User login
The pendulum swings in favor of corticosteroids
Critical Care Network
Sepsis/Shock Section
The pendulum swings in favor of corticosteroids and endorses the colloquialism among intensivists that no patient shall die without steroids, especially as it relates to sepsis and septic shock.
In 2018, we saw divergence among randomized controlled trials in the use of glucocorticoids for adults with septic shock such that hydrocortisone without the use of fludrocortisone showed no 90-day mortality benefit; however, hydrocortisone with fludrocortisone showed a 90-day mortality benefit.1,2 The Surviving Sepsis Guidelines in 2021 favored using low-dose corticosteroids in those with persistent vasopressor requirements in whom other core interventions had been instituted.
In 2023, a patient-level meta-analysis of low-dose hydrocortisone in adults with septic shock included seven trials and failed to demonstrate a mortality benefit by relative risk in those who received hydrocortisone compared with placebo. Separately, a network meta-analysis with hydrocortisone plus enteral fludrocortisone was associated with a 90-day all-cause mortality. Of the secondary outcomes, these results offered a possible association of hydrocortisone with a decreased risk of ICU mortality and with increased vasopressor-free days.3
The 2024 Society of Critical Care Medicine recently shared an update of focused guidelines on the use of corticosteroids in sepsis, acute respiratory distress syndrome, and community-acquired pneumonia. These included a conditional recommendation to administer corticosteroids for patients with septic shock but recommended against high-dose/short-duration administration of corticosteroids in these patients. These guidelines were supported by data from 46 randomized controlled trials, which showed that corticosteroid use may reduce hospital/long-term mortality and ICU/short-term mortality, as well as result in higher rates of shock reversal and reduced organ dysfunction.
With the results of these meta-analyses and randomized controlled trials, clinicians should consider low-dose corticosteroids paired with fludrocortisone as a tool in treating patients with septic shock given that the short- and long-term benefits may exceed any risks.
References
1. Venkatesh B, et al. Adjunctive glucocorticoid therapy in patients with septic shock. N Engl J Med. 2018;378:797-808.
2. Annane D, et al. Hydrocortisone plus fludrocortisone for adults with septic shock. N Engl J Med. 2018;378:809-818.
3. Pirracchio R, et al. Patient-level meta-analysis of low-dose hydrocortisone in adults with septic shock. NEJM Evid. 2023;2(6).
Critical Care Network
Sepsis/Shock Section
The pendulum swings in favor of corticosteroids and endorses the colloquialism among intensivists that no patient shall die without steroids, especially as it relates to sepsis and septic shock.
In 2018, we saw divergence among randomized controlled trials in the use of glucocorticoids for adults with septic shock such that hydrocortisone without the use of fludrocortisone showed no 90-day mortality benefit; however, hydrocortisone with fludrocortisone showed a 90-day mortality benefit.1,2 The Surviving Sepsis Guidelines in 2021 favored using low-dose corticosteroids in those with persistent vasopressor requirements in whom other core interventions had been instituted.
In 2023, a patient-level meta-analysis of low-dose hydrocortisone in adults with septic shock included seven trials and failed to demonstrate a mortality benefit by relative risk in those who received hydrocortisone compared with placebo. Separately, a network meta-analysis with hydrocortisone plus enteral fludrocortisone was associated with a 90-day all-cause mortality. Of the secondary outcomes, these results offered a possible association of hydrocortisone with a decreased risk of ICU mortality and with increased vasopressor-free days.3
The 2024 Society of Critical Care Medicine recently shared an update of focused guidelines on the use of corticosteroids in sepsis, acute respiratory distress syndrome, and community-acquired pneumonia. These included a conditional recommendation to administer corticosteroids for patients with septic shock but recommended against high-dose/short-duration administration of corticosteroids in these patients. These guidelines were supported by data from 46 randomized controlled trials, which showed that corticosteroid use may reduce hospital/long-term mortality and ICU/short-term mortality, as well as result in higher rates of shock reversal and reduced organ dysfunction.
With the results of these meta-analyses and randomized controlled trials, clinicians should consider low-dose corticosteroids paired with fludrocortisone as a tool in treating patients with septic shock given that the short- and long-term benefits may exceed any risks.
References
1. Venkatesh B, et al. Adjunctive glucocorticoid therapy in patients with septic shock. N Engl J Med. 2018;378:797-808.
2. Annane D, et al. Hydrocortisone plus fludrocortisone for adults with septic shock. N Engl J Med. 2018;378:809-818.
3. Pirracchio R, et al. Patient-level meta-analysis of low-dose hydrocortisone in adults with septic shock. NEJM Evid. 2023;2(6).
Critical Care Network
Sepsis/Shock Section
The pendulum swings in favor of corticosteroids and endorses the colloquialism among intensivists that no patient shall die without steroids, especially as it relates to sepsis and septic shock.
In 2018, we saw divergence among randomized controlled trials in the use of glucocorticoids for adults with septic shock such that hydrocortisone without the use of fludrocortisone showed no 90-day mortality benefit; however, hydrocortisone with fludrocortisone showed a 90-day mortality benefit.1,2 The Surviving Sepsis Guidelines in 2021 favored using low-dose corticosteroids in those with persistent vasopressor requirements in whom other core interventions had been instituted.
In 2023, a patient-level meta-analysis of low-dose hydrocortisone in adults with septic shock included seven trials and failed to demonstrate a mortality benefit by relative risk in those who received hydrocortisone compared with placebo. Separately, a network meta-analysis with hydrocortisone plus enteral fludrocortisone was associated with a 90-day all-cause mortality. Of the secondary outcomes, these results offered a possible association of hydrocortisone with a decreased risk of ICU mortality and with increased vasopressor-free days.3
The 2024 Society of Critical Care Medicine recently shared an update of focused guidelines on the use of corticosteroids in sepsis, acute respiratory distress syndrome, and community-acquired pneumonia. These included a conditional recommendation to administer corticosteroids for patients with septic shock but recommended against high-dose/short-duration administration of corticosteroids in these patients. These guidelines were supported by data from 46 randomized controlled trials, which showed that corticosteroid use may reduce hospital/long-term mortality and ICU/short-term mortality, as well as result in higher rates of shock reversal and reduced organ dysfunction.
With the results of these meta-analyses and randomized controlled trials, clinicians should consider low-dose corticosteroids paired with fludrocortisone as a tool in treating patients with septic shock given that the short- and long-term benefits may exceed any risks.
References
1. Venkatesh B, et al. Adjunctive glucocorticoid therapy in patients with septic shock. N Engl J Med. 2018;378:797-808.
2. Annane D, et al. Hydrocortisone plus fludrocortisone for adults with septic shock. N Engl J Med. 2018;378:809-818.
3. Pirracchio R, et al. Patient-level meta-analysis of low-dose hydrocortisone in adults with septic shock. NEJM Evid. 2023;2(6).
Is it time to embrace a multinight sleep study?
Sleep Medicine Network
Respiratory-Related Sleep Disorders Section
Since the 1960s, sleep researchers have been intrigued by the first-night effect (FNE) in polysomnography (PSG) studies. A meta-analysis by Ding and colleagues revealed FNE’s impact on sleep metrics, like total sleep time and REM sleep, without affecting the apnea-hypopnea index, highlighting PSG’s limitations in simulating natural sleep patterns.1
Lechat and colleagues conducted a study using a home-based sleep analyzer on more than 67,000 individuals, averaging 170 nights each.2 This study found that single-night studies could lead to a 20% misdiagnosis rate in OSA, attributed to overlooking real sleep factors such as body posture, environmental effects, alcohol, and medication. Despite this, the wider use of multinight studies for accurate diagnosis is limited by insurance coverage issues.3
The last decade has seen substantial advances in health technology, particularly in consumer wearables capable of detecting various medical conditions. Devices employing techniques like actigraphy and accelerometry have reached a level of performance comparable with US Food and Drug Administration-approved clinical tools. However, these technologies are still in development for the diagnosis and classification of sleep-disordered breathing.
Tech companies are actively innovating sleep sensing technologies, smartwatches, bed sensors, wireless EEG, radiofrequency, and ultrasound sensors. With significant investments in this sector, these technologies could be ready for widespread use in the next 5 to 10 years. Health care professionals should consider data from sleep-tracking wearables when there are inconsistencies between a patient’s sleep study results and symptoms. The insights from these devices could provide crucial diagnostic information, enhancing the accuracy of sleep disorder diagnoses.
References
1. Ding L, Chen B, Dai Y, Li Y. A meta-analysis of the first-night effect in healthy individuals for the full age spectrum. Sleep Med. 2022;89:159-165. Preprint. Posted online December 17, 2021. PMID: 34998093. doi: 10.1016/j.sleep.2021.12.007
2. Lechat B, Naik G, Reynolds A, et al. Multinight prevalence, variability, and diagnostic misclassification of obstructive sleep apnea. Am J Respir Crit Care Med. 2022;205(5):563-569. PMID: 34904935; PMCID: PMC8906484. doi: 10.1164/rccm.202107-1761OC
3. Abreu A, Punjabi NM. How many nights are really needed to diagnose obstructive sleep apnea? Am J Respir Crit Care Med. 2022;206(1):125-126. PMID: 35476613; PMCID: PMC9954337. doi: 10.1164/rccm.202112-2837LE
Sleep Medicine Network
Respiratory-Related Sleep Disorders Section
Since the 1960s, sleep researchers have been intrigued by the first-night effect (FNE) in polysomnography (PSG) studies. A meta-analysis by Ding and colleagues revealed FNE’s impact on sleep metrics, like total sleep time and REM sleep, without affecting the apnea-hypopnea index, highlighting PSG’s limitations in simulating natural sleep patterns.1
Lechat and colleagues conducted a study using a home-based sleep analyzer on more than 67,000 individuals, averaging 170 nights each.2 This study found that single-night studies could lead to a 20% misdiagnosis rate in OSA, attributed to overlooking real sleep factors such as body posture, environmental effects, alcohol, and medication. Despite this, the wider use of multinight studies for accurate diagnosis is limited by insurance coverage issues.3
The last decade has seen substantial advances in health technology, particularly in consumer wearables capable of detecting various medical conditions. Devices employing techniques like actigraphy and accelerometry have reached a level of performance comparable with US Food and Drug Administration-approved clinical tools. However, these technologies are still in development for the diagnosis and classification of sleep-disordered breathing.
Tech companies are actively innovating sleep sensing technologies, smartwatches, bed sensors, wireless EEG, radiofrequency, and ultrasound sensors. With significant investments in this sector, these technologies could be ready for widespread use in the next 5 to 10 years. Health care professionals should consider data from sleep-tracking wearables when there are inconsistencies between a patient’s sleep study results and symptoms. The insights from these devices could provide crucial diagnostic information, enhancing the accuracy of sleep disorder diagnoses.
References
1. Ding L, Chen B, Dai Y, Li Y. A meta-analysis of the first-night effect in healthy individuals for the full age spectrum. Sleep Med. 2022;89:159-165. Preprint. Posted online December 17, 2021. PMID: 34998093. doi: 10.1016/j.sleep.2021.12.007
2. Lechat B, Naik G, Reynolds A, et al. Multinight prevalence, variability, and diagnostic misclassification of obstructive sleep apnea. Am J Respir Crit Care Med. 2022;205(5):563-569. PMID: 34904935; PMCID: PMC8906484. doi: 10.1164/rccm.202107-1761OC
3. Abreu A, Punjabi NM. How many nights are really needed to diagnose obstructive sleep apnea? Am J Respir Crit Care Med. 2022;206(1):125-126. PMID: 35476613; PMCID: PMC9954337. doi: 10.1164/rccm.202112-2837LE
Sleep Medicine Network
Respiratory-Related Sleep Disorders Section
Since the 1960s, sleep researchers have been intrigued by the first-night effect (FNE) in polysomnography (PSG) studies. A meta-analysis by Ding and colleagues revealed FNE’s impact on sleep metrics, like total sleep time and REM sleep, without affecting the apnea-hypopnea index, highlighting PSG’s limitations in simulating natural sleep patterns.1
Lechat and colleagues conducted a study using a home-based sleep analyzer on more than 67,000 individuals, averaging 170 nights each.2 This study found that single-night studies could lead to a 20% misdiagnosis rate in OSA, attributed to overlooking real sleep factors such as body posture, environmental effects, alcohol, and medication. Despite this, the wider use of multinight studies for accurate diagnosis is limited by insurance coverage issues.3
The last decade has seen substantial advances in health technology, particularly in consumer wearables capable of detecting various medical conditions. Devices employing techniques like actigraphy and accelerometry have reached a level of performance comparable with US Food and Drug Administration-approved clinical tools. However, these technologies are still in development for the diagnosis and classification of sleep-disordered breathing.
Tech companies are actively innovating sleep sensing technologies, smartwatches, bed sensors, wireless EEG, radiofrequency, and ultrasound sensors. With significant investments in this sector, these technologies could be ready for widespread use in the next 5 to 10 years. Health care professionals should consider data from sleep-tracking wearables when there are inconsistencies between a patient’s sleep study results and symptoms. The insights from these devices could provide crucial diagnostic information, enhancing the accuracy of sleep disorder diagnoses.
References
1. Ding L, Chen B, Dai Y, Li Y. A meta-analysis of the first-night effect in healthy individuals for the full age spectrum. Sleep Med. 2022;89:159-165. Preprint. Posted online December 17, 2021. PMID: 34998093. doi: 10.1016/j.sleep.2021.12.007
2. Lechat B, Naik G, Reynolds A, et al. Multinight prevalence, variability, and diagnostic misclassification of obstructive sleep apnea. Am J Respir Crit Care Med. 2022;205(5):563-569. PMID: 34904935; PMCID: PMC8906484. doi: 10.1164/rccm.202107-1761OC
3. Abreu A, Punjabi NM. How many nights are really needed to diagnose obstructive sleep apnea? Am J Respir Crit Care Med. 2022;206(1):125-126. PMID: 35476613; PMCID: PMC9954337. doi: 10.1164/rccm.202112-2837LE
Novel Treatment Options for Epilepsy
DENVER — , according to new data presented at the 2024 annual meeting of the American Academy of Neurology.
Of the two drugs evaluated in phase 2 trials, one is a highly targeted TARP-8–dependent AMPA receptor antagonist known as ES-481. The other is XEN1101, a novel potassium channel opener that was well tolerated as well as effective.
TARP inhibitors, which act on transmembrane AMPA (alpha-amino-3-hydroxy-5-methyl-4 isoxazolepropionic acid) receptor regulatory proteins, are already available for the control of epilepsy, but ES-481 might be different, according to Terrence J. O’Brien, MD, department of neuroscience, Monash University, Melbourne, Australia.
First-in-Class TARP Inhibitor Is Tested
As a “first-in-class, potent and selective antagonist of the TARP-γ8 AMPA receptor,” ES-481 is “predicted to effectively suppress focal seizures arising from the hippocampus and limbic system,” he said. Dr. O’Brien claims that this specificity of action appears to circumvent central nervous system side effects in studies so far.
In the phase 2a multicenter, randomized trial, 22 patients with drug-resistant epilepsy of any type (focal, generalized, or mixed) were randomized to ES-481 or placebo. In the ES-481 arm, the dose was escalated each week, climbing from 25 mg once-daily, to 25 mg twice-daily, 50 mg twice-daily, and then to 75 mg twice daily. At the end of 4 weeks and after a 7-day washout, the randomized groups were crossed over to the opposite therapy for another 4 weeks.
When data were confined to the first treatment period to avoid a carry-over effect, there was a consistent advantage for active treatment over placebo. At the highest 75-mg twice-daily dose of ES-481, the reduction in seizure frequency was 80% vs 49% on placebo ( P < .05).
The rate of complete remission at the end of the study was not greater for ES-481, but higher proportions of patients on active therapy achieved reductions from baseline in seizure activity when defined as greater than 30% (72.77% vs 36.4%) or greater than 50% (36.4% vs 18.2%). P values for these differences were not provided.
Differences in EEG were not observed, but Dr. O’Brien reported that 18 of the subjects had no EEG activity at baseline, diminishing the opportunity to show a difference.
Open-Label Study Supports Controlled Data
Sixteen patients have entered an open-label extension with sustained suppression of seizure activity relative to baseline observed so far, Dr. O’Brien reported.
ES-481 was well tolerated. There were no significant changes in lab values, and all four of the adverse events leading to discontinuation occurred on placebo. There were higher rates of dizziness, insomnia, gait disturbance, and dysarthria associated with ES-481 than placebo, but the rate of serious adverse events was lower (4.8% vs 14.3%).
These response rates are noteworthy because patients had severe disease with diminishing therapeutic options, according to Dr. O’Brien. For entry, patients were required to be taking one to four antiseizure medications while still experiencing seizure activity. The patients averaged one interictal epileptiform discharge and/or seizure per hour on EEG.
Large-scale, double-blind, controlled studies are planned and warranted on the basis of these data, according to Dr. O’Brien, who emphasized that benefit was achieved with a low relative risk of significant adverse events.
New Potassium Channel Opener Shows Promise
Data with the selective potassium channel opener XEN1101 from the previously published phase 2b X-TOLE trial were reported in two parts. The first set of data involved an analysis of response by baseline activity. The other set of data were generated by an ongoing open-label extension (OLE) of X-TOLE.
In X-TOLE, which randomized 325 patients with treatment-resistant focal-onset seizures (FOS) to one of three doses of XEN-1011 or placebo, the median reduction in FOS at the highest dose of 25 mg once-daily XEN-1011 was 52.8% vs placebo (P < .001).
In the new analysis, the goal was to look at efficacy of the 25-mg dose across differences in baseline severity, reported Roger J. Porter, MD, adjunct professor of neurology, University of Pennsylvania, Philadelphia.
Generally, a greater response was observed for those with less severe disease. For example, the response rate defined as greater than 50% reduction in seizure frequency on XEN1101 was higher for those with a baseline seizure activity of 8.5 seizures/month or fewer relative to more (65.5% vs 50.6%) and six or fewer antiseizure medications relative to more (64.2% vs 40.0%).
Pointing out that the study enrolled a challenging group of patients, Dr. Porter said that the data do not rule out efficacy “across the spectrum of epilepsy severity,” but he did suggest that these data will provide context for the coming phase 3 trials.
In the OLE data presented by Jacqueline French, MD, professor of neurology at the Langone School of Medicine of New York University, the efficacy and safety of XEN1101 taken with food has been consistent with what was observed in the double-blind trial. With up to 2 years of follow-up in the planned 5-year OLE, which is evaluating 20 mg once-daily taken with food, 60% are still on therapy,
For those followed for 24 months, 23.6% are completely seizure free, according to Dr. French. For those followed at least 12 months, 31.5% have achieved a median percent reduction in monthly seizure activity of 90% or more; 41.8% a reduction of 75% or more; and 69.7% a reduction of 50% or more.
The side-effect profile has also been consistent with that seen in the phase 2b trial. Dizziness and other mild to moderate side effects that often accompany antiseizure medications have been observed, along with modest weight gain, but there have been no new safety signals over long-term use.
If a planned phase 3 study enrolling patients with localized and general epilepsy confirms these phase 2 data, Dr. French indicated that it has the potential to advance a potassium channel opener that is both efficacious and well tolerated.
First-in-Man Study Performed With Stem Cell Product
The investigational product for treatment-resistant epilepsy has data on just five patients. Yet, the two patients followed the longest, both of which had highly treatment-resistant epilepsy, have had reductions in seizure activity exceeding 95%, according to Cory Nicholas, PhD, the chief executive officer of Neurona Therapeutics.
NRTX-100 is a GABAergic interneuron product derived from human pluripotent stem cells. The NRTX cells are surgically transplanted into the head and body of the hippocampus in patients with unilateral temporal lobe epilepsy with hippocampal sclerosis. The procedure is performed with MRI guidance, and patients are placed on immunosuppression that starts 1 week before surgery and is tapered 1 year later.
In this first-in-man study, the primary outcome of interest was safety. There have been no adverse events associated with this stem cell product in follow-up so far, according to Dr. Nicholas, who presented data on the first 5 of 10 procedures that have been completed so far.
Consistent with the prior work in animal models, it takes several months for the reduction in seizures to be achieved and, in animal models, to see improved functionality. It is notable that the reductions in seizure activity observed over time in those patients followed the longest have been accompanied by evidence of neurocognitive improvement, Dr. Nicholas reported.
“The efficacy has seemed durable so far, and we expect incremental improvement in clinical response over time,” said Dr. Nicholas, who reported that the Food and Drug Administration has already approved a second clinical study.
Are New Antiseizure Therapies Needed?
The value of this and the other emerging therapies is that “no treatment for epilepsy works well in every patient. We continue to need a wide array of treatments to find the one right for the patient in front of us,” said Nassim Zecavati, MD, director of Epilepsy, Children’s Hospital, Virginia Commonwealth University, Richmond.
Asked to comment on the promise of these three therapies, Dr. Zecavati suggested each is intriguing for different reasons. AMPA receptor antagonists have proven to be a promising drug class so far, suggesting that “another could be helpful.” Potassium channel openers appear to have “a great mechanism of action,” but Dr. Zecavati said drugs in this class with a more favorable safety profile are needed.
As for NRTX-1001, she was intrigued with its novelty. She speculated that it might have particular promise for intractable drug-resistant epilepsy in patients who are not candidates for standard surgical strategies but might tolerate a less invasive procedure.
“My question might be who is going to perform this procedure,” Dr. Zecavati said. Noting that experience and skill might be needed to achieve an optimal result with cell transplantation into the brain, she said she will be waiting for more studies that might answer this question and to determine where, if effective, it would fit among current options.
Dr. O’Brien reported financial relationships with Eisai, Kinoxis, Livanova, Supernus, and UCB Pharma. Dr. Porter reported financial relationships with Axonis, Engrail, Longboard, Neurocrine, and Xenon, which provided funding for the study he discussed. Dr. French has financial relationships with more than 20 pharmaceutical companies, including Xenon, which provided funding for the study she discussed. Dr. Nicholas is chief executive officer of Neurona Therapeutics. Dr. Zecavati reported no potential conflicts of interest.
DENVER — , according to new data presented at the 2024 annual meeting of the American Academy of Neurology.
Of the two drugs evaluated in phase 2 trials, one is a highly targeted TARP-8–dependent AMPA receptor antagonist known as ES-481. The other is XEN1101, a novel potassium channel opener that was well tolerated as well as effective.
TARP inhibitors, which act on transmembrane AMPA (alpha-amino-3-hydroxy-5-methyl-4 isoxazolepropionic acid) receptor regulatory proteins, are already available for the control of epilepsy, but ES-481 might be different, according to Terrence J. O’Brien, MD, department of neuroscience, Monash University, Melbourne, Australia.
First-in-Class TARP Inhibitor Is Tested
As a “first-in-class, potent and selective antagonist of the TARP-γ8 AMPA receptor,” ES-481 is “predicted to effectively suppress focal seizures arising from the hippocampus and limbic system,” he said. Dr. O’Brien claims that this specificity of action appears to circumvent central nervous system side effects in studies so far.
In the phase 2a multicenter, randomized trial, 22 patients with drug-resistant epilepsy of any type (focal, generalized, or mixed) were randomized to ES-481 or placebo. In the ES-481 arm, the dose was escalated each week, climbing from 25 mg once-daily, to 25 mg twice-daily, 50 mg twice-daily, and then to 75 mg twice daily. At the end of 4 weeks and after a 7-day washout, the randomized groups were crossed over to the opposite therapy for another 4 weeks.
When data were confined to the first treatment period to avoid a carry-over effect, there was a consistent advantage for active treatment over placebo. At the highest 75-mg twice-daily dose of ES-481, the reduction in seizure frequency was 80% vs 49% on placebo ( P < .05).
The rate of complete remission at the end of the study was not greater for ES-481, but higher proportions of patients on active therapy achieved reductions from baseline in seizure activity when defined as greater than 30% (72.77% vs 36.4%) or greater than 50% (36.4% vs 18.2%). P values for these differences were not provided.
Differences in EEG were not observed, but Dr. O’Brien reported that 18 of the subjects had no EEG activity at baseline, diminishing the opportunity to show a difference.
Open-Label Study Supports Controlled Data
Sixteen patients have entered an open-label extension with sustained suppression of seizure activity relative to baseline observed so far, Dr. O’Brien reported.
ES-481 was well tolerated. There were no significant changes in lab values, and all four of the adverse events leading to discontinuation occurred on placebo. There were higher rates of dizziness, insomnia, gait disturbance, and dysarthria associated with ES-481 than placebo, but the rate of serious adverse events was lower (4.8% vs 14.3%).
These response rates are noteworthy because patients had severe disease with diminishing therapeutic options, according to Dr. O’Brien. For entry, patients were required to be taking one to four antiseizure medications while still experiencing seizure activity. The patients averaged one interictal epileptiform discharge and/or seizure per hour on EEG.
Large-scale, double-blind, controlled studies are planned and warranted on the basis of these data, according to Dr. O’Brien, who emphasized that benefit was achieved with a low relative risk of significant adverse events.
New Potassium Channel Opener Shows Promise
Data with the selective potassium channel opener XEN1101 from the previously published phase 2b X-TOLE trial were reported in two parts. The first set of data involved an analysis of response by baseline activity. The other set of data were generated by an ongoing open-label extension (OLE) of X-TOLE.
In X-TOLE, which randomized 325 patients with treatment-resistant focal-onset seizures (FOS) to one of three doses of XEN-1011 or placebo, the median reduction in FOS at the highest dose of 25 mg once-daily XEN-1011 was 52.8% vs placebo (P < .001).
In the new analysis, the goal was to look at efficacy of the 25-mg dose across differences in baseline severity, reported Roger J. Porter, MD, adjunct professor of neurology, University of Pennsylvania, Philadelphia.
Generally, a greater response was observed for those with less severe disease. For example, the response rate defined as greater than 50% reduction in seizure frequency on XEN1101 was higher for those with a baseline seizure activity of 8.5 seizures/month or fewer relative to more (65.5% vs 50.6%) and six or fewer antiseizure medications relative to more (64.2% vs 40.0%).
Pointing out that the study enrolled a challenging group of patients, Dr. Porter said that the data do not rule out efficacy “across the spectrum of epilepsy severity,” but he did suggest that these data will provide context for the coming phase 3 trials.
In the OLE data presented by Jacqueline French, MD, professor of neurology at the Langone School of Medicine of New York University, the efficacy and safety of XEN1101 taken with food has been consistent with what was observed in the double-blind trial. With up to 2 years of follow-up in the planned 5-year OLE, which is evaluating 20 mg once-daily taken with food, 60% are still on therapy,
For those followed for 24 months, 23.6% are completely seizure free, according to Dr. French. For those followed at least 12 months, 31.5% have achieved a median percent reduction in monthly seizure activity of 90% or more; 41.8% a reduction of 75% or more; and 69.7% a reduction of 50% or more.
The side-effect profile has also been consistent with that seen in the phase 2b trial. Dizziness and other mild to moderate side effects that often accompany antiseizure medications have been observed, along with modest weight gain, but there have been no new safety signals over long-term use.
If a planned phase 3 study enrolling patients with localized and general epilepsy confirms these phase 2 data, Dr. French indicated that it has the potential to advance a potassium channel opener that is both efficacious and well tolerated.
First-in-Man Study Performed With Stem Cell Product
The investigational product for treatment-resistant epilepsy has data on just five patients. Yet, the two patients followed the longest, both of which had highly treatment-resistant epilepsy, have had reductions in seizure activity exceeding 95%, according to Cory Nicholas, PhD, the chief executive officer of Neurona Therapeutics.
NRTX-100 is a GABAergic interneuron product derived from human pluripotent stem cells. The NRTX cells are surgically transplanted into the head and body of the hippocampus in patients with unilateral temporal lobe epilepsy with hippocampal sclerosis. The procedure is performed with MRI guidance, and patients are placed on immunosuppression that starts 1 week before surgery and is tapered 1 year later.
In this first-in-man study, the primary outcome of interest was safety. There have been no adverse events associated with this stem cell product in follow-up so far, according to Dr. Nicholas, who presented data on the first 5 of 10 procedures that have been completed so far.
Consistent with the prior work in animal models, it takes several months for the reduction in seizures to be achieved and, in animal models, to see improved functionality. It is notable that the reductions in seizure activity observed over time in those patients followed the longest have been accompanied by evidence of neurocognitive improvement, Dr. Nicholas reported.
“The efficacy has seemed durable so far, and we expect incremental improvement in clinical response over time,” said Dr. Nicholas, who reported that the Food and Drug Administration has already approved a second clinical study.
Are New Antiseizure Therapies Needed?
The value of this and the other emerging therapies is that “no treatment for epilepsy works well in every patient. We continue to need a wide array of treatments to find the one right for the patient in front of us,” said Nassim Zecavati, MD, director of Epilepsy, Children’s Hospital, Virginia Commonwealth University, Richmond.
Asked to comment on the promise of these three therapies, Dr. Zecavati suggested each is intriguing for different reasons. AMPA receptor antagonists have proven to be a promising drug class so far, suggesting that “another could be helpful.” Potassium channel openers appear to have “a great mechanism of action,” but Dr. Zecavati said drugs in this class with a more favorable safety profile are needed.
As for NRTX-1001, she was intrigued with its novelty. She speculated that it might have particular promise for intractable drug-resistant epilepsy in patients who are not candidates for standard surgical strategies but might tolerate a less invasive procedure.
“My question might be who is going to perform this procedure,” Dr. Zecavati said. Noting that experience and skill might be needed to achieve an optimal result with cell transplantation into the brain, she said she will be waiting for more studies that might answer this question and to determine where, if effective, it would fit among current options.
Dr. O’Brien reported financial relationships with Eisai, Kinoxis, Livanova, Supernus, and UCB Pharma. Dr. Porter reported financial relationships with Axonis, Engrail, Longboard, Neurocrine, and Xenon, which provided funding for the study he discussed. Dr. French has financial relationships with more than 20 pharmaceutical companies, including Xenon, which provided funding for the study she discussed. Dr. Nicholas is chief executive officer of Neurona Therapeutics. Dr. Zecavati reported no potential conflicts of interest.
DENVER — , according to new data presented at the 2024 annual meeting of the American Academy of Neurology.
Of the two drugs evaluated in phase 2 trials, one is a highly targeted TARP-8–dependent AMPA receptor antagonist known as ES-481. The other is XEN1101, a novel potassium channel opener that was well tolerated as well as effective.
TARP inhibitors, which act on transmembrane AMPA (alpha-amino-3-hydroxy-5-methyl-4 isoxazolepropionic acid) receptor regulatory proteins, are already available for the control of epilepsy, but ES-481 might be different, according to Terrence J. O’Brien, MD, department of neuroscience, Monash University, Melbourne, Australia.
First-in-Class TARP Inhibitor Is Tested
As a “first-in-class, potent and selective antagonist of the TARP-γ8 AMPA receptor,” ES-481 is “predicted to effectively suppress focal seizures arising from the hippocampus and limbic system,” he said. Dr. O’Brien claims that this specificity of action appears to circumvent central nervous system side effects in studies so far.
In the phase 2a multicenter, randomized trial, 22 patients with drug-resistant epilepsy of any type (focal, generalized, or mixed) were randomized to ES-481 or placebo. In the ES-481 arm, the dose was escalated each week, climbing from 25 mg once-daily, to 25 mg twice-daily, 50 mg twice-daily, and then to 75 mg twice daily. At the end of 4 weeks and after a 7-day washout, the randomized groups were crossed over to the opposite therapy for another 4 weeks.
When data were confined to the first treatment period to avoid a carry-over effect, there was a consistent advantage for active treatment over placebo. At the highest 75-mg twice-daily dose of ES-481, the reduction in seizure frequency was 80% vs 49% on placebo ( P < .05).
The rate of complete remission at the end of the study was not greater for ES-481, but higher proportions of patients on active therapy achieved reductions from baseline in seizure activity when defined as greater than 30% (72.77% vs 36.4%) or greater than 50% (36.4% vs 18.2%). P values for these differences were not provided.
Differences in EEG were not observed, but Dr. O’Brien reported that 18 of the subjects had no EEG activity at baseline, diminishing the opportunity to show a difference.
Open-Label Study Supports Controlled Data
Sixteen patients have entered an open-label extension with sustained suppression of seizure activity relative to baseline observed so far, Dr. O’Brien reported.
ES-481 was well tolerated. There were no significant changes in lab values, and all four of the adverse events leading to discontinuation occurred on placebo. There were higher rates of dizziness, insomnia, gait disturbance, and dysarthria associated with ES-481 than placebo, but the rate of serious adverse events was lower (4.8% vs 14.3%).
These response rates are noteworthy because patients had severe disease with diminishing therapeutic options, according to Dr. O’Brien. For entry, patients were required to be taking one to four antiseizure medications while still experiencing seizure activity. The patients averaged one interictal epileptiform discharge and/or seizure per hour on EEG.
Large-scale, double-blind, controlled studies are planned and warranted on the basis of these data, according to Dr. O’Brien, who emphasized that benefit was achieved with a low relative risk of significant adverse events.
New Potassium Channel Opener Shows Promise
Data with the selective potassium channel opener XEN1101 from the previously published phase 2b X-TOLE trial were reported in two parts. The first set of data involved an analysis of response by baseline activity. The other set of data were generated by an ongoing open-label extension (OLE) of X-TOLE.
In X-TOLE, which randomized 325 patients with treatment-resistant focal-onset seizures (FOS) to one of three doses of XEN-1011 or placebo, the median reduction in FOS at the highest dose of 25 mg once-daily XEN-1011 was 52.8% vs placebo (P < .001).
In the new analysis, the goal was to look at efficacy of the 25-mg dose across differences in baseline severity, reported Roger J. Porter, MD, adjunct professor of neurology, University of Pennsylvania, Philadelphia.
Generally, a greater response was observed for those with less severe disease. For example, the response rate defined as greater than 50% reduction in seizure frequency on XEN1101 was higher for those with a baseline seizure activity of 8.5 seizures/month or fewer relative to more (65.5% vs 50.6%) and six or fewer antiseizure medications relative to more (64.2% vs 40.0%).
Pointing out that the study enrolled a challenging group of patients, Dr. Porter said that the data do not rule out efficacy “across the spectrum of epilepsy severity,” but he did suggest that these data will provide context for the coming phase 3 trials.
In the OLE data presented by Jacqueline French, MD, professor of neurology at the Langone School of Medicine of New York University, the efficacy and safety of XEN1101 taken with food has been consistent with what was observed in the double-blind trial. With up to 2 years of follow-up in the planned 5-year OLE, which is evaluating 20 mg once-daily taken with food, 60% are still on therapy,
For those followed for 24 months, 23.6% are completely seizure free, according to Dr. French. For those followed at least 12 months, 31.5% have achieved a median percent reduction in monthly seizure activity of 90% or more; 41.8% a reduction of 75% or more; and 69.7% a reduction of 50% or more.
The side-effect profile has also been consistent with that seen in the phase 2b trial. Dizziness and other mild to moderate side effects that often accompany antiseizure medications have been observed, along with modest weight gain, but there have been no new safety signals over long-term use.
If a planned phase 3 study enrolling patients with localized and general epilepsy confirms these phase 2 data, Dr. French indicated that it has the potential to advance a potassium channel opener that is both efficacious and well tolerated.
First-in-Man Study Performed With Stem Cell Product
The investigational product for treatment-resistant epilepsy has data on just five patients. Yet, the two patients followed the longest, both of which had highly treatment-resistant epilepsy, have had reductions in seizure activity exceeding 95%, according to Cory Nicholas, PhD, the chief executive officer of Neurona Therapeutics.
NRTX-100 is a GABAergic interneuron product derived from human pluripotent stem cells. The NRTX cells are surgically transplanted into the head and body of the hippocampus in patients with unilateral temporal lobe epilepsy with hippocampal sclerosis. The procedure is performed with MRI guidance, and patients are placed on immunosuppression that starts 1 week before surgery and is tapered 1 year later.
In this first-in-man study, the primary outcome of interest was safety. There have been no adverse events associated with this stem cell product in follow-up so far, according to Dr. Nicholas, who presented data on the first 5 of 10 procedures that have been completed so far.
Consistent with the prior work in animal models, it takes several months for the reduction in seizures to be achieved and, in animal models, to see improved functionality. It is notable that the reductions in seizure activity observed over time in those patients followed the longest have been accompanied by evidence of neurocognitive improvement, Dr. Nicholas reported.
“The efficacy has seemed durable so far, and we expect incremental improvement in clinical response over time,” said Dr. Nicholas, who reported that the Food and Drug Administration has already approved a second clinical study.
Are New Antiseizure Therapies Needed?
The value of this and the other emerging therapies is that “no treatment for epilepsy works well in every patient. We continue to need a wide array of treatments to find the one right for the patient in front of us,” said Nassim Zecavati, MD, director of Epilepsy, Children’s Hospital, Virginia Commonwealth University, Richmond.
Asked to comment on the promise of these three therapies, Dr. Zecavati suggested each is intriguing for different reasons. AMPA receptor antagonists have proven to be a promising drug class so far, suggesting that “another could be helpful.” Potassium channel openers appear to have “a great mechanism of action,” but Dr. Zecavati said drugs in this class with a more favorable safety profile are needed.
As for NRTX-1001, she was intrigued with its novelty. She speculated that it might have particular promise for intractable drug-resistant epilepsy in patients who are not candidates for standard surgical strategies but might tolerate a less invasive procedure.
“My question might be who is going to perform this procedure,” Dr. Zecavati said. Noting that experience and skill might be needed to achieve an optimal result with cell transplantation into the brain, she said she will be waiting for more studies that might answer this question and to determine where, if effective, it would fit among current options.
Dr. O’Brien reported financial relationships with Eisai, Kinoxis, Livanova, Supernus, and UCB Pharma. Dr. Porter reported financial relationships with Axonis, Engrail, Longboard, Neurocrine, and Xenon, which provided funding for the study he discussed. Dr. French has financial relationships with more than 20 pharmaceutical companies, including Xenon, which provided funding for the study she discussed. Dr. Nicholas is chief executive officer of Neurona Therapeutics. Dr. Zecavati reported no potential conflicts of interest.
FROM AAN 2024
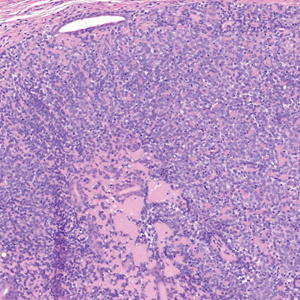
MicroRNAs May Predict Pancreatic Cancer Risk Years Before Diagnosis
TOPLINE:
METHODOLOGY:
- Early detection of pancreatic cancer could improve patient prognosis, but clinically viable biomarkers are lacking. In a two-stage study, researchers screened and validated circulating miRNAs as biomarkers for early detection using prediagnostic plasma samples from 462 case-control pairs across multiple cohorts.
- The discovery stage included 185 pairs from the PLCO Cancer Screening Trial, and the replication stage included 277 pairs from Shanghai Women’s/Men’s Health Study, Southern Community Cohort Study, and Multiethnic Cohort Study.
- Overall, 798 plasma microRNAs were measured using the NanoString nCounter Analysis System, and odds ratios (ORs) for pancreatic cancer risk were calculated on the basis of miRNA concentrations.
- Statistical analysis involved conditional logistic regression, stratified by age and time from sample collection to diagnosis.
TAKEAWAY:
- In the discovery stage, the researchers identified 120 miRNAs significantly associated with pancreatic cancer risk.
- Three of these miRNAs showed consistent significant associations in the replication stage. Specifically, hsa-miR-199a-3p/hsa-miR-199b-3p and hsa-miR-191-5p were associated with a 10%-11% lower risk for pancreatic cancer (OR, 0.89 and 0.90, respectively), and hsa-miR-767-5p was associated with an 8% higher risk for pancreatic cancer (OR, 1.08) within 5 years of the blood draw.
- In age-stratified analyses, hsa-miR-767-5p (OR, 1.23) along with four other miRNAs — hsa-miR-640 (OR, 1.33), hsa-miR-874-5p (OR, 1.25), hsa-miR-1299 (OR, 1.28), and hsa-miR-449b-5p (OR, 1.22) — were associated with an increased risk for pancreatic cancer among patients diagnosed at age 65 or older.
- One miRNA, hsa-miR-22-3p (OR, 0.76), was associated with a lower risk for pancreatic cancer in this older age group.
IN PRACTICE:
The findings provide evidence that miRNAs “have a potential utilization in clinical practice” to help “identify high-risk individuals who could subsequently undergo a more definitive but invasive diagnostic procedure,” the authors said. “Such a multistep strategy for pancreatic cancer screening and early detection, likely cost-efficient and low-risk, could be critical to improve survival.”
SOURCE:
The study, with first author Cong Wang, Vanderbilt University Medical Center, Nashville, Tennessee, was published online in the International Journal of Cancer.
LIMITATIONS:
The researchers lacked miRNA profiles of patients with pancreatic cancer at diagnosis and were not able to track the miRNA changes among pancreatic cancer cases at the time of clinical diagnosis. Sample collection protocols differed across study cohorts, and the researchers changed assay panels during the study.
DISCLOSURES:
The research was funded by grants from the National Cancer Institute. The authors declared no conflicts of interest.
A version of this article appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- Early detection of pancreatic cancer could improve patient prognosis, but clinically viable biomarkers are lacking. In a two-stage study, researchers screened and validated circulating miRNAs as biomarkers for early detection using prediagnostic plasma samples from 462 case-control pairs across multiple cohorts.
- The discovery stage included 185 pairs from the PLCO Cancer Screening Trial, and the replication stage included 277 pairs from Shanghai Women’s/Men’s Health Study, Southern Community Cohort Study, and Multiethnic Cohort Study.
- Overall, 798 plasma microRNAs were measured using the NanoString nCounter Analysis System, and odds ratios (ORs) for pancreatic cancer risk were calculated on the basis of miRNA concentrations.
- Statistical analysis involved conditional logistic regression, stratified by age and time from sample collection to diagnosis.
TAKEAWAY:
- In the discovery stage, the researchers identified 120 miRNAs significantly associated with pancreatic cancer risk.
- Three of these miRNAs showed consistent significant associations in the replication stage. Specifically, hsa-miR-199a-3p/hsa-miR-199b-3p and hsa-miR-191-5p were associated with a 10%-11% lower risk for pancreatic cancer (OR, 0.89 and 0.90, respectively), and hsa-miR-767-5p was associated with an 8% higher risk for pancreatic cancer (OR, 1.08) within 5 years of the blood draw.
- In age-stratified analyses, hsa-miR-767-5p (OR, 1.23) along with four other miRNAs — hsa-miR-640 (OR, 1.33), hsa-miR-874-5p (OR, 1.25), hsa-miR-1299 (OR, 1.28), and hsa-miR-449b-5p (OR, 1.22) — were associated with an increased risk for pancreatic cancer among patients diagnosed at age 65 or older.
- One miRNA, hsa-miR-22-3p (OR, 0.76), was associated with a lower risk for pancreatic cancer in this older age group.
IN PRACTICE:
The findings provide evidence that miRNAs “have a potential utilization in clinical practice” to help “identify high-risk individuals who could subsequently undergo a more definitive but invasive diagnostic procedure,” the authors said. “Such a multistep strategy for pancreatic cancer screening and early detection, likely cost-efficient and low-risk, could be critical to improve survival.”
SOURCE:
The study, with first author Cong Wang, Vanderbilt University Medical Center, Nashville, Tennessee, was published online in the International Journal of Cancer.
LIMITATIONS:
The researchers lacked miRNA profiles of patients with pancreatic cancer at diagnosis and were not able to track the miRNA changes among pancreatic cancer cases at the time of clinical diagnosis. Sample collection protocols differed across study cohorts, and the researchers changed assay panels during the study.
DISCLOSURES:
The research was funded by grants from the National Cancer Institute. The authors declared no conflicts of interest.
A version of this article appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- Early detection of pancreatic cancer could improve patient prognosis, but clinically viable biomarkers are lacking. In a two-stage study, researchers screened and validated circulating miRNAs as biomarkers for early detection using prediagnostic plasma samples from 462 case-control pairs across multiple cohorts.
- The discovery stage included 185 pairs from the PLCO Cancer Screening Trial, and the replication stage included 277 pairs from Shanghai Women’s/Men’s Health Study, Southern Community Cohort Study, and Multiethnic Cohort Study.
- Overall, 798 plasma microRNAs were measured using the NanoString nCounter Analysis System, and odds ratios (ORs) for pancreatic cancer risk were calculated on the basis of miRNA concentrations.
- Statistical analysis involved conditional logistic regression, stratified by age and time from sample collection to diagnosis.
TAKEAWAY:
- In the discovery stage, the researchers identified 120 miRNAs significantly associated with pancreatic cancer risk.
- Three of these miRNAs showed consistent significant associations in the replication stage. Specifically, hsa-miR-199a-3p/hsa-miR-199b-3p and hsa-miR-191-5p were associated with a 10%-11% lower risk for pancreatic cancer (OR, 0.89 and 0.90, respectively), and hsa-miR-767-5p was associated with an 8% higher risk for pancreatic cancer (OR, 1.08) within 5 years of the blood draw.
- In age-stratified analyses, hsa-miR-767-5p (OR, 1.23) along with four other miRNAs — hsa-miR-640 (OR, 1.33), hsa-miR-874-5p (OR, 1.25), hsa-miR-1299 (OR, 1.28), and hsa-miR-449b-5p (OR, 1.22) — were associated with an increased risk for pancreatic cancer among patients diagnosed at age 65 or older.
- One miRNA, hsa-miR-22-3p (OR, 0.76), was associated with a lower risk for pancreatic cancer in this older age group.
IN PRACTICE:
The findings provide evidence that miRNAs “have a potential utilization in clinical practice” to help “identify high-risk individuals who could subsequently undergo a more definitive but invasive diagnostic procedure,” the authors said. “Such a multistep strategy for pancreatic cancer screening and early detection, likely cost-efficient and low-risk, could be critical to improve survival.”
SOURCE:
The study, with first author Cong Wang, Vanderbilt University Medical Center, Nashville, Tennessee, was published online in the International Journal of Cancer.
LIMITATIONS:
The researchers lacked miRNA profiles of patients with pancreatic cancer at diagnosis and were not able to track the miRNA changes among pancreatic cancer cases at the time of clinical diagnosis. Sample collection protocols differed across study cohorts, and the researchers changed assay panels during the study.
DISCLOSURES:
The research was funded by grants from the National Cancer Institute. The authors declared no conflicts of interest.
A version of this article appeared on Medscape.com.
‘Bread and Butter’: Societies Issue T2D Management Guidance
Two professional societies have issued new guidance for type 2 diabetes management in primary care, with one focused specifically on the use of the newer medications.
On April 19, 2024, the American College of Physicians (ACP) published Newer Pharmacologic Treatments in Adults With Type 2 Diabetes: A Clinical Guideline From the American College of Physicians. The internal medicine group recommends the use of glucagon-like peptide-1 (GLP-1) agonists, and sodium–glucose cotransporter-2 (SGLT2) inhibitors as second-line treatment after metformin. They also advise against the use of dipeptidyl peptidase-4 (DPP-4) inhibitors.
The document was also presented simultaneously at the ACP annual meeting.
And on April 15, the American Diabetes Association (ADA) posted its comprehensive Standards of Care in Diabetes—2024 Abridged for Primary Care Professionals as a follow-up to the December 2023 publication of its full-length Standards. Section 9, Pharmacologic Approaches to Glycemic Treatment, covers the same ground as the ACP guidelines.
General Agreement but Some Differences
The recommendations generally agree regarding medication use, although there are some differences. Both societies continue to endorse metformin and lifestyle modification as first-line therapy for glycemic management in type 2 diabetes. However, while ADA also gives the option of initial combination therapy with prioritization of avoiding hypoglycemia, ACP advises adding new medications only if glycemic goals aren’t met with lifestyle and metformin alone.
The new ACP document gives two general recommendations:
1. Add an SGLT2 inhibitor or a GLP-1 agonist to metformin and lifestyle modifications in adults with type 2 diabetes and inadequate glycemic control.
*Use an SGLT2 inhibitor to reduce the risk for all-cause mortality, major adverse cardiovascular events, progression of chronic kidney disease, and hospitalization due to congestive heart failure.
*Use a GLP-1 agonist to reduce the risk for all-cause mortality, major adverse cardiovascular events, and stroke.
2. ACP recommends against adding a DPP-4 inhibitor to metformin and lifestyle modifications in adults with type 2 diabetes and inadequate glycemic control to reduce morbidity and all-cause mortality.
Both ADA and ACP advise using SGLT2 inhibitors in patients with congestive heart failure and/or chronic kidney disease, and using GLP-1 agonists in patients for whom weight management is a priority. The ADA also advises using agents of either drug class with proven cardiovascular benefit for people with type 2 diabetes who have established cardiovascular disease or who are at high risk.
ADA doesn’t advise against the use of DPP-4 inhibitors but doesn’t prioritize them either. Both insulin and sulfonylureas remain options for both, but they also are lower priority due to their potential for causing hypoglycemia. ACP says that sulfonylureas and long-acting insulin are “inferior to SGLT2 inhibitors and GLP-1 agonists in reducing all-cause mortality and morbidity but may still have some limited value for glycemic control.”
The two groups continue to differ regarding A1c goals, although both recommend individualization. The ACP generally advises levels between 7% and 8% for most adults with type 2 diabetes, and de-intensification of pharmacologic agents for those with A1c levels below 6.5%. On the other hand, ADA recommends A1c levels < 7% as long as that can be achieved safely.
This is the first time ACP has addressed this topic in a guideline, panel chair Carolyn J. Crandall, MD, told this news organization. “Diabetes treatment, of course, is our bread and butter…but what we had done before was based on the need to identify a target, like glycosylated hemoglobin. What patients and physicians really want to know now is, who should receive these new drugs? Should they receive these new drugs? And what benefits do they have?”
Added Dr. Crandall, who is professor of medicine at the David Geffen School of Medicine at the University of California, Los Angeles. “At ACP we have a complicated process that I’m actually very proud of, where we’ve asked a lay public panel, as well at the members of our guideline committee, to rank what’s most important in terms of the health outcomes for this condition…And then we look at how to balance those risks and benefits to make the recommendations.”
In the same Annals of Internal Medicine issue are two systematic reviews/meta-analyses that informed the new document, one on drug effectiveness and the other on cost-effectiveness.
In the accompanying editorial from Fatima Z. Syed, MD, an internist and medical weight management specialist at Duke University Division of General Internal Medicine, Durham, North Carolina, she notes, “the potential added benefits of these newer medications, including weight loss and cardiovascular and renal benefits, motivate their prescription, but cost and prior authorization hurdles can bar their use.”
Dr. Syed cites as “missing” from the ACP guidelines an analysis of comorbidities, including obesity. The reason for that, according to the document, is that “weight loss, as measured by percentage of participants who achieved at least 10% total body weight loss, was a prioritized outcome, but data were insufficient for network meta-analysis.”
However, Dr. Syed notes that factoring in weight loss could improve the cost-effectiveness of the newer medications. She points out that the ADA Standards suggest a GLP-1 agonist with or without metformin as initial therapy options for people with newly diagnosed type 2 diabetes who might benefit from weight loss.
“The ACP guidelines strengthen the case for metformin as first-line medication for diabetes when comorbid conditions are not present. Metformin is cost-effective and has excellent hemoglobin A1c reduction. The accompanying economic analysis tells us that in the absence of comorbidity, the newer medication classes do not seem to be cost-effective. However, given that many patients with type 2 diabetes have obesity or existing cardiovascular or renal disease, the choice and accessibility of newer medications can be nuanced. The cost-effectiveness of GLP1 agonists and SGLT2 inhibitors as initial diabetes therapy in the setting of various comorbid conditions warrants careful exploration.”
Dr. Crandall has no disclosures. Dr. Syed disclosed that her husband is employed by Blue Cross Blue Shield of North Carolina.
A version of this article first appeared on Medscape.com.
Two professional societies have issued new guidance for type 2 diabetes management in primary care, with one focused specifically on the use of the newer medications.
On April 19, 2024, the American College of Physicians (ACP) published Newer Pharmacologic Treatments in Adults With Type 2 Diabetes: A Clinical Guideline From the American College of Physicians. The internal medicine group recommends the use of glucagon-like peptide-1 (GLP-1) agonists, and sodium–glucose cotransporter-2 (SGLT2) inhibitors as second-line treatment after metformin. They also advise against the use of dipeptidyl peptidase-4 (DPP-4) inhibitors.
The document was also presented simultaneously at the ACP annual meeting.
And on April 15, the American Diabetes Association (ADA) posted its comprehensive Standards of Care in Diabetes—2024 Abridged for Primary Care Professionals as a follow-up to the December 2023 publication of its full-length Standards. Section 9, Pharmacologic Approaches to Glycemic Treatment, covers the same ground as the ACP guidelines.
General Agreement but Some Differences
The recommendations generally agree regarding medication use, although there are some differences. Both societies continue to endorse metformin and lifestyle modification as first-line therapy for glycemic management in type 2 diabetes. However, while ADA also gives the option of initial combination therapy with prioritization of avoiding hypoglycemia, ACP advises adding new medications only if glycemic goals aren’t met with lifestyle and metformin alone.
The new ACP document gives two general recommendations:
1. Add an SGLT2 inhibitor or a GLP-1 agonist to metformin and lifestyle modifications in adults with type 2 diabetes and inadequate glycemic control.
*Use an SGLT2 inhibitor to reduce the risk for all-cause mortality, major adverse cardiovascular events, progression of chronic kidney disease, and hospitalization due to congestive heart failure.
*Use a GLP-1 agonist to reduce the risk for all-cause mortality, major adverse cardiovascular events, and stroke.
2. ACP recommends against adding a DPP-4 inhibitor to metformin and lifestyle modifications in adults with type 2 diabetes and inadequate glycemic control to reduce morbidity and all-cause mortality.
Both ADA and ACP advise using SGLT2 inhibitors in patients with congestive heart failure and/or chronic kidney disease, and using GLP-1 agonists in patients for whom weight management is a priority. The ADA also advises using agents of either drug class with proven cardiovascular benefit for people with type 2 diabetes who have established cardiovascular disease or who are at high risk.
ADA doesn’t advise against the use of DPP-4 inhibitors but doesn’t prioritize them either. Both insulin and sulfonylureas remain options for both, but they also are lower priority due to their potential for causing hypoglycemia. ACP says that sulfonylureas and long-acting insulin are “inferior to SGLT2 inhibitors and GLP-1 agonists in reducing all-cause mortality and morbidity but may still have some limited value for glycemic control.”
The two groups continue to differ regarding A1c goals, although both recommend individualization. The ACP generally advises levels between 7% and 8% for most adults with type 2 diabetes, and de-intensification of pharmacologic agents for those with A1c levels below 6.5%. On the other hand, ADA recommends A1c levels < 7% as long as that can be achieved safely.
This is the first time ACP has addressed this topic in a guideline, panel chair Carolyn J. Crandall, MD, told this news organization. “Diabetes treatment, of course, is our bread and butter…but what we had done before was based on the need to identify a target, like glycosylated hemoglobin. What patients and physicians really want to know now is, who should receive these new drugs? Should they receive these new drugs? And what benefits do they have?”
Added Dr. Crandall, who is professor of medicine at the David Geffen School of Medicine at the University of California, Los Angeles. “At ACP we have a complicated process that I’m actually very proud of, where we’ve asked a lay public panel, as well at the members of our guideline committee, to rank what’s most important in terms of the health outcomes for this condition…And then we look at how to balance those risks and benefits to make the recommendations.”
In the same Annals of Internal Medicine issue are two systematic reviews/meta-analyses that informed the new document, one on drug effectiveness and the other on cost-effectiveness.
In the accompanying editorial from Fatima Z. Syed, MD, an internist and medical weight management specialist at Duke University Division of General Internal Medicine, Durham, North Carolina, she notes, “the potential added benefits of these newer medications, including weight loss and cardiovascular and renal benefits, motivate their prescription, but cost and prior authorization hurdles can bar their use.”
Dr. Syed cites as “missing” from the ACP guidelines an analysis of comorbidities, including obesity. The reason for that, according to the document, is that “weight loss, as measured by percentage of participants who achieved at least 10% total body weight loss, was a prioritized outcome, but data were insufficient for network meta-analysis.”
However, Dr. Syed notes that factoring in weight loss could improve the cost-effectiveness of the newer medications. She points out that the ADA Standards suggest a GLP-1 agonist with or without metformin as initial therapy options for people with newly diagnosed type 2 diabetes who might benefit from weight loss.
“The ACP guidelines strengthen the case for metformin as first-line medication for diabetes when comorbid conditions are not present. Metformin is cost-effective and has excellent hemoglobin A1c reduction. The accompanying economic analysis tells us that in the absence of comorbidity, the newer medication classes do not seem to be cost-effective. However, given that many patients with type 2 diabetes have obesity or existing cardiovascular or renal disease, the choice and accessibility of newer medications can be nuanced. The cost-effectiveness of GLP1 agonists and SGLT2 inhibitors as initial diabetes therapy in the setting of various comorbid conditions warrants careful exploration.”
Dr. Crandall has no disclosures. Dr. Syed disclosed that her husband is employed by Blue Cross Blue Shield of North Carolina.
A version of this article first appeared on Medscape.com.
Two professional societies have issued new guidance for type 2 diabetes management in primary care, with one focused specifically on the use of the newer medications.
On April 19, 2024, the American College of Physicians (ACP) published Newer Pharmacologic Treatments in Adults With Type 2 Diabetes: A Clinical Guideline From the American College of Physicians. The internal medicine group recommends the use of glucagon-like peptide-1 (GLP-1) agonists, and sodium–glucose cotransporter-2 (SGLT2) inhibitors as second-line treatment after metformin. They also advise against the use of dipeptidyl peptidase-4 (DPP-4) inhibitors.
The document was also presented simultaneously at the ACP annual meeting.
And on April 15, the American Diabetes Association (ADA) posted its comprehensive Standards of Care in Diabetes—2024 Abridged for Primary Care Professionals as a follow-up to the December 2023 publication of its full-length Standards. Section 9, Pharmacologic Approaches to Glycemic Treatment, covers the same ground as the ACP guidelines.
General Agreement but Some Differences
The recommendations generally agree regarding medication use, although there are some differences. Both societies continue to endorse metformin and lifestyle modification as first-line therapy for glycemic management in type 2 diabetes. However, while ADA also gives the option of initial combination therapy with prioritization of avoiding hypoglycemia, ACP advises adding new medications only if glycemic goals aren’t met with lifestyle and metformin alone.
The new ACP document gives two general recommendations:
1. Add an SGLT2 inhibitor or a GLP-1 agonist to metformin and lifestyle modifications in adults with type 2 diabetes and inadequate glycemic control.
*Use an SGLT2 inhibitor to reduce the risk for all-cause mortality, major adverse cardiovascular events, progression of chronic kidney disease, and hospitalization due to congestive heart failure.
*Use a GLP-1 agonist to reduce the risk for all-cause mortality, major adverse cardiovascular events, and stroke.
2. ACP recommends against adding a DPP-4 inhibitor to metformin and lifestyle modifications in adults with type 2 diabetes and inadequate glycemic control to reduce morbidity and all-cause mortality.
Both ADA and ACP advise using SGLT2 inhibitors in patients with congestive heart failure and/or chronic kidney disease, and using GLP-1 agonists in patients for whom weight management is a priority. The ADA also advises using agents of either drug class with proven cardiovascular benefit for people with type 2 diabetes who have established cardiovascular disease or who are at high risk.
ADA doesn’t advise against the use of DPP-4 inhibitors but doesn’t prioritize them either. Both insulin and sulfonylureas remain options for both, but they also are lower priority due to their potential for causing hypoglycemia. ACP says that sulfonylureas and long-acting insulin are “inferior to SGLT2 inhibitors and GLP-1 agonists in reducing all-cause mortality and morbidity but may still have some limited value for glycemic control.”
The two groups continue to differ regarding A1c goals, although both recommend individualization. The ACP generally advises levels between 7% and 8% for most adults with type 2 diabetes, and de-intensification of pharmacologic agents for those with A1c levels below 6.5%. On the other hand, ADA recommends A1c levels < 7% as long as that can be achieved safely.
This is the first time ACP has addressed this topic in a guideline, panel chair Carolyn J. Crandall, MD, told this news organization. “Diabetes treatment, of course, is our bread and butter…but what we had done before was based on the need to identify a target, like glycosylated hemoglobin. What patients and physicians really want to know now is, who should receive these new drugs? Should they receive these new drugs? And what benefits do they have?”
Added Dr. Crandall, who is professor of medicine at the David Geffen School of Medicine at the University of California, Los Angeles. “At ACP we have a complicated process that I’m actually very proud of, where we’ve asked a lay public panel, as well at the members of our guideline committee, to rank what’s most important in terms of the health outcomes for this condition…And then we look at how to balance those risks and benefits to make the recommendations.”
In the same Annals of Internal Medicine issue are two systematic reviews/meta-analyses that informed the new document, one on drug effectiveness and the other on cost-effectiveness.
In the accompanying editorial from Fatima Z. Syed, MD, an internist and medical weight management specialist at Duke University Division of General Internal Medicine, Durham, North Carolina, she notes, “the potential added benefits of these newer medications, including weight loss and cardiovascular and renal benefits, motivate their prescription, but cost and prior authorization hurdles can bar their use.”
Dr. Syed cites as “missing” from the ACP guidelines an analysis of comorbidities, including obesity. The reason for that, according to the document, is that “weight loss, as measured by percentage of participants who achieved at least 10% total body weight loss, was a prioritized outcome, but data were insufficient for network meta-analysis.”
However, Dr. Syed notes that factoring in weight loss could improve the cost-effectiveness of the newer medications. She points out that the ADA Standards suggest a GLP-1 agonist with or without metformin as initial therapy options for people with newly diagnosed type 2 diabetes who might benefit from weight loss.
“The ACP guidelines strengthen the case for metformin as first-line medication for diabetes when comorbid conditions are not present. Metformin is cost-effective and has excellent hemoglobin A1c reduction. The accompanying economic analysis tells us that in the absence of comorbidity, the newer medication classes do not seem to be cost-effective. However, given that many patients with type 2 diabetes have obesity or existing cardiovascular or renal disease, the choice and accessibility of newer medications can be nuanced. The cost-effectiveness of GLP1 agonists and SGLT2 inhibitors as initial diabetes therapy in the setting of various comorbid conditions warrants careful exploration.”
Dr. Crandall has no disclosures. Dr. Syed disclosed that her husband is employed by Blue Cross Blue Shield of North Carolina.
A version of this article first appeared on Medscape.com.
Pediatric Clinic Doubles Well-Visit Data Capture by Going Completely Paperless
TORONTO — , and, of course, it eliminated the work associated with managing paper records.
Due to the efficacy of the pre-visit capture, “all of the information — including individual responses and total scores — is available to the provider at a glance” by the time of the examination encounter, according to Brian T. Ketterman, MD, a third-year resident in pediatrics at the Monroe Carell Jr. Children’s Hospital, Vanderbilt University, Nashville, Tennessee.
Urgent issues, such as suicidality risk, “are flagged so that they are seen first,” he added.
The goal of going paperless was to improve the amount and the consistency of data captured with each well visit, according to Dr. Ketterman. He described a major undertaking involving fundamental changes in the electronic medical record (EMR) system to accommodate the new approach.
Characterizing screening at well visits as “one of the most important jobs of a pediatrician” when he presented these data at the Pediatric Academic Societies annual meeting, he added that a paperless approach comes with many advantages.
Raising the Rate of Complete Data Capture
The American Academy of Pediatrics (AAP) has identified more than 30 screening elements at well-child visits. At his institution several additional screening elements have been added. Prior to going paperless, about 45.6% on average of this well-visit data was being captured in any specific patient encounter, even if the institution was doing well overall in capturing essential information, such as key laboratory values and immunizations.
The vast majority of the information that was missing depended on patient or family input, such as social determinants of health (SDoH), which includes the aspects of home environment, such as nutrition and safety. Dr. Ketterman said that going paperless was inspired by positive experiences reported elsewhere.
“We wanted to build on this work,” he said.
The goal of the program was to raise the rate of complete data capture at well visits to at least 80% and do this across all languages. The first step was to create digital forms in English and Spanish for completion unique to each milestone well-child visit defined by age. For those who speak English or Spanish, these forms were supplied through the patient portal several days before the visit.
For those who spoke one of the more than 40 other languages encountered among patients at Dr. Ketterman’s institution, an interpreter was supplied. If patients arrived at the clinic without completing the digital entry, they completed it on a tablet with the help of an interpreter if one was needed.
Prior to going paperless, all screening data were captured on paper forms completed in the waiting room. The provider then manually reviewed and scored the forms before they were then scanned into the medical records. The paperless approach has eliminated all of these steps and the information is already available for review by the time the pediatrician enters the examination room.
With more than 50,000 well-child checkups captured over a recent 15-month period of paperless questionnaires, the proportion with 100% data capture using what Dr. Ketterman characterized as “strict criteria” was 84%, surpassing the goal at the initiation of the program.
“We improved in almost every screening measure,” Dr. Ketterman said, providing P values that were mostly < .001 for a range of standard tests such as M-CHAT-R (Modified Checklist for Autism in Toddlers), QPA (Quick Parenting Assessment), and PSC-17 (Pediatric Symptom Checklist) when compared to the baseline period.
Additional Advantages
The improvement in well-visit data capture was the goal, but the list of other advantages of the paperless system is long. For one, the EMR system now automatically uses the data to offer guidance that might improve patient outcomes. For example, if the family reports that the child does not see a dentist or does not know how to swim, these lead prompt the EMR to provide resources, such as the names of dentists of swim programs, to address the problem.
As another example, screening questions that reveal food insecurity automatically trigger guidance for enrolling in the U.S. Department of Agriculture’s WIC (Women, Infants, and Children) food program. According to Dr. Ketterman, the proportion of children now enrolled in WIC has increased 10-fold from baseline. He also reported there was a twofold increase in the proportion of patients enrolled in a free book program as a result of a screening questions that ask about reading at home.
The improvement in well-visit data capture was seen across all languages. Even if the gains were not quite as good in languages other than English or Spanish, they were still highly significant relative to baseline.
A ‘Life-Changing’ Improvement
The discussion following his talk made it clear that similar approaches are being actively pursued nationwide. Several in the audience working on similar programs identified such challenges as getting electronic medical record (EMR) systems to cooperate, ensuring patient enrollment in the portals, and avoiding form completion fatigue, but the comments were uniformly supportive of the benefits of this approach.
“This has been life-changing for us,” said Katie E. McPeak, MD, a primary care pediatrician and Medical Director for Health Equity at the Children’s Hospital of Philadelphia. “The information is more accurate in the digital format and it reduces time for the clinician reviewing the data in the exam room.” She also agreed that paperless completion of screen captures better information on more topics, like sleep, nutrition, and mood disorders.
However, Dr. McPeak was one of those who was concerned about form fatigue. Patients have to enter extensive information over multiple screens for each well-child visit. She said this problem might need to be addressed if the success of paperless screening leads to even greater expansion of data requested.
In addressing the work behind creating a system of the depth and scope of the one he described, Dr. Ketterman acknowledged that it involved a daunting development process with substantial coding and testing. Referring to the EMR system used at his hospital, he said the preparation required an “epic guru,” but he said that input fatigue has not yet arisen as a major issue.
“Many of the screens are mandatory, so you cannot advance without completing them, but some are optional,” he noted. “However, we are seeing a high rate of response even on the screens they could click past.”
Dr. Ketterman and Dr. McPeak report no potential conflicts of interest.
TORONTO — , and, of course, it eliminated the work associated with managing paper records.
Due to the efficacy of the pre-visit capture, “all of the information — including individual responses and total scores — is available to the provider at a glance” by the time of the examination encounter, according to Brian T. Ketterman, MD, a third-year resident in pediatrics at the Monroe Carell Jr. Children’s Hospital, Vanderbilt University, Nashville, Tennessee.
Urgent issues, such as suicidality risk, “are flagged so that they are seen first,” he added.
The goal of going paperless was to improve the amount and the consistency of data captured with each well visit, according to Dr. Ketterman. He described a major undertaking involving fundamental changes in the electronic medical record (EMR) system to accommodate the new approach.
Characterizing screening at well visits as “one of the most important jobs of a pediatrician” when he presented these data at the Pediatric Academic Societies annual meeting, he added that a paperless approach comes with many advantages.
Raising the Rate of Complete Data Capture
The American Academy of Pediatrics (AAP) has identified more than 30 screening elements at well-child visits. At his institution several additional screening elements have been added. Prior to going paperless, about 45.6% on average of this well-visit data was being captured in any specific patient encounter, even if the institution was doing well overall in capturing essential information, such as key laboratory values and immunizations.
The vast majority of the information that was missing depended on patient or family input, such as social determinants of health (SDoH), which includes the aspects of home environment, such as nutrition and safety. Dr. Ketterman said that going paperless was inspired by positive experiences reported elsewhere.
“We wanted to build on this work,” he said.
The goal of the program was to raise the rate of complete data capture at well visits to at least 80% and do this across all languages. The first step was to create digital forms in English and Spanish for completion unique to each milestone well-child visit defined by age. For those who speak English or Spanish, these forms were supplied through the patient portal several days before the visit.
For those who spoke one of the more than 40 other languages encountered among patients at Dr. Ketterman’s institution, an interpreter was supplied. If patients arrived at the clinic without completing the digital entry, they completed it on a tablet with the help of an interpreter if one was needed.
Prior to going paperless, all screening data were captured on paper forms completed in the waiting room. The provider then manually reviewed and scored the forms before they were then scanned into the medical records. The paperless approach has eliminated all of these steps and the information is already available for review by the time the pediatrician enters the examination room.
With more than 50,000 well-child checkups captured over a recent 15-month period of paperless questionnaires, the proportion with 100% data capture using what Dr. Ketterman characterized as “strict criteria” was 84%, surpassing the goal at the initiation of the program.
“We improved in almost every screening measure,” Dr. Ketterman said, providing P values that were mostly < .001 for a range of standard tests such as M-CHAT-R (Modified Checklist for Autism in Toddlers), QPA (Quick Parenting Assessment), and PSC-17 (Pediatric Symptom Checklist) when compared to the baseline period.
Additional Advantages
The improvement in well-visit data capture was the goal, but the list of other advantages of the paperless system is long. For one, the EMR system now automatically uses the data to offer guidance that might improve patient outcomes. For example, if the family reports that the child does not see a dentist or does not know how to swim, these lead prompt the EMR to provide resources, such as the names of dentists of swim programs, to address the problem.
As another example, screening questions that reveal food insecurity automatically trigger guidance for enrolling in the U.S. Department of Agriculture’s WIC (Women, Infants, and Children) food program. According to Dr. Ketterman, the proportion of children now enrolled in WIC has increased 10-fold from baseline. He also reported there was a twofold increase in the proportion of patients enrolled in a free book program as a result of a screening questions that ask about reading at home.
The improvement in well-visit data capture was seen across all languages. Even if the gains were not quite as good in languages other than English or Spanish, they were still highly significant relative to baseline.
A ‘Life-Changing’ Improvement
The discussion following his talk made it clear that similar approaches are being actively pursued nationwide. Several in the audience working on similar programs identified such challenges as getting electronic medical record (EMR) systems to cooperate, ensuring patient enrollment in the portals, and avoiding form completion fatigue, but the comments were uniformly supportive of the benefits of this approach.
“This has been life-changing for us,” said Katie E. McPeak, MD, a primary care pediatrician and Medical Director for Health Equity at the Children’s Hospital of Philadelphia. “The information is more accurate in the digital format and it reduces time for the clinician reviewing the data in the exam room.” She also agreed that paperless completion of screen captures better information on more topics, like sleep, nutrition, and mood disorders.
However, Dr. McPeak was one of those who was concerned about form fatigue. Patients have to enter extensive information over multiple screens for each well-child visit. She said this problem might need to be addressed if the success of paperless screening leads to even greater expansion of data requested.
In addressing the work behind creating a system of the depth and scope of the one he described, Dr. Ketterman acknowledged that it involved a daunting development process with substantial coding and testing. Referring to the EMR system used at his hospital, he said the preparation required an “epic guru,” but he said that input fatigue has not yet arisen as a major issue.
“Many of the screens are mandatory, so you cannot advance without completing them, but some are optional,” he noted. “However, we are seeing a high rate of response even on the screens they could click past.”
Dr. Ketterman and Dr. McPeak report no potential conflicts of interest.
TORONTO — , and, of course, it eliminated the work associated with managing paper records.
Due to the efficacy of the pre-visit capture, “all of the information — including individual responses and total scores — is available to the provider at a glance” by the time of the examination encounter, according to Brian T. Ketterman, MD, a third-year resident in pediatrics at the Monroe Carell Jr. Children’s Hospital, Vanderbilt University, Nashville, Tennessee.
Urgent issues, such as suicidality risk, “are flagged so that they are seen first,” he added.
The goal of going paperless was to improve the amount and the consistency of data captured with each well visit, according to Dr. Ketterman. He described a major undertaking involving fundamental changes in the electronic medical record (EMR) system to accommodate the new approach.
Characterizing screening at well visits as “one of the most important jobs of a pediatrician” when he presented these data at the Pediatric Academic Societies annual meeting, he added that a paperless approach comes with many advantages.
Raising the Rate of Complete Data Capture
The American Academy of Pediatrics (AAP) has identified more than 30 screening elements at well-child visits. At his institution several additional screening elements have been added. Prior to going paperless, about 45.6% on average of this well-visit data was being captured in any specific patient encounter, even if the institution was doing well overall in capturing essential information, such as key laboratory values and immunizations.
The vast majority of the information that was missing depended on patient or family input, such as social determinants of health (SDoH), which includes the aspects of home environment, such as nutrition and safety. Dr. Ketterman said that going paperless was inspired by positive experiences reported elsewhere.
“We wanted to build on this work,” he said.
The goal of the program was to raise the rate of complete data capture at well visits to at least 80% and do this across all languages. The first step was to create digital forms in English and Spanish for completion unique to each milestone well-child visit defined by age. For those who speak English or Spanish, these forms were supplied through the patient portal several days before the visit.
For those who spoke one of the more than 40 other languages encountered among patients at Dr. Ketterman’s institution, an interpreter was supplied. If patients arrived at the clinic without completing the digital entry, they completed it on a tablet with the help of an interpreter if one was needed.
Prior to going paperless, all screening data were captured on paper forms completed in the waiting room. The provider then manually reviewed and scored the forms before they were then scanned into the medical records. The paperless approach has eliminated all of these steps and the information is already available for review by the time the pediatrician enters the examination room.
With more than 50,000 well-child checkups captured over a recent 15-month period of paperless questionnaires, the proportion with 100% data capture using what Dr. Ketterman characterized as “strict criteria” was 84%, surpassing the goal at the initiation of the program.
“We improved in almost every screening measure,” Dr. Ketterman said, providing P values that were mostly < .001 for a range of standard tests such as M-CHAT-R (Modified Checklist for Autism in Toddlers), QPA (Quick Parenting Assessment), and PSC-17 (Pediatric Symptom Checklist) when compared to the baseline period.
Additional Advantages
The improvement in well-visit data capture was the goal, but the list of other advantages of the paperless system is long. For one, the EMR system now automatically uses the data to offer guidance that might improve patient outcomes. For example, if the family reports that the child does not see a dentist or does not know how to swim, these lead prompt the EMR to provide resources, such as the names of dentists of swim programs, to address the problem.
As another example, screening questions that reveal food insecurity automatically trigger guidance for enrolling in the U.S. Department of Agriculture’s WIC (Women, Infants, and Children) food program. According to Dr. Ketterman, the proportion of children now enrolled in WIC has increased 10-fold from baseline. He also reported there was a twofold increase in the proportion of patients enrolled in a free book program as a result of a screening questions that ask about reading at home.
The improvement in well-visit data capture was seen across all languages. Even if the gains were not quite as good in languages other than English or Spanish, they were still highly significant relative to baseline.
A ‘Life-Changing’ Improvement
The discussion following his talk made it clear that similar approaches are being actively pursued nationwide. Several in the audience working on similar programs identified such challenges as getting electronic medical record (EMR) systems to cooperate, ensuring patient enrollment in the portals, and avoiding form completion fatigue, but the comments were uniformly supportive of the benefits of this approach.
“This has been life-changing for us,” said Katie E. McPeak, MD, a primary care pediatrician and Medical Director for Health Equity at the Children’s Hospital of Philadelphia. “The information is more accurate in the digital format and it reduces time for the clinician reviewing the data in the exam room.” She also agreed that paperless completion of screen captures better information on more topics, like sleep, nutrition, and mood disorders.
However, Dr. McPeak was one of those who was concerned about form fatigue. Patients have to enter extensive information over multiple screens for each well-child visit. She said this problem might need to be addressed if the success of paperless screening leads to even greater expansion of data requested.
In addressing the work behind creating a system of the depth and scope of the one he described, Dr. Ketterman acknowledged that it involved a daunting development process with substantial coding and testing. Referring to the EMR system used at his hospital, he said the preparation required an “epic guru,” but he said that input fatigue has not yet arisen as a major issue.
“Many of the screens are mandatory, so you cannot advance without completing them, but some are optional,” he noted. “However, we are seeing a high rate of response even on the screens they could click past.”
Dr. Ketterman and Dr. McPeak report no potential conflicts of interest.
FROM PAS 2024
Multiple Asymptomatic Dome-Shaped Papules on the Scalp
The Diagnosis: Spiradenocylindroma
Shave biopsies of our patient’s lesions showed wellcircumscribed dermal nodules resembling a spiradenoma with 3 cell populations: those with lighter nuclei, darker nuclei, and scattered lymphocytes. However, the conspicuous globules of basement membrane material were reminiscent of a cylindroma. These overlapping features and the patient’s history of cylindroma were suggestive of a diagnosis of spiradenocylindroma.
Spiradenocylindroma is an uncommon dermal tumor with features that overlap with spiradenoma and cylindroma.1 It may manifest as a solitary lesion or multiple lesions and can occur sporadically or in the context of a family history. Histologically, it must be distinguished from other intradermal basaloid neoplasms including conventional cylindroma and spiradenoma, dermal duct tumor, hidradenoma, and trichoblastoma.
When patients present with multiple cylindromas, spiradenomas, or spiradenocylindromas, physicians should consider genetic testing and review of the family history to assess for cylindromatosis gene mutations or Brooke-Spiegler syndrome. Biopsy and histologic examination are important because malignant tumors can evolve from pre-existing spiradenocylindromas, cylindromas, and spiradenomas,2 with an increased risk in patients with Brooke-Spiegler syndrome.1 Our patient declined further genetic workup but continues to follow up with dermatology for monitoring of lesions.
Dermal duct tumors are morphologic variants of poromas that are derived from sweat gland lineage and usually manifest as solitary dome-shaped papules, plaques, or nodules most often seen on acral surfaces as well as the head and neck.3 Clinically, they may be indistinguishable from spiradenocylindromas and require biopsy for histologic evaluation. They can be distinguished from spiradenocylindroma by the presence of small dermal nodules composed of cuboidal cells with ample pink cytoplasm and cuticle-lined ducts (Figure 1).
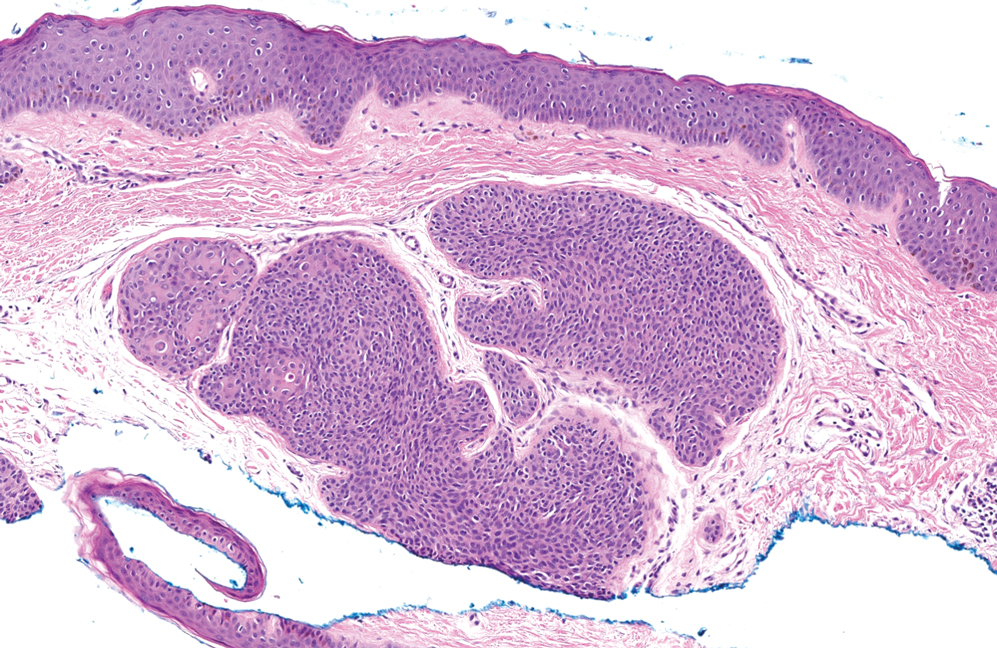
Trichoblastomas typically are deep-seated basaloid follicular neoplasms on the scalp with papillary mesenchyme resembling the normal fibrous sheath of the hair follicle, often replete with papillary mesenchymal bodies (Figure 2). There generally are no retraction spaces between its basaloid nests and the surrounding stroma, which is unlikely to contain mucin relative to basal cell carcinoma (BCC).4,5
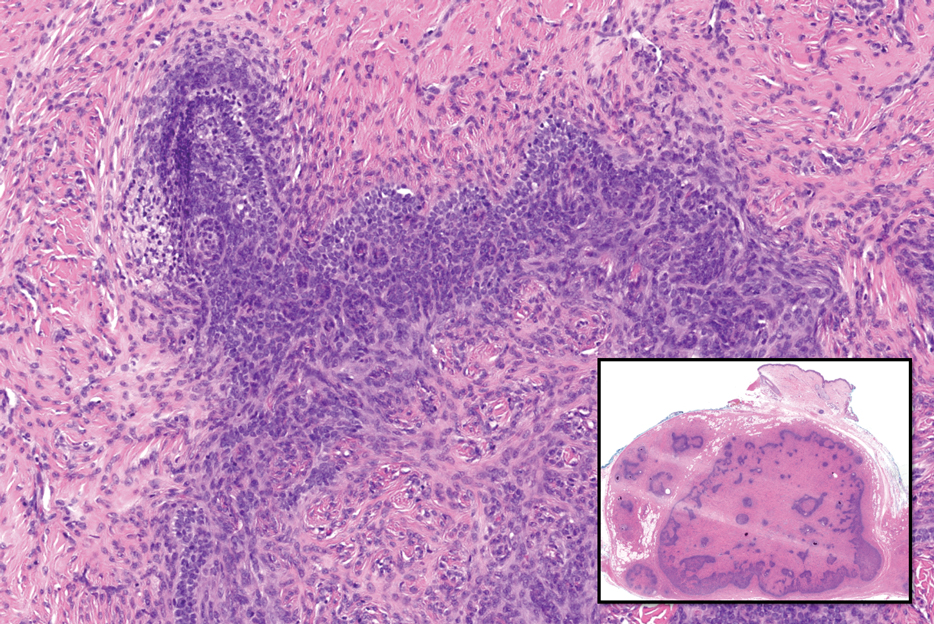
Adenoid cystic carcinoma is a rare salivary gland tumor that can metastasize to the skin and rarely arises as a primary skin adnexal tumor. It manifests as a slowgrowing mass that can be tender to palpation.6 Histologic examination shows dermal islands with cribriform blue and pink spaces. Compared to BCC, adenoid cystic carcinoma cells are enlarged and epithelioid with relatively scarce cytoplasm (Figure 3).6,7 Adenoid cystic carcinoma can show variable growth patterns including infiltrative nests and trabeculae. Perineural invasion is common, and there is a high risk for local recurrence.7 First-line therapy usually is surgical, and postoperative radiotherapy may be required.6,7
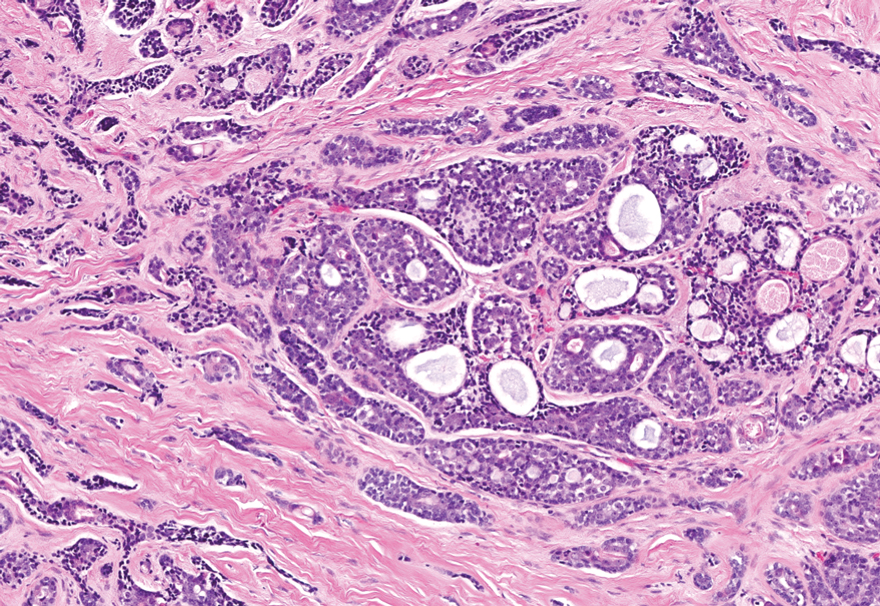
Nodular BCC commonly manifests as an enlarging nonhealing lesion on sun-exposed skin and has many subtypes, typically with arborizing telangiectases on dermoscopy. Histopathologic examination of nodular BCC reveals a nest of basaloid follicular germinative cells in the dermis with peripheral palisading and a fibromyxoid stroma (Figure 4).8 Patients with Brooke-Spiegler syndrome are at increased risk for nodular BCC, which may be clinically indistinguishable from spiradenoma, cylindroma, and spiradenocylindroma, necessitating histologic assessment.
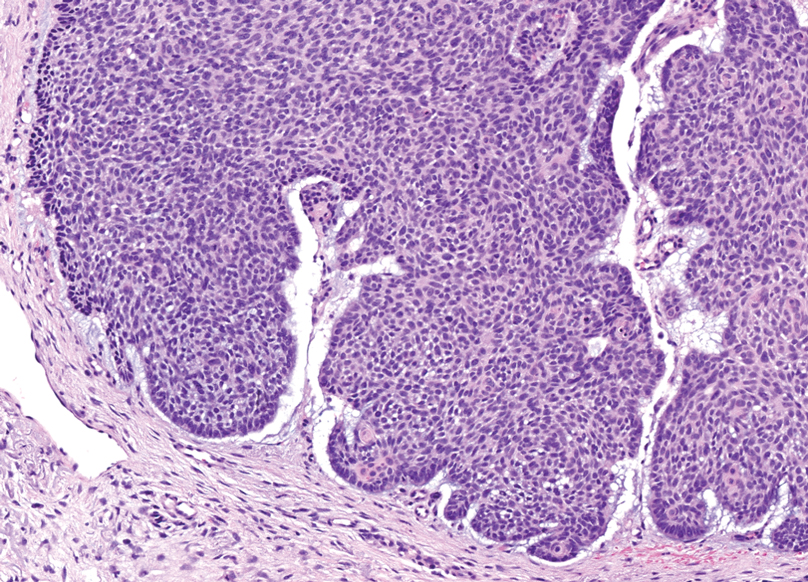
- Facchini V, Colangeli W, Bozza F, et al. A rare histopathological spiradenocylindroma: a case report. Clin Ter. 2022;173:292-294. doi:10.7417/ CT.2022.2433
- Kazakov DV. Brooke-Spiegler syndrome and phenotypic variants: an update [published online March 14, 2016]. Head Neck Pathol. 2016;10:125-30. doi:10.1007/s12105-016-0705-x
- Miller AC, Adjei S, Temiz LA, et al. Dermal duct tumor: a diagnostic dilemma. Dermatopathology (Basel). 2022;9:36-47. doi:10.3390/dermatopathology9010007
- Elston DM. Pilar and sebaceous neoplasms. In: Elston DM, Ferringer T, Ko C, et al. Dermatopathology. 3rd ed. Elsevier; 2018:71-85.
- McCalmont TH, Pincus LB. Adnexal neoplasms. In: Bolognia J, Schaffer J, Cerroni, L. Dermatology. 4th ed. Elsevier; 2017:1930-1953.
- Coca-Pelaz A, Rodrigo JP, Bradley PJ, et al. Adenoid cystic carcinoma of the head and neck—an update [published online May 2, 2015]. Oral Oncol. 2015;51:652-661. doi:10.1016/j.oraloncology.2015.04.005
- Tonev ID, Pirgova YS, Conev NV. Primary adenoid cystic carcinoma of the skin with multiple local recurrences. Case Rep Oncol. 2015;8:251- 255. doi:10.1159/000431082
- Cameron MC, Lee E, Hibler BP, et al. Basal cell carcinoma: epidemiology; pathophysiology; clinical and histological subtypes; and disease associations [published online May 18, 2018]. J Am Acad Dermatol. 2019;80:303-317. doi:10.1016/j.jaad.2018.03.060
The Diagnosis: Spiradenocylindroma
Shave biopsies of our patient’s lesions showed wellcircumscribed dermal nodules resembling a spiradenoma with 3 cell populations: those with lighter nuclei, darker nuclei, and scattered lymphocytes. However, the conspicuous globules of basement membrane material were reminiscent of a cylindroma. These overlapping features and the patient’s history of cylindroma were suggestive of a diagnosis of spiradenocylindroma.
Spiradenocylindroma is an uncommon dermal tumor with features that overlap with spiradenoma and cylindroma.1 It may manifest as a solitary lesion or multiple lesions and can occur sporadically or in the context of a family history. Histologically, it must be distinguished from other intradermal basaloid neoplasms including conventional cylindroma and spiradenoma, dermal duct tumor, hidradenoma, and trichoblastoma.
When patients present with multiple cylindromas, spiradenomas, or spiradenocylindromas, physicians should consider genetic testing and review of the family history to assess for cylindromatosis gene mutations or Brooke-Spiegler syndrome. Biopsy and histologic examination are important because malignant tumors can evolve from pre-existing spiradenocylindromas, cylindromas, and spiradenomas,2 with an increased risk in patients with Brooke-Spiegler syndrome.1 Our patient declined further genetic workup but continues to follow up with dermatology for monitoring of lesions.
Dermal duct tumors are morphologic variants of poromas that are derived from sweat gland lineage and usually manifest as solitary dome-shaped papules, plaques, or nodules most often seen on acral surfaces as well as the head and neck.3 Clinically, they may be indistinguishable from spiradenocylindromas and require biopsy for histologic evaluation. They can be distinguished from spiradenocylindroma by the presence of small dermal nodules composed of cuboidal cells with ample pink cytoplasm and cuticle-lined ducts (Figure 1).

Trichoblastomas typically are deep-seated basaloid follicular neoplasms on the scalp with papillary mesenchyme resembling the normal fibrous sheath of the hair follicle, often replete with papillary mesenchymal bodies (Figure 2). There generally are no retraction spaces between its basaloid nests and the surrounding stroma, which is unlikely to contain mucin relative to basal cell carcinoma (BCC).4,5

Adenoid cystic carcinoma is a rare salivary gland tumor that can metastasize to the skin and rarely arises as a primary skin adnexal tumor. It manifests as a slowgrowing mass that can be tender to palpation.6 Histologic examination shows dermal islands with cribriform blue and pink spaces. Compared to BCC, adenoid cystic carcinoma cells are enlarged and epithelioid with relatively scarce cytoplasm (Figure 3).6,7 Adenoid cystic carcinoma can show variable growth patterns including infiltrative nests and trabeculae. Perineural invasion is common, and there is a high risk for local recurrence.7 First-line therapy usually is surgical, and postoperative radiotherapy may be required.6,7

Nodular BCC commonly manifests as an enlarging nonhealing lesion on sun-exposed skin and has many subtypes, typically with arborizing telangiectases on dermoscopy. Histopathologic examination of nodular BCC reveals a nest of basaloid follicular germinative cells in the dermis with peripheral palisading and a fibromyxoid stroma (Figure 4).8 Patients with Brooke-Spiegler syndrome are at increased risk for nodular BCC, which may be clinically indistinguishable from spiradenoma, cylindroma, and spiradenocylindroma, necessitating histologic assessment.

The Diagnosis: Spiradenocylindroma
Shave biopsies of our patient’s lesions showed wellcircumscribed dermal nodules resembling a spiradenoma with 3 cell populations: those with lighter nuclei, darker nuclei, and scattered lymphocytes. However, the conspicuous globules of basement membrane material were reminiscent of a cylindroma. These overlapping features and the patient’s history of cylindroma were suggestive of a diagnosis of spiradenocylindroma.
Spiradenocylindroma is an uncommon dermal tumor with features that overlap with spiradenoma and cylindroma.1 It may manifest as a solitary lesion or multiple lesions and can occur sporadically or in the context of a family history. Histologically, it must be distinguished from other intradermal basaloid neoplasms including conventional cylindroma and spiradenoma, dermal duct tumor, hidradenoma, and trichoblastoma.
When patients present with multiple cylindromas, spiradenomas, or spiradenocylindromas, physicians should consider genetic testing and review of the family history to assess for cylindromatosis gene mutations or Brooke-Spiegler syndrome. Biopsy and histologic examination are important because malignant tumors can evolve from pre-existing spiradenocylindromas, cylindromas, and spiradenomas,2 with an increased risk in patients with Brooke-Spiegler syndrome.1 Our patient declined further genetic workup but continues to follow up with dermatology for monitoring of lesions.
Dermal duct tumors are morphologic variants of poromas that are derived from sweat gland lineage and usually manifest as solitary dome-shaped papules, plaques, or nodules most often seen on acral surfaces as well as the head and neck.3 Clinically, they may be indistinguishable from spiradenocylindromas and require biopsy for histologic evaluation. They can be distinguished from spiradenocylindroma by the presence of small dermal nodules composed of cuboidal cells with ample pink cytoplasm and cuticle-lined ducts (Figure 1).

Trichoblastomas typically are deep-seated basaloid follicular neoplasms on the scalp with papillary mesenchyme resembling the normal fibrous sheath of the hair follicle, often replete with papillary mesenchymal bodies (Figure 2). There generally are no retraction spaces between its basaloid nests and the surrounding stroma, which is unlikely to contain mucin relative to basal cell carcinoma (BCC).4,5

Adenoid cystic carcinoma is a rare salivary gland tumor that can metastasize to the skin and rarely arises as a primary skin adnexal tumor. It manifests as a slowgrowing mass that can be tender to palpation.6 Histologic examination shows dermal islands with cribriform blue and pink spaces. Compared to BCC, adenoid cystic carcinoma cells are enlarged and epithelioid with relatively scarce cytoplasm (Figure 3).6,7 Adenoid cystic carcinoma can show variable growth patterns including infiltrative nests and trabeculae. Perineural invasion is common, and there is a high risk for local recurrence.7 First-line therapy usually is surgical, and postoperative radiotherapy may be required.6,7

Nodular BCC commonly manifests as an enlarging nonhealing lesion on sun-exposed skin and has many subtypes, typically with arborizing telangiectases on dermoscopy. Histopathologic examination of nodular BCC reveals a nest of basaloid follicular germinative cells in the dermis with peripheral palisading and a fibromyxoid stroma (Figure 4).8 Patients with Brooke-Spiegler syndrome are at increased risk for nodular BCC, which may be clinically indistinguishable from spiradenoma, cylindroma, and spiradenocylindroma, necessitating histologic assessment.

- Facchini V, Colangeli W, Bozza F, et al. A rare histopathological spiradenocylindroma: a case report. Clin Ter. 2022;173:292-294. doi:10.7417/ CT.2022.2433
- Kazakov DV. Brooke-Spiegler syndrome and phenotypic variants: an update [published online March 14, 2016]. Head Neck Pathol. 2016;10:125-30. doi:10.1007/s12105-016-0705-x
- Miller AC, Adjei S, Temiz LA, et al. Dermal duct tumor: a diagnostic dilemma. Dermatopathology (Basel). 2022;9:36-47. doi:10.3390/dermatopathology9010007
- Elston DM. Pilar and sebaceous neoplasms. In: Elston DM, Ferringer T, Ko C, et al. Dermatopathology. 3rd ed. Elsevier; 2018:71-85.
- McCalmont TH, Pincus LB. Adnexal neoplasms. In: Bolognia J, Schaffer J, Cerroni, L. Dermatology. 4th ed. Elsevier; 2017:1930-1953.
- Coca-Pelaz A, Rodrigo JP, Bradley PJ, et al. Adenoid cystic carcinoma of the head and neck—an update [published online May 2, 2015]. Oral Oncol. 2015;51:652-661. doi:10.1016/j.oraloncology.2015.04.005
- Tonev ID, Pirgova YS, Conev NV. Primary adenoid cystic carcinoma of the skin with multiple local recurrences. Case Rep Oncol. 2015;8:251- 255. doi:10.1159/000431082
- Cameron MC, Lee E, Hibler BP, et al. Basal cell carcinoma: epidemiology; pathophysiology; clinical and histological subtypes; and disease associations [published online May 18, 2018]. J Am Acad Dermatol. 2019;80:303-317. doi:10.1016/j.jaad.2018.03.060
- Facchini V, Colangeli W, Bozza F, et al. A rare histopathological spiradenocylindroma: a case report. Clin Ter. 2022;173:292-294. doi:10.7417/ CT.2022.2433
- Kazakov DV. Brooke-Spiegler syndrome and phenotypic variants: an update [published online March 14, 2016]. Head Neck Pathol. 2016;10:125-30. doi:10.1007/s12105-016-0705-x
- Miller AC, Adjei S, Temiz LA, et al. Dermal duct tumor: a diagnostic dilemma. Dermatopathology (Basel). 2022;9:36-47. doi:10.3390/dermatopathology9010007
- Elston DM. Pilar and sebaceous neoplasms. In: Elston DM, Ferringer T, Ko C, et al. Dermatopathology. 3rd ed. Elsevier; 2018:71-85.
- McCalmont TH, Pincus LB. Adnexal neoplasms. In: Bolognia J, Schaffer J, Cerroni, L. Dermatology. 4th ed. Elsevier; 2017:1930-1953.
- Coca-Pelaz A, Rodrigo JP, Bradley PJ, et al. Adenoid cystic carcinoma of the head and neck—an update [published online May 2, 2015]. Oral Oncol. 2015;51:652-661. doi:10.1016/j.oraloncology.2015.04.005
- Tonev ID, Pirgova YS, Conev NV. Primary adenoid cystic carcinoma of the skin with multiple local recurrences. Case Rep Oncol. 2015;8:251- 255. doi:10.1159/000431082
- Cameron MC, Lee E, Hibler BP, et al. Basal cell carcinoma: epidemiology; pathophysiology; clinical and histological subtypes; and disease associations [published online May 18, 2018]. J Am Acad Dermatol. 2019;80:303-317. doi:10.1016/j.jaad.2018.03.060
A 62-year-old man with a history of cylindromas presented to our clinic with multiple asymptomatic, 3- to 4-mm, nonmobile, dome-shaped, telangiectatic, pink papules over the parietal and vertex scalp that had been present for more than 10 years without change. Several family members had similar lesions that had not been evaluated by a physician, and there had been no genetic evaluation. Shave biopsies of several lesions were performed.
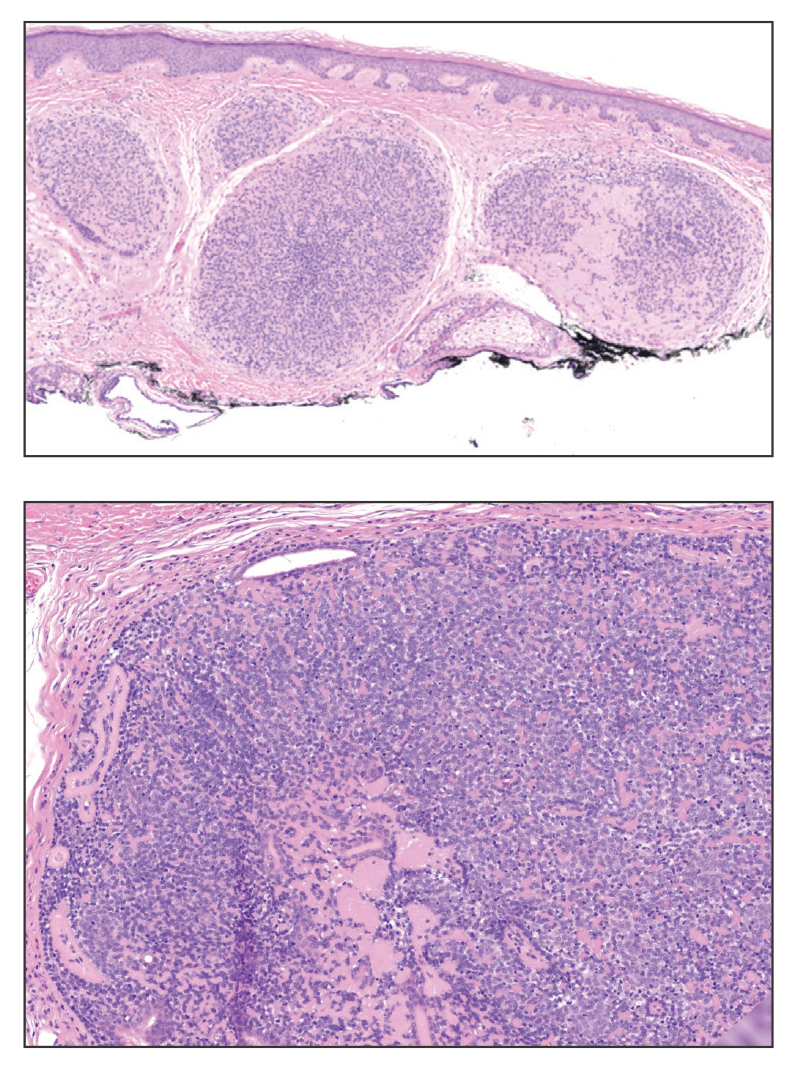
Impact of the COVID-19 Pandemic on Care for Patients With Skin Cancer
To the Editor:
The most common malignancy in the United States is skin cancer, with melanoma accounting for the majority of skin cancer deaths.1 Despite the lack of established guidelines for routine total-body skin examinations, many patients regularly visit their dermatologist for assessment of pigmented skin lesions.2 During the COVID-19 pandemic, many patients were unable to attend in-person dermatology visits, which resulted in many high-risk individuals not receiving care or alternatively seeking virtual care for cutaneous lesions.3 There has been a lack of research in the United States exploring the utilization of teledermatology during the pandemic and its overall impact on the care of patients with a history of skin cancer. We explored the impact of the COVID-19 pandemic on care for patients with skin cancer in a large US population.
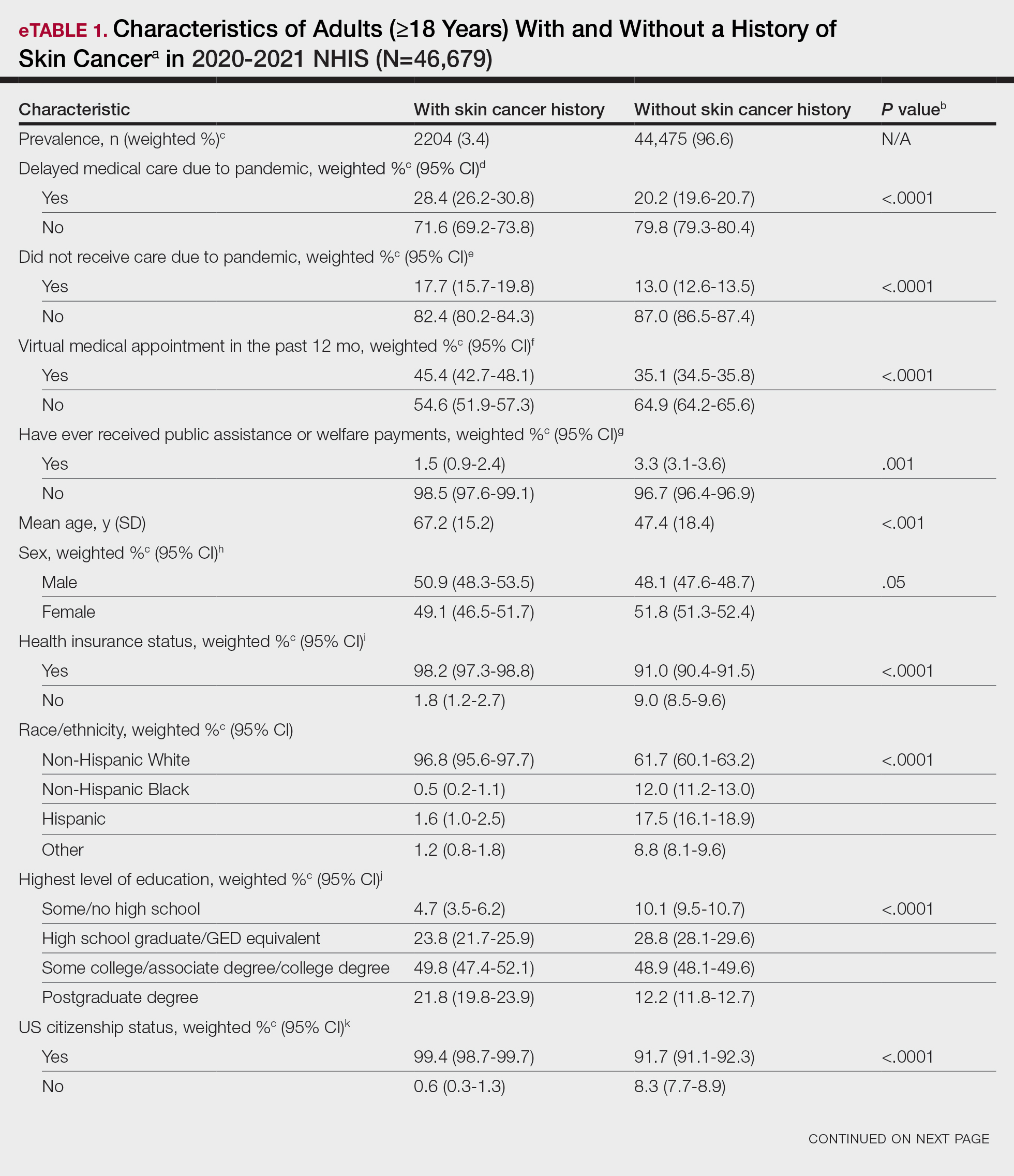

Using anonymous survey data from the 2020-2021 National Health Interview Survey,4 we conducted a population-based, cross-sectional study to evaluate access to care during the COVID-19 pandemic for patients with a self-reported history of skin cancer—melanoma, nonmelanoma skin cancer, or unknown skin cancer. The 3 outcome variables included having a virtual medical appointment in the past 12 months (yes/no), delaying medical care due to the COVID-19 pandemic (yes/no), and not receiving care due to the COVID-19 pandemic (yes/no). Multivariable logistic regression models evaluating the relationship between a history of skin cancer and access to care were constructed using Stata/MP 17.0 (StataCorp LLC). We controlled for patient age; education; race/ethnicity; received public assistance or welfare payments; sex; region; US citizenship status; health insurance status; comorbidities including history of hypertension, diabetes, and hypercholesterolemia; and birthplace in the United States in the logistic regression models.
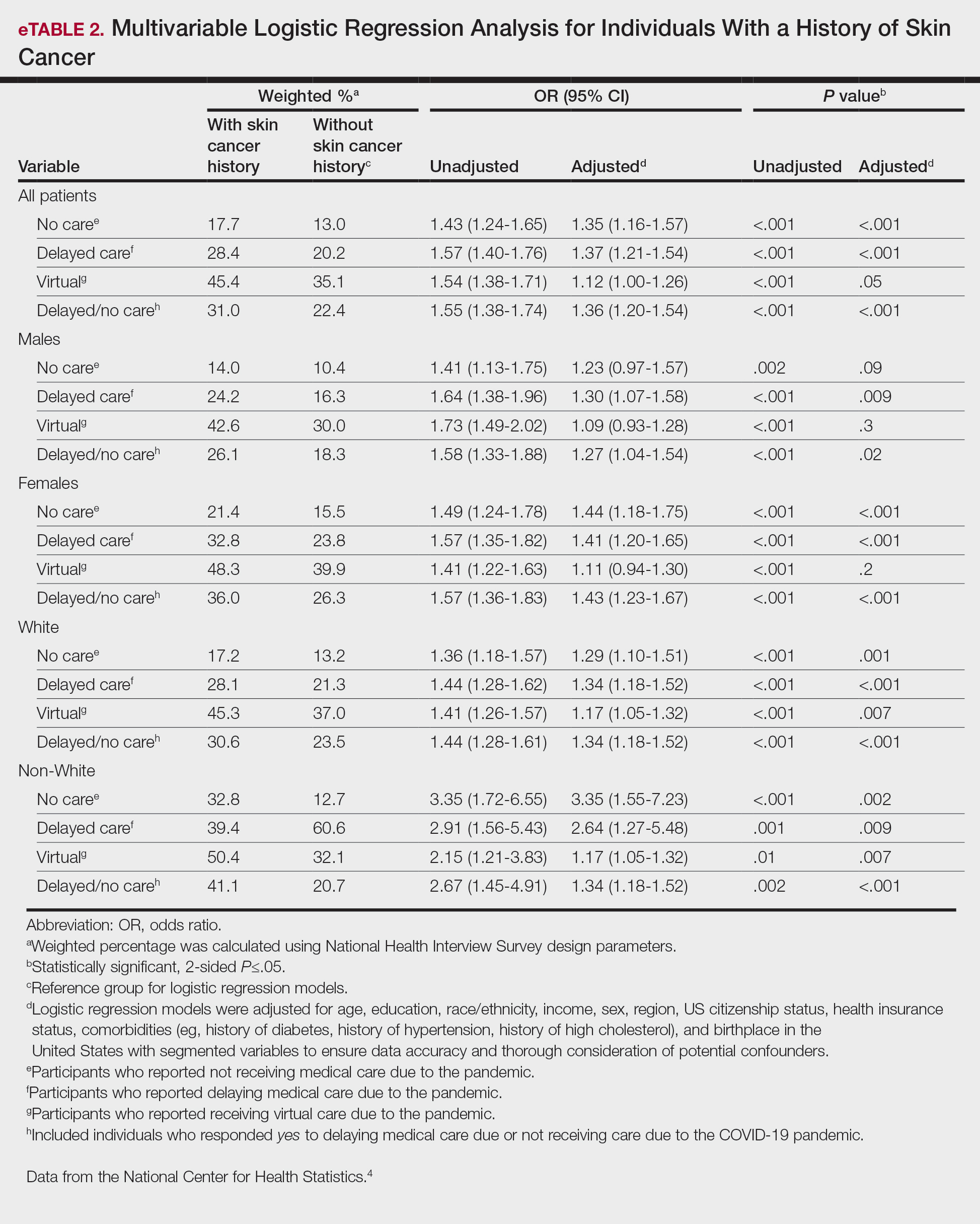
Our analysis included 46,679 patients aged 18 years or older, of whom 3.4% (weighted)(n=2204) reported a history of skin cancer (eTable 1). The weighted percentage was calculated using National Health Interview Survey design parameters (accounting for the multistage sampling design) to represent the general US population. Compared with those with no history of skin cancer, patients with a history of skin cancer were significantly more likely to delay medical care (adjusted odds ratio [AOR], 1.37; 95% CI, 1.21-1.54; P<.001) or not receive care (AOR, 1.35; 95% CI, 1.16-1.57; P<.001) due to the pandemic and were more likely to have had a virtual medical visit in the past 12 months (AOR, 1.12; 95% CI, 1.00-1.26; P=.05). Additionally, subgroup analysis revealed that females were more likely than males to forego medical care (eTable 2). β Coefficients for independent and dependent variables were further analyzed using logistic regression (eTable 3).
After adjusting for various potential confounders including comorbidities, our results revealed that patients with a history of skin cancer reported that they were less likely to receive in-person medical care due to the COVID-19 pandemic, as high-risk individuals with a history of skin cancer may have stopped receiving total-body skin examinations and dermatology care during the pandemic. Our findings showed that patients with a history of skin cancer were more likely than those without skin cancer to delay or forego care due to the pandemic, which may contribute to a higher incidence of advanced-stage melanomas postpandemic. Trepanowski et al5 reported an increased incidence of patients presenting with more advanced melanomas during the pandemic. Telemedicine was more commonly utilized by patients with a history of skin cancer during the pandemic.
In the future, virtual care may help limit advanced stages of skin cancer by serving as a viable alternative to in-person care.6 It has been reported that telemedicine can serve as a useful triage service reducing patient wait times.7 Teledermatology should not replace in-person care, as there is no evidence of the diagnostic accuracy of this service and many patients still will need to be seen in-person for confirmation of their diagnosis and potential biopsy. Further studies are needed to assess for missed skin cancer diagnoses due to the utilization of telemedicine.
Limitations of this study included a self-reported history of skin cancer, β coefficients that may suggest a high degree of collinearity, and lack of specific survey questions regarding dermatologic care during the COVID-19 pandemic. Further long-term studies exploring the clinical applicability and diagnostic accuracy of virtual medicine visits for cutaneous malignancies are vital, as teledermatology may play an essential role in curbing rising skin cancer rates even beyond the pandemic.
- Guy GP Jr, Thomas CC, Thompson T, et al. Vital signs: melanoma incidence and mortality trends and projections—United States, 1982-2030. MMWR Morb Mortal Wkly Rep. 2015;64:591-596.
- Whiteman DC, Olsen CM, MacGregor S, et al; QSkin Study. The effect of screening on melanoma incidence and biopsy rates. Br J Dermatol. 2022;187:515-522. doi:10.1111/bjd.21649
- Jobbágy A, Kiss N, Meznerics FA, et al. Emergency use and efficacy of an asynchronous teledermatology system as a novel tool for early diagnosis of skin cancer during the first wave of COVID-19 pandemic. Int J Environ Res Public Health. 2022;19:2699. doi:10.3390/ijerph19052699
- National Center for Health Statistics. NHIS Data, Questionnaires and Related Documentation. Centers for Disease Control and Prevention website. Accessed April 19, 2023. https://www.cdc.gov/nchs/nhis/data-questionnaires-documentation.htm
- Trepanowski N, Chang MS, Zhou G, et al. Delays in melanoma presentation during the COVID-19 pandemic: a nationwide multi-institutional cohort study. J Am Acad Dermatol. 2022;87:1217-1219. doi:10.1016/j.jaad.2022.06.031
- Chiru MR, Hindocha S, Burova E, et al. Management of the two-week wait pathway for skin cancer patients, before and during the pandemic: is virtual consultation an option? J Pers Med. 2022;12:1258. doi:10.3390/jpm12081258
- Finnane A Dallest K Janda M et al. Teledermatology for the diagnosis and management of skin cancer: a systematic review. JAMA Dermatol. 2017;153:319-327. doi:10.1001/jamadermatol.2016.4361
To the Editor:
The most common malignancy in the United States is skin cancer, with melanoma accounting for the majority of skin cancer deaths.1 Despite the lack of established guidelines for routine total-body skin examinations, many patients regularly visit their dermatologist for assessment of pigmented skin lesions.2 During the COVID-19 pandemic, many patients were unable to attend in-person dermatology visits, which resulted in many high-risk individuals not receiving care or alternatively seeking virtual care for cutaneous lesions.3 There has been a lack of research in the United States exploring the utilization of teledermatology during the pandemic and its overall impact on the care of patients with a history of skin cancer. We explored the impact of the COVID-19 pandemic on care for patients with skin cancer in a large US population.


Using anonymous survey data from the 2020-2021 National Health Interview Survey,4 we conducted a population-based, cross-sectional study to evaluate access to care during the COVID-19 pandemic for patients with a self-reported history of skin cancer—melanoma, nonmelanoma skin cancer, or unknown skin cancer. The 3 outcome variables included having a virtual medical appointment in the past 12 months (yes/no), delaying medical care due to the COVID-19 pandemic (yes/no), and not receiving care due to the COVID-19 pandemic (yes/no). Multivariable logistic regression models evaluating the relationship between a history of skin cancer and access to care were constructed using Stata/MP 17.0 (StataCorp LLC). We controlled for patient age; education; race/ethnicity; received public assistance or welfare payments; sex; region; US citizenship status; health insurance status; comorbidities including history of hypertension, diabetes, and hypercholesterolemia; and birthplace in the United States in the logistic regression models.

Our analysis included 46,679 patients aged 18 years or older, of whom 3.4% (weighted)(n=2204) reported a history of skin cancer (eTable 1). The weighted percentage was calculated using National Health Interview Survey design parameters (accounting for the multistage sampling design) to represent the general US population. Compared with those with no history of skin cancer, patients with a history of skin cancer were significantly more likely to delay medical care (adjusted odds ratio [AOR], 1.37; 95% CI, 1.21-1.54; P<.001) or not receive care (AOR, 1.35; 95% CI, 1.16-1.57; P<.001) due to the pandemic and were more likely to have had a virtual medical visit in the past 12 months (AOR, 1.12; 95% CI, 1.00-1.26; P=.05). Additionally, subgroup analysis revealed that females were more likely than males to forego medical care (eTable 2). β Coefficients for independent and dependent variables were further analyzed using logistic regression (eTable 3).

After adjusting for various potential confounders including comorbidities, our results revealed that patients with a history of skin cancer reported that they were less likely to receive in-person medical care due to the COVID-19 pandemic, as high-risk individuals with a history of skin cancer may have stopped receiving total-body skin examinations and dermatology care during the pandemic. Our findings showed that patients with a history of skin cancer were more likely than those without skin cancer to delay or forego care due to the pandemic, which may contribute to a higher incidence of advanced-stage melanomas postpandemic. Trepanowski et al5 reported an increased incidence of patients presenting with more advanced melanomas during the pandemic. Telemedicine was more commonly utilized by patients with a history of skin cancer during the pandemic.
In the future, virtual care may help limit advanced stages of skin cancer by serving as a viable alternative to in-person care.6 It has been reported that telemedicine can serve as a useful triage service reducing patient wait times.7 Teledermatology should not replace in-person care, as there is no evidence of the diagnostic accuracy of this service and many patients still will need to be seen in-person for confirmation of their diagnosis and potential biopsy. Further studies are needed to assess for missed skin cancer diagnoses due to the utilization of telemedicine.
Limitations of this study included a self-reported history of skin cancer, β coefficients that may suggest a high degree of collinearity, and lack of specific survey questions regarding dermatologic care during the COVID-19 pandemic. Further long-term studies exploring the clinical applicability and diagnostic accuracy of virtual medicine visits for cutaneous malignancies are vital, as teledermatology may play an essential role in curbing rising skin cancer rates even beyond the pandemic.
To the Editor:
The most common malignancy in the United States is skin cancer, with melanoma accounting for the majority of skin cancer deaths.1 Despite the lack of established guidelines for routine total-body skin examinations, many patients regularly visit their dermatologist for assessment of pigmented skin lesions.2 During the COVID-19 pandemic, many patients were unable to attend in-person dermatology visits, which resulted in many high-risk individuals not receiving care or alternatively seeking virtual care for cutaneous lesions.3 There has been a lack of research in the United States exploring the utilization of teledermatology during the pandemic and its overall impact on the care of patients with a history of skin cancer. We explored the impact of the COVID-19 pandemic on care for patients with skin cancer in a large US population.


Using anonymous survey data from the 2020-2021 National Health Interview Survey,4 we conducted a population-based, cross-sectional study to evaluate access to care during the COVID-19 pandemic for patients with a self-reported history of skin cancer—melanoma, nonmelanoma skin cancer, or unknown skin cancer. The 3 outcome variables included having a virtual medical appointment in the past 12 months (yes/no), delaying medical care due to the COVID-19 pandemic (yes/no), and not receiving care due to the COVID-19 pandemic (yes/no). Multivariable logistic regression models evaluating the relationship between a history of skin cancer and access to care were constructed using Stata/MP 17.0 (StataCorp LLC). We controlled for patient age; education; race/ethnicity; received public assistance or welfare payments; sex; region; US citizenship status; health insurance status; comorbidities including history of hypertension, diabetes, and hypercholesterolemia; and birthplace in the United States in the logistic regression models.

Our analysis included 46,679 patients aged 18 years or older, of whom 3.4% (weighted)(n=2204) reported a history of skin cancer (eTable 1). The weighted percentage was calculated using National Health Interview Survey design parameters (accounting for the multistage sampling design) to represent the general US population. Compared with those with no history of skin cancer, patients with a history of skin cancer were significantly more likely to delay medical care (adjusted odds ratio [AOR], 1.37; 95% CI, 1.21-1.54; P<.001) or not receive care (AOR, 1.35; 95% CI, 1.16-1.57; P<.001) due to the pandemic and were more likely to have had a virtual medical visit in the past 12 months (AOR, 1.12; 95% CI, 1.00-1.26; P=.05). Additionally, subgroup analysis revealed that females were more likely than males to forego medical care (eTable 2). β Coefficients for independent and dependent variables were further analyzed using logistic regression (eTable 3).

After adjusting for various potential confounders including comorbidities, our results revealed that patients with a history of skin cancer reported that they were less likely to receive in-person medical care due to the COVID-19 pandemic, as high-risk individuals with a history of skin cancer may have stopped receiving total-body skin examinations and dermatology care during the pandemic. Our findings showed that patients with a history of skin cancer were more likely than those without skin cancer to delay or forego care due to the pandemic, which may contribute to a higher incidence of advanced-stage melanomas postpandemic. Trepanowski et al5 reported an increased incidence of patients presenting with more advanced melanomas during the pandemic. Telemedicine was more commonly utilized by patients with a history of skin cancer during the pandemic.
In the future, virtual care may help limit advanced stages of skin cancer by serving as a viable alternative to in-person care.6 It has been reported that telemedicine can serve as a useful triage service reducing patient wait times.7 Teledermatology should not replace in-person care, as there is no evidence of the diagnostic accuracy of this service and many patients still will need to be seen in-person for confirmation of their diagnosis and potential biopsy. Further studies are needed to assess for missed skin cancer diagnoses due to the utilization of telemedicine.
Limitations of this study included a self-reported history of skin cancer, β coefficients that may suggest a high degree of collinearity, and lack of specific survey questions regarding dermatologic care during the COVID-19 pandemic. Further long-term studies exploring the clinical applicability and diagnostic accuracy of virtual medicine visits for cutaneous malignancies are vital, as teledermatology may play an essential role in curbing rising skin cancer rates even beyond the pandemic.
- Guy GP Jr, Thomas CC, Thompson T, et al. Vital signs: melanoma incidence and mortality trends and projections—United States, 1982-2030. MMWR Morb Mortal Wkly Rep. 2015;64:591-596.
- Whiteman DC, Olsen CM, MacGregor S, et al; QSkin Study. The effect of screening on melanoma incidence and biopsy rates. Br J Dermatol. 2022;187:515-522. doi:10.1111/bjd.21649
- Jobbágy A, Kiss N, Meznerics FA, et al. Emergency use and efficacy of an asynchronous teledermatology system as a novel tool for early diagnosis of skin cancer during the first wave of COVID-19 pandemic. Int J Environ Res Public Health. 2022;19:2699. doi:10.3390/ijerph19052699
- National Center for Health Statistics. NHIS Data, Questionnaires and Related Documentation. Centers for Disease Control and Prevention website. Accessed April 19, 2023. https://www.cdc.gov/nchs/nhis/data-questionnaires-documentation.htm
- Trepanowski N, Chang MS, Zhou G, et al. Delays in melanoma presentation during the COVID-19 pandemic: a nationwide multi-institutional cohort study. J Am Acad Dermatol. 2022;87:1217-1219. doi:10.1016/j.jaad.2022.06.031
- Chiru MR, Hindocha S, Burova E, et al. Management of the two-week wait pathway for skin cancer patients, before and during the pandemic: is virtual consultation an option? J Pers Med. 2022;12:1258. doi:10.3390/jpm12081258
- Finnane A Dallest K Janda M et al. Teledermatology for the diagnosis and management of skin cancer: a systematic review. JAMA Dermatol. 2017;153:319-327. doi:10.1001/jamadermatol.2016.4361
- Guy GP Jr, Thomas CC, Thompson T, et al. Vital signs: melanoma incidence and mortality trends and projections—United States, 1982-2030. MMWR Morb Mortal Wkly Rep. 2015;64:591-596.
- Whiteman DC, Olsen CM, MacGregor S, et al; QSkin Study. The effect of screening on melanoma incidence and biopsy rates. Br J Dermatol. 2022;187:515-522. doi:10.1111/bjd.21649
- Jobbágy A, Kiss N, Meznerics FA, et al. Emergency use and efficacy of an asynchronous teledermatology system as a novel tool for early diagnosis of skin cancer during the first wave of COVID-19 pandemic. Int J Environ Res Public Health. 2022;19:2699. doi:10.3390/ijerph19052699
- National Center for Health Statistics. NHIS Data, Questionnaires and Related Documentation. Centers for Disease Control and Prevention website. Accessed April 19, 2023. https://www.cdc.gov/nchs/nhis/data-questionnaires-documentation.htm
- Trepanowski N, Chang MS, Zhou G, et al. Delays in melanoma presentation during the COVID-19 pandemic: a nationwide multi-institutional cohort study. J Am Acad Dermatol. 2022;87:1217-1219. doi:10.1016/j.jaad.2022.06.031
- Chiru MR, Hindocha S, Burova E, et al. Management of the two-week wait pathway for skin cancer patients, before and during the pandemic: is virtual consultation an option? J Pers Med. 2022;12:1258. doi:10.3390/jpm12081258
- Finnane A Dallest K Janda M et al. Teledermatology for the diagnosis and management of skin cancer: a systematic review. JAMA Dermatol. 2017;153:319-327. doi:10.1001/jamadermatol.2016.4361
PRACTICE POINTS
- The COVID-19 pandemic has altered the landscape of medicine, as many individuals are now utilizing telemedicine to receive care.
- Many individuals will continue to receive telemedicine moving forward, making it crucial to understand access to care.
Comment on “Skin Cancer Screening: The Paradox of Melanoma and Improved All-Cause Mortality”
To the Editor:
I was unsurprised and gratified by the information presented in the Viewpoint on skin cancer screening by Ngo1 (Cutis. 2024;113:94-96). In my 30 years as a community dermatologist, I have observed that patients who opt to have periodic full-body skin examinations usually are more health literate, more likely to have a primary care physician (PCP) who has encouraged them to do so (ie, a conscientious practitioner directing their preventive care), more likely to have a strong will to live, and less likely to have multiple stressors that preclude self-care (eg, may be less likely to have a spouse for whom they are a caregiver) compared to those who do not get screened.
Findings on a full-body skin examination may impact patients in many ways, not only by the detection of skin cancers. I have discovered the following:
- evidence of diabetes/insulin resistance in the form of acanthosis nigricans, tinea corporis, erythrasma;
- evidence of rosacea associated with excessive alcohol intake;
- evidence of smoking-related issues such as psoriasis or hidradenitis suppurativa;
- cutaneous evidence of other systemic diseases (eg, autoimmune disease, cancer);
- elucidation of other chronic health problems (eg, psoriasis of the skin as a clue for undiagnosed psoriatic arthritis); and
- detection of parasites on the skin (eg, ticks) or signs of infection that may have notable ramifications (eg, interdigital maceration of a diabetic patient with tinea pedis).
I even saw a patient who had been sent for magnetic resonance imaging for back pain by her internist without any physical examination when she actually had an erosion over the sacrum from a rug burn!
When conducting full-body skin examinations, dermatologists should not underestimate these principles:
- The “magic” of using a relatively noninvasive and sensitive screening tool—comfort and stress reduction for the patient from a thorough visual, tactile, olfactory, and auditory examination.
- Human interaction—especially when the patient is seen annually or even more frequently over a period of years or decades, and especially when an excellent patient-physician rapport has been established.
- The impact of improving a patient’s appearance on their overall sense of well-being (eg, by controlling rosacea).
- The opportunity to introduce concepts (ie, educate patients) such as alcohol avoidance, smoking cessation, weight reduction, hygiene, diet, and exercise in a more tangential way than a PCP, as well as to consider with patients the idea that lifestyle modification may be an adjunct, if not a replacement, for prescription treatments.
- The stress reduction that ensues when a variety of self-identified health issues are addressed, for which the only treatment may be reassurance.
I would add to Dr. Ngo’s argument that stratifying patients into skin cancer risk categories may be a useful measure if the only goal of periodic dermatologic evaluation is skin cancer detection. One size rarely fits all when it comes to health recommendations.
In sum, I believe that periodic full-body skin examination is absolutely beneficial to patient care, and I am not at all surprised that all-cause mortality was lower in patients who have those examinations. Furthermore, when I offer my healthy, low-risk patients the option to return in 2 years rather than 1, the vast majority insist on 1 year. My mother used to say, “It’s better to be looked over than to be overlooked,” and I tell my patients that, too—but it seems they already know that instinctively.
- Ngo BT. Skin cancer screening: the paradox of melanoma and improved all-cause mortality. Cutis. 2024;113:94-96. doi:10.12788/cutis.0948
To the Editor:
I was unsurprised and gratified by the information presented in the Viewpoint on skin cancer screening by Ngo1 (Cutis. 2024;113:94-96). In my 30 years as a community dermatologist, I have observed that patients who opt to have periodic full-body skin examinations usually are more health literate, more likely to have a primary care physician (PCP) who has encouraged them to do so (ie, a conscientious practitioner directing their preventive care), more likely to have a strong will to live, and less likely to have multiple stressors that preclude self-care (eg, may be less likely to have a spouse for whom they are a caregiver) compared to those who do not get screened.
Findings on a full-body skin examination may impact patients in many ways, not only by the detection of skin cancers. I have discovered the following:
- evidence of diabetes/insulin resistance in the form of acanthosis nigricans, tinea corporis, erythrasma;
- evidence of rosacea associated with excessive alcohol intake;
- evidence of smoking-related issues such as psoriasis or hidradenitis suppurativa;
- cutaneous evidence of other systemic diseases (eg, autoimmune disease, cancer);
- elucidation of other chronic health problems (eg, psoriasis of the skin as a clue for undiagnosed psoriatic arthritis); and
- detection of parasites on the skin (eg, ticks) or signs of infection that may have notable ramifications (eg, interdigital maceration of a diabetic patient with tinea pedis).
I even saw a patient who had been sent for magnetic resonance imaging for back pain by her internist without any physical examination when she actually had an erosion over the sacrum from a rug burn!
When conducting full-body skin examinations, dermatologists should not underestimate these principles:
- The “magic” of using a relatively noninvasive and sensitive screening tool—comfort and stress reduction for the patient from a thorough visual, tactile, olfactory, and auditory examination.
- Human interaction—especially when the patient is seen annually or even more frequently over a period of years or decades, and especially when an excellent patient-physician rapport has been established.
- The impact of improving a patient’s appearance on their overall sense of well-being (eg, by controlling rosacea).
- The opportunity to introduce concepts (ie, educate patients) such as alcohol avoidance, smoking cessation, weight reduction, hygiene, diet, and exercise in a more tangential way than a PCP, as well as to consider with patients the idea that lifestyle modification may be an adjunct, if not a replacement, for prescription treatments.
- The stress reduction that ensues when a variety of self-identified health issues are addressed, for which the only treatment may be reassurance.
I would add to Dr. Ngo’s argument that stratifying patients into skin cancer risk categories may be a useful measure if the only goal of periodic dermatologic evaluation is skin cancer detection. One size rarely fits all when it comes to health recommendations.
In sum, I believe that periodic full-body skin examination is absolutely beneficial to patient care, and I am not at all surprised that all-cause mortality was lower in patients who have those examinations. Furthermore, when I offer my healthy, low-risk patients the option to return in 2 years rather than 1, the vast majority insist on 1 year. My mother used to say, “It’s better to be looked over than to be overlooked,” and I tell my patients that, too—but it seems they already know that instinctively.
To the Editor:
I was unsurprised and gratified by the information presented in the Viewpoint on skin cancer screening by Ngo1 (Cutis. 2024;113:94-96). In my 30 years as a community dermatologist, I have observed that patients who opt to have periodic full-body skin examinations usually are more health literate, more likely to have a primary care physician (PCP) who has encouraged them to do so (ie, a conscientious practitioner directing their preventive care), more likely to have a strong will to live, and less likely to have multiple stressors that preclude self-care (eg, may be less likely to have a spouse for whom they are a caregiver) compared to those who do not get screened.
Findings on a full-body skin examination may impact patients in many ways, not only by the detection of skin cancers. I have discovered the following:
- evidence of diabetes/insulin resistance in the form of acanthosis nigricans, tinea corporis, erythrasma;
- evidence of rosacea associated with excessive alcohol intake;
- evidence of smoking-related issues such as psoriasis or hidradenitis suppurativa;
- cutaneous evidence of other systemic diseases (eg, autoimmune disease, cancer);
- elucidation of other chronic health problems (eg, psoriasis of the skin as a clue for undiagnosed psoriatic arthritis); and
- detection of parasites on the skin (eg, ticks) or signs of infection that may have notable ramifications (eg, interdigital maceration of a diabetic patient with tinea pedis).
I even saw a patient who had been sent for magnetic resonance imaging for back pain by her internist without any physical examination when she actually had an erosion over the sacrum from a rug burn!
When conducting full-body skin examinations, dermatologists should not underestimate these principles:
- The “magic” of using a relatively noninvasive and sensitive screening tool—comfort and stress reduction for the patient from a thorough visual, tactile, olfactory, and auditory examination.
- Human interaction—especially when the patient is seen annually or even more frequently over a period of years or decades, and especially when an excellent patient-physician rapport has been established.
- The impact of improving a patient’s appearance on their overall sense of well-being (eg, by controlling rosacea).
- The opportunity to introduce concepts (ie, educate patients) such as alcohol avoidance, smoking cessation, weight reduction, hygiene, diet, and exercise in a more tangential way than a PCP, as well as to consider with patients the idea that lifestyle modification may be an adjunct, if not a replacement, for prescription treatments.
- The stress reduction that ensues when a variety of self-identified health issues are addressed, for which the only treatment may be reassurance.
I would add to Dr. Ngo’s argument that stratifying patients into skin cancer risk categories may be a useful measure if the only goal of periodic dermatologic evaluation is skin cancer detection. One size rarely fits all when it comes to health recommendations.
In sum, I believe that periodic full-body skin examination is absolutely beneficial to patient care, and I am not at all surprised that all-cause mortality was lower in patients who have those examinations. Furthermore, when I offer my healthy, low-risk patients the option to return in 2 years rather than 1, the vast majority insist on 1 year. My mother used to say, “It’s better to be looked over than to be overlooked,” and I tell my patients that, too—but it seems they already know that instinctively.
- Ngo BT. Skin cancer screening: the paradox of melanoma and improved all-cause mortality. Cutis. 2024;113:94-96. doi:10.12788/cutis.0948
- Ngo BT. Skin cancer screening: the paradox of melanoma and improved all-cause mortality. Cutis. 2024;113:94-96. doi:10.12788/cutis.0948
Understanding the Evaluation and Management Add-on Complexity Code
On January 1, 2024, a new add-on complexity code, G2211, was implemented to the documentation of evaluation and management (E/M) visits.1 Created by the Centers for Medicare & Medicaid Services (CMS), G2211 is defined as “visit complexity inherent to evaluation and management associated with medical care services that serve as the continuing focal point for all needed health care services and/or with medical care services that are part of ongoing care related to a patient’s single, serious, or complex condition.”2 It is an add-on code, meaning that it must be listed with either a new or established outpatient E/M visit.
G2211 originally was introduced in the 2021 Proposed Rule but was delayed via a congressional mandate for 3 years.1 It originally was estimated that this code would be billed with 90% of all office visit claims, accounting for an approximately $3.3 billion increase in physician fee schedule spending; however, this estimate was revised with its reintroduction in the 2024 Final Rule, and it currently is estimated that it will be billed with 38% of all office visit claims.3,4
This add-on code was created to capture the inherent complexity of an E/M visit that is derived from the longitudinal nature of the physician-patient relationship and to better account for the additional resources of these outpatient E/M visits.5 Although these criteria often are met in the setting of an E/M visit within a primary care specialty (eg, family practice, internal medicine, obstetrics/gynecology, pediatrics), this code is not restricted to medical professionals based on specialties. The CMS noted that “the most important information used to determine whether the add-on code could be billed is the relationship between the practitioner and the patient,” specifically if they are fulfilling one of the following roles: “the continuing focal point for all needed health care services” or “ongoing care related to a patient's single, serious and complex condition.”6
Of note, further definitions regarding what constitutes a single, serious or complex condition have not yet been provided by CMS. The code should not be utilized when the relationship with the patient is of a discrete, routine, or time-limited nature. The resulting care should be personalized and should result in a comprehensive, longitudinal, and continuous relationship with the patient and should involve delivery of team-based care that is accessible, coordinated with other practitioners and providers, and integrated with the broader health care landscape.6
Herein, 5 examples are provided of scenarios when G2211 might be utilized as well as when it would not be appropriate to bill for this code.
Example 1
A 48-year-old man (an established patient) with a history of psoriasis and psoriatic arthritis presents to a dermatologist for follow-up. The dermatologist has been managing both conditions for 3 years with methotrexate. The patient’s disease is well controlled at the current visit, and he presents for follow-up of disease activity and laboratory monitoring every 3 months. The dermatologist continues the patient on methotrexate after reviewing the risks, benefits, and adverse effects and orders a complete blood cell count and comprehensive metabolic panel.
Would use of G2211 be appropriate for this visit?—Yes, in this case it would be appropriate to bill for G2211. In this example, the physician is providing longitudinal ongoing medical care related to a patient’s single, serious or complex condition—specifically psoriasis and psoriatic arthritis—via managing methotrexate therapy.
Example 2
Let’s alter the previous example slightly: A 48-year-old man (an established patient) with a history of psoriasis and psoriatic arthritis presents to a dermatologist for follow-up. He is being followed by both a dermatologist and a rheumatologist. The patient is on methotrexate, which was prescribed by the rheumatologist, who also conducts the appropriate laboratory monitoring. The patient’s skin disease currently is well controlled, and the dermatologist discusses this with the patient and advises that he continue to follow up with rheumatology.
Would use of G2211 be appropriate for this visit?—No, in this case it would not be appropriate to utilize G2211. In this example, the dermatologist is providing longitudinal ongoing medical care; however, unlike in the first example, much of the ongoing medical care—in particular the management of the patient’s methotrexate therapy—is being performed by the rheumatologist. Therefore, although these conditions are serious or complex, the dermatologist is not the primary manager of treatment, and it would not be appropriate to bill for G2211.
Example 3
A 35-year-old woman (an established patient) presents to a dermatologist for follow-up of hidradenitis suppurativa. She currently is receiving infliximab infusions that are managed by the dermatologist. At the current presentation, physical examination reveals several persistent active lesions. After discussing possible treatment options, the dermatologist elects to continue infliximab therapy and schedule a deroofing procedure of the persistent areas.
Would use of G2211 be appropriate for this visit?—Yes, in this example it would be appropriate to utilize G2211. The patient has hidradenitis suppurativa, which would be considered a single, serious or complex condition. Additionally, the dermatologist is the primary manager of this condition by prescribing infliximab as well as counseling the patient on the appropriateness of procedural interventions and scheduling for these procedures; the dermatologist also is providing ongoing longitudinal care.
Example 4
Let’s alter the previous example slightly: A 35-year-old woman (an established patient) presents to a dermatologist for follow-up of hidradenitis suppurativa. She currently is receiving infliximab infusions, which are managed by the dermatologist. At the current presentation, physical examination reveals several persistent active lesions. After discussing possible treatment options, the dermatologist elects to perform intralesional triamcinolone injections to active areas during the current visit.
Would use of G2211 be appropriate for this visit?—No, in this case it would not be appropriate to bill for G2211. Similar to Example 3, the dermatologist is treating a single, serious and complex condition and is primarily managing the disease and providing longitudinal care; however, in this case the dermatologist also is performing a minor procedure during the visit: injection of intralesional triamcinolone.
Importantly, G2211 cannot be utilized when modifier -25 is being appended to an outpatient E/M visit. Modifier -25 is defined as a “significant, separately identifiable evaluation and management service by the same physician or other qualified health care professional on the same day of the procedure or other service.”7 Modifier -25 is utilized when a minor procedure is performed by a qualified health care professional on the same day (generally during the same visit) as an E/M visit. Therefore, G2211 cannot be utilized when a minor procedure (eg, a tangential biopsy, punch biopsy, destruction or intralesional injection into skin) is performed during a visit.
Example 5
A 6-year-old girl presents to a dermatologist for a new rash on the trunk that started 5 days after an upper respiratory infection. The dermatologist evaluates the patient and identifies a blanchable macular eruption on the trunk; the patient is diagnosed with a viral exanthem. Because the patient reported associated pruritus, topical triamcinolone is prescribed.
Would use of G2211 be appropriate for this visit?—No, in this case it would not be appropriate to bill for G2211. A viral exanthem would not be considered an ongoing single, serious or complex condition and would be more consistent with a discrete condition; therefore, even though the dermatologist is primarily managing the disease process, it still would not fulfill the criteria necessary to bill for G2211.
Final Thoughts
G2211 is an add-on code created by the CMS that can be utilized in conjunction with an outpatient E/M visit when certain requirements are fulfilled. Specifically, this code can be utilized when the dermatologist is the primary provider of care for a patient’s ongoing single, serious or complex condition or serves as the continuing focal point for all of the patient’s health care needs. Understanding the nuances associated with this code are critical for correct billing.
- Centers for Medicare & Medicaid Services. Calendar Year (CY) 2024 Medicare physician fee schedule final rule. Published November 2, 2023. Accessed April 15, 2024. https://www.cms.gov/newsroom/fact-sheets/calendar-year-cy-2024-medicare-physician-fee-schedule-final-rule
- Centers for Medicare & Medicaid Services. Fact Sheet—Physician Fee Schedule (PFS) payment for office/outpatient evaluation and management (E/M) visits. Published January 11, 2021. Accessed April 15, 2024. https://www.cms.gov/files/document/physician-fee-schedule-pfs-payment-officeoutpatient-evaluation-and-management-em-visits-fact-sheet.pdf
- American Society of Anesthesiologists. Broken Medicare system results in CMS proposing reduced physician payments in 2024. Published July 13, 2023. Accessed April 15, 2024. https://www.asahq.org/advocacy-and-asapac/fda-and-washington-alerts/washington-alerts/2023/07/broken-medicare-system-results-in-cms-proposing-reduced-physician-payments-in-2024
- American Medical Association. CY 2024 Medicare physician payment schedule and quality payment program (QPP) final rule summary. Accessed April 15, 2024. https://www.ama-assn.org/system/files/ama-summary-2024-mfs-proposed-rule.pdf
- Centers for Medicare & Medicaid Services. How to use the office & outpatient evaluation and management visit complexity add-on code G2211. MM13473. MLN Matters. Updated January 18, 2024. Accessed April 15, 2024. https://www.cms.gov/files/document/mm13473-how-use-office-and-outpatient-evaluation-and-management-visit-complexity-add-code-g2211.pdf
- Centers for Medicare & Medicaid Services. CMS manual system. Published January 18, 2024. Accessed April 15, 2024. https://www.cms.gov/files/document/r12461cp.pdf
- American Medical Association. Reporting CPT Modifier 25. CPT Assistant (Online). 2023;33:1-12. Accessed April 15, 2024. https://www.ama-assn.org/system/files/reporting-CPT-modifier-25.pdf
On January 1, 2024, a new add-on complexity code, G2211, was implemented to the documentation of evaluation and management (E/M) visits.1 Created by the Centers for Medicare & Medicaid Services (CMS), G2211 is defined as “visit complexity inherent to evaluation and management associated with medical care services that serve as the continuing focal point for all needed health care services and/or with medical care services that are part of ongoing care related to a patient’s single, serious, or complex condition.”2 It is an add-on code, meaning that it must be listed with either a new or established outpatient E/M visit.
G2211 originally was introduced in the 2021 Proposed Rule but was delayed via a congressional mandate for 3 years.1 It originally was estimated that this code would be billed with 90% of all office visit claims, accounting for an approximately $3.3 billion increase in physician fee schedule spending; however, this estimate was revised with its reintroduction in the 2024 Final Rule, and it currently is estimated that it will be billed with 38% of all office visit claims.3,4
This add-on code was created to capture the inherent complexity of an E/M visit that is derived from the longitudinal nature of the physician-patient relationship and to better account for the additional resources of these outpatient E/M visits.5 Although these criteria often are met in the setting of an E/M visit within a primary care specialty (eg, family practice, internal medicine, obstetrics/gynecology, pediatrics), this code is not restricted to medical professionals based on specialties. The CMS noted that “the most important information used to determine whether the add-on code could be billed is the relationship between the practitioner and the patient,” specifically if they are fulfilling one of the following roles: “the continuing focal point for all needed health care services” or “ongoing care related to a patient's single, serious and complex condition.”6
Of note, further definitions regarding what constitutes a single, serious or complex condition have not yet been provided by CMS. The code should not be utilized when the relationship with the patient is of a discrete, routine, or time-limited nature. The resulting care should be personalized and should result in a comprehensive, longitudinal, and continuous relationship with the patient and should involve delivery of team-based care that is accessible, coordinated with other practitioners and providers, and integrated with the broader health care landscape.6
Herein, 5 examples are provided of scenarios when G2211 might be utilized as well as when it would not be appropriate to bill for this code.
Example 1
A 48-year-old man (an established patient) with a history of psoriasis and psoriatic arthritis presents to a dermatologist for follow-up. The dermatologist has been managing both conditions for 3 years with methotrexate. The patient’s disease is well controlled at the current visit, and he presents for follow-up of disease activity and laboratory monitoring every 3 months. The dermatologist continues the patient on methotrexate after reviewing the risks, benefits, and adverse effects and orders a complete blood cell count and comprehensive metabolic panel.
Would use of G2211 be appropriate for this visit?—Yes, in this case it would be appropriate to bill for G2211. In this example, the physician is providing longitudinal ongoing medical care related to a patient’s single, serious or complex condition—specifically psoriasis and psoriatic arthritis—via managing methotrexate therapy.
Example 2
Let’s alter the previous example slightly: A 48-year-old man (an established patient) with a history of psoriasis and psoriatic arthritis presents to a dermatologist for follow-up. He is being followed by both a dermatologist and a rheumatologist. The patient is on methotrexate, which was prescribed by the rheumatologist, who also conducts the appropriate laboratory monitoring. The patient’s skin disease currently is well controlled, and the dermatologist discusses this with the patient and advises that he continue to follow up with rheumatology.
Would use of G2211 be appropriate for this visit?—No, in this case it would not be appropriate to utilize G2211. In this example, the dermatologist is providing longitudinal ongoing medical care; however, unlike in the first example, much of the ongoing medical care—in particular the management of the patient’s methotrexate therapy—is being performed by the rheumatologist. Therefore, although these conditions are serious or complex, the dermatologist is not the primary manager of treatment, and it would not be appropriate to bill for G2211.
Example 3
A 35-year-old woman (an established patient) presents to a dermatologist for follow-up of hidradenitis suppurativa. She currently is receiving infliximab infusions that are managed by the dermatologist. At the current presentation, physical examination reveals several persistent active lesions. After discussing possible treatment options, the dermatologist elects to continue infliximab therapy and schedule a deroofing procedure of the persistent areas.
Would use of G2211 be appropriate for this visit?—Yes, in this example it would be appropriate to utilize G2211. The patient has hidradenitis suppurativa, which would be considered a single, serious or complex condition. Additionally, the dermatologist is the primary manager of this condition by prescribing infliximab as well as counseling the patient on the appropriateness of procedural interventions and scheduling for these procedures; the dermatologist also is providing ongoing longitudinal care.
Example 4
Let’s alter the previous example slightly: A 35-year-old woman (an established patient) presents to a dermatologist for follow-up of hidradenitis suppurativa. She currently is receiving infliximab infusions, which are managed by the dermatologist. At the current presentation, physical examination reveals several persistent active lesions. After discussing possible treatment options, the dermatologist elects to perform intralesional triamcinolone injections to active areas during the current visit.
Would use of G2211 be appropriate for this visit?—No, in this case it would not be appropriate to bill for G2211. Similar to Example 3, the dermatologist is treating a single, serious and complex condition and is primarily managing the disease and providing longitudinal care; however, in this case the dermatologist also is performing a minor procedure during the visit: injection of intralesional triamcinolone.
Importantly, G2211 cannot be utilized when modifier -25 is being appended to an outpatient E/M visit. Modifier -25 is defined as a “significant, separately identifiable evaluation and management service by the same physician or other qualified health care professional on the same day of the procedure or other service.”7 Modifier -25 is utilized when a minor procedure is performed by a qualified health care professional on the same day (generally during the same visit) as an E/M visit. Therefore, G2211 cannot be utilized when a minor procedure (eg, a tangential biopsy, punch biopsy, destruction or intralesional injection into skin) is performed during a visit.
Example 5
A 6-year-old girl presents to a dermatologist for a new rash on the trunk that started 5 days after an upper respiratory infection. The dermatologist evaluates the patient and identifies a blanchable macular eruption on the trunk; the patient is diagnosed with a viral exanthem. Because the patient reported associated pruritus, topical triamcinolone is prescribed.
Would use of G2211 be appropriate for this visit?—No, in this case it would not be appropriate to bill for G2211. A viral exanthem would not be considered an ongoing single, serious or complex condition and would be more consistent with a discrete condition; therefore, even though the dermatologist is primarily managing the disease process, it still would not fulfill the criteria necessary to bill for G2211.
Final Thoughts
G2211 is an add-on code created by the CMS that can be utilized in conjunction with an outpatient E/M visit when certain requirements are fulfilled. Specifically, this code can be utilized when the dermatologist is the primary provider of care for a patient’s ongoing single, serious or complex condition or serves as the continuing focal point for all of the patient’s health care needs. Understanding the nuances associated with this code are critical for correct billing.
On January 1, 2024, a new add-on complexity code, G2211, was implemented to the documentation of evaluation and management (E/M) visits.1 Created by the Centers for Medicare & Medicaid Services (CMS), G2211 is defined as “visit complexity inherent to evaluation and management associated with medical care services that serve as the continuing focal point for all needed health care services and/or with medical care services that are part of ongoing care related to a patient’s single, serious, or complex condition.”2 It is an add-on code, meaning that it must be listed with either a new or established outpatient E/M visit.
G2211 originally was introduced in the 2021 Proposed Rule but was delayed via a congressional mandate for 3 years.1 It originally was estimated that this code would be billed with 90% of all office visit claims, accounting for an approximately $3.3 billion increase in physician fee schedule spending; however, this estimate was revised with its reintroduction in the 2024 Final Rule, and it currently is estimated that it will be billed with 38% of all office visit claims.3,4
This add-on code was created to capture the inherent complexity of an E/M visit that is derived from the longitudinal nature of the physician-patient relationship and to better account for the additional resources of these outpatient E/M visits.5 Although these criteria often are met in the setting of an E/M visit within a primary care specialty (eg, family practice, internal medicine, obstetrics/gynecology, pediatrics), this code is not restricted to medical professionals based on specialties. The CMS noted that “the most important information used to determine whether the add-on code could be billed is the relationship between the practitioner and the patient,” specifically if they are fulfilling one of the following roles: “the continuing focal point for all needed health care services” or “ongoing care related to a patient's single, serious and complex condition.”6
Of note, further definitions regarding what constitutes a single, serious or complex condition have not yet been provided by CMS. The code should not be utilized when the relationship with the patient is of a discrete, routine, or time-limited nature. The resulting care should be personalized and should result in a comprehensive, longitudinal, and continuous relationship with the patient and should involve delivery of team-based care that is accessible, coordinated with other practitioners and providers, and integrated with the broader health care landscape.6
Herein, 5 examples are provided of scenarios when G2211 might be utilized as well as when it would not be appropriate to bill for this code.
Example 1
A 48-year-old man (an established patient) with a history of psoriasis and psoriatic arthritis presents to a dermatologist for follow-up. The dermatologist has been managing both conditions for 3 years with methotrexate. The patient’s disease is well controlled at the current visit, and he presents for follow-up of disease activity and laboratory monitoring every 3 months. The dermatologist continues the patient on methotrexate after reviewing the risks, benefits, and adverse effects and orders a complete blood cell count and comprehensive metabolic panel.
Would use of G2211 be appropriate for this visit?—Yes, in this case it would be appropriate to bill for G2211. In this example, the physician is providing longitudinal ongoing medical care related to a patient’s single, serious or complex condition—specifically psoriasis and psoriatic arthritis—via managing methotrexate therapy.
Example 2
Let’s alter the previous example slightly: A 48-year-old man (an established patient) with a history of psoriasis and psoriatic arthritis presents to a dermatologist for follow-up. He is being followed by both a dermatologist and a rheumatologist. The patient is on methotrexate, which was prescribed by the rheumatologist, who also conducts the appropriate laboratory monitoring. The patient’s skin disease currently is well controlled, and the dermatologist discusses this with the patient and advises that he continue to follow up with rheumatology.
Would use of G2211 be appropriate for this visit?—No, in this case it would not be appropriate to utilize G2211. In this example, the dermatologist is providing longitudinal ongoing medical care; however, unlike in the first example, much of the ongoing medical care—in particular the management of the patient’s methotrexate therapy—is being performed by the rheumatologist. Therefore, although these conditions are serious or complex, the dermatologist is not the primary manager of treatment, and it would not be appropriate to bill for G2211.
Example 3
A 35-year-old woman (an established patient) presents to a dermatologist for follow-up of hidradenitis suppurativa. She currently is receiving infliximab infusions that are managed by the dermatologist. At the current presentation, physical examination reveals several persistent active lesions. After discussing possible treatment options, the dermatologist elects to continue infliximab therapy and schedule a deroofing procedure of the persistent areas.
Would use of G2211 be appropriate for this visit?—Yes, in this example it would be appropriate to utilize G2211. The patient has hidradenitis suppurativa, which would be considered a single, serious or complex condition. Additionally, the dermatologist is the primary manager of this condition by prescribing infliximab as well as counseling the patient on the appropriateness of procedural interventions and scheduling for these procedures; the dermatologist also is providing ongoing longitudinal care.
Example 4
Let’s alter the previous example slightly: A 35-year-old woman (an established patient) presents to a dermatologist for follow-up of hidradenitis suppurativa. She currently is receiving infliximab infusions, which are managed by the dermatologist. At the current presentation, physical examination reveals several persistent active lesions. After discussing possible treatment options, the dermatologist elects to perform intralesional triamcinolone injections to active areas during the current visit.
Would use of G2211 be appropriate for this visit?—No, in this case it would not be appropriate to bill for G2211. Similar to Example 3, the dermatologist is treating a single, serious and complex condition and is primarily managing the disease and providing longitudinal care; however, in this case the dermatologist also is performing a minor procedure during the visit: injection of intralesional triamcinolone.
Importantly, G2211 cannot be utilized when modifier -25 is being appended to an outpatient E/M visit. Modifier -25 is defined as a “significant, separately identifiable evaluation and management service by the same physician or other qualified health care professional on the same day of the procedure or other service.”7 Modifier -25 is utilized when a minor procedure is performed by a qualified health care professional on the same day (generally during the same visit) as an E/M visit. Therefore, G2211 cannot be utilized when a minor procedure (eg, a tangential biopsy, punch biopsy, destruction or intralesional injection into skin) is performed during a visit.
Example 5
A 6-year-old girl presents to a dermatologist for a new rash on the trunk that started 5 days after an upper respiratory infection. The dermatologist evaluates the patient and identifies a blanchable macular eruption on the trunk; the patient is diagnosed with a viral exanthem. Because the patient reported associated pruritus, topical triamcinolone is prescribed.
Would use of G2211 be appropriate for this visit?—No, in this case it would not be appropriate to bill for G2211. A viral exanthem would not be considered an ongoing single, serious or complex condition and would be more consistent with a discrete condition; therefore, even though the dermatologist is primarily managing the disease process, it still would not fulfill the criteria necessary to bill for G2211.
Final Thoughts
G2211 is an add-on code created by the CMS that can be utilized in conjunction with an outpatient E/M visit when certain requirements are fulfilled. Specifically, this code can be utilized when the dermatologist is the primary provider of care for a patient’s ongoing single, serious or complex condition or serves as the continuing focal point for all of the patient’s health care needs. Understanding the nuances associated with this code are critical for correct billing.
- Centers for Medicare & Medicaid Services. Calendar Year (CY) 2024 Medicare physician fee schedule final rule. Published November 2, 2023. Accessed April 15, 2024. https://www.cms.gov/newsroom/fact-sheets/calendar-year-cy-2024-medicare-physician-fee-schedule-final-rule
- Centers for Medicare & Medicaid Services. Fact Sheet—Physician Fee Schedule (PFS) payment for office/outpatient evaluation and management (E/M) visits. Published January 11, 2021. Accessed April 15, 2024. https://www.cms.gov/files/document/physician-fee-schedule-pfs-payment-officeoutpatient-evaluation-and-management-em-visits-fact-sheet.pdf
- American Society of Anesthesiologists. Broken Medicare system results in CMS proposing reduced physician payments in 2024. Published July 13, 2023. Accessed April 15, 2024. https://www.asahq.org/advocacy-and-asapac/fda-and-washington-alerts/washington-alerts/2023/07/broken-medicare-system-results-in-cms-proposing-reduced-physician-payments-in-2024
- American Medical Association. CY 2024 Medicare physician payment schedule and quality payment program (QPP) final rule summary. Accessed April 15, 2024. https://www.ama-assn.org/system/files/ama-summary-2024-mfs-proposed-rule.pdf
- Centers for Medicare & Medicaid Services. How to use the office & outpatient evaluation and management visit complexity add-on code G2211. MM13473. MLN Matters. Updated January 18, 2024. Accessed April 15, 2024. https://www.cms.gov/files/document/mm13473-how-use-office-and-outpatient-evaluation-and-management-visit-complexity-add-code-g2211.pdf
- Centers for Medicare & Medicaid Services. CMS manual system. Published January 18, 2024. Accessed April 15, 2024. https://www.cms.gov/files/document/r12461cp.pdf
- American Medical Association. Reporting CPT Modifier 25. CPT Assistant (Online). 2023;33:1-12. Accessed April 15, 2024. https://www.ama-assn.org/system/files/reporting-CPT-modifier-25.pdf
- Centers for Medicare & Medicaid Services. Calendar Year (CY) 2024 Medicare physician fee schedule final rule. Published November 2, 2023. Accessed April 15, 2024. https://www.cms.gov/newsroom/fact-sheets/calendar-year-cy-2024-medicare-physician-fee-schedule-final-rule
- Centers for Medicare & Medicaid Services. Fact Sheet—Physician Fee Schedule (PFS) payment for office/outpatient evaluation and management (E/M) visits. Published January 11, 2021. Accessed April 15, 2024. https://www.cms.gov/files/document/physician-fee-schedule-pfs-payment-officeoutpatient-evaluation-and-management-em-visits-fact-sheet.pdf
- American Society of Anesthesiologists. Broken Medicare system results in CMS proposing reduced physician payments in 2024. Published July 13, 2023. Accessed April 15, 2024. https://www.asahq.org/advocacy-and-asapac/fda-and-washington-alerts/washington-alerts/2023/07/broken-medicare-system-results-in-cms-proposing-reduced-physician-payments-in-2024
- American Medical Association. CY 2024 Medicare physician payment schedule and quality payment program (QPP) final rule summary. Accessed April 15, 2024. https://www.ama-assn.org/system/files/ama-summary-2024-mfs-proposed-rule.pdf
- Centers for Medicare & Medicaid Services. How to use the office & outpatient evaluation and management visit complexity add-on code G2211. MM13473. MLN Matters. Updated January 18, 2024. Accessed April 15, 2024. https://www.cms.gov/files/document/mm13473-how-use-office-and-outpatient-evaluation-and-management-visit-complexity-add-code-g2211.pdf
- Centers for Medicare & Medicaid Services. CMS manual system. Published January 18, 2024. Accessed April 15, 2024. https://www.cms.gov/files/document/r12461cp.pdf
- American Medical Association. Reporting CPT Modifier 25. CPT Assistant (Online). 2023;33:1-12. Accessed April 15, 2024. https://www.ama-assn.org/system/files/reporting-CPT-modifier-25.pdf
PRACTICE POINTS
- The add-on code G2211 went into effect on January 1, 2024, and can be applied to outpatient evaluation and management visits that fulfill certain criteria.
- This code should be utilized when one is serving as the continuing focal point for all of the patient's health care needs or providing ongoing medical care related to a patient’s single, serious or complex condition.