumnalis [article in German]. Acta Trop. 1955;12:308-326.
- Jones BM. The penetration of the host tissue by the harvest mite, Trombicula autumnalis Shaw. Parasitology. 1950;40:247-260.
- Farkas J. Concerning the predilected localisation of the manifestations of trombidiosis. predilected localisation and its relation to the ways of invasion [article in German]. Dermatol Monatsschr. 1979;165:858-861.
- Jones JG. Chiggers. Am Fam Physician. 1987;36:149-152.
- Shatrov AB. Stylostome formation in trombiculid mites (Acariformes: Trombiculidae). Exp Appl Acarol. 2009;49:261-280.
- Potts J. Eradication of ectoparasites in children. how to treat infestations of lice, scabies, and chiggers. Postgrad Med. 2001;110:57-59, 63-64.
- Elston DM. Arthropods and infestations. Infectious Diseases of the Skin. Boca Raton, FL; CRC Press; 2009:112-116.
- Smith GA, Sharma V, Knapp JF, et al. The summer penile syndrome: seasonal acute hypersensitivity reaction caused by chigger bites on the penis. Pediatr Emerg Care. 1998;14:116-118.
- Young GD, Evans S. Safety of DEET and permethrin in the prevention of arthropod attack. Military Med. 1998;163:324-330.
- Watt G, Parola P. Scrub typhus and tropical rickettsioses. Curr Opin Infect Dis. 2003;16:429-436.
- Panpanich R, Garner P. Antibiotics for treating scrub typhus. Cochrane Database Syst Rev. 2000;2:CD002150.
- Fernández-Soto P, Pérez-Sánchez R, Encinas-Grandes A. Molecular detection of Ehrlichia phagocytophila genogroup organisms in larvae of Neotrombicula autumnalis (Acari: Trombiculidae) captured in Spain. J Parasitol. 2001;87:1482-1483.
- Literak I, Stekolnikov AA, Sychra O, et al. Larvae of chigger mites Neotrombicula spp. (Acari: Trombiculidae) exhibited Borrelia but no Anaplasma infections: a field study including birds from the Czech Carpathians as hosts of chiggers. Exp Appl Acarol. 2008;44:307-314.
- Kampen H, Schöler A, Metzen M, et al. Neotrombicula autumnalis (Acari, Trombiculidae) as a vector for Borrelia burgdorferi sensu lato? Exp Appl Acarol. 2004;33:93-102.
- Houck MA, Qin H, Roberts HR. Hantavirus transmission: potential role of ectoparasites. Vector Borne Zoonotic Dis. 2001;1:75-79.
- Yu XJ, Tesh RB. The role of mites in the transmission and maintenance of Hantaan virus (Hantavirus: Bunyaviridae). J Infect Dis. 2014;210:1693-1699.
Practice Points
- The classic clinical presentation of chigger bites includes severe pruritus, cutaneous swelling, and erythematous papules and papulovesicles appearing in groups, most commonly affecting the legs and waistline.
- Because itching generally subsides within 72 hours of the chigger bite and cutaneous lesions typically heal within 1 to 2 weeks, treatment is focused on symptomatic relief.
- Symptomatic relief may be achieved by means of topical antipruritics or oral antihistamines as well as potent topical corticosteroids or an intralesional triamcinolone acetonide injection in severe cases.
What’s Eating You? Cheyletiella Mites
Identifying Characteristics and Disease Transmission
Cheyletiella are nonburrowing mites characterized by hooklike anterior palps (Figure 1) that have a worldwide distribution. Human dermatitis is the result of contact with an affected animal and may present as papular or bullous lesions. Cheyletiella blakei affects cats, Cheyletiella parasitovorax is found on rabbits, and Cheyletiella yasguri is found on dogs. The mites live in the outer layer of the epidermis of the host animal and feed on surface debris and tissue fluids.1 They complete an entire 35-day life cycle on a single animal host. The larval, nymph, and adult male mites die within 48 hours of separation from a host. The female mite and possibly the eggs can live up to 10 days off the host, which makes environmental decontamination a critical part of pest control.2 In animals, the mite often produces a subtle dermatitis sometimes called walking dandruff (Figure 2).3 Affected animals also can be asymptomatic, and up to 50% of rabbits in commercial colonies may harbor Cheyletiella or other mites.4
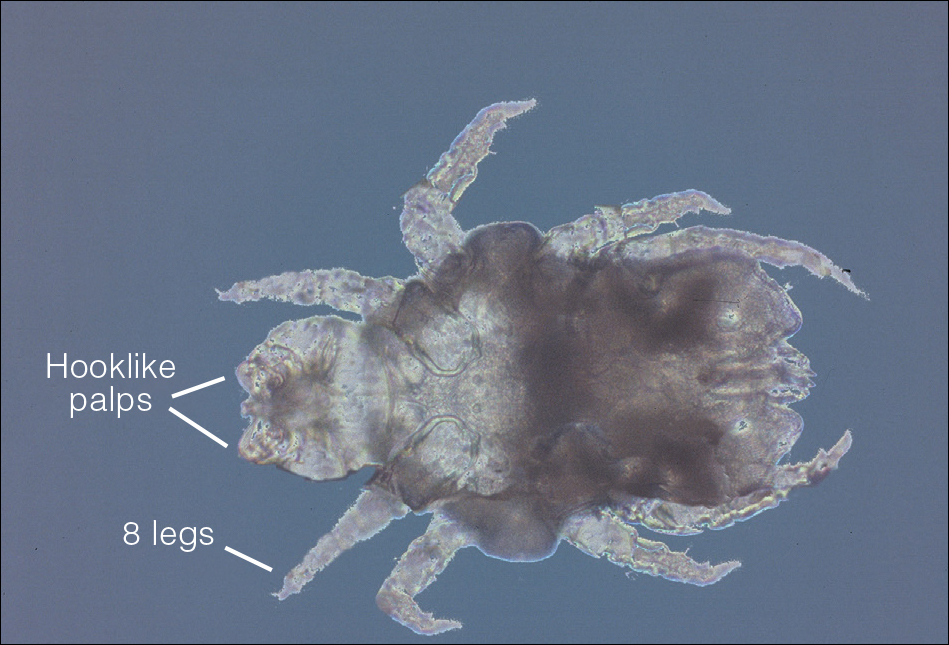
The typical human patient with Cheyletiella-associated dermatitis is a female 40 years or younger who presents with grouped pruritic papules.5 Although papules usually are grouped on exposed areas, they also may be widespread.6,7 Bullous eruptions caused by Cheyletiella mites may mimic those found in immunobullous diseases (Figure 3).8 Children may experience widespread dermatitis after taking a nap where a dog has slept.9 Pet owners, farmers, and veterinarians frequently present with zoonotic mite-induced dermatitis.10 Arthralgia and peripheral eosinophilia caused by Cheyletiella infestation also has been reported.11
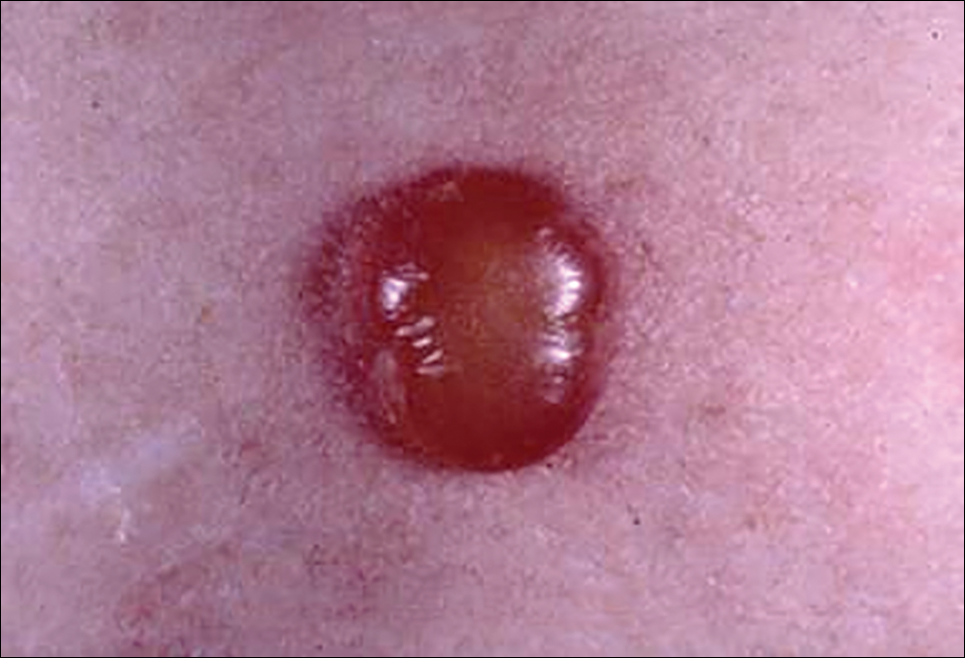
Management of Affected Pets
In a case of human infestation resulting from an affected pet, the implicated pet should be evaluated by a qualified veterinarian. Various diagnostic techniques for animals have been used, including adhesive tape preparations.12 A rapid knockdown insecticidal spray marketed for use on animals has been used to facilitate collection of mites, but some pets may be susceptible to toxicity from insecticides. The scaly area should be carefully brushed with a toothbrush or fine-tooth comb, and all scales, crust, and hair collected should be placed in a resealable plastic storage bag. When alcohol is added to the bag, most contents will sink, but the mites tend to float. Vacuum cleaners fitted with in-line filters also have been used to collect mites. The filter samples can be treated with hot potassium hydroxide, then floated in a concentrated sugar solution to collect the ectoparasites.13 Often, a straightforward approach using a #10 blade to provide a skin scraping from the animal in question is effective.14
Various treatment modalities may be employed by the veterinarian, including dips or shampoos, as well as fipronil.15,16 A single application of fipronil 10% has been shown to be highly effective in the elimination of mites after a single application in cats.17 Oral ivermectin and topical amitraz also have been used.18,19 A veterinarian should treat the animals, as some are more susceptible to toxicity from topical or systemic agents.
Treatment in Humans
Cheyletiella infestations in humans usually are self-limited and resolve within a few weeks after treatment of the source animal. Symptomatic treatment with antipruritic medications and topical steroids may be of use while awaiting resolution. Identification and treatment of the vector is key to eliminating the infestation and preventing recurrence.
- Angarano DW, Parish LC. Comparative dermatology: parasitic disorders. Clin Dermatol. 1994;12:543-550.
- Kunkle GA, Miller WH Jr. Cheyletiella infestation in humans. Arch Dermatol. 1980;116:1345.
- Rivers JK, Martin J, Pukay B. Walking dandruff and Cheyletiella dermatitis. J Am Acad Dermatol. 1986;15:1130-1133.
- Flatt RE, Wiemers J. A survey of fur mites in domestic rabbits. Lab Animal Sci. 1976;26:758-761.
- Lee BW. Cheyletiella dermatitis: a report of fourteen cases. Cutis. 1991;47:111-114.
- Cohen SR. Cheyletiella dermatitis. A mite infestation of rabbit, cat, dog and man. Arch Dermatol. 1980;116:435-437.
- Bradrup F, Andersen KE, Kristensen S. Infection in man and dog with the mite, Cheyletiella yasguri Smiley [in German]. Hautarzt. 1979;30:497-500.
- Cvancara JL, Elston DM. Bullous eruption in a patient with systemic lupus erythematosus: mite dermatitis caused by Cheyletiella blakei. J Am Acad Dermatol. 1997;37:265-267.
- Shelley ED, Shelley WB, Pula JF, et al. The diagnostic challenge of nonburrowing mite bites. Cheyletiella yasguri. JAMA. 1984;251:2690-2691.
- Beck W. Farm animals as disease vectors of parasitic epizoonoses and zoophilic dermatophytes and their importance in dermatology [in German]. Hautartz. 1999;50:621-628.
- Dobrosavljevic DD, Popovic ND, Radovanovic SS. Systemic manifestations of Cheyletiella infestation in man. Int J Dermatol. 2007;46:397-399.
- Ottenschot TR, Gil D. Cheyletiellosis in long-haired cats. Tijdschr Diergeneeskd. 1978;103:1104-1108.
- Klayman E, Schillhorn van Veen TW. Diagnosis of ectoparasitism. Mod Vet Pract. 1981;62:767-771.
- Milley C, Dryden M, Rosenkrantz W, et al. Comparison of parasitic mite retrieval methods in a population of community cats [published online Jun 3, 2016]. J Feline Med Surg. pii:1098612X16650717.
- McKeever PJ, Allen SK. Dermatitis associated with Cheyletiella infestation in cats. J Am Vet Med Assoc. 1979;174:718-720.
- Chadwick AJ. Use of a 0.25 per cent fipronil pump spray formulation to treat canine cheyletiellosis. J Small Anim Pract. 1997;38:261-262.
- Scarampella F, Pollmeier M, Visser M, et al. Efficacy of fipronil in the treatment of feline cheyletiellosis. Vet Parasitol. 2005;129:333-339.
- Folz SD, Kakuk TJ, Henke CL, et al. Clinical evaluation of amitraz for treatment of canine scabies. Mod Vet Pract. 1984;65:597-600.
- Dourmishev AL, Dourmishev LA, Schwartz RA. Ivermectin: pharmacology and application in dermatology. Int J Dermatol. 2005;44:981-988.
Identifying Characteristics and Disease Transmission
Cheyletiella are nonburrowing mites characterized by hooklike anterior palps (Figure 1) that have a worldwide distribution. Human dermatitis is the result of contact with an affected animal and may present as papular or bullous lesions. Cheyletiella blakei affects cats, Cheyletiella parasitovorax is found on rabbits, and Cheyletiella yasguri is found on dogs. The mites live in the outer layer of the epidermis of the host animal and feed on surface debris and tissue fluids.1 They complete an entire 35-day life cycle on a single animal host. The larval, nymph, and adult male mites die within 48 hours of separation from a host. The female mite and possibly the eggs can live up to 10 days off the host, which makes environmental decontamination a critical part of pest control.2 In animals, the mite often produces a subtle dermatitis sometimes called walking dandruff (Figure 2).3 Affected animals also can be asymptomatic, and up to 50% of rabbits in commercial colonies may harbor Cheyletiella or other mites.4


The typical human patient with Cheyletiella-associated dermatitis is a female 40 years or younger who presents with grouped pruritic papules.5 Although papules usually are grouped on exposed areas, they also may be widespread.6,7 Bullous eruptions caused by Cheyletiella mites may mimic those found in immunobullous diseases (Figure 3).8 Children may experience widespread dermatitis after taking a nap where a dog has slept.9 Pet owners, farmers, and veterinarians frequently present with zoonotic mite-induced dermatitis.10 Arthralgia and peripheral eosinophilia caused by Cheyletiella infestation also has been reported.11

Management of Affected Pets
In a case of human infestation resulting from an affected pet, the implicated pet should be evaluated by a qualified veterinarian. Various diagnostic techniques for animals have been used, including adhesive tape preparations.12 A rapid knockdown insecticidal spray marketed for use on animals has been used to facilitate collection of mites, but some pets may be susceptible to toxicity from insecticides. The scaly area should be carefully brushed with a toothbrush or fine-tooth comb, and all scales, crust, and hair collected should be placed in a resealable plastic storage bag. When alcohol is added to the bag, most contents will sink, but the mites tend to float. Vacuum cleaners fitted with in-line filters also have been used to collect mites. The filter samples can be treated with hot potassium hydroxide, then floated in a concentrated sugar solution to collect the ectoparasites.13 Often, a straightforward approach using a #10 blade to provide a skin scraping from the animal in question is effective.14
Various treatment modalities may be employed by the veterinarian, including dips or shampoos, as well as fipronil.15,16 A single application of fipronil 10% has been shown to be highly effective in the elimination of mites after a single application in cats.17 Oral ivermectin and topical amitraz also have been used.18,19 A veterinarian should treat the animals, as some are more susceptible to toxicity from topical or systemic agents.
Treatment in Humans
Cheyletiella infestations in humans usually are self-limited and resolve within a few weeks after treatment of the source animal. Symptomatic treatment with antipruritic medications and topical steroids may be of use while awaiting resolution. Identification and treatment of the vector is key to eliminating the infestation and preventing recurrence.
Identifying Characteristics and Disease Transmission
Cheyletiella are nonburrowing mites characterized by hooklike anterior palps (Figure 1) that have a worldwide distribution. Human dermatitis is the result of contact with an affected animal and may present as papular or bullous lesions. Cheyletiella blakei affects cats, Cheyletiella parasitovorax is found on rabbits, and Cheyletiella yasguri is found on dogs. The mites live in the outer layer of the epidermis of the host animal and feed on surface debris and tissue fluids.1 They complete an entire 35-day life cycle on a single animal host. The larval, nymph, and adult male mites die within 48 hours of separation from a host. The female mite and possibly the eggs can live up to 10 days off the host, which makes environmental decontamination a critical part of pest control.2 In animals, the mite often produces a subtle dermatitis sometimes called walking dandruff (Figure 2).3 Affected animals also can be asymptomatic, and up to 50% of rabbits in commercial colonies may harbor Cheyletiella or other mites.4


The typical human patient with Cheyletiella-associated dermatitis is a female 40 years or younger who presents with grouped pruritic papules.5 Although papules usually are grouped on exposed areas, they also may be widespread.6,7 Bullous eruptions caused by Cheyletiella mites may mimic those found in immunobullous diseases (Figure 3).8 Children may experience widespread dermatitis after taking a nap where a dog has slept.9 Pet owners, farmers, and veterinarians frequently present with zoonotic mite-induced dermatitis.10 Arthralgia and peripheral eosinophilia caused by Cheyletiella infestation also has been reported.11

Management of Affected Pets
In a case of human infestation resulting from an affected pet, the implicated pet should be evaluated by a qualified veterinarian. Various diagnostic techniques for animals have been used, including adhesive tape preparations.12 A rapid knockdown insecticidal spray marketed for use on animals has been used to facilitate collection of mites, but some pets may be susceptible to toxicity from insecticides. The scaly area should be carefully brushed with a toothbrush or fine-tooth comb, and all scales, crust, and hair collected should be placed in a resealable plastic storage bag. When alcohol is added to the bag, most contents will sink, but the mites tend to float. Vacuum cleaners fitted with in-line filters also have been used to collect mites. The filter samples can be treated with hot potassium hydroxide, then floated in a concentrated sugar solution to collect the ectoparasites.13 Often, a straightforward approach using a #10 blade to provide a skin scraping from the animal in question is effective.14
Various treatment modalities may be employed by the veterinarian, including dips or shampoos, as well as fipronil.15,16 A single application of fipronil 10% has been shown to be highly effective in the elimination of mites after a single application in cats.17 Oral ivermectin and topical amitraz also have been used.18,19 A veterinarian should treat the animals, as some are more susceptible to toxicity from topical or systemic agents.
Treatment in Humans
Cheyletiella infestations in humans usually are self-limited and resolve within a few weeks after treatment of the source animal. Symptomatic treatment with antipruritic medications and topical steroids may be of use while awaiting resolution. Identification and treatment of the vector is key to eliminating the infestation and preventing recurrence.
- Angarano DW, Parish LC. Comparative dermatology: parasitic disorders. Clin Dermatol. 1994;12:543-550.
- Kunkle GA, Miller WH Jr. Cheyletiella infestation in humans. Arch Dermatol. 1980;116:1345.
- Rivers JK, Martin J, Pukay B. Walking dandruff and Cheyletiella dermatitis. J Am Acad Dermatol. 1986;15:1130-1133.
- Flatt RE, Wiemers J. A survey of fur mites in domestic rabbits. Lab Animal Sci. 1976;26:758-761.
- Lee BW. Cheyletiella dermatitis: a report of fourteen cases. Cutis. 1991;47:111-114.
- Cohen SR. Cheyletiella dermatitis. A mite infestation of rabbit, cat, dog and man. Arch Dermatol. 1980;116:435-437.
- Bradrup F, Andersen KE, Kristensen S. Infection in man and dog with the mite, Cheyletiella yasguri Smiley [in German]. Hautarzt. 1979;30:497-500.
- Cvancara JL, Elston DM. Bullous eruption in a patient with systemic lupus erythematosus: mite dermatitis caused by Cheyletiella blakei. J Am Acad Dermatol. 1997;37:265-267.
- Shelley ED, Shelley WB, Pula JF, et al. The diagnostic challenge of nonburrowing mite bites. Cheyletiella yasguri. JAMA. 1984;251:2690-2691.
- Beck W. Farm animals as disease vectors of parasitic epizoonoses and zoophilic dermatophytes and their importance in dermatology [in German]. Hautartz. 1999;50:621-628.
- Dobrosavljevic DD, Popovic ND, Radovanovic SS. Systemic manifestations of Cheyletiella infestation in man. Int J Dermatol. 2007;46:397-399.
- Ottenschot TR, Gil D. Cheyletiellosis in long-haired cats. Tijdschr Diergeneeskd. 1978;103:1104-1108.
- Klayman E, Schillhorn van Veen TW. Diagnosis of ectoparasitism. Mod Vet Pract. 1981;62:767-771.
- Milley C, Dryden M, Rosenkrantz W, et al. Comparison of parasitic mite retrieval methods in a population of community cats [published online Jun 3, 2016]. J Feline Med Surg. pii:1098612X16650717.
- McKeever PJ, Allen SK. Dermatitis associated with Cheyletiella infestation in cats. J Am Vet Med Assoc. 1979;174:718-720.
- Chadwick AJ. Use of a 0.25 per cent fipronil pump spray formulation to treat canine cheyletiellosis. J Small Anim Pract. 1997;38:261-262.
- Scarampella F, Pollmeier M, Visser M, et al. Efficacy of fipronil in the treatment of feline cheyletiellosis. Vet Parasitol. 2005;129:333-339.
- Folz SD, Kakuk TJ, Henke CL, et al. Clinical evaluation of amitraz for treatment of canine scabies. Mod Vet Pract. 1984;65:597-600.
- Dourmishev AL, Dourmishev LA, Schwartz RA. Ivermectin: pharmacology and application in dermatology. Int J Dermatol. 2005;44:981-988.
- Angarano DW, Parish LC. Comparative dermatology: parasitic disorders. Clin Dermatol. 1994;12:543-550.
- Kunkle GA, Miller WH Jr. Cheyletiella infestation in humans. Arch Dermatol. 1980;116:1345.
- Rivers JK, Martin J, Pukay B. Walking dandruff and Cheyletiella dermatitis. J Am Acad Dermatol. 1986;15:1130-1133.
- Flatt RE, Wiemers J. A survey of fur mites in domestic rabbits. Lab Animal Sci. 1976;26:758-761.
- Lee BW. Cheyletiella dermatitis: a report of fourteen cases. Cutis. 1991;47:111-114.
- Cohen SR. Cheyletiella dermatitis. A mite infestation of rabbit, cat, dog and man. Arch Dermatol. 1980;116:435-437.
- Bradrup F, Andersen KE, Kristensen S. Infection in man and dog with the mite, Cheyletiella yasguri Smiley [in German]. Hautarzt. 1979;30:497-500.
- Cvancara JL, Elston DM. Bullous eruption in a patient with systemic lupus erythematosus: mite dermatitis caused by Cheyletiella blakei. J Am Acad Dermatol. 1997;37:265-267.
- Shelley ED, Shelley WB, Pula JF, et al. The diagnostic challenge of nonburrowing mite bites. Cheyletiella yasguri. JAMA. 1984;251:2690-2691.
- Beck W. Farm animals as disease vectors of parasitic epizoonoses and zoophilic dermatophytes and their importance in dermatology [in German]. Hautartz. 1999;50:621-628.
- Dobrosavljevic DD, Popovic ND, Radovanovic SS. Systemic manifestations of Cheyletiella infestation in man. Int J Dermatol. 2007;46:397-399.
- Ottenschot TR, Gil D. Cheyletiellosis in long-haired cats. Tijdschr Diergeneeskd. 1978;103:1104-1108.
- Klayman E, Schillhorn van Veen TW. Diagnosis of ectoparasitism. Mod Vet Pract. 1981;62:767-771.
- Milley C, Dryden M, Rosenkrantz W, et al. Comparison of parasitic mite retrieval methods in a population of community cats [published online Jun 3, 2016]. J Feline Med Surg. pii:1098612X16650717.
- McKeever PJ, Allen SK. Dermatitis associated with Cheyletiella infestation in cats. J Am Vet Med Assoc. 1979;174:718-720.
- Chadwick AJ. Use of a 0.25 per cent fipronil pump spray formulation to treat canine cheyletiellosis. J Small Anim Pract. 1997;38:261-262.
- Scarampella F, Pollmeier M, Visser M, et al. Efficacy of fipronil in the treatment of feline cheyletiellosis. Vet Parasitol. 2005;129:333-339.
- Folz SD, Kakuk TJ, Henke CL, et al. Clinical evaluation of amitraz for treatment of canine scabies. Mod Vet Pract. 1984;65:597-600.
- Dourmishev AL, Dourmishev LA, Schwartz RA. Ivermectin: pharmacology and application in dermatology. Int J Dermatol. 2005;44:981-988.
Practice Points
- Cheyletiella mites can cause a range of cutaneous and systemic symptoms in affected individuals.
- Diagnosis can be difficult and requires a high level of suspicion, with inquiries directed at animal exposures.
- Identification of the animal vector and treatment by a knowledgeable veterinarian is necessary to prevent recurrence in humans.