User login
Erectile dysfunction drugs linked to ocular conditions
, researchers say.
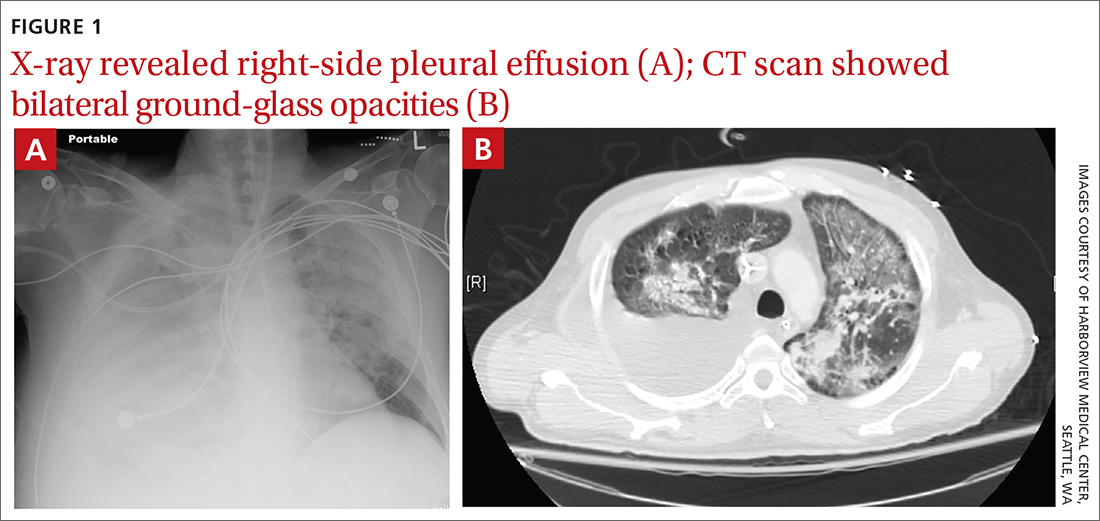
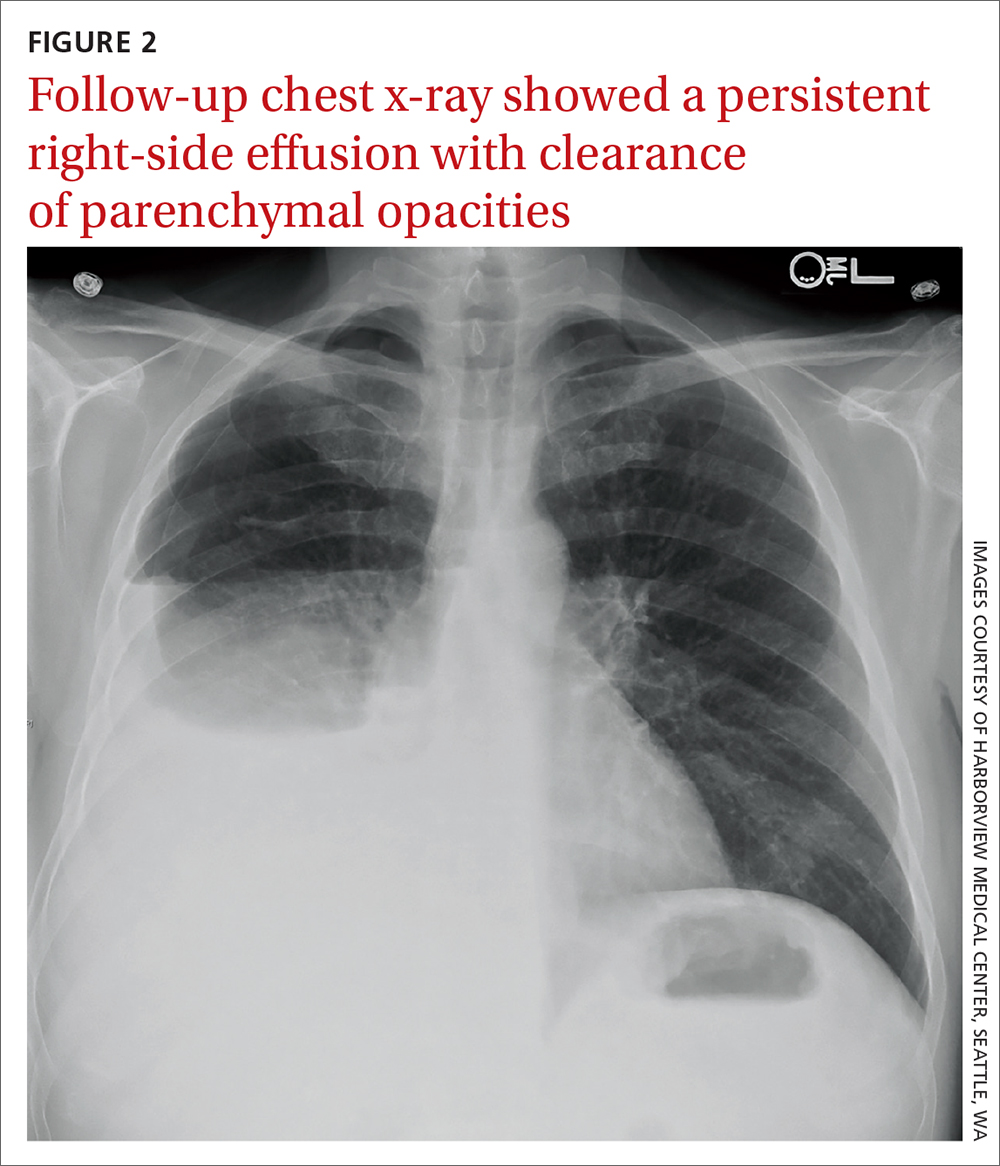
Patients in an insurance database who were prescribed sildenafil (Viagra), tadalafil (Cialis), vardenafil (Levitra), or avanafil (Stendra) were almost twice as likely as were patients not prescribed the drugs to have ischemic optic neuropathy, retinal vascular occlusion, or serous retinal detachment.
In 2020, physicians wrote about 20 million monthly prescriptions for PDE5Is in the United States alone, said Mahyar Etminan, PharmD, associate professor of ophthalmology at the University of British Columbia, Vancouver.
“We don’t want to alarm people taking them, but generally speaking, if they experience visual problems or changes in vision, then these drugs may be the culprits, and they should check it out,” he said in an interview.
The study was published in JAMA Ophthalmology.
Previous reports, including postmarketing studies by the drug makers, have documented ocular events. The monographs for sildenafil, tadalafil, vardenafil, and avanafil warn users about ischemic optic neuropathy, the researchers found.
The monographs for sildenafil, tadalafil, and vardenafil list retinal vascular occlusion as a potential adverse event but do not quantify the risk. None of the drug monographs mention serous retinal detachment.
Previous research has associated PDE5Is with compromised perfusion of the optic nerve. Some researchers have speculated that the choroid blood vessels can undergo smooth muscle relaxation through a cyclic guanosine monophosphate pathway that can lead to choroidal congestion.
To get a better handle on the ocular risks of PDE51s, Dr. Etminan and his colleagues analyzed health insurance claim records from the PharMetrics Plus database of 213,033 men who had not experienced any of the three ocular conditions in the year before they became regular users of the medications.
They identified 1,146 patients who had been diagnosed with at least one of the three conditions.
The overall number of conditions diagnosed was small relative to the size of the population, 15.5 cases per 10,000 person-years. “So that’s still relatively rare, but the problem is that these are very heavily used medications,” Dr. Etminan said.
For each man diagnosed with one of the ocular conditions, the researchers matched four control persons who were the same age and could be followed for the same length of time. There was a total of 4,584 control persons.
The researchers compared regular users of PDE5Is (those who had received at least one prescription for a PDE5I every 3 months in the year before the ocular diagnosis) with nonusers (those who had not received a PDE5I prescription during that time).
Patients with the ocular conditions were more likely than were those in the control group to have hypertension, diabetes, cardiovascular disease, or sleep apnea. After controlling for these covariates, the researchers found that the users were overall 85% more likely to be diagnosed with one or more of them (incidence rate ratio [IRR], 1.85).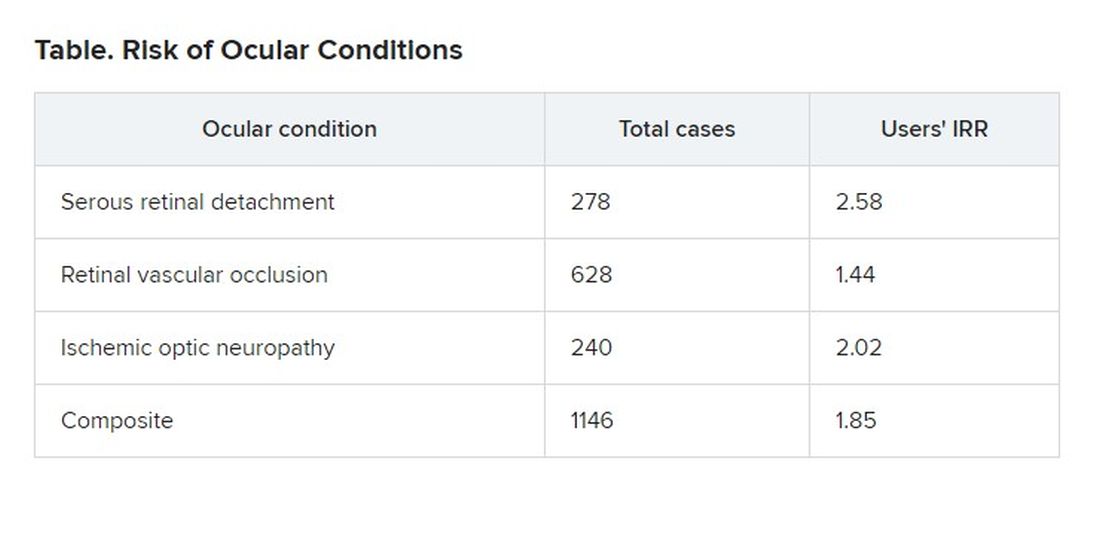
The researchers also found that the risk was even greater for those patients who were given five or more prescriptions of PDE5Is, compared to those given fewer than five prescriptions, suggesting a dose response.
On the basis of these findings, Dr. Etminan thinks drug companies should add warnings about serous retinal detachment and retinal vascular occlusion to the drug monographs.
Asked to comment, Pfizer, which developed Viagra, referred questions to its spinoff company, Viatris, which did not respond. Eli Lilly, which makes Cialis, also did not respond to a request for comment. Vivus, which makes Stendra, could not be reached by press time.
Bayer, which makes Levitra, declined to provide anyone who could answer questions, but it provided a statement noting that the occurrence of ocular adverse events is already known among PDE5I users and that retinal vascular occlusion and ischemic optic neuropathy are mentioned in the product information.
“For example, non-arteritic anterior ischemic optic neuropathy (NAION) is a very rare condition which occurs with an overall higher risk in the population usually suffering from erectile dysfunction (ED) – that is, elderly men with concomitant diseases such as diabetes, dyslipidemia, and hypertension – compared to the general population,” the statement said.
Because of the retrospective nature of the analysis, Dr. Etminan acknowledged that researchers could not prove that the increased risk of ocular disease was associated with use of the drugs rather than some underlying condition. But in addition to adjusting for known risk factors, they also separately analyzed men without hypertension, diabetes, or coronary artery disease and still found that the risk of the ocular conditions was roughly double for men with PDE5I prescriptions.
Howard Pomeranz, MD, PhD, professor of ophthalmology at Northwell Health in Great Neck, N.Y., who was not involved in this study, said its findings confirmed similar research that he conducted on ischemic optic neuropathy.
He told this news organization that people taking PDE5Is should weigh the risk against the benefit, but added that the calculation might be different for people who use them to treat pulmonary hypertension rather than erectile dysfunction.
Although people taking the drugs should discuss any changes in their vision with their practitioners, he said they should not be concerned about a “bluish type of tint to the vision that may occur transiently for anywhere from a few minutes up to 40 or 45 minutes.”
Drug companies and regulators should consider changing the monographs in light of this new evidence, Dr. Pomeranz said. “Perhaps this data might drive the warning to be perhaps a little bit stronger, now that there’s more data to suggest maybe a bit of a stronger association and not just some chance association between using these drugs and these visual events.”
The study was funded by the University of British Columbia. Dr. Etminan and Dr. Pomeranz have disclosed no relevant financial interests.
A version of this article first appeared on Medscape.com
, researchers say.
Patients in an insurance database who were prescribed sildenafil (Viagra), tadalafil (Cialis), vardenafil (Levitra), or avanafil (Stendra) were almost twice as likely as were patients not prescribed the drugs to have ischemic optic neuropathy, retinal vascular occlusion, or serous retinal detachment.
In 2020, physicians wrote about 20 million monthly prescriptions for PDE5Is in the United States alone, said Mahyar Etminan, PharmD, associate professor of ophthalmology at the University of British Columbia, Vancouver.
“We don’t want to alarm people taking them, but generally speaking, if they experience visual problems or changes in vision, then these drugs may be the culprits, and they should check it out,” he said in an interview.
The study was published in JAMA Ophthalmology.
Previous reports, including postmarketing studies by the drug makers, have documented ocular events. The monographs for sildenafil, tadalafil, vardenafil, and avanafil warn users about ischemic optic neuropathy, the researchers found.
The monographs for sildenafil, tadalafil, and vardenafil list retinal vascular occlusion as a potential adverse event but do not quantify the risk. None of the drug monographs mention serous retinal detachment.
Previous research has associated PDE5Is with compromised perfusion of the optic nerve. Some researchers have speculated that the choroid blood vessels can undergo smooth muscle relaxation through a cyclic guanosine monophosphate pathway that can lead to choroidal congestion.
To get a better handle on the ocular risks of PDE51s, Dr. Etminan and his colleagues analyzed health insurance claim records from the PharMetrics Plus database of 213,033 men who had not experienced any of the three ocular conditions in the year before they became regular users of the medications.
They identified 1,146 patients who had been diagnosed with at least one of the three conditions.
The overall number of conditions diagnosed was small relative to the size of the population, 15.5 cases per 10,000 person-years. “So that’s still relatively rare, but the problem is that these are very heavily used medications,” Dr. Etminan said.
For each man diagnosed with one of the ocular conditions, the researchers matched four control persons who were the same age and could be followed for the same length of time. There was a total of 4,584 control persons.
The researchers compared regular users of PDE5Is (those who had received at least one prescription for a PDE5I every 3 months in the year before the ocular diagnosis) with nonusers (those who had not received a PDE5I prescription during that time).
Patients with the ocular conditions were more likely than were those in the control group to have hypertension, diabetes, cardiovascular disease, or sleep apnea. After controlling for these covariates, the researchers found that the users were overall 85% more likely to be diagnosed with one or more of them (incidence rate ratio [IRR], 1.85).
The researchers also found that the risk was even greater for those patients who were given five or more prescriptions of PDE5Is, compared to those given fewer than five prescriptions, suggesting a dose response.
On the basis of these findings, Dr. Etminan thinks drug companies should add warnings about serous retinal detachment and retinal vascular occlusion to the drug monographs.
Asked to comment, Pfizer, which developed Viagra, referred questions to its spinoff company, Viatris, which did not respond. Eli Lilly, which makes Cialis, also did not respond to a request for comment. Vivus, which makes Stendra, could not be reached by press time.
Bayer, which makes Levitra, declined to provide anyone who could answer questions, but it provided a statement noting that the occurrence of ocular adverse events is already known among PDE5I users and that retinal vascular occlusion and ischemic optic neuropathy are mentioned in the product information.
“For example, non-arteritic anterior ischemic optic neuropathy (NAION) is a very rare condition which occurs with an overall higher risk in the population usually suffering from erectile dysfunction (ED) – that is, elderly men with concomitant diseases such as diabetes, dyslipidemia, and hypertension – compared to the general population,” the statement said.
Because of the retrospective nature of the analysis, Dr. Etminan acknowledged that researchers could not prove that the increased risk of ocular disease was associated with use of the drugs rather than some underlying condition. But in addition to adjusting for known risk factors, they also separately analyzed men without hypertension, diabetes, or coronary artery disease and still found that the risk of the ocular conditions was roughly double for men with PDE5I prescriptions.
Howard Pomeranz, MD, PhD, professor of ophthalmology at Northwell Health in Great Neck, N.Y., who was not involved in this study, said its findings confirmed similar research that he conducted on ischemic optic neuropathy.
He told this news organization that people taking PDE5Is should weigh the risk against the benefit, but added that the calculation might be different for people who use them to treat pulmonary hypertension rather than erectile dysfunction.
Although people taking the drugs should discuss any changes in their vision with their practitioners, he said they should not be concerned about a “bluish type of tint to the vision that may occur transiently for anywhere from a few minutes up to 40 or 45 minutes.”
Drug companies and regulators should consider changing the monographs in light of this new evidence, Dr. Pomeranz said. “Perhaps this data might drive the warning to be perhaps a little bit stronger, now that there’s more data to suggest maybe a bit of a stronger association and not just some chance association between using these drugs and these visual events.”
The study was funded by the University of British Columbia. Dr. Etminan and Dr. Pomeranz have disclosed no relevant financial interests.
A version of this article first appeared on Medscape.com
, researchers say.
Patients in an insurance database who were prescribed sildenafil (Viagra), tadalafil (Cialis), vardenafil (Levitra), or avanafil (Stendra) were almost twice as likely as were patients not prescribed the drugs to have ischemic optic neuropathy, retinal vascular occlusion, or serous retinal detachment.
In 2020, physicians wrote about 20 million monthly prescriptions for PDE5Is in the United States alone, said Mahyar Etminan, PharmD, associate professor of ophthalmology at the University of British Columbia, Vancouver.
“We don’t want to alarm people taking them, but generally speaking, if they experience visual problems or changes in vision, then these drugs may be the culprits, and they should check it out,” he said in an interview.
The study was published in JAMA Ophthalmology.
Previous reports, including postmarketing studies by the drug makers, have documented ocular events. The monographs for sildenafil, tadalafil, vardenafil, and avanafil warn users about ischemic optic neuropathy, the researchers found.
The monographs for sildenafil, tadalafil, and vardenafil list retinal vascular occlusion as a potential adverse event but do not quantify the risk. None of the drug monographs mention serous retinal detachment.
Previous research has associated PDE5Is with compromised perfusion of the optic nerve. Some researchers have speculated that the choroid blood vessels can undergo smooth muscle relaxation through a cyclic guanosine monophosphate pathway that can lead to choroidal congestion.
To get a better handle on the ocular risks of PDE51s, Dr. Etminan and his colleagues analyzed health insurance claim records from the PharMetrics Plus database of 213,033 men who had not experienced any of the three ocular conditions in the year before they became regular users of the medications.
They identified 1,146 patients who had been diagnosed with at least one of the three conditions.
The overall number of conditions diagnosed was small relative to the size of the population, 15.5 cases per 10,000 person-years. “So that’s still relatively rare, but the problem is that these are very heavily used medications,” Dr. Etminan said.
For each man diagnosed with one of the ocular conditions, the researchers matched four control persons who were the same age and could be followed for the same length of time. There was a total of 4,584 control persons.
The researchers compared regular users of PDE5Is (those who had received at least one prescription for a PDE5I every 3 months in the year before the ocular diagnosis) with nonusers (those who had not received a PDE5I prescription during that time).
Patients with the ocular conditions were more likely than were those in the control group to have hypertension, diabetes, cardiovascular disease, or sleep apnea. After controlling for these covariates, the researchers found that the users were overall 85% more likely to be diagnosed with one or more of them (incidence rate ratio [IRR], 1.85).
The researchers also found that the risk was even greater for those patients who were given five or more prescriptions of PDE5Is, compared to those given fewer than five prescriptions, suggesting a dose response.
On the basis of these findings, Dr. Etminan thinks drug companies should add warnings about serous retinal detachment and retinal vascular occlusion to the drug monographs.
Asked to comment, Pfizer, which developed Viagra, referred questions to its spinoff company, Viatris, which did not respond. Eli Lilly, which makes Cialis, also did not respond to a request for comment. Vivus, which makes Stendra, could not be reached by press time.
Bayer, which makes Levitra, declined to provide anyone who could answer questions, but it provided a statement noting that the occurrence of ocular adverse events is already known among PDE5I users and that retinal vascular occlusion and ischemic optic neuropathy are mentioned in the product information.
“For example, non-arteritic anterior ischemic optic neuropathy (NAION) is a very rare condition which occurs with an overall higher risk in the population usually suffering from erectile dysfunction (ED) – that is, elderly men with concomitant diseases such as diabetes, dyslipidemia, and hypertension – compared to the general population,” the statement said.
Because of the retrospective nature of the analysis, Dr. Etminan acknowledged that researchers could not prove that the increased risk of ocular disease was associated with use of the drugs rather than some underlying condition. But in addition to adjusting for known risk factors, they also separately analyzed men without hypertension, diabetes, or coronary artery disease and still found that the risk of the ocular conditions was roughly double for men with PDE5I prescriptions.
Howard Pomeranz, MD, PhD, professor of ophthalmology at Northwell Health in Great Neck, N.Y., who was not involved in this study, said its findings confirmed similar research that he conducted on ischemic optic neuropathy.
He told this news organization that people taking PDE5Is should weigh the risk against the benefit, but added that the calculation might be different for people who use them to treat pulmonary hypertension rather than erectile dysfunction.
Although people taking the drugs should discuss any changes in their vision with their practitioners, he said they should not be concerned about a “bluish type of tint to the vision that may occur transiently for anywhere from a few minutes up to 40 or 45 minutes.”
Drug companies and regulators should consider changing the monographs in light of this new evidence, Dr. Pomeranz said. “Perhaps this data might drive the warning to be perhaps a little bit stronger, now that there’s more data to suggest maybe a bit of a stronger association and not just some chance association between using these drugs and these visual events.”
The study was funded by the University of British Columbia. Dr. Etminan and Dr. Pomeranz have disclosed no relevant financial interests.
A version of this article first appeared on Medscape.com
Breakthrough COVID dangerous for vaccinated cancer patients
, according to a study published in JAMA Oncology.
The risks were highest among patients who had certain cancers and those who had received cancer treatment within the past year.
“These results emphasize the need for patients with cancer to maintain mitigation practice, especially with the emergence of different virus variants and the waning immunity of vaccines,” the study authors wrote.
Researchers at Case Western Reserve University in Cleveland analyzed electronic health record data for more than 636,000 vaccinated patients, including more than 45,000 vaccinated patients with cancer. They looked for the time trends, risks, and outcomes of breakthrough COVID-19 infections for vaccinated cancer patients in the United States between December 2020 and November 2021.
Overall, the cumulative risk of breakthrough infections in vaccinated cancer patients was 13.6%, with the highest risk for pancreatic (24.7%), liver (22.8%), lung (20.4%), and colorectal (17.5%) cancers and the lowest risk for thyroid (10.3%), endometrial (11.9%), and breast (11.9%) cancers, versus 4.9% in vaccinated patients without cancer.
Patients who had medical encounters for their cancer within the past year had a higher risk for a breakthrough infection, particularly those with breast cancer, blood cancers, colorectal cancer, bladder cancer, and pancreatic cancer.
Among patients with cancer, the overall risk for hospitalization after a breakthrough infection was 31.6%, as compared with 3.9% in those without a breakthrough infection. In addition, the risk of death was 6.7% after a breakthrough infection, as compared with 1.3% in those without a breakthrough infection.
Among patients who didn’t have cancer, the overall hospitalization risk was 25.9% in patients with a breakthrough infection, as compared with 3% in those without a breakthrough infection. The overall risk of death was 2.7% after a breakthrough infection, as compared with 0.5% in those without a breakthrough infection.
In addition, breakthrough infections continuously increased for all patients from December 2020 to November 2021, with the numbers consistently higher among patients with cancer.
“This increasing time trend may reflect waning immunity of vaccines, the emergence of different virus variants, and varied measures taken by individuals and communities over time during the pandemic,” the study authors wrote.
Vaccines are likely less protective against coronavirus infection in cancer patients, and in turn, cancer patients may be more susceptible to COVID-19 infections, the researchers wrote. As breakthrough infections continue to increase for everyone, patients with cancer will face increased risks for severe breakthroughs, hospitalization, and death, they concluded.
A version of this article first appeared on WebMD.com.
, according to a study published in JAMA Oncology.
The risks were highest among patients who had certain cancers and those who had received cancer treatment within the past year.
“These results emphasize the need for patients with cancer to maintain mitigation practice, especially with the emergence of different virus variants and the waning immunity of vaccines,” the study authors wrote.
Researchers at Case Western Reserve University in Cleveland analyzed electronic health record data for more than 636,000 vaccinated patients, including more than 45,000 vaccinated patients with cancer. They looked for the time trends, risks, and outcomes of breakthrough COVID-19 infections for vaccinated cancer patients in the United States between December 2020 and November 2021.
Overall, the cumulative risk of breakthrough infections in vaccinated cancer patients was 13.6%, with the highest risk for pancreatic (24.7%), liver (22.8%), lung (20.4%), and colorectal (17.5%) cancers and the lowest risk for thyroid (10.3%), endometrial (11.9%), and breast (11.9%) cancers, versus 4.9% in vaccinated patients without cancer.
Patients who had medical encounters for their cancer within the past year had a higher risk for a breakthrough infection, particularly those with breast cancer, blood cancers, colorectal cancer, bladder cancer, and pancreatic cancer.
Among patients with cancer, the overall risk for hospitalization after a breakthrough infection was 31.6%, as compared with 3.9% in those without a breakthrough infection. In addition, the risk of death was 6.7% after a breakthrough infection, as compared with 1.3% in those without a breakthrough infection.
Among patients who didn’t have cancer, the overall hospitalization risk was 25.9% in patients with a breakthrough infection, as compared with 3% in those without a breakthrough infection. The overall risk of death was 2.7% after a breakthrough infection, as compared with 0.5% in those without a breakthrough infection.
In addition, breakthrough infections continuously increased for all patients from December 2020 to November 2021, with the numbers consistently higher among patients with cancer.
“This increasing time trend may reflect waning immunity of vaccines, the emergence of different virus variants, and varied measures taken by individuals and communities over time during the pandemic,” the study authors wrote.
Vaccines are likely less protective against coronavirus infection in cancer patients, and in turn, cancer patients may be more susceptible to COVID-19 infections, the researchers wrote. As breakthrough infections continue to increase for everyone, patients with cancer will face increased risks for severe breakthroughs, hospitalization, and death, they concluded.
A version of this article first appeared on WebMD.com.
, according to a study published in JAMA Oncology.
The risks were highest among patients who had certain cancers and those who had received cancer treatment within the past year.
“These results emphasize the need for patients with cancer to maintain mitigation practice, especially with the emergence of different virus variants and the waning immunity of vaccines,” the study authors wrote.
Researchers at Case Western Reserve University in Cleveland analyzed electronic health record data for more than 636,000 vaccinated patients, including more than 45,000 vaccinated patients with cancer. They looked for the time trends, risks, and outcomes of breakthrough COVID-19 infections for vaccinated cancer patients in the United States between December 2020 and November 2021.
Overall, the cumulative risk of breakthrough infections in vaccinated cancer patients was 13.6%, with the highest risk for pancreatic (24.7%), liver (22.8%), lung (20.4%), and colorectal (17.5%) cancers and the lowest risk for thyroid (10.3%), endometrial (11.9%), and breast (11.9%) cancers, versus 4.9% in vaccinated patients without cancer.
Patients who had medical encounters for their cancer within the past year had a higher risk for a breakthrough infection, particularly those with breast cancer, blood cancers, colorectal cancer, bladder cancer, and pancreatic cancer.
Among patients with cancer, the overall risk for hospitalization after a breakthrough infection was 31.6%, as compared with 3.9% in those without a breakthrough infection. In addition, the risk of death was 6.7% after a breakthrough infection, as compared with 1.3% in those without a breakthrough infection.
Among patients who didn’t have cancer, the overall hospitalization risk was 25.9% in patients with a breakthrough infection, as compared with 3% in those without a breakthrough infection. The overall risk of death was 2.7% after a breakthrough infection, as compared with 0.5% in those without a breakthrough infection.
In addition, breakthrough infections continuously increased for all patients from December 2020 to November 2021, with the numbers consistently higher among patients with cancer.
“This increasing time trend may reflect waning immunity of vaccines, the emergence of different virus variants, and varied measures taken by individuals and communities over time during the pandemic,” the study authors wrote.
Vaccines are likely less protective against coronavirus infection in cancer patients, and in turn, cancer patients may be more susceptible to COVID-19 infections, the researchers wrote. As breakthrough infections continue to increase for everyone, patients with cancer will face increased risks for severe breakthroughs, hospitalization, and death, they concluded.
A version of this article first appeared on WebMD.com.
FROM JAMA ONCOLOGY
Nontuberculous mycobacterial lung disease can be challenging to treat
Living in coastal areas of Florida and California has great appeal for many, with the warm, sunny climate and nearby fresh water and salt water.
But, unknown to many, those balmy coasts also carry the risk of infection from nontuberculous (atypical) mycobacteria (NTM). Unlike its relative, tuberculosis, NTM is not transmitted from person to person, with one exception: patients with cystic fibrosis.
It is estimated that there were 181,000 people with NTM lung disease in the U.S. in 2015, and according to one study, the incidence is increasing by 8.2% annually among those aged 65 years and older. But NTM doesn’t only affect the elderly; it’s estimated that 31% of all NTM patients are younger than 65 years.
With the warm, moist soil and water, NTM is most commonly found in Florida, California, Hawaii, and the Gulf Coast states. The incidence is somewhat lower in states along the Great Lakes. Other states are not without risk – but NTM is perhaps even more likely to be overlooked in these states by physicians because of a lack of awareness of the disease.
Rebecca Prevots, PhD, MPH, chief of the epidemiology and population studies unit of the Division of Intramural Research at the National Institute of Allergy and Infectious Diseases, told this news organization that “why NTM is increasing is one of the most common questions” she gets, followed by whether it is due to climate change. “The short answer is, we don’t know.”
She suggests that the increase in diagnoses is due to a combination of increased awareness, host susceptibility, and perhaps environmental changes. One problem is that NTM is not a reportable disease. Also, public health resources have been decimated, both through funding cuts and loss of personnel. Dr. Prevots said, “It’s not just NTM surveillance that is important, but you can’t just make a certain condition reportable and expect to have good data without putting resources to it. ... Diseases are made reportable at the state level. There’s no mandated reporting up to CDC. So CDC is piloting reporting events through their emerging infectious program.”
Anthony Cannella, MD, assistant professor of infectious diseases at the University of South Florida (USF), is in the midst of NTM. He told this news organization that “there’s a huge circle with big old dots right over the center of the state.” He is adamant that “a soil-water survey has to occur. We need to know what the devil is happening.”
Florida legislators agreed to allocate $519,000 for NTM testing and surveillance in 2019. But Florida Governor Ron DeSantis vetoed that line item in the budget. WUSF (a National Public Radio affiliate on the USF campus) was unable to get a response to their query about this from the governor’s office.
Who gets NTM?
Mycobacterium avium complex primarily causes lung disease, which presents as two clinical syndromes.
“These infections don’t affect everyone,” Kenneth Olivier, MD, MPH, chief of pulmonary clinical medicine, Cardiovascular Pulmonary Branch of the National Heart, Lung, and Blood Institute, said in an interview. They affect “patients that have underlying genetic conditions that cause abnormalities in the airway clearance mechanisms, particularly cystic fibrosis and primary ciliary dyskinesia [and], to some extent, patients with COPD.”
The second group is “comprised mainly of postmenopausal women, many of whom have had no predisposing medical problems prior to onset of generally frequent throat clearing or chronic cough, which is what brings them to medical attention.” Dr. Olivier added that “many of these patients have a fairly unique appearance. They tend to have a high prevalence of curvature of the spine, scoliosis, indentation of the chest wall (pectus excavatum), and physical characteristics that overlap heritable connective tissue disorders like Marfan syndrome or Ehlers-Danlos syndrome.”
Dr. Olivier pointed out a major problem in NTM diagnosis and treatment: “The guidelines-based approach to chronic cough generally calls for treating postnasal drip, airway reactivity, asthma type symptoms first empirically, before doing different diagnostic studies. That generally causes a delay in obtaining things like CT scan, where you can see the characteristic changes.”
Dr. Cannella added, “People are starting to become more aware of it. It’s kind of like pneumocystis back in the 80s. ... We’ve had patients who have had long periods of febrile neutropenia, and NTM wasn’t on the radar. Now we’ve picked up at least seven or eight.”
In addition to pulmonary infections, nosocomial outbreaks have occurred, owing to contaminated heater-cooler units, catheter infections, nail salons, or to medical tourism. These more commonly involve rapidly growing species, such as M abscessus, M chelonae, and M fortuitum. Clinicians should also be aware of skin infections from M marinum, which come from wounds from aquariums, fish, or shellfish. Incubation can occur over months, highlighting the importance of a detailed history and special cultures.
Diagnostics
The diagnosis of NTM is delayed for several reasons. One is the lack of awareness among clinicians about NTM and its risk factors, including hobbies such as gardening or working in places where dirt is aerosolized, such as on road crews, or even from hot tubs. A thorough history is critical.
Another is not recognizing the need for an acid-fast bacilli (AFB) culture, which requires specialized media. Fortunately, NTM can be picked up on fungal cultures, Dr. Cannella noted. Clinicians are sometimes discouraged from culturing AFB because doing so may not be cost-effective. And many hospital laboratories are increasingly sending cultures to outside labs, and it can take days – sometimes even more than a week – to receive a report of results.
Charles Daley, MD, chief of the Division of Mycobacterial and Respiratory Infections at National Jewish Health, expressed his frustration about labs in an interview, saying diagnostics is “an important hole in the U.S., as our laboratories do not provide clinicians with the results that they need to make good decisions. Most laboratories in the U.S. just don’t speciate the organisms or subspeciate in the setting of abscesses. They don’t tell the clinician enough about the susceptibility, particularly whether there’s inducible resistance. As a clinician, you just don’t have the information to make the right decisions. ... We need to improve diagnostics in NTM. Everything is there and available. They just don’t want to do it because it increases the costs.”
Men tend to have fibrocavitary disease, which shows on ordinary chest x-rays, but CT scans are essential for women because women tend to have either nodular disease or bronchiectasis, which does not show on a plain film.
Treatment
A standard treatment for NTM lung disease includes three or four medications – clarithromycin or azithromycin, rifampin or rifabutin, ethambutol, and streptomycin or amikacin. In vitro resistance is important in predicting the clinical response to a macrolide or amikacin.
For bronchiectatic disease, National Jewish Hospital recommends treatment three times per week rather than daily therapy, as it is better tolerated. Azithromycin is preferred over clarithromycin. Amikacin should be added if there is cavitary or severe disease, and the macrolide is then given daily.
Dr. Olivier suggested that physicians stagger the initiation of those drugs to improve the tolerability of the difficult regimen. Generally, treatment is for 18 months – a year after sputum cultures become negative.
If therapy fails – that is, sputum is persistently positive at 6 months – amikacin liposomal inhalation solution (Arikayce) is likely to be added. Patients should be monitored with monthly safety labs, sputum cultures, and an audiogram (if receiving amikacin). Every 3 months, vestibular tests, eye exams, and spirometry should be conducted, and every 6 months, physicians should order a CT, an audiogram, and an electrocardiogram.
Despite completing such a rigorous regimen, about half of patients experience reinfection because of their underlying host susceptibility. Genomic sequencing shows that these are new infections, not relapses, Dr. Prevots said. She also noted that gastroesophageal reflux disease is a significant risk factor because of chronic aspiration.
Dr. Daley outlined the newer treatments being studied. They include Arikayce, omadocycline, and bedaquiline. He added, “There’s a neutrophil elastase inhibitor trial that’s ongoing, a huge trial. There’s another one looking at basically eosinophilic inflammation.”
Other trials are in the offing, he said, all focusing on the inflammatory response – a development he described as exciting, because for the longest time, there were few if any NTM trials.
Dr. Cannella is also buoyed by the potential synergy of dual beta-lactam therapy with ceftaroline and a carbapenem for M abscessus infections, which are notoriously difficult to treat.
There are unique problems facing drug development for NTM because, for approval, the U.S. Food and Drug Administration requires the drug to “improve how a patient feels, functions, or survives.” NTM is associated with low mortality, so that “is off the table,” Dr. Daley explained. It’s hard to quantify improvement in function. The top two symptoms to measure are coughing and fatigue, he said. But both are difficult to measure, and some of the medicines worsen cough. Some research groups are now trying to validate patient-reported outcome instruments to satisfy the FDA’s requirements.
Tips for patients and physicians
The experts this news organization spoke to had very consistent recommendations for patients:
- NTM is resistant to chlorine and bromine, so tap water is a major source of infection. Patients should consider to greater than 130° F and using metal showerheads or bathing rather than showering.
- Good bathroom ventilation helps.
- Patients should consider using a water filter that filters entities less than 5 mcm in size – but not carbon filters, which concentrate the organisms.
- Humidifiers and hot tubs should be avoided.
- A good face mask, such as an N95, should be worn when gardening or repotting plants.
Dr. Olivier stressed that clinicians should familiarize themselves with the guidelines for diagnosing and treating NTM. In particular, clinicians should be aware that using azithromycin for bronchitis might cause resistance in NTM. “Macrolide resistance turns what may be a slowly progressive or bothersome infection into a lethal infection with a 1-year mortality of 35%.”
He concluded, “I would just urge that if the patient’s on their second or third Z-Pak within a year, it’s probably time to look for other causes of what might be happening.”
Dr. Cannella, Dr. Prevots, and Dr. Olivier reported no relevant financial relationships. Dr. Cannella adds, “My views are not those of my employers, the U.S. Dept of VA, or the University of South Florida Morsani College of Medicine.” Dr. Daley reports research grants/contracts with AN2, Beyond Air, Bugworks, Insmed, and Paratek and service on advisory boards or as a consultant for AN2, AstraZeneca, Genentech, Insmed, Matinas, Paratek, Pfizer, and Spero.
A version of this article first appeared on Medscape.com.
Living in coastal areas of Florida and California has great appeal for many, with the warm, sunny climate and nearby fresh water and salt water.
But, unknown to many, those balmy coasts also carry the risk of infection from nontuberculous (atypical) mycobacteria (NTM). Unlike its relative, tuberculosis, NTM is not transmitted from person to person, with one exception: patients with cystic fibrosis.
It is estimated that there were 181,000 people with NTM lung disease in the U.S. in 2015, and according to one study, the incidence is increasing by 8.2% annually among those aged 65 years and older. But NTM doesn’t only affect the elderly; it’s estimated that 31% of all NTM patients are younger than 65 years.
With the warm, moist soil and water, NTM is most commonly found in Florida, California, Hawaii, and the Gulf Coast states. The incidence is somewhat lower in states along the Great Lakes. Other states are not without risk – but NTM is perhaps even more likely to be overlooked in these states by physicians because of a lack of awareness of the disease.
Rebecca Prevots, PhD, MPH, chief of the epidemiology and population studies unit of the Division of Intramural Research at the National Institute of Allergy and Infectious Diseases, told this news organization that “why NTM is increasing is one of the most common questions” she gets, followed by whether it is due to climate change. “The short answer is, we don’t know.”
She suggests that the increase in diagnoses is due to a combination of increased awareness, host susceptibility, and perhaps environmental changes. One problem is that NTM is not a reportable disease. Also, public health resources have been decimated, both through funding cuts and loss of personnel. Dr. Prevots said, “It’s not just NTM surveillance that is important, but you can’t just make a certain condition reportable and expect to have good data without putting resources to it. ... Diseases are made reportable at the state level. There’s no mandated reporting up to CDC. So CDC is piloting reporting events through their emerging infectious program.”
Anthony Cannella, MD, assistant professor of infectious diseases at the University of South Florida (USF), is in the midst of NTM. He told this news organization that “there’s a huge circle with big old dots right over the center of the state.” He is adamant that “a soil-water survey has to occur. We need to know what the devil is happening.”
Florida legislators agreed to allocate $519,000 for NTM testing and surveillance in 2019. But Florida Governor Ron DeSantis vetoed that line item in the budget. WUSF (a National Public Radio affiliate on the USF campus) was unable to get a response to their query about this from the governor’s office.
Who gets NTM?
Mycobacterium avium complex primarily causes lung disease, which presents as two clinical syndromes.
“These infections don’t affect everyone,” Kenneth Olivier, MD, MPH, chief of pulmonary clinical medicine, Cardiovascular Pulmonary Branch of the National Heart, Lung, and Blood Institute, said in an interview. They affect “patients that have underlying genetic conditions that cause abnormalities in the airway clearance mechanisms, particularly cystic fibrosis and primary ciliary dyskinesia [and], to some extent, patients with COPD.”
The second group is “comprised mainly of postmenopausal women, many of whom have had no predisposing medical problems prior to onset of generally frequent throat clearing or chronic cough, which is what brings them to medical attention.” Dr. Olivier added that “many of these patients have a fairly unique appearance. They tend to have a high prevalence of curvature of the spine, scoliosis, indentation of the chest wall (pectus excavatum), and physical characteristics that overlap heritable connective tissue disorders like Marfan syndrome or Ehlers-Danlos syndrome.”
Dr. Olivier pointed out a major problem in NTM diagnosis and treatment: “The guidelines-based approach to chronic cough generally calls for treating postnasal drip, airway reactivity, asthma type symptoms first empirically, before doing different diagnostic studies. That generally causes a delay in obtaining things like CT scan, where you can see the characteristic changes.”
Dr. Cannella added, “People are starting to become more aware of it. It’s kind of like pneumocystis back in the 80s. ... We’ve had patients who have had long periods of febrile neutropenia, and NTM wasn’t on the radar. Now we’ve picked up at least seven or eight.”
In addition to pulmonary infections, nosocomial outbreaks have occurred, owing to contaminated heater-cooler units, catheter infections, nail salons, or to medical tourism. These more commonly involve rapidly growing species, such as M abscessus, M chelonae, and M fortuitum. Clinicians should also be aware of skin infections from M marinum, which come from wounds from aquariums, fish, or shellfish. Incubation can occur over months, highlighting the importance of a detailed history and special cultures.
Diagnostics
The diagnosis of NTM is delayed for several reasons. One is the lack of awareness among clinicians about NTM and its risk factors, including hobbies such as gardening or working in places where dirt is aerosolized, such as on road crews, or even from hot tubs. A thorough history is critical.
Another is not recognizing the need for an acid-fast bacilli (AFB) culture, which requires specialized media. Fortunately, NTM can be picked up on fungal cultures, Dr. Cannella noted. Clinicians are sometimes discouraged from culturing AFB because doing so may not be cost-effective. And many hospital laboratories are increasingly sending cultures to outside labs, and it can take days – sometimes even more than a week – to receive a report of results.
Charles Daley, MD, chief of the Division of Mycobacterial and Respiratory Infections at National Jewish Health, expressed his frustration about labs in an interview, saying diagnostics is “an important hole in the U.S., as our laboratories do not provide clinicians with the results that they need to make good decisions. Most laboratories in the U.S. just don’t speciate the organisms or subspeciate in the setting of abscesses. They don’t tell the clinician enough about the susceptibility, particularly whether there’s inducible resistance. As a clinician, you just don’t have the information to make the right decisions. ... We need to improve diagnostics in NTM. Everything is there and available. They just don’t want to do it because it increases the costs.”
Men tend to have fibrocavitary disease, which shows on ordinary chest x-rays, but CT scans are essential for women because women tend to have either nodular disease or bronchiectasis, which does not show on a plain film.
Treatment
A standard treatment for NTM lung disease includes three or four medications – clarithromycin or azithromycin, rifampin or rifabutin, ethambutol, and streptomycin or amikacin. In vitro resistance is important in predicting the clinical response to a macrolide or amikacin.
For bronchiectatic disease, National Jewish Hospital recommends treatment three times per week rather than daily therapy, as it is better tolerated. Azithromycin is preferred over clarithromycin. Amikacin should be added if there is cavitary or severe disease, and the macrolide is then given daily.
Dr. Olivier suggested that physicians stagger the initiation of those drugs to improve the tolerability of the difficult regimen. Generally, treatment is for 18 months – a year after sputum cultures become negative.
If therapy fails – that is, sputum is persistently positive at 6 months – amikacin liposomal inhalation solution (Arikayce) is likely to be added. Patients should be monitored with monthly safety labs, sputum cultures, and an audiogram (if receiving amikacin). Every 3 months, vestibular tests, eye exams, and spirometry should be conducted, and every 6 months, physicians should order a CT, an audiogram, and an electrocardiogram.
Despite completing such a rigorous regimen, about half of patients experience reinfection because of their underlying host susceptibility. Genomic sequencing shows that these are new infections, not relapses, Dr. Prevots said. She also noted that gastroesophageal reflux disease is a significant risk factor because of chronic aspiration.
Dr. Daley outlined the newer treatments being studied. They include Arikayce, omadocycline, and bedaquiline. He added, “There’s a neutrophil elastase inhibitor trial that’s ongoing, a huge trial. There’s another one looking at basically eosinophilic inflammation.”
Other trials are in the offing, he said, all focusing on the inflammatory response – a development he described as exciting, because for the longest time, there were few if any NTM trials.
Dr. Cannella is also buoyed by the potential synergy of dual beta-lactam therapy with ceftaroline and a carbapenem for M abscessus infections, which are notoriously difficult to treat.
There are unique problems facing drug development for NTM because, for approval, the U.S. Food and Drug Administration requires the drug to “improve how a patient feels, functions, or survives.” NTM is associated with low mortality, so that “is off the table,” Dr. Daley explained. It’s hard to quantify improvement in function. The top two symptoms to measure are coughing and fatigue, he said. But both are difficult to measure, and some of the medicines worsen cough. Some research groups are now trying to validate patient-reported outcome instruments to satisfy the FDA’s requirements.
Tips for patients and physicians
The experts this news organization spoke to had very consistent recommendations for patients:
- NTM is resistant to chlorine and bromine, so tap water is a major source of infection. Patients should consider to greater than 130° F and using metal showerheads or bathing rather than showering.
- Good bathroom ventilation helps.
- Patients should consider using a water filter that filters entities less than 5 mcm in size – but not carbon filters, which concentrate the organisms.
- Humidifiers and hot tubs should be avoided.
- A good face mask, such as an N95, should be worn when gardening or repotting plants.
Dr. Olivier stressed that clinicians should familiarize themselves with the guidelines for diagnosing and treating NTM. In particular, clinicians should be aware that using azithromycin for bronchitis might cause resistance in NTM. “Macrolide resistance turns what may be a slowly progressive or bothersome infection into a lethal infection with a 1-year mortality of 35%.”
He concluded, “I would just urge that if the patient’s on their second or third Z-Pak within a year, it’s probably time to look for other causes of what might be happening.”
Dr. Cannella, Dr. Prevots, and Dr. Olivier reported no relevant financial relationships. Dr. Cannella adds, “My views are not those of my employers, the U.S. Dept of VA, or the University of South Florida Morsani College of Medicine.” Dr. Daley reports research grants/contracts with AN2, Beyond Air, Bugworks, Insmed, and Paratek and service on advisory boards or as a consultant for AN2, AstraZeneca, Genentech, Insmed, Matinas, Paratek, Pfizer, and Spero.
A version of this article first appeared on Medscape.com.
Living in coastal areas of Florida and California has great appeal for many, with the warm, sunny climate and nearby fresh water and salt water.
But, unknown to many, those balmy coasts also carry the risk of infection from nontuberculous (atypical) mycobacteria (NTM). Unlike its relative, tuberculosis, NTM is not transmitted from person to person, with one exception: patients with cystic fibrosis.
It is estimated that there were 181,000 people with NTM lung disease in the U.S. in 2015, and according to one study, the incidence is increasing by 8.2% annually among those aged 65 years and older. But NTM doesn’t only affect the elderly; it’s estimated that 31% of all NTM patients are younger than 65 years.
With the warm, moist soil and water, NTM is most commonly found in Florida, California, Hawaii, and the Gulf Coast states. The incidence is somewhat lower in states along the Great Lakes. Other states are not without risk – but NTM is perhaps even more likely to be overlooked in these states by physicians because of a lack of awareness of the disease.
Rebecca Prevots, PhD, MPH, chief of the epidemiology and population studies unit of the Division of Intramural Research at the National Institute of Allergy and Infectious Diseases, told this news organization that “why NTM is increasing is one of the most common questions” she gets, followed by whether it is due to climate change. “The short answer is, we don’t know.”
She suggests that the increase in diagnoses is due to a combination of increased awareness, host susceptibility, and perhaps environmental changes. One problem is that NTM is not a reportable disease. Also, public health resources have been decimated, both through funding cuts and loss of personnel. Dr. Prevots said, “It’s not just NTM surveillance that is important, but you can’t just make a certain condition reportable and expect to have good data without putting resources to it. ... Diseases are made reportable at the state level. There’s no mandated reporting up to CDC. So CDC is piloting reporting events through their emerging infectious program.”
Anthony Cannella, MD, assistant professor of infectious diseases at the University of South Florida (USF), is in the midst of NTM. He told this news organization that “there’s a huge circle with big old dots right over the center of the state.” He is adamant that “a soil-water survey has to occur. We need to know what the devil is happening.”
Florida legislators agreed to allocate $519,000 for NTM testing and surveillance in 2019. But Florida Governor Ron DeSantis vetoed that line item in the budget. WUSF (a National Public Radio affiliate on the USF campus) was unable to get a response to their query about this from the governor’s office.
Who gets NTM?
Mycobacterium avium complex primarily causes lung disease, which presents as two clinical syndromes.
“These infections don’t affect everyone,” Kenneth Olivier, MD, MPH, chief of pulmonary clinical medicine, Cardiovascular Pulmonary Branch of the National Heart, Lung, and Blood Institute, said in an interview. They affect “patients that have underlying genetic conditions that cause abnormalities in the airway clearance mechanisms, particularly cystic fibrosis and primary ciliary dyskinesia [and], to some extent, patients with COPD.”
The second group is “comprised mainly of postmenopausal women, many of whom have had no predisposing medical problems prior to onset of generally frequent throat clearing or chronic cough, which is what brings them to medical attention.” Dr. Olivier added that “many of these patients have a fairly unique appearance. They tend to have a high prevalence of curvature of the spine, scoliosis, indentation of the chest wall (pectus excavatum), and physical characteristics that overlap heritable connective tissue disorders like Marfan syndrome or Ehlers-Danlos syndrome.”
Dr. Olivier pointed out a major problem in NTM diagnosis and treatment: “The guidelines-based approach to chronic cough generally calls for treating postnasal drip, airway reactivity, asthma type symptoms first empirically, before doing different diagnostic studies. That generally causes a delay in obtaining things like CT scan, where you can see the characteristic changes.”
Dr. Cannella added, “People are starting to become more aware of it. It’s kind of like pneumocystis back in the 80s. ... We’ve had patients who have had long periods of febrile neutropenia, and NTM wasn’t on the radar. Now we’ve picked up at least seven or eight.”
In addition to pulmonary infections, nosocomial outbreaks have occurred, owing to contaminated heater-cooler units, catheter infections, nail salons, or to medical tourism. These more commonly involve rapidly growing species, such as M abscessus, M chelonae, and M fortuitum. Clinicians should also be aware of skin infections from M marinum, which come from wounds from aquariums, fish, or shellfish. Incubation can occur over months, highlighting the importance of a detailed history and special cultures.
Diagnostics
The diagnosis of NTM is delayed for several reasons. One is the lack of awareness among clinicians about NTM and its risk factors, including hobbies such as gardening or working in places where dirt is aerosolized, such as on road crews, or even from hot tubs. A thorough history is critical.
Another is not recognizing the need for an acid-fast bacilli (AFB) culture, which requires specialized media. Fortunately, NTM can be picked up on fungal cultures, Dr. Cannella noted. Clinicians are sometimes discouraged from culturing AFB because doing so may not be cost-effective. And many hospital laboratories are increasingly sending cultures to outside labs, and it can take days – sometimes even more than a week – to receive a report of results.
Charles Daley, MD, chief of the Division of Mycobacterial and Respiratory Infections at National Jewish Health, expressed his frustration about labs in an interview, saying diagnostics is “an important hole in the U.S., as our laboratories do not provide clinicians with the results that they need to make good decisions. Most laboratories in the U.S. just don’t speciate the organisms or subspeciate in the setting of abscesses. They don’t tell the clinician enough about the susceptibility, particularly whether there’s inducible resistance. As a clinician, you just don’t have the information to make the right decisions. ... We need to improve diagnostics in NTM. Everything is there and available. They just don’t want to do it because it increases the costs.”
Men tend to have fibrocavitary disease, which shows on ordinary chest x-rays, but CT scans are essential for women because women tend to have either nodular disease or bronchiectasis, which does not show on a plain film.
Treatment
A standard treatment for NTM lung disease includes three or four medications – clarithromycin or azithromycin, rifampin or rifabutin, ethambutol, and streptomycin or amikacin. In vitro resistance is important in predicting the clinical response to a macrolide or amikacin.
For bronchiectatic disease, National Jewish Hospital recommends treatment three times per week rather than daily therapy, as it is better tolerated. Azithromycin is preferred over clarithromycin. Amikacin should be added if there is cavitary or severe disease, and the macrolide is then given daily.
Dr. Olivier suggested that physicians stagger the initiation of those drugs to improve the tolerability of the difficult regimen. Generally, treatment is for 18 months – a year after sputum cultures become negative.
If therapy fails – that is, sputum is persistently positive at 6 months – amikacin liposomal inhalation solution (Arikayce) is likely to be added. Patients should be monitored with monthly safety labs, sputum cultures, and an audiogram (if receiving amikacin). Every 3 months, vestibular tests, eye exams, and spirometry should be conducted, and every 6 months, physicians should order a CT, an audiogram, and an electrocardiogram.
Despite completing such a rigorous regimen, about half of patients experience reinfection because of their underlying host susceptibility. Genomic sequencing shows that these are new infections, not relapses, Dr. Prevots said. She also noted that gastroesophageal reflux disease is a significant risk factor because of chronic aspiration.
Dr. Daley outlined the newer treatments being studied. They include Arikayce, omadocycline, and bedaquiline. He added, “There’s a neutrophil elastase inhibitor trial that’s ongoing, a huge trial. There’s another one looking at basically eosinophilic inflammation.”
Other trials are in the offing, he said, all focusing on the inflammatory response – a development he described as exciting, because for the longest time, there were few if any NTM trials.
Dr. Cannella is also buoyed by the potential synergy of dual beta-lactam therapy with ceftaroline and a carbapenem for M abscessus infections, which are notoriously difficult to treat.
There are unique problems facing drug development for NTM because, for approval, the U.S. Food and Drug Administration requires the drug to “improve how a patient feels, functions, or survives.” NTM is associated with low mortality, so that “is off the table,” Dr. Daley explained. It’s hard to quantify improvement in function. The top two symptoms to measure are coughing and fatigue, he said. But both are difficult to measure, and some of the medicines worsen cough. Some research groups are now trying to validate patient-reported outcome instruments to satisfy the FDA’s requirements.
Tips for patients and physicians
The experts this news organization spoke to had very consistent recommendations for patients:
- NTM is resistant to chlorine and bromine, so tap water is a major source of infection. Patients should consider to greater than 130° F and using metal showerheads or bathing rather than showering.
- Good bathroom ventilation helps.
- Patients should consider using a water filter that filters entities less than 5 mcm in size – but not carbon filters, which concentrate the organisms.
- Humidifiers and hot tubs should be avoided.
- A good face mask, such as an N95, should be worn when gardening or repotting plants.
Dr. Olivier stressed that clinicians should familiarize themselves with the guidelines for diagnosing and treating NTM. In particular, clinicians should be aware that using azithromycin for bronchitis might cause resistance in NTM. “Macrolide resistance turns what may be a slowly progressive or bothersome infection into a lethal infection with a 1-year mortality of 35%.”
He concluded, “I would just urge that if the patient’s on their second or third Z-Pak within a year, it’s probably time to look for other causes of what might be happening.”
Dr. Cannella, Dr. Prevots, and Dr. Olivier reported no relevant financial relationships. Dr. Cannella adds, “My views are not those of my employers, the U.S. Dept of VA, or the University of South Florida Morsani College of Medicine.” Dr. Daley reports research grants/contracts with AN2, Beyond Air, Bugworks, Insmed, and Paratek and service on advisory boards or as a consultant for AN2, AstraZeneca, Genentech, Insmed, Matinas, Paratek, Pfizer, and Spero.
A version of this article first appeared on Medscape.com.
Long-term smell loss in COVID-19 tied to damage in the brain’s olfactory bulb
Patients with COVID-19, especially those with an altered sense of smell, have significantly more axon and microvasculopathy damage in the brain’s olfactory tissue versus non-COVID patients. These new findings from a postmortem study may explain long-term loss of smell in some patients with the virus.
“The striking axonal pathology in some cases indicates that olfactory dysfunction in COVID-19 may be severe and permanent,” the investigators led by Cheng-Ying Ho, MD, PhD, associate professor, department of pathology, Johns Hopkins University School of Medicine, Baltimore, write.
“The results show the damage caused by COVID can extend beyond the nasal cavity and involve the brain,” Dr. Ho told this news organization.
The study was published online April 11 in JAMA Neurology.
A more thorough investigation
Patients infected with SARS-CoV-2, which causes COVID-19, present with a wide range of symptoms. In addition to respiratory illnesses, they may exhibit various nonrespiratory manifestations of COVID-19.
One of the most prevalent of these is olfactory dysfunction. Research shows such dysfunction, including anosmia (loss of smell), hyposmia (reduced sense of smell), and parosmia (smells that are distorted or unpleasant), affects 30%-60% of patients with COVID-19, said Dr. Ho.
However, these statistics come from research before the advent of the Omicron variant, which evidence suggests causes less smell loss in patients with COVID, she said.
Previous studies in this area mainly focused on the lining of the nasal cavity. “We wanted to go a step beyond to see how the olfactory bulb was affected by COVID infection,” said Dr. Ho.
The study included 23 deceased patients with confirmed COVID-19 ranging in age from 28 to 93 years at death (median 62 years, 60.9% men). It also included 14 controls who tested negative for COVID-19, ranging in age from 20 to 77 years (median 53.5 years, 50% men).
Researchers collected postmortem tissue from the brain, lung, and other organs and reviewed pertinent clinical information.
Most patients with COVID died of COVID pneumonia or related complications, although some died from a different cause. Some had an active COVID infection and others were “post infection, meaning they were in the recovery stage,” said Dr. Ho.
Six patients with COVID-19 and eight controls had significant brain pathology.
Compared with controls, those with COVID-19 showed significantly worse olfactory axonal damage. The mean axon pathology score (range 1-3 with 3 the worst) was 1.921 in patients with COVID-19 and 1.198 in controls (95% confidence interval, 0.444-1.002; P < .001).
The mean axon density in the lateral olfactory tract was significantly less in patients with COVID-19 than in controls (P = .002), indicating a 23% loss of olfactory axons in the COVID group.
Comparing COVID patients with and without reported loss of smell, researchers found those with an altered sense of smell had significantly more severe olfactory axon pathology.
Vascular damage
Patients with COVID also had worse vascular damage. The mean microvasculopathy score (range, 1-3) was 1.907 in patients with COVID-19 and 1.405 in controls (95% CI, 0.259-0.745; P < .001).
There was no evidence of the virus in the olfactory tissue of most patients, suggesting the olfactory pathology was likely caused by vascular damage, said Dr. Ho.
What’s unique about SARS-CoV-2 is that, although it’s a respiratory virus, it’s capable of infecting endothelial cells lining vessels.
“Other respiratory viruses only attack the airways and won’t attack vessels, but vascular damage has been seen in the heart and lung in COVID patients, and our study showed the same findings in the olfactory bulb,” Dr. Ho explained.
The researchers divided patients with COVID by infection severity: mild, moderate, severe, and critical. Interestingly, those with the most severe olfactory pathology were the ones with milder infections, said Dr. Ho.
She noted other studies have reported patients with mild infection are more likely to lose the sense of smell than those with severe infection, but she’s skeptical about this finding.
“Patients with severe COVID are usually hospitalized and intubated, so it’s hard to get them to tell you whether they’ve lost smell or not; they have other more important issues to deal with like respiratory failure,” said Dr. Ho.
Advanced age is associated with neuropathologic changes, such as tau deposits, so the researchers conducted an analysis factoring in age-related brain changes. They found a COVID-19 diagnosis remained associated with increased axonal pathology, reduced axonal density, and increased vascular pathology.
“This means that the COVID patients had more severe olfactory pathology not just because they had more tau pathology,” Dr. Ho added.
New guidance for patients
Commenting for this news organization, Davangere P. Devanand, MD, professor of psychiatry and neurology and director of geriatric psychiatry, Columbia University Irving Medical Center, New York, said the findings indicate the damage from COVID in the olfactory pathway may not be reversible as was previously thought.
“This has been suggested before as a possibility, but the autopsy findings in this case series indicate clearly that there may be permanent damage,” he said.
The results highlight the need to monitor patients with COVID for a smell deficit, said Dr. Devanand.
“Assuring patients of a full recovery in smell and taste may not be sound advice, although recovery does occur in many patients,” he added.
He praised the study design, especially the blinding of raters, but noted a number of weaknesses, including the small sample size and the age and gender discrepancies between the groups.
Another possible limitation was inclusion of patients with Alzheimer’s and Lewy body pathology, said Dr. Devanand.
“These patients typically already have pathology in the olfactory pathways, which means we don’t know if it was COVID or the underlying brain pathology contributing to smell difficulties in these patients,” he said.
He noted that, unlike deceased COVID cases in the study, patients who survive COVID may not experience axonal and microvascular injury in olfactory neurons and pathways and their sense of smell may make a full return.
Dr. Devanand said he would have liked more detailed information on the clinical history and course of study participants and whether these factors affected the pathology findings.
The study was supported by grants from the National Institutes of Health.
Dr. Ho and Dr. Devanand have reported no relevant financial disclosures.
A version of this article first appeared on Medscape.com.
Patients with COVID-19, especially those with an altered sense of smell, have significantly more axon and microvasculopathy damage in the brain’s olfactory tissue versus non-COVID patients. These new findings from a postmortem study may explain long-term loss of smell in some patients with the virus.
“The striking axonal pathology in some cases indicates that olfactory dysfunction in COVID-19 may be severe and permanent,” the investigators led by Cheng-Ying Ho, MD, PhD, associate professor, department of pathology, Johns Hopkins University School of Medicine, Baltimore, write.
“The results show the damage caused by COVID can extend beyond the nasal cavity and involve the brain,” Dr. Ho told this news organization.
The study was published online April 11 in JAMA Neurology.
A more thorough investigation
Patients infected with SARS-CoV-2, which causes COVID-19, present with a wide range of symptoms. In addition to respiratory illnesses, they may exhibit various nonrespiratory manifestations of COVID-19.
One of the most prevalent of these is olfactory dysfunction. Research shows such dysfunction, including anosmia (loss of smell), hyposmia (reduced sense of smell), and parosmia (smells that are distorted or unpleasant), affects 30%-60% of patients with COVID-19, said Dr. Ho.
However, these statistics come from research before the advent of the Omicron variant, which evidence suggests causes less smell loss in patients with COVID, she said.
Previous studies in this area mainly focused on the lining of the nasal cavity. “We wanted to go a step beyond to see how the olfactory bulb was affected by COVID infection,” said Dr. Ho.
The study included 23 deceased patients with confirmed COVID-19 ranging in age from 28 to 93 years at death (median 62 years, 60.9% men). It also included 14 controls who tested negative for COVID-19, ranging in age from 20 to 77 years (median 53.5 years, 50% men).
Researchers collected postmortem tissue from the brain, lung, and other organs and reviewed pertinent clinical information.
Most patients with COVID died of COVID pneumonia or related complications, although some died from a different cause. Some had an active COVID infection and others were “post infection, meaning they were in the recovery stage,” said Dr. Ho.
Six patients with COVID-19 and eight controls had significant brain pathology.
Compared with controls, those with COVID-19 showed significantly worse olfactory axonal damage. The mean axon pathology score (range 1-3 with 3 the worst) was 1.921 in patients with COVID-19 and 1.198 in controls (95% confidence interval, 0.444-1.002; P < .001).
The mean axon density in the lateral olfactory tract was significantly less in patients with COVID-19 than in controls (P = .002), indicating a 23% loss of olfactory axons in the COVID group.
Comparing COVID patients with and without reported loss of smell, researchers found those with an altered sense of smell had significantly more severe olfactory axon pathology.
Vascular damage
Patients with COVID also had worse vascular damage. The mean microvasculopathy score (range, 1-3) was 1.907 in patients with COVID-19 and 1.405 in controls (95% CI, 0.259-0.745; P < .001).
There was no evidence of the virus in the olfactory tissue of most patients, suggesting the olfactory pathology was likely caused by vascular damage, said Dr. Ho.
What’s unique about SARS-CoV-2 is that, although it’s a respiratory virus, it’s capable of infecting endothelial cells lining vessels.
“Other respiratory viruses only attack the airways and won’t attack vessels, but vascular damage has been seen in the heart and lung in COVID patients, and our study showed the same findings in the olfactory bulb,” Dr. Ho explained.
The researchers divided patients with COVID by infection severity: mild, moderate, severe, and critical. Interestingly, those with the most severe olfactory pathology were the ones with milder infections, said Dr. Ho.
She noted other studies have reported patients with mild infection are more likely to lose the sense of smell than those with severe infection, but she’s skeptical about this finding.
“Patients with severe COVID are usually hospitalized and intubated, so it’s hard to get them to tell you whether they’ve lost smell or not; they have other more important issues to deal with like respiratory failure,” said Dr. Ho.
Advanced age is associated with neuropathologic changes, such as tau deposits, so the researchers conducted an analysis factoring in age-related brain changes. They found a COVID-19 diagnosis remained associated with increased axonal pathology, reduced axonal density, and increased vascular pathology.
“This means that the COVID patients had more severe olfactory pathology not just because they had more tau pathology,” Dr. Ho added.
New guidance for patients
Commenting for this news organization, Davangere P. Devanand, MD, professor of psychiatry and neurology and director of geriatric psychiatry, Columbia University Irving Medical Center, New York, said the findings indicate the damage from COVID in the olfactory pathway may not be reversible as was previously thought.
“This has been suggested before as a possibility, but the autopsy findings in this case series indicate clearly that there may be permanent damage,” he said.
The results highlight the need to monitor patients with COVID for a smell deficit, said Dr. Devanand.
“Assuring patients of a full recovery in smell and taste may not be sound advice, although recovery does occur in many patients,” he added.
He praised the study design, especially the blinding of raters, but noted a number of weaknesses, including the small sample size and the age and gender discrepancies between the groups.
Another possible limitation was inclusion of patients with Alzheimer’s and Lewy body pathology, said Dr. Devanand.
“These patients typically already have pathology in the olfactory pathways, which means we don’t know if it was COVID or the underlying brain pathology contributing to smell difficulties in these patients,” he said.
He noted that, unlike deceased COVID cases in the study, patients who survive COVID may not experience axonal and microvascular injury in olfactory neurons and pathways and their sense of smell may make a full return.
Dr. Devanand said he would have liked more detailed information on the clinical history and course of study participants and whether these factors affected the pathology findings.
The study was supported by grants from the National Institutes of Health.
Dr. Ho and Dr. Devanand have reported no relevant financial disclosures.
A version of this article first appeared on Medscape.com.
Patients with COVID-19, especially those with an altered sense of smell, have significantly more axon and microvasculopathy damage in the brain’s olfactory tissue versus non-COVID patients. These new findings from a postmortem study may explain long-term loss of smell in some patients with the virus.
“The striking axonal pathology in some cases indicates that olfactory dysfunction in COVID-19 may be severe and permanent,” the investigators led by Cheng-Ying Ho, MD, PhD, associate professor, department of pathology, Johns Hopkins University School of Medicine, Baltimore, write.
“The results show the damage caused by COVID can extend beyond the nasal cavity and involve the brain,” Dr. Ho told this news organization.
The study was published online April 11 in JAMA Neurology.
A more thorough investigation
Patients infected with SARS-CoV-2, which causes COVID-19, present with a wide range of symptoms. In addition to respiratory illnesses, they may exhibit various nonrespiratory manifestations of COVID-19.
One of the most prevalent of these is olfactory dysfunction. Research shows such dysfunction, including anosmia (loss of smell), hyposmia (reduced sense of smell), and parosmia (smells that are distorted or unpleasant), affects 30%-60% of patients with COVID-19, said Dr. Ho.
However, these statistics come from research before the advent of the Omicron variant, which evidence suggests causes less smell loss in patients with COVID, she said.
Previous studies in this area mainly focused on the lining of the nasal cavity. “We wanted to go a step beyond to see how the olfactory bulb was affected by COVID infection,” said Dr. Ho.
The study included 23 deceased patients with confirmed COVID-19 ranging in age from 28 to 93 years at death (median 62 years, 60.9% men). It also included 14 controls who tested negative for COVID-19, ranging in age from 20 to 77 years (median 53.5 years, 50% men).
Researchers collected postmortem tissue from the brain, lung, and other organs and reviewed pertinent clinical information.
Most patients with COVID died of COVID pneumonia or related complications, although some died from a different cause. Some had an active COVID infection and others were “post infection, meaning they were in the recovery stage,” said Dr. Ho.
Six patients with COVID-19 and eight controls had significant brain pathology.
Compared with controls, those with COVID-19 showed significantly worse olfactory axonal damage. The mean axon pathology score (range 1-3 with 3 the worst) was 1.921 in patients with COVID-19 and 1.198 in controls (95% confidence interval, 0.444-1.002; P < .001).
The mean axon density in the lateral olfactory tract was significantly less in patients with COVID-19 than in controls (P = .002), indicating a 23% loss of olfactory axons in the COVID group.
Comparing COVID patients with and without reported loss of smell, researchers found those with an altered sense of smell had significantly more severe olfactory axon pathology.
Vascular damage
Patients with COVID also had worse vascular damage. The mean microvasculopathy score (range, 1-3) was 1.907 in patients with COVID-19 and 1.405 in controls (95% CI, 0.259-0.745; P < .001).
There was no evidence of the virus in the olfactory tissue of most patients, suggesting the olfactory pathology was likely caused by vascular damage, said Dr. Ho.
What’s unique about SARS-CoV-2 is that, although it’s a respiratory virus, it’s capable of infecting endothelial cells lining vessels.
“Other respiratory viruses only attack the airways and won’t attack vessels, but vascular damage has been seen in the heart and lung in COVID patients, and our study showed the same findings in the olfactory bulb,” Dr. Ho explained.
The researchers divided patients with COVID by infection severity: mild, moderate, severe, and critical. Interestingly, those with the most severe olfactory pathology were the ones with milder infections, said Dr. Ho.
She noted other studies have reported patients with mild infection are more likely to lose the sense of smell than those with severe infection, but she’s skeptical about this finding.
“Patients with severe COVID are usually hospitalized and intubated, so it’s hard to get them to tell you whether they’ve lost smell or not; they have other more important issues to deal with like respiratory failure,” said Dr. Ho.
Advanced age is associated with neuropathologic changes, such as tau deposits, so the researchers conducted an analysis factoring in age-related brain changes. They found a COVID-19 diagnosis remained associated with increased axonal pathology, reduced axonal density, and increased vascular pathology.
“This means that the COVID patients had more severe olfactory pathology not just because they had more tau pathology,” Dr. Ho added.
New guidance for patients
Commenting for this news organization, Davangere P. Devanand, MD, professor of psychiatry and neurology and director of geriatric psychiatry, Columbia University Irving Medical Center, New York, said the findings indicate the damage from COVID in the olfactory pathway may not be reversible as was previously thought.
“This has been suggested before as a possibility, but the autopsy findings in this case series indicate clearly that there may be permanent damage,” he said.
The results highlight the need to monitor patients with COVID for a smell deficit, said Dr. Devanand.
“Assuring patients of a full recovery in smell and taste may not be sound advice, although recovery does occur in many patients,” he added.
He praised the study design, especially the blinding of raters, but noted a number of weaknesses, including the small sample size and the age and gender discrepancies between the groups.
Another possible limitation was inclusion of patients with Alzheimer’s and Lewy body pathology, said Dr. Devanand.
“These patients typically already have pathology in the olfactory pathways, which means we don’t know if it was COVID or the underlying brain pathology contributing to smell difficulties in these patients,” he said.
He noted that, unlike deceased COVID cases in the study, patients who survive COVID may not experience axonal and microvascular injury in olfactory neurons and pathways and their sense of smell may make a full return.
Dr. Devanand said he would have liked more detailed information on the clinical history and course of study participants and whether these factors affected the pathology findings.
The study was supported by grants from the National Institutes of Health.
Dr. Ho and Dr. Devanand have reported no relevant financial disclosures.
A version of this article first appeared on Medscape.com.
FROM JAMA NEUROLOGY
Meningococcal vaccine shows moderate protective effect against gonorrhea
A widely approved vaccine for meningitis may provide up to 40% protection against gonorrhea in young adults and adolescents, according to new research. This moderate efficacy paired with a targeted risk-based approach could reduce cases as well as lead to health care savings over 10 years, an additional modeling study showed.
The results – in three linked papers – were published in The Lancet Infectious Diseases.
Gonorrhea, caused by the bacterium Neisseria gonorrhoeae, is the second most commonly reported sexually transmitted infection in the United States, according to the Centers for Disease Control and Prevention. Globally, the World Health Organization estimates that there were 82.4 million new cases in people aged 15-49 in 2020. At the same time, it is becoming more difficult to treat the infection because of the increasing prevalence of drug-resistant strains of N. gonorrhoeae.
“New approaches, such as vaccination, are needed as long-term strategies to prevent gonorrhea and address the emerging threat of antimicrobial resistance,” Winston Abara, MD, PhD, Division of STD Prevention, Centers for Disease Control and Prevention, and colleagues wrote.
While there is currently no vaccine for gonorrhea, observational studies have found an association between a meningococcal serogroup B vaccine and reduced gonorrhea cases. One study in New Zealand found that people vaccinated with the MeNZB vaccine, which was produced to control an outbreak of meningococcal disease in the country, were 31% less likely to contract gonorrhea.
This cross-reactivity comes about because Neisseria meningitidis, the bacterium that can cause meningitis, is closely related to N. gonorrhoeae, Joseph Alex Duncan, MD, PhD, associate professor of medicine, Division of Infectious Diseases, University of North Carolina School of Medicine, Chapel Hill, said in an interview. He was not involved with the research. The thought is that “a large proportion of the proteins that are in the vaccine also recognize proteins from Neisseria gonorrhea, because the bacteria are so similar at the genetic level,” he said.
To see if this association was still found for the four-component serogroup B meningococcal vaccine (MenB-4C), which is now widely available, Dr. Abara and colleagues looked through health records to identify laboratory-confirmed gonorrhea and chlamydia infections in adolescents and young adults in New York City and Philadelphia. All individuals included in the analysis were age 16-23 and all infections occurred between Jan. 1, 2016, and Dec. 31, 2018. These infections were then linked to vaccination records to determine individuals’ MenB-4C vaccination status. Complete vaccination was defined as two MenB-4C doses, delivered 30-180 days apart.
The research team identified over 167,700 infections, including 18,099 gonococcal infections, 124,876 chlamydial infections, and 24,731 coinfections, among 109,737 individuals. A total of 7,692 individuals had received at least one shot of the vaccine, and 3,660 people were fully vaccinated. Full MenB-4C vaccination was estimated to be 40% protective (APR 0.60; P < .0001) against gonorrhea, and partial vaccination was 26% protective (APR 0.74; P = .0012).
“The findings of our study add to the body of evidence that demonstrates that the MenB-4C may offer cross-protection against Neisseria gonorrhoeae, and it supports feasibility of an effective gonococcal vaccine with implications for gonorrhea prevention and control,” Dr. Abara told this news organization.
A second study conducted in South Australia looked at the effectiveness of the MenB-4C vaccine against meningitis and gonorrhea as part of a vaccination program. Using infection data from the Government of South Australia and vaccination records from the Australian Immunization Register, researchers identified individuals born between Feb. 1, 1998, and Feb. 1, 2005, with a documented gonorrhea or chlamydia infection between Feb. 1, 2019, and Jan. 31, 2021. Individuals with chlamydia served as the controls to account for similar sexual behavioral risks.
The analysis included 512 individuals with 575 cases of gonorrhea and 3,140 individuals with 3,847 episodes of chlamydia. In this group, the estimated vaccine effectiveness against gonorrhea was 32.7% (95% confidence interval, 8.3-50.6) in individuals who were fully vaccinated and 32.6% (95% CI, 10.6-49.1) in those who had received at least one dose of the MenB-4C.
While these findings are “confirmatory” because they showed results similar to those in previous observational studies, they are still exciting, Dr. Duncan said. “Up until now, we really haven’t had any real progress in knowing what type of immune responses could actually be protective from the disease,” he said. “These observational studies have really reinvigorated the Neisseria gonorrhea vaccine research community.”
A vaccine with moderate efficacy – like the protection demonstrated in both studies – could lead to a significant reduction in cases, he noted. A 2015 Australian modeling study estimated that a nonwaning vaccine with 20% efficacy could reduce cases by 40% over 20 years. Focusing on vaccinating higher-risk groups could also have an “outsize impact,” said Jeanne Marrazzo, MD, director, Division of Infectious Diseases, UAB Medicine, Birmingham, Alabama, in an interview. In the third study published in The Lancet, researchers estimated the possible reduction of cases and the potential health care cost savings in England in a vaccination effort focusing on men who have sex with men (MSM) at high risk for gonorrhea infection. They predicted that a vaccine with 31% efficacy could prevent 110,200 cases in MSM and save about £8 million ($10.4 million) over 10 years.
Both Dr. Duncan and Dr. Marrazzo agreed that clinical trials are needed to tease out whether the decrease in gonorrhea cases is due to the MenB-4C vaccine or the association is incidental. There are two ongoing clinical trials, one in Australia and one in the United States. Dr. Marrazzo leads the U.S. multicenter study, which also has two locations in Bangkok. The trial will also look at whether vaccination protection varies by the location of gonococcal infection: urethra, rectum, cervix, or pharynx. The two new observational studies did not distinguish the different sites of infection.
Dr. Marrazzo’s trial has enrolled almost 500 individuals so far, with the goal of enrolling over 2,000 participants in total. She hopes to see results by late 2023. “It’s a pretty ambitious effort, but I’m hoping it will give us not only a definitive answer in terms of reduction in infection by anatomic site,” she said, but “also give us a lot of information about how the immune response works to protect you from getting gonorrhea if you do get the vaccine.”
Dr. Duncan has received research grants from the National Institutes of Health. Dr. Marrazzo leads a clinical trial of the MenB-4C vaccine sponsored by the National Institute of Allergy and Infectious Diseases.
A version of this article first appeared on Medscape.com.
A widely approved vaccine for meningitis may provide up to 40% protection against gonorrhea in young adults and adolescents, according to new research. This moderate efficacy paired with a targeted risk-based approach could reduce cases as well as lead to health care savings over 10 years, an additional modeling study showed.
The results – in three linked papers – were published in The Lancet Infectious Diseases.
Gonorrhea, caused by the bacterium Neisseria gonorrhoeae, is the second most commonly reported sexually transmitted infection in the United States, according to the Centers for Disease Control and Prevention. Globally, the World Health Organization estimates that there were 82.4 million new cases in people aged 15-49 in 2020. At the same time, it is becoming more difficult to treat the infection because of the increasing prevalence of drug-resistant strains of N. gonorrhoeae.
“New approaches, such as vaccination, are needed as long-term strategies to prevent gonorrhea and address the emerging threat of antimicrobial resistance,” Winston Abara, MD, PhD, Division of STD Prevention, Centers for Disease Control and Prevention, and colleagues wrote.
While there is currently no vaccine for gonorrhea, observational studies have found an association between a meningococcal serogroup B vaccine and reduced gonorrhea cases. One study in New Zealand found that people vaccinated with the MeNZB vaccine, which was produced to control an outbreak of meningococcal disease in the country, were 31% less likely to contract gonorrhea.
This cross-reactivity comes about because Neisseria meningitidis, the bacterium that can cause meningitis, is closely related to N. gonorrhoeae, Joseph Alex Duncan, MD, PhD, associate professor of medicine, Division of Infectious Diseases, University of North Carolina School of Medicine, Chapel Hill, said in an interview. He was not involved with the research. The thought is that “a large proportion of the proteins that are in the vaccine also recognize proteins from Neisseria gonorrhea, because the bacteria are so similar at the genetic level,” he said.
To see if this association was still found for the four-component serogroup B meningococcal vaccine (MenB-4C), which is now widely available, Dr. Abara and colleagues looked through health records to identify laboratory-confirmed gonorrhea and chlamydia infections in adolescents and young adults in New York City and Philadelphia. All individuals included in the analysis were age 16-23 and all infections occurred between Jan. 1, 2016, and Dec. 31, 2018. These infections were then linked to vaccination records to determine individuals’ MenB-4C vaccination status. Complete vaccination was defined as two MenB-4C doses, delivered 30-180 days apart.
The research team identified over 167,700 infections, including 18,099 gonococcal infections, 124,876 chlamydial infections, and 24,731 coinfections, among 109,737 individuals. A total of 7,692 individuals had received at least one shot of the vaccine, and 3,660 people were fully vaccinated. Full MenB-4C vaccination was estimated to be 40% protective (APR 0.60; P < .0001) against gonorrhea, and partial vaccination was 26% protective (APR 0.74; P = .0012).
“The findings of our study add to the body of evidence that demonstrates that the MenB-4C may offer cross-protection against Neisseria gonorrhoeae, and it supports feasibility of an effective gonococcal vaccine with implications for gonorrhea prevention and control,” Dr. Abara told this news organization.
A second study conducted in South Australia looked at the effectiveness of the MenB-4C vaccine against meningitis and gonorrhea as part of a vaccination program. Using infection data from the Government of South Australia and vaccination records from the Australian Immunization Register, researchers identified individuals born between Feb. 1, 1998, and Feb. 1, 2005, with a documented gonorrhea or chlamydia infection between Feb. 1, 2019, and Jan. 31, 2021. Individuals with chlamydia served as the controls to account for similar sexual behavioral risks.
The analysis included 512 individuals with 575 cases of gonorrhea and 3,140 individuals with 3,847 episodes of chlamydia. In this group, the estimated vaccine effectiveness against gonorrhea was 32.7% (95% confidence interval, 8.3-50.6) in individuals who were fully vaccinated and 32.6% (95% CI, 10.6-49.1) in those who had received at least one dose of the MenB-4C.
While these findings are “confirmatory” because they showed results similar to those in previous observational studies, they are still exciting, Dr. Duncan said. “Up until now, we really haven’t had any real progress in knowing what type of immune responses could actually be protective from the disease,” he said. “These observational studies have really reinvigorated the Neisseria gonorrhea vaccine research community.”
A vaccine with moderate efficacy – like the protection demonstrated in both studies – could lead to a significant reduction in cases, he noted. A 2015 Australian modeling study estimated that a nonwaning vaccine with 20% efficacy could reduce cases by 40% over 20 years. Focusing on vaccinating higher-risk groups could also have an “outsize impact,” said Jeanne Marrazzo, MD, director, Division of Infectious Diseases, UAB Medicine, Birmingham, Alabama, in an interview. In the third study published in The Lancet, researchers estimated the possible reduction of cases and the potential health care cost savings in England in a vaccination effort focusing on men who have sex with men (MSM) at high risk for gonorrhea infection. They predicted that a vaccine with 31% efficacy could prevent 110,200 cases in MSM and save about £8 million ($10.4 million) over 10 years.
Both Dr. Duncan and Dr. Marrazzo agreed that clinical trials are needed to tease out whether the decrease in gonorrhea cases is due to the MenB-4C vaccine or the association is incidental. There are two ongoing clinical trials, one in Australia and one in the United States. Dr. Marrazzo leads the U.S. multicenter study, which also has two locations in Bangkok. The trial will also look at whether vaccination protection varies by the location of gonococcal infection: urethra, rectum, cervix, or pharynx. The two new observational studies did not distinguish the different sites of infection.
Dr. Marrazzo’s trial has enrolled almost 500 individuals so far, with the goal of enrolling over 2,000 participants in total. She hopes to see results by late 2023. “It’s a pretty ambitious effort, but I’m hoping it will give us not only a definitive answer in terms of reduction in infection by anatomic site,” she said, but “also give us a lot of information about how the immune response works to protect you from getting gonorrhea if you do get the vaccine.”
Dr. Duncan has received research grants from the National Institutes of Health. Dr. Marrazzo leads a clinical trial of the MenB-4C vaccine sponsored by the National Institute of Allergy and Infectious Diseases.
A version of this article first appeared on Medscape.com.
A widely approved vaccine for meningitis may provide up to 40% protection against gonorrhea in young adults and adolescents, according to new research. This moderate efficacy paired with a targeted risk-based approach could reduce cases as well as lead to health care savings over 10 years, an additional modeling study showed.
The results – in three linked papers – were published in The Lancet Infectious Diseases.
Gonorrhea, caused by the bacterium Neisseria gonorrhoeae, is the second most commonly reported sexually transmitted infection in the United States, according to the Centers for Disease Control and Prevention. Globally, the World Health Organization estimates that there were 82.4 million new cases in people aged 15-49 in 2020. At the same time, it is becoming more difficult to treat the infection because of the increasing prevalence of drug-resistant strains of N. gonorrhoeae.
“New approaches, such as vaccination, are needed as long-term strategies to prevent gonorrhea and address the emerging threat of antimicrobial resistance,” Winston Abara, MD, PhD, Division of STD Prevention, Centers for Disease Control and Prevention, and colleagues wrote.
While there is currently no vaccine for gonorrhea, observational studies have found an association between a meningococcal serogroup B vaccine and reduced gonorrhea cases. One study in New Zealand found that people vaccinated with the MeNZB vaccine, which was produced to control an outbreak of meningococcal disease in the country, were 31% less likely to contract gonorrhea.
This cross-reactivity comes about because Neisseria meningitidis, the bacterium that can cause meningitis, is closely related to N. gonorrhoeae, Joseph Alex Duncan, MD, PhD, associate professor of medicine, Division of Infectious Diseases, University of North Carolina School of Medicine, Chapel Hill, said in an interview. He was not involved with the research. The thought is that “a large proportion of the proteins that are in the vaccine also recognize proteins from Neisseria gonorrhea, because the bacteria are so similar at the genetic level,” he said.
To see if this association was still found for the four-component serogroup B meningococcal vaccine (MenB-4C), which is now widely available, Dr. Abara and colleagues looked through health records to identify laboratory-confirmed gonorrhea and chlamydia infections in adolescents and young adults in New York City and Philadelphia. All individuals included in the analysis were age 16-23 and all infections occurred between Jan. 1, 2016, and Dec. 31, 2018. These infections were then linked to vaccination records to determine individuals’ MenB-4C vaccination status. Complete vaccination was defined as two MenB-4C doses, delivered 30-180 days apart.
The research team identified over 167,700 infections, including 18,099 gonococcal infections, 124,876 chlamydial infections, and 24,731 coinfections, among 109,737 individuals. A total of 7,692 individuals had received at least one shot of the vaccine, and 3,660 people were fully vaccinated. Full MenB-4C vaccination was estimated to be 40% protective (APR 0.60; P < .0001) against gonorrhea, and partial vaccination was 26% protective (APR 0.74; P = .0012).
“The findings of our study add to the body of evidence that demonstrates that the MenB-4C may offer cross-protection against Neisseria gonorrhoeae, and it supports feasibility of an effective gonococcal vaccine with implications for gonorrhea prevention and control,” Dr. Abara told this news organization.
A second study conducted in South Australia looked at the effectiveness of the MenB-4C vaccine against meningitis and gonorrhea as part of a vaccination program. Using infection data from the Government of South Australia and vaccination records from the Australian Immunization Register, researchers identified individuals born between Feb. 1, 1998, and Feb. 1, 2005, with a documented gonorrhea or chlamydia infection between Feb. 1, 2019, and Jan. 31, 2021. Individuals with chlamydia served as the controls to account for similar sexual behavioral risks.
The analysis included 512 individuals with 575 cases of gonorrhea and 3,140 individuals with 3,847 episodes of chlamydia. In this group, the estimated vaccine effectiveness against gonorrhea was 32.7% (95% confidence interval, 8.3-50.6) in individuals who were fully vaccinated and 32.6% (95% CI, 10.6-49.1) in those who had received at least one dose of the MenB-4C.
While these findings are “confirmatory” because they showed results similar to those in previous observational studies, they are still exciting, Dr. Duncan said. “Up until now, we really haven’t had any real progress in knowing what type of immune responses could actually be protective from the disease,” he said. “These observational studies have really reinvigorated the Neisseria gonorrhea vaccine research community.”
A vaccine with moderate efficacy – like the protection demonstrated in both studies – could lead to a significant reduction in cases, he noted. A 2015 Australian modeling study estimated that a nonwaning vaccine with 20% efficacy could reduce cases by 40% over 20 years. Focusing on vaccinating higher-risk groups could also have an “outsize impact,” said Jeanne Marrazzo, MD, director, Division of Infectious Diseases, UAB Medicine, Birmingham, Alabama, in an interview. In the third study published in The Lancet, researchers estimated the possible reduction of cases and the potential health care cost savings in England in a vaccination effort focusing on men who have sex with men (MSM) at high risk for gonorrhea infection. They predicted that a vaccine with 31% efficacy could prevent 110,200 cases in MSM and save about £8 million ($10.4 million) over 10 years.
Both Dr. Duncan and Dr. Marrazzo agreed that clinical trials are needed to tease out whether the decrease in gonorrhea cases is due to the MenB-4C vaccine or the association is incidental. There are two ongoing clinical trials, one in Australia and one in the United States. Dr. Marrazzo leads the U.S. multicenter study, which also has two locations in Bangkok. The trial will also look at whether vaccination protection varies by the location of gonococcal infection: urethra, rectum, cervix, or pharynx. The two new observational studies did not distinguish the different sites of infection.
Dr. Marrazzo’s trial has enrolled almost 500 individuals so far, with the goal of enrolling over 2,000 participants in total. She hopes to see results by late 2023. “It’s a pretty ambitious effort, but I’m hoping it will give us not only a definitive answer in terms of reduction in infection by anatomic site,” she said, but “also give us a lot of information about how the immune response works to protect you from getting gonorrhea if you do get the vaccine.”
Dr. Duncan has received research grants from the National Institutes of Health. Dr. Marrazzo leads a clinical trial of the MenB-4C vaccine sponsored by the National Institute of Allergy and Infectious Diseases.
A version of this article first appeared on Medscape.com.
FROM THE LANCET INFECTIOUS DISEASES
Children with RMDs not at high risk for severe COVID-19, study finds
The of short-term COVID-19 outcomes in this patient group to date.
In the study, only 1 in 15 (7%) children and young people (younger than 19 years) with RMDs and COVID-19 were hospitalized, and even then, they experienced only mild symptoms; 4 of 5 of those hospitalized did not require supplemental oxygen or ventilatory support.
The study also found that those with severe systemic RMDs and obesity were more likely to be hospitalized than children with juvenile idiopathic arthritis (JIA).
Treatment with biologics, such as tumor necrosis factor inhibitors, did not appear to be associated with more severe COVID-19; however, the study found that children and young people with obesity (body mass index ≥ 30) were more likely to be hospitalized, although only 6% of patients in this study had a BMI in this category. Three patients died – two from areas of lower resources who were diagnosed with systemic lupus erythematosus (SLE) at approximately the same time they were diagnosed with COVID-19, and one with a preexisting autoinflammatory syndrome who was being treated with low-dose glucocorticoids and methotrexate.
Published in Annals of the Rheumatic Diseases, the study was led by Kimme L. Hyrich, MD, PhD, and Lianne Kearsley-Fleet, PhD, both from the University of Manchester (England). Dr. Hyrich is also a consultant rheumatologist at Manchester University Hospitals NHS Foundation Trust.
In an interview, Dr. Hyrich explained that overall these data are reassuring and show that the majority of children and young people with RMDs are not at high risk of severe COVID-19.
“Many parents and families with children who have RMDs have lived with great fear over the pandemic about whether or not their children are at an increased risk of severe COVID-19,” said Dr. Hyrich. “Many are immunosuppressed or take other immunomodulatory medications. This has also had a great impact on schooling and children’s well-being.”
In the study, children with SLE, mixed connective tissue disease (MCTD), or vasculitis were more likely to have severe COVID-19. “[This] is not surprising given the typically greater systemic involvement and need for more aggressive immunosuppressive therapy than the majority of individuals with JIA,” the researchers wrote.
Dr. Hyrich added: “There may be times when children are on particularly high doses of immunosuppression or their disease is particularly active, when they may need more protection, and rheumatology teams can advise parents and young people about this.”
Studies such as those by Zimmerman and Curtis and Viner and colleagues have found that generally, children with no underlying disease are less susceptible to symptomatic COVID-19 and that reports of death are rare. Findings show that the younger the child, the less likely they will be symptomatic.
Adult data suggest a higher risk of COVID-related death among patients with arthritis, lupus, or psoriasis. A recent systematic review of the literature suggested that increased risk of COVID-related death only applies to subgroups of people with RMDs.
However, whether children and young people with RMDs are likely to have more severe COVID-19 and whether there is additional risk attributable to either their underlying disease or its therapy remain unknown. The goal of the study by Dr. Hyrich and colleagues was to address these questions.
The global analysis aimed to describe characteristics of those children and young people (younger than 19 years) with preexisting RMDs who also had COVID-19; to describe outcomes following COVID-19; and to identify characteristics associated with more severe COVID-19 outcomes.
Data were drawn from the European Alliance of Associations for Rheumatology COVID-19 Registry, the Childhood Arthritis and Rheumatology Research Alliance Registry, and the CARRA-sponsored COVID-19 Global Paediatric Rheumatology Database.
Demographic information included primary RMD diagnosis; RMD disease activity (remission, low, moderate, high, or unknown); RMD treatments, including glucocorticoid use and which disease-modifying antirheumatic drug (DMARD) the patient was taking at the time of COVID-19; and comorbidities (none, ocular inflammation, interstitial lung disease, asthma, diabetes, obesity, hypertension, cerebrovascular accident, renal disease, inflammatory bowel disease, and heart disease).
With respect to COVID-19, information collected included diagnosis date, whether the case was presumptive or confirmed, clinical symptoms, hospitalization and/or death because of COVID-19, and whether the patient stopped receiving rheumatic therapies.
Rheumatology diagnoses were categorized into four groups: JIA; SLE, MCTD, vasculitis, or other RMD; autoinflammatory syndromes; and “other,” including chronic recurrent multifocal osteomyelitis, sarcoidosis, or ocular inflammation.
Of the 607 children and young people with reported SARS-CoV-2 infection from 25 different countries (464 from the EULAR COVID-19 Registry), 499 (82%) cases were polymerase chain reaction confirmed, and 399 (66%) patients were female (median age, 14 years). Most (62%) had JIA: 37%, polyarticular JIA; 30%, oligoarticular JIA; 12%, enthesitis-related JIA; 9%, systemic JIA; 4%, psoriatic JIA; and 9%, JIA of unknown subcategory. Furthermore, 13% of patients had autoinflammatory syndromes, 8% with SLE or MCTD, 3% with vasculitis, and 2% with inflammatory myopathy.
No associations were seen between DMARD treatment (conventional-synthetic, biologic/targeted-synthetic, or combination therapy), compared with no DMARD treatment, glucocorticoid use, and hospitalization.
Owing to substantial differences in reporting of race and ethnicity between data sources, the researchers were unable to analyze whether Black, Asian, and minority ethnic groups with pediatric RMDs are at higher risk of COVID-19–related death, compared with those of White ethnicity, as has been reported for the general population.
The study also did not account for variants of SARS-CoV-2 other than to note that data were collected prior to the spread of the Omicron variant. Also, the registries did not capture vaccination status (though very few children had received vaccines at the time of data collection) or information on long COVID or multisystem inflammatory syndrome in children.
Dr. Hyrich and Dr. Kearsley-Fleet have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The of short-term COVID-19 outcomes in this patient group to date.
In the study, only 1 in 15 (7%) children and young people (younger than 19 years) with RMDs and COVID-19 were hospitalized, and even then, they experienced only mild symptoms; 4 of 5 of those hospitalized did not require supplemental oxygen or ventilatory support.
The study also found that those with severe systemic RMDs and obesity were more likely to be hospitalized than children with juvenile idiopathic arthritis (JIA).
Treatment with biologics, such as tumor necrosis factor inhibitors, did not appear to be associated with more severe COVID-19; however, the study found that children and young people with obesity (body mass index ≥ 30) were more likely to be hospitalized, although only 6% of patients in this study had a BMI in this category. Three patients died – two from areas of lower resources who were diagnosed with systemic lupus erythematosus (SLE) at approximately the same time they were diagnosed with COVID-19, and one with a preexisting autoinflammatory syndrome who was being treated with low-dose glucocorticoids and methotrexate.
Published in Annals of the Rheumatic Diseases, the study was led by Kimme L. Hyrich, MD, PhD, and Lianne Kearsley-Fleet, PhD, both from the University of Manchester (England). Dr. Hyrich is also a consultant rheumatologist at Manchester University Hospitals NHS Foundation Trust.
In an interview, Dr. Hyrich explained that overall these data are reassuring and show that the majority of children and young people with RMDs are not at high risk of severe COVID-19.
“Many parents and families with children who have RMDs have lived with great fear over the pandemic about whether or not their children are at an increased risk of severe COVID-19,” said Dr. Hyrich. “Many are immunosuppressed or take other immunomodulatory medications. This has also had a great impact on schooling and children’s well-being.”
In the study, children with SLE, mixed connective tissue disease (MCTD), or vasculitis were more likely to have severe COVID-19. “[This] is not surprising given the typically greater systemic involvement and need for more aggressive immunosuppressive therapy than the majority of individuals with JIA,” the researchers wrote.
Dr. Hyrich added: “There may be times when children are on particularly high doses of immunosuppression or their disease is particularly active, when they may need more protection, and rheumatology teams can advise parents and young people about this.”
Studies such as those by Zimmerman and Curtis and Viner and colleagues have found that generally, children with no underlying disease are less susceptible to symptomatic COVID-19 and that reports of death are rare. Findings show that the younger the child, the less likely they will be symptomatic.
Adult data suggest a higher risk of COVID-related death among patients with arthritis, lupus, or psoriasis. A recent systematic review of the literature suggested that increased risk of COVID-related death only applies to subgroups of people with RMDs.
However, whether children and young people with RMDs are likely to have more severe COVID-19 and whether there is additional risk attributable to either their underlying disease or its therapy remain unknown. The goal of the study by Dr. Hyrich and colleagues was to address these questions.
The global analysis aimed to describe characteristics of those children and young people (younger than 19 years) with preexisting RMDs who also had COVID-19; to describe outcomes following COVID-19; and to identify characteristics associated with more severe COVID-19 outcomes.
Data were drawn from the European Alliance of Associations for Rheumatology COVID-19 Registry, the Childhood Arthritis and Rheumatology Research Alliance Registry, and the CARRA-sponsored COVID-19 Global Paediatric Rheumatology Database.
Demographic information included primary RMD diagnosis; RMD disease activity (remission, low, moderate, high, or unknown); RMD treatments, including glucocorticoid use and which disease-modifying antirheumatic drug (DMARD) the patient was taking at the time of COVID-19; and comorbidities (none, ocular inflammation, interstitial lung disease, asthma, diabetes, obesity, hypertension, cerebrovascular accident, renal disease, inflammatory bowel disease, and heart disease).
With respect to COVID-19, information collected included diagnosis date, whether the case was presumptive or confirmed, clinical symptoms, hospitalization and/or death because of COVID-19, and whether the patient stopped receiving rheumatic therapies.
Rheumatology diagnoses were categorized into four groups: JIA; SLE, MCTD, vasculitis, or other RMD; autoinflammatory syndromes; and “other,” including chronic recurrent multifocal osteomyelitis, sarcoidosis, or ocular inflammation.
Of the 607 children and young people with reported SARS-CoV-2 infection from 25 different countries (464 from the EULAR COVID-19 Registry), 499 (82%) cases were polymerase chain reaction confirmed, and 399 (66%) patients were female (median age, 14 years). Most (62%) had JIA: 37%, polyarticular JIA; 30%, oligoarticular JIA; 12%, enthesitis-related JIA; 9%, systemic JIA; 4%, psoriatic JIA; and 9%, JIA of unknown subcategory. Furthermore, 13% of patients had autoinflammatory syndromes, 8% with SLE or MCTD, 3% with vasculitis, and 2% with inflammatory myopathy.
No associations were seen between DMARD treatment (conventional-synthetic, biologic/targeted-synthetic, or combination therapy), compared with no DMARD treatment, glucocorticoid use, and hospitalization.
Owing to substantial differences in reporting of race and ethnicity between data sources, the researchers were unable to analyze whether Black, Asian, and minority ethnic groups with pediatric RMDs are at higher risk of COVID-19–related death, compared with those of White ethnicity, as has been reported for the general population.
The study also did not account for variants of SARS-CoV-2 other than to note that data were collected prior to the spread of the Omicron variant. Also, the registries did not capture vaccination status (though very few children had received vaccines at the time of data collection) or information on long COVID or multisystem inflammatory syndrome in children.
Dr. Hyrich and Dr. Kearsley-Fleet have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The of short-term COVID-19 outcomes in this patient group to date.
In the study, only 1 in 15 (7%) children and young people (younger than 19 years) with RMDs and COVID-19 were hospitalized, and even then, they experienced only mild symptoms; 4 of 5 of those hospitalized did not require supplemental oxygen or ventilatory support.
The study also found that those with severe systemic RMDs and obesity were more likely to be hospitalized than children with juvenile idiopathic arthritis (JIA).
Treatment with biologics, such as tumor necrosis factor inhibitors, did not appear to be associated with more severe COVID-19; however, the study found that children and young people with obesity (body mass index ≥ 30) were more likely to be hospitalized, although only 6% of patients in this study had a BMI in this category. Three patients died – two from areas of lower resources who were diagnosed with systemic lupus erythematosus (SLE) at approximately the same time they were diagnosed with COVID-19, and one with a preexisting autoinflammatory syndrome who was being treated with low-dose glucocorticoids and methotrexate.
Published in Annals of the Rheumatic Diseases, the study was led by Kimme L. Hyrich, MD, PhD, and Lianne Kearsley-Fleet, PhD, both from the University of Manchester (England). Dr. Hyrich is also a consultant rheumatologist at Manchester University Hospitals NHS Foundation Trust.
In an interview, Dr. Hyrich explained that overall these data are reassuring and show that the majority of children and young people with RMDs are not at high risk of severe COVID-19.
“Many parents and families with children who have RMDs have lived with great fear over the pandemic about whether or not their children are at an increased risk of severe COVID-19,” said Dr. Hyrich. “Many are immunosuppressed or take other immunomodulatory medications. This has also had a great impact on schooling and children’s well-being.”
In the study, children with SLE, mixed connective tissue disease (MCTD), or vasculitis were more likely to have severe COVID-19. “[This] is not surprising given the typically greater systemic involvement and need for more aggressive immunosuppressive therapy than the majority of individuals with JIA,” the researchers wrote.
Dr. Hyrich added: “There may be times when children are on particularly high doses of immunosuppression or their disease is particularly active, when they may need more protection, and rheumatology teams can advise parents and young people about this.”
Studies such as those by Zimmerman and Curtis and Viner and colleagues have found that generally, children with no underlying disease are less susceptible to symptomatic COVID-19 and that reports of death are rare. Findings show that the younger the child, the less likely they will be symptomatic.
Adult data suggest a higher risk of COVID-related death among patients with arthritis, lupus, or psoriasis. A recent systematic review of the literature suggested that increased risk of COVID-related death only applies to subgroups of people with RMDs.
However, whether children and young people with RMDs are likely to have more severe COVID-19 and whether there is additional risk attributable to either their underlying disease or its therapy remain unknown. The goal of the study by Dr. Hyrich and colleagues was to address these questions.
The global analysis aimed to describe characteristics of those children and young people (younger than 19 years) with preexisting RMDs who also had COVID-19; to describe outcomes following COVID-19; and to identify characteristics associated with more severe COVID-19 outcomes.
Data were drawn from the European Alliance of Associations for Rheumatology COVID-19 Registry, the Childhood Arthritis and Rheumatology Research Alliance Registry, and the CARRA-sponsored COVID-19 Global Paediatric Rheumatology Database.
Demographic information included primary RMD diagnosis; RMD disease activity (remission, low, moderate, high, or unknown); RMD treatments, including glucocorticoid use and which disease-modifying antirheumatic drug (DMARD) the patient was taking at the time of COVID-19; and comorbidities (none, ocular inflammation, interstitial lung disease, asthma, diabetes, obesity, hypertension, cerebrovascular accident, renal disease, inflammatory bowel disease, and heart disease).
With respect to COVID-19, information collected included diagnosis date, whether the case was presumptive or confirmed, clinical symptoms, hospitalization and/or death because of COVID-19, and whether the patient stopped receiving rheumatic therapies.
Rheumatology diagnoses were categorized into four groups: JIA; SLE, MCTD, vasculitis, or other RMD; autoinflammatory syndromes; and “other,” including chronic recurrent multifocal osteomyelitis, sarcoidosis, or ocular inflammation.
Of the 607 children and young people with reported SARS-CoV-2 infection from 25 different countries (464 from the EULAR COVID-19 Registry), 499 (82%) cases were polymerase chain reaction confirmed, and 399 (66%) patients were female (median age, 14 years). Most (62%) had JIA: 37%, polyarticular JIA; 30%, oligoarticular JIA; 12%, enthesitis-related JIA; 9%, systemic JIA; 4%, psoriatic JIA; and 9%, JIA of unknown subcategory. Furthermore, 13% of patients had autoinflammatory syndromes, 8% with SLE or MCTD, 3% with vasculitis, and 2% with inflammatory myopathy.
No associations were seen between DMARD treatment (conventional-synthetic, biologic/targeted-synthetic, or combination therapy), compared with no DMARD treatment, glucocorticoid use, and hospitalization.
Owing to substantial differences in reporting of race and ethnicity between data sources, the researchers were unable to analyze whether Black, Asian, and minority ethnic groups with pediatric RMDs are at higher risk of COVID-19–related death, compared with those of White ethnicity, as has been reported for the general population.
The study also did not account for variants of SARS-CoV-2 other than to note that data were collected prior to the spread of the Omicron variant. Also, the registries did not capture vaccination status (though very few children had received vaccines at the time of data collection) or information on long COVID or multisystem inflammatory syndrome in children.
Dr. Hyrich and Dr. Kearsley-Fleet have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM ANNALS OF THE RHEUMATIC DISEASES
Locoregional therapy lowers wait-list dropout in HCC
The use of bridging locoregional therapy (LRT) before liver transplantation in patients with hepatocellular carcinoma (HCC) has significantly increased in the United States within the past 15 years, a recent analysis suggests. Data show that liver transplant candidates with HCC who have elevated tumor burden and patients with more compensated liver disease have received a greater number of treatments while awaiting transplant.
According to the researchers, led by Allison Kwong, MD, of Stanford (Calif.) University, liver transplant remains a curative option for individuals with unresectable HCC who meet prespecified size criteria. In the United States, a mandated waiting period of 6 months prior “to gaining exception points has been implemented” in an effort “to allow for consideration of tumor biology and reduce the disparities in wait-list dropout between HCC and non-HCC patients,” the researchers wrote.
Several forms of LRT are now available for HCC, including chemoembolization, external beam radiation, radioembolization, and radiofrequency or microwave ablation. In the liver transplant setting, these LRT options enable management of intrahepatic disease in patients who are waiting for liver transplant, Dr. Kwong and colleagues explained.
The researchers, who published their study findings in the May issue of Clinical Gastroenterology and Hepatology, sought to examine the national temporal trends and wait-list outcomes of LRT in 31,609 patients eligible for liver transplant with greater than or equal to one approved HCC exception application in the United States.
Patient data were obtained from the Organ Procurement and Transplantation Network database and comprised primary adult LT candidates who were listed from the years 2003 to 2018. The investigators assessed explant histology and performed multivariable competing risk analysis to examine the relationship between the type of first LRT and time to wait-list dropout.
The wait-list dropout variable was defined by list removal because of death or excessive illness. The researchers noted that list removal likely represents disease progression “beyond transplantable criteria and beyond which patients were unlikely to benefit from or be eligible for further LRT.”
In the study population, the median age was 59 years, and approximately 77% of patients were male. More than half (53.1%) of the cohort had hepatitis C as the predominant liver disease etiology. Patients had a median follow-up period of 214 days on the waiting list.
Most patients (79%) received deceased or living-donor transplants, and 18.6% of patients were removed from the waiting list. Between the 2003 and 2006 period, the median wait-list time was 123 days, but this median wait-list duration increased to 257 days for patients listed between 2015 and 2018.
A total of 34,610 LRTs were performed among 24,145 liver transplant candidates during the study period. From 2003 to 2018, the proportion of patients with greater than or equal to 1 LRT recorded in the database rose from 42.3% to 92.4%, respectively. Most patients (67.8%) who received liver-directed therapy had a single LRT, while 23.8% of patients had two LRTs, 6.2% had three LRTs, and 2.2% had greater than or equal to four LRTs.
The most frequent type of LRT performed was chemoembolization, followed by thermal ablation. Radioembolization increased from less than 5% in 2013 to 19% in 2018. Moreover, in 2018, chemoembolization accounted for 50% of LRTs, while thermal ablation accounted for 22% of LRTs.
The incidence rates of LRT per 100 wait-list days was above average in patients who had an initial tumor burden beyond the Milan criteria (0.188), an alpha-fetoprotein level of 21-40 (0.171) or 41-500 ng/mL (0.179), Child-Pugh class A (0.160), patients in short (0.151) and medium (0.154) wait-time regions, as well as patients who were listed following implementation of cap-and-delay in October 2015 (0.192).
In the multivariable competing-risk analysis for wait-list dropout, adjusting for initial tumor burden and AFP, Child-Pugh class, wait region, and listing era, no locoregional therapy was associated with an increased risk of wait-list dropout versus chemoembolization as the first LRT in a multivariable competing-risk analysis (subhazard ratio, 1.37; 95% CI, 1.28-1.47). The inverse probability of treatment weighting–adjusted analysis found an association between radioembolization, when compared with chemoembolization, and a reduced risk of wait-list dropout (sHR, 0.85; 95% CI, 0.81-0.89). Thermal ablation was also associated with a reduced risk of wait-list dropout, compared with chemoembolization (sHR, 0.95; 95% CI, 0.91-0.99). “Radioembolization and thermal ablation may be superior to chemoembolization and prove to be more cost-effective options, depending on the clinical context,” the researchers wrote.
The researchers noted that they were unable to distinguish patients who were removed from the waiting list between those with disease progression versus liver failure.
The researchers reported no conflicts of interest with the pharmaceutical industry. The study received no industry funding.
In 1996, Mazzaferro and colleagues reported the results of a cohort of 48 patients with cirrhosis who had small, unresectable hepatocellular carcinoma (HCC). The actuarial survival rate was 75% at 4 years, and 83% of these patients had no recurrence, so, orthotopic liver transplantation became one of the standard options with curative intent for the treatment HCC. Because of HCC biology, some of these tumors grow or, worst-case scenario, are outside the Milan criteria. Locoregional therapies (LRT) were applied to arrest or downsize the tumor(s) to be within the liver transplantation criteria.
Kwong and colleagues, using the data of the Organ Procurement and Transplantation Network database, showed an exponential increase of LRT over 15 years: from 32.5% in 2003 to 92.4% in 2018. The Barcelona Clinic Liver Cancer staging system classifies chemoembolization, the most common LRT modality used in this cohort, as a palliative treatment rather than curative. Not surprisingly, the authors found that radioembolization was independently associated with a 15% reduction in the wait-list dropout rate, compared with chemoembolization. Further, listing in longer wait-time regions and more recent years was independently associated with a higher likelihood of wait-list dropout.
These data may be worrisome for patients listed for HCC. The median Model for End-Stage Liver Disease at Transplant Minus 3 National Policy, introduced in May 2019, decreases the transplantation rates in patients with HCC. Consequently, longer wait-list time leads to increase utilization of LRT to keep these patients within criteria. Radioembolization could become the preferred LRT therapy to stop tumor growth than chemoembolization and, probably, will be more cost effective. Future work should address explant outcomes and outcome on downstaging with external radiation therapy and adjuvant use of immunotherapy.
Ruben Hernaez, MD, MPH, PhD, is an assistant professor at the Michael E. DeBakey Veterans Affairs Medical Center and Baylor College of Medicine, both in Houston. He has no relevant conflicts to disclose.
In 1996, Mazzaferro and colleagues reported the results of a cohort of 48 patients with cirrhosis who had small, unresectable hepatocellular carcinoma (HCC). The actuarial survival rate was 75% at 4 years, and 83% of these patients had no recurrence, so, orthotopic liver transplantation became one of the standard options with curative intent for the treatment HCC. Because of HCC biology, some of these tumors grow or, worst-case scenario, are outside the Milan criteria. Locoregional therapies (LRT) were applied to arrest or downsize the tumor(s) to be within the liver transplantation criteria.
Kwong and colleagues, using the data of the Organ Procurement and Transplantation Network database, showed an exponential increase of LRT over 15 years: from 32.5% in 2003 to 92.4% in 2018. The Barcelona Clinic Liver Cancer staging system classifies chemoembolization, the most common LRT modality used in this cohort, as a palliative treatment rather than curative. Not surprisingly, the authors found that radioembolization was independently associated with a 15% reduction in the wait-list dropout rate, compared with chemoembolization. Further, listing in longer wait-time regions and more recent years was independently associated with a higher likelihood of wait-list dropout.
These data may be worrisome for patients listed for HCC. The median Model for End-Stage Liver Disease at Transplant Minus 3 National Policy, introduced in May 2019, decreases the transplantation rates in patients with HCC. Consequently, longer wait-list time leads to increase utilization of LRT to keep these patients within criteria. Radioembolization could become the preferred LRT therapy to stop tumor growth than chemoembolization and, probably, will be more cost effective. Future work should address explant outcomes and outcome on downstaging with external radiation therapy and adjuvant use of immunotherapy.
Ruben Hernaez, MD, MPH, PhD, is an assistant professor at the Michael E. DeBakey Veterans Affairs Medical Center and Baylor College of Medicine, both in Houston. He has no relevant conflicts to disclose.
In 1996, Mazzaferro and colleagues reported the results of a cohort of 48 patients with cirrhosis who had small, unresectable hepatocellular carcinoma (HCC). The actuarial survival rate was 75% at 4 years, and 83% of these patients had no recurrence, so, orthotopic liver transplantation became one of the standard options with curative intent for the treatment HCC. Because of HCC biology, some of these tumors grow or, worst-case scenario, are outside the Milan criteria. Locoregional therapies (LRT) were applied to arrest or downsize the tumor(s) to be within the liver transplantation criteria.
Kwong and colleagues, using the data of the Organ Procurement and Transplantation Network database, showed an exponential increase of LRT over 15 years: from 32.5% in 2003 to 92.4% in 2018. The Barcelona Clinic Liver Cancer staging system classifies chemoembolization, the most common LRT modality used in this cohort, as a palliative treatment rather than curative. Not surprisingly, the authors found that radioembolization was independently associated with a 15% reduction in the wait-list dropout rate, compared with chemoembolization. Further, listing in longer wait-time regions and more recent years was independently associated with a higher likelihood of wait-list dropout.
These data may be worrisome for patients listed for HCC. The median Model for End-Stage Liver Disease at Transplant Minus 3 National Policy, introduced in May 2019, decreases the transplantation rates in patients with HCC. Consequently, longer wait-list time leads to increase utilization of LRT to keep these patients within criteria. Radioembolization could become the preferred LRT therapy to stop tumor growth than chemoembolization and, probably, will be more cost effective. Future work should address explant outcomes and outcome on downstaging with external radiation therapy and adjuvant use of immunotherapy.
Ruben Hernaez, MD, MPH, PhD, is an assistant professor at the Michael E. DeBakey Veterans Affairs Medical Center and Baylor College of Medicine, both in Houston. He has no relevant conflicts to disclose.
The use of bridging locoregional therapy (LRT) before liver transplantation in patients with hepatocellular carcinoma (HCC) has significantly increased in the United States within the past 15 years, a recent analysis suggests. Data show that liver transplant candidates with HCC who have elevated tumor burden and patients with more compensated liver disease have received a greater number of treatments while awaiting transplant.
According to the researchers, led by Allison Kwong, MD, of Stanford (Calif.) University, liver transplant remains a curative option for individuals with unresectable HCC who meet prespecified size criteria. In the United States, a mandated waiting period of 6 months prior “to gaining exception points has been implemented” in an effort “to allow for consideration of tumor biology and reduce the disparities in wait-list dropout between HCC and non-HCC patients,” the researchers wrote.
Several forms of LRT are now available for HCC, including chemoembolization, external beam radiation, radioembolization, and radiofrequency or microwave ablation. In the liver transplant setting, these LRT options enable management of intrahepatic disease in patients who are waiting for liver transplant, Dr. Kwong and colleagues explained.
The researchers, who published their study findings in the May issue of Clinical Gastroenterology and Hepatology, sought to examine the national temporal trends and wait-list outcomes of LRT in 31,609 patients eligible for liver transplant with greater than or equal to one approved HCC exception application in the United States.
Patient data were obtained from the Organ Procurement and Transplantation Network database and comprised primary adult LT candidates who were listed from the years 2003 to 2018. The investigators assessed explant histology and performed multivariable competing risk analysis to examine the relationship between the type of first LRT and time to wait-list dropout.
The wait-list dropout variable was defined by list removal because of death or excessive illness. The researchers noted that list removal likely represents disease progression “beyond transplantable criteria and beyond which patients were unlikely to benefit from or be eligible for further LRT.”
In the study population, the median age was 59 years, and approximately 77% of patients were male. More than half (53.1%) of the cohort had hepatitis C as the predominant liver disease etiology. Patients had a median follow-up period of 214 days on the waiting list.
Most patients (79%) received deceased or living-donor transplants, and 18.6% of patients were removed from the waiting list. Between the 2003 and 2006 period, the median wait-list time was 123 days, but this median wait-list duration increased to 257 days for patients listed between 2015 and 2018.
A total of 34,610 LRTs were performed among 24,145 liver transplant candidates during the study period. From 2003 to 2018, the proportion of patients with greater than or equal to 1 LRT recorded in the database rose from 42.3% to 92.4%, respectively. Most patients (67.8%) who received liver-directed therapy had a single LRT, while 23.8% of patients had two LRTs, 6.2% had three LRTs, and 2.2% had greater than or equal to four LRTs.
The most frequent type of LRT performed was chemoembolization, followed by thermal ablation. Radioembolization increased from less than 5% in 2013 to 19% in 2018. Moreover, in 2018, chemoembolization accounted for 50% of LRTs, while thermal ablation accounted for 22% of LRTs.
The incidence rates of LRT per 100 wait-list days was above average in patients who had an initial tumor burden beyond the Milan criteria (0.188), an alpha-fetoprotein level of 21-40 (0.171) or 41-500 ng/mL (0.179), Child-Pugh class A (0.160), patients in short (0.151) and medium (0.154) wait-time regions, as well as patients who were listed following implementation of cap-and-delay in October 2015 (0.192).
In the multivariable competing-risk analysis for wait-list dropout, adjusting for initial tumor burden and AFP, Child-Pugh class, wait region, and listing era, no locoregional therapy was associated with an increased risk of wait-list dropout versus chemoembolization as the first LRT in a multivariable competing-risk analysis (subhazard ratio, 1.37; 95% CI, 1.28-1.47). The inverse probability of treatment weighting–adjusted analysis found an association between radioembolization, when compared with chemoembolization, and a reduced risk of wait-list dropout (sHR, 0.85; 95% CI, 0.81-0.89). Thermal ablation was also associated with a reduced risk of wait-list dropout, compared with chemoembolization (sHR, 0.95; 95% CI, 0.91-0.99). “Radioembolization and thermal ablation may be superior to chemoembolization and prove to be more cost-effective options, depending on the clinical context,” the researchers wrote.
The researchers noted that they were unable to distinguish patients who were removed from the waiting list between those with disease progression versus liver failure.
The researchers reported no conflicts of interest with the pharmaceutical industry. The study received no industry funding.
The use of bridging locoregional therapy (LRT) before liver transplantation in patients with hepatocellular carcinoma (HCC) has significantly increased in the United States within the past 15 years, a recent analysis suggests. Data show that liver transplant candidates with HCC who have elevated tumor burden and patients with more compensated liver disease have received a greater number of treatments while awaiting transplant.
According to the researchers, led by Allison Kwong, MD, of Stanford (Calif.) University, liver transplant remains a curative option for individuals with unresectable HCC who meet prespecified size criteria. In the United States, a mandated waiting period of 6 months prior “to gaining exception points has been implemented” in an effort “to allow for consideration of tumor biology and reduce the disparities in wait-list dropout between HCC and non-HCC patients,” the researchers wrote.
Several forms of LRT are now available for HCC, including chemoembolization, external beam radiation, radioembolization, and radiofrequency or microwave ablation. In the liver transplant setting, these LRT options enable management of intrahepatic disease in patients who are waiting for liver transplant, Dr. Kwong and colleagues explained.
The researchers, who published their study findings in the May issue of Clinical Gastroenterology and Hepatology, sought to examine the national temporal trends and wait-list outcomes of LRT in 31,609 patients eligible for liver transplant with greater than or equal to one approved HCC exception application in the United States.
Patient data were obtained from the Organ Procurement and Transplantation Network database and comprised primary adult LT candidates who were listed from the years 2003 to 2018. The investigators assessed explant histology and performed multivariable competing risk analysis to examine the relationship between the type of first LRT and time to wait-list dropout.
The wait-list dropout variable was defined by list removal because of death or excessive illness. The researchers noted that list removal likely represents disease progression “beyond transplantable criteria and beyond which patients were unlikely to benefit from or be eligible for further LRT.”
In the study population, the median age was 59 years, and approximately 77% of patients were male. More than half (53.1%) of the cohort had hepatitis C as the predominant liver disease etiology. Patients had a median follow-up period of 214 days on the waiting list.
Most patients (79%) received deceased or living-donor transplants, and 18.6% of patients were removed from the waiting list. Between the 2003 and 2006 period, the median wait-list time was 123 days, but this median wait-list duration increased to 257 days for patients listed between 2015 and 2018.
A total of 34,610 LRTs were performed among 24,145 liver transplant candidates during the study period. From 2003 to 2018, the proportion of patients with greater than or equal to 1 LRT recorded in the database rose from 42.3% to 92.4%, respectively. Most patients (67.8%) who received liver-directed therapy had a single LRT, while 23.8% of patients had two LRTs, 6.2% had three LRTs, and 2.2% had greater than or equal to four LRTs.
The most frequent type of LRT performed was chemoembolization, followed by thermal ablation. Radioembolization increased from less than 5% in 2013 to 19% in 2018. Moreover, in 2018, chemoembolization accounted for 50% of LRTs, while thermal ablation accounted for 22% of LRTs.
The incidence rates of LRT per 100 wait-list days was above average in patients who had an initial tumor burden beyond the Milan criteria (0.188), an alpha-fetoprotein level of 21-40 (0.171) or 41-500 ng/mL (0.179), Child-Pugh class A (0.160), patients in short (0.151) and medium (0.154) wait-time regions, as well as patients who were listed following implementation of cap-and-delay in October 2015 (0.192).
In the multivariable competing-risk analysis for wait-list dropout, adjusting for initial tumor burden and AFP, Child-Pugh class, wait region, and listing era, no locoregional therapy was associated with an increased risk of wait-list dropout versus chemoembolization as the first LRT in a multivariable competing-risk analysis (subhazard ratio, 1.37; 95% CI, 1.28-1.47). The inverse probability of treatment weighting–adjusted analysis found an association between radioembolization, when compared with chemoembolization, and a reduced risk of wait-list dropout (sHR, 0.85; 95% CI, 0.81-0.89). Thermal ablation was also associated with a reduced risk of wait-list dropout, compared with chemoembolization (sHR, 0.95; 95% CI, 0.91-0.99). “Radioembolization and thermal ablation may be superior to chemoembolization and prove to be more cost-effective options, depending on the clinical context,” the researchers wrote.
The researchers noted that they were unable to distinguish patients who were removed from the waiting list between those with disease progression versus liver failure.
The researchers reported no conflicts of interest with the pharmaceutical industry. The study received no industry funding.
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
‘Forever chemicals’ exposures may compound diabetes risk
Women in midlife exposed to combinations of perfluoroalkyl and polyfluoroalkyl substances (PFASs), dubbed “forever and everywhere chemicals”, are at increased risk of developing diabetes, similar to the magnitude of risk associated with overweight and even greater than the risk associated with smoking, new research shows.
“This is the first study to examine the joint effect of PFAS on incident diabetes,” first author Sung Kyun Park, ScD, MPH, told this news organization.
“We showed that multiple PFAS as mixtures have larger effects than individual PFAS,” said Dr. Park, of the department of epidemiology, School of Public Health, University of Michigan, Ann Arbor.
The results suggest that, “given that 1.5 million Americans are newly diagnosed with diabetes each year in the USA, approximately 370,000 new cases of diabetes annually in the U.S. are attributable to PFAS exposure,” Dr. Park and authors note in the study, published in Diabetologia.
However, Kevin McConway, PhD, emeritus professor of applied statistics, The Open University, U.K., told the UK Science Media Centre: “[Some] doubt about cause still remains. Yes, this study does show that PFAS may increase diabetes risk in middle-aged women, but it certainly can’t rule out other explanations for its findings.”
Is there any way to reduce exposure?
PFASs, known to be ubiquitous in the environment and also often dubbed “endocrine-disrupting” chemicals, have structures similar to fatty acids. They have been detected in the blood of most people and linked to health concerns including pre-eclampsia, altered levels of liver enzymes, inflammation, and altered lipid and glucose metabolism.
Sources of PFAS exposure can run the gamut from nonstick cookware, food wrappers, and waterproof fabrics to cosmetics and even drinking water.
The authors note a recent Consumer Reports investigation of 118 food packaging products, for instance, which reported finding PFAS chemicals in the packaging of every fast-food chain and retailer examined, including Burger King, McDonald’s, and even more health-focused chains, such as Trader Joe’s.
While efforts to pressure industry to limit PFAS in products are ongoing, Dr. Park asserted that “PFAS exposure reduction at the individual-level is very limited, so a more important way is to change policies and to limit PFAS in the air, drinking water, and foods, etc.”
“It is impossible to completely avoid exposure to PFAS, but I think it is important to acknowledge such sources and change our mindset,” he said.
In terms of clinical practice, the authors add that “it is also important for clinicians to be aware of PFAS as unrecognized risk factors for diabetes and to be prepared to counsel patients in terms of sources of exposure and potential health effects.”
Prospective findings from the SWAN-MPS study
The findings come from a prospective study of 1,237 women, with a median age of 49.4 years, who were diabetes-free upon entering the Study of Women’s Health Across the Nation – Multi-Pollutant Study (SWAN-MPS) between 1999 and 2000 and followed until 2017.
Blood samples taken throughout the study were analyzed for serum concentrations of seven PFASs.
Over the study period, there were 102 cases of incident diabetes, representing a rate of 6 cases per 1,000 person-years. Type of diabetes was not determined, but given the age of study participants, most were assumed to have type 2 diabetes, Dr. Park and colleagues note.
After adjustment for key confounders including race/ethnicity, smoking status, alcohol consumption, total energy intake, physical activity, menopausal status, and body mass index (BMI), those in the highest tertile of exposure to a combination of all seven of the PFASs were significantly more likely to develop diabetes, compared with those in the lowest tertile for exposure (hazard ratio, 2.62).
This risk was greater than that seen with individual PFASs (HR, 1.36-1.85), suggesting a potential additive or synergistic effect of multiple PFASs on diabetes risk.
The association between the combined exposure to PFASs among the highest versus lowest tertile was similar to the risk of diabetes developing among those with overweight (BMI 25-< 30 kg/m2) versus normal weight (HR, 2.89) and higher than the risk among current versus never smokers (HR, 2.30).
“Our findings suggest that PFAS may be an important risk factor for diabetes that has a substantial public health impact,” the authors say.
“Given the widespread exposure to PFAS in the general population, the expected benefit of reducing exposure to these ubiquitous chemicals might be considerable,” they emphasize.
The authors have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Women in midlife exposed to combinations of perfluoroalkyl and polyfluoroalkyl substances (PFASs), dubbed “forever and everywhere chemicals”, are at increased risk of developing diabetes, similar to the magnitude of risk associated with overweight and even greater than the risk associated with smoking, new research shows.
“This is the first study to examine the joint effect of PFAS on incident diabetes,” first author Sung Kyun Park, ScD, MPH, told this news organization.
“We showed that multiple PFAS as mixtures have larger effects than individual PFAS,” said Dr. Park, of the department of epidemiology, School of Public Health, University of Michigan, Ann Arbor.
The results suggest that, “given that 1.5 million Americans are newly diagnosed with diabetes each year in the USA, approximately 370,000 new cases of diabetes annually in the U.S. are attributable to PFAS exposure,” Dr. Park and authors note in the study, published in Diabetologia.
However, Kevin McConway, PhD, emeritus professor of applied statistics, The Open University, U.K., told the UK Science Media Centre: “[Some] doubt about cause still remains. Yes, this study does show that PFAS may increase diabetes risk in middle-aged women, but it certainly can’t rule out other explanations for its findings.”
Is there any way to reduce exposure?
PFASs, known to be ubiquitous in the environment and also often dubbed “endocrine-disrupting” chemicals, have structures similar to fatty acids. They have been detected in the blood of most people and linked to health concerns including pre-eclampsia, altered levels of liver enzymes, inflammation, and altered lipid and glucose metabolism.
Sources of PFAS exposure can run the gamut from nonstick cookware, food wrappers, and waterproof fabrics to cosmetics and even drinking water.
The authors note a recent Consumer Reports investigation of 118 food packaging products, for instance, which reported finding PFAS chemicals in the packaging of every fast-food chain and retailer examined, including Burger King, McDonald’s, and even more health-focused chains, such as Trader Joe’s.
While efforts to pressure industry to limit PFAS in products are ongoing, Dr. Park asserted that “PFAS exposure reduction at the individual-level is very limited, so a more important way is to change policies and to limit PFAS in the air, drinking water, and foods, etc.”
“It is impossible to completely avoid exposure to PFAS, but I think it is important to acknowledge such sources and change our mindset,” he said.
In terms of clinical practice, the authors add that “it is also important for clinicians to be aware of PFAS as unrecognized risk factors for diabetes and to be prepared to counsel patients in terms of sources of exposure and potential health effects.”
Prospective findings from the SWAN-MPS study
The findings come from a prospective study of 1,237 women, with a median age of 49.4 years, who were diabetes-free upon entering the Study of Women’s Health Across the Nation – Multi-Pollutant Study (SWAN-MPS) between 1999 and 2000 and followed until 2017.
Blood samples taken throughout the study were analyzed for serum concentrations of seven PFASs.
Over the study period, there were 102 cases of incident diabetes, representing a rate of 6 cases per 1,000 person-years. Type of diabetes was not determined, but given the age of study participants, most were assumed to have type 2 diabetes, Dr. Park and colleagues note.
After adjustment for key confounders including race/ethnicity, smoking status, alcohol consumption, total energy intake, physical activity, menopausal status, and body mass index (BMI), those in the highest tertile of exposure to a combination of all seven of the PFASs were significantly more likely to develop diabetes, compared with those in the lowest tertile for exposure (hazard ratio, 2.62).
This risk was greater than that seen with individual PFASs (HR, 1.36-1.85), suggesting a potential additive or synergistic effect of multiple PFASs on diabetes risk.
The association between the combined exposure to PFASs among the highest versus lowest tertile was similar to the risk of diabetes developing among those with overweight (BMI 25-< 30 kg/m2) versus normal weight (HR, 2.89) and higher than the risk among current versus never smokers (HR, 2.30).
“Our findings suggest that PFAS may be an important risk factor for diabetes that has a substantial public health impact,” the authors say.
“Given the widespread exposure to PFAS in the general population, the expected benefit of reducing exposure to these ubiquitous chemicals might be considerable,” they emphasize.
The authors have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Women in midlife exposed to combinations of perfluoroalkyl and polyfluoroalkyl substances (PFASs), dubbed “forever and everywhere chemicals”, are at increased risk of developing diabetes, similar to the magnitude of risk associated with overweight and even greater than the risk associated with smoking, new research shows.
“This is the first study to examine the joint effect of PFAS on incident diabetes,” first author Sung Kyun Park, ScD, MPH, told this news organization.
“We showed that multiple PFAS as mixtures have larger effects than individual PFAS,” said Dr. Park, of the department of epidemiology, School of Public Health, University of Michigan, Ann Arbor.
The results suggest that, “given that 1.5 million Americans are newly diagnosed with diabetes each year in the USA, approximately 370,000 new cases of diabetes annually in the U.S. are attributable to PFAS exposure,” Dr. Park and authors note in the study, published in Diabetologia.
However, Kevin McConway, PhD, emeritus professor of applied statistics, The Open University, U.K., told the UK Science Media Centre: “[Some] doubt about cause still remains. Yes, this study does show that PFAS may increase diabetes risk in middle-aged women, but it certainly can’t rule out other explanations for its findings.”
Is there any way to reduce exposure?
PFASs, known to be ubiquitous in the environment and also often dubbed “endocrine-disrupting” chemicals, have structures similar to fatty acids. They have been detected in the blood of most people and linked to health concerns including pre-eclampsia, altered levels of liver enzymes, inflammation, and altered lipid and glucose metabolism.
Sources of PFAS exposure can run the gamut from nonstick cookware, food wrappers, and waterproof fabrics to cosmetics and even drinking water.
The authors note a recent Consumer Reports investigation of 118 food packaging products, for instance, which reported finding PFAS chemicals in the packaging of every fast-food chain and retailer examined, including Burger King, McDonald’s, and even more health-focused chains, such as Trader Joe’s.
While efforts to pressure industry to limit PFAS in products are ongoing, Dr. Park asserted that “PFAS exposure reduction at the individual-level is very limited, so a more important way is to change policies and to limit PFAS in the air, drinking water, and foods, etc.”
“It is impossible to completely avoid exposure to PFAS, but I think it is important to acknowledge such sources and change our mindset,” he said.
In terms of clinical practice, the authors add that “it is also important for clinicians to be aware of PFAS as unrecognized risk factors for diabetes and to be prepared to counsel patients in terms of sources of exposure and potential health effects.”
Prospective findings from the SWAN-MPS study
The findings come from a prospective study of 1,237 women, with a median age of 49.4 years, who were diabetes-free upon entering the Study of Women’s Health Across the Nation – Multi-Pollutant Study (SWAN-MPS) between 1999 and 2000 and followed until 2017.
Blood samples taken throughout the study were analyzed for serum concentrations of seven PFASs.
Over the study period, there were 102 cases of incident diabetes, representing a rate of 6 cases per 1,000 person-years. Type of diabetes was not determined, but given the age of study participants, most were assumed to have type 2 diabetes, Dr. Park and colleagues note.
After adjustment for key confounders including race/ethnicity, smoking status, alcohol consumption, total energy intake, physical activity, menopausal status, and body mass index (BMI), those in the highest tertile of exposure to a combination of all seven of the PFASs were significantly more likely to develop diabetes, compared with those in the lowest tertile for exposure (hazard ratio, 2.62).
This risk was greater than that seen with individual PFASs (HR, 1.36-1.85), suggesting a potential additive or synergistic effect of multiple PFASs on diabetes risk.
The association between the combined exposure to PFASs among the highest versus lowest tertile was similar to the risk of diabetes developing among those with overweight (BMI 25-< 30 kg/m2) versus normal weight (HR, 2.89) and higher than the risk among current versus never smokers (HR, 2.30).
“Our findings suggest that PFAS may be an important risk factor for diabetes that has a substantial public health impact,” the authors say.
“Given the widespread exposure to PFAS in the general population, the expected benefit of reducing exposure to these ubiquitous chemicals might be considerable,” they emphasize.
The authors have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM DIABETOLOGIA
52-year-old man • hematemesis • history of cirrhosis • persistent fevers • Dx?
THE CASE
A 52-year-old man presented to the emergency department after vomiting a large volume of blood and was admitted to the intensive care unit. His past medical history was remarkable for untreated chronic hepatitis C resulting from injection drug use and cirrhosis without prior history of esophageal varices.
Due to ongoing hematemesis, he was intubated for airway protection and underwent esophagogastroduodenoscopy with banding of large esophageal varices on hospital day (HD) 1. He was extubated on HD 2 after clinical stability was achieved; however, he became encephalopathic over the subsequent days despite treatment with lactulose. On HD 4, the patient required re-intubation for progressive respiratory failure. Chest imaging revealed a large, simple-appearing right pleural effusion and extensive bilateral patchy ground-glass opacities (FIGURE 1).
Thoracentesis was ordered and revealed transudative pleural fluid; this finding, along with negative infectious studies, was consistent with hepatic hydrothorax. In the setting of initial decompensation, empiric treatment with vancomycin and meropenem was started for suspected hospital-acquired pneumonia.
The patient had persistent fevers that had developed during his hospital stay and pulmonary opacities, despite 72 hours of treatment with broad-spectrum antibiotics. Thus, a diagnostic bronchoscopy with bronchoalveolar lavage (BAL) was performed. BAL cell count and differential revealed 363 nucleated cells/µL, with profound eosinophilia (42% eosinophils, 44% macrophages, 14% neutrophils).
Bacterial and fungal cultures and a viral polymerase chain reaction panel were negative. HIV antibody-antigen and RNA testing were also negative. The patient had no evidence or history of underlying malignancy, autoimmune disease, or recent immunosuppressive therapy, including corticosteroids. Due to consistent imaging findings and lack of improvement with appropriate treatment for bacterial pneumonia, further work-up was pursued.
THE DIAGNOSIS
Given the consistent radiographic pattern, the differential diagnosis for this patient included pneumocystis pneumonia (PCP), a potentially life-threatening opportunistic infection. Work-up therefore included direct fluorescent antibody testing, which was positive for Pneumocystis jirovecii, a fungus that can cause PCP.
Of note, the patient’s white blood cell count was elevated on admission (11.44 × 103/µL) but low for much of his hospital stay (nadir = 1.97 × 103/µL), with associated lymphopenia (nadir = 0.22 × 103/µl). No peripheral eosinophilia was noted.
Continue to: DISCUSSION
DISCUSSION
PCP typically occurs in immunocompromised individuals and may be related to HIV infection, malignancy, or exposure to immunosuppressive therapies.1,2 While rare cases of PCP have been described in adults without predisposing factors, many of these cases occurred at the beginning of the AIDS epidemic, prior to reliable HIV testing.3-5
Uncharted territory. We were confident in our diagnosis because immunofluorescence testing has very few false-positives and a high specificity.6-8 But there were informational gaps. The eosinophilia recorded on BAL is poorly described in HIV-negative patients with PCP but well-described in HIV-positive patients, with the level of eosinophilia associated with disease severity.9,10 Eosinophils are thought to contribute to pulmonary inflammation, which may explain the severity of our patient’s course.10
A first of its kind case?
To our knowledge, this is the first report of PCP in a patient with cirrhosis from chronic hepatitis C virus infection and no other predisposing conditions or preceding immunosuppressive therapy. We suspect that his lymphopenia, which was noted during his critical illness, predisposed him to PCP.
Lymphocytes (in particular CD4+ T cells) have been shown to play an important role, along with alveolar macrophages and neutrophils, in directing the host defense against
Typical risk factors for lymphopenia had not been observed in this patient. However, cirrhosis has been associated with low CD4+ T-cell counts and disruption of cell-mediated immunity, even in HIV-seronegative patients.14,15 There are several postulated mechanisms for low CD4+ T-cell counts in cirrhosis, including splenic sequestration, impaired T-cell production (due to impaired thymopoiesis), increased T-cell consumption, and apoptosis (due to persistent immune system activation from bacterial translocation and an overall pro-inflammatory state).16,17
Continue to: Predisposing factors guide treatment
Predisposing factors guide treatment
Routine treatment for PCP in patients without HIV is a 21-day course of trimethoprim/sulfamethoxazole (Bactrim). Dosing for patients with normal renal function is 15 to 20 mg/kg orally or intravenously per day. Patients with allergy to trimethoprim/sulfamethoxazole should ideally undergo desensitization, given its effectiveness against PCP.
Due to a sulfonamide allergy, our patient was started on primaquine 30 mg/d, clindamycin 600 mg tid, and prednisone 40 mg bid. (The corticosteroid was added because of the severity of the disease.) Three days after starting treatment—and 10 days into his hospital stay—the patient had significant improvement in his respiratory status and was successfully extubated. He underwent trimethoprim/sulfamethoxazole desensitization and completed a 21-day course of treatment for PCP with complete resolution of respiratory symptoms. Follow-up chest radiograph 2 months later (FIGURE 2) confirmed clearance of opacities.
THE TAKEAWAY
PCP remains a rare disease in patients without the typical immunosuppressive risk factors. However, it should be considered in patients with cirrhosis who develop respiratory failure, especially those with compatible radiographic findings and negative microbiologic evaluation for other, more typical, organisms.
CORRESPONDENCE
Tyler Albert, MD, VA Puget Sound Healthcare System, 1660 South Columbian Way, S-111-Pulm, Seattle, WA 98108; talbert@uw.edu
1. Thomas CF Jr, Limper AH. Pneumocystis pneumonia. N Engl J Med. 2004;350:2487-2498. doi: 10.1056/NEJMra032588
2. Walzer PD, Perl DP, Krogstad DJ, et al. Pneumocystis carinii pneumonia in the United States. Epidemiologic, diagnostic, and clinical features. Ann Intern Med. 1974;80:83-93. doi: 10.7326/0003-4819-80-1-83
3. Sepkowitz KA. Pneumocystis carinii pneumonia in patients without AIDS. Clin Infect Dis. 1993;17 suppl 2:S416-422. doi: 10.1093/clinids/17.supplement_2.s416
4. Al Soub H, Taha RY, El Deeb Y, et al. Pneumocystis carinii pneumonia in a patient without a predisposing illness: case report and review. Scand J Infect Dis. 2004;36:618-621. doi: 10.1080/00365540410017608
5. Jacobs JL, Libby DM, Winters RA, et al. A cluster of Pneumocystis carinii pneumonia in adults without predisposing illnesses. N Engl J Med. 1991;324:246-250. doi: 10.1056/NEJM199101243240407
6. Ng VL, Yajko DM, McPhaul LW, et al. Evaluation of an indirect fluorescent-antibody stain for detection of Pneumocystis carinii in respiratory specimens. J Clin Microbiol. 1990;28:975-979. doi: 10.1128/jcm.28.5.975-979.1990
7. Cregan P, Yamamoto A, Lum A, et al. Comparison of four methods for rapid detection of Pneumocystis carinii in respiratory specimens. J Clin Microbiol. 1990;28:2432-2436. doi: 10.1128/jcm.28.11.2432-2436.1990
8. Turner D, Schwarz Y, Yust I. Induced sputum for diagnosing Pneumocystis carinii pneumonia in HIV patients: new data, new issues. Eur Respir J. 2003;21:204-208. doi: 10.1183/09031936.03.00035303
9. Smith RL, el-Sadr WM, Lewis ML. Correlation of bronchoalveolar lavage cell populations with clinical severity of Pneumocystis carinii pneumonia. Chest. 1988;93:60-64. doi: 10.1378/chest.93.1.60
10. Fleury-Feith J, Van Nhieu JT, Picard C, et al. Bronchoalveolar lavage eosinophilia associated with Pneumocystis carinii pneumonitis in AIDS patients. Comparative study with non-AIDS patients. Chest. 1989;95:1198-1201. doi: 10.1378/chest.95.6.1198
11. Thomas CF Jr, Limper AH. Current insights into the biology and pathogenesis of Pneumocystis pneumonia. Nat Rev Microbiol. 2007;5:298-308. doi: 10.1038/nrmicro1621
12. Toh BH, Roberts-Thomson IC, Mathews JD, et al. Depression of cell-mediated immunity in old age and the immunopathic diseases, lupus erythematosus, chronic hepatitis and rheumatoid arthritis. Clin Exp Immunol. 1973;14:193-202.
13. Mansharamani NG, Balachandran D, Vernovsky I, et al. Peripheral blood CD4 + T-lymphocyte counts during Pneumocystis carinii pneumonia in immunocompromised patients without HIV infection. Chest. 2000;118:712-720. doi: 10.1378/chest.118.3.712
14. McGovern BH, Golan Y, Lopez M, et al. The impact of cirrhosis on CD4+ T cell counts in HIV-seronegative patients. Clin Infect Dis. 2007;44:431-437. doi: 10.1086/509580
15. Bienvenu AL, Traore K, Plekhanova I, et al. Pneumocystis pneumonia suspected cases in 604 non-HIV and HIV patients. Int J Infect Dis. 2016;46:11-17. doi: 10.1016/j.ijid.2016.03.018
16. Albillos A, Lario M, Álvarez-Mon M. Cirrhosis-associated immune dysfunction: distinctive features and clinical relevance. J Hepatol. 2014;61:1385-1396. doi: 10.1016/j.jhep.2014.08.010
17. Lario M, Muñoz L, Ubeda M, et al. Defective thymopoiesis and poor peripheral homeostatic replenishment of T-helper cells cause T-cell lymphopenia in cirrhosis. J Hepatol. 2013;59:723-730. doi: 10.1016/j.jhep.2013.05.042
THE CASE
A 52-year-old man presented to the emergency department after vomiting a large volume of blood and was admitted to the intensive care unit. His past medical history was remarkable for untreated chronic hepatitis C resulting from injection drug use and cirrhosis without prior history of esophageal varices.
Due to ongoing hematemesis, he was intubated for airway protection and underwent esophagogastroduodenoscopy with banding of large esophageal varices on hospital day (HD) 1. He was extubated on HD 2 after clinical stability was achieved; however, he became encephalopathic over the subsequent days despite treatment with lactulose. On HD 4, the patient required re-intubation for progressive respiratory failure. Chest imaging revealed a large, simple-appearing right pleural effusion and extensive bilateral patchy ground-glass opacities (FIGURE 1).
Thoracentesis was ordered and revealed transudative pleural fluid; this finding, along with negative infectious studies, was consistent with hepatic hydrothorax. In the setting of initial decompensation, empiric treatment with vancomycin and meropenem was started for suspected hospital-acquired pneumonia.
The patient had persistent fevers that had developed during his hospital stay and pulmonary opacities, despite 72 hours of treatment with broad-spectrum antibiotics. Thus, a diagnostic bronchoscopy with bronchoalveolar lavage (BAL) was performed. BAL cell count and differential revealed 363 nucleated cells/µL, with profound eosinophilia (42% eosinophils, 44% macrophages, 14% neutrophils).
Bacterial and fungal cultures and a viral polymerase chain reaction panel were negative. HIV antibody-antigen and RNA testing were also negative. The patient had no evidence or history of underlying malignancy, autoimmune disease, or recent immunosuppressive therapy, including corticosteroids. Due to consistent imaging findings and lack of improvement with appropriate treatment for bacterial pneumonia, further work-up was pursued.
THE DIAGNOSIS
Given the consistent radiographic pattern, the differential diagnosis for this patient included pneumocystis pneumonia (PCP), a potentially life-threatening opportunistic infection. Work-up therefore included direct fluorescent antibody testing, which was positive for Pneumocystis jirovecii, a fungus that can cause PCP.
Of note, the patient’s white blood cell count was elevated on admission (11.44 × 103/µL) but low for much of his hospital stay (nadir = 1.97 × 103/µL), with associated lymphopenia (nadir = 0.22 × 103/µl). No peripheral eosinophilia was noted.
Continue to: DISCUSSION
DISCUSSION
PCP typically occurs in immunocompromised individuals and may be related to HIV infection, malignancy, or exposure to immunosuppressive therapies.1,2 While rare cases of PCP have been described in adults without predisposing factors, many of these cases occurred at the beginning of the AIDS epidemic, prior to reliable HIV testing.3-5
Uncharted territory. We were confident in our diagnosis because immunofluorescence testing has very few false-positives and a high specificity.6-8 But there were informational gaps. The eosinophilia recorded on BAL is poorly described in HIV-negative patients with PCP but well-described in HIV-positive patients, with the level of eosinophilia associated with disease severity.9,10 Eosinophils are thought to contribute to pulmonary inflammation, which may explain the severity of our patient’s course.10
A first of its kind case?
To our knowledge, this is the first report of PCP in a patient with cirrhosis from chronic hepatitis C virus infection and no other predisposing conditions or preceding immunosuppressive therapy. We suspect that his lymphopenia, which was noted during his critical illness, predisposed him to PCP.
Lymphocytes (in particular CD4+ T cells) have been shown to play an important role, along with alveolar macrophages and neutrophils, in directing the host defense against
Typical risk factors for lymphopenia had not been observed in this patient. However, cirrhosis has been associated with low CD4+ T-cell counts and disruption of cell-mediated immunity, even in HIV-seronegative patients.14,15 There are several postulated mechanisms for low CD4+ T-cell counts in cirrhosis, including splenic sequestration, impaired T-cell production (due to impaired thymopoiesis), increased T-cell consumption, and apoptosis (due to persistent immune system activation from bacterial translocation and an overall pro-inflammatory state).16,17
Continue to: Predisposing factors guide treatment
Predisposing factors guide treatment
Routine treatment for PCP in patients without HIV is a 21-day course of trimethoprim/sulfamethoxazole (Bactrim). Dosing for patients with normal renal function is 15 to 20 mg/kg orally or intravenously per day. Patients with allergy to trimethoprim/sulfamethoxazole should ideally undergo desensitization, given its effectiveness against PCP.
Due to a sulfonamide allergy, our patient was started on primaquine 30 mg/d, clindamycin 600 mg tid, and prednisone 40 mg bid. (The corticosteroid was added because of the severity of the disease.) Three days after starting treatment—and 10 days into his hospital stay—the patient had significant improvement in his respiratory status and was successfully extubated. He underwent trimethoprim/sulfamethoxazole desensitization and completed a 21-day course of treatment for PCP with complete resolution of respiratory symptoms. Follow-up chest radiograph 2 months later (FIGURE 2) confirmed clearance of opacities.
THE TAKEAWAY
PCP remains a rare disease in patients without the typical immunosuppressive risk factors. However, it should be considered in patients with cirrhosis who develop respiratory failure, especially those with compatible radiographic findings and negative microbiologic evaluation for other, more typical, organisms.
CORRESPONDENCE
Tyler Albert, MD, VA Puget Sound Healthcare System, 1660 South Columbian Way, S-111-Pulm, Seattle, WA 98108; talbert@uw.edu
THE CASE
A 52-year-old man presented to the emergency department after vomiting a large volume of blood and was admitted to the intensive care unit. His past medical history was remarkable for untreated chronic hepatitis C resulting from injection drug use and cirrhosis without prior history of esophageal varices.
Due to ongoing hematemesis, he was intubated for airway protection and underwent esophagogastroduodenoscopy with banding of large esophageal varices on hospital day (HD) 1. He was extubated on HD 2 after clinical stability was achieved; however, he became encephalopathic over the subsequent days despite treatment with lactulose. On HD 4, the patient required re-intubation for progressive respiratory failure. Chest imaging revealed a large, simple-appearing right pleural effusion and extensive bilateral patchy ground-glass opacities (FIGURE 1).
Thoracentesis was ordered and revealed transudative pleural fluid; this finding, along with negative infectious studies, was consistent with hepatic hydrothorax. In the setting of initial decompensation, empiric treatment with vancomycin and meropenem was started for suspected hospital-acquired pneumonia.
The patient had persistent fevers that had developed during his hospital stay and pulmonary opacities, despite 72 hours of treatment with broad-spectrum antibiotics. Thus, a diagnostic bronchoscopy with bronchoalveolar lavage (BAL) was performed. BAL cell count and differential revealed 363 nucleated cells/µL, with profound eosinophilia (42% eosinophils, 44% macrophages, 14% neutrophils).
Bacterial and fungal cultures and a viral polymerase chain reaction panel were negative. HIV antibody-antigen and RNA testing were also negative. The patient had no evidence or history of underlying malignancy, autoimmune disease, or recent immunosuppressive therapy, including corticosteroids. Due to consistent imaging findings and lack of improvement with appropriate treatment for bacterial pneumonia, further work-up was pursued.
THE DIAGNOSIS
Given the consistent radiographic pattern, the differential diagnosis for this patient included pneumocystis pneumonia (PCP), a potentially life-threatening opportunistic infection. Work-up therefore included direct fluorescent antibody testing, which was positive for Pneumocystis jirovecii, a fungus that can cause PCP.
Of note, the patient’s white blood cell count was elevated on admission (11.44 × 103/µL) but low for much of his hospital stay (nadir = 1.97 × 103/µL), with associated lymphopenia (nadir = 0.22 × 103/µl). No peripheral eosinophilia was noted.
Continue to: DISCUSSION
DISCUSSION
PCP typically occurs in immunocompromised individuals and may be related to HIV infection, malignancy, or exposure to immunosuppressive therapies.1,2 While rare cases of PCP have been described in adults without predisposing factors, many of these cases occurred at the beginning of the AIDS epidemic, prior to reliable HIV testing.3-5
Uncharted territory. We were confident in our diagnosis because immunofluorescence testing has very few false-positives and a high specificity.6-8 But there were informational gaps. The eosinophilia recorded on BAL is poorly described in HIV-negative patients with PCP but well-described in HIV-positive patients, with the level of eosinophilia associated with disease severity.9,10 Eosinophils are thought to contribute to pulmonary inflammation, which may explain the severity of our patient’s course.10
A first of its kind case?
To our knowledge, this is the first report of PCP in a patient with cirrhosis from chronic hepatitis C virus infection and no other predisposing conditions or preceding immunosuppressive therapy. We suspect that his lymphopenia, which was noted during his critical illness, predisposed him to PCP.
Lymphocytes (in particular CD4+ T cells) have been shown to play an important role, along with alveolar macrophages and neutrophils, in directing the host defense against
Typical risk factors for lymphopenia had not been observed in this patient. However, cirrhosis has been associated with low CD4+ T-cell counts and disruption of cell-mediated immunity, even in HIV-seronegative patients.14,15 There are several postulated mechanisms for low CD4+ T-cell counts in cirrhosis, including splenic sequestration, impaired T-cell production (due to impaired thymopoiesis), increased T-cell consumption, and apoptosis (due to persistent immune system activation from bacterial translocation and an overall pro-inflammatory state).16,17
Continue to: Predisposing factors guide treatment
Predisposing factors guide treatment
Routine treatment for PCP in patients without HIV is a 21-day course of trimethoprim/sulfamethoxazole (Bactrim). Dosing for patients with normal renal function is 15 to 20 mg/kg orally or intravenously per day. Patients with allergy to trimethoprim/sulfamethoxazole should ideally undergo desensitization, given its effectiveness against PCP.
Due to a sulfonamide allergy, our patient was started on primaquine 30 mg/d, clindamycin 600 mg tid, and prednisone 40 mg bid. (The corticosteroid was added because of the severity of the disease.) Three days after starting treatment—and 10 days into his hospital stay—the patient had significant improvement in his respiratory status and was successfully extubated. He underwent trimethoprim/sulfamethoxazole desensitization and completed a 21-day course of treatment for PCP with complete resolution of respiratory symptoms. Follow-up chest radiograph 2 months later (FIGURE 2) confirmed clearance of opacities.
THE TAKEAWAY
PCP remains a rare disease in patients without the typical immunosuppressive risk factors. However, it should be considered in patients with cirrhosis who develop respiratory failure, especially those with compatible radiographic findings and negative microbiologic evaluation for other, more typical, organisms.
CORRESPONDENCE
Tyler Albert, MD, VA Puget Sound Healthcare System, 1660 South Columbian Way, S-111-Pulm, Seattle, WA 98108; talbert@uw.edu
1. Thomas CF Jr, Limper AH. Pneumocystis pneumonia. N Engl J Med. 2004;350:2487-2498. doi: 10.1056/NEJMra032588
2. Walzer PD, Perl DP, Krogstad DJ, et al. Pneumocystis carinii pneumonia in the United States. Epidemiologic, diagnostic, and clinical features. Ann Intern Med. 1974;80:83-93. doi: 10.7326/0003-4819-80-1-83
3. Sepkowitz KA. Pneumocystis carinii pneumonia in patients without AIDS. Clin Infect Dis. 1993;17 suppl 2:S416-422. doi: 10.1093/clinids/17.supplement_2.s416
4. Al Soub H, Taha RY, El Deeb Y, et al. Pneumocystis carinii pneumonia in a patient without a predisposing illness: case report and review. Scand J Infect Dis. 2004;36:618-621. doi: 10.1080/00365540410017608
5. Jacobs JL, Libby DM, Winters RA, et al. A cluster of Pneumocystis carinii pneumonia in adults without predisposing illnesses. N Engl J Med. 1991;324:246-250. doi: 10.1056/NEJM199101243240407
6. Ng VL, Yajko DM, McPhaul LW, et al. Evaluation of an indirect fluorescent-antibody stain for detection of Pneumocystis carinii in respiratory specimens. J Clin Microbiol. 1990;28:975-979. doi: 10.1128/jcm.28.5.975-979.1990
7. Cregan P, Yamamoto A, Lum A, et al. Comparison of four methods for rapid detection of Pneumocystis carinii in respiratory specimens. J Clin Microbiol. 1990;28:2432-2436. doi: 10.1128/jcm.28.11.2432-2436.1990
8. Turner D, Schwarz Y, Yust I. Induced sputum for diagnosing Pneumocystis carinii pneumonia in HIV patients: new data, new issues. Eur Respir J. 2003;21:204-208. doi: 10.1183/09031936.03.00035303
9. Smith RL, el-Sadr WM, Lewis ML. Correlation of bronchoalveolar lavage cell populations with clinical severity of Pneumocystis carinii pneumonia. Chest. 1988;93:60-64. doi: 10.1378/chest.93.1.60
10. Fleury-Feith J, Van Nhieu JT, Picard C, et al. Bronchoalveolar lavage eosinophilia associated with Pneumocystis carinii pneumonitis in AIDS patients. Comparative study with non-AIDS patients. Chest. 1989;95:1198-1201. doi: 10.1378/chest.95.6.1198
11. Thomas CF Jr, Limper AH. Current insights into the biology and pathogenesis of Pneumocystis pneumonia. Nat Rev Microbiol. 2007;5:298-308. doi: 10.1038/nrmicro1621
12. Toh BH, Roberts-Thomson IC, Mathews JD, et al. Depression of cell-mediated immunity in old age and the immunopathic diseases, lupus erythematosus, chronic hepatitis and rheumatoid arthritis. Clin Exp Immunol. 1973;14:193-202.
13. Mansharamani NG, Balachandran D, Vernovsky I, et al. Peripheral blood CD4 + T-lymphocyte counts during Pneumocystis carinii pneumonia in immunocompromised patients without HIV infection. Chest. 2000;118:712-720. doi: 10.1378/chest.118.3.712
14. McGovern BH, Golan Y, Lopez M, et al. The impact of cirrhosis on CD4+ T cell counts in HIV-seronegative patients. Clin Infect Dis. 2007;44:431-437. doi: 10.1086/509580
15. Bienvenu AL, Traore K, Plekhanova I, et al. Pneumocystis pneumonia suspected cases in 604 non-HIV and HIV patients. Int J Infect Dis. 2016;46:11-17. doi: 10.1016/j.ijid.2016.03.018
16. Albillos A, Lario M, Álvarez-Mon M. Cirrhosis-associated immune dysfunction: distinctive features and clinical relevance. J Hepatol. 2014;61:1385-1396. doi: 10.1016/j.jhep.2014.08.010
17. Lario M, Muñoz L, Ubeda M, et al. Defective thymopoiesis and poor peripheral homeostatic replenishment of T-helper cells cause T-cell lymphopenia in cirrhosis. J Hepatol. 2013;59:723-730. doi: 10.1016/j.jhep.2013.05.042
1. Thomas CF Jr, Limper AH. Pneumocystis pneumonia. N Engl J Med. 2004;350:2487-2498. doi: 10.1056/NEJMra032588
2. Walzer PD, Perl DP, Krogstad DJ, et al. Pneumocystis carinii pneumonia in the United States. Epidemiologic, diagnostic, and clinical features. Ann Intern Med. 1974;80:83-93. doi: 10.7326/0003-4819-80-1-83
3. Sepkowitz KA. Pneumocystis carinii pneumonia in patients without AIDS. Clin Infect Dis. 1993;17 suppl 2:S416-422. doi: 10.1093/clinids/17.supplement_2.s416
4. Al Soub H, Taha RY, El Deeb Y, et al. Pneumocystis carinii pneumonia in a patient without a predisposing illness: case report and review. Scand J Infect Dis. 2004;36:618-621. doi: 10.1080/00365540410017608
5. Jacobs JL, Libby DM, Winters RA, et al. A cluster of Pneumocystis carinii pneumonia in adults without predisposing illnesses. N Engl J Med. 1991;324:246-250. doi: 10.1056/NEJM199101243240407
6. Ng VL, Yajko DM, McPhaul LW, et al. Evaluation of an indirect fluorescent-antibody stain for detection of Pneumocystis carinii in respiratory specimens. J Clin Microbiol. 1990;28:975-979. doi: 10.1128/jcm.28.5.975-979.1990
7. Cregan P, Yamamoto A, Lum A, et al. Comparison of four methods for rapid detection of Pneumocystis carinii in respiratory specimens. J Clin Microbiol. 1990;28:2432-2436. doi: 10.1128/jcm.28.11.2432-2436.1990
8. Turner D, Schwarz Y, Yust I. Induced sputum for diagnosing Pneumocystis carinii pneumonia in HIV patients: new data, new issues. Eur Respir J. 2003;21:204-208. doi: 10.1183/09031936.03.00035303
9. Smith RL, el-Sadr WM, Lewis ML. Correlation of bronchoalveolar lavage cell populations with clinical severity of Pneumocystis carinii pneumonia. Chest. 1988;93:60-64. doi: 10.1378/chest.93.1.60
10. Fleury-Feith J, Van Nhieu JT, Picard C, et al. Bronchoalveolar lavage eosinophilia associated with Pneumocystis carinii pneumonitis in AIDS patients. Comparative study with non-AIDS patients. Chest. 1989;95:1198-1201. doi: 10.1378/chest.95.6.1198
11. Thomas CF Jr, Limper AH. Current insights into the biology and pathogenesis of Pneumocystis pneumonia. Nat Rev Microbiol. 2007;5:298-308. doi: 10.1038/nrmicro1621
12. Toh BH, Roberts-Thomson IC, Mathews JD, et al. Depression of cell-mediated immunity in old age and the immunopathic diseases, lupus erythematosus, chronic hepatitis and rheumatoid arthritis. Clin Exp Immunol. 1973;14:193-202.
13. Mansharamani NG, Balachandran D, Vernovsky I, et al. Peripheral blood CD4 + T-lymphocyte counts during Pneumocystis carinii pneumonia in immunocompromised patients without HIV infection. Chest. 2000;118:712-720. doi: 10.1378/chest.118.3.712
14. McGovern BH, Golan Y, Lopez M, et al. The impact of cirrhosis on CD4+ T cell counts in HIV-seronegative patients. Clin Infect Dis. 2007;44:431-437. doi: 10.1086/509580
15. Bienvenu AL, Traore K, Plekhanova I, et al. Pneumocystis pneumonia suspected cases in 604 non-HIV and HIV patients. Int J Infect Dis. 2016;46:11-17. doi: 10.1016/j.ijid.2016.03.018
16. Albillos A, Lario M, Álvarez-Mon M. Cirrhosis-associated immune dysfunction: distinctive features and clinical relevance. J Hepatol. 2014;61:1385-1396. doi: 10.1016/j.jhep.2014.08.010
17. Lario M, Muñoz L, Ubeda M, et al. Defective thymopoiesis and poor peripheral homeostatic replenishment of T-helper cells cause T-cell lymphopenia in cirrhosis. J Hepatol. 2013;59:723-730. doi: 10.1016/j.jhep.2013.05.042
Somatic symptom disorder in primary care: A collaborative approach
THE CASE
James R* is a 30-year-old man who presented for a primary care walk-in visit due to dizziness, 2 days after he visited an emergency department (ED) for the same concern. He reported episodic symptoms lasting seconds to minutes, specifically when lying down. He said he had not fallen or experienced other physical trauma, did not have blurred vision or hearing loss, and was taking no medications. He also reported panic attacks, during which he experienced palpitations, trembling, paresthesia, and fear of dying. He stated that dizziness did not occur exclusively during panic episodes. His medical history was significant for hypertension; however, he reported significant anxiety related to medical visits. All home blood pressure readings he reported were within normal limits.
Upon examination, the patient had a blood pressure reading of 142/90 mm Hg and no evidence of nystagmus at rest. A neurologic exam was normal and a Dix-Hallpike maneuver reproduced subjective vertigo without nystagmus. Laboratory findings from the patient’s ED visit were negative for troponin and drug use, and blood oxygenation levels were within normal limits. At the time of this current visit, an electrocardiogram was unremarkable, with the exception of some tachycardia.
The presumptive diagnosis was benign paroxysmal positional vertigo (BPPV). An Epley maneuver was performed in the clinic and resulted in minimal symptom improvement. The physician taught Mr. R how to perform the Epley maneuver himself, prescribed a short course of meclizine, and referred him to the integrated mental health care service to address his panic attacks and anxiety.
Over the next few months, Mr. R continued to report significant distress about the dizzy spells, which persisted even after performing the Epley maneuver, and he reported that the meclizine was causing worsening vertigo. He received an ear-nose-and-throat consultation and cognitive behavioral therapy (CBT)–based consultation/interventions. He also reported avoiding multiple activities due to concerns about his dizziness.
●
*The patient’s name and other personally identifying information have been changed to protect his identity.
Somatic symptom disorder (SSD) is characterized by one or more physical symptoms associated with “excessive thoughts, feelings, or behaviors that result in distress and/or functional impairment.”1 Individuals with SSD are preoccupied with symptom-related severity, experience high symptom-related anxiety, or devote significant time and energy to the symptoms or heath concerns. With a diagnosis of SSD, physical symptoms experienced by the patient may or may not be medically explained. The same symptom need not be continuously present as long as the overall symptomatic presentation lasts 6 months or longer.
The specifier “with predominant pain” is used when pain dominates the presentation.1 Estimated prevalence of SSD in primary care ranges from 5% to 35%.2 The true scope of SSD is difficult to assess accurately since research tends to focus on medically unexplained symptoms, rather than excessive symptom-related concerns. Furthermore, terms such as “medically unexplained symptoms” and “functional syndromes” (including fibromyalgia and irritable bowel syndrome) are frequently used when describing SSD.3
One or more factors may contribute to unexplained symptoms: limitations of medical procedures and techniques, partial clinical information, patients’ inability to follow management recommendations, challenges in differential diagnostics, and access-to-care limitations preventing regular care and appropriate diagnostic work up.
What’s important to remember is that it’s the patient’s reaction to physical symptoms, rather than the presence of symptoms per se, that defines SSD.
Considerations in the differential diagnosis
When making a diagnosis of SSD, symptoms cannot:4
- be feigned or deliberately produced as in malingering or factitious disorder.
- result from physiologic effects of a substance (eg, intoxication, withdrawal, or adverse medication effects).
- constitute somatic delusions, as occur in psychotic disorders.
- constitute symptoms or deficits affecting voluntary motor or sensory function that are better explained by neurologic, medical, or psychiatric conditions (consider conversion disorder).
- be preoccupations with physical appearance flaws, as in body dysmorphic disorder.
- be accounted for by an anxiety disorder (eg, palpitations associated with panic attacks).
Continue to: Illness anxiety disorder...
Illness anxiety disorder is also characterized by significant health-related concerns; however, physical symptoms are either mild or absent.
Possible causes of SSD are varied and complex, including genetic and biological factors, family dynamics, behavioral modeling/learning, personality traits, difficulties with emotional regulation, and awareness.5 Patients may present with ongoing requests for symptom explanations, feelings of helplessness, fear of having concerns dismissed, and low motivation for change.3
Aids in supporting a diagnosis of SSD
It’s not appropriate to rely solely on questionnaires to make the diagnosis of SSD. However, brief screening tools are a time-efficient way to capture patients’ experiences and perceptions.6 Along with other components of clinical evaluation, brief symptom screens can both support the diagnosis and help in longitudinal symptom assessment.
Patient Health Questionnaire-15 (PHQ-15), developed for self-report screening in primary care, has desirable psychometric properties including appropriate internal reliability; convergent validity with measures of functional status, disability days, and symptom-related burden; and discriminant validity from measures of depressive symptoms.7 The PHQ-15 is an open access tool that is available in several languages. The respondent is asked to rate the extent of being bothered by a range of medical symptoms in the proceeding 4 weeks. Total scores range from 0 to 30, with higher scores indicating greater symptom aggravation. Cutoffs of 5, 10, and 15 correspond to mild, moderate, and severe symptom levels.8
Somatic Symptom Disorder - B Criteria Scale (SSD-12) aims to capture SSD symptoms in line with Diagnostic and Statistical Manual of Mental Disorders (DSM-5) diagnostic criteria. It assesses cognitive, affective, and behavioral aspects of SSD.9 The SSD-12 is copyrighted and its use requires registration and purchase. Cutoffs by age and gender are available. SSD-12 has demonstrated appropriate reliability and validity.9
Continue to: Structured Clinical Interview for DSM Disorders
Structured Clinical Interview for DSM Disorders (SCID)10,11 is perhaps the most rigorous differential diagnostic tool. However, SCID administration requires training and skill; time for administration and cost of the materials may be prohibitive in primary care.
Finally, SSD symptoms are highly associated with depression and anxiety. Ongoing elevated screening scores for anxiety and depression refractory to interventions may indicate the possibility of overlooked SSD. Furthermore, use of SSD screening tools with anxiety and depression screening tools can provide a more comprehensive picture of impairment, as well as symptom progress.
Treatment: Avoid a split approach
Diagnosing and treating SSD can be challenging for physicians who focus on biomedically based approaches in patient care. Additional tests, studies, and prescriptions are likely to fuel (rather than pacify) patients’ concerns, as such steps divert attention from the underlying psychological needs and mechanisms which maintain SSD. Avoid using a split biopsychosocial approach—ie, beginning the inquiry and treatment planning from a biomedical perspective, and then falling back on psychosocial formulation when treatment efforts have been ineffective. Such an approach leads to understandable patient dissatisfaction and can be interpreted by them as the caregiver suggesting that physical symptoms are “all in [their] head.”12
These 4 tips can help
1. Use a biopsychosocial formulation when initiating treatment. Be familiar with biopsychosocial factors in SSD and develop a narrative for discussing this formulation with patients. For example: “Mr. R, we are going to use the following [medical tests/studies/medications] to understand the cause of your symptoms and better manage them. We also need to think about the role of stress and distress in your symptoms because these can also be at play with dizziness.” This may be particularly beneficial for a functional disorder, such as chronic pain. Incorporating patient education resources is an important step toward shared understanding (see Hunter Integrated Pain Service for chronic pain educational videos; www.tga.gov.au/chronic-pain-management-video-resource-brainman13).
2. Combine education about pathophysiology with patient-centered interviewing. Significant SSD symptom improvements were noted following a single 30-minute educational session, while motivational interviewing techniques were used to probe patients’ concerns.2
Continue to: Maintain professionalism and good clinical practice
3. Maintain professionalism and good clinical practice. Consider SSD a medical matter and address it accordingly: explore concerns fully, provide evidence-based responses, communicate empathy, and employ objective management strategies.14
4. Do not overlook the value of the relationship. A recent systematic review concluded that the relationship between the patient and care provider was central to the success of the interventions for symptom reduction.15
A controversial approach. Pharmacotherapy for SSD is controversial. While several trials of antidepressants and St. John’s wort have been positive and some authors have stated that all classes of antidepressants are effective for SSD, others maintain that questions regarding dosing, treatment duration, and sustainability of improvement have not been sufficiently addressed in research.16,17
Coordination of care issues
Primary care continues to be the de facto mental health system, and specialty services may be unavailable or declined by patients.18 CBT delivered in person or online is empirically supported as a treatment approach to medically unexplained symptoms and SSD.17,19-22
A recent meta-analysis of randomized controlled trials published by Jing and colleagues23 reported that CBT was effective for SSD symptom reduction, and that treatment gains were maintained 3 to 12 months post treatment. However, concerns about the practical implementation of CBT in primary care were raised because CBT was not shown to be effective in improving social functioning or reducing the number of medical visits. Symptom improvement was maximized with longer durations of treatment (> 10 sessions) and greater session lengths (> 50 minutes). Additionally, Abbass and colleagues24 brought up several methodologic (sampling and analysis) concerns related to Jing et al’s work.
Continue to: Overally, CBT's effect sizes...
Overall, CBT’s effect sizes are small, and patients who are open to biopsychosocial explanations for their symptoms and to receiving psychological services may differ from most patients seen in primary care practices.21 Furthermore, mental health providers may hesitate to diagnose SSD because they are concerned about missing a somatic illness.3 Therefore, when coordinating care with mental health providers, it may be beneficial to discuss the treatment approach, assess familiarity with the SSD diagnosis, and closely coordinate and collaborate on the treatment plan.
While physicians cannot be expected to function as psychotherapists, an understanding of CBT and techniques for SSD treatment can be beneficial. Integrated mental health services may hold promise in addressing SSD in primary care. Onsite availability of a behavioral health provider competent in providing evidence-based care can target SSD symptoms and support both patients and physicians.
THE CASE
Mr. R’s treatment course included multiple primary care appointments (scheduled and walk in), ED visits, and specialist visits (ENT/vestibular rehabilitation). He sought care as symptoms intensified, lasted longer, or occurred in new circumstances. He reported persistent fear of the symptoms and anxiety that serious medical causes had been overlooked. He also described distress associated with vertigo and his anxiety sensitivity (anxiety about being anxious).
The behavioral health consultant (BHC; psychologist) and physician talked to the patient about the biopsychosocial antecedents of his condition and the factors that perpetuate the anxiety and stress response. The BHC described the fight/flight/freeze response to the patient and explained its role in the physiologic stress response associated with somatic symptoms and panic. Educational materials (videos and handouts) were also provided to the patient to further illustrate these concepts. The BHC also discussed the role of interoceptive and situational avoidance and active coping (eg, engaging in safe activities); taught the patient relaxation and grounding techniques; and used cognitive disputation aimed at challenging catastrophic symptom interpretations.
The BHC and the patient’s physician established joint treatment goals that included improving functioning, promoting active coping, and decreasing distress associated with symptoms. After the initial medical and BHC visits, both vertigo and anxiety symptoms appeared to abate somewhat, but symptoms have been ongoing and distress and impairment have been variable. The patient’s family physician and BHC continue to work with him to optimize the care plan and treatment goals.
CORRESPONDENCE
Nataliya Pilipenko, PhD, ABPP, Center for Family and Community Medicine, Columbia University Vagelos College of Physicians and Surgeons, 630 West 168th Street, New York, NY 10032; np2615@cumc.columbia.edu
ACKNOWLEDGEMENT
The author thanks Dr. Molly Warren for her collaboration and guidance.
1. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th edition (DSM-5). American Psychiatric Publishing; 2013.
2. Johnson KK, Bennett C, Rochani H. Significant improvement of somatic symptom disorder with brief psychoeducational intervention by PMHNP in primary care. J Am Psychiatr Nurses Assoc. 2020;28:171-180. doi: 10.1177/1078390320960524
3. Weigel A, Maehder K, Witt M, et al. Psychotherapists’ perspective on the treatment of patients with somatic symptom disorders. J Psychosom Res. 2020;138:110228. doi: 10.1016/j.jpsychores.2020.110228
4. American Psychiatric Association. Handbook of Differential Diagnosis. American Psychiatric Publishing; 2014;234-235.
5. Mayo Clinic. Somatic symptom disorder. Accessed February 21, 2022. www.mayoclinic.org/diseases-conditions/somatic-symptom-disorder/symptoms-causes/syc-20377776?p=1
6. Toussaint A, Riedl B, Kehrer S, et al. Validity of the Somatic Symptom Disorder-B Criteria Scale (SSD-12) in primary care. Fam Pract. 2018;35:342-347. doi: 10.1093/fampra/cmx116
7. Kroenke K, Spitzer RL, Williams JB. The PHQ-15: validity of a new measure for evaluating the severity of somatic symptoms. Psychosom Med. 2002;64:258-66. doi: 10.1097/00006842-200203000-00008
8. Kroenke K, Spitzer RL, Williams JB, et al. The Patient Health Questionnaire Somatic, Anxiety, and Depressive Symptom Scales: a systematic review. Gen Hosp Psychiatry. 2010;32:345-359. doi: 10.1016/j.genhosppsych.2010.03.006
9. Toussaint A, Löwe B, Brähler E, et al. The Somatic Symptom Disorder - B Criteria Scale (SSD-12): factorial structure, validity and population-based norms. J Psychosom Res. 2017;97:9-17. doi: 10.1016/j.jpsychores.2017.03.017
10. First MB, Williams JBW, Karg RS, Spitzer RL, eds. Structured Clinical Interview for DSM-5 Disorders, Research Version. American Psychiatric Association, 2015.
11. First MB, Williams JBW, Karg RS, Spitzer RL, eds. Structured Clinical Interview for DSM-5 Disorders, Clinician Version. American Psychiatric Publishing; 2016.
12. McDaniel SH, Hepworth J, Campbell TL, et al, eds. Family Oriented Primary Care. Springer Publishing Co; 2005:1-15.
13. Hunter Integrated Pain Service (2016). Brainman videos. Hunter New England Local Health District. New South Wales, Australia. Accessed February 21, 2022. www.tga.gov.au/chronic-pain-management-video-resource-brainman
14. Murray AM, Toussaint A, Althaus A, et al. The challenge of diagnosing non-specific, functional, and somatoform disorders: a systematic review of barriers to diagnosis in primary care. J Psychosom Res. 2016;80:1-10. doi: 10.1016/j.jpsychores.2015.11.002
15. Leaviss J, Davis S, Ren S, et al. Behavioral modification interventions for medically unexplained symptoms in primary care: systematic reviews and economic evaluation. Health Technol Assess. 2020;24:1-490. doi: 10.3310/hta24460
16. Kroenke K. Efficacy of treatment for somatoform disorders: a review of randomized controlled trials. Psychosom Med. 2007;69:881-888. doi: 10.1097/PSY.0b013e31815b00c4
17. Steinbrecher N, Koerber S, Frieser D, et al. The prevalence of medically unexplained symptoms in primary care. Psychosomatics. 2011;52:263-271. doi: 10.1016/j.psym.2011.01.007
18. Kessler R, Stafford D. Primary care is the de facto mental health system. In Kessler R, Stafford D, eds. Collaborative Medicine Case Studies: Evidence in Practice. Springer Publishing Co, 2008; 9-21.
19. Barsky AJ, Ahern DK, Bauer MR, et al. A randomized trial of treatments for high-utilizing somatizing patients. J Gen Intern Med. 2013;28:1396-1404. doi: 10.1007/s11606-013-2392-6
20. Newby JM, Smith J, Uppal S, et al. Internet-based cognitive behavioral therapy versus psychoeducation control for illness anxiety disorder and somatic symptom disorder: A randomized controlled trial. J Consult Clin Psychol. 2018;86:89-98. doi: 10.1037/ccp0000248
21. van Dessel N, den Boeft M, van der Wouden JC, et al. Non-pharmacological interventions for somatoform disorders and medically unexplained physical symptoms (MUPS) in adults. Cochrane Database Syst Rev. 2014(11):CD011142. doi: 10.1002/14651858.CD011142.pub2
22. Verdurmen MJ, Videler AC, Kamperman AM, et al. Cognitive behavioral therapy for somatic symptom disorders in later life: a prospective comparative explorative pilot study in two clinical populations. Neuropsychiatr Dis Treat. 2017;13:2331-2339. doi: 10.2147/NDT.S141208
23. Liu J, Gill NS, Teodorczuk A, et al. The efficacy of cognitive behavioural therapy in somatoform disorders and medically unexplained physical symptoms: a meta-analysis of randomized controlled trials. J Affect Disord. 2019;245:98-112. doi: 10.1016/j.jad.2018.10.114
24. Abbass A, Leichsenring F, Steinert C. Re: Jing et al., the efficacy of cognitive behavioural therapy in somatoform disorders and medically unexplained physical symptoms: a meta-analysis of randomized controlled trials. J Affect Disord. 2019;255:S0165-0327(18)33197-5. doi: 10.1016/j.jad.2019.02.055
THE CASE
James R* is a 30-year-old man who presented for a primary care walk-in visit due to dizziness, 2 days after he visited an emergency department (ED) for the same concern. He reported episodic symptoms lasting seconds to minutes, specifically when lying down. He said he had not fallen or experienced other physical trauma, did not have blurred vision or hearing loss, and was taking no medications. He also reported panic attacks, during which he experienced palpitations, trembling, paresthesia, and fear of dying. He stated that dizziness did not occur exclusively during panic episodes. His medical history was significant for hypertension; however, he reported significant anxiety related to medical visits. All home blood pressure readings he reported were within normal limits.
Upon examination, the patient had a blood pressure reading of 142/90 mm Hg and no evidence of nystagmus at rest. A neurologic exam was normal and a Dix-Hallpike maneuver reproduced subjective vertigo without nystagmus. Laboratory findings from the patient’s ED visit were negative for troponin and drug use, and blood oxygenation levels were within normal limits. At the time of this current visit, an electrocardiogram was unremarkable, with the exception of some tachycardia.
The presumptive diagnosis was benign paroxysmal positional vertigo (BPPV). An Epley maneuver was performed in the clinic and resulted in minimal symptom improvement. The physician taught Mr. R how to perform the Epley maneuver himself, prescribed a short course of meclizine, and referred him to the integrated mental health care service to address his panic attacks and anxiety.
Over the next few months, Mr. R continued to report significant distress about the dizzy spells, which persisted even after performing the Epley maneuver, and he reported that the meclizine was causing worsening vertigo. He received an ear-nose-and-throat consultation and cognitive behavioral therapy (CBT)–based consultation/interventions. He also reported avoiding multiple activities due to concerns about his dizziness.
●
*The patient’s name and other personally identifying information have been changed to protect his identity.
Somatic symptom disorder (SSD) is characterized by one or more physical symptoms associated with “excessive thoughts, feelings, or behaviors that result in distress and/or functional impairment.”1 Individuals with SSD are preoccupied with symptom-related severity, experience high symptom-related anxiety, or devote significant time and energy to the symptoms or heath concerns. With a diagnosis of SSD, physical symptoms experienced by the patient may or may not be medically explained. The same symptom need not be continuously present as long as the overall symptomatic presentation lasts 6 months or longer.
The specifier “with predominant pain” is used when pain dominates the presentation.1 Estimated prevalence of SSD in primary care ranges from 5% to 35%.2 The true scope of SSD is difficult to assess accurately since research tends to focus on medically unexplained symptoms, rather than excessive symptom-related concerns. Furthermore, terms such as “medically unexplained symptoms” and “functional syndromes” (including fibromyalgia and irritable bowel syndrome) are frequently used when describing SSD.3
One or more factors may contribute to unexplained symptoms: limitations of medical procedures and techniques, partial clinical information, patients’ inability to follow management recommendations, challenges in differential diagnostics, and access-to-care limitations preventing regular care and appropriate diagnostic work up.
What’s important to remember is that it’s the patient’s reaction to physical symptoms, rather than the presence of symptoms per se, that defines SSD.
Considerations in the differential diagnosis
When making a diagnosis of SSD, symptoms cannot:4
- be feigned or deliberately produced as in malingering or factitious disorder.
- result from physiologic effects of a substance (eg, intoxication, withdrawal, or adverse medication effects).
- constitute somatic delusions, as occur in psychotic disorders.
- constitute symptoms or deficits affecting voluntary motor or sensory function that are better explained by neurologic, medical, or psychiatric conditions (consider conversion disorder).
- be preoccupations with physical appearance flaws, as in body dysmorphic disorder.
- be accounted for by an anxiety disorder (eg, palpitations associated with panic attacks).
Continue to: Illness anxiety disorder...
Illness anxiety disorder is also characterized by significant health-related concerns; however, physical symptoms are either mild or absent.
Possible causes of SSD are varied and complex, including genetic and biological factors, family dynamics, behavioral modeling/learning, personality traits, difficulties with emotional regulation, and awareness.5 Patients may present with ongoing requests for symptom explanations, feelings of helplessness, fear of having concerns dismissed, and low motivation for change.3
Aids in supporting a diagnosis of SSD
It’s not appropriate to rely solely on questionnaires to make the diagnosis of SSD. However, brief screening tools are a time-efficient way to capture patients’ experiences and perceptions.6 Along with other components of clinical evaluation, brief symptom screens can both support the diagnosis and help in longitudinal symptom assessment.
Patient Health Questionnaire-15 (PHQ-15), developed for self-report screening in primary care, has desirable psychometric properties including appropriate internal reliability; convergent validity with measures of functional status, disability days, and symptom-related burden; and discriminant validity from measures of depressive symptoms.7 The PHQ-15 is an open access tool that is available in several languages. The respondent is asked to rate the extent of being bothered by a range of medical symptoms in the proceeding 4 weeks. Total scores range from 0 to 30, with higher scores indicating greater symptom aggravation. Cutoffs of 5, 10, and 15 correspond to mild, moderate, and severe symptom levels.8
Somatic Symptom Disorder - B Criteria Scale (SSD-12) aims to capture SSD symptoms in line with Diagnostic and Statistical Manual of Mental Disorders (DSM-5) diagnostic criteria. It assesses cognitive, affective, and behavioral aspects of SSD.9 The SSD-12 is copyrighted and its use requires registration and purchase. Cutoffs by age and gender are available. SSD-12 has demonstrated appropriate reliability and validity.9
Continue to: Structured Clinical Interview for DSM Disorders
Structured Clinical Interview for DSM Disorders (SCID)10,11 is perhaps the most rigorous differential diagnostic tool. However, SCID administration requires training and skill; time for administration and cost of the materials may be prohibitive in primary care.
Finally, SSD symptoms are highly associated with depression and anxiety. Ongoing elevated screening scores for anxiety and depression refractory to interventions may indicate the possibility of overlooked SSD. Furthermore, use of SSD screening tools with anxiety and depression screening tools can provide a more comprehensive picture of impairment, as well as symptom progress.
Treatment: Avoid a split approach
Diagnosing and treating SSD can be challenging for physicians who focus on biomedically based approaches in patient care. Additional tests, studies, and prescriptions are likely to fuel (rather than pacify) patients’ concerns, as such steps divert attention from the underlying psychological needs and mechanisms which maintain SSD. Avoid using a split biopsychosocial approach—ie, beginning the inquiry and treatment planning from a biomedical perspective, and then falling back on psychosocial formulation when treatment efforts have been ineffective. Such an approach leads to understandable patient dissatisfaction and can be interpreted by them as the caregiver suggesting that physical symptoms are “all in [their] head.”12
These 4 tips can help
1. Use a biopsychosocial formulation when initiating treatment. Be familiar with biopsychosocial factors in SSD and develop a narrative for discussing this formulation with patients. For example: “Mr. R, we are going to use the following [medical tests/studies/medications] to understand the cause of your symptoms and better manage them. We also need to think about the role of stress and distress in your symptoms because these can also be at play with dizziness.” This may be particularly beneficial for a functional disorder, such as chronic pain. Incorporating patient education resources is an important step toward shared understanding (see Hunter Integrated Pain Service for chronic pain educational videos; www.tga.gov.au/chronic-pain-management-video-resource-brainman13).
2. Combine education about pathophysiology with patient-centered interviewing. Significant SSD symptom improvements were noted following a single 30-minute educational session, while motivational interviewing techniques were used to probe patients’ concerns.2
Continue to: Maintain professionalism and good clinical practice
3. Maintain professionalism and good clinical practice. Consider SSD a medical matter and address it accordingly: explore concerns fully, provide evidence-based responses, communicate empathy, and employ objective management strategies.14
4. Do not overlook the value of the relationship. A recent systematic review concluded that the relationship between the patient and care provider was central to the success of the interventions for symptom reduction.15
A controversial approach. Pharmacotherapy for SSD is controversial. While several trials of antidepressants and St. John’s wort have been positive and some authors have stated that all classes of antidepressants are effective for SSD, others maintain that questions regarding dosing, treatment duration, and sustainability of improvement have not been sufficiently addressed in research.16,17
Coordination of care issues
Primary care continues to be the de facto mental health system, and specialty services may be unavailable or declined by patients.18 CBT delivered in person or online is empirically supported as a treatment approach to medically unexplained symptoms and SSD.17,19-22
A recent meta-analysis of randomized controlled trials published by Jing and colleagues23 reported that CBT was effective for SSD symptom reduction, and that treatment gains were maintained 3 to 12 months post treatment. However, concerns about the practical implementation of CBT in primary care were raised because CBT was not shown to be effective in improving social functioning or reducing the number of medical visits. Symptom improvement was maximized with longer durations of treatment (> 10 sessions) and greater session lengths (> 50 minutes). Additionally, Abbass and colleagues24 brought up several methodologic (sampling and analysis) concerns related to Jing et al’s work.
Continue to: Overally, CBT's effect sizes...
Overall, CBT’s effect sizes are small, and patients who are open to biopsychosocial explanations for their symptoms and to receiving psychological services may differ from most patients seen in primary care practices.21 Furthermore, mental health providers may hesitate to diagnose SSD because they are concerned about missing a somatic illness.3 Therefore, when coordinating care with mental health providers, it may be beneficial to discuss the treatment approach, assess familiarity with the SSD diagnosis, and closely coordinate and collaborate on the treatment plan.
While physicians cannot be expected to function as psychotherapists, an understanding of CBT and techniques for SSD treatment can be beneficial. Integrated mental health services may hold promise in addressing SSD in primary care. Onsite availability of a behavioral health provider competent in providing evidence-based care can target SSD symptoms and support both patients and physicians.
THE CASE
Mr. R’s treatment course included multiple primary care appointments (scheduled and walk in), ED visits, and specialist visits (ENT/vestibular rehabilitation). He sought care as symptoms intensified, lasted longer, or occurred in new circumstances. He reported persistent fear of the symptoms and anxiety that serious medical causes had been overlooked. He also described distress associated with vertigo and his anxiety sensitivity (anxiety about being anxious).
The behavioral health consultant (BHC; psychologist) and physician talked to the patient about the biopsychosocial antecedents of his condition and the factors that perpetuate the anxiety and stress response. The BHC described the fight/flight/freeze response to the patient and explained its role in the physiologic stress response associated with somatic symptoms and panic. Educational materials (videos and handouts) were also provided to the patient to further illustrate these concepts. The BHC also discussed the role of interoceptive and situational avoidance and active coping (eg, engaging in safe activities); taught the patient relaxation and grounding techniques; and used cognitive disputation aimed at challenging catastrophic symptom interpretations.
The BHC and the patient’s physician established joint treatment goals that included improving functioning, promoting active coping, and decreasing distress associated with symptoms. After the initial medical and BHC visits, both vertigo and anxiety symptoms appeared to abate somewhat, but symptoms have been ongoing and distress and impairment have been variable. The patient’s family physician and BHC continue to work with him to optimize the care plan and treatment goals.
CORRESPONDENCE
Nataliya Pilipenko, PhD, ABPP, Center for Family and Community Medicine, Columbia University Vagelos College of Physicians and Surgeons, 630 West 168th Street, New York, NY 10032; np2615@cumc.columbia.edu
ACKNOWLEDGEMENT
The author thanks Dr. Molly Warren for her collaboration and guidance.
THE CASE
James R* is a 30-year-old man who presented for a primary care walk-in visit due to dizziness, 2 days after he visited an emergency department (ED) for the same concern. He reported episodic symptoms lasting seconds to minutes, specifically when lying down. He said he had not fallen or experienced other physical trauma, did not have blurred vision or hearing loss, and was taking no medications. He also reported panic attacks, during which he experienced palpitations, trembling, paresthesia, and fear of dying. He stated that dizziness did not occur exclusively during panic episodes. His medical history was significant for hypertension; however, he reported significant anxiety related to medical visits. All home blood pressure readings he reported were within normal limits.
Upon examination, the patient had a blood pressure reading of 142/90 mm Hg and no evidence of nystagmus at rest. A neurologic exam was normal and a Dix-Hallpike maneuver reproduced subjective vertigo without nystagmus. Laboratory findings from the patient’s ED visit were negative for troponin and drug use, and blood oxygenation levels were within normal limits. At the time of this current visit, an electrocardiogram was unremarkable, with the exception of some tachycardia.
The presumptive diagnosis was benign paroxysmal positional vertigo (BPPV). An Epley maneuver was performed in the clinic and resulted in minimal symptom improvement. The physician taught Mr. R how to perform the Epley maneuver himself, prescribed a short course of meclizine, and referred him to the integrated mental health care service to address his panic attacks and anxiety.
Over the next few months, Mr. R continued to report significant distress about the dizzy spells, which persisted even after performing the Epley maneuver, and he reported that the meclizine was causing worsening vertigo. He received an ear-nose-and-throat consultation and cognitive behavioral therapy (CBT)–based consultation/interventions. He also reported avoiding multiple activities due to concerns about his dizziness.
●
*The patient’s name and other personally identifying information have been changed to protect his identity.
Somatic symptom disorder (SSD) is characterized by one or more physical symptoms associated with “excessive thoughts, feelings, or behaviors that result in distress and/or functional impairment.”1 Individuals with SSD are preoccupied with symptom-related severity, experience high symptom-related anxiety, or devote significant time and energy to the symptoms or heath concerns. With a diagnosis of SSD, physical symptoms experienced by the patient may or may not be medically explained. The same symptom need not be continuously present as long as the overall symptomatic presentation lasts 6 months or longer.
The specifier “with predominant pain” is used when pain dominates the presentation.1 Estimated prevalence of SSD in primary care ranges from 5% to 35%.2 The true scope of SSD is difficult to assess accurately since research tends to focus on medically unexplained symptoms, rather than excessive symptom-related concerns. Furthermore, terms such as “medically unexplained symptoms” and “functional syndromes” (including fibromyalgia and irritable bowel syndrome) are frequently used when describing SSD.3
One or more factors may contribute to unexplained symptoms: limitations of medical procedures and techniques, partial clinical information, patients’ inability to follow management recommendations, challenges in differential diagnostics, and access-to-care limitations preventing regular care and appropriate diagnostic work up.
What’s important to remember is that it’s the patient’s reaction to physical symptoms, rather than the presence of symptoms per se, that defines SSD.
Considerations in the differential diagnosis
When making a diagnosis of SSD, symptoms cannot:4
- be feigned or deliberately produced as in malingering or factitious disorder.
- result from physiologic effects of a substance (eg, intoxication, withdrawal, or adverse medication effects).
- constitute somatic delusions, as occur in psychotic disorders.
- constitute symptoms or deficits affecting voluntary motor or sensory function that are better explained by neurologic, medical, or psychiatric conditions (consider conversion disorder).
- be preoccupations with physical appearance flaws, as in body dysmorphic disorder.
- be accounted for by an anxiety disorder (eg, palpitations associated with panic attacks).
Continue to: Illness anxiety disorder...
Illness anxiety disorder is also characterized by significant health-related concerns; however, physical symptoms are either mild or absent.
Possible causes of SSD are varied and complex, including genetic and biological factors, family dynamics, behavioral modeling/learning, personality traits, difficulties with emotional regulation, and awareness.5 Patients may present with ongoing requests for symptom explanations, feelings of helplessness, fear of having concerns dismissed, and low motivation for change.3
Aids in supporting a diagnosis of SSD
It’s not appropriate to rely solely on questionnaires to make the diagnosis of SSD. However, brief screening tools are a time-efficient way to capture patients’ experiences and perceptions.6 Along with other components of clinical evaluation, brief symptom screens can both support the diagnosis and help in longitudinal symptom assessment.
Patient Health Questionnaire-15 (PHQ-15), developed for self-report screening in primary care, has desirable psychometric properties including appropriate internal reliability; convergent validity with measures of functional status, disability days, and symptom-related burden; and discriminant validity from measures of depressive symptoms.7 The PHQ-15 is an open access tool that is available in several languages. The respondent is asked to rate the extent of being bothered by a range of medical symptoms in the proceeding 4 weeks. Total scores range from 0 to 30, with higher scores indicating greater symptom aggravation. Cutoffs of 5, 10, and 15 correspond to mild, moderate, and severe symptom levels.8
Somatic Symptom Disorder - B Criteria Scale (SSD-12) aims to capture SSD symptoms in line with Diagnostic and Statistical Manual of Mental Disorders (DSM-5) diagnostic criteria. It assesses cognitive, affective, and behavioral aspects of SSD.9 The SSD-12 is copyrighted and its use requires registration and purchase. Cutoffs by age and gender are available. SSD-12 has demonstrated appropriate reliability and validity.9
Continue to: Structured Clinical Interview for DSM Disorders
Structured Clinical Interview for DSM Disorders (SCID)10,11 is perhaps the most rigorous differential diagnostic tool. However, SCID administration requires training and skill; time for administration and cost of the materials may be prohibitive in primary care.
Finally, SSD symptoms are highly associated with depression and anxiety. Ongoing elevated screening scores for anxiety and depression refractory to interventions may indicate the possibility of overlooked SSD. Furthermore, use of SSD screening tools with anxiety and depression screening tools can provide a more comprehensive picture of impairment, as well as symptom progress.
Treatment: Avoid a split approach
Diagnosing and treating SSD can be challenging for physicians who focus on biomedically based approaches in patient care. Additional tests, studies, and prescriptions are likely to fuel (rather than pacify) patients’ concerns, as such steps divert attention from the underlying psychological needs and mechanisms which maintain SSD. Avoid using a split biopsychosocial approach—ie, beginning the inquiry and treatment planning from a biomedical perspective, and then falling back on psychosocial formulation when treatment efforts have been ineffective. Such an approach leads to understandable patient dissatisfaction and can be interpreted by them as the caregiver suggesting that physical symptoms are “all in [their] head.”12
These 4 tips can help
1. Use a biopsychosocial formulation when initiating treatment. Be familiar with biopsychosocial factors in SSD and develop a narrative for discussing this formulation with patients. For example: “Mr. R, we are going to use the following [medical tests/studies/medications] to understand the cause of your symptoms and better manage them. We also need to think about the role of stress and distress in your symptoms because these can also be at play with dizziness.” This may be particularly beneficial for a functional disorder, such as chronic pain. Incorporating patient education resources is an important step toward shared understanding (see Hunter Integrated Pain Service for chronic pain educational videos; www.tga.gov.au/chronic-pain-management-video-resource-brainman13).
2. Combine education about pathophysiology with patient-centered interviewing. Significant SSD symptom improvements were noted following a single 30-minute educational session, while motivational interviewing techniques were used to probe patients’ concerns.2
Continue to: Maintain professionalism and good clinical practice
3. Maintain professionalism and good clinical practice. Consider SSD a medical matter and address it accordingly: explore concerns fully, provide evidence-based responses, communicate empathy, and employ objective management strategies.14
4. Do not overlook the value of the relationship. A recent systematic review concluded that the relationship between the patient and care provider was central to the success of the interventions for symptom reduction.15
A controversial approach. Pharmacotherapy for SSD is controversial. While several trials of antidepressants and St. John’s wort have been positive and some authors have stated that all classes of antidepressants are effective for SSD, others maintain that questions regarding dosing, treatment duration, and sustainability of improvement have not been sufficiently addressed in research.16,17
Coordination of care issues
Primary care continues to be the de facto mental health system, and specialty services may be unavailable or declined by patients.18 CBT delivered in person or online is empirically supported as a treatment approach to medically unexplained symptoms and SSD.17,19-22
A recent meta-analysis of randomized controlled trials published by Jing and colleagues23 reported that CBT was effective for SSD symptom reduction, and that treatment gains were maintained 3 to 12 months post treatment. However, concerns about the practical implementation of CBT in primary care were raised because CBT was not shown to be effective in improving social functioning or reducing the number of medical visits. Symptom improvement was maximized with longer durations of treatment (> 10 sessions) and greater session lengths (> 50 minutes). Additionally, Abbass and colleagues24 brought up several methodologic (sampling and analysis) concerns related to Jing et al’s work.
Continue to: Overally, CBT's effect sizes...
Overall, CBT’s effect sizes are small, and patients who are open to biopsychosocial explanations for their symptoms and to receiving psychological services may differ from most patients seen in primary care practices.21 Furthermore, mental health providers may hesitate to diagnose SSD because they are concerned about missing a somatic illness.3 Therefore, when coordinating care with mental health providers, it may be beneficial to discuss the treatment approach, assess familiarity with the SSD diagnosis, and closely coordinate and collaborate on the treatment plan.
While physicians cannot be expected to function as psychotherapists, an understanding of CBT and techniques for SSD treatment can be beneficial. Integrated mental health services may hold promise in addressing SSD in primary care. Onsite availability of a behavioral health provider competent in providing evidence-based care can target SSD symptoms and support both patients and physicians.
THE CASE
Mr. R’s treatment course included multiple primary care appointments (scheduled and walk in), ED visits, and specialist visits (ENT/vestibular rehabilitation). He sought care as symptoms intensified, lasted longer, or occurred in new circumstances. He reported persistent fear of the symptoms and anxiety that serious medical causes had been overlooked. He also described distress associated with vertigo and his anxiety sensitivity (anxiety about being anxious).
The behavioral health consultant (BHC; psychologist) and physician talked to the patient about the biopsychosocial antecedents of his condition and the factors that perpetuate the anxiety and stress response. The BHC described the fight/flight/freeze response to the patient and explained its role in the physiologic stress response associated with somatic symptoms and panic. Educational materials (videos and handouts) were also provided to the patient to further illustrate these concepts. The BHC also discussed the role of interoceptive and situational avoidance and active coping (eg, engaging in safe activities); taught the patient relaxation and grounding techniques; and used cognitive disputation aimed at challenging catastrophic symptom interpretations.
The BHC and the patient’s physician established joint treatment goals that included improving functioning, promoting active coping, and decreasing distress associated with symptoms. After the initial medical and BHC visits, both vertigo and anxiety symptoms appeared to abate somewhat, but symptoms have been ongoing and distress and impairment have been variable. The patient’s family physician and BHC continue to work with him to optimize the care plan and treatment goals.
CORRESPONDENCE
Nataliya Pilipenko, PhD, ABPP, Center for Family and Community Medicine, Columbia University Vagelos College of Physicians and Surgeons, 630 West 168th Street, New York, NY 10032; np2615@cumc.columbia.edu
ACKNOWLEDGEMENT
The author thanks Dr. Molly Warren for her collaboration and guidance.
1. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th edition (DSM-5). American Psychiatric Publishing; 2013.
2. Johnson KK, Bennett C, Rochani H. Significant improvement of somatic symptom disorder with brief psychoeducational intervention by PMHNP in primary care. J Am Psychiatr Nurses Assoc. 2020;28:171-180. doi: 10.1177/1078390320960524
3. Weigel A, Maehder K, Witt M, et al. Psychotherapists’ perspective on the treatment of patients with somatic symptom disorders. J Psychosom Res. 2020;138:110228. doi: 10.1016/j.jpsychores.2020.110228
4. American Psychiatric Association. Handbook of Differential Diagnosis. American Psychiatric Publishing; 2014;234-235.
5. Mayo Clinic. Somatic symptom disorder. Accessed February 21, 2022. www.mayoclinic.org/diseases-conditions/somatic-symptom-disorder/symptoms-causes/syc-20377776?p=1
6. Toussaint A, Riedl B, Kehrer S, et al. Validity of the Somatic Symptom Disorder-B Criteria Scale (SSD-12) in primary care. Fam Pract. 2018;35:342-347. doi: 10.1093/fampra/cmx116
7. Kroenke K, Spitzer RL, Williams JB. The PHQ-15: validity of a new measure for evaluating the severity of somatic symptoms. Psychosom Med. 2002;64:258-66. doi: 10.1097/00006842-200203000-00008
8. Kroenke K, Spitzer RL, Williams JB, et al. The Patient Health Questionnaire Somatic, Anxiety, and Depressive Symptom Scales: a systematic review. Gen Hosp Psychiatry. 2010;32:345-359. doi: 10.1016/j.genhosppsych.2010.03.006
9. Toussaint A, Löwe B, Brähler E, et al. The Somatic Symptom Disorder - B Criteria Scale (SSD-12): factorial structure, validity and population-based norms. J Psychosom Res. 2017;97:9-17. doi: 10.1016/j.jpsychores.2017.03.017
10. First MB, Williams JBW, Karg RS, Spitzer RL, eds. Structured Clinical Interview for DSM-5 Disorders, Research Version. American Psychiatric Association, 2015.
11. First MB, Williams JBW, Karg RS, Spitzer RL, eds. Structured Clinical Interview for DSM-5 Disorders, Clinician Version. American Psychiatric Publishing; 2016.
12. McDaniel SH, Hepworth J, Campbell TL, et al, eds. Family Oriented Primary Care. Springer Publishing Co; 2005:1-15.
13. Hunter Integrated Pain Service (2016). Brainman videos. Hunter New England Local Health District. New South Wales, Australia. Accessed February 21, 2022. www.tga.gov.au/chronic-pain-management-video-resource-brainman
14. Murray AM, Toussaint A, Althaus A, et al. The challenge of diagnosing non-specific, functional, and somatoform disorders: a systematic review of barriers to diagnosis in primary care. J Psychosom Res. 2016;80:1-10. doi: 10.1016/j.jpsychores.2015.11.002
15. Leaviss J, Davis S, Ren S, et al. Behavioral modification interventions for medically unexplained symptoms in primary care: systematic reviews and economic evaluation. Health Technol Assess. 2020;24:1-490. doi: 10.3310/hta24460
16. Kroenke K. Efficacy of treatment for somatoform disorders: a review of randomized controlled trials. Psychosom Med. 2007;69:881-888. doi: 10.1097/PSY.0b013e31815b00c4
17. Steinbrecher N, Koerber S, Frieser D, et al. The prevalence of medically unexplained symptoms in primary care. Psychosomatics. 2011;52:263-271. doi: 10.1016/j.psym.2011.01.007
18. Kessler R, Stafford D. Primary care is the de facto mental health system. In Kessler R, Stafford D, eds. Collaborative Medicine Case Studies: Evidence in Practice. Springer Publishing Co, 2008; 9-21.
19. Barsky AJ, Ahern DK, Bauer MR, et al. A randomized trial of treatments for high-utilizing somatizing patients. J Gen Intern Med. 2013;28:1396-1404. doi: 10.1007/s11606-013-2392-6
20. Newby JM, Smith J, Uppal S, et al. Internet-based cognitive behavioral therapy versus psychoeducation control for illness anxiety disorder and somatic symptom disorder: A randomized controlled trial. J Consult Clin Psychol. 2018;86:89-98. doi: 10.1037/ccp0000248
21. van Dessel N, den Boeft M, van der Wouden JC, et al. Non-pharmacological interventions for somatoform disorders and medically unexplained physical symptoms (MUPS) in adults. Cochrane Database Syst Rev. 2014(11):CD011142. doi: 10.1002/14651858.CD011142.pub2
22. Verdurmen MJ, Videler AC, Kamperman AM, et al. Cognitive behavioral therapy for somatic symptom disorders in later life: a prospective comparative explorative pilot study in two clinical populations. Neuropsychiatr Dis Treat. 2017;13:2331-2339. doi: 10.2147/NDT.S141208
23. Liu J, Gill NS, Teodorczuk A, et al. The efficacy of cognitive behavioural therapy in somatoform disorders and medically unexplained physical symptoms: a meta-analysis of randomized controlled trials. J Affect Disord. 2019;245:98-112. doi: 10.1016/j.jad.2018.10.114
24. Abbass A, Leichsenring F, Steinert C. Re: Jing et al., the efficacy of cognitive behavioural therapy in somatoform disorders and medically unexplained physical symptoms: a meta-analysis of randomized controlled trials. J Affect Disord. 2019;255:S0165-0327(18)33197-5. doi: 10.1016/j.jad.2019.02.055
1. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th edition (DSM-5). American Psychiatric Publishing; 2013.
2. Johnson KK, Bennett C, Rochani H. Significant improvement of somatic symptom disorder with brief psychoeducational intervention by PMHNP in primary care. J Am Psychiatr Nurses Assoc. 2020;28:171-180. doi: 10.1177/1078390320960524
3. Weigel A, Maehder K, Witt M, et al. Psychotherapists’ perspective on the treatment of patients with somatic symptom disorders. J Psychosom Res. 2020;138:110228. doi: 10.1016/j.jpsychores.2020.110228
4. American Psychiatric Association. Handbook of Differential Diagnosis. American Psychiatric Publishing; 2014;234-235.
5. Mayo Clinic. Somatic symptom disorder. Accessed February 21, 2022. www.mayoclinic.org/diseases-conditions/somatic-symptom-disorder/symptoms-causes/syc-20377776?p=1
6. Toussaint A, Riedl B, Kehrer S, et al. Validity of the Somatic Symptom Disorder-B Criteria Scale (SSD-12) in primary care. Fam Pract. 2018;35:342-347. doi: 10.1093/fampra/cmx116
7. Kroenke K, Spitzer RL, Williams JB. The PHQ-15: validity of a new measure for evaluating the severity of somatic symptoms. Psychosom Med. 2002;64:258-66. doi: 10.1097/00006842-200203000-00008
8. Kroenke K, Spitzer RL, Williams JB, et al. The Patient Health Questionnaire Somatic, Anxiety, and Depressive Symptom Scales: a systematic review. Gen Hosp Psychiatry. 2010;32:345-359. doi: 10.1016/j.genhosppsych.2010.03.006
9. Toussaint A, Löwe B, Brähler E, et al. The Somatic Symptom Disorder - B Criteria Scale (SSD-12): factorial structure, validity and population-based norms. J Psychosom Res. 2017;97:9-17. doi: 10.1016/j.jpsychores.2017.03.017
10. First MB, Williams JBW, Karg RS, Spitzer RL, eds. Structured Clinical Interview for DSM-5 Disorders, Research Version. American Psychiatric Association, 2015.
11. First MB, Williams JBW, Karg RS, Spitzer RL, eds. Structured Clinical Interview for DSM-5 Disorders, Clinician Version. American Psychiatric Publishing; 2016.
12. McDaniel SH, Hepworth J, Campbell TL, et al, eds. Family Oriented Primary Care. Springer Publishing Co; 2005:1-15.
13. Hunter Integrated Pain Service (2016). Brainman videos. Hunter New England Local Health District. New South Wales, Australia. Accessed February 21, 2022. www.tga.gov.au/chronic-pain-management-video-resource-brainman
14. Murray AM, Toussaint A, Althaus A, et al. The challenge of diagnosing non-specific, functional, and somatoform disorders: a systematic review of barriers to diagnosis in primary care. J Psychosom Res. 2016;80:1-10. doi: 10.1016/j.jpsychores.2015.11.002
15. Leaviss J, Davis S, Ren S, et al. Behavioral modification interventions for medically unexplained symptoms in primary care: systematic reviews and economic evaluation. Health Technol Assess. 2020;24:1-490. doi: 10.3310/hta24460
16. Kroenke K. Efficacy of treatment for somatoform disorders: a review of randomized controlled trials. Psychosom Med. 2007;69:881-888. doi: 10.1097/PSY.0b013e31815b00c4
17. Steinbrecher N, Koerber S, Frieser D, et al. The prevalence of medically unexplained symptoms in primary care. Psychosomatics. 2011;52:263-271. doi: 10.1016/j.psym.2011.01.007
18. Kessler R, Stafford D. Primary care is the de facto mental health system. In Kessler R, Stafford D, eds. Collaborative Medicine Case Studies: Evidence in Practice. Springer Publishing Co, 2008; 9-21.
19. Barsky AJ, Ahern DK, Bauer MR, et al. A randomized trial of treatments for high-utilizing somatizing patients. J Gen Intern Med. 2013;28:1396-1404. doi: 10.1007/s11606-013-2392-6
20. Newby JM, Smith J, Uppal S, et al. Internet-based cognitive behavioral therapy versus psychoeducation control for illness anxiety disorder and somatic symptom disorder: A randomized controlled trial. J Consult Clin Psychol. 2018;86:89-98. doi: 10.1037/ccp0000248
21. van Dessel N, den Boeft M, van der Wouden JC, et al. Non-pharmacological interventions for somatoform disorders and medically unexplained physical symptoms (MUPS) in adults. Cochrane Database Syst Rev. 2014(11):CD011142. doi: 10.1002/14651858.CD011142.pub2
22. Verdurmen MJ, Videler AC, Kamperman AM, et al. Cognitive behavioral therapy for somatic symptom disorders in later life: a prospective comparative explorative pilot study in two clinical populations. Neuropsychiatr Dis Treat. 2017;13:2331-2339. doi: 10.2147/NDT.S141208
23. Liu J, Gill NS, Teodorczuk A, et al. The efficacy of cognitive behavioural therapy in somatoform disorders and medically unexplained physical symptoms: a meta-analysis of randomized controlled trials. J Affect Disord. 2019;245:98-112. doi: 10.1016/j.jad.2018.10.114
24. Abbass A, Leichsenring F, Steinert C. Re: Jing et al., the efficacy of cognitive behavioural therapy in somatoform disorders and medically unexplained physical symptoms: a meta-analysis of randomized controlled trials. J Affect Disord. 2019;255:S0165-0327(18)33197-5. doi: 10.1016/j.jad.2019.02.055