User login
CRISPR-Based Gene Therapy Earns Beta Thalassemia Approval
The approval, which comes more than 2 months ahead of a target action date of March 30, marks the second for the landmark therapy. The FDA greenlit the CRISPR gene therapy to treat sickle cell disease last December.
The autologous, ex vivo, CRISPR/Cas9 gene-edited therapy from Vertex and CRISPR Therapeutics is the first to use the gene-editing tool CRISPR.
The transfusion-dependent beta thalassemia approval is based on data from pivotal studies showing “consistent and durable response to treatment” in 52 patients who received an infusion and followed for up to 4 years. Treatment conferred transfusion independence in patients with transfusion-dependent beta thalassemia, according to a press release from Vertex late last year.
Vertex noted in a new press statement that expanded approval means about 1000 patients aged 12 years or older will be eligible for the one-time treatment for this indication.
Exa-cel requires administration at authorized treatment centers experienced in stem cell transplantation.
The therapy, which has a list price of $2.2 million in the United States, should be available initially at nine authorized treatment centers early this year, with more to come, according to Vertex.
A version of this article appeared on Medscape.com.
The approval, which comes more than 2 months ahead of a target action date of March 30, marks the second for the landmark therapy. The FDA greenlit the CRISPR gene therapy to treat sickle cell disease last December.
The autologous, ex vivo, CRISPR/Cas9 gene-edited therapy from Vertex and CRISPR Therapeutics is the first to use the gene-editing tool CRISPR.
The transfusion-dependent beta thalassemia approval is based on data from pivotal studies showing “consistent and durable response to treatment” in 52 patients who received an infusion and followed for up to 4 years. Treatment conferred transfusion independence in patients with transfusion-dependent beta thalassemia, according to a press release from Vertex late last year.
Vertex noted in a new press statement that expanded approval means about 1000 patients aged 12 years or older will be eligible for the one-time treatment for this indication.
Exa-cel requires administration at authorized treatment centers experienced in stem cell transplantation.
The therapy, which has a list price of $2.2 million in the United States, should be available initially at nine authorized treatment centers early this year, with more to come, according to Vertex.
A version of this article appeared on Medscape.com.
The approval, which comes more than 2 months ahead of a target action date of March 30, marks the second for the landmark therapy. The FDA greenlit the CRISPR gene therapy to treat sickle cell disease last December.
The autologous, ex vivo, CRISPR/Cas9 gene-edited therapy from Vertex and CRISPR Therapeutics is the first to use the gene-editing tool CRISPR.
The transfusion-dependent beta thalassemia approval is based on data from pivotal studies showing “consistent and durable response to treatment” in 52 patients who received an infusion and followed for up to 4 years. Treatment conferred transfusion independence in patients with transfusion-dependent beta thalassemia, according to a press release from Vertex late last year.
Vertex noted in a new press statement that expanded approval means about 1000 patients aged 12 years or older will be eligible for the one-time treatment for this indication.
Exa-cel requires administration at authorized treatment centers experienced in stem cell transplantation.
The therapy, which has a list price of $2.2 million in the United States, should be available initially at nine authorized treatment centers early this year, with more to come, according to Vertex.
A version of this article appeared on Medscape.com.
New Federal Rule for Prior Authorizations a ‘Major Win’ for Patients, Doctors
Physicians groups on January 17 hailed a new federal rule requiring health insurers to streamline and disclose more information about their prior authorization processes, saying it will improve patient care and reduce doctors’ administrative burden.
Health insurers participating in federal programs, including Medicare Advantage and Medicaid, must now respond to expedited prior authorization requests within 72 hours and other requests within 7 days under the long-awaited final rule, released on January 17 by the Centers for Medicare & Medicaid Services (CMS).
Insurers also must include their reasons for denying a prior authorization request and will be required to publicly release data on denial and approval rates for medical treatment. They’ll also need to give patients more information about their decisions to deny care. Insurers must comply with some of the rule’s provisions by January 2026 and others by January 2027.
The final rule “is an important step forward” toward the Medical Group Management Association’s goal of reducing the overall volume of prior authorization requests, said Anders Gilberg, the group’s senior vice president for government affairs, in a statement.
“Only then will medical groups find meaningful reprieve from these onerous, ill-intentioned administrative requirements that dangerously impede patient care,” Mr. Gilberg said.
Health insurers have long lobbied against increased regulation of prior authorization, arguing that it’s needed to rein in healthcare costs and prevent unnecessary treatment.
“We appreciate CMS’s announcement of enforcement discretion that will permit plans to use one standard, rather than mixing and matching, to reduce costs and speed implementation,” said America’s Health Insurance Plans, an insurers’ lobbying group, in an unsigned statement. “However, we must remember that the CMS rule is only half the picture; the Office of the Coordinator for Health Information Technology (ONC) should swiftly require vendors to build electronic prior authorization capabilities into the electronic health record so that providers can do their part, or plans will build a bridge to nowhere.”
The rule comes as health insurers have increasingly been criticized for onerous and time-consuming prior authorization procedures that physicians say unfairly delay or deny the medical treatment that their patients need. With federal legislation to rein in prior authorization overuse at a standstill, 30 states have introduced their own bills to address the problem. Regulators and lawsuits also have called attention to insurers’ increasing use of artificial intelligence and algorithms to deny claims without human review.
“Family physicians know firsthand how prior authorizations divert valuable time and resources away from direct patient care. We also know that these types of administrative requirements are driving physicians away from the workforce and worsening physician shortages,” said Steven P. Furr, MD, president of the American Academy of Family Physicians, in a statement praising the new rule.
Jesse M. Ehrenfeld, MD, MPH, president of the American Medical Association, called the final rule “ a major win” for patients and physicians, adding that its requirements for health insurers to integrate their prior authorization procedures into physicians’ electronic health records systems will also help make “the current time-consuming, manual workflow” more efficient.
A version of this article first appeared on Medscape.com.
Physicians groups on January 17 hailed a new federal rule requiring health insurers to streamline and disclose more information about their prior authorization processes, saying it will improve patient care and reduce doctors’ administrative burden.
Health insurers participating in federal programs, including Medicare Advantage and Medicaid, must now respond to expedited prior authorization requests within 72 hours and other requests within 7 days under the long-awaited final rule, released on January 17 by the Centers for Medicare & Medicaid Services (CMS).
Insurers also must include their reasons for denying a prior authorization request and will be required to publicly release data on denial and approval rates for medical treatment. They’ll also need to give patients more information about their decisions to deny care. Insurers must comply with some of the rule’s provisions by January 2026 and others by January 2027.
The final rule “is an important step forward” toward the Medical Group Management Association’s goal of reducing the overall volume of prior authorization requests, said Anders Gilberg, the group’s senior vice president for government affairs, in a statement.
“Only then will medical groups find meaningful reprieve from these onerous, ill-intentioned administrative requirements that dangerously impede patient care,” Mr. Gilberg said.
Health insurers have long lobbied against increased regulation of prior authorization, arguing that it’s needed to rein in healthcare costs and prevent unnecessary treatment.
“We appreciate CMS’s announcement of enforcement discretion that will permit plans to use one standard, rather than mixing and matching, to reduce costs and speed implementation,” said America’s Health Insurance Plans, an insurers’ lobbying group, in an unsigned statement. “However, we must remember that the CMS rule is only half the picture; the Office of the Coordinator for Health Information Technology (ONC) should swiftly require vendors to build electronic prior authorization capabilities into the electronic health record so that providers can do their part, or plans will build a bridge to nowhere.”
The rule comes as health insurers have increasingly been criticized for onerous and time-consuming prior authorization procedures that physicians say unfairly delay or deny the medical treatment that their patients need. With federal legislation to rein in prior authorization overuse at a standstill, 30 states have introduced their own bills to address the problem. Regulators and lawsuits also have called attention to insurers’ increasing use of artificial intelligence and algorithms to deny claims without human review.
“Family physicians know firsthand how prior authorizations divert valuable time and resources away from direct patient care. We also know that these types of administrative requirements are driving physicians away from the workforce and worsening physician shortages,” said Steven P. Furr, MD, president of the American Academy of Family Physicians, in a statement praising the new rule.
Jesse M. Ehrenfeld, MD, MPH, president of the American Medical Association, called the final rule “ a major win” for patients and physicians, adding that its requirements for health insurers to integrate their prior authorization procedures into physicians’ electronic health records systems will also help make “the current time-consuming, manual workflow” more efficient.
A version of this article first appeared on Medscape.com.
Physicians groups on January 17 hailed a new federal rule requiring health insurers to streamline and disclose more information about their prior authorization processes, saying it will improve patient care and reduce doctors’ administrative burden.
Health insurers participating in federal programs, including Medicare Advantage and Medicaid, must now respond to expedited prior authorization requests within 72 hours and other requests within 7 days under the long-awaited final rule, released on January 17 by the Centers for Medicare & Medicaid Services (CMS).
Insurers also must include their reasons for denying a prior authorization request and will be required to publicly release data on denial and approval rates for medical treatment. They’ll also need to give patients more information about their decisions to deny care. Insurers must comply with some of the rule’s provisions by January 2026 and others by January 2027.
The final rule “is an important step forward” toward the Medical Group Management Association’s goal of reducing the overall volume of prior authorization requests, said Anders Gilberg, the group’s senior vice president for government affairs, in a statement.
“Only then will medical groups find meaningful reprieve from these onerous, ill-intentioned administrative requirements that dangerously impede patient care,” Mr. Gilberg said.
Health insurers have long lobbied against increased regulation of prior authorization, arguing that it’s needed to rein in healthcare costs and prevent unnecessary treatment.
“We appreciate CMS’s announcement of enforcement discretion that will permit plans to use one standard, rather than mixing and matching, to reduce costs and speed implementation,” said America’s Health Insurance Plans, an insurers’ lobbying group, in an unsigned statement. “However, we must remember that the CMS rule is only half the picture; the Office of the Coordinator for Health Information Technology (ONC) should swiftly require vendors to build electronic prior authorization capabilities into the electronic health record so that providers can do their part, or plans will build a bridge to nowhere.”
The rule comes as health insurers have increasingly been criticized for onerous and time-consuming prior authorization procedures that physicians say unfairly delay or deny the medical treatment that their patients need. With federal legislation to rein in prior authorization overuse at a standstill, 30 states have introduced their own bills to address the problem. Regulators and lawsuits also have called attention to insurers’ increasing use of artificial intelligence and algorithms to deny claims without human review.
“Family physicians know firsthand how prior authorizations divert valuable time and resources away from direct patient care. We also know that these types of administrative requirements are driving physicians away from the workforce and worsening physician shortages,” said Steven P. Furr, MD, president of the American Academy of Family Physicians, in a statement praising the new rule.
Jesse M. Ehrenfeld, MD, MPH, president of the American Medical Association, called the final rule “ a major win” for patients and physicians, adding that its requirements for health insurers to integrate their prior authorization procedures into physicians’ electronic health records systems will also help make “the current time-consuming, manual workflow” more efficient.
A version of this article first appeared on Medscape.com.
Why Is Kidney Disease So Often Missed?
Nearly 37 million Americans, or 15%, have chronic kidney disease (CKD), but 9 in 10 adults with the condition are not aware of their diagnosis. A recent study from Stanford University found that
What should primary care providers be doing differently?
The current standard of care is to screen people with underlying conditions that put them at higher risk of developing CKD, most commonly diabetes and hypertension. That’s why the American Diabetes Association recommends annual screening for CKD in patients with type 1 diabetes as well as those with type 2 diabetes.
And the American Heart Association (AHA) released an advisory last year that defined cardiovascular-kidney-metabolic (CKM) syndrome, a constellation of conditions that often occur together: obesity, diabetes, CKD, and cardiovascular disease. They propose a staged approach to identifying and monitoring CKM throughout the lifespan, which includes regular monitoring of the urine albumin-creatinine ratio in patients who have developed diabetes, hypertension, metabolic syndrome, or any signs of kidney disease.
But despite recognition from the subspecialty professional societies of the importance of screening persons with risk factors — additional conditions are obesity and family history of CKD — real-world implementation lags.
Sylvia Rosas, MD, is a nephrologist and associate professor of medicine at Harvard University in Cambridge, Massachusetts, who also serves as president of the National Kidney Foundation. In an interview with this news organization, she cited several alarming facts about the state of CKD screening in the United States.
“Of people with diabetes who have insurance, only 40% get both the glomerular filtration rate (GFR) and the albumin performed, and for those who have hypertension, only 10%,” Dr. Rosas said. She is referring to a urine spot test that measures the amount of albumin in the urine, which is then paired with a serum measurement of creatinine to estimate the glomerular filtration rate. Both tests are needed to detect the asymptomatic stages of CKD, because the presence of albumin in the urine usually precedes drops in the GFR, which indicates more serious disease.
Dr. Rosas said she is frustrated by the low rate of testing compared with other commonly recommended preventive screenings, given the low cost and simplicity of assessment. Serum creatinine often is obtained as part of a routine chemistry panel, and the albumin test requires a single spot urine test. Yet, in 2018, 61% of US adults aged 50-75 years had received a colonoscopy in the past 10 years. Compared with the high price and inconvenience of undergoing colonoscopy, Dr. Rosas has trouble believing that “we cannot get more than 40% of people [with diabetes] to pee in a cup.”
But the biggest issue is that if people with risk factors don’t get screened before they develop symptoms of CKD, it is often too late to avoid dialysis or the need for transplantation.
The early warning symptoms are few, according to Nisha Bansal, MD, a professor in the department of nephrology at the University of Washington in Seattle. “New hypertension is a really important early sign,” Dr. Bansal said. “We know kidney disease almost certainly causes hypertension, so I would definitely think about screening for kidney disease.” Other findings on exam are the appearance of new edema or signs of fluid retention in the hands or around the eyes, along with findings in the urine of albumin, protein, or blood.
But most patients don’t have any symptoms in the early stages, and they can be nonspecific. “It is fatigue and some nausea,” Dr. Rosas said. “It’s only way at the end that you start vomiting, get itchy, or have hiccups.” Data from the Centers for Disease Control and Prevention have shown that over one third of patients at high risk for kidney failure are unaware of their disease. According to Dr. Rosas, these are patients who often receive the diagnosis of CKD and start dialysis the same day.
Why Not Screen Everyone?
For many conditions, like HIV or different types of cancer, the US Preventive Services Task Force (USPSTF) recommends broad screening of asymptomatic individuals so that early treatment can improve outcomes.
But when the USPSTF considered the question in 2012 of whether adults should be screened for CKD regardless of symptoms, it found little evidence that early detection could change the course of their illness. At that time, the standard of care for treating early stages of CKD generally focused on treating the comorbid conditions, such as diabetes, hypertension, and cardiovascular disease.
But the equation has changed with the availability of new drugs to treat CKD, such as sodium-glucose cotransporter 2 (SGLT2) inhibitors and mineralocorticoid receptor antagonists (MRAs).
“I consider these blockbuster drugs,” Dr. Bansal said. “For the first time in decades, we’re showing that this class of medications, the SGLT2 inhibitors, substantially reduce risk of loss of kidney function.”
Expressed in the lumen of the proximal renal tubules, SGLT2 reabsorbs filtered glucose from the tubular lumen. Inhibition of SGLT2 promotes urinary glucose excretion and reduces sodium reabsorption, increasing delivery of sodium to the distal tubule. The first SGLT2 inhibitor, canagliflozin, was approved in 2013 for use as an antihyperglycemic agent but subsequently was shown to have serendipitous benefits for the heart and kidneys.
Clinical trials have documented reductions in the risk for cardiovascular events in patients with type 2 diabetes, as well as decreases in the risk for progression to end-stage renal disease, cardiovascular mortality, and hospitalization for heart failure. Updated international guidelines from 2022 recommend treating all patients with type 2 diabetes and CKD with an estimated GFR ≥ 20 mL/min/1.73 m2 with an SGLT2 inhibitor.
But several trials of SGLT2 inhibitors also demonstrated benefits in reducing the risk for cardiovascular-related death or hospitalization for heart failure, even in patients without diabetes. Although initial approval from the US Food and Drug Administration was limited to patients with diabetes and heart failure, the agency has recently expanded its indications to include adults with CKD who do not have diabetes.
Dr. Bansal said she was happy to see this widening of the indications, which makes more patients eligible to receive SGLT2 inhibitors. “I really think this early CKD group is a great group to consider for those medications,” she said.
Dr. Bansal also pointed out that MRAs are another class of drugs with an interesting history. Earlier steroidal MRAs were found to have anti-inflammatory and antifibrotic properties, and in 1960 spironolactone was approved for use as a diuretic for the management of edema, primary aldosteronism, and hypertension. But even as their use in cardiology rose, MRAs had less utility for CKD, given adverse events such as hyperkalemia and hormonal effects like gynecomastia.
But the latest generation of nonsteroidal MRAs (nsMRAs) has higher selectivity for the mineralocorticoid receptor than sex-steroid hormone receptors, reducing androgenic side effects and preventing elevated potassium. Finerenone, the only nsMRA approved in the United States, has been shown in clinical trials to reduce the incidence of cardiovascular events (death from cardiovascular causes, nonfatal myocardial infarction, nonfatal stroke, or hospitalization for heart failure) and CKD outcomes, including kidney failure, decrease in estimated GFR, or death from renal causes.
EPIC Changes Coming?
In light of treatment advances that offer hope of preventing progression of CKD in patients identified early, both the National Kidney Foundation and the American Kidney Fund lobbied the USPSTF in 2022 to conduct a fresh review of recent data to evaluate the need for updated screening recommendations.
The task force completed development of a research plan and collection of public comments in early 2023 and is now reviewing evidence before developing a draft recommendation.
A team of health policy researchers from Stanford is hoping that some of their recently published work will attract the panel’s attention. The first study, published in 2022, evaluated the cost-effectiveness of dapagliflozin, an SGLT2 inhibitor that has been shown to reduce mortality by 48% in CKD patients without diabetes.
The Stanford team found that adding dapagliflozin to standard care for these patients improved life expectancy by 2 years and reduced the percentage of those who needed dialysis or kidney transplant from 17% to 11%.
More recently, Marika Cusick, a doctoral candidate in health policy at the Stanford School of Medicine in Stanford, California, served as first author of an evaluation of the cost-effectiveness of screening asymptomatic adults. “We assessed screening for albuminuria in conjunction with conventional CKD therapy in addition to this new SGLT2 inhibitor class of drugs,” she said. They projected how this might change CKD progression in US adults who are aged 35 or older compared with standard therapy alone.
The findings were favorable. “A one-time screening would result in a reduction of 398,000 cases of kidney replacement therapy [defined as needing either dialysis or renal transplant] among 158 million US adults who are currently aged 35-75 years,” Ms. Cusick told this news organization.
In terms of quality-adjusted life years (QALYs), a one-time screening at age 55 years yielded an incremental cost-effectiveness ratio of $86,300 per QALY. Screening every 10 years between the ages of 35 and 75 years cost less than $100,000 per QALY gained.
According to Doug Owens, MD, professor and chair of the department of health policy at Stanford School of Medicine, “There’s a societal decision about how much are we willing to pay for additional length and quality of life. And this fits within what is generally considered reasonable for the US.”
For example, in the United States, screening for breast cancer among women aged 40-64 years costs $51,000 per QALY, whereas screening for lung cancer using USPSTF guidelines ranges from $72,639 to $156,774 per QALY.
A former member of the USPSTF, Owens predicted that the current review process would take at least another year. Meanwhile, he and Ms. Cusick are hoping that their work influences the USPSTF to recommend screening asymptomatic adults. “Increasing the awareness of these drugs and their effectiveness is a crucial first step,” he said.
Although adherence to current recommendations for screening of people at risk is poor, Dr. Rosas suggested that the USPSTF guidelines would be more influential in changing practice among primary care physicians than subspecialty guidelines would.
“When you have a recommendation like that, they’re putting it in the electronic health record,” she said. By adding best practice alerts to their electronic health record systems, health systems can make it easier for primary care doctors to check all the boxes.
In line with the AHA’s holistic approach towards managing cardiovascular illnesses, CKD, and metabolic disease, Dr. Bansal suggested an additional strategy: “I think we’re moving toward more interdisciplinary care models, where primary care doctors, nephrologist, cardiologists, and endocrinologists — all of us — should be working together in a collaborative care model, to help break down some of these barriers in terms of screening as well as implementation of these therapies.”
Dr. Bansal, Ms. Cusick, and Dr. Owens reported no financial conflicts of interest. Dr. Rosas receives funding from AstraZeneca and Bayer for serving on advisory boards and clinical research funding, as well as funding from the National Institute of Diabetes and Digestive and Kidney Diseases for clinical trials.
Dr. Thomas is a pediatrician and epidemiologist living in Portland, Oregon.
A version of this article appeared on Medscape.com.
Nearly 37 million Americans, or 15%, have chronic kidney disease (CKD), but 9 in 10 adults with the condition are not aware of their diagnosis. A recent study from Stanford University found that
What should primary care providers be doing differently?
The current standard of care is to screen people with underlying conditions that put them at higher risk of developing CKD, most commonly diabetes and hypertension. That’s why the American Diabetes Association recommends annual screening for CKD in patients with type 1 diabetes as well as those with type 2 diabetes.
And the American Heart Association (AHA) released an advisory last year that defined cardiovascular-kidney-metabolic (CKM) syndrome, a constellation of conditions that often occur together: obesity, diabetes, CKD, and cardiovascular disease. They propose a staged approach to identifying and monitoring CKM throughout the lifespan, which includes regular monitoring of the urine albumin-creatinine ratio in patients who have developed diabetes, hypertension, metabolic syndrome, or any signs of kidney disease.
But despite recognition from the subspecialty professional societies of the importance of screening persons with risk factors — additional conditions are obesity and family history of CKD — real-world implementation lags.
Sylvia Rosas, MD, is a nephrologist and associate professor of medicine at Harvard University in Cambridge, Massachusetts, who also serves as president of the National Kidney Foundation. In an interview with this news organization, she cited several alarming facts about the state of CKD screening in the United States.
“Of people with diabetes who have insurance, only 40% get both the glomerular filtration rate (GFR) and the albumin performed, and for those who have hypertension, only 10%,” Dr. Rosas said. She is referring to a urine spot test that measures the amount of albumin in the urine, which is then paired with a serum measurement of creatinine to estimate the glomerular filtration rate. Both tests are needed to detect the asymptomatic stages of CKD, because the presence of albumin in the urine usually precedes drops in the GFR, which indicates more serious disease.
Dr. Rosas said she is frustrated by the low rate of testing compared with other commonly recommended preventive screenings, given the low cost and simplicity of assessment. Serum creatinine often is obtained as part of a routine chemistry panel, and the albumin test requires a single spot urine test. Yet, in 2018, 61% of US adults aged 50-75 years had received a colonoscopy in the past 10 years. Compared with the high price and inconvenience of undergoing colonoscopy, Dr. Rosas has trouble believing that “we cannot get more than 40% of people [with diabetes] to pee in a cup.”
But the biggest issue is that if people with risk factors don’t get screened before they develop symptoms of CKD, it is often too late to avoid dialysis or the need for transplantation.
The early warning symptoms are few, according to Nisha Bansal, MD, a professor in the department of nephrology at the University of Washington in Seattle. “New hypertension is a really important early sign,” Dr. Bansal said. “We know kidney disease almost certainly causes hypertension, so I would definitely think about screening for kidney disease.” Other findings on exam are the appearance of new edema or signs of fluid retention in the hands or around the eyes, along with findings in the urine of albumin, protein, or blood.
But most patients don’t have any symptoms in the early stages, and they can be nonspecific. “It is fatigue and some nausea,” Dr. Rosas said. “It’s only way at the end that you start vomiting, get itchy, or have hiccups.” Data from the Centers for Disease Control and Prevention have shown that over one third of patients at high risk for kidney failure are unaware of their disease. According to Dr. Rosas, these are patients who often receive the diagnosis of CKD and start dialysis the same day.
Why Not Screen Everyone?
For many conditions, like HIV or different types of cancer, the US Preventive Services Task Force (USPSTF) recommends broad screening of asymptomatic individuals so that early treatment can improve outcomes.
But when the USPSTF considered the question in 2012 of whether adults should be screened for CKD regardless of symptoms, it found little evidence that early detection could change the course of their illness. At that time, the standard of care for treating early stages of CKD generally focused on treating the comorbid conditions, such as diabetes, hypertension, and cardiovascular disease.
But the equation has changed with the availability of new drugs to treat CKD, such as sodium-glucose cotransporter 2 (SGLT2) inhibitors and mineralocorticoid receptor antagonists (MRAs).
“I consider these blockbuster drugs,” Dr. Bansal said. “For the first time in decades, we’re showing that this class of medications, the SGLT2 inhibitors, substantially reduce risk of loss of kidney function.”
Expressed in the lumen of the proximal renal tubules, SGLT2 reabsorbs filtered glucose from the tubular lumen. Inhibition of SGLT2 promotes urinary glucose excretion and reduces sodium reabsorption, increasing delivery of sodium to the distal tubule. The first SGLT2 inhibitor, canagliflozin, was approved in 2013 for use as an antihyperglycemic agent but subsequently was shown to have serendipitous benefits for the heart and kidneys.
Clinical trials have documented reductions in the risk for cardiovascular events in patients with type 2 diabetes, as well as decreases in the risk for progression to end-stage renal disease, cardiovascular mortality, and hospitalization for heart failure. Updated international guidelines from 2022 recommend treating all patients with type 2 diabetes and CKD with an estimated GFR ≥ 20 mL/min/1.73 m2 with an SGLT2 inhibitor.
But several trials of SGLT2 inhibitors also demonstrated benefits in reducing the risk for cardiovascular-related death or hospitalization for heart failure, even in patients without diabetes. Although initial approval from the US Food and Drug Administration was limited to patients with diabetes and heart failure, the agency has recently expanded its indications to include adults with CKD who do not have diabetes.
Dr. Bansal said she was happy to see this widening of the indications, which makes more patients eligible to receive SGLT2 inhibitors. “I really think this early CKD group is a great group to consider for those medications,” she said.
Dr. Bansal also pointed out that MRAs are another class of drugs with an interesting history. Earlier steroidal MRAs were found to have anti-inflammatory and antifibrotic properties, and in 1960 spironolactone was approved for use as a diuretic for the management of edema, primary aldosteronism, and hypertension. But even as their use in cardiology rose, MRAs had less utility for CKD, given adverse events such as hyperkalemia and hormonal effects like gynecomastia.
But the latest generation of nonsteroidal MRAs (nsMRAs) has higher selectivity for the mineralocorticoid receptor than sex-steroid hormone receptors, reducing androgenic side effects and preventing elevated potassium. Finerenone, the only nsMRA approved in the United States, has been shown in clinical trials to reduce the incidence of cardiovascular events (death from cardiovascular causes, nonfatal myocardial infarction, nonfatal stroke, or hospitalization for heart failure) and CKD outcomes, including kidney failure, decrease in estimated GFR, or death from renal causes.
EPIC Changes Coming?
In light of treatment advances that offer hope of preventing progression of CKD in patients identified early, both the National Kidney Foundation and the American Kidney Fund lobbied the USPSTF in 2022 to conduct a fresh review of recent data to evaluate the need for updated screening recommendations.
The task force completed development of a research plan and collection of public comments in early 2023 and is now reviewing evidence before developing a draft recommendation.
A team of health policy researchers from Stanford is hoping that some of their recently published work will attract the panel’s attention. The first study, published in 2022, evaluated the cost-effectiveness of dapagliflozin, an SGLT2 inhibitor that has been shown to reduce mortality by 48% in CKD patients without diabetes.
The Stanford team found that adding dapagliflozin to standard care for these patients improved life expectancy by 2 years and reduced the percentage of those who needed dialysis or kidney transplant from 17% to 11%.
More recently, Marika Cusick, a doctoral candidate in health policy at the Stanford School of Medicine in Stanford, California, served as first author of an evaluation of the cost-effectiveness of screening asymptomatic adults. “We assessed screening for albuminuria in conjunction with conventional CKD therapy in addition to this new SGLT2 inhibitor class of drugs,” she said. They projected how this might change CKD progression in US adults who are aged 35 or older compared with standard therapy alone.
The findings were favorable. “A one-time screening would result in a reduction of 398,000 cases of kidney replacement therapy [defined as needing either dialysis or renal transplant] among 158 million US adults who are currently aged 35-75 years,” Ms. Cusick told this news organization.
In terms of quality-adjusted life years (QALYs), a one-time screening at age 55 years yielded an incremental cost-effectiveness ratio of $86,300 per QALY. Screening every 10 years between the ages of 35 and 75 years cost less than $100,000 per QALY gained.
According to Doug Owens, MD, professor and chair of the department of health policy at Stanford School of Medicine, “There’s a societal decision about how much are we willing to pay for additional length and quality of life. And this fits within what is generally considered reasonable for the US.”
For example, in the United States, screening for breast cancer among women aged 40-64 years costs $51,000 per QALY, whereas screening for lung cancer using USPSTF guidelines ranges from $72,639 to $156,774 per QALY.
A former member of the USPSTF, Owens predicted that the current review process would take at least another year. Meanwhile, he and Ms. Cusick are hoping that their work influences the USPSTF to recommend screening asymptomatic adults. “Increasing the awareness of these drugs and their effectiveness is a crucial first step,” he said.
Although adherence to current recommendations for screening of people at risk is poor, Dr. Rosas suggested that the USPSTF guidelines would be more influential in changing practice among primary care physicians than subspecialty guidelines would.
“When you have a recommendation like that, they’re putting it in the electronic health record,” she said. By adding best practice alerts to their electronic health record systems, health systems can make it easier for primary care doctors to check all the boxes.
In line with the AHA’s holistic approach towards managing cardiovascular illnesses, CKD, and metabolic disease, Dr. Bansal suggested an additional strategy: “I think we’re moving toward more interdisciplinary care models, where primary care doctors, nephrologist, cardiologists, and endocrinologists — all of us — should be working together in a collaborative care model, to help break down some of these barriers in terms of screening as well as implementation of these therapies.”
Dr. Bansal, Ms. Cusick, and Dr. Owens reported no financial conflicts of interest. Dr. Rosas receives funding from AstraZeneca and Bayer for serving on advisory boards and clinical research funding, as well as funding from the National Institute of Diabetes and Digestive and Kidney Diseases for clinical trials.
Dr. Thomas is a pediatrician and epidemiologist living in Portland, Oregon.
A version of this article appeared on Medscape.com.
Nearly 37 million Americans, or 15%, have chronic kidney disease (CKD), but 9 in 10 adults with the condition are not aware of their diagnosis. A recent study from Stanford University found that
What should primary care providers be doing differently?
The current standard of care is to screen people with underlying conditions that put them at higher risk of developing CKD, most commonly diabetes and hypertension. That’s why the American Diabetes Association recommends annual screening for CKD in patients with type 1 diabetes as well as those with type 2 diabetes.
And the American Heart Association (AHA) released an advisory last year that defined cardiovascular-kidney-metabolic (CKM) syndrome, a constellation of conditions that often occur together: obesity, diabetes, CKD, and cardiovascular disease. They propose a staged approach to identifying and monitoring CKM throughout the lifespan, which includes regular monitoring of the urine albumin-creatinine ratio in patients who have developed diabetes, hypertension, metabolic syndrome, or any signs of kidney disease.
But despite recognition from the subspecialty professional societies of the importance of screening persons with risk factors — additional conditions are obesity and family history of CKD — real-world implementation lags.
Sylvia Rosas, MD, is a nephrologist and associate professor of medicine at Harvard University in Cambridge, Massachusetts, who also serves as president of the National Kidney Foundation. In an interview with this news organization, she cited several alarming facts about the state of CKD screening in the United States.
“Of people with diabetes who have insurance, only 40% get both the glomerular filtration rate (GFR) and the albumin performed, and for those who have hypertension, only 10%,” Dr. Rosas said. She is referring to a urine spot test that measures the amount of albumin in the urine, which is then paired with a serum measurement of creatinine to estimate the glomerular filtration rate. Both tests are needed to detect the asymptomatic stages of CKD, because the presence of albumin in the urine usually precedes drops in the GFR, which indicates more serious disease.
Dr. Rosas said she is frustrated by the low rate of testing compared with other commonly recommended preventive screenings, given the low cost and simplicity of assessment. Serum creatinine often is obtained as part of a routine chemistry panel, and the albumin test requires a single spot urine test. Yet, in 2018, 61% of US adults aged 50-75 years had received a colonoscopy in the past 10 years. Compared with the high price and inconvenience of undergoing colonoscopy, Dr. Rosas has trouble believing that “we cannot get more than 40% of people [with diabetes] to pee in a cup.”
But the biggest issue is that if people with risk factors don’t get screened before they develop symptoms of CKD, it is often too late to avoid dialysis or the need for transplantation.
The early warning symptoms are few, according to Nisha Bansal, MD, a professor in the department of nephrology at the University of Washington in Seattle. “New hypertension is a really important early sign,” Dr. Bansal said. “We know kidney disease almost certainly causes hypertension, so I would definitely think about screening for kidney disease.” Other findings on exam are the appearance of new edema or signs of fluid retention in the hands or around the eyes, along with findings in the urine of albumin, protein, or blood.
But most patients don’t have any symptoms in the early stages, and they can be nonspecific. “It is fatigue and some nausea,” Dr. Rosas said. “It’s only way at the end that you start vomiting, get itchy, or have hiccups.” Data from the Centers for Disease Control and Prevention have shown that over one third of patients at high risk for kidney failure are unaware of their disease. According to Dr. Rosas, these are patients who often receive the diagnosis of CKD and start dialysis the same day.
Why Not Screen Everyone?
For many conditions, like HIV or different types of cancer, the US Preventive Services Task Force (USPSTF) recommends broad screening of asymptomatic individuals so that early treatment can improve outcomes.
But when the USPSTF considered the question in 2012 of whether adults should be screened for CKD regardless of symptoms, it found little evidence that early detection could change the course of their illness. At that time, the standard of care for treating early stages of CKD generally focused on treating the comorbid conditions, such as diabetes, hypertension, and cardiovascular disease.
But the equation has changed with the availability of new drugs to treat CKD, such as sodium-glucose cotransporter 2 (SGLT2) inhibitors and mineralocorticoid receptor antagonists (MRAs).
“I consider these blockbuster drugs,” Dr. Bansal said. “For the first time in decades, we’re showing that this class of medications, the SGLT2 inhibitors, substantially reduce risk of loss of kidney function.”
Expressed in the lumen of the proximal renal tubules, SGLT2 reabsorbs filtered glucose from the tubular lumen. Inhibition of SGLT2 promotes urinary glucose excretion and reduces sodium reabsorption, increasing delivery of sodium to the distal tubule. The first SGLT2 inhibitor, canagliflozin, was approved in 2013 for use as an antihyperglycemic agent but subsequently was shown to have serendipitous benefits for the heart and kidneys.
Clinical trials have documented reductions in the risk for cardiovascular events in patients with type 2 diabetes, as well as decreases in the risk for progression to end-stage renal disease, cardiovascular mortality, and hospitalization for heart failure. Updated international guidelines from 2022 recommend treating all patients with type 2 diabetes and CKD with an estimated GFR ≥ 20 mL/min/1.73 m2 with an SGLT2 inhibitor.
But several trials of SGLT2 inhibitors also demonstrated benefits in reducing the risk for cardiovascular-related death or hospitalization for heart failure, even in patients without diabetes. Although initial approval from the US Food and Drug Administration was limited to patients with diabetes and heart failure, the agency has recently expanded its indications to include adults with CKD who do not have diabetes.
Dr. Bansal said she was happy to see this widening of the indications, which makes more patients eligible to receive SGLT2 inhibitors. “I really think this early CKD group is a great group to consider for those medications,” she said.
Dr. Bansal also pointed out that MRAs are another class of drugs with an interesting history. Earlier steroidal MRAs were found to have anti-inflammatory and antifibrotic properties, and in 1960 spironolactone was approved for use as a diuretic for the management of edema, primary aldosteronism, and hypertension. But even as their use in cardiology rose, MRAs had less utility for CKD, given adverse events such as hyperkalemia and hormonal effects like gynecomastia.
But the latest generation of nonsteroidal MRAs (nsMRAs) has higher selectivity for the mineralocorticoid receptor than sex-steroid hormone receptors, reducing androgenic side effects and preventing elevated potassium. Finerenone, the only nsMRA approved in the United States, has been shown in clinical trials to reduce the incidence of cardiovascular events (death from cardiovascular causes, nonfatal myocardial infarction, nonfatal stroke, or hospitalization for heart failure) and CKD outcomes, including kidney failure, decrease in estimated GFR, or death from renal causes.
EPIC Changes Coming?
In light of treatment advances that offer hope of preventing progression of CKD in patients identified early, both the National Kidney Foundation and the American Kidney Fund lobbied the USPSTF in 2022 to conduct a fresh review of recent data to evaluate the need for updated screening recommendations.
The task force completed development of a research plan and collection of public comments in early 2023 and is now reviewing evidence before developing a draft recommendation.
A team of health policy researchers from Stanford is hoping that some of their recently published work will attract the panel’s attention. The first study, published in 2022, evaluated the cost-effectiveness of dapagliflozin, an SGLT2 inhibitor that has been shown to reduce mortality by 48% in CKD patients without diabetes.
The Stanford team found that adding dapagliflozin to standard care for these patients improved life expectancy by 2 years and reduced the percentage of those who needed dialysis or kidney transplant from 17% to 11%.
More recently, Marika Cusick, a doctoral candidate in health policy at the Stanford School of Medicine in Stanford, California, served as first author of an evaluation of the cost-effectiveness of screening asymptomatic adults. “We assessed screening for albuminuria in conjunction with conventional CKD therapy in addition to this new SGLT2 inhibitor class of drugs,” she said. They projected how this might change CKD progression in US adults who are aged 35 or older compared with standard therapy alone.
The findings were favorable. “A one-time screening would result in a reduction of 398,000 cases of kidney replacement therapy [defined as needing either dialysis or renal transplant] among 158 million US adults who are currently aged 35-75 years,” Ms. Cusick told this news organization.
In terms of quality-adjusted life years (QALYs), a one-time screening at age 55 years yielded an incremental cost-effectiveness ratio of $86,300 per QALY. Screening every 10 years between the ages of 35 and 75 years cost less than $100,000 per QALY gained.
According to Doug Owens, MD, professor and chair of the department of health policy at Stanford School of Medicine, “There’s a societal decision about how much are we willing to pay for additional length and quality of life. And this fits within what is generally considered reasonable for the US.”
For example, in the United States, screening for breast cancer among women aged 40-64 years costs $51,000 per QALY, whereas screening for lung cancer using USPSTF guidelines ranges from $72,639 to $156,774 per QALY.
A former member of the USPSTF, Owens predicted that the current review process would take at least another year. Meanwhile, he and Ms. Cusick are hoping that their work influences the USPSTF to recommend screening asymptomatic adults. “Increasing the awareness of these drugs and their effectiveness is a crucial first step,” he said.
Although adherence to current recommendations for screening of people at risk is poor, Dr. Rosas suggested that the USPSTF guidelines would be more influential in changing practice among primary care physicians than subspecialty guidelines would.
“When you have a recommendation like that, they’re putting it in the electronic health record,” she said. By adding best practice alerts to their electronic health record systems, health systems can make it easier for primary care doctors to check all the boxes.
In line with the AHA’s holistic approach towards managing cardiovascular illnesses, CKD, and metabolic disease, Dr. Bansal suggested an additional strategy: “I think we’re moving toward more interdisciplinary care models, where primary care doctors, nephrologist, cardiologists, and endocrinologists — all of us — should be working together in a collaborative care model, to help break down some of these barriers in terms of screening as well as implementation of these therapies.”
Dr. Bansal, Ms. Cusick, and Dr. Owens reported no financial conflicts of interest. Dr. Rosas receives funding from AstraZeneca and Bayer for serving on advisory boards and clinical research funding, as well as funding from the National Institute of Diabetes and Digestive and Kidney Diseases for clinical trials.
Dr. Thomas is a pediatrician and epidemiologist living in Portland, Oregon.
A version of this article appeared on Medscape.com.
Tips and Techniques to Boost Colonoscopy Quality
This transcript has been edited for clarity.
When it comes to the use of colonoscopy to reduce the risk for cancer, quality is key.
There are a number of performance improvements we can make in our practices so that we can do better. This is evident in several recently published studies and a recent review article on the topic, which I’d like to profile for you; many of these key quality indicators you can implement now.
Even though it may take more time before they’re supported in the guidelines, you’ll see that the evidence behind these is extraordinarily strong.
Increasing the Adenoma Detection Rate
Certainly, we all do what we can to increase the adenoma detection rate (ADR).
However, at the moment, the nationally recommended benchmark is to achieve an ADR of 25%, which is inordinately low. The ADR rate reported in the GIQuIC registry data is closer to 39%, and in high-level detectors, it’s actually in the greater-than-50% range.
There’s no question that we can do more, and there are a number of ways to do that.
First, This may actually decrease your withdrawal time because you don’t spend so much time trying to face these folds.
In considering tools to aid ADR, don’t forget electronic chromoendoscopy (eg, narrow-band imaging).
There are a number of new artificial intelligence options out there as well, which have been reported to increase the ADR by approximately 10%. Of importance, this improvement even occurs among expert endoscopists.
There’s also important emerging data about ADR in fecal immunochemical test (FIT)–positive patients. FIT-positive status increases the ADR threshold by 15%-20%. This places you in an ADR range of approximately 50%, which is really the norm when screening patients that present for colonoscopy because of FIT positivity.
Adenoma Per Colonoscopy: A Possible ADR Substitute
Growing evidence supports the use of adenoma per colonoscopy (APC) as a substitute to ADR. This would allow you to record every adenoma and attribute it to that index colonoscopy.
A high-quality paper showed that the APC value should be around 0.6 to achieve the current ADR minimum threshold of 25%. Having the APC < 0.6 seems to be associated with an increased risk for residual polyp. Sessile serrated lesions also increased the hazard ratio for interval colorectal cancer. This was evaluated recently with data from the New Hampshire Colonoscopy Registry, which Dr Joseph Anderson has led for so long. They showed that 21% of endoscopists had an ADR of 25% or greater but still had APCs < 0.6.
Therefore, when it comes to remedial corrective work, doctors need to be reevaluated, retrained, and educated in the ways that they can incorporate this. The APC in high-level detectors is > 1.0.
APC may be something you want to consider using internally. It does require that you place each polyp into an individual jar, which can increase incremental cost. Nonetheless, there is clear evidence that APC positively changes outcomes.
Including Sessile Serrated Lesions in ADR Detectors
Unfortunately, some of the high-level ADR detectors aren’t so “high level” when it comes to detecting sessile serrated lesions. It’s not quite as concordant as we had previously thought.
Nonetheless, there are many adjunctive things you can do with sessile serrated lesions, including narrow-band imaging and chromoendoscopy.
When it comes to establishing a discriminant, the numbers should be 5%-6% if we’re going to set a quality ratio and an index. However, this is somewhat dependent on your pathologist because they have to read these correctly. Lesions that are ≥ 6 mm above the sigmoid colon and anything in the right colon should be evaluated really closely as a sessile serrated lesion.
I’ve had indications where the pathologist says the lesion is hyperplastic, to which I say, “I’m going to follow as a sessile serrated lesion.” This is because it’s apparent to me in the endoscopic appearance and the narrow-band imaging appearance that it was characteristic of sessile serrated lesions.
Best Practices in Bowel Preparations
The US Multi-Society Task Force recommends that adequate bowel preparation should occur in 85% or more of outpatients, and for the European Society for Gastrointestinal Endoscopy, it’s 90% or more.
I’ll pass along a tip I use in my patients undergoing bowel preparation: I make them aware that during this process, they want to see a clear, yellow, urine-like color to their stool. Otherwise, many patients will think if they’ve had some diarrhea, they don’t need to finish prep. Setting that expectation for them upfront is really important.
The nurses also should be aware of this because if there’s a murky brown effluent upon presentation for the colonoscopy, there’s a greater than 50% chance that they’re going to have had an inadequate preparation. In such cases, you would want to preempt the colonoscopy and perhaps send them out for a re-prep that day or bring them back for a rescheduled appointment.
Resection Considerations
There is substantial variation when it comes to lesion resection, which makes it an important quality indicator on which to focus: High-level detectors aren’t always high-level resectors.
There are two validated instruments that you can use to gauge the adequacy of resection. Those aren’t really ready for prime time in every practice, though they may be seen in fellowship programs.
The idea here is that you want to get a ≥ 2 mm margin for cold snare polypectomy in lesions 1-10 mm in size. This has been a challenge, as findings indicate we don’t do this that well.
Joseph Anderson and colleagues recently published a study using a 2-mm resection margin. They reported that this was only possible in approximately 28% of polyps. For a 1-mm margin, the rate was 84%.
We simply need to set clearer margins when setting our snare. Make sure you’re close enough to the polyp, push down on the snare, and get a good margin of tissue.
When the sample contracts are placed into the formalin, it’s not quite so simple to define the margin at the time of the surgical resection. This often requires an audit evaluation by the pathologist.
There are two other considerations regarding resection.
The first is about the referral for surgery. Referral should not occur for any benign lesions ascribed by your endoscopic advanced imaging techniques and classifications that are not thought to have intramucosal carcinoma. These should be referred to an expert endoscopic evaluation. If you can’t do it, then somebody else should. And you shouldn’t attempt it unless you can get it totally because resection of partially resected lesions is much more complicated. The European Society of Gastrointestinal Endoscopy says this applies to any benign lesion of any size, which I think really is the emerging standard of care. You should consider and offer that to the patient. It may require a referral for outside of your institution.
The second additional consideration is around the minimization of cold forceps for removal of polyps. The US Multi-Society Task Force says cold forceps shouldn’t be used for any lesions > 2 mm, whereas for the European Society of Gastrointestinal Endoscopy, it is > 3 mm. However, it’s still done very commonly in clinical practice. Nibbling the polyp is not an option. Cold snare is actually quicker, more effective, has better outcomes, and is something that you can bill for when you look at the coding.
In summary, there are a lot of things that we can do now to improve colonoscopy. Quality indicators continue to emerge with a compelling, excellent evidence base that strongly supports their use. Given that, I think most of these are actionable now, and it’s not necessary to wait for the guidelines to begin using them. I’d therefore challenge all of us to incorporate them in our continual efforts to do better.
Dr. Johnson is professor of medicine and chief of gastroenterology at Eastern Virginia Medical School in Norfolk, and a past president of the American College of Gastroenterology. His primary focus is the clinical practice of gastroenterology. He has published extensively in the internal medicine/gastroenterology literature, with principal research interests in esophageal and colon disease, and more recently in sleep and microbiome effects on gastrointestinal health and disease. He has disclosed ties with ISOTHRIVE and Johnson & Johnson.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
When it comes to the use of colonoscopy to reduce the risk for cancer, quality is key.
There are a number of performance improvements we can make in our practices so that we can do better. This is evident in several recently published studies and a recent review article on the topic, which I’d like to profile for you; many of these key quality indicators you can implement now.
Even though it may take more time before they’re supported in the guidelines, you’ll see that the evidence behind these is extraordinarily strong.
Increasing the Adenoma Detection Rate
Certainly, we all do what we can to increase the adenoma detection rate (ADR).
However, at the moment, the nationally recommended benchmark is to achieve an ADR of 25%, which is inordinately low. The ADR rate reported in the GIQuIC registry data is closer to 39%, and in high-level detectors, it’s actually in the greater-than-50% range.
There’s no question that we can do more, and there are a number of ways to do that.
First, This may actually decrease your withdrawal time because you don’t spend so much time trying to face these folds.
In considering tools to aid ADR, don’t forget electronic chromoendoscopy (eg, narrow-band imaging).
There are a number of new artificial intelligence options out there as well, which have been reported to increase the ADR by approximately 10%. Of importance, this improvement even occurs among expert endoscopists.
There’s also important emerging data about ADR in fecal immunochemical test (FIT)–positive patients. FIT-positive status increases the ADR threshold by 15%-20%. This places you in an ADR range of approximately 50%, which is really the norm when screening patients that present for colonoscopy because of FIT positivity.
Adenoma Per Colonoscopy: A Possible ADR Substitute
Growing evidence supports the use of adenoma per colonoscopy (APC) as a substitute to ADR. This would allow you to record every adenoma and attribute it to that index colonoscopy.
A high-quality paper showed that the APC value should be around 0.6 to achieve the current ADR minimum threshold of 25%. Having the APC < 0.6 seems to be associated with an increased risk for residual polyp. Sessile serrated lesions also increased the hazard ratio for interval colorectal cancer. This was evaluated recently with data from the New Hampshire Colonoscopy Registry, which Dr Joseph Anderson has led for so long. They showed that 21% of endoscopists had an ADR of 25% or greater but still had APCs < 0.6.
Therefore, when it comes to remedial corrective work, doctors need to be reevaluated, retrained, and educated in the ways that they can incorporate this. The APC in high-level detectors is > 1.0.
APC may be something you want to consider using internally. It does require that you place each polyp into an individual jar, which can increase incremental cost. Nonetheless, there is clear evidence that APC positively changes outcomes.
Including Sessile Serrated Lesions in ADR Detectors
Unfortunately, some of the high-level ADR detectors aren’t so “high level” when it comes to detecting sessile serrated lesions. It’s not quite as concordant as we had previously thought.
Nonetheless, there are many adjunctive things you can do with sessile serrated lesions, including narrow-band imaging and chromoendoscopy.
When it comes to establishing a discriminant, the numbers should be 5%-6% if we’re going to set a quality ratio and an index. However, this is somewhat dependent on your pathologist because they have to read these correctly. Lesions that are ≥ 6 mm above the sigmoid colon and anything in the right colon should be evaluated really closely as a sessile serrated lesion.
I’ve had indications where the pathologist says the lesion is hyperplastic, to which I say, “I’m going to follow as a sessile serrated lesion.” This is because it’s apparent to me in the endoscopic appearance and the narrow-band imaging appearance that it was characteristic of sessile serrated lesions.
Best Practices in Bowel Preparations
The US Multi-Society Task Force recommends that adequate bowel preparation should occur in 85% or more of outpatients, and for the European Society for Gastrointestinal Endoscopy, it’s 90% or more.
I’ll pass along a tip I use in my patients undergoing bowel preparation: I make them aware that during this process, they want to see a clear, yellow, urine-like color to their stool. Otherwise, many patients will think if they’ve had some diarrhea, they don’t need to finish prep. Setting that expectation for them upfront is really important.
The nurses also should be aware of this because if there’s a murky brown effluent upon presentation for the colonoscopy, there’s a greater than 50% chance that they’re going to have had an inadequate preparation. In such cases, you would want to preempt the colonoscopy and perhaps send them out for a re-prep that day or bring them back for a rescheduled appointment.
Resection Considerations
There is substantial variation when it comes to lesion resection, which makes it an important quality indicator on which to focus: High-level detectors aren’t always high-level resectors.
There are two validated instruments that you can use to gauge the adequacy of resection. Those aren’t really ready for prime time in every practice, though they may be seen in fellowship programs.
The idea here is that you want to get a ≥ 2 mm margin for cold snare polypectomy in lesions 1-10 mm in size. This has been a challenge, as findings indicate we don’t do this that well.
Joseph Anderson and colleagues recently published a study using a 2-mm resection margin. They reported that this was only possible in approximately 28% of polyps. For a 1-mm margin, the rate was 84%.
We simply need to set clearer margins when setting our snare. Make sure you’re close enough to the polyp, push down on the snare, and get a good margin of tissue.
When the sample contracts are placed into the formalin, it’s not quite so simple to define the margin at the time of the surgical resection. This often requires an audit evaluation by the pathologist.
There are two other considerations regarding resection.
The first is about the referral for surgery. Referral should not occur for any benign lesions ascribed by your endoscopic advanced imaging techniques and classifications that are not thought to have intramucosal carcinoma. These should be referred to an expert endoscopic evaluation. If you can’t do it, then somebody else should. And you shouldn’t attempt it unless you can get it totally because resection of partially resected lesions is much more complicated. The European Society of Gastrointestinal Endoscopy says this applies to any benign lesion of any size, which I think really is the emerging standard of care. You should consider and offer that to the patient. It may require a referral for outside of your institution.
The second additional consideration is around the minimization of cold forceps for removal of polyps. The US Multi-Society Task Force says cold forceps shouldn’t be used for any lesions > 2 mm, whereas for the European Society of Gastrointestinal Endoscopy, it is > 3 mm. However, it’s still done very commonly in clinical practice. Nibbling the polyp is not an option. Cold snare is actually quicker, more effective, has better outcomes, and is something that you can bill for when you look at the coding.
In summary, there are a lot of things that we can do now to improve colonoscopy. Quality indicators continue to emerge with a compelling, excellent evidence base that strongly supports their use. Given that, I think most of these are actionable now, and it’s not necessary to wait for the guidelines to begin using them. I’d therefore challenge all of us to incorporate them in our continual efforts to do better.
Dr. Johnson is professor of medicine and chief of gastroenterology at Eastern Virginia Medical School in Norfolk, and a past president of the American College of Gastroenterology. His primary focus is the clinical practice of gastroenterology. He has published extensively in the internal medicine/gastroenterology literature, with principal research interests in esophageal and colon disease, and more recently in sleep and microbiome effects on gastrointestinal health and disease. He has disclosed ties with ISOTHRIVE and Johnson & Johnson.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
When it comes to the use of colonoscopy to reduce the risk for cancer, quality is key.
There are a number of performance improvements we can make in our practices so that we can do better. This is evident in several recently published studies and a recent review article on the topic, which I’d like to profile for you; many of these key quality indicators you can implement now.
Even though it may take more time before they’re supported in the guidelines, you’ll see that the evidence behind these is extraordinarily strong.
Increasing the Adenoma Detection Rate
Certainly, we all do what we can to increase the adenoma detection rate (ADR).
However, at the moment, the nationally recommended benchmark is to achieve an ADR of 25%, which is inordinately low. The ADR rate reported in the GIQuIC registry data is closer to 39%, and in high-level detectors, it’s actually in the greater-than-50% range.
There’s no question that we can do more, and there are a number of ways to do that.
First, This may actually decrease your withdrawal time because you don’t spend so much time trying to face these folds.
In considering tools to aid ADR, don’t forget electronic chromoendoscopy (eg, narrow-band imaging).
There are a number of new artificial intelligence options out there as well, which have been reported to increase the ADR by approximately 10%. Of importance, this improvement even occurs among expert endoscopists.
There’s also important emerging data about ADR in fecal immunochemical test (FIT)–positive patients. FIT-positive status increases the ADR threshold by 15%-20%. This places you in an ADR range of approximately 50%, which is really the norm when screening patients that present for colonoscopy because of FIT positivity.
Adenoma Per Colonoscopy: A Possible ADR Substitute
Growing evidence supports the use of adenoma per colonoscopy (APC) as a substitute to ADR. This would allow you to record every adenoma and attribute it to that index colonoscopy.
A high-quality paper showed that the APC value should be around 0.6 to achieve the current ADR minimum threshold of 25%. Having the APC < 0.6 seems to be associated with an increased risk for residual polyp. Sessile serrated lesions also increased the hazard ratio for interval colorectal cancer. This was evaluated recently with data from the New Hampshire Colonoscopy Registry, which Dr Joseph Anderson has led for so long. They showed that 21% of endoscopists had an ADR of 25% or greater but still had APCs < 0.6.
Therefore, when it comes to remedial corrective work, doctors need to be reevaluated, retrained, and educated in the ways that they can incorporate this. The APC in high-level detectors is > 1.0.
APC may be something you want to consider using internally. It does require that you place each polyp into an individual jar, which can increase incremental cost. Nonetheless, there is clear evidence that APC positively changes outcomes.
Including Sessile Serrated Lesions in ADR Detectors
Unfortunately, some of the high-level ADR detectors aren’t so “high level” when it comes to detecting sessile serrated lesions. It’s not quite as concordant as we had previously thought.
Nonetheless, there are many adjunctive things you can do with sessile serrated lesions, including narrow-band imaging and chromoendoscopy.
When it comes to establishing a discriminant, the numbers should be 5%-6% if we’re going to set a quality ratio and an index. However, this is somewhat dependent on your pathologist because they have to read these correctly. Lesions that are ≥ 6 mm above the sigmoid colon and anything in the right colon should be evaluated really closely as a sessile serrated lesion.
I’ve had indications where the pathologist says the lesion is hyperplastic, to which I say, “I’m going to follow as a sessile serrated lesion.” This is because it’s apparent to me in the endoscopic appearance and the narrow-band imaging appearance that it was characteristic of sessile serrated lesions.
Best Practices in Bowel Preparations
The US Multi-Society Task Force recommends that adequate bowel preparation should occur in 85% or more of outpatients, and for the European Society for Gastrointestinal Endoscopy, it’s 90% or more.
I’ll pass along a tip I use in my patients undergoing bowel preparation: I make them aware that during this process, they want to see a clear, yellow, urine-like color to their stool. Otherwise, many patients will think if they’ve had some diarrhea, they don’t need to finish prep. Setting that expectation for them upfront is really important.
The nurses also should be aware of this because if there’s a murky brown effluent upon presentation for the colonoscopy, there’s a greater than 50% chance that they’re going to have had an inadequate preparation. In such cases, you would want to preempt the colonoscopy and perhaps send them out for a re-prep that day or bring them back for a rescheduled appointment.
Resection Considerations
There is substantial variation when it comes to lesion resection, which makes it an important quality indicator on which to focus: High-level detectors aren’t always high-level resectors.
There are two validated instruments that you can use to gauge the adequacy of resection. Those aren’t really ready for prime time in every practice, though they may be seen in fellowship programs.
The idea here is that you want to get a ≥ 2 mm margin for cold snare polypectomy in lesions 1-10 mm in size. This has been a challenge, as findings indicate we don’t do this that well.
Joseph Anderson and colleagues recently published a study using a 2-mm resection margin. They reported that this was only possible in approximately 28% of polyps. For a 1-mm margin, the rate was 84%.
We simply need to set clearer margins when setting our snare. Make sure you’re close enough to the polyp, push down on the snare, and get a good margin of tissue.
When the sample contracts are placed into the formalin, it’s not quite so simple to define the margin at the time of the surgical resection. This often requires an audit evaluation by the pathologist.
There are two other considerations regarding resection.
The first is about the referral for surgery. Referral should not occur for any benign lesions ascribed by your endoscopic advanced imaging techniques and classifications that are not thought to have intramucosal carcinoma. These should be referred to an expert endoscopic evaluation. If you can’t do it, then somebody else should. And you shouldn’t attempt it unless you can get it totally because resection of partially resected lesions is much more complicated. The European Society of Gastrointestinal Endoscopy says this applies to any benign lesion of any size, which I think really is the emerging standard of care. You should consider and offer that to the patient. It may require a referral for outside of your institution.
The second additional consideration is around the minimization of cold forceps for removal of polyps. The US Multi-Society Task Force says cold forceps shouldn’t be used for any lesions > 2 mm, whereas for the European Society of Gastrointestinal Endoscopy, it is > 3 mm. However, it’s still done very commonly in clinical practice. Nibbling the polyp is not an option. Cold snare is actually quicker, more effective, has better outcomes, and is something that you can bill for when you look at the coding.
In summary, there are a lot of things that we can do now to improve colonoscopy. Quality indicators continue to emerge with a compelling, excellent evidence base that strongly supports their use. Given that, I think most of these are actionable now, and it’s not necessary to wait for the guidelines to begin using them. I’d therefore challenge all of us to incorporate them in our continual efforts to do better.
Dr. Johnson is professor of medicine and chief of gastroenterology at Eastern Virginia Medical School in Norfolk, and a past president of the American College of Gastroenterology. His primary focus is the clinical practice of gastroenterology. He has published extensively in the internal medicine/gastroenterology literature, with principal research interests in esophageal and colon disease, and more recently in sleep and microbiome effects on gastrointestinal health and disease. He has disclosed ties with ISOTHRIVE and Johnson & Johnson.
A version of this article appeared on Medscape.com.
Superficial Vascular Anomaly of the Glabella Mimicking a Cutaneous Cyst
To the Editor:
Cutaneous cysts commonly are treated by dermatologists and typically are diagnosed clinically, followed by intraoperative or histologic confirmation; however, cyst mimickers can be misdiagnosed due to similar appearance and limited diagnostic guidelines.1 Vascular anomalies (VAs) of the face such as a facial aneurysm are rare.2 Preoperative assessment of findings suggestive of vascular etiology vs other common cutaneous tumors such as an epidermal inclusion cyst (EIC) and lipoma can help guide dermatologic management. We present a case of a VA of the glabella manifesting as a flesh-colored nodule that clinically mimicked a cyst and discuss the subsequent surgical management.
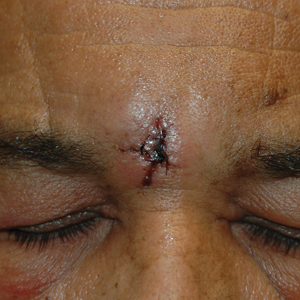
A 61-year-old man with a history of benign prostatic hyperplasia was evaluated at our dermatology clinic for an enlarging forehead mass of 1 year’s duration. Physical examination yielded a soft, flesh-colored, 2.5-cm nodule located superficially in the midline glabellar region without pulsation or palpable thrill. The differential diagnosis at the time included lipoma or EIC.
Excision of the lesion was performed utilizing superficial incisions with a descending depth of 1-mm increments to safely reach the target, identify the type of tumor, and prevent rupture of the suspected EIC. After the third incision to the level of the dermis, nonpulsatile bleeding was more than expected for a cyst. Digital pressure was applied, and the area was explored with blunt dissection to identify the source of bleeding. A fusiform, thin-walled aneurysm was identified in the dermal plane with additional tributaries coursing deep into the subcutaneous plane. The visualized tributaries were ligated with 3-0 polyglactin, figure-of-eight sutures resulting in hemostasis. The wound was closed with 5-0 nylon simple interrupted sutures. The patient was closely followed postoperatively for 1 week (Figure) and was referred for head imaging to evaluate for a possible associated intracranial aneurysm. Based on the thin vessel wall and continuous nonpulsatile hemorrhage, this VA was most consistent with venous aneurysm.
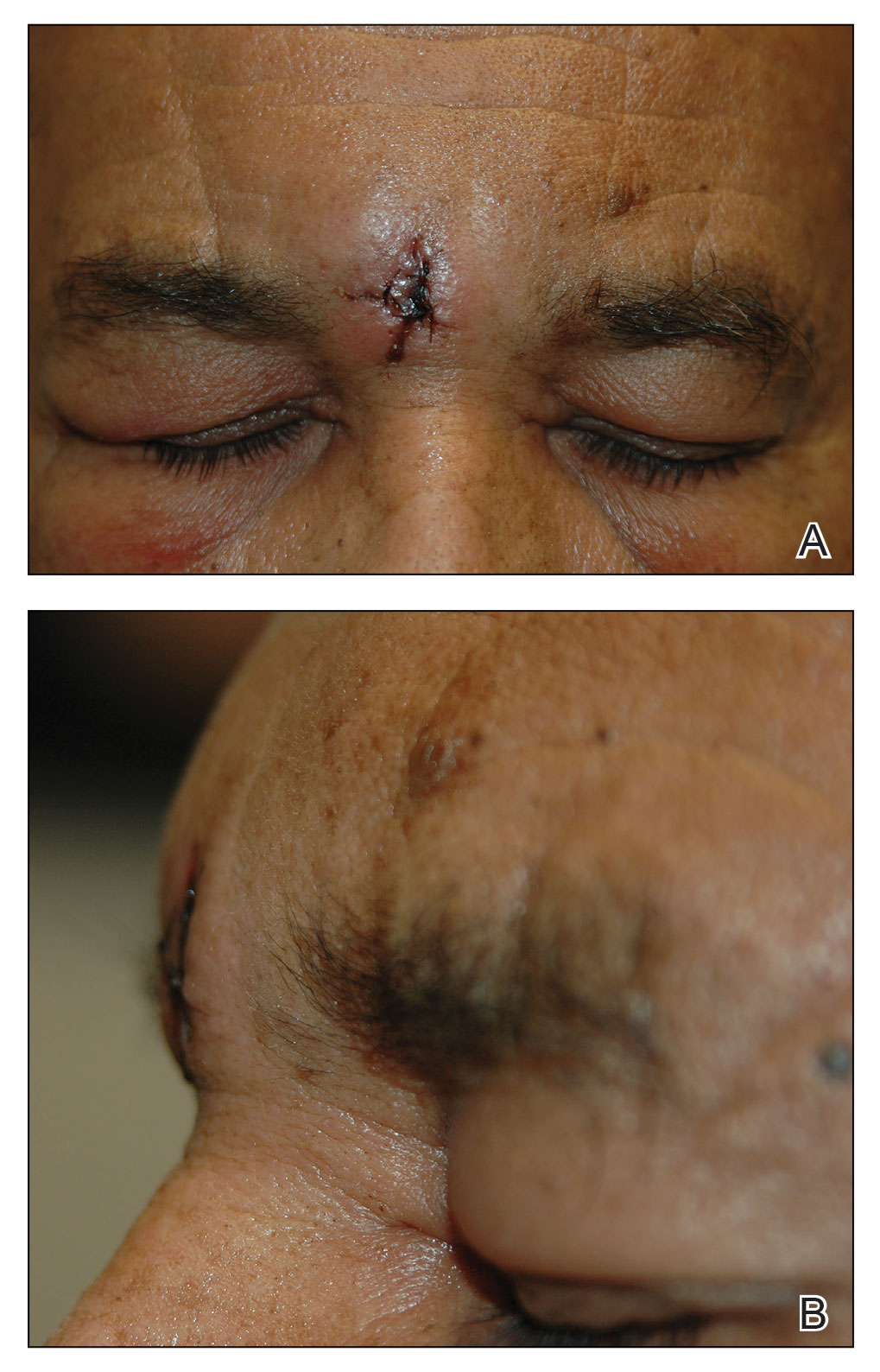
A VA can be encountered unexpectedly during dermatologic surgery. An aneurysm is a type of VA and is defined as an abnormal dilatation of a blood vessel that can be arterial, venous, or an arteriovenous malformation. Most reported aneurysms of the head and neck are cirsoid aneurysms or involve the superficial temporal artery.2,3 Reports of superficial venous aneurysms are rare.4 Preoperatively, cutaneous nodules can be evaluated for findings suggestive of a VA in the dermatologist’s office through physical examination. Arterial aneurysms may reveal palpable pulsation and audible bruit, while a venous aneurysm may exhibit a blue color, a size reduction with compression, and variable size with Valsalva maneuver.
The gold standard diagnostic tool for most dermatologic conditions is histopathology; however, dermatologic ultrasonography can provide noninvasive, real-time, important diagnostic characteristics of cutaneous pathologies as well as VA.5-7 Crisan et al6 outlined specific sonographic findings of lipomas, EICs, trichilemmal cysts, and other dermatologic conditions as well as the associated surgical pertinence. Ultrasonography of a venous aneurysm may show a heterogeneous, contiguous, echoic lesion with an adjacent superficial vein, which may be easily compressed by the probe.8 Advanced imaging such as computed tomography with contrast or magnetic resonance imaging may be performed, but these are more costly than ultrasonography. Additionally, point-of-care ultrasonography is becoming more popular and accessible for physicians to carry at bedside with portable tablet options available. Dermatologists may want to consider incorporating it into the outpatient setting to improve procedural planning.9
In conclusion, VAs should be included in the differential diagnosis of soft cutaneous nodules, as management differs from a cyst or lipoma. Dermatologists should use their clinical judgment preoperatively—including a comprehensive history, physical examination, and consideration of color Doppler ultrasonography to assess for findings of VA. We do not recommend intentional surgical exploration of cutaneous aneurysms in the ambulatory setting due to risk for hemorrhage. Furthermore, when clinical suspicion of EIC or lipoma is high, it still is preferable to descend the incision slowly at 1 to 2 mm per cut until the tumor is visualized.
- Ring CM, Kornreich DA, Lee JB. Clinical simulators of cysts. J Am Acad Dermatol. 2016;75:1255-1257.
- Evans CC, Larson MJ, Eichhorn PJ, et al. Traumatic pseudoaneurysm of the superficial temporal artery: two cases and review of the literature. J Am Acad Dermatol. 2003;49(5 suppl):S286-S288.
- Sofela A, Osunronbi T, Hettige S. Scalp cirsoid aneurysms: case illustration and systematic review of literature. Neurosurgery. 2020;86:E98-E107.
- McKesey J, Cohen PR. Spontaneous venous aneurysm: report of a non-traumatic superficial venous aneurysm on the distal arm. Cureus. 2018;10:E2641.
- Wortsman X, Alfageme F, Roustan G, et al. Guidelines for performing dermatologic ultrasound examinations by the DERMUS Group. J Ultrasound Med. 2016;35:577-580.
- Crisan D, Wortsman X, Alfageme F, et al. Ultrasonography in dermatologic surgery: revealing the unseen for improved surgical planning [published online May 26, 2022]. J Dtsch Dermatol Ges. doi:10.1111/ddg.14781
- Corvino A, Catalano O, Corvino F, et al. Superficial temporal artery pseudoaneurysm: what is the role of ultrasound. J Ultrasound. 2016;19:197-201.
- Lee HY, Lee W, Cho YK, et al. Superficial venous aneurysm: reports of 3 cases and literature review. J Ultrasound Med. 2006;25:771-776.
- Hadian Y, Link D, Dahle SE, et al. Ultrasound as a diagnostic and interventional aid at point-of-care in dermatology clinic: a case report. J Dermatolog Treat. 2020;31:74-76.
To the Editor:
Cutaneous cysts commonly are treated by dermatologists and typically are diagnosed clinically, followed by intraoperative or histologic confirmation; however, cyst mimickers can be misdiagnosed due to similar appearance and limited diagnostic guidelines.1 Vascular anomalies (VAs) of the face such as a facial aneurysm are rare.2 Preoperative assessment of findings suggestive of vascular etiology vs other common cutaneous tumors such as an epidermal inclusion cyst (EIC) and lipoma can help guide dermatologic management. We present a case of a VA of the glabella manifesting as a flesh-colored nodule that clinically mimicked a cyst and discuss the subsequent surgical management.
A 61-year-old man with a history of benign prostatic hyperplasia was evaluated at our dermatology clinic for an enlarging forehead mass of 1 year’s duration. Physical examination yielded a soft, flesh-colored, 2.5-cm nodule located superficially in the midline glabellar region without pulsation or palpable thrill. The differential diagnosis at the time included lipoma or EIC.
Excision of the lesion was performed utilizing superficial incisions with a descending depth of 1-mm increments to safely reach the target, identify the type of tumor, and prevent rupture of the suspected EIC. After the third incision to the level of the dermis, nonpulsatile bleeding was more than expected for a cyst. Digital pressure was applied, and the area was explored with blunt dissection to identify the source of bleeding. A fusiform, thin-walled aneurysm was identified in the dermal plane with additional tributaries coursing deep into the subcutaneous plane. The visualized tributaries were ligated with 3-0 polyglactin, figure-of-eight sutures resulting in hemostasis. The wound was closed with 5-0 nylon simple interrupted sutures. The patient was closely followed postoperatively for 1 week (Figure) and was referred for head imaging to evaluate for a possible associated intracranial aneurysm. Based on the thin vessel wall and continuous nonpulsatile hemorrhage, this VA was most consistent with venous aneurysm.

A VA can be encountered unexpectedly during dermatologic surgery. An aneurysm is a type of VA and is defined as an abnormal dilatation of a blood vessel that can be arterial, venous, or an arteriovenous malformation. Most reported aneurysms of the head and neck are cirsoid aneurysms or involve the superficial temporal artery.2,3 Reports of superficial venous aneurysms are rare.4 Preoperatively, cutaneous nodules can be evaluated for findings suggestive of a VA in the dermatologist’s office through physical examination. Arterial aneurysms may reveal palpable pulsation and audible bruit, while a venous aneurysm may exhibit a blue color, a size reduction with compression, and variable size with Valsalva maneuver.
The gold standard diagnostic tool for most dermatologic conditions is histopathology; however, dermatologic ultrasonography can provide noninvasive, real-time, important diagnostic characteristics of cutaneous pathologies as well as VA.5-7 Crisan et al6 outlined specific sonographic findings of lipomas, EICs, trichilemmal cysts, and other dermatologic conditions as well as the associated surgical pertinence. Ultrasonography of a venous aneurysm may show a heterogeneous, contiguous, echoic lesion with an adjacent superficial vein, which may be easily compressed by the probe.8 Advanced imaging such as computed tomography with contrast or magnetic resonance imaging may be performed, but these are more costly than ultrasonography. Additionally, point-of-care ultrasonography is becoming more popular and accessible for physicians to carry at bedside with portable tablet options available. Dermatologists may want to consider incorporating it into the outpatient setting to improve procedural planning.9
In conclusion, VAs should be included in the differential diagnosis of soft cutaneous nodules, as management differs from a cyst or lipoma. Dermatologists should use their clinical judgment preoperatively—including a comprehensive history, physical examination, and consideration of color Doppler ultrasonography to assess for findings of VA. We do not recommend intentional surgical exploration of cutaneous aneurysms in the ambulatory setting due to risk for hemorrhage. Furthermore, when clinical suspicion of EIC or lipoma is high, it still is preferable to descend the incision slowly at 1 to 2 mm per cut until the tumor is visualized.
To the Editor:
Cutaneous cysts commonly are treated by dermatologists and typically are diagnosed clinically, followed by intraoperative or histologic confirmation; however, cyst mimickers can be misdiagnosed due to similar appearance and limited diagnostic guidelines.1 Vascular anomalies (VAs) of the face such as a facial aneurysm are rare.2 Preoperative assessment of findings suggestive of vascular etiology vs other common cutaneous tumors such as an epidermal inclusion cyst (EIC) and lipoma can help guide dermatologic management. We present a case of a VA of the glabella manifesting as a flesh-colored nodule that clinically mimicked a cyst and discuss the subsequent surgical management.
A 61-year-old man with a history of benign prostatic hyperplasia was evaluated at our dermatology clinic for an enlarging forehead mass of 1 year’s duration. Physical examination yielded a soft, flesh-colored, 2.5-cm nodule located superficially in the midline glabellar region without pulsation or palpable thrill. The differential diagnosis at the time included lipoma or EIC.
Excision of the lesion was performed utilizing superficial incisions with a descending depth of 1-mm increments to safely reach the target, identify the type of tumor, and prevent rupture of the suspected EIC. After the third incision to the level of the dermis, nonpulsatile bleeding was more than expected for a cyst. Digital pressure was applied, and the area was explored with blunt dissection to identify the source of bleeding. A fusiform, thin-walled aneurysm was identified in the dermal plane with additional tributaries coursing deep into the subcutaneous plane. The visualized tributaries were ligated with 3-0 polyglactin, figure-of-eight sutures resulting in hemostasis. The wound was closed with 5-0 nylon simple interrupted sutures. The patient was closely followed postoperatively for 1 week (Figure) and was referred for head imaging to evaluate for a possible associated intracranial aneurysm. Based on the thin vessel wall and continuous nonpulsatile hemorrhage, this VA was most consistent with venous aneurysm.

A VA can be encountered unexpectedly during dermatologic surgery. An aneurysm is a type of VA and is defined as an abnormal dilatation of a blood vessel that can be arterial, venous, or an arteriovenous malformation. Most reported aneurysms of the head and neck are cirsoid aneurysms or involve the superficial temporal artery.2,3 Reports of superficial venous aneurysms are rare.4 Preoperatively, cutaneous nodules can be evaluated for findings suggestive of a VA in the dermatologist’s office through physical examination. Arterial aneurysms may reveal palpable pulsation and audible bruit, while a venous aneurysm may exhibit a blue color, a size reduction with compression, and variable size with Valsalva maneuver.
The gold standard diagnostic tool for most dermatologic conditions is histopathology; however, dermatologic ultrasonography can provide noninvasive, real-time, important diagnostic characteristics of cutaneous pathologies as well as VA.5-7 Crisan et al6 outlined specific sonographic findings of lipomas, EICs, trichilemmal cysts, and other dermatologic conditions as well as the associated surgical pertinence. Ultrasonography of a venous aneurysm may show a heterogeneous, contiguous, echoic lesion with an adjacent superficial vein, which may be easily compressed by the probe.8 Advanced imaging such as computed tomography with contrast or magnetic resonance imaging may be performed, but these are more costly than ultrasonography. Additionally, point-of-care ultrasonography is becoming more popular and accessible for physicians to carry at bedside with portable tablet options available. Dermatologists may want to consider incorporating it into the outpatient setting to improve procedural planning.9
In conclusion, VAs should be included in the differential diagnosis of soft cutaneous nodules, as management differs from a cyst or lipoma. Dermatologists should use their clinical judgment preoperatively—including a comprehensive history, physical examination, and consideration of color Doppler ultrasonography to assess for findings of VA. We do not recommend intentional surgical exploration of cutaneous aneurysms in the ambulatory setting due to risk for hemorrhage. Furthermore, when clinical suspicion of EIC or lipoma is high, it still is preferable to descend the incision slowly at 1 to 2 mm per cut until the tumor is visualized.
- Ring CM, Kornreich DA, Lee JB. Clinical simulators of cysts. J Am Acad Dermatol. 2016;75:1255-1257.
- Evans CC, Larson MJ, Eichhorn PJ, et al. Traumatic pseudoaneurysm of the superficial temporal artery: two cases and review of the literature. J Am Acad Dermatol. 2003;49(5 suppl):S286-S288.
- Sofela A, Osunronbi T, Hettige S. Scalp cirsoid aneurysms: case illustration and systematic review of literature. Neurosurgery. 2020;86:E98-E107.
- McKesey J, Cohen PR. Spontaneous venous aneurysm: report of a non-traumatic superficial venous aneurysm on the distal arm. Cureus. 2018;10:E2641.
- Wortsman X, Alfageme F, Roustan G, et al. Guidelines for performing dermatologic ultrasound examinations by the DERMUS Group. J Ultrasound Med. 2016;35:577-580.
- Crisan D, Wortsman X, Alfageme F, et al. Ultrasonography in dermatologic surgery: revealing the unseen for improved surgical planning [published online May 26, 2022]. J Dtsch Dermatol Ges. doi:10.1111/ddg.14781
- Corvino A, Catalano O, Corvino F, et al. Superficial temporal artery pseudoaneurysm: what is the role of ultrasound. J Ultrasound. 2016;19:197-201.
- Lee HY, Lee W, Cho YK, et al. Superficial venous aneurysm: reports of 3 cases and literature review. J Ultrasound Med. 2006;25:771-776.
- Hadian Y, Link D, Dahle SE, et al. Ultrasound as a diagnostic and interventional aid at point-of-care in dermatology clinic: a case report. J Dermatolog Treat. 2020;31:74-76.
- Ring CM, Kornreich DA, Lee JB. Clinical simulators of cysts. J Am Acad Dermatol. 2016;75:1255-1257.
- Evans CC, Larson MJ, Eichhorn PJ, et al. Traumatic pseudoaneurysm of the superficial temporal artery: two cases and review of the literature. J Am Acad Dermatol. 2003;49(5 suppl):S286-S288.
- Sofela A, Osunronbi T, Hettige S. Scalp cirsoid aneurysms: case illustration and systematic review of literature. Neurosurgery. 2020;86:E98-E107.
- McKesey J, Cohen PR. Spontaneous venous aneurysm: report of a non-traumatic superficial venous aneurysm on the distal arm. Cureus. 2018;10:E2641.
- Wortsman X, Alfageme F, Roustan G, et al. Guidelines for performing dermatologic ultrasound examinations by the DERMUS Group. J Ultrasound Med. 2016;35:577-580.
- Crisan D, Wortsman X, Alfageme F, et al. Ultrasonography in dermatologic surgery: revealing the unseen for improved surgical planning [published online May 26, 2022]. J Dtsch Dermatol Ges. doi:10.1111/ddg.14781
- Corvino A, Catalano O, Corvino F, et al. Superficial temporal artery pseudoaneurysm: what is the role of ultrasound. J Ultrasound. 2016;19:197-201.
- Lee HY, Lee W, Cho YK, et al. Superficial venous aneurysm: reports of 3 cases and literature review. J Ultrasound Med. 2006;25:771-776.
- Hadian Y, Link D, Dahle SE, et al. Ultrasound as a diagnostic and interventional aid at point-of-care in dermatology clinic: a case report. J Dermatolog Treat. 2020;31:74-76.
Practice Points
- Vascular anomalies should be included in the differential diagnosis of soft cutaneous nodules, as management differs from cysts or lipomas.
- Preoperative evaluation for a cutaneous cyst excision on the head and neck should include ruling out findings of a vascular lesion through history, physical examination, and consideration of color Doppler ultrasonography in unclear cases.
- Surgical technique should involve sequential superficial incisions, descending at 1 to 2 mm per cut, until the suspected capsule is identified to minimize the risk for inadvertent injury to a cyst mimicker such as a vascular anomaly.
Continued Caution Needed Combining Nitrates With ED Drugs
New research supports continued caution in prescribing a phosphodiesterase-5 inhibitor (PDE5i) to treat erectile dysfunction (ED) in men with heart disease using nitrate medications.
In a large Swedish population study of men with stable coronary artery disease (CAD), the combined use of a PDE5i and nitrates was associated with a higher risk for cardiovascular (CV) morbidity and mortality.
“According to current recommendations, PDE5i are contraindicated in patients taking organic nitrates; however, in clinical practice, both are commonly prescribed, and concomitant use has increased,” first author Ylva Trolle Lagerros, MD, PhD, with Karolinska Institutet, Stockholm, Sweden, told this news organization.
and weigh the benefits of the medication against the possible increased risk for cardiovascular morbidity and mortality given by this combination,” Dr. Lagerros said.
The study was published online in the Journal of the American College of Cardiology (JACC).
The researchers used the Swedish Patient Register and the Prescribed Drug Register to assess the association between PDE5i treatment and CV outcomes in men with stable CAD treated with nitrate medication.
Among 55,777 men with a history of previous myocardial infarction (MI) or coronary revascularization who had filled at least two nitrate prescriptions (sublingual, oral, or both), 5710 also had at least two filled prescriptions of a PDE5i.
In multivariate-adjusted analysis, the combined use of PDE5i treatment with nitrates was associated with an increased relative risk for all studied outcomes, including all-cause mortality, CV and non-CV mortality, MI, heart failure, cardiac revascularization (hazard ratio), and major adverse cardiovascular events.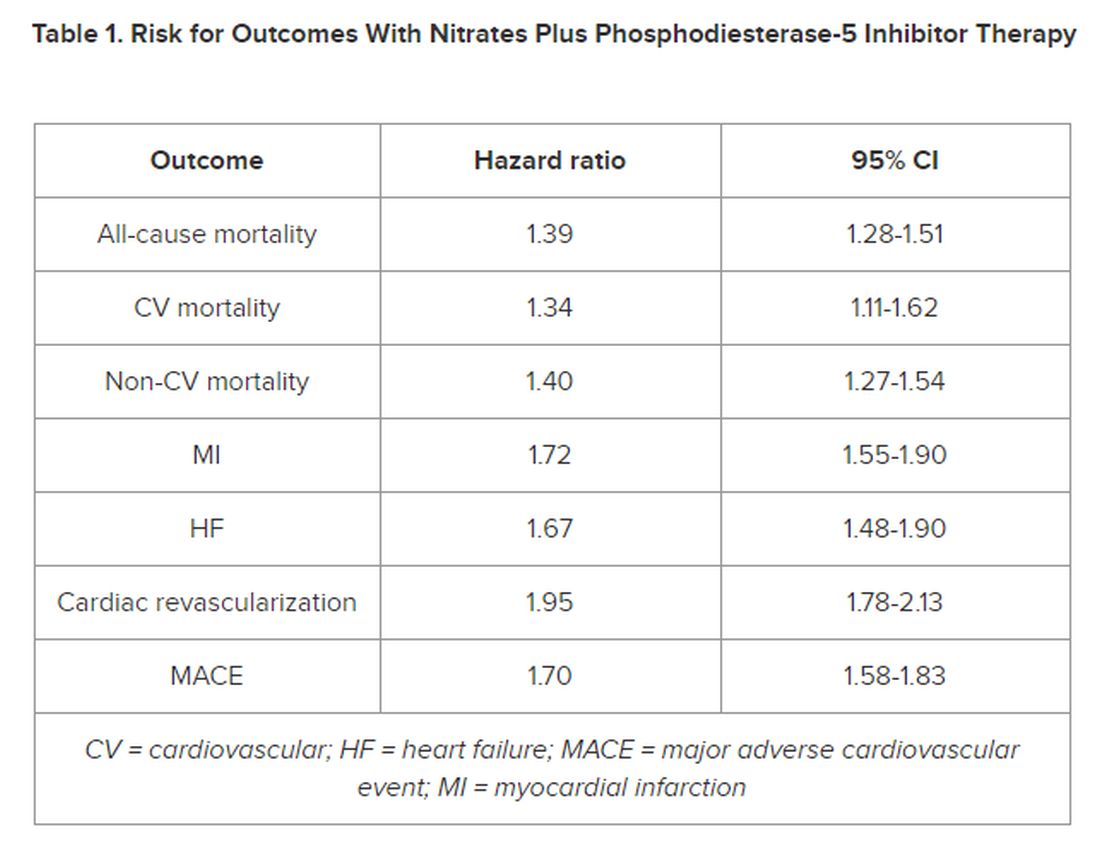
However, the number of events 28 days following a PDE5i prescription fill was “few, with lower incidence rates than in subjects taking nitrates only, indicating a low immediate risk for any event,” the authors noted in their article.
‘Common Bedfellows’
In a JACC editorial, Glenn N. Levine, MD, with Baylor College of Medicine, Houston, Texas, noted that, “ED and CAD are unfortunate, and all too common, bedfellows. But, as with most relationships, assuming proper precautions and care, they can coexist together for many years, perhaps even a lifetime.”
Dr. Levine noted that PDE5is are “reasonably safe” in most patients with stable CAD and only mild angina if not on chronic nitrate therapy. For those on chronic oral nitrate therapy, the use of PDE5is should continue to be regarded as “ill-advised at best and generally contraindicated.”
In some patients on oral nitrate therapy who want to use a PDE5i, particularly those who have undergone revascularization and have minimal or no angina, Dr. Levine said it may be reasonable to initiate a several-week trial of the nitrate therapy (or on a different class of antianginal therapy) and assess if the patient remains relatively angina-free.
In those patients with just rare exertional angina at generally higher levels of activity or those prescribed sublingual nitroglycerin “just in case,” it may be reasonable to prescribe PDE5i after a “clear and detailed” discussion with the patient of the risks for temporarily combining PDE5i and sublingual nitroglycerin.
Dr. Levine said these patients should be instructed not to take nitroglycerin within 24 hours of using a shorter-acting PDE5i and within 48 hours of using the longer-acting PDE5i tadalafil.
They should also be told to call 9-1-1 if angina develops during sexual intercourse and does not resolve upon cessation of such sexual activity, as well as to make medical personnel aware that they have recently used a PDE5i.
The study was funded by Region Stockholm, the Center for Innovative Medicine, and Karolinska Institutet. The researchers and editorial writer had declared no relevant conflicts of interest.
A version of this article appeared on Medscape.com.
New research supports continued caution in prescribing a phosphodiesterase-5 inhibitor (PDE5i) to treat erectile dysfunction (ED) in men with heart disease using nitrate medications.
In a large Swedish population study of men with stable coronary artery disease (CAD), the combined use of a PDE5i and nitrates was associated with a higher risk for cardiovascular (CV) morbidity and mortality.
“According to current recommendations, PDE5i are contraindicated in patients taking organic nitrates; however, in clinical practice, both are commonly prescribed, and concomitant use has increased,” first author Ylva Trolle Lagerros, MD, PhD, with Karolinska Institutet, Stockholm, Sweden, told this news organization.
and weigh the benefits of the medication against the possible increased risk for cardiovascular morbidity and mortality given by this combination,” Dr. Lagerros said.
The study was published online in the Journal of the American College of Cardiology (JACC).
The researchers used the Swedish Patient Register and the Prescribed Drug Register to assess the association between PDE5i treatment and CV outcomes in men with stable CAD treated with nitrate medication.
Among 55,777 men with a history of previous myocardial infarction (MI) or coronary revascularization who had filled at least two nitrate prescriptions (sublingual, oral, or both), 5710 also had at least two filled prescriptions of a PDE5i.
In multivariate-adjusted analysis, the combined use of PDE5i treatment with nitrates was associated with an increased relative risk for all studied outcomes, including all-cause mortality, CV and non-CV mortality, MI, heart failure, cardiac revascularization (hazard ratio), and major adverse cardiovascular events.
However, the number of events 28 days following a PDE5i prescription fill was “few, with lower incidence rates than in subjects taking nitrates only, indicating a low immediate risk for any event,” the authors noted in their article.
‘Common Bedfellows’
In a JACC editorial, Glenn N. Levine, MD, with Baylor College of Medicine, Houston, Texas, noted that, “ED and CAD are unfortunate, and all too common, bedfellows. But, as with most relationships, assuming proper precautions and care, they can coexist together for many years, perhaps even a lifetime.”
Dr. Levine noted that PDE5is are “reasonably safe” in most patients with stable CAD and only mild angina if not on chronic nitrate therapy. For those on chronic oral nitrate therapy, the use of PDE5is should continue to be regarded as “ill-advised at best and generally contraindicated.”
In some patients on oral nitrate therapy who want to use a PDE5i, particularly those who have undergone revascularization and have minimal or no angina, Dr. Levine said it may be reasonable to initiate a several-week trial of the nitrate therapy (or on a different class of antianginal therapy) and assess if the patient remains relatively angina-free.
In those patients with just rare exertional angina at generally higher levels of activity or those prescribed sublingual nitroglycerin “just in case,” it may be reasonable to prescribe PDE5i after a “clear and detailed” discussion with the patient of the risks for temporarily combining PDE5i and sublingual nitroglycerin.
Dr. Levine said these patients should be instructed not to take nitroglycerin within 24 hours of using a shorter-acting PDE5i and within 48 hours of using the longer-acting PDE5i tadalafil.
They should also be told to call 9-1-1 if angina develops during sexual intercourse and does not resolve upon cessation of such sexual activity, as well as to make medical personnel aware that they have recently used a PDE5i.
The study was funded by Region Stockholm, the Center for Innovative Medicine, and Karolinska Institutet. The researchers and editorial writer had declared no relevant conflicts of interest.
A version of this article appeared on Medscape.com.
New research supports continued caution in prescribing a phosphodiesterase-5 inhibitor (PDE5i) to treat erectile dysfunction (ED) in men with heart disease using nitrate medications.
In a large Swedish population study of men with stable coronary artery disease (CAD), the combined use of a PDE5i and nitrates was associated with a higher risk for cardiovascular (CV) morbidity and mortality.
“According to current recommendations, PDE5i are contraindicated in patients taking organic nitrates; however, in clinical practice, both are commonly prescribed, and concomitant use has increased,” first author Ylva Trolle Lagerros, MD, PhD, with Karolinska Institutet, Stockholm, Sweden, told this news organization.
and weigh the benefits of the medication against the possible increased risk for cardiovascular morbidity and mortality given by this combination,” Dr. Lagerros said.
The study was published online in the Journal of the American College of Cardiology (JACC).
The researchers used the Swedish Patient Register and the Prescribed Drug Register to assess the association between PDE5i treatment and CV outcomes in men with stable CAD treated with nitrate medication.
Among 55,777 men with a history of previous myocardial infarction (MI) or coronary revascularization who had filled at least two nitrate prescriptions (sublingual, oral, or both), 5710 also had at least two filled prescriptions of a PDE5i.
In multivariate-adjusted analysis, the combined use of PDE5i treatment with nitrates was associated with an increased relative risk for all studied outcomes, including all-cause mortality, CV and non-CV mortality, MI, heart failure, cardiac revascularization (hazard ratio), and major adverse cardiovascular events.
However, the number of events 28 days following a PDE5i prescription fill was “few, with lower incidence rates than in subjects taking nitrates only, indicating a low immediate risk for any event,” the authors noted in their article.
‘Common Bedfellows’
In a JACC editorial, Glenn N. Levine, MD, with Baylor College of Medicine, Houston, Texas, noted that, “ED and CAD are unfortunate, and all too common, bedfellows. But, as with most relationships, assuming proper precautions and care, they can coexist together for many years, perhaps even a lifetime.”
Dr. Levine noted that PDE5is are “reasonably safe” in most patients with stable CAD and only mild angina if not on chronic nitrate therapy. For those on chronic oral nitrate therapy, the use of PDE5is should continue to be regarded as “ill-advised at best and generally contraindicated.”
In some patients on oral nitrate therapy who want to use a PDE5i, particularly those who have undergone revascularization and have minimal or no angina, Dr. Levine said it may be reasonable to initiate a several-week trial of the nitrate therapy (or on a different class of antianginal therapy) and assess if the patient remains relatively angina-free.
In those patients with just rare exertional angina at generally higher levels of activity or those prescribed sublingual nitroglycerin “just in case,” it may be reasonable to prescribe PDE5i after a “clear and detailed” discussion with the patient of the risks for temporarily combining PDE5i and sublingual nitroglycerin.
Dr. Levine said these patients should be instructed not to take nitroglycerin within 24 hours of using a shorter-acting PDE5i and within 48 hours of using the longer-acting PDE5i tadalafil.
They should also be told to call 9-1-1 if angina develops during sexual intercourse and does not resolve upon cessation of such sexual activity, as well as to make medical personnel aware that they have recently used a PDE5i.
The study was funded by Region Stockholm, the Center for Innovative Medicine, and Karolinska Institutet. The researchers and editorial writer had declared no relevant conflicts of interest.
A version of this article appeared on Medscape.com.
ADHD Symptoms Linked With Physical Comorbidities
Investigators from the French Health and Medical Research Institute (INSERM), University of Bordeaux, and Charles Perrens Hospital, alongside their Canadian, British, and Swedish counterparts, have shown that attention-deficit/hyperactivity disorder (ADHD) or attention-deficit disorder without hyperactivity is linked with physical health problems. Cédric Galéra, MD, PhD, child and adolescent psychiatrist and epidemiologist at the Bordeaux Population Health Research Center (INSERM/University of Bordeaux) and the Charles Perrens Hospital, explained these findings to this news organization.
A Bilateral Association
ADHD is a neurodevelopmental condition that develops in childhood and is characterized by high levels of inattention or agitation and impulsiveness. Some studies have revealed a link between ADHD and medical comorbidities, but these studies were carried out on small patient samples and were cross-sectional.
A new longitudinal study published in Lancet Child and Adolescent Health has shown a reciprocal link between ADHD and physical health problems. The researchers conducted statistical analyses to measure the links between ADHD symptoms and subsequent development of certain physical conditions and, conversely, between physical problems during childhood and subsequent development of ADHD symptoms.
Children From Quebec
The study was conducted by a team headed by Dr. Galéra in collaboration with teams from Britain, Sweden, and Canada. “We studied a Quebec-based cohort of 2000 children aged between 5 months and 17 years,” said Dr. Galéra.
“The researchers in Quebec sent interviewers to question parents at home. And once the children were able to answer for themselves, from adolescence, they were asked to answer the questions directly,” he added.
The children were assessed on the severity of their ADHD symptoms as well as their physical condition (general well-being, any conditions diagnosed, etc.).
Dental Caries, Excess Weight
“We were able to show links between ADHD in childhood and physical health problems in adolescence. There is a greater risk for dental caries, infections, injuries, wounds, sleep disorders, and excess weight.
“Accounting for socioeconomic status and mental health problems such as anxiety and depression or medical treatments, we observed that dental caries, wounds, excess weight, and restless legs syndrome were the conditions that cropped up time and time again,” said Dr. Galéra.
On the other hand, the researchers noted that certain physical health issues in childhood were linked with the onset of ADHD at a later stage. “We discovered that asthma in early childhood, injuries, sleep disturbances, epilepsy, and excess weight were associated with ADHD. Taking all above-referenced features into account, we were left with just wounds and injuries as well as restless legs syndrome as being linked to ADHD,” Dr. Galéra concluded.
For Dr. Galéra, the study illustrates the direction and timing of the links between physical problems and ADHD. “This reflects the link between physical and mental health. It’s important that all healthcare professionals be alert to this. Psychiatrists and mental health professionals must be vigilant about the physical health risks, and pediatricians and family physicians must be aware of the fact that children can present with physical conditions that will later be linked with ADHD. Each of them must be able to refer their young patients to their medical colleagues to ensure that these people receive the best care,” he emphasized.
The team will continue to study this cohort to see which problems emerge in adulthood. They also wish to study the Elfe cohort, a French longitudinal study of children.
This article was translated from the Medscape French edition. A version of this article appeared on Medscape.com.
Investigators from the French Health and Medical Research Institute (INSERM), University of Bordeaux, and Charles Perrens Hospital, alongside their Canadian, British, and Swedish counterparts, have shown that attention-deficit/hyperactivity disorder (ADHD) or attention-deficit disorder without hyperactivity is linked with physical health problems. Cédric Galéra, MD, PhD, child and adolescent psychiatrist and epidemiologist at the Bordeaux Population Health Research Center (INSERM/University of Bordeaux) and the Charles Perrens Hospital, explained these findings to this news organization.
A Bilateral Association
ADHD is a neurodevelopmental condition that develops in childhood and is characterized by high levels of inattention or agitation and impulsiveness. Some studies have revealed a link between ADHD and medical comorbidities, but these studies were carried out on small patient samples and were cross-sectional.
A new longitudinal study published in Lancet Child and Adolescent Health has shown a reciprocal link between ADHD and physical health problems. The researchers conducted statistical analyses to measure the links between ADHD symptoms and subsequent development of certain physical conditions and, conversely, between physical problems during childhood and subsequent development of ADHD symptoms.
Children From Quebec
The study was conducted by a team headed by Dr. Galéra in collaboration with teams from Britain, Sweden, and Canada. “We studied a Quebec-based cohort of 2000 children aged between 5 months and 17 years,” said Dr. Galéra.
“The researchers in Quebec sent interviewers to question parents at home. And once the children were able to answer for themselves, from adolescence, they were asked to answer the questions directly,” he added.
The children were assessed on the severity of their ADHD symptoms as well as their physical condition (general well-being, any conditions diagnosed, etc.).
Dental Caries, Excess Weight
“We were able to show links between ADHD in childhood and physical health problems in adolescence. There is a greater risk for dental caries, infections, injuries, wounds, sleep disorders, and excess weight.
“Accounting for socioeconomic status and mental health problems such as anxiety and depression or medical treatments, we observed that dental caries, wounds, excess weight, and restless legs syndrome were the conditions that cropped up time and time again,” said Dr. Galéra.
On the other hand, the researchers noted that certain physical health issues in childhood were linked with the onset of ADHD at a later stage. “We discovered that asthma in early childhood, injuries, sleep disturbances, epilepsy, and excess weight were associated with ADHD. Taking all above-referenced features into account, we were left with just wounds and injuries as well as restless legs syndrome as being linked to ADHD,” Dr. Galéra concluded.
For Dr. Galéra, the study illustrates the direction and timing of the links between physical problems and ADHD. “This reflects the link between physical and mental health. It’s important that all healthcare professionals be alert to this. Psychiatrists and mental health professionals must be vigilant about the physical health risks, and pediatricians and family physicians must be aware of the fact that children can present with physical conditions that will later be linked with ADHD. Each of them must be able to refer their young patients to their medical colleagues to ensure that these people receive the best care,” he emphasized.
The team will continue to study this cohort to see which problems emerge in adulthood. They also wish to study the Elfe cohort, a French longitudinal study of children.
This article was translated from the Medscape French edition. A version of this article appeared on Medscape.com.
Investigators from the French Health and Medical Research Institute (INSERM), University of Bordeaux, and Charles Perrens Hospital, alongside their Canadian, British, and Swedish counterparts, have shown that attention-deficit/hyperactivity disorder (ADHD) or attention-deficit disorder without hyperactivity is linked with physical health problems. Cédric Galéra, MD, PhD, child and adolescent psychiatrist and epidemiologist at the Bordeaux Population Health Research Center (INSERM/University of Bordeaux) and the Charles Perrens Hospital, explained these findings to this news organization.
A Bilateral Association
ADHD is a neurodevelopmental condition that develops in childhood and is characterized by high levels of inattention or agitation and impulsiveness. Some studies have revealed a link between ADHD and medical comorbidities, but these studies were carried out on small patient samples and were cross-sectional.
A new longitudinal study published in Lancet Child and Adolescent Health has shown a reciprocal link between ADHD and physical health problems. The researchers conducted statistical analyses to measure the links between ADHD symptoms and subsequent development of certain physical conditions and, conversely, between physical problems during childhood and subsequent development of ADHD symptoms.
Children From Quebec
The study was conducted by a team headed by Dr. Galéra in collaboration with teams from Britain, Sweden, and Canada. “We studied a Quebec-based cohort of 2000 children aged between 5 months and 17 years,” said Dr. Galéra.
“The researchers in Quebec sent interviewers to question parents at home. And once the children were able to answer for themselves, from adolescence, they were asked to answer the questions directly,” he added.
The children were assessed on the severity of their ADHD symptoms as well as their physical condition (general well-being, any conditions diagnosed, etc.).
Dental Caries, Excess Weight
“We were able to show links between ADHD in childhood and physical health problems in adolescence. There is a greater risk for dental caries, infections, injuries, wounds, sleep disorders, and excess weight.
“Accounting for socioeconomic status and mental health problems such as anxiety and depression or medical treatments, we observed that dental caries, wounds, excess weight, and restless legs syndrome were the conditions that cropped up time and time again,” said Dr. Galéra.
On the other hand, the researchers noted that certain physical health issues in childhood were linked with the onset of ADHD at a later stage. “We discovered that asthma in early childhood, injuries, sleep disturbances, epilepsy, and excess weight were associated with ADHD. Taking all above-referenced features into account, we were left with just wounds and injuries as well as restless legs syndrome as being linked to ADHD,” Dr. Galéra concluded.
For Dr. Galéra, the study illustrates the direction and timing of the links between physical problems and ADHD. “This reflects the link between physical and mental health. It’s important that all healthcare professionals be alert to this. Psychiatrists and mental health professionals must be vigilant about the physical health risks, and pediatricians and family physicians must be aware of the fact that children can present with physical conditions that will later be linked with ADHD. Each of them must be able to refer their young patients to their medical colleagues to ensure that these people receive the best care,” he emphasized.
The team will continue to study this cohort to see which problems emerge in adulthood. They also wish to study the Elfe cohort, a French longitudinal study of children.
This article was translated from the Medscape French edition. A version of this article appeared on Medscape.com.
FDA Clears AI-Powered Device for Noninvasive Skin Cancer Testing
The handheld wireless tool, which was developed by Miami-based DermaSensor Inc., operates on battery power, uses spectroscopy and algorithms to evaluate skin lesions for potential cancer in a matter of seconds, and is intended for use by primary care physicians. After the device completes the scan of a lesion, a result of “investigate further” (positive result) suggests further evaluation through a referral to a dermatologist, while “monitor” (negative result) suggests that there is no immediate need for a referral to a dermatologist.
In a pivotal trial of the device that evaluated 224 high risk lesions at 18 primary care study sites in the United States and 4 in Australia, the device had an overall sensitivity of 95.5% for detecting malignancy.
In a more recent validation study funded by DermaSensor, investigators tested 333 lesions at four U.S. dermatology offices and found that the overall device sensitivity was 97.04%, with subgroup sensitivity of 96.67% for melanoma, 97.22% for basal cell carcinoma, and 97.01% for squamous cell carcinoma. Overall specificity of the device was 26.22%.
The study authors, led by Tallahassee, Fla.–based dermatologist Armand B. Cognetta Jr., MD, concluded that DermaSensor’s rapid clinical analysis of lesions “allows for its easy integration into clinical practice infrastructures. Proper use of this device may aid in the reduction of morbidity and mortality associated with skin cancer through expedited and enhanced detection and intervention.”
According to marketing material from the DermaSensor website, the device’s AI algorithm was developed and validated with more than 20,000 scans, composed of more than 4,000 benign and malignant lesions. In a statement about the clearance, the FDA emphasized that the device “should not be used as the sole diagnostic criterion nor to confirm a diagnosis of skin cancer.” The agency is requiring that the manufacturer “conduct additional post-market clinical validation performance testing of the DermaSensor device in patients from demographic groups representative of the U.S. population, including populations who had limited representation of melanomas in the premarket studies, due to their having a relatively low incidence of the disease.”
According to a spokesperson for DermaSensor, pricing for the device is based on a subscription model: $199 per month for five patients or $399 per month for unlimited use. DermaSensor is currently commercially available in Europe and Australia.
Asked to comment, Vishal A. Patel, MD, director of cutaneous oncology at the George Washington Cancer Center, Washington, said that the FDA clearance of DermaSensor highlights the growing appreciation of AI-driven diagnostic support for primary care providers and dermatologists. "Skin cancers are a growing epidemic in the US and the ability to accurately identify potential suspicious lesions without immediately reaching for the scalpel is invaluable," Patel told this news organization. He was not involved with DermSensor studies.
"Furthermore, this tool can help address the shortage of dermatologists and long wait times by helping primary care providers accurately risk-stratify patients and identify those who need to be seen immediately for potential biopsy and expert care," he added. "However, just like with any new technology, we must use caution to not overutilize this tool," which he said, could "lead to overdiagnosis and overtreatment of early or innocuous lesions that are better managed with empiric field treatments."
Dr. Cognetta was a paid investigator for the study.
Dr. Patel disclosed that he is chief medical officer for Lazarus AI.
The handheld wireless tool, which was developed by Miami-based DermaSensor Inc., operates on battery power, uses spectroscopy and algorithms to evaluate skin lesions for potential cancer in a matter of seconds, and is intended for use by primary care physicians. After the device completes the scan of a lesion, a result of “investigate further” (positive result) suggests further evaluation through a referral to a dermatologist, while “monitor” (negative result) suggests that there is no immediate need for a referral to a dermatologist.
In a pivotal trial of the device that evaluated 224 high risk lesions at 18 primary care study sites in the United States and 4 in Australia, the device had an overall sensitivity of 95.5% for detecting malignancy.
In a more recent validation study funded by DermaSensor, investigators tested 333 lesions at four U.S. dermatology offices and found that the overall device sensitivity was 97.04%, with subgroup sensitivity of 96.67% for melanoma, 97.22% for basal cell carcinoma, and 97.01% for squamous cell carcinoma. Overall specificity of the device was 26.22%.
The study authors, led by Tallahassee, Fla.–based dermatologist Armand B. Cognetta Jr., MD, concluded that DermaSensor’s rapid clinical analysis of lesions “allows for its easy integration into clinical practice infrastructures. Proper use of this device may aid in the reduction of morbidity and mortality associated with skin cancer through expedited and enhanced detection and intervention.”
According to marketing material from the DermaSensor website, the device’s AI algorithm was developed and validated with more than 20,000 scans, composed of more than 4,000 benign and malignant lesions. In a statement about the clearance, the FDA emphasized that the device “should not be used as the sole diagnostic criterion nor to confirm a diagnosis of skin cancer.” The agency is requiring that the manufacturer “conduct additional post-market clinical validation performance testing of the DermaSensor device in patients from demographic groups representative of the U.S. population, including populations who had limited representation of melanomas in the premarket studies, due to their having a relatively low incidence of the disease.”
According to a spokesperson for DermaSensor, pricing for the device is based on a subscription model: $199 per month for five patients or $399 per month for unlimited use. DermaSensor is currently commercially available in Europe and Australia.
Asked to comment, Vishal A. Patel, MD, director of cutaneous oncology at the George Washington Cancer Center, Washington, said that the FDA clearance of DermaSensor highlights the growing appreciation of AI-driven diagnostic support for primary care providers and dermatologists. "Skin cancers are a growing epidemic in the US and the ability to accurately identify potential suspicious lesions without immediately reaching for the scalpel is invaluable," Patel told this news organization. He was not involved with DermSensor studies.
"Furthermore, this tool can help address the shortage of dermatologists and long wait times by helping primary care providers accurately risk-stratify patients and identify those who need to be seen immediately for potential biopsy and expert care," he added. "However, just like with any new technology, we must use caution to not overutilize this tool," which he said, could "lead to overdiagnosis and overtreatment of early or innocuous lesions that are better managed with empiric field treatments."
Dr. Cognetta was a paid investigator for the study.
Dr. Patel disclosed that he is chief medical officer for Lazarus AI.
The handheld wireless tool, which was developed by Miami-based DermaSensor Inc., operates on battery power, uses spectroscopy and algorithms to evaluate skin lesions for potential cancer in a matter of seconds, and is intended for use by primary care physicians. After the device completes the scan of a lesion, a result of “investigate further” (positive result) suggests further evaluation through a referral to a dermatologist, while “monitor” (negative result) suggests that there is no immediate need for a referral to a dermatologist.
In a pivotal trial of the device that evaluated 224 high risk lesions at 18 primary care study sites in the United States and 4 in Australia, the device had an overall sensitivity of 95.5% for detecting malignancy.
In a more recent validation study funded by DermaSensor, investigators tested 333 lesions at four U.S. dermatology offices and found that the overall device sensitivity was 97.04%, with subgroup sensitivity of 96.67% for melanoma, 97.22% for basal cell carcinoma, and 97.01% for squamous cell carcinoma. Overall specificity of the device was 26.22%.
The study authors, led by Tallahassee, Fla.–based dermatologist Armand B. Cognetta Jr., MD, concluded that DermaSensor’s rapid clinical analysis of lesions “allows for its easy integration into clinical practice infrastructures. Proper use of this device may aid in the reduction of morbidity and mortality associated with skin cancer through expedited and enhanced detection and intervention.”
According to marketing material from the DermaSensor website, the device’s AI algorithm was developed and validated with more than 20,000 scans, composed of more than 4,000 benign and malignant lesions. In a statement about the clearance, the FDA emphasized that the device “should not be used as the sole diagnostic criterion nor to confirm a diagnosis of skin cancer.” The agency is requiring that the manufacturer “conduct additional post-market clinical validation performance testing of the DermaSensor device in patients from demographic groups representative of the U.S. population, including populations who had limited representation of melanomas in the premarket studies, due to their having a relatively low incidence of the disease.”
According to a spokesperson for DermaSensor, pricing for the device is based on a subscription model: $199 per month for five patients or $399 per month for unlimited use. DermaSensor is currently commercially available in Europe and Australia.
Asked to comment, Vishal A. Patel, MD, director of cutaneous oncology at the George Washington Cancer Center, Washington, said that the FDA clearance of DermaSensor highlights the growing appreciation of AI-driven diagnostic support for primary care providers and dermatologists. "Skin cancers are a growing epidemic in the US and the ability to accurately identify potential suspicious lesions without immediately reaching for the scalpel is invaluable," Patel told this news organization. He was not involved with DermSensor studies.
"Furthermore, this tool can help address the shortage of dermatologists and long wait times by helping primary care providers accurately risk-stratify patients and identify those who need to be seen immediately for potential biopsy and expert care," he added. "However, just like with any new technology, we must use caution to not overutilize this tool," which he said, could "lead to overdiagnosis and overtreatment of early or innocuous lesions that are better managed with empiric field treatments."
Dr. Cognetta was a paid investigator for the study.
Dr. Patel disclosed that he is chief medical officer for Lazarus AI.
With Proper Training, AI Can Be a Useful Tool in Epilepsy Management
ORLANDO — Experts shed light on the applications, benefits, and pitfalls of artificial intelligence (AI) during the Merrit-Putnam Symposium at the annual meeting of the American Epilepsy Society (AES).
In a session titled “Artificial Intelligence Fundamentals and Breakthrough Applications in Epilepsy,” University of Pittsburgh neurologist and assistant professor Wesley Kerr, MD, PhD, provided an overview of AI as well its applications in neurology. He began by addressing perhaps one of the most controversial topics regarding AI in the medical community: clinicians’ fear of being replaced by technology.
he told the audience.
To Optimize AI, Clinicians Must Lay the Proper Foundation
Dr. Kerr’s presentation focused on providing audience members with tools to help them evaluate new technologies, recognize benefits, and identify key costs and limitations associated with AI implementation and integration into clinical practice.
Before delving deeper, one must first understand basic terminology regarding AI. Without this knowledge, clinicians may inadvertently introduce bias or errata or fail to understand how to best leverage the technology to enhance the quality of the practice while improving patient outcomes.
Machine learning (ML) describes the process of using data to learn a specific task. Deep learning (DL) stacks multiple layers of ML to improve performance on the task. Lastly, generative AI generates content such as text, images, and media.
Utilizing AI effectively in clinical applications involves tapping into select features most related to prediction (for example, disease factors) and grouping features into categories based on measuring commonalities such as factor composition in a population. This information should be used in training data only.
Fully understanding ML/AI allows clinicians to use it as a diagnostic test by exploiting a combination of accuracy, sensitivity, and specificity, along with positive and negative predictive values.
Data Fidelity and Integrity Hinge on Optimal Data Inputs
In the case of epilepsy, calibration curves can provide practical guidance in terms of predicting impending seizures.
“ML/AI needs gold-standard labels for evaluation,” Dr. Kerr said. He went on to stress the importance of quality data inputs to optimize the fidelity of AI’s predictive analytics.
“If you input garbage, you’ll get garbage out,” he said. “So a lot of garbage going in means a lot of garbage out.”
Such “garbage” can result in missed or erroneous diagnoses, or even faulty predictions. Even when the data are complete, AI can draw incorrect conclusions based on trends for which it lacks proper context.
Dr. Kerr used epilepsy trends in the Black population to illustrate this problem.
“One potential bias is that AI can figure out a patient is Black without being told, and based on data that Black patients are less likely to get epilepsy surgery,” he said, “AI would say they don’t need it because they’re Black, which isn’t true.”
In other words, ML/AI can use systematic determinants of health, such as race, to learn what Dr. Kerr referred to as an “inappropriate association.”
For that reason, ML/AI users must test for bias.
Such data are often retrieved from electronic health records (EHR), which serve as an important source of data ML/AI input. Using EHR makes sense, as they are a major source of missed potential in improving prompt treatment. According to Dr. Kerr, 20% of academic neurologists’ notes miss seizure frequency, and 30% miss the age of onset.
In addition, International Classification of Diseases (ICD) codes create another hurdle depending on the type of code used. For example, epilepsy with G40 or 2 codes of R56 is reliable, while focal to bilateral versus generalized epilepsy proves more challenging.
AI Improves Efficiency in National Language Generation
Large language models (LLM) look at first drafts and can save time on formatting, image selection, and construction. Perhaps ChatGPT is the most famous LLM, but other tools in this category include Open AI and Bard. LLMs are trained on “the whole internet” and use publicly accessible text.
In these cases, prompts serve as input data. Output data are predictions of the first and subsequent words.
Many users appreciate the foundation LLMs provide in terms of facilitating and collating research and summarizing ideas. The LLM-generated text actually serves as a first draft, saving users time on more clerical tasks such as formatting, image selection, and structure. Notwithstanding, these tools still require human supervision to screen for hallucinations or to add specialized content.
“LLMs are a great starting place to save time but are loaded with errors,” Dr. Kerr said.
Even if the tools could produce error-free content, ethics still come into play when using AI-generated content without any alterations. Any ML/AI that has not been modified or supervised is considered plagiarism.
Yet, interestingly enough, Dr. Kerr found that patients respond more positively to AI than physicians when interacting.
“Patients felt that AI was more sensitive and compassionate because it was longer-winded and humans are short,” he said. He went on to argue that AI might actually prove useful in helping physicians to improve the quality of their patient interactions.
Dr. Kerr left the audience with these key takeaways:
- ML/AI is just one type of clinical tool with benefits and limitations. The technology conveys the advantages of freeing up the clinician’s time to focus on more human-centered tasks, improving clinical decisions in challenging situations, and improving efficiency.
- However, healthcare systems should understand that ML/AI is not 100% foolproof, as the software’s knowledge is limited to its training exposure, and proper use requires supervision.
ORLANDO — Experts shed light on the applications, benefits, and pitfalls of artificial intelligence (AI) during the Merrit-Putnam Symposium at the annual meeting of the American Epilepsy Society (AES).
In a session titled “Artificial Intelligence Fundamentals and Breakthrough Applications in Epilepsy,” University of Pittsburgh neurologist and assistant professor Wesley Kerr, MD, PhD, provided an overview of AI as well its applications in neurology. He began by addressing perhaps one of the most controversial topics regarding AI in the medical community: clinicians’ fear of being replaced by technology.
he told the audience.
To Optimize AI, Clinicians Must Lay the Proper Foundation
Dr. Kerr’s presentation focused on providing audience members with tools to help them evaluate new technologies, recognize benefits, and identify key costs and limitations associated with AI implementation and integration into clinical practice.
Before delving deeper, one must first understand basic terminology regarding AI. Without this knowledge, clinicians may inadvertently introduce bias or errata or fail to understand how to best leverage the technology to enhance the quality of the practice while improving patient outcomes.
Machine learning (ML) describes the process of using data to learn a specific task. Deep learning (DL) stacks multiple layers of ML to improve performance on the task. Lastly, generative AI generates content such as text, images, and media.
Utilizing AI effectively in clinical applications involves tapping into select features most related to prediction (for example, disease factors) and grouping features into categories based on measuring commonalities such as factor composition in a population. This information should be used in training data only.
Fully understanding ML/AI allows clinicians to use it as a diagnostic test by exploiting a combination of accuracy, sensitivity, and specificity, along with positive and negative predictive values.
Data Fidelity and Integrity Hinge on Optimal Data Inputs
In the case of epilepsy, calibration curves can provide practical guidance in terms of predicting impending seizures.
“ML/AI needs gold-standard labels for evaluation,” Dr. Kerr said. He went on to stress the importance of quality data inputs to optimize the fidelity of AI’s predictive analytics.
“If you input garbage, you’ll get garbage out,” he said. “So a lot of garbage going in means a lot of garbage out.”
Such “garbage” can result in missed or erroneous diagnoses, or even faulty predictions. Even when the data are complete, AI can draw incorrect conclusions based on trends for which it lacks proper context.
Dr. Kerr used epilepsy trends in the Black population to illustrate this problem.
“One potential bias is that AI can figure out a patient is Black without being told, and based on data that Black patients are less likely to get epilepsy surgery,” he said, “AI would say they don’t need it because they’re Black, which isn’t true.”
In other words, ML/AI can use systematic determinants of health, such as race, to learn what Dr. Kerr referred to as an “inappropriate association.”
For that reason, ML/AI users must test for bias.
Such data are often retrieved from electronic health records (EHR), which serve as an important source of data ML/AI input. Using EHR makes sense, as they are a major source of missed potential in improving prompt treatment. According to Dr. Kerr, 20% of academic neurologists’ notes miss seizure frequency, and 30% miss the age of onset.
In addition, International Classification of Diseases (ICD) codes create another hurdle depending on the type of code used. For example, epilepsy with G40 or 2 codes of R56 is reliable, while focal to bilateral versus generalized epilepsy proves more challenging.
AI Improves Efficiency in National Language Generation
Large language models (LLM) look at first drafts and can save time on formatting, image selection, and construction. Perhaps ChatGPT is the most famous LLM, but other tools in this category include Open AI and Bard. LLMs are trained on “the whole internet” and use publicly accessible text.
In these cases, prompts serve as input data. Output data are predictions of the first and subsequent words.
Many users appreciate the foundation LLMs provide in terms of facilitating and collating research and summarizing ideas. The LLM-generated text actually serves as a first draft, saving users time on more clerical tasks such as formatting, image selection, and structure. Notwithstanding, these tools still require human supervision to screen for hallucinations or to add specialized content.
“LLMs are a great starting place to save time but are loaded with errors,” Dr. Kerr said.
Even if the tools could produce error-free content, ethics still come into play when using AI-generated content without any alterations. Any ML/AI that has not been modified or supervised is considered plagiarism.
Yet, interestingly enough, Dr. Kerr found that patients respond more positively to AI than physicians when interacting.
“Patients felt that AI was more sensitive and compassionate because it was longer-winded and humans are short,” he said. He went on to argue that AI might actually prove useful in helping physicians to improve the quality of their patient interactions.
Dr. Kerr left the audience with these key takeaways:
- ML/AI is just one type of clinical tool with benefits and limitations. The technology conveys the advantages of freeing up the clinician’s time to focus on more human-centered tasks, improving clinical decisions in challenging situations, and improving efficiency.
- However, healthcare systems should understand that ML/AI is not 100% foolproof, as the software’s knowledge is limited to its training exposure, and proper use requires supervision.
ORLANDO — Experts shed light on the applications, benefits, and pitfalls of artificial intelligence (AI) during the Merrit-Putnam Symposium at the annual meeting of the American Epilepsy Society (AES).
In a session titled “Artificial Intelligence Fundamentals and Breakthrough Applications in Epilepsy,” University of Pittsburgh neurologist and assistant professor Wesley Kerr, MD, PhD, provided an overview of AI as well its applications in neurology. He began by addressing perhaps one of the most controversial topics regarding AI in the medical community: clinicians’ fear of being replaced by technology.
he told the audience.
To Optimize AI, Clinicians Must Lay the Proper Foundation
Dr. Kerr’s presentation focused on providing audience members with tools to help them evaluate new technologies, recognize benefits, and identify key costs and limitations associated with AI implementation and integration into clinical practice.
Before delving deeper, one must first understand basic terminology regarding AI. Without this knowledge, clinicians may inadvertently introduce bias or errata or fail to understand how to best leverage the technology to enhance the quality of the practice while improving patient outcomes.
Machine learning (ML) describes the process of using data to learn a specific task. Deep learning (DL) stacks multiple layers of ML to improve performance on the task. Lastly, generative AI generates content such as text, images, and media.
Utilizing AI effectively in clinical applications involves tapping into select features most related to prediction (for example, disease factors) and grouping features into categories based on measuring commonalities such as factor composition in a population. This information should be used in training data only.
Fully understanding ML/AI allows clinicians to use it as a diagnostic test by exploiting a combination of accuracy, sensitivity, and specificity, along with positive and negative predictive values.
Data Fidelity and Integrity Hinge on Optimal Data Inputs
In the case of epilepsy, calibration curves can provide practical guidance in terms of predicting impending seizures.
“ML/AI needs gold-standard labels for evaluation,” Dr. Kerr said. He went on to stress the importance of quality data inputs to optimize the fidelity of AI’s predictive analytics.
“If you input garbage, you’ll get garbage out,” he said. “So a lot of garbage going in means a lot of garbage out.”
Such “garbage” can result in missed or erroneous diagnoses, or even faulty predictions. Even when the data are complete, AI can draw incorrect conclusions based on trends for which it lacks proper context.
Dr. Kerr used epilepsy trends in the Black population to illustrate this problem.
“One potential bias is that AI can figure out a patient is Black without being told, and based on data that Black patients are less likely to get epilepsy surgery,” he said, “AI would say they don’t need it because they’re Black, which isn’t true.”
In other words, ML/AI can use systematic determinants of health, such as race, to learn what Dr. Kerr referred to as an “inappropriate association.”
For that reason, ML/AI users must test for bias.
Such data are often retrieved from electronic health records (EHR), which serve as an important source of data ML/AI input. Using EHR makes sense, as they are a major source of missed potential in improving prompt treatment. According to Dr. Kerr, 20% of academic neurologists’ notes miss seizure frequency, and 30% miss the age of onset.
In addition, International Classification of Diseases (ICD) codes create another hurdle depending on the type of code used. For example, epilepsy with G40 or 2 codes of R56 is reliable, while focal to bilateral versus generalized epilepsy proves more challenging.
AI Improves Efficiency in National Language Generation
Large language models (LLM) look at first drafts and can save time on formatting, image selection, and construction. Perhaps ChatGPT is the most famous LLM, but other tools in this category include Open AI and Bard. LLMs are trained on “the whole internet” and use publicly accessible text.
In these cases, prompts serve as input data. Output data are predictions of the first and subsequent words.
Many users appreciate the foundation LLMs provide in terms of facilitating and collating research and summarizing ideas. The LLM-generated text actually serves as a first draft, saving users time on more clerical tasks such as formatting, image selection, and structure. Notwithstanding, these tools still require human supervision to screen for hallucinations or to add specialized content.
“LLMs are a great starting place to save time but are loaded with errors,” Dr. Kerr said.
Even if the tools could produce error-free content, ethics still come into play when using AI-generated content without any alterations. Any ML/AI that has not been modified or supervised is considered plagiarism.
Yet, interestingly enough, Dr. Kerr found that patients respond more positively to AI than physicians when interacting.
“Patients felt that AI was more sensitive and compassionate because it was longer-winded and humans are short,” he said. He went on to argue that AI might actually prove useful in helping physicians to improve the quality of their patient interactions.
Dr. Kerr left the audience with these key takeaways:
- ML/AI is just one type of clinical tool with benefits and limitations. The technology conveys the advantages of freeing up the clinician’s time to focus on more human-centered tasks, improving clinical decisions in challenging situations, and improving efficiency.
- However, healthcare systems should understand that ML/AI is not 100% foolproof, as the software’s knowledge is limited to its training exposure, and proper use requires supervision.
FROM AES 2023
Magic Wand Initiative Empowers Dermatologists to Innovate
NEW YORK – .
The program was founded in 2013 by two Harvard Medical School dermatologists, Lilit Garibyan, MD, PhD, the program director, and her mentor R. Rox Anderson MD, director of the Wellman Center for Photomedicine at Massachusetts General Hospital (MGH), Boston. It was based on the idea that clinicians are in a unique position to identify gaps in patient care and should be active in developing medical solutions to address those gaps.
“I truly believe that if we do a better job educating, training, and empowering our clinicians to become innovators, this will benefit patients and hospitals and physicians,” Dr. Garibyan said at the 26th annual Mount Sinai Winter Symposium — Advances in Medical and Surgical Dermatology.
One of the seeds for the project was her own experience with cryolipolysis which involves topical cooling, a noninvasive method of removing subcutaneous fat for body contouring, which relies on conducting heat from subcutaneous fat across the skin and therefore, does not reach fat far from the dermis. With Dr. Anderson’s mentorship, she developed injectable cooling technology (ICT), a procedure where “ice slurry,” composed of normal saline and glycerol, is directly injected into adipose tissue, possibly leading to more efficient and effective cryolipolysis.
After nearly 10 years of animal studies at MGH, led by Dr. Garibyan as proof of concept trials, ice slurry (Coolio Therapy) recently received FDA breakthrough designation for long-term pain control and early-stage human trials of clinical applications are underway, she noted.
Magic Wand Program
In the Magic Wand program, participating physicians start by recording areas of unmet needs in their day-to-day practices, and in groups, engage in clinician-only brainstorming sessions to screen ideas, define problems, and generate lists of specifications and tools needed to address clinical problems. After working together to define challenges and possible solutions, they take their ideas to a development team, where scientists, engineers, regulatory experts, and industry professionals meet and help clinicians start pilot proof-of-concept projects, develop prototypes, and gain support for studies, followed by pilot feasibility studies.
Part of the project is the Virtual Magic Wand (VMW) Initiative, a 10-month online instructive and interactive course open to clinicians in the United States and Europe, designed to bring together dermatologists “interested in deeply understanding a dermatologic clinical problem worth solving,” according to Dr. Garibyan. Currently, there are more than 86 VMW scholars from 46 institutions, and military and private practice sites in the United States. The VMW was expanded to Europe in 2021 and there are plans to expand to Asia as well, she said.
The success of the program is not only attributed to its clinical methods but the fact that it provides a benefit to doctors at all stages of their careers, patients, and industry. “This is the only program that aims to engage in innovation from resident to full professor. We provide ideas that industry can then support and bring to market. Everyone including patients, doctors, and healthcare companies can benefit from active, engaged, and innovative physicians,” Dr. Garibyan said.
One of the success stories is that of Veradermics, a company founded by Kansas City dermatologist, Reid A. Waldman, MD, the company’s CEO, and Tim Durso, MD, the president, who met while participating in the VMW program in 2020, which eventually led them to start a company addressing an unmet need in dermatology, a kid-friendly treatment of warts.
In an interview with this news organization, Dr. Waldman explained how the program informed his company’s ethos. “Magic Wand Initiative is about identifying problems worth solving,” he said. At the company, “we find problems or unmet needs that are large enough to motivate prescribing changes, so we’ve really taken the philosophy I learned in the program into this company and building our portfolio.”
One of the first needs that Veradermics addressed was the fact that treatment for common warts, cryotherapy with liquid nitrogen, is painful and can frighten children, and, with a response rate of “at best, 50%,” Dr. Waldman said. Veradermics is in the process of creating a nearly painless, child-friendly wart treatment: an “immunostimulatory dissolvable microarray” patch that contains Candida antigen extract, which is currently being evaluated for treating warts in a phase 2 clinical trial started in 2023.
Although the Magic Wand Initiative was initially restricted to dermatologists at MGH, stories like that of Veradermics have made the program so popular that it has branched out to include anesthesiologists and otolaryngologists, as well as general and orthopedic surgeons at MGH, Dr. Garibyan said at the Mount Sinai meeting.
Dr. Garibyan disclosed that she is a cofounder of and has equity in Brixton Biosciences and EyeCool, and is a consultant for and/or investor in Brixton and Clarity Cosmetics. Royalties/inventorship are assigned to MGH.
NEW YORK – .
The program was founded in 2013 by two Harvard Medical School dermatologists, Lilit Garibyan, MD, PhD, the program director, and her mentor R. Rox Anderson MD, director of the Wellman Center for Photomedicine at Massachusetts General Hospital (MGH), Boston. It was based on the idea that clinicians are in a unique position to identify gaps in patient care and should be active in developing medical solutions to address those gaps.
“I truly believe that if we do a better job educating, training, and empowering our clinicians to become innovators, this will benefit patients and hospitals and physicians,” Dr. Garibyan said at the 26th annual Mount Sinai Winter Symposium — Advances in Medical and Surgical Dermatology.
One of the seeds for the project was her own experience with cryolipolysis which involves topical cooling, a noninvasive method of removing subcutaneous fat for body contouring, which relies on conducting heat from subcutaneous fat across the skin and therefore, does not reach fat far from the dermis. With Dr. Anderson’s mentorship, she developed injectable cooling technology (ICT), a procedure where “ice slurry,” composed of normal saline and glycerol, is directly injected into adipose tissue, possibly leading to more efficient and effective cryolipolysis.
After nearly 10 years of animal studies at MGH, led by Dr. Garibyan as proof of concept trials, ice slurry (Coolio Therapy) recently received FDA breakthrough designation for long-term pain control and early-stage human trials of clinical applications are underway, she noted.
Magic Wand Program
In the Magic Wand program, participating physicians start by recording areas of unmet needs in their day-to-day practices, and in groups, engage in clinician-only brainstorming sessions to screen ideas, define problems, and generate lists of specifications and tools needed to address clinical problems. After working together to define challenges and possible solutions, they take their ideas to a development team, where scientists, engineers, regulatory experts, and industry professionals meet and help clinicians start pilot proof-of-concept projects, develop prototypes, and gain support for studies, followed by pilot feasibility studies.
Part of the project is the Virtual Magic Wand (VMW) Initiative, a 10-month online instructive and interactive course open to clinicians in the United States and Europe, designed to bring together dermatologists “interested in deeply understanding a dermatologic clinical problem worth solving,” according to Dr. Garibyan. Currently, there are more than 86 VMW scholars from 46 institutions, and military and private practice sites in the United States. The VMW was expanded to Europe in 2021 and there are plans to expand to Asia as well, she said.
The success of the program is not only attributed to its clinical methods but the fact that it provides a benefit to doctors at all stages of their careers, patients, and industry. “This is the only program that aims to engage in innovation from resident to full professor. We provide ideas that industry can then support and bring to market. Everyone including patients, doctors, and healthcare companies can benefit from active, engaged, and innovative physicians,” Dr. Garibyan said.
One of the success stories is that of Veradermics, a company founded by Kansas City dermatologist, Reid A. Waldman, MD, the company’s CEO, and Tim Durso, MD, the president, who met while participating in the VMW program in 2020, which eventually led them to start a company addressing an unmet need in dermatology, a kid-friendly treatment of warts.
In an interview with this news organization, Dr. Waldman explained how the program informed his company’s ethos. “Magic Wand Initiative is about identifying problems worth solving,” he said. At the company, “we find problems or unmet needs that are large enough to motivate prescribing changes, so we’ve really taken the philosophy I learned in the program into this company and building our portfolio.”
One of the first needs that Veradermics addressed was the fact that treatment for common warts, cryotherapy with liquid nitrogen, is painful and can frighten children, and, with a response rate of “at best, 50%,” Dr. Waldman said. Veradermics is in the process of creating a nearly painless, child-friendly wart treatment: an “immunostimulatory dissolvable microarray” patch that contains Candida antigen extract, which is currently being evaluated for treating warts in a phase 2 clinical trial started in 2023.
Although the Magic Wand Initiative was initially restricted to dermatologists at MGH, stories like that of Veradermics have made the program so popular that it has branched out to include anesthesiologists and otolaryngologists, as well as general and orthopedic surgeons at MGH, Dr. Garibyan said at the Mount Sinai meeting.
Dr. Garibyan disclosed that she is a cofounder of and has equity in Brixton Biosciences and EyeCool, and is a consultant for and/or investor in Brixton and Clarity Cosmetics. Royalties/inventorship are assigned to MGH.
NEW YORK – .
The program was founded in 2013 by two Harvard Medical School dermatologists, Lilit Garibyan, MD, PhD, the program director, and her mentor R. Rox Anderson MD, director of the Wellman Center for Photomedicine at Massachusetts General Hospital (MGH), Boston. It was based on the idea that clinicians are in a unique position to identify gaps in patient care and should be active in developing medical solutions to address those gaps.
“I truly believe that if we do a better job educating, training, and empowering our clinicians to become innovators, this will benefit patients and hospitals and physicians,” Dr. Garibyan said at the 26th annual Mount Sinai Winter Symposium — Advances in Medical and Surgical Dermatology.
One of the seeds for the project was her own experience with cryolipolysis which involves topical cooling, a noninvasive method of removing subcutaneous fat for body contouring, which relies on conducting heat from subcutaneous fat across the skin and therefore, does not reach fat far from the dermis. With Dr. Anderson’s mentorship, she developed injectable cooling technology (ICT), a procedure where “ice slurry,” composed of normal saline and glycerol, is directly injected into adipose tissue, possibly leading to more efficient and effective cryolipolysis.
After nearly 10 years of animal studies at MGH, led by Dr. Garibyan as proof of concept trials, ice slurry (Coolio Therapy) recently received FDA breakthrough designation for long-term pain control and early-stage human trials of clinical applications are underway, she noted.
Magic Wand Program
In the Magic Wand program, participating physicians start by recording areas of unmet needs in their day-to-day practices, and in groups, engage in clinician-only brainstorming sessions to screen ideas, define problems, and generate lists of specifications and tools needed to address clinical problems. After working together to define challenges and possible solutions, they take their ideas to a development team, where scientists, engineers, regulatory experts, and industry professionals meet and help clinicians start pilot proof-of-concept projects, develop prototypes, and gain support for studies, followed by pilot feasibility studies.
Part of the project is the Virtual Magic Wand (VMW) Initiative, a 10-month online instructive and interactive course open to clinicians in the United States and Europe, designed to bring together dermatologists “interested in deeply understanding a dermatologic clinical problem worth solving,” according to Dr. Garibyan. Currently, there are more than 86 VMW scholars from 46 institutions, and military and private practice sites in the United States. The VMW was expanded to Europe in 2021 and there are plans to expand to Asia as well, she said.
The success of the program is not only attributed to its clinical methods but the fact that it provides a benefit to doctors at all stages of their careers, patients, and industry. “This is the only program that aims to engage in innovation from resident to full professor. We provide ideas that industry can then support and bring to market. Everyone including patients, doctors, and healthcare companies can benefit from active, engaged, and innovative physicians,” Dr. Garibyan said.
One of the success stories is that of Veradermics, a company founded by Kansas City dermatologist, Reid A. Waldman, MD, the company’s CEO, and Tim Durso, MD, the president, who met while participating in the VMW program in 2020, which eventually led them to start a company addressing an unmet need in dermatology, a kid-friendly treatment of warts.
In an interview with this news organization, Dr. Waldman explained how the program informed his company’s ethos. “Magic Wand Initiative is about identifying problems worth solving,” he said. At the company, “we find problems or unmet needs that are large enough to motivate prescribing changes, so we’ve really taken the philosophy I learned in the program into this company and building our portfolio.”
One of the first needs that Veradermics addressed was the fact that treatment for common warts, cryotherapy with liquid nitrogen, is painful and can frighten children, and, with a response rate of “at best, 50%,” Dr. Waldman said. Veradermics is in the process of creating a nearly painless, child-friendly wart treatment: an “immunostimulatory dissolvable microarray” patch that contains Candida antigen extract, which is currently being evaluated for treating warts in a phase 2 clinical trial started in 2023.
Although the Magic Wand Initiative was initially restricted to dermatologists at MGH, stories like that of Veradermics have made the program so popular that it has branched out to include anesthesiologists and otolaryngologists, as well as general and orthopedic surgeons at MGH, Dr. Garibyan said at the Mount Sinai meeting.
Dr. Garibyan disclosed that she is a cofounder of and has equity in Brixton Biosciences and EyeCool, and is a consultant for and/or investor in Brixton and Clarity Cosmetics. Royalties/inventorship are assigned to MGH.